User login
Congress opens investigation into FDA’s handling of a problematic heart device
A congressional oversight subcommittee is investigating the Food and Drug Administration’s regulation of a high-risk heart pump, citing safety issues detailed by ProPublica.
The HeartWare Ventricular Assist Device, created to treat patients with severe heart failure, stopped meeting key federal standards as early as 2014. But the FDA took no decisive action even as those problems persisted, and thousands of Americans continued to be implanted with the pump.
By the end of 2020, the FDA had received more than 3,000 reports of deaths related to the HeartWare device, according to a ProPublica data analysis. A father of four died as his children tried to resuscitate him when his device suddenly stopped. A teenager died after vomiting blood in the middle of the night, while his mother struggled to restart a faulty pump.
“I am concerned by FDA’s slow action, over multiple administrations, to protect patients from this product despite early warning signs,” Rep. Raja Krishnamoorthi, D-Ill., said in a scathing letter sent March 22 to the agency’s commissioner, Robert Califf, MD.
Mr. Krishnamoorthi, the chairman of the U.S. House Committee on Oversight and Reform’s Subcommittee on Economic and Consumer Policy, requested information on how the FDA made regulatory decisions related to the HeartWare device and why it didn’t take further action.
The FDA did not provide comment to ProPublica on the subcommittee’s investigation and said it would respond directly to Mr. Krishnamoorthi. It also reiterated its response to ProPublica’s findings and said the agency had been closely overseeing the HeartWare device since 2012, with patient safety as its “highest priority.”
Medtronic, the company that acquired HeartWare in 2016, took the device off the market in June 2021. The company said that new data showed a competing heart pump had better outcomes. In response to the ProPublica investigation 2 months later, the company said it took the FDA’s inspections seriously and had worked closely with the agency to address issues with the device.
Medtronic declined to comment on the subcommittee’s investigation.
Mr. Krishnamoorthi asked in the letter if any steps were being taken to address how patients, doctors and other federal agencies are notified of problems that the FDA finds with medical devices.
Many patients told ProPublica they were never informed of issues with the HeartWare pump before or after their implants. Some people who still have the device said they weren’t told when it was taken off the market. Medtronic said in December it had confirmed 90% of U.S. patients had received notification of the HeartWare discontinuation, but that it was still working to reach the other 10%.
About 2,000 patients still had HeartWare pumps as of last year. The FDA and Medtronic recommended against removing those devices barring medical necessity because the surgery to do so carries a high risk.
In his letter, Mr. Krishnamoorthi gave the FDA a deadline of April 5 to respond.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
A congressional oversight subcommittee is investigating the Food and Drug Administration’s regulation of a high-risk heart pump, citing safety issues detailed by ProPublica.
The HeartWare Ventricular Assist Device, created to treat patients with severe heart failure, stopped meeting key federal standards as early as 2014. But the FDA took no decisive action even as those problems persisted, and thousands of Americans continued to be implanted with the pump.
By the end of 2020, the FDA had received more than 3,000 reports of deaths related to the HeartWare device, according to a ProPublica data analysis. A father of four died as his children tried to resuscitate him when his device suddenly stopped. A teenager died after vomiting blood in the middle of the night, while his mother struggled to restart a faulty pump.
“I am concerned by FDA’s slow action, over multiple administrations, to protect patients from this product despite early warning signs,” Rep. Raja Krishnamoorthi, D-Ill., said in a scathing letter sent March 22 to the agency’s commissioner, Robert Califf, MD.
Mr. Krishnamoorthi, the chairman of the U.S. House Committee on Oversight and Reform’s Subcommittee on Economic and Consumer Policy, requested information on how the FDA made regulatory decisions related to the HeartWare device and why it didn’t take further action.
The FDA did not provide comment to ProPublica on the subcommittee’s investigation and said it would respond directly to Mr. Krishnamoorthi. It also reiterated its response to ProPublica’s findings and said the agency had been closely overseeing the HeartWare device since 2012, with patient safety as its “highest priority.”
Medtronic, the company that acquired HeartWare in 2016, took the device off the market in June 2021. The company said that new data showed a competing heart pump had better outcomes. In response to the ProPublica investigation 2 months later, the company said it took the FDA’s inspections seriously and had worked closely with the agency to address issues with the device.
Medtronic declined to comment on the subcommittee’s investigation.
Mr. Krishnamoorthi asked in the letter if any steps were being taken to address how patients, doctors and other federal agencies are notified of problems that the FDA finds with medical devices.
Many patients told ProPublica they were never informed of issues with the HeartWare pump before or after their implants. Some people who still have the device said they weren’t told when it was taken off the market. Medtronic said in December it had confirmed 90% of U.S. patients had received notification of the HeartWare discontinuation, but that it was still working to reach the other 10%.
About 2,000 patients still had HeartWare pumps as of last year. The FDA and Medtronic recommended against removing those devices barring medical necessity because the surgery to do so carries a high risk.
In his letter, Mr. Krishnamoorthi gave the FDA a deadline of April 5 to respond.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
A congressional oversight subcommittee is investigating the Food and Drug Administration’s regulation of a high-risk heart pump, citing safety issues detailed by ProPublica.
The HeartWare Ventricular Assist Device, created to treat patients with severe heart failure, stopped meeting key federal standards as early as 2014. But the FDA took no decisive action even as those problems persisted, and thousands of Americans continued to be implanted with the pump.
By the end of 2020, the FDA had received more than 3,000 reports of deaths related to the HeartWare device, according to a ProPublica data analysis. A father of four died as his children tried to resuscitate him when his device suddenly stopped. A teenager died after vomiting blood in the middle of the night, while his mother struggled to restart a faulty pump.
“I am concerned by FDA’s slow action, over multiple administrations, to protect patients from this product despite early warning signs,” Rep. Raja Krishnamoorthi, D-Ill., said in a scathing letter sent March 22 to the agency’s commissioner, Robert Califf, MD.
Mr. Krishnamoorthi, the chairman of the U.S. House Committee on Oversight and Reform’s Subcommittee on Economic and Consumer Policy, requested information on how the FDA made regulatory decisions related to the HeartWare device and why it didn’t take further action.
The FDA did not provide comment to ProPublica on the subcommittee’s investigation and said it would respond directly to Mr. Krishnamoorthi. It also reiterated its response to ProPublica’s findings and said the agency had been closely overseeing the HeartWare device since 2012, with patient safety as its “highest priority.”
Medtronic, the company that acquired HeartWare in 2016, took the device off the market in June 2021. The company said that new data showed a competing heart pump had better outcomes. In response to the ProPublica investigation 2 months later, the company said it took the FDA’s inspections seriously and had worked closely with the agency to address issues with the device.
Medtronic declined to comment on the subcommittee’s investigation.
Mr. Krishnamoorthi asked in the letter if any steps were being taken to address how patients, doctors and other federal agencies are notified of problems that the FDA finds with medical devices.
Many patients told ProPublica they were never informed of issues with the HeartWare pump before or after their implants. Some people who still have the device said they weren’t told when it was taken off the market. Medtronic said in December it had confirmed 90% of U.S. patients had received notification of the HeartWare discontinuation, but that it was still working to reach the other 10%.
About 2,000 patients still had HeartWare pumps as of last year. The FDA and Medtronic recommended against removing those devices barring medical necessity because the surgery to do so carries a high risk.
In his letter, Mr. Krishnamoorthi gave the FDA a deadline of April 5 to respond.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
Death of pig heart transplant patient is more a beginning than an end
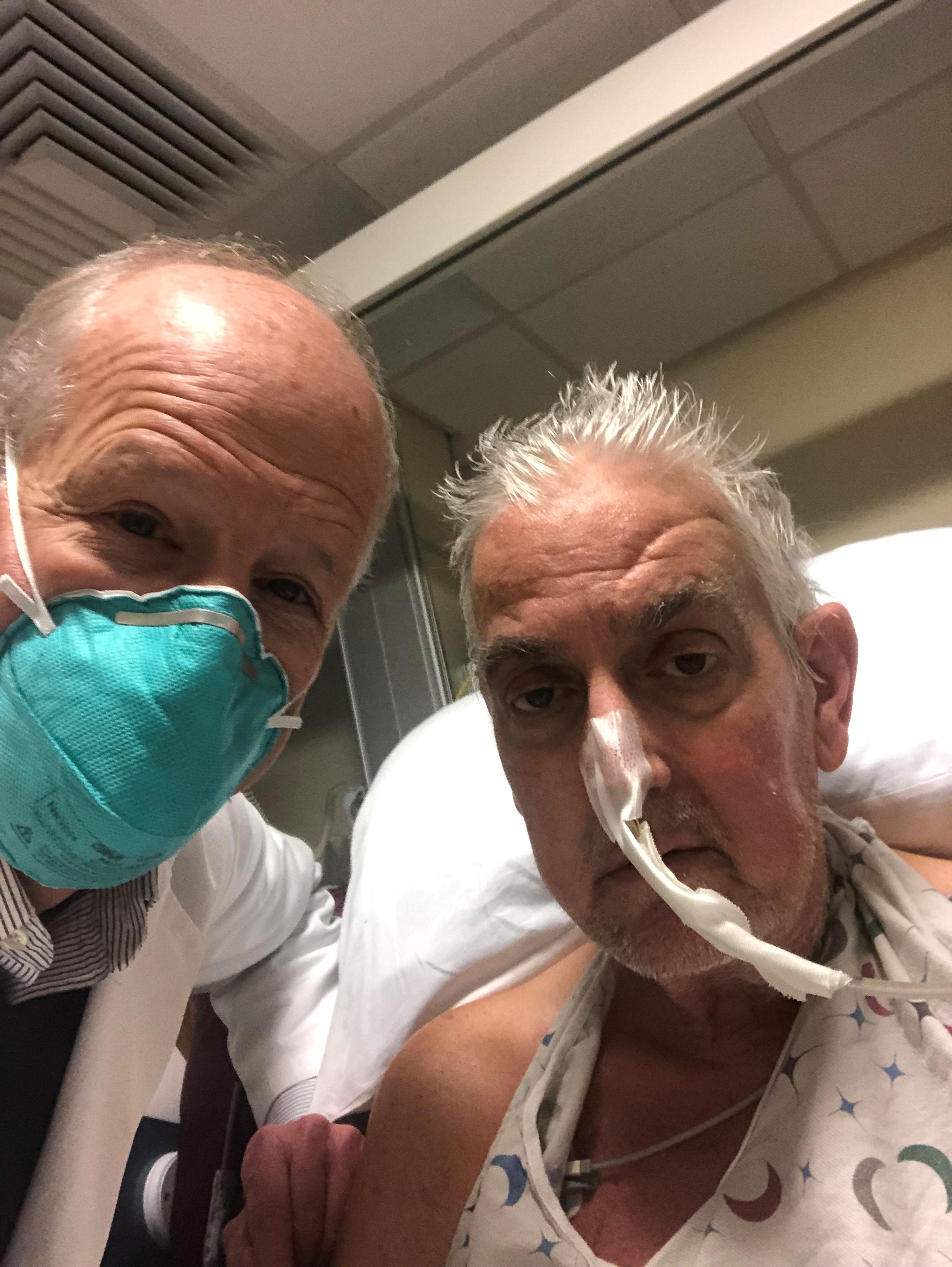
The genetically altered pig’s heart “worked like a rock star, beautifully functioning,” the surgeon who performed the pioneering Jan. 7 xenotransplant procedure said in a press statement on the death of the patient, David Bennett Sr.
“He wasn’t able to overcome what turned out to be devastating – the debilitation from his previous period of heart failure, which was extreme,” said Bartley P. Griffith, MD, clinical director of the cardiac xenotransplantation program at the University of Maryland, Baltimore.
Representatives of the institution aren’t offering many details on the cause of Mr. Bennett’s death on March 8, 60 days after his operation, but said they will elaborate when their findings are formally published. But their comments seem to downplay the unique nature of the implanted heart itself as a culprit and instead implicate the patient’s diminished overall clinical condition and what grew into an ongoing battle with infections.
The 57-year-old Bennett, bedridden with end-stage heart failure, judged a poor candidate for a ventricular assist device, and on extracorporeal membrane oxygenation (ECMO), reportedly was offered the extraordinary surgery after being turned down for a conventional transplant at several major centers.
“Until day 45 or 50, he was doing very well,” Muhammad M. Mohiuddin, MD, the xenotransplantation program’s scientific director, observed in the statement. But infections soon took advantage of his hobbled immune system.
Given his “preexisting condition and how frail his body was,” Dr. Mohiuddin said, “we were having difficulty maintaining a balance between his immunosuppression and controlling his infection.” Mr. Bennett went into multiple organ failure and “I think that resulted in his passing away.”
Beyond wildest dreams
The surgeons confidently framed Mr. Bennett’s experience as a milestone for heart xenotransplantation. “The demonstration that it was possible, beyond the wildest dreams of most people in the field, even, at this point – that we were able to take a genetically engineered organ and watch it function flawlessly for 9 weeks – is pretty positive in terms of the potential of this therapy,” Dr. Griffith said.
But enough questions linger that others were more circumspect, even as they praised the accomplishment. “There’s no question that this is a historic event,” Mandeep R. Mehra, MD, of Harvard Medical School, and director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital, both in Boston, said in an interview.
Still, “I don’t think we should just conclude that it was the patient’s frailty or death from infection,” Dr. Mehra said. With so few details available, “I would be very careful in prematurely concluding that the problem did not reside with the heart but with the patient. We cannot be sure.”
For example, he noted, “6 to 8 weeks is right around the time when some cardiac complications, like accelerated forms of vasculopathy, could become evident.” Immune-mediated cardiac allograft vasculopathy is a common cause of heart transplant failure.
Or, “it could as easily have been the fact that immunosuppression was modified at 6 to 7 weeks in response to potential infection, which could have led to a cardiac compromise,” Dr. Mehra said. “We just don’t know.”
“It’s really important that this be reported in a scientifically accurate way, because we will all learn from this,” Lori J. West, MD, DPhil, said in an interview.
Little seems to be known for sure about the actual cause of death, “but the fact there was not hyperacute rejection is itself a big step forward. And we know, at least from the limited information we have, that it did not occur,” observed Dr. West, who directs the Alberta Transplant Institute, Edmonton, and the Canadian Donation and Transplantation Research Program. She is a professor of pediatrics with adjunct positions in the departments of surgery and microbiology/immunology.
Dr. West also sees Mr. Bennett’s struggle with infections and adjustments to his unique immunosuppressive regimen, at least as characterized by his care team, as in line with the experience of many heart transplant recipients facing the same threat.
“We already walk this tightrope with every transplant patient,” she said. Typically, they’re put on a somewhat standardized immunosuppressant regimen, “and then we modify it a bit, either increasing or decreasing it, depending on the posttransplant course.” The regimen can become especially intense in response to new signs of rejection, “and you know that that’s going to have an impact on susceptibility to all kinds of infections.”
Full circle
The porcine heart was protected along two fronts against assault from Mr. Bennett’s immune system and other inhospitable aspects of his physiology, either of which could also have been obstacles to success: Genetic modification (Revivicor) of the pig that provided the heart, and a singularly aggressive antirejection drug regimen for the patient.
The knockout of three genes targeting specific porcine cell-surface carbohydrates that provoke a strong human antibody response reportedly averted a hyperacute rejection response that would have caused the graft to fail almost immediately.
Other genetic manipulations, some using CRISPR technology, silenced genes encoded for porcine endogenous retroviruses. Others were aimed at controlling myocardial growth and stemming graft microangiopathy.
Mr. Bennett himself was treated with powerful immunosuppressants, including an investigational anti-CD40 monoclonal antibody (KPL-404, Kiniksa Pharmaceuticals) that, according to UMSOM, inhibits a well-recognized pathway critical to B-cell proliferation, T-cell activation, and antibody production.
“I suspect the patient may not have had rejection, but unfortunately, that intense immunosuppression really set him up – even if he had been half that age – for a very difficult time,” David A. Baran, MD, a cardiologist from Sentara Advanced Heart Failure Center, Norfolk, Va., who studies transplant immunology, said in an interview.
“This is in some ways like the original heart transplant in 1967, when the ability to do the surgery evolved before understanding of the immunosuppression needed. Four or 5 years later, heart transplantation almost died out, before the development of better immunosuppressants like cyclosporine and later tacrolimus,” Dr. Baran said.
“The current age, when we use less immunosuppression than ever, is based on 30 years of progressive success,” he noted. This landmark xenotransplantation “basically turns back the clock to a time when the intensity of immunosuppression by definition had to be extremely high, because we really didn’t know what to expect.”
