User login
Kicking the can
Medicare, like any other business (regardless of how you want to view it, it’s as much a business as any other insurance company), is dependent on cash flow. Money comes in from young people and their employers through withholding and taxes, and goes back out again in payments to doctors, hospitals, and all the others who bill Medicare for services and supplies in providing health care.
Unlike other businesses, it’s hampered by regulations and competing interests that affect its viability and capacity to adapt to changing markets and circumstances.
For a while, estimates were that Medicare would run out of cash in 2026, but with a stronger-then-expected COVID recovery, it’s been pushed back all the way to ... 2028.
Yeah.
The trouble here is that nobody wants to fix the system to keep it from happening. It’s easier to blame the other side for losing the game than it is to work together to win it. This isn’t a Republican or Democrat issue. Both of them are the problem.
Pushing it back 2 years doesn’t keep it from happening, though it does give more time to find a solution. But that’s only if you have people willing to do so.
Currently politicians favor a strategy of kicking the can down the road for the next congress to deal with. But we’re running out of road to kick it down, and the odds of the next generation of politicians being reasonable, functioning, adults seem to get lower each year.
Can you run your practice like that? In such a way that you know that in a few years your expenses will outweigh your income? And just figure that at some point you’ll get it figured out before your creditors come knocking?
Me neither.
If you were like me, or any other small business owner, you’d sit down and figure out what changes are needed so you’ll still have a viable business down the road.
Of course, that’s part of the issue. The people making these decisions for Medicare don’t have a vested interest in it. If it fails, they have other jobs and income sources to move on to, not to mention some pension-funded health insurance plan. It’s not their problem.
But for the patients, doctors, and other health care professionals who will be depending on it in 6 years, it is a problem, and a serious one.
Hopefully someone will listen before then.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Medicare, like any other business (regardless of how you want to view it, it’s as much a business as any other insurance company), is dependent on cash flow. Money comes in from young people and their employers through withholding and taxes, and goes back out again in payments to doctors, hospitals, and all the others who bill Medicare for services and supplies in providing health care.
Unlike other businesses, it’s hampered by regulations and competing interests that affect its viability and capacity to adapt to changing markets and circumstances.
For a while, estimates were that Medicare would run out of cash in 2026, but with a stronger-then-expected COVID recovery, it’s been pushed back all the way to ... 2028.
Yeah.
The trouble here is that nobody wants to fix the system to keep it from happening. It’s easier to blame the other side for losing the game than it is to work together to win it. This isn’t a Republican or Democrat issue. Both of them are the problem.
Pushing it back 2 years doesn’t keep it from happening, though it does give more time to find a solution. But that’s only if you have people willing to do so.
Currently politicians favor a strategy of kicking the can down the road for the next congress to deal with. But we’re running out of road to kick it down, and the odds of the next generation of politicians being reasonable, functioning, adults seem to get lower each year.
Can you run your practice like that? In such a way that you know that in a few years your expenses will outweigh your income? And just figure that at some point you’ll get it figured out before your creditors come knocking?
Me neither.
If you were like me, or any other small business owner, you’d sit down and figure out what changes are needed so you’ll still have a viable business down the road.
Of course, that’s part of the issue. The people making these decisions for Medicare don’t have a vested interest in it. If it fails, they have other jobs and income sources to move on to, not to mention some pension-funded health insurance plan. It’s not their problem.
But for the patients, doctors, and other health care professionals who will be depending on it in 6 years, it is a problem, and a serious one.
Hopefully someone will listen before then.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Medicare, like any other business (regardless of how you want to view it, it’s as much a business as any other insurance company), is dependent on cash flow. Money comes in from young people and their employers through withholding and taxes, and goes back out again in payments to doctors, hospitals, and all the others who bill Medicare for services and supplies in providing health care.
Unlike other businesses, it’s hampered by regulations and competing interests that affect its viability and capacity to adapt to changing markets and circumstances.
For a while, estimates were that Medicare would run out of cash in 2026, but with a stronger-then-expected COVID recovery, it’s been pushed back all the way to ... 2028.
Yeah.
The trouble here is that nobody wants to fix the system to keep it from happening. It’s easier to blame the other side for losing the game than it is to work together to win it. This isn’t a Republican or Democrat issue. Both of them are the problem.
Pushing it back 2 years doesn’t keep it from happening, though it does give more time to find a solution. But that’s only if you have people willing to do so.
Currently politicians favor a strategy of kicking the can down the road for the next congress to deal with. But we’re running out of road to kick it down, and the odds of the next generation of politicians being reasonable, functioning, adults seem to get lower each year.
Can you run your practice like that? In such a way that you know that in a few years your expenses will outweigh your income? And just figure that at some point you’ll get it figured out before your creditors come knocking?
Me neither.
If you were like me, or any other small business owner, you’d sit down and figure out what changes are needed so you’ll still have a viable business down the road.
Of course, that’s part of the issue. The people making these decisions for Medicare don’t have a vested interest in it. If it fails, they have other jobs and income sources to move on to, not to mention some pension-funded health insurance plan. It’s not their problem.
But for the patients, doctors, and other health care professionals who will be depending on it in 6 years, it is a problem, and a serious one.
Hopefully someone will listen before then.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Infographic: Is physician behavior on social media really so bad?
The medical profession is held to a high standard of personal conduct, so physicians keep a sharp eye out for how fellow doctors behave. That goes for social media as well as in-person conduct.
(and it’s not as egregious as you might think). If you’re interested in delving deeper into the data, check out the Medscape Physicians Behaving Badly Report 2022.
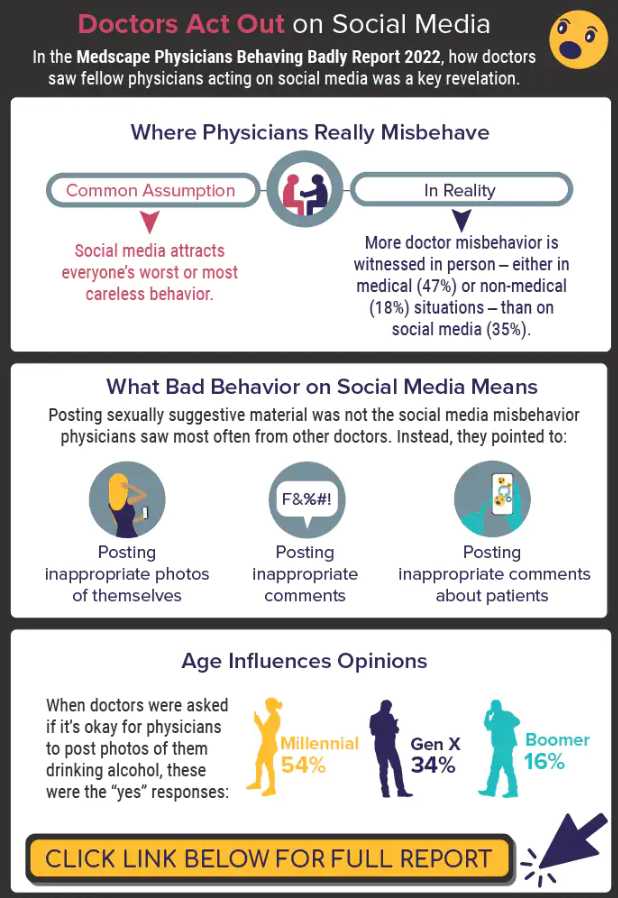
A version of this article first appeared on Medscape.com.
The medical profession is held to a high standard of personal conduct, so physicians keep a sharp eye out for how fellow doctors behave. That goes for social media as well as in-person conduct.
(and it’s not as egregious as you might think). If you’re interested in delving deeper into the data, check out the Medscape Physicians Behaving Badly Report 2022.

A version of this article first appeared on Medscape.com.
The medical profession is held to a high standard of personal conduct, so physicians keep a sharp eye out for how fellow doctors behave. That goes for social media as well as in-person conduct.
(and it’s not as egregious as you might think). If you’re interested in delving deeper into the data, check out the Medscape Physicians Behaving Badly Report 2022.

A version of this article first appeared on Medscape.com.
Congressman’s wife died after taking herbal remedy marketed for diabetes and weight loss
The wife of a Northern California congressman died late in 2021 after ingesting a plant that is generally considered safe and is used as an herbal remedy for a variety of ailments, including diabetes, obesity, and high cholesterol.
Lori McClintock, the wife of U.S. Rep. Tom McClintock, died from dehydration due to gastroenteritis – an inflammation of the stomach and intestines – that was caused by “adverse effects of white mulberry leaf ingestion,” according to a report from the Sacramento County coroner that is dated March 10 but was not immediately released to the public. KHN obtained that report – in addition to the autopsy report and an amended death certificate containing an updated cause of death – in July.
The coroner’s office ruled her death an accident. The original death certificate, dated Dec. 20, 2021, listed the cause of death as “pending.”
Tom McClintock, a Republican who represents a district that spans multiple counties in northern and central California, found his 61-year-old wife unresponsive at their Elk Grove, Calif., home on Dec. 15, 2021, according to the coroner’s report. He had just returned from Washington after voting in Congress the night before.
It’s unclear from the autopsy report whether Lori McClintock took a dietary supplement containing white mulberry leaf, ate fresh or dried leaves, or drank them in a tea, but a “partially intact” white mulberry leaf was found in her stomach, according to the report.
Ms. McClintock’s death underscores the risks of the vast, booming market of dietary supplements and herbal remedies, which have grown into a $54 billion industry in the United States – one that both lawmakers and health care experts say needs more government scrutiny.
“Many people assume if that product is sold in the United States of America, somebody has inspected it, and it must be safe. Unfortunately, that’s not always true,” U.S. Sen. Richard Durbin (D-Ill.) said on the Senate floor this spring when he introduced legislation to strengthen oversight of dietary supplements.
Daniel Fabricant, CEO and president of the Natural Products Association, which represents the dietary supplements industry, questioned whether Ms. McClintock’s death was related to a supplement.
“It’s completely speculative. There’s a science to this. It’s not just what a coroner feels,” said Mr. Fabricant, who oversaw dietary supplements at the Food and Drug Administration during the Obama administration. “People unfortunately pass from dehydration every day, and there’s a lot of different reasons and a lot of different causes.”
Mr. Fabricant said it would have been ideal had the coroner or the family reported her death to the FDA so the agency could have launched an investigation.
Such reports are voluntary, and it’s not clear whether anyone reported her death to the agency. FDA spokesperson Courtney Rhodes said the agency does not discuss possible or ongoing investigations.
The FDA, Mr. Fabricant added, has a system in place to investigate deaths that might be linked to a supplement or drug. “It’s casework,” he said. “It’s good, old-fashioned police work that needs to be done.”
Tom McClintock has remained mostly silent about his wife’s death since he released a statement on Dec. 19, 2021, announcing it and gave a tribute to her at her Jan. 4 funeral. Until now, the cause of death had not been reported.
Mr. McClintock, contacted multiple times by phone and email Wednesday, was not immediately available for comment.
At his wife’s funeral, McClintock told mourners that she was fine when he spoke with her the day before he returned. She had told a friend that “she was on a roll” at a new job she loved in a Sacramento real estate office, he said, and “she was carefully dieting.”
“She just joined a gym,” he said. “At home, she was counting down the days to Christmas, wrapping all the gifts and making all the plans to make it the best family Christmas ever, and it would have been.”
According to the coroner’s report, however, the day before her death, “she had complaints of an upset stomach.”
Sacramento County spokesperson Kim Nava said via email Wednesday that the law prohibits the coroner’s office from discussing many details of specific cases. As part of any death investigation, the office “attempts to locate and review medical records and speak to family/witnesses to establish events leading up to and surrounding a death,” she said.
If any medications or supplements are found at the scene or if pertinent information is in the person’s medical records, those are passed along to the pathologist to help establish cause of death, Ms. Nava said.
“Any information the office obtains from medical records can’t be disseminated to a third party except by court order,” she said.
The leaves and fruit of the white mulberry tree, which is native to China, have been used for centuries in traditional medicine. Academic studies over the past decade have found that the extract from its leaves can lower blood sugar levels and help with weight loss. People take it in capsule or pill form, as an extract or powder. They can also brew the leaves as an herbal tea.
Lori McClintock’s reaction seems unusual. No deaths from the white mulberry plant have been reported to poison control officials in the past 10 years, according to the American Association of Poison Control Centers.
Since 2012, 148 cases of white mulberry plant ingestion were voluntarily reported to poison control officials nationally, most involving accidental ingestion by children 12 and under, said Kaitlyn Brown, clinical managing director for the association. Only one case required medical follow-up, she said.
While poison control centers track exposures to the white mulberry plant, the FDA oversees dietary supplements, such as products that contain white mulberry leaf extract. Since 2004, two cases of people sickened by mulberry supplements have been reported to the FDA, according to its database that tracks “adverse events.” It relies heavily on voluntary reports from health care professionals and consumers. At least one of those cases led to hospitalization.
White mulberry leaf can have side effects, including nausea and diarrhea, according to research. Independent lab tests ordered by the coroner’s office showed Ms. McClintock’s body had elevated levels of nitrogen, sodium, and creatinine – all signs of dehydration, according to three pathologists who reviewed the coroner’s documents, which KHN redacted to remove Ms. McClintock’s name.
White mulberry leaves “do tend to cause dehydration, and part of the uses for that can be to help someone lose weight, mostly through fluid loss, which in this case was just kind of excessive,” said D’Michelle DuPre, MD, a retired forensic pathologist and a former medical examiner in South Carolina who reviewed the documents.
Dietary supplements, which include a broad range of vitamins, herbs, and minerals, are regulated by the FDA. However, they are classified as food and don’t undergo the rigorous scientific and safety testing the government requires of prescription drugs and over-the-counter medicines.
Lawmakers aren’t proposing to put supplements into the same category as pharmaceuticals, but some say they are alarmed that neither the FDA nor the industry knows how many dietary supplements are out there – making it almost impossible for the government to oversee them and punish bad actors.
The FDA estimates 40,000 to 80,000 supplement products are on the market in the United States, and industry surveys estimate 80% of Americans use them.
Legislation by Sen. Durbin and U.S. Sen. Mike Braun (R-Ind.) would require manufacturers to register with the FDA and provide a public list of ingredients in their products, two provisions that are backed by the Council for Responsible Nutrition, another industry group that represents supplement makers.
