User login
Is salpingectomy (vs standard tubal ligation) for sterilization a feasible option at cesarean delivery?
Concerns over discretion, efficacy lead teen females to use emergency contraception
Emergency contraception is perceived as “easy, effective, and discrete,” especially when compared with nonemergent contraception and condoms, according to a qualitative study of contraceptive behaviors and decision making among adolescent females who had previously used emergency contraception (EC) or planned to use it.
“Three main themes emerged from our interviews: There are multiple perceived benefits to using EC, nonemergent contraception (NEC) use is challenging, and the decision to use NEC is multifactorial,” lead author Geetha N. Fink, MD, MPH, and her coauthors wrote in the Journal of Pediatric and Adolescent Gynecology.
The investigators reviewed interview transcripts and questionnaire responses from 28 adolescent females who had all used or were planning to use EC. The participants, who were recruited from school-based health centers (SBHC) in New York, reported having used EC a mean of 3.5 times (range 0-30 times), noted Dr. Fink of the department of obstetrics, gynecology and reproductive sciences at the Icahn School of Medicine at Mount Sinai in New York and her colleagues.
SBHCs in New York can distribute EC for free and – once general consent to care at the SBHC is provided at the start of each school year– without parental notification. This ease of access contributed to EC use, along with its minimal side effects. EC also can “be used discretely without the involvement of the partner,” Dr. Fink and her coauthors noted. Although the majority of participants stated being comfortable discussing their EC use, “they still appreciated that EC does not require partner involvement or awareness, unlike condoms or withdrawal.”
The participants’ decision making often was influenced by misperception; 65% incorrectly stated that EC was 90%-99% effective, and NEC use was ascribed to beliefs that “excess EC decreases efficacy or is detrimental to health and social interactions.” At the same time, Dr. Fink and her colleagues found that NEC use was associated with participants who had more sexual experience or who correctly identified it as more effective than EC.
“Our findings suggest that as adolescents gained more experience with sex and counseling, and also matured, they appeared to be more likely to utilize NEC,” they wrote.
Dr. Fink and her associates shared limitations of their study, including the uniqueness of SBHCs in New York City in providing comprehensive health care options, compared with those in the rest of the United States. However, they also noted the value in interviewing adolescent EC users and therefore better understanding why they’ve made these contraceptive decisions.
“We suspect many more students would benefit from access to EC and the SBHC, but may be unaware of these resources. We recommend increased efforts to promote awareness of these resources in schools, especially incorporated into sexual education. EC should be readily available for all adolescents,” they wrote.
The study was funded through a grant from the Society of Family Planning. No conflicts of interest were reported.
SOURCE: Fink GN et al. J Pediatr Adolesc Gynecol. 2018. doi: 10.1016/j.jpag.2018.10.005.
This study was very well conducted and provides genuine insight into adolescents who access contraception and their views about the difference between emergency contraception and nonemergent contraception. It also highlights that we have so much more work to do, from a public health perspective, when it comes to educating youth about the efficacy of contraception. If young people who have easier access to emergency contraception still believe incorrect information, what about those people who have minimal access?
Catherine Cansino, MD, MPH , is an associate clinical professor in the department of obstetrics and gynecology at the University of California, Davis. She was asked to comment on the article by Fink et al.
This study was very well conducted and provides genuine insight into adolescents who access contraception and their views about the difference between emergency contraception and nonemergent contraception. It also highlights that we have so much more work to do, from a public health perspective, when it comes to educating youth about the efficacy of contraception. If young people who have easier access to emergency contraception still believe incorrect information, what about those people who have minimal access?
Catherine Cansino, MD, MPH , is an associate clinical professor in the department of obstetrics and gynecology at the University of California, Davis. She was asked to comment on the article by Fink et al.
This study was very well conducted and provides genuine insight into adolescents who access contraception and their views about the difference between emergency contraception and nonemergent contraception. It also highlights that we have so much more work to do, from a public health perspective, when it comes to educating youth about the efficacy of contraception. If young people who have easier access to emergency contraception still believe incorrect information, what about those people who have minimal access?
Catherine Cansino, MD, MPH , is an associate clinical professor in the department of obstetrics and gynecology at the University of California, Davis. She was asked to comment on the article by Fink et al.
Emergency contraception is perceived as “easy, effective, and discrete,” especially when compared with nonemergent contraception and condoms, according to a qualitative study of contraceptive behaviors and decision making among adolescent females who had previously used emergency contraception (EC) or planned to use it.
“Three main themes emerged from our interviews: There are multiple perceived benefits to using EC, nonemergent contraception (NEC) use is challenging, and the decision to use NEC is multifactorial,” lead author Geetha N. Fink, MD, MPH, and her coauthors wrote in the Journal of Pediatric and Adolescent Gynecology.
The investigators reviewed interview transcripts and questionnaire responses from 28 adolescent females who had all used or were planning to use EC. The participants, who were recruited from school-based health centers (SBHC) in New York, reported having used EC a mean of 3.5 times (range 0-30 times), noted Dr. Fink of the department of obstetrics, gynecology and reproductive sciences at the Icahn School of Medicine at Mount Sinai in New York and her colleagues.
SBHCs in New York can distribute EC for free and – once general consent to care at the SBHC is provided at the start of each school year– without parental notification. This ease of access contributed to EC use, along with its minimal side effects. EC also can “be used discretely without the involvement of the partner,” Dr. Fink and her coauthors noted. Although the majority of participants stated being comfortable discussing their EC use, “they still appreciated that EC does not require partner involvement or awareness, unlike condoms or withdrawal.”
The participants’ decision making often was influenced by misperception; 65% incorrectly stated that EC was 90%-99% effective, and NEC use was ascribed to beliefs that “excess EC decreases efficacy or is detrimental to health and social interactions.” At the same time, Dr. Fink and her colleagues found that NEC use was associated with participants who had more sexual experience or who correctly identified it as more effective than EC.
“Our findings suggest that as adolescents gained more experience with sex and counseling, and also matured, they appeared to be more likely to utilize NEC,” they wrote.
Dr. Fink and her associates shared limitations of their study, including the uniqueness of SBHCs in New York City in providing comprehensive health care options, compared with those in the rest of the United States. However, they also noted the value in interviewing adolescent EC users and therefore better understanding why they’ve made these contraceptive decisions.
“We suspect many more students would benefit from access to EC and the SBHC, but may be unaware of these resources. We recommend increased efforts to promote awareness of these resources in schools, especially incorporated into sexual education. EC should be readily available for all adolescents,” they wrote.
The study was funded through a grant from the Society of Family Planning. No conflicts of interest were reported.
SOURCE: Fink GN et al. J Pediatr Adolesc Gynecol. 2018. doi: 10.1016/j.jpag.2018.10.005.
Emergency contraception is perceived as “easy, effective, and discrete,” especially when compared with nonemergent contraception and condoms, according to a qualitative study of contraceptive behaviors and decision making among adolescent females who had previously used emergency contraception (EC) or planned to use it.
“Three main themes emerged from our interviews: There are multiple perceived benefits to using EC, nonemergent contraception (NEC) use is challenging, and the decision to use NEC is multifactorial,” lead author Geetha N. Fink, MD, MPH, and her coauthors wrote in the Journal of Pediatric and Adolescent Gynecology.
The investigators reviewed interview transcripts and questionnaire responses from 28 adolescent females who had all used or were planning to use EC. The participants, who were recruited from school-based health centers (SBHC) in New York, reported having used EC a mean of 3.5 times (range 0-30 times), noted Dr. Fink of the department of obstetrics, gynecology and reproductive sciences at the Icahn School of Medicine at Mount Sinai in New York and her colleagues.
SBHCs in New York can distribute EC for free and – once general consent to care at the SBHC is provided at the start of each school year– without parental notification. This ease of access contributed to EC use, along with its minimal side effects. EC also can “be used discretely without the involvement of the partner,” Dr. Fink and her coauthors noted. Although the majority of participants stated being comfortable discussing their EC use, “they still appreciated that EC does not require partner involvement or awareness, unlike condoms or withdrawal.”
The participants’ decision making often was influenced by misperception; 65% incorrectly stated that EC was 90%-99% effective, and NEC use was ascribed to beliefs that “excess EC decreases efficacy or is detrimental to health and social interactions.” At the same time, Dr. Fink and her colleagues found that NEC use was associated with participants who had more sexual experience or who correctly identified it as more effective than EC.
“Our findings suggest that as adolescents gained more experience with sex and counseling, and also matured, they appeared to be more likely to utilize NEC,” they wrote.
Dr. Fink and her associates shared limitations of their study, including the uniqueness of SBHCs in New York City in providing comprehensive health care options, compared with those in the rest of the United States. However, they also noted the value in interviewing adolescent EC users and therefore better understanding why they’ve made these contraceptive decisions.
“We suspect many more students would benefit from access to EC and the SBHC, but may be unaware of these resources. We recommend increased efforts to promote awareness of these resources in schools, especially incorporated into sexual education. EC should be readily available for all adolescents,” they wrote.
The study was funded through a grant from the Society of Family Planning. No conflicts of interest were reported.
SOURCE: Fink GN et al. J Pediatr Adolesc Gynecol. 2018. doi: 10.1016/j.jpag.2018.10.005.
FROM THE JOURNAL OF PEDIATRIC AND ADOLESCENT GYNECOLOGY
Key clinical point: 65% of adolescent females who were interviewed incorrectly believed that emergency contraception is 90%-99% effective.
Major finding: Adolescents who use emergency contraception prefer it over nonemergent contraception because it is perceived as easy to use and a more private alternative.
Study details: A study of 28 interviews of adolescent females who self-reported emergency contraception use.
Disclosures: The study was funded through a grant from the Society of Family Planning. No conflicts of interest were reported.
Source: Fink GN et al. J Pediatr Adolesc Gynecol. 2018. doi: 10.1016/j.jpag.2018.10.005.
Trump administration rule erodes ACA contraceptive mandate
More employers can opt out of providing contraception coverage to their employees under final regulations from the Trump administration that narrow the Affordable Care Act’s contraceptive mandate.
The two regulations, released Nov. 7, allow an expanded group of employers and insurers to get out of covering contraception methods by objecting on either religious or moral grounds.
The first rule broadens exemptions to the ACA’s contraceptive mandate to entities that object to services covered by the mandate on the basis of sincerely held religious beliefs. The second rule protects nonprofit organizations and small businesses that have nonreligious moral convictions that oppose services covered by the mandate. The religious and moral exemptions apply to institutions of education, issuers, and individuals, but not to governmental entities.
When first proposed in 2017, Trump administration officials said the new policies would “better balance the government’s interest in promoting coverage for contraceptive and sterilization services with the government’s interests in providing conscience protections for entities with sincerely held moral convictions.” The U.S. Department of Health & Human Services estimates that the rules, which take effect in January 2019, will affect no more than 200 employers.
The American College of Obstetricians and Gynecologists expressed concern that the final rules will restrict patient access to meaningful contraceptive methods and will erode decades of progress in increasing women’s reproductive autonomy and restrict patient access to contraception.
“Women, families and our nation all benefit from seamless, affordable access to contraception,” ACOG President Lisa M. Hollier, MD, said in a statement. “Contraception improves women’s health and well-being, reduces unintended pregnancy, enables pregnancy spacing for safer pregnancies and deliveries, and empowers women’s engagement in the workforce and economic self-sufficiency. A woman’s employer should not determine whether or not she has this access.”
Marjorie Dannenfelser, president of Susan B. Anthony List, an anti-abortion group, praised the final rules, calling them needed protections from the burdensome Obama-era ACA abortifacient drug mandate.
“President Trump and HHS Secretary Azar delivered a huge victory for conscience rights and religious liberty in America,” Ms. Dannenfelser said in a statement. “No longer will Catholic nuns who care for the elderly poor be forced by the government to provide abortion-inducing drugs in their health care plans. Not only that, moral objectors such as Susan B. Anthony List, will also no longer have to pay for life-ending drugs that are antithetical to their mission and for which we have argued there is certainly no compelling state interest.”
The ACA initially required all employers to cover birth control for employees with no copayments, except for group health plans of religious employers, which were deemed exempt. Those religious employers were primarily churches and other houses of worship. After a number of complaints and legal challenges, the Obama administration created a workaround for nonprofit religious employers to opt out of the mandate.
However, critics argued the process itself was a violation of their religious freedom. The issue led to the case of Zubik v. Burwell, a legal challenge over the mandate exemption that went before the U.S. Supreme Court in March 2016. The issue was never resolved. In May 2016, the Supreme Court vacated the lower court rulings related to Zubik v. Burwell and remanded the case back to the four appeals courts that had originally ruled on the issue.
Under the approved regulations, employers or insurers can stop their coverage of contraceptive services if they have religious beliefs or moral convictions against covering birth control. Exempted entities and individuals also can choose to cover some, but not all, contraceptive services, depending on their specific religious or moral objection, according to an HHS fact sheet.
The agency emphasized that the regulations leave in place government programs that provide free or subsidized contraceptive coverage to low-income women, such as through community health centers, and that the rules do not ban any employer from covering contraceptives.
The regulations become effective 60 days after they are published in the Federal Register.
More employers can opt out of providing contraception coverage to their employees under final regulations from the Trump administration that narrow the Affordable Care Act’s contraceptive mandate.
The two regulations, released Nov. 7, allow an expanded group of employers and insurers to get out of covering contraception methods by objecting on either religious or moral grounds.
The first rule broadens exemptions to the ACA’s contraceptive mandate to entities that object to services covered by the mandate on the basis of sincerely held religious beliefs. The second rule protects nonprofit organizations and small businesses that have nonreligious moral convictions that oppose services covered by the mandate. The religious and moral exemptions apply to institutions of education, issuers, and individuals, but not to governmental entities.
When first proposed in 2017, Trump administration officials said the new policies would “better balance the government’s interest in promoting coverage for contraceptive and sterilization services with the government’s interests in providing conscience protections for entities with sincerely held moral convictions.” The U.S. Department of Health & Human Services estimates that the rules, which take effect in January 2019, will affect no more than 200 employers.
The American College of Obstetricians and Gynecologists expressed concern that the final rules will restrict patient access to meaningful contraceptive methods and will erode decades of progress in increasing women’s reproductive autonomy and restrict patient access to contraception.
“Women, families and our nation all benefit from seamless, affordable access to contraception,” ACOG President Lisa M. Hollier, MD, said in a statement. “Contraception improves women’s health and well-being, reduces unintended pregnancy, enables pregnancy spacing for safer pregnancies and deliveries, and empowers women’s engagement in the workforce and economic self-sufficiency. A woman’s employer should not determine whether or not she has this access.”
Marjorie Dannenfelser, president of Susan B. Anthony List, an anti-abortion group, praised the final rules, calling them needed protections from the burdensome Obama-era ACA abortifacient drug mandate.
“President Trump and HHS Secretary Azar delivered a huge victory for conscience rights and religious liberty in America,” Ms. Dannenfelser said in a statement. “No longer will Catholic nuns who care for the elderly poor be forced by the government to provide abortion-inducing drugs in their health care plans. Not only that, moral objectors such as Susan B. Anthony List, will also no longer have to pay for life-ending drugs that are antithetical to their mission and for which we have argued there is certainly no compelling state interest.”
The ACA initially required all employers to cover birth control for employees with no copayments, except for group health plans of religious employers, which were deemed exempt. Those religious employers were primarily churches and other houses of worship. After a number of complaints and legal challenges, the Obama administration created a workaround for nonprofit religious employers to opt out of the mandate.
However, critics argued the process itself was a violation of their religious freedom. The issue led to the case of Zubik v. Burwell, a legal challenge over the mandate exemption that went before the U.S. Supreme Court in March 2016. The issue was never resolved. In May 2016, the Supreme Court vacated the lower court rulings related to Zubik v. Burwell and remanded the case back to the four appeals courts that had originally ruled on the issue.
Under the approved regulations, employers or insurers can stop their coverage of contraceptive services if they have religious beliefs or moral convictions against covering birth control. Exempted entities and individuals also can choose to cover some, but not all, contraceptive services, depending on their specific religious or moral objection, according to an HHS fact sheet.
The agency emphasized that the regulations leave in place government programs that provide free or subsidized contraceptive coverage to low-income women, such as through community health centers, and that the rules do not ban any employer from covering contraceptives.
The regulations become effective 60 days after they are published in the Federal Register.
More employers can opt out of providing contraception coverage to their employees under final regulations from the Trump administration that narrow the Affordable Care Act’s contraceptive mandate.
The two regulations, released Nov. 7, allow an expanded group of employers and insurers to get out of covering contraception methods by objecting on either religious or moral grounds.
The first rule broadens exemptions to the ACA’s contraceptive mandate to entities that object to services covered by the mandate on the basis of sincerely held religious beliefs. The second rule protects nonprofit organizations and small businesses that have nonreligious moral convictions that oppose services covered by the mandate. The religious and moral exemptions apply to institutions of education, issuers, and individuals, but not to governmental entities.
When first proposed in 2017, Trump administration officials said the new policies would “better balance the government’s interest in promoting coverage for contraceptive and sterilization services with the government’s interests in providing conscience protections for entities with sincerely held moral convictions.” The U.S. Department of Health & Human Services estimates that the rules, which take effect in January 2019, will affect no more than 200 employers.
The American College of Obstetricians and Gynecologists expressed concern that the final rules will restrict patient access to meaningful contraceptive methods and will erode decades of progress in increasing women’s reproductive autonomy and restrict patient access to contraception.
“Women, families and our nation all benefit from seamless, affordable access to contraception,” ACOG President Lisa M. Hollier, MD, said in a statement. “Contraception improves women’s health and well-being, reduces unintended pregnancy, enables pregnancy spacing for safer pregnancies and deliveries, and empowers women’s engagement in the workforce and economic self-sufficiency. A woman’s employer should not determine whether or not she has this access.”