Emerging role of xeno-organs
Xenotransplantation has been touted as potential strategy for expanding the pool of organs available for transplantation. Mr. Bennett’s “breakthrough surgery” takes the world “one step closer to solving the organ shortage crisis,” his surgeon, Dr. Griffith, announced soon after the procedure. “There are simply not enough donor human hearts available to meet the long list of potential recipients.”
But it’s not the only proposed approach. Measures could be taken, for example, to make more efficient use of the human organs that become available, partly by opening the field to additional less-than-ideal hearts and loosening regulatory mandates for projected graft survival.
“Every year, more than two-thirds of donor organs in the United States are discarded. So it’s not actually that we don’t have enough organs, it’s that we don’t have enough organs that people are willing to take,” Dr. Baran said. Still, it’s important to pursue all promising avenues, and “the genetic manipulation pathway is remarkable.”
But “honestly, organs such as kidneys probably make the most sense” for early study of xenotransplantation from pigs, he said. “The waiting list for kidneys is also very long, but if the kidney graft were to fail, the patient wouldn’t die. It would allow us to work out the immunosuppression without putting patients’ lives at risk.”
Often overlooked in assessments of organ demand, Dr. West said, is that “a lot of patients who could benefit from a transplant will never even be listed for a transplant.” It’s not clear why; perhaps they have multiple comorbidities, live too far from a transplant center, “or they’re too big or too small. Even if there were unlimited organs, you could never meet the needs of people who could benefit from transplantation.”
So even if more available donor organs were used, she said, there would still be a gap that xenotransplantation could help fill. “I’m very much in favor of research that allows us to continue to try to find a pathway to xenotransplantation. I think it’s critically important.”
Unquestionably, “we now need to have a dialogue to entertain how a technology like this, using modern medicine with gene editing, is really going to be utilized,” Dr. Mehra said. The Bennett case “does open up the field, but it also raises caution.” There should be broad participation to move the field forward, “coordinated through either societies or nationally allocated advisory committees that oversee the movement of this technology, to the next step.”
Ideally, that next step “would be to do a safety clinical trial in the right patient,” he said. “And the right patient, by definition, would be one who does not have a life-prolonging option, either mechanical circulatory support or allograft transplantation. That would be the goal.”
Dr. Mehra has reported receiving payments to his institution from Abbott for consulting; consulting fees from Janssen, Mesoblast, Broadview Ventures, Natera, Paragonix, Moderna, and the Baim Institute for Clinical Research; and serving on a scientific advisory board NuPulseCV, Leviticus, and FineHeart. Dr. Baran disclosed consulting for Getinge and LivaNova; speaking for Pfizer; and serving on trial steering committees for CareDx and Procyrion, all unrelated to xenotransplantation. Dr. West has declared no relevant conflicts.
A version of this article first appeared on Medscape.com.
The genetically altered pig’s heart “worked like a rock star, beautifully functioning,” the surgeon who performed the pioneering Jan. 7 xenotransplant procedure said in a press statement on the death of the patient, David Bennett Sr.
“He wasn’t able to overcome what turned out to be devastating – the debilitation from his previous period of heart failure, which was extreme,” said Bartley P. Griffith, MD, clinical director of the cardiac xenotransplantation program at the University of Maryland, Baltimore.
Representatives of the institution aren’t offering many details on the cause of Mr. Bennett’s death on March 8, 60 days after his operation, but said they will elaborate when their findings are formally published. But their comments seem to downplay the unique nature of the implanted heart itself as a culprit and instead implicate the patient’s diminished overall clinical condition and what grew into an ongoing battle with infections.
The 57-year-old Bennett, bedridden with end-stage heart failure, judged a poor candidate for a ventricular assist device, and on extracorporeal membrane oxygenation (ECMO), reportedly was offered the extraordinary surgery after being turned down for a conventional transplant at several major centers.
“Until day 45 or 50, he was doing very well,” Muhammad M. Mohiuddin, MD, the xenotransplantation program’s scientific director, observed in the statement. But infections soon took advantage of his hobbled immune system.
Given his “preexisting condition and how frail his body was,” Dr. Mohiuddin said, “we were having difficulty maintaining a balance between his immunosuppression and controlling his infection.” Mr. Bennett went into multiple organ failure and “I think that resulted in his passing away.”
Beyond wildest dreams
The surgeons confidently framed Mr. Bennett’s experience as a milestone for heart xenotransplantation. “The demonstration that it was possible, beyond the wildest dreams of most people in the field, even, at this point – that we were able to take a genetically engineered organ and watch it function flawlessly for 9 weeks – is pretty positive in terms of the potential of this therapy,” Dr. Griffith said.
But enough questions linger that others were more circumspect, even as they praised the accomplishment. “There’s no question that this is a historic event,” Mandeep R. Mehra, MD, of Harvard Medical School, and director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital, both in Boston, said in an interview.
Still, “I don’t think we should just conclude that it was the patient’s frailty or death from infection,” Dr. Mehra said. With so few details available, “I would be very careful in prematurely concluding that the problem did not reside with the heart but with the patient. We cannot be sure.”
For example, he noted, “6 to 8 weeks is right around the time when some cardiac complications, like accelerated forms of vasculopathy, could become evident.” Immune-mediated cardiac allograft vasculopathy is a common cause of heart transplant failure.
Or, “it could as easily have been the fact that immunosuppression was modified at 6 to 7 weeks in response to potential infection, which could have led to a cardiac compromise,” Dr. Mehra said. “We just don’t know.”
“It’s really important that this be reported in a scientifically accurate way, because we will all learn from this,” Lori J. West, MD, DPhil, said in an interview.
Little seems to be known for sure about the actual cause of death, “but the fact there was not hyperacute rejection is itself a big step forward. And we know, at least from the limited information we have, that it did not occur,” observed Dr. West, who directs the Alberta Transplant Institute, Edmonton, and the Canadian Donation and Transplantation Research Program. She is a professor of pediatrics with adjunct positions in the departments of surgery and microbiology/immunology.
Dr. West also sees Mr. Bennett’s struggle with infections and adjustments to his unique immunosuppressive regimen, at least as characterized by his care team, as in line with the experience of many heart transplant recipients facing the same threat.
“We already walk this tightrope with every transplant patient,” she said. Typically, they’re put on a somewhat standardized immunosuppressant regimen, “and then we modify it a bit, either increasing or decreasing it, depending on the posttransplant course.” The regimen can become especially intense in response to new signs of rejection, “and you know that that’s going to have an impact on susceptibility to all kinds of infections.”
Full circle
The porcine heart was protected along two fronts against assault from Mr. Bennett’s immune system and other inhospitable aspects of his physiology, either of which could also have been obstacles to success: Genetic modification (Revivicor) of the pig that provided the heart, and a singularly aggressive antirejection drug regimen for the patient.
The knockout of three genes targeting specific porcine cell-surface carbohydrates that provoke a strong human antibody response reportedly averted a hyperacute rejection response that would have caused the graft to fail almost immediately.
Other genetic manipulations, some using CRISPR technology, silenced genes encoded for porcine endogenous retroviruses. Others were aimed at controlling myocardial growth and stemming graft microangiopathy.
Mr. Bennett himself was treated with powerful immunosuppressants, including an investigational anti-CD40 monoclonal antibody (KPL-404, Kiniksa Pharmaceuticals) that, according to UMSOM, inhibits a well-recognized pathway critical to B-cell proliferation, T-cell activation, and antibody production.
“I suspect the patient may not have had rejection, but unfortunately, that intense immunosuppression really set him up – even if he had been half that age – for a very difficult time,” David A. Baran, MD, a cardiologist from Sentara Advanced Heart Failure Center, Norfolk, Va., who studies transplant immunology, said in an interview.
“This is in some ways like the original heart transplant in 1967, when the ability to do the surgery evolved before understanding of the immunosuppression needed. Four or 5 years later, heart transplantation almost died out, before the development of better immunosuppressants like cyclosporine and later tacrolimus,” Dr. Baran said.
“The current age, when we use less immunosuppression than ever, is based on 30 years of progressive success,” he noted. This landmark xenotransplantation “basically turns back the clock to a time when the intensity of immunosuppression by definition had to be extremely high, because we really didn’t know what to expect.”
Emerging role of xeno-organs
Xenotransplantation has been touted as potential strategy for expanding the pool of organs available for transplantation. Mr. Bennett’s “breakthrough surgery” takes the world “one step closer to solving the organ shortage crisis,” his surgeon, Dr. Griffith, announced soon after the procedure. “There are simply not enough donor human hearts available to meet the long list of potential recipients.”
But it’s not the only proposed approach. Measures could be taken, for example, to make more efficient use of the human organs that become available, partly by opening the field to additional less-than-ideal hearts and loosening regulatory mandates for projected graft survival.
“Every year, more than two-thirds of donor organs in the United States are discarded. So it’s not actually that we don’t have enough organs, it’s that we don’t have enough organs that people are willing to take,” Dr. Baran said. Still, it’s important to pursue all promising avenues, and “the genetic manipulation pathway is remarkable.”
But “honestly, organs such as kidneys probably make the most sense” for early study of xenotransplantation from pigs, he said. “The waiting list for kidneys is also very long, but if the kidney graft were to fail, the patient wouldn’t die. It would allow us to work out the immunosuppression without putting patients’ lives at risk.”
Often overlooked in assessments of organ demand, Dr. West said, is that “a lot of patients who could benefit from a transplant will never even be listed for a transplant.” It’s not clear why; perhaps they have multiple comorbidities, live too far from a transplant center, “or they’re too big or too small. Even if there were unlimited organs, you could never meet the needs of people who could benefit from transplantation.”
So even if more available donor organs were used, she said, there would still be a gap that xenotransplantation could help fill. “I’m very much in favor of research that allows us to continue to try to find a pathway to xenotransplantation. I think it’s critically important.”
Unquestionably, “we now need to have a dialogue to entertain how a technology like this, using modern medicine with gene editing, is really going to be utilized,” Dr. Mehra said. The Bennett case “does open up the field, but it also raises caution.” There should be broad participation to move the field forward, “coordinated through either societies or nationally allocated advisory committees that oversee the movement of this technology, to the next step.”
Ideally, that next step “would be to do a safety clinical trial in the right patient,” he said. “And the right patient, by definition, would be one who does not have a life-prolonging option, either mechanical circulatory support or allograft transplantation. That would be the goal.”
Dr. Mehra has reported receiving payments to his institution from Abbott for consulting; consulting fees from Janssen, Mesoblast, Broadview Ventures, Natera, Paragonix, Moderna, and the Baim Institute for Clinical Research; and serving on a scientific advisory board NuPulseCV, Leviticus, and FineHeart. Dr. Baran disclosed consulting for Getinge and LivaNova; speaking for Pfizer; and serving on trial steering committees for CareDx and Procyrion, all unrelated to xenotransplantation. Dr. West has declared no relevant conflicts.
A version of this article first appeared on Medscape.com.
The genetically altered pig’s heart “worked like a rock star, beautifully functioning,” the surgeon who performed the pioneering Jan. 7 xenotransplant procedure said in a press statement on the death of the patient, David Bennett Sr.
“He wasn’t able to overcome what turned out to be devastating – the debilitation from his previous period of heart failure, which was extreme,” said Bartley P. Griffith, MD, clinical director of the cardiac xenotransplantation program at the University of Maryland, Baltimore.
Representatives of the institution aren’t offering many details on the cause of Mr. Bennett’s death on March 8, 60 days after his operation, but said they will elaborate when their findings are formally published. But their comments seem to downplay the unique nature of the implanted heart itself as a culprit and instead implicate the patient’s diminished overall clinical condition and what grew into an ongoing battle with infections.
The 57-year-old Bennett, bedridden with end-stage heart failure, judged a poor candidate for a ventricular assist device, and on extracorporeal membrane oxygenation (ECMO), reportedly was offered the extraordinary surgery after being turned down for a conventional transplant at several major centers.
“Until day 45 or 50, he was doing very well,” Muhammad M. Mohiuddin, MD, the xenotransplantation program’s scientific director, observed in the statement. But infections soon took advantage of his hobbled immune system.
Given his “preexisting condition and how frail his body was,” Dr. Mohiuddin said, “we were having difficulty maintaining a balance between his immunosuppression and controlling his infection.” Mr. Bennett went into multiple organ failure and “I think that resulted in his passing away.”
Beyond wildest dreams
The surgeons confidently framed Mr. Bennett’s experience as a milestone for heart xenotransplantation. “The demonstration that it was possible, beyond the wildest dreams of most people in the field, even, at this point – that we were able to take a genetically engineered organ and watch it function flawlessly for 9 weeks – is pretty positive in terms of the potential of this therapy,” Dr. Griffith said.
But enough questions linger that others were more circumspect, even as they praised the accomplishment. “There’s no question that this is a historic event,” Mandeep R. Mehra, MD, of Harvard Medical School, and director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital, both in Boston, said in an interview.
Still, “I don’t think we should just conclude that it was the patient’s frailty or death from infection,” Dr. Mehra said. With so few details available, “I would be very careful in prematurely concluding that the problem did not reside with the heart but with the patient. We cannot be sure.”
For example, he noted, “6 to 8 weeks is right around the time when some cardiac complications, like accelerated forms of vasculopathy, could become evident.” Immune-mediated cardiac allograft vasculopathy is a common cause of heart transplant failure.
Or, “it could as easily have been the fact that immunosuppression was modified at 6 to 7 weeks in response to potential infection, which could have led to a cardiac compromise,” Dr. Mehra said. “We just don’t know.”
“It’s really important that this be reported in a scientifically accurate way, because we will all learn from this,” Lori J. West, MD, DPhil, said in an interview.
Little seems to be known for sure about the actual cause of death, “but the fact there was not hyperacute rejection is itself a big step forward. And we know, at least from the limited information we have, that it did not occur,” observed Dr. West, who directs the Alberta Transplant Institute, Edmonton, and the Canadian Donation and Transplantation Research Program. She is a professor of pediatrics with adjunct positions in the departments of surgery and microbiology/immunology.
Dr. West also sees Mr. Bennett’s struggle with infections and adjustments to his unique immunosuppressive regimen, at least as characterized by his care team, as in line with the experience of many heart transplant recipients facing the same threat.
“We already walk this tightrope with every transplant patient,” she said. Typically, they’re put on a somewhat standardized immunosuppressant regimen, “and then we modify it a bit, either increasing or decreasing it, depending on the posttransplant course.” The regimen can become especially intense in response to new signs of rejection, “and you know that that’s going to have an impact on susceptibility to all kinds of infections.”
Full circle
The porcine heart was protected along two fronts against assault from Mr. Bennett’s immune system and other inhospitable aspects of his physiology, either of which could also have been obstacles to success: Genetic modification (Revivicor) of the pig that provided the heart, and a singularly aggressive antirejection drug regimen for the patient.
The knockout of three genes targeting specific porcine cell-surface carbohydrates that provoke a strong human antibody response reportedly averted a hyperacute rejection response that would have caused the graft to fail almost immediately.
Other genetic manipulations, some using CRISPR technology, silenced genes encoded for porcine endogenous retroviruses. Others were aimed at controlling myocardial growth and stemming graft microangiopathy.
Mr. Bennett himself was treated with powerful immunosuppressants, including an investigational anti-CD40 monoclonal antibody (KPL-404, Kiniksa Pharmaceuticals) that, according to UMSOM, inhibits a well-recognized pathway critical to B-cell proliferation, T-cell activation, and antibody production.
“I suspect the patient may not have had rejection, but unfortunately, that intense immunosuppression really set him up – even if he had been half that age – for a very difficult time,” David A. Baran, MD, a cardiologist from Sentara Advanced Heart Failure Center, Norfolk, Va., who studies transplant immunology, said in an interview.
“This is in some ways like the original heart transplant in 1967, when the ability to do the surgery evolved before understanding of the immunosuppression needed. Four or 5 years later, heart transplantation almost died out, before the development of better immunosuppressants like cyclosporine and later tacrolimus,” Dr. Baran said.
“The current age, when we use less immunosuppression than ever, is based on 30 years of progressive success,” he noted. This landmark xenotransplantation “basically turns back the clock to a time when the intensity of immunosuppression by definition had to be extremely high, because we really didn’t know what to expect.”
Emerging role of xeno-organs
Xenotransplantation has been touted as potential strategy for expanding the pool of organs available for transplantation. Mr. Bennett’s “breakthrough surgery” takes the world “one step closer to solving the organ shortage crisis,” his surgeon, Dr. Griffith, announced soon after the procedure. “There are simply not enough donor human hearts available to meet the long list of potential recipients.”
