User login
Your 15-year-old patient requests an IUD without parental knowledge
CASE Adolescent seeks care without parent
A 15-year-old patient (G0) presents to the gynecology clinic requesting birth control. She reports being sexually active over the past 6 months and having several male partners over the past 2 years. She and her current male partner use condoms inconsistently. She reports being active in school sports, and her academic performance has been noteworthy. Her peers have encouraged her to seek out birth control; one of her good friends recently became pregnant and dropped out of school. She states that her best friend went to a similar clinic and received a “gynecologic encounter” that included information regarding safe sex and contraception, with no pelvic exam required for her to receive birth control pills.
The patient insists that her parents are not to know of her request for contraception due to sexual activity or that she is a patient at the clinic. The gynecologist covering the clinic is aware of the American College of Obstetricians and Gynecologists Committee on Adolescent Health Care and their many publications. The patient is counseled regarding human papillomavirus (HPV) vaccination and screened for sexually transmitted infections. In addition, the gynecologist discusses contraceptive options with the patient, ranging from oral contraceptives, vaginal rings, subdermal implants, depomedroxyprogesterone acetate, as well as intrauterine devices (IUDs). The gynecologist emphasizes safe sex and advises that her partner consider use of condoms independent of her method of birth control. The patient asks for oral contraceptives and is given information about their use and risks, and she indicates that she understands.
A few months later the patient requests an IUD, as she would like to have lighter menses and not have to remember to take a pill every day. The provider obtains informed consent for the insertion procedure; the patient signs the appropriate forms.
The IUD is inserted, with difficulty, by a resident physician in the clinic. The patient experiences severe pelvic pain during and immediately following the insertion. She is sent home and told to contact the clinic or another health care provider or proceed to the local emergency department should pain persist or if fever develops.
The patient returns 72 hours later in pain. Pelvic ultrasonography shows the IUD out of place and at risk of perforating the fundus of the uterus. Later that day the patient’s mother calls the clinic, saying that she found a statement of service with the clinic’s number on it in her daughter’s bedroom. She wants to know if her daughter is there, what is going on, and what services have been or are being provided. In passing she remarks that she has no intention of paying (or allowing her insurance to pay for) any care that was provided.
What are the provider’s obligations at this point, both medically and legally?

Medical and legal considerations
One of the most difficult and important health law questions in adolescent medicine is the ability of minors to consent to treatment and to control the health care information resulting from treatment. (“Minor” describes a child or adolescent who has not obtained the age of legal consent, generally 18 years old, to lawfully enter into a legal transaction.)
Continue to: The consent of minor patients...
The consent of minor patients
The traditional legal rule is that parents or guardians (“parent” refers to both) must consent to medical treatment for minor children. There is an exception for emergency situations but generally minors do not provide consent for medical care, a parent does.1 The parent typically is obliged to provide payment (often through insurance) for those services.
This traditional rule has some exceptions—the emergency exception already noted and the case of emancipated minors, notably an adolescent who is living almost entirely independent of her parents (for example, she is married or not relying on parents in a meaningful way). In recent times there has been increasing authority for “mature minors” to make some medical decisions.2 A mature minor is one who has sufficient understanding and judgment to appreciate the consequences, benefits, and risks of accepting proposed medical intervention.
No circumstance involving adolescent treatment has been more contentious than services related to abortion and, to a lesser degree, contraception.3 Both the law of consent to services and the rights of parents to obtain information about contraceptive and abortion services have been a matter of strong, continuing debate. The law in these areas varies greatly from state-to-state, and includes a mix of state law (statutes and court decisions) with an overlay of federal constitutional law related to reproduction-related decisions of adolescents. In addition, the law in this area of consent and information changes relatively frequently.4 Clinicians, of course, must focus on the consent laws of the state in which they practice.
STI counseling and treatment
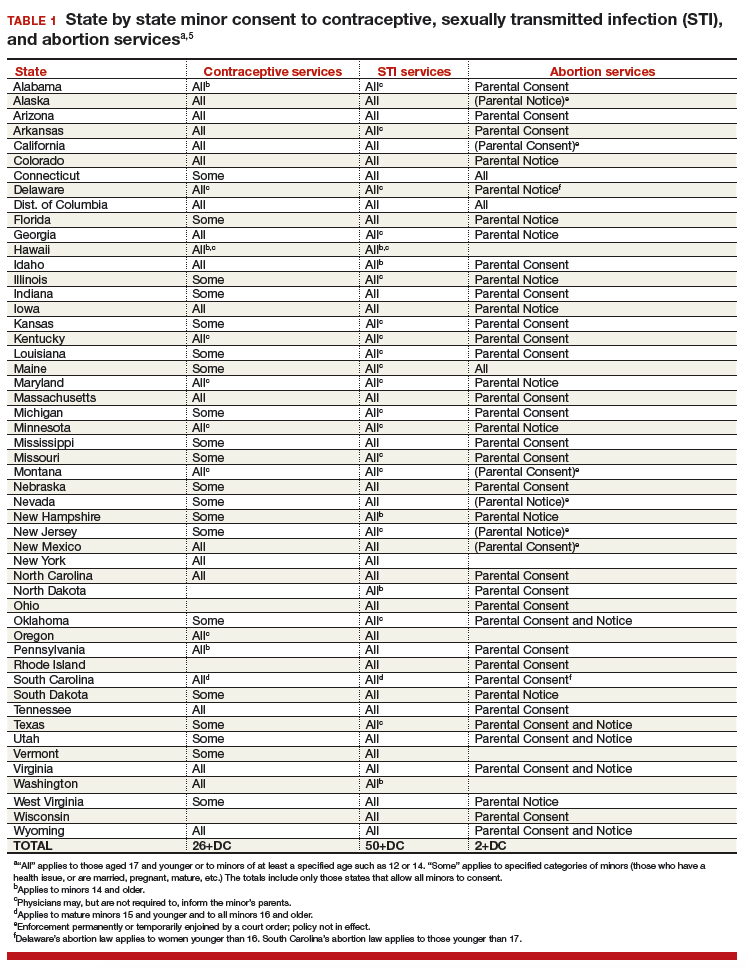
All states permit a minor patient to consent to treatment for an STI (TABLE 1).5 A number of states expressly permit, but do not require, health care providers to inform parents of treatment when a physician determines it would be in the best interest of the minor. Thus, the clinic would not be required to provide proactively the information to our case patient’s mother (regarding any STI issues) when she called.6
Contraception
Consent for contraception is more complicated. About half the states allow minors who have reached a certain age (12, 14, or 16 years) to consent to contraception. About 20 other states allow some minors to consent to contraceptive services, but the “allowed group” may be fairly narrow (eg, be married, have a health issue, or be “mature”). In 4 states there is currently no clear legal authority to provide contraceptive services to minors, yet those states do not specifically prohibit it. The US Supreme Court has held that a state cannot completely prohibit the availability of contraception to minors.7 The reach of that decision, however, is not clear and may not extend beyond what the states currently permit.
The ability of minors to consent to contraception services does not mean that there is a right to consent to all contraceptive options. As contraception becomes more irreversible, permanent, or risky, it is more problematic. For example, consent to sterilization would not ordinarily be within a minor’s recognized ability to consent. Standard, low risk, reversible contraception generally is covered by these state laws.8
In our case here, the patient likely was able to consent to contraception—initially to the oral contraception and later to the IUD. The risks and reversibility of both are probably within her ability to consent.9,10 Of course, if the care was provided in a state that does not include the patient within the groups that can give consent to contraception, it is possible that she might not have the legal authority to consent.
Continue to: General requirements of consent...
General requirements of consent
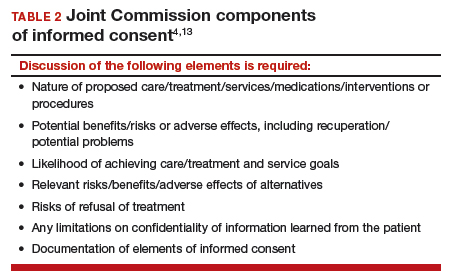
Even when adolescent consent is permitted for treatment, including in cases of contraception, it is essential that all of the legal and ethical requirements related to informed consent are met.
1. The adolescent has the capacity to consent. This means not only that the state-mandated requirements are met (age, for example) but also that the patient can and does understand the various elements of consent, and can make a sensible, informed decision.
The bottom line is “adolescent capacity is a complex process dependent upon the development of maturity of the adolescent, degree of intervention, expected benefit of the medical procedure, and the sociocultural context surrounding the decision.”11 Other items of interest include the “evolving capacity” of the child,12 which is the concept of increasing ability of the teen to process information and provide more appropriate informed consent. Central nervous system (CNS) maturation allows the adolescent to become increasingly more capable of decision making and has awareness of consequences of such decisions. Abstract thinking capabilities is a reflection of this CNS maturing process. If this competency is not established, the adolescent patient cannot give legitimate consent.
2. The patient must be given appropriate information (be “informed”). The discussion should include information relevant to the condition being treated (and the disease process if relevant). In addition, information about the treatment or intervention proposed and its risks and alternatives must be provided to the patient and in a way that is understandable.
3. As with all patients, consent must be voluntary and free of coercion or manipulation. These elements of informed consent are expanded on by the Joint Commission, which has established a number of components of informed consent (TABLE 2).4,13
Confidentiality and release of information to parents and others
Similar to consent, parents historically have had the authority to obtain medical information about their minor children. This right generally continues today, with some limitations. The right to give consent generally carries with it the right to medical information. There are some times when parents may access medical information even if they have not given consent.
This right adds complexity to minor consent and is an important treatment issue and legal consideration because confidentiality for adolescents affects quality of care. Adolescents report that “confidentiality is an important factor in their decision to seek [medical] care.”14 Many parents are under the assumption that the health care provider will automatically inform them independent of whether or not the adolescent expressed precise instruction not to inform.15,16
Of course if a minor patient authorizes the physician to provide information to her parents, that is consent and the health care provider may then provide the information. If the patient instructs the provider to convey the information, the practitioner would ordinarily be expected to be proactive in providing the information to the parent. The issue of “voluntariness” of the waiver of confidentiality can be a question, and the physician may discuss that question with the patient. Ordinarily, however, once a minor has authorized disclosure to the parent, the clinician has the authority to disclose the information to the parent, but not to others.
All of the usual considerations of confidentiality in health care apply to adolescent ObGyn services and care. This includes the general obligation not to disclose information without consent and to ensure that health care information is protected from accidental release as required by the Health Insurance Portability and Accountability Act (HIPAA) and other health information privacy laws.17
It is important to emphasize that the issues of consent to abortion are much different than those for contraception and sexually transmitted infections. As our case presentation does not deal with abortion, we will address this complex but important discussion in the future--as there are an estimated 90,000 abortions in adolescent girls annually.1
Given that abortion consent and notification laws are often complex, any physician providing abortion services to any minor should have sound legal advice on the requirements of the pertinent state law. In earlier publications of this section in OBG Management we have discussed the importance of practitioners having an ongoing relationship with a health law attorney. We make this point again, as this person can provide advice on consent and the rights of parents to have information about their minor children.
Reference
- Henshaw SK. U.S. teenage pregnancy statistics with comparative statistics for women age 20-24. New York, New York: Alan Guttmacher Institute; May 2003.
Continue to: How and when to protect minor confidentiality...
How and when to protect minor confidentiality
A clinician cannot assure minors of absolute confidentiality and should not agree to do so or imply that they are doing so.18 In our hypothetical case, when the patient told the physician that her parents were not to know of any of her treatment or communications, the provider should not have acquiesced by silence. He/she might have responded along these lines: “I have a strong commitment to confidentiality of your information, and we take many steps to protect that information. The law also allows some special protection of health care information. Despite the commitment to privacy, there are circumstances in which the law requires disclosure of information—and that might even be to parents. In addition, if you want any of your care covered by insurance, we would have to disclose that. While I expect that we can do as you ask about maintaining your confidentiality, no health care provider can absolutely guarantee it.”
Proactive vs reactive disclosure. There is “proactive” disclosure of information and “reactive” disclosure. Proactive is when the provider (without being asked) contacts a parent or others and provides information. Some states require proactive information about specific kinds of treatment (especially abortion services). For the most part, in states where a minor can legally consent to treatment, health care providers are not required to proactively disclose information.19
Clinicians may be required to respond to parental requests for information, which is reactive disclosure and is reflected in our case presentation. Even in such circumstances, however, the individual providing care may seek to avoid disclosure. In many states, the law would not require the release of this information (but would permit it if it is in the best interest of the patient). In addition, there are practical ways of avoiding the release of information. For example, the health care provider might acknowledge the interest and desire of the parent to have the information, but might humbly explain that in the experience of many clinicians protecting the confidentiality of patients is very important to successful treatment and it is the policy of the office/clinic not to breach the expectation of patient confidentiality except where that is clearly in the best interest of the patient or required by law.
In response to the likely question, “Well, isn’t that required by law?” the clinician can honestly reply, “I don’t know. There are many complex factors in the law regarding disclosure of medical information and as I am not an attorney I do not know how they all apply in this instance.” In some cases the parent may push the matter or take some kind of legal action. It is in this type of situation that an attorney familiar with health law and the clinician’s practice can be invaluable.
When parents are involved in the minor’s treatment (bringing the patient to the office/clinic, for example), there is an opportunity for an understanding, or agreement, among the patient, provider, and parent about what information the parent will receive. Ordinarily the agreement should not create the expectation of detailed information for the parent. Perhaps, for example, the physician will provide information only when he or she believes that doing so will be in the best interest of the patient. Even with parental agreement, complete confidentiality cannot be assured for minor patients. There may, for example, be another parent who will not feel bound by the established understanding, and the law requires some disclosures (in the case of child abuse or a court order).20
Continue to: Accidental disclosure...
Accidental disclosure. Health care providers also should make sure that office procedures do not unnecessarily or accidentally disclose information about patients. For example, routinely gathering information about insurance coverage may well trigger the release of information to the policy holder (often a parent). Thus, there should be clear understandings about billing, insurance, and related issues before information is divulged by the patient. This should be part of the process of obtaining informed consent to treatment. It should be up front and honest. Developing a clear understanding of the legal requirements of the state is essential, so that assurance of confidentiality is on legal, solid ground.
As the pediatric and adolescent segment of gynecologic care continues to evolve, it is noteworthy that the American Board of Obstetrics and Gynecology recently has established a "Focused Practice" designation in pediatric adolescent gynecology. This allows ObGyns to have an ongoing level of professional education in this specialized area. Additional information can be obtained at www.abog.org or [email protected].
More resources for adolescent contraceptive care include:
- The American College of Obstetricians and Gynecologists (ACOG) "Birth Control (Especially for Teens)" frequently asked questions information series (https://www.acog.org/Patients/FAQs/Birth-Control-Especially-for-Teens)
- ACOG's Adolescent Healthcare Committee Opinions address adolescent pregnancy, contraception, and sexual activity (https://www.acog.org/-/media/List-of-Titles/COListOfTitles.pdf)
- ACOG statement on teen pregnancy and contraception, April 7, 2015 (https://www.acog.org/About-ACOG/News-Room/Statements/2015/ACOG-Statement-on-Teen-Pregnancy-and-Contraception?IsMobileSet=false)
- North American Society for Pediatric and Adolescent Gynecology resources for patients (https://www.naspag.org/page/patienttools)
- Society for Adolescent Health and Medicine statement regarding contraceptive access policies (https://www.adolescenthealth.org)
- The Guttmacher Institute's overview of state laws relevant to minor consent, as of January 1, 2019 (https://www.guttmacher.org/state-policy/explore/overview-minors-consent-law). It is updated frequently.
Abuse reporting obligations
All states have mandatory child abuse reporting laws. These laws require medical professionals (and others) to report known, and often suspected, abuse of children. Abuse includes physical, sexual, or emotional, and generally also includes neglect that is harming a child. When there is apparent sexual or physical abuse, the health care provider is obligated to report it to designated state authorities, generally child protective services. Reporting laws vary from state to state based on the relationship between the suspected abuser and the minor, the nature of the harm, and how strong the suspicion of abuse needs to be. The failure to make required reports is a crime in most states and also may result in civil liability or licensure discipline. Criminal charges seldom result from the failure to report, but in some cases the failure to report may have serious consequences for the professional.