Marjorie Dannenfelser, president of Susan B. Anthony List, an anti-abortion group, praised the final rules, calling them needed protections from the burdensome Obama-era ACA abortifacient drug mandate.
“President Trump and HHS Secretary Azar delivered a huge victory for conscience rights and religious liberty in America,” Ms. Dannenfelser said in a statement. “No longer will Catholic nuns who care for the elderly poor be forced by the government to provide abortion-inducing drugs in their health care plans. Not only that, moral objectors such as Susan B. Anthony List, will also no longer have to pay for life-ending drugs that are antithetical to their mission and for which we have argued there is certainly no compelling state interest.”
The ACA initially required all employers to cover birth control for employees with no copayments, except for group health plans of religious employers, which were deemed exempt. Those religious employers were primarily churches and other houses of worship. After a number of complaints and legal challenges, the Obama administration created a workaround for nonprofit religious employers to opt out of the mandate.
However, critics argued the process itself was a violation of their religious freedom. The issue led to the case of Zubik v. Burwell, a legal challenge over the mandate exemption that went before the U.S. Supreme Court in March 2016. The issue was never resolved. In May 2016, the Supreme Court vacated the lower court rulings related to Zubik v. Burwell and remanded the case back to the four appeals courts that had originally ruled on the issue.
Under the approved regulations, employers or insurers can stop their coverage of contraceptive services if they have religious beliefs or moral convictions against covering birth control. Exempted entities and individuals also can choose to cover some, but not all, contraceptive services, depending on their specific religious or moral objection, according to an HHS fact sheet.
The agency emphasized that the regulations leave in place government programs that provide free or subsidized contraceptive coverage to low-income women, such as through community health centers, and that the rules do not ban any employer from covering contraceptives.
The regulations become effective 60 days after they are published in the Federal Register.
ACR readies first-ever guidelines on managing reproductive health in rheumatology
CHICAGO – Help is on the way for rheumatologists who may feel out of their depth regarding reproductive health issues in their patients.
for internal review in draft form. Lisa R. Sammaritano, MD, a leader of the expert panel that developed the evidence-based recommendations, shared highlights of the forthcoming guidelines at the annual meeting of the American College of Rheumatology.
“Our patients, fortunately, are pursuing pregnancy more often now than in years past. One of the key messages of the guidelines is that patients really do want to discuss these topics with their rheumatologist, even though that often does not happen now. What patients told us [in the guideline-development process] is their rheumatologist knows them better than their gynecologist or any of their other doctors because we have followed them for a long period of time and we understand their disease and their symptoms. They really want our input on questions about contraception, when to plan a pregnancy, and medication use,” said Dr. Sammaritano of the Hospital for Special Surgery and Cornell University in New York.
The guidelines were created over the course of a year and a half with extensive input from ob.gyns., as well as a patient panel. The project included a systematic review of more than 300 published studies in which guideline panelists attempt to find answers to an initial list of 370 questions. Dr. Sammaritano predicted that the guidelines will prove to be useful not only for rheumatologists, but for their colleagues in ob.gyn. as well. Just as rheumatologists likely haven’t kept up with the sea changes that have occurred in ob.gyn. since their medical school days, most ob.gyns. know little about rheumatic diseases.
“There’s room for education on both sides,” she observed in an interview. “I have had to write letters to gynecologists to get them to put my patients with antiphospholipid antibodies on a contraceptive that includes a progestin because the labeling says, ‘May increase risk of thrombosis.’ And yet if you look at the literature, most of the progestins do not increase the risk of thrombosis, even in patients who are already at increased risk because of a genetic prothrombotic abnormality. I practically had to sign my life away to get a gynecologist to put a progestin-containing IUD in my patient, whereas the risk of thrombosis to my patient with an unplanned pregnancy would have been 10-fold or 100-fold higher. Unplanned pregnancy is dangerous for patients with our diseases.”
And yet, she noted, half of all pregnancies in the United States are unplanned. Among women with rheumatic diseases, the proportion may well be even higher in light of their documented low rate of utilization of effective contraception.
A publication date for the guidelines won’t be set until the review is completed, but the plan is to issue three separate documents. One will address reproductive health outside of pregnancy, with key topics to include contraception, fertility preservation, menopause, and hormone replacement therapy. The second document will focus on pregnancy management, with special attention devoted to women with lupus or antiphospholipid antibodies because they are at particularly high risk of adverse pregnancy outcomes. The third document will be devoted to medications, covering issues including which medications can be continued during pregnancy and when to safely stop the ones that can’t. This section will address both maternal and paternal use of rheumatologic medications, the latter being a topic below the radar of ob.gyns.
The three medications whose paternal use in pregnancy generate the most questions in clinical practice are methotrexate, cyclophosphamide, and sulfasalazine.
“I cannot tell you how many times I’ve been asked whether male patients with rheumatic diseases need to stop their methotrexate before they plan to father a child – that’s been a big one. The answer is they don’t need to stop, but that’s a conditional recommendation because the product label still says to stop it 3 months before. But that’s based on theoretical concerns, and all the data support a lack of teratogenicity for men using methotrexate prior to and during pregnancy,” Dr. Sammaritano said.
Men on cyclophosphamide absolutely have to stop the drug 3 months before pregnancy because the drug causes DNA fragmentation in the sperm. Sulfasalazine is known to impair male fertility. The ACR guidelines will recommend that men continue the drug, but if pregnancy doesn’t occur within a reasonable time, then it’s appropriate to get a semen analysis rather than stopping sulfasalazine unnecessarily.
American College of Obstetricians and Gynecologists guidelines now recommend long-acting reversible contraception, including IUDs and progestin implants, as first-line contraception for all women. The ACR draft guidelines strongly recommend the same.
“That is new. The use of this form of contraception in women with rheumatic diseases is quite low. In general, our patients don’t use contraception as often as other women, and when they do, they don’t use effective contraception. There are many theories as to why that may be: perhaps it’s a focus on the more immediate issues of their rheumatic disease that doesn’t allow their rheumatologist to get to the point of discussing contraception,” according to Dr. Sammaritano.
Many rheumatologists will be pleasantly surprised to learn that the problem of increased risk of pelvic inflammatory disease associated with earlier-generation IUDs is no longer an issue with the current devices. And contrary to a misconception among some ob.gyns., autoimmune disease will not cause a woman to reject her IUD.
The ACR guidelines recommend continuing hydroxychloroquine in lupus patients during pregnancy – and considering starting the drug in those not already on it – because of strong evidence supporting both safety and benefit for mother and baby.
“We are recommending the use of low-dose aspirin for patients with lupus and antiphospholipid antibodies because those two conditions increase the risk for preeclampsia, and the ob.gyns. routinely use low-dose aspirin starting toward the end of the first trimester as preventive therapy. Large studies show that it reduces the risk,” she continued.
Dr. Sammaritano cautioned that the literature on the use of rheumatologic medications in pregnancy and breast feeding is generally weak – and in the case of the new oral small molecule JAK inhibitors, essentially nonexistent.
“A lot of our recommendations are conditional because we did not feel that the data support a strong recommendation. But you have to do something. As long as you communicate the idea that we’re doing the best we can with what information is available, I think patients will respond to that,” the rheumatologist said.
She reported having no financial conflicts regarding her presentation.
CHICAGO – Help is on the way for rheumatologists who may feel out of their depth regarding reproductive health issues in their patients.
for internal review in draft form. Lisa R. Sammaritano, MD, a leader of the expert panel that developed the evidence-based recommendations, shared highlights of the forthcoming guidelines at the annual meeting of the American College of Rheumatology.
“Our patients, fortunately, are pursuing pregnancy more often now than in years past. One of the key messages of the guidelines is that patients really do want to discuss these topics with their rheumatologist, even though that often does not happen now. What patients told us [in the guideline-development process] is their rheumatologist knows them better than their gynecologist or any of their other doctors because we have followed them for a long period of time and we understand their disease and their symptoms. They really want our input on questions about contraception, when to plan a pregnancy, and medication use,” said Dr. Sammaritano of the Hospital for Special Surgery and Cornell University in New York.
The guidelines were created over the course of a year and a half with extensive input from ob.gyns., as well as a patient panel. The project included a systematic review of more than 300 published studies in which guideline panelists attempt to find answers to an initial list of 370 questions. Dr. Sammaritano predicted that the guidelines will prove to be useful not only for rheumatologists, but for their colleagues in ob.gyn. as well. Just as rheumatologists likely haven’t kept up with the sea changes that have occurred in ob.gyn. since their medical school days, most ob.gyns. know little about rheumatic diseases.
“There’s room for education on both sides,” she observed in an interview. “I have had to write letters to gynecologists to get them to put my patients with antiphospholipid antibodies on a contraceptive that includes a progestin because the labeling says, ‘May increase risk of thrombosis.’ And yet if you look at the literature, most of the progestins do not increase the risk of thrombosis, even in patients who are already at increased risk because of a genetic prothrombotic abnormality. I practically had to sign my life away to get a gynecologist to put a progestin-containing IUD in my patient, whereas the risk of thrombosis to my patient with an unplanned pregnancy would have been 10-fold or 100-fold higher. Unplanned pregnancy is dangerous for patients with our diseases.”
And yet, she noted, half of all pregnancies in the United States are unplanned. Among women with rheumatic diseases, the proportion may well be even higher in light of their documented low rate of utilization of effective contraception.
A publication date for the guidelines won’t be set until the review is completed, but the plan is to issue three separate documents. One will address reproductive health outside of pregnancy, with key topics to include contraception, fertility preservation, menopause, and hormone replacement therapy. The second document will focus on pregnancy management, with special attention devoted to women with lupus or antiphospholipid antibodies because they are at particularly high risk of adverse pregnancy outcomes. The third document will be devoted to medications, covering issues including which medications can be continued during pregnancy and when to safely stop the ones that can’t. This section will address both maternal and paternal use of rheumatologic medications, the latter being a topic below the radar of ob.gyns.
The three medications whose paternal use in pregnancy generate the most questions in clinical practice are methotrexate, cyclophosphamide, and sulfasalazine.
“I cannot tell you how many times I’ve been asked whether male patients with rheumatic diseases need to stop their methotrexate before they plan to father a child – that’s been a big one. The answer is they don’t need to stop, but that’s a conditional recommendation because the product label still says to stop it 3 months before. But that’s based on theoretical concerns, and all the data support a lack of teratogenicity for men using methotrexate prior to and during pregnancy,” Dr. Sammaritano said.
Men on cyclophosphamide absolutely have to stop the drug 3 months before pregnancy because the drug causes DNA fragmentation in the sperm. Sulfasalazine is known to impair male fertility. The ACR guidelines will recommend that men continue the drug, but if pregnancy doesn’t occur within a reasonable time, then it’s appropriate to get a semen analysis rather than stopping sulfasalazine unnecessarily.
American College of Obstetricians and Gynecologists guidelines now recommend long-acting reversible contraception, including IUDs and progestin implants, as first-line contraception for all women. The ACR draft guidelines strongly recommend the same.
“That is new. The use of this form of contraception in women with rheumatic diseases is quite low. In general, our patients don’t use contraception as often as other women, and when they do, they don’t use effective contraception. There are many theories as to why that may be: perhaps it’s a focus on the more immediate issues of their rheumatic disease that doesn’t allow their rheumatologist to get to the point of discussing contraception,” according to Dr. Sammaritano.
Many rheumatologists will be pleasantly surprised to learn that the problem of increased risk of pelvic inflammatory disease associated with earlier-generation IUDs is no longer an issue with the current devices. And contrary to a misconception among some ob.gyns., autoimmune disease will not cause a woman to reject her IUD.
The ACR guidelines recommend continuing hydroxychloroquine in lupus patients during pregnancy – and considering starting the drug in those not already on it – because of strong evidence supporting both safety and benefit for mother and baby.
“We are recommending the use of low-dose aspirin for patients with lupus and antiphospholipid antibodies because those two conditions increase the risk for preeclampsia, and the ob.gyns. routinely use low-dose aspirin starting toward the end of the first trimester as preventive therapy. Large studies show that it reduces the risk,” she continued.
Dr. Sammaritano cautioned that the literature on the use of rheumatologic medications in pregnancy and breast feeding is generally weak – and in the case of the new oral small molecule JAK inhibitors, essentially nonexistent.
“A lot of our recommendations are conditional because we did not feel that the data support a strong recommendation. But you have to do something. As long as you communicate the idea that we’re doing the best we can with what information is available, I think patients will respond to that,” the rheumatologist said.
She reported having no financial conflicts regarding her presentation.
CHICAGO – Help is on the way for rheumatologists who may feel out of their depth regarding reproductive health issues in their patients.
for internal review in draft form. Lisa R. Sammaritano, MD, a leader of the expert panel that developed the evidence-based recommendations, shared highlights of the forthcoming guidelines at the annual meeting of the American College of Rheumatology.
“Our patients, fortunately, are pursuing pregnancy more often now than in years past. One of the key messages of the guidelines is that patients really do want to discuss these topics with their rheumatologist, even though that often does not happen now. What patients told us [in the guideline-development process] is their rheumatologist knows them better than their gynecologist or any of their other doctors because we have followed them for a long period of time and we understand their disease and their symptoms. They really want our input on questions about contraception, when to plan a pregnancy, and medication use,” said Dr. Sammaritano of the Hospital for Special Surgery and Cornell University in New York.
The guidelines were created over the course of a year and a half with extensive input from ob.gyns., as well as a patient panel. The project included a systematic review of more than 300 published studies in which guideline panelists attempt to find answers to an initial list of 370 questions. Dr. Sammaritano predicted that the guidelines will prove to be useful not only for rheumatologists, but for their colleagues in ob.gyn. as well. Just as rheumatologists likely haven’t kept up with the sea changes that have occurred in ob.gyn. since their medical school days, most ob.gyns. know little about rheumatic diseases.
“There’s room for education on both sides,” she observed in an interview. “I have had to write letters to gynecologists to get them to put my patients with antiphospholipid antibodies on a contraceptive that includes a progestin because the labeling says, ‘May increase risk of thrombosis.’ And yet if you look at the literature, most of the progestins do not increase the risk of thrombosis, even in patients who are already at increased risk because of a genetic prothrombotic abnormality. I practically had to sign my life away to get a gynecologist to put a progestin-containing IUD in my patient, whereas the risk of thrombosis to my patient with an unplanned pregnancy would have been 10-fold or 100-fold higher. Unplanned pregnancy is dangerous for patients with our diseases.”
And yet, she noted, half of all pregnancies in the United States are unplanned. Among women with rheumatic diseases, the proportion may well be even higher in light of their documented low rate of utilization of effective contraception.
A publication date for the guidelines won’t be set until the review is completed, but the plan is to issue three separate documents. One will address reproductive health outside of pregnancy, with key topics to include contraception, fertility preservation, menopause, and hormone replacement therapy. The second document will focus on pregnancy management, with special attention devoted to women with lupus or antiphospholipid antibodies because they are at particularly high risk of adverse pregnancy outcomes. The third document will be devoted to medications, covering issues including which medications can be continued during pregnancy and when to safely stop the ones that can’t. This section will address both maternal and paternal use of rheumatologic medications, the latter being a topic below the radar of ob.gyns.
The three medications whose paternal use in pregnancy generate the most questions in clinical practice are methotrexate, cyclophosphamide, and sulfasalazine.
“I cannot tell you how many times I’ve been asked whether male patients with rheumatic diseases need to stop their methotrexate before they plan to father a child – that’s been a big one. The answer is they don’t need to stop, but that’s a conditional recommendation because the product label still says to stop it 3 months before. But that’s based on theoretical concerns, and all the data support a lack of teratogenicity for men using methotrexate prior to and during pregnancy,” Dr. Sammaritano said.
Men on cyclophosphamide absolutely have to stop the drug 3 months before pregnancy because the drug causes DNA fragmentation in the sperm. Sulfasalazine is known to impair male fertility. The ACR guidelines will recommend that men continue the drug, but if pregnancy doesn’t occur within a reasonable time, then it’s appropriate to get a semen analysis rather than stopping sulfasalazine unnecessarily.
American College of Obstetricians and Gynecologists guidelines now recommend long-acting reversible contraception, including IUDs and progestin implants, as first-line contraception for all women. The ACR draft guidelines strongly recommend the same.
“That is new. The use of this form of contraception in women with rheumatic diseases is quite low. In general, our patients don’t use contraception as often as other women, and when they do, they don’t use effective contraception. There are many theories as to why that may be: perhaps it’s a focus on the more immediate issues of their rheumatic disease that doesn’t allow their rheumatologist to get to the point of discussing contraception,” according to Dr. Sammaritano.
Many rheumatologists will be pleasantly surprised to learn that the problem of increased risk of pelvic inflammatory disease associated with earlier-generation IUDs is no longer an issue with the current devices. And contrary to a misconception among some ob.gyns., autoimmune disease will not cause a woman to reject her IUD.
The ACR guidelines recommend continuing hydroxychloroquine in lupus patients during pregnancy – and considering starting the drug in those not already on it – because of strong evidence supporting both safety and benefit for mother and baby.
“We are recommending the use of low-dose aspirin for patients with lupus and antiphospholipid antibodies because those two conditions increase the risk for preeclampsia, and the ob.gyns. routinely use low-dose aspirin starting toward the end of the first trimester as preventive therapy. Large studies show that it reduces the risk,” she continued.
Dr. Sammaritano cautioned that the literature on the use of rheumatologic medications in pregnancy and breast feeding is generally weak – and in the case of the new oral small molecule JAK inhibitors, essentially nonexistent.
“A lot of our recommendations are conditional because we did not feel that the data support a strong recommendation. But you have to do something. As long as you communicate the idea that we’re doing the best we can with what information is available, I think patients will respond to that,” the rheumatologist said.
She reported having no financial conflicts regarding her presentation.
REPORTING FROM THE ACR ANNUAL MEETING
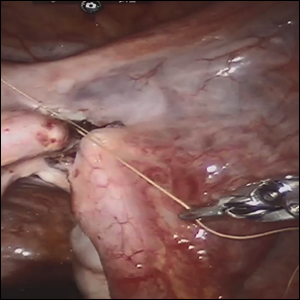
Robot-assisted laparoscopic tubal anastomosis following sterilization

