User login
Breakthrough Blood Test for Colorectal Cancer Gets Green Light
The FDA on July 29 approved the test, called Shield, which can accurately detect tumors in the colon or rectum about 87% of the time when the cancer is in treatable early stages. The approval was announced July 29 by the test’s maker, Guardant Health, and comes just months after promising clinical trial results were published in The New England Journal of Medicine.
Colorectal cancer is among the most common types of cancer diagnosed in the United States each year, along with being one of the leading causes of cancer deaths. The condition is treatable in early stages, but about 1 in 3 people don’t stay up to date on regular screenings, which should begin at age 45.
The simplicity of a blood test could make it more likely for people to be screened for and, ultimately, survive the disease. Other primary screening options include feces-based tests or colonoscopy. The 5-year survival rate for colorectal cancer is 64%.
While highly accurate at detecting DNA shed by tumors during treatable stages of colorectal cancer, the Shield test was not as effective at detecting precancerous areas of tissue, which are typically removed after being detected.
In its news release, Guardant Health officials said they anticipate the test to be covered under Medicare. The out-of-pocket cost for people whose insurance does not cover the test has not yet been announced. The test is expected to be available by next week, The New York Times reported.
If someone’s Shield test comes back positive, the person would then get more tests to confirm the result. Shield was shown in trials to have a 10% false positive rate.
“I was in for a routine physical, and my doctor asked when I had my last colonoscopy,” said John Gormly, a 77-year-old business executive in Newport Beach, California, according to a Guardant Health news release. “I said it’s been a long time, so he offered to give me the Shield blood test. A few days later, the result came back positive, so he referred me for a colonoscopy. It turned out I had stage II colon cancer. The tumor was removed, and I recovered very quickly. Thank God I had taken that blood test.”
A version of this article appeared on WebMD.com.
The FDA on July 29 approved the test, called Shield, which can accurately detect tumors in the colon or rectum about 87% of the time when the cancer is in treatable early stages. The approval was announced July 29 by the test’s maker, Guardant Health, and comes just months after promising clinical trial results were published in The New England Journal of Medicine.
Colorectal cancer is among the most common types of cancer diagnosed in the United States each year, along with being one of the leading causes of cancer deaths. The condition is treatable in early stages, but about 1 in 3 people don’t stay up to date on regular screenings, which should begin at age 45.
The simplicity of a blood test could make it more likely for people to be screened for and, ultimately, survive the disease. Other primary screening options include feces-based tests or colonoscopy. The 5-year survival rate for colorectal cancer is 64%.
While highly accurate at detecting DNA shed by tumors during treatable stages of colorectal cancer, the Shield test was not as effective at detecting precancerous areas of tissue, which are typically removed after being detected.
In its news release, Guardant Health officials said they anticipate the test to be covered under Medicare. The out-of-pocket cost for people whose insurance does not cover the test has not yet been announced. The test is expected to be available by next week, The New York Times reported.
If someone’s Shield test comes back positive, the person would then get more tests to confirm the result. Shield was shown in trials to have a 10% false positive rate.
“I was in for a routine physical, and my doctor asked when I had my last colonoscopy,” said John Gormly, a 77-year-old business executive in Newport Beach, California, according to a Guardant Health news release. “I said it’s been a long time, so he offered to give me the Shield blood test. A few days later, the result came back positive, so he referred me for a colonoscopy. It turned out I had stage II colon cancer. The tumor was removed, and I recovered very quickly. Thank God I had taken that blood test.”
A version of this article appeared on WebMD.com.
The FDA on July 29 approved the test, called Shield, which can accurately detect tumors in the colon or rectum about 87% of the time when the cancer is in treatable early stages. The approval was announced July 29 by the test’s maker, Guardant Health, and comes just months after promising clinical trial results were published in The New England Journal of Medicine.
Colorectal cancer is among the most common types of cancer diagnosed in the United States each year, along with being one of the leading causes of cancer deaths. The condition is treatable in early stages, but about 1 in 3 people don’t stay up to date on regular screenings, which should begin at age 45.
The simplicity of a blood test could make it more likely for people to be screened for and, ultimately, survive the disease. Other primary screening options include feces-based tests or colonoscopy. The 5-year survival rate for colorectal cancer is 64%.
While highly accurate at detecting DNA shed by tumors during treatable stages of colorectal cancer, the Shield test was not as effective at detecting precancerous areas of tissue, which are typically removed after being detected.
In its news release, Guardant Health officials said they anticipate the test to be covered under Medicare. The out-of-pocket cost for people whose insurance does not cover the test has not yet been announced. The test is expected to be available by next week, The New York Times reported.
If someone’s Shield test comes back positive, the person would then get more tests to confirm the result. Shield was shown in trials to have a 10% false positive rate.
“I was in for a routine physical, and my doctor asked when I had my last colonoscopy,” said John Gormly, a 77-year-old business executive in Newport Beach, California, according to a Guardant Health news release. “I said it’s been a long time, so he offered to give me the Shield blood test. A few days later, the result came back positive, so he referred me for a colonoscopy. It turned out I had stage II colon cancer. The tumor was removed, and I recovered very quickly. Thank God I had taken that blood test.”
A version of this article appeared on WebMD.com.
Paclitaxel Drug-Drug Interactions in the Military Health System
Background
Paclitaxel was first derived from the bark of the yew tree (Taxus brevifolia). It was discovered as part of a National Cancer Institute program screen of plants and natural products with putative anticancer activity during the 1960s.1-9 Paclitaxel works by suppressing spindle microtube dynamics, which results in the blockage of the metaphase-anaphase transitions, inhibition of mitosis, and induction of apoptosis in a broad spectrum of cancer cells. Paclitaxel also displayed additional anticancer activities, including the suppression of cell proliferation and antiangiogenic effects. However, since the growth of normal body cells may also be affected, other adverse effects (AEs) will also occur.8-18
Two different chemotherapy drugs contain paclitaxel—paclitaxel and nab-paclitaxel—and the US Food and Drug Administration (FDA) recognizes them as separate entities.19-21 Taxol (paclitaxel) was approved by the FDA in 1992 for treating advanced ovarian cancer.20 It has since been approved for the treatment of metastatic breast cancer, AIDS-related Kaposi sarcoma (as an orphan drug), non-small cell lung cancer (NSCLC), and cervical cancers (in combination withbevacizumab) in 1994, 1997, 1999, and 2014, respectively.21 Since 2002, a generic version of Taxol, known as paclitaxel injectable, has been FDA-approved from different manufacturers. According to the National Cancer Institute, a combination of carboplatin and Taxol is approved to treat carcinoma of unknown primary, cervical, endometrial, NSCLC, ovarian, and thymoma cancers.19 Abraxane (nab-paclitaxel) was FDA-approved to treat metastatic breast cancer in 2005. It was later approved for first-line treatment of advanced NSCLC and late-stage pancreatic cancer in 2012 and 2013, respectively. In 2018 and 2020, both Taxol and Abraxane were approved for first-line treatment of metastatic squamous cell NSCLC in combination with carboplatin and pembrolizumab and metastatic triple-negative breast cancer in combination with pembrolizumab, respectively.22-26 In 2019, Abraxane was approved with atezolizumab to treat metastatic triple-negative breast cancer, but this approval was withdrawn in 2021. In 2022, a generic version of Abraxane, known as paclitaxel protein-bound, was released in the United States. Furthermore, paclitaxel-containing formulations also are being studied in the treatment of other types of cancer.19-32
One of the main limitations of paclitaxel is its low solubility in water, which complicates its drug supply. To distribute this hydrophobic anticancer drug efficiently, paclitaxel is formulated and administered to patients via polyethoxylated castor oil or albumin-bound (nab-paclitaxel). However, polyethoxylated castor oil induces complement activation and is the cause of common hypersensitivity reactions related to paclitaxel use.2,17,33-38 Therefore, many alternatives to polyethoxylated castor oil have been researched.
Since 2000, new paclitaxel formulations have emerged using nanomedicine techniques. The difference between these formulations is the drug vehicle. Different paclitaxel-based nanotechnological vehicles have been developed and approved, such as albumin-based nanoparticles, polymeric lipidic nanoparticles, polymeric micelles, and liposomes, with many others in clinical trial phases.3,37 Albumin-based nanoparticles have a high response rate (33%), whereas the response rate for polyethoxylated castor oil is 25% in patients with metastatic breast cancer.33,39-52 The use of paclitaxel dimer nanoparticles also has been proposed as a method for increasing drug solubility.33,53
Paclitaxel is metabolized by cytochrome P450 (CYP) isoenzymes 2C8 and 3A4. When administering paclitaxel with known inhibitors, inducers, or substrates of CYP2C8 or CYP3A4, caution is required.19-22 Regulations for CYP research were not issued until 2008, so potential interactions between paclitaxel and other drugs have not been extensively evaluated in clinical trials. A study of 12 kinase inhibitors showed strong inhibition of CYP2C8 and/or CYP3A4 pathways by these inhibitors, which could alter the ratio of paclitaxel metabolites in vivo, leading to clinically relevant changes.54 Differential metabolism has been linked to paclitaxel-induced neurotoxicity in patients with cancer.55 Nonetheless, variants in the CYP2C8, CYP3A4, CYP3A5, and ABCB1 genes do not account for significant interindividual variability in paclitaxel pharmacokinetics.56 In liver microsomes, losartan inhibited paclitaxel metabolism when used at concentrations > 50 µmol/L.57 Many drug-drug interaction (DDI) studies of CYP2C8 and CYP3A4 have shown similar results for paclitaxel.58-64
The goals of this study are to investigate prescribed drugs used with paclitaxel and determine patient outcomes through several Military Health System (MHS) databases. The investigation focused on (1) the functions of paclitaxel; (2) identifying AEs that patients experienced; (3) evaluating differences when paclitaxel is used alone vs concomitantly and between the completed vs discontinued treatment groups; (4) identifying all drugs used during paclitaxel treatment; and (5) evaluating DDIs with antidepressants (that have an FDA boxed warning and are known to have DDIs confirmed in previous publications) and other drugs.65-67
The Walter Reed National Military Medical Center in Bethesda, Maryland, institutionalreview board approved the study protocol and ensured compliance with the Health Insurance Portability and Accountability Act as an exempt protocol. The Joint Pathology Center (JPC) of the US Department of Defense (DoD) Cancer Registry Program and MHS data experts from the Comprehensive Ambulatory/Professional Encounter Record (CAPER) and the Pharmacy Data Transaction Service (PDTS) provided data for the analysis.
METHODS
The DoD Cancer Registry Program was established in 1986 and currently contains data from 1998 to 2024. CAPER and PDTS are part of the MHS Data Repository/Management Analysis and Reporting Tool database. Each observation in the CAPER record represents an ambulatory encounter at a military treatment facility (MTF). CAPER includes data from 2003 to 2024.
Each observation in the PDTS record represents a prescription filled for an MHS beneficiary at an MTF through the TRICARE mail-order program or a US retail pharmacy. Missing from this record are prescriptions filled at international civilian pharmacies and inpatient pharmacy prescriptions. The MHS Data Repository PDTS record is available from 2002 to 2024. The legacy Composite Health Care System is being replaced by GENESIS at MTFs.
Data Extraction Design
The study design involved a cross-sectional analysis. We requested data extraction for paclitaxel from 1998 to 2022. Data from the DoD Cancer Registry Program were used to identify patients who received cancer treatment. Once patients were identified, the CAPER database was searched for diagnoses to identify other health conditions, whereas the PDTS database was used to populate a list of prescription medications filled during chemotherapy treatment.
Data collected from the JPC included cancer treatment, cancer information, demographics, and physicians’ comments on AEs. Collected data from the MHS include diagnosis and filled prescription history from initiation to completion of the therapy period (or 2 years after the diagnosis date). For the analysis of the DoD Cancer Registry Program and CAPER databases, we used all collected data without excluding any. When analyzing PDTS data, we excluded patients with PDTS data but without a record of paclitaxel being filled, or medications filled outside the chemotherapy period (by evaluating the dispensed date and day of supply).
Data Extraction Analysis
The Surveillance, Epidemiology, and End Results Program Coding and Staging Manual 2016 and the International Classification of Diseases for Oncology, 3rd edition, 1st revision, were used to decode disease and cancer types.68,69 Data sorting and analysis were performed using Microsoft Excel. The percentage for the total was calculated by using the number of patients or data available within the paclitaxel groups divided by the total number of patients or data variables. The subgroup percentage was calculated by using the number of patients or data available within the subgroup divided by the total number of patients in that subgroup.
In alone vs concomitant and completed vs discontinued treatment groups, a 2-tailed, 2-sample z test was used to statistical significance (P < .05) using a statistics website.70 Concomitant was defined as paclitaxel taken with other antineoplastic agent(s) before, after, or at the same time as cancer therapy. For the retrospective data analysis, physicians’ notes with a period, comma, forward slash, semicolon, or space between medication names were interpreted as concurrent, whereas plus (+), minus/plus (-/+), or “and” between drug names that were dispensed on the same day were interpreted as combined with known common combinations: 2 drugs (DM886 paclitaxel and carboplatin and DM881-TC-1 paclitaxel and cisplatin) or 3 drugs (DM887-ACT doxorubicin, cyclophosphamide, and paclitaxel). Completed treatment was defined as paclitaxel as the last medication the patient took without recorded AEs; switching or experiencing AEs was defined as discontinued treatment.
RESULTS
The JPC provided 702 entries for 687 patients with a mean age of 56 years (range, 2 months to 88 years) who were treated with paclitaxel from March 1996 to October 2021. Fifteen patients had duplicate entries because they had multiple cancer sites or occurrences. There were 623 patients (89%) who received paclitaxel for FDA-approved indications. The most common types of cancer identified were 344 patients with breast cancer (49%), 91 patients with lung cancer (13%), 79 patients with ovarian cancer (11%), and 75 patients with endometrial cancer (11%) (Table 1). Seventy-nine patients (11%) received paclitaxel for cancers that were not for FDA-approved indications, including 19 for cancers of the fallopian tube (3%) and 17 for esophageal cancer (2%) (Table 2).
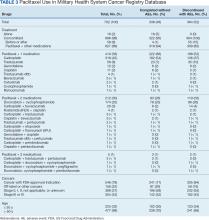
There were 477 patients (68%) aged > 50 years. A total of 304 patients (43%) had a stage III or IV cancer diagnosis and 398 (57%) had stage II or lower (combination of data for stages 0, I, and II; not applicable; and unknown) cancer diagnosis. For systemic treatment, 16 patients (2%) were treated with paclitaxel alone and 686 patients (98%) received paclitaxel concomitantly with additional chemotherapy: 59 patients (9%) in the before or after group, 410 patients (58%) had a 2-drug combination, 212 patients (30%) had a 3-drug combination, and 5 patients (1%) had a 4-drug combination. In addition, for doublet therapies, paclitaxel combined with carboplatin, trastuzumab, gemcitabine, or cisplatin had more patients (318, 58, 12, and 11, respectively) than other combinations (≤ 4 patients). For triplet therapies, paclitaxel combined withdoxorubicin plus cyclophosphamide or carboplatin plus bevacizumab had more patients (174 and 20, respectively) than other combinations, including quadruplet therapies (≤ 4 patients) (Table 3).
Patients were more likely to discontinue paclitaxel if they received concomitant treatment. None of the 16 patients receiving paclitaxel monotherapy experienced AEs, whereas 364 of 686 patients (53%) treated concomitantly discontinued (P < .001). Comparisons of 1 drug vs combination (2 to 4 drugs) and use for treating cancers that were FDA-approved indications vs off-label use were significant (P < .001), whereas comparisons of stage II or lower vs stage III and IV cancer and of those aged ≤ 50 years vs aged > 50 years were not significant (P = .50 andP = .30, respectively) (Table 4).
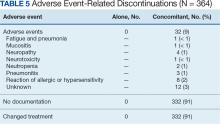
Among the 364 patients who had concomitant treatment and had discontinued their treatment, 332 (91%) switched treatments with no AEs documented and 32 (9%) experienced fatigue with pneumonia, mucositis, neuropathy, neurotoxicity, neutropenia, pneumonitis, allergic or hypersensitivity reaction, or an unknown AE. Patients who discontinued treatment because of unknown AEs had a physician’s note that detailed progressive disease, a significant decline in performance status, and another unknown adverse effect due to a previous sinus tract infection and infectious colitis (Table 5).
Management Analysis and Reporting Tool Database
MHS data analysts provided data on diagnoses for 639 patients among 687 submitteddiagnoses, with 294 patients completing and 345 discontinuing paclitaxel treatment. Patients in the completed treatment group had 3 to 258 unique health conditions documented, while patients in the discontinued treatment group had 4 to 181 unique health conditions documented. The MHS reported 3808 unique diagnosis conditions for the completed group and 3714 for the discontinued group (P = .02).
The mean (SD) number of diagnoses was 51 (31) for the completed and 55 (28) for the discontinued treatment groups (Figure). Among 639 patients who received paclitaxel, the top 5 diagnoses were administrative, including encounters for other administrative examinations; antineoplastic chemotherapy; administrative examination for unspecified; other specified counseling; and adjustment and management of vascular access device. The database does not differentiate between administrative and clinically significant diagnoses.
MHS data analysts provided data for 336 of 687 submitted patients who were prescribed paclitaxel; 46 patients had no PDTS data, and 305 patients had PDTS data without paclitaxel, Taxol, or Abraxane dispensed. Medications that were filled outside the chemotherapy period were removed by evaluating the dispensed date and day of supply. Among these 336 patients, 151 completed the treatment and 185 discontinued, with 14 patients experiencing documented AEs. Patients in the completed treatment group filled 9 to 56 prescriptions while patients in the discontinued treatment group filled 6 to 70 prescriptions.Patients in the discontinued group filled more prescriptions than those who completed treatment: 793 vs 591, respectively (P = .34).
The mean (SD) number of filled prescription drugs was 24 (9) for the completed and 34 (12) for the discontinued treatment group. The 5 most filled prescriptions with paclitaxel from 336 patients with PDTS data were dexamethasone (324 prescriptions with 14 recorded AEs), diphenhydramine (296 prescriptions with 12 recorded AEs), ondansetron (277 prescriptions with 11 recorded AEs), prochlorperazine (265 prescriptions with 12 recorded AEs), and sodium chloride (232 prescriptions with 11 recorded AEs).
DISCUSSION
As a retrospective review, this study is more limited in the strength of its conclusions when compared to randomized control trials. The DoD Cancer Registry Program only contains information about cancer types, stages, treatment regimens, and physicians’ notes. Therefore, noncancer drugs are based solely on the PDTS database. In most cases, physicians' notes on AEs were not detailed. There was no distinction between initial vs later lines of therapy and dosage reductions. The change in status or appearance of a new medical condition did not indicate whether paclitaxel caused the changes to develop or directly worsen a pre-existing condition. The PDTS records prescriptions filled, but that may not reflect patients taking prescriptions.
Paclitaxel
Paclitaxel has a long list of both approved and off-label uses in malignancies as a primary agent and in conjunction with other drugs. The FDA prescribing information for Taxol and Abraxane was last updated in April 2011 and September 2020, respectively.20,21 The National Institutes of Health National Library of Medicine has the current update for paclitaxel on July 2023.19,22 Thus, the prescribed information for paclitaxel referenced in the database may not always be up to date. The combinations of paclitaxel with bevacizumab, carboplatin, or carboplatin and pembrolizumab were not in the Taxol prescribing information. Likewise, a combination of nab-paclitaxel with atezolizumab or carboplatin and pembrolizumab is missing in the Abraxane prescribing information.22-27
The generic name is not the same as a generic drug, which may have slight differences from the brand name product.71 The generic drug versions of Taxol and Abraxane have been approved by the FDA as paclitaxel injectable and paclitaxel-protein bound, respectively. There was a global shortage of nab-paclitaxel from October 2021 to June 2022 because of a manufacturing problem.72 During this shortage, data showed similar comments from physician documents that treatment switched to Taxol due to the Abraxane shortage.
Of 336 patients in the PDTS database with dispensed paclitaxel prescriptions, 276 received paclitaxel (year dispensed, 2013-2022), 27 received Abraxane (year dispensed, 2013-2022), 47 received Taxol (year dispensed, 2004-2015), 8 received both Abraxane and paclitaxel, and 6 received both Taxol and paclitaxel. Based on this information, it appears that the distinction between the drugs was not made in the PDTS until after 2015, 10 years after Abraxane received FDA approval. Abraxane was prescribed in the MHS in 2013, 8 years after FDA approval. There were a few comparison studies of Abraxane and Taxol.73-76
Safety and effectiveness in pediatric patients have not been established for paclitaxel. According to the DoD Cancer Registry Program, the youngest patient was aged 2 months. In 2021, this patient was diagnosed with corpus uteri and treated with carboplatin and Taxol in course 1; in course 2, the patient reacted to Taxol; in course 3, Taxol was replaced with Abraxane; in courses 4 to 7, the patient was treated with carboplatin only.
Discontinued Treatment
Ten patients had prescribed Taxol that was changed due to AEs: 1 was switched to Abraxane and atezolizumab, 3 switched to Abraxane, 2 switched to docetaxel, 1 switched to doxorubicin, and 3 switched to pembrolizumab (based on physician’s comments). Of the 10 patients, 7 had Taxol reaction, 2 experienced disease progression, and 1 experienced high programmed death–ligand 1 expression (this patient with breast cancer was switched to Abraxane and atezolizumab during the accelerated FDA approval phase for atezolizumab, which was later revoked). Five patients were treated with carboplatin and Taxol for cancer of the anal canal (changed to pembrolizumab after disease progression), lung not otherwise specified (changed to carboplatin and pembrolizumab due to Taxol reaction), lower inner quadrant of the breast (changed to doxorubicin due to hypersensitivity reaction), corpus uteri (changed to Abraxane due to Taxol reaction), and ovary (changed to docetaxel due to Taxol reaction). Three patients were treated with doxorubicin, cyclophosphamide, and Taxol for breast cancer; 2 patients with breast cancer not otherwise specified switched to Abraxane due to cardiopulmonary hypersensitivity and Taxol reaction and 1 patient with cancer of the upper outer quadrant of the breast changed to docetaxel due to allergic reaction. One patient, who was treated with paclitaxel, ifosfamide, and cisplatin for metastasis of the lower lobe of the lung and kidney cancer, experienced complications due to infectious colitis (treated with ciprofloxacin) and then switched to pembrolizumab after the disease progressed. These AEs are known in paclitaxel medical literature on paclitaxel AEs.19-24,77-81
Combining 2 or more treatments to target cancer-inducing or cell-sustaining pathways is a cornerstone of chemotherapy.82-84 Most combinations are given on the same day, but some are not. For 3- or 4-drug combinations, doxorubicin and cyclophosphamide were given first, followed by paclitaxel with or withouttrastuzumab, carboplatin, or pembrolizumab. Only 16 patients (2%) were treated with paclitaxel alone; therefore, the completed and discontinued treatment groups are mostly concomitant treatment. As a result, the comparisons of the completed and discontinued treatment groups were almost the same for the diagnosis. The PDTS data have a better result because 2 exclusion criteria were applied before narrowing the analysis down to paclitaxel treatment specifically.
Antidepressants and Other Drugs
Drug response can vary from person to person and can lead to treatment failure related to AEs. One major factor in drug metabolism is CYP.85 CYP2C8 is the major pathway for paclitaxel and CYP3A4 is the minor pathway. When evaluating the noncancer drugs, there were no reports of CYP2C8 inhibition or induction. Over the years, many DDI warnings have been issued for paclitaxel with different drugs in various electronic resources.
Oncologists follow guidelines to prevent DDIs, as paclitaxel is known to have severe, moderate, and minor interactions with other drugs. Among 687 patients, 261 (38%) were prescribed any of 14 antidepressants. Eight of these antidepressants (amitriptyline, citalopram, desipramine, doxepin, venlafaxine, escitalopram, nortriptyline, and trazodone) are metabolized, 3 (mirtazapine, sertraline, and fluoxetine) are metabolized and inhibited, 2 (bupropion and duloxetine) are neither metabolized nor inhibited, and 1 (paroxetine) is inhibited by CYP3A4. Duloxetine, venlafaxine, and trazodone were more commonly dispensed (84, 78, and 42 patients, respectively) than others (≤ 33 patients).
Of 32 patients with documented AEs,14 (44%) had 168 dispensed drugs in the PDTS database. Six patients (19%) were treated with doxorubicin and cyclophosphamide followed by paclitaxel for breast cancer; 6 (19%) were treated with carboplatin and paclitaxel for cancer of the lung (n = 3), corpus uteri (n = 2), and ovary (n = 1); 1 patient (3%) was treated with carboplatin and paclitaxel, then switched to carboplatin, bevacizumab, and paclitaxel, and then completed treatment with carboplatin and paclitaxel for an unspecified female genital cancer; and 1 patient (3%) was treated with cisplatin, ifosfamide, and paclitaxel for metastasis of the lower lobe lung and kidney cancer.
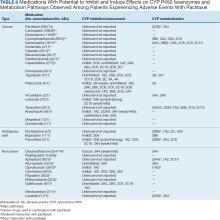
The 14 patients with PDTS data had 18 cancer drugs dispensed. Eleven had moderate interaction reports and 7 had no interaction reports. A total of 165 noncancer drugs were dispensed, of which 3 were antidepressants and had no interactions reported, 8 had moderate interactions reported, and 2 had minor interactions with Taxol and Abraxane, respectively (Table 6).86-129
Of 3 patients who were dispensed bupropion, nortriptyline, or paroxetine, 1 patient with breast cancer was treated with doxorubicin andcyclophosphamide, followed by paclitaxel with bupropion, nortriptyline, pegfilgrastim,dexamethasone, and 17 other noncancer drugs that had no interaction report dispensed during paclitaxel treatment. Of 2 patients with lung cancer, 1 patient was treated with carboplatin and paclitaxel with nortriptyline, dexamethasone, and 13 additional medications, and the second patient was treated with paroxetine, cimetidine, dexamethasone, and 12 other medications. Patients were dispensed up to6 noncancer medications on the same day as paclitaxel administration to control the AEs, not including the prodrugs filled before the treatments. Paroxetine and cimetidine have weak inhibition, and dexamethasone has weak induction of CYP3A4. Therefore, while 1:1 DDIs might have little or no effect with weak inhibit/induce CYP3A4 drugs, 1:1:1 or more combinations could have a different outcome (confirmed in previous publications).65-67
Dispensed on the same day may not mean taken at the same time. One patient experienced an AE with dispensed 50 mg losartan, carboplatin plus paclitaxel, dexamethasone, and 6 other noncancer drugs. Losartan inhibits paclitaxel, which can lead to negative AEs.57,66,67 However, there were no blood or plasma samples taken to confirm the losartan was taken at the same time as the paclitaxel given this was not a clinical trial.
Conclusions
This retrospective study discusses the use of paclitaxel in the MHS and the potential DDIs associated with it. The study population consisted mostly of active-duty personnel, who are required to be healthy or have controlled or nonactive medical diagnoses and be physically fit. This group is mixed with dependents and retirees that are more reflective of the average US population. As a result, this patient population is healthier than the general population, with a lower prevalence of common illnesses such as diabetes and obesity. The study aimed to identify drugs used alongside paclitaxel treatment. While further research is needed to identify potential DDIs among patients who experienced AEs, in vitro testing will need to be conducted before confirming causality. The low number of AEs experienced by only 32 of 702 patients (5%), with no deaths during paclitaxel treatment, indicates that the drug is generally well tolerated. Although this study cannot conclude that concomitant use with noncancer drugs led to the discontinuation of paclitaxel, we can conclude that there seems to be no significant DDIsidentified between paclitaxel and antidepressants. This comprehensive overview provides clinicians with a complete picture of paclitaxel use for 27 years (1996-2022), enabling them to make informed decisions about paclitaxel treatment.
Acknowledgments
The Department of Research Program funds at Walter Reed National Military Medical Center supported this protocol. We sincerely appreciate the contribution of data extraction from the Joint Pathology Center teams (Francisco J. Rentas, John D. McGeeney, Beatriz A. Hallo, and Johnny P. Beason) and the MHS database personnel (Maj Ryan Costantino, Brandon E. Jenkins, and Alexander G. Rittel). We gratefully thank you for the protocol support from the Department of Research programs: CDR Martin L. Boese, CDR Wesley R. Campbell, Maj. Abhimanyu Chandel, CDR Ling Ye, Chelsea N. Powers, Yaling Zhou, Elizabeth Schafer, Micah Stretch, Diane Beaner, and Adrienne Woodard.
1. American Chemical Society. Discovery of camptothecin and taxol. acs.org. Accessed June 4, 2024. https://www.acs.org/education/whatischemistry/landmarks/camptothecintaxol.html
2. Bocci G, Di Paolo A, Danesi R. The pharmacological bases of the antiangiogenic activity of paclitaxel. Angiogenesis. 2013;16(3):481-492. doi:10.1007/s10456-013-9334-0.
3. Meštrovic T. Paclitaxel history. News Medical Life Sciences. Updated March 11, 2023. Accessed June 4, 2024. https://www.news-medical.net/health/Paclitaxel-History.aspx
4. Rowinsky EK, Donehower RC. Paclitaxel (taxol). N Engl J Med. 1995;332(15):1004-1014. doi:10.1056/NEJM199504133321507
5. Walsh V, Goodman J. The billion dollar molecule: Taxol in historical and theoretical perspective. Clio Med. 2002;66:245-267. doi:10.1163/9789004333499_013
6. Perdue RE, Jr, Hartwell JL. The search for plant sources of anticancer drugs. Morris Arboretum Bull. 1969;20:35-53.
7. Wall ME, Wani MC. Camptothecin and taxol: discovery to clinic—thirteenth Bruce F. Cain Memorial Award lecture. Cancer Res. 1995;55:753-760.
8. Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from taxus brevifolia. J Am Chem Soc. 1971;93(9):2325-2327. doi:10.1021/ja00738a045
9. Weaver BA. How taxol/paclitaxel kills cancer cells. Mol Biol Cell. 2014;25(18):2677-2681. doi:10.1091/mbc.E14-04-0916
10. Chen JG, Horwitz SB. Differential mitotic responses to microtubule-stabilizing and-destabilizing drugs. Cancer Res. 2002;62(7):1935-1938.
11. Singh S, Dash AK. Paclitaxel in cancer treatment: perspectives and prospects of its delivery challenges. Crit Rev Ther Drug Carrier Syst. 2009;26(4):333-372. doi:10.1615/critrevtherdrugcarriersyst.v26.i4.10
12. Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277(5698):665-667. doi:10.1038/277665a0
13. Fuchs DA, Johnson RK. Cytologic evidence that taxol, an antineoplastic agent from taxus brevifolia, acts as a mitotic spindle poison. Cancer Treat Rep. 1978;62(8):1219-1222.
14. Walsh V, Goodman J. From taxol to taxol: the changing identities and ownership of an anti-cancer drug. Med Anthropol. 2002;21(3-4):307-336. doi:10.1080/01459740214074
15. Walsh V, Goodman J. Cancer chemotherapy, biodiversity, public and private property: the case of the anti-cancer drug taxol. Soc Sci Med. 1999;49(9):1215-1225. doi:10.1016/s0277-9536(99)00161-6
16. Jordan MA, Wendell K, Gardiner S, Derry WB, Copp H, Wilson L. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 1996;56(4):816-825.
17. Picard M, Castells MC. Re-visiting hypersensitivity reactions to taxanes: a comprehensive review. Clin Rev Allergy Immunol. 2015;49(2):177-191. doi:10.1007/s12016-014-8416-0
18. Zasadil LM, Andersen KA, Yeum D, et al. Cytotoxicity of paclitaxel in breast cancer is due to chromosome missegregation on multipolar spindles. Sci Transl Med. 2014;6:229ra243. doi:10.1126/scitranslmed.3007965
19. National Cancer Institute. Carboplatin-Taxol. Published May 30, 2012. Updated March 22, 2023. Accessed June 4, 2024. https://www.cancer.gov/about-cancer/treatment/drugs/carboplatin-taxol
20. Taxol (paclitaxel). Prescribing information. Bristol-Myers Squibb; 2011. Accessed June 4, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
21. Abraxane (paclitaxel). Prescribing information. Celgene Corporation; 2021. Accessed June 4, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
22. Awosika AO, Farrar MC, Jacobs TF. Paclitaxel. StatPearls. Updated November 18, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK536917/
23. Gerriets V, Kasi A. Bevacizumab. StatPearls. Updated September 1, 2022. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482126/
24. American Cancer Society. Chemotherapy for endometrial cancer. Updated March 27, 2019. Accessed June 4, 2024. https://www.cancer.org/cancer/types/endometrial-cancer/treating/chemotherapy.html
25. US Food and Drug Administration. FDA approves pembrolizumab in combination with chemotherapy for first-line treatment of metastatic squamous NSCLC. October 30, 2018. Updated December 14, 2018. Accessed June 4, 2024. https://www.fda.gov/drugs/fda-approves-pembrolizumab-combination-chemotherapy-first-line-treatment-metastatic-squamous-nsclc
26. US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for locally recurrent unresectable or metastatic triple negative breast cancer. November 13, 2020. Accessed June 4, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-locally-recurrent-unresectable-or-metastatic-triple
27. US Food and Drug Administration. FDA approves atezolizumab for PD-L1 positive unresectable locally advanced or metastatic triple-negative breast. March 8, 2019. Updated March 18, 2019. Accessed June 5, 2024. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-atezolizumab-pd-l1-positive-unresectable-locally-advanced-or-metastatic-triple-negative
28. US Food and Drug Administration. FDA issues alert about efficacy and potential safety concerns with atezolizumab in combination with paclitaxel for treatment of breast cancer. September 8, 2020. Accessed June 5, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-issues-alert-about-efficacy-and-potential-safety-concerns-atezolizumab-combination-paclitaxel
29. Tan AR. Chemoimmunotherapy: still the standard of care for metastatic triple-negative breast cancer. ASCO Daily News. February 23, 2022. Accessed June 5, 2024. https://dailynews.ascopubs.org/do/chemoimmunotherapy-still-standard-care-metastatic-triple-negative-breast-cancer
30. McGuire WP, Rowinsky EK, Rosenshein NB, et al. Taxol: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Ann Intern Med. 1989;111(4):273-279. doi:10.7326/0003-4819-111-4-273
31. Milas L, Hunter NR, Kurdoglu B, et al. Kinetics of mitotic arrest and apoptosis in murine mammary and ovarian tumors treated with taxol. Cancer Chemother Pharmacol. 1995;35(4):297-303. doi:10.1007/BF00689448
32. Searle J, Collins DJ, Harmon B, Kerr JF. The spontaneous occurrence of apoptosis in squamous carcinomas of the uterine cervix. Pathology. 1973;5(2):163-169. doi:10.3109/00313027309060831
33. Gallego-Jara J, Lozano-Terol G, Sola-Martínez RA, Cánovas-Díaz M, de Diego Puente T. A compressive review about taxol®: history and future challenges. Molecules. 2020;25(24):5986. doi:10.3390/molecules25245986
34. Bernabeu E, Cagel M, Lagomarsino E, Moretton M, Chiappetta DA. Paclitaxel: What has been done and the challenges remain ahead. Int J Pharm. 2017;526(1-2):474-495. doi:10.1016/j.ijpharm.2017.05.016
35. Nehate C, Jain S, Saneja A, et al. Paclitaxel formulations: challenges and novel delivery options. Curr Drug Deliv. 2014;11(6):666-686. doi:10.2174/1567201811666140609154949
36. Gelderblom H, Verweij J, Nooter K, Sparreboom A, Cremophor EL. The drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37(13):1590-1598. doi:10.1016/S0959-8049(01)00171-x
37. Chowdhury MR, Moshikur RM, Wakabayashi R, et al. In vivo biocompatibility, pharmacokinetics, antitumor efficacy, and hypersensitivity evaluation of ionic liquid-mediated paclitaxel formulations. Int J Pharm. 2019;565:219-226. doi:10.1016/j.ijpharm.2019.05.020
38. Borgå O, Henriksson R, Bjermo H, Lilienberg E, Heldring N, Loman N. Maximum tolerated dose and pharmacokinetics of paclitaxel micellar in patients with recurrent malignant solid tumours: a dose-escalation study. Adv Ther. 2019;36(5):1150-1163. doi:10.1007/s12325-019-00909-6
39. Rouzier R, Rajan R, Wagner P, et al. Microtubule-associated protein tau: a marker of paclitaxel sensitivity in breast cancer. Proc Natl Acad Sci USA. 2005;102(23):8315-8320. doi:10.1073/pnas.0408974102
40. Choudhury H, Gorain B, Tekade RK, Pandey M, Karmakar S, Pal TK. Safety against nephrotoxicity in paclitaxel treatment: oral nanocarrier as an effective tool in preclinical evaluation with marked in vivo antitumor activity. Regul Toxicol Pharmacol. 2017;91:179-189. doi:10.1016/j.yrtph.2017.10.023
41. Barkat MA, Beg S, Pottoo FH, Ahmad FJ. Nanopaclitaxel therapy: an evidence based review on the battle for next-generation formulation challenges. Nanomedicine (Lond). 2019;14(10):1323-1341. doi:10.2217/nnm-2018-0313
42. Sofias AM, Dunne M, Storm G, Allen C. The battle of “nano” paclitaxel. Adv Drug Deliv Rev. 2017;122:20-30. doi:10.1016/j.addr.2017.02.003
43. Yang N, Wang C, Wang J, et al. Aurora inase a stabilizes FOXM1 to enhance paclitaxel resistance in triple-negative breast cancer. J Cell Mol Med. 2019;23(9):6442-6453. doi:10.1111/jcmm.14538
44. Chowdhury MR, Moshikur RM, Wakabayashi R, et al. Ionic-liquid-based paclitaxel preparation: a new potential formulation for cancer treatment. Mol Pharm. 2018;15(16):2484-2488. doi:10.1021/acs.molpharmaceut.8b00305
45. Chung HJ, Kim HJ, Hong ST. Tumor-specific delivery of a paclitaxel-loading HSA-haemin nanoparticle for cancer treatment. Nanomedicine. 2020;23:102089. doi:10.1016/j.nano.2019.102089
46. Ye L, He J, Hu Z, et al. Antitumor effect and toxicity of lipusu in rat ovarian cancer xenografts. Food Chem Toxicol. 2013;52:200-206. doi:10.1016/j.fct.2012.11.004
47. Ma WW, Lam ET, Dy GK, et al. A pharmacokinetic and dose-escalating study of paclitaxel injection concentrate for nano-dispersion (PICN) alone and with arboplatin in patients with advanced solid tumors. J Clin Oncol. 2013;31:2557. doi:10.1200/jco.2013.31.15_suppl.2557
48. Micha JP, Goldstein BH, Birk CL, Rettenmaier MA, Brown JV. Abraxane in the treatment of ovarian cancer: the absence of hypersensitivity reactions. Gynecol Oncol. 2006;100(2):437-438. doi:10.1016/j.ygyno.2005.09.012
49. Ingle SG, Pai RV, Monpara JD, Vavia PR. Liposils: an effective strategy for stabilizing paclitaxel loaded liposomes by surface coating with silica. Eur J Pharm Sci. 2018;122:51-63. doi:10.1016/j.ejps.2018.06.025
50. Abriata JP, Turatti RC, Luiz MT, et al. Development, characterization and biological in vitro assays of paclitaxel-loaded PCL polymeric nanoparticles. Mater Sci Eng C Mater Biol Appl. 2019;96:347-355. doi:10.1016/j.msec.2018.11.035
51. Hu J, Fu S, Peng Q, et al. Paclitaxel-loaded polymeric nanoparticles combined with chronomodulated chemotherapy on lung cancer: in vitro and in vivo evaluation. Int J Pharm. 2017;516(1-2):313-322. doi:10.1016/j.ijpharm.2016.11.047
52. Dranitsaris G, Yu B, Wang L, et al. Abraxane® vs Taxol® for patients with advanced breast cancer: a prospective time and motion analysis from a chinese health care perspective. J Oncol Pharm Pract. 2016;22(2):205-211. doi:10.1177/1078155214556008
53. Pei Q, Hu X, Liu S, Li Y, Xie Z, Jing X. Paclitaxel dimers assembling nanomedicines for treatment of cervix carcinoma. J Control Release. 2017;254:23-33. doi:10.1016/j.jconrel.2017.03.391
54. Wang Y, Wang M, Qi H, et al. Pathway-dependent inhibition of paclitaxel hydroxylation by kinase inhibitors and assessment of drug-drug interaction potentials. Drug Metab Dispos. 2014;42(4):782-795. doi:10.1124/dmd.113.053793
55. Shen F, Jiang G, Philips S, et al. Cytochrome P450 oxidoreductase (POR) associated with severe paclitaxel-induced peripheral neuropathy in patients of european ancestry from ECOG-ACRIN E5103. Clin Cancer Res. 2023;29(13):2494-2500. doi:10.1158/1078-0432.CCR-22-2431
56. Henningsson A, Marsh S, Loos WJ, et al. Association of CYP2C8, CYP3A4, CYP3A5, and ABCB1 polymorphisms with the pharmacokinetics of paclitaxel. Clin Cancer Res. 2005;11(22):8097-8104. doi:10.1158/1078-0432.CCR-05-1152
57. Mukai Y, Senda A, Toda T, et al. Drug-drug interaction between losartan and paclitaxel in human liver microsomes with different CYP2C8 genotypes. Basic Clin Pharmacol Toxicol. 2015;116(6):493-498. doi:10.1111/bcpt.12355
58. Kawahara B, Faull KF, Janzen C, Mascharak PK. Carbon monoxide inhibits cytochrome P450 enzymes CYP3A4/2C8 in human breast cancer cells, increasing sensitivity to paclitaxel. J Med Chem. 2021;64(12):8437-8446. doi:10.1021/acs.jmedchem.1c00404
59. Cresteil T, Monsarrat B, Dubois J, Sonnier M, Alvinerie P, Gueritte F. Regioselective metabolism of taxoids by human CYP3A4 and 2C8: structure-activity relationship. Drug Metab Dispos. 2002;30(4):438-445. doi:10.1124/dmd.30.4.438
60. Taniguchi R, Kumai T, Matsumoto N, et al. Utilization of human liver microsomes to explain individual differences in paclitaxel metabolism by CYP2C8 and CYP3A4. J Pharmacol Sci. 2005;97(1):83-90. doi:10.1254/jphs.fp0040603
61. Nakayama A, Tsuchiya K, Xu L, Matsumoto T, Makino T. Drug-interaction between paclitaxel and goshajinkigan extract and its constituents. J Nat Med. 2022;76(1):59-67. doi:10.1007/s11418-021-01552-8
62. Monsarrat B, Chatelut E, Royer I, et al. Modification of paclitaxel metabolism in a cancer patient by induction of cytochrome P450 3A4. Drug Metab Dispos. 1998;26(3):229-233.
63. Walle T. Assays of CYP2C8- and CYP3A4-mediated metabolism of taxol in vivo and in vitro. Methods Enzymol. 1996;272:145-151. doi:10.1016/s0076-6879(96)72018-9
64. Hanioka N, Matsumoto K, Saito Y, Narimatsu S. Functional characterization of CYP2C8.13 and CYP2C8.14: catalytic activities toward paclitaxel. Basic Clin Pharmacol Toxicol. 2010;107(1):565-569. doi:10.1111/j.1742-7843.2010.00543.x
65. Luong TT, Powers CN, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interactions (DDIs) of gefitinib with/without losartan and selective serotonin reuptake inhibitors (SSRIs): citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and venlafaxine. Curr Res Pharmacol Drug Discov. 2022;3:100112. doi:10.1016/j.crphar.2022.100112
66. Luong TT, McAnulty MJ, Evers DL, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interaction (DDI) of gefitinib or erlotinib with Cytochrome P450 (CYP) inhibiting drugs, fluoxetine and/or losartan. Curr Res Toxicol. 2021;2:217-224. doi:10.1016/j.crtox.2021.05.006
67. Luong TT, Powers CN, Reinhardt BJ, et al. Retrospective evaluation of drug-drug interactions with erlotinib and gefitinib use in the military health system. Fed Pract. 2023;40(suppl 3):S24-S34. doi:10.12788/fp.0401
68. Adamo M, Dickie L, Ruhl J. SEER program coding and staging manual 2016. National Cancer Institute. Accessed June 5, 2024. https://seer.cancer.gov/archive/manuals/2016/SPCSM_2016_maindoc.pdf
69. World Health Organization. International classification of diseases for oncology (ICD-O) 3rd ed, 1st revision. World Health Organization; 2013. Accessed June 5, 2024. https://apps.who.int/iris/handle/10665/96612
70. Z score calculator for 2 population proportions. Social science statistics. Accessed June 5, 2024. https://www.socscistatistics.com/tests/ztest/default2.aspx
71. US Food and Drug Administration. Generic drugs: question & answers. FDA.gov. Accessed June 5, 2024. https://www.fda.gov/drugs/frequently-asked-questions-popular-topics/generic-drugs-questions-answers
72. Oura M, Saito H, Nishikawa Y. Shortage of nab-paclitaxel in Japan and around the world: issues in global information sharing. JMA J. 2023;6(2):192-195. doi:10.31662/jmaj.2022-0179
73. Yuan H, Guo H, Luan X, et al. Albumin nanoparticle of paclitaxel (abraxane) decreases while taxol increases breast cancer stem cells in treatment of triple negative breast cancer. Mol Pharm. 2020;17(7):2275-2286. doi:10.1021/acs.molpharmaceut.9b01221
74. Dranitsaris G, Yu B, Wang L, et al. Abraxane® versus Taxol® for patients with advanced breast cancer: a prospective time and motion analysis from a Chinese health care perspective. J Oncol Pharm Pract. 2016;22(2):205-211. doi:10.1177/1078155214556008
75. Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794-7803. doi:10.1200/JCO.2005.04.
76. Liu M, Liu S, Yang L, Wang S. Comparison between nab-paclitaxel and solvent-based taxanes as neoadjuvant therapy in breast cancer: a systematic review and meta-analysis. BMC Cancer. 2021;21(1):118. doi:10.1186/s12885-021-07831-7
77. Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (taxol). Semin Oncol. 1993;20(4 Suppl 3):1-15.
78. Banerji A, Lax T, Guyer A, Hurwitz S, Camargo CA Jr, Long AA. Management of hypersensitivity reactions to carboplatin and paclitaxel in an outpatient oncology infusion center: a 5-year review. J Allergy Clin Immunol Pract. 2014;2(4):428-433. doi:10.1016/j.jaip.2014.04.010
79. Staff NP, Fehrenbacher JC, Caillaud M, Damaj MI, Segal RA, Rieger S. Pathogenesis of paclitaxel-induced peripheral neuropathy: a current review of in vitro and in vivo findings using rodent and human model systems. Exp Neurol. 2020;324:113121. doi:10.1016/j.expneurol.2019.113121
80. Postma TJ, Vermorken JB, Liefting AJ, Pinedo HM, Heimans JJ. Paclitaxel-induced neuropathy. Ann Oncol. 1995;6(5):489-494. doi:10.1093/oxfordjournals.annonc.a059220
81. Liu JM, Chen YM, Chao Y, et al. Paclitaxel-induced severe neuropathy in patients with previous radiotherapy to the head and neck region. J Natl Cancer Inst. 1996;88(14):1000-1002. doi:10.1093/jnci/88.14.1000-a
82. Bayat Mokhtari R, Homayouni TS, Baluch N, et al. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022-38043. doi:10.18632/oncotarget.16723
83. Blagosklonny MV. Analysis of FDA approved anticancer drugs reveals the future of cancer therapy. Cell Cycle. 2004;3(8):1035-1042.
84. Yap TA, Omlin A, de Bono JS. Development of therapeutic combinations targeting major cancer signaling pathways. J Clin Oncol. 2013;31(12):1592-1605. doi:10.1200/JCO.2011.37.6418
85. Gilani B, Cassagnol M. Biochemistry, Cytochrome P450. StatPearls. Updated April 24, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557698/
86. LiverTox: clinical and research information on drug-induced liver injury; 2012. Carboplatin. Updated September 15, 2020. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548565/
87. Carboplatin. Prescribing information. Teva Parenteral Medicines; 2012. Accessed June 5, 204. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/077139Orig1s016lbl.pdf
88. Johnson-Arbor K, Dubey R. Doxorubicin. StatPearls. Updated August 8, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459232/
89. Doxorubicin hydrochloride injection. Prescribing information. Pfizer; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/050467s078,050629s030lbl.pdf
90. Gor, PP, Su, HI, Gray, RJ, et al. Cyclophosphamide-metabolizing enzyme polymorphisms and survival outcomes after adjuvant chemotherapy for node-positive breast cancer: a retrospective cohort study. Breast Cancer Res. 2010;12(3):R26. doi:10.1186/bcr2570
91. Cyclophosphamide. Prescribing information. Ingenus Pharmaceuticals; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212501s000lbl.pdf
92. Gemcitabine. Prescribing information. Hospira; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/200795Orig1s010lbl.pdf
93. Ifex (ifosfamide). Prescribing information. Baxter; 2012. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/019763s017lbl.pdf
94. Cisplatin. Prescribing information. WG Critical Care; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/018057s089lbl.pdf
95. Gerriets V, Kasi A. Bevacizumab. StatPearls. Updated August 28, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482126/
96. Avastin (bevacizumab). Prescribing information. Genentech; 2022. Accessed June 5, 2024. https://www.accessdata .fda.gov/drugsatfda_docs/label/2022/125085s340lbl.pdf
 97. Keytruda (pembrolizumab). Prescribing information. Merck; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf
97. Keytruda (pembrolizumab). Prescribing information. Merck; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf
98. Dean L, Kane M. Capecitabine therapy and DPYD genotype. National Center for Biotechnology Information (US); 2012. Updated November 2, 2020. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK385155/
99. Xeloda (capecitabine). Prescribing information. Roche; 2000. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2000/20896lbl.pdf
100. Pemetrexed injection. Prescribing information. Fareva Unterach; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214657s000lbl.pdf
101. Topotecan Injection. Prescribing information. Zydus Hospira Oncology; 2014. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/200582s001lbl.pdf
102. Ibrance (palbociclib). Prescribing information. Pfizer; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/207103s008lbl.pdf
103. Navelbine (vinorelbine) injection. Prescribing information. Pierre Fabre Médicament; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020388s037lbl.pdf
104. LiverTox: clinical and research information on drug-induced liver injury; 2012. Letrozole. Updated July 25, 2017. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548381/
105. Femara (letrozole). Prescribing information. Novartis; 2014. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020726s027lbl.pdf
106. Soltamox (tamoxifen citrate). Prescribing information. Rosemont Pharmaceuticals; 2018. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021807s005lbl.pdf
107. LiverTox: clinical and research information on drug-induced liver injury; 2012. Anastrozole. Updated July 25, 2017. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548189/
108. Grimm SW, Dyroff MC. Inhibition of human drug metabolizing cytochromes P450 by anastrozole, a potent and selective inhibitor of aromatase. Drug Metab Dispos. 1997;25(5):598-602.
109. Arimidex (anastrozole). Prescribing information. AstraZeneca; 2010. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020541s026lbl.pdf
110. Megace (megestrol acetate). Prescribing information. Endo Pharmaceuticals; 2018. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021778s024lbl.pdf
111. Imfinzi (durvalumab). Prescribing information. AstraZeneca; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761069s018lbl.pdf
112. Merwar G, Gibbons JR, Hosseini SA, et al. Nortriptyline. StatPearls. Updated June 5, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482214/
113. Pamelor (nortriptyline HCl). Prescribing information. Patheon Inc.; 2012. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/018012s029,018013s061lbl.pdf
114. Wellbutrin (bupropion hydrochloride). Prescribing information. GlaxoSmithKline; 2017. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018644s052lbl.pdf
115. Paxil (paroxetine). Prescribing information. Apotex Inc.; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020031s077lbl.pdf
116. Johnson DB, Lopez MJ, Kelley B. Dexamethasone. StatPearls. Updated May 2, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482130/
117. Hemady (dexamethasone). Prescribing information. Dexcel Pharma; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211379s000lbl.pdf
118. Parker SD, King N, Jacobs TF. Pegfilgrastim. StatPearls. Updated May 9, 2024. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK532893/
119. Fylnetra (pegfilgrastim-pbbk). Prescribing information. Kashiv BioSciences; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761084s000lbl.pdf
120. Emend (aprepitant). Prescribing information. Merck; 2015. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207865lbl.pdf
121. Lipitor (atorvastatin calcium). Prescribing information. Viatris Specialty; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020702Orig1s079correctedlbl.pdf
122. Cipro (ciprofloxacin hydrochloride). Prescribing information. Bayer HealthCare Pharmaceuticals Inc.; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/019537s090,020780s047lbl.pdf
123. Pino MA, Azer SA. Cimetidine. StatPearls. Updated March 6, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK544255/
124. Tagament (Cimetidine). Prescribing information. Mylan; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020238Orig1s024lbl.pdf
125. Neupogen (filgrastim). Prescribing information. Amgen Inc.; 2015. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/103353s5184lbl.pdf
126. Flagyl (metronidazole). Prescribing information. Pfizer; 2013. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020334s008lbl.pdf
127. Zymaxid (gatifloxacin ophthalmic solution). Prescribing information. Allergan; 2016. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/022548s002lbl.pdf
128. Macrobid (nitrofurantoin monohydrate). Prescribing information. Procter and Gamble Pharmaceutical Inc.; 2009. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020064s019lbl.pdf
129. Hyzaar (losartan). Prescribing information. Merck; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020387s067lbl.pdf
Background
Paclitaxel was first derived from the bark of the yew tree (Taxus brevifolia). It was discovered as part of a National Cancer Institute program screen of plants and natural products with putative anticancer activity during the 1960s.1-9 Paclitaxel works by suppressing spindle microtube dynamics, which results in the blockage of the metaphase-anaphase transitions, inhibition of mitosis, and induction of apoptosis in a broad spectrum of cancer cells. Paclitaxel also displayed additional anticancer activities, including the suppression of cell proliferation and antiangiogenic effects. However, since the growth of normal body cells may also be affected, other adverse effects (AEs) will also occur.8-18
Two different chemotherapy drugs contain paclitaxel—paclitaxel and nab-paclitaxel—and the US Food and Drug Administration (FDA) recognizes them as separate entities.19-21 Taxol (paclitaxel) was approved by the FDA in 1992 for treating advanced ovarian cancer.20 It has since been approved for the treatment of metastatic breast cancer, AIDS-related Kaposi sarcoma (as an orphan drug), non-small cell lung cancer (NSCLC), and cervical cancers (in combination withbevacizumab) in 1994, 1997, 1999, and 2014, respectively.21 Since 2002, a generic version of Taxol, known as paclitaxel injectable, has been FDA-approved from different manufacturers. According to the National Cancer Institute, a combination of carboplatin and Taxol is approved to treat carcinoma of unknown primary, cervical, endometrial, NSCLC, ovarian, and thymoma cancers.19 Abraxane (nab-paclitaxel) was FDA-approved to treat metastatic breast cancer in 2005. It was later approved for first-line treatment of advanced NSCLC and late-stage pancreatic cancer in 2012 and 2013, respectively. In 2018 and 2020, both Taxol and Abraxane were approved for first-line treatment of metastatic squamous cell NSCLC in combination with carboplatin and pembrolizumab and metastatic triple-negative breast cancer in combination with pembrolizumab, respectively.22-26 In 2019, Abraxane was approved with atezolizumab to treat metastatic triple-negative breast cancer, but this approval was withdrawn in 2021. In 2022, a generic version of Abraxane, known as paclitaxel protein-bound, was released in the United States. Furthermore, paclitaxel-containing formulations also are being studied in the treatment of other types of cancer.19-32
One of the main limitations of paclitaxel is its low solubility in water, which complicates its drug supply. To distribute this hydrophobic anticancer drug efficiently, paclitaxel is formulated and administered to patients via polyethoxylated castor oil or albumin-bound (nab-paclitaxel). However, polyethoxylated castor oil induces complement activation and is the cause of common hypersensitivity reactions related to paclitaxel use.2,17,33-38 Therefore, many alternatives to polyethoxylated castor oil have been researched.
Since 2000, new paclitaxel formulations have emerged using nanomedicine techniques. The difference between these formulations is the drug vehicle. Different paclitaxel-based nanotechnological vehicles have been developed and approved, such as albumin-based nanoparticles, polymeric lipidic nanoparticles, polymeric micelles, and liposomes, with many others in clinical trial phases.3,37 Albumin-based nanoparticles have a high response rate (33%), whereas the response rate for polyethoxylated castor oil is 25% in patients with metastatic breast cancer.33,39-52 The use of paclitaxel dimer nanoparticles also has been proposed as a method for increasing drug solubility.33,53
Paclitaxel is metabolized by cytochrome P450 (CYP) isoenzymes 2C8 and 3A4. When administering paclitaxel with known inhibitors, inducers, or substrates of CYP2C8 or CYP3A4, caution is required.19-22 Regulations for CYP research were not issued until 2008, so potential interactions between paclitaxel and other drugs have not been extensively evaluated in clinical trials. A study of 12 kinase inhibitors showed strong inhibition of CYP2C8 and/or CYP3A4 pathways by these inhibitors, which could alter the ratio of paclitaxel metabolites in vivo, leading to clinically relevant changes.54 Differential metabolism has been linked to paclitaxel-induced neurotoxicity in patients with cancer.55 Nonetheless, variants in the CYP2C8, CYP3A4, CYP3A5, and ABCB1 genes do not account for significant interindividual variability in paclitaxel pharmacokinetics.56 In liver microsomes, losartan inhibited paclitaxel metabolism when used at concentrations > 50 µmol/L.57 Many drug-drug interaction (DDI) studies of CYP2C8 and CYP3A4 have shown similar results for paclitaxel.58-64
The goals of this study are to investigate prescribed drugs used with paclitaxel and determine patient outcomes through several Military Health System (MHS) databases. The investigation focused on (1) the functions of paclitaxel; (2) identifying AEs that patients experienced; (3) evaluating differences when paclitaxel is used alone vs concomitantly and between the completed vs discontinued treatment groups; (4) identifying all drugs used during paclitaxel treatment; and (5) evaluating DDIs with antidepressants (that have an FDA boxed warning and are known to have DDIs confirmed in previous publications) and other drugs.65-67
The Walter Reed National Military Medical Center in Bethesda, Maryland, institutionalreview board approved the study protocol and ensured compliance with the Health Insurance Portability and Accountability Act as an exempt protocol. The Joint Pathology Center (JPC) of the US Department of Defense (DoD) Cancer Registry Program and MHS data experts from the Comprehensive Ambulatory/Professional Encounter Record (CAPER) and the Pharmacy Data Transaction Service (PDTS) provided data for the analysis.
