User login
RV dysfunction slams survival in acute COVID, flu, pneumonia
The study covered in this summary was published in medRxiv.org as a preprint and has not yet been peer reviewed.
Key takeaways
- Right ventricular (RV) dilation or dysfunction in patients hospitalized with acute COVID-19 is associated with an elevated risk for in-hospital death.
- The impact of RV dilation or dysfunction on in-hospital mortality is similar for patients with acute COVID-19 and those with influenza, pneumonia, or acute respiratory distress syndrome (ARDS), but COVID-19 patients have greater absolute in-hospital mortality.
- RV dilatation or dysfunction in patients with acute COVID-19 is associated with a diagnosis of venous thromboembolism and subsequent intubation and mechanical ventilation.
Why this matters
- Right ventricular dysfunction increases mortality risk in acute COVID-19, and this study shows that
- The findings suggest that abnormal RV findings should be considered a mortality risk marker in patients with acute respiratory illness, especially COVID-19.
Study design
- The retrospective study involved 225 consecutive patients admitted for acute COVID-19 from March 2020 to February 2021 at four major hospitals in the same metropolitan region and a control group of 6,150 adults admitted to the hospital for influenza, pneumonia, or ARDS; mean age in the study cohort was 63 years.
- All participants underwent echocardiography during their hospitalization, including evaluation of any RV dilation or dysfunction.
- Associations between RV measurements and in-hospital mortality, the primary outcome, were adjusted for potential confounders.
Key results
- Patients in the COVID-19 group were more likely than were those in the control group to be male (66% vs. 54%; P < .001), to identify as Hispanic (38% vs. 15%; P < .001), and to have a higher mean body mass index (29.4 vs. 27.9 kg/m2; P = .008).
- Compared with the control group, patients in the COVID-19 group more often required admission to the intensive care unit (75% vs. 54%; P < .001), mechanical ventilation (P < .001), and initiation of renal replacement therapy (P = .002), and more often were diagnosed with deep-vein thrombosis or pulmonary embolism (25% vs. 14%; P < .001). The median length of hospital stay was 20 days in the COVID-19 group, compared with 10 days in the control group (P < .001).
- In-hospital mortality was 21.3% in the COVID-19 group and 11.8% in the control group (P = .001). Those hospitalized with COVID-19 had an adjusted relative risk (RR) of 1.54 (95% confidence interval [CI], 1.06-2.24; P = .02) for in-hospital mortality, compared with those hospitalized for other respiratory illnesses.
- Mild RV dilation was associated with an adjusted RR of 1.4 (95% CI, 1.17-1.69; P = .0003) for in-hospital death, and moderate to severe RV dilation was associated with an adjusted RR of 2.0 (95% CI, 1.62-2.47; P < .0001).
- The corresponding adjusted risks for mild RV dysfunction and greater-than-mild RV dysfunction were, respectively, 1.39 (95% CI, 1.10-1.77; P = .007) and 1.68 (95% CI, 1.17-2.42; P = .005).
- The RR for in-hospital mortality associated with RV dilation and dysfunction was similar in those with COVID-19 and those with other respiratory illness, but the former had a higher baseline risk that yielded a greater absolute risk in the COVID-19 group.
Limitations
- The study was based primarily on a retrospective review of electronic health records, which poses a risk for misclassification.
- Echocardiography was performed without blinding operators to patient clinical status, and echocardiograms were interpreted in a single university hospital system, so were not externally validated.
- Because echocardiograms obtained during hospitalization could not be compared with previous echocardiograms, it could not be determined whether any of the patients had preexisting RV dilation or dysfunction.
- Strain imaging was not feasible in many cases.
Disclosures
- The study received no commercial funding.
- The authors disclosed no financial relationships.
This is a summary of a preprint research study, Association of Right Ventricular Dilation and Dysfunction on Echocardiogram With In-Hospital Mortality Among Patients Hospitalized with COVID-19 Compared With Other Acute Respiratory Illness, written by researchers at the University of California, San Francisco, department of medicine, and Zuckerberg San Francisco General Hospital, division of cardiology. A version of this article first appeared on Medscape.com.
The study covered in this summary was published in medRxiv.org as a preprint and has not yet been peer reviewed.
Key takeaways
- Right ventricular (RV) dilation or dysfunction in patients hospitalized with acute COVID-19 is associated with an elevated risk for in-hospital death.
- The impact of RV dilation or dysfunction on in-hospital mortality is similar for patients with acute COVID-19 and those with influenza, pneumonia, or acute respiratory distress syndrome (ARDS), but COVID-19 patients have greater absolute in-hospital mortality.
- RV dilatation or dysfunction in patients with acute COVID-19 is associated with a diagnosis of venous thromboembolism and subsequent intubation and mechanical ventilation.
Why this matters
- Right ventricular dysfunction increases mortality risk in acute COVID-19, and this study shows that
- The findings suggest that abnormal RV findings should be considered a mortality risk marker in patients with acute respiratory illness, especially COVID-19.
Study design
- The retrospective study involved 225 consecutive patients admitted for acute COVID-19 from March 2020 to February 2021 at four major hospitals in the same metropolitan region and a control group of 6,150 adults admitted to the hospital for influenza, pneumonia, or ARDS; mean age in the study cohort was 63 years.
- All participants underwent echocardiography during their hospitalization, including evaluation of any RV dilation or dysfunction.
- Associations between RV measurements and in-hospital mortality, the primary outcome, were adjusted for potential confounders.
Key results
- Patients in the COVID-19 group were more likely than were those in the control group to be male (66% vs. 54%; P < .001), to identify as Hispanic (38% vs. 15%; P < .001), and to have a higher mean body mass index (29.4 vs. 27.9 kg/m2; P = .008).
- Compared with the control group, patients in the COVID-19 group more often required admission to the intensive care unit (75% vs. 54%; P < .001), mechanical ventilation (P < .001), and initiation of renal replacement therapy (P = .002), and more often were diagnosed with deep-vein thrombosis or pulmonary embolism (25% vs. 14%; P < .001). The median length of hospital stay was 20 days in the COVID-19 group, compared with 10 days in the control group (P < .001).
- In-hospital mortality was 21.3% in the COVID-19 group and 11.8% in the control group (P = .001). Those hospitalized with COVID-19 had an adjusted relative risk (RR) of 1.54 (95% confidence interval [CI], 1.06-2.24; P = .02) for in-hospital mortality, compared with those hospitalized for other respiratory illnesses.
- Mild RV dilation was associated with an adjusted RR of 1.4 (95% CI, 1.17-1.69; P = .0003) for in-hospital death, and moderate to severe RV dilation was associated with an adjusted RR of 2.0 (95% CI, 1.62-2.47; P < .0001).
- The corresponding adjusted risks for mild RV dysfunction and greater-than-mild RV dysfunction were, respectively, 1.39 (95% CI, 1.10-1.77; P = .007) and 1.68 (95% CI, 1.17-2.42; P = .005).
- The RR for in-hospital mortality associated with RV dilation and dysfunction was similar in those with COVID-19 and those with other respiratory illness, but the former had a higher baseline risk that yielded a greater absolute risk in the COVID-19 group.
Limitations
- The study was based primarily on a retrospective review of electronic health records, which poses a risk for misclassification.
- Echocardiography was performed without blinding operators to patient clinical status, and echocardiograms were interpreted in a single university hospital system, so were not externally validated.
- Because echocardiograms obtained during hospitalization could not be compared with previous echocardiograms, it could not be determined whether any of the patients had preexisting RV dilation or dysfunction.
- Strain imaging was not feasible in many cases.
Disclosures
- The study received no commercial funding.
- The authors disclosed no financial relationships.
This is a summary of a preprint research study, Association of Right Ventricular Dilation and Dysfunction on Echocardiogram With In-Hospital Mortality Among Patients Hospitalized with COVID-19 Compared With Other Acute Respiratory Illness, written by researchers at the University of California, San Francisco, department of medicine, and Zuckerberg San Francisco General Hospital, division of cardiology. A version of this article first appeared on Medscape.com.
The study covered in this summary was published in medRxiv.org as a preprint and has not yet been peer reviewed.
Key takeaways
- Right ventricular (RV) dilation or dysfunction in patients hospitalized with acute COVID-19 is associated with an elevated risk for in-hospital death.
- The impact of RV dilation or dysfunction on in-hospital mortality is similar for patients with acute COVID-19 and those with influenza, pneumonia, or acute respiratory distress syndrome (ARDS), but COVID-19 patients have greater absolute in-hospital mortality.
- RV dilatation or dysfunction in patients with acute COVID-19 is associated with a diagnosis of venous thromboembolism and subsequent intubation and mechanical ventilation.
Why this matters
- Right ventricular dysfunction increases mortality risk in acute COVID-19, and this study shows that
- The findings suggest that abnormal RV findings should be considered a mortality risk marker in patients with acute respiratory illness, especially COVID-19.
Study design
- The retrospective study involved 225 consecutive patients admitted for acute COVID-19 from March 2020 to February 2021 at four major hospitals in the same metropolitan region and a control group of 6,150 adults admitted to the hospital for influenza, pneumonia, or ARDS; mean age in the study cohort was 63 years.
- All participants underwent echocardiography during their hospitalization, including evaluation of any RV dilation or dysfunction.
- Associations between RV measurements and in-hospital mortality, the primary outcome, were adjusted for potential confounders.
Key results
- Patients in the COVID-19 group were more likely than were those in the control group to be male (66% vs. 54%; P < .001), to identify as Hispanic (38% vs. 15%; P < .001), and to have a higher mean body mass index (29.4 vs. 27.9 kg/m2; P = .008).
- Compared with the control group, patients in the COVID-19 group more often required admission to the intensive care unit (75% vs. 54%; P < .001), mechanical ventilation (P < .001), and initiation of renal replacement therapy (P = .002), and more often were diagnosed with deep-vein thrombosis or pulmonary embolism (25% vs. 14%; P < .001). The median length of hospital stay was 20 days in the COVID-19 group, compared with 10 days in the control group (P < .001).
- In-hospital mortality was 21.3% in the COVID-19 group and 11.8% in the control group (P = .001). Those hospitalized with COVID-19 had an adjusted relative risk (RR) of 1.54 (95% confidence interval [CI], 1.06-2.24; P = .02) for in-hospital mortality, compared with those hospitalized for other respiratory illnesses.
- Mild RV dilation was associated with an adjusted RR of 1.4 (95% CI, 1.17-1.69; P = .0003) for in-hospital death, and moderate to severe RV dilation was associated with an adjusted RR of 2.0 (95% CI, 1.62-2.47; P < .0001).
- The corresponding adjusted risks for mild RV dysfunction and greater-than-mild RV dysfunction were, respectively, 1.39 (95% CI, 1.10-1.77; P = .007) and 1.68 (95% CI, 1.17-2.42; P = .005).
- The RR for in-hospital mortality associated with RV dilation and dysfunction was similar in those with COVID-19 and those with other respiratory illness, but the former had a higher baseline risk that yielded a greater absolute risk in the COVID-19 group.
Limitations
- The study was based primarily on a retrospective review of electronic health records, which poses a risk for misclassification.
- Echocardiography was performed without blinding operators to patient clinical status, and echocardiograms were interpreted in a single university hospital system, so were not externally validated.
- Because echocardiograms obtained during hospitalization could not be compared with previous echocardiograms, it could not be determined whether any of the patients had preexisting RV dilation or dysfunction.
- Strain imaging was not feasible in many cases.
Disclosures
- The study received no commercial funding.
- The authors disclosed no financial relationships.
This is a summary of a preprint research study, Association of Right Ventricular Dilation and Dysfunction on Echocardiogram With In-Hospital Mortality Among Patients Hospitalized with COVID-19 Compared With Other Acute Respiratory Illness, written by researchers at the University of California, San Francisco, department of medicine, and Zuckerberg San Francisco General Hospital, division of cardiology. A version of this article first appeared on Medscape.com.
Methotrexate’s impact on COVID-19 vaccination: New insights made
Patients who take methotrexate for a variety of immune-mediated inflammatory diseases and pause taking the drug following receipt of a COVID-19 vaccine dose did not have a higher risk of disease flare and had higher antireceptor binding domain (anti-RBD) antibody titers and increased immunogenicity when compared with continuing the drug, three recent studies suggest.
In one study, British researchers examined the effects of a 2-week break in methotrexate therapy on anti-RBD titers following receipt of a third COVID-19 vaccine dose. In their paper published in The Lancet: Respiratory Medicine, they reported results from a randomized, open-label, superiority trial that suggested pausing the drug improved immunogenicity, compared with no break.
In two trials presented at the European Alliance of Associations for Rheumatology (EULAR) 2022 Congress, a team from India set out to determine whether holding methotrexate after receiving both doses of a COVID-19 vaccine, or holding it only after the second dose, was safe and effective. They found that pausing methotrexate only following the second dose contributed to a lower flare risk, and that patients had higher anti-RBD titers when holding methotrexate for 2 weeks following each dose.
Pausing methotrexate after booster
The 2-week methotrexate break and booster vaccine dose data in the Vaccine Response On Off Methotrexate (VROOM) trial showed that after a month, the geometric mean antispike 1 (S1)-RBD antibody titer was 10,798 U/mL (95% confidence interval [CI], 8,970-12,997) in the group that continued methotrexate and 22,750 U/mL (95% CI, 19,314-26,796) in the group that suspended methotrexate; the geometric mean ratio was 2.19 (P < .0001; mixed-effects model), reported Abhishek Abhishek, MD, PhD, professor of rheumatology at the University of Nottingham in Nottingham, England, and colleagues.
Prior research showed that stopping methotrexate therapy for 2 weeks following the seasonal influenza vaccine contributed to better vaccine immunity among patients with rheumatoid arthritis, but there was no impact of stopping the drug for up to 4 weeks before vaccination on vaccine-related immunity, the researchers noted.
It is crucial in maximizing long-lasting vaccine protection in people who are possibly susceptible through immune suppression at this point in the COVID-19 vaccination regimen, the study team noted.
“Evidence from this study will be useful for policymakers, national immunization advisory committees, and specialist societies formulating recommendations on the use of methotrexate around the time of COVID-19 vaccination. This evidence will help patients and clinicians make informed choices about the risks and benefits of interrupting methotrexate treatment around the time of COVID-19 vaccination, with implications for the potential to extend such approaches to other therapeutics,” they wrote.
In American College of Rheumatology (ACR) guidance for COVID-19 vaccination, the organization advised against using standard synthetic disease-modifying antirheumatic medicines such as methotrexate “for 1-2 weeks (as disease activity allows) after each COVID-19 vaccine dose,” given the at-risk population and public health concerns, Jeffrey A. Sparks, MD, MMSc, assistant professor of medicine and associate physician at Brigham and Women’s Hospital and Harvard Medical School, Boston, and Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School, noted in an accompanying editorial in The Lancet: Respiratory Medicine.
However, when the ACR developed this statement, there was only one trial involving patients with rheumatoid arthritis who paused methotrexate following seasonal influenza vaccination, the editorialists said.
“Although this finding adds to the evidence base to support interruption of methotrexate after vaccination, a shared decision process is needed to weigh the possible benefit of optimizing protection from COVID-19 and the possible risk of underlying disease flare,” they added.
Dr. Abhishek and colleagues assessed 254 patients with immune-mediated inflammatory disease from dermatology and rheumatology clinics across 26 hospitals in the United Kingdom. Participants had been diagnosed with systemic lupus erythematosus, rheumatoid arthritis, atopic dermatitis, polymyalgia rheumatica, axial spondyloarthritis, and psoriasis without or with arthritis. They had also been taking up to 25 mg of methotrexate per week for 3 months or longer and had received two doses of either the Pfizer/BioNTech BNT162b2 vaccine or AstraZeneca/Oxford viral vector vaccine. The booster dose was most often the Pfizer BNT162b2 vaccine (82%). The patients’ mean age was 59 years, with females comprising 61% of the cohort. Participants were randomly assigned 1:1 to either group.
Investigators performing laboratory analysis were masked to cohort assignment, and clinical research staff, data analysts, participants, and researchers were unmasked.
The elevated antibody response of patients who suspended methotrexate was the same across different kinds of immune-mediated inflammatory disease, primary vaccination platform, SARS-CoV-2 infection history, and age.
Notably, no intervention-associated adverse events were reported, the study team noted.
The conclusions that could be drawn from the booster-dose study were limited by the trial’s modest cohort size, the small number of patients in exploratory subgroup analyses, a lack of information about differences in prescription drug behavior, and early termination’s effect on the researchers’ ability to identify differences between subgroups and in secondary outcomes, the authors noted.
Other limitations included a lack of generalizability to patients with active disease who couldn’t stop therapy and were not included in the investigation, and participants were not blinded to what group they were in, the researchers said.
Expert commentary
This current study is consistent with other studies over the last several months showing that methotrexate harms both humoral and cell-mediated COVID-19 responses, noted Kevin Winthrop, MD, MPH, professor of infectious disease and public health at Oregon Health & Science University, Portland, who was not involved in the study. “And so now the new wave of studies are like this one, where they are holding methotrexate experimentally and seeing if it makes a difference,” he said.
“The one shortcoming of this study – and so far, the studies to date – is that no one has looked at whether the experimental hold has resulted in a change in T-cell responses, which ... we are [now] recognizing [the importance of] more and more in long-term protection, particularly in severe disease. Theoretically, holding [methotrexate] might help enhance T-cell responses, but that hasn’t been shown experimentally.”
Dr. Winthrop pointed out that one might get the same benefit from holding methotrexate for 1 week instead of 2 and that there likely is a reduced risk of flare-up from underlying autoimmune disease.
It is still not certain that this benefit extends to other vaccines, Dr. Winthrop noted. “It is probably true for most vaccines that if you hold methotrexate for 1 or 2 weeks, you might see some short-term benefit in responsiveness, but you don’t know that there is any clinical meaningfulness of this. That’s going to take other long-term studies. You don’t know how long this benefit lasts.”