Female sterilization is the most common method of contraception worldwide, and the second most common contraceptive method used in the United States. Approximately 643,000 sterilization procedures are performed annually.1 Approximately 1% to 3% of women who undergo sterilization will subsequently undergo a sterilization reversal.2 Although multiple variables have been identified, change in marital status is the most commonly cited reason for desiring a tubal reversal.3,4 Tubal anastomosis can be a technically challenging surgical procedure when done by laparoscopy, especially given the microsurgical elements that are required. Several modifications, including limiting the number of sutures, have evolved as a result of its tedious nature.5 By leveraging 3D magnification, articulating instruments, and tremor filtration, it is only natural that robotic surgery has been applied to tubal anastomosis.
In this video, we review some background information surrounding a tubal reversal, followed by demonstration of a robotic interpretation of a 2-stitch anastomosis technique in a patient who successfully conceived and delivered.6 Overall robot-assisted laparoscopic tubal anastomosis is a feasible and safe option for women who desire reversal of surgical sterilization, with pregnancy and live-birth rates comparable to those observed when an open technique is utilized.7 I hope that you will find this video beneficial to your clinical practice.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94:1-6.
- Moss CC. Sterilization: a review and update. Obstet Gynecol Clin North Am. 2015-12-01;42:713-724.
- Gordts S, Campo R, Puttemans P, Gordts S. Clinical factors determining pregnancy outcome after microsurgical tubal anastomosis. Fertil Steril. 2009;92:1198-1202.
- Chi I-C, Jones DB. Incidence, risk factors, and prevention of poststerilization regret in women. Obstet Gynecol Surv. 1994;49:722-732.
- Dubuisson JB, Swolin K. Laparoscopic tubal anastomosis (the one stitch technique): preliminary results. Human Reprod. 1995;10:2044-2046.
- Bissonnette FCA, Lapensee L, Bouzayen R. Outpatient laparoscopic tubal anastomosis and subsequent fertility. Fertil Steril. 1999;72:549-552.
- Caillet M, Vandromme J, Rozenberg S, Paesmans M, Germay O, Degueldre M. Robotically assisted laparoscopic microsurgical tubal anastomosis: a retrospective study. Fertil Steril. 2010;94:1844-1847.

