User login
VA foster program helps older vets manage COVID challenges
Susan Snead used to live in an apartment complex for older adults. The complex had a nice dayroom, and neighbors would knock on her door every now and then to check in.
But despite not being lonely, Ms. Snead, 89, did live alone in downtown Charleston, S.C. Eventually, that became dangerous.
“I fell a few times,” she says. “I had to call somebody to come and get me up.”
Sometimes help would come from the apartment complex’s office. Sometimes it came with a police escort.
Over time, needing to make those calls became a burden. Making and keeping appointments with her doctor, something she had to do regularly, as she has diabetes, got harder, too.
“It kind of wore me out,” she says. “Like you’re going up a hill.”
As she was beginning to accept she could no longer live alone, Ms. Snead, an Air Force veteran, learned about a program run by the Department of Veterans Affairs called Medical Foster Home.
Caregivers help aging veterans with activities of daily living like bathing, cooking, making and getting to appointments, getting dressed, and taking daily medication.
Caregivers can take care of up to three residents in their home at a time. While most residents are veterans, caregivers sometimes care for non-veteran residents, such as a veteran’s spouse or a caregiver’s family member.
Veterans typically pay about $1,500 to $3,000 out-of-pocket per month for the service, depending on location.
According to the VA, the concept of medical foster homes has been around since 1999, when VA hospitals across the country began reaching out to people willing to provide live-in care for veterans. The option is led by local VA hospitals, which approve caregivers and provide administrative services. There are now 517 medical foster homes, the VA says.
Much like other residential care facilities, medical foster homes get regular inspections for safety, nutrition, and more.
In 2019, Ms. Snead signed up for the program. She expected to be cared for, but she found a sense of family with her caregiver, Wilhelmina Brown, and another veteran in the home.
Ms. Brown started taking care of people – but not necessarily veterans – in 1997 when her grandmother was unable to care for herself, she says.
“My grandmama carried me to church every Sunday, she carried me to the beach – everywhere she went, she took me with her,” Ms. Brown says. As her grandmother got older, “I said, ‘I’m going to take care of her in my home.’ ”
Caring for others must come from the heart, Ms. Brown says.
She cooks her residents’ meals three times a day with dietary restrictions in mind, washes their dishes, does their laundry, remembers birthdays, and plans little parties.
“That’s my family,” Ms. Brown says.
In 2020, the COVID-19 pandemic upended the world – but at the same time, it highlighted the advantages of the medical foster home model.
Home-based primary care keeps veterans out of nursing homes – something that became particularly important as COVID-19 hit nursing homes and long-term care facilities.
Caregivers in the system were also able to help veterans, often living in rural areas, pivot and adapt to telehealth during a time of crisis.
One study, published in the journal Geriatrics, set out to identify how medical foster homes were able to deliver safe, effective health care during the early stages of the pandemic.
Researchers interviewed 37 VA care providers at 16 rural medical foster home programs across the country. The interviews took place between December 2020 and February 2021. They found medical foster home caregivers, coordinators, and health care providers communicated to move office visits to the home, helped veterans navigate telehealth, advocated to get veterans vaccinated in-home, and relied on each other to fight social isolation.
Caregivers also adapted quickly to telehealth, according to Leah Haverhals, PhD, a health research scientist and communications director for the Seattle-Denver Center of Innovation for Veteran Centered and Value Driven Care, who led the study.
Most veterans in the foster home program are older and find new technology difficult to use.
Caregivers, coordinators, and health care providers were largely new to the technology, too.
While the study found that most veterans and caregivers preferred in-person care, they were able to work together to make the best of telehealth.
“That speaks to the nature of the care being given, being able to pivot in a crisis like that,” Dr. Haverhals says.
If caregivers didn’t already have computers or telehealth-compatible devices, the VA provided iPads that would connect to the internet using cellular signals. According to the study, this helped to overcome connectivity issues that may have caused problems in rural areas.
Ms. Snead says Ms. Brown helped a lot with her telehealth calls.
“If we had to do things over the phone or with video, she was able to set that up to work with the person on the other end. She knows a lot about that stuff – about computers and things like that,” Ms. Snead says, adding that she hadn’t worked with computers since retirement in 1998.
Telehealth helped health care providers identify infections and quickly prescribe antibiotics to veterans in rural areas and provide other care that was more safely delivered in private homes.
“The findings from our study highlighted that when working together for the common goal of keeping vulnerable populations like veterans in MFHs [medical foster homes] safe during times of crisis, adaptation and collaboration facilitated the ongoing provision of high-quality care,” Dr. Haverhals’s group wrote. “Such collaboration has been shown to be critical in recent research in the United States on supporting older adults during the pandemic.”
Cari Levy, MD, PhD, a professor at the University of Colorado at Denver, Aurora, and a co-author of the study, specializes in palliative and telenursing home care for the VA.
Dr. Levy, who has worked for the VA for about 20 years, says how medical foster homes provided care during the pandemic carries lessons for civilian clinics. One of the most important lessons, she says, is that medical professionals will need to provide more care where people are, especially in populations that are too sick to get to the clinic.
“For years, there was all this hope that telehealth would expand,” but it took a pandemic to authorize approval from federal agencies to explode, she says. “I shudder to think what would have happened if we didn’t have telehealth. Fortunately, it was the right time to be able to flip a switch.”
Crisis aside, Dr. Levy says her dream would be for health care providers to do more home-based care. The model allows people to preserve the relational aspects of medicine, which can counteract a lot of the moral injury and burnout in the field, she says, adding:
“I see this as the kind of medicine many people intended to do when they got into medicine.”
A version of this article first appeared on WebMD.com.
Susan Snead used to live in an apartment complex for older adults. The complex had a nice dayroom, and neighbors would knock on her door every now and then to check in.
But despite not being lonely, Ms. Snead, 89, did live alone in downtown Charleston, S.C. Eventually, that became dangerous.
“I fell a few times,” she says. “I had to call somebody to come and get me up.”
Sometimes help would come from the apartment complex’s office. Sometimes it came with a police escort.
Over time, needing to make those calls became a burden. Making and keeping appointments with her doctor, something she had to do regularly, as she has diabetes, got harder, too.
“It kind of wore me out,” she says. “Like you’re going up a hill.”
As she was beginning to accept she could no longer live alone, Ms. Snead, an Air Force veteran, learned about a program run by the Department of Veterans Affairs called Medical Foster Home.
Caregivers help aging veterans with activities of daily living like bathing, cooking, making and getting to appointments, getting dressed, and taking daily medication.
Caregivers can take care of up to three residents in their home at a time. While most residents are veterans, caregivers sometimes care for non-veteran residents, such as a veteran’s spouse or a caregiver’s family member.
Veterans typically pay about $1,500 to $3,000 out-of-pocket per month for the service, depending on location.
According to the VA, the concept of medical foster homes has been around since 1999, when VA hospitals across the country began reaching out to people willing to provide live-in care for veterans. The option is led by local VA hospitals, which approve caregivers and provide administrative services. There are now 517 medical foster homes, the VA says.
Much like other residential care facilities, medical foster homes get regular inspections for safety, nutrition, and more.
In 2019, Ms. Snead signed up for the program. She expected to be cared for, but she found a sense of family with her caregiver, Wilhelmina Brown, and another veteran in the home.
Ms. Brown started taking care of people – but not necessarily veterans – in 1997 when her grandmother was unable to care for herself, she says.
“My grandmama carried me to church every Sunday, she carried me to the beach – everywhere she went, she took me with her,” Ms. Brown says. As her grandmother got older, “I said, ‘I’m going to take care of her in my home.’ ”
Caring for others must come from the heart, Ms. Brown says.
She cooks her residents’ meals three times a day with dietary restrictions in mind, washes their dishes, does their laundry, remembers birthdays, and plans little parties.
“That’s my family,” Ms. Brown says.
In 2020, the COVID-19 pandemic upended the world – but at the same time, it highlighted the advantages of the medical foster home model.
Home-based primary care keeps veterans out of nursing homes – something that became particularly important as COVID-19 hit nursing homes and long-term care facilities.
Caregivers in the system were also able to help veterans, often living in rural areas, pivot and adapt to telehealth during a time of crisis.
One study, published in the journal Geriatrics, set out to identify how medical foster homes were able to deliver safe, effective health care during the early stages of the pandemic.
Researchers interviewed 37 VA care providers at 16 rural medical foster home programs across the country. The interviews took place between December 2020 and February 2021. They found medical foster home caregivers, coordinators, and health care providers communicated to move office visits to the home, helped veterans navigate telehealth, advocated to get veterans vaccinated in-home, and relied on each other to fight social isolation.
Caregivers also adapted quickly to telehealth, according to Leah Haverhals, PhD, a health research scientist and communications director for the Seattle-Denver Center of Innovation for Veteran Centered and Value Driven Care, who led the study.
Most veterans in the foster home program are older and find new technology difficult to use.
Caregivers, coordinators, and health care providers were largely new to the technology, too.
While the study found that most veterans and caregivers preferred in-person care, they were able to work together to make the best of telehealth.
“That speaks to the nature of the care being given, being able to pivot in a crisis like that,” Dr. Haverhals says.
If caregivers didn’t already have computers or telehealth-compatible devices, the VA provided iPads that would connect to the internet using cellular signals. According to the study, this helped to overcome connectivity issues that may have caused problems in rural areas.
Ms. Snead says Ms. Brown helped a lot with her telehealth calls.
“If we had to do things over the phone or with video, she was able to set that up to work with the person on the other end. She knows a lot about that stuff – about computers and things like that,” Ms. Snead says, adding that she hadn’t worked with computers since retirement in 1998.
Telehealth helped health care providers identify infections and quickly prescribe antibiotics to veterans in rural areas and provide other care that was more safely delivered in private homes.
“The findings from our study highlighted that when working together for the common goal of keeping vulnerable populations like veterans in MFHs [medical foster homes] safe during times of crisis, adaptation and collaboration facilitated the ongoing provision of high-quality care,” Dr. Haverhals’s group wrote. “Such collaboration has been shown to be critical in recent research in the United States on supporting older adults during the pandemic.”
Cari Levy, MD, PhD, a professor at the University of Colorado at Denver, Aurora, and a co-author of the study, specializes in palliative and telenursing home care for the VA.
Dr. Levy, who has worked for the VA for about 20 years, says how medical foster homes provided care during the pandemic carries lessons for civilian clinics. One of the most important lessons, she says, is that medical professionals will need to provide more care where people are, especially in populations that are too sick to get to the clinic.
“For years, there was all this hope that telehealth would expand,” but it took a pandemic to authorize approval from federal agencies to explode, she says. “I shudder to think what would have happened if we didn’t have telehealth. Fortunately, it was the right time to be able to flip a switch.”
Crisis aside, Dr. Levy says her dream would be for health care providers to do more home-based care. The model allows people to preserve the relational aspects of medicine, which can counteract a lot of the moral injury and burnout in the field, she says, adding:
“I see this as the kind of medicine many people intended to do when they got into medicine.”
A version of this article first appeared on WebMD.com.
Susan Snead used to live in an apartment complex for older adults. The complex had a nice dayroom, and neighbors would knock on her door every now and then to check in.
But despite not being lonely, Ms. Snead, 89, did live alone in downtown Charleston, S.C. Eventually, that became dangerous.
“I fell a few times,” she says. “I had to call somebody to come and get me up.”
Sometimes help would come from the apartment complex’s office. Sometimes it came with a police escort.
Over time, needing to make those calls became a burden. Making and keeping appointments with her doctor, something she had to do regularly, as she has diabetes, got harder, too.
“It kind of wore me out,” she says. “Like you’re going up a hill.”
As she was beginning to accept she could no longer live alone, Ms. Snead, an Air Force veteran, learned about a program run by the Department of Veterans Affairs called Medical Foster Home.
Caregivers help aging veterans with activities of daily living like bathing, cooking, making and getting to appointments, getting dressed, and taking daily medication.
Caregivers can take care of up to three residents in their home at a time. While most residents are veterans, caregivers sometimes care for non-veteran residents, such as a veteran’s spouse or a caregiver’s family member.
Veterans typically pay about $1,500 to $3,000 out-of-pocket per month for the service, depending on location.
According to the VA, the concept of medical foster homes has been around since 1999, when VA hospitals across the country began reaching out to people willing to provide live-in care for veterans. The option is led by local VA hospitals, which approve caregivers and provide administrative services. There are now 517 medical foster homes, the VA says.
Much like other residential care facilities, medical foster homes get regular inspections for safety, nutrition, and more.
In 2019, Ms. Snead signed up for the program. She expected to be cared for, but she found a sense of family with her caregiver, Wilhelmina Brown, and another veteran in the home.
Ms. Brown started taking care of people – but not necessarily veterans – in 1997 when her grandmother was unable to care for herself, she says.
“My grandmama carried me to church every Sunday, she carried me to the beach – everywhere she went, she took me with her,” Ms. Brown says. As her grandmother got older, “I said, ‘I’m going to take care of her in my home.’ ”
Caring for others must come from the heart, Ms. Brown says.
She cooks her residents’ meals three times a day with dietary restrictions in mind, washes their dishes, does their laundry, remembers birthdays, and plans little parties.
“That’s my family,” Ms. Brown says.
In 2020, the COVID-19 pandemic upended the world – but at the same time, it highlighted the advantages of the medical foster home model.
Home-based primary care keeps veterans out of nursing homes – something that became particularly important as COVID-19 hit nursing homes and long-term care facilities.
Caregivers in the system were also able to help veterans, often living in rural areas, pivot and adapt to telehealth during a time of crisis.
One study, published in the journal Geriatrics, set out to identify how medical foster homes were able to deliver safe, effective health care during the early stages of the pandemic.
Researchers interviewed 37 VA care providers at 16 rural medical foster home programs across the country. The interviews took place between December 2020 and February 2021. They found medical foster home caregivers, coordinators, and health care providers communicated to move office visits to the home, helped veterans navigate telehealth, advocated to get veterans vaccinated in-home, and relied on each other to fight social isolation.
Caregivers also adapted quickly to telehealth, according to Leah Haverhals, PhD, a health research scientist and communications director for the Seattle-Denver Center of Innovation for Veteran Centered and Value Driven Care, who led the study.
Most veterans in the foster home program are older and find new technology difficult to use.
Caregivers, coordinators, and health care providers were largely new to the technology, too.
While the study found that most veterans and caregivers preferred in-person care, they were able to work together to make the best of telehealth.
“That speaks to the nature of the care being given, being able to pivot in a crisis like that,” Dr. Haverhals says.
If caregivers didn’t already have computers or telehealth-compatible devices, the VA provided iPads that would connect to the internet using cellular signals. According to the study, this helped to overcome connectivity issues that may have caused problems in rural areas.
Ms. Snead says Ms. Brown helped a lot with her telehealth calls.
“If we had to do things over the phone or with video, she was able to set that up to work with the person on the other end. She knows a lot about that stuff – about computers and things like that,” Ms. Snead says, adding that she hadn’t worked with computers since retirement in 1998.
Telehealth helped health care providers identify infections and quickly prescribe antibiotics to veterans in rural areas and provide other care that was more safely delivered in private homes.
