User login
Protective hypothermia during arch surgery lacked benefit, study shows
NEW YORK – Deep hypothermia may affect long-term survival in individuals who have aortic arch surgery with antegrade cerebral perfusion (ACP), but not short-term outcomes in terms of death and major morbidities, according to a Baylor College of Medicine study.
The study evaluated outcomes of 544 consecutive patients who had proximal and total aortic arch surgery and received ACP for more than 30 minutes over a 10-year period, said lead investigator Ourania Preventza, MD, of the division of cardiothoracic surgery at the college in Houston. The researchers compared results of three different hypothermia levels: deep hypothermia at 14.1°-20° C; low-moderate at 20.1°-23.9° C; and high-moderate at 24°-28° C. The study also classified ACP time in two levels: 31-45 minutes for 238 patients (43.8%); and 45 minutes or more in 306 patients (56.3%).
“The different temperature levels did not significantly affect the short-term mortality and major morbidity rates,” Dr. Preventza said. “Reoperation for bleeding was associated with lower temperature (14.1°-20° C). The long-term survival rate in patients who underwent proximal arch surgery involving ACP for more than 30 minutes and use of moderate hypothermia (20.1°-28° C) were actually improved.”
While the outcomes showed small variations between the three groups, with deep hypothermia being associated with a higher percentage of adverse outcomes, Dr. Preventza said the differences were not statistically significant. The overall operative mortality rate was 12.5% (68 patients): 15.5% (18 patients) in the deep-hypothermia group; 11.8% (31 patients) in the low-moderate group; and 11.5% (19 patients) in the high-moderate group (P = 0.54).
The patients who underwent deep hypothermia were more likely to receive unilateral ACP, and those who underwent moderate hypothermia were more likely to have bilateral ACP, Dr. Preventza said at the meeting sponsored by the American Association for Thoracic Surgery. The deep-hypothermia group had higher transfusion rates, but, again, the researchers did not consider this variation to be statistically significant.
In the deep-hypothermia group, 20.9% of patients had a reoperation for bleeding, compared with 11.3% in the overall group and 7.7% and 10.2% in the low- and high-moderate groups, respectively, Dr. Preventza reported. Multivariate analysis revealed that higher temperature was associated with less bleeding, with an odds ratio of 0.61 (P = 0.015).
Deep hypothermia was related to statistically significant differences in the rates of permanent stroke and permanent neurologic events in the univariate analysis only, Dr. Preventza said: 6.3% and 7.2%, respectively, in the overall analysis vs. 12.2% for both events in the deep-hypothermia group. In the propensity score analysis, the rates of permanent stroke and permanent neurologic events in the moderate-hypothermia group were 7.6% and 8.5%, respectively, vs. 11.3% for both events in the deep-hypothermia group, a nonsignificant difference.
“With regard to permanent stoke and permanent neurological events, the multivariate analysis showed that preoperatively a neurologic deficit as well as acute type I aortic dissection were associated with adverse neurological events,” she said.
“However,” Dr. Preventza added, “the surprising thing is that when we looked at long-term survival for the entire cohort, we saw that the patients with moderate hypothermia did better.”
Kaplan-Meier analysis for the propensity pairs showed that the probability of survival at 8 years was 55.3% for the deep-hypothermia group vs. 68.5% for the moderate-hypothermia group.
The approach the Baylor researchers used involved cannulating the axillary or innominate artery in most patients before administering ACP, although a few patients had femoral or direct aortic cannulation, Dr. Preventza said. For bilateral ACP, the researchers delivered cerebral perfusion via a 9-French Pruitt balloon-tip catheter (LeMaitre Vascular) in the left common carotid artery. To protect the brain, they administered perfusion at 8-12 cc/kg per min and maintained a perfusion pressure of 50-70 mm Hg, as measured via the radial arterial line and guided by near-infrared spectroscopy.
Dr. Preventza had no relevant disclosures.
NEW YORK – Deep hypothermia may affect long-term survival in individuals who have aortic arch surgery with antegrade cerebral perfusion (ACP), but not short-term outcomes in terms of death and major morbidities, according to a Baylor College of Medicine study.
The study evaluated outcomes of 544 consecutive patients who had proximal and total aortic arch surgery and received ACP for more than 30 minutes over a 10-year period, said lead investigator Ourania Preventza, MD, of the division of cardiothoracic surgery at the college in Houston. The researchers compared results of three different hypothermia levels: deep hypothermia at 14.1°-20° C; low-moderate at 20.1°-23.9° C; and high-moderate at 24°-28° C. The study also classified ACP time in two levels: 31-45 minutes for 238 patients (43.8%); and 45 minutes or more in 306 patients (56.3%).
“The different temperature levels did not significantly affect the short-term mortality and major morbidity rates,” Dr. Preventza said. “Reoperation for bleeding was associated with lower temperature (14.1°-20° C). The long-term survival rate in patients who underwent proximal arch surgery involving ACP for more than 30 minutes and use of moderate hypothermia (20.1°-28° C) were actually improved.”
While the outcomes showed small variations between the three groups, with deep hypothermia being associated with a higher percentage of adverse outcomes, Dr. Preventza said the differences were not statistically significant. The overall operative mortality rate was 12.5% (68 patients): 15.5% (18 patients) in the deep-hypothermia group; 11.8% (31 patients) in the low-moderate group; and 11.5% (19 patients) in the high-moderate group (P = 0.54).
The patients who underwent deep hypothermia were more likely to receive unilateral ACP, and those who underwent moderate hypothermia were more likely to have bilateral ACP, Dr. Preventza said at the meeting sponsored by the American Association for Thoracic Surgery. The deep-hypothermia group had higher transfusion rates, but, again, the researchers did not consider this variation to be statistically significant.
In the deep-hypothermia group, 20.9% of patients had a reoperation for bleeding, compared with 11.3% in the overall group and 7.7% and 10.2% in the low- and high-moderate groups, respectively, Dr. Preventza reported. Multivariate analysis revealed that higher temperature was associated with less bleeding, with an odds ratio of 0.61 (P = 0.015).
Deep hypothermia was related to statistically significant differences in the rates of permanent stroke and permanent neurologic events in the univariate analysis only, Dr. Preventza said: 6.3% and 7.2%, respectively, in the overall analysis vs. 12.2% for both events in the deep-hypothermia group. In the propensity score analysis, the rates of permanent stroke and permanent neurologic events in the moderate-hypothermia group were 7.6% and 8.5%, respectively, vs. 11.3% for both events in the deep-hypothermia group, a nonsignificant difference.
“With regard to permanent stoke and permanent neurological events, the multivariate analysis showed that preoperatively a neurologic deficit as well as acute type I aortic dissection were associated with adverse neurological events,” she said.
“However,” Dr. Preventza added, “the surprising thing is that when we looked at long-term survival for the entire cohort, we saw that the patients with moderate hypothermia did better.”
Kaplan-Meier analysis for the propensity pairs showed that the probability of survival at 8 years was 55.3% for the deep-hypothermia group vs. 68.5% for the moderate-hypothermia group.
The approach the Baylor researchers used involved cannulating the axillary or innominate artery in most patients before administering ACP, although a few patients had femoral or direct aortic cannulation, Dr. Preventza said. For bilateral ACP, the researchers delivered cerebral perfusion via a 9-French Pruitt balloon-tip catheter (LeMaitre Vascular) in the left common carotid artery. To protect the brain, they administered perfusion at 8-12 cc/kg per min and maintained a perfusion pressure of 50-70 mm Hg, as measured via the radial arterial line and guided by near-infrared spectroscopy.
Dr. Preventza had no relevant disclosures.
NEW YORK – Deep hypothermia may affect long-term survival in individuals who have aortic arch surgery with antegrade cerebral perfusion (ACP), but not short-term outcomes in terms of death and major morbidities, according to a Baylor College of Medicine study.
The study evaluated outcomes of 544 consecutive patients who had proximal and total aortic arch surgery and received ACP for more than 30 minutes over a 10-year period, said lead investigator Ourania Preventza, MD, of the division of cardiothoracic surgery at the college in Houston. The researchers compared results of three different hypothermia levels: deep hypothermia at 14.1°-20° C; low-moderate at 20.1°-23.9° C; and high-moderate at 24°-28° C. The study also classified ACP time in two levels: 31-45 minutes for 238 patients (43.8%); and 45 minutes or more in 306 patients (56.3%).
“The different temperature levels did not significantly affect the short-term mortality and major morbidity rates,” Dr. Preventza said. “Reoperation for bleeding was associated with lower temperature (14.1°-20° C). The long-term survival rate in patients who underwent proximal arch surgery involving ACP for more than 30 minutes and use of moderate hypothermia (20.1°-28° C) were actually improved.”
While the outcomes showed small variations between the three groups, with deep hypothermia being associated with a higher percentage of adverse outcomes, Dr. Preventza said the differences were not statistically significant. The overall operative mortality rate was 12.5% (68 patients): 15.5% (18 patients) in the deep-hypothermia group; 11.8% (31 patients) in the low-moderate group; and 11.5% (19 patients) in the high-moderate group (P = 0.54).
The patients who underwent deep hypothermia were more likely to receive unilateral ACP, and those who underwent moderate hypothermia were more likely to have bilateral ACP, Dr. Preventza said at the meeting sponsored by the American Association for Thoracic Surgery. The deep-hypothermia group had higher transfusion rates, but, again, the researchers did not consider this variation to be statistically significant.
In the deep-hypothermia group, 20.9% of patients had a reoperation for bleeding, compared with 11.3% in the overall group and 7.7% and 10.2% in the low- and high-moderate groups, respectively, Dr. Preventza reported. Multivariate analysis revealed that higher temperature was associated with less bleeding, with an odds ratio of 0.61 (P = 0.015).
Deep hypothermia was related to statistically significant differences in the rates of permanent stroke and permanent neurologic events in the univariate analysis only, Dr. Preventza said: 6.3% and 7.2%, respectively, in the overall analysis vs. 12.2% for both events in the deep-hypothermia group. In the propensity score analysis, the rates of permanent stroke and permanent neurologic events in the moderate-hypothermia group were 7.6% and 8.5%, respectively, vs. 11.3% for both events in the deep-hypothermia group, a nonsignificant difference.
“With regard to permanent stoke and permanent neurological events, the multivariate analysis showed that preoperatively a neurologic deficit as well as acute type I aortic dissection were associated with adverse neurological events,” she said.
“However,” Dr. Preventza added, “the surprising thing is that when we looked at long-term survival for the entire cohort, we saw that the patients with moderate hypothermia did better.”
Kaplan-Meier analysis for the propensity pairs showed that the probability of survival at 8 years was 55.3% for the deep-hypothermia group vs. 68.5% for the moderate-hypothermia group.
The approach the Baylor researchers used involved cannulating the axillary or innominate artery in most patients before administering ACP, although a few patients had femoral or direct aortic cannulation, Dr. Preventza said. For bilateral ACP, the researchers delivered cerebral perfusion via a 9-French Pruitt balloon-tip catheter (LeMaitre Vascular) in the left common carotid artery. To protect the brain, they administered perfusion at 8-12 cc/kg per min and maintained a perfusion pressure of 50-70 mm Hg, as measured via the radial arterial line and guided by near-infrared spectroscopy.
Dr. Preventza had no relevant disclosures.
AT AATS AORTIC SYMPOSIUM 2016
Key clinical point: Differing temperatures of hypothermia did not affect death or morbidity in patients who had aortic arch surgery with more than 30 minutes of antegrade cerebral perfusion.
Major finding: The overall operative death rate in the study was 12.4% with no statistically significant differences between three different hypothermia groups.
Data source: Series of 510 consecutive patients who had proximal and total arch surgery and received antegrade cerebral perfusion for more than 30 minutes over a 10-year period.
Disclosures: Dr. Preventza reported having no financial disclosures.
Surgery for acute type A dissection shows 20-year shift to valve sparing, biological valves
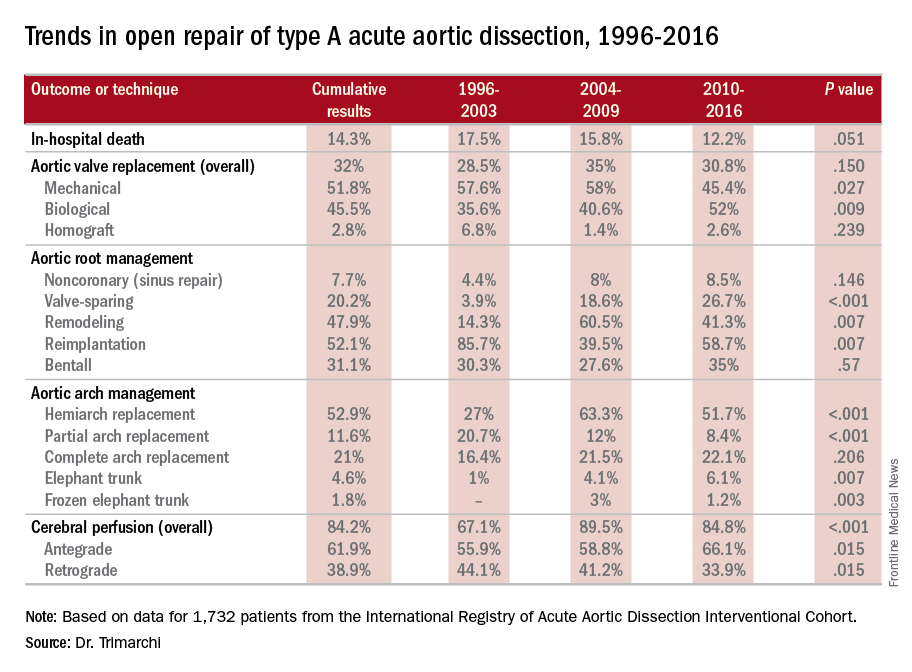
NEW YORK – A study of an international database of individuals who have had open repair for acute type A aortic dissection (ATAAD) has revealed that in the past 20 years, cardiovascular surgeons have widely embraced valve-sparing procedures, bioprosthetic valves, and cerebral profusion strategies, according to a report here on the latest analysis of the database.
The most telling result is the decline in overall mortality, Santi Trimarchi, MD, PhD, of the University of Milan IRCCS Policlinico San Donato in Italy reported on behalf of the International Registry of Acute Aortic Dissection (IRAD) Interventional Cohort (IVC). The cohort analyzed surgery techniques and outcomes of 1,732 patients who had open repair from 1996 to 2016, clustering results in three time intervals: 1996-2003; 2004-2009; and 2010-2015.