Female sterilization is the most common method of contraception worldwide, and the second most common contraceptive method used in the United States. Approximately 643,000 sterilization procedures are performed annually.1 Approximately 1% to 3% of women who undergo sterilization will subsequently undergo a sterilization reversal.2 Although multiple variables have been identified, change in marital status is the most commonly cited reason for desiring a tubal reversal.3,4 Tubal anastomosis can be a technically challenging surgical procedure when done by laparoscopy, especially given the microsurgical elements that are required. Several modifications, including limiting the number of sutures, have evolved as a result of its tedious nature.5 By leveraging 3D magnification, articulating instruments, and tremor filtration, it is only natural that robotic surgery has been applied to tubal anastomosis.
In this video, we review some background information surrounding a tubal reversal, followed by demonstration of a robotic interpretation of a 2-stitch anastomosis technique in a patient who successfully conceived and delivered.6 Overall robot-assisted laparoscopic tubal anastomosis is a feasible and safe option for women who desire reversal of surgical sterilization, with pregnancy and live-birth rates comparable to those observed when an open technique is utilized.7 I hope that you will find this video beneficial to your clinical practice.

Female sterilization is the most common method of contraception worldwide, and the second most common contraceptive method used in the United States. Approximately 643,000 sterilization procedures are performed annually.1 Approximately 1% to 3% of women who undergo sterilization will subsequently undergo a sterilization reversal.2 Although multiple variables have been identified, change in marital status is the most commonly cited reason for desiring a tubal reversal.3,4 Tubal anastomosis can be a technically challenging surgical procedure when done by laparoscopy, especially given the microsurgical elements that are required. Several modifications, including limiting the number of sutures, have evolved as a result of its tedious nature.5 By leveraging 3D magnification, articulating instruments, and tremor filtration, it is only natural that robotic surgery has been applied to tubal anastomosis.
In this video, we review some background information surrounding a tubal reversal, followed by demonstration of a robotic interpretation of a 2-stitch anastomosis technique in a patient who successfully conceived and delivered.6 Overall robot-assisted laparoscopic tubal anastomosis is a feasible and safe option for women who desire reversal of surgical sterilization, with pregnancy and live-birth rates comparable to those observed when an open technique is utilized.7 I hope that you will find this video beneficial to your clinical practice.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94:1-6.
- Moss CC. Sterilization: a review and update. Obstet Gynecol Clin North Am. 2015-12-01;42:713-724.
- Gordts S, Campo R, Puttemans P, Gordts S. Clinical factors determining pregnancy outcome after microsurgical tubal anastomosis. Fertil Steril. 2009;92:1198-1202.
- Chi I-C, Jones DB. Incidence, risk factors, and prevention of poststerilization regret in women. Obstet Gynecol Surv. 1994;49:722-732.
- Dubuisson JB, Swolin K. Laparoscopic tubal anastomosis (the one stitch technique): preliminary results. Human Reprod. 1995;10:2044-2046.
- Bissonnette FCA, Lapensee L, Bouzayen R. Outpatient laparoscopic tubal anastomosis and subsequent fertility. Fertil Steril. 1999;72:549-552.
- Caillet M, Vandromme J, Rozenberg S, Paesmans M, Germay O, Degueldre M. Robotically assisted laparoscopic microsurgical tubal anastomosis: a retrospective study. Fertil Steril. 2010;94:1844-1847.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94:1-6.
- Moss CC. Sterilization: a review and update. Obstet Gynecol Clin North Am. 2015-12-01;42:713-724.
- Gordts S, Campo R, Puttemans P, Gordts S. Clinical factors determining pregnancy outcome after microsurgical tubal anastomosis. Fertil Steril. 2009;92:1198-1202.
- Chi I-C, Jones DB. Incidence, risk factors, and prevention of poststerilization regret in women. Obstet Gynecol Surv. 1994;49:722-732.
- Dubuisson JB, Swolin K. Laparoscopic tubal anastomosis (the one stitch technique): preliminary results. Human Reprod. 1995;10:2044-2046.
- Bissonnette FCA, Lapensee L, Bouzayen R. Outpatient laparoscopic tubal anastomosis and subsequent fertility. Fertil Steril. 1999;72:549-552.
- Caillet M, Vandromme J, Rozenberg S, Paesmans M, Germay O, Degueldre M. Robotically assisted laparoscopic microsurgical tubal anastomosis: a retrospective study. Fertil Steril. 2010;94:1844-1847.
Are women seeking short-acting contraception satisfied with LARC after giving it a try?
EXPERT COMMENTARY
Because of women’s personal preference and aversion, for various reasons, to LARC methods, the current estimated use rate of 17% for LARC methods would increase only to 24% to 29% even if major barriers, such as cost and availability, were removed.1 To gain more insight into this issue, Hubacher and colleagues sought to determine if LARC methods would meet the contraceptive needs and be acceptable to a population of women who were not seeking these methods actively and who might have some reservation about using them.
Details of the study
The authors approached women actively seeking 1 of the 2 SARC methods but not a LARC method for contraception. They enrolled 524 women into a cohort study in which they received their desired SARC method. In addition, 392 women agreed to be enrolled in a randomized clinical trial comparing women beginning a LARC method for the first time with a group receiving 1 of the 2 SARC methods.
Importance of covered costs. Of note, the women in the randomized trial had the costs of the insertion or removal of the LARC method covered; those randomly assigned to the comparative SARC arm had the costs of their oral contraceptives (OCs) or depot medroxyprogesterone acetate (DMPA) covered for the first year of use. Underwriting the costs in the randomized study was likely important for study recruitment, since 47% of participants who were randomized to the LARC group cited cost as one of the reasons they did not try a LARC method previously.
Satisfaction with contraceptive method. In addition to the differences in continuation rates and pregnancy rates noted, it is interesting that, among women who tried a LARC method and who had some persistent negative feelings about the method, 65.9% would try the method again.
Satisfaction levels were estimated using 3 choices, with “happiness” being the highest level of satisfaction, followed by “neutral” and “unhappy.” At 24 months, the number of women indicating happiness was similar among the 3 study groups: 71.4% for the LARC randomized group, 75.0% for the randomized SARC group, and 77.6% for the preferred SARC cohort group.
Among women who discontinued their LARC method, occurrence of adverse effects was the reason given 74.2% of the time, while among SARC method users in both groups there was no dominant reason for discontinuation. Also, among women who discontinued their method, the percentage indicating happiness was 32.2% for the LARC randomized group compared with 69.9% and 68.2% for the randomized and preference cohort SARC groups, respectively.
Study strengths and weaknesses
This study had several strengths. The population from which the study groups were obtained was demographically diverse and was appropriate for determining if women with reservations about LARC methods could have satisfactory outcomes similar to women who self-select LARC methods. Further, the 24 months of observations indicate that, for the most part, satisfaction persisted.
One of the study’s shortcomings is the limited data on the subsets, that is, the specific method chosen, within each of the study groups.
-- Ronald T. Burkman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Foster DG, Barar R, Gould H, et al. Projections and opinions from 100 experts in long-acting reversible contraception. Contraception. 2015;92:543-552.
EXPERT COMMENTARY
Because of women’s personal preference and aversion, for various reasons, to LARC methods, the current estimated use rate of 17% for LARC methods would increase only to 24% to 29% even if major barriers, such as cost and availability, were removed.1 To gain more insight into this issue, Hubacher and colleagues sought to determine if LARC methods would meet the contraceptive needs and be acceptable to a population of women who were not seeking these methods actively and who might have some reservation about using them.
Details of the study
The authors approached women actively seeking 1 of the 2 SARC methods but not a LARC method for contraception. They enrolled 524 women into a cohort study in which they received their desired SARC method. In addition, 392 women agreed to be enrolled in a randomized clinical trial comparing women beginning a LARC method for the first time with a group receiving 1 of the 2 SARC methods.
Importance of covered costs. Of note, the women in the randomized trial had the costs of the insertion or removal of the LARC method covered; those randomly assigned to the comparative SARC arm had the costs of their oral contraceptives (OCs) or depot medroxyprogesterone acetate (DMPA) covered for the first year of use. Underwriting the costs in the randomized study was likely important for study recruitment, since 47% of participants who were randomized to the LARC group cited cost as one of the reasons they did not try a LARC method previously.
Satisfaction with contraceptive method. In addition to the differences in continuation rates and pregnancy rates noted, it is interesting that, among women who tried a LARC method and who had some persistent negative feelings about the method, 65.9% would try the method again.
Satisfaction levels were estimated using 3 choices, with “happiness” being the highest level of satisfaction, followed by “neutral” and “unhappy.” At 24 months, the number of women indicating happiness was similar among the 3 study groups: 71.4% for the LARC randomized group, 75.0% for the randomized SARC group, and 77.6% for the preferred SARC cohort group.
Among women who discontinued their LARC method, occurrence of adverse effects was the reason given 74.2% of the time, while among SARC method users in both groups there was no dominant reason for discontinuation. Also, among women who discontinued their method, the percentage indicating happiness was 32.2% for the LARC randomized group compared with 69.9% and 68.2% for the randomized and preference cohort SARC groups, respectively.
Study strengths and weaknesses
This study had several strengths. The population from which the study groups were obtained was demographically diverse and was appropriate for determining if women with reservations about LARC methods could have satisfactory outcomes similar to women who self-select LARC methods. Further, the 24 months of observations indicate that, for the most part, satisfaction persisted.
One of the study’s shortcomings is the limited data on the subsets, that is, the specific method chosen, within each of the study groups.
-- Ronald T. Burkman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Because of women’s personal preference and aversion, for various reasons, to LARC methods, the current estimated use rate of 17% for LARC methods would increase only to 24% to 29% even if major barriers, such as cost and availability, were removed.1 To gain more insight into this issue, Hubacher and colleagues sought to determine if LARC methods would meet the contraceptive needs and be acceptable to a population of women who were not seeking these methods actively and who might have some reservation about using them.
Details of the study
The authors approached women actively seeking 1 of the 2 SARC methods but not a LARC method for contraception. They enrolled 524 women into a cohort study in which they received their desired SARC method. In addition, 392 women agreed to be enrolled in a randomized clinical trial comparing women beginning a LARC method for the first time with a group receiving 1 of the 2 SARC methods.
Importance of covered costs. Of note, the women in the randomized trial had the costs of the insertion or removal of the LARC method covered; those randomly assigned to the comparative SARC arm had the costs of their oral contraceptives (OCs) or depot medroxyprogesterone acetate (DMPA) covered for the first year of use. Underwriting the costs in the randomized study was likely important for study recruitment, since 47% of participants who were randomized to the LARC group cited cost as one of the reasons they did not try a LARC method previously.
Satisfaction with contraceptive method. In addition to the differences in continuation rates and pregnancy rates noted, it is interesting that, among women who tried a LARC method and who had some persistent negative feelings about the method, 65.9% would try the method again.
Satisfaction levels were estimated using 3 choices, with “happiness” being the highest level of satisfaction, followed by “neutral” and “unhappy.” At 24 months, the number of women indicating happiness was similar among the 3 study groups: 71.4% for the LARC randomized group, 75.0% for the randomized SARC group, and 77.6% for the preferred SARC cohort group.
Among women who discontinued their LARC method, occurrence of adverse effects was the reason given 74.2% of the time, while among SARC method users in both groups there was no dominant reason for discontinuation. Also, among women who discontinued their method, the percentage indicating happiness was 32.2% for the LARC randomized group compared with 69.9% and 68.2% for the randomized and preference cohort SARC groups, respectively.
Study strengths and weaknesses
This study had several strengths. The population from which the study groups were obtained was demographically diverse and was appropriate for determining if women with reservations about LARC methods could have satisfactory outcomes similar to women who self-select LARC methods. Further, the 24 months of observations indicate that, for the most part, satisfaction persisted.
One of the study’s shortcomings is the limited data on the subsets, that is, the specific method chosen, within each of the study groups.
-- Ronald T. Burkman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Foster DG, Barar R, Gould H, et al. Projections and opinions from 100 experts in long-acting reversible contraception. Contraception. 2015;92:543-552.
- Foster DG, Barar R, Gould H, et al. Projections and opinions from 100 experts in long-acting reversible contraception. Contraception. 2015;92:543-552.
Should return to fertility be a concern for nulliparous patients using an IUD?
Investigators from the University of Texas Southwestern are dispelling the myth that you shouldn’t recommend intrauterine devices (IUDs) for nulliparous women because the devices might make it more difficult for them to become pregnant after discontinuation. They found that nulliparous women can just as easily get pregnant after using a progestin intrauterine system (IUS) as parous women,1 according to results of a study presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6–10, Denver, Colorado).
Bruce R. Carr, MD, lead investigator of the study, explained in an interview with OBG Management, “There have been a number of studies—maybe 10 to 15 years ago—that looked at pregnancy rates when patients stopped using IUDs, but most of these studies were done in women who were multiparous. There is almost no data on patients who are nulliparous stopping an IUD and trying to get pregnant.”
Participants and methods. This prospective, multicenter, clinical trial, which is still ongoing, is evaluating the efficacy and safety for up to 10 years of the Liletta levonorgestrel 52-mg IUS in nulliparous and parous women ages 16 to 45 years. Every 3 months for up to 1 year, the investigators contacted the women who discontinued the IUS during the first 5 years of use and who were trying to become pregnant to determine pregnancy status.
Outcomes. The primary outcome was time to pregnancy among nulliparous vs parous women after discontinuation of a progestin IUS.
Findings. Overall, 132 (87%) of 152 women ages 16 to 35 years at the beginning of the study who attempted to become pregnant did so within 1 year of discontinuing the IUS, and there was no difference in pregnancy rates between nulliparous and parous women (87.5% vs 86.1%, respectively; P<.82) or between nulligravid and gravid women (88.2% vs 85.7%, respectively; P<.81). High percentages of women became pregnant by the end of 3 months (43.4%) and 6 months (69.7%), with a median time to conception of 91.5 days. The women used the IUS for a median of 34 months before discontinuation. Length of IUS use and age of the women at IUS discontinuation did not affect pregnancy rates at 12 months postdiscontinuation in either nulliparous or parous women (TABLE).1
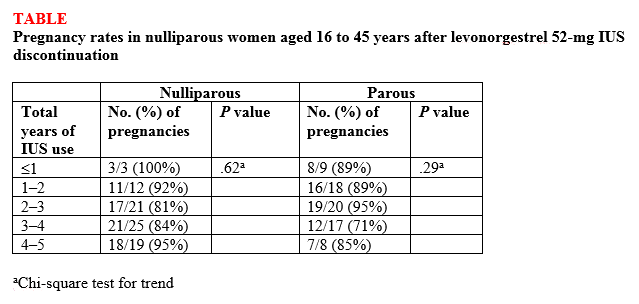
“The bottom line,” according to Dr. Carr, is that the “pregnancy rates were the same in women who had never been pregnant compared with women who had previously been pregnant.” He continued, “People worried that if a patient who had never been pregnant used an IUD that maybe she was going to have a harder time getting pregnant after discontinuing, and now we know that is not true. It [the study] reinforces the option of using progestin IUDs and not having to worry about future pregnancy.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
This article was updated October 15, 2018.
- Carr BR, Thomas MA, Gangestad A, Eisenberg DL, Olariu AI, Creinin MD. Return of fertility in nulliparous and parous women after levonorgestrel 52 mg intrauterine system discontinuation [ASRM abstract O-104]. Fertil Steril. 2018;110(45 suppl):e46.
Investigators from the University of Texas Southwestern are dispelling the myth that you shouldn’t recommend intrauterine devices (IUDs) for nulliparous women because the devices might make it more difficult for them to become pregnant after discontinuation. They found that nulliparous women can just as easily get pregnant after using a progestin intrauterine system (IUS) as parous women,1 according to results of a study presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6–10, Denver, Colorado).
Bruce R. Carr, MD, lead investigator of the study, explained in an interview with OBG Management, “There have been a number of studies—maybe 10 to 15 years ago—that looked at pregnancy rates when patients stopped using IUDs, but most of these studies were done in women who were multiparous. There is almost no data on patients who are nulliparous stopping an IUD and trying to get pregnant.”
Participants and methods. This prospective, multicenter, clinical trial, which is still ongoing, is evaluating the efficacy and safety for up to 10 years of the Liletta levonorgestrel 52-mg IUS in nulliparous and parous women ages 16 to 45 years. Every 3 months for up to 1 year, the investigators contacted the women who discontinued the IUS during the first 5 years of use and who were trying to become pregnant to determine pregnancy status.
Outcomes. The primary outcome was time to pregnancy among nulliparous vs parous women after discontinuation of a progestin IUS.
Findings. Overall, 132 (87%) of 152 women ages 16 to 35 years at the beginning of the study who attempted to become pregnant did so within 1 year of discontinuing the IUS, and there was no difference in pregnancy rates between nulliparous and parous women (87.5% vs 86.1%, respectively; P<.82) or between nulligravid and gravid women (88.2% vs 85.7%, respectively; P<.81). High percentages of women became pregnant by the end of 3 months (43.4%) and 6 months (69.7%), with a median time to conception of 91.5 days. The women used the IUS for a median of 34 months before discontinuation. Length of IUS use and age of the women at IUS discontinuation did not affect pregnancy rates at 12 months postdiscontinuation in either nulliparous or parous women (TABLE).1

“The bottom line,” according to Dr. Carr, is that the “pregnancy rates were the same in women who had never been pregnant compared with women who had previously been pregnant.” He continued, “People worried that if a patient who had never been pregnant used an IUD that maybe she was going to have a harder time getting pregnant after discontinuing, and now we know that is not true. It [the study] reinforces the option of using progestin IUDs and not having to worry about future pregnancy.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
This article was updated October 15, 2018.
Investigators from the University of Texas Southwestern are dispelling the myth that you shouldn’t recommend intrauterine devices (IUDs) for nulliparous women because the devices might make it more difficult for them to become pregnant after discontinuation. They found that nulliparous women can just as easily get pregnant after using a progestin intrauterine system (IUS) as parous women,1 according to results of a study presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6–10, Denver, Colorado).
Bruce R. Carr, MD, lead investigator of the study, explained in an interview with OBG Management, “There have been a number of studies—maybe 10 to 15 years ago—that looked at pregnancy rates when patients stopped using IUDs, but most of these studies were done in women who were multiparous. There is almost no data on patients who are nulliparous stopping an IUD and trying to get pregnant.”
Participants and methods. This prospective, multicenter, clinical trial, which is still ongoing, is evaluating the efficacy and safety for up to 10 years of the Liletta levonorgestrel 52-mg IUS in nulliparous and parous women ages 16 to 45 years. Every 3 months for up to 1 year, the investigators contacted the women who discontinued the IUS during the first 5 years of use and who were trying to become pregnant to determine pregnancy status.
Outcomes. The primary outcome was time to pregnancy among nulliparous vs parous women after discontinuation of a progestin IUS.
Findings. Overall, 132 (87%) of 152 women ages 16 to 35 years at the beginning of the study who attempted to become pregnant did so within 1 year of discontinuing the IUS, and there was no difference in pregnancy rates between nulliparous and parous women (87.5% vs 86.1%, respectively; P<.82) or between nulligravid and gravid women (88.2% vs 85.7%, respectively; P<.81). High percentages of women became pregnant by the end of 3 months (43.4%) and 6 months (69.7%), with a median time to conception of 91.5 days. The women used the IUS for a median of 34 months before discontinuation. Length of IUS use and age of the women at IUS discontinuation did not affect pregnancy rates at 12 months postdiscontinuation in either nulliparous or parous women (TABLE).1

“The bottom line,” according to Dr. Carr, is that the “pregnancy rates were the same in women who had never been pregnant compared with women who had previously been pregnant.” He continued, “People worried that if a patient who had never been pregnant used an IUD that maybe she was going to have a harder time getting pregnant after discontinuing, and now we know that is not true. It [the study] reinforces the option of using progestin IUDs and not having to worry about future pregnancy.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
This article was updated October 15, 2018.
- Carr BR, Thomas MA, Gangestad A, Eisenberg DL, Olariu AI, Creinin MD. Return of fertility in nulliparous and parous women after levonorgestrel 52 mg intrauterine system discontinuation [ASRM abstract O-104]. Fertil Steril. 2018;110(45 suppl):e46.
- Carr BR, Thomas MA, Gangestad A, Eisenberg DL, Olariu AI, Creinin MD. Return of fertility in nulliparous and parous women after levonorgestrel 52 mg intrauterine system discontinuation [ASRM abstract O-104]. Fertil Steril. 2018;110(45 suppl):e46.
Abortion, the travel ban, and other top Supreme Court rulings affecting your practice
The 2017−2018 term of the Supreme Court of the United States (SCOTUS) was momentous. Justice Anthony Kennedy, who had been the deciding vote in most of the 5 to 4 cases for a generation, announced his retirement as of July 31, 2018. In addition, the Court decided a number of cases of interest to ObGyns. In this article we review some of those cases, as well as consider the future of the Court without Justice Kennedy. In selecting cases, we have given special attention to those in which national medical organizations filed amicus briefs. These “amicus curiae” or “friend of the court” briefs are filed by an entity who is not party to a case but wants to provide information or views to the court.

