User login
Synthetic lethality: Triple combination is a viable strategy for B-cell malignancies
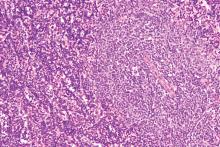
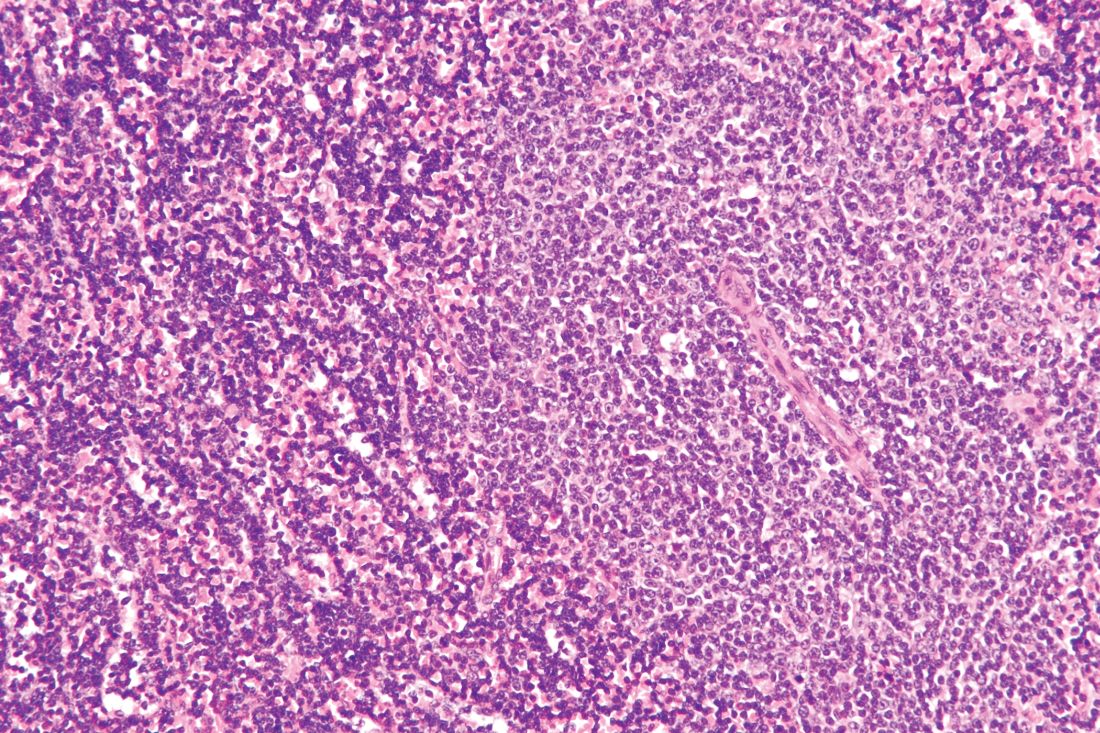
For B-cell malignancies, synthetic lethality is a viable treatment approach, according to preliminary clinical trial data with once-daily oral DTRM-555. The triple combination therapy, DTRM-555, combines a Bruton’s tyrosine kinase (BTK) inhibitor, a mammalian target of rapamycin (mTOR) inhibitor and pomalidomide, an immunomodulatory imide drug (IMiD), according to Anthony R. Mato, MD, in a presentation at the annual meeting of the American Society of Hematology, which was held virtually.
Richter’s transformation, a rare event
Dr. Mato’s phase 1 clinical trial included 13 patients with Richter’s transformation (RT) and 11 with diffuse large B-cell lymphoma (DLBCL). Richter’s transformation, a rare event occurring in 5%-7% of chronic lymphocytic leukemia (CLL) cases, has no clear standard of care and universally poor outcomes (overall survival, 3-12 months) once it becomes refractory to anthracycline-based chemotherapy, according to Dr. Mato.
Despite great progress in treating DLBCL, cure rates with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone), the standard of care, are in the 50%-60% range and much lower (30%-40%) with poor-risk features. Furthermore, most (60%-70%) patients receiving autologous stem cell transplant or CAR-T still require additional lines of therapy.
The “synthetic lethality” (SL) strategy, which has become a focus of cancer treatment in the last decade, identifies multiple disease primary aberrant and compensatory pathways and then inhibits them together in a manner lethal to cell survival. Preclinical studies have shown low doses of a BTK inhibitor/mTOR inhibitor/IMiD to synergistically kill malignant B cells. DTRM-555 is an optimized, oral, once-daily triplet combination of a novel and clinically differentiated irreversible BTK inhibitor (DTRM-12), everolimus and pomalidomide, Dr. Mato explained.
Individuals (38% women) included in the trial had a median of 2 (1-10) prior lines of therapy, with a CD20 monoclonal antibody as one of them in all cases, and 83% with R-CHOP. All patients had life expectancy >12 weeks, with 0-1 performance status and adequate organ and hematologic function.
DTRM-12 plasma concentrations, Dr. Mato noted, were unaffected by coadministration with everolimus with or without pomalidomide.
Manageable adverse events
Among adverse events, neutropenia (grade 3-4, 33%/21%) and thrombocytopenia (grade 3-4, 29%/8%) were most common. One patient had grade 4 leukopenia (4%). No patients discontinued treatment on account of adverse events, however, and nonhematologic adverse event rates were low, without grade 4 events. Eight different grade 3 adverse events (atrial fibrillation [with prior history], diarrhea, hyponatremia pneumonia, pulmonary opportunistic infection, rash maculopapular, rash acneiform, skin ulceration) were reported, each in one patient. Pharmacokinetic data supported once-daily dosing for DTRM-12, with an estimated half-life of 5-9 hours that was comparable with that of once-daily ibrutinib, and longer than that of other agents of the same class. The recommended phase 2 dose going forward was 200 mg for DTRM-12, 5 mg for everolimus and 2 mg for pomalidomide.
Favorable responses
In efficacy analysis for 22 evaluable patients (11 in the RT group, 11 in the DLBCL ), there was 1 complete response in the RT group and 2 in the DLBCL group, with partial responses in 4 and 3, respectively, giving overall response rates of 46% in the RT group and 45% in the DLBCL group. Two and four patients, respectively, in the RT and DLBCL groups, had stable disease, Dr. Mato said, and most patients (71%) had SPD (sum of the product of the diameters) lymph node reductions, with lymph node reductions of 50% or more in 43%.
“Encouraging clinical activity was observed in high-risk, heavily pretreated Richter’s transformation and diffuse large B-cell lymphoma patients,” Dr. Mato concluded. He also noted that the main safety findings were “expected and manageable.”
The session moderator, Chaitra S. Ujjani, MD, of the Seattle Health Care Alliance, asked if the DTRM-555 regimen should be considered definitive therapy in patients who are responding, or if moving on to cellular therapies or a consolidative approach should be considered.
“If they are responding, it is reasonable to consider consolidating with a cellular therapy at this point in time,” Dr. Mato replied. He did observe, however, that many of the included patients had tried experimental therapies, including cellular therapy. “Without [data from] a much larger patient population and longer-term follow-up, I think that, for responding patients with a durable remission who have a [chimeric antigen receptor] T or transplant option, these, at the least, have to be discussed with them.”
To an additional question as to whether any of the subjects had prior exposure to BTK inhibitors, Dr. Mato responded, “There is a high exposure to BTK inhibitors, and almost universally these patients were progressors. So again, this is supportive of the hypothesis that hitting multiple pathways simultaneously is somewhat different from hitting just BTK by itself, even in the setting of progression.”
A DTRM-555 triple fixed-dose combination tablet is under development, and a double fixed-dose tablet (DTRM-505) is ready for the ongoing phase 2 U.S. study (NCT04030544) among patients with relapsed/refractory CLL or non-Hodgkin lymphoma (RT, DLBCL or transformed follicular lymphoma) with prior exposure to a novel agent.
Dr. Mato, disclosed consultancy and research funding relationships with multiple pharmaceutical and biotechnology companies.
SOURCE: Mato AR et al. ASH 2020, Abstract 126.
For B-cell malignancies, synthetic lethality is a viable treatment approach, according to preliminary clinical trial data with once-daily oral DTRM-555. The triple combination therapy, DTRM-555, combines a Bruton’s tyrosine kinase (BTK) inhibitor, a mammalian target of rapamycin (mTOR) inhibitor and pomalidomide, an immunomodulatory imide drug (IMiD), according to Anthony R. Mato, MD, in a presentation at the annual meeting of the American Society of Hematology, which was held virtually.
Richter’s transformation, a rare event
Dr. Mato’s phase 1 clinical trial included 13 patients with Richter’s transformation (RT) and 11 with diffuse large B-cell lymphoma (DLBCL). Richter’s transformation, a rare event occurring in 5%-7% of chronic lymphocytic leukemia (CLL) cases, has no clear standard of care and universally poor outcomes (overall survival, 3-12 months) once it becomes refractory to anthracycline-based chemotherapy, according to Dr. Mato.
Despite great progress in treating DLBCL, cure rates with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone), the standard of care, are in the 50%-60% range and much lower (30%-40%) with poor-risk features. Furthermore, most (60%-70%) patients receiving autologous stem cell transplant or CAR-T still require additional lines of therapy.
The “synthetic lethality” (SL) strategy, which has become a focus of cancer treatment in the last decade, identifies multiple disease primary aberrant and compensatory pathways and then inhibits them together in a manner lethal to cell survival. Preclinical studies have shown low doses of a BTK inhibitor/mTOR inhibitor/IMiD to synergistically kill malignant B cells. DTRM-555 is an optimized, oral, once-daily triplet combination of a novel and clinically differentiated irreversible BTK inhibitor (DTRM-12), everolimus and pomalidomide, Dr. Mato explained.
Individuals (38% women) included in the trial had a median of 2 (1-10) prior lines of therapy, with a CD20 monoclonal antibody as one of them in all cases, and 83% with R-CHOP. All patients had life expectancy >12 weeks, with 0-1 performance status and adequate organ and hematologic function.
DTRM-12 plasma concentrations, Dr. Mato noted, were unaffected by coadministration with everolimus with or without pomalidomide.
Manageable adverse events
Among adverse events, neutropenia (grade 3-4, 33%/21%) and thrombocytopenia (grade 3-4, 29%/8%) were most common. One patient had grade 4 leukopenia (4%). No patients discontinued treatment on account of adverse events, however, and nonhematologic adverse event rates were low, without grade 4 events. Eight different grade 3 adverse events (atrial fibrillation [with prior history], diarrhea, hyponatremia pneumonia, pulmonary opportunistic infection, rash maculopapular, rash acneiform, skin ulceration) were reported, each in one patient. Pharmacokinetic data supported once-daily dosing for DTRM-12, with an estimated half-life of 5-9 hours that was comparable with that of once-daily ibrutinib, and longer than that of other agents of the same class. The recommended phase 2 dose going forward was 200 mg for DTRM-12, 5 mg for everolimus and 2 mg for pomalidomide.
Favorable responses
In efficacy analysis for 22 evaluable patients (11 in the RT group, 11 in the DLBCL ), there was 1 complete response in the RT group and 2 in the DLBCL group, with partial responses in 4 and 3, respectively, giving overall response rates of 46% in the RT group and 45% in the DLBCL group. Two and four patients, respectively, in the RT and DLBCL groups, had stable disease, Dr. Mato said, and most patients (71%) had SPD (sum of the product of the diameters) lymph node reductions, with lymph node reductions of 50% or more in 43%.
“Encouraging clinical activity was observed in high-risk, heavily pretreated Richter’s transformation and diffuse large B-cell lymphoma patients,” Dr. Mato concluded. He also noted that the main safety findings were “expected and manageable.”
The session moderator, Chaitra S. Ujjani, MD, of the Seattle Health Care Alliance, asked if the DTRM-555 regimen should be considered definitive therapy in patients who are responding, or if moving on to cellular therapies or a consolidative approach should be considered.
“If they are responding, it is reasonable to consider consolidating with a cellular therapy at this point in time,” Dr. Mato replied. He did observe, however, that many of the included patients had tried experimental therapies, including cellular therapy. “Without [data from] a much larger patient population and longer-term follow-up, I think that, for responding patients with a durable remission who have a [chimeric antigen receptor] T or transplant option, these, at the least, have to be discussed with them.”
To an additional question as to whether any of the subjects had prior exposure to BTK inhibitors, Dr. Mato responded, “There is a high exposure to BTK inhibitors, and almost universally these patients were progressors. So again, this is supportive of the hypothesis that hitting multiple pathways simultaneously is somewhat different from hitting just BTK by itself, even in the setting of progression.”
A DTRM-555 triple fixed-dose combination tablet is under development, and a double fixed-dose tablet (DTRM-505) is ready for the ongoing phase 2 U.S. study (NCT04030544) among patients with relapsed/refractory CLL or non-Hodgkin lymphoma (RT, DLBCL or transformed follicular lymphoma) with prior exposure to a novel agent.
