User login
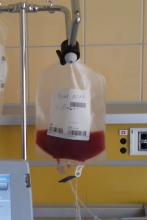
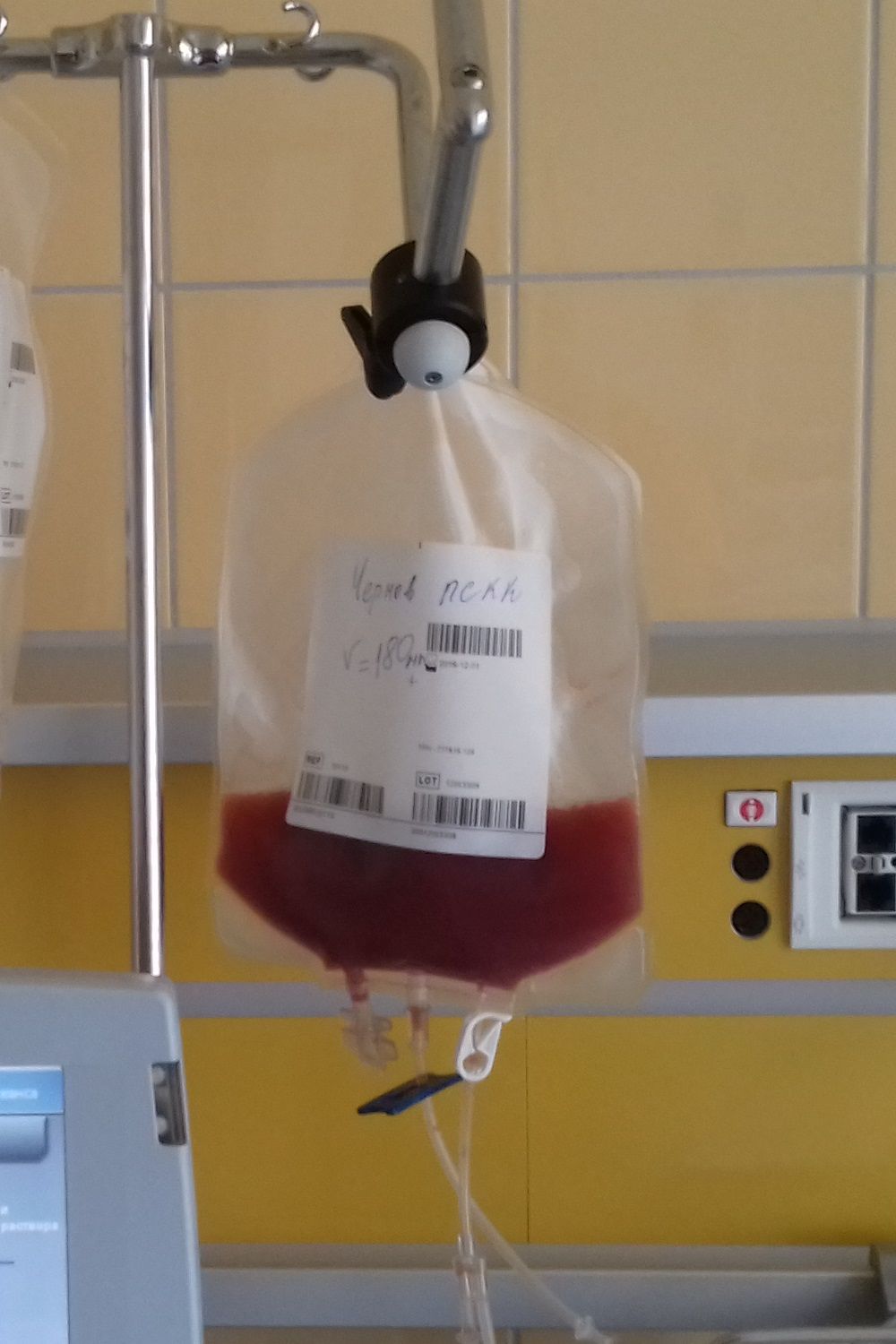
Outpatient CAR T infusions feasible using liso-cel
SALT LAKE CITY – A CD19-directed 4-1BB chimeric antigen receptor (CAR) T cell product showed efficacy and a low rate of cytokine release syndrome and neurotoxicity in patients with aggressive lymphomas and poor prognoses, raising the possibility of outpatient administration and fewer hospitalization days in this high-risk group.
A total of 86 patients who received inpatient infusions of lisocabtagene maraleucel (liso-cel, also known as JCAR017) had a mean 15.6 days of hospitalization, compared with 9.3 days for 8 outpatient recipients, said Jeremy Abramson, MD, speaking at a top abstracts session of the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
As of October 2017, eight patients had received liso-cel infusion as outpatients with at least 28 days of postinfusion data, Dr. Abramson said.
Although all but one required hospital admission, at a median of 5 days postinfusion (range, 4-22 days), there had been no intensive care unit admissions, and no outpatient recipients had experienced severe cytokine release syndrome (CRS) or neurotoxicity. All admitted patients presented with fever.
Among the study population, “Cytokine release syndrome was only seen in 35% of our entire dataset,” with neurologic toxicity seen in 19% of participants, Dr. Abramson said. “The majority of subjects had no CRS and no toxicity,” he said. Severe CRS occurred in 1% of the study population, and severe neurotoxicity in 12%. There were no deaths related to either complication.
Dr. Abramson reported these results from the TRANSCEND NHL 001 trial, a seamless design phase 1 pivotal trial of liso-cel enrolling patients with relapsed and refractory aggressive B cell non-Hodgkin lymphoma (NHL). Liso-cel delivers CD19-directed CD4 and CD8 CAR T cells in a 1:1 ratio, said Dr. Abramson, director of the lymphoma program at the Massachusetts General Hospital Cancer Center, Boston.
A total of 91 patients were randomized to one of the three dose-finding cohorts of the multicenter trial of liso-cel. One cohort received 5 x 107 cells in a single dose; a second cohort received the same number of cells but in two doses administered 14 days apart; the third cohort received a single dose of 1 x 108 cells.
The seamless trial design then moved to dose expansion, using the two single doses established in the dose-finding phase of the study. Ultimately, Dr. Abramson said, the third and pivotal diffuse large B-cell lymphoma (DLBCL) cohort received the higher single dose, since a dose-response relationship was seen in the earlier cohorts. No increase in cytokine release syndrome or neurotoxicity has been seen with the higher dose in patients evaluated to date.
Patients (median age, 61 years) were eligible to participate in the trial if they had relapsed or refractory DLBCL, primary mediastinal B-cell lymphoma, grade 3B follicular lymphoma, or mantle cell lymphoma. Patients with a failed prior allogeneic stem cell transplant or secondary central nervous system involvement were eligible, but all patients had to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2.
As the trial moved to the core pivotal phase, eligibility requirements shifted slightly to include patients with ECOG status 0 or 1, and lymphoma diagnoses narrowed to include only DLBCL not otherwise specified (NOS), transformed follicular lymphoma, and high-grade B-cell lymphoma with double- and triple-hit cytogenetics. The core group was nearing completion of accrual at the time of the presentation, which presented preliminary results from this phase of the trial.
Among the 88 evaluable patients in the initial population with DLBCL receiving any of three dose levels, the best overall response rate (ORR) was 74% (95% confidence interval, 63%-83%); 52% of these patients achieved complete response (CR; 95% CI, 41%-63%).
For patients receiving the higher dose of liso-cel, the ORR was 81% (95% CI, 62%-94%), with a 63% CR rate (95% CI, 42%-81%), bearing out the dose-response rate that had been seen earlier in the trial, Dr. Abramson said.
The median duration of response in all TRANSCEND patients was 9.2 months; the median duration of remission has not been reached, he said. “We see evidence of durable response at 3 months in all our high-risk subsets, and that includes double- and triple-hit lymphomas, double-expresser lymphomas, patients who’ve never achieved prior complete remission, and patients with refractory disease.”
“The overall results are similarly encouraging,” Dr. Abramson said, with 86% of all patients alive at 6 months. Among the complete responders, 94% are alive at the 6-month mark. “The median duration of complete responders has not been reached in this cohort,” he said.
These results are notable, Dr. Abramson said, since about 90% of study participants have at least one disease risk factor that would predict median overall survival of 3-6 months.
During the period after leukapheresis while the CAR T cells were in production, patients could have ongoing treatment, but received PET scans to confirm disease before continuing enrollment in the trial and receiving liso-cel. The time from apheresis to product release for the pivotal cohort is now under 21 days, he said.
The study was supported by Juno Therapeutics, which plans to market liso-cel. Dr. Abramson reported ties with Celgene, Gilead, Seattle Genetics, Novartis, and Genentech.
SOURCE: Abramson J et al. Abstract 5.
SALT LAKE CITY – A CD19-directed 4-1BB chimeric antigen receptor (CAR) T cell product showed efficacy and a low rate of cytokine release syndrome and neurotoxicity in patients with aggressive lymphomas and poor prognoses, raising the possibility of outpatient administration and fewer hospitalization days in this high-risk group.
A total of 86 patients who received inpatient infusions of lisocabtagene maraleucel (liso-cel, also known as JCAR017) had a mean 15.6 days of hospitalization, compared with 9.3 days for 8 outpatient recipients, said Jeremy Abramson, MD, speaking at a top abstracts session of the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
As of October 2017, eight patients had received liso-cel infusion as outpatients with at least 28 days of postinfusion data, Dr. Abramson said.
Although all but one required hospital admission, at a median of 5 days postinfusion (range, 4-22 days), there had been no intensive care unit admissions, and no outpatient recipients had experienced severe cytokine release syndrome (CRS) or neurotoxicity. All admitted patients presented with fever.
Among the study population, “Cytokine release syndrome was only seen in 35% of our entire dataset,” with neurologic toxicity seen in 19% of participants, Dr. Abramson said. “The majority of subjects had no CRS and no toxicity,” he said. Severe CRS occurred in 1% of the study population, and severe neurotoxicity in 12%. There were no deaths related to either complication.
Dr. Abramson reported these results from the TRANSCEND NHL 001 trial, a seamless design phase 1 pivotal trial of liso-cel enrolling patients with relapsed and refractory aggressive B cell non-Hodgkin lymphoma (NHL). Liso-cel delivers CD19-directed CD4 and CD8 CAR T cells in a 1:1 ratio, said Dr. Abramson, director of the lymphoma program at the Massachusetts General Hospital Cancer Center, Boston.
A total of 91 patients were randomized to one of the three dose-finding cohorts of the multicenter trial of liso-cel. One cohort received 5 x 107 cells in a single dose; a second cohort received the same number of cells but in two doses administered 14 days apart; the third cohort received a single dose of 1 x 108 cells.
The seamless trial design then moved to dose expansion, using the two single doses established in the dose-finding phase of the study. Ultimately, Dr. Abramson said, the third and pivotal diffuse large B-cell lymphoma (DLBCL) cohort received the higher single dose, since a dose-response relationship was seen in the earlier cohorts. No increase in cytokine release syndrome or neurotoxicity has been seen with the higher dose in patients evaluated to date.
Patients (median age, 61 years) were eligible to participate in the trial if they had relapsed or refractory DLBCL, primary mediastinal B-cell lymphoma, grade 3B follicular lymphoma, or mantle cell lymphoma. Patients with a failed prior allogeneic stem cell transplant or secondary central nervous system involvement were eligible, but all patients had to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2.
As the trial moved to the core pivotal phase, eligibility requirements shifted slightly to include patients with ECOG status 0 or 1, and lymphoma diagnoses narrowed to include only DLBCL not otherwise specified (NOS), transformed follicular lymphoma, and high-grade B-cell lymphoma with double- and triple-hit cytogenetics. The core group was nearing completion of accrual at the time of the presentation, which presented preliminary results from this phase of the trial.
Among the 88 evaluable patients in the initial population with DLBCL receiving any of three dose levels, the best overall response rate (ORR) was 74% (95% confidence interval, 63%-83%); 52% of these patients achieved complete response (CR; 95% CI, 41%-63%).
For patients receiving the higher dose of liso-cel, the ORR was 81% (95% CI, 62%-94%), with a 63% CR rate (95% CI, 42%-81%), bearing out the dose-response rate that had been seen earlier in the trial, Dr. Abramson said.
The median duration of response in all TRANSCEND patients was 9.2 months; the median duration of remission has not been reached, he said. “We see evidence of durable response at 3 months in all our high-risk subsets, and that includes double- and triple-hit lymphomas, double-expresser lymphomas, patients who’ve never achieved prior complete remission, and patients with refractory disease.”
“The overall results are similarly encouraging,” Dr. Abramson said, with 86% of all patients alive at 6 months. Among the complete responders, 94% are alive at the 6-month mark. “The median duration of complete responders has not been reached in this cohort,” he said.
These results are notable, Dr. Abramson said, since about 90% of study participants have at least one disease risk factor that would predict median overall survival of 3-6 months.
During the period after leukapheresis while the CAR T cells were in production, patients could have ongoing treatment, but received PET scans to confirm disease before continuing enrollment in the trial and receiving liso-cel. The time from apheresis to product release for the pivotal cohort is now under 21 days, he said.
The study was supported by Juno Therapeutics, which plans to market liso-cel. Dr. Abramson reported ties with Celgene, Gilead, Seattle Genetics, Novartis, and Genentech.
SOURCE: Abramson J et al. Abstract 5.
SALT LAKE CITY – A CD19-directed 4-1BB chimeric antigen receptor (CAR) T cell product showed efficacy and a low rate of cytokine release syndrome and neurotoxicity in patients with aggressive lymphomas and poor prognoses, raising the possibility of outpatient administration and fewer hospitalization days in this high-risk group.
A total of 86 patients who received inpatient infusions of lisocabtagene maraleucel (liso-cel, also known as JCAR017) had a mean 15.6 days of hospitalization, compared with 9.3 days for 8 outpatient recipients, said Jeremy Abramson, MD, speaking at a top abstracts session of the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
As of October 2017, eight patients had received liso-cel infusion as outpatients with at least 28 days of postinfusion data, Dr. Abramson said.
Although all but one required hospital admission, at a median of 5 days postinfusion (range, 4-22 days), there had been no intensive care unit admissions, and no outpatient recipients had experienced severe cytokine release syndrome (CRS) or neurotoxicity. All admitted patients presented with fever.