“We noted in the registry that the overall in-hospital mortality rate was 14.3%, and this mortality decreased over time from 17.5% in the first six-year time span to 12.2% in the last six years,” Dr. Trimarchi said.

Among other trends the study identified are greater reliance on biological vs. mechanical valves, an increase in valve-sparing procedures, and steady use of Bentall procedures throughout the study period. “Operative techniques for redo aortic valve repair have been improving over the time, and that’s why we see more frequent use of biologic valves,” he said at the meeting, sponsored by the American Association for Thoracic Surgery.
“Cerebral profusion management has been widely adopted,” Dr. Trimarchi said. “Also there is an important trend showing an increasing utilization of antegrade cerebral profusion while we see a negative trend of the utilization of retrograde brain protection.”
Dr. Trimarchi attributed the detail the study generated to the survey form sent to the 26 IRAD-IVC sites around the world. The form measures 131 different variables, he said.
“Using this new specific surgical data form, we think we can address some surgical issues and report better data from the IRAD registry results on acute dissection,” he said. “These analyses have shown there have been significant changes in operative strategy over time in terms of managing such patients, and more importantly, a significant decrease in in-hospital mortality was observed in a 20-year time period.”
Dr. Trimarchi disclosed that he has received speaking and consulting fees and research support from W.L. Gore & Associates and Medtronic. IRAD is supported by W.L. Gore, Active Sites, Medtronic, Varbedian Aortic Research Fund, the Hewlett Foundation, the Mardigian Foundation, UM Faculty Group Practice, Terumo, and Ann and Bob Aikens.
NEW YORK – A study of an international database of individuals who have had open repair for acute type A aortic dissection (ATAAD) has revealed that in the past 20 years, cardiovascular surgeons have widely embraced valve-sparing procedures, bioprosthetic valves, and cerebral profusion strategies, according to a report here on the latest analysis of the database.
The most telling result is the decline in overall mortality, Santi Trimarchi, MD, PhD, of the University of Milan IRCCS Policlinico San Donato in Italy reported on behalf of the International Registry of Acute Aortic Dissection (IRAD) Interventional Cohort (IVC). The cohort analyzed surgery techniques and outcomes of 1,732 patients who had open repair from 1996 to 2016, clustering results in three time intervals: 1996-2003; 2004-2009; and 2010-2015.
“We noted in the registry that the overall in-hospital mortality rate was 14.3%, and this mortality decreased over time from 17.5% in the first six-year time span to 12.2% in the last six years,” Dr. Trimarchi said.

Among other trends the study identified are greater reliance on biological vs. mechanical valves, an increase in valve-sparing procedures, and steady use of Bentall procedures throughout the study period. “Operative techniques for redo aortic valve repair have been improving over the time, and that’s why we see more frequent use of biologic valves,” he said at the meeting, sponsored by the American Association for Thoracic Surgery.
“Cerebral profusion management has been widely adopted,” Dr. Trimarchi said. “Also there is an important trend showing an increasing utilization of antegrade cerebral profusion while we see a negative trend of the utilization of retrograde brain protection.”
Dr. Trimarchi attributed the detail the study generated to the survey form sent to the 26 IRAD-IVC sites around the world. The form measures 131 different variables, he said.
“Using this new specific surgical data form, we think we can address some surgical issues and report better data from the IRAD registry results on acute dissection,” he said. “These analyses have shown there have been significant changes in operative strategy over time in terms of managing such patients, and more importantly, a significant decrease in in-hospital mortality was observed in a 20-year time period.”
Dr. Trimarchi disclosed that he has received speaking and consulting fees and research support from W.L. Gore & Associates and Medtronic. IRAD is supported by W.L. Gore, Active Sites, Medtronic, Varbedian Aortic Research Fund, the Hewlett Foundation, the Mardigian Foundation, UM Faculty Group Practice, Terumo, and Ann and Bob Aikens.
NEW YORK – A study of an international database of individuals who have had open repair for acute type A aortic dissection (ATAAD) has revealed that in the past 20 years, cardiovascular surgeons have widely embraced valve-sparing procedures, bioprosthetic valves, and cerebral profusion strategies, according to a report here on the latest analysis of the database.
The most telling result is the decline in overall mortality, Santi Trimarchi, MD, PhD, of the University of Milan IRCCS Policlinico San Donato in Italy reported on behalf of the International Registry of Acute Aortic Dissection (IRAD) Interventional Cohort (IVC). The cohort analyzed surgery techniques and outcomes of 1,732 patients who had open repair from 1996 to 2016, clustering results in three time intervals: 1996-2003; 2004-2009; and 2010-2015.
“We noted in the registry that the overall in-hospital mortality rate was 14.3%, and this mortality decreased over time from 17.5% in the first six-year time span to 12.2% in the last six years,” Dr. Trimarchi said.

Among other trends the study identified are greater reliance on biological vs. mechanical valves, an increase in valve-sparing procedures, and steady use of Bentall procedures throughout the study period. “Operative techniques for redo aortic valve repair have been improving over the time, and that’s why we see more frequent use of biologic valves,” he said at the meeting, sponsored by the American Association for Thoracic Surgery.
“Cerebral profusion management has been widely adopted,” Dr. Trimarchi said. “Also there is an important trend showing an increasing utilization of antegrade cerebral profusion while we see a negative trend of the utilization of retrograde brain protection.”
Dr. Trimarchi attributed the detail the study generated to the survey form sent to the 26 IRAD-IVC sites around the world. The form measures 131 different variables, he said.
“Using this new specific surgical data form, we think we can address some surgical issues and report better data from the IRAD registry results on acute dissection,” he said. “These analyses have shown there have been significant changes in operative strategy over time in terms of managing such patients, and more importantly, a significant decrease in in-hospital mortality was observed in a 20-year time period.”
Dr. Trimarchi disclosed that he has received speaking and consulting fees and research support from W.L. Gore & Associates and Medtronic. IRAD is supported by W.L. Gore, Active Sites, Medtronic, Varbedian Aortic Research Fund, the Hewlett Foundation, the Mardigian Foundation, UM Faculty Group Practice, Terumo, and Ann and Bob Aikens.
AT AATS AORTIC SYMPOSIUM 2016
Key clinical point: Operations for acute type A aortic dissection (ATAAD) have seen significant changes in technique over the past 20 years.
Major finding: Use of biological valves increased from 35.6% of procedures to 52% over the study period while reliance of mechanical valves declined from 57.6% to 45.4%.
Data source: Interventional Cohort database of 1,732 patients enrolled in the International Registry of Acute Aortic Dissection database who had open surgery for ATAAD from February 1996 to March 2015.
Disclosures: Dr. Trimarchi disclosed having receive speaking and consulting fees from W.L. Gore & Associates and Medtronic as well as research support from the two companies. IRAD is supported by W.L. Gore, Active Sites, Medtronic, Varbedian Aortic Research Fund, the Hewlett Foundation, the Mardigian Foundation, UM Faculty Group Practice, Terumo, and Ann and Bob Aikens.
Study quantifies volume disparities for ATAD repair in the U.K.
NEW YORK – Mastery is the product of repetition, and it has long been taken for granted that surgeons and centers that perform a high volume of an operation will have better results than those who don’t do the operation as often, but a study out of the United Kingdom has determined just how much better high-volume centers are when it comes to repair of acute type A aortic dissection (ATAD) – and what the in-hospital mortality odds ratio is for lower-volume surgeons.
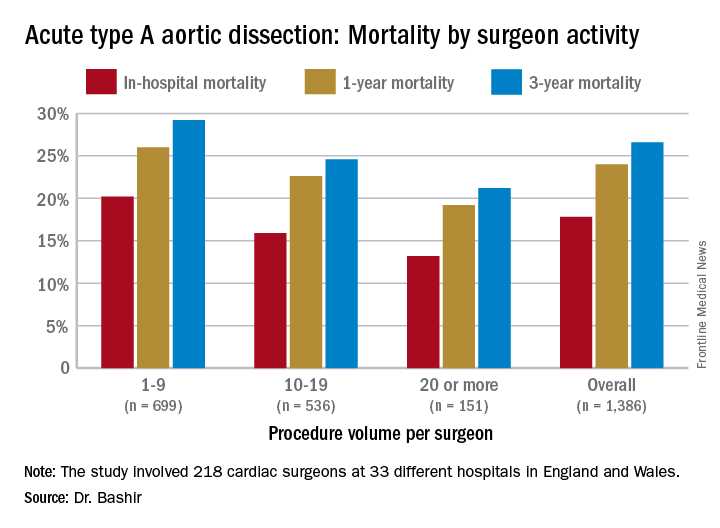
Specifically, that odds ratio is 1.64 (P = .030), Mohamad Bashir, MD, PhD, MRCS, a research fellow at Liverpool Heart and Chest Hospital, said in reporting early results of the study here. Lower-volume surgeons had worse outcomes in 12 of 14 different operative metrics the study evaluated, most notably in-hospital mortality: 20.2% for lower-volume surgeons vs. 15.2% for higher-volume surgeons. “There is an initiative in the U.K. to change the trend,” Dr. Bashir said. Full study results will be published in an upcoming issue of BMJ, he said.
“In-hospital mortality for surgeons who operate on 20 or more procedures is very good at 13.2%, and the same follows for 90-day mortality, one-year mortality and three-year mortality,” Dr. Bashir said.
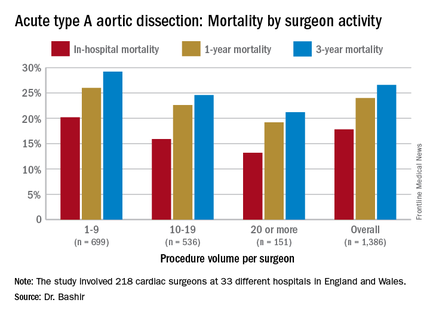
The study evaluated 1,386 ATAD procedures in the National Institute for Cardiovascular Outcomes Research database by 218 different cardiac surgeons at 33 different hospitals in England and Wales from April 2007 to March 2013. That would make the average number of procedures per surgeon 6.4, Dr. Bashir said, but a closer look at each surgeon’s case load reveals some disconcerting trends: almost 80% of the surgeons performed fewer than 10 ATAD repairs in the 6-year span of the study, and 34 surgeons, or about 15%, just did a single procedure in that time. The highest-volume surgeon did 32 procedures. The minimum hospital volume was 8 ATAD operations and the maximum was 103.
The study stratified lower- and higher-volume surgeon groups by characteristics of the patients they operated on. “The differences between these two groups are pretty interesting because we noticed that the lower-volume surgeons are actually operating on patients who are diabetic, who are smokers, who use inotropic support prior to anesthesia and who also have an injection fraction that is significant,” Dr. Bashir said.
In drilling down into those characteristics, people with diabetes made up 6% of the lower-volume surgeons’ cases vs. 3.1% of the higher-volume surgeons’ cases, despite an almost 50-50 split in share of procedures between the two surgeon groups. Current smokers comprised 20.5% of the lower-volume surgeons’ patients vs. 15.5% of their high-volume counterparts’ patients. Operative characteristics in terms of urgency of surgery were similar between the two groups. However Dr. Bashir noted, lower-volume surgeons had longer times for cardiopulmonary bypass, aortic cross-clamping, and circulatory arrest.
The study investigators applied a multivariable logistic regression model to determine predictors of in-hospital mortality for ATAD. “The odds ratio (OR) of mortality for lower-volume surgeons is 1.64, which is statistically significant,” Dr. Bashir said. Odds ratios for other predictors are: previous cardiac surgery, 2.51; peripheral vascular disease, 2.15; preoperative cardiogenic shock, 2.05; salvage operation, 5.57; and concomitant coronary artery bypass procedure, 2.98. For 5-year mortality, the odds ratio was 1.37 for the lower-volume surgeons.
Dr. Bashir laid out how the National Health Service can use the study results. “Concentration of expertise and volume to the appropriate surgeons and centers who perform increasingly more work and more complex aortic cases would be required to change the paradigm of acute type A aortic dissection outcomes in the U.K.,” he said. “It is reasonable to suggest that there should be a national standardization mandate and a quality-improvement framework of acute aortic dissection treatment.”
Dr. Bashir had no financial relationships to disclose.
NEW YORK – Mastery is the product of repetition, and it has long been taken for granted that surgeons and centers that perform a high volume of an operation will have better results than those who don’t do the operation as often, but a study out of the United Kingdom has determined just how much better high-volume centers are when it comes to repair of acute type A aortic dissection (ATAD) – and what the in-hospital mortality odds ratio is for lower-volume surgeons.
Specifically, that odds ratio is 1.64 (P = .030), Mohamad Bashir, MD, PhD, MRCS, a research fellow at Liverpool Heart and Chest Hospital, said in reporting early results of the study here. Lower-volume surgeons had worse outcomes in 12 of 14 different operative metrics the study evaluated, most notably in-hospital mortality: 20.2% for lower-volume surgeons vs. 15.2% for higher-volume surgeons. “There is an initiative in the U.K. to change the trend,” Dr. Bashir said. Full study results will be published in an upcoming issue of BMJ, he said.
“In-hospital mortality for surgeons who operate on 20 or more procedures is very good at 13.2%, and the same follows for 90-day mortality, one-year mortality and three-year mortality,” Dr. Bashir said.

The study evaluated 1,386 ATAD procedures in the National Institute for Cardiovascular Outcomes Research database by 218 different cardiac surgeons at 33 different hospitals in England and Wales from April 2007 to March 2013. That would make the average number of procedures per surgeon 6.4, Dr. Bashir said, but a closer look at each surgeon’s case load reveals some disconcerting trends: almost 80% of the surgeons performed fewer than 10 ATAD repairs in the 6-year span of the study, and 34 surgeons, or about 15%, just did a single procedure in that time. The highest-volume surgeon did 32 procedures. The minimum hospital volume was 8 ATAD operations and the maximum was 103.
The study stratified lower- and higher-volume surgeon groups by characteristics of the patients they operated on. “The differences between these two groups are pretty interesting because we noticed that the lower-volume surgeons are actually operating on patients who are diabetic, who are smokers, who use inotropic support prior to anesthesia and who also have an injection fraction that is significant,” Dr. Bashir said.