An ObGyn example of the complexity of reporting laws, and variation from state to state, is in the area of “statutory rape” reporting. Those state laws, which define serious criminal offenses, set out the age below which an individual is not legally capable of consenting to sexual activity. It varies among states, but may be an absolute age of consent, the age differential between the parties, or some combination of age and age differential.21 The question of reporting is further complicated by the issue of when statutory rape must be reported—for example, the circumstances when the harm to the underage person is sufficient to require reporting.22
Laws are complex, as is practice navigation
It is apparent that navigating these issues makes it essential for an ObGyn practice to have clear policies and practices regarding reporting, yet the overall complexity is also why it is so difficult to develop those policies in the first place. Of course, they must be tailored to the state in which the practice resides. Once again, the need is clear for health care professionals to have an ongoing relationship with a health attorney who can help navigate ongoing questions.
- Benjamin L, Ishimine P, Joseph M, et al. Evaluation and treatment of minors. Ann Emerg Med. 2018;71(2):225-232.
- Coleman D, Rosoff P. The legal authority of mature minors to consent to general medical treatment. Pediatrics. 2013;13:786-793.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 699. Adolescent pregnancy, contraception, and sexual activity. Obstet Gynecol. 2017;129:e142-e149.
- Tillett J. Adolescents and informed consent. J Perinat Neonat Nurs. 2005;19:112-121.
- An overview of minor's consent law. Guttmacher Institute's website. https://www.guttmacher.org/state-policy/explore/overview-minors-consent-law. Accessed February 14, 2019.
- Chelmow D, Karjane N, Ricciotti HA, et al, eds. A 16-year-old adolescent requesting confidential treatment for chlamydia exposure (understanding state laws regarding minors and resources). Office Gynecology: A Case-Based Approach. Cambridge, United Kingdom: Cambridge University Press; January 31, 2019:39.
- Carey v Population Services, 431 US 678 (1977).
- Williams RL, Meredith AH, Ott MA. Expanding adolescent access to hormonal contraception: an update on over-the-counter, pharmacist prescribing, and web-based telehealth approaches. Curr Opin Obstet Gynecol. 2018;30:458-464.
- McClellan K, Temples H, Miller L. The latest in teen pregnancy prevention: long-acting reversible contraception. J Pediatr Health Care. 2018;32:e91-e97.
- Behmer Hansen RT, Arora KS. Consenting to invasive contraceptives: an ethical analysis of adolescent decision-making authority for long-acting reversible contraception. J Med Ethics. 2018;44:585-588.
- Robertson D. Opinions in pediatric and adolescent gynecology. J Pediatr Adolesc Gynecol. 2008:21:47-51.
- Lansdown G. The evolving capacities of the child. Florence, Italy: UNICEF Innocenti Research Centre, Innocenti Insight; 2005. https://www.unicef-irc.org/publications/384-the-evolving-capacities-of-the-child.html. Accessed February 15, 2019.
- Clapp JT, Fleisher LA. What is the realistic scope of informed consent? Jt Comm J Qual Patient Saf. 2018;44(6):341-342.
- Berlan E, Bravender T. Confidentiality, consent and caring for the adolescent. Curr Opin Pediatr. 2009;21:450-456.
- Schantz K. Who Needs to Know? Confidentiality in Adolescent Sexual Health Care. Act for Youth website. http://www.actforyouth.net/resources/rf/rf_confidentiality_1118.pdf. Accessed February 14, 2019.
- Lynn A, Kodish E, Lazebnik R, et al. Understanding confidentiality: perspectives of African American adolescents and their parents. J Adolesc Health. 2006;39:261-265.
- English A, Ford CA. The HIPAA privacy rule and adolescents: legal questions and clinical challenges. Perspect Sex Reprod Health. 2004;36:80-86.
- Schapiro NA, Mejia J. Adolescent confidentiality and women's health: history, rationale, and current threats. Nurs Clin North Am. 2018;53:145-156.
- Scott NL, Alderman EM, 2018. Case of a girl with a secret. In: Adolescent Gynecology: A Clinical Casebook. New York, New York: Springer International; 2017:3-11.
- Cullitan CM. Please don't tell my mom--a minor's right to informational privacy. JL & Educ. 2011;40:417-460.
- Bierie DM, Budd KM. Romeo, Juliet, and statutory rape. Sex Abuse. 2018;30:296-321.
- Mathews B. A taxonomy of duties to report child sexual abuse: legal developments offer new ways to facilitate disclosure. Child Abuse Negl. 2019;88:337-347.
CASE Adolescent seeks care without parent
A 15-year-old patient (G0) presents to the gynecology clinic requesting birth control. She reports being sexually active over the past 6 months and having several male partners over the past 2 years. She and her current male partner use condoms inconsistently. She reports being active in school sports, and her academic performance has been noteworthy. Her peers have encouraged her to seek out birth control; one of her good friends recently became pregnant and dropped out of school. She states that her best friend went to a similar clinic and received a “gynecologic encounter” that included information regarding safe sex and contraception, with no pelvic exam required for her to receive birth control pills.
The patient insists that her parents are not to know of her request for contraception due to sexual activity or that she is a patient at the clinic. The gynecologist covering the clinic is aware of the American College of Obstetricians and Gynecologists Committee on Adolescent Health Care and their many publications. The patient is counseled regarding human papillomavirus (HPV) vaccination and screened for sexually transmitted infections. In addition, the gynecologist discusses contraceptive options with the patient, ranging from oral contraceptives, vaginal rings, subdermal implants, depomedroxyprogesterone acetate, as well as intrauterine devices (IUDs). The gynecologist emphasizes safe sex and advises that her partner consider use of condoms independent of her method of birth control. The patient asks for oral contraceptives and is given information about their use and risks, and she indicates that she understands.
A few months later the patient requests an IUD, as she would like to have lighter menses and not have to remember to take a pill every day. The provider obtains informed consent for the insertion procedure; the patient signs the appropriate forms.
The IUD is inserted, with difficulty, by a resident physician in the clinic. The patient experiences severe pelvic pain during and immediately following the insertion. She is sent home and told to contact the clinic or another health care provider or proceed to the local emergency department should pain persist or if fever develops.
The patient returns 72 hours later in pain. Pelvic ultrasonography shows the IUD out of place and at risk of perforating the fundus of the uterus. Later that day the patient’s mother calls the clinic, saying that she found a statement of service with the clinic’s number on it in her daughter’s bedroom. She wants to know if her daughter is there, what is going on, and what services have been or are being provided. In passing she remarks that she has no intention of paying (or allowing her insurance to pay for) any care that was provided.
What are the provider’s obligations at this point, both medically and legally?

Medical and legal considerations
One of the most difficult and important health law questions in adolescent medicine is the ability of minors to consent to treatment and to control the health care information resulting from treatment. (“Minor” describes a child or adolescent who has not obtained the age of legal consent, generally 18 years old, to lawfully enter into a legal transaction.)
Continue to: The consent of minor patients...
The consent of minor patients
The traditional legal rule is that parents or guardians (“parent” refers to both) must consent to medical treatment for minor children. There is an exception for emergency situations but generally minors do not provide consent for medical care, a parent does.1 The parent typically is obliged to provide payment (often through insurance) for those services.
This traditional rule has some exceptions—the emergency exception already noted and the case of emancipated minors, notably an adolescent who is living almost entirely independent of her parents (for example, she is married or not relying on parents in a meaningful way). In recent times there has been increasing authority for “mature minors” to make some medical decisions.2 A mature minor is one who has sufficient understanding and judgment to appreciate the consequences, benefits, and risks of accepting proposed medical intervention.
No circumstance involving adolescent treatment has been more contentious than services related to abortion and, to a lesser degree, contraception.3 Both the law of consent to services and the rights of parents to obtain information about contraceptive and abortion services have been a matter of strong, continuing debate. The law in these areas varies greatly from state-to-state, and includes a mix of state law (statutes and court decisions) with an overlay of federal constitutional law related to reproduction-related decisions of adolescents. In addition, the law in this area of consent and information changes relatively frequently.4 Clinicians, of course, must focus on the consent laws of the state in which they practice.
STI counseling and treatment
All states permit a minor patient to consent to treatment for an STI (TABLE 1).5 A number of states expressly permit, but do not require, health care providers to inform parents of treatment when a physician determines it would be in the best interest of the minor. Thus, the clinic would not be required to provide proactively the information to our case patient’s mother (regarding any STI issues) when she called.6

Contraception
Consent for contraception is more complicated. About half the states allow minors who have reached a certain age (12, 14, or 16 years) to consent to contraception. About 20 other states allow some minors to consent to contraceptive services, but the “allowed group” may be fairly narrow (eg, be married, have a health issue, or be “mature”). In 4 states there is currently no clear legal authority to provide contraceptive services to minors, yet those states do not specifically prohibit it. The US Supreme Court has held that a state cannot completely prohibit the availability of contraception to minors.7 The reach of that decision, however, is not clear and may not extend beyond what the states currently permit.
The ability of minors to consent to contraception services does not mean that there is a right to consent to all contraceptive options. As contraception becomes more irreversible, permanent, or risky, it is more problematic. For example, consent to sterilization would not ordinarily be within a minor’s recognized ability to consent. Standard, low risk, reversible contraception generally is covered by these state laws.8
In our case here, the patient likely was able to consent to contraception—initially to the oral contraception and later to the IUD. The risks and reversibility of both are probably within her ability to consent.9,10 Of course, if the care was provided in a state that does not include the patient within the groups that can give consent to contraception, it is possible that she might not have the legal authority to consent.
Continue to: General requirements of consent...
General requirements of consent
Even when adolescent consent is permitted for treatment, including in cases of contraception, it is essential that all of the legal and ethical requirements related to informed consent are met.
1. The adolescent has the capacity to consent. This means not only that the state-mandated requirements are met (age, for example) but also that the patient can and does understand the various elements of consent, and can make a sensible, informed decision.
The bottom line is “adolescent capacity is a complex process dependent upon the development of maturity of the adolescent, degree of intervention, expected benefit of the medical procedure, and the sociocultural context surrounding the decision.”11 Other items of interest include the “evolving capacity” of the child,12 which is the concept of increasing ability of the teen to process information and provide more appropriate informed consent. Central nervous system (CNS) maturation allows the adolescent to become increasingly more capable of decision making and has awareness of consequences of such decisions. Abstract thinking capabilities is a reflection of this CNS maturing process. If this competency is not established, the adolescent patient cannot give legitimate consent.
2. The patient must be given appropriate information (be “informed”). The discussion should include information relevant to the condition being treated (and the disease process if relevant). In addition, information about the treatment or intervention proposed and its risks and alternatives must be provided to the patient and in a way that is understandable.
3. As with all patients, consent must be voluntary and free of coercion or manipulation. These elements of informed consent are expanded on by the Joint Commission, which has established a number of components of informed consent (TABLE 2).4,13

Confidentiality and release of information to parents and others
Similar to consent, parents historically have had the authority to obtain medical information about their minor children. This right generally continues today, with some limitations. The right to give consent generally carries with it the right to medical information. There are some times when parents may access medical information even if they have not given consent.
This right adds complexity to minor consent and is an important treatment issue and legal consideration because confidentiality for adolescents affects quality of care. Adolescents report that “confidentiality is an important factor in their decision to seek [medical] care.”14 Many parents are under the assumption that the health care provider will automatically inform them independent of whether or not the adolescent expressed precise instruction not to inform.15,16
Of course if a minor patient authorizes the physician to provide information to her parents, that is consent and the health care provider may then provide the information. If the patient instructs the provider to convey the information, the practitioner would ordinarily be expected to be proactive in providing the information to the parent. The issue of “voluntariness” of the waiver of confidentiality can be a question, and the physician may discuss that question with the patient. Ordinarily, however, once a minor has authorized disclosure to the parent, the clinician has the authority to disclose the information to the parent, but not to others.
All of the usual considerations of confidentiality in health care apply to adolescent ObGyn services and care. This includes the general obligation not to disclose information without consent and to ensure that health care information is protected from accidental release as required by the Health Insurance Portability and Accountability Act (HIPAA) and other health information privacy laws.17
It is important to emphasize that the issues of consent to abortion are much different than those for contraception and sexually transmitted infections. As our case presentation does not deal with abortion, we will address this complex but important discussion in the future--as there are an estimated 90,000 abortions in adolescent girls annually.1
Given that abortion consent and notification laws are often complex, any physician providing abortion services to any minor should have sound legal advice on the requirements of the pertinent state law. In earlier publications of this section in OBG Management we have discussed the importance of practitioners having an ongoing relationship with a health law attorney. We make this point again, as this person can provide advice on consent and the rights of parents to have information about their minor children.
Reference
- Henshaw SK. U.S. teenage pregnancy statistics with comparative statistics for women age 20-24. New York, New York: Alan Guttmacher Institute; May 2003.
Continue to: How and when to protect minor confidentiality...
How and when to protect minor confidentiality
A clinician cannot assure minors of absolute confidentiality and should not agree to do so or imply that they are doing so.18 In our hypothetical case, when the patient told the physician that her parents were not to know of any of her treatment or communications, the provider should not have acquiesced by silence. He/she might have responded along these lines: “I have a strong commitment to confidentiality of your information, and we take many steps to protect that information. The law also allows some special protection of health care information. Despite the commitment to privacy, there are circumstances in which the law requires disclosure of information—and that might even be to parents. In addition, if you want any of your care covered by insurance, we would have to disclose that. While I expect that we can do as you ask about maintaining your confidentiality, no health care provider can absolutely guarantee it.”
Proactive vs reactive disclosure. There is “proactive” disclosure of information and “reactive” disclosure. Proactive is when the provider (without being asked) contacts a parent or others and provides information. Some states require proactive information about specific kinds of treatment (especially abortion services). For the most part, in states where a minor can legally consent to treatment, health care providers are not required to proactively disclose information.19
Clinicians may be required to respond to parental requests for information, which is reactive disclosure and is reflected in our case presentation. Even in such circumstances, however, the individual providing care may seek to avoid disclosure. In many states, the law would not require the release of this information (but would permit it if it is in the best interest of the patient). In addition, there are practical ways of avoiding the release of information. For example, the health care provider might acknowledge the interest and desire of the parent to have the information, but might humbly explain that in the experience of many clinicians protecting the confidentiality of patients is very important to successful treatment and it is the policy of the office/clinic not to breach the expectation of patient confidentiality except where that is clearly in the best interest of the patient or required by law.
In response to the likely question, “Well, isn’t that required by law?” the clinician can honestly reply, “I don’t know. There are many complex factors in the law regarding disclosure of medical information and as I am not an attorney I do not know how they all apply in this instance.” In some cases the parent may push the matter or take some kind of legal action. It is in this type of situation that an attorney familiar with health law and the clinician’s practice can be invaluable.
When parents are involved in the minor’s treatment (bringing the patient to the office/clinic, for example), there is an opportunity for an understanding, or agreement, among the patient, provider, and parent about what information the parent will receive. Ordinarily the agreement should not create the expectation of detailed information for the parent. Perhaps, for example, the physician will provide information only when he or she believes that doing so will be in the best interest of the patient. Even with parental agreement, complete confidentiality cannot be assured for minor patients. There may, for example, be another parent who will not feel bound by the established understanding, and the law requires some disclosures (in the case of child abuse or a court order).20
Continue to: Accidental disclosure...
Accidental disclosure. Health care providers also should make sure that office procedures do not unnecessarily or accidentally disclose information about patients. For example, routinely gathering information about insurance coverage may well trigger the release of information to the policy holder (often a parent). Thus, there should be clear understandings about billing, insurance, and related issues before information is divulged by the patient. This should be part of the process of obtaining informed consent to treatment. It should be up front and honest. Developing a clear understanding of the legal requirements of the state is essential, so that assurance of confidentiality is on legal, solid ground.
As the pediatric and adolescent segment of gynecologic care continues to evolve, it is noteworthy that the American Board of Obstetrics and Gynecology recently has established a "Focused Practice" designation in pediatric adolescent gynecology. This allows ObGyns to have an ongoing level of professional education in this specialized area. Additional information can be obtained at www.abog.org or [email protected].
More resources for adolescent contraceptive care include:
- The American College of Obstetricians and Gynecologists (ACOG) "Birth Control (Especially for Teens)" frequently asked questions information series (https://www.acog.org/Patients/FAQs/Birth-Control-Especially-for-Teens)
- ACOG's Adolescent Healthcare Committee Opinions address adolescent pregnancy, contraception, and sexual activity (https://www.acog.org/-/media/List-of-Titles/COListOfTitles.pdf)
- ACOG statement on teen pregnancy and contraception, April 7, 2015 (https://www.acog.org/About-ACOG/News-Room/Statements/2015/ACOG-Statement-on-Teen-Pregnancy-and-Contraception?IsMobileSet=false)
- North American Society for Pediatric and Adolescent Gynecology resources for patients (https://www.naspag.org/page/patienttools)
- Society for Adolescent Health and Medicine statement regarding contraceptive access policies (https://www.adolescenthealth.org)
- The Guttmacher Institute's overview of state laws relevant to minor consent, as of January 1, 2019 (https://www.guttmacher.org/state-policy/explore/overview-minors-consent-law). It is updated frequently.