But it’s not the only proposed approach. Measures could be taken, for example, to make more efficient use of the human organs that become available, partly by opening the field to additional less-than-ideal hearts and loosening regulatory mandates for projected graft survival.
“Every year, more than two-thirds of donor organs in the United States are discarded. So it’s not actually that we don’t have enough organs, it’s that we don’t have enough organs that people are willing to take,” Dr. Baran said. Still, it’s important to pursue all promising avenues, and “the genetic manipulation pathway is remarkable.”
But “honestly, organs such as kidneys probably make the most sense” for early study of xenotransplantation from pigs, he said. “The waiting list for kidneys is also very long, but if the kidney graft were to fail, the patient wouldn’t die. It would allow us to work out the immunosuppression without putting patients’ lives at risk.”
Often overlooked in assessments of organ demand, Dr. West said, is that “a lot of patients who could benefit from a transplant will never even be listed for a transplant.” It’s not clear why; perhaps they have multiple comorbidities, live too far from a transplant center, “or they’re too big or too small. Even if there were unlimited organs, you could never meet the needs of people who could benefit from transplantation.”
So even if more available donor organs were used, she said, there would still be a gap that xenotransplantation could help fill. “I’m very much in favor of research that allows us to continue to try to find a pathway to xenotransplantation. I think it’s critically important.”
Unquestionably, “we now need to have a dialogue to entertain how a technology like this, using modern medicine with gene editing, is really going to be utilized,” Dr. Mehra said. The Bennett case “does open up the field, but it also raises caution.” There should be broad participation to move the field forward, “coordinated through either societies or nationally allocated advisory committees that oversee the movement of this technology, to the next step.”
Ideally, that next step “would be to do a safety clinical trial in the right patient,” he said. “And the right patient, by definition, would be one who does not have a life-prolonging option, either mechanical circulatory support or allograft transplantation. That would be the goal.”
Dr. Mehra has reported receiving payments to his institution from Abbott for consulting; consulting fees from Janssen, Mesoblast, Broadview Ventures, Natera, Paragonix, Moderna, and the Baim Institute for Clinical Research; and serving on a scientific advisory board NuPulseCV, Leviticus, and FineHeart. Dr. Baran disclosed consulting for Getinge and LivaNova; speaking for Pfizer; and serving on trial steering committees for CareDx and Procyrion, all unrelated to xenotransplantation. Dr. West has declared no relevant conflicts.
A version of this article first appeared on Medscape.com.
No COVID vax, no transplant: Unfair or good medicine?
Right now, more than 106,600 people in the United States are on the national transplant waiting list, each hoping to hear soon that a lung, kidney, heart, or other vital organ has been found for them. It’s the promise not just of a new organ, but a new life.
Well before they are placed on that list, transplant candidates, as they’re known, are evaluated with a battery of tests and exams to be sure they are infection free, their other organs are healthy, and that all their vaccinations are up to date.
In January, a 31-year-old Boston father of two declined to get the COVID-19 vaccine, and Brigham and Women’s Hospital officials removed him from the heart transplant waiting list. And in North Carolina, a 38-year-old man in need of a kidney transplant said he, too, was denied the organ when he declined to get the vaccination.
Those are just two of the most recent cases. The decisions by the transplant centers to remove the candidates from the waiting list have set off a national debate among ethicists, family members, doctors, patients, and others.
On social media and in conversation, the question persists: Is removing them from the list unfair and cruel, or simply business as usual to keep the patient as healthy as possible and the transplant as successful as possible?
Two recent tweets sum up the debate.
“The people responsible for this should be charged with attempted homicide,” one Twitter user said, while another suggested that the more accurate way to headline the news about a transplant candidate refusing the COVID-19 vaccine would be: “Patient voluntarily forfeits donor organ.”
Doctors and ethics experts, as well as other patients on the waiting list, say it’s simply good medicine to require the COVID vaccine, along with a host of other pretransplant requirements.
Transplant protocols
“Transplant medicine has always been a strong promoter of vaccination,” said Silas Prescod Norman, MD, a clinical associate professor of nephrology and internal medicine at the University of Michigan, Ann Arbor. He is a kidney specialist who works in the university’s transplant clinic.
Requiring the COVID vaccine is in line with requirements to get numerous other vaccines, he said.“Promoting the COVID vaccine among our transplant candidates and recipients is just an extension of our usual practice.
“In transplantation, first and foremost is patient safety,” Dr. Norman said. “And we know that solid organ transplant patients are at substantially higher risk of contracting COVID than nontransplant patients.”
After the transplant, they are placed on immunosuppressant drugs, that weaken the immune system while also decreasing the body’s ability to reject the new organ.
“We know now, because there is good data about the vaccine to show that people who are on transplant medications are less likely to make detectable antibodies after vaccination,” said Dr. Norman, who’s also a medical adviser for the American Kidney Fund, a nonprofit that provides kidney health information and financial assistance for dialysis.
And this is not a surprise because of the immunosuppressive effects, he said. “So it only makes sense to get people vaccinated before transplantation.”
Researchers compared the cases of more than 17,000 people who had received organ transplants and were hospitalized from April to November 2020, either for COVID (1,682 of them) or other health issues. Those who had COVID were more likely to have complications and to die in the hospital than those who did not have it.
Vaccination guidelines, policies
Federal COVID-19 treatment guidelines from the National Institutes of Health state that transplant patients on immunosuppressant drugs used after the procedure should be considered at a higher risk of getting severe COVID if infected.
In a joint statement from the American Society of Transplant Surgeons, the American Society of Transplantation, and the International Society for Heart and Lung Transplantation, the organizations say they “strongly recommend that all eligible children and adult transplant candidates and recipients be vaccinated with a COVID-19 vaccine [and booster] that is approved or authorized in their jurisdiction. Whenever possible, vaccination should occur prior to transplantation.” Ideally, it should be completed at least 2 weeks before the transplant.
The organizations also “support the development of institutional policies regarding pretransplant vaccination. We believe that this is in the best interest of the transplant candidate, optimizing their chances of getting through the perioperative and posttransplant periods without severe COVID-19 disease, especially at times of greater infection prevalence.”
Officials at Brigham and Women’s Hospital, where the 31-year-old father was removed from the list, issued a statement that reads, in part: “Our Mass General Brigham health care system requires several [Centers for Disease Control and Prevention]-recommended vaccines, including the COVID-19 vaccine, and lifestyle behaviors for transplant candidates to create both the best chance for a successful operation and to optimize the patient’s survival after transplantation, given that their immune system is drastically suppressed. Patients are not active on the wait list without this.”
Ethics amid organ shortage
“Organs are scarce,” said Arthur L. Caplan, PhD, director of the division of medical ethics at New York University Langone Medical Center. That makes the goal of choosing the very best candidates for success even more crucial.
“You try to maximize the chance the organ will work,” he said. Pretransplant vaccination is one way.
The shortage is most severe for kidney transplants. In 2020, according to federal statistics, more than 91,000 kidney transplants were needed, but fewer than 23,000 were received. During 2021, 41,354 transplants were done, an increase of nearly 6% over the previous year. The total includes kidneys, hearts, lungs, and other organs, with kidneys accounting for more than 24,000 of the total.
Even with the rise in transplant numbers, supply does not meet demand. According to federal statistics, 17 people in the United States die each day waiting for an organ transplant. Every 9 minutes, someone is added to the waiting list.
“This isn’t and it shouldn’t be a fight about the COVID vaccine,” Dr. Caplan said. “This isn’t an issue about punishing non-COVID vaccinators. It’s deciding who is going to get a scarce organ.”
“A lot of people [opposed to removing the nonvaccinated from the list] think: ‘Oh, they are just killing those people who won’t take a COVID vaccine.’ That’s not what is going on.”
The transplant candidate must be in the best possible shape overall, Dr. Caplan and doctors agreed. Someone who is smoking, drinking heavily, or abusing drugs isn’t going to the top of the list either. And for other procedures, such as bariatric surgery or knee surgery, some patients are told first to lose weight before a surgeon will operate.
The worry about side effects from the vaccine, which some patients have cited as a concern, is misplaced, Dr. Caplan said. What transplant candidates who refuse the COVID vaccine may not be thinking about is that they are facing a serious operation and will be on numerous anti-rejection drugs, with side effects, after the surgery.
“So to be worried about the side effects of a COVID vaccine is irrational,” he said.
Transplants: The process
The patients who were recently removed from the transplant list could seek care and a transplant at an alternate center, said Anne Paschke, a spokesperson for the United Network for Organ Sharing, a nonprofit group that is under contract with the federal government and operates the national Organ Procurement and Transplantation Network (OPTN).
“Transplant hospitals decide which patients to add to the wait list based on their own criteria and medical judgment to create the best chance for a positive transplant outcome,” she said. That’s done with the understanding that patients will help with their medical care.
So, if one program won’t accept a patient, another may. But, if a patient turned down at one center due to refusing to get the COVID vaccine tries another center, the requirements at that hospital may be the same, she said.
OPTN maintains a list of transplant centers. As of Jan. 28, there were 251 transplant centers, according to UNOS, which manages the waiting list, matches donors and recipients, and strives for equity, among other duties.
Pretransplant refusers not typical
“The cases we are seeing are outliers,” Dr. Caplan said of the handful of known candidates who have refused the vaccine. Most ask their doctor exactly what they need to do to live and follow those instructions.
Dr. Norman agreed. Most of the kidney patients he cares for who are hoping for a transplant have been on dialysis, “which they do not like. They are doing whatever they can to make sure they don’t go back on dialysis. As a group, they tend to be very adherent, very safety conscious because they understand their risk and they understand the gift they have received [or will receive] through transplantation. They want to do everything they can to respect and protect that gift.”
Not surprisingly, some on the transplant list who are vaccinated have strong opinions about those who refuse to get the vaccine. Dana J. Ufkes, 61, a Seattle realtor, has been on the kidney transplant list – this time – since 2003, hoping for her third transplant. When asked if potential recipients should be removed from the list if they refuse the COVID vaccine, her answer was immediate: “Absolutely.”
At age 17, Ms. Ufkes got a serious kidney infection that went undiagnosed and untreated. Her kidney health worsened, and she needed a transplant. She got her first one in 1986, then again in 1992.
“They last longer than they used to,” she said. But not forever. (According to the American Kidney Fund, transplants from a living kidney donor last about 15-20 years; from a deceased donor, 10-15.)
The decision to decline the vaccine is, of course, each person’s choice, Ms. Ufkes said. But “if they don’t want to be vaccinated [and still want to be on the list], I think that’s BS.”
Citing the lack of organs, “it’s not like they are handing these out like jellybeans.”
A version of this article first appeared on WebMD.com.
Right now, more than 106,600 people in the United States are on the national transplant waiting list, each hoping to hear soon that a lung, kidney, heart, or other vital organ has been found for them. It’s the promise not just of a new organ, but a new life.
Well before they are placed on that list, transplant candidates, as they’re known, are evaluated with a battery of tests and exams to be sure they are infection free, their other organs are healthy, and that all their vaccinations are up to date.
In January, a 31-year-old Boston father of two declined to get the COVID-19 vaccine, and Brigham and Women’s Hospital officials removed him from the heart transplant waiting list. And in North Carolina, a 38-year-old man in need of a kidney transplant said he, too, was denied the organ when he declined to get the vaccination.
Those are just two of the most recent cases. The decisions by the transplant centers to remove the candidates from the waiting list have set off a national debate among ethicists, family members, doctors, patients, and others.
On social media and in conversation, the question persists: Is removing them from the list unfair and cruel, or simply business as usual to keep the patient as healthy as possible and the transplant as successful as possible?
Two recent tweets sum up the debate.
“The people responsible for this should be charged with attempted homicide,” one Twitter user said, while another suggested that the more accurate way to headline the news about a transplant candidate refusing the COVID-19 vaccine would be: “Patient voluntarily forfeits donor organ.”
Doctors and ethics experts, as well as other patients on the waiting list, say it’s simply good medicine to require the COVID vaccine, along with a host of other pretransplant requirements.
Transplant protocols
“Transplant medicine has always been a strong promoter of vaccination,” said Silas Prescod Norman, MD, a clinical associate professor of nephrology and internal medicine at the University of Michigan, Ann Arbor. He is a kidney specialist who works in the university’s transplant clinic.
Requiring the COVID vaccine is in line with requirements to get numerous other vaccines, he said.“Promoting the COVID vaccine among our transplant candidates and recipients is just an extension of our usual practice.
“In transplantation, first and foremost is patient safety,” Dr. Norman said. “And we know that solid organ transplant patients are at substantially higher risk of contracting COVID than nontransplant patients.”
After the transplant, they are placed on immunosuppressant drugs, that weaken the immune system while also decreasing the body’s ability to reject the new organ.
“We know now, because there is good data about the vaccine to show that people who are on transplant medications are less likely to make detectable antibodies after vaccination,” said Dr. Norman, who’s also a medical adviser for the American Kidney Fund, a nonprofit that provides kidney health information and financial assistance for dialysis.
And this is not a surprise because of the immunosuppressive effects, he said. “So it only makes sense to get people vaccinated before transplantation.”
Researchers compared the cases of more than 17,000 people who had received organ transplants and were hospitalized from April to November 2020, either for COVID (1,682 of them) or other health issues. Those who had COVID were more likely to have complications and to die in the hospital than those who did not have it.
Vaccination guidelines, policies
Federal COVID-19 treatment guidelines from the National Institutes of Health state that transplant patients on immunosuppressant drugs used after the procedure should be considered at a higher risk of getting severe COVID if infected.
In a joint statement from the American Society of Transplant Surgeons, the American Society of Transplantation, and the International Society for Heart and Lung Transplantation, the organizations say they “strongly recommend that all eligible children and adult transplant candidates and recipients be vaccinated with a COVID-19 vaccine [and booster] that is approved or authorized in their jurisdiction. Whenever possible, vaccination should occur prior to transplantation.” Ideally, it should be completed at least 2 weeks before the transplant.
The organizations also “support the development of institutional policies regarding pretransplant vaccination. We believe that this is in the best interest of the transplant candidate, optimizing their chances of getting through the perioperative and posttransplant periods without severe COVID-19 disease, especially at times of greater infection prevalence.”
Officials at Brigham and Women’s Hospital, where the 31-year-old father was removed from the list, issued a statement that reads, in part: “Our Mass General Brigham health care system requires several [Centers for Disease Control and Prevention]-recommended vaccines, including the COVID-19 vaccine, and lifestyle behaviors for transplant candidates to create both the best chance for a successful operation and to optimize the patient’s survival after transplantation, given that their immune system is drastically suppressed. Patients are not active on the wait list without this.”
Ethics amid organ shortage
“Organs are scarce,” said Arthur L. Caplan, PhD, director of the division of medical ethics at New York University Langone Medical Center. That makes the goal of choosing the very best candidates for success even more crucial.
“You try to maximize the chance the organ will work,” he said. Pretransplant vaccination is one way.
The shortage is most severe for kidney transplants. In 2020, according to federal statistics, more than 91,000 kidney transplants were needed, but fewer than 23,000 were received. During 2021, 41,354 transplants were done, an increase of nearly 6% over the previous year. The total includes kidneys, hearts, lungs, and other organs, with kidneys accounting for more than 24,000 of the total.
Even with the rise in transplant numbers, supply does not meet demand. According to federal statistics, 17 people in the United States die each day waiting for an organ transplant. Every 9 minutes, someone is added to the waiting list.
“This isn’t and it shouldn’t be a fight about the COVID vaccine,” Dr. Caplan said. “This isn’t an issue about punishing non-COVID vaccinators. It’s deciding who is going to get a scarce organ.”
“A lot of people [opposed to removing the nonvaccinated from the list] think: ‘Oh, they are just killing those people who won’t take a COVID vaccine.’ That’s not what is going on.”
The transplant candidate must be in the best possible shape overall, Dr. Caplan and doctors agreed. Someone who is smoking, drinking heavily, or abusing drugs isn’t going to the top of the list either. And for other procedures, such as bariatric surgery or knee surgery, some patients are told first to lose weight before a surgeon will operate.
The worry about side effects from the vaccine, which some patients have cited as a concern, is misplaced, Dr. Caplan said. What transplant candidates who refuse the COVID vaccine may not be thinking about is that they are facing a serious operation and will be on numerous anti-rejection drugs, with side effects, after the surgery.
“So to be worried about the side effects of a COVID vaccine is irrational,” he said.
