User login
FFR-guided PCI in stable CAD beats medical management
DENVER – Percutaneous coronary intervention plus optimal medical therapy in stable coronary artery disease (CAD) patients with at least one coronary lesion having an abnormal fractional flow reserve measurement resulted in superior clinical outcomes, better quality of life, and virtually identical cost, compared with optimal medical management alone over 3 years of follow-up in the FAME 2 trial.
“These results reinforce the point that the greater the burden of ischemia, the greater the benefit of revascularization with PCI [percutaneous coronary intervention],” William F. Fearon, MD, said while presenting the FAME 2 findings at the Transcatheter Cardiovascular Therapeutics annual meeting.
FAME 2 was a randomized, multicenter trial designed to help bring clarity regarding the optimal treatment strategy for patients with stable angina and CAD. This is an issue surrounded by considerable controversy. The fog descended a decade ago, when the COURAGE trial created a stir with its conclusion that optimal medical therapy (OMT) alone was as good as PCI plus OMT in terms of clinical outcome and quality of life – and was considerably less expensive as well. And the British ORBITA trial, presented earlier in the same session at TCT 2017 as Dr. Fearon’s report on FAME 2, caused an uproar with its finding that PCI plus OMT was no more effective than sham PCI plus OMT in the setting of stable CAD.
However, FAME 2 (Fractional Flow Reserve versus Angiography for Multivessel Evaluation) differed from those studies in a crucial aspect: randomization in FAME 2 was restricted to patients with physiologically significant cardiac ischemia as evidenced by a fractional flow reserve (FFR) measurement of 0.80 or less.
In contrast, COURAGE and ORBITA randomized patients without FFR guidance. As a result, in those trials PCI was performed in a substantial proportion of patients who actually should not have undergone the intervention because they didn’t have physiologic evidence of clinically important ischemia. The non–physiologically based approach to PCI utilized in COURAGE and ORBITA – disappointingly commonplace in daily clinical practice – diluted any true benefit of the procedure when applied appropriately, explained Dr. Fearon, professor of medicine and director of interventional cardiology at Stanford (Calif.) University.
FAME 2 randomized 888 patients with stable single- or multivessel CAD and an FFR of 0.80 or less to PCI plus OMT or an initial strategy of OMT alone at 28 European and North American sites. The primary outcome was the rate of major adverse cardiac events – death, MI, and urgent revascularization – at 3 years. The rate was 10.1% in the PCI group, compared with 22% in the medically managed cohort, Dr. Fearon reported at the meeting sponsored by the Cardiovascular Research Foundation.
Death or MI occurred in 8.3% of the PCI group versus 10.4% in the OMT group, a trend that didn’t reach significance. Of note, however, fully 44% of patients in the OMT group crossed over to PCI during the 3-year study. In the prespecified intent-to-treat analysis they were counted in the OMT group, whereas an as-treated analysis might well have shown statistically significant reductions in death and MI in the PCI group.
The proportion of patients with class II-IV angina was significantly lower in the PCI plus medical therapy group at every time point, including 5.9% versus 15.2% for OMT alone at 1 year, 5.9% versus 12% at 2 years, and 5.2% versus 9.7% at 3 years. This was the case even though the OMT group received significantly more antianginal therapy in an effort to control symptoms.
FAME 2 featured a first-of-its-kind comprehensive cost-effectiveness analysis of OMT vs. PCI over a 3-year period. It showed that, while mean initial costs were as expected higher in the PCI group ($9,944 versus $4,440), by 3 years the cumulative costs were near identical at $16,792 in the PCI group and $16,737 in the initial OMT group. The incremental cost-effectiveness ratio for PCI, compared with OMT at 3 years was attractive at $1,600 per quality-adjusted life-year gained.
The question on TCT attendees’ minds following presentation of the bombshell ORBITA findings was, what would have happened had FAME 2 featured a sham PCI arm? Could the advantageous outcomes for the initial PCI strategy seen in FAME 2 possibly have been due to a placebo effect?
Extremely unlikely, according to Dr. Fearon. For one thing, when he and his coinvestigators broke down the FFR values in the OMT group into quintiles, they saw a clear dose-response effect: The clinical event rate rose further with worsening quintile of FFR. Also, the study endpoints were death, MI, and urgent revascularization – triggered by ACS in half of cases – which are less susceptible to a placebo effect than, say, treadmill exercise time, the primary endpoint in ORBITA.
Moreover, as noted by Gary S. Mintz, MD, who moderated a press conference highlighting the ORBITA and FAME 2 results, placebo effects don’t last for years.
“Most people would say the placebo effect wanes over time. That’s why these 3-year data, analyzed by intent-to-treat, which allow for crossovers to still be analyzed in the medical therapy arm, are pretty compelling to me,” commented Dr. Mintz, chief medical officer for the Cardiovascular Research Foundation in New York.
FAME 2 was supported by St. Jude Medical. Dr. Fearon reported receiving institutional research support from Medtronic, Abbott Vascular, ACIST Medical, CathWorks, and Edwards LifeSciences.
DENVER – Percutaneous coronary intervention plus optimal medical therapy in stable coronary artery disease (CAD) patients with at least one coronary lesion having an abnormal fractional flow reserve measurement resulted in superior clinical outcomes, better quality of life, and virtually identical cost, compared with optimal medical management alone over 3 years of follow-up in the FAME 2 trial.
“These results reinforce the point that the greater the burden of ischemia, the greater the benefit of revascularization with PCI [percutaneous coronary intervention],” William F. Fearon, MD, said while presenting the FAME 2 findings at the Transcatheter Cardiovascular Therapeutics annual meeting.
FAME 2 was a randomized, multicenter trial designed to help bring clarity regarding the optimal treatment strategy for patients with stable angina and CAD. This is an issue surrounded by considerable controversy. The fog descended a decade ago, when the COURAGE trial created a stir with its conclusion that optimal medical therapy (OMT) alone was as good as PCI plus OMT in terms of clinical outcome and quality of life – and was considerably less expensive as well. And the British ORBITA trial, presented earlier in the same session at TCT 2017 as Dr. Fearon’s report on FAME 2, caused an uproar with its finding that PCI plus OMT was no more effective than sham PCI plus OMT in the setting of stable CAD.
However, FAME 2 (Fractional Flow Reserve versus Angiography for Multivessel Evaluation) differed from those studies in a crucial aspect: randomization in FAME 2 was restricted to patients with physiologically significant cardiac ischemia as evidenced by a fractional flow reserve (FFR) measurement of 0.80 or less.
In contrast, COURAGE and ORBITA randomized patients without FFR guidance. As a result, in those trials PCI was performed in a substantial proportion of patients who actually should not have undergone the intervention because they didn’t have physiologic evidence of clinically important ischemia. The non–physiologically based approach to PCI utilized in COURAGE and ORBITA – disappointingly commonplace in daily clinical practice – diluted any true benefit of the procedure when applied appropriately, explained Dr. Fearon, professor of medicine and director of interventional cardiology at Stanford (Calif.) University.
FAME 2 randomized 888 patients with stable single- or multivessel CAD and an FFR of 0.80 or less to PCI plus OMT or an initial strategy of OMT alone at 28 European and North American sites. The primary outcome was the rate of major adverse cardiac events – death, MI, and urgent revascularization – at 3 years. The rate was 10.1% in the PCI group, compared with 22% in the medically managed cohort, Dr. Fearon reported at the meeting sponsored by the Cardiovascular Research Foundation.
Death or MI occurred in 8.3% of the PCI group versus 10.4% in the OMT group, a trend that didn’t reach significance. Of note, however, fully 44% of patients in the OMT group crossed over to PCI during the 3-year study. In the prespecified intent-to-treat analysis they were counted in the OMT group, whereas an as-treated analysis might well have shown statistically significant reductions in death and MI in the PCI group.
The proportion of patients with class II-IV angina was significantly lower in the PCI plus medical therapy group at every time point, including 5.9% versus 15.2% for OMT alone at 1 year, 5.9% versus 12% at 2 years, and 5.2% versus 9.7% at 3 years. This was the case even though the OMT group received significantly more antianginal therapy in an effort to control symptoms.
FAME 2 featured a first-of-its-kind comprehensive cost-effectiveness analysis of OMT vs. PCI over a 3-year period. It showed that, while mean initial costs were as expected higher in the PCI group ($9,944 versus $4,440), by 3 years the cumulative costs were near identical at $16,792 in the PCI group and $16,737 in the initial OMT group. The incremental cost-effectiveness ratio for PCI, compared with OMT at 3 years was attractive at $1,600 per quality-adjusted life-year gained.
The question on TCT attendees’ minds following presentation of the bombshell ORBITA findings was, what would have happened had FAME 2 featured a sham PCI arm? Could the advantageous outcomes for the initial PCI strategy seen in FAME 2 possibly have been due to a placebo effect?
Extremely unlikely, according to Dr. Fearon. For one thing, when he and his coinvestigators broke down the FFR values in the OMT group into quintiles, they saw a clear dose-response effect: The clinical event rate rose further with worsening quintile of FFR. Also, the study endpoints were death, MI, and urgent revascularization – triggered by ACS in half of cases – which are less susceptible to a placebo effect than, say, treadmill exercise time, the primary endpoint in ORBITA.
Moreover, as noted by Gary S. Mintz, MD, who moderated a press conference highlighting the ORBITA and FAME 2 results, placebo effects don’t last for years.
“Most people would say the placebo effect wanes over time. That’s why these 3-year data, analyzed by intent-to-treat, which allow for crossovers to still be analyzed in the medical therapy arm, are pretty compelling to me,” commented Dr. Mintz, chief medical officer for the Cardiovascular Research Foundation in New York.
FAME 2 was supported by St. Jude Medical. Dr. Fearon reported receiving institutional research support from Medtronic, Abbott Vascular, ACIST Medical, CathWorks, and Edwards LifeSciences.
DENVER – Percutaneous coronary intervention plus optimal medical therapy in stable coronary artery disease (CAD) patients with at least one coronary lesion having an abnormal fractional flow reserve measurement resulted in superior clinical outcomes, better quality of life, and virtually identical cost, compared with optimal medical management alone over 3 years of follow-up in the FAME 2 trial.
“These results reinforce the point that the greater the burden of ischemia, the greater the benefit of revascularization with PCI [percutaneous coronary intervention],” William F. Fearon, MD, said while presenting the FAME 2 findings at the Transcatheter Cardiovascular Therapeutics annual meeting.
FAME 2 was a randomized, multicenter trial designed to help bring clarity regarding the optimal treatment strategy for patients with stable angina and CAD. This is an issue surrounded by considerable controversy. The fog descended a decade ago, when the COURAGE trial created a stir with its conclusion that optimal medical therapy (OMT) alone was as good as PCI plus OMT in terms of clinical outcome and quality of life – and was considerably less expensive as well. And the British ORBITA trial, presented earlier in the same session at TCT 2017 as Dr. Fearon’s report on FAME 2, caused an uproar with its finding that PCI plus OMT was no more effective than sham PCI plus OMT in the setting of stable CAD.
However, FAME 2 (Fractional Flow Reserve versus Angiography for Multivessel Evaluation) differed from those studies in a crucial aspect: randomization in FAME 2 was restricted to patients with physiologically significant cardiac ischemia as evidenced by a fractional flow reserve (FFR) measurement of 0.80 or less.
In contrast, COURAGE and ORBITA randomized patients without FFR guidance. As a result, in those trials PCI was performed in a substantial proportion of patients who actually should not have undergone the intervention because they didn’t have physiologic evidence of clinically important ischemia. The non–physiologically based approach to PCI utilized in COURAGE and ORBITA – disappointingly commonplace in daily clinical practice – diluted any true benefit of the procedure when applied appropriately, explained Dr. Fearon, professor of medicine and director of interventional cardiology at Stanford (Calif.) University.
FAME 2 randomized 888 patients with stable single- or multivessel CAD and an FFR of 0.80 or less to PCI plus OMT or an initial strategy of OMT alone at 28 European and North American sites. The primary outcome was the rate of major adverse cardiac events – death, MI, and urgent revascularization – at 3 years. The rate was 10.1% in the PCI group, compared with 22% in the medically managed cohort, Dr. Fearon reported at the meeting sponsored by the Cardiovascular Research Foundation.
Death or MI occurred in 8.3% of the PCI group versus 10.4% in the OMT group, a trend that didn’t reach significance. Of note, however, fully 44% of patients in the OMT group crossed over to PCI during the 3-year study. In the prespecified intent-to-treat analysis they were counted in the OMT group, whereas an as-treated analysis might well have shown statistically significant reductions in death and MI in the PCI group.
The proportion of patients with class II-IV angina was significantly lower in the PCI plus medical therapy group at every time point, including 5.9% versus 15.2% for OMT alone at 1 year, 5.9% versus 12% at 2 years, and 5.2% versus 9.7% at 3 years. This was the case even though the OMT group received significantly more antianginal therapy in an effort to control symptoms.
FAME 2 featured a first-of-its-kind comprehensive cost-effectiveness analysis of OMT vs. PCI over a 3-year period. It showed that, while mean initial costs were as expected higher in the PCI group ($9,944 versus $4,440), by 3 years the cumulative costs were near identical at $16,792 in the PCI group and $16,737 in the initial OMT group. The incremental cost-effectiveness ratio for PCI, compared with OMT at 3 years was attractive at $1,600 per quality-adjusted life-year gained.
