User login
Spike in Schizophrenia-Related ED Visits During COVID
TOPLINE:
, a new study showed. Researchers said the findings suggested a need for social policies that strengthen mental health prevention systems.
METHODOLOGY:
Investigators obtained data from the University of California (UC) Health Data Warehouse on ED visits at five large UC health systems.
They captured the ICD-10 codes relating to schizophrenia spectrum disorders for ED visits from January 2016 to December 2021 for patients aged 18 years and older.
TAKEAWAY:
Between January 2016 and December 2021, there were 377,800 psychiatric ED visits, 10% of which involved schizophrenia spectrum disorders.
The mean number of visits per month for schizophrenia spectrum disorders rose from 520 before the pandemic to 558 visits per month after March 2020.
Compared to prepandemic numbers and after controlling for visits for other psychiatric disorders, there were 70.5 additional visits (P = .02) for schizophrenia spectrum disorders at 1 month and 74.9 additional visits (P = .005) at 3 months following the initial phase of the COVID-19 pandemic in California.
Investigators noted that prior studies indicated that COVID-19 infections may induce psychosis in some individuals, which could have been one underlying factor in the spike in cases.
IN PRACTICE:
“The COVID-19 pandemic draws attention to the vulnerability of patients with schizophrenia to macrosocial shocks, underscoring the importance of social policies related to income support, housing, and health insurance for future emergency preparedness and the need to strengthen mental healthcare systems,” the authors wrote.
SOURCE:
Parvita Singh, PhD, of The Ohio State University in Columbus, led the study, which was published online in JAMA Network Open.
LIMITATIONS:
Data used in the study excluded patients younger than 18 years. In addition, there was no analysis for trends by age or sex, which could have added valuable information to the study, the authors wrote. There was also no way to identify patients with newly diagnosed schizophrenia.
DISCLOSURES:
The study was funded through the Coronavirus Response and Relief Supplemental Appropriations Act and the Ohio Department of Mental Health and Addiction Services. Study disclosures are noted in the original study.
A version of this article appeared on Medscape.com.
TOPLINE:
, a new study showed. Researchers said the findings suggested a need for social policies that strengthen mental health prevention systems.
METHODOLOGY:
Investigators obtained data from the University of California (UC) Health Data Warehouse on ED visits at five large UC health systems.
They captured the ICD-10 codes relating to schizophrenia spectrum disorders for ED visits from January 2016 to December 2021 for patients aged 18 years and older.
TAKEAWAY:
Between January 2016 and December 2021, there were 377,800 psychiatric ED visits, 10% of which involved schizophrenia spectrum disorders.
The mean number of visits per month for schizophrenia spectrum disorders rose from 520 before the pandemic to 558 visits per month after March 2020.
Compared to prepandemic numbers and after controlling for visits for other psychiatric disorders, there were 70.5 additional visits (P = .02) for schizophrenia spectrum disorders at 1 month and 74.9 additional visits (P = .005) at 3 months following the initial phase of the COVID-19 pandemic in California.
Investigators noted that prior studies indicated that COVID-19 infections may induce psychosis in some individuals, which could have been one underlying factor in the spike in cases.
IN PRACTICE:
“The COVID-19 pandemic draws attention to the vulnerability of patients with schizophrenia to macrosocial shocks, underscoring the importance of social policies related to income support, housing, and health insurance for future emergency preparedness and the need to strengthen mental healthcare systems,” the authors wrote.
SOURCE:
Parvita Singh, PhD, of The Ohio State University in Columbus, led the study, which was published online in JAMA Network Open.
LIMITATIONS:
Data used in the study excluded patients younger than 18 years. In addition, there was no analysis for trends by age or sex, which could have added valuable information to the study, the authors wrote. There was also no way to identify patients with newly diagnosed schizophrenia.
DISCLOSURES:
The study was funded through the Coronavirus Response and Relief Supplemental Appropriations Act and the Ohio Department of Mental Health and Addiction Services. Study disclosures are noted in the original study.
A version of this article appeared on Medscape.com.
TOPLINE:
, a new study showed. Researchers said the findings suggested a need for social policies that strengthen mental health prevention systems.
METHODOLOGY:
Investigators obtained data from the University of California (UC) Health Data Warehouse on ED visits at five large UC health systems.
They captured the ICD-10 codes relating to schizophrenia spectrum disorders for ED visits from January 2016 to December 2021 for patients aged 18 years and older.
TAKEAWAY:
Between January 2016 and December 2021, there were 377,800 psychiatric ED visits, 10% of which involved schizophrenia spectrum disorders.
The mean number of visits per month for schizophrenia spectrum disorders rose from 520 before the pandemic to 558 visits per month after March 2020.
Compared to prepandemic numbers and after controlling for visits for other psychiatric disorders, there were 70.5 additional visits (P = .02) for schizophrenia spectrum disorders at 1 month and 74.9 additional visits (P = .005) at 3 months following the initial phase of the COVID-19 pandemic in California.
Investigators noted that prior studies indicated that COVID-19 infections may induce psychosis in some individuals, which could have been one underlying factor in the spike in cases.
IN PRACTICE:
“The COVID-19 pandemic draws attention to the vulnerability of patients with schizophrenia to macrosocial shocks, underscoring the importance of social policies related to income support, housing, and health insurance for future emergency preparedness and the need to strengthen mental healthcare systems,” the authors wrote.
SOURCE:
Parvita Singh, PhD, of The Ohio State University in Columbus, led the study, which was published online in JAMA Network Open.
LIMITATIONS:
Data used in the study excluded patients younger than 18 years. In addition, there was no analysis for trends by age or sex, which could have added valuable information to the study, the authors wrote. There was also no way to identify patients with newly diagnosed schizophrenia.
DISCLOSURES:
The study was funded through the Coronavirus Response and Relief Supplemental Appropriations Act and the Ohio Department of Mental Health and Addiction Services. Study disclosures are noted in the original study.
A version of this article appeared on Medscape.com.
Long COVID Has Caused Thousands of US Deaths: New CDC Data
While COVID has now claimed more than 1 million lives in the United States alone, these aren’t the only fatalities caused at least in part by the virus. A small but growing number of Americans are surviving acute infections only to succumb months later to the lingering health problems caused by long COVID.
Much of the attention on long COVID has centered on the sometimes debilitating symptoms that strike people with the condition, with no formal diagnostic tests or standard treatments available, and the effect it has on quality of life. But new figures from the US Centers for Disease Control and Prevention (CDC) show that long COVID can also be deadly.
More than 5000 Americans have died from long COVID since the start of the pandemic, according to new estimates from the CDC.
This total, based on death certificate data collected by the CDC, includes a preliminary tally of 1491 long COVID deaths in 2023 in addition to 3544 fatalities previously reported from January 2020 through June 2022.
Guidance issued in 2023 on how to formally report long COVID as a cause of death on death certificates should help get a more accurate count of these fatalities going forward, said Robert Anderson, PhD, chief mortality statistician for the CDC, Atlanta, Georgia.
“We hope that the guidance will help cause of death certifiers be more aware of the impact of long COVID and more likely to report long COVID as a cause of death when appropriate,” Dr. Anderson said. “That said, we do not expect that this guidance will have a dramatic impact on the trend.”
There’s no standard definition or diagnostic test for long COVID. It’s typically diagnosed when people have symptoms at least 3 months after an acute infection that weren’t present before they got sick. As of the end of last year, about 7% of American adults had experienced long COVID at some point, the CDC estimated in September 2023.
The new death tally indicates long COVID remains a significant public health threat and is likely to grow in the years ahead, even though the pandemic may no longer be considered a global health crisis, experts said.
For example, the death certificate figures indicate:
COVID-19 was the third leading cause of American deaths in 2020 and 2021, and the fourth leading cause of death in the United States in 2023.
Nearly 1% of the more than one million deaths related to COVID-19 since the start of the pandemic have been attributed to long COVID, according to data released by the CDC.
The proportion of COVID-related deaths from long COVID peaked in June 2021 at 1.2% and again in April 2022 at 3.8%, according to the CDC. Both of these peaks coincided with periods of declining fatalities from acute infections.
“I do expect that deaths associated with long COVID will make up an increasingly larger proportion of total deaths associated with COVID-19,” said Mark Czeisler, PhD, a researcher at Harvard Medical School, Boston, Massachusetts, who has studied long COVID fatalities.
Months and even years after an acute infection, long COVID can contribute to serious and potentially life-threatening conditions that impact nearly every major system in the body, according to the CDC guidelines for identifying the condition on death certificates.
This means long COVID may often be listed as an underlying cause of death when people with this condition die of issues related to their heart, lungs, brain or kidneys, the CDC guidelines noted.
The risk for long COVID fatalities remains elevated for at least 6 months for people with milder acute infections and for at least 2 years in severe cases that require hospitalization, some previous research suggested.
As happens with other acute infections, certain people are more at risk for fatal case of long COVID. Age, race, and ethnicity have all been cited as risk factors by researchers who have been tracking the condition since the start of the pandemic.
Half of long COVID fatalities from July 2021 to June 2022 occurred in people aged 65 years and older, and another 23% were recorded among people aged 50-64 years old, according a report from CDC.
Long COVID death rates also varied by race and ethnicity, from a high of 14.1 cases per million among America Indian and Alaskan natives to a low of 1.5 cases per million among Asian people, the CDC found. Death rates per million were 6.7 for White individuals, 6.4 for Black people, and 4.7 for Hispanic people.
The disproportionate share of Black and Hispanic people who developed and died from severe acute infections may have left fewer survivors to develop long COVID, limiting long COVID fatalities among these groups, the CDC report concluded.
It’s also possible that long COVID fatalities were undercounted in these populations because they faced challenges accessing healthcare or seeing providers who could recognize the hallmark symptoms of long COVID.
It’s also difficult to distinguish between how many deaths related to the virus ultimately occur as a result of long COVID rather than acute infections. That’s because it may depend on a variety of factors, including how consistently medical examiners follow the CDC guidelines, said Ziyad Al-Aly, MD, chief of research at the Veterans Affairs, St. Louis Health Care System and a senior clinical epidemiologist at Washington University in St. Louis.
“Long COVID remains massively underdiagnosed, and death in people with long COVID is misattributed to other things,” Dr. Al-Aly said.
An accurate test for long COVID could help lead to a more accurate count of these fatalities, Dr. Czeisler said. Some preliminary research suggests that it might one day be possible to diagnose long COVID with a blood test.
“The timeline for such a test and the extent to which it would be widely applied is uncertain,” Dr. Czeisler noted, “though that would certainly be a gamechanger.”
A version of this article appeared on Medscape.com.
While COVID has now claimed more than 1 million lives in the United States alone, these aren’t the only fatalities caused at least in part by the virus. A small but growing number of Americans are surviving acute infections only to succumb months later to the lingering health problems caused by long COVID.
Much of the attention on long COVID has centered on the sometimes debilitating symptoms that strike people with the condition, with no formal diagnostic tests or standard treatments available, and the effect it has on quality of life. But new figures from the US Centers for Disease Control and Prevention (CDC) show that long COVID can also be deadly.
More than 5000 Americans have died from long COVID since the start of the pandemic, according to new estimates from the CDC.
This total, based on death certificate data collected by the CDC, includes a preliminary tally of 1491 long COVID deaths in 2023 in addition to 3544 fatalities previously reported from January 2020 through June 2022.
Guidance issued in 2023 on how to formally report long COVID as a cause of death on death certificates should help get a more accurate count of these fatalities going forward, said Robert Anderson, PhD, chief mortality statistician for the CDC, Atlanta, Georgia.
“We hope that the guidance will help cause of death certifiers be more aware of the impact of long COVID and more likely to report long COVID as a cause of death when appropriate,” Dr. Anderson said. “That said, we do not expect that this guidance will have a dramatic impact on the trend.”
There’s no standard definition or diagnostic test for long COVID. It’s typically diagnosed when people have symptoms at least 3 months after an acute infection that weren’t present before they got sick. As of the end of last year, about 7% of American adults had experienced long COVID at some point, the CDC estimated in September 2023.
The new death tally indicates long COVID remains a significant public health threat and is likely to grow in the years ahead, even though the pandemic may no longer be considered a global health crisis, experts said.
For example, the death certificate figures indicate:
COVID-19 was the third leading cause of American deaths in 2020 and 2021, and the fourth leading cause of death in the United States in 2023.
Nearly 1% of the more than one million deaths related to COVID-19 since the start of the pandemic have been attributed to long COVID, according to data released by the CDC.
The proportion of COVID-related deaths from long COVID peaked in June 2021 at 1.2% and again in April 2022 at 3.8%, according to the CDC. Both of these peaks coincided with periods of declining fatalities from acute infections.
“I do expect that deaths associated with long COVID will make up an increasingly larger proportion of total deaths associated with COVID-19,” said Mark Czeisler, PhD, a researcher at Harvard Medical School, Boston, Massachusetts, who has studied long COVID fatalities.
Months and even years after an acute infection, long COVID can contribute to serious and potentially life-threatening conditions that impact nearly every major system in the body, according to the CDC guidelines for identifying the condition on death certificates.
This means long COVID may often be listed as an underlying cause of death when people with this condition die of issues related to their heart, lungs, brain or kidneys, the CDC guidelines noted.
The risk for long COVID fatalities remains elevated for at least 6 months for people with milder acute infections and for at least 2 years in severe cases that require hospitalization, some previous research suggested.
As happens with other acute infections, certain people are more at risk for fatal case of long COVID. Age, race, and ethnicity have all been cited as risk factors by researchers who have been tracking the condition since the start of the pandemic.
Half of long COVID fatalities from July 2021 to June 2022 occurred in people aged 65 years and older, and another 23% were recorded among people aged 50-64 years old, according a report from CDC.
Long COVID death rates also varied by race and ethnicity, from a high of 14.1 cases per million among America Indian and Alaskan natives to a low of 1.5 cases per million among Asian people, the CDC found. Death rates per million were 6.7 for White individuals, 6.4 for Black people, and 4.7 for Hispanic people.
The disproportionate share of Black and Hispanic people who developed and died from severe acute infections may have left fewer survivors to develop long COVID, limiting long COVID fatalities among these groups, the CDC report concluded.
It’s also possible that long COVID fatalities were undercounted in these populations because they faced challenges accessing healthcare or seeing providers who could recognize the hallmark symptoms of long COVID.
It’s also difficult to distinguish between how many deaths related to the virus ultimately occur as a result of long COVID rather than acute infections. That’s because it may depend on a variety of factors, including how consistently medical examiners follow the CDC guidelines, said Ziyad Al-Aly, MD, chief of research at the Veterans Affairs, St. Louis Health Care System and a senior clinical epidemiologist at Washington University in St. Louis.
“Long COVID remains massively underdiagnosed, and death in people with long COVID is misattributed to other things,” Dr. Al-Aly said.
An accurate test for long COVID could help lead to a more accurate count of these fatalities, Dr. Czeisler said. Some preliminary research suggests that it might one day be possible to diagnose long COVID with a blood test.
“The timeline for such a test and the extent to which it would be widely applied is uncertain,” Dr. Czeisler noted, “though that would certainly be a gamechanger.”
A version of this article appeared on Medscape.com.
While COVID has now claimed more than 1 million lives in the United States alone, these aren’t the only fatalities caused at least in part by the virus. A small but growing number of Americans are surviving acute infections only to succumb months later to the lingering health problems caused by long COVID.
Much of the attention on long COVID has centered on the sometimes debilitating symptoms that strike people with the condition, with no formal diagnostic tests or standard treatments available, and the effect it has on quality of life. But new figures from the US Centers for Disease Control and Prevention (CDC) show that long COVID can also be deadly.
More than 5000 Americans have died from long COVID since the start of the pandemic, according to new estimates from the CDC.
This total, based on death certificate data collected by the CDC, includes a preliminary tally of 1491 long COVID deaths in 2023 in addition to 3544 fatalities previously reported from January 2020 through June 2022.
Guidance issued in 2023 on how to formally report long COVID as a cause of death on death certificates should help get a more accurate count of these fatalities going forward, said Robert Anderson, PhD, chief mortality statistician for the CDC, Atlanta, Georgia.
“We hope that the guidance will help cause of death certifiers be more aware of the impact of long COVID and more likely to report long COVID as a cause of death when appropriate,” Dr. Anderson said. “That said, we do not expect that this guidance will have a dramatic impact on the trend.”
There’s no standard definition or diagnostic test for long COVID. It’s typically diagnosed when people have symptoms at least 3 months after an acute infection that weren’t present before they got sick. As of the end of last year, about 7% of American adults had experienced long COVID at some point, the CDC estimated in September 2023.
The new death tally indicates long COVID remains a significant public health threat and is likely to grow in the years ahead, even though the pandemic may no longer be considered a global health crisis, experts said.
For example, the death certificate figures indicate:
COVID-19 was the third leading cause of American deaths in 2020 and 2021, and the fourth leading cause of death in the United States in 2023.
Nearly 1% of the more than one million deaths related to COVID-19 since the start of the pandemic have been attributed to long COVID, according to data released by the CDC.
The proportion of COVID-related deaths from long COVID peaked in June 2021 at 1.2% and again in April 2022 at 3.8%, according to the CDC. Both of these peaks coincided with periods of declining fatalities from acute infections.
“I do expect that deaths associated with long COVID will make up an increasingly larger proportion of total deaths associated with COVID-19,” said Mark Czeisler, PhD, a researcher at Harvard Medical School, Boston, Massachusetts, who has studied long COVID fatalities.
Months and even years after an acute infection, long COVID can contribute to serious and potentially life-threatening conditions that impact nearly every major system in the body, according to the CDC guidelines for identifying the condition on death certificates.
This means long COVID may often be listed as an underlying cause of death when people with this condition die of issues related to their heart, lungs, brain or kidneys, the CDC guidelines noted.
The risk for long COVID fatalities remains elevated for at least 6 months for people with milder acute infections and for at least 2 years in severe cases that require hospitalization, some previous research suggested.
As happens with other acute infections, certain people are more at risk for fatal case of long COVID. Age, race, and ethnicity have all been cited as risk factors by researchers who have been tracking the condition since the start of the pandemic.
Half of long COVID fatalities from July 2021 to June 2022 occurred in people aged 65 years and older, and another 23% were recorded among people aged 50-64 years old, according a report from CDC.
Long COVID death rates also varied by race and ethnicity, from a high of 14.1 cases per million among America Indian and Alaskan natives to a low of 1.5 cases per million among Asian people, the CDC found. Death rates per million were 6.7 for White individuals, 6.4 for Black people, and 4.7 for Hispanic people.
The disproportionate share of Black and Hispanic people who developed and died from severe acute infections may have left fewer survivors to develop long COVID, limiting long COVID fatalities among these groups, the CDC report concluded.
It’s also possible that long COVID fatalities were undercounted in these populations because they faced challenges accessing healthcare or seeing providers who could recognize the hallmark symptoms of long COVID.
It’s also difficult to distinguish between how many deaths related to the virus ultimately occur as a result of long COVID rather than acute infections. That’s because it may depend on a variety of factors, including how consistently medical examiners follow the CDC guidelines, said Ziyad Al-Aly, MD, chief of research at the Veterans Affairs, St. Louis Health Care System and a senior clinical epidemiologist at Washington University in St. Louis.
“Long COVID remains massively underdiagnosed, and death in people with long COVID is misattributed to other things,” Dr. Al-Aly said.
An accurate test for long COVID could help lead to a more accurate count of these fatalities, Dr. Czeisler said. Some preliminary research suggests that it might one day be possible to diagnose long COVID with a blood test.
“The timeline for such a test and the extent to which it would be widely applied is uncertain,” Dr. Czeisler noted, “though that would certainly be a gamechanger.”
A version of this article appeared on Medscape.com.
COVID Strain JN.1 Is Now a ‘Variant of Interest,’ WHO Says
the global health agency has announced.
JN.1 was previously grouped with its relative, BA.2.86, but has increased so much in the past 4 weeks that the WHO moved it to standalone status, according to a summary published by the agency. The prevalence of JN.1 worldwide jumped from 3% for the week ending November 5 to 27% for the week ending December 3. During that same period, JN.1 rose from 1% to 66% of cases in the Western Pacific, which stretches across 37 countries, from China and Mongolia to Australia and New Zealand.
In the United States, JN.1 has been increasing rapidly. The variant accounted for an estimated 21% of cases for the 2-week period ending December 9, up from 8% during the 2 weeks prior.
SARS-CoV-2 is the virus that causes COVID, and like other viruses, it evolves over time, sometimes changing how the virus affects people or how well existing treatments and vaccines work against it.
The WHO and CDC have said the current COVID vaccine appears to protect people against severe symptoms due to JN.1, and the WHO called the rising variant’s public health risk “low.”
“As we observe the rise of the JN.1 variant, it’s important to note that while it may be spreading more widely, there is currently no significant evidence suggesting it is more severe or that it poses a substantial public health risk,” John Brownstein, PhD, chief innovation officer at Boston Children’s Hospital, told ABC News.
In its recent risk analysis, the WHO did acknowledge that it’s not certain whether JN.1 has a higher risk of evading immunity or causing more severe symptoms than other strains. The WHO advised countries to further study how much JN.1 can evade existing antibodies and whether the variant results in more severe disease.
The latest CDC data show that 11% of COVID tests reported to the agency are positive, and 23,432 people were hospitalized with severe symptoms within a 7-day period. The CDC urgently asked people to get vaccinated against respiratory illnesses like the flu and COVID-19 ahead of the holidays as cases rise nationwide.
“Getting vaccinated now can help prevent hospitalizations and save lives,” the agency advised.
A version of this article originally appeared on WebMD.com.
the global health agency has announced.
JN.1 was previously grouped with its relative, BA.2.86, but has increased so much in the past 4 weeks that the WHO moved it to standalone status, according to a summary published by the agency. The prevalence of JN.1 worldwide jumped from 3% for the week ending November 5 to 27% for the week ending December 3. During that same period, JN.1 rose from 1% to 66% of cases in the Western Pacific, which stretches across 37 countries, from China and Mongolia to Australia and New Zealand.
