User login
Risk factors identified for thrombosis in pediatric SLE
Pediatric patients with systemic lupus erythematosus may have greater odds for developing thrombosis if they have a history of vasculitis, antiphospholipid antibody positivity (aPL), and/or avascular necrosis (AVN), according to Dr. Kyla Driest and her associates.
Among 974 pediatric systemic lupus erythematosus (pSLE) patients in the CARRA (Childhood Arthritis & Rheumatology Research Alliance) registry cohort who had available data on thrombosis history, 24 (2.5%) had a history of arterial thrombosis and 35 (3.6%) had a history of venous thrombosis. The researchers conducted a multivariable analysis that found statistically higher odds of thrombosis (P less than .10) among patients with histories of AVN (odds ratio, 4.24; 95% confidence interval, 1.53-11.74), aPL (OR, 2.95; 95% CI, 1.38-6.28), and vasculitis (OR, 2.19; 95% CI, 1.03-4.77), whereas significantly lower odds occurred in patients with a history of renal disease (OR, 0.47; 95% CI, 0.24-0.92). Gender and body-mass index were not statistically significant.
“This study adds to our understanding of which pSLE patients are at the most risk for thrombosis,” the researchers concluded. “These results may prompt discussion concerning potential measures to prevent thrombosis in high-risk patients.”
Find the full study in Lupus (doi: 10.1177/0961203316638164).
Pediatric patients with systemic lupus erythematosus may have greater odds for developing thrombosis if they have a history of vasculitis, antiphospholipid antibody positivity (aPL), and/or avascular necrosis (AVN), according to Dr. Kyla Driest and her associates.
Among 974 pediatric systemic lupus erythematosus (pSLE) patients in the CARRA (Childhood Arthritis & Rheumatology Research Alliance) registry cohort who had available data on thrombosis history, 24 (2.5%) had a history of arterial thrombosis and 35 (3.6%) had a history of venous thrombosis. The researchers conducted a multivariable analysis that found statistically higher odds of thrombosis (P less than .10) among patients with histories of AVN (odds ratio, 4.24; 95% confidence interval, 1.53-11.74), aPL (OR, 2.95; 95% CI, 1.38-6.28), and vasculitis (OR, 2.19; 95% CI, 1.03-4.77), whereas significantly lower odds occurred in patients with a history of renal disease (OR, 0.47; 95% CI, 0.24-0.92). Gender and body-mass index were not statistically significant.
“This study adds to our understanding of which pSLE patients are at the most risk for thrombosis,” the researchers concluded. “These results may prompt discussion concerning potential measures to prevent thrombosis in high-risk patients.”
Find the full study in Lupus (doi: 10.1177/0961203316638164).
Pediatric patients with systemic lupus erythematosus may have greater odds for developing thrombosis if they have a history of vasculitis, antiphospholipid antibody positivity (aPL), and/or avascular necrosis (AVN), according to Dr. Kyla Driest and her associates.
Among 974 pediatric systemic lupus erythematosus (pSLE) patients in the CARRA (Childhood Arthritis & Rheumatology Research Alliance) registry cohort who had available data on thrombosis history, 24 (2.5%) had a history of arterial thrombosis and 35 (3.6%) had a history of venous thrombosis. The researchers conducted a multivariable analysis that found statistically higher odds of thrombosis (P less than .10) among patients with histories of AVN (odds ratio, 4.24; 95% confidence interval, 1.53-11.74), aPL (OR, 2.95; 95% CI, 1.38-6.28), and vasculitis (OR, 2.19; 95% CI, 1.03-4.77), whereas significantly lower odds occurred in patients with a history of renal disease (OR, 0.47; 95% CI, 0.24-0.92). Gender and body-mass index were not statistically significant.
“This study adds to our understanding of which pSLE patients are at the most risk for thrombosis,” the researchers concluded. “These results may prompt discussion concerning potential measures to prevent thrombosis in high-risk patients.”
Find the full study in Lupus (doi: 10.1177/0961203316638164).
FROM LUPUS
FDA approves two new hemophilia therapies
The Food and Drug Administration has approved Idelvion, the first hemophilia B therapy with up to 14-day dosing intervals, and Kovaltry, an unmodified, full-length factor VIII compound for the treatment of hemophilia A.
Idelvion is a novel long-acting recombinant albumin fusion protein administered intravenously. In clinical trials from the PROLONG-9FP clinical development program – including phase I through III open-label, multicenter studies – the biotherapeutic agent maintained factor IX activity levels above 5% over 14 days at a dose of 75 IU/kg, resulting in a median annualized spontaneous bleeding rate of zero in patients, according to a statement by Idelvion’s manufacturer, CSL Behring. That “reduces the monthly number of units needed for prophylaxis therapy,” the company noted.
Idelvion is indicated in children and adults with hemophilia B for routine prophylaxis, as well as for on-demand control and prevention of bleeding episodes. It is also indicated for the perioperative management of bleeding. With on-demand treatment, 94% of bleeds were controlled with one infusion, and 99% were controlled with one or two infusions.
Appropriate patients 12 years and older can go up to 14 days between infusions, according to CSL Behring. The most common adverse reaction in the clinical trials was headache.
Idelvion is expected to be available later this month.
“The approval of this long-acting recombinant factor IX therapy for hemophilia B is vital, as physicians need more options to help their patients effectively and safely manage their bleeding disorder,” said Elena Santagostino, M.D., Ph.D., of the University of Milan/IRCCS Maggiore Hospital, and lead investigator of PROLONG-9FP, in a statement. “This provides them with greater freedom from frequent infusions.”
The FDA approved Kovaltry for children and adults with hemophilia A based on the results of the LEOPOLD (Long-Term Efficacy Open-Label Program in Severe Hemophilia A Disease) clinical trials. Kovaltry received approval in Europe and Canada earlier this year.
The LEOPOLD findings supported the approval for routine prophylaxis to reduce the frequency of bleeding episodes.
“In the LEOPOLD trials, Kovaltry reduced bleeding episodes in patients with hemophilia A when infused twice to three times per week with routine prophylaxis,” said Dr. Sanjay P. Ahuja, LEOPOLD investigator and director of the hemostasis and thrombosis center at University Hospitals Rainbow Babies & Children’s Hospital, Cleveland, in a statement by Kovaltry manufacturer Bayer. “Kovaltry may offer appropriate patients a twice-weekly prophylaxis dosing option.”
Dosing is 20-40 IU/kg of body weight 2-3 times per week in adolescents and adults, and 25-50 IU/kg of body weight 2-3 times per week or every other day in children aged 12 years or younger.
The most common adverse events associated with Kovaltry in the clinical trials were headache, fever, and pruritus.
The Food and Drug Administration has approved Idelvion, the first hemophilia B therapy with up to 14-day dosing intervals, and Kovaltry, an unmodified, full-length factor VIII compound for the treatment of hemophilia A.
Idelvion is a novel long-acting recombinant albumin fusion protein administered intravenously. In clinical trials from the PROLONG-9FP clinical development program – including phase I through III open-label, multicenter studies – the biotherapeutic agent maintained factor IX activity levels above 5% over 14 days at a dose of 75 IU/kg, resulting in a median annualized spontaneous bleeding rate of zero in patients, according to a statement by Idelvion’s manufacturer, CSL Behring. That “reduces the monthly number of units needed for prophylaxis therapy,” the company noted.
Idelvion is indicated in children and adults with hemophilia B for routine prophylaxis, as well as for on-demand control and prevention of bleeding episodes. It is also indicated for the perioperative management of bleeding. With on-demand treatment, 94% of bleeds were controlled with one infusion, and 99% were controlled with one or two infusions.
Appropriate patients 12 years and older can go up to 14 days between infusions, according to CSL Behring. The most common adverse reaction in the clinical trials was headache.
Idelvion is expected to be available later this month.
“The approval of this long-acting recombinant factor IX therapy for hemophilia B is vital, as physicians need more options to help their patients effectively and safely manage their bleeding disorder,” said Elena Santagostino, M.D., Ph.D., of the University of Milan/IRCCS Maggiore Hospital, and lead investigator of PROLONG-9FP, in a statement. “This provides them with greater freedom from frequent infusions.”
The FDA approved Kovaltry for children and adults with hemophilia A based on the results of the LEOPOLD (Long-Term Efficacy Open-Label Program in Severe Hemophilia A Disease) clinical trials. Kovaltry received approval in Europe and Canada earlier this year.
The LEOPOLD findings supported the approval for routine prophylaxis to reduce the frequency of bleeding episodes.
“In the LEOPOLD trials, Kovaltry reduced bleeding episodes in patients with hemophilia A when infused twice to three times per week with routine prophylaxis,” said Dr. Sanjay P. Ahuja, LEOPOLD investigator and director of the hemostasis and thrombosis center at University Hospitals Rainbow Babies & Children’s Hospital, Cleveland, in a statement by Kovaltry manufacturer Bayer. “Kovaltry may offer appropriate patients a twice-weekly prophylaxis dosing option.”
Dosing is 20-40 IU/kg of body weight 2-3 times per week in adolescents and adults, and 25-50 IU/kg of body weight 2-3 times per week or every other day in children aged 12 years or younger.
The most common adverse events associated with Kovaltry in the clinical trials were headache, fever, and pruritus.
The Food and Drug Administration has approved Idelvion, the first hemophilia B therapy with up to 14-day dosing intervals, and Kovaltry, an unmodified, full-length factor VIII compound for the treatment of hemophilia A.