The question on TCT attendees’ minds following presentation of the bombshell ORBITA findings was, what would have happened had FAME 2 featured a sham PCI arm? Could the advantageous outcomes for the initial PCI strategy seen in FAME 2 possibly have been due to a placebo effect?
Extremely unlikely, according to Dr. Fearon. For one thing, when he and his coinvestigators broke down the FFR values in the OMT group into quintiles, they saw a clear dose-response effect: The clinical event rate rose further with worsening quintile of FFR. Also, the study endpoints were death, MI, and urgent revascularization – triggered by ACS in half of cases – which are less susceptible to a placebo effect than, say, treadmill exercise time, the primary endpoint in ORBITA.
Moreover, as noted by Gary S. Mintz, MD, who moderated a press conference highlighting the ORBITA and FAME 2 results, placebo effects don’t last for years.
“Most people would say the placebo effect wanes over time. That’s why these 3-year data, analyzed by intent-to-treat, which allow for crossovers to still be analyzed in the medical therapy arm, are pretty compelling to me,” commented Dr. Mintz, chief medical officer for the Cardiovascular Research Foundation in New York.
FAME 2 was supported by St. Jude Medical. Dr. Fearon reported receiving institutional research support from Medtronic, Abbott Vascular, ACIST Medical, CathWorks, and Edwards LifeSciences.
REPORTING FROM TCT 2017
Key clinical point:
Major finding: The rate of major adverse cardiac events at 3 years was 10.1% in the PCI group and 22% in the medically managed cohort.
Data source: The FAME 2 trial randomized 888 patients with stable single- or multivessel CAD and an FFR of 0.80 or less to PCI plus optimal medical therapy or an initial strategy of optimal medical management alone.
Disclosures: The trial was supported by St. Jude Medical. The presenter reported receiving institutional research support from Medtronic, Abbott Vascular, ACIST Medical, CathWorks, and Edwards LifeSciences.
New anticoagulants pose challenges for surgeons
SAN DIEGO – A new class of is now commonly seen in patients and can require new strategies for management in the surgical setting. New drugs to reverse anticoagulation agents now need to be routinely considered in advance of surgery.
“They’re all over the place. It feels like everyone I see is on an anticoagulant, especially the new anticoagulants,” said Carlos V.R. Brown, MD, FACS, associate professor of surgery and chief of the division of acute care surgery at the University of Texas at Austin. “There’s a lot more learning that has to take place into how these medications work and how to take care of patients who use them.”
“Warfarin is slow, unpredictable, and requires monitoring and dose adjustment,” he said. In addition, interactions with food and other medications can be problematic, he said. The injectable drug heparin, meanwhile, requires monitoring and frequent dose adjustments, he said. “We’re in search of the ideal anticoagulant – one that’s oral, has a wide therapeutic window, is very predictable with rapid onset, and has minimal interaction with other food or drugs.”
Here’s the hitch, he said: “It probably doesn’t exist.”
There are now several alternatives to the old standbys on the market. One class, the direct thrombin inhibitor, is led by dabigatran etexilate (Pradaxa). Another class, the factor Xa inhibitors, includes rivaroxaban (Xarelto), apixaban (Eliquis), and edoxaban (Savaysa). From an elective surgery standpoint, Dr. Brown said in an interview, it has become important to understand how to reverse the effects of anticoagulants before a procedure.
To determine levels of the drugs, a TT (thrombin time) screening test is recommended for dabigatran and an anti-Xa test for rivaroxaban, apixaban, and edoxaban said Dr. Brown, referring to a 2017 study published in Critical Care Clinics. The paper summarized the available evidence and provided the optimal reversal strategy for bleeding patients with trauma on novel oral anticoagulants. The report also noted that newer blood thinners have a half-life of 7 or 12 hours and reach peak plasma level at 1-4 hours, depending on the medication (Crit Care Clin. 2017;33[1]135-52).
There may be no time to determine blood thinner levels in emergency situations. In those cases, patient or caregiver history about recent doses can be crucial, Dr. Brown said. “Knowing the patient’s history is going to be a key component,” he said.
Surgeons can turn to a variety of options to reverse the newer anticoagulants in an emergent setting, but only dabigatran has a Food and Drug Administration–approved reversal agent. Activated charcoal, PCC (Kcentra) and aPCC (FEIBA) can reverse dabigatran and oral factor Xa inhibitors, Dr. Brown said. Dialysis is also an option for dabigatran.
Another option to reverse dabigatran may be idarucizumab (Praxbind), a reversal agent. A 2017 industry-funded, open-label study reported successful results. It has been shown to work rapidly, Dr. Brown said, and the drug is now FDA approved (N Engl J Med. 2017 Aug 3;377[5]:431-41).
For oral factor Xa inhibitors, Dr. Brown said, andexanet alfa is now in a trial and doesn’t yet have FDA approval. “Presumably, it will provide a benefit over PCC because it’s directed at that specific medication,” he said.
Dr. Brown cautioned about the risks of reversing anticoagulants. “Any time you’re reversing an anticoagulant, the side effect is going to be clotting,” boosting the likelihood of events such as heart attack or stroke, he said. “You’re always weighing the risk versus the benefit of reversing.”
Dr. Brown has no relevant disclosures.
SAN DIEGO – A new class of is now commonly seen in patients and can require new strategies for management in the surgical setting. New drugs to reverse anticoagulation agents now need to be routinely considered in advance of surgery.
“They’re all over the place. It feels like everyone I see is on an anticoagulant, especially the new anticoagulants,” said Carlos V.R. Brown, MD, FACS, associate professor of surgery and chief of the division of acute care surgery at the University of Texas at Austin. “There’s a lot more learning that has to take place into how these medications work and how to take care of patients who use them.”
“Warfarin is slow, unpredictable, and requires monitoring and dose adjustment,” he said. In addition, interactions with food and other medications can be problematic, he said. The injectable drug heparin, meanwhile, requires monitoring and frequent dose adjustments, he said. “We’re in search of the ideal anticoagulant – one that’s oral, has a wide therapeutic window, is very predictable with rapid onset, and has minimal interaction with other food or drugs.”
Here’s the hitch, he said: “It probably doesn’t exist.”
There are now several alternatives to the old standbys on the market. One class, the direct thrombin inhibitor, is led by dabigatran etexilate (Pradaxa). Another class, the factor Xa inhibitors, includes rivaroxaban (Xarelto), apixaban (Eliquis), and edoxaban (Savaysa). From an elective surgery standpoint, Dr. Brown said in an interview, it has become important to understand how to reverse the effects of anticoagulants before a procedure.
To determine levels of the drugs, a TT (thrombin time) screening test is recommended for dabigatran and an anti-Xa test for rivaroxaban, apixaban, and edoxaban said Dr. Brown, referring to a 2017 study published in Critical Care Clinics. The paper summarized the available evidence and provided the optimal reversal strategy for bleeding patients with trauma on novel oral anticoagulants. The report also noted that newer blood thinners have a half-life of 7 or 12 hours and reach peak plasma level at 1-4 hours, depending on the medication (Crit Care Clin. 2017;33[1]135-52).
There may be no time to determine blood thinner levels in emergency situations. In those cases, patient or caregiver history about recent doses can be crucial, Dr. Brown said. “Knowing the patient’s history is going to be a key component,” he said.
Surgeons can turn to a variety of options to reverse the newer anticoagulants in an emergent setting, but only dabigatran has a Food and Drug Administration–approved reversal agent. Activated charcoal, PCC (Kcentra) and aPCC (FEIBA) can reverse dabigatran and oral factor Xa inhibitors, Dr. Brown said. Dialysis is also an option for dabigatran.
Another option to reverse dabigatran may be idarucizumab (Praxbind), a reversal agent. A 2017 industry-funded, open-label study reported successful results. It has been shown to work rapidly, Dr. Brown said, and the drug is now FDA approved (N Engl J Med. 2017 Aug 3;377[5]:431-41).
For oral factor Xa inhibitors, Dr. Brown said, andexanet alfa is now in a trial and doesn’t yet have FDA approval. “Presumably, it will provide a benefit over PCC because it’s directed at that specific medication,” he said.
Dr. Brown cautioned about the risks of reversing anticoagulants. “Any time you’re reversing an anticoagulant, the side effect is going to be clotting,” boosting the likelihood of events such as heart attack or stroke, he said. “You’re always weighing the risk versus the benefit of reversing.”
Dr. Brown has no relevant disclosures.
SAN DIEGO – A new class of is now commonly seen in patients and can require new strategies for management in the surgical setting. New drugs to reverse anticoagulation agents now need to be routinely considered in advance of surgery.
“They’re all over the place. It feels like everyone I see is on an anticoagulant, especially the new anticoagulants,” said Carlos V.R. Brown, MD, FACS, associate professor of surgery and chief of the division of acute care surgery at the University of Texas at Austin. “There’s a lot more learning that has to take place into how these medications work and how to take care of patients who use them.”
“Warfarin is slow, unpredictable, and requires monitoring and dose adjustment,” he said. In addition, interactions with food and other medications can be problematic, he said. The injectable drug heparin, meanwhile, requires monitoring and frequent dose adjustments, he said. “We’re in search of the ideal anticoagulant – one that’s oral, has a wide therapeutic window, is very predictable with rapid onset, and has minimal interaction with other food or drugs.”
Here’s the hitch, he said: “It probably doesn’t exist.”
There are now several alternatives to the old standbys on the market. One class, the direct thrombin inhibitor, is led by dabigatran etexilate (Pradaxa). Another class, the factor Xa inhibitors, includes rivaroxaban (Xarelto), apixaban (Eliquis), and edoxaban (Savaysa). From an elective surgery standpoint, Dr. Brown said in an interview, it has become important to understand how to reverse the effects of anticoagulants before a procedure.
To determine levels of the drugs, a TT (thrombin time) screening test is recommended for dabigatran and an anti-Xa test for rivaroxaban, apixaban, and edoxaban said Dr. Brown, referring to a 2017 study published in Critical Care Clinics. The paper summarized the available evidence and provided the optimal reversal strategy for bleeding patients with trauma on novel oral anticoagulants. The report also noted that newer blood thinners have a half-life of 7 or 12 hours and reach peak plasma level at 1-4 hours, depending on the medication (Crit Care Clin. 2017;33[1]135-52).
There may be no time to determine blood thinner levels in emergency situations. In those cases, patient or caregiver history about recent doses can be crucial, Dr. Brown said. “Knowing the patient’s history is going to be a key component,” he said.
Surgeons can turn to a variety of options to reverse the newer anticoagulants in an emergent setting, but only dabigatran has a Food and Drug Administration–approved reversal agent. Activated charcoal, PCC (Kcentra) and aPCC (FEIBA) can reverse dabigatran and oral factor Xa inhibitors, Dr. Brown said. Dialysis is also an option for dabigatran.
Another option to reverse dabigatran may be idarucizumab (Praxbind), a reversal agent. A 2017 industry-funded, open-label study reported successful results. It has been shown to work rapidly, Dr. Brown said, and the drug is now FDA approved (N Engl J Med. 2017 Aug 3;377[5]:431-41).
For oral factor Xa inhibitors, Dr. Brown said, andexanet alfa is now in a trial and doesn’t yet have FDA approval. “Presumably, it will provide a benefit over PCC because it’s directed at that specific medication,” he said.
Dr. Brown cautioned about the risks of reversing anticoagulants. “Any time you’re reversing an anticoagulant, the side effect is going to be clotting,” boosting the likelihood of events such as heart attack or stroke, he said. “You’re always weighing the risk versus the benefit of reversing.”
Dr. Brown has no relevant disclosures.
EXPERT OPINION FROM THE ACS CLINICAL CONGRESS
Benefit of dabigatran over warfarin persists in AF patient subgroups undergoing PCI
ANAHEIM, CALIF. – The benefit of dabigatran dual therapy versus warfarin triple therapy after percutaneous coronary intervention in patients with atrial fibrillation was consistent whether patients had drug-eluting or bare-metal stents, concomitant treatment with ticagrelor or clopidogrel, or acute coronary syndrome or stable disease as the indication for PCI, according to a subgroup analysis of the RE-DUAL PCI trial.
The trial, presented at the American Heart Association scientific sessions, randomized 2,725 patients to triple therapy with warfarin plus a P2Y12 inhibitor (clopidogrel or ticagrelor) and aspirin – the triple therapy group – or dabigatran 110 mg or 150 mg twice daily plus clopidogrel or ticagrelor – the dual therapy groups (N Engl J Med. 2017 Oct 19;377[16]:1513-24).
After a mean follow-up 14 months, the incidence of the major or clinically relevant nonmajor bleeding was 15.4% in the 110-mg dual-therapy group (hazard ratio, 0.52; 95% CI, 0.42-0.63; P less than .001) and 20.2% in the 150-mg dual-therapy group (HR, 0.72; 95% CI, 0.58-0.88; P less than .001), versus about 26% with triple-therapy.
The incidence of the composite efficacy endpoint – death, unplanned revascularization, myocardial infarction, stroke, or systemic embolism – was 13.7% in the two dual-therapy groups versus 13.4% with triple-therapy (HR, 1.04; 95% CI, 0.84-1.29; P = .005).
The investigators found consistent results when they analyzed their prespecified subgroups.