In the United States, JN.1 has been increasing rapidly. The variant accounted for an estimated 21% of cases for the 2-week period ending December 9, up from 8% during the 2 weeks prior.
SARS-CoV-2 is the virus that causes COVID, and like other viruses, it evolves over time, sometimes changing how the virus affects people or how well existing treatments and vaccines work against it.
The WHO and CDC have said the current COVID vaccine appears to protect people against severe symptoms due to JN.1, and the WHO called the rising variant’s public health risk “low.”
“As we observe the rise of the JN.1 variant, it’s important to note that while it may be spreading more widely, there is currently no significant evidence suggesting it is more severe or that it poses a substantial public health risk,” John Brownstein, PhD, chief innovation officer at Boston Children’s Hospital, told ABC News.
In its recent risk analysis, the WHO did acknowledge that it’s not certain whether JN.1 has a higher risk of evading immunity or causing more severe symptoms than other strains. The WHO advised countries to further study how much JN.1 can evade existing antibodies and whether the variant results in more severe disease.
The latest CDC data show that 11% of COVID tests reported to the agency are positive, and 23,432 people were hospitalized with severe symptoms within a 7-day period. The CDC urgently asked people to get vaccinated against respiratory illnesses like the flu and COVID-19 ahead of the holidays as cases rise nationwide.
“Getting vaccinated now can help prevent hospitalizations and save lives,” the agency advised.
A version of this article originally appeared on WebMD.com.
the global health agency has announced.
JN.1 was previously grouped with its relative, BA.2.86, but has increased so much in the past 4 weeks that the WHO moved it to standalone status, according to a summary published by the agency. The prevalence of JN.1 worldwide jumped from 3% for the week ending November 5 to 27% for the week ending December 3. During that same period, JN.1 rose from 1% to 66% of cases in the Western Pacific, which stretches across 37 countries, from China and Mongolia to Australia and New Zealand.
In the United States, JN.1 has been increasing rapidly. The variant accounted for an estimated 21% of cases for the 2-week period ending December 9, up from 8% during the 2 weeks prior.
SARS-CoV-2 is the virus that causes COVID, and like other viruses, it evolves over time, sometimes changing how the virus affects people or how well existing treatments and vaccines work against it.
The WHO and CDC have said the current COVID vaccine appears to protect people against severe symptoms due to JN.1, and the WHO called the rising variant’s public health risk “low.”
“As we observe the rise of the JN.1 variant, it’s important to note that while it may be spreading more widely, there is currently no significant evidence suggesting it is more severe or that it poses a substantial public health risk,” John Brownstein, PhD, chief innovation officer at Boston Children’s Hospital, told ABC News.
In its recent risk analysis, the WHO did acknowledge that it’s not certain whether JN.1 has a higher risk of evading immunity or causing more severe symptoms than other strains. The WHO advised countries to further study how much JN.1 can evade existing antibodies and whether the variant results in more severe disease.
The latest CDC data show that 11% of COVID tests reported to the agency are positive, and 23,432 people were hospitalized with severe symptoms within a 7-day period. The CDC urgently asked people to get vaccinated against respiratory illnesses like the flu and COVID-19 ahead of the holidays as cases rise nationwide.
“Getting vaccinated now can help prevent hospitalizations and save lives,” the agency advised.
A version of this article originally appeared on WebMD.com.
Reactive Angioendotheliomatosis Following Ad26.COV2.S Vaccination
To the Editor:
Reactive angioendotheliomatosis (RAE) is a rare self-limited cutaneous vascular proliferation of endothelial cells within blood vessels that manifests clinically as infiltrated red-blue patches and plaques with purpura that can progress to occlude vascular lumina. The etiology of RAE is mostly idiopathic; however, the disorder typically occurs in association with a range of systemic diseases, including infection, cryoglobulinemia, leukemia, antiphospholipid syndrome, peripheral vascular disease, and arteriovenous fistula. Histopathologic examination of these lesions shows marked proliferation of endothelial cells, including occlusion of the lumen of blood vessels over wide areas.
After ruling out malignancy, treatment of RAE focuses on targeting the underlying cause or disease, if any is present; 75% of reported cases occur in association with systemic disease.1 Onset can occur at any age without predilection for sex. Reactive angioendotheliomatosis commonly manifests on the extremities but may occur on the head and neck in rare instances.2
The rarity of the condition and its poorly defined clinical characteristics make it difficult to develop a treatment plan. There are no standardized treatment guidelines for the reactive form of angiomatosis. We report a case of RAE that developed 2 weeks after vaccination with the Ad26.COV2.S vaccine (Johnson & Johnson Innovative Medicine [formerly Janssen Pharmaceutical Companies of Johnson & Johnson]) that improved following 2 weeks of treatment with a topical corticosteroid and an oral antihistamine.
A 58-year-old man presented to an outpatient dermatology clinic with pruritus and occasional paresthesia associated with a rash over the left arm of 1 month’s duration. The patient suspected that the rash may have formed secondary to the bite of oak mites on the arms and chest while he was carrying milled wood. Further inquiry into the patient’s history revealed that he received the Ad26.COV2.S vaccine 2 weeks prior to the appearance of the rash. He denied mechanical trauma. His medical history included hypercholesterolemia and a mild COVID-19 infection 8 months prior to the appearance of the rash that did not require hospitalization. He denied fever or chills during the 2 weeks following vaccination. The pruritus was minimally relieved for short periods with over-the-counter calamine lotion. The patient’s medication regimen included daily pravastatin and loratadine at the time of the initial visit. He used acetaminophen as needed for knee pain.
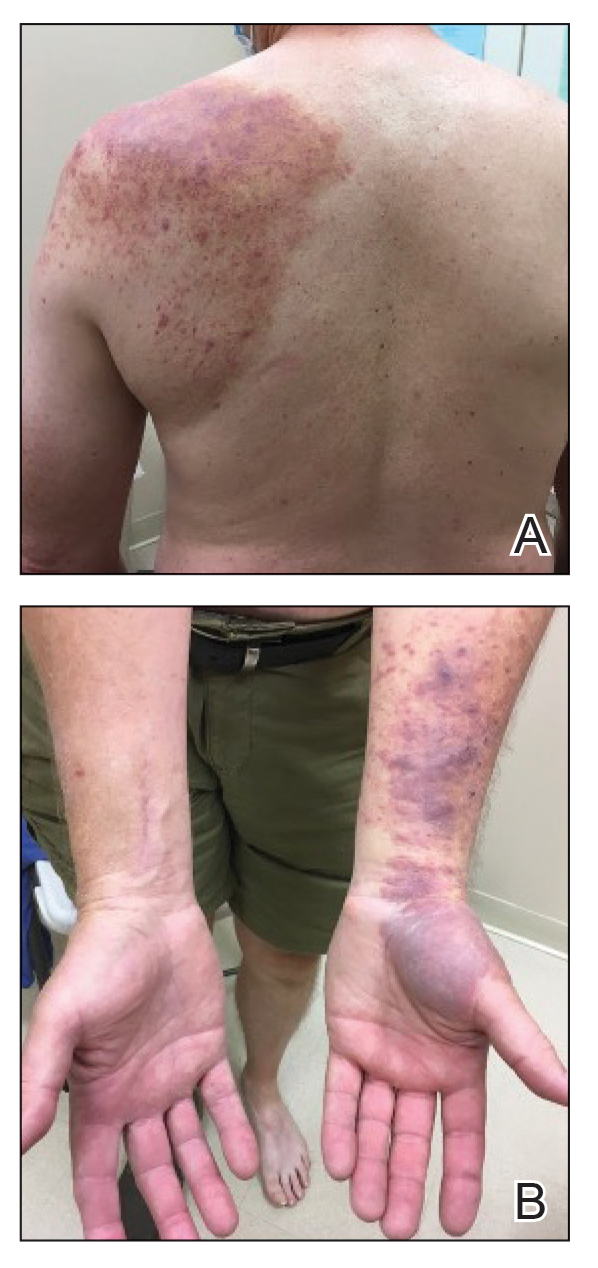
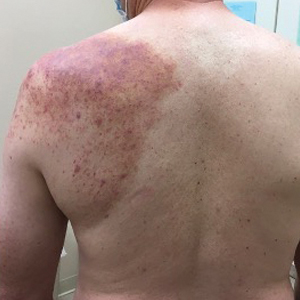
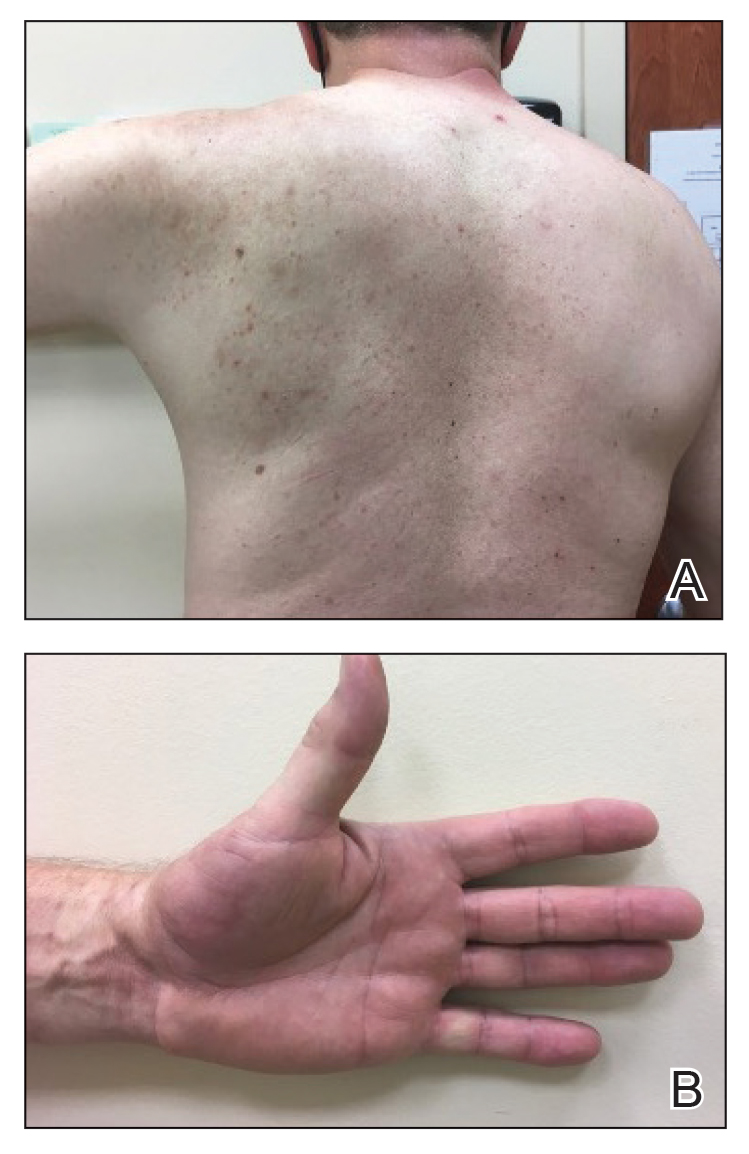
Physical examination revealed palpable purpura in a dermatomal distribution with nonpitting edema over the left scapula (Figure 1A), left anterolateral shoulder, left lateral volar forearm, and thenar eminence of the left hand (Figure 1B). Notably, the entire right arm, conjunctivae, tongue, lips, and bilateral fingernails were clear. Three 4-mm punch biopsies were performed at the initial presentation: 1 perilesional biopsy for direct immunofluorescence testing and 2 lesional biopsies for routine histologic evaluation. An extensive serologic workup failed to reveal abnormalities. An activated partial thromboplastin time, dilute Russell viper venom time, serum protein electrophoresis, and levels of rheumatoid factor and angiotensin-converting enzyme were within reference range. Anticardiolipin antibodies IgA, IgM, and IgG were negative. A cryoglobulin test was negative.
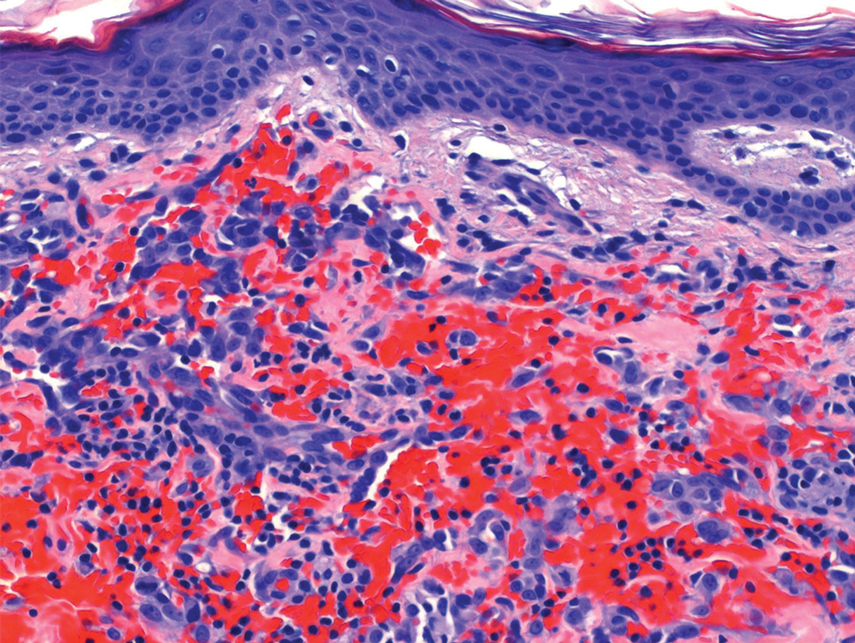
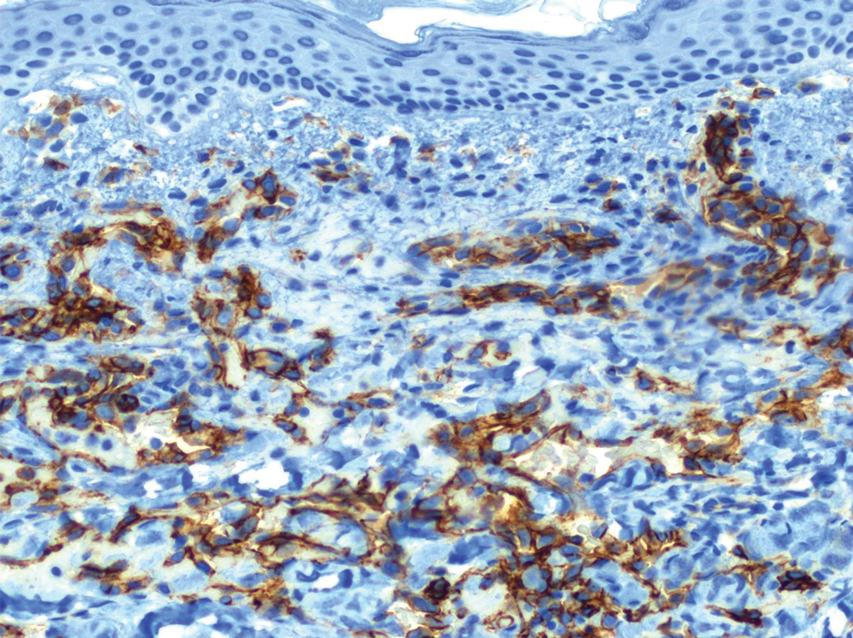
Histopathology revealed a proliferation of irregularly shaped vascular spaces with plump endothelium in the papillary dermis (Figure 2). Scattered leukocyte common antigen-positive lymphocytes were noted within lesions. The epidermis appeared normal, without evidence of spongiosis or alteration of the stratum corneum. Immunohistochemical studies of the perilesional skin biopsy revealed positivity for CD31 and D2-40 (Figure 3). Specimens were negative for CD20 and human herpesvirus 8. Direct immunofluorescence of the perilesional biopsy was negative.
A diagnosis of RAE was made based on clinical and histologic findings. Treatment with triamcinolone ointment 0.1% twice daily and oral cetirizine 10 mg twice daily was initiated. Re-evaluation 2 weeks later revealed notable improvement in the affected areas, including decreased edema, improvement of the purpura, and absence of pruritus. The patient noted no further spread or blister formation while the active areas were being treated with the topical steroid. The treatment regimen was modified to triamcinolone ointment 0.1% once daily, and cetirizine was discontinued. At 3-month follow-up, active areas had completely resolved (Figure 4) and triamcinolone was discontinued. To date, the patient has not had recurrence of symptoms and remains healthy.
Gottron and Nikolowski3 reported the first case of RAE in an adult patient who presented with purpuric patches secondary to skin infarction. Current definitions use the umbrella term cutaneous reactive angiomatosis to cover 3 major subtypes: reactive angioendotheliomatosis, diffuse dermal angioendotheliomatosis, and acroangiodermatitis (pseudo-Kaposi sarcoma [KS]). The manifestation of these subgroups is clinically similar, and they must be differentiated through histologic evaluation.4
Reactive angioendotheliomatosis has an unknown pathogenesis and is poorly defined clinically. The exact pathophysiology is unknown but likely is linked to vaso-occlusion and hypoxia.1 A PubMed search of articles indexed for MEDLINE, as well as a review of Science Direct, Google Scholar, and Cochrane Library, using the terms reactive angioendotheliomatosis, COVID, vaccine, Ad26.COV2.S, and RAE in any combination revealed no prior cases of RAE in association with Ad26.COV2.S vaccination.
By the late 1980s, systemic angioendotheliomatosis was segregated into 2 distinct entities: malignant and reactive.4 The differential diagnosis of malignant systemic angioendotheliomatosis includes KS and angiosarcoma; nonmalignant causes are the variants of cutaneous reactive angiomatosis. It is important to rule out KS because of its malignant and deceptive nature. It is unknown if KS originates in blood vessels or lymphatic endothelial cells; however, evidence is strongly in favor of blood vessel origin using CD31 and CD34 endothelial markers.5 CD34 positivity is more reliable than CD31 in diagnosing KS, but the absence of both markers does not offer enough evidence to rule out KS on its own.6
In our patient, histopathology revealed cells positive for CD31 and D2-40; the latter is a lymphatic endothelial cell marker that stains the endothelium of lymphatic channels but not blood vessels.7 Positive D2-40 can be indicative of KS and non-KS lesions, each with a distinct staining pattern. D2-40 staining on non-KS lesions is confined to lymphatic vessels, as it was in our patient; in contrast, spindle-shaped cells also will be stained in KS lesions.8
Another cell marker, CD20, is a B cell–specific protein that can be measured to help diagnose malignant diseases such as B-cell lymphoma and leukemia. Human herpesvirus 8 (also known as KS-associated herpesvirus) is the infectious cause of KS and traditionally has been detected using methods such as the polymerase chain reaction.9,10
Most cases of RAE are idiopathic and occur in association with systemic disease, which was not the case in our patient. We speculated that his reaction was most likely triggered by vascular transfection of endothelial cells secondary to Ad26.COV2.S vaccination. Alternatively, vaccination may have caused vascular occlusion, though the lack of cyanosis, nail changes, and route of inoculant make this less likely.
All approved COVID-19 vaccines are designed solely for intramuscular injection. In comparison to other types of tissue, muscles have superior vascularity, allowing for enhanced mobilization of compounds, which results in faster systemic circulation.11 Alternative methods of injection, including intravascular, subcutaneous, and intradermal, may lead to decreased efficacy or adverse events, or both.
Prior cases of RAE have been treated with laser therapy, topical or systemic corticosteroids, excisional removal, or topical β-blockers, such as timolol.12 β-Blocking agents act on β-adrenergic receptors on endothelial cells to inhibit angiogenesis by reducing release of blood vessel growth-signaling molecules and triggering apoptosis. In this patient, topical steroids and oral antihistamines were sufficient treatment.
Vaccine-related adverse events have been reported but remain rare. The benefits of Ad26.COV2.S vaccination for protection against COVID-19 outweigh the extremely low risk for adverse events.13 For that reason, the Centers for Disease Control and Prevention recommends a booster for individuals who are eligible to maximize protection. Intramuscular injection of Ad26.COV2.S resulted in a lower incidence of moderate to severe COVID-19 cases in all age groups vs the placebo group. Hypersensitivity adverse events were reported in 0.4% of Ad26.COV2.S-vaccinated patients vs 0.4% of patients who received a placebo; the more common reactions were nonanaphylactic.13
There have been 12 reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, which sparked nationwide controversy over the safety of the Ad26.COV2.S vaccine.14 After further investigation into those reports, the US Food and Drug Administration and the Centers for Disease Control and Prevention concluded that the benefits of the Ad26.COV2.S vaccine outweigh the low risk for associated thrombosis.15
Although adverse reactions are rare, it is important that health care providers take proper safety measures before and while administering any COVID-19 vaccine. Patients should be screened for contraindications to the COVID-19 vaccine to mitigate adverse effects seen in the small percentage of patients who may need to take alternative precautions.
The broad tissue tropism and high transmissibility of SARS-CoV-2 are the main contributors to its infection having reached pandemic scale. The spike (S) protein on SARS-CoV-2 binds to ACE2, the most thoroughly studied SARS-CoV-2 receptor, which is found in a range of tissues, including arterial endothelial cells, leading to its transfection. Several studies have proposed that expression of the S protein causes endothelial dysfunction through cytokine release, activation of complement, and ultimately microvascular occlusion.16
Recent developments in the use of viral-like particles, such as vesicular stomatitis virus, may mitigate future cases of RAE that are associated with endothelial cell transfection. Vesicular stomatitis virus is a popular model virus for research applications due to its glycoprotein and matrix protein contributing to its broad tropism. Recent efforts to alter these proteins have successfully limited the broad tropism of vesicular stomatitis virus.17
The SARS-CoV-2 virus must be handled in a Biosafety Level 3 laboratory. Conversely, pseudoviruses can be handled in lower containment facilities due to their safe and efficacious nature, offering an avenue to expedite vaccine development against many viral outbreaks, including SARS-CoV-2.18
An increasing number of cutaneous manifestations have been associated with COVID-19 infection and vaccination. Eruptive pseudoangiomatosis, a rare self-limiting exanthem, has been reported in association with COVID-19 vaccination.19 Eruptive pseudoangiomatosis manifests as erythematous blanchable papules that resemble angiomas, typically in a widespread distribution. Eruptive pseudoangiomatosis has striking similarities to RAE histologically; both manifest as dilated dermal blood vessels with plump endothelial cells.
