User login
Facial Temperature Can Reveal Age and Disease
This transcript has been edited for clarity.
My oldest daughter is at sleepaway camp for a couple of weeks, and the camp has a photographer who goes around all day taking pictures of the kids, which get uploaded to a private Facebook group. In the past, I would go online every day (or, okay, several times a day) and scroll through all those pictures looking for one that features my kid.
I don’t have to do that anymore. This year, I simply uploaded a picture of my daughter to an app and artificial intelligence (AI) takes care of the rest, recognizing her face amidst the sea of smiling children, and flagging just those photos for me to peruse. It’s amazing, really. And a bit scary.
The fact that facial recognition has penetrated the summer camp market should tell you that the tech is truly ubiquitous. But today we’re going to think a bit more about what AI can do with a picture of your face, because the power of facial recognition is not just skin deep.
What’s got me hot and bothered about facial images is this paper, appearing in Cell Metabolism, which adds a new layer to the standard facial-analysis playbook: facial temperature.
To understand this paper, you need to understand a whole field of research that is developing various different “clocks” for age.
It turns out that age really is just a number. Our cells, our proteins, our biochemistry can be analyzed to give different numbers. These “clocks,” as distinct from the calendar we usually use to measure our age, might have more predictive power than the number itself.
There are numerous molecular clocks, such as telomere length, that not only correlate with calendar age but are superior to calendar age in predicting age-related complications. Testing telomere length typically requires a blood sample — and remains costly. But we can use other sources to estimate age; how about a photo?
I mean, we do this all the time when we meet someone new or, as a physician, when we meet a new patient. I have often written that a patient “appears younger than their stated age,” and we’ve all had the experience of hearing how old someone is and being shocked. I mean, have you seen Sharon Stone recently? She’s 66 years old. Okay — to be fair, there might be some outside help there. But you get the point.
Back to the Cell Metabolism paper. Researchers report on multiple algorithms to obtain an “age” from a picture of an individual’s face.
The first algorithm is pretty straightforward. Researchers collected 2811 images, all of Han Chinese individuals ranging in age from 20 to 90 years, and reconstructed a 3D facial map from those. 
They then trained a convolutional neural network to predict the individuals’ ages from the pictures. It was quite accurate, as you can see here.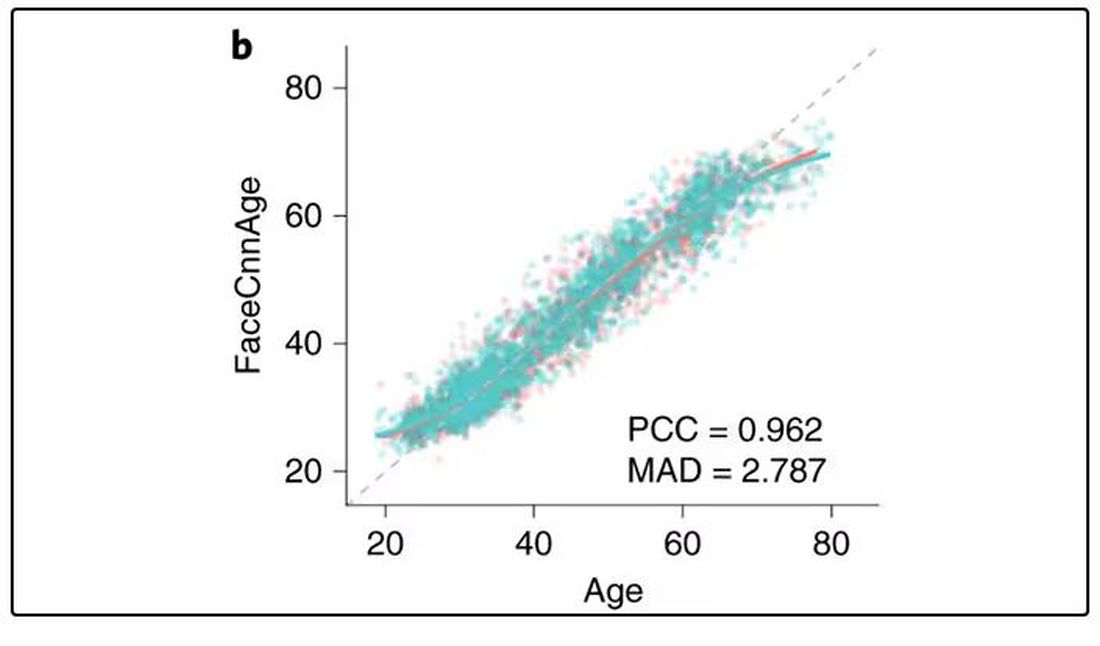
In the AI age, this may not seem that impressive. A brief search online turned up dozens of apps that promised to guess my age from a photo.
I sent this rather unflattering picture of myself to ChatGPT which, after initially demurring and saying it was not designed to guess ages, pegged me at somewhere between 35 and 45, which I am taking as a major victory.
But the Cell Metabolism paper goes deeper. Literally. 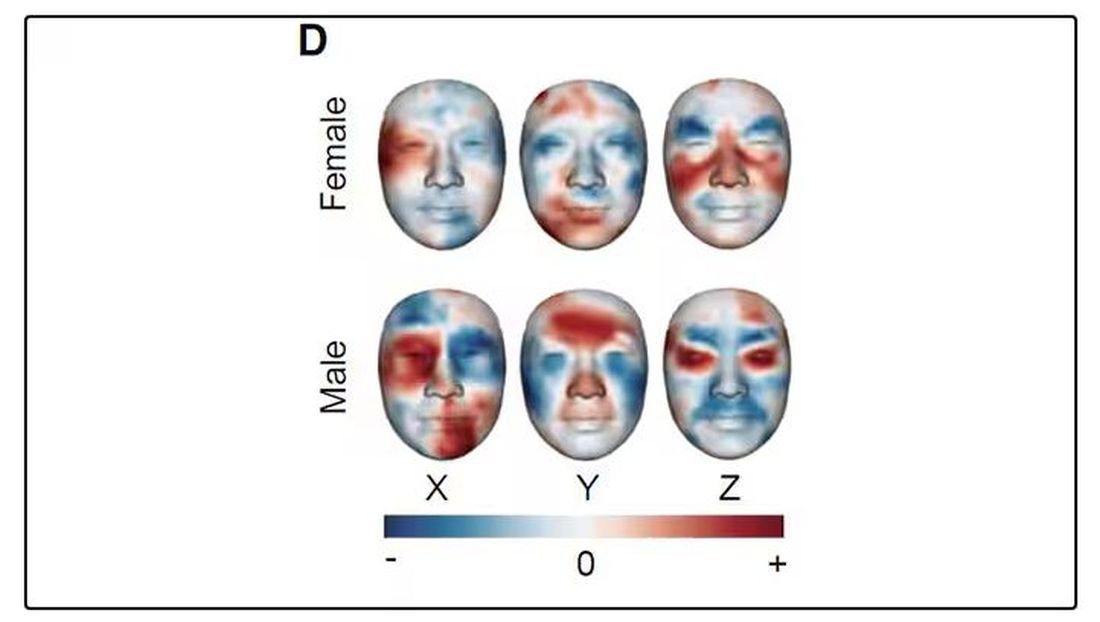
And this is where things start to get interesting. Because sure, the visible part of your face can change depending on makeup, expression, plastic surgery, and the like. But the temperature? That’s harder to fake.
It turns out that the temperature distribution in your face changes as you get older. There is a cooling of the nose and the cheeks, for example.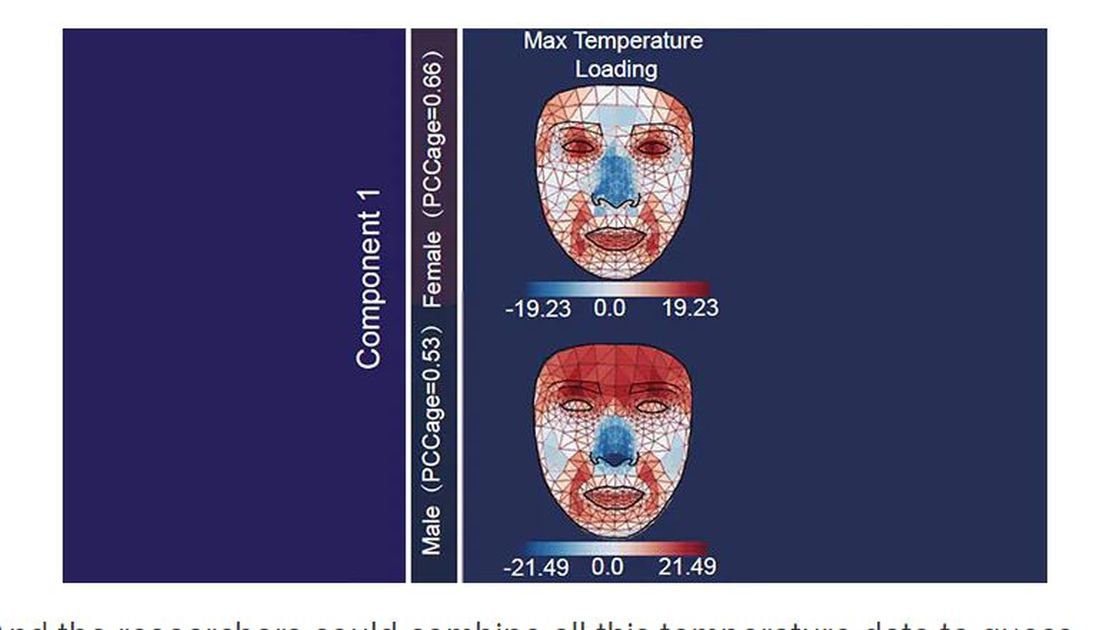
And the researchers could combine all this temperature data to guess someone’s calendar age fairly accurately, though notably not as accurately as the model that just looks at the pictures.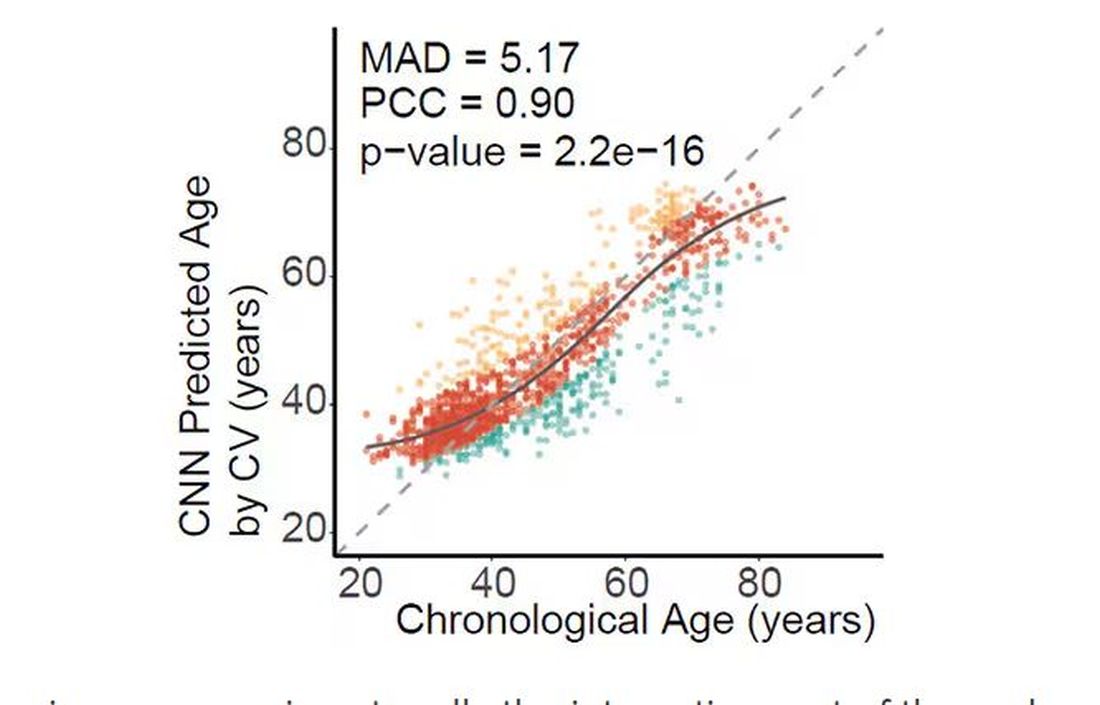
But guessing your age is not really the interesting part of thermal imaging of the face. It’s guessing — or, rather, predicting — the state of your metabolism. All these study participants had extensive metabolic testing performed, as well as detailed analysis of their lifestyle behaviors. And facial images could be used to predict those factors.
For example, the 3D reconstruction of the faces could predict who ate seafood (they tend to look younger than their actual age) compared with who ate poultry and meat (they tend to look older). The thermal imaging could predict who got more sleep (they look younger from a temperature perspective) and who ate more yogurt (also younger-appearing, temperature-wise). Facial temperature patterns could identify those with higher BMI, higher blood pressure, higher fasting glucose.
The researchers used the difference between actual and predicted age as a metric to measure illness as well. You can see here how, on average, individuals with hypertension, diabetes, and even liver cysts are “older,” at least by face temperature.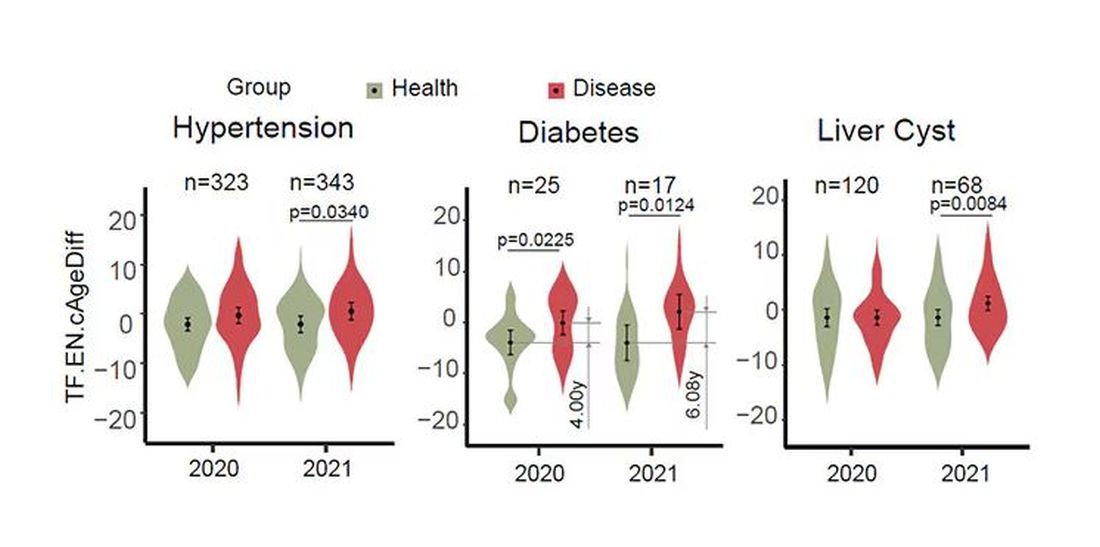
It may even be possible to use facial temperature as biofeedback. In a small study, the researchers measured the difference between facial temperature age and real age before and after 2 weeks of jump-roping. It turns out that 2 weeks of jump-roping can make you look about 5 years younger, at least as judged by a thermal camera. Or like the Predator.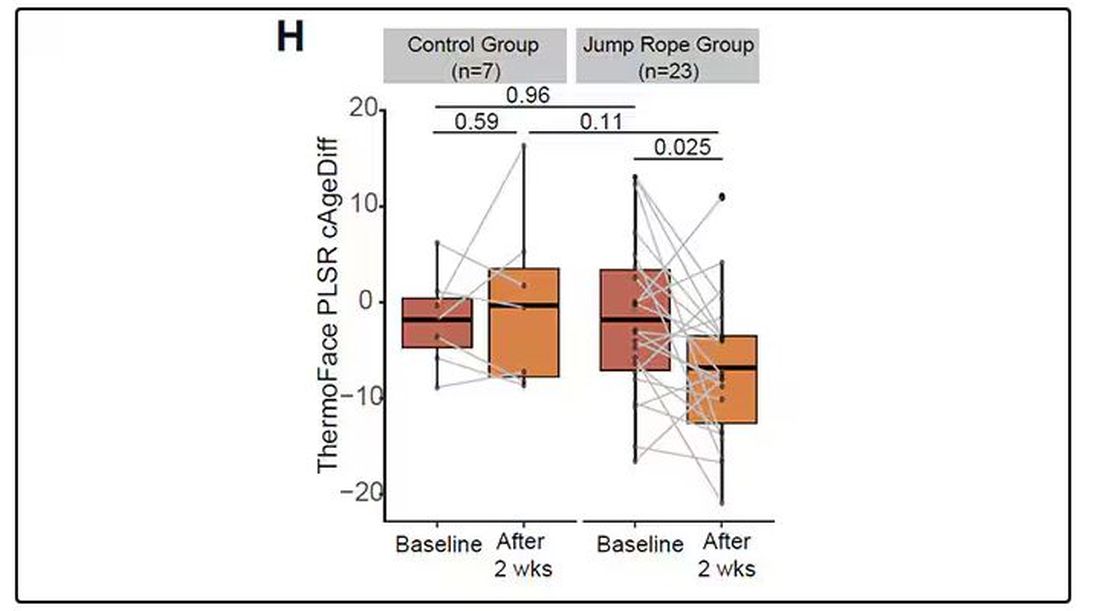
Okay, this is all very cool, but I’m not saying we’ll all be doing facial temperature tests in the near future. No; what this study highlights for me is how much information about ourselves is available to those who know how to decode it. Maybe those data come from the wrinkles in our faces, or the angles of our smiles, or the speed with which we type, or the temperature of our elbows. The data have always been there, actually, but we’ve never had the tools powerful enough to analyze them until now.