In drilling down into those characteristics, people with diabetes made up 6% of the lower-volume surgeons’ cases vs. 3.1% of the higher-volume surgeons’ cases, despite an almost 50-50 split in share of procedures between the two surgeon groups. Current smokers comprised 20.5% of the lower-volume surgeons’ patients vs. 15.5% of their high-volume counterparts’ patients. Operative characteristics in terms of urgency of surgery were similar between the two groups. However Dr. Bashir noted, lower-volume surgeons had longer times for cardiopulmonary bypass, aortic cross-clamping, and circulatory arrest.
The study investigators applied a multivariable logistic regression model to determine predictors of in-hospital mortality for ATAD. “The odds ratio (OR) of mortality for lower-volume surgeons is 1.64, which is statistically significant,” Dr. Bashir said. Odds ratios for other predictors are: previous cardiac surgery, 2.51; peripheral vascular disease, 2.15; preoperative cardiogenic shock, 2.05; salvage operation, 5.57; and concomitant coronary artery bypass procedure, 2.98. For 5-year mortality, the odds ratio was 1.37 for the lower-volume surgeons.
Dr. Bashir laid out how the National Health Service can use the study results. “Concentration of expertise and volume to the appropriate surgeons and centers who perform increasingly more work and more complex aortic cases would be required to change the paradigm of acute type A aortic dissection outcomes in the U.K.,” he said. “It is reasonable to suggest that there should be a national standardization mandate and a quality-improvement framework of acute aortic dissection treatment.”
Dr. Bashir had no financial relationships to disclose.
NEW YORK – Mastery is the product of repetition, and it has long been taken for granted that surgeons and centers that perform a high volume of an operation will have better results than those who don’t do the operation as often, but a study out of the United Kingdom has determined just how much better high-volume centers are when it comes to repair of acute type A aortic dissection (ATAD) – and what the in-hospital mortality odds ratio is for lower-volume surgeons.
Specifically, that odds ratio is 1.64 (P = .030), Mohamad Bashir, MD, PhD, MRCS, a research fellow at Liverpool Heart and Chest Hospital, said in reporting early results of the study here. Lower-volume surgeons had worse outcomes in 12 of 14 different operative metrics the study evaluated, most notably in-hospital mortality: 20.2% for lower-volume surgeons vs. 15.2% for higher-volume surgeons. “There is an initiative in the U.K. to change the trend,” Dr. Bashir said. Full study results will be published in an upcoming issue of BMJ, he said.
“In-hospital mortality for surgeons who operate on 20 or more procedures is very good at 13.2%, and the same follows for 90-day mortality, one-year mortality and three-year mortality,” Dr. Bashir said.

The study evaluated 1,386 ATAD procedures in the National Institute for Cardiovascular Outcomes Research database by 218 different cardiac surgeons at 33 different hospitals in England and Wales from April 2007 to March 2013. That would make the average number of procedures per surgeon 6.4, Dr. Bashir said, but a closer look at each surgeon’s case load reveals some disconcerting trends: almost 80% of the surgeons performed fewer than 10 ATAD repairs in the 6-year span of the study, and 34 surgeons, or about 15%, just did a single procedure in that time. The highest-volume surgeon did 32 procedures. The minimum hospital volume was 8 ATAD operations and the maximum was 103.
The study stratified lower- and higher-volume surgeon groups by characteristics of the patients they operated on. “The differences between these two groups are pretty interesting because we noticed that the lower-volume surgeons are actually operating on patients who are diabetic, who are smokers, who use inotropic support prior to anesthesia and who also have an injection fraction that is significant,” Dr. Bashir said.
In drilling down into those characteristics, people with diabetes made up 6% of the lower-volume surgeons’ cases vs. 3.1% of the higher-volume surgeons’ cases, despite an almost 50-50 split in share of procedures between the two surgeon groups. Current smokers comprised 20.5% of the lower-volume surgeons’ patients vs. 15.5% of their high-volume counterparts’ patients. Operative characteristics in terms of urgency of surgery were similar between the two groups. However Dr. Bashir noted, lower-volume surgeons had longer times for cardiopulmonary bypass, aortic cross-clamping, and circulatory arrest.
The study investigators applied a multivariable logistic regression model to determine predictors of in-hospital mortality for ATAD. “The odds ratio (OR) of mortality for lower-volume surgeons is 1.64, which is statistically significant,” Dr. Bashir said. Odds ratios for other predictors are: previous cardiac surgery, 2.51; peripheral vascular disease, 2.15; preoperative cardiogenic shock, 2.05; salvage operation, 5.57; and concomitant coronary artery bypass procedure, 2.98. For 5-year mortality, the odds ratio was 1.37 for the lower-volume surgeons.
Dr. Bashir laid out how the National Health Service can use the study results. “Concentration of expertise and volume to the appropriate surgeons and centers who perform increasingly more work and more complex aortic cases would be required to change the paradigm of acute type A aortic dissection outcomes in the U.K.,” he said. “It is reasonable to suggest that there should be a national standardization mandate and a quality-improvement framework of acute aortic dissection treatment.”
Dr. Bashir had no financial relationships to disclose.
AT THE AMERICAN ASSOCIATION FOR THORACIC SURGERY AORTIC SYMPOSIUM
Key clinical point: Patients undergoing repair of acute type A aortic dissection (ATAD) by lower-volume surgeons have high mortality in comparison with those undergoing repair by the highest-volume surgeons.
Major finding: In-hospital mortality for ATAD repair was 20.2% for lower-volume surgeons and 15.3% for higher-volume surgeons.
Data source: Analysis of 1,386 ATAD procedures from April 2007 to March 2013 in the National Institute for Cardiovascular Outcomes Research data.
Disclosures: Dr. Bashir reported having no financial disclosures.
Guideline tweak addresses conflicting recommendations on BAV
NEW YORK – While overall guidelines for aortic repair surgery have not changed significantly in the past 5 years, guidelines for the timing of surgery in patients with bicuspid aortic valves and enlarged aortas have undergone some updating in an attempt to clear up disparities in different guidelines on when to operate on those patients.
Lars G. Svensson, MD, PhD, chairman of the Cleveland Clinic Heart and Vascular Institute, coauthor of the clarification statement by the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines (J Thorac Cardiovasc Surg. 2016;151:959-66), reported on the guidelines clarification at the meeting sponsored by the American Association for Thoracic Surgery. He noted that five different clinical guidelines between 2010 and 2014 recommended five different size thresholds for prophylactic aortic root or ascending aortic surgery in the setting of bicuspid aortic valve (BAV), ranging from 4 cm to greater than 5.5 cm. “This created a bit of a quandary and controversy between different guidelines and time periods,” he said.
Dr. Svensson and Loren Hiratzka, MD, medical director of cardiac surgery for TriHealth in Cincinnati, and their colleagues drafted the guideline clarification that makes the following recommendations for aortic root and ascending aorta repair or replacement when patients have BAV (strength of recommendation):
• Surgery is indicated to replace the aortic root or ascending aorta in asymptomatic patients with BAV if the diameter of the aortic root or ascending aorta is 5.5 cm or greater (Class 1).
• Surgical repair is indicated for asymptomatic patients with BAV if the root or ascending aorta diameter is 5 cm or greater in two scenarios: if the patient has an additional risk factor for dissection, such as family history or excessive aortic growth rate; or if the patient is a low surgical risk and has access to an experienced surgeon at a high-volume center (Class IIa).
The guideline update also addresses BAV in patients with Turner syndrome. The 2010 joint guidelines of 10 societies left some questions with regard to surgery in these patients, Dr. Svensson said. The established guidelines included a Class IIb recommendation for imaging of the heart and aorta to help determine the aorta risk in patients with Turner syndrome who had additional risk factors, including BAV, aortic coarctation and/or hypertension, or were planning a pregnancy.
The updated guideline includes Class IIa recommendation that in short-statured patients with Turner syndrome and BAV, measurement of the aortic root or ascending aorta diameter may not predict the dissection risk as well as aortic diameter index greater than 2.5 cm/m2. The updated recommendations also draw on one study that reported that in patients with BAV, a maximum aortic cross-sectional area-to-height ratio of 10 cm2/m or greater was also predictive of aortic dissection. (Ann Thorac Surg. 2015;100:1666-73)
The updated recommendations for open surgery for ascending aortic aneurysm include separate valve and ascending aortic replacement in patients without significant aortic root dilatation or in elderly patients, or in younger patients with minimal dilatation who have aortic valve disease; and excision of the sinuses of Valsalva with a modified David reimplantation when technically feasible in patients with connective tissue disease and others with dilatation of the aortic root and sinuses. For patients in whom the latter procedure is not feasible, root replacement with valved graft conduit would be indicated, Dr. Svensson said.
Dr. Svensson also reported on recent studies that validated recommendations in established guidelines.
Studies of circulatory arrest practices in aortic arch surgery as prescribed by established guidelines showed confirmatory results, he said. “The one point I would make about circulatory arrest is that we found in a fairly large study of 1,352 circulatory arrest patients that we reduced the risk of stroke by 40% when we used the axillary artery with a side a graft,” he said (Ann Thorac Surg. 2004;78:1274-84). His own institution’s clinical trial of 121 patients who received antegrade or retrograde brain perfusion showed rates of 0.8% for each stroke and operative death, he said (J Thorac Cardiovasc Surg. 2015;150:1140-7).
“What was also of interest there was no difference in outcomes with antegrade vs. retrograde brain profusion,” he said. “I think protection of the brain is pretty good if you follow the fundamental principles of brain protection.”
He also reported on a recent study at his institution that documented the benefits of intrathecal papaverine (IP) for spinal cord protection during descending open and endovascular aortic repairs. In 398 aortic repairs from 2001-2009, the rates of spinal cord injury were 23% in the non-IP group vs. 7% in the IP group (P = .07) in a matched cohort.
He noted that the clinical guidelines of the American Association for Thoracic Surgery as well as AATS/Society of Thoracic Surgeons joint guidelines are open to input. “If you have areas where you think guideline should be written about, please let me or other members of the committee know,” he said.
Dr. Svensson had no disclosures relevant to his presentation.
NEW YORK – While overall guidelines for aortic repair surgery have not changed significantly in the past 5 years, guidelines for the timing of surgery in patients with bicuspid aortic valves and enlarged aortas have undergone some updating in an attempt to clear up disparities in different guidelines on when to operate on those patients.
Lars G. Svensson, MD, PhD, chairman of the Cleveland Clinic Heart and Vascular Institute, coauthor of the clarification statement by the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines (J Thorac Cardiovasc Surg. 2016;151:959-66), reported on the guidelines clarification at the meeting sponsored by the American Association for Thoracic Surgery. He noted that five different clinical guidelines between 2010 and 2014 recommended five different size thresholds for prophylactic aortic root or ascending aortic surgery in the setting of bicuspid aortic valve (BAV), ranging from 4 cm to greater than 5.5 cm. “This created a bit of a quandary and controversy between different guidelines and time periods,” he said.
Dr. Svensson and Loren Hiratzka, MD, medical director of cardiac surgery for TriHealth in Cincinnati, and their colleagues drafted the guideline clarification that makes the following recommendations for aortic root and ascending aorta repair or replacement when patients have BAV (strength of recommendation):
• Surgery is indicated to replace the aortic root or ascending aorta in asymptomatic patients with BAV if the diameter of the aortic root or ascending aorta is 5.5 cm or greater (Class 1).
• Surgical repair is indicated for asymptomatic patients with BAV if the root or ascending aorta diameter is 5 cm or greater in two scenarios: if the patient has an additional risk factor for dissection, such as family history or excessive aortic growth rate; or if the patient is a low surgical risk and has access to an experienced surgeon at a high-volume center (Class IIa).
The guideline update also addresses BAV in patients with Turner syndrome. The 2010 joint guidelines of 10 societies left some questions with regard to surgery in these patients, Dr. Svensson said. The established guidelines included a Class IIb recommendation for imaging of the heart and aorta to help determine the aorta risk in patients with Turner syndrome who had additional risk factors, including BAV, aortic coarctation and/or hypertension, or were planning a pregnancy.
The updated guideline includes Class IIa recommendation that in short-statured patients with Turner syndrome and BAV, measurement of the aortic root or ascending aorta diameter may not predict the dissection risk as well as aortic diameter index greater than 2.5 cm/m2. The updated recommendations also draw on one study that reported that in patients with BAV, a maximum aortic cross-sectional area-to-height ratio of 10 cm2/m or greater was also predictive of aortic dissection. (Ann Thorac Surg. 2015;100:1666-73)
The updated recommendations for open surgery for ascending aortic aneurysm include separate valve and ascending aortic replacement in patients without significant aortic root dilatation or in elderly patients, or in younger patients with minimal dilatation who have aortic valve disease; and excision of the sinuses of Valsalva with a modified David reimplantation when technically feasible in patients with connective tissue disease and others with dilatation of the aortic root and sinuses. For patients in whom the latter procedure is not feasible, root replacement with valved graft conduit would be indicated, Dr. Svensson said.
Dr. Svensson also reported on recent studies that validated recommendations in established guidelines.
Studies of circulatory arrest practices in aortic arch surgery as prescribed by established guidelines showed confirmatory results, he said. “The one point I would make about circulatory arrest is that we found in a fairly large study of 1,352 circulatory arrest patients that we reduced the risk of stroke by 40% when we used the axillary artery with a side a graft,” he said (Ann Thorac Surg. 2004;78:1274-84). His own institution’s clinical trial of 121 patients who received antegrade or retrograde brain perfusion showed rates of 0.8% for each stroke and operative death, he said (J Thorac Cardiovasc Surg. 2015;150:1140-7).
“What was also of interest there was no difference in outcomes with antegrade vs. retrograde brain profusion,” he said. “I think protection of the brain is pretty good if you follow the fundamental principles of brain protection.”
He also reported on a recent study at his institution that documented the benefits of intrathecal papaverine (IP) for spinal cord protection during descending open and endovascular aortic repairs. In 398 aortic repairs from 2001-2009, the rates of spinal cord injury were 23% in the non-IP group vs. 7% in the IP group (P = .07) in a matched cohort.
He noted that the clinical guidelines of the American Association for Thoracic Surgery as well as AATS/Society of Thoracic Surgeons joint guidelines are open to input. “If you have areas where you think guideline should be written about, please let me or other members of the committee know,” he said.
Dr. Svensson had no disclosures relevant to his presentation.
