User login
Tips for helping children improve adherence to asthma treatment
NEW ORLEANS – Up to 50% of children with asthma struggle to control their condition, yet fewer than 5% of pediatric asthma is severe and truly resistant to therapy, according to Susan Laubach, MD.
Other factors may make asthma difficult to control and may be modifiable, especially nonadherence to recommended treatment. In fact, up to 70% of patients report poor adherence to recommended treatment, Dr. Laubach said at the annual meeting of the American Academy of Pediatrics.
“Barriers to adherence may be related to the treatments themselves,” she said. “These include complex treatment schedules, lack of an immediately discernible beneficial effect, adverse effects of the medication, and prohibitive costs.”
Dr. Laubach, who directs the allergy clinic at Rady Children’s Hospital in San Diego, said that clinician-related barriers also influence patient adherence to recommended treatment, including difficulty scheduling appointments or seeing the same physician, a perceived lack of empathy, or failure to discuss the family’s concerns or answer questions. Common patient-related barriers include poor understanding of how the medication may help or how to use the inhalers.
“Some families have a lack of trust in the health care system, or certain beliefs about illness or medication that may hamper motivation to adhere,” she added. “Social issues such as poverty, lack of insurance, or a chaotic home environment may make it difficult for a patient to adhere to recommended treatment.”
In 2013, researchers led by Ted Klok, MD, PhD, of Princess Amalia Children’s Clinic in the Netherlands, explored practical ways to improve treatment adherence in children with pediatric respiratory disease (Breathe. 2013;9:268-77). One of their recommendations involves “five E’s” of ensuring optimal adherence. They include:
Ensure close and repeated follow-up to help build trust and partnership. “I’ll often follow up every month until I know a patient has gained good control of his or her asthma,” said Dr. Laubach, who was not involved in developing the recommendations. “Then I’ll follow up every 3 months.”
Explore the patient’s views, beliefs, and preferences. “You can do this by inviting questions or following up on comments or remarks made about the treatment plan,” she said. “This doesn’t have to take long. You can simply ask, ‘What are you concerned might happen if your child uses an inhaled corticosteroid?’ Or, ‘What have you heard about inhaled steroids?’ ”
Express empathy using active listening techniques tailored to the patient’s needs. Consider phrasing like, “I understand what you’re saying. In a perfect world, your child would not have to use any medications. But when he can’t sleep because he’s coughing so much, the benefit of this medication probably outweighs any potential risks.”
Exercise shared decision making. For example, if the parent of one of your patients has to leave for work very early in the morning, “maybe find a way to adjust to once-daily dosing so that appropriate doses can be given at bedtime when the parent is consistently available,” Dr. Laubach said.
Evaluate adherence in a nonjudgmental fashion. Evidence suggests that most patients with asthma miss a couple of medication doses now and then. She makes it a point to ask patients, “If you’re supposed to take 14 doses a week, how many do you think you actually take?” Their response “gives me an idea about their level of adherence and it opens a discussion into why they may miss doses, so that we can find a solution to help improve adherence.”
The Childhood Asthma Management Program (CAMP) study found a significant reduction in height velocity in patients treated with budesonide, compared with placebo (N Engl J Med. 2012;367[10]:904-12). “However, most of this reduction occurred in the first year of treatment, was not additive over time, and led in average to a 1-cm difference in height as an adult,” said Dr. Laubach, who is also of the department of pediatrics at the University of California, San Diego. “So while it must be acknowledged that high-dose inhaled corticosteroids may affect growth, who do we put on inhaled corticosteroids? People who can’t breathe.”
Studies have demonstrated that the regular use of inhaled corticosteroids is associated with a decreased risk of death from asthma (N Engl J Med. 2000;343:332-6). “I suspect that most parents would trade 1 cm of height to reduce the risk of death in their child,” Dr. Laubach said.
She reported having no financial disclosures.
NEW ORLEANS – Up to 50% of children with asthma struggle to control their condition, yet fewer than 5% of pediatric asthma is severe and truly resistant to therapy, according to Susan Laubach, MD.
Other factors may make asthma difficult to control and may be modifiable, especially nonadherence to recommended treatment. In fact, up to 70% of patients report poor adherence to recommended treatment, Dr. Laubach said at the annual meeting of the American Academy of Pediatrics.
“Barriers to adherence may be related to the treatments themselves,” she said. “These include complex treatment schedules, lack of an immediately discernible beneficial effect, adverse effects of the medication, and prohibitive costs.”
Dr. Laubach, who directs the allergy clinic at Rady Children’s Hospital in San Diego, said that clinician-related barriers also influence patient adherence to recommended treatment, including difficulty scheduling appointments or seeing the same physician, a perceived lack of empathy, or failure to discuss the family’s concerns or answer questions. Common patient-related barriers include poor understanding of how the medication may help or how to use the inhalers.
“Some families have a lack of trust in the health care system, or certain beliefs about illness or medication that may hamper motivation to adhere,” she added. “Social issues such as poverty, lack of insurance, or a chaotic home environment may make it difficult for a patient to adhere to recommended treatment.”
In 2013, researchers led by Ted Klok, MD, PhD, of Princess Amalia Children’s Clinic in the Netherlands, explored practical ways to improve treatment adherence in children with pediatric respiratory disease (Breathe. 2013;9:268-77). One of their recommendations involves “five E’s” of ensuring optimal adherence. They include:
Ensure close and repeated follow-up to help build trust and partnership. “I’ll often follow up every month until I know a patient has gained good control of his or her asthma,” said Dr. Laubach, who was not involved in developing the recommendations. “Then I’ll follow up every 3 months.”
Explore the patient’s views, beliefs, and preferences. “You can do this by inviting questions or following up on comments or remarks made about the treatment plan,” she said. “This doesn’t have to take long. You can simply ask, ‘What are you concerned might happen if your child uses an inhaled corticosteroid?’ Or, ‘What have you heard about inhaled steroids?’ ”
Express empathy using active listening techniques tailored to the patient’s needs. Consider phrasing like, “I understand what you’re saying. In a perfect world, your child would not have to use any medications. But when he can’t sleep because he’s coughing so much, the benefit of this medication probably outweighs any potential risks.”
Exercise shared decision making. For example, if the parent of one of your patients has to leave for work very early in the morning, “maybe find a way to adjust to once-daily dosing so that appropriate doses can be given at bedtime when the parent is consistently available,” Dr. Laubach said.
Evaluate adherence in a nonjudgmental fashion. Evidence suggests that most patients with asthma miss a couple of medication doses now and then. She makes it a point to ask patients, “If you’re supposed to take 14 doses a week, how many do you think you actually take?” Their response “gives me an idea about their level of adherence and it opens a discussion into why they may miss doses, so that we can find a solution to help improve adherence.”
The Childhood Asthma Management Program (CAMP) study found a significant reduction in height velocity in patients treated with budesonide, compared with placebo (N Engl J Med. 2012;367[10]:904-12). “However, most of this reduction occurred in the first year of treatment, was not additive over time, and led in average to a 1-cm difference in height as an adult,” said Dr. Laubach, who is also of the department of pediatrics at the University of California, San Diego. “So while it must be acknowledged that high-dose inhaled corticosteroids may affect growth, who do we put on inhaled corticosteroids? People who can’t breathe.”
Studies have demonstrated that the regular use of inhaled corticosteroids is associated with a decreased risk of death from asthma (N Engl J Med. 2000;343:332-6). “I suspect that most parents would trade 1 cm of height to reduce the risk of death in their child,” Dr. Laubach said.
She reported having no financial disclosures.
NEW ORLEANS – Up to 50% of children with asthma struggle to control their condition, yet fewer than 5% of pediatric asthma is severe and truly resistant to therapy, according to Susan Laubach, MD.
Other factors may make asthma difficult to control and may be modifiable, especially nonadherence to recommended treatment. In fact, up to 70% of patients report poor adherence to recommended treatment, Dr. Laubach said at the annual meeting of the American Academy of Pediatrics.
“Barriers to adherence may be related to the treatments themselves,” she said. “These include complex treatment schedules, lack of an immediately discernible beneficial effect, adverse effects of the medication, and prohibitive costs.”
Dr. Laubach, who directs the allergy clinic at Rady Children’s Hospital in San Diego, said that clinician-related barriers also influence patient adherence to recommended treatment, including difficulty scheduling appointments or seeing the same physician, a perceived lack of empathy, or failure to discuss the family’s concerns or answer questions. Common patient-related barriers include poor understanding of how the medication may help or how to use the inhalers.
“Some families have a lack of trust in the health care system, or certain beliefs about illness or medication that may hamper motivation to adhere,” she added. “Social issues such as poverty, lack of insurance, or a chaotic home environment may make it difficult for a patient to adhere to recommended treatment.”
In 2013, researchers led by Ted Klok, MD, PhD, of Princess Amalia Children’s Clinic in the Netherlands, explored practical ways to improve treatment adherence in children with pediatric respiratory disease (Breathe. 2013;9:268-77). One of their recommendations involves “five E’s” of ensuring optimal adherence. They include:
Ensure close and repeated follow-up to help build trust and partnership. “I’ll often follow up every month until I know a patient has gained good control of his or her asthma,” said Dr. Laubach, who was not involved in developing the recommendations. “Then I’ll follow up every 3 months.”
Explore the patient’s views, beliefs, and preferences. “You can do this by inviting questions or following up on comments or remarks made about the treatment plan,” she said. “This doesn’t have to take long. You can simply ask, ‘What are you concerned might happen if your child uses an inhaled corticosteroid?’ Or, ‘What have you heard about inhaled steroids?’ ”
Express empathy using active listening techniques tailored to the patient’s needs. Consider phrasing like, “I understand what you’re saying. In a perfect world, your child would not have to use any medications. But when he can’t sleep because he’s coughing so much, the benefit of this medication probably outweighs any potential risks.”
Exercise shared decision making. For example, if the parent of one of your patients has to leave for work very early in the morning, “maybe find a way to adjust to once-daily dosing so that appropriate doses can be given at bedtime when the parent is consistently available,” Dr. Laubach said.
Evaluate adherence in a nonjudgmental fashion. Evidence suggests that most patients with asthma miss a couple of medication doses now and then. She makes it a point to ask patients, “If you’re supposed to take 14 doses a week, how many do you think you actually take?” Their response “gives me an idea about their level of adherence and it opens a discussion into why they may miss doses, so that we can find a solution to help improve adherence.”
The Childhood Asthma Management Program (CAMP) study found a significant reduction in height velocity in patients treated with budesonide, compared with placebo (N Engl J Med. 2012;367[10]:904-12). “However, most of this reduction occurred in the first year of treatment, was not additive over time, and led in average to a 1-cm difference in height as an adult,” said Dr. Laubach, who is also of the department of pediatrics at the University of California, San Diego. “So while it must be acknowledged that high-dose inhaled corticosteroids may affect growth, who do we put on inhaled corticosteroids? People who can’t breathe.”
Studies have demonstrated that the regular use of inhaled corticosteroids is associated with a decreased risk of death from asthma (N Engl J Med. 2000;343:332-6). “I suspect that most parents would trade 1 cm of height to reduce the risk of death in their child,” Dr. Laubach said.
She reported having no financial disclosures.
EXPERT ANALYSIS FROM AAP 2019
Omalizumab results for asthma varied with fixed airflow obstruction, reversibility
NEW ORLEANS – A new analysis suggests However, exacerbation reductions were greatest in patients with high reversibility, and omalizumab only improved lung function significantly in FAO-negative patients with high reversibility.
Nicola Hanania, MD, of Baylor College of Medicine, Houston, presented these findings at the annual meeting of the American College of Chest Physicians.
The findings are from a post hoc analysis of the phase 3 EXTRA study (NCT00314574). This 48-week study enrolled patients who had inadequately controlled, severe asthma despite receiving high-dose inhaled corticosteroids and long-acting beta-agonists.
The patients were randomized to receive omalizumab (n = 427) or placebo (n = 421). Baseline characteristics were similar between the treatment arms.
FAO presence was defined as a postbronchodilator FEV1/FVC (forced expiratory volume in 1 second/forced vital capacity) ratio less than 70%. High reversibility was defined as an increase in FEV1 of 12% or greater after albuterol administration.Omalizumab reduced exacerbations regardless of FAO, but the exacerbation relative rate reductions were greatest in FAO-positive and -negative subgroups with high reversibility.
The exacerbation relative rate reductions with omalizumab versus placebo were as follows:
- 24.8% in the overall population.
- 6.0% in FAO-positive patients with low reversibility.
- 59.8% in FAO-positive patients with high reversibility.
- 17.4% in FAO-negative patients with low reversibility.
- 44.3% in FAO-negative patients with high reversibility.
“So bronchodilator reversibility at baseline was … a correlate of more significant exacerbation reduction than … low reversibility,” Dr. Hanania said. “But the fixed airflow obstruction, whether it was present or not, did not really matter.”
As for lung function improvement, omalizumab conferred a marginal benefit for the overall population, but the improvement was “much more significant” in the FAO-negative patients with high reversibility, according to Dr. Hanania.
At week 48, the least-square mean treatment difference (omalizumab vs. placebo) for absolute FEV1 change from baseline was:
- 68 mL in the overall population.
- 17 mL in FAO-positive patients with low reversibility.