Transplants: The process
The patients who were recently removed from the transplant list could seek care and a transplant at an alternate center, said Anne Paschke, a spokesperson for the United Network for Organ Sharing, a nonprofit group that is under contract with the federal government and operates the national Organ Procurement and Transplantation Network (OPTN).
“Transplant hospitals decide which patients to add to the wait list based on their own criteria and medical judgment to create the best chance for a positive transplant outcome,” she said. That’s done with the understanding that patients will help with their medical care.
So, if one program won’t accept a patient, another may. But, if a patient turned down at one center due to refusing to get the COVID vaccine tries another center, the requirements at that hospital may be the same, she said.
OPTN maintains a list of transplant centers. As of Jan. 28, there were 251 transplant centers, according to UNOS, which manages the waiting list, matches donors and recipients, and strives for equity, among other duties.
Pretransplant refusers not typical
“The cases we are seeing are outliers,” Dr. Caplan said of the handful of known candidates who have refused the vaccine. Most ask their doctor exactly what they need to do to live and follow those instructions.
Dr. Norman agreed. Most of the kidney patients he cares for who are hoping for a transplant have been on dialysis, “which they do not like. They are doing whatever they can to make sure they don’t go back on dialysis. As a group, they tend to be very adherent, very safety conscious because they understand their risk and they understand the gift they have received [or will receive] through transplantation. They want to do everything they can to respect and protect that gift.”
Not surprisingly, some on the transplant list who are vaccinated have strong opinions about those who refuse to get the vaccine. Dana J. Ufkes, 61, a Seattle realtor, has been on the kidney transplant list – this time – since 2003, hoping for her third transplant. When asked if potential recipients should be removed from the list if they refuse the COVID vaccine, her answer was immediate: “Absolutely.”
At age 17, Ms. Ufkes got a serious kidney infection that went undiagnosed and untreated. Her kidney health worsened, and she needed a transplant. She got her first one in 1986, then again in 1992.
“They last longer than they used to,” she said. But not forever. (According to the American Kidney Fund, transplants from a living kidney donor last about 15-20 years; from a deceased donor, 10-15.)
The decision to decline the vaccine is, of course, each person’s choice, Ms. Ufkes said. But “if they don’t want to be vaccinated [and still want to be on the list], I think that’s BS.”
Citing the lack of organs, “it’s not like they are handing these out like jellybeans.”
A version of this article first appeared on WebMD.com.
Right now, more than 106,600 people in the United States are on the national transplant waiting list, each hoping to hear soon that a lung, kidney, heart, or other vital organ has been found for them. It’s the promise not just of a new organ, but a new life.
Well before they are placed on that list, transplant candidates, as they’re known, are evaluated with a battery of tests and exams to be sure they are infection free, their other organs are healthy, and that all their vaccinations are up to date.
In January, a 31-year-old Boston father of two declined to get the COVID-19 vaccine, and Brigham and Women’s Hospital officials removed him from the heart transplant waiting list. And in North Carolina, a 38-year-old man in need of a kidney transplant said he, too, was denied the organ when he declined to get the vaccination.
Those are just two of the most recent cases. The decisions by the transplant centers to remove the candidates from the waiting list have set off a national debate among ethicists, family members, doctors, patients, and others.
On social media and in conversation, the question persists: Is removing them from the list unfair and cruel, or simply business as usual to keep the patient as healthy as possible and the transplant as successful as possible?
Two recent tweets sum up the debate.
“The people responsible for this should be charged with attempted homicide,” one Twitter user said, while another suggested that the more accurate way to headline the news about a transplant candidate refusing the COVID-19 vaccine would be: “Patient voluntarily forfeits donor organ.”
Doctors and ethics experts, as well as other patients on the waiting list, say it’s simply good medicine to require the COVID vaccine, along with a host of other pretransplant requirements.
Transplant protocols
“Transplant medicine has always been a strong promoter of vaccination,” said Silas Prescod Norman, MD, a clinical associate professor of nephrology and internal medicine at the University of Michigan, Ann Arbor. He is a kidney specialist who works in the university’s transplant clinic.
Requiring the COVID vaccine is in line with requirements to get numerous other vaccines, he said.“Promoting the COVID vaccine among our transplant candidates and recipients is just an extension of our usual practice.
“In transplantation, first and foremost is patient safety,” Dr. Norman said. “And we know that solid organ transplant patients are at substantially higher risk of contracting COVID than nontransplant patients.”
After the transplant, they are placed on immunosuppressant drugs, that weaken the immune system while also decreasing the body’s ability to reject the new organ.
“We know now, because there is good data about the vaccine to show that people who are on transplant medications are less likely to make detectable antibodies after vaccination,” said Dr. Norman, who’s also a medical adviser for the American Kidney Fund, a nonprofit that provides kidney health information and financial assistance for dialysis.
And this is not a surprise because of the immunosuppressive effects, he said. “So it only makes sense to get people vaccinated before transplantation.”
Researchers compared the cases of more than 17,000 people who had received organ transplants and were hospitalized from April to November 2020, either for COVID (1,682 of them) or other health issues. Those who had COVID were more likely to have complications and to die in the hospital than those who did not have it.
Vaccination guidelines, policies
Federal COVID-19 treatment guidelines from the National Institutes of Health state that transplant patients on immunosuppressant drugs used after the procedure should be considered at a higher risk of getting severe COVID if infected.
In a joint statement from the American Society of Transplant Surgeons, the American Society of Transplantation, and the International Society for Heart and Lung Transplantation, the organizations say they “strongly recommend that all eligible children and adult transplant candidates and recipients be vaccinated with a COVID-19 vaccine [and booster] that is approved or authorized in their jurisdiction. Whenever possible, vaccination should occur prior to transplantation.” Ideally, it should be completed at least 2 weeks before the transplant.
The organizations also “support the development of institutional policies regarding pretransplant vaccination. We believe that this is in the best interest of the transplant candidate, optimizing their chances of getting through the perioperative and posttransplant periods without severe COVID-19 disease, especially at times of greater infection prevalence.”
Officials at Brigham and Women’s Hospital, where the 31-year-old father was removed from the list, issued a statement that reads, in part: “Our Mass General Brigham health care system requires several [Centers for Disease Control and Prevention]-recommended vaccines, including the COVID-19 vaccine, and lifestyle behaviors for transplant candidates to create both the best chance for a successful operation and to optimize the patient’s survival after transplantation, given that their immune system is drastically suppressed. Patients are not active on the wait list without this.”
Ethics amid organ shortage
“Organs are scarce,” said Arthur L. Caplan, PhD, director of the division of medical ethics at New York University Langone Medical Center. That makes the goal of choosing the very best candidates for success even more crucial.
“You try to maximize the chance the organ will work,” he said. Pretransplant vaccination is one way.
The shortage is most severe for kidney transplants. In 2020, according to federal statistics, more than 91,000 kidney transplants were needed, but fewer than 23,000 were received. During 2021, 41,354 transplants were done, an increase of nearly 6% over the previous year. The total includes kidneys, hearts, lungs, and other organs, with kidneys accounting for more than 24,000 of the total.
Even with the rise in transplant numbers, supply does not meet demand. According to federal statistics, 17 people in the United States die each day waiting for an organ transplant. Every 9 minutes, someone is added to the waiting list.
“This isn’t and it shouldn’t be a fight about the COVID vaccine,” Dr. Caplan said. “This isn’t an issue about punishing non-COVID vaccinators. It’s deciding who is going to get a scarce organ.”
“A lot of people [opposed to removing the nonvaccinated from the list] think: ‘Oh, they are just killing those people who won’t take a COVID vaccine.’ That’s not what is going on.”
The transplant candidate must be in the best possible shape overall, Dr. Caplan and doctors agreed. Someone who is smoking, drinking heavily, or abusing drugs isn’t going to the top of the list either. And for other procedures, such as bariatric surgery or knee surgery, some patients are told first to lose weight before a surgeon will operate.
The worry about side effects from the vaccine, which some patients have cited as a concern, is misplaced, Dr. Caplan said. What transplant candidates who refuse the COVID vaccine may not be thinking about is that they are facing a serious operation and will be on numerous anti-rejection drugs, with side effects, after the surgery.
“So to be worried about the side effects of a COVID vaccine is irrational,” he said.
Transplants: The process
The patients who were recently removed from the transplant list could seek care and a transplant at an alternate center, said Anne Paschke, a spokesperson for the United Network for Organ Sharing, a nonprofit group that is under contract with the federal government and operates the national Organ Procurement and Transplantation Network (OPTN).
“Transplant hospitals decide which patients to add to the wait list based on their own criteria and medical judgment to create the best chance for a positive transplant outcome,” she said. That’s done with the understanding that patients will help with their medical care.
So, if one program won’t accept a patient, another may. But, if a patient turned down at one center due to refusing to get the COVID vaccine tries another center, the requirements at that hospital may be the same, she said.
OPTN maintains a list of transplant centers. As of Jan. 28, there were 251 transplant centers, according to UNOS, which manages the waiting list, matches donors and recipients, and strives for equity, among other duties.
Pretransplant refusers not typical
“The cases we are seeing are outliers,” Dr. Caplan said of the handful of known candidates who have refused the vaccine. Most ask their doctor exactly what they need to do to live and follow those instructions.
Dr. Norman agreed. Most of the kidney patients he cares for who are hoping for a transplant have been on dialysis, “which they do not like. They are doing whatever they can to make sure they don’t go back on dialysis. As a group, they tend to be very adherent, very safety conscious because they understand their risk and they understand the gift they have received [or will receive] through transplantation. They want to do everything they can to respect and protect that gift.”
Not surprisingly, some on the transplant list who are vaccinated have strong opinions about those who refuse to get the vaccine. Dana J. Ufkes, 61, a Seattle realtor, has been on the kidney transplant list – this time – since 2003, hoping for her third transplant. When asked if potential recipients should be removed from the list if they refuse the COVID vaccine, her answer was immediate: “Absolutely.”
At age 17, Ms. Ufkes got a serious kidney infection that went undiagnosed and untreated. Her kidney health worsened, and she needed a transplant. She got her first one in 1986, then again in 1992.
“They last longer than they used to,” she said. But not forever. (According to the American Kidney Fund, transplants from a living kidney donor last about 15-20 years; from a deceased donor, 10-15.)
The decision to decline the vaccine is, of course, each person’s choice, Ms. Ufkes said. But “if they don’t want to be vaccinated [and still want to be on the list], I think that’s BS.”
Citing the lack of organs, “it’s not like they are handing these out like jellybeans.”
A version of this article first appeared on WebMD.com.
What does a pig-to-human heart transplant mean for medicine?
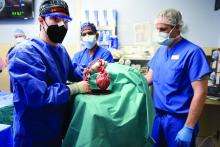
Scientific achievements usually raise big new questions, and the remarkable surgery that took place on Jan. 7, when Maryland resident David Bennett was transplanted with a genetically modified heart from a pig, has been no different.
The 57-year-old with end-stage heart failure had been repeatedly turned down for a standard transplant and was judged a poor candidate for a ventricular assist device. Now his new heart is beating soundly and apparently accepted by his immune system as Mr. Bennett, his physicians at the University of Maryland where the procedure took place, and indeed the world set out on a journey with far more unknowns than knowns.
“I think even just a couple of years ago, people felt that xenotransplantation for the heart and other organs was still a long way off. And it seems like it’s started to move very quickly,” Larry A. Allen, MD, University of Colorado, Aurora, said in an interview.
Demand for donor hearts far outstrips supply, and despite advances in the development of ventricular assist pumps and artificial hearts, “there are still significant limitations to them in terms of clotting, stroke, and infection. We’ve seen the use of those devices plateau,” Dr. Allen said. “So, the concept of a nonhuman source of organs is exciting and very much in need, if people can get it to work.”
“I really credit the surgeons at the University of Maryland for courageous clinical work and a brilliant scientific innovation,” Clyde W. Yancy, MD, MSc, Northwestern University, Chicago, said in an interview. “But it’s always in the implementation that we have to hold our breath.” Heart xenotransplantation is an old idea that “has never before been successful,” he said. And standard heart transplantation has set a high bar, with a 1-year survival of about 90% and low 1-year risk for rejection. Whether the new procedure can meet that standard is unknown, as is its potential for complications, such as chronic rejection or cancers due to long-term immunosuppression. Those are “major questions requiring more time and careful follow-up.”
‘Still a nascent technology’
“This is an exciting and courageous step forward in heart transplantation, and kudos to the team at the University of Maryland,” said Mandeep R. Mehra, MD, Brigham and Woman’s Hospital, Boston. But “there are many challenges here.”
The procedure’s 10 gene modifications were reportedly aimed at preventing hyperacute rejection of the heart and its excessive growth after transplantation, and making the organ less immunogenic, Dr. Mehra said in an interview. But even if those goals are met, could the same changes potentially impede the heart’s adaptation to human physiology, such as during ambulation or stress?
That kind of adaptation may become important. For example, Dr. Mehra observed, normally a pig heart “provides flow in a four-footed configuration, and pig temperature is inherently higher than humans by several degrees, so it will be functioning in a relatively hypothermic environment.”
Transplantation remains the gold standard for patients with advanced heart failure despite modern medical and device therapy, Dr. Allen agreed. But “if we can raise pig hearts that provide the organ, and it can be implanted with a surgery that’s been done for 50 years, and rejection can be managed with gene editing and tailored immunosuppression, then it’s not hard to think about this very rapidly replacing a lot of what we do in the advanced heart failure and transplantation world.”
Certainly, it would be a major advance if the gene editing technique successfully improves the heart’s immunologic compatibility, Dr. Yancy noted. But do we have enough genomic knowledge to select gene deletions and insertions in the safest way for a successful outcome? “We have to appreciate that this is still a nascent technology, and we should be careful that there might be consequences that we haven’t anticipated.”
For example, he said, the xenotransplantation and gene-modifying techniques should be explored in a range of patients, including older and younger people, women and men, and people of different ethnicities and races.
“There may be some differences based on ancestry, based on gender, based on aging, that will influence the way in which these engineered donor hearts are experienced clinically,” Dr. Yancy said.
The xenotransplantation technique’s potential impact on health equity should also be considered, as it “almost assuredly will be a very expensive technology that will be utilized in a very select population,” he noted. “We need to have a really wide lens to think about all of the potential ramifications.”
‘This field needs to evolve’
Dr. Mehra also flagged the procedure’s potential cost should it become mainstream. Perhaps that would promote dialogue on how to primarily use it “after legitimately exhausting all available options, such as total artificial heart support.”
It might also teach the field to take greater advantage of the many donated hearts discarded as suboptimal. “The general usage rate for offered organs is around a third,” despite opportunities to expand use of those that are “less than perfect,” Dr. Mehra said. “I think that the field will grow with the community focusing on reduced discards of current available heart organs, and not necessarily grow because of the availability of ‘xeno-organs.’ ”
“This field needs to evolve because we’re actively transplanting patients today. But in my mind, the real future is to have such a sufficient understanding of the biology of left ventricular dysfunction that transplantation is a rare event,” Dr. Yancy proposed.
“I’m not certain that heart transplantation per se is the endgame. I think the avoidance of transplantation is the real endgame,” he said. “This may be controversial, but my vision of the future is not one where we have a supply of animals that we can use for transplantation. My vision of the future is that heart transplantation becomes obsolete.”
A version of this article first appeared on Medscape.com.
Scientific achievements usually raise big new questions, and the remarkable surgery that took place on Jan. 7, when Maryland resident David Bennett was transplanted with a genetically modified heart from a pig, has been no different.
The 57-year-old with end-stage heart failure had been repeatedly turned down for a standard transplant and was judged a poor candidate for a ventricular assist device. Now his new heart is beating soundly and apparently accepted by his immune system as Mr. Bennett, his physicians at the University of Maryland where the procedure took place, and indeed the world set out on a journey with far more unknowns than knowns.
“I think even just a couple of years ago, people felt that xenotransplantation for the heart and other organs was still a long way off. And it seems like it’s started to move very quickly,” Larry A. Allen, MD, University of Colorado, Aurora, said in an interview.
Demand for donor hearts far outstrips supply, and despite advances in the development of ventricular assist pumps and artificial hearts, “there are still significant limitations to them in terms of clotting, stroke, and infection. We’ve seen the use of those devices plateau,” Dr. Allen said. “So, the concept of a nonhuman source of organs is exciting and very much in need, if people can get it to work.”
“I really credit the surgeons at the University of Maryland for courageous clinical work and a brilliant scientific innovation,” Clyde W. Yancy, MD, MSc, Northwestern University, Chicago, said in an interview. “But it’s always in the implementation that we have to hold our breath.” Heart xenotransplantation is an old idea that “has never before been successful,” he said. And standard heart transplantation has set a high bar, with a 1-year survival of about 90% and low 1-year risk for rejection. Whether the new procedure can meet that standard is unknown, as is its potential for complications, such as chronic rejection or cancers due to long-term immunosuppression. Those are “major questions requiring more time and careful follow-up.”