Our case is unique because of the vasculitic palpable nature of the lesions, which were localized to the left arm. Eruptive pseudoangiomatosis formation after COVID-19 infection or SARS-CoV-2 vaccination may suggest alteration of ACE2 by binding of S protein.20 Such alteration of the ACE2 pathway would lead to inflammation of angiotensin II, causing proliferation of endothelial cells in the formation of angiomalike lesions. This hypothesis suggests a paraviral eruption secondary to an immunologic reaction, not a classical virtual eruption from direct contact of the virus on blood vessels. Although EPA and RAE are harmless and self-limiting, these reports will spread awareness of the increasing number of skin manifestations related to COVID-19 and SARS-CoV-2 virus vaccination.
Acknowledgment—Thoughtful insights and comments on this manuscript were provided by Christine J. Ko, MD (New Haven, Connecticut); Christine L. Egan, MD (Glen Mills, Pennsylvania); Howard A. Bueller, MD (Delray Beach, Florida); and Juan Pablo Robles, PhD (Juriquilla, Mexico).
- McMenamin ME, Fletcher CDM. Reactive angioendotheliomatosis: a study of 15 cases demonstrating a wide clinicopathologic spectrum. Am J Surg Pathol. 2002;26:686-697. doi:10.1097/00000478-200206000-00001
- Khan S, Pujani M, Jetley S, et al. Angiomatosis: a rare vascular proliferation of head and neck region. J Cutan Aesthet Surg. 2015;8:108-110. doi:10.4103/0974-2077.158448
- Gottron HA, Nikolowski W. Extrarenal Lohlein focal nephritis of the skin in endocarditis. Arch Klin Exp Dermatol. 1958;207:156-176.
- Cooper PH. Angioendotheliomatosis: two separate diseases. J Cutan Pathol. 1988;15:259. doi:10.1111/j.1600-0560.1988.tb00556.x
- Cancian L, Hansen A, Boshoff C. Cellular origin of Kaposi’s sarcoma and Kaposi’s sarcoma-associated herpesvirus-induced cell reprogramming. Trends Cell Biol. Sep 2013;23:421-32. doi:10.1016/j.tcb.2013.04.001
- Russell Jones R, Orchard G, Zelger B, et al. Immunostaining for CD31 and CD34 in Kaposi sarcoma. J Clin Pathol. 1995;48:1011-1016. doi:10.1136/jcp.48.11.1011
- Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15:434-440. doi:10.1038/modpathol.3880543
- Genedy RM, Hamza AM, Abdel Latef AA, et al. Sensitivity and specificity of D2-40 in differentiating Kaposi sarcoma from its mimickers. J Egyptian Womens Dermatolog Soc. 2021;18:67-74. doi:10.4103/jewd.jewd_61_20
- Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10:707-719. doi:10.1038/nrc2888
- Patel RM, Goldblum JR, Hsi ED. Immunohistochemical detection of human herpes virus-8 latent nuclear antigen-1 is useful in the diagnosis of Kaposi sarcoma. Mod Pathol. 2004;17:456-460. doi:10.1038/modpathol.3800061
- Zuckerman JN. The importance of injecting vaccines into muscle. Different patients need different needle sizes. BMJ. 2000;321:1237-1238. doi:10.1136/bmj.321.7271.1237
- Bhatia R, Hazarika N, Chandrasekaran D, et al. Treatment of posttraumatic reactive angioendotheliomatosis with topical timolol maleate. JAMA Dermatol. 2021;157:1002-1004. doi:10.1001/jamadermatol.2021.1770
- Sadoff J, Gray G, Vandebosch A, et al; ENSEMBLE Study Group. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187-2201. doi:10.1056/NEJMoa2101544
- See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448-2456. doi:10.1001/jama.2021.7517
- Berry CT, Eliliwi M, Gallagher S, et al. Cutaneous small vessel vasculitis following single-dose Janssen Ad26.COV2.S vaccination. JAAD Case Rep. 2021;15:11-14. doi:10.1016/j.jdcr.2021.07.002
- Flaumenhaft R, Enjyoji K, Schmaier AA. Vasculopathy in COVID-19. Blood. 2022;140:222-235. doi:10.1182/blood.2021012250
- Hastie E, Cataldi M, Marriott I, et al. Understanding and altering cell tropism of vesicular stomatitis virus. Virus Res. 2013;176:16-32. doi:10.1016/j.virusres.2013.06.003
- Xiong H-L, Wu Y-T, Cao J-L, et al. Robust neutralization assay based on SARS-CoV-2 S-protein-bearing vesicular stomatitis virus (VSV) pseudovirus and ACE2-overexpressing BHK21 cells. Emerg Microbes Infect. 2020;9:2105-2113. doi:10.1080/22221751.2020.1815589
- Mohta A, Jain SK, Mehta RD, et al. Development of eruptive pseudoangiomatosis following COVID-19 immunization – apropos of 5 cases. J Eur Acad Dermatol Venereol. 2021;35:e722-e725. doi:10.1111/jdv.17499
- Angeli F, Spanevello A, Reboldi G, et al. SARS-CoV-2 vaccines: lights and shadows. Eur J Intern Med. 2021;88:1-8. doi:10.1016/j.ejim.2021.04.019
To the Editor:
Reactive angioendotheliomatosis (RAE) is a rare self-limited cutaneous vascular proliferation of endothelial cells within blood vessels that manifests clinically as infiltrated red-blue patches and plaques with purpura that can progress to occlude vascular lumina. The etiology of RAE is mostly idiopathic; however, the disorder typically occurs in association with a range of systemic diseases, including infection, cryoglobulinemia, leukemia, antiphospholipid syndrome, peripheral vascular disease, and arteriovenous fistula. Histopathologic examination of these lesions shows marked proliferation of endothelial cells, including occlusion of the lumen of blood vessels over wide areas.
After ruling out malignancy, treatment of RAE focuses on targeting the underlying cause or disease, if any is present; 75% of reported cases occur in association with systemic disease.1 Onset can occur at any age without predilection for sex. Reactive angioendotheliomatosis commonly manifests on the extremities but may occur on the head and neck in rare instances.2
The rarity of the condition and its poorly defined clinical characteristics make it difficult to develop a treatment plan. There are no standardized treatment guidelines for the reactive form of angiomatosis. We report a case of RAE that developed 2 weeks after vaccination with the Ad26.COV2.S vaccine (Johnson & Johnson Innovative Medicine [formerly Janssen Pharmaceutical Companies of Johnson & Johnson]) that improved following 2 weeks of treatment with a topical corticosteroid and an oral antihistamine.
A 58-year-old man presented to an outpatient dermatology clinic with pruritus and occasional paresthesia associated with a rash over the left arm of 1 month’s duration. The patient suspected that the rash may have formed secondary to the bite of oak mites on the arms and chest while he was carrying milled wood. Further inquiry into the patient’s history revealed that he received the Ad26.COV2.S vaccine 2 weeks prior to the appearance of the rash. He denied mechanical trauma. His medical history included hypercholesterolemia and a mild COVID-19 infection 8 months prior to the appearance of the rash that did not require hospitalization. He denied fever or chills during the 2 weeks following vaccination. The pruritus was minimally relieved for short periods with over-the-counter calamine lotion. The patient’s medication regimen included daily pravastatin and loratadine at the time of the initial visit. He used acetaminophen as needed for knee pain.

Physical examination revealed palpable purpura in a dermatomal distribution with nonpitting edema over the left scapula (Figure 1A), left anterolateral shoulder, left lateral volar forearm, and thenar eminence of the left hand (Figure 1B). Notably, the entire right arm, conjunctivae, tongue, lips, and bilateral fingernails were clear. Three 4-mm punch biopsies were performed at the initial presentation: 1 perilesional biopsy for direct immunofluorescence testing and 2 lesional biopsies for routine histologic evaluation. An extensive serologic workup failed to reveal abnormalities. An activated partial thromboplastin time, dilute Russell viper venom time, serum protein electrophoresis, and levels of rheumatoid factor and angiotensin-converting enzyme were within reference range. Anticardiolipin antibodies IgA, IgM, and IgG were negative. A cryoglobulin test was negative.

Histopathology revealed a proliferation of irregularly shaped vascular spaces with plump endothelium in the papillary dermis (Figure 2). Scattered leukocyte common antigen-positive lymphocytes were noted within lesions. The epidermis appeared normal, without evidence of spongiosis or alteration of the stratum corneum. Immunohistochemical studies of the perilesional skin biopsy revealed positivity for CD31 and D2-40 (Figure 3). Specimens were negative for CD20 and human herpesvirus 8. Direct immunofluorescence of the perilesional biopsy was negative.

A diagnosis of RAE was made based on clinical and histologic findings. Treatment with triamcinolone ointment 0.1% twice daily and oral cetirizine 10 mg twice daily was initiated. Re-evaluation 2 weeks later revealed notable improvement in the affected areas, including decreased edema, improvement of the purpura, and absence of pruritus. The patient noted no further spread or blister formation while the active areas were being treated with the topical steroid. The treatment regimen was modified to triamcinolone ointment 0.1% once daily, and cetirizine was discontinued. At 3-month follow-up, active areas had completely resolved (Figure 4) and triamcinolone was discontinued. To date, the patient has not had recurrence of symptoms and remains healthy.

Gottron and Nikolowski3 reported the first case of RAE in an adult patient who presented with purpuric patches secondary to skin infarction. Current definitions use the umbrella term cutaneous reactive angiomatosis to cover 3 major subtypes: reactive angioendotheliomatosis, diffuse dermal angioendotheliomatosis, and acroangiodermatitis (pseudo-Kaposi sarcoma [KS]). The manifestation of these subgroups is clinically similar, and they must be differentiated through histologic evaluation.4
Reactive angioendotheliomatosis has an unknown pathogenesis and is poorly defined clinically. The exact pathophysiology is unknown but likely is linked to vaso-occlusion and hypoxia.1 A PubMed search of articles indexed for MEDLINE, as well as a review of Science Direct, Google Scholar, and Cochrane Library, using the terms reactive angioendotheliomatosis, COVID, vaccine, Ad26.COV2.S, and RAE in any combination revealed no prior cases of RAE in association with Ad26.COV2.S vaccination.
By the late 1980s, systemic angioendotheliomatosis was segregated into 2 distinct entities: malignant and reactive.4 The differential diagnosis of malignant systemic angioendotheliomatosis includes KS and angiosarcoma; nonmalignant causes are the variants of cutaneous reactive angiomatosis. It is important to rule out KS because of its malignant and deceptive nature. It is unknown if KS originates in blood vessels or lymphatic endothelial cells; however, evidence is strongly in favor of blood vessel origin using CD31 and CD34 endothelial markers.5 CD34 positivity is more reliable than CD31 in diagnosing KS, but the absence of both markers does not offer enough evidence to rule out KS on its own.6
In our patient, histopathology revealed cells positive for CD31 and D2-40; the latter is a lymphatic endothelial cell marker that stains the endothelium of lymphatic channels but not blood vessels.7 Positive D2-40 can be indicative of KS and non-KS lesions, each with a distinct staining pattern. D2-40 staining on non-KS lesions is confined to lymphatic vessels, as it was in our patient; in contrast, spindle-shaped cells also will be stained in KS lesions.8
Another cell marker, CD20, is a B cell–specific protein that can be measured to help diagnose malignant diseases such as B-cell lymphoma and leukemia. Human herpesvirus 8 (also known as KS-associated herpesvirus) is the infectious cause of KS and traditionally has been detected using methods such as the polymerase chain reaction.9,10
Most cases of RAE are idiopathic and occur in association with systemic disease, which was not the case in our patient. We speculated that his reaction was most likely triggered by vascular transfection of endothelial cells secondary to Ad26.COV2.S vaccination. Alternatively, vaccination may have caused vascular occlusion, though the lack of cyanosis, nail changes, and route of inoculant make this less likely.
All approved COVID-19 vaccines are designed solely for intramuscular injection. In comparison to other types of tissue, muscles have superior vascularity, allowing for enhanced mobilization of compounds, which results in faster systemic circulation.11 Alternative methods of injection, including intravascular, subcutaneous, and intradermal, may lead to decreased efficacy or adverse events, or both.
Prior cases of RAE have been treated with laser therapy, topical or systemic corticosteroids, excisional removal, or topical β-blockers, such as timolol.12 β-Blocking agents act on β-adrenergic receptors on endothelial cells to inhibit angiogenesis by reducing release of blood vessel growth-signaling molecules and triggering apoptosis. In this patient, topical steroids and oral antihistamines were sufficient treatment.
Vaccine-related adverse events have been reported but remain rare. The benefits of Ad26.COV2.S vaccination for protection against COVID-19 outweigh the extremely low risk for adverse events.13 For that reason, the Centers for Disease Control and Prevention recommends a booster for individuals who are eligible to maximize protection. Intramuscular injection of Ad26.COV2.S resulted in a lower incidence of moderate to severe COVID-19 cases in all age groups vs the placebo group. Hypersensitivity adverse events were reported in 0.4% of Ad26.COV2.S-vaccinated patients vs 0.4% of patients who received a placebo; the more common reactions were nonanaphylactic.13
There have been 12 reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, which sparked nationwide controversy over the safety of the Ad26.COV2.S vaccine.14 After further investigation into those reports, the US Food and Drug Administration and the Centers for Disease Control and Prevention concluded that the benefits of the Ad26.COV2.S vaccine outweigh the low risk for associated thrombosis.15
Although adverse reactions are rare, it is important that health care providers take proper safety measures before and while administering any COVID-19 vaccine. Patients should be screened for contraindications to the COVID-19 vaccine to mitigate adverse effects seen in the small percentage of patients who may need to take alternative precautions.
The broad tissue tropism and high transmissibility of SARS-CoV-2 are the main contributors to its infection having reached pandemic scale. The spike (S) protein on SARS-CoV-2 binds to ACE2, the most thoroughly studied SARS-CoV-2 receptor, which is found in a range of tissues, including arterial endothelial cells, leading to its transfection. Several studies have proposed that expression of the S protein causes endothelial dysfunction through cytokine release, activation of complement, and ultimately microvascular occlusion.16
Recent developments in the use of viral-like particles, such as vesicular stomatitis virus, may mitigate future cases of RAE that are associated with endothelial cell transfection. Vesicular stomatitis virus is a popular model virus for research applications due to its glycoprotein and matrix protein contributing to its broad tropism. Recent efforts to alter these proteins have successfully limited the broad tropism of vesicular stomatitis virus.17
The SARS-CoV-2 virus must be handled in a Biosafety Level 3 laboratory. Conversely, pseudoviruses can be handled in lower containment facilities due to their safe and efficacious nature, offering an avenue to expedite vaccine development against many viral outbreaks, including SARS-CoV-2.18
An increasing number of cutaneous manifestations have been associated with COVID-19 infection and vaccination. Eruptive pseudoangiomatosis, a rare self-limiting exanthem, has been reported in association with COVID-19 vaccination.19 Eruptive pseudoangiomatosis manifests as erythematous blanchable papules that resemble angiomas, typically in a widespread distribution. Eruptive pseudoangiomatosis has striking similarities to RAE histologically; both manifest as dilated dermal blood vessels with plump endothelial cells.
Our case is unique because of the vasculitic palpable nature of the lesions, which were localized to the left arm. Eruptive pseudoangiomatosis formation after COVID-19 infection or SARS-CoV-2 vaccination may suggest alteration of ACE2 by binding of S protein.20 Such alteration of the ACE2 pathway would lead to inflammation of angiotensin II, causing proliferation of endothelial cells in the formation of angiomalike lesions. This hypothesis suggests a paraviral eruption secondary to an immunologic reaction, not a classical virtual eruption from direct contact of the virus on blood vessels. Although EPA and RAE are harmless and self-limiting, these reports will spread awareness of the increasing number of skin manifestations related to COVID-19 and SARS-CoV-2 virus vaccination.
Acknowledgment—Thoughtful insights and comments on this manuscript were provided by Christine J. Ko, MD (New Haven, Connecticut); Christine L. Egan, MD (Glen Mills, Pennsylvania); Howard A. Bueller, MD (Delray Beach, Florida); and Juan Pablo Robles, PhD (Juriquilla, Mexico).
To the Editor:
Reactive angioendotheliomatosis (RAE) is a rare self-limited cutaneous vascular proliferation of endothelial cells within blood vessels that manifests clinically as infiltrated red-blue patches and plaques with purpura that can progress to occlude vascular lumina. The etiology of RAE is mostly idiopathic; however, the disorder typically occurs in association with a range of systemic diseases, including infection, cryoglobulinemia, leukemia, antiphospholipid syndrome, peripheral vascular disease, and arteriovenous fistula. Histopathologic examination of these lesions shows marked proliferation of endothelial cells, including occlusion of the lumen of blood vessels over wide areas.
After ruling out malignancy, treatment of RAE focuses on targeting the underlying cause or disease, if any is present; 75% of reported cases occur in association with systemic disease.1 Onset can occur at any age without predilection for sex. Reactive angioendotheliomatosis commonly manifests on the extremities but may occur on the head and neck in rare instances.2
The rarity of the condition and its poorly defined clinical characteristics make it difficult to develop a treatment plan. There are no standardized treatment guidelines for the reactive form of angiomatosis. We report a case of RAE that developed 2 weeks after vaccination with the Ad26.COV2.S vaccine (Johnson & Johnson Innovative Medicine [formerly Janssen Pharmaceutical Companies of Johnson & Johnson]) that improved following 2 weeks of treatment with a topical corticosteroid and an oral antihistamine.
A 58-year-old man presented to an outpatient dermatology clinic with pruritus and occasional paresthesia associated with a rash over the left arm of 1 month’s duration. The patient suspected that the rash may have formed secondary to the bite of oak mites on the arms and chest while he was carrying milled wood. Further inquiry into the patient’s history revealed that he received the Ad26.COV2.S vaccine 2 weeks prior to the appearance of the rash. He denied mechanical trauma. His medical history included hypercholesterolemia and a mild COVID-19 infection 8 months prior to the appearance of the rash that did not require hospitalization. He denied fever or chills during the 2 weeks following vaccination. The pruritus was minimally relieved for short periods with over-the-counter calamine lotion. The patient’s medication regimen included daily pravastatin and loratadine at the time of the initial visit. He used acetaminophen as needed for knee pain.

Physical examination revealed palpable purpura in a dermatomal distribution with nonpitting edema over the left scapula (Figure 1A), left anterolateral shoulder, left lateral volar forearm, and thenar eminence of the left hand (Figure 1B). Notably, the entire right arm, conjunctivae, tongue, lips, and bilateral fingernails were clear. Three 4-mm punch biopsies were performed at the initial presentation: 1 perilesional biopsy for direct immunofluorescence testing and 2 lesional biopsies for routine histologic evaluation. An extensive serologic workup failed to reveal abnormalities. An activated partial thromboplastin time, dilute Russell viper venom time, serum protein electrophoresis, and levels of rheumatoid factor and angiotensin-converting enzyme were within reference range. Anticardiolipin antibodies IgA, IgM, and IgG were negative. A cryoglobulin test was negative.

Histopathology revealed a proliferation of irregularly shaped vascular spaces with plump endothelium in the papillary dermis (Figure 2). Scattered leukocyte common antigen-positive lymphocytes were noted within lesions. The epidermis appeared normal, without evidence of spongiosis or alteration of the stratum corneum. Immunohistochemical studies of the perilesional skin biopsy revealed positivity for CD31 and D2-40 (Figure 3). Specimens were negative for CD20 and human herpesvirus 8. Direct immunofluorescence of the perilesional biopsy was negative.

A diagnosis of RAE was made based on clinical and histologic findings. Treatment with triamcinolone ointment 0.1% twice daily and oral cetirizine 10 mg twice daily was initiated. Re-evaluation 2 weeks later revealed notable improvement in the affected areas, including decreased edema, improvement of the purpura, and absence of pruritus. The patient noted no further spread or blister formation while the active areas were being treated with the topical steroid. The treatment regimen was modified to triamcinolone ointment 0.1% once daily, and cetirizine was discontinued. At 3-month follow-up, active areas had completely resolved (Figure 4) and triamcinolone was discontinued. To date, the patient has not had recurrence of symptoms and remains healthy.

Gottron and Nikolowski3 reported the first case of RAE in an adult patient who presented with purpuric patches secondary to skin infarction. Current definitions use the umbrella term cutaneous reactive angiomatosis to cover 3 major subtypes: reactive angioendotheliomatosis, diffuse dermal angioendotheliomatosis, and acroangiodermatitis (pseudo-Kaposi sarcoma [KS]). The manifestation of these subgroups is clinically similar, and they must be differentiated through histologic evaluation.4
Reactive angioendotheliomatosis has an unknown pathogenesis and is poorly defined clinically. The exact pathophysiology is unknown but likely is linked to vaso-occlusion and hypoxia.1 A PubMed search of articles indexed for MEDLINE, as well as a review of Science Direct, Google Scholar, and Cochrane Library, using the terms reactive angioendotheliomatosis, COVID, vaccine, Ad26.COV2.S, and RAE in any combination revealed no prior cases of RAE in association with Ad26.COV2.S vaccination.