Pausing methotrexate during initial COVID vaccine doses
Patients with either rheumatoid arthritis or psoriatic arthritis had higher anti-RBD antibody titers when methotrexate was stopped after both doses of the AstraZeneca vaccine, or simply after the second dose, than when methotrexate was continued, according to results from two single-center, randomized controlled trials called MIVAC I and II, Anu Sreekanth, MD, of Sree Sudheendra Medical Mission in Kochi, Kerala, India, and colleagues reported at EULAR 2022.
Results from MIVAC I indicated that there was a higher flare rate when methotrexate was stopped after both vaccine doses, but there was no difference in flare rate in MIVAC II when methotrexate was stopped only after the second dose as opposed to stopping it after both doses.
In the MIVAC I trial, 158 unvaccinated patients were randomized 1:1 to a cohort in which methotrexate was held for 2 weeks after both doses and a cohort in which methotrexate was continued despite the vaccine. In MIVAC II, 157 patients continued methotrexate while receiving the first vaccine dose. These patients were subsequently randomized either to continue or to stop methotrexate for 2 weeks following the second dose.
The findings from MIVAC I demonstrated the flare rate was lower in the methotrexate-continue group than in the methotrexate-pause group (8% vs. 25%; P = .005) and that the median anti-RBD titer was significantly higher for the methotrexate-pause group than the methotrexate-continue group (2,484 vs. 1,147; P = .001).
The results from MIVAC II trial indicated that there was no difference in flare rates between the two study groups (7.9% vs. 11.8%; P = .15). Yet, the median anti-RBD titer was significantly higher in the methotrexate-pause cohort than in the methotrexate-continue cohort (2,553 vs. 990; P = .001).
The report suggests there is a flare risk when methotrexate is stopped, Dr. Sreekanth noted. “It appears more logical to hold only after the second dose, as comparable anti-RBD titers are generated” with either approach, Dr. Sreekanth said.
Expert commentary: MIVAC I and II
Inés Colmegna, MD, associate professor at McGill University in Montreal, noted that it was intriguing that the risk of flares in MIVAC II is half of that reported after each of the doses of MIVAC I. “It is also worth emphasizing that despite the reported frequency of flares, the actual disease activity [as measured by the Disease Activity Score in 28 joints] in patients who did or did not withhold methotrexate was similar.
“MIVAC I and II have practical implications as they help to adequately inform patients about the risk and benefit trade of withholding methotrexate post–COVID-19 vaccination,” Dr. Colmegna told this news organization.
“Additional information would help to [further] interpret the findings of these studies, including whether any of the participants were taking any other DMARDs; data on the severity of the flares and functional impact; analysis of factors that predict the risk of flares, such as higher doses of methotrexate; [and change in] disease activity scores pre- and postvaccination,” Dr. Colmegna concluded.
Dr. Abhishek disclosed relationships with Springer, UpTodate, Oxford, Immunotec, AstraZeneca, Inflazome, NGM Biopharmaceuticals, Menarini Pharmaceuticals, and Cadila Pharmaceuticals. Dr. Abhishek is cochair of the ACR/EULAR CPPD Classification Criteria Working Group and the OMERACT CPPD Working Group. Dr. Sparks disclosed relationships with Gilead, Boehringer Ingelheim, Amgen, Bristol-Myers Squibb, and AbbVie, unrelated to this study. Dr. Tedeschi disclosed relationships with ModernaTx and NGM Biopharmaceuticals. Dr. Winthrop disclosed a research grant and serving as a scientific consultant for Pfizer. Dr. Sreekanth and Dr. Colmegna have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients who take methotrexate for a variety of immune-mediated inflammatory diseases and pause taking the drug following receipt of a COVID-19 vaccine dose did not have a higher risk of disease flare and had higher antireceptor binding domain (anti-RBD) antibody titers and increased immunogenicity when compared with continuing the drug, three recent studies suggest.
In one study, British researchers examined the effects of a 2-week break in methotrexate therapy on anti-RBD titers following receipt of a third COVID-19 vaccine dose. In their paper published in The Lancet: Respiratory Medicine, they reported results from a randomized, open-label, superiority trial that suggested pausing the drug improved immunogenicity, compared with no break.
In two trials presented at the European Alliance of Associations for Rheumatology (EULAR) 2022 Congress, a team from India set out to determine whether holding methotrexate after receiving both doses of a COVID-19 vaccine, or holding it only after the second dose, was safe and effective. They found that pausing methotrexate only following the second dose contributed to a lower flare risk, and that patients had higher anti-RBD titers when holding methotrexate for 2 weeks following each dose.
Pausing methotrexate after booster
The 2-week methotrexate break and booster vaccine dose data in the Vaccine Response On Off Methotrexate (VROOM) trial showed that after a month, the geometric mean antispike 1 (S1)-RBD antibody titer was 10,798 U/mL (95% confidence interval [CI], 8,970-12,997) in the group that continued methotrexate and 22,750 U/mL (95% CI, 19,314-26,796) in the group that suspended methotrexate; the geometric mean ratio was 2.19 (P < .0001; mixed-effects model), reported Abhishek Abhishek, MD, PhD, professor of rheumatology at the University of Nottingham in Nottingham, England, and colleagues.
Prior research showed that stopping methotrexate therapy for 2 weeks following the seasonal influenza vaccine contributed to better vaccine immunity among patients with rheumatoid arthritis, but there was no impact of stopping the drug for up to 4 weeks before vaccination on vaccine-related immunity, the researchers noted.
It is crucial in maximizing long-lasting vaccine protection in people who are possibly susceptible through immune suppression at this point in the COVID-19 vaccination regimen, the study team noted.
“Evidence from this study will be useful for policymakers, national immunization advisory committees, and specialist societies formulating recommendations on the use of methotrexate around the time of COVID-19 vaccination. This evidence will help patients and clinicians make informed choices about the risks and benefits of interrupting methotrexate treatment around the time of COVID-19 vaccination, with implications for the potential to extend such approaches to other therapeutics,” they wrote.
In American College of Rheumatology (ACR) guidance for COVID-19 vaccination, the organization advised against using standard synthetic disease-modifying antirheumatic medicines such as methotrexate “for 1-2 weeks (as disease activity allows) after each COVID-19 vaccine dose,” given the at-risk population and public health concerns, Jeffrey A. Sparks, MD, MMSc, assistant professor of medicine and associate physician at Brigham and Women’s Hospital and Harvard Medical School, Boston, and Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School, noted in an accompanying editorial in The Lancet: Respiratory Medicine.
However, when the ACR developed this statement, there was only one trial involving patients with rheumatoid arthritis who paused methotrexate following seasonal influenza vaccination, the editorialists said.
“Although this finding adds to the evidence base to support interruption of methotrexate after vaccination, a shared decision process is needed to weigh the possible benefit of optimizing protection from COVID-19 and the possible risk of underlying disease flare,” they added.
Dr. Abhishek and colleagues assessed 254 patients with immune-mediated inflammatory disease from dermatology and rheumatology clinics across 26 hospitals in the United Kingdom. Participants had been diagnosed with systemic lupus erythematosus, rheumatoid arthritis, atopic dermatitis, polymyalgia rheumatica, axial spondyloarthritis, and psoriasis without or with arthritis. They had also been taking up to 25 mg of methotrexate per week for 3 months or longer and had received two doses of either the Pfizer/BioNTech BNT162b2 vaccine or AstraZeneca/Oxford viral vector vaccine. The booster dose was most often the Pfizer BNT162b2 vaccine (82%). The patients’ mean age was 59 years, with females comprising 61% of the cohort. Participants were randomly assigned 1:1 to either group.
Investigators performing laboratory analysis were masked to cohort assignment, and clinical research staff, data analysts, participants, and researchers were unmasked.
The elevated antibody response of patients who suspended methotrexate was the same across different kinds of immune-mediated inflammatory disease, primary vaccination platform, SARS-CoV-2 infection history, and age.
Notably, no intervention-associated adverse events were reported, the study team noted.
The conclusions that could be drawn from the booster-dose study were limited by the trial’s modest cohort size, the small number of patients in exploratory subgroup analyses, a lack of information about differences in prescription drug behavior, and early termination’s effect on the researchers’ ability to identify differences between subgroups and in secondary outcomes, the authors noted.
Other limitations included a lack of generalizability to patients with active disease who couldn’t stop therapy and were not included in the investigation, and participants were not blinded to what group they were in, the researchers said.
Expert commentary
This current study is consistent with other studies over the last several months showing that methotrexate harms both humoral and cell-mediated COVID-19 responses, noted Kevin Winthrop, MD, MPH, professor of infectious disease and public health at Oregon Health & Science University, Portland, who was not involved in the study. “And so now the new wave of studies are like this one, where they are holding methotrexate experimentally and seeing if it makes a difference,” he said.
“The one shortcoming of this study – and so far, the studies to date – is that no one has looked at whether the experimental hold has resulted in a change in T-cell responses, which ... we are [now] recognizing [the importance of] more and more in long-term protection, particularly in severe disease. Theoretically, holding [methotrexate] might help enhance T-cell responses, but that hasn’t been shown experimentally.”
Dr. Winthrop pointed out that one might get the same benefit from holding methotrexate for 1 week instead of 2 and that there likely is a reduced risk of flare-up from underlying autoimmune disease.
It is still not certain that this benefit extends to other vaccines, Dr. Winthrop noted. “It is probably true for most vaccines that if you hold methotrexate for 1 or 2 weeks, you might see some short-term benefit in responsiveness, but you don’t know that there is any clinical meaningfulness of this. That’s going to take other long-term studies. You don’t know how long this benefit lasts.”
Pausing methotrexate during initial COVID vaccine doses
Patients with either rheumatoid arthritis or psoriatic arthritis had higher anti-RBD antibody titers when methotrexate was stopped after both doses of the AstraZeneca vaccine, or simply after the second dose, than when methotrexate was continued, according to results from two single-center, randomized controlled trials called MIVAC I and II, Anu Sreekanth, MD, of Sree Sudheendra Medical Mission in Kochi, Kerala, India, and colleagues reported at EULAR 2022.
Results from MIVAC I indicated that there was a higher flare rate when methotrexate was stopped after both vaccine doses, but there was no difference in flare rate in MIVAC II when methotrexate was stopped only after the second dose as opposed to stopping it after both doses.
In the MIVAC I trial, 158 unvaccinated patients were randomized 1:1 to a cohort in which methotrexate was held for 2 weeks after both doses and a cohort in which methotrexate was continued despite the vaccine. In MIVAC II, 157 patients continued methotrexate while receiving the first vaccine dose. These patients were subsequently randomized either to continue or to stop methotrexate for 2 weeks following the second dose.
The findings from MIVAC I demonstrated the flare rate was lower in the methotrexate-continue group than in the methotrexate-pause group (8% vs. 25%; P = .005) and that the median anti-RBD titer was significantly higher for the methotrexate-pause group than the methotrexate-continue group (2,484 vs. 1,147; P = .001).
The results from MIVAC II trial indicated that there was no difference in flare rates between the two study groups (7.9% vs. 11.8%; P = .15). Yet, the median anti-RBD titer was significantly higher in the methotrexate-pause cohort than in the methotrexate-continue cohort (2,553 vs. 990; P = .001).
The report suggests there is a flare risk when methotrexate is stopped, Dr. Sreekanth noted. “It appears more logical to hold only after the second dose, as comparable anti-RBD titers are generated” with either approach, Dr. Sreekanth said.
Expert commentary: MIVAC I and II
Inés Colmegna, MD, associate professor at McGill University in Montreal, noted that it was intriguing that the risk of flares in MIVAC II is half of that reported after each of the doses of MIVAC I. “It is also worth emphasizing that despite the reported frequency of flares, the actual disease activity [as measured by the Disease Activity Score in 28 joints] in patients who did or did not withhold methotrexate was similar.
“MIVAC I and II have practical implications as they help to adequately inform patients about the risk and benefit trade of withholding methotrexate post–COVID-19 vaccination,” Dr. Colmegna told this news organization.
“Additional information would help to [further] interpret the findings of these studies, including whether any of the participants were taking any other DMARDs; data on the severity of the flares and functional impact; analysis of factors that predict the risk of flares, such as higher doses of methotrexate; [and change in] disease activity scores pre- and postvaccination,” Dr. Colmegna concluded.
Dr. Abhishek disclosed relationships with Springer, UpTodate, Oxford, Immunotec, AstraZeneca, Inflazome, NGM Biopharmaceuticals, Menarini Pharmaceuticals, and Cadila Pharmaceuticals. Dr. Abhishek is cochair of the ACR/EULAR CPPD Classification Criteria Working Group and the OMERACT CPPD Working Group. Dr. Sparks disclosed relationships with Gilead, Boehringer Ingelheim, Amgen, Bristol-Myers Squibb, and AbbVie, unrelated to this study. Dr. Tedeschi disclosed relationships with ModernaTx and NGM Biopharmaceuticals. Dr. Winthrop disclosed a research grant and serving as a scientific consultant for Pfizer. Dr. Sreekanth and Dr. Colmegna have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients who take methotrexate for a variety of immune-mediated inflammatory diseases and pause taking the drug following receipt of a COVID-19 vaccine dose did not have a higher risk of disease flare and had higher antireceptor binding domain (anti-RBD) antibody titers and increased immunogenicity when compared with continuing the drug, three recent studies suggest.
In one study, British researchers examined the effects of a 2-week break in methotrexate therapy on anti-RBD titers following receipt of a third COVID-19 vaccine dose. In their paper published in The Lancet: Respiratory Medicine, they reported results from a randomized, open-label, superiority trial that suggested pausing the drug improved immunogenicity, compared with no break.
In two trials presented at the European Alliance of Associations for Rheumatology (EULAR) 2022 Congress, a team from India set out to determine whether holding methotrexate after receiving both doses of a COVID-19 vaccine, or holding it only after the second dose, was safe and effective. They found that pausing methotrexate only following the second dose contributed to a lower flare risk, and that patients had higher anti-RBD titers when holding methotrexate for 2 weeks following each dose.
Pausing methotrexate after booster
The 2-week methotrexate break and booster vaccine dose data in the Vaccine Response On Off Methotrexate (VROOM) trial showed that after a month, the geometric mean antispike 1 (S1)-RBD antibody titer was 10,798 U/mL (95% confidence interval [CI], 8,970-12,997) in the group that continued methotrexate and 22,750 U/mL (95% CI, 19,314-26,796) in the group that suspended methotrexate; the geometric mean ratio was 2.19 (P < .0001; mixed-effects model), reported Abhishek Abhishek, MD, PhD, professor of rheumatology at the University of Nottingham in Nottingham, England, and colleagues.
Prior research showed that stopping methotrexate therapy for 2 weeks following the seasonal influenza vaccine contributed to better vaccine immunity among patients with rheumatoid arthritis, but there was no impact of stopping the drug for up to 4 weeks before vaccination on vaccine-related immunity, the researchers noted.
It is crucial in maximizing long-lasting vaccine protection in people who are possibly susceptible through immune suppression at this point in the COVID-19 vaccination regimen, the study team noted.
“Evidence from this study will be useful for policymakers, national immunization advisory committees, and specialist societies formulating recommendations on the use of methotrexate around the time of COVID-19 vaccination. This evidence will help patients and clinicians make informed choices about the risks and benefits of interrupting methotrexate treatment around the time of COVID-19 vaccination, with implications for the potential to extend such approaches to other therapeutics,” they wrote.
In American College of Rheumatology (ACR) guidance for COVID-19 vaccination, the organization advised against using standard synthetic disease-modifying antirheumatic medicines such as methotrexate “for 1-2 weeks (as disease activity allows) after each COVID-19 vaccine dose,” given the at-risk population and public health concerns, Jeffrey A. Sparks, MD, MMSc, assistant professor of medicine and associate physician at Brigham and Women’s Hospital and Harvard Medical School, Boston, and Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School, noted in an accompanying editorial in The Lancet: Respiratory Medicine.
However, when the ACR developed this statement, there was only one trial involving patients with rheumatoid arthritis who paused methotrexate following seasonal influenza vaccination, the editorialists said.
“Although this finding adds to the evidence base to support interruption of methotrexate after vaccination, a shared decision process is needed to weigh the possible benefit of optimizing protection from COVID-19 and the possible risk of underlying disease flare,” they added.
Dr. Abhishek and colleagues assessed 254 patients with immune-mediated inflammatory disease from dermatology and rheumatology clinics across 26 hospitals in the United Kingdom. Participants had been diagnosed with systemic lupus erythematosus, rheumatoid arthritis, atopic dermatitis, polymyalgia rheumatica, axial spondyloarthritis, and psoriasis without or with arthritis. They had also been taking up to 25 mg of methotrexate per week for 3 months or longer and had received two doses of either the Pfizer/BioNTech BNT162b2 vaccine or AstraZeneca/Oxford viral vector vaccine. The booster dose was most often the Pfizer BNT162b2 vaccine (82%). The patients’ mean age was 59 years, with females comprising 61% of the cohort. Participants were randomly assigned 1:1 to either group.
Investigators performing laboratory analysis were masked to cohort assignment, and clinical research staff, data analysts, participants, and researchers were unmasked.
The elevated antibody response of patients who suspended methotrexate was the same across different kinds of immune-mediated inflammatory disease, primary vaccination platform, SARS-CoV-2 infection history, and age.
Notably, no intervention-associated adverse events were reported, the study team noted.
The conclusions that could be drawn from the booster-dose study were limited by the trial’s modest cohort size, the small number of patients in exploratory subgroup analyses, a lack of information about differences in prescription drug behavior, and early termination’s effect on the researchers’ ability to identify differences between subgroups and in secondary outcomes, the authors noted.
Other limitations included a lack of generalizability to patients with active disease who couldn’t stop therapy and were not included in the investigation, and participants were not blinded to what group they were in, the researchers said.
Expert commentary
This current study is consistent with other studies over the last several months showing that methotrexate harms both humoral and cell-mediated COVID-19 responses, noted Kevin Winthrop, MD, MPH, professor of infectious disease and public health at Oregon Health & Science University, Portland, who was not involved in the study. “And so now the new wave of studies are like this one, where they are holding methotrexate experimentally and seeing if it makes a difference,” he said.
