User login
COVID vaccination protects B cell–deficient patients through T-cell responses
TOPLINE:
In individuals with low B-cell counts, T cells have enhanced responses to COVID-19 vaccination and may help prevent severe disease after infection.
METHODOLOGY:
- How the immune systems of B cell–deficient patients respond to SARS-CoV-2 infection and vaccination is not fully understood.
- Researchers evaluated anti–SARS-CoV-2 T-cell responses in 33 patients treated with rituximab (RTX), 12 patients with common variable immune deficiency, and 44 controls.
- The study analyzed effector and memory CD4+ and CD8+ T-cell responses to SARS-CoV-2 after infection and vaccination.
TAKEAWAY:
- All B cell–deficient individuals (those treated with RTX or those with a diagnosis of common variable immune deficiency) had increased effector and memory T-cell responses after SARS-CoV-2 vaccination, compared with controls.
- Patients treated with RTX who were vaccinated against COVID-19 had 4.8-fold reduced odds of moderate or severe disease. (These data were not available for patients with common variable immune deficiency.)
- RTX treatment was associated with a decrease in preexisting T-cell immunity in unvaccinated patients, regardless of prior infection with SARS-CoV-2.
- This association was not found in vaccinated patients treated with RTX.
IN PRACTICE:
“[These findings] provide support for vaccination in this vulnerable population and demonstrate the potential benefit of vaccine-induced CD8+ T-cell responses on reducing disease severity from SARS-CoV-2 infection in the absence of spike protein–specific antibodies,” the authors wrote.
SOURCE:
The study was published online on November 29 in Science Translational Medicine. The first author is Reza Zonozi, MD, who conducted the research while at Massachusetts General Hospital, Boston, and is now in private practice in northern Virginia.
LIMITATIONS:
Researchers did not obtain specimens from patients with common variable immune deficiency after SARS-CoV-2 infection. Only a small subset of immunophenotyped participants had subsequent SARS-CoV-2 infection.
DISCLOSURES:
The research was supported by grants from the National Institutes of Health, the Centers for Disease Control and Prevention, the Howard Hughes Medical Institute, the Ragon Institute of Massachusetts General Hospital, Massachusetts Institute of Technology, and Harvard Medical School, the Mark and Lisa Schwartz Foundation and E. Schwartz; the Lambertus Family Foundation; and S. Edgerly and P. Edgerly. Four authors reported relationships with pharmaceutical companies including AbbVie, Bristol-Myers Squibb, Boehringer Ingelheim, Gilead Sciences, Merck, and Pfizer.
A version of this article first appeared on Medscape.com.
TOPLINE:
In individuals with low B-cell counts, T cells have enhanced responses to COVID-19 vaccination and may help prevent severe disease after infection.
METHODOLOGY:
- How the immune systems of B cell–deficient patients respond to SARS-CoV-2 infection and vaccination is not fully understood.
- Researchers evaluated anti–SARS-CoV-2 T-cell responses in 33 patients treated with rituximab (RTX), 12 patients with common variable immune deficiency, and 44 controls.
- The study analyzed effector and memory CD4+ and CD8+ T-cell responses to SARS-CoV-2 after infection and vaccination.
TAKEAWAY:
- All B cell–deficient individuals (those treated with RTX or those with a diagnosis of common variable immune deficiency) had increased effector and memory T-cell responses after SARS-CoV-2 vaccination, compared with controls.
- Patients treated with RTX who were vaccinated against COVID-19 had 4.8-fold reduced odds of moderate or severe disease. (These data were not available for patients with common variable immune deficiency.)
- RTX treatment was associated with a decrease in preexisting T-cell immunity in unvaccinated patients, regardless of prior infection with SARS-CoV-2.
- This association was not found in vaccinated patients treated with RTX.
IN PRACTICE:
“[These findings] provide support for vaccination in this vulnerable population and demonstrate the potential benefit of vaccine-induced CD8+ T-cell responses on reducing disease severity from SARS-CoV-2 infection in the absence of spike protein–specific antibodies,” the authors wrote.
SOURCE:
The study was published online on November 29 in Science Translational Medicine. The first author is Reza Zonozi, MD, who conducted the research while at Massachusetts General Hospital, Boston, and is now in private practice in northern Virginia.
LIMITATIONS:
Researchers did not obtain specimens from patients with common variable immune deficiency after SARS-CoV-2 infection. Only a small subset of immunophenotyped participants had subsequent SARS-CoV-2 infection.
DISCLOSURES:
The research was supported by grants from the National Institutes of Health, the Centers for Disease Control and Prevention, the Howard Hughes Medical Institute, the Ragon Institute of Massachusetts General Hospital, Massachusetts Institute of Technology, and Harvard Medical School, the Mark and Lisa Schwartz Foundation and E. Schwartz; the Lambertus Family Foundation; and S. Edgerly and P. Edgerly. Four authors reported relationships with pharmaceutical companies including AbbVie, Bristol-Myers Squibb, Boehringer Ingelheim, Gilead Sciences, Merck, and Pfizer.
A version of this article first appeared on Medscape.com.
TOPLINE:
In individuals with low B-cell counts, T cells have enhanced responses to COVID-19 vaccination and may help prevent severe disease after infection.
METHODOLOGY:
- How the immune systems of B cell–deficient patients respond to SARS-CoV-2 infection and vaccination is not fully understood.
- Researchers evaluated anti–SARS-CoV-2 T-cell responses in 33 patients treated with rituximab (RTX), 12 patients with common variable immune deficiency, and 44 controls.
- The study analyzed effector and memory CD4+ and CD8+ T-cell responses to SARS-CoV-2 after infection and vaccination.
TAKEAWAY:
- All B cell–deficient individuals (those treated with RTX or those with a diagnosis of common variable immune deficiency) had increased effector and memory T-cell responses after SARS-CoV-2 vaccination, compared with controls.
- Patients treated with RTX who were vaccinated against COVID-19 had 4.8-fold reduced odds of moderate or severe disease. (These data were not available for patients with common variable immune deficiency.)
- RTX treatment was associated with a decrease in preexisting T-cell immunity in unvaccinated patients, regardless of prior infection with SARS-CoV-2.
- This association was not found in vaccinated patients treated with RTX.
IN PRACTICE:
“[These findings] provide support for vaccination in this vulnerable population and demonstrate the potential benefit of vaccine-induced CD8+ T-cell responses on reducing disease severity from SARS-CoV-2 infection in the absence of spike protein–specific antibodies,” the authors wrote.
SOURCE:
The study was published online on November 29 in Science Translational Medicine. The first author is Reza Zonozi, MD, who conducted the research while at Massachusetts General Hospital, Boston, and is now in private practice in northern Virginia.
LIMITATIONS:
Researchers did not obtain specimens from patients with common variable immune deficiency after SARS-CoV-2 infection. Only a small subset of immunophenotyped participants had subsequent SARS-CoV-2 infection.
DISCLOSURES:
The research was supported by grants from the National Institutes of Health, the Centers for Disease Control and Prevention, the Howard Hughes Medical Institute, the Ragon Institute of Massachusetts General Hospital, Massachusetts Institute of Technology, and Harvard Medical School, the Mark and Lisa Schwartz Foundation and E. Schwartz; the Lambertus Family Foundation; and S. Edgerly and P. Edgerly. Four authors reported relationships with pharmaceutical companies including AbbVie, Bristol-Myers Squibb, Boehringer Ingelheim, Gilead Sciences, Merck, and Pfizer.
A version of this article first appeared on Medscape.com.
New CDC advisory once again flags BA.2.86 COVID variant
An emerging variant of COVID-19 called BA.2.86 that caused alarm in the summer of 2023 has landed on the Center for Disease Control and Prevention’s radar again.
The variant accounted for nearly 9% of cases during the 2-week period ending Nov. 25, up from 3% during the previous 2 weeks, according to data published Nov. 27 by the CDC. The estimates are not exact, and the CDC indicated the actual percentage of cases may range from 5% to 15%.
The CDC took the unusual step of publishing a specific statement about the rise in BA.2.86 cases. The variant drew worldwide attention during the summer because of how different its makeup is, compared with other prominent variants of the virus that causes COVID-19, raising the potential for the new variant to be more capable of causing infection. But after a flurry of interest in BA.2.86, it didn’t end up being as widespread as expected, so for months it wasn’t listed as a standalone variant on the CDC’s variant tracker list.
“At this time, BA.2.86 does not appear to be driving increases in infections or hospitalizations in the United States,” the CDC wrote in its advisory. “It is not possible at this time to know whether BA.2.86 infection produces different symptoms from other variants. In general, symptoms of COVID-19 tend to be similar across variants. The types of symptoms and how severe they are usually depend more on a person’s immunity than which variant causes the infection.”
BA.2.86 is now the third-most prominent variant circulating the United States, behind HV.1 and EG.5, which combined account for about 45% of all U.S. COVID-19 cases. All three are from the Omicron lineage of the virus.
About 8% of all COVID tests reported to the CDC were positive for the week ending Nov. 18, which is a decline, compared with recent weeks. But indicators for severe cases of the illness have ticked up lately, including rises among ED visits for COVID, hospitalizations, and deaths.
A version of this article appeared on WebMD.com.
An emerging variant of COVID-19 called BA.2.86 that caused alarm in the summer of 2023 has landed on the Center for Disease Control and Prevention’s radar again.
The variant accounted for nearly 9% of cases during the 2-week period ending Nov. 25, up from 3% during the previous 2 weeks, according to data published Nov. 27 by the CDC. The estimates are not exact, and the CDC indicated the actual percentage of cases may range from 5% to 15%.
The CDC took the unusual step of publishing a specific statement about the rise in BA.2.86 cases. The variant drew worldwide attention during the summer because of how different its makeup is, compared with other prominent variants of the virus that causes COVID-19, raising the potential for the new variant to be more capable of causing infection. But after a flurry of interest in BA.2.86, it didn’t end up being as widespread as expected, so for months it wasn’t listed as a standalone variant on the CDC’s variant tracker list.
“At this time, BA.2.86 does not appear to be driving increases in infections or hospitalizations in the United States,” the CDC wrote in its advisory. “It is not possible at this time to know whether BA.2.86 infection produces different symptoms from other variants. In general, symptoms of COVID-19 tend to be similar across variants. The types of symptoms and how severe they are usually depend more on a person’s immunity than which variant causes the infection.”
BA.2.86 is now the third-most prominent variant circulating the United States, behind HV.1 and EG.5, which combined account for about 45% of all U.S. COVID-19 cases. All three are from the Omicron lineage of the virus.
About 8% of all COVID tests reported to the CDC were positive for the week ending Nov. 18, which is a decline, compared with recent weeks. But indicators for severe cases of the illness have ticked up lately, including rises among ED visits for COVID, hospitalizations, and deaths.
A version of this article appeared on WebMD.com.
An emerging variant of COVID-19 called BA.2.86 that caused alarm in the summer of 2023 has landed on the Center for Disease Control and Prevention’s radar again.
The variant accounted for nearly 9% of cases during the 2-week period ending Nov. 25, up from 3% during the previous 2 weeks, according to data published Nov. 27 by the CDC. The estimates are not exact, and the CDC indicated the actual percentage of cases may range from 5% to 15%.
The CDC took the unusual step of publishing a specific statement about the rise in BA.2.86 cases. The variant drew worldwide attention during the summer because of how different its makeup is, compared with other prominent variants of the virus that causes COVID-19, raising the potential for the new variant to be more capable of causing infection. But after a flurry of interest in BA.2.86, it didn’t end up being as widespread as expected, so for months it wasn’t listed as a standalone variant on the CDC’s variant tracker list.
“At this time, BA.2.86 does not appear to be driving increases in infections or hospitalizations in the United States,” the CDC wrote in its advisory. “It is not possible at this time to know whether BA.2.86 infection produces different symptoms from other variants. In general, symptoms of COVID-19 tend to be similar across variants. The types of symptoms and how severe they are usually depend more on a person’s immunity than which variant causes the infection.”
BA.2.86 is now the third-most prominent variant circulating the United States, behind HV.1 and EG.5, which combined account for about 45% of all U.S. COVID-19 cases. All three are from the Omicron lineage of the virus.
About 8% of all COVID tests reported to the CDC were positive for the week ending Nov. 18, which is a decline, compared with recent weeks. But indicators for severe cases of the illness have ticked up lately, including rises among ED visits for COVID, hospitalizations, and deaths.
A version of this article appeared on WebMD.com.
Chest pain with long COVID common but undertreated
And chronic chest discomfort may persist in some individuals for years after COVID, warranting future studies of reliable treatments and pain management in this population, a new study shows.