Dr. Mato, disclosed consultancy and research funding relationships with multiple pharmaceutical and biotechnology companies.
SOURCE: Mato AR et al. ASH 2020, Abstract 126.
For B-cell malignancies, synthetic lethality is a viable treatment approach, according to preliminary clinical trial data with once-daily oral DTRM-555. The triple combination therapy, DTRM-555, combines a Bruton’s tyrosine kinase (BTK) inhibitor, a mammalian target of rapamycin (mTOR) inhibitor and pomalidomide, an immunomodulatory imide drug (IMiD), according to Anthony R. Mato, MD, in a presentation at the annual meeting of the American Society of Hematology, which was held virtually.
Richter’s transformation, a rare event
Dr. Mato’s phase 1 clinical trial included 13 patients with Richter’s transformation (RT) and 11 with diffuse large B-cell lymphoma (DLBCL). Richter’s transformation, a rare event occurring in 5%-7% of chronic lymphocytic leukemia (CLL) cases, has no clear standard of care and universally poor outcomes (overall survival, 3-12 months) once it becomes refractory to anthracycline-based chemotherapy, according to Dr. Mato.
Despite great progress in treating DLBCL, cure rates with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone), the standard of care, are in the 50%-60% range and much lower (30%-40%) with poor-risk features. Furthermore, most (60%-70%) patients receiving autologous stem cell transplant or CAR-T still require additional lines of therapy.
The “synthetic lethality” (SL) strategy, which has become a focus of cancer treatment in the last decade, identifies multiple disease primary aberrant and compensatory pathways and then inhibits them together in a manner lethal to cell survival. Preclinical studies have shown low doses of a BTK inhibitor/mTOR inhibitor/IMiD to synergistically kill malignant B cells. DTRM-555 is an optimized, oral, once-daily triplet combination of a novel and clinically differentiated irreversible BTK inhibitor (DTRM-12), everolimus and pomalidomide, Dr. Mato explained.
Individuals (38% women) included in the trial had a median of 2 (1-10) prior lines of therapy, with a CD20 monoclonal antibody as one of them in all cases, and 83% with R-CHOP. All patients had life expectancy >12 weeks, with 0-1 performance status and adequate organ and hematologic function.
DTRM-12 plasma concentrations, Dr. Mato noted, were unaffected by coadministration with everolimus with or without pomalidomide.
Manageable adverse events
Among adverse events, neutropenia (grade 3-4, 33%/21%) and thrombocytopenia (grade 3-4, 29%/8%) were most common. One patient had grade 4 leukopenia (4%). No patients discontinued treatment on account of adverse events, however, and nonhematologic adverse event rates were low, without grade 4 events. Eight different grade 3 adverse events (atrial fibrillation [with prior history], diarrhea, hyponatremia pneumonia, pulmonary opportunistic infection, rash maculopapular, rash acneiform, skin ulceration) were reported, each in one patient. Pharmacokinetic data supported once-daily dosing for DTRM-12, with an estimated half-life of 5-9 hours that was comparable with that of once-daily ibrutinib, and longer than that of other agents of the same class. The recommended phase 2 dose going forward was 200 mg for DTRM-12, 5 mg for everolimus and 2 mg for pomalidomide.
Favorable responses
In efficacy analysis for 22 evaluable patients (11 in the RT group, 11 in the DLBCL ), there was 1 complete response in the RT group and 2 in the DLBCL group, with partial responses in 4 and 3, respectively, giving overall response rates of 46% in the RT group and 45% in the DLBCL group. Two and four patients, respectively, in the RT and DLBCL groups, had stable disease, Dr. Mato said, and most patients (71%) had SPD (sum of the product of the diameters) lymph node reductions, with lymph node reductions of 50% or more in 43%.
“Encouraging clinical activity was observed in high-risk, heavily pretreated Richter’s transformation and diffuse large B-cell lymphoma patients,” Dr. Mato concluded. He also noted that the main safety findings were “expected and manageable.”
The session moderator, Chaitra S. Ujjani, MD, of the Seattle Health Care Alliance, asked if the DTRM-555 regimen should be considered definitive therapy in patients who are responding, or if moving on to cellular therapies or a consolidative approach should be considered.
“If they are responding, it is reasonable to consider consolidating with a cellular therapy at this point in time,” Dr. Mato replied. He did observe, however, that many of the included patients had tried experimental therapies, including cellular therapy. “Without [data from] a much larger patient population and longer-term follow-up, I think that, for responding patients with a durable remission who have a [chimeric antigen receptor] T or transplant option, these, at the least, have to be discussed with them.”
To an additional question as to whether any of the subjects had prior exposure to BTK inhibitors, Dr. Mato responded, “There is a high exposure to BTK inhibitors, and almost universally these patients were progressors. So again, this is supportive of the hypothesis that hitting multiple pathways simultaneously is somewhat different from hitting just BTK by itself, even in the setting of progression.”
A DTRM-555 triple fixed-dose combination tablet is under development, and a double fixed-dose tablet (DTRM-505) is ready for the ongoing phase 2 U.S. study (NCT04030544) among patients with relapsed/refractory CLL or non-Hodgkin lymphoma (RT, DLBCL or transformed follicular lymphoma) with prior exposure to a novel agent.
Dr. Mato, disclosed consultancy and research funding relationships with multiple pharmaceutical and biotechnology companies.
SOURCE: Mato AR et al. ASH 2020, Abstract 126.
FROM ASH 2020
Fixed duration ibrutinib/venetoclax appears feasible for some CLL/SLL patients
Among chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) patients in the minimal residual disease (MRD) cohort of the phase 2 CAPTIVATE trial, a 1-year disease-free survival (DFS) rate of 95% in those randomized to placebo after 12 cycles of combined ibrutinib plus venetoclax supports a fixed-duration treatment approach, according to William G. Wierda, MD, PhD, University of Texas, MD Anderson Cancer Center, Houston.
Ibrutinib, a once-daily Bruton kinase inhibitor, is the only targeted therapy for first-line treatment of CLL that has demonstrated significant overall survival benefit in randomized phase 3 studies, Dr. Wierda said at the American Society of Hematology annual meeting, held virtually.
Ibrutinib and venetoclax have synergistic and complementary antitumor activity, he noted, through mobilizing and clearing CLL cells from protective niches and disease compartments beyond blood and bone marrow.
Fixed-duration study
CAPTIVATE (PCYC-1142), an international phase 2 study, evaluated first-line treatment with 12 cycles of the ibrutinib/venetoclax combination in MRD and fixed-duration cohorts. The current primary analysis of 1-year DFS from the MRD cohort tested whether the regimen allows for treatment-free remission in the setting of confirmed undetectable MRD (uMRD).
Patients (n = 164, median age 58 years) in the CAPTIVATE study MRD cohort had previously untreated active CLL/SLL requiring treatment per International Workshop on Chronic Lymphocytic Leukemia criteria.
They received 3 cycles of lead-in ibrutinib (420 mg once daily) followed by 12 cycles of ibrutinib (420 mg once daily plus venetoclax ramp-up to 400 mg once daily). Thereafter, in an MRD-guided 1:1 randomization stratified by immunoglobulin heavy chain (IGHV) mutational status, those with confirmed uMRD received either placebo or ibrutinib, and those with uMRD not confirmed received either ibrutinib or ibrutinib plus venetoclax (both open-label).
Among high-risk features in CAPTIVATE subjects, 60% of patients had unmutated IGHV, with del(17p)/TP53 mutation in 20%, del(11Q) in 17%, complex karyotype in 19%, cytopenias in 36%, bulky lymph nodes in 32%, and absolute neutrophil count ≥25x109/L in 76%.
Response findings
The ibrutinib lead-in, Dr. Wierda said, reduced tumor lysis syndrome (TLS) risk, shifting 90% of patients with high baseline TLS risk to medium or low-risk categories (from 77 to 51 patients), precluding need for hospitalization with venetoclax initiation.
The rate for best response of uMRD (defined as uMRD over at least 3 cycles in both peripheral blood and bone marrow) in evaluable patients was 75% in peripheral blood (n = 163) and 72% in bone marrow (n = 155).
Confirmed uMRD was achieved in 86/149 (58%), with uMRD not confirmed in 63/149 (uMRD 32% in bone marrow and 48% in peripheral blood). One-year DFS after the further randomization to placebo or ibrutinib in the confirmed uMRD group was 95.3% in the placebo group and 100% in the ibrutinib group (P = .1475). In the uMRD not confirmed group, 30-month progression-free survival (PFS) was 95.2% and 96.7% in the ibrutinib and ibrutinib plus venetoclax groups, respectively. Thirty-month PFS rates in the confirmed uMRD placebo and ibrutinib arms were 95.3% and 100%. “Thirty-month PFS rates were greater than 95% across all randomized arms,” Dr. Wierda stated.
In patients without confirmed uMRD after 12 cycles of combined ibrutinib plus venetoclax, additional randomized treatment led to greater increases in uMRD in the ibrutinib plus venetoclax group than in the ibrutinib alone group (bone marrow additional 10% ibrutinib alone, 34% ibrutinib plus venetoclax; peripheral blood 0% ibrutinib, 19% ibrutinib plus venetoclax).
Adverse events generally decreased after the first 6 months of ibrutinib plus venetoclax treatment, with no new safety signals emerging over time. “There were no safety concerns with this highly active combination of first-line ibrutinib plus venetoclax. It’s an oral, once-daily fixed duration regimen that achieves undetectable MRD in blood or bone marrow in three-fourths of patients after 12 cycles of combined treatment.”
When asked, in a question-and-answer session after his presentation, if the findings were “practice changing,” Dr. Wierda responded: “We need additional data from ongoing studies looking at various combinations of targeted therapy. But this study does clearly show efficacy in terms of depth of remission, and it supports the concept of fixed duration treatment, particularly for those patients who achieved undetectable MRD status.”
SOURCE: William G. Wierda, MD, PhD. ASH 2020, Abstract 123.
Among chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) patients in the minimal residual disease (MRD) cohort of the phase 2 CAPTIVATE trial, a 1-year disease-free survival (DFS) rate of 95% in those randomized to placebo after 12 cycles of combined ibrutinib plus venetoclax supports a fixed-duration treatment approach, according to William G. Wierda, MD, PhD, University of Texas, MD Anderson Cancer Center, Houston.
Ibrutinib, a once-daily Bruton kinase inhibitor, is the only targeted therapy for first-line treatment of CLL that has demonstrated significant overall survival benefit in randomized phase 3 studies, Dr. Wierda said at the American Society of Hematology annual meeting, held virtually.
Ibrutinib and venetoclax have synergistic and complementary antitumor activity, he noted, through mobilizing and clearing CLL cells from protective niches and disease compartments beyond blood and bone marrow.
Fixed-duration study
CAPTIVATE (PCYC-1142), an international phase 2 study, evaluated first-line treatment with 12 cycles of the ibrutinib/venetoclax combination in MRD and fixed-duration cohorts. The current primary analysis of 1-year DFS from the MRD cohort tested whether the regimen allows for treatment-free remission in the setting of confirmed undetectable MRD (uMRD).
Patients (n = 164, median age 58 years) in the CAPTIVATE study MRD cohort had previously untreated active CLL/SLL requiring treatment per International Workshop on Chronic Lymphocytic Leukemia criteria.
They received 3 cycles of lead-in ibrutinib (420 mg once daily) followed by 12 cycles of ibrutinib (420 mg once daily plus venetoclax ramp-up to 400 mg once daily). Thereafter, in an MRD-guided 1:1 randomization stratified by immunoglobulin heavy chain (IGHV) mutational status, those with confirmed uMRD received either placebo or ibrutinib, and those with uMRD not confirmed received either ibrutinib or ibrutinib plus venetoclax (both open-label).
Among high-risk features in CAPTIVATE subjects, 60% of patients had unmutated IGHV, with del(17p)/TP53 mutation in 20%, del(11Q) in 17%, complex karyotype in 19%, cytopenias in 36%, bulky lymph nodes in 32%, and absolute neutrophil count ≥25x109/L in 76%.
Response findings
The ibrutinib lead-in, Dr. Wierda said, reduced tumor lysis syndrome (TLS) risk, shifting 90% of patients with high baseline TLS risk to medium or low-risk categories (from 77 to 51 patients), precluding need for hospitalization with venetoclax initiation.
The rate for best response of uMRD (defined as uMRD over at least 3 cycles in both peripheral blood and bone marrow) in evaluable patients was 75% in peripheral blood (n = 163) and 72% in bone marrow (n = 155).
Confirmed uMRD was achieved in 86/149 (58%), with uMRD not confirmed in 63/149 (uMRD 32% in bone marrow and 48% in peripheral blood). One-year DFS after the further randomization to placebo or ibrutinib in the confirmed uMRD group was 95.3% in the placebo group and 100% in the ibrutinib group (P = .1475). In the uMRD not confirmed group, 30-month progression-free survival (PFS) was 95.2% and 96.7% in the ibrutinib and ibrutinib plus venetoclax groups, respectively. Thirty-month PFS rates in the confirmed uMRD placebo and ibrutinib arms were 95.3% and 100%. “Thirty-month PFS rates were greater than 95% across all randomized arms,” Dr. Wierda stated.