Among the study population, “Cytokine release syndrome was only seen in 35% of our entire dataset,” with neurologic toxicity seen in 19% of participants, Dr. Abramson said. “The majority of subjects had no CRS and no toxicity,” he said. Severe CRS occurred in 1% of the study population, and severe neurotoxicity in 12%. There were no deaths related to either complication.
Dr. Abramson reported these results from the TRANSCEND NHL 001 trial, a seamless design phase 1 pivotal trial of liso-cel enrolling patients with relapsed and refractory aggressive B cell non-Hodgkin lymphoma (NHL). Liso-cel delivers CD19-directed CD4 and CD8 CAR T cells in a 1:1 ratio, said Dr. Abramson, director of the lymphoma program at the Massachusetts General Hospital Cancer Center, Boston.
A total of 91 patients were randomized to one of the three dose-finding cohorts of the multicenter trial of liso-cel. One cohort received 5 x 107 cells in a single dose; a second cohort received the same number of cells but in two doses administered 14 days apart; the third cohort received a single dose of 1 x 108 cells.
The seamless trial design then moved to dose expansion, using the two single doses established in the dose-finding phase of the study. Ultimately, Dr. Abramson said, the third and pivotal diffuse large B-cell lymphoma (DLBCL) cohort received the higher single dose, since a dose-response relationship was seen in the earlier cohorts. No increase in cytokine release syndrome or neurotoxicity has been seen with the higher dose in patients evaluated to date.
Patients (median age, 61 years) were eligible to participate in the trial if they had relapsed or refractory DLBCL, primary mediastinal B-cell lymphoma, grade 3B follicular lymphoma, or mantle cell lymphoma. Patients with a failed prior allogeneic stem cell transplant or secondary central nervous system involvement were eligible, but all patients had to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2.
As the trial moved to the core pivotal phase, eligibility requirements shifted slightly to include patients with ECOG status 0 or 1, and lymphoma diagnoses narrowed to include only DLBCL not otherwise specified (NOS), transformed follicular lymphoma, and high-grade B-cell lymphoma with double- and triple-hit cytogenetics. The core group was nearing completion of accrual at the time of the presentation, which presented preliminary results from this phase of the trial.
Among the 88 evaluable patients in the initial population with DLBCL receiving any of three dose levels, the best overall response rate (ORR) was 74% (95% confidence interval, 63%-83%); 52% of these patients achieved complete response (CR; 95% CI, 41%-63%).
For patients receiving the higher dose of liso-cel, the ORR was 81% (95% CI, 62%-94%), with a 63% CR rate (95% CI, 42%-81%), bearing out the dose-response rate that had been seen earlier in the trial, Dr. Abramson said.
The median duration of response in all TRANSCEND patients was 9.2 months; the median duration of remission has not been reached, he said. “We see evidence of durable response at 3 months in all our high-risk subsets, and that includes double- and triple-hit lymphomas, double-expresser lymphomas, patients who’ve never achieved prior complete remission, and patients with refractory disease.”
“The overall results are similarly encouraging,” Dr. Abramson said, with 86% of all patients alive at 6 months. Among the complete responders, 94% are alive at the 6-month mark. “The median duration of complete responders has not been reached in this cohort,” he said.
These results are notable, Dr. Abramson said, since about 90% of study participants have at least one disease risk factor that would predict median overall survival of 3-6 months.
During the period after leukapheresis while the CAR T cells were in production, patients could have ongoing treatment, but received PET scans to confirm disease before continuing enrollment in the trial and receiving liso-cel. The time from apheresis to product release for the pivotal cohort is now under 21 days, he said.
The study was supported by Juno Therapeutics, which plans to market liso-cel. Dr. Abramson reported ties with Celgene, Gilead, Seattle Genetics, Novartis, and Genentech.
SOURCE: Abramson J et al. Abstract 5.
REPORTING FROM THE 2018 BMT TANDEM MEETINGS
Key clinical point:
Major finding: High-risk lymphoma patients had more than 6 fewer inpatient days with outpatient CAR T infusion.
Study details: Seamless phase 1 trial initially evaluating 91 patients with relapsed/refractory diffuse large B cell lymphoma.
Disclosures: Juno Therapeutics sponsored the study. Dr. Abramson reported ties with Celgene, Gilead, Seattle Genetics, Novartis, and Genentech.
Source: Abramson J et al. Abstract 5.
Cancer groups offer guidance on immune-related adverse events
The American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) have released new guidelines designed to help clinicians manage the unique and sometimes severe side effects associated with cancer immunotherapy agents.
These guidelines meet a growing need to help practicing clinicians identify and best manage immune-related adverse events, according to Bryan J. Schneider, MD, of the University of Michigan Comprehensive Cancer Center, and vice chair of the NCCN Panel on Management of Immunotherapy-Related Toxicities.
“The mechanism of action of these anticancer therapies is so much different from anything that we’re used to,” Dr. Schneider said in an interview.
Critical need for guidance
The ASCO and NCCN guidelines are “critically important” to ensure uniform management of common immune-related adverse events, according to Stephen M. Ansell, MD, PhD, professor of medicine and chair of the Lymphoma Group at Mayo Clinic, Rochester, Minn.
“I think it also specifically highlights a few side effects that many people may not necessarily think about, from eye toxicities to thyroid effects, or the type of things that the average oncologist who is now using this in their practice quite regularly may not necessarily think about,” Dr. Ansell said. “Those kind of effects are now clearly outlined with clear guidance about what should be done, and I think that allows oncologists a resource to go and look at this carefully so that they do the right thing.”
The spectrum of adverse effects associated with checkpoint inhibitors is markedly different from what is seen with cytotoxic chemotherapy, the guidelines note. Most often, the side effects are seen in the skin, GI tract, and lungs, as well as the endocrine, adrenal, nervous, thyroid, pituitary, musculoskeletal, cardiovascular, ocular, and hematologic systems.
Stepwise approach
Side effects of checkpoint inhibitors are typically mild, but they can be severe and sometimes life-threatening, according to ASCO and NCCN.
If immune-related adverse events are mild (i.e., grade 1), treatment can continue with close monitoring, according to the guidelines. By contrast, moderate to severe immune-related adverse events can lead to severe declines in organ function and quality of life, or even fatal outcomes, so early detection and proper management are needed.
Grade 2 toxicities warrant suspending immune checkpoint inhibitor treatment, and resuming it once symptoms subside to grade 1 or less, according to the guidelines. Grade 3 toxicities should also prompt suspension of treatment, plus initiation of high-dose corticosteroids tapered over at least 4-6 weeks.
For most toxicities that reach grade 4, permanent discontinuation of checkpoint inhibitors is recommended.
A thoughtful discussion of potential risks and benefits is needed before using immune checkpoint inhibitors in patients who have autoimmune disease or prior organ transplant, according to the guidelines.
Vigilance required
Checkpoint inhibitors have been approved by the Food and Drug Administration to treat a variety of cancers, including melanoma, lung cancer, and Hodgkin lymphoma, as well as lung, liver, kidney, and bladder cancers.
Clinicians managing patients on checkpoint inhibitors should always be vigilant because immune-related adverse event symptoms can be subtle, according to Julie Brahmer, MD, of The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins in Baltimore.
“Everyone has to work as a team, which includes being educated on possible side effects to immunotherapy prior to prescribing it,” said Dr. Brahmer, chair of the ASCO panel and vice chair of the NCCN panel that developed the guidelines.
The guidelines were published Feb. 14 in two documents that are similar in content, but different in format. The ASCO guideline was published in the Journal of Clinical Oncology (doi: 10.1200/JCO.2017.77.6385) and the NCCN Clinical Practice Guidelines in Oncology were posted on the NCCN website.
While the first edition of the guidelines focuses specifically on immune checkpoint inhibitors, an update anticipated for 2019 will include guidance on chimeric antigen receptor (CAR) T-cell therapy, which is associated with several important side effects, notably cytokine release syndrome.
The American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) have released new guidelines designed to help clinicians manage the unique and sometimes severe side effects associated with cancer immunotherapy agents.
These guidelines meet a growing need to help practicing clinicians identify and best manage immune-related adverse events, according to Bryan J. Schneider, MD, of the University of Michigan Comprehensive Cancer Center, and vice chair of the NCCN Panel on Management of Immunotherapy-Related Toxicities.
“The mechanism of action of these anticancer therapies is so much different from anything that we’re used to,” Dr. Schneider said in an interview.
Critical need for guidance
The ASCO and NCCN guidelines are “critically important” to ensure uniform management of common immune-related adverse events, according to Stephen M. Ansell, MD, PhD, professor of medicine and chair of the Lymphoma Group at Mayo Clinic, Rochester, Minn.
“I think it also specifically highlights a few side effects that many people may not necessarily think about, from eye toxicities to thyroid effects, or the type of things that the average oncologist who is now using this in their practice quite regularly may not necessarily think about,” Dr. Ansell said. “Those kind of effects are now clearly outlined with clear guidance about what should be done, and I think that allows oncologists a resource to go and look at this carefully so that they do the right thing.”
The spectrum of adverse effects associated with checkpoint inhibitors is markedly different from what is seen with cytotoxic chemotherapy, the guidelines note. Most often, the side effects are seen in the skin, GI tract, and lungs, as well as the endocrine, adrenal, nervous, thyroid, pituitary, musculoskeletal, cardiovascular, ocular, and hematologic systems.
Stepwise approach
Side effects of checkpoint inhibitors are typically mild, but they can be severe and sometimes life-threatening, according to ASCO and NCCN.
If immune-related adverse events are mild (i.e., grade 1), treatment can continue with close monitoring, according to the guidelines. By contrast, moderate to severe immune-related adverse events can lead to severe declines in organ function and quality of life, or even fatal outcomes, so early detection and proper management are needed.
Grade 2 toxicities warrant suspending immune checkpoint inhibitor treatment, and resuming it once symptoms subside to grade 1 or less, according to the guidelines. Grade 3 toxicities should also prompt suspension of treatment, plus initiation of high-dose corticosteroids tapered over at least 4-6 weeks.
For most toxicities that reach grade 4, permanent discontinuation of checkpoint inhibitors is recommended.
A thoughtful discussion of potential risks and benefits is needed before using immune checkpoint inhibitors in patients who have autoimmune disease or prior organ transplant, according to the guidelines.
Vigilance required
Checkpoint inhibitors have been approved by the Food and Drug Administration to treat a variety of cancers, including melanoma, lung cancer, and Hodgkin lymphoma, as well as lung, liver, kidney, and bladder cancers.
Clinicians managing patients on checkpoint inhibitors should always be vigilant because immune-related adverse event symptoms can be subtle, according to Julie Brahmer, MD, of The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins in Baltimore.
“Everyone has to work as a team, which includes being educated on possible side effects to immunotherapy prior to prescribing it,” said Dr. Brahmer, chair of the ASCO panel and vice chair of the NCCN panel that developed the guidelines.
The guidelines were published Feb. 14 in two documents that are similar in content, but different in format. The ASCO guideline was published in the Journal of Clinical Oncology (doi: 10.1200/JCO.2017.77.6385) and the NCCN Clinical Practice Guidelines in Oncology were posted on the NCCN website.
While the first edition of the guidelines focuses specifically on immune checkpoint inhibitors, an update anticipated for 2019 will include guidance on chimeric antigen receptor (CAR) T-cell therapy, which is associated with several important side effects, notably cytokine release syndrome.
The American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) have released new guidelines designed to help clinicians manage the unique and sometimes severe side effects associated with cancer immunotherapy agents.
These guidelines meet a growing need to help practicing clinicians identify and best manage immune-related adverse events, according to Bryan J. Schneider, MD, of the University of Michigan Comprehensive Cancer Center, and vice chair of the NCCN Panel on Management of Immunotherapy-Related Toxicities.
“The mechanism of action of these anticancer therapies is so much different from anything that we’re used to,” Dr. Schneider said in an interview.
Critical need for guidance
The ASCO and NCCN guidelines are “critically important” to ensure uniform management of common immune-related adverse events, according to Stephen M. Ansell, MD, PhD, professor of medicine and chair of the Lymphoma Group at Mayo Clinic, Rochester, Minn.
“I think it also specifically highlights a few side effects that many people may not necessarily think about, from eye toxicities to thyroid effects, or the type of things that the average oncologist who is now using this in their practice quite regularly may not necessarily think about,” Dr. Ansell said. “Those kind of effects are now clearly outlined with clear guidance about what should be done, and I think that allows oncologists a resource to go and look at this carefully so that they do the right thing.”
The spectrum of adverse effects associated with checkpoint inhibitors is markedly different from what is seen with cytotoxic chemotherapy, the guidelines note. Most often, the side effects are seen in the skin, GI tract, and lungs, as well as the endocrine, adrenal, nervous, thyroid, pituitary, musculoskeletal, cardiovascular, ocular, and hematologic systems.
Stepwise approach
Side effects of checkpoint inhibitors are typically mild, but they can be severe and sometimes life-threatening, according to ASCO and NCCN.
If immune-related adverse events are mild (i.e., grade 1), treatment can continue with close monitoring, according to the guidelines. By contrast, moderate to severe immune-related adverse events can lead to severe declines in organ function and quality of life, or even fatal outcomes, so early detection and proper management are needed.
Grade 2 toxicities warrant suspending immune checkpoint inhibitor treatment, and resuming it once symptoms subside to grade 1 or less, according to the guidelines. Grade 3 toxicities should also prompt suspension of treatment, plus initiation of high-dose corticosteroids tapered over at least 4-6 weeks.
For most toxicities that reach grade 4, permanent discontinuation of checkpoint inhibitors is recommended.
A thoughtful discussion of potential risks and benefits is needed before using immune checkpoint inhibitors in patients who have autoimmune disease or prior organ transplant, according to the guidelines.
Vigilance required
Checkpoint inhibitors have been approved by the Food and Drug Administration to treat a variety of cancers, including melanoma, lung cancer, and Hodgkin lymphoma, as well as lung, liver, kidney, and bladder cancers.
Clinicians managing patients on checkpoint inhibitors should always be vigilant because immune-related adverse event symptoms can be subtle, according to Julie Brahmer, MD, of The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins in Baltimore.