- 2 mL in FAO-positive patients with high reversibility.
- 34 mL in FAO-negative patients with low reversibility.
- 104 mL in FAO-negative patients with high reversibility.
“As lung function improvement by omalizumab appeared to be driven by reversibility, asthma with lower reversibility and fixed airflow obstruction may represent a different phenotype,” Dr. Hanania said. “I think this needs to be looked at.”
This research was funded by Genentech and Novartis. Dr. Hanania disclosed relationships with Genentech, Novartis, AstraZeneca, Boehringer Ingelheim, GSK, Regeneron, and Sanofi.
SOURCE: Hanania N et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.869.
NEW ORLEANS – A new analysis suggests However, exacerbation reductions were greatest in patients with high reversibility, and omalizumab only improved lung function significantly in FAO-negative patients with high reversibility.
Nicola Hanania, MD, of Baylor College of Medicine, Houston, presented these findings at the annual meeting of the American College of Chest Physicians.
The findings are from a post hoc analysis of the phase 3 EXTRA study (NCT00314574). This 48-week study enrolled patients who had inadequately controlled, severe asthma despite receiving high-dose inhaled corticosteroids and long-acting beta-agonists.
The patients were randomized to receive omalizumab (n = 427) or placebo (n = 421). Baseline characteristics were similar between the treatment arms.
FAO presence was defined as a postbronchodilator FEV1/FVC (forced expiratory volume in 1 second/forced vital capacity) ratio less than 70%. High reversibility was defined as an increase in FEV1 of 12% or greater after albuterol administration.Omalizumab reduced exacerbations regardless of FAO, but the exacerbation relative rate reductions were greatest in FAO-positive and -negative subgroups with high reversibility.
The exacerbation relative rate reductions with omalizumab versus placebo were as follows:
- 24.8% in the overall population.
- 6.0% in FAO-positive patients with low reversibility.
- 59.8% in FAO-positive patients with high reversibility.
- 17.4% in FAO-negative patients with low reversibility.
- 44.3% in FAO-negative patients with high reversibility.
“So bronchodilator reversibility at baseline was … a correlate of more significant exacerbation reduction than … low reversibility,” Dr. Hanania said. “But the fixed airflow obstruction, whether it was present or not, did not really matter.”
As for lung function improvement, omalizumab conferred a marginal benefit for the overall population, but the improvement was “much more significant” in the FAO-negative patients with high reversibility, according to Dr. Hanania.
At week 48, the least-square mean treatment difference (omalizumab vs. placebo) for absolute FEV1 change from baseline was:
- 68 mL in the overall population.
- 17 mL in FAO-positive patients with low reversibility.
- 2 mL in FAO-positive patients with high reversibility.
- 34 mL in FAO-negative patients with low reversibility.
- 104 mL in FAO-negative patients with high reversibility.
“As lung function improvement by omalizumab appeared to be driven by reversibility, asthma with lower reversibility and fixed airflow obstruction may represent a different phenotype,” Dr. Hanania said. “I think this needs to be looked at.”
This research was funded by Genentech and Novartis. Dr. Hanania disclosed relationships with Genentech, Novartis, AstraZeneca, Boehringer Ingelheim, GSK, Regeneron, and Sanofi.
SOURCE: Hanania N et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.869.
NEW ORLEANS – A new analysis suggests However, exacerbation reductions were greatest in patients with high reversibility, and omalizumab only improved lung function significantly in FAO-negative patients with high reversibility.
Nicola Hanania, MD, of Baylor College of Medicine, Houston, presented these findings at the annual meeting of the American College of Chest Physicians.
The findings are from a post hoc analysis of the phase 3 EXTRA study (NCT00314574). This 48-week study enrolled patients who had inadequately controlled, severe asthma despite receiving high-dose inhaled corticosteroids and long-acting beta-agonists.
The patients were randomized to receive omalizumab (n = 427) or placebo (n = 421). Baseline characteristics were similar between the treatment arms.
FAO presence was defined as a postbronchodilator FEV1/FVC (forced expiratory volume in 1 second/forced vital capacity) ratio less than 70%. High reversibility was defined as an increase in FEV1 of 12% or greater after albuterol administration.Omalizumab reduced exacerbations regardless of FAO, but the exacerbation relative rate reductions were greatest in FAO-positive and -negative subgroups with high reversibility.
The exacerbation relative rate reductions with omalizumab versus placebo were as follows:
- 24.8% in the overall population.
- 6.0% in FAO-positive patients with low reversibility.
- 59.8% in FAO-positive patients with high reversibility.
- 17.4% in FAO-negative patients with low reversibility.
- 44.3% in FAO-negative patients with high reversibility.
“So bronchodilator reversibility at baseline was … a correlate of more significant exacerbation reduction than … low reversibility,” Dr. Hanania said. “But the fixed airflow obstruction, whether it was present or not, did not really matter.”
As for lung function improvement, omalizumab conferred a marginal benefit for the overall population, but the improvement was “much more significant” in the FAO-negative patients with high reversibility, according to Dr. Hanania.
At week 48, the least-square mean treatment difference (omalizumab vs. placebo) for absolute FEV1 change from baseline was:
- 68 mL in the overall population.
- 17 mL in FAO-positive patients with low reversibility.
- 2 mL in FAO-positive patients with high reversibility.
- 34 mL in FAO-negative patients with low reversibility.
- 104 mL in FAO-negative patients with high reversibility.
“As lung function improvement by omalizumab appeared to be driven by reversibility, asthma with lower reversibility and fixed airflow obstruction may represent a different phenotype,” Dr. Hanania said. “I think this needs to be looked at.”
This research was funded by Genentech and Novartis. Dr. Hanania disclosed relationships with Genentech, Novartis, AstraZeneca, Boehringer Ingelheim, GSK, Regeneron, and Sanofi.
SOURCE: Hanania N et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.869.
REPORTING FROM CHEST 2019
Digital inhaler reveals uncontrolled asthma
NEW ORLEANS – Data collected by the ProAir Digihaler suggest .
Researchers studied asthma patients who had experienced exacerbations in the previous year. Patients who also had exacerbations while on study used the ProAir Digihaler about twice a day, on average. Patients without on-study exacerbations used the ProAir Digihaler an average of 1.14 times per day.
The daily use among patients without exacerbations suggests their asthma is “still quite uncontrolled,” and, according to guidelines, they may require additional therapy, said Roy Pleasants, PharmD, of the University of North Carolina at Chapel Hill.
Dr. Pleasants presented these findings at the annual meeting of the American College of Chest Physicians.
He and his colleagues conducted a phase 3 study (NCT02969408) of ProAir Digihaler use in adults who had at least one severe clinical asthma exacerbation in the previous 12 months. They had an Asthma Control Questionnaire score of 1.5 or greater, were on moderate-dose inhaled corticosteroids (with or without a long-acting beta-agonist), and had stable asthma controller dosing for at least 3 months.
For this study, the ProAir Digihaler replaced patients’ other rescue medications. The ProAir Digihaler is a digital inhaler that delivers 90 mcg of albuterol per dose, detects the date and time a dose was prepared, and records the inhalation profile. Over a 12-week period, the ProAir Digihaler recorded each use, which was defined as consecutive inhalations within 60 seconds.
Of the 381 patients enrolled in the study, 360 (94.5%) made at least one valid inhalation. The mean age of these patients was 50 years, and 80.6% were female. Of the 360 patients, 64 experienced 78 exacerbations while on study.
Most episodes of inhaler use consisted of a single inhalation (58.9%), although 35.8% consisted of two inhalations, 3.5% consisted of three inhalations, and 1.8% consisted of four or more inhalations.
The mean peak inspiratory flow was 73.18 L/min (standard deviation [SD] 20.33) in patients without exacerbations. Among patients with exacerbations, the mean peak inspiratory flow was 71.36 (SD 23.80) during exacerbation and 74.71 L/min (SD 22.46) outside the exacerbation window, which was 14 days before and after the exacerbation peak.
The mean inhalation volume was 1.45 L (SD 0.75) among patients without exacerbations, 1.44 L (SD 0.66) outside the exacerbation window, and 1.44 L (SD 0.76) during exacerbation. The mean inhalation duration was 1.62 sec (SD 0.88), 1.59 sec (SD 0.77), and 1.61 sec (SD 0.82), respectively.
“If you look at the inhalation volume in the 64 patients who exacerbated, it really didn’t change during exacerbation,” Dr. Pleasants noted. “Essentially, you can say the same thing about inhalation duration.”
Patients without exacerbations used the ProAir Digihaler an average of 1.14 (SD 2.35) times per day. Patients who had at least one exacerbation used the ProAir Digihaler an average of 1.87 (SD 2.78) times per day outside the exacerbation window and 2.43 (SD 3.67) times during exacerbation.
“As you would predict, those exacerbating patients were using more albuterol than patients who were not exacerbating,” Dr. Pleasants said. “Even when they weren’t exacerbating, that frequency of daily albuterol use is pretty much indicating these patients were not so well controlled.”
Dr. Pleasants went on to say that data from the ProAir Digihaler could help identify, in real time, patients with poor asthma control and those with impending exacerbations.
This study was sponsored by Teva, makers of the ProAir Digihaler. Dr. Pleasants disclosed relationships with Teva, Grifols, Sunovion, and Boehringer Ingelheim.
SOURCE: Pleasants R et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.273.
NEW ORLEANS – Data collected by the ProAir Digihaler suggest .
Researchers studied asthma patients who had experienced exacerbations in the previous year. Patients who also had exacerbations while on study used the ProAir Digihaler about twice a day, on average. Patients without on-study exacerbations used the ProAir Digihaler an average of 1.14 times per day.
The daily use among patients without exacerbations suggests their asthma is “still quite uncontrolled,” and, according to guidelines, they may require additional therapy, said Roy Pleasants, PharmD, of the University of North Carolina at Chapel Hill.
Dr. Pleasants presented these findings at the annual meeting of the American College of Chest Physicians.
He and his colleagues conducted a phase 3 study (NCT02969408) of ProAir Digihaler use in adults who had at least one severe clinical asthma exacerbation in the previous 12 months. They had an Asthma Control Questionnaire score of 1.5 or greater, were on moderate-dose inhaled corticosteroids (with or without a long-acting beta-agonist), and had stable asthma controller dosing for at least 3 months.
For this study, the ProAir Digihaler replaced patients’ other rescue medications. The ProAir Digihaler is a digital inhaler that delivers 90 mcg of albuterol per dose, detects the date and time a dose was prepared, and records the inhalation profile. Over a 12-week period, the ProAir Digihaler recorded each use, which was defined as consecutive inhalations within 60 seconds.
Of the 381 patients enrolled in the study, 360 (94.5%) made at least one valid inhalation. The mean age of these patients was 50 years, and 80.6% were female. Of the 360 patients, 64 experienced 78 exacerbations while on study.
Most episodes of inhaler use consisted of a single inhalation (58.9%), although 35.8% consisted of two inhalations, 3.5% consisted of three inhalations, and 1.8% consisted of four or more inhalations.
The mean peak inspiratory flow was 73.18 L/min (standard deviation [SD] 20.33) in patients without exacerbations. Among patients with exacerbations, the mean peak inspiratory flow was 71.36 (SD 23.80) during exacerbation and 74.71 L/min (SD 22.46) outside the exacerbation window, which was 14 days before and after the exacerbation peak.
The mean inhalation volume was 1.45 L (SD 0.75) among patients without exacerbations, 1.44 L (SD 0.66) outside the exacerbation window, and 1.44 L (SD 0.76) during exacerbation. The mean inhalation duration was 1.62 sec (SD 0.88), 1.59 sec (SD 0.77), and 1.61 sec (SD 0.82), respectively.
“If you look at the inhalation volume in the 64 patients who exacerbated, it really didn’t change during exacerbation,” Dr. Pleasants noted. “Essentially, you can say the same thing about inhalation duration.”
Patients without exacerbations used the ProAir Digihaler an average of 1.14 (SD 2.35) times per day. Patients who had at least one exacerbation used the ProAir Digihaler an average of 1.87 (SD 2.78) times per day outside the exacerbation window and 2.43 (SD 3.67) times during exacerbation.
“As you would predict, those exacerbating patients were using more albuterol than patients who were not exacerbating,” Dr. Pleasants said. “Even when they weren’t exacerbating, that frequency of daily albuterol use is pretty much indicating these patients were not so well controlled.”
Dr. Pleasants went on to say that data from the ProAir Digihaler could help identify, in real time, patients with poor asthma control and those with impending exacerbations.
This study was sponsored by Teva, makers of the ProAir Digihaler. Dr. Pleasants disclosed relationships with Teva, Grifols, Sunovion, and Boehringer Ingelheim.
SOURCE: Pleasants R et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.273.
NEW ORLEANS – Data collected by the ProAir Digihaler suggest .
Researchers studied asthma patients who had experienced exacerbations in the previous year. Patients who also had exacerbations while on study used the ProAir Digihaler about twice a day, on average. Patients without on-study exacerbations used the ProAir Digihaler an average of 1.14 times per day.
The daily use among patients without exacerbations suggests their asthma is “still quite uncontrolled,” and, according to guidelines, they may require additional therapy, said Roy Pleasants, PharmD, of the University of North Carolina at Chapel Hill.