In patients without confirmed uMRD after 12 cycles of combined ibrutinib plus venetoclax, additional randomized treatment led to greater increases in uMRD in the ibrutinib plus venetoclax group than in the ibrutinib alone group (bone marrow additional 10% ibrutinib alone, 34% ibrutinib plus venetoclax; peripheral blood 0% ibrutinib, 19% ibrutinib plus venetoclax).
Adverse events generally decreased after the first 6 months of ibrutinib plus venetoclax treatment, with no new safety signals emerging over time. “There were no safety concerns with this highly active combination of first-line ibrutinib plus venetoclax. It’s an oral, once-daily fixed duration regimen that achieves undetectable MRD in blood or bone marrow in three-fourths of patients after 12 cycles of combined treatment.”
When asked, in a question-and-answer session after his presentation, if the findings were “practice changing,” Dr. Wierda responded: “We need additional data from ongoing studies looking at various combinations of targeted therapy. But this study does clearly show efficacy in terms of depth of remission, and it supports the concept of fixed duration treatment, particularly for those patients who achieved undetectable MRD status.”
SOURCE: William G. Wierda, MD, PhD. ASH 2020, Abstract 123.
Among chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) patients in the minimal residual disease (MRD) cohort of the phase 2 CAPTIVATE trial, a 1-year disease-free survival (DFS) rate of 95% in those randomized to placebo after 12 cycles of combined ibrutinib plus venetoclax supports a fixed-duration treatment approach, according to William G. Wierda, MD, PhD, University of Texas, MD Anderson Cancer Center, Houston.
Ibrutinib, a once-daily Bruton kinase inhibitor, is the only targeted therapy for first-line treatment of CLL that has demonstrated significant overall survival benefit in randomized phase 3 studies, Dr. Wierda said at the American Society of Hematology annual meeting, held virtually.
Ibrutinib and venetoclax have synergistic and complementary antitumor activity, he noted, through mobilizing and clearing CLL cells from protective niches and disease compartments beyond blood and bone marrow.
Fixed-duration study
CAPTIVATE (PCYC-1142), an international phase 2 study, evaluated first-line treatment with 12 cycles of the ibrutinib/venetoclax combination in MRD and fixed-duration cohorts. The current primary analysis of 1-year DFS from the MRD cohort tested whether the regimen allows for treatment-free remission in the setting of confirmed undetectable MRD (uMRD).
Patients (n = 164, median age 58 years) in the CAPTIVATE study MRD cohort had previously untreated active CLL/SLL requiring treatment per International Workshop on Chronic Lymphocytic Leukemia criteria.
They received 3 cycles of lead-in ibrutinib (420 mg once daily) followed by 12 cycles of ibrutinib (420 mg once daily plus venetoclax ramp-up to 400 mg once daily). Thereafter, in an MRD-guided 1:1 randomization stratified by immunoglobulin heavy chain (IGHV) mutational status, those with confirmed uMRD received either placebo or ibrutinib, and those with uMRD not confirmed received either ibrutinib or ibrutinib plus venetoclax (both open-label).
Among high-risk features in CAPTIVATE subjects, 60% of patients had unmutated IGHV, with del(17p)/TP53 mutation in 20%, del(11Q) in 17%, complex karyotype in 19%, cytopenias in 36%, bulky lymph nodes in 32%, and absolute neutrophil count ≥25x109/L in 76%.
Response findings
The ibrutinib lead-in, Dr. Wierda said, reduced tumor lysis syndrome (TLS) risk, shifting 90% of patients with high baseline TLS risk to medium or low-risk categories (from 77 to 51 patients), precluding need for hospitalization with venetoclax initiation.
The rate for best response of uMRD (defined as uMRD over at least 3 cycles in both peripheral blood and bone marrow) in evaluable patients was 75% in peripheral blood (n = 163) and 72% in bone marrow (n = 155).
Confirmed uMRD was achieved in 86/149 (58%), with uMRD not confirmed in 63/149 (uMRD 32% in bone marrow and 48% in peripheral blood). One-year DFS after the further randomization to placebo or ibrutinib in the confirmed uMRD group was 95.3% in the placebo group and 100% in the ibrutinib group (P = .1475). In the uMRD not confirmed group, 30-month progression-free survival (PFS) was 95.2% and 96.7% in the ibrutinib and ibrutinib plus venetoclax groups, respectively. Thirty-month PFS rates in the confirmed uMRD placebo and ibrutinib arms were 95.3% and 100%. “Thirty-month PFS rates were greater than 95% across all randomized arms,” Dr. Wierda stated.
In patients without confirmed uMRD after 12 cycles of combined ibrutinib plus venetoclax, additional randomized treatment led to greater increases in uMRD in the ibrutinib plus venetoclax group than in the ibrutinib alone group (bone marrow additional 10% ibrutinib alone, 34% ibrutinib plus venetoclax; peripheral blood 0% ibrutinib, 19% ibrutinib plus venetoclax).
Adverse events generally decreased after the first 6 months of ibrutinib plus venetoclax treatment, with no new safety signals emerging over time. “There were no safety concerns with this highly active combination of first-line ibrutinib plus venetoclax. It’s an oral, once-daily fixed duration regimen that achieves undetectable MRD in blood or bone marrow in three-fourths of patients after 12 cycles of combined treatment.”
When asked, in a question-and-answer session after his presentation, if the findings were “practice changing,” Dr. Wierda responded: “We need additional data from ongoing studies looking at various combinations of targeted therapy. But this study does clearly show efficacy in terms of depth of remission, and it supports the concept of fixed duration treatment, particularly for those patients who achieved undetectable MRD status.”
SOURCE: William G. Wierda, MD, PhD. ASH 2020, Abstract 123.
FROM ASH 2020
Key clinical point: A favorable 1-year DFS in patients after 12 cycles of ibrutinib plus venetoclax in the MRD cohort of the phase 2 CAPTIVATE trial supports fixed-duration treatment for chronic lymphocytic leukemia/small lymphocytic lymphoma.
Major finding: One-year DFS after randomization to placebo or ibrutinib in the confirmed undetectable MRD group was 95.3% in the placebo group and 100.0 percent in the ibrutinib group (P = .1475).
Study details: The phase 2 CAPTIVATE study included 164 patients with previously untreated active chronic lymphocytic leukemia/small lymphocytic lymphoma requiring treatment per International Workshop on Chronic Lymphocytic Leukemia criteria.
Disclosures: Dr. Wierda disclosed consultancy and research funding with multiple pharmaceutical companies.
Source: William G. Wierda, MD, PhD. ASH 2020 Abstract 123.
Pigment traits, sun sensitivity associated with risk of non-Hodgkin lymphomas and chronic lymphocytic leukemia
Risk factors for keratinocyte carcinomas, primarily pigment traits and sun sensitivity, were associated with the risk of developing non-Hodgkin lymphomas (NHL) and chronic lymphocytic leukemia (CLL) in an analysis of 92,097 women in France.
The presence of “many or very many nevi [moles]” was particularly associated with the risk of CLL among individuals in the E3N cohort, according to a report published online in Cancer Medicine. E3N is a prospective cohort of French women aged 40-65 years at inclusion in 1990. Researchers collected cancer data at baseline and every 2-3 years.
Hazard ratios and 95% confidence intervals for associations between patients pigmentary traits and sun exposure and their risk for CLL/NHL were estimated using Cox models, according to study author Louis-Marie Garcin, MD, of the Université Paris-Saclay, Villejuif, and colleagues.
Common etiology?
Among the 92,097 women included in the study, 622 incident cases of CLL/NHL were observed over a median of 24-years’ follow-up.
The presence of nevi was associated with CLL/NHL risk. The HR for “many or very many nevi” relative to “no nevi” was 1.56. The association with number of nevi was strongest for the risk of CLL, with an HR for “many or very many nevi” of 3.00 vs. 1.32 for NHL. In addition, the researchers found that women whose skin was highly sensitive to sunburn also had a higher risk of CLL (HR, 1.96), while no increased risk of NHL was observed. All HR values were within their respective 95% confidence intervals.
Relevant characteristics that were found to not be associated with added CLL/NHL risk were skin or hair color, number of freckles, and average daily UV dose during spring and summer in the location of residence at birth or at inclusion.
These observations suggest that CLL in particular may share some constitutional risk factors with keratinocyte cancers, according to the researchers.
“We report an association between nevi frequency and CLL/NHL risk, suggesting a partly common genetic etiology of these tumors. Future research should investigate common pathophysiological pathways that could promote the development of both skin carcinoma and CLL/NHL,” the researchers concluded.
The study was sponsored by the French government. The authors stated that they had no conflicts of interest.
SOURCE: Garcin L-M et al. Cancer Med. 2020. doi: 10.1002/cam4.3586.
Risk factors for keratinocyte carcinomas, primarily pigment traits and sun sensitivity, were associated with the risk of developing non-Hodgkin lymphomas (NHL) and chronic lymphocytic leukemia (CLL) in an analysis of 92,097 women in France.
The presence of “many or very many nevi [moles]” was particularly associated with the risk of CLL among individuals in the E3N cohort, according to a report published online in Cancer Medicine. E3N is a prospective cohort of French women aged 40-65 years at inclusion in 1990. Researchers collected cancer data at baseline and every 2-3 years.
Hazard ratios and 95% confidence intervals for associations between patients pigmentary traits and sun exposure and their risk for CLL/NHL were estimated using Cox models, according to study author Louis-Marie Garcin, MD, of the Université Paris-Saclay, Villejuif, and colleagues.
Common etiology?
Among the 92,097 women included in the study, 622 incident cases of CLL/NHL were observed over a median of 24-years’ follow-up.
The presence of nevi was associated with CLL/NHL risk. The HR for “many or very many nevi” relative to “no nevi” was 1.56. The association with number of nevi was strongest for the risk of CLL, with an HR for “many or very many nevi” of 3.00 vs. 1.32 for NHL. In addition, the researchers found that women whose skin was highly sensitive to sunburn also had a higher risk of CLL (HR, 1.96), while no increased risk of NHL was observed. All HR values were within their respective 95% confidence intervals.
Relevant characteristics that were found to not be associated with added CLL/NHL risk were skin or hair color, number of freckles, and average daily UV dose during spring and summer in the location of residence at birth or at inclusion.
These observations suggest that CLL in particular may share some constitutional risk factors with keratinocyte cancers, according to the researchers.
“We report an association between nevi frequency and CLL/NHL risk, suggesting a partly common genetic etiology of these tumors. Future research should investigate common pathophysiological pathways that could promote the development of both skin carcinoma and CLL/NHL,” the researchers concluded.
The study was sponsored by the French government. The authors stated that they had no conflicts of interest.
SOURCE: Garcin L-M et al. Cancer Med. 2020. doi: 10.1002/cam4.3586.
Risk factors for keratinocyte carcinomas, primarily pigment traits and sun sensitivity, were associated with the risk of developing non-Hodgkin lymphomas (NHL) and chronic lymphocytic leukemia (CLL) in an analysis of 92,097 women in France.
The presence of “many or very many nevi [moles]” was particularly associated with the risk of CLL among individuals in the E3N cohort, according to a report published online in Cancer Medicine. E3N is a prospective cohort of French women aged 40-65 years at inclusion in 1990. Researchers collected cancer data at baseline and every 2-3 years.
Hazard ratios and 95% confidence intervals for associations between patients pigmentary traits and sun exposure and their risk for CLL/NHL were estimated using Cox models, according to study author Louis-Marie Garcin, MD, of the Université Paris-Saclay, Villejuif, and colleagues.
Common etiology?
Among the 92,097 women included in the study, 622 incident cases of CLL/NHL were observed over a median of 24-years’ follow-up.
The presence of nevi was associated with CLL/NHL risk. The HR for “many or very many nevi” relative to “no nevi” was 1.56. The association with number of nevi was strongest for the risk of CLL, with an HR for “many or very many nevi” of 3.00 vs. 1.32 for NHL. In addition, the researchers found that women whose skin was highly sensitive to sunburn also had a higher risk of CLL (HR, 1.96), while no increased risk of NHL was observed. All HR values were within their respective 95% confidence intervals.
Relevant characteristics that were found to not be associated with added CLL/NHL risk were skin or hair color, number of freckles, and average daily UV dose during spring and summer in the location of residence at birth or at inclusion.
These observations suggest that CLL in particular may share some constitutional risk factors with keratinocyte cancers, according to the researchers.
“We report an association between nevi frequency and CLL/NHL risk, suggesting a partly common genetic etiology of these tumors. Future research should investigate common pathophysiological pathways that could promote the development of both skin carcinoma and CLL/NHL,” the researchers concluded.
The study was sponsored by the French government. The authors stated that they had no conflicts of interest.