‘Still a nascent technology’
“This is an exciting and courageous step forward in heart transplantation, and kudos to the team at the University of Maryland,” said Mandeep R. Mehra, MD, Brigham and Woman’s Hospital, Boston. But “there are many challenges here.”
The procedure’s 10 gene modifications were reportedly aimed at preventing hyperacute rejection of the heart and its excessive growth after transplantation, and making the organ less immunogenic, Dr. Mehra said in an interview. But even if those goals are met, could the same changes potentially impede the heart’s adaptation to human physiology, such as during ambulation or stress?
That kind of adaptation may become important. For example, Dr. Mehra observed, normally a pig heart “provides flow in a four-footed configuration, and pig temperature is inherently higher than humans by several degrees, so it will be functioning in a relatively hypothermic environment.”
Transplantation remains the gold standard for patients with advanced heart failure despite modern medical and device therapy, Dr. Allen agreed. But “if we can raise pig hearts that provide the organ, and it can be implanted with a surgery that’s been done for 50 years, and rejection can be managed with gene editing and tailored immunosuppression, then it’s not hard to think about this very rapidly replacing a lot of what we do in the advanced heart failure and transplantation world.”
Certainly, it would be a major advance if the gene editing technique successfully improves the heart’s immunologic compatibility, Dr. Yancy noted. But do we have enough genomic knowledge to select gene deletions and insertions in the safest way for a successful outcome? “We have to appreciate that this is still a nascent technology, and we should be careful that there might be consequences that we haven’t anticipated.”
For example, he said, the xenotransplantation and gene-modifying techniques should be explored in a range of patients, including older and younger people, women and men, and people of different ethnicities and races.
“There may be some differences based on ancestry, based on gender, based on aging, that will influence the way in which these engineered donor hearts are experienced clinically,” Dr. Yancy said.
The xenotransplantation technique’s potential impact on health equity should also be considered, as it “almost assuredly will be a very expensive technology that will be utilized in a very select population,” he noted. “We need to have a really wide lens to think about all of the potential ramifications.”
‘This field needs to evolve’
Dr. Mehra also flagged the procedure’s potential cost should it become mainstream. Perhaps that would promote dialogue on how to primarily use it “after legitimately exhausting all available options, such as total artificial heart support.”
It might also teach the field to take greater advantage of the many donated hearts discarded as suboptimal. “The general usage rate for offered organs is around a third,” despite opportunities to expand use of those that are “less than perfect,” Dr. Mehra said. “I think that the field will grow with the community focusing on reduced discards of current available heart organs, and not necessarily grow because of the availability of ‘xeno-organs.’ ”
“This field needs to evolve because we’re actively transplanting patients today. But in my mind, the real future is to have such a sufficient understanding of the biology of left ventricular dysfunction that transplantation is a rare event,” Dr. Yancy proposed.
“I’m not certain that heart transplantation per se is the endgame. I think the avoidance of transplantation is the real endgame,” he said. “This may be controversial, but my vision of the future is not one where we have a supply of animals that we can use for transplantation. My vision of the future is that heart transplantation becomes obsolete.”
A version of this article first appeared on Medscape.com.
Scientific achievements usually raise big new questions, and the remarkable surgery that took place on Jan. 7, when Maryland resident David Bennett was transplanted with a genetically modified heart from a pig, has been no different.
The 57-year-old with end-stage heart failure had been repeatedly turned down for a standard transplant and was judged a poor candidate for a ventricular assist device. Now his new heart is beating soundly and apparently accepted by his immune system as Mr. Bennett, his physicians at the University of Maryland where the procedure took place, and indeed the world set out on a journey with far more unknowns than knowns.
“I think even just a couple of years ago, people felt that xenotransplantation for the heart and other organs was still a long way off. And it seems like it’s started to move very quickly,” Larry A. Allen, MD, University of Colorado, Aurora, said in an interview.
Demand for donor hearts far outstrips supply, and despite advances in the development of ventricular assist pumps and artificial hearts, “there are still significant limitations to them in terms of clotting, stroke, and infection. We’ve seen the use of those devices plateau,” Dr. Allen said. “So, the concept of a nonhuman source of organs is exciting and very much in need, if people can get it to work.”
“I really credit the surgeons at the University of Maryland for courageous clinical work and a brilliant scientific innovation,” Clyde W. Yancy, MD, MSc, Northwestern University, Chicago, said in an interview. “But it’s always in the implementation that we have to hold our breath.” Heart xenotransplantation is an old idea that “has never before been successful,” he said. And standard heart transplantation has set a high bar, with a 1-year survival of about 90% and low 1-year risk for rejection. Whether the new procedure can meet that standard is unknown, as is its potential for complications, such as chronic rejection or cancers due to long-term immunosuppression. Those are “major questions requiring more time and careful follow-up.”
‘Still a nascent technology’
“This is an exciting and courageous step forward in heart transplantation, and kudos to the team at the University of Maryland,” said Mandeep R. Mehra, MD, Brigham and Woman’s Hospital, Boston. But “there are many challenges here.”
The procedure’s 10 gene modifications were reportedly aimed at preventing hyperacute rejection of the heart and its excessive growth after transplantation, and making the organ less immunogenic, Dr. Mehra said in an interview. But even if those goals are met, could the same changes potentially impede the heart’s adaptation to human physiology, such as during ambulation or stress?
That kind of adaptation may become important. For example, Dr. Mehra observed, normally a pig heart “provides flow in a four-footed configuration, and pig temperature is inherently higher than humans by several degrees, so it will be functioning in a relatively hypothermic environment.”
Transplantation remains the gold standard for patients with advanced heart failure despite modern medical and device therapy, Dr. Allen agreed. But “if we can raise pig hearts that provide the organ, and it can be implanted with a surgery that’s been done for 50 years, and rejection can be managed with gene editing and tailored immunosuppression, then it’s not hard to think about this very rapidly replacing a lot of what we do in the advanced heart failure and transplantation world.”
Certainly, it would be a major advance if the gene editing technique successfully improves the heart’s immunologic compatibility, Dr. Yancy noted. But do we have enough genomic knowledge to select gene deletions and insertions in the safest way for a successful outcome? “We have to appreciate that this is still a nascent technology, and we should be careful that there might be consequences that we haven’t anticipated.”
For example, he said, the xenotransplantation and gene-modifying techniques should be explored in a range of patients, including older and younger people, women and men, and people of different ethnicities and races.
“There may be some differences based on ancestry, based on gender, based on aging, that will influence the way in which these engineered donor hearts are experienced clinically,” Dr. Yancy said.
The xenotransplantation technique’s potential impact on health equity should also be considered, as it “almost assuredly will be a very expensive technology that will be utilized in a very select population,” he noted. “We need to have a really wide lens to think about all of the potential ramifications.”
‘This field needs to evolve’
Dr. Mehra also flagged the procedure’s potential cost should it become mainstream. Perhaps that would promote dialogue on how to primarily use it “after legitimately exhausting all available options, such as total artificial heart support.”
It might also teach the field to take greater advantage of the many donated hearts discarded as suboptimal. “The general usage rate for offered organs is around a third,” despite opportunities to expand use of those that are “less than perfect,” Dr. Mehra said. “I think that the field will grow with the community focusing on reduced discards of current available heart organs, and not necessarily grow because of the availability of ‘xeno-organs.’ ”
“This field needs to evolve because we’re actively transplanting patients today. But in my mind, the real future is to have such a sufficient understanding of the biology of left ventricular dysfunction that transplantation is a rare event,” Dr. Yancy proposed.
“I’m not certain that heart transplantation per se is the endgame. I think the avoidance of transplantation is the real endgame,” he said. “This may be controversial, but my vision of the future is not one where we have a supply of animals that we can use for transplantation. My vision of the future is that heart transplantation becomes obsolete.”
A version of this article first appeared on Medscape.com.
Pig heart successfully transplanted to man
A genetically modified pig heart has been successfully transplanted into a 57-year-old man who had no other treatment options but is “doing well” 3 days after the procedure, officials at the University of Maryland Medical Center (UMMC), Baltimore, announced Jan. 10.
“This organ transplant demonstrated for the first time that a genetically modified animal heart can function like a human heart without immediate rejection by the body,” they said.
Three genes associated with antibody-mediated rejection had been knocked out in the pig supplying the transplanted heart, and six human genes associated with immune acceptance of the organ had been inserted into the pig’s genome, notes a UMMC press release.
“Lastly, one additional gene in the pig was knocked out to prevent excessive growth of the pig heart tissue, which totaled 10 unique gene edits made in the donor pig,” the release states.
The patient, Maryland resident David Bennett, had required mechanical circulatory support to stay alive but was rejected for standard heart transplantation at UMMC and other centers. He was ineligible for an implanted ventricular assist device due to ventricular arrhythmias.
Mr. Bennett “is being carefully monitored over the next days and weeks to determine whether the transplant provides lifesaving benefits,” the announcement says.
“We are proceeding cautiously, but we are also optimistic that this first-in-the-world surgery will provide an important new option for patients in the future,” notes a quote from Bartley P. Griffith, MD, the UMMC surgeon who performed the procedure.
The pig supplying the heart was provided to the center by Revivicor (Blacksburg, Virginia), a regenerative medicine company. An experimental antirejection medication (Kiniksa Pharmaceuticals; Lexington, Massachusetts) was also used, in addition to standard immunosuppressants.
A version of this article first appeared on Medscape.com.
A genetically modified pig heart has been successfully transplanted into a 57-year-old man who had no other treatment options but is “doing well” 3 days after the procedure, officials at the University of Maryland Medical Center (UMMC), Baltimore, announced Jan. 10.
“This organ transplant demonstrated for the first time that a genetically modified animal heart can function like a human heart without immediate rejection by the body,” they said.
Three genes associated with antibody-mediated rejection had been knocked out in the pig supplying the transplanted heart, and six human genes associated with immune acceptance of the organ had been inserted into the pig’s genome, notes a UMMC press release.
“Lastly, one additional gene in the pig was knocked out to prevent excessive growth of the pig heart tissue, which totaled 10 unique gene edits made in the donor pig,” the release states.
The patient, Maryland resident David Bennett, had required mechanical circulatory support to stay alive but was rejected for standard heart transplantation at UMMC and other centers. He was ineligible for an implanted ventricular assist device due to ventricular arrhythmias.
Mr. Bennett “is being carefully monitored over the next days and weeks to determine whether the transplant provides lifesaving benefits,” the announcement says.
“We are proceeding cautiously, but we are also optimistic that this first-in-the-world surgery will provide an important new option for patients in the future,” notes a quote from Bartley P. Griffith, MD, the UMMC surgeon who performed the procedure.
The pig supplying the heart was provided to the center by Revivicor (Blacksburg, Virginia), a regenerative medicine company. An experimental antirejection medication (Kiniksa Pharmaceuticals; Lexington, Massachusetts) was also used, in addition to standard immunosuppressants.
A version of this article first appeared on Medscape.com.
A genetically modified pig heart has been successfully transplanted into a 57-year-old man who had no other treatment options but is “doing well” 3 days after the procedure, officials at the University of Maryland Medical Center (UMMC), Baltimore, announced Jan. 10.
“This organ transplant demonstrated for the first time that a genetically modified animal heart can function like a human heart without immediate rejection by the body,” they said.
Three genes associated with antibody-mediated rejection had been knocked out in the pig supplying the transplanted heart, and six human genes associated with immune acceptance of the organ had been inserted into the pig’s genome, notes a UMMC press release.
“Lastly, one additional gene in the pig was knocked out to prevent excessive growth of the pig heart tissue, which totaled 10 unique gene edits made in the donor pig,” the release states.
The patient, Maryland resident David Bennett, had required mechanical circulatory support to stay alive but was rejected for standard heart transplantation at UMMC and other centers. He was ineligible for an implanted ventricular assist device due to ventricular arrhythmias.
Mr. Bennett “is being carefully monitored over the next days and weeks to determine whether the transplant provides lifesaving benefits,” the announcement says.
“We are proceeding cautiously, but we are also optimistic that this first-in-the-world surgery will provide an important new option for patients in the future,” notes a quote from Bartley P. Griffith, MD, the UMMC surgeon who performed the procedure.
The pig supplying the heart was provided to the center by Revivicor (Blacksburg, Virginia), a regenerative medicine company. An experimental antirejection medication (Kiniksa Pharmaceuticals; Lexington, Massachusetts) was also used, in addition to standard immunosuppressants.
A version of this article first appeared on Medscape.com.
Female patients fare worse with male surgeons, study finds
, according to a new analysis of over 1.3 million surgery patients. The study found no difference in adverse outcomes in male patients treated by surgeons of either sex.
While the effect of patient and provider sex discordance (female patient/male physician or male patient/female physician) on care has been explored before, “to the best of our knowledge, this is the first study to assess this in patients undergoing surgery,” Christopher Wallis, MD, PhD, an assistant professor in the division of urology at the University of Toronto, said in an email. The study was published online December 8 in JAMA Surgery.
Past studies in primary care settings have found that sex discordance between a physician and patient can result in “worse rapport, lower certainty of diagnosis, lower likelihood of assessing patient’s conditions as being of high severity, concerns of a hidden agenda, and disagreements regarding advice provided,” the authors write in the paper. Gender discordance has also been shown to negatively affect cancer screening rates and survival after heart attack. Given these past findings, Dr. Wallis and colleagues postulated that gender match between patients and surgeons could affect postoperative outcomes.
To find out, researchers analyzed data from over 1,320,100 patients undergoing one of 21 common elective and emergent surgical procedures in Ontario, Canada, from January 1, 2007, through December 31, 2019. Procedures were performed across the following specialties: cardiothoracic surgery, general surgery, neurosurgery, orthopedic surgery, otolaryngology, plastic surgery, thoracic surgery, urology, and vascular surgery. The investigators compared adverse postoperative outcomes — death, readmission, or complications within 30 days after surgery — in patients of both sexes when treated by male or female surgeons.
The study included 2,937 surgeons, and nearly 46% of patients included in the study were the same sex as their surgeon. Of the remaining 717,548 sex-discordant pairings, 93% were female patients with male surgeons, and 7% were male patients with female surgeons.
Among all patients, 14.9% experienced at least one adverse outcome. The researchers found that sex discordance between patient and surgeon was associated with higher odds of complications (adjusted odds ratio [aOR], 1.09; 95% CI, 1.07 – 1.11) and death (aOR, 1.07; 95% CI, 1.02 – 1.13). There was no statistically significant relationship between sex discordance and readmission in the study.
Using multivariable modeling, the researchers then teased out how patient sex affected this association. They found that female patients treated by male surgeons, compared to those treated by female surgeons, were more likely to have worse outcomes (aOR, 1.15; 95% CI, 1.10 – 1.20); however, there was no difference in outcomes in male patients treated by female surgeons compared with those with male surgeons (aOR, 0.99; 95% CI, 0.95 – 1.03).
While the study did not look at the underlying reasons for this disparity, communication differences between surgeons and patients could be one factor, Dr. Wallis noted. “Prior research has suggested differences in communication style between male and female physicians. Further, there is evidence that female physicians, including surgeons, spend more time with patients,” he wrote in an email. “This, coupled with evidence that female patients may have disparities in the management of their pain, suggest that communication differences may underpin the observed disparity.”
The finding “sounds the alarm for urgent action,” write Andrea Riner, MD, MPH, and Amalia Cochran, MD, both from the department of surgery at the University of Florida College of Medicine in Gainesville, in an accompanying commentary. While recruiting more women into surgical specialties is one way to address this disparity, both Dr. Riner and Dr. Cochran note the importance of identifying unconscious biases in patient care. “Surgeons likely believe they provide the same quality of care to patients irrespective of identity,” they write. “However, these data underscore an underappreciated phenomenon and highlight a measurable repercussion of implicit bias.”
Training programs that work with surgeons to improve communication and care with diverse patients may help counter these biases, they suggest, and incorporating patient identity in surgical outcome metrics could help identify biases. “Female patients with surgical disease should not be disadvantaged because there simply are not enough female surgeons or surgeons who are competent in the care of female patients,” they note. “We owe it to patients to provide them with the best outcomes, regardless of how their identities may align with ours.”
Dr. Riner reports grants from the National Human Genome Research Institute and the National Cancer Institute.Dr. Cochran is a section editor for UpToDate. Dr. Wallis reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a new analysis of over 1.3 million surgery patients. The study found no difference in adverse outcomes in male patients treated by surgeons of either sex.