But the council is lobbying against a provision that would require supplement makers to provide consumers with the ingredient amounts – or the blend – in their products, something they say is akin to giving a recipe to competitors. That’s proprietary information only government regulators should have access to, said Megan Olsen, the group’s senior vice president and general counsel.
Ms. Olsen explained that supplement manufacturers are regulated just like other food companies and are subject to strict labeling requirements and inspections by the FDA. They also must inform the agency about any adverse effects reported by consumers or doctors.
“Companies are testing products throughout the process, are reviewing how they’re being manufactured and what’s going into them,” Ms. Olsen said. “All of that is overseen and dictated by FDA regulation.”
The dietary supplement provisions were rolled into a larger Senate health committee bill that reauthorizes FDA programs, and senators are currently in negotiations with the House of Representatives. The Natural Products Association opposes all of the dietary supplement provisions.
Because dietary pills, teas, and other supplements are regulated as food products, manufacturers can’t advertise them as treatments or cures for health issues. But they can make claims about how the supplements affect the body. So someone who wants to lose weight or get their diabetes under control might reach for a bottle of white mulberry leaf extract because some supplement makers advertise it as a natural remedy that can lower blood sugar levels and promote weight loss.
Those kinds of claims are appealing to Americans and have been especially potent during the pandemic, as people sought to boost their immune systems and fend off COVID-19, said Debbie Petitpain, a registered dietitian nutritionist and a spokesperson for the Academy of Nutrition and Dietetics.
But dietary supplements can be dangerous and don’t affect everyone the same way. Mixing supplements and prescription medicines can compound the problem, according to the FDA.
“I think a lot of people are thinking, ‘Oh, it’s a plant.’ Or, ‘Oh, it’s just a vitamin. Certainly, that means that it’s not going to hurt me,’ ” Ms. Petitpain said. “But there’s always a risk for taking anything.”
It’s not clear why Lori McClintock was taking white mulberry leaf. Friends and family who gathered for her funeral described a vibrant, happy woman who loved her family and her work and already had wrapped Christmas presents under the tree in mid-December. She was planning to buy a recreational vehicle with her husband in retirement.
“We grieve the loss because of all the things she was looking forward to doing and all the years yet ahead,” Tom McClintock told mourners. “And we grieve for something else, because we’ve all lost a genuinely good person in our lives.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The wife of a Northern California congressman died late in 2021 after ingesting a plant that is generally considered safe and is used as an herbal remedy for a variety of ailments, including diabetes, obesity, and high cholesterol.
Lori McClintock, the wife of U.S. Rep. Tom McClintock, died from dehydration due to gastroenteritis – an inflammation of the stomach and intestines – that was caused by “adverse effects of white mulberry leaf ingestion,” according to a report from the Sacramento County coroner that is dated March 10 but was not immediately released to the public. KHN obtained that report – in addition to the autopsy report and an amended death certificate containing an updated cause of death – in July.
The coroner’s office ruled her death an accident. The original death certificate, dated Dec. 20, 2021, listed the cause of death as “pending.”
Tom McClintock, a Republican who represents a district that spans multiple counties in northern and central California, found his 61-year-old wife unresponsive at their Elk Grove, Calif., home on Dec. 15, 2021, according to the coroner’s report. He had just returned from Washington after voting in Congress the night before.
It’s unclear from the autopsy report whether Lori McClintock took a dietary supplement containing white mulberry leaf, ate fresh or dried leaves, or drank them in a tea, but a “partially intact” white mulberry leaf was found in her stomach, according to the report.
Ms. McClintock’s death underscores the risks of the vast, booming market of dietary supplements and herbal remedies, which have grown into a $54 billion industry in the United States – one that both lawmakers and health care experts say needs more government scrutiny.
“Many people assume if that product is sold in the United States of America, somebody has inspected it, and it must be safe. Unfortunately, that’s not always true,” U.S. Sen. Richard Durbin (D-Ill.) said on the Senate floor this spring when he introduced legislation to strengthen oversight of dietary supplements.
Daniel Fabricant, CEO and president of the Natural Products Association, which represents the dietary supplements industry, questioned whether Ms. McClintock’s death was related to a supplement.
“It’s completely speculative. There’s a science to this. It’s not just what a coroner feels,” said Mr. Fabricant, who oversaw dietary supplements at the Food and Drug Administration during the Obama administration. “People unfortunately pass from dehydration every day, and there’s a lot of different reasons and a lot of different causes.”
Mr. Fabricant said it would have been ideal had the coroner or the family reported her death to the FDA so the agency could have launched an investigation.
Such reports are voluntary, and it’s not clear whether anyone reported her death to the agency. FDA spokesperson Courtney Rhodes said the agency does not discuss possible or ongoing investigations.
The FDA, Mr. Fabricant added, has a system in place to investigate deaths that might be linked to a supplement or drug. “It’s casework,” he said. “It’s good, old-fashioned police work that needs to be done.”
Tom McClintock has remained mostly silent about his wife’s death since he released a statement on Dec. 19, 2021, announcing it and gave a tribute to her at her Jan. 4 funeral. Until now, the cause of death had not been reported.
Mr. McClintock, contacted multiple times by phone and email Wednesday, was not immediately available for comment.
At his wife’s funeral, McClintock told mourners that she was fine when he spoke with her the day before he returned. She had told a friend that “she was on a roll” at a new job she loved in a Sacramento real estate office, he said, and “she was carefully dieting.”
“She just joined a gym,” he said. “At home, she was counting down the days to Christmas, wrapping all the gifts and making all the plans to make it the best family Christmas ever, and it would have been.”
According to the coroner’s report, however, the day before her death, “she had complaints of an upset stomach.”
Sacramento County spokesperson Kim Nava said via email Wednesday that the law prohibits the coroner’s office from discussing many details of specific cases. As part of any death investigation, the office “attempts to locate and review medical records and speak to family/witnesses to establish events leading up to and surrounding a death,” she said.
If any medications or supplements are found at the scene or if pertinent information is in the person’s medical records, those are passed along to the pathologist to help establish cause of death, Ms. Nava said.
“Any information the office obtains from medical records can’t be disseminated to a third party except by court order,” she said.
The leaves and fruit of the white mulberry tree, which is native to China, have been used for centuries in traditional medicine. Academic studies over the past decade have found that the extract from its leaves can lower blood sugar levels and help with weight loss. People take it in capsule or pill form, as an extract or powder. They can also brew the leaves as an herbal tea.
Lori McClintock’s reaction seems unusual. No deaths from the white mulberry plant have been reported to poison control officials in the past 10 years, according to the American Association of Poison Control Centers.
Since 2012, 148 cases of white mulberry plant ingestion were voluntarily reported to poison control officials nationally, most involving accidental ingestion by children 12 and under, said Kaitlyn Brown, clinical managing director for the association. Only one case required medical follow-up, she said.
While poison control centers track exposures to the white mulberry plant, the FDA oversees dietary supplements, such as products that contain white mulberry leaf extract. Since 2004, two cases of people sickened by mulberry supplements have been reported to the FDA, according to its database that tracks “adverse events.” It relies heavily on voluntary reports from health care professionals and consumers. At least one of those cases led to hospitalization.
White mulberry leaf can have side effects, including nausea and diarrhea, according to research. Independent lab tests ordered by the coroner’s office showed Ms. McClintock’s body had elevated levels of nitrogen, sodium, and creatinine – all signs of dehydration, according to three pathologists who reviewed the coroner’s documents, which KHN redacted to remove Ms. McClintock’s name.
White mulberry leaves “do tend to cause dehydration, and part of the uses for that can be to help someone lose weight, mostly through fluid loss, which in this case was just kind of excessive,” said D’Michelle DuPre, MD, a retired forensic pathologist and a former medical examiner in South Carolina who reviewed the documents.
Dietary supplements, which include a broad range of vitamins, herbs, and minerals, are regulated by the FDA. However, they are classified as food and don’t undergo the rigorous scientific and safety testing the government requires of prescription drugs and over-the-counter medicines.
Lawmakers aren’t proposing to put supplements into the same category as pharmaceuticals, but some say they are alarmed that neither the FDA nor the industry knows how many dietary supplements are out there – making it almost impossible for the government to oversee them and punish bad actors.
The FDA estimates 40,000 to 80,000 supplement products are on the market in the United States, and industry surveys estimate 80% of Americans use them.
Legislation by Sen. Durbin and U.S. Sen. Mike Braun (R-Ind.) would require manufacturers to register with the FDA and provide a public list of ingredients in their products, two provisions that are backed by the Council for Responsible Nutrition, another industry group that represents supplement makers.
But the council is lobbying against a provision that would require supplement makers to provide consumers with the ingredient amounts – or the blend – in their products, something they say is akin to giving a recipe to competitors. That’s proprietary information only government regulators should have access to, said Megan Olsen, the group’s senior vice president and general counsel.
Ms. Olsen explained that supplement manufacturers are regulated just like other food companies and are subject to strict labeling requirements and inspections by the FDA. They also must inform the agency about any adverse effects reported by consumers or doctors.
“Companies are testing products throughout the process, are reviewing how they’re being manufactured and what’s going into them,” Ms. Olsen said. “All of that is overseen and dictated by FDA regulation.”
The dietary supplement provisions were rolled into a larger Senate health committee bill that reauthorizes FDA programs, and senators are currently in negotiations with the House of Representatives. The Natural Products Association opposes all of the dietary supplement provisions.
Because dietary pills, teas, and other supplements are regulated as food products, manufacturers can’t advertise them as treatments or cures for health issues. But they can make claims about how the supplements affect the body. So someone who wants to lose weight or get their diabetes under control might reach for a bottle of white mulberry leaf extract because some supplement makers advertise it as a natural remedy that can lower blood sugar levels and promote weight loss.
Those kinds of claims are appealing to Americans and have been especially potent during the pandemic, as people sought to boost their immune systems and fend off COVID-19, said Debbie Petitpain, a registered dietitian nutritionist and a spokesperson for the Academy of Nutrition and Dietetics.
But dietary supplements can be dangerous and don’t affect everyone the same way. Mixing supplements and prescription medicines can compound the problem, according to the FDA.
“I think a lot of people are thinking, ‘Oh, it’s a plant.’ Or, ‘Oh, it’s just a vitamin. Certainly, that means that it’s not going to hurt me,’ ” Ms. Petitpain said. “But there’s always a risk for taking anything.”
It’s not clear why Lori McClintock was taking white mulberry leaf. Friends and family who gathered for her funeral described a vibrant, happy woman who loved her family and her work and already had wrapped Christmas presents under the tree in mid-December. She was planning to buy a recreational vehicle with her husband in retirement.
“We grieve the loss because of all the things she was looking forward to doing and all the years yet ahead,” Tom McClintock told mourners. “And we grieve for something else, because we’ve all lost a genuinely good person in our lives.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The wife of a Northern California congressman died late in 2021 after ingesting a plant that is generally considered safe and is used as an herbal remedy for a variety of ailments, including diabetes, obesity, and high cholesterol.
Lori McClintock, the wife of U.S. Rep. Tom McClintock, died from dehydration due to gastroenteritis – an inflammation of the stomach and intestines – that was caused by “adverse effects of white mulberry leaf ingestion,” according to a report from the Sacramento County coroner that is dated March 10 but was not immediately released to the public. KHN obtained that report – in addition to the autopsy report and an amended death certificate containing an updated cause of death – in July.
The coroner’s office ruled her death an accident. The original death certificate, dated Dec. 20, 2021, listed the cause of death as “pending.”
Tom McClintock, a Republican who represents a district that spans multiple counties in northern and central California, found his 61-year-old wife unresponsive at their Elk Grove, Calif., home on Dec. 15, 2021, according to the coroner’s report. He had just returned from Washington after voting in Congress the night before.
It’s unclear from the autopsy report whether Lori McClintock took a dietary supplement containing white mulberry leaf, ate fresh or dried leaves, or drank them in a tea, but a “partially intact” white mulberry leaf was found in her stomach, according to the report.
Ms. McClintock’s death underscores the risks of the vast, booming market of dietary supplements and herbal remedies, which have grown into a $54 billion industry in the United States – one that both lawmakers and health care experts say needs more government scrutiny.
“Many people assume if that product is sold in the United States of America, somebody has inspected it, and it must be safe. Unfortunately, that’s not always true,” U.S. Sen. Richard Durbin (D-Ill.) said on the Senate floor this spring when he introduced legislation to strengthen oversight of dietary supplements.
Daniel Fabricant, CEO and president of the Natural Products Association, which represents the dietary supplements industry, questioned whether Ms. McClintock’s death was related to a supplement.
“It’s completely speculative. There’s a science to this. It’s not just what a coroner feels,” said Mr. Fabricant, who oversaw dietary supplements at the Food and Drug Administration during the Obama administration. “People unfortunately pass from dehydration every day, and there’s a lot of different reasons and a lot of different causes.”
Mr. Fabricant said it would have been ideal had the coroner or the family reported her death to the FDA so the agency could have launched an investigation.
Such reports are voluntary, and it’s not clear whether anyone reported her death to the agency. FDA spokesperson Courtney Rhodes said the agency does not discuss possible or ongoing investigations.
The FDA, Mr. Fabricant added, has a system in place to investigate deaths that might be linked to a supplement or drug. “It’s casework,” he said. “It’s good, old-fashioned police work that needs to be done.”
Tom McClintock has remained mostly silent about his wife’s death since he released a statement on Dec. 19, 2021, announcing it and gave a tribute to her at her Jan. 4 funeral. Until now, the cause of death had not been reported.
Mr. McClintock, contacted multiple times by phone and email Wednesday, was not immediately available for comment.
At his wife’s funeral, McClintock told mourners that she was fine when he spoke with her the day before he returned. She had told a friend that “she was on a roll” at a new job she loved in a Sacramento real estate office, he said, and “she was carefully dieting.”
“She just joined a gym,” he said. “At home, she was counting down the days to Christmas, wrapping all the gifts and making all the plans to make it the best family Christmas ever, and it would have been.”
According to the coroner’s report, however, the day before her death, “she had complaints of an upset stomach.”
Sacramento County spokesperson Kim Nava said via email Wednesday that the law prohibits the coroner’s office from discussing many details of specific cases. As part of any death investigation, the office “attempts to locate and review medical records and speak to family/witnesses to establish events leading up to and surrounding a death,” she said.
If any medications or supplements are found at the scene or if pertinent information is in the person’s medical records, those are passed along to the pathologist to help establish cause of death, Ms. Nava said.
“Any information the office obtains from medical records can’t be disseminated to a third party except by court order,” she said.