METHODS
The DoD Cancer Registry Program was established in 1986 and currently contains data from 1998 to 2024. CAPER and PDTS are part of the MHS Data Repository/Management Analysis and Reporting Tool database. Each observation in the CAPER record represents an ambulatory encounter at a military treatment facility (MTF). CAPER includes data from 2003 to 2024.
Each observation in the PDTS record represents a prescription filled for an MHS beneficiary at an MTF through the TRICARE mail-order program or a US retail pharmacy. Missing from this record are prescriptions filled at international civilian pharmacies and inpatient pharmacy prescriptions. The MHS Data Repository PDTS record is available from 2002 to 2024. The legacy Composite Health Care System is being replaced by GENESIS at MTFs.
Data Extraction Design
The study design involved a cross-sectional analysis. We requested data extraction for paclitaxel from 1998 to 2022. Data from the DoD Cancer Registry Program were used to identify patients who received cancer treatment. Once patients were identified, the CAPER database was searched for diagnoses to identify other health conditions, whereas the PDTS database was used to populate a list of prescription medications filled during chemotherapy treatment.
Data collected from the JPC included cancer treatment, cancer information, demographics, and physicians’ comments on AEs. Collected data from the MHS include diagnosis and filled prescription history from initiation to completion of the therapy period (or 2 years after the diagnosis date). For the analysis of the DoD Cancer Registry Program and CAPER databases, we used all collected data without excluding any. When analyzing PDTS data, we excluded patients with PDTS data but without a record of paclitaxel being filled, or medications filled outside the chemotherapy period (by evaluating the dispensed date and day of supply).
Data Extraction Analysis
The Surveillance, Epidemiology, and End Results Program Coding and Staging Manual 2016 and the International Classification of Diseases for Oncology, 3rd edition, 1st revision, were used to decode disease and cancer types.68,69 Data sorting and analysis were performed using Microsoft Excel. The percentage for the total was calculated by using the number of patients or data available within the paclitaxel groups divided by the total number of patients or data variables. The subgroup percentage was calculated by using the number of patients or data available within the subgroup divided by the total number of patients in that subgroup.
In alone vs concomitant and completed vs discontinued treatment groups, a 2-tailed, 2-sample z test was used to statistical significance (P < .05) using a statistics website.70 Concomitant was defined as paclitaxel taken with other antineoplastic agent(s) before, after, or at the same time as cancer therapy. For the retrospective data analysis, physicians’ notes with a period, comma, forward slash, semicolon, or space between medication names were interpreted as concurrent, whereas plus (+), minus/plus (-/+), or “and” between drug names that were dispensed on the same day were interpreted as combined with known common combinations: 2 drugs (DM886 paclitaxel and carboplatin and DM881-TC-1 paclitaxel and cisplatin) or 3 drugs (DM887-ACT doxorubicin, cyclophosphamide, and paclitaxel). Completed treatment was defined as paclitaxel as the last medication the patient took without recorded AEs; switching or experiencing AEs was defined as discontinued treatment.
RESULTS
The JPC provided 702 entries for 687 patients with a mean age of 56 years (range, 2 months to 88 years) who were treated with paclitaxel from March 1996 to October 2021. Fifteen patients had duplicate entries because they had multiple cancer sites or occurrences. There were 623 patients (89%) who received paclitaxel for FDA-approved indications. The most common types of cancer identified were 344 patients with breast cancer (49%), 91 patients with lung cancer (13%), 79 patients with ovarian cancer (11%), and 75 patients with endometrial cancer (11%) (Table 1). Seventy-nine patients (11%) received paclitaxel for cancers that were not for FDA-approved indications, including 19 for cancers of the fallopian tube (3%) and 17 for esophageal cancer (2%) (Table 2).
There were 477 patients (68%) aged > 50 years. A total of 304 patients (43%) had a stage III or IV cancer diagnosis and 398 (57%) had stage II or lower (combination of data for stages 0, I, and II; not applicable; and unknown) cancer diagnosis. For systemic treatment, 16 patients (2%) were treated with paclitaxel alone and 686 patients (98%) received paclitaxel concomitantly with additional chemotherapy: 59 patients (9%) in the before or after group, 410 patients (58%) had a 2-drug combination, 212 patients (30%) had a 3-drug combination, and 5 patients (1%) had a 4-drug combination. In addition, for doublet therapies, paclitaxel combined with carboplatin, trastuzumab, gemcitabine, or cisplatin had more patients (318, 58, 12, and 11, respectively) than other combinations (≤ 4 patients). For triplet therapies, paclitaxel combined withdoxorubicin plus cyclophosphamide or carboplatin plus bevacizumab had more patients (174 and 20, respectively) than other combinations, including quadruplet therapies (≤ 4 patients) (Table 3).
Patients were more likely to discontinue paclitaxel if they received concomitant treatment. None of the 16 patients receiving paclitaxel monotherapy experienced AEs, whereas 364 of 686 patients (53%) treated concomitantly discontinued (P < .001). Comparisons of 1 drug vs combination (2 to 4 drugs) and use for treating cancers that were FDA-approved indications vs off-label use were significant (P < .001), whereas comparisons of stage II or lower vs stage III and IV cancer and of those aged ≤ 50 years vs aged > 50 years were not significant (P = .50 andP = .30, respectively) (Table 4).
Among the 364 patients who had concomitant treatment and had discontinued their treatment, 332 (91%) switched treatments with no AEs documented and 32 (9%) experienced fatigue with pneumonia, mucositis, neuropathy, neurotoxicity, neutropenia, pneumonitis, allergic or hypersensitivity reaction, or an unknown AE. Patients who discontinued treatment because of unknown AEs had a physician’s note that detailed progressive disease, a significant decline in performance status, and another unknown adverse effect due to a previous sinus tract infection and infectious colitis (Table 5).
Management Analysis and Reporting Tool Database
MHS data analysts provided data on diagnoses for 639 patients among 687 submitteddiagnoses, with 294 patients completing and 345 discontinuing paclitaxel treatment. Patients in the completed treatment group had 3 to 258 unique health conditions documented, while patients in the discontinued treatment group had 4 to 181 unique health conditions documented. The MHS reported 3808 unique diagnosis conditions for the completed group and 3714 for the discontinued group (P = .02).
The mean (SD) number of diagnoses was 51 (31) for the completed and 55 (28) for the discontinued treatment groups (Figure). Among 639 patients who received paclitaxel, the top 5 diagnoses were administrative, including encounters for other administrative examinations; antineoplastic chemotherapy; administrative examination for unspecified; other specified counseling; and adjustment and management of vascular access device. The database does not differentiate between administrative and clinically significant diagnoses.
MHS data analysts provided data for 336 of 687 submitted patients who were prescribed paclitaxel; 46 patients had no PDTS data, and 305 patients had PDTS data without paclitaxel, Taxol, or Abraxane dispensed. Medications that were filled outside the chemotherapy period were removed by evaluating the dispensed date and day of supply. Among these 336 patients, 151 completed the treatment and 185 discontinued, with 14 patients experiencing documented AEs. Patients in the completed treatment group filled 9 to 56 prescriptions while patients in the discontinued treatment group filled 6 to 70 prescriptions.Patients in the discontinued group filled more prescriptions than those who completed treatment: 793 vs 591, respectively (P = .34).
The mean (SD) number of filled prescription drugs was 24 (9) for the completed and 34 (12) for the discontinued treatment group. The 5 most filled prescriptions with paclitaxel from 336 patients with PDTS data were dexamethasone (324 prescriptions with 14 recorded AEs), diphenhydramine (296 prescriptions with 12 recorded AEs), ondansetron (277 prescriptions with 11 recorded AEs), prochlorperazine (265 prescriptions with 12 recorded AEs), and sodium chloride (232 prescriptions with 11 recorded AEs).
DISCUSSION
As a retrospective review, this study is more limited in the strength of its conclusions when compared to randomized control trials. The DoD Cancer Registry Program only contains information about cancer types, stages, treatment regimens, and physicians’ notes. Therefore, noncancer drugs are based solely on the PDTS database. In most cases, physicians' notes on AEs were not detailed. There was no distinction between initial vs later lines of therapy and dosage reductions. The change in status or appearance of a new medical condition did not indicate whether paclitaxel caused the changes to develop or directly worsen a pre-existing condition. The PDTS records prescriptions filled, but that may not reflect patients taking prescriptions.
Paclitaxel
Paclitaxel has a long list of both approved and off-label uses in malignancies as a primary agent and in conjunction with other drugs. The FDA prescribing information for Taxol and Abraxane was last updated in April 2011 and September 2020, respectively.20,21 The National Institutes of Health National Library of Medicine has the current update for paclitaxel on July 2023.19,22 Thus, the prescribed information for paclitaxel referenced in the database may not always be up to date. The combinations of paclitaxel with bevacizumab, carboplatin, or carboplatin and pembrolizumab were not in the Taxol prescribing information. Likewise, a combination of nab-paclitaxel with atezolizumab or carboplatin and pembrolizumab is missing in the Abraxane prescribing information.22-27
The generic name is not the same as a generic drug, which may have slight differences from the brand name product.71 The generic drug versions of Taxol and Abraxane have been approved by the FDA as paclitaxel injectable and paclitaxel-protein bound, respectively. There was a global shortage of nab-paclitaxel from October 2021 to June 2022 because of a manufacturing problem.72 During this shortage, data showed similar comments from physician documents that treatment switched to Taxol due to the Abraxane shortage.
Of 336 patients in the PDTS database with dispensed paclitaxel prescriptions, 276 received paclitaxel (year dispensed, 2013-2022), 27 received Abraxane (year dispensed, 2013-2022), 47 received Taxol (year dispensed, 2004-2015), 8 received both Abraxane and paclitaxel, and 6 received both Taxol and paclitaxel. Based on this information, it appears that the distinction between the drugs was not made in the PDTS until after 2015, 10 years after Abraxane received FDA approval. Abraxane was prescribed in the MHS in 2013, 8 years after FDA approval. There were a few comparison studies of Abraxane and Taxol.73-76
Safety and effectiveness in pediatric patients have not been established for paclitaxel. According to the DoD Cancer Registry Program, the youngest patient was aged 2 months. In 2021, this patient was diagnosed with corpus uteri and treated with carboplatin and Taxol in course 1; in course 2, the patient reacted to Taxol; in course 3, Taxol was replaced with Abraxane; in courses 4 to 7, the patient was treated with carboplatin only.
Discontinued Treatment
Ten patients had prescribed Taxol that was changed due to AEs: 1 was switched to Abraxane and atezolizumab, 3 switched to Abraxane, 2 switched to docetaxel, 1 switched to doxorubicin, and 3 switched to pembrolizumab (based on physician’s comments). Of the 10 patients, 7 had Taxol reaction, 2 experienced disease progression, and 1 experienced high programmed death–ligand 1 expression (this patient with breast cancer was switched to Abraxane and atezolizumab during the accelerated FDA approval phase for atezolizumab, which was later revoked). Five patients were treated with carboplatin and Taxol for cancer of the anal canal (changed to pembrolizumab after disease progression), lung not otherwise specified (changed to carboplatin and pembrolizumab due to Taxol reaction), lower inner quadrant of the breast (changed to doxorubicin due to hypersensitivity reaction), corpus uteri (changed to Abraxane due to Taxol reaction), and ovary (changed to docetaxel due to Taxol reaction). Three patients were treated with doxorubicin, cyclophosphamide, and Taxol for breast cancer; 2 patients with breast cancer not otherwise specified switched to Abraxane due to cardiopulmonary hypersensitivity and Taxol reaction and 1 patient with cancer of the upper outer quadrant of the breast changed to docetaxel due to allergic reaction. One patient, who was treated with paclitaxel, ifosfamide, and cisplatin for metastasis of the lower lobe of the lung and kidney cancer, experienced complications due to infectious colitis (treated with ciprofloxacin) and then switched to pembrolizumab after the disease progressed. These AEs are known in paclitaxel medical literature on paclitaxel AEs.19-24,77-81
Combining 2 or more treatments to target cancer-inducing or cell-sustaining pathways is a cornerstone of chemotherapy.82-84 Most combinations are given on the same day, but some are not. For 3- or 4-drug combinations, doxorubicin and cyclophosphamide were given first, followed by paclitaxel with or withouttrastuzumab, carboplatin, or pembrolizumab. Only 16 patients (2%) were treated with paclitaxel alone; therefore, the completed and discontinued treatment groups are mostly concomitant treatment. As a result, the comparisons of the completed and discontinued treatment groups were almost the same for the diagnosis. The PDTS data have a better result because 2 exclusion criteria were applied before narrowing the analysis down to paclitaxel treatment specifically.
Antidepressants and Other Drugs
Drug response can vary from person to person and can lead to treatment failure related to AEs. One major factor in drug metabolism is CYP.85 CYP2C8 is the major pathway for paclitaxel and CYP3A4 is the minor pathway. When evaluating the noncancer drugs, there were no reports of CYP2C8 inhibition or induction. Over the years, many DDI warnings have been issued for paclitaxel with different drugs in various electronic resources.
Oncologists follow guidelines to prevent DDIs, as paclitaxel is known to have severe, moderate, and minor interactions with other drugs. Among 687 patients, 261 (38%) were prescribed any of 14 antidepressants. Eight of these antidepressants (amitriptyline, citalopram, desipramine, doxepin, venlafaxine, escitalopram, nortriptyline, and trazodone) are metabolized, 3 (mirtazapine, sertraline, and fluoxetine) are metabolized and inhibited, 2 (bupropion and duloxetine) are neither metabolized nor inhibited, and 1 (paroxetine) is inhibited by CYP3A4. Duloxetine, venlafaxine, and trazodone were more commonly dispensed (84, 78, and 42 patients, respectively) than others (≤ 33 patients).
Of 32 patients with documented AEs,14 (44%) had 168 dispensed drugs in the PDTS database. Six patients (19%) were treated with doxorubicin and cyclophosphamide followed by paclitaxel for breast cancer; 6 (19%) were treated with carboplatin and paclitaxel for cancer of the lung (n = 3), corpus uteri (n = 2), and ovary (n = 1); 1 patient (3%) was treated with carboplatin and paclitaxel, then switched to carboplatin, bevacizumab, and paclitaxel, and then completed treatment with carboplatin and paclitaxel for an unspecified female genital cancer; and 1 patient (3%) was treated with cisplatin, ifosfamide, and paclitaxel for metastasis of the lower lobe lung and kidney cancer.
The 14 patients with PDTS data had 18 cancer drugs dispensed. Eleven had moderate interaction reports and 7 had no interaction reports. A total of 165 noncancer drugs were dispensed, of which 3 were antidepressants and had no interactions reported, 8 had moderate interactions reported, and 2 had minor interactions with Taxol and Abraxane, respectively (Table 6).86-129
Of 3 patients who were dispensed bupropion, nortriptyline, or paroxetine, 1 patient with breast cancer was treated with doxorubicin andcyclophosphamide, followed by paclitaxel with bupropion, nortriptyline, pegfilgrastim,dexamethasone, and 17 other noncancer drugs that had no interaction report dispensed during paclitaxel treatment. Of 2 patients with lung cancer, 1 patient was treated with carboplatin and paclitaxel with nortriptyline, dexamethasone, and 13 additional medications, and the second patient was treated with paroxetine, cimetidine, dexamethasone, and 12 other medications. Patients were dispensed up to6 noncancer medications on the same day as paclitaxel administration to control the AEs, not including the prodrugs filled before the treatments. Paroxetine and cimetidine have weak inhibition, and dexamethasone has weak induction of CYP3A4. Therefore, while 1:1 DDIs might have little or no effect with weak inhibit/induce CYP3A4 drugs, 1:1:1 or more combinations could have a different outcome (confirmed in previous publications).65-67
Dispensed on the same day may not mean taken at the same time. One patient experienced an AE with dispensed 50 mg losartan, carboplatin plus paclitaxel, dexamethasone, and 6 other noncancer drugs. Losartan inhibits paclitaxel, which can lead to negative AEs.57,66,67 However, there were no blood or plasma samples taken to confirm the losartan was taken at the same time as the paclitaxel given this was not a clinical trial.
Conclusions
This retrospective study discusses the use of paclitaxel in the MHS and the potential DDIs associated with it. The study population consisted mostly of active-duty personnel, who are required to be healthy or have controlled or nonactive medical diagnoses and be physically fit. This group is mixed with dependents and retirees that are more reflective of the average US population. As a result, this patient population is healthier than the general population, with a lower prevalence of common illnesses such as diabetes and obesity. The study aimed to identify drugs used alongside paclitaxel treatment. While further research is needed to identify potential DDIs among patients who experienced AEs, in vitro testing will need to be conducted before confirming causality. The low number of AEs experienced by only 32 of 702 patients (5%), with no deaths during paclitaxel treatment, indicates that the drug is generally well tolerated. Although this study cannot conclude that concomitant use with noncancer drugs led to the discontinuation of paclitaxel, we can conclude that there seems to be no significant DDIsidentified between paclitaxel and antidepressants. This comprehensive overview provides clinicians with a complete picture of paclitaxel use for 27 years (1996-2022), enabling them to make informed decisions about paclitaxel treatment.
Acknowledgments
The Department of Research Program funds at Walter Reed National Military Medical Center supported this protocol. We sincerely appreciate the contribution of data extraction from the Joint Pathology Center teams (Francisco J. Rentas, John D. McGeeney, Beatriz A. Hallo, and Johnny P. Beason) and the MHS database personnel (Maj Ryan Costantino, Brandon E. Jenkins, and Alexander G. Rittel). We gratefully thank you for the protocol support from the Department of Research programs: CDR Martin L. Boese, CDR Wesley R. Campbell, Maj. Abhimanyu Chandel, CDR Ling Ye, Chelsea N. Powers, Yaling Zhou, Elizabeth Schafer, Micah Stretch, Diane Beaner, and Adrienne Woodard.
Background
Paclitaxel was first derived from the bark of the yew tree (Taxus brevifolia). It was discovered as part of a National Cancer Institute program screen of plants and natural products with putative anticancer activity during the 1960s.1-9 Paclitaxel works by suppressing spindle microtube dynamics, which results in the blockage of the metaphase-anaphase transitions, inhibition of mitosis, and induction of apoptosis in a broad spectrum of cancer cells. Paclitaxel also displayed additional anticancer activities, including the suppression of cell proliferation and antiangiogenic effects. However, since the growth of normal body cells may also be affected, other adverse effects (AEs) will also occur.8-18
Two different chemotherapy drugs contain paclitaxel—paclitaxel and nab-paclitaxel—and the US Food and Drug Administration (FDA) recognizes them as separate entities.19-21 Taxol (paclitaxel) was approved by the FDA in 1992 for treating advanced ovarian cancer.20 It has since been approved for the treatment of metastatic breast cancer, AIDS-related Kaposi sarcoma (as an orphan drug), non-small cell lung cancer (NSCLC), and cervical cancers (in combination withbevacizumab) in 1994, 1997, 1999, and 2014, respectively.21 Since 2002, a generic version of Taxol, known as paclitaxel injectable, has been FDA-approved from different manufacturers. According to the National Cancer Institute, a combination of carboplatin and Taxol is approved to treat carcinoma of unknown primary, cervical, endometrial, NSCLC, ovarian, and thymoma cancers.19 Abraxane (nab-paclitaxel) was FDA-approved to treat metastatic breast cancer in 2005. It was later approved for first-line treatment of advanced NSCLC and late-stage pancreatic cancer in 2012 and 2013, respectively. In 2018 and 2020, both Taxol and Abraxane were approved for first-line treatment of metastatic squamous cell NSCLC in combination with carboplatin and pembrolizumab and metastatic triple-negative breast cancer in combination with pembrolizumab, respectively.22-26 In 2019, Abraxane was approved with atezolizumab to treat metastatic triple-negative breast cancer, but this approval was withdrawn in 2021. In 2022, a generic version of Abraxane, known as paclitaxel protein-bound, was released in the United States. Furthermore, paclitaxel-containing formulations also are being studied in the treatment of other types of cancer.19-32
One of the main limitations of paclitaxel is its low solubility in water, which complicates its drug supply. To distribute this hydrophobic anticancer drug efficiently, paclitaxel is formulated and administered to patients via polyethoxylated castor oil or albumin-bound (nab-paclitaxel). However, polyethoxylated castor oil induces complement activation and is the cause of common hypersensitivity reactions related to paclitaxel use.2,17,33-38 Therefore, many alternatives to polyethoxylated castor oil have been researched.
Since 2000, new paclitaxel formulations have emerged using nanomedicine techniques. The difference between these formulations is the drug vehicle. Different paclitaxel-based nanotechnological vehicles have been developed and approved, such as albumin-based nanoparticles, polymeric lipidic nanoparticles, polymeric micelles, and liposomes, with many others in clinical trial phases.3,37 Albumin-based nanoparticles have a high response rate (33%), whereas the response rate for polyethoxylated castor oil is 25% in patients with metastatic breast cancer.33,39-52 The use of paclitaxel dimer nanoparticles also has been proposed as a method for increasing drug solubility.33,53
Paclitaxel is metabolized by cytochrome P450 (CYP) isoenzymes 2C8 and 3A4. When administering paclitaxel with known inhibitors, inducers, or substrates of CYP2C8 or CYP3A4, caution is required.19-22 Regulations for CYP research were not issued until 2008, so potential interactions between paclitaxel and other drugs have not been extensively evaluated in clinical trials. A study of 12 kinase inhibitors showed strong inhibition of CYP2C8 and/or CYP3A4 pathways by these inhibitors, which could alter the ratio of paclitaxel metabolites in vivo, leading to clinically relevant changes.54 Differential metabolism has been linked to paclitaxel-induced neurotoxicity in patients with cancer.55 Nonetheless, variants in the CYP2C8, CYP3A4, CYP3A5, and ABCB1 genes do not account for significant interindividual variability in paclitaxel pharmacokinetics.56 In liver microsomes, losartan inhibited paclitaxel metabolism when used at concentrations > 50 µmol/L.57 Many drug-drug interaction (DDI) studies of CYP2C8 and CYP3A4 have shown similar results for paclitaxel.58-64
The goals of this study are to investigate prescribed drugs used with paclitaxel and determine patient outcomes through several Military Health System (MHS) databases. The investigation focused on (1) the functions of paclitaxel; (2) identifying AEs that patients experienced; (3) evaluating differences when paclitaxel is used alone vs concomitantly and between the completed vs discontinued treatment groups; (4) identifying all drugs used during paclitaxel treatment; and (5) evaluating DDIs with antidepressants (that have an FDA boxed warning and are known to have DDIs confirmed in previous publications) and other drugs.65-67
The Walter Reed National Military Medical Center in Bethesda, Maryland, institutionalreview board approved the study protocol and ensured compliance with the Health Insurance Portability and Accountability Act as an exempt protocol. The Joint Pathology Center (JPC) of the US Department of Defense (DoD) Cancer Registry Program and MHS data experts from the Comprehensive Ambulatory/Professional Encounter Record (CAPER) and the Pharmacy Data Transaction Service (PDTS) provided data for the analysis.
METHODS
The DoD Cancer Registry Program was established in 1986 and currently contains data from 1998 to 2024. CAPER and PDTS are part of the MHS Data Repository/Management Analysis and Reporting Tool database. Each observation in the CAPER record represents an ambulatory encounter at a military treatment facility (MTF). CAPER includes data from 2003 to 2024.
Each observation in the PDTS record represents a prescription filled for an MHS beneficiary at an MTF through the TRICARE mail-order program or a US retail pharmacy. Missing from this record are prescriptions filled at international civilian pharmacies and inpatient pharmacy prescriptions. The MHS Data Repository PDTS record is available from 2002 to 2024. The legacy Composite Health Care System is being replaced by GENESIS at MTFs.
Data Extraction Design
The study design involved a cross-sectional analysis. We requested data extraction for paclitaxel from 1998 to 2022. Data from the DoD Cancer Registry Program were used to identify patients who received cancer treatment. Once patients were identified, the CAPER database was searched for diagnoses to identify other health conditions, whereas the PDTS database was used to populate a list of prescription medications filled during chemotherapy treatment.
Data collected from the JPC included cancer treatment, cancer information, demographics, and physicians’ comments on AEs. Collected data from the MHS include diagnosis and filled prescription history from initiation to completion of the therapy period (or 2 years after the diagnosis date). For the analysis of the DoD Cancer Registry Program and CAPER databases, we used all collected data without excluding any. When analyzing PDTS data, we excluded patients with PDTS data but without a record of paclitaxel being filled, or medications filled outside the chemotherapy period (by evaluating the dispensed date and day of supply).
Data Extraction Analysis
The Surveillance, Epidemiology, and End Results Program Coding and Staging Manual 2016 and the International Classification of Diseases for Oncology, 3rd edition, 1st revision, were used to decode disease and cancer types.68,69 Data sorting and analysis were performed using Microsoft Excel. The percentage for the total was calculated by using the number of patients or data available within the paclitaxel groups divided by the total number of patients or data variables. The subgroup percentage was calculated by using the number of patients or data available within the subgroup divided by the total number of patients in that subgroup.
In alone vs concomitant and completed vs discontinued treatment groups, a 2-tailed, 2-sample z test was used to statistical significance (P < .05) using a statistics website.70 Concomitant was defined as paclitaxel taken with other antineoplastic agent(s) before, after, or at the same time as cancer therapy. For the retrospective data analysis, physicians’ notes with a period, comma, forward slash, semicolon, or space between medication names were interpreted as concurrent, whereas plus (+), minus/plus (-/+), or “and” between drug names that were dispensed on the same day were interpreted as combined with known common combinations: 2 drugs (DM886 paclitaxel and carboplatin and DM881-TC-1 paclitaxel and cisplatin) or 3 drugs (DM887-ACT doxorubicin, cyclophosphamide, and paclitaxel). Completed treatment was defined as paclitaxel as the last medication the patient took without recorded AEs; switching or experiencing AEs was defined as discontinued treatment.
RESULTS
The JPC provided 702 entries for 687 patients with a mean age of 56 years (range, 2 months to 88 years) who were treated with paclitaxel from March 1996 to October 2021. Fifteen patients had duplicate entries because they had multiple cancer sites or occurrences. There were 623 patients (89%) who received paclitaxel for FDA-approved indications. The most common types of cancer identified were 344 patients with breast cancer (49%), 91 patients with lung cancer (13%), 79 patients with ovarian cancer (11%), and 75 patients with endometrial cancer (11%) (Table 1). Seventy-nine patients (11%) received paclitaxel for cancers that were not for FDA-approved indications, including 19 for cancers of the fallopian tube (3%) and 17 for esophageal cancer (2%) (Table 2).
There were 477 patients (68%) aged > 50 years. A total of 304 patients (43%) had a stage III or IV cancer diagnosis and 398 (57%) had stage II or lower (combination of data for stages 0, I, and II; not applicable; and unknown) cancer diagnosis. For systemic treatment, 16 patients (2%) were treated with paclitaxel alone and 686 patients (98%) received paclitaxel concomitantly with additional chemotherapy: 59 patients (9%) in the before or after group, 410 patients (58%) had a 2-drug combination, 212 patients (30%) had a 3-drug combination, and 5 patients (1%) had a 4-drug combination. In addition, for doublet therapies, paclitaxel combined with carboplatin, trastuzumab, gemcitabine, or cisplatin had more patients (318, 58, 12, and 11, respectively) than other combinations (≤ 4 patients). For triplet therapies, paclitaxel combined withdoxorubicin plus cyclophosphamide or carboplatin plus bevacizumab had more patients (174 and 20, respectively) than other combinations, including quadruplet therapies (≤ 4 patients) (Table 3).
Patients were more likely to discontinue paclitaxel if they received concomitant treatment. None of the 16 patients receiving paclitaxel monotherapy experienced AEs, whereas 364 of 686 patients (53%) treated concomitantly discontinued (P < .001). Comparisons of 1 drug vs combination (2 to 4 drugs) and use for treating cancers that were FDA-approved indications vs off-label use were significant (P < .001), whereas comparisons of stage II or lower vs stage III and IV cancer and of those aged ≤ 50 years vs aged > 50 years were not significant (P = .50 andP = .30, respectively) (Table 4).
Among the 364 patients who had concomitant treatment and had discontinued their treatment, 332 (91%) switched treatments with no AEs documented and 32 (9%) experienced fatigue with pneumonia, mucositis, neuropathy, neurotoxicity, neutropenia, pneumonitis, allergic or hypersensitivity reaction, or an unknown AE. Patients who discontinued treatment because of unknown AEs had a physician’s note that detailed progressive disease, a significant decline in performance status, and another unknown adverse effect due to a previous sinus tract infection and infectious colitis (Table 5).
Management Analysis and Reporting Tool Database
MHS data analysts provided data on diagnoses for 639 patients among 687 submitteddiagnoses, with 294 patients completing and 345 discontinuing paclitaxel treatment. Patients in the completed treatment group had 3 to 258 unique health conditions documented, while patients in the discontinued treatment group had 4 to 181 unique health conditions documented. The MHS reported 3808 unique diagnosis conditions for the completed group and 3714 for the discontinued group (P = .02).
The mean (SD) number of diagnoses was 51 (31) for the completed and 55 (28) for the discontinued treatment groups (Figure). Among 639 patients who received paclitaxel, the top 5 diagnoses were administrative, including encounters for other administrative examinations; antineoplastic chemotherapy; administrative examination for unspecified; other specified counseling; and adjustment and management of vascular access device. The database does not differentiate between administrative and clinically significant diagnoses.
