User login
Early evidence supports psilocybin for bipolar depression
, a small, nonrandomized clinical trial shows. However, the investigators and other outside experts warn these results should not be overinterpreted.
Three weeks after receiving psilocybin and psychotherapy, all 15 participants’ depression scores dropped by an average of 24 points. Twelve met criteria for response and 11 for remission.
This benefit lasted until the 12-week mark, with 12 participants (80%) meeting criteria for both response and remission. There were no reports of mixed or manic symptoms, psychotic symptoms, or suicidal ideation.
Given the study’s small, open-label design, the findings should be interpreted cautiously, but the investigators say the results are promising.
“The results we saw from this trial are encouraging and further support the clinical study of psychedelics in patients with treatment-resistant bipolar II,” lead investigator Scott T. Aaronson, MD, chief science officer of the Institute for Advanced Diagnostics and Therapeutics at Sheppard Pratt Health System in Baltimore, Maryland, remarked in a press release. “One participant compared the transformation she experienced to taking a deep breath after breathing through a straw for years.”
The findings were published online on December 6, 2023, in JAMA Psychiatry.
Underserved Population
Previous studies show that psilocybin is effective in reducing the symptoms of treatment-resistant depression, major depressive disorder, and anorexia nervosa, most with only mild to moderate adverse effects.
Individuals with bipolar disorder (BPD) have been excluded from psilocybin studies in the last two decades. Investigators attribute this to anecdotal evidence that psychedelics may result in manic episodes in patients with BPD, even though empirical evidence of those effects is limited.
This study included 15 participants (9 female; mean age, 37.8 years) with BDII who had experienced an episode for more than 3 months and failed at least two medications within the current episode.
Participants stopped all psychotropic medications at least 2 weeks prior to the trial and received 25 mg of synthetic COMP360 psilocybin in a controlled setting. Psychotherapy included three sessions before dosing, one during the 8-hour dosing day, and three integration sessions posttreatment.
Depression was measured with the Montgomery Ǻsberg Depression Rating Scale (MADRS) at six points during the 12-week study.
By week 3, all 15 participants had lower MADRS scores, with a mean decrease of 24 points (P < .001). Twelve participants met the response criteria of ≥ 50% reduction in MADRS scores, and 11 met the criteria for remission of a MADRS score of ≤ 10 (both P < .001).
MADRS scores at each posttreatment time point were significantly lower than they were at baseline and the improvement persisted at 12 weeks.
Participants were also monitored for mania and suicidality at various time points during the study, and no significant changes were found from baseline.
“As a first open-label foray into this underserved and treatment-resistant population, care should be taken not to overinterpret the findings,” the authors note, adding that the findings may not apply to patients with BDI or BDII in a mixed or hypomanic phase of their illness.
No Definitive Conclusion
In an accompanying editorial, David B. Yaden, PhD, Johns Hopkins University School of Medicine, Baltimore, Maryland, and colleagues write that the findings are “tantalizingly suggestive,” but ultimately say nothing definitive about the efficacy of psilocybin for BDII.
“The danger is that some individuals will see the large (yet uncontrolled) effect size and believe that a new treatment of bipolar II has been discovered that is substantially better than all other treatments, while neglecting to mention the lack of control condition and substantial psychosocial support included in the study,” Dr. Yaden and colleagues wrote.
They also cautioned that due to the study’s limitations, which include its small sample size and lack of a control group, “it is imperative that the large effect size before to after the study is not overinterpreted.”
However, Dr. Yaden and colleagues also characterized the safety data as “compelling,” noting the safety profile could affect exclusion criteria in future studies involving people with BPD.
“The results of the present study provide preliminary evidence that perhaps those with bipolar II can be safely included in study samples without undue risk of triggering hypomanic episodes,” they wrote. “It also suggests re-evaluation of the need to exclude individuals with mere family history of bipolar II, which several studies do.”
The study was funded by COMPASS Pathways, who provided the study drug. Dr. Aaronson reported grants and nonfinancial support (supply of drug) from COMPASS Pathways during the conduct of the study and personal fees from LivaNova, Neuronetics, Genomind, and Sage Therapeutics outside the submitted work. Other disclosures are noted in the original article.
A version of this article appeared on Medscape.com.
, a small, nonrandomized clinical trial shows. However, the investigators and other outside experts warn these results should not be overinterpreted.