NEW YORK – While overall guidelines for aortic repair surgery have not changed significantly in the past 5 years, guidelines for the timing of surgery in patients with bicuspid aortic valves and enlarged aortas have undergone some updating in an attempt to clear up disparities in different guidelines on when to operate on those patients.
Lars G. Svensson, MD, PhD, chairman of the Cleveland Clinic Heart and Vascular Institute, coauthor of the clarification statement by the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines (J Thorac Cardiovasc Surg. 2016;151:959-66), reported on the guidelines clarification at the meeting sponsored by the American Association for Thoracic Surgery. He noted that five different clinical guidelines between 2010 and 2014 recommended five different size thresholds for prophylactic aortic root or ascending aortic surgery in the setting of bicuspid aortic valve (BAV), ranging from 4 cm to greater than 5.5 cm. “This created a bit of a quandary and controversy between different guidelines and time periods,” he said.
Dr. Svensson and Loren Hiratzka, MD, medical director of cardiac surgery for TriHealth in Cincinnati, and their colleagues drafted the guideline clarification that makes the following recommendations for aortic root and ascending aorta repair or replacement when patients have BAV (strength of recommendation):
• Surgery is indicated to replace the aortic root or ascending aorta in asymptomatic patients with BAV if the diameter of the aortic root or ascending aorta is 5.5 cm or greater (Class 1).
• Surgical repair is indicated for asymptomatic patients with BAV if the root or ascending aorta diameter is 5 cm or greater in two scenarios: if the patient has an additional risk factor for dissection, such as family history or excessive aortic growth rate; or if the patient is a low surgical risk and has access to an experienced surgeon at a high-volume center (Class IIa).
The guideline update also addresses BAV in patients with Turner syndrome. The 2010 joint guidelines of 10 societies left some questions with regard to surgery in these patients, Dr. Svensson said. The established guidelines included a Class IIb recommendation for imaging of the heart and aorta to help determine the aorta risk in patients with Turner syndrome who had additional risk factors, including BAV, aortic coarctation and/or hypertension, or were planning a pregnancy.
The updated guideline includes Class IIa recommendation that in short-statured patients with Turner syndrome and BAV, measurement of the aortic root or ascending aorta diameter may not predict the dissection risk as well as aortic diameter index greater than 2.5 cm/m2. The updated recommendations also draw on one study that reported that in patients with BAV, a maximum aortic cross-sectional area-to-height ratio of 10 cm2/m or greater was also predictive of aortic dissection. (Ann Thorac Surg. 2015;100:1666-73)
The updated recommendations for open surgery for ascending aortic aneurysm include separate valve and ascending aortic replacement in patients without significant aortic root dilatation or in elderly patients, or in younger patients with minimal dilatation who have aortic valve disease; and excision of the sinuses of Valsalva with a modified David reimplantation when technically feasible in patients with connective tissue disease and others with dilatation of the aortic root and sinuses. For patients in whom the latter procedure is not feasible, root replacement with valved graft conduit would be indicated, Dr. Svensson said.
Dr. Svensson also reported on recent studies that validated recommendations in established guidelines.
Studies of circulatory arrest practices in aortic arch surgery as prescribed by established guidelines showed confirmatory results, he said. “The one point I would make about circulatory arrest is that we found in a fairly large study of 1,352 circulatory arrest patients that we reduced the risk of stroke by 40% when we used the axillary artery with a side a graft,” he said (Ann Thorac Surg. 2004;78:1274-84). His own institution’s clinical trial of 121 patients who received antegrade or retrograde brain perfusion showed rates of 0.8% for each stroke and operative death, he said (J Thorac Cardiovasc Surg. 2015;150:1140-7).
“What was also of interest there was no difference in outcomes with antegrade vs. retrograde brain profusion,” he said. “I think protection of the brain is pretty good if you follow the fundamental principles of brain protection.”
He also reported on a recent study at his institution that documented the benefits of intrathecal papaverine (IP) for spinal cord protection during descending open and endovascular aortic repairs. In 398 aortic repairs from 2001-2009, the rates of spinal cord injury were 23% in the non-IP group vs. 7% in the IP group (P = .07) in a matched cohort.
He noted that the clinical guidelines of the American Association for Thoracic Surgery as well as AATS/Society of Thoracic Surgeons joint guidelines are open to input. “If you have areas where you think guideline should be written about, please let me or other members of the committee know,” he said.
Dr. Svensson had no disclosures relevant to his presentation.
AT THE AATS AORTIC SYMPOSIUM 2016
Key clinical point: Various clinical guidelines provided five different recommendations for the timing of aortic repair surgery in patients with bicuspid aortic valves.
Major finding: Recent updates in guidelines provide clarity on when an aortic repair is needed in the setting of aortic bicuspid valve.
Data source: American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines.
Disclosures: Dr. Svensson had no relevant financial relationships to disclose.
The ‘guilty’ associates of silent thoracic aneurysm fingered
NEW YORK – Aortic aneurysm ranks as one of the top 20 causes of death in the United States. Most of these aneurysms are clinically silent until they rupture, but Yale cardiovascular surgeon John A. Elefteriades, MD, has developed a clinical paradigm that identifies eight markers that physicians can use to detect the disease before it strikes.
Dr. Elefteriades calls his paradigm “Guilt by Association.” It is based on an article he published online last year in the journal Open Heart (2015;2:e000169).
“What we need is for our colleagues in affiliated disciplines to recognize the importance of these offenders in indicating the presence of thoracic aortic aneurysm,” he said, reporting on the paradigm at the meeting sponsored by the American Association for Thoracic Surgery. He noted that studies from Japan of people who had died from out-of-hospital cardiac arrest found that 8% of them had a type A aortic dissection (Am J Cardiol 2016;117:1826-30).
He outlined eight “associates” of thoracic aortic aneurysm (TAA): intracranial aneurysm; bovine aortic arch; abdominal aortic aneurysm; simple renal cysts; bicuspid aortic valve; family history; positive thumb-palm test; and temporal arteritis and other autoimmune disorders.
A patient with TAA has a 10% likelihood of harboring an intracranial aneurysm (Am J Cardiol 2010;105:417-20). “It’s even more common in the descending, compared to the ascending group in examples that we’ve identified,” Dr. Elefteriades said. Particularly vulnerable are patients over age 70 and those with an intracranial aneurysm larger than 4 mm: the former has a 9% chance of harboring a TAA, the latter a 6% chance, he said.
The bovine arch had been thought to be benign, but, Dr. Elefteriades said, “We don’t think it is.”
Bovine arch refers to a group of congenital aortic arch vessels with an aberrant origin of the left common carotid artery. “We recently looked at this as a marker for thoracic aortic disease, and please note that 20% of our TAA patients have a bovine arch,” he said. “This is much higher than in the general population.”
Abdominal aortic aneurysm has long been associated with TAA, he said. “When these aneurysms are identified by ultrasound, it’s important that the thoracic aorta be checked as well,” he said.
“This is a message for internists and our vascular colleagues.”
Simple renal cysts have been found in patients with TAA at a “much higher” rate than the general population, as high as 57% of those with descending aortic aneurysms vs. 11%-13.7% of the general population, Dr. Elefteriades said (J. Am. Heart Assoc. 2016;5:e002248). Simple renal cysts are detected by abdominal CT scan. “It’s just a matter of a few more slices with the CT scan to get the entirety of the thoracic aorta evaluated,” he said.
“We encourage our radiology colleagues to do this when a renal cyst is detected.”
Bicuspid aortic valve mandates “support from our cardiac colleagues when they find one of these to let the patient know he has to be monitored lifelong for later development of this aneurysm,” Dr. Elefteriades said.
Family history of TAA has been known as a strong predictor, but genetic studies have provided clarity on the association (Arch Surg. 1999;134:361‐7). “If the proband has a thoracic aortic aneurysm, there’s 21% likelihood there’s a family member who is affected with an aneurysm somewhere in the body,” he said.
Location of aneurysms in family members is also important, Dr. Elefteriades said. “If the proband has a ascending aortic aneurysm, the kindred also have an ascending aortic aneurysm; but if the proband has a descending aneurysm, the likelihood is that the kindred will have an abdominal aortic aneurysm,” he said. “To identify silent disease, it’s very important we check siblings and children, and now, of course, we’re using whole-exome sequencing.” So far, Dr. Elefteriades has obtained whole-exome sequencing in 200 patients.
The thumb-palm test involves the patient touching the thumb to the palm; the thumb crossing the edge of the flat palm is an indicator of connective tissue disease. “It doesn’t cost anything,” Dr. Elefteriades said. “It is a very simple thing for internists to do to identify those connective tissue diseases.”
Temporal arteritis has become increasingly common in elderly women. “They have a markedly increased likelihood of having a thoracic aortic aneurysm – about 8% in some studies,’ Dr. Elefteriades said. “So we want our neurology colleagues to be aware of this and to look for thoracic aortic aneurysm.”
Dr. Elefteriades disclosed he has received consulting fees from Baxter, Covidien, Datascope, and CryoLife, and a research grant from Medtronic.
NEW YORK – Aortic aneurysm ranks as one of the top 20 causes of death in the United States. Most of these aneurysms are clinically silent until they rupture, but Yale cardiovascular surgeon John A. Elefteriades, MD, has developed a clinical paradigm that identifies eight markers that physicians can use to detect the disease before it strikes.
Dr. Elefteriades calls his paradigm “Guilt by Association.” It is based on an article he published online last year in the journal Open Heart (2015;2:e000169).
“What we need is for our colleagues in affiliated disciplines to recognize the importance of these offenders in indicating the presence of thoracic aortic aneurysm,” he said, reporting on the paradigm at the meeting sponsored by the American Association for Thoracic Surgery. He noted that studies from Japan of people who had died from out-of-hospital cardiac arrest found that 8% of them had a type A aortic dissection (Am J Cardiol 2016;117:1826-30).
He outlined eight “associates” of thoracic aortic aneurysm (TAA): intracranial aneurysm; bovine aortic arch; abdominal aortic aneurysm; simple renal cysts; bicuspid aortic valve; family history; positive thumb-palm test; and temporal arteritis and other autoimmune disorders.
A patient with TAA has a 10% likelihood of harboring an intracranial aneurysm (Am J Cardiol 2010;105:417-20). “It’s even more common in the descending, compared to the ascending group in examples that we’ve identified,” Dr. Elefteriades said. Particularly vulnerable are patients over age 70 and those with an intracranial aneurysm larger than 4 mm: the former has a 9% chance of harboring a TAA, the latter a 6% chance, he said.
The bovine arch had been thought to be benign, but, Dr. Elefteriades said, “We don’t think it is.”
Bovine arch refers to a group of congenital aortic arch vessels with an aberrant origin of the left common carotid artery. “We recently looked at this as a marker for thoracic aortic disease, and please note that 20% of our TAA patients have a bovine arch,” he said. “This is much higher than in the general population.”
Abdominal aortic aneurysm has long been associated with TAA, he said. “When these aneurysms are identified by ultrasound, it’s important that the thoracic aorta be checked as well,” he said.
“This is a message for internists and our vascular colleagues.”
Simple renal cysts have been found in patients with TAA at a “much higher” rate than the general population, as high as 57% of those with descending aortic aneurysms vs. 11%-13.7% of the general population, Dr. Elefteriades said (J. Am. Heart Assoc. 2016;5:e002248). Simple renal cysts are detected by abdominal CT scan. “It’s just a matter of a few more slices with the CT scan to get the entirety of the thoracic aorta evaluated,” he said.
“We encourage our radiology colleagues to do this when a renal cyst is detected.”
Bicuspid aortic valve mandates “support from our cardiac colleagues when they find one of these to let the patient know he has to be monitored lifelong for later development of this aneurysm,” Dr. Elefteriades said.
Family history of TAA has been known as a strong predictor, but genetic studies have provided clarity on the association (Arch Surg. 1999;134:361‐7). “If the proband has a thoracic aortic aneurysm, there’s 21% likelihood there’s a family member who is affected with an aneurysm somewhere in the body,” he said.
Location of aneurysms in family members is also important, Dr. Elefteriades said. “If the proband has a ascending aortic aneurysm, the kindred also have an ascending aortic aneurysm; but if the proband has a descending aneurysm, the likelihood is that the kindred will have an abdominal aortic aneurysm,” he said. “To identify silent disease, it’s very important we check siblings and children, and now, of course, we’re using whole-exome sequencing.” So far, Dr. Elefteriades has obtained whole-exome sequencing in 200 patients.
The thumb-palm test involves the patient touching the thumb to the palm; the thumb crossing the edge of the flat palm is an indicator of connective tissue disease. “It doesn’t cost anything,” Dr. Elefteriades said. “It is a very simple thing for internists to do to identify those connective tissue diseases.”
Temporal arteritis has become increasingly common in elderly women. “They have a markedly increased likelihood of having a thoracic aortic aneurysm – about 8% in some studies,’ Dr. Elefteriades said. “So we want our neurology colleagues to be aware of this and to look for thoracic aortic aneurysm.”
Dr. Elefteriades disclosed he has received consulting fees from Baxter, Covidien, Datascope, and CryoLife, and a research grant from Medtronic.
NEW YORK – Aortic aneurysm ranks as one of the top 20 causes of death in the United States. Most of these aneurysms are clinically silent until they rupture, but Yale cardiovascular surgeon John A. Elefteriades, MD, has developed a clinical paradigm that identifies eight markers that physicians can use to detect the disease before it strikes.
Dr. Elefteriades calls his paradigm “Guilt by Association.” It is based on an article he published online last year in the journal Open Heart (2015;2:e000169).
“What we need is for our colleagues in affiliated disciplines to recognize the importance of these offenders in indicating the presence of thoracic aortic aneurysm,” he said, reporting on the paradigm at the meeting sponsored by the American Association for Thoracic Surgery. He noted that studies from Japan of people who had died from out-of-hospital cardiac arrest found that 8% of them had a type A aortic dissection (Am J Cardiol 2016;117:1826-30).
He outlined eight “associates” of thoracic aortic aneurysm (TAA): intracranial aneurysm; bovine aortic arch; abdominal aortic aneurysm; simple renal cysts; bicuspid aortic valve; family history; positive thumb-palm test; and temporal arteritis and other autoimmune disorders.