MHS data analysts provided data for 336 of 687 submitted patients who were prescribed paclitaxel; 46 patients had no PDTS data, and 305 patients had PDTS data without paclitaxel, Taxol, or Abraxane dispensed. Medications that were filled outside the chemotherapy period were removed by evaluating the dispensed date and day of supply. Among these 336 patients, 151 completed the treatment and 185 discontinued, with 14 patients experiencing documented AEs. Patients in the completed treatment group filled 9 to 56 prescriptions while patients in the discontinued treatment group filled 6 to 70 prescriptions.Patients in the discontinued group filled more prescriptions than those who completed treatment: 793 vs 591, respectively (P = .34).
The mean (SD) number of filled prescription drugs was 24 (9) for the completed and 34 (12) for the discontinued treatment group. The 5 most filled prescriptions with paclitaxel from 336 patients with PDTS data were dexamethasone (324 prescriptions with 14 recorded AEs), diphenhydramine (296 prescriptions with 12 recorded AEs), ondansetron (277 prescriptions with 11 recorded AEs), prochlorperazine (265 prescriptions with 12 recorded AEs), and sodium chloride (232 prescriptions with 11 recorded AEs).
DISCUSSION
As a retrospective review, this study is more limited in the strength of its conclusions when compared to randomized control trials. The DoD Cancer Registry Program only contains information about cancer types, stages, treatment regimens, and physicians’ notes. Therefore, noncancer drugs are based solely on the PDTS database. In most cases, physicians' notes on AEs were not detailed. There was no distinction between initial vs later lines of therapy and dosage reductions. The change in status or appearance of a new medical condition did not indicate whether paclitaxel caused the changes to develop or directly worsen a pre-existing condition. The PDTS records prescriptions filled, but that may not reflect patients taking prescriptions.
Paclitaxel
Paclitaxel has a long list of both approved and off-label uses in malignancies as a primary agent and in conjunction with other drugs. The FDA prescribing information for Taxol and Abraxane was last updated in April 2011 and September 2020, respectively.20,21 The National Institutes of Health National Library of Medicine has the current update for paclitaxel on July 2023.19,22 Thus, the prescribed information for paclitaxel referenced in the database may not always be up to date. The combinations of paclitaxel with bevacizumab, carboplatin, or carboplatin and pembrolizumab were not in the Taxol prescribing information. Likewise, a combination of nab-paclitaxel with atezolizumab or carboplatin and pembrolizumab is missing in the Abraxane prescribing information.22-27
The generic name is not the same as a generic drug, which may have slight differences from the brand name product.71 The generic drug versions of Taxol and Abraxane have been approved by the FDA as paclitaxel injectable and paclitaxel-protein bound, respectively. There was a global shortage of nab-paclitaxel from October 2021 to June 2022 because of a manufacturing problem.72 During this shortage, data showed similar comments from physician documents that treatment switched to Taxol due to the Abraxane shortage.
Of 336 patients in the PDTS database with dispensed paclitaxel prescriptions, 276 received paclitaxel (year dispensed, 2013-2022), 27 received Abraxane (year dispensed, 2013-2022), 47 received Taxol (year dispensed, 2004-2015), 8 received both Abraxane and paclitaxel, and 6 received both Taxol and paclitaxel. Based on this information, it appears that the distinction between the drugs was not made in the PDTS until after 2015, 10 years after Abraxane received FDA approval. Abraxane was prescribed in the MHS in 2013, 8 years after FDA approval. There were a few comparison studies of Abraxane and Taxol.73-76
Safety and effectiveness in pediatric patients have not been established for paclitaxel. According to the DoD Cancer Registry Program, the youngest patient was aged 2 months. In 2021, this patient was diagnosed with corpus uteri and treated with carboplatin and Taxol in course 1; in course 2, the patient reacted to Taxol; in course 3, Taxol was replaced with Abraxane; in courses 4 to 7, the patient was treated with carboplatin only.
Discontinued Treatment
Ten patients had prescribed Taxol that was changed due to AEs: 1 was switched to Abraxane and atezolizumab, 3 switched to Abraxane, 2 switched to docetaxel, 1 switched to doxorubicin, and 3 switched to pembrolizumab (based on physician’s comments). Of the 10 patients, 7 had Taxol reaction, 2 experienced disease progression, and 1 experienced high programmed death–ligand 1 expression (this patient with breast cancer was switched to Abraxane and atezolizumab during the accelerated FDA approval phase for atezolizumab, which was later revoked). Five patients were treated with carboplatin and Taxol for cancer of the anal canal (changed to pembrolizumab after disease progression), lung not otherwise specified (changed to carboplatin and pembrolizumab due to Taxol reaction), lower inner quadrant of the breast (changed to doxorubicin due to hypersensitivity reaction), corpus uteri (changed to Abraxane due to Taxol reaction), and ovary (changed to docetaxel due to Taxol reaction). Three patients were treated with doxorubicin, cyclophosphamide, and Taxol for breast cancer; 2 patients with breast cancer not otherwise specified switched to Abraxane due to cardiopulmonary hypersensitivity and Taxol reaction and 1 patient with cancer of the upper outer quadrant of the breast changed to docetaxel due to allergic reaction. One patient, who was treated with paclitaxel, ifosfamide, and cisplatin for metastasis of the lower lobe of the lung and kidney cancer, experienced complications due to infectious colitis (treated with ciprofloxacin) and then switched to pembrolizumab after the disease progressed. These AEs are known in paclitaxel medical literature on paclitaxel AEs.19-24,77-81
Combining 2 or more treatments to target cancer-inducing or cell-sustaining pathways is a cornerstone of chemotherapy.82-84 Most combinations are given on the same day, but some are not. For 3- or 4-drug combinations, doxorubicin and cyclophosphamide were given first, followed by paclitaxel with or withouttrastuzumab, carboplatin, or pembrolizumab. Only 16 patients (2%) were treated with paclitaxel alone; therefore, the completed and discontinued treatment groups are mostly concomitant treatment. As a result, the comparisons of the completed and discontinued treatment groups were almost the same for the diagnosis. The PDTS data have a better result because 2 exclusion criteria were applied before narrowing the analysis down to paclitaxel treatment specifically.
Antidepressants and Other Drugs
Drug response can vary from person to person and can lead to treatment failure related to AEs. One major factor in drug metabolism is CYP.85 CYP2C8 is the major pathway for paclitaxel and CYP3A4 is the minor pathway. When evaluating the noncancer drugs, there were no reports of CYP2C8 inhibition or induction. Over the years, many DDI warnings have been issued for paclitaxel with different drugs in various electronic resources.
Oncologists follow guidelines to prevent DDIs, as paclitaxel is known to have severe, moderate, and minor interactions with other drugs. Among 687 patients, 261 (38%) were prescribed any of 14 antidepressants. Eight of these antidepressants (amitriptyline, citalopram, desipramine, doxepin, venlafaxine, escitalopram, nortriptyline, and trazodone) are metabolized, 3 (mirtazapine, sertraline, and fluoxetine) are metabolized and inhibited, 2 (bupropion and duloxetine) are neither metabolized nor inhibited, and 1 (paroxetine) is inhibited by CYP3A4. Duloxetine, venlafaxine, and trazodone were more commonly dispensed (84, 78, and 42 patients, respectively) than others (≤ 33 patients).
Of 32 patients with documented AEs,14 (44%) had 168 dispensed drugs in the PDTS database. Six patients (19%) were treated with doxorubicin and cyclophosphamide followed by paclitaxel for breast cancer; 6 (19%) were treated with carboplatin and paclitaxel for cancer of the lung (n = 3), corpus uteri (n = 2), and ovary (n = 1); 1 patient (3%) was treated with carboplatin and paclitaxel, then switched to carboplatin, bevacizumab, and paclitaxel, and then completed treatment with carboplatin and paclitaxel for an unspecified female genital cancer; and 1 patient (3%) was treated with cisplatin, ifosfamide, and paclitaxel for metastasis of the lower lobe lung and kidney cancer.
The 14 patients with PDTS data had 18 cancer drugs dispensed. Eleven had moderate interaction reports and 7 had no interaction reports. A total of 165 noncancer drugs were dispensed, of which 3 were antidepressants and had no interactions reported, 8 had moderate interactions reported, and 2 had minor interactions with Taxol and Abraxane, respectively (Table 6).86-129
Of 3 patients who were dispensed bupropion, nortriptyline, or paroxetine, 1 patient with breast cancer was treated with doxorubicin andcyclophosphamide, followed by paclitaxel with bupropion, nortriptyline, pegfilgrastim,dexamethasone, and 17 other noncancer drugs that had no interaction report dispensed during paclitaxel treatment. Of 2 patients with lung cancer, 1 patient was treated with carboplatin and paclitaxel with nortriptyline, dexamethasone, and 13 additional medications, and the second patient was treated with paroxetine, cimetidine, dexamethasone, and 12 other medications. Patients were dispensed up to6 noncancer medications on the same day as paclitaxel administration to control the AEs, not including the prodrugs filled before the treatments. Paroxetine and cimetidine have weak inhibition, and dexamethasone has weak induction of CYP3A4. Therefore, while 1:1 DDIs might have little or no effect with weak inhibit/induce CYP3A4 drugs, 1:1:1 or more combinations could have a different outcome (confirmed in previous publications).65-67
Dispensed on the same day may not mean taken at the same time. One patient experienced an AE with dispensed 50 mg losartan, carboplatin plus paclitaxel, dexamethasone, and 6 other noncancer drugs. Losartan inhibits paclitaxel, which can lead to negative AEs.57,66,67 However, there were no blood or plasma samples taken to confirm the losartan was taken at the same time as the paclitaxel given this was not a clinical trial.
Conclusions
This retrospective study discusses the use of paclitaxel in the MHS and the potential DDIs associated with it. The study population consisted mostly of active-duty personnel, who are required to be healthy or have controlled or nonactive medical diagnoses and be physically fit. This group is mixed with dependents and retirees that are more reflective of the average US population. As a result, this patient population is healthier than the general population, with a lower prevalence of common illnesses such as diabetes and obesity. The study aimed to identify drugs used alongside paclitaxel treatment. While further research is needed to identify potential DDIs among patients who experienced AEs, in vitro testing will need to be conducted before confirming causality. The low number of AEs experienced by only 32 of 702 patients (5%), with no deaths during paclitaxel treatment, indicates that the drug is generally well tolerated. Although this study cannot conclude that concomitant use with noncancer drugs led to the discontinuation of paclitaxel, we can conclude that there seems to be no significant DDIsidentified between paclitaxel and antidepressants. This comprehensive overview provides clinicians with a complete picture of paclitaxel use for 27 years (1996-2022), enabling them to make informed decisions about paclitaxel treatment.
Acknowledgments
The Department of Research Program funds at Walter Reed National Military Medical Center supported this protocol. We sincerely appreciate the contribution of data extraction from the Joint Pathology Center teams (Francisco J. Rentas, John D. McGeeney, Beatriz A. Hallo, and Johnny P. Beason) and the MHS database personnel (Maj Ryan Costantino, Brandon E. Jenkins, and Alexander G. Rittel). We gratefully thank you for the protocol support from the Department of Research programs: CDR Martin L. Boese, CDR Wesley R. Campbell, Maj. Abhimanyu Chandel, CDR Ling Ye, Chelsea N. Powers, Yaling Zhou, Elizabeth Schafer, Micah Stretch, Diane Beaner, and Adrienne Woodard.
1. American Chemical Society. Discovery of camptothecin and taxol. acs.org. Accessed June 4, 2024. https://www.acs.org/education/whatischemistry/landmarks/camptothecintaxol.html
2. Bocci G, Di Paolo A, Danesi R. The pharmacological bases of the antiangiogenic activity of paclitaxel. Angiogenesis. 2013;16(3):481-492. doi:10.1007/s10456-013-9334-0.
3. Meštrovic T. Paclitaxel history. News Medical Life Sciences. Updated March 11, 2023. Accessed June 4, 2024. https://www.news-medical.net/health/Paclitaxel-History.aspx
4. Rowinsky EK, Donehower RC. Paclitaxel (taxol). N Engl J Med. 1995;332(15):1004-1014. doi:10.1056/NEJM199504133321507
5. Walsh V, Goodman J. The billion dollar molecule: Taxol in historical and theoretical perspective. Clio Med. 2002;66:245-267. doi:10.1163/9789004333499_013
6. Perdue RE, Jr, Hartwell JL. The search for plant sources of anticancer drugs. Morris Arboretum Bull. 1969;20:35-53.
7. Wall ME, Wani MC. Camptothecin and taxol: discovery to clinic—thirteenth Bruce F. Cain Memorial Award lecture. Cancer Res. 1995;55:753-760.
8. Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from taxus brevifolia. J Am Chem Soc. 1971;93(9):2325-2327. doi:10.1021/ja00738a045
9. Weaver BA. How taxol/paclitaxel kills cancer cells. Mol Biol Cell. 2014;25(18):2677-2681. doi:10.1091/mbc.E14-04-0916
10. Chen JG, Horwitz SB. Differential mitotic responses to microtubule-stabilizing and-destabilizing drugs. Cancer Res. 2002;62(7):1935-1938.
11. Singh S, Dash AK. Paclitaxel in cancer treatment: perspectives and prospects of its delivery challenges. Crit Rev Ther Drug Carrier Syst. 2009;26(4):333-372. doi:10.1615/critrevtherdrugcarriersyst.v26.i4.10
12. Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277(5698):665-667. doi:10.1038/277665a0
13. Fuchs DA, Johnson RK. Cytologic evidence that taxol, an antineoplastic agent from taxus brevifolia, acts as a mitotic spindle poison. Cancer Treat Rep. 1978;62(8):1219-1222.
14. Walsh V, Goodman J. From taxol to taxol: the changing identities and ownership of an anti-cancer drug. Med Anthropol. 2002;21(3-4):307-336. doi:10.1080/01459740214074
15. Walsh V, Goodman J. Cancer chemotherapy, biodiversity, public and private property: the case of the anti-cancer drug taxol. Soc Sci Med. 1999;49(9):1215-1225. doi:10.1016/s0277-9536(99)00161-6
16. Jordan MA, Wendell K, Gardiner S, Derry WB, Copp H, Wilson L. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 1996;56(4):816-825.
17. Picard M, Castells MC. Re-visiting hypersensitivity reactions to taxanes: a comprehensive review. Clin Rev Allergy Immunol. 2015;49(2):177-191. doi:10.1007/s12016-014-8416-0
18. Zasadil LM, Andersen KA, Yeum D, et al. Cytotoxicity of paclitaxel in breast cancer is due to chromosome missegregation on multipolar spindles. Sci Transl Med. 2014;6:229ra243. doi:10.1126/scitranslmed.3007965
19. National Cancer Institute. Carboplatin-Taxol. Published May 30, 2012. Updated March 22, 2023. Accessed June 4, 2024. https://www.cancer.gov/about-cancer/treatment/drugs/carboplatin-taxol
20. Taxol (paclitaxel). Prescribing information. Bristol-Myers Squibb; 2011. Accessed June 4, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
21. Abraxane (paclitaxel). Prescribing information. Celgene Corporation; 2021. Accessed June 4, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
22. Awosika AO, Farrar MC, Jacobs TF. Paclitaxel. StatPearls. Updated November 18, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK536917/
23. Gerriets V, Kasi A. Bevacizumab. StatPearls. Updated September 1, 2022. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482126/
24. American Cancer Society. Chemotherapy for endometrial cancer. Updated March 27, 2019. Accessed June 4, 2024. https://www.cancer.org/cancer/types/endometrial-cancer/treating/chemotherapy.html
25. US Food and Drug Administration. FDA approves pembrolizumab in combination with chemotherapy for first-line treatment of metastatic squamous NSCLC. October 30, 2018. Updated December 14, 2018. Accessed June 4, 2024. https://www.fda.gov/drugs/fda-approves-pembrolizumab-combination-chemotherapy-first-line-treatment-metastatic-squamous-nsclc
26. US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for locally recurrent unresectable or metastatic triple negative breast cancer. November 13, 2020. Accessed June 4, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-locally-recurrent-unresectable-or-metastatic-triple
27. US Food and Drug Administration. FDA approves atezolizumab for PD-L1 positive unresectable locally advanced or metastatic triple-negative breast. March 8, 2019. Updated March 18, 2019. Accessed June 5, 2024. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-atezolizumab-pd-l1-positive-unresectable-locally-advanced-or-metastatic-triple-negative
28. US Food and Drug Administration. FDA issues alert about efficacy and potential safety concerns with atezolizumab in combination with paclitaxel for treatment of breast cancer. September 8, 2020. Accessed June 5, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-issues-alert-about-efficacy-and-potential-safety-concerns-atezolizumab-combination-paclitaxel
29. Tan AR. Chemoimmunotherapy: still the standard of care for metastatic triple-negative breast cancer. ASCO Daily News. February 23, 2022. Accessed June 5, 2024. https://dailynews.ascopubs.org/do/chemoimmunotherapy-still-standard-care-metastatic-triple-negative-breast-cancer
30. McGuire WP, Rowinsky EK, Rosenshein NB, et al. Taxol: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Ann Intern Med. 1989;111(4):273-279. doi:10.7326/0003-4819-111-4-273
31. Milas L, Hunter NR, Kurdoglu B, et al. Kinetics of mitotic arrest and apoptosis in murine mammary and ovarian tumors treated with taxol. Cancer Chemother Pharmacol. 1995;35(4):297-303. doi:10.1007/BF00689448
32. Searle J, Collins DJ, Harmon B, Kerr JF. The spontaneous occurrence of apoptosis in squamous carcinomas of the uterine cervix. Pathology. 1973;5(2):163-169. doi:10.3109/00313027309060831
33. Gallego-Jara J, Lozano-Terol G, Sola-Martínez RA, Cánovas-Díaz M, de Diego Puente T. A compressive review about taxol®: history and future challenges. Molecules. 2020;25(24):5986. doi:10.3390/molecules25245986
34. Bernabeu E, Cagel M, Lagomarsino E, Moretton M, Chiappetta DA. Paclitaxel: What has been done and the challenges remain ahead. Int J Pharm. 2017;526(1-2):474-495. doi:10.1016/j.ijpharm.2017.05.016
35. Nehate C, Jain S, Saneja A, et al. Paclitaxel formulations: challenges and novel delivery options. Curr Drug Deliv. 2014;11(6):666-686. doi:10.2174/1567201811666140609154949
36. Gelderblom H, Verweij J, Nooter K, Sparreboom A, Cremophor EL. The drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37(13):1590-1598. doi:10.1016/S0959-8049(01)00171-x
37. Chowdhury MR, Moshikur RM, Wakabayashi R, et al. In vivo biocompatibility, pharmacokinetics, antitumor efficacy, and hypersensitivity evaluation of ionic liquid-mediated paclitaxel formulations. Int J Pharm. 2019;565:219-226. doi:10.1016/j.ijpharm.2019.05.020
38. Borgå O, Henriksson R, Bjermo H, Lilienberg E, Heldring N, Loman N. Maximum tolerated dose and pharmacokinetics of paclitaxel micellar in patients with recurrent malignant solid tumours: a dose-escalation study. Adv Ther. 2019;36(5):1150-1163. doi:10.1007/s12325-019-00909-6
39. Rouzier R, Rajan R, Wagner P, et al. Microtubule-associated protein tau: a marker of paclitaxel sensitivity in breast cancer. Proc Natl Acad Sci USA. 2005;102(23):8315-8320. doi:10.1073/pnas.0408974102
40. Choudhury H, Gorain B, Tekade RK, Pandey M, Karmakar S, Pal TK. Safety against nephrotoxicity in paclitaxel treatment: oral nanocarrier as an effective tool in preclinical evaluation with marked in vivo antitumor activity. Regul Toxicol Pharmacol. 2017;91:179-189. doi:10.1016/j.yrtph.2017.10.023
41. Barkat MA, Beg S, Pottoo FH, Ahmad FJ. Nanopaclitaxel therapy: an evidence based review on the battle for next-generation formulation challenges. Nanomedicine (Lond). 2019;14(10):1323-1341. doi:10.2217/nnm-2018-0313
42. Sofias AM, Dunne M, Storm G, Allen C. The battle of “nano” paclitaxel. Adv Drug Deliv Rev. 2017;122:20-30. doi:10.1016/j.addr.2017.02.003
43. Yang N, Wang C, Wang J, et al. Aurora inase a stabilizes FOXM1 to enhance paclitaxel resistance in triple-negative breast cancer. J Cell Mol Med. 2019;23(9):6442-6453. doi:10.1111/jcmm.14538
44. Chowdhury MR, Moshikur RM, Wakabayashi R, et al. Ionic-liquid-based paclitaxel preparation: a new potential formulation for cancer treatment. Mol Pharm. 2018;15(16):2484-2488. doi:10.1021/acs.molpharmaceut.8b00305
45. Chung HJ, Kim HJ, Hong ST. Tumor-specific delivery of a paclitaxel-loading HSA-haemin nanoparticle for cancer treatment. Nanomedicine. 2020;23:102089. doi:10.1016/j.nano.2019.102089
46. Ye L, He J, Hu Z, et al. Antitumor effect and toxicity of lipusu in rat ovarian cancer xenografts. Food Chem Toxicol. 2013;52:200-206. doi:10.1016/j.fct.2012.11.004
47. Ma WW, Lam ET, Dy GK, et al. A pharmacokinetic and dose-escalating study of paclitaxel injection concentrate for nano-dispersion (PICN) alone and with arboplatin in patients with advanced solid tumors. J Clin Oncol. 2013;31:2557. doi:10.1200/jco.2013.31.15_suppl.2557
48. Micha JP, Goldstein BH, Birk CL, Rettenmaier MA, Brown JV. Abraxane in the treatment of ovarian cancer: the absence of hypersensitivity reactions. Gynecol Oncol. 2006;100(2):437-438. doi:10.1016/j.ygyno.2005.09.012
49. Ingle SG, Pai RV, Monpara JD, Vavia PR. Liposils: an effective strategy for stabilizing paclitaxel loaded liposomes by surface coating with silica. Eur J Pharm Sci. 2018;122:51-63. doi:10.1016/j.ejps.2018.06.025
50. Abriata JP, Turatti RC, Luiz MT, et al. Development, characterization and biological in vitro assays of paclitaxel-loaded PCL polymeric nanoparticles. Mater Sci Eng C Mater Biol Appl. 2019;96:347-355. doi:10.1016/j.msec.2018.11.035
51. Hu J, Fu S, Peng Q, et al. Paclitaxel-loaded polymeric nanoparticles combined with chronomodulated chemotherapy on lung cancer: in vitro and in vivo evaluation. Int J Pharm. 2017;516(1-2):313-322. doi:10.1016/j.ijpharm.2016.11.047
52. Dranitsaris G, Yu B, Wang L, et al. Abraxane® vs Taxol® for patients with advanced breast cancer: a prospective time and motion analysis from a chinese health care perspective. J Oncol Pharm Pract. 2016;22(2):205-211. doi:10.1177/1078155214556008
53. Pei Q, Hu X, Liu S, Li Y, Xie Z, Jing X. Paclitaxel dimers assembling nanomedicines for treatment of cervix carcinoma. J Control Release. 2017;254:23-33. doi:10.1016/j.jconrel.2017.03.391
54. Wang Y, Wang M, Qi H, et al. Pathway-dependent inhibition of paclitaxel hydroxylation by kinase inhibitors and assessment of drug-drug interaction potentials. Drug Metab Dispos. 2014;42(4):782-795. doi:10.1124/dmd.113.053793
55. Shen F, Jiang G, Philips S, et al. Cytochrome P450 oxidoreductase (POR) associated with severe paclitaxel-induced peripheral neuropathy in patients of european ancestry from ECOG-ACRIN E5103. Clin Cancer Res. 2023;29(13):2494-2500. doi:10.1158/1078-0432.CCR-22-2431
56. Henningsson A, Marsh S, Loos WJ, et al. Association of CYP2C8, CYP3A4, CYP3A5, and ABCB1 polymorphisms with the pharmacokinetics of paclitaxel. Clin Cancer Res. 2005;11(22):8097-8104. doi:10.1158/1078-0432.CCR-05-1152
57. Mukai Y, Senda A, Toda T, et al. Drug-drug interaction between losartan and paclitaxel in human liver microsomes with different CYP2C8 genotypes. Basic Clin Pharmacol Toxicol. 2015;116(6):493-498. doi:10.1111/bcpt.12355
58. Kawahara B, Faull KF, Janzen C, Mascharak PK. Carbon monoxide inhibits cytochrome P450 enzymes CYP3A4/2C8 in human breast cancer cells, increasing sensitivity to paclitaxel. J Med Chem. 2021;64(12):8437-8446. doi:10.1021/acs.jmedchem.1c00404
59. Cresteil T, Monsarrat B, Dubois J, Sonnier M, Alvinerie P, Gueritte F. Regioselective metabolism of taxoids by human CYP3A4 and 2C8: structure-activity relationship. Drug Metab Dispos. 2002;30(4):438-445. doi:10.1124/dmd.30.4.438
60. Taniguchi R, Kumai T, Matsumoto N, et al. Utilization of human liver microsomes to explain individual differences in paclitaxel metabolism by CYP2C8 and CYP3A4. J Pharmacol Sci. 2005;97(1):83-90. doi:10.1254/jphs.fp0040603
61. Nakayama A, Tsuchiya K, Xu L, Matsumoto T, Makino T. Drug-interaction between paclitaxel and goshajinkigan extract and its constituents. J Nat Med. 2022;76(1):59-67. doi:10.1007/s11418-021-01552-8
62. Monsarrat B, Chatelut E, Royer I, et al. Modification of paclitaxel metabolism in a cancer patient by induction of cytochrome P450 3A4. Drug Metab Dispos. 1998;26(3):229-233.
63. Walle T. Assays of CYP2C8- and CYP3A4-mediated metabolism of taxol in vivo and in vitro. Methods Enzymol. 1996;272:145-151. doi:10.1016/s0076-6879(96)72018-9
64. Hanioka N, Matsumoto K, Saito Y, Narimatsu S. Functional characterization of CYP2C8.13 and CYP2C8.14: catalytic activities toward paclitaxel. Basic Clin Pharmacol Toxicol. 2010;107(1):565-569. doi:10.1111/j.1742-7843.2010.00543.x
65. Luong TT, Powers CN, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interactions (DDIs) of gefitinib with/without losartan and selective serotonin reuptake inhibitors (SSRIs): citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and venlafaxine. Curr Res Pharmacol Drug Discov. 2022;3:100112. doi:10.1016/j.crphar.2022.100112
66. Luong TT, McAnulty MJ, Evers DL, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interaction (DDI) of gefitinib or erlotinib with Cytochrome P450 (CYP) inhibiting drugs, fluoxetine and/or losartan. Curr Res Toxicol. 2021;2:217-224. doi:10.1016/j.crtox.2021.05.006
67. Luong TT, Powers CN, Reinhardt BJ, et al. Retrospective evaluation of drug-drug interactions with erlotinib and gefitinib use in the military health system. Fed Pract. 2023;40(suppl 3):S24-S34. doi:10.12788/fp.0401
68. Adamo M, Dickie L, Ruhl J. SEER program coding and staging manual 2016. National Cancer Institute. Accessed June 5, 2024. https://seer.cancer.gov/archive/manuals/2016/SPCSM_2016_maindoc.pdf
69. World Health Organization. International classification of diseases for oncology (ICD-O) 3rd ed, 1st revision. World Health Organization; 2013. Accessed June 5, 2024. https://apps.who.int/iris/handle/10665/96612
70. Z score calculator for 2 population proportions. Social science statistics. Accessed June 5, 2024. https://www.socscistatistics.com/tests/ztest/default2.aspx
71. US Food and Drug Administration. Generic drugs: question & answers. FDA.gov. Accessed June 5, 2024. https://www.fda.gov/drugs/frequently-asked-questions-popular-topics/generic-drugs-questions-answers
72. Oura M, Saito H, Nishikawa Y. Shortage of nab-paclitaxel in Japan and around the world: issues in global information sharing. JMA J. 2023;6(2):192-195. doi:10.31662/jmaj.2022-0179
73. Yuan H, Guo H, Luan X, et al. Albumin nanoparticle of paclitaxel (abraxane) decreases while taxol increases breast cancer stem cells in treatment of triple negative breast cancer. Mol Pharm. 2020;17(7):2275-2286. doi:10.1021/acs.molpharmaceut.9b01221
74. Dranitsaris G, Yu B, Wang L, et al. Abraxane® versus Taxol® for patients with advanced breast cancer: a prospective time and motion analysis from a Chinese health care perspective. J Oncol Pharm Pract. 2016;22(2):205-211. doi:10.1177/1078155214556008
75. Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794-7803. doi:10.1200/JCO.2005.04.
76. Liu M, Liu S, Yang L, Wang S. Comparison between nab-paclitaxel and solvent-based taxanes as neoadjuvant therapy in breast cancer: a systematic review and meta-analysis. BMC Cancer. 2021;21(1):118. doi:10.1186/s12885-021-07831-7
77. Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (taxol). Semin Oncol. 1993;20(4 Suppl 3):1-15.