Drug-eluting stents were placed in 83% of patients; the rest had bare metal stents (BMS). The groups were well-balanced, except BMS patients were again more likely to be new to oral anticoagulation. Bleeding, thromboembolic events, and mortality were consistent with the main results regardless of the stent type, Most of the subjects were on clopidogrel, with just 12% on ticagrelor in both the dabigatran and warfarin groups. Ticagrelor patients were more likely to have ACS as their PCI indication and be new to oral anticoagulation. Ticagrelor patients were also more clinically complex, with a higher bleeding risk. Even so, they had relative bleeding risk reduction and efficacy results with dabigatran that were consistent with the overall finding, Dr. Oldgren said.
Patients were eligible for RE-DUAL PCI (Evaluation of Dual Therapy with Dabigatran vs. Triple Therapy with Warfarin in Patients with AF That Undergo a PCI with Stenting) if they had nonvalvular atrial fibrillation and a successful PCI within 120 hours. Those with bioprosthetic or mechanical heart valves, severe renal insufficiency, or other major comorbidities were excluded.
The trial was funded by Boehringer Ingelheim, the maker of dabigatran. Several investigators were employees. Dr. Oldgren is an adviser to Boehringer Ingelheim. Other authors reported financial ties to the company as well.
ANAHEIM, CALIF. – The benefit of dabigatran dual therapy versus warfarin triple therapy after percutaneous coronary intervention in patients with atrial fibrillation was consistent whether patients had drug-eluting or bare-metal stents, concomitant treatment with ticagrelor or clopidogrel, or acute coronary syndrome or stable disease as the indication for PCI, according to a subgroup analysis of the RE-DUAL PCI trial.
The trial, presented at the American Heart Association scientific sessions, randomized 2,725 patients to triple therapy with warfarin plus a P2Y12 inhibitor (clopidogrel or ticagrelor) and aspirin – the triple therapy group – or dabigatran 110 mg or 150 mg twice daily plus clopidogrel or ticagrelor – the dual therapy groups (N Engl J Med. 2017 Oct 19;377[16]:1513-24).
After a mean follow-up 14 months, the incidence of the major or clinically relevant nonmajor bleeding was 15.4% in the 110-mg dual-therapy group (hazard ratio, 0.52; 95% CI, 0.42-0.63; P less than .001) and 20.2% in the 150-mg dual-therapy group (HR, 0.72; 95% CI, 0.58-0.88; P less than .001), versus about 26% with triple-therapy.
The incidence of the composite efficacy endpoint – death, unplanned revascularization, myocardial infarction, stroke, or systemic embolism – was 13.7% in the two dual-therapy groups versus 13.4% with triple-therapy (HR, 1.04; 95% CI, 0.84-1.29; P = .005).
The investigators found consistent results when they analyzed their prespecified subgroups.
Drug-eluting stents were placed in 83% of patients; the rest had bare metal stents (BMS). The groups were well-balanced, except BMS patients were again more likely to be new to oral anticoagulation. Bleeding, thromboembolic events, and mortality were consistent with the main results regardless of the stent type, Most of the subjects were on clopidogrel, with just 12% on ticagrelor in both the dabigatran and warfarin groups. Ticagrelor patients were more likely to have ACS as their PCI indication and be new to oral anticoagulation. Ticagrelor patients were also more clinically complex, with a higher bleeding risk. Even so, they had relative bleeding risk reduction and efficacy results with dabigatran that were consistent with the overall finding, Dr. Oldgren said.
Patients were eligible for RE-DUAL PCI (Evaluation of Dual Therapy with Dabigatran vs. Triple Therapy with Warfarin in Patients with AF That Undergo a PCI with Stenting) if they had nonvalvular atrial fibrillation and a successful PCI within 120 hours. Those with bioprosthetic or mechanical heart valves, severe renal insufficiency, or other major comorbidities were excluded.
The trial was funded by Boehringer Ingelheim, the maker of dabigatran. Several investigators were employees. Dr. Oldgren is an adviser to Boehringer Ingelheim. Other authors reported financial ties to the company as well.
ANAHEIM, CALIF. – The benefit of dabigatran dual therapy versus warfarin triple therapy after percutaneous coronary intervention in patients with atrial fibrillation was consistent whether patients had drug-eluting or bare-metal stents, concomitant treatment with ticagrelor or clopidogrel, or acute coronary syndrome or stable disease as the indication for PCI, according to a subgroup analysis of the RE-DUAL PCI trial.
The trial, presented at the American Heart Association scientific sessions, randomized 2,725 patients to triple therapy with warfarin plus a P2Y12 inhibitor (clopidogrel or ticagrelor) and aspirin – the triple therapy group – or dabigatran 110 mg or 150 mg twice daily plus clopidogrel or ticagrelor – the dual therapy groups (N Engl J Med. 2017 Oct 19;377[16]:1513-24).
After a mean follow-up 14 months, the incidence of the major or clinically relevant nonmajor bleeding was 15.4% in the 110-mg dual-therapy group (hazard ratio, 0.52; 95% CI, 0.42-0.63; P less than .001) and 20.2% in the 150-mg dual-therapy group (HR, 0.72; 95% CI, 0.58-0.88; P less than .001), versus about 26% with triple-therapy.
The incidence of the composite efficacy endpoint – death, unplanned revascularization, myocardial infarction, stroke, or systemic embolism – was 13.7% in the two dual-therapy groups versus 13.4% with triple-therapy (HR, 1.04; 95% CI, 0.84-1.29; P = .005).
The investigators found consistent results when they analyzed their prespecified subgroups.
Drug-eluting stents were placed in 83% of patients; the rest had bare metal stents (BMS). The groups were well-balanced, except BMS patients were again more likely to be new to oral anticoagulation. Bleeding, thromboembolic events, and mortality were consistent with the main results regardless of the stent type, Most of the subjects were on clopidogrel, with just 12% on ticagrelor in both the dabigatran and warfarin groups. Ticagrelor patients were more likely to have ACS as their PCI indication and be new to oral anticoagulation. Ticagrelor patients were also more clinically complex, with a higher bleeding risk. Even so, they had relative bleeding risk reduction and efficacy results with dabigatran that were consistent with the overall finding, Dr. Oldgren said.
Patients were eligible for RE-DUAL PCI (Evaluation of Dual Therapy with Dabigatran vs. Triple Therapy with Warfarin in Patients with AF That Undergo a PCI with Stenting) if they had nonvalvular atrial fibrillation and a successful PCI within 120 hours. Those with bioprosthetic or mechanical heart valves, severe renal insufficiency, or other major comorbidities were excluded.
The trial was funded by Boehringer Ingelheim, the maker of dabigatran. Several investigators were employees. Dr. Oldgren is an adviser to Boehringer Ingelheim. Other authors reported financial ties to the company as well.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: After a mean follow-up of 14 months, the incidence of major or clinically relevant nonmajor bleeding was 15.4% in the 110-mg dual-therapy group (HR, 0.52; 95% CI, 0.42-0.63, P less than .001) and 20.2% in the 150-mg dual-therapy group (HR, 0.72; 95% CI, 0.58-0.88; P less than .001), versus about 26% with triple-therapy.
Data source: Subgroup analysis of RE-DUAL PCI trial
Disclosures: The trial was funded by Boehringer Ingelheim, the maker of dabigatran. Several investigators were employees. Authors disclosed various financial ties to the company.
Bilateral ACP shown similar to unilateral in arch replacement study
What may be the largest study comparing unilateral and bilateral antegrade cerebral perfusion during total arch replacement for type A aortic dissection has reported that outcomes between the two approaches are comparable, although the bilateral approach showed some advantages during the operation itself, investigators from China reported in the Journal of Thoracic and Cardiovascular Surgery (2017;154:767-75).
The effectiveness of bilateral antegrade cerebral perfusion (b-ACP) vs. unilateral antegrade cerebral perfusion (u-ACP) has been the focus of extensive debate, lead study author Guang Tong, MD, of the Guangzhou (China) General Hospital, and coauthors said. They compared outcomes in six different metrics, ranging from cardiopulmonary bypass time to length of stay (LOS) in the ICU and hospital, in 203 patients with type A aortic dissection who had total aortic arch replacement with hypothermic circulatory arrest over an 8-year period ending in August 2014; 121 had b-ACP and 82 had u-ACP. “The issue of u-ACP vs. b-ACP has been examined in aortic arch surgery, but few reports have focused on type A aortic dissection,” Dr. Tong and coauthors wrote.
They acknowledged that some surgeons are reluctant to use b-ACP because of its complexity, but their study found no increase in cross-clamp time, cardiopulmonary bypass time, or surgery time in the b-ACP group. They cited another reason surgeons give for avoiding b-ACP: the risk of embolic injury caused by canulating the left common carotid artery in an atheromatous aorta. “In the present study, this risk was avoided by attaching the left common carotid artery to the four-branched prosthetic graft for left hemisphere perfusion,” Dr. Tong and coauthors wrote.
Key outcomes that the researchers found not statistically significant were:
- Overall 30-day mortality (11.6% for b-ACP vs. 20.7% for u-ACP; P = .075).
- Prevalence of postoperative permanent neurologic dysfunction (8.4% vs. 16.9%; P = .091).
- Average ICU LOS (16 ± 17.75 days vs. 17 ± 11.5 days, P =.454).
- Average hospital LOS (26.5 ± 20.6 days vs. 24.8 ± 10.3 days, P = .434).
However, average ventilation time was lower in the b-ACP group (95.5 hours vs. 147 hours; P less than or equal to.001).
Dr. Tong and coauthors used an aggressive approach, as advocated by Dhaval Trivedi, MD, and colleagues (Ann Thorac Surg. 2016;101:896-903), and had a total arch replacement rate of 57.8%. This rate is higher than most published series in the west but comparable to other studies from China, perhaps because of the relatively young age of this study cohort – an average age of 51 years – compared to data sets other studies have used. Dr. Tong and coauthors used a b-ACP strategy that established both cerebral perfusion routes before circulatory arrest.
Rates of the following complications were also not significantly different across the study population: paraplegia (2.8% for b-ACP vs. 3.1% for u-ACP), temporary neurologic dysfunction (4.7% vs. 9.2%), permanent neurologic dysfunction (8.4% vs. 16.9%), renal failure (18% vs. 23.1%), reoperation for bleeding (2.8% vs. 4.6%), and mediastinal infection (3.7% vs. 6.2%).
While b-ACP patients did not have a statistically significant lower incidence of TND, Dr. Tong and coauthors noted the shorter time on ventilation and significantly lower tracheostomy rates for the b-ACP patients, 3.7% vs. 16.9% for the u-ACP group (P = .003). “In our institute, protocols to wean patients from ventilation were normally initiated as soon as consciousness was regained,” Dr. Tong and coauthors wrote.
Among the study limits Dr. Tong and coauthors acknowledged were its retrospective, nonrandomized, single-center nature, and the fact that the surgeries were performed over an 8-year period representing different eras.
The investigators reported having no relevant financial disclosures.
The study by Dr. Tong and coauthors adds to the discussion between the “bilateralists” and “unilateralists,” as Jean Bachet, MD, called the two prevailing camps on cerebral perfusion strategies in his invited commentary (J Thorac Cardiovasc Surg. 2017;154:765-6). And while most clinical reports find outcomes similar between the two approaches, the evidence favors the bilateral approach for total arch replacement.
Citing how the study implied mortality and neurologic morbidity rates almost half those for unilateral perfusion, but not reaching statistical significance, Dr. Bachet said, “The statisticians would say that this is only a trend and no proof, but some trends might be indicative, and significance might only be a matter of number in each arm of the comparison.”
Dr. Bachet raised a question about the unilateral approach – that once the arch is opened it takes a minute or so to insert the small balloon canula into the origin of the left carotid artery or divided vessel and start bilateral perfusion. “A major question arises,” said Dr. Bachet: “Why should we expose our patients to any undue risk just to avoid a simple maneuver, to spare a little time, or for any other fancy and questionable reason?”
Cardiologists have raised that question for more than 20 years. Said Dr. Bachet, “We still wait for the answer.”
Dr. Bachet is a cardiac surgeon in Surgenes, France. He reported having no financial relationships to disclose.
The study by Dr. Tong and coauthors adds to the discussion between the “bilateralists” and “unilateralists,” as Jean Bachet, MD, called the two prevailing camps on cerebral perfusion strategies in his invited commentary (J Thorac Cardiovasc Surg. 2017;154:765-6). And while most clinical reports find outcomes similar between the two approaches, the evidence favors the bilateral approach for total arch replacement.
Citing how the study implied mortality and neurologic morbidity rates almost half those for unilateral perfusion, but not reaching statistical significance, Dr. Bachet said, “The statisticians would say that this is only a trend and no proof, but some trends might be indicative, and significance might only be a matter of number in each arm of the comparison.”
Dr. Bachet raised a question about the unilateral approach – that once the arch is opened it takes a minute or so to insert the small balloon canula into the origin of the left carotid artery or divided vessel and start bilateral perfusion. “A major question arises,” said Dr. Bachet: “Why should we expose our patients to any undue risk just to avoid a simple maneuver, to spare a little time, or for any other fancy and questionable reason?”
Cardiologists have raised that question for more than 20 years. Said Dr. Bachet, “We still wait for the answer.”
Dr. Bachet is a cardiac surgeon in Surgenes, France. He reported having no financial relationships to disclose.
The study by Dr. Tong and coauthors adds to the discussion between the “bilateralists” and “unilateralists,” as Jean Bachet, MD, called the two prevailing camps on cerebral perfusion strategies in his invited commentary (J Thorac Cardiovasc Surg. 2017;154:765-6). And while most clinical reports find outcomes similar between the two approaches, the evidence favors the bilateral approach for total arch replacement.