A patient with TAA has a 10% likelihood of harboring an intracranial aneurysm (Am J Cardiol 2010;105:417-20). “It’s even more common in the descending, compared to the ascending group in examples that we’ve identified,” Dr. Elefteriades said. Particularly vulnerable are patients over age 70 and those with an intracranial aneurysm larger than 4 mm: the former has a 9% chance of harboring a TAA, the latter a 6% chance, he said.
The bovine arch had been thought to be benign, but, Dr. Elefteriades said, “We don’t think it is.”
Bovine arch refers to a group of congenital aortic arch vessels with an aberrant origin of the left common carotid artery. “We recently looked at this as a marker for thoracic aortic disease, and please note that 20% of our TAA patients have a bovine arch,” he said. “This is much higher than in the general population.”
Abdominal aortic aneurysm has long been associated with TAA, he said. “When these aneurysms are identified by ultrasound, it’s important that the thoracic aorta be checked as well,” he said.
“This is a message for internists and our vascular colleagues.”
Simple renal cysts have been found in patients with TAA at a “much higher” rate than the general population, as high as 57% of those with descending aortic aneurysms vs. 11%-13.7% of the general population, Dr. Elefteriades said (J. Am. Heart Assoc. 2016;5:e002248). Simple renal cysts are detected by abdominal CT scan. “It’s just a matter of a few more slices with the CT scan to get the entirety of the thoracic aorta evaluated,” he said.
“We encourage our radiology colleagues to do this when a renal cyst is detected.”
Bicuspid aortic valve mandates “support from our cardiac colleagues when they find one of these to let the patient know he has to be monitored lifelong for later development of this aneurysm,” Dr. Elefteriades said.
Family history of TAA has been known as a strong predictor, but genetic studies have provided clarity on the association (Arch Surg. 1999;134:361‐7). “If the proband has a thoracic aortic aneurysm, there’s 21% likelihood there’s a family member who is affected with an aneurysm somewhere in the body,” he said.
Location of aneurysms in family members is also important, Dr. Elefteriades said. “If the proband has a ascending aortic aneurysm, the kindred also have an ascending aortic aneurysm; but if the proband has a descending aneurysm, the likelihood is that the kindred will have an abdominal aortic aneurysm,” he said. “To identify silent disease, it’s very important we check siblings and children, and now, of course, we’re using whole-exome sequencing.” So far, Dr. Elefteriades has obtained whole-exome sequencing in 200 patients.
The thumb-palm test involves the patient touching the thumb to the palm; the thumb crossing the edge of the flat palm is an indicator of connective tissue disease. “It doesn’t cost anything,” Dr. Elefteriades said. “It is a very simple thing for internists to do to identify those connective tissue diseases.”
Temporal arteritis has become increasingly common in elderly women. “They have a markedly increased likelihood of having a thoracic aortic aneurysm – about 8% in some studies,’ Dr. Elefteriades said. “So we want our neurology colleagues to be aware of this and to look for thoracic aortic aneurysm.”
Dr. Elefteriades disclosed he has received consulting fees from Baxter, Covidien, Datascope, and CryoLife, and a research grant from Medtronic.
EXPERT ANALYSIS FROM AATS AORTIC SYMPOSIUM 2016
Key clinical point: Eight markers may detect silent thoracic aneurysms before rupture.
Major finding: The eight “associates” of TAA include intracranial aneurysm; bovine aortic arch; abdominal aortic aneurysm; simple renal cysts; and bicuspid aortic valve.
Data source: The “Guilt by Association” paradigm was based upon a review of the literature by Dr. Elefteriades and his own published reports.
Disclosures: Dr. Elefteriades disclosed he has received consulting fees from Baxter, Covidien, Datascope, and CryoLife, and a research grant from Medtroni
Sex-mismatched RBCs associated with increased mortality after cardiac surgery
Transfusing sex-mismatched red blood cells (RBCs) was associated with an increased risk of death in people undergoing heart bypass surgery or aortic valve replacement, based on results of a retrospective single-center study of almost 10,000 transfusions in cardiac surgery patients.
Each unit of sex-mismatched red blood cells (RBCs) transfused was associated with an increased risk of death (hazard ratio, 1.083; 95% confidence interval, 1.028-1.140; P = .003). In addition, transfusing 1-2 units of non–leukocyte depleted RBCs was associated with a significant increase in the risk of death during the first year after surgery (HR, 1.426; 95% CI, 1.004-2.024; P = .047).
Transfusion of 1-2 units of leukocyte-depleted RBCs and the age of blood products was not associated with increased mortality (J Thorac Cardiovasc Surg. 2016;152:223-32.e1).
“Factors such as ABO group, Rh profile and sex of the PRBC [packed RBC] donor generally have been overlooked, as has the variation in postdonation treatment of blood,” in the outcomes of cardiac surgery patients, researchers led by Henrik Bjursten, MD, PhD, of Lund (Sweden) University, reported.
The study involved 9,907 patients at Lund University from 2002 to 2012: 7,696 had coronary artery bypass grafting (CABG); 1,216 had aortic valve replacement (AVR); and 995 concomitantly had both procedures. PRBC transfusions were given to nearly 51% of the patients. Compared with the group that did not receive PRBC transfusions, the transfused group had significantly higher rates of heart attack after surgery (1.5% vs. 0.6%), infection (0.6% vs. 0.3%), reoperation for bleeding (4.3% vs. 0.2%), 30-day death (0.7% vs. 0.2%), and overall death (25.9% vs. 12.6%).
Based on an analysis that factored in 24 different variables, transfusion of 1-2 units of non–leukocyte depleted PRBCs was associated with a HR of 1.426, but the same amount of leukocyte-depleted PRBCs did not increase risk (HR, 0.981). However, transfusion of 5-7 units of leukocyte-depleted RBCs was associated with decreased survival, as was transfusion of sex-mismatched PRBCs, associated with a HR of 1.046-1.133 per unit, Dr. Bjursten and colleagues wrote. “In this cohort, 58% of transfusions were sex mismatched, and thus we interpret the result as relatively robust and clinically relevant.”
Patients having combined CABG and AVR were more likely to have PRBC transfusions than patients who had a single procedure. Additionally, the increased death rate in the PRBC transfusion group may have been related to age and comorbidities such as diabetes, chronic obstructive pulmonary disease, and cardiac insufficiency. “Blood transfusion in part is a biomarker for advanced disease,” Dr. Bjursten and coauthors said. While patient who received PRBC transfusions may have been sicker, they did not require greater use of the ICU than patients who did not receive transfusions.
Dr. Bjursten disclosed receiving consulting fees from Boston Scientific. Coauthor Lars Algotsson, MD, PhD, disclosed receiving lecture fees from Abbott. All other authors had no financial disclosures.
The study results expand on the knowledge of potential sex-mismatch risks and reiterates the potential benefits of limiting transfusion to leukocyte-depleted PRBC.
Strengths of the study are its size and its use of the Swedish national tax registry to accurately count deaths. Weaknesses include its retrospective design and inherent issues with advanced statistical analysis, and the failure to address secondary morbidity outcomes. Cardiac surgery’s mortality is multifactorial and secondary outcomes would have strengthened the results.
Unlike previous studies, this study showed that 1 or 2 units of leukocyte-depleted PRBCs did not increase mortality.
This study suggests that sex-mismatched blood transfusions may create a high enough risk to necessitate a change in transfusion protocols. Further, many countries already have universal strategies to use leukocyte-depleted PRBC, and perhaps this study should call for the United States to pursue the same policy.
Jennifer Banayan, MD, and Mark Chaney, MD, of the University of Chicago made their remarks in a commentary (J Thorac Cardiovasc Surg. 2016;152:18-9) that accompanied the study.
The study results expand on the knowledge of potential sex-mismatch risks and reiterates the potential benefits of limiting transfusion to leukocyte-depleted PRBC.
Strengths of the study are its size and its use of the Swedish national tax registry to accurately count deaths. Weaknesses include its retrospective design and inherent issues with advanced statistical analysis, and the failure to address secondary morbidity outcomes. Cardiac surgery’s mortality is multifactorial and secondary outcomes would have strengthened the results.
Unlike previous studies, this study showed that 1 or 2 units of leukocyte-depleted PRBCs did not increase mortality.
This study suggests that sex-mismatched blood transfusions may create a high enough risk to necessitate a change in transfusion protocols. Further, many countries already have universal strategies to use leukocyte-depleted PRBC, and perhaps this study should call for the United States to pursue the same policy.
Jennifer Banayan, MD, and Mark Chaney, MD, of the University of Chicago made their remarks in a commentary (J Thorac Cardiovasc Surg. 2016;152:18-9) that accompanied the study.
The study results expand on the knowledge of potential sex-mismatch risks and reiterates the potential benefits of limiting transfusion to leukocyte-depleted PRBC.
Strengths of the study are its size and its use of the Swedish national tax registry to accurately count deaths. Weaknesses include its retrospective design and inherent issues with advanced statistical analysis, and the failure to address secondary morbidity outcomes. Cardiac surgery’s mortality is multifactorial and secondary outcomes would have strengthened the results.
Unlike previous studies, this study showed that 1 or 2 units of leukocyte-depleted PRBCs did not increase mortality.
This study suggests that sex-mismatched blood transfusions may create a high enough risk to necessitate a change in transfusion protocols. Further, many countries already have universal strategies to use leukocyte-depleted PRBC, and perhaps this study should call for the United States to pursue the same policy.
Jennifer Banayan, MD, and Mark Chaney, MD, of the University of Chicago made their remarks in a commentary (J Thorac Cardiovasc Surg. 2016;152:18-9) that accompanied the study.
Transfusing sex-mismatched red blood cells (RBCs) was associated with an increased risk of death in people undergoing heart bypass surgery or aortic valve replacement, based on results of a retrospective single-center study of almost 10,000 transfusions in cardiac surgery patients.
Each unit of sex-mismatched red blood cells (RBCs) transfused was associated with an increased risk of death (hazard ratio, 1.083; 95% confidence interval, 1.028-1.140; P = .003). In addition, transfusing 1-2 units of non–leukocyte depleted RBCs was associated with a significant increase in the risk of death during the first year after surgery (HR, 1.426; 95% CI, 1.004-2.024; P = .047).
Transfusion of 1-2 units of leukocyte-depleted RBCs and the age of blood products was not associated with increased mortality (J Thorac Cardiovasc Surg. 2016;152:223-32.e1).
“Factors such as ABO group, Rh profile and sex of the PRBC [packed RBC] donor generally have been overlooked, as has the variation in postdonation treatment of blood,” in the outcomes of cardiac surgery patients, researchers led by Henrik Bjursten, MD, PhD, of Lund (Sweden) University, reported.
The study involved 9,907 patients at Lund University from 2002 to 2012: 7,696 had coronary artery bypass grafting (CABG); 1,216 had aortic valve replacement (AVR); and 995 concomitantly had both procedures. PRBC transfusions were given to nearly 51% of the patients. Compared with the group that did not receive PRBC transfusions, the transfused group had significantly higher rates of heart attack after surgery (1.5% vs. 0.6%), infection (0.6% vs. 0.3%), reoperation for bleeding (4.3% vs. 0.2%), 30-day death (0.7% vs. 0.2%), and overall death (25.9% vs. 12.6%).
Based on an analysis that factored in 24 different variables, transfusion of 1-2 units of non–leukocyte depleted PRBCs was associated with a HR of 1.426, but the same amount of leukocyte-depleted PRBCs did not increase risk (HR, 0.981). However, transfusion of 5-7 units of leukocyte-depleted RBCs was associated with decreased survival, as was transfusion of sex-mismatched PRBCs, associated with a HR of 1.046-1.133 per unit, Dr. Bjursten and colleagues wrote. “In this cohort, 58% of transfusions were sex mismatched, and thus we interpret the result as relatively robust and clinically relevant.”
Patients having combined CABG and AVR were more likely to have PRBC transfusions than patients who had a single procedure. Additionally, the increased death rate in the PRBC transfusion group may have been related to age and comorbidities such as diabetes, chronic obstructive pulmonary disease, and cardiac insufficiency. “Blood transfusion in part is a biomarker for advanced disease,” Dr. Bjursten and coauthors said. While patient who received PRBC transfusions may have been sicker, they did not require greater use of the ICU than patients who did not receive transfusions.
Dr. Bjursten disclosed receiving consulting fees from Boston Scientific. Coauthor Lars Algotsson, MD, PhD, disclosed receiving lecture fees from Abbott. All other authors had no financial disclosures.
Transfusing sex-mismatched red blood cells (RBCs) was associated with an increased risk of death in people undergoing heart bypass surgery or aortic valve replacement, based on results of a retrospective single-center study of almost 10,000 transfusions in cardiac surgery patients.
Each unit of sex-mismatched red blood cells (RBCs) transfused was associated with an increased risk of death (hazard ratio, 1.083; 95% confidence interval, 1.028-1.140; P = .003). In addition, transfusing 1-2 units of non–leukocyte depleted RBCs was associated with a significant increase in the risk of death during the first year after surgery (HR, 1.426; 95% CI, 1.004-2.024; P = .047).
Transfusion of 1-2 units of leukocyte-depleted RBCs and the age of blood products was not associated with increased mortality (J Thorac Cardiovasc Surg. 2016;152:223-32.e1).
“Factors such as ABO group, Rh profile and sex of the PRBC [packed RBC] donor generally have been overlooked, as has the variation in postdonation treatment of blood,” in the outcomes of cardiac surgery patients, researchers led by Henrik Bjursten, MD, PhD, of Lund (Sweden) University, reported.
The study involved 9,907 patients at Lund University from 2002 to 2012: 7,696 had coronary artery bypass grafting (CABG); 1,216 had aortic valve replacement (AVR); and 995 concomitantly had both procedures. PRBC transfusions were given to nearly 51% of the patients. Compared with the group that did not receive PRBC transfusions, the transfused group had significantly higher rates of heart attack after surgery (1.5% vs. 0.6%), infection (0.6% vs. 0.3%), reoperation for bleeding (4.3% vs. 0.2%), 30-day death (0.7% vs. 0.2%), and overall death (25.9% vs. 12.6%).
Based on an analysis that factored in 24 different variables, transfusion of 1-2 units of non–leukocyte depleted PRBCs was associated with a HR of 1.426, but the same amount of leukocyte-depleted PRBCs did not increase risk (HR, 0.981). However, transfusion of 5-7 units of leukocyte-depleted RBCs was associated with decreased survival, as was transfusion of sex-mismatched PRBCs, associated with a HR of 1.046-1.133 per unit, Dr. Bjursten and colleagues wrote. “In this cohort, 58% of transfusions were sex mismatched, and thus we interpret the result as relatively robust and clinically relevant.”