When I was a kid, I was obsessed with Star Trek — I know, you’re shocked — and, of course, the famous tricorder, a scanner that could tell everything about someone’s state of health in 5 seconds from 3 feet away. That’s how I thought medicine really would be in the future. Once I got to medical school, I was disabused of that notion. But the age of data, the age of AI, may mean the tricorder age is not actually that far away.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
My oldest daughter is at sleepaway camp for a couple of weeks, and the camp has a photographer who goes around all day taking pictures of the kids, which get uploaded to a private Facebook group. In the past, I would go online every day (or, okay, several times a day) and scroll through all those pictures looking for one that features my kid.
I don’t have to do that anymore. This year, I simply uploaded a picture of my daughter to an app and artificial intelligence (AI) takes care of the rest, recognizing her face amidst the sea of smiling children, and flagging just those photos for me to peruse. It’s amazing, really. And a bit scary.
The fact that facial recognition has penetrated the summer camp market should tell you that the tech is truly ubiquitous. But today we’re going to think a bit more about what AI can do with a picture of your face, because the power of facial recognition is not just skin deep.
What’s got me hot and bothered about facial images is this paper, appearing in Cell Metabolism, which adds a new layer to the standard facial-analysis playbook: facial temperature.
To understand this paper, you need to understand a whole field of research that is developing various different “clocks” for age.
It turns out that age really is just a number. Our cells, our proteins, our biochemistry can be analyzed to give different numbers. These “clocks,” as distinct from the calendar we usually use to measure our age, might have more predictive power than the number itself.
There are numerous molecular clocks, such as telomere length, that not only correlate with calendar age but are superior to calendar age in predicting age-related complications. Testing telomere length typically requires a blood sample — and remains costly. But we can use other sources to estimate age; how about a photo?
I mean, we do this all the time when we meet someone new or, as a physician, when we meet a new patient. I have often written that a patient “appears younger than their stated age,” and we’ve all had the experience of hearing how old someone is and being shocked. I mean, have you seen Sharon Stone recently? She’s 66 years old. Okay — to be fair, there might be some outside help there. But you get the point.
Back to the Cell Metabolism paper. Researchers report on multiple algorithms to obtain an “age” from a picture of an individual’s face.
The first algorithm is pretty straightforward. Researchers collected 2811 images, all of Han Chinese individuals ranging in age from 20 to 90 years, and reconstructed a 3D facial map from those. 
They then trained a convolutional neural network to predict the individuals’ ages from the pictures. It was quite accurate, as you can see here.
In the AI age, this may not seem that impressive. A brief search online turned up dozens of apps that promised to guess my age from a photo.
I sent this rather unflattering picture of myself to ChatGPT which, after initially demurring and saying it was not designed to guess ages, pegged me at somewhere between 35 and 45, which I am taking as a major victory.
But the Cell Metabolism paper goes deeper. Literally. 
And this is where things start to get interesting. Because sure, the visible part of your face can change depending on makeup, expression, plastic surgery, and the like. But the temperature? That’s harder to fake.
It turns out that the temperature distribution in your face changes as you get older. There is a cooling of the nose and the cheeks, for example.
And the researchers could combine all this temperature data to guess someone’s calendar age fairly accurately, though notably not as accurately as the model that just looks at the pictures.
But guessing your age is not really the interesting part of thermal imaging of the face. It’s guessing — or, rather, predicting — the state of your metabolism. All these study participants had extensive metabolic testing performed, as well as detailed analysis of their lifestyle behaviors. And facial images could be used to predict those factors.
For example, the 3D reconstruction of the faces could predict who ate seafood (they tend to look younger than their actual age) compared with who ate poultry and meat (they tend to look older). The thermal imaging could predict who got more sleep (they look younger from a temperature perspective) and who ate more yogurt (also younger-appearing, temperature-wise). Facial temperature patterns could identify those with higher BMI, higher blood pressure, higher fasting glucose.
The researchers used the difference between actual and predicted age as a metric to measure illness as well. You can see here how, on average, individuals with hypertension, diabetes, and even liver cysts are “older,” at least by face temperature.
It may even be possible to use facial temperature as biofeedback. In a small study, the researchers measured the difference between facial temperature age and real age before and after 2 weeks of jump-roping. It turns out that 2 weeks of jump-roping can make you look about 5 years younger, at least as judged by a thermal camera. Or like the Predator.
Okay, this is all very cool, but I’m not saying we’ll all be doing facial temperature tests in the near future. No; what this study highlights for me is how much information about ourselves is available to those who know how to decode it. Maybe those data come from the wrinkles in our faces, or the angles of our smiles, or the speed with which we type, or the temperature of our elbows. The data have always been there, actually, but we’ve never had the tools powerful enough to analyze them until now.
When I was a kid, I was obsessed with Star Trek — I know, you’re shocked — and, of course, the famous tricorder, a scanner that could tell everything about someone’s state of health in 5 seconds from 3 feet away. That’s how I thought medicine really would be in the future. Once I got to medical school, I was disabused of that notion. But the age of data, the age of AI, may mean the tricorder age is not actually that far away.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
My oldest daughter is at sleepaway camp for a couple of weeks, and the camp has a photographer who goes around all day taking pictures of the kids, which get uploaded to a private Facebook group. In the past, I would go online every day (or, okay, several times a day) and scroll through all those pictures looking for one that features my kid.
I don’t have to do that anymore. This year, I simply uploaded a picture of my daughter to an app and artificial intelligence (AI) takes care of the rest, recognizing her face amidst the sea of smiling children, and flagging just those photos for me to peruse. It’s amazing, really. And a bit scary.
The fact that facial recognition has penetrated the summer camp market should tell you that the tech is truly ubiquitous. But today we’re going to think a bit more about what AI can do with a picture of your face, because the power of facial recognition is not just skin deep.
What’s got me hot and bothered about facial images is this paper, appearing in Cell Metabolism, which adds a new layer to the standard facial-analysis playbook: facial temperature.
To understand this paper, you need to understand a whole field of research that is developing various different “clocks” for age.
It turns out that age really is just a number. Our cells, our proteins, our biochemistry can be analyzed to give different numbers. These “clocks,” as distinct from the calendar we usually use to measure our age, might have more predictive power than the number itself.
There are numerous molecular clocks, such as telomere length, that not only correlate with calendar age but are superior to calendar age in predicting age-related complications. Testing telomere length typically requires a blood sample — and remains costly. But we can use other sources to estimate age; how about a photo?
I mean, we do this all the time when we meet someone new or, as a physician, when we meet a new patient. I have often written that a patient “appears younger than their stated age,” and we’ve all had the experience of hearing how old someone is and being shocked. I mean, have you seen Sharon Stone recently? She’s 66 years old. Okay — to be fair, there might be some outside help there. But you get the point.
Back to the Cell Metabolism paper. Researchers report on multiple algorithms to obtain an “age” from a picture of an individual’s face.
The first algorithm is pretty straightforward. Researchers collected 2811 images, all of Han Chinese individuals ranging in age from 20 to 90 years, and reconstructed a 3D facial map from those. 
They then trained a convolutional neural network to predict the individuals’ ages from the pictures. It was quite accurate, as you can see here.
In the AI age, this may not seem that impressive. A brief search online turned up dozens of apps that promised to guess my age from a photo.
I sent this rather unflattering picture of myself to ChatGPT which, after initially demurring and saying it was not designed to guess ages, pegged me at somewhere between 35 and 45, which I am taking as a major victory.
But the Cell Metabolism paper goes deeper. Literally. 
And this is where things start to get interesting. Because sure, the visible part of your face can change depending on makeup, expression, plastic surgery, and the like. But the temperature? That’s harder to fake.
It turns out that the temperature distribution in your face changes as you get older. There is a cooling of the nose and the cheeks, for example.
And the researchers could combine all this temperature data to guess someone’s calendar age fairly accurately, though notably not as accurately as the model that just looks at the pictures.
But guessing your age is not really the interesting part of thermal imaging of the face. It’s guessing — or, rather, predicting — the state of your metabolism. All these study participants had extensive metabolic testing performed, as well as detailed analysis of their lifestyle behaviors. And facial images could be used to predict those factors.
For example, the 3D reconstruction of the faces could predict who ate seafood (they tend to look younger than their actual age) compared with who ate poultry and meat (they tend to look older). The thermal imaging could predict who got more sleep (they look younger from a temperature perspective) and who ate more yogurt (also younger-appearing, temperature-wise). Facial temperature patterns could identify those with higher BMI, higher blood pressure, higher fasting glucose.
The researchers used the difference between actual and predicted age as a metric to measure illness as well. You can see here how, on average, individuals with hypertension, diabetes, and even liver cysts are “older,” at least by face temperature.
It may even be possible to use facial temperature as biofeedback. In a small study, the researchers measured the difference between facial temperature age and real age before and after 2 weeks of jump-roping. It turns out that 2 weeks of jump-roping can make you look about 5 years younger, at least as judged by a thermal camera. Or like the Predator.
Okay, this is all very cool, but I’m not saying we’ll all be doing facial temperature tests in the near future. No; what this study highlights for me is how much information about ourselves is available to those who know how to decode it. Maybe those data come from the wrinkles in our faces, or the angles of our smiles, or the speed with which we type, or the temperature of our elbows. The data have always been there, actually, but we’ve never had the tools powerful enough to analyze them until now.
When I was a kid, I was obsessed with Star Trek — I know, you’re shocked — and, of course, the famous tricorder, a scanner that could tell everything about someone’s state of health in 5 seconds from 3 feet away. That’s how I thought medicine really would be in the future. Once I got to medical school, I was disabused of that notion. But the age of data, the age of AI, may mean the tricorder age is not actually that far away.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Does Semaglutide Reduce Inflammation?
LYON, FRANCE — The anti-obesity drug semaglutide is associated with significant reductions in the inflammatory marker high-sensitivity C-reactive protein (CRP), even in patients who do not lose substantial amounts of weight with the drug, according to data from the SELECT clinical trial.
The research, presented at the European Atherosclerosis Society 2024, involved over 17,600 patients with overweight or obesity and had established cardiovascular disease but not diabetes.
“Weight loss was associated with greater high-sensitivity CRP reduction in both treatment groups,” said study presenter Jorge Plutzky, MD, director of Preventive Cardiology at Brigham and Women’s Hospital, Boston, but “with increased high-sensitivity CRP reductions in those receiving semaglutide.”
The drug also “significantly reduced high-sensitivity CRP early,” he said, “prior to major weight loss and in those who did not lose significant amounts of weight.” The reductions reached approximately 12% at 4 weeks and around 20% at 8 weeks, when the weight loss “was still quite modest,” at 2% and 3% of body weight, respectively. Even among patients who achieved weight loss of less than 2% body weight, semaglutide was associated with a reduction in high-sensitivity CRP levels.
In the SELECT trial, semaglutide also resulted in a consistent reduction of around 20% vs placebo in major adverse cardiovascular events such as cardiovascular mortality, nonfatal myocardial infarction, or nonfatal stroke.
But Naveed Sattar, MD, PhD, professor of cardiometabolic medicine at the University of Glasgow, Scotland, said in an interview that body weight “is probably the major driver” of CRP levels in the population, accounting for between 20% and 30% of the variation.
Dr. Sattar, who was not involved in the study, said that because drugs like semaglutide lower weight but also have anti-inflammatory effects, the question becomes: “Could the anti-inflammatory effects be part of the mechanisms by which these drugs affect the risk of major adverse cardiovascular events?”
Reducing Cardiovascular Events
The current analysis, however, cannot answer the question, he said. “All it tells us is about associations.”
“What we do know is semaglutide, predominantly by lowering weight, is lowering CRP levels and equally, we know that when you lose weight, you improve blood pressure, you improve lipids, and you reduce the risk of diabetes,” he said.
Dr. Sattar also took issue with the researchers’ conclusion that the high-sensitivity CRP reductions seen in SELECT occurred prior to major weight loss because the “pattern of CRP reduction and weight reduction is almost identical.”
Dr. Sattar also pointed out in a recent editorial that the drug appears to have a direct effect on blood vessels and the heart, which may lead to improvements in systemic inflammation. Consequently, he said, any assertion that semaglutide is genuinely anti-inflammatory is, at this stage, “speculation.”
Dr. Plutzky said that “systemic, chronic inflammation is implicated as a potential mechanism and therapeutic target in atherosclerosis and major adverse cardiovascular events, as well as obesity,” and high-sensitivity CRP levels are an “established biomarker of inflammation and have been shown to predict cardiovascular risk.”
However, the relationship between high-sensitivity CRP, responses to glucagon-like peptide 1 receptor agonists like semaglutide, and cardiovascular outcomes in obesity “remains incompletely understood,” said Dr. Plutzky.
A version of this article appeared on Medscape.com.
LYON, FRANCE — The anti-obesity drug semaglutide is associated with significant reductions in the inflammatory marker high-sensitivity C-reactive protein (CRP), even in patients who do not lose substantial amounts of weight with the drug, according to data from the SELECT clinical trial.
The research, presented at the European Atherosclerosis Society 2024, involved over 17,600 patients with overweight or obesity and had established cardiovascular disease but not diabetes.