1. Abortion rulings

The Court decided 2 abortion cases and rejected a request to hear a third.
National Institute of Family and Life Advocates v Becerra
In this case,1 the Court struck down a California law that required pregnancy crisis centers not offering abortions (generally operated by pro-life groups) to provide special notices to clients.2
At stake. These notices would inform clients that California provides free or low-cost services, including abortions, and provide a phone number to call for those services.
There were many amicus briefs filed in this case, including those by the American College of Obstetricians and Gynecologists (ACOG) and other specialty boards,3 as well as the American Association of Pro-Life Obstetricians and Gynecologists and other pro-life organizations.4 ACOG’s brief argued that the California-required notice facilitates the goal of allowing women to receive medical services without harmful delay.
Final ruling. The Court held that the law required clinics to engage in speech with which the clinics disagreed (known as “compelled speech”). It also noted that California disclosure requirements were “wildly underinclusive” because they apply only to some clinics. The majority felt that there was no strong state interest in compelling this speech because there were other alternatives for the state to provide information about the availability of abortion and other services. The Court found that the clinics were likely to succeed on the merits of their claims of a First Amendment (free speech) violation.
Right to abortion for illegal immigrants in custody
A very unusual abortion case involved “Jane Doe,” a minor who was at 8 weeks’ gestation when she illegally crossed the border into the United States.5 She was placed in a federally-funded shelter where she requested an abortion. The facility denied that request.
At stake. Legal argument ensued about releasing her to another facility for an abortion, as the argument was made that pregnant minors who are apprehended crossing into the United States illegally and placed into the custody of federal officials should have abortion access. A lower Court of Appeals ruled against the Trump Administration’s policy of denying abortions to undocumented minors in federal custody. During the process of the federal government taking the case to the Supreme Court, the attorneys for Doe moved appointments around and, without notice, the abortion was performed. Government attorneys said that Doe’s attorneys made “what appear to be material misrepresentations and omissions” designed to “thwart [the Supreme Court’s] review” of the case.5 The government requested that the Court vacate the order of the Court of Appeals so that it could not be used as precedent.
Final ruling. The Court granted the governments request to vacate the lower court’s order because the minor was no longer pregnant and the order was therefore moot. The basic issue in this case (the right of in-custody minors to access abortions) remains unresolved. It is likely to appear before the Court in the future.
Continue to: Access to medical abortions
Access to medical abortions
An Arkansas law requires that a physician administering medical abortions contract with a physician who has admitting privileges at a hospital (a “contracted physician”).
At stake. Planned Parenthood filed suit challenging the requirement as unnecessary and harmful because it would result in the closure of 2 of the 3 abortion providers in Arkansas. ACOG filed an amicus brief urging the Supreme Court to consider the case.6 (Technically this was a petition for a Writ of Certiorari, the procedure by which the Court accepts cases. It accepts only about 1% of applications.) ACOG argued that there was no medical reason for the contracted physician requirement, and noted the harm it would do to women who would not have access to abortions.
Final ruling. On May 29, 2018, the Court declined to hear the case. This case is still active in the lower courts and may eventually return to the Supreme Court.

2. The patent system

The medical profession depends on the patent system to encourage the discovery of new patents efficiently and effectively. In 2012, Congress passed the America Invents Act7 that authorizes a petition by anyone other than the patent holder to the Patent and Trademark Office (PTO) for an “inter partes review” to assess a challenge to the patent’s legitimacy. If the PTO determines that there may be merit to the claim, the Patent Trial and Appeal Board undertakes a trial-like review process that may validate, invalidate, or amend the patent. The Board’s decision is subject to appellate court review.
At stake. This term, the inter partes review was challenged as unconstitutional on technical bases.8
Final ruling. The Court rejected this claim and approved the current administrative inter partes review process. The Court determined that once the Patent Office takes a petition challenging a patent, it must decide all of the claims against the patent, not pick and choose which elements of the challenge to evaluate.9 The Court’s decision upheld patent-review reform, but will require the Patent Office to tweak its procedures.

3. The travel ban

ACOG, the American Medical Association (AMA), the Association of American Medical Colleges, and more than 30 other health care and specialty associations filed an amicus brief regarding one of the most anticipated cases of the term—the “travel ban.”10
At stake. The essential argument of these organizations was that the US health care system depends on professionals from other countries. An efficient and fair immigration program is, therefore, important to advance the nation’s “health security.” During the 2016−2017 term, the Court considered but then removed the issue from its calendar when the Trump Administration issued a revised travel ban.11
In September 2017, President Trump’s proclamation imposed a range of entry restrictions on the citizens of 8 countries, most (but not all) of which are predominantly Muslim. The government indicated that, in a study by Homeland Security and the State Department, these countries were identified as having especially deficient information-sharing practices and presented national security concerns. Trump v Hawaii12 challenged this proclamation.
Final ruling. The majority of the Court upheld the travel ban. For the 5-Justice majority led by Chief Justice Roberts, the case came down to 3 things:
- The Constitution and the laws passed by Congress of necessity give the President great authority to engage in foreign policy, including policies regarding entry into the country.
- The courts are very reluctant to get into the substance of foreign affairs—they are not equipped to know in detail what the facts are, and things change very fast.
- If courts start tinkering with foreign policy and things turn bad, it will appear that the courts are to blame and were interfering in an area about which they are not competent.
Continue to: 4. Did a credit card case add risk to health insurance markets?

It was just a credit card case, but one in which the AMA saw a real risk to regulation of the health insurance markets.
At stake. Technically, Ohio v American Express concerned a claim that American Express (AmEx) violated antitrust laws when it prohibited merchants taking its credit card from “steering” customers to cards with lower fees.13 AmEx maintained that, because credit cards were a special kind of “2-sided” market (connecting merchants on one side and customers on the other), antitrust laws should not be strictly enforced.
The AMA noticed that special rules regarding 2-sided markets might apply to health insurance, and it submitted an amicus brief14 that noted: “dominant health insurance networks … have imposed and could further impose rules or effectively erect barriers that prohibit physicians from referring patients to certain specialists, particularly out-of-network specialists, for innovative and even necessary medical tests.”14 It concluded that the antitrust rule AmEx was suggesting would make it nearly impossible to challenge these unfair provisions in health insurance arrangements.
Final ruling. The Court, however, accepted the AmEx position, making it very difficult to develop an antitrust case against 2-sided markets. It remains to be seen the degree to which the AMA concern about health insurance markets will be realized.

5. Gay wedding and a bakeshop

At stake. In Masterpiece Cakeshop v Colorado, a cakemaker declined to design a cake for a gay wedding and had been disciplined under Colorado law for discriminating against the couple based on sexual orientation.15
Final ruling. The Court, however, found that the Colorado regulators had, ironically, shown such religious animus in the way they treated the baker that the regulators themselves had discriminated on the basis of religion. As a result, the Court reversed the sanctions against the baker.
This decision was fairly narrow. It does not, for example, stand for the proposition that there may be a general religious exception to antidiscrimination laws. The question of broader religious or free-speech objections to antidiscrimination laws remains for another time.
Amicus brief. It was interesting that the American College of Pediatricians, American Association of Pro-Life Obstetricians and Gynecologists, and others, filed an amicus brief to report with concern the “demands that individual medical professionals must perform, assist with, or facilitate abortions, without regard to the teachings of their own faiths, consciences, and convictions.”16 The brief also noted that “issues in the present case implicate the fundamental rights of health care professionals, and to respectfully urge that the Court should by no means permit any weakening or qualification of well-established protections against compelled speech, and of free exercise” of religion.16
Arbitration. The Court upheld, as it has in most recent terms, another arbitration agreement.1 This case concerned an employment agreement in which employees consented to submit to arbitration rather than file lawsuits and not use class action claims.
Search of cell-phone location. Cell phones, whenever turned on, connect with cell towers that record the phone’s location several times a minute. Cell companies store this information, creating a virtual map of where the owner is at all times. The Federal Bureau of Investigation asked a cell company for location information for several people during a 127-day period in which robberies were committed.2 The Court held that the search was illegal in the absence of a warrant.
Public employee unions. The Court held that agency (fair share) fees, in which public employees who are not union members can be required to pay dues for the bargaining and grievance activities (from which they generally benefit), violate the First Amendment. The majority held that forcing public employees to pay fees to unions requires the employees, through those fees, to engage in political activities with which they disagree.3 This is a form of compelled speech, which the Court found violates the First Amendment. Health care professionals who are public employees in positions that have union representation will probably have the opportunity to opt out of agency agreements.
Internet sales tax. The Court permitted states to charge sales tax on out-of-state Internet purchases.4 In doing so, a state may require out-of-state companies to collect taxes on sales to its residents.
References
- Epic Systems Corp. v Lewis, 584 US 16 285 (2018).
- Carpenter v United States, 585 US 16 402 (2018).
- Janus v State, County, and Municipal Employees, 585 US 16 1466 (2018).
- South Dakota v Wayfair, Inc, 585 US 17 494 (2018).
Clues to the future
During the term that ran from October 2, 2017, through June 27, 2018, the Court issued 72 decisions. An unusually high proportion of cases (26%; 19 cases) were decided on a 5 to 4 vote. Last term, the rate of 5 to 4 decisions was 10%; the 6-year average was 18%. The unanimous decision rate was 39% this term, compared with 59% last term, and 50% on average.
The rate of 5 to 4 cases provides a clue about the Court’s general direction. The number of times each Justice was in the majority in those nineteen 5 to 4 decisions included: Chief Justice Roberts, 17; and Justices Kennedy, 16; Gorsuch, 16; Thomas, 15; and Alito, 15; compared with Justices Ginsburg, 5; Breyer, 4; Sotomayor, 4; and Kagan, 3.
The Court convened on October 1, 2018. At this writing, whether the new term starts with 8 or 9 justices remains a question. President Trump nominated Brett Kavanaugh, JD, to take Justice Kennedy’s place on the Court. His professional qualifications and experience appear to make him qualified for a position on the Court, but as we have seen, there are many other elements that go into confirming a Justice’s nomination.
Justice Anthony Kennedy was the deciding vote in the overwhelming majority of the 5 to 4 decisions in 20 of his 30 years on the Court. The areas in which he had an especially important impact include1:
- Gay rights. Justice Kennedy wrote the opinions (usually 5 to 4 decisions) in a number of groundbreaking gay-rights cases, including decriminalizing homosexual conduct, striking down the Defense of Marriage Act, and finding that the Constitution requires states to recognize gay marriage.
- The death penalty. Justice Kennedy wrote decisions that prohibited states from imposing the death penalty for any crime other than murder, for defendants who were under 18 when they committed the crime, and for defendants with serious developmental disabilities. He expressed reservations about long-term solitary confinement, but did not have a case that allowed him to decide its constitutionality.
- The First Amendment. Early in his service on the Court, he held that the First Amendment protected flag burning as a form of speech. He decided many important freespeech and freedom-of-religion cases that have set a standard for protecting those fundamental freedoms.
- Use of health and social science data. Justice Kennedy was more open to mental health information and cited it more often than most other Justices.
- Abortion rights? Many commentators would add protecting the right to choose to have an abortion to the above list. Justice Kennedy was a central figure in one case that declined to back away from Roe v Wade, and joined a more recent decision that struck down a Texas law that created an undue burden on women seeking abortion. Plus, he also voted to uphold abortion restrictions, such as “partial-birth-abortion laws.” So there is a good argument for including abortion rights on the list, although he did not break new ground.
Justice Kennedy as a person
Outside the courtroom, Justice Kennedy is a person of great warmth and compassion. He is a natural teacher and spends a great deal of time with students. When asked how he would like to be remembered, Justice Kennedy once replied, “Somebody who’s decent, and honest, and fair, and who’s absolutely committed to the proposition that freedom is America’s gift to the rest of the world.” I agree with that assessment.
STEVEN R. SMITH, MS, JD
Reference
- South Dakota v Wayfair, Inc, 585 US (2018)
Next term, the Court is scheduled to hear cases regarding pharmaceutical liability, double jeopardy, sex-offender registration, expert witnesses, Social Security disability benefits, and the Age Discrimination in Employment Act. There will be at least 3 arbitration cases. Health care and reproductive rights will continue to be an important part of the Court’s docket.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- National Institute of Family and Life Advocates v Becerra, 585 US 16 1140 (2018).
- California Reproductive Freedom, Accountability, Comprehensive Care, and Transparency Act (FACT Act), Cal. Health & Safety Code Ann. §123470 et seq. (West 2018).
- Brief amici curiae of American Academy of Pediatrics, et al. in National Institute of Family and Life Advocates v Becerra, February 27, 2018.
- Brief amici curiae of American Association of Pro-Life Obstetricians & Gynecologists, et al. in National Institute of Family and Life Advocates v Becerra, January 16, 2018.
- Azar v Garza, 584 US 17 654 (2018).
- Brief amici curiae of American College of Obstetricians and Gynecologists and American Public Health Association in Planned Parenthood of Arkansas and Eastern Oklahoma v Jegley, February 1, 2018.
- Chapter 31, Inter Partes Review. United States Code. Title 35: Patents. Part III, Patents and protection of patents. 2012 Ed. 35 USC 311–319.
- Oil States Energy Services, LLC v Greene’s Energy Group, LLC, 584 US 16 712 (2018).
- SAS Institute Inc. v Iancu, 584 US 16 969 (2018).
- Brief for Association of American Medical Colleges and Others as Amici Curiae Supporting Respondents, Trump v Hawaii. https://www.supremecourt.gov/Docket PDF/17/ 17-965/40128/20180327105855912_17-965%20Amicus%20Br.%20Proclamation.pdf. Accessed September 21, 2018.
- Smith SR, Sanfilippo JS. Supreme Court decisions in 2017 that affected your practice. OBG Manag. 2017;29(12)44–47.
- Trump v Hawaii, 585 US 17 965 (2018).
- Ohio v American Express Co, 585 US 16 1454 (2018).
- Brief amici curiae of American Medical Association and Ohio State Medical Association in Ohio v American Express, December 24, 2017.
- Masterpiece Cakeshop, Ltd. v Colorado Civil Rights Commission, 584 US 16 111 (2018).
- Brief amici curiae of American College of Pediatricians, et al. in Masterpiece Cakeshop v Colorado Civil Rights Commission, September 7, 2017.
The 2017−2018 term of the Supreme Court of the United States (SCOTUS) was momentous. Justice Anthony Kennedy, who had been the deciding vote in most of the 5 to 4 cases for a generation, announced his retirement as of July 31, 2018. In addition, the Court decided a number of cases of interest to ObGyns. In this article we review some of those cases, as well as consider the future of the Court without Justice Kennedy. In selecting cases, we have given special attention to those in which national medical organizations filed amicus briefs. These “amicus curiae” or “friend of the court” briefs are filed by an entity who is not party to a case but wants to provide information or views to the court.