Abuse reporting obligations
All states have mandatory child abuse reporting laws. These laws require medical professionals (and others) to report known, and often suspected, abuse of children. Abuse includes physical, sexual, or emotional, and generally also includes neglect that is harming a child. When there is apparent sexual or physical abuse, the health care provider is obligated to report it to designated state authorities, generally child protective services. Reporting laws vary from state to state based on the relationship between the suspected abuser and the minor, the nature of the harm, and how strong the suspicion of abuse needs to be. The failure to make required reports is a crime in most states and also may result in civil liability or licensure discipline. Criminal charges seldom result from the failure to report, but in some cases the failure to report may have serious consequences for the professional.
An ObGyn example of the complexity of reporting laws, and variation from state to state, is in the area of “statutory rape” reporting. Those state laws, which define serious criminal offenses, set out the age below which an individual is not legally capable of consenting to sexual activity. It varies among states, but may be an absolute age of consent, the age differential between the parties, or some combination of age and age differential.21 The question of reporting is further complicated by the issue of when statutory rape must be reported—for example, the circumstances when the harm to the underage person is sufficient to require reporting.22
Laws are complex, as is practice navigation
It is apparent that navigating these issues makes it essential for an ObGyn practice to have clear policies and practices regarding reporting, yet the overall complexity is also why it is so difficult to develop those policies in the first place. Of course, they must be tailored to the state in which the practice resides. Once again, the need is clear for health care professionals to have an ongoing relationship with a health attorney who can help navigate ongoing questions.
CASE Adolescent seeks care without parent
A 15-year-old patient (G0) presents to the gynecology clinic requesting birth control. She reports being sexually active over the past 6 months and having several male partners over the past 2 years. She and her current male partner use condoms inconsistently. She reports being active in school sports, and her academic performance has been noteworthy. Her peers have encouraged her to seek out birth control; one of her good friends recently became pregnant and dropped out of school. She states that her best friend went to a similar clinic and received a “gynecologic encounter” that included information regarding safe sex and contraception, with no pelvic exam required for her to receive birth control pills.
The patient insists that her parents are not to know of her request for contraception due to sexual activity or that she is a patient at the clinic. The gynecologist covering the clinic is aware of the American College of Obstetricians and Gynecologists Committee on Adolescent Health Care and their many publications. The patient is counseled regarding human papillomavirus (HPV) vaccination and screened for sexually transmitted infections. In addition, the gynecologist discusses contraceptive options with the patient, ranging from oral contraceptives, vaginal rings, subdermal implants, depomedroxyprogesterone acetate, as well as intrauterine devices (IUDs). The gynecologist emphasizes safe sex and advises that her partner consider use of condoms independent of her method of birth control. The patient asks for oral contraceptives and is given information about their use and risks, and she indicates that she understands.
A few months later the patient requests an IUD, as she would like to have lighter menses and not have to remember to take a pill every day. The provider obtains informed consent for the insertion procedure; the patient signs the appropriate forms.
The IUD is inserted, with difficulty, by a resident physician in the clinic. The patient experiences severe pelvic pain during and immediately following the insertion. She is sent home and told to contact the clinic or another health care provider or proceed to the local emergency department should pain persist or if fever develops.
The patient returns 72 hours later in pain. Pelvic ultrasonography shows the IUD out of place and at risk of perforating the fundus of the uterus. Later that day the patient’s mother calls the clinic, saying that she found a statement of service with the clinic’s number on it in her daughter’s bedroom. She wants to know if her daughter is there, what is going on, and what services have been or are being provided. In passing she remarks that she has no intention of paying (or allowing her insurance to pay for) any care that was provided.
What are the provider’s obligations at this point, both medically and legally?

Medical and legal considerations
One of the most difficult and important health law questions in adolescent medicine is the ability of minors to consent to treatment and to control the health care information resulting from treatment. (“Minor” describes a child or adolescent who has not obtained the age of legal consent, generally 18 years old, to lawfully enter into a legal transaction.)
Continue to: The consent of minor patients...
The consent of minor patients
The traditional legal rule is that parents or guardians (“parent” refers to both) must consent to medical treatment for minor children. There is an exception for emergency situations but generally minors do not provide consent for medical care, a parent does.1 The parent typically is obliged to provide payment (often through insurance) for those services.
This traditional rule has some exceptions—the emergency exception already noted and the case of emancipated minors, notably an adolescent who is living almost entirely independent of her parents (for example, she is married or not relying on parents in a meaningful way). In recent times there has been increasing authority for “mature minors” to make some medical decisions.2 A mature minor is one who has sufficient understanding and judgment to appreciate the consequences, benefits, and risks of accepting proposed medical intervention.
No circumstance involving adolescent treatment has been more contentious than services related to abortion and, to a lesser degree, contraception.3 Both the law of consent to services and the rights of parents to obtain information about contraceptive and abortion services have been a matter of strong, continuing debate. The law in these areas varies greatly from state-to-state, and includes a mix of state law (statutes and court decisions) with an overlay of federal constitutional law related to reproduction-related decisions of adolescents. In addition, the law in this area of consent and information changes relatively frequently.4 Clinicians, of course, must focus on the consent laws of the state in which they practice.
STI counseling and treatment
All states permit a minor patient to consent to treatment for an STI (TABLE 1).5 A number of states expressly permit, but do not require, health care providers to inform parents of treatment when a physician determines it would be in the best interest of the minor. Thus, the clinic would not be required to provide proactively the information to our case patient’s mother (regarding any STI issues) when she called.6

Contraception
Consent for contraception is more complicated. About half the states allow minors who have reached a certain age (12, 14, or 16 years) to consent to contraception. About 20 other states allow some minors to consent to contraceptive services, but the “allowed group” may be fairly narrow (eg, be married, have a health issue, or be “mature”). In 4 states there is currently no clear legal authority to provide contraceptive services to minors, yet those states do not specifically prohibit it. The US Supreme Court has held that a state cannot completely prohibit the availability of contraception to minors.7 The reach of that decision, however, is not clear and may not extend beyond what the states currently permit.
The ability of minors to consent to contraception services does not mean that there is a right to consent to all contraceptive options. As contraception becomes more irreversible, permanent, or risky, it is more problematic. For example, consent to sterilization would not ordinarily be within a minor’s recognized ability to consent. Standard, low risk, reversible contraception generally is covered by these state laws.8
In our case here, the patient likely was able to consent to contraception—initially to the oral contraception and later to the IUD. The risks and reversibility of both are probably within her ability to consent.9,10 Of course, if the care was provided in a state that does not include the patient within the groups that can give consent to contraception, it is possible that she might not have the legal authority to consent.
Continue to: General requirements of consent...
General requirements of consent
Even when adolescent consent is permitted for treatment, including in cases of contraception, it is essential that all of the legal and ethical requirements related to informed consent are met.
1. The adolescent has the capacity to consent. This means not only that the state-mandated requirements are met (age, for example) but also that the patient can and does understand the various elements of consent, and can make a sensible, informed decision.
The bottom line is “adolescent capacity is a complex process dependent upon the development of maturity of the adolescent, degree of intervention, expected benefit of the medical procedure, and the sociocultural context surrounding the decision.”11 Other items of interest include the “evolving capacity” of the child,12 which is the concept of increasing ability of the teen to process information and provide more appropriate informed consent. Central nervous system (CNS) maturation allows the adolescent to become increasingly more capable of decision making and has awareness of consequences of such decisions. Abstract thinking capabilities is a reflection of this CNS maturing process. If this competency is not established, the adolescent patient cannot give legitimate consent.
2. The patient must be given appropriate information (be “informed”). The discussion should include information relevant to the condition being treated (and the disease process if relevant). In addition, information about the treatment or intervention proposed and its risks and alternatives must be provided to the patient and in a way that is understandable.
3. As with all patients, consent must be voluntary and free of coercion or manipulation. These elements of informed consent are expanded on by the Joint Commission, which has established a number of components of informed consent (TABLE 2).4,13

Confidentiality and release of information to parents and others
Similar to consent, parents historically have had the authority to obtain medical information about their minor children. This right generally continues today, with some limitations. The right to give consent generally carries with it the right to medical information. There are some times when parents may access medical information even if they have not given consent.
This right adds complexity to minor consent and is an important treatment issue and legal consideration because confidentiality for adolescents affects quality of care. Adolescents report that “confidentiality is an important factor in their decision to seek [medical] care.”14 Many parents are under the assumption that the health care provider will automatically inform them independent of whether or not the adolescent expressed precise instruction not to inform.15,16
Of course if a minor patient authorizes the physician to provide information to her parents, that is consent and the health care provider may then provide the information. If the patient instructs the provider to convey the information, the practitioner would ordinarily be expected to be proactive in providing the information to the parent. The issue of “voluntariness” of the waiver of confidentiality can be a question, and the physician may discuss that question with the patient. Ordinarily, however, once a minor has authorized disclosure to the parent, the clinician has the authority to disclose the information to the parent, but not to others.
All of the usual considerations of confidentiality in health care apply to adolescent ObGyn services and care. This includes the general obligation not to disclose information without consent and to ensure that health care information is protected from accidental release as required by the Health Insurance Portability and Accountability Act (HIPAA) and other health information privacy laws.17
It is important to emphasize that the issues of consent to abortion are much different than those for contraception and sexually transmitted infections. As our case presentation does not deal with abortion, we will address this complex but important discussion in the future--as there are an estimated 90,000 abortions in adolescent girls annually.1
Given that abortion consent and notification laws are often complex, any physician providing abortion services to any minor should have sound legal advice on the requirements of the pertinent state law. In earlier publications of this section in OBG Management we have discussed the importance of practitioners having an ongoing relationship with a health law attorney. We make this point again, as this person can provide advice on consent and the rights of parents to have information about their minor children.
Reference
- Henshaw SK. U.S. teenage pregnancy statistics with comparative statistics for women age 20-24. New York, New York: Alan Guttmacher Institute; May 2003.
Continue to: How and when to protect minor confidentiality...
How and when to protect minor confidentiality
A clinician cannot assure minors of absolute confidentiality and should not agree to do so or imply that they are doing so.18 In our hypothetical case, when the patient told the physician that her parents were not to know of any of her treatment or communications, the provider should not have acquiesced by silence. He/she might have responded along these lines: “I have a strong commitment to confidentiality of your information, and we take many steps to protect that information. The law also allows some special protection of health care information. Despite the commitment to privacy, there are circumstances in which the law requires disclosure of information—and that might even be to parents. In addition, if you want any of your care covered by insurance, we would have to disclose that. While I expect that we can do as you ask about maintaining your confidentiality, no health care provider can absolutely guarantee it.”
Proactive vs reactive disclosure. There is “proactive” disclosure of information and “reactive” disclosure. Proactive is when the provider (without being asked) contacts a parent or others and provides information. Some states require proactive information about specific kinds of treatment (especially abortion services). For the most part, in states where a minor can legally consent to treatment, health care providers are not required to proactively disclose information.19
Clinicians may be required to respond to parental requests for information, which is reactive disclosure and is reflected in our case presentation. Even in such circumstances, however, the individual providing care may seek to avoid disclosure. In many states, the law would not require the release of this information (but would permit it if it is in the best interest of the patient). In addition, there are practical ways of avoiding the release of information. For example, the health care provider might acknowledge the interest and desire of the parent to have the information, but might humbly explain that in the experience of many clinicians protecting the confidentiality of patients is very important to successful treatment and it is the policy of the office/clinic not to breach the expectation of patient confidentiality except where that is clearly in the best interest of the patient or required by law.
In response to the likely question, “Well, isn’t that required by law?” the clinician can honestly reply, “I don’t know. There are many complex factors in the law regarding disclosure of medical information and as I am not an attorney I do not know how they all apply in this instance.” In some cases the parent may push the matter or take some kind of legal action. It is in this type of situation that an attorney familiar with health law and the clinician’s practice can be invaluable.
When parents are involved in the minor’s treatment (bringing the patient to the office/clinic, for example), there is an opportunity for an understanding, or agreement, among the patient, provider, and parent about what information the parent will receive. Ordinarily the agreement should not create the expectation of detailed information for the parent. Perhaps, for example, the physician will provide information only when he or she believes that doing so will be in the best interest of the patient. Even with parental agreement, complete confidentiality cannot be assured for minor patients. There may, for example, be another parent who will not feel bound by the established understanding, and the law requires some disclosures (in the case of child abuse or a court order).20
Continue to: Accidental disclosure...
Accidental disclosure. Health care providers also should make sure that office procedures do not unnecessarily or accidentally disclose information about patients. For example, routinely gathering information about insurance coverage may well trigger the release of information to the policy holder (often a parent). Thus, there should be clear understandings about billing, insurance, and related issues before information is divulged by the patient. This should be part of the process of obtaining informed consent to treatment. It should be up front and honest. Developing a clear understanding of the legal requirements of the state is essential, so that assurance of confidentiality is on legal, solid ground.
As the pediatric and adolescent segment of gynecologic care continues to evolve, it is noteworthy that the American Board of Obstetrics and Gynecology recently has established a "Focused Practice" designation in pediatric adolescent gynecology. This allows ObGyns to have an ongoing level of professional education in this specialized area. Additional information can be obtained at www.abog.org or [email protected].
More resources for adolescent contraceptive care include:
- The American College of Obstetricians and Gynecologists (ACOG) "Birth Control (Especially for Teens)" frequently asked questions information series (https://www.acog.org/Patients/FAQs/Birth-Control-Especially-for-Teens)
- ACOG's Adolescent Healthcare Committee Opinions address adolescent pregnancy, contraception, and sexual activity (https://www.acog.org/-/media/List-of-Titles/COListOfTitles.pdf)
- ACOG statement on teen pregnancy and contraception, April 7, 2015 (https://www.acog.org/About-ACOG/News-Room/Statements/2015/ACOG-Statement-on-Teen-Pregnancy-and-Contraception?IsMobileSet=false)
- North American Society for Pediatric and Adolescent Gynecology resources for patients (https://www.naspag.org/page/patienttools)
- Society for Adolescent Health and Medicine statement regarding contraceptive access policies (https://www.adolescenthealth.org)
- The Guttmacher Institute's overview of state laws relevant to minor consent, as of January 1, 2019 (https://www.guttmacher.org/state-policy/explore/overview-minors-consent-law). It is updated frequently.
Abuse reporting obligations
All states have mandatory child abuse reporting laws. These laws require medical professionals (and others) to report known, and often suspected, abuse of children. Abuse includes physical, sexual, or emotional, and generally also includes neglect that is harming a child. When there is apparent sexual or physical abuse, the health care provider is obligated to report it to designated state authorities, generally child protective services. Reporting laws vary from state to state based on the relationship between the suspected abuser and the minor, the nature of the harm, and how strong the suspicion of abuse needs to be. The failure to make required reports is a crime in most states and also may result in civil liability or licensure discipline. Criminal charges seldom result from the failure to report, but in some cases the failure to report may have serious consequences for the professional.
An ObGyn example of the complexity of reporting laws, and variation from state to state, is in the area of “statutory rape” reporting. Those state laws, which define serious criminal offenses, set out the age below which an individual is not legally capable of consenting to sexual activity. It varies among states, but may be an absolute age of consent, the age differential between the parties, or some combination of age and age differential.21 The question of reporting is further complicated by the issue of when statutory rape must be reported—for example, the circumstances when the harm to the underage person is sufficient to require reporting.22
Laws are complex, as is practice navigation
It is apparent that navigating these issues makes it essential for an ObGyn practice to have clear policies and practices regarding reporting, yet the overall complexity is also why it is so difficult to develop those policies in the first place. Of course, they must be tailored to the state in which the practice resides. Once again, the need is clear for health care professionals to have an ongoing relationship with a health attorney who can help navigate ongoing questions.
- Benjamin L, Ishimine P, Joseph M, et al. Evaluation and treatment of minors. Ann Emerg Med. 2018;71(2):225-232.
- Coleman D, Rosoff P. The legal authority of mature minors to consent to general medical treatment. Pediatrics. 2013;13:786-793.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 699. Adolescent pregnancy, contraception, and sexual activity. Obstet Gynecol. 2017;129:e142-e149.
- Tillett J. Adolescents and informed consent. J Perinat Neonat Nurs. 2005;19:112-121.
- An overview of minor's consent law. Guttmacher Institute's website. https://www.guttmacher.org/state-policy/explore/overview-minors-consent-law. Accessed February 14, 2019.