Three weeks after receiving psilocybin and psychotherapy, all 15 participants’ depression scores dropped by an average of 24 points. Twelve met criteria for response and 11 for remission.
This benefit lasted until the 12-week mark, with 12 participants (80%) meeting criteria for both response and remission. There were no reports of mixed or manic symptoms, psychotic symptoms, or suicidal ideation.
Given the study’s small, open-label design, the findings should be interpreted cautiously, but the investigators say the results are promising.
“The results we saw from this trial are encouraging and further support the clinical study of psychedelics in patients with treatment-resistant bipolar II,” lead investigator Scott T. Aaronson, MD, chief science officer of the Institute for Advanced Diagnostics and Therapeutics at Sheppard Pratt Health System in Baltimore, Maryland, remarked in a press release. “One participant compared the transformation she experienced to taking a deep breath after breathing through a straw for years.”
The findings were published online on December 6, 2023, in JAMA Psychiatry.
Underserved Population
Previous studies show that psilocybin is effective in reducing the symptoms of treatment-resistant depression, major depressive disorder, and anorexia nervosa, most with only mild to moderate adverse effects.
Individuals with bipolar disorder (BPD) have been excluded from psilocybin studies in the last two decades. Investigators attribute this to anecdotal evidence that psychedelics may result in manic episodes in patients with BPD, even though empirical evidence of those effects is limited.
This study included 15 participants (9 female; mean age, 37.8 years) with BDII who had experienced an episode for more than 3 months and failed at least two medications within the current episode.
Participants stopped all psychotropic medications at least 2 weeks prior to the trial and received 25 mg of synthetic COMP360 psilocybin in a controlled setting. Psychotherapy included three sessions before dosing, one during the 8-hour dosing day, and three integration sessions posttreatment.
Depression was measured with the Montgomery Ǻsberg Depression Rating Scale (MADRS) at six points during the 12-week study.
By week 3, all 15 participants had lower MADRS scores, with a mean decrease of 24 points (P < .001). Twelve participants met the response criteria of ≥ 50% reduction in MADRS scores, and 11 met the criteria for remission of a MADRS score of ≤ 10 (both P < .001).
MADRS scores at each posttreatment time point were significantly lower than they were at baseline and the improvement persisted at 12 weeks.
Participants were also monitored for mania and suicidality at various time points during the study, and no significant changes were found from baseline.
“As a first open-label foray into this underserved and treatment-resistant population, care should be taken not to overinterpret the findings,” the authors note, adding that the findings may not apply to patients with BDI or BDII in a mixed or hypomanic phase of their illness.
No Definitive Conclusion
In an accompanying editorial, David B. Yaden, PhD, Johns Hopkins University School of Medicine, Baltimore, Maryland, and colleagues write that the findings are “tantalizingly suggestive,” but ultimately say nothing definitive about the efficacy of psilocybin for BDII.
“The danger is that some individuals will see the large (yet uncontrolled) effect size and believe that a new treatment of bipolar II has been discovered that is substantially better than all other treatments, while neglecting to mention the lack of control condition and substantial psychosocial support included in the study,” Dr. Yaden and colleagues wrote.
They also cautioned that due to the study’s limitations, which include its small sample size and lack of a control group, “it is imperative that the large effect size before to after the study is not overinterpreted.”
However, Dr. Yaden and colleagues also characterized the safety data as “compelling,” noting the safety profile could affect exclusion criteria in future studies involving people with BPD.
“The results of the present study provide preliminary evidence that perhaps those with bipolar II can be safely included in study samples without undue risk of triggering hypomanic episodes,” they wrote. “It also suggests re-evaluation of the need to exclude individuals with mere family history of bipolar II, which several studies do.”
The study was funded by COMPASS Pathways, who provided the study drug. Dr. Aaronson reported grants and nonfinancial support (supply of drug) from COMPASS Pathways during the conduct of the study and personal fees from LivaNova, Neuronetics, Genomind, and Sage Therapeutics outside the submitted work. Other disclosures are noted in the original article.
A version of this article appeared on Medscape.com.
, a small, nonrandomized clinical trial shows. However, the investigators and other outside experts warn these results should not be overinterpreted.
Three weeks after receiving psilocybin and psychotherapy, all 15 participants’ depression scores dropped by an average of 24 points. Twelve met criteria for response and 11 for remission.