SOURCE: Garcin L-M et al. Cancer Med. 2020. doi: 10.1002/cam4.3586.
FROM CANCER MEDICINE
Ibrutinib associated with decreased circulating malignant cells and restored T-cell function in CLL patients
Ibrutinib showed significant impact on circulating malignant and nonmalignant immune cells and was found to restore healthy T-cell function in patients with chronic lymphocytic leukemia (CLL), according to the results of a comparative study of CLL patients and healthy controls.
Researchers compared circulating counts of 21 immune blood cell subsets throughout the first year of treatment in 55 patients with relapsed/refractory (R/R) CLL from the RESONATE trial and 50 previously untreated CLL patients from the RESONATE-2 trial with 20 untreated age-matched healthy donors, according to a report published online in Leukemia Research.
In addition, T-cell function was assessed in response to T-cell–receptor stimulation in 21 patients with R/R CLL, compared with 18 age-matched healthy donors, according to Isabelle G. Solman, MS, an employee of Translational Medicine, Pharmacyclics, Sunnyvale, Calif. and colleagues.
Positive indicators
Ibrutinib significantly decreased pathologically high circulating B cells, regulatory T cells, effector/memory CD4+ and CD8+ T cells (including exhausted and chronically activated T cells), natural killer (NK) T cells, and myeloid-derived suppressor cells; preserved naive T cells and NK cells; and increased circulating classical monocytes, according to the researchers.
Ibrutinib also significantly restored T-cell proliferative ability, degranulation, and cytokine secretion. Over the same period, ofatumumab or chlorambucil did not confer the same spectrum of normalization as ibrutinib in multiple immune subsets that were examined, they added.
“These results establish that ibrutinib has a significant and likely positive impact on circulating malignant and nonmalignant immune cells and restores healthy T-cell function,” the researchers indicated.
“Ibrutinib has a significant, progressively positive impact on both malignant and nonmalignant immune cells in CLL. These positive effects on circulating nonmalignant immune cells may contribute to long-term CLL disease control, overall health status, and decreased susceptibility to infection,” they concluded.
The study was funded by Pharmacyclics, an AbbVie Company. Ms. Solman is an employee of Translational Medicine, Pharmacyclics, Sunnyvale, Calif. as were several other authors.
SOURCE: Solman IG et al. Leuk Res. 2020;97. doi: 10.1016/j.leukres.2020.106432.
Ibrutinib showed significant impact on circulating malignant and nonmalignant immune cells and was found to restore healthy T-cell function in patients with chronic lymphocytic leukemia (CLL), according to the results of a comparative study of CLL patients and healthy controls.
Researchers compared circulating counts of 21 immune blood cell subsets throughout the first year of treatment in 55 patients with relapsed/refractory (R/R) CLL from the RESONATE trial and 50 previously untreated CLL patients from the RESONATE-2 trial with 20 untreated age-matched healthy donors, according to a report published online in Leukemia Research.
In addition, T-cell function was assessed in response to T-cell–receptor stimulation in 21 patients with R/R CLL, compared with 18 age-matched healthy donors, according to Isabelle G. Solman, MS, an employee of Translational Medicine, Pharmacyclics, Sunnyvale, Calif. and colleagues.
Positive indicators
Ibrutinib significantly decreased pathologically high circulating B cells, regulatory T cells, effector/memory CD4+ and CD8+ T cells (including exhausted and chronically activated T cells), natural killer (NK) T cells, and myeloid-derived suppressor cells; preserved naive T cells and NK cells; and increased circulating classical monocytes, according to the researchers.
Ibrutinib also significantly restored T-cell proliferative ability, degranulation, and cytokine secretion. Over the same period, ofatumumab or chlorambucil did not confer the same spectrum of normalization as ibrutinib in multiple immune subsets that were examined, they added.
“These results establish that ibrutinib has a significant and likely positive impact on circulating malignant and nonmalignant immune cells and restores healthy T-cell function,” the researchers indicated.
“Ibrutinib has a significant, progressively positive impact on both malignant and nonmalignant immune cells in CLL. These positive effects on circulating nonmalignant immune cells may contribute to long-term CLL disease control, overall health status, and decreased susceptibility to infection,” they concluded.
The study was funded by Pharmacyclics, an AbbVie Company. Ms. Solman is an employee of Translational Medicine, Pharmacyclics, Sunnyvale, Calif. as were several other authors.
SOURCE: Solman IG et al. Leuk Res. 2020;97. doi: 10.1016/j.leukres.2020.106432.
Ibrutinib showed significant impact on circulating malignant and nonmalignant immune cells and was found to restore healthy T-cell function in patients with chronic lymphocytic leukemia (CLL), according to the results of a comparative study of CLL patients and healthy controls.
Researchers compared circulating counts of 21 immune blood cell subsets throughout the first year of treatment in 55 patients with relapsed/refractory (R/R) CLL from the RESONATE trial and 50 previously untreated CLL patients from the RESONATE-2 trial with 20 untreated age-matched healthy donors, according to a report published online in Leukemia Research.
In addition, T-cell function was assessed in response to T-cell–receptor stimulation in 21 patients with R/R CLL, compared with 18 age-matched healthy donors, according to Isabelle G. Solman, MS, an employee of Translational Medicine, Pharmacyclics, Sunnyvale, Calif. and colleagues.
Positive indicators
Ibrutinib significantly decreased pathologically high circulating B cells, regulatory T cells, effector/memory CD4+ and CD8+ T cells (including exhausted and chronically activated T cells), natural killer (NK) T cells, and myeloid-derived suppressor cells; preserved naive T cells and NK cells; and increased circulating classical monocytes, according to the researchers.
Ibrutinib also significantly restored T-cell proliferative ability, degranulation, and cytokine secretion. Over the same period, ofatumumab or chlorambucil did not confer the same spectrum of normalization as ibrutinib in multiple immune subsets that were examined, they added.
“These results establish that ibrutinib has a significant and likely positive impact on circulating malignant and nonmalignant immune cells and restores healthy T-cell function,” the researchers indicated.
“Ibrutinib has a significant, progressively positive impact on both malignant and nonmalignant immune cells in CLL. These positive effects on circulating nonmalignant immune cells may contribute to long-term CLL disease control, overall health status, and decreased susceptibility to infection,” they concluded.
The study was funded by Pharmacyclics, an AbbVie Company. Ms. Solman is an employee of Translational Medicine, Pharmacyclics, Sunnyvale, Calif. as were several other authors.
SOURCE: Solman IG et al. Leuk Res. 2020;97. doi: 10.1016/j.leukres.2020.106432.
FROM LEUKEMIA RESEARCH
Final ASCEND study data: Acalabrutinib beat standard of care for r/r CLL
Acalabrutinib, a next-generation Bruton tyrosine kinase inhibitor, provides prolonged progression-free survival and better tolerability, compared with standard-of-care regimens for relapsed or refractory chronic lymphocytic leukemia (CLL), according to final results from the phase 3 ASCEND study.
The estimated 18-month progression-free survival (PFS) at a median of 22 months was 82% in 155 patients treated with acalabrutinib, compared with 48% in 155 treated with investigator’s choice of either idelalisib-rituximab (IdR) or bendamustine-rituximab (BR), which were given in 119 and 36 patients, respectively, Paolo Ghia, MD, PhD, reported at the Society of Hematologic Oncology virtual meeting.
The benefits of acalabrutinib were apparent regardless of high-risk genetic characteristics: Those with and without both del(17p) and TP53 mutations had similarly good PFS outcomes with acalabrutinib versus IdR/BR (HRs, 0.11 and 0.29, respectively), as did those with versus without unmutated IgVH (HRs, 0.28 and 0.30, respectively), said Dr. Ghia, professor of medical oncology at the Università Vita-Salute San Raffaele and IRCCS Ospedale San Raffaele, Milan.
The median overall survival was not reached in either arm, but estimated 18-month OS was 88% in both groups, likely because of the crossover being allowed for nonresponders in the IdR/BR groups, he noted.
Overall responses
The investigator-assessed overall response rates, including partial response or better, were also similar in the groups at 80% and 84%, respectively, and ORR, including partial response with lymphocytosis, was 92% versus 88%.
The duration of response was not reached in the acalabrutinib arm versus 18 months with IdR/BR, and estimated duration of response was 85% versus 49%.
The median drug exposure with acalabrutinib was approximately double that with IdR and about four times that of BR, Dr. Ghia said, noting that the difference between acalabrutinib and BR is explained by the short 6-month duration of treatment with BR, but the difference between acalabrutinib and IdR is because of adverse events (AEs).
Adverse events
AEs were the most common reason for treatment discontinuation in all three groups, but they led to discontinuation in only 16% with acalabrutinib versus 56% with IdR, he added.
The rates of AEs and AEs of clinical interest were generally similar to those reported at the interim analysis as presented in 2019 at the European Hematology Association annual meeting and published in the Journal of Clinical Oncology, despite the additional 6 months of follow up, he said.
Additionally, the incidence of grade 3 or higher AEs, serious AEs, and treatment-related AEs were all greater with IdR than with acalabrutinib or BR. The most common AEs with acalabrutinib were headache, neutropenia, diarrhea, and upper-respiratory infection, which were mostly grade 1 or 2. The most common grade 3 or higher AEs were neutropenia, anemia, and pneumonia, which were reported in 12%, 17%, and 7% of patients.
Confirmatory results
“The final results from the ASCEND study confirm the findings at the interim analysis and support the favorable efficacy and safety of acalabrutinib versus standard-of-care regimens ... in patients with relapsed/refractory CLL,” Dr. Ghia said.
“Overall, these final results from ASCENT support the use of acalabrutinib in patients with relapsed/refractory CLL, including those with high-risk genetic features.”
This study was sponsored by Acerta Pharma. Dr. Ghia reported consulting or advisory roles, grant or research funding, and/or honoraria from Abbvie, BeiGene, Janssen, Gilead Sciences, Sunesis Pharmaceuticals, Juno Therapeutics, ArQule, Adaptive Biotechnologies, Dynamo Therapeutics, MEI Pharma, and Novartis.
SOURCE: Ghia P et al. SOHO 2020, Abstract CLL-091.
Acalabrutinib, a next-generation Bruton tyrosine kinase inhibitor, provides prolonged progression-free survival and better tolerability, compared with standard-of-care regimens for relapsed or refractory chronic lymphocytic leukemia (CLL), according to final results from the phase 3 ASCEND study.
The estimated 18-month progression-free survival (PFS) at a median of 22 months was 82% in 155 patients treated with acalabrutinib, compared with 48% in 155 treated with investigator’s choice of either idelalisib-rituximab (IdR) or bendamustine-rituximab (BR), which were given in 119 and 36 patients, respectively, Paolo Ghia, MD, PhD, reported at the Society of Hematologic Oncology virtual meeting.
The benefits of acalabrutinib were apparent regardless of high-risk genetic characteristics: Those with and without both del(17p) and TP53 mutations had similarly good PFS outcomes with acalabrutinib versus IdR/BR (HRs, 0.11 and 0.29, respectively), as did those with versus without unmutated IgVH (HRs, 0.28 and 0.30, respectively), said Dr. Ghia, professor of medical oncology at the Università Vita-Salute San Raffaele and IRCCS Ospedale San Raffaele, Milan.
The median overall survival was not reached in either arm, but estimated 18-month OS was 88% in both groups, likely because of the crossover being allowed for nonresponders in the IdR/BR groups, he noted.
Overall responses
The investigator-assessed overall response rates, including partial response or better, were also similar in the groups at 80% and 84%, respectively, and ORR, including partial response with lymphocytosis, was 92% versus 88%.
The duration of response was not reached in the acalabrutinib arm versus 18 months with IdR/BR, and estimated duration of response was 85% versus 49%.
The median drug exposure with acalabrutinib was approximately double that with IdR and about four times that of BR, Dr. Ghia said, noting that the difference between acalabrutinib and BR is explained by the short 6-month duration of treatment with BR, but the difference between acalabrutinib and IdR is because of adverse events (AEs).
Adverse events
AEs were the most common reason for treatment discontinuation in all three groups, but they led to discontinuation in only 16% with acalabrutinib versus 56% with IdR, he added.
The rates of AEs and AEs of clinical interest were generally similar to those reported at the interim analysis as presented in 2019 at the European Hematology Association annual meeting and published in the Journal of Clinical Oncology, despite the additional 6 months of follow up, he said.
Additionally, the incidence of grade 3 or higher AEs, serious AEs, and treatment-related AEs were all greater with IdR than with acalabrutinib or BR. The most common AEs with acalabrutinib were headache, neutropenia, diarrhea, and upper-respiratory infection, which were mostly grade 1 or 2. The most common grade 3 or higher AEs were neutropenia, anemia, and pneumonia, which were reported in 12%, 17%, and 7% of patients.
Confirmatory results
“The final results from the ASCEND study confirm the findings at the interim analysis and support the favorable efficacy and safety of acalabrutinib versus standard-of-care regimens ... in patients with relapsed/refractory CLL,” Dr. Ghia said.