- Chelmow D, Karjane N, Ricciotti HA, et al, eds. A 16-year-old adolescent requesting confidential treatment for chlamydia exposure (understanding state laws regarding minors and resources). Office Gynecology: A Case-Based Approach. Cambridge, United Kingdom: Cambridge University Press; January 31, 2019:39.
- Carey v Population Services, 431 US 678 (1977).
- Williams RL, Meredith AH, Ott MA. Expanding adolescent access to hormonal contraception: an update on over-the-counter, pharmacist prescribing, and web-based telehealth approaches. Curr Opin Obstet Gynecol. 2018;30:458-464.
- McClellan K, Temples H, Miller L. The latest in teen pregnancy prevention: long-acting reversible contraception. J Pediatr Health Care. 2018;32:e91-e97.
- Behmer Hansen RT, Arora KS. Consenting to invasive contraceptives: an ethical analysis of adolescent decision-making authority for long-acting reversible contraception. J Med Ethics. 2018;44:585-588.
- Robertson D. Opinions in pediatric and adolescent gynecology. J Pediatr Adolesc Gynecol. 2008:21:47-51.
- Lansdown G. The evolving capacities of the child. Florence, Italy: UNICEF Innocenti Research Centre, Innocenti Insight; 2005. https://www.unicef-irc.org/publications/384-the-evolving-capacities-of-the-child.html. Accessed February 15, 2019.
- Clapp JT, Fleisher LA. What is the realistic scope of informed consent? Jt Comm J Qual Patient Saf. 2018;44(6):341-342.
- Berlan E, Bravender T. Confidentiality, consent and caring for the adolescent. Curr Opin Pediatr. 2009;21:450-456.
- Schantz K. Who Needs to Know? Confidentiality in Adolescent Sexual Health Care. Act for Youth website. http://www.actforyouth.net/resources/rf/rf_confidentiality_1118.pdf. Accessed February 14, 2019.
- Lynn A, Kodish E, Lazebnik R, et al. Understanding confidentiality: perspectives of African American adolescents and their parents. J Adolesc Health. 2006;39:261-265.
- English A, Ford CA. The HIPAA privacy rule and adolescents: legal questions and clinical challenges. Perspect Sex Reprod Health. 2004;36:80-86.
- Schapiro NA, Mejia J. Adolescent confidentiality and women's health: history, rationale, and current threats. Nurs Clin North Am. 2018;53:145-156.
- Scott NL, Alderman EM, 2018. Case of a girl with a secret. In: Adolescent Gynecology: A Clinical Casebook. New York, New York: Springer International; 2017:3-11.
- Cullitan CM. Please don't tell my mom--a minor's right to informational privacy. JL & Educ. 2011;40:417-460.
- Bierie DM, Budd KM. Romeo, Juliet, and statutory rape. Sex Abuse. 2018;30:296-321.
- Mathews B. A taxonomy of duties to report child sexual abuse: legal developments offer new ways to facilitate disclosure. Child Abuse Negl. 2019;88:337-347.
- Benjamin L, Ishimine P, Joseph M, et al. Evaluation and treatment of minors. Ann Emerg Med. 2018;71(2):225-232.
- Coleman D, Rosoff P. The legal authority of mature minors to consent to general medical treatment. Pediatrics. 2013;13:786-793.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 699. Adolescent pregnancy, contraception, and sexual activity. Obstet Gynecol. 2017;129:e142-e149.
- Tillett J. Adolescents and informed consent. J Perinat Neonat Nurs. 2005;19:112-121.
- An overview of minor's consent law. Guttmacher Institute's website. https://www.guttmacher.org/state-policy/explore/overview-minors-consent-law. Accessed February 14, 2019.
- Chelmow D, Karjane N, Ricciotti HA, et al, eds. A 16-year-old adolescent requesting confidential treatment for chlamydia exposure (understanding state laws regarding minors and resources). Office Gynecology: A Case-Based Approach. Cambridge, United Kingdom: Cambridge University Press; January 31, 2019:39.
- Carey v Population Services, 431 US 678 (1977).
- Williams RL, Meredith AH, Ott MA. Expanding adolescent access to hormonal contraception: an update on over-the-counter, pharmacist prescribing, and web-based telehealth approaches. Curr Opin Obstet Gynecol. 2018;30:458-464.
- McClellan K, Temples H, Miller L. The latest in teen pregnancy prevention: long-acting reversible contraception. J Pediatr Health Care. 2018;32:e91-e97.
- Behmer Hansen RT, Arora KS. Consenting to invasive contraceptives: an ethical analysis of adolescent decision-making authority for long-acting reversible contraception. J Med Ethics. 2018;44:585-588.
- Robertson D. Opinions in pediatric and adolescent gynecology. J Pediatr Adolesc Gynecol. 2008:21:47-51.
- Lansdown G. The evolving capacities of the child. Florence, Italy: UNICEF Innocenti Research Centre, Innocenti Insight; 2005. https://www.unicef-irc.org/publications/384-the-evolving-capacities-of-the-child.html. Accessed February 15, 2019.
- Clapp JT, Fleisher LA. What is the realistic scope of informed consent? Jt Comm J Qual Patient Saf. 2018;44(6):341-342.
- Berlan E, Bravender T. Confidentiality, consent and caring for the adolescent. Curr Opin Pediatr. 2009;21:450-456.
- Schantz K. Who Needs to Know? Confidentiality in Adolescent Sexual Health Care. Act for Youth website. http://www.actforyouth.net/resources/rf/rf_confidentiality_1118.pdf. Accessed February 14, 2019.
- Lynn A, Kodish E, Lazebnik R, et al. Understanding confidentiality: perspectives of African American adolescents and their parents. J Adolesc Health. 2006;39:261-265.
- English A, Ford CA. The HIPAA privacy rule and adolescents: legal questions and clinical challenges. Perspect Sex Reprod Health. 2004;36:80-86.
- Schapiro NA, Mejia J. Adolescent confidentiality and women's health: history, rationale, and current threats. Nurs Clin North Am. 2018;53:145-156.
- Scott NL, Alderman EM, 2018. Case of a girl with a secret. In: Adolescent Gynecology: A Clinical Casebook. New York, New York: Springer International; 2017:3-11.
- Cullitan CM. Please don't tell my mom--a minor's right to informational privacy. JL & Educ. 2011;40:417-460.
- Bierie DM, Budd KM. Romeo, Juliet, and statutory rape. Sex Abuse. 2018;30:296-321.
- Mathews B. A taxonomy of duties to report child sexual abuse: legal developments offer new ways to facilitate disclosure. Child Abuse Negl. 2019;88:337-347.
Two free contraception apps for providers of family planning
Evidence-based research and guidelines regarding contraception are continually changing. Health care providers often have difficulty memorizing and staying up-to-date on all the important developments around family planning. Those who provide contraceptive counseling may not all use guidelines to inform their choices, and some may have misperceptions about patient eligibility for certain methods.1,2 Mobile health applications (apps) that present this information in an easily accessible fashion have the potential to improve family planning services.
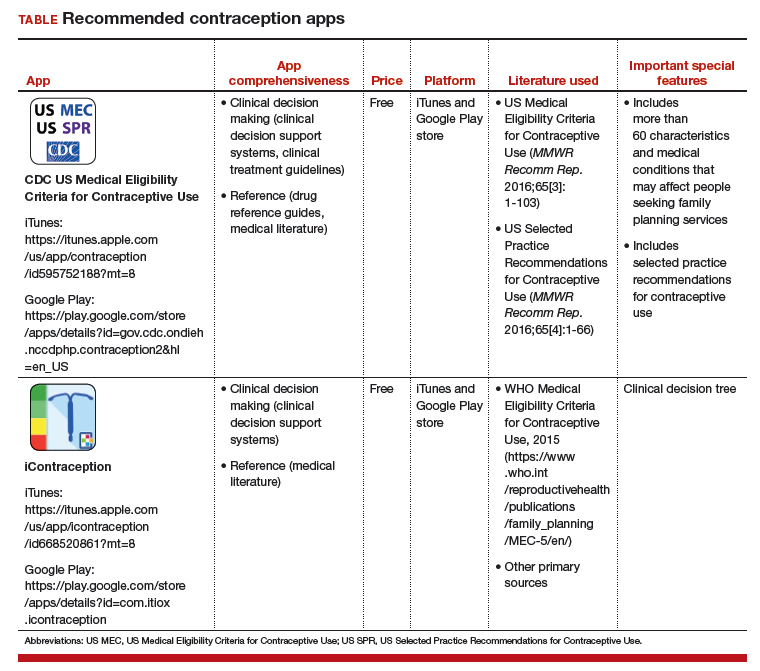
In a search for contraception apps, Dr. Rachel Perry and colleagues identified two contraception apps that were evaluated highly: 1) the Centers for Disease Control and Prevention (CDC) US Medical Eligibility Criteria for Contraceptive Use (MEC) app and 2) the iContraception app.3
Two free contraception apps for clinician use. Both the CDC Contraception and iContraception apps are based on CDC MEC information and provide guidance on contraceptive initiation and maintenance.4 Notably, the American College of Obstetricians and Gynecologists (ACOG) endorses the use of the CDC MEC.5
These two apps can aid physicians in prescribing appropriate and safe contraceptive methods and can help them tailor the extensive CDC MEC guidelines for an individual patient. Additionally, the iContraception app allows a user to input multiple clinical and demographic characteristics to determine an individual patient’s eligibility for a specific contraceptive method (that is, it incorporates a clinical decision tree).
The recommended contraception apps are listed in the TABLE and are detailed with a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature used, and important special features).6 I hope that the apps described here will assist you in managing patients who need contraception counseling.
- Russo JA, Chen BA, Creinin MD. Primary care physician familiarity with US medical eligibility for contraceptive use. Fam Med. 2015;47:15-21.
- Dehlendorf C, Levy K, Ruskin R, et al. Health care providers' knowledge about contraceptive evidence: a barrier to quality family planning care? Contraception. 2010;81:292-298.
- Perry R, Lunde B, Chen KT. An evaluation of contraception mobile applications for providers of family planning services. Contraception. 2016;93:539-544.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65(3):1-103.
- American College of Obstetricians and Gynecologists. Committee opinion no. 505: understanding and using the US Medical Eligibility Criteria for Contraceptive Use. Obstet Gynecol. 2011;118:754-760.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
Evidence-based research and guidelines regarding contraception are continually changing. Health care providers often have difficulty memorizing and staying up-to-date on all the important developments around family planning. Those who provide contraceptive counseling may not all use guidelines to inform their choices, and some may have misperceptions about patient eligibility for certain methods.1,2 Mobile health applications (apps) that present this information in an easily accessible fashion have the potential to improve family planning services.
In a search for contraception apps, Dr. Rachel Perry and colleagues identified two contraception apps that were evaluated highly: 1) the Centers for Disease Control and Prevention (CDC) US Medical Eligibility Criteria for Contraceptive Use (MEC) app and 2) the iContraception app.3
Two free contraception apps for clinician use. Both the CDC Contraception and iContraception apps are based on CDC MEC information and provide guidance on contraceptive initiation and maintenance.4 Notably, the American College of Obstetricians and Gynecologists (ACOG) endorses the use of the CDC MEC.5
These two apps can aid physicians in prescribing appropriate and safe contraceptive methods and can help them tailor the extensive CDC MEC guidelines for an individual patient. Additionally, the iContraception app allows a user to input multiple clinical and demographic characteristics to determine an individual patient’s eligibility for a specific contraceptive method (that is, it incorporates a clinical decision tree).
The recommended contraception apps are listed in the TABLE and are detailed with a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature used, and important special features).6 I hope that the apps described here will assist you in managing patients who need contraception counseling.

Evidence-based research and guidelines regarding contraception are continually changing. Health care providers often have difficulty memorizing and staying up-to-date on all the important developments around family planning. Those who provide contraceptive counseling may not all use guidelines to inform their choices, and some may have misperceptions about patient eligibility for certain methods.1,2 Mobile health applications (apps) that present this information in an easily accessible fashion have the potential to improve family planning services.
In a search for contraception apps, Dr. Rachel Perry and colleagues identified two contraception apps that were evaluated highly: 1) the Centers for Disease Control and Prevention (CDC) US Medical Eligibility Criteria for Contraceptive Use (MEC) app and 2) the iContraception app.3
Two free contraception apps for clinician use. Both the CDC Contraception and iContraception apps are based on CDC MEC information and provide guidance on contraceptive initiation and maintenance.4 Notably, the American College of Obstetricians and Gynecologists (ACOG) endorses the use of the CDC MEC.5
These two apps can aid physicians in prescribing appropriate and safe contraceptive methods and can help them tailor the extensive CDC MEC guidelines for an individual patient. Additionally, the iContraception app allows a user to input multiple clinical and demographic characteristics to determine an individual patient’s eligibility for a specific contraceptive method (that is, it incorporates a clinical decision tree).
The recommended contraception apps are listed in the TABLE and are detailed with a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature used, and important special features).6 I hope that the apps described here will assist you in managing patients who need contraception counseling.

- Russo JA, Chen BA, Creinin MD. Primary care physician familiarity with US medical eligibility for contraceptive use. Fam Med. 2015;47:15-21.
- Dehlendorf C, Levy K, Ruskin R, et al. Health care providers' knowledge about contraceptive evidence: a barrier to quality family planning care? Contraception. 2010;81:292-298.
- Perry R, Lunde B, Chen KT. An evaluation of contraception mobile applications for providers of family planning services. Contraception. 2016;93:539-544.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65(3):1-103.
- American College of Obstetricians and Gynecologists. Committee opinion no. 505: understanding and using the US Medical Eligibility Criteria for Contraceptive Use. Obstet Gynecol. 2011;118:754-760.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
- Russo JA, Chen BA, Creinin MD. Primary care physician familiarity with US medical eligibility for contraceptive use. Fam Med. 2015;47:15-21.
- Dehlendorf C, Levy K, Ruskin R, et al. Health care providers' knowledge about contraceptive evidence: a barrier to quality family planning care? Contraception. 2010;81:292-298.
- Perry R, Lunde B, Chen KT. An evaluation of contraception mobile applications for providers of family planning services. Contraception. 2016;93:539-544.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65(3):1-103.
- American College of Obstetricians and Gynecologists. Committee opinion no. 505: understanding and using the US Medical Eligibility Criteria for Contraceptive Use. Obstet Gynecol. 2011;118:754-760.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
Supreme Court halts Louisiana abortion law from taking effect
The U.S. Supreme Court has temporarily barred a Louisiana law that would require stricter requirements for physicians who provide abortion care, the first abortion-related decision for the current conservative-leaning high court.
In a Feb. 7, 2019, order, Supreme Court justices stopped the law from moving forward until they can decide whether to accept the case for oral argument. The law in question would require Louisiana physicians who perform abortions to have admitting privileges at a hospital within 30 miles of the clinic where they offer abortion care.
A group of health professionals sued over the Louisiana statute after it was enacted in 2014, arguing that the requirement was unconstitutional because it placed an undue burden on women seeking abortions. A federal court agreed, concluding that the law would leave a significant number of Louisiana women unable to get an abortion. The state appealed to the 5th U.S. Circuit Court of Appeals, which reversed the decision in January 2019. The physician plaintiffs then urged the Supreme Court to stop the law, scheduled to take effect on Feb. 4 while the case continued through the courts. The health professionals argue that no physicians in Louisiana would be available to perform abortions after 17 weeks of pregnancy if the law proceeds and that only one physician in the state would be available to provide an abortion in the earlier stages of pregnancy. Attorneys for the state countered that health providers are overestimating the law’s effect and requested that the measure be allowed to go forward.
In a Feb. 1 order, the Supreme Court provided the plaintiffs a short stay while they reviewed briefs in the case. Then, in a 5-4 decision on Feb. 7, the majority court halted the legal challenge indefinitely until they can decide whether to take up the case.
Four justices – Clarence Thomas, Samuel Alito, Neil Gorsuch, and Brett Kavanaugh – dissented from the majority, writing that they would have allowed Louisiana to enforce the law. Chief Justice John Roberts joined the high court’s four more liberal justices in stopping the law’s enactment.
The plaintiffs’ petition to the Supreme Court is due in April. If the case is accepted, oral arguments would likely be scheduled for fall 2019 or winter 2020, according to court analysts.
The U.S. Supreme Court has temporarily barred a Louisiana law that would require stricter requirements for physicians who provide abortion care, the first abortion-related decision for the current conservative-leaning high court.
In a Feb. 7, 2019, order, Supreme Court justices stopped the law from moving forward until they can decide whether to accept the case for oral argument. The law in question would require Louisiana physicians who perform abortions to have admitting privileges at a hospital within 30 miles of the clinic where they offer abortion care.