Dr. Pleasants presented these findings at the annual meeting of the American College of Chest Physicians.
He and his colleagues conducted a phase 3 study (NCT02969408) of ProAir Digihaler use in adults who had at least one severe clinical asthma exacerbation in the previous 12 months. They had an Asthma Control Questionnaire score of 1.5 or greater, were on moderate-dose inhaled corticosteroids (with or without a long-acting beta-agonist), and had stable asthma controller dosing for at least 3 months.
For this study, the ProAir Digihaler replaced patients’ other rescue medications. The ProAir Digihaler is a digital inhaler that delivers 90 mcg of albuterol per dose, detects the date and time a dose was prepared, and records the inhalation profile. Over a 12-week period, the ProAir Digihaler recorded each use, which was defined as consecutive inhalations within 60 seconds.
Of the 381 patients enrolled in the study, 360 (94.5%) made at least one valid inhalation. The mean age of these patients was 50 years, and 80.6% were female. Of the 360 patients, 64 experienced 78 exacerbations while on study.
Most episodes of inhaler use consisted of a single inhalation (58.9%), although 35.8% consisted of two inhalations, 3.5% consisted of three inhalations, and 1.8% consisted of four or more inhalations.
The mean peak inspiratory flow was 73.18 L/min (standard deviation [SD] 20.33) in patients without exacerbations. Among patients with exacerbations, the mean peak inspiratory flow was 71.36 (SD 23.80) during exacerbation and 74.71 L/min (SD 22.46) outside the exacerbation window, which was 14 days before and after the exacerbation peak.
The mean inhalation volume was 1.45 L (SD 0.75) among patients without exacerbations, 1.44 L (SD 0.66) outside the exacerbation window, and 1.44 L (SD 0.76) during exacerbation. The mean inhalation duration was 1.62 sec (SD 0.88), 1.59 sec (SD 0.77), and 1.61 sec (SD 0.82), respectively.
“If you look at the inhalation volume in the 64 patients who exacerbated, it really didn’t change during exacerbation,” Dr. Pleasants noted. “Essentially, you can say the same thing about inhalation duration.”
Patients without exacerbations used the ProAir Digihaler an average of 1.14 (SD 2.35) times per day. Patients who had at least one exacerbation used the ProAir Digihaler an average of 1.87 (SD 2.78) times per day outside the exacerbation window and 2.43 (SD 3.67) times during exacerbation.
“As you would predict, those exacerbating patients were using more albuterol than patients who were not exacerbating,” Dr. Pleasants said. “Even when they weren’t exacerbating, that frequency of daily albuterol use is pretty much indicating these patients were not so well controlled.”
Dr. Pleasants went on to say that data from the ProAir Digihaler could help identify, in real time, patients with poor asthma control and those with impending exacerbations.
This study was sponsored by Teva, makers of the ProAir Digihaler. Dr. Pleasants disclosed relationships with Teva, Grifols, Sunovion, and Boehringer Ingelheim.
SOURCE: Pleasants R et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.273.
REPORTING FROM CHEST 2019
Study suggests physicians and patients overestimate asthma control
NEW ORLEANS – according to an observational study.
More than half (53%) of cases physicians rated as controlled were actually uncontrolled according to the Asthma Control Test (ACT), and 30% of patients who considered their asthma controlled actually had uncontrolled asthma according to the ACT.
Reynold A. Panettieri Jr., MD, of Rutgers University in New Brunswick, N.J., presented these findings at the annual meeting of the American College of Chest Physicians.
The findings are from the CHRONICLE study, an ongoing observational study of adults with severe asthma who are being treated by U.S. allergists or pulmonologists. The study enrolled 796 patients from Feb. 2018 to Feb. 2019, and 482 of them were evaluable because they completed the necessary surveys.
The patients’ median age at enrollment was 55 years, and 68% of patients were female. Most were white (82%), 12% were black, 6% were an “other” race, and 7% had Hispanic ethnicity. The median body mass index was 31 kg/m2.
Patients received care from an allergist (49%), a pulmonologist (38%), or both (13%). Patients were treated with biologics (n = 370), maintenance systemic corticosteroids (n = 64), or high-dosage inhaled corticosteroids with additional controllers (n = 90).
At patient enrollment, physicians reported their assessment of patients’ asthma control and completed the 5-point Global Evaluation of Treatment Effectiveness (GETE). The physicians’ assessments of patients were informed by patients’ verbal reports (50%), lung function testing (44%), in-office ACT (41%), and recent exacerbations (39%).
Patients also completed the ACT and GETE online at the time of enrollment. Neither patients nor physicians were privy to the other group’s responses.
Overall, physicians said 279 patients had controlled asthma. However, according to the ACT, 27% of these cases were very poorly controlled, 26% were not well controlled, and 47% were well controlled.
“So [when] we as a provider say the patient’s controlled, we’re wrong half the time,” Dr. Panettieri said.
However, physicians were more accurate when deeming patients’ asthma uncontrolled. Physicians said 201 cases of asthma were uncontrolled, and the ACT said 64% of these cases were very poorly controlled, 22% were not well controlled, and 13% were well controlled.
Compared with the physicians’ results, the patients’ reports were more in line with ACT results. However, the patients still overestimated control.
In all, 222 patients said their asthma was controlled. According to the ACT, 70% of these cases were well controlled, 23% were not well controlled, and 7% were very poorly controlled.
Patients were even more accurate when deeming their asthma uncontrolled. A total of 258 patients said their asthma was uncontrolled. According to the ACT, 74% of these cases were very poorly controlled, 25% were not well controlled, and 1% were well controlled.
“About 99% of the time, when a patient tells you they’re uncontrolled, they’re uncontrolled by the ACT,” Dr. Panettieri said.
Though patients were fairly accurate when assessing asthma control, they were less accurate when gauging treatment effectiveness. A majority of patients overestimated the effectiveness of treatment.
There were 124 patients who did not have any improvement after treatment, according to physicians. Although 23% of the patients concurred with this assessment, 77% said they did experience some improvement.
On the other hand, there were 355 patients who had some improvement after treatment according to physicians, and most of these patients (96%) agreed that they had some improvement.
Dr. Panettieri said these results support use of the ACT and similar tools. When using these tools isn’t feasible, Dr. Panettieri recommends simply asking patients how they are feeling. However, he said, providers should not rely on a patient’s report of treatment effectiveness to assess asthma control.
This study is supported by AstraZeneca. Dr. Panettieri disclosed relationships with AstraZeneca, Sanofi, Regeneron, Genentech, and Novartis.
SOURCE: Panettieri R et al. CHEST 2019. Abstract, doi. 10.1016/j.chest.2019.08.272.
NEW ORLEANS – according to an observational study.
More than half (53%) of cases physicians rated as controlled were actually uncontrolled according to the Asthma Control Test (ACT), and 30% of patients who considered their asthma controlled actually had uncontrolled asthma according to the ACT.
Reynold A. Panettieri Jr., MD, of Rutgers University in New Brunswick, N.J., presented these findings at the annual meeting of the American College of Chest Physicians.
The findings are from the CHRONICLE study, an ongoing observational study of adults with severe asthma who are being treated by U.S. allergists or pulmonologists. The study enrolled 796 patients from Feb. 2018 to Feb. 2019, and 482 of them were evaluable because they completed the necessary surveys.
The patients’ median age at enrollment was 55 years, and 68% of patients were female. Most were white (82%), 12% were black, 6% were an “other” race, and 7% had Hispanic ethnicity. The median body mass index was 31 kg/m2.
Patients received care from an allergist (49%), a pulmonologist (38%), or both (13%). Patients were treated with biologics (n = 370), maintenance systemic corticosteroids (n = 64), or high-dosage inhaled corticosteroids with additional controllers (n = 90).
At patient enrollment, physicians reported their assessment of patients’ asthma control and completed the 5-point Global Evaluation of Treatment Effectiveness (GETE). The physicians’ assessments of patients were informed by patients’ verbal reports (50%), lung function testing (44%), in-office ACT (41%), and recent exacerbations (39%).
Patients also completed the ACT and GETE online at the time of enrollment. Neither patients nor physicians were privy to the other group’s responses.
Overall, physicians said 279 patients had controlled asthma. However, according to the ACT, 27% of these cases were very poorly controlled, 26% were not well controlled, and 47% were well controlled.
“So [when] we as a provider say the patient’s controlled, we’re wrong half the time,” Dr. Panettieri said.
However, physicians were more accurate when deeming patients’ asthma uncontrolled. Physicians said 201 cases of asthma were uncontrolled, and the ACT said 64% of these cases were very poorly controlled, 22% were not well controlled, and 13% were well controlled.
Compared with the physicians’ results, the patients’ reports were more in line with ACT results. However, the patients still overestimated control.
In all, 222 patients said their asthma was controlled. According to the ACT, 70% of these cases were well controlled, 23% were not well controlled, and 7% were very poorly controlled.
Patients were even more accurate when deeming their asthma uncontrolled. A total of 258 patients said their asthma was uncontrolled. According to the ACT, 74% of these cases were very poorly controlled, 25% were not well controlled, and 1% were well controlled.
“About 99% of the time, when a patient tells you they’re uncontrolled, they’re uncontrolled by the ACT,” Dr. Panettieri said.
Though patients were fairly accurate when assessing asthma control, they were less accurate when gauging treatment effectiveness. A majority of patients overestimated the effectiveness of treatment.
There were 124 patients who did not have any improvement after treatment, according to physicians. Although 23% of the patients concurred with this assessment, 77% said they did experience some improvement.
On the other hand, there were 355 patients who had some improvement after treatment according to physicians, and most of these patients (96%) agreed that they had some improvement.
Dr. Panettieri said these results support use of the ACT and similar tools. When using these tools isn’t feasible, Dr. Panettieri recommends simply asking patients how they are feeling. However, he said, providers should not rely on a patient’s report of treatment effectiveness to assess asthma control.
This study is supported by AstraZeneca. Dr. Panettieri disclosed relationships with AstraZeneca, Sanofi, Regeneron, Genentech, and Novartis.
SOURCE: Panettieri R et al. CHEST 2019. Abstract, doi. 10.1016/j.chest.2019.08.272.
NEW ORLEANS – according to an observational study.
More than half (53%) of cases physicians rated as controlled were actually uncontrolled according to the Asthma Control Test (ACT), and 30% of patients who considered their asthma controlled actually had uncontrolled asthma according to the ACT.
Reynold A. Panettieri Jr., MD, of Rutgers University in New Brunswick, N.J., presented these findings at the annual meeting of the American College of Chest Physicians.
The findings are from the CHRONICLE study, an ongoing observational study of adults with severe asthma who are being treated by U.S. allergists or pulmonologists. The study enrolled 796 patients from Feb. 2018 to Feb. 2019, and 482 of them were evaluable because they completed the necessary surveys.
The patients’ median age at enrollment was 55 years, and 68% of patients were female. Most were white (82%), 12% were black, 6% were an “other” race, and 7% had Hispanic ethnicity. The median body mass index was 31 kg/m2.
Patients received care from an allergist (49%), a pulmonologist (38%), or both (13%). Patients were treated with biologics (n = 370), maintenance systemic corticosteroids (n = 64), or high-dosage inhaled corticosteroids with additional controllers (n = 90).
At patient enrollment, physicians reported their assessment of patients’ asthma control and completed the 5-point Global Evaluation of Treatment Effectiveness (GETE). The physicians’ assessments of patients were informed by patients’ verbal reports (50%), lung function testing (44%), in-office ACT (41%), and recent exacerbations (39%).
Patients also completed the ACT and GETE online at the time of enrollment. Neither patients nor physicians were privy to the other group’s responses.
Overall, physicians said 279 patients had controlled asthma. However, according to the ACT, 27% of these cases were very poorly controlled, 26% were not well controlled, and 47% were well controlled.
“So [when] we as a provider say the patient’s controlled, we’re wrong half the time,” Dr. Panettieri said.
However, physicians were more accurate when deeming patients’ asthma uncontrolled. Physicians said 201 cases of asthma were uncontrolled, and the ACT said 64% of these cases were very poorly controlled, 22% were not well controlled, and 13% were well controlled.
Compared with the physicians’ results, the patients’ reports were more in line with ACT results. However, the patients still overestimated control.
In all, 222 patients said their asthma was controlled. According to the ACT, 70% of these cases were well controlled, 23% were not well controlled, and 7% were very poorly controlled.
Patients were even more accurate when deeming their asthma uncontrolled. A total of 258 patients said their asthma was uncontrolled. According to the ACT, 74% of these cases were very poorly controlled, 25% were not well controlled, and 1% were well controlled.
“About 99% of the time, when a patient tells you they’re uncontrolled, they’re uncontrolled by the ACT,” Dr. Panettieri said.
Though patients were fairly accurate when assessing asthma control, they were less accurate when gauging treatment effectiveness. A majority of patients overestimated the effectiveness of treatment.
There were 124 patients who did not have any improvement after treatment, according to physicians. Although 23% of the patients concurred with this assessment, 77% said they did experience some improvement.
On the other hand, there were 355 patients who had some improvement after treatment according to physicians, and most of these patients (96%) agreed that they had some improvement.
Dr. Panettieri said these results support use of the ACT and similar tools. When using these tools isn’t feasible, Dr. Panettieri recommends simply asking patients how they are feeling. However, he said, providers should not rely on a patient’s report of treatment effectiveness to assess asthma control.