By the late 1980s, systemic angioendotheliomatosis was segregated into 2 distinct entities: malignant and reactive.4 The differential diagnosis of malignant systemic angioendotheliomatosis includes KS and angiosarcoma; nonmalignant causes are the variants of cutaneous reactive angiomatosis. It is important to rule out KS because of its malignant and deceptive nature. It is unknown if KS originates in blood vessels or lymphatic endothelial cells; however, evidence is strongly in favor of blood vessel origin using CD31 and CD34 endothelial markers.5 CD34 positivity is more reliable than CD31 in diagnosing KS, but the absence of both markers does not offer enough evidence to rule out KS on its own.6
In our patient, histopathology revealed cells positive for CD31 and D2-40; the latter is a lymphatic endothelial cell marker that stains the endothelium of lymphatic channels but not blood vessels.7 Positive D2-40 can be indicative of KS and non-KS lesions, each with a distinct staining pattern. D2-40 staining on non-KS lesions is confined to lymphatic vessels, as it was in our patient; in contrast, spindle-shaped cells also will be stained in KS lesions.8
Another cell marker, CD20, is a B cell–specific protein that can be measured to help diagnose malignant diseases such as B-cell lymphoma and leukemia. Human herpesvirus 8 (also known as KS-associated herpesvirus) is the infectious cause of KS and traditionally has been detected using methods such as the polymerase chain reaction.9,10
Most cases of RAE are idiopathic and occur in association with systemic disease, which was not the case in our patient. We speculated that his reaction was most likely triggered by vascular transfection of endothelial cells secondary to Ad26.COV2.S vaccination. Alternatively, vaccination may have caused vascular occlusion, though the lack of cyanosis, nail changes, and route of inoculant make this less likely.
All approved COVID-19 vaccines are designed solely for intramuscular injection. In comparison to other types of tissue, muscles have superior vascularity, allowing for enhanced mobilization of compounds, which results in faster systemic circulation.11 Alternative methods of injection, including intravascular, subcutaneous, and intradermal, may lead to decreased efficacy or adverse events, or both.
Prior cases of RAE have been treated with laser therapy, topical or systemic corticosteroids, excisional removal, or topical β-blockers, such as timolol.12 β-Blocking agents act on β-adrenergic receptors on endothelial cells to inhibit angiogenesis by reducing release of blood vessel growth-signaling molecules and triggering apoptosis. In this patient, topical steroids and oral antihistamines were sufficient treatment.
Vaccine-related adverse events have been reported but remain rare. The benefits of Ad26.COV2.S vaccination for protection against COVID-19 outweigh the extremely low risk for adverse events.13 For that reason, the Centers for Disease Control and Prevention recommends a booster for individuals who are eligible to maximize protection. Intramuscular injection of Ad26.COV2.S resulted in a lower incidence of moderate to severe COVID-19 cases in all age groups vs the placebo group. Hypersensitivity adverse events were reported in 0.4% of Ad26.COV2.S-vaccinated patients vs 0.4% of patients who received a placebo; the more common reactions were nonanaphylactic.13
There have been 12 reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, which sparked nationwide controversy over the safety of the Ad26.COV2.S vaccine.14 After further investigation into those reports, the US Food and Drug Administration and the Centers for Disease Control and Prevention concluded that the benefits of the Ad26.COV2.S vaccine outweigh the low risk for associated thrombosis.15
Although adverse reactions are rare, it is important that health care providers take proper safety measures before and while administering any COVID-19 vaccine. Patients should be screened for contraindications to the COVID-19 vaccine to mitigate adverse effects seen in the small percentage of patients who may need to take alternative precautions.
The broad tissue tropism and high transmissibility of SARS-CoV-2 are the main contributors to its infection having reached pandemic scale. The spike (S) protein on SARS-CoV-2 binds to ACE2, the most thoroughly studied SARS-CoV-2 receptor, which is found in a range of tissues, including arterial endothelial cells, leading to its transfection. Several studies have proposed that expression of the S protein causes endothelial dysfunction through cytokine release, activation of complement, and ultimately microvascular occlusion.16
Recent developments in the use of viral-like particles, such as vesicular stomatitis virus, may mitigate future cases of RAE that are associated with endothelial cell transfection. Vesicular stomatitis virus is a popular model virus for research applications due to its glycoprotein and matrix protein contributing to its broad tropism. Recent efforts to alter these proteins have successfully limited the broad tropism of vesicular stomatitis virus.17
The SARS-CoV-2 virus must be handled in a Biosafety Level 3 laboratory. Conversely, pseudoviruses can be handled in lower containment facilities due to their safe and efficacious nature, offering an avenue to expedite vaccine development against many viral outbreaks, including SARS-CoV-2.18
An increasing number of cutaneous manifestations have been associated with COVID-19 infection and vaccination. Eruptive pseudoangiomatosis, a rare self-limiting exanthem, has been reported in association with COVID-19 vaccination.19 Eruptive pseudoangiomatosis manifests as erythematous blanchable papules that resemble angiomas, typically in a widespread distribution. Eruptive pseudoangiomatosis has striking similarities to RAE histologically; both manifest as dilated dermal blood vessels with plump endothelial cells.
Our case is unique because of the vasculitic palpable nature of the lesions, which were localized to the left arm. Eruptive pseudoangiomatosis formation after COVID-19 infection or SARS-CoV-2 vaccination may suggest alteration of ACE2 by binding of S protein.20 Such alteration of the ACE2 pathway would lead to inflammation of angiotensin II, causing proliferation of endothelial cells in the formation of angiomalike lesions. This hypothesis suggests a paraviral eruption secondary to an immunologic reaction, not a classical virtual eruption from direct contact of the virus on blood vessels. Although EPA and RAE are harmless and self-limiting, these reports will spread awareness of the increasing number of skin manifestations related to COVID-19 and SARS-CoV-2 virus vaccination.
Acknowledgment—Thoughtful insights and comments on this manuscript were provided by Christine J. Ko, MD (New Haven, Connecticut); Christine L. Egan, MD (Glen Mills, Pennsylvania); Howard A. Bueller, MD (Delray Beach, Florida); and Juan Pablo Robles, PhD (Juriquilla, Mexico).
- McMenamin ME, Fletcher CDM. Reactive angioendotheliomatosis: a study of 15 cases demonstrating a wide clinicopathologic spectrum. Am J Surg Pathol. 2002;26:686-697. doi:10.1097/00000478-200206000-00001
- Khan S, Pujani M, Jetley S, et al. Angiomatosis: a rare vascular proliferation of head and neck region. J Cutan Aesthet Surg. 2015;8:108-110. doi:10.4103/0974-2077.158448
- Gottron HA, Nikolowski W. Extrarenal Lohlein focal nephritis of the skin in endocarditis. Arch Klin Exp Dermatol. 1958;207:156-176.
- Cooper PH. Angioendotheliomatosis: two separate diseases. J Cutan Pathol. 1988;15:259. doi:10.1111/j.1600-0560.1988.tb00556.x
- Cancian L, Hansen A, Boshoff C. Cellular origin of Kaposi’s sarcoma and Kaposi’s sarcoma-associated herpesvirus-induced cell reprogramming. Trends Cell Biol. Sep 2013;23:421-32. doi:10.1016/j.tcb.2013.04.001
- Russell Jones R, Orchard G, Zelger B, et al. Immunostaining for CD31 and CD34 in Kaposi sarcoma. J Clin Pathol. 1995;48:1011-1016. doi:10.1136/jcp.48.11.1011
- Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15:434-440. doi:10.1038/modpathol.3880543
- Genedy RM, Hamza AM, Abdel Latef AA, et al. Sensitivity and specificity of D2-40 in differentiating Kaposi sarcoma from its mimickers. J Egyptian Womens Dermatolog Soc. 2021;18:67-74. doi:10.4103/jewd.jewd_61_20
- Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10:707-719. doi:10.1038/nrc2888
- Patel RM, Goldblum JR, Hsi ED. Immunohistochemical detection of human herpes virus-8 latent nuclear antigen-1 is useful in the diagnosis of Kaposi sarcoma. Mod Pathol. 2004;17:456-460. doi:10.1038/modpathol.3800061
- Zuckerman JN. The importance of injecting vaccines into muscle. Different patients need different needle sizes. BMJ. 2000;321:1237-1238. doi:10.1136/bmj.321.7271.1237
- Bhatia R, Hazarika N, Chandrasekaran D, et al. Treatment of posttraumatic reactive angioendotheliomatosis with topical timolol maleate. JAMA Dermatol. 2021;157:1002-1004. doi:10.1001/jamadermatol.2021.1770
- Sadoff J, Gray G, Vandebosch A, et al; ENSEMBLE Study Group. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187-2201. doi:10.1056/NEJMoa2101544
- See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448-2456. doi:10.1001/jama.2021.7517
- Berry CT, Eliliwi M, Gallagher S, et al. Cutaneous small vessel vasculitis following single-dose Janssen Ad26.COV2.S vaccination. JAAD Case Rep. 2021;15:11-14. doi:10.1016/j.jdcr.2021.07.002
- Flaumenhaft R, Enjyoji K, Schmaier AA. Vasculopathy in COVID-19. Blood. 2022;140:222-235. doi:10.1182/blood.2021012250
- Hastie E, Cataldi M, Marriott I, et al. Understanding and altering cell tropism of vesicular stomatitis virus. Virus Res. 2013;176:16-32. doi:10.1016/j.virusres.2013.06.003
- Xiong H-L, Wu Y-T, Cao J-L, et al. Robust neutralization assay based on SARS-CoV-2 S-protein-bearing vesicular stomatitis virus (VSV) pseudovirus and ACE2-overexpressing BHK21 cells. Emerg Microbes Infect. 2020;9:2105-2113. doi:10.1080/22221751.2020.1815589
- Mohta A, Jain SK, Mehta RD, et al. Development of eruptive pseudoangiomatosis following COVID-19 immunization – apropos of 5 cases. J Eur Acad Dermatol Venereol. 2021;35:e722-e725. doi:10.1111/jdv.17499
- Angeli F, Spanevello A, Reboldi G, et al. SARS-CoV-2 vaccines: lights and shadows. Eur J Intern Med. 2021;88:1-8. doi:10.1016/j.ejim.2021.04.019
- McMenamin ME, Fletcher CDM. Reactive angioendotheliomatosis: a study of 15 cases demonstrating a wide clinicopathologic spectrum. Am J Surg Pathol. 2002;26:686-697. doi:10.1097/00000478-200206000-00001
- Khan S, Pujani M, Jetley S, et al. Angiomatosis: a rare vascular proliferation of head and neck region. J Cutan Aesthet Surg. 2015;8:108-110. doi:10.4103/0974-2077.158448
- Gottron HA, Nikolowski W. Extrarenal Lohlein focal nephritis of the skin in endocarditis. Arch Klin Exp Dermatol. 1958;207:156-176.
- Cooper PH. Angioendotheliomatosis: two separate diseases. J Cutan Pathol. 1988;15:259. doi:10.1111/j.1600-0560.1988.tb00556.x
- Cancian L, Hansen A, Boshoff C. Cellular origin of Kaposi’s sarcoma and Kaposi’s sarcoma-associated herpesvirus-induced cell reprogramming. Trends Cell Biol. Sep 2013;23:421-32. doi:10.1016/j.tcb.2013.04.001
- Russell Jones R, Orchard G, Zelger B, et al. Immunostaining for CD31 and CD34 in Kaposi sarcoma. J Clin Pathol. 1995;48:1011-1016. doi:10.1136/jcp.48.11.1011
- Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15:434-440. doi:10.1038/modpathol.3880543
- Genedy RM, Hamza AM, Abdel Latef AA, et al. Sensitivity and specificity of D2-40 in differentiating Kaposi sarcoma from its mimickers. J Egyptian Womens Dermatolog Soc. 2021;18:67-74. doi:10.4103/jewd.jewd_61_20
- Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10:707-719. doi:10.1038/nrc2888
- Patel RM, Goldblum JR, Hsi ED. Immunohistochemical detection of human herpes virus-8 latent nuclear antigen-1 is useful in the diagnosis of Kaposi sarcoma. Mod Pathol. 2004;17:456-460. doi:10.1038/modpathol.3800061
- Zuckerman JN. The importance of injecting vaccines into muscle. Different patients need different needle sizes. BMJ. 2000;321:1237-1238. doi:10.1136/bmj.321.7271.1237
- Bhatia R, Hazarika N, Chandrasekaran D, et al. Treatment of posttraumatic reactive angioendotheliomatosis with topical timolol maleate. JAMA Dermatol. 2021;157:1002-1004. doi:10.1001/jamadermatol.2021.1770
- Sadoff J, Gray G, Vandebosch A, et al; ENSEMBLE Study Group. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187-2201. doi:10.1056/NEJMoa2101544
- See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448-2456. doi:10.1001/jama.2021.7517
- Berry CT, Eliliwi M, Gallagher S, et al. Cutaneous small vessel vasculitis following single-dose Janssen Ad26.COV2.S vaccination. JAAD Case Rep. 2021;15:11-14. doi:10.1016/j.jdcr.2021.07.002
- Flaumenhaft R, Enjyoji K, Schmaier AA. Vasculopathy in COVID-19. Blood. 2022;140:222-235. doi:10.1182/blood.2021012250
- Hastie E, Cataldi M, Marriott I, et al. Understanding and altering cell tropism of vesicular stomatitis virus. Virus Res. 2013;176:16-32. doi:10.1016/j.virusres.2013.06.003
- Xiong H-L, Wu Y-T, Cao J-L, et al. Robust neutralization assay based on SARS-CoV-2 S-protein-bearing vesicular stomatitis virus (VSV) pseudovirus and ACE2-overexpressing BHK21 cells. Emerg Microbes Infect. 2020;9:2105-2113. doi:10.1080/22221751.2020.1815589
- Mohta A, Jain SK, Mehta RD, et al. Development of eruptive pseudoangiomatosis following COVID-19 immunization – apropos of 5 cases. J Eur Acad Dermatol Venereol. 2021;35:e722-e725. doi:10.1111/jdv.17499
- Angeli F, Spanevello A, Reboldi G, et al. SARS-CoV-2 vaccines: lights and shadows. Eur J Intern Med. 2021;88:1-8. doi:10.1016/j.ejim.2021.04.019
Practice points
- Reactive angioendotheliomatosis (RAE) is a rare benign vascular proliferation of endothelial cells lining blood vessels that clinically appears similar to Kaposi sarcoma and must be differentiated by microscopic evaluation.
- An increasing number of reports link SARS-CoV-2 viral infection or vaccination against this virus with various cutaneous manifestations. Our case offers a link between RAE and Ad26.COV2.S vaccination.
Federal program offers free COVID, flu at-home tests, treatments
The U.S. government has expanded a program offering free COVID-19 and flu tests and treatment.
The Home Test to Treat program is virtual and offers at-home rapid tests, telehealth sessions, and at-home treatments to people nationwide. The program is a collaboration among the National Institutes of Health, the Administration for Strategic Preparedness and Response, and the CDC. It began as a pilot program in some locations this year.
“With its expansion, the Home Test to Treat program will now offer free testing, telehealth and treatment for both COVID-19 and for influenza (flu) A and B,” the NIH said in a press release. “It is the first public health program that includes home testing technology at such a scale for both COVID-19 and flu.”
The news release says that anyone 18 or over with a current positive test for COVID-19 or flu can get free telehealth care and medicine delivered to their home.
Adults who don’t have COVID-19 or the flu can get free tests if they are uninsured or are enrolled in Medicare, Medicaid, the Veterans Affairs health care system, or Indian Health Services. If they test positive later, they can get free telehealth care and, if prescribed, treatment.
“I think that these [telehealth] delivery mechanisms are going to be absolutely crucial to unburden the in-person offices and the lines that we have and wait times,” said Michael Mina, MD, chief science officer at eMed, the company that helped implement the new Home Test to Treat program, to ABC News.
ABC notes that COVID tests can also be ordered at covidtests.gov – four tests per household or eight for those who have yet to order any this fall.
A version of this article appeared on WebMD.com .
The U.S. government has expanded a program offering free COVID-19 and flu tests and treatment.
The Home Test to Treat program is virtual and offers at-home rapid tests, telehealth sessions, and at-home treatments to people nationwide. The program is a collaboration among the National Institutes of Health, the Administration for Strategic Preparedness and Response, and the CDC. It began as a pilot program in some locations this year.
“With its expansion, the Home Test to Treat program will now offer free testing, telehealth and treatment for both COVID-19 and for influenza (flu) A and B,” the NIH said in a press release. “It is the first public health program that includes home testing technology at such a scale for both COVID-19 and flu.”
The news release says that anyone 18 or over with a current positive test for COVID-19 or flu can get free telehealth care and medicine delivered to their home.
Adults who don’t have COVID-19 or the flu can get free tests if they are uninsured or are enrolled in Medicare, Medicaid, the Veterans Affairs health care system, or Indian Health Services. If they test positive later, they can get free telehealth care and, if prescribed, treatment.
“I think that these [telehealth] delivery mechanisms are going to be absolutely crucial to unburden the in-person offices and the lines that we have and wait times,” said Michael Mina, MD, chief science officer at eMed, the company that helped implement the new Home Test to Treat program, to ABC News.
ABC notes that COVID tests can also be ordered at covidtests.gov – four tests per household or eight for those who have yet to order any this fall.
A version of this article appeared on WebMD.com .
The U.S. government has expanded a program offering free COVID-19 and flu tests and treatment.
The Home Test to Treat program is virtual and offers at-home rapid tests, telehealth sessions, and at-home treatments to people nationwide. The program is a collaboration among the National Institutes of Health, the Administration for Strategic Preparedness and Response, and the CDC. It began as a pilot program in some locations this year.
“With its expansion, the Home Test to Treat program will now offer free testing, telehealth and treatment for both COVID-19 and for influenza (flu) A and B,” the NIH said in a press release. “It is the first public health program that includes home testing technology at such a scale for both COVID-19 and flu.”
The news release says that anyone 18 or over with a current positive test for COVID-19 or flu can get free telehealth care and medicine delivered to their home.
Adults who don’t have COVID-19 or the flu can get free tests if they are uninsured or are enrolled in Medicare, Medicaid, the Veterans Affairs health care system, or Indian Health Services. If they test positive later, they can get free telehealth care and, if prescribed, treatment.
“I think that these [telehealth] delivery mechanisms are going to be absolutely crucial to unburden the in-person offices and the lines that we have and wait times,” said Michael Mina, MD, chief science officer at eMed, the company that helped implement the new Home Test to Treat program, to ABC News.
ABC notes that COVID tests can also be ordered at covidtests.gov – four tests per household or eight for those who have yet to order any this fall.
A version of this article appeared on WebMD.com .
Focus on long-COVID: Perimenopause and post-COVID chronic fatigue
Long COVID (postacute sequelae of SARS-CoV-2 infection, or PASC) is an emerging syndrome that affects 50% to 70% of people who survive COVID-19 for up to 3 months or longer after acute disease.1 It is a multisystem condition that causes dysfunction of respiratory, cardiac, and nervous tissue, at least in part likely due to alterations in cellular energy metabolism and reduced oxygen supply to tissue.3 Patients who have had SARS-CoV-2 infection report persistent symptoms and signs that affect their quality of life. These may include neurocognitive, cardiorespiratory, gastrointestinal, and musculoskeletal symptoms; loss of taste and smell; and constitutional symptoms.2 There is no one test to determine if symptoms are due to COVID-19.3
Acute COVID-19 mortality risk factors include increasing age, chronic comorbidities, and male sex. However, long COVID risk factors are quite different. A meta-analysis and review of 20 articles that met inclusion criteria (n = 13,340 study participants), limited by pooling of crude estimates, found that risk factors were female sex and severity of acute disease.4 A second meta-analysis of 37 studies with 1 preprint found that female sex and comorbidities such as pulmonary disease, diabetes, and obesity were risk factors for long COVID.5 Qualitative analysis of single studies (n = 18 study participants) suggested that older adults can develop more long COVID symptoms than younger adults, but this association between advancing age and long COVID was not supported when data were pooled into a meta-analysis.3 However, both single studies (n = 16 study participants) and the meta-analysis (n = 7 study participants) did support female sex as a risk factor for long COVID, along with single studies suggesting increased risk with medical comorbidities for pulmonary disease, diabetes, and organ transplantation.
Perimenopause
Perimenopause: A temporary disruption to physiologic ovarian steroid hormone production following COVID could acutely exacerbate symptoms of perimenopause and menopause.
JoAnn V. Pinkerton, MD, MSCP
The higher prevalence of long COVID in women younger than 50 years6 supports the overlap that studies have shown between symptoms of long COVID and perimenopause,7 as the median age of natural menopause is 51 years. Thus, health care providers need to differentiate between long COVID and other conditions, such as perimenopause, which share similar symptoms (FIGURE). Perimenopause might be diagnosed as long COVID, or the 2 might affect each other.
Symptoms of long COVID include fatigue, brain fog, and increased heart rate after recovering from COVID-19 and may continue or increase after an initial infection.8 Common symptoms of perimenopause and menopause, which also could be seen with long COVID, include typical menopausal symptoms such as hot flashes, night sweats, or disrupted sleep; changes in mood including dysthymia, depression, anxiety, or emotional lability; cognitive concerns such as brain fog or decreased concentration; and decreased stamina, fatigue, joint and muscle pains, or more frequent headaches. Therefore, women in their 40s or 50s with persistent symptoms after having COVID-19 without an alternative diagnosis, and who present with menstrual irregularity,9 hot flashes, or night sweats, could be having an exacerbation of perimenopausal symptoms, or they could be experiencing a combination of long COVID and perimenopausal symptoms.
- Consider long COVID, versus perimenopause, or both, in women aged younger than 50 years
- Estradiol, which has been shown to alleviate perimenopausal and menopausal symptoms, also has been shown to have beneficial effects during acute COVID-19 infection
- Hormone therapy could improve symptoms of perimenopause and long COVID if some of the symptoms are due to changes in ovary function
Continue to: Potential pathophysiology...