Idelvion is a novel long-acting recombinant albumin fusion protein administered intravenously. In clinical trials from the PROLONG-9FP clinical development program – including phase I through III open-label, multicenter studies – the biotherapeutic agent maintained factor IX activity levels above 5% over 14 days at a dose of 75 IU/kg, resulting in a median annualized spontaneous bleeding rate of zero in patients, according to a statement by Idelvion’s manufacturer, CSL Behring. That “reduces the monthly number of units needed for prophylaxis therapy,” the company noted.
Idelvion is indicated in children and adults with hemophilia B for routine prophylaxis, as well as for on-demand control and prevention of bleeding episodes. It is also indicated for the perioperative management of bleeding. With on-demand treatment, 94% of bleeds were controlled with one infusion, and 99% were controlled with one or two infusions.
Appropriate patients 12 years and older can go up to 14 days between infusions, according to CSL Behring. The most common adverse reaction in the clinical trials was headache.
Idelvion is expected to be available later this month.
“The approval of this long-acting recombinant factor IX therapy for hemophilia B is vital, as physicians need more options to help their patients effectively and safely manage their bleeding disorder,” said Elena Santagostino, M.D., Ph.D., of the University of Milan/IRCCS Maggiore Hospital, and lead investigator of PROLONG-9FP, in a statement. “This provides them with greater freedom from frequent infusions.”
The FDA approved Kovaltry for children and adults with hemophilia A based on the results of the LEOPOLD (Long-Term Efficacy Open-Label Program in Severe Hemophilia A Disease) clinical trials. Kovaltry received approval in Europe and Canada earlier this year.
The LEOPOLD findings supported the approval for routine prophylaxis to reduce the frequency of bleeding episodes.
“In the LEOPOLD trials, Kovaltry reduced bleeding episodes in patients with hemophilia A when infused twice to three times per week with routine prophylaxis,” said Dr. Sanjay P. Ahuja, LEOPOLD investigator and director of the hemostasis and thrombosis center at University Hospitals Rainbow Babies & Children’s Hospital, Cleveland, in a statement by Kovaltry manufacturer Bayer. “Kovaltry may offer appropriate patients a twice-weekly prophylaxis dosing option.”
Dosing is 20-40 IU/kg of body weight 2-3 times per week in adolescents and adults, and 25-50 IU/kg of body weight 2-3 times per week or every other day in children aged 12 years or younger.
The most common adverse events associated with Kovaltry in the clinical trials were headache, fever, and pruritus.
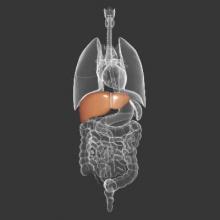
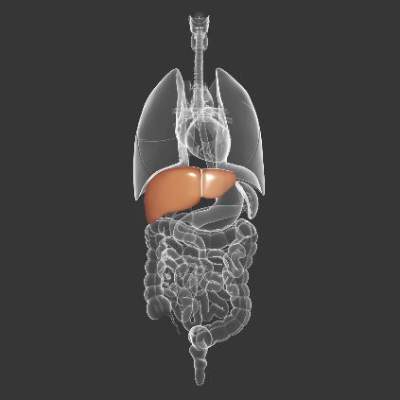
Thrombocytopenia signals multiorgan system failure in acute liver failure
In patients with acute liver failure (ALF), decreasing platelet counts after hospital admission signaled systemic inflammation and a greater likelihood of systemic complications, such as high-grade hepatic encephalopathy, cardiovascular collapse, the need for liver transplant, and death, according to researchers.
Patients with systemic inflammatory response syndrome (SIRS) had significantly lower platelet counts at admission, compared with those without SIRS, and their platelet counts decreased dramatically (from 182 plus or minus 27 times 109/L on admission to 103 plus or minus 3.20 times 109/L on day 6) compared with stable platelet counts in patients without SIRS. For days 2-7 postadmission, lower platelet counts were associated with high-grade hepatic encephalopathy, as well as the need for vasopressor support and renal replacement therapy (P less than or equal to .001 for all).
“We hypothesize that the decrease in platelet count represents an integral event in the pathogenesis of the ALF syndrome rather than a nonspecific marker of suppressed bone marrow production. Further studies will be needed to prove the pivotal role of platelets in mediating the proinflammatory and prothrombotic features of the ALF syndrome,” wrote Dr. R. Todd Stravitz, professor of medicine, section of hepatology, Virginia Commonwealth University, Richmond, and his colleagues (Clin Gastroenterol Hepatol. 2016 Feb 25. doi: 10.1016/j.cgh.2015.09.029).
Results showed that SIRS and multiorgan system failure (MOSF) were more closely linked to a decrease in platelet counts than to an increase in the international normalized ratio (INR), a laboratory marker for liver injury. The INR was similar in patients with and without SIRS, in high-grade and low-grade hepatic encephalopathy, and in patients with and without requirements for vasopressor support and renal replacement therapy (indicators of MOSF). Given that both platelet counts and INR were associated with prognosis, the investigators suggest that these laboratory parameters may reflect different types of injury, with INR signaling primary liver injury and platelets reflecting the severity of systemic inflammation secondary to liver injury.
Platelet counts varied according to outcome on day 21. Spontaneous survivors had higher mean platelet counts than patients who underwent liver transplant, and patients who died had the lowest platelet counts. Platelet counts in spontaneous survivors decreased from day 1 to 2, then subsequently recovered; platelets decreased progressively in patients who underwent liver transplant or died. The INR trend over time according to 21-day outcome was similar to that of platelet counts.
The retrospective study evaluated data from 1598 patients who enrolled in the ALF Study Group from 1998 to 2012. The mean age was 41 years, 76% were Caucasian, and 70% were female. Nearly one-half of participants (47%) had ALF due to acetaminophen overdose, 85% had at least one positive element of SIRS on admission, 32% required vasopressors, 33% required renal replacement therapy, and 50% developed high-grade (3 or 4) hepatic encephalopathy. In total, 47% of patients recovered without liver transplant, 24% underwent liver transplant, and 32% died.
The mechanism underlying development of thrombocytopenia in patients with ALF is poorly understood. Previous findings by the investigators showed that platelet-derived, prothrombotic microparticles increased in proportion to SIRS severity, and concentration of microparticles increased in parallel with laboratory markers of poor outcome. Current results suggest the converse to be true as well: platelet counts decreased in proportion to the severity of SIRS, the development of MOSF and poor outcome at 21 days.
The researchers proposed that deficiencies in the number of platelets or in liver-derived, prohemostatic coagulation factors may be compensated by systemic inflammation. Increased levels of platelet-derived microparticles in patients with ALF may overcompensate for thrombocytopenia due to a nearly 40-fold higher prothrombotic potential in tissue factor–dependent assays, compared with healthy control populations.
The findings point to the importance of platelet count in signaling impending complications in patients with ALF.
Dr. Stravitz and his coauthors reported having no disclosures.
In patients with acute liver failure (ALF), decreasing platelet counts after hospital admission signaled systemic inflammation and a greater likelihood of systemic complications, such as high-grade hepatic encephalopathy, cardiovascular collapse, the need for liver transplant, and death, according to researchers.
Patients with systemic inflammatory response syndrome (SIRS) had significantly lower platelet counts at admission, compared with those without SIRS, and their platelet counts decreased dramatically (from 182 plus or minus 27 times 109/L on admission to 103 plus or minus 3.20 times 109/L on day 6) compared with stable platelet counts in patients without SIRS. For days 2-7 postadmission, lower platelet counts were associated with high-grade hepatic encephalopathy, as well as the need for vasopressor support and renal replacement therapy (P less than or equal to .001 for all).
“We hypothesize that the decrease in platelet count represents an integral event in the pathogenesis of the ALF syndrome rather than a nonspecific marker of suppressed bone marrow production. Further studies will be needed to prove the pivotal role of platelets in mediating the proinflammatory and prothrombotic features of the ALF syndrome,” wrote Dr. R. Todd Stravitz, professor of medicine, section of hepatology, Virginia Commonwealth University, Richmond, and his colleagues (Clin Gastroenterol Hepatol. 2016 Feb 25. doi: 10.1016/j.cgh.2015.09.029).
Results showed that SIRS and multiorgan system failure (MOSF) were more closely linked to a decrease in platelet counts than to an increase in the international normalized ratio (INR), a laboratory marker for liver injury. The INR was similar in patients with and without SIRS, in high-grade and low-grade hepatic encephalopathy, and in patients with and without requirements for vasopressor support and renal replacement therapy (indicators of MOSF). Given that both platelet counts and INR were associated with prognosis, the investigators suggest that these laboratory parameters may reflect different types of injury, with INR signaling primary liver injury and platelets reflecting the severity of systemic inflammation secondary to liver injury.
Platelet counts varied according to outcome on day 21. Spontaneous survivors had higher mean platelet counts than patients who underwent liver transplant, and patients who died had the lowest platelet counts. Platelet counts in spontaneous survivors decreased from day 1 to 2, then subsequently recovered; platelets decreased progressively in patients who underwent liver transplant or died. The INR trend over time according to 21-day outcome was similar to that of platelet counts.
The retrospective study evaluated data from 1598 patients who enrolled in the ALF Study Group from 1998 to 2012. The mean age was 41 years, 76% were Caucasian, and 70% were female. Nearly one-half of participants (47%) had ALF due to acetaminophen overdose, 85% had at least one positive element of SIRS on admission, 32% required vasopressors, 33% required renal replacement therapy, and 50% developed high-grade (3 or 4) hepatic encephalopathy. In total, 47% of patients recovered without liver transplant, 24% underwent liver transplant, and 32% died.