This study is supported by AstraZeneca. Dr. Panettieri disclosed relationships with AstraZeneca, Sanofi, Regeneron, Genentech, and Novartis.
SOURCE: Panettieri R et al. CHEST 2019. Abstract, doi. 10.1016/j.chest.2019.08.272.
REPORTING FROM CHEST 2019
Wildfire smoke impact, part 2: Resources, advice for patients
Wildfires are on the move in California and communities from the Bay Area to Los Angeles County are once again coping with evacuation, possible destruction of homes, and health concerns related to poor air quality and smoke.
What can doctors tell their patients with cardiovascular and pulmonary conditions about the risks of smoke from wildfires?
EPA resources online
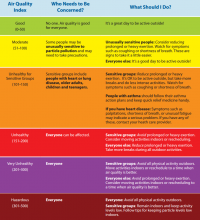
AirNow, a website managed by the Environmental Protection Agency, provides a variety of resources for the public and for health providers, including links to online tutorials, printable health fact sheets, and the newly updated document “Wildfire Smoke: Guide for Public Health Officials 2019.” When wildfire smoke generates an Air Quality Index (AQI) from 101-150, at-risk subgroups like people with asthma, chronic obstructive pulmonary disease (COPD), or heart disease should take precautions.
“An AQI of 151-200 is unhealthy for everyone, and an AQI above 200 is very unhealthy,” John R. Balmes, MD, a pulmonologist at the University of California, San Francisco, and an expert on the respiratory and cardiovascular effects of air pollutants, said in an interview. “That does not mean that everybody is going to die, though. You’re going to have some symptoms of scratchy throat, and you may cough once or twice an hour [from exposure to wildfire smoke], but people who don’t have any preexisting health problems are probably going to be fine and don’t necessarily have to wear an N95 mask. People should wear one if they need to feel comfortable.”
Masks – How much protection?
Wayne Cascio, MD, who directs the EPA’s National Health and Environmental Effects Research Laboratory, notes that some public health officials don’t recommend wearing N95 masks during wildfire smoke events. “There’s a lot of concern that people won’t use them correctly and will therefore feel like they’re protected and will spend more time outdoors than they should and still not get the benefit,” he said. “The masks also pose a challenge for children and for people with severe asthma and COPD.”
Masks also have to fit properly, which can be problematic for kids, added Dr. Balmes, one of the authors of “Wildfires Disaster Guidance: Tips for Staying Healthy During Wildfires” (Am J Respir Crit Care Med. 2019;199[2]:3-4).
“Even the small versions don’t necessarily fit kids well, so they’re not recommended for kids,” he said. “It doesn’t mean a kid couldn’t wear them, but it’s not officially recommended. The actual physiologic work of breathing isn’t much increased by using the N95 mask, but if you’re already struggling to get your breath, or experience dyspnea, then it might be hard to wear one. People with milder COPD can wear an N95 mask just like people with mild asthma if they have to go out.”
The EPA published a tip sheet about where and when to use an N95 or P100 mask, with warnings about the limited protection these devices offer, especially if not used correctly. Most masks do not protect the wearer from harmful gases that can be in wildfire smoke.
Ventilation systems
The EPA also recommends that people with more severe disease should stay indoors and avoid using air conditioning units that only draw in air from the outside or do not have a recirculating option. “If you have to bring in outside air because that’s how your system works, you should have a MERV 13 or better filter to filter out the fine particles,” Dr. Balmes said. “Not every ventilation system can handle it, but most can. That will help the house.”
Dr. Cascio pointed out that the instruction to close all windows and doors is a difficult proposition for people who live in states with moderate weather climates such as Montana and Colorado, where few homes have central air conditioning. “The treatment may be worse than the disease in this case, because it may exacerbate heat stress,” he said. “Try to find a place that has cleaner air. That might be a public building, a school, a fitness center, or a library. Yet we don’t know a lot about whether those areas are cleaner or not. That is currently the subject of some research on our part.”
Traveling away from an area affected by wildfire smoke to ride out the conditions is one option, but that can backfire. One weekend when smoke from the 2008 North Carolina peat fire was particularly troublesome, Dr. Cascio and his family drove about 60 miles west of Greenville to the town of Zebulon, where a minor league baseball game was being played and the air quality was good.
“My thought was to get the family to a better environment for at least a few hours,” Dr. Cascio recalled. “When we arrived in Zebulon the air quality was good as advertised. However, the direction of the wind shifted and the smoke started to move due west and within a short time you could barely see the players on the field. This experience also pointed out one of the lessons of wildfire smoke. That is, in the short term, it is sometimes difficult to predict where it will be present because of the nature of changes in weather and wind.”
Consumer tools to monitor air quality
Colleen E. Reid, PhD, of the department of geography at the University of Colorado, Boulder, an expert on the impact of wildfire smoke on human health, has observed in increase in consumer action to counter smoke inhalation during wildfire events. She said that consumers are buying personal laser particle counters, like the ones made by PurpleAir, to provide a real-time assessment of air quality.
“There is a lot of error with these smaller, cheaper monitors, but I think they give you a sense of trends over time,” Dr. Reid said. “People are trying to figure out how we can work with this sort of real-time data along with the high-quality EPA monitors. If everybody has their own monitor, or ways to better calibrate them to the high-quality data, that would be amazing. Researchers are trying to see how they can use that data to inform our understanding of the spatial and temporal patterning of air pollution.”
The EPA’s Smoke Sense app also holds promise. Characterized on its website as “a citizen science project,” the study uses a free mobile app to engage people living in affected communities to monitor their air quality and their cardiorespiratory symptoms. “Through engagement over time, you learn what the effects on your body are and what the expected effects are, so you can recognize the hazards and change the behavior when you’re experiencing it,” said Ana G. Rappold, PhD, who is the app’s principal investigator at the National Health and Environmental Effects Research Laboratory. One component of the app is time of last measurement of fine particulate matter and ozone based on the user’s location. Another is a module called Be Smoke Smart, which tests the user’s knowledge of wildfire smoke exposure. For example, one question is: “How likely are you to reduce your exposure on an Orange AQI alert day?” (which indicates that sensitive populations may experience health effects).
“Through gamification, they’re engaging with the issue,” Dr. Rappold said. “Then they learn about what others are reporting. In that part we also study how different messages change individuals’ perspective on how likely they are to make a change the next time they’re impacted by smoke.”
Wildfires are on the move in California and communities from the Bay Area to Los Angeles County are once again coping with evacuation, possible destruction of homes, and health concerns related to poor air quality and smoke.
What can doctors tell their patients with cardiovascular and pulmonary conditions about the risks of smoke from wildfires?
EPA resources online
AirNow, a website managed by the Environmental Protection Agency, provides a variety of resources for the public and for health providers, including links to online tutorials, printable health fact sheets, and the newly updated document “Wildfire Smoke: Guide for Public Health Officials 2019.” When wildfire smoke generates an Air Quality Index (AQI) from 101-150, at-risk subgroups like people with asthma, chronic obstructive pulmonary disease (COPD), or heart disease should take precautions.
“An AQI of 151-200 is unhealthy for everyone, and an AQI above 200 is very unhealthy,” John R. Balmes, MD, a pulmonologist at the University of California, San Francisco, and an expert on the respiratory and cardiovascular effects of air pollutants, said in an interview. “That does not mean that everybody is going to die, though. You’re going to have some symptoms of scratchy throat, and you may cough once or twice an hour [from exposure to wildfire smoke], but people who don’t have any preexisting health problems are probably going to be fine and don’t necessarily have to wear an N95 mask. People should wear one if they need to feel comfortable.”
Masks – How much protection?
Wayne Cascio, MD, who directs the EPA’s National Health and Environmental Effects Research Laboratory, notes that some public health officials don’t recommend wearing N95 masks during wildfire smoke events. “There’s a lot of concern that people won’t use them correctly and will therefore feel like they’re protected and will spend more time outdoors than they should and still not get the benefit,” he said. “The masks also pose a challenge for children and for people with severe asthma and COPD.”
Masks also have to fit properly, which can be problematic for kids, added Dr. Balmes, one of the authors of “Wildfires Disaster Guidance: Tips for Staying Healthy During Wildfires” (Am J Respir Crit Care Med. 2019;199[2]:3-4).
“Even the small versions don’t necessarily fit kids well, so they’re not recommended for kids,” he said. “It doesn’t mean a kid couldn’t wear them, but it’s not officially recommended. The actual physiologic work of breathing isn’t much increased by using the N95 mask, but if you’re already struggling to get your breath, or experience dyspnea, then it might be hard to wear one. People with milder COPD can wear an N95 mask just like people with mild asthma if they have to go out.”
The EPA published a tip sheet about where and when to use an N95 or P100 mask, with warnings about the limited protection these devices offer, especially if not used correctly. Most masks do not protect the wearer from harmful gases that can be in wildfire smoke.
Ventilation systems
The EPA also recommends that people with more severe disease should stay indoors and avoid using air conditioning units that only draw in air from the outside or do not have a recirculating option. “If you have to bring in outside air because that’s how your system works, you should have a MERV 13 or better filter to filter out the fine particles,” Dr. Balmes said. “Not every ventilation system can handle it, but most can. That will help the house.”
Dr. Cascio pointed out that the instruction to close all windows and doors is a difficult proposition for people who live in states with moderate weather climates such as Montana and Colorado, where few homes have central air conditioning. “The treatment may be worse than the disease in this case, because it may exacerbate heat stress,” he said. “Try to find a place that has cleaner air. That might be a public building, a school, a fitness center, or a library. Yet we don’t know a lot about whether those areas are cleaner or not. That is currently the subject of some research on our part.”
Traveling away from an area affected by wildfire smoke to ride out the conditions is one option, but that can backfire. One weekend when smoke from the 2008 North Carolina peat fire was particularly troublesome, Dr. Cascio and his family drove about 60 miles west of Greenville to the town of Zebulon, where a minor league baseball game was being played and the air quality was good.
“My thought was to get the family to a better environment for at least a few hours,” Dr. Cascio recalled. “When we arrived in Zebulon the air quality was good as advertised. However, the direction of the wind shifted and the smoke started to move due west and within a short time you could barely see the players on the field. This experience also pointed out one of the lessons of wildfire smoke. That is, in the short term, it is sometimes difficult to predict where it will be present because of the nature of changes in weather and wind.”
Consumer tools to monitor air quality
Colleen E. Reid, PhD, of the department of geography at the University of Colorado, Boulder, an expert on the impact of wildfire smoke on human health, has observed in increase in consumer action to counter smoke inhalation during wildfire events. She said that consumers are buying personal laser particle counters, like the ones made by PurpleAir, to provide a real-time assessment of air quality.
“There is a lot of error with these smaller, cheaper monitors, but I think they give you a sense of trends over time,” Dr. Reid said. “People are trying to figure out how we can work with this sort of real-time data along with the high-quality EPA monitors. If everybody has their own monitor, or ways to better calibrate them to the high-quality data, that would be amazing. Researchers are trying to see how they can use that data to inform our understanding of the spatial and temporal patterning of air pollution.”
The EPA’s Smoke Sense app also holds promise. Characterized on its website as “a citizen science project,” the study uses a free mobile app to engage people living in affected communities to monitor their air quality and their cardiorespiratory symptoms. “Through engagement over time, you learn what the effects on your body are and what the expected effects are, so you can recognize the hazards and change the behavior when you’re experiencing it,” said Ana G. Rappold, PhD, who is the app’s principal investigator at the National Health and Environmental Effects Research Laboratory. One component of the app is time of last measurement of fine particulate matter and ozone based on the user’s location. Another is a module called Be Smoke Smart, which tests the user’s knowledge of wildfire smoke exposure. For example, one question is: “How likely are you to reduce your exposure on an Orange AQI alert day?” (which indicates that sensitive populations may experience health effects).
“Through gamification, they’re engaging with the issue,” Dr. Rappold said. “Then they learn about what others are reporting. In that part we also study how different messages change individuals’ perspective on how likely they are to make a change the next time they’re impacted by smoke.”
Wildfires are on the move in California and communities from the Bay Area to Los Angeles County are once again coping with evacuation, possible destruction of homes, and health concerns related to poor air quality and smoke.
What can doctors tell their patients with cardiovascular and pulmonary conditions about the risks of smoke from wildfires?
EPA resources online
AirNow, a website managed by the Environmental Protection Agency, provides a variety of resources for the public and for health providers, including links to online tutorials, printable health fact sheets, and the newly updated document “Wildfire Smoke: Guide for Public Health Officials 2019.” When wildfire smoke generates an Air Quality Index (AQI) from 101-150, at-risk subgroups like people with asthma, chronic obstructive pulmonary disease (COPD), or heart disease should take precautions.
“An AQI of 151-200 is unhealthy for everyone, and an AQI above 200 is very unhealthy,” John R. Balmes, MD, a pulmonologist at the University of California, San Francisco, and an expert on the respiratory and cardiovascular effects of air pollutants, said in an interview. “That does not mean that everybody is going to die, though. You’re going to have some symptoms of scratchy throat, and you may cough once or twice an hour [from exposure to wildfire smoke], but people who don’t have any preexisting health problems are probably going to be fine and don’t necessarily have to wear an N95 mask. People should wear one if they need to feel comfortable.”