“Everyone has to work as a team, which includes being educated on possible side effects to immunotherapy prior to prescribing it,” said Dr. Brahmer, chair of the ASCO panel and vice chair of the NCCN panel that developed the guidelines.
The guidelines were published Feb. 14 in two documents that are similar in content, but different in format. The ASCO guideline was published in the Journal of Clinical Oncology (doi: 10.1200/JCO.2017.77.6385) and the NCCN Clinical Practice Guidelines in Oncology were posted on the NCCN website.
While the first edition of the guidelines focuses specifically on immune checkpoint inhibitors, an update anticipated for 2019 will include guidance on chimeric antigen receptor (CAR) T-cell therapy, which is associated with several important side effects, notably cytokine release syndrome.
High efficacy, no safety signals for herpes zoster vaccine post-HSCT
SALT LAKE CITY – A recently approved adjuvanted herpes zoster vaccine)(Shingrix) effectively and safely prevented herpes zoster in a population of patients with multiple myeloma and other hematologic malignancies who received autologous hematopoietic stem cell transplantation.
The use of recombinant varicella zoster virus glycoprotein E in combination with an adjuvant system gives immunosuppressed individuals who have received hematopoietic stem cell transplantation (HSCT) a safe option for prevention of herpes zoster (HZ), said Javier de la Serna, MD, PhD, speaking at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
Presenting the findings at a late-breaking abstract session, Dr. de la Serna said that for the 1,721 participants in a placebo-controlled multicenter trial who received both doses of the vaccine, the incidence of HZ for vaccine recipients was 3.0%, compared with 9.4% of placebo recipients, for a vaccine efficacy of 68.2% (95% confidence interval, 55.6-77.5; P less than 0.0001). These results met the study’s primary objective.
Postherpetic neuralgia (PHN) prevention efficacy – a secondary endpoint – was 89.3% for those receiving the vaccine (HZ/su); the incidence of PHN was 0.5% in the HZ/su study arm, compared with 4.9% for those who received placebo (95% CI, 22.5-99.8). The study also tracked other HZ complications as a secondary endpoint, finding efficacy of 77.8% (95% CI, 19.1–95.0). “The vaccine was highly efficacious in preventing all the secondary outcomes,” said Dr. de la Serna of the Hospital Universitario 12 de Octubre, Madrid.
The randomized, observer-blind phase 3 trial was conducted in 28 countries.Adults who received autologous HSCT were randomized 1:1 to receive HZ/su (n = 922) or placebo (n = 924) within 50-70 days of their transplant. Patients were excluded if they were expected to receive more than 6 months of anti–varicella zoster prophylaxis posttransplant, Dr. de la Serna said.
Participants received the first dose of HZ/su at the first study visit, and the second dose 30-60 days later. Patients were seen 1 month after the last vaccine dose, and then again at months 13 and 25, with telephone follow-up between the later visits. All participants were followed for at least 1 year, Dr. de la Serna said.
Episodes of HZ were confirmed by polymerase chain reaction assay, or, when samples were lacking or indeterminate, by agreement of at least three members of an ascertainment committee.
Of the two components of the HZ/su vaccine, glycoprotein E triggers both humoral immunity and activity of varicella zoster–specific CD4+ T cells; the adjuvant system – dubbed ASO1 – boosts immune response. The vaccine was approved by the Food and Drug Administration in October 2017 for use in adults aged 50 years and older.
In addition to the primary endpoint of vaccine efficacy in prevention of HZ cases during the study period, secondary objectives included monitoring vaccine reactogenicity and safety, and evaluating vaccine efficacy for the prevention of PHN and other complications of HZ.
Tertiary objectives included vaccine efficacy in preventing HZ during the first year posttransplant (vaccine efficacy 84.7%; 95% CI, 32.2-96.6), as well as efficacy in preventing hospitalizations related to HZ (vaccine efficacy 76.2%, 95% CI 61.1-86.0).
An exploratory analysis found vaccine efficacy of 71.8% for participants younger than 50 years (95% CI, 38.8 – 88.3). For patients aged 50 years and older, vaccine efficacy was 67.3% (95% CI, 52.6–77.9).
The safety of HZ/su was determined by analyzing data for all participants, but efficacy data included only those who received the second dose and did not develop HZ within a month of receiving the second vaccine dose.
In the efficacy group (n = 1,721), patients were mostly (n = 1,296) aged 50 years or older. Most patients (n = 937) received HSCT for multiple myeloma. Overall, participants were about 63% male, and 77% were of Caucasian/European ancestry.
Adverse events, solicited for the first 7 days after injections, were mostly mild and related to the local site pain and inflammation expected with an adjuvanted vaccine; HZ/su recipients also experienced more fatigue and muscle aches than did those receiving placebo. Median duration of symptoms was up to 3 days, with grade 3 events lasting up to 2 days.
Unsolicited and serious adverse events were similar between study arms, with a median safety follow-up period of 29 months. The investigators judged that no deaths were related to the vaccine, and there were no signals for increased rate of relapse or immune-mediated diseases.
The study was funded by GlaxoSmithKline; HZ/su(Shingrix) is marketed by GlaxoSmithKline. Dr. de la Serna reported being on the advisory board or receiving honoraria from multiple pharmaceutical companies.
SOURCE: de la Serna J et al. 2018 BMT Tandem Meetings, Abstract LBA2.
SALT LAKE CITY – A recently approved adjuvanted herpes zoster vaccine)(Shingrix) effectively and safely prevented herpes zoster in a population of patients with multiple myeloma and other hematologic malignancies who received autologous hematopoietic stem cell transplantation.
The use of recombinant varicella zoster virus glycoprotein E in combination with an adjuvant system gives immunosuppressed individuals who have received hematopoietic stem cell transplantation (HSCT) a safe option for prevention of herpes zoster (HZ), said Javier de la Serna, MD, PhD, speaking at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
Presenting the findings at a late-breaking abstract session, Dr. de la Serna said that for the 1,721 participants in a placebo-controlled multicenter trial who received both doses of the vaccine, the incidence of HZ for vaccine recipients was 3.0%, compared with 9.4% of placebo recipients, for a vaccine efficacy of 68.2% (95% confidence interval, 55.6-77.5; P less than 0.0001). These results met the study’s primary objective.
Postherpetic neuralgia (PHN) prevention efficacy – a secondary endpoint – was 89.3% for those receiving the vaccine (HZ/su); the incidence of PHN was 0.5% in the HZ/su study arm, compared with 4.9% for those who received placebo (95% CI, 22.5-99.8). The study also tracked other HZ complications as a secondary endpoint, finding efficacy of 77.8% (95% CI, 19.1–95.0). “The vaccine was highly efficacious in preventing all the secondary outcomes,” said Dr. de la Serna of the Hospital Universitario 12 de Octubre, Madrid.
The randomized, observer-blind phase 3 trial was conducted in 28 countries.Adults who received autologous HSCT were randomized 1:1 to receive HZ/su (n = 922) or placebo (n = 924) within 50-70 days of their transplant. Patients were excluded if they were expected to receive more than 6 months of anti–varicella zoster prophylaxis posttransplant, Dr. de la Serna said.
Participants received the first dose of HZ/su at the first study visit, and the second dose 30-60 days later. Patients were seen 1 month after the last vaccine dose, and then again at months 13 and 25, with telephone follow-up between the later visits. All participants were followed for at least 1 year, Dr. de la Serna said.
Episodes of HZ were confirmed by polymerase chain reaction assay, or, when samples were lacking or indeterminate, by agreement of at least three members of an ascertainment committee.
Of the two components of the HZ/su vaccine, glycoprotein E triggers both humoral immunity and activity of varicella zoster–specific CD4+ T cells; the adjuvant system – dubbed ASO1 – boosts immune response. The vaccine was approved by the Food and Drug Administration in October 2017 for use in adults aged 50 years and older.
In addition to the primary endpoint of vaccine efficacy in prevention of HZ cases during the study period, secondary objectives included monitoring vaccine reactogenicity and safety, and evaluating vaccine efficacy for the prevention of PHN and other complications of HZ.
Tertiary objectives included vaccine efficacy in preventing HZ during the first year posttransplant (vaccine efficacy 84.7%; 95% CI, 32.2-96.6), as well as efficacy in preventing hospitalizations related to HZ (vaccine efficacy 76.2%, 95% CI 61.1-86.0).
An exploratory analysis found vaccine efficacy of 71.8% for participants younger than 50 years (95% CI, 38.8 – 88.3). For patients aged 50 years and older, vaccine efficacy was 67.3% (95% CI, 52.6–77.9).
The safety of HZ/su was determined by analyzing data for all participants, but efficacy data included only those who received the second dose and did not develop HZ within a month of receiving the second vaccine dose.
In the efficacy group (n = 1,721), patients were mostly (n = 1,296) aged 50 years or older. Most patients (n = 937) received HSCT for multiple myeloma. Overall, participants were about 63% male, and 77% were of Caucasian/European ancestry.
Adverse events, solicited for the first 7 days after injections, were mostly mild and related to the local site pain and inflammation expected with an adjuvanted vaccine; HZ/su recipients also experienced more fatigue and muscle aches than did those receiving placebo. Median duration of symptoms was up to 3 days, with grade 3 events lasting up to 2 days.
Unsolicited and serious adverse events were similar between study arms, with a median safety follow-up period of 29 months. The investigators judged that no deaths were related to the vaccine, and there were no signals for increased rate of relapse or immune-mediated diseases.
The study was funded by GlaxoSmithKline; HZ/su(Shingrix) is marketed by GlaxoSmithKline. Dr. de la Serna reported being on the advisory board or receiving honoraria from multiple pharmaceutical companies.
SOURCE: de la Serna J et al. 2018 BMT Tandem Meetings, Abstract LBA2.
SALT LAKE CITY – A recently approved adjuvanted herpes zoster vaccine)(Shingrix) effectively and safely prevented herpes zoster in a population of patients with multiple myeloma and other hematologic malignancies who received autologous hematopoietic stem cell transplantation.
The use of recombinant varicella zoster virus glycoprotein E in combination with an adjuvant system gives immunosuppressed individuals who have received hematopoietic stem cell transplantation (HSCT) a safe option for prevention of herpes zoster (HZ), said Javier de la Serna, MD, PhD, speaking at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
Presenting the findings at a late-breaking abstract session, Dr. de la Serna said that for the 1,721 participants in a placebo-controlled multicenter trial who received both doses of the vaccine, the incidence of HZ for vaccine recipients was 3.0%, compared with 9.4% of placebo recipients, for a vaccine efficacy of 68.2% (95% confidence interval, 55.6-77.5; P less than 0.0001). These results met the study’s primary objective.
Postherpetic neuralgia (PHN) prevention efficacy – a secondary endpoint – was 89.3% for those receiving the vaccine (HZ/su); the incidence of PHN was 0.5% in the HZ/su study arm, compared with 4.9% for those who received placebo (95% CI, 22.5-99.8). The study also tracked other HZ complications as a secondary endpoint, finding efficacy of 77.8% (95% CI, 19.1–95.0). “The vaccine was highly efficacious in preventing all the secondary outcomes,” said Dr. de la Serna of the Hospital Universitario 12 de Octubre, Madrid.
The randomized, observer-blind phase 3 trial was conducted in 28 countries.Adults who received autologous HSCT were randomized 1:1 to receive HZ/su (n = 922) or placebo (n = 924) within 50-70 days of their transplant. Patients were excluded if they were expected to receive more than 6 months of anti–varicella zoster prophylaxis posttransplant, Dr. de la Serna said.
Participants received the first dose of HZ/su at the first study visit, and the second dose 30-60 days later. Patients were seen 1 month after the last vaccine dose, and then again at months 13 and 25, with telephone follow-up between the later visits. All participants were followed for at least 1 year, Dr. de la Serna said.
Episodes of HZ were confirmed by polymerase chain reaction assay, or, when samples were lacking or indeterminate, by agreement of at least three members of an ascertainment committee.
Of the two components of the HZ/su vaccine, glycoprotein E triggers both humoral immunity and activity of varicella zoster–specific CD4+ T cells; the adjuvant system – dubbed ASO1 – boosts immune response. The vaccine was approved by the Food and Drug Administration in October 2017 for use in adults aged 50 years and older.
In addition to the primary endpoint of vaccine efficacy in prevention of HZ cases during the study period, secondary objectives included monitoring vaccine reactogenicity and safety, and evaluating vaccine efficacy for the prevention of PHN and other complications of HZ.
Tertiary objectives included vaccine efficacy in preventing HZ during the first year posttransplant (vaccine efficacy 84.7%; 95% CI, 32.2-96.6), as well as efficacy in preventing hospitalizations related to HZ (vaccine efficacy 76.2%, 95% CI 61.1-86.0).
An exploratory analysis found vaccine efficacy of 71.8% for participants younger than 50 years (95% CI, 38.8 – 88.3). For patients aged 50 years and older, vaccine efficacy was 67.3% (95% CI, 52.6–77.9).
The safety of HZ/su was determined by analyzing data for all participants, but efficacy data included only those who received the second dose and did not develop HZ within a month of receiving the second vaccine dose.
In the efficacy group (n = 1,721), patients were mostly (n = 1,296) aged 50 years or older. Most patients (n = 937) received HSCT for multiple myeloma. Overall, participants were about 63% male, and 77% were of Caucasian/European ancestry.
Adverse events, solicited for the first 7 days after injections, were mostly mild and related to the local site pain and inflammation expected with an adjuvanted vaccine; HZ/su recipients also experienced more fatigue and muscle aches than did those receiving placebo. Median duration of symptoms was up to 3 days, with grade 3 events lasting up to 2 days.
Unsolicited and serious adverse events were similar between study arms, with a median safety follow-up period of 29 months. The investigators judged that no deaths were related to the vaccine, and there were no signals for increased rate of relapse or immune-mediated diseases.
The study was funded by GlaxoSmithKline; HZ/su(Shingrix) is marketed by GlaxoSmithKline. Dr. de la Serna reported being on the advisory board or receiving honoraria from multiple pharmaceutical companies.
SOURCE: de la Serna J et al. 2018 BMT Tandem Meetings, Abstract LBA2.
REPORTING FROM THE 2018 BMT TANDEM MEETINGS
Key clinical point:
Major finding: Efficacy was 68.17% for preventing herpes zoster among HSCT recipients.
Study details: A randomized, observer blind, placebo-controlled phase 3 study of 1,846 post-HSCT recipients.