“Weight loss was associated with greater high-sensitivity CRP reduction in both treatment groups,” said study presenter Jorge Plutzky, MD, director of Preventive Cardiology at Brigham and Women’s Hospital, Boston, but “with increased high-sensitivity CRP reductions in those receiving semaglutide.”
The drug also “significantly reduced high-sensitivity CRP early,” he said, “prior to major weight loss and in those who did not lose significant amounts of weight.” The reductions reached approximately 12% at 4 weeks and around 20% at 8 weeks, when the weight loss “was still quite modest,” at 2% and 3% of body weight, respectively. Even among patients who achieved weight loss of less than 2% body weight, semaglutide was associated with a reduction in high-sensitivity CRP levels.
In the SELECT trial, semaglutide also resulted in a consistent reduction of around 20% vs placebo in major adverse cardiovascular events such as cardiovascular mortality, nonfatal myocardial infarction, or nonfatal stroke.
But Naveed Sattar, MD, PhD, professor of cardiometabolic medicine at the University of Glasgow, Scotland, said in an interview that body weight “is probably the major driver” of CRP levels in the population, accounting for between 20% and 30% of the variation.
Dr. Sattar, who was not involved in the study, said that because drugs like semaglutide lower weight but also have anti-inflammatory effects, the question becomes: “Could the anti-inflammatory effects be part of the mechanisms by which these drugs affect the risk of major adverse cardiovascular events?”
Reducing Cardiovascular Events
The current analysis, however, cannot answer the question, he said. “All it tells us is about associations.”
“What we do know is semaglutide, predominantly by lowering weight, is lowering CRP levels and equally, we know that when you lose weight, you improve blood pressure, you improve lipids, and you reduce the risk of diabetes,” he said.
Dr. Sattar also took issue with the researchers’ conclusion that the high-sensitivity CRP reductions seen in SELECT occurred prior to major weight loss because the “pattern of CRP reduction and weight reduction is almost identical.”
Dr. Sattar also pointed out in a recent editorial that the drug appears to have a direct effect on blood vessels and the heart, which may lead to improvements in systemic inflammation. Consequently, he said, any assertion that semaglutide is genuinely anti-inflammatory is, at this stage, “speculation.”
Dr. Plutzky said that “systemic, chronic inflammation is implicated as a potential mechanism and therapeutic target in atherosclerosis and major adverse cardiovascular events, as well as obesity,” and high-sensitivity CRP levels are an “established biomarker of inflammation and have been shown to predict cardiovascular risk.”
However, the relationship between high-sensitivity CRP, responses to glucagon-like peptide 1 receptor agonists like semaglutide, and cardiovascular outcomes in obesity “remains incompletely understood,” said Dr. Plutzky.
A version of this article appeared on Medscape.com.
LYON, FRANCE — The anti-obesity drug semaglutide is associated with significant reductions in the inflammatory marker high-sensitivity C-reactive protein (CRP), even in patients who do not lose substantial amounts of weight with the drug, according to data from the SELECT clinical trial.
The research, presented at the European Atherosclerosis Society 2024, involved over 17,600 patients with overweight or obesity and had established cardiovascular disease but not diabetes.
“Weight loss was associated with greater high-sensitivity CRP reduction in both treatment groups,” said study presenter Jorge Plutzky, MD, director of Preventive Cardiology at Brigham and Women’s Hospital, Boston, but “with increased high-sensitivity CRP reductions in those receiving semaglutide.”
The drug also “significantly reduced high-sensitivity CRP early,” he said, “prior to major weight loss and in those who did not lose significant amounts of weight.” The reductions reached approximately 12% at 4 weeks and around 20% at 8 weeks, when the weight loss “was still quite modest,” at 2% and 3% of body weight, respectively. Even among patients who achieved weight loss of less than 2% body weight, semaglutide was associated with a reduction in high-sensitivity CRP levels.
In the SELECT trial, semaglutide also resulted in a consistent reduction of around 20% vs placebo in major adverse cardiovascular events such as cardiovascular mortality, nonfatal myocardial infarction, or nonfatal stroke.
But Naveed Sattar, MD, PhD, professor of cardiometabolic medicine at the University of Glasgow, Scotland, said in an interview that body weight “is probably the major driver” of CRP levels in the population, accounting for between 20% and 30% of the variation.
Dr. Sattar, who was not involved in the study, said that because drugs like semaglutide lower weight but also have anti-inflammatory effects, the question becomes: “Could the anti-inflammatory effects be part of the mechanisms by which these drugs affect the risk of major adverse cardiovascular events?”
Reducing Cardiovascular Events
The current analysis, however, cannot answer the question, he said. “All it tells us is about associations.”
“What we do know is semaglutide, predominantly by lowering weight, is lowering CRP levels and equally, we know that when you lose weight, you improve blood pressure, you improve lipids, and you reduce the risk of diabetes,” he said.
Dr. Sattar also took issue with the researchers’ conclusion that the high-sensitivity CRP reductions seen in SELECT occurred prior to major weight loss because the “pattern of CRP reduction and weight reduction is almost identical.”
Dr. Sattar also pointed out in a recent editorial that the drug appears to have a direct effect on blood vessels and the heart, which may lead to improvements in systemic inflammation. Consequently, he said, any assertion that semaglutide is genuinely anti-inflammatory is, at this stage, “speculation.”
Dr. Plutzky said that “systemic, chronic inflammation is implicated as a potential mechanism and therapeutic target in atherosclerosis and major adverse cardiovascular events, as well as obesity,” and high-sensitivity CRP levels are an “established biomarker of inflammation and have been shown to predict cardiovascular risk.”
However, the relationship between high-sensitivity CRP, responses to glucagon-like peptide 1 receptor agonists like semaglutide, and cardiovascular outcomes in obesity “remains incompletely understood,” said Dr. Plutzky.
A version of this article appeared on Medscape.com.
Top reads from the CHEST journal portfolio
Understanding RA with COPD, lung cancer prediction models, and chronic cardiac dysfunction
Journal CHEST®
Does Rheumatoid Arthritis Increase the Risk of COPD?
By: Chiwook Chung, MD, and colleagues
Notably, individuals with seropositive RA exhibit a greater risk of COPD onset than those with seronegative RA. Although smoking history didn’t affect the relationship between RA and COPD, monitoring respiratory symptoms and pulmonary function in patients with RA, especially patients who are seropositive, is crucial. These findings underscore the importance of interdisciplinary collaboration between rheumatologists and pulmonologists to enhance early detection and management strategies for pulmonary complications in patients with RA.
– Commentary by Corinne Young, MSN, FNP-C, FCCP, Member of the CHEST Physician® Editorial Board
CHEST Pulmonary®
The Lung Cancer Prediction Model “Stress Test”
By: Brent E. Heideman, MD, and colleagues
Current lung cancer prediction models have limited utility in high-risk patients referred for diagnostic biopsy. In a study of 322 indeterminate pulmonary nodules, the Brock, Mayo Clinic, Herder, and Veterans Affairs models showed modest discrimination between benign and malignant nodules (AUCs 0.67-0.77). The models performed poorly for low-risk patients (negative predictive values 63%-71%) and suboptimally for high-risk patients (positive predictive values 73%-87%), suggesting referring physicians use additional clinical information not captured in these models to identify high-risk patients needing biopsy. New prediction models and biomarkers specifically developed and calibrated for high-risk populations are needed to better inform clinical decision-making. Incorporating interval imaging to assess changes in nodule characteristics could potentially improve model performance. Tailored risk assessment tools are crucial for optimizing management and reducing unnecessary invasive procedures in this challenging patient population.
– Commentary by Russell Miller, MD, Member of the CHEST Physician Editorial Board
CHEST Critical Care ®
Characterizing Cardiac Function in ICU Survivors of Sepsis
By: Kevin Garrity, MBChB, and colleagues
While chronic cardiac dysfunction is one of the proposed mechanisms of long-term impairment post critical illness, its prevalence, mechanisms, and associations with disability following admission for sepsis are not well understood. Garrity and colleagues describe the Characterization of Cardiovascular Function in ICU Survivors of Sepsis (CONDUCT-ICU) protocol, a prospective study including two ICUs in Scotland aimed to better define cardiovascular dysfunction in survivors of sepsis. Designed to enroll 69 patients, demographics, cardiac and inflammatory biomarkers, and echocardiograms will be obtained on ICU discharge with additional laboratory data, cardiac magnetic resonance imaging, and patient-reported outcome measures to be obtained at 6 to 10 weeks. This novel multimodal approach will provide understanding into the role of cardiovascular dysfunction following critical illness as well as offer mechanistic insights. The investigators hope to obtain operational and pilot data for larger future studies.
– Commentary by Eugene Yuriditsky, MD, FCCP, Member of the CHEST Physician Editorial Board
Understanding RA with COPD, lung cancer prediction models, and chronic cardiac dysfunction
Understanding RA with COPD, lung cancer prediction models, and chronic cardiac dysfunction
Journal CHEST®
Does Rheumatoid Arthritis Increase the Risk of COPD?
By: Chiwook Chung, MD, and colleagues
Notably, individuals with seropositive RA exhibit a greater risk of COPD onset than those with seronegative RA. Although smoking history didn’t affect the relationship between RA and COPD, monitoring respiratory symptoms and pulmonary function in patients with RA, especially patients who are seropositive, is crucial. These findings underscore the importance of interdisciplinary collaboration between rheumatologists and pulmonologists to enhance early detection and management strategies for pulmonary complications in patients with RA.
– Commentary by Corinne Young, MSN, FNP-C, FCCP, Member of the CHEST Physician® Editorial Board
CHEST Pulmonary®
The Lung Cancer Prediction Model “Stress Test”
By: Brent E. Heideman, MD, and colleagues
Current lung cancer prediction models have limited utility in high-risk patients referred for diagnostic biopsy. In a study of 322 indeterminate pulmonary nodules, the Brock, Mayo Clinic, Herder, and Veterans Affairs models showed modest discrimination between benign and malignant nodules (AUCs 0.67-0.77). The models performed poorly for low-risk patients (negative predictive values 63%-71%) and suboptimally for high-risk patients (positive predictive values 73%-87%), suggesting referring physicians use additional clinical information not captured in these models to identify high-risk patients needing biopsy. New prediction models and biomarkers specifically developed and calibrated for high-risk populations are needed to better inform clinical decision-making. Incorporating interval imaging to assess changes in nodule characteristics could potentially improve model performance. Tailored risk assessment tools are crucial for optimizing management and reducing unnecessary invasive procedures in this challenging patient population.
– Commentary by Russell Miller, MD, Member of the CHEST Physician Editorial Board
CHEST Critical Care ®
Characterizing Cardiac Function in ICU Survivors of Sepsis
By: Kevin Garrity, MBChB, and colleagues
While chronic cardiac dysfunction is one of the proposed mechanisms of long-term impairment post critical illness, its prevalence, mechanisms, and associations with disability following admission for sepsis are not well understood. Garrity and colleagues describe the Characterization of Cardiovascular Function in ICU Survivors of Sepsis (CONDUCT-ICU) protocol, a prospective study including two ICUs in Scotland aimed to better define cardiovascular dysfunction in survivors of sepsis. Designed to enroll 69 patients, demographics, cardiac and inflammatory biomarkers, and echocardiograms will be obtained on ICU discharge with additional laboratory data, cardiac magnetic resonance imaging, and patient-reported outcome measures to be obtained at 6 to 10 weeks. This novel multimodal approach will provide understanding into the role of cardiovascular dysfunction following critical illness as well as offer mechanistic insights. The investigators hope to obtain operational and pilot data for larger future studies.
– Commentary by Eugene Yuriditsky, MD, FCCP, Member of the CHEST Physician Editorial Board
Journal CHEST®
Does Rheumatoid Arthritis Increase the Risk of COPD?
By: Chiwook Chung, MD, and colleagues
Notably, individuals with seropositive RA exhibit a greater risk of COPD onset than those with seronegative RA. Although smoking history didn’t affect the relationship between RA and COPD, monitoring respiratory symptoms and pulmonary function in patients with RA, especially patients who are seropositive, is crucial. These findings underscore the importance of interdisciplinary collaboration between rheumatologists and pulmonologists to enhance early detection and management strategies for pulmonary complications in patients with RA.
– Commentary by Corinne Young, MSN, FNP-C, FCCP, Member of the CHEST Physician® Editorial Board
CHEST Pulmonary®
The Lung Cancer Prediction Model “Stress Test”
By: Brent E. Heideman, MD, and colleagues
Current lung cancer prediction models have limited utility in high-risk patients referred for diagnostic biopsy. In a study of 322 indeterminate pulmonary nodules, the Brock, Mayo Clinic, Herder, and Veterans Affairs models showed modest discrimination between benign and malignant nodules (AUCs 0.67-0.77). The models performed poorly for low-risk patients (negative predictive values 63%-71%) and suboptimally for high-risk patients (positive predictive values 73%-87%), suggesting referring physicians use additional clinical information not captured in these models to identify high-risk patients needing biopsy. New prediction models and biomarkers specifically developed and calibrated for high-risk populations are needed to better inform clinical decision-making. Incorporating interval imaging to assess changes in nodule characteristics could potentially improve model performance. Tailored risk assessment tools are crucial for optimizing management and reducing unnecessary invasive procedures in this challenging patient population.
– Commentary by Russell Miller, MD, Member of the CHEST Physician Editorial Board
CHEST Critical Care ®
Characterizing Cardiac Function in ICU Survivors of Sepsis
By: Kevin Garrity, MBChB, and colleagues
While chronic cardiac dysfunction is one of the proposed mechanisms of long-term impairment post critical illness, its prevalence, mechanisms, and associations with disability following admission for sepsis are not well understood. Garrity and colleagues describe the Characterization of Cardiovascular Function in ICU Survivors of Sepsis (CONDUCT-ICU) protocol, a prospective study including two ICUs in Scotland aimed to better define cardiovascular dysfunction in survivors of sepsis. Designed to enroll 69 patients, demographics, cardiac and inflammatory biomarkers, and echocardiograms will be obtained on ICU discharge with additional laboratory data, cardiac magnetic resonance imaging, and patient-reported outcome measures to be obtained at 6 to 10 weeks. This novel multimodal approach will provide understanding into the role of cardiovascular dysfunction following critical illness as well as offer mechanistic insights. The investigators hope to obtain operational and pilot data for larger future studies.
– Commentary by Eugene Yuriditsky, MD, FCCP, Member of the CHEST Physician Editorial Board
Cardiovascular Health Becoming a Major Risk Factor for Dementia
That’s according to researchers from University College London (UCL) in the United Kingdom who analyzed 27 papers about dementia that had data collected over more than 70 years. They calculated what share of dementia cases were due to different risk factors. Their findings were recently published in the Lancet Public Health.
Top risk factors for dementia over the years have been hypertension, obesity, diabetes, education, and smoking, according to a news release on the findings. But the prevalence of risk factors has changed over the decades.
Researchers said smoking and education have become less important risk factors because of “population-level interventions,” such as stop-smoking campaigns and compulsory public education. On the other hand, obesity and diabetes rates have increased and become bigger risk factors.
Hypertension remains the greatest risk factor, even though doctors and public health groups are putting more emphasis on managing the condition, the study said.
“Cardiovascular risk factors may have contributed more to dementia risk over time, so these deserve more targeted action for future dementia prevention efforts,” said Naaheed Mukadam, PhD, an associate professor at UCL and the lead author of the study.
Eliminating modifiable risk factors could theoretically prevent 40% of dementia cases, the release said.
The CDC says that an estimated 5.8 million people in the United States have Alzheimer’s disease and related dementias, including 5.6 million people ages 65 and older and about 200,000 under age 65. The UCL release said an estimated 944,000 in the U.K. have dementia.