Masks – How much protection?
Wayne Cascio, MD, who directs the EPA’s National Health and Environmental Effects Research Laboratory, notes that some public health officials don’t recommend wearing N95 masks during wildfire smoke events. “There’s a lot of concern that people won’t use them correctly and will therefore feel like they’re protected and will spend more time outdoors than they should and still not get the benefit,” he said. “The masks also pose a challenge for children and for people with severe asthma and COPD.”
Masks also have to fit properly, which can be problematic for kids, added Dr. Balmes, one of the authors of “Wildfires Disaster Guidance: Tips for Staying Healthy During Wildfires” (Am J Respir Crit Care Med. 2019;199[2]:3-4).
“Even the small versions don’t necessarily fit kids well, so they’re not recommended for kids,” he said. “It doesn’t mean a kid couldn’t wear them, but it’s not officially recommended. The actual physiologic work of breathing isn’t much increased by using the N95 mask, but if you’re already struggling to get your breath, or experience dyspnea, then it might be hard to wear one. People with milder COPD can wear an N95 mask just like people with mild asthma if they have to go out.”
The EPA published a tip sheet about where and when to use an N95 or P100 mask, with warnings about the limited protection these devices offer, especially if not used correctly. Most masks do not protect the wearer from harmful gases that can be in wildfire smoke.
Ventilation systems
The EPA also recommends that people with more severe disease should stay indoors and avoid using air conditioning units that only draw in air from the outside or do not have a recirculating option. “If you have to bring in outside air because that’s how your system works, you should have a MERV 13 or better filter to filter out the fine particles,” Dr. Balmes said. “Not every ventilation system can handle it, but most can. That will help the house.”
Dr. Cascio pointed out that the instruction to close all windows and doors is a difficult proposition for people who live in states with moderate weather climates such as Montana and Colorado, where few homes have central air conditioning. “The treatment may be worse than the disease in this case, because it may exacerbate heat stress,” he said. “Try to find a place that has cleaner air. That might be a public building, a school, a fitness center, or a library. Yet we don’t know a lot about whether those areas are cleaner or not. That is currently the subject of some research on our part.”
Traveling away from an area affected by wildfire smoke to ride out the conditions is one option, but that can backfire. One weekend when smoke from the 2008 North Carolina peat fire was particularly troublesome, Dr. Cascio and his family drove about 60 miles west of Greenville to the town of Zebulon, where a minor league baseball game was being played and the air quality was good.
“My thought was to get the family to a better environment for at least a few hours,” Dr. Cascio recalled. “When we arrived in Zebulon the air quality was good as advertised. However, the direction of the wind shifted and the smoke started to move due west and within a short time you could barely see the players on the field. This experience also pointed out one of the lessons of wildfire smoke. That is, in the short term, it is sometimes difficult to predict where it will be present because of the nature of changes in weather and wind.”
Consumer tools to monitor air quality
Colleen E. Reid, PhD, of the department of geography at the University of Colorado, Boulder, an expert on the impact of wildfire smoke on human health, has observed in increase in consumer action to counter smoke inhalation during wildfire events. She said that consumers are buying personal laser particle counters, like the ones made by PurpleAir, to provide a real-time assessment of air quality.
“There is a lot of error with these smaller, cheaper monitors, but I think they give you a sense of trends over time,” Dr. Reid said. “People are trying to figure out how we can work with this sort of real-time data along with the high-quality EPA monitors. If everybody has their own monitor, or ways to better calibrate them to the high-quality data, that would be amazing. Researchers are trying to see how they can use that data to inform our understanding of the spatial and temporal patterning of air pollution.”
The EPA’s Smoke Sense app also holds promise. Characterized on its website as “a citizen science project,” the study uses a free mobile app to engage people living in affected communities to monitor their air quality and their cardiorespiratory symptoms. “Through engagement over time, you learn what the effects on your body are and what the expected effects are, so you can recognize the hazards and change the behavior when you’re experiencing it,” said Ana G. Rappold, PhD, who is the app’s principal investigator at the National Health and Environmental Effects Research Laboratory. One component of the app is time of last measurement of fine particulate matter and ozone based on the user’s location. Another is a module called Be Smoke Smart, which tests the user’s knowledge of wildfire smoke exposure. For example, one question is: “How likely are you to reduce your exposure on an Orange AQI alert day?” (which indicates that sensitive populations may experience health effects).
“Through gamification, they’re engaging with the issue,” Dr. Rappold said. “Then they learn about what others are reporting. In that part we also study how different messages change individuals’ perspective on how likely they are to make a change the next time they’re impacted by smoke.”
Mesh nebulizer worked faster to control acute asthma
MADRID – Consistent with previous evidence of higher relative rates of drug delivery, mesh nebulizers offer several advantages over jet nebulizers for treatment of acute asthma in children presenting to an emergency department, according to results of a randomized trial presented at the annual congress of the European Respiratory Society.
For the primary outcome of hospital admission, the advantage of the mesh over the jet nebulizer only reached significance when used with a mask, rather than a valve, but trial results overall support the conclusion that the mesh device delivers drug more efficiently, according to Gerald Moody, RRT-NPS, clinical research coordinator at Children’s Medical Center, Dallas.
In this multicenter, single-blinded trial, 217 children presenting to an ED with acute asthma of moderate or greater severity were randomized to receive bronchodilator treatment delivered with a mesh device or a jet device. For drug delivery, aerosol masks or mouthpiece valves were permitted and selected at the discretion of the clinician administrating treatment. Masks were used in 80% of cases.
Patients remained in the study until either symptom control was achieved or a decision was reached to advise hospital admission. Patients with complex comorbidities or who had received oral corticosteroids within the previous 24 hours were excluded.
For the primary outcome of hospital discharge, the 31% reduction (P = .22) in hospitalization in favor of the mesh nebulizer failed to reach statistical significance. Although the study is likely to have been underpowered, Mr. Moody also pointed out an uneven distribution in severity of disease at baseline. In addition to a significantly higher median asthma score (9.0 vs. 8.0; P = .042) in the mesh nebulizer group, there was also a significantly higher percentage with severe disease (57% vs. 42%; P = .025).
“There were no significant differences in any of the other variables we evaluated, such as age, gender, race, or body mass index,” Mr. Moody reported.
Despite the higher disease burden in the mesh nebulizer group, there was a 48% reduction (P = .03) in hospital admissions among those randomized to the mesh nebulizer when both groups received treatment through a mask.
In addition, those treated with the mask required on average only two treatments before achieving symptom control whether they met criteria for moderate or severe asthma at baseline. The median numbers of treatments in the jet nebulizer group for moderate and severe asthma were 3 and 3.5, respectively.
In previous experimental studies, which ultimately provided the rationale for this trial, the estimated amount of drug reaching the airways with a mesh nebulizer was approximately twice as great as that estimated in the model when delivery was performed with a jet device, according to Mr. Moody.
This study appeared to corroborate that advantage. Both the median doses of albuterol (10 mg vs. 15 mg) and ipratropium (1,000 mcg vs. 1,500 mcg) were significantly lower (P less than .001 for both) among the patients randomized to the mesh nebulizer.
Although the jet nebulizers are widely employed “for their ease of use and low cost,” Mr. Moody characterized mesh nebulizers as an advance in technology. In this study, which Mr. Moody said is the first to evaluate whether the experimental evidence of greater drug delivery efficiency translates into a clinical advantage, the primary endpoint was missed, but Mr. Moody indicated that the overall findings support the potential for a difference.
The ERS-invited discussant on this study, Celeste Michala Porsbjerg, MD, Bispebjerg Hospital, Copenhagen University, expressed a concern that might deserve attention in a larger trial. Based on the premise that more efficient delivery increases drug exposure, she questioned whether it might not also increase risks.
There were no significant treatment-related adverse events reported in either arm of this study, Mr. Moody responded, but he conceded that this is an appropriate focus of attention for future studies.
Mr. Moody reported a financial relationship with Aerogen, which produces the mesh device tested in this trial.
MADRID – Consistent with previous evidence of higher relative rates of drug delivery, mesh nebulizers offer several advantages over jet nebulizers for treatment of acute asthma in children presenting to an emergency department, according to results of a randomized trial presented at the annual congress of the European Respiratory Society.
For the primary outcome of hospital admission, the advantage of the mesh over the jet nebulizer only reached significance when used with a mask, rather than a valve, but trial results overall support the conclusion that the mesh device delivers drug more efficiently, according to Gerald Moody, RRT-NPS, clinical research coordinator at Children’s Medical Center, Dallas.
In this multicenter, single-blinded trial, 217 children presenting to an ED with acute asthma of moderate or greater severity were randomized to receive bronchodilator treatment delivered with a mesh device or a jet device. For drug delivery, aerosol masks or mouthpiece valves were permitted and selected at the discretion of the clinician administrating treatment. Masks were used in 80% of cases.
Patients remained in the study until either symptom control was achieved or a decision was reached to advise hospital admission. Patients with complex comorbidities or who had received oral corticosteroids within the previous 24 hours were excluded.
For the primary outcome of hospital discharge, the 31% reduction (P = .22) in hospitalization in favor of the mesh nebulizer failed to reach statistical significance. Although the study is likely to have been underpowered, Mr. Moody also pointed out an uneven distribution in severity of disease at baseline. In addition to a significantly higher median asthma score (9.0 vs. 8.0; P = .042) in the mesh nebulizer group, there was also a significantly higher percentage with severe disease (57% vs. 42%; P = .025).
“There were no significant differences in any of the other variables we evaluated, such as age, gender, race, or body mass index,” Mr. Moody reported.
Despite the higher disease burden in the mesh nebulizer group, there was a 48% reduction (P = .03) in hospital admissions among those randomized to the mesh nebulizer when both groups received treatment through a mask.
In addition, those treated with the mask required on average only two treatments before achieving symptom control whether they met criteria for moderate or severe asthma at baseline. The median numbers of treatments in the jet nebulizer group for moderate and severe asthma were 3 and 3.5, respectively.
In previous experimental studies, which ultimately provided the rationale for this trial, the estimated amount of drug reaching the airways with a mesh nebulizer was approximately twice as great as that estimated in the model when delivery was performed with a jet device, according to Mr. Moody.
This study appeared to corroborate that advantage. Both the median doses of albuterol (10 mg vs. 15 mg) and ipratropium (1,000 mcg vs. 1,500 mcg) were significantly lower (P less than .001 for both) among the patients randomized to the mesh nebulizer.
Although the jet nebulizers are widely employed “for their ease of use and low cost,” Mr. Moody characterized mesh nebulizers as an advance in technology. In this study, which Mr. Moody said is the first to evaluate whether the experimental evidence of greater drug delivery efficiency translates into a clinical advantage, the primary endpoint was missed, but Mr. Moody indicated that the overall findings support the potential for a difference.
The ERS-invited discussant on this study, Celeste Michala Porsbjerg, MD, Bispebjerg Hospital, Copenhagen University, expressed a concern that might deserve attention in a larger trial. Based on the premise that more efficient delivery increases drug exposure, she questioned whether it might not also increase risks.
There were no significant treatment-related adverse events reported in either arm of this study, Mr. Moody responded, but he conceded that this is an appropriate focus of attention for future studies.
Mr. Moody reported a financial relationship with Aerogen, which produces the mesh device tested in this trial.
MADRID – Consistent with previous evidence of higher relative rates of drug delivery, mesh nebulizers offer several advantages over jet nebulizers for treatment of acute asthma in children presenting to an emergency department, according to results of a randomized trial presented at the annual congress of the European Respiratory Society.
For the primary outcome of hospital admission, the advantage of the mesh over the jet nebulizer only reached significance when used with a mask, rather than a valve, but trial results overall support the conclusion that the mesh device delivers drug more efficiently, according to Gerald Moody, RRT-NPS, clinical research coordinator at Children’s Medical Center, Dallas.
In this multicenter, single-blinded trial, 217 children presenting to an ED with acute asthma of moderate or greater severity were randomized to receive bronchodilator treatment delivered with a mesh device or a jet device. For drug delivery, aerosol masks or mouthpiece valves were permitted and selected at the discretion of the clinician administrating treatment. Masks were used in 80% of cases.
Patients remained in the study until either symptom control was achieved or a decision was reached to advise hospital admission. Patients with complex comorbidities or who had received oral corticosteroids within the previous 24 hours were excluded.
For the primary outcome of hospital discharge, the 31% reduction (P = .22) in hospitalization in favor of the mesh nebulizer failed to reach statistical significance. Although the study is likely to have been underpowered, Mr. Moody also pointed out an uneven distribution in severity of disease at baseline. In addition to a significantly higher median asthma score (9.0 vs. 8.0; P = .042) in the mesh nebulizer group, there was also a significantly higher percentage with severe disease (57% vs. 42%; P = .025).
“There were no significant differences in any of the other variables we evaluated, such as age, gender, race, or body mass index,” Mr. Moody reported.
Despite the higher disease burden in the mesh nebulizer group, there was a 48% reduction (P = .03) in hospital admissions among those randomized to the mesh nebulizer when both groups received treatment through a mask.
In addition, those treated with the mask required on average only two treatments before achieving symptom control whether they met criteria for moderate or severe asthma at baseline. The median numbers of treatments in the jet nebulizer group for moderate and severe asthma were 3 and 3.5, respectively.