A group of health professionals sued over the Louisiana statute after it was enacted in 2014, arguing that the requirement was unconstitutional because it placed an undue burden on women seeking abortions. A federal court agreed, concluding that the law would leave a significant number of Louisiana women unable to get an abortion. The state appealed to the 5th U.S. Circuit Court of Appeals, which reversed the decision in January 2019. The physician plaintiffs then urged the Supreme Court to stop the law, scheduled to take effect on Feb. 4 while the case continued through the courts. The health professionals argue that no physicians in Louisiana would be available to perform abortions after 17 weeks of pregnancy if the law proceeds and that only one physician in the state would be available to provide an abortion in the earlier stages of pregnancy. Attorneys for the state countered that health providers are overestimating the law’s effect and requested that the measure be allowed to go forward.
In a Feb. 1 order, the Supreme Court provided the plaintiffs a short stay while they reviewed briefs in the case. Then, in a 5-4 decision on Feb. 7, the majority court halted the legal challenge indefinitely until they can decide whether to take up the case.
Four justices – Clarence Thomas, Samuel Alito, Neil Gorsuch, and Brett Kavanaugh – dissented from the majority, writing that they would have allowed Louisiana to enforce the law. Chief Justice John Roberts joined the high court’s four more liberal justices in stopping the law’s enactment.
The plaintiffs’ petition to the Supreme Court is due in April. If the case is accepted, oral arguments would likely be scheduled for fall 2019 or winter 2020, according to court analysts.
The U.S. Supreme Court has temporarily barred a Louisiana law that would require stricter requirements for physicians who provide abortion care, the first abortion-related decision for the current conservative-leaning high court.
In a Feb. 7, 2019, order, Supreme Court justices stopped the law from moving forward until they can decide whether to accept the case for oral argument. The law in question would require Louisiana physicians who perform abortions to have admitting privileges at a hospital within 30 miles of the clinic where they offer abortion care.
A group of health professionals sued over the Louisiana statute after it was enacted in 2014, arguing that the requirement was unconstitutional because it placed an undue burden on women seeking abortions. A federal court agreed, concluding that the law would leave a significant number of Louisiana women unable to get an abortion. The state appealed to the 5th U.S. Circuit Court of Appeals, which reversed the decision in January 2019. The physician plaintiffs then urged the Supreme Court to stop the law, scheduled to take effect on Feb. 4 while the case continued through the courts. The health professionals argue that no physicians in Louisiana would be available to perform abortions after 17 weeks of pregnancy if the law proceeds and that only one physician in the state would be available to provide an abortion in the earlier stages of pregnancy. Attorneys for the state countered that health providers are overestimating the law’s effect and requested that the measure be allowed to go forward.
In a Feb. 1 order, the Supreme Court provided the plaintiffs a short stay while they reviewed briefs in the case. Then, in a 5-4 decision on Feb. 7, the majority court halted the legal challenge indefinitely until they can decide whether to take up the case.
Four justices – Clarence Thomas, Samuel Alito, Neil Gorsuch, and Brett Kavanaugh – dissented from the majority, writing that they would have allowed Louisiana to enforce the law. Chief Justice John Roberts joined the high court’s four more liberal justices in stopping the law’s enactment.
The plaintiffs’ petition to the Supreme Court is due in April. If the case is accepted, oral arguments would likely be scheduled for fall 2019 or winter 2020, according to court analysts.
Product Update: Bijuva; Liletta; Aegea Vapor System; Natural Cycles
TREATMENT FOR VASOMOTOR SYMPTOMS
BIJUVA™ has been US Food and Drug Administration (FDA)–approved as the first oral treatment for moderate-to-severe vasomotor symptoms due to menopause in women with a uterus. BIJUVA offers a combination of bioidentical estradiol to reduce moderate-to-severe hot flashes and bioidentical progesterone to reduce the risk for endometrial hyperplasia.
TherapeuticsMD says that BIJUVA will be available Spring 2019 and is a proven treatment option for women who are experiencing bothersome symptoms of menopause, with clinical trial data demonstrating a statistically significant reduction in both the frequency and severity of moderate-to-severe vasomotor symptoms. The manufacturer also says that BIJUVA is developed to be identical in molecular structure to the hormones already produced by the body and is designed to help women restore what is lost during menopause.
BIJUVA estradiol and progesterone combination (1 mg/100 mg) will be available in capsule form.
FOR MORE INFORMATION, VISIT:https://bijuva.com/discover/
LILETTA USE EXTENDED
The FDA has approved LILETTA® (levonorgestrel-releasing intrauterine system) 52 mg for 5-year use. This approval is based on efficacy and safety data from ACCESS IUS, the largest ongoing intrauterine device (IUD) Phase 3 clinical trial in the United States. Previously, LILETTA was indicated for use up to 4 years.
LILETTA continues to be greater than 99% effective in preventing pregnancy in a broad range of women, regardless of age, race, body mass index, or parity, according to Allergan and Medicines360. The extended duration and proven efficacy across a diverse population enables more women in the United States to obtain effective birth control, as the IUD is now available for a low cost at public health clinics.
FOR MORE INFORMATION, VISIT: https://www.liletta.com
Continue to: ENDOMETRIAL ABLATION TECHNOLOGY
ENDOMETRIAL ABLATION TECHNOLOGY
AEGEA Medical introduces the AEGEA Vapor SystemTM, an innovative solution for endometrial ablation to treat menorrhagia.
The system uses Adaptive Vapor Ablation and is the first endometrial ablation system specifically designed for use in the doctor’s office, allowing minimal anesthesia/analgesia and rapid recovery, says AEGEA Medical.
AEGEA Medical describes the AEGEA Vapor System as a fully automated safety monitoring and vapor delivery system that uses a slender, flexible Vapor Probe with SmartSealTM technology and the Integrity ProTM safety feature, for an added level of confidence. The 4-minute procedure time includes 2 minutes of vapor treatment and can be performed in patients with a wider range of uterine anatomies than indicated for use with currently available treatments, says AEGEA Medical.
FOR MORE INFORMATION, VISIT: http://aegeamedical.com/
NATURAL CYCLES
The FDA has cleared Natural Cycles as the first digital method of birth control in the United States. Delivered in the form of an app, Natural Cycles is a fertility awareness–based contraceptive that uses a sophisticated algorithm to accurately and conveniently determine a woman’s daily fertility based on basal body temperature.
That data builds into a personalized fertility indicator that informs her when she needs to use protection to minimize the chance of conception. The app also can be used to help plan a pregnancy when the time is right, according to Natural Cycles.
A clinical study showed that the efficacy of a contraceptive mobile application is higher than usually reported for traditional fertility awareness–based methods. The application may contribute to reducing the unmet need for contraception, says Natural Cycles.
FOR MORE INFORMATION, VISIT: https://www.naturalcycles.com/en/hcp
TREATMENT FOR VASOMOTOR SYMPTOMS
BIJUVA™ has been US Food and Drug Administration (FDA)–approved as the first oral treatment for moderate-to-severe vasomotor symptoms due to menopause in women with a uterus. BIJUVA offers a combination of bioidentical estradiol to reduce moderate-to-severe hot flashes and bioidentical progesterone to reduce the risk for endometrial hyperplasia.
TherapeuticsMD says that BIJUVA will be available Spring 2019 and is a proven treatment option for women who are experiencing bothersome symptoms of menopause, with clinical trial data demonstrating a statistically significant reduction in both the frequency and severity of moderate-to-severe vasomotor symptoms. The manufacturer also says that BIJUVA is developed to be identical in molecular structure to the hormones already produced by the body and is designed to help women restore what is lost during menopause.
BIJUVA estradiol and progesterone combination (1 mg/100 mg) will be available in capsule form.
FOR MORE INFORMATION, VISIT:https://bijuva.com/discover/
LILETTA USE EXTENDED
The FDA has approved LILETTA® (levonorgestrel-releasing intrauterine system) 52 mg for 5-year use. This approval is based on efficacy and safety data from ACCESS IUS, the largest ongoing intrauterine device (IUD) Phase 3 clinical trial in the United States. Previously, LILETTA was indicated for use up to 4 years.
LILETTA continues to be greater than 99% effective in preventing pregnancy in a broad range of women, regardless of age, race, body mass index, or parity, according to Allergan and Medicines360. The extended duration and proven efficacy across a diverse population enables more women in the United States to obtain effective birth control, as the IUD is now available for a low cost at public health clinics.
FOR MORE INFORMATION, VISIT: https://www.liletta.com
Continue to: ENDOMETRIAL ABLATION TECHNOLOGY
ENDOMETRIAL ABLATION TECHNOLOGY
AEGEA Medical introduces the AEGEA Vapor SystemTM, an innovative solution for endometrial ablation to treat menorrhagia.
The system uses Adaptive Vapor Ablation and is the first endometrial ablation system specifically designed for use in the doctor’s office, allowing minimal anesthesia/analgesia and rapid recovery, says AEGEA Medical.
AEGEA Medical describes the AEGEA Vapor System as a fully automated safety monitoring and vapor delivery system that uses a slender, flexible Vapor Probe with SmartSealTM technology and the Integrity ProTM safety feature, for an added level of confidence. The 4-minute procedure time includes 2 minutes of vapor treatment and can be performed in patients with a wider range of uterine anatomies than indicated for use with currently available treatments, says AEGEA Medical.
FOR MORE INFORMATION, VISIT: http://aegeamedical.com/
NATURAL CYCLES
The FDA has cleared Natural Cycles as the first digital method of birth control in the United States. Delivered in the form of an app, Natural Cycles is a fertility awareness–based contraceptive that uses a sophisticated algorithm to accurately and conveniently determine a woman’s daily fertility based on basal body temperature.
That data builds into a personalized fertility indicator that informs her when she needs to use protection to minimize the chance of conception. The app also can be used to help plan a pregnancy when the time is right, according to Natural Cycles.
A clinical study showed that the efficacy of a contraceptive mobile application is higher than usually reported for traditional fertility awareness–based methods. The application may contribute to reducing the unmet need for contraception, says Natural Cycles.
FOR MORE INFORMATION, VISIT: https://www.naturalcycles.com/en/hcp
TREATMENT FOR VASOMOTOR SYMPTOMS
BIJUVA™ has been US Food and Drug Administration (FDA)–approved as the first oral treatment for moderate-to-severe vasomotor symptoms due to menopause in women with a uterus. BIJUVA offers a combination of bioidentical estradiol to reduce moderate-to-severe hot flashes and bioidentical progesterone to reduce the risk for endometrial hyperplasia.
TherapeuticsMD says that BIJUVA will be available Spring 2019 and is a proven treatment option for women who are experiencing bothersome symptoms of menopause, with clinical trial data demonstrating a statistically significant reduction in both the frequency and severity of moderate-to-severe vasomotor symptoms. The manufacturer also says that BIJUVA is developed to be identical in molecular structure to the hormones already produced by the body and is designed to help women restore what is lost during menopause.
BIJUVA estradiol and progesterone combination (1 mg/100 mg) will be available in capsule form.
FOR MORE INFORMATION, VISIT:https://bijuva.com/discover/
LILETTA USE EXTENDED
The FDA has approved LILETTA® (levonorgestrel-releasing intrauterine system) 52 mg for 5-year use. This approval is based on efficacy and safety data from ACCESS IUS, the largest ongoing intrauterine device (IUD) Phase 3 clinical trial in the United States. Previously, LILETTA was indicated for use up to 4 years.
LILETTA continues to be greater than 99% effective in preventing pregnancy in a broad range of women, regardless of age, race, body mass index, or parity, according to Allergan and Medicines360. The extended duration and proven efficacy across a diverse population enables more women in the United States to obtain effective birth control, as the IUD is now available for a low cost at public health clinics.
FOR MORE INFORMATION, VISIT: https://www.liletta.com
Continue to: ENDOMETRIAL ABLATION TECHNOLOGY
ENDOMETRIAL ABLATION TECHNOLOGY
AEGEA Medical introduces the AEGEA Vapor SystemTM, an innovative solution for endometrial ablation to treat menorrhagia.
The system uses Adaptive Vapor Ablation and is the first endometrial ablation system specifically designed for use in the doctor’s office, allowing minimal anesthesia/analgesia and rapid recovery, says AEGEA Medical.
AEGEA Medical describes the AEGEA Vapor System as a fully automated safety monitoring and vapor delivery system that uses a slender, flexible Vapor Probe with SmartSealTM technology and the Integrity ProTM safety feature, for an added level of confidence. The 4-minute procedure time includes 2 minutes of vapor treatment and can be performed in patients with a wider range of uterine anatomies than indicated for use with currently available treatments, says AEGEA Medical.
FOR MORE INFORMATION, VISIT: http://aegeamedical.com/
NATURAL CYCLES
The FDA has cleared Natural Cycles as the first digital method of birth control in the United States. Delivered in the form of an app, Natural Cycles is a fertility awareness–based contraceptive that uses a sophisticated algorithm to accurately and conveniently determine a woman’s daily fertility based on basal body temperature.
That data builds into a personalized fertility indicator that informs her when she needs to use protection to minimize the chance of conception. The app also can be used to help plan a pregnancy when the time is right, according to Natural Cycles.
A clinical study showed that the efficacy of a contraceptive mobile application is higher than usually reported for traditional fertility awareness–based methods. The application may contribute to reducing the unmet need for contraception, says Natural Cycles.
FOR MORE INFORMATION, VISIT: https://www.naturalcycles.com/en/hcp
Delayed first contraception use raises unwanted pregnancy risk
Young women who delay starting contraception when they start sexual activity are at increased risk of unwanted pregnancy, according to data from a cross-sectional study of more than 26,000 women in the United States.
Unintended pregnancy in the United States is associated with delayed prenatal care, premature birth, and low birth weight and remains more common among African American and Hispanic women than among white women, and it also is more common among low-income women than among high income women, wrote Mara E. Murray Horwitz, MD, of Harvard Pilgrim Health Care Institute in Boston and her colleagues.
“Reducing unintended pregnancy and the associated socioeconomic disparities is a national public health priority,” they wrote.
In a study published in Pediatrics, the researchers reviewed data from four cycles of the National Survey of Family Growth between 2002 and 2015. They examined self-reported responses from 26,359 women aged 15-44 years with sexual debuts during 1970-2014, including the dates of sexual debut, initiation of contraceptives, and rates of unwanted pregnancy. Timely contraceptive initiation was defined as use within a month of starting sexual activity.
Overall, one in five women reported delayed initiation of contraception. This delay was significantly associated with an increased unwanted pregnancy risk within 3 months of starting sexual activity, compared with timely use of contraception (adjusted risk ratio, 3.7). The average age of sexual debut was 17 years.
When the researchers examined subgroups, they found that one in four respondents who were African American, Hispanic, or low income reported delayed contraceptive initiation.
No association with unwanted pregnancy was found between effective versus less effective contraception methods. Timely contraceptive use increased during the study period from less than 10% in the 1970s to more than 25% in the 2000s, but condoms accounted for most of this increase. Use of other methods including long-acting reversible and short-acting hormonal options was low, especially among African American, Hispanic, and low-income women, Dr. Murray Horwitz and her colleagues noted.
The study was limited by several factors including the use of self-reports, lack of data on the exact start of contraceptive initiation, and the lack of association between contraceptive method and unwanted pregnancy, the researchers noted. However, the findings suggest that clinicians can help by intervening with young patients and educating them about early adoption of pregnancy prevention strategies.
The study was funded by the National Institutes of Health; Dr. Murray Horwitz was supported by an award from the NIH and Harvard Pilgrim Health Care Institute. Another researcher received support from Harvard Pilgrim Health Care Institute to provide mentorship for the study. The remaining researcher had no relevant financial disclosures.
SOURCE: Murray Horwitz M et al. Pediatrics. 2019;143(2):e20182463.
Despite a declining teen birth rate in the last several decades, the United States has the highest teen birth rate among industrialized nations. While many factors play into this rate, we know that, in many European countries with low teen birth rates, adolescents often initiate contraceptive methods before their sexual debut. As we often tell teenagers, they can become pregnant the “first time,” which makes initiating contraception early – and preferably before sexual debut – an important strategy to preventing unplanned pregnancy.
This study identifies the trends over time in the initiation of contraception in relationship to sexual debut and examines its effects on unplanned teen pregnancy. Understanding these trends can help clinicians more effectively target teen pregnancy.
I was pleasantly surprised to see that rates of timely contraceptive initiation have increased since 1970. Sadly, this rise is largely because of condom use. Use of effective forms of contraception – especially long-acting reversible forms of contraception (LARC), such as the IUD or the etonogestrel rod – still remain low at the time of sexual debut. While we continue to encourage LARCs as first line for pregnancy prevention, many patients are not getting the message about these highly effective, safe methods. Unsurprisingly, there are significant differences based on race/ethnicity and socioeconomic status on timely initiation of contraceptive methods, especially highly effective methods. This supports prior research which has shown significant barriers in access to contraception to these groups, which leads to higher rates of unplanned pregnancies.