“Recent studies have shown that chest pain occurs in as many as 89% of patients who qualify as having long COVID,” said Ansley Poole, an undergraduate student at the University of South Florida, Tampa, who conducted the research under the supervision of Christine Hunt, DO, and her colleagues at Mayo Clinic, Jacksonville, Fla.
The findings, though preliminary, shed light on the prevalence, current treatments, and ongoing challenges in managing symptoms of long COVID, said Ms. Poole, who presented the research at the annual Pain Medicine Meeting sponsored by the American Society of Regional Anesthesia and Pain Medicine.
Long COVID, which affects an estimated 18 million Americans, manifests approximately 12 weeks after the initial infection and can persist for 2 months or more. Ms. Poole and her team set out to identify risk factors, treatment options, and outcomes for patients dealing with post-COVID chest discomfort.
The study involved a retrospective chart review of 520 patients from the Mayo Clinic network, narrowed down to a final sample of 104. To be included, patients had to report chest discomfort 3-6 months post COVID that continued for 3-6 months after presentation, with no history of chronic chest pain before the infection.
The researchers identified no standardized method for the treatment or management of chest pain linked to long COVID. “Patients were prescribed multiple different treatments, including opioids, post-COVID treatment programs, anticoagulants, steroids, and even psychological programs,” Ms. Poole said.
The median age of the patients was around 50 years; more than 65% were female and over 90% identified as White. More than half (55%) had received one or more vaccine doses at the time of infection. The majority were classified as overweight or obese at the time of their SARS-CoV-2 infection.
Of the 104 patients analyzed, 30 were referred to one or more subspecialties within the pain medicine department, 23 were hospitalized, and 9 were admitted to the intensive care unit or critical care.
“Fifty-three of our patients visited the ER one or more times after COVID because of chest discomfort; however, only six were admitted for over 24 hours, indicating possible overuse of emergency services,” Ms. Poole noted.
Overall, chest pain was described as intermittent instead of constant, which may have been a barrier to providing adequate and timely treatment. The inconsistent presence of pain contributed to the prolonged suffering some patients experienced, Ms. Poole noted.
The study identified several comorbidities, potentially complicating the treatment and etiology of chest pain. These comorbidities – when combined with COVID-related chest pain – contributed to the wide array of prescribed treatments, including steroids, anticoagulants, beta blockers, and physical therapy. Chest pain also seldom stood alone; it was often accompanied by other long COVID–related symptoms, such as shortness of breath.
“Our current analysis indicates that chest pain continues on for years in many individuals, suggesting that COVID-related chest pain may be resistant to treatment,” Ms. Poole reported.
The observed heterogeneity in treatments and outcomes in patients experiencing long-term chest discomfort after COVID infection underscores the need for future studies to establish reliable treatment and management protocols for this population, said Dalia Elmofty, MD, an associate professor of anesthesia and critical care at the University of Chicago, who was not involved in the study. “There are things about COVID that we don’t fully understand. As we’re seeing its consequences and trying to understand its etiology, we recognize the need for further research,” Dr. Elmofty said.
“So many different disease pathologies came out of COVID, whether it’s organ pathology, myofascial pathology, or autoimmune pathology, and all of that is obviously linked to pain,” Dr. Elmofty told this news organization. “It’s an area of research that we are going to have to devote a lot of time to in order to understand, but I think we’re still in the very early phases, trying to fit the pieces of the puzzle together.”
Ms. Poole and Dr. Elmofty report no relevant financial relationships.
A version of this article appeared on Medscape.com.
And chronic chest discomfort may persist in some individuals for years after COVID, warranting future studies of reliable treatments and pain management in this population, a new study shows.
“Recent studies have shown that chest pain occurs in as many as 89% of patients who qualify as having long COVID,” said Ansley Poole, an undergraduate student at the University of South Florida, Tampa, who conducted the research under the supervision of Christine Hunt, DO, and her colleagues at Mayo Clinic, Jacksonville, Fla.
The findings, though preliminary, shed light on the prevalence, current treatments, and ongoing challenges in managing symptoms of long COVID, said Ms. Poole, who presented the research at the annual Pain Medicine Meeting sponsored by the American Society of Regional Anesthesia and Pain Medicine.
Long COVID, which affects an estimated 18 million Americans, manifests approximately 12 weeks after the initial infection and can persist for 2 months or more. Ms. Poole and her team set out to identify risk factors, treatment options, and outcomes for patients dealing with post-COVID chest discomfort.
The study involved a retrospective chart review of 520 patients from the Mayo Clinic network, narrowed down to a final sample of 104. To be included, patients had to report chest discomfort 3-6 months post COVID that continued for 3-6 months after presentation, with no history of chronic chest pain before the infection.
The researchers identified no standardized method for the treatment or management of chest pain linked to long COVID. “Patients were prescribed multiple different treatments, including opioids, post-COVID treatment programs, anticoagulants, steroids, and even psychological programs,” Ms. Poole said.
The median age of the patients was around 50 years; more than 65% were female and over 90% identified as White. More than half (55%) had received one or more vaccine doses at the time of infection. The majority were classified as overweight or obese at the time of their SARS-CoV-2 infection.
Of the 104 patients analyzed, 30 were referred to one or more subspecialties within the pain medicine department, 23 were hospitalized, and 9 were admitted to the intensive care unit or critical care.
“Fifty-three of our patients visited the ER one or more times after COVID because of chest discomfort; however, only six were admitted for over 24 hours, indicating possible overuse of emergency services,” Ms. Poole noted.
Overall, chest pain was described as intermittent instead of constant, which may have been a barrier to providing adequate and timely treatment. The inconsistent presence of pain contributed to the prolonged suffering some patients experienced, Ms. Poole noted.
The study identified several comorbidities, potentially complicating the treatment and etiology of chest pain. These comorbidities – when combined with COVID-related chest pain – contributed to the wide array of prescribed treatments, including steroids, anticoagulants, beta blockers, and physical therapy. Chest pain also seldom stood alone; it was often accompanied by other long COVID–related symptoms, such as shortness of breath.
“Our current analysis indicates that chest pain continues on for years in many individuals, suggesting that COVID-related chest pain may be resistant to treatment,” Ms. Poole reported.
The observed heterogeneity in treatments and outcomes in patients experiencing long-term chest discomfort after COVID infection underscores the need for future studies to establish reliable treatment and management protocols for this population, said Dalia Elmofty, MD, an associate professor of anesthesia and critical care at the University of Chicago, who was not involved in the study. “There are things about COVID that we don’t fully understand. As we’re seeing its consequences and trying to understand its etiology, we recognize the need for further research,” Dr. Elmofty said.
“So many different disease pathologies came out of COVID, whether it’s organ pathology, myofascial pathology, or autoimmune pathology, and all of that is obviously linked to pain,” Dr. Elmofty told this news organization. “It’s an area of research that we are going to have to devote a lot of time to in order to understand, but I think we’re still in the very early phases, trying to fit the pieces of the puzzle together.”
Ms. Poole and Dr. Elmofty report no relevant financial relationships.
A version of this article appeared on Medscape.com.
And chronic chest discomfort may persist in some individuals for years after COVID, warranting future studies of reliable treatments and pain management in this population, a new study shows.
“Recent studies have shown that chest pain occurs in as many as 89% of patients who qualify as having long COVID,” said Ansley Poole, an undergraduate student at the University of South Florida, Tampa, who conducted the research under the supervision of Christine Hunt, DO, and her colleagues at Mayo Clinic, Jacksonville, Fla.
The findings, though preliminary, shed light on the prevalence, current treatments, and ongoing challenges in managing symptoms of long COVID, said Ms. Poole, who presented the research at the annual Pain Medicine Meeting sponsored by the American Society of Regional Anesthesia and Pain Medicine.
Long COVID, which affects an estimated 18 million Americans, manifests approximately 12 weeks after the initial infection and can persist for 2 months or more. Ms. Poole and her team set out to identify risk factors, treatment options, and outcomes for patients dealing with post-COVID chest discomfort.
The study involved a retrospective chart review of 520 patients from the Mayo Clinic network, narrowed down to a final sample of 104. To be included, patients had to report chest discomfort 3-6 months post COVID that continued for 3-6 months after presentation, with no history of chronic chest pain before the infection.
The researchers identified no standardized method for the treatment or management of chest pain linked to long COVID. “Patients were prescribed multiple different treatments, including opioids, post-COVID treatment programs, anticoagulants, steroids, and even psychological programs,” Ms. Poole said.
The median age of the patients was around 50 years; more than 65% were female and over 90% identified as White. More than half (55%) had received one or more vaccine doses at the time of infection. The majority were classified as overweight or obese at the time of their SARS-CoV-2 infection.
Of the 104 patients analyzed, 30 were referred to one or more subspecialties within the pain medicine department, 23 were hospitalized, and 9 were admitted to the intensive care unit or critical care.
“Fifty-three of our patients visited the ER one or more times after COVID because of chest discomfort; however, only six were admitted for over 24 hours, indicating possible overuse of emergency services,” Ms. Poole noted.
Overall, chest pain was described as intermittent instead of constant, which may have been a barrier to providing adequate and timely treatment. The inconsistent presence of pain contributed to the prolonged suffering some patients experienced, Ms. Poole noted.
The study identified several comorbidities, potentially complicating the treatment and etiology of chest pain. These comorbidities – when combined with COVID-related chest pain – contributed to the wide array of prescribed treatments, including steroids, anticoagulants, beta blockers, and physical therapy. Chest pain also seldom stood alone; it was often accompanied by other long COVID–related symptoms, such as shortness of breath.
“Our current analysis indicates that chest pain continues on for years in many individuals, suggesting that COVID-related chest pain may be resistant to treatment,” Ms. Poole reported.
The observed heterogeneity in treatments and outcomes in patients experiencing long-term chest discomfort after COVID infection underscores the need for future studies to establish reliable treatment and management protocols for this population, said Dalia Elmofty, MD, an associate professor of anesthesia and critical care at the University of Chicago, who was not involved in the study. “There are things about COVID that we don’t fully understand. As we’re seeing its consequences and trying to understand its etiology, we recognize the need for further research,” Dr. Elmofty said.
“So many different disease pathologies came out of COVID, whether it’s organ pathology, myofascial pathology, or autoimmune pathology, and all of that is obviously linked to pain,” Dr. Elmofty told this news organization. “It’s an area of research that we are going to have to devote a lot of time to in order to understand, but I think we’re still in the very early phases, trying to fit the pieces of the puzzle together.”
Ms. Poole and Dr. Elmofty report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Unexplained collapse unveils rare blood disorder
This case report was published in the New England Journal of Medicine.
Noting the patient’s confusion and aphasia, emergency medical services were alerted, and she was taken to the emergency department of Massachusetts General Hospital. Initial examination revealed aphasia and coordination difficulties. However, imaging studies, including CT angiography, showed no signs of stroke or other neurological abnormalities.
The patient’s coworkers had observed that she appeared “unwell.” Her medical history included hypertension, which was managed with amlodipine, and there was no known family history of neurologic disorders.
During the examination, her vital signs were within normal ranges.
The patient’s potassium level of 2.5 mmol/L was noteworthy, indicating hypokalemia. Additionally, the patient presented with anemia and thrombocytopenia. Additional laboratory results unveiled thrombotic thrombocytopenic purpura (TTP), a rare blood disorder characterized by microangiopathic hemolytic anemia. The microscopic examination of a peripheral blood smear confirmed the extent of thrombocytopenia and was particularly notable for the increased number of schistocytes. The patient’s peripheral blood smear revealed five or six schistocytes per high-power field, constituting approximately 5% of the red cells. This significant number of schistocytes aligned with the severity of anemia and thrombocytopenia, confirming the diagnosis of microangiopathic hemolytic anemia.
Acquired TTP is an autoimmune condition driven by antibody-mediated clearance of the plasma enzyme ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin motif 13). Confirmatory laboratory testing for ADAMTS13 takes 1-3 days; therefore, therapeutic plasma exchange with glucocorticoid therapy and rituximab was initiated, which promptly improved her condition.
In this patient, the ADAMTS13 activity level was severely reduced (< 5%; reference value > 67%), and the inhibitor was present (1.4 inhibitor units; reference value ≤ 0.4).
Rectal cancer was diagnosed in this patient 2 months after the diagnosis of acquired TTP.
After undergoing four weekly infusions of rituximab and a 2-month tapering course of glucocorticoids, the patient experienced a relapse, approximately 6 months following the acquired TTP diagnosis. In response, therapeutic plasma exchange and glucocorticoid therapy were administered. There is a possibility that the underlying cancer played a role in the relapse. To minimize the risk for recurrence, the patient also received a second round of rituximab.