The leaves and fruit of the white mulberry tree, which is native to China, have been used for centuries in traditional medicine. Academic studies over the past decade have found that the extract from its leaves can lower blood sugar levels and help with weight loss. People take it in capsule or pill form, as an extract or powder. They can also brew the leaves as an herbal tea.
Lori McClintock’s reaction seems unusual. No deaths from the white mulberry plant have been reported to poison control officials in the past 10 years, according to the American Association of Poison Control Centers.
Since 2012, 148 cases of white mulberry plant ingestion were voluntarily reported to poison control officials nationally, most involving accidental ingestion by children 12 and under, said Kaitlyn Brown, clinical managing director for the association. Only one case required medical follow-up, she said.
While poison control centers track exposures to the white mulberry plant, the FDA oversees dietary supplements, such as products that contain white mulberry leaf extract. Since 2004, two cases of people sickened by mulberry supplements have been reported to the FDA, according to its database that tracks “adverse events.” It relies heavily on voluntary reports from health care professionals and consumers. At least one of those cases led to hospitalization.
White mulberry leaf can have side effects, including nausea and diarrhea, according to research. Independent lab tests ordered by the coroner’s office showed Ms. McClintock’s body had elevated levels of nitrogen, sodium, and creatinine – all signs of dehydration, according to three pathologists who reviewed the coroner’s documents, which KHN redacted to remove Ms. McClintock’s name.
White mulberry leaves “do tend to cause dehydration, and part of the uses for that can be to help someone lose weight, mostly through fluid loss, which in this case was just kind of excessive,” said D’Michelle DuPre, MD, a retired forensic pathologist and a former medical examiner in South Carolina who reviewed the documents.
Dietary supplements, which include a broad range of vitamins, herbs, and minerals, are regulated by the FDA. However, they are classified as food and don’t undergo the rigorous scientific and safety testing the government requires of prescription drugs and over-the-counter medicines.
Lawmakers aren’t proposing to put supplements into the same category as pharmaceuticals, but some say they are alarmed that neither the FDA nor the industry knows how many dietary supplements are out there – making it almost impossible for the government to oversee them and punish bad actors.
The FDA estimates 40,000 to 80,000 supplement products are on the market in the United States, and industry surveys estimate 80% of Americans use them.
Legislation by Sen. Durbin and U.S. Sen. Mike Braun (R-Ind.) would require manufacturers to register with the FDA and provide a public list of ingredients in their products, two provisions that are backed by the Council for Responsible Nutrition, another industry group that represents supplement makers.
But the council is lobbying against a provision that would require supplement makers to provide consumers with the ingredient amounts – or the blend – in their products, something they say is akin to giving a recipe to competitors. That’s proprietary information only government regulators should have access to, said Megan Olsen, the group’s senior vice president and general counsel.
Ms. Olsen explained that supplement manufacturers are regulated just like other food companies and are subject to strict labeling requirements and inspections by the FDA. They also must inform the agency about any adverse effects reported by consumers or doctors.
“Companies are testing products throughout the process, are reviewing how they’re being manufactured and what’s going into them,” Ms. Olsen said. “All of that is overseen and dictated by FDA regulation.”
The dietary supplement provisions were rolled into a larger Senate health committee bill that reauthorizes FDA programs, and senators are currently in negotiations with the House of Representatives. The Natural Products Association opposes all of the dietary supplement provisions.
Because dietary pills, teas, and other supplements are regulated as food products, manufacturers can’t advertise them as treatments or cures for health issues. But they can make claims about how the supplements affect the body. So someone who wants to lose weight or get their diabetes under control might reach for a bottle of white mulberry leaf extract because some supplement makers advertise it as a natural remedy that can lower blood sugar levels and promote weight loss.
Those kinds of claims are appealing to Americans and have been especially potent during the pandemic, as people sought to boost their immune systems and fend off COVID-19, said Debbie Petitpain, a registered dietitian nutritionist and a spokesperson for the Academy of Nutrition and Dietetics.
But dietary supplements can be dangerous and don’t affect everyone the same way. Mixing supplements and prescription medicines can compound the problem, according to the FDA.
“I think a lot of people are thinking, ‘Oh, it’s a plant.’ Or, ‘Oh, it’s just a vitamin. Certainly, that means that it’s not going to hurt me,’ ” Ms. Petitpain said. “But there’s always a risk for taking anything.”
It’s not clear why Lori McClintock was taking white mulberry leaf. Friends and family who gathered for her funeral described a vibrant, happy woman who loved her family and her work and already had wrapped Christmas presents under the tree in mid-December. She was planning to buy a recreational vehicle with her husband in retirement.
“We grieve the loss because of all the things she was looking forward to doing and all the years yet ahead,” Tom McClintock told mourners. “And we grieve for something else, because we’ve all lost a genuinely good person in our lives.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Well-child visits rise, but disparities remain
Adherence to well-child visits in the United States increased overall over a 10-year period, but a gap of up to 20% persisted between the highest and lowest adherence groups, reflecting disparities by race and ethnicity, poverty level, geography, and insurance status.
Well-child visits are recommended to provide children with preventive health and development services, ensure immunizations, and allow parents to discuss health concerns, wrote Salam Abdus, PhD, and Thomas M. Selden, PhD, of the Agency for Healthcare Research and Quality, Rockville, Md.
“We know from prior studies that as of 2008, well-child visits were trending upward, but often fell short of recommendations among key socioeconomic groups,” they wrote.
To examine recent trends in well-child visits, the researchers conducted a cross-sectional study of data from the Medical Expenditure Panel Survey (MEPS) on children aged 0 to 18 years. The findings were published in JAMA Pediatrics.
The study population included 19,018 children in 2006 and 2007 and 17,533 children in 2016 and 2017.
Adherence was defined as the ratio of reported well-child visits divided by the recommended number of visits in a calendar year.
Overall, the mean adherence increased from 47.9% in 2006-2007 to 62.3% in 2016-2017.
However, significant gaps persisted across race and ethnicity. Notably, adherence in the Hispanic population increased by nearly 22% between the study dates, compared to a 15.3% increase among White non-Hispanic children. However, Hispanic children still trailed White children overall in 2016-2017 (58% vs. 67.8%).
The smallest increase in adherence occurred among Black non-Hispanic children (5.6%) which further widened the gap between Black and White non-Hispanic children in 2016-2017 (52.5% vs. 67.8%).
Adherence rates increased similarly for children with public and private insurance (15.5% and 13.9%, respectively), but the adherence rates for uninsured children remained stable. Adherence in 2016-2017 for children with private, public, and no insurance were 66.3%, 58.7%, and 31.1%.
Also, despite overall increases in adherence across regions, a gap of more than 20% separated the region with the highest adherence (Northeast) from the lowest (West) in both the 2006-2007 and 2016-2017 periods (69.3% vs. 38.4%, and 79.3% vs. 55.2%, respectively).
The findings show an increase in well-child visits that spanned a time period of increased recommendations, economic changes, and the impact of the Affordable Care Act, but unaddressed disparities remain, the researchers noted.
Reducing disparities and improving adherence, “will require the combined efforts of researchers, policymakers, and clinicians to improve our understanding of adherence, to implement policies improving access to care, and to increase health care professional engagement with disadvantaged communities,” they concluded.
Overall increases are encouraging, but barriers need attention
“Demographic data are critical to determine which groups of children need the most support for recommended well child care,” Susan Boulter, MD, of the Geisel School of Medicine at Dartmouth, Hanover, N.H., said in an interview. In the current study, “it was encouraging to see how either public or private insurance significantly increased the percentage of children receiving well child care,” she said.
The level of increased adherence to AAP-recommended guidelines for well-child visits was surprising, said Dr. Boulter. The overall increase is likely attributable in part to the increased coverage for well-child visits in the wake of the Affordable Care Act, as the study authors mention, she said.
“The gains experienced by Hispanic families were especially encouraging,” she added.
However, ongoing barriers to well-child care include “lack of adequate provider numbers and mix, transportation difficulties for patients, and lack of child care and time away from work for parents so they can complete the recommended well child visit schedule,” Dr. Boulter noted. “Provider schedules and locations of care should be improved so families would have easier access. Also, social media should have more positive well-child messages to counteract the negative messaging.”
More research is needed to examine the impact of COVID-19 on well-child visits, Dr. Boulter emphasized. “Most likely, the percentages in all groups will have changed since COVID-19 has impacted office practices,” she said. “Anxiety about COVID-19 transmissibility in the pediatric office decreased routine office visits, and skepticism about vaccines, including vaccine refusal, has significantly changed the percentage of children who have received the AAP recommended vaccines,” she explained. Ideally, the study authors will review the MEPS data again to examine changes since the COVID-19 pandemic began, she told this news organization.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Boulter had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
Adherence to well-child visits in the United States increased overall over a 10-year period, but a gap of up to 20% persisted between the highest and lowest adherence groups, reflecting disparities by race and ethnicity, poverty level, geography, and insurance status.
Well-child visits are recommended to provide children with preventive health and development services, ensure immunizations, and allow parents to discuss health concerns, wrote Salam Abdus, PhD, and Thomas M. Selden, PhD, of the Agency for Healthcare Research and Quality, Rockville, Md.
“We know from prior studies that as of 2008, well-child visits were trending upward, but often fell short of recommendations among key socioeconomic groups,” they wrote.
To examine recent trends in well-child visits, the researchers conducted a cross-sectional study of data from the Medical Expenditure Panel Survey (MEPS) on children aged 0 to 18 years. The findings were published in JAMA Pediatrics.
The study population included 19,018 children in 2006 and 2007 and 17,533 children in 2016 and 2017.
Adherence was defined as the ratio of reported well-child visits divided by the recommended number of visits in a calendar year.
Overall, the mean adherence increased from 47.9% in 2006-2007 to 62.3% in 2016-2017.
However, significant gaps persisted across race and ethnicity. Notably, adherence in the Hispanic population increased by nearly 22% between the study dates, compared to a 15.3% increase among White non-Hispanic children. However, Hispanic children still trailed White children overall in 2016-2017 (58% vs. 67.8%).
The smallest increase in adherence occurred among Black non-Hispanic children (5.6%) which further widened the gap between Black and White non-Hispanic children in 2016-2017 (52.5% vs. 67.8%).
Adherence rates increased similarly for children with public and private insurance (15.5% and 13.9%, respectively), but the adherence rates for uninsured children remained stable. Adherence in 2016-2017 for children with private, public, and no insurance were 66.3%, 58.7%, and 31.1%.
Also, despite overall increases in adherence across regions, a gap of more than 20% separated the region with the highest adherence (Northeast) from the lowest (West) in both the 2006-2007 and 2016-2017 periods (69.3% vs. 38.4%, and 79.3% vs. 55.2%, respectively).
The findings show an increase in well-child visits that spanned a time period of increased recommendations, economic changes, and the impact of the Affordable Care Act, but unaddressed disparities remain, the researchers noted.
Reducing disparities and improving adherence, “will require the combined efforts of researchers, policymakers, and clinicians to improve our understanding of adherence, to implement policies improving access to care, and to increase health care professional engagement with disadvantaged communities,” they concluded.
Overall increases are encouraging, but barriers need attention
“Demographic data are critical to determine which groups of children need the most support for recommended well child care,” Susan Boulter, MD, of the Geisel School of Medicine at Dartmouth, Hanover, N.H., said in an interview. In the current study, “it was encouraging to see how either public or private insurance significantly increased the percentage of children receiving well child care,” she said.
The level of increased adherence to AAP-recommended guidelines for well-child visits was surprising, said Dr. Boulter. The overall increase is likely attributable in part to the increased coverage for well-child visits in the wake of the Affordable Care Act, as the study authors mention, she said.
“The gains experienced by Hispanic families were especially encouraging,” she added.
However, ongoing barriers to well-child care include “lack of adequate provider numbers and mix, transportation difficulties for patients, and lack of child care and time away from work for parents so they can complete the recommended well child visit schedule,” Dr. Boulter noted. “Provider schedules and locations of care should be improved so families would have easier access. Also, social media should have more positive well-child messages to counteract the negative messaging.”
More research is needed to examine the impact of COVID-19 on well-child visits, Dr. Boulter emphasized. “Most likely, the percentages in all groups will have changed since COVID-19 has impacted office practices,” she said. “Anxiety about COVID-19 transmissibility in the pediatric office decreased routine office visits, and skepticism about vaccines, including vaccine refusal, has significantly changed the percentage of children who have received the AAP recommended vaccines,” she explained. Ideally, the study authors will review the MEPS data again to examine changes since the COVID-19 pandemic began, she told this news organization.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Boulter had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
Adherence to well-child visits in the United States increased overall over a 10-year period, but a gap of up to 20% persisted between the highest and lowest adherence groups, reflecting disparities by race and ethnicity, poverty level, geography, and insurance status.
Well-child visits are recommended to provide children with preventive health and development services, ensure immunizations, and allow parents to discuss health concerns, wrote Salam Abdus, PhD, and Thomas M. Selden, PhD, of the Agency for Healthcare Research and Quality, Rockville, Md.
“We know from prior studies that as of 2008, well-child visits were trending upward, but often fell short of recommendations among key socioeconomic groups,” they wrote.
To examine recent trends in well-child visits, the researchers conducted a cross-sectional study of data from the Medical Expenditure Panel Survey (MEPS) on children aged 0 to 18 years. The findings were published in JAMA Pediatrics.
The study population included 19,018 children in 2006 and 2007 and 17,533 children in 2016 and 2017.
Adherence was defined as the ratio of reported well-child visits divided by the recommended number of visits in a calendar year.
Overall, the mean adherence increased from 47.9% in 2006-2007 to 62.3% in 2016-2017.
However, significant gaps persisted across race and ethnicity. Notably, adherence in the Hispanic population increased by nearly 22% between the study dates, compared to a 15.3% increase among White non-Hispanic children. However, Hispanic children still trailed White children overall in 2016-2017 (58% vs. 67.8%).
The smallest increase in adherence occurred among Black non-Hispanic children (5.6%) which further widened the gap between Black and White non-Hispanic children in 2016-2017 (52.5% vs. 67.8%).
Adherence rates increased similarly for children with public and private insurance (15.5% and 13.9%, respectively), but the adherence rates for uninsured children remained stable. Adherence in 2016-2017 for children with private, public, and no insurance were 66.3%, 58.7%, and 31.1%.