“The findings from our study highlighted that when working together for the common goal of keeping vulnerable populations like veterans in MFHs [medical foster homes] safe during times of crisis, adaptation and collaboration facilitated the ongoing provision of high-quality care,” Dr. Haverhals’s group wrote. “Such collaboration has been shown to be critical in recent research in the United States on supporting older adults during the pandemic.”
Cari Levy, MD, PhD, a professor at the University of Colorado at Denver, Aurora, and a co-author of the study, specializes in palliative and telenursing home care for the VA.
Dr. Levy, who has worked for the VA for about 20 years, says how medical foster homes provided care during the pandemic carries lessons for civilian clinics. One of the most important lessons, she says, is that medical professionals will need to provide more care where people are, especially in populations that are too sick to get to the clinic.
“For years, there was all this hope that telehealth would expand,” but it took a pandemic to authorize approval from federal agencies to explode, she says. “I shudder to think what would have happened if we didn’t have telehealth. Fortunately, it was the right time to be able to flip a switch.”
Crisis aside, Dr. Levy says her dream would be for health care providers to do more home-based care. The model allows people to preserve the relational aspects of medicine, which can counteract a lot of the moral injury and burnout in the field, she says, adding:
“I see this as the kind of medicine many people intended to do when they got into medicine.”
A version of this article first appeared on WebMD.com.
Children and COVID: Many parents see vaccine as the greater risk
New COVID-19 cases rose for the second week in a row as cumulative cases among U.S. children passed the 14-million mark, but a recent survey shows that more than half of parents believe that the vaccine is a greater risk to children under age 5 years than the virus.
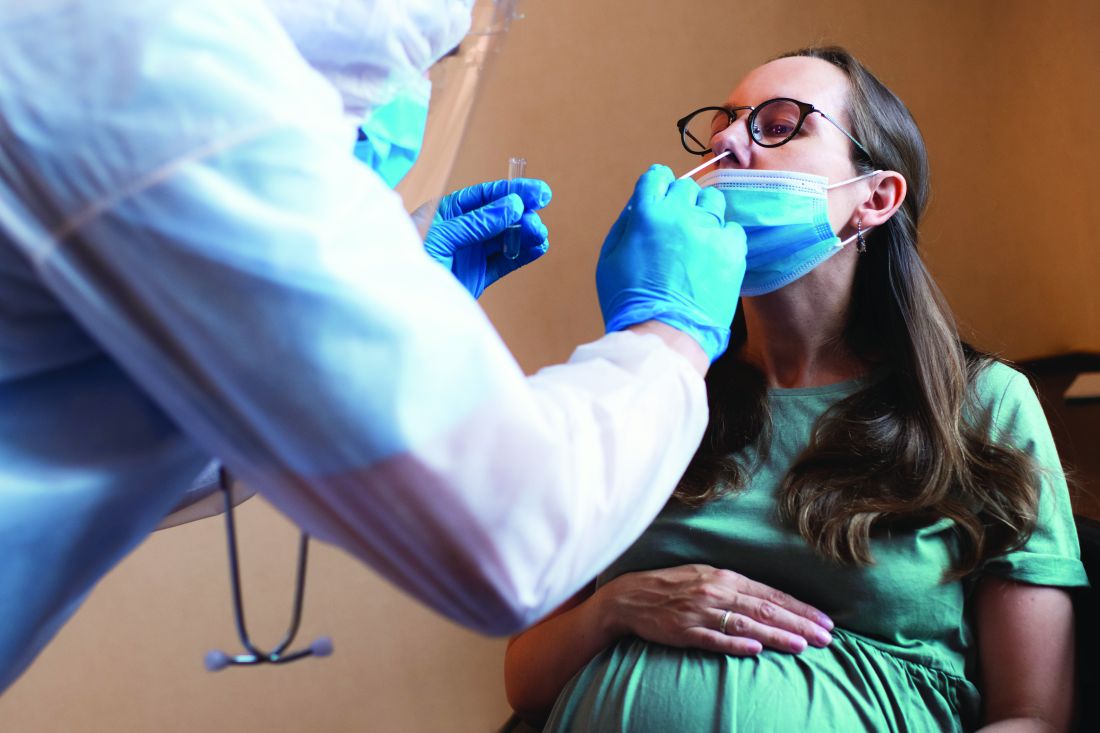
In a Kaiser Family Foundation survey conducted July 7-17, 53% of parents with children aged 6 months to 5 years said that the vaccine is “a bigger risk to their child’s health than getting infected with COVID-19, compared to 44% who say getting infected is the bigger risk,” KFF reported July 26.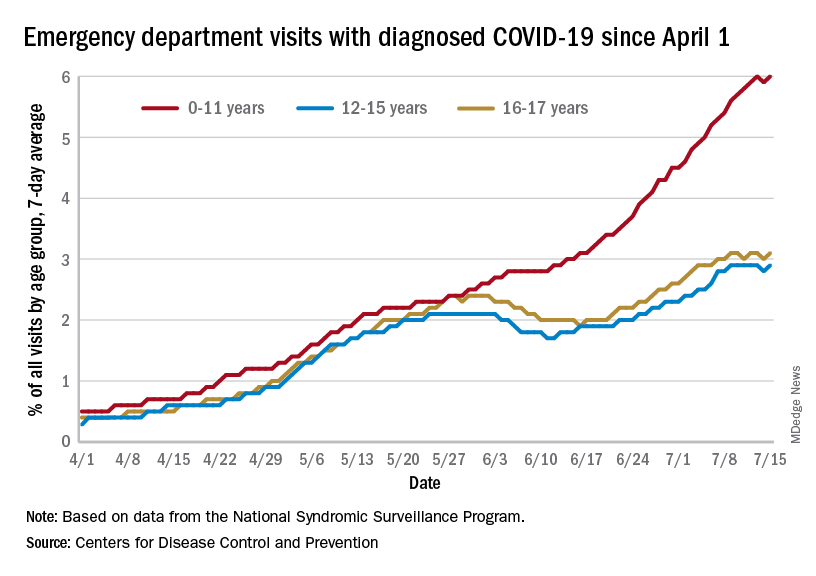
More than 4 out of 10 of respondents (43%) said that they will “definitely not” get their eligible children vaccinated, while only 7% said that their children had already received it and 10% said their children would get it as soon as possible, according to the KFF survey, which had an overall sample size of 1,847 adults, including an oversample of 471 parents of children under age 5.
Vaccine initiation has been slow in the first month since it was approved for the youngest children. Just 2.8% of all eligible children under age 5 had received an initial dose as of July 19, compared with first-month uptake figures of more than 18% for the 5- to 11-year-olds and 27% for those aged 12-15, based on data from the Centers for Disease Control and Prevention.
The current rates for vaccination in those aged 5 and older look like this: 70.2% of 12- to 17-year-olds have received at least one dose, versus 37.1% of those aged 5-11. Just over 60% of the older children were fully vaccinated as of July 19, as were 30.2% of the 5- to 11-year-olds, the CDC reported on its COVID Data Tracker.
Number of new cases hits 2-month high
Despite the vaccine, SARS-CoV-2 and its various mutations have continued with their summer travels. With 92,000 newly infected children added for the week of July 15-21, there have now been a total of 14,003,497 pediatric cases reported since the start of the pandemic, which works out to 18.6% of cases in all ages, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
The 92,000 new cases represent an increase of almost 22% over the previous week and mark the highest 1-week count since May, when the total passed 100,000 for 2 consecutive weeks. More recently the trend had seemed more stable as weekly cases dropped twice and rose twice as the total hovered around 70,000, based on the data collected by the AAP and CHA from state and territorial health departments.
A different scenario has played out for emergency department visits and hospital admissions, which have risen steadily since the beginning of April. The admission rate for children aged 0-17, which was just 0.13 new patients per 100,000 population on April 11, was up to 0.44 per 100,000 on July 21. By comparison, the highest rate reached last year during the Delta surge was 0.47 per 100,000, based on CDC data.
The 7-day average of emergency dept. visits among the youngest age group, 0-11 years, shows the same general increase as hospital admissions, but the older children have diverged form that path (see graph). For those aged 12-15 and 16-17, hospitalizations started dropping in late May and into mid-June before climbing again, although more slowly than for the youngest group, the CDC data show.
The ED visit rate with diagnosed COVID among those aged 0-11, measured at 6.1% of all visits on July 19, is, in fact, considerably higher than at any time during the Delta surge last year, when it never passed 4.0%, although much lower than peak Omicron (14.1%). That 6.1% was also higher than any other age group on that day, adults included, the CDC said.
New COVID-19 cases rose for the second week in a row as cumulative cases among U.S. children passed the 14-million mark, but a recent survey shows that more than half of parents believe that the vaccine is a greater risk to children under age 5 years than the virus.
In a Kaiser Family Foundation survey conducted July 7-17, 53% of parents with children aged 6 months to 5 years said that the vaccine is “a bigger risk to their child’s health than getting infected with COVID-19, compared to 44% who say getting infected is the bigger risk,” KFF reported July 26.
More than 4 out of 10 of respondents (43%) said that they will “definitely not” get their eligible children vaccinated, while only 7% said that their children had already received it and 10% said their children would get it as soon as possible, according to the KFF survey, which had an overall sample size of 1,847 adults, including an oversample of 471 parents of children under age 5.
Vaccine initiation has been slow in the first month since it was approved for the youngest children. Just 2.8% of all eligible children under age 5 had received an initial dose as of July 19, compared with first-month uptake figures of more than 18% for the 5- to 11-year-olds and 27% for those aged 12-15, based on data from the Centers for Disease Control and Prevention.
The current rates for vaccination in those aged 5 and older look like this: 70.2% of 12- to 17-year-olds have received at least one dose, versus 37.1% of those aged 5-11. Just over 60% of the older children were fully vaccinated as of July 19, as were 30.2% of the 5- to 11-year-olds, the CDC reported on its COVID Data Tracker.
Number of new cases hits 2-month high
Despite the vaccine, SARS-CoV-2 and its various mutations have continued with their summer travels. With 92,000 newly infected children added for the week of July 15-21, there have now been a total of 14,003,497 pediatric cases reported since the start of the pandemic, which works out to 18.6% of cases in all ages, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
The 92,000 new cases represent an increase of almost 22% over the previous week and mark the highest 1-week count since May, when the total passed 100,000 for 2 consecutive weeks. More recently the trend had seemed more stable as weekly cases dropped twice and rose twice as the total hovered around 70,000, based on the data collected by the AAP and CHA from state and territorial health departments.
A different scenario has played out for emergency department visits and hospital admissions, which have risen steadily since the beginning of April. The admission rate for children aged 0-17, which was just 0.13 new patients per 100,000 population on April 11, was up to 0.44 per 100,000 on July 21. By comparison, the highest rate reached last year during the Delta surge was 0.47 per 100,000, based on CDC data.
The 7-day average of emergency dept. visits among the youngest age group, 0-11 years, shows the same general increase as hospital admissions, but the older children have diverged form that path (see graph). For those aged 12-15 and 16-17, hospitalizations started dropping in late May and into mid-June before climbing again, although more slowly than for the youngest group, the CDC data show.
The ED visit rate with diagnosed COVID among those aged 0-11, measured at 6.1% of all visits on July 19, is, in fact, considerably higher than at any time during the Delta surge last year, when it never passed 4.0%, although much lower than peak Omicron (14.1%). That 6.1% was also higher than any other age group on that day, adults included, the CDC said.
New COVID-19 cases rose for the second week in a row as cumulative cases among U.S. children passed the 14-million mark, but a recent survey shows that more than half of parents believe that the vaccine is a greater risk to children under age 5 years than the virus.
In a Kaiser Family Foundation survey conducted July 7-17, 53% of parents with children aged 6 months to 5 years said that the vaccine is “a bigger risk to their child’s health than getting infected with COVID-19, compared to 44% who say getting infected is the bigger risk,” KFF reported July 26.
More than 4 out of 10 of respondents (43%) said that they will “definitely not” get their eligible children vaccinated, while only 7% said that their children had already received it and 10% said their children would get it as soon as possible, according to the KFF survey, which had an overall sample size of 1,847 adults, including an oversample of 471 parents of children under age 5.
Vaccine initiation has been slow in the first month since it was approved for the youngest children. Just 2.8% of all eligible children under age 5 had received an initial dose as of July 19, compared with first-month uptake figures of more than 18% for the 5- to 11-year-olds and 27% for those aged 12-15, based on data from the Centers for Disease Control and Prevention.
The current rates for vaccination in those aged 5 and older look like this: 70.2% of 12- to 17-year-olds have received at least one dose, versus 37.1% of those aged 5-11. Just over 60% of the older children were fully vaccinated as of July 19, as were 30.2% of the 5- to 11-year-olds, the CDC reported on its COVID Data Tracker.
Number of new cases hits 2-month high
Despite the vaccine, SARS-CoV-2 and its various mutations have continued with their summer travels. With 92,000 newly infected children added for the week of July 15-21, there have now been a total of 14,003,497 pediatric cases reported since the start of the pandemic, which works out to 18.6% of cases in all ages, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
The 92,000 new cases represent an increase of almost 22% over the previous week and mark the highest 1-week count since May, when the total passed 100,000 for 2 consecutive weeks. More recently the trend had seemed more stable as weekly cases dropped twice and rose twice as the total hovered around 70,000, based on the data collected by the AAP and CHA from state and territorial health departments.
A different scenario has played out for emergency department visits and hospital admissions, which have risen steadily since the beginning of April. The admission rate for children aged 0-17, which was just 0.13 new patients per 100,000 population on April 11, was up to 0.44 per 100,000 on July 21. By comparison, the highest rate reached last year during the Delta surge was 0.47 per 100,000, based on CDC data.
The 7-day average of emergency dept. visits among the youngest age group, 0-11 years, shows the same general increase as hospital admissions, but the older children have diverged form that path (see graph). For those aged 12-15 and 16-17, hospitalizations started dropping in late May and into mid-June before climbing again, although more slowly than for the youngest group, the CDC data show.
The ED visit rate with diagnosed COVID among those aged 0-11, measured at 6.1% of all visits on July 19, is, in fact, considerably higher than at any time during the Delta surge last year, when it never passed 4.0%, although much lower than peak Omicron (14.1%). That 6.1% was also higher than any other age group on that day, adults included, the CDC said.
Two distinct phenotypes of COVID-related myocarditis emerge
Researchers from France have identified two distinct phenotypes of fulminant COVID-19–related myocarditis in adults, with different clinical presentations, immunologic profiles, and outcomes.
Differentiation between the two bioclinical entities is important to understand for patient management and further pathophysiological studies, they said.
The first phenotype occurs early (within a few days) in acute SARS-CoV-2 infection, with active viral replication (polymerase chain reaction positive) in adults who meet criteria for multisystem inflammatory syndrome (MIS-A+).
In this early phenotype, there is “limited systemic inflammation without skin and mucosal involvement, but myocardial dysfunction is fulminant and frequently associated with large pericardial effusions. These cases more often require extracorporeal membrane oxygenation [ECMO],” Guy Gorochov, MD, PhD, Sorbonne University, Paris, said in an interview.