Dr. Kelly Curran, MD, specializes in adolescent medicine at the University of Oklahoma, Oklahoma City. She is a member of the Pediatric News editorial advisory board and was asked to comment on the study by Murray Horwitz et al. Dr. Curran had no relevant financial disclosures.
Despite a declining teen birth rate in the last several decades, the United States has the highest teen birth rate among industrialized nations. While many factors play into this rate, we know that, in many European countries with low teen birth rates, adolescents often initiate contraceptive methods before their sexual debut. As we often tell teenagers, they can become pregnant the “first time,” which makes initiating contraception early – and preferably before sexual debut – an important strategy to preventing unplanned pregnancy.
This study identifies the trends over time in the initiation of contraception in relationship to sexual debut and examines its effects on unplanned teen pregnancy. Understanding these trends can help clinicians more effectively target teen pregnancy.
I was pleasantly surprised to see that rates of timely contraceptive initiation have increased since 1970. Sadly, this rise is largely because of condom use. Use of effective forms of contraception – especially long-acting reversible forms of contraception (LARC), such as the IUD or the etonogestrel rod – still remain low at the time of sexual debut. While we continue to encourage LARCs as first line for pregnancy prevention, many patients are not getting the message about these highly effective, safe methods. Unsurprisingly, there are significant differences based on race/ethnicity and socioeconomic status on timely initiation of contraceptive methods, especially highly effective methods. This supports prior research which has shown significant barriers in access to contraception to these groups, which leads to higher rates of unplanned pregnancies.
Dr. Kelly Curran, MD, specializes in adolescent medicine at the University of Oklahoma, Oklahoma City. She is a member of the Pediatric News editorial advisory board and was asked to comment on the study by Murray Horwitz et al. Dr. Curran had no relevant financial disclosures.
Despite a declining teen birth rate in the last several decades, the United States has the highest teen birth rate among industrialized nations. While many factors play into this rate, we know that, in many European countries with low teen birth rates, adolescents often initiate contraceptive methods before their sexual debut. As we often tell teenagers, they can become pregnant the “first time,” which makes initiating contraception early – and preferably before sexual debut – an important strategy to preventing unplanned pregnancy.
This study identifies the trends over time in the initiation of contraception in relationship to sexual debut and examines its effects on unplanned teen pregnancy. Understanding these trends can help clinicians more effectively target teen pregnancy.
I was pleasantly surprised to see that rates of timely contraceptive initiation have increased since 1970. Sadly, this rise is largely because of condom use. Use of effective forms of contraception – especially long-acting reversible forms of contraception (LARC), such as the IUD or the etonogestrel rod – still remain low at the time of sexual debut. While we continue to encourage LARCs as first line for pregnancy prevention, many patients are not getting the message about these highly effective, safe methods. Unsurprisingly, there are significant differences based on race/ethnicity and socioeconomic status on timely initiation of contraceptive methods, especially highly effective methods. This supports prior research which has shown significant barriers in access to contraception to these groups, which leads to higher rates of unplanned pregnancies.
Dr. Kelly Curran, MD, specializes in adolescent medicine at the University of Oklahoma, Oklahoma City. She is a member of the Pediatric News editorial advisory board and was asked to comment on the study by Murray Horwitz et al. Dr. Curran had no relevant financial disclosures.
Young women who delay starting contraception when they start sexual activity are at increased risk of unwanted pregnancy, according to data from a cross-sectional study of more than 26,000 women in the United States.
Unintended pregnancy in the United States is associated with delayed prenatal care, premature birth, and low birth weight and remains more common among African American and Hispanic women than among white women, and it also is more common among low-income women than among high income women, wrote Mara E. Murray Horwitz, MD, of Harvard Pilgrim Health Care Institute in Boston and her colleagues.
“Reducing unintended pregnancy and the associated socioeconomic disparities is a national public health priority,” they wrote.
In a study published in Pediatrics, the researchers reviewed data from four cycles of the National Survey of Family Growth between 2002 and 2015. They examined self-reported responses from 26,359 women aged 15-44 years with sexual debuts during 1970-2014, including the dates of sexual debut, initiation of contraceptives, and rates of unwanted pregnancy. Timely contraceptive initiation was defined as use within a month of starting sexual activity.
Overall, one in five women reported delayed initiation of contraception. This delay was significantly associated with an increased unwanted pregnancy risk within 3 months of starting sexual activity, compared with timely use of contraception (adjusted risk ratio, 3.7). The average age of sexual debut was 17 years.
When the researchers examined subgroups, they found that one in four respondents who were African American, Hispanic, or low income reported delayed contraceptive initiation.
No association with unwanted pregnancy was found between effective versus less effective contraception methods. Timely contraceptive use increased during the study period from less than 10% in the 1970s to more than 25% in the 2000s, but condoms accounted for most of this increase. Use of other methods including long-acting reversible and short-acting hormonal options was low, especially among African American, Hispanic, and low-income women, Dr. Murray Horwitz and her colleagues noted.
The study was limited by several factors including the use of self-reports, lack of data on the exact start of contraceptive initiation, and the lack of association between contraceptive method and unwanted pregnancy, the researchers noted. However, the findings suggest that clinicians can help by intervening with young patients and educating them about early adoption of pregnancy prevention strategies.
The study was funded by the National Institutes of Health; Dr. Murray Horwitz was supported by an award from the NIH and Harvard Pilgrim Health Care Institute. Another researcher received support from Harvard Pilgrim Health Care Institute to provide mentorship for the study. The remaining researcher had no relevant financial disclosures.
SOURCE: Murray Horwitz M et al. Pediatrics. 2019;143(2):e20182463.
Young women who delay starting contraception when they start sexual activity are at increased risk of unwanted pregnancy, according to data from a cross-sectional study of more than 26,000 women in the United States.
Unintended pregnancy in the United States is associated with delayed prenatal care, premature birth, and low birth weight and remains more common among African American and Hispanic women than among white women, and it also is more common among low-income women than among high income women, wrote Mara E. Murray Horwitz, MD, of Harvard Pilgrim Health Care Institute in Boston and her colleagues.
“Reducing unintended pregnancy and the associated socioeconomic disparities is a national public health priority,” they wrote.
In a study published in Pediatrics, the researchers reviewed data from four cycles of the National Survey of Family Growth between 2002 and 2015. They examined self-reported responses from 26,359 women aged 15-44 years with sexual debuts during 1970-2014, including the dates of sexual debut, initiation of contraceptives, and rates of unwanted pregnancy. Timely contraceptive initiation was defined as use within a month of starting sexual activity.
Overall, one in five women reported delayed initiation of contraception. This delay was significantly associated with an increased unwanted pregnancy risk within 3 months of starting sexual activity, compared with timely use of contraception (adjusted risk ratio, 3.7). The average age of sexual debut was 17 years.
When the researchers examined subgroups, they found that one in four respondents who were African American, Hispanic, or low income reported delayed contraceptive initiation.
No association with unwanted pregnancy was found between effective versus less effective contraception methods. Timely contraceptive use increased during the study period from less than 10% in the 1970s to more than 25% in the 2000s, but condoms accounted for most of this increase. Use of other methods including long-acting reversible and short-acting hormonal options was low, especially among African American, Hispanic, and low-income women, Dr. Murray Horwitz and her colleagues noted.
The study was limited by several factors including the use of self-reports, lack of data on the exact start of contraceptive initiation, and the lack of association between contraceptive method and unwanted pregnancy, the researchers noted. However, the findings suggest that clinicians can help by intervening with young patients and educating them about early adoption of pregnancy prevention strategies.
The study was funded by the National Institutes of Health; Dr. Murray Horwitz was supported by an award from the NIH and Harvard Pilgrim Health Care Institute. Another researcher received support from Harvard Pilgrim Health Care Institute to provide mentorship for the study. The remaining researcher had no relevant financial disclosures.
SOURCE: Murray Horwitz M et al. Pediatrics. 2019;143(2):e20182463.
FROM PEDIATRICS
Key clinical point: Women who delayed using contraception were significantly more likely to become pregnant within 3 months of starting sexual activity than were those who had initiated contraception use, especially black, Hispanic, and low-income women.
Major finding: Unwanted pregnancy within 3 months of sexual debut was 3.7 times more likely in women who delayed initial contraception use, compared with those who had timely initiation.
Study details: The data come from a cross-sectional study including 26,359 women with sexual debuts between 1970 and 2014.
Disclosures: The study was funded by the National Institutes of Health; Dr. Murray Horwitz was supported by an award from the NIH and Harvard Pilgrim Health Care Institute. Another researcher received support from Harvard Pilgrim Health Care Institute to provide mentorship for the study. The remaining researcher had no relevant financial disclosures.
Source: Horwitz M et al. Pediatrics. 2019;143(2):e20182463.
Courts block Trump from eroding contraceptive mandate
Federal judges have blocked the Trump administration from weakening the Affordable Care Act’s contraceptive mandate in two separate orders that bar the President from letting more entities claim exemptions.
On Jan. 14, U.S. District Court Judge Wendy Beetlestone for the Eastern District of Pennsylvania issued a temporary nationwide ban on two rules that would have allowed an expanded group of employers and insurers to object to providing contraception coverage on either religious or moral grounds. The regulations, announced Nov. 7, 2018, were scheduled to take effect Jan. 14. The day before, U.S. District Judge Haywood Gilliam for the Northern District of California issued a similar temporary ban, but his order applied only to the 13 plaintiff states in the case, plus the District of Columbia.
While Pennsylvania and New Jersey are the only plaintiffs in the Judge Beetlestone case, she wrote that a nationwide injunction is required to protect numerous citizens from losing contraceptive coverage and resulting in “significant, direct, and proprietary harm” to states in the form of increased state-funded contraceptive services and increased costs associated with unintended pregnancies. Judge Gilliam provided similar reasoning in his Jan. 13 order, writing that the 13 plaintiff states have proven that rules promulgated by the U.S. Department of Health and Human Services would cause women to lose employer-sponsored contraceptive coverage, resulting in economic harm to the states.
California Attorney General Xavier Becerra, a plaintiff in the second case, said Judge Gilliam’s ruling will stop the Trump administration from denying millions of women and families access to co-pay birth control guaranteed by the Affordable Care Act.
“The law couldn’t be clearer – employers have no business interfering in women’s health care decisions,” Mr. Becerra said in the statement. “[The] court ruling stops another attempt by the Trump administration to trample on women’s access to basic reproductive care.”
At press time, the Trump administration officials had responded publicly to the court orders. The administration previously said the new policies would “better balance the government’s interest in promoting coverage for contraceptive and sterilization services with the government’s interests in providing conscience protections for entities with sincerely held moral convictions.” HHS estimated that the rules would affect no more than 200 employers.
Mark Rienzi, president of the Becket Fund for Religious Liberty, a legal institute that defends religious freedoms, expressed disappointment at the court orders. Becket represents the Little Sisters of the Poor, an organization that has been fighting for several years for an exemption to the contraceptive mandate.
“Government bureaucrats should not be allowed to threaten the rights of the Little Sisters of the Poor to serve according to their Catholic beliefs,” Mr. Rienzi said in a statement. “Now the nuns are forced to keep fighting this unnecessary lawsuit to protect their ability to focus on caring for the poor. We are confident these decisions will be overturned.”
The ACA initially required all employers to cover birth control for employees with no copayments, except for group health plans of religious employers, which were deemed exempt. Those religious employers were primarily churches and other houses of worship. After a number of complaints and legal challenges, the Obama administration created a workaround for nonprofit religious employers to opt out of the mandate.
However, critics argued the process itself was a violation of their religious freedom. The issue led to the case of Zubik v. Burwell, a legal challenge over the mandate exemption that went before the U.S. Supreme Court in March 2016. The issue was never resolved. In May 2016, the Supreme Court vacated the lower court rulings related to Zubik v. Burwell and remanded the case back to the four appeals courts that had originally ruled on the issue.
The Trump administration then announced new rules aimed at broadening exemptions to the ACA’s contraceptive mandate to entities that object to services covered by the mandate on the basis of sincerely held religious beliefs. A second rule would protect nonprofit organizations and small businesses that have nonreligious moral convictions that oppose services covered by the mandate. The religious and moral exemptions would apply to institutions of education, issuers, and individuals, but not to governmental entities.
Thirteen states and the District of Columbia then sued the Trump administration over the rules as well as Pennsylvania and New Jersey in a separate case.
The nationwide ban against the rules will remain in effect while the cases continue through the court system.
Federal judges have blocked the Trump administration from weakening the Affordable Care Act’s contraceptive mandate in two separate orders that bar the President from letting more entities claim exemptions.
On Jan. 14, U.S. District Court Judge Wendy Beetlestone for the Eastern District of Pennsylvania issued a temporary nationwide ban on two rules that would have allowed an expanded group of employers and insurers to object to providing contraception coverage on either religious or moral grounds. The regulations, announced Nov. 7, 2018, were scheduled to take effect Jan. 14. The day before, U.S. District Judge Haywood Gilliam for the Northern District of California issued a similar temporary ban, but his order applied only to the 13 plaintiff states in the case, plus the District of Columbia.
While Pennsylvania and New Jersey are the only plaintiffs in the Judge Beetlestone case, she wrote that a nationwide injunction is required to protect numerous citizens from losing contraceptive coverage and resulting in “significant, direct, and proprietary harm” to states in the form of increased state-funded contraceptive services and increased costs associated with unintended pregnancies. Judge Gilliam provided similar reasoning in his Jan. 13 order, writing that the 13 plaintiff states have proven that rules promulgated by the U.S. Department of Health and Human Services would cause women to lose employer-sponsored contraceptive coverage, resulting in economic harm to the states.
California Attorney General Xavier Becerra, a plaintiff in the second case, said Judge Gilliam’s ruling will stop the Trump administration from denying millions of women and families access to co-pay birth control guaranteed by the Affordable Care Act.
“The law couldn’t be clearer – employers have no business interfering in women’s health care decisions,” Mr. Becerra said in the statement. “[The] court ruling stops another attempt by the Trump administration to trample on women’s access to basic reproductive care.”
At press time, the Trump administration officials had responded publicly to the court orders. The administration previously said the new policies would “better balance the government’s interest in promoting coverage for contraceptive and sterilization services with the government’s interests in providing conscience protections for entities with sincerely held moral convictions.” HHS estimated that the rules would affect no more than 200 employers.
Mark Rienzi, president of the Becket Fund for Religious Liberty, a legal institute that defends religious freedoms, expressed disappointment at the court orders. Becket represents the Little Sisters of the Poor, an organization that has been fighting for several years for an exemption to the contraceptive mandate.
“Government bureaucrats should not be allowed to threaten the rights of the Little Sisters of the Poor to serve according to their Catholic beliefs,” Mr. Rienzi said in a statement. “Now the nuns are forced to keep fighting this unnecessary lawsuit to protect their ability to focus on caring for the poor. We are confident these decisions will be overturned.”
The ACA initially required all employers to cover birth control for employees with no copayments, except for group health plans of religious employers, which were deemed exempt. Those religious employers were primarily churches and other houses of worship. After a number of complaints and legal challenges, the Obama administration created a workaround for nonprofit religious employers to opt out of the mandate.
However, critics argued the process itself was a violation of their religious freedom. The issue led to the case of Zubik v. Burwell, a legal challenge over the mandate exemption that went before the U.S. Supreme Court in March 2016. The issue was never resolved. In May 2016, the Supreme Court vacated the lower court rulings related to Zubik v. Burwell and remanded the case back to the four appeals courts that had originally ruled on the issue.
The Trump administration then announced new rules aimed at broadening exemptions to the ACA’s contraceptive mandate to entities that object to services covered by the mandate on the basis of sincerely held religious beliefs. A second rule would protect nonprofit organizations and small businesses that have nonreligious moral convictions that oppose services covered by the mandate. The religious and moral exemptions would apply to institutions of education, issuers, and individuals, but not to governmental entities.
Thirteen states and the District of Columbia then sued the Trump administration over the rules as well as Pennsylvania and New Jersey in a separate case.
The nationwide ban against the rules will remain in effect while the cases continue through the court system.
Federal judges have blocked the Trump administration from weakening the Affordable Care Act’s contraceptive mandate in two separate orders that bar the President from letting more entities claim exemptions.