Also, despite overall increases in adherence across regions, a gap of more than 20% separated the region with the highest adherence (Northeast) from the lowest (West) in both the 2006-2007 and 2016-2017 periods (69.3% vs. 38.4%, and 79.3% vs. 55.2%, respectively).
The findings show an increase in well-child visits that spanned a time period of increased recommendations, economic changes, and the impact of the Affordable Care Act, but unaddressed disparities remain, the researchers noted.
Reducing disparities and improving adherence, “will require the combined efforts of researchers, policymakers, and clinicians to improve our understanding of adherence, to implement policies improving access to care, and to increase health care professional engagement with disadvantaged communities,” they concluded.
Overall increases are encouraging, but barriers need attention
“Demographic data are critical to determine which groups of children need the most support for recommended well child care,” Susan Boulter, MD, of the Geisel School of Medicine at Dartmouth, Hanover, N.H., said in an interview. In the current study, “it was encouraging to see how either public or private insurance significantly increased the percentage of children receiving well child care,” she said.
The level of increased adherence to AAP-recommended guidelines for well-child visits was surprising, said Dr. Boulter. The overall increase is likely attributable in part to the increased coverage for well-child visits in the wake of the Affordable Care Act, as the study authors mention, she said.
“The gains experienced by Hispanic families were especially encouraging,” she added.
However, ongoing barriers to well-child care include “lack of adequate provider numbers and mix, transportation difficulties for patients, and lack of child care and time away from work for parents so they can complete the recommended well child visit schedule,” Dr. Boulter noted. “Provider schedules and locations of care should be improved so families would have easier access. Also, social media should have more positive well-child messages to counteract the negative messaging.”
More research is needed to examine the impact of COVID-19 on well-child visits, Dr. Boulter emphasized. “Most likely, the percentages in all groups will have changed since COVID-19 has impacted office practices,” she said. “Anxiety about COVID-19 transmissibility in the pediatric office decreased routine office visits, and skepticism about vaccines, including vaccine refusal, has significantly changed the percentage of children who have received the AAP recommended vaccines,” she explained. Ideally, the study authors will review the MEPS data again to examine changes since the COVID-19 pandemic began, she told this news organization.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Boulter had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
FROM JAMA PEDIATRICS
How did cancer survivors fare early in the COVID-19 pandemic?
In addition, the prevalence of unhealthy behaviors, including smoking and poor sleep habits, appeared to decline among cancer survivors as well as adults who had no history of cancer during this period.
“Our findings suggest that the pandemic may have motivated people to adopt certain healthier behaviors,” Xuesong Han, PhD, American Cancer Society, Atlanta, said in a statement. In addition, policies implemented in response to the pandemic regarding insurance coverage, unemployment benefits, and financial assistance “may have contributed to the observed positive changes.”
Dr. Han and colleagues noted that “to the best of our knowledge, our study provides the first nationally representative estimates of the effects of the first year of the COVID-19 pandemic on cancer survivors in the United States.”
The study was published online in Cancer.
Given the considerable upheaval caused by the COVID-19 pandemic, Dr. Han and colleagues wanted to explore how cancer survivors, in particular, were affected during the first year.
The analysis included 57,132 cancer survivors and 1,044,585 adults without cancer who were involved in the Behavioral Risk Factor Surveillance System.
The researchers found that the unemployment rate in 2020 increased by 43% among cancer survivors and by 57% among adults without a cancer history compared with the previous 2 years.
However, the rate of uninsured cancer survivors aged 18-64 years remained relatively stable in 2020 at 8%, compared with 8.8% in 2017-2019.
Notably, the prevalence of insufficient sleep decreased among cancer survivors (43% to 39%), as did smoking (22% to 19%). Among adults without a history of cancer, there was a decline in insufficient sleep (37% to 34.3%) and smoking (16% to 15%). The prevalence of binge drinking decreased among adults with and those without a history of cancer as well.
Obesity rates, however, increased during the first year of the pandemic among cancer survivors (36.5% to 40%) as well as among those with no cancer history (30.8% to 32.7%). In addition, more adults without a cancer history reported an increase in mental distress in 2020 compared with before the COVID-19 pandemic.
The authors suggest that some of the positive trends observed could be explained, in part, by increased enrollment in the Affordable Care Act and by the Families First Coronavirus Response Act, which increased the federal government’s share of Medicaid costs and prevented states from terminating Medicaid coverage during the pandemic.
“These provisions likely compensated for the loss in employer-sponsored insurance,” the authors noted.
But, they added, “as policies related to the public health emergency expire, ongoing monitoring of long-term effects of the COVID-19 pandemic on cancer survivorship is warranted.”
Dr. Han has received a grant from AstraZeneca outside of the current study.
A version of this article first appeared on Medscape.com.
In addition, the prevalence of unhealthy behaviors, including smoking and poor sleep habits, appeared to decline among cancer survivors as well as adults who had no history of cancer during this period.
“Our findings suggest that the pandemic may have motivated people to adopt certain healthier behaviors,” Xuesong Han, PhD, American Cancer Society, Atlanta, said in a statement. In addition, policies implemented in response to the pandemic regarding insurance coverage, unemployment benefits, and financial assistance “may have contributed to the observed positive changes.”
Dr. Han and colleagues noted that “to the best of our knowledge, our study provides the first nationally representative estimates of the effects of the first year of the COVID-19 pandemic on cancer survivors in the United States.”
The study was published online in Cancer.
Given the considerable upheaval caused by the COVID-19 pandemic, Dr. Han and colleagues wanted to explore how cancer survivors, in particular, were affected during the first year.
The analysis included 57,132 cancer survivors and 1,044,585 adults without cancer who were involved in the Behavioral Risk Factor Surveillance System.
The researchers found that the unemployment rate in 2020 increased by 43% among cancer survivors and by 57% among adults without a cancer history compared with the previous 2 years.
However, the rate of uninsured cancer survivors aged 18-64 years remained relatively stable in 2020 at 8%, compared with 8.8% in 2017-2019.
Notably, the prevalence of insufficient sleep decreased among cancer survivors (43% to 39%), as did smoking (22% to 19%). Among adults without a history of cancer, there was a decline in insufficient sleep (37% to 34.3%) and smoking (16% to 15%). The prevalence of binge drinking decreased among adults with and those without a history of cancer as well.
Obesity rates, however, increased during the first year of the pandemic among cancer survivors (36.5% to 40%) as well as among those with no cancer history (30.8% to 32.7%). In addition, more adults without a cancer history reported an increase in mental distress in 2020 compared with before the COVID-19 pandemic.
The authors suggest that some of the positive trends observed could be explained, in part, by increased enrollment in the Affordable Care Act and by the Families First Coronavirus Response Act, which increased the federal government’s share of Medicaid costs and prevented states from terminating Medicaid coverage during the pandemic.
“These provisions likely compensated for the loss in employer-sponsored insurance,” the authors noted.
But, they added, “as policies related to the public health emergency expire, ongoing monitoring of long-term effects of the COVID-19 pandemic on cancer survivorship is warranted.”
Dr. Han has received a grant from AstraZeneca outside of the current study.
A version of this article first appeared on Medscape.com.
In addition, the prevalence of unhealthy behaviors, including smoking and poor sleep habits, appeared to decline among cancer survivors as well as adults who had no history of cancer during this period.
“Our findings suggest that the pandemic may have motivated people to adopt certain healthier behaviors,” Xuesong Han, PhD, American Cancer Society, Atlanta, said in a statement. In addition, policies implemented in response to the pandemic regarding insurance coverage, unemployment benefits, and financial assistance “may have contributed to the observed positive changes.”
Dr. Han and colleagues noted that “to the best of our knowledge, our study provides the first nationally representative estimates of the effects of the first year of the COVID-19 pandemic on cancer survivors in the United States.”
The study was published online in Cancer.
Given the considerable upheaval caused by the COVID-19 pandemic, Dr. Han and colleagues wanted to explore how cancer survivors, in particular, were affected during the first year.
The analysis included 57,132 cancer survivors and 1,044,585 adults without cancer who were involved in the Behavioral Risk Factor Surveillance System.
The researchers found that the unemployment rate in 2020 increased by 43% among cancer survivors and by 57% among adults without a cancer history compared with the previous 2 years.
However, the rate of uninsured cancer survivors aged 18-64 years remained relatively stable in 2020 at 8%, compared with 8.8% in 2017-2019.
Notably, the prevalence of insufficient sleep decreased among cancer survivors (43% to 39%), as did smoking (22% to 19%). Among adults without a history of cancer, there was a decline in insufficient sleep (37% to 34.3%) and smoking (16% to 15%). The prevalence of binge drinking decreased among adults with and those without a history of cancer as well.
Obesity rates, however, increased during the first year of the pandemic among cancer survivors (36.5% to 40%) as well as among those with no cancer history (30.8% to 32.7%). In addition, more adults without a cancer history reported an increase in mental distress in 2020 compared with before the COVID-19 pandemic.
The authors suggest that some of the positive trends observed could be explained, in part, by increased enrollment in the Affordable Care Act and by the Families First Coronavirus Response Act, which increased the federal government’s share of Medicaid costs and prevented states from terminating Medicaid coverage during the pandemic.
“These provisions likely compensated for the loss in employer-sponsored insurance,” the authors noted.
But, they added, “as policies related to the public health emergency expire, ongoing monitoring of long-term effects of the COVID-19 pandemic on cancer survivorship is warranted.”
Dr. Han has received a grant from AstraZeneca outside of the current study.
A version of this article first appeared on Medscape.com.
FROM CANCER
Former nurse charged with murder in death of 97-year-old war veteran
A former Kentucky nurse was charged with murder stemming from an incident in which she gave “something special” to a 97-year-old patient who died 5 days later, according to multiple sources, including police and nursing records.
Ms. Hunter allegedly gave lorazepam, typically used for anxiety, to Mr. Morris on April 30. He subsequently developed pneumonia and died on May 5.
Ms. Hunter “intentionally performed actions of medical maltreatment,” according to the Lexington Police Department’s report.
A Baptist Health Lexington spokeswoman told this news organization that the nurse who was charged hasn’t worked at the hospital since the April incident. “We have learned that a former nurse at our hospital has been arrested yesterday on criminal charges,” spokeswoman Ruth Ann Childers stated. “The hospital has fully cooperated with the police investigation. Patient care and safety are always our top priorities. Out of respect for the patient’s family and because this is criminal matter, we are not able to talk about the investigation.”
According to the Kentucky Board of Nursing, which suspended Ms. Hunter’s RN license on a temporary basis on Aug. 22, she allegedly asked the on-duty physician and a nurse practitioner separately for a medication order to calm Mr. Morris, who had become agitated and aggressive. They denied Ms. Hunter’s request, so she withdrew lorazepam intended for another patient and administered it to Mr. Morris, the nursing board suspension order states. “When asked what was administered, she replied ‘something special,’ “ the order states.
Another RN found the patient with labored breathing and “it was determined that respondent had disarmed/lowered the oxygen monitoring system several times as to not set off an alarm at the bedside,” the order continued. “The RN discussed with charge nurse that the patient had been given something intravenously that was causing his decline.”
When the charge nurse entered the room later, she found the patient in “respiratory distress with labored breathing and poor oxygen saturation. ... X-rays would show that the aspiration from the substances ingested by the patient while in his condition caused the patient to develop aspirational pneumonia,” the order continues.
“Despite the rapidly declining condition of the patient, respondent never called for rapid response nor acted with any sense of urgency. Respondent did however edit documentation of administration of Ativan on ‘patient B’ to state ‘not given.’ ”
Mr. Morris’ condition never improved. He was taken to hospice care on May 3 and died 2 days later, the order states.
Ms. Hunter was being held in the Lexington Jail on $100,000 bond, according to jail records.
A version of this article first appeared on Medscape.com.
A former Kentucky nurse was charged with murder stemming from an incident in which she gave “something special” to a 97-year-old patient who died 5 days later, according to multiple sources, including police and nursing records.
Ms. Hunter allegedly gave lorazepam, typically used for anxiety, to Mr. Morris on April 30. He subsequently developed pneumonia and died on May 5.
Ms. Hunter “intentionally performed actions of medical maltreatment,” according to the Lexington Police Department’s report.
A Baptist Health Lexington spokeswoman told this news organization that the nurse who was charged hasn’t worked at the hospital since the April incident. “We have learned that a former nurse at our hospital has been arrested yesterday on criminal charges,” spokeswoman Ruth Ann Childers stated. “The hospital has fully cooperated with the police investigation. Patient care and safety are always our top priorities. Out of respect for the patient’s family and because this is criminal matter, we are not able to talk about the investigation.”
According to the Kentucky Board of Nursing, which suspended Ms. Hunter’s RN license on a temporary basis on Aug. 22, she allegedly asked the on-duty physician and a nurse practitioner separately for a medication order to calm Mr. Morris, who had become agitated and aggressive. They denied Ms. Hunter’s request, so she withdrew lorazepam intended for another patient and administered it to Mr. Morris, the nursing board suspension order states. “When asked what was administered, she replied ‘something special,’ “ the order states.
Another RN found the patient with labored breathing and “it was determined that respondent had disarmed/lowered the oxygen monitoring system several times as to not set off an alarm at the bedside,” the order continued. “The RN discussed with charge nurse that the patient had been given something intravenously that was causing his decline.”
When the charge nurse entered the room later, she found the patient in “respiratory distress with labored breathing and poor oxygen saturation. ... X-rays would show that the aspiration from the substances ingested by the patient while in his condition caused the patient to develop aspirational pneumonia,” the order continues.
“Despite the rapidly declining condition of the patient, respondent never called for rapid response nor acted with any sense of urgency. Respondent did however edit documentation of administration of Ativan on ‘patient B’ to state ‘not given.’ ”
Mr. Morris’ condition never improved. He was taken to hospice care on May 3 and died 2 days later, the order states.
Ms. Hunter was being held in the Lexington Jail on $100,000 bond, according to jail records.
A version of this article first appeared on Medscape.com.
A former Kentucky nurse was charged with murder stemming from an incident in which she gave “something special” to a 97-year-old patient who died 5 days later, according to multiple sources, including police and nursing records.
Ms. Hunter allegedly gave lorazepam, typically used for anxiety, to Mr. Morris on April 30. He subsequently developed pneumonia and died on May 5.
Ms. Hunter “intentionally performed actions of medical maltreatment,” according to the Lexington Police Department’s report.