78. Banerji A, Lax T, Guyer A, Hurwitz S, Camargo CA Jr, Long AA. Management of hypersensitivity reactions to carboplatin and paclitaxel in an outpatient oncology infusion center: a 5-year review. J Allergy Clin Immunol Pract. 2014;2(4):428-433. doi:10.1016/j.jaip.2014.04.010
79. Staff NP, Fehrenbacher JC, Caillaud M, Damaj MI, Segal RA, Rieger S. Pathogenesis of paclitaxel-induced peripheral neuropathy: a current review of in vitro and in vivo findings using rodent and human model systems. Exp Neurol. 2020;324:113121. doi:10.1016/j.expneurol.2019.113121
80. Postma TJ, Vermorken JB, Liefting AJ, Pinedo HM, Heimans JJ. Paclitaxel-induced neuropathy. Ann Oncol. 1995;6(5):489-494. doi:10.1093/oxfordjournals.annonc.a059220
81. Liu JM, Chen YM, Chao Y, et al. Paclitaxel-induced severe neuropathy in patients with previous radiotherapy to the head and neck region. J Natl Cancer Inst. 1996;88(14):1000-1002. doi:10.1093/jnci/88.14.1000-a
82. Bayat Mokhtari R, Homayouni TS, Baluch N, et al. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022-38043. doi:10.18632/oncotarget.16723
83. Blagosklonny MV. Analysis of FDA approved anticancer drugs reveals the future of cancer therapy. Cell Cycle. 2004;3(8):1035-1042.
84. Yap TA, Omlin A, de Bono JS. Development of therapeutic combinations targeting major cancer signaling pathways. J Clin Oncol. 2013;31(12):1592-1605. doi:10.1200/JCO.2011.37.6418
85. Gilani B, Cassagnol M. Biochemistry, Cytochrome P450. StatPearls. Updated April 24, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557698/
86. LiverTox: clinical and research information on drug-induced liver injury; 2012. Carboplatin. Updated September 15, 2020. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548565/
87. Carboplatin. Prescribing information. Teva Parenteral Medicines; 2012. Accessed June 5, 204. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/077139Orig1s016lbl.pdf
88. Johnson-Arbor K, Dubey R. Doxorubicin. StatPearls. Updated August 8, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459232/
89. Doxorubicin hydrochloride injection. Prescribing information. Pfizer; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/050467s078,050629s030lbl.pdf
90. Gor, PP, Su, HI, Gray, RJ, et al. Cyclophosphamide-metabolizing enzyme polymorphisms and survival outcomes after adjuvant chemotherapy for node-positive breast cancer: a retrospective cohort study. Breast Cancer Res. 2010;12(3):R26. doi:10.1186/bcr2570
91. Cyclophosphamide. Prescribing information. Ingenus Pharmaceuticals; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212501s000lbl.pdf
92. Gemcitabine. Prescribing information. Hospira; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/200795Orig1s010lbl.pdf
93. Ifex (ifosfamide). Prescribing information. Baxter; 2012. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/019763s017lbl.pdf
94. Cisplatin. Prescribing information. WG Critical Care; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/018057s089lbl.pdf
95. Gerriets V, Kasi A. Bevacizumab. StatPearls. Updated August 28, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482126/
96. Avastin (bevacizumab). Prescribing information. Genentech; 2022. Accessed June 5, 2024. https://www.accessdata .fda.gov/drugsatfda_docs/label/2022/125085s340lbl.pdf
 97. Keytruda (pembrolizumab). Prescribing information. Merck; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf
97. Keytruda (pembrolizumab). Prescribing information. Merck; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf
98. Dean L, Kane M. Capecitabine therapy and DPYD genotype. National Center for Biotechnology Information (US); 2012. Updated November 2, 2020. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK385155/
99. Xeloda (capecitabine). Prescribing information. Roche; 2000. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2000/20896lbl.pdf
100. Pemetrexed injection. Prescribing information. Fareva Unterach; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214657s000lbl.pdf
101. Topotecan Injection. Prescribing information. Zydus Hospira Oncology; 2014. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/200582s001lbl.pdf
102. Ibrance (palbociclib). Prescribing information. Pfizer; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/207103s008lbl.pdf
103. Navelbine (vinorelbine) injection. Prescribing information. Pierre Fabre Médicament; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020388s037lbl.pdf
104. LiverTox: clinical and research information on drug-induced liver injury; 2012. Letrozole. Updated July 25, 2017. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548381/
105. Femara (letrozole). Prescribing information. Novartis; 2014. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020726s027lbl.pdf
106. Soltamox (tamoxifen citrate). Prescribing information. Rosemont Pharmaceuticals; 2018. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021807s005lbl.pdf
107. LiverTox: clinical and research information on drug-induced liver injury; 2012. Anastrozole. Updated July 25, 2017. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548189/
108. Grimm SW, Dyroff MC. Inhibition of human drug metabolizing cytochromes P450 by anastrozole, a potent and selective inhibitor of aromatase. Drug Metab Dispos. 1997;25(5):598-602.
109. Arimidex (anastrozole). Prescribing information. AstraZeneca; 2010. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020541s026lbl.pdf
110. Megace (megestrol acetate). Prescribing information. Endo Pharmaceuticals; 2018. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021778s024lbl.pdf
111. Imfinzi (durvalumab). Prescribing information. AstraZeneca; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761069s018lbl.pdf
112. Merwar G, Gibbons JR, Hosseini SA, et al. Nortriptyline. StatPearls. Updated June 5, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482214/
113. Pamelor (nortriptyline HCl). Prescribing information. Patheon Inc.; 2012. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/018012s029,018013s061lbl.pdf
114. Wellbutrin (bupropion hydrochloride). Prescribing information. GlaxoSmithKline; 2017. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018644s052lbl.pdf
115. Paxil (paroxetine). Prescribing information. Apotex Inc.; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020031s077lbl.pdf
116. Johnson DB, Lopez MJ, Kelley B. Dexamethasone. StatPearls. Updated May 2, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482130/
117. Hemady (dexamethasone). Prescribing information. Dexcel Pharma; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211379s000lbl.pdf
118. Parker SD, King N, Jacobs TF. Pegfilgrastim. StatPearls. Updated May 9, 2024. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK532893/
119. Fylnetra (pegfilgrastim-pbbk). Prescribing information. Kashiv BioSciences; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761084s000lbl.pdf
120. Emend (aprepitant). Prescribing information. Merck; 2015. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207865lbl.pdf
121. Lipitor (atorvastatin calcium). Prescribing information. Viatris Specialty; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020702Orig1s079correctedlbl.pdf
122. Cipro (ciprofloxacin hydrochloride). Prescribing information. Bayer HealthCare Pharmaceuticals Inc.; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/019537s090,020780s047lbl.pdf
123. Pino MA, Azer SA. Cimetidine. StatPearls. Updated March 6, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK544255/
124. Tagament (Cimetidine). Prescribing information. Mylan; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020238Orig1s024lbl.pdf
125. Neupogen (filgrastim). Prescribing information. Amgen Inc.; 2015. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/103353s5184lbl.pdf
126. Flagyl (metronidazole). Prescribing information. Pfizer; 2013. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020334s008lbl.pdf
127. Zymaxid (gatifloxacin ophthalmic solution). Prescribing information. Allergan; 2016. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/022548s002lbl.pdf
128. Macrobid (nitrofurantoin monohydrate). Prescribing information. Procter and Gamble Pharmaceutical Inc.; 2009. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020064s019lbl.pdf
129. Hyzaar (losartan). Prescribing information. Merck; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020387s067lbl.pdf
1. American Chemical Society. Discovery of camptothecin and taxol. acs.org. Accessed June 4, 2024. https://www.acs.org/education/whatischemistry/landmarks/camptothecintaxol.html
2. Bocci G, Di Paolo A, Danesi R. The pharmacological bases of the antiangiogenic activity of paclitaxel. Angiogenesis. 2013;16(3):481-492. doi:10.1007/s10456-013-9334-0.
3. Meštrovic T. Paclitaxel history. News Medical Life Sciences. Updated March 11, 2023. Accessed June 4, 2024. https://www.news-medical.net/health/Paclitaxel-History.aspx
4. Rowinsky EK, Donehower RC. Paclitaxel (taxol). N Engl J Med. 1995;332(15):1004-1014. doi:10.1056/NEJM199504133321507
5. Walsh V, Goodman J. The billion dollar molecule: Taxol in historical and theoretical perspective. Clio Med. 2002;66:245-267. doi:10.1163/9789004333499_013
6. Perdue RE, Jr, Hartwell JL. The search for plant sources of anticancer drugs. Morris Arboretum Bull. 1969;20:35-53.
7. Wall ME, Wani MC. Camptothecin and taxol: discovery to clinic—thirteenth Bruce F. Cain Memorial Award lecture. Cancer Res. 1995;55:753-760.
8. Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from taxus brevifolia. J Am Chem Soc. 1971;93(9):2325-2327. doi:10.1021/ja00738a045
9. Weaver BA. How taxol/paclitaxel kills cancer cells. Mol Biol Cell. 2014;25(18):2677-2681. doi:10.1091/mbc.E14-04-0916
10. Chen JG, Horwitz SB. Differential mitotic responses to microtubule-stabilizing and-destabilizing drugs. Cancer Res. 2002;62(7):1935-1938.
11. Singh S, Dash AK. Paclitaxel in cancer treatment: perspectives and prospects of its delivery challenges. Crit Rev Ther Drug Carrier Syst. 2009;26(4):333-372. doi:10.1615/critrevtherdrugcarriersyst.v26.i4.10
12. Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277(5698):665-667. doi:10.1038/277665a0
13. Fuchs DA, Johnson RK. Cytologic evidence that taxol, an antineoplastic agent from taxus brevifolia, acts as a mitotic spindle poison. Cancer Treat Rep. 1978;62(8):1219-1222.
14. Walsh V, Goodman J. From taxol to taxol: the changing identities and ownership of an anti-cancer drug. Med Anthropol. 2002;21(3-4):307-336. doi:10.1080/01459740214074
15. Walsh V, Goodman J. Cancer chemotherapy, biodiversity, public and private property: the case of the anti-cancer drug taxol. Soc Sci Med. 1999;49(9):1215-1225. doi:10.1016/s0277-9536(99)00161-6
16. Jordan MA, Wendell K, Gardiner S, Derry WB, Copp H, Wilson L. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 1996;56(4):816-825.
17. Picard M, Castells MC. Re-visiting hypersensitivity reactions to taxanes: a comprehensive review. Clin Rev Allergy Immunol. 2015;49(2):177-191. doi:10.1007/s12016-014-8416-0
18. Zasadil LM, Andersen KA, Yeum D, et al. Cytotoxicity of paclitaxel in breast cancer is due to chromosome missegregation on multipolar spindles. Sci Transl Med. 2014;6:229ra243. doi:10.1126/scitranslmed.3007965
19. National Cancer Institute. Carboplatin-Taxol. Published May 30, 2012. Updated March 22, 2023. Accessed June 4, 2024. https://www.cancer.gov/about-cancer/treatment/drugs/carboplatin-taxol
20. Taxol (paclitaxel). Prescribing information. Bristol-Myers Squibb; 2011. Accessed June 4, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
21. Abraxane (paclitaxel). Prescribing information. Celgene Corporation; 2021. Accessed June 4, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
22. Awosika AO, Farrar MC, Jacobs TF. Paclitaxel. StatPearls. Updated November 18, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK536917/
23. Gerriets V, Kasi A. Bevacizumab. StatPearls. Updated September 1, 2022. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482126/
24. American Cancer Society. Chemotherapy for endometrial cancer. Updated March 27, 2019. Accessed June 4, 2024. https://www.cancer.org/cancer/types/endometrial-cancer/treating/chemotherapy.html
25. US Food and Drug Administration. FDA approves pembrolizumab in combination with chemotherapy for first-line treatment of metastatic squamous NSCLC. October 30, 2018. Updated December 14, 2018. Accessed June 4, 2024. https://www.fda.gov/drugs/fda-approves-pembrolizumab-combination-chemotherapy-first-line-treatment-metastatic-squamous-nsclc
26. US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for locally recurrent unresectable or metastatic triple negative breast cancer. November 13, 2020. Accessed June 4, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-locally-recurrent-unresectable-or-metastatic-triple
27. US Food and Drug Administration. FDA approves atezolizumab for PD-L1 positive unresectable locally advanced or metastatic triple-negative breast. March 8, 2019. Updated March 18, 2019. Accessed June 5, 2024. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-atezolizumab-pd-l1-positive-unresectable-locally-advanced-or-metastatic-triple-negative
28. US Food and Drug Administration. FDA issues alert about efficacy and potential safety concerns with atezolizumab in combination with paclitaxel for treatment of breast cancer. September 8, 2020. Accessed June 5, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-issues-alert-about-efficacy-and-potential-safety-concerns-atezolizumab-combination-paclitaxel
29. Tan AR. Chemoimmunotherapy: still the standard of care for metastatic triple-negative breast cancer. ASCO Daily News. February 23, 2022. Accessed June 5, 2024. https://dailynews.ascopubs.org/do/chemoimmunotherapy-still-standard-care-metastatic-triple-negative-breast-cancer
30. McGuire WP, Rowinsky EK, Rosenshein NB, et al. Taxol: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Ann Intern Med. 1989;111(4):273-279. doi:10.7326/0003-4819-111-4-273
31. Milas L, Hunter NR, Kurdoglu B, et al. Kinetics of mitotic arrest and apoptosis in murine mammary and ovarian tumors treated with taxol. Cancer Chemother Pharmacol. 1995;35(4):297-303. doi:10.1007/BF00689448
32. Searle J, Collins DJ, Harmon B, Kerr JF. The spontaneous occurrence of apoptosis in squamous carcinomas of the uterine cervix. Pathology. 1973;5(2):163-169. doi:10.3109/00313027309060831
33. Gallego-Jara J, Lozano-Terol G, Sola-Martínez RA, Cánovas-Díaz M, de Diego Puente T. A compressive review about taxol®: history and future challenges. Molecules. 2020;25(24):5986. doi:10.3390/molecules25245986
34. Bernabeu E, Cagel M, Lagomarsino E, Moretton M, Chiappetta DA. Paclitaxel: What has been done and the challenges remain ahead. Int J Pharm. 2017;526(1-2):474-495. doi:10.1016/j.ijpharm.2017.05.016
35. Nehate C, Jain S, Saneja A, et al. Paclitaxel formulations: challenges and novel delivery options. Curr Drug Deliv. 2014;11(6):666-686. doi:10.2174/1567201811666140609154949
36. Gelderblom H, Verweij J, Nooter K, Sparreboom A, Cremophor EL. The drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37(13):1590-1598. doi:10.1016/S0959-8049(01)00171-x
37. Chowdhury MR, Moshikur RM, Wakabayashi R, et al. In vivo biocompatibility, pharmacokinetics, antitumor efficacy, and hypersensitivity evaluation of ionic liquid-mediated paclitaxel formulations. Int J Pharm. 2019;565:219-226. doi:10.1016/j.ijpharm.2019.05.020
38. Borgå O, Henriksson R, Bjermo H, Lilienberg E, Heldring N, Loman N. Maximum tolerated dose and pharmacokinetics of paclitaxel micellar in patients with recurrent malignant solid tumours: a dose-escalation study. Adv Ther. 2019;36(5):1150-1163. doi:10.1007/s12325-019-00909-6
39. Rouzier R, Rajan R, Wagner P, et al. Microtubule-associated protein tau: a marker of paclitaxel sensitivity in breast cancer. Proc Natl Acad Sci USA. 2005;102(23):8315-8320. doi:10.1073/pnas.0408974102
40. Choudhury H, Gorain B, Tekade RK, Pandey M, Karmakar S, Pal TK. Safety against nephrotoxicity in paclitaxel treatment: oral nanocarrier as an effective tool in preclinical evaluation with marked in vivo antitumor activity. Regul Toxicol Pharmacol. 2017;91:179-189. doi:10.1016/j.yrtph.2017.10.023
41. Barkat MA, Beg S, Pottoo FH, Ahmad FJ. Nanopaclitaxel therapy: an evidence based review on the battle for next-generation formulation challenges. Nanomedicine (Lond). 2019;14(10):1323-1341. doi:10.2217/nnm-2018-0313
42. Sofias AM, Dunne M, Storm G, Allen C. The battle of “nano” paclitaxel. Adv Drug Deliv Rev. 2017;122:20-30. doi:10.1016/j.addr.2017.02.003
43. Yang N, Wang C, Wang J, et al. Aurora inase a stabilizes FOXM1 to enhance paclitaxel resistance in triple-negative breast cancer. J Cell Mol Med. 2019;23(9):6442-6453. doi:10.1111/jcmm.14538
44. Chowdhury MR, Moshikur RM, Wakabayashi R, et al. Ionic-liquid-based paclitaxel preparation: a new potential formulation for cancer treatment. Mol Pharm. 2018;15(16):2484-2488. doi:10.1021/acs.molpharmaceut.8b00305
45. Chung HJ, Kim HJ, Hong ST. Tumor-specific delivery of a paclitaxel-loading HSA-haemin nanoparticle for cancer treatment. Nanomedicine. 2020;23:102089. doi:10.1016/j.nano.2019.102089
46. Ye L, He J, Hu Z, et al. Antitumor effect and toxicity of lipusu in rat ovarian cancer xenografts. Food Chem Toxicol. 2013;52:200-206. doi:10.1016/j.fct.2012.11.004
47. Ma WW, Lam ET, Dy GK, et al. A pharmacokinetic and dose-escalating study of paclitaxel injection concentrate for nano-dispersion (PICN) alone and with arboplatin in patients with advanced solid tumors. J Clin Oncol. 2013;31:2557. doi:10.1200/jco.2013.31.15_suppl.2557
48. Micha JP, Goldstein BH, Birk CL, Rettenmaier MA, Brown JV. Abraxane in the treatment of ovarian cancer: the absence of hypersensitivity reactions. Gynecol Oncol. 2006;100(2):437-438. doi:10.1016/j.ygyno.2005.09.012
49. Ingle SG, Pai RV, Monpara JD, Vavia PR. Liposils: an effective strategy for stabilizing paclitaxel loaded liposomes by surface coating with silica. Eur J Pharm Sci. 2018;122:51-63. doi:10.1016/j.ejps.2018.06.025
50. Abriata JP, Turatti RC, Luiz MT, et al. Development, characterization and biological in vitro assays of paclitaxel-loaded PCL polymeric nanoparticles. Mater Sci Eng C Mater Biol Appl. 2019;96:347-355. doi:10.1016/j.msec.2018.11.035
51. Hu J, Fu S, Peng Q, et al. Paclitaxel-loaded polymeric nanoparticles combined with chronomodulated chemotherapy on lung cancer: in vitro and in vivo evaluation. Int J Pharm. 2017;516(1-2):313-322. doi:10.1016/j.ijpharm.2016.11.047
52. Dranitsaris G, Yu B, Wang L, et al. Abraxane® vs Taxol® for patients with advanced breast cancer: a prospective time and motion analysis from a chinese health care perspective. J Oncol Pharm Pract. 2016;22(2):205-211. doi:10.1177/1078155214556008
53. Pei Q, Hu X, Liu S, Li Y, Xie Z, Jing X. Paclitaxel dimers assembling nanomedicines for treatment of cervix carcinoma. J Control Release. 2017;254:23-33. doi:10.1016/j.jconrel.2017.03.391
54. Wang Y, Wang M, Qi H, et al. Pathway-dependent inhibition of paclitaxel hydroxylation by kinase inhibitors and assessment of drug-drug interaction potentials. Drug Metab Dispos. 2014;42(4):782-795. doi:10.1124/dmd.113.053793
55. Shen F, Jiang G, Philips S, et al. Cytochrome P450 oxidoreductase (POR) associated with severe paclitaxel-induced peripheral neuropathy in patients of european ancestry from ECOG-ACRIN E5103. Clin Cancer Res. 2023;29(13):2494-2500. doi:10.1158/1078-0432.CCR-22-2431
56. Henningsson A, Marsh S, Loos WJ, et al. Association of CYP2C8, CYP3A4, CYP3A5, and ABCB1 polymorphisms with the pharmacokinetics of paclitaxel. Clin Cancer Res. 2005;11(22):8097-8104. doi:10.1158/1078-0432.CCR-05-1152
57. Mukai Y, Senda A, Toda T, et al. Drug-drug interaction between losartan and paclitaxel in human liver microsomes with different CYP2C8 genotypes. Basic Clin Pharmacol Toxicol. 2015;116(6):493-498. doi:10.1111/bcpt.12355
58. Kawahara B, Faull KF, Janzen C, Mascharak PK. Carbon monoxide inhibits cytochrome P450 enzymes CYP3A4/2C8 in human breast cancer cells, increasing sensitivity to paclitaxel. J Med Chem. 2021;64(12):8437-8446. doi:10.1021/acs.jmedchem.1c00404
59. Cresteil T, Monsarrat B, Dubois J, Sonnier M, Alvinerie P, Gueritte F. Regioselective metabolism of taxoids by human CYP3A4 and 2C8: structure-activity relationship. Drug Metab Dispos. 2002;30(4):438-445. doi:10.1124/dmd.30.4.438
60. Taniguchi R, Kumai T, Matsumoto N, et al. Utilization of human liver microsomes to explain individual differences in paclitaxel metabolism by CYP2C8 and CYP3A4. J Pharmacol Sci. 2005;97(1):83-90. doi:10.1254/jphs.fp0040603
61. Nakayama A, Tsuchiya K, Xu L, Matsumoto T, Makino T. Drug-interaction between paclitaxel and goshajinkigan extract and its constituents. J Nat Med. 2022;76(1):59-67. doi:10.1007/s11418-021-01552-8
62. Monsarrat B, Chatelut E, Royer I, et al. Modification of paclitaxel metabolism in a cancer patient by induction of cytochrome P450 3A4. Drug Metab Dispos. 1998;26(3):229-233.
63. Walle T. Assays of CYP2C8- and CYP3A4-mediated metabolism of taxol in vivo and in vitro. Methods Enzymol. 1996;272:145-151. doi:10.1016/s0076-6879(96)72018-9
64. Hanioka N, Matsumoto K, Saito Y, Narimatsu S. Functional characterization of CYP2C8.13 and CYP2C8.14: catalytic activities toward paclitaxel. Basic Clin Pharmacol Toxicol. 2010;107(1):565-569. doi:10.1111/j.1742-7843.2010.00543.x
65. Luong TT, Powers CN, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interactions (DDIs) of gefitinib with/without losartan and selective serotonin reuptake inhibitors (SSRIs): citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and venlafaxine. Curr Res Pharmacol Drug Discov. 2022;3:100112. doi:10.1016/j.crphar.2022.100112
66. Luong TT, McAnulty MJ, Evers DL, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interaction (DDI) of gefitinib or erlotinib with Cytochrome P450 (CYP) inhibiting drugs, fluoxetine and/or losartan. Curr Res Toxicol. 2021;2:217-224. doi:10.1016/j.crtox.2021.05.006
67. Luong TT, Powers CN, Reinhardt BJ, et al. Retrospective evaluation of drug-drug interactions with erlotinib and gefitinib use in the military health system. Fed Pract. 2023;40(suppl 3):S24-S34. doi:10.12788/fp.0401
68. Adamo M, Dickie L, Ruhl J. SEER program coding and staging manual 2016. National Cancer Institute. Accessed June 5, 2024. https://seer.cancer.gov/archive/manuals/2016/SPCSM_2016_maindoc.pdf
69. World Health Organization. International classification of diseases for oncology (ICD-O) 3rd ed, 1st revision. World Health Organization; 2013. Accessed June 5, 2024. https://apps.who.int/iris/handle/10665/96612
70. Z score calculator for 2 population proportions. Social science statistics. Accessed June 5, 2024. https://www.socscistatistics.com/tests/ztest/default2.aspx
71. US Food and Drug Administration. Generic drugs: question & answers. FDA.gov. Accessed June 5, 2024. https://www.fda.gov/drugs/frequently-asked-questions-popular-topics/generic-drugs-questions-answers
72. Oura M, Saito H, Nishikawa Y. Shortage of nab-paclitaxel in Japan and around the world: issues in global information sharing. JMA J. 2023;6(2):192-195. doi:10.31662/jmaj.2022-0179
73. Yuan H, Guo H, Luan X, et al. Albumin nanoparticle of paclitaxel (abraxane) decreases while taxol increases breast cancer stem cells in treatment of triple negative breast cancer. Mol Pharm. 2020;17(7):2275-2286. doi:10.1021/acs.molpharmaceut.9b01221
74. Dranitsaris G, Yu B, Wang L, et al. Abraxane® versus Taxol® for patients with advanced breast cancer: a prospective time and motion analysis from a Chinese health care perspective. J Oncol Pharm Pract. 2016;22(2):205-211. doi:10.1177/1078155214556008
75. Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794-7803. doi:10.1200/JCO.2005.04.
76. Liu M, Liu S, Yang L, Wang S. Comparison between nab-paclitaxel and solvent-based taxanes as neoadjuvant therapy in breast cancer: a systematic review and meta-analysis. BMC Cancer. 2021;21(1):118. doi:10.1186/s12885-021-07831-7
77. Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (taxol). Semin Oncol. 1993;20(4 Suppl 3):1-15.
78. Banerji A, Lax T, Guyer A, Hurwitz S, Camargo CA Jr, Long AA. Management of hypersensitivity reactions to carboplatin and paclitaxel in an outpatient oncology infusion center: a 5-year review. J Allergy Clin Immunol Pract. 2014;2(4):428-433. doi:10.1016/j.jaip.2014.04.010
79. Staff NP, Fehrenbacher JC, Caillaud M, Damaj MI, Segal RA, Rieger S. Pathogenesis of paclitaxel-induced peripheral neuropathy: a current review of in vitro and in vivo findings using rodent and human model systems. Exp Neurol. 2020;324:113121. doi:10.1016/j.expneurol.2019.113121
80. Postma TJ, Vermorken JB, Liefting AJ, Pinedo HM, Heimans JJ. Paclitaxel-induced neuropathy. Ann Oncol. 1995;6(5):489-494. doi:10.1093/oxfordjournals.annonc.a059220
81. Liu JM, Chen YM, Chao Y, et al. Paclitaxel-induced severe neuropathy in patients with previous radiotherapy to the head and neck region. J Natl Cancer Inst. 1996;88(14):1000-1002. doi:10.1093/jnci/88.14.1000-a
82. Bayat Mokhtari R, Homayouni TS, Baluch N, et al. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022-38043. doi:10.18632/oncotarget.16723
83. Blagosklonny MV. Analysis of FDA approved anticancer drugs reveals the future of cancer therapy. Cell Cycle. 2004;3(8):1035-1042.
84. Yap TA, Omlin A, de Bono JS. Development of therapeutic combinations targeting major cancer signaling pathways. J Clin Oncol. 2013;31(12):1592-1605. doi:10.1200/JCO.2011.37.6418
85. Gilani B, Cassagnol M. Biochemistry, Cytochrome P450. StatPearls. Updated April 24, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557698/
86. LiverTox: clinical and research information on drug-induced liver injury; 2012. Carboplatin. Updated September 15, 2020. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548565/
87. Carboplatin. Prescribing information. Teva Parenteral Medicines; 2012. Accessed June 5, 204. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/077139Orig1s016lbl.pdf
88. Johnson-Arbor K, Dubey R. Doxorubicin. StatPearls. Updated August 8, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459232/
89. Doxorubicin hydrochloride injection. Prescribing information. Pfizer; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/050467s078,050629s030lbl.pdf
90. Gor, PP, Su, HI, Gray, RJ, et al. Cyclophosphamide-metabolizing enzyme polymorphisms and survival outcomes after adjuvant chemotherapy for node-positive breast cancer: a retrospective cohort study. Breast Cancer Res. 2010;12(3):R26. doi:10.1186/bcr2570
91. Cyclophosphamide. Prescribing information. Ingenus Pharmaceuticals; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212501s000lbl.pdf
92. Gemcitabine. Prescribing information. Hospira; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/200795Orig1s010lbl.pdf
93. Ifex (ifosfamide). Prescribing information. Baxter; 2012. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/019763s017lbl.pdf
94. Cisplatin. Prescribing information. WG Critical Care; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/018057s089lbl.pdf
95. Gerriets V, Kasi A. Bevacizumab. StatPearls. Updated August 28, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482126/
96. Avastin (bevacizumab). Prescribing information. Genentech; 2022. Accessed June 5, 2024. https://www.accessdata .fda.gov/drugsatfda_docs/label/2022/125085s340lbl.pdf
 97. Keytruda (pembrolizumab). Prescribing information. Merck; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf
97. Keytruda (pembrolizumab). Prescribing information. Merck; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf
98. Dean L, Kane M. Capecitabine therapy and DPYD genotype. National Center for Biotechnology Information (US); 2012. Updated November 2, 2020. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK385155/
99. Xeloda (capecitabine). Prescribing information. Roche; 2000. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2000/20896lbl.pdf
100. Pemetrexed injection. Prescribing information. Fareva Unterach; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214657s000lbl.pdf
101. Topotecan Injection. Prescribing information. Zydus Hospira Oncology; 2014. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/200582s001lbl.pdf
102. Ibrance (palbociclib). Prescribing information. Pfizer; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/207103s008lbl.pdf
103. Navelbine (vinorelbine) injection. Prescribing information. Pierre Fabre Médicament; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020388s037lbl.pdf
104. LiverTox: clinical and research information on drug-induced liver injury; 2012. Letrozole. Updated July 25, 2017. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548381/
105. Femara (letrozole). Prescribing information. Novartis; 2014. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020726s027lbl.pdf
106. Soltamox (tamoxifen citrate). Prescribing information. Rosemont Pharmaceuticals; 2018. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021807s005lbl.pdf
107. LiverTox: clinical and research information on drug-induced liver injury; 2012. Anastrozole. Updated July 25, 2017. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548189/
108. Grimm SW, Dyroff MC. Inhibition of human drug metabolizing cytochromes P450 by anastrozole, a potent and selective inhibitor of aromatase. Drug Metab Dispos. 1997;25(5):598-602.