Potential pathophysiology
Inflammation is likely to be critical in the pathogenesis of postacute sequelae of SARS-CoV-2 infection, or PASC. Individuals with long COVID have elevated inflammatory markers for several months.10 The chronic inflammation associated with long COVID could cause disturbances in the ovary and ovarian hormone production.2,10,11
During perimenopause, the ovary is more sensitive to illnesses such as COVID-19and to stress. The current theory is that COVID-19 affects the ovary with declines in ovarian reserve and ovarian function7 and with potential disruptions to the menstrual cycle, gonadal function, and ovarian sufficiency that lead to issues with menopause or fertility, as well as symptom exacerbation around menstruation.12 Another theory is that SARS-CoV-2 infection affects ovary hormone production, as there is an abundance of angiotensin-converting enzyme-2 receptors on ovarian and endometrial tissue.11 Thus, it makes sense that long COVID could bring on symptoms of perimenopause or menopause more acutely or more severely or lengthen the duration of perimenopausal symptoms.
Perimenopause is the transitional period prior to menopause, when the ovaries gradually produce fewer hormones and is associated with erratic hormonal fluctuations. The length of this transitional period varies from 4 to 10 years. Ethnic variations in the duration of hot flashes have been found, noting that Black and Hispanic women have them for an average of 8 to 10 years (longer), White women for an average of 7 years, and Asian, Japanese, and Chinese women for an average of 5 to 6 years (shorter).17
What should health care providers ask?
Distinguishing perimenopause from long COVID. It is important to try to differentiate between perimenopause and long COVID, and it is possible to have both, with long COVID exacerbating the menopausal symptoms.7,8 Health care providers should be alert to consider perimenopause if women present with shorter or longer cycles (21-40 days), missed periods (particularly 60 days or 2 months), or worsening perimenopausal mood, migraines, insomnia, or hot flashes. Clinicians should actively enquire about all of these symptoms.
Moreover, if a perimenopausal woman reports acutely worsening symptoms after COVID-19, health care providers should address the perimenopausal symptoms and determine whether hormone therapy is appropriate and could improve their symptoms. Women do not need to wait until they go 1 year without a period to be treated with hormone therapy to improve perimenopausal and menopausal symptoms. If women with long COVID have perimenopause or menopause symptoms, they should have access to evidence-based information and discuss menopausal hormone therapy if appropriate. Hormone therapy could improve both perimenopausal symptoms and the long COVID symptoms if some of the symptoms are due to changes in ovary function. Health care providers could consider progesterone or antidepressants during the second half of the cycle (luteal phase) or estrogen combined with progesterone for the entire cycle.18
For health care providers working in long COVID clinics, in addition to asking when symptoms started, what makes symptoms worse, the frequency of symptoms, and which activities are affected, ask about perimenopausal and menopausal symptoms. If a woman has irregular periods, sleep disturbances, fatigue, or mood changes, consider that these could be related to long COVID, perimenopause, or both.8,18 Be able to offer treatment or refer patients to a women’s health specialist who can assess and offer treatment.
A role for vitamin D? A recent retrospective case-matched study found that 6 months after hospital discharge, patients with long COVID had lower levels of 25(OH) vitamin D with the most notable symptom being brain fog.19 Thus, there may be a role for vitamin D supplementation as a preventive strategy in those being discharged after hospitalization. Vitamin D levels and supplementation have not been otherwise evaluated to date.
Lifestyle strategies for women with perimenopause and long COVID
Lifestyle strategies should be encouraged for women during perimenopause and long COVID. This includes good nutrition (avoiding carbs and sweets, particularly before menses), getting at least 7 hours of sleep and using sleep hygiene (regular bedtimes, sleep regimen, no late screens), getting regular exercise 5 days per week, reducing stress, avoiding excess alcohol, and not smoking. All of these factors can help women and their ovarian function during this period of ovarian fluctuations.
The timing of menopause and COVID may coincide with midlife stressors, including relationship issues (separations or divorce), health issues for the individual or their partner, widowhood, parenting challenges (care of young children, struggles with adolescents, grown children returning home), being childless, concerns about aging parents and caregiving responsibilities, as well as midlife career, community, or education issues—all of which make both long COVID and perimenopause more challenging to navigate.
Need for research
There is a need for future research to understand the epidemiologic basis and underlying biological mechanisms of sex differences seen in women with long COVID. Studying the effects of COVID-19 on ovarian function could lead to a better understanding of perimenopause, what causes ovarian failure to speed up, and possibly ways to slow it down8 since there are health risks of early menopause.16
References
- Fernández-de-Las-Peñas C, Palacios-Ceña D, GómezMayordomo V, et al. Defining post-COVID symptoms (postacute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health. 2021;18:2621. doi: 10.3390/ijerph18052621
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601-615. doi: 10.1038/s41591 -021-01283-z
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022-00846-2
- Maglietta G, Diodati F, Puntoni M, et al. Prognostic factors for post-COVID-19 syndrome: a systematic review and meta-analysis. J Clin Med. 2022;11:1541. doi: 10.3390 /jcm11061541
- Notarte KI, de Oliveira MHS, Peligro PJ, et al. Age, sex and previous comorbidities as risk factors not associated with SARS-CoV-2 infection for long COVID-19: a systematic review and meta-analysis. J Clin Med. 2022;11:7314. doi: 10.3390 /jcm11247314
- Sigfrid L, Drake TM, Pauley E, et al. Long COVID in adults discharged from UK hospitals after COVID-19: a prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Health Eur. 2021;8:100186. doi: 10.1016/j.lanepe.2021.100186
- Pollack B, von Saltza E, McCorkell L, et al. Female reproductive health impacts of long COVID and associated illnesses including ME/CFS, POTS, and connective tissue disorders: a literature review. Front Rehabil Sci. 2023;4:1122673. doi: 10.3389/fresc.2023.1122673
- Stewart S, Newson L, Briggs TA, et al. Long COVID risk - a signal to address sex hormones and women’s health. Lancet Reg Health Eur. 2021;11:100242. doi: 10.1016 /j.lanepe.2021.100242
- Li K, Chen G, Hou H, et al. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod Biomed Online. 2021;42:260-267. doi: 10.1016 /j.rbmo.2020.09.020
- Phetsouphanh C, Darley DR, Wilson DB, et al. Immunological dysfunction persists for 8 months following initial mild-tomoderate SARS-CoV-2 infection. Nat Immunol. 2022;23:210216. doi: 10.1038/s41590-021-01113-x
- Sharp GC, Fraser A, Sawyer G, et al. The COVID-19 pandemic and the menstrual cycle: research gaps and opportunities. Int J Epidemiol. 2022;51:691-700. doi: 10.1093/ije/dyab239
- Ding T, Wang T, Zhang J, et al. Analysis of ovarian injury associated with COVID-19 disease in reproductive-aged women in Wuhan, China: an observational study. Front Med (Lausanne). 2021;8:635255. doi: 10.3389/fmed.2021.635255
- Huang B, Cai Y, Li N, et al. Sex-based clinical and immunological differences in COVID-19. BMC Infect Dis. 2021;21:647. doi: 10.1186/s12879-021-06313-2
- Connor J, Madhavan S, Mokashi M, et al. Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: a review. Soc Sci Med. 2020;266:113364. doi: 10.1016/j.socscimed.2020.113364
- Mauvais-Jarvis F, Klein SL, Levin ER. Estradiol, progesterone, immunomodulation, and COVID-19 outcomes. Endocrinology. 2020;161:bqaa127. doi:10.1210/endocr/bqaa127
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi: 10.1097/GME.0000000000002028
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531-539. doi:10.1001 /jamainternmed.2014.8063
- Newson L, Lewis R, O’Hara M. Long COVID and menopause - the important role of hormones in long COVID must be considered. Maturitas. 2021;152:74. doi: 10.1016 /j.maturitas.2021.08.026
- di Filippo L, Frara S, Nannipieri F, et al. Low Vitamin D levels are associated with long COVID syndrome in COVID-19 survivors. J Clin Endocrinol Metab. 2023;108:e1106-e1116. doi: 10.1210/clinem/dgad207
Continue to: Chronic fatigue syndrome...
Chronic fatigue syndrome
Chronic fatigue syndrome: A large number of patients have “post-COVID conditions” affecting everyday function, including depression/anxiety, insomnia, and chronic fatigue (with a 3:1 female predominance)
Alexandra Kadl, MD
After 3 years battling acute COVID-19 infections, we encounter now a large number of patients with PASC— also known as “long COVID,” “COVID long-hauler syndrome,” and “post-COVID conditions”—a persistent multisystem syndrome that impacts everyday function.1 As of October 2023, there are more than 100 million COVID-19 survivors reported in the United States; 10% to 85% of COVID survivors2-4 may show lingering, life-altering symptoms after recovery. Common reported symptoms include fatigue, depression/ anxiety, insomnia, and brain fog/difficulty concentrating, which are particularly high in women who often had experienced only mild acute COVID-19 disease and were not even hospitalized. More recently, chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as major component of PASC5 with a 3:1 female predominance.6 Up to 75% of patients with this diagnosis are not able to maintain their jobs and normal life, and up to 25% are so disabled that they are bedbound.6
Diagnosis
Although illnesses resembling CFS have been reported for more than 200 years,7 the diagnosis of CFS/ME remains difficult to make. There is a likely underreporting due to fear of being labeled as malingering when reaching out to health care providers, and there is a reporting bias toward higher socioeconomic groups due to better access to health care. The current criteria for the diagnosis of CFS/ME include the following 3 components8:
- substantial impairment in the ability to function for more than 6 months, accompanied by profound fatigue, not alleviated by rest
- post-exertional malaise (PEM; prolonged, disabling exacerbation of the patient’s baseline symptoms after exercise)
- non-refreshing sleep, PLUS either cognitive impairment or orthostatic intolerance.
Pathophysiology
Originally found to evolve in a small patient population with Epstein-Barr virus infection and Lyme disease, CFS/ME has moved to centerstage after the COVID-19 pandemic. While the diagnosis of COVID-19–related CFS/ME has advanced in the field, a clear mechanistic explanation of why it occurs is still missing. Certain risk factors have been identified for the development of CFS/ME, including female sex, reactivation of herpesviruses, and presence of connective tissue disorders; however, about one-third of patients with CFS/ME do not have identifiable risk factors.9,10 Persistence of viral particles11 and prolonged inflammatory states are speculated to affect the nervous system and mitochondrial function and metabolism. Interestingly, there is no correlation between severity of initial COVID-19 illness and the development of CFS/ME, similar to observations in non–COVID-19–related CFS/ME.
Proposed therapy
There is currently no proven therapy for CFS/ME. At this time, several immunomodulatory, antiviral, and neuromodulator drugs are being tested in clinical trial networks around the world.12 Usual physical therapy with near maximum intensity has been shown to exacerbate symptoms and often results in PEM, which is described as a “crash” or “full collapse” by patients. The time for recovery after such episodes can be several days.13
Instead, the focus should be on addressing “treatable” concomitant symptoms, such as sleep disorders, anxiety and depression, and chronic pain. Lifestyle changes, avoidance of triggers, and exercise without over exertion are currently recommended to avoid incapacitating PEM.
Gaps in knowledge
There is a large knowledge gap regarding the pathophysiology, prevention, and therapy for CFS/ME. Many health care practitioners are not familiar with the disease and have focused on measurable parameters of exercise limitations and fatigue, such as anemias and lung and cardiac impairments, thus treating CFS/ME as a form of deconditioning. Given the large number of patients who recovered from acute COVID-19 that are now disabled due to CFS/ME, a patient-centered research opportunity has arisen. Biomedical/mechanistic research is ongoing, and well-designed clinical trials evaluating pharmacologic intervention as well as tailored exercise programs are needed.
Conclusion
General practitioners and women’s health specialists need to be aware of CFS/ME, especially when managing patients with long COVID. They also need to know that typical physical therapy may worsen symptoms. Furthermore, clinicians should shy away from trial drugs with a theoretical benefit outside of a clinical trial. ●
- Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as a major component of PASC
- Typical physical therapy has been shown to exacerbate symptoms of CFS/ME
- Treatment should focus on addressing “treatable” concomitant symptoms, lifestyle changes, avoidance of triggers, and exercise without over exertion
References
- Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102-e107. doi: 10.1016 /S1473-3099(21)00703-9
- Chen C, Haupert SR, Zimmermann L, et al. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022;226:1593-1607. doi: 10.1093/infdis/jiac136
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022 -00846-2
- Pavli A, Theodoridou M, Maltezou HC. Post-COVID syndrome: incidence, clinical spectrum, and challenges for primary healthcare professionals. Arch Med Res. 2021;52:575-581. doi: 10.1016/j.arcmed.2021.03.010
- Kedor C, Freitag H, Meyer-Arndt L, et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun. 2022;13:5104. doi: 10.1038/s41467-022-32507-6
- Bateman L, Bested AC, Bonilla HF, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: essentials of diagnosis and management. Mayo Clin Proc. 2021;96:28612878. doi: 10.1016/j.mayocp.2021.07.004
- Wessely S. History of postviral fatigue syndrome. Br Med Bull. 1991;47:919-941. doi: 10.1093/oxfordjournals.bmb.a072521
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. National Academies Press; 2015. doi: 10.17226/19012
- Ceban F, Ling S, Lui LMW, et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav Immun. 2022;101:93135. doi: 10.1016/j.bbi.2021.12.020
- Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
- Hanson MR. The viral origin of myalgic encephalomyelitis/ chronic fatigue syndrome. PLoS Pathog. 2023;19:e1011523. doi: 10.1371/journal.ppat.1011523
- Scheibenbogen C, Bellmann-Strobl JT, Heindrich C, et al. Fighting post-COVID and ME/CFS—development of curative therapies. Front Med (Lausanne). 2023;10:1194754. doi: 10.3389/fmed.2023.1194754
- Stussman B, Williams A, Snow J, et al. Characterization of post-exertional malaise in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Front Neurol. 2020;11:1025. doi: 10.3389/fneur.2020.01025
Long COVID (postacute sequelae of SARS-CoV-2 infection, or PASC) is an emerging syndrome that affects 50% to 70% of people who survive COVID-19 for up to 3 months or longer after acute disease.1 It is a multisystem condition that causes dysfunction of respiratory, cardiac, and nervous tissue, at least in part likely due to alterations in cellular energy metabolism and reduced oxygen supply to tissue.3 Patients who have had SARS-CoV-2 infection report persistent symptoms and signs that affect their quality of life. These may include neurocognitive, cardiorespiratory, gastrointestinal, and musculoskeletal symptoms; loss of taste and smell; and constitutional symptoms.2 There is no one test to determine if symptoms are due to COVID-19.3
Acute COVID-19 mortality risk factors include increasing age, chronic comorbidities, and male sex. However, long COVID risk factors are quite different. A meta-analysis and review of 20 articles that met inclusion criteria (n = 13,340 study participants), limited by pooling of crude estimates, found that risk factors were female sex and severity of acute disease.4 A second meta-analysis of 37 studies with 1 preprint found that female sex and comorbidities such as pulmonary disease, diabetes, and obesity were risk factors for long COVID.5 Qualitative analysis of single studies (n = 18 study participants) suggested that older adults can develop more long COVID symptoms than younger adults, but this association between advancing age and long COVID was not supported when data were pooled into a meta-analysis.3 However, both single studies (n = 16 study participants) and the meta-analysis (n = 7 study participants) did support female sex as a risk factor for long COVID, along with single studies suggesting increased risk with medical comorbidities for pulmonary disease, diabetes, and organ transplantation.
Perimenopause
Perimenopause: A temporary disruption to physiologic ovarian steroid hormone production following COVID could acutely exacerbate symptoms of perimenopause and menopause.
JoAnn V. Pinkerton, MD, MSCP
The higher prevalence of long COVID in women younger than 50 years6 supports the overlap that studies have shown between symptoms of long COVID and perimenopause,7 as the median age of natural menopause is 51 years. Thus, health care providers need to differentiate between long COVID and other conditions, such as perimenopause, which share similar symptoms (FIGURE). Perimenopause might be diagnosed as long COVID, or the 2 might affect each other.

Symptoms of long COVID include fatigue, brain fog, and increased heart rate after recovering from COVID-19 and may continue or increase after an initial infection.8 Common symptoms of perimenopause and menopause, which also could be seen with long COVID, include typical menopausal symptoms such as hot flashes, night sweats, or disrupted sleep; changes in mood including dysthymia, depression, anxiety, or emotional lability; cognitive concerns such as brain fog or decreased concentration; and decreased stamina, fatigue, joint and muscle pains, or more frequent headaches. Therefore, women in their 40s or 50s with persistent symptoms after having COVID-19 without an alternative diagnosis, and who present with menstrual irregularity,9 hot flashes, or night sweats, could be having an exacerbation of perimenopausal symptoms, or they could be experiencing a combination of long COVID and perimenopausal symptoms.
- Consider long COVID, versus perimenopause, or both, in women aged younger than 50 years
- Estradiol, which has been shown to alleviate perimenopausal and menopausal symptoms, also has been shown to have beneficial effects during acute COVID-19 infection
- Hormone therapy could improve symptoms of perimenopause and long COVID if some of the symptoms are due to changes in ovary function
Continue to: Potential pathophysiology...
Potential pathophysiology
Inflammation is likely to be critical in the pathogenesis of postacute sequelae of SARS-CoV-2 infection, or PASC. Individuals with long COVID have elevated inflammatory markers for several months.10 The chronic inflammation associated with long COVID could cause disturbances in the ovary and ovarian hormone production.2,10,11
During perimenopause, the ovary is more sensitive to illnesses such as COVID-19and to stress. The current theory is that COVID-19 affects the ovary with declines in ovarian reserve and ovarian function7 and with potential disruptions to the menstrual cycle, gonadal function, and ovarian sufficiency that lead to issues with menopause or fertility, as well as symptom exacerbation around menstruation.12 Another theory is that SARS-CoV-2 infection affects ovary hormone production, as there is an abundance of angiotensin-converting enzyme-2 receptors on ovarian and endometrial tissue.11 Thus, it makes sense that long COVID could bring on symptoms of perimenopause or menopause more acutely or more severely or lengthen the duration of perimenopausal symptoms.
Perimenopause is the transitional period prior to menopause, when the ovaries gradually produce fewer hormones and is associated with erratic hormonal fluctuations. The length of this transitional period varies from 4 to 10 years. Ethnic variations in the duration of hot flashes have been found, noting that Black and Hispanic women have them for an average of 8 to 10 years (longer), White women for an average of 7 years, and Asian, Japanese, and Chinese women for an average of 5 to 6 years (shorter).17
What should health care providers ask?
Distinguishing perimenopause from long COVID. It is important to try to differentiate between perimenopause and long COVID, and it is possible to have both, with long COVID exacerbating the menopausal symptoms.7,8 Health care providers should be alert to consider perimenopause if women present with shorter or longer cycles (21-40 days), missed periods (particularly 60 days or 2 months), or worsening perimenopausal mood, migraines, insomnia, or hot flashes. Clinicians should actively enquire about all of these symptoms.
Moreover, if a perimenopausal woman reports acutely worsening symptoms after COVID-19, health care providers should address the perimenopausal symptoms and determine whether hormone therapy is appropriate and could improve their symptoms. Women do not need to wait until they go 1 year without a period to be treated with hormone therapy to improve perimenopausal and menopausal symptoms. If women with long COVID have perimenopause or menopause symptoms, they should have access to evidence-based information and discuss menopausal hormone therapy if appropriate. Hormone therapy could improve both perimenopausal symptoms and the long COVID symptoms if some of the symptoms are due to changes in ovary function. Health care providers could consider progesterone or antidepressants during the second half of the cycle (luteal phase) or estrogen combined with progesterone for the entire cycle.18
For health care providers working in long COVID clinics, in addition to asking when symptoms started, what makes symptoms worse, the frequency of symptoms, and which activities are affected, ask about perimenopausal and menopausal symptoms. If a woman has irregular periods, sleep disturbances, fatigue, or mood changes, consider that these could be related to long COVID, perimenopause, or both.8,18 Be able to offer treatment or refer patients to a women’s health specialist who can assess and offer treatment.
A role for vitamin D? A recent retrospective case-matched study found that 6 months after hospital discharge, patients with long COVID had lower levels of 25(OH) vitamin D with the most notable symptom being brain fog.19 Thus, there may be a role for vitamin D supplementation as a preventive strategy in those being discharged after hospitalization. Vitamin D levels and supplementation have not been otherwise evaluated to date.
Lifestyle strategies for women with perimenopause and long COVID
Lifestyle strategies should be encouraged for women during perimenopause and long COVID. This includes good nutrition (avoiding carbs and sweets, particularly before menses), getting at least 7 hours of sleep and using sleep hygiene (regular bedtimes, sleep regimen, no late screens), getting regular exercise 5 days per week, reducing stress, avoiding excess alcohol, and not smoking. All of these factors can help women and their ovarian function during this period of ovarian fluctuations.
The timing of menopause and COVID may coincide with midlife stressors, including relationship issues (separations or divorce), health issues for the individual or their partner, widowhood, parenting challenges (care of young children, struggles with adolescents, grown children returning home), being childless, concerns about aging parents and caregiving responsibilities, as well as midlife career, community, or education issues—all of which make both long COVID and perimenopause more challenging to navigate.