The mechanism underlying development of thrombocytopenia in patients with ALF is poorly understood. Previous findings by the investigators showed that platelet-derived, prothrombotic microparticles increased in proportion to SIRS severity, and concentration of microparticles increased in parallel with laboratory markers of poor outcome. Current results suggest the converse to be true as well: platelet counts decreased in proportion to the severity of SIRS, the development of MOSF and poor outcome at 21 days.
The researchers proposed that deficiencies in the number of platelets or in liver-derived, prohemostatic coagulation factors may be compensated by systemic inflammation. Increased levels of platelet-derived microparticles in patients with ALF may overcompensate for thrombocytopenia due to a nearly 40-fold higher prothrombotic potential in tissue factor–dependent assays, compared with healthy control populations.
The findings point to the importance of platelet count in signaling impending complications in patients with ALF.
Dr. Stravitz and his coauthors reported having no disclosures.
In patients with acute liver failure (ALF), decreasing platelet counts after hospital admission signaled systemic inflammation and a greater likelihood of systemic complications, such as high-grade hepatic encephalopathy, cardiovascular collapse, the need for liver transplant, and death, according to researchers.
Patients with systemic inflammatory response syndrome (SIRS) had significantly lower platelet counts at admission, compared with those without SIRS, and their platelet counts decreased dramatically (from 182 plus or minus 27 times 109/L on admission to 103 plus or minus 3.20 times 109/L on day 6) compared with stable platelet counts in patients without SIRS. For days 2-7 postadmission, lower platelet counts were associated with high-grade hepatic encephalopathy, as well as the need for vasopressor support and renal replacement therapy (P less than or equal to .001 for all).
“We hypothesize that the decrease in platelet count represents an integral event in the pathogenesis of the ALF syndrome rather than a nonspecific marker of suppressed bone marrow production. Further studies will be needed to prove the pivotal role of platelets in mediating the proinflammatory and prothrombotic features of the ALF syndrome,” wrote Dr. R. Todd Stravitz, professor of medicine, section of hepatology, Virginia Commonwealth University, Richmond, and his colleagues (Clin Gastroenterol Hepatol. 2016 Feb 25. doi: 10.1016/j.cgh.2015.09.029).
Results showed that SIRS and multiorgan system failure (MOSF) were more closely linked to a decrease in platelet counts than to an increase in the international normalized ratio (INR), a laboratory marker for liver injury. The INR was similar in patients with and without SIRS, in high-grade and low-grade hepatic encephalopathy, and in patients with and without requirements for vasopressor support and renal replacement therapy (indicators of MOSF). Given that both platelet counts and INR were associated with prognosis, the investigators suggest that these laboratory parameters may reflect different types of injury, with INR signaling primary liver injury and platelets reflecting the severity of systemic inflammation secondary to liver injury.
Platelet counts varied according to outcome on day 21. Spontaneous survivors had higher mean platelet counts than patients who underwent liver transplant, and patients who died had the lowest platelet counts. Platelet counts in spontaneous survivors decreased from day 1 to 2, then subsequently recovered; platelets decreased progressively in patients who underwent liver transplant or died. The INR trend over time according to 21-day outcome was similar to that of platelet counts.
The retrospective study evaluated data from 1598 patients who enrolled in the ALF Study Group from 1998 to 2012. The mean age was 41 years, 76% were Caucasian, and 70% were female. Nearly one-half of participants (47%) had ALF due to acetaminophen overdose, 85% had at least one positive element of SIRS on admission, 32% required vasopressors, 33% required renal replacement therapy, and 50% developed high-grade (3 or 4) hepatic encephalopathy. In total, 47% of patients recovered without liver transplant, 24% underwent liver transplant, and 32% died.
The mechanism underlying development of thrombocytopenia in patients with ALF is poorly understood. Previous findings by the investigators showed that platelet-derived, prothrombotic microparticles increased in proportion to SIRS severity, and concentration of microparticles increased in parallel with laboratory markers of poor outcome. Current results suggest the converse to be true as well: platelet counts decreased in proportion to the severity of SIRS, the development of MOSF and poor outcome at 21 days.
The researchers proposed that deficiencies in the number of platelets or in liver-derived, prohemostatic coagulation factors may be compensated by systemic inflammation. Increased levels of platelet-derived microparticles in patients with ALF may overcompensate for thrombocytopenia due to a nearly 40-fold higher prothrombotic potential in tissue factor–dependent assays, compared with healthy control populations.
The findings point to the importance of platelet count in signaling impending complications in patients with ALF.
Dr. Stravitz and his coauthors reported having no disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: In patients with acute liver failure (ALF), decreasing platelet counts were associated with systemic inflammation and greater likelihood of serious systemic complications.
Major finding: Platelet counts decreased dramatically in patients with systemic inflammatory response syndrome (SIRS) (182 plus or minus 27 times 109/L on admission to 103 plus or minus 3.20 times 109/L on day 6) but remained stable in patients without SIRS; lower platelet counts were associated with high-grade hepatic encephalopathy, as well as the need for vasopressor support and renal replacement therapy (P less than or equal to .001 for all).
Data source: From 1998 to 2012 the ALF Study Group included 1,598 patients of mean age 41 years; 76% were Caucasian and 70% were female.
Disclosures: Dr. Stravitz and his coauthors reported having no disclosures.
Caplacizumab induces rapid resolution of acute TTP
Caplacizumab induces faster resolution of acute episodes of acquired thrombotic thrombocytopenic purpura than does conventional therapy by blocking further platelet aggregation mediated by von Willebrand factor, according to a report published online Feb. 11 in the New England Journal of Medicine.
Faster normalization of the platelet count “prevents further consumption of platelets into microthrombi, and the consequent progression of tissue ischemia.” This in turn should prevent further ischemic injury to the brain, heart, and kidneys in both the short and the long term, said Dr. Flora Peyvandi of the Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Ospedale Maggiore Policlinico, Milan, and her associates.
The investigators assessed caplacizumab, an anti–von Willebrand factor immunoglobulin, as a potential treatment for acquired TTP in a manufacturer-sponsored phase II trial involving 75 patients treated at 56 medical centers worldwide. All the study participants received standard treatment – daily plasma exchange and immunosuppressive therapy. In addition, they were randomly assigned to receive daily caplacizumab (36 patients) or placebo injections (39 patients) after each plasma exchange procedure and for 30 days following the final procedure, for a maximum of 90 days.
“Caplacizumab rapidly neutralized its target as indicated by suppression of von Willebrand factor–ristocetin cofactor activity to a mean of less than 20% by day 1 and throughout the treatment period,” Dr. Peyvandi and her associates said. These values returned to baseline levels within 1 week of treatment cessation.
The primary endpoint of the study, median time to normalization of the platelet count, was significantly reduced by 39% with caplacizumab compared with placebo. Among the 69 patients who had not undergone an initial plasma-exchange session before study enrollment, the median time to response was 3.0 days with caplacizumab and 4.9 days with placebo. And among the six patients who had undergone an initial plasma-exchange session before enrollment, the median time to response was 2.4 days with caplacizumab and 4.3 days with placebo, Dr. Peyvandi and her associates said (N Engl J Med. 2016 Feb 11;374[6]. doi:10.1056/NEJMoa1505533).
At 1-month follow-up, 81% of the caplacizumab group showed complete remission, compared with 46% of the placebo group. Three patients in the caplacizumab group had TTP exacerbations, compared with 11 in the placebo group. Post hoc analyses showed that the mean number of plasma-exchange days (5.9 vs. 7.9) and the mean volume of plasma administered (19.9 liters vs. 28.3 liters) were lower with caplacizumab than with placebo. And post hoc analyses of markers of end-organ damage, such as lactate dehydrogenase, troponin T, troponin 1, and creatinine levels, showed more rapid normalization with caplacizumab.
As expected, the number of patients who had bleeding-related adverse events was higher with the active treatment (19 patients) than with placebo (14 patients), but these events “were generally mild” and didn’t require treatment. Serious bleeding events occurred in 2 patients in each study group and included subarachnoid hemorrhage, retinal hemorrhage, and metrorrhagia in the caplacizumab group and cerebral hemorrhage and hematuria in the placebo group.
The study was supported by Ablynx, maker of caplacizumab, which also designed and conducted the study, analyzed the data, and prepared the manuscript. Dr. Peyvandi reported ties to Ablynx, Alexion, Biotest, Kedrion Biopharma, Novo Nordisk, Baxter, Bayer, CSL Behring, Grifols, LFB, Roche, and Sobi; her associates’ financial disclosures are available at NEJM.org.
These study findings prompt speculation that effective treatment of TTP will require a combination of interventions that target different aspects of the disorder’s pathophysiology.
First, using caplacizumab or similar agents to interfere with the binding of von Willebrand factor to platelets will prevent formation of new microthrombi. Second, replacing the specific cleaving metalloprotease ADAMTS13 using normal plasma will restore von Willebrand factor multimers to the appropriate size. And third, inducing disaggregation of platelet-rich thrombi will be the final step, but we haven’t yet found the means to accomplish that.
Dr. Agnes Veyradier is at the Institute of Hematology at the French Reference Center for Thrombotic Microangiopathies, Paris, and University Paris Diderot. Dr. Veyradier reported having no relevant financial disclosures and made these remarks in an editorial accompanying Dr. Peyvandi’s report (N Engl J Med. 2016 Feb 11;374[6]. doi:10.1056/NEJMe151876).