Citing how the study implied mortality and neurologic morbidity rates almost half those for unilateral perfusion, but not reaching statistical significance, Dr. Bachet said, “The statisticians would say that this is only a trend and no proof, but some trends might be indicative, and significance might only be a matter of number in each arm of the comparison.”
Dr. Bachet raised a question about the unilateral approach – that once the arch is opened it takes a minute or so to insert the small balloon canula into the origin of the left carotid artery or divided vessel and start bilateral perfusion. “A major question arises,” said Dr. Bachet: “Why should we expose our patients to any undue risk just to avoid a simple maneuver, to spare a little time, or for any other fancy and questionable reason?”
Cardiologists have raised that question for more than 20 years. Said Dr. Bachet, “We still wait for the answer.”
Dr. Bachet is a cardiac surgeon in Surgenes, France. He reported having no financial relationships to disclose.
What may be the largest study comparing unilateral and bilateral antegrade cerebral perfusion during total arch replacement for type A aortic dissection has reported that outcomes between the two approaches are comparable, although the bilateral approach showed some advantages during the operation itself, investigators from China reported in the Journal of Thoracic and Cardiovascular Surgery (2017;154:767-75).
The effectiveness of bilateral antegrade cerebral perfusion (b-ACP) vs. unilateral antegrade cerebral perfusion (u-ACP) has been the focus of extensive debate, lead study author Guang Tong, MD, of the Guangzhou (China) General Hospital, and coauthors said. They compared outcomes in six different metrics, ranging from cardiopulmonary bypass time to length of stay (LOS) in the ICU and hospital, in 203 patients with type A aortic dissection who had total aortic arch replacement with hypothermic circulatory arrest over an 8-year period ending in August 2014; 121 had b-ACP and 82 had u-ACP. “The issue of u-ACP vs. b-ACP has been examined in aortic arch surgery, but few reports have focused on type A aortic dissection,” Dr. Tong and coauthors wrote.
They acknowledged that some surgeons are reluctant to use b-ACP because of its complexity, but their study found no increase in cross-clamp time, cardiopulmonary bypass time, or surgery time in the b-ACP group. They cited another reason surgeons give for avoiding b-ACP: the risk of embolic injury caused by canulating the left common carotid artery in an atheromatous aorta. “In the present study, this risk was avoided by attaching the left common carotid artery to the four-branched prosthetic graft for left hemisphere perfusion,” Dr. Tong and coauthors wrote.
Key outcomes that the researchers found not statistically significant were:
- Overall 30-day mortality (11.6% for b-ACP vs. 20.7% for u-ACP; P = .075).
- Prevalence of postoperative permanent neurologic dysfunction (8.4% vs. 16.9%; P = .091).
- Average ICU LOS (16 ± 17.75 days vs. 17 ± 11.5 days, P =.454).
- Average hospital LOS (26.5 ± 20.6 days vs. 24.8 ± 10.3 days, P = .434).
However, average ventilation time was lower in the b-ACP group (95.5 hours vs. 147 hours; P less than or equal to.001).
Dr. Tong and coauthors used an aggressive approach, as advocated by Dhaval Trivedi, MD, and colleagues (Ann Thorac Surg. 2016;101:896-903), and had a total arch replacement rate of 57.8%. This rate is higher than most published series in the west but comparable to other studies from China, perhaps because of the relatively young age of this study cohort – an average age of 51 years – compared to data sets other studies have used. Dr. Tong and coauthors used a b-ACP strategy that established both cerebral perfusion routes before circulatory arrest.
Rates of the following complications were also not significantly different across the study population: paraplegia (2.8% for b-ACP vs. 3.1% for u-ACP), temporary neurologic dysfunction (4.7% vs. 9.2%), permanent neurologic dysfunction (8.4% vs. 16.9%), renal failure (18% vs. 23.1%), reoperation for bleeding (2.8% vs. 4.6%), and mediastinal infection (3.7% vs. 6.2%).
While b-ACP patients did not have a statistically significant lower incidence of TND, Dr. Tong and coauthors noted the shorter time on ventilation and significantly lower tracheostomy rates for the b-ACP patients, 3.7% vs. 16.9% for the u-ACP group (P = .003). “In our institute, protocols to wean patients from ventilation were normally initiated as soon as consciousness was regained,” Dr. Tong and coauthors wrote.
Among the study limits Dr. Tong and coauthors acknowledged were its retrospective, nonrandomized, single-center nature, and the fact that the surgeries were performed over an 8-year period representing different eras.
The investigators reported having no relevant financial disclosures.
What may be the largest study comparing unilateral and bilateral antegrade cerebral perfusion during total arch replacement for type A aortic dissection has reported that outcomes between the two approaches are comparable, although the bilateral approach showed some advantages during the operation itself, investigators from China reported in the Journal of Thoracic and Cardiovascular Surgery (2017;154:767-75).
The effectiveness of bilateral antegrade cerebral perfusion (b-ACP) vs. unilateral antegrade cerebral perfusion (u-ACP) has been the focus of extensive debate, lead study author Guang Tong, MD, of the Guangzhou (China) General Hospital, and coauthors said. They compared outcomes in six different metrics, ranging from cardiopulmonary bypass time to length of stay (LOS) in the ICU and hospital, in 203 patients with type A aortic dissection who had total aortic arch replacement with hypothermic circulatory arrest over an 8-year period ending in August 2014; 121 had b-ACP and 82 had u-ACP. “The issue of u-ACP vs. b-ACP has been examined in aortic arch surgery, but few reports have focused on type A aortic dissection,” Dr. Tong and coauthors wrote.
They acknowledged that some surgeons are reluctant to use b-ACP because of its complexity, but their study found no increase in cross-clamp time, cardiopulmonary bypass time, or surgery time in the b-ACP group. They cited another reason surgeons give for avoiding b-ACP: the risk of embolic injury caused by canulating the left common carotid artery in an atheromatous aorta. “In the present study, this risk was avoided by attaching the left common carotid artery to the four-branched prosthetic graft for left hemisphere perfusion,” Dr. Tong and coauthors wrote.
Key outcomes that the researchers found not statistically significant were:
- Overall 30-day mortality (11.6% for b-ACP vs. 20.7% for u-ACP; P = .075).
- Prevalence of postoperative permanent neurologic dysfunction (8.4% vs. 16.9%; P = .091).
- Average ICU LOS (16 ± 17.75 days vs. 17 ± 11.5 days, P =.454).
- Average hospital LOS (26.5 ± 20.6 days vs. 24.8 ± 10.3 days, P = .434).
However, average ventilation time was lower in the b-ACP group (95.5 hours vs. 147 hours; P less than or equal to.001).
Dr. Tong and coauthors used an aggressive approach, as advocated by Dhaval Trivedi, MD, and colleagues (Ann Thorac Surg. 2016;101:896-903), and had a total arch replacement rate of 57.8%. This rate is higher than most published series in the west but comparable to other studies from China, perhaps because of the relatively young age of this study cohort – an average age of 51 years – compared to data sets other studies have used. Dr. Tong and coauthors used a b-ACP strategy that established both cerebral perfusion routes before circulatory arrest.
Rates of the following complications were also not significantly different across the study population: paraplegia (2.8% for b-ACP vs. 3.1% for u-ACP), temporary neurologic dysfunction (4.7% vs. 9.2%), permanent neurologic dysfunction (8.4% vs. 16.9%), renal failure (18% vs. 23.1%), reoperation for bleeding (2.8% vs. 4.6%), and mediastinal infection (3.7% vs. 6.2%).
While b-ACP patients did not have a statistically significant lower incidence of TND, Dr. Tong and coauthors noted the shorter time on ventilation and significantly lower tracheostomy rates for the b-ACP patients, 3.7% vs. 16.9% for the u-ACP group (P = .003). “In our institute, protocols to wean patients from ventilation were normally initiated as soon as consciousness was regained,” Dr. Tong and coauthors wrote.
Among the study limits Dr. Tong and coauthors acknowledged were its retrospective, nonrandomized, single-center nature, and the fact that the surgeries were performed over an 8-year period representing different eras.
The investigators reported having no relevant financial disclosures.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Clinical outcomes were comparable between groups who underwent unilateral or bilateral antegrade cerebral perfusion in total arch replacement for type A aortic dissection.
Major finding: Overall 30-day mortality was 11.6% in the bilateral ACP group vs. 20.7% for unilateral ACP (P =.075).
Data source: Population of 203 patients who had aortic arch replacement surgery for type A aortic dissection between September 2006 and August 2014.
Disclosures: Dr. Tong and coauthors reported having no relevant financial disclosures.
Heart transplantation: Preop LVAD erases adverse impact of pulmonary hypertension
COLORADO SPRINGS – Reconsideration of the role of pulmonary hypertension in heart transplant outcomes is appropriate in the emerging era of the use of left ventricular assist devices (LVADs) as bridge to transplant, according to Ann C. Gaffey, MD, of the University of Pennsylvania, Philadelphia.
“Pulmonary hypertension secondary to congestive heart failure more than likely can be reversed to the values acceptable for heart transplant by the use of an LVAD. For bridge-to-transplant patients, pretransplant pulmonary hypertension does not affect recipient outcomes post transplantation,” she said at the annual meeting of the Western Thoracic Surgical Association.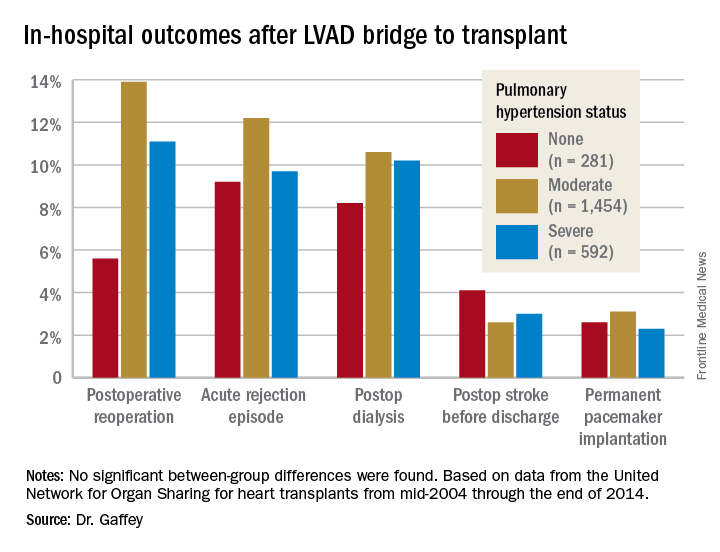
Vasodilators are prescribed in an effort to reduce PH; however, 40% of patients with PH are unresponsive to the medications and have therefore been excluded from consideration as potential candidates for a donor heart.
But the growing use of LVADs as a bridge to transplant has changed all that, Dr. Gaffey said. As supporting evidence, she presented a retrospective analysis of the United Network for Organ Sharing database on adult heart transplants from mid-2004 through the end of 2014.
The review turned up 3,951 heart transplant recipients who had been bridged to transplant with an LVAD. Dr. Gaffey and her coinvestigators divided them into three groups: 281 patients without pretransplant PH; 1,454 with moderate PH as defined by 1-3 Wood units; and 592 with severe PH and more than 3 Wood units.
The three groups didn’t differ in terms of age, sex, wait-list time, or the prevalence of diabetes or renal, liver, or cerebrovascular disease. Nor did their donors differ in age, sex, left ventricular function, or allograft ischemic time.
Key in-hospital outcomes were similar between the groups with no, mild, and severe PH.
Moreover, there was no between-group difference in the rate of rejection at 1 year. Five-year survival rates were closely similar in the three groups, in the mid-70s.
Audience member Nahush A. Mokadam, MD, rose to praise Dr. Gaffey’s report.
“This is a great and important study. I think as a group we have been too conservative with pulmonary hypertension, so thank you for shining a good light on it,” said Dr. Mokadam of the University of Washington, Seattle.
Dr. Gaffey reported having no financial conflicts regarding the study, which was conducted free of commercial support.
COLORADO SPRINGS – Reconsideration of the role of pulmonary hypertension in heart transplant outcomes is appropriate in the emerging era of the use of left ventricular assist devices (LVADs) as bridge to transplant, according to Ann C. Gaffey, MD, of the University of Pennsylvania, Philadelphia.
“Pulmonary hypertension secondary to congestive heart failure more than likely can be reversed to the values acceptable for heart transplant by the use of an LVAD. For bridge-to-transplant patients, pretransplant pulmonary hypertension does not affect recipient outcomes post transplantation,” she said at the annual meeting of the Western Thoracic Surgical Association.
Vasodilators are prescribed in an effort to reduce PH; however, 40% of patients with PH are unresponsive to the medications and have therefore been excluded from consideration as potential candidates for a donor heart.
But the growing use of LVADs as a bridge to transplant has changed all that, Dr. Gaffey said. As supporting evidence, she presented a retrospective analysis of the United Network for Organ Sharing database on adult heart transplants from mid-2004 through the end of 2014.
The review turned up 3,951 heart transplant recipients who had been bridged to transplant with an LVAD. Dr. Gaffey and her coinvestigators divided them into three groups: 281 patients without pretransplant PH; 1,454 with moderate PH as defined by 1-3 Wood units; and 592 with severe PH and more than 3 Wood units.
The three groups didn’t differ in terms of age, sex, wait-list time, or the prevalence of diabetes or renal, liver, or cerebrovascular disease. Nor did their donors differ in age, sex, left ventricular function, or allograft ischemic time.