109. Arimidex (anastrozole). Prescribing information. AstraZeneca; 2010. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020541s026lbl.pdf
110. Megace (megestrol acetate). Prescribing information. Endo Pharmaceuticals; 2018. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021778s024lbl.pdf
111. Imfinzi (durvalumab). Prescribing information. AstraZeneca; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761069s018lbl.pdf
112. Merwar G, Gibbons JR, Hosseini SA, et al. Nortriptyline. StatPearls. Updated June 5, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482214/
113. Pamelor (nortriptyline HCl). Prescribing information. Patheon Inc.; 2012. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/018012s029,018013s061lbl.pdf
114. Wellbutrin (bupropion hydrochloride). Prescribing information. GlaxoSmithKline; 2017. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018644s052lbl.pdf
115. Paxil (paroxetine). Prescribing information. Apotex Inc.; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020031s077lbl.pdf
116. Johnson DB, Lopez MJ, Kelley B. Dexamethasone. StatPearls. Updated May 2, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482130/
117. Hemady (dexamethasone). Prescribing information. Dexcel Pharma; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211379s000lbl.pdf
118. Parker SD, King N, Jacobs TF. Pegfilgrastim. StatPearls. Updated May 9, 2024. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK532893/
119. Fylnetra (pegfilgrastim-pbbk). Prescribing information. Kashiv BioSciences; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761084s000lbl.pdf
120. Emend (aprepitant). Prescribing information. Merck; 2015. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207865lbl.pdf
121. Lipitor (atorvastatin calcium). Prescribing information. Viatris Specialty; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020702Orig1s079correctedlbl.pdf
122. Cipro (ciprofloxacin hydrochloride). Prescribing information. Bayer HealthCare Pharmaceuticals Inc.; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/019537s090,020780s047lbl.pdf
123. Pino MA, Azer SA. Cimetidine. StatPearls. Updated March 6, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK544255/
124. Tagament (Cimetidine). Prescribing information. Mylan; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020238Orig1s024lbl.pdf
125. Neupogen (filgrastim). Prescribing information. Amgen Inc.; 2015. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/103353s5184lbl.pdf
126. Flagyl (metronidazole). Prescribing information. Pfizer; 2013. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020334s008lbl.pdf
127. Zymaxid (gatifloxacin ophthalmic solution). Prescribing information. Allergan; 2016. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/022548s002lbl.pdf
128. Macrobid (nitrofurantoin monohydrate). Prescribing information. Procter and Gamble Pharmaceutical Inc.; 2009. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020064s019lbl.pdf
129. Hyzaar (losartan). Prescribing information. Merck; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020387s067lbl.pdf
Risk Stratification May Work Well for FIT-Based CRC Screening in Elderly
WASHINGTON — , according to a study presented at the annual Digestive Disease Week® (DDW).
In particular, interval CRC risk can vary substantially based on the fecal hemoglobin (f-Hb) concentration in the patient’s last fecal immunochemical test (FIT), as well as the number of prior screening rounds.
“Less is known about what happens after the upper age limit has been reached and individuals are not invited to participate in more screening rounds. This is important as life expectancy is increasing, and it is increasingly important to consider the most efficient way of screening the elderly,” said lead author Brenda van Stigt, a PhD candidate focused on cancer screening at Erasmus University Medical Center in Rotterdam, the Netherlands.
In the Netherlands, adults between ages 55 and 75 are invited to participate in stool-based CRC screening every 2 years. Based on a fecal immunochemical testing (FIT) threshold of 47 μg Hb/g, those who test positive are referred to colonoscopy, and those who test negative are invited to participate again after a 2-year period.
FIT can play a major role in risk stratification, Ms. van Stigt noted, along with other factors that influence CRC risk, such as age, sex, and CRC screening history. Although this is documented for ages 55-75, she and colleagues wanted to know more about what happens after age 75.
Ms. Van Stigt and colleagues conducted a population-based study by analyzing Dutch national cancer registry data and FIT results around the final screening at age 75, looking at those who were diagnosed with CRC within 24 months of their last negative FIT. The researchers assessed interval CRC risk and cancer stage, accounting for sex, last f-Hb concentration, and the number of screening rounds.
Among 305,761 people with a complete 24-month follow-up after a negative FIT, 661 patients were diagnosed with interval CRC, indicating an overall interval CRC risk of 21.6 per 10,000 individuals with a negative FIT. There were no significant differences by sex.
However, there were differences by screening rounds, with those who had participated in three or four screening rounds having a lower risk than those who participated only once (HR, .49).
In addition, those with detectable f-Hb (>0 μg Hb/g) in their last screening round had a much higher interval CRC risk (HR, 4.87), at 65.8 per 10,000 negative FITs, compared with 13.8 per 10,000 among those without detectable f-Hb. Interval CRC risk also increased over time for those with detectable f-Hb.
About 15% of the total population had detectable f-Hb, whereas 46% of those with interval CRC had detectable f-Hb, Ms. van Stigt said, meaning that nearly half of patients who were diagnosed with interval CRC already had detectable f-Hb in their prior FIT.
In a survival analysis, there was no association between interval CRC risk and sex. However, those who participated in three or four screening rounds were half as likely to be diagnosed than those who participated once or twice, and those with detectable f-Hb were five times as likely to be diagnosed.
For late-stage CRC, there was no association with sex or the number of screening rounds. Detectable f-Hb was associated with not only a higher risk of interval CRC but also a late-stage diagnosis.
“These findings indicate that one uniform age to stop screening is suboptimal,” Ms. van Stigt said. “Personalized screening strategies should, therefore, also ideally incorporate a risk-stratified age to stop screening.”
The US Preventive Services Task Force recommends that clinicians personalize screening for ages 76-85, accounting for overall health, prior screening history, and patient preferences.
“But we have no clear guidance on how to quantify or weigh these factors. This interesting study highlights how one of these factors (prior screening history) and fecal hemoglobin level (an emerging factor) are powerful stratifiers of subsequent colorectal cancer risk,” said Sameer D. Saini, MD, AGAF, director and research investigator at the VA Ann Arbor Healthcare System’s Center for Clinical Management Research. Dr. Saini wasn’t involved with the study.
At the clinical level, Dr. Saini said, sophisticated modeling is needed to understand the interaction with competing risks and identify the optimal screening strategies for patients at varying levels of cancer risk and life expectancy. Models could also help to quantify the population benefits and cost-effectiveness of personalized screening.
“Finally, it is important to note that, in many health systems, access to quantitative FIT may be limited,” he said. “These data may be less informative if colonoscopy is the primary mode of screening.”
Ms. van Stigt and Dr. Saini reported no relevant disclosures.
WASHINGTON — , according to a study presented at the annual Digestive Disease Week® (DDW).
In particular, interval CRC risk can vary substantially based on the fecal hemoglobin (f-Hb) concentration in the patient’s last fecal immunochemical test (FIT), as well as the number of prior screening rounds.
“Less is known about what happens after the upper age limit has been reached and individuals are not invited to participate in more screening rounds. This is important as life expectancy is increasing, and it is increasingly important to consider the most efficient way of screening the elderly,” said lead author Brenda van Stigt, a PhD candidate focused on cancer screening at Erasmus University Medical Center in Rotterdam, the Netherlands.
In the Netherlands, adults between ages 55 and 75 are invited to participate in stool-based CRC screening every 2 years. Based on a fecal immunochemical testing (FIT) threshold of 47 μg Hb/g, those who test positive are referred to colonoscopy, and those who test negative are invited to participate again after a 2-year period.
FIT can play a major role in risk stratification, Ms. van Stigt noted, along with other factors that influence CRC risk, such as age, sex, and CRC screening history. Although this is documented for ages 55-75, she and colleagues wanted to know more about what happens after age 75.
Ms. Van Stigt and colleagues conducted a population-based study by analyzing Dutch national cancer registry data and FIT results around the final screening at age 75, looking at those who were diagnosed with CRC within 24 months of their last negative FIT. The researchers assessed interval CRC risk and cancer stage, accounting for sex, last f-Hb concentration, and the number of screening rounds.
Among 305,761 people with a complete 24-month follow-up after a negative FIT, 661 patients were diagnosed with interval CRC, indicating an overall interval CRC risk of 21.6 per 10,000 individuals with a negative FIT. There were no significant differences by sex.
However, there were differences by screening rounds, with those who had participated in three or four screening rounds having a lower risk than those who participated only once (HR, .49).
In addition, those with detectable f-Hb (>0 μg Hb/g) in their last screening round had a much higher interval CRC risk (HR, 4.87), at 65.8 per 10,000 negative FITs, compared with 13.8 per 10,000 among those without detectable f-Hb. Interval CRC risk also increased over time for those with detectable f-Hb.
About 15% of the total population had detectable f-Hb, whereas 46% of those with interval CRC had detectable f-Hb, Ms. van Stigt said, meaning that nearly half of patients who were diagnosed with interval CRC already had detectable f-Hb in their prior FIT.
In a survival analysis, there was no association between interval CRC risk and sex. However, those who participated in three or four screening rounds were half as likely to be diagnosed than those who participated once or twice, and those with detectable f-Hb were five times as likely to be diagnosed.
For late-stage CRC, there was no association with sex or the number of screening rounds. Detectable f-Hb was associated with not only a higher risk of interval CRC but also a late-stage diagnosis.
“These findings indicate that one uniform age to stop screening is suboptimal,” Ms. van Stigt said. “Personalized screening strategies should, therefore, also ideally incorporate a risk-stratified age to stop screening.”
The US Preventive Services Task Force recommends that clinicians personalize screening for ages 76-85, accounting for overall health, prior screening history, and patient preferences.
“But we have no clear guidance on how to quantify or weigh these factors. This interesting study highlights how one of these factors (prior screening history) and fecal hemoglobin level (an emerging factor) are powerful stratifiers of subsequent colorectal cancer risk,” said Sameer D. Saini, MD, AGAF, director and research investigator at the VA Ann Arbor Healthcare System’s Center for Clinical Management Research. Dr. Saini wasn’t involved with the study.
At the clinical level, Dr. Saini said, sophisticated modeling is needed to understand the interaction with competing risks and identify the optimal screening strategies for patients at varying levels of cancer risk and life expectancy. Models could also help to quantify the population benefits and cost-effectiveness of personalized screening.
“Finally, it is important to note that, in many health systems, access to quantitative FIT may be limited,” he said. “These data may be less informative if colonoscopy is the primary mode of screening.”
Ms. van Stigt and Dr. Saini reported no relevant disclosures.
WASHINGTON — , according to a study presented at the annual Digestive Disease Week® (DDW).
In particular, interval CRC risk can vary substantially based on the fecal hemoglobin (f-Hb) concentration in the patient’s last fecal immunochemical test (FIT), as well as the number of prior screening rounds.
“Less is known about what happens after the upper age limit has been reached and individuals are not invited to participate in more screening rounds. This is important as life expectancy is increasing, and it is increasingly important to consider the most efficient way of screening the elderly,” said lead author Brenda van Stigt, a PhD candidate focused on cancer screening at Erasmus University Medical Center in Rotterdam, the Netherlands.
In the Netherlands, adults between ages 55 and 75 are invited to participate in stool-based CRC screening every 2 years. Based on a fecal immunochemical testing (FIT) threshold of 47 μg Hb/g, those who test positive are referred to colonoscopy, and those who test negative are invited to participate again after a 2-year period.
FIT can play a major role in risk stratification, Ms. van Stigt noted, along with other factors that influence CRC risk, such as age, sex, and CRC screening history. Although this is documented for ages 55-75, she and colleagues wanted to know more about what happens after age 75.
Ms. Van Stigt and colleagues conducted a population-based study by analyzing Dutch national cancer registry data and FIT results around the final screening at age 75, looking at those who were diagnosed with CRC within 24 months of their last negative FIT. The researchers assessed interval CRC risk and cancer stage, accounting for sex, last f-Hb concentration, and the number of screening rounds.
Among 305,761 people with a complete 24-month follow-up after a negative FIT, 661 patients were diagnosed with interval CRC, indicating an overall interval CRC risk of 21.6 per 10,000 individuals with a negative FIT. There were no significant differences by sex.
However, there were differences by screening rounds, with those who had participated in three or four screening rounds having a lower risk than those who participated only once (HR, .49).
In addition, those with detectable f-Hb (>0 μg Hb/g) in their last screening round had a much higher interval CRC risk (HR, 4.87), at 65.8 per 10,000 negative FITs, compared with 13.8 per 10,000 among those without detectable f-Hb. Interval CRC risk also increased over time for those with detectable f-Hb.
About 15% of the total population had detectable f-Hb, whereas 46% of those with interval CRC had detectable f-Hb, Ms. van Stigt said, meaning that nearly half of patients who were diagnosed with interval CRC already had detectable f-Hb in their prior FIT.
In a survival analysis, there was no association between interval CRC risk and sex. However, those who participated in three or four screening rounds were half as likely to be diagnosed than those who participated once or twice, and those with detectable f-Hb were five times as likely to be diagnosed.
For late-stage CRC, there was no association with sex or the number of screening rounds. Detectable f-Hb was associated with not only a higher risk of interval CRC but also a late-stage diagnosis.
“These findings indicate that one uniform age to stop screening is suboptimal,” Ms. van Stigt said. “Personalized screening strategies should, therefore, also ideally incorporate a risk-stratified age to stop screening.”
The US Preventive Services Task Force recommends that clinicians personalize screening for ages 76-85, accounting for overall health, prior screening history, and patient preferences.
“But we have no clear guidance on how to quantify or weigh these factors. This interesting study highlights how one of these factors (prior screening history) and fecal hemoglobin level (an emerging factor) are powerful stratifiers of subsequent colorectal cancer risk,” said Sameer D. Saini, MD, AGAF, director and research investigator at the VA Ann Arbor Healthcare System’s Center for Clinical Management Research. Dr. Saini wasn’t involved with the study.
At the clinical level, Dr. Saini said, sophisticated modeling is needed to understand the interaction with competing risks and identify the optimal screening strategies for patients at varying levels of cancer risk and life expectancy. Models could also help to quantify the population benefits and cost-effectiveness of personalized screening.
“Finally, it is important to note that, in many health systems, access to quantitative FIT may be limited,” he said. “These data may be less informative if colonoscopy is the primary mode of screening.”
Ms. van Stigt and Dr. Saini reported no relevant disclosures.
FROM DDW 2024
Does Extended Postop Follow-Up Improve Survival in Gastric Cancer?
TOPLINE:
METHODOLOGY:
- Currently, postgastrectomy cancer surveillance typically lasts 5 years, although some centers now monitor patients beyond this point.
- To investigate the potential benefit of extended surveillance, researchers used Korean National Health Insurance claims data to identify 40,468 patients with gastric cancer who were disease free 5 years after gastrectomy — 14,294 received extended regular follow-up visits and 26,174 did not.
- The extended regular follow-up group was defined as having endoscopy or abdominopelvic CT between 2 months and 2 years before diagnosis of late recurrence or gastric remnant cancer and having two or more examinations between 5.5 and 8.5 years after gastrectomy. Late recurrence was a recurrence diagnosed 5 years after gastrectomy.
- Researchers used Cox proportional hazards regression to evaluate the independent association between follow-up and overall and postrecurrence survival rates.
TAKEAWAY:
- Overall, 5 years postgastrectomy, the incidence of late recurrence or gastric remnant cancer was 7.8% — 4.0% between 5 and 10 years (1610 of 40,468 patients) and 9.4% after 10 years (1528 of 16,287 patients).
- Regular follow-up beyond 5 years was associated with a significant reduction in overall mortality — from 49.4% to 36.9% at 15 years (P < .001). Overall survival after late recurrence or gastric remnant cancer also improved significantly with extended regular follow-up, with the 5-year postrecurrence survival rate increasing from 32.7% to 71.1% (P < .001).
- The combination of endoscopy and abdominopelvic CT provided the highest 5-year postrecurrence survival rate (74.5%), compared with endoscopy alone (54.5%) or CT alone (47.1%).
- A time interval of more than 2 years between a previous endoscopy or abdominopelvic CT and diagnosis of late recurrence or gastric remnant cancer significantly decreased postrecurrence survival (hazard ratio [HR], 1.72 for endoscopy and HR, 1.48 for abdominopelvic CT).
IN PRACTICE:
“These findings suggest that extended regular follow-up after 5 years post gastrectomy should be implemented clinically and that current practice and value of follow-up protocols in postoperative care of patients with gastric cancer be reconsidered,” the authors concluded.
The authors of an accompanying commentary cautioned that, while the study “successfully establishes groundwork for extending surveillance of gastric cancer in high-risk populations, more work is needed to strategically identify those who would benefit most from extended surveillance.”
SOURCE:
The study, with first author Ju-Hee Lee, MD, PhD, Department of Surgery, Hanyang University College of Medicine, Seoul, South Korea, and accompanying commentary were published online on June 18 in JAMA Surgery.
LIMITATIONS:
Recurrent cancer and gastric remnant cancer could not be distinguished from each other because clinical records were not analyzed. The claims database lacked detailed clinical information on individual patients, including cancer stages, and a separate analysis of tumor markers could not be performed.
DISCLOSURES:
The study was funded by a grant from the Korean Gastric Cancer Association. The study authors and commentary authors reported no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Currently, postgastrectomy cancer surveillance typically lasts 5 years, although some centers now monitor patients beyond this point.
- To investigate the potential benefit of extended surveillance, researchers used Korean National Health Insurance claims data to identify 40,468 patients with gastric cancer who were disease free 5 years after gastrectomy — 14,294 received extended regular follow-up visits and 26,174 did not.
- The extended regular follow-up group was defined as having endoscopy or abdominopelvic CT between 2 months and 2 years before diagnosis of late recurrence or gastric remnant cancer and having two or more examinations between 5.5 and 8.5 years after gastrectomy. Late recurrence was a recurrence diagnosed 5 years after gastrectomy.
- Researchers used Cox proportional hazards regression to evaluate the independent association between follow-up and overall and postrecurrence survival rates.
TAKEAWAY:
- Overall, 5 years postgastrectomy, the incidence of late recurrence or gastric remnant cancer was 7.8% — 4.0% between 5 and 10 years (1610 of 40,468 patients) and 9.4% after 10 years (1528 of 16,287 patients).
- Regular follow-up beyond 5 years was associated with a significant reduction in overall mortality — from 49.4% to 36.9% at 15 years (P < .001). Overall survival after late recurrence or gastric remnant cancer also improved significantly with extended regular follow-up, with the 5-year postrecurrence survival rate increasing from 32.7% to 71.1% (P < .001).
- The combination of endoscopy and abdominopelvic CT provided the highest 5-year postrecurrence survival rate (74.5%), compared with endoscopy alone (54.5%) or CT alone (47.1%).
- A time interval of more than 2 years between a previous endoscopy or abdominopelvic CT and diagnosis of late recurrence or gastric remnant cancer significantly decreased postrecurrence survival (hazard ratio [HR], 1.72 for endoscopy and HR, 1.48 for abdominopelvic CT).
IN PRACTICE:
“These findings suggest that extended regular follow-up after 5 years post gastrectomy should be implemented clinically and that current practice and value of follow-up protocols in postoperative care of patients with gastric cancer be reconsidered,” the authors concluded.
The authors of an accompanying commentary cautioned that, while the study “successfully establishes groundwork for extending surveillance of gastric cancer in high-risk populations, more work is needed to strategically identify those who would benefit most from extended surveillance.”
SOURCE:
The study, with first author Ju-Hee Lee, MD, PhD, Department of Surgery, Hanyang University College of Medicine, Seoul, South Korea, and accompanying commentary were published online on June 18 in JAMA Surgery.
LIMITATIONS:
Recurrent cancer and gastric remnant cancer could not be distinguished from each other because clinical records were not analyzed. The claims database lacked detailed clinical information on individual patients, including cancer stages, and a separate analysis of tumor markers could not be performed.
DISCLOSURES:
The study was funded by a grant from the Korean Gastric Cancer Association. The study authors and commentary authors reported no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Currently, postgastrectomy cancer surveillance typically lasts 5 years, although some centers now monitor patients beyond this point.
- To investigate the potential benefit of extended surveillance, researchers used Korean National Health Insurance claims data to identify 40,468 patients with gastric cancer who were disease free 5 years after gastrectomy — 14,294 received extended regular follow-up visits and 26,174 did not.
- The extended regular follow-up group was defined as having endoscopy or abdominopelvic CT between 2 months and 2 years before diagnosis of late recurrence or gastric remnant cancer and having two or more examinations between 5.5 and 8.5 years after gastrectomy. Late recurrence was a recurrence diagnosed 5 years after gastrectomy.
- Researchers used Cox proportional hazards regression to evaluate the independent association between follow-up and overall and postrecurrence survival rates.
TAKEAWAY:
- Overall, 5 years postgastrectomy, the incidence of late recurrence or gastric remnant cancer was 7.8% — 4.0% between 5 and 10 years (1610 of 40,468 patients) and 9.4% after 10 years (1528 of 16,287 patients).
- Regular follow-up beyond 5 years was associated with a significant reduction in overall mortality — from 49.4% to 36.9% at 15 years (P < .001). Overall survival after late recurrence or gastric remnant cancer also improved significantly with extended regular follow-up, with the 5-year postrecurrence survival rate increasing from 32.7% to 71.1% (P < .001).
- The combination of endoscopy and abdominopelvic CT provided the highest 5-year postrecurrence survival rate (74.5%), compared with endoscopy alone (54.5%) or CT alone (47.1%).
- A time interval of more than 2 years between a previous endoscopy or abdominopelvic CT and diagnosis of late recurrence or gastric remnant cancer significantly decreased postrecurrence survival (hazard ratio [HR], 1.72 for endoscopy and HR, 1.48 for abdominopelvic CT).
IN PRACTICE:
“These findings suggest that extended regular follow-up after 5 years post gastrectomy should be implemented clinically and that current practice and value of follow-up protocols in postoperative care of patients with gastric cancer be reconsidered,” the authors concluded.
The authors of an accompanying commentary cautioned that, while the study “successfully establishes groundwork for extending surveillance of gastric cancer in high-risk populations, more work is needed to strategically identify those who would benefit most from extended surveillance.”
SOURCE:
The study, with first author Ju-Hee Lee, MD, PhD, Department of Surgery, Hanyang University College of Medicine, Seoul, South Korea, and accompanying commentary were published online on June 18 in JAMA Surgery.
LIMITATIONS:
Recurrent cancer and gastric remnant cancer could not be distinguished from each other because clinical records were not analyzed. The claims database lacked detailed clinical information on individual patients, including cancer stages, and a separate analysis of tumor markers could not be performed.
DISCLOSURES:
The study was funded by a grant from the Korean Gastric Cancer Association. The study authors and commentary authors reported no conflicts of interest.
A version of this article appeared on Medscape.com.
How Aspirin May Lower Risk for Colorectal Cancer
A 2020 meta-analysis, for instance, found that 325 mg of daily aspirin — the typical dose in a single tablet — conferred a 35% reduced risk of developing CRC, and a highly cited The Lancet study from 2010 found that a low dose of daily aspirin reduced the incidence of colon cancer by 24% and colon cancer deaths by 35% over 20 years.
The evidence surrounding aspirin and CRC is so intriguing that more than 70,000 people are currently participating in more than two dozen clinical studies worldwide, putting aspirin through its paces as an intervention in CRC.
But what, exactly, is aspirin doing?
We know that aspirin inhibits cyclooxygenase (COX) enzymes — COX-1 and COX-2, specifically — and that the COX-2 pathway is implicated in the development and progression of CRC, explained Marco Scarpa, MD, PhD, staff surgeon at the University of Padova in Padova, Italy.
“However, the new thing we’ve found is that aspirin may have a direct role in enhancing immunosurveillance,” Dr. Scarpa said in an interview.
In April, Dr. Scarpa’s team published a paper in Cancer describing a mechanism that provides deeper insight into the aspirin-CRC connection.
Dr. Scarpa heads up the IMMUNOREACT study group, a collaboration of dozens of researchers across Italy running studies on immunosurveillance in rectal cancer. In the baseline study, IMMUNOREACT 1, the team created and analyzed a database of records from 238 patients who underwent surgery for CRC at the Azienda Ospedale Università di Padova, Padova, Italy, from 2015 to 2019.
Using the same database, the latest findings from IMMUNOREACT 7 focused on the fate of the 31 patients (13%) who used aspirin regularly.
The researchers found that regular aspirin use did not appear to affect colorectal tumor stage at diagnosis, but tumor grading was significantly lower overall, especially in patients with BRAF mutations. Regular aspirin users were also less likely to have nodal metastases and metastatic lymph nodes, and this effect was more pronounced in patients with proximal (right-sided) colon cancer vs distal (left-sided).
Most notably, IMMUNOREACT 7 revealed that aspirin has beneficial effects on the CRC immune microenvironment.
The team found that aspirin directly boosts the presence of antigens on gastrointestinal epithelial tumor cells, which can direct the body’s immune response to combat the cancer.
At a macro level, the aspirin users in the study were more likely to have high levels of tumor-infiltrating lymphocytes (TILs). Dr. Scarpa’s team had previously shown that high levels of CD8+ and CD3+ TILs were predictive of successful neoadjuvant therapy in rectal cancer.
Cytotoxic CD8+ T cells are central to the anticancer immune response, and in the latest study, a high ratio of CD8+/CD3+ T cells was more common in aspirin users, suggesting a stronger presence of cancer-killing CD8+ cells. Expression of CD8 beta+, an activation marker of CD8+ cells, was also enhanced in aspirin users.
The most significant discovery, according to Dr. Scarpa, was that aspirin users were more likely to show high expression of CD80 on the surface of their rectal epithelial cells.
CD80 is a molecule that allows T cells to identify the tumor cell as foreign and kill it. Although cancer cells can downregulate their CD80 to avoid detection by T cells, the study suggests that aspirin appears to help foil this strategy by boosting the production of CD80 on the surface of the tumor cells.
The researchers confirmed the clinical findings by showing that aspirin increased CD80 gene expression in lab-cultivated CRC cells.
“We didn’t expect the activation through CD80,” said Dr. Scarpa. “This means that aspirin can act on this very first interaction between the epithelial cell and the CD8+ lymphocyte.”
Overall, these new data suggest that aspirin helps activate the immune system, which helps explain its potential chemopreventive effect in CRC.
However, one puzzling result was that aspirin boosted expression of PD-L1 genes in the CRC cells, said Joanna Davies, DPhil, an immunologist who heads up the San Diego Biomedical Research Institute, San Diego, California, and was not involved in the study.
PD-L1 serves as an “off” switch for patrolling T cells, which protects the tumor cell from being recognized.
“If aspirin is inducing PD-L1 on cancer cells, that is a potential problem,” said Dr. Davies. “An ideal therapy might be the combination of aspirin to enhance the CD8 T cells in the tumor and immune checkpoint blockade to block PD-L1.”
David Kerr, CBE, MD, DSc, agreed that high-dose aspirin plus immunotherapy might be “a wee bit more effective.” However, the combination would be blocked by the economics of drug development: “Will anybody ever do a trial of 10,000 patients to prove that? Not on your nelly,” said Dr. Kerr, professor of cancer medicine at the University of Oxford, Oxford, England.
Despite the small patient numbers in the study, Dr. Kerr felt encouraged by the IMMUNOREACT analysis. “It’s a plausible piece of science and some quite promising work on the tumor immune microenvironment and the effects of aspirin on it,” Dr. Kerr said in a recent commentary for this news organization.
Dr. Scarpa and Dr. Davies had no conflicts of interest to declare.
A version of this article appeared on Medscape.com .
A 2020 meta-analysis, for instance, found that 325 mg of daily aspirin — the typical dose in a single tablet — conferred a 35% reduced risk of developing CRC, and a highly cited The Lancet study from 2010 found that a low dose of daily aspirin reduced the incidence of colon cancer by 24% and colon cancer deaths by 35% over 20 years.
The evidence surrounding aspirin and CRC is so intriguing that more than 70,000 people are currently participating in more than two dozen clinical studies worldwide, putting aspirin through its paces as an intervention in CRC.
But what, exactly, is aspirin doing?
We know that aspirin inhibits cyclooxygenase (COX) enzymes — COX-1 and COX-2, specifically — and that the COX-2 pathway is implicated in the development and progression of CRC, explained Marco Scarpa, MD, PhD, staff surgeon at the University of Padova in Padova, Italy.
“However, the new thing we’ve found is that aspirin may have a direct role in enhancing immunosurveillance,” Dr. Scarpa said in an interview.
In April, Dr. Scarpa’s team published a paper in Cancer describing a mechanism that provides deeper insight into the aspirin-CRC connection.
Dr. Scarpa heads up the IMMUNOREACT study group, a collaboration of dozens of researchers across Italy running studies on immunosurveillance in rectal cancer. In the baseline study, IMMUNOREACT 1, the team created and analyzed a database of records from 238 patients who underwent surgery for CRC at the Azienda Ospedale Università di Padova, Padova, Italy, from 2015 to 2019.
Using the same database, the latest findings from IMMUNOREACT 7 focused on the fate of the 31 patients (13%) who used aspirin regularly.
The researchers found that regular aspirin use did not appear to affect colorectal tumor stage at diagnosis, but tumor grading was significantly lower overall, especially in patients with BRAF mutations. Regular aspirin users were also less likely to have nodal metastases and metastatic lymph nodes, and this effect was more pronounced in patients with proximal (right-sided) colon cancer vs distal (left-sided).
Most notably, IMMUNOREACT 7 revealed that aspirin has beneficial effects on the CRC immune microenvironment.
The team found that aspirin directly boosts the presence of antigens on gastrointestinal epithelial tumor cells, which can direct the body’s immune response to combat the cancer.