While establishing a clear cause is difficult, acquired TTP often appears to arise in connection with either an immune trigger, such as a viral infection, or immune dysregulation associated with another autoimmune disease or ongoing cancer. In this case, 4 weeks before the acquired TTP diagnosis, the patient had experienced COVID-19, which was likely to be the most probable trigger. However, rectal cancer was also identified in the patient, and whether these conditions are directly linked remains unclear.
A version of this article first appeared on Medscape.com.
This case report was published in the New England Journal of Medicine.
Noting the patient’s confusion and aphasia, emergency medical services were alerted, and she was taken to the emergency department of Massachusetts General Hospital. Initial examination revealed aphasia and coordination difficulties. However, imaging studies, including CT angiography, showed no signs of stroke or other neurological abnormalities.
The patient’s coworkers had observed that she appeared “unwell.” Her medical history included hypertension, which was managed with amlodipine, and there was no known family history of neurologic disorders.
During the examination, her vital signs were within normal ranges.
The patient’s potassium level of 2.5 mmol/L was noteworthy, indicating hypokalemia. Additionally, the patient presented with anemia and thrombocytopenia. Additional laboratory results unveiled thrombotic thrombocytopenic purpura (TTP), a rare blood disorder characterized by microangiopathic hemolytic anemia. The microscopic examination of a peripheral blood smear confirmed the extent of thrombocytopenia and was particularly notable for the increased number of schistocytes. The patient’s peripheral blood smear revealed five or six schistocytes per high-power field, constituting approximately 5% of the red cells. This significant number of schistocytes aligned with the severity of anemia and thrombocytopenia, confirming the diagnosis of microangiopathic hemolytic anemia.
Acquired TTP is an autoimmune condition driven by antibody-mediated clearance of the plasma enzyme ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin motif 13). Confirmatory laboratory testing for ADAMTS13 takes 1-3 days; therefore, therapeutic plasma exchange with glucocorticoid therapy and rituximab was initiated, which promptly improved her condition.
In this patient, the ADAMTS13 activity level was severely reduced (< 5%; reference value > 67%), and the inhibitor was present (1.4 inhibitor units; reference value ≤ 0.4).
Rectal cancer was diagnosed in this patient 2 months after the diagnosis of acquired TTP.
After undergoing four weekly infusions of rituximab and a 2-month tapering course of glucocorticoids, the patient experienced a relapse, approximately 6 months following the acquired TTP diagnosis. In response, therapeutic plasma exchange and glucocorticoid therapy were administered. There is a possibility that the underlying cancer played a role in the relapse. To minimize the risk for recurrence, the patient also received a second round of rituximab.
While establishing a clear cause is difficult, acquired TTP often appears to arise in connection with either an immune trigger, such as a viral infection, or immune dysregulation associated with another autoimmune disease or ongoing cancer. In this case, 4 weeks before the acquired TTP diagnosis, the patient had experienced COVID-19, which was likely to be the most probable trigger. However, rectal cancer was also identified in the patient, and whether these conditions are directly linked remains unclear.
A version of this article first appeared on Medscape.com.
This case report was published in the New England Journal of Medicine.
Noting the patient’s confusion and aphasia, emergency medical services were alerted, and she was taken to the emergency department of Massachusetts General Hospital. Initial examination revealed aphasia and coordination difficulties. However, imaging studies, including CT angiography, showed no signs of stroke or other neurological abnormalities.
The patient’s coworkers had observed that she appeared “unwell.” Her medical history included hypertension, which was managed with amlodipine, and there was no known family history of neurologic disorders.
During the examination, her vital signs were within normal ranges.
The patient’s potassium level of 2.5 mmol/L was noteworthy, indicating hypokalemia. Additionally, the patient presented with anemia and thrombocytopenia. Additional laboratory results unveiled thrombotic thrombocytopenic purpura (TTP), a rare blood disorder characterized by microangiopathic hemolytic anemia. The microscopic examination of a peripheral blood smear confirmed the extent of thrombocytopenia and was particularly notable for the increased number of schistocytes. The patient’s peripheral blood smear revealed five or six schistocytes per high-power field, constituting approximately 5% of the red cells. This significant number of schistocytes aligned with the severity of anemia and thrombocytopenia, confirming the diagnosis of microangiopathic hemolytic anemia.
Acquired TTP is an autoimmune condition driven by antibody-mediated clearance of the plasma enzyme ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin motif 13). Confirmatory laboratory testing for ADAMTS13 takes 1-3 days; therefore, therapeutic plasma exchange with glucocorticoid therapy and rituximab was initiated, which promptly improved her condition.
In this patient, the ADAMTS13 activity level was severely reduced (< 5%; reference value > 67%), and the inhibitor was present (1.4 inhibitor units; reference value ≤ 0.4).
Rectal cancer was diagnosed in this patient 2 months after the diagnosis of acquired TTP.
After undergoing four weekly infusions of rituximab and a 2-month tapering course of glucocorticoids, the patient experienced a relapse, approximately 6 months following the acquired TTP diagnosis. In response, therapeutic plasma exchange and glucocorticoid therapy were administered. There is a possibility that the underlying cancer played a role in the relapse. To minimize the risk for recurrence, the patient also received a second round of rituximab.
While establishing a clear cause is difficult, acquired TTP often appears to arise in connection with either an immune trigger, such as a viral infection, or immune dysregulation associated with another autoimmune disease or ongoing cancer. In this case, 4 weeks before the acquired TTP diagnosis, the patient had experienced COVID-19, which was likely to be the most probable trigger. However, rectal cancer was also identified in the patient, and whether these conditions are directly linked remains unclear.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Impact of the COVID-19 Pandemic on Care for Patients With Atopic Dermatitis
To the Editor:
Atopic dermatitis (AD) is a widely prevalent dermatologic condition that can severely impact a patient’s quality of life.1 Individuals with AD have been substantially affected during the COVID-19 pandemic due to the increased use of irritants, decreased access to care, and rise in psychological stress.1,2 These factors have resulted in lower quality of life and worsening dermatologic symptoms for many AD patients over the last few years.1 One major potential contributory component of these findings is decreased accessibility to in-office care during the pandemic, with a shift to telemedicine instead. Accessibility to care during the COVID-19 pandemic for AD patients compared to those without AD remains unknown. Therefore, we explored the impact of the COVID-19 pandemic on care for patients with AD in a large US population.
Using anonymous survey data from the 2021 National Health Interview Survey,3 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with AD compared to those without AD. We assigned the following 3 survey questions as outcome variables to assess access to care: delayed medical care due to COVID-19 pandemic (yes/no), did not get care due to COVID-19 pandemic (yes/no), and virtual medical appointment in the last 12 months (yes/no). In Table 1, numerous categorical survey variables, including sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region, were analyzed using χ2 testing to evaluate for differences among individuals with and without AD. Multivariable logistic regression models evaluating the relationship between AD and access to care were constructed using Stata/MP 17 (StataCorp LLC). In our analysis we controlled for age, sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region.
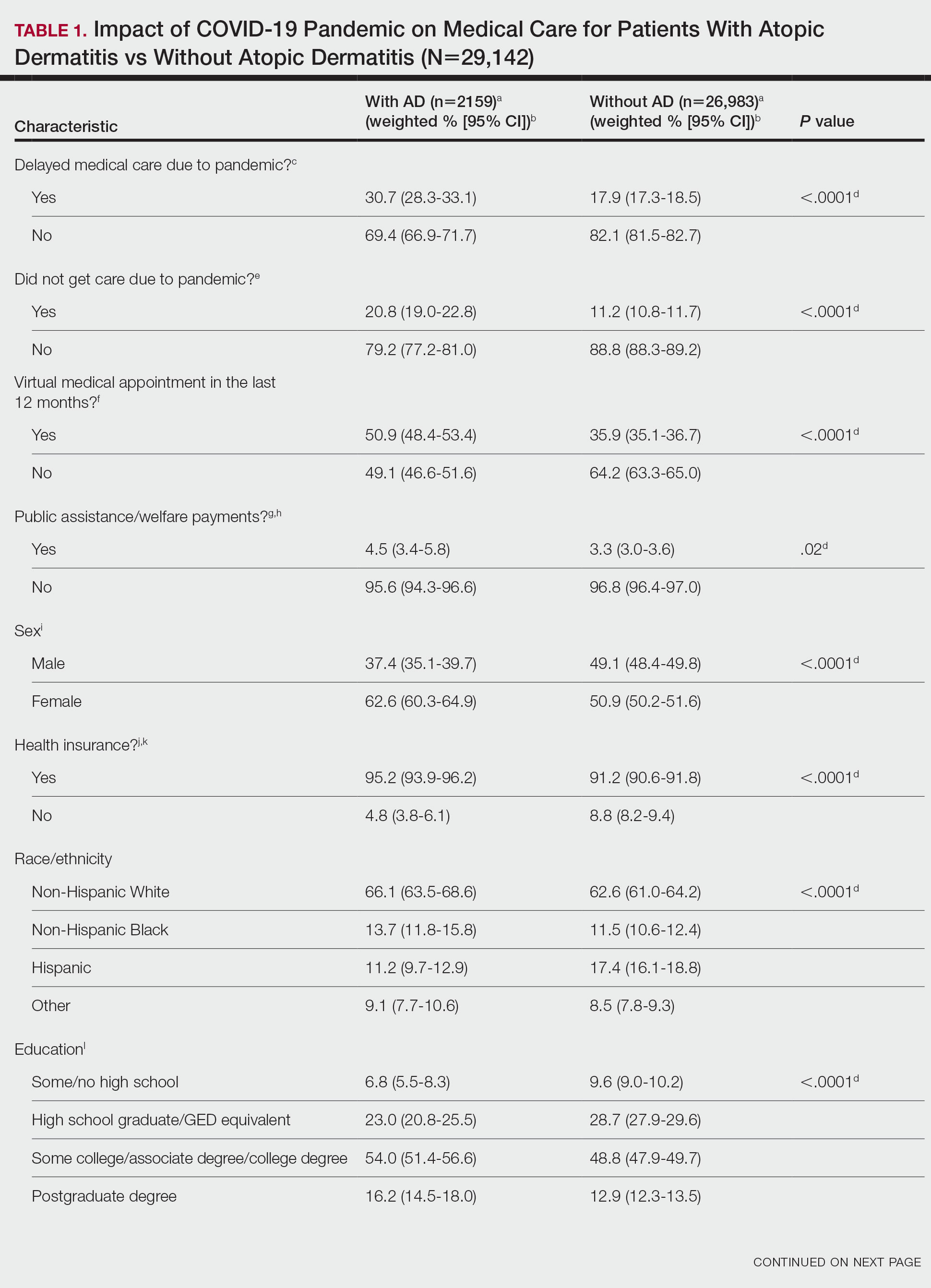
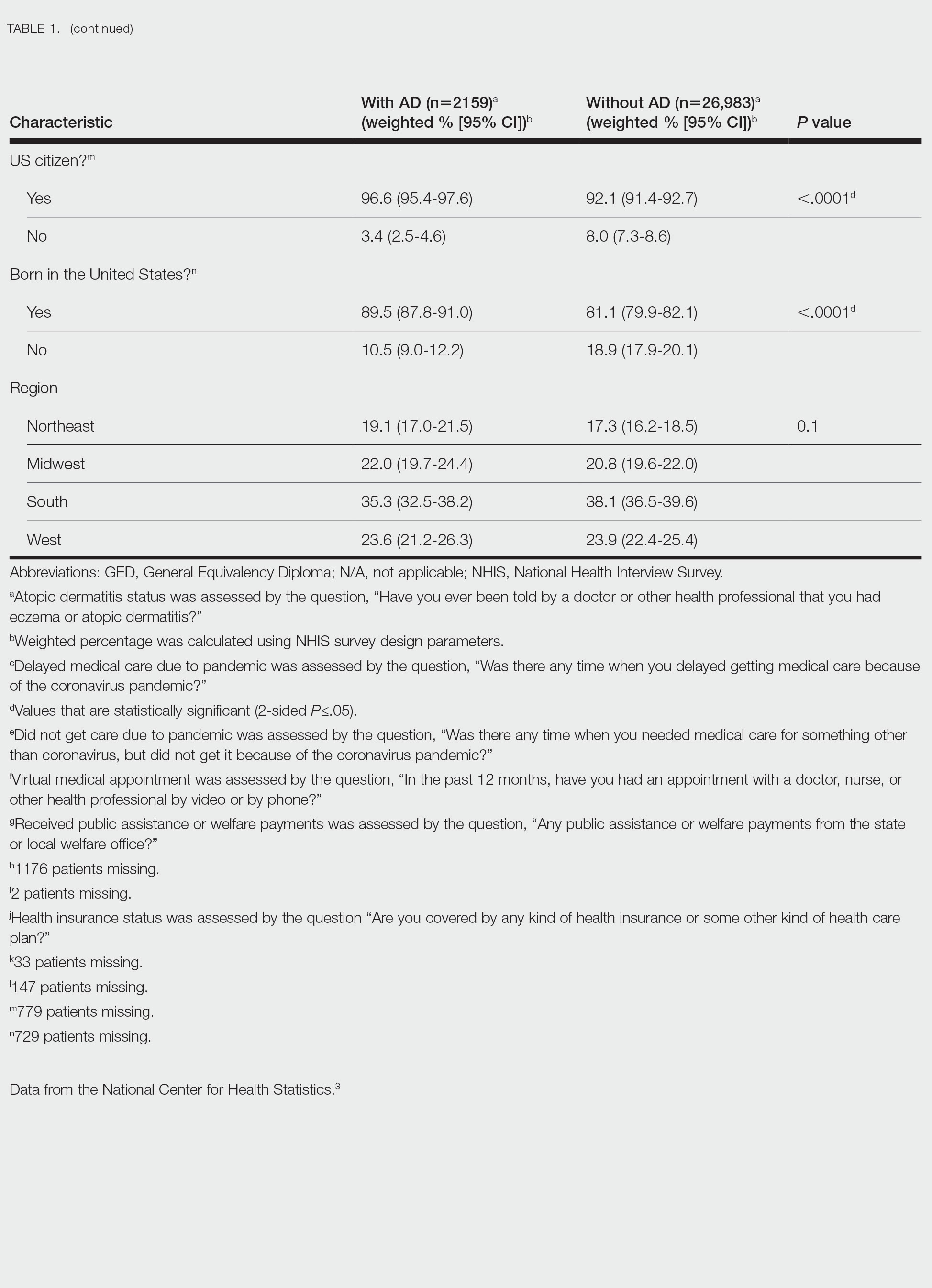
There were 29,142 adult patients (aged ≥18 years) included in our analysis. Approximately 7.4% (weighted) of individuals had AD (Table 1). After adjusting for confounding variables, patients with AD had a higher odds of delaying medical care due to the COVID-19 pandemic (adjusted odds ratio [AOR], 1.91; 95% CI, 1.69-2.16; P<.001), not receiving care due to the COVID-19 pandemic (AOR, 1.94; 95% CI, 1.71-2.22; P<.001), and having a virtual medical visit in the last 12 months (AOR, 1.72; 95% CI, 1.54-1.93; P<.001)(Table 2) compared with patients without AD.