Key in-hospital outcomes were similar between the groups with no, mild, and severe PH.
Moreover, there was no between-group difference in the rate of rejection at 1 year. Five-year survival rates were closely similar in the three groups, in the mid-70s.
Audience member Nahush A. Mokadam, MD, rose to praise Dr. Gaffey’s report.
“This is a great and important study. I think as a group we have been too conservative with pulmonary hypertension, so thank you for shining a good light on it,” said Dr. Mokadam of the University of Washington, Seattle.
Dr. Gaffey reported having no financial conflicts regarding the study, which was conducted free of commercial support.
COLORADO SPRINGS – Reconsideration of the role of pulmonary hypertension in heart transplant outcomes is appropriate in the emerging era of the use of left ventricular assist devices (LVADs) as bridge to transplant, according to Ann C. Gaffey, MD, of the University of Pennsylvania, Philadelphia.
“Pulmonary hypertension secondary to congestive heart failure more than likely can be reversed to the values acceptable for heart transplant by the use of an LVAD. For bridge-to-transplant patients, pretransplant pulmonary hypertension does not affect recipient outcomes post transplantation,” she said at the annual meeting of the Western Thoracic Surgical Association.
Vasodilators are prescribed in an effort to reduce PH; however, 40% of patients with PH are unresponsive to the medications and have therefore been excluded from consideration as potential candidates for a donor heart.
But the growing use of LVADs as a bridge to transplant has changed all that, Dr. Gaffey said. As supporting evidence, she presented a retrospective analysis of the United Network for Organ Sharing database on adult heart transplants from mid-2004 through the end of 2014.
The review turned up 3,951 heart transplant recipients who had been bridged to transplant with an LVAD. Dr. Gaffey and her coinvestigators divided them into three groups: 281 patients without pretransplant PH; 1,454 with moderate PH as defined by 1-3 Wood units; and 592 with severe PH and more than 3 Wood units.
The three groups didn’t differ in terms of age, sex, wait-list time, or the prevalence of diabetes or renal, liver, or cerebrovascular disease. Nor did their donors differ in age, sex, left ventricular function, or allograft ischemic time.
Key in-hospital outcomes were similar between the groups with no, mild, and severe PH.
Moreover, there was no between-group difference in the rate of rejection at 1 year. Five-year survival rates were closely similar in the three groups, in the mid-70s.
Audience member Nahush A. Mokadam, MD, rose to praise Dr. Gaffey’s report.
“This is a great and important study. I think as a group we have been too conservative with pulmonary hypertension, so thank you for shining a good light on it,” said Dr. Mokadam of the University of Washington, Seattle.
Dr. Gaffey reported having no financial conflicts regarding the study, which was conducted free of commercial support.
AT THE WTSA ANNUAL MEETING
Key clinical point:
Major finding: It’s time to reconsider the practice of excluding patients with pulmonary hypertension from consideration for a donor heart.
Data source: A retrospective analysis of the United Network for Organ Sharing database including outcomes out to 5 years on 3,951 heart transplant recipients who had been bridged to transplant with an LVAD, most of whom had moderate or severe pulmonary hypertension before transplant.
Disclosures: This study was conducted free of commercial support. The presenter reported having no relevant financial conflicts of interest.
Remimazolam surpasses midazolam for bronchoscopy sedation
TORONTO – An investigational sedative, remimazolam, that’s similar to midazolam but with faster onset and offset, resulted in significantly better procedural success compared with midazolam in a multicenter, phase III trial with 431 patients.
The results also showed that remimazolam was as safe as midazolam (Versed), with a very similar adverse event profile, said Gerard A. Silvestri, MD, FCCP, at the CHEST annual meeting.
The bronchoscopy trial enrolled patients at any of 15 U.S. centers with an American Society of Anesthesiologists (ASA) physical status classification of I-III and scheduled for diagnostic or therapeutic bronchoscopy. The enrolled patients averaged 62 years of age, and 38% were in ASA class III.
All patients received initial sedation treatment with fentanyl, followed by a three-to-one randomization to blinded remimazolam, blinded placebo that included midazolam rescue, or open-label midazolam. The study’s primary efficacy endpoint was procedural success, defined as patients who underwent the complete procedure without need for an alternative sedative and without need for more than five doses of the patient’s assigned medication within any 15 minute period during the procedure or need for more than three midazolam doses within any 12-minute period in the patients randomized to receive midazolam.
This primary endpoint occurred in 83% of 303 patients in the remimazolam arm, 5% of 59 patients in the placebo arm, and 34% of 69 patients in the midazolam arm, a statistically significant difference between the remimazolam patients and each of the comparator groups, reported Dr. Silvestri, a professor of medicine and a lung cancer pulmonologist at the Medical University of South Carolina in Charleston.
The results also demonstrated the faster inset and offset of remimazolam. Treatment achieved adequate sedation to start the procedure after a median of 5 minutes with remimazolam, a median of 15.5 minutes with midazolam, and a median of 17 minutes among patients in the placebo group. Once sedation finished, patients returned to being fully alert after a median of 6 minutes with remimazolam, a median of 12 minutes with midazolam, and a median of 13.5 minutes for patients in the placebo arm.
“What’s nice about remimazolam is that the adverse event profile is exactly the same as with placebo and midazolam, and you have a reversal agent,” the same as what’s used for midazolam, he said.
Dr. Silvestri suggested several additional studies he would like to see run on remimazolam to better understand its clinical performance and role. These include studying the drug in the elderly, patients with an ASA classification of IV, obese patients, and those on high narcotic doses. He also suggested comparing remimazolam directly with propofol, testing remimazolam as a stand-alone agent without fentanyl co-administration, and trying the drug during other pulmonary procedures such as pleural-catheter placement and other invasive procedures, and in ICU patients.
The trial was funded by Paion, the company developing remimazolam. Dr. Silvestri and Dr. Stanbrook had no relevant disclosures.
[email protected]
On Twitter @mitchelzoler
TORONTO – An investigational sedative, remimazolam, that’s similar to midazolam but with faster onset and offset, resulted in significantly better procedural success compared with midazolam in a multicenter, phase III trial with 431 patients.
The results also showed that remimazolam was as safe as midazolam (Versed), with a very similar adverse event profile, said Gerard A. Silvestri, MD, FCCP, at the CHEST annual meeting.
The bronchoscopy trial enrolled patients at any of 15 U.S. centers with an American Society of Anesthesiologists (ASA) physical status classification of I-III and scheduled for diagnostic or therapeutic bronchoscopy. The enrolled patients averaged 62 years of age, and 38% were in ASA class III.
All patients received initial sedation treatment with fentanyl, followed by a three-to-one randomization to blinded remimazolam, blinded placebo that included midazolam rescue, or open-label midazolam. The study’s primary efficacy endpoint was procedural success, defined as patients who underwent the complete procedure without need for an alternative sedative and without need for more than five doses of the patient’s assigned medication within any 15 minute period during the procedure or need for more than three midazolam doses within any 12-minute period in the patients randomized to receive midazolam.
This primary endpoint occurred in 83% of 303 patients in the remimazolam arm, 5% of 59 patients in the placebo arm, and 34% of 69 patients in the midazolam arm, a statistically significant difference between the remimazolam patients and each of the comparator groups, reported Dr. Silvestri, a professor of medicine and a lung cancer pulmonologist at the Medical University of South Carolina in Charleston.
The results also demonstrated the faster inset and offset of remimazolam. Treatment achieved adequate sedation to start the procedure after a median of 5 minutes with remimazolam, a median of 15.5 minutes with midazolam, and a median of 17 minutes among patients in the placebo group. Once sedation finished, patients returned to being fully alert after a median of 6 minutes with remimazolam, a median of 12 minutes with midazolam, and a median of 13.5 minutes for patients in the placebo arm.
“What’s nice about remimazolam is that the adverse event profile is exactly the same as with placebo and midazolam, and you have a reversal agent,” the same as what’s used for midazolam, he said.
Dr. Silvestri suggested several additional studies he would like to see run on remimazolam to better understand its clinical performance and role. These include studying the drug in the elderly, patients with an ASA classification of IV, obese patients, and those on high narcotic doses. He also suggested comparing remimazolam directly with propofol, testing remimazolam as a stand-alone agent without fentanyl co-administration, and trying the drug during other pulmonary procedures such as pleural-catheter placement and other invasive procedures, and in ICU patients.
The trial was funded by Paion, the company developing remimazolam. Dr. Silvestri and Dr. Stanbrook had no relevant disclosures.
[email protected]
On Twitter @mitchelzoler
TORONTO – An investigational sedative, remimazolam, that’s similar to midazolam but with faster onset and offset, resulted in significantly better procedural success compared with midazolam in a multicenter, phase III trial with 431 patients.
The results also showed that remimazolam was as safe as midazolam (Versed), with a very similar adverse event profile, said Gerard A. Silvestri, MD, FCCP, at the CHEST annual meeting.
The bronchoscopy trial enrolled patients at any of 15 U.S. centers with an American Society of Anesthesiologists (ASA) physical status classification of I-III and scheduled for diagnostic or therapeutic bronchoscopy. The enrolled patients averaged 62 years of age, and 38% were in ASA class III.
All patients received initial sedation treatment with fentanyl, followed by a three-to-one randomization to blinded remimazolam, blinded placebo that included midazolam rescue, or open-label midazolam. The study’s primary efficacy endpoint was procedural success, defined as patients who underwent the complete procedure without need for an alternative sedative and without need for more than five doses of the patient’s assigned medication within any 15 minute period during the procedure or need for more than three midazolam doses within any 12-minute period in the patients randomized to receive midazolam.
This primary endpoint occurred in 83% of 303 patients in the remimazolam arm, 5% of 59 patients in the placebo arm, and 34% of 69 patients in the midazolam arm, a statistically significant difference between the remimazolam patients and each of the comparator groups, reported Dr. Silvestri, a professor of medicine and a lung cancer pulmonologist at the Medical University of South Carolina in Charleston.
The results also demonstrated the faster inset and offset of remimazolam. Treatment achieved adequate sedation to start the procedure after a median of 5 minutes with remimazolam, a median of 15.5 minutes with midazolam, and a median of 17 minutes among patients in the placebo group. Once sedation finished, patients returned to being fully alert after a median of 6 minutes with remimazolam, a median of 12 minutes with midazolam, and a median of 13.5 minutes for patients in the placebo arm.
“What’s nice about remimazolam is that the adverse event profile is exactly the same as with placebo and midazolam, and you have a reversal agent,” the same as what’s used for midazolam, he said.
Dr. Silvestri suggested several additional studies he would like to see run on remimazolam to better understand its clinical performance and role. These include studying the drug in the elderly, patients with an ASA classification of IV, obese patients, and those on high narcotic doses. He also suggested comparing remimazolam directly with propofol, testing remimazolam as a stand-alone agent without fentanyl co-administration, and trying the drug during other pulmonary procedures such as pleural-catheter placement and other invasive procedures, and in ICU patients.
The trial was funded by Paion, the company developing remimazolam. Dr. Silvestri and Dr. Stanbrook had no relevant disclosures.
[email protected]
On Twitter @mitchelzoler
AT CHEST 2017
Key clinical point:
Major finding: The rate of successful bronchoscopies was 83% with remimazolam, 34% with midazolam, and 5% with placebo.
Data source: A multicenter, phase III trial with 431 patients.
Disclosures: The trial was funded by Paion, the company developing remimazolam. Dr. Silvestri and Dr. Stanbrook had no relevant disclosures.
Why VADS is gaining ground in pediatrics
The miniaturization of continuous-flow ventricular assist devices has launched the era of continuous-flow VAD support in pediatric patients, and the trend may accelerate with the introduction of a continuous-flow device designed specifically for small children. In an expert opinion in the Journal of Thoracic and Cardiovascular Surgery, Iki Adachi, MD, of Baylor College of Medicine in Houston, said the emerging science of continuous-flow VADs in children promises to solve problems like device size mismatch, hospital-only VADs, and chronic therapy (J Thorac Cardiovasc Surg. 2017 Oct;154:1358-61). “With ongoing device miniaturization, enthusiasm has been growing among pediatric physicians for the use of continuous-flow VADs in children,” Dr. Adachi said. He noted the introduction of a continuous-flow device for small children, the Infant Jarvik 2015, “may further accelerate the trend.”
Dr. Adachi cited PediMACS reports that stated that more than half of the long-term devices now registered are continuous-flow devices, and that continuous-flow VADs comprised 62% of all durable VAD implants in the third quarter of 2016. “With the encouraging results recorded to date, the use of continuous-flow devices in the pediatric population is rapidly increasing,” he said.
Miniaturization has addressed the problem of size mismatch when using continuous-flow VAD devices in children, he said, noting that use of the Infant Jarvik device may be expanded even further to children as small at 8 kg or less. The PumpKIN trial(Pumps for Kids, Infants, and Neonates), which is evaluating the Infant Jarvik 2015 vs. the Berlin Heart EXCOR, could provide answers on the feasibility of continuous-flow VADs in small children.
“Based on experience with the chronic animal model, I believe that the Infant Jarvik device will properly fit the patients included in the trial,” he said.
Continuous-flow VAD in children also holds potential for managing these patients outside the hospital setting. “Outpatient management of children with continuous-flow VADs has been shown to be feasible,” he said, adding that the PediMACS registry has reported that only 45% of patients have been managed this way. “Nonetheless, with maturation of the pediatric field, outpatient management will become routine rather than the exception,” he said.