“Overall, these final results from ASCENT support the use of acalabrutinib in patients with relapsed/refractory CLL, including those with high-risk genetic features.”
This study was sponsored by Acerta Pharma. Dr. Ghia reported consulting or advisory roles, grant or research funding, and/or honoraria from Abbvie, BeiGene, Janssen, Gilead Sciences, Sunesis Pharmaceuticals, Juno Therapeutics, ArQule, Adaptive Biotechnologies, Dynamo Therapeutics, MEI Pharma, and Novartis.
SOURCE: Ghia P et al. SOHO 2020, Abstract CLL-091.
Acalabrutinib, a next-generation Bruton tyrosine kinase inhibitor, provides prolonged progression-free survival and better tolerability, compared with standard-of-care regimens for relapsed or refractory chronic lymphocytic leukemia (CLL), according to final results from the phase 3 ASCEND study.
The estimated 18-month progression-free survival (PFS) at a median of 22 months was 82% in 155 patients treated with acalabrutinib, compared with 48% in 155 treated with investigator’s choice of either idelalisib-rituximab (IdR) or bendamustine-rituximab (BR), which were given in 119 and 36 patients, respectively, Paolo Ghia, MD, PhD, reported at the Society of Hematologic Oncology virtual meeting.
The benefits of acalabrutinib were apparent regardless of high-risk genetic characteristics: Those with and without both del(17p) and TP53 mutations had similarly good PFS outcomes with acalabrutinib versus IdR/BR (HRs, 0.11 and 0.29, respectively), as did those with versus without unmutated IgVH (HRs, 0.28 and 0.30, respectively), said Dr. Ghia, professor of medical oncology at the Università Vita-Salute San Raffaele and IRCCS Ospedale San Raffaele, Milan.
The median overall survival was not reached in either arm, but estimated 18-month OS was 88% in both groups, likely because of the crossover being allowed for nonresponders in the IdR/BR groups, he noted.
Overall responses
The investigator-assessed overall response rates, including partial response or better, were also similar in the groups at 80% and 84%, respectively, and ORR, including partial response with lymphocytosis, was 92% versus 88%.
The duration of response was not reached in the acalabrutinib arm versus 18 months with IdR/BR, and estimated duration of response was 85% versus 49%.
The median drug exposure with acalabrutinib was approximately double that with IdR and about four times that of BR, Dr. Ghia said, noting that the difference between acalabrutinib and BR is explained by the short 6-month duration of treatment with BR, but the difference between acalabrutinib and IdR is because of adverse events (AEs).
Adverse events
AEs were the most common reason for treatment discontinuation in all three groups, but they led to discontinuation in only 16% with acalabrutinib versus 56% with IdR, he added.
The rates of AEs and AEs of clinical interest were generally similar to those reported at the interim analysis as presented in 2019 at the European Hematology Association annual meeting and published in the Journal of Clinical Oncology, despite the additional 6 months of follow up, he said.
Additionally, the incidence of grade 3 or higher AEs, serious AEs, and treatment-related AEs were all greater with IdR than with acalabrutinib or BR. The most common AEs with acalabrutinib were headache, neutropenia, diarrhea, and upper-respiratory infection, which were mostly grade 1 or 2. The most common grade 3 or higher AEs were neutropenia, anemia, and pneumonia, which were reported in 12%, 17%, and 7% of patients.
Confirmatory results
“The final results from the ASCEND study confirm the findings at the interim analysis and support the favorable efficacy and safety of acalabrutinib versus standard-of-care regimens ... in patients with relapsed/refractory CLL,” Dr. Ghia said.
“Overall, these final results from ASCENT support the use of acalabrutinib in patients with relapsed/refractory CLL, including those with high-risk genetic features.”
This study was sponsored by Acerta Pharma. Dr. Ghia reported consulting or advisory roles, grant or research funding, and/or honoraria from Abbvie, BeiGene, Janssen, Gilead Sciences, Sunesis Pharmaceuticals, Juno Therapeutics, ArQule, Adaptive Biotechnologies, Dynamo Therapeutics, MEI Pharma, and Novartis.
SOURCE: Ghia P et al. SOHO 2020, Abstract CLL-091.
FROM SOHO 2020
ESMO offers new clinical practice guideline for CLL
An updated European Society for Medical Oncology (ESMO) clinical practice guidelines were released to provide key recommendations on the management of chronic lymphocytic leukemia (CLL).
The guidelines were developed by a multidisciplinary group of experts from different institutions and countries in Europe and provide levels of evidence and grades of recommendation where applicable for issues regarding prognosis and treatment decisions in CLL. Such decisions depend on genetic and clinical factors such as age, stage, and comorbidities. The guidelines also focus on new therapies targeting B-cell-receptor pathways or defect mechanism of apoptosis, which have been found to induce long-lasting remissions. The guidelines were endorsed by the European Hematology Association (EHA) through the Scientific Working Group on CLL/European Research Initiative on CLL (ERIC), according to the report published online the Annals of Oncology.
These clinical practice guidelines were developed in accordance with the ESMO standard operating procedures for clinical practice guidelines development with use of relevant literature selected by the expert authors. Statements without grading were considered justified as standard clinical practice by the experts and the ESMO faculty.
Below are some highlights of the guidelines, which cover a wide array of topics regarding the diagnosis, staging, treatment, and progression of CLL disease.
Diagnosis
The guidelines indicate that CLL diagnosis is usually possible by immunophenotyping of peripheral blood only and that lymph node (LN) biopsy and/or bone marrow biopsy may be helpful if immunophenotyping is not conclusive for the diagnosis of CLL, according to Barbara Eichhorst, MD, of the University of Cologne (Germany) and colleagues on behalf of the ESMO guidelines committee.
Staging and risk assessment
Early asymptomatic-stage disease does not need further risk assessment, but after the first year, when all patients should be seen at 3-monthly intervals, patients can be followed every 3-12 months. The interval would depend on burden and dynamics of the disease obtained by the using history and physical examinations, including a careful palpation of all LN areas, spleen, and liver, as well as assessing complete blood cell count and differential count, according to the report.
Advanced- and symptomatic-stage disease requires a broader examination including imaging, history and status of relevant infections, and fluorescent in situ hybridization (FISH) assays for the detection of deletion of the chromosome 17 (del[17p]) affecting the tumor protein p53 expression and, in the absence of del(17p), TP53 sequencing for detection of TP53 gene mutation, according to the authors.
Prognostication
Two clinical staging systems are typically used in CLL. Both Binet and Rai staging systems separate three groups of patients with different prognosis, although “as a consequence of more effective therapy, the overall survival (OS) of patients with advanced stage has improved and the relevance of the staging systems for prognostication has decreased,” according to the report.
The recent addition of genetic markers has also proved highly relevant to identifying patients with different prognoses and to guide treatment.
Therapy
Although CLL is an incurable disease, choice and application of treatment are strongly tied to the length of survival, according to the authors. The guidelines recommend Binet and Rai staging with clinical symptoms as relevant for treatment indication. In addition, the identification of del(17p), TP53 mutations, and IGHV status are relevant for choice of therapy and should be assessed prior to treatment.
The guidelines discuss specific treatment modalities for various stages of the disease, from early stages to relapse.
For frontline therapy, different treatment strategies are available including continuous treatment with Bruton tyrosine kinase (BTK)–inhibitors, such as ibrutinib, until progression or time-limited therapy with ChT backbone and CD20 antibodies. In addition, the Food and Drug Administration and European Medicines Agency have recently approved the combination of venetoclax plus obinutuzumab for first-line therapy of CLL.
Treatment decisions should include an assessment of IGHV and TP53 status, as well as patient-related factors such as comedication, comorbidities, preferences, drug availability, and potential of treatment adherence, according to the guidelines.
In case of symptomatic relapse within 3 years after fixed-duration therapy or nonresponse to therapy, the guidelines recommend that the therapeutic regimen should be changed, regardless of the type of first-line either to venetoclax plus rituximab for 24 months or to ibrutinib, acalabrutinib, or other BTK inhibitors (if available) as continuous therapy.
The guidelines also discuss the possible roles for hematopoietic stem cell transplantation and cellular therapies, as well as the treatment of the various complications that can arise in patients with CLL, and dealing with various aspects of disease progression.
No external funds were provided for the production of the guidelines. The authors of the report and members of the ESMO Guidelines Committee reported numerous disclosures regarding pharmaceutical and biotechnology companies.
SOURCE: Eichhorst B et al. Ann Oncol. 2020 Oct 19. doi: 10.1016/j.annonc.2020.09.019.
An updated European Society for Medical Oncology (ESMO) clinical practice guidelines were released to provide key recommendations on the management of chronic lymphocytic leukemia (CLL).
The guidelines were developed by a multidisciplinary group of experts from different institutions and countries in Europe and provide levels of evidence and grades of recommendation where applicable for issues regarding prognosis and treatment decisions in CLL. Such decisions depend on genetic and clinical factors such as age, stage, and comorbidities. The guidelines also focus on new therapies targeting B-cell-receptor pathways or defect mechanism of apoptosis, which have been found to induce long-lasting remissions. The guidelines were endorsed by the European Hematology Association (EHA) through the Scientific Working Group on CLL/European Research Initiative on CLL (ERIC), according to the report published online the Annals of Oncology.
These clinical practice guidelines were developed in accordance with the ESMO standard operating procedures for clinical practice guidelines development with use of relevant literature selected by the expert authors. Statements without grading were considered justified as standard clinical practice by the experts and the ESMO faculty.
Below are some highlights of the guidelines, which cover a wide array of topics regarding the diagnosis, staging, treatment, and progression of CLL disease.
Diagnosis
The guidelines indicate that CLL diagnosis is usually possible by immunophenotyping of peripheral blood only and that lymph node (LN) biopsy and/or bone marrow biopsy may be helpful if immunophenotyping is not conclusive for the diagnosis of CLL, according to Barbara Eichhorst, MD, of the University of Cologne (Germany) and colleagues on behalf of the ESMO guidelines committee.
Staging and risk assessment
Early asymptomatic-stage disease does not need further risk assessment, but after the first year, when all patients should be seen at 3-monthly intervals, patients can be followed every 3-12 months. The interval would depend on burden and dynamics of the disease obtained by the using history and physical examinations, including a careful palpation of all LN areas, spleen, and liver, as well as assessing complete blood cell count and differential count, according to the report.
Advanced- and symptomatic-stage disease requires a broader examination including imaging, history and status of relevant infections, and fluorescent in situ hybridization (FISH) assays for the detection of deletion of the chromosome 17 (del[17p]) affecting the tumor protein p53 expression and, in the absence of del(17p), TP53 sequencing for detection of TP53 gene mutation, according to the authors.
Prognostication
Two clinical staging systems are typically used in CLL. Both Binet and Rai staging systems separate three groups of patients with different prognosis, although “as a consequence of more effective therapy, the overall survival (OS) of patients with advanced stage has improved and the relevance of the staging systems for prognostication has decreased,” according to the report.
The recent addition of genetic markers has also proved highly relevant to identifying patients with different prognoses and to guide treatment.
Therapy
Although CLL is an incurable disease, choice and application of treatment are strongly tied to the length of survival, according to the authors. The guidelines recommend Binet and Rai staging with clinical symptoms as relevant for treatment indication. In addition, the identification of del(17p), TP53 mutations, and IGHV status are relevant for choice of therapy and should be assessed prior to treatment.
The guidelines discuss specific treatment modalities for various stages of the disease, from early stages to relapse.
For frontline therapy, different treatment strategies are available including continuous treatment with Bruton tyrosine kinase (BTK)–inhibitors, such as ibrutinib, until progression or time-limited therapy with ChT backbone and CD20 antibodies. In addition, the Food and Drug Administration and European Medicines Agency have recently approved the combination of venetoclax plus obinutuzumab for first-line therapy of CLL.
Treatment decisions should include an assessment of IGHV and TP53 status, as well as patient-related factors such as comedication, comorbidities, preferences, drug availability, and potential of treatment adherence, according to the guidelines.
In case of symptomatic relapse within 3 years after fixed-duration therapy or nonresponse to therapy, the guidelines recommend that the therapeutic regimen should be changed, regardless of the type of first-line either to venetoclax plus rituximab for 24 months or to ibrutinib, acalabrutinib, or other BTK inhibitors (if available) as continuous therapy.
The guidelines also discuss the possible roles for hematopoietic stem cell transplantation and cellular therapies, as well as the treatment of the various complications that can arise in patients with CLL, and dealing with various aspects of disease progression.
No external funds were provided for the production of the guidelines. The authors of the report and members of the ESMO Guidelines Committee reported numerous disclosures regarding pharmaceutical and biotechnology companies.
SOURCE: Eichhorst B et al. Ann Oncol. 2020 Oct 19. doi: 10.1016/j.annonc.2020.09.019.