These study findings prompt speculation that effective treatment of TTP will require a combination of interventions that target different aspects of the disorder’s pathophysiology.
First, using caplacizumab or similar agents to interfere with the binding of von Willebrand factor to platelets will prevent formation of new microthrombi. Second, replacing the specific cleaving metalloprotease ADAMTS13 using normal plasma will restore von Willebrand factor multimers to the appropriate size. And third, inducing disaggregation of platelet-rich thrombi will be the final step, but we haven’t yet found the means to accomplish that.
Dr. Agnes Veyradier is at the Institute of Hematology at the French Reference Center for Thrombotic Microangiopathies, Paris, and University Paris Diderot. Dr. Veyradier reported having no relevant financial disclosures and made these remarks in an editorial accompanying Dr. Peyvandi’s report (N Engl J Med. 2016 Feb 11;374[6]. doi:10.1056/NEJMe151876).
These study findings prompt speculation that effective treatment of TTP will require a combination of interventions that target different aspects of the disorder’s pathophysiology.
First, using caplacizumab or similar agents to interfere with the binding of von Willebrand factor to platelets will prevent formation of new microthrombi. Second, replacing the specific cleaving metalloprotease ADAMTS13 using normal plasma will restore von Willebrand factor multimers to the appropriate size. And third, inducing disaggregation of platelet-rich thrombi will be the final step, but we haven’t yet found the means to accomplish that.
Dr. Agnes Veyradier is at the Institute of Hematology at the French Reference Center for Thrombotic Microangiopathies, Paris, and University Paris Diderot. Dr. Veyradier reported having no relevant financial disclosures and made these remarks in an editorial accompanying Dr. Peyvandi’s report (N Engl J Med. 2016 Feb 11;374[6]. doi:10.1056/NEJMe151876).
Caplacizumab induces faster resolution of acute episodes of acquired thrombotic thrombocytopenic purpura than does conventional therapy by blocking further platelet aggregation mediated by von Willebrand factor, according to a report published online Feb. 11 in the New England Journal of Medicine.
Faster normalization of the platelet count “prevents further consumption of platelets into microthrombi, and the consequent progression of tissue ischemia.” This in turn should prevent further ischemic injury to the brain, heart, and kidneys in both the short and the long term, said Dr. Flora Peyvandi of the Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Ospedale Maggiore Policlinico, Milan, and her associates.
The investigators assessed caplacizumab, an anti–von Willebrand factor immunoglobulin, as a potential treatment for acquired TTP in a manufacturer-sponsored phase II trial involving 75 patients treated at 56 medical centers worldwide. All the study participants received standard treatment – daily plasma exchange and immunosuppressive therapy. In addition, they were randomly assigned to receive daily caplacizumab (36 patients) or placebo injections (39 patients) after each plasma exchange procedure and for 30 days following the final procedure, for a maximum of 90 days.
“Caplacizumab rapidly neutralized its target as indicated by suppression of von Willebrand factor–ristocetin cofactor activity to a mean of less than 20% by day 1 and throughout the treatment period,” Dr. Peyvandi and her associates said. These values returned to baseline levels within 1 week of treatment cessation.
The primary endpoint of the study, median time to normalization of the platelet count, was significantly reduced by 39% with caplacizumab compared with placebo. Among the 69 patients who had not undergone an initial plasma-exchange session before study enrollment, the median time to response was 3.0 days with caplacizumab and 4.9 days with placebo. And among the six patients who had undergone an initial plasma-exchange session before enrollment, the median time to response was 2.4 days with caplacizumab and 4.3 days with placebo, Dr. Peyvandi and her associates said (N Engl J Med. 2016 Feb 11;374[6]. doi:10.1056/NEJMoa1505533).
At 1-month follow-up, 81% of the caplacizumab group showed complete remission, compared with 46% of the placebo group. Three patients in the caplacizumab group had TTP exacerbations, compared with 11 in the placebo group. Post hoc analyses showed that the mean number of plasma-exchange days (5.9 vs. 7.9) and the mean volume of plasma administered (19.9 liters vs. 28.3 liters) were lower with caplacizumab than with placebo. And post hoc analyses of markers of end-organ damage, such as lactate dehydrogenase, troponin T, troponin 1, and creatinine levels, showed more rapid normalization with caplacizumab.
As expected, the number of patients who had bleeding-related adverse events was higher with the active treatment (19 patients) than with placebo (14 patients), but these events “were generally mild” and didn’t require treatment. Serious bleeding events occurred in 2 patients in each study group and included subarachnoid hemorrhage, retinal hemorrhage, and metrorrhagia in the caplacizumab group and cerebral hemorrhage and hematuria in the placebo group.
The study was supported by Ablynx, maker of caplacizumab, which also designed and conducted the study, analyzed the data, and prepared the manuscript. Dr. Peyvandi reported ties to Ablynx, Alexion, Biotest, Kedrion Biopharma, Novo Nordisk, Baxter, Bayer, CSL Behring, Grifols, LFB, Roche, and Sobi; her associates’ financial disclosures are available at NEJM.org.
Caplacizumab induces faster resolution of acute episodes of acquired thrombotic thrombocytopenic purpura than does conventional therapy by blocking further platelet aggregation mediated by von Willebrand factor, according to a report published online Feb. 11 in the New England Journal of Medicine.
Faster normalization of the platelet count “prevents further consumption of platelets into microthrombi, and the consequent progression of tissue ischemia.” This in turn should prevent further ischemic injury to the brain, heart, and kidneys in both the short and the long term, said Dr. Flora Peyvandi of the Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Ospedale Maggiore Policlinico, Milan, and her associates.
The investigators assessed caplacizumab, an anti–von Willebrand factor immunoglobulin, as a potential treatment for acquired TTP in a manufacturer-sponsored phase II trial involving 75 patients treated at 56 medical centers worldwide. All the study participants received standard treatment – daily plasma exchange and immunosuppressive therapy. In addition, they were randomly assigned to receive daily caplacizumab (36 patients) or placebo injections (39 patients) after each plasma exchange procedure and for 30 days following the final procedure, for a maximum of 90 days.
“Caplacizumab rapidly neutralized its target as indicated by suppression of von Willebrand factor–ristocetin cofactor activity to a mean of less than 20% by day 1 and throughout the treatment period,” Dr. Peyvandi and her associates said. These values returned to baseline levels within 1 week of treatment cessation.
The primary endpoint of the study, median time to normalization of the platelet count, was significantly reduced by 39% with caplacizumab compared with placebo. Among the 69 patients who had not undergone an initial plasma-exchange session before study enrollment, the median time to response was 3.0 days with caplacizumab and 4.9 days with placebo. And among the six patients who had undergone an initial plasma-exchange session before enrollment, the median time to response was 2.4 days with caplacizumab and 4.3 days with placebo, Dr. Peyvandi and her associates said (N Engl J Med. 2016 Feb 11;374[6]. doi:10.1056/NEJMoa1505533).
At 1-month follow-up, 81% of the caplacizumab group showed complete remission, compared with 46% of the placebo group. Three patients in the caplacizumab group had TTP exacerbations, compared with 11 in the placebo group. Post hoc analyses showed that the mean number of plasma-exchange days (5.9 vs. 7.9) and the mean volume of plasma administered (19.9 liters vs. 28.3 liters) were lower with caplacizumab than with placebo. And post hoc analyses of markers of end-organ damage, such as lactate dehydrogenase, troponin T, troponin 1, and creatinine levels, showed more rapid normalization with caplacizumab.
As expected, the number of patients who had bleeding-related adverse events was higher with the active treatment (19 patients) than with placebo (14 patients), but these events “were generally mild” and didn’t require treatment. Serious bleeding events occurred in 2 patients in each study group and included subarachnoid hemorrhage, retinal hemorrhage, and metrorrhagia in the caplacizumab group and cerebral hemorrhage and hematuria in the placebo group.
The study was supported by Ablynx, maker of caplacizumab, which also designed and conducted the study, analyzed the data, and prepared the manuscript. Dr. Peyvandi reported ties to Ablynx, Alexion, Biotest, Kedrion Biopharma, Novo Nordisk, Baxter, Bayer, CSL Behring, Grifols, LFB, Roche, and Sobi; her associates’ financial disclosures are available at NEJM.org.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Caplacizumab induces more rapid resolution of an acute TTP episode than does conventional therapy.
Major finding: At 1-month follow-up, 81% of the caplacizumab group showed complete remission, compared with 46% of the placebo group.
Data source: An international single-blind placebo-controlled phase II trial involving 75 patients treated over a 3.5-year period.
Disclosures: This study was supported by Ablynx, maker of caplacizumab, which also designed and conducted the study, analyzed the data, and prepared the manuscript. Dr. Peyvandi reported ties to Ablynx, Alexion, Biotest, Kedrion Biopharma, Novo Nordisk, Baxter, Bayer, CSL Behring, Grifols, LFB, Roche, and Sobi; her associates’ financial disclosures are available at NEJM.org.
Defibrotide offers benefit for severe veno-occlusive disease and multiorgan failure
Defibrotide improved survival at 100 days after hematopoietic stem cell transplantation (HSCT) in patients with hepatic veno-occlusive disease, based on an open-label trial that compared trial participants with historical controls.