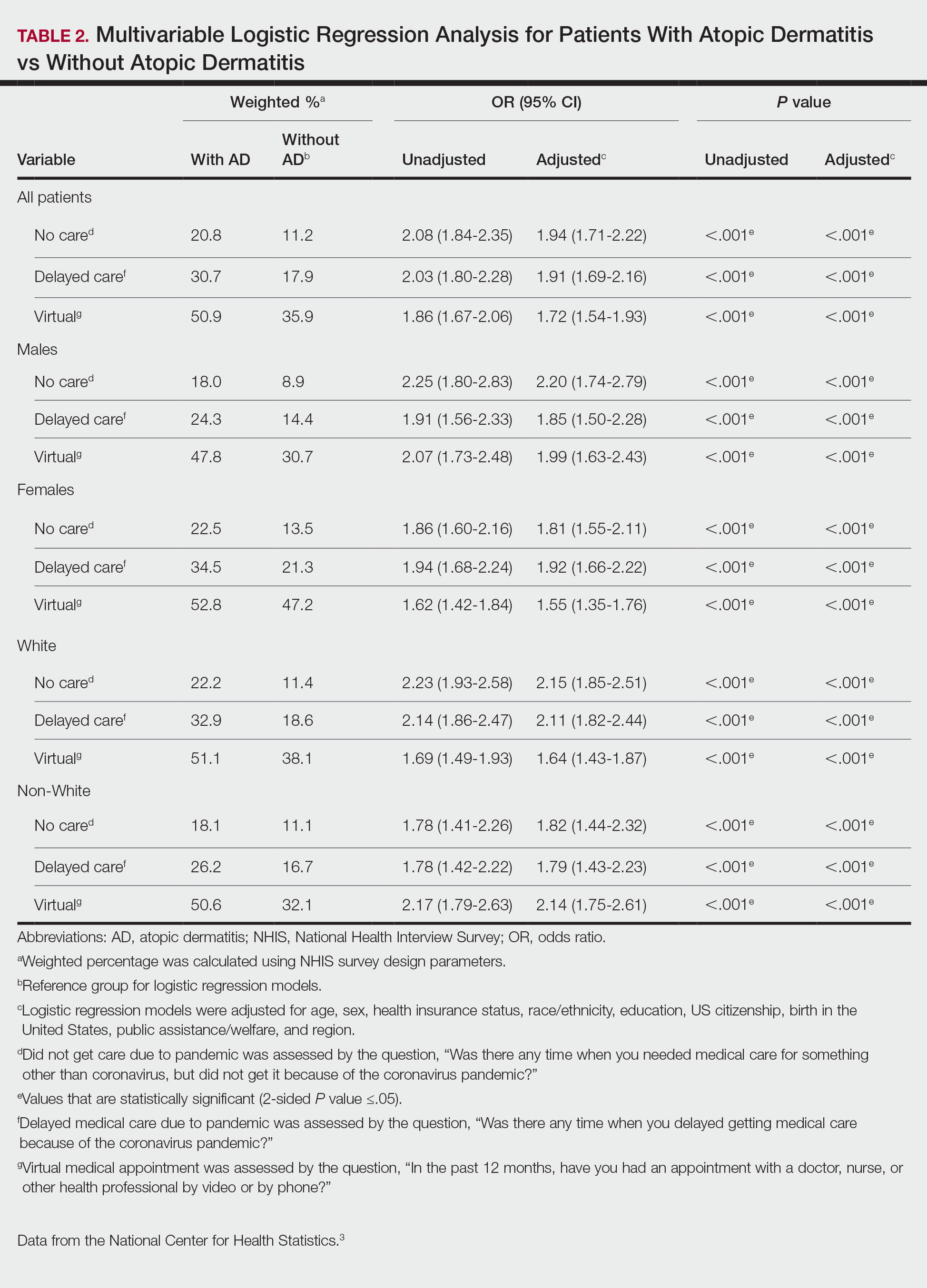
Our findings support the association between AD and decreased access to in-person care due to the COVID-19 pandemic. Moreover, telemedicine was utilized more among individuals with AD, possibly due to the accessibility of diagnostic tools for dermatologic diagnoses, such as high-quality photographs.4 According to Trinidad et al,4 telemedicine became an invaluable tool for dermatology hospitalists during the COVID-19 pandemic, as many physicians were able to comfortably diagnose patients with cutaneous diseases without an in-person visit. Utilizing telemedicine for patient care can help reduce the risk for COVID-19 transmission while also providing quality care for individuals living in rural areas.5 Chiricozzi et al6 discussed the importance of telemedicine in Italy during the pandemic, as many AD patients were able to maintain control of their disease while on systemic treatments.
Limitations of this study include self-reported measures; inability to compare patients with AD to individuals with other cutaneous diseases; and additional potential confounders, such as chronic comorbidities. Future studies should evaluate the use of telemedicine and access to care among individuals with other common skin diseases and help determine why such discrepancies exist. Understanding the difficulties in access to care and the viable alternatives in place may increase awareness and assist clinicians with adequate management of patients with AD.
1. Sieniawska J, Lesiak A, Cia˛z˙yn´ski K, et al. Impact of the COVID-19 pandemic on atopic dermatitis patients. Int J Environ Res Public Health. 2022;19:1734. doi:10.3390/ijerph19031734
2. Pourani MR, Ganji R, Dashti T, et al. Impact of COVID-19 pandemic on patients with atopic dermatitis [in Spanish]. Actas Dermosifiliogr. 2022;113:T286-T293. doi:10.1016/j.ad.2021.08.004
3. National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
4. Trinidad J, Gabel CK, Han JJ, et al. Telemedicine and dermatology hospital consultations during the COVID-19 pandemic: a multi-centre observational study on resource utilization and conversion to in-person consultations during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2022;36:E323-E325. doi:10.1111/jdv.17898
5. Marasca C, Annunziata MC, Camela E, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med. 2022;11:1511. doi:10.3390/jcm11061511
6. Chiricozzi A, Talamonti M, De Simone C, et al. Management of patients with atopic dermatitis undergoing systemic therapy during COVID-19 pandemic in Italy: data from the DA-COVID-19 registry. Allergy. 2021;76:1813-1824. doi:10.1111/all.14767
To the Editor:
Atopic dermatitis (AD) is a widely prevalent dermatologic condition that can severely impact a patient’s quality of life.1 Individuals with AD have been substantially affected during the COVID-19 pandemic due to the increased use of irritants, decreased access to care, and rise in psychological stress.1,2 These factors have resulted in lower quality of life and worsening dermatologic symptoms for many AD patients over the last few years.1 One major potential contributory component of these findings is decreased accessibility to in-office care during the pandemic, with a shift to telemedicine instead. Accessibility to care during the COVID-19 pandemic for AD patients compared to those without AD remains unknown. Therefore, we explored the impact of the COVID-19 pandemic on care for patients with AD in a large US population.
Using anonymous survey data from the 2021 National Health Interview Survey,3 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with AD compared to those without AD. We assigned the following 3 survey questions as outcome variables to assess access to care: delayed medical care due to COVID-19 pandemic (yes/no), did not get care due to COVID-19 pandemic (yes/no), and virtual medical appointment in the last 12 months (yes/no). In Table 1, numerous categorical survey variables, including sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region, were analyzed using χ2 testing to evaluate for differences among individuals with and without AD. Multivariable logistic regression models evaluating the relationship between AD and access to care were constructed using Stata/MP 17 (StataCorp LLC). In our analysis we controlled for age, sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region.


There were 29,142 adult patients (aged ≥18 years) included in our analysis. Approximately 7.4% (weighted) of individuals had AD (Table 1). After adjusting for confounding variables, patients with AD had a higher odds of delaying medical care due to the COVID-19 pandemic (adjusted odds ratio [AOR], 1.91; 95% CI, 1.69-2.16; P<.001), not receiving care due to the COVID-19 pandemic (AOR, 1.94; 95% CI, 1.71-2.22; P<.001), and having a virtual medical visit in the last 12 months (AOR, 1.72; 95% CI, 1.54-1.93; P<.001)(Table 2) compared with patients without AD.

Our findings support the association between AD and decreased access to in-person care due to the COVID-19 pandemic. Moreover, telemedicine was utilized more among individuals with AD, possibly due to the accessibility of diagnostic tools for dermatologic diagnoses, such as high-quality photographs.4 According to Trinidad et al,4 telemedicine became an invaluable tool for dermatology hospitalists during the COVID-19 pandemic, as many physicians were able to comfortably diagnose patients with cutaneous diseases without an in-person visit. Utilizing telemedicine for patient care can help reduce the risk for COVID-19 transmission while also providing quality care for individuals living in rural areas.5 Chiricozzi et al6 discussed the importance of telemedicine in Italy during the pandemic, as many AD patients were able to maintain control of their disease while on systemic treatments.
Limitations of this study include self-reported measures; inability to compare patients with AD to individuals with other cutaneous diseases; and additional potential confounders, such as chronic comorbidities. Future studies should evaluate the use of telemedicine and access to care among individuals with other common skin diseases and help determine why such discrepancies exist. Understanding the difficulties in access to care and the viable alternatives in place may increase awareness and assist clinicians with adequate management of patients with AD.
To the Editor:
Atopic dermatitis (AD) is a widely prevalent dermatologic condition that can severely impact a patient’s quality of life.1 Individuals with AD have been substantially affected during the COVID-19 pandemic due to the increased use of irritants, decreased access to care, and rise in psychological stress.1,2 These factors have resulted in lower quality of life and worsening dermatologic symptoms for many AD patients over the last few years.1 One major potential contributory component of these findings is decreased accessibility to in-office care during the pandemic, with a shift to telemedicine instead. Accessibility to care during the COVID-19 pandemic for AD patients compared to those without AD remains unknown. Therefore, we explored the impact of the COVID-19 pandemic on care for patients with AD in a large US population.