Disclosures: The study was sponsored by GlaxoSmithKline. Dr. de la Serna reported relationships with multiple pharmaceutical companies.
Source: de la Serna J et al. 2018 BMT Tandem Meetings, Abstract LBA2.
HCT-CI score may predict mortality for nonmalignant disease
SALT LAKE CITY – Scores of 3 or higher on the Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI) are associated with an increased risk of posttransplant mortality in certain patients undergoing allogeneic HCT for nonmalignant disease, according to findings from a review of more than 4,000 patients.
The exception was in patients undergoing HCT for hemoglobinopathies, Larisa Broglie, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The findings of the study, which is the largest to date to validate the usefulness of the HCT-CI for risk assessment in HCT patients with nonmalignant disease, have important implications for patient counseling and decision making, said Dr. Broglie of the Medical College of Wisconsin, Milwaukee.
Of 4,083 children and adults who underwent a first allogeneic HCT for a nonmalignant disease between 2007 and 2014 and who had sufficient follow-up (median, 39 months), 61% had an HCT-CI score of 0, 20% had a score of 1-2, and 19% had a score of 3 or higher.
After adjustment for age, disease, donor, graft source, recipient cytomegalovirus status, and performance status, scores of 3 or greater were associated with an overall increased hazard ratio for poor survival (HRs of 1.33 for scores of 3-4 and 2.31 for scores of 5 or greater, vs. 1.0 and 1.127 for scores of 0 or 1-2, respectively), she said.
Patients with an HCT-CI score of 0 had estimated 2-year overall survival of 82.7%, compared with 80.3% for scores 1-2, 74.0% for scores 3-4, and 55.8% for scores of 5 or greater.
Patients included in this study were identified from the Center for International Blood & Marrow Transplant Research database. They ranged in age from under 1 year to 77 years but most were young; the median age was 9 years and 78% of patients were under age 20.
HCT was performed for acquired aplastic anemia in 33% of patients, immune deficiencies in 19%, hemoglobinopathies in 16%, bone marrow failure in 12%, histiocytic disorders in 11%, metabolic disease in 9%, or autoimmune disease in less than 1%, she said, noting that the most frequent comorbidities seen within the entire cohort were moderate pulmonary disease, hepatic disease, and infection requiring ongoing treatment.
The effect of HCT-CI score on survival was present regardless of conditioning intensity and graft-versus-host disease prophylaxis, but scores predicted mortality risk differently based on underlying disease, and different comorbidities predominated in each disease category, she said, explaining that this was apparent when patients were stratified by the seven represented nonmalignant disease categories to account for disease heterogeneity.
For example, HCT-CI score predicted mortality risk in patients with aplastic anemia (HRs of 1.00, 1.19, and 2.06 for scores of 0, 1-2, and 3 or greater, respectively), and in patients with immune deficiency (HRs of 1.00, 1.37, and 1.87 for scores of 0, 1-2, and 3 or greater, respectively), and the distribution of comorbidities in patients in these two disease categories was similar to that of the overall cohort.
However, HCT-CI score did not predict mortality risk in those undergoing HCT for hemoglobinopathies (HRs of 1.00, 0.46, and 0.59 for scores of 0, 1-2, and 3 or greater, respectively), Dr. Broglie said, noting that these patients had overall high survival rates regardless of HCT-CI scores, and they had comorbidities that differed from the overall cohort.
HCT is a curative treatment strategy for many patients with nonmalignant diseases but transplant-related mortality remains a concern, she said. While HCT-CI has been shown to be useful for discriminating the risks of nonrelapse and overall survival among patients with hematologic malignancies who undergo allogeneic HCT, its usefulness in patients undergoing HCT for nonmalignant diseases has been less clear.
The distinction is important, as patients with nonmalignant diseases have different pretransplant exposures and may have comorbidities specific to their underlying disease. Furthermore, transplantation approaches – including conditioning regimens and intensities – differ, she said.
“And the HCT-CI was developed to predict risk of nonrelapse mortality, but relapse in nonmalignant diseases can often be difficult to define,” she added.
The current findings demonstrate the value of the HCT-CI in nonmalignant diseases, and “offer the potential to intervene with transplantation prior to the onset of comorbidities, or with efforts to prevent comorbidities prior to transplantation,” she said, concluding that “future efforts could focus on further refining pretransplant risk assessment for patients with nonmalignant diseases, especially for patients with hemoglobinopathies and HCT-CI scores of less than 3.”
Dr. Broglie reported having no financial disclosures.
SOURCE: Broglie L et al. Abstract 16.
SALT LAKE CITY – Scores of 3 or higher on the Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI) are associated with an increased risk of posttransplant mortality in certain patients undergoing allogeneic HCT for nonmalignant disease, according to findings from a review of more than 4,000 patients.
The exception was in patients undergoing HCT for hemoglobinopathies, Larisa Broglie, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The findings of the study, which is the largest to date to validate the usefulness of the HCT-CI for risk assessment in HCT patients with nonmalignant disease, have important implications for patient counseling and decision making, said Dr. Broglie of the Medical College of Wisconsin, Milwaukee.
Of 4,083 children and adults who underwent a first allogeneic HCT for a nonmalignant disease between 2007 and 2014 and who had sufficient follow-up (median, 39 months), 61% had an HCT-CI score of 0, 20% had a score of 1-2, and 19% had a score of 3 or higher.
After adjustment for age, disease, donor, graft source, recipient cytomegalovirus status, and performance status, scores of 3 or greater were associated with an overall increased hazard ratio for poor survival (HRs of 1.33 for scores of 3-4 and 2.31 for scores of 5 or greater, vs. 1.0 and 1.127 for scores of 0 or 1-2, respectively), she said.
Patients with an HCT-CI score of 0 had estimated 2-year overall survival of 82.7%, compared with 80.3% for scores 1-2, 74.0% for scores 3-4, and 55.8% for scores of 5 or greater.
Patients included in this study were identified from the Center for International Blood & Marrow Transplant Research database. They ranged in age from under 1 year to 77 years but most were young; the median age was 9 years and 78% of patients were under age 20.
HCT was performed for acquired aplastic anemia in 33% of patients, immune deficiencies in 19%, hemoglobinopathies in 16%, bone marrow failure in 12%, histiocytic disorders in 11%, metabolic disease in 9%, or autoimmune disease in less than 1%, she said, noting that the most frequent comorbidities seen within the entire cohort were moderate pulmonary disease, hepatic disease, and infection requiring ongoing treatment.
The effect of HCT-CI score on survival was present regardless of conditioning intensity and graft-versus-host disease prophylaxis, but scores predicted mortality risk differently based on underlying disease, and different comorbidities predominated in each disease category, she said, explaining that this was apparent when patients were stratified by the seven represented nonmalignant disease categories to account for disease heterogeneity.
For example, HCT-CI score predicted mortality risk in patients with aplastic anemia (HRs of 1.00, 1.19, and 2.06 for scores of 0, 1-2, and 3 or greater, respectively), and in patients with immune deficiency (HRs of 1.00, 1.37, and 1.87 for scores of 0, 1-2, and 3 or greater, respectively), and the distribution of comorbidities in patients in these two disease categories was similar to that of the overall cohort.
However, HCT-CI score did not predict mortality risk in those undergoing HCT for hemoglobinopathies (HRs of 1.00, 0.46, and 0.59 for scores of 0, 1-2, and 3 or greater, respectively), Dr. Broglie said, noting that these patients had overall high survival rates regardless of HCT-CI scores, and they had comorbidities that differed from the overall cohort.
HCT is a curative treatment strategy for many patients with nonmalignant diseases but transplant-related mortality remains a concern, she said. While HCT-CI has been shown to be useful for discriminating the risks of nonrelapse and overall survival among patients with hematologic malignancies who undergo allogeneic HCT, its usefulness in patients undergoing HCT for nonmalignant diseases has been less clear.
The distinction is important, as patients with nonmalignant diseases have different pretransplant exposures and may have comorbidities specific to their underlying disease. Furthermore, transplantation approaches – including conditioning regimens and intensities – differ, she said.
“And the HCT-CI was developed to predict risk of nonrelapse mortality, but relapse in nonmalignant diseases can often be difficult to define,” she added.
The current findings demonstrate the value of the HCT-CI in nonmalignant diseases, and “offer the potential to intervene with transplantation prior to the onset of comorbidities, or with efforts to prevent comorbidities prior to transplantation,” she said, concluding that “future efforts could focus on further refining pretransplant risk assessment for patients with nonmalignant diseases, especially for patients with hemoglobinopathies and HCT-CI scores of less than 3.”
Dr. Broglie reported having no financial disclosures.
SOURCE: Broglie L et al. Abstract 16.
SALT LAKE CITY – Scores of 3 or higher on the Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI) are associated with an increased risk of posttransplant mortality in certain patients undergoing allogeneic HCT for nonmalignant disease, according to findings from a review of more than 4,000 patients.
The exception was in patients undergoing HCT for hemoglobinopathies, Larisa Broglie, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The findings of the study, which is the largest to date to validate the usefulness of the HCT-CI for risk assessment in HCT patients with nonmalignant disease, have important implications for patient counseling and decision making, said Dr. Broglie of the Medical College of Wisconsin, Milwaukee.
Of 4,083 children and adults who underwent a first allogeneic HCT for a nonmalignant disease between 2007 and 2014 and who had sufficient follow-up (median, 39 months), 61% had an HCT-CI score of 0, 20% had a score of 1-2, and 19% had a score of 3 or higher.
After adjustment for age, disease, donor, graft source, recipient cytomegalovirus status, and performance status, scores of 3 or greater were associated with an overall increased hazard ratio for poor survival (HRs of 1.33 for scores of 3-4 and 2.31 for scores of 5 or greater, vs. 1.0 and 1.127 for scores of 0 or 1-2, respectively), she said.
Patients with an HCT-CI score of 0 had estimated 2-year overall survival of 82.7%, compared with 80.3% for scores 1-2, 74.0% for scores 3-4, and 55.8% for scores of 5 or greater.
Patients included in this study were identified from the Center for International Blood & Marrow Transplant Research database. They ranged in age from under 1 year to 77 years but most were young; the median age was 9 years and 78% of patients were under age 20.
HCT was performed for acquired aplastic anemia in 33% of patients, immune deficiencies in 19%, hemoglobinopathies in 16%, bone marrow failure in 12%, histiocytic disorders in 11%, metabolic disease in 9%, or autoimmune disease in less than 1%, she said, noting that the most frequent comorbidities seen within the entire cohort were moderate pulmonary disease, hepatic disease, and infection requiring ongoing treatment.
The effect of HCT-CI score on survival was present regardless of conditioning intensity and graft-versus-host disease prophylaxis, but scores predicted mortality risk differently based on underlying disease, and different comorbidities predominated in each disease category, she said, explaining that this was apparent when patients were stratified by the seven represented nonmalignant disease categories to account for disease heterogeneity.
For example, HCT-CI score predicted mortality risk in patients with aplastic anemia (HRs of 1.00, 1.19, and 2.06 for scores of 0, 1-2, and 3 or greater, respectively), and in patients with immune deficiency (HRs of 1.00, 1.37, and 1.87 for scores of 0, 1-2, and 3 or greater, respectively), and the distribution of comorbidities in patients in these two disease categories was similar to that of the overall cohort.
However, HCT-CI score did not predict mortality risk in those undergoing HCT for hemoglobinopathies (HRs of 1.00, 0.46, and 0.59 for scores of 0, 1-2, and 3 or greater, respectively), Dr. Broglie said, noting that these patients had overall high survival rates regardless of HCT-CI scores, and they had comorbidities that differed from the overall cohort.
HCT is a curative treatment strategy for many patients with nonmalignant diseases but transplant-related mortality remains a concern, she said. While HCT-CI has been shown to be useful for discriminating the risks of nonrelapse and overall survival among patients with hematologic malignancies who undergo allogeneic HCT, its usefulness in patients undergoing HCT for nonmalignant diseases has been less clear.
The distinction is important, as patients with nonmalignant diseases have different pretransplant exposures and may have comorbidities specific to their underlying disease. Furthermore, transplantation approaches – including conditioning regimens and intensities – differ, she said.
“And the HCT-CI was developed to predict risk of nonrelapse mortality, but relapse in nonmalignant diseases can often be difficult to define,” she added.
The current findings demonstrate the value of the HCT-CI in nonmalignant diseases, and “offer the potential to intervene with transplantation prior to the onset of comorbidities, or with efforts to prevent comorbidities prior to transplantation,” she said, concluding that “future efforts could focus on further refining pretransplant risk assessment for patients with nonmalignant diseases, especially for patients with hemoglobinopathies and HCT-CI scores of less than 3.”
Dr. Broglie reported having no financial disclosures.
SOURCE: Broglie L et al. Abstract 16.
REPORTING FROM THE 2018 BMT TANDEM MEETINGS
Key clinical point:
Major finding: Hazard ratios for poor survival were 1.33 for scores of 3-4 and 2.31 for scores of 5 or greater, compared with 1.0 and 1.127 for scores of 0 or 1-2, respectively.
Study details: A review of 4,083 patients from the CIBMTR database.
Disclosures: Dr. Broglie reported having no financial disclosures.
Source: Broglie L et al. Abstract 16.
Tisagenlecleucel looks effective in phase 2 study of young ALL patients
Tisagenlecleucel was associated with durable remission and long-term persistence for younger patients with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL), according to the results of a multicenter, multicontinent, phase 2 trial published in the New England Journal of Medicine.
Shannon L. Maude, MD, PhD, of the Children’s Hospital of Philadelphia and her coauthors reported that the anti-CD19 chimeric antigen receptor (CAR) therapy was highly toxic, but the effects were usually mitigated. Additionally, the investigators showed feasibility of a global supply chain for distribution of the therapy.
The investigators evaluated data from 75 patients with at least 5% lymphoblasts in their bone marrow at the time of screening. Patients were aged 3 years or older at the time of screening but were no older than 21 years of age at the time of diagnosis.
For 50 patients evaluated at the interim analysis, the primary endpoint of overall remission at 3 months was met, and the overall remission rate was 82% (P less than .001).
An updated analysis showed that 81% of 75 patients who had at least 3 months of follow-up experienced overall remission (95% confidence interval, 71-89). A total of 45 of those patients experienced complete remission, and 16 had complete remission with incomplete hematologic recovery.