The second is a delayed, postinfectious, immune-driven phenotype that occurs in adults who fail to meet the criteria for MIS-A (MIS-A–).
This phenotype occurs weeks after SARS-CoV-2 infection, usually beyond detectable active viral replication (PCR–) in the context of specific immune response and severe systemic inflammation with skin and mucosal involvement. Myocardial dysfunction is more progressive and rarely associated with large pericardial effusions, Dr. Gorochov explained.
The study was published in the Journal of the American College of Cardiology.
Evolving understanding
The findings are based on a retrospective analysis of 38 patients without a history of COVID-19 vaccination who were admitted to the intensive care unit from March 2020 to June 2021 for suspected fulminant COVID-19 myocarditis.
Patients were confirmed to have SARS-CoV-2 infection by PCR and/or by serologic testing. As noted in other studies, the patients were predominantly young men (66%; median age, 27.5 years). Twenty-five (66%) patients were MIS-A+ and 13 (34%) were MIS-A–.
In general, the MIS-A– patients were sicker and had worse outcomes.
Specifically, compared with the MIS-A+ patients, MIS-A– patients had a shorter time between the onset of COVID-19 symptoms and the development of myocarditis, a shorter time to ICU admission, and more severe presentations assessed using lower left ventricular ejection fraction and sequential organ failure assessment scores.
MIS-A– patients also had higher lactate levels, were more likely to need venoarterial ECMO (92% vs 16%), had higher ICU mortality (31% vs. 4%), and a had lower probability of survival at 3 months (68% vs. 96%), compared with their MIS-A+ peers.
Immunologic differences
The immunologic profiles of these two distinct clinical phenotypes also differed.
In MIS-A– early-type COVID-19 myocarditis, RNA polymerase III autoantibodies are frequently positive and serum levels of antiviral interferon-alpha and granulocyte-attracting interleukin-8 are elevated.
In contrast, in MIS-A+ delayed-type COVID-19 myocarditis, RNA polymerase III autoantibodies are negative and serum levels of IL-17 and IL-22 are highly elevated.
“We suggest that IL-17 and IL-22 are novel criteria that should help to assess in adults the recently recognized MIS-A,” Dr. Gorochov told this news organization. “It should be tested whether IL-17 and IL-22 are also elevated in children with MIS-C.”
The researchers also observed “extremely” high serum IL-10 levels in both patient groups. This has been previously associated with severe myocardial injury and an increase in the risk for death in severe COVID-19 patients.
The researchers said the phenotypic clustering of patients with fulminant COVID-19–related myocarditis “seems relevant” for their management.
MIS-A– cases, owing to the high risk for evolution toward refractory cardiogenic shock, should be “urgently” referred to a center with venoarterial ECMO and closely monitored to prevent a “too-late” cannulation, especially under cardiopulmonary resuscitation, known to be associated with poor outcomes, they advised.
They noted that the five patients who died in their series had late venoarterial ECMO implantation, while undergoing multiple organ failures or resuscitation.
Conversely, the risk for evolution to refractory cardiogenic shock is lower in MIS-A+ cases. However, identifying MIS-A+ cases is “all the more important given that numerous data support the efficacy of corticosteroids and/or intravenous immunoglobulins in MIS-C,” Dr. Gorochov and colleagues wrote.
The authors of a linked editorial said the French team should be “commended on their work in furthering our understanding of fulminant myocarditis related to COVID-19 infection.”
Ajith Nair, MD, Baylor College of Medicine, and Anita Deswal, MD, MPH, University of Texas M.D. Anderson Cancer Center, both in Houston, noted that fulminant myocarditis is rare and can result from either of two mechanisms: viral tropism or an immune-mediated mechanism.
“It remains to be seen whether using antiviral therapy versus immunomodulatory therapy on the basis of clinical and cytokine profiles will yield benefits,” they wrote.
“Fulminant myocarditis invariably requires hemodynamic support and carries a high mortality risk if it is recognized late. However, the long-term prognosis in patients who survive the critical period is favorable, with recovery of myocardial function,” they added.
“This study highlights the ever-shifting understanding of the pathophysiology and therapeutic approaches to fulminant myocarditis,” Dr. Nair and Dr. Deswal concluded.
This research was supported in part by the Foundation of France, French National Research Agency, Sorbonne University, and Clinical Research Hospital. The researchers have filed a patent application based on these results. Dr. Nair and Dr. Deswal have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Researchers from France have identified two distinct phenotypes of fulminant COVID-19–related myocarditis in adults, with different clinical presentations, immunologic profiles, and outcomes.
Differentiation between the two bioclinical entities is important to understand for patient management and further pathophysiological studies, they said.
The first phenotype occurs early (within a few days) in acute SARS-CoV-2 infection, with active viral replication (polymerase chain reaction positive) in adults who meet criteria for multisystem inflammatory syndrome (MIS-A+).
In this early phenotype, there is “limited systemic inflammation without skin and mucosal involvement, but myocardial dysfunction is fulminant and frequently associated with large pericardial effusions. These cases more often require extracorporeal membrane oxygenation [ECMO],” Guy Gorochov, MD, PhD, Sorbonne University, Paris, said in an interview.
The second is a delayed, postinfectious, immune-driven phenotype that occurs in adults who fail to meet the criteria for MIS-A (MIS-A–).
This phenotype occurs weeks after SARS-CoV-2 infection, usually beyond detectable active viral replication (PCR–) in the context of specific immune response and severe systemic inflammation with skin and mucosal involvement. Myocardial dysfunction is more progressive and rarely associated with large pericardial effusions, Dr. Gorochov explained.
The study was published in the Journal of the American College of Cardiology.
Evolving understanding
The findings are based on a retrospective analysis of 38 patients without a history of COVID-19 vaccination who were admitted to the intensive care unit from March 2020 to June 2021 for suspected fulminant COVID-19 myocarditis.
Patients were confirmed to have SARS-CoV-2 infection by PCR and/or by serologic testing. As noted in other studies, the patients were predominantly young men (66%; median age, 27.5 years). Twenty-five (66%) patients were MIS-A+ and 13 (34%) were MIS-A–.
In general, the MIS-A– patients were sicker and had worse outcomes.
Specifically, compared with the MIS-A+ patients, MIS-A– patients had a shorter time between the onset of COVID-19 symptoms and the development of myocarditis, a shorter time to ICU admission, and more severe presentations assessed using lower left ventricular ejection fraction and sequential organ failure assessment scores.
MIS-A– patients also had higher lactate levels, were more likely to need venoarterial ECMO (92% vs 16%), had higher ICU mortality (31% vs. 4%), and a had lower probability of survival at 3 months (68% vs. 96%), compared with their MIS-A+ peers.
Immunologic differences
The immunologic profiles of these two distinct clinical phenotypes also differed.
In MIS-A– early-type COVID-19 myocarditis, RNA polymerase III autoantibodies are frequently positive and serum levels of antiviral interferon-alpha and granulocyte-attracting interleukin-8 are elevated.
In contrast, in MIS-A+ delayed-type COVID-19 myocarditis, RNA polymerase III autoantibodies are negative and serum levels of IL-17 and IL-22 are highly elevated.
“We suggest that IL-17 and IL-22 are novel criteria that should help to assess in adults the recently recognized MIS-A,” Dr. Gorochov told this news organization. “It should be tested whether IL-17 and IL-22 are also elevated in children with MIS-C.”
The researchers also observed “extremely” high serum IL-10 levels in both patient groups. This has been previously associated with severe myocardial injury and an increase in the risk for death in severe COVID-19 patients.
The researchers said the phenotypic clustering of patients with fulminant COVID-19–related myocarditis “seems relevant” for their management.
MIS-A– cases, owing to the high risk for evolution toward refractory cardiogenic shock, should be “urgently” referred to a center with venoarterial ECMO and closely monitored to prevent a “too-late” cannulation, especially under cardiopulmonary resuscitation, known to be associated with poor outcomes, they advised.
They noted that the five patients who died in their series had late venoarterial ECMO implantation, while undergoing multiple organ failures or resuscitation.
Conversely, the risk for evolution to refractory cardiogenic shock is lower in MIS-A+ cases. However, identifying MIS-A+ cases is “all the more important given that numerous data support the efficacy of corticosteroids and/or intravenous immunoglobulins in MIS-C,” Dr. Gorochov and colleagues wrote.
The authors of a linked editorial said the French team should be “commended on their work in furthering our understanding of fulminant myocarditis related to COVID-19 infection.”
Ajith Nair, MD, Baylor College of Medicine, and Anita Deswal, MD, MPH, University of Texas M.D. Anderson Cancer Center, both in Houston, noted that fulminant myocarditis is rare and can result from either of two mechanisms: viral tropism or an immune-mediated mechanism.
“It remains to be seen whether using antiviral therapy versus immunomodulatory therapy on the basis of clinical and cytokine profiles will yield benefits,” they wrote.
“Fulminant myocarditis invariably requires hemodynamic support and carries a high mortality risk if it is recognized late. However, the long-term prognosis in patients who survive the critical period is favorable, with recovery of myocardial function,” they added.
“This study highlights the ever-shifting understanding of the pathophysiology and therapeutic approaches to fulminant myocarditis,” Dr. Nair and Dr. Deswal concluded.
This research was supported in part by the Foundation of France, French National Research Agency, Sorbonne University, and Clinical Research Hospital. The researchers have filed a patent application based on these results. Dr. Nair and Dr. Deswal have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Researchers from France have identified two distinct phenotypes of fulminant COVID-19–related myocarditis in adults, with different clinical presentations, immunologic profiles, and outcomes.
Differentiation between the two bioclinical entities is important to understand for patient management and further pathophysiological studies, they said.
The first phenotype occurs early (within a few days) in acute SARS-CoV-2 infection, with active viral replication (polymerase chain reaction positive) in adults who meet criteria for multisystem inflammatory syndrome (MIS-A+).
In this early phenotype, there is “limited systemic inflammation without skin and mucosal involvement, but myocardial dysfunction is fulminant and frequently associated with large pericardial effusions. These cases more often require extracorporeal membrane oxygenation [ECMO],” Guy Gorochov, MD, PhD, Sorbonne University, Paris, said in an interview.
The second is a delayed, postinfectious, immune-driven phenotype that occurs in adults who fail to meet the criteria for MIS-A (MIS-A–).
This phenotype occurs weeks after SARS-CoV-2 infection, usually beyond detectable active viral replication (PCR–) in the context of specific immune response and severe systemic inflammation with skin and mucosal involvement. Myocardial dysfunction is more progressive and rarely associated with large pericardial effusions, Dr. Gorochov explained.
The study was published in the Journal of the American College of Cardiology.
Evolving understanding
The findings are based on a retrospective analysis of 38 patients without a history of COVID-19 vaccination who were admitted to the intensive care unit from March 2020 to June 2021 for suspected fulminant COVID-19 myocarditis.
Patients were confirmed to have SARS-CoV-2 infection by PCR and/or by serologic testing. As noted in other studies, the patients were predominantly young men (66%; median age, 27.5 years). Twenty-five (66%) patients were MIS-A+ and 13 (34%) were MIS-A–.
In general, the MIS-A– patients were sicker and had worse outcomes.
Specifically, compared with the MIS-A+ patients, MIS-A– patients had a shorter time between the onset of COVID-19 symptoms and the development of myocarditis, a shorter time to ICU admission, and more severe presentations assessed using lower left ventricular ejection fraction and sequential organ failure assessment scores.
MIS-A– patients also had higher lactate levels, were more likely to need venoarterial ECMO (92% vs 16%), had higher ICU mortality (31% vs. 4%), and a had lower probability of survival at 3 months (68% vs. 96%), compared with their MIS-A+ peers.
Immunologic differences
The immunologic profiles of these two distinct clinical phenotypes also differed.
In MIS-A– early-type COVID-19 myocarditis, RNA polymerase III autoantibodies are frequently positive and serum levels of antiviral interferon-alpha and granulocyte-attracting interleukin-8 are elevated.
In contrast, in MIS-A+ delayed-type COVID-19 myocarditis, RNA polymerase III autoantibodies are negative and serum levels of IL-17 and IL-22 are highly elevated.
“We suggest that IL-17 and IL-22 are novel criteria that should help to assess in adults the recently recognized MIS-A,” Dr. Gorochov told this news organization. “It should be tested whether IL-17 and IL-22 are also elevated in children with MIS-C.”
The researchers also observed “extremely” high serum IL-10 levels in both patient groups. This has been previously associated with severe myocardial injury and an increase in the risk for death in severe COVID-19 patients.
The researchers said the phenotypic clustering of patients with fulminant COVID-19–related myocarditis “seems relevant” for their management.
MIS-A– cases, owing to the high risk for evolution toward refractory cardiogenic shock, should be “urgently” referred to a center with venoarterial ECMO and closely monitored to prevent a “too-late” cannulation, especially under cardiopulmonary resuscitation, known to be associated with poor outcomes, they advised.
They noted that the five patients who died in their series had late venoarterial ECMO implantation, while undergoing multiple organ failures or resuscitation.
Conversely, the risk for evolution to refractory cardiogenic shock is lower in MIS-A+ cases. However, identifying MIS-A+ cases is “all the more important given that numerous data support the efficacy of corticosteroids and/or intravenous immunoglobulins in MIS-C,” Dr. Gorochov and colleagues wrote.
The authors of a linked editorial said the French team should be “commended on their work in furthering our understanding of fulminant myocarditis related to COVID-19 infection.”
Ajith Nair, MD, Baylor College of Medicine, and Anita Deswal, MD, MPH, University of Texas M.D. Anderson Cancer Center, both in Houston, noted that fulminant myocarditis is rare and can result from either of two mechanisms: viral tropism or an immune-mediated mechanism.
“It remains to be seen whether using antiviral therapy versus immunomodulatory therapy on the basis of clinical and cytokine profiles will yield benefits,” they wrote.
“Fulminant myocarditis invariably requires hemodynamic support and carries a high mortality risk if it is recognized late. However, the long-term prognosis in patients who survive the critical period is favorable, with recovery of myocardial function,” they added.
“This study highlights the ever-shifting understanding of the pathophysiology and therapeutic approaches to fulminant myocarditis,” Dr. Nair and Dr. Deswal concluded.
This research was supported in part by the Foundation of France, French National Research Agency, Sorbonne University, and Clinical Research Hospital. The researchers have filed a patent application based on these results. Dr. Nair and Dr. Deswal have no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Hypertension heightens risk for severe COVID-19, even in the fully vaxxed
Adults with hypertension who were vaccinated for COVID-19 with at least one booster were more than twice as likely as vaccinated and boosted individuals without hypertension to be hospitalized for severe COVID-19, according to data from more than 900 individuals.
“We were surprised to learn that many people who were hospitalized with COVID-19 had hypertension and no other risk factors,” said Susan Cheng, MD, MPH, director of the Institute for Research on Healthy Aging in the department of cardiology at the Smidt Heart Institute, Los Angeles, and a senior author of the study. “This is concerning when you consider that almost half of American adults have high blood pressure.”