Using anonymous survey data from the 2021 National Health Interview Survey,3 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with AD compared to those without AD. We assigned the following 3 survey questions as outcome variables to assess access to care: delayed medical care due to COVID-19 pandemic (yes/no), did not get care due to COVID-19 pandemic (yes/no), and virtual medical appointment in the last 12 months (yes/no). In Table 1, numerous categorical survey variables, including sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region, were analyzed using χ2 testing to evaluate for differences among individuals with and without AD. Multivariable logistic regression models evaluating the relationship between AD and access to care were constructed using Stata/MP 17 (StataCorp LLC). In our analysis we controlled for age, sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region.


There were 29,142 adult patients (aged ≥18 years) included in our analysis. Approximately 7.4% (weighted) of individuals had AD (Table 1). After adjusting for confounding variables, patients with AD had a higher odds of delaying medical care due to the COVID-19 pandemic (adjusted odds ratio [AOR], 1.91; 95% CI, 1.69-2.16; P<.001), not receiving care due to the COVID-19 pandemic (AOR, 1.94; 95% CI, 1.71-2.22; P<.001), and having a virtual medical visit in the last 12 months (AOR, 1.72; 95% CI, 1.54-1.93; P<.001)(Table 2) compared with patients without AD.

Our findings support the association between AD and decreased access to in-person care due to the COVID-19 pandemic. Moreover, telemedicine was utilized more among individuals with AD, possibly due to the accessibility of diagnostic tools for dermatologic diagnoses, such as high-quality photographs.4 According to Trinidad et al,4 telemedicine became an invaluable tool for dermatology hospitalists during the COVID-19 pandemic, as many physicians were able to comfortably diagnose patients with cutaneous diseases without an in-person visit. Utilizing telemedicine for patient care can help reduce the risk for COVID-19 transmission while also providing quality care for individuals living in rural areas.5 Chiricozzi et al6 discussed the importance of telemedicine in Italy during the pandemic, as many AD patients were able to maintain control of their disease while on systemic treatments.
Limitations of this study include self-reported measures; inability to compare patients with AD to individuals with other cutaneous diseases; and additional potential confounders, such as chronic comorbidities. Future studies should evaluate the use of telemedicine and access to care among individuals with other common skin diseases and help determine why such discrepancies exist. Understanding the difficulties in access to care and the viable alternatives in place may increase awareness and assist clinicians with adequate management of patients with AD.
1. Sieniawska J, Lesiak A, Cia˛z˙yn´ski K, et al. Impact of the COVID-19 pandemic on atopic dermatitis patients. Int J Environ Res Public Health. 2022;19:1734. doi:10.3390/ijerph19031734
2. Pourani MR, Ganji R, Dashti T, et al. Impact of COVID-19 pandemic on patients with atopic dermatitis [in Spanish]. Actas Dermosifiliogr. 2022;113:T286-T293. doi:10.1016/j.ad.2021.08.004
3. National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
4. Trinidad J, Gabel CK, Han JJ, et al. Telemedicine and dermatology hospital consultations during the COVID-19 pandemic: a multi-centre observational study on resource utilization and conversion to in-person consultations during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2022;36:E323-E325. doi:10.1111/jdv.17898
5. Marasca C, Annunziata MC, Camela E, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med. 2022;11:1511. doi:10.3390/jcm11061511
6. Chiricozzi A, Talamonti M, De Simone C, et al. Management of patients with atopic dermatitis undergoing systemic therapy during COVID-19 pandemic in Italy: data from the DA-COVID-19 registry. Allergy. 2021;76:1813-1824. doi:10.1111/all.14767
1. Sieniawska J, Lesiak A, Cia˛z˙yn´ski K, et al. Impact of the COVID-19 pandemic on atopic dermatitis patients. Int J Environ Res Public Health. 2022;19:1734. doi:10.3390/ijerph19031734
2. Pourani MR, Ganji R, Dashti T, et al. Impact of COVID-19 pandemic on patients with atopic dermatitis [in Spanish]. Actas Dermosifiliogr. 2022;113:T286-T293. doi:10.1016/j.ad.2021.08.004
3. National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
4. Trinidad J, Gabel CK, Han JJ, et al. Telemedicine and dermatology hospital consultations during the COVID-19 pandemic: a multi-centre observational study on resource utilization and conversion to in-person consultations during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2022;36:E323-E325. doi:10.1111/jdv.17898
5. Marasca C, Annunziata MC, Camela E, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med. 2022;11:1511. doi:10.3390/jcm11061511
6. Chiricozzi A, Talamonti M, De Simone C, et al. Management of patients with atopic dermatitis undergoing systemic therapy during COVID-19 pandemic in Italy: data from the DA-COVID-19 registry. Allergy. 2021;76:1813-1824. doi:10.1111/all.14767
Practice Points
- The landscape of dermatology has seen major changes due to the COVID-19 pandemic, as many patients now utilize telemedicine to receive care.
- Understanding accessibility to in-person care for patients with atopic dermatitis during the COVID-19 pandemic can assist with the development of methods to enhance management.
Saltwater gargling may help avoid COVID hospitalization
ANAHEIM, CALIF. –
“The hypothesis was that interventions that target the upper respiratory tract may reduce the frequency and duration of upper respiratory symptoms associated with COVID-19,” said Sebastian Espinoza, first author of the study; he is with Trinity University, San Antonio.
Adults aged 18-65 years who tested positive for SARS-CoV-2 on polymerase chain reaction (PCR) testing between 2020 and 2022 were randomly selected to use low- or high-dose saltwater regimens for 14 days at the Harris Health System, Houston. For patients to be included in the study, 14 days had to have elapsed since the onset of any symptoms associated with COVID.
The low dose was 2.13 grams of salt dissolved in 8 ounces of warm water, and the high dose was 6 grams. Participants gargled the saltwater and used it as a nasal rinse for 5 minutes four times a day.
Primary outcomes included frequency and duration of symptoms associated with SARS-CoV-2 infection; secondary outcomes included admission to the hospital or the intensive care unit, mechanical ventilatory support, or death.
The findings were presented in a poster at the annual meeting of the American College of Allergy, Asthma, and Immunology.
Fifty-eight people were randomly assigned to either the low-saline (n = 27) or the high-saline (n = 28) group; three patients were lost to follow-up in both these groups. The reference control population consisted of 9,398 people with confirmed SARS-CoV-2 infection. Rates of vaccination were similar for all participants.
Hospitalization rates in the low- (18.5%) and high- (21.4%) saline groups were significantly lower than in the reference control population (58.8%; P < .001). No significant differences were noted in other outcomes among these groups.
The average age of patients in the control population (n = 9,398) was 45 years. The average age was similar in the low- and high-saline groups. In the low-saline group (n = 27), the average age was 39, and in the high-saline group, the average age was 41.
In all three groups, body mass index was between 29.6 and 31.7.
Exclusion criteria included chronic hypertension or participation in another interventional study.
‘Low risk, small potential benefit’
Allergist Zach Rubin, MD, a spokesperson for the ACAAI, said in an interview that the findings are in line with other small studies that previously reported some benefit in using nasal saline irrigation and gargling to treat a SARS-CoV-2 infection.
“This is a type of intervention that is low risk with some small potential benefit,” he said.
The researchers did not evaluate the potential reason for the saline regimen’s association with fewer hospitalizations, but Dr. Rubin said, “It may be possible that nasal saline irrigation and gargling help improve viral clearance and reduce the risk of microaspiration into the lungs, so it may be possible that this intervention could reduce the risk of pneumonia, which is a major cause of hospitalization.”
Dr. Rubin, who is an allergist at Oak Brook Allergists, Ill., said, “I generally recommend nasal saline irrigation to my patients for allergic rhinitis and viral upper respiratory infections already. It can help reduce symptoms such as nasal congestion, rhinorrhea, postnasal drip, and sinus pain and pressure.”
The intervention may be reasonable beyond an adult population, he said.
“This could be used for pediatric patients as well, if they are developmentally ready to try this intervention,” he said.
Mr. Espinoza said further study is warranted, but he said that if confirmed in later trials, the simple intervention may be particularly helpful in low-resource settings.
Mr. Espinoza and Dr. Rubin have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
ANAHEIM, CALIF. –
“The hypothesis was that interventions that target the upper respiratory tract may reduce the frequency and duration of upper respiratory symptoms associated with COVID-19,” said Sebastian Espinoza, first author of the study; he is with Trinity University, San Antonio.
Adults aged 18-65 years who tested positive for SARS-CoV-2 on polymerase chain reaction (PCR) testing between 2020 and 2022 were randomly selected to use low- or high-dose saltwater regimens for 14 days at the Harris Health System, Houston. For patients to be included in the study, 14 days had to have elapsed since the onset of any symptoms associated with COVID.
The low dose was 2.13 grams of salt dissolved in 8 ounces of warm water, and the high dose was 6 grams. Participants gargled the saltwater and used it as a nasal rinse for 5 minutes four times a day.
Primary outcomes included frequency and duration of symptoms associated with SARS-CoV-2 infection; secondary outcomes included admission to the hospital or the intensive care unit, mechanical ventilatory support, or death.
The findings were presented in a poster at the annual meeting of the American College of Allergy, Asthma, and Immunology.
Fifty-eight people were randomly assigned to either the low-saline (n = 27) or the high-saline (n = 28) group; three patients were lost to follow-up in both these groups. The reference control population consisted of 9,398 people with confirmed SARS-CoV-2 infection. Rates of vaccination were similar for all participants.
Hospitalization rates in the low- (18.5%) and high- (21.4%) saline groups were significantly lower than in the reference control population (58.8%; P < .001). No significant differences were noted in other outcomes among these groups.
The average age of patients in the control population (n = 9,398) was 45 years. The average age was similar in the low- and high-saline groups. In the low-saline group (n = 27), the average age was 39, and in the high-saline group, the average age was 41.
In all three groups, body mass index was between 29.6 and 31.7.
Exclusion criteria included chronic hypertension or participation in another interventional study.
‘Low risk, small potential benefit’
Allergist Zach Rubin, MD, a spokesperson for the ACAAI, said in an interview that the findings are in line with other small studies that previously reported some benefit in using nasal saline irrigation and gargling to treat a SARS-CoV-2 infection.
“This is a type of intervention that is low risk with some small potential benefit,” he said.
The researchers did not evaluate the potential reason for the saline regimen’s association with fewer hospitalizations, but Dr. Rubin said, “It may be possible that nasal saline irrigation and gargling help improve viral clearance and reduce the risk of microaspiration into the lungs, so it may be possible that this intervention could reduce the risk of pneumonia, which is a major cause of hospitalization.”
Dr. Rubin, who is an allergist at Oak Brook Allergists, Ill., said, “I generally recommend nasal saline irrigation to my patients for allergic rhinitis and viral upper respiratory infections already. It can help reduce symptoms such as nasal congestion, rhinorrhea, postnasal drip, and sinus pain and pressure.”
The intervention may be reasonable beyond an adult population, he said.
“This could be used for pediatric patients as well, if they are developmentally ready to try this intervention,” he said.
Mr. Espinoza said further study is warranted, but he said that if confirmed in later trials, the simple intervention may be particularly helpful in low-resource settings.
Mr. Espinoza and Dr. Rubin have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
ANAHEIM, CALIF. –
“The hypothesis was that interventions that target the upper respiratory tract may reduce the frequency and duration of upper respiratory symptoms associated with COVID-19,” said Sebastian Espinoza, first author of the study; he is with Trinity University, San Antonio.
Adults aged 18-65 years who tested positive for SARS-CoV-2 on polymerase chain reaction (PCR) testing between 2020 and 2022 were randomly selected to use low- or high-dose saltwater regimens for 14 days at the Harris Health System, Houston. For patients to be included in the study, 14 days had to have elapsed since the onset of any symptoms associated with COVID.
The low dose was 2.13 grams of salt dissolved in 8 ounces of warm water, and the high dose was 6 grams. Participants gargled the saltwater and used it as a nasal rinse for 5 minutes four times a day.
Primary outcomes included frequency and duration of symptoms associated with SARS-CoV-2 infection; secondary outcomes included admission to the hospital or the intensive care unit, mechanical ventilatory support, or death.
The findings were presented in a poster at the annual meeting of the American College of Allergy, Asthma, and Immunology.
Fifty-eight people were randomly assigned to either the low-saline (n = 27) or the high-saline (n = 28) group; three patients were lost to follow-up in both these groups. The reference control population consisted of 9,398 people with confirmed SARS-CoV-2 infection. Rates of vaccination were similar for all participants.