1. Abortion rulings

The Court decided 2 abortion cases and rejected a request to hear a third.
National Institute of Family and Life Advocates v Becerra
In this case,1 the Court struck down a California law that required pregnancy crisis centers not offering abortions (generally operated by pro-life groups) to provide special notices to clients.2
At stake. These notices would inform clients that California provides free or low-cost services, including abortions, and provide a phone number to call for those services.
There were many amicus briefs filed in this case, including those by the American College of Obstetricians and Gynecologists (ACOG) and other specialty boards,3 as well as the American Association of Pro-Life Obstetricians and Gynecologists and other pro-life organizations.4 ACOG’s brief argued that the California-required notice facilitates the goal of allowing women to receive medical services without harmful delay.
Final ruling. The Court held that the law required clinics to engage in speech with which the clinics disagreed (known as “compelled speech”). It also noted that California disclosure requirements were “wildly underinclusive” because they apply only to some clinics. The majority felt that there was no strong state interest in compelling this speech because there were other alternatives for the state to provide information about the availability of abortion and other services. The Court found that the clinics were likely to succeed on the merits of their claims of a First Amendment (free speech) violation.
Right to abortion for illegal immigrants in custody
A very unusual abortion case involved “Jane Doe,” a minor who was at 8 weeks’ gestation when she illegally crossed the border into the United States.5 She was placed in a federally-funded shelter where she requested an abortion. The facility denied that request.
At stake. Legal argument ensued about releasing her to another facility for an abortion, as the argument was made that pregnant minors who are apprehended crossing into the United States illegally and placed into the custody of federal officials should have abortion access. A lower Court of Appeals ruled against the Trump Administration’s policy of denying abortions to undocumented minors in federal custody. During the process of the federal government taking the case to the Supreme Court, the attorneys for Doe moved appointments around and, without notice, the abortion was performed. Government attorneys said that Doe’s attorneys made “what appear to be material misrepresentations and omissions” designed to “thwart [the Supreme Court’s] review” of the case.5 The government requested that the Court vacate the order of the Court of Appeals so that it could not be used as precedent.
Final ruling. The Court granted the governments request to vacate the lower court’s order because the minor was no longer pregnant and the order was therefore moot. The basic issue in this case (the right of in-custody minors to access abortions) remains unresolved. It is likely to appear before the Court in the future.
Continue to: Access to medical abortions
Access to medical abortions
An Arkansas law requires that a physician administering medical abortions contract with a physician who has admitting privileges at a hospital (a “contracted physician”).
At stake. Planned Parenthood filed suit challenging the requirement as unnecessary and harmful because it would result in the closure of 2 of the 3 abortion providers in Arkansas. ACOG filed an amicus brief urging the Supreme Court to consider the case.6 (Technically this was a petition for a Writ of Certiorari, the procedure by which the Court accepts cases. It accepts only about 1% of applications.) ACOG argued that there was no medical reason for the contracted physician requirement, and noted the harm it would do to women who would not have access to abortions.
Final ruling. On May 29, 2018, the Court declined to hear the case. This case is still active in the lower courts and may eventually return to the Supreme Court.

2. The patent system

The medical profession depends on the patent system to encourage the discovery of new patents efficiently and effectively. In 2012, Congress passed the America Invents Act7 that authorizes a petition by anyone other than the patent holder to the Patent and Trademark Office (PTO) for an “inter partes review” to assess a challenge to the patent’s legitimacy. If the PTO determines that there may be merit to the claim, the Patent Trial and Appeal Board undertakes a trial-like review process that may validate, invalidate, or amend the patent. The Board’s decision is subject to appellate court review.
At stake. This term, the inter partes review was challenged as unconstitutional on technical bases.8
Final ruling. The Court rejected this claim and approved the current administrative inter partes review process. The Court determined that once the Patent Office takes a petition challenging a patent, it must decide all of the claims against the patent, not pick and choose which elements of the challenge to evaluate.9 The Court’s decision upheld patent-review reform, but will require the Patent Office to tweak its procedures.

3. The travel ban

ACOG, the American Medical Association (AMA), the Association of American Medical Colleges, and more than 30 other health care and specialty associations filed an amicus brief regarding one of the most anticipated cases of the term—the “travel ban.”10
At stake. The essential argument of these organizations was that the US health care system depends on professionals from other countries. An efficient and fair immigration program is, therefore, important to advance the nation’s “health security.” During the 2016−2017 term, the Court considered but then removed the issue from its calendar when the Trump Administration issued a revised travel ban.11
In September 2017, President Trump’s proclamation imposed a range of entry restrictions on the citizens of 8 countries, most (but not all) of which are predominantly Muslim. The government indicated that, in a study by Homeland Security and the State Department, these countries were identified as having especially deficient information-sharing practices and presented national security concerns. Trump v Hawaii12 challenged this proclamation.
Final ruling. The majority of the Court upheld the travel ban. For the 5-Justice majority led by Chief Justice Roberts, the case came down to 3 things:
- The Constitution and the laws passed by Congress of necessity give the President great authority to engage in foreign policy, including policies regarding entry into the country.
- The courts are very reluctant to get into the substance of foreign affairs—they are not equipped to know in detail what the facts are, and things change very fast.
- If courts start tinkering with foreign policy and things turn bad, it will appear that the courts are to blame and were interfering in an area about which they are not competent.
Continue to: 4. Did a credit card case add risk to health insurance markets?

It was just a credit card case, but one in which the AMA saw a real risk to regulation of the health insurance markets.
At stake. Technically, Ohio v American Express concerned a claim that American Express (AmEx) violated antitrust laws when it prohibited merchants taking its credit card from “steering” customers to cards with lower fees.13 AmEx maintained that, because credit cards were a special kind of “2-sided” market (connecting merchants on one side and customers on the other), antitrust laws should not be strictly enforced.
The AMA noticed that special rules regarding 2-sided markets might apply to health insurance, and it submitted an amicus brief14 that noted: “dominant health insurance networks … have imposed and could further impose rules or effectively erect barriers that prohibit physicians from referring patients to certain specialists, particularly out-of-network specialists, for innovative and even necessary medical tests.”14 It concluded that the antitrust rule AmEx was suggesting would make it nearly impossible to challenge these unfair provisions in health insurance arrangements.
Final ruling. The Court, however, accepted the AmEx position, making it very difficult to develop an antitrust case against 2-sided markets. It remains to be seen the degree to which the AMA concern about health insurance markets will be realized.

5. Gay wedding and a bakeshop

At stake. In Masterpiece Cakeshop v Colorado, a cakemaker declined to design a cake for a gay wedding and had been disciplined under Colorado law for discriminating against the couple based on sexual orientation.15
Final ruling. The Court, however, found that the Colorado regulators had, ironically, shown such religious animus in the way they treated the baker that the regulators themselves had discriminated on the basis of religion. As a result, the Court reversed the sanctions against the baker.
This decision was fairly narrow. It does not, for example, stand for the proposition that there may be a general religious exception to antidiscrimination laws. The question of broader religious or free-speech objections to antidiscrimination laws remains for another time.
Amicus brief. It was interesting that the American College of Pediatricians, American Association of Pro-Life Obstetricians and Gynecologists, and others, filed an amicus brief to report with concern the “demands that individual medical professionals must perform, assist with, or facilitate abortions, without regard to the teachings of their own faiths, consciences, and convictions.”16 The brief also noted that “issues in the present case implicate the fundamental rights of health care professionals, and to respectfully urge that the Court should by no means permit any weakening or qualification of well-established protections against compelled speech, and of free exercise” of religion.16
Arbitration. The Court upheld, as it has in most recent terms, another arbitration agreement.1 This case concerned an employment agreement in which employees consented to submit to arbitration rather than file lawsuits and not use class action claims.
Search of cell-phone location. Cell phones, whenever turned on, connect with cell towers that record the phone’s location several times a minute. Cell companies store this information, creating a virtual map of where the owner is at all times. The Federal Bureau of Investigation asked a cell company for location information for several people during a 127-day period in which robberies were committed.2 The Court held that the search was illegal in the absence of a warrant.
Public employee unions. The Court held that agency (fair share) fees, in which public employees who are not union members can be required to pay dues for the bargaining and grievance activities (from which they generally benefit), violate the First Amendment. The majority held that forcing public employees to pay fees to unions requires the employees, through those fees, to engage in political activities with which they disagree.3 This is a form of compelled speech, which the Court found violates the First Amendment. Health care professionals who are public employees in positions that have union representation will probably have the opportunity to opt out of agency agreements.
Internet sales tax. The Court permitted states to charge sales tax on out-of-state Internet purchases.4 In doing so, a state may require out-of-state companies to collect taxes on sales to its residents.
References
- Epic Systems Corp. v Lewis, 584 US 16 285 (2018).
- Carpenter v United States, 585 US 16 402 (2018).
- Janus v State, County, and Municipal Employees, 585 US 16 1466 (2018).
- South Dakota v Wayfair, Inc, 585 US 17 494 (2018).
Clues to the future
During the term that ran from October 2, 2017, through June 27, 2018, the Court issued 72 decisions. An unusually high proportion of cases (26%; 19 cases) were decided on a 5 to 4 vote. Last term, the rate of 5 to 4 decisions was 10%; the 6-year average was 18%. The unanimous decision rate was 39% this term, compared with 59% last term, and 50% on average.
The rate of 5 to 4 cases provides a clue about the Court’s general direction. The number of times each Justice was in the majority in those nineteen 5 to 4 decisions included: Chief Justice Roberts, 17; and Justices Kennedy, 16; Gorsuch, 16; Thomas, 15; and Alito, 15; compared with Justices Ginsburg, 5; Breyer, 4; Sotomayor, 4; and Kagan, 3.
The Court convened on October 1, 2018. At this writing, whether the new term starts with 8 or 9 justices remains a question. President Trump nominated Brett Kavanaugh, JD, to take Justice Kennedy’s place on the Court. His professional qualifications and experience appear to make him qualified for a position on the Court, but as we have seen, there are many other elements that go into confirming a Justice’s nomination.
Justice Anthony Kennedy was the deciding vote in the overwhelming majority of the 5 to 4 decisions in 20 of his 30 years on the Court. The areas in which he had an especially important impact include1:
- Gay rights. Justice Kennedy wrote the opinions (usually 5 to 4 decisions) in a number of groundbreaking gay-rights cases, including decriminalizing homosexual conduct, striking down the Defense of Marriage Act, and finding that the Constitution requires states to recognize gay marriage.
- The death penalty. Justice Kennedy wrote decisions that prohibited states from imposing the death penalty for any crime other than murder, for defendants who were under 18 when they committed the crime, and for defendants with serious developmental disabilities. He expressed reservations about long-term solitary confinement, but did not have a case that allowed him to decide its constitutionality.
- The First Amendment. Early in his service on the Court, he held that the First Amendment protected flag burning as a form of speech. He decided many important freespeech and freedom-of-religion cases that have set a standard for protecting those fundamental freedoms.
- Use of health and social science data. Justice Kennedy was more open to mental health information and cited it more often than most other Justices.
- Abortion rights? Many commentators would add protecting the right to choose to have an abortion to the above list. Justice Kennedy was a central figure in one case that declined to back away from Roe v Wade, and joined a more recent decision that struck down a Texas law that created an undue burden on women seeking abortion. Plus, he also voted to uphold abortion restrictions, such as “partial-birth-abortion laws.” So there is a good argument for including abortion rights on the list, although he did not break new ground.
Justice Kennedy as a person
Outside the courtroom, Justice Kennedy is a person of great warmth and compassion. He is a natural teacher and spends a great deal of time with students. When asked how he would like to be remembered, Justice Kennedy once replied, “Somebody who’s decent, and honest, and fair, and who’s absolutely committed to the proposition that freedom is America’s gift to the rest of the world.” I agree with that assessment.
STEVEN R. SMITH, MS, JD
Reference
- South Dakota v Wayfair, Inc, 585 US (2018)
Next term, the Court is scheduled to hear cases regarding pharmaceutical liability, double jeopardy, sex-offender registration, expert witnesses, Social Security disability benefits, and the Age Discrimination in Employment Act. There will be at least 3 arbitration cases. Health care and reproductive rights will continue to be an important part of the Court’s docket.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The 2017−2018 term of the Supreme Court of the United States (SCOTUS) was momentous. Justice Anthony Kennedy, who had been the deciding vote in most of the 5 to 4 cases for a generation, announced his retirement as of July 31, 2018. In addition, the Court decided a number of cases of interest to ObGyns. In this article we review some of those cases, as well as consider the future of the Court without Justice Kennedy. In selecting cases, we have given special attention to those in which national medical organizations filed amicus briefs. These “amicus curiae” or “friend of the court” briefs are filed by an entity who is not party to a case but wants to provide information or views to the court.