A Baptist Health Lexington spokeswoman told this news organization that the nurse who was charged hasn’t worked at the hospital since the April incident. “We have learned that a former nurse at our hospital has been arrested yesterday on criminal charges,” spokeswoman Ruth Ann Childers stated. “The hospital has fully cooperated with the police investigation. Patient care and safety are always our top priorities. Out of respect for the patient’s family and because this is criminal matter, we are not able to talk about the investigation.”
According to the Kentucky Board of Nursing, which suspended Ms. Hunter’s RN license on a temporary basis on Aug. 22, she allegedly asked the on-duty physician and a nurse practitioner separately for a medication order to calm Mr. Morris, who had become agitated and aggressive. They denied Ms. Hunter’s request, so she withdrew lorazepam intended for another patient and administered it to Mr. Morris, the nursing board suspension order states. “When asked what was administered, she replied ‘something special,’ “ the order states.
Another RN found the patient with labored breathing and “it was determined that respondent had disarmed/lowered the oxygen monitoring system several times as to not set off an alarm at the bedside,” the order continued. “The RN discussed with charge nurse that the patient had been given something intravenously that was causing his decline.”
When the charge nurse entered the room later, she found the patient in “respiratory distress with labored breathing and poor oxygen saturation. ... X-rays would show that the aspiration from the substances ingested by the patient while in his condition caused the patient to develop aspirational pneumonia,” the order continues.
“Despite the rapidly declining condition of the patient, respondent never called for rapid response nor acted with any sense of urgency. Respondent did however edit documentation of administration of Ativan on ‘patient B’ to state ‘not given.’ ”
Mr. Morris’ condition never improved. He was taken to hospice care on May 3 and died 2 days later, the order states.
Ms. Hunter was being held in the Lexington Jail on $100,000 bond, according to jail records.
A version of this article first appeared on Medscape.com.
California wants to snip costs for vasectomies and condoms
SACRAMENTO – California is trying to ease the pain of vasectomies by making them free for millions of residents.
Federal law and state law require most health insurers to cover prescription contraceptives at no cost to the patient. But those provisions apply to only 18 Food and Drug Administration–approved birth control options for women, so anyone with testicles is out of luck.
California lawmakers are now considering a bill that would expand that requirement to male sterilization and non-prescription birth control, including condoms and contraceptive sponges. If the Contraceptive Equity Act of 2022 passes, commercial insurance plans regulated by the state won’t be allowed to impose out-of-pocket costs, like copays, coinsurance, and deductibles, on those modes of birth control.
“It’s pretty groundbreaking in that way – it’s a whole new framework to think about contraception as something that is relevant for people of all genders,” said Liz McCaman Taylor, a senior attorney with the National Health Law Program, a group that advocates for the health rights of low-income people.
A vasectomy is an outpatient surgical procedure in which the patient’s supply of sperm is cut off from his semen by sealing or snipping the tubes that transport sperm from the testes to the penis. Most men need to recover on the couch with an ice pack for a day or 2, and a test a few months later determines whether the procedure worked.
Because vasectomies are elective procedures and usually not urgent, price can be a deciding factor.
For Nathan Songne, cost was the most stressful part of the procedure. For several years, the 31-year-old had known he didn’t want to have kids biologically. Better to adopt a 4-year-old and skip the diaper stage, he thought. He was adopted by his stepfather as a child and knew he didn’t need to be genetically related to his children to love them.
“My only concern was that I had no idea how much it was going to cost me because nobody told me,” said Mr. Songne, who lives in Mission Viejo, in Orange County. If the procedure cost $1,000, as he expected, he wouldn’t be able to afford it.
Mr. Songne’s insurance, which he gets through his work assembling guitars, covered 70% of the Aug. 8 procedure, leaving him with a bill of just under $200. “Cost did affect my decision, but because it was only $200, it made me feel a lot more relieved about continuing on with the vasectomy.”
There are two hot times of year in the vasectomy business, according to Mary Samplaski, MD, an associate professor of urology at the University of Southern California, Los Angeles. First, she sees an uptick during the March Madness college basketball tournament, when men choose to recover on the couch watching hoops.
The end of the year is also busy, she said, because many patients have finally met their annual insurance deductible and can afford the procedure.
Patients discuss out-of-pocket costs in about 20% of her vasectomy consultations. “It’s obviously a nerve-wracking procedure,” Dr. Samplaski said. “And on top of that, if your copay is high, there’s even less reason to want to do it.”
In April, Jacob Elert comparison-shopped for a vasectomy near his home in Sacramento because his health plan doesn’t cover the procedure. He had hoped to schedule one with his regular urologist, but that would have come with a $1,500 price tag.
Instead, he found a chain of vasectomy clinics where he could get the procedure for $850. Three months later, a test confirmed the vasectomy was a success.
Mr. Elert has no regrets, but had price not been a factor, he would have preferred to go to his regular urologist. “That’s the doctor I trust,” Mr. Elert said. “But it was just way too expensive.”
In November, California voters will decide whether to lock rights to abortion and contraception into the state constitution. But Proposition 1 doesn’t address issues such as cost and coverage, said Amy Moy, a spokesperson for Essential Access Health, a group that runs California’s Title X family planning program.
“The constitutional amendment is kind of the long-term protection, and we are still working to reduce barriers for Californians on the short-term and day-to-day level regardless of their gender,” she said.
SB 523 has sailed through preliminary votes in the state legislature, which faces an end-of-August deadline to act on bills. If the measure passes, it would take effect in 2024, and California would join a handful of states that require plans they regulate to completely cover vasectomies or nonprescription birth control.
The California Association of Health Plans is still evaluating the measure, which may be amended in the final days of the legislative session. But the association generally opposes bills that require additional insurance benefits because they could lead to higher premiums, spokesperson Mary Ellen Grant said.
SB 523 applies to more than 14 million Californians who work for the state, have a student health plan through a university, or have state-regulated commercial health plans. They would become eligible to receive free over-the-counter birth control – such as emergency contraception, condoms, spermicide, and contraceptive sponges – in addition to vasectomies. The bill would not apply to the millions of Californians whose health insurance plans are regulated by the federal government.
The specifics of how the benefit would work, including the frequency and amount of birth control that insurers must cover and whether patients would have to pay upfront and be reimbursed later, would be hammered out after the measure is adopted. Ms. McCaman Taylor said allowing people to simply present their insurance card at a pharmacy counter and walk away with the birth control they need would be preferable.
“We kind of learned from the national experiment with COVID over-the-counter tests that reimbursement wasn’t the best model,” she said. “If people can’t afford to pay out of pocket for it, they’re just not going to get it.”
The California Health Benefits Review Program, which analyzes legislation, projected that roughly 14,200 people with state-regulated commercial insurance would get vasectomies in California in 2022. Eliminating cost sharing would increase the number of vasectomies by 252 in the law’s first year, the program estimated.
It’s a small increase. But that, plus a jump in the use of other contraceptives covered by the bill, particularly condoms, could add up to a big reduction in unintended pregnancies. Roughly 12,300 unplanned pregnancies might be averted each year if the mandate takes effect, a reduction of more than 11%, according to the analysis.
This story was produced by KHN, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
SACRAMENTO – California is trying to ease the pain of vasectomies by making them free for millions of residents.
Federal law and state law require most health insurers to cover prescription contraceptives at no cost to the patient. But those provisions apply to only 18 Food and Drug Administration–approved birth control options for women, so anyone with testicles is out of luck.
California lawmakers are now considering a bill that would expand that requirement to male sterilization and non-prescription birth control, including condoms and contraceptive sponges. If the Contraceptive Equity Act of 2022 passes, commercial insurance plans regulated by the state won’t be allowed to impose out-of-pocket costs, like copays, coinsurance, and deductibles, on those modes of birth control.
“It’s pretty groundbreaking in that way – it’s a whole new framework to think about contraception as something that is relevant for people of all genders,” said Liz McCaman Taylor, a senior attorney with the National Health Law Program, a group that advocates for the health rights of low-income people.
A vasectomy is an outpatient surgical procedure in which the patient’s supply of sperm is cut off from his semen by sealing or snipping the tubes that transport sperm from the testes to the penis. Most men need to recover on the couch with an ice pack for a day or 2, and a test a few months later determines whether the procedure worked.
Because vasectomies are elective procedures and usually not urgent, price can be a deciding factor.
For Nathan Songne, cost was the most stressful part of the procedure. For several years, the 31-year-old had known he didn’t want to have kids biologically. Better to adopt a 4-year-old and skip the diaper stage, he thought. He was adopted by his stepfather as a child and knew he didn’t need to be genetically related to his children to love them.
“My only concern was that I had no idea how much it was going to cost me because nobody told me,” said Mr. Songne, who lives in Mission Viejo, in Orange County. If the procedure cost $1,000, as he expected, he wouldn’t be able to afford it.
Mr. Songne’s insurance, which he gets through his work assembling guitars, covered 70% of the Aug. 8 procedure, leaving him with a bill of just under $200. “Cost did affect my decision, but because it was only $200, it made me feel a lot more relieved about continuing on with the vasectomy.”
There are two hot times of year in the vasectomy business, according to Mary Samplaski, MD, an associate professor of urology at the University of Southern California, Los Angeles. First, she sees an uptick during the March Madness college basketball tournament, when men choose to recover on the couch watching hoops.
The end of the year is also busy, she said, because many patients have finally met their annual insurance deductible and can afford the procedure.
Patients discuss out-of-pocket costs in about 20% of her vasectomy consultations. “It’s obviously a nerve-wracking procedure,” Dr. Samplaski said. “And on top of that, if your copay is high, there’s even less reason to want to do it.”
In April, Jacob Elert comparison-shopped for a vasectomy near his home in Sacramento because his health plan doesn’t cover the procedure. He had hoped to schedule one with his regular urologist, but that would have come with a $1,500 price tag.
Instead, he found a chain of vasectomy clinics where he could get the procedure for $850. Three months later, a test confirmed the vasectomy was a success.
Mr. Elert has no regrets, but had price not been a factor, he would have preferred to go to his regular urologist. “That’s the doctor I trust,” Mr. Elert said. “But it was just way too expensive.”
In November, California voters will decide whether to lock rights to abortion and contraception into the state constitution. But Proposition 1 doesn’t address issues such as cost and coverage, said Amy Moy, a spokesperson for Essential Access Health, a group that runs California’s Title X family planning program.
“The constitutional amendment is kind of the long-term protection, and we are still working to reduce barriers for Californians on the short-term and day-to-day level regardless of their gender,” she said.
SB 523 has sailed through preliminary votes in the state legislature, which faces an end-of-August deadline to act on bills. If the measure passes, it would take effect in 2024, and California would join a handful of states that require plans they regulate to completely cover vasectomies or nonprescription birth control.
The California Association of Health Plans is still evaluating the measure, which may be amended in the final days of the legislative session. But the association generally opposes bills that require additional insurance benefits because they could lead to higher premiums, spokesperson Mary Ellen Grant said.
SB 523 applies to more than 14 million Californians who work for the state, have a student health plan through a university, or have state-regulated commercial health plans. They would become eligible to receive free over-the-counter birth control – such as emergency contraception, condoms, spermicide, and contraceptive sponges – in addition to vasectomies. The bill would not apply to the millions of Californians whose health insurance plans are regulated by the federal government.
The specifics of how the benefit would work, including the frequency and amount of birth control that insurers must cover and whether patients would have to pay upfront and be reimbursed later, would be hammered out after the measure is adopted. Ms. McCaman Taylor said allowing people to simply present their insurance card at a pharmacy counter and walk away with the birth control they need would be preferable.
“We kind of learned from the national experiment with COVID over-the-counter tests that reimbursement wasn’t the best model,” she said. “If people can’t afford to pay out of pocket for it, they’re just not going to get it.”
The California Health Benefits Review Program, which analyzes legislation, projected that roughly 14,200 people with state-regulated commercial insurance would get vasectomies in California in 2022. Eliminating cost sharing would increase the number of vasectomies by 252 in the law’s first year, the program estimated.
It’s a small increase. But that, plus a jump in the use of other contraceptives covered by the bill, particularly condoms, could add up to a big reduction in unintended pregnancies. Roughly 12,300 unplanned pregnancies might be averted each year if the mandate takes effect, a reduction of more than 11%, according to the analysis.
This story was produced by KHN, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
SACRAMENTO – California is trying to ease the pain of vasectomies by making them free for millions of residents.
Federal law and state law require most health insurers to cover prescription contraceptives at no cost to the patient. But those provisions apply to only 18 Food and Drug Administration–approved birth control options for women, so anyone with testicles is out of luck.
California lawmakers are now considering a bill that would expand that requirement to male sterilization and non-prescription birth control, including condoms and contraceptive sponges. If the Contraceptive Equity Act of 2022 passes, commercial insurance plans regulated by the state won’t be allowed to impose out-of-pocket costs, like copays, coinsurance, and deductibles, on those modes of birth control.
“It’s pretty groundbreaking in that way – it’s a whole new framework to think about contraception as something that is relevant for people of all genders,” said Liz McCaman Taylor, a senior attorney with the National Health Law Program, a group that advocates for the health rights of low-income people.
A vasectomy is an outpatient surgical procedure in which the patient’s supply of sperm is cut off from his semen by sealing or snipping the tubes that transport sperm from the testes to the penis. Most men need to recover on the couch with an ice pack for a day or 2, and a test a few months later determines whether the procedure worked.
Because vasectomies are elective procedures and usually not urgent, price can be a deciding factor.
For Nathan Songne, cost was the most stressful part of the procedure. For several years, the 31-year-old had known he didn’t want to have kids biologically. Better to adopt a 4-year-old and skip the diaper stage, he thought. He was adopted by his stepfather as a child and knew he didn’t need to be genetically related to his children to love them.
“My only concern was that I had no idea how much it was going to cost me because nobody told me,” said Mr. Songne, who lives in Mission Viejo, in Orange County. If the procedure cost $1,000, as he expected, he wouldn’t be able to afford it.
Mr. Songne’s insurance, which he gets through his work assembling guitars, covered 70% of the Aug. 8 procedure, leaving him with a bill of just under $200. “Cost did affect my decision, but because it was only $200, it made me feel a lot more relieved about continuing on with the vasectomy.”
There are two hot times of year in the vasectomy business, according to Mary Samplaski, MD, an associate professor of urology at the University of Southern California, Los Angeles. First, she sees an uptick during the March Madness college basketball tournament, when men choose to recover on the couch watching hoops.
The end of the year is also busy, she said, because many patients have finally met their annual insurance deductible and can afford the procedure.