Greater use of continuous-flow VADs also may create opportunities to improve the status and suitability for transplantation of children with severe heart failure, he said. He gave as an example his group’s practice at Houston’s Texas Children’s Hospital, which is to deactivate patients on the transplant wait list for 3 months once they start continuous-flow VAD support. “A postoperative ‘grace period’ affords protected opportunities for both physical and psychological recovery,” he said. This timeout of sorts also affords the care team time to assess the myocardium for possible functional recovery.
In patients who are not good candidates for transplantation, durable continuous-flow VADs may provide chronic therapy, and in time, these patients may become suitable transplant candidates, said Dr. Adachi. “Bypassing such an unfavorable period for transplantation with prolonged VAD support may be a reasonable approach,” he said.
Patients with failing single-ventricle circulation also may benefit from VAD support, although the challenges facing this population are more profound than in other groups, Dr. Adachi said. VAD support for single-ventricle disease is sparse, but these patients require careful evaluation of the nature of their condition. “If systolic dysfunction is the predominant cause of circulatory failure, then VAD support for the failing systemic ventricle will likely improve hemodynamics,” said Dr. Adachi. VAD support also could help the patient move through the various stages of palliation.
“Again, the emphasis is not just on simply keeping the patient alive until a donor organ becomes available; rather, attention is refocused on overall health beyond survival, which may eventually affect transplantation candidacy and even post transplantation outcome,” Dr. Adachi concluded.
Dr. Adachi serves as a consultant and proctor for Berlin Heart and HeartWare, and as a consultant for the New England Research Institute related to the PumpKIN trial.
The miniaturization of continuous-flow ventricular assist devices has launched the era of continuous-flow VAD support in pediatric patients, and the trend may accelerate with the introduction of a continuous-flow device designed specifically for small children. In an expert opinion in the Journal of Thoracic and Cardiovascular Surgery, Iki Adachi, MD, of Baylor College of Medicine in Houston, said the emerging science of continuous-flow VADs in children promises to solve problems like device size mismatch, hospital-only VADs, and chronic therapy (J Thorac Cardiovasc Surg. 2017 Oct;154:1358-61). “With ongoing device miniaturization, enthusiasm has been growing among pediatric physicians for the use of continuous-flow VADs in children,” Dr. Adachi said. He noted the introduction of a continuous-flow device for small children, the Infant Jarvik 2015, “may further accelerate the trend.”
Dr. Adachi cited PediMACS reports that stated that more than half of the long-term devices now registered are continuous-flow devices, and that continuous-flow VADs comprised 62% of all durable VAD implants in the third quarter of 2016. “With the encouraging results recorded to date, the use of continuous-flow devices in the pediatric population is rapidly increasing,” he said.
Miniaturization has addressed the problem of size mismatch when using continuous-flow VAD devices in children, he said, noting that use of the Infant Jarvik device may be expanded even further to children as small at 8 kg or less. The PumpKIN trial(Pumps for Kids, Infants, and Neonates), which is evaluating the Infant Jarvik 2015 vs. the Berlin Heart EXCOR, could provide answers on the feasibility of continuous-flow VADs in small children.
“Based on experience with the chronic animal model, I believe that the Infant Jarvik device will properly fit the patients included in the trial,” he said.
Continuous-flow VAD in children also holds potential for managing these patients outside the hospital setting. “Outpatient management of children with continuous-flow VADs has been shown to be feasible,” he said, adding that the PediMACS registry has reported that only 45% of patients have been managed this way. “Nonetheless, with maturation of the pediatric field, outpatient management will become routine rather than the exception,” he said.
Greater use of continuous-flow VADs also may create opportunities to improve the status and suitability for transplantation of children with severe heart failure, he said. He gave as an example his group’s practice at Houston’s Texas Children’s Hospital, which is to deactivate patients on the transplant wait list for 3 months once they start continuous-flow VAD support. “A postoperative ‘grace period’ affords protected opportunities for both physical and psychological recovery,” he said. This timeout of sorts also affords the care team time to assess the myocardium for possible functional recovery.
In patients who are not good candidates for transplantation, durable continuous-flow VADs may provide chronic therapy, and in time, these patients may become suitable transplant candidates, said Dr. Adachi. “Bypassing such an unfavorable period for transplantation with prolonged VAD support may be a reasonable approach,” he said.
Patients with failing single-ventricle circulation also may benefit from VAD support, although the challenges facing this population are more profound than in other groups, Dr. Adachi said. VAD support for single-ventricle disease is sparse, but these patients require careful evaluation of the nature of their condition. “If systolic dysfunction is the predominant cause of circulatory failure, then VAD support for the failing systemic ventricle will likely improve hemodynamics,” said Dr. Adachi. VAD support also could help the patient move through the various stages of palliation.
“Again, the emphasis is not just on simply keeping the patient alive until a donor organ becomes available; rather, attention is refocused on overall health beyond survival, which may eventually affect transplantation candidacy and even post transplantation outcome,” Dr. Adachi concluded.
Dr. Adachi serves as a consultant and proctor for Berlin Heart and HeartWare, and as a consultant for the New England Research Institute related to the PumpKIN trial.
The miniaturization of continuous-flow ventricular assist devices has launched the era of continuous-flow VAD support in pediatric patients, and the trend may accelerate with the introduction of a continuous-flow device designed specifically for small children. In an expert opinion in the Journal of Thoracic and Cardiovascular Surgery, Iki Adachi, MD, of Baylor College of Medicine in Houston, said the emerging science of continuous-flow VADs in children promises to solve problems like device size mismatch, hospital-only VADs, and chronic therapy (J Thorac Cardiovasc Surg. 2017 Oct;154:1358-61). “With ongoing device miniaturization, enthusiasm has been growing among pediatric physicians for the use of continuous-flow VADs in children,” Dr. Adachi said. He noted the introduction of a continuous-flow device for small children, the Infant Jarvik 2015, “may further accelerate the trend.”
Dr. Adachi cited PediMACS reports that stated that more than half of the long-term devices now registered are continuous-flow devices, and that continuous-flow VADs comprised 62% of all durable VAD implants in the third quarter of 2016. “With the encouraging results recorded to date, the use of continuous-flow devices in the pediatric population is rapidly increasing,” he said.
Miniaturization has addressed the problem of size mismatch when using continuous-flow VAD devices in children, he said, noting that use of the Infant Jarvik device may be expanded even further to children as small at 8 kg or less. The PumpKIN trial(Pumps for Kids, Infants, and Neonates), which is evaluating the Infant Jarvik 2015 vs. the Berlin Heart EXCOR, could provide answers on the feasibility of continuous-flow VADs in small children.
“Based on experience with the chronic animal model, I believe that the Infant Jarvik device will properly fit the patients included in the trial,” he said.
Continuous-flow VAD in children also holds potential for managing these patients outside the hospital setting. “Outpatient management of children with continuous-flow VADs has been shown to be feasible,” he said, adding that the PediMACS registry has reported that only 45% of patients have been managed this way. “Nonetheless, with maturation of the pediatric field, outpatient management will become routine rather than the exception,” he said.
Greater use of continuous-flow VADs also may create opportunities to improve the status and suitability for transplantation of children with severe heart failure, he said. He gave as an example his group’s practice at Houston’s Texas Children’s Hospital, which is to deactivate patients on the transplant wait list for 3 months once they start continuous-flow VAD support. “A postoperative ‘grace period’ affords protected opportunities for both physical and psychological recovery,” he said. This timeout of sorts also affords the care team time to assess the myocardium for possible functional recovery.
In patients who are not good candidates for transplantation, durable continuous-flow VADs may provide chronic therapy, and in time, these patients may become suitable transplant candidates, said Dr. Adachi. “Bypassing such an unfavorable period for transplantation with prolonged VAD support may be a reasonable approach,” he said.
Patients with failing single-ventricle circulation also may benefit from VAD support, although the challenges facing this population are more profound than in other groups, Dr. Adachi said. VAD support for single-ventricle disease is sparse, but these patients require careful evaluation of the nature of their condition. “If systolic dysfunction is the predominant cause of circulatory failure, then VAD support for the failing systemic ventricle will likely improve hemodynamics,” said Dr. Adachi. VAD support also could help the patient move through the various stages of palliation.
“Again, the emphasis is not just on simply keeping the patient alive until a donor organ becomes available; rather, attention is refocused on overall health beyond survival, which may eventually affect transplantation candidacy and even post transplantation outcome,” Dr. Adachi concluded.
Dr. Adachi serves as a consultant and proctor for Berlin Heart and HeartWare, and as a consultant for the New England Research Institute related to the PumpKIN trial.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Advances in continuous-flow ventricular assist devices (VADs) promise a paradigm shift in pediatrics.
Major finding: Device miniaturization is solving problems such as size mismatch, inpatients on VADs, and chronic therapy.
Data source: Expert opinion drawing on PediMACS reports and published trials of continuous-flow VAD.
Disclosures: Dr. Adachi serves as a consultant and proctor for Berlin Heart and HeartWare and as a consultant for the New England Research Institute related to the PumpKIN trial.
TAVR wallops SAVR in cost-effectiveness for intermediate-risk patients
DENVER – A formal cost-effectiveness analysis indicates that transcatheter aortic valve replacement (TAVR) is substantially more cost effective than surgical valve replacement in patients at intermediate surgical risk similar to those enrolled in the landmark PARTNER 2 trial.
The analysis demonstrated that over a 1- and 2-year follow-up period, as well as with projected lifetime follow-up, TAVR entails both lower long-term costs and greater quality-adjusted life expectancy, David J. Cohen, MD, reported at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
His two-part, patient-level economic analysis examined data from nearly 2,000 participants in the PARTNER 2A randomized trial comparing TAVR, using the Sapien XT valve, with surgical aortic valve replacement (SAVR), as well as the experience with the current-generation Sapien 3 TAVR valve in 1,077 intermediate–surgical risk TAVR patients in the S3i registry. The analysis utilized Medicare claims data on the costs of the index hospitalization and follow-up care.
In PARTNER 2A, the average total cost of the index hospitalization for valve replacement was $61,433 with TAVR. That was just $2,888 more than the SAVR hospitalization, despite the far higher acquisition cost of the Sapien 3 valve, which was roughly $32,500, compared with $5,000 for the surgical valve. Most of this additional cost of the TAVR valve was counterbalanced by TAVR’s 2-hour shorter procedural duration, the 6.4-day average length of stay, compared with 10.9 days for SAVR, and the fact that TAVR patients spent only 2.4 days in intensive care while SAVR patients averaged 4.6 days, Dr. Cohen explained at the meeting sponsored by the Cardiovascular Research Foundation.
During 24 months of postdischarge follow-up in the PARTNER 2A trial, SAVR patients racked up an average of $9,303 more in costs than TAVR patients. This was mainly because of their much higher rates of rehospitalization and time spent in skilled nursing facilities and rehabilitation centers, mainly during months 2-6 post discharge. The result was that 2-year total costs including the index hospitalization averaged $107,716 per TAVR patient and $114,132 per SAVR patient.
“One of the really remarkable findings of this study was what happened during follow-up,” the cardiologist observed.
Extrapolating to projected remaining lifetime years, TAVR using the Sapien XT valve resulted in a cost savings of $7,949 per patient and a 0.15-year increase in quality-adjusted life expectancy compared with SAVR.
But since the time of PARTNER 2A, the Sapien XT valve has been replaced by the updated Sapien 3 valve. The analysis of the S3i registry showed that the economic dominance of TAVR over SAVR was even greater owing to improved valve technology and contemporary care patterns. For this analysis, because there has been no randomized trial of TAVR with the Sapien 3 valve versus SAVR, patients in the SAVR of arm of PARTNER 2A served as the comparison group.
The cost of the index hospitalization was more than $4,000 less with TAVR in the S3i registry than with SAVR. The total cost of TAVR through 1 year of follow-up averaged $80,977, which was $15,511 less than the $96,489 for SAVR. The cost post discharge out to 1 year was more than $11,000 less per TAVR patient, driven by sharply lower rates of both cardiovascular and noncardiovascular hospitalizations as well as a greater than 50% reduction in days spent in rehab centers and skilled nursing facilities, compared with SAVR patients.
Projected over estimated remaining years of life, TAVR with the Sapien 3 valve yielded a cost savings of $9,692 per patient compared with SAVR, as well as a 0.27-year gain in quality-adjusted life-years.
Eighty-eight percent of patients in the S3i registry received their Sapien 3 valve via a transfemoral approach. When Dr. Cohen and his coinvestigators compared their costs and clinical outcomes to the subset of PARTNER 2A TAVR patients who got the Sapien XT valve transfemorally, the outcomes were “virtually identical,” he said.
“These findings are reassuring with regard to the S3i results and also suggest that the primary mechanism of benefit of the Sapien 3 valve over the XT valve is its lower profile, which allows roughly 90% of patients to be treated via a transfemoral approach,” according to Dr. Cohen.
He predicted the new cost-effectiveness findings will not substantially increase patient demand for TAVR, which is already high.
“By far what’s driving patients to TAVR today are the quality of life advantages. They love the idea of recovering quickly,” he said.
Michael Mack, MD, commented that this analysis probably underestimates the true cost advantage of TAVR by a fair amount, since the average hospital length of stay for TAVR patients in PARTNER 2A was 6.4 days.
“We now know that half of U.S. TAVR patients in many centers go home the day after the procedure, so you would expect that TAVR would look even more favorable based on current practice,” said Dr. Mack, medical director of cardiovascular surgery for the Baylor Health Care System and chairman of the Heart Hospital Baylor Plano (Tex.) Research Center.