A version of this article first appeared on WebMD.com.
That’s according to researchers from University College London (UCL) in the United Kingdom who analyzed 27 papers about dementia that had data collected over more than 70 years. They calculated what share of dementia cases were due to different risk factors. Their findings were recently published in the Lancet Public Health.
Top risk factors for dementia over the years have been hypertension, obesity, diabetes, education, and smoking, according to a news release on the findings. But the prevalence of risk factors has changed over the decades.
Researchers said smoking and education have become less important risk factors because of “population-level interventions,” such as stop-smoking campaigns and compulsory public education. On the other hand, obesity and diabetes rates have increased and become bigger risk factors.
Hypertension remains the greatest risk factor, even though doctors and public health groups are putting more emphasis on managing the condition, the study said.
“Cardiovascular risk factors may have contributed more to dementia risk over time, so these deserve more targeted action for future dementia prevention efforts,” said Naaheed Mukadam, PhD, an associate professor at UCL and the lead author of the study.
Eliminating modifiable risk factors could theoretically prevent 40% of dementia cases, the release said.
The CDC says that an estimated 5.8 million people in the United States have Alzheimer’s disease and related dementias, including 5.6 million people ages 65 and older and about 200,000 under age 65. The UCL release said an estimated 944,000 in the U.K. have dementia.
A version of this article first appeared on WebMD.com.
That’s according to researchers from University College London (UCL) in the United Kingdom who analyzed 27 papers about dementia that had data collected over more than 70 years. They calculated what share of dementia cases were due to different risk factors. Their findings were recently published in the Lancet Public Health.
Top risk factors for dementia over the years have been hypertension, obesity, diabetes, education, and smoking, according to a news release on the findings. But the prevalence of risk factors has changed over the decades.
Researchers said smoking and education have become less important risk factors because of “population-level interventions,” such as stop-smoking campaigns and compulsory public education. On the other hand, obesity and diabetes rates have increased and become bigger risk factors.
Hypertension remains the greatest risk factor, even though doctors and public health groups are putting more emphasis on managing the condition, the study said.
“Cardiovascular risk factors may have contributed more to dementia risk over time, so these deserve more targeted action for future dementia prevention efforts,” said Naaheed Mukadam, PhD, an associate professor at UCL and the lead author of the study.
Eliminating modifiable risk factors could theoretically prevent 40% of dementia cases, the release said.
The CDC says that an estimated 5.8 million people in the United States have Alzheimer’s disease and related dementias, including 5.6 million people ages 65 and older and about 200,000 under age 65. The UCL release said an estimated 944,000 in the U.K. have dementia.
A version of this article first appeared on WebMD.com.
FROM THE LANCET PUBLIC HEALTH
Similar Outcomes With Labetalol, Nifedipine for Chronic Hypertension in Pregnancy
Treatment for chronic hypertension in pregnancy with labetalol showed no significant differences in maternal or neonatal outcomes, compared with treatment with nifedipine, new research indicates.
The open-label, multicenter, randomized CHAP (Chronic Hypertension in Pregnancy) trial showed that treating mild chronic hypertension was better than delaying treatment until severe hypertension developed, but still unclear was whether, or to what extent, the choice of first-line treatment affected outcomes.
Researchers, led by Ayodeji A. Sanusi, MD, MPH, with the Division of Maternal and Fetal Medicine at the University of Alabama at Birmingham, conducted a secondary analysis of CHAP to compare the primary treatments. Mild chronic hypertension in the study was defined as blood pressure of 140-159/90-104 mmHg before 20 weeks of gestation.
Three Comparisons
Three comparisons were performed in 2292 participants based on medications prescribed at enrollment: 720 (31.4%) received labetalol; 417 (18.2%) initially received nifedipine; and 1155 (50.4%) had standard care. Labetalol was compared with standard care; nifedipine was compared with standard care; and labetalol was compared with nifedipine.
The primary outcome was occurrence of superimposed preeclampsia with severe features; preterm birth before 35 weeks of gestation; placental abruption; or fetal or neonatal death. The key secondary outcome was a small-for-gestational age neonate. Researchers also compared adverse effects between groups.
Among the results were the following:
- The primary outcome occurred in 30.1% in the labetalol group; 31.2% in the nifedipine group; and 37% in the standard care group.
- Risk of the primary outcome was lower among those receiving treatment. For labetalol vs standard care, the adjusted relative risk (RR) was 0.82; 95% confidence interval (CI), 0.72-0.94. For nifedipine vs standard care, the adjusted RR was 0.84; 95% CI, 0.71-0.99. There was no significant difference in risk when labetalol was compared with nifedipine (adjusted RR, 0.98; 95% CI, 0.82-1.18).
- There were no significant differences in numbers of small-for-gestational age neonates or serious adverse events between those who received labetalol and those using nifedipine.
Any adverse events were significantly more common with nifedipine, compared with labetalol (35.7% vs 28.3%, P = .009), and with nifedipine, compared with standard care (35.7% vs 26.3%, P = .0003). Adverse event rates were not significantly higher with labetalol when compared with standard care (28.3% vs 26.3%, P = .34). The most frequently reported adverse events were headache, medication intolerance, dizziness, nausea, dyspepsia, neonatal jaundice, and vomiting.
“Thus, labetalol compared with nifedipine appeared to have fewer adverse events and to be better tolerated,” the authors write. They note that labetalol, a third-generation mixed alpha- and beta-adrenergic antagonist, is contraindicated for those who have obstructive pulmonary disease and nifedipine, a dihydropyridine calcium channel blocker, is contraindicated in people with tachycardia.
The authors write that their results align with other studies that have not found differences between labetalol and nifedipine. “[O]ur findings support the use of either labetalol or nifedipine as initial first-line agents for the management of mild chronic hypertension in pregnancy to reduce the risk of adverse maternal and other perinatal outcomes with no increased risk of fetal harm,” the authors write.
Dr. Sanusi reports no relevant financial relationships. Full coauthor disclosures are available with the full text of the paper.
Treatment for chronic hypertension in pregnancy with labetalol showed no significant differences in maternal or neonatal outcomes, compared with treatment with nifedipine, new research indicates.
The open-label, multicenter, randomized CHAP (Chronic Hypertension in Pregnancy) trial showed that treating mild chronic hypertension was better than delaying treatment until severe hypertension developed, but still unclear was whether, or to what extent, the choice of first-line treatment affected outcomes.
Researchers, led by Ayodeji A. Sanusi, MD, MPH, with the Division of Maternal and Fetal Medicine at the University of Alabama at Birmingham, conducted a secondary analysis of CHAP to compare the primary treatments. Mild chronic hypertension in the study was defined as blood pressure of 140-159/90-104 mmHg before 20 weeks of gestation.
Three Comparisons
Three comparisons were performed in 2292 participants based on medications prescribed at enrollment: 720 (31.4%) received labetalol; 417 (18.2%) initially received nifedipine; and 1155 (50.4%) had standard care. Labetalol was compared with standard care; nifedipine was compared with standard care; and labetalol was compared with nifedipine.
The primary outcome was occurrence of superimposed preeclampsia with severe features; preterm birth before 35 weeks of gestation; placental abruption; or fetal or neonatal death. The key secondary outcome was a small-for-gestational age neonate. Researchers also compared adverse effects between groups.
Among the results were the following:
- The primary outcome occurred in 30.1% in the labetalol group; 31.2% in the nifedipine group; and 37% in the standard care group.
- Risk of the primary outcome was lower among those receiving treatment. For labetalol vs standard care, the adjusted relative risk (RR) was 0.82; 95% confidence interval (CI), 0.72-0.94. For nifedipine vs standard care, the adjusted RR was 0.84; 95% CI, 0.71-0.99. There was no significant difference in risk when labetalol was compared with nifedipine (adjusted RR, 0.98; 95% CI, 0.82-1.18).
- There were no significant differences in numbers of small-for-gestational age neonates or serious adverse events between those who received labetalol and those using nifedipine.
Any adverse events were significantly more common with nifedipine, compared with labetalol (35.7% vs 28.3%, P = .009), and with nifedipine, compared with standard care (35.7% vs 26.3%, P = .0003). Adverse event rates were not significantly higher with labetalol when compared with standard care (28.3% vs 26.3%, P = .34). The most frequently reported adverse events were headache, medication intolerance, dizziness, nausea, dyspepsia, neonatal jaundice, and vomiting.
“Thus, labetalol compared with nifedipine appeared to have fewer adverse events and to be better tolerated,” the authors write. They note that labetalol, a third-generation mixed alpha- and beta-adrenergic antagonist, is contraindicated for those who have obstructive pulmonary disease and nifedipine, a dihydropyridine calcium channel blocker, is contraindicated in people with tachycardia.
The authors write that their results align with other studies that have not found differences between labetalol and nifedipine. “[O]ur findings support the use of either labetalol or nifedipine as initial first-line agents for the management of mild chronic hypertension in pregnancy to reduce the risk of adverse maternal and other perinatal outcomes with no increased risk of fetal harm,” the authors write.
Dr. Sanusi reports no relevant financial relationships. Full coauthor disclosures are available with the full text of the paper.
Treatment for chronic hypertension in pregnancy with labetalol showed no significant differences in maternal or neonatal outcomes, compared with treatment with nifedipine, new research indicates.
The open-label, multicenter, randomized CHAP (Chronic Hypertension in Pregnancy) trial showed that treating mild chronic hypertension was better than delaying treatment until severe hypertension developed, but still unclear was whether, or to what extent, the choice of first-line treatment affected outcomes.
Researchers, led by Ayodeji A. Sanusi, MD, MPH, with the Division of Maternal and Fetal Medicine at the University of Alabama at Birmingham, conducted a secondary analysis of CHAP to compare the primary treatments. Mild chronic hypertension in the study was defined as blood pressure of 140-159/90-104 mmHg before 20 weeks of gestation.
Three Comparisons
Three comparisons were performed in 2292 participants based on medications prescribed at enrollment: 720 (31.4%) received labetalol; 417 (18.2%) initially received nifedipine; and 1155 (50.4%) had standard care. Labetalol was compared with standard care; nifedipine was compared with standard care; and labetalol was compared with nifedipine.
The primary outcome was occurrence of superimposed preeclampsia with severe features; preterm birth before 35 weeks of gestation; placental abruption; or fetal or neonatal death. The key secondary outcome was a small-for-gestational age neonate. Researchers also compared adverse effects between groups.
Among the results were the following:
- The primary outcome occurred in 30.1% in the labetalol group; 31.2% in the nifedipine group; and 37% in the standard care group.
- Risk of the primary outcome was lower among those receiving treatment. For labetalol vs standard care, the adjusted relative risk (RR) was 0.82; 95% confidence interval (CI), 0.72-0.94. For nifedipine vs standard care, the adjusted RR was 0.84; 95% CI, 0.71-0.99. There was no significant difference in risk when labetalol was compared with nifedipine (adjusted RR, 0.98; 95% CI, 0.82-1.18).
- There were no significant differences in numbers of small-for-gestational age neonates or serious adverse events between those who received labetalol and those using nifedipine.
Any adverse events were significantly more common with nifedipine, compared with labetalol (35.7% vs 28.3%, P = .009), and with nifedipine, compared with standard care (35.7% vs 26.3%, P = .0003). Adverse event rates were not significantly higher with labetalol when compared with standard care (28.3% vs 26.3%, P = .34). The most frequently reported adverse events were headache, medication intolerance, dizziness, nausea, dyspepsia, neonatal jaundice, and vomiting.
“Thus, labetalol compared with nifedipine appeared to have fewer adverse events and to be better tolerated,” the authors write. They note that labetalol, a third-generation mixed alpha- and beta-adrenergic antagonist, is contraindicated for those who have obstructive pulmonary disease and nifedipine, a dihydropyridine calcium channel blocker, is contraindicated in people with tachycardia.
The authors write that their results align with other studies that have not found differences between labetalol and nifedipine. “[O]ur findings support the use of either labetalol or nifedipine as initial first-line agents for the management of mild chronic hypertension in pregnancy to reduce the risk of adverse maternal and other perinatal outcomes with no increased risk of fetal harm,” the authors write.
Dr. Sanusi reports no relevant financial relationships. Full coauthor disclosures are available with the full text of the paper.
FROM OBSTETRICS & GYNECOLOGY
New Insight Into CVD, Stroke Risk in Migraine
SAN DIEGO – Researchers are unraveling the complex relationship between cardiovascular (CV)- and stroke-related outcomes in migraine with, and without, aura.
“We confirmed that aura increases the risk for these cerebrovascular and cardiovascular outcomes in people with migraine and that there’s an increased risk of these MACE events in men with migraine,” said study investigator Gina Dumkrieger, PhD, principal data science analyst and assistant professor of neurology, Mayo Clinic, Phoenix, Arizona.
The findings were presented at the annual meeting of the American Headache Society.
Few Data on Migraine and Stroke Risk
The extent to which migraine increases the risk for stroke CV outcomes has not been extensively studied.
“We’re trying to find out whether migraine-related factors make it more likely that you’re going to have one of these events,” said Dr. Dumkrieger. “Knowing a particular factor increases the risk is something patients and medical providers would want to know.”
Using Mayo Clinic electronic health records, which cover all three sites (Florida, Minnesota, and Arizona), researchers identified individuals with migraine using diagnostic codes. They also looked at data on sex, race, and the presence of aura.
They investigated whether a history of MACE risk factors — including atrial fibrillation, diabetes, hyperlipidemia, hypertension, and tobacco use — affected risk and the potential interaction of aura with these risk factors.
MACE events included cerebral infarction, intracerebral hemorrhage, and acute myocardial infarction.
The analysis included 130,126 participants (80% women, 95% White individuals). Of these, 6% experienced a MACE event, and 94% did not.
“We confirmed that aura does increase the risk for a MACE event, and all of the known risk factors that we included were also significant,” said Dr. Dumkrieger.
Odds ratios (ORs) were 3.82 for atrial fibrillation, 3.11 for hypertension, and 3.06 for hyperlipidemia.
It was surprising, said Dr. Dumkrieger, that male sex was tied to an increased risk for a MACE event (OR, 1.40). “This is not something that was known before,” she said.
The link between migraine and ischemic stroke, particularly with aura, was stronger in women — particularly young women.
Investigators also found an interaction between male sex and aura, when it comes to MACE outcomes, said Dr. Dumkrieger. “Males in general are at higher risk, and people with aura are at higher risk. Males with aura are also at higher risk, but maybe not as much as you would think they would be. It’s not a purely additive thing. This is something we need to look into more,” she said.
The study also revealed an interaction between aura and hypertension as well as aura and tobacco use, but here too, it was not an additive risk, said Dr. Dumkrieger. However, she added, the presence of aura does not moderate the risk for hyperlipidemia, diabetes, or atrial fibrillation.
The research also showed a significant interaction between male sex and Black race which was additive. “There’s apparently increased risk if you are male and Black or African American that’s greater than what you would expect. We should be especially concerned about these individuals,” she said.
Unanswered Questions
The current analysis is part of a larger study that will more closely examine these relationships. “We want to learn, for example, why aura moderates some of the risk factors but not others,” said Dr. Dumkrieger.
The researchers also plan to investigate other migraine features, including headache frequency, and headache sensations such as pulsating or throbbing.
Dr. Dumkrieger was an investigator of another study, also presented at the AHS meeting, that’s investigating the role of migraine-specific features and imaging results in the complex interrelationship between migraine and MACE risk.
That study, which also used the Mayo Clinic electronic health record data, included 60,454 migraine patients diagnosed with migraine after 2010.