On Jan. 14, U.S. District Court Judge Wendy Beetlestone for the Eastern District of Pennsylvania issued a temporary nationwide ban on two rules that would have allowed an expanded group of employers and insurers to object to providing contraception coverage on either religious or moral grounds. The regulations, announced Nov. 7, 2018, were scheduled to take effect Jan. 14. The day before, U.S. District Judge Haywood Gilliam for the Northern District of California issued a similar temporary ban, but his order applied only to the 13 plaintiff states in the case, plus the District of Columbia.
While Pennsylvania and New Jersey are the only plaintiffs in the Judge Beetlestone case, she wrote that a nationwide injunction is required to protect numerous citizens from losing contraceptive coverage and resulting in “significant, direct, and proprietary harm” to states in the form of increased state-funded contraceptive services and increased costs associated with unintended pregnancies. Judge Gilliam provided similar reasoning in his Jan. 13 order, writing that the 13 plaintiff states have proven that rules promulgated by the U.S. Department of Health and Human Services would cause women to lose employer-sponsored contraceptive coverage, resulting in economic harm to the states.
California Attorney General Xavier Becerra, a plaintiff in the second case, said Judge Gilliam’s ruling will stop the Trump administration from denying millions of women and families access to co-pay birth control guaranteed by the Affordable Care Act.
“The law couldn’t be clearer – employers have no business interfering in women’s health care decisions,” Mr. Becerra said in the statement. “[The] court ruling stops another attempt by the Trump administration to trample on women’s access to basic reproductive care.”
At press time, the Trump administration officials had responded publicly to the court orders. The administration previously said the new policies would “better balance the government’s interest in promoting coverage for contraceptive and sterilization services with the government’s interests in providing conscience protections for entities with sincerely held moral convictions.” HHS estimated that the rules would affect no more than 200 employers.
Mark Rienzi, president of the Becket Fund for Religious Liberty, a legal institute that defends religious freedoms, expressed disappointment at the court orders. Becket represents the Little Sisters of the Poor, an organization that has been fighting for several years for an exemption to the contraceptive mandate.
“Government bureaucrats should not be allowed to threaten the rights of the Little Sisters of the Poor to serve according to their Catholic beliefs,” Mr. Rienzi said in a statement. “Now the nuns are forced to keep fighting this unnecessary lawsuit to protect their ability to focus on caring for the poor. We are confident these decisions will be overturned.”
The ACA initially required all employers to cover birth control for employees with no copayments, except for group health plans of religious employers, which were deemed exempt. Those religious employers were primarily churches and other houses of worship. After a number of complaints and legal challenges, the Obama administration created a workaround for nonprofit religious employers to opt out of the mandate.
However, critics argued the process itself was a violation of their religious freedom. The issue led to the case of Zubik v. Burwell, a legal challenge over the mandate exemption that went before the U.S. Supreme Court in March 2016. The issue was never resolved. In May 2016, the Supreme Court vacated the lower court rulings related to Zubik v. Burwell and remanded the case back to the four appeals courts that had originally ruled on the issue.
The Trump administration then announced new rules aimed at broadening exemptions to the ACA’s contraceptive mandate to entities that object to services covered by the mandate on the basis of sincerely held religious beliefs. A second rule would protect nonprofit organizations and small businesses that have nonreligious moral convictions that oppose services covered by the mandate. The religious and moral exemptions would apply to institutions of education, issuers, and individuals, but not to governmental entities.
Thirteen states and the District of Columbia then sued the Trump administration over the rules as well as Pennsylvania and New Jersey in a separate case.
The nationwide ban against the rules will remain in effect while the cases continue through the court system.
FDA expands Essure’s postmarketing surveillance study
The study, ordered in 2016, will now run 5 years instead of 3, and the cohort will be enlarged to add any women who elect implantation while the device is still on the market, FDA Commissioner Scott Gottlieb, MD, announced in a press statement. The agency also added a key biological measure: All patients with Essure will undergo regular blood work to evaluate proinflammatory markers that could be device related.
“We’re requiring additional blood testing of patients enrolled in follow-up visits during the study to learn more about patients’ levels of certain inflammatory markers that can be indicators of increased inflammation,” Dr. Gottlieb said. “This could help us better evaluate potential immune reactions to the device and whether these findings are associated with symptoms that patients have reported related to Essure.”
The device has been associated with severe problems in some patients, he noted.
“I personally had the opportunity to meet with women who have been adversely affected by Essure to listen and learn about their concerns. Some of the women I spoke with developed significant medical problems that they ascribe to their use of the product. We remain committed to these women and to improving how we monitor the safety of medical devices, including those related to women’s health.”
The study expansion comes as Bayer is facing more than 16,000 lawsuits over adverse events associated with Essure implantation.
Since its approval, Essure is estimated to have been used by more than 750,000 patients worldwide. Bayer claims the device is 99% effective in preventing pregnancy, but it’s also been associated with some serious risks, including persistent pain, perforation of the uterus and fallopian tubes, and migration of the coils into the pelvis or abdomen. In view of these – and more than 15,000 adverse events reported to the FDA – the agency announced new restrictions on Essure earlier this year. Those restrictions, plus a prior boxed warning on the label, contributed to about a 70% decline in U.S. sales, which Bayer says prompted the discontinuation.
The open-label prospective observational study will compare women who have the Essure device to a matched cohort that underwent laparoscopic tubal ligation. The main safety endpoints are chronic pelvic pain and abnormal uterine bleeding, as well as the new measure of inflammatory markers. As of Dec. 3, 791 patients have been enrolled (293 in the Essure arm and 498 in the laparoscopic tubal ligation arm).
Women who have the implant now and remain free of any adverse events should probably keep the device, Dr. Gottlieb advised.
“We believe women who’ve been using Essure successfully to prevent pregnancy can and should continue to do so. Women who suspect the device may be related to symptoms they are experiencing, such as persistent pain, should talk to their doctor on what steps may be appropriate. Device removal has its own risks. Patients should discuss the benefits and risks of any procedure with their health care providers before deciding on the best option for them.”
The study, ordered in 2016, will now run 5 years instead of 3, and the cohort will be enlarged to add any women who elect implantation while the device is still on the market, FDA Commissioner Scott Gottlieb, MD, announced in a press statement. The agency also added a key biological measure: All patients with Essure will undergo regular blood work to evaluate proinflammatory markers that could be device related.
“We’re requiring additional blood testing of patients enrolled in follow-up visits during the study to learn more about patients’ levels of certain inflammatory markers that can be indicators of increased inflammation,” Dr. Gottlieb said. “This could help us better evaluate potential immune reactions to the device and whether these findings are associated with symptoms that patients have reported related to Essure.”
The device has been associated with severe problems in some patients, he noted.
“I personally had the opportunity to meet with women who have been adversely affected by Essure to listen and learn about their concerns. Some of the women I spoke with developed significant medical problems that they ascribe to their use of the product. We remain committed to these women and to improving how we monitor the safety of medical devices, including those related to women’s health.”
The study expansion comes as Bayer is facing more than 16,000 lawsuits over adverse events associated with Essure implantation.
Since its approval, Essure is estimated to have been used by more than 750,000 patients worldwide. Bayer claims the device is 99% effective in preventing pregnancy, but it’s also been associated with some serious risks, including persistent pain, perforation of the uterus and fallopian tubes, and migration of the coils into the pelvis or abdomen. In view of these – and more than 15,000 adverse events reported to the FDA – the agency announced new restrictions on Essure earlier this year. Those restrictions, plus a prior boxed warning on the label, contributed to about a 70% decline in U.S. sales, which Bayer says prompted the discontinuation.
The open-label prospective observational study will compare women who have the Essure device to a matched cohort that underwent laparoscopic tubal ligation. The main safety endpoints are chronic pelvic pain and abnormal uterine bleeding, as well as the new measure of inflammatory markers. As of Dec. 3, 791 patients have been enrolled (293 in the Essure arm and 498 in the laparoscopic tubal ligation arm).
Women who have the implant now and remain free of any adverse events should probably keep the device, Dr. Gottlieb advised.
“We believe women who’ve been using Essure successfully to prevent pregnancy can and should continue to do so. Women who suspect the device may be related to symptoms they are experiencing, such as persistent pain, should talk to their doctor on what steps may be appropriate. Device removal has its own risks. Patients should discuss the benefits and risks of any procedure with their health care providers before deciding on the best option for them.”
The study, ordered in 2016, will now run 5 years instead of 3, and the cohort will be enlarged to add any women who elect implantation while the device is still on the market, FDA Commissioner Scott Gottlieb, MD, announced in a press statement. The agency also added a key biological measure: All patients with Essure will undergo regular blood work to evaluate proinflammatory markers that could be device related.
“We’re requiring additional blood testing of patients enrolled in follow-up visits during the study to learn more about patients’ levels of certain inflammatory markers that can be indicators of increased inflammation,” Dr. Gottlieb said. “This could help us better evaluate potential immune reactions to the device and whether these findings are associated with symptoms that patients have reported related to Essure.”
The device has been associated with severe problems in some patients, he noted.
“I personally had the opportunity to meet with women who have been adversely affected by Essure to listen and learn about their concerns. Some of the women I spoke with developed significant medical problems that they ascribe to their use of the product. We remain committed to these women and to improving how we monitor the safety of medical devices, including those related to women’s health.”
The study expansion comes as Bayer is facing more than 16,000 lawsuits over adverse events associated with Essure implantation.
Since its approval, Essure is estimated to have been used by more than 750,000 patients worldwide. Bayer claims the device is 99% effective in preventing pregnancy, but it’s also been associated with some serious risks, including persistent pain, perforation of the uterus and fallopian tubes, and migration of the coils into the pelvis or abdomen. In view of these – and more than 15,000 adverse events reported to the FDA – the agency announced new restrictions on Essure earlier this year. Those restrictions, plus a prior boxed warning on the label, contributed to about a 70% decline in U.S. sales, which Bayer says prompted the discontinuation.
The open-label prospective observational study will compare women who have the Essure device to a matched cohort that underwent laparoscopic tubal ligation. The main safety endpoints are chronic pelvic pain and abnormal uterine bleeding, as well as the new measure of inflammatory markers. As of Dec. 3, 791 patients have been enrolled (293 in the Essure arm and 498 in the laparoscopic tubal ligation arm).
Women who have the implant now and remain free of any adverse events should probably keep the device, Dr. Gottlieb advised.
“We believe women who’ve been using Essure successfully to prevent pregnancy can and should continue to do so. Women who suspect the device may be related to symptoms they are experiencing, such as persistent pain, should talk to their doctor on what steps may be appropriate. Device removal has its own risks. Patients should discuss the benefits and risks of any procedure with their health care providers before deciding on the best option for them.”
Is an IUD a good contraceptive choice for a never sexually active teen?
Expert Commentary
Data demonstrate efficacy and safety of the IUD in adolescents. In addition, IUDs (particularly the levonorgestrel-containing IUD) have many noncontraceptive benefits. There is still reluctance, however, among clinicians to use IUDs in adolescents. In a sample of fellows of the American College of Obstetricians and Gynecologists, only 43% considered adolescents appropriate candidates for use of an IUD.1
Study details
In this retrospective chart review, Kebodeaux and Schwartz sought to compare successful IUD insertion rates on first attempt in 120 sexually active (SA) and 82 never sexually active (NSA) adolescents. The IUD type used for all women was the 52-mg levonorgestrel IUD (Mirena), except for 3 copper IUDs (Paragard) used in the SA group. The primary indications for IUD use were contraception (85.2%) in the SA group and abnormal uterine bleeding (43.9%) and menstrual suppression (24.4%) in the NSA group.
In the NSA group, 82.9% of adolescents had had some type of prior treatment affecting the menstrual cycle, compared with 60.9% in the SA group (P = .001).
Non–office insertion. Either a sedation unit or operating room was utilized in 5.5% of the IUD insertions in the SA group and 47.6% of the NSA group. Among the 39 adolescents in the NSA group undergoing non–office insertion, 19 (48.7%) had special needs (learning or intellectual disabilities, autism/autism spectrum, or physical disabilities, such as cerebral palsy). Only 1 adolescent with special needs in the NSA group had an office insertion compared with 5 out of 6 in the SA group.
The performance of another procedure other than the IUD insertion (including diagnostic laparoscopy and hymenectomy) was common among adolescents undergoing procedures in the sedation unit or operating room who did not have special needs. It is also important to note that adolescents with special needs were routinely offered insertion under anesthesia while SA adolescents were offered insertion under anesthesia only if they were undergoing another procedure as well.
Study strengths and weaknesses
The study’s strengths include IUD insertions performed at a children’s hospital by providers with experience working with adolescent populations. This likely accounts for the high rates of “tolerance of the procedure well” (93.8% in the SA group vs 81.7% in the NSA group; P = .006). The study also included a patient population—adolescents with special needs—that has not been studied relative to IUD use previously.
A significant weakness of the study, however, is that there are no long-term follow-up data, particularly related to continuation rates.
These study findings provides further support to combat the myth that adolescents, particularly if nulliparous or not sexually active, are not suitable candidates for IUD use. However, if they have never been sexually active or have special needs, IUD insertion under sedation or in an operating room may be necessary. It is also likely that selection of the IUD as an option by an adolescent and overall tolerance of the insertion procedure requires providers with experience in caring for adolescents as well as providers possessing good counseling skills.
—Ronald T. Burkman, MD
1. Luchowski AT, Anderson BL, Power ML, Reglan GB, Espey E, Shulkin J. Obstetrician-gynecologists and contraception: practice and opinions about the use of IUDs in nulliparous women, adolescents and other patient populations. Contraception. 2014;89:572-577.
Expert Commentary
Data demonstrate efficacy and safety of the IUD in adolescents. In addition, IUDs (particularly the levonorgestrel-containing IUD) have many noncontraceptive benefits. There is still reluctance, however, among clinicians to use IUDs in adolescents. In a sample of fellows of the American College of Obstetricians and Gynecologists, only 43% considered adolescents appropriate candidates for use of an IUD.1
Study details
In this retrospective chart review, Kebodeaux and Schwartz sought to compare successful IUD insertion rates on first attempt in 120 sexually active (SA) and 82 never sexually active (NSA) adolescents. The IUD type used for all women was the 52-mg levonorgestrel IUD (Mirena), except for 3 copper IUDs (Paragard) used in the SA group. The primary indications for IUD use were contraception (85.2%) in the SA group and abnormal uterine bleeding (43.9%) and menstrual suppression (24.4%) in the NSA group.
In the NSA group, 82.9% of adolescents had had some type of prior treatment affecting the menstrual cycle, compared with 60.9% in the SA group (P = .001).
Non–office insertion. Either a sedation unit or operating room was utilized in 5.5% of the IUD insertions in the SA group and 47.6% of the NSA group. Among the 39 adolescents in the NSA group undergoing non–office insertion, 19 (48.7%) had special needs (learning or intellectual disabilities, autism/autism spectrum, or physical disabilities, such as cerebral palsy). Only 1 adolescent with special needs in the NSA group had an office insertion compared with 5 out of 6 in the SA group.
The performance of another procedure other than the IUD insertion (including diagnostic laparoscopy and hymenectomy) was common among adolescents undergoing procedures in the sedation unit or operating room who did not have special needs. It is also important to note that adolescents with special needs were routinely offered insertion under anesthesia while SA adolescents were offered insertion under anesthesia only if they were undergoing another procedure as well.
Study strengths and weaknesses
The study’s strengths include IUD insertions performed at a children’s hospital by providers with experience working with adolescent populations. This likely accounts for the high rates of “tolerance of the procedure well” (93.8% in the SA group vs 81.7% in the NSA group; P = .006). The study also included a patient population—adolescents with special needs—that has not been studied relative to IUD use previously.
A significant weakness of the study, however, is that there are no long-term follow-up data, particularly related to continuation rates.
These study findings provides further support to combat the myth that adolescents, particularly if nulliparous or not sexually active, are not suitable candidates for IUD use. However, if they have never been sexually active or have special needs, IUD insertion under sedation or in an operating room may be necessary. It is also likely that selection of the IUD as an option by an adolescent and overall tolerance of the insertion procedure requires providers with experience in caring for adolescents as well as providers possessing good counseling skills.
—Ronald T. Burkman, MD
Expert Commentary
Data demonstrate efficacy and safety of the IUD in adolescents. In addition, IUDs (particularly the levonorgestrel-containing IUD) have many noncontraceptive benefits. There is still reluctance, however, among clinicians to use IUDs in adolescents. In a sample of fellows of the American College of Obstetricians and Gynecologists, only 43% considered adolescents appropriate candidates for use of an IUD.1
Study details
In this retrospective chart review, Kebodeaux and Schwartz sought to compare successful IUD insertion rates on first attempt in 120 sexually active (SA) and 82 never sexually active (NSA) adolescents. The IUD type used for all women was the 52-mg levonorgestrel IUD (Mirena), except for 3 copper IUDs (Paragard) used in the SA group. The primary indications for IUD use were contraception (85.2%) in the SA group and abnormal uterine bleeding (43.9%) and menstrual suppression (24.4%) in the NSA group.