Session moderator Patrick W. Serruys, MD, of Imperial College, London, observed that the cost differential between TAVR and SAVR will grow even larger once the sky-high cost of TAVR valves comes down. He predicted that’s likely to happen as a result of increased competition once a third valve receives marketing approval, just as occurred after a third drug-eluting stent hit the market.
Several physicians grumbled about the unfairness of current reimbursement for TAVR, which in effect penalizes hospitals. Dr. Cohen said that situation will change.
“I think the future of health care financing in the U.S. is bundled payment and accountable care organizations. In the setting of bundled payment for a 6-month period or even for 90 days, TAVR would look fantastic to a hospital or an health maintenance organization due to avoidance of rehospitalizations and rehabilitation and skilled nursing facility stays,” the cardiologist said.
The PARTNER 2A trial, the S3i registry, and the cost-effectiveness analysis were funded by Edwards Lifesciences. Dr. Cohen reported receiving research funding from and serving as a consultant to Edwards Lifesciences and other device companies.
DENVER – A formal cost-effectiveness analysis indicates that transcatheter aortic valve replacement (TAVR) is substantially more cost effective than surgical valve replacement in patients at intermediate surgical risk similar to those enrolled in the landmark PARTNER 2 trial.
The analysis demonstrated that over a 1- and 2-year follow-up period, as well as with projected lifetime follow-up, TAVR entails both lower long-term costs and greater quality-adjusted life expectancy, David J. Cohen, MD, reported at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
His two-part, patient-level economic analysis examined data from nearly 2,000 participants in the PARTNER 2A randomized trial comparing TAVR, using the Sapien XT valve, with surgical aortic valve replacement (SAVR), as well as the experience with the current-generation Sapien 3 TAVR valve in 1,077 intermediate–surgical risk TAVR patients in the S3i registry. The analysis utilized Medicare claims data on the costs of the index hospitalization and follow-up care.
In PARTNER 2A, the average total cost of the index hospitalization for valve replacement was $61,433 with TAVR. That was just $2,888 more than the SAVR hospitalization, despite the far higher acquisition cost of the Sapien 3 valve, which was roughly $32,500, compared with $5,000 for the surgical valve. Most of this additional cost of the TAVR valve was counterbalanced by TAVR’s 2-hour shorter procedural duration, the 6.4-day average length of stay, compared with 10.9 days for SAVR, and the fact that TAVR patients spent only 2.4 days in intensive care while SAVR patients averaged 4.6 days, Dr. Cohen explained at the meeting sponsored by the Cardiovascular Research Foundation.
During 24 months of postdischarge follow-up in the PARTNER 2A trial, SAVR patients racked up an average of $9,303 more in costs than TAVR patients. This was mainly because of their much higher rates of rehospitalization and time spent in skilled nursing facilities and rehabilitation centers, mainly during months 2-6 post discharge. The result was that 2-year total costs including the index hospitalization averaged $107,716 per TAVR patient and $114,132 per SAVR patient.
“One of the really remarkable findings of this study was what happened during follow-up,” the cardiologist observed.
Extrapolating to projected remaining lifetime years, TAVR using the Sapien XT valve resulted in a cost savings of $7,949 per patient and a 0.15-year increase in quality-adjusted life expectancy compared with SAVR.
But since the time of PARTNER 2A, the Sapien XT valve has been replaced by the updated Sapien 3 valve. The analysis of the S3i registry showed that the economic dominance of TAVR over SAVR was even greater owing to improved valve technology and contemporary care patterns. For this analysis, because there has been no randomized trial of TAVR with the Sapien 3 valve versus SAVR, patients in the SAVR of arm of PARTNER 2A served as the comparison group.
The cost of the index hospitalization was more than $4,000 less with TAVR in the S3i registry than with SAVR. The total cost of TAVR through 1 year of follow-up averaged $80,977, which was $15,511 less than the $96,489 for SAVR. The cost post discharge out to 1 year was more than $11,000 less per TAVR patient, driven by sharply lower rates of both cardiovascular and noncardiovascular hospitalizations as well as a greater than 50% reduction in days spent in rehab centers and skilled nursing facilities, compared with SAVR patients.
Projected over estimated remaining years of life, TAVR with the Sapien 3 valve yielded a cost savings of $9,692 per patient compared with SAVR, as well as a 0.27-year gain in quality-adjusted life-years.
Eighty-eight percent of patients in the S3i registry received their Sapien 3 valve via a transfemoral approach. When Dr. Cohen and his coinvestigators compared their costs and clinical outcomes to the subset of PARTNER 2A TAVR patients who got the Sapien XT valve transfemorally, the outcomes were “virtually identical,” he said.
“These findings are reassuring with regard to the S3i results and also suggest that the primary mechanism of benefit of the Sapien 3 valve over the XT valve is its lower profile, which allows roughly 90% of patients to be treated via a transfemoral approach,” according to Dr. Cohen.
He predicted the new cost-effectiveness findings will not substantially increase patient demand for TAVR, which is already high.
“By far what’s driving patients to TAVR today are the quality of life advantages. They love the idea of recovering quickly,” he said.
Michael Mack, MD, commented that this analysis probably underestimates the true cost advantage of TAVR by a fair amount, since the average hospital length of stay for TAVR patients in PARTNER 2A was 6.4 days.
“We now know that half of U.S. TAVR patients in many centers go home the day after the procedure, so you would expect that TAVR would look even more favorable based on current practice,” said Dr. Mack, medical director of cardiovascular surgery for the Baylor Health Care System and chairman of the Heart Hospital Baylor Plano (Tex.) Research Center.
Session moderator Patrick W. Serruys, MD, of Imperial College, London, observed that the cost differential between TAVR and SAVR will grow even larger once the sky-high cost of TAVR valves comes down. He predicted that’s likely to happen as a result of increased competition once a third valve receives marketing approval, just as occurred after a third drug-eluting stent hit the market.
Several physicians grumbled about the unfairness of current reimbursement for TAVR, which in effect penalizes hospitals. Dr. Cohen said that situation will change.
“I think the future of health care financing in the U.S. is bundled payment and accountable care organizations. In the setting of bundled payment for a 6-month period or even for 90 days, TAVR would look fantastic to a hospital or an health maintenance organization due to avoidance of rehospitalizations and rehabilitation and skilled nursing facility stays,” the cardiologist said.
The PARTNER 2A trial, the S3i registry, and the cost-effectiveness analysis were funded by Edwards Lifesciences. Dr. Cohen reported receiving research funding from and serving as a consultant to Edwards Lifesciences and other device companies.
DENVER – A formal cost-effectiveness analysis indicates that transcatheter aortic valve replacement (TAVR) is substantially more cost effective than surgical valve replacement in patients at intermediate surgical risk similar to those enrolled in the landmark PARTNER 2 trial.
The analysis demonstrated that over a 1- and 2-year follow-up period, as well as with projected lifetime follow-up, TAVR entails both lower long-term costs and greater quality-adjusted life expectancy, David J. Cohen, MD, reported at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
His two-part, patient-level economic analysis examined data from nearly 2,000 participants in the PARTNER 2A randomized trial comparing TAVR, using the Sapien XT valve, with surgical aortic valve replacement (SAVR), as well as the experience with the current-generation Sapien 3 TAVR valve in 1,077 intermediate–surgical risk TAVR patients in the S3i registry. The analysis utilized Medicare claims data on the costs of the index hospitalization and follow-up care.
In PARTNER 2A, the average total cost of the index hospitalization for valve replacement was $61,433 with TAVR. That was just $2,888 more than the SAVR hospitalization, despite the far higher acquisition cost of the Sapien 3 valve, which was roughly $32,500, compared with $5,000 for the surgical valve. Most of this additional cost of the TAVR valve was counterbalanced by TAVR’s 2-hour shorter procedural duration, the 6.4-day average length of stay, compared with 10.9 days for SAVR, and the fact that TAVR patients spent only 2.4 days in intensive care while SAVR patients averaged 4.6 days, Dr. Cohen explained at the meeting sponsored by the Cardiovascular Research Foundation.
During 24 months of postdischarge follow-up in the PARTNER 2A trial, SAVR patients racked up an average of $9,303 more in costs than TAVR patients. This was mainly because of their much higher rates of rehospitalization and time spent in skilled nursing facilities and rehabilitation centers, mainly during months 2-6 post discharge. The result was that 2-year total costs including the index hospitalization averaged $107,716 per TAVR patient and $114,132 per SAVR patient.
“One of the really remarkable findings of this study was what happened during follow-up,” the cardiologist observed.
Extrapolating to projected remaining lifetime years, TAVR using the Sapien XT valve resulted in a cost savings of $7,949 per patient and a 0.15-year increase in quality-adjusted life expectancy compared with SAVR.
But since the time of PARTNER 2A, the Sapien XT valve has been replaced by the updated Sapien 3 valve. The analysis of the S3i registry showed that the economic dominance of TAVR over SAVR was even greater owing to improved valve technology and contemporary care patterns. For this analysis, because there has been no randomized trial of TAVR with the Sapien 3 valve versus SAVR, patients in the SAVR of arm of PARTNER 2A served as the comparison group.
The cost of the index hospitalization was more than $4,000 less with TAVR in the S3i registry than with SAVR. The total cost of TAVR through 1 year of follow-up averaged $80,977, which was $15,511 less than the $96,489 for SAVR. The cost post discharge out to 1 year was more than $11,000 less per TAVR patient, driven by sharply lower rates of both cardiovascular and noncardiovascular hospitalizations as well as a greater than 50% reduction in days spent in rehab centers and skilled nursing facilities, compared with SAVR patients.
Projected over estimated remaining years of life, TAVR with the Sapien 3 valve yielded a cost savings of $9,692 per patient compared with SAVR, as well as a 0.27-year gain in quality-adjusted life-years.
Eighty-eight percent of patients in the S3i registry received their Sapien 3 valve via a transfemoral approach. When Dr. Cohen and his coinvestigators compared their costs and clinical outcomes to the subset of PARTNER 2A TAVR patients who got the Sapien XT valve transfemorally, the outcomes were “virtually identical,” he said.
“These findings are reassuring with regard to the S3i results and also suggest that the primary mechanism of benefit of the Sapien 3 valve over the XT valve is its lower profile, which allows roughly 90% of patients to be treated via a transfemoral approach,” according to Dr. Cohen.
He predicted the new cost-effectiveness findings will not substantially increase patient demand for TAVR, which is already high.
“By far what’s driving patients to TAVR today are the quality of life advantages. They love the idea of recovering quickly,” he said.
Michael Mack, MD, commented that this analysis probably underestimates the true cost advantage of TAVR by a fair amount, since the average hospital length of stay for TAVR patients in PARTNER 2A was 6.4 days.
“We now know that half of U.S. TAVR patients in many centers go home the day after the procedure, so you would expect that TAVR would look even more favorable based on current practice,” said Dr. Mack, medical director of cardiovascular surgery for the Baylor Health Care System and chairman of the Heart Hospital Baylor Plano (Tex.) Research Center.
Session moderator Patrick W. Serruys, MD, of Imperial College, London, observed that the cost differential between TAVR and SAVR will grow even larger once the sky-high cost of TAVR valves comes down. He predicted that’s likely to happen as a result of increased competition once a third valve receives marketing approval, just as occurred after a third drug-eluting stent hit the market.
Several physicians grumbled about the unfairness of current reimbursement for TAVR, which in effect penalizes hospitals. Dr. Cohen said that situation will change.
“I think the future of health care financing in the U.S. is bundled payment and accountable care organizations. In the setting of bundled payment for a 6-month period or even for 90 days, TAVR would look fantastic to a hospital or an health maintenance organization due to avoidance of rehospitalizations and rehabilitation and skilled nursing facility stays,” the cardiologist said.
The PARTNER 2A trial, the S3i registry, and the cost-effectiveness analysis were funded by Edwards Lifesciences. Dr. Cohen reported receiving research funding from and serving as a consultant to Edwards Lifesciences and other device companies.
AT TCT 2017
Key clinical point:
Major finding: The total cost of TAVR with the Sapien 3 valve in intermediate-risk patients, including the index hospitalization and costs incurred during the first year after, averaged $80,977, compared with $96,489 per SAVR patient.
Data source: This patient-level formal cost-effectiveness analysis included nearly 2,000 patients in the PARTNER 2A trial and more than 1,700 in a registry of recipients of the Sapien 3 TAVR valve.
Disclosures: The cost-effectiveness analysis was funded by Edwards Lifesciences. The presenter reported receiving research funding from and serving as a consultant to Edwards Lifesciences and other device companies.
VIDEO: Rapid influenza test obviates empiric antivirals
TORONTO – A test that only requires a maximum 2-hour wait for results was highly accurate at detecting influenza and respiratory syncytial virus infection in lung transplant patients, according to research presented at the CHEST annual meeting on Oct. 30.
This rapid and highly accurate test for detecting three common respiratory viruses has dramatically cut the need for empiric treatments and the risk for causing nosocomial infections in lung transplant patients who develop severe upper respiratory infections, Macé M. Schuurmans, MD, noted during the presentation.
This study involved 100 consecutive lung transplant patients who presented at Zurich University Hospital with signs of severe upper respiratory infection. The researchers ran the rapid and standard diagnostic tests for each patient and found that, relative to the standard test, the rapid test had positive and negative predictive values of 95%.