Event-free survival was experienced by 73% of patients at 6 months and 50% of patients at 12 months. Overall survival was 90% at 6 months and 76% at 12 months, the investigators reported.
Before tisagenlecleucel infusion, 96% of patients received lymphodepleting chemotherapy. The administration of chemotherapy was not done at the discretion of the investigator if a patient had leukopenia.
The median duration of remission was not reached, and the persistence of tisagenlecleucel in the blood was observed for as long as 20 months.
“The remissions were durable, with a 6-month relapse-free survival rate of 80%,” the investigators wrote. “The durability of the clinical response was associated with persistence of tisagenlecleucel in peripheral blood and with persistent B-cell aplasia.”
The phase 1 study of tisagenlecleucel infusion therapy for younger patients with B-cell ALL showed the toxic nature of the therapy, so investigators were not surprised by the safety data they found. Nearly three-quarters of patients who were evaluated in the study experienced a grade 3 or 4 tisagenlecleucel-related adverse event. Cytokine release syndrome occurred in 77% of patients.
Previously reported data regarding anti-CD19 CAR T-cell therapy for ALL came from single-center studies where manufacturing occurred on site, but the current study employed a global, multicenter supply chain, according to the investigators.
“The toxicity and efficacy of tisagenlecleucel [in this study] were consistent with those in the single-center study, and the feasibility of a global supply chain was demonstrated,” they wrote. “Because this study used cryopreserved leukapheresis product, it did not require fresh product and an open manufacture slot for enrollment.”
This research was sponsored and designed by Novartis Pharmaceuticals. Dr. Maude reported having received personal fees from Novartis as well as grant funding from St. Baldrick’s Foundation.
SOURCE: Maude SL, et al. N Engl J Med. 2018;378:439-48.
Tisagenlecleucel was associated with durable remission and long-term persistence for younger patients with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL), according to the results of a multicenter, multicontinent, phase 2 trial published in the New England Journal of Medicine.
Shannon L. Maude, MD, PhD, of the Children’s Hospital of Philadelphia and her coauthors reported that the anti-CD19 chimeric antigen receptor (CAR) therapy was highly toxic, but the effects were usually mitigated. Additionally, the investigators showed feasibility of a global supply chain for distribution of the therapy.
The investigators evaluated data from 75 patients with at least 5% lymphoblasts in their bone marrow at the time of screening. Patients were aged 3 years or older at the time of screening but were no older than 21 years of age at the time of diagnosis.
For 50 patients evaluated at the interim analysis, the primary endpoint of overall remission at 3 months was met, and the overall remission rate was 82% (P less than .001).
An updated analysis showed that 81% of 75 patients who had at least 3 months of follow-up experienced overall remission (95% confidence interval, 71-89). A total of 45 of those patients experienced complete remission, and 16 had complete remission with incomplete hematologic recovery.
Event-free survival was experienced by 73% of patients at 6 months and 50% of patients at 12 months. Overall survival was 90% at 6 months and 76% at 12 months, the investigators reported.
Before tisagenlecleucel infusion, 96% of patients received lymphodepleting chemotherapy. The administration of chemotherapy was not done at the discretion of the investigator if a patient had leukopenia.
The median duration of remission was not reached, and the persistence of tisagenlecleucel in the blood was observed for as long as 20 months.
“The remissions were durable, with a 6-month relapse-free survival rate of 80%,” the investigators wrote. “The durability of the clinical response was associated with persistence of tisagenlecleucel in peripheral blood and with persistent B-cell aplasia.”
The phase 1 study of tisagenlecleucel infusion therapy for younger patients with B-cell ALL showed the toxic nature of the therapy, so investigators were not surprised by the safety data they found. Nearly three-quarters of patients who were evaluated in the study experienced a grade 3 or 4 tisagenlecleucel-related adverse event. Cytokine release syndrome occurred in 77% of patients.
Previously reported data regarding anti-CD19 CAR T-cell therapy for ALL came from single-center studies where manufacturing occurred on site, but the current study employed a global, multicenter supply chain, according to the investigators.
“The toxicity and efficacy of tisagenlecleucel [in this study] were consistent with those in the single-center study, and the feasibility of a global supply chain was demonstrated,” they wrote. “Because this study used cryopreserved leukapheresis product, it did not require fresh product and an open manufacture slot for enrollment.”
This research was sponsored and designed by Novartis Pharmaceuticals. Dr. Maude reported having received personal fees from Novartis as well as grant funding from St. Baldrick’s Foundation.
SOURCE: Maude SL, et al. N Engl J Med. 2018;378:439-48.
Tisagenlecleucel was associated with durable remission and long-term persistence for younger patients with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL), according to the results of a multicenter, multicontinent, phase 2 trial published in the New England Journal of Medicine.
Shannon L. Maude, MD, PhD, of the Children’s Hospital of Philadelphia and her coauthors reported that the anti-CD19 chimeric antigen receptor (CAR) therapy was highly toxic, but the effects were usually mitigated. Additionally, the investigators showed feasibility of a global supply chain for distribution of the therapy.
The investigators evaluated data from 75 patients with at least 5% lymphoblasts in their bone marrow at the time of screening. Patients were aged 3 years or older at the time of screening but were no older than 21 years of age at the time of diagnosis.
For 50 patients evaluated at the interim analysis, the primary endpoint of overall remission at 3 months was met, and the overall remission rate was 82% (P less than .001).
An updated analysis showed that 81% of 75 patients who had at least 3 months of follow-up experienced overall remission (95% confidence interval, 71-89). A total of 45 of those patients experienced complete remission, and 16 had complete remission with incomplete hematologic recovery.
Event-free survival was experienced by 73% of patients at 6 months and 50% of patients at 12 months. Overall survival was 90% at 6 months and 76% at 12 months, the investigators reported.
Before tisagenlecleucel infusion, 96% of patients received lymphodepleting chemotherapy. The administration of chemotherapy was not done at the discretion of the investigator if a patient had leukopenia.
The median duration of remission was not reached, and the persistence of tisagenlecleucel in the blood was observed for as long as 20 months.
“The remissions were durable, with a 6-month relapse-free survival rate of 80%,” the investigators wrote. “The durability of the clinical response was associated with persistence of tisagenlecleucel in peripheral blood and with persistent B-cell aplasia.”
The phase 1 study of tisagenlecleucel infusion therapy for younger patients with B-cell ALL showed the toxic nature of the therapy, so investigators were not surprised by the safety data they found. Nearly three-quarters of patients who were evaluated in the study experienced a grade 3 or 4 tisagenlecleucel-related adverse event. Cytokine release syndrome occurred in 77% of patients.
Previously reported data regarding anti-CD19 CAR T-cell therapy for ALL came from single-center studies where manufacturing occurred on site, but the current study employed a global, multicenter supply chain, according to the investigators.
“The toxicity and efficacy of tisagenlecleucel [in this study] were consistent with those in the single-center study, and the feasibility of a global supply chain was demonstrated,” they wrote. “Because this study used cryopreserved leukapheresis product, it did not require fresh product and an open manufacture slot for enrollment.”
This research was sponsored and designed by Novartis Pharmaceuticals. Dr. Maude reported having received personal fees from Novartis as well as grant funding from St. Baldrick’s Foundation.
SOURCE: Maude SL, et al. N Engl J Med. 2018;378:439-48.
Key clinical point:
Major finding: The overall remission rate was 81% at 3 months, and 73% of patients experienced grade 3 or 4 adverse events.
Study details: A multicenter, phase 2 study of 75 patients.
Disclosures: Novartis designed and sponsored this research. Dr. Maude reported receiving fees from Novartis and grant funding from St. Baldrick’s Foundation.
Source: Maude SL et al. N Engl J Med. 2018;378:439-48.
Checkpoint inhibitors look safe in rheumatology patients
People with rheumatologic diseases and cancer appear to be at no higher risk of having an adverse event or disease flare if they receive checkpoint inhibitor therapy, compared with the general population, experience from the Mayo Clinic suggests.
In a brief report published in Arthritis and Rheumatology, a team from the Mayo Clinic in Rochester, Minn., reported on 16 patients with rheumatologic diseases who received cancer immunotherapy. They found that only a minority experienced a flare of their disease or another immune-related event.
The rate of severe immune-related adverse effects (IRAEs) with a single immune checkpoint inhibitor (ICI) has been reported to be less than 2% among the average population. However, less is known about patients with underlying rheumatologic disease, largely because initial trials of ICIs had excluded patients with autoimmune diseases for fear the treatment would induce a disease flare, the researchers noted.
Small studies have suggested that people with inflammatory arthritis or connective tissue diseases have higher rates of IRAEs with immunotherapy, but it is unclear how often these events represented flares of their disease or new autoimmune events, and whether the events had any predictive significance for cancer survival.
In this study, researchers performed a retrospective review of medical records and identified 16 patients with rheumatologic diseases who had received checkpoint inhibitor therapy at the Mayo Clinic between 2011 and 2016.
The most common rheumatologic diseases among the 16 patients were rheumatoid arthritis, polymyalgia rheumatica, Sjögren’s syndrome, and systemic lupus erythematosus, and the most common cancers were malignant melanoma, pulmonary malignancies, and non-Hodgkin lymphoma. Seven of the patients were receiving immunosuppressive therapy or glucocorticoids for their rheumatologic disease upon initiation of a checkpoint inhibitor.
Ten patients had received a prior disease-modifying antirheumatic drug, but only two patients were still taking this at the time of ICI initiation.
Results showed that six of the patients (38%) had an IRAE or flare of their rheumatologic disease, two were graded as mild. All of the patients responded well to glucocorticoids and discontinuation of therapy. The most common event was colitis and just one patient had a flare of rheumatologic disease.
“This is consistent with what is currently known about the management of IRAEs,” the research team wrote. “This study adds further support to the emerging notion that the rate of IRAEs is not necessarily higher in this group compared to the general population.”
The type and severity of rheumatologic disease may play an important role in both the risk of disease flare and IRAEs, a factor that they were unable to assess in the current study, the researchers wrote.
“Further large, prospective studies are needed to address the link between the type, severity, and concurrent rheumatologic disease activity on the risk of flare and IRAE. It is possible that patients with more severe or active disease are at higher risk for these complications,” they wrote.
While patients in the study did not appear to have significantly increased incidence or severity of adverse effects, the research team advised that “treatment decisions must factor in clinical judgement.”
They noted that some studies had proposed predictive biomarkers, pretreatment workup, and monitoring, but this advice was based on a small body of evidence.
“Larger, prospective studies will be necessary to validate these findings and establish evidence-based guidelines for appropriate identification and rating of the rheumatologic IRAEs as well as their treatment, such that patients can continue to receive potentially life-saving cancer treatments,” they wrote.
One of the researchers reported advisory board membership with Bristol-Myers Squibb.
SOURCE: Richter M et al. Arthritis Rheumatol. 2018 Jan 24. doi: 10.1002/art.40397.
People with rheumatologic diseases and cancer appear to be at no higher risk of having an adverse event or disease flare if they receive checkpoint inhibitor therapy, compared with the general population, experience from the Mayo Clinic suggests.
In a brief report published in Arthritis and Rheumatology, a team from the Mayo Clinic in Rochester, Minn., reported on 16 patients with rheumatologic diseases who received cancer immunotherapy. They found that only a minority experienced a flare of their disease or another immune-related event.
The rate of severe immune-related adverse effects (IRAEs) with a single immune checkpoint inhibitor (ICI) has been reported to be less than 2% among the average population. However, less is known about patients with underlying rheumatologic disease, largely because initial trials of ICIs had excluded patients with autoimmune diseases for fear the treatment would induce a disease flare, the researchers noted.
Small studies have suggested that people with inflammatory arthritis or connective tissue diseases have higher rates of IRAEs with immunotherapy, but it is unclear how often these events represented flares of their disease or new autoimmune events, and whether the events had any predictive significance for cancer survival.
In this study, researchers performed a retrospective review of medical records and identified 16 patients with rheumatologic diseases who had received checkpoint inhibitor therapy at the Mayo Clinic between 2011 and 2016.
The most common rheumatologic diseases among the 16 patients were rheumatoid arthritis, polymyalgia rheumatica, Sjögren’s syndrome, and systemic lupus erythematosus, and the most common cancers were malignant melanoma, pulmonary malignancies, and non-Hodgkin lymphoma. Seven of the patients were receiving immunosuppressive therapy or glucocorticoids for their rheumatologic disease upon initiation of a checkpoint inhibitor.
Ten patients had received a prior disease-modifying antirheumatic drug, but only two patients were still taking this at the time of ICI initiation.
Results showed that six of the patients (38%) had an IRAE or flare of their rheumatologic disease, two were graded as mild. All of the patients responded well to glucocorticoids and discontinuation of therapy. The most common event was colitis and just one patient had a flare of rheumatologic disease.
“This is consistent with what is currently known about the management of IRAEs,” the research team wrote. “This study adds further support to the emerging notion that the rate of IRAEs is not necessarily higher in this group compared to the general population.”
The type and severity of rheumatologic disease may play an important role in both the risk of disease flare and IRAEs, a factor that they were unable to assess in the current study, the researchers wrote.
“Further large, prospective studies are needed to address the link between the type, severity, and concurrent rheumatologic disease activity on the risk of flare and IRAE. It is possible that patients with more severe or active disease are at higher risk for these complications,” they wrote.
While patients in the study did not appear to have significantly increased incidence or severity of adverse effects, the research team advised that “treatment decisions must factor in clinical judgement.”
They noted that some studies had proposed predictive biomarkers, pretreatment workup, and monitoring, but this advice was based on a small body of evidence.
“Larger, prospective studies will be necessary to validate these findings and establish evidence-based guidelines for appropriate identification and rating of the rheumatologic IRAEs as well as their treatment, such that patients can continue to receive potentially life-saving cancer treatments,” they wrote.
One of the researchers reported advisory board membership with Bristol-Myers Squibb.
SOURCE: Richter M et al. Arthritis Rheumatol. 2018 Jan 24. doi: 10.1002/art.40397.
People with rheumatologic diseases and cancer appear to be at no higher risk of having an adverse event or disease flare if they receive checkpoint inhibitor therapy, compared with the general population, experience from the Mayo Clinic suggests.