COVID-19 vaccines demonstrated ability to reduce death and some of the most severe side effects from the infection in the early stages of the pandemic. Although the Omicron surge prompted recommendations for a third mRNA vaccine dose, “a proportion of individuals who received three mRNA vaccine doses still required hospitalization for COVID-19 during the Omicron surge,” and the characteristics associated with severe illness in vaccinated and boosted patients have not been explored, Joseph Ebinger, MD, of Cedars-Sinai Medical Center, Los Angeles, and colleagues wrote.
Previous research has shown an association between high blood pressure an increased risk for developing severe COVID-19 compared to several other chronic health conditions, including kidney disease, type 2 diabetes, chronic obstructive pulmonary disease, and heart failure, the researchers noted.
In a study published in Hypertension, the researchers identified 912 adults who received at least three doses of mRNA COVID-19 vaccine and were later diagnosed with COVID-19 during the surge in infections from the Omicron variant between December 2021 and April 2022.
A total of 145 of the individuals were hospitalized (16%); of these, 125 (86%) had hypertension.
Patients with hypertension were the most likely to be hospitalized, with an odds ratio of 2.9. In addition to high blood pressure, factors including older age (OR, 1.3), chronic kidney disease (OR, 2.2), prior myocardial infarction or heart failure (OR, 2.2), and longer time since the last vaccination and COVID-19 infection were associated with increased risk of hospitalization in a multivariate analysis.
However, the increased risk of severe illness and hospitalization associated with high blood pressure persisted, with an OR of 2.6, in the absence of comorbid conditions such as type 2 diabetes, kidney disease, and heart failure, the researchers emphasized.
“Although the mechanism for hypertension-associated COVID-19 risk remains unclear, prior studies have identified delayed SARS-CoV-2 viral clearance and prolonged inflammatory response among hypertensive patients, which may contribute to greater disease severity,” they wrote.
The findings were limited by several factors, including the use of data from a single center and lack of information on which Omicron variants and subvariants were behind the infections, the researchers noted.
However, the results highlight the need for more research on how to reduce the risks of severe COVID-19 in vulnerable populations, and on the mechanism for a potential connection between high blood pressure and severe COVID-19, they said.
Given the high prevalence of hypertension worldwide, increased understanding of the hypertension-specific risks and identification of individual and population-level risk reduction strategies will be important to the transition of COVID-19 from pandemic to endemic, they concluded.
Omicron changes the game
“When the pandemic initially started, many conditions were seen to increase risk for more severe COVID illness, and hypertension was one of those factors – and then things changed,” lead author Dr. Ebinger said in an interview. “First, vaccines arrived on the scene and substantially reduced risk of severe COVID for everyone who received them. Second, Omicron arrived and, while more transmissible, this variant has been less likely to cause severe COVID. On the one hand, we have vaccines and boosters that we want to think of as ‘the great equalizer’ when it comes to preexisting conditions. On the other hand, we have a dominant set of SARS-CoV-2 subvariants that seem less virulent in most people.
“Taken together, we have been hoping and even assuming that we have been doing pretty well with minimizing risks. Unfortunately, our study results indicate this is not exactly the case,” he said.
“Although vaccines and boosters appear to have equalized or minimized the risks of severe COVID for some people, this has not happened for others – even in the setting of the milder Omicron variant. Of individuals who were fully vaccinated and boosted, having hypertension increased the odds of needing to be hospitalized after getting infected with Omicron by 2.6-fold, even when accounting for or in the absence of having any major chronic disease that might otherwise predispose to more severe COVID-19 illness,” Dr. Ebinger added.
“So, while the originally seen risks of having obesity or diabetes seem to have been minimized during this current era of pandemic, the risk of having hypertension has persisted. We found this both surprising and concerning, because hypertension is very common and present in over half of people over age 50.”
Surprisingly, “we found that a fair number of people, even after being fully vaccinated plus a having gotten a booster, will not only catch Omicron but get sick enough to need hospital care,” Dr. Ebinger emphasized. “Moreover, it is not just older adults with major comorbid conditions who are vulnerable. Our data show that this can happen to an adult of any age and especially if a person has only hypertension and otherwise no major chronic disease.”
The first takeaway message for clinicians at this time is to raise awareness, Dr. Ebinger stressed in the interview. “We need to raise understanding around the fact that receiving three doses of vaccine may not prevent severe COVID-19 illness in everyone, even when the circulating viral variant is presumed to be causing only mild disease in most people. Moreover, the people who are most at risk are not whom we might think they are. They are not the sickest of the sick. They include people who might not have major conditions such as heart disease or kidney disease, but they do have hypertension.”
Second, “we need more research to understand out why there is this link between hypertension and excess risk for the more severe forms of COVID-19, despite it arising from a supposedly milder variant,” said Dr. Ebinger.
“Third, we need to determine how to reduce these risks, whether through more tailored vaccine regimens or novel therapeutics or a combination approach,” he said.
Looking ahead, “the biological mechanism underpinning the association between hypertension and severe COVID-19 remains underexplored. Future work should focus on understanding the factors linking hypertension to severe COVID-19, as this may elucidate both information on how SARS-CoV-2 effects the body and potential targets for intervention,” Dr. Ebinger added.
The study was supported in part by Cedars-Sinai Medical Center, the Erika J. Glazer Family Foundation and the National Institutes of Health. The researchers had no financial conflicts to disclose.
Adults with hypertension who were vaccinated for COVID-19 with at least one booster were more than twice as likely as vaccinated and boosted individuals without hypertension to be hospitalized for severe COVID-19, according to data from more than 900 individuals.
“We were surprised to learn that many people who were hospitalized with COVID-19 had hypertension and no other risk factors,” said Susan Cheng, MD, MPH, director of the Institute for Research on Healthy Aging in the department of cardiology at the Smidt Heart Institute, Los Angeles, and a senior author of the study. “This is concerning when you consider that almost half of American adults have high blood pressure.”
COVID-19 vaccines demonstrated ability to reduce death and some of the most severe side effects from the infection in the early stages of the pandemic. Although the Omicron surge prompted recommendations for a third mRNA vaccine dose, “a proportion of individuals who received three mRNA vaccine doses still required hospitalization for COVID-19 during the Omicron surge,” and the characteristics associated with severe illness in vaccinated and boosted patients have not been explored, Joseph Ebinger, MD, of Cedars-Sinai Medical Center, Los Angeles, and colleagues wrote.
Previous research has shown an association between high blood pressure an increased risk for developing severe COVID-19 compared to several other chronic health conditions, including kidney disease, type 2 diabetes, chronic obstructive pulmonary disease, and heart failure, the researchers noted.
In a study published in Hypertension, the researchers identified 912 adults who received at least three doses of mRNA COVID-19 vaccine and were later diagnosed with COVID-19 during the surge in infections from the Omicron variant between December 2021 and April 2022.
A total of 145 of the individuals were hospitalized (16%); of these, 125 (86%) had hypertension.
Patients with hypertension were the most likely to be hospitalized, with an odds ratio of 2.9. In addition to high blood pressure, factors including older age (OR, 1.3), chronic kidney disease (OR, 2.2), prior myocardial infarction or heart failure (OR, 2.2), and longer time since the last vaccination and COVID-19 infection were associated with increased risk of hospitalization in a multivariate analysis.
However, the increased risk of severe illness and hospitalization associated with high blood pressure persisted, with an OR of 2.6, in the absence of comorbid conditions such as type 2 diabetes, kidney disease, and heart failure, the researchers emphasized.
“Although the mechanism for hypertension-associated COVID-19 risk remains unclear, prior studies have identified delayed SARS-CoV-2 viral clearance and prolonged inflammatory response among hypertensive patients, which may contribute to greater disease severity,” they wrote.
The findings were limited by several factors, including the use of data from a single center and lack of information on which Omicron variants and subvariants were behind the infections, the researchers noted.
However, the results highlight the need for more research on how to reduce the risks of severe COVID-19 in vulnerable populations, and on the mechanism for a potential connection between high blood pressure and severe COVID-19, they said.
Given the high prevalence of hypertension worldwide, increased understanding of the hypertension-specific risks and identification of individual and population-level risk reduction strategies will be important to the transition of COVID-19 from pandemic to endemic, they concluded.
Omicron changes the game
“When the pandemic initially started, many conditions were seen to increase risk for more severe COVID illness, and hypertension was one of those factors – and then things changed,” lead author Dr. Ebinger said in an interview. “First, vaccines arrived on the scene and substantially reduced risk of severe COVID for everyone who received them. Second, Omicron arrived and, while more transmissible, this variant has been less likely to cause severe COVID. On the one hand, we have vaccines and boosters that we want to think of as ‘the great equalizer’ when it comes to preexisting conditions. On the other hand, we have a dominant set of SARS-CoV-2 subvariants that seem less virulent in most people.
“Taken together, we have been hoping and even assuming that we have been doing pretty well with minimizing risks. Unfortunately, our study results indicate this is not exactly the case,” he said.
“Although vaccines and boosters appear to have equalized or minimized the risks of severe COVID for some people, this has not happened for others – even in the setting of the milder Omicron variant. Of individuals who were fully vaccinated and boosted, having hypertension increased the odds of needing to be hospitalized after getting infected with Omicron by 2.6-fold, even when accounting for or in the absence of having any major chronic disease that might otherwise predispose to more severe COVID-19 illness,” Dr. Ebinger added.
“So, while the originally seen risks of having obesity or diabetes seem to have been minimized during this current era of pandemic, the risk of having hypertension has persisted. We found this both surprising and concerning, because hypertension is very common and present in over half of people over age 50.”
Surprisingly, “we found that a fair number of people, even after being fully vaccinated plus a having gotten a booster, will not only catch Omicron but get sick enough to need hospital care,” Dr. Ebinger emphasized. “Moreover, it is not just older adults with major comorbid conditions who are vulnerable. Our data show that this can happen to an adult of any age and especially if a person has only hypertension and otherwise no major chronic disease.”
The first takeaway message for clinicians at this time is to raise awareness, Dr. Ebinger stressed in the interview. “We need to raise understanding around the fact that receiving three doses of vaccine may not prevent severe COVID-19 illness in everyone, even when the circulating viral variant is presumed to be causing only mild disease in most people. Moreover, the people who are most at risk are not whom we might think they are. They are not the sickest of the sick. They include people who might not have major conditions such as heart disease or kidney disease, but they do have hypertension.”
Second, “we need more research to understand out why there is this link between hypertension and excess risk for the more severe forms of COVID-19, despite it arising from a supposedly milder variant,” said Dr. Ebinger.
“Third, we need to determine how to reduce these risks, whether through more tailored vaccine regimens or novel therapeutics or a combination approach,” he said.
Looking ahead, “the biological mechanism underpinning the association between hypertension and severe COVID-19 remains underexplored. Future work should focus on understanding the factors linking hypertension to severe COVID-19, as this may elucidate both information on how SARS-CoV-2 effects the body and potential targets for intervention,” Dr. Ebinger added.
The study was supported in part by Cedars-Sinai Medical Center, the Erika J. Glazer Family Foundation and the National Institutes of Health. The researchers had no financial conflicts to disclose.
Adults with hypertension who were vaccinated for COVID-19 with at least one booster were more than twice as likely as vaccinated and boosted individuals without hypertension to be hospitalized for severe COVID-19, according to data from more than 900 individuals.
“We were surprised to learn that many people who were hospitalized with COVID-19 had hypertension and no other risk factors,” said Susan Cheng, MD, MPH, director of the Institute for Research on Healthy Aging in the department of cardiology at the Smidt Heart Institute, Los Angeles, and a senior author of the study. “This is concerning when you consider that almost half of American adults have high blood pressure.”
COVID-19 vaccines demonstrated ability to reduce death and some of the most severe side effects from the infection in the early stages of the pandemic. Although the Omicron surge prompted recommendations for a third mRNA vaccine dose, “a proportion of individuals who received three mRNA vaccine doses still required hospitalization for COVID-19 during the Omicron surge,” and the characteristics associated with severe illness in vaccinated and boosted patients have not been explored, Joseph Ebinger, MD, of Cedars-Sinai Medical Center, Los Angeles, and colleagues wrote.
Previous research has shown an association between high blood pressure an increased risk for developing severe COVID-19 compared to several other chronic health conditions, including kidney disease, type 2 diabetes, chronic obstructive pulmonary disease, and heart failure, the researchers noted.
In a study published in Hypertension, the researchers identified 912 adults who received at least three doses of mRNA COVID-19 vaccine and were later diagnosed with COVID-19 during the surge in infections from the Omicron variant between December 2021 and April 2022.
A total of 145 of the individuals were hospitalized (16%); of these, 125 (86%) had hypertension.
Patients with hypertension were the most likely to be hospitalized, with an odds ratio of 2.9. In addition to high blood pressure, factors including older age (OR, 1.3), chronic kidney disease (OR, 2.2), prior myocardial infarction or heart failure (OR, 2.2), and longer time since the last vaccination and COVID-19 infection were associated with increased risk of hospitalization in a multivariate analysis.
However, the increased risk of severe illness and hospitalization associated with high blood pressure persisted, with an OR of 2.6, in the absence of comorbid conditions such as type 2 diabetes, kidney disease, and heart failure, the researchers emphasized.
“Although the mechanism for hypertension-associated COVID-19 risk remains unclear, prior studies have identified delayed SARS-CoV-2 viral clearance and prolonged inflammatory response among hypertensive patients, which may contribute to greater disease severity,” they wrote.
The findings were limited by several factors, including the use of data from a single center and lack of information on which Omicron variants and subvariants were behind the infections, the researchers noted.
However, the results highlight the need for more research on how to reduce the risks of severe COVID-19 in vulnerable populations, and on the mechanism for a potential connection between high blood pressure and severe COVID-19, they said.
Given the high prevalence of hypertension worldwide, increased understanding of the hypertension-specific risks and identification of individual and population-level risk reduction strategies will be important to the transition of COVID-19 from pandemic to endemic, they concluded.
Omicron changes the game
“When the pandemic initially started, many conditions were seen to increase risk for more severe COVID illness, and hypertension was one of those factors – and then things changed,” lead author Dr. Ebinger said in an interview. “First, vaccines arrived on the scene and substantially reduced risk of severe COVID for everyone who received them. Second, Omicron arrived and, while more transmissible, this variant has been less likely to cause severe COVID. On the one hand, we have vaccines and boosters that we want to think of as ‘the great equalizer’ when it comes to preexisting conditions. On the other hand, we have a dominant set of SARS-CoV-2 subvariants that seem less virulent in most people.
“Taken together, we have been hoping and even assuming that we have been doing pretty well with minimizing risks. Unfortunately, our study results indicate this is not exactly the case,” he said.
“Although vaccines and boosters appear to have equalized or minimized the risks of severe COVID for some people, this has not happened for others – even in the setting of the milder Omicron variant. Of individuals who were fully vaccinated and boosted, having hypertension increased the odds of needing to be hospitalized after getting infected with Omicron by 2.6-fold, even when accounting for or in the absence of having any major chronic disease that might otherwise predispose to more severe COVID-19 illness,” Dr. Ebinger added.