Researchers divided participants into those with a MACE outcome (1107) and those without such an outcome (59,347) after at least 2 years of follow-up. They created a propensity cohort of individuals matched for age and risk factors for MACE outcome.
The final cohort consisted of 575 patients with and 652 patients without MACE outcome.
One of the most interesting early results from this study was that those with a MACE outcome had significantly more white matter hyperintensities than those with no MACE outcome, at 64% versus 51%, respectively.
This and other findings need to be validated in a different cohort with an electronic health records database from another institution. In future, the team plans to focus on identifying specific migraine features and medications and their relative contributions to MACE risk in migraine patients.
Yet another study featured at the AHS meeting confirmed the increased risk for stroke among migraine patients using a large database with over 410,000 subjects.
Results showed stroke was more than three times more common in those with a migraine diagnosis than in those without (risk ratio, [RR] 3.23; P < .001). The RR for hemorrhagic stroke (3.15) was comparable with that of ischemic stroke (3.20).
The overall stroke RR for chronic migraine versus controls without migraine was 3.68 (P < .001). The RR for migraine with aura versus migraine without aura was 1.37 (P < .001).
Useful Data
Commenting on the research, Juliana VanderPluym, MD, a headache specialist at the Mayo Clinic, Phoenix, Arizona, described this new information as “very useful.”
The fact that there are more white matter lesions on MRI scans in migraine patients with MACE needs further exploration, said Dr. VanderPluym.
“Understanding how much of that relates to migraine, how much relates to other comorbid conditions, and what this all means together, is very important, particularly because MACE can be life-threatening and life-altering,” she added.
Learning how migraine medications may impact MACE risk is also something that needs to be examined in greater depth, she said. “I would think that migraines that are controlled might have a different risk for MACE than uncontrolled migraine,” she said.
The investigators reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Researchers are unraveling the complex relationship between cardiovascular (CV)- and stroke-related outcomes in migraine with, and without, aura.
“We confirmed that aura increases the risk for these cerebrovascular and cardiovascular outcomes in people with migraine and that there’s an increased risk of these MACE events in men with migraine,” said study investigator Gina Dumkrieger, PhD, principal data science analyst and assistant professor of neurology, Mayo Clinic, Phoenix, Arizona.
The findings were presented at the annual meeting of the American Headache Society.
Few Data on Migraine and Stroke Risk
The extent to which migraine increases the risk for stroke CV outcomes has not been extensively studied.
“We’re trying to find out whether migraine-related factors make it more likely that you’re going to have one of these events,” said Dr. Dumkrieger. “Knowing a particular factor increases the risk is something patients and medical providers would want to know.”
Using Mayo Clinic electronic health records, which cover all three sites (Florida, Minnesota, and Arizona), researchers identified individuals with migraine using diagnostic codes. They also looked at data on sex, race, and the presence of aura.
They investigated whether a history of MACE risk factors — including atrial fibrillation, diabetes, hyperlipidemia, hypertension, and tobacco use — affected risk and the potential interaction of aura with these risk factors.
MACE events included cerebral infarction, intracerebral hemorrhage, and acute myocardial infarction.
The analysis included 130,126 participants (80% women, 95% White individuals). Of these, 6% experienced a MACE event, and 94% did not.
“We confirmed that aura does increase the risk for a MACE event, and all of the known risk factors that we included were also significant,” said Dr. Dumkrieger.
Odds ratios (ORs) were 3.82 for atrial fibrillation, 3.11 for hypertension, and 3.06 for hyperlipidemia.
It was surprising, said Dr. Dumkrieger, that male sex was tied to an increased risk for a MACE event (OR, 1.40). “This is not something that was known before,” she said.
The link between migraine and ischemic stroke, particularly with aura, was stronger in women — particularly young women.
Investigators also found an interaction between male sex and aura, when it comes to MACE outcomes, said Dr. Dumkrieger. “Males in general are at higher risk, and people with aura are at higher risk. Males with aura are also at higher risk, but maybe not as much as you would think they would be. It’s not a purely additive thing. This is something we need to look into more,” she said.
The study also revealed an interaction between aura and hypertension as well as aura and tobacco use, but here too, it was not an additive risk, said Dr. Dumkrieger. However, she added, the presence of aura does not moderate the risk for hyperlipidemia, diabetes, or atrial fibrillation.
The research also showed a significant interaction between male sex and Black race which was additive. “There’s apparently increased risk if you are male and Black or African American that’s greater than what you would expect. We should be especially concerned about these individuals,” she said.
Unanswered Questions
The current analysis is part of a larger study that will more closely examine these relationships. “We want to learn, for example, why aura moderates some of the risk factors but not others,” said Dr. Dumkrieger.
The researchers also plan to investigate other migraine features, including headache frequency, and headache sensations such as pulsating or throbbing.
Dr. Dumkrieger was an investigator of another study, also presented at the AHS meeting, that’s investigating the role of migraine-specific features and imaging results in the complex interrelationship between migraine and MACE risk.
That study, which also used the Mayo Clinic electronic health record data, included 60,454 migraine patients diagnosed with migraine after 2010.
Researchers divided participants into those with a MACE outcome (1107) and those without such an outcome (59,347) after at least 2 years of follow-up. They created a propensity cohort of individuals matched for age and risk factors for MACE outcome.
The final cohort consisted of 575 patients with and 652 patients without MACE outcome.
One of the most interesting early results from this study was that those with a MACE outcome had significantly more white matter hyperintensities than those with no MACE outcome, at 64% versus 51%, respectively.
This and other findings need to be validated in a different cohort with an electronic health records database from another institution. In future, the team plans to focus on identifying specific migraine features and medications and their relative contributions to MACE risk in migraine patients.
Yet another study featured at the AHS meeting confirmed the increased risk for stroke among migraine patients using a large database with over 410,000 subjects.
Results showed stroke was more than three times more common in those with a migraine diagnosis than in those without (risk ratio, [RR] 3.23; P < .001). The RR for hemorrhagic stroke (3.15) was comparable with that of ischemic stroke (3.20).
The overall stroke RR for chronic migraine versus controls without migraine was 3.68 (P < .001). The RR for migraine with aura versus migraine without aura was 1.37 (P < .001).
Useful Data
Commenting on the research, Juliana VanderPluym, MD, a headache specialist at the Mayo Clinic, Phoenix, Arizona, described this new information as “very useful.”
The fact that there are more white matter lesions on MRI scans in migraine patients with MACE needs further exploration, said Dr. VanderPluym.
“Understanding how much of that relates to migraine, how much relates to other comorbid conditions, and what this all means together, is very important, particularly because MACE can be life-threatening and life-altering,” she added.
Learning how migraine medications may impact MACE risk is also something that needs to be examined in greater depth, she said. “I would think that migraines that are controlled might have a different risk for MACE than uncontrolled migraine,” she said.
The investigators reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Researchers are unraveling the complex relationship between cardiovascular (CV)- and stroke-related outcomes in migraine with, and without, aura.
“We confirmed that aura increases the risk for these cerebrovascular and cardiovascular outcomes in people with migraine and that there’s an increased risk of these MACE events in men with migraine,” said study investigator Gina Dumkrieger, PhD, principal data science analyst and assistant professor of neurology, Mayo Clinic, Phoenix, Arizona.
The findings were presented at the annual meeting of the American Headache Society.
Few Data on Migraine and Stroke Risk
The extent to which migraine increases the risk for stroke CV outcomes has not been extensively studied.
“We’re trying to find out whether migraine-related factors make it more likely that you’re going to have one of these events,” said Dr. Dumkrieger. “Knowing a particular factor increases the risk is something patients and medical providers would want to know.”
Using Mayo Clinic electronic health records, which cover all three sites (Florida, Minnesota, and Arizona), researchers identified individuals with migraine using diagnostic codes. They also looked at data on sex, race, and the presence of aura.
They investigated whether a history of MACE risk factors — including atrial fibrillation, diabetes, hyperlipidemia, hypertension, and tobacco use — affected risk and the potential interaction of aura with these risk factors.
MACE events included cerebral infarction, intracerebral hemorrhage, and acute myocardial infarction.
The analysis included 130,126 participants (80% women, 95% White individuals). Of these, 6% experienced a MACE event, and 94% did not.
“We confirmed that aura does increase the risk for a MACE event, and all of the known risk factors that we included were also significant,” said Dr. Dumkrieger.
Odds ratios (ORs) were 3.82 for atrial fibrillation, 3.11 for hypertension, and 3.06 for hyperlipidemia.
It was surprising, said Dr. Dumkrieger, that male sex was tied to an increased risk for a MACE event (OR, 1.40). “This is not something that was known before,” she said.
The link between migraine and ischemic stroke, particularly with aura, was stronger in women — particularly young women.
Investigators also found an interaction between male sex and aura, when it comes to MACE outcomes, said Dr. Dumkrieger. “Males in general are at higher risk, and people with aura are at higher risk. Males with aura are also at higher risk, but maybe not as much as you would think they would be. It’s not a purely additive thing. This is something we need to look into more,” she said.
The study also revealed an interaction between aura and hypertension as well as aura and tobacco use, but here too, it was not an additive risk, said Dr. Dumkrieger. However, she added, the presence of aura does not moderate the risk for hyperlipidemia, diabetes, or atrial fibrillation.
The research also showed a significant interaction between male sex and Black race which was additive. “There’s apparently increased risk if you are male and Black or African American that’s greater than what you would expect. We should be especially concerned about these individuals,” she said.
Unanswered Questions
The current analysis is part of a larger study that will more closely examine these relationships. “We want to learn, for example, why aura moderates some of the risk factors but not others,” said Dr. Dumkrieger.
The researchers also plan to investigate other migraine features, including headache frequency, and headache sensations such as pulsating or throbbing.
Dr. Dumkrieger was an investigator of another study, also presented at the AHS meeting, that’s investigating the role of migraine-specific features and imaging results in the complex interrelationship between migraine and MACE risk.
That study, which also used the Mayo Clinic electronic health record data, included 60,454 migraine patients diagnosed with migraine after 2010.
Researchers divided participants into those with a MACE outcome (1107) and those without such an outcome (59,347) after at least 2 years of follow-up. They created a propensity cohort of individuals matched for age and risk factors for MACE outcome.
The final cohort consisted of 575 patients with and 652 patients without MACE outcome.
One of the most interesting early results from this study was that those with a MACE outcome had significantly more white matter hyperintensities than those with no MACE outcome, at 64% versus 51%, respectively.
This and other findings need to be validated in a different cohort with an electronic health records database from another institution. In future, the team plans to focus on identifying specific migraine features and medications and their relative contributions to MACE risk in migraine patients.
Yet another study featured at the AHS meeting confirmed the increased risk for stroke among migraine patients using a large database with over 410,000 subjects.
Results showed stroke was more than three times more common in those with a migraine diagnosis than in those without (risk ratio, [RR] 3.23; P < .001). The RR for hemorrhagic stroke (3.15) was comparable with that of ischemic stroke (3.20).
The overall stroke RR for chronic migraine versus controls without migraine was 3.68 (P < .001). The RR for migraine with aura versus migraine without aura was 1.37 (P < .001).
Useful Data
Commenting on the research, Juliana VanderPluym, MD, a headache specialist at the Mayo Clinic, Phoenix, Arizona, described this new information as “very useful.”
The fact that there are more white matter lesions on MRI scans in migraine patients with MACE needs further exploration, said Dr. VanderPluym.
“Understanding how much of that relates to migraine, how much relates to other comorbid conditions, and what this all means together, is very important, particularly because MACE can be life-threatening and life-altering,” she added.
Learning how migraine medications may impact MACE risk is also something that needs to be examined in greater depth, she said. “I would think that migraines that are controlled might have a different risk for MACE than uncontrolled migraine,” she said.
The investigators reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM AHS 2024
Novel PCSK9 Drives High-Risk Patients to Target LDL
LYON, France – Lerodalcibep, a novel, third-generation anti-proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor, helps high-risk patients already receiving maximally tolerated statins to achieve guideline lipid targets, reported investigators.
In the randomized, placebo-controlled LIBerate-CVD trial of more than 900 patients, lerodalcibep led to reductions from baseline in low-density lipoprotein (LDL) cholesterol levels of more than 60%.
“We believe that lerodalcibep offers a novel, effective alternative to current PCSK9 inhibitors for patients with cardiovascular disease or at very high risk for cardiovascular disease,” said Evan Stein, MD, PhD, chief scientific officer and cofounder of LIB Therapeutics in Chicago, who presented the findings at the European Atherosclerosis Society (EAS) 2024.
Moreover, it leads to “substantial additional LDL cholesterol reductions on top of existing oral agents” and allows more than 90% of patients to achieve the latest European Society of Cardiology (ESC) guideline targets, he said.
Lerodalcibep has “tolerability and safety similar to placebo,” Dr. Stein said, and requires only “a small monthly injection, which takes about 12 seconds.”
“The drug doesn’t require refrigeration” and is “stable, so far, over 9 months,” he reported.
The latest data “confirm the efficacy of lerodalcibep,” said Giuseppe Danilo Norata, PhD, from the Department of Pharmacological and Biomolecular Sciences at the University of Milan, Milan, Italy, who was not involved in the study.
The LDL cholesterol reduction in this phase 3 trial is “in line with what was observed in LIBerate-FH,” and the high proportion of patients achieving their LDL cholesterol target is “impressive,” he added.
Effective and Well Tolerated
The safety results are “suggestive of a drug that is well tolerated, with injection-site reactions being the only remarkable adverse event increased in the treatment group,” Dr. Norata reported.
Only a “limited number” of patients developed neutralizing antidrug antibodies, which did not affect the efficacy of lerodalcibep. However, “given that the therapy is expected to be administered for years,” a longer analysis is needed to exclude the concern that a small percentage of neutralizing antidrug antibodies could reduce the efficacy, he said.
If approved, lerodalcibep could end up as a first-line option in the treatment pathway for high-risk cardiovascular disease because the efficacy “is similar to that of other injectable PCSK9 inhibitors,” he said, adding that its position in the market will “largely depend on the price.”
As the mechanism of action is similar to that of other monoclonal antibodies, “there is no pharmacological rationale to use it after another PSCK9 inhibitor,” he explained.
Lerodalcibep is a small recombinant fusion protein that combines a PCSK9-binding domain with human serum albumin.
The binding domain blocks the interaction between PCSK9 and the LDL cholesterol receptor, and the albumin linkage increases the half-life to 12-15 days, allowing low-volume injections to be given every 4 weeks.
A prior phase 2 study suggested that lerodalcibep substantially decreases LDL cholesterol levels in patients already taking maximally tolerated statins. The 300-mg dose was associated with an average reduction from baseline in LDL cholesterol levels of 77% over 12 weeks, whereas free PCSK9 levels decreased by 88%.
The current phase 3 study enrolled individuals at 65 centers in 100 countries who had or were at a very high risk for cardiovascular disease and who had an LDL cholesterol level of ≥ 1.8 mmol/L despite being on maximally tolerated statins.
Study participants were randomized in a 2:1 ratio to receive monthly subcutaneous lerodalcibep (n = 614) or placebo (n = 308) for 52 weeks and were assessed for the co-primary endpoints of the percentage change in LDL cholesterol levels from baseline to week 52 and the mean of levels at weeks 50 and 52.
The mean age was similar in the lerodalcibep and placebo groups (63.3 vs 64.5 years), as were the proportion of female (30% vs 30%) and White (80% vs 79%) participants.