An updated European Society for Medical Oncology (ESMO) clinical practice guidelines were released to provide key recommendations on the management of chronic lymphocytic leukemia (CLL).
The guidelines were developed by a multidisciplinary group of experts from different institutions and countries in Europe and provide levels of evidence and grades of recommendation where applicable for issues regarding prognosis and treatment decisions in CLL. Such decisions depend on genetic and clinical factors such as age, stage, and comorbidities. The guidelines also focus on new therapies targeting B-cell-receptor pathways or defect mechanism of apoptosis, which have been found to induce long-lasting remissions. The guidelines were endorsed by the European Hematology Association (EHA) through the Scientific Working Group on CLL/European Research Initiative on CLL (ERIC), according to the report published online the Annals of Oncology.
These clinical practice guidelines were developed in accordance with the ESMO standard operating procedures for clinical practice guidelines development with use of relevant literature selected by the expert authors. Statements without grading were considered justified as standard clinical practice by the experts and the ESMO faculty.
Below are some highlights of the guidelines, which cover a wide array of topics regarding the diagnosis, staging, treatment, and progression of CLL disease.
Diagnosis
The guidelines indicate that CLL diagnosis is usually possible by immunophenotyping of peripheral blood only and that lymph node (LN) biopsy and/or bone marrow biopsy may be helpful if immunophenotyping is not conclusive for the diagnosis of CLL, according to Barbara Eichhorst, MD, of the University of Cologne (Germany) and colleagues on behalf of the ESMO guidelines committee.
Staging and risk assessment
Early asymptomatic-stage disease does not need further risk assessment, but after the first year, when all patients should be seen at 3-monthly intervals, patients can be followed every 3-12 months. The interval would depend on burden and dynamics of the disease obtained by the using history and physical examinations, including a careful palpation of all LN areas, spleen, and liver, as well as assessing complete blood cell count and differential count, according to the report.
Advanced- and symptomatic-stage disease requires a broader examination including imaging, history and status of relevant infections, and fluorescent in situ hybridization (FISH) assays for the detection of deletion of the chromosome 17 (del[17p]) affecting the tumor protein p53 expression and, in the absence of del(17p), TP53 sequencing for detection of TP53 gene mutation, according to the authors.
Prognostication
Two clinical staging systems are typically used in CLL. Both Binet and Rai staging systems separate three groups of patients with different prognosis, although “as a consequence of more effective therapy, the overall survival (OS) of patients with advanced stage has improved and the relevance of the staging systems for prognostication has decreased,” according to the report.
The recent addition of genetic markers has also proved highly relevant to identifying patients with different prognoses and to guide treatment.
Therapy
Although CLL is an incurable disease, choice and application of treatment are strongly tied to the length of survival, according to the authors. The guidelines recommend Binet and Rai staging with clinical symptoms as relevant for treatment indication. In addition, the identification of del(17p), TP53 mutations, and IGHV status are relevant for choice of therapy and should be assessed prior to treatment.
The guidelines discuss specific treatment modalities for various stages of the disease, from early stages to relapse.
For frontline therapy, different treatment strategies are available including continuous treatment with Bruton tyrosine kinase (BTK)–inhibitors, such as ibrutinib, until progression or time-limited therapy with ChT backbone and CD20 antibodies. In addition, the Food and Drug Administration and European Medicines Agency have recently approved the combination of venetoclax plus obinutuzumab for first-line therapy of CLL.
Treatment decisions should include an assessment of IGHV and TP53 status, as well as patient-related factors such as comedication, comorbidities, preferences, drug availability, and potential of treatment adherence, according to the guidelines.
In case of symptomatic relapse within 3 years after fixed-duration therapy or nonresponse to therapy, the guidelines recommend that the therapeutic regimen should be changed, regardless of the type of first-line either to venetoclax plus rituximab for 24 months or to ibrutinib, acalabrutinib, or other BTK inhibitors (if available) as continuous therapy.
The guidelines also discuss the possible roles for hematopoietic stem cell transplantation and cellular therapies, as well as the treatment of the various complications that can arise in patients with CLL, and dealing with various aspects of disease progression.
No external funds were provided for the production of the guidelines. The authors of the report and members of the ESMO Guidelines Committee reported numerous disclosures regarding pharmaceutical and biotechnology companies.
SOURCE: Eichhorst B et al. Ann Oncol. 2020 Oct 19. doi: 10.1016/j.annonc.2020.09.019.
FROM ANNALS OF ONCOLOGY
Meta-analysis: Acalabrutinib showed better PFS and OS than other frontline CLL therapies
Acalabrutinib, given alone or in combination with obinutuzumab, showed favorable progression-free survival (PFS) and overall survival (OS), compared with other frontline therapies for chronic lymphocytic leukemia (CLL) in fludarabine-ineligible patients, according to the results of a meta-analysis comparing clinical trial results.
Researchers conducted a systematic literature review for applicable CLL studies that examined frontline treatments in order to compare the results with data on acalabrutinib (monotherapy and in combination with obinutuzumab) from patients in the ELEVATE-TN study (NCT02475681), according to a report published in Clinical Therapeutics.
Matthew S. Davids, MD, MMSc, of the Dana-Farber Cancer Institute in Boston, and colleagues performed a network meta-analysis (NMA) comparing acalabrutinib versus other standard frontline therapies for CLL in patients for whom fludarabine-based treatment is not appropriate.
“In the absence of head-to-head trial data, NMAs allow for simultaneous comparisons of a number of interventions with multiple comparators, by synthesizing direct and indirect evidence,” the authors stated.
Eight randomized controlled trials (RCTs) met the criteria for comparison.
The researchers constructed two evidence networks: Network A comprised solely RCTs that met the inclusion criteria, and Network B comprised seven RCTs and a published cross-trial comparison of ibrutinib from RESONATE-2 and chlorambucil plus obinutuzumab from iLLUMINATE. PFS and OS results were reported by using hazard ratios and 95% credible intervals.
Overall benefit
Both networks showed a significant improvement in PFS for acalabrutinib plus obinutuzumab over all comparators, according to the researchers. Both networks also showed a significant improvement in PFS for acalabrutinib monotherapy versus most comparators, with a significant difference to ibrutinib monotherapy found in Network A but not Network B.
Conversely, a significant difference in PFS was observed for acalabrutinib monotherapy versus venetoclax plus obinutuzumab in Network B but not Network A.
Overall survival hazard ratios ranged from 0.18 to 0.65 in favor of acalabrutinib-based treatment, but not all were significant. Acalabrutinib plus obinutuzumab ranked highest in terms of PFS and OS improvement, followed by acalabrutinib monotherapy.
“Although our NMAs provide useful insights into the relative efficacy of acalabrutinib, compared with other frontline treatments of CLL, the results cannot be considered confirmatory, and head-to-head randomized trials are needed, especially to compare the efficacy of acalabrutinib versus other targeted agents,” the researchers concluded.
AstraZeneca sponsored the study. The authors reported funding from AstraZeneca and numerous other pharmaceutical companies.
SOURCE: Davids MS et al. Clin Ther. 2020 Oct 5. doi: 10.1016/j.clinthera.2020.08.017.
Acalabrutinib, given alone or in combination with obinutuzumab, showed favorable progression-free survival (PFS) and overall survival (OS), compared with other frontline therapies for chronic lymphocytic leukemia (CLL) in fludarabine-ineligible patients, according to the results of a meta-analysis comparing clinical trial results.
Researchers conducted a systematic literature review for applicable CLL studies that examined frontline treatments in order to compare the results with data on acalabrutinib (monotherapy and in combination with obinutuzumab) from patients in the ELEVATE-TN study (NCT02475681), according to a report published in Clinical Therapeutics.
Matthew S. Davids, MD, MMSc, of the Dana-Farber Cancer Institute in Boston, and colleagues performed a network meta-analysis (NMA) comparing acalabrutinib versus other standard frontline therapies for CLL in patients for whom fludarabine-based treatment is not appropriate.
“In the absence of head-to-head trial data, NMAs allow for simultaneous comparisons of a number of interventions with multiple comparators, by synthesizing direct and indirect evidence,” the authors stated.
Eight randomized controlled trials (RCTs) met the criteria for comparison.
The researchers constructed two evidence networks: Network A comprised solely RCTs that met the inclusion criteria, and Network B comprised seven RCTs and a published cross-trial comparison of ibrutinib from RESONATE-2 and chlorambucil plus obinutuzumab from iLLUMINATE. PFS and OS results were reported by using hazard ratios and 95% credible intervals.
Overall benefit
Both networks showed a significant improvement in PFS for acalabrutinib plus obinutuzumab over all comparators, according to the researchers. Both networks also showed a significant improvement in PFS for acalabrutinib monotherapy versus most comparators, with a significant difference to ibrutinib monotherapy found in Network A but not Network B.
Conversely, a significant difference in PFS was observed for acalabrutinib monotherapy versus venetoclax plus obinutuzumab in Network B but not Network A.
Overall survival hazard ratios ranged from 0.18 to 0.65 in favor of acalabrutinib-based treatment, but not all were significant. Acalabrutinib plus obinutuzumab ranked highest in terms of PFS and OS improvement, followed by acalabrutinib monotherapy.
“Although our NMAs provide useful insights into the relative efficacy of acalabrutinib, compared with other frontline treatments of CLL, the results cannot be considered confirmatory, and head-to-head randomized trials are needed, especially to compare the efficacy of acalabrutinib versus other targeted agents,” the researchers concluded.
AstraZeneca sponsored the study. The authors reported funding from AstraZeneca and numerous other pharmaceutical companies.
SOURCE: Davids MS et al. Clin Ther. 2020 Oct 5. doi: 10.1016/j.clinthera.2020.08.017.
Acalabrutinib, given alone or in combination with obinutuzumab, showed favorable progression-free survival (PFS) and overall survival (OS), compared with other frontline therapies for chronic lymphocytic leukemia (CLL) in fludarabine-ineligible patients, according to the results of a meta-analysis comparing clinical trial results.
Researchers conducted a systematic literature review for applicable CLL studies that examined frontline treatments in order to compare the results with data on acalabrutinib (monotherapy and in combination with obinutuzumab) from patients in the ELEVATE-TN study (NCT02475681), according to a report published in Clinical Therapeutics.
Matthew S. Davids, MD, MMSc, of the Dana-Farber Cancer Institute in Boston, and colleagues performed a network meta-analysis (NMA) comparing acalabrutinib versus other standard frontline therapies for CLL in patients for whom fludarabine-based treatment is not appropriate.
“In the absence of head-to-head trial data, NMAs allow for simultaneous comparisons of a number of interventions with multiple comparators, by synthesizing direct and indirect evidence,” the authors stated.
Eight randomized controlled trials (RCTs) met the criteria for comparison.
The researchers constructed two evidence networks: Network A comprised solely RCTs that met the inclusion criteria, and Network B comprised seven RCTs and a published cross-trial comparison of ibrutinib from RESONATE-2 and chlorambucil plus obinutuzumab from iLLUMINATE. PFS and OS results were reported by using hazard ratios and 95% credible intervals.
Overall benefit
Both networks showed a significant improvement in PFS for acalabrutinib plus obinutuzumab over all comparators, according to the researchers. Both networks also showed a significant improvement in PFS for acalabrutinib monotherapy versus most comparators, with a significant difference to ibrutinib monotherapy found in Network A but not Network B.
Conversely, a significant difference in PFS was observed for acalabrutinib monotherapy versus venetoclax plus obinutuzumab in Network B but not Network A.
Overall survival hazard ratios ranged from 0.18 to 0.65 in favor of acalabrutinib-based treatment, but not all were significant. Acalabrutinib plus obinutuzumab ranked highest in terms of PFS and OS improvement, followed by acalabrutinib monotherapy.
“Although our NMAs provide useful insights into the relative efficacy of acalabrutinib, compared with other frontline treatments of CLL, the results cannot be considered confirmatory, and head-to-head randomized trials are needed, especially to compare the efficacy of acalabrutinib versus other targeted agents,” the researchers concluded.
AstraZeneca sponsored the study. The authors reported funding from AstraZeneca and numerous other pharmaceutical companies.
SOURCE: Davids MS et al. Clin Ther. 2020 Oct 5. doi: 10.1016/j.clinthera.2020.08.017.
FROM CLINICAL THERAPEUTICS
Two new protein biomarkers may serve as prognostic indicators for outcomes in CLL
Two new protein biomarkers may serve as prognostic indicators for outcomes in chronic lymphocytic leukemia (CLL) patients, according to the results of a proteomic assessment of patients’ serum compared to their event-free survival (EFS).
The results were published in Experimental Hematology.
The study attempted to validate the prognostic ability of known proteomic markers measured pretreatment and to search for new proteomic markers that might be related to treatment response in CLL, according to Fatemeh Saberi Hosnijeh, MD, of Erasmus MC, University Medical Center, Rotterdam, The Netherlands, and colleagues.
Baseline serum samples were taken from 51 CLL patients who were then treated with chemoimmunotherapy. The samples were analyzed for 360 proteomic markers, and those results were compared with patient EFS.