“So, while the originally seen risks of having obesity or diabetes seem to have been minimized during this current era of pandemic, the risk of having hypertension has persisted. We found this both surprising and concerning, because hypertension is very common and present in over half of people over age 50.”
Surprisingly, “we found that a fair number of people, even after being fully vaccinated plus a having gotten a booster, will not only catch Omicron but get sick enough to need hospital care,” Dr. Ebinger emphasized. “Moreover, it is not just older adults with major comorbid conditions who are vulnerable. Our data show that this can happen to an adult of any age and especially if a person has only hypertension and otherwise no major chronic disease.”
The first takeaway message for clinicians at this time is to raise awareness, Dr. Ebinger stressed in the interview. “We need to raise understanding around the fact that receiving three doses of vaccine may not prevent severe COVID-19 illness in everyone, even when the circulating viral variant is presumed to be causing only mild disease in most people. Moreover, the people who are most at risk are not whom we might think they are. They are not the sickest of the sick. They include people who might not have major conditions such as heart disease or kidney disease, but they do have hypertension.”
Second, “we need more research to understand out why there is this link between hypertension and excess risk for the more severe forms of COVID-19, despite it arising from a supposedly milder variant,” said Dr. Ebinger.
“Third, we need to determine how to reduce these risks, whether through more tailored vaccine regimens or novel therapeutics or a combination approach,” he said.
Looking ahead, “the biological mechanism underpinning the association between hypertension and severe COVID-19 remains underexplored. Future work should focus on understanding the factors linking hypertension to severe COVID-19, as this may elucidate both information on how SARS-CoV-2 effects the body and potential targets for intervention,” Dr. Ebinger added.
The study was supported in part by Cedars-Sinai Medical Center, the Erika J. Glazer Family Foundation and the National Institutes of Health. The researchers had no financial conflicts to disclose.
FROM HYPERTENSION
Science lags behind for kids with long COVID
Emma Sherman, a 13-year-old girl in Ascot, England, woke up to a dizzying aura of blind spots and flashing lights in her field of vision. It was May 2020, and she also had crippling nausea and headaches. By August, her dizziness was so overwhelming, she couldn’t hold her head up, lying in her mother’s lap for hours, too fatigued to attend school.
The former competitive gymnast, who had hoped to try out for the cheerleading squad, now used a wheelchair and was a shadow of her former self. She had been diagnosed with COVID-induced postural orthostatic tachycardia syndrome, a condition often caused by an infection that results in a higher heart rate, extreme nausea, dizziness, and fatigue.
“I was so into sports before I got long COVID, and afterwards I could barely walk,” Emma said.
Even minor movements sent her heart rate sky-high. Her long chestnut hair turned gray and fell out in clumps. In the hospital, she was pricked and prodded, her blood tested for numerous conditions.
“They ran every scan known to man and took an MRI of her brain,” said Emma’s mother, Marie Sherman. “All was clear.”
Emma’s pediatrician determined that the teen had long COVID after having had a mild case of the virus in March, about 2 months before her puzzling symptoms began. But beyond a positive antibody test, doctors have found little evidence of what was causing Emma’s symptoms.
For Emma and others with long COVID, there are no medications shown to directly target the condition. Instead, caregivers target their symptoms, which include nausea, dizziness, fatigue, headaches, and a racing heart, said Laura Malone, MD, codirector of the Johns Hopkins Kennedy Krieger Pediatric Post–COVID-19 Rehabilitation Clinic in Baltimore.
“Right now, it’s a rehabilitation-based approach focused on improving symptoms and functioning so that kids can go back to their usual activities as much as possible,” she says.
Depression and anxiety are common, although doctors are struggling to figure out whether COVID is changing the brain or whether mental health symptoms result from all the life disruptions. There’s little research to show how may kids have depression because of long COVID. Dr. Malone said about half of her patients at the Kennedy Krieger Institute›s long COVID clinic are also dealing with mental health issues.
Patients with headaches, dizziness, and nausea are given pain and nausea medications and recommendations for a healthy diet with added fruits and vegetables, monounsaturated fats, lower sodium, unprocessed foods, and whole grains. Kids with irregular or racing heart rates are referred to cardiologists and potentially prescribed beta-blockers to treat their heart arrhythmias, while children with breathing problems may be referred to pulmonologists and those with depression to a psychiatrist.
Still, many patients like Emma go to their doctors with phantom symptoms that don’t show up on scans or blood tests.
“We’re not seeing any evidence of structural damage to the brain, for example,” said Dr. Malone. “When we do MRIs, they often come out normal.”
It’s possible that the virus lingers in some patients, said Rajeev Fernando, MD, an infectious disease specialist and a fellow at Harvard Medical School, Boston. Kids’ strong immune systems often fend off problems that can be noticed. But on the inside, dead fragments of the virus persist, floating in hidden parts of the body and activating the immune system long after the threat has passed.
The virus can be in the gut and in the brain, which may help explain why symptoms like brain fog and nausea can linger in children.
“The immune system doesn’t recognize whether fragments of the virus are dead or alive. It continues to think it’s fighting active COVID,” said Dr. Fernando.
There is little data on how long symptoms last, Dr. Fernando said, as well as how many kids get them and why some are more vulnerable than others. Some research has found that about 5%-15% of children with COVID may get long COVID, but the statistics vary globally.
“Children with long COVID have largely been ignored. And while we’re talking about it now, we’ve got some work to do,” said Dr. Fernando.
As for Emma, she recovered in January of 2021, heading back to school and her friends, although her cardiologist advised her to skip gym classes.
“For the first time in months, I was feeling like myself again,” she said.
But the coronavirus found its way to Emma again. Although she was fully vaccinated in the fall of 2021, when the Omicron variant swept the world late that year, she was infected again.
“When the wave of Omicron descended, Emma was like a sitting duck,” her mother said.
She was bedridden with a high fever and cough. The cold-like symptoms eventually went away, but the issues in her gut stuck around. Since then, Emma has had extreme nausea, losing most of the weight she had gained back.
For her part, Ms. Sherman has found solace in a group called Long COVID Kids, a nonprofit in Europe and the United States. The group is raising awareness about the condition in kids to increase funding, boost understanding, and improve treatment and outcomes.
“There’s nothing worse than watching your child suffer and not being able to do anything about it,” she said. “I tell Emma all the time: If I could just crawl in your body and take it, I would do it in a second.”
Emma is hoping for a fresh start with her family’s move in the coming weeks to Sotogrande in southern Spain.
“I miss the simplest things like going for a run, going to the fair with my friends, and just feeling well,” she said. “I have a long list of things I’ll do once this is all done.”
A version of this article first appeared on WebMD.com.
Emma Sherman, a 13-year-old girl in Ascot, England, woke up to a dizzying aura of blind spots and flashing lights in her field of vision. It was May 2020, and she also had crippling nausea and headaches. By August, her dizziness was so overwhelming, she couldn’t hold her head up, lying in her mother’s lap for hours, too fatigued to attend school.
The former competitive gymnast, who had hoped to try out for the cheerleading squad, now used a wheelchair and was a shadow of her former self. She had been diagnosed with COVID-induced postural orthostatic tachycardia syndrome, a condition often caused by an infection that results in a higher heart rate, extreme nausea, dizziness, and fatigue.
“I was so into sports before I got long COVID, and afterwards I could barely walk,” Emma said.
Even minor movements sent her heart rate sky-high. Her long chestnut hair turned gray and fell out in clumps. In the hospital, she was pricked and prodded, her blood tested for numerous conditions.
“They ran every scan known to man and took an MRI of her brain,” said Emma’s mother, Marie Sherman. “All was clear.”
Emma’s pediatrician determined that the teen had long COVID after having had a mild case of the virus in March, about 2 months before her puzzling symptoms began. But beyond a positive antibody test, doctors have found little evidence of what was causing Emma’s symptoms.
For Emma and others with long COVID, there are no medications shown to directly target the condition. Instead, caregivers target their symptoms, which include nausea, dizziness, fatigue, headaches, and a racing heart, said Laura Malone, MD, codirector of the Johns Hopkins Kennedy Krieger Pediatric Post–COVID-19 Rehabilitation Clinic in Baltimore.
“Right now, it’s a rehabilitation-based approach focused on improving symptoms and functioning so that kids can go back to their usual activities as much as possible,” she says.
Depression and anxiety are common, although doctors are struggling to figure out whether COVID is changing the brain or whether mental health symptoms result from all the life disruptions. There’s little research to show how may kids have depression because of long COVID. Dr. Malone said about half of her patients at the Kennedy Krieger Institute›s long COVID clinic are also dealing with mental health issues.
Patients with headaches, dizziness, and nausea are given pain and nausea medications and recommendations for a healthy diet with added fruits and vegetables, monounsaturated fats, lower sodium, unprocessed foods, and whole grains. Kids with irregular or racing heart rates are referred to cardiologists and potentially prescribed beta-blockers to treat their heart arrhythmias, while children with breathing problems may be referred to pulmonologists and those with depression to a psychiatrist.
Still, many patients like Emma go to their doctors with phantom symptoms that don’t show up on scans or blood tests.
“We’re not seeing any evidence of structural damage to the brain, for example,” said Dr. Malone. “When we do MRIs, they often come out normal.”
It’s possible that the virus lingers in some patients, said Rajeev Fernando, MD, an infectious disease specialist and a fellow at Harvard Medical School, Boston. Kids’ strong immune systems often fend off problems that can be noticed. But on the inside, dead fragments of the virus persist, floating in hidden parts of the body and activating the immune system long after the threat has passed.
The virus can be in the gut and in the brain, which may help explain why symptoms like brain fog and nausea can linger in children.
“The immune system doesn’t recognize whether fragments of the virus are dead or alive. It continues to think it’s fighting active COVID,” said Dr. Fernando.
There is little data on how long symptoms last, Dr. Fernando said, as well as how many kids get them and why some are more vulnerable than others. Some research has found that about 5%-15% of children with COVID may get long COVID, but the statistics vary globally.
“Children with long COVID have largely been ignored. And while we’re talking about it now, we’ve got some work to do,” said Dr. Fernando.
As for Emma, she recovered in January of 2021, heading back to school and her friends, although her cardiologist advised her to skip gym classes.
“For the first time in months, I was feeling like myself again,” she said.
But the coronavirus found its way to Emma again. Although she was fully vaccinated in the fall of 2021, when the Omicron variant swept the world late that year, she was infected again.
“When the wave of Omicron descended, Emma was like a sitting duck,” her mother said.
She was bedridden with a high fever and cough. The cold-like symptoms eventually went away, but the issues in her gut stuck around. Since then, Emma has had extreme nausea, losing most of the weight she had gained back.
For her part, Ms. Sherman has found solace in a group called Long COVID Kids, a nonprofit in Europe and the United States. The group is raising awareness about the condition in kids to increase funding, boost understanding, and improve treatment and outcomes.
“There’s nothing worse than watching your child suffer and not being able to do anything about it,” she said. “I tell Emma all the time: If I could just crawl in your body and take it, I would do it in a second.”
Emma is hoping for a fresh start with her family’s move in the coming weeks to Sotogrande in southern Spain.
“I miss the simplest things like going for a run, going to the fair with my friends, and just feeling well,” she said. “I have a long list of things I’ll do once this is all done.”
A version of this article first appeared on WebMD.com.
Emma Sherman, a 13-year-old girl in Ascot, England, woke up to a dizzying aura of blind spots and flashing lights in her field of vision. It was May 2020, and she also had crippling nausea and headaches. By August, her dizziness was so overwhelming, she couldn’t hold her head up, lying in her mother’s lap for hours, too fatigued to attend school.
The former competitive gymnast, who had hoped to try out for the cheerleading squad, now used a wheelchair and was a shadow of her former self. She had been diagnosed with COVID-induced postural orthostatic tachycardia syndrome, a condition often caused by an infection that results in a higher heart rate, extreme nausea, dizziness, and fatigue.
“I was so into sports before I got long COVID, and afterwards I could barely walk,” Emma said.
Even minor movements sent her heart rate sky-high. Her long chestnut hair turned gray and fell out in clumps. In the hospital, she was pricked and prodded, her blood tested for numerous conditions.
“They ran every scan known to man and took an MRI of her brain,” said Emma’s mother, Marie Sherman. “All was clear.”
Emma’s pediatrician determined that the teen had long COVID after having had a mild case of the virus in March, about 2 months before her puzzling symptoms began. But beyond a positive antibody test, doctors have found little evidence of what was causing Emma’s symptoms.
For Emma and others with long COVID, there are no medications shown to directly target the condition. Instead, caregivers target their symptoms, which include nausea, dizziness, fatigue, headaches, and a racing heart, said Laura Malone, MD, codirector of the Johns Hopkins Kennedy Krieger Pediatric Post–COVID-19 Rehabilitation Clinic in Baltimore.
“Right now, it’s a rehabilitation-based approach focused on improving symptoms and functioning so that kids can go back to their usual activities as much as possible,” she says.
Depression and anxiety are common, although doctors are struggling to figure out whether COVID is changing the brain or whether mental health symptoms result from all the life disruptions. There’s little research to show how may kids have depression because of long COVID. Dr. Malone said about half of her patients at the Kennedy Krieger Institute›s long COVID clinic are also dealing with mental health issues.
Patients with headaches, dizziness, and nausea are given pain and nausea medications and recommendations for a healthy diet with added fruits and vegetables, monounsaturated fats, lower sodium, unprocessed foods, and whole grains. Kids with irregular or racing heart rates are referred to cardiologists and potentially prescribed beta-blockers to treat their heart arrhythmias, while children with breathing problems may be referred to pulmonologists and those with depression to a psychiatrist.
Still, many patients like Emma go to their doctors with phantom symptoms that don’t show up on scans or blood tests.
“We’re not seeing any evidence of structural damage to the brain, for example,” said Dr. Malone. “When we do MRIs, they often come out normal.”
It’s possible that the virus lingers in some patients, said Rajeev Fernando, MD, an infectious disease specialist and a fellow at Harvard Medical School, Boston. Kids’ strong immune systems often fend off problems that can be noticed. But on the inside, dead fragments of the virus persist, floating in hidden parts of the body and activating the immune system long after the threat has passed.
The virus can be in the gut and in the brain, which may help explain why symptoms like brain fog and nausea can linger in children.
“The immune system doesn’t recognize whether fragments of the virus are dead or alive. It continues to think it’s fighting active COVID,” said Dr. Fernando.
There is little data on how long symptoms last, Dr. Fernando said, as well as how many kids get them and why some are more vulnerable than others. Some research has found that about 5%-15% of children with COVID may get long COVID, but the statistics vary globally.
“Children with long COVID have largely been ignored. And while we’re talking about it now, we’ve got some work to do,” said Dr. Fernando.
As for Emma, she recovered in January of 2021, heading back to school and her friends, although her cardiologist advised her to skip gym classes.
“For the first time in months, I was feeling like myself again,” she said.
But the coronavirus found its way to Emma again. Although she was fully vaccinated in the fall of 2021, when the Omicron variant swept the world late that year, she was infected again.