1. Abortion rulings

The Court decided 2 abortion cases and rejected a request to hear a third.
National Institute of Family and Life Advocates v Becerra
In this case,1 the Court struck down a California law that required pregnancy crisis centers not offering abortions (generally operated by pro-life groups) to provide special notices to clients.2
At stake. These notices would inform clients that California provides free or low-cost services, including abortions, and provide a phone number to call for those services.
There were many amicus briefs filed in this case, including those by the American College of Obstetricians and Gynecologists (ACOG) and other specialty boards,3 as well as the American Association of Pro-Life Obstetricians and Gynecologists and other pro-life organizations.4 ACOG’s brief argued that the California-required notice facilitates the goal of allowing women to receive medical services without harmful delay.
Final ruling. The Court held that the law required clinics to engage in speech with which the clinics disagreed (known as “compelled speech”). It also noted that California disclosure requirements were “wildly underinclusive” because they apply only to some clinics. The majority felt that there was no strong state interest in compelling this speech because there were other alternatives for the state to provide information about the availability of abortion and other services. The Court found that the clinics were likely to succeed on the merits of their claims of a First Amendment (free speech) violation.
Right to abortion for illegal immigrants in custody
A very unusual abortion case involved “Jane Doe,” a minor who was at 8 weeks’ gestation when she illegally crossed the border into the United States.5 She was placed in a federally-funded shelter where she requested an abortion. The facility denied that request.
At stake. Legal argument ensued about releasing her to another facility for an abortion, as the argument was made that pregnant minors who are apprehended crossing into the United States illegally and placed into the custody of federal officials should have abortion access. A lower Court of Appeals ruled against the Trump Administration’s policy of denying abortions to undocumented minors in federal custody. During the process of the federal government taking the case to the Supreme Court, the attorneys for Doe moved appointments around and, without notice, the abortion was performed. Government attorneys said that Doe’s attorneys made “what appear to be material misrepresentations and omissions” designed to “thwart [the Supreme Court’s] review” of the case.5 The government requested that the Court vacate the order of the Court of Appeals so that it could not be used as precedent.
Final ruling. The Court granted the governments request to vacate the lower court’s order because the minor was no longer pregnant and the order was therefore moot. The basic issue in this case (the right of in-custody minors to access abortions) remains unresolved. It is likely to appear before the Court in the future.
Continue to: Access to medical abortions
Access to medical abortions
An Arkansas law requires that a physician administering medical abortions contract with a physician who has admitting privileges at a hospital (a “contracted physician”).
At stake. Planned Parenthood filed suit challenging the requirement as unnecessary and harmful because it would result in the closure of 2 of the 3 abortion providers in Arkansas. ACOG filed an amicus brief urging the Supreme Court to consider the case.6 (Technically this was a petition for a Writ of Certiorari, the procedure by which the Court accepts cases. It accepts only about 1% of applications.) ACOG argued that there was no medical reason for the contracted physician requirement, and noted the harm it would do to women who would not have access to abortions.
Final ruling. On May 29, 2018, the Court declined to hear the case. This case is still active in the lower courts and may eventually return to the Supreme Court.

2. The patent system

The medical profession depends on the patent system to encourage the discovery of new patents efficiently and effectively. In 2012, Congress passed the America Invents Act7 that authorizes a petition by anyone other than the patent holder to the Patent and Trademark Office (PTO) for an “inter partes review” to assess a challenge to the patent’s legitimacy. If the PTO determines that there may be merit to the claim, the Patent Trial and Appeal Board undertakes a trial-like review process that may validate, invalidate, or amend the patent. The Board’s decision is subject to appellate court review.
At stake. This term, the inter partes review was challenged as unconstitutional on technical bases.8
Final ruling. The Court rejected this claim and approved the current administrative inter partes review process. The Court determined that once the Patent Office takes a petition challenging a patent, it must decide all of the claims against the patent, not pick and choose which elements of the challenge to evaluate.9 The Court’s decision upheld patent-review reform, but will require the Patent Office to tweak its procedures.

3. The travel ban

ACOG, the American Medical Association (AMA), the Association of American Medical Colleges, and more than 30 other health care and specialty associations filed an amicus brief regarding one of the most anticipated cases of the term—the “travel ban.”10
At stake. The essential argument of these organizations was that the US health care system depends on professionals from other countries. An efficient and fair immigration program is, therefore, important to advance the nation’s “health security.” During the 2016−2017 term, the Court considered but then removed the issue from its calendar when the Trump Administration issued a revised travel ban.11
In September 2017, President Trump’s proclamation imposed a range of entry restrictions on the citizens of 8 countries, most (but not all) of which are predominantly Muslim. The government indicated that, in a study by Homeland Security and the State Department, these countries were identified as having especially deficient information-sharing practices and presented national security concerns. Trump v Hawaii12 challenged this proclamation.
Final ruling. The majority of the Court upheld the travel ban. For the 5-Justice majority led by Chief Justice Roberts, the case came down to 3 things:
- The Constitution and the laws passed by Congress of necessity give the President great authority to engage in foreign policy, including policies regarding entry into the country.
- The courts are very reluctant to get into the substance of foreign affairs—they are not equipped to know in detail what the facts are, and things change very fast.
- If courts start tinkering with foreign policy and things turn bad, it will appear that the courts are to blame and were interfering in an area about which they are not competent.
Continue to: 4. Did a credit card case add risk to health insurance markets?

It was just a credit card case, but one in which the AMA saw a real risk to regulation of the health insurance markets.
At stake. Technically, Ohio v American Express concerned a claim that American Express (AmEx) violated antitrust laws when it prohibited merchants taking its credit card from “steering” customers to cards with lower fees.13 AmEx maintained that, because credit cards were a special kind of “2-sided” market (connecting merchants on one side and customers on the other), antitrust laws should not be strictly enforced.
The AMA noticed that special rules regarding 2-sided markets might apply to health insurance, and it submitted an amicus brief14 that noted: “dominant health insurance networks … have imposed and could further impose rules or effectively erect barriers that prohibit physicians from referring patients to certain specialists, particularly out-of-network specialists, for innovative and even necessary medical tests.”14 It concluded that the antitrust rule AmEx was suggesting would make it nearly impossible to challenge these unfair provisions in health insurance arrangements.
Final ruling. The Court, however, accepted the AmEx position, making it very difficult to develop an antitrust case against 2-sided markets. It remains to be seen the degree to which the AMA concern about health insurance markets will be realized.