Hospitalization rates in the low- (18.5%) and high- (21.4%) saline groups were significantly lower than in the reference control population (58.8%; P < .001). No significant differences were noted in other outcomes among these groups.
The average age of patients in the control population (n = 9,398) was 45 years. The average age was similar in the low- and high-saline groups. In the low-saline group (n = 27), the average age was 39, and in the high-saline group, the average age was 41.
In all three groups, body mass index was between 29.6 and 31.7.
Exclusion criteria included chronic hypertension or participation in another interventional study.
‘Low risk, small potential benefit’
Allergist Zach Rubin, MD, a spokesperson for the ACAAI, said in an interview that the findings are in line with other small studies that previously reported some benefit in using nasal saline irrigation and gargling to treat a SARS-CoV-2 infection.
“This is a type of intervention that is low risk with some small potential benefit,” he said.
The researchers did not evaluate the potential reason for the saline regimen’s association with fewer hospitalizations, but Dr. Rubin said, “It may be possible that nasal saline irrigation and gargling help improve viral clearance and reduce the risk of microaspiration into the lungs, so it may be possible that this intervention could reduce the risk of pneumonia, which is a major cause of hospitalization.”
Dr. Rubin, who is an allergist at Oak Brook Allergists, Ill., said, “I generally recommend nasal saline irrigation to my patients for allergic rhinitis and viral upper respiratory infections already. It can help reduce symptoms such as nasal congestion, rhinorrhea, postnasal drip, and sinus pain and pressure.”
The intervention may be reasonable beyond an adult population, he said.
“This could be used for pediatric patients as well, if they are developmentally ready to try this intervention,” he said.
Mr. Espinoza said further study is warranted, but he said that if confirmed in later trials, the simple intervention may be particularly helpful in low-resource settings.
Mr. Espinoza and Dr. Rubin have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM ACAAI 2023
AI tool perfect in study of inflammatory diseases
Artificial intelligence can distinguish overlapping inflammatory conditions with total accuracy, according to a new study presented at the annual meeting of the American College of Rheumatology.
Texas pediatricians faced a conundrum during the pandemic. Endemic typhus, a flea-borne tropical infection common to the region, is nearly indistinguishable from multisystem inflammatory syndrome in children (MIS-C), a rare condition set in motion by SARS-CoV-2 infection. Children with either ailment had seemingly identical symptoms: fever, rash, gastrointestinal issues, and in need of swift treatment. A diagnosis of endemic typhus can take 4-6 days to confirm.
Tiphanie Vogel, MD, PhD, a pediatric rheumatologist at Texas Children’s Hospital, Houston, and colleagues sought to create a tool to hasten diagnosis and, ideally, treatment. To do so, they incorporated machine learning and clinical factors available within the first 6 hours of the onset of symptoms.
The team analyzed 49 demographic, clinical, and laboratory measures from the medical records of 133 children with MIS-C and 87 with endemic typhus. Using deep learning, they narrowed the model to 30 essential features that became the backbone of AI-MET, a two-phase clinical-decision support system.
Phase 1 uses 17 clinical factors and can be performed on paper. If a patient’s score in phase 1 is not determinative, clinicians proceed to phase 2, which uses an additional 13 weighted factors and machine learning.
In testing, the two-part tool classified each of the 220 test patients perfectly. And it diagnosed a second group of 111 patients with MIS-C with 99% (110/111) accuracy.
Of note, “that first step classifies [a patient] correctly half of the time,” Dr. Vogel said, so the second, AI phase of the tool was necessary for only half of cases. Dr. Vogel said that’s a good sign; it means that the tool is useful in settings where AI may not always be feasible, like in a busy ED.
Melissa Mizesko, MD, a pediatric rheumatologist at Driscoll Children’s Hospital in Corpus Christi, Tex., said that the new tool could help clinicians streamline care. When cases of MIS-C peaked in Texas, clinicians often would start sick children on doxycycline and treat for MIS-C at the same time, then wait to see whether the antibiotic brought the fever down.
“This [new tool] is helpful if you live in a part of the country that has typhus,” said Jane Burns, MD, director of the Kawasaki Disease Research Center at the University of California, San Diego, who helped develop a similar AI-based tool to distinguish MIS-C from Kawasaki disease. But she encouraged the researchers to expand their testing to include other conditions. Although the AI model Dr. Vogel’s group developed can pinpoint MIS-C or endemic typhus, what if a child has neither condition? “It’s not often you’re dealing with a diagnosis between just two specific diseases,” Dr. Burns said.
Dr. Vogel is also interested in making AI-MET more efficient. “This go-round we prioritized perfect accuracy,” she said. But 30 clinical factors, with 17 of them recorded and calculated by hand, is a lot. “Could we still get this to be very accurate, maybe not perfect, with less inputs?”
In addition to refining AI-MET, which Texas Children’s eventually hopes to make available to other institutions, Dr. Vogel and associates are also considering other use cases for AI. Lupus is one option. “Maybe with machine learning we could identify clues at diagnosis that would help recommend targeted treatment,” she said
Dr. Vogel disclosed potential conflicts of interest with Moderna, Novartis, Pfizer, and SOBI. Dr. Burns and Dr. Mizesko disclosed no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
Artificial intelligence can distinguish overlapping inflammatory conditions with total accuracy, according to a new study presented at the annual meeting of the American College of Rheumatology.
Texas pediatricians faced a conundrum during the pandemic. Endemic typhus, a flea-borne tropical infection common to the region, is nearly indistinguishable from multisystem inflammatory syndrome in children (MIS-C), a rare condition set in motion by SARS-CoV-2 infection. Children with either ailment had seemingly identical symptoms: fever, rash, gastrointestinal issues, and in need of swift treatment. A diagnosis of endemic typhus can take 4-6 days to confirm.
Tiphanie Vogel, MD, PhD, a pediatric rheumatologist at Texas Children’s Hospital, Houston, and colleagues sought to create a tool to hasten diagnosis and, ideally, treatment. To do so, they incorporated machine learning and clinical factors available within the first 6 hours of the onset of symptoms.
The team analyzed 49 demographic, clinical, and laboratory measures from the medical records of 133 children with MIS-C and 87 with endemic typhus. Using deep learning, they narrowed the model to 30 essential features that became the backbone of AI-MET, a two-phase clinical-decision support system.
Phase 1 uses 17 clinical factors and can be performed on paper. If a patient’s score in phase 1 is not determinative, clinicians proceed to phase 2, which uses an additional 13 weighted factors and machine learning.
In testing, the two-part tool classified each of the 220 test patients perfectly. And it diagnosed a second group of 111 patients with MIS-C with 99% (110/111) accuracy.
Of note, “that first step classifies [a patient] correctly half of the time,” Dr. Vogel said, so the second, AI phase of the tool was necessary for only half of cases. Dr. Vogel said that’s a good sign; it means that the tool is useful in settings where AI may not always be feasible, like in a busy ED.
Melissa Mizesko, MD, a pediatric rheumatologist at Driscoll Children’s Hospital in Corpus Christi, Tex., said that the new tool could help clinicians streamline care. When cases of MIS-C peaked in Texas, clinicians often would start sick children on doxycycline and treat for MIS-C at the same time, then wait to see whether the antibiotic brought the fever down.
“This [new tool] is helpful if you live in a part of the country that has typhus,” said Jane Burns, MD, director of the Kawasaki Disease Research Center at the University of California, San Diego, who helped develop a similar AI-based tool to distinguish MIS-C from Kawasaki disease. But she encouraged the researchers to expand their testing to include other conditions. Although the AI model Dr. Vogel’s group developed can pinpoint MIS-C or endemic typhus, what if a child has neither condition? “It’s not often you’re dealing with a diagnosis between just two specific diseases,” Dr. Burns said.
Dr. Vogel is also interested in making AI-MET more efficient. “This go-round we prioritized perfect accuracy,” she said. But 30 clinical factors, with 17 of them recorded and calculated by hand, is a lot. “Could we still get this to be very accurate, maybe not perfect, with less inputs?”
In addition to refining AI-MET, which Texas Children’s eventually hopes to make available to other institutions, Dr. Vogel and associates are also considering other use cases for AI. Lupus is one option. “Maybe with machine learning we could identify clues at diagnosis that would help recommend targeted treatment,” she said
Dr. Vogel disclosed potential conflicts of interest with Moderna, Novartis, Pfizer, and SOBI. Dr. Burns and Dr. Mizesko disclosed no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
Artificial intelligence can distinguish overlapping inflammatory conditions with total accuracy, according to a new study presented at the annual meeting of the American College of Rheumatology.
Texas pediatricians faced a conundrum during the pandemic. Endemic typhus, a flea-borne tropical infection common to the region, is nearly indistinguishable from multisystem inflammatory syndrome in children (MIS-C), a rare condition set in motion by SARS-CoV-2 infection. Children with either ailment had seemingly identical symptoms: fever, rash, gastrointestinal issues, and in need of swift treatment. A diagnosis of endemic typhus can take 4-6 days to confirm.
Tiphanie Vogel, MD, PhD, a pediatric rheumatologist at Texas Children’s Hospital, Houston, and colleagues sought to create a tool to hasten diagnosis and, ideally, treatment. To do so, they incorporated machine learning and clinical factors available within the first 6 hours of the onset of symptoms.
The team analyzed 49 demographic, clinical, and laboratory measures from the medical records of 133 children with MIS-C and 87 with endemic typhus. Using deep learning, they narrowed the model to 30 essential features that became the backbone of AI-MET, a two-phase clinical-decision support system.
Phase 1 uses 17 clinical factors and can be performed on paper. If a patient’s score in phase 1 is not determinative, clinicians proceed to phase 2, which uses an additional 13 weighted factors and machine learning.
In testing, the two-part tool classified each of the 220 test patients perfectly. And it diagnosed a second group of 111 patients with MIS-C with 99% (110/111) accuracy.
Of note, “that first step classifies [a patient] correctly half of the time,” Dr. Vogel said, so the second, AI phase of the tool was necessary for only half of cases. Dr. Vogel said that’s a good sign; it means that the tool is useful in settings where AI may not always be feasible, like in a busy ED.
Melissa Mizesko, MD, a pediatric rheumatologist at Driscoll Children’s Hospital in Corpus Christi, Tex., said that the new tool could help clinicians streamline care. When cases of MIS-C peaked in Texas, clinicians often would start sick children on doxycycline and treat for MIS-C at the same time, then wait to see whether the antibiotic brought the fever down.
“This [new tool] is helpful if you live in a part of the country that has typhus,” said Jane Burns, MD, director of the Kawasaki Disease Research Center at the University of California, San Diego, who helped develop a similar AI-based tool to distinguish MIS-C from Kawasaki disease. But she encouraged the researchers to expand their testing to include other conditions. Although the AI model Dr. Vogel’s group developed can pinpoint MIS-C or endemic typhus, what if a child has neither condition? “It’s not often you’re dealing with a diagnosis between just two specific diseases,” Dr. Burns said.
Dr. Vogel is also interested in making AI-MET more efficient. “This go-round we prioritized perfect accuracy,” she said. But 30 clinical factors, with 17 of them recorded and calculated by hand, is a lot. “Could we still get this to be very accurate, maybe not perfect, with less inputs?”
In addition to refining AI-MET, which Texas Children’s eventually hopes to make available to other institutions, Dr. Vogel and associates are also considering other use cases for AI. Lupus is one option. “Maybe with machine learning we could identify clues at diagnosis that would help recommend targeted treatment,” she said
Dr. Vogel disclosed potential conflicts of interest with Moderna, Novartis, Pfizer, and SOBI. Dr. Burns and Dr. Mizesko disclosed no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FROM ACR 2023
CDC says child vaccination exemptions hit all-time high
– the highest exemption rate ever reported in the United States.
Of the 3% of children who got exemptions, 0.2% were for medical reasons and 2.8% for nonmedical reasons, the CDC report said. The overall exemption rate was 2.6% for the previous school year.
Though more children received exemptions, the overall national vaccination rate remained steady at 93% for children entering kindergarten for the 2022-2023 school year. Before the COVID-19 pandemic, the overall rate was 95%, the CDC said.
“The bad news is that it’s gone down since the pandemic and still hasn’t rebounded,” Sean O’Leary, MD, a University of Colorado pediatric infectious diseases specialist, told The Associated Press. “The good news is that the vast majority of parents are still vaccinating their kids according to the recommended schedule.”
The CDC report did not offer a specific reason for higher vaccine exemptions. But it did note that the increase could be caused by the COVID-19 pandemic and COVID vaccine hesitancy.