“When the wave of Omicron descended, Emma was like a sitting duck,” her mother said.
She was bedridden with a high fever and cough. The cold-like symptoms eventually went away, but the issues in her gut stuck around. Since then, Emma has had extreme nausea, losing most of the weight she had gained back.
For her part, Ms. Sherman has found solace in a group called Long COVID Kids, a nonprofit in Europe and the United States. The group is raising awareness about the condition in kids to increase funding, boost understanding, and improve treatment and outcomes.
“There’s nothing worse than watching your child suffer and not being able to do anything about it,” she said. “I tell Emma all the time: If I could just crawl in your body and take it, I would do it in a second.”
Emma is hoping for a fresh start with her family’s move in the coming weeks to Sotogrande in southern Spain.
“I miss the simplest things like going for a run, going to the fair with my friends, and just feeling well,” she said. “I have a long list of things I’ll do once this is all done.”
A version of this article first appeared on WebMD.com.
Immune response may explain brain damage after COVID-19
It seems that the virus does not infect the brain directly. The scientists found evidence that antibodies – proteins produced by the immune system in response to viruses and other invaders – are involved in an attack on the cells lining the brain’s blood vessels, leading to inflammation and damage. The study was published in the journal Brain.
Brain tissue autopsy
“Patients often develop neurological complications with COVID-19, but the underlying pathophysiological process is not well understood,” Avindra Nath, MD, stated in a National Institutes of Health news release. Dr. Nath, who specializes in neuroimmunology, is the clinical director at the National Institute of Neurological Disorders and Stroke (NINDS) and the senior author of the study. “We had previously shown blood vessel damage and inflammation in patients’ brains at autopsy, but we didn’t understand the cause of the damage. I think in this paper we’ve gained important insight into the cascade of events.”
In this study, Dr. Nath and his team examined brain tissue from a subset of patients from their previous study. The nine individuals, ages 24-73 years, died shortly after contracting COVID-19. They were chosen because structural brain scans showed signs of blood vessel damage in the brain. The samples were compared with those from 10 controls. The team looked at neuroinflammation and immune responses using immunohistochemistry.
As in their earlier study, researchers found signs of leaky blood vessels based on the presence of blood proteins that normally do not cross the blood-brain barrier. This suggests that the tight junctions between the endothelial cells in the blood-brain barrier have been damaged.
Neurologic symptoms’ molecular basis
Dr. Nath and his colleagues discovered deposits of immune complexes on the surface of the cells. This finding is evidence that damage to endothelial cells was likely due to an immune response.
These observations suggest an antibody-mediated attack that activates endothelial cells. When endothelial cells are activated, they express proteins called adhesion molecules that cause platelets to stick together.
“Activation of the endothelial cells brings platelets that stick to the blood vessel walls, causing clots to form and leakage to occur. At the same time, the tight junctions between the endothelial cells get disrupted, causing them to leak,” Dr. Nath explained. “Once leakage occurs, immune cells such as macrophages may come to repair the damage, setting up inflammation. This, in turn, causes damage to neurons.”
Researchers found that in areas with damage to the endothelial cells, more than 300 genes showed decreased expression, whereas six genes were increased. These genes were associated with oxidative stress, DNA damage, and metabolic dysregulation. As the NIH news release notes, this may provide clues to the molecular basis of neurologic symptoms related to COVID-19 and offer potential therapeutic targets.
Together, these findings give insight into the immune response damaging the brain after COVID-19 infection. But it remains unclear what antigen the immune response is targeting, because the virus itself was not detected in the brain. It is possible that antibodies against the SARS-CoV-2 spike protein could bind to the angiotensin-converting enzyme 2 receptor used by the virus to enter cells. More research is needed to explore this hypothesis.
‘Brain fog’ explained?
The study may also have implications for understanding and treating long-term neurologic symptoms after COVID-19, which include headache, fatigue, loss of taste and smell, sleep problems, and “brain fog.” Had the patients in the study survived, the researchers believe they would likely have developed long COVID.
“It is quite possible that this same immune response persists in long COVID patients, resulting in neuronal injury,” said Dr. Nath. “There could be a small, indolent immune response that is continuing, which means that immune-modulating therapies might help these patients. So, these findings have very important therapeutic implications.”
The results suggest that treatments designed to prevent the development of the immune complexes observed in the study could be potential therapies for post-COVID neurologic symptoms.
This study was supported by the NINDS Division of Intramural Research (NS003130) and K23NS109284, the Roy J. Carver Foundation, and the Iowa Neuroscience Institute.
A version of this article first appeared on Medscape.com. This article was translated from Medscape French edition.
It seems that the virus does not infect the brain directly. The scientists found evidence that antibodies – proteins produced by the immune system in response to viruses and other invaders – are involved in an attack on the cells lining the brain’s blood vessels, leading to inflammation and damage. The study was published in the journal Brain.
Brain tissue autopsy
“Patients often develop neurological complications with COVID-19, but the underlying pathophysiological process is not well understood,” Avindra Nath, MD, stated in a National Institutes of Health news release. Dr. Nath, who specializes in neuroimmunology, is the clinical director at the National Institute of Neurological Disorders and Stroke (NINDS) and the senior author of the study. “We had previously shown blood vessel damage and inflammation in patients’ brains at autopsy, but we didn’t understand the cause of the damage. I think in this paper we’ve gained important insight into the cascade of events.”
In this study, Dr. Nath and his team examined brain tissue from a subset of patients from their previous study. The nine individuals, ages 24-73 years, died shortly after contracting COVID-19. They were chosen because structural brain scans showed signs of blood vessel damage in the brain. The samples were compared with those from 10 controls. The team looked at neuroinflammation and immune responses using immunohistochemistry.
As in their earlier study, researchers found signs of leaky blood vessels based on the presence of blood proteins that normally do not cross the blood-brain barrier. This suggests that the tight junctions between the endothelial cells in the blood-brain barrier have been damaged.
Neurologic symptoms’ molecular basis
Dr. Nath and his colleagues discovered deposits of immune complexes on the surface of the cells. This finding is evidence that damage to endothelial cells was likely due to an immune response.
These observations suggest an antibody-mediated attack that activates endothelial cells. When endothelial cells are activated, they express proteins called adhesion molecules that cause platelets to stick together.
“Activation of the endothelial cells brings platelets that stick to the blood vessel walls, causing clots to form and leakage to occur. At the same time, the tight junctions between the endothelial cells get disrupted, causing them to leak,” Dr. Nath explained. “Once leakage occurs, immune cells such as macrophages may come to repair the damage, setting up inflammation. This, in turn, causes damage to neurons.”
Researchers found that in areas with damage to the endothelial cells, more than 300 genes showed decreased expression, whereas six genes were increased. These genes were associated with oxidative stress, DNA damage, and metabolic dysregulation. As the NIH news release notes, this may provide clues to the molecular basis of neurologic symptoms related to COVID-19 and offer potential therapeutic targets.
Together, these findings give insight into the immune response damaging the brain after COVID-19 infection. But it remains unclear what antigen the immune response is targeting, because the virus itself was not detected in the brain. It is possible that antibodies against the SARS-CoV-2 spike protein could bind to the angiotensin-converting enzyme 2 receptor used by the virus to enter cells. More research is needed to explore this hypothesis.
‘Brain fog’ explained?
The study may also have implications for understanding and treating long-term neurologic symptoms after COVID-19, which include headache, fatigue, loss of taste and smell, sleep problems, and “brain fog.” Had the patients in the study survived, the researchers believe they would likely have developed long COVID.
“It is quite possible that this same immune response persists in long COVID patients, resulting in neuronal injury,” said Dr. Nath. “There could be a small, indolent immune response that is continuing, which means that immune-modulating therapies might help these patients. So, these findings have very important therapeutic implications.”
The results suggest that treatments designed to prevent the development of the immune complexes observed in the study could be potential therapies for post-COVID neurologic symptoms.
This study was supported by the NINDS Division of Intramural Research (NS003130) and K23NS109284, the Roy J. Carver Foundation, and the Iowa Neuroscience Institute.
A version of this article first appeared on Medscape.com. This article was translated from Medscape French edition.
It seems that the virus does not infect the brain directly. The scientists found evidence that antibodies – proteins produced by the immune system in response to viruses and other invaders – are involved in an attack on the cells lining the brain’s blood vessels, leading to inflammation and damage. The study was published in the journal Brain.
Brain tissue autopsy
“Patients often develop neurological complications with COVID-19, but the underlying pathophysiological process is not well understood,” Avindra Nath, MD, stated in a National Institutes of Health news release. Dr. Nath, who specializes in neuroimmunology, is the clinical director at the National Institute of Neurological Disorders and Stroke (NINDS) and the senior author of the study. “We had previously shown blood vessel damage and inflammation in patients’ brains at autopsy, but we didn’t understand the cause of the damage. I think in this paper we’ve gained important insight into the cascade of events.”
In this study, Dr. Nath and his team examined brain tissue from a subset of patients from their previous study. The nine individuals, ages 24-73 years, died shortly after contracting COVID-19. They were chosen because structural brain scans showed signs of blood vessel damage in the brain. The samples were compared with those from 10 controls. The team looked at neuroinflammation and immune responses using immunohistochemistry.
As in their earlier study, researchers found signs of leaky blood vessels based on the presence of blood proteins that normally do not cross the blood-brain barrier. This suggests that the tight junctions between the endothelial cells in the blood-brain barrier have been damaged.
Neurologic symptoms’ molecular basis
Dr. Nath and his colleagues discovered deposits of immune complexes on the surface of the cells. This finding is evidence that damage to endothelial cells was likely due to an immune response.
These observations suggest an antibody-mediated attack that activates endothelial cells. When endothelial cells are activated, they express proteins called adhesion molecules that cause platelets to stick together.
“Activation of the endothelial cells brings platelets that stick to the blood vessel walls, causing clots to form and leakage to occur. At the same time, the tight junctions between the endothelial cells get disrupted, causing them to leak,” Dr. Nath explained. “Once leakage occurs, immune cells such as macrophages may come to repair the damage, setting up inflammation. This, in turn, causes damage to neurons.”
Researchers found that in areas with damage to the endothelial cells, more than 300 genes showed decreased expression, whereas six genes were increased. These genes were associated with oxidative stress, DNA damage, and metabolic dysregulation. As the NIH news release notes, this may provide clues to the molecular basis of neurologic symptoms related to COVID-19 and offer potential therapeutic targets.
Together, these findings give insight into the immune response damaging the brain after COVID-19 infection. But it remains unclear what antigen the immune response is targeting, because the virus itself was not detected in the brain. It is possible that antibodies against the SARS-CoV-2 spike protein could bind to the angiotensin-converting enzyme 2 receptor used by the virus to enter cells. More research is needed to explore this hypothesis.
‘Brain fog’ explained?
The study may also have implications for understanding and treating long-term neurologic symptoms after COVID-19, which include headache, fatigue, loss of taste and smell, sleep problems, and “brain fog.” Had the patients in the study survived, the researchers believe they would likely have developed long COVID.
“It is quite possible that this same immune response persists in long COVID patients, resulting in neuronal injury,” said Dr. Nath. “There could be a small, indolent immune response that is continuing, which means that immune-modulating therapies might help these patients. So, these findings have very important therapeutic implications.”
The results suggest that treatments designed to prevent the development of the immune complexes observed in the study could be potential therapies for post-COVID neurologic symptoms.
This study was supported by the NINDS Division of Intramural Research (NS003130) and K23NS109284, the Roy J. Carver Foundation, and the Iowa Neuroscience Institute.
A version of this article first appeared on Medscape.com. This article was translated from Medscape French edition.
Biden tests positive for COVID-19: White House
Biden, 79, is experiencing “very mild” symptoms, White House Press Secretary Karine Jean-Pierre said in a statement. The president is fully vaccinated and has been boosted twice and has started taking the antiviral Paxlovid since testing positive, Ms. Jean-Pierre said.
President Biden plans to isolate at the White House and “will continue to carry out all of his duties fully during that time,” the statement said.
“He has been in contact with members of the White House staff by phone this morning, and will participate in his planned meetings at the White House this morning via phone and Zoom from the residence.”
President Biden will return to in-person work after he tests negative.
This is a developing story. Please check back for updates. A version of this article first appeared on WebMD.com .
Biden, 79, is experiencing “very mild” symptoms, White House Press Secretary Karine Jean-Pierre said in a statement. The president is fully vaccinated and has been boosted twice and has started taking the antiviral Paxlovid since testing positive, Ms. Jean-Pierre said.
President Biden plans to isolate at the White House and “will continue to carry out all of his duties fully during that time,” the statement said.
“He has been in contact with members of the White House staff by phone this morning, and will participate in his planned meetings at the White House this morning via phone and Zoom from the residence.”
President Biden will return to in-person work after he tests negative.
This is a developing story. Please check back for updates. A version of this article first appeared on WebMD.com .
Biden, 79, is experiencing “very mild” symptoms, White House Press Secretary Karine Jean-Pierre said in a statement. The president is fully vaccinated and has been boosted twice and has started taking the antiviral Paxlovid since testing positive, Ms. Jean-Pierre said.
President Biden plans to isolate at the White House and “will continue to carry out all of his duties fully during that time,” the statement said.
“He has been in contact with members of the White House staff by phone this morning, and will participate in his planned meetings at the White House this morning via phone and Zoom from the residence.”
President Biden will return to in-person work after he tests negative.
This is a developing story. Please check back for updates. A version of this article first appeared on WebMD.com .
COVID-19 infection late in pregnancy linked to sevenfold risk of preterm birth
Pregnant women who get infected with SARS-CoV-2 in their third trimester are almost three times as likely to have a preterm birth, while infection after 34 weeks’ gestation raises this risk sevenfold, based on the largest matched population-based cohort study published to date.
These findings support previous studies, underscoring the need for pregnant women and their families to take preventive measures against infection, lead author Noga Fallach, MA, of the Kahn-Sagol-Maccabi Research and Innovation Center, Tel Aviv, and colleagues reported.
Past research has suggested that COVID-19 may cause low birth weights and preterm birth in pregnant women, but those studies didn’t report outcomes for each trimester, the investigators wrote in PLoS ONE, noting that “timing of viral infection during fetal development may affect birth and other health outcomes.”
To address this knowledge gap, the investigators looked back at data from 2,703 pregnant women in Israel who tested positive for SARS-CoV-2 from Feb. 21, 2020, to July 2, 2021. Pregnancy outcomes in these women were compared with outcomes in an equal number of uninfected pregnant women. Vaccination status was not reported.
Comparing the two groups showed that catching COVID-19 in the third trimester was linked with nearly triple the risk of preterm birth (odds ratio, 2.76; 95% confidence interval, 1.63-4.67), and more than quadruple the risk if COVID-19 symptoms were present (OR, 4.28; 95% CI, 1.94-9.41). Women who tested positive for SARS-CoV-2 after 34 weeks’ gestation were seven times more likely than uninfected women to deliver early (OR, 7.10; 95% CI, 2.44-20.61).
Pregnant women who caught COVID-19 in the first two trimesters were not significantly more likely to have a preterm birth. Infection was not associated with abnormally low birth rates, or pregnancy loss, in any trimester.