Patients discuss out-of-pocket costs in about 20% of her vasectomy consultations. “It’s obviously a nerve-wracking procedure,” Dr. Samplaski said. “And on top of that, if your copay is high, there’s even less reason to want to do it.”
In April, Jacob Elert comparison-shopped for a vasectomy near his home in Sacramento because his health plan doesn’t cover the procedure. He had hoped to schedule one with his regular urologist, but that would have come with a $1,500 price tag.
Instead, he found a chain of vasectomy clinics where he could get the procedure for $850. Three months later, a test confirmed the vasectomy was a success.
Mr. Elert has no regrets, but had price not been a factor, he would have preferred to go to his regular urologist. “That’s the doctor I trust,” Mr. Elert said. “But it was just way too expensive.”
In November, California voters will decide whether to lock rights to abortion and contraception into the state constitution. But Proposition 1 doesn’t address issues such as cost and coverage, said Amy Moy, a spokesperson for Essential Access Health, a group that runs California’s Title X family planning program.
“The constitutional amendment is kind of the long-term protection, and we are still working to reduce barriers for Californians on the short-term and day-to-day level regardless of their gender,” she said.
SB 523 has sailed through preliminary votes in the state legislature, which faces an end-of-August deadline to act on bills. If the measure passes, it would take effect in 2024, and California would join a handful of states that require plans they regulate to completely cover vasectomies or nonprescription birth control.
The California Association of Health Plans is still evaluating the measure, which may be amended in the final days of the legislative session. But the association generally opposes bills that require additional insurance benefits because they could lead to higher premiums, spokesperson Mary Ellen Grant said.
SB 523 applies to more than 14 million Californians who work for the state, have a student health plan through a university, or have state-regulated commercial health plans. They would become eligible to receive free over-the-counter birth control – such as emergency contraception, condoms, spermicide, and contraceptive sponges – in addition to vasectomies. The bill would not apply to the millions of Californians whose health insurance plans are regulated by the federal government.
The specifics of how the benefit would work, including the frequency and amount of birth control that insurers must cover and whether patients would have to pay upfront and be reimbursed later, would be hammered out after the measure is adopted. Ms. McCaman Taylor said allowing people to simply present their insurance card at a pharmacy counter and walk away with the birth control they need would be preferable.
“We kind of learned from the national experiment with COVID over-the-counter tests that reimbursement wasn’t the best model,” she said. “If people can’t afford to pay out of pocket for it, they’re just not going to get it.”
The California Health Benefits Review Program, which analyzes legislation, projected that roughly 14,200 people with state-regulated commercial insurance would get vasectomies in California in 2022. Eliminating cost sharing would increase the number of vasectomies by 252 in the law’s first year, the program estimated.
It’s a small increase. But that, plus a jump in the use of other contraceptives covered by the bill, particularly condoms, could add up to a big reduction in unintended pregnancies. Roughly 12,300 unplanned pregnancies might be averted each year if the mandate takes effect, a reduction of more than 11%, according to the analysis.
This story was produced by KHN, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Does DTC heart drug advertising discourage lifestyle changes?
A 5-minute bout of direct-to-consumer advertising (DTCA) for prescription heart drugs was associated with favorable perceptions of both medication use and pharmaceutical companies, but did not seem to negate intentions to use lifestyle interventions, a survey study shows.
Participants who watched ads for various prescription heart drugs, with or without price disclosure, were more likely to report positive perceptions of drug companies and intentions to take actions such as switching medications.
The ads did not seem to affect intentions to eat healthfully and exercise.
The study was published online in JAMA Health Forum.
DTCA ‘unlikely to have an adverse effect’
“Increasing prevalence of DTCA may promote an overreliance on medication over healthy lifestyle choices to manage chronic conditions,” coauthor Yashaswini Singh, MPA, a PhD candidate at the Johns Hopkins University, Baltimore, told this news organization. “Thus, we hypothesized that DTCA exposure would reduce the likelihood of individuals engaging in preventive health behaviors.”
“However,” she said, “our results did not support this hypothesis, suggesting that exposure to DTCA for heart disease medication is unlikely to have an adverse effect on individuals’ intentions to engage in diet and exercise.”
That said, she added, “DTCA of prescription drugs can contribute to rising drug costs due to overprescribing of both inappropriate and brand-name drugs over cheaper generic alternatives. While we do not examine this mechanism in our paper, this remains an important question for future research.”
For the study, the team recruited 2,874 individuals (mean age, 53.8 years; 54% men; 83% White) from a U.S. nationally representative sample of people at high risk of cardiovascular disease, the Ipsos Public Affairs KnowledgePanel.
Participants were randomly assigned to one of three interventions: DTCA for heart disease medications, DTCA for heart disease medications with price disclosure, or nonpharmaceutical advertising (control). Each group watched five 1-minute videos for a total of 5 minutes of advertising exposure.
One group viewed ads for four heart disease medications – two ads for sacubitril/valsartan (Entresto, Novartis) and one each for rivaroxaban (Xarelto, Bayer), evolocumab (Repatha, Amgen), and ticagrelor (Brilinta, AstraZeneca); the second group saw the same ads, but with prices spliced in; and controls watched videos for nondrug products, such as consumer electronics.
Participants then completed a questionnaire to measure medication- and lifestyle-related intentions, as well as health-related beliefs and perceptions. Using a scale of 1 (highly unlikely) to 5 (highly likely), they rated the likelihood of their switching medication, asking a physician or insurer about a medication, searching for the drug online, or taking it as directed. The same scale was used to rate the likelihood of their being more physically active or eating more healthfully.
On a scale of 1 (always disagree) to 5 (always agree), they also related their perceptions of pharmaceutical manufacturers as being competent, innovative, and trustworthy.
To measure the magnitude of DTCA associations, the researchers calculated marginal effects (MEs) of treatment – that is, the difference in probability of an outcome between the treatment and control arms.
They found a positive association between DTCA and medication-related behavioral intentions, including intention to switch medication (ME, 0.004; P = .002) and engage in information-seeking behaviors (ME, 0.02; P = .01).
There was no evidence suggesting that pharmaceutical DTCA discouraged use of nonpharmacologic lifestyle interventions to help manage heart disease. DTCA also was positively associated with consumers’ favorable perceptions of pharmaceutical manufacturers (competence: ME, 0.03; P = .01; innovative: ME, 0.03; P = .008).
No differential associations were seen for price disclosures in DTCA.
Questions remain
The authors acknowledged that the study focused on short-term behavioral intentions and that “future research should focus on the long-term effects of advertising in a real-world randomized setting.”
Ms. Singh said additional questions, some of which her team is investigating, include “understanding the interaction between government policies [such as] drug pricing reforms and firms’ advertising decisions; understanding whether observed changes in individuals’ health beliefs translate into actual changes to information-seeking behavior and health care utilization; and whether the demographic, political, and social characteristics of individuals shape their behavioral responses to advertising.”
Johanna Contreras, MD, an advanced heart failure and transplantation cardiologist at Mount Sinai Hospital, New York, said in an interview that the findings don’t surprise her. “The caveat is that this study was an online survey, so it only captured the beliefs and intentions, but not patient demand for the product and use of the product.”
“I do believe DTCA can create positive intentions towards the product ... and could make people more receptive to interventions,” she said. However, the information must be presented in a balanced way.
In addition, she noted, “price is still important. I think people take pricing into account when deciding to proceed with an intervention. If the price is ‘right’ or a little lower than expected, then they will likely consider the product. But if the price is significantly lower, then they may not trust that it is a good product. Generic drugs are an example. Even though they are approved and far cheaper than brand names, patients are often skeptical to take them.”
The study was funded with a grant from the Blue Cross Blue Shield of Illinois Affordability Cures Consortium. Ms. Singh and coauthors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A 5-minute bout of direct-to-consumer advertising (DTCA) for prescription heart drugs was associated with favorable perceptions of both medication use and pharmaceutical companies, but did not seem to negate intentions to use lifestyle interventions, a survey study shows.
Participants who watched ads for various prescription heart drugs, with or without price disclosure, were more likely to report positive perceptions of drug companies and intentions to take actions such as switching medications.
The ads did not seem to affect intentions to eat healthfully and exercise.
The study was published online in JAMA Health Forum.
DTCA ‘unlikely to have an adverse effect’
“Increasing prevalence of DTCA may promote an overreliance on medication over healthy lifestyle choices to manage chronic conditions,” coauthor Yashaswini Singh, MPA, a PhD candidate at the Johns Hopkins University, Baltimore, told this news organization. “Thus, we hypothesized that DTCA exposure would reduce the likelihood of individuals engaging in preventive health behaviors.”
“However,” she said, “our results did not support this hypothesis, suggesting that exposure to DTCA for heart disease medication is unlikely to have an adverse effect on individuals’ intentions to engage in diet and exercise.”
That said, she added, “DTCA of prescription drugs can contribute to rising drug costs due to overprescribing of both inappropriate and brand-name drugs over cheaper generic alternatives. While we do not examine this mechanism in our paper, this remains an important question for future research.”
For the study, the team recruited 2,874 individuals (mean age, 53.8 years; 54% men; 83% White) from a U.S. nationally representative sample of people at high risk of cardiovascular disease, the Ipsos Public Affairs KnowledgePanel.
Participants were randomly assigned to one of three interventions: DTCA for heart disease medications, DTCA for heart disease medications with price disclosure, or nonpharmaceutical advertising (control). Each group watched five 1-minute videos for a total of 5 minutes of advertising exposure.
One group viewed ads for four heart disease medications – two ads for sacubitril/valsartan (Entresto, Novartis) and one each for rivaroxaban (Xarelto, Bayer), evolocumab (Repatha, Amgen), and ticagrelor (Brilinta, AstraZeneca); the second group saw the same ads, but with prices spliced in; and controls watched videos for nondrug products, such as consumer electronics.
Participants then completed a questionnaire to measure medication- and lifestyle-related intentions, as well as health-related beliefs and perceptions. Using a scale of 1 (highly unlikely) to 5 (highly likely), they rated the likelihood of their switching medication, asking a physician or insurer about a medication, searching for the drug online, or taking it as directed. The same scale was used to rate the likelihood of their being more physically active or eating more healthfully.
On a scale of 1 (always disagree) to 5 (always agree), they also related their perceptions of pharmaceutical manufacturers as being competent, innovative, and trustworthy.
To measure the magnitude of DTCA associations, the researchers calculated marginal effects (MEs) of treatment – that is, the difference in probability of an outcome between the treatment and control arms.
They found a positive association between DTCA and medication-related behavioral intentions, including intention to switch medication (ME, 0.004; P = .002) and engage in information-seeking behaviors (ME, 0.02; P = .01).
There was no evidence suggesting that pharmaceutical DTCA discouraged use of nonpharmacologic lifestyle interventions to help manage heart disease. DTCA also was positively associated with consumers’ favorable perceptions of pharmaceutical manufacturers (competence: ME, 0.03; P = .01; innovative: ME, 0.03; P = .008).
No differential associations were seen for price disclosures in DTCA.
Questions remain
The authors acknowledged that the study focused on short-term behavioral intentions and that “future research should focus on the long-term effects of advertising in a real-world randomized setting.”
Ms. Singh said additional questions, some of which her team is investigating, include “understanding the interaction between government policies [such as] drug pricing reforms and firms’ advertising decisions; understanding whether observed changes in individuals’ health beliefs translate into actual changes to information-seeking behavior and health care utilization; and whether the demographic, political, and social characteristics of individuals shape their behavioral responses to advertising.”
Johanna Contreras, MD, an advanced heart failure and transplantation cardiologist at Mount Sinai Hospital, New York, said in an interview that the findings don’t surprise her. “The caveat is that this study was an online survey, so it only captured the beliefs and intentions, but not patient demand for the product and use of the product.”
“I do believe DTCA can create positive intentions towards the product ... and could make people more receptive to interventions,” she said. However, the information must be presented in a balanced way.
In addition, she noted, “price is still important. I think people take pricing into account when deciding to proceed with an intervention. If the price is ‘right’ or a little lower than expected, then they will likely consider the product. But if the price is significantly lower, then they may not trust that it is a good product. Generic drugs are an example. Even though they are approved and far cheaper than brand names, patients are often skeptical to take them.”
The study was funded with a grant from the Blue Cross Blue Shield of Illinois Affordability Cures Consortium. Ms. Singh and coauthors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A 5-minute bout of direct-to-consumer advertising (DTCA) for prescription heart drugs was associated with favorable perceptions of both medication use and pharmaceutical companies, but did not seem to negate intentions to use lifestyle interventions, a survey study shows.
Participants who watched ads for various prescription heart drugs, with or without price disclosure, were more likely to report positive perceptions of drug companies and intentions to take actions such as switching medications.
The ads did not seem to affect intentions to eat healthfully and exercise.
The study was published online in JAMA Health Forum.
DTCA ‘unlikely to have an adverse effect’
“Increasing prevalence of DTCA may promote an overreliance on medication over healthy lifestyle choices to manage chronic conditions,” coauthor Yashaswini Singh, MPA, a PhD candidate at the Johns Hopkins University, Baltimore, told this news organization. “Thus, we hypothesized that DTCA exposure would reduce the likelihood of individuals engaging in preventive health behaviors.”
“However,” she said, “our results did not support this hypothesis, suggesting that exposure to DTCA for heart disease medication is unlikely to have an adverse effect on individuals’ intentions to engage in diet and exercise.”
That said, she added, “DTCA of prescription drugs can contribute to rising drug costs due to overprescribing of both inappropriate and brand-name drugs over cheaper generic alternatives. While we do not examine this mechanism in our paper, this remains an important question for future research.”
For the study, the team recruited 2,874 individuals (mean age, 53.8 years; 54% men; 83% White) from a U.S. nationally representative sample of people at high risk of cardiovascular disease, the Ipsos Public Affairs KnowledgePanel.
Participants were randomly assigned to one of three interventions: DTCA for heart disease medications, DTCA for heart disease medications with price disclosure, or nonpharmaceutical advertising (control). Each group watched five 1-minute videos for a total of 5 minutes of advertising exposure.
One group viewed ads for four heart disease medications – two ads for sacubitril/valsartan (Entresto, Novartis) and one each for rivaroxaban (Xarelto, Bayer), evolocumab (Repatha, Amgen), and ticagrelor (Brilinta, AstraZeneca); the second group saw the same ads, but with prices spliced in; and controls watched videos for nondrug products, such as consumer electronics.