In previous experimental studies, which ultimately provided the rationale for this trial, the estimated amount of drug reaching the airways with a mesh nebulizer was approximately twice as great as that estimated in the model when delivery was performed with a jet device, according to Mr. Moody.
This study appeared to corroborate that advantage. Both the median doses of albuterol (10 mg vs. 15 mg) and ipratropium (1,000 mcg vs. 1,500 mcg) were significantly lower (P less than .001 for both) among the patients randomized to the mesh nebulizer.
Although the jet nebulizers are widely employed “for their ease of use and low cost,” Mr. Moody characterized mesh nebulizers as an advance in technology. In this study, which Mr. Moody said is the first to evaluate whether the experimental evidence of greater drug delivery efficiency translates into a clinical advantage, the primary endpoint was missed, but Mr. Moody indicated that the overall findings support the potential for a difference.
The ERS-invited discussant on this study, Celeste Michala Porsbjerg, MD, Bispebjerg Hospital, Copenhagen University, expressed a concern that might deserve attention in a larger trial. Based on the premise that more efficient delivery increases drug exposure, she questioned whether it might not also increase risks.
There were no significant treatment-related adverse events reported in either arm of this study, Mr. Moody responded, but he conceded that this is an appropriate focus of attention for future studies.
Mr. Moody reported a financial relationship with Aerogen, which produces the mesh device tested in this trial.
REPORTING FROM ERS 2019
Trial confirms as-needed inhalers suffice for mild to moderate asthma
MADRID – In the context of three previous trials, a new phase 3 trial demonstrates that the efficacy of as-needed inhaled corticosteroids (ICS) plus a long-acting beta agonist (LABA) is at least comparable to maintenance ICS for preventing severe exacerbations in the routine care of patients with mild to moderate asthma, according to a presentation at the 2019 ERS International Congress.
This “real-world” study, called PRACTICAL, produced results similar to those of the previous three studies. It showed similar or modestly improved efficacy for the as-needed approach in patients enrolled with mild to moderate asthma, according to Joanna Hardy, MD, a research fellow at the Medical Research Institute of New Zealand, Wellington.
Currently, the Global Initiative for Asthma (GINA) guidelines identify either of the two strategies tested in this trial as acceptable for patients eligible for step 2 asthma control. This study, as in the three trials published previously, provided reassurance that an as-needed approach is adequate for patients insufficiently adherent to daily maintenance therapy.
In PRACTICAL, the results of which were published just prior to the 2019 ERS Congress (Lancet 2019;394:919-28), 890 patients were randomized to use of a single inhaler containing 200 mcg budesonide plus 6 mcg formoterol as needed for symptoms or to a maintenance regimen with the same dose of budesonide taken twice daily. The protocol allowed 250 mcg terbutaline as needed for symptom control in the maintenance arm. The patients were followed for 52 weeks.
For the primary endpoint of the per-patient number of severe exacerbations, defined as need for 3 consecutive days of oral corticosteroids or an emergency department visit to receive oral corticosteroids, the as-needed approach reduced the relative risk by 31% (hazard ratio, 0.69; P = .049). The per-patient rates of exacerbations for the as-needed and maintenance arms were 0.0119 and 0.172, respectively.
The time to first exacerbation, a secondary endpoint, approached significance in favor of as-needed treatment (HR 0.6; P = .05). There was no difference in asthma control as measured with the Asthma Control Questionnaire or in lung function as measured with forced expiratory volume in 1 second (FEV1) at any visit or at the end of the study.
Two SYGMA trials (SYGMA 1 and SYGMA 2), both published in the New England Journal of Medicine in 2018, addressed the same question. Most like PRACTICAL, SYGMA 2 randomized 4,215 patients with mild asthma and found as-needed budesonide/formoterol noninferior to budesonide maintenance for preventing severe exacerbations.
In SYGMA 1, which included an as-needed terbutaline arm, 3,849 patients were randomized. Although as-needed budesonide-formoterol was inferior to budesonide maintenance in that study (but superior to as-needed to terbutaline), the adherence to budesonide maintenance was 78.9%, which Dr. Hardy said does not reflect real-world patient behavior.
“The problem is that we have a lot of data to show that adherence to maintenance asthma therapy in mild asthma is poor,” Dr. Hardy said. In PRACTICAL, all patients were provided with an asthma action plan but no strategies were offered to improve compliance over those employed in usual practice.
In the open-label Novel START trial, published in 2019 in the New England Journal of Medicine, the question posed was different. In that study, which randomized 675 patients, as-needed budesonide/formoterol was superior to as-needed albuterol for prevention of asthma exacerbations at 52 weeks, the time point employed in all four studies. The results, while confirming the importance of the ICS component, have been generally interpreted as supporting the as-needed therapy in mild asthma.
At the ERS 2019 Congress, one of the moderators of the session in which Dr. Hardy spoke, Guy Brusselle, MD, Ghent (Belgium) University, agreed that the available evidence supports as-needed therapy as a viable strategy in mild asthma, but expressed concern about applying this conclusion to patients who have asthma requiring therapy beyond GINA step 2.
“These data might put patients who need GINA step 3 or 4 therapy at risk of not receiving the maintenance therapy they need for disease control,” Dr. Brusselle said.
In light of the challenge of separating those with moderate from mild asthma, Dr. Brusselle suggested another arm to add to real-world clinical trials attempting to identify the most effective approach.
“The optimal arm might be maintenance budesonide with as-needed ICS/LABA,” Dr. Brusselle said. He explained that even if compliance is low, at least some patients will be receiving a maintenance therapy, and this approach might ultimately offer more benefit than one in which patients do not even consider maintenance.
Dr. Hardy reports no potential conflicts of interest.
MADRID – In the context of three previous trials, a new phase 3 trial demonstrates that the efficacy of as-needed inhaled corticosteroids (ICS) plus a long-acting beta agonist (LABA) is at least comparable to maintenance ICS for preventing severe exacerbations in the routine care of patients with mild to moderate asthma, according to a presentation at the 2019 ERS International Congress.
This “real-world” study, called PRACTICAL, produced results similar to those of the previous three studies. It showed similar or modestly improved efficacy for the as-needed approach in patients enrolled with mild to moderate asthma, according to Joanna Hardy, MD, a research fellow at the Medical Research Institute of New Zealand, Wellington.
Currently, the Global Initiative for Asthma (GINA) guidelines identify either of the two strategies tested in this trial as acceptable for patients eligible for step 2 asthma control. This study, as in the three trials published previously, provided reassurance that an as-needed approach is adequate for patients insufficiently adherent to daily maintenance therapy.
In PRACTICAL, the results of which were published just prior to the 2019 ERS Congress (Lancet 2019;394:919-28), 890 patients were randomized to use of a single inhaler containing 200 mcg budesonide plus 6 mcg formoterol as needed for symptoms or to a maintenance regimen with the same dose of budesonide taken twice daily. The protocol allowed 250 mcg terbutaline as needed for symptom control in the maintenance arm. The patients were followed for 52 weeks.
For the primary endpoint of the per-patient number of severe exacerbations, defined as need for 3 consecutive days of oral corticosteroids or an emergency department visit to receive oral corticosteroids, the as-needed approach reduced the relative risk by 31% (hazard ratio, 0.69; P = .049). The per-patient rates of exacerbations for the as-needed and maintenance arms were 0.0119 and 0.172, respectively.
The time to first exacerbation, a secondary endpoint, approached significance in favor of as-needed treatment (HR 0.6; P = .05). There was no difference in asthma control as measured with the Asthma Control Questionnaire or in lung function as measured with forced expiratory volume in 1 second (FEV1) at any visit or at the end of the study.
Two SYGMA trials (SYGMA 1 and SYGMA 2), both published in the New England Journal of Medicine in 2018, addressed the same question. Most like PRACTICAL, SYGMA 2 randomized 4,215 patients with mild asthma and found as-needed budesonide/formoterol noninferior to budesonide maintenance for preventing severe exacerbations.
In SYGMA 1, which included an as-needed terbutaline arm, 3,849 patients were randomized. Although as-needed budesonide-formoterol was inferior to budesonide maintenance in that study (but superior to as-needed to terbutaline), the adherence to budesonide maintenance was 78.9%, which Dr. Hardy said does not reflect real-world patient behavior.
“The problem is that we have a lot of data to show that adherence to maintenance asthma therapy in mild asthma is poor,” Dr. Hardy said. In PRACTICAL, all patients were provided with an asthma action plan but no strategies were offered to improve compliance over those employed in usual practice.
In the open-label Novel START trial, published in 2019 in the New England Journal of Medicine, the question posed was different. In that study, which randomized 675 patients, as-needed budesonide/formoterol was superior to as-needed albuterol for prevention of asthma exacerbations at 52 weeks, the time point employed in all four studies. The results, while confirming the importance of the ICS component, have been generally interpreted as supporting the as-needed therapy in mild asthma.
At the ERS 2019 Congress, one of the moderators of the session in which Dr. Hardy spoke, Guy Brusselle, MD, Ghent (Belgium) University, agreed that the available evidence supports as-needed therapy as a viable strategy in mild asthma, but expressed concern about applying this conclusion to patients who have asthma requiring therapy beyond GINA step 2.
“These data might put patients who need GINA step 3 or 4 therapy at risk of not receiving the maintenance therapy they need for disease control,” Dr. Brusselle said.
In light of the challenge of separating those with moderate from mild asthma, Dr. Brusselle suggested another arm to add to real-world clinical trials attempting to identify the most effective approach.
“The optimal arm might be maintenance budesonide with as-needed ICS/LABA,” Dr. Brusselle said. He explained that even if compliance is low, at least some patients will be receiving a maintenance therapy, and this approach might ultimately offer more benefit than one in which patients do not even consider maintenance.
Dr. Hardy reports no potential conflicts of interest.
MADRID – In the context of three previous trials, a new phase 3 trial demonstrates that the efficacy of as-needed inhaled corticosteroids (ICS) plus a long-acting beta agonist (LABA) is at least comparable to maintenance ICS for preventing severe exacerbations in the routine care of patients with mild to moderate asthma, according to a presentation at the 2019 ERS International Congress.
This “real-world” study, called PRACTICAL, produced results similar to those of the previous three studies. It showed similar or modestly improved efficacy for the as-needed approach in patients enrolled with mild to moderate asthma, according to Joanna Hardy, MD, a research fellow at the Medical Research Institute of New Zealand, Wellington.
Currently, the Global Initiative for Asthma (GINA) guidelines identify either of the two strategies tested in this trial as acceptable for patients eligible for step 2 asthma control. This study, as in the three trials published previously, provided reassurance that an as-needed approach is adequate for patients insufficiently adherent to daily maintenance therapy.
In PRACTICAL, the results of which were published just prior to the 2019 ERS Congress (Lancet 2019;394:919-28), 890 patients were randomized to use of a single inhaler containing 200 mcg budesonide plus 6 mcg formoterol as needed for symptoms or to a maintenance regimen with the same dose of budesonide taken twice daily. The protocol allowed 250 mcg terbutaline as needed for symptom control in the maintenance arm. The patients were followed for 52 weeks.
For the primary endpoint of the per-patient number of severe exacerbations, defined as need for 3 consecutive days of oral corticosteroids or an emergency department visit to receive oral corticosteroids, the as-needed approach reduced the relative risk by 31% (hazard ratio, 0.69; P = .049). The per-patient rates of exacerbations for the as-needed and maintenance arms were 0.0119 and 0.172, respectively.
The time to first exacerbation, a secondary endpoint, approached significance in favor of as-needed treatment (HR 0.6; P = .05). There was no difference in asthma control as measured with the Asthma Control Questionnaire or in lung function as measured with forced expiratory volume in 1 second (FEV1) at any visit or at the end of the study.
Two SYGMA trials (SYGMA 1 and SYGMA 2), both published in the New England Journal of Medicine in 2018, addressed the same question. Most like PRACTICAL, SYGMA 2 randomized 4,215 patients with mild asthma and found as-needed budesonide/formoterol noninferior to budesonide maintenance for preventing severe exacerbations.
In SYGMA 1, which included an as-needed terbutaline arm, 3,849 patients were randomized. Although as-needed budesonide-formoterol was inferior to budesonide maintenance in that study (but superior to as-needed to terbutaline), the adherence to budesonide maintenance was 78.9%, which Dr. Hardy said does not reflect real-world patient behavior.
“The problem is that we have a lot of data to show that adherence to maintenance asthma therapy in mild asthma is poor,” Dr. Hardy said. In PRACTICAL, all patients were provided with an asthma action plan but no strategies were offered to improve compliance over those employed in usual practice.
In the open-label Novel START trial, published in 2019 in the New England Journal of Medicine, the question posed was different. In that study, which randomized 675 patients, as-needed budesonide/formoterol was superior to as-needed albuterol for prevention of asthma exacerbations at 52 weeks, the time point employed in all four studies. The results, while confirming the importance of the ICS component, have been generally interpreted as supporting the as-needed therapy in mild asthma.
At the ERS 2019 Congress, one of the moderators of the session in which Dr. Hardy spoke, Guy Brusselle, MD, Ghent (Belgium) University, agreed that the available evidence supports as-needed therapy as a viable strategy in mild asthma, but expressed concern about applying this conclusion to patients who have asthma requiring therapy beyond GINA step 2.