Tal Patalon, MD, coauthor and head of the Kahn-Sagol-Maccabi Research and Innovation Center, focused on these more optimistic findings in an interview.
“The results are encouraging, and reassuring that COVID-19 infection during pregnancy is not associated with any type of pregnancy loss,” Dr. Patalon said.
She also pointed out that the women in the study were infected with SARS-CoV-2 variants that are no longer common.
“It should be remembered that the research group tested the COVID-19 pre-Delta variants, and does not refer to the dominant variant today, which is Omicron,” Dr. Patalon said.
Still, the investigators concluded that the “results underline the importance of preventive measures taken against SARS-CoV-2 infection among pregnant women and their families.”
Sonja A. Rasmussen, MD, of the University of Florida, Gainesville, said that the issue with out-of-date variants in published research has been one of the “real challenges” in studying the ever-evolving COVID-19 pandemic; however, it’s not a good enough reason to dismiss this study.
“I think at this point, we need to assume that it applies to Omicron too,” Dr. Rasmussen said, noting that other respiratory viruses, like influenza, have also been shown to increase the risk of preterm birth when contracted in late pregnancy.
While the present findings highlight the risk of infection in the third trimester, Dr. Rasmussen advised women in all stages of pregnancy to protect themselves against COVID-19, based on the knowledge that illness in a mother can affect normal growth and development in a fetus, even if it doesn’t lead to preterm birth.
“A mom getting sick during pregnancy is not good for the baby,” Dr. Rasmussen said. “The baby’s really dependent on the mom. So you want that baby to have good nutrition throughout the pregnancy. It’s just as important earlier on as later. And you want that baby to get good oxygenation no matter what time [in the pregnancy]. I know that people want a little bit of a break [from preventive measures]. But I would emphasize that if you’re pregnant, we do all sorts of things during pregnancy to make sure that our babies are safe and healthy, and I would continue that for the whole pregnancy.”
Specifically, Dr. Rasmussen advised social distancing, use of an N95 mask, and vaccination. Getting vaccinated during pregnancy helps newborns fight off infection until 6 months of age, she added, when they become eligible for vaccination themselves. This added benefit was recently reported in a study published in the New England Journal of Medicine , for which Dr. Rasmussen cowrote an editorial .
“Vaccines have been approved for 6 months and older,” Dr. Rasmussen said. “But what do you do in those first 6 months of life? That’s a high-risk time for kids.”
Despite these risks, convincing pregnant women to get vaccinated remains a key challenge for health care providers, according to Dr. Rasmussen, even with an abundance of safety data. “Early on [in the pandemic], we said we didn’t know a lot about risks. We knew that other vaccines were safe during pregnancy, but we didn’t have a lot of information about a COVID-19 vaccine. But now we have a lot of data on safety during pregnancy, and these vaccines appear to be completely safe, based on the information we have. There have been many, many pregnant women vaccinated in the United States and in other countries.”
For reluctant expecting mothers, Dr. Rasmussen offered some words of advice: “I know that you worry about anything you do when you’re pregnant. But this is something that you can do to help your baby – now, to make a preterm birth less likely, and later, after the baby is born.
“The most important thing is for the pregnant person to hear this [vaccine recommendation] from their doctor,” she added. “If they’re going to listen to anybody, they’re going to listen to their physician. That’s what the data have shown for a long time.”
The investigators and Dr. Rasmussen disclosed no conflicts of interest.
Pregnant women who get infected with SARS-CoV-2 in their third trimester are almost three times as likely to have a preterm birth, while infection after 34 weeks’ gestation raises this risk sevenfold, based on the largest matched population-based cohort study published to date.
These findings support previous studies, underscoring the need for pregnant women and their families to take preventive measures against infection, lead author Noga Fallach, MA, of the Kahn-Sagol-Maccabi Research and Innovation Center, Tel Aviv, and colleagues reported.
Past research has suggested that COVID-19 may cause low birth weights and preterm birth in pregnant women, but those studies didn’t report outcomes for each trimester, the investigators wrote in PLoS ONE, noting that “timing of viral infection during fetal development may affect birth and other health outcomes.”
To address this knowledge gap, the investigators looked back at data from 2,703 pregnant women in Israel who tested positive for SARS-CoV-2 from Feb. 21, 2020, to July 2, 2021. Pregnancy outcomes in these women were compared with outcomes in an equal number of uninfected pregnant women. Vaccination status was not reported.
Comparing the two groups showed that catching COVID-19 in the third trimester was linked with nearly triple the risk of preterm birth (odds ratio, 2.76; 95% confidence interval, 1.63-4.67), and more than quadruple the risk if COVID-19 symptoms were present (OR, 4.28; 95% CI, 1.94-9.41). Women who tested positive for SARS-CoV-2 after 34 weeks’ gestation were seven times more likely than uninfected women to deliver early (OR, 7.10; 95% CI, 2.44-20.61).
Pregnant women who caught COVID-19 in the first two trimesters were not significantly more likely to have a preterm birth. Infection was not associated with abnormally low birth rates, or pregnancy loss, in any trimester.
Tal Patalon, MD, coauthor and head of the Kahn-Sagol-Maccabi Research and Innovation Center, focused on these more optimistic findings in an interview.
“The results are encouraging, and reassuring that COVID-19 infection during pregnancy is not associated with any type of pregnancy loss,” Dr. Patalon said.
She also pointed out that the women in the study were infected with SARS-CoV-2 variants that are no longer common.
“It should be remembered that the research group tested the COVID-19 pre-Delta variants, and does not refer to the dominant variant today, which is Omicron,” Dr. Patalon said.
Still, the investigators concluded that the “results underline the importance of preventive measures taken against SARS-CoV-2 infection among pregnant women and their families.”
Sonja A. Rasmussen, MD, of the University of Florida, Gainesville, said that the issue with out-of-date variants in published research has been one of the “real challenges” in studying the ever-evolving COVID-19 pandemic; however, it’s not a good enough reason to dismiss this study.
“I think at this point, we need to assume that it applies to Omicron too,” Dr. Rasmussen said, noting that other respiratory viruses, like influenza, have also been shown to increase the risk of preterm birth when contracted in late pregnancy.
While the present findings highlight the risk of infection in the third trimester, Dr. Rasmussen advised women in all stages of pregnancy to protect themselves against COVID-19, based on the knowledge that illness in a mother can affect normal growth and development in a fetus, even if it doesn’t lead to preterm birth.
“A mom getting sick during pregnancy is not good for the baby,” Dr. Rasmussen said. “The baby’s really dependent on the mom. So you want that baby to have good nutrition throughout the pregnancy. It’s just as important earlier on as later. And you want that baby to get good oxygenation no matter what time [in the pregnancy]. I know that people want a little bit of a break [from preventive measures]. But I would emphasize that if you’re pregnant, we do all sorts of things during pregnancy to make sure that our babies are safe and healthy, and I would continue that for the whole pregnancy.”
Specifically, Dr. Rasmussen advised social distancing, use of an N95 mask, and vaccination. Getting vaccinated during pregnancy helps newborns fight off infection until 6 months of age, she added, when they become eligible for vaccination themselves. This added benefit was recently reported in a study published in the New England Journal of Medicine , for which Dr. Rasmussen cowrote an editorial .
“Vaccines have been approved for 6 months and older,” Dr. Rasmussen said. “But what do you do in those first 6 months of life? That’s a high-risk time for kids.”
Despite these risks, convincing pregnant women to get vaccinated remains a key challenge for health care providers, according to Dr. Rasmussen, even with an abundance of safety data. “Early on [in the pandemic], we said we didn’t know a lot about risks. We knew that other vaccines were safe during pregnancy, but we didn’t have a lot of information about a COVID-19 vaccine. But now we have a lot of data on safety during pregnancy, and these vaccines appear to be completely safe, based on the information we have. There have been many, many pregnant women vaccinated in the United States and in other countries.”
For reluctant expecting mothers, Dr. Rasmussen offered some words of advice: “I know that you worry about anything you do when you’re pregnant. But this is something that you can do to help your baby – now, to make a preterm birth less likely, and later, after the baby is born.
“The most important thing is for the pregnant person to hear this [vaccine recommendation] from their doctor,” she added. “If they’re going to listen to anybody, they’re going to listen to their physician. That’s what the data have shown for a long time.”
The investigators and Dr. Rasmussen disclosed no conflicts of interest.
Pregnant women who get infected with SARS-CoV-2 in their third trimester are almost three times as likely to have a preterm birth, while infection after 34 weeks’ gestation raises this risk sevenfold, based on the largest matched population-based cohort study published to date.
These findings support previous studies, underscoring the need for pregnant women and their families to take preventive measures against infection, lead author Noga Fallach, MA, of the Kahn-Sagol-Maccabi Research and Innovation Center, Tel Aviv, and colleagues reported.
Past research has suggested that COVID-19 may cause low birth weights and preterm birth in pregnant women, but those studies didn’t report outcomes for each trimester, the investigators wrote in PLoS ONE, noting that “timing of viral infection during fetal development may affect birth and other health outcomes.”
To address this knowledge gap, the investigators looked back at data from 2,703 pregnant women in Israel who tested positive for SARS-CoV-2 from Feb. 21, 2020, to July 2, 2021. Pregnancy outcomes in these women were compared with outcomes in an equal number of uninfected pregnant women. Vaccination status was not reported.
Comparing the two groups showed that catching COVID-19 in the third trimester was linked with nearly triple the risk of preterm birth (odds ratio, 2.76; 95% confidence interval, 1.63-4.67), and more than quadruple the risk if COVID-19 symptoms were present (OR, 4.28; 95% CI, 1.94-9.41). Women who tested positive for SARS-CoV-2 after 34 weeks’ gestation were seven times more likely than uninfected women to deliver early (OR, 7.10; 95% CI, 2.44-20.61).
Pregnant women who caught COVID-19 in the first two trimesters were not significantly more likely to have a preterm birth. Infection was not associated with abnormally low birth rates, or pregnancy loss, in any trimester.
Tal Patalon, MD, coauthor and head of the Kahn-Sagol-Maccabi Research and Innovation Center, focused on these more optimistic findings in an interview.
“The results are encouraging, and reassuring that COVID-19 infection during pregnancy is not associated with any type of pregnancy loss,” Dr. Patalon said.
She also pointed out that the women in the study were infected with SARS-CoV-2 variants that are no longer common.
“It should be remembered that the research group tested the COVID-19 pre-Delta variants, and does not refer to the dominant variant today, which is Omicron,” Dr. Patalon said.
Still, the investigators concluded that the “results underline the importance of preventive measures taken against SARS-CoV-2 infection among pregnant women and their families.”
Sonja A. Rasmussen, MD, of the University of Florida, Gainesville, said that the issue with out-of-date variants in published research has been one of the “real challenges” in studying the ever-evolving COVID-19 pandemic; however, it’s not a good enough reason to dismiss this study.
“I think at this point, we need to assume that it applies to Omicron too,” Dr. Rasmussen said, noting that other respiratory viruses, like influenza, have also been shown to increase the risk of preterm birth when contracted in late pregnancy.
While the present findings highlight the risk of infection in the third trimester, Dr. Rasmussen advised women in all stages of pregnancy to protect themselves against COVID-19, based on the knowledge that illness in a mother can affect normal growth and development in a fetus, even if it doesn’t lead to preterm birth.
“A mom getting sick during pregnancy is not good for the baby,” Dr. Rasmussen said. “The baby’s really dependent on the mom. So you want that baby to have good nutrition throughout the pregnancy. It’s just as important earlier on as later. And you want that baby to get good oxygenation no matter what time [in the pregnancy]. I know that people want a little bit of a break [from preventive measures]. But I would emphasize that if you’re pregnant, we do all sorts of things during pregnancy to make sure that our babies are safe and healthy, and I would continue that for the whole pregnancy.”
Specifically, Dr. Rasmussen advised social distancing, use of an N95 mask, and vaccination. Getting vaccinated during pregnancy helps newborns fight off infection until 6 months of age, she added, when they become eligible for vaccination themselves. This added benefit was recently reported in a study published in the New England Journal of Medicine , for which Dr. Rasmussen cowrote an editorial .
“Vaccines have been approved for 6 months and older,” Dr. Rasmussen said. “But what do you do in those first 6 months of life? That’s a high-risk time for kids.”
Despite these risks, convincing pregnant women to get vaccinated remains a key challenge for health care providers, according to Dr. Rasmussen, even with an abundance of safety data. “Early on [in the pandemic], we said we didn’t know a lot about risks. We knew that other vaccines were safe during pregnancy, but we didn’t have a lot of information about a COVID-19 vaccine. But now we have a lot of data on safety during pregnancy, and these vaccines appear to be completely safe, based on the information we have. There have been many, many pregnant women vaccinated in the United States and in other countries.”
For reluctant expecting mothers, Dr. Rasmussen offered some words of advice: “I know that you worry about anything you do when you’re pregnant. But this is something that you can do to help your baby – now, to make a preterm birth less likely, and later, after the baby is born.
“The most important thing is for the pregnant person to hear this [vaccine recommendation] from their doctor,” she added. “If they’re going to listen to anybody, they’re going to listen to their physician. That’s what the data have shown for a long time.”
The investigators and Dr. Rasmussen disclosed no conflicts of interest.
FROM PLOS ONE
Pre-endoscopy COVID-19 testing may not be needed
Pre-endoscopy viral testing may not be necessary to prevent coronavirus transmission from patients to endoscopy staff members, according to a new study published in Gut.
Instead, using personal protective equipment (PPE) and ensuring up-to-date COVID-19 vaccination among the medical team was found to be enough to substantially reduce the risk of spreading SARS-CoV-2, wrote Alexander Hann, Dr.med., gastroenterologist at University Hospital W
“We suggest that pre-selection of patients using respective questionnaires, vaccination, and particularly PPE appears to be sufficient for the prevention of SARS-CoV-2 transmission in GI endoscopy,” they wrote.
Dr. Hann and colleagues analyzed 15,750 endoscopies performed by 29 staff members during the period between May 2020 and December 2021. The researchers looked at three test approaches: No testing (4,543 patients), rapid antigen testing (682 patients), and real-time PCR testing (10,465 patients). In addition, 60 endoscopies were performed in patients with known COVID-19. Overall, no staff members became infected with SARS-CoV-2 during the study period. In all three scenarios, staff used PPE, and the vaccination rate of the team was 97%.
University Hospital W
All patients were interviewed before admission for COVID-19 symptoms, close contact with infected people, and recent travel to high-risk countries. Moreover, some endoscopies were performed even if a patient had positive markers for COVID-19.
The clinical team wore recommended PPE, including a high-filter FFP2 mask, one pair of gloves, protective eyewear, and disposable gowns. For patients with known COVID-19, staff wore two pairs of gloves, a disposable hairnet, and a water-resistant disposable gown. In addition, endoscopies were performed in negative pressure intervention rooms.