“The one shortcoming of this study – and so far, the studies to date – is that no one has looked at whether the experimental hold has resulted in a change in T-cell responses, which ... we are [now] recognizing [the importance of] more and more in long-term protection, particularly in severe disease. Theoretically, holding [methotrexate] might help enhance T-cell responses, but that hasn’t been shown experimentally.”
Dr. Winthrop pointed out that one might get the same benefit from holding methotrexate for 1 week instead of 2 and that there likely is a reduced risk of flare-up from underlying autoimmune disease.
It is still not certain that this benefit extends to other vaccines, Dr. Winthrop noted. “It is probably true for most vaccines that if you hold methotrexate for 1 or 2 weeks, you might see some short-term benefit in responsiveness, but you don’t know that there is any clinical meaningfulness of this. That’s going to take other long-term studies. You don’t know how long this benefit lasts.”
Pausing methotrexate during initial COVID vaccine doses
Patients with either rheumatoid arthritis or psoriatic arthritis had higher anti-RBD antibody titers when methotrexate was stopped after both doses of the AstraZeneca vaccine, or simply after the second dose, than when methotrexate was continued, according to results from two single-center, randomized controlled trials called MIVAC I and II, Anu Sreekanth, MD, of Sree Sudheendra Medical Mission in Kochi, Kerala, India, and colleagues reported at EULAR 2022.
Results from MIVAC I indicated that there was a higher flare rate when methotrexate was stopped after both vaccine doses, but there was no difference in flare rate in MIVAC II when methotrexate was stopped only after the second dose as opposed to stopping it after both doses.
In the MIVAC I trial, 158 unvaccinated patients were randomized 1:1 to a cohort in which methotrexate was held for 2 weeks after both doses and a cohort in which methotrexate was continued despite the vaccine. In MIVAC II, 157 patients continued methotrexate while receiving the first vaccine dose. These patients were subsequently randomized either to continue or to stop methotrexate for 2 weeks following the second dose.
The findings from MIVAC I demonstrated the flare rate was lower in the methotrexate-continue group than in the methotrexate-pause group (8% vs. 25%; P = .005) and that the median anti-RBD titer was significantly higher for the methotrexate-pause group than the methotrexate-continue group (2,484 vs. 1,147; P = .001).
The results from MIVAC II trial indicated that there was no difference in flare rates between the two study groups (7.9% vs. 11.8%; P = .15). Yet, the median anti-RBD titer was significantly higher in the methotrexate-pause cohort than in the methotrexate-continue cohort (2,553 vs. 990; P = .001).
The report suggests there is a flare risk when methotrexate is stopped, Dr. Sreekanth noted. “It appears more logical to hold only after the second dose, as comparable anti-RBD titers are generated” with either approach, Dr. Sreekanth said.
Expert commentary: MIVAC I and II
Inés Colmegna, MD, associate professor at McGill University in Montreal, noted that it was intriguing that the risk of flares in MIVAC II is half of that reported after each of the doses of MIVAC I. “It is also worth emphasizing that despite the reported frequency of flares, the actual disease activity [as measured by the Disease Activity Score in 28 joints] in patients who did or did not withhold methotrexate was similar.
“MIVAC I and II have practical implications as they help to adequately inform patients about the risk and benefit trade of withholding methotrexate post–COVID-19 vaccination,” Dr. Colmegna told this news organization.
“Additional information would help to [further] interpret the findings of these studies, including whether any of the participants were taking any other DMARDs; data on the severity of the flares and functional impact; analysis of factors that predict the risk of flares, such as higher doses of methotrexate; [and change in] disease activity scores pre- and postvaccination,” Dr. Colmegna concluded.
Dr. Abhishek disclosed relationships with Springer, UpTodate, Oxford, Immunotec, AstraZeneca, Inflazome, NGM Biopharmaceuticals, Menarini Pharmaceuticals, and Cadila Pharmaceuticals. Dr. Abhishek is cochair of the ACR/EULAR CPPD Classification Criteria Working Group and the OMERACT CPPD Working Group. Dr. Sparks disclosed relationships with Gilead, Boehringer Ingelheim, Amgen, Bristol-Myers Squibb, and AbbVie, unrelated to this study. Dr. Tedeschi disclosed relationships with ModernaTx and NGM Biopharmaceuticals. Dr. Winthrop disclosed a research grant and serving as a scientific consultant for Pfizer. Dr. Sreekanth and Dr. Colmegna have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Many people becoming reinfected as BA.5 dominates new COVID-19 cases
When the COVID-19 pandemic first began, the general thought was that once people were infected, they were then protected from the virus.
It’s hard to say how many. The ABC News analysis found at least 1.6 million reinfections in 24 states, but the actual number is probably a lot higher.
“These are not the real numbers because many people are not reporting cases,” Ali Mokdad, MD, an epidemiologist with the University of Washington, Seattle, told ABC.
The latest variant, BA.5, has become the dominant strain in the United States, making up more than 65% of all COVID-19 cases as of July 13, according to data from the CDC.
Prior infections and vaccines aren’t providing as much protection against the newly dominant BA.5 strain as they did against earlier variants.
But evidence doesn’t show this subvariant of Omicron to be more harmful than earlier, less transmissible versions.
Several factors are contributing to rising reinfections, experts say. For example, fewer people are wearing masks than in the first year or so of the pandemic. Dr. Mokdad said just 18% of Americans reported always wearing a mask in public at the end of May, down from 44% the year before.
The emergence of the Omicron variant, of which BA.5 is a subvariant, is indicating that less protection is being offered by prior infections.
A version of this article first appeared on WebMD.com.
When the COVID-19 pandemic first began, the general thought was that once people were infected, they were then protected from the virus.
It’s hard to say how many. The ABC News analysis found at least 1.6 million reinfections in 24 states, but the actual number is probably a lot higher.
“These are not the real numbers because many people are not reporting cases,” Ali Mokdad, MD, an epidemiologist with the University of Washington, Seattle, told ABC.
The latest variant, BA.5, has become the dominant strain in the United States, making up more than 65% of all COVID-19 cases as of July 13, according to data from the CDC.
Prior infections and vaccines aren’t providing as much protection against the newly dominant BA.5 strain as they did against earlier variants.
But evidence doesn’t show this subvariant of Omicron to be more harmful than earlier, less transmissible versions.
Several factors are contributing to rising reinfections, experts say. For example, fewer people are wearing masks than in the first year or so of the pandemic. Dr. Mokdad said just 18% of Americans reported always wearing a mask in public at the end of May, down from 44% the year before.
The emergence of the Omicron variant, of which BA.5 is a subvariant, is indicating that less protection is being offered by prior infections.
A version of this article first appeared on WebMD.com.
When the COVID-19 pandemic first began, the general thought was that once people were infected, they were then protected from the virus.
It’s hard to say how many. The ABC News analysis found at least 1.6 million reinfections in 24 states, but the actual number is probably a lot higher.
“These are not the real numbers because many people are not reporting cases,” Ali Mokdad, MD, an epidemiologist with the University of Washington, Seattle, told ABC.
The latest variant, BA.5, has become the dominant strain in the United States, making up more than 65% of all COVID-19 cases as of July 13, according to data from the CDC.
Prior infections and vaccines aren’t providing as much protection against the newly dominant BA.5 strain as they did against earlier variants.
But evidence doesn’t show this subvariant of Omicron to be more harmful than earlier, less transmissible versions.
Several factors are contributing to rising reinfections, experts say. For example, fewer people are wearing masks than in the first year or so of the pandemic. Dr. Mokdad said just 18% of Americans reported always wearing a mask in public at the end of May, down from 44% the year before.
The emergence of the Omicron variant, of which BA.5 is a subvariant, is indicating that less protection is being offered by prior infections.
A version of this article first appeared on WebMD.com.
Some have heavier periods after COVID vaccine
Many women who got a COVID-19 vaccine have reported heavier bleeding during their periods since they had the shots.
A team of researchers investigated the trend and set out to find out who among the vaccinated were more likely to experience the menstruation changes.
The researchers were led by Katharine M.N. Lee, PhD, MS, of the division of public health sciences at Washington University in St. Louis. Their findings were published ahead of print in Science Advances.
The investigators analyzed more than 139,000 responses from an online survey from both currently and formerly menstruating women.
They found that, among people who have regular periods, about the same percentage had heavier bleeding after they got a COVID vaccine as had no change in bleeding after the vaccine (44% vs. 42%, respectively).
“A much smaller portion had lighter periods,” they write.
The phenomenon has been difficult to study because questions about changes in menstruation are not a standard part of vaccine trials.
Date of last period is often tracked in clinical trials to make sure a participant is not pregnant, but the questions about periods often stop there.
Additionally, periods are different for everyone and can be influenced by all sorts of environmental factors, so making associations regarding exposures is problematic.
No changes found to fertility
The authors emphasized that, generally, changes to menstrual bleeding are not uncommon nor dangerous. They also emphasized that the changes in bleeding don’t mean changes to fertility.
The uterine reproductive system is flexible when the body is under stress, they note.
“We know that running a marathon may influence hormone concentrations in the short term while not rendering that person infertile,” the authors write.
However, they acknowledge that investigating these reports is critical in building trust in medicine.
This report includes information that hasn’t been available through the clinical trial follow-up process.
For instance, the authors write, “To the best of our knowledge, our work is the first to examine breakthrough bleeding after vaccination in either pre- or postmenopausal people.”
Reports of changes to periods after vaccination started emerging in 2021. But without data, reports were largely dismissed, fueling criticism from those waging campaigns against COVID vaccines.
Dr. Lee and colleagues gathered data from those who responded to the online survey and detailed some trends.
People who were bleeding more heavily after vaccination were more likely to be older, Hispanic, had vaccine side effects of fever and fatigue, had been pregnant at some point, or had given birth.
People with regular periods who had endometriosis, prolonged bleeding during their periods, polycystic ovarian syndrome (PCOS) or fibroids were also more likely to have increased bleeding after a COVID vaccine.
Breakthrough bleeding
For people who don’t menstruate, but have not reached menopause, breakthrough bleeding happened more often in women who had been pregnant and/or had given birth.
Among respondents who were postmenopausal, breakthrough bleeding happened more often in younger people and/or those who are Hispanic.
More than a third of the respondents (39%) who use gender-affirming hormones that eliminate menstruation reported breakthrough bleeding after vaccination.
The majority of premenopausal people on long-acting, reversible contraception (71%) and the majority of postmenopausal respondents (66%) had breakthrough bleeding as well.
The authors note that you can’t compare the percentages who report these experiences in the survey with the incidence of those who would experience changes in menstrual bleeding in the general population.
The nature of the online survey means it may be naturally biased because the people who responded may be more often those who noted some change in their own menstrual experiences, particularly if that involved discomfort, pain, or fear.
Researchers also acknowledge that Black, Indigenous, Latinx, and other respondents of color are underrepresented in this research and that represents a limitation in the work.
Alison Edelman, MD, MPH, with the department of obstetrics and gynecology at Oregon Health & Science University in Portland, was not involved with Dr. Lee and associates’ study but has also studied the relationship between COVID vaccines and menstruation.
Her team’s study found that COVID vaccination is associated with a small change in time between periods but not length of periods.
She said about the work by Dr. Lee and colleagues, “This work really elevates the voices of the public and what they’re experiencing.”
The association makes sense, Dr. Edelman says, in that the reproductive system and the immune system talk to each other and inflammation in the immune system is going to be noticed by the system governing periods.
Lack of data on the relationship between exposures and menstruation didn’t start with COVID. “There has been a signal in the population before with other vaccines that’s been dismissed,” she said.
Tracking menstruation information in clinical trials can help physicians counsel women on what may be coming with any vaccine and alleviate fears and vaccine hesitancy, Dr. Edelman explained. It can also help vaccine developers know what to include in information about their product.
“When you are counseled about what to expect, it’s not as scary. That provides trust in the system,” she said. She likened it to original lack of data on whether COVID-19 vaccines would affect pregnancy.
“We have great science now that COVID vaccine does not affect fertility and [vaccine] does not impact pregnancy.”
Another important aspect of this paper is that it included subgroups not studied before regarding menstruation and breakthrough bleeding, such as those taking gender-affirming hormones, she added.
Menstruation has been often overlooked as important in clinical trial exposures but Dr. Edelman hopes this recent attention and question will escalate and prompt more research.
“I’m hoping with the immense outpouring from the public about how important this is, that future studies will look at this a little bit better,” she says.
She said when the National Institutes of Health opened up funding for trials on COVID-19 vaccines and menstruation, researchers got flooded with requests from women to share their stories.
“As a researcher – I’ve been doing research for over 20 years – that’s not something that usually happens. I would love to have that happen for every research project.”
The authors and Dr. Edelman declare that they have no competing interests. This research was supported in part by the University of Illinois Beckman Institute for Advanced Science and Technology, the University of Illinois Interdisciplinary Health Sciences Institute, the National Institutes of Health, the Foundation for Barnes-Jewish Hospital, and the Siteman Cancer Center.
Many women who got a COVID-19 vaccine have reported heavier bleeding during their periods since they had the shots.
A team of researchers investigated the trend and set out to find out who among the vaccinated were more likely to experience the menstruation changes.
The researchers were led by Katharine M.N. Lee, PhD, MS, of the division of public health sciences at Washington University in St. Louis. Their findings were published ahead of print in Science Advances.
The investigators analyzed more than 139,000 responses from an online survey from both currently and formerly menstruating women.
They found that, among people who have regular periods, about the same percentage had heavier bleeding after they got a COVID vaccine as had no change in bleeding after the vaccine (44% vs. 42%, respectively).
“A much smaller portion had lighter periods,” they write.
The phenomenon has been difficult to study because questions about changes in menstruation are not a standard part of vaccine trials.
Date of last period is often tracked in clinical trials to make sure a participant is not pregnant, but the questions about periods often stop there.
Additionally, periods are different for everyone and can be influenced by all sorts of environmental factors, so making associations regarding exposures is problematic.
No changes found to fertility
The authors emphasized that, generally, changes to menstrual bleeding are not uncommon nor dangerous. They also emphasized that the changes in bleeding don’t mean changes to fertility.
The uterine reproductive system is flexible when the body is under stress, they note.
“We know that running a marathon may influence hormone concentrations in the short term while not rendering that person infertile,” the authors write.
However, they acknowledge that investigating these reports is critical in building trust in medicine.
This report includes information that hasn’t been available through the clinical trial follow-up process.
For instance, the authors write, “To the best of our knowledge, our work is the first to examine breakthrough bleeding after vaccination in either pre- or postmenopausal people.”
Reports of changes to periods after vaccination started emerging in 2021. But without data, reports were largely dismissed, fueling criticism from those waging campaigns against COVID vaccines.
Dr. Lee and colleagues gathered data from those who responded to the online survey and detailed some trends.
People who were bleeding more heavily after vaccination were more likely to be older, Hispanic, had vaccine side effects of fever and fatigue, had been pregnant at some point, or had given birth.
People with regular periods who had endometriosis, prolonged bleeding during their periods, polycystic ovarian syndrome (PCOS) or fibroids were also more likely to have increased bleeding after a COVID vaccine.
Breakthrough bleeding
For people who don’t menstruate, but have not reached menopause, breakthrough bleeding happened more often in women who had been pregnant and/or had given birth.
Among respondents who were postmenopausal, breakthrough bleeding happened more often in younger people and/or those who are Hispanic.
More than a third of the respondents (39%) who use gender-affirming hormones that eliminate menstruation reported breakthrough bleeding after vaccination.
The majority of premenopausal people on long-acting, reversible contraception (71%) and the majority of postmenopausal respondents (66%) had breakthrough bleeding as well.
The authors note that you can’t compare the percentages who report these experiences in the survey with the incidence of those who would experience changes in menstrual bleeding in the general population.
The nature of the online survey means it may be naturally biased because the people who responded may be more often those who noted some change in their own menstrual experiences, particularly if that involved discomfort, pain, or fear.
Researchers also acknowledge that Black, Indigenous, Latinx, and other respondents of color are underrepresented in this research and that represents a limitation in the work.
Alison Edelman, MD, MPH, with the department of obstetrics and gynecology at Oregon Health & Science University in Portland, was not involved with Dr. Lee and associates’ study but has also studied the relationship between COVID vaccines and menstruation.
Her team’s study found that COVID vaccination is associated with a small change in time between periods but not length of periods.
She said about the work by Dr. Lee and colleagues, “This work really elevates the voices of the public and what they’re experiencing.”
The association makes sense, Dr. Edelman says, in that the reproductive system and the immune system talk to each other and inflammation in the immune system is going to be noticed by the system governing periods.
Lack of data on the relationship between exposures and menstruation didn’t start with COVID. “There has been a signal in the population before with other vaccines that’s been dismissed,” she said.
Tracking menstruation information in clinical trials can help physicians counsel women on what may be coming with any vaccine and alleviate fears and vaccine hesitancy, Dr. Edelman explained. It can also help vaccine developers know what to include in information about their product.
“When you are counseled about what to expect, it’s not as scary. That provides trust in the system,” she said. She likened it to original lack of data on whether COVID-19 vaccines would affect pregnancy.
“We have great science now that COVID vaccine does not affect fertility and [vaccine] does not impact pregnancy.”
Another important aspect of this paper is that it included subgroups not studied before regarding menstruation and breakthrough bleeding, such as those taking gender-affirming hormones, she added.
Menstruation has been often overlooked as important in clinical trial exposures but Dr. Edelman hopes this recent attention and question will escalate and prompt more research.
“I’m hoping with the immense outpouring from the public about how important this is, that future studies will look at this a little bit better,” she says.
She said when the National Institutes of Health opened up funding for trials on COVID-19 vaccines and menstruation, researchers got flooded with requests from women to share their stories.
“As a researcher – I’ve been doing research for over 20 years – that’s not something that usually happens. I would love to have that happen for every research project.”
The authors and Dr. Edelman declare that they have no competing interests. This research was supported in part by the University of Illinois Beckman Institute for Advanced Science and Technology, the University of Illinois Interdisciplinary Health Sciences Institute, the National Institutes of Health, the Foundation for Barnes-Jewish Hospital, and the Siteman Cancer Center.