“These data might put patients who need GINA step 3 or 4 therapy at risk of not receiving the maintenance therapy they need for disease control,” Dr. Brusselle said.
In light of the challenge of separating those with moderate from mild asthma, Dr. Brusselle suggested another arm to add to real-world clinical trials attempting to identify the most effective approach.
“The optimal arm might be maintenance budesonide with as-needed ICS/LABA,” Dr. Brusselle said. He explained that even if compliance is low, at least some patients will be receiving a maintenance therapy, and this approach might ultimately offer more benefit than one in which patients do not even consider maintenance.
Dr. Hardy reports no potential conflicts of interest.
REPORTING FROM ERS 2019
FDA approves benralizumab autoinjector for eosinophilic asthma
according to a press release from AstraZeneca. Benralizumab is already approved as add-on maintenance for this form of asthma, but not for other eosinophilic conditions or for acute bronchospasm or status asthmaticus.
The autoinjector “pen” was tested for usability and pharmacokinetic exposure in two studies, the phase 3 GRECO trial and the phase 1 AMES trial, respectively. The multicenter, open-label GRECO trial was designed to assess patient- or caregiver-reported functionality, and it found that 97% of at-home administrations were successful at week 12 and week 16. The multicenter, randomized, open-label, parallel-group AMES trial compared pharmacokinetic exposure with the subcutaneous administration using either prefilled syringe or prefilled autoinjector; it found that the eosinophils were rapidly depleted in patients with use of either device.
The safety profiles in both trials were comparable to those seen in previous trials. Hypersensitivity reactions have been sometimes observed in the hours following administration of benralizumab; discontinuation is advised in case of any hypersensitivity reaction. The therapy should not be used to treat acute asthma symptoms, such as exacerbations, or bronchospasm, and any reduction in corticosteroid therapy should be gradual and performed under careful supervision of a health care professional. Although benralizumab’s effects on helminth infections are currently unknown, care should be taken with preexisting or incident infections.
Full prescribing information can be found on the AstraZeneca website.
according to a press release from AstraZeneca. Benralizumab is already approved as add-on maintenance for this form of asthma, but not for other eosinophilic conditions or for acute bronchospasm or status asthmaticus.
The autoinjector “pen” was tested for usability and pharmacokinetic exposure in two studies, the phase 3 GRECO trial and the phase 1 AMES trial, respectively. The multicenter, open-label GRECO trial was designed to assess patient- or caregiver-reported functionality, and it found that 97% of at-home administrations were successful at week 12 and week 16. The multicenter, randomized, open-label, parallel-group AMES trial compared pharmacokinetic exposure with the subcutaneous administration using either prefilled syringe or prefilled autoinjector; it found that the eosinophils were rapidly depleted in patients with use of either device.
The safety profiles in both trials were comparable to those seen in previous trials. Hypersensitivity reactions have been sometimes observed in the hours following administration of benralizumab; discontinuation is advised in case of any hypersensitivity reaction. The therapy should not be used to treat acute asthma symptoms, such as exacerbations, or bronchospasm, and any reduction in corticosteroid therapy should be gradual and performed under careful supervision of a health care professional. Although benralizumab’s effects on helminth infections are currently unknown, care should be taken with preexisting or incident infections.
Full prescribing information can be found on the AstraZeneca website.
according to a press release from AstraZeneca. Benralizumab is already approved as add-on maintenance for this form of asthma, but not for other eosinophilic conditions or for acute bronchospasm or status asthmaticus.
The autoinjector “pen” was tested for usability and pharmacokinetic exposure in two studies, the phase 3 GRECO trial and the phase 1 AMES trial, respectively. The multicenter, open-label GRECO trial was designed to assess patient- or caregiver-reported functionality, and it found that 97% of at-home administrations were successful at week 12 and week 16. The multicenter, randomized, open-label, parallel-group AMES trial compared pharmacokinetic exposure with the subcutaneous administration using either prefilled syringe or prefilled autoinjector; it found that the eosinophils were rapidly depleted in patients with use of either device.
The safety profiles in both trials were comparable to those seen in previous trials. Hypersensitivity reactions have been sometimes observed in the hours following administration of benralizumab; discontinuation is advised in case of any hypersensitivity reaction. The therapy should not be used to treat acute asthma symptoms, such as exacerbations, or bronchospasm, and any reduction in corticosteroid therapy should be gradual and performed under careful supervision of a health care professional. Although benralizumab’s effects on helminth infections are currently unknown, care should be taken with preexisting or incident infections.
Full prescribing information can be found on the AstraZeneca website.
One-third of patients with severe asthma are overusing corticosteroids
MADRID – if data from a Dutch study presented at the annual congress of the European Respiratory Society are representative of practice elsewhere.
“The main message from our study is that OCS overuse is common and unnecessary in the majority of asthma patients,” reported Katrien A.B. Eger, MD, Amsterdam University Medical Centre.
In this study, 5,002 patients on high doses of inhaled corticosteroids (ICS), defined as at least 500 mcg/day, were identified in a pharmacy database in the Netherlands. These patients were asked to complete a questionnaire to determine how many had severe asthma and had received rescue or maintenance OCS in the past year.
Drawing from the pharmacy database, it could be determined that 29% of the 2,312 patients who responded to the questionnaire were taking harmfully high doses of OCS as well as high doses of ICS. For this study, harmful exposure was defined as a cumulative intake of 420 mg of prednisone-equivalent OCS over a 1-year period. The median cumulative 1-year exposure, according to Dr. Eger, was 750 mg of prednisone equivalent.
In this population, the investigators then calculated ICS medication adherence based on prescription refills. In addition, a subset of this population was evaluated for inhaler technique.
On the basis of these calculations, 47.4% of patients with harmful OCS exposure were found not to be adherent to their prescribed ICS. Of those who were adherent, 53.9% were found not be taking their inhaled steroids appropriately,
When these numbers are put together, the data suggest “78.1% of high OCS users are either nonadherent or using poor inhalation techniques, which means there is a big potential for treatment optimization,” Dr. Eger said.
Yet even among the 21.9% who were adherent and using good inhaler technique, identifying a group who presumably require OCS for exacerbations, the study found that only 46.1% had been prescribed a biologic, which Dr. Eger considers an important steroid-sparing option. She conceded that many of those not on a biologic might not be candidates, but she believes this is another missed opportunity for reducing OCS exposure.
“In the Netherlands, we have very good access to health care, and biologics are available to anyone who needs them,” said Dr. Eger, explaining that access to these drugs is not a barrier.
The evidence overall is that not enough is being done to ensure that asthma patients are being protected from the risks of OCS, according to Dr. Eger. Citing evidence that adverse events associated with OCS begin with a cumulative lifetime prednisone-equivalent exposure of only 500 mg, she believes that clinicians should be more aggressive in intervening.
“We know that there are both acute and chronic complications associated with OCS that involve a range of organ systems,” Dr. Eger said. She listed osteoporosis, diabetes mellitus, hypertension, and adrenal insufficiency as examples. Rescue OCS, even if used sparingly, can drive risk of OCS complications attributable to the importance of cumulative exposure.
In the session where these data were presented, the moderator, Guy Brusselle, MD, professor of asthma and immunology, Ghent (Belgium) University, labeled them “important.” However, he quibbled with Dr. Eger’s assertion that biologics represent a major opportunity to reduce OCS exposure.
“By suggesting that biologics are not being used often enough, there is an assumption that all of these patients have type 2 inflammatory asthma,” Dr. Brusselle said. “I think it makes more sense to emphasize steroid-sparing strategies, not just biologics.”
Dr. Eger did not disagree, but she emphasized that steroid-sparing alternatives are just one strategy to reduce OCS exposure, and ensuring that patients are adherent to prescribed ICS therapies and are using them correctly might have an even greater impact.
Dr. Eger reports no potential conflicts of interest.
MADRID – if data from a Dutch study presented at the annual congress of the European Respiratory Society are representative of practice elsewhere.
“The main message from our study is that OCS overuse is common and unnecessary in the majority of asthma patients,” reported Katrien A.B. Eger, MD, Amsterdam University Medical Centre.
In this study, 5,002 patients on high doses of inhaled corticosteroids (ICS), defined as at least 500 mcg/day, were identified in a pharmacy database in the Netherlands. These patients were asked to complete a questionnaire to determine how many had severe asthma and had received rescue or maintenance OCS in the past year.
Drawing from the pharmacy database, it could be determined that 29% of the 2,312 patients who responded to the questionnaire were taking harmfully high doses of OCS as well as high doses of ICS. For this study, harmful exposure was defined as a cumulative intake of 420 mg of prednisone-equivalent OCS over a 1-year period. The median cumulative 1-year exposure, according to Dr. Eger, was 750 mg of prednisone equivalent.
In this population, the investigators then calculated ICS medication adherence based on prescription refills. In addition, a subset of this population was evaluated for inhaler technique.
On the basis of these calculations, 47.4% of patients with harmful OCS exposure were found not to be adherent to their prescribed ICS. Of those who were adherent, 53.9% were found not be taking their inhaled steroids appropriately,
When these numbers are put together, the data suggest “78.1% of high OCS users are either nonadherent or using poor inhalation techniques, which means there is a big potential for treatment optimization,” Dr. Eger said.
Yet even among the 21.9% who were adherent and using good inhaler technique, identifying a group who presumably require OCS for exacerbations, the study found that only 46.1% had been prescribed a biologic, which Dr. Eger considers an important steroid-sparing option. She conceded that many of those not on a biologic might not be candidates, but she believes this is another missed opportunity for reducing OCS exposure.
“In the Netherlands, we have very good access to health care, and biologics are available to anyone who needs them,” said Dr. Eger, explaining that access to these drugs is not a barrier.
The evidence overall is that not enough is being done to ensure that asthma patients are being protected from the risks of OCS, according to Dr. Eger. Citing evidence that adverse events associated with OCS begin with a cumulative lifetime prednisone-equivalent exposure of only 500 mg, she believes that clinicians should be more aggressive in intervening.
“We know that there are both acute and chronic complications associated with OCS that involve a range of organ systems,” Dr. Eger said. She listed osteoporosis, diabetes mellitus, hypertension, and adrenal insufficiency as examples. Rescue OCS, even if used sparingly, can drive risk of OCS complications attributable to the importance of cumulative exposure.
In the session where these data were presented, the moderator, Guy Brusselle, MD, professor of asthma and immunology, Ghent (Belgium) University, labeled them “important.” However, he quibbled with Dr. Eger’s assertion that biologics represent a major opportunity to reduce OCS exposure.
“By suggesting that biologics are not being used often enough, there is an assumption that all of these patients have type 2 inflammatory asthma,” Dr. Brusselle said. “I think it makes more sense to emphasize steroid-sparing strategies, not just biologics.”
Dr. Eger did not disagree, but she emphasized that steroid-sparing alternatives are just one strategy to reduce OCS exposure, and ensuring that patients are adherent to prescribed ICS therapies and are using them correctly might have an even greater impact.
Dr. Eger reports no potential conflicts of interest.
MADRID – if data from a Dutch study presented at the annual congress of the European Respiratory Society are representative of practice elsewhere.
“The main message from our study is that OCS overuse is common and unnecessary in the majority of asthma patients,” reported Katrien A.B. Eger, MD, Amsterdam University Medical Centre.
In this study, 5,002 patients on high doses of inhaled corticosteroids (ICS), defined as at least 500 mcg/day, were identified in a pharmacy database in the Netherlands. These patients were asked to complete a questionnaire to determine how many had severe asthma and had received rescue or maintenance OCS in the past year.
Drawing from the pharmacy database, it could be determined that 29% of the 2,312 patients who responded to the questionnaire were taking harmfully high doses of OCS as well as high doses of ICS. For this study, harmful exposure was defined as a cumulative intake of 420 mg of prednisone-equivalent OCS over a 1-year period. The median cumulative 1-year exposure, according to Dr. Eger, was 750 mg of prednisone equivalent.
In this population, the investigators then calculated ICS medication adherence based on prescription refills. In addition, a subset of this population was evaluated for inhaler technique.
On the basis of these calculations, 47.4% of patients with harmful OCS exposure were found not to be adherent to their prescribed ICS. Of those who were adherent, 53.9% were found not be taking their inhaled steroids appropriately,
When these numbers are put together, the data suggest “78.1% of high OCS users are either nonadherent or using poor inhalation techniques, which means there is a big potential for treatment optimization,” Dr. Eger said.
Yet even among the 21.9% who were adherent and using good inhaler technique, identifying a group who presumably require OCS for exacerbations, the study found that only 46.1% had been prescribed a biologic, which Dr. Eger considers an important steroid-sparing option. She conceded that many of those not on a biologic might not be candidates, but she believes this is another missed opportunity for reducing OCS exposure.
“In the Netherlands, we have very good access to health care, and biologics are available to anyone who needs them,” said Dr. Eger, explaining that access to these drugs is not a barrier.
The evidence overall is that not enough is being done to ensure that asthma patients are being protected from the risks of OCS, according to Dr. Eger. Citing evidence that adverse events associated with OCS begin with a cumulative lifetime prednisone-equivalent exposure of only 500 mg, she believes that clinicians should be more aggressive in intervening.