Among the 29 staff members involved, 16 physicians and 13 assistants worked in the endoscopy unit for at least 2 days per week for at least 6 months. The hospital’s internal policy required medical staff to undergo PCR testing if a rapid antigen test was positive or symptoms developed. Staff were vaccinated with two doses of the Pfizer-BioNTech vaccine in January and February 2021. A single booster dose of the Pfizer or Moderna vaccine was administered in November and December 2021.
The clinical team was not tested routinely, so asymptomatic infections may have existed. Moreover, the relatively low COVID-19 incidence in the local area might have influenced the risk of transmission. “However, even at the end of 2021, when the incidence was increasing, we did not see any higher risk of transmission,” the researchers explained.
“An important limitation of our study relates to the new variant Omicron that was dominant in our local area after the analyzed time frame.” Additional studies may be needed to understand the risk of transmission with the latest Omicron variants, and given the additional costs and implications on routine activity, current testing guidelines may need to be reconsidered.
“Although our data were not part of a randomized prospective study, we were able to demonstrate on a fairly high number of patients that PPE measures in addition to a short interview for assessment of a patient’s individual risks appear to be highly effective to control transmission of SARS-CoV-2 during an endoscopy. ... Pre-procedural RT-PCR testing or RA testing did not show any additional benefit,” Dr. Hann and colleagues concluded.
The authors reported no conflicts of interest.
Pre-endoscopy viral testing may not be necessary to prevent coronavirus transmission from patients to endoscopy staff members, according to a new study published in Gut.
Instead, using personal protective equipment (PPE) and ensuring up-to-date COVID-19 vaccination among the medical team was found to be enough to substantially reduce the risk of spreading SARS-CoV-2, wrote Alexander Hann, Dr.med., gastroenterologist at University Hospital W
“We suggest that pre-selection of patients using respective questionnaires, vaccination, and particularly PPE appears to be sufficient for the prevention of SARS-CoV-2 transmission in GI endoscopy,” they wrote.
Dr. Hann and colleagues analyzed 15,750 endoscopies performed by 29 staff members during the period between May 2020 and December 2021. The researchers looked at three test approaches: No testing (4,543 patients), rapid antigen testing (682 patients), and real-time PCR testing (10,465 patients). In addition, 60 endoscopies were performed in patients with known COVID-19. Overall, no staff members became infected with SARS-CoV-2 during the study period. In all three scenarios, staff used PPE, and the vaccination rate of the team was 97%.
University Hospital W
All patients were interviewed before admission for COVID-19 symptoms, close contact with infected people, and recent travel to high-risk countries. Moreover, some endoscopies were performed even if a patient had positive markers for COVID-19.
The clinical team wore recommended PPE, including a high-filter FFP2 mask, one pair of gloves, protective eyewear, and disposable gowns. For patients with known COVID-19, staff wore two pairs of gloves, a disposable hairnet, and a water-resistant disposable gown. In addition, endoscopies were performed in negative pressure intervention rooms.
Among the 29 staff members involved, 16 physicians and 13 assistants worked in the endoscopy unit for at least 2 days per week for at least 6 months. The hospital’s internal policy required medical staff to undergo PCR testing if a rapid antigen test was positive or symptoms developed. Staff were vaccinated with two doses of the Pfizer-BioNTech vaccine in January and February 2021. A single booster dose of the Pfizer or Moderna vaccine was administered in November and December 2021.
The clinical team was not tested routinely, so asymptomatic infections may have existed. Moreover, the relatively low COVID-19 incidence in the local area might have influenced the risk of transmission. “However, even at the end of 2021, when the incidence was increasing, we did not see any higher risk of transmission,” the researchers explained.
“An important limitation of our study relates to the new variant Omicron that was dominant in our local area after the analyzed time frame.” Additional studies may be needed to understand the risk of transmission with the latest Omicron variants, and given the additional costs and implications on routine activity, current testing guidelines may need to be reconsidered.
“Although our data were not part of a randomized prospective study, we were able to demonstrate on a fairly high number of patients that PPE measures in addition to a short interview for assessment of a patient’s individual risks appear to be highly effective to control transmission of SARS-CoV-2 during an endoscopy. ... Pre-procedural RT-PCR testing or RA testing did not show any additional benefit,” Dr. Hann and colleagues concluded.
The authors reported no conflicts of interest.
Pre-endoscopy viral testing may not be necessary to prevent coronavirus transmission from patients to endoscopy staff members, according to a new study published in Gut.
Instead, using personal protective equipment (PPE) and ensuring up-to-date COVID-19 vaccination among the medical team was found to be enough to substantially reduce the risk of spreading SARS-CoV-2, wrote Alexander Hann, Dr.med., gastroenterologist at University Hospital W
“We suggest that pre-selection of patients using respective questionnaires, vaccination, and particularly PPE appears to be sufficient for the prevention of SARS-CoV-2 transmission in GI endoscopy,” they wrote.
Dr. Hann and colleagues analyzed 15,750 endoscopies performed by 29 staff members during the period between May 2020 and December 2021. The researchers looked at three test approaches: No testing (4,543 patients), rapid antigen testing (682 patients), and real-time PCR testing (10,465 patients). In addition, 60 endoscopies were performed in patients with known COVID-19. Overall, no staff members became infected with SARS-CoV-2 during the study period. In all three scenarios, staff used PPE, and the vaccination rate of the team was 97%.
University Hospital W
All patients were interviewed before admission for COVID-19 symptoms, close contact with infected people, and recent travel to high-risk countries. Moreover, some endoscopies were performed even if a patient had positive markers for COVID-19.
The clinical team wore recommended PPE, including a high-filter FFP2 mask, one pair of gloves, protective eyewear, and disposable gowns. For patients with known COVID-19, staff wore two pairs of gloves, a disposable hairnet, and a water-resistant disposable gown. In addition, endoscopies were performed in negative pressure intervention rooms.
Among the 29 staff members involved, 16 physicians and 13 assistants worked in the endoscopy unit for at least 2 days per week for at least 6 months. The hospital’s internal policy required medical staff to undergo PCR testing if a rapid antigen test was positive or symptoms developed. Staff were vaccinated with two doses of the Pfizer-BioNTech vaccine in January and February 2021. A single booster dose of the Pfizer or Moderna vaccine was administered in November and December 2021.
The clinical team was not tested routinely, so asymptomatic infections may have existed. Moreover, the relatively low COVID-19 incidence in the local area might have influenced the risk of transmission. “However, even at the end of 2021, when the incidence was increasing, we did not see any higher risk of transmission,” the researchers explained.
“An important limitation of our study relates to the new variant Omicron that was dominant in our local area after the analyzed time frame.” Additional studies may be needed to understand the risk of transmission with the latest Omicron variants, and given the additional costs and implications on routine activity, current testing guidelines may need to be reconsidered.
“Although our data were not part of a randomized prospective study, we were able to demonstrate on a fairly high number of patients that PPE measures in addition to a short interview for assessment of a patient’s individual risks appear to be highly effective to control transmission of SARS-CoV-2 during an endoscopy. ... Pre-procedural RT-PCR testing or RA testing did not show any additional benefit,” Dr. Hann and colleagues concluded.
The authors reported no conflicts of interest.
FROM GUT
Children and COVID: Does latest rise in new cases point toward stabilization?
New COVID-19 cases rose for the second time in 3 weeks, as the effort to vaccinate the youngest children continued to slow after just 3 full weeks.
Nationally, over 75,000 children under age 5 years received their first dose of COVID-19 vaccine during the week of July 7-13. That number is down from the previous week – 118,000 from June 30 to July 6 – which, in turn, was lower than the 206,000 doses administered through the first 10 days after approval, based on data from the Centers for Disease Control and Prevention. That all adds up to just under 400,000 vaccinated children, or 2% of the eligible population under age 5, as of July 13.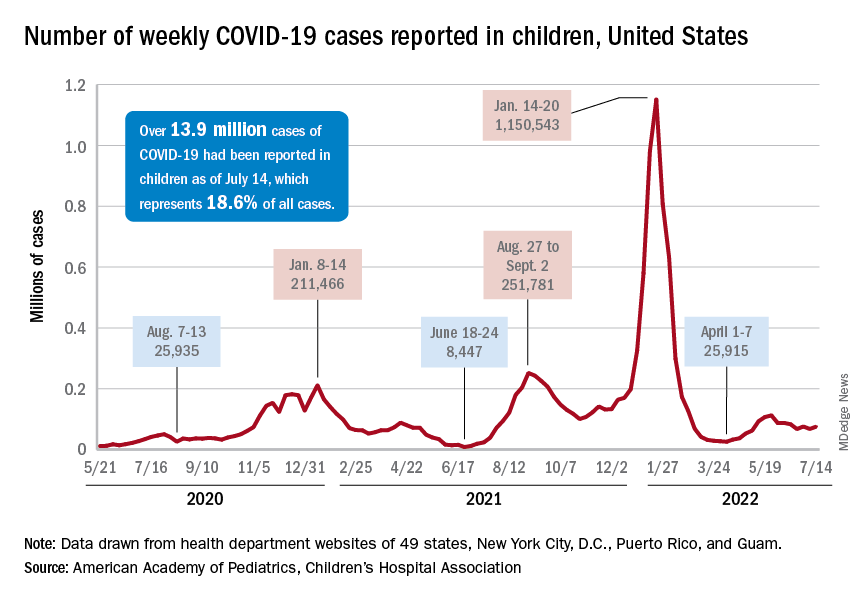
State-level data, meanwhile, show considerable variation, the American Academy of Pediatrics noted in its weekly analysis of the CDC vaccine data. Vermont has already vaccinated 10.0% of children under age 5 years, and Massachusetts is at 9.5%, while Mississippi (0.3%), Alabama (0.5%), and Louisiana (0.8%) are still below 1%, the AAP said.
New cases show signs of steadying
The national count was up by 11.1% for the week of July 8-14, rising to 75,000 new cases, compared with 68,000 the previous week, but the recent trend seems to be leaning toward steadiness. The overall number has been between 67,000 and 76,000 over the past 4 weeks, alternating between rising and falling in that time span, according to data gathered by the AAP and the Children’s Hospital Association from state and territorial health departments.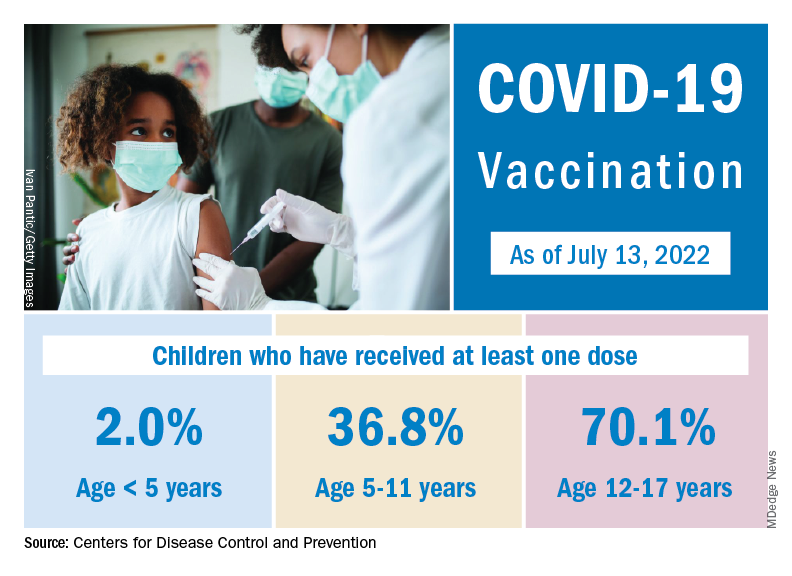
the two groups said, also noting that several states have stopped updating their online dashboards over the past year, making the current total artificially low in comparison.
Taken with that grain of salt, the cumulative number of child cases since the start of the pandemic is just over 13.9 million, which represents 18.6% of all cases in the United States. That proportion has been declining in recent weeks and was as high as 19.0% as late as mid-May. “While COVID-19 cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases,” the AAP and CHA said.
New COVID-19 cases rose for the second time in 3 weeks, as the effort to vaccinate the youngest children continued to slow after just 3 full weeks.
Nationally, over 75,000 children under age 5 years received their first dose of COVID-19 vaccine during the week of July 7-13. That number is down from the previous week – 118,000 from June 30 to July 6 – which, in turn, was lower than the 206,000 doses administered through the first 10 days after approval, based on data from the Centers for Disease Control and Prevention. That all adds up to just under 400,000 vaccinated children, or 2% of the eligible population under age 5, as of July 13.
State-level data, meanwhile, show considerable variation, the American Academy of Pediatrics noted in its weekly analysis of the CDC vaccine data. Vermont has already vaccinated 10.0% of children under age 5 years, and Massachusetts is at 9.5%, while Mississippi (0.3%), Alabama (0.5%), and Louisiana (0.8%) are still below 1%, the AAP said.
New cases show signs of steadying
The national count was up by 11.1% for the week of July 8-14, rising to 75,000 new cases, compared with 68,000 the previous week, but the recent trend seems to be leaning toward steadiness. The overall number has been between 67,000 and 76,000 over the past 4 weeks, alternating between rising and falling in that time span, according to data gathered by the AAP and the Children’s Hospital Association from state and territorial health departments.
the two groups said, also noting that several states have stopped updating their online dashboards over the past year, making the current total artificially low in comparison.
Taken with that grain of salt, the cumulative number of child cases since the start of the pandemic is just over 13.9 million, which represents 18.6% of all cases in the United States. That proportion has been declining in recent weeks and was as high as 19.0% as late as mid-May. “While COVID-19 cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases,” the AAP and CHA said.
New COVID-19 cases rose for the second time in 3 weeks, as the effort to vaccinate the youngest children continued to slow after just 3 full weeks.
Nationally, over 75,000 children under age 5 years received their first dose of COVID-19 vaccine during the week of July 7-13. That number is down from the previous week – 118,000 from June 30 to July 6 – which, in turn, was lower than the 206,000 doses administered through the first 10 days after approval, based on data from the Centers for Disease Control and Prevention. That all adds up to just under 400,000 vaccinated children, or 2% of the eligible population under age 5, as of July 13.
State-level data, meanwhile, show considerable variation, the American Academy of Pediatrics noted in its weekly analysis of the CDC vaccine data. Vermont has already vaccinated 10.0% of children under age 5 years, and Massachusetts is at 9.5%, while Mississippi (0.3%), Alabama (0.5%), and Louisiana (0.8%) are still below 1%, the AAP said.
New cases show signs of steadying
The national count was up by 11.1% for the week of July 8-14, rising to 75,000 new cases, compared with 68,000 the previous week, but the recent trend seems to be leaning toward steadiness. The overall number has been between 67,000 and 76,000 over the past 4 weeks, alternating between rising and falling in that time span, according to data gathered by the AAP and the Children’s Hospital Association from state and territorial health departments.
the two groups said, also noting that several states have stopped updating their online dashboards over the past year, making the current total artificially low in comparison.
Taken with that grain of salt, the cumulative number of child cases since the start of the pandemic is just over 13.9 million, which represents 18.6% of all cases in the United States. That proportion has been declining in recent weeks and was as high as 19.0% as late as mid-May. “While COVID-19 cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases,” the AAP and CHA said.