Many women who got a COVID-19 vaccine have reported heavier bleeding during their periods since they had the shots.
A team of researchers investigated the trend and set out to find out who among the vaccinated were more likely to experience the menstruation changes.
The researchers were led by Katharine M.N. Lee, PhD, MS, of the division of public health sciences at Washington University in St. Louis. Their findings were published ahead of print in Science Advances.
The investigators analyzed more than 139,000 responses from an online survey from both currently and formerly menstruating women.
They found that, among people who have regular periods, about the same percentage had heavier bleeding after they got a COVID vaccine as had no change in bleeding after the vaccine (44% vs. 42%, respectively).
“A much smaller portion had lighter periods,” they write.
The phenomenon has been difficult to study because questions about changes in menstruation are not a standard part of vaccine trials.
Date of last period is often tracked in clinical trials to make sure a participant is not pregnant, but the questions about periods often stop there.
Additionally, periods are different for everyone and can be influenced by all sorts of environmental factors, so making associations regarding exposures is problematic.
No changes found to fertility
The authors emphasized that, generally, changes to menstrual bleeding are not uncommon nor dangerous. They also emphasized that the changes in bleeding don’t mean changes to fertility.
The uterine reproductive system is flexible when the body is under stress, they note.
“We know that running a marathon may influence hormone concentrations in the short term while not rendering that person infertile,” the authors write.
However, they acknowledge that investigating these reports is critical in building trust in medicine.
This report includes information that hasn’t been available through the clinical trial follow-up process.
For instance, the authors write, “To the best of our knowledge, our work is the first to examine breakthrough bleeding after vaccination in either pre- or postmenopausal people.”
Reports of changes to periods after vaccination started emerging in 2021. But without data, reports were largely dismissed, fueling criticism from those waging campaigns against COVID vaccines.
Dr. Lee and colleagues gathered data from those who responded to the online survey and detailed some trends.
People who were bleeding more heavily after vaccination were more likely to be older, Hispanic, had vaccine side effects of fever and fatigue, had been pregnant at some point, or had given birth.
People with regular periods who had endometriosis, prolonged bleeding during their periods, polycystic ovarian syndrome (PCOS) or fibroids were also more likely to have increased bleeding after a COVID vaccine.
Breakthrough bleeding
For people who don’t menstruate, but have not reached menopause, breakthrough bleeding happened more often in women who had been pregnant and/or had given birth.
Among respondents who were postmenopausal, breakthrough bleeding happened more often in younger people and/or those who are Hispanic.
More than a third of the respondents (39%) who use gender-affirming hormones that eliminate menstruation reported breakthrough bleeding after vaccination.
The majority of premenopausal people on long-acting, reversible contraception (71%) and the majority of postmenopausal respondents (66%) had breakthrough bleeding as well.
The authors note that you can’t compare the percentages who report these experiences in the survey with the incidence of those who would experience changes in menstrual bleeding in the general population.
The nature of the online survey means it may be naturally biased because the people who responded may be more often those who noted some change in their own menstrual experiences, particularly if that involved discomfort, pain, or fear.
Researchers also acknowledge that Black, Indigenous, Latinx, and other respondents of color are underrepresented in this research and that represents a limitation in the work.
Alison Edelman, MD, MPH, with the department of obstetrics and gynecology at Oregon Health & Science University in Portland, was not involved with Dr. Lee and associates’ study but has also studied the relationship between COVID vaccines and menstruation.
Her team’s study found that COVID vaccination is associated with a small change in time between periods but not length of periods.
She said about the work by Dr. Lee and colleagues, “This work really elevates the voices of the public and what they’re experiencing.”
The association makes sense, Dr. Edelman says, in that the reproductive system and the immune system talk to each other and inflammation in the immune system is going to be noticed by the system governing periods.
Lack of data on the relationship between exposures and menstruation didn’t start with COVID. “There has been a signal in the population before with other vaccines that’s been dismissed,” she said.
Tracking menstruation information in clinical trials can help physicians counsel women on what may be coming with any vaccine and alleviate fears and vaccine hesitancy, Dr. Edelman explained. It can also help vaccine developers know what to include in information about their product.
“When you are counseled about what to expect, it’s not as scary. That provides trust in the system,” she said. She likened it to original lack of data on whether COVID-19 vaccines would affect pregnancy.
“We have great science now that COVID vaccine does not affect fertility and [vaccine] does not impact pregnancy.”
Another important aspect of this paper is that it included subgroups not studied before regarding menstruation and breakthrough bleeding, such as those taking gender-affirming hormones, she added.
Menstruation has been often overlooked as important in clinical trial exposures but Dr. Edelman hopes this recent attention and question will escalate and prompt more research.
“I’m hoping with the immense outpouring from the public about how important this is, that future studies will look at this a little bit better,” she says.
She said when the National Institutes of Health opened up funding for trials on COVID-19 vaccines and menstruation, researchers got flooded with requests from women to share their stories.
“As a researcher – I’ve been doing research for over 20 years – that’s not something that usually happens. I would love to have that happen for every research project.”
The authors and Dr. Edelman declare that they have no competing interests. This research was supported in part by the University of Illinois Beckman Institute for Advanced Science and Technology, the University of Illinois Interdisciplinary Health Sciences Institute, the National Institutes of Health, the Foundation for Barnes-Jewish Hospital, and the Siteman Cancer Center.
FROM SCIENCE ADVANCES
SARS-CoV-2: A Novel Precipitant of Ischemic Priapism
Priapism is a disorder that occurs when the penis maintains a prolonged erection in the absence of appropriate stimulation. The disorder is typically divided into subgroups based on arterial flow: low flow (ischemic) and high flow (nonischemic). Ischemic priapism is the most common form and results from venous congestion due to obstructed outflow and inability of cavernous smooth muscle to contract, resulting in compartment syndrome, tissue hypoxia, hypercapnia, and acidosis.1 Conditions that result in hypercoagulable states and hyperviscosity are associated with ischemic priapism. COVID-19 is well known to cause an acute respiratory illness and systemic inflammatory response and has been increasingly associated with coagulopathy. Studies have shown that 20% to 55% of patients admitted to the hospital for COVID-19 show objective laboratory evidence of a hypercoagulable state.2
To date, there are 6 reported cases of priapism occurring in the setting of COVID-19 with all cases demonstrating the ischemic subtype. The onset of priapism from the beginning of infectious symptoms ranged from 2 days to more than a month. Five of the cases occurred in patients with critical COVID-19 and 1 in the setting of mild disease.3-8 Two critically ill patients did not receive treatment for their ischemic priapism as they were transitioned to expectant management and/or comfort measures.Most were treated with cavernosal blood aspiration and intracavernosal injections of phenylephrine or ethylephrine. Some patients were managed with prophylactic doses of anticoagulation after the identification of priapism; others were transitioned to therapeutic doses. Two patients were followed postdischarge; one patient reported normal nighttime erections with sexual desire 2 weeks postdischarge, and another patient, who underwent a bilateral T-shunt procedure after unsuccessful phenylephrine injections, reported complete erectile dysfunction at 3 months postdischarge.4,7 There was a potentially confounding variable in 2 cases in which propofol infusions were used for sedation management in the setting of mechanical ventilation.6,8 Propofol has been linked to priapism through its blockade of sympathetic activation resulting in persistent relaxation of cavernosal smooth muscle.9 We present a unique case of COVID-19–associated ischemic priapism as our patient had moderate rather than critical COVID-19.
Case Presentation
A 67-year-old male patient presented to the emergency department for a painful erection of 34-hour duration. The patient had been exposed to COVID-19 roughly 2 months prior. Since the exposure, he had experienced headache, nonproductive cough, sore throat, and decreased appetite with weight loss. His medical history included hypertension, thoracic aortic aneurysm, B-cell type chronic lymphocytic leukemia (CLL), and obstructive sleep apnea. Daily outpatient medications included atenolol 100 mg, hydrochlorothiazide 25 mg, and omeprazole 20 mg. The patient stopped tobacco use about 30 years previously. He reported no alcohol consumption or illicit drug use and had no previous episodes of prolonged erection.
The patient was afebrile, hemodynamically stable, and had an oxygen saturation of 92% on room air. Physical examination revealed clear breath sounds and an erect circumcised penis without any lesions, discoloration, or skin necrosis. Laboratory data were remarkable for the following values: 125,660 cells/μL white blood cells (WBCs), 13.82 × 103/ μL neutrophils, 110.58 × 103/μL lymphocytes, 1.26 × 103/μL monocytes, no blasts, 9.4 gm/dL hemoglobin, 100.3 fl mean corpuscular volume, 417,000 cells/μL platelets, 23,671 ng/mL D-dimer, 29.6 seconds activated partial thromboplastin time (aPTT), 16.3 seconds prothrombin time, 743 mg/dL fibrinogen, 474 U/L lactate dehydrogenase, and 202.1 mg/dL haptoglobin. A nasopharyngeal reverse transcription polymerase chain reaction test resulted positive for the SARS-CoV-2 virus, and subsequent chest X-ray revealed bilateral, hazy opacities predominantly in a peripheral distribution. Computed tomography (CT) angiogram of the chest did not reveal pulmonary emboli, pneumothorax, effusions, or lobar consolidation. However, it displayed bilateral ground-glass opacities with interstitial consolidation worst in the upper lobes. Corporal aspiration and blood gas analysis revealed a pH of 7.05, P
Differential Diagnosis
The first consideration in the differential diagnosis of priapism is to differentiate between ischemic and nonischemic. Based on the abnormal blood gas results above, this case clearly falls within the ischemic spectrum. Ischemic priapism secondary to CLL-induced hyperleukocytosis was considered. It has been noted that up to 20% of priapism cases in adults are related to hematologic disorders.10 While it is not uncommon to see hyperleukocytosis (total WBC count > 100 × 109/L) in CLL, leukostasis is rare with most reports demonstrating WBC counts > 1000 × 109/L.11 Hematology, vascular surgery, and urology services were consulted and agreed that ischemic priapism was due to microthrombi or pelvic vein thrombosis secondary to COVID-19–associated coagulopathy (CAC) was the most likely etiology.
Treatment
After corporal aspiration, intracorporal phenylephrine was administered. Diluted phenylephrine (100 ug/mL) was injected every 5 to 10 minutes while intermittently aspirating and irrigating multiple sites along the lateral length of the penile shaft. This initial procedure reduced the erection from 100% to 30% rigidity, with repeat blood gas analysis revealing minimal improvement. CT of the abdomen and pelvis with IV contrast revealed no evidence of pelvic thrombi. A second round of phenylephrine injections were administered, resulting in detumescence. The patient was treated with 2 to 3 L/min of oxygen supplementation via nasal cannula, a 5-day course of remdesivir and low-intensity heparin drip. Following the initial low-intensity heparin drip, the patient transitioned to therapeutic enoxaparin and subsequently was discharged on apixaban for a 3-month course. Since discharge, the patient followed up with hematology. He tolerated and completed the anticoagulation regimen without any recurrences of priapism or residual deficits.
Discussion
Recent studies have overwhelmingly analyzed the incidence and presentation of thrombotic complications in critically ill patients with COVID-19. CAC has been postulated to result from endotheliopathy along with immune cell activation and propagation of coagulation. While COVID-19 has been noted to create lung injury through binding angiotensin-converting enzyme 2 receptors expressed on alveolar pneumocytes, it increasingly has been found to affect endothelial cells throughout the body. Recent postmortem analyses have demonstrated direct viral infection of endothelial cells with consequent diffuse endothelial inflammation, as evidenced by viral inclusions, sequestered immune cells, and endothelial apoptosis.12,13 Manifestations of this endotheliopathy have been delineated through various studies.
An early retrospective study in Wuhan, China, illustrated that 36% of the first 99 patients hospitalized with COVID-19 demonstrated an elevated D-dimer, 6% an elevated aPTT, and 5% an elevated prothrombin time.14 Another retrospective study conducted in Wuhan found a 25% incidence of venous thromboembolic complications in critically ill patients with severe COVID-19.15 In the Netherlands, a study reported the incidence of arterial and venous thrombotic complications to be 31% in 184 critically ill patients with COVID-19, with 81% of these cases involving pulmonary emboli.16
To our knowledge, our patient is the seventh reported case of ischemic priapism occurring in the setting of a COVID-19 infection, and the first to have occurred in its moderate form. Ischemic priapism is often a consequence of penile venous outflow obstruction and resultant stasis of hypoxic blood.7 The prothrombotic state induced by CAC has been proposed to cause the obstruction of small emissary veins in the subtunical space and in turn lead to venous stasis, which propagates the formation of ischemic priapism.8 Furthermore, 4 of the previously reported cases shared laboratory data on their patients, and all demonstrated elevated D-dimer and fibrinogen levels, which strengthens this hypothesis.3,5,7,8 CLL presents a potential confounding variable in this case; however, as we have reviewed earlier, the risk of leukostasis at WBC counts < 1000 × 109/L is very low.11 It is also probable that the patient had some level of immune dysregulation secondary to CLL, leading to his prolonged course and slow clearance of the virus.
Conclusions
Although only a handful of CAC cases leading to ischemic priapism have been reported, the true incidence may be much higher. While our case highlights the importance of considering COVID-19 infection in the differential diagnosis of ischemic priapism, more research is needed to understand incidence and definitively establish a causative relationship.
1. Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med. 2004;1(1):116-120. doi:10.1111/j.1743-6109.2004.10117.x
2. Lee SG, Fralick M, Sholzberg M. Coagulopathy associated with COVID-19. CMAJ. 2020;192(21):E583. doi:10.1503/cmaj.200685
3. Lam G, McCarthy R, Haider R. A peculiar case of priapism: the hypercoagulable state in patients with severe COVID-19 infection. Eur J Case Rep Intern Med. 2020;7(8):001779. doi:10.12890/2020_001779
4. Addar A, Al Fraidi O, Nazer A, Althonayan N, Ghazwani Y. Priapism for 10 days in a patient with SARS-CoV-2 pneumonia: a case report. J Surg Case Rep. 2021;2021(4):rjab020. doi:10.1093/jscr/rjab020
5. Lamamri M, Chebbi A, Mamane J, et al. Priapism in a patient with coronavirus disease 2019 (COVID-19). Am J Emerg Med. 2021;39:251.e5-251.e7. doi:10.1016/j.ajem.2020.06.027
6. Silverman ML, VanDerVeer SJ, Donnelly TJ. Priapism in COVID-19: a thromboembolic complication. Am J Emerg Med. 2021;45:686.e5-686.e6. doi:10.1016/j.ajem.2020.12.072
7. Giuliano AFM, Vulpi M, Passerini F, et al. SARS-CoV-2 infection as a determining factor to the precipitation of ischemic priapism in a young patient with asymptomatic COVID-19. Case Rep Urol. 2021;2021:9936891. doi:10.1155/2021/9936891
8. Carreno BD, Perez CP, Vasquez D, Oyola JA, Suarez O, Bedoya C. Veno-occlusive priapism in COVID-19 disease. Urol Int. 2021;105(9-10):916-919. doi:10.1159/000514421
9. Senthilkumaran S, Shah S, Ganapathysubramanian, Balamurgan N, Thirumalaikolundusubramanian P. Propofol and priapism. Indian J Pharmacol. 2010;42(4):238-239. doi:10.4103/0253-7613.68430
10. Qu M, Lu X, Wang L, Liu Z, Sun Y, Gao X. Priapism secondary to chronic myeloid leukemia treated by a surgical cavernosa-corpus spongiosum shunt: case report. Asian J Urol. 2019;6(4):373-376. doi:10.1016/j.ajur.2018.12.004
11. Singh N, Singh Lubana S, Dabrowski L, Sidhu G. Leukostasis in chronic lymphocytic leukemia. Am J Case Rep. 2020;21:e924798. doi:10.12659/AJCR.924798
12. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417-1418. doi:10.1016/S0140-6736(20)30937-5
13. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033-2040. doi:10.1182/blood.2020006000
14. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi:10.1016/S0140-6736(20)30211-7
15. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421-1424. doi:10.1111/jth.14830
16. Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
Priapism is a disorder that occurs when the penis maintains a prolonged erection in the absence of appropriate stimulation. The disorder is typically divided into subgroups based on arterial flow: low flow (ischemic) and high flow (nonischemic). Ischemic priapism is the most common form and results from venous congestion due to obstructed outflow and inability of cavernous smooth muscle to contract, resulting in compartment syndrome, tissue hypoxia, hypercapnia, and acidosis.1 Conditions that result in hypercoagulable states and hyperviscosity are associated with ischemic priapism. COVID-19 is well known to cause an acute respiratory illness and systemic inflammatory response and has been increasingly associated with coagulopathy. Studies have shown that 20% to 55% of patients admitted to the hospital for COVID-19 show objective laboratory evidence of a hypercoagulable state.2
To date, there are 6 reported cases of priapism occurring in the setting of COVID-19 with all cases demonstrating the ischemic subtype. The onset of priapism from the beginning of infectious symptoms ranged from 2 days to more than a month. Five of the cases occurred in patients with critical COVID-19 and 1 in the setting of mild disease.3-8 Two critically ill patients did not receive treatment for their ischemic priapism as they were transitioned to expectant management and/or comfort measures.Most were treated with cavernosal blood aspiration and intracavernosal injections of phenylephrine or ethylephrine. Some patients were managed with prophylactic doses of anticoagulation after the identification of priapism; others were transitioned to therapeutic doses. Two patients were followed postdischarge; one patient reported normal nighttime erections with sexual desire 2 weeks postdischarge, and another patient, who underwent a bilateral T-shunt procedure after unsuccessful phenylephrine injections, reported complete erectile dysfunction at 3 months postdischarge.4,7 There was a potentially confounding variable in 2 cases in which propofol infusions were used for sedation management in the setting of mechanical ventilation.6,8 Propofol has been linked to priapism through its blockade of sympathetic activation resulting in persistent relaxation of cavernosal smooth muscle.9 We present a unique case of COVID-19–associated ischemic priapism as our patient had moderate rather than critical COVID-19.