At a macro level, the aspirin users in the study were more likely to have high levels of tumor-infiltrating lymphocytes (TILs). Dr. Scarpa’s team had previously shown that high levels of CD8+ and CD3+ TILs were predictive of successful neoadjuvant therapy in rectal cancer.
Cytotoxic CD8+ T cells are central to the anticancer immune response, and in the latest study, a high ratio of CD8+/CD3+ T cells was more common in aspirin users, suggesting a stronger presence of cancer-killing CD8+ cells. Expression of CD8 beta+, an activation marker of CD8+ cells, was also enhanced in aspirin users.
The most significant discovery, according to Dr. Scarpa, was that aspirin users were more likely to show high expression of CD80 on the surface of their rectal epithelial cells.
CD80 is a molecule that allows T cells to identify the tumor cell as foreign and kill it. Although cancer cells can downregulate their CD80 to avoid detection by T cells, the study suggests that aspirin appears to help foil this strategy by boosting the production of CD80 on the surface of the tumor cells.
The researchers confirmed the clinical findings by showing that aspirin increased CD80 gene expression in lab-cultivated CRC cells.
“We didn’t expect the activation through CD80,” said Dr. Scarpa. “This means that aspirin can act on this very first interaction between the epithelial cell and the CD8+ lymphocyte.”
Overall, these new data suggest that aspirin helps activate the immune system, which helps explain its potential chemopreventive effect in CRC.
However, one puzzling result was that aspirin boosted expression of PD-L1 genes in the CRC cells, said Joanna Davies, DPhil, an immunologist who heads up the San Diego Biomedical Research Institute, San Diego, California, and was not involved in the study.
PD-L1 serves as an “off” switch for patrolling T cells, which protects the tumor cell from being recognized.
“If aspirin is inducing PD-L1 on cancer cells, that is a potential problem,” said Dr. Davies. “An ideal therapy might be the combination of aspirin to enhance the CD8 T cells in the tumor and immune checkpoint blockade to block PD-L1.”
David Kerr, CBE, MD, DSc, agreed that high-dose aspirin plus immunotherapy might be “a wee bit more effective.” However, the combination would be blocked by the economics of drug development: “Will anybody ever do a trial of 10,000 patients to prove that? Not on your nelly,” said Dr. Kerr, professor of cancer medicine at the University of Oxford, Oxford, England.
Despite the small patient numbers in the study, Dr. Kerr felt encouraged by the IMMUNOREACT analysis. “It’s a plausible piece of science and some quite promising work on the tumor immune microenvironment and the effects of aspirin on it,” Dr. Kerr said in a recent commentary for this news organization.
Dr. Scarpa and Dr. Davies had no conflicts of interest to declare.
A version of this article appeared on Medscape.com .
A 2020 meta-analysis, for instance, found that 325 mg of daily aspirin — the typical dose in a single tablet — conferred a 35% reduced risk of developing CRC, and a highly cited The Lancet study from 2010 found that a low dose of daily aspirin reduced the incidence of colon cancer by 24% and colon cancer deaths by 35% over 20 years.
The evidence surrounding aspirin and CRC is so intriguing that more than 70,000 people are currently participating in more than two dozen clinical studies worldwide, putting aspirin through its paces as an intervention in CRC.
But what, exactly, is aspirin doing?
We know that aspirin inhibits cyclooxygenase (COX) enzymes — COX-1 and COX-2, specifically — and that the COX-2 pathway is implicated in the development and progression of CRC, explained Marco Scarpa, MD, PhD, staff surgeon at the University of Padova in Padova, Italy.
“However, the new thing we’ve found is that aspirin may have a direct role in enhancing immunosurveillance,” Dr. Scarpa said in an interview.
In April, Dr. Scarpa’s team published a paper in Cancer describing a mechanism that provides deeper insight into the aspirin-CRC connection.
Dr. Scarpa heads up the IMMUNOREACT study group, a collaboration of dozens of researchers across Italy running studies on immunosurveillance in rectal cancer. In the baseline study, IMMUNOREACT 1, the team created and analyzed a database of records from 238 patients who underwent surgery for CRC at the Azienda Ospedale Università di Padova, Padova, Italy, from 2015 to 2019.
Using the same database, the latest findings from IMMUNOREACT 7 focused on the fate of the 31 patients (13%) who used aspirin regularly.
The researchers found that regular aspirin use did not appear to affect colorectal tumor stage at diagnosis, but tumor grading was significantly lower overall, especially in patients with BRAF mutations. Regular aspirin users were also less likely to have nodal metastases and metastatic lymph nodes, and this effect was more pronounced in patients with proximal (right-sided) colon cancer vs distal (left-sided).
Most notably, IMMUNOREACT 7 revealed that aspirin has beneficial effects on the CRC immune microenvironment.
The team found that aspirin directly boosts the presence of antigens on gastrointestinal epithelial tumor cells, which can direct the body’s immune response to combat the cancer.
At a macro level, the aspirin users in the study were more likely to have high levels of tumor-infiltrating lymphocytes (TILs). Dr. Scarpa’s team had previously shown that high levels of CD8+ and CD3+ TILs were predictive of successful neoadjuvant therapy in rectal cancer.
Cytotoxic CD8+ T cells are central to the anticancer immune response, and in the latest study, a high ratio of CD8+/CD3+ T cells was more common in aspirin users, suggesting a stronger presence of cancer-killing CD8+ cells. Expression of CD8 beta+, an activation marker of CD8+ cells, was also enhanced in aspirin users.
The most significant discovery, according to Dr. Scarpa, was that aspirin users were more likely to show high expression of CD80 on the surface of their rectal epithelial cells.
CD80 is a molecule that allows T cells to identify the tumor cell as foreign and kill it. Although cancer cells can downregulate their CD80 to avoid detection by T cells, the study suggests that aspirin appears to help foil this strategy by boosting the production of CD80 on the surface of the tumor cells.
The researchers confirmed the clinical findings by showing that aspirin increased CD80 gene expression in lab-cultivated CRC cells.
“We didn’t expect the activation through CD80,” said Dr. Scarpa. “This means that aspirin can act on this very first interaction between the epithelial cell and the CD8+ lymphocyte.”
Overall, these new data suggest that aspirin helps activate the immune system, which helps explain its potential chemopreventive effect in CRC.
However, one puzzling result was that aspirin boosted expression of PD-L1 genes in the CRC cells, said Joanna Davies, DPhil, an immunologist who heads up the San Diego Biomedical Research Institute, San Diego, California, and was not involved in the study.
PD-L1 serves as an “off” switch for patrolling T cells, which protects the tumor cell from being recognized.
“If aspirin is inducing PD-L1 on cancer cells, that is a potential problem,” said Dr. Davies. “An ideal therapy might be the combination of aspirin to enhance the CD8 T cells in the tumor and immune checkpoint blockade to block PD-L1.”
David Kerr, CBE, MD, DSc, agreed that high-dose aspirin plus immunotherapy might be “a wee bit more effective.” However, the combination would be blocked by the economics of drug development: “Will anybody ever do a trial of 10,000 patients to prove that? Not on your nelly,” said Dr. Kerr, professor of cancer medicine at the University of Oxford, Oxford, England.
Despite the small patient numbers in the study, Dr. Kerr felt encouraged by the IMMUNOREACT analysis. “It’s a plausible piece of science and some quite promising work on the tumor immune microenvironment and the effects of aspirin on it,” Dr. Kerr said in a recent commentary for this news organization.
Dr. Scarpa and Dr. Davies had no conflicts of interest to declare.
A version of this article appeared on Medscape.com .
The Appendix: Is It ’Useless,’ or a Safe House and Immune Training Ground?
When doctors and patients consider the appendix, it’s often with urgency. In cases of appendicitis, the clock could be ticking down to a life-threatening burst. Thus, despite recent research suggesting antibiotics could be an alternative therapy, appendectomy remains standard for uncomplicated appendicitis.
But what if removing the appendix could raise the risk for gastrointestinal (GI) diseases like irritable bowel syndrome and colorectal cancer? That’s what some emerging science suggests. And though the research is early and mixed, it’s enough to give some health professionals pause.
“If there’s no reason to remove the appendix, then it’s better to have one,” said Heather Smith, PhD, a comparative anatomist at Midwestern University, Glendale, Arizona. Preemptive removal is not supported by the evidence, she said.
To be fair, we’ve come a long way since 1928, when American physician Miles Breuer, MD, suggested that people with infected appendixes should be left to perish, so as to remove their inferior DNA from the gene pool (he called such people “uncivilized” and “candidates for extinction”). Charles Darwin, while less radical, believed the appendix was at best useless — a mere vestige of our ancestors switching diets from leaves to fruits.
What we know now is that the appendix isn’t just a troublesome piece of worthless flesh. Instead, it may act as a safe house for friendly gut bacteria and a training camp for the immune system. It also appears to play a role in several medical conditions, from ulcerative colitis and colorectal cancer to Parkinson’s disease and lupus. The roughly 300,000 Americans who undergo appendectomy each year should be made aware of this, some experts say. But the frustrating truth is, scientists are still trying to figure out in which cases having an appendix is protective and in which we may be better off without it.
A ‘Worm’ as Intestinal Protection
The appendix is a blind pouch (meaning its ending is closed off) that extends from the large intestine. Not all mammals have one; it’s been found in several species of primates and rodents, as well as in rabbits, wombats, and Florida manatees, among others (dogs and cats don’t have it). While a human appendix “looks like a little worm,” Dr. Smith said, these anatomical structures come in various sizes and shapes. Some are thick, as in a beaver, while others are long and spiraling, like a rabbit’s.
Comparative anatomy studies reveal that the appendix has evolved independently at least 29 times throughout mammalian evolution. This suggests that “it has some kind of an adaptive function,” Dr. Smith said. When French scientists analyzed data from 258 species of mammals, they discovered that those that possess an appendix live longer than those without one. A possible explanation, the researchers wrote, may lie with the appendix’s role in preventing diarrhea.
Their 2023 study supported this hypothesis. Based on veterinary records of 45 different species of primates housed in a French zoo, the scientists established that primates with appendixes are far less likely to suffer severe diarrhea than those that don’t possess this organ. The appendix, it appears, might be our tiny weapon against bowel troubles.
For immunologist William Parker, PhD, a visiting scholar at the University of North Carolina at Chapel Hill, these data are “about as good as we could hope for” in support of the idea that the appendix might protect mammals from GI problems. An experiment on humans would be unethical, Dr. Parker said. But observational studies offer clues.
One study showed that compared with people with an intact appendix, young adults with a history of appendectomy have more than double the risk of developing a serious infection with non-typhoidal Salmonella of the kind that would require hospitalization.
A ‘Safe House’ for Bacteria
Such studies add weight to a theory that Dr. Parker and his colleagues developed back in 2007: That the appendix acts as a “safe house” for beneficial gut bacteria.
Think of the colon as a wide pipe, Dr. Parker said, that may become contaminated with a pathogen such as Salmonella. Diarrhea follows, and the pipe gets repeatedly flushed, wiping everything clean, including your friendly gut microbiome. Luckily, “you’ve got this little offshoot of that pipe,” where the flow can’t really get in “because it’s so constricted,” Dr. Parker said. The friendly gut microbes can survive inside the appendix and repopulate the colon once diarrhea is over. Dr. Parker and his colleagues found that the human appendix contains a thick layer of beneficial bacteria. “They were right where we predicted they would be,” he said.
This safe house hypothesis could explain why the gut microbiome may be different in people who no longer have an appendix. In one small study, people who’d had an appendectomy had a less diverse microbiome, with a lower abundance of beneficial strains such as Butyricicoccus and Barnesiella, than did those with intact appendixes.
The appendix likely has a second function, too, Dr. Smith said: It may serve as a training camp for the immune system. “When there is an invading pathogen in the gut, it helps the GI system to mount the immune response,” she said. The human appendix is rich in special cells known as M cells. These act as scouts, detecting and capturing invasive bacteria and viruses and presenting them to the body’s defense team, such as the T lymphocytes.
If the appendix shelters beneficial bacteria and boosts immune response, that may explain its links to various diseases. According to an epidemiological study from Taiwan,patients who underwent an appendectomy have a 46% higher risk of developing irritable bowel syndrome (IBS) — a disease associated with a low abundance of Butyricicoccus bacteria. This is why, the study authors wrote, doctors should pay careful attention to people who’ve had their appendixes removed, monitoring them for potential symptoms of IBS.
The same database helped uncover other connections between appendectomy and disease. For one, there was type 2 diabetes: Within 3 years of the surgery, patients under 30 had double the risk of developing this disorder. Then there was lupus: While those who underwent appendectomy generally had higher risk for this autoimmune disease, women were particularly affected.
The Contentious Connections
The most heated scientific discussion surrounds the links between the appendix and conditions such as Parkinson’s disease, ulcerative colitis, and colorectal cancer. A small 2019 study showed, for example, that appendectomy may improve symptoms of certain forms of ulcerative colitis that don’t respond to standard medical treatments. A third of patients improved after their appendix was removed, and 17% fully recovered.
Why? According to Dr. Parker, appendectomy may work for ulcerative colitis because it’s “a way of suppressing the immune system, especially in the lower intestinal areas.” A 2023 meta-analysis found that people who’d had their appendix removed before being diagnosed with ulcerative colitis were less likely to need their colon removed later on.
Such a procedure may have a serious side effect, however: Colorectal cancer. French scientists discovered that removing the appendix may reduce the numbers of certain immune cells called CD3+ and CD8+ T cells, causing a weakened immune surveillance. As a result, tumor cells might escape detection.
Yet the links between appendix removal and cancer are far from clear. A recent meta-analysis found that while people with appendectomies generally had a higher risk for colorectal cancer, for Europeans, these effects were insignificant. In fact, removal of the appendix actually protected European women from this particular form of cancer. For Parker, such mixed results may stem from the fact that treatments and populations vary widely. The issue “may depend on complex social and medical factors,” Dr. Parker said.
Things also appear complicated with Parkinson’s disease — another condition linked to the appendix. A large epidemiological study showed that appendectomy is associated with a lower risk for Parkinson’s disease and a delayed age of Parkinson’s onset. It also found that a normal appendix contains α-synuclein, a protein that may accumulate in the brain and contribute to the development of Parkinson’s. “Although α-synuclein is toxic when in the brain, it appears to be quite normal when present in the appendix,” said Luis Vitetta, PhD, MD, a clinical epidemiologist at the University of Sydney, Camperdown, Australia. Yet, not all studies find that removing the appendix lowers the risk for Parkinson’s. In fact, some show the opposite results.
How Should Doctors View the Appendix?
Even with these mysteries and contradictions, Dr. Vitetta said, a healthy appendix in a healthy body appears to be protective. This is why, he said, when someone is diagnosed with appendicitis, careful assessment is essential before surgery is performed.
“Perhaps an antibiotic can actually help fix it,” he said. A 2020 study published in The New England Journal of Medicine showed that antibiotics may indeed be a good alternative to surgery for the treatment of appendicitis. “We don’t want necessarily to remove an appendix that could be beneficial,” Dr. Smith said.
The many links between the appendix and various diseases mean that doctors should be more vigilant when treating patients who’ve had this organ removed, Dr. Parker said. “When a patient loses an appendix, depending on their environment, there may be effects on infection and cancer. So they might need more regular checkups,” he said. This could include monitoring for IBS and colorectal cancer.
What’s more, Dr. Parker believes that research on the appendix puts even more emphasis on the need to protect the gut microbiome — such as taking probiotics with antibiotics. And while we are still a long way from understanding how exactly this worm-like structure affects various diseases, one thing appears quite certain: The appendix is not useless. “If Darwin had the information that we have, he would not have drawn these conclusions,” Dr. Parker said.
A version of this article first appeared on Medscape.com.
When doctors and patients consider the appendix, it’s often with urgency. In cases of appendicitis, the clock could be ticking down to a life-threatening burst. Thus, despite recent research suggesting antibiotics could be an alternative therapy, appendectomy remains standard for uncomplicated appendicitis.
But what if removing the appendix could raise the risk for gastrointestinal (GI) diseases like irritable bowel syndrome and colorectal cancer? That’s what some emerging science suggests. And though the research is early and mixed, it’s enough to give some health professionals pause.
“If there’s no reason to remove the appendix, then it’s better to have one,” said Heather Smith, PhD, a comparative anatomist at Midwestern University, Glendale, Arizona. Preemptive removal is not supported by the evidence, she said.
To be fair, we’ve come a long way since 1928, when American physician Miles Breuer, MD, suggested that people with infected appendixes should be left to perish, so as to remove their inferior DNA from the gene pool (he called such people “uncivilized” and “candidates for extinction”). Charles Darwin, while less radical, believed the appendix was at best useless — a mere vestige of our ancestors switching diets from leaves to fruits.
What we know now is that the appendix isn’t just a troublesome piece of worthless flesh. Instead, it may act as a safe house for friendly gut bacteria and a training camp for the immune system. It also appears to play a role in several medical conditions, from ulcerative colitis and colorectal cancer to Parkinson’s disease and lupus. The roughly 300,000 Americans who undergo appendectomy each year should be made aware of this, some experts say. But the frustrating truth is, scientists are still trying to figure out in which cases having an appendix is protective and in which we may be better off without it.
A ‘Worm’ as Intestinal Protection
The appendix is a blind pouch (meaning its ending is closed off) that extends from the large intestine. Not all mammals have one; it’s been found in several species of primates and rodents, as well as in rabbits, wombats, and Florida manatees, among others (dogs and cats don’t have it). While a human appendix “looks like a little worm,” Dr. Smith said, these anatomical structures come in various sizes and shapes. Some are thick, as in a beaver, while others are long and spiraling, like a rabbit’s.
Comparative anatomy studies reveal that the appendix has evolved independently at least 29 times throughout mammalian evolution. This suggests that “it has some kind of an adaptive function,” Dr. Smith said. When French scientists analyzed data from 258 species of mammals, they discovered that those that possess an appendix live longer than those without one. A possible explanation, the researchers wrote, may lie with the appendix’s role in preventing diarrhea.
Their 2023 study supported this hypothesis. Based on veterinary records of 45 different species of primates housed in a French zoo, the scientists established that primates with appendixes are far less likely to suffer severe diarrhea than those that don’t possess this organ. The appendix, it appears, might be our tiny weapon against bowel troubles.
For immunologist William Parker, PhD, a visiting scholar at the University of North Carolina at Chapel Hill, these data are “about as good as we could hope for” in support of the idea that the appendix might protect mammals from GI problems. An experiment on humans would be unethical, Dr. Parker said. But observational studies offer clues.
One study showed that compared with people with an intact appendix, young adults with a history of appendectomy have more than double the risk of developing a serious infection with non-typhoidal Salmonella of the kind that would require hospitalization.
A ‘Safe House’ for Bacteria
Such studies add weight to a theory that Dr. Parker and his colleagues developed back in 2007: That the appendix acts as a “safe house” for beneficial gut bacteria.
Think of the colon as a wide pipe, Dr. Parker said, that may become contaminated with a pathogen such as Salmonella. Diarrhea follows, and the pipe gets repeatedly flushed, wiping everything clean, including your friendly gut microbiome. Luckily, “you’ve got this little offshoot of that pipe,” where the flow can’t really get in “because it’s so constricted,” Dr. Parker said. The friendly gut microbes can survive inside the appendix and repopulate the colon once diarrhea is over. Dr. Parker and his colleagues found that the human appendix contains a thick layer of beneficial bacteria. “They were right where we predicted they would be,” he said.
This safe house hypothesis could explain why the gut microbiome may be different in people who no longer have an appendix. In one small study, people who’d had an appendectomy had a less diverse microbiome, with a lower abundance of beneficial strains such as Butyricicoccus and Barnesiella, than did those with intact appendixes.
The appendix likely has a second function, too, Dr. Smith said: It may serve as a training camp for the immune system. “When there is an invading pathogen in the gut, it helps the GI system to mount the immune response,” she said. The human appendix is rich in special cells known as M cells. These act as scouts, detecting and capturing invasive bacteria and viruses and presenting them to the body’s defense team, such as the T lymphocytes.
If the appendix shelters beneficial bacteria and boosts immune response, that may explain its links to various diseases. According to an epidemiological study from Taiwan,patients who underwent an appendectomy have a 46% higher risk of developing irritable bowel syndrome (IBS) — a disease associated with a low abundance of Butyricicoccus bacteria. This is why, the study authors wrote, doctors should pay careful attention to people who’ve had their appendixes removed, monitoring them for potential symptoms of IBS.
The same database helped uncover other connections between appendectomy and disease. For one, there was type 2 diabetes: Within 3 years of the surgery, patients under 30 had double the risk of developing this disorder. Then there was lupus: While those who underwent appendectomy generally had higher risk for this autoimmune disease, women were particularly affected.
The Contentious Connections
The most heated scientific discussion surrounds the links between the appendix and conditions such as Parkinson’s disease, ulcerative colitis, and colorectal cancer. A small 2019 study showed, for example, that appendectomy may improve symptoms of certain forms of ulcerative colitis that don’t respond to standard medical treatments. A third of patients improved after their appendix was removed, and 17% fully recovered.
Why? According to Dr. Parker, appendectomy may work for ulcerative colitis because it’s “a way of suppressing the immune system, especially in the lower intestinal areas.” A 2023 meta-analysis found that people who’d had their appendix removed before being diagnosed with ulcerative colitis were less likely to need their colon removed later on.
Such a procedure may have a serious side effect, however: Colorectal cancer. French scientists discovered that removing the appendix may reduce the numbers of certain immune cells called CD3+ and CD8+ T cells, causing a weakened immune surveillance. As a result, tumor cells might escape detection.
Yet the links between appendix removal and cancer are far from clear. A recent meta-analysis found that while people with appendectomies generally had a higher risk for colorectal cancer, for Europeans, these effects were insignificant. In fact, removal of the appendix actually protected European women from this particular form of cancer. For Parker, such mixed results may stem from the fact that treatments and populations vary widely. The issue “may depend on complex social and medical factors,” Dr. Parker said.
Things also appear complicated with Parkinson’s disease — another condition linked to the appendix. A large epidemiological study showed that appendectomy is associated with a lower risk for Parkinson’s disease and a delayed age of Parkinson’s onset. It also found that a normal appendix contains α-synuclein, a protein that may accumulate in the brain and contribute to the development of Parkinson’s. “Although α-synuclein is toxic when in the brain, it appears to be quite normal when present in the appendix,” said Luis Vitetta, PhD, MD, a clinical epidemiologist at the University of Sydney, Camperdown, Australia. Yet, not all studies find that removing the appendix lowers the risk for Parkinson’s. In fact, some show the opposite results.
How Should Doctors View the Appendix?
Even with these mysteries and contradictions, Dr. Vitetta said, a healthy appendix in a healthy body appears to be protective. This is why, he said, when someone is diagnosed with appendicitis, careful assessment is essential before surgery is performed.
“Perhaps an antibiotic can actually help fix it,” he said. A 2020 study published in The New England Journal of Medicine showed that antibiotics may indeed be a good alternative to surgery for the treatment of appendicitis. “We don’t want necessarily to remove an appendix that could be beneficial,” Dr. Smith said.
The many links between the appendix and various diseases mean that doctors should be more vigilant when treating patients who’ve had this organ removed, Dr. Parker said. “When a patient loses an appendix, depending on their environment, there may be effects on infection and cancer. So they might need more regular checkups,” he said. This could include monitoring for IBS and colorectal cancer.
What’s more, Dr. Parker believes that research on the appendix puts even more emphasis on the need to protect the gut microbiome — such as taking probiotics with antibiotics. And while we are still a long way from understanding how exactly this worm-like structure affects various diseases, one thing appears quite certain: The appendix is not useless. “If Darwin had the information that we have, he would not have drawn these conclusions,” Dr. Parker said.
A version of this article first appeared on Medscape.com.
When doctors and patients consider the appendix, it’s often with urgency. In cases of appendicitis, the clock could be ticking down to a life-threatening burst. Thus, despite recent research suggesting antibiotics could be an alternative therapy, appendectomy remains standard for uncomplicated appendicitis.
But what if removing the appendix could raise the risk for gastrointestinal (GI) diseases like irritable bowel syndrome and colorectal cancer? That’s what some emerging science suggests. And though the research is early and mixed, it’s enough to give some health professionals pause.
“If there’s no reason to remove the appendix, then it’s better to have one,” said Heather Smith, PhD, a comparative anatomist at Midwestern University, Glendale, Arizona. Preemptive removal is not supported by the evidence, she said.
To be fair, we’ve come a long way since 1928, when American physician Miles Breuer, MD, suggested that people with infected appendixes should be left to perish, so as to remove their inferior DNA from the gene pool (he called such people “uncivilized” and “candidates for extinction”). Charles Darwin, while less radical, believed the appendix was at best useless — a mere vestige of our ancestors switching diets from leaves to fruits.
What we know now is that the appendix isn’t just a troublesome piece of worthless flesh. Instead, it may act as a safe house for friendly gut bacteria and a training camp for the immune system. It also appears to play a role in several medical conditions, from ulcerative colitis and colorectal cancer to Parkinson’s disease and lupus. The roughly 300,000 Americans who undergo appendectomy each year should be made aware of this, some experts say. But the frustrating truth is, scientists are still trying to figure out in which cases having an appendix is protective and in which we may be better off without it.
A ‘Worm’ as Intestinal Protection
The appendix is a blind pouch (meaning its ending is closed off) that extends from the large intestine. Not all mammals have one; it’s been found in several species of primates and rodents, as well as in rabbits, wombats, and Florida manatees, among others (dogs and cats don’t have it). While a human appendix “looks like a little worm,” Dr. Smith said, these anatomical structures come in various sizes and shapes. Some are thick, as in a beaver, while others are long and spiraling, like a rabbit’s.
Comparative anatomy studies reveal that the appendix has evolved independently at least 29 times throughout mammalian evolution. This suggests that “it has some kind of an adaptive function,” Dr. Smith said. When French scientists analyzed data from 258 species of mammals, they discovered that those that possess an appendix live longer than those without one. A possible explanation, the researchers wrote, may lie with the appendix’s role in preventing diarrhea.
Their 2023 study supported this hypothesis. Based on veterinary records of 45 different species of primates housed in a French zoo, the scientists established that primates with appendixes are far less likely to suffer severe diarrhea than those that don’t possess this organ. The appendix, it appears, might be our tiny weapon against bowel troubles.
For immunologist William Parker, PhD, a visiting scholar at the University of North Carolina at Chapel Hill, these data are “about as good as we could hope for” in support of the idea that the appendix might protect mammals from GI problems. An experiment on humans would be unethical, Dr. Parker said. But observational studies offer clues.
One study showed that compared with people with an intact appendix, young adults with a history of appendectomy have more than double the risk of developing a serious infection with non-typhoidal Salmonella of the kind that would require hospitalization.
A ‘Safe House’ for Bacteria
Such studies add weight to a theory that Dr. Parker and his colleagues developed back in 2007: That the appendix acts as a “safe house” for beneficial gut bacteria.
Think of the colon as a wide pipe, Dr. Parker said, that may become contaminated with a pathogen such as Salmonella. Diarrhea follows, and the pipe gets repeatedly flushed, wiping everything clean, including your friendly gut microbiome. Luckily, “you’ve got this little offshoot of that pipe,” where the flow can’t really get in “because it’s so constricted,” Dr. Parker said. The friendly gut microbes can survive inside the appendix and repopulate the colon once diarrhea is over. Dr. Parker and his colleagues found that the human appendix contains a thick layer of beneficial bacteria. “They were right where we predicted they would be,” he said.
This safe house hypothesis could explain why the gut microbiome may be different in people who no longer have an appendix. In one small study, people who’d had an appendectomy had a less diverse microbiome, with a lower abundance of beneficial strains such as Butyricicoccus and Barnesiella, than did those with intact appendixes.
The appendix likely has a second function, too, Dr. Smith said: It may serve as a training camp for the immune system. “When there is an invading pathogen in the gut, it helps the GI system to mount the immune response,” she said. The human appendix is rich in special cells known as M cells. These act as scouts, detecting and capturing invasive bacteria and viruses and presenting them to the body’s defense team, such as the T lymphocytes.
If the appendix shelters beneficial bacteria and boosts immune response, that may explain its links to various diseases. According to an epidemiological study from Taiwan,patients who underwent an appendectomy have a 46% higher risk of developing irritable bowel syndrome (IBS) — a disease associated with a low abundance of Butyricicoccus bacteria. This is why, the study authors wrote, doctors should pay careful attention to people who’ve had their appendixes removed, monitoring them for potential symptoms of IBS.
The same database helped uncover other connections between appendectomy and disease. For one, there was type 2 diabetes: Within 3 years of the surgery, patients under 30 had double the risk of developing this disorder. Then there was lupus: While those who underwent appendectomy generally had higher risk for this autoimmune disease, women were particularly affected.
The Contentious Connections
The most heated scientific discussion surrounds the links between the appendix and conditions such as Parkinson’s disease, ulcerative colitis, and colorectal cancer. A small 2019 study showed, for example, that appendectomy may improve symptoms of certain forms of ulcerative colitis that don’t respond to standard medical treatments. A third of patients improved after their appendix was removed, and 17% fully recovered.
Why? According to Dr. Parker, appendectomy may work for ulcerative colitis because it’s “a way of suppressing the immune system, especially in the lower intestinal areas.” A 2023 meta-analysis found that people who’d had their appendix removed before being diagnosed with ulcerative colitis were less likely to need their colon removed later on.