This benefit lasted until the 12-week mark, with 12 participants (80%) meeting criteria for both response and remission. There were no reports of mixed or manic symptoms, psychotic symptoms, or suicidal ideation.
Given the study’s small, open-label design, the findings should be interpreted cautiously, but the investigators say the results are promising.
“The results we saw from this trial are encouraging and further support the clinical study of psychedelics in patients with treatment-resistant bipolar II,” lead investigator Scott T. Aaronson, MD, chief science officer of the Institute for Advanced Diagnostics and Therapeutics at Sheppard Pratt Health System in Baltimore, Maryland, remarked in a press release. “One participant compared the transformation she experienced to taking a deep breath after breathing through a straw for years.”
The findings were published online on December 6, 2023, in JAMA Psychiatry.
Underserved Population
Previous studies show that psilocybin is effective in reducing the symptoms of treatment-resistant depression, major depressive disorder, and anorexia nervosa, most with only mild to moderate adverse effects.
Individuals with bipolar disorder (BPD) have been excluded from psilocybin studies in the last two decades. Investigators attribute this to anecdotal evidence that psychedelics may result in manic episodes in patients with BPD, even though empirical evidence of those effects is limited.
This study included 15 participants (9 female; mean age, 37.8 years) with BDII who had experienced an episode for more than 3 months and failed at least two medications within the current episode.
Participants stopped all psychotropic medications at least 2 weeks prior to the trial and received 25 mg of synthetic COMP360 psilocybin in a controlled setting. Psychotherapy included three sessions before dosing, one during the 8-hour dosing day, and three integration sessions posttreatment.
Depression was measured with the Montgomery Ǻsberg Depression Rating Scale (MADRS) at six points during the 12-week study.
By week 3, all 15 participants had lower MADRS scores, with a mean decrease of 24 points (P < .001). Twelve participants met the response criteria of ≥ 50% reduction in MADRS scores, and 11 met the criteria for remission of a MADRS score of ≤ 10 (both P < .001).
MADRS scores at each posttreatment time point were significantly lower than they were at baseline and the improvement persisted at 12 weeks.
Participants were also monitored for mania and suicidality at various time points during the study, and no significant changes were found from baseline.
“As a first open-label foray into this underserved and treatment-resistant population, care should be taken not to overinterpret the findings,” the authors note, adding that the findings may not apply to patients with BDI or BDII in a mixed or hypomanic phase of their illness.
No Definitive Conclusion
In an accompanying editorial, David B. Yaden, PhD, Johns Hopkins University School of Medicine, Baltimore, Maryland, and colleagues write that the findings are “tantalizingly suggestive,” but ultimately say nothing definitive about the efficacy of psilocybin for BDII.
“The danger is that some individuals will see the large (yet uncontrolled) effect size and believe that a new treatment of bipolar II has been discovered that is substantially better than all other treatments, while neglecting to mention the lack of control condition and substantial psychosocial support included in the study,” Dr. Yaden and colleagues wrote.
They also cautioned that due to the study’s limitations, which include its small sample size and lack of a control group, “it is imperative that the large effect size before to after the study is not overinterpreted.”
However, Dr. Yaden and colleagues also characterized the safety data as “compelling,” noting the safety profile could affect exclusion criteria in future studies involving people with BPD.
“The results of the present study provide preliminary evidence that perhaps those with bipolar II can be safely included in study samples without undue risk of triggering hypomanic episodes,” they wrote. “It also suggests re-evaluation of the need to exclude individuals with mere family history of bipolar II, which several studies do.”
The study was funded by COMPASS Pathways, who provided the study drug. Dr. Aaronson reported grants and nonfinancial support (supply of drug) from COMPASS Pathways during the conduct of the study and personal fees from LivaNova, Neuronetics, Genomind, and Sage Therapeutics outside the submitted work. Other disclosures are noted in the original article.
A version of this article appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Sometimes well-intended mental health treatment hurts
We love psychiatry. We love the idea that someone can come to receive care from a physician to alleviate psychological suffering.
Some people experience such severe anguish that they are unable to relate to others. Some are so despondent that they are unable to make decisions. Some are so distressed that their thoughts become inconsistent with reality. We want all those people, and many more, to have access to effective psychiatric care. However, there are reasonable expectations that one should be able to have that a treatment will help, and that appropriate informed consent is given.
One recent article reminded us of this in a particularly poignant way.