Of the 102 patients in the defibrotide group, 39 were alive 100 days after HSCT (38.2%), compared with 8 of 32 (25.0%) in the historical control group. The propensity-adjusted, between-group difference was 23.0% (95.1% confidence interval, 5.2%-40.8%; P = .0109). At 180 days post-HSCT, the difference in survival between the groups was not significant (Blood. 2016 Feb 3. doi: 10.1182/blood-2015-10-676924).
Defibrotide has Fast Track designation from the FDA and the new drug application is currently under Priority Review with a decision expected by March 31, 2016. A potentially fatal complication of HSCT, hepatic veno-occlusive disease is characterized by hepatomegaly, jaundice, rapid weight gain, fluid retention, and ascites. There are no approved therapies.
“In this context, defibrotide provides a promising treatment option for patients with a high unmet medical need,” wrote Dr. Paul G. Richardson of Dana-Farber Cancer Institute in Boston and his colleagues.
At day 100 post-HSCT, complete response was seen in 25.5% of the defibrotide group and in 12.5% of the historical control group. The propensity-adjusted, between-group difference was 19% (95.1% CI, 3.5-34.6%; P = .0160). The complete response was durable in 22 of the 26 patients. In the control group, complete response was limited in two patients, impossible to assess in one patient, and durable in one patient.
The multicenter, open-label, phase III trial prospectively enrolled 102 patients with hepatic veno-occlusive disease from 2006 to 2008. Defibrotide was administered intravenously at 25 mg/kg/day in 4 divided doses for a minimum of 21 days. Treatment continued beyond 21 days until resolution of veno-occlusive disease or until the patient was discharged from the hospital.
To identify the historical controls, 6,867 medical charts of HSCT patients hospitalized from 1995 to 2007 were reviewed, and 32 historical control patients were selected. Most (21 of 32) were diagnosed with during 2000-2006, and 11 were diagnosed before 2000. The historical controls were selected by an independent medical review committee, and met the same entry criteria as the defibrotide group.
Because recruiting for the defibrotide group and screening for historical controls occurred at the same institutions during similar time periods, patient management and supportive care were likely similar for the two groups. Propensity scores were included in the analysis to adjust for prognostic factors that were unbalanced between treatment and control groups, including ventilator and dialysis dependency at study entry, age greater or less than 16 years, prior HSCT (0 vs. 1), and allogeneic or autologous transplant.
Hypotension was the most common adverse event reported in the defibrotide and control groups (39% and 50%, respectively), followed by diarrhea (23.5% and 37.5%, respectively). The defibrotide and control groups had similar incidences of common hemorrhagic adverse events (64% and 75%, respectively). Fatal adverse events occurred in 64% of the defibrotide group and 69% of the control group, and fatal hemorrhagic events occurred in 14.7% of the defibrotide group and 6.3% of the control group.
Approved by the European Union, defibrotide is a single-stranded, deoxyribonucleic acid derivative that stabilizes damaged endothelial cells and prevents further endothelial cell damage.
Dr. Richardson reported consulting or advisory roles with Gentium/Jazz Pharmaceuticals, the maker of defibrotide.
Defibrotide improved survival at 100 days after hematopoietic stem cell transplantation (HSCT) in patients with hepatic veno-occlusive disease, based on an open-label trial that compared trial participants with historical controls.
Of the 102 patients in the defibrotide group, 39 were alive 100 days after HSCT (38.2%), compared with 8 of 32 (25.0%) in the historical control group. The propensity-adjusted, between-group difference was 23.0% (95.1% confidence interval, 5.2%-40.8%; P = .0109). At 180 days post-HSCT, the difference in survival between the groups was not significant (Blood. 2016 Feb 3. doi: 10.1182/blood-2015-10-676924).
Defibrotide has Fast Track designation from the FDA and the new drug application is currently under Priority Review with a decision expected by March 31, 2016. A potentially fatal complication of HSCT, hepatic veno-occlusive disease is characterized by hepatomegaly, jaundice, rapid weight gain, fluid retention, and ascites. There are no approved therapies.
“In this context, defibrotide provides a promising treatment option for patients with a high unmet medical need,” wrote Dr. Paul G. Richardson of Dana-Farber Cancer Institute in Boston and his colleagues.
At day 100 post-HSCT, complete response was seen in 25.5% of the defibrotide group and in 12.5% of the historical control group. The propensity-adjusted, between-group difference was 19% (95.1% CI, 3.5-34.6%; P = .0160). The complete response was durable in 22 of the 26 patients. In the control group, complete response was limited in two patients, impossible to assess in one patient, and durable in one patient.
The multicenter, open-label, phase III trial prospectively enrolled 102 patients with hepatic veno-occlusive disease from 2006 to 2008. Defibrotide was administered intravenously at 25 mg/kg/day in 4 divided doses for a minimum of 21 days. Treatment continued beyond 21 days until resolution of veno-occlusive disease or until the patient was discharged from the hospital.
To identify the historical controls, 6,867 medical charts of HSCT patients hospitalized from 1995 to 2007 were reviewed, and 32 historical control patients were selected. Most (21 of 32) were diagnosed with during 2000-2006, and 11 were diagnosed before 2000. The historical controls were selected by an independent medical review committee, and met the same entry criteria as the defibrotide group.
Because recruiting for the defibrotide group and screening for historical controls occurred at the same institutions during similar time periods, patient management and supportive care were likely similar for the two groups. Propensity scores were included in the analysis to adjust for prognostic factors that were unbalanced between treatment and control groups, including ventilator and dialysis dependency at study entry, age greater or less than 16 years, prior HSCT (0 vs. 1), and allogeneic or autologous transplant.
Hypotension was the most common adverse event reported in the defibrotide and control groups (39% and 50%, respectively), followed by diarrhea (23.5% and 37.5%, respectively). The defibrotide and control groups had similar incidences of common hemorrhagic adverse events (64% and 75%, respectively). Fatal adverse events occurred in 64% of the defibrotide group and 69% of the control group, and fatal hemorrhagic events occurred in 14.7% of the defibrotide group and 6.3% of the control group.
Approved by the European Union, defibrotide is a single-stranded, deoxyribonucleic acid derivative that stabilizes damaged endothelial cells and prevents further endothelial cell damage.
Dr. Richardson reported consulting or advisory roles with Gentium/Jazz Pharmaceuticals, the maker of defibrotide.
Defibrotide improved survival at 100 days after hematopoietic stem cell transplantation (HSCT) in patients with hepatic veno-occlusive disease, based on an open-label trial that compared trial participants with historical controls.
Of the 102 patients in the defibrotide group, 39 were alive 100 days after HSCT (38.2%), compared with 8 of 32 (25.0%) in the historical control group. The propensity-adjusted, between-group difference was 23.0% (95.1% confidence interval, 5.2%-40.8%; P = .0109). At 180 days post-HSCT, the difference in survival between the groups was not significant (Blood. 2016 Feb 3. doi: 10.1182/blood-2015-10-676924).
Defibrotide has Fast Track designation from the FDA and the new drug application is currently under Priority Review with a decision expected by March 31, 2016. A potentially fatal complication of HSCT, hepatic veno-occlusive disease is characterized by hepatomegaly, jaundice, rapid weight gain, fluid retention, and ascites. There are no approved therapies.
“In this context, defibrotide provides a promising treatment option for patients with a high unmet medical need,” wrote Dr. Paul G. Richardson of Dana-Farber Cancer Institute in Boston and his colleagues.
At day 100 post-HSCT, complete response was seen in 25.5% of the defibrotide group and in 12.5% of the historical control group. The propensity-adjusted, between-group difference was 19% (95.1% CI, 3.5-34.6%; P = .0160). The complete response was durable in 22 of the 26 patients. In the control group, complete response was limited in two patients, impossible to assess in one patient, and durable in one patient.
The multicenter, open-label, phase III trial prospectively enrolled 102 patients with hepatic veno-occlusive disease from 2006 to 2008. Defibrotide was administered intravenously at 25 mg/kg/day in 4 divided doses for a minimum of 21 days. Treatment continued beyond 21 days until resolution of veno-occlusive disease or until the patient was discharged from the hospital.
To identify the historical controls, 6,867 medical charts of HSCT patients hospitalized from 1995 to 2007 were reviewed, and 32 historical control patients were selected. Most (21 of 32) were diagnosed with during 2000-2006, and 11 were diagnosed before 2000. The historical controls were selected by an independent medical review committee, and met the same entry criteria as the defibrotide group.
Because recruiting for the defibrotide group and screening for historical controls occurred at the same institutions during similar time periods, patient management and supportive care were likely similar for the two groups. Propensity scores were included in the analysis to adjust for prognostic factors that were unbalanced between treatment and control groups, including ventilator and dialysis dependency at study entry, age greater or less than 16 years, prior HSCT (0 vs. 1), and allogeneic or autologous transplant.
Hypotension was the most common adverse event reported in the defibrotide and control groups (39% and 50%, respectively), followed by diarrhea (23.5% and 37.5%, respectively). The defibrotide and control groups had similar incidences of common hemorrhagic adverse events (64% and 75%, respectively). Fatal adverse events occurred in 64% of the defibrotide group and 69% of the control group, and fatal hemorrhagic events occurred in 14.7% of the defibrotide group and 6.3% of the control group.
Approved by the European Union, defibrotide is a single-stranded, deoxyribonucleic acid derivative that stabilizes damaged endothelial cells and prevents further endothelial cell damage.