Study subjects were selected from patients enrolled in the HOVON 109 clinical trial, a phase 1/2 trial designed to assess the efficacy and safety of first-line therapy involving chlorambucil, rituximab,and lenalidomide in elderly patients and young frail patients with advanced CLL.
The patients assessed comprised 30 men and 21 women, and the median EFS for all patients was 23 months (ranging from 1.25 to 60.9 months).
Promising biomarkers
The researchers found that patients who had high serum levels of the proteins sCD23 (P = .026), sCD27 (P = .04), the serine peptidase inhibitor SPINT1 (P = .001), and the surface antigen protein LY9 (P = .0003) had a shorter EFS than those with marker levels below the median.
“Taken together, our results validate the prognostic impact of sCD23 and highlight SPINT1 and LY9 as possible promising markers for treatment response in CLL patients,” the researchers stated.
“Despite the relatively small number of available cases, which had an impact on statistical power, our pilot study identified SPINT1 and LY9 as promising independent prognostic proteomic markers next to sCD23 and sCD27 in patients treated for CLL. Further studies with larger sample sizes are required to validate these results,” the researchers concluded.
This research was supported by a grant from Gilead Sciences and an EU TRANSCAN/Dutch Cancer Society grant. The authors declared that they had no conflicts of interest.
SOURCE: Hosnijeh FS et al. Exp Hematol. 2020;89:55-60.
Two new protein biomarkers may serve as prognostic indicators for outcomes in chronic lymphocytic leukemia (CLL) patients, according to the results of a proteomic assessment of patients’ serum compared to their event-free survival (EFS).
The results were published in Experimental Hematology.
The study attempted to validate the prognostic ability of known proteomic markers measured pretreatment and to search for new proteomic markers that might be related to treatment response in CLL, according to Fatemeh Saberi Hosnijeh, MD, of Erasmus MC, University Medical Center, Rotterdam, The Netherlands, and colleagues.
Baseline serum samples were taken from 51 CLL patients who were then treated with chemoimmunotherapy. The samples were analyzed for 360 proteomic markers, and those results were compared with patient EFS.
Study subjects were selected from patients enrolled in the HOVON 109 clinical trial, a phase 1/2 trial designed to assess the efficacy and safety of first-line therapy involving chlorambucil, rituximab,and lenalidomide in elderly patients and young frail patients with advanced CLL.
The patients assessed comprised 30 men and 21 women, and the median EFS for all patients was 23 months (ranging from 1.25 to 60.9 months).
Promising biomarkers
The researchers found that patients who had high serum levels of the proteins sCD23 (P = .026), sCD27 (P = .04), the serine peptidase inhibitor SPINT1 (P = .001), and the surface antigen protein LY9 (P = .0003) had a shorter EFS than those with marker levels below the median.
“Taken together, our results validate the prognostic impact of sCD23 and highlight SPINT1 and LY9 as possible promising markers for treatment response in CLL patients,” the researchers stated.
“Despite the relatively small number of available cases, which had an impact on statistical power, our pilot study identified SPINT1 and LY9 as promising independent prognostic proteomic markers next to sCD23 and sCD27 in patients treated for CLL. Further studies with larger sample sizes are required to validate these results,” the researchers concluded.
This research was supported by a grant from Gilead Sciences and an EU TRANSCAN/Dutch Cancer Society grant. The authors declared that they had no conflicts of interest.
SOURCE: Hosnijeh FS et al. Exp Hematol. 2020;89:55-60.
Two new protein biomarkers may serve as prognostic indicators for outcomes in chronic lymphocytic leukemia (CLL) patients, according to the results of a proteomic assessment of patients’ serum compared to their event-free survival (EFS).
The results were published in Experimental Hematology.
The study attempted to validate the prognostic ability of known proteomic markers measured pretreatment and to search for new proteomic markers that might be related to treatment response in CLL, according to Fatemeh Saberi Hosnijeh, MD, of Erasmus MC, University Medical Center, Rotterdam, The Netherlands, and colleagues.
Baseline serum samples were taken from 51 CLL patients who were then treated with chemoimmunotherapy. The samples were analyzed for 360 proteomic markers, and those results were compared with patient EFS.
Study subjects were selected from patients enrolled in the HOVON 109 clinical trial, a phase 1/2 trial designed to assess the efficacy and safety of first-line therapy involving chlorambucil, rituximab,and lenalidomide in elderly patients and young frail patients with advanced CLL.
The patients assessed comprised 30 men and 21 women, and the median EFS for all patients was 23 months (ranging from 1.25 to 60.9 months).
Promising biomarkers
The researchers found that patients who had high serum levels of the proteins sCD23 (P = .026), sCD27 (P = .04), the serine peptidase inhibitor SPINT1 (P = .001), and the surface antigen protein LY9 (P = .0003) had a shorter EFS than those with marker levels below the median.
“Taken together, our results validate the prognostic impact of sCD23 and highlight SPINT1 and LY9 as possible promising markers for treatment response in CLL patients,” the researchers stated.
“Despite the relatively small number of available cases, which had an impact on statistical power, our pilot study identified SPINT1 and LY9 as promising independent prognostic proteomic markers next to sCD23 and sCD27 in patients treated for CLL. Further studies with larger sample sizes are required to validate these results,” the researchers concluded.
This research was supported by a grant from Gilead Sciences and an EU TRANSCAN/Dutch Cancer Society grant. The authors declared that they had no conflicts of interest.
SOURCE: Hosnijeh FS et al. Exp Hematol. 2020;89:55-60.
FROM EXPERIMENTAL HEMATOLOGY
Survey quantifies COVID-19’s impact on oncology
An international survey provides new insights into how COVID-19 has affected, and may continue to affect, the field of oncology.
The survey showed that “COVID-19 has had a major impact on the organization of patient care, on the well-being of caregivers, on continued medical education, and on clinical trial activities in oncology,” stated Guy Jerusalem, MD, PhD, of Centre Hospitalier Universitaire de Liège (Belgium).
Dr. Jerusalem presented these findings at the European Society for Medical Oncology Virtual Congress 2020.
The survey was distributed by 20 oncologists from 10 of the countries most affected by COVID-19. Responses were obtained from 109 oncologists representing centers in 18 countries. The responses were recorded between June 17 and July 14, 2020.
The survey consisted of 95 items intended to evaluate the impact of COVID-19 on the organization of oncologic care. Questions encompassed the capacity and service offered at each center, the magnitude of COVID-19–based care interruptions and the reasons for them, the ensuing challenges faced, interventions implemented, and the estimated harms to patients during the pandemic.
The 109 oncologists surveyed had a median of 20 years of oncology experience. A majority of respondents were men (61.5%), and the median age was 48.5 years.
The respondents had worked predominantly (62.4%) at academic hospitals, with 29.6% at community hospitals. Most respondents worked at general hospitals with an oncology unit (66.1%) rather than a specialized separate cancer center (32.1%).
The most common specialty was breast cancer (60.6%), followed by gastrointestinal cancer (10.1%), urogenital cancer (9.2%), and lung cancer (8.3%).
Impact on treatment
The treatment modalities affected by the pandemic – through cancellations or delays in more than 10% of patients – included surgery (in 34% of centers), chemotherapy (22%), radiotherapy (13.7%), checkpoint inhibitor therapy (9.1%), monoclonal antibodies (9%), and oral targeted therapy (3.7%).
Among oncologists treating breast cancer, cancellations/delays in more than 10% of patients were reported for everolimus (18%), CDK4/6 inhibitors (8.9%), and endocrine therapy (2.2%).
Overall, 34.8% of respondents reported increased use of granulocyte colony–stimulating factor, and 6.4% reported increased use of erythropoietin.
On the other hand, 11.1% of respondents reported a decrease in the use of double immunotherapy, and 21.9% reported decreased use of corticosteroids.
Not only can the immunosuppressive effects of steroid use increase infection risks, Dr. Jerusalem noted, fever suppression can lead to a delayed diagnosis of COVID-19.
“To circumvent potential higher infection risks or greater disease severity, we use lower doses of steroids, but this is not based on studies,” he said.
“Previous exposure to steroids or being on steroids at the time of COVID-19 infection is a detrimental factor for complications and mortality,” commented ESMO President Solange Peters, MD, PhD, of Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland.
Dr. Peters noted that the observation was based on lung cancer registry findings. Furthermore, because data from smaller outbreaks of other coronavirus infections suggested worse prognosis and increased mortality, steroid use was already feared in the very early days of the COVID-19 pandemic.
Lastly, earlier cessation of palliative treatment was observed in 32.1% of centers, and 64.2% of respondents agreed that undertreatment because of COVID-19 is a major concern.
Dr. Jerusalem noted that the survey data do not explain the early cessation of palliative treatment. “I suspect that many patients died at home rather than alone in institutions because it was the only way they could die with their families around them.”
Telehealth, meetings, and trials
The survey also revealed rationales for the use of teleconsultation, including follow-up (94.5%), oral therapy (92.7%), immunotherapy (57.8%), and chemotherapy (55%).
Most respondents reported more frequent use of virtual meetings for continuing medical education (94%), oncologic team meetings (92%), and tumor boards (82%).
While about 82% of respondents said they were likely to continue the use of telemedicine, 45% said virtual conferences are not an acceptable alternative to live international conferences such as ESMO, Dr. Jerusalem said.
Finally, nearly three-quarters of respondents (72.5%) said all clinical trial activities are or will soon be activated, or never stopped, at their centers. On the other hand, 27.5% of respondents reported that their centers had major protocol violations or deviations, and 37% of respondents said they expect significant reductions in clinical trial activities this year.
Dr. Jerusalem concluded that COVID-19 is having a major, long-term impact on the organization of patient care, caregivers, continued medical education, and clinical trial activities in oncology.
He cautioned that “the risk of a delayed diagnosis of new cancers and economic consequences of COVID-19 on access to health care and cancer treatments have to be carefully evaluated.”
This research was funded by Fondation Léon Fredericq. Dr. Jerusalem disclosed relationships with Novartis, Roche, Lilly, Pfizer, Amgen, Bristol-Myers Squibb, AstraZeneca, Daiichi Sankyo, AbbVie, MedImmune, and Merck. Dr. Peters disclosed relationships with AbbVie, Amgen, AstraZeneca, and many other companies.
SOURCE: Jerusalem G et al. ESMO 2020, Abstract LBA76.
An international survey provides new insights into how COVID-19 has affected, and may continue to affect, the field of oncology.
The survey showed that “COVID-19 has had a major impact on the organization of patient care, on the well-being of caregivers, on continued medical education, and on clinical trial activities in oncology,” stated Guy Jerusalem, MD, PhD, of Centre Hospitalier Universitaire de Liège (Belgium).
Dr. Jerusalem presented these findings at the European Society for Medical Oncology Virtual Congress 2020.
The survey was distributed by 20 oncologists from 10 of the countries most affected by COVID-19. Responses were obtained from 109 oncologists representing centers in 18 countries. The responses were recorded between June 17 and July 14, 2020.
The survey consisted of 95 items intended to evaluate the impact of COVID-19 on the organization of oncologic care. Questions encompassed the capacity and service offered at each center, the magnitude of COVID-19–based care interruptions and the reasons for them, the ensuing challenges faced, interventions implemented, and the estimated harms to patients during the pandemic.
The 109 oncologists surveyed had a median of 20 years of oncology experience. A majority of respondents were men (61.5%), and the median age was 48.5 years.
The respondents had worked predominantly (62.4%) at academic hospitals, with 29.6% at community hospitals. Most respondents worked at general hospitals with an oncology unit (66.1%) rather than a specialized separate cancer center (32.1%).
The most common specialty was breast cancer (60.6%), followed by gastrointestinal cancer (10.1%), urogenital cancer (9.2%), and lung cancer (8.3%).
Impact on treatment
The treatment modalities affected by the pandemic – through cancellations or delays in more than 10% of patients – included surgery (in 34% of centers), chemotherapy (22%), radiotherapy (13.7%), checkpoint inhibitor therapy (9.1%), monoclonal antibodies (9%), and oral targeted therapy (3.7%).
Among oncologists treating breast cancer, cancellations/delays in more than 10% of patients were reported for everolimus (18%), CDK4/6 inhibitors (8.9%), and endocrine therapy (2.2%).
Overall, 34.8% of respondents reported increased use of granulocyte colony–stimulating factor, and 6.4% reported increased use of erythropoietin.
On the other hand, 11.1% of respondents reported a decrease in the use of double immunotherapy, and 21.9% reported decreased use of corticosteroids.
Not only can the immunosuppressive effects of steroid use increase infection risks, Dr. Jerusalem noted, fever suppression can lead to a delayed diagnosis of COVID-19.
“To circumvent potential higher infection risks or greater disease severity, we use lower doses of steroids, but this is not based on studies,” he said.
“Previous exposure to steroids or being on steroids at the time of COVID-19 infection is a detrimental factor for complications and mortality,” commented ESMO President Solange Peters, MD, PhD, of Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland.