The study in question is a recent publication looking at the universal use of psychotherapy for teenagers.1 At face value, we would have certainly considered this to be a benevolent and well-meaning intervention. Anyone who has been a teenager or has talked to one, is aware of the emotional instability punctuated by episodes of intense anxiety or irritability. It is age appropriate for a teenager to question and explore their identity. Teenagers are notoriously impulsive with a deep desire for validating interpersonal relationships. One could continue to list the symptoms of borderline personality disorder (BPD) and find a lot of similarity with the condition of transitioning from a child to an adult.
It is thus common sense to consider applying the most established therapy for BPD, dialectical behavioral therapy (DBT), to teenagers. The basics of DBT would seem to be helpful to anyone but appear particularly appropriate to this population. Mindfulness, the practice of paying attention to your present experience, allows one to realize that they are trapped in past or hypothetical future moments. Emotional regulation provides the tools that offer a frame for our feelings and involves recognizing feelings and understanding what they mean. Interpersonal work allows one to recognize and adapt to the feelings of others, while learning how to have a healthy voice with others. Distress tolerance is the exercise of learning to experience and contain our feelings.
The study looked at about 1,000 young adolescents, around 13 years old across high schools in Sydney, Australia: 598 adolescents were allocated to the intervention, and 566 to the control. The intervention consisted of eight weekly sessions of DBT lasting about 50 minutes. The results were “contrary to predictions.” Participants who received DBT “reported significantly increased total difficulties,” and “significant increases in depression and anxiety.” The effects were worse in males yet significant in both genders. The study concludes with “a reminder that present enthusiasm for universal dissemination of short-term DBT-based group skills training within schools, specifically in early adolescence, is ahead of the research evidence.”
We can’t help but wonder why the outcomes of the study were this way; here are some ideas:
• Society has natural ways of developing interpersonal skills, emotional regulation, and the ability to appreciate the present. Interpersonal skills are consistently fostered and tested in schools. Navigating high school parties, the process of organizing them, and getting invited to them requires significant social dexterity. Rejection from romantic interest, alienation from peers, rewards for accomplishment, and acceptance by other peers are some of the daily emotional obstacles that teenagers face. Being constantly taught by older individuals and scolded by parents is its own course in mindfulness. Those are few of the many natural processes of interpersonal growth that formalized therapy may impede.
• The universal discussion of psychological terms and psychiatric symptoms may not only destigmatize mental illness, but also normalize and possibly even promote it. While punishing or stigmatizing a child for having mental illness is obviously unacceptable and cruel, we do wonder if the compulsory psychotherapy may provide negative effects. Psychotherapies, especially manualized ones, were developed to alleviate mental suffering. It seems possible that this format normalizes pathology.
In 1961, Erving Goffman described the concept of sane people appearing insane in an asylum as “mortification.” In 2023, we have much improved, but have we done something to internalize patterns of suffering and alienation rather than dispel them? They are given forms that explain what the feeling of depression is when they may have never considered it. They are given tools to handle distress, when distress may not be present.
• Many human beings live on a fairly tight rope of suppression and the less adaptive repression. Suppression is the defense mechanism by which individuals make an effort to put distressing thoughts out of conscious awareness. After a difficult breakup a teenager may ask some friends to go out and watch a movie, making efforts to put negative feelings out of conscious awareness until there is an opportunity to cope adaptively with those stressors.
Repression is the defense mechanism by which individuals make an effort to prevent distressing thoughts from entering conscious awareness in the first place. After a difficult breakup a teenager acts like nothing happened. While not particularly adaptive, many people live with significant repression and without particular anguish. It is possible that uncovering all of those repressed and suppressed feelings through the exploratory work of therapy may destabilize individuals from their tight rope.
• A less problematic explanation could also be what was previously referred to as therapeutic regression. In psychoanalytic theory, patients are generally thought to have a compromise formation, a psychological strategy used to reconcile conflicting drives. The compromise formation is the way a patient balances their desires against moral expectations and the realities of the external world. In therapy, that compromise formation can be challenged, leading to therapeutic regression.
By uncovering and confronting deeply rooted feelings, a patient may find that their symptoms temporarily intensify. This may not be a problem, but a necessary step to growth in some patients. It is possible that a program longer than 8 weeks would have overcome a temporary worsening in outcome measures.