Participants then completed a questionnaire to measure medication- and lifestyle-related intentions, as well as health-related beliefs and perceptions. Using a scale of 1 (highly unlikely) to 5 (highly likely), they rated the likelihood of their switching medication, asking a physician or insurer about a medication, searching for the drug online, or taking it as directed. The same scale was used to rate the likelihood of their being more physically active or eating more healthfully.
On a scale of 1 (always disagree) to 5 (always agree), they also related their perceptions of pharmaceutical manufacturers as being competent, innovative, and trustworthy.
To measure the magnitude of DTCA associations, the researchers calculated marginal effects (MEs) of treatment – that is, the difference in probability of an outcome between the treatment and control arms.
They found a positive association between DTCA and medication-related behavioral intentions, including intention to switch medication (ME, 0.004; P = .002) and engage in information-seeking behaviors (ME, 0.02; P = .01).
There was no evidence suggesting that pharmaceutical DTCA discouraged use of nonpharmacologic lifestyle interventions to help manage heart disease. DTCA also was positively associated with consumers’ favorable perceptions of pharmaceutical manufacturers (competence: ME, 0.03; P = .01; innovative: ME, 0.03; P = .008).
No differential associations were seen for price disclosures in DTCA.
Questions remain
The authors acknowledged that the study focused on short-term behavioral intentions and that “future research should focus on the long-term effects of advertising in a real-world randomized setting.”
Ms. Singh said additional questions, some of which her team is investigating, include “understanding the interaction between government policies [such as] drug pricing reforms and firms’ advertising decisions; understanding whether observed changes in individuals’ health beliefs translate into actual changes to information-seeking behavior and health care utilization; and whether the demographic, political, and social characteristics of individuals shape their behavioral responses to advertising.”
Johanna Contreras, MD, an advanced heart failure and transplantation cardiologist at Mount Sinai Hospital, New York, said in an interview that the findings don’t surprise her. “The caveat is that this study was an online survey, so it only captured the beliefs and intentions, but not patient demand for the product and use of the product.”
“I do believe DTCA can create positive intentions towards the product ... and could make people more receptive to interventions,” she said. However, the information must be presented in a balanced way.
In addition, she noted, “price is still important. I think people take pricing into account when deciding to proceed with an intervention. If the price is ‘right’ or a little lower than expected, then they will likely consider the product. But if the price is significantly lower, then they may not trust that it is a good product. Generic drugs are an example. Even though they are approved and far cheaper than brand names, patients are often skeptical to take them.”
The study was funded with a grant from the Blue Cross Blue Shield of Illinois Affordability Cures Consortium. Ms. Singh and coauthors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA HEALTH FORUM
Mondegreens
Recently I was reading an article on the histories behind great songs, and one section featured Procol Harem’s “A Whiter Shade of Pale.” It mentioned the verse that incorporated a reference to Chaucer (“As the Miller told his tale”).
This surprised me, as, since I’d first heard the song (1983, in “The Big Chill”) until I read this piece, I thought the line was “As the mirror told its tale.” The idea that it was a misheard Chaucer reference had never occurred to me.
These are called mondegreens. The brain translates the phrase into what it hears, often giving it an entirely different meaning. Manfred Mann’s version of “Blinded by the Light” is absolutely full of them. Even the national anthem isn’t immune (“José can you see by the donzerly light?”)
I’m sure there’s an interesting study idea about the brain and mondegreens, probably involving PET scans, somewhere in there.
The whole thing reminded me of an incident early in residency, I suppose you could call it a medical mondegreen.
During training I never went anywhere without a clipboard and notepad, frantically scribbling tidbits down during rounds, lectures, meetings, whatever. I’d go home and reread them over dinner, trying to commit them to memory.
And somewhere, on rounds early in my first year of training, an attending told me that you can sometimes see a Bell’s palsy cause a mild ipsilateral hemiparesis. This surprised me, but hey, I was the newly minted doctor, there to learn. So I wrote it down, memorized it, and moved on.
Even then, though, it made no sense to me. Of course, I was too afraid to ask other residents about it, for fear they’d think I was an idiot (a point that’s still debatable). And questioning the attending involved seemed unthinkable.
But I wandered through my hospital library (back then, young ones, we used paper textbooks and journals) trying to figure out why a peripheral VII palsy could cause an ipsilateral hemiparesis. It would not let me be.
Nothing.
Finally, one day after a lecture, I asked the attending involved. He had no recollection of having tossed the point out a few months ago, and said there was no reason. This confirmed what I’d already realized – a standard Bell’s palsy couldn’t possibly cause an ipsilateral hemiparesis (I’m not going into the crossed-brainstem syndromes here).
Maybe he’d misspoken and not realized it. Maybe I hadn’t heard him correctly. Maybe a little of both. Hospital hallways are anything but quiet. He also had a pending vacation to the coast which could have distracted him.
Like mondegreens in songs, it was just an error, and looking back on it with 30 years perspective, it’s kind of funny. Fortunately I never sent anyone with a hemiparesis home from the ER thinking they had a Bell’s palsy.
But it makes you realize how flawed human communication can be. By the time I asked the attending about it I’d realized it couldn’t possibly be right. It still leaves me wondering about how much we think we heard correctly but we didn’t – and that we don’t notice.
Sometimes you may think your ears are open, but they might just as well be closed if you don’t hear correctly. In medicine the consequences of such can be a lot worse than screwing up on karaoke night.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Recently I was reading an article on the histories behind great songs, and one section featured Procol Harem’s “A Whiter Shade of Pale.” It mentioned the verse that incorporated a reference to Chaucer (“As the Miller told his tale”).
This surprised me, as, since I’d first heard the song (1983, in “The Big Chill”) until I read this piece, I thought the line was “As the mirror told its tale.” The idea that it was a misheard Chaucer reference had never occurred to me.
These are called mondegreens. The brain translates the phrase into what it hears, often giving it an entirely different meaning. Manfred Mann’s version of “Blinded by the Light” is absolutely full of them. Even the national anthem isn’t immune (“José can you see by the donzerly light?”)
I’m sure there’s an interesting study idea about the brain and mondegreens, probably involving PET scans, somewhere in there.
The whole thing reminded me of an incident early in residency, I suppose you could call it a medical mondegreen.
During training I never went anywhere without a clipboard and notepad, frantically scribbling tidbits down during rounds, lectures, meetings, whatever. I’d go home and reread them over dinner, trying to commit them to memory.
And somewhere, on rounds early in my first year of training, an attending told me that you can sometimes see a Bell’s palsy cause a mild ipsilateral hemiparesis. This surprised me, but hey, I was the newly minted doctor, there to learn. So I wrote it down, memorized it, and moved on.
Even then, though, it made no sense to me. Of course, I was too afraid to ask other residents about it, for fear they’d think I was an idiot (a point that’s still debatable). And questioning the attending involved seemed unthinkable.
But I wandered through my hospital library (back then, young ones, we used paper textbooks and journals) trying to figure out why a peripheral VII palsy could cause an ipsilateral hemiparesis. It would not let me be.
Nothing.
Finally, one day after a lecture, I asked the attending involved. He had no recollection of having tossed the point out a few months ago, and said there was no reason. This confirmed what I’d already realized – a standard Bell’s palsy couldn’t possibly cause an ipsilateral hemiparesis (I’m not going into the crossed-brainstem syndromes here).
Maybe he’d misspoken and not realized it. Maybe I hadn’t heard him correctly. Maybe a little of both. Hospital hallways are anything but quiet. He also had a pending vacation to the coast which could have distracted him.
Like mondegreens in songs, it was just an error, and looking back on it with 30 years perspective, it’s kind of funny. Fortunately I never sent anyone with a hemiparesis home from the ER thinking they had a Bell’s palsy.
But it makes you realize how flawed human communication can be. By the time I asked the attending about it I’d realized it couldn’t possibly be right. It still leaves me wondering about how much we think we heard correctly but we didn’t – and that we don’t notice.
Sometimes you may think your ears are open, but they might just as well be closed if you don’t hear correctly. In medicine the consequences of such can be a lot worse than screwing up on karaoke night.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Recently I was reading an article on the histories behind great songs, and one section featured Procol Harem’s “A Whiter Shade of Pale.” It mentioned the verse that incorporated a reference to Chaucer (“As the Miller told his tale”).
This surprised me, as, since I’d first heard the song (1983, in “The Big Chill”) until I read this piece, I thought the line was “As the mirror told its tale.” The idea that it was a misheard Chaucer reference had never occurred to me.
These are called mondegreens. The brain translates the phrase into what it hears, often giving it an entirely different meaning. Manfred Mann’s version of “Blinded by the Light” is absolutely full of them. Even the national anthem isn’t immune (“José can you see by the donzerly light?”)
I’m sure there’s an interesting study idea about the brain and mondegreens, probably involving PET scans, somewhere in there.
The whole thing reminded me of an incident early in residency, I suppose you could call it a medical mondegreen.
During training I never went anywhere without a clipboard and notepad, frantically scribbling tidbits down during rounds, lectures, meetings, whatever. I’d go home and reread them over dinner, trying to commit them to memory.
And somewhere, on rounds early in my first year of training, an attending told me that you can sometimes see a Bell’s palsy cause a mild ipsilateral hemiparesis. This surprised me, but hey, I was the newly minted doctor, there to learn. So I wrote it down, memorized it, and moved on.
Even then, though, it made no sense to me. Of course, I was too afraid to ask other residents about it, for fear they’d think I was an idiot (a point that’s still debatable). And questioning the attending involved seemed unthinkable.
But I wandered through my hospital library (back then, young ones, we used paper textbooks and journals) trying to figure out why a peripheral VII palsy could cause an ipsilateral hemiparesis. It would not let me be.
Nothing.
Finally, one day after a lecture, I asked the attending involved. He had no recollection of having tossed the point out a few months ago, and said there was no reason. This confirmed what I’d already realized – a standard Bell’s palsy couldn’t possibly cause an ipsilateral hemiparesis (I’m not going into the crossed-brainstem syndromes here).
Maybe he’d misspoken and not realized it. Maybe I hadn’t heard him correctly. Maybe a little of both. Hospital hallways are anything but quiet. He also had a pending vacation to the coast which could have distracted him.
Like mondegreens in songs, it was just an error, and looking back on it with 30 years perspective, it’s kind of funny. Fortunately I never sent anyone with a hemiparesis home from the ER thinking they had a Bell’s palsy.
But it makes you realize how flawed human communication can be. By the time I asked the attending about it I’d realized it couldn’t possibly be right. It still leaves me wondering about how much we think we heard correctly but we didn’t – and that we don’t notice.
Sometimes you may think your ears are open, but they might just as well be closed if you don’t hear correctly. In medicine the consequences of such can be a lot worse than screwing up on karaoke night.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Indiana’s new abortion ban may drive some young ob.gyns. to leave a state where they’re needed
On a Monday morning, a group of obstetrics and gynecology residents, dressed in blue scrubs and white coats, gathered in an auditorium at Indiana University, Indianapolis. After the usual updates and announcements, Nicole Scott, MD, the residency program director, addressed the elephant in the room. “Any more abortion care questions?” she asked the trainees.
After a few moments of silence, one resident asked: “How’s Dr. Bernard doing?”
“Bernard is actually in really good spirits – I mean, relatively,” Dr. Scott answered. “She has 24/7 security, has her own lawyer.”
They were talking about Caitlin Bernard, MD, an Indiana ob.gyn. who provides abortions and trains residents at the university hospital. Dr. Bernard was recently caught in a political whirlwind after she spoke about an abortion she provided to a 10-year-old rape victim from Ohio. Dr. Bernard was the target of false accusations made on national television by pundits and political leaders, including Indiana’s attorney general.
The doctors interviewed for this article said that they are not speaking on behalf of their school of medicine but rather about their personal experiences during a tumultuous moment that they worry will affect the way they care for their patients.
The vitriol directed at Dr. Bernard hit home for this group of residents. She has mentored most of them for years. Many of the young doctors were certain they wanted to practice in Indiana after their training. But lately, some have been ambivalent about that prospect.
Beatrice Soderholm, DO, a fourth-year ob.gyn. resident, said watching what Dr. Bernard went through was “scary.” “I think that was part of the point for those who were putting her through that,” Dr. Soderholm said. They were trying “to scare other people out of doing the work that she does.”
In early August, Gov. Eric Holcomb, a Republican, signed a near-total abortion ban into law, making Indiana the first state to adopt new restrictions on abortion access since the Supreme Court struck down Roe v. Wade in June. When the ban takes effect Sept. 15, medical providers who violate the law risk losing their licenses or serving up to six years in prison.
These days, Dr. Scott, the residency program director, uses some meeting time with residents to fill them in on political updates and available mental health services. She also reminds them that legal counsel is on call round the clock to help if they’re ever unsure about the care they should provide a patient.
“Our residents are devastated,” Dr. Scott said, holding back tears. “They signed up to provide comprehensive health care to women, and they are being told that they can’t do that.”
She expects this will “deeply impact” how Indiana hospitals recruit and retain medical professionals.
A 2018 report from the March of Dimes found that 27% of Indiana counties are considered maternity care deserts, with no or limited access to maternal care. The state has one of the nation’s highest maternal mortality rates.
Dr. Scott said new laws restricting abortion will only worsen those statistics.
Dr. Scott shared results from a recent survey of nearly 1,400 residents and fellows across all specialties at IU, nearly 80% of the trainees said they were less likely to stay and practice in Indiana after the abortion ban.
Wendy Tian, MD, a third-year resident, said she is worried about her safety. Dr. Tian grew up and went to medical school in Chicago and chose to do her residency in Indiana because the program has a strong family-planning focus. She was open to practicing in Indiana when she completed her training.
But that’s changed.
“I, for sure, don’t know if I would be able to stay in Indiana post graduation with what’s going on,” Dr. Tian said.
Still, she feels guilty for “giving up” on Indiana’s most vulnerable patients.
Even before Roe fell, Dr. Tian said, the climate in Indiana could be hostile and frustrating for ob.gyns. Indiana, like other states with abortion restrictions, allows nearly all health care providers to opt out of providing care to patients having an abortion.
“We encounter other people who we work with on a daily basis who are opposed to what we do,” Dr. Tian said, adding that she and her colleagues have had to cancel scheduled procedures because the nurses on call were not comfortable assisting during an abortion.