“We know that there are both acute and chronic complications associated with OCS that involve a range of organ systems,” Dr. Eger said. She listed osteoporosis, diabetes mellitus, hypertension, and adrenal insufficiency as examples. Rescue OCS, even if used sparingly, can drive risk of OCS complications attributable to the importance of cumulative exposure.
In the session where these data were presented, the moderator, Guy Brusselle, MD, professor of asthma and immunology, Ghent (Belgium) University, labeled them “important.” However, he quibbled with Dr. Eger’s assertion that biologics represent a major opportunity to reduce OCS exposure.
“By suggesting that biologics are not being used often enough, there is an assumption that all of these patients have type 2 inflammatory asthma,” Dr. Brusselle said. “I think it makes more sense to emphasize steroid-sparing strategies, not just biologics.”
Dr. Eger did not disagree, but she emphasized that steroid-sparing alternatives are just one strategy to reduce OCS exposure, and ensuring that patients are adherent to prescribed ICS therapies and are using them correctly might have an even greater impact.
Dr. Eger reports no potential conflicts of interest.
REPORTING FROM ERS 2019
Six factors predicted benefit from asthma triple therapy
MADRID – Two newly published but previously reported phase 3 trials associated triple therapy in a single inhaler with a 23% reduction (P = .008) in asthma exacerbations relative to a two-drug inhaler, but fresh data from a prespecified analysis presented at the annual congress of the European Respiratory Society has identified those patients most likely to benefit.
“Six easily identifiable factors appear to be associated with the most prominent response to treatment and may help in the treatment step-up decision at the point of care,” reported Dave Singh, MD, professor in the division of infection, immunity, and respiratory medicine, University of Manchester (England).
The primary results of these trials were presented several months ago at the 2019 American Thoracic Society (ATS) meeting, but the full data were published on the day that Dr. Singh spoke at the ERS.
To identify predictors of response, the pooled analysis of TRIMARIN and TRIGGER was prespecified. Both of these trials, which were similarly designed, compared a single inhaler of inhaled corticosteroids (ICS), long-acting beta agonist (LABA), and long-acting muscarinic antagonist (LAMA) to a single ICS/LABA inhaler.
On the basis of risk for severe exacerbations, greater protection from triple therapy relative to a conventional ICS/LABA inhaler was identified for those with a high degree of reversibility (defined as greater than 400 mL) relative to those with a lower degree (RR, 0.729; P = .024), those with a body mass index less than 25 kg/m2 relative to a higher BMI (RR, 0.570; P = .005), those with only one exacerbation in the previous 12 months relative to those with more (RR, 0.731; P = .009), never-smokers relative to those with smoking history (RR, 0.764; P = .013), those younger than age 65 years relative to older (RR, 0.770; P = .17), and males relative to females (RR, 0.651; P = .009).
“This gives us six factors to consider when you are thinking about stepping up to triple therapy and are trying to determine which patients would benefit the most,” Dr. Singh said.
Both the TRIMARIN and the TRIGGER trials were double blind and placebo controlled. In both, the experimental arm was a single inhaler triple therapy of the ICS beclomethasone, the LABA formoterol, and the LAMA glycopyrronium. The control arm was a single inhaler combination of beclomethasone and formoterol. All inhalers were used twice daily.
TRIMARIN, with 171 participating sites in 16 countries, randomized 1,155 patients to the triple-drug inhaler with a moderate dose of ICS (100 mcg) or to the ICS/LABA inhaler. In TRIGGER, with 221 sites in 17 countries, 1,437 patients were randomized to one of three arms. Both the triple-drug inhaler arm and the ICS/LABA arm contained a higher dose of ICS (200 mcg) than in TRIMARIN. In an open-label third arm, patients also received the higher dose of ICS plus LABA and a second inhaler with tiotropium. The formoterol dose in all arms of both studies was 6 mcg.
As reported at the ATS and now published in the Lancet, the reduction in exacerbations on single inhaler triple therapy relative to ICS/LABA was significant when the data were pooled (even though the reduction in the TRIGGER study fell short of statistical significance). The median improvement in lung function for single inhaler triple therapy relative to ICS/LABA was significant in both TRIMARIN (57 mL; P = .008) and TRIGGER (73 mL; P = .0025).
In discussing the new pooled analysis of response predictors in TRIMARIN/TRIGGER, the ERS-invited discussant, Celeste M. Porsbjerg, MD, Bispebjerg Hospital, Copenhagen, expressed particular interest in reversibility. A positive reversibility test to salbutamol was an entry criterion for both trials, but Dr. Porsbjerg pointed out that a greater response in those with the highest reversibility suggests these patients have a phenotype in which bronchodilation is a more important driver of disease than is inflammation.
While conceding that this was possible, Dr. Singh cautioned that he considers these predictors of response to be “exploratory.” He believes that the TRIMARIN/TRIGGER studies were not designed to tease out the relative importance of mechanisms of asthma in response to the assigned therapies. However, he believes the response predictor analysis is a step in this direction, which might be valuable for better individualizing therapy.
The studies were funded by Chiesi Farmaceutici. Dr. Singh reports no potential conflicts of interest.
Virchow JC et al. Lancet. 2019 Sep 30. doi. org/10.1016/S0140-6736(19)32215-9.
MADRID – Two newly published but previously reported phase 3 trials associated triple therapy in a single inhaler with a 23% reduction (P = .008) in asthma exacerbations relative to a two-drug inhaler, but fresh data from a prespecified analysis presented at the annual congress of the European Respiratory Society has identified those patients most likely to benefit.
“Six easily identifiable factors appear to be associated with the most prominent response to treatment and may help in the treatment step-up decision at the point of care,” reported Dave Singh, MD, professor in the division of infection, immunity, and respiratory medicine, University of Manchester (England).
The primary results of these trials were presented several months ago at the 2019 American Thoracic Society (ATS) meeting, but the full data were published on the day that Dr. Singh spoke at the ERS.
To identify predictors of response, the pooled analysis of TRIMARIN and TRIGGER was prespecified. Both of these trials, which were similarly designed, compared a single inhaler of inhaled corticosteroids (ICS), long-acting beta agonist (LABA), and long-acting muscarinic antagonist (LAMA) to a single ICS/LABA inhaler.
On the basis of risk for severe exacerbations, greater protection from triple therapy relative to a conventional ICS/LABA inhaler was identified for those with a high degree of reversibility (defined as greater than 400 mL) relative to those with a lower degree (RR, 0.729; P = .024), those with a body mass index less than 25 kg/m2 relative to a higher BMI (RR, 0.570; P = .005), those with only one exacerbation in the previous 12 months relative to those with more (RR, 0.731; P = .009), never-smokers relative to those with smoking history (RR, 0.764; P = .013), those younger than age 65 years relative to older (RR, 0.770; P = .17), and males relative to females (RR, 0.651; P = .009).
“This gives us six factors to consider when you are thinking about stepping up to triple therapy and are trying to determine which patients would benefit the most,” Dr. Singh said.
Both the TRIMARIN and the TRIGGER trials were double blind and placebo controlled. In both, the experimental arm was a single inhaler triple therapy of the ICS beclomethasone, the LABA formoterol, and the LAMA glycopyrronium. The control arm was a single inhaler combination of beclomethasone and formoterol. All inhalers were used twice daily.
TRIMARIN, with 171 participating sites in 16 countries, randomized 1,155 patients to the triple-drug inhaler with a moderate dose of ICS (100 mcg) or to the ICS/LABA inhaler. In TRIGGER, with 221 sites in 17 countries, 1,437 patients were randomized to one of three arms. Both the triple-drug inhaler arm and the ICS/LABA arm contained a higher dose of ICS (200 mcg) than in TRIMARIN. In an open-label third arm, patients also received the higher dose of ICS plus LABA and a second inhaler with tiotropium. The formoterol dose in all arms of both studies was 6 mcg.
As reported at the ATS and now published in the Lancet, the reduction in exacerbations on single inhaler triple therapy relative to ICS/LABA was significant when the data were pooled (even though the reduction in the TRIGGER study fell short of statistical significance). The median improvement in lung function for single inhaler triple therapy relative to ICS/LABA was significant in both TRIMARIN (57 mL; P = .008) and TRIGGER (73 mL; P = .0025).
In discussing the new pooled analysis of response predictors in TRIMARIN/TRIGGER, the ERS-invited discussant, Celeste M. Porsbjerg, MD, Bispebjerg Hospital, Copenhagen, expressed particular interest in reversibility. A positive reversibility test to salbutamol was an entry criterion for both trials, but Dr. Porsbjerg pointed out that a greater response in those with the highest reversibility suggests these patients have a phenotype in which bronchodilation is a more important driver of disease than is inflammation.
While conceding that this was possible, Dr. Singh cautioned that he considers these predictors of response to be “exploratory.” He believes that the TRIMARIN/TRIGGER studies were not designed to tease out the relative importance of mechanisms of asthma in response to the assigned therapies. However, he believes the response predictor analysis is a step in this direction, which might be valuable for better individualizing therapy.
The studies were funded by Chiesi Farmaceutici. Dr. Singh reports no potential conflicts of interest.
Virchow JC et al. Lancet. 2019 Sep 30. doi. org/10.1016/S0140-6736(19)32215-9.
MADRID – Two newly published but previously reported phase 3 trials associated triple therapy in a single inhaler with a 23% reduction (P = .008) in asthma exacerbations relative to a two-drug inhaler, but fresh data from a prespecified analysis presented at the annual congress of the European Respiratory Society has identified those patients most likely to benefit.
“Six easily identifiable factors appear to be associated with the most prominent response to treatment and may help in the treatment step-up decision at the point of care,” reported Dave Singh, MD, professor in the division of infection, immunity, and respiratory medicine, University of Manchester (England).
The primary results of these trials were presented several months ago at the 2019 American Thoracic Society (ATS) meeting, but the full data were published on the day that Dr. Singh spoke at the ERS.
To identify predictors of response, the pooled analysis of TRIMARIN and TRIGGER was prespecified. Both of these trials, which were similarly designed, compared a single inhaler of inhaled corticosteroids (ICS), long-acting beta agonist (LABA), and long-acting muscarinic antagonist (LAMA) to a single ICS/LABA inhaler.
On the basis of risk for severe exacerbations, greater protection from triple therapy relative to a conventional ICS/LABA inhaler was identified for those with a high degree of reversibility (defined as greater than 400 mL) relative to those with a lower degree (RR, 0.729; P = .024), those with a body mass index less than 25 kg/m2 relative to a higher BMI (RR, 0.570; P = .005), those with only one exacerbation in the previous 12 months relative to those with more (RR, 0.731; P = .009), never-smokers relative to those with smoking history (RR, 0.764; P = .013), those younger than age 65 years relative to older (RR, 0.770; P = .17), and males relative to females (RR, 0.651; P = .009).
“This gives us six factors to consider when you are thinking about stepping up to triple therapy and are trying to determine which patients would benefit the most,” Dr. Singh said.
Both the TRIMARIN and the TRIGGER trials were double blind and placebo controlled. In both, the experimental arm was a single inhaler triple therapy of the ICS beclomethasone, the LABA formoterol, and the LAMA glycopyrronium. The control arm was a single inhaler combination of beclomethasone and formoterol. All inhalers were used twice daily.
TRIMARIN, with 171 participating sites in 16 countries, randomized 1,155 patients to the triple-drug inhaler with a moderate dose of ICS (100 mcg) or to the ICS/LABA inhaler. In TRIGGER, with 221 sites in 17 countries, 1,437 patients were randomized to one of three arms. Both the triple-drug inhaler arm and the ICS/LABA arm contained a higher dose of ICS (200 mcg) than in TRIMARIN. In an open-label third arm, patients also received the higher dose of ICS plus LABA and a second inhaler with tiotropium. The formoterol dose in all arms of both studies was 6 mcg.
As reported at the ATS and now published in the Lancet, the reduction in exacerbations on single inhaler triple therapy relative to ICS/LABA was significant when the data were pooled (even though the reduction in the TRIGGER study fell short of statistical significance). The median improvement in lung function for single inhaler triple therapy relative to ICS/LABA was significant in both TRIMARIN (57 mL; P = .008) and TRIGGER (73 mL; P = .0025).
In discussing the new pooled analysis of response predictors in TRIMARIN/TRIGGER, the ERS-invited discussant, Celeste M. Porsbjerg, MD, Bispebjerg Hospital, Copenhagen, expressed particular interest in reversibility. A positive reversibility test to salbutamol was an entry criterion for both trials, but Dr. Porsbjerg pointed out that a greater response in those with the highest reversibility suggests these patients have a phenotype in which bronchodilation is a more important driver of disease than is inflammation.
While conceding that this was possible, Dr. Singh cautioned that he considers these predictors of response to be “exploratory.” He believes that the TRIMARIN/TRIGGER studies were not designed to tease out the relative importance of mechanisms of asthma in response to the assigned therapies. However, he believes the response predictor analysis is a step in this direction, which might be valuable for better individualizing therapy.
The studies were funded by Chiesi Farmaceutici. Dr. Singh reports no potential conflicts of interest.
Virchow JC et al. Lancet. 2019 Sep 30. doi. org/10.1016/S0140-6736(19)32215-9.
REPORTING FROM ERS 2019