While it’s easy to highlight the darker moments in psychiatric history, psychiatry has grown into a field which offers well-accepted and uncontroversially promoted forms of treatment. This is evolution, exemplified by the mere consideration of the universal use of psychotherapy for teenagers. But this raises important questions about the potential unintended consequences of normalizing and formalizing therapy. It prompted us to reflect on whether psychiatric treatment is always the best solution and if it might, at times, impede natural processes of growth and coping.
In this context, the study on universal DBT-based group skills training for teenagers challenged our assumptions. The unexpected outcomes suggest that societal and educational systems may naturally foster many of the skills that formalized therapy seeks to provide, and may do so with greater efficacy than that which prescriptive psychiatric treatments have to offer. Moreover, the universal discussion of psychiatric symptoms may not only destigmatize mental illness but also normalize it, potentially leading to unnecessary pathology.
Finally, the study prompted us to consider the fine balance that people find themselves in, questioning whether we should be so certain that our interventions can always provide a better outcome than an individual’s current coping mechanisms. These findings serve as a valuable reminder that our enthusiasm for widespread psychiatric interventions should be tempered by rigorous research and a nuanced understanding of human psychology and development.
This study could be an example of the grandiose stance psychiatry has at times taken of late, suggesting the field has an intervention for all that ails you and can serve as a corrective to society’s maladaptive deviations. Rising rates of mental illness in the community are not interpreted as a failing of the field of psychiatry, but as evidence that we need more psychiatrists. Acts of gun violence, ever increasing rates suicides, and even political disagreements are met with the idea that if only we had more mental health capacity, this could be avoided.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. He has no conflicts of interest. Dr. ZoBell is a fourth-year senior resident at UCSD Psychiatry Residency Program. She is currently serving as the program’s Chief Resident at the VA San Diego on the inpatient psychiatric unit. Dr. ZoBell is interested in outpatient and emergency psychiatry as well as psychotherapy. Dr. Lehman is a professor of psychiatry at the University of California, San Diego. He is codirector of all acute and intensive psychiatric treatment at the Veterans Affairs Medical Center in San Diego, where he practices clinical psychiatry. He has no conflicts of interest.
Reference
1. Harvey, LJ, et al. Investigating the efficacy of a Dialectical behaviour therapy-based universal intervention on adolescent social and emotional well-being outcomes. Behav Res Ther. 2023 Oct. doi: 10.1016/j.brat.2023.104408.
We love psychiatry. We love the idea that someone can come to receive care from a physician to alleviate psychological suffering.
Some people experience such severe anguish that they are unable to relate to others. Some are so despondent that they are unable to make decisions. Some are so distressed that their thoughts become inconsistent with reality. We want all those people, and many more, to have access to effective psychiatric care. However, there are reasonable expectations that one should be able to have that a treatment will help, and that appropriate informed consent is given.
One recent article reminded us of this in a particularly poignant way.
The study in question is a recent publication looking at the universal use of psychotherapy for teenagers.1 At face value, we would have certainly considered this to be a benevolent and well-meaning intervention. Anyone who has been a teenager or has talked to one, is aware of the emotional instability punctuated by episodes of intense anxiety or irritability. It is age appropriate for a teenager to question and explore their identity. Teenagers are notoriously impulsive with a deep desire for validating interpersonal relationships. One could continue to list the symptoms of borderline personality disorder (BPD) and find a lot of similarity with the condition of transitioning from a child to an adult.
It is thus common sense to consider applying the most established therapy for BPD, dialectical behavioral therapy (DBT), to teenagers. The basics of DBT would seem to be helpful to anyone but appear particularly appropriate to this population. Mindfulness, the practice of paying attention to your present experience, allows one to realize that they are trapped in past or hypothetical future moments. Emotional regulation provides the tools that offer a frame for our feelings and involves recognizing feelings and understanding what they mean. Interpersonal work allows one to recognize and adapt to the feelings of others, while learning how to have a healthy voice with others. Distress tolerance is the exercise of learning to experience and contain our feelings.
The study looked at about 1,000 young adolescents, around 13 years old across high schools in Sydney, Australia: 598 adolescents were allocated to the intervention, and 566 to the control. The intervention consisted of eight weekly sessions of DBT lasting about 50 minutes. The results were “contrary to predictions.” Participants who received DBT “reported significantly increased total difficulties,” and “significant increases in depression and anxiety.” The effects were worse in males yet significant in both genders. The study concludes with “a reminder that present enthusiasm for universal dissemination of short-term DBT-based group skills training within schools, specifically in early adolescence, is ahead of the research evidence.”