Need for research
There is a need for future research to understand the epidemiologic basis and underlying biological mechanisms of sex differences seen in women with long COVID. Studying the effects of COVID-19 on ovarian function could lead to a better understanding of perimenopause, what causes ovarian failure to speed up, and possibly ways to slow it down8 since there are health risks of early menopause.16
References
- Fernández-de-Las-Peñas C, Palacios-Ceña D, GómezMayordomo V, et al. Defining post-COVID symptoms (postacute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health. 2021;18:2621. doi: 10.3390/ijerph18052621
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601-615. doi: 10.1038/s41591 -021-01283-z
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022-00846-2
- Maglietta G, Diodati F, Puntoni M, et al. Prognostic factors for post-COVID-19 syndrome: a systematic review and meta-analysis. J Clin Med. 2022;11:1541. doi: 10.3390 /jcm11061541
- Notarte KI, de Oliveira MHS, Peligro PJ, et al. Age, sex and previous comorbidities as risk factors not associated with SARS-CoV-2 infection for long COVID-19: a systematic review and meta-analysis. J Clin Med. 2022;11:7314. doi: 10.3390 /jcm11247314
- Sigfrid L, Drake TM, Pauley E, et al. Long COVID in adults discharged from UK hospitals after COVID-19: a prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Health Eur. 2021;8:100186. doi: 10.1016/j.lanepe.2021.100186
- Pollack B, von Saltza E, McCorkell L, et al. Female reproductive health impacts of long COVID and associated illnesses including ME/CFS, POTS, and connective tissue disorders: a literature review. Front Rehabil Sci. 2023;4:1122673. doi: 10.3389/fresc.2023.1122673
- Stewart S, Newson L, Briggs TA, et al. Long COVID risk - a signal to address sex hormones and women’s health. Lancet Reg Health Eur. 2021;11:100242. doi: 10.1016 /j.lanepe.2021.100242
- Li K, Chen G, Hou H, et al. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod Biomed Online. 2021;42:260-267. doi: 10.1016 /j.rbmo.2020.09.020
- Phetsouphanh C, Darley DR, Wilson DB, et al. Immunological dysfunction persists for 8 months following initial mild-tomoderate SARS-CoV-2 infection. Nat Immunol. 2022;23:210216. doi: 10.1038/s41590-021-01113-x
- Sharp GC, Fraser A, Sawyer G, et al. The COVID-19 pandemic and the menstrual cycle: research gaps and opportunities. Int J Epidemiol. 2022;51:691-700. doi: 10.1093/ije/dyab239
- Ding T, Wang T, Zhang J, et al. Analysis of ovarian injury associated with COVID-19 disease in reproductive-aged women in Wuhan, China: an observational study. Front Med (Lausanne). 2021;8:635255. doi: 10.3389/fmed.2021.635255
- Huang B, Cai Y, Li N, et al. Sex-based clinical and immunological differences in COVID-19. BMC Infect Dis. 2021;21:647. doi: 10.1186/s12879-021-06313-2
- Connor J, Madhavan S, Mokashi M, et al. Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: a review. Soc Sci Med. 2020;266:113364. doi: 10.1016/j.socscimed.2020.113364
- Mauvais-Jarvis F, Klein SL, Levin ER. Estradiol, progesterone, immunomodulation, and COVID-19 outcomes. Endocrinology. 2020;161:bqaa127. doi:10.1210/endocr/bqaa127
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi: 10.1097/GME.0000000000002028
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531-539. doi:10.1001 /jamainternmed.2014.8063
- Newson L, Lewis R, O’Hara M. Long COVID and menopause - the important role of hormones in long COVID must be considered. Maturitas. 2021;152:74. doi: 10.1016 /j.maturitas.2021.08.026
- di Filippo L, Frara S, Nannipieri F, et al. Low Vitamin D levels are associated with long COVID syndrome in COVID-19 survivors. J Clin Endocrinol Metab. 2023;108:e1106-e1116. doi: 10.1210/clinem/dgad207
Continue to: Chronic fatigue syndrome...
Chronic fatigue syndrome
Chronic fatigue syndrome: A large number of patients have “post-COVID conditions” affecting everyday function, including depression/anxiety, insomnia, and chronic fatigue (with a 3:1 female predominance)
Alexandra Kadl, MD
After 3 years battling acute COVID-19 infections, we encounter now a large number of patients with PASC— also known as “long COVID,” “COVID long-hauler syndrome,” and “post-COVID conditions”—a persistent multisystem syndrome that impacts everyday function.1 As of October 2023, there are more than 100 million COVID-19 survivors reported in the United States; 10% to 85% of COVID survivors2-4 may show lingering, life-altering symptoms after recovery. Common reported symptoms include fatigue, depression/ anxiety, insomnia, and brain fog/difficulty concentrating, which are particularly high in women who often had experienced only mild acute COVID-19 disease and were not even hospitalized. More recently, chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as major component of PASC5 with a 3:1 female predominance.6 Up to 75% of patients with this diagnosis are not able to maintain their jobs and normal life, and up to 25% are so disabled that they are bedbound.6
Diagnosis
Although illnesses resembling CFS have been reported for more than 200 years,7 the diagnosis of CFS/ME remains difficult to make. There is a likely underreporting due to fear of being labeled as malingering when reaching out to health care providers, and there is a reporting bias toward higher socioeconomic groups due to better access to health care. The current criteria for the diagnosis of CFS/ME include the following 3 components8:
- substantial impairment in the ability to function for more than 6 months, accompanied by profound fatigue, not alleviated by rest
- post-exertional malaise (PEM; prolonged, disabling exacerbation of the patient’s baseline symptoms after exercise)
- non-refreshing sleep, PLUS either cognitive impairment or orthostatic intolerance.
Pathophysiology
Originally found to evolve in a small patient population with Epstein-Barr virus infection and Lyme disease, CFS/ME has moved to centerstage after the COVID-19 pandemic. While the diagnosis of COVID-19–related CFS/ME has advanced in the field, a clear mechanistic explanation of why it occurs is still missing. Certain risk factors have been identified for the development of CFS/ME, including female sex, reactivation of herpesviruses, and presence of connective tissue disorders; however, about one-third of patients with CFS/ME do not have identifiable risk factors.9,10 Persistence of viral particles11 and prolonged inflammatory states are speculated to affect the nervous system and mitochondrial function and metabolism. Interestingly, there is no correlation between severity of initial COVID-19 illness and the development of CFS/ME, similar to observations in non–COVID-19–related CFS/ME.
Proposed therapy
There is currently no proven therapy for CFS/ME. At this time, several immunomodulatory, antiviral, and neuromodulator drugs are being tested in clinical trial networks around the world.12 Usual physical therapy with near maximum intensity has been shown to exacerbate symptoms and often results in PEM, which is described as a “crash” or “full collapse” by patients. The time for recovery after such episodes can be several days.13
Instead, the focus should be on addressing “treatable” concomitant symptoms, such as sleep disorders, anxiety and depression, and chronic pain. Lifestyle changes, avoidance of triggers, and exercise without over exertion are currently recommended to avoid incapacitating PEM.
Gaps in knowledge
There is a large knowledge gap regarding the pathophysiology, prevention, and therapy for CFS/ME. Many health care practitioners are not familiar with the disease and have focused on measurable parameters of exercise limitations and fatigue, such as anemias and lung and cardiac impairments, thus treating CFS/ME as a form of deconditioning. Given the large number of patients who recovered from acute COVID-19 that are now disabled due to CFS/ME, a patient-centered research opportunity has arisen. Biomedical/mechanistic research is ongoing, and well-designed clinical trials evaluating pharmacologic intervention as well as tailored exercise programs are needed.
Conclusion
General practitioners and women’s health specialists need to be aware of CFS/ME, especially when managing patients with long COVID. They also need to know that typical physical therapy may worsen symptoms. Furthermore, clinicians should shy away from trial drugs with a theoretical benefit outside of a clinical trial. ●
- Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as a major component of PASC
- Typical physical therapy has been shown to exacerbate symptoms of CFS/ME
- Treatment should focus on addressing “treatable” concomitant symptoms, lifestyle changes, avoidance of triggers, and exercise without over exertion
References
- Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102-e107. doi: 10.1016 /S1473-3099(21)00703-9
- Chen C, Haupert SR, Zimmermann L, et al. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022;226:1593-1607. doi: 10.1093/infdis/jiac136
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022 -00846-2
- Pavli A, Theodoridou M, Maltezou HC. Post-COVID syndrome: incidence, clinical spectrum, and challenges for primary healthcare professionals. Arch Med Res. 2021;52:575-581. doi: 10.1016/j.arcmed.2021.03.010
- Kedor C, Freitag H, Meyer-Arndt L, et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun. 2022;13:5104. doi: 10.1038/s41467-022-32507-6
- Bateman L, Bested AC, Bonilla HF, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: essentials of diagnosis and management. Mayo Clin Proc. 2021;96:28612878. doi: 10.1016/j.mayocp.2021.07.004
- Wessely S. History of postviral fatigue syndrome. Br Med Bull. 1991;47:919-941. doi: 10.1093/oxfordjournals.bmb.a072521
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. National Academies Press; 2015. doi: 10.17226/19012
- Ceban F, Ling S, Lui LMW, et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav Immun. 2022;101:93135. doi: 10.1016/j.bbi.2021.12.020
- Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
- Hanson MR. The viral origin of myalgic encephalomyelitis/ chronic fatigue syndrome. PLoS Pathog. 2023;19:e1011523. doi: 10.1371/journal.ppat.1011523
- Scheibenbogen C, Bellmann-Strobl JT, Heindrich C, et al. Fighting post-COVID and ME/CFS—development of curative therapies. Front Med (Lausanne). 2023;10:1194754. doi: 10.3389/fmed.2023.1194754
- Stussman B, Williams A, Snow J, et al. Characterization of post-exertional malaise in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Front Neurol. 2020;11:1025. doi: 10.3389/fneur.2020.01025
Long COVID (postacute sequelae of SARS-CoV-2 infection, or PASC) is an emerging syndrome that affects 50% to 70% of people who survive COVID-19 for up to 3 months or longer after acute disease.1 It is a multisystem condition that causes dysfunction of respiratory, cardiac, and nervous tissue, at least in part likely due to alterations in cellular energy metabolism and reduced oxygen supply to tissue.3 Patients who have had SARS-CoV-2 infection report persistent symptoms and signs that affect their quality of life. These may include neurocognitive, cardiorespiratory, gastrointestinal, and musculoskeletal symptoms; loss of taste and smell; and constitutional symptoms.2 There is no one test to determine if symptoms are due to COVID-19.3
Acute COVID-19 mortality risk factors include increasing age, chronic comorbidities, and male sex. However, long COVID risk factors are quite different. A meta-analysis and review of 20 articles that met inclusion criteria (n = 13,340 study participants), limited by pooling of crude estimates, found that risk factors were female sex and severity of acute disease.4 A second meta-analysis of 37 studies with 1 preprint found that female sex and comorbidities such as pulmonary disease, diabetes, and obesity were risk factors for long COVID.5 Qualitative analysis of single studies (n = 18 study participants) suggested that older adults can develop more long COVID symptoms than younger adults, but this association between advancing age and long COVID was not supported when data were pooled into a meta-analysis.3 However, both single studies (n = 16 study participants) and the meta-analysis (n = 7 study participants) did support female sex as a risk factor for long COVID, along with single studies suggesting increased risk with medical comorbidities for pulmonary disease, diabetes, and organ transplantation.
Perimenopause
Perimenopause: A temporary disruption to physiologic ovarian steroid hormone production following COVID could acutely exacerbate symptoms of perimenopause and menopause.
JoAnn V. Pinkerton, MD, MSCP
The higher prevalence of long COVID in women younger than 50 years6 supports the overlap that studies have shown between symptoms of long COVID and perimenopause,7 as the median age of natural menopause is 51 years. Thus, health care providers need to differentiate between long COVID and other conditions, such as perimenopause, which share similar symptoms (FIGURE). Perimenopause might be diagnosed as long COVID, or the 2 might affect each other.

Symptoms of long COVID include fatigue, brain fog, and increased heart rate after recovering from COVID-19 and may continue or increase after an initial infection.8 Common symptoms of perimenopause and menopause, which also could be seen with long COVID, include typical menopausal symptoms such as hot flashes, night sweats, or disrupted sleep; changes in mood including dysthymia, depression, anxiety, or emotional lability; cognitive concerns such as brain fog or decreased concentration; and decreased stamina, fatigue, joint and muscle pains, or more frequent headaches. Therefore, women in their 40s or 50s with persistent symptoms after having COVID-19 without an alternative diagnosis, and who present with menstrual irregularity,9 hot flashes, or night sweats, could be having an exacerbation of perimenopausal symptoms, or they could be experiencing a combination of long COVID and perimenopausal symptoms.
- Consider long COVID, versus perimenopause, or both, in women aged younger than 50 years
- Estradiol, which has been shown to alleviate perimenopausal and menopausal symptoms, also has been shown to have beneficial effects during acute COVID-19 infection
- Hormone therapy could improve symptoms of perimenopause and long COVID if some of the symptoms are due to changes in ovary function
Continue to: Potential pathophysiology...
Potential pathophysiology
Inflammation is likely to be critical in the pathogenesis of postacute sequelae of SARS-CoV-2 infection, or PASC. Individuals with long COVID have elevated inflammatory markers for several months.10 The chronic inflammation associated with long COVID could cause disturbances in the ovary and ovarian hormone production.2,10,11
During perimenopause, the ovary is more sensitive to illnesses such as COVID-19and to stress. The current theory is that COVID-19 affects the ovary with declines in ovarian reserve and ovarian function7 and with potential disruptions to the menstrual cycle, gonadal function, and ovarian sufficiency that lead to issues with menopause or fertility, as well as symptom exacerbation around menstruation.12 Another theory is that SARS-CoV-2 infection affects ovary hormone production, as there is an abundance of angiotensin-converting enzyme-2 receptors on ovarian and endometrial tissue.11 Thus, it makes sense that long COVID could bring on symptoms of perimenopause or menopause more acutely or more severely or lengthen the duration of perimenopausal symptoms.
Perimenopause is the transitional period prior to menopause, when the ovaries gradually produce fewer hormones and is associated with erratic hormonal fluctuations. The length of this transitional period varies from 4 to 10 years. Ethnic variations in the duration of hot flashes have been found, noting that Black and Hispanic women have them for an average of 8 to 10 years (longer), White women for an average of 7 years, and Asian, Japanese, and Chinese women for an average of 5 to 6 years (shorter).17
What should health care providers ask?
Distinguishing perimenopause from long COVID. It is important to try to differentiate between perimenopause and long COVID, and it is possible to have both, with long COVID exacerbating the menopausal symptoms.7,8 Health care providers should be alert to consider perimenopause if women present with shorter or longer cycles (21-40 days), missed periods (particularly 60 days or 2 months), or worsening perimenopausal mood, migraines, insomnia, or hot flashes. Clinicians should actively enquire about all of these symptoms.
Moreover, if a perimenopausal woman reports acutely worsening symptoms after COVID-19, health care providers should address the perimenopausal symptoms and determine whether hormone therapy is appropriate and could improve their symptoms. Women do not need to wait until they go 1 year without a period to be treated with hormone therapy to improve perimenopausal and menopausal symptoms. If women with long COVID have perimenopause or menopause symptoms, they should have access to evidence-based information and discuss menopausal hormone therapy if appropriate. Hormone therapy could improve both perimenopausal symptoms and the long COVID symptoms if some of the symptoms are due to changes in ovary function. Health care providers could consider progesterone or antidepressants during the second half of the cycle (luteal phase) or estrogen combined with progesterone for the entire cycle.18
For health care providers working in long COVID clinics, in addition to asking when symptoms started, what makes symptoms worse, the frequency of symptoms, and which activities are affected, ask about perimenopausal and menopausal symptoms. If a woman has irregular periods, sleep disturbances, fatigue, or mood changes, consider that these could be related to long COVID, perimenopause, or both.8,18 Be able to offer treatment or refer patients to a women’s health specialist who can assess and offer treatment.
A role for vitamin D? A recent retrospective case-matched study found that 6 months after hospital discharge, patients with long COVID had lower levels of 25(OH) vitamin D with the most notable symptom being brain fog.19 Thus, there may be a role for vitamin D supplementation as a preventive strategy in those being discharged after hospitalization. Vitamin D levels and supplementation have not been otherwise evaluated to date.
Lifestyle strategies for women with perimenopause and long COVID
Lifestyle strategies should be encouraged for women during perimenopause and long COVID. This includes good nutrition (avoiding carbs and sweets, particularly before menses), getting at least 7 hours of sleep and using sleep hygiene (regular bedtimes, sleep regimen, no late screens), getting regular exercise 5 days per week, reducing stress, avoiding excess alcohol, and not smoking. All of these factors can help women and their ovarian function during this period of ovarian fluctuations.
The timing of menopause and COVID may coincide with midlife stressors, including relationship issues (separations or divorce), health issues for the individual or their partner, widowhood, parenting challenges (care of young children, struggles with adolescents, grown children returning home), being childless, concerns about aging parents and caregiving responsibilities, as well as midlife career, community, or education issues—all of which make both long COVID and perimenopause more challenging to navigate.
Need for research
There is a need for future research to understand the epidemiologic basis and underlying biological mechanisms of sex differences seen in women with long COVID. Studying the effects of COVID-19 on ovarian function could lead to a better understanding of perimenopause, what causes ovarian failure to speed up, and possibly ways to slow it down8 since there are health risks of early menopause.16
References
- Fernández-de-Las-Peñas C, Palacios-Ceña D, GómezMayordomo V, et al. Defining post-COVID symptoms (postacute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health. 2021;18:2621. doi: 10.3390/ijerph18052621
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601-615. doi: 10.1038/s41591 -021-01283-z
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022-00846-2
- Maglietta G, Diodati F, Puntoni M, et al. Prognostic factors for post-COVID-19 syndrome: a systematic review and meta-analysis. J Clin Med. 2022;11:1541. doi: 10.3390 /jcm11061541
- Notarte KI, de Oliveira MHS, Peligro PJ, et al. Age, sex and previous comorbidities as risk factors not associated with SARS-CoV-2 infection for long COVID-19: a systematic review and meta-analysis. J Clin Med. 2022;11:7314. doi: 10.3390 /jcm11247314
- Sigfrid L, Drake TM, Pauley E, et al. Long COVID in adults discharged from UK hospitals after COVID-19: a prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Health Eur. 2021;8:100186. doi: 10.1016/j.lanepe.2021.100186
- Pollack B, von Saltza E, McCorkell L, et al. Female reproductive health impacts of long COVID and associated illnesses including ME/CFS, POTS, and connective tissue disorders: a literature review. Front Rehabil Sci. 2023;4:1122673. doi: 10.3389/fresc.2023.1122673
- Stewart S, Newson L, Briggs TA, et al. Long COVID risk - a signal to address sex hormones and women’s health. Lancet Reg Health Eur. 2021;11:100242. doi: 10.1016 /j.lanepe.2021.100242
- Li K, Chen G, Hou H, et al. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod Biomed Online. 2021;42:260-267. doi: 10.1016 /j.rbmo.2020.09.020
- Phetsouphanh C, Darley DR, Wilson DB, et al. Immunological dysfunction persists for 8 months following initial mild-tomoderate SARS-CoV-2 infection. Nat Immunol. 2022;23:210216. doi: 10.1038/s41590-021-01113-x
- Sharp GC, Fraser A, Sawyer G, et al. The COVID-19 pandemic and the menstrual cycle: research gaps and opportunities. Int J Epidemiol. 2022;51:691-700. doi: 10.1093/ije/dyab239
- Ding T, Wang T, Zhang J, et al. Analysis of ovarian injury associated with COVID-19 disease in reproductive-aged women in Wuhan, China: an observational study. Front Med (Lausanne). 2021;8:635255. doi: 10.3389/fmed.2021.635255
- Huang B, Cai Y, Li N, et al. Sex-based clinical and immunological differences in COVID-19. BMC Infect Dis. 2021;21:647. doi: 10.1186/s12879-021-06313-2
- Connor J, Madhavan S, Mokashi M, et al. Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: a review. Soc Sci Med. 2020;266:113364. doi: 10.1016/j.socscimed.2020.113364
- Mauvais-Jarvis F, Klein SL, Levin ER. Estradiol, progesterone, immunomodulation, and COVID-19 outcomes. Endocrinology. 2020;161:bqaa127. doi:10.1210/endocr/bqaa127
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi: 10.1097/GME.0000000000002028
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531-539. doi:10.1001 /jamainternmed.2014.8063
- Newson L, Lewis R, O’Hara M. Long COVID and menopause - the important role of hormones in long COVID must be considered. Maturitas. 2021;152:74. doi: 10.1016 /j.maturitas.2021.08.026
- di Filippo L, Frara S, Nannipieri F, et al. Low Vitamin D levels are associated with long COVID syndrome in COVID-19 survivors. J Clin Endocrinol Metab. 2023;108:e1106-e1116. doi: 10.1210/clinem/dgad207
Continue to: Chronic fatigue syndrome...
Chronic fatigue syndrome
Chronic fatigue syndrome: A large number of patients have “post-COVID conditions” affecting everyday function, including depression/anxiety, insomnia, and chronic fatigue (with a 3:1 female predominance)
Alexandra Kadl, MD
After 3 years battling acute COVID-19 infections, we encounter now a large number of patients with PASC— also known as “long COVID,” “COVID long-hauler syndrome,” and “post-COVID conditions”—a persistent multisystem syndrome that impacts everyday function.1 As of October 2023, there are more than 100 million COVID-19 survivors reported in the United States; 10% to 85% of COVID survivors2-4 may show lingering, life-altering symptoms after recovery. Common reported symptoms include fatigue, depression/ anxiety, insomnia, and brain fog/difficulty concentrating, which are particularly high in women who often had experienced only mild acute COVID-19 disease and were not even hospitalized. More recently, chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as major component of PASC5 with a 3:1 female predominance.6 Up to 75% of patients with this diagnosis are not able to maintain their jobs and normal life, and up to 25% are so disabled that they are bedbound.6
Diagnosis
Although illnesses resembling CFS have been reported for more than 200 years,7 the diagnosis of CFS/ME remains difficult to make. There is a likely underreporting due to fear of being labeled as malingering when reaching out to health care providers, and there is a reporting bias toward higher socioeconomic groups due to better access to health care. The current criteria for the diagnosis of CFS/ME include the following 3 components8:
- substantial impairment in the ability to function for more than 6 months, accompanied by profound fatigue, not alleviated by rest
- post-exertional malaise (PEM; prolonged, disabling exacerbation of the patient’s baseline symptoms after exercise)
- non-refreshing sleep, PLUS either cognitive impairment or orthostatic intolerance.