“There is a rising distrust in the health care system,” Amna Husain, MD, a pediatrician in private practice in North Carolina and a spokesperson for the American Academy of Pediatrics, told NBC News. Vaccine exemptions “have unfortunately trended upward with it.”
Exemption rates varied across the nation. The CDC said 40 states reported a rise in exemptions and that the exemption rate went over 5% in 10 states: Alaska, Arizona, Hawaii, Idaho, Michigan, Nevada, North Dakota, Oregon, Utah, and Wisconsin. Idaho had the highest exemption rate in 2022 with 12%.
While requirements vary from state to state, most states require students entering kindergarten to receive four vaccines: MMR, DTaP, polio, and chickenpox.
A version of this article first appeared on WebMD.com.
– the highest exemption rate ever reported in the United States.
Of the 3% of children who got exemptions, 0.2% were for medical reasons and 2.8% for nonmedical reasons, the CDC report said. The overall exemption rate was 2.6% for the previous school year.
Though more children received exemptions, the overall national vaccination rate remained steady at 93% for children entering kindergarten for the 2022-2023 school year. Before the COVID-19 pandemic, the overall rate was 95%, the CDC said.
“The bad news is that it’s gone down since the pandemic and still hasn’t rebounded,” Sean O’Leary, MD, a University of Colorado pediatric infectious diseases specialist, told The Associated Press. “The good news is that the vast majority of parents are still vaccinating their kids according to the recommended schedule.”
The CDC report did not offer a specific reason for higher vaccine exemptions. But it did note that the increase could be caused by the COVID-19 pandemic and COVID vaccine hesitancy.
“There is a rising distrust in the health care system,” Amna Husain, MD, a pediatrician in private practice in North Carolina and a spokesperson for the American Academy of Pediatrics, told NBC News. Vaccine exemptions “have unfortunately trended upward with it.”
Exemption rates varied across the nation. The CDC said 40 states reported a rise in exemptions and that the exemption rate went over 5% in 10 states: Alaska, Arizona, Hawaii, Idaho, Michigan, Nevada, North Dakota, Oregon, Utah, and Wisconsin. Idaho had the highest exemption rate in 2022 with 12%.
While requirements vary from state to state, most states require students entering kindergarten to receive four vaccines: MMR, DTaP, polio, and chickenpox.
A version of this article first appeared on WebMD.com.
– the highest exemption rate ever reported in the United States.
Of the 3% of children who got exemptions, 0.2% were for medical reasons and 2.8% for nonmedical reasons, the CDC report said. The overall exemption rate was 2.6% for the previous school year.
Though more children received exemptions, the overall national vaccination rate remained steady at 93% for children entering kindergarten for the 2022-2023 school year. Before the COVID-19 pandemic, the overall rate was 95%, the CDC said.
“The bad news is that it’s gone down since the pandemic and still hasn’t rebounded,” Sean O’Leary, MD, a University of Colorado pediatric infectious diseases specialist, told The Associated Press. “The good news is that the vast majority of parents are still vaccinating their kids according to the recommended schedule.”
The CDC report did not offer a specific reason for higher vaccine exemptions. But it did note that the increase could be caused by the COVID-19 pandemic and COVID vaccine hesitancy.
“There is a rising distrust in the health care system,” Amna Husain, MD, a pediatrician in private practice in North Carolina and a spokesperson for the American Academy of Pediatrics, told NBC News. Vaccine exemptions “have unfortunately trended upward with it.”
Exemption rates varied across the nation. The CDC said 40 states reported a rise in exemptions and that the exemption rate went over 5% in 10 states: Alaska, Arizona, Hawaii, Idaho, Michigan, Nevada, North Dakota, Oregon, Utah, and Wisconsin. Idaho had the highest exemption rate in 2022 with 12%.
While requirements vary from state to state, most states require students entering kindergarten to receive four vaccines: MMR, DTaP, polio, and chickenpox.
A version of this article first appeared on WebMD.com.
Long COVID and mental illness: New guidance
The consensus guidance statement on the assessment and treatment of mental health symptoms in patients with post-acute sequelae of SARS-CoV-2 infection (PASC), also known as long COVID, was published online in Physical Medicine and Rehabilitation, the journal of the American Academy of Physical Medicine and Rehabilitation (AAPM&R).
The statement was developed by a task force that included experts from physical medicine, neurology, neuropsychiatry, neuropsychology, rehabilitation psychology, and primary care. It is the eighth guidance statement on long COVID published by AAPM&R).
“Many of our patients have reported experiences in which their symptoms of long COVID have been dismissed either by loved ones in the community, or also amongst health care providers, and they’ve been told their symptoms are in their head or due to a mental health condition, but that’s simply not true,” Abby L. Cheng, MD, a physiatrist at Barnes Jewish Hospital in St. Louis and a coauthor of the new guidance, said in a press briefing.
“Long COVID is real, and mental health conditions do not cause long COVID,” Dr. Cheng added.
Millions of Americans affected
Anxiety and depression have been reported as the second and third most common symptoms of long COVID, according to the guidance statement.
There is some evidence that the body’s inflammatory response – specifically, circulating cytokines – may contribute to the worsening of mental health symptoms or may bring on new symptoms of anxiety or depression, said Dr. Cheng. Cytokines may also affect levels of brain chemicals, such as serotonin, she said.
Researchers are also exploring whether the persistence of virus in the body, miniature blood clots in the body and brain, and changes to the gut microbiome affect the mental health of people with long COVID.
Some mental health symptoms – such as fatigue, brain fog, sleep disturbances, and tachycardia – can mimic long COVID symptoms, said Dr. Cheng.
The treatment is the same for someone with or without long COVID who has anxiety, depression, posttraumatic stress disorder, or other mental health conditions and includes treatment of coexisting medical conditions, supportive therapy and cognitive-behavioral therapy, and pharmacologic interventions, she said.
“Group therapy may have a particular role in the long COVID population because it really provides that social connection and awareness of additional resources in addition to validation of their experiences,” Dr. Cheng said.
The guidance suggests that primary care practitioners – if it’s within their comfort zone and they have the training – can be the first line for managing mental health symptoms.
But for patients whose symptoms are interfering with functioning and their ability to interact with the community, the guidance urges primary care clinicians to refer the patient to a specialist.
“It leaves the door open to them to practice within their scope but also gives guidance as to how, why, and who should be referred to the next level of care,” said Dr. Cheng.
Coauthor Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine at UT Health San Antonio, Texas, said that although fewer people are now getting long COVID, “it’s still an impactful number.”
The Centers for Disease Control and Prevention recently estimated that about 7% of American adults (18 million) and 1.3% of children had experienced long COVID.
Dr. Gutierrez said that it’s an evolving number, as some patients who have a second or third or fourth SARS-CoV-2 infection experience exacerbations of previous bouts of long COVID or develop long COVID for the first time.
“We are still getting new patients on a regular basis with long COVID,” said AAPM&R President Steven R. Flanagan, MD, a physical medicine specialist.
“This is a problem that really is not going away. It is still real and still ever-present,” said Dr. Flanagan, chair of rehabilitation medicine at NYU Langone Health.
A version of this article first appeared on Medscape.com.
The consensus guidance statement on the assessment and treatment of mental health symptoms in patients with post-acute sequelae of SARS-CoV-2 infection (PASC), also known as long COVID, was published online in Physical Medicine and Rehabilitation, the journal of the American Academy of Physical Medicine and Rehabilitation (AAPM&R).
The statement was developed by a task force that included experts from physical medicine, neurology, neuropsychiatry, neuropsychology, rehabilitation psychology, and primary care. It is the eighth guidance statement on long COVID published by AAPM&R).
“Many of our patients have reported experiences in which their symptoms of long COVID have been dismissed either by loved ones in the community, or also amongst health care providers, and they’ve been told their symptoms are in their head or due to a mental health condition, but that’s simply not true,” Abby L. Cheng, MD, a physiatrist at Barnes Jewish Hospital in St. Louis and a coauthor of the new guidance, said in a press briefing.
“Long COVID is real, and mental health conditions do not cause long COVID,” Dr. Cheng added.
Millions of Americans affected
Anxiety and depression have been reported as the second and third most common symptoms of long COVID, according to the guidance statement.
There is some evidence that the body’s inflammatory response – specifically, circulating cytokines – may contribute to the worsening of mental health symptoms or may bring on new symptoms of anxiety or depression, said Dr. Cheng. Cytokines may also affect levels of brain chemicals, such as serotonin, she said.
Researchers are also exploring whether the persistence of virus in the body, miniature blood clots in the body and brain, and changes to the gut microbiome affect the mental health of people with long COVID.
Some mental health symptoms – such as fatigue, brain fog, sleep disturbances, and tachycardia – can mimic long COVID symptoms, said Dr. Cheng.
The treatment is the same for someone with or without long COVID who has anxiety, depression, posttraumatic stress disorder, or other mental health conditions and includes treatment of coexisting medical conditions, supportive therapy and cognitive-behavioral therapy, and pharmacologic interventions, she said.
“Group therapy may have a particular role in the long COVID population because it really provides that social connection and awareness of additional resources in addition to validation of their experiences,” Dr. Cheng said.
The guidance suggests that primary care practitioners – if it’s within their comfort zone and they have the training – can be the first line for managing mental health symptoms.
But for patients whose symptoms are interfering with functioning and their ability to interact with the community, the guidance urges primary care clinicians to refer the patient to a specialist.
“It leaves the door open to them to practice within their scope but also gives guidance as to how, why, and who should be referred to the next level of care,” said Dr. Cheng.
Coauthor Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine at UT Health San Antonio, Texas, said that although fewer people are now getting long COVID, “it’s still an impactful number.”
The Centers for Disease Control and Prevention recently estimated that about 7% of American adults (18 million) and 1.3% of children had experienced long COVID.
Dr. Gutierrez said that it’s an evolving number, as some patients who have a second or third or fourth SARS-CoV-2 infection experience exacerbations of previous bouts of long COVID or develop long COVID for the first time.
“We are still getting new patients on a regular basis with long COVID,” said AAPM&R President Steven R. Flanagan, MD, a physical medicine specialist.
“This is a problem that really is not going away. It is still real and still ever-present,” said Dr. Flanagan, chair of rehabilitation medicine at NYU Langone Health.
A version of this article first appeared on Medscape.com.
The consensus guidance statement on the assessment and treatment of mental health symptoms in patients with post-acute sequelae of SARS-CoV-2 infection (PASC), also known as long COVID, was published online in Physical Medicine and Rehabilitation, the journal of the American Academy of Physical Medicine and Rehabilitation (AAPM&R).
The statement was developed by a task force that included experts from physical medicine, neurology, neuropsychiatry, neuropsychology, rehabilitation psychology, and primary care. It is the eighth guidance statement on long COVID published by AAPM&R).
“Many of our patients have reported experiences in which their symptoms of long COVID have been dismissed either by loved ones in the community, or also amongst health care providers, and they’ve been told their symptoms are in their head or due to a mental health condition, but that’s simply not true,” Abby L. Cheng, MD, a physiatrist at Barnes Jewish Hospital in St. Louis and a coauthor of the new guidance, said in a press briefing.
“Long COVID is real, and mental health conditions do not cause long COVID,” Dr. Cheng added.
Millions of Americans affected
Anxiety and depression have been reported as the second and third most common symptoms of long COVID, according to the guidance statement.
There is some evidence that the body’s inflammatory response – specifically, circulating cytokines – may contribute to the worsening of mental health symptoms or may bring on new symptoms of anxiety or depression, said Dr. Cheng. Cytokines may also affect levels of brain chemicals, such as serotonin, she said.
Researchers are also exploring whether the persistence of virus in the body, miniature blood clots in the body and brain, and changes to the gut microbiome affect the mental health of people with long COVID.
Some mental health symptoms – such as fatigue, brain fog, sleep disturbances, and tachycardia – can mimic long COVID symptoms, said Dr. Cheng.
The treatment is the same for someone with or without long COVID who has anxiety, depression, posttraumatic stress disorder, or other mental health conditions and includes treatment of coexisting medical conditions, supportive therapy and cognitive-behavioral therapy, and pharmacologic interventions, she said.
“Group therapy may have a particular role in the long COVID population because it really provides that social connection and awareness of additional resources in addition to validation of their experiences,” Dr. Cheng said.
The guidance suggests that primary care practitioners – if it’s within their comfort zone and they have the training – can be the first line for managing mental health symptoms.
But for patients whose symptoms are interfering with functioning and their ability to interact with the community, the guidance urges primary care clinicians to refer the patient to a specialist.