We can’t help but wonder why the outcomes of the study were this way; here are some ideas:
• Society has natural ways of developing interpersonal skills, emotional regulation, and the ability to appreciate the present. Interpersonal skills are consistently fostered and tested in schools. Navigating high school parties, the process of organizing them, and getting invited to them requires significant social dexterity. Rejection from romantic interest, alienation from peers, rewards for accomplishment, and acceptance by other peers are some of the daily emotional obstacles that teenagers face. Being constantly taught by older individuals and scolded by parents is its own course in mindfulness. Those are few of the many natural processes of interpersonal growth that formalized therapy may impede.
• The universal discussion of psychological terms and psychiatric symptoms may not only destigmatize mental illness, but also normalize and possibly even promote it. While punishing or stigmatizing a child for having mental illness is obviously unacceptable and cruel, we do wonder if the compulsory psychotherapy may provide negative effects. Psychotherapies, especially manualized ones, were developed to alleviate mental suffering. It seems possible that this format normalizes pathology.
In 1961, Erving Goffman described the concept of sane people appearing insane in an asylum as “mortification.” In 2023, we have much improved, but have we done something to internalize patterns of suffering and alienation rather than dispel them? They are given forms that explain what the feeling of depression is when they may have never considered it. They are given tools to handle distress, when distress may not be present.
• Many human beings live on a fairly tight rope of suppression and the less adaptive repression. Suppression is the defense mechanism by which individuals make an effort to put distressing thoughts out of conscious awareness. After a difficult breakup a teenager may ask some friends to go out and watch a movie, making efforts to put negative feelings out of conscious awareness until there is an opportunity to cope adaptively with those stressors.
Repression is the defense mechanism by which individuals make an effort to prevent distressing thoughts from entering conscious awareness in the first place. After a difficult breakup a teenager acts like nothing happened. While not particularly adaptive, many people live with significant repression and without particular anguish. It is possible that uncovering all of those repressed and suppressed feelings through the exploratory work of therapy may destabilize individuals from their tight rope.
• A less problematic explanation could also be what was previously referred to as therapeutic regression. In psychoanalytic theory, patients are generally thought to have a compromise formation, a psychological strategy used to reconcile conflicting drives. The compromise formation is the way a patient balances their desires against moral expectations and the realities of the external world. In therapy, that compromise formation can be challenged, leading to therapeutic regression.
By uncovering and confronting deeply rooted feelings, a patient may find that their symptoms temporarily intensify. This may not be a problem, but a necessary step to growth in some patients. It is possible that a program longer than 8 weeks would have overcome a temporary worsening in outcome measures.
While it’s easy to highlight the darker moments in psychiatric history, psychiatry has grown into a field which offers well-accepted and uncontroversially promoted forms of treatment. This is evolution, exemplified by the mere consideration of the universal use of psychotherapy for teenagers. But this raises important questions about the potential unintended consequences of normalizing and formalizing therapy. It prompted us to reflect on whether psychiatric treatment is always the best solution and if it might, at times, impede natural processes of growth and coping.
In this context, the study on universal DBT-based group skills training for teenagers challenged our assumptions. The unexpected outcomes suggest that societal and educational systems may naturally foster many of the skills that formalized therapy seeks to provide, and may do so with greater efficacy than that which prescriptive psychiatric treatments have to offer. Moreover, the universal discussion of psychiatric symptoms may not only destigmatize mental illness but also normalize it, potentially leading to unnecessary pathology.
Finally, the study prompted us to consider the fine balance that people find themselves in, questioning whether we should be so certain that our interventions can always provide a better outcome than an individual’s current coping mechanisms. These findings serve as a valuable reminder that our enthusiasm for widespread psychiatric interventions should be tempered by rigorous research and a nuanced understanding of human psychology and development.