Pathophysiology
Originally found to evolve in a small patient population with Epstein-Barr virus infection and Lyme disease, CFS/ME has moved to centerstage after the COVID-19 pandemic. While the diagnosis of COVID-19–related CFS/ME has advanced in the field, a clear mechanistic explanation of why it occurs is still missing. Certain risk factors have been identified for the development of CFS/ME, including female sex, reactivation of herpesviruses, and presence of connective tissue disorders; however, about one-third of patients with CFS/ME do not have identifiable risk factors.9,10 Persistence of viral particles11 and prolonged inflammatory states are speculated to affect the nervous system and mitochondrial function and metabolism. Interestingly, there is no correlation between severity of initial COVID-19 illness and the development of CFS/ME, similar to observations in non–COVID-19–related CFS/ME.
Proposed therapy
There is currently no proven therapy for CFS/ME. At this time, several immunomodulatory, antiviral, and neuromodulator drugs are being tested in clinical trial networks around the world.12 Usual physical therapy with near maximum intensity has been shown to exacerbate symptoms and often results in PEM, which is described as a “crash” or “full collapse” by patients. The time for recovery after such episodes can be several days.13
Instead, the focus should be on addressing “treatable” concomitant symptoms, such as sleep disorders, anxiety and depression, and chronic pain. Lifestyle changes, avoidance of triggers, and exercise without over exertion are currently recommended to avoid incapacitating PEM.
Gaps in knowledge
There is a large knowledge gap regarding the pathophysiology, prevention, and therapy for CFS/ME. Many health care practitioners are not familiar with the disease and have focused on measurable parameters of exercise limitations and fatigue, such as anemias and lung and cardiac impairments, thus treating CFS/ME as a form of deconditioning. Given the large number of patients who recovered from acute COVID-19 that are now disabled due to CFS/ME, a patient-centered research opportunity has arisen. Biomedical/mechanistic research is ongoing, and well-designed clinical trials evaluating pharmacologic intervention as well as tailored exercise programs are needed.
Conclusion
General practitioners and women’s health specialists need to be aware of CFS/ME, especially when managing patients with long COVID. They also need to know that typical physical therapy may worsen symptoms. Furthermore, clinicians should shy away from trial drugs with a theoretical benefit outside of a clinical trial. ●
- Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as a major component of PASC
- Typical physical therapy has been shown to exacerbate symptoms of CFS/ME
- Treatment should focus on addressing “treatable” concomitant symptoms, lifestyle changes, avoidance of triggers, and exercise without over exertion
References
- Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102-e107. doi: 10.1016 /S1473-3099(21)00703-9
- Chen C, Haupert SR, Zimmermann L, et al. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022;226:1593-1607. doi: 10.1093/infdis/jiac136
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022 -00846-2
- Pavli A, Theodoridou M, Maltezou HC. Post-COVID syndrome: incidence, clinical spectrum, and challenges for primary healthcare professionals. Arch Med Res. 2021;52:575-581. doi: 10.1016/j.arcmed.2021.03.010
- Kedor C, Freitag H, Meyer-Arndt L, et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun. 2022;13:5104. doi: 10.1038/s41467-022-32507-6
- Bateman L, Bested AC, Bonilla HF, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: essentials of diagnosis and management. Mayo Clin Proc. 2021;96:28612878. doi: 10.1016/j.mayocp.2021.07.004
- Wessely S. History of postviral fatigue syndrome. Br Med Bull. 1991;47:919-941. doi: 10.1093/oxfordjournals.bmb.a072521
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. National Academies Press; 2015. doi: 10.17226/19012
- Ceban F, Ling S, Lui LMW, et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav Immun. 2022;101:93135. doi: 10.1016/j.bbi.2021.12.020
- Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
- Hanson MR. The viral origin of myalgic encephalomyelitis/ chronic fatigue syndrome. PLoS Pathog. 2023;19:e1011523. doi: 10.1371/journal.ppat.1011523
- Scheibenbogen C, Bellmann-Strobl JT, Heindrich C, et al. Fighting post-COVID and ME/CFS—development of curative therapies. Front Med (Lausanne). 2023;10:1194754. doi: 10.3389/fmed.2023.1194754
- Stussman B, Williams A, Snow J, et al. Characterization of post-exertional malaise in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Front Neurol. 2020;11:1025. doi: 10.3389/fneur.2020.01025
New COVID variant JN.1 could disrupt holiday plans
No one planning holiday gatherings or travel wants to hear this, but the rise of a new COVID-19 variant, JN.1, is concerning experts, who say it may threaten those good times.
The good news is recent research suggests the 2023-2024 COVID-19 vaccine appears to work against this newest variant. But so few people have gotten the latest vaccine — less than 16% of U.S. adults — that some experts suggest it’s time for the CDC to urge the public who haven’t it to do so now, so the antibodies can kick in before the festivities.
“A significant wave [of JN.1] has started here and could be blunted with a high booster rate and mitigation measures,” said Eric Topol, MD, professor and executive vice president of Scripps Research in La Jolla, CA, and editor-in-chief of Medscape, a sister site of this news organization.
COVID metrics, meanwhile, have started to climb again. Nearly 10,000 people were hospitalized for COVID in the U.S. for the week ending Nov. 25, the CDC said, a 10% increase over the previous week.
Who’s Who in the Family Tree
JN.1, an Omicron subvariant, was first detected in the U.S. in September and is termed “a notable descendent lineage” of Omicron subvariant BA.2.86 by the World Health Organization. When BA.2.86, also known as Pirola, was first identified in August, it appeared very different from other variants, the CDC said. That triggered concerns it might be more infectious than previous ones, even for people with immunity from vaccination and previous infections.
“JN.1 is Pirola’s kid,” said Rajendram Rajnarayanan, PhD, assistant dean of research and associate professor at the New York Institute of Technology at Arkansas State University, who maintains a COVID-19 variant database. The variant BA.2.86 and offspring are worrisome due to the mutations, he said.
How Widespread Is JN.1?
As of Nov. 27, the CDC says, BA.2.86 is projected to comprise 5%-15% of circulating variants in the U.S. “The expected public health risk of this variant, including its offshoot JN.1, is low,” the agency said.
Currently, JN.1 is reported more often in Europe, Dr. Rajnarayanan said, but some countries have better reporting data than others. “It has probably spread to every country tracking COVID,’’ he said, due to the mutations in the spike protein that make it easier for it to bind and infect.
Wastewater data suggest the variant’s rise is helping to fuel a wave, Dr. Topol said.
Vaccine Effectiveness Against JN.1, Other New Variants
The new XBB.1.5 monovalent vaccine, protects against XBB.1.5, another Omicron subvariant, but also JN.1 and other “emergent” viruses, a team of researchers reported Nov. 26 in a study on bioRxiv that has not yet been certified by peer review.
The updated vaccine, when given to uninfected people, boosted antibodies about 27-fold against XBB.1.5 and about 13- to 27-fold against JN.1 and other emergent viruses, the researchers reported.
While even primary doses of the COVID vaccine will likely help protect against the new JN.1 subvariant, “if you got the XBB.1.5 booster, it is going to be protecting you better against this new variant,” Dr. Rajnarayanan said.
2023-2024 Vaccine Uptake Low
In November, the CDC posted the first detailed estimates of who did. As of Nov. 18, less than 16% of U.S. adults had, with nearly 15% saying they planned to get it.
Coverage among children is lower, with just 6.3% of children up to date on the newest vaccine and 19% of parents saying they planned to get the 2023-2024 vaccine for their children.
Predictions, Mitigation
While some experts say a peak due to JN.1 is expected in the weeks ahead, Dr. Topol said it’s impossible to predict exactly how JN.1 will play out.
“It’s not going to be a repeat of November 2021,” when Omicron surfaced, Dr. Rajnarayanan predicted. Within 4 weeks of the World Health Organization declaring Omicron as a virus of concern, it spread around the world.
Mitigation measures can help, Dr. Rajnarayanan said. He suggested:
Get the new vaccine, and especially encourage vulnerable family and friends to do so.
If you are gathering inside for holiday festivities, improve circulation in the house, if possible.
Wear masks in airports and on planes and other public transportation.
A version of this article appeared on WebMD.com.
No one planning holiday gatherings or travel wants to hear this, but the rise of a new COVID-19 variant, JN.1, is concerning experts, who say it may threaten those good times.
The good news is recent research suggests the 2023-2024 COVID-19 vaccine appears to work against this newest variant. But so few people have gotten the latest vaccine — less than 16% of U.S. adults — that some experts suggest it’s time for the CDC to urge the public who haven’t it to do so now, so the antibodies can kick in before the festivities.
“A significant wave [of JN.1] has started here and could be blunted with a high booster rate and mitigation measures,” said Eric Topol, MD, professor and executive vice president of Scripps Research in La Jolla, CA, and editor-in-chief of Medscape, a sister site of this news organization.
COVID metrics, meanwhile, have started to climb again. Nearly 10,000 people were hospitalized for COVID in the U.S. for the week ending Nov. 25, the CDC said, a 10% increase over the previous week.
Who’s Who in the Family Tree
JN.1, an Omicron subvariant, was first detected in the U.S. in September and is termed “a notable descendent lineage” of Omicron subvariant BA.2.86 by the World Health Organization. When BA.2.86, also known as Pirola, was first identified in August, it appeared very different from other variants, the CDC said. That triggered concerns it might be more infectious than previous ones, even for people with immunity from vaccination and previous infections.
“JN.1 is Pirola’s kid,” said Rajendram Rajnarayanan, PhD, assistant dean of research and associate professor at the New York Institute of Technology at Arkansas State University, who maintains a COVID-19 variant database. The variant BA.2.86 and offspring are worrisome due to the mutations, he said.
How Widespread Is JN.1?
As of Nov. 27, the CDC says, BA.2.86 is projected to comprise 5%-15% of circulating variants in the U.S. “The expected public health risk of this variant, including its offshoot JN.1, is low,” the agency said.
Currently, JN.1 is reported more often in Europe, Dr. Rajnarayanan said, but some countries have better reporting data than others. “It has probably spread to every country tracking COVID,’’ he said, due to the mutations in the spike protein that make it easier for it to bind and infect.
Wastewater data suggest the variant’s rise is helping to fuel a wave, Dr. Topol said.
Vaccine Effectiveness Against JN.1, Other New Variants
The new XBB.1.5 monovalent vaccine, protects against XBB.1.5, another Omicron subvariant, but also JN.1 and other “emergent” viruses, a team of researchers reported Nov. 26 in a study on bioRxiv that has not yet been certified by peer review.
The updated vaccine, when given to uninfected people, boosted antibodies about 27-fold against XBB.1.5 and about 13- to 27-fold against JN.1 and other emergent viruses, the researchers reported.
While even primary doses of the COVID vaccine will likely help protect against the new JN.1 subvariant, “if you got the XBB.1.5 booster, it is going to be protecting you better against this new variant,” Dr. Rajnarayanan said.
2023-2024 Vaccine Uptake Low
In November, the CDC posted the first detailed estimates of who did. As of Nov. 18, less than 16% of U.S. adults had, with nearly 15% saying they planned to get it.
Coverage among children is lower, with just 6.3% of children up to date on the newest vaccine and 19% of parents saying they planned to get the 2023-2024 vaccine for their children.
Predictions, Mitigation
While some experts say a peak due to JN.1 is expected in the weeks ahead, Dr. Topol said it’s impossible to predict exactly how JN.1 will play out.
“It’s not going to be a repeat of November 2021,” when Omicron surfaced, Dr. Rajnarayanan predicted. Within 4 weeks of the World Health Organization declaring Omicron as a virus of concern, it spread around the world.
Mitigation measures can help, Dr. Rajnarayanan said. He suggested:
Get the new vaccine, and especially encourage vulnerable family and friends to do so.
If you are gathering inside for holiday festivities, improve circulation in the house, if possible.
Wear masks in airports and on planes and other public transportation.
A version of this article appeared on WebMD.com.
No one planning holiday gatherings or travel wants to hear this, but the rise of a new COVID-19 variant, JN.1, is concerning experts, who say it may threaten those good times.
The good news is recent research suggests the 2023-2024 COVID-19 vaccine appears to work against this newest variant. But so few people have gotten the latest vaccine — less than 16% of U.S. adults — that some experts suggest it’s time for the CDC to urge the public who haven’t it to do so now, so the antibodies can kick in before the festivities.
“A significant wave [of JN.1] has started here and could be blunted with a high booster rate and mitigation measures,” said Eric Topol, MD, professor and executive vice president of Scripps Research in La Jolla, CA, and editor-in-chief of Medscape, a sister site of this news organization.
COVID metrics, meanwhile, have started to climb again. Nearly 10,000 people were hospitalized for COVID in the U.S. for the week ending Nov. 25, the CDC said, a 10% increase over the previous week.
Who’s Who in the Family Tree
JN.1, an Omicron subvariant, was first detected in the U.S. in September and is termed “a notable descendent lineage” of Omicron subvariant BA.2.86 by the World Health Organization. When BA.2.86, also known as Pirola, was first identified in August, it appeared very different from other variants, the CDC said. That triggered concerns it might be more infectious than previous ones, even for people with immunity from vaccination and previous infections.
“JN.1 is Pirola’s kid,” said Rajendram Rajnarayanan, PhD, assistant dean of research and associate professor at the New York Institute of Technology at Arkansas State University, who maintains a COVID-19 variant database. The variant BA.2.86 and offspring are worrisome due to the mutations, he said.
How Widespread Is JN.1?
As of Nov. 27, the CDC says, BA.2.86 is projected to comprise 5%-15% of circulating variants in the U.S. “The expected public health risk of this variant, including its offshoot JN.1, is low,” the agency said.
Currently, JN.1 is reported more often in Europe, Dr. Rajnarayanan said, but some countries have better reporting data than others. “It has probably spread to every country tracking COVID,’’ he said, due to the mutations in the spike protein that make it easier for it to bind and infect.
Wastewater data suggest the variant’s rise is helping to fuel a wave, Dr. Topol said.
Vaccine Effectiveness Against JN.1, Other New Variants
The new XBB.1.5 monovalent vaccine, protects against XBB.1.5, another Omicron subvariant, but also JN.1 and other “emergent” viruses, a team of researchers reported Nov. 26 in a study on bioRxiv that has not yet been certified by peer review.
The updated vaccine, when given to uninfected people, boosted antibodies about 27-fold against XBB.1.5 and about 13- to 27-fold against JN.1 and other emergent viruses, the researchers reported.
While even primary doses of the COVID vaccine will likely help protect against the new JN.1 subvariant, “if you got the XBB.1.5 booster, it is going to be protecting you better against this new variant,” Dr. Rajnarayanan said.
2023-2024 Vaccine Uptake Low
In November, the CDC posted the first detailed estimates of who did. As of Nov. 18, less than 16% of U.S. adults had, with nearly 15% saying they planned to get it.
Coverage among children is lower, with just 6.3% of children up to date on the newest vaccine and 19% of parents saying they planned to get the 2023-2024 vaccine for their children.
Predictions, Mitigation
While some experts say a peak due to JN.1 is expected in the weeks ahead, Dr. Topol said it’s impossible to predict exactly how JN.1 will play out.
“It’s not going to be a repeat of November 2021,” when Omicron surfaced, Dr. Rajnarayanan predicted. Within 4 weeks of the World Health Organization declaring Omicron as a virus of concern, it spread around the world.
Mitigation measures can help, Dr. Rajnarayanan said. He suggested:
Get the new vaccine, and especially encourage vulnerable family and friends to do so.
If you are gathering inside for holiday festivities, improve circulation in the house, if possible.
Wear masks in airports and on planes and other public transportation.
A version of this article appeared on WebMD.com.
Some reasons to get off the fence about COVID booster
Though many people remain on the fence about getting the latest COVID vaccine booster, new research suggests a strong argument for getting the shot this winter: It sharply reduces the risk for COVID.
The risk reduction was 37% for those who received two doses. Experts say the research provides a strong argument for getting the vaccine, noting that about 10% of people infected with COVID go on to have long COVID, which can be debilitating for one quarter of those with long-lasting symptoms.
The data come from a systematic literature review and meta-analysis published in October in Antimicrobial Stewardship & Epidemiology. Researchers examined 32 studies published between December 2019 and June 2023, involving 775,931 adults. Twenty-four studies, encompassing 620,221 individuals, were included in the meta-analysis.
“The body of evidence from all these different studies converge on one single reality — that vaccines reduce the risk of long COVID, and people who keep up to date on their vaccinations also fared better than people who got it once or twice and didn’t follow up,” said Ziyad Al-Aly, MD, a clinical epidemiologist at Washington University in St Louis.
Researchers have reported similar results for children. The National Institutes of Health RECOVER Initiative team found that vaccines are up to 42% effective in preventing long COVID in children, said Dr. Carlos Oliveira, MD, a pediatric infectious diseases specialist and Yale researcher who contributed to the study, which is in preprint.
Vaccines also protect children from multisystem inflammatory syndrome, a condition that can happen after COVID, as well as protect against other COVID-related problems, such as missed school days, Oliveira said. “Even if the vaccine doesn’t completely stop long COVID, it’s still good for kids to get vaccinated for all these other reasons.”
However, uptake for the latest boosters has been slow: the Centers for Disease Control and Prevention reported that by mid-November, less than 16% of people aged 18 years or older had received a shot. For children, the number was closer to 6%. A recent Kaiser Family Foundation survey found that booster rates for adults are similar to what it was 1 year ago.
The survey results suggest that people are no longer as worried about COVID, which is why there is less concerned about keeping up with boosters. Though the current mutation of the virus is not as debilitating as its predecessors, long COVID continues to be a problem: as of January 2023, 28% of people who had contracted the virus had experienced long-COVID symptoms. And though the mechanisms are still not fully understood, and researchers have yet to agree on a definition of long COVID, they are certain about this much: The best way to avoid it is to avoid getting infected to begin with.
The lack of a diagnostic test for long COVID and the fact that the symptoms mimic those of other diseases lead to inconsistency that can make studies hard to replicate. In the papers reviewed for the Antimicrobial Stewardship & Epidemiology study, long COVID was defined as having symptoms lasting from more than 4 weeks to more than 6 months. Alexandre Marra, MD, the lead author and a researcher at the Hospital Israelita Albert Einstein, in São Paulo, Brazil, and at the University of Iowa, said that a clear standard definition is needed to better understand the actual prevalence and evaluate vaccine effectiveness.
Al-Aly noted that there is a logical explanation for one finding in the paper: The percentage of individuals who had COVID and reported that long-COVID symptoms declined from 19% in June 2022 to 11% in January 2023.
Because a pandemic is a dynamic event, constantly producing different variants with different phenotypes, the prevalence of disease is naturally going to be affected. “People who got infected early in the pandemic may have a different long COVID profile and long COVID risk than people who got infected in the second or third year of the pandemic,” Al-Aly said.
Most of the studies reported data from before the Omicron-variant era. Only eight reported data during that era. Omicron was not as lethal as previous variants, and consequently, fewer patients developed long COVID during that time.
One of those who did is Yeng Chang, age 40 years, a family doctor who lives in Sherwood Park, Alberta, Canada. Chang developed long COVID during fall 2022 after getting the virus in June. By then, she’d been vaccinated three times, but she isn’t surprised that she got sick because each vaccine she had was developed before Omicron.
“When I had COVID I was really sick, but I was well enough to stay home,” she said. “I think if I didn’t have my immunizations, I might have been hospitalized, and I don’t know what would have happened.”
Long COVID has left Chang with brain fog, fatigue, and a lack of physical stamina that forced her to pause her medical practice. For the past year and a half, she’s spent more time as a patient than a physician.
Chang had her fifth COVID vaccination in the fall and recommends that others do the same. “The booster you got however many years ago was effective for the COVID of that time but there is a new COVID now. You can’t just say, ‘I had one and I’m fine forever.’”
A version of this article appeared on Medscape.com.
Though many people remain on the fence about getting the latest COVID vaccine booster, new research suggests a strong argument for getting the shot this winter: It sharply reduces the risk for COVID.
The risk reduction was 37% for those who received two doses. Experts say the research provides a strong argument for getting the vaccine, noting that about 10% of people infected with COVID go on to have long COVID, which can be debilitating for one quarter of those with long-lasting symptoms.
The data come from a systematic literature review and meta-analysis published in October in Antimicrobial Stewardship & Epidemiology. Researchers examined 32 studies published between December 2019 and June 2023, involving 775,931 adults. Twenty-four studies, encompassing 620,221 individuals, were included in the meta-analysis.
“The body of evidence from all these different studies converge on one single reality — that vaccines reduce the risk of long COVID, and people who keep up to date on their vaccinations also fared better than people who got it once or twice and didn’t follow up,” said Ziyad Al-Aly, MD, a clinical epidemiologist at Washington University in St Louis.
Researchers have reported similar results for children. The National Institutes of Health RECOVER Initiative team found that vaccines are up to 42% effective in preventing long COVID in children, said Dr. Carlos Oliveira, MD, a pediatric infectious diseases specialist and Yale researcher who contributed to the study, which is in preprint.
Vaccines also protect children from multisystem inflammatory syndrome, a condition that can happen after COVID, as well as protect against other COVID-related problems, such as missed school days, Oliveira said. “Even if the vaccine doesn’t completely stop long COVID, it’s still good for kids to get vaccinated for all these other reasons.”