Case Presentation
A 67-year-old male patient presented to the emergency department for a painful erection of 34-hour duration. The patient had been exposed to COVID-19 roughly 2 months prior. Since the exposure, he had experienced headache, nonproductive cough, sore throat, and decreased appetite with weight loss. His medical history included hypertension, thoracic aortic aneurysm, B-cell type chronic lymphocytic leukemia (CLL), and obstructive sleep apnea. Daily outpatient medications included atenolol 100 mg, hydrochlorothiazide 25 mg, and omeprazole 20 mg. The patient stopped tobacco use about 30 years previously. He reported no alcohol consumption or illicit drug use and had no previous episodes of prolonged erection.
The patient was afebrile, hemodynamically stable, and had an oxygen saturation of 92% on room air. Physical examination revealed clear breath sounds and an erect circumcised penis without any lesions, discoloration, or skin necrosis. Laboratory data were remarkable for the following values: 125,660 cells/μL white blood cells (WBCs), 13.82 × 103/ μL neutrophils, 110.58 × 103/μL lymphocytes, 1.26 × 103/μL monocytes, no blasts, 9.4 gm/dL hemoglobin, 100.3 fl mean corpuscular volume, 417,000 cells/μL platelets, 23,671 ng/mL D-dimer, 29.6 seconds activated partial thromboplastin time (aPTT), 16.3 seconds prothrombin time, 743 mg/dL fibrinogen, 474 U/L lactate dehydrogenase, and 202.1 mg/dL haptoglobin. A nasopharyngeal reverse transcription polymerase chain reaction test resulted positive for the SARS-CoV-2 virus, and subsequent chest X-ray revealed bilateral, hazy opacities predominantly in a peripheral distribution. Computed tomography (CT) angiogram of the chest did not reveal pulmonary emboli, pneumothorax, effusions, or lobar consolidation. However, it displayed bilateral ground-glass opacities with interstitial consolidation worst in the upper lobes. Corporal aspiration and blood gas analysis revealed a pH of 7.05, P
Differential Diagnosis
The first consideration in the differential diagnosis of priapism is to differentiate between ischemic and nonischemic. Based on the abnormal blood gas results above, this case clearly falls within the ischemic spectrum. Ischemic priapism secondary to CLL-induced hyperleukocytosis was considered. It has been noted that up to 20% of priapism cases in adults are related to hematologic disorders.10 While it is not uncommon to see hyperleukocytosis (total WBC count > 100 × 109/L) in CLL, leukostasis is rare with most reports demonstrating WBC counts > 1000 × 109/L.11 Hematology, vascular surgery, and urology services were consulted and agreed that ischemic priapism was due to microthrombi or pelvic vein thrombosis secondary to COVID-19–associated coagulopathy (CAC) was the most likely etiology.
Treatment
After corporal aspiration, intracorporal phenylephrine was administered. Diluted phenylephrine (100 ug/mL) was injected every 5 to 10 minutes while intermittently aspirating and irrigating multiple sites along the lateral length of the penile shaft. This initial procedure reduced the erection from 100% to 30% rigidity, with repeat blood gas analysis revealing minimal improvement. CT of the abdomen and pelvis with IV contrast revealed no evidence of pelvic thrombi. A second round of phenylephrine injections were administered, resulting in detumescence. The patient was treated with 2 to 3 L/min of oxygen supplementation via nasal cannula, a 5-day course of remdesivir and low-intensity heparin drip. Following the initial low-intensity heparin drip, the patient transitioned to therapeutic enoxaparin and subsequently was discharged on apixaban for a 3-month course. Since discharge, the patient followed up with hematology. He tolerated and completed the anticoagulation regimen without any recurrences of priapism or residual deficits.
Discussion
Recent studies have overwhelmingly analyzed the incidence and presentation of thrombotic complications in critically ill patients with COVID-19. CAC has been postulated to result from endotheliopathy along with immune cell activation and propagation of coagulation. While COVID-19 has been noted to create lung injury through binding angiotensin-converting enzyme 2 receptors expressed on alveolar pneumocytes, it increasingly has been found to affect endothelial cells throughout the body. Recent postmortem analyses have demonstrated direct viral infection of endothelial cells with consequent diffuse endothelial inflammation, as evidenced by viral inclusions, sequestered immune cells, and endothelial apoptosis.12,13 Manifestations of this endotheliopathy have been delineated through various studies.
An early retrospective study in Wuhan, China, illustrated that 36% of the first 99 patients hospitalized with COVID-19 demonstrated an elevated D-dimer, 6% an elevated aPTT, and 5% an elevated prothrombin time.14 Another retrospective study conducted in Wuhan found a 25% incidence of venous thromboembolic complications in critically ill patients with severe COVID-19.15 In the Netherlands, a study reported the incidence of arterial and venous thrombotic complications to be 31% in 184 critically ill patients with COVID-19, with 81% of these cases involving pulmonary emboli.16
To our knowledge, our patient is the seventh reported case of ischemic priapism occurring in the setting of a COVID-19 infection, and the first to have occurred in its moderate form. Ischemic priapism is often a consequence of penile venous outflow obstruction and resultant stasis of hypoxic blood.7 The prothrombotic state induced by CAC has been proposed to cause the obstruction of small emissary veins in the subtunical space and in turn lead to venous stasis, which propagates the formation of ischemic priapism.8 Furthermore, 4 of the previously reported cases shared laboratory data on their patients, and all demonstrated elevated D-dimer and fibrinogen levels, which strengthens this hypothesis.3,5,7,8 CLL presents a potential confounding variable in this case; however, as we have reviewed earlier, the risk of leukostasis at WBC counts < 1000 × 109/L is very low.11 It is also probable that the patient had some level of immune dysregulation secondary to CLL, leading to his prolonged course and slow clearance of the virus.
Conclusions
Although only a handful of CAC cases leading to ischemic priapism have been reported, the true incidence may be much higher. While our case highlights the importance of considering COVID-19 infection in the differential diagnosis of ischemic priapism, more research is needed to understand incidence and definitively establish a causative relationship.
Priapism is a disorder that occurs when the penis maintains a prolonged erection in the absence of appropriate stimulation. The disorder is typically divided into subgroups based on arterial flow: low flow (ischemic) and high flow (nonischemic). Ischemic priapism is the most common form and results from venous congestion due to obstructed outflow and inability of cavernous smooth muscle to contract, resulting in compartment syndrome, tissue hypoxia, hypercapnia, and acidosis.1 Conditions that result in hypercoagulable states and hyperviscosity are associated with ischemic priapism. COVID-19 is well known to cause an acute respiratory illness and systemic inflammatory response and has been increasingly associated with coagulopathy. Studies have shown that 20% to 55% of patients admitted to the hospital for COVID-19 show objective laboratory evidence of a hypercoagulable state.2
To date, there are 6 reported cases of priapism occurring in the setting of COVID-19 with all cases demonstrating the ischemic subtype. The onset of priapism from the beginning of infectious symptoms ranged from 2 days to more than a month. Five of the cases occurred in patients with critical COVID-19 and 1 in the setting of mild disease.3-8 Two critically ill patients did not receive treatment for their ischemic priapism as they were transitioned to expectant management and/or comfort measures.Most were treated with cavernosal blood aspiration and intracavernosal injections of phenylephrine or ethylephrine. Some patients were managed with prophylactic doses of anticoagulation after the identification of priapism; others were transitioned to therapeutic doses. Two patients were followed postdischarge; one patient reported normal nighttime erections with sexual desire 2 weeks postdischarge, and another patient, who underwent a bilateral T-shunt procedure after unsuccessful phenylephrine injections, reported complete erectile dysfunction at 3 months postdischarge.4,7 There was a potentially confounding variable in 2 cases in which propofol infusions were used for sedation management in the setting of mechanical ventilation.6,8 Propofol has been linked to priapism through its blockade of sympathetic activation resulting in persistent relaxation of cavernosal smooth muscle.9 We present a unique case of COVID-19–associated ischemic priapism as our patient had moderate rather than critical COVID-19.
Case Presentation
A 67-year-old male patient presented to the emergency department for a painful erection of 34-hour duration. The patient had been exposed to COVID-19 roughly 2 months prior. Since the exposure, he had experienced headache, nonproductive cough, sore throat, and decreased appetite with weight loss. His medical history included hypertension, thoracic aortic aneurysm, B-cell type chronic lymphocytic leukemia (CLL), and obstructive sleep apnea. Daily outpatient medications included atenolol 100 mg, hydrochlorothiazide 25 mg, and omeprazole 20 mg. The patient stopped tobacco use about 30 years previously. He reported no alcohol consumption or illicit drug use and had no previous episodes of prolonged erection.
The patient was afebrile, hemodynamically stable, and had an oxygen saturation of 92% on room air. Physical examination revealed clear breath sounds and an erect circumcised penis without any lesions, discoloration, or skin necrosis. Laboratory data were remarkable for the following values: 125,660 cells/μL white blood cells (WBCs), 13.82 × 103/ μL neutrophils, 110.58 × 103/μL lymphocytes, 1.26 × 103/μL monocytes, no blasts, 9.4 gm/dL hemoglobin, 100.3 fl mean corpuscular volume, 417,000 cells/μL platelets, 23,671 ng/mL D-dimer, 29.6 seconds activated partial thromboplastin time (aPTT), 16.3 seconds prothrombin time, 743 mg/dL fibrinogen, 474 U/L lactate dehydrogenase, and 202.1 mg/dL haptoglobin. A nasopharyngeal reverse transcription polymerase chain reaction test resulted positive for the SARS-CoV-2 virus, and subsequent chest X-ray revealed bilateral, hazy opacities predominantly in a peripheral distribution. Computed tomography (CT) angiogram of the chest did not reveal pulmonary emboli, pneumothorax, effusions, or lobar consolidation. However, it displayed bilateral ground-glass opacities with interstitial consolidation worst in the upper lobes. Corporal aspiration and blood gas analysis revealed a pH of 7.05, P
Differential Diagnosis
The first consideration in the differential diagnosis of priapism is to differentiate between ischemic and nonischemic. Based on the abnormal blood gas results above, this case clearly falls within the ischemic spectrum. Ischemic priapism secondary to CLL-induced hyperleukocytosis was considered. It has been noted that up to 20% of priapism cases in adults are related to hematologic disorders.10 While it is not uncommon to see hyperleukocytosis (total WBC count > 100 × 109/L) in CLL, leukostasis is rare with most reports demonstrating WBC counts > 1000 × 109/L.11 Hematology, vascular surgery, and urology services were consulted and agreed that ischemic priapism was due to microthrombi or pelvic vein thrombosis secondary to COVID-19–associated coagulopathy (CAC) was the most likely etiology.
Treatment
After corporal aspiration, intracorporal phenylephrine was administered. Diluted phenylephrine (100 ug/mL) was injected every 5 to 10 minutes while intermittently aspirating and irrigating multiple sites along the lateral length of the penile shaft. This initial procedure reduced the erection from 100% to 30% rigidity, with repeat blood gas analysis revealing minimal improvement. CT of the abdomen and pelvis with IV contrast revealed no evidence of pelvic thrombi. A second round of phenylephrine injections were administered, resulting in detumescence. The patient was treated with 2 to 3 L/min of oxygen supplementation via nasal cannula, a 5-day course of remdesivir and low-intensity heparin drip. Following the initial low-intensity heparin drip, the patient transitioned to therapeutic enoxaparin and subsequently was discharged on apixaban for a 3-month course. Since discharge, the patient followed up with hematology. He tolerated and completed the anticoagulation regimen without any recurrences of priapism or residual deficits.
Discussion
Recent studies have overwhelmingly analyzed the incidence and presentation of thrombotic complications in critically ill patients with COVID-19. CAC has been postulated to result from endotheliopathy along with immune cell activation and propagation of coagulation. While COVID-19 has been noted to create lung injury through binding angiotensin-converting enzyme 2 receptors expressed on alveolar pneumocytes, it increasingly has been found to affect endothelial cells throughout the body. Recent postmortem analyses have demonstrated direct viral infection of endothelial cells with consequent diffuse endothelial inflammation, as evidenced by viral inclusions, sequestered immune cells, and endothelial apoptosis.12,13 Manifestations of this endotheliopathy have been delineated through various studies.
An early retrospective study in Wuhan, China, illustrated that 36% of the first 99 patients hospitalized with COVID-19 demonstrated an elevated D-dimer, 6% an elevated aPTT, and 5% an elevated prothrombin time.14 Another retrospective study conducted in Wuhan found a 25% incidence of venous thromboembolic complications in critically ill patients with severe COVID-19.15 In the Netherlands, a study reported the incidence of arterial and venous thrombotic complications to be 31% in 184 critically ill patients with COVID-19, with 81% of these cases involving pulmonary emboli.16
To our knowledge, our patient is the seventh reported case of ischemic priapism occurring in the setting of a COVID-19 infection, and the first to have occurred in its moderate form. Ischemic priapism is often a consequence of penile venous outflow obstruction and resultant stasis of hypoxic blood.7 The prothrombotic state induced by CAC has been proposed to cause the obstruction of small emissary veins in the subtunical space and in turn lead to venous stasis, which propagates the formation of ischemic priapism.8 Furthermore, 4 of the previously reported cases shared laboratory data on their patients, and all demonstrated elevated D-dimer and fibrinogen levels, which strengthens this hypothesis.3,5,7,8 CLL presents a potential confounding variable in this case; however, as we have reviewed earlier, the risk of leukostasis at WBC counts < 1000 × 109/L is very low.11 It is also probable that the patient had some level of immune dysregulation secondary to CLL, leading to his prolonged course and slow clearance of the virus.
Conclusions
Although only a handful of CAC cases leading to ischemic priapism have been reported, the true incidence may be much higher. While our case highlights the importance of considering COVID-19 infection in the differential diagnosis of ischemic priapism, more research is needed to understand incidence and definitively establish a causative relationship.
1. Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med. 2004;1(1):116-120. doi:10.1111/j.1743-6109.2004.10117.x
2. Lee SG, Fralick M, Sholzberg M. Coagulopathy associated with COVID-19. CMAJ. 2020;192(21):E583. doi:10.1503/cmaj.200685
3. Lam G, McCarthy R, Haider R. A peculiar case of priapism: the hypercoagulable state in patients with severe COVID-19 infection. Eur J Case Rep Intern Med. 2020;7(8):001779. doi:10.12890/2020_001779
4. Addar A, Al Fraidi O, Nazer A, Althonayan N, Ghazwani Y. Priapism for 10 days in a patient with SARS-CoV-2 pneumonia: a case report. J Surg Case Rep. 2021;2021(4):rjab020. doi:10.1093/jscr/rjab020
5. Lamamri M, Chebbi A, Mamane J, et al. Priapism in a patient with coronavirus disease 2019 (COVID-19). Am J Emerg Med. 2021;39:251.e5-251.e7. doi:10.1016/j.ajem.2020.06.027
6. Silverman ML, VanDerVeer SJ, Donnelly TJ. Priapism in COVID-19: a thromboembolic complication. Am J Emerg Med. 2021;45:686.e5-686.e6. doi:10.1016/j.ajem.2020.12.072
7. Giuliano AFM, Vulpi M, Passerini F, et al. SARS-CoV-2 infection as a determining factor to the precipitation of ischemic priapism in a young patient with asymptomatic COVID-19. Case Rep Urol. 2021;2021:9936891. doi:10.1155/2021/9936891
8. Carreno BD, Perez CP, Vasquez D, Oyola JA, Suarez O, Bedoya C. Veno-occlusive priapism in COVID-19 disease. Urol Int. 2021;105(9-10):916-919. doi:10.1159/000514421
9. Senthilkumaran S, Shah S, Ganapathysubramanian, Balamurgan N, Thirumalaikolundusubramanian P. Propofol and priapism. Indian J Pharmacol. 2010;42(4):238-239. doi:10.4103/0253-7613.68430
10. Qu M, Lu X, Wang L, Liu Z, Sun Y, Gao X. Priapism secondary to chronic myeloid leukemia treated by a surgical cavernosa-corpus spongiosum shunt: case report. Asian J Urol. 2019;6(4):373-376. doi:10.1016/j.ajur.2018.12.004
11. Singh N, Singh Lubana S, Dabrowski L, Sidhu G. Leukostasis in chronic lymphocytic leukemia. Am J Case Rep. 2020;21:e924798. doi:10.12659/AJCR.924798
12. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417-1418. doi:10.1016/S0140-6736(20)30937-5
13. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033-2040. doi:10.1182/blood.2020006000
14. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi:10.1016/S0140-6736(20)30211-7
15. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421-1424. doi:10.1111/jth.14830
16. Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
1. Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med. 2004;1(1):116-120. doi:10.1111/j.1743-6109.2004.10117.x
2. Lee SG, Fralick M, Sholzberg M. Coagulopathy associated with COVID-19. CMAJ. 2020;192(21):E583. doi:10.1503/cmaj.200685
3. Lam G, McCarthy R, Haider R. A peculiar case of priapism: the hypercoagulable state in patients with severe COVID-19 infection. Eur J Case Rep Intern Med. 2020;7(8):001779. doi:10.12890/2020_001779
4. Addar A, Al Fraidi O, Nazer A, Althonayan N, Ghazwani Y. Priapism for 10 days in a patient with SARS-CoV-2 pneumonia: a case report. J Surg Case Rep. 2021;2021(4):rjab020. doi:10.1093/jscr/rjab020
5. Lamamri M, Chebbi A, Mamane J, et al. Priapism in a patient with coronavirus disease 2019 (COVID-19). Am J Emerg Med. 2021;39:251.e5-251.e7. doi:10.1016/j.ajem.2020.06.027
6. Silverman ML, VanDerVeer SJ, Donnelly TJ. Priapism in COVID-19: a thromboembolic complication. Am J Emerg Med. 2021;45:686.e5-686.e6. doi:10.1016/j.ajem.2020.12.072
7. Giuliano AFM, Vulpi M, Passerini F, et al. SARS-CoV-2 infection as a determining factor to the precipitation of ischemic priapism in a young patient with asymptomatic COVID-19. Case Rep Urol. 2021;2021:9936891. doi:10.1155/2021/9936891
8. Carreno BD, Perez CP, Vasquez D, Oyola JA, Suarez O, Bedoya C. Veno-occlusive priapism in COVID-19 disease. Urol Int. 2021;105(9-10):916-919. doi:10.1159/000514421
9. Senthilkumaran S, Shah S, Ganapathysubramanian, Balamurgan N, Thirumalaikolundusubramanian P. Propofol and priapism. Indian J Pharmacol. 2010;42(4):238-239. doi:10.4103/0253-7613.68430
10. Qu M, Lu X, Wang L, Liu Z, Sun Y, Gao X. Priapism secondary to chronic myeloid leukemia treated by a surgical cavernosa-corpus spongiosum shunt: case report. Asian J Urol. 2019;6(4):373-376. doi:10.1016/j.ajur.2018.12.004
11. Singh N, Singh Lubana S, Dabrowski L, Sidhu G. Leukostasis in chronic lymphocytic leukemia. Am J Case Rep. 2020;21:e924798. doi:10.12659/AJCR.924798
12. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417-1418. doi:10.1016/S0140-6736(20)30937-5
13. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033-2040. doi:10.1182/blood.2020006000
14. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi:10.1016/S0140-6736(20)30211-7
15. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421-1424. doi:10.1111/jth.14830
16. Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
Cancer drug significantly cuts risk for COVID-19 death
, an interim analysis of a phase 3 placebo-controlled trial found.