Dr. Richardson reported consulting or advisory roles with Gentium/Jazz Pharmaceuticals, the maker of defibrotide.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Defibrotide improved survival at 100 days after hematopoietic stem cell transplantation (HSCT) in patients with hepatic veno-occlusive disease, based on an open-label trial that compared trial participants with historical controls.
Major finding: At day 100 post-HSCT, survival was 38.2% for the treatment group and 25.0% for historical controls; propensity-adjusted between-group difference, 23.0%; 95.1% CI, 5.2%-40.8%; P = .0109).
Data source: The multicenter phase III trial included 102 patients with hepatic veno-occlusive disease in the defibrotide group and 32 patients selected for the historical control group.
Disclosures: Dr. Richardson reported consulting or advisory roles with Gentium/Jazz Pharmaceuticals, the maker of defibrotide.
FDA approves first recombinant von Willebrand treatment
The first recombinant von Willebrand factor has been approved for von Willebrand disease, the Food and Drug Administration announced.
Dr. Karen Midthun, director of the FDA’s Center for Biologics Evaluation and Research, said in the Dec. 8 statement that the approval “provides an additional therapeutic option for the treatment of bleeding episodes in patients with von Willebrand disease.”
The product was approved for on-demand therapy for bleeding episodes in adults with the disorder, which affects up to 1% of the U.S. population. Current treatment options include desmopressin and plasma-derived von Willebrand factor/factor VIII concentrates. The latter are limited by donor availability and are variable in the factor VIII levels they contain.
The new treatment, marketed as Vonvendi (Baxalta), is administered by infusion. The product contains only trace amounts of factor VIII, allowing for administration of factor VIII only as needed.
In a phase III manufacturer-sponsored nonrandomized trial of 22 subjects, bleeding was controlled with a single infusion in 82% of 192 bleeding episodes, according to a Baxalta press release. Patients with major bleeding required as many as four infusions, but treatment was effective in all subjects and 97% had an excellent efficacy rating and the remaining 3% had a good efficacy rating.
The most common adverse event was pruritus. No thrombotic events or severe allergic reactions were reported. Patients did not develop neutralizing antibodies against von Willebrand factor or factor VIII during the course of the study.
In its news release, the manufacturer said it is building a clinical development program to increase patient access to the treatment and evaluate its use in “prophylaxis, surgical, and pediatric indications.” Vonvendi is expected to be available in late 2016.
The first recombinant von Willebrand factor has been approved for von Willebrand disease, the Food and Drug Administration announced.
Dr. Karen Midthun, director of the FDA’s Center for Biologics Evaluation and Research, said in the Dec. 8 statement that the approval “provides an additional therapeutic option for the treatment of bleeding episodes in patients with von Willebrand disease.”
The product was approved for on-demand therapy for bleeding episodes in adults with the disorder, which affects up to 1% of the U.S. population. Current treatment options include desmopressin and plasma-derived von Willebrand factor/factor VIII concentrates. The latter are limited by donor availability and are variable in the factor VIII levels they contain.
The new treatment, marketed as Vonvendi (Baxalta), is administered by infusion. The product contains only trace amounts of factor VIII, allowing for administration of factor VIII only as needed.
In a phase III manufacturer-sponsored nonrandomized trial of 22 subjects, bleeding was controlled with a single infusion in 82% of 192 bleeding episodes, according to a Baxalta press release. Patients with major bleeding required as many as four infusions, but treatment was effective in all subjects and 97% had an excellent efficacy rating and the remaining 3% had a good efficacy rating.
The most common adverse event was pruritus. No thrombotic events or severe allergic reactions were reported. Patients did not develop neutralizing antibodies against von Willebrand factor or factor VIII during the course of the study.
In its news release, the manufacturer said it is building a clinical development program to increase patient access to the treatment and evaluate its use in “prophylaxis, surgical, and pediatric indications.” Vonvendi is expected to be available in late 2016.
The first recombinant von Willebrand factor has been approved for von Willebrand disease, the Food and Drug Administration announced.
Dr. Karen Midthun, director of the FDA’s Center for Biologics Evaluation and Research, said in the Dec. 8 statement that the approval “provides an additional therapeutic option for the treatment of bleeding episodes in patients with von Willebrand disease.”
The product was approved for on-demand therapy for bleeding episodes in adults with the disorder, which affects up to 1% of the U.S. population. Current treatment options include desmopressin and plasma-derived von Willebrand factor/factor VIII concentrates. The latter are limited by donor availability and are variable in the factor VIII levels they contain.
The new treatment, marketed as Vonvendi (Baxalta), is administered by infusion. The product contains only trace amounts of factor VIII, allowing for administration of factor VIII only as needed.
In a phase III manufacturer-sponsored nonrandomized trial of 22 subjects, bleeding was controlled with a single infusion in 82% of 192 bleeding episodes, according to a Baxalta press release. Patients with major bleeding required as many as four infusions, but treatment was effective in all subjects and 97% had an excellent efficacy rating and the remaining 3% had a good efficacy rating.
The most common adverse event was pruritus. No thrombotic events or severe allergic reactions were reported. Patients did not develop neutralizing antibodies against von Willebrand factor or factor VIII during the course of the study.
In its news release, the manufacturer said it is building a clinical development program to increase patient access to the treatment and evaluate its use in “prophylaxis, surgical, and pediatric indications.” Vonvendi is expected to be available in late 2016.
Video: Eltrombopag boosted standard therapy in severe, newly-diagnosed aplastic anemia
ORLANDO – Adding eltrombopag to standard immunosuppressive treatment for newly-diagnosed severe aplastic anemia boosted overall response rates from around 65% to over 90%, based on a late-breaker study presented by Dr. Danielle M. Townsley at the annual meeting of the American Society of Hematology.
Dr. Townsley said that starting the combination treatment immediately at diagnosis, rather than waiting to introduce eltrombopag at either 2 weeks or 3 months after initiating standard immunosuppressive therapy, was associated with better outcomes. As a result of the findings, a cohort extension of the trial will continue and is enrolling patients.
In our video interview, Dr. Townsley discusses the top-level results and says that it’s too early to introduce the protocol into practice outside a clinical trial. She urges hematologists to enroll their patients in the ongoing cohort study.
On Twitter @maryjodales
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Adding eltrombopag to standard immunosuppressive treatment for newly-diagnosed severe aplastic anemia boosted overall response rates from around 65% to over 90%, based on a late-breaker study presented by Dr. Danielle M. Townsley at the annual meeting of the American Society of Hematology.
Dr. Townsley said that starting the combination treatment immediately at diagnosis, rather than waiting to introduce eltrombopag at either 2 weeks or 3 months after initiating standard immunosuppressive therapy, was associated with better outcomes. As a result of the findings, a cohort extension of the trial will continue and is enrolling patients.
In our video interview, Dr. Townsley discusses the top-level results and says that it’s too early to introduce the protocol into practice outside a clinical trial. She urges hematologists to enroll their patients in the ongoing cohort study.
On Twitter @maryjodales
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Adding eltrombopag to standard immunosuppressive treatment for newly-diagnosed severe aplastic anemia boosted overall response rates from around 65% to over 90%, based on a late-breaker study presented by Dr. Danielle M. Townsley at the annual meeting of the American Society of Hematology.
Dr. Townsley said that starting the combination treatment immediately at diagnosis, rather than waiting to introduce eltrombopag at either 2 weeks or 3 months after initiating standard immunosuppressive therapy, was associated with better outcomes. As a result of the findings, a cohort extension of the trial will continue and is enrolling patients.
In our video interview, Dr. Townsley discusses the top-level results and says that it’s too early to introduce the protocol into practice outside a clinical trial. She urges hematologists to enroll their patients in the ongoing cohort study.
On Twitter @maryjodales
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2015
VIDEO: ASH highlights five Choosing Wisely initiatives
ORLANDO – Five “Choosing Wisely” initiatives selected from other specialties were featured at the annual meeting of the American Society of Hematology.
Dr. Lisa Hicks of St. Michael’s Hospital in Toronto led ASH’s Choosing Wisely list and moderated their presentation and the discussion of this year’s recommendations at the meeting. In a video interview, Dr. Hicks discussed the five recommendations and how hematologists can influence better patient care through cross-specialty collaborations. The complete Choosing Wisely list is available at www.hematology.org/choosingwisely
The 2015 Choosing Wisely recommendations, selected from recommendations made previously by other organizations, are:
• Don’t image for suspected pulmonary embolism (PE) without moderate or high pre-test probability of PE. (American College of Radiology)
• Don’t perform repetitive CBC and chemistry testing in the face of clinical and lab stability. (Society of Hospital Medicine)
• Don’t routinely order thrombophilia testing on patients undergoing a routine infertility evaluation. (American Society of Reproductive Medicine)
• Don’t transfuse red blood cells for iron deficiency without hemodynamic instability. (American Association of Blood Banks)
• Avoid using PET or PET-CT scanning as part of routine follow-up care to monitor for a cancer recurrence in asymptomatic patients who have finished initial treatment to eliminate the cancer unless there is high-level evidence that such imaging will change the outcome. (American Society of Clinical Oncology)
Choosing Wisely is an initiative of the ABIM Foundation. Dr. Hicks had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Five “Choosing Wisely” initiatives selected from other specialties were featured at the annual meeting of the American Society of Hematology.