In a brief report published in Arthritis and Rheumatology, a team from the Mayo Clinic in Rochester, Minn., reported on 16 patients with rheumatologic diseases who received cancer immunotherapy. They found that only a minority experienced a flare of their disease or another immune-related event.
The rate of severe immune-related adverse effects (IRAEs) with a single immune checkpoint inhibitor (ICI) has been reported to be less than 2% among the average population. However, less is known about patients with underlying rheumatologic disease, largely because initial trials of ICIs had excluded patients with autoimmune diseases for fear the treatment would induce a disease flare, the researchers noted.
Small studies have suggested that people with inflammatory arthritis or connective tissue diseases have higher rates of IRAEs with immunotherapy, but it is unclear how often these events represented flares of their disease or new autoimmune events, and whether the events had any predictive significance for cancer survival.
In this study, researchers performed a retrospective review of medical records and identified 16 patients with rheumatologic diseases who had received checkpoint inhibitor therapy at the Mayo Clinic between 2011 and 2016.
The most common rheumatologic diseases among the 16 patients were rheumatoid arthritis, polymyalgia rheumatica, Sjögren’s syndrome, and systemic lupus erythematosus, and the most common cancers were malignant melanoma, pulmonary malignancies, and non-Hodgkin lymphoma. Seven of the patients were receiving immunosuppressive therapy or glucocorticoids for their rheumatologic disease upon initiation of a checkpoint inhibitor.
Ten patients had received a prior disease-modifying antirheumatic drug, but only two patients were still taking this at the time of ICI initiation.
Results showed that six of the patients (38%) had an IRAE or flare of their rheumatologic disease, two were graded as mild. All of the patients responded well to glucocorticoids and discontinuation of therapy. The most common event was colitis and just one patient had a flare of rheumatologic disease.
“This is consistent with what is currently known about the management of IRAEs,” the research team wrote. “This study adds further support to the emerging notion that the rate of IRAEs is not necessarily higher in this group compared to the general population.”
The type and severity of rheumatologic disease may play an important role in both the risk of disease flare and IRAEs, a factor that they were unable to assess in the current study, the researchers wrote.
“Further large, prospective studies are needed to address the link between the type, severity, and concurrent rheumatologic disease activity on the risk of flare and IRAE. It is possible that patients with more severe or active disease are at higher risk for these complications,” they wrote.
While patients in the study did not appear to have significantly increased incidence or severity of adverse effects, the research team advised that “treatment decisions must factor in clinical judgement.”
They noted that some studies had proposed predictive biomarkers, pretreatment workup, and monitoring, but this advice was based on a small body of evidence.
“Larger, prospective studies will be necessary to validate these findings and establish evidence-based guidelines for appropriate identification and rating of the rheumatologic IRAEs as well as their treatment, such that patients can continue to receive potentially life-saving cancer treatments,” they wrote.
One of the researchers reported advisory board membership with Bristol-Myers Squibb.
SOURCE: Richter M et al. Arthritis Rheumatol. 2018 Jan 24. doi: 10.1002/art.40397.
FROM ARTHRITIS AND RHEUMATOLOGY
Key clinical point:
Major finding: Six of 16 patients (38%) with rheumatologic disease and cancer had an IRAE or flare of their rheumatologic disease.
Study details: A single-center, retrospective records review to identify patients with rheumatologic diseases who had received checkpoint inhibitor therapy at Mayo Clinic between 2011 and 2016.
Disclosures: One of the authors reported advisory board membership with Bristol-Myers Squibb.
Source: Richter M et al. Arthritis Rheumatol. 2018 Jan 24. doi: 10.1002/art.40397.
A mismatched haplotype may improve outcomes in second BMT
Undergoing a second allogeneic bone or marrow transplant (BMT) is a feasible strategy for patients who have relapsed after their first transplantation, according to findings reported in Biology of Blood and Marrow Transplantation.
Specifically, there may be an advantage to selecting a donor with a new mismatched haplotype after a failed HLA-matched allograft. Among patients who had a second graft transplanted that did not harbor a new mismatched haplotype, median survival was 552 days (95% CI, 376-2,950+). But median survival was not reached in the cohort whose allograft contained a new mismatched haplotype (hazard ratio, 0.36; 95% confidence interval, 0.14-0.9; P = .02).
“In the case of a failed HLA-matched transplantation, when haplotype loss would not be favored through selective pressure and thus would not provide a mechanism of relapse, switching to a different haploidentical donor may be beneficial by increasing major histocompatibility mismatch,” Philip H. Imus, MD, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, and colleagues wrote.
For this retrospective review, the researchers examined 40 consecutive patients who underwent a second BMT for a relapsed hematologic malignancy at a single institution between 2005 and 2014. In all, 21 patients had their first allograft from a haploidentical donor, with 14 of these patients receiving a second haploidentical transplant (8 from a donor sharing the same shared haplotype as the first donor and 6 from a second donor sharing the other haplotype).
The other 19 patients received their first allograft from a fully matched donor, with 14 patients receiving a haploidentical allograft at the second transplantation and 5 receiving a second matched allograft. Overall, a total of 20 patients had a second allograft with a new mismatched haplotype.
The median overall survival for the six patients who were retransplantated with an HLA-haploidentical donor sharing the different haplotype from the first haploidentical donor had not been reached, compared with 502 days (95% CI, 317-2,950+) for the eight patients with a second haplo allograft that shared the same haplotype (HR, 0.37; 95% CI, 0.08 to 1.7; P = .16).
The median event free survival was also superior among those retransplanted with a new mismatched haplotype (not reached vs. 401 days; HR, .50; 95% CI, .22-1.14, P = .09).
A higher risk of mortality was observed in patients who had relapsed within 6 months of their first transplantation (HR, 2.49; 95% CI, 1.0-6.2; P = .07), as well as in those who had progressive or refractory disease prior to their second alloBMT (HR, 2.56; 95% CI, 1.03-6.25; P = .06).
“For patients who relapse more than 6 months after a BMT and who achieve remission with salvage therapy, results are very encouraging,” the researchers wrote. “Although a randomized trial to study selecting donors with a new mismatched haplotype may be impractical, larger numbers should provide additional information on the effectiveness of such an approach.”
The researchers reported having no financial disclosures.
SOURCE: Imus P et al. Biol Blood Marrow Transplant. 2017;23:1887-94.
Undergoing a second allogeneic bone or marrow transplant (BMT) is a feasible strategy for patients who have relapsed after their first transplantation, according to findings reported in Biology of Blood and Marrow Transplantation.
Specifically, there may be an advantage to selecting a donor with a new mismatched haplotype after a failed HLA-matched allograft. Among patients who had a second graft transplanted that did not harbor a new mismatched haplotype, median survival was 552 days (95% CI, 376-2,950+). But median survival was not reached in the cohort whose allograft contained a new mismatched haplotype (hazard ratio, 0.36; 95% confidence interval, 0.14-0.9; P = .02).
“In the case of a failed HLA-matched transplantation, when haplotype loss would not be favored through selective pressure and thus would not provide a mechanism of relapse, switching to a different haploidentical donor may be beneficial by increasing major histocompatibility mismatch,” Philip H. Imus, MD, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, and colleagues wrote.
For this retrospective review, the researchers examined 40 consecutive patients who underwent a second BMT for a relapsed hematologic malignancy at a single institution between 2005 and 2014. In all, 21 patients had their first allograft from a haploidentical donor, with 14 of these patients receiving a second haploidentical transplant (8 from a donor sharing the same shared haplotype as the first donor and 6 from a second donor sharing the other haplotype).
The other 19 patients received their first allograft from a fully matched donor, with 14 patients receiving a haploidentical allograft at the second transplantation and 5 receiving a second matched allograft. Overall, a total of 20 patients had a second allograft with a new mismatched haplotype.
The median overall survival for the six patients who were retransplantated with an HLA-haploidentical donor sharing the different haplotype from the first haploidentical donor had not been reached, compared with 502 days (95% CI, 317-2,950+) for the eight patients with a second haplo allograft that shared the same haplotype (HR, 0.37; 95% CI, 0.08 to 1.7; P = .16).
The median event free survival was also superior among those retransplanted with a new mismatched haplotype (not reached vs. 401 days; HR, .50; 95% CI, .22-1.14, P = .09).
A higher risk of mortality was observed in patients who had relapsed within 6 months of their first transplantation (HR, 2.49; 95% CI, 1.0-6.2; P = .07), as well as in those who had progressive or refractory disease prior to their second alloBMT (HR, 2.56; 95% CI, 1.03-6.25; P = .06).
“For patients who relapse more than 6 months after a BMT and who achieve remission with salvage therapy, results are very encouraging,” the researchers wrote. “Although a randomized trial to study selecting donors with a new mismatched haplotype may be impractical, larger numbers should provide additional information on the effectiveness of such an approach.”
The researchers reported having no financial disclosures.
SOURCE: Imus P et al. Biol Blood Marrow Transplant. 2017;23:1887-94.
Undergoing a second allogeneic bone or marrow transplant (BMT) is a feasible strategy for patients who have relapsed after their first transplantation, according to findings reported in Biology of Blood and Marrow Transplantation.
Specifically, there may be an advantage to selecting a donor with a new mismatched haplotype after a failed HLA-matched allograft. Among patients who had a second graft transplanted that did not harbor a new mismatched haplotype, median survival was 552 days (95% CI, 376-2,950+). But median survival was not reached in the cohort whose allograft contained a new mismatched haplotype (hazard ratio, 0.36; 95% confidence interval, 0.14-0.9; P = .02).
“In the case of a failed HLA-matched transplantation, when haplotype loss would not be favored through selective pressure and thus would not provide a mechanism of relapse, switching to a different haploidentical donor may be beneficial by increasing major histocompatibility mismatch,” Philip H. Imus, MD, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, and colleagues wrote.
For this retrospective review, the researchers examined 40 consecutive patients who underwent a second BMT for a relapsed hematologic malignancy at a single institution between 2005 and 2014. In all, 21 patients had their first allograft from a haploidentical donor, with 14 of these patients receiving a second haploidentical transplant (8 from a donor sharing the same shared haplotype as the first donor and 6 from a second donor sharing the other haplotype).
The other 19 patients received their first allograft from a fully matched donor, with 14 patients receiving a haploidentical allograft at the second transplantation and 5 receiving a second matched allograft. Overall, a total of 20 patients had a second allograft with a new mismatched haplotype.
The median overall survival for the six patients who were retransplantated with an HLA-haploidentical donor sharing the different haplotype from the first haploidentical donor had not been reached, compared with 502 days (95% CI, 317-2,950+) for the eight patients with a second haplo allograft that shared the same haplotype (HR, 0.37; 95% CI, 0.08 to 1.7; P = .16).
The median event free survival was also superior among those retransplanted with a new mismatched haplotype (not reached vs. 401 days; HR, .50; 95% CI, .22-1.14, P = .09).
A higher risk of mortality was observed in patients who had relapsed within 6 months of their first transplantation (HR, 2.49; 95% CI, 1.0-6.2; P = .07), as well as in those who had progressive or refractory disease prior to their second alloBMT (HR, 2.56; 95% CI, 1.03-6.25; P = .06).
“For patients who relapse more than 6 months after a BMT and who achieve remission with salvage therapy, results are very encouraging,” the researchers wrote. “Although a randomized trial to study selecting donors with a new mismatched haplotype may be impractical, larger numbers should provide additional information on the effectiveness of such an approach.”
The researchers reported having no financial disclosures.
SOURCE: Imus P et al. Biol Blood Marrow Transplant. 2017;23:1887-94.
FROM BIOLOGY OF BLOOD AND MARROW TRANSPLANTATION
Key clinical point:
Major finding: Median survival was 552 days (95% CI, 376-2,950+) for those without a new mismatched haplotype vs. not being reached for patients with a new mismatched haplotype on second transplant (HR, 0.36; 95% CI, 0.14-0.9; P = .02).
Study details: Retrospective review in a single institution that included 40 patients who underwent a second BMT between 2005 and 2014.
Disclosures: The researchers reported having no financial disclosures.
Source: Imus P et al. Biol Blood Marrow Transplant. 2017;23:1887-94.
Autologous stem-cell transplantation for scleroderma beats cyclophosphamide in long term
with improved event-free and overall survival over intravenous cyclophosphamide, according to Keith M. Sullivan, MD, and his associates.
A total of 75 patients with severe scleroderma (diffuse cutaneous systemic sclerosis) underwent randomization to open-label treatment with either transplantation or intravenous cyclophosphamide at an initial dose of 500 mg/m2 of body-surface area, followed by 11 monthly infusions of 750 mg/m2 with mesna prophylaxis.
Additionally in the per-protocol population, the rate of event-free survival at 54 months was 79% in the transplantation group and 50% in the cyclophosphamide group, while the Kaplan-Meier estimated rate of event-free survival at 72 months was 74% in the transplantation group and 47% in the cyclophosphamide group (P = .03), and the rate of overall survival was 86% and 51% (P = .02). Also, in the per-protocol population at 54 months, the percentage of participants who had initiated disease-modifying antirheumatic drugs was lower in the transplantation group than in the cyclophosphamide group (9% vs. 44%; P = .001).
The researchers noted that 21 deaths occurred over a period of 72 months, including 7 participants in the transplantation group, of whom 3 died without receiving a transplant. The percentage of participants who had serious adverse events in the 72-month period was lower in the cyclophosphamide group than in the transplantation group (51% vs. 74%).
“In conclusion, at 54 months after randomization, myeloablative CD34-positive selected autologous hematopoietic stem-cell transplantation resulted in significantly better clinical outcomes than 12 months of cyclophosphamide,” the researchers wrote. “Although there was greater hematopoietic toxicity and an unquantified risk of second cancers from exposure to total body irradiation, toxic effects should be weighed against the beneficial results of treatment and the seriousness of the underlying disease.”
with improved event-free and overall survival over intravenous cyclophosphamide, according to Keith M. Sullivan, MD, and his associates.
A total of 75 patients with severe scleroderma (diffuse cutaneous systemic sclerosis) underwent randomization to open-label treatment with either transplantation or intravenous cyclophosphamide at an initial dose of 500 mg/m2 of body-surface area, followed by 11 monthly infusions of 750 mg/m2 with mesna prophylaxis.