Sabizabulin treatment consistently and significantly reduced deaths across patient subgroups “regardless of standard of care treatment received, baseline World Health Organization scores, age, comorbidities, vaccination status, COVID-19 variant, or geography,” study investigator Mitchell Steiner, MD, chairman, president, and CEO of Veru, said in a news release.
The company has submitted an emergency use authorization request to the U.S. Food and Drug Administration to use sabizabulin to treat COVID-19.
The analysis was published online in NEJM Evidence.
Sabizabulin, originally developed to treat metastatic castration-resistant prostate cancer, is a novel, investigational, oral microtubule disruptor with dual antiviral and anti-inflammatory activities. Given the drug’s mechanism, researchers at Veru thought that sabizabulin could help treat lung inflammation in patients with COVID-19 as well.
Findings of the interim analysis are based on 150 adults hospitalized with moderate to severe COVID-19 at high risk for acute respiratory distress syndrome and death. The patients were randomly allocated to receive 9 mg oral sabizabulin (n = 98) or placebo (n = 52) once daily for up to 21 days.
Overall, the mortality rate was 20.2% in the sabizabulin group vs. 45.1% in the placebo group. Compared with placebo, treatment with sabizabulin led to a 24.9–percentage point absolute reduction and a 55.2% relative reduction in death (odds ratio, 3.23; P = .0042).
The key secondary endpoint of mortality through day 29 also favored sabizabulin over placebo, with a mortality rate of 17% vs. 35.3%. In this scenario, treatment with sabizabulin resulted in an absolute reduction in deaths of 18.3 percentage points and a relative reduction of 51.8%.
Sabizabulin led to a significant 43% relative reduction in ICU days, a 49% relative reduction in days on mechanical ventilation, and a 26% relative reduction in days in the hospital, compared with placebo.
Adverse and serious adverse events were also lower in the sabizabulin group (61.5%) than the placebo group (78.3%).
The data are “pretty impressive and in a group of patients that we really have limited things to offer,” Aaron Glatt, MD, a spokesperson for the Infectious Diseases Society of America and chief of infectious diseases and hospital epidemiologist at Mount Sinai South Nassau in Oceanside, N.Y., said in an interview. “This is an interim analysis and obviously we’d like to see more data, but it certainly is something that is novel and quite interesting.”
David Boulware, MD, MPH, an infectious disease expert at the University of Minnesota, Minneapolis, told the New York Times that the large number of deaths in the placebo group seemed “rather high” and that the final analysis might reveal a more modest benefit for sabizabulin.
“I would be skeptical” that the reduced risk for death remains 55%, he noted.
The study was funded by Veru Pharmaceuticals. Several authors are employed by the company or have financial relationships with the company.
A version of this article first appeared on Medscape.com.
, an interim analysis of a phase 3 placebo-controlled trial found.
Sabizabulin treatment consistently and significantly reduced deaths across patient subgroups “regardless of standard of care treatment received, baseline World Health Organization scores, age, comorbidities, vaccination status, COVID-19 variant, or geography,” study investigator Mitchell Steiner, MD, chairman, president, and CEO of Veru, said in a news release.
The company has submitted an emergency use authorization request to the U.S. Food and Drug Administration to use sabizabulin to treat COVID-19.
The analysis was published online in NEJM Evidence.
Sabizabulin, originally developed to treat metastatic castration-resistant prostate cancer, is a novel, investigational, oral microtubule disruptor with dual antiviral and anti-inflammatory activities. Given the drug’s mechanism, researchers at Veru thought that sabizabulin could help treat lung inflammation in patients with COVID-19 as well.
Findings of the interim analysis are based on 150 adults hospitalized with moderate to severe COVID-19 at high risk for acute respiratory distress syndrome and death. The patients were randomly allocated to receive 9 mg oral sabizabulin (n = 98) or placebo (n = 52) once daily for up to 21 days.
Overall, the mortality rate was 20.2% in the sabizabulin group vs. 45.1% in the placebo group. Compared with placebo, treatment with sabizabulin led to a 24.9–percentage point absolute reduction and a 55.2% relative reduction in death (odds ratio, 3.23; P = .0042).
The key secondary endpoint of mortality through day 29 also favored sabizabulin over placebo, with a mortality rate of 17% vs. 35.3%. In this scenario, treatment with sabizabulin resulted in an absolute reduction in deaths of 18.3 percentage points and a relative reduction of 51.8%.
Sabizabulin led to a significant 43% relative reduction in ICU days, a 49% relative reduction in days on mechanical ventilation, and a 26% relative reduction in days in the hospital, compared with placebo.
Adverse and serious adverse events were also lower in the sabizabulin group (61.5%) than the placebo group (78.3%).
The data are “pretty impressive and in a group of patients that we really have limited things to offer,” Aaron Glatt, MD, a spokesperson for the Infectious Diseases Society of America and chief of infectious diseases and hospital epidemiologist at Mount Sinai South Nassau in Oceanside, N.Y., said in an interview. “This is an interim analysis and obviously we’d like to see more data, but it certainly is something that is novel and quite interesting.”
David Boulware, MD, MPH, an infectious disease expert at the University of Minnesota, Minneapolis, told the New York Times that the large number of deaths in the placebo group seemed “rather high” and that the final analysis might reveal a more modest benefit for sabizabulin.
“I would be skeptical” that the reduced risk for death remains 55%, he noted.
The study was funded by Veru Pharmaceuticals. Several authors are employed by the company or have financial relationships with the company.
A version of this article first appeared on Medscape.com.
, an interim analysis of a phase 3 placebo-controlled trial found.
Sabizabulin treatment consistently and significantly reduced deaths across patient subgroups “regardless of standard of care treatment received, baseline World Health Organization scores, age, comorbidities, vaccination status, COVID-19 variant, or geography,” study investigator Mitchell Steiner, MD, chairman, president, and CEO of Veru, said in a news release.
The company has submitted an emergency use authorization request to the U.S. Food and Drug Administration to use sabizabulin to treat COVID-19.
The analysis was published online in NEJM Evidence.
Sabizabulin, originally developed to treat metastatic castration-resistant prostate cancer, is a novel, investigational, oral microtubule disruptor with dual antiviral and anti-inflammatory activities. Given the drug’s mechanism, researchers at Veru thought that sabizabulin could help treat lung inflammation in patients with COVID-19 as well.
Findings of the interim analysis are based on 150 adults hospitalized with moderate to severe COVID-19 at high risk for acute respiratory distress syndrome and death. The patients were randomly allocated to receive 9 mg oral sabizabulin (n = 98) or placebo (n = 52) once daily for up to 21 days.
Overall, the mortality rate was 20.2% in the sabizabulin group vs. 45.1% in the placebo group. Compared with placebo, treatment with sabizabulin led to a 24.9–percentage point absolute reduction and a 55.2% relative reduction in death (odds ratio, 3.23; P = .0042).
The key secondary endpoint of mortality through day 29 also favored sabizabulin over placebo, with a mortality rate of 17% vs. 35.3%. In this scenario, treatment with sabizabulin resulted in an absolute reduction in deaths of 18.3 percentage points and a relative reduction of 51.8%.
Sabizabulin led to a significant 43% relative reduction in ICU days, a 49% relative reduction in days on mechanical ventilation, and a 26% relative reduction in days in the hospital, compared with placebo.
Adverse and serious adverse events were also lower in the sabizabulin group (61.5%) than the placebo group (78.3%).
The data are “pretty impressive and in a group of patients that we really have limited things to offer,” Aaron Glatt, MD, a spokesperson for the Infectious Diseases Society of America and chief of infectious diseases and hospital epidemiologist at Mount Sinai South Nassau in Oceanside, N.Y., said in an interview. “This is an interim analysis and obviously we’d like to see more data, but it certainly is something that is novel and quite interesting.”
David Boulware, MD, MPH, an infectious disease expert at the University of Minnesota, Minneapolis, told the New York Times that the large number of deaths in the placebo group seemed “rather high” and that the final analysis might reveal a more modest benefit for sabizabulin.
“I would be skeptical” that the reduced risk for death remains 55%, he noted.
The study was funded by Veru Pharmaceuticals. Several authors are employed by the company or have financial relationships with the company.
A version of this article first appeared on Medscape.com.
FROM NEJM EVIDENCE
Shift schedule today could worsen that stroke tomorrow
Body clocks and the shifting risks of stroke
Health care professionals, we’re sure, are no strangers to rotating shifts. And, as practitioners of the shiftly arts, you should know new research shows that working those kinds of hours can have lasting effects on your health. And it’s all based on your sleep-wake cycle.
In a study published in Neurobiology of Sleep and Circadian Rhythms, investigators at Texas A&M University looked at the effects of working these kinds of shifts for a long period of time and then returning to a regular 24-hour cycle later in life. The study piggybacks on a previous study, which showed that rats on shift schedules had more severe stroke outcomes than those who were on a 24-hour cycle.
The current study demonstrates that working rotating shifts does have a lasting effect, by way of messing with the sleep-wake cycle. Based on the research, the rats that performed those kinds of shifts never got back to a normal schedule. When strokes occurred, outcomes were much worse, and the females had a higher mortality rate and more severe functional deficits than the males.
Now for the “good” news: Even if you’re among those who haven’t worked a rotating shift, you may not be safe either.
People who have regular working hours have a tendency to take work home and stay up late, especially with so many moving to a remote-work model. And if you’re staying up late on the weekends you’re producing what lead author David J. Earnest, PhD, called “social jet lag,” which messes with your circadian rhythm to wind you down for sleep. All of these things can lead to the same kind of effects that working rotating shifts has on your health, he said in a written statement.
How do you combat this? Dr. Earnest recommended creating a sleep schedule and setting regular mealtimes. Also ease up on high-fat foods, drinking, and smoking. The connection between your brain and gut also could play a part in how severe a stroke can be.
So continue to work hard, but not too hard.
Got 3 minutes? You got time for culture
Much like a Krabby Patty, art is good for your soul. Seriously, staring at a 500-year-old painting may not seem like much, but research has proven time and again that going to a museum and looking at paintings by long-dead artists you probably know better as pizza-eating superhero turtles improves mood, stress, and well-being.
A couple of years ago, however, museums and art galleries ran into a big virus-shaped problem. You may have heard of it. All of a sudden it became a very bad idea for people to gather together in one building and huddle around the Mona Lisa, which, by the way, is a lot smaller in person than you might expect. But, rather than sit around with a bunch of priceless art for an indeterminate amount of time, museums brought their exhibits to the Internet so that people from all over the world could see great works from their couches.
This is absolutely a good thing for public access, but do these virtual art exhibits provide the same health benefits as going to a museum in person? That’s what a group of European researchers aimed to find out, and in a study published in Frontiers of Psychology, that’s exactly what they found.
Their directive to the 84 study participants was simple: Take a well-being survey, engage with either of a pair of online exhibits (a Monet painting and a display of Japanese culinary traditions) for just 3 minutes, then take another well-being assessment. The results were quite clear: Even just a couple of minutes of viewing art online improved all the well-being categories on the survey, such as lowering anxiety, negative mood, and loneliness, as well as increasing subjective well-being. Also, the more beautiful or meaningful a person found the art, the more their mood and well-being improved.
The researchers noted that these results could help access in places where access to art is limited, such as waiting rooms, hospitals, and rural areas. Let’s just hope it sticks to that, and that big businesses don’t take notice. Just imagine them plastering ads with classic Renaissance artworks. After all, art makes you feel good, and you know what else feels good on a hot summer day? An ice-cold Coca-Cola! By the way, we’re taking offers, advertising agencies. The LOTME staff can absolutely be bought.
Appetite for etymology
Today on “It’s a Thing,” we examine various states of hunger and what they should be called. Our first guest is that historically hungry royal person, King Henry VIII of England. Your majesty, have you ever been “hangry?”
KH8: First, let me thank you for inviting me on the show, Maurice. I’m a huge fan. A recent study done in the United Kingdom and Austria showed that “hunger is associated with greater levels of anger and irritability, as well as lower levels of pleasure,” according to a Eurekalert statement. So, yes, I have been “hangry.”
Maurice: Now to our next guest. Martha Stewart, can you add anything about that study?
Martha: Happy to, Maurice. The 64 participants used a smartphone app to record their hunger levels and emotional states five times a day for 21 days. It’s the first time that “hanger” was studied outside a lab, and it showed that hunger “was associated with 37% of the variance in irritability, 34% of the variance in anger, and 38% of the variance in pleasure recorded by the participants,” the investigators said in that statement.
Maurice: It’s official, then. Hangry is a thing, and we don’t need to put it in quotes anymore. Now let’s meet our third and final guest, Betty Crocker. Betty, I’m told you have a study to plug.
Betty: That’s right, Mo. Researchers at Tel Aviv University looked at survey data from almost 3,000 men and women and found that men ate 17% more food during the warmer months (March to September) than they did the rest of the year. Among women, however, caloric intake did not change.
KH8: I saw that study. Didn’t they put 27 people out in the sun and then take blood samples?
Betty: Indeed they did, Hank. After 25 minutes of sun exposure, the 13 men felt hungrier than before, but the 14 women did not. The men also had higher levels of ghrelin, an appetite-stimulating hormone, than the women.
Maurice: To sum all this up, then, we’ve got angry and hungry officially combining to make hangry, and now it looks like the sun is causing hunger in men, which makes them … sungry?
Martha: It’s a thing.
Chicken cutlets with a side of COVID
You stopped at the drive through at McDonald’s on the way home from work, and while you’re looking for something sweet in the refrigerator for dessert, you see that chicken breast that expires today.
Freezing meat that’s about to expire might be your go-to so it doesn’t go to waste, but it’s been found that SARS-CoV-2 can live in meat that’s been in the refrigerator or freezer for more than a month.
Researchers exposed chicken, beef, pork, and salmon to surrogate viruses that are similar to COVID but not as harmful and stored them in freezers at –4° F and in the refrigerator at 39.2° F. “We even found that the viruses could be cultured after [being frozen for] that length of time,” lead author Emily Bailey, PhD, of Campbell University in Buies Creek, N.C., said in Study Finds.
The team began its research after hearing of COVID-19 outbreaks where there were no reports of community transmission, such as in Southeast Asia. Tracing eventually led to packaged meats as the culprits in those cases. SARS-CoV-2 is able to replicate in the gut, as well as the respiratory tract, so it could affect the gut before respiratory symptoms start. It is crucial to ensure cross contamination doesn’t occur, and inadequate sanitation prior to packaging needs to be addressed, the investigators said.
Honestly, we didn’t think anything could survive in a freezer for that long, but SARS-CoV-2 is a fighter.
Body clocks and the shifting risks of stroke
Health care professionals, we’re sure, are no strangers to rotating shifts. And, as practitioners of the shiftly arts, you should know new research shows that working those kinds of hours can have lasting effects on your health. And it’s all based on your sleep-wake cycle.
In a study published in Neurobiology of Sleep and Circadian Rhythms, investigators at Texas A&M University looked at the effects of working these kinds of shifts for a long period of time and then returning to a regular 24-hour cycle later in life. The study piggybacks on a previous study, which showed that rats on shift schedules had more severe stroke outcomes than those who were on a 24-hour cycle.
The current study demonstrates that working rotating shifts does have a lasting effect, by way of messing with the sleep-wake cycle. Based on the research, the rats that performed those kinds of shifts never got back to a normal schedule. When strokes occurred, outcomes were much worse, and the females had a higher mortality rate and more severe functional deficits than the males.
Now for the “good” news: Even if you’re among those who haven’t worked a rotating shift, you may not be safe either.
People who have regular working hours have a tendency to take work home and stay up late, especially with so many moving to a remote-work model. And if you’re staying up late on the weekends you’re producing what lead author David J. Earnest, PhD, called “social jet lag,” which messes with your circadian rhythm to wind you down for sleep. All of these things can lead to the same kind of effects that working rotating shifts has on your health, he said in a written statement.
How do you combat this? Dr. Earnest recommended creating a sleep schedule and setting regular mealtimes. Also ease up on high-fat foods, drinking, and smoking. The connection between your brain and gut also could play a part in how severe a stroke can be.
So continue to work hard, but not too hard.
Got 3 minutes? You got time for culture
Much like a Krabby Patty, art is good for your soul. Seriously, staring at a 500-year-old painting may not seem like much, but research has proven time and again that going to a museum and looking at paintings by long-dead artists you probably know better as pizza-eating superhero turtles improves mood, stress, and well-being.
A couple of years ago, however, museums and art galleries ran into a big virus-shaped problem. You may have heard of it. All of a sudden it became a very bad idea for people to gather together in one building and huddle around the Mona Lisa, which, by the way, is a lot smaller in person than you might expect. But, rather than sit around with a bunch of priceless art for an indeterminate amount of time, museums brought their exhibits to the Internet so that people from all over the world could see great works from their couches.