However, uptake for the latest boosters has been slow: the Centers for Disease Control and Prevention reported that by mid-November, less than 16% of people aged 18 years or older had received a shot. For children, the number was closer to 6%. A recent Kaiser Family Foundation survey found that booster rates for adults are similar to what it was 1 year ago.
The survey results suggest that people are no longer as worried about COVID, which is why there is less concerned about keeping up with boosters. Though the current mutation of the virus is not as debilitating as its predecessors, long COVID continues to be a problem: as of January 2023, 28% of people who had contracted the virus had experienced long-COVID symptoms. And though the mechanisms are still not fully understood, and researchers have yet to agree on a definition of long COVID, they are certain about this much: The best way to avoid it is to avoid getting infected to begin with.
The lack of a diagnostic test for long COVID and the fact that the symptoms mimic those of other diseases lead to inconsistency that can make studies hard to replicate. In the papers reviewed for the Antimicrobial Stewardship & Epidemiology study, long COVID was defined as having symptoms lasting from more than 4 weeks to more than 6 months. Alexandre Marra, MD, the lead author and a researcher at the Hospital Israelita Albert Einstein, in São Paulo, Brazil, and at the University of Iowa, said that a clear standard definition is needed to better understand the actual prevalence and evaluate vaccine effectiveness.
Al-Aly noted that there is a logical explanation for one finding in the paper: The percentage of individuals who had COVID and reported that long-COVID symptoms declined from 19% in June 2022 to 11% in January 2023.
Because a pandemic is a dynamic event, constantly producing different variants with different phenotypes, the prevalence of disease is naturally going to be affected. “People who got infected early in the pandemic may have a different long COVID profile and long COVID risk than people who got infected in the second or third year of the pandemic,” Al-Aly said.
Most of the studies reported data from before the Omicron-variant era. Only eight reported data during that era. Omicron was not as lethal as previous variants, and consequently, fewer patients developed long COVID during that time.
One of those who did is Yeng Chang, age 40 years, a family doctor who lives in Sherwood Park, Alberta, Canada. Chang developed long COVID during fall 2022 after getting the virus in June. By then, she’d been vaccinated three times, but she isn’t surprised that she got sick because each vaccine she had was developed before Omicron.
“When I had COVID I was really sick, but I was well enough to stay home,” she said. “I think if I didn’t have my immunizations, I might have been hospitalized, and I don’t know what would have happened.”
Long COVID has left Chang with brain fog, fatigue, and a lack of physical stamina that forced her to pause her medical practice. For the past year and a half, she’s spent more time as a patient than a physician.
Chang had her fifth COVID vaccination in the fall and recommends that others do the same. “The booster you got however many years ago was effective for the COVID of that time but there is a new COVID now. You can’t just say, ‘I had one and I’m fine forever.’”
A version of this article appeared on Medscape.com.
Though many people remain on the fence about getting the latest COVID vaccine booster, new research suggests a strong argument for getting the shot this winter: It sharply reduces the risk for COVID.
The risk reduction was 37% for those who received two doses. Experts say the research provides a strong argument for getting the vaccine, noting that about 10% of people infected with COVID go on to have long COVID, which can be debilitating for one quarter of those with long-lasting symptoms.
The data come from a systematic literature review and meta-analysis published in October in Antimicrobial Stewardship & Epidemiology. Researchers examined 32 studies published between December 2019 and June 2023, involving 775,931 adults. Twenty-four studies, encompassing 620,221 individuals, were included in the meta-analysis.
“The body of evidence from all these different studies converge on one single reality — that vaccines reduce the risk of long COVID, and people who keep up to date on their vaccinations also fared better than people who got it once or twice and didn’t follow up,” said Ziyad Al-Aly, MD, a clinical epidemiologist at Washington University in St Louis.
Researchers have reported similar results for children. The National Institutes of Health RECOVER Initiative team found that vaccines are up to 42% effective in preventing long COVID in children, said Dr. Carlos Oliveira, MD, a pediatric infectious diseases specialist and Yale researcher who contributed to the study, which is in preprint.
Vaccines also protect children from multisystem inflammatory syndrome, a condition that can happen after COVID, as well as protect against other COVID-related problems, such as missed school days, Oliveira said. “Even if the vaccine doesn’t completely stop long COVID, it’s still good for kids to get vaccinated for all these other reasons.”
However, uptake for the latest boosters has been slow: the Centers for Disease Control and Prevention reported that by mid-November, less than 16% of people aged 18 years or older had received a shot. For children, the number was closer to 6%. A recent Kaiser Family Foundation survey found that booster rates for adults are similar to what it was 1 year ago.
The survey results suggest that people are no longer as worried about COVID, which is why there is less concerned about keeping up with boosters. Though the current mutation of the virus is not as debilitating as its predecessors, long COVID continues to be a problem: as of January 2023, 28% of people who had contracted the virus had experienced long-COVID symptoms. And though the mechanisms are still not fully understood, and researchers have yet to agree on a definition of long COVID, they are certain about this much: The best way to avoid it is to avoid getting infected to begin with.
The lack of a diagnostic test for long COVID and the fact that the symptoms mimic those of other diseases lead to inconsistency that can make studies hard to replicate. In the papers reviewed for the Antimicrobial Stewardship & Epidemiology study, long COVID was defined as having symptoms lasting from more than 4 weeks to more than 6 months. Alexandre Marra, MD, the lead author and a researcher at the Hospital Israelita Albert Einstein, in São Paulo, Brazil, and at the University of Iowa, said that a clear standard definition is needed to better understand the actual prevalence and evaluate vaccine effectiveness.
Al-Aly noted that there is a logical explanation for one finding in the paper: The percentage of individuals who had COVID and reported that long-COVID symptoms declined from 19% in June 2022 to 11% in January 2023.
Because a pandemic is a dynamic event, constantly producing different variants with different phenotypes, the prevalence of disease is naturally going to be affected. “People who got infected early in the pandemic may have a different long COVID profile and long COVID risk than people who got infected in the second or third year of the pandemic,” Al-Aly said.
Most of the studies reported data from before the Omicron-variant era. Only eight reported data during that era. Omicron was not as lethal as previous variants, and consequently, fewer patients developed long COVID during that time.
One of those who did is Yeng Chang, age 40 years, a family doctor who lives in Sherwood Park, Alberta, Canada. Chang developed long COVID during fall 2022 after getting the virus in June. By then, she’d been vaccinated three times, but she isn’t surprised that she got sick because each vaccine she had was developed before Omicron.
“When I had COVID I was really sick, but I was well enough to stay home,” she said. “I think if I didn’t have my immunizations, I might have been hospitalized, and I don’t know what would have happened.”
Long COVID has left Chang with brain fog, fatigue, and a lack of physical stamina that forced her to pause her medical practice. For the past year and a half, she’s spent more time as a patient than a physician.
Chang had her fifth COVID vaccination in the fall and recommends that others do the same. “The booster you got however many years ago was effective for the COVID of that time but there is a new COVID now. You can’t just say, ‘I had one and I’m fine forever.’”
A version of this article appeared on Medscape.com.
COVID livers are safe for transplant
, based on a national study with the longest follow-up to date.
Using livers from deceased patients with COVID-19 could be an opportunity expand organ availability, reported principal investigator Nadim Mahmud, MD, of the University of Pennsylvania, Philadelphia, and colleagues.
Findings were presented in November at the annual meeting of the American Association for the Study of Liver Diseases.
“During the COVID-19 pandemic, a few centers trialed transplanting solid organs from COVID-19 positive donors with promising initial results,” presenting author Roy X. Wang, MD, of the University of Pennsylvania, said in a written comment. “However, these were smaller experiences with short follow-up that were not exclusively focused on liver transplantation. We wanted to explore the safety of liver transplantation from COVID-19 positive donors using a large national dataset with the longest follow up time to date.”
The dataset included 13,096 COVID-negative donors and 299 COVID-positive donors who died between July 2020 and July 2022, with cases and controls matched via propensity scoring. COVID-positive donors were significantly more likely to be younger and have died of brain death. Beyond this difference in age, no significant demographic differences were detected.
After 1 year of follow-up, no statistically significant differences in patient survival (subhazard ratio, 1.11; log-rank P = .70) or allograft survival (hazard ratio, 1.44; log-rank P = .14) were detected when comparing livers transplanted from positive versus negative donors.
“Our findings support and expand upon the results from earlier studies,” Dr. Wang concluded. “Liver transplant from COVID-19-positive donors has acceptable short-term outcomes and may represent an opportunity to expand organ access.”
Still, more work is needed to assess other clinical metrics and long-term outcomes, he added.
“While we were able to show similar patient and graft survival post-transplant between COVID-19-positive and negative donors, rates of other complications were not investigated such as episodes of rejection, liver injury, and hospitalizations,” Dr. Wang said. “Due to data limitations, we are only able to report on outcomes up to 1 year post transplant. Additional investigation will be needed to continue monitoring future outcomes and identifying any differences between recipients of COVID-19-positive and negative donors.”
Timucin Taner, MD, PhD, division chair of transplant surgery at Mayo Clinic, Rochester, Minnesota, said the study is important because it reaffirms the majority opinion among transplant physicians: These livers are safe.
In an interview, Dr. Taner suggested that Dr. Wang’s call for longer term data is “mostly science speak,” since 1 year of follow-up should be sufficient to determine liver viability.
“If a liver from a COVID-19 donor behaved well for a year, then chances are it’s not going to behave badly [later on] because of the virus at the time of donation,” Dr. Taner said.
He said the reported trends in usage of COVID-positive livers reflect early hesitancy that waned with rising vaccination rates, and recognition that the virus could not be spread via liver donation.
“To date, the only transmission [of SARS-CoV-2] from a transplant has been from a lung transplant,” Dr. Taner said, “and that was back in the days that we didn’t know about this. Other organs don’t transmit the disease, so they are easily usable.”
These new data should further increase confidence among both health care providers and patients, he added.
“[This study is] reassuring to the patients on the waitlist that these organs are very safe to use,” Dr. Taner said. “We as the transplant society are comfortable using them without any hesitation.”
The investigators and Dr. Taner disclosed no conflicts of interest.
, based on a national study with the longest follow-up to date.
Using livers from deceased patients with COVID-19 could be an opportunity expand organ availability, reported principal investigator Nadim Mahmud, MD, of the University of Pennsylvania, Philadelphia, and colleagues.
Findings were presented in November at the annual meeting of the American Association for the Study of Liver Diseases.
“During the COVID-19 pandemic, a few centers trialed transplanting solid organs from COVID-19 positive donors with promising initial results,” presenting author Roy X. Wang, MD, of the University of Pennsylvania, said in a written comment. “However, these were smaller experiences with short follow-up that were not exclusively focused on liver transplantation. We wanted to explore the safety of liver transplantation from COVID-19 positive donors using a large national dataset with the longest follow up time to date.”
The dataset included 13,096 COVID-negative donors and 299 COVID-positive donors who died between July 2020 and July 2022, with cases and controls matched via propensity scoring. COVID-positive donors were significantly more likely to be younger and have died of brain death. Beyond this difference in age, no significant demographic differences were detected.
After 1 year of follow-up, no statistically significant differences in patient survival (subhazard ratio, 1.11; log-rank P = .70) or allograft survival (hazard ratio, 1.44; log-rank P = .14) were detected when comparing livers transplanted from positive versus negative donors.
“Our findings support and expand upon the results from earlier studies,” Dr. Wang concluded. “Liver transplant from COVID-19-positive donors has acceptable short-term outcomes and may represent an opportunity to expand organ access.”
Still, more work is needed to assess other clinical metrics and long-term outcomes, he added.
“While we were able to show similar patient and graft survival post-transplant between COVID-19-positive and negative donors, rates of other complications were not investigated such as episodes of rejection, liver injury, and hospitalizations,” Dr. Wang said. “Due to data limitations, we are only able to report on outcomes up to 1 year post transplant. Additional investigation will be needed to continue monitoring future outcomes and identifying any differences between recipients of COVID-19-positive and negative donors.”
Timucin Taner, MD, PhD, division chair of transplant surgery at Mayo Clinic, Rochester, Minnesota, said the study is important because it reaffirms the majority opinion among transplant physicians: These livers are safe.
In an interview, Dr. Taner suggested that Dr. Wang’s call for longer term data is “mostly science speak,” since 1 year of follow-up should be sufficient to determine liver viability.
“If a liver from a COVID-19 donor behaved well for a year, then chances are it’s not going to behave badly [later on] because of the virus at the time of donation,” Dr. Taner said.
He said the reported trends in usage of COVID-positive livers reflect early hesitancy that waned with rising vaccination rates, and recognition that the virus could not be spread via liver donation.
“To date, the only transmission [of SARS-CoV-2] from a transplant has been from a lung transplant,” Dr. Taner said, “and that was back in the days that we didn’t know about this. Other organs don’t transmit the disease, so they are easily usable.”
These new data should further increase confidence among both health care providers and patients, he added.
“[This study is] reassuring to the patients on the waitlist that these organs are very safe to use,” Dr. Taner said. “We as the transplant society are comfortable using them without any hesitation.”
The investigators and Dr. Taner disclosed no conflicts of interest.
, based on a national study with the longest follow-up to date.
Using livers from deceased patients with COVID-19 could be an opportunity expand organ availability, reported principal investigator Nadim Mahmud, MD, of the University of Pennsylvania, Philadelphia, and colleagues.
Findings were presented in November at the annual meeting of the American Association for the Study of Liver Diseases.
“During the COVID-19 pandemic, a few centers trialed transplanting solid organs from COVID-19 positive donors with promising initial results,” presenting author Roy X. Wang, MD, of the University of Pennsylvania, said in a written comment. “However, these were smaller experiences with short follow-up that were not exclusively focused on liver transplantation. We wanted to explore the safety of liver transplantation from COVID-19 positive donors using a large national dataset with the longest follow up time to date.”
The dataset included 13,096 COVID-negative donors and 299 COVID-positive donors who died between July 2020 and July 2022, with cases and controls matched via propensity scoring. COVID-positive donors were significantly more likely to be younger and have died of brain death. Beyond this difference in age, no significant demographic differences were detected.
After 1 year of follow-up, no statistically significant differences in patient survival (subhazard ratio, 1.11; log-rank P = .70) or allograft survival (hazard ratio, 1.44; log-rank P = .14) were detected when comparing livers transplanted from positive versus negative donors.
“Our findings support and expand upon the results from earlier studies,” Dr. Wang concluded. “Liver transplant from COVID-19-positive donors has acceptable short-term outcomes and may represent an opportunity to expand organ access.”
Still, more work is needed to assess other clinical metrics and long-term outcomes, he added.
“While we were able to show similar patient and graft survival post-transplant between COVID-19-positive and negative donors, rates of other complications were not investigated such as episodes of rejection, liver injury, and hospitalizations,” Dr. Wang said. “Due to data limitations, we are only able to report on outcomes up to 1 year post transplant. Additional investigation will be needed to continue monitoring future outcomes and identifying any differences between recipients of COVID-19-positive and negative donors.”
Timucin Taner, MD, PhD, division chair of transplant surgery at Mayo Clinic, Rochester, Minnesota, said the study is important because it reaffirms the majority opinion among transplant physicians: These livers are safe.
In an interview, Dr. Taner suggested that Dr. Wang’s call for longer term data is “mostly science speak,” since 1 year of follow-up should be sufficient to determine liver viability.
“If a liver from a COVID-19 donor behaved well for a year, then chances are it’s not going to behave badly [later on] because of the virus at the time of donation,” Dr. Taner said.
He said the reported trends in usage of COVID-positive livers reflect early hesitancy that waned with rising vaccination rates, and recognition that the virus could not be spread via liver donation.
“To date, the only transmission [of SARS-CoV-2] from a transplant has been from a lung transplant,” Dr. Taner said, “and that was back in the days that we didn’t know about this. Other organs don’t transmit the disease, so they are easily usable.”
These new data should further increase confidence among both health care providers and patients, he added.
“[This study is] reassuring to the patients on the waitlist that these organs are very safe to use,” Dr. Taner said. “We as the transplant society are comfortable using them without any hesitation.”
The investigators and Dr. Taner disclosed no conflicts of interest.
AT THE LIVER MEETING
COVID vaccines lower risk of serious illness in children
TOPLINE:
, according to a new study by the Centers for Disease Control and Prevention (CDC).
METHODOLOGY:
- SARS-CoV-2 infection can severely affect children who have certain chronic conditions.
- Researchers assessed the effectiveness of COVID-19 vaccines in preventing emergency ED visits and hospitalizations associated with the illness from July 2022 to September 2023.
- They drew data from the New Vaccine Surveillance Network, which conducts population-based, prospective surveillance for acute respiratory illness in children at seven pediatric medical centers.
- The period assessed was the first year vaccines were authorized for children aged 6 months to 4 years; during that period, several Omicron subvariants arose.
- Researchers used data from 7,434 infants and children; data included patients’ vaccine status and their test results for SARS-CoV-2.
TAKEAWAY:
- Of the 7,434 infants and children who had an acute respiratory illness and were hospitalized or visited the ED, 387 had COVID-19.
- Children who received two doses of a COVID-19 vaccine were 40% less likely to have a COVID-19-associated hospitalization or ED visit compared with unvaccinated youth.
- One dose of a COVID-19 vaccine reduced ED visits and hospitalizations by 31%.
IN PRACTICE:
“The findings in this report support the recommendation for COVID-19 vaccination for all children aged ≥6 months and highlight the importance of completion of a primary series for young children,” the researchers reported.
SOURCE:
The study was led by Heidi L. Moline, MD, of the CDC.
LIMITATIONS:
Because the number of children with antibodies and immunity against SARS-CoV-2 has grown, vaccine effectiveness rates in the study may no longer be as relevant. Children with preexisting chronic conditions may be more likely to be vaccinated and receive medical attention. The low rates of vaccination may have prevented researchers from conducting a more detailed analysis. The Pfizer-BioNTech vaccine requires three doses, whereas Moderna’s requires two doses; this may have skewed the estimated efficacy of the Pfizer-BioNTech vaccine.
DISCLOSURES:
The authors report a variety of potential conflicts of interest, which are detailed in the article.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to a new study by the Centers for Disease Control and Prevention (CDC).
METHODOLOGY:
- SARS-CoV-2 infection can severely affect children who have certain chronic conditions.
- Researchers assessed the effectiveness of COVID-19 vaccines in preventing emergency ED visits and hospitalizations associated with the illness from July 2022 to September 2023.
- They drew data from the New Vaccine Surveillance Network, which conducts population-based, prospective surveillance for acute respiratory illness in children at seven pediatric medical centers.
- The period assessed was the first year vaccines were authorized for children aged 6 months to 4 years; during that period, several Omicron subvariants arose.
- Researchers used data from 7,434 infants and children; data included patients’ vaccine status and their test results for SARS-CoV-2.
TAKEAWAY:
- Of the 7,434 infants and children who had an acute respiratory illness and were hospitalized or visited the ED, 387 had COVID-19.
- Children who received two doses of a COVID-19 vaccine were 40% less likely to have a COVID-19-associated hospitalization or ED visit compared with unvaccinated youth.
- One dose of a COVID-19 vaccine reduced ED visits and hospitalizations by 31%.
IN PRACTICE:
“The findings in this report support the recommendation for COVID-19 vaccination for all children aged ≥6 months and highlight the importance of completion of a primary series for young children,” the researchers reported.
SOURCE:
The study was led by Heidi L. Moline, MD, of the CDC.
LIMITATIONS:
Because the number of children with antibodies and immunity against SARS-CoV-2 has grown, vaccine effectiveness rates in the study may no longer be as relevant. Children with preexisting chronic conditions may be more likely to be vaccinated and receive medical attention. The low rates of vaccination may have prevented researchers from conducting a more detailed analysis. The Pfizer-BioNTech vaccine requires three doses, whereas Moderna’s requires two doses; this may have skewed the estimated efficacy of the Pfizer-BioNTech vaccine.
DISCLOSURES:
The authors report a variety of potential conflicts of interest, which are detailed in the article.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to a new study by the Centers for Disease Control and Prevention (CDC).
METHODOLOGY:
- SARS-CoV-2 infection can severely affect children who have certain chronic conditions.
- Researchers assessed the effectiveness of COVID-19 vaccines in preventing emergency ED visits and hospitalizations associated with the illness from July 2022 to September 2023.
- They drew data from the New Vaccine Surveillance Network, which conducts population-based, prospective surveillance for acute respiratory illness in children at seven pediatric medical centers.
- The period assessed was the first year vaccines were authorized for children aged 6 months to 4 years; during that period, several Omicron subvariants arose.
- Researchers used data from 7,434 infants and children; data included patients’ vaccine status and their test results for SARS-CoV-2.
TAKEAWAY:
- Of the 7,434 infants and children who had an acute respiratory illness and were hospitalized or visited the ED, 387 had COVID-19.
- Children who received two doses of a COVID-19 vaccine were 40% less likely to have a COVID-19-associated hospitalization or ED visit compared with unvaccinated youth.
- One dose of a COVID-19 vaccine reduced ED visits and hospitalizations by 31%.
IN PRACTICE:
“The findings in this report support the recommendation for COVID-19 vaccination for all children aged ≥6 months and highlight the importance of completion of a primary series for young children,” the researchers reported.
SOURCE:
The study was led by Heidi L. Moline, MD, of the CDC.
LIMITATIONS:
Because the number of children with antibodies and immunity against SARS-CoV-2 has grown, vaccine effectiveness rates in the study may no longer be as relevant. Children with preexisting chronic conditions may be more likely to be vaccinated and receive medical attention. The low rates of vaccination may have prevented researchers from conducting a more detailed analysis. The Pfizer-BioNTech vaccine requires three doses, whereas Moderna’s requires two doses; this may have skewed the estimated efficacy of the Pfizer-BioNTech vaccine.
DISCLOSURES:
The authors report a variety of potential conflicts of interest, which are detailed in the article.
A version of this article appeared on Medscape.com.