Patients having combined CABG and AVR were more likely to have PRBC transfusions than patients who had a single procedure. Additionally, the increased death rate in the PRBC transfusion group may have been related to age and comorbidities such as diabetes, chronic obstructive pulmonary disease, and cardiac insufficiency. “Blood transfusion in part is a biomarker for advanced disease,” Dr. Bjursten and coauthors said. While patient who received PRBC transfusions may have been sicker, they did not require greater use of the ICU than patients who did not receive transfusions.
Dr. Bjursten disclosed receiving consulting fees from Boston Scientific. Coauthor Lars Algotsson, MD, PhD, disclosed receiving lecture fees from Abbott. All other authors had no financial disclosures.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Transfusion of sex-mismatched and non–leukocyte depleted PRBCs may impact survival after cardiac surgery.
Major finding: Transfusion of 1-2 units of non–leukocyte depleted PRBCs has a hazard ratio of 1.426.
Data source: Single-center, retrospective study of 9,007 patients who had CABG or AVR, or both, from 2002 to 2012. The study used the Swedish national tax registry to track deaths.
Disclosures: Lead author Dr. Bjursten disclosed receiving consulting fees from Boston Scientific. Coauthor Lars Algotsson, MD, PhD, disclosed receiving lecture fees from Abbott. All other authors have nothing to disclose with regard to commercial support.
Permanent pacemaker in TAVR: Earlier implantation costs much less
PARIS – When a patient undergoing transcatheter aortic valve replacement needs a permanent pacemaker, the additional hospital costs are significantly less if the device is implanted within 24 hours post TAVR rather than later, Seth Clancy reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Not only the need for permanent pacemaker implantation but also the timing of the procedure as well as the management and monitoring of conduction disturbances have important resource use implications for TAVR,” observed Mr. Clancy of Edwards Lifesciences of Irvine, Calif.
He presented an economic analysis of all 12,148 TAVR hospitalizations included in the Medicare database for 2014. A key finding: The mean cost of TAVR hospitalizations with no permanent pacemaker implantation was $63,136, while for the 12% of TAVRs that did include permanent pacemaker implantation, the mean cost shot up to $80,441, for a difference of $17,305.
The additional cost of putting in a permanent pacemaker included nearly $8,000 for supplies, more than $2,600 for additional time in the operating room and/or catheterization laboratory, and in excess of $2,100 worth of extra ICU or cardiac care unit time.
Patients who received a permanent pacemaker during their TAVR hospitalization spent an average of 2.3 days longer in the hospital than the mean 6.6 days for patients who didn’t get a permanent pacemaker.
Drilling down further into the data, Mr. Clancy found that 41% of permanent pacemakers implanted during hospitalization for TAVR went in within 24 hours of the TAVR procedure. In a multivariate regression analysis adjusted for differences in patient demographics, comorbid conditions, and complications, those patients generated an average of $9,843 more in hospital costs than patients who didn’t get a permanent pacemaker during their TAVR hospitalization. However, patients who received a permanent pacemaker more than 24 hours after TAVR cost an average of $17,681 more and had a 2.72-day longer stay than patients who didn’t get a permanent pacemaker.
The need for a permanent pacemaker is a common complication following TAVR. This has been a sticking point for many cardiothoracic surgeons, who note that rates of permanent pacemaker implantation following surgical aortic valve replacement are far lower. Still, rates in TAVR patients have come down over time with advances in valve technology. Currently, permanent pacemaker implantation rates in TAVR patients are 5%-25%, depending upon the valve system, according to Mr. Clancy.
Advances in device design and techniques aimed at reducing the permanent pacemaker implantation rate substantially below the 12% figure seen in 2014 have the potential to generate substantial cost savings, he observed.
Session chair Mohammad Abdelghani, MD, of the Academic Medical Center at Amsterdam questioned whether the study results are relevant to European practice because of the large differences in health care costs.
Discussant Sonia Petronio, MD, expressed a more fundamental reservation.
“This is a very important subject – and a very dangerous one,” said Dr. Petronio of the University of Pisa (Italy). “It’s easier and less costly for a hospital to encourage increasing early permanent pacemaker implantation because the patient can go home earlier.”
“We don’t want to put in a pacemaker earlier to save money,” agreed Dr. Abdelghani. “This is not a cost-effectiveness analysis, it’s purely a cost analysis. Cost-effectiveness would take into account the long-term clinical outcomes and welfare of the patients. We would like to see that from you next year.”
Mr. Clancy is an employee of Edwards Lifesciences, which funded the study.
PARIS – When a patient undergoing transcatheter aortic valve replacement needs a permanent pacemaker, the additional hospital costs are significantly less if the device is implanted within 24 hours post TAVR rather than later, Seth Clancy reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Not only the need for permanent pacemaker implantation but also the timing of the procedure as well as the management and monitoring of conduction disturbances have important resource use implications for TAVR,” observed Mr. Clancy of Edwards Lifesciences of Irvine, Calif.
He presented an economic analysis of all 12,148 TAVR hospitalizations included in the Medicare database for 2014. A key finding: The mean cost of TAVR hospitalizations with no permanent pacemaker implantation was $63,136, while for the 12% of TAVRs that did include permanent pacemaker implantation, the mean cost shot up to $80,441, for a difference of $17,305.
The additional cost of putting in a permanent pacemaker included nearly $8,000 for supplies, more than $2,600 for additional time in the operating room and/or catheterization laboratory, and in excess of $2,100 worth of extra ICU or cardiac care unit time.
Patients who received a permanent pacemaker during their TAVR hospitalization spent an average of 2.3 days longer in the hospital than the mean 6.6 days for patients who didn’t get a permanent pacemaker.
Drilling down further into the data, Mr. Clancy found that 41% of permanent pacemakers implanted during hospitalization for TAVR went in within 24 hours of the TAVR procedure. In a multivariate regression analysis adjusted for differences in patient demographics, comorbid conditions, and complications, those patients generated an average of $9,843 more in hospital costs than patients who didn’t get a permanent pacemaker during their TAVR hospitalization. However, patients who received a permanent pacemaker more than 24 hours after TAVR cost an average of $17,681 more and had a 2.72-day longer stay than patients who didn’t get a permanent pacemaker.
The need for a permanent pacemaker is a common complication following TAVR. This has been a sticking point for many cardiothoracic surgeons, who note that rates of permanent pacemaker implantation following surgical aortic valve replacement are far lower. Still, rates in TAVR patients have come down over time with advances in valve technology. Currently, permanent pacemaker implantation rates in TAVR patients are 5%-25%, depending upon the valve system, according to Mr. Clancy.
Advances in device design and techniques aimed at reducing the permanent pacemaker implantation rate substantially below the 12% figure seen in 2014 have the potential to generate substantial cost savings, he observed.
Session chair Mohammad Abdelghani, MD, of the Academic Medical Center at Amsterdam questioned whether the study results are relevant to European practice because of the large differences in health care costs.
Discussant Sonia Petronio, MD, expressed a more fundamental reservation.
“This is a very important subject – and a very dangerous one,” said Dr. Petronio of the University of Pisa (Italy). “It’s easier and less costly for a hospital to encourage increasing early permanent pacemaker implantation because the patient can go home earlier.”
“We don’t want to put in a pacemaker earlier to save money,” agreed Dr. Abdelghani. “This is not a cost-effectiveness analysis, it’s purely a cost analysis. Cost-effectiveness would take into account the long-term clinical outcomes and welfare of the patients. We would like to see that from you next year.”
Mr. Clancy is an employee of Edwards Lifesciences, which funded the study.
PARIS – When a patient undergoing transcatheter aortic valve replacement needs a permanent pacemaker, the additional hospital costs are significantly less if the device is implanted within 24 hours post TAVR rather than later, Seth Clancy reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Not only the need for permanent pacemaker implantation but also the timing of the procedure as well as the management and monitoring of conduction disturbances have important resource use implications for TAVR,” observed Mr. Clancy of Edwards Lifesciences of Irvine, Calif.
He presented an economic analysis of all 12,148 TAVR hospitalizations included in the Medicare database for 2014. A key finding: The mean cost of TAVR hospitalizations with no permanent pacemaker implantation was $63,136, while for the 12% of TAVRs that did include permanent pacemaker implantation, the mean cost shot up to $80,441, for a difference of $17,305.
The additional cost of putting in a permanent pacemaker included nearly $8,000 for supplies, more than $2,600 for additional time in the operating room and/or catheterization laboratory, and in excess of $2,100 worth of extra ICU or cardiac care unit time.
Patients who received a permanent pacemaker during their TAVR hospitalization spent an average of 2.3 days longer in the hospital than the mean 6.6 days for patients who didn’t get a permanent pacemaker.
Drilling down further into the data, Mr. Clancy found that 41% of permanent pacemakers implanted during hospitalization for TAVR went in within 24 hours of the TAVR procedure. In a multivariate regression analysis adjusted for differences in patient demographics, comorbid conditions, and complications, those patients generated an average of $9,843 more in hospital costs than patients who didn’t get a permanent pacemaker during their TAVR hospitalization. However, patients who received a permanent pacemaker more than 24 hours after TAVR cost an average of $17,681 more and had a 2.72-day longer stay than patients who didn’t get a permanent pacemaker.
The need for a permanent pacemaker is a common complication following TAVR. This has been a sticking point for many cardiothoracic surgeons, who note that rates of permanent pacemaker implantation following surgical aortic valve replacement are far lower. Still, rates in TAVR patients have come down over time with advances in valve technology. Currently, permanent pacemaker implantation rates in TAVR patients are 5%-25%, depending upon the valve system, according to Mr. Clancy.
Advances in device design and techniques aimed at reducing the permanent pacemaker implantation rate substantially below the 12% figure seen in 2014 have the potential to generate substantial cost savings, he observed.
Session chair Mohammad Abdelghani, MD, of the Academic Medical Center at Amsterdam questioned whether the study results are relevant to European practice because of the large differences in health care costs.
Discussant Sonia Petronio, MD, expressed a more fundamental reservation.
“This is a very important subject – and a very dangerous one,” said Dr. Petronio of the University of Pisa (Italy). “It’s easier and less costly for a hospital to encourage increasing early permanent pacemaker implantation because the patient can go home earlier.”
“We don’t want to put in a pacemaker earlier to save money,” agreed Dr. Abdelghani. “This is not a cost-effectiveness analysis, it’s purely a cost analysis. Cost-effectiveness would take into account the long-term clinical outcomes and welfare of the patients. We would like to see that from you next year.”
Mr. Clancy is an employee of Edwards Lifesciences, which funded the study.
AT EUROPCR 2016
Key clinical point: The incremental cost of permanent pacemaker implantation more than 24 hours after transcatheter aortic valve replacement is almost twice as great as if the pacemaker goes in within 24 hours.
Major finding: The mean cost of hospitalization for transcatheter aortic valve replacement without permanent pacemaker implication in Medicare patients in 2014 was $63,136, compared with $80,441 if they needed a pacemaker.
Data source: This was a retrospective study of the health care costs and lengths of stay for all 12,148 hospitalizations for transcatheter aortic valve replacement in the Medicare inpatient database for 2014.
Disclosures: The presenter is an employee of Edwards Lifesciences, which funded the study.
CABG tops PCI for nondiabetic patients with multivessel CAD
Coronary artery bypass graft surgery was associated with a 35% lower rate of all-cause mortality rate and a 59% lower rate of cardiac death than was percutaneous coronary intervention in a pooled analysis of nondiabetic patients with multivessel coronary artery disease in two international randomized trials.
“The superiority of CABG over PCI was consistent across all major clinical subgroups. Likewise, the rate of myocardial infarction was remarkably lower after CABG than after PCI,” Mineok Chang, MD, of the University of Ulsan in Seoul, South Korea, and her associates reported online June 27 in the Journal of the American College of Cardiology.
The advent of drug-eluting stents has led to widespread use of PCI, but controversy persists regarding the best revascularization strategy for nondiabetic patients with multivessel CAD, the researchers said. All-cause mortality “is undoubtedly the most unbiased endpoint to determine treatment strategy,” but individual trials have lacked the power to evaluate this endpoint, they added. Accordingly, they pooled data from the SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) trial – which included 1,800 patients with three-vessel or left main CAD from Europe and the United States – and from the BEST (Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients With Multivessel Coronary Artery Disease) trial, which included 880 patients from Asia with two- or three-vessel CAD. Excluding patients with left main CAD or diabetes left 1,275 patients for analysis (J Am Coll Cardiol. 2016 Jun 27. 68:29-36).
After a median follow-up of 61 months, 38 (6%) CABG patients had died, compared with 59 (9.3%) PCI patients, for a statistically significant hazard ratio of 0.65 (95% confidence interval, 0.25-0.78; P = .039). Likewise, cardiac death was significantly less likely after CABG than PCI (HR, 0.41; 95% CI, 0.25-0.78; P = .005). “The statistical difference between the two groups was pronounced after 2 years of randomization for both all-cause and cardiac mortality,” the researchers reported. Although the latest drug-eluting stents “are reported to improve clinical outcomes,” they did not find a significant interaction between older and more recently developed stents when considering all-cause mortality, they noted.
The study was supported by a research grant from the CardioVascular Research Foundation in Seoul, South Korea. Dr. Chang had no disclosures. Senior author, Dr. Seung-Jung Park, disclosed research support from Abbott Vascular, Cordis, Boston Scientific, and Medtronic.
It is important to consider some limitations of the current study. First, the majority of patients were derived from the SYNTAX trial, which used first-generation paclitaxel drug-eluting stents (DES) that exhibit higher stent complication rates compared with current second- and third-generation everolimus-eluting stents. Second, although complete revascularization was more frequently obtained with CABG in the SYNTAX and BEST trials, current PCI approaches are narrowing this gap with chronic total occlusion PCI techniques and temporary hemodynamic support when needed. Third, selection bias may limit the applicability of these results to patients with more complex disease who are often excluded from clinical trials and may not be eligible for CABG.