This is absolutely a good thing for public access, but do these virtual art exhibits provide the same health benefits as going to a museum in person? That’s what a group of European researchers aimed to find out, and in a study published in Frontiers of Psychology, that’s exactly what they found.
Their directive to the 84 study participants was simple: Take a well-being survey, engage with either of a pair of online exhibits (a Monet painting and a display of Japanese culinary traditions) for just 3 minutes, then take another well-being assessment. The results were quite clear: Even just a couple of minutes of viewing art online improved all the well-being categories on the survey, such as lowering anxiety, negative mood, and loneliness, as well as increasing subjective well-being. Also, the more beautiful or meaningful a person found the art, the more their mood and well-being improved.
The researchers noted that these results could help access in places where access to art is limited, such as waiting rooms, hospitals, and rural areas. Let’s just hope it sticks to that, and that big businesses don’t take notice. Just imagine them plastering ads with classic Renaissance artworks. After all, art makes you feel good, and you know what else feels good on a hot summer day? An ice-cold Coca-Cola! By the way, we’re taking offers, advertising agencies. The LOTME staff can absolutely be bought.
Appetite for etymology
Today on “It’s a Thing,” we examine various states of hunger and what they should be called. Our first guest is that historically hungry royal person, King Henry VIII of England. Your majesty, have you ever been “hangry?”
KH8: First, let me thank you for inviting me on the show, Maurice. I’m a huge fan. A recent study done in the United Kingdom and Austria showed that “hunger is associated with greater levels of anger and irritability, as well as lower levels of pleasure,” according to a Eurekalert statement. So, yes, I have been “hangry.”
Maurice: Now to our next guest. Martha Stewart, can you add anything about that study?
Martha: Happy to, Maurice. The 64 participants used a smartphone app to record their hunger levels and emotional states five times a day for 21 days. It’s the first time that “hanger” was studied outside a lab, and it showed that hunger “was associated with 37% of the variance in irritability, 34% of the variance in anger, and 38% of the variance in pleasure recorded by the participants,” the investigators said in that statement.
Maurice: It’s official, then. Hangry is a thing, and we don’t need to put it in quotes anymore. Now let’s meet our third and final guest, Betty Crocker. Betty, I’m told you have a study to plug.
Betty: That’s right, Mo. Researchers at Tel Aviv University looked at survey data from almost 3,000 men and women and found that men ate 17% more food during the warmer months (March to September) than they did the rest of the year. Among women, however, caloric intake did not change.
KH8: I saw that study. Didn’t they put 27 people out in the sun and then take blood samples?
Betty: Indeed they did, Hank. After 25 minutes of sun exposure, the 13 men felt hungrier than before, but the 14 women did not. The men also had higher levels of ghrelin, an appetite-stimulating hormone, than the women.
Maurice: To sum all this up, then, we’ve got angry and hungry officially combining to make hangry, and now it looks like the sun is causing hunger in men, which makes them … sungry?
Martha: It’s a thing.
Chicken cutlets with a side of COVID
You stopped at the drive through at McDonald’s on the way home from work, and while you’re looking for something sweet in the refrigerator for dessert, you see that chicken breast that expires today.
Freezing meat that’s about to expire might be your go-to so it doesn’t go to waste, but it’s been found that SARS-CoV-2 can live in meat that’s been in the refrigerator or freezer for more than a month.
Researchers exposed chicken, beef, pork, and salmon to surrogate viruses that are similar to COVID but not as harmful and stored them in freezers at –4° F and in the refrigerator at 39.2° F. “We even found that the viruses could be cultured after [being frozen for] that length of time,” lead author Emily Bailey, PhD, of Campbell University in Buies Creek, N.C., said in Study Finds.
The team began its research after hearing of COVID-19 outbreaks where there were no reports of community transmission, such as in Southeast Asia. Tracing eventually led to packaged meats as the culprits in those cases. SARS-CoV-2 is able to replicate in the gut, as well as the respiratory tract, so it could affect the gut before respiratory symptoms start. It is crucial to ensure cross contamination doesn’t occur, and inadequate sanitation prior to packaging needs to be addressed, the investigators said.
Honestly, we didn’t think anything could survive in a freezer for that long, but SARS-CoV-2 is a fighter.
Body clocks and the shifting risks of stroke
Health care professionals, we’re sure, are no strangers to rotating shifts. And, as practitioners of the shiftly arts, you should know new research shows that working those kinds of hours can have lasting effects on your health. And it’s all based on your sleep-wake cycle.
In a study published in Neurobiology of Sleep and Circadian Rhythms, investigators at Texas A&M University looked at the effects of working these kinds of shifts for a long period of time and then returning to a regular 24-hour cycle later in life. The study piggybacks on a previous study, which showed that rats on shift schedules had more severe stroke outcomes than those who were on a 24-hour cycle.
The current study demonstrates that working rotating shifts does have a lasting effect, by way of messing with the sleep-wake cycle. Based on the research, the rats that performed those kinds of shifts never got back to a normal schedule. When strokes occurred, outcomes were much worse, and the females had a higher mortality rate and more severe functional deficits than the males.
Now for the “good” news: Even if you’re among those who haven’t worked a rotating shift, you may not be safe either.
People who have regular working hours have a tendency to take work home and stay up late, especially with so many moving to a remote-work model. And if you’re staying up late on the weekends you’re producing what lead author David J. Earnest, PhD, called “social jet lag,” which messes with your circadian rhythm to wind you down for sleep. All of these things can lead to the same kind of effects that working rotating shifts has on your health, he said in a written statement.
How do you combat this? Dr. Earnest recommended creating a sleep schedule and setting regular mealtimes. Also ease up on high-fat foods, drinking, and smoking. The connection between your brain and gut also could play a part in how severe a stroke can be.
So continue to work hard, but not too hard.
Got 3 minutes? You got time for culture
Much like a Krabby Patty, art is good for your soul. Seriously, staring at a 500-year-old painting may not seem like much, but research has proven time and again that going to a museum and looking at paintings by long-dead artists you probably know better as pizza-eating superhero turtles improves mood, stress, and well-being.
A couple of years ago, however, museums and art galleries ran into a big virus-shaped problem. You may have heard of it. All of a sudden it became a very bad idea for people to gather together in one building and huddle around the Mona Lisa, which, by the way, is a lot smaller in person than you might expect. But, rather than sit around with a bunch of priceless art for an indeterminate amount of time, museums brought their exhibits to the Internet so that people from all over the world could see great works from their couches.
This is absolutely a good thing for public access, but do these virtual art exhibits provide the same health benefits as going to a museum in person? That’s what a group of European researchers aimed to find out, and in a study published in Frontiers of Psychology, that’s exactly what they found.
Their directive to the 84 study participants was simple: Take a well-being survey, engage with either of a pair of online exhibits (a Monet painting and a display of Japanese culinary traditions) for just 3 minutes, then take another well-being assessment. The results were quite clear: Even just a couple of minutes of viewing art online improved all the well-being categories on the survey, such as lowering anxiety, negative mood, and loneliness, as well as increasing subjective well-being. Also, the more beautiful or meaningful a person found the art, the more their mood and well-being improved.
The researchers noted that these results could help access in places where access to art is limited, such as waiting rooms, hospitals, and rural areas. Let’s just hope it sticks to that, and that big businesses don’t take notice. Just imagine them plastering ads with classic Renaissance artworks. After all, art makes you feel good, and you know what else feels good on a hot summer day? An ice-cold Coca-Cola! By the way, we’re taking offers, advertising agencies. The LOTME staff can absolutely be bought.
Appetite for etymology
Today on “It’s a Thing,” we examine various states of hunger and what they should be called. Our first guest is that historically hungry royal person, King Henry VIII of England. Your majesty, have you ever been “hangry?”
KH8: First, let me thank you for inviting me on the show, Maurice. I’m a huge fan. A recent study done in the United Kingdom and Austria showed that “hunger is associated with greater levels of anger and irritability, as well as lower levels of pleasure,” according to a Eurekalert statement. So, yes, I have been “hangry.”
Maurice: Now to our next guest. Martha Stewart, can you add anything about that study?
Martha: Happy to, Maurice. The 64 participants used a smartphone app to record their hunger levels and emotional states five times a day for 21 days. It’s the first time that “hanger” was studied outside a lab, and it showed that hunger “was associated with 37% of the variance in irritability, 34% of the variance in anger, and 38% of the variance in pleasure recorded by the participants,” the investigators said in that statement.
Maurice: It’s official, then. Hangry is a thing, and we don’t need to put it in quotes anymore. Now let’s meet our third and final guest, Betty Crocker. Betty, I’m told you have a study to plug.
Betty: That’s right, Mo. Researchers at Tel Aviv University looked at survey data from almost 3,000 men and women and found that men ate 17% more food during the warmer months (March to September) than they did the rest of the year. Among women, however, caloric intake did not change.
KH8: I saw that study. Didn’t they put 27 people out in the sun and then take blood samples?
Betty: Indeed they did, Hank. After 25 minutes of sun exposure, the 13 men felt hungrier than before, but the 14 women did not. The men also had higher levels of ghrelin, an appetite-stimulating hormone, than the women.
Maurice: To sum all this up, then, we’ve got angry and hungry officially combining to make hangry, and now it looks like the sun is causing hunger in men, which makes them … sungry?
Martha: It’s a thing.
Chicken cutlets with a side of COVID
You stopped at the drive through at McDonald’s on the way home from work, and while you’re looking for something sweet in the refrigerator for dessert, you see that chicken breast that expires today.
Freezing meat that’s about to expire might be your go-to so it doesn’t go to waste, but it’s been found that SARS-CoV-2 can live in meat that’s been in the refrigerator or freezer for more than a month.
Researchers exposed chicken, beef, pork, and salmon to surrogate viruses that are similar to COVID but not as harmful and stored them in freezers at –4° F and in the refrigerator at 39.2° F. “We even found that the viruses could be cultured after [being frozen for] that length of time,” lead author Emily Bailey, PhD, of Campbell University in Buies Creek, N.C., said in Study Finds.
The team began its research after hearing of COVID-19 outbreaks where there were no reports of community transmission, such as in Southeast Asia. Tracing eventually led to packaged meats as the culprits in those cases. SARS-CoV-2 is able to replicate in the gut, as well as the respiratory tract, so it could affect the gut before respiratory symptoms start. It is crucial to ensure cross contamination doesn’t occur, and inadequate sanitation prior to packaging needs to be addressed, the investigators said.
Honestly, we didn’t think anything could survive in a freezer for that long, but SARS-CoV-2 is a fighter.
FDA grants emergency authorization for Novavax COVID vaccine
on July 13.
The vaccine is authorized for adults only. Should the Centers for Disease Control and Prevention follow suit and approve its use, Novavax would join Moderna, Pfizer and Johnson & Johnson on the U.S. market. A CDC panel of advisors is expected to consider the new entry on July 19.
The Novavax vaccine is only for those who have not yet been vaccinated at all.
“Today’s authorization offers adults in the United States who have not yet received a COVID-19 vaccine another option that meets the FDA’s rigorous standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization,” FDA Commissioner Robert Califf, MD, said in a statement. “COVID-19 vaccines remain the best preventive measure against severe disease caused by COVID-19 and I encourage anyone who is eligible for, but has not yet received a COVID-19 vaccine, to consider doing so.”
The Novavax vaccine is protein-based, making it different than mRNA vaccines from Pfizer and Moderna. It contains harmless elements of actual coronavirus spike protein and an ingredient known as a adjuvant that enhances the patient’s immune response.
Clinical trials found the vaccine to be 90.4% effective in preventing mild, moderate or severe COVID-19. Only 17 patients out of 17,200 developed COVID-19 after receiving both doses.
The FDA said, however, that Novavax’s vaccine did show evidence of increased risk of myocarditis – inflammation of the heart – and pericarditis, inflammation of tissue surrounding the heart. In most people both disorders began within 10 days.
A version of this article first appeared on WebMD.com.
on July 13.
The vaccine is authorized for adults only. Should the Centers for Disease Control and Prevention follow suit and approve its use, Novavax would join Moderna, Pfizer and Johnson & Johnson on the U.S. market. A CDC panel of advisors is expected to consider the new entry on July 19.
The Novavax vaccine is only for those who have not yet been vaccinated at all.
“Today’s authorization offers adults in the United States who have not yet received a COVID-19 vaccine another option that meets the FDA’s rigorous standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization,” FDA Commissioner Robert Califf, MD, said in a statement. “COVID-19 vaccines remain the best preventive measure against severe disease caused by COVID-19 and I encourage anyone who is eligible for, but has not yet received a COVID-19 vaccine, to consider doing so.”
The Novavax vaccine is protein-based, making it different than mRNA vaccines from Pfizer and Moderna. It contains harmless elements of actual coronavirus spike protein and an ingredient known as a adjuvant that enhances the patient’s immune response.
Clinical trials found the vaccine to be 90.4% effective in preventing mild, moderate or severe COVID-19. Only 17 patients out of 17,200 developed COVID-19 after receiving both doses.
The FDA said, however, that Novavax’s vaccine did show evidence of increased risk of myocarditis – inflammation of the heart – and pericarditis, inflammation of tissue surrounding the heart. In most people both disorders began within 10 days.
A version of this article first appeared on WebMD.com.
on July 13.
The vaccine is authorized for adults only. Should the Centers for Disease Control and Prevention follow suit and approve its use, Novavax would join Moderna, Pfizer and Johnson & Johnson on the U.S. market. A CDC panel of advisors is expected to consider the new entry on July 19.
The Novavax vaccine is only for those who have not yet been vaccinated at all.
“Today’s authorization offers adults in the United States who have not yet received a COVID-19 vaccine another option that meets the FDA’s rigorous standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization,” FDA Commissioner Robert Califf, MD, said in a statement. “COVID-19 vaccines remain the best preventive measure against severe disease caused by COVID-19 and I encourage anyone who is eligible for, but has not yet received a COVID-19 vaccine, to consider doing so.”
The Novavax vaccine is protein-based, making it different than mRNA vaccines from Pfizer and Moderna. It contains harmless elements of actual coronavirus spike protein and an ingredient known as a adjuvant that enhances the patient’s immune response.
Clinical trials found the vaccine to be 90.4% effective in preventing mild, moderate or severe COVID-19. Only 17 patients out of 17,200 developed COVID-19 after receiving both doses.
The FDA said, however, that Novavax’s vaccine did show evidence of increased risk of myocarditis – inflammation of the heart – and pericarditis, inflammation of tissue surrounding the heart. In most people both disorders began within 10 days.
A version of this article first appeared on WebMD.com.
Children and COVID: Vaccination a harder sell in the summer
The COVID-19 vaccination effort in the youngest children has begun much more slowly than the most recent rollout for older children, according to the Centers for Disease Control and Prevention.
in early November of 2021, based on CDC data last updated on July 7.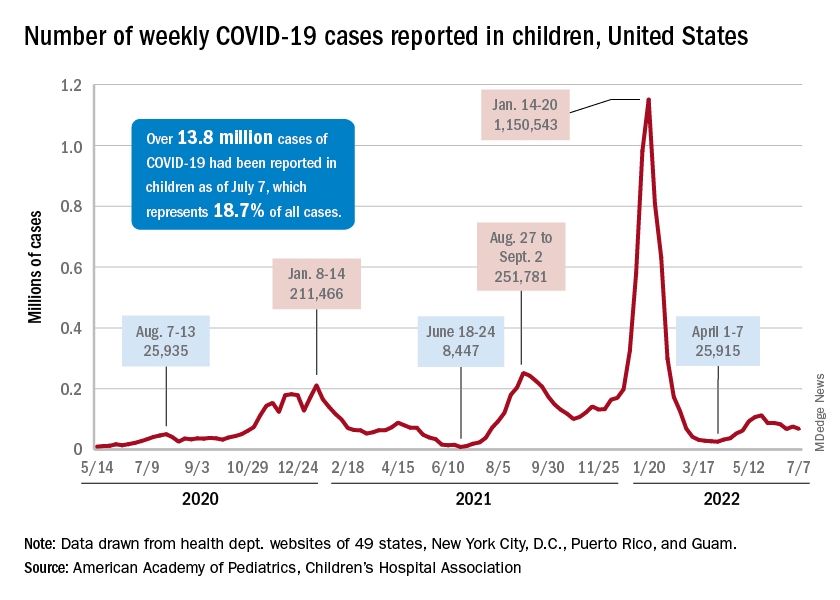
That approval, of course, came between the Delta and Omicron surges, when awareness was higher. The low initial uptake among those under age 5, however, was not unexpected by the Biden administration. “That number in and of itself is very much in line with our expectation, and we’re eager to continue working closely with partners to build on this start,” a senior administration official told ABC News.
With approval of the vaccine occurring after the school year was over, parents’ thoughts have been focused more on vacations and less on vaccinations. “Even before these vaccines officially became available, this was going to be a different rollout; it was going to take more time,” the official explained.
Incidence measures continue on different paths
New COVID-19 cases dropped during the latest reporting week (July 1-7), returning to the downward trend that began in late May and then stopped for 1 week (June 24-30), when cases were up by 12.4%, according to the American Academy of Pediatrics and the Children’s Hospital Association.
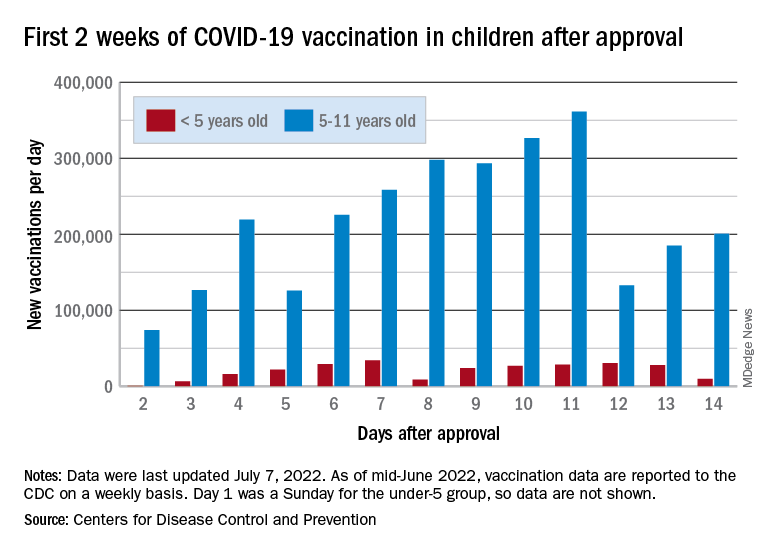
Children also represent a smaller share of cases, probably because of underreporting. “There has been a notable decline in the portion of reported weekly COVID-19 cases that are children,” the two groups said in their weekly COVID report. Although “cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases.”