Additionally in the per-protocol population, the rate of event-free survival at 54 months was 79% in the transplantation group and 50% in the cyclophosphamide group, while the Kaplan-Meier estimated rate of event-free survival at 72 months was 74% in the transplantation group and 47% in the cyclophosphamide group (P = .03), and the rate of overall survival was 86% and 51% (P = .02). Also, in the per-protocol population at 54 months, the percentage of participants who had initiated disease-modifying antirheumatic drugs was lower in the transplantation group than in the cyclophosphamide group (9% vs. 44%; P = .001).
The researchers noted that 21 deaths occurred over a period of 72 months, including 7 participants in the transplantation group, of whom 3 died without receiving a transplant. The percentage of participants who had serious adverse events in the 72-month period was lower in the cyclophosphamide group than in the transplantation group (51% vs. 74%).
“In conclusion, at 54 months after randomization, myeloablative CD34-positive selected autologous hematopoietic stem-cell transplantation resulted in significantly better clinical outcomes than 12 months of cyclophosphamide,” the researchers wrote. “Although there was greater hematopoietic toxicity and an unquantified risk of second cancers from exposure to total body irradiation, toxic effects should be weighed against the beneficial results of treatment and the seriousness of the underlying disease.”
with improved event-free and overall survival over intravenous cyclophosphamide, according to Keith M. Sullivan, MD, and his associates.
A total of 75 patients with severe scleroderma (diffuse cutaneous systemic sclerosis) underwent randomization to open-label treatment with either transplantation or intravenous cyclophosphamide at an initial dose of 500 mg/m2 of body-surface area, followed by 11 monthly infusions of 750 mg/m2 with mesna prophylaxis.
Additionally in the per-protocol population, the rate of event-free survival at 54 months was 79% in the transplantation group and 50% in the cyclophosphamide group, while the Kaplan-Meier estimated rate of event-free survival at 72 months was 74% in the transplantation group and 47% in the cyclophosphamide group (P = .03), and the rate of overall survival was 86% and 51% (P = .02). Also, in the per-protocol population at 54 months, the percentage of participants who had initiated disease-modifying antirheumatic drugs was lower in the transplantation group than in the cyclophosphamide group (9% vs. 44%; P = .001).
The researchers noted that 21 deaths occurred over a period of 72 months, including 7 participants in the transplantation group, of whom 3 died without receiving a transplant. The percentage of participants who had serious adverse events in the 72-month period was lower in the cyclophosphamide group than in the transplantation group (51% vs. 74%).
“In conclusion, at 54 months after randomization, myeloablative CD34-positive selected autologous hematopoietic stem-cell transplantation resulted in significantly better clinical outcomes than 12 months of cyclophosphamide,” the researchers wrote. “Although there was greater hematopoietic toxicity and an unquantified risk of second cancers from exposure to total body irradiation, toxic effects should be weighed against the beneficial results of treatment and the seriousness of the underlying disease.”
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
How to manage cytokine release syndrome
ATLANTA – Closely monitoring for cytokine release syndrome (CRS) and starting anticytokine therapy early can prevent life-threatening organ toxicities in recipients of chimeric antigen receptor (CAR) T-cell therapy, according to Daniel W. Lee III, MD.
There’s no evidence that early anticytokine therapy impairs antitumor response, he noted. “If you wait to give anticytokine therapy until a patient is intubated, it’s too late,” Dr. Lee said at the annual meeting of the American Society of Hematology. “If you wait until a patient has been hypotensive for days, it’s probably too late. If you intervene earlier, you can avoid intubation.”
Treating CRS is a clinical decision that shouldn’t hinge on cytokine levels, according to Dr. Lee. He and his colleagues base treatment on their revised severity grading assessment, which spans mild, moderate, severe, and life-threatening syndromes (Blood. 2014;124:188-95).
Using a consistent CRS severity grading system enables physicians to treat rationally across trials and CAR T-cell therapies, he said. His system defines grade 1 CRS as flu-like symptoms and fever up to 41.5 degrees Celsius. Patients with grade 1 CRS should receive antipyretics and analgesia as needed and should be monitored closely for infections and fluid imbalances, Dr. Lee said.
Hypotension signifies progression beyond grade 1 CRS. Affected patients should receive no more than two to three IV fluid boluses and then should “quickly move on to vasopressors,” such as norepinephrine, he emphasized.
His and his team implemented this important change after one of their patients, a 14-year-old boy with severe hypotensive grade 4 CRS, died of a cardiovascular event after receiving multiple IV fluid boluses. “We had not appreciated the extent of this patient’s ventricular strain,” Dr. Lee said. The patient was heavily pretreated and had an “extremely high disease burden” (more than 99% marrow involvement, hepatosplenomegaly, and pronounced blastic leukocytosis), which increased his risk of severe CRS, he noted. “Admittedly, we pushed the envelope a little bit, and we learned you should start anticytokine therapies much earlier. Earlier seems to be better, although we do not yet know if prophylactic tocilizumab or corticosteroids can prevent CRS symptoms before they start.”
For hypotensive patients on pressors, Dr. Lee recommends vigilant supportive care and daily echocardiograms to monitor ejection fraction and ventricular wall mobility. His system defines grade 2 CRS as hypotension responsive to one low-dose pressor or to fluid therapy and hypoxia responsive to less than 40% oxygen therapy. Patients with grade 2 CRS who also have comorbidities should receive tocilizumab – with or without corticosteroids – both of which “remain the standard of care for managing CRS,” he said. Severe CRS often stems from supraphysiologic release of interleukin 6, which induces not only classic IL-6 signaling but also proinflammatory trans-signaling across many cell types. Tocilizumab reverses this process by binding and blocking the IL-6 receptor, Dr. Lee noted.
Patients with grade 3 CRS have hypotension requiring multiple or high-dose pressors and hypoxia requiring at least 40% oxygen therapy. These patients have grade 3 organ toxicity and grade 4 transaminitis, Dr. Lee said. Even if they lack comorbidities, they need vigilant supportive care, tocilizumab, and possibly corticosteroids, he added. The ultimate goal is to avoid grade 4 CRS, he said, which involves grade 4 organ toxicity, requires mechanical ventilation, and yields a poor prognosis despite vigilant supportive care, tocilizumab, and corticosteroids.
Dr. Lee reported having no relevant conflicts of interest.
ATLANTA – Closely monitoring for cytokine release syndrome (CRS) and starting anticytokine therapy early can prevent life-threatening organ toxicities in recipients of chimeric antigen receptor (CAR) T-cell therapy, according to Daniel W. Lee III, MD.
There’s no evidence that early anticytokine therapy impairs antitumor response, he noted. “If you wait to give anticytokine therapy until a patient is intubated, it’s too late,” Dr. Lee said at the annual meeting of the American Society of Hematology. “If you wait until a patient has been hypotensive for days, it’s probably too late. If you intervene earlier, you can avoid intubation.”
Treating CRS is a clinical decision that shouldn’t hinge on cytokine levels, according to Dr. Lee. He and his colleagues base treatment on their revised severity grading assessment, which spans mild, moderate, severe, and life-threatening syndromes (Blood. 2014;124:188-95).
Using a consistent CRS severity grading system enables physicians to treat rationally across trials and CAR T-cell therapies, he said. His system defines grade 1 CRS as flu-like symptoms and fever up to 41.5 degrees Celsius. Patients with grade 1 CRS should receive antipyretics and analgesia as needed and should be monitored closely for infections and fluid imbalances, Dr. Lee said.
Hypotension signifies progression beyond grade 1 CRS. Affected patients should receive no more than two to three IV fluid boluses and then should “quickly move on to vasopressors,” such as norepinephrine, he emphasized.
His and his team implemented this important change after one of their patients, a 14-year-old boy with severe hypotensive grade 4 CRS, died of a cardiovascular event after receiving multiple IV fluid boluses. “We had not appreciated the extent of this patient’s ventricular strain,” Dr. Lee said. The patient was heavily pretreated and had an “extremely high disease burden” (more than 99% marrow involvement, hepatosplenomegaly, and pronounced blastic leukocytosis), which increased his risk of severe CRS, he noted. “Admittedly, we pushed the envelope a little bit, and we learned you should start anticytokine therapies much earlier. Earlier seems to be better, although we do not yet know if prophylactic tocilizumab or corticosteroids can prevent CRS symptoms before they start.”
For hypotensive patients on pressors, Dr. Lee recommends vigilant supportive care and daily echocardiograms to monitor ejection fraction and ventricular wall mobility. His system defines grade 2 CRS as hypotension responsive to one low-dose pressor or to fluid therapy and hypoxia responsive to less than 40% oxygen therapy. Patients with grade 2 CRS who also have comorbidities should receive tocilizumab – with or without corticosteroids – both of which “remain the standard of care for managing CRS,” he said. Severe CRS often stems from supraphysiologic release of interleukin 6, which induces not only classic IL-6 signaling but also proinflammatory trans-signaling across many cell types. Tocilizumab reverses this process by binding and blocking the IL-6 receptor, Dr. Lee noted.
Patients with grade 3 CRS have hypotension requiring multiple or high-dose pressors and hypoxia requiring at least 40% oxygen therapy. These patients have grade 3 organ toxicity and grade 4 transaminitis, Dr. Lee said. Even if they lack comorbidities, they need vigilant supportive care, tocilizumab, and possibly corticosteroids, he added. The ultimate goal is to avoid grade 4 CRS, he said, which involves grade 4 organ toxicity, requires mechanical ventilation, and yields a poor prognosis despite vigilant supportive care, tocilizumab, and corticosteroids.
Dr. Lee reported having no relevant conflicts of interest.
ATLANTA – Closely monitoring for cytokine release syndrome (CRS) and starting anticytokine therapy early can prevent life-threatening organ toxicities in recipients of chimeric antigen receptor (CAR) T-cell therapy, according to Daniel W. Lee III, MD.
There’s no evidence that early anticytokine therapy impairs antitumor response, he noted. “If you wait to give anticytokine therapy until a patient is intubated, it’s too late,” Dr. Lee said at the annual meeting of the American Society of Hematology. “If you wait until a patient has been hypotensive for days, it’s probably too late. If you intervene earlier, you can avoid intubation.”
Treating CRS is a clinical decision that shouldn’t hinge on cytokine levels, according to Dr. Lee. He and his colleagues base treatment on their revised severity grading assessment, which spans mild, moderate, severe, and life-threatening syndromes (Blood. 2014;124:188-95).
Using a consistent CRS severity grading system enables physicians to treat rationally across trials and CAR T-cell therapies, he said. His system defines grade 1 CRS as flu-like symptoms and fever up to 41.5 degrees Celsius. Patients with grade 1 CRS should receive antipyretics and analgesia as needed and should be monitored closely for infections and fluid imbalances, Dr. Lee said.
Hypotension signifies progression beyond grade 1 CRS. Affected patients should receive no more than two to three IV fluid boluses and then should “quickly move on to vasopressors,” such as norepinephrine, he emphasized.
His and his team implemented this important change after one of their patients, a 14-year-old boy with severe hypotensive grade 4 CRS, died of a cardiovascular event after receiving multiple IV fluid boluses. “We had not appreciated the extent of this patient’s ventricular strain,” Dr. Lee said. The patient was heavily pretreated and had an “extremely high disease burden” (more than 99% marrow involvement, hepatosplenomegaly, and pronounced blastic leukocytosis), which increased his risk of severe CRS, he noted. “Admittedly, we pushed the envelope a little bit, and we learned you should start anticytokine therapies much earlier. Earlier seems to be better, although we do not yet know if prophylactic tocilizumab or corticosteroids can prevent CRS symptoms before they start.”
For hypotensive patients on pressors, Dr. Lee recommends vigilant supportive care and daily echocardiograms to monitor ejection fraction and ventricular wall mobility. His system defines grade 2 CRS as hypotension responsive to one low-dose pressor or to fluid therapy and hypoxia responsive to less than 40% oxygen therapy. Patients with grade 2 CRS who also have comorbidities should receive tocilizumab – with or without corticosteroids – both of which “remain the standard of care for managing CRS,” he said. Severe CRS often stems from supraphysiologic release of interleukin 6, which induces not only classic IL-6 signaling but also proinflammatory trans-signaling across many cell types. Tocilizumab reverses this process by binding and blocking the IL-6 receptor, Dr. Lee noted.
Patients with grade 3 CRS have hypotension requiring multiple or high-dose pressors and hypoxia requiring at least 40% oxygen therapy. These patients have grade 3 organ toxicity and grade 4 transaminitis, Dr. Lee said. Even if they lack comorbidities, they need vigilant supportive care, tocilizumab, and possibly corticosteroids, he added. The ultimate goal is to avoid grade 4 CRS, he said, which involves grade 4 organ toxicity, requires mechanical ventilation, and yields a poor prognosis despite vigilant supportive care, tocilizumab, and corticosteroids.
Dr. Lee reported having no relevant conflicts of interest.
EXPERT ANALYSIS FROM ASH 2017
Updated ZUMA-1 data show durable CAR-T responses in B-cell lymphomas
ATLANTA – More than one-third of patients with refractory large B-cell lymphomas treated with the chimeric antigen receptor (CAR) T-cell product axicabtagene ciloleucel (Yescarta), often called axi-cel, had durable responses, with some patients having complete responses lasting more than 1 year after a single infusion, according to investigators in the ZUMA-1 trial.
Updated combined phase 1 and phase 2 results in 108 patients with diffuse large B-cell lymphoma (DLBCL), primary mediastinal B-cell lymphoma (PMBCL), or transformed follicular lymphoma (TFL) showed an objective response rate (ORR) of 82%, including 58% complete responses, after a median follow-up of 15.4 months, reported Sattva S. Neelapu, MD, from the University of Texas MD Anderson Cancer Center in Houston.
“Axi-cel is highly effective in patients with large B-cell lymphoma who otherwise have no curative treatment options,” he said in a briefing at the annual meeting of the American Society of Hematology, prior to his presentation of the data in an oral session.
The trial results were also published simultaneously in the New England Journal of Medicine.As previously reported, in the multicenter phase 2 ZUMA-1 trial, 111 patients with treatment refractory DLBCL, PMBCL, or TFL were enrolled and treated with axi-cel at a target dose of 2 x 106 cells/kg, following a conditioning regimen with low-dose cyclophosphamide and fludarabine.