5. Gay wedding and a bakeshop

At stake. In Masterpiece Cakeshop v Colorado, a cakemaker declined to design a cake for a gay wedding and had been disciplined under Colorado law for discriminating against the couple based on sexual orientation.15
Final ruling. The Court, however, found that the Colorado regulators had, ironically, shown such religious animus in the way they treated the baker that the regulators themselves had discriminated on the basis of religion. As a result, the Court reversed the sanctions against the baker.
This decision was fairly narrow. It does not, for example, stand for the proposition that there may be a general religious exception to antidiscrimination laws. The question of broader religious or free-speech objections to antidiscrimination laws remains for another time.
Amicus brief. It was interesting that the American College of Pediatricians, American Association of Pro-Life Obstetricians and Gynecologists, and others, filed an amicus brief to report with concern the “demands that individual medical professionals must perform, assist with, or facilitate abortions, without regard to the teachings of their own faiths, consciences, and convictions.”16 The brief also noted that “issues in the present case implicate the fundamental rights of health care professionals, and to respectfully urge that the Court should by no means permit any weakening or qualification of well-established protections against compelled speech, and of free exercise” of religion.16
Arbitration. The Court upheld, as it has in most recent terms, another arbitration agreement.1 This case concerned an employment agreement in which employees consented to submit to arbitration rather than file lawsuits and not use class action claims.
Search of cell-phone location. Cell phones, whenever turned on, connect with cell towers that record the phone’s location several times a minute. Cell companies store this information, creating a virtual map of where the owner is at all times. The Federal Bureau of Investigation asked a cell company for location information for several people during a 127-day period in which robberies were committed.2 The Court held that the search was illegal in the absence of a warrant.
Public employee unions. The Court held that agency (fair share) fees, in which public employees who are not union members can be required to pay dues for the bargaining and grievance activities (from which they generally benefit), violate the First Amendment. The majority held that forcing public employees to pay fees to unions requires the employees, through those fees, to engage in political activities with which they disagree.3 This is a form of compelled speech, which the Court found violates the First Amendment. Health care professionals who are public employees in positions that have union representation will probably have the opportunity to opt out of agency agreements.
Internet sales tax. The Court permitted states to charge sales tax on out-of-state Internet purchases.4 In doing so, a state may require out-of-state companies to collect taxes on sales to its residents.
References
- Epic Systems Corp. v Lewis, 584 US 16 285 (2018).
- Carpenter v United States, 585 US 16 402 (2018).
- Janus v State, County, and Municipal Employees, 585 US 16 1466 (2018).
- South Dakota v Wayfair, Inc, 585 US 17 494 (2018).
Clues to the future
During the term that ran from October 2, 2017, through June 27, 2018, the Court issued 72 decisions. An unusually high proportion of cases (26%; 19 cases) were decided on a 5 to 4 vote. Last term, the rate of 5 to 4 decisions was 10%; the 6-year average was 18%. The unanimous decision rate was 39% this term, compared with 59% last term, and 50% on average.
The rate of 5 to 4 cases provides a clue about the Court’s general direction. The number of times each Justice was in the majority in those nineteen 5 to 4 decisions included: Chief Justice Roberts, 17; and Justices Kennedy, 16; Gorsuch, 16; Thomas, 15; and Alito, 15; compared with Justices Ginsburg, 5; Breyer, 4; Sotomayor, 4; and Kagan, 3.
The Court convened on October 1, 2018. At this writing, whether the new term starts with 8 or 9 justices remains a question. President Trump nominated Brett Kavanaugh, JD, to take Justice Kennedy’s place on the Court. His professional qualifications and experience appear to make him qualified for a position on the Court, but as we have seen, there are many other elements that go into confirming a Justice’s nomination.
Justice Anthony Kennedy was the deciding vote in the overwhelming majority of the 5 to 4 decisions in 20 of his 30 years on the Court. The areas in which he had an especially important impact include1:
- Gay rights. Justice Kennedy wrote the opinions (usually 5 to 4 decisions) in a number of groundbreaking gay-rights cases, including decriminalizing homosexual conduct, striking down the Defense of Marriage Act, and finding that the Constitution requires states to recognize gay marriage.
- The death penalty. Justice Kennedy wrote decisions that prohibited states from imposing the death penalty for any crime other than murder, for defendants who were under 18 when they committed the crime, and for defendants with serious developmental disabilities. He expressed reservations about long-term solitary confinement, but did not have a case that allowed him to decide its constitutionality.
- The First Amendment. Early in his service on the Court, he held that the First Amendment protected flag burning as a form of speech. He decided many important freespeech and freedom-of-religion cases that have set a standard for protecting those fundamental freedoms.
- Use of health and social science data. Justice Kennedy was more open to mental health information and cited it more often than most other Justices.
- Abortion rights? Many commentators would add protecting the right to choose to have an abortion to the above list. Justice Kennedy was a central figure in one case that declined to back away from Roe v Wade, and joined a more recent decision that struck down a Texas law that created an undue burden on women seeking abortion. Plus, he also voted to uphold abortion restrictions, such as “partial-birth-abortion laws.” So there is a good argument for including abortion rights on the list, although he did not break new ground.
Justice Kennedy as a person
Outside the courtroom, Justice Kennedy is a person of great warmth and compassion. He is a natural teacher and spends a great deal of time with students. When asked how he would like to be remembered, Justice Kennedy once replied, “Somebody who’s decent, and honest, and fair, and who’s absolutely committed to the proposition that freedom is America’s gift to the rest of the world.” I agree with that assessment.
STEVEN R. SMITH, MS, JD
Reference
- South Dakota v Wayfair, Inc, 585 US (2018)
Next term, the Court is scheduled to hear cases regarding pharmaceutical liability, double jeopardy, sex-offender registration, expert witnesses, Social Security disability benefits, and the Age Discrimination in Employment Act. There will be at least 3 arbitration cases. Health care and reproductive rights will continue to be an important part of the Court’s docket.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- National Institute of Family and Life Advocates v Becerra, 585 US 16 1140 (2018).
- California Reproductive Freedom, Accountability, Comprehensive Care, and Transparency Act (FACT Act), Cal. Health & Safety Code Ann. §123470 et seq. (West 2018).
- Brief amici curiae of American Academy of Pediatrics, et al. in National Institute of Family and Life Advocates v Becerra, February 27, 2018.
- Brief amici curiae of American Association of Pro-Life Obstetricians & Gynecologists, et al. in National Institute of Family and Life Advocates v Becerra, January 16, 2018.
- Azar v Garza, 584 US 17 654 (2018).
- Brief amici curiae of American College of Obstetricians and Gynecologists and American Public Health Association in Planned Parenthood of Arkansas and Eastern Oklahoma v Jegley, February 1, 2018.
- Chapter 31, Inter Partes Review. United States Code. Title 35: Patents. Part III, Patents and protection of patents. 2012 Ed. 35 USC 311–319.
- Oil States Energy Services, LLC v Greene’s Energy Group, LLC, 584 US 16 712 (2018).
- SAS Institute Inc. v Iancu, 584 US 16 969 (2018).
- Brief for Association of American Medical Colleges and Others as Amici Curiae Supporting Respondents, Trump v Hawaii. https://www.supremecourt.gov/Docket PDF/17/ 17-965/40128/20180327105855912_17-965%20Amicus%20Br.%20Proclamation.pdf. Accessed September 21, 2018.
- Smith SR, Sanfilippo JS. Supreme Court decisions in 2017 that affected your practice. OBG Manag. 2017;29(12)44–47.
- Trump v Hawaii, 585 US 17 965 (2018).
- Ohio v American Express Co, 585 US 16 1454 (2018).
- Brief amici curiae of American Medical Association and Ohio State Medical Association in Ohio v American Express, December 24, 2017.
- Masterpiece Cakeshop, Ltd. v Colorado Civil Rights Commission, 584 US 16 111 (2018).
- Brief amici curiae of American College of Pediatricians, et al. in Masterpiece Cakeshop v Colorado Civil Rights Commission, September 7, 2017.
- National Institute of Family and Life Advocates v Becerra, 585 US 16 1140 (2018).
- California Reproductive Freedom, Accountability, Comprehensive Care, and Transparency Act (FACT Act), Cal. Health & Safety Code Ann. §123470 et seq. (West 2018).
- Brief amici curiae of American Academy of Pediatrics, et al. in National Institute of Family and Life Advocates v Becerra, February 27, 2018.
- Brief amici curiae of American Association of Pro-Life Obstetricians & Gynecologists, et al. in National Institute of Family and Life Advocates v Becerra, January 16, 2018.
- Azar v Garza, 584 US 17 654 (2018).
- Brief amici curiae of American College of Obstetricians and Gynecologists and American Public Health Association in Planned Parenthood of Arkansas and Eastern Oklahoma v Jegley, February 1, 2018.
- Chapter 31, Inter Partes Review. United States Code. Title 35: Patents. Part III, Patents and protection of patents. 2012 Ed. 35 USC 311–319.
- Oil States Energy Services, LLC v Greene’s Energy Group, LLC, 584 US 16 712 (2018).
- SAS Institute Inc. v Iancu, 584 US 16 969 (2018).
- Brief for Association of American Medical Colleges and Others as Amici Curiae Supporting Respondents, Trump v Hawaii. https://www.supremecourt.gov/Docket PDF/17/ 17-965/40128/20180327105855912_17-965%20Amicus%20Br.%20Proclamation.pdf. Accessed September 21, 2018.
- Smith SR, Sanfilippo JS. Supreme Court decisions in 2017 that affected your practice. OBG Manag. 2017;29(12)44–47.
- Trump v Hawaii, 585 US 17 965 (2018).
- Ohio v American Express Co, 585 US 16 1454 (2018).
- Brief amici curiae of American Medical Association and Ohio State Medical Association in Ohio v American Express, December 24, 2017.
- Masterpiece Cakeshop, Ltd. v Colorado Civil Rights Commission, 584 US 16 111 (2018).
- Brief amici curiae of American College of Pediatricians, et al. in Masterpiece Cakeshop v Colorado Civil Rights Commission, September 7, 2017.
Agrees that OC use clearly reduces mortality
Agrees that OC use clearly reduces mortality
Recent evidence from long-term observations of hundreds of thousands of women, in 10 European countries, clearly demonstrated that the use of oral contraceptives (OCs) reduced mortality by roughly 10%.1,2 Newer OCs increase women’s overall survival.
In comparison, reducing obesity by 5 body mass index points would reduce mortality by only 5%, from 1.05 to 1.3
Dr. Stavros Saripanidis
Thessaloniki, Greece
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Merritt MA, Riboli E, Murphy N, et al. Reproductive factors and risk of mortality in the European Prospective Investigation into Cancer and Nutrition: a cohort study. BMC Med. 2015;13:252.
- Iversen L, Sivasubramaniam S, Lee AJ, Fielding S, Hannaford PC. Lifetime cancer risk and combined oral contraceptives: the Royal College of General Practitioners’ Oral Contraception Study. Am J Obstet Gynecol. 2017;216(6):580.e1–580.e9.
- Aune D, Sen A, Prasad M, et al. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:i2156.
Agrees that OC use clearly reduces mortality
Recent evidence from long-term observations of hundreds of thousands of women, in 10 European countries, clearly demonstrated that the use of oral contraceptives (OCs) reduced mortality by roughly 10%.1,2 Newer OCs increase women’s overall survival.
In comparison, reducing obesity by 5 body mass index points would reduce mortality by only 5%, from 1.05 to 1.3
Dr. Stavros Saripanidis
Thessaloniki, Greece
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Agrees that OC use clearly reduces mortality
Recent evidence from long-term observations of hundreds of thousands of women, in 10 European countries, clearly demonstrated that the use of oral contraceptives (OCs) reduced mortality by roughly 10%.1,2 Newer OCs increase women’s overall survival.
In comparison, reducing obesity by 5 body mass index points would reduce mortality by only 5%, from 1.05 to 1.3
Dr. Stavros Saripanidis
Thessaloniki, Greece
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Merritt MA, Riboli E, Murphy N, et al. Reproductive factors and risk of mortality in the European Prospective Investigation into Cancer and Nutrition: a cohort study. BMC Med. 2015;13:252.
- Iversen L, Sivasubramaniam S, Lee AJ, Fielding S, Hannaford PC. Lifetime cancer risk and combined oral contraceptives: the Royal College of General Practitioners’ Oral Contraception Study. Am J Obstet Gynecol. 2017;216(6):580.e1–580.e9.
- Aune D, Sen A, Prasad M, et al. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:i2156.
- Merritt MA, Riboli E, Murphy N, et al. Reproductive factors and risk of mortality in the European Prospective Investigation into Cancer and Nutrition: a cohort study. BMC Med. 2015;13:252.
- Iversen L, Sivasubramaniam S, Lee AJ, Fielding S, Hannaford PC. Lifetime cancer risk and combined oral contraceptives: the Royal College of General Practitioners’ Oral Contraception Study. Am J Obstet Gynecol. 2017;216(6):580.e1–580.e9.
- Aune D, Sen A, Prasad M, et al. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:i2156.
No significant VTE risk for women taking noncyclic COCs
Women who use combined oral contraceptives (COC) without hormone-free or low-dose hormone intervals have a slightly elevated, but not statistically significant, risk of venous thromboembolism (VTE), compared with women who use cyclic COCs, according to research published in JAMA Internal Medicine.
Jie Li, PhD, from the Center for Drug Evaluation and Research, and colleagues performed a retrospective cohort study of 733,007 women aged 18-50 years in the Sentinel Distributed Database from 2007 to 2015 who received low-dose extended and continuous cycle (210,691 women; mean age, 30 years) COCs or cyclic COCs (522,316 women; mean age, 29 years). Continuous cycle COCs were defined as an 84/7 cycle or a 365/0 cycle, while cyclic COCs were 21/7 cycles.
The researchers noted some baseline differences between the two groups, with gynecologic conditions occurring in 40% of the noncyclic group, compared with 32% in the cyclic group; cardiovascular and metabolic conditions occurring in 7% of noncyclic women, compared with 5% of cyclic women; inflammatory disease occurring in 3% of noncyclic women, compared with 2% of cyclic women; and a slightly higher rate of health care services use in the noncyclic group, compared with the cyclic group.
Dr. Li and associates found 228 cases of VTE in the noncyclic group and 297 cases in the cyclic group, with an incidence rate of 1.54 (95% confidence interval, 1.34-1.74) per 1,000 person-years for noncyclic users and 0.83 (95% CI, 0.74-0.93) per 1,000 person-years for cyclic users (crude hazard ratio, 1.84; 95% CI, 1.53-2.21).
However, propensity score matching lowered the incidence rate to 1.44 (95% CI, 1.24-1.64) per 1,000 person-years for the noncyclic group and raised it to 1.09 (95% CI, 0.92-1.27) per 1,000 person-years for the cyclic group, for an adjusted hazard ratio of 1.32 (95% CI, 1.07-1.64), which does not show “strong evidence” of VTE risk based on a small absolute risk difference of 0.27 cases per 1,000 persons, the researchers said. They added that there might be residual or unmeasured confounding, perhaps for potential concurrent medication use or incompletely measured covariates.
“Accordingly, we do not recommend selective prescribing of COCs based on the cyclic and continuous/extended type,” Dr. Li and colleagues wrote. “Clinicians should prescribe COCs based on patients’ individual risk factors and preferences.”
The Sentinel Initiative is funded by a contract from the Department of Health and Human Services. The authors reported no relevant conflicts of interest.
SOURCE: Li J et al. JAMA Intern Med. 2018 Oct 1. doi: 10.1001/jamainternmed.2018.4251.
Women who use combined oral contraceptives (COC) without hormone-free or low-dose hormone intervals have a slightly elevated, but not statistically significant, risk of venous thromboembolism (VTE), compared with women who use cyclic COCs, according to research published in JAMA Internal Medicine.
Jie Li, PhD, from the Center for Drug Evaluation and Research, and colleagues performed a retrospective cohort study of 733,007 women aged 18-50 years in the Sentinel Distributed Database from 2007 to 2015 who received low-dose extended and continuous cycle (210,691 women; mean age, 30 years) COCs or cyclic COCs (522,316 women; mean age, 29 years). Continuous cycle COCs were defined as an 84/7 cycle or a 365/0 cycle, while cyclic COCs were 21/7 cycles.
The researchers noted some baseline differences between the two groups, with gynecologic conditions occurring in 40% of the noncyclic group, compared with 32% in the cyclic group; cardiovascular and metabolic conditions occurring in 7% of noncyclic women, compared with 5% of cyclic women; inflammatory disease occurring in 3% of noncyclic women, compared with 2% of cyclic women; and a slightly higher rate of health care services use in the noncyclic group, compared with the cyclic group.
Dr. Li and associates found 228 cases of VTE in the noncyclic group and 297 cases in the cyclic group, with an incidence rate of 1.54 (95% confidence interval, 1.34-1.74) per 1,000 person-years for noncyclic users and 0.83 (95% CI, 0.74-0.93) per 1,000 person-years for cyclic users (crude hazard ratio, 1.84; 95% CI, 1.53-2.21).
However, propensity score matching lowered the incidence rate to 1.44 (95% CI, 1.24-1.64) per 1,000 person-years for the noncyclic group and raised it to 1.09 (95% CI, 0.92-1.27) per 1,000 person-years for the cyclic group, for an adjusted hazard ratio of 1.32 (95% CI, 1.07-1.64), which does not show “strong evidence” of VTE risk based on a small absolute risk difference of 0.27 cases per 1,000 persons, the researchers said. They added that there might be residual or unmeasured confounding, perhaps for potential concurrent medication use or incompletely measured covariates.
“Accordingly, we do not recommend selective prescribing of COCs based on the cyclic and continuous/extended type,” Dr. Li and colleagues wrote. “Clinicians should prescribe COCs based on patients’ individual risk factors and preferences.”
The Sentinel Initiative is funded by a contract from the Department of Health and Human Services. The authors reported no relevant conflicts of interest.
SOURCE: Li J et al. JAMA Intern Med. 2018 Oct 1. doi: 10.1001/jamainternmed.2018.4251.
Women who use combined oral contraceptives (COC) without hormone-free or low-dose hormone intervals have a slightly elevated, but not statistically significant, risk of venous thromboembolism (VTE), compared with women who use cyclic COCs, according to research published in JAMA Internal Medicine.
Jie Li, PhD, from the Center for Drug Evaluation and Research, and colleagues performed a retrospective cohort study of 733,007 women aged 18-50 years in the Sentinel Distributed Database from 2007 to 2015 who received low-dose extended and continuous cycle (210,691 women; mean age, 30 years) COCs or cyclic COCs (522,316 women; mean age, 29 years). Continuous cycle COCs were defined as an 84/7 cycle or a 365/0 cycle, while cyclic COCs were 21/7 cycles.
The researchers noted some baseline differences between the two groups, with gynecologic conditions occurring in 40% of the noncyclic group, compared with 32% in the cyclic group; cardiovascular and metabolic conditions occurring in 7% of noncyclic women, compared with 5% of cyclic women; inflammatory disease occurring in 3% of noncyclic women, compared with 2% of cyclic women; and a slightly higher rate of health care services use in the noncyclic group, compared with the cyclic group.
Dr. Li and associates found 228 cases of VTE in the noncyclic group and 297 cases in the cyclic group, with an incidence rate of 1.54 (95% confidence interval, 1.34-1.74) per 1,000 person-years for noncyclic users and 0.83 (95% CI, 0.74-0.93) per 1,000 person-years for cyclic users (crude hazard ratio, 1.84; 95% CI, 1.53-2.21).
However, propensity score matching lowered the incidence rate to 1.44 (95% CI, 1.24-1.64) per 1,000 person-years for the noncyclic group and raised it to 1.09 (95% CI, 0.92-1.27) per 1,000 person-years for the cyclic group, for an adjusted hazard ratio of 1.32 (95% CI, 1.07-1.64), which does not show “strong evidence” of VTE risk based on a small absolute risk difference of 0.27 cases per 1,000 persons, the researchers said. They added that there might be residual or unmeasured confounding, perhaps for potential concurrent medication use or incompletely measured covariates.
“Accordingly, we do not recommend selective prescribing of COCs based on the cyclic and continuous/extended type,” Dr. Li and colleagues wrote. “Clinicians should prescribe COCs based on patients’ individual risk factors and preferences.”
The Sentinel Initiative is funded by a contract from the Department of Health and Human Services. The authors reported no relevant conflicts of interest.
SOURCE: Li J et al. JAMA Intern Med. 2018 Oct 1. doi: 10.1001/jamainternmed.2018.4251.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Continuous or extended cycle combined oral contraceptive (COC) use was associated with a slightly elevated, but not statistically significant, risk of venous thromboembolism.
Major finding: The adjusted hazard ratio for women taking continuous/extended COCs was 1.32 (95% confidence interval, 1.07-1.74), compared with women taking noncyclic COCs, but the absolute risk difference between the two groups was low (0.27 per 1,000 persons).
Study details: A retrospective cohort study of 210,691 women with continuous/extended COC use and 522,316 women with cyclic COC use.
Disclosures: The Sentinel Initiative is funded by a contract from the Department of Health and Human Services. The authors reported no relevant conflicts of interest.
Source: Li J et al. JAMA Intern Med. 2018 Oct 1. doi:10.1001/jamainternmed.2018.4251.