Other measures, however, have been rising slowly but steadily since the spring. New admissions of patients aged 0-17 years with confirmed COVID, which were down to 0.13 per 100,000 population in early April, had climbed to 0.39 per 100,000 by July 7, the CDC said on its COVID Data Tracker.
Emergency department visits continue to show the same upward trend, despite a small decline in early June. A COVID diagnosis was involved in just 0.5% of ED visits in children aged 0-11 years on March 26, but by July 6 the rate was 4.7%. Increases were not as high among older children: From 0.3% on March 26 to 2.5% on July 6 for those aged 12-15 and from 0.3% to 2.4% for 16- and 17-year-olds, according to the CDC.
The COVID-19 vaccination effort in the youngest children has begun much more slowly than the most recent rollout for older children, according to the Centers for Disease Control and Prevention.
in early November of 2021, based on CDC data last updated on July 7.
That approval, of course, came between the Delta and Omicron surges, when awareness was higher. The low initial uptake among those under age 5, however, was not unexpected by the Biden administration. “That number in and of itself is very much in line with our expectation, and we’re eager to continue working closely with partners to build on this start,” a senior administration official told ABC News.
With approval of the vaccine occurring after the school year was over, parents’ thoughts have been focused more on vacations and less on vaccinations. “Even before these vaccines officially became available, this was going to be a different rollout; it was going to take more time,” the official explained.
Incidence measures continue on different paths
New COVID-19 cases dropped during the latest reporting week (July 1-7), returning to the downward trend that began in late May and then stopped for 1 week (June 24-30), when cases were up by 12.4%, according to the American Academy of Pediatrics and the Children’s Hospital Association.

Children also represent a smaller share of cases, probably because of underreporting. “There has been a notable decline in the portion of reported weekly COVID-19 cases that are children,” the two groups said in their weekly COVID report. Although “cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases.”
Other measures, however, have been rising slowly but steadily since the spring. New admissions of patients aged 0-17 years with confirmed COVID, which were down to 0.13 per 100,000 population in early April, had climbed to 0.39 per 100,000 by July 7, the CDC said on its COVID Data Tracker.
Emergency department visits continue to show the same upward trend, despite a small decline in early June. A COVID diagnosis was involved in just 0.5% of ED visits in children aged 0-11 years on March 26, but by July 6 the rate was 4.7%. Increases were not as high among older children: From 0.3% on March 26 to 2.5% on July 6 for those aged 12-15 and from 0.3% to 2.4% for 16- and 17-year-olds, according to the CDC.
The COVID-19 vaccination effort in the youngest children has begun much more slowly than the most recent rollout for older children, according to the Centers for Disease Control and Prevention.
in early November of 2021, based on CDC data last updated on July 7.
That approval, of course, came between the Delta and Omicron surges, when awareness was higher. The low initial uptake among those under age 5, however, was not unexpected by the Biden administration. “That number in and of itself is very much in line with our expectation, and we’re eager to continue working closely with partners to build on this start,” a senior administration official told ABC News.
With approval of the vaccine occurring after the school year was over, parents’ thoughts have been focused more on vacations and less on vaccinations. “Even before these vaccines officially became available, this was going to be a different rollout; it was going to take more time,” the official explained.
Incidence measures continue on different paths
New COVID-19 cases dropped during the latest reporting week (July 1-7), returning to the downward trend that began in late May and then stopped for 1 week (June 24-30), when cases were up by 12.4%, according to the American Academy of Pediatrics and the Children’s Hospital Association.

Children also represent a smaller share of cases, probably because of underreporting. “There has been a notable decline in the portion of reported weekly COVID-19 cases that are children,” the two groups said in their weekly COVID report. Although “cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases.”
Other measures, however, have been rising slowly but steadily since the spring. New admissions of patients aged 0-17 years with confirmed COVID, which were down to 0.13 per 100,000 population in early April, had climbed to 0.39 per 100,000 by July 7, the CDC said on its COVID Data Tracker.
Emergency department visits continue to show the same upward trend, despite a small decline in early June. A COVID diagnosis was involved in just 0.5% of ED visits in children aged 0-11 years on March 26, but by July 6 the rate was 4.7%. Increases were not as high among older children: From 0.3% on March 26 to 2.5% on July 6 for those aged 12-15 and from 0.3% to 2.4% for 16- and 17-year-olds, according to the CDC.
BA.4 and BA.5 subvariants are more evasive of antibodies, but not of cellular immunity
The picture around the BA.4 and BA.5 subvariants of Omicron has been really confusing in that the pair is driving up cases but global COVID-19 deaths remain at their lowest level since the beginning of the pandemic. Explaining the two components of the immune response – antibodies versus cellular immune responses – can help us understand where we are in the pandemic and future booster options.
These two subvariants of Omicron, as of July 5, make up more than half of the COVID-19 strains in the United States and are expected to keep increasing. One of two reasons can lead to a variant or subvariant becoming dominant strain: increased transmissibility or evasion of antibodies.
Although BA.4 and BA.5 could be more transmissible than other subvariants of Omicron (which is already very transmissible), this has not yet been established in experiments showing increased affinity for the human receptor or in animal models. What we do know is that BA.4 and BA.5 seem to evade neutralizing antibodies conferred by the vaccines or even prior BA.1 infection (an earlier subvariant of Omicron), which could be the reason we are seeing so many reinfections now. Of note, BA.1 infection conferred antibodies that protected against subsequent BA.2 infection, so we did not see the same spike in cases in the United States with BA.2 (after a large BA.1 spike over the winter) earlier this spring.
Okay, so isn’t evasion of antibodies a bad thing? Of course it is but, luckily, our immune system is “redundant” and doesn›t just rely on antibodies to protect us from infection. In fact, antibodies (such as IgA, which is the mucosal antibody most prevalent in the nose and mouth, and IgG, which is the most prevalent antibody in the bloodstream) are our first line of COVID-19 defense in the nasal mucosa. Therefore, mild upper respiratory infections will be common as BA.4/BA.5 evade our nasal antibodies. Luckily, the rate of severe disease is remaining low throughout the world, probably because of the high amounts of cellular immunity to the virus. B and T cells are our protectors from severe disease.
For instance, two-dose vaccines are still conferring high rates of protection from severe disease with the BA.4 and BA.5 variants, with 87% protection against hospitalization per South Africa data. This is probably attributable to the fact that T-cell immunity from the vaccines remains protective across variants “from Alpha to Omicron,” as described by a recent and elegant paper.
Data from Qatar show that natural infection (even occurring up to 14 months ago) remains very protective (97.3%) against severe disease with the current circulating subvariants, including BA.4 and BA.5. Again, this is probably attributable to T cells which specifically amplify in response to a piece of the virus and help recruit cells to attack the pathogen directly.
The original BA.1 subvariant of Omicron has 26-32 mutations along its spike protein that differ from the “ancestral strain,” and BA.4 and BA.5 variants have a few more. Our T-cell response, even across a mutated spike protein, is so robust that we have not seen Omicron yet able to evade the many T cells (which we produce from the vaccines or infection) that descend upon the mutated virus to fight severe disease. Antibody-producing memory B cells, generated by the vaccines (or prior infection), have been shown to actually adapt their immune response to the variant to which they are exposed.
Therefore, the story of the BA.4 and BA.5 subvariants seems to remain about antibodies vs. cellular immunity. Our immunity in the United States is growing and is from both vaccination and natural infection, with 78.3% of the population having had at least one dose of the vaccine and at least 60% of adults (and 75% of children 0-18) having been exposed to the virus by February 2022, per the Centers for Disease Control and Prevention (with exposure probably much higher now in July 2022 after subsequent Omicron subvariants waves).
So, what about Omicron-specific boosters? A booster shot will just raise antibodies temporarily, but their effectiveness wanes several months later. Moreover, a booster shot against the ancestral strain is not very effective in neutralizing BA.4 and BA.5 (with a prior BA.1 Omicron infection being more effective than a booster). Luckily, Pfizer has promised a BA.4/BA.5-specific mRNA vaccine by October, and Moderna has promised a bivalent vaccine containing BA.4/BA.5 mRNA sequences around the same time. A vaccine that specifically increases antibodies against the most prevalent circulating strain should be important as a booster for those who are predisposed to severe breakthrough infections (for example, those with immunocompromise or older individuals with multiple comorbidities). Moreover, BA.4/BA.5–specific booster vaccines may help prevent mild infections for many individuals. Finally, any booster (or exposure) should diversify and broaden T-cell responses to the virus, and a booster shot will also expand the potency of B cells, making them better able to respond to the newest subvariants as we continue to live with COVID-19.
Monica Gandhi, MD, MPH, is an infectious diseases doctor, professor of medicine, and associate chief in the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco.
A version of this article first appeared on Medscape.com.
The picture around the BA.4 and BA.5 subvariants of Omicron has been really confusing in that the pair is driving up cases but global COVID-19 deaths remain at their lowest level since the beginning of the pandemic. Explaining the two components of the immune response – antibodies versus cellular immune responses – can help us understand where we are in the pandemic and future booster options.
These two subvariants of Omicron, as of July 5, make up more than half of the COVID-19 strains in the United States and are expected to keep increasing. One of two reasons can lead to a variant or subvariant becoming dominant strain: increased transmissibility or evasion of antibodies.
Although BA.4 and BA.5 could be more transmissible than other subvariants of Omicron (which is already very transmissible), this has not yet been established in experiments showing increased affinity for the human receptor or in animal models. What we do know is that BA.4 and BA.5 seem to evade neutralizing antibodies conferred by the vaccines or even prior BA.1 infection (an earlier subvariant of Omicron), which could be the reason we are seeing so many reinfections now. Of note, BA.1 infection conferred antibodies that protected against subsequent BA.2 infection, so we did not see the same spike in cases in the United States with BA.2 (after a large BA.1 spike over the winter) earlier this spring.
Okay, so isn’t evasion of antibodies a bad thing? Of course it is but, luckily, our immune system is “redundant” and doesn›t just rely on antibodies to protect us from infection. In fact, antibodies (such as IgA, which is the mucosal antibody most prevalent in the nose and mouth, and IgG, which is the most prevalent antibody in the bloodstream) are our first line of COVID-19 defense in the nasal mucosa. Therefore, mild upper respiratory infections will be common as BA.4/BA.5 evade our nasal antibodies. Luckily, the rate of severe disease is remaining low throughout the world, probably because of the high amounts of cellular immunity to the virus. B and T cells are our protectors from severe disease.
For instance, two-dose vaccines are still conferring high rates of protection from severe disease with the BA.4 and BA.5 variants, with 87% protection against hospitalization per South Africa data. This is probably attributable to the fact that T-cell immunity from the vaccines remains protective across variants “from Alpha to Omicron,” as described by a recent and elegant paper.
Data from Qatar show that natural infection (even occurring up to 14 months ago) remains very protective (97.3%) against severe disease with the current circulating subvariants, including BA.4 and BA.5. Again, this is probably attributable to T cells which specifically amplify in response to a piece of the virus and help recruit cells to attack the pathogen directly.
The original BA.1 subvariant of Omicron has 26-32 mutations along its spike protein that differ from the “ancestral strain,” and BA.4 and BA.5 variants have a few more. Our T-cell response, even across a mutated spike protein, is so robust that we have not seen Omicron yet able to evade the many T cells (which we produce from the vaccines or infection) that descend upon the mutated virus to fight severe disease. Antibody-producing memory B cells, generated by the vaccines (or prior infection), have been shown to actually adapt their immune response to the variant to which they are exposed.
Therefore, the story of the BA.4 and BA.5 subvariants seems to remain about antibodies vs. cellular immunity. Our immunity in the United States is growing and is from both vaccination and natural infection, with 78.3% of the population having had at least one dose of the vaccine and at least 60% of adults (and 75% of children 0-18) having been exposed to the virus by February 2022, per the Centers for Disease Control and Prevention (with exposure probably much higher now in July 2022 after subsequent Omicron subvariants waves).
So, what about Omicron-specific boosters? A booster shot will just raise antibodies temporarily, but their effectiveness wanes several months later. Moreover, a booster shot against the ancestral strain is not very effective in neutralizing BA.4 and BA.5 (with a prior BA.1 Omicron infection being more effective than a booster). Luckily, Pfizer has promised a BA.4/BA.5-specific mRNA vaccine by October, and Moderna has promised a bivalent vaccine containing BA.4/BA.5 mRNA sequences around the same time. A vaccine that specifically increases antibodies against the most prevalent circulating strain should be important as a booster for those who are predisposed to severe breakthrough infections (for example, those with immunocompromise or older individuals with multiple comorbidities). Moreover, BA.4/BA.5–specific booster vaccines may help prevent mild infections for many individuals. Finally, any booster (or exposure) should diversify and broaden T-cell responses to the virus, and a booster shot will also expand the potency of B cells, making them better able to respond to the newest subvariants as we continue to live with COVID-19.
Monica Gandhi, MD, MPH, is an infectious diseases doctor, professor of medicine, and associate chief in the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco.
A version of this article first appeared on Medscape.com.
The picture around the BA.4 and BA.5 subvariants of Omicron has been really confusing in that the pair is driving up cases but global COVID-19 deaths remain at their lowest level since the beginning of the pandemic. Explaining the two components of the immune response – antibodies versus cellular immune responses – can help us understand where we are in the pandemic and future booster options.
These two subvariants of Omicron, as of July 5, make up more than half of the COVID-19 strains in the United States and are expected to keep increasing. One of two reasons can lead to a variant or subvariant becoming dominant strain: increased transmissibility or evasion of antibodies.
Although BA.4 and BA.5 could be more transmissible than other subvariants of Omicron (which is already very transmissible), this has not yet been established in experiments showing increased affinity for the human receptor or in animal models. What we do know is that BA.4 and BA.5 seem to evade neutralizing antibodies conferred by the vaccines or even prior BA.1 infection (an earlier subvariant of Omicron), which could be the reason we are seeing so many reinfections now. Of note, BA.1 infection conferred antibodies that protected against subsequent BA.2 infection, so we did not see the same spike in cases in the United States with BA.2 (after a large BA.1 spike over the winter) earlier this spring.
Okay, so isn’t evasion of antibodies a bad thing? Of course it is but, luckily, our immune system is “redundant” and doesn›t just rely on antibodies to protect us from infection. In fact, antibodies (such as IgA, which is the mucosal antibody most prevalent in the nose and mouth, and IgG, which is the most prevalent antibody in the bloodstream) are our first line of COVID-19 defense in the nasal mucosa. Therefore, mild upper respiratory infections will be common as BA.4/BA.5 evade our nasal antibodies. Luckily, the rate of severe disease is remaining low throughout the world, probably because of the high amounts of cellular immunity to the virus. B and T cells are our protectors from severe disease.
For instance, two-dose vaccines are still conferring high rates of protection from severe disease with the BA.4 and BA.5 variants, with 87% protection against hospitalization per South Africa data. This is probably attributable to the fact that T-cell immunity from the vaccines remains protective across variants “from Alpha to Omicron,” as described by a recent and elegant paper.
Data from Qatar show that natural infection (even occurring up to 14 months ago) remains very protective (97.3%) against severe disease with the current circulating subvariants, including BA.4 and BA.5. Again, this is probably attributable to T cells which specifically amplify in response to a piece of the virus and help recruit cells to attack the pathogen directly.
The original BA.1 subvariant of Omicron has 26-32 mutations along its spike protein that differ from the “ancestral strain,” and BA.4 and BA.5 variants have a few more. Our T-cell response, even across a mutated spike protein, is so robust that we have not seen Omicron yet able to evade the many T cells (which we produce from the vaccines or infection) that descend upon the mutated virus to fight severe disease. Antibody-producing memory B cells, generated by the vaccines (or prior infection), have been shown to actually adapt their immune response to the variant to which they are exposed.
Therefore, the story of the BA.4 and BA.5 subvariants seems to remain about antibodies vs. cellular immunity. Our immunity in the United States is growing and is from both vaccination and natural infection, with 78.3% of the population having had at least one dose of the vaccine and at least 60% of adults (and 75% of children 0-18) having been exposed to the virus by February 2022, per the Centers for Disease Control and Prevention (with exposure probably much higher now in July 2022 after subsequent Omicron subvariants waves).
So, what about Omicron-specific boosters? A booster shot will just raise antibodies temporarily, but their effectiveness wanes several months later. Moreover, a booster shot against the ancestral strain is not very effective in neutralizing BA.4 and BA.5 (with a prior BA.1 Omicron infection being more effective than a booster). Luckily, Pfizer has promised a BA.4/BA.5-specific mRNA vaccine by October, and Moderna has promised a bivalent vaccine containing BA.4/BA.5 mRNA sequences around the same time. A vaccine that specifically increases antibodies against the most prevalent circulating strain should be important as a booster for those who are predisposed to severe breakthrough infections (for example, those with immunocompromise or older individuals with multiple comorbidities). Moreover, BA.4/BA.5–specific booster vaccines may help prevent mild infections for many individuals. Finally, any booster (or exposure) should diversify and broaden T-cell responses to the virus, and a booster shot will also expand the potency of B cells, making them better able to respond to the newest subvariants as we continue to live with COVID-19.
Monica Gandhi, MD, MPH, is an infectious diseases doctor, professor of medicine, and associate chief in the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco.
A version of this article first appeared on Medscape.com.