In the NSA group, 82.9% of adolescents had had some type of prior treatment affecting the menstrual cycle, compared with 60.9% in the SA group (P = .001).
Non–office insertion. Either a sedation unit or operating room was utilized in 5.5% of the IUD insertions in the SA group and 47.6% of the NSA group. Among the 39 adolescents in the NSA group undergoing non–office insertion, 19 (48.7%) had special needs (learning or intellectual disabilities, autism/autism spectrum, or physical disabilities, such as cerebral palsy). Only 1 adolescent with special needs in the NSA group had an office insertion compared with 5 out of 6 in the SA group.
The performance of another procedure other than the IUD insertion (including diagnostic laparoscopy and hymenectomy) was common among adolescents undergoing procedures in the sedation unit or operating room who did not have special needs. It is also important to note that adolescents with special needs were routinely offered insertion under anesthesia while SA adolescents were offered insertion under anesthesia only if they were undergoing another procedure as well.
Study strengths and weaknesses
The study’s strengths include IUD insertions performed at a children’s hospital by providers with experience working with adolescent populations. This likely accounts for the high rates of “tolerance of the procedure well” (93.8% in the SA group vs 81.7% in the NSA group; P = .006). The study also included a patient population—adolescents with special needs—that has not been studied relative to IUD use previously.
A significant weakness of the study, however, is that there are no long-term follow-up data, particularly related to continuation rates.
These study findings provides further support to combat the myth that adolescents, particularly if nulliparous or not sexually active, are not suitable candidates for IUD use. However, if they have never been sexually active or have special needs, IUD insertion under sedation or in an operating room may be necessary. It is also likely that selection of the IUD as an option by an adolescent and overall tolerance of the insertion procedure requires providers with experience in caring for adolescents as well as providers possessing good counseling skills.
—Ronald T. Burkman, MD
1. Luchowski AT, Anderson BL, Power ML, Reglan GB, Espey E, Shulkin J. Obstetrician-gynecologists and contraception: practice and opinions about the use of IUDs in nulliparous women, adolescents and other patient populations. Contraception. 2014;89:572-577.
1. Luchowski AT, Anderson BL, Power ML, Reglan GB, Espey E, Shulkin J. Obstetrician-gynecologists and contraception: practice and opinions about the use of IUDs in nulliparous women, adolescents and other patient populations. Contraception. 2014;89:572-577.
Abortion measures continue to fall
Three important national measures of abortion dropped by at least 19% from 2006 to 2015, according to the Centers for Disease Control and Prevention.
Surgical and medical abortions reported to the CDC dropped by 24%, going from almost 843,000 in 2006 to 638,000 in 2015, with declines occurring every year, Tara C. Jatlaoui, MD, and her associates at the CDC’s National Center for Chronic Disease Prevention and Health Promotion, Atlanta, reported in Morbidity and Mortality Weekly Report Surveillance Summaries.
Over that same time period, the abortion rate fell from 15.9 per 1,000 women aged 15-44 years to 11.8 per 1,000 – a decline of 26%. Abortion ratio – the number of abortions per 1,000 live births within a given population – declined by 19%, dropping from 233 abortions per 1,000 live births in 2006 to 188 abortions per 1,000 live births in 2015, the investigators reported. The findings were based on data from 49 areas that continuously reported over the study period (excludes California, Maryland, and New Hampshire but includes the District of Columbia and New York City).
Abortion rates were highest for women aged 20-29 years for the study period, and this age group accounted for the largest share among the 44 reporting areas that provided data by maternal age each year. Those under age 15 years had the largest drops by age in total number of abortions (40%) and abortion rate (58%) but had the highest, by far, abortion ratio for each year of the study (700 per 1,000 live births in that age group in 2015. The abortion ratio for 15- to 19-year-olds was 289 per 1,000 live births).
The percentage of abortions performed before 14 weeks’ gestation changed little, going from 91.5% in 2006 to 91% in 2015, but “a shift occurred toward earlier gestational ages,” they noted. The percentage of abortions performed before or at 8 weeks increased 3% and those done at 9-13 weeks dropped almost 9% among the 33 areas that reported gestational age every year. Abortions done after 13 weeks gestation represented 9% of all abortions during the study period, with an increase of 7% occurring from 2006 to 2015, Dr. Jatlaoui and her associates said.
Removing barriers such as cost, “insufficient provider reimbursement and training, inadequate client-centered counseling, lack of youth-friendly services, and low client awareness ... might help improve contraceptive use, potentially reducing the number of unintended pregnancies and the number of abortions performed in the United States,” the researchers wrote.
SOURCE: Jatlaoui TC et al. MMWR Surveill Summ. 2018 Nov 23(13):1-45.
Three important national measures of abortion dropped by at least 19% from 2006 to 2015, according to the Centers for Disease Control and Prevention.
Surgical and medical abortions reported to the CDC dropped by 24%, going from almost 843,000 in 2006 to 638,000 in 2015, with declines occurring every year, Tara C. Jatlaoui, MD, and her associates at the CDC’s National Center for Chronic Disease Prevention and Health Promotion, Atlanta, reported in Morbidity and Mortality Weekly Report Surveillance Summaries.
Over that same time period, the abortion rate fell from 15.9 per 1,000 women aged 15-44 years to 11.8 per 1,000 – a decline of 26%. Abortion ratio – the number of abortions per 1,000 live births within a given population – declined by 19%, dropping from 233 abortions per 1,000 live births in 2006 to 188 abortions per 1,000 live births in 2015, the investigators reported. The findings were based on data from 49 areas that continuously reported over the study period (excludes California, Maryland, and New Hampshire but includes the District of Columbia and New York City).
Abortion rates were highest for women aged 20-29 years for the study period, and this age group accounted for the largest share among the 44 reporting areas that provided data by maternal age each year. Those under age 15 years had the largest drops by age in total number of abortions (40%) and abortion rate (58%) but had the highest, by far, abortion ratio for each year of the study (700 per 1,000 live births in that age group in 2015. The abortion ratio for 15- to 19-year-olds was 289 per 1,000 live births).
The percentage of abortions performed before 14 weeks’ gestation changed little, going from 91.5% in 2006 to 91% in 2015, but “a shift occurred toward earlier gestational ages,” they noted. The percentage of abortions performed before or at 8 weeks increased 3% and those done at 9-13 weeks dropped almost 9% among the 33 areas that reported gestational age every year. Abortions done after 13 weeks gestation represented 9% of all abortions during the study period, with an increase of 7% occurring from 2006 to 2015, Dr. Jatlaoui and her associates said.
Removing barriers such as cost, “insufficient provider reimbursement and training, inadequate client-centered counseling, lack of youth-friendly services, and low client awareness ... might help improve contraceptive use, potentially reducing the number of unintended pregnancies and the number of abortions performed in the United States,” the researchers wrote.
SOURCE: Jatlaoui TC et al. MMWR Surveill Summ. 2018 Nov 23(13):1-45.
Three important national measures of abortion dropped by at least 19% from 2006 to 2015, according to the Centers for Disease Control and Prevention.
Surgical and medical abortions reported to the CDC dropped by 24%, going from almost 843,000 in 2006 to 638,000 in 2015, with declines occurring every year, Tara C. Jatlaoui, MD, and her associates at the CDC’s National Center for Chronic Disease Prevention and Health Promotion, Atlanta, reported in Morbidity and Mortality Weekly Report Surveillance Summaries.
Over that same time period, the abortion rate fell from 15.9 per 1,000 women aged 15-44 years to 11.8 per 1,000 – a decline of 26%. Abortion ratio – the number of abortions per 1,000 live births within a given population – declined by 19%, dropping from 233 abortions per 1,000 live births in 2006 to 188 abortions per 1,000 live births in 2015, the investigators reported. The findings were based on data from 49 areas that continuously reported over the study period (excludes California, Maryland, and New Hampshire but includes the District of Columbia and New York City).
Abortion rates were highest for women aged 20-29 years for the study period, and this age group accounted for the largest share among the 44 reporting areas that provided data by maternal age each year. Those under age 15 years had the largest drops by age in total number of abortions (40%) and abortion rate (58%) but had the highest, by far, abortion ratio for each year of the study (700 per 1,000 live births in that age group in 2015. The abortion ratio for 15- to 19-year-olds was 289 per 1,000 live births).
The percentage of abortions performed before 14 weeks’ gestation changed little, going from 91.5% in 2006 to 91% in 2015, but “a shift occurred toward earlier gestational ages,” they noted. The percentage of abortions performed before or at 8 weeks increased 3% and those done at 9-13 weeks dropped almost 9% among the 33 areas that reported gestational age every year. Abortions done after 13 weeks gestation represented 9% of all abortions during the study period, with an increase of 7% occurring from 2006 to 2015, Dr. Jatlaoui and her associates said.
Removing barriers such as cost, “insufficient provider reimbursement and training, inadequate client-centered counseling, lack of youth-friendly services, and low client awareness ... might help improve contraceptive use, potentially reducing the number of unintended pregnancies and the number of abortions performed in the United States,” the researchers wrote.
SOURCE: Jatlaoui TC et al. MMWR Surveill Summ. 2018 Nov 23(13):1-45.
FROM MMWR SURVEILLANCE SUMMARIES
Levonorgestrel implant right after delivery does not affect breastfeeding, infant growth
Researchers found no significant differences in infant growth, changes in breastfeeding initiation or breastfeeding continuation at 3-month and 6-month follow-up among women who received a levonorgestrel contraception implant very soon after delivery, compared with women who waited to receive the implant.
“These findings are consistent with the preponderance of literature supporting the hypothesis that progestin-containing contraceptives do not compromise a woman’s ability to initiate or sustain breastfeeding and do not adversely affect infant growth,” Sarah Averbach, MD, of the University of California, San Francisco, and her colleagues wrote in their study published in Contraception.
Dr. Averbach and her colleagues randomized 96 women to receive a two-rod levonorgestrel (LNG)–releasing subdermal contraceptive implant within 5 days of delivery (mean time, 36 hours post delivery) and 87 women to delay the implant to between 6 and 8 weeks at a postpartum follow-up visit (mean time, 68 days). The women were a minimum of 18 years old with a recent vaginal or cesarean section delivery at a Ugandan hospital; 55% of the women had at least three children, and 73% said they had prior experience breastfeeding. The researchers then examined infant weight change and infant head circumference change at 6 months from birth, time to lactogenesis, and whether mothers continued to breastfeed at 3 months and 6 months after birth.
Infant weight was similar in the immediate-implant group (4,632 g), compared with the delayed-implant group (4,407 g; P = .26); infant head circumference was similar between both groups (9.3 cm vs. 9.5 cm; P = .70) at 6 months as well. The time to lactogenesis was not significantly different in the immediate-implant (65 hours) and delayed-implant (63 hours; P = .84) groups. At 3 months, 74% of immediate-implant participants and 71% of delayed-implant participants said they were breastfeeding exclusively (P = .74); at 6 months, 48% of immediate implant participants and 52% of delayed implant participants reported exclusive breastfeeding (P equals .58).
Limitations of the study included follow-up to only 6 months and selection of participants with previous breastfeeding experience. Researchers also noted better measurements of infant and maternal breast milk intake also could be used and limit generalization of the results.
This study was funded by the Society of Family Planning Research Fund. Dr. Averbach is supported by an award from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The other authors had no relevant financial disclosures.
SOURCE: Averbach S et al. Contraception. 2018. doi: 10.1016/j.contraception.2018.10.008.
Researchers found no significant differences in infant growth, changes in breastfeeding initiation or breastfeeding continuation at 3-month and 6-month follow-up among women who received a levonorgestrel contraception implant very soon after delivery, compared with women who waited to receive the implant.
“These findings are consistent with the preponderance of literature supporting the hypothesis that progestin-containing contraceptives do not compromise a woman’s ability to initiate or sustain breastfeeding and do not adversely affect infant growth,” Sarah Averbach, MD, of the University of California, San Francisco, and her colleagues wrote in their study published in Contraception.
Dr. Averbach and her colleagues randomized 96 women to receive a two-rod levonorgestrel (LNG)–releasing subdermal contraceptive implant within 5 days of delivery (mean time, 36 hours post delivery) and 87 women to delay the implant to between 6 and 8 weeks at a postpartum follow-up visit (mean time, 68 days). The women were a minimum of 18 years old with a recent vaginal or cesarean section delivery at a Ugandan hospital; 55% of the women had at least three children, and 73% said they had prior experience breastfeeding. The researchers then examined infant weight change and infant head circumference change at 6 months from birth, time to lactogenesis, and whether mothers continued to breastfeed at 3 months and 6 months after birth.
Infant weight was similar in the immediate-implant group (4,632 g), compared with the delayed-implant group (4,407 g; P = .26); infant head circumference was similar between both groups (9.3 cm vs. 9.5 cm; P = .70) at 6 months as well. The time to lactogenesis was not significantly different in the immediate-implant (65 hours) and delayed-implant (63 hours; P = .84) groups. At 3 months, 74% of immediate-implant participants and 71% of delayed-implant participants said they were breastfeeding exclusively (P = .74); at 6 months, 48% of immediate implant participants and 52% of delayed implant participants reported exclusive breastfeeding (P equals .58).
Limitations of the study included follow-up to only 6 months and selection of participants with previous breastfeeding experience. Researchers also noted better measurements of infant and maternal breast milk intake also could be used and limit generalization of the results.
This study was funded by the Society of Family Planning Research Fund. Dr. Averbach is supported by an award from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The other authors had no relevant financial disclosures.
SOURCE: Averbach S et al. Contraception. 2018. doi: 10.1016/j.contraception.2018.10.008.
Researchers found no significant differences in infant growth, changes in breastfeeding initiation or breastfeeding continuation at 3-month and 6-month follow-up among women who received a levonorgestrel contraception implant very soon after delivery, compared with women who waited to receive the implant.
“These findings are consistent with the preponderance of literature supporting the hypothesis that progestin-containing contraceptives do not compromise a woman’s ability to initiate or sustain breastfeeding and do not adversely affect infant growth,” Sarah Averbach, MD, of the University of California, San Francisco, and her colleagues wrote in their study published in Contraception.
Dr. Averbach and her colleagues randomized 96 women to receive a two-rod levonorgestrel (LNG)–releasing subdermal contraceptive implant within 5 days of delivery (mean time, 36 hours post delivery) and 87 women to delay the implant to between 6 and 8 weeks at a postpartum follow-up visit (mean time, 68 days). The women were a minimum of 18 years old with a recent vaginal or cesarean section delivery at a Ugandan hospital; 55% of the women had at least three children, and 73% said they had prior experience breastfeeding. The researchers then examined infant weight change and infant head circumference change at 6 months from birth, time to lactogenesis, and whether mothers continued to breastfeed at 3 months and 6 months after birth.
Infant weight was similar in the immediate-implant group (4,632 g), compared with the delayed-implant group (4,407 g; P = .26); infant head circumference was similar between both groups (9.3 cm vs. 9.5 cm; P = .70) at 6 months as well. The time to lactogenesis was not significantly different in the immediate-implant (65 hours) and delayed-implant (63 hours; P = .84) groups. At 3 months, 74% of immediate-implant participants and 71% of delayed-implant participants said they were breastfeeding exclusively (P = .74); at 6 months, 48% of immediate implant participants and 52% of delayed implant participants reported exclusive breastfeeding (P equals .58).
Limitations of the study included follow-up to only 6 months and selection of participants with previous breastfeeding experience. Researchers also noted better measurements of infant and maternal breast milk intake also could be used and limit generalization of the results.
This study was funded by the Society of Family Planning Research Fund. Dr. Averbach is supported by an award from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The other authors had no relevant financial disclosures.
SOURCE: Averbach S et al. Contraception. 2018. doi: 10.1016/j.contraception.2018.10.008.
FROM CONTRACEPTION
Key clinical point:
Major finding: Infant weight was similar in the immediate-implant group (4,632 g), compared with the delayed-implant group (4,407 g; P = .26); infant head circumference was similar between both groups (9.3 cm vs. 9.5 cm; P = .70) at 6 months as well.
Study details: A randomized trial of 96 women in Uganda who received a contraceptive implant less than 5 days after delivery and 86 women who received the implant between 6 and 8 weeks post partum.
Disclosures: This study was funded by the Society of Family Planning Research Fund. Dr. Averbach is supported by an award from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The other authors had no relevant financial disclosures.
Source: Averbach S et al. Contraception. 2018. doi: 10.1016/j.contraception.2018.10.008.