This study could be an example of the grandiose stance psychiatry has at times taken of late, suggesting the field has an intervention for all that ails you and can serve as a corrective to society’s maladaptive deviations. Rising rates of mental illness in the community are not interpreted as a failing of the field of psychiatry, but as evidence that we need more psychiatrists. Acts of gun violence, ever increasing rates suicides, and even political disagreements are met with the idea that if only we had more mental health capacity, this could be avoided.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. He has no conflicts of interest. Dr. ZoBell is a fourth-year senior resident at UCSD Psychiatry Residency Program. She is currently serving as the program’s Chief Resident at the VA San Diego on the inpatient psychiatric unit. Dr. ZoBell is interested in outpatient and emergency psychiatry as well as psychotherapy. Dr. Lehman is a professor of psychiatry at the University of California, San Diego. He is codirector of all acute and intensive psychiatric treatment at the Veterans Affairs Medical Center in San Diego, where he practices clinical psychiatry. He has no conflicts of interest.
Reference
1. Harvey, LJ, et al. Investigating the efficacy of a Dialectical behaviour therapy-based universal intervention on adolescent social and emotional well-being outcomes. Behav Res Ther. 2023 Oct. doi: 10.1016/j.brat.2023.104408.
We love psychiatry. We love the idea that someone can come to receive care from a physician to alleviate psychological suffering.
Some people experience such severe anguish that they are unable to relate to others. Some are so despondent that they are unable to make decisions. Some are so distressed that their thoughts become inconsistent with reality. We want all those people, and many more, to have access to effective psychiatric care. However, there are reasonable expectations that one should be able to have that a treatment will help, and that appropriate informed consent is given.
One recent article reminded us of this in a particularly poignant way.
The study in question is a recent publication looking at the universal use of psychotherapy for teenagers.1 At face value, we would have certainly considered this to be a benevolent and well-meaning intervention. Anyone who has been a teenager or has talked to one, is aware of the emotional instability punctuated by episodes of intense anxiety or irritability. It is age appropriate for a teenager to question and explore their identity. Teenagers are notoriously impulsive with a deep desire for validating interpersonal relationships. One could continue to list the symptoms of borderline personality disorder (BPD) and find a lot of similarity with the condition of transitioning from a child to an adult.
It is thus common sense to consider applying the most established therapy for BPD, dialectical behavioral therapy (DBT), to teenagers. The basics of DBT would seem to be helpful to anyone but appear particularly appropriate to this population. Mindfulness, the practice of paying attention to your present experience, allows one to realize that they are trapped in past or hypothetical future moments. Emotional regulation provides the tools that offer a frame for our feelings and involves recognizing feelings and understanding what they mean. Interpersonal work allows one to recognize and adapt to the feelings of others, while learning how to have a healthy voice with others. Distress tolerance is the exercise of learning to experience and contain our feelings.
The study looked at about 1,000 young adolescents, around 13 years old across high schools in Sydney, Australia: 598 adolescents were allocated to the intervention, and 566 to the control. The intervention consisted of eight weekly sessions of DBT lasting about 50 minutes. The results were “contrary to predictions.” Participants who received DBT “reported significantly increased total difficulties,” and “significant increases in depression and anxiety.” The effects were worse in males yet significant in both genders. The study concludes with “a reminder that present enthusiasm for universal dissemination of short-term DBT-based group skills training within schools, specifically in early adolescence, is ahead of the research evidence.”
We can’t help but wonder why the outcomes of the study were this way; here are some ideas:
• Society has natural ways of developing interpersonal skills, emotional regulation, and the ability to appreciate the present. Interpersonal skills are consistently fostered and tested in schools. Navigating high school parties, the process of organizing them, and getting invited to them requires significant social dexterity. Rejection from romantic interest, alienation from peers, rewards for accomplishment, and acceptance by other peers are some of the daily emotional obstacles that teenagers face. Being constantly taught by older individuals and scolded by parents is its own course in mindfulness. Those are few of the many natural processes of interpersonal growth that formalized therapy may impede.
• The universal discussion of psychological terms and psychiatric symptoms may not only destigmatize mental illness, but also normalize and possibly even promote it. While punishing or stigmatizing a child for having mental illness is obviously unacceptable and cruel, we do wonder if the compulsory psychotherapy may provide negative effects. Psychotherapies, especially manualized ones, were developed to alleviate mental suffering. It seems possible that this format normalizes pathology.
In 1961, Erving Goffman described the concept of sane people appearing insane in an asylum as “mortification.” In 2023, we have much improved, but have we done something to internalize patterns of suffering and alienation rather than dispel them? They are given forms that explain what the feeling of depression is when they may have never considered it. They are given tools to handle distress, when distress may not be present.