Dr. Lisa Hicks of St. Michael’s Hospital in Toronto led ASH’s Choosing Wisely list and moderated their presentation and the discussion of this year’s recommendations at the meeting. In a video interview, Dr. Hicks discussed the five recommendations and how hematologists can influence better patient care through cross-specialty collaborations. The complete Choosing Wisely list is available at www.hematology.org/choosingwisely
The 2015 Choosing Wisely recommendations, selected from recommendations made previously by other organizations, are:
• Don’t image for suspected pulmonary embolism (PE) without moderate or high pre-test probability of PE. (American College of Radiology)
• Don’t perform repetitive CBC and chemistry testing in the face of clinical and lab stability. (Society of Hospital Medicine)
• Don’t routinely order thrombophilia testing on patients undergoing a routine infertility evaluation. (American Society of Reproductive Medicine)
• Don’t transfuse red blood cells for iron deficiency without hemodynamic instability. (American Association of Blood Banks)
• Avoid using PET or PET-CT scanning as part of routine follow-up care to monitor for a cancer recurrence in asymptomatic patients who have finished initial treatment to eliminate the cancer unless there is high-level evidence that such imaging will change the outcome. (American Society of Clinical Oncology)
Choosing Wisely is an initiative of the ABIM Foundation. Dr. Hicks had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Five “Choosing Wisely” initiatives selected from other specialties were featured at the annual meeting of the American Society of Hematology.
Dr. Lisa Hicks of St. Michael’s Hospital in Toronto led ASH’s Choosing Wisely list and moderated their presentation and the discussion of this year’s recommendations at the meeting. In a video interview, Dr. Hicks discussed the five recommendations and how hematologists can influence better patient care through cross-specialty collaborations. The complete Choosing Wisely list is available at www.hematology.org/choosingwisely
The 2015 Choosing Wisely recommendations, selected from recommendations made previously by other organizations, are:
• Don’t image for suspected pulmonary embolism (PE) without moderate or high pre-test probability of PE. (American College of Radiology)
• Don’t perform repetitive CBC and chemistry testing in the face of clinical and lab stability. (Society of Hospital Medicine)
• Don’t routinely order thrombophilia testing on patients undergoing a routine infertility evaluation. (American Society of Reproductive Medicine)
• Don’t transfuse red blood cells for iron deficiency without hemodynamic instability. (American Association of Blood Banks)
• Avoid using PET or PET-CT scanning as part of routine follow-up care to monitor for a cancer recurrence in asymptomatic patients who have finished initial treatment to eliminate the cancer unless there is high-level evidence that such imaging will change the outcome. (American Society of Clinical Oncology)
Choosing Wisely is an initiative of the ABIM Foundation. Dr. Hicks had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2015
ASH: Genes affecting risk, severity of chronic ITP are identified
ORLANDO – Children with chronic immune thrombocytopenia (ITP) have an increased frequency of damaging variants in genes associated with cellular immunity, notably IFNA17 and IFNLR1, based on the results of whole genome sequencing.
The links to IFNA17 and IFNLR1 genes, which are involved in T cell pathways, remain significant when patients are stratified according to disease severity, Dr. Jenny M. Despotovic reported at the annual meeting of the American Sociey of Hematology. The finding is further evidence for the role of T cell abnormalities in the pathophysiology of chronic ITP.
These may be important candidate genes involved in immune regulation and in sustained autoimmunity, which appears to be due to generalized immune dysregulation that includes altered T cell balance with a shift toward immune activation (increased Th1/Th2 ratio) as well as decreased number and impaired function of regulatory T cells, said Dr. Despotovic, of Texas Children’s Cancer and Hematology Centers, Baylor College of Medicine, Houston.
In their study, Dr. Despotovic and her colleagues performed whole exome sequencing on 262 samples with robust phenotype data from children with chronic ITP in the North American Chronic ITP Registry and the Platelet Disorders Center at the Weill-Cornell Medical Center. All but three patients were less than 20 years old at diagnosis; 83% had primary ITP, 10% had Evans syndrome, and 7% had other autoimmune disorders.
To identify candidate genes associated with ITP susceptibility, sequencing data were compared for 172 ITP cases of European American ancestry and 5,664 controls of European American ancestry with platelet levels over 150 x 109/L in the Atherosclerosis Risk in Communities (ARIC) Study. In a separate analysis, phenotype data for ITP cases were reviewed and cases were stratified by disease severity according to the need for second line treatment.
A significant increase in the frequency of several damaging variants were identified in genes in the ITP cohort. The most significant associations were detected in the IFNA17 gene, which is involved in transforming growth factor beta secretion and could affect number and function of regulatory T cells.
IFNA17 rs9298814 was identified in 26% of cases in the ITP cohort compared to less than 0.01% of controls. In all, 43% of ITP patients had at a presumed deleterious variant of IFNA17.
IFNA17 gene variants remained significant in the most severely affected patients, specifically those requiring second line therapy, providing further evidence for this gene’s functional relevance in the pathogenesis and pathophysiology of ITP, Dr. Despotovic said.
Other genes with known impact on T cell number or function, including DGCR14, SMAD2 and CD83 also contained an increased frequency of variants in the European American ITP cohort. IFNLR1 and REL genes were also significantly associated with need for second line ITP therapy.
Analysis of this large cohort did not validate any of over 20 variants that have been previously published as candidates for ITP susceptibility or evolution to chronic ITP, she added.
On Twitter @maryjodales
ORLANDO – Children with chronic immune thrombocytopenia (ITP) have an increased frequency of damaging variants in genes associated with cellular immunity, notably IFNA17 and IFNLR1, based on the results of whole genome sequencing.
The links to IFNA17 and IFNLR1 genes, which are involved in T cell pathways, remain significant when patients are stratified according to disease severity, Dr. Jenny M. Despotovic reported at the annual meeting of the American Sociey of Hematology. The finding is further evidence for the role of T cell abnormalities in the pathophysiology of chronic ITP.
These may be important candidate genes involved in immune regulation and in sustained autoimmunity, which appears to be due to generalized immune dysregulation that includes altered T cell balance with a shift toward immune activation (increased Th1/Th2 ratio) as well as decreased number and impaired function of regulatory T cells, said Dr. Despotovic, of Texas Children’s Cancer and Hematology Centers, Baylor College of Medicine, Houston.
In their study, Dr. Despotovic and her colleagues performed whole exome sequencing on 262 samples with robust phenotype data from children with chronic ITP in the North American Chronic ITP Registry and the Platelet Disorders Center at the Weill-Cornell Medical Center. All but three patients were less than 20 years old at diagnosis; 83% had primary ITP, 10% had Evans syndrome, and 7% had other autoimmune disorders.
To identify candidate genes associated with ITP susceptibility, sequencing data were compared for 172 ITP cases of European American ancestry and 5,664 controls of European American ancestry with platelet levels over 150 x 109/L in the Atherosclerosis Risk in Communities (ARIC) Study. In a separate analysis, phenotype data for ITP cases were reviewed and cases were stratified by disease severity according to the need for second line treatment.
A significant increase in the frequency of several damaging variants were identified in genes in the ITP cohort. The most significant associations were detected in the IFNA17 gene, which is involved in transforming growth factor beta secretion and could affect number and function of regulatory T cells.
IFNA17 rs9298814 was identified in 26% of cases in the ITP cohort compared to less than 0.01% of controls. In all, 43% of ITP patients had at a presumed deleterious variant of IFNA17.
IFNA17 gene variants remained significant in the most severely affected patients, specifically those requiring second line therapy, providing further evidence for this gene’s functional relevance in the pathogenesis and pathophysiology of ITP, Dr. Despotovic said.
Other genes with known impact on T cell number or function, including DGCR14, SMAD2 and CD83 also contained an increased frequency of variants in the European American ITP cohort. IFNLR1 and REL genes were also significantly associated with need for second line ITP therapy.
Analysis of this large cohort did not validate any of over 20 variants that have been previously published as candidates for ITP susceptibility or evolution to chronic ITP, she added.
On Twitter @maryjodales
ORLANDO – Children with chronic immune thrombocytopenia (ITP) have an increased frequency of damaging variants in genes associated with cellular immunity, notably IFNA17 and IFNLR1, based on the results of whole genome sequencing.
The links to IFNA17 and IFNLR1 genes, which are involved in T cell pathways, remain significant when patients are stratified according to disease severity, Dr. Jenny M. Despotovic reported at the annual meeting of the American Sociey of Hematology. The finding is further evidence for the role of T cell abnormalities in the pathophysiology of chronic ITP.
These may be important candidate genes involved in immune regulation and in sustained autoimmunity, which appears to be due to generalized immune dysregulation that includes altered T cell balance with a shift toward immune activation (increased Th1/Th2 ratio) as well as decreased number and impaired function of regulatory T cells, said Dr. Despotovic, of Texas Children’s Cancer and Hematology Centers, Baylor College of Medicine, Houston.
In their study, Dr. Despotovic and her colleagues performed whole exome sequencing on 262 samples with robust phenotype data from children with chronic ITP in the North American Chronic ITP Registry and the Platelet Disorders Center at the Weill-Cornell Medical Center. All but three patients were less than 20 years old at diagnosis; 83% had primary ITP, 10% had Evans syndrome, and 7% had other autoimmune disorders.
To identify candidate genes associated with ITP susceptibility, sequencing data were compared for 172 ITP cases of European American ancestry and 5,664 controls of European American ancestry with platelet levels over 150 x 109/L in the Atherosclerosis Risk in Communities (ARIC) Study. In a separate analysis, phenotype data for ITP cases were reviewed and cases were stratified by disease severity according to the need for second line treatment.