While the effect of patient and provider sex discordance (female patient/male physician or male patient/female physician) on care has been explored before, “to the best of our knowledge, this is the first study to assess this in patients undergoing surgery,” Christopher Wallis, MD, PhD, an assistant professor in the division of urology at the University of Toronto, said in an email. The study was published online December 8 in JAMA Surgery.
Past studies in primary care settings have found that sex discordance between a physician and patient can result in “worse rapport, lower certainty of diagnosis, lower likelihood of assessing patient’s conditions as being of high severity, concerns of a hidden agenda, and disagreements regarding advice provided,” the authors write in the paper. Gender discordance has also been shown to negatively affect cancer screening rates and survival after heart attack. Given these past findings, Dr. Wallis and colleagues postulated that gender match between patients and surgeons could affect postoperative outcomes.
To find out, researchers analyzed data from over 1,320,100 patients undergoing one of 21 common elective and emergent surgical procedures in Ontario, Canada, from January 1, 2007, through December 31, 2019. Procedures were performed across the following specialties: cardiothoracic surgery, general surgery, neurosurgery, orthopedic surgery, otolaryngology, plastic surgery, thoracic surgery, urology, and vascular surgery. The investigators compared adverse postoperative outcomes — death, readmission, or complications within 30 days after surgery — in patients of both sexes when treated by male or female surgeons.
The study included 2,937 surgeons, and nearly 46% of patients included in the study were the same sex as their surgeon. Of the remaining 717,548 sex-discordant pairings, 93% were female patients with male surgeons, and 7% were male patients with female surgeons.
Among all patients, 14.9% experienced at least one adverse outcome. The researchers found that sex discordance between patient and surgeon was associated with higher odds of complications (adjusted odds ratio [aOR], 1.09; 95% CI, 1.07 – 1.11) and death (aOR, 1.07; 95% CI, 1.02 – 1.13). There was no statistically significant relationship between sex discordance and readmission in the study.
Using multivariable modeling, the researchers then teased out how patient sex affected this association. They found that female patients treated by male surgeons, compared to those treated by female surgeons, were more likely to have worse outcomes (aOR, 1.15; 95% CI, 1.10 – 1.20); however, there was no difference in outcomes in male patients treated by female surgeons compared with those with male surgeons (aOR, 0.99; 95% CI, 0.95 – 1.03).
While the study did not look at the underlying reasons for this disparity, communication differences between surgeons and patients could be one factor, Dr. Wallis noted. “Prior research has suggested differences in communication style between male and female physicians. Further, there is evidence that female physicians, including surgeons, spend more time with patients,” he wrote in an email. “This, coupled with evidence that female patients may have disparities in the management of their pain, suggest that communication differences may underpin the observed disparity.”
The finding “sounds the alarm for urgent action,” write Andrea Riner, MD, MPH, and Amalia Cochran, MD, both from the department of surgery at the University of Florida College of Medicine in Gainesville, in an accompanying commentary. While recruiting more women into surgical specialties is one way to address this disparity, both Dr. Riner and Dr. Cochran note the importance of identifying unconscious biases in patient care. “Surgeons likely believe they provide the same quality of care to patients irrespective of identity,” they write. “However, these data underscore an underappreciated phenomenon and highlight a measurable repercussion of implicit bias.”
Training programs that work with surgeons to improve communication and care with diverse patients may help counter these biases, they suggest, and incorporating patient identity in surgical outcome metrics could help identify biases. “Female patients with surgical disease should not be disadvantaged because there simply are not enough female surgeons or surgeons who are competent in the care of female patients,” they note. “We owe it to patients to provide them with the best outcomes, regardless of how their identities may align with ours.”
Dr. Riner reports grants from the National Human Genome Research Institute and the National Cancer Institute.Dr. Cochran is a section editor for UpToDate. Dr. Wallis reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a new analysis of over 1.3 million surgery patients. The study found no difference in adverse outcomes in male patients treated by surgeons of either sex.
While the effect of patient and provider sex discordance (female patient/male physician or male patient/female physician) on care has been explored before, “to the best of our knowledge, this is the first study to assess this in patients undergoing surgery,” Christopher Wallis, MD, PhD, an assistant professor in the division of urology at the University of Toronto, said in an email. The study was published online December 8 in JAMA Surgery.
Past studies in primary care settings have found that sex discordance between a physician and patient can result in “worse rapport, lower certainty of diagnosis, lower likelihood of assessing patient’s conditions as being of high severity, concerns of a hidden agenda, and disagreements regarding advice provided,” the authors write in the paper. Gender discordance has also been shown to negatively affect cancer screening rates and survival after heart attack. Given these past findings, Dr. Wallis and colleagues postulated that gender match between patients and surgeons could affect postoperative outcomes.
To find out, researchers analyzed data from over 1,320,100 patients undergoing one of 21 common elective and emergent surgical procedures in Ontario, Canada, from January 1, 2007, through December 31, 2019. Procedures were performed across the following specialties: cardiothoracic surgery, general surgery, neurosurgery, orthopedic surgery, otolaryngology, plastic surgery, thoracic surgery, urology, and vascular surgery. The investigators compared adverse postoperative outcomes — death, readmission, or complications within 30 days after surgery — in patients of both sexes when treated by male or female surgeons.
The study included 2,937 surgeons, and nearly 46% of patients included in the study were the same sex as their surgeon. Of the remaining 717,548 sex-discordant pairings, 93% were female patients with male surgeons, and 7% were male patients with female surgeons.
Among all patients, 14.9% experienced at least one adverse outcome. The researchers found that sex discordance between patient and surgeon was associated with higher odds of complications (adjusted odds ratio [aOR], 1.09; 95% CI, 1.07 – 1.11) and death (aOR, 1.07; 95% CI, 1.02 – 1.13). There was no statistically significant relationship between sex discordance and readmission in the study.
Using multivariable modeling, the researchers then teased out how patient sex affected this association. They found that female patients treated by male surgeons, compared to those treated by female surgeons, were more likely to have worse outcomes (aOR, 1.15; 95% CI, 1.10 – 1.20); however, there was no difference in outcomes in male patients treated by female surgeons compared with those with male surgeons (aOR, 0.99; 95% CI, 0.95 – 1.03).
While the study did not look at the underlying reasons for this disparity, communication differences between surgeons and patients could be one factor, Dr. Wallis noted. “Prior research has suggested differences in communication style between male and female physicians. Further, there is evidence that female physicians, including surgeons, spend more time with patients,” he wrote in an email. “This, coupled with evidence that female patients may have disparities in the management of their pain, suggest that communication differences may underpin the observed disparity.”
The finding “sounds the alarm for urgent action,” write Andrea Riner, MD, MPH, and Amalia Cochran, MD, both from the department of surgery at the University of Florida College of Medicine in Gainesville, in an accompanying commentary. While recruiting more women into surgical specialties is one way to address this disparity, both Dr. Riner and Dr. Cochran note the importance of identifying unconscious biases in patient care. “Surgeons likely believe they provide the same quality of care to patients irrespective of identity,” they write. “However, these data underscore an underappreciated phenomenon and highlight a measurable repercussion of implicit bias.”
Training programs that work with surgeons to improve communication and care with diverse patients may help counter these biases, they suggest, and incorporating patient identity in surgical outcome metrics could help identify biases. “Female patients with surgical disease should not be disadvantaged because there simply are not enough female surgeons or surgeons who are competent in the care of female patients,” they note. “We owe it to patients to provide them with the best outcomes, regardless of how their identities may align with ours.”
Dr. Riner reports grants from the National Human Genome Research Institute and the National Cancer Institute.Dr. Cochran is a section editor for UpToDate. Dr. Wallis reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA SURGERY
Cement found in man’s heart after spinal surgery
, according to a new report published in the New England Journal of Medicine.
The 56-year-old man, who was not identified in the report, went to the emergency room after experiencing 2 days of chest pain and shortness of breath. Imaging scans showed that the chest pain was caused by a foreign object, and he was rushed to surgery.
Surgeons then located and removed a thin, sharp, cylindrical piece of cement and repaired the damage to the patient’s heart. The cement had pierced the upper right chamber of his heart and his right lung, according to the report authors from the Yale University School of Medicine.
A week before, the man had undergone a spinal surgery known as kyphoplasty. The procedure treats spine injuries by injecting a special type of medical cement into damaged vertebrae, according to USA Today. The cement had leaked into the patient’s body, hardened, and traveled to his heart.
The man has now “nearly recovered” since the heart surgery and cement removal, which occurred about a month ago, the journal report stated. He experienced no additional complications.
Cement leakage after kyphoplasty can happen but is an extremely rare complication. Less than 2% of patients who undergo the procedure for osteoporosis or brittle bones have complications, according to patient information from the American Association of Neurological Surgeons.
A version of this article first appeared on WebMD.com.
, according to a new report published in the New England Journal of Medicine.
The 56-year-old man, who was not identified in the report, went to the emergency room after experiencing 2 days of chest pain and shortness of breath. Imaging scans showed that the chest pain was caused by a foreign object, and he was rushed to surgery.
Surgeons then located and removed a thin, sharp, cylindrical piece of cement and repaired the damage to the patient’s heart. The cement had pierced the upper right chamber of his heart and his right lung, according to the report authors from the Yale University School of Medicine.
A week before, the man had undergone a spinal surgery known as kyphoplasty. The procedure treats spine injuries by injecting a special type of medical cement into damaged vertebrae, according to USA Today. The cement had leaked into the patient’s body, hardened, and traveled to his heart.
The man has now “nearly recovered” since the heart surgery and cement removal, which occurred about a month ago, the journal report stated. He experienced no additional complications.
Cement leakage after kyphoplasty can happen but is an extremely rare complication. Less than 2% of patients who undergo the procedure for osteoporosis or brittle bones have complications, according to patient information from the American Association of Neurological Surgeons.
A version of this article first appeared on WebMD.com.
, according to a new report published in the New England Journal of Medicine.
The 56-year-old man, who was not identified in the report, went to the emergency room after experiencing 2 days of chest pain and shortness of breath. Imaging scans showed that the chest pain was caused by a foreign object, and he was rushed to surgery.
Surgeons then located and removed a thin, sharp, cylindrical piece of cement and repaired the damage to the patient’s heart. The cement had pierced the upper right chamber of his heart and his right lung, according to the report authors from the Yale University School of Medicine.
A week before, the man had undergone a spinal surgery known as kyphoplasty. The procedure treats spine injuries by injecting a special type of medical cement into damaged vertebrae, according to USA Today. The cement had leaked into the patient’s body, hardened, and traveled to his heart.
The man has now “nearly recovered” since the heart surgery and cement removal, which occurred about a month ago, the journal report stated. He experienced no additional complications.
Cement leakage after kyphoplasty can happen but is an extremely rare complication. Less than 2% of patients who undergo the procedure for osteoporosis or brittle bones have complications, according to patient information from the American Association of Neurological Surgeons.
A version of this article first appeared on WebMD.com.
Feds slap UPMC, lead cardiothoracic surgeon with fraud lawsuit
Following a 2-year investigation, the U.S. government has filed suit against the University of Pittsburgh Medical Center (UPMC), University of Pittsburgh Physicians (UPP), and James Luketich, MD, for billing related to concurrent surgeries performed by the long-time chair of cardiothoracic surgery.
The lawsuit alleges that UPMC “knowingly allowed” Dr. Luketich to “book and perform three surgeries at the same time, to miss the surgical time outs at the outset of those procedures, to go back-and-forth between operating rooms and even hospital facilities while his surgical patients remain under general anesthesia...”
UPMC, the lawsuit claims, also allowed Dr. Luketich to falsely attest that “he was with his patients throughout the entirety of their surgical procedures or during all ‘key and critical’ portions of those procedures and to unlawfully bill Government Health Benefit Programs for those procedures, all in order to increase surgical volume, maximize UPMC and UPP’s revenue, and/or appease Dr. Luketich.”
These practices violate the statutes and regulations governing the defendants, including those that prohibit “teaching physicians” like Dr. Luketich from performing and billing the U.S. for concurrent surgeries, the Department of Justice said in news release.
The Justice Department contends the defendants “knowingly submitted hundreds of materially false claims for payment” to Medicare, Medicaid, and other government programs over the past 6 years.
“The laws prohibiting ‘concurrent surgeries’ are in place for a reason: To protect patients and ensure they receive appropriate and focused medical care,” Stephen R. Kaufman, Acting U.S. Attorney for the Western District of Pennsylvania, said in the release.
According to the lawsuit, “some of Dr. Luketich’s patients were forced to endure additional surgical procedures and/or extended hospital stays as a result of his unlawful conduct. Numerous patients developed painful pressure ulcers. A few were diagnosed with compartment syndrome. And at least two had to undergo amputations.”
The allegations were originally brought forward under the federal False Claims Act’s whistleblower provisions by Jonathan D’Cunha, MD, PhD, who worked closely with Dr. Luketich from 2012 to 2019 and now chairs the department of cardiothoracic surgery at the Mayo Clinic, Phoenix.
The charges cited in the lawsuit include three counts of violating the False Claims Act, one count of unjust enrichment, and one count of payment by mistake.
The 56-page lawsuit includes numerous case examples and cites an October 2015 Boston Globe Spotlight Team report on the safety of running concurrent operations, which reportedly prompted UPMC to reevaluate its policies and identify physicians or departments in potential violation.
Hospital officials met with Dr. Luketich in March 2016 and devised a “plan” to ensure his availability and “compliance with concurrency rules,” it alleges, but also highlights an email that notes “continued problems” with Dr. Luketich’s schedule.
“UPMC has persistently ignored or minimized complaints by employees and staff regarding Dr. Luketich, his hyper-busy schedule, his refusal to delegate surgeries and surgical tasks” and “protected him from meaningful sanction; refused to curtail his surgical practice; and continued to allow Dr. Luketich to skirt the rules and endanger his patients,” according to the lawsuit.
The suit notes that Dr. Luketich is one of UPMC and UPP’s highest sources of revenue and that UPMC advertises him as a “life-saving pioneer” who routinely performs dramatic, last-ditch procedures on patients who are otherwise hopeless.
In response to an interview request from this news organization, a UPMC spokesperson wrote: “As the government itself concedes in its complaint, many of Dr. Luketich’s surgical patients are elderly, frail, and/or very ill. They include the ‘hopeless’ patients ... who suffer from chronic illness or metastatic cancer, and/or have extensive surgical histories and choose UPMC and Dr. Luketich when other physicians and health care providers have turned them down.”
“Dr. Luketich always performs the most critical portions of every operation he undertakes,” the spokesperson said, adding that no law or regulation prohibits overlapping surgeries or billing for those surgeries, “let alone surgeries conducted by teams of surgeons like those led by Dr. Luketich.”
“The government’s claims are, rather, based on a misapplication or misinterpretation of UPMC’s internal policies and [Centers for Medicare & Medicaid Services] guidance, neither of which can support a claim for fraudulent billing. UPMC and Dr. Luketich plan to vigorously defend against the government’s claims,” the spokesperson concluded.
The claims asserted against the defendants are allegations only; there has been no determination of liability. The government is seeking three times the amount of actual damages suffered as a result of the alleged false claims and/or fraud; a sum of $23,331 (or the maximum penalty, whichever is greater) for each false claim submitted by UPMC, UPP, and/or Dr. Luketich; and costs and expenses associated with the civil suit.
A version of this article first appeared on Medscape.com.
Following a 2-year investigation, the U.S. government has filed suit against the University of Pittsburgh Medical Center (UPMC), University of Pittsburgh Physicians (UPP), and James Luketich, MD, for billing related to concurrent surgeries performed by the long-time chair of cardiothoracic surgery.
The lawsuit alleges that UPMC “knowingly allowed” Dr. Luketich to “book and perform three surgeries at the same time, to miss the surgical time outs at the outset of those procedures, to go back-and-forth between operating rooms and even hospital facilities while his surgical patients remain under general anesthesia...”
UPMC, the lawsuit claims, also allowed Dr. Luketich to falsely attest that “he was with his patients throughout the entirety of their surgical procedures or during all ‘key and critical’ portions of those procedures and to unlawfully bill Government Health Benefit Programs for those procedures, all in order to increase surgical volume, maximize UPMC and UPP’s revenue, and/or appease Dr. Luketich.”
These practices violate the statutes and regulations governing the defendants, including those that prohibit “teaching physicians” like Dr. Luketich from performing and billing the U.S. for concurrent surgeries, the Department of Justice said in news release.