The vast majority of participants in the lerodalcibep and placebo groups had a documented cardiovascular event (85.3% vs 86.4%) and were receiving secondary prevention, and 87% and 82%, respectively, were receiving a statin (any dose).
In a modified intention-to-treat analysis, the mean placebo-adjusted reduction in LDL cholesterol levels from baseline with lerodalcibep was 62% at week 52 (P < .0001), and the mean of levels at weeks 50 and 52 was 69.4% (P < .0001).
Similar results were seen in a per protocol analysis and an intention-to-treat analysis with imputation, which is a US Food and Drug Administration measure introduced in 2021 that assumes patients who discontinue the study treatment have an outcome similar to that in the placebo patients.
Moreover, 98.2% of patients in the lerodalcibep group achieved the ESC and European Atherosclerosis Society recommended reduction in LDL cholesterol levels of ≥ 50%, whereas only 8.8% in the placebo group did.
Hitting the LDL Cholesterol Target
More patients in the lerodalcibep group than in the placebo group achieved the LDL cholesterol target of < 1.4 mmol/L (95.3% vs 18.5%), and more patients in the lerodalcibep group achieved both that target and the ≥ 50% target (94.5% and 6.8%).
Lerodalcibep was also associated with significant reductions from baseline in levels of non–high-density lipoprotein (HDL) cholesterol, apolipoprotein B, very LDL cholesterol, and triglycerides, as well as an increase in HDL cholesterol levels (P < .0001 for all).
In terms of safety, lerodalcibep was associated with an adverse event rate leading to withdrawal similar to that seen with placebo (4.2% vs 3.6%), and 15.9% and 14.8% of patients, respectively, experienced at least one serious adverse event.
In-stent restenosis occurred more often in the lerodalcibep group than in the placebo group (5.4% vs 2.0%).
The study drug was associated with low levels of transient and sporadic antidrug antibodies and a low rate of neutralizing antidrug antibodies (0.9%), which were not associated with restenosis, a reduction in free PCSK9 levels, or the ability of lerodalcibep to lower LDL cholesterol levels.
A version of this article first appeared on Medscape.com.
LYON, France – Lerodalcibep, a novel, third-generation anti-proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor, helps high-risk patients already receiving maximally tolerated statins to achieve guideline lipid targets, reported investigators.
In the randomized, placebo-controlled LIBerate-CVD trial of more than 900 patients, lerodalcibep led to reductions from baseline in low-density lipoprotein (LDL) cholesterol levels of more than 60%.
“We believe that lerodalcibep offers a novel, effective alternative to current PCSK9 inhibitors for patients with cardiovascular disease or at very high risk for cardiovascular disease,” said Evan Stein, MD, PhD, chief scientific officer and cofounder of LIB Therapeutics in Chicago, who presented the findings at the European Atherosclerosis Society (EAS) 2024.
Moreover, it leads to “substantial additional LDL cholesterol reductions on top of existing oral agents” and allows more than 90% of patients to achieve the latest European Society of Cardiology (ESC) guideline targets, he said.
Lerodalcibep has “tolerability and safety similar to placebo,” Dr. Stein said, and requires only “a small monthly injection, which takes about 12 seconds.”
“The drug doesn’t require refrigeration” and is “stable, so far, over 9 months,” he reported.
The latest data “confirm the efficacy of lerodalcibep,” said Giuseppe Danilo Norata, PhD, from the Department of Pharmacological and Biomolecular Sciences at the University of Milan, Milan, Italy, who was not involved in the study.
The LDL cholesterol reduction in this phase 3 trial is “in line with what was observed in LIBerate-FH,” and the high proportion of patients achieving their LDL cholesterol target is “impressive,” he added.
Effective and Well Tolerated
The safety results are “suggestive of a drug that is well tolerated, with injection-site reactions being the only remarkable adverse event increased in the treatment group,” Dr. Norata reported.
Only a “limited number” of patients developed neutralizing antidrug antibodies, which did not affect the efficacy of lerodalcibep. However, “given that the therapy is expected to be administered for years,” a longer analysis is needed to exclude the concern that a small percentage of neutralizing antidrug antibodies could reduce the efficacy, he said.
If approved, lerodalcibep could end up as a first-line option in the treatment pathway for high-risk cardiovascular disease because the efficacy “is similar to that of other injectable PCSK9 inhibitors,” he said, adding that its position in the market will “largely depend on the price.”
As the mechanism of action is similar to that of other monoclonal antibodies, “there is no pharmacological rationale to use it after another PSCK9 inhibitor,” he explained.
Lerodalcibep is a small recombinant fusion protein that combines a PCSK9-binding domain with human serum albumin.
The binding domain blocks the interaction between PCSK9 and the LDL cholesterol receptor, and the albumin linkage increases the half-life to 12-15 days, allowing low-volume injections to be given every 4 weeks.
A prior phase 2 study suggested that lerodalcibep substantially decreases LDL cholesterol levels in patients already taking maximally tolerated statins. The 300-mg dose was associated with an average reduction from baseline in LDL cholesterol levels of 77% over 12 weeks, whereas free PCSK9 levels decreased by 88%.
The current phase 3 study enrolled individuals at 65 centers in 100 countries who had or were at a very high risk for cardiovascular disease and who had an LDL cholesterol level of ≥ 1.8 mmol/L despite being on maximally tolerated statins.
Study participants were randomized in a 2:1 ratio to receive monthly subcutaneous lerodalcibep (n = 614) or placebo (n = 308) for 52 weeks and were assessed for the co-primary endpoints of the percentage change in LDL cholesterol levels from baseline to week 52 and the mean of levels at weeks 50 and 52.
The mean age was similar in the lerodalcibep and placebo groups (63.3 vs 64.5 years), as were the proportion of female (30% vs 30%) and White (80% vs 79%) participants.
The vast majority of participants in the lerodalcibep and placebo groups had a documented cardiovascular event (85.3% vs 86.4%) and were receiving secondary prevention, and 87% and 82%, respectively, were receiving a statin (any dose).
In a modified intention-to-treat analysis, the mean placebo-adjusted reduction in LDL cholesterol levels from baseline with lerodalcibep was 62% at week 52 (P < .0001), and the mean of levels at weeks 50 and 52 was 69.4% (P < .0001).
Similar results were seen in a per protocol analysis and an intention-to-treat analysis with imputation, which is a US Food and Drug Administration measure introduced in 2021 that assumes patients who discontinue the study treatment have an outcome similar to that in the placebo patients.
Moreover, 98.2% of patients in the lerodalcibep group achieved the ESC and European Atherosclerosis Society recommended reduction in LDL cholesterol levels of ≥ 50%, whereas only 8.8% in the placebo group did.
Hitting the LDL Cholesterol Target
More patients in the lerodalcibep group than in the placebo group achieved the LDL cholesterol target of < 1.4 mmol/L (95.3% vs 18.5%), and more patients in the lerodalcibep group achieved both that target and the ≥ 50% target (94.5% and 6.8%).
Lerodalcibep was also associated with significant reductions from baseline in levels of non–high-density lipoprotein (HDL) cholesterol, apolipoprotein B, very LDL cholesterol, and triglycerides, as well as an increase in HDL cholesterol levels (P < .0001 for all).
In terms of safety, lerodalcibep was associated with an adverse event rate leading to withdrawal similar to that seen with placebo (4.2% vs 3.6%), and 15.9% and 14.8% of patients, respectively, experienced at least one serious adverse event.
In-stent restenosis occurred more often in the lerodalcibep group than in the placebo group (5.4% vs 2.0%).
The study drug was associated with low levels of transient and sporadic antidrug antibodies and a low rate of neutralizing antidrug antibodies (0.9%), which were not associated with restenosis, a reduction in free PCSK9 levels, or the ability of lerodalcibep to lower LDL cholesterol levels.
A version of this article first appeared on Medscape.com.
LYON, France – Lerodalcibep, a novel, third-generation anti-proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor, helps high-risk patients already receiving maximally tolerated statins to achieve guideline lipid targets, reported investigators.
In the randomized, placebo-controlled LIBerate-CVD trial of more than 900 patients, lerodalcibep led to reductions from baseline in low-density lipoprotein (LDL) cholesterol levels of more than 60%.
“We believe that lerodalcibep offers a novel, effective alternative to current PCSK9 inhibitors for patients with cardiovascular disease or at very high risk for cardiovascular disease,” said Evan Stein, MD, PhD, chief scientific officer and cofounder of LIB Therapeutics in Chicago, who presented the findings at the European Atherosclerosis Society (EAS) 2024.
Moreover, it leads to “substantial additional LDL cholesterol reductions on top of existing oral agents” and allows more than 90% of patients to achieve the latest European Society of Cardiology (ESC) guideline targets, he said.
Lerodalcibep has “tolerability and safety similar to placebo,” Dr. Stein said, and requires only “a small monthly injection, which takes about 12 seconds.”
“The drug doesn’t require refrigeration” and is “stable, so far, over 9 months,” he reported.
The latest data “confirm the efficacy of lerodalcibep,” said Giuseppe Danilo Norata, PhD, from the Department of Pharmacological and Biomolecular Sciences at the University of Milan, Milan, Italy, who was not involved in the study.
The LDL cholesterol reduction in this phase 3 trial is “in line with what was observed in LIBerate-FH,” and the high proportion of patients achieving their LDL cholesterol target is “impressive,” he added.
Effective and Well Tolerated
The safety results are “suggestive of a drug that is well tolerated, with injection-site reactions being the only remarkable adverse event increased in the treatment group,” Dr. Norata reported.
Only a “limited number” of patients developed neutralizing antidrug antibodies, which did not affect the efficacy of lerodalcibep. However, “given that the therapy is expected to be administered for years,” a longer analysis is needed to exclude the concern that a small percentage of neutralizing antidrug antibodies could reduce the efficacy, he said.
If approved, lerodalcibep could end up as a first-line option in the treatment pathway for high-risk cardiovascular disease because the efficacy “is similar to that of other injectable PCSK9 inhibitors,” he said, adding that its position in the market will “largely depend on the price.”
As the mechanism of action is similar to that of other monoclonal antibodies, “there is no pharmacological rationale to use it after another PSCK9 inhibitor,” he explained.
Lerodalcibep is a small recombinant fusion protein that combines a PCSK9-binding domain with human serum albumin.
The binding domain blocks the interaction between PCSK9 and the LDL cholesterol receptor, and the albumin linkage increases the half-life to 12-15 days, allowing low-volume injections to be given every 4 weeks.
A prior phase 2 study suggested that lerodalcibep substantially decreases LDL cholesterol levels in patients already taking maximally tolerated statins. The 300-mg dose was associated with an average reduction from baseline in LDL cholesterol levels of 77% over 12 weeks, whereas free PCSK9 levels decreased by 88%.
The current phase 3 study enrolled individuals at 65 centers in 100 countries who had or were at a very high risk for cardiovascular disease and who had an LDL cholesterol level of ≥ 1.8 mmol/L despite being on maximally tolerated statins.
Study participants were randomized in a 2:1 ratio to receive monthly subcutaneous lerodalcibep (n = 614) or placebo (n = 308) for 52 weeks and were assessed for the co-primary endpoints of the percentage change in LDL cholesterol levels from baseline to week 52 and the mean of levels at weeks 50 and 52.
The mean age was similar in the lerodalcibep and placebo groups (63.3 vs 64.5 years), as were the proportion of female (30% vs 30%) and White (80% vs 79%) participants.
The vast majority of participants in the lerodalcibep and placebo groups had a documented cardiovascular event (85.3% vs 86.4%) and were receiving secondary prevention, and 87% and 82%, respectively, were receiving a statin (any dose).
In a modified intention-to-treat analysis, the mean placebo-adjusted reduction in LDL cholesterol levels from baseline with lerodalcibep was 62% at week 52 (P < .0001), and the mean of levels at weeks 50 and 52 was 69.4% (P < .0001).
Similar results were seen in a per protocol analysis and an intention-to-treat analysis with imputation, which is a US Food and Drug Administration measure introduced in 2021 that assumes patients who discontinue the study treatment have an outcome similar to that in the placebo patients.
Moreover, 98.2% of patients in the lerodalcibep group achieved the ESC and European Atherosclerosis Society recommended reduction in LDL cholesterol levels of ≥ 50%, whereas only 8.8% in the placebo group did.
Hitting the LDL Cholesterol Target
More patients in the lerodalcibep group than in the placebo group achieved the LDL cholesterol target of < 1.4 mmol/L (95.3% vs 18.5%), and more patients in the lerodalcibep group achieved both that target and the ≥ 50% target (94.5% and 6.8%).
Lerodalcibep was also associated with significant reductions from baseline in levels of non–high-density lipoprotein (HDL) cholesterol, apolipoprotein B, very LDL cholesterol, and triglycerides, as well as an increase in HDL cholesterol levels (P < .0001 for all).
In terms of safety, lerodalcibep was associated with an adverse event rate leading to withdrawal similar to that seen with placebo (4.2% vs 3.6%), and 15.9% and 14.8% of patients, respectively, experienced at least one serious adverse event.
In-stent restenosis occurred more often in the lerodalcibep group than in the placebo group (5.4% vs 2.0%).
The study drug was associated with low levels of transient and sporadic antidrug antibodies and a low rate of neutralizing antidrug antibodies (0.9%), which were not associated with restenosis, a reduction in free PCSK9 levels, or the ability of lerodalcibep to lower LDL cholesterol levels.
A version of this article first appeared on Medscape.com.
FROM EAS 2024
Chronic Loneliness Tied to Increased Stroke Risk
Adults older than 50 years who report experiencing persistently high levels of loneliness have a 56% increased risk for stroke, a new study showed.
The increased stroke risk did not apply to individuals who reported experiencing situational loneliness, a finding that investigators believe bolsters the hypothesis that chronic loneliness is driving the association.
“Our findings suggest that individuals who experience chronic loneliness are at higher risk for incident stroke,” lead investigator Yenee Soh, ScD, research associate of social and behavioral sciences in the Harvard T.H. Chan School of Public Health, Boston, told this news organization. “It is important to routinely assess loneliness, as the consequences may be worse if unidentified and/or ignored.”
The findings were published online in eClinicalMedicine.
Significant, Chronic Health Consequences
Exacerbated by the COVID-19 pandemic, loneliness is at an all-time high. A 2023 Surgeon General’s report highlighted the fact that loneliness and social isolation are linked to significant and chronic health consequences.
Previous research has linked loneliness to cardiovascular disease, yet few studies have examined the association between loneliness and stroke risk. The current study is one of the first to examine the association between changes in loneliness and stroke risk over time.
Using data from the 2006-2018 Health and Retirement Study, researchers assessed the link between loneliness and incident stroke over time. Between 2006 and 2008, 12,161 study participants, who were all older than 50 years with no history of stroke, responded to questions from the Revised UCLA Loneliness Scale. From these responses, researchers created summary loneliness scores.
Four years later, from 2010 to 2012, the 8936 remaining study participants responded to the same 20 questions again. Based on loneliness scores across the two time points, participants were divided into four groups:
- Consistently low (those who scored low on the loneliness scale at both baseline and follow-up).
- Remitting (those who scored high at baseline and low at follow-up).
- Recent onset (those who scored low at baseline and high at follow-up).
- Consistently high (those who scored high at both baseline and follow-up).
Incident stroke was determined by participant report and medical record data.
Among participants whose loneliness was measured at baseline only, 1237 strokes occurred during the 2006-2018 follow-up period. Among those who provided two loneliness assessments over time, 601 strokes occurred during the follow-up period.