A significant increase in the frequency of several damaging variants were identified in genes in the ITP cohort. The most significant associations were detected in the IFNA17 gene, which is involved in transforming growth factor beta secretion and could affect number and function of regulatory T cells.
IFNA17 rs9298814 was identified in 26% of cases in the ITP cohort compared to less than 0.01% of controls. In all, 43% of ITP patients had at a presumed deleterious variant of IFNA17.
IFNA17 gene variants remained significant in the most severely affected patients, specifically those requiring second line therapy, providing further evidence for this gene’s functional relevance in the pathogenesis and pathophysiology of ITP, Dr. Despotovic said.
Other genes with known impact on T cell number or function, including DGCR14, SMAD2 and CD83 also contained an increased frequency of variants in the European American ITP cohort. IFNLR1 and REL genes were also significantly associated with need for second line ITP therapy.
Analysis of this large cohort did not validate any of over 20 variants that have been previously published as candidates for ITP susceptibility or evolution to chronic ITP, she added.
On Twitter @maryjodales
AT ASH 2015
Key clinical point: IFNA17 and IFNLR1 may be important candidate genes involved in immune regulation and sustained autoimmunity in immune thrombocytopenia.
Major finding: In all, 43% of ITP patients had a presumed deleterious variant of IFNA17.
Data source: Whole exome sequencing on 262 samples with robust phenotype data from children with chronic ITP.
Disclosures: Dr. Despotovic had no relevant financial disclosures.
Lusutrombopag is effective for thrombocytopenia in liver disease
Lusutrombopag, an oral thrombopoietin receptor agonist, was found to reduce the need for platelet transfusion in patients with chronic liver disease with a planned invasive procedure, according to results in an abstract of a phase III trial that will be presented as a latebreaker at the annual meeting of the American Association for the Study of Liver Disease in San Francisco.
The novel therapy, which upregulates platelet production, was “efficacious and well tolerated,” producing a reduced risk of overall adverse events, including bleeding events, according to Dr. Namiki Izumi, Musashino Red Cross Hospital, Tokyo.
In this ongoing global phase III trial, called L-PLUS 2, 96 patients with chronic liver disease, a platelet count less than 50,000/microL, and a planned invasive procedure were randomized to receive a once-daily 3-mg tablet of lusutrombopag or a matching placebo for 7 days. The primary endpoint was the need for a preoperative platelet transfusion.
“The proportion of patients who required no preoperative platelet transfusion was significantly greater with lusutrombopag [29.2% vs. 12.5%; P less than .0001],” Dr. Izumi reported. The proportion of responders, defined by a platelet count greater than or equal to 50,000/microL and a greater than or equal to 20,000/microL-increase from baseline, was also significantly greater in the lusutrombopag arm (77.1% vs. 6.3%; P less than .0001).
In addition, the median time with a platelet count greater than or equal to 50,000/microL was 22.1 days in those who received lusutrombopag but no platelet transfusion versus 3.3 days in the placebo patients who did receive transfusion (P less than 0.0001).
Many adverse events occurred less frequently in the arm randomized to lusutrombopag. This included bleeding events (14.6% vs. 27.1%) and postoperative fever (39.6% vs. 56.3%). The rates of procedural hypertension (41.7% vs. 37.5%) and procedural pain (45.8% vs. 41.7%) were slightly greater in the group randomized to lusutrombopag, but elevations in liver enzymes, such as aspartate aminotransferase (22.9% vs. 31.3%) were somewhat lower.
“Protocol-required imaging revealed one thromboembolic event of the portal venous system in each study arm, neither of which was related to platelets,” according to Dr. Izumi, who reported that no patient discontinued therapy as a result of an adverse event.
Because of the frequency with which thrombocytopenia is observed in patients with chronic liver disease, platelet transfusion is considered a standard procedure when an invasive intervention is planned, according to Dr. Izumi. The data from this trial suggest that preoperative treatment with lusutrombopag may be an alternative.
Dr. Izumi reported financial relationships with Bayer, Daiichi Sankyo, Gilead, Merck, and Shionogi.
Lusutrombopag, an oral thrombopoietin receptor agonist, was found to reduce the need for platelet transfusion in patients with chronic liver disease with a planned invasive procedure, according to results in an abstract of a phase III trial that will be presented as a latebreaker at the annual meeting of the American Association for the Study of Liver Disease in San Francisco.
The novel therapy, which upregulates platelet production, was “efficacious and well tolerated,” producing a reduced risk of overall adverse events, including bleeding events, according to Dr. Namiki Izumi, Musashino Red Cross Hospital, Tokyo.
In this ongoing global phase III trial, called L-PLUS 2, 96 patients with chronic liver disease, a platelet count less than 50,000/microL, and a planned invasive procedure were randomized to receive a once-daily 3-mg tablet of lusutrombopag or a matching placebo for 7 days. The primary endpoint was the need for a preoperative platelet transfusion.
“The proportion of patients who required no preoperative platelet transfusion was significantly greater with lusutrombopag [29.2% vs. 12.5%; P less than .0001],” Dr. Izumi reported. The proportion of responders, defined by a platelet count greater than or equal to 50,000/microL and a greater than or equal to 20,000/microL-increase from baseline, was also significantly greater in the lusutrombopag arm (77.1% vs. 6.3%; P less than .0001).
In addition, the median time with a platelet count greater than or equal to 50,000/microL was 22.1 days in those who received lusutrombopag but no platelet transfusion versus 3.3 days in the placebo patients who did receive transfusion (P less than 0.0001).
Many adverse events occurred less frequently in the arm randomized to lusutrombopag. This included bleeding events (14.6% vs. 27.1%) and postoperative fever (39.6% vs. 56.3%). The rates of procedural hypertension (41.7% vs. 37.5%) and procedural pain (45.8% vs. 41.7%) were slightly greater in the group randomized to lusutrombopag, but elevations in liver enzymes, such as aspartate aminotransferase (22.9% vs. 31.3%) were somewhat lower.
“Protocol-required imaging revealed one thromboembolic event of the portal venous system in each study arm, neither of which was related to platelets,” according to Dr. Izumi, who reported that no patient discontinued therapy as a result of an adverse event.
Because of the frequency with which thrombocytopenia is observed in patients with chronic liver disease, platelet transfusion is considered a standard procedure when an invasive intervention is planned, according to Dr. Izumi. The data from this trial suggest that preoperative treatment with lusutrombopag may be an alternative.
Dr. Izumi reported financial relationships with Bayer, Daiichi Sankyo, Gilead, Merck, and Shionogi.
Lusutrombopag, an oral thrombopoietin receptor agonist, was found to reduce the need for platelet transfusion in patients with chronic liver disease with a planned invasive procedure, according to results in an abstract of a phase III trial that will be presented as a latebreaker at the annual meeting of the American Association for the Study of Liver Disease in San Francisco.
The novel therapy, which upregulates platelet production, was “efficacious and well tolerated,” producing a reduced risk of overall adverse events, including bleeding events, according to Dr. Namiki Izumi, Musashino Red Cross Hospital, Tokyo.
In this ongoing global phase III trial, called L-PLUS 2, 96 patients with chronic liver disease, a platelet count less than 50,000/microL, and a planned invasive procedure were randomized to receive a once-daily 3-mg tablet of lusutrombopag or a matching placebo for 7 days. The primary endpoint was the need for a preoperative platelet transfusion.
“The proportion of patients who required no preoperative platelet transfusion was significantly greater with lusutrombopag [29.2% vs. 12.5%; P less than .0001],” Dr. Izumi reported. The proportion of responders, defined by a platelet count greater than or equal to 50,000/microL and a greater than or equal to 20,000/microL-increase from baseline, was also significantly greater in the lusutrombopag arm (77.1% vs. 6.3%; P less than .0001).
In addition, the median time with a platelet count greater than or equal to 50,000/microL was 22.1 days in those who received lusutrombopag but no platelet transfusion versus 3.3 days in the placebo patients who did receive transfusion (P less than 0.0001).
Many adverse events occurred less frequently in the arm randomized to lusutrombopag. This included bleeding events (14.6% vs. 27.1%) and postoperative fever (39.6% vs. 56.3%). The rates of procedural hypertension (41.7% vs. 37.5%) and procedural pain (45.8% vs. 41.7%) were slightly greater in the group randomized to lusutrombopag, but elevations in liver enzymes, such as aspartate aminotransferase (22.9% vs. 31.3%) were somewhat lower.
“Protocol-required imaging revealed one thromboembolic event of the portal venous system in each study arm, neither of which was related to platelets,” according to Dr. Izumi, who reported that no patient discontinued therapy as a result of an adverse event.
Because of the frequency with which thrombocytopenia is observed in patients with chronic liver disease, platelet transfusion is considered a standard procedure when an invasive intervention is planned, according to Dr. Izumi. The data from this trial suggest that preoperative treatment with lusutrombopag may be an alternative.
Dr. Izumi reported financial relationships with Bayer, Daiichi Sankyo, Gilead, Merck, and Shionogi.
FROM THE LIVER MEETING 2015
Key clinical point: In a phase III trial, lusutrombopag was found to reduce the need for platelet transfusion in chronic liver disease patients requiring surgery.
Major finding: Prior to a planned invasive procedure, only 20.8% of lusutrombopag versus 87.5% of placebo patients (P less than .0001) required platelet transfusion.
Data source: A multicenter, double-blind phase III trial.
Disclosures: Dr. Izumi reported financial relationships with Bayer, Daiichi Sankyo, Gilead, Merck, and Shionogi.