Such a procedure may have a serious side effect, however: Colorectal cancer. French scientists discovered that removing the appendix may reduce the numbers of certain immune cells called CD3+ and CD8+ T cells, causing a weakened immune surveillance. As a result, tumor cells might escape detection.
Yet the links between appendix removal and cancer are far from clear. A recent meta-analysis found that while people with appendectomies generally had a higher risk for colorectal cancer, for Europeans, these effects were insignificant. In fact, removal of the appendix actually protected European women from this particular form of cancer. For Parker, such mixed results may stem from the fact that treatments and populations vary widely. The issue “may depend on complex social and medical factors,” Dr. Parker said.
Things also appear complicated with Parkinson’s disease — another condition linked to the appendix. A large epidemiological study showed that appendectomy is associated with a lower risk for Parkinson’s disease and a delayed age of Parkinson’s onset. It also found that a normal appendix contains α-synuclein, a protein that may accumulate in the brain and contribute to the development of Parkinson’s. “Although α-synuclein is toxic when in the brain, it appears to be quite normal when present in the appendix,” said Luis Vitetta, PhD, MD, a clinical epidemiologist at the University of Sydney, Camperdown, Australia. Yet, not all studies find that removing the appendix lowers the risk for Parkinson’s. In fact, some show the opposite results.
How Should Doctors View the Appendix?
Even with these mysteries and contradictions, Dr. Vitetta said, a healthy appendix in a healthy body appears to be protective. This is why, he said, when someone is diagnosed with appendicitis, careful assessment is essential before surgery is performed.
“Perhaps an antibiotic can actually help fix it,” he said. A 2020 study published in The New England Journal of Medicine showed that antibiotics may indeed be a good alternative to surgery for the treatment of appendicitis. “We don’t want necessarily to remove an appendix that could be beneficial,” Dr. Smith said.
The many links between the appendix and various diseases mean that doctors should be more vigilant when treating patients who’ve had this organ removed, Dr. Parker said. “When a patient loses an appendix, depending on their environment, there may be effects on infection and cancer. So they might need more regular checkups,” he said. This could include monitoring for IBS and colorectal cancer.
What’s more, Dr. Parker believes that research on the appendix puts even more emphasis on the need to protect the gut microbiome — such as taking probiotics with antibiotics. And while we are still a long way from understanding how exactly this worm-like structure affects various diseases, one thing appears quite certain: The appendix is not useless. “If Darwin had the information that we have, he would not have drawn these conclusions,” Dr. Parker said.
A version of this article first appeared on Medscape.com.
Young People’s Gut Bacteria May Drive Colorectal Cancer Risk
CHICAGO — Genetics and diet have been among the top theories for what may be fueling the troubling rise of colorectal cancer in young adults. Now,
The findings were presented at the annual meeting of the American Society for Clinical Oncology (ASCO) by researchers from Ohio State University. For the analysis, they analyzed genetic data on tumors.
The researchers found signs that a high-fat, low-fiber diet may increase inflammation in the gut that prevents it from naturally suppressing tumors. The cells of young people with colorectal cancer also appeared to have aged more quickly — by 15 years on average — than a person’s actual age. That’s unusual, because older people with colorectal cancer don’t have the same boost in cellular aging.
The rate of colorectal cancer among young people has been rising at an alarming rate, according to a 2023 report from the American Cancer Society. In 2019, one in five colorectal cancer cases were among people younger than 55. That’s up from 1 in 10 in 1995, which means the rate has doubled in less than 30 years.
Need Colon Cancer Screening?
Who needs a colorectal cancer screening? Ask colorectal cancer specialist Nancy Kemeny, MD.
A 2017 analysis estimated that a person’s risk of colorectal cancer increased 12% by eating 3.5 ounces of red or processed meat daily, which is the equivalent of the size of a deck of playing cards. The same study also linked colorectal cancer risk to alcohol intake, citing its ethanol content. Eating a diet high in fiber can reduce a person’s risk.
This latest study aligned with previous findings that link bacteria called Fusobacterium to colorectal cancer. It’s not unusual for Fusobacterium to be present in a person’s mouth, but it is more likely to be found in the intestines of colorectal cancer patients, compared with those of healthy people. One study even found that people with colorectal cancer were five times more likely to have Fusobacterium in their stool, compared with healthy people.
Colorectal cancer is more common among men than women, “likely reflecting differences in risk factor prevalence, such as excess body weight and processed meat consumption,” the authors of the 2023 American Cancer Society report explained.
People younger than 45 should alert their medical provider if they have constipation, rectal bleeding, or sudden changes in bowel movements, which can be symptoms of colorectal cancer. Screening for colorectal cancer should begin for most people at age 45.
A version of this article appeared on WebMD.com.
CHICAGO — Genetics and diet have been among the top theories for what may be fueling the troubling rise of colorectal cancer in young adults. Now,
The findings were presented at the annual meeting of the American Society for Clinical Oncology (ASCO) by researchers from Ohio State University. For the analysis, they analyzed genetic data on tumors.
The researchers found signs that a high-fat, low-fiber diet may increase inflammation in the gut that prevents it from naturally suppressing tumors. The cells of young people with colorectal cancer also appeared to have aged more quickly — by 15 years on average — than a person’s actual age. That’s unusual, because older people with colorectal cancer don’t have the same boost in cellular aging.
The rate of colorectal cancer among young people has been rising at an alarming rate, according to a 2023 report from the American Cancer Society. In 2019, one in five colorectal cancer cases were among people younger than 55. That’s up from 1 in 10 in 1995, which means the rate has doubled in less than 30 years.
Need Colon Cancer Screening?
Who needs a colorectal cancer screening? Ask colorectal cancer specialist Nancy Kemeny, MD.
A 2017 analysis estimated that a person’s risk of colorectal cancer increased 12% by eating 3.5 ounces of red or processed meat daily, which is the equivalent of the size of a deck of playing cards. The same study also linked colorectal cancer risk to alcohol intake, citing its ethanol content. Eating a diet high in fiber can reduce a person’s risk.
This latest study aligned with previous findings that link bacteria called Fusobacterium to colorectal cancer. It’s not unusual for Fusobacterium to be present in a person’s mouth, but it is more likely to be found in the intestines of colorectal cancer patients, compared with those of healthy people. One study even found that people with colorectal cancer were five times more likely to have Fusobacterium in their stool, compared with healthy people.
Colorectal cancer is more common among men than women, “likely reflecting differences in risk factor prevalence, such as excess body weight and processed meat consumption,” the authors of the 2023 American Cancer Society report explained.
People younger than 45 should alert their medical provider if they have constipation, rectal bleeding, or sudden changes in bowel movements, which can be symptoms of colorectal cancer. Screening for colorectal cancer should begin for most people at age 45.
A version of this article appeared on WebMD.com.
CHICAGO — Genetics and diet have been among the top theories for what may be fueling the troubling rise of colorectal cancer in young adults. Now,
The findings were presented at the annual meeting of the American Society for Clinical Oncology (ASCO) by researchers from Ohio State University. For the analysis, they analyzed genetic data on tumors.
The researchers found signs that a high-fat, low-fiber diet may increase inflammation in the gut that prevents it from naturally suppressing tumors. The cells of young people with colorectal cancer also appeared to have aged more quickly — by 15 years on average — than a person’s actual age. That’s unusual, because older people with colorectal cancer don’t have the same boost in cellular aging.
The rate of colorectal cancer among young people has been rising at an alarming rate, according to a 2023 report from the American Cancer Society. In 2019, one in five colorectal cancer cases were among people younger than 55. That’s up from 1 in 10 in 1995, which means the rate has doubled in less than 30 years.
Need Colon Cancer Screening?
Who needs a colorectal cancer screening? Ask colorectal cancer specialist Nancy Kemeny, MD.
A 2017 analysis estimated that a person’s risk of colorectal cancer increased 12% by eating 3.5 ounces of red or processed meat daily, which is the equivalent of the size of a deck of playing cards. The same study also linked colorectal cancer risk to alcohol intake, citing its ethanol content. Eating a diet high in fiber can reduce a person’s risk.
This latest study aligned with previous findings that link bacteria called Fusobacterium to colorectal cancer. It’s not unusual for Fusobacterium to be present in a person’s mouth, but it is more likely to be found in the intestines of colorectal cancer patients, compared with those of healthy people. One study even found that people with colorectal cancer were five times more likely to have Fusobacterium in their stool, compared with healthy people.
Colorectal cancer is more common among men than women, “likely reflecting differences in risk factor prevalence, such as excess body weight and processed meat consumption,” the authors of the 2023 American Cancer Society report explained.
People younger than 45 should alert their medical provider if they have constipation, rectal bleeding, or sudden changes in bowel movements, which can be symptoms of colorectal cancer. Screening for colorectal cancer should begin for most people at age 45.
A version of this article appeared on WebMD.com.
FROM ASCO 2024
Red Flags for Early-Onset Colorectal Cancer Identified
TOPLINE:
Patients with early-onset colorectal cancer (EOCRC) often present with hematochezia or abdominal pain, symptoms frequently overlooked in younger populations, leading to delays in diagnosis of 4-6 months, a new analysis showed.
METHODOLOGY:
- As the number of cases of EOCRC, defined as colorectal cancer (CRC) diagnosed before age 50, continues to rise, early detection has become increasingly important. Improved recognition of presenting signs and symptoms associated with EOCRC could lead to a more timely diagnosis and better clinical outcomes.
- In a systematic review and meta-analysis of 81 studies with 24.9 million EOCRC cases, researchers sought to determine the most common presenting signs and symptoms, their association with EOCRC risk, and the time from presentation to diagnosis.
- Data extraction and quality assessment were performed independently in duplicate using PRISMA guidelines, and Joanna Briggs Institute critical appraisal tools were used to measure the risk of bias.
TAKEAWAY:
- Hematochezia was the most common presenting sign/symptom, with a pooled prevalence of 45%, followed by abdominal pain, with a pooled prevalence of 40%.
- Altered bowel habits, which included constipation, diarrhea, and alternating bowel habits, were the third most common presenting sign/symptom (pooled prevalence of 27%), followed by unexplained weight loss (pooled prevalence of 17%).
- The likelihood of EOCRC was estimated to be fivefold to 54-fold higher with hematochezia and 1.3-fold to sixfold higher with abdominal pain.
- The mean time from sign or symptom onset to EOCRC diagnosis was 6.4 months (range, 1.8-13.7 months).
IN PRACTICE:
“These findings and the increasing risk of CRC in individuals younger than 50 years highlight the urgent need to educate clinicians and patients about these signs and symptoms to ensure that diagnostic workup and resolution are not delayed. Adapting current clinical practice to identify and address these signs and symptoms through careful clinical triage and follow-up could help limit morbidity and mortality associated with EOCRC,” the authors wrote.
SOURCE:
The study, with Joshua Demb, PhD, MPH, division of gastroenterology, department of medicine, University of California, San Diego, was published online May 24 in JAMA Network Open.
LIMITATIONS:
Significant heterogeneity across studies affected the ability to meta-analyze some results. The cross-sectional data limited the ability to stratify by age, sex, race and ethnicity, or genetic ancestry. It was not possible to evaluate the impact of time to diagnosis on CRC outcomes due to a limited number of studies answering this question. Researchers were unable to examine the constellation of signs and symptoms because they lacked individual-level data from each study.
DISCLOSURES:
The authors disclosed no relevant conflicts of interest. No specific funding was disclosed.
A version of this article appeared on Medscape.com.
TOPLINE:
Patients with early-onset colorectal cancer (EOCRC) often present with hematochezia or abdominal pain, symptoms frequently overlooked in younger populations, leading to delays in diagnosis of 4-6 months, a new analysis showed.
METHODOLOGY:
- As the number of cases of EOCRC, defined as colorectal cancer (CRC) diagnosed before age 50, continues to rise, early detection has become increasingly important. Improved recognition of presenting signs and symptoms associated with EOCRC could lead to a more timely diagnosis and better clinical outcomes.
- In a systematic review and meta-analysis of 81 studies with 24.9 million EOCRC cases, researchers sought to determine the most common presenting signs and symptoms, their association with EOCRC risk, and the time from presentation to diagnosis.
- Data extraction and quality assessment were performed independently in duplicate using PRISMA guidelines, and Joanna Briggs Institute critical appraisal tools were used to measure the risk of bias.
TAKEAWAY:
- Hematochezia was the most common presenting sign/symptom, with a pooled prevalence of 45%, followed by abdominal pain, with a pooled prevalence of 40%.
- Altered bowel habits, which included constipation, diarrhea, and alternating bowel habits, were the third most common presenting sign/symptom (pooled prevalence of 27%), followed by unexplained weight loss (pooled prevalence of 17%).
- The likelihood of EOCRC was estimated to be fivefold to 54-fold higher with hematochezia and 1.3-fold to sixfold higher with abdominal pain.
- The mean time from sign or symptom onset to EOCRC diagnosis was 6.4 months (range, 1.8-13.7 months).
IN PRACTICE:
“These findings and the increasing risk of CRC in individuals younger than 50 years highlight the urgent need to educate clinicians and patients about these signs and symptoms to ensure that diagnostic workup and resolution are not delayed. Adapting current clinical practice to identify and address these signs and symptoms through careful clinical triage and follow-up could help limit morbidity and mortality associated with EOCRC,” the authors wrote.
SOURCE:
The study, with Joshua Demb, PhD, MPH, division of gastroenterology, department of medicine, University of California, San Diego, was published online May 24 in JAMA Network Open.
LIMITATIONS:
Significant heterogeneity across studies affected the ability to meta-analyze some results. The cross-sectional data limited the ability to stratify by age, sex, race and ethnicity, or genetic ancestry. It was not possible to evaluate the impact of time to diagnosis on CRC outcomes due to a limited number of studies answering this question. Researchers were unable to examine the constellation of signs and symptoms because they lacked individual-level data from each study.
DISCLOSURES:
The authors disclosed no relevant conflicts of interest. No specific funding was disclosed.
A version of this article appeared on Medscape.com.
TOPLINE:
Patients with early-onset colorectal cancer (EOCRC) often present with hematochezia or abdominal pain, symptoms frequently overlooked in younger populations, leading to delays in diagnosis of 4-6 months, a new analysis showed.
METHODOLOGY:
- As the number of cases of EOCRC, defined as colorectal cancer (CRC) diagnosed before age 50, continues to rise, early detection has become increasingly important. Improved recognition of presenting signs and symptoms associated with EOCRC could lead to a more timely diagnosis and better clinical outcomes.
- In a systematic review and meta-analysis of 81 studies with 24.9 million EOCRC cases, researchers sought to determine the most common presenting signs and symptoms, their association with EOCRC risk, and the time from presentation to diagnosis.
- Data extraction and quality assessment were performed independently in duplicate using PRISMA guidelines, and Joanna Briggs Institute critical appraisal tools were used to measure the risk of bias.
TAKEAWAY:
- Hematochezia was the most common presenting sign/symptom, with a pooled prevalence of 45%, followed by abdominal pain, with a pooled prevalence of 40%.
- Altered bowel habits, which included constipation, diarrhea, and alternating bowel habits, were the third most common presenting sign/symptom (pooled prevalence of 27%), followed by unexplained weight loss (pooled prevalence of 17%).
- The likelihood of EOCRC was estimated to be fivefold to 54-fold higher with hematochezia and 1.3-fold to sixfold higher with abdominal pain.
- The mean time from sign or symptom onset to EOCRC diagnosis was 6.4 months (range, 1.8-13.7 months).
IN PRACTICE:
“These findings and the increasing risk of CRC in individuals younger than 50 years highlight the urgent need to educate clinicians and patients about these signs and symptoms to ensure that diagnostic workup and resolution are not delayed. Adapting current clinical practice to identify and address these signs and symptoms through careful clinical triage and follow-up could help limit morbidity and mortality associated with EOCRC,” the authors wrote.
SOURCE:
The study, with Joshua Demb, PhD, MPH, division of gastroenterology, department of medicine, University of California, San Diego, was published online May 24 in JAMA Network Open.
LIMITATIONS:
Significant heterogeneity across studies affected the ability to meta-analyze some results. The cross-sectional data limited the ability to stratify by age, sex, race and ethnicity, or genetic ancestry. It was not possible to evaluate the impact of time to diagnosis on CRC outcomes due to a limited number of studies answering this question. Researchers were unable to examine the constellation of signs and symptoms because they lacked individual-level data from each study.
DISCLOSURES:
The authors disclosed no relevant conflicts of interest. No specific funding was disclosed.
A version of this article appeared on Medscape.com.
Late-Night Eaters May Have Increased Risk for Colorectal Cancer
WASHINGTON — , according to the results of research presented at the annual Digestive Disease Week® (DDW).
Investigators in a new study questioned 664 people getting a colonoscopy to screen for cancer, and 42% said they were late eaters. This group was 46% more likely than non–late eaters to have an adenoma found during colonoscopy. An estimated 5% to 10% of them become cancerous over time.
“A lot of other studies are about what we eat but not when we eat,” said Edena Khoshaba, lead investigator and a medical student at Rush University Medical College in Chicago. “The common advice includes not eating red meat, eating more fruits and vegetables — which is great, of course — but we wanted to see if the timing affects us at all.”
Ms. Khoshaba and colleagues found it did. Late eaters were 5.5 times more likely to have three or more tubular adenomas compared to non–late eaters, even after adjusting for what people were eating. Tubular adenomas are the most common type of polyp found in the colon.
So, what’s the possible connection between late eating and the risk for colorectal cancer?
Resetting Your Internal Clock
Eating close to bedtime could be throwing off the body’s circadian rhythm. But in this case, it’s not the central circadian center located in the brain — the one that releases melatonin. Instead, late eating could disrupt the peripheral circadian rhythm, part of which is found in the GI tract. For example, if a person is eating late at night, the brain thinks it is nighttime while the gut thinks it is daytime, Ms. Khoshaba said in an interview.
This is an interesting study, said Amy Bragagnini, MS, RD, spokesperson for the Academy of Nutrition and Dietetics, when asked to comment on the research. “It is true that eating later at night can disrupt your circadian rhythm.”
“In addition, many of my patients have told me that when they do eat later at night, they don’t always make the healthiest food choices,” Ms. Bragagnini said. “Their late-night food choices are generally higher in added sugar and fat. This may cause them to consume far more calories than their body needs.” So, eating late at night can also lead to unwanted weight gain.
An unanswered question is if late eating is connected in any way at all to increasing rates of colorectal cancer seen in younger patients.
This was an observational study, and another possible limitation, Ms. Khoshaba said, is that people were asked to recall their diets over 24 hours, which may not always be accurate.
Some of the organisms in the gut have their own internal clocks that follow a daily rhythm, and what someone eat determines how many different kinds of these organisms are active, Ms. Bragagnini said.
“So, if your late-night eating consists of foods high in sugar and fat, you may be negatively impacting your microbiome.” she said.
The next step for Ms. Khoshaba and colleagues is a study examining the peripheral circadian rhythm, changes in the gut microbiome, and the risk for developing metabolic syndrome. Ms. Khoshaba and Ms. Bragagnini had no relevant disclosures.
A version of this article appeared on Medscape.com.
WASHINGTON — , according to the results of research presented at the annual Digestive Disease Week® (DDW).
Investigators in a new study questioned 664 people getting a colonoscopy to screen for cancer, and 42% said they were late eaters. This group was 46% more likely than non–late eaters to have an adenoma found during colonoscopy. An estimated 5% to 10% of them become cancerous over time.
“A lot of other studies are about what we eat but not when we eat,” said Edena Khoshaba, lead investigator and a medical student at Rush University Medical College in Chicago. “The common advice includes not eating red meat, eating more fruits and vegetables — which is great, of course — but we wanted to see if the timing affects us at all.”
Ms. Khoshaba and colleagues found it did. Late eaters were 5.5 times more likely to have three or more tubular adenomas compared to non–late eaters, even after adjusting for what people were eating. Tubular adenomas are the most common type of polyp found in the colon.
So, what’s the possible connection between late eating and the risk for colorectal cancer?
Resetting Your Internal Clock
Eating close to bedtime could be throwing off the body’s circadian rhythm. But in this case, it’s not the central circadian center located in the brain — the one that releases melatonin. Instead, late eating could disrupt the peripheral circadian rhythm, part of which is found in the GI tract. For example, if a person is eating late at night, the brain thinks it is nighttime while the gut thinks it is daytime, Ms. Khoshaba said in an interview.
This is an interesting study, said Amy Bragagnini, MS, RD, spokesperson for the Academy of Nutrition and Dietetics, when asked to comment on the research. “It is true that eating later at night can disrupt your circadian rhythm.”
“In addition, many of my patients have told me that when they do eat later at night, they don’t always make the healthiest food choices,” Ms. Bragagnini said. “Their late-night food choices are generally higher in added sugar and fat. This may cause them to consume far more calories than their body needs.” So, eating late at night can also lead to unwanted weight gain.
An unanswered question is if late eating is connected in any way at all to increasing rates of colorectal cancer seen in younger patients.
This was an observational study, and another possible limitation, Ms. Khoshaba said, is that people were asked to recall their diets over 24 hours, which may not always be accurate.
Some of the organisms in the gut have their own internal clocks that follow a daily rhythm, and what someone eat determines how many different kinds of these organisms are active, Ms. Bragagnini said.
“So, if your late-night eating consists of foods high in sugar and fat, you may be negatively impacting your microbiome.” she said.
The next step for Ms. Khoshaba and colleagues is a study examining the peripheral circadian rhythm, changes in the gut microbiome, and the risk for developing metabolic syndrome. Ms. Khoshaba and Ms. Bragagnini had no relevant disclosures.
A version of this article appeared on Medscape.com.
WASHINGTON — , according to the results of research presented at the annual Digestive Disease Week® (DDW).
Investigators in a new study questioned 664 people getting a colonoscopy to screen for cancer, and 42% said they were late eaters. This group was 46% more likely than non–late eaters to have an adenoma found during colonoscopy. An estimated 5% to 10% of them become cancerous over time.
“A lot of other studies are about what we eat but not when we eat,” said Edena Khoshaba, lead investigator and a medical student at Rush University Medical College in Chicago. “The common advice includes not eating red meat, eating more fruits and vegetables — which is great, of course — but we wanted to see if the timing affects us at all.”
Ms. Khoshaba and colleagues found it did. Late eaters were 5.5 times more likely to have three or more tubular adenomas compared to non–late eaters, even after adjusting for what people were eating. Tubular adenomas are the most common type of polyp found in the colon.
So, what’s the possible connection between late eating and the risk for colorectal cancer?
Resetting Your Internal Clock
Eating close to bedtime could be throwing off the body’s circadian rhythm. But in this case, it’s not the central circadian center located in the brain — the one that releases melatonin. Instead, late eating could disrupt the peripheral circadian rhythm, part of which is found in the GI tract. For example, if a person is eating late at night, the brain thinks it is nighttime while the gut thinks it is daytime, Ms. Khoshaba said in an interview.
This is an interesting study, said Amy Bragagnini, MS, RD, spokesperson for the Academy of Nutrition and Dietetics, when asked to comment on the research. “It is true that eating later at night can disrupt your circadian rhythm.”
“In addition, many of my patients have told me that when they do eat later at night, they don’t always make the healthiest food choices,” Ms. Bragagnini said. “Their late-night food choices are generally higher in added sugar and fat. This may cause them to consume far more calories than their body needs.” So, eating late at night can also lead to unwanted weight gain.
An unanswered question is if late eating is connected in any way at all to increasing rates of colorectal cancer seen in younger patients.
This was an observational study, and another possible limitation, Ms. Khoshaba said, is that people were asked to recall their diets over 24 hours, which may not always be accurate.
Some of the organisms in the gut have their own internal clocks that follow a daily rhythm, and what someone eat determines how many different kinds of these organisms are active, Ms. Bragagnini said.
“So, if your late-night eating consists of foods high in sugar and fat, you may be negatively impacting your microbiome.” she said.
The next step for Ms. Khoshaba and colleagues is a study examining the peripheral circadian rhythm, changes in the gut microbiome, and the risk for developing metabolic syndrome. Ms. Khoshaba and Ms. Bragagnini had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM DDW 2024
Negative Colonoscopy? 15-Year Screening Interval May Be Safe
TOPLINE:
a population-based study suggests.
METHODOLOGY:
- Using Swedish nationwide registry data, researchers compared 110,074 individuals who had a first colonoscopy with negative findings for CRC at age 45-69 years (exposed group) with more than 1.9 million matched controls who either did not have a colonoscopy during the study period or underwent colonoscopy that led to a CRC diagnosis.
- They calculated 10-year standardized incidence ratio (SIR) and standardized mortality ratio (SMR) to compare risks for CRC and CRC-specific death in the exposed and control groups based on different follow-up screening intervals.
TAKEAWAY:
- During up to 29 years of follow-up, 484 incident CRCs and 112 CRC deaths occurred in the group with a negative initial colonoscopy.
- Up to 15 years after negative colonoscopy, the 10-year cumulative risk for CRC and CRC mortality was lower than in the control group, with an SIR of 0.72 and SMR of 0.55, respectively.
- Extending the screening interval from 10 to 15 years would miss early detection of only two CRC cases and prevention of only one CRC death per 1000 individuals, while potentially avoiding 1000 colonoscopies.
IN PRACTICE:
“This study provides evidence for recommending a longer colonoscopy screening interval than what is currently recommended in most guidelines for populations with no familial risk of CRC,” the authors wrote. “A longer interval between colonoscopy screenings could be beneficial in avoiding unnecessary invasive examinations.”
SOURCE:
The study, with first author Qunfeng Liang, MSc, with the German Cancer Research Center, Heidelberg, Germany, was published online on May 2 in JAMA Oncology.
LIMITATIONS:
The study population primarily included White individuals, particularly ethnic Swedish individuals, so external validation would be necessary to generalize the recommendation to other populations. The researchers lacked data on non-endoscopic tests, such as fecal occult blood tests, which could have been performed as a substitution for colonoscopy during the interval between colonoscopy screenings.
DISCLOSURES:
The study had no specific funding. The authors had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
a population-based study suggests.
METHODOLOGY:
- Using Swedish nationwide registry data, researchers compared 110,074 individuals who had a first colonoscopy with negative findings for CRC at age 45-69 years (exposed group) with more than 1.9 million matched controls who either did not have a colonoscopy during the study period or underwent colonoscopy that led to a CRC diagnosis.
- They calculated 10-year standardized incidence ratio (SIR) and standardized mortality ratio (SMR) to compare risks for CRC and CRC-specific death in the exposed and control groups based on different follow-up screening intervals.
TAKEAWAY:
- During up to 29 years of follow-up, 484 incident CRCs and 112 CRC deaths occurred in the group with a negative initial colonoscopy.
- Up to 15 years after negative colonoscopy, the 10-year cumulative risk for CRC and CRC mortality was lower than in the control group, with an SIR of 0.72 and SMR of 0.55, respectively.
- Extending the screening interval from 10 to 15 years would miss early detection of only two CRC cases and prevention of only one CRC death per 1000 individuals, while potentially avoiding 1000 colonoscopies.
IN PRACTICE:
“This study provides evidence for recommending a longer colonoscopy screening interval than what is currently recommended in most guidelines for populations with no familial risk of CRC,” the authors wrote. “A longer interval between colonoscopy screenings could be beneficial in avoiding unnecessary invasive examinations.”
SOURCE:
The study, with first author Qunfeng Liang, MSc, with the German Cancer Research Center, Heidelberg, Germany, was published online on May 2 in JAMA Oncology.
LIMITATIONS:
The study population primarily included White individuals, particularly ethnic Swedish individuals, so external validation would be necessary to generalize the recommendation to other populations. The researchers lacked data on non-endoscopic tests, such as fecal occult blood tests, which could have been performed as a substitution for colonoscopy during the interval between colonoscopy screenings.
DISCLOSURES:
The study had no specific funding. The authors had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
a population-based study suggests.
METHODOLOGY:
- Using Swedish nationwide registry data, researchers compared 110,074 individuals who had a first colonoscopy with negative findings for CRC at age 45-69 years (exposed group) with more than 1.9 million matched controls who either did not have a colonoscopy during the study period or underwent colonoscopy that led to a CRC diagnosis.
- They calculated 10-year standardized incidence ratio (SIR) and standardized mortality ratio (SMR) to compare risks for CRC and CRC-specific death in the exposed and control groups based on different follow-up screening intervals.
TAKEAWAY:
- During up to 29 years of follow-up, 484 incident CRCs and 112 CRC deaths occurred in the group with a negative initial colonoscopy.
- Up to 15 years after negative colonoscopy, the 10-year cumulative risk for CRC and CRC mortality was lower than in the control group, with an SIR of 0.72 and SMR of 0.55, respectively.
- Extending the screening interval from 10 to 15 years would miss early detection of only two CRC cases and prevention of only one CRC death per 1000 individuals, while potentially avoiding 1000 colonoscopies.
IN PRACTICE:
“This study provides evidence for recommending a longer colonoscopy screening interval than what is currently recommended in most guidelines for populations with no familial risk of CRC,” the authors wrote. “A longer interval between colonoscopy screenings could be beneficial in avoiding unnecessary invasive examinations.”
SOURCE:
The study, with first author Qunfeng Liang, MSc, with the German Cancer Research Center, Heidelberg, Germany, was published online on May 2 in JAMA Oncology.
LIMITATIONS:
The study population primarily included White individuals, particularly ethnic Swedish individuals, so external validation would be necessary to generalize the recommendation to other populations. The researchers lacked data on non-endoscopic tests, such as fecal occult blood tests, which could have been performed as a substitution for colonoscopy during the interval between colonoscopy screenings.
DISCLOSURES:
The study had no specific funding. The authors had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.