Dr. Scott said the ob.gyn. program at the IU has provided residents with comprehensive training, including on abortion care and family planning. Since miscarriages are managed the same way as first-trimester abortions, she said, the training gives residents lots of hands-on experience. “What termination procedures allow you to do is that kind of repetition and that understanding of the female anatomy and how to manage complications that may happen with miscarriages.”
The ban on abortions dramatically reduces the hands-on opportunities for ob.gyn. residents, and that’s a huge concern, she said.
The program is exploring ways to offer training. One option is to send residents to learn in states without abortion restrictions, but Dr. Scott said that would be a logistical nightmare. “This is not as simple as just showing up to an office and saying: ‘Can I observe?’ This includes getting a medical license for out-of-state trainees. This includes funding for travel and lodging,” Dr. Scott said. “It adds a lot to what we already do to educate future ob.gyns.”
Four in 10 of all ob.gyn. residents in the United States are in states where abortion is banned or likely to be banned, so there could be a surge of residents looking to go out of state to make up for lost training opportunities. The Accreditation Council for Graduate Medical Education, the body that accredits residency programs, proposed modifications to the graduation requirements for ob.gyn. residents to account for the changing landscape.
For some of the Indiana ob.gyn. residents – including Veronica Santana, MD, a first-year resident – these political hurdles are a challenge they’re more than willing to take on. Dr. Santana is Latina, grew up in Seattle, and has been involved in community organizing since she was a teenager. One reason she chose obstetrics and gynecology was because of how the field intersects with social justice. “It’s political. It always has been, and it continues to be,” she said, “And, obviously, especially now.”
After Roe was overturned, Dr. Santana, alongside other residents and mentors, took to the streets of Indianapolis to participate in rallies in support of abortion rights.
Indiana could be the perfect battleground for Dr. Santana’s advocacy and social activism. But lately, she said, she is “very unsure” whether staying in Indiana to practice after residency makes sense, since she wants to provide the entire range of ob.gyn. services.
Dr. Soderholm, who grew up in Minnesota, has felt a strong connection to patients at the county hospital in Indianapolis. She had been certain she wanted to practice in Indiana. But her family in Minnesota – where abortion remains largely protected – has recently questioned why she would stay in a state with such a hostile climate for ob.gyns. “There’s been a lot of hesitation,” she said. But the patients make leaving difficult. “Sorry,” she said, starting to cry.
It’s for those patients that Dr. Soderholm decided she’ll likely stay. Other young doctors may make a different decision.
This story is part of a partnership that includes Side Effects Public Media, NPR, and KHN. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
On a Monday morning, a group of obstetrics and gynecology residents, dressed in blue scrubs and white coats, gathered in an auditorium at Indiana University, Indianapolis. After the usual updates and announcements, Nicole Scott, MD, the residency program director, addressed the elephant in the room. “Any more abortion care questions?” she asked the trainees.
After a few moments of silence, one resident asked: “How’s Dr. Bernard doing?”
“Bernard is actually in really good spirits – I mean, relatively,” Dr. Scott answered. “She has 24/7 security, has her own lawyer.”
They were talking about Caitlin Bernard, MD, an Indiana ob.gyn. who provides abortions and trains residents at the university hospital. Dr. Bernard was recently caught in a political whirlwind after she spoke about an abortion she provided to a 10-year-old rape victim from Ohio. Dr. Bernard was the target of false accusations made on national television by pundits and political leaders, including Indiana’s attorney general.
The doctors interviewed for this article said that they are not speaking on behalf of their school of medicine but rather about their personal experiences during a tumultuous moment that they worry will affect the way they care for their patients.
The vitriol directed at Dr. Bernard hit home for this group of residents. She has mentored most of them for years. Many of the young doctors were certain they wanted to practice in Indiana after their training. But lately, some have been ambivalent about that prospect.
Beatrice Soderholm, DO, a fourth-year ob.gyn. resident, said watching what Dr. Bernard went through was “scary.” “I think that was part of the point for those who were putting her through that,” Dr. Soderholm said. They were trying “to scare other people out of doing the work that she does.”
In early August, Gov. Eric Holcomb, a Republican, signed a near-total abortion ban into law, making Indiana the first state to adopt new restrictions on abortion access since the Supreme Court struck down Roe v. Wade in June. When the ban takes effect Sept. 15, medical providers who violate the law risk losing their licenses or serving up to six years in prison.
These days, Dr. Scott, the residency program director, uses some meeting time with residents to fill them in on political updates and available mental health services. She also reminds them that legal counsel is on call round the clock to help if they’re ever unsure about the care they should provide a patient.
“Our residents are devastated,” Dr. Scott said, holding back tears. “They signed up to provide comprehensive health care to women, and they are being told that they can’t do that.”
She expects this will “deeply impact” how Indiana hospitals recruit and retain medical professionals.
A 2018 report from the March of Dimes found that 27% of Indiana counties are considered maternity care deserts, with no or limited access to maternal care. The state has one of the nation’s highest maternal mortality rates.
Dr. Scott said new laws restricting abortion will only worsen those statistics.
Dr. Scott shared results from a recent survey of nearly 1,400 residents and fellows across all specialties at IU, nearly 80% of the trainees said they were less likely to stay and practice in Indiana after the abortion ban.
Wendy Tian, MD, a third-year resident, said she is worried about her safety. Dr. Tian grew up and went to medical school in Chicago and chose to do her residency in Indiana because the program has a strong family-planning focus. She was open to practicing in Indiana when she completed her training.
But that’s changed.
“I, for sure, don’t know if I would be able to stay in Indiana post graduation with what’s going on,” Dr. Tian said.
Still, she feels guilty for “giving up” on Indiana’s most vulnerable patients.
Even before Roe fell, Dr. Tian said, the climate in Indiana could be hostile and frustrating for ob.gyns. Indiana, like other states with abortion restrictions, allows nearly all health care providers to opt out of providing care to patients having an abortion.
“We encounter other people who we work with on a daily basis who are opposed to what we do,” Dr. Tian said, adding that she and her colleagues have had to cancel scheduled procedures because the nurses on call were not comfortable assisting during an abortion.
Dr. Scott said the ob.gyn. program at the IU has provided residents with comprehensive training, including on abortion care and family planning. Since miscarriages are managed the same way as first-trimester abortions, she said, the training gives residents lots of hands-on experience. “What termination procedures allow you to do is that kind of repetition and that understanding of the female anatomy and how to manage complications that may happen with miscarriages.”
The ban on abortions dramatically reduces the hands-on opportunities for ob.gyn. residents, and that’s a huge concern, she said.
The program is exploring ways to offer training. One option is to send residents to learn in states without abortion restrictions, but Dr. Scott said that would be a logistical nightmare. “This is not as simple as just showing up to an office and saying: ‘Can I observe?’ This includes getting a medical license for out-of-state trainees. This includes funding for travel and lodging,” Dr. Scott said. “It adds a lot to what we already do to educate future ob.gyns.”
Four in 10 of all ob.gyn. residents in the United States are in states where abortion is banned or likely to be banned, so there could be a surge of residents looking to go out of state to make up for lost training opportunities. The Accreditation Council for Graduate Medical Education, the body that accredits residency programs, proposed modifications to the graduation requirements for ob.gyn. residents to account for the changing landscape.
For some of the Indiana ob.gyn. residents – including Veronica Santana, MD, a first-year resident – these political hurdles are a challenge they’re more than willing to take on. Dr. Santana is Latina, grew up in Seattle, and has been involved in community organizing since she was a teenager. One reason she chose obstetrics and gynecology was because of how the field intersects with social justice. “It’s political. It always has been, and it continues to be,” she said, “And, obviously, especially now.”
After Roe was overturned, Dr. Santana, alongside other residents and mentors, took to the streets of Indianapolis to participate in rallies in support of abortion rights.
Indiana could be the perfect battleground for Dr. Santana’s advocacy and social activism. But lately, she said, she is “very unsure” whether staying in Indiana to practice after residency makes sense, since she wants to provide the entire range of ob.gyn. services.
Dr. Soderholm, who grew up in Minnesota, has felt a strong connection to patients at the county hospital in Indianapolis. She had been certain she wanted to practice in Indiana. But her family in Minnesota – where abortion remains largely protected – has recently questioned why she would stay in a state with such a hostile climate for ob.gyns. “There’s been a lot of hesitation,” she said. But the patients make leaving difficult. “Sorry,” she said, starting to cry.
It’s for those patients that Dr. Soderholm decided she’ll likely stay. Other young doctors may make a different decision.
This story is part of a partnership that includes Side Effects Public Media, NPR, and KHN. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
On a Monday morning, a group of obstetrics and gynecology residents, dressed in blue scrubs and white coats, gathered in an auditorium at Indiana University, Indianapolis. After the usual updates and announcements, Nicole Scott, MD, the residency program director, addressed the elephant in the room. “Any more abortion care questions?” she asked the trainees.
After a few moments of silence, one resident asked: “How’s Dr. Bernard doing?”
“Bernard is actually in really good spirits – I mean, relatively,” Dr. Scott answered. “She has 24/7 security, has her own lawyer.”
They were talking about Caitlin Bernard, MD, an Indiana ob.gyn. who provides abortions and trains residents at the university hospital. Dr. Bernard was recently caught in a political whirlwind after she spoke about an abortion she provided to a 10-year-old rape victim from Ohio. Dr. Bernard was the target of false accusations made on national television by pundits and political leaders, including Indiana’s attorney general.
The doctors interviewed for this article said that they are not speaking on behalf of their school of medicine but rather about their personal experiences during a tumultuous moment that they worry will affect the way they care for their patients.
The vitriol directed at Dr. Bernard hit home for this group of residents. She has mentored most of them for years. Many of the young doctors were certain they wanted to practice in Indiana after their training. But lately, some have been ambivalent about that prospect.
Beatrice Soderholm, DO, a fourth-year ob.gyn. resident, said watching what Dr. Bernard went through was “scary.” “I think that was part of the point for those who were putting her through that,” Dr. Soderholm said. They were trying “to scare other people out of doing the work that she does.”
In early August, Gov. Eric Holcomb, a Republican, signed a near-total abortion ban into law, making Indiana the first state to adopt new restrictions on abortion access since the Supreme Court struck down Roe v. Wade in June. When the ban takes effect Sept. 15, medical providers who violate the law risk losing their licenses or serving up to six years in prison.
These days, Dr. Scott, the residency program director, uses some meeting time with residents to fill them in on political updates and available mental health services. She also reminds them that legal counsel is on call round the clock to help if they’re ever unsure about the care they should provide a patient.
“Our residents are devastated,” Dr. Scott said, holding back tears. “They signed up to provide comprehensive health care to women, and they are being told that they can’t do that.”
She expects this will “deeply impact” how Indiana hospitals recruit and retain medical professionals.
A 2018 report from the March of Dimes found that 27% of Indiana counties are considered maternity care deserts, with no or limited access to maternal care. The state has one of the nation’s highest maternal mortality rates.
Dr. Scott said new laws restricting abortion will only worsen those statistics.
Dr. Scott shared results from a recent survey of nearly 1,400 residents and fellows across all specialties at IU, nearly 80% of the trainees said they were less likely to stay and practice in Indiana after the abortion ban.
Wendy Tian, MD, a third-year resident, said she is worried about her safety. Dr. Tian grew up and went to medical school in Chicago and chose to do her residency in Indiana because the program has a strong family-planning focus. She was open to practicing in Indiana when she completed her training.
But that’s changed.
“I, for sure, don’t know if I would be able to stay in Indiana post graduation with what’s going on,” Dr. Tian said.
Still, she feels guilty for “giving up” on Indiana’s most vulnerable patients.
Even before Roe fell, Dr. Tian said, the climate in Indiana could be hostile and frustrating for ob.gyns. Indiana, like other states with abortion restrictions, allows nearly all health care providers to opt out of providing care to patients having an abortion.
“We encounter other people who we work with on a daily basis who are opposed to what we do,” Dr. Tian said, adding that she and her colleagues have had to cancel scheduled procedures because the nurses on call were not comfortable assisting during an abortion.
Dr. Scott said the ob.gyn. program at the IU has provided residents with comprehensive training, including on abortion care and family planning. Since miscarriages are managed the same way as first-trimester abortions, she said, the training gives residents lots of hands-on experience. “What termination procedures allow you to do is that kind of repetition and that understanding of the female anatomy and how to manage complications that may happen with miscarriages.”
The ban on abortions dramatically reduces the hands-on opportunities for ob.gyn. residents, and that’s a huge concern, she said.
The program is exploring ways to offer training. One option is to send residents to learn in states without abortion restrictions, but Dr. Scott said that would be a logistical nightmare. “This is not as simple as just showing up to an office and saying: ‘Can I observe?’ This includes getting a medical license for out-of-state trainees. This includes funding for travel and lodging,” Dr. Scott said. “It adds a lot to what we already do to educate future ob.gyns.”
Four in 10 of all ob.gyn. residents in the United States are in states where abortion is banned or likely to be banned, so there could be a surge of residents looking to go out of state to make up for lost training opportunities. The Accreditation Council for Graduate Medical Education, the body that accredits residency programs, proposed modifications to the graduation requirements for ob.gyn. residents to account for the changing landscape.
For some of the Indiana ob.gyn. residents – including Veronica Santana, MD, a first-year resident – these political hurdles are a challenge they’re more than willing to take on. Dr. Santana is Latina, grew up in Seattle, and has been involved in community organizing since she was a teenager. One reason she chose obstetrics and gynecology was because of how the field intersects with social justice. “It’s political. It always has been, and it continues to be,” she said, “And, obviously, especially now.”
After Roe was overturned, Dr. Santana, alongside other residents and mentors, took to the streets of Indianapolis to participate in rallies in support of abortion rights.
Indiana could be the perfect battleground for Dr. Santana’s advocacy and social activism. But lately, she said, she is “very unsure” whether staying in Indiana to practice after residency makes sense, since she wants to provide the entire range of ob.gyn. services.
Dr. Soderholm, who grew up in Minnesota, has felt a strong connection to patients at the county hospital in Indianapolis. She had been certain she wanted to practice in Indiana. But her family in Minnesota – where abortion remains largely protected – has recently questioned why she would stay in a state with such a hostile climate for ob.gyns. “There’s been a lot of hesitation,” she said. But the patients make leaving difficult. “Sorry,” she said, starting to cry.
It’s for those patients that Dr. Soderholm decided she’ll likely stay. Other young doctors may make a different decision.
This story is part of a partnership that includes Side Effects Public Media, NPR, and KHN. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.