Therefore, an adequately powered study of contemporary DES PCI versus CABG is warranted for real-world, nondiabetic patients with multivessel CAD. Guidelines and meta-analyses provide valuable recommendations for populations at large, but decision making for nondiabetic patients with multivessel CAD should be individualized. If equipoise between CABG and PCI exists, decision-making can be improved by engaging a local multidisciplinary CAD heart team. Well-informed cardiologists, interventionalists, and cardiac surgeons who engage in multidisciplinary heart team evaluations and patient-centered shared decision making will provide optimal guidance for patients with multivessel CAD.
Farouc A. Jaffer, MD, is at Massachusetts General Hospital; Patrick T. O’Gara, MD, is at Brigham and Women’s Hospital, both in Boston. Dr. Jaffer disclosed research grants from Siemens and Kowa and consulting relationships with Boston Scientific and Abbott Vascular. Dr. O’Gara had no disclosures. These comments are from their editorial (J Am Coll Cardiol. 2016 Jun 27. doi: 10.1016/j.jacc.2016.05.008).
It is important to consider some limitations of the current study. First, the majority of patients were derived from the SYNTAX trial, which used first-generation paclitaxel drug-eluting stents (DES) that exhibit higher stent complication rates compared with current second- and third-generation everolimus-eluting stents. Second, although complete revascularization was more frequently obtained with CABG in the SYNTAX and BEST trials, current PCI approaches are narrowing this gap with chronic total occlusion PCI techniques and temporary hemodynamic support when needed. Third, selection bias may limit the applicability of these results to patients with more complex disease who are often excluded from clinical trials and may not be eligible for CABG.
Therefore, an adequately powered study of contemporary DES PCI versus CABG is warranted for real-world, nondiabetic patients with multivessel CAD. Guidelines and meta-analyses provide valuable recommendations for populations at large, but decision making for nondiabetic patients with multivessel CAD should be individualized. If equipoise between CABG and PCI exists, decision-making can be improved by engaging a local multidisciplinary CAD heart team. Well-informed cardiologists, interventionalists, and cardiac surgeons who engage in multidisciplinary heart team evaluations and patient-centered shared decision making will provide optimal guidance for patients with multivessel CAD.
Farouc A. Jaffer, MD, is at Massachusetts General Hospital; Patrick T. O’Gara, MD, is at Brigham and Women’s Hospital, both in Boston. Dr. Jaffer disclosed research grants from Siemens and Kowa and consulting relationships with Boston Scientific and Abbott Vascular. Dr. O’Gara had no disclosures. These comments are from their editorial (J Am Coll Cardiol. 2016 Jun 27. doi: 10.1016/j.jacc.2016.05.008).
It is important to consider some limitations of the current study. First, the majority of patients were derived from the SYNTAX trial, which used first-generation paclitaxel drug-eluting stents (DES) that exhibit higher stent complication rates compared with current second- and third-generation everolimus-eluting stents. Second, although complete revascularization was more frequently obtained with CABG in the SYNTAX and BEST trials, current PCI approaches are narrowing this gap with chronic total occlusion PCI techniques and temporary hemodynamic support when needed. Third, selection bias may limit the applicability of these results to patients with more complex disease who are often excluded from clinical trials and may not be eligible for CABG.
Therefore, an adequately powered study of contemporary DES PCI versus CABG is warranted for real-world, nondiabetic patients with multivessel CAD. Guidelines and meta-analyses provide valuable recommendations for populations at large, but decision making for nondiabetic patients with multivessel CAD should be individualized. If equipoise between CABG and PCI exists, decision-making can be improved by engaging a local multidisciplinary CAD heart team. Well-informed cardiologists, interventionalists, and cardiac surgeons who engage in multidisciplinary heart team evaluations and patient-centered shared decision making will provide optimal guidance for patients with multivessel CAD.
Farouc A. Jaffer, MD, is at Massachusetts General Hospital; Patrick T. O’Gara, MD, is at Brigham and Women’s Hospital, both in Boston. Dr. Jaffer disclosed research grants from Siemens and Kowa and consulting relationships with Boston Scientific and Abbott Vascular. Dr. O’Gara had no disclosures. These comments are from their editorial (J Am Coll Cardiol. 2016 Jun 27. doi: 10.1016/j.jacc.2016.05.008).
Coronary artery bypass graft surgery was associated with a 35% lower rate of all-cause mortality rate and a 59% lower rate of cardiac death than was percutaneous coronary intervention in a pooled analysis of nondiabetic patients with multivessel coronary artery disease in two international randomized trials.
“The superiority of CABG over PCI was consistent across all major clinical subgroups. Likewise, the rate of myocardial infarction was remarkably lower after CABG than after PCI,” Mineok Chang, MD, of the University of Ulsan in Seoul, South Korea, and her associates reported online June 27 in the Journal of the American College of Cardiology.
The advent of drug-eluting stents has led to widespread use of PCI, but controversy persists regarding the best revascularization strategy for nondiabetic patients with multivessel CAD, the researchers said. All-cause mortality “is undoubtedly the most unbiased endpoint to determine treatment strategy,” but individual trials have lacked the power to evaluate this endpoint, they added. Accordingly, they pooled data from the SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) trial – which included 1,800 patients with three-vessel or left main CAD from Europe and the United States – and from the BEST (Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients With Multivessel Coronary Artery Disease) trial, which included 880 patients from Asia with two- or three-vessel CAD. Excluding patients with left main CAD or diabetes left 1,275 patients for analysis (J Am Coll Cardiol. 2016 Jun 27. 68:29-36).
After a median follow-up of 61 months, 38 (6%) CABG patients had died, compared with 59 (9.3%) PCI patients, for a statistically significant hazard ratio of 0.65 (95% confidence interval, 0.25-0.78; P = .039). Likewise, cardiac death was significantly less likely after CABG than PCI (HR, 0.41; 95% CI, 0.25-0.78; P = .005). “The statistical difference between the two groups was pronounced after 2 years of randomization for both all-cause and cardiac mortality,” the researchers reported. Although the latest drug-eluting stents “are reported to improve clinical outcomes,” they did not find a significant interaction between older and more recently developed stents when considering all-cause mortality, they noted.
The study was supported by a research grant from the CardioVascular Research Foundation in Seoul, South Korea. Dr. Chang had no disclosures. Senior author, Dr. Seung-Jung Park, disclosed research support from Abbott Vascular, Cordis, Boston Scientific, and Medtronic.
Coronary artery bypass graft surgery was associated with a 35% lower rate of all-cause mortality rate and a 59% lower rate of cardiac death than was percutaneous coronary intervention in a pooled analysis of nondiabetic patients with multivessel coronary artery disease in two international randomized trials.
“The superiority of CABG over PCI was consistent across all major clinical subgroups. Likewise, the rate of myocardial infarction was remarkably lower after CABG than after PCI,” Mineok Chang, MD, of the University of Ulsan in Seoul, South Korea, and her associates reported online June 27 in the Journal of the American College of Cardiology.
The advent of drug-eluting stents has led to widespread use of PCI, but controversy persists regarding the best revascularization strategy for nondiabetic patients with multivessel CAD, the researchers said. All-cause mortality “is undoubtedly the most unbiased endpoint to determine treatment strategy,” but individual trials have lacked the power to evaluate this endpoint, they added. Accordingly, they pooled data from the SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) trial – which included 1,800 patients with three-vessel or left main CAD from Europe and the United States – and from the BEST (Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients With Multivessel Coronary Artery Disease) trial, which included 880 patients from Asia with two- or three-vessel CAD. Excluding patients with left main CAD or diabetes left 1,275 patients for analysis (J Am Coll Cardiol. 2016 Jun 27. 68:29-36).
After a median follow-up of 61 months, 38 (6%) CABG patients had died, compared with 59 (9.3%) PCI patients, for a statistically significant hazard ratio of 0.65 (95% confidence interval, 0.25-0.78; P = .039). Likewise, cardiac death was significantly less likely after CABG than PCI (HR, 0.41; 95% CI, 0.25-0.78; P = .005). “The statistical difference between the two groups was pronounced after 2 years of randomization for both all-cause and cardiac mortality,” the researchers reported. Although the latest drug-eluting stents “are reported to improve clinical outcomes,” they did not find a significant interaction between older and more recently developed stents when considering all-cause mortality, they noted.
The study was supported by a research grant from the CardioVascular Research Foundation in Seoul, South Korea. Dr. Chang had no disclosures. Senior author, Dr. Seung-Jung Park, disclosed research support from Abbott Vascular, Cordis, Boston Scientific, and Medtronic.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point: Coronary artery bypass graft is superior to percutaneous coronary intervention for nondiabetic patients with multivessel coronary artery disease.
Major finding: All-cause mortality rates were 6% for CABG and 9.3% for PCI (HR, 0.65; P = .039).
Data source: A pooled analysis of 1,275 nondiabetic patients with two- or three-vessel CAD from the SYNTAX and BEST trials.
Disclosures: The study was supported by a research grant from the CardioVascular Research Foundation in Seoul, South Korea. Dr. Chang had no disclosures. Senior author, Dr. Seung-Jung Park, disclosed research support from Abbott Vascular, Cordis, Boston Scientific, and Medtronic.
Esophagectomy 30-day readmission rate pegged at 19%
BALTIMORE – Approximately one in five patients is readmitted after esophagectomy, and leading risk factors for readmission are longer operative time, post-surgical ICU admission, and preoperative blood transfusions, according to a single-center study of 86 patients.
As one of the first reports on readmissions following esophagectomy with complete follow-up, this study, conducted at the Mayo Clinic in Rochester, Minn., demonstrates that even in a high volume center with specialization in esophageal and foregut surgery, readmission after esophagectomy is not uncommon, researchers reported at the annual meeting of the American Association for Thoracic Surgery.
“In the context of increasing pressures to reduce length of stay, we must also put in the effort to better understand our readmission rates and the important factors that affect them,” said study investigator Dr. Stephen Cassivi. “Reporting on ‘improved’ lengths of stay without accompanying data on readmission rates is not telling the whole story.”
According to the Mayo Clinic research team, identifying risk factors that predict readmissions might permit improved patient management and outcomes.
“Careful collection of data regarding patient outcomes, including unplanned hospital readmissions is essential to improve the quality of patient care since national databases can leave gaps in data regarding follow-up of these patients by failing to identify all readmissions after their surgery,” said Dr. Karen J. Dickinson, who presented the study at the meeting.
The study was designed such that all patients undergoing an elective esophagectomy between August 2013 and July 2014 were contacted directly to follow up on whether they had been readmitted to any medical institution within 30 days of dismissal from the Mayo Clinic. Among all patients who underwent esophagectomy during the one-year study period, 86 patients met the study inclusion criteria. Follow-up was complete in 100% of patients, according to Dr. Dickinson.
Median age of the patients at the time of surgery was 63 years, and the majority of patients were men (70 patients). The most common operative approach was transthoracic (Ivor Lewis) esophagectomy (72%); 7% of cases were performed using a minimally invasive approach. Overall 30-day mortality was 2% (2/86), and anastomotic leak occurred in 8% of the patients.
Median length of stay was 9 days, and the rate of unplanned 30-day readmission was 19% (16 patients). Of these patients, 88% were readmitted to the Mayo Clinic and 12% were readmitted to other medical institutions.
The most common reasons for readmission were due to respiratory causes such as dyspnea, pleural effusions or pneumonia and gastrointestinal causes, including bowel obstruction and anastomotic complications.
Using multivariable analysis, the researchers found that the factors significantly associated with unplanned readmission were postoperative ICU admission (13% in non-readmitted, 38% in admitted), perioperative blood transfusion (12% vs. 38%), and operative length (368 vs. 460 minutes). Importantly, initial hospital length of stay was not associated with the need for readmission. Furthermore, ASA score, sex, BMI, neoadjuvant therapy, and postoperative pain scores also were not associated with unplanned readmission.
“Identifying these risk factors in the perioperative and postoperative setting may provide opportunities for decreasing morbidity, improving readmission rates, and enhancing overall patient outcomes,” Dr. Dickinson concluded.
A video of this presentation at the AATS Annual Meeting is available online.
Dr. Dickinson and her colleagues reported having no relevant disclosures.
On Twitter @ThoracicTweets
BALTIMORE – Approximately one in five patients is readmitted after esophagectomy, and leading risk factors for readmission are longer operative time, post-surgical ICU admission, and preoperative blood transfusions, according to a single-center study of 86 patients.
As one of the first reports on readmissions following esophagectomy with complete follow-up, this study, conducted at the Mayo Clinic in Rochester, Minn., demonstrates that even in a high volume center with specialization in esophageal and foregut surgery, readmission after esophagectomy is not uncommon, researchers reported at the annual meeting of the American Association for Thoracic Surgery.
“In the context of increasing pressures to reduce length of stay, we must also put in the effort to better understand our readmission rates and the important factors that affect them,” said study investigator Dr. Stephen Cassivi. “Reporting on ‘improved’ lengths of stay without accompanying data on readmission rates is not telling the whole story.”
According to the Mayo Clinic research team, identifying risk factors that predict readmissions might permit improved patient management and outcomes.
“Careful collection of data regarding patient outcomes, including unplanned hospital readmissions is essential to improve the quality of patient care since national databases can leave gaps in data regarding follow-up of these patients by failing to identify all readmissions after their surgery,” said Dr. Karen J. Dickinson, who presented the study at the meeting.
The study was designed such that all patients undergoing an elective esophagectomy between August 2013 and July 2014 were contacted directly to follow up on whether they had been readmitted to any medical institution within 30 days of dismissal from the Mayo Clinic. Among all patients who underwent esophagectomy during the one-year study period, 86 patients met the study inclusion criteria. Follow-up was complete in 100% of patients, according to Dr. Dickinson.
Median age of the patients at the time of surgery was 63 years, and the majority of patients were men (70 patients). The most common operative approach was transthoracic (Ivor Lewis) esophagectomy (72%); 7% of cases were performed using a minimally invasive approach. Overall 30-day mortality was 2% (2/86), and anastomotic leak occurred in 8% of the patients.
Median length of stay was 9 days, and the rate of unplanned 30-day readmission was 19% (16 patients). Of these patients, 88% were readmitted to the Mayo Clinic and 12% were readmitted to other medical institutions.
The most common reasons for readmission were due to respiratory causes such as dyspnea, pleural effusions or pneumonia and gastrointestinal causes, including bowel obstruction and anastomotic complications.