The median patient age was 58 years. Patients had stage III or IV disease, 48% had International Prognostic Index scores of 3-4, 76% had disease that was refractory to third-line therapies or beyond, and 21% had disease that relapsed within 12 months of an autologous bone marrow transplant
Axi-cel was successfully manufactured with sufficient cells for transfusion in all but one of the 111 patients, and 101 patients eventually received infusions in phase 2 (modified intention-to-treat population). The average turnaround time from apheresis to the clinical site was 17 days.
Dr. Neelapu also presented data on seven patients enrolled in phase 1; the data were combined with the phase 2 results for an updated analysis of those patients who had at least 1 year of follow-up.
The phase 2 trial met its primary endpoint at the time of the primary analysis, with an 82% ORR, consisting of 54% complete responses and 28% partial responses at a median follow-up of 8.7 months.
In the updated analysis, the ORR and respective remission rates were 82%, 58%, and 34%, at a median of 15.4 months follow-up.
The median duration of response in the updated analysis was 11.1 months. The median duration of complete responses had not been reached at the time of data cutoff in August 2017. The median duration of partial responses was 1.9 months.
At the 15.4-month mark, 42% of patients remained free of disease progression, and 56% were alive, with the median overall survival not yet reached.
The treatment had generally acceptable toxicities, with only 13% of patients in phase 2 experiencing grade 3 or greater cytokine release syndrome (CRS), although one patient with CRS died from hemophagocytic lymphohistiocytosis, and one with CRS died from cardiac arrest. Grade 3 or greater neurologic events occurred in 28% of patients, and included encephalopathy, confusional state, aphasia, and somnolence.
The events were generally reversible, and the rates of each declined over time. The use of tocilizumab or steroids to control adverse events did not have a negative effect on responses.
Since the primary analysis with at least 6 months of follow-up, there have been no new axi-cel–related cases of CRS, neurologic events, or deaths.
Dr. Neelapu also presented safety data on serious adverse events occurring more than 6 months after therapy in 10 patients who developed symptoms after the data cutoff.
Grade 3 events in these patients included lung infection, recurrent upper respiratory viral infection, and rotavirus infection, pneumonias, atrial fibrillation with rapid ventricular response, lung infection, febrile neutropenia, and influenza B infection. One patient had grade 4 sepsis.
In an editorial accompanying the study in the New England Journal of Medicine, Eric Tran, PhD, and Walter J. Urba, MD, PhD, from the Earle A. Chiles Research Institute and the Providence Portland (Ore.) Medical Center, and Dan L. Longo, MD, deputy editor of the journal, praised ZUMA-1 as “a landmark study because it involved 22 institutions and showed that a personalized gene-engineered T-cell product could be rapidly generated at a centralized cell-manufacturing facility and safely administered to patients at transplantation-capable medical centers.”
They noted, however, that about half of all patients with relapsed or refractory large B-cell lymphomas will not have durable responses to CAR T-cell therapy directed against CD19, and that new strategies will be needed to improve responses (N Engl J Med. 2017 Dec 10; doi: 10.1056/NEJMe1714680).
In the question and answer session at the end of the briefing, Dr. Neelapu said the preliminary observations of mechanisms of relapse or disease progression in some patients may be related to the loss of the CD19 antigen, which occurs in about one-third of patients who experience relapse, and to high expression of the programmed death ligand-1, which can potentially inhibit CAR-T cell function. A clinical trial is currently underway to evaluate potential strategies for improving response rates to CAR-T therapies, he said.
ZUMA-1 is supported by Kite Pharma and the Leukemia and Lymphoma Society Therapy Acceleration Program. Dr. Neelapu reported receiving advisory board fees from the company. Myriad coauthors also reported financial relationship with multiple companies.
SOURCE: Neelapu S et al. ASH 2017 Abstract 578.
ATLANTA – More than one-third of patients with refractory large B-cell lymphomas treated with the chimeric antigen receptor (CAR) T-cell product axicabtagene ciloleucel (Yescarta), often called axi-cel, had durable responses, with some patients having complete responses lasting more than 1 year after a single infusion, according to investigators in the ZUMA-1 trial.
Updated combined phase 1 and phase 2 results in 108 patients with diffuse large B-cell lymphoma (DLBCL), primary mediastinal B-cell lymphoma (PMBCL), or transformed follicular lymphoma (TFL) showed an objective response rate (ORR) of 82%, including 58% complete responses, after a median follow-up of 15.4 months, reported Sattva S. Neelapu, MD, from the University of Texas MD Anderson Cancer Center in Houston.
“Axi-cel is highly effective in patients with large B-cell lymphoma who otherwise have no curative treatment options,” he said in a briefing at the annual meeting of the American Society of Hematology, prior to his presentation of the data in an oral session.
The trial results were also published simultaneously in the New England Journal of Medicine.As previously reported, in the multicenter phase 2 ZUMA-1 trial, 111 patients with treatment refractory DLBCL, PMBCL, or TFL were enrolled and treated with axi-cel at a target dose of 2 x 106 cells/kg, following a conditioning regimen with low-dose cyclophosphamide and fludarabine.
The median patient age was 58 years. Patients had stage III or IV disease, 48% had International Prognostic Index scores of 3-4, 76% had disease that was refractory to third-line therapies or beyond, and 21% had disease that relapsed within 12 months of an autologous bone marrow transplant
Axi-cel was successfully manufactured with sufficient cells for transfusion in all but one of the 111 patients, and 101 patients eventually received infusions in phase 2 (modified intention-to-treat population). The average turnaround time from apheresis to the clinical site was 17 days.
Dr. Neelapu also presented data on seven patients enrolled in phase 1; the data were combined with the phase 2 results for an updated analysis of those patients who had at least 1 year of follow-up.
The phase 2 trial met its primary endpoint at the time of the primary analysis, with an 82% ORR, consisting of 54% complete responses and 28% partial responses at a median follow-up of 8.7 months.
In the updated analysis, the ORR and respective remission rates were 82%, 58%, and 34%, at a median of 15.4 months follow-up.
The median duration of response in the updated analysis was 11.1 months. The median duration of complete responses had not been reached at the time of data cutoff in August 2017. The median duration of partial responses was 1.9 months.
At the 15.4-month mark, 42% of patients remained free of disease progression, and 56% were alive, with the median overall survival not yet reached.
The treatment had generally acceptable toxicities, with only 13% of patients in phase 2 experiencing grade 3 or greater cytokine release syndrome (CRS), although one patient with CRS died from hemophagocytic lymphohistiocytosis, and one with CRS died from cardiac arrest. Grade 3 or greater neurologic events occurred in 28% of patients, and included encephalopathy, confusional state, aphasia, and somnolence.
The events were generally reversible, and the rates of each declined over time. The use of tocilizumab or steroids to control adverse events did not have a negative effect on responses.
Since the primary analysis with at least 6 months of follow-up, there have been no new axi-cel–related cases of CRS, neurologic events, or deaths.
Dr. Neelapu also presented safety data on serious adverse events occurring more than 6 months after therapy in 10 patients who developed symptoms after the data cutoff.
Grade 3 events in these patients included lung infection, recurrent upper respiratory viral infection, and rotavirus infection, pneumonias, atrial fibrillation with rapid ventricular response, lung infection, febrile neutropenia, and influenza B infection. One patient had grade 4 sepsis.
In an editorial accompanying the study in the New England Journal of Medicine, Eric Tran, PhD, and Walter J. Urba, MD, PhD, from the Earle A. Chiles Research Institute and the Providence Portland (Ore.) Medical Center, and Dan L. Longo, MD, deputy editor of the journal, praised ZUMA-1 as “a landmark study because it involved 22 institutions and showed that a personalized gene-engineered T-cell product could be rapidly generated at a centralized cell-manufacturing facility and safely administered to patients at transplantation-capable medical centers.”
They noted, however, that about half of all patients with relapsed or refractory large B-cell lymphomas will not have durable responses to CAR T-cell therapy directed against CD19, and that new strategies will be needed to improve responses (N Engl J Med. 2017 Dec 10; doi: 10.1056/NEJMe1714680).
In the question and answer session at the end of the briefing, Dr. Neelapu said the preliminary observations of mechanisms of relapse or disease progression in some patients may be related to the loss of the CD19 antigen, which occurs in about one-third of patients who experience relapse, and to high expression of the programmed death ligand-1, which can potentially inhibit CAR-T cell function. A clinical trial is currently underway to evaluate potential strategies for improving response rates to CAR-T therapies, he said.
ZUMA-1 is supported by Kite Pharma and the Leukemia and Lymphoma Society Therapy Acceleration Program. Dr. Neelapu reported receiving advisory board fees from the company. Myriad coauthors also reported financial relationship with multiple companies.
SOURCE: Neelapu S et al. ASH 2017 Abstract 578.
ATLANTA – More than one-third of patients with refractory large B-cell lymphomas treated with the chimeric antigen receptor (CAR) T-cell product axicabtagene ciloleucel (Yescarta), often called axi-cel, had durable responses, with some patients having complete responses lasting more than 1 year after a single infusion, according to investigators in the ZUMA-1 trial.
Updated combined phase 1 and phase 2 results in 108 patients with diffuse large B-cell lymphoma (DLBCL), primary mediastinal B-cell lymphoma (PMBCL), or transformed follicular lymphoma (TFL) showed an objective response rate (ORR) of 82%, including 58% complete responses, after a median follow-up of 15.4 months, reported Sattva S. Neelapu, MD, from the University of Texas MD Anderson Cancer Center in Houston.
“Axi-cel is highly effective in patients with large B-cell lymphoma who otherwise have no curative treatment options,” he said in a briefing at the annual meeting of the American Society of Hematology, prior to his presentation of the data in an oral session.
The trial results were also published simultaneously in the New England Journal of Medicine.As previously reported, in the multicenter phase 2 ZUMA-1 trial, 111 patients with treatment refractory DLBCL, PMBCL, or TFL were enrolled and treated with axi-cel at a target dose of 2 x 106 cells/kg, following a conditioning regimen with low-dose cyclophosphamide and fludarabine.
The median patient age was 58 years. Patients had stage III or IV disease, 48% had International Prognostic Index scores of 3-4, 76% had disease that was refractory to third-line therapies or beyond, and 21% had disease that relapsed within 12 months of an autologous bone marrow transplant
Axi-cel was successfully manufactured with sufficient cells for transfusion in all but one of the 111 patients, and 101 patients eventually received infusions in phase 2 (modified intention-to-treat population). The average turnaround time from apheresis to the clinical site was 17 days.
Dr. Neelapu also presented data on seven patients enrolled in phase 1; the data were combined with the phase 2 results for an updated analysis of those patients who had at least 1 year of follow-up.
The phase 2 trial met its primary endpoint at the time of the primary analysis, with an 82% ORR, consisting of 54% complete responses and 28% partial responses at a median follow-up of 8.7 months.
In the updated analysis, the ORR and respective remission rates were 82%, 58%, and 34%, at a median of 15.4 months follow-up.
The median duration of response in the updated analysis was 11.1 months. The median duration of complete responses had not been reached at the time of data cutoff in August 2017. The median duration of partial responses was 1.9 months.
At the 15.4-month mark, 42% of patients remained free of disease progression, and 56% were alive, with the median overall survival not yet reached.
The treatment had generally acceptable toxicities, with only 13% of patients in phase 2 experiencing grade 3 or greater cytokine release syndrome (CRS), although one patient with CRS died from hemophagocytic lymphohistiocytosis, and one with CRS died from cardiac arrest. Grade 3 or greater neurologic events occurred in 28% of patients, and included encephalopathy, confusional state, aphasia, and somnolence.
The events were generally reversible, and the rates of each declined over time. The use of tocilizumab or steroids to control adverse events did not have a negative effect on responses.
Since the primary analysis with at least 6 months of follow-up, there have been no new axi-cel–related cases of CRS, neurologic events, or deaths.
Dr. Neelapu also presented safety data on serious adverse events occurring more than 6 months after therapy in 10 patients who developed symptoms after the data cutoff.
Grade 3 events in these patients included lung infection, recurrent upper respiratory viral infection, and rotavirus infection, pneumonias, atrial fibrillation with rapid ventricular response, lung infection, febrile neutropenia, and influenza B infection. One patient had grade 4 sepsis.
In an editorial accompanying the study in the New England Journal of Medicine, Eric Tran, PhD, and Walter J. Urba, MD, PhD, from the Earle A. Chiles Research Institute and the Providence Portland (Ore.) Medical Center, and Dan L. Longo, MD, deputy editor of the journal, praised ZUMA-1 as “a landmark study because it involved 22 institutions and showed that a personalized gene-engineered T-cell product could be rapidly generated at a centralized cell-manufacturing facility and safely administered to patients at transplantation-capable medical centers.”
They noted, however, that about half of all patients with relapsed or refractory large B-cell lymphomas will not have durable responses to CAR T-cell therapy directed against CD19, and that new strategies will be needed to improve responses (N Engl J Med. 2017 Dec 10; doi: 10.1056/NEJMe1714680).
In the question and answer session at the end of the briefing, Dr. Neelapu said the preliminary observations of mechanisms of relapse or disease progression in some patients may be related to the loss of the CD19 antigen, which occurs in about one-third of patients who experience relapse, and to high expression of the programmed death ligand-1, which can potentially inhibit CAR-T cell function. A clinical trial is currently underway to evaluate potential strategies for improving response rates to CAR-T therapies, he said.
ZUMA-1 is supported by Kite Pharma and the Leukemia and Lymphoma Society Therapy Acceleration Program. Dr. Neelapu reported receiving advisory board fees from the company. Myriad coauthors also reported financial relationship with multiple companies.
SOURCE: Neelapu S et al. ASH 2017 Abstract 578.
REPORTING FROM ASH 2017
Key clinical point:.
Major finding: The objective response rate was 82%, including 58% complete responses at a median of 15.4 months of follow-up.
Data source: Update analysis of phase 1 and 2 data from the ZUMA-1 trial in 108 patients with large B-cell lymphomas.
Disclosures: ZUMA-1 is supported by Kite Pharma and the Leukemia and Lymphoma Society Therapy Acceleration Program. Dr. Neelapu reported receiving advisory board fees from the company. Myriad coauthors also reported financial relationship with multiple companies.
Source: Neelapu S et al. ASH 2017 Abstract 578