The number of empiric treatments with oseltamivir (Tamiflu) and ribavirin to treat a suspected influenza or respiratory syncytial virus infection (RSV) has “strongly diminished” by about two-thirds, noted Dr. Schuurmans, who is a pulmonologist at the hospital.
Until the rapid test became available, Dr. Shuurmans and his associates used a standard polymerase chain reaction test that takes 36-48 hours to yield a result. Using this test made treating patients empirically with oseltamivir and oral antibiotics for a couple of days a necessity, he said in a video interview. The older test also required isolating patients to avoid the potential spread of influenza or RSV in the hospital.
The rapid test, which became available for U.S. use in early 2017, covers influenza A and B and RSV in a single test with a single mouth-swab specimen.
“We now routinely use the rapid test and don’t prescribe empiric antivirals or antibiotics as often,” Dr. Schuurmans said. “There is much less drug cost and fewer potential adverse effects from empiric treatment.” Specimens still also undergo conventional testing, however, because that can identify eight additional viruses that the rapid test doesn’t cover.
Dr. Schuurmans acknowledged that further study needs to assess the cost-benefit of the rapid test to confirm that its added expense is offset by reduced expenses for empiric treatment and hospital isolation.
He had no disclosures. The study received no commercial support.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
TORONTO – A test that only requires a maximum 2-hour wait for results was highly accurate at detecting influenza and respiratory syncytial virus infection in lung transplant patients, according to research presented at the CHEST annual meeting on Oct. 30.
This rapid and highly accurate test for detecting three common respiratory viruses has dramatically cut the need for empiric treatments and the risk for causing nosocomial infections in lung transplant patients who develop severe upper respiratory infections, Macé M. Schuurmans, MD, noted during the presentation.
This study involved 100 consecutive lung transplant patients who presented at Zurich University Hospital with signs of severe upper respiratory infection. The researchers ran the rapid and standard diagnostic tests for each patient and found that, relative to the standard test, the rapid test had positive and negative predictive values of 95%.
The number of empiric treatments with oseltamivir (Tamiflu) and ribavirin to treat a suspected influenza or respiratory syncytial virus infection (RSV) has “strongly diminished” by about two-thirds, noted Dr. Schuurmans, who is a pulmonologist at the hospital.
Until the rapid test became available, Dr. Shuurmans and his associates used a standard polymerase chain reaction test that takes 36-48 hours to yield a result. Using this test made treating patients empirically with oseltamivir and oral antibiotics for a couple of days a necessity, he said in a video interview. The older test also required isolating patients to avoid the potential spread of influenza or RSV in the hospital.
The rapid test, which became available for U.S. use in early 2017, covers influenza A and B and RSV in a single test with a single mouth-swab specimen.
“We now routinely use the rapid test and don’t prescribe empiric antivirals or antibiotics as often,” Dr. Schuurmans said. “There is much less drug cost and fewer potential adverse effects from empiric treatment.” Specimens still also undergo conventional testing, however, because that can identify eight additional viruses that the rapid test doesn’t cover.
Dr. Schuurmans acknowledged that further study needs to assess the cost-benefit of the rapid test to confirm that its added expense is offset by reduced expenses for empiric treatment and hospital isolation.
He had no disclosures. The study received no commercial support.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
TORONTO – A test that only requires a maximum 2-hour wait for results was highly accurate at detecting influenza and respiratory syncytial virus infection in lung transplant patients, according to research presented at the CHEST annual meeting on Oct. 30.
This rapid and highly accurate test for detecting three common respiratory viruses has dramatically cut the need for empiric treatments and the risk for causing nosocomial infections in lung transplant patients who develop severe upper respiratory infections, Macé M. Schuurmans, MD, noted during the presentation.
This study involved 100 consecutive lung transplant patients who presented at Zurich University Hospital with signs of severe upper respiratory infection. The researchers ran the rapid and standard diagnostic tests for each patient and found that, relative to the standard test, the rapid test had positive and negative predictive values of 95%.
The number of empiric treatments with oseltamivir (Tamiflu) and ribavirin to treat a suspected influenza or respiratory syncytial virus infection (RSV) has “strongly diminished” by about two-thirds, noted Dr. Schuurmans, who is a pulmonologist at the hospital.
Until the rapid test became available, Dr. Shuurmans and his associates used a standard polymerase chain reaction test that takes 36-48 hours to yield a result. Using this test made treating patients empirically with oseltamivir and oral antibiotics for a couple of days a necessity, he said in a video interview. The older test also required isolating patients to avoid the potential spread of influenza or RSV in the hospital.
The rapid test, which became available for U.S. use in early 2017, covers influenza A and B and RSV in a single test with a single mouth-swab specimen.
“We now routinely use the rapid test and don’t prescribe empiric antivirals or antibiotics as often,” Dr. Schuurmans said. “There is much less drug cost and fewer potential adverse effects from empiric treatment.” Specimens still also undergo conventional testing, however, because that can identify eight additional viruses that the rapid test doesn’t cover.
Dr. Schuurmans acknowledged that further study needs to assess the cost-benefit of the rapid test to confirm that its added expense is offset by reduced expenses for empiric treatment and hospital isolation.
He had no disclosures. The study received no commercial support.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
AT CHEST 2017
Key clinical point:
Major finding: The rapid test had positive and negative predictive values of 95%.
Data source: A single-center observational study of 100 consecutive lung transplant recipients who presented with severe, acute respiratory infection.
Disclosures: Dr. Schuurmans had no disclosures. The study received no commercial support.
Cold stored platelets control bleeding after complex cardiac surgery
SAN DIEGO – Cold stored leukoreduced apheresis platelets in platelet additive solution were effective for controlling bleeding in a small study of patients undergoing complex cardiothoracic surgery, according to findings presented at the annual meeting of the American Association of Blood Banks.
The volume of postoperative bleeding was significantly lower among patients who received cold stored platelets compared with those who received standard room temperature storage platelets. Thromboembolic events did not differ between the two groups, nor did measures of coagulation at varying time points. Platelet counts and blood usage were also similar in the two groups. The study was small, however, and further studies are needed to confirm the findings.
“These patients are undergoing major surgery and are at high risk in every aspect,” said Torunn Oveland Apelseth, MD, PhD, of the Laboratory of Clinical Biochemistry, Haukeland (Norway) University Hospital. “They are at high risk for bleeding, at high risk for thromboembolic events and high blood usage, and there is a need for optimized blood components.”
There has been debate over the use of cold stored platelets, she noted. While storage at 4° C shortens platelet circulation time, some research shows that cold stored platelets have better hemostatic function.
In this study, one patient cohort was transfused with leukoreduced apheresis platelets stored at 4° C in platelet additive solution for up to 7 days under constant agitation, while the other group received platelets stored at standard room temperature. The study endpoints were comparisons between the two groups of postoperative bleeding, total blood usage, and laboratory measures of coagulation and blood cell counts within the first postoperative day. Thromboembolic events in the 28 days after surgery were also evaluated.
The study evaluated 17 patients who received cold stored platelets and 22 who received room temperature storage platelets. Patient demographics for the two groups were similar – as were their international normalized ratios, activated partial thromboplastin times, and fibrinogen levels – before surgery, immediately after heparin reversal, and the morning following the procedure.
Platelet counts and hemoglobin levels also did not significantly differ between groups.
As measured by chest drain output after chest closure, patients who received cold stored platelets had a significantly lower median amount of bleeding in the postoperative period compared with patients given room temperature storage platelets: 576 mL vs. 838 mL. Average chest drain output after chest closure was 594 mL in those who did not receive any transfusions.
Thromboembolic events occurred in 3 patients (18%) who received cold stored platelets and 7 (31%) of those given room temperature storage platelets. The difference was not statistically significant. In addition, blood usage – platelets, red blood cells, and solvent/detergent-treated pooled plasma – was similar for the two cohorts.
“There were also no differences in the number of thromboembolic episodes or length of stay in ICU,” said Dr. Apelseth, who recommended larger studies to explore the use of use of cold stored platelet transfusion in the critical care setting.
SAN DIEGO – Cold stored leukoreduced apheresis platelets in platelet additive solution were effective for controlling bleeding in a small study of patients undergoing complex cardiothoracic surgery, according to findings presented at the annual meeting of the American Association of Blood Banks.
The volume of postoperative bleeding was significantly lower among patients who received cold stored platelets compared with those who received standard room temperature storage platelets. Thromboembolic events did not differ between the two groups, nor did measures of coagulation at varying time points. Platelet counts and blood usage were also similar in the two groups. The study was small, however, and further studies are needed to confirm the findings.
“These patients are undergoing major surgery and are at high risk in every aspect,” said Torunn Oveland Apelseth, MD, PhD, of the Laboratory of Clinical Biochemistry, Haukeland (Norway) University Hospital. “They are at high risk for bleeding, at high risk for thromboembolic events and high blood usage, and there is a need for optimized blood components.”
There has been debate over the use of cold stored platelets, she noted. While storage at 4° C shortens platelet circulation time, some research shows that cold stored platelets have better hemostatic function.
In this study, one patient cohort was transfused with leukoreduced apheresis platelets stored at 4° C in platelet additive solution for up to 7 days under constant agitation, while the other group received platelets stored at standard room temperature. The study endpoints were comparisons between the two groups of postoperative bleeding, total blood usage, and laboratory measures of coagulation and blood cell counts within the first postoperative day. Thromboembolic events in the 28 days after surgery were also evaluated.
The study evaluated 17 patients who received cold stored platelets and 22 who received room temperature storage platelets. Patient demographics for the two groups were similar – as were their international normalized ratios, activated partial thromboplastin times, and fibrinogen levels – before surgery, immediately after heparin reversal, and the morning following the procedure.
Platelet counts and hemoglobin levels also did not significantly differ between groups.
As measured by chest drain output after chest closure, patients who received cold stored platelets had a significantly lower median amount of bleeding in the postoperative period compared with patients given room temperature storage platelets: 576 mL vs. 838 mL. Average chest drain output after chest closure was 594 mL in those who did not receive any transfusions.
Thromboembolic events occurred in 3 patients (18%) who received cold stored platelets and 7 (31%) of those given room temperature storage platelets. The difference was not statistically significant. In addition, blood usage – platelets, red blood cells, and solvent/detergent-treated pooled plasma – was similar for the two cohorts.
“There were also no differences in the number of thromboembolic episodes or length of stay in ICU,” said Dr. Apelseth, who recommended larger studies to explore the use of use of cold stored platelet transfusion in the critical care setting.
SAN DIEGO – Cold stored leukoreduced apheresis platelets in platelet additive solution were effective for controlling bleeding in a small study of patients undergoing complex cardiothoracic surgery, according to findings presented at the annual meeting of the American Association of Blood Banks.
The volume of postoperative bleeding was significantly lower among patients who received cold stored platelets compared with those who received standard room temperature storage platelets. Thromboembolic events did not differ between the two groups, nor did measures of coagulation at varying time points. Platelet counts and blood usage were also similar in the two groups. The study was small, however, and further studies are needed to confirm the findings.
“These patients are undergoing major surgery and are at high risk in every aspect,” said Torunn Oveland Apelseth, MD, PhD, of the Laboratory of Clinical Biochemistry, Haukeland (Norway) University Hospital. “They are at high risk for bleeding, at high risk for thromboembolic events and high blood usage, and there is a need for optimized blood components.”
There has been debate over the use of cold stored platelets, she noted. While storage at 4° C shortens platelet circulation time, some research shows that cold stored platelets have better hemostatic function.
In this study, one patient cohort was transfused with leukoreduced apheresis platelets stored at 4° C in platelet additive solution for up to 7 days under constant agitation, while the other group received platelets stored at standard room temperature. The study endpoints were comparisons between the two groups of postoperative bleeding, total blood usage, and laboratory measures of coagulation and blood cell counts within the first postoperative day. Thromboembolic events in the 28 days after surgery were also evaluated.
The study evaluated 17 patients who received cold stored platelets and 22 who received room temperature storage platelets. Patient demographics for the two groups were similar – as were their international normalized ratios, activated partial thromboplastin times, and fibrinogen levels – before surgery, immediately after heparin reversal, and the morning following the procedure.
Platelet counts and hemoglobin levels also did not significantly differ between groups.
As measured by chest drain output after chest closure, patients who received cold stored platelets had a significantly lower median amount of bleeding in the postoperative period compared with patients given room temperature storage platelets: 576 mL vs. 838 mL. Average chest drain output after chest closure was 594 mL in those who did not receive any transfusions.
Thromboembolic events occurred in 3 patients (18%) who received cold stored platelets and 7 (31%) of those given room temperature storage platelets. The difference was not statistically significant. In addition, blood usage – platelets, red blood cells, and solvent/detergent-treated pooled plasma – was similar for the two cohorts.
“There were also no differences in the number of thromboembolic episodes or length of stay in ICU,” said Dr. Apelseth, who recommended larger studies to explore the use of use of cold stored platelet transfusion in the critical care setting.
AT AABB17
Key clinical point: Cold stored leukoreduced apheresis platelets in platelet additive solution are effective for treating bleeding in patients undergoing complex cardiothoracic surgery.
Major finding: Patients who underwent procedures requiring cardiopulmonary bypass circulation had a significantly lower median amount of bleeding in the postoperative period with cold stored platelets compared with standard room temperature platelets: 576 mL vs. 838 mL.
Data source: Randomized two-arm pilot trial of cardiothoracic surgery patients.
Disclosures: The authors have no relevant financial disclosures.