Using multivariable analysis, the researchers found that the factors significantly associated with unplanned readmission were postoperative ICU admission (13% in non-readmitted, 38% in admitted), perioperative blood transfusion (12% vs. 38%), and operative length (368 vs. 460 minutes). Importantly, initial hospital length of stay was not associated with the need for readmission. Furthermore, ASA score, sex, BMI, neoadjuvant therapy, and postoperative pain scores also were not associated with unplanned readmission.
“Identifying these risk factors in the perioperative and postoperative setting may provide opportunities for decreasing morbidity, improving readmission rates, and enhancing overall patient outcomes,” Dr. Dickinson concluded.
A video of this presentation at the AATS Annual Meeting is available online.
Dr. Dickinson and her colleagues reported having no relevant disclosures.
On Twitter @ThoracicTweets
BALTIMORE – Approximately one in five patients is readmitted after esophagectomy, and leading risk factors for readmission are longer operative time, post-surgical ICU admission, and preoperative blood transfusions, according to a single-center study of 86 patients.
As one of the first reports on readmissions following esophagectomy with complete follow-up, this study, conducted at the Mayo Clinic in Rochester, Minn., demonstrates that even in a high volume center with specialization in esophageal and foregut surgery, readmission after esophagectomy is not uncommon, researchers reported at the annual meeting of the American Association for Thoracic Surgery.
“In the context of increasing pressures to reduce length of stay, we must also put in the effort to better understand our readmission rates and the important factors that affect them,” said study investigator Dr. Stephen Cassivi. “Reporting on ‘improved’ lengths of stay without accompanying data on readmission rates is not telling the whole story.”
According to the Mayo Clinic research team, identifying risk factors that predict readmissions might permit improved patient management and outcomes.
“Careful collection of data regarding patient outcomes, including unplanned hospital readmissions is essential to improve the quality of patient care since national databases can leave gaps in data regarding follow-up of these patients by failing to identify all readmissions after their surgery,” said Dr. Karen J. Dickinson, who presented the study at the meeting.
The study was designed such that all patients undergoing an elective esophagectomy between August 2013 and July 2014 were contacted directly to follow up on whether they had been readmitted to any medical institution within 30 days of dismissal from the Mayo Clinic. Among all patients who underwent esophagectomy during the one-year study period, 86 patients met the study inclusion criteria. Follow-up was complete in 100% of patients, according to Dr. Dickinson.
Median age of the patients at the time of surgery was 63 years, and the majority of patients were men (70 patients). The most common operative approach was transthoracic (Ivor Lewis) esophagectomy (72%); 7% of cases were performed using a minimally invasive approach. Overall 30-day mortality was 2% (2/86), and anastomotic leak occurred in 8% of the patients.
Median length of stay was 9 days, and the rate of unplanned 30-day readmission was 19% (16 patients). Of these patients, 88% were readmitted to the Mayo Clinic and 12% were readmitted to other medical institutions.
The most common reasons for readmission were due to respiratory causes such as dyspnea, pleural effusions or pneumonia and gastrointestinal causes, including bowel obstruction and anastomotic complications.
Using multivariable analysis, the researchers found that the factors significantly associated with unplanned readmission were postoperative ICU admission (13% in non-readmitted, 38% in admitted), perioperative blood transfusion (12% vs. 38%), and operative length (368 vs. 460 minutes). Importantly, initial hospital length of stay was not associated with the need for readmission. Furthermore, ASA score, sex, BMI, neoadjuvant therapy, and postoperative pain scores also were not associated with unplanned readmission.
“Identifying these risk factors in the perioperative and postoperative setting may provide opportunities for decreasing morbidity, improving readmission rates, and enhancing overall patient outcomes,” Dr. Dickinson concluded.
A video of this presentation at the AATS Annual Meeting is available online.
Dr. Dickinson and her colleagues reported having no relevant disclosures.
On Twitter @ThoracicTweets
AT THE AATS ANNUAL MEETING
Key clinical point: Operative length, perioperative blood transfusions, and postoperative ICU admission were significant risk factors for readmission after esophagectomy.
Major finding: The rate of unplanned 30-day readmission was 19%.
Data source: The study assessed 86 patients who underwent esophagectomy at the Mayo Clinic between August 2012 and July 2014.
Disclosures: Dr. Dickinson and her colleagues reported having no relevant disclosures.
Additional antibiotics needed when implanting cryopreserved human aortic grafts
Infections of aortic prosthetic grafts can be a devastating complication, and while cryopreserved human allografts (CHA) can continue to possess antibacterial activity even after 5 years or more in storage, cardiac surgeons may want to apply additional antibiotic agents during implantation to boost bacterial resistance, investigators from Germany reported in the May issue of the Journal of Thoracic and Cardiovascular Surgery (2016;151:1251-9).
The researchers compared three different antibiotic regimens used in processing CHA aortic tissue and valves to determine the impact each can have on long-term bacterial resistance.
“Antibiotic combinations applied during CHA processing have a significant influence on their infection resistance,” said Dr. Viola Steffen of Hannover (Germany) Medical School and her colleagues. The average storage time of CHAs was 8.5 years, with the longest having been stored for 10 years.
The study involved microbiologic tests in vitro of three different antibiotic regimens used in processing CHA: gentamicin-piperacillin-vancomycin-metronidazole-amphotericin B (group A); gentamicin-piperacillin-flucloxacillin-metronidazole-amphotericin B (group B); and meropenem-vancomycin-tobramycin-colistin-amphotericin B (group C). The combinations are used to counteract Staphylococcus epidermidis and Staphylococcus aureus.
The study exposed pieces of 10 CHAs to different microbes and determined that regimen groups B and C were more effective than group A in eradicating gram-positive organisms. Specifically, group C was most resistant to Escherichia coli, whereas group B was most effective against Pseudomonas aeruginosa. Aortic tissue showed significantly less contamination with staphylococcal bacteria than valve grafts, the study reported.
Dr. Steffen and her colleagues said that tissue banks use antibiotic protocols during CHA processing, but they differ substantially. “Our results support the hypothesis that infection resistance of CHAs depends on the antibiotic pretreatment during processing and their residual activity,” they said.
The study had four key findings:
• The infection resistance of aortic wall and valve tissue differed significantly.
• In aortic wall specimens, group A specimens exhibited increased adherence of S. epidermidis, with vancomycin in group A and flucloxacillin in group B being the only differentiating agents between the two.
• Cryopreserved aortic vessels had a propensity toward reduced infection resistance against P. aeruginosa.
• Morphologic changes occurred in the microorganisms, especially rod-shaped E. coli, indicating that regional antibiotic release alters bacterial growth without eliminating all adherent bacteria.
Dr. Steffen and her colleagues noted that while previous studies determined that residual concentrations of antibiotics used in processing CHA heart valves and blood vessels are still present after they are prepared for implantation, they neither measured the antibacterial affect, clarified the period of cryopreservation nor differentiated between valve and vessel tissue (PLoS One. 2014;9:e112679; Transfus Med Hemother. 2011;38:379-86).
The Hannover researchers found that only a few gram-positive microorganisms adhered to aortic wall specimens, although they found “extensive adherence” of S. epidermidis in group A specimens. Valves, however, “were completely colonized” with both strains of staphylococcal bacteria, although the severity of contamination varied depending on the regimen used.
The findings raise some questions about the antibiotic properties of CHAs. “Because their infection resistance depends on the antibiotic combination selected during processing, further investigations concerning this treatment are necessary to improve the antimicrobial activity against frequent and highly virulent infection-causing bacteria,” Dr. Steffen and her colleagues said.
Dr. Steffen and her coauthors had no financial relationships to disclose.
Infections of aortic prosthetic grafts can be a devastating complication, and while cryopreserved human allografts (CHA) can continue to possess antibacterial activity even after 5 years or more in storage, cardiac surgeons may want to apply additional antibiotic agents during implantation to boost bacterial resistance, investigators from Germany reported in the May issue of the Journal of Thoracic and Cardiovascular Surgery (2016;151:1251-9).
The researchers compared three different antibiotic regimens used in processing CHA aortic tissue and valves to determine the impact each can have on long-term bacterial resistance.
“Antibiotic combinations applied during CHA processing have a significant influence on their infection resistance,” said Dr. Viola Steffen of Hannover (Germany) Medical School and her colleagues. The average storage time of CHAs was 8.5 years, with the longest having been stored for 10 years.
The study involved microbiologic tests in vitro of three different antibiotic regimens used in processing CHA: gentamicin-piperacillin-vancomycin-metronidazole-amphotericin B (group A); gentamicin-piperacillin-flucloxacillin-metronidazole-amphotericin B (group B); and meropenem-vancomycin-tobramycin-colistin-amphotericin B (group C). The combinations are used to counteract Staphylococcus epidermidis and Staphylococcus aureus.
The study exposed pieces of 10 CHAs to different microbes and determined that regimen groups B and C were more effective than group A in eradicating gram-positive organisms. Specifically, group C was most resistant to Escherichia coli, whereas group B was most effective against Pseudomonas aeruginosa. Aortic tissue showed significantly less contamination with staphylococcal bacteria than valve grafts, the study reported.
Dr. Steffen and her colleagues said that tissue banks use antibiotic protocols during CHA processing, but they differ substantially. “Our results support the hypothesis that infection resistance of CHAs depends on the antibiotic pretreatment during processing and their residual activity,” they said.
The study had four key findings:
• The infection resistance of aortic wall and valve tissue differed significantly.
• In aortic wall specimens, group A specimens exhibited increased adherence of S. epidermidis, with vancomycin in group A and flucloxacillin in group B being the only differentiating agents between the two.
• Cryopreserved aortic vessels had a propensity toward reduced infection resistance against P. aeruginosa.
• Morphologic changes occurred in the microorganisms, especially rod-shaped E. coli, indicating that regional antibiotic release alters bacterial growth without eliminating all adherent bacteria.
Dr. Steffen and her colleagues noted that while previous studies determined that residual concentrations of antibiotics used in processing CHA heart valves and blood vessels are still present after they are prepared for implantation, they neither measured the antibacterial affect, clarified the period of cryopreservation nor differentiated between valve and vessel tissue (PLoS One. 2014;9:e112679; Transfus Med Hemother. 2011;38:379-86).
The Hannover researchers found that only a few gram-positive microorganisms adhered to aortic wall specimens, although they found “extensive adherence” of S. epidermidis in group A specimens. Valves, however, “were completely colonized” with both strains of staphylococcal bacteria, although the severity of contamination varied depending on the regimen used.
The findings raise some questions about the antibiotic properties of CHAs. “Because their infection resistance depends on the antibiotic combination selected during processing, further investigations concerning this treatment are necessary to improve the antimicrobial activity against frequent and highly virulent infection-causing bacteria,” Dr. Steffen and her colleagues said.
Dr. Steffen and her coauthors had no financial relationships to disclose.
Infections of aortic prosthetic grafts can be a devastating complication, and while cryopreserved human allografts (CHA) can continue to possess antibacterial activity even after 5 years or more in storage, cardiac surgeons may want to apply additional antibiotic agents during implantation to boost bacterial resistance, investigators from Germany reported in the May issue of the Journal of Thoracic and Cardiovascular Surgery (2016;151:1251-9).
The researchers compared three different antibiotic regimens used in processing CHA aortic tissue and valves to determine the impact each can have on long-term bacterial resistance.
“Antibiotic combinations applied during CHA processing have a significant influence on their infection resistance,” said Dr. Viola Steffen of Hannover (Germany) Medical School and her colleagues. The average storage time of CHAs was 8.5 years, with the longest having been stored for 10 years.
The study involved microbiologic tests in vitro of three different antibiotic regimens used in processing CHA: gentamicin-piperacillin-vancomycin-metronidazole-amphotericin B (group A); gentamicin-piperacillin-flucloxacillin-metronidazole-amphotericin B (group B); and meropenem-vancomycin-tobramycin-colistin-amphotericin B (group C). The combinations are used to counteract Staphylococcus epidermidis and Staphylococcus aureus.
The study exposed pieces of 10 CHAs to different microbes and determined that regimen groups B and C were more effective than group A in eradicating gram-positive organisms. Specifically, group C was most resistant to Escherichia coli, whereas group B was most effective against Pseudomonas aeruginosa. Aortic tissue showed significantly less contamination with staphylococcal bacteria than valve grafts, the study reported.
Dr. Steffen and her colleagues said that tissue banks use antibiotic protocols during CHA processing, but they differ substantially. “Our results support the hypothesis that infection resistance of CHAs depends on the antibiotic pretreatment during processing and their residual activity,” they said.
The study had four key findings:
• The infection resistance of aortic wall and valve tissue differed significantly.
• In aortic wall specimens, group A specimens exhibited increased adherence of S. epidermidis, with vancomycin in group A and flucloxacillin in group B being the only differentiating agents between the two.
• Cryopreserved aortic vessels had a propensity toward reduced infection resistance against P. aeruginosa.
• Morphologic changes occurred in the microorganisms, especially rod-shaped E. coli, indicating that regional antibiotic release alters bacterial growth without eliminating all adherent bacteria.
Dr. Steffen and her colleagues noted that while previous studies determined that residual concentrations of antibiotics used in processing CHA heart valves and blood vessels are still present after they are prepared for implantation, they neither measured the antibacterial affect, clarified the period of cryopreservation nor differentiated between valve and vessel tissue (PLoS One. 2014;9:e112679; Transfus Med Hemother. 2011;38:379-86).
The Hannover researchers found that only a few gram-positive microorganisms adhered to aortic wall specimens, although they found “extensive adherence” of S. epidermidis in group A specimens. Valves, however, “were completely colonized” with both strains of staphylococcal bacteria, although the severity of contamination varied depending on the regimen used.
The findings raise some questions about the antibiotic properties of CHAs. “Because their infection resistance depends on the antibiotic combination selected during processing, further investigations concerning this treatment are necessary to improve the antimicrobial activity against frequent and highly virulent infection-causing bacteria,” Dr. Steffen and her colleagues said.
Dr. Steffen and her coauthors had no financial relationships to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: The infection resistance of cryopreserved human allografts (CHAs) depends on antibiotic pretreatment during processing.
Major finding: Cardiac surgeons can recommend CHAs to patients either with active destructive infections or at high risk of reinfection, but they may want to apply additional antibiotic agents during implantation to enhance bacterial resistance.
Data source: Pieces of 10 CHAs were microbiologically tested in vitro and exposed to bacterial contamination and the number of attached bacteria quantified.
Disclosures: Dr. Steffen and her coauthors had no financial relationships to disclose.