Even after adjusting for social isolation, depressive symptoms, physical activity, body mass index, and other health conditions, investigators found that participants who reported being lonely at baseline only had a 25% increased stroke risk, compared with those who did not report being lonely at baseline (hazard ratio [HR], 1.25; 95% confidence interval (CI), 1.06-1.47).
Participants who reported having consistently high loneliness across both time points had a 56% increased risk for incident stroke vs those who did not report loneliness at both time points after adjusting for social isolation and depression (HR, 1.56; 95% CI, 1.11-2.18).
The researchers did not investigate any of the underlying issues that may contribute to the association between loneliness and stroke risk, but speculated there may be physiological factors at play. These could include inflammation caused by increased hypothalamic pituitary-adrenocortical activity, behavioral factors such as poor medication adherence, smoking and/or alcohol use, and psychosocial issues.
Those who experience chronic loneliness may represent individuals that are unable to develop or maintain satisfying social relationships, which may result in longer-term interpersonal difficulties, Dr. Soh noted.
“Since loneliness is a highly subjective experience, seeking help to address and intervene to address a patient’s specific personal needs is important. It’s important to distinguish loneliness from social isolation,” said Dr. Soh.
She added that “by screening for loneliness and providing care or referring patients to relevant behavioral healthcare providers, clinicians can play a crucial role in addressing loneliness and its associated health risks early on to help reduce the population burden of loneliness.”
Progressive Research
Commenting on the findings for this news organization, Elaine Jones, MD, medical director of Access TeleCare, who was not involved in the research, applauded the investigators for “advancing the topic by looking at the chronicity aspect of loneliness.”
She said more research is needed to investigate loneliness as a stroke risk factor and noted that there may be something inherently different among respondents who reported loneliness at both study time points.
“Personality types may play a role here. We know people with positive attitudes and outlooks can do better in challenging health situations than people who are negative in their attitudes, regardless of depression. Perhaps those who feel lonely initially decided to do something about it and join groups, take up a hobby, or re-engage with family or friends. Perhaps the people who are chronically lonely don’t, or can’t, do this,” Dr. Jones said.
Chronic loneliness can cause stress, she added, “and we know that stress chemicals and hormones can be harmful to health over long durations of time.”
The study was funded by the National Institute on Aging. There were no conflicts of interest noted.
A version of this article first appeared on Medscape.com.
Adults older than 50 years who report experiencing persistently high levels of loneliness have a 56% increased risk for stroke, a new study showed.
The increased stroke risk did not apply to individuals who reported experiencing situational loneliness, a finding that investigators believe bolsters the hypothesis that chronic loneliness is driving the association.
“Our findings suggest that individuals who experience chronic loneliness are at higher risk for incident stroke,” lead investigator Yenee Soh, ScD, research associate of social and behavioral sciences in the Harvard T.H. Chan School of Public Health, Boston, told this news organization. “It is important to routinely assess loneliness, as the consequences may be worse if unidentified and/or ignored.”
The findings were published online in eClinicalMedicine.
Significant, Chronic Health Consequences
Exacerbated by the COVID-19 pandemic, loneliness is at an all-time high. A 2023 Surgeon General’s report highlighted the fact that loneliness and social isolation are linked to significant and chronic health consequences.
Previous research has linked loneliness to cardiovascular disease, yet few studies have examined the association between loneliness and stroke risk. The current study is one of the first to examine the association between changes in loneliness and stroke risk over time.
Using data from the 2006-2018 Health and Retirement Study, researchers assessed the link between loneliness and incident stroke over time. Between 2006 and 2008, 12,161 study participants, who were all older than 50 years with no history of stroke, responded to questions from the Revised UCLA Loneliness Scale. From these responses, researchers created summary loneliness scores.
Four years later, from 2010 to 2012, the 8936 remaining study participants responded to the same 20 questions again. Based on loneliness scores across the two time points, participants were divided into four groups:
- Consistently low (those who scored low on the loneliness scale at both baseline and follow-up).
- Remitting (those who scored high at baseline and low at follow-up).
- Recent onset (those who scored low at baseline and high at follow-up).
- Consistently high (those who scored high at both baseline and follow-up).
Incident stroke was determined by participant report and medical record data.
Among participants whose loneliness was measured at baseline only, 1237 strokes occurred during the 2006-2018 follow-up period. Among those who provided two loneliness assessments over time, 601 strokes occurred during the follow-up period.
Even after adjusting for social isolation, depressive symptoms, physical activity, body mass index, and other health conditions, investigators found that participants who reported being lonely at baseline only had a 25% increased stroke risk, compared with those who did not report being lonely at baseline (hazard ratio [HR], 1.25; 95% confidence interval (CI), 1.06-1.47).
Participants who reported having consistently high loneliness across both time points had a 56% increased risk for incident stroke vs those who did not report loneliness at both time points after adjusting for social isolation and depression (HR, 1.56; 95% CI, 1.11-2.18).
The researchers did not investigate any of the underlying issues that may contribute to the association between loneliness and stroke risk, but speculated there may be physiological factors at play. These could include inflammation caused by increased hypothalamic pituitary-adrenocortical activity, behavioral factors such as poor medication adherence, smoking and/or alcohol use, and psychosocial issues.
Those who experience chronic loneliness may represent individuals that are unable to develop or maintain satisfying social relationships, which may result in longer-term interpersonal difficulties, Dr. Soh noted.
“Since loneliness is a highly subjective experience, seeking help to address and intervene to address a patient’s specific personal needs is important. It’s important to distinguish loneliness from social isolation,” said Dr. Soh.
She added that “by screening for loneliness and providing care or referring patients to relevant behavioral healthcare providers, clinicians can play a crucial role in addressing loneliness and its associated health risks early on to help reduce the population burden of loneliness.”
Progressive Research
Commenting on the findings for this news organization, Elaine Jones, MD, medical director of Access TeleCare, who was not involved in the research, applauded the investigators for “advancing the topic by looking at the chronicity aspect of loneliness.”
She said more research is needed to investigate loneliness as a stroke risk factor and noted that there may be something inherently different among respondents who reported loneliness at both study time points.
“Personality types may play a role here. We know people with positive attitudes and outlooks can do better in challenging health situations than people who are negative in their attitudes, regardless of depression. Perhaps those who feel lonely initially decided to do something about it and join groups, take up a hobby, or re-engage with family or friends. Perhaps the people who are chronically lonely don’t, or can’t, do this,” Dr. Jones said.
Chronic loneliness can cause stress, she added, “and we know that stress chemicals and hormones can be harmful to health over long durations of time.”
The study was funded by the National Institute on Aging. There were no conflicts of interest noted.
A version of this article first appeared on Medscape.com.
Adults older than 50 years who report experiencing persistently high levels of loneliness have a 56% increased risk for stroke, a new study showed.
The increased stroke risk did not apply to individuals who reported experiencing situational loneliness, a finding that investigators believe bolsters the hypothesis that chronic loneliness is driving the association.
“Our findings suggest that individuals who experience chronic loneliness are at higher risk for incident stroke,” lead investigator Yenee Soh, ScD, research associate of social and behavioral sciences in the Harvard T.H. Chan School of Public Health, Boston, told this news organization. “It is important to routinely assess loneliness, as the consequences may be worse if unidentified and/or ignored.”
The findings were published online in eClinicalMedicine.
Significant, Chronic Health Consequences
Exacerbated by the COVID-19 pandemic, loneliness is at an all-time high. A 2023 Surgeon General’s report highlighted the fact that loneliness and social isolation are linked to significant and chronic health consequences.
Previous research has linked loneliness to cardiovascular disease, yet few studies have examined the association between loneliness and stroke risk. The current study is one of the first to examine the association between changes in loneliness and stroke risk over time.
Using data from the 2006-2018 Health and Retirement Study, researchers assessed the link between loneliness and incident stroke over time. Between 2006 and 2008, 12,161 study participants, who were all older than 50 years with no history of stroke, responded to questions from the Revised UCLA Loneliness Scale. From these responses, researchers created summary loneliness scores.
Four years later, from 2010 to 2012, the 8936 remaining study participants responded to the same 20 questions again. Based on loneliness scores across the two time points, participants were divided into four groups:
- Consistently low (those who scored low on the loneliness scale at both baseline and follow-up).
- Remitting (those who scored high at baseline and low at follow-up).
- Recent onset (those who scored low at baseline and high at follow-up).
- Consistently high (those who scored high at both baseline and follow-up).
Incident stroke was determined by participant report and medical record data.
Among participants whose loneliness was measured at baseline only, 1237 strokes occurred during the 2006-2018 follow-up period. Among those who provided two loneliness assessments over time, 601 strokes occurred during the follow-up period.
Even after adjusting for social isolation, depressive symptoms, physical activity, body mass index, and other health conditions, investigators found that participants who reported being lonely at baseline only had a 25% increased stroke risk, compared with those who did not report being lonely at baseline (hazard ratio [HR], 1.25; 95% confidence interval (CI), 1.06-1.47).
Participants who reported having consistently high loneliness across both time points had a 56% increased risk for incident stroke vs those who did not report loneliness at both time points after adjusting for social isolation and depression (HR, 1.56; 95% CI, 1.11-2.18).
The researchers did not investigate any of the underlying issues that may contribute to the association between loneliness and stroke risk, but speculated there may be physiological factors at play. These could include inflammation caused by increased hypothalamic pituitary-adrenocortical activity, behavioral factors such as poor medication adherence, smoking and/or alcohol use, and psychosocial issues.
Those who experience chronic loneliness may represent individuals that are unable to develop or maintain satisfying social relationships, which may result in longer-term interpersonal difficulties, Dr. Soh noted.
“Since loneliness is a highly subjective experience, seeking help to address and intervene to address a patient’s specific personal needs is important. It’s important to distinguish loneliness from social isolation,” said Dr. Soh.
She added that “by screening for loneliness and providing care or referring patients to relevant behavioral healthcare providers, clinicians can play a crucial role in addressing loneliness and its associated health risks early on to help reduce the population burden of loneliness.”
Progressive Research
Commenting on the findings for this news organization, Elaine Jones, MD, medical director of Access TeleCare, who was not involved in the research, applauded the investigators for “advancing the topic by looking at the chronicity aspect of loneliness.”
She said more research is needed to investigate loneliness as a stroke risk factor and noted that there may be something inherently different among respondents who reported loneliness at both study time points.
“Personality types may play a role here. We know people with positive attitudes and outlooks can do better in challenging health situations than people who are negative in their attitudes, regardless of depression. Perhaps those who feel lonely initially decided to do something about it and join groups, take up a hobby, or re-engage with family or friends. Perhaps the people who are chronically lonely don’t, or can’t, do this,” Dr. Jones said.
Chronic loneliness can cause stress, she added, “and we know that stress chemicals and hormones can be harmful to health over long durations of time.”
The study was funded by the National Institute on Aging. There were no conflicts of interest noted.
A version of this article first appeared on Medscape.com.
BP Disorder in Pregnancy Tied to Young-Onset Dementia Risk
TOPLINE:
A new analysis showed that preeclampsia is associated with an increased risk for young-onset dementia.
METHODOLOGY:
- Researchers analyzed data from the French Conception study, a nationwide prospective cohort study of more than 1.9 million pregnancies.
- Mothers were followed for an average of 9 years.
TAKEAWAY:
- Nearly 3% of the mothers had preeclampsia, and 128 developed young-onset dementia.
- Preeclampsia was associated with a 2.65-fold increased risk for young-onset dementia after adjusting for obesity, diabetes, smoking, drug or alcohol addiction, and social deprivation.
- The risk was greater when preeclampsia occurred before 34 weeks of gestation (hazard ratio [HR], 4.15) or was superimposed on chronic hypertension (HR, 4.76).
- Prior research has found an association between preeclampsia and vascular dementia, but this analysis “is the first to show an increase in early-onset dementia risk,” the authors of the study wrote.
IN PRACTICE:
“Individuals who have had preeclampsia should be reassured that young-onset dementia remains a very rare condition. Their absolute risk increases only imperceptibly,” Stephen Tong, PhD, and Roxanne Hastie, PhD, both with the University of Melbourne, Melbourne, Australia, wrote in a related commentary about the findings.
“Individuals who have been affected by preeclampsia in a prior pregnancy might instead focus on reducing their risk of developing the many chronic health ailments that are far more common,” they added. “Although it is yet to be proven in clinical trials, it is plausible that after an episode of preeclampsia, adopting a healthy lifestyle may improve vascular health and reduce the risk of many serious cardiovascular conditions.”
SOURCE:
Valérie Olié, PhD, of the Santé Publique France in Saint-Maurice, France, was the corresponding author on the paper. The research letter was published online in JAMA Network Open.
LIMITATIONS:
The investigators relied on hospital records to identify cases of dementia, which may have led to underestimation of incidence of the disease.
DISCLOSURES:
The study was funded by the French Hypertension Society, the French Hypertension Research Foundation, and the French Cardiology Federation. A co-author disclosed personal fees from pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
A new analysis showed that preeclampsia is associated with an increased risk for young-onset dementia.
METHODOLOGY:
- Researchers analyzed data from the French Conception study, a nationwide prospective cohort study of more than 1.9 million pregnancies.
- Mothers were followed for an average of 9 years.
TAKEAWAY:
- Nearly 3% of the mothers had preeclampsia, and 128 developed young-onset dementia.
- Preeclampsia was associated with a 2.65-fold increased risk for young-onset dementia after adjusting for obesity, diabetes, smoking, drug or alcohol addiction, and social deprivation.
- The risk was greater when preeclampsia occurred before 34 weeks of gestation (hazard ratio [HR], 4.15) or was superimposed on chronic hypertension (HR, 4.76).
- Prior research has found an association between preeclampsia and vascular dementia, but this analysis “is the first to show an increase in early-onset dementia risk,” the authors of the study wrote.
IN PRACTICE:
“Individuals who have had preeclampsia should be reassured that young-onset dementia remains a very rare condition. Their absolute risk increases only imperceptibly,” Stephen Tong, PhD, and Roxanne Hastie, PhD, both with the University of Melbourne, Melbourne, Australia, wrote in a related commentary about the findings.
“Individuals who have been affected by preeclampsia in a prior pregnancy might instead focus on reducing their risk of developing the many chronic health ailments that are far more common,” they added. “Although it is yet to be proven in clinical trials, it is plausible that after an episode of preeclampsia, adopting a healthy lifestyle may improve vascular health and reduce the risk of many serious cardiovascular conditions.”
SOURCE:
Valérie Olié, PhD, of the Santé Publique France in Saint-Maurice, France, was the corresponding author on the paper. The research letter was published online in JAMA Network Open.
LIMITATIONS:
The investigators relied on hospital records to identify cases of dementia, which may have led to underestimation of incidence of the disease.
DISCLOSURES:
The study was funded by the French Hypertension Society, the French Hypertension Research Foundation, and the French Cardiology Federation. A co-author disclosed personal fees from pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
A new analysis showed that preeclampsia is associated with an increased risk for young-onset dementia.
METHODOLOGY:
- Researchers analyzed data from the French Conception study, a nationwide prospective cohort study of more than 1.9 million pregnancies.
- Mothers were followed for an average of 9 years.
TAKEAWAY:
- Nearly 3% of the mothers had preeclampsia, and 128 developed young-onset dementia.
- Preeclampsia was associated with a 2.65-fold increased risk for young-onset dementia after adjusting for obesity, diabetes, smoking, drug or alcohol addiction, and social deprivation.
- The risk was greater when preeclampsia occurred before 34 weeks of gestation (hazard ratio [HR], 4.15) or was superimposed on chronic hypertension (HR, 4.76).
- Prior research has found an association between preeclampsia and vascular dementia, but this analysis “is the first to show an increase in early-onset dementia risk,” the authors of the study wrote.