The Justice Department contends the defendants “knowingly submitted hundreds of materially false claims for payment” to Medicare, Medicaid, and other government programs over the past 6 years.
“The laws prohibiting ‘concurrent surgeries’ are in place for a reason: To protect patients and ensure they receive appropriate and focused medical care,” Stephen R. Kaufman, Acting U.S. Attorney for the Western District of Pennsylvania, said in the release.
According to the lawsuit, “some of Dr. Luketich’s patients were forced to endure additional surgical procedures and/or extended hospital stays as a result of his unlawful conduct. Numerous patients developed painful pressure ulcers. A few were diagnosed with compartment syndrome. And at least two had to undergo amputations.”
The allegations were originally brought forward under the federal False Claims Act’s whistleblower provisions by Jonathan D’Cunha, MD, PhD, who worked closely with Dr. Luketich from 2012 to 2019 and now chairs the department of cardiothoracic surgery at the Mayo Clinic, Phoenix.
The charges cited in the lawsuit include three counts of violating the False Claims Act, one count of unjust enrichment, and one count of payment by mistake.
The 56-page lawsuit includes numerous case examples and cites an October 2015 Boston Globe Spotlight Team report on the safety of running concurrent operations, which reportedly prompted UPMC to reevaluate its policies and identify physicians or departments in potential violation.
Hospital officials met with Dr. Luketich in March 2016 and devised a “plan” to ensure his availability and “compliance with concurrency rules,” it alleges, but also highlights an email that notes “continued problems” with Dr. Luketich’s schedule.
“UPMC has persistently ignored or minimized complaints by employees and staff regarding Dr. Luketich, his hyper-busy schedule, his refusal to delegate surgeries and surgical tasks” and “protected him from meaningful sanction; refused to curtail his surgical practice; and continued to allow Dr. Luketich to skirt the rules and endanger his patients,” according to the lawsuit.
The suit notes that Dr. Luketich is one of UPMC and UPP’s highest sources of revenue and that UPMC advertises him as a “life-saving pioneer” who routinely performs dramatic, last-ditch procedures on patients who are otherwise hopeless.
In response to an interview request from this news organization, a UPMC spokesperson wrote: “As the government itself concedes in its complaint, many of Dr. Luketich’s surgical patients are elderly, frail, and/or very ill. They include the ‘hopeless’ patients ... who suffer from chronic illness or metastatic cancer, and/or have extensive surgical histories and choose UPMC and Dr. Luketich when other physicians and health care providers have turned them down.”
“Dr. Luketich always performs the most critical portions of every operation he undertakes,” the spokesperson said, adding that no law or regulation prohibits overlapping surgeries or billing for those surgeries, “let alone surgeries conducted by teams of surgeons like those led by Dr. Luketich.”
“The government’s claims are, rather, based on a misapplication or misinterpretation of UPMC’s internal policies and [Centers for Medicare & Medicaid Services] guidance, neither of which can support a claim for fraudulent billing. UPMC and Dr. Luketich plan to vigorously defend against the government’s claims,” the spokesperson concluded.
The claims asserted against the defendants are allegations only; there has been no determination of liability. The government is seeking three times the amount of actual damages suffered as a result of the alleged false claims and/or fraud; a sum of $23,331 (or the maximum penalty, whichever is greater) for each false claim submitted by UPMC, UPP, and/or Dr. Luketich; and costs and expenses associated with the civil suit.
A version of this article first appeared on Medscape.com.
Following a 2-year investigation, the U.S. government has filed suit against the University of Pittsburgh Medical Center (UPMC), University of Pittsburgh Physicians (UPP), and James Luketich, MD, for billing related to concurrent surgeries performed by the long-time chair of cardiothoracic surgery.
The lawsuit alleges that UPMC “knowingly allowed” Dr. Luketich to “book and perform three surgeries at the same time, to miss the surgical time outs at the outset of those procedures, to go back-and-forth between operating rooms and even hospital facilities while his surgical patients remain under general anesthesia...”
UPMC, the lawsuit claims, also allowed Dr. Luketich to falsely attest that “he was with his patients throughout the entirety of their surgical procedures or during all ‘key and critical’ portions of those procedures and to unlawfully bill Government Health Benefit Programs for those procedures, all in order to increase surgical volume, maximize UPMC and UPP’s revenue, and/or appease Dr. Luketich.”
These practices violate the statutes and regulations governing the defendants, including those that prohibit “teaching physicians” like Dr. Luketich from performing and billing the U.S. for concurrent surgeries, the Department of Justice said in news release.
The Justice Department contends the defendants “knowingly submitted hundreds of materially false claims for payment” to Medicare, Medicaid, and other government programs over the past 6 years.
“The laws prohibiting ‘concurrent surgeries’ are in place for a reason: To protect patients and ensure they receive appropriate and focused medical care,” Stephen R. Kaufman, Acting U.S. Attorney for the Western District of Pennsylvania, said in the release.
According to the lawsuit, “some of Dr. Luketich’s patients were forced to endure additional surgical procedures and/or extended hospital stays as a result of his unlawful conduct. Numerous patients developed painful pressure ulcers. A few were diagnosed with compartment syndrome. And at least two had to undergo amputations.”
The allegations were originally brought forward under the federal False Claims Act’s whistleblower provisions by Jonathan D’Cunha, MD, PhD, who worked closely with Dr. Luketich from 2012 to 2019 and now chairs the department of cardiothoracic surgery at the Mayo Clinic, Phoenix.
The charges cited in the lawsuit include three counts of violating the False Claims Act, one count of unjust enrichment, and one count of payment by mistake.
The 56-page lawsuit includes numerous case examples and cites an October 2015 Boston Globe Spotlight Team report on the safety of running concurrent operations, which reportedly prompted UPMC to reevaluate its policies and identify physicians or departments in potential violation.
Hospital officials met with Dr. Luketich in March 2016 and devised a “plan” to ensure his availability and “compliance with concurrency rules,” it alleges, but also highlights an email that notes “continued problems” with Dr. Luketich’s schedule.
“UPMC has persistently ignored or minimized complaints by employees and staff regarding Dr. Luketich, his hyper-busy schedule, his refusal to delegate surgeries and surgical tasks” and “protected him from meaningful sanction; refused to curtail his surgical practice; and continued to allow Dr. Luketich to skirt the rules and endanger his patients,” according to the lawsuit.
The suit notes that Dr. Luketich is one of UPMC and UPP’s highest sources of revenue and that UPMC advertises him as a “life-saving pioneer” who routinely performs dramatic, last-ditch procedures on patients who are otherwise hopeless.
In response to an interview request from this news organization, a UPMC spokesperson wrote: “As the government itself concedes in its complaint, many of Dr. Luketich’s surgical patients are elderly, frail, and/or very ill. They include the ‘hopeless’ patients ... who suffer from chronic illness or metastatic cancer, and/or have extensive surgical histories and choose UPMC and Dr. Luketich when other physicians and health care providers have turned them down.”
“Dr. Luketich always performs the most critical portions of every operation he undertakes,” the spokesperson said, adding that no law or regulation prohibits overlapping surgeries or billing for those surgeries, “let alone surgeries conducted by teams of surgeons like those led by Dr. Luketich.”
“The government’s claims are, rather, based on a misapplication or misinterpretation of UPMC’s internal policies and [Centers for Medicare & Medicaid Services] guidance, neither of which can support a claim for fraudulent billing. UPMC and Dr. Luketich plan to vigorously defend against the government’s claims,” the spokesperson concluded.
The claims asserted against the defendants are allegations only; there has been no determination of liability. The government is seeking three times the amount of actual damages suffered as a result of the alleged false claims and/or fraud; a sum of $23,331 (or the maximum penalty, whichever is greater) for each false claim submitted by UPMC, UPP, and/or Dr. Luketich; and costs and expenses associated with the civil suit.
A version of this article first appeared on Medscape.com.
Third COVID-19 vaccine dose helped some transplant recipients
All of those with low titers before the third dose had high titers after receiving the additional shot, but only about 33% of those with negative initial responses had detectable antibodies after the third dose, according to the paper, published in Annals of Internal Medicine.
Researchers at Johns Hopkins, Baltimore, who keep a COVID-19 vaccine registry, perform antibody tests on all registry subjects and inform them of their results. Registry participants were asked to inform the research team if they received a third dose, and, the research team tracked the immune responses of those who did.
The participants in this case series had low antibody levels and received a third dose of the vaccine on their own between March 20 and May 10 of 2021.
Third dose results
In this cases series – thought to be the first to look at third vaccine shots in this type of patient group – all six of those who had low antibody titers before the third dose had high-positive titers after the third dose.
Of the 24 individuals who had negative antibody titers before the third dose, just 6 had high titers after the third dose.
Two of the participants had low-positive titers, and 16 were negative.
“Several of those boosted very nicely into ranges seen, using these assays, in healthy persons,” said William Werbel, MD, a fellow in infectious disease at Johns Hopkins Medicine, Baltimore, who helped lead the study. Those with negative levels, even if they responded, tended to have lower titers, he said.
“The benefits at least from an antibody perspective were not the same for everybody and so this is obviously something that needs to be considered when thinking about selecting patients” for a COVID-19 prevention strategy, he said.
Reactions to the vaccine were low to moderate, such as some arm pain and fatigue.
“Showing that something is safe in that special, vulnerable population is important,” Dr. Werbel said. “We’re all wanting to make sure that we’re doing no harm.”
Dr. Werbel noted that there was no pattern in the small series based on the organ transplanted or in the vaccines used. As their third shot, 15 of the patients received the Johnson & Johnson vaccine; 9 received Moderna; and 6 received Pfizer-BioNTech.
Welcome news, but larger studies needed
“To think that a third dose could confer protection for a significant number of people is of course extremely welcome news,” said Christian Larsen, MD, DPhil, professor of surgery in the transplantation division at Emory University, Atlanta, who was not involved in the study. “It’s the easiest conceivable next intervention.”
He added, “We just want studies to confirm that – larger studies.”
Dr. Werbel stressed the importance of looking at third doses in these patients in a more controlled fashion in a randomized trial, to more carefully monitor safety and how patients fare when starting with one type of vaccine and switching to another, for example.
Richard Wender, MD, chair of family medicine and community health at the University of Pennsylvania, Philadelphia, said the findings are a reminder that there is still a lot that is unknown about COVID-19 and vaccination.
“We still don’t know who will or will not benefit from a third dose,” he said. “And our knowledge is evolving. For example, a recent study suggested that people with previous infection and who are vaccinated may have better and longer protection than people with vaccination alone. We’re still learning.”
He added that specialists, not primary care clinicians, should be relied upon to respond to this emerging vaccination data. Primary care doctors are very busy in other ways – such as in getting children caught up on vaccinations and helping adults return to managing their chronic diseases, Dr. Wender noted.
“Their focus needs to be on helping to overcome hesitancy, mistrust, lack of information, or antivaccination sentiment to help more people feel comfortable being vaccinated – this is a lot of work and needs constant focus. In short, primary care clinicians need to focus chiefly on the unvaccinated,” he said.
“Monitoring immunization recommendations for unique at-risk populations should be the chief responsibility of teams providing subspecialty care, [such as for] transplant patients, people with chronic kidney disease, cancer patients, and people with other chronic illnesses. This will allow primary care clinicians to tackle their many complex jobs.”
Possible solutions for those with low antibody responses
Dr. Larsen said that those with ongoing low antibody responses might still have other immune responses, such as a T-cell response. Such patients also could consider changing their vaccine type, he said.
“At the more significant intervention level, there may be circumstances where one could change the immunosuppressive drugs in a controlled way that might allow a better response,” suggested Dr. Larsen. “That’s obviously going to be something that requires a lot more thought and careful study.”
Dr. Werbel said that other options might need to be considered for those having no response following a third dose. One possibility is trying a vaccine with an adjuvant, such as the Novavax version, which might be more widely available soon.
“If you’re given a third dose of a very immunogenic vaccine – something that should work – and you just have no antibody development, it seems relatively unlikely that doing the same thing again is going to help you from that perspective, and for all we know might expose you to more risk,” Dr. Werbel noted.
Participant details
None of the 30 patients were thought to have ever had COVID-19. On average, patients had received their transplant 4.5 years before their original vaccination. In 25 patients, maintenance immunosuppression included tacrolimus or cyclosporine along with mycophenolate. Corticosteroids were also used for 24 patients, sirolimus was used for one patient, and belatacept was used for another patient.
Fifty-seven percent of patients had received the Pfizer/BioNTech vaccine originally, and 43% the Moderna vaccine. Most of the patients were kidney recipients, with two heart, three liver, one lung, one pancreas and one kidney-pancreas.
Dr. Werbel, Dr. Wender, and Dr. Larsen reported no relevant disclosures.
All of those with low titers before the third dose had high titers after receiving the additional shot, but only about 33% of those with negative initial responses had detectable antibodies after the third dose, according to the paper, published in Annals of Internal Medicine.
Researchers at Johns Hopkins, Baltimore, who keep a COVID-19 vaccine registry, perform antibody tests on all registry subjects and inform them of their results. Registry participants were asked to inform the research team if they received a third dose, and, the research team tracked the immune responses of those who did.
The participants in this case series had low antibody levels and received a third dose of the vaccine on their own between March 20 and May 10 of 2021.
Third dose results
In this cases series – thought to be the first to look at third vaccine shots in this type of patient group – all six of those who had low antibody titers before the third dose had high-positive titers after the third dose.
Of the 24 individuals who had negative antibody titers before the third dose, just 6 had high titers after the third dose.
Two of the participants had low-positive titers, and 16 were negative.
“Several of those boosted very nicely into ranges seen, using these assays, in healthy persons,” said William Werbel, MD, a fellow in infectious disease at Johns Hopkins Medicine, Baltimore, who helped lead the study. Those with negative levels, even if they responded, tended to have lower titers, he said.
“The benefits at least from an antibody perspective were not the same for everybody and so this is obviously something that needs to be considered when thinking about selecting patients” for a COVID-19 prevention strategy, he said.
Reactions to the vaccine were low to moderate, such as some arm pain and fatigue.
“Showing that something is safe in that special, vulnerable population is important,” Dr. Werbel said. “We’re all wanting to make sure that we’re doing no harm.”
Dr. Werbel noted that there was no pattern in the small series based on the organ transplanted or in the vaccines used. As their third shot, 15 of the patients received the Johnson & Johnson vaccine; 9 received Moderna; and 6 received Pfizer-BioNTech.
Welcome news, but larger studies needed
“To think that a third dose could confer protection for a significant number of people is of course extremely welcome news,” said Christian Larsen, MD, DPhil, professor of surgery in the transplantation division at Emory University, Atlanta, who was not involved in the study. “It’s the easiest conceivable next intervention.”
He added, “We just want studies to confirm that – larger studies.”
Dr. Werbel stressed the importance of looking at third doses in these patients in a more controlled fashion in a randomized trial, to more carefully monitor safety and how patients fare when starting with one type of vaccine and switching to another, for example.
Richard Wender, MD, chair of family medicine and community health at the University of Pennsylvania, Philadelphia, said the findings are a reminder that there is still a lot that is unknown about COVID-19 and vaccination.
“We still don’t know who will or will not benefit from a third dose,” he said. “And our knowledge is evolving. For example, a recent study suggested that people with previous infection and who are vaccinated may have better and longer protection than people with vaccination alone. We’re still learning.”
He added that specialists, not primary care clinicians, should be relied upon to respond to this emerging vaccination data. Primary care doctors are very busy in other ways – such as in getting children caught up on vaccinations and helping adults return to managing their chronic diseases, Dr. Wender noted.
“Their focus needs to be on helping to overcome hesitancy, mistrust, lack of information, or antivaccination sentiment to help more people feel comfortable being vaccinated – this is a lot of work and needs constant focus. In short, primary care clinicians need to focus chiefly on the unvaccinated,” he said.
“Monitoring immunization recommendations for unique at-risk populations should be the chief responsibility of teams providing subspecialty care, [such as for] transplant patients, people with chronic kidney disease, cancer patients, and people with other chronic illnesses. This will allow primary care clinicians to tackle their many complex jobs.”
Possible solutions for those with low antibody responses
Dr. Larsen said that those with ongoing low antibody responses might still have other immune responses, such as a T-cell response. Such patients also could consider changing their vaccine type, he said.
“At the more significant intervention level, there may be circumstances where one could change the immunosuppressive drugs in a controlled way that might allow a better response,” suggested Dr. Larsen. “That’s obviously going to be something that requires a lot more thought and careful study.”