“It leaves the door open to them to practice within their scope but also gives guidance as to how, why, and who should be referred to the next level of care,” said Dr. Cheng.
Coauthor Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine at UT Health San Antonio, Texas, said that although fewer people are now getting long COVID, “it’s still an impactful number.”
The Centers for Disease Control and Prevention recently estimated that about 7% of American adults (18 million) and 1.3% of children had experienced long COVID.
Dr. Gutierrez said that it’s an evolving number, as some patients who have a second or third or fourth SARS-CoV-2 infection experience exacerbations of previous bouts of long COVID or develop long COVID for the first time.
“We are still getting new patients on a regular basis with long COVID,” said AAPM&R President Steven R. Flanagan, MD, a physical medicine specialist.
“This is a problem that really is not going away. It is still real and still ever-present,” said Dr. Flanagan, chair of rehabilitation medicine at NYU Langone Health.
A version of this article first appeared on Medscape.com.
FROM PHYSICAL MEDICINE AND REHABILITATION
Sensory comeback: New findings show the path to smell and taste recovery after COVID
Good news for people struggling with sensory problems after a bout of COVID-19. Although mild cases of the disease often impair the ability to taste and smell, and the problem can drag on for months, a new study from Italy shows that most people return to their senses, as it were, within 3 years.
said Paolo Boscolo-Rizzo, MD, a professor of medicine, surgery, and health sciences at the University of Trieste (Italy), and a co-author of the study, published as a research letter in JAMA Otolaryngology–Head & Neck Surgery.
Dr. Boscolo-Rizzo and his colleagues analyzed data from 88 adults with mild COVID-19, which was defined as having no lower respiratory disease and blood oxygen saturation of 94% or greater. Another group of 88 adults who never contracted the virus but sometimes had difficulties with smell and taste were also studied. In both groups, the average age was 49 years, all participants were White, and 58% were women.
The researchers tested participants’ sense of smell with sticks that contained different odors and checked their sense of taste with strips that had different tastes. Over time, fewer people had difficulty distinguishing odors. Three years after developing COVID-19, only 12 people had impaired smell, compared with 36 people at year 1 and 24 people at year 2. And at the 3-year mark, all participants had at least a partial ability to smell.
The story was similar with sense of taste, with 10 of 88 people reporting impairments 3 years later. By then, people with COVID-19 were no more likely to have trouble with smell or taste than people who did not get the virus.
A study this past June showed a strong correlation between severity of COVID-19 symptoms and impaired sense of taste and smell and estimated that millions of Americans maintained altered senses. More than 10% of people in the Italian study still had trouble with smell or taste 3 years later.
Emerging treatments, psychological concerns
“We’re seeing fewer people with this problem, but there are still people suffering from it,” said Fernando Carnavali, MD, an internal medicine physician and a site director for the Center for Post-COVID Care at the Icahn School of Medicine at Mount Sinai, New York City.
Dr. Carnavali wasn’t part of this study, but he did find the new results encouraging, and he called for similar studies in diverse populations that have experienced COVID-19. He also noted that an impaired sense of smell is distressing.
“It really has a significant psychological impact,” Dr. Carnavali said.
He recalled a patient crying in his office because her inability to smell made it impossible for her to cook. Dr. Carnavali recommended clinicians refer patients facing protracted loss of smell or taste to mental health professionals for support.
Treatments are emerging for COVID-19 smell loss. One approach is to inject platelet-rich plasma into a patient’s nasal cavities to help neurons related to smell repair themselves.
A randomized trial showed platelet-rich plasma significantly outperformed placebo in patients with smell loss up to a year after getting COVID-19.
“I wish more people would do it,” said Zara Patel, MD, an otolaryngologist at Stanford (Calif.) Medicine, who helped conduct that trial. She said some physicians may be nervous about injecting plasma so close to the skull and are therefore hesitant to try this approach.
Another technique may help to address the olfactory condition known as parosmia, in which patients generally experience a benign odor as rancid, according to otolaryngologist Nyssa Farrell, MD, of Washington University School of Medicine, St. Louis. Dr. Farrell said around two-thirds of patients who contract COVID-19 develop the condition, and the rates of long-term parosmia range from 10%-50% depending on various studies.
“It is almost always foul; this can profoundly affect someone’s quality of life,” impairing their ability to eat or to be intimate with a partner who now smells unpleasant, said Dr. Farrell, who wasn’t associated with this research.
The treatment, called a stellate ganglion block, is provided through a shot into nerves in the neck. People with parosmia associated with COVID-19 often report that this method cures them. Dr. Patel said that may be because their psychological health is improving, not their sense of smell, because the area of the body where the stellate ganglion block is applied is not part of the olfactory system.
Earlier this year, Dr. Farrell and colleagues reported that parosmia linked to COVID-19 is associated with an increased risk for depression, anxiety, and suicidal ideation.
One coauthor reported receiving grants from Smell and Taste Lab, Takasago, Baia Foods, and Frequency Therapeutics. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Good news for people struggling with sensory problems after a bout of COVID-19. Although mild cases of the disease often impair the ability to taste and smell, and the problem can drag on for months, a new study from Italy shows that most people return to their senses, as it were, within 3 years.
said Paolo Boscolo-Rizzo, MD, a professor of medicine, surgery, and health sciences at the University of Trieste (Italy), and a co-author of the study, published as a research letter in JAMA Otolaryngology–Head & Neck Surgery.
Dr. Boscolo-Rizzo and his colleagues analyzed data from 88 adults with mild COVID-19, which was defined as having no lower respiratory disease and blood oxygen saturation of 94% or greater. Another group of 88 adults who never contracted the virus but sometimes had difficulties with smell and taste were also studied. In both groups, the average age was 49 years, all participants were White, and 58% were women.
The researchers tested participants’ sense of smell with sticks that contained different odors and checked their sense of taste with strips that had different tastes. Over time, fewer people had difficulty distinguishing odors. Three years after developing COVID-19, only 12 people had impaired smell, compared with 36 people at year 1 and 24 people at year 2. And at the 3-year mark, all participants had at least a partial ability to smell.
The story was similar with sense of taste, with 10 of 88 people reporting impairments 3 years later. By then, people with COVID-19 were no more likely to have trouble with smell or taste than people who did not get the virus.
A study this past June showed a strong correlation between severity of COVID-19 symptoms and impaired sense of taste and smell and estimated that millions of Americans maintained altered senses. More than 10% of people in the Italian study still had trouble with smell or taste 3 years later.
Emerging treatments, psychological concerns
“We’re seeing fewer people with this problem, but there are still people suffering from it,” said Fernando Carnavali, MD, an internal medicine physician and a site director for the Center for Post-COVID Care at the Icahn School of Medicine at Mount Sinai, New York City.
Dr. Carnavali wasn’t part of this study, but he did find the new results encouraging, and he called for similar studies in diverse populations that have experienced COVID-19. He also noted that an impaired sense of smell is distressing.
“It really has a significant psychological impact,” Dr. Carnavali said.
He recalled a patient crying in his office because her inability to smell made it impossible for her to cook. Dr. Carnavali recommended clinicians refer patients facing protracted loss of smell or taste to mental health professionals for support.
Treatments are emerging for COVID-19 smell loss. One approach is to inject platelet-rich plasma into a patient’s nasal cavities to help neurons related to smell repair themselves.
A randomized trial showed platelet-rich plasma significantly outperformed placebo in patients with smell loss up to a year after getting COVID-19.
“I wish more people would do it,” said Zara Patel, MD, an otolaryngologist at Stanford (Calif.) Medicine, who helped conduct that trial. She said some physicians may be nervous about injecting plasma so close to the skull and are therefore hesitant to try this approach.
Another technique may help to address the olfactory condition known as parosmia, in which patients generally experience a benign odor as rancid, according to otolaryngologist Nyssa Farrell, MD, of Washington University School of Medicine, St. Louis. Dr. Farrell said around two-thirds of patients who contract COVID-19 develop the condition, and the rates of long-term parosmia range from 10%-50% depending on various studies.
“It is almost always foul; this can profoundly affect someone’s quality of life,” impairing their ability to eat or to be intimate with a partner who now smells unpleasant, said Dr. Farrell, who wasn’t associated with this research.
The treatment, called a stellate ganglion block, is provided through a shot into nerves in the neck. People with parosmia associated with COVID-19 often report that this method cures them. Dr. Patel said that may be because their psychological health is improving, not their sense of smell, because the area of the body where the stellate ganglion block is applied is not part of the olfactory system.
Earlier this year, Dr. Farrell and colleagues reported that parosmia linked to COVID-19 is associated with an increased risk for depression, anxiety, and suicidal ideation.
One coauthor reported receiving grants from Smell and Taste Lab, Takasago, Baia Foods, and Frequency Therapeutics. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Good news for people struggling with sensory problems after a bout of COVID-19. Although mild cases of the disease often impair the ability to taste and smell, and the problem can drag on for months, a new study from Italy shows that most people return to their senses, as it were, within 3 years.
said Paolo Boscolo-Rizzo, MD, a professor of medicine, surgery, and health sciences at the University of Trieste (Italy), and a co-author of the study, published as a research letter in JAMA Otolaryngology–Head & Neck Surgery.
Dr. Boscolo-Rizzo and his colleagues analyzed data from 88 adults with mild COVID-19, which was defined as having no lower respiratory disease and blood oxygen saturation of 94% or greater. Another group of 88 adults who never contracted the virus but sometimes had difficulties with smell and taste were also studied. In both groups, the average age was 49 years, all participants were White, and 58% were women.
The researchers tested participants’ sense of smell with sticks that contained different odors and checked their sense of taste with strips that had different tastes. Over time, fewer people had difficulty distinguishing odors. Three years after developing COVID-19, only 12 people had impaired smell, compared with 36 people at year 1 and 24 people at year 2. And at the 3-year mark, all participants had at least a partial ability to smell.
The story was similar with sense of taste, with 10 of 88 people reporting impairments 3 years later. By then, people with COVID-19 were no more likely to have trouble with smell or taste than people who did not get the virus.
A study this past June showed a strong correlation between severity of COVID-19 symptoms and impaired sense of taste and smell and estimated that millions of Americans maintained altered senses. More than 10% of people in the Italian study still had trouble with smell or taste 3 years later.
Emerging treatments, psychological concerns
“We’re seeing fewer people with this problem, but there are still people suffering from it,” said Fernando Carnavali, MD, an internal medicine physician and a site director for the Center for Post-COVID Care at the Icahn School of Medicine at Mount Sinai, New York City.
Dr. Carnavali wasn’t part of this study, but he did find the new results encouraging, and he called for similar studies in diverse populations that have experienced COVID-19. He also noted that an impaired sense of smell is distressing.
“It really has a significant psychological impact,” Dr. Carnavali said.
He recalled a patient crying in his office because her inability to smell made it impossible for her to cook. Dr. Carnavali recommended clinicians refer patients facing protracted loss of smell or taste to mental health professionals for support.
Treatments are emerging for COVID-19 smell loss. One approach is to inject platelet-rich plasma into a patient’s nasal cavities to help neurons related to smell repair themselves.
A randomized trial showed platelet-rich plasma significantly outperformed placebo in patients with smell loss up to a year after getting COVID-19.
“I wish more people would do it,” said Zara Patel, MD, an otolaryngologist at Stanford (Calif.) Medicine, who helped conduct that trial. She said some physicians may be nervous about injecting plasma so close to the skull and are therefore hesitant to try this approach.
Another technique may help to address the olfactory condition known as parosmia, in which patients generally experience a benign odor as rancid, according to otolaryngologist Nyssa Farrell, MD, of Washington University School of Medicine, St. Louis. Dr. Farrell said around two-thirds of patients who contract COVID-19 develop the condition, and the rates of long-term parosmia range from 10%-50% depending on various studies.
“It is almost always foul; this can profoundly affect someone’s quality of life,” impairing their ability to eat or to be intimate with a partner who now smells unpleasant, said Dr. Farrell, who wasn’t associated with this research.
The treatment, called a stellate ganglion block, is provided through a shot into nerves in the neck. People with parosmia associated with COVID-19 often report that this method cures them. Dr. Patel said that may be because their psychological health is improving, not their sense of smell, because the area of the body where the stellate ganglion block is applied is not part of the olfactory system.
Earlier this year, Dr. Farrell and colleagues reported that parosmia linked to COVID-19 is associated with an increased risk for depression, anxiety, and suicidal ideation.
One coauthor reported receiving grants from Smell and Taste Lab, Takasago, Baia Foods, and Frequency Therapeutics. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA OTOLARYNGOLOGY–HEAD & NECK SURGERY