IN PRACTICE:
“Individuals who have had preeclampsia should be reassured that young-onset dementia remains a very rare condition. Their absolute risk increases only imperceptibly,” Stephen Tong, PhD, and Roxanne Hastie, PhD, both with the University of Melbourne, Melbourne, Australia, wrote in a related commentary about the findings.
“Individuals who have been affected by preeclampsia in a prior pregnancy might instead focus on reducing their risk of developing the many chronic health ailments that are far more common,” they added. “Although it is yet to be proven in clinical trials, it is plausible that after an episode of preeclampsia, adopting a healthy lifestyle may improve vascular health and reduce the risk of many serious cardiovascular conditions.”
SOURCE:
Valérie Olié, PhD, of the Santé Publique France in Saint-Maurice, France, was the corresponding author on the paper. The research letter was published online in JAMA Network Open.
LIMITATIONS:
The investigators relied on hospital records to identify cases of dementia, which may have led to underestimation of incidence of the disease.
DISCLOSURES:
The study was funded by the French Hypertension Society, the French Hypertension Research Foundation, and the French Cardiology Federation. A co-author disclosed personal fees from pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Compounded Semaglutide: How to Better Ensure Its Safety
Glucagon-like peptide 1 (GLP-1) receptor agonists such as semaglutide (marketed as Ozempic and Rybelsus for type 2 diabetes and as Wegovy for obesity) slow down digestion and curb hunger by working on the brain’s dopamine reward center. They are prescribed to promote weight loss, metabolic health in type 2 diabetes, and heart health in coronary artery disease.
Semaglutide can be prescribed in two forms: the brand-name version, which is approved and confirmed as safe and effective by the US Food and Drug Administration (FDA), and the versions that can be obtained from a compounding pharmacy. Compounding pharmacies are permitted by the FDA to produce what is “ essentially a copy” of approved medications when there’s an official shortage, which is currently the case with semaglutide and other GLP-1 receptor agonists.
Patients are often drawn to compounding pharmacies for pricing-related reasons. If semaglutide is prescribed for a clear indication like diabetes and is covered by insurance, the brand-name version is commonly dispensed. However, if it’s not covered, patients need to pay out of pocket for branded versions, which carry a monthly cost of $1000 or more. Alternatively, their doctors can prescribe compounded semaglutide, which some telehealth companies advertise at costs of approximately $150-$300 per month.
Potential Issues With Compounded Semaglutide
Compounding pharmacies produce drugs from raw materials containing active pharmaceutical ingredients (APIs). Although compounders use many of the same ingredients found in brand-name medications, for drugs like semaglutide, they may opt for specific salts that are not identical to those involved in the production of the standard versions. These salts are typically reserved for research purposes and may not be suitable for general use.
In late 2023, the FDA issued a letter asking the public to exercise caution when using compounded products containing semaglutide or semaglutide salts. This was followed in January 2024 by an FDA communication citing adverse events reported with the use of compounded semaglutide and advising patients to avoid these versions if an approved form of the drug is available.
Compound Pharmacies: A Closer Look
Compounding pharmacies have exploded in popularity in the past several decades. The compounding pharmacy market is expected to grow at 7.8% per year over the next decade.
Historically, compounding pharmacies have filled a niche for specialty vitamins for intravenous administration as well as chemotherapy medications. They also offer controlled substances, such as ketamine lozenges and nasal sprays, which are unavailable or are in short supply from traditional manufacturers.
Compounding pharmacies fall into two categories. First are compounding pharmacies covered under Section 503A of the Federal Food, Drug and Cosmetic Act; these drugs are neither tested nor monitored. Such facilities do not have to report adverse events to the FDA. The second category is Section 503B outsourcing facilities. These pharmacies choose to be tested by, to be inspected by, and to report adverse events to the FDA.
The FDA’s Latest Update on This Issue
This news organization contacted the FDA for an update on the adverse events reported about compounded semaglutide. From August 8, 2021, to March 31, 2024, they received more than 20,000 adverse events reports for FDA-approved semaglutide. Comparatively, there were 210 adverse events reported on compounded semaglutide products.
The FDA went on to describe that many of the adverse events reported were consistent with known reactions in the labeling, like nausea, diarrhea, and headache. Yet, they added that, “the FDA is unable to determine how, or if, other factors may have contributed to these adverse events, such as differences in ingredients and formulation between FDA-approved and compounded semaglutide products.” They also noted there was variation in the data quality in the reports they have received, which came only from 503B compounding pharmacies.
In conclusion, given the concerns about compounded semaglutide, it is prudent for the prescribing physicians as well as the patients taking the medication to know that risks are “higher” according to the FDA. We eagerly await more specific information from the FDA to better understand reported adverse events.
How to Help Patients Receive Safe Compounded Semaglutide
For clinicians considering prescribing semaglutide from compounding pharmacies, there are several questions worth asking, according to the Alliance for Pharmacy Compounding. First, find out whether the pharmacy complies with United States Pharmacopeia compounding standards and whether they source their APIs from FDA-registered facilities, the latter being required by federal law. It’s also important to ensure that these facilities undergo periodic third-party testing to verify medication purity and dosing.
Ask whether the pharmacy is accredited by the Pharmacy Compounding Accreditation Board (PCAB). Accreditation from the PCAB means that pharmacies have been assessed for processes related to continuous quality improvement. In addition, ask whether the pharmacy is designated as a 503B compounder and if not, why.
Finally, interviewing the pharmacist themselves can provide useful information about staffing, training, and their methods of preparing medications. For example, if they are preparing a sterile eye drop, it is important to ask about sterility testing.
Jesse M. Pines, MD, MBA, MSCE, is a clinical professor of emergency medicine at George Washington University in Washington, and a professor in the department of emergency medicine at Drexel University College of Medicine in Philadelphia, Pennsylvania. Dr. Pines is also the chief of clinical innovation at US Acute Care Solutions in Canton, Ohio. Robert D. Glatter, MD, is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. Dr. Pines reported conflicts of interest with CSL Behring and Abbott Point-of-Care. Dr. Glatter reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Glucagon-like peptide 1 (GLP-1) receptor agonists such as semaglutide (marketed as Ozempic and Rybelsus for type 2 diabetes and as Wegovy for obesity) slow down digestion and curb hunger by working on the brain’s dopamine reward center. They are prescribed to promote weight loss, metabolic health in type 2 diabetes, and heart health in coronary artery disease.
Semaglutide can be prescribed in two forms: the brand-name version, which is approved and confirmed as safe and effective by the US Food and Drug Administration (FDA), and the versions that can be obtained from a compounding pharmacy. Compounding pharmacies are permitted by the FDA to produce what is “ essentially a copy” of approved medications when there’s an official shortage, which is currently the case with semaglutide and other GLP-1 receptor agonists.
Patients are often drawn to compounding pharmacies for pricing-related reasons. If semaglutide is prescribed for a clear indication like diabetes and is covered by insurance, the brand-name version is commonly dispensed. However, if it’s not covered, patients need to pay out of pocket for branded versions, which carry a monthly cost of $1000 or more. Alternatively, their doctors can prescribe compounded semaglutide, which some telehealth companies advertise at costs of approximately $150-$300 per month.
Potential Issues With Compounded Semaglutide
Compounding pharmacies produce drugs from raw materials containing active pharmaceutical ingredients (APIs). Although compounders use many of the same ingredients found in brand-name medications, for drugs like semaglutide, they may opt for specific salts that are not identical to those involved in the production of the standard versions. These salts are typically reserved for research purposes and may not be suitable for general use.
In late 2023, the FDA issued a letter asking the public to exercise caution when using compounded products containing semaglutide or semaglutide salts. This was followed in January 2024 by an FDA communication citing adverse events reported with the use of compounded semaglutide and advising patients to avoid these versions if an approved form of the drug is available.
Compound Pharmacies: A Closer Look
Compounding pharmacies have exploded in popularity in the past several decades. The compounding pharmacy market is expected to grow at 7.8% per year over the next decade.
Historically, compounding pharmacies have filled a niche for specialty vitamins for intravenous administration as well as chemotherapy medications. They also offer controlled substances, such as ketamine lozenges and nasal sprays, which are unavailable or are in short supply from traditional manufacturers.
Compounding pharmacies fall into two categories. First are compounding pharmacies covered under Section 503A of the Federal Food, Drug and Cosmetic Act; these drugs are neither tested nor monitored. Such facilities do not have to report adverse events to the FDA. The second category is Section 503B outsourcing facilities. These pharmacies choose to be tested by, to be inspected by, and to report adverse events to the FDA.
The FDA’s Latest Update on This Issue
This news organization contacted the FDA for an update on the adverse events reported about compounded semaglutide. From August 8, 2021, to March 31, 2024, they received more than 20,000 adverse events reports for FDA-approved semaglutide. Comparatively, there were 210 adverse events reported on compounded semaglutide products.
The FDA went on to describe that many of the adverse events reported were consistent with known reactions in the labeling, like nausea, diarrhea, and headache. Yet, they added that, “the FDA is unable to determine how, or if, other factors may have contributed to these adverse events, such as differences in ingredients and formulation between FDA-approved and compounded semaglutide products.” They also noted there was variation in the data quality in the reports they have received, which came only from 503B compounding pharmacies.
In conclusion, given the concerns about compounded semaglutide, it is prudent for the prescribing physicians as well as the patients taking the medication to know that risks are “higher” according to the FDA. We eagerly await more specific information from the FDA to better understand reported adverse events.
How to Help Patients Receive Safe Compounded Semaglutide
For clinicians considering prescribing semaglutide from compounding pharmacies, there are several questions worth asking, according to the Alliance for Pharmacy Compounding. First, find out whether the pharmacy complies with United States Pharmacopeia compounding standards and whether they source their APIs from FDA-registered facilities, the latter being required by federal law. It’s also important to ensure that these facilities undergo periodic third-party testing to verify medication purity and dosing.
Ask whether the pharmacy is accredited by the Pharmacy Compounding Accreditation Board (PCAB). Accreditation from the PCAB means that pharmacies have been assessed for processes related to continuous quality improvement. In addition, ask whether the pharmacy is designated as a 503B compounder and if not, why.
Finally, interviewing the pharmacist themselves can provide useful information about staffing, training, and their methods of preparing medications. For example, if they are preparing a sterile eye drop, it is important to ask about sterility testing.
Jesse M. Pines, MD, MBA, MSCE, is a clinical professor of emergency medicine at George Washington University in Washington, and a professor in the department of emergency medicine at Drexel University College of Medicine in Philadelphia, Pennsylvania. Dr. Pines is also the chief of clinical innovation at US Acute Care Solutions in Canton, Ohio. Robert D. Glatter, MD, is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. Dr. Pines reported conflicts of interest with CSL Behring and Abbott Point-of-Care. Dr. Glatter reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Glucagon-like peptide 1 (GLP-1) receptor agonists such as semaglutide (marketed as Ozempic and Rybelsus for type 2 diabetes and as Wegovy for obesity) slow down digestion and curb hunger by working on the brain’s dopamine reward center. They are prescribed to promote weight loss, metabolic health in type 2 diabetes, and heart health in coronary artery disease.
Semaglutide can be prescribed in two forms: the brand-name version, which is approved and confirmed as safe and effective by the US Food and Drug Administration (FDA), and the versions that can be obtained from a compounding pharmacy. Compounding pharmacies are permitted by the FDA to produce what is “ essentially a copy” of approved medications when there’s an official shortage, which is currently the case with semaglutide and other GLP-1 receptor agonists.
Patients are often drawn to compounding pharmacies for pricing-related reasons. If semaglutide is prescribed for a clear indication like diabetes and is covered by insurance, the brand-name version is commonly dispensed. However, if it’s not covered, patients need to pay out of pocket for branded versions, which carry a monthly cost of $1000 or more. Alternatively, their doctors can prescribe compounded semaglutide, which some telehealth companies advertise at costs of approximately $150-$300 per month.
Potential Issues With Compounded Semaglutide
Compounding pharmacies produce drugs from raw materials containing active pharmaceutical ingredients (APIs). Although compounders use many of the same ingredients found in brand-name medications, for drugs like semaglutide, they may opt for specific salts that are not identical to those involved in the production of the standard versions. These salts are typically reserved for research purposes and may not be suitable for general use.
In late 2023, the FDA issued a letter asking the public to exercise caution when using compounded products containing semaglutide or semaglutide salts. This was followed in January 2024 by an FDA communication citing adverse events reported with the use of compounded semaglutide and advising patients to avoid these versions if an approved form of the drug is available.
Compound Pharmacies: A Closer Look
Compounding pharmacies have exploded in popularity in the past several decades. The compounding pharmacy market is expected to grow at 7.8% per year over the next decade.
Historically, compounding pharmacies have filled a niche for specialty vitamins for intravenous administration as well as chemotherapy medications. They also offer controlled substances, such as ketamine lozenges and nasal sprays, which are unavailable or are in short supply from traditional manufacturers.
Compounding pharmacies fall into two categories. First are compounding pharmacies covered under Section 503A of the Federal Food, Drug and Cosmetic Act; these drugs are neither tested nor monitored. Such facilities do not have to report adverse events to the FDA. The second category is Section 503B outsourcing facilities. These pharmacies choose to be tested by, to be inspected by, and to report adverse events to the FDA.
The FDA’s Latest Update on This Issue
This news organization contacted the FDA for an update on the adverse events reported about compounded semaglutide. From August 8, 2021, to March 31, 2024, they received more than 20,000 adverse events reports for FDA-approved semaglutide. Comparatively, there were 210 adverse events reported on compounded semaglutide products.
The FDA went on to describe that many of the adverse events reported were consistent with known reactions in the labeling, like nausea, diarrhea, and headache. Yet, they added that, “the FDA is unable to determine how, or if, other factors may have contributed to these adverse events, such as differences in ingredients and formulation between FDA-approved and compounded semaglutide products.” They also noted there was variation in the data quality in the reports they have received, which came only from 503B compounding pharmacies.
In conclusion, given the concerns about compounded semaglutide, it is prudent for the prescribing physicians as well as the patients taking the medication to know that risks are “higher” according to the FDA. We eagerly await more specific information from the FDA to better understand reported adverse events.
How to Help Patients Receive Safe Compounded Semaglutide
For clinicians considering prescribing semaglutide from compounding pharmacies, there are several questions worth asking, according to the Alliance for Pharmacy Compounding. First, find out whether the pharmacy complies with United States Pharmacopeia compounding standards and whether they source their APIs from FDA-registered facilities, the latter being required by federal law. It’s also important to ensure that these facilities undergo periodic third-party testing to verify medication purity and dosing.
Ask whether the pharmacy is accredited by the Pharmacy Compounding Accreditation Board (PCAB). Accreditation from the PCAB means that pharmacies have been assessed for processes related to continuous quality improvement. In addition, ask whether the pharmacy is designated as a 503B compounder and if not, why.
Finally, interviewing the pharmacist themselves can provide useful information about staffing, training, and their methods of preparing medications. For example, if they are preparing a sterile eye drop, it is important to ask about sterility testing.
Jesse M. Pines, MD, MBA, MSCE, is a clinical professor of emergency medicine at George Washington University in Washington, and a professor in the department of emergency medicine at Drexel University College of Medicine in Philadelphia, Pennsylvania. Dr. Pines is also the chief of clinical innovation at US Acute Care Solutions in Canton, Ohio. Robert D. Glatter, MD, is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. Dr. Pines reported conflicts of interest with CSL Behring and Abbott Point-of-Care. Dr. Glatter reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.