Dr. Werbel said that other options might need to be considered for those having no response following a third dose. One possibility is trying a vaccine with an adjuvant, such as the Novavax version, which might be more widely available soon.
“If you’re given a third dose of a very immunogenic vaccine – something that should work – and you just have no antibody development, it seems relatively unlikely that doing the same thing again is going to help you from that perspective, and for all we know might expose you to more risk,” Dr. Werbel noted.
Participant details
None of the 30 patients were thought to have ever had COVID-19. On average, patients had received their transplant 4.5 years before their original vaccination. In 25 patients, maintenance immunosuppression included tacrolimus or cyclosporine along with mycophenolate. Corticosteroids were also used for 24 patients, sirolimus was used for one patient, and belatacept was used for another patient.
Fifty-seven percent of patients had received the Pfizer/BioNTech vaccine originally, and 43% the Moderna vaccine. Most of the patients were kidney recipients, with two heart, three liver, one lung, one pancreas and one kidney-pancreas.
Dr. Werbel, Dr. Wender, and Dr. Larsen reported no relevant disclosures.
All of those with low titers before the third dose had high titers after receiving the additional shot, but only about 33% of those with negative initial responses had detectable antibodies after the third dose, according to the paper, published in Annals of Internal Medicine.
Researchers at Johns Hopkins, Baltimore, who keep a COVID-19 vaccine registry, perform antibody tests on all registry subjects and inform them of their results. Registry participants were asked to inform the research team if they received a third dose, and, the research team tracked the immune responses of those who did.
The participants in this case series had low antibody levels and received a third dose of the vaccine on their own between March 20 and May 10 of 2021.
Third dose results
In this cases series – thought to be the first to look at third vaccine shots in this type of patient group – all six of those who had low antibody titers before the third dose had high-positive titers after the third dose.
Of the 24 individuals who had negative antibody titers before the third dose, just 6 had high titers after the third dose.
Two of the participants had low-positive titers, and 16 were negative.
“Several of those boosted very nicely into ranges seen, using these assays, in healthy persons,” said William Werbel, MD, a fellow in infectious disease at Johns Hopkins Medicine, Baltimore, who helped lead the study. Those with negative levels, even if they responded, tended to have lower titers, he said.
“The benefits at least from an antibody perspective were not the same for everybody and so this is obviously something that needs to be considered when thinking about selecting patients” for a COVID-19 prevention strategy, he said.
Reactions to the vaccine were low to moderate, such as some arm pain and fatigue.
“Showing that something is safe in that special, vulnerable population is important,” Dr. Werbel said. “We’re all wanting to make sure that we’re doing no harm.”
Dr. Werbel noted that there was no pattern in the small series based on the organ transplanted or in the vaccines used. As their third shot, 15 of the patients received the Johnson & Johnson vaccine; 9 received Moderna; and 6 received Pfizer-BioNTech.
Welcome news, but larger studies needed
“To think that a third dose could confer protection for a significant number of people is of course extremely welcome news,” said Christian Larsen, MD, DPhil, professor of surgery in the transplantation division at Emory University, Atlanta, who was not involved in the study. “It’s the easiest conceivable next intervention.”
He added, “We just want studies to confirm that – larger studies.”
Dr. Werbel stressed the importance of looking at third doses in these patients in a more controlled fashion in a randomized trial, to more carefully monitor safety and how patients fare when starting with one type of vaccine and switching to another, for example.
Richard Wender, MD, chair of family medicine and community health at the University of Pennsylvania, Philadelphia, said the findings are a reminder that there is still a lot that is unknown about COVID-19 and vaccination.
“We still don’t know who will or will not benefit from a third dose,” he said. “And our knowledge is evolving. For example, a recent study suggested that people with previous infection and who are vaccinated may have better and longer protection than people with vaccination alone. We’re still learning.”
He added that specialists, not primary care clinicians, should be relied upon to respond to this emerging vaccination data. Primary care doctors are very busy in other ways – such as in getting children caught up on vaccinations and helping adults return to managing their chronic diseases, Dr. Wender noted.
“Their focus needs to be on helping to overcome hesitancy, mistrust, lack of information, or antivaccination sentiment to help more people feel comfortable being vaccinated – this is a lot of work and needs constant focus. In short, primary care clinicians need to focus chiefly on the unvaccinated,” he said.
“Monitoring immunization recommendations for unique at-risk populations should be the chief responsibility of teams providing subspecialty care, [such as for] transplant patients, people with chronic kidney disease, cancer patients, and people with other chronic illnesses. This will allow primary care clinicians to tackle their many complex jobs.”
Possible solutions for those with low antibody responses
Dr. Larsen said that those with ongoing low antibody responses might still have other immune responses, such as a T-cell response. Such patients also could consider changing their vaccine type, he said.
“At the more significant intervention level, there may be circumstances where one could change the immunosuppressive drugs in a controlled way that might allow a better response,” suggested Dr. Larsen. “That’s obviously going to be something that requires a lot more thought and careful study.”
Dr. Werbel said that other options might need to be considered for those having no response following a third dose. One possibility is trying a vaccine with an adjuvant, such as the Novavax version, which might be more widely available soon.
“If you’re given a third dose of a very immunogenic vaccine – something that should work – and you just have no antibody development, it seems relatively unlikely that doing the same thing again is going to help you from that perspective, and for all we know might expose you to more risk,” Dr. Werbel noted.
Participant details
None of the 30 patients were thought to have ever had COVID-19. On average, patients had received their transplant 4.5 years before their original vaccination. In 25 patients, maintenance immunosuppression included tacrolimus or cyclosporine along with mycophenolate. Corticosteroids were also used for 24 patients, sirolimus was used for one patient, and belatacept was used for another patient.
Fifty-seven percent of patients had received the Pfizer/BioNTech vaccine originally, and 43% the Moderna vaccine. Most of the patients were kidney recipients, with two heart, three liver, one lung, one pancreas and one kidney-pancreas.
Dr. Werbel, Dr. Wender, and Dr. Larsen reported no relevant disclosures.
Medtronic yanks Heartware VAD, calls for halt to new implants
Medtronic has stopped the sale of its Heartware Ventricular Assist Device (HVAD) system and is advising that physicians cease implanting the device because problems with an internal pump can lead to death or serious injuries.
“There is an increased risk of neurological adverse events and mortality associated with the internal pump,” the U.S. Food and Drug Administration announced today.
There is also a potential for the internal pump to stop, and there may be delay or failure to restart. “Both problems may lead to death or serious injuries,” the agency said.
Between January 2009 and April 22, 2021, Medtronic received a total of 106 complaints involving delay or failure to restart with the HVAD pump. Of these, 26 complaints involved HVAD devices operating under normal conditions (dual stator mode) and 80 involved devices operating in a back-up mode (single stator mode) that allows for continued pump function if electrical continuity between the pump and controller is interrupted.
Of the 26 complaints that occurred under normal conditions, four resulted in patient death and five led to urgent explant. Of the 80 complaints that occurred in single stator mode, 10 deaths and eight explants were reported to Medtronic, according to an urgent medical device communication letter issued by the company today.
“Considering these findings and given the availability of alternative devices such as the Abbott HeartMate 3, Medtronic has made the decision to stop the distribution and sale of the HVAD System,” the letter says. “Medtronic advises that there be no further implantations of the HVAD System.”
Medtronic undertook a previous recall of the Heartware HVAD system in February, focusing on batteries, power, datalink cables, and other peripheral equipment, because of the “risk of wear and tear of the connector plugs (power sources, data cable, and alarm adapter), which could cause damage to the controller port metal pins (for example, bent pins).” The FDA deemed that recall Class I, the most serious category of safety alert, in April.
The company noted that patients who currently have an HVAD implant “may require support for many years,” and that it is moving as quickly as possible to create a plan to guide the ongoing support for patients, caregivers, and health care professionals.
In response to the restart failure issue and evolving data about neurologic risks associated with the HVAD pump, Medtronic said it engaged an Independent Practitioner Quality Panel (IPQP), composed of cardiologists, surgeons, and VAD coordinators, to advise on recommendations for appropriate patient management. Based on information collected to date and IPQP input, Medtronic is recommending that physicians continue following best clinical practices and manage patients implanted with the HVAD pump according to the recommendations in the Instructions for Use (IFU).
“Prophylactic explant of the HVAD™ device is not recommended, as risks associated with explantation may outweigh the potential benefits,” the letter says. “The decision regarding explant and exchange of the HVAD™ pump should be made by physicians on a case-by-case basis, considering the patient’s clinical condition and surgical risks. If a physician determines that pump exchange is appropriate, we recommend exchanging to an alternative commercial LVAD.”
For patients in urgent need of an LVAD, Medtronic said physicians should use an alternative commercial LVAD or, if one is not available, that “a Patient Information form is required to be completed by you and your patient to acknowledge the risks of an HVAD implant prior to implanting your HVAD inventory.”
Today’s letter also provides recommendations on blood pressure management goals and anticoagulation. For any other questions or concerns, physicians should contact the Medtronic Office of Medical Affairs at: [email protected].
Medtronic issued another urgent letter in December 2020, warning physicians that a subset of HVAD devices included an internal pump component from three specific lots that increased the risk for restart failure. At that time, the company had not been able to pinpoint a root cause of the pump restart failure.
Consistent with the December 2020 notice, the rate of failure among pumps outside of the subset of three specific lots currently remains at about 0.4%, according to today’s notice.
Although Medtronic has identified the root cause and mitigations for pumps within the three specific lots, it has not been able to identify a root cause of the other restart failures reported with the HVAD pumps, the company said.
A version of this article first appeared on Medscape.com.
Medtronic has stopped the sale of its Heartware Ventricular Assist Device (HVAD) system and is advising that physicians cease implanting the device because problems with an internal pump can lead to death or serious injuries.
“There is an increased risk of neurological adverse events and mortality associated with the internal pump,” the U.S. Food and Drug Administration announced today.
There is also a potential for the internal pump to stop, and there may be delay or failure to restart. “Both problems may lead to death or serious injuries,” the agency said.
Between January 2009 and April 22, 2021, Medtronic received a total of 106 complaints involving delay or failure to restart with the HVAD pump. Of these, 26 complaints involved HVAD devices operating under normal conditions (dual stator mode) and 80 involved devices operating in a back-up mode (single stator mode) that allows for continued pump function if electrical continuity between the pump and controller is interrupted.
Of the 26 complaints that occurred under normal conditions, four resulted in patient death and five led to urgent explant. Of the 80 complaints that occurred in single stator mode, 10 deaths and eight explants were reported to Medtronic, according to an urgent medical device communication letter issued by the company today.
“Considering these findings and given the availability of alternative devices such as the Abbott HeartMate 3, Medtronic has made the decision to stop the distribution and sale of the HVAD System,” the letter says. “Medtronic advises that there be no further implantations of the HVAD System.”
Medtronic undertook a previous recall of the Heartware HVAD system in February, focusing on batteries, power, datalink cables, and other peripheral equipment, because of the “risk of wear and tear of the connector plugs (power sources, data cable, and alarm adapter), which could cause damage to the controller port metal pins (for example, bent pins).” The FDA deemed that recall Class I, the most serious category of safety alert, in April.
The company noted that patients who currently have an HVAD implant “may require support for many years,” and that it is moving as quickly as possible to create a plan to guide the ongoing support for patients, caregivers, and health care professionals.
In response to the restart failure issue and evolving data about neurologic risks associated with the HVAD pump, Medtronic said it engaged an Independent Practitioner Quality Panel (IPQP), composed of cardiologists, surgeons, and VAD coordinators, to advise on recommendations for appropriate patient management. Based on information collected to date and IPQP input, Medtronic is recommending that physicians continue following best clinical practices and manage patients implanted with the HVAD pump according to the recommendations in the Instructions for Use (IFU).
“Prophylactic explant of the HVAD™ device is not recommended, as risks associated with explantation may outweigh the potential benefits,” the letter says. “The decision regarding explant and exchange of the HVAD™ pump should be made by physicians on a case-by-case basis, considering the patient’s clinical condition and surgical risks. If a physician determines that pump exchange is appropriate, we recommend exchanging to an alternative commercial LVAD.”
For patients in urgent need of an LVAD, Medtronic said physicians should use an alternative commercial LVAD or, if one is not available, that “a Patient Information form is required to be completed by you and your patient to acknowledge the risks of an HVAD implant prior to implanting your HVAD inventory.”
Today’s letter also provides recommendations on blood pressure management goals and anticoagulation. For any other questions or concerns, physicians should contact the Medtronic Office of Medical Affairs at: [email protected].
Medtronic issued another urgent letter in December 2020, warning physicians that a subset of HVAD devices included an internal pump component from three specific lots that increased the risk for restart failure. At that time, the company had not been able to pinpoint a root cause of the pump restart failure.
Consistent with the December 2020 notice, the rate of failure among pumps outside of the subset of three specific lots currently remains at about 0.4%, according to today’s notice.
Although Medtronic has identified the root cause and mitigations for pumps within the three specific lots, it has not been able to identify a root cause of the other restart failures reported with the HVAD pumps, the company said.
A version of this article first appeared on Medscape.com.
Medtronic has stopped the sale of its Heartware Ventricular Assist Device (HVAD) system and is advising that physicians cease implanting the device because problems with an internal pump can lead to death or serious injuries.
“There is an increased risk of neurological adverse events and mortality associated with the internal pump,” the U.S. Food and Drug Administration announced today.
There is also a potential for the internal pump to stop, and there may be delay or failure to restart. “Both problems may lead to death or serious injuries,” the agency said.
Between January 2009 and April 22, 2021, Medtronic received a total of 106 complaints involving delay or failure to restart with the HVAD pump. Of these, 26 complaints involved HVAD devices operating under normal conditions (dual stator mode) and 80 involved devices operating in a back-up mode (single stator mode) that allows for continued pump function if electrical continuity between the pump and controller is interrupted.
Of the 26 complaints that occurred under normal conditions, four resulted in patient death and five led to urgent explant. Of the 80 complaints that occurred in single stator mode, 10 deaths and eight explants were reported to Medtronic, according to an urgent medical device communication letter issued by the company today.
“Considering these findings and given the availability of alternative devices such as the Abbott HeartMate 3, Medtronic has made the decision to stop the distribution and sale of the HVAD System,” the letter says. “Medtronic advises that there be no further implantations of the HVAD System.”
Medtronic undertook a previous recall of the Heartware HVAD system in February, focusing on batteries, power, datalink cables, and other peripheral equipment, because of the “risk of wear and tear of the connector plugs (power sources, data cable, and alarm adapter), which could cause damage to the controller port metal pins (for example, bent pins).” The FDA deemed that recall Class I, the most serious category of safety alert, in April.
The company noted that patients who currently have an HVAD implant “may require support for many years,” and that it is moving as quickly as possible to create a plan to guide the ongoing support for patients, caregivers, and health care professionals.
In response to the restart failure issue and evolving data about neurologic risks associated with the HVAD pump, Medtronic said it engaged an Independent Practitioner Quality Panel (IPQP), composed of cardiologists, surgeons, and VAD coordinators, to advise on recommendations for appropriate patient management. Based on information collected to date and IPQP input, Medtronic is recommending that physicians continue following best clinical practices and manage patients implanted with the HVAD pump according to the recommendations in the Instructions for Use (IFU).
“Prophylactic explant of the HVAD™ device is not recommended, as risks associated with explantation may outweigh the potential benefits,” the letter says. “The decision regarding explant and exchange of the HVAD™ pump should be made by physicians on a case-by-case basis, considering the patient’s clinical condition and surgical risks. If a physician determines that pump exchange is appropriate, we recommend exchanging to an alternative commercial LVAD.”
For patients in urgent need of an LVAD, Medtronic said physicians should use an alternative commercial LVAD or, if one is not available, that “a Patient Information form is required to be completed by you and your patient to acknowledge the risks of an HVAD implant prior to implanting your HVAD inventory.”
Today’s letter also provides recommendations on blood pressure management goals and anticoagulation. For any other questions or concerns, physicians should contact the Medtronic Office of Medical Affairs at: [email protected].
Medtronic issued another urgent letter in December 2020, warning physicians that a subset of HVAD devices included an internal pump component from three specific lots that increased the risk for restart failure. At that time, the company had not been able to pinpoint a root cause of the pump restart failure.
Consistent with the December 2020 notice, the rate of failure among pumps outside of the subset of three specific lots currently remains at about 0.4%, according to today’s notice.
Although Medtronic has identified the root cause and mitigations for pumps within the three specific lots, it has not been able to identify a root cause of the other restart failures reported with the HVAD pumps, the company said.
A version of this article first appeared on Medscape.com.