Dr. Peters noted that the observation was based on lung cancer registry findings. Furthermore, because data from smaller outbreaks of other coronavirus infections suggested worse prognosis and increased mortality, steroid use was already feared in the very early days of the COVID-19 pandemic.
Lastly, earlier cessation of palliative treatment was observed in 32.1% of centers, and 64.2% of respondents agreed that undertreatment because of COVID-19 is a major concern.
Dr. Jerusalem noted that the survey data do not explain the early cessation of palliative treatment. “I suspect that many patients died at home rather than alone in institutions because it was the only way they could die with their families around them.”
Telehealth, meetings, and trials
The survey also revealed rationales for the use of teleconsultation, including follow-up (94.5%), oral therapy (92.7%), immunotherapy (57.8%), and chemotherapy (55%).
Most respondents reported more frequent use of virtual meetings for continuing medical education (94%), oncologic team meetings (92%), and tumor boards (82%).
While about 82% of respondents said they were likely to continue the use of telemedicine, 45% said virtual conferences are not an acceptable alternative to live international conferences such as ESMO, Dr. Jerusalem said.
Finally, nearly three-quarters of respondents (72.5%) said all clinical trial activities are or will soon be activated, or never stopped, at their centers. On the other hand, 27.5% of respondents reported that their centers had major protocol violations or deviations, and 37% of respondents said they expect significant reductions in clinical trial activities this year.
Dr. Jerusalem concluded that COVID-19 is having a major, long-term impact on the organization of patient care, caregivers, continued medical education, and clinical trial activities in oncology.
He cautioned that “the risk of a delayed diagnosis of new cancers and economic consequences of COVID-19 on access to health care and cancer treatments have to be carefully evaluated.”
This research was funded by Fondation Léon Fredericq. Dr. Jerusalem disclosed relationships with Novartis, Roche, Lilly, Pfizer, Amgen, Bristol-Myers Squibb, AstraZeneca, Daiichi Sankyo, AbbVie, MedImmune, and Merck. Dr. Peters disclosed relationships with AbbVie, Amgen, AstraZeneca, and many other companies.
SOURCE: Jerusalem G et al. ESMO 2020, Abstract LBA76.
An international survey provides new insights into how COVID-19 has affected, and may continue to affect, the field of oncology.
The survey showed that “COVID-19 has had a major impact on the organization of patient care, on the well-being of caregivers, on continued medical education, and on clinical trial activities in oncology,” stated Guy Jerusalem, MD, PhD, of Centre Hospitalier Universitaire de Liège (Belgium).
Dr. Jerusalem presented these findings at the European Society for Medical Oncology Virtual Congress 2020.
The survey was distributed by 20 oncologists from 10 of the countries most affected by COVID-19. Responses were obtained from 109 oncologists representing centers in 18 countries. The responses were recorded between June 17 and July 14, 2020.
The survey consisted of 95 items intended to evaluate the impact of COVID-19 on the organization of oncologic care. Questions encompassed the capacity and service offered at each center, the magnitude of COVID-19–based care interruptions and the reasons for them, the ensuing challenges faced, interventions implemented, and the estimated harms to patients during the pandemic.
The 109 oncologists surveyed had a median of 20 years of oncology experience. A majority of respondents were men (61.5%), and the median age was 48.5 years.
The respondents had worked predominantly (62.4%) at academic hospitals, with 29.6% at community hospitals. Most respondents worked at general hospitals with an oncology unit (66.1%) rather than a specialized separate cancer center (32.1%).
The most common specialty was breast cancer (60.6%), followed by gastrointestinal cancer (10.1%), urogenital cancer (9.2%), and lung cancer (8.3%).
Impact on treatment
The treatment modalities affected by the pandemic – through cancellations or delays in more than 10% of patients – included surgery (in 34% of centers), chemotherapy (22%), radiotherapy (13.7%), checkpoint inhibitor therapy (9.1%), monoclonal antibodies (9%), and oral targeted therapy (3.7%).
Among oncologists treating breast cancer, cancellations/delays in more than 10% of patients were reported for everolimus (18%), CDK4/6 inhibitors (8.9%), and endocrine therapy (2.2%).
Overall, 34.8% of respondents reported increased use of granulocyte colony–stimulating factor, and 6.4% reported increased use of erythropoietin.
On the other hand, 11.1% of respondents reported a decrease in the use of double immunotherapy, and 21.9% reported decreased use of corticosteroids.
Not only can the immunosuppressive effects of steroid use increase infection risks, Dr. Jerusalem noted, fever suppression can lead to a delayed diagnosis of COVID-19.
“To circumvent potential higher infection risks or greater disease severity, we use lower doses of steroids, but this is not based on studies,” he said.
“Previous exposure to steroids or being on steroids at the time of COVID-19 infection is a detrimental factor for complications and mortality,” commented ESMO President Solange Peters, MD, PhD, of Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland.
Dr. Peters noted that the observation was based on lung cancer registry findings. Furthermore, because data from smaller outbreaks of other coronavirus infections suggested worse prognosis and increased mortality, steroid use was already feared in the very early days of the COVID-19 pandemic.
Lastly, earlier cessation of palliative treatment was observed in 32.1% of centers, and 64.2% of respondents agreed that undertreatment because of COVID-19 is a major concern.
Dr. Jerusalem noted that the survey data do not explain the early cessation of palliative treatment. “I suspect that many patients died at home rather than alone in institutions because it was the only way they could die with their families around them.”
Telehealth, meetings, and trials
The survey also revealed rationales for the use of teleconsultation, including follow-up (94.5%), oral therapy (92.7%), immunotherapy (57.8%), and chemotherapy (55%).
Most respondents reported more frequent use of virtual meetings for continuing medical education (94%), oncologic team meetings (92%), and tumor boards (82%).
While about 82% of respondents said they were likely to continue the use of telemedicine, 45% said virtual conferences are not an acceptable alternative to live international conferences such as ESMO, Dr. Jerusalem said.
Finally, nearly three-quarters of respondents (72.5%) said all clinical trial activities are or will soon be activated, or never stopped, at their centers. On the other hand, 27.5% of respondents reported that their centers had major protocol violations or deviations, and 37% of respondents said they expect significant reductions in clinical trial activities this year.
Dr. Jerusalem concluded that COVID-19 is having a major, long-term impact on the organization of patient care, caregivers, continued medical education, and clinical trial activities in oncology.
He cautioned that “the risk of a delayed diagnosis of new cancers and economic consequences of COVID-19 on access to health care and cancer treatments have to be carefully evaluated.”
This research was funded by Fondation Léon Fredericq. Dr. Jerusalem disclosed relationships with Novartis, Roche, Lilly, Pfizer, Amgen, Bristol-Myers Squibb, AstraZeneca, Daiichi Sankyo, AbbVie, MedImmune, and Merck. Dr. Peters disclosed relationships with AbbVie, Amgen, AstraZeneca, and many other companies.
SOURCE: Jerusalem G et al. ESMO 2020, Abstract LBA76.
FROM ESMO 2020
Oxidative stress linked to cytogenetic abnormalities in CLL
Oxidative stress may play a role in pathogenesis of B-cell chronic lymphocytic leukemia (B-CLL), according to the results of a biochemical and cytogenetic study of patients published online in Experimental and Molecular Pathology.
The study evaluated the serum levels of oxidative stress biomarkers [conjugated dienes (CD), malondialdehyde (MDA), and nitrite levels] and the levels of antioxidant biomarkers [ceruloplasmin (CP) and glutathione peroxidase (GPx)] in 64 B-CLL patients. The relationship between these biomarkers and the presence of cytogenetic abnormalities was examined, according to Tatiana Zhevak, MD, of Sechenov First Moscow (Russia) State Medical University, and colleagues.
Cytogenetic abnormalities have previously been determined to be linked to a poorer prognosis in CLL patients, and factors that increase the frequency of CA have been shown to increase the risk of rapid tumor progression, Dr. Zhevak and her colleagues stated.
Oxidative stress connection
Enhanced oxidative stress was detected in B-CLL patients as shown by their increased levels of serum CD, MDA, and nitrite, as well as a demonstrated imbalance in the antioxidant defense system as shown by an increased serum CP level and decreased serum GPx activity, according to the researchers.
In addition, these metabolic changes were found to be greater in those patients whose lymphocytes harbored specific cytogenetic abnormalities, and could be predicted by the serum levels of CD. Specifically, the odds of harboring a cytogenetic abnormality increased by a factor of 1.88 (P = .004) for every one-unit increase in serum CD level (mcmol/L), according to the authors.
“Collectively, the results support our hypothesis that oxidative stress and resulting lipid peroxidation play a role in pathogenesis of B-CLL and provide a rational basis for the use of agents regulating the pro-oxidant and antioxidant activity in the treatment of B-CLL patients,” the researchers concluded.
The research was unsponsored and the authors reported having no conflicts.
SOURCE: Zhevak T et al. Exp Mol Patholo. 2020 Oct;16:104524 doi: 10.1016/j.yexmp.2020.104524.
Oxidative stress may play a role in pathogenesis of B-cell chronic lymphocytic leukemia (B-CLL), according to the results of a biochemical and cytogenetic study of patients published online in Experimental and Molecular Pathology.
The study evaluated the serum levels of oxidative stress biomarkers [conjugated dienes (CD), malondialdehyde (MDA), and nitrite levels] and the levels of antioxidant biomarkers [ceruloplasmin (CP) and glutathione peroxidase (GPx)] in 64 B-CLL patients. The relationship between these biomarkers and the presence of cytogenetic abnormalities was examined, according to Tatiana Zhevak, MD, of Sechenov First Moscow (Russia) State Medical University, and colleagues.
Cytogenetic abnormalities have previously been determined to be linked to a poorer prognosis in CLL patients, and factors that increase the frequency of CA have been shown to increase the risk of rapid tumor progression, Dr. Zhevak and her colleagues stated.
Oxidative stress connection
Enhanced oxidative stress was detected in B-CLL patients as shown by their increased levels of serum CD, MDA, and nitrite, as well as a demonstrated imbalance in the antioxidant defense system as shown by an increased serum CP level and decreased serum GPx activity, according to the researchers.
In addition, these metabolic changes were found to be greater in those patients whose lymphocytes harbored specific cytogenetic abnormalities, and could be predicted by the serum levels of CD. Specifically, the odds of harboring a cytogenetic abnormality increased by a factor of 1.88 (P = .004) for every one-unit increase in serum CD level (mcmol/L), according to the authors.
“Collectively, the results support our hypothesis that oxidative stress and resulting lipid peroxidation play a role in pathogenesis of B-CLL and provide a rational basis for the use of agents regulating the pro-oxidant and antioxidant activity in the treatment of B-CLL patients,” the researchers concluded.
The research was unsponsored and the authors reported having no conflicts.
SOURCE: Zhevak T et al. Exp Mol Patholo. 2020 Oct;16:104524 doi: 10.1016/j.yexmp.2020.104524.
Oxidative stress may play a role in pathogenesis of B-cell chronic lymphocytic leukemia (B-CLL), according to the results of a biochemical and cytogenetic study of patients published online in Experimental and Molecular Pathology.
The study evaluated the serum levels of oxidative stress biomarkers [conjugated dienes (CD), malondialdehyde (MDA), and nitrite levels] and the levels of antioxidant biomarkers [ceruloplasmin (CP) and glutathione peroxidase (GPx)] in 64 B-CLL patients. The relationship between these biomarkers and the presence of cytogenetic abnormalities was examined, according to Tatiana Zhevak, MD, of Sechenov First Moscow (Russia) State Medical University, and colleagues.
Cytogenetic abnormalities have previously been determined to be linked to a poorer prognosis in CLL patients, and factors that increase the frequency of CA have been shown to increase the risk of rapid tumor progression, Dr. Zhevak and her colleagues stated.
Oxidative stress connection
Enhanced oxidative stress was detected in B-CLL patients as shown by their increased levels of serum CD, MDA, and nitrite, as well as a demonstrated imbalance in the antioxidant defense system as shown by an increased serum CP level and decreased serum GPx activity, according to the researchers.
In addition, these metabolic changes were found to be greater in those patients whose lymphocytes harbored specific cytogenetic abnormalities, and could be predicted by the serum levels of CD. Specifically, the odds of harboring a cytogenetic abnormality increased by a factor of 1.88 (P = .004) for every one-unit increase in serum CD level (mcmol/L), according to the authors.
“Collectively, the results support our hypothesis that oxidative stress and resulting lipid peroxidation play a role in pathogenesis of B-CLL and provide a rational basis for the use of agents regulating the pro-oxidant and antioxidant activity in the treatment of B-CLL patients,” the researchers concluded.
The research was unsponsored and the authors reported having no conflicts.
SOURCE: Zhevak T et al. Exp Mol Patholo. 2020 Oct;16:104524 doi: 10.1016/j.yexmp.2020.104524.
FROM Experimental and Molecular Pathology