• Many human beings live on a fairly tight rope of suppression and the less adaptive repression. Suppression is the defense mechanism by which individuals make an effort to put distressing thoughts out of conscious awareness. After a difficult breakup a teenager may ask some friends to go out and watch a movie, making efforts to put negative feelings out of conscious awareness until there is an opportunity to cope adaptively with those stressors.
Repression is the defense mechanism by which individuals make an effort to prevent distressing thoughts from entering conscious awareness in the first place. After a difficult breakup a teenager acts like nothing happened. While not particularly adaptive, many people live with significant repression and without particular anguish. It is possible that uncovering all of those repressed and suppressed feelings through the exploratory work of therapy may destabilize individuals from their tight rope.
• A less problematic explanation could also be what was previously referred to as therapeutic regression. In psychoanalytic theory, patients are generally thought to have a compromise formation, a psychological strategy used to reconcile conflicting drives. The compromise formation is the way a patient balances their desires against moral expectations and the realities of the external world. In therapy, that compromise formation can be challenged, leading to therapeutic regression.
By uncovering and confronting deeply rooted feelings, a patient may find that their symptoms temporarily intensify. This may not be a problem, but a necessary step to growth in some patients. It is possible that a program longer than 8 weeks would have overcome a temporary worsening in outcome measures.
While it’s easy to highlight the darker moments in psychiatric history, psychiatry has grown into a field which offers well-accepted and uncontroversially promoted forms of treatment. This is evolution, exemplified by the mere consideration of the universal use of psychotherapy for teenagers. But this raises important questions about the potential unintended consequences of normalizing and formalizing therapy. It prompted us to reflect on whether psychiatric treatment is always the best solution and if it might, at times, impede natural processes of growth and coping.
In this context, the study on universal DBT-based group skills training for teenagers challenged our assumptions. The unexpected outcomes suggest that societal and educational systems may naturally foster many of the skills that formalized therapy seeks to provide, and may do so with greater efficacy than that which prescriptive psychiatric treatments have to offer. Moreover, the universal discussion of psychiatric symptoms may not only destigmatize mental illness but also normalize it, potentially leading to unnecessary pathology.
Finally, the study prompted us to consider the fine balance that people find themselves in, questioning whether we should be so certain that our interventions can always provide a better outcome than an individual’s current coping mechanisms. These findings serve as a valuable reminder that our enthusiasm for widespread psychiatric interventions should be tempered by rigorous research and a nuanced understanding of human psychology and development.
This study could be an example of the grandiose stance psychiatry has at times taken of late, suggesting the field has an intervention for all that ails you and can serve as a corrective to society’s maladaptive deviations. Rising rates of mental illness in the community are not interpreted as a failing of the field of psychiatry, but as evidence that we need more psychiatrists. Acts of gun violence, ever increasing rates suicides, and even political disagreements are met with the idea that if only we had more mental health capacity, this could be avoided.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. He has no conflicts of interest. Dr. ZoBell is a fourth-year senior resident at UCSD Psychiatry Residency Program. She is currently serving as the program’s Chief Resident at the VA San Diego on the inpatient psychiatric unit. Dr. ZoBell is interested in outpatient and emergency psychiatry as well as psychotherapy. Dr. Lehman is a professor of psychiatry at the University of California, San Diego. He is codirector of all acute and intensive psychiatric treatment at the Veterans Affairs Medical Center in San Diego, where he practices clinical psychiatry. He has no conflicts of interest.
Reference
1. Harvey, LJ, et al. Investigating the efficacy of a Dialectical behaviour therapy-based universal intervention on adolescent social and emotional well-being outcomes. Behav Res Ther. 2023 Oct. doi: 10.1016/j.brat.2023.104408.
Light therapy a beacon of hope for Alzheimer’s?
TOPLINE:
Light therapy leads to significant improvement in several sleep measures and helps alleviate depression and agitation in patients with Alzheimer’s disease (AD), a meta-analysis of 15 high-quality trials shows.
METHODOLOGY:
- This meta-analysis included 15 randomized controlled trials involving 598 patients with mild to moderate AD.
- The included trials were written in English, published between 2005 and 2022, and performed in seven countries. A fixed-effects model was used for data analysis.
TAKEAWAY:
- Light therapy significantly improved sleep efficiency (mean difference [MD], −2.42; P < .00001), increased interdaily stability (MD, −0.04; P < .00001), and reduced intradaily variability (MD, −0.04; P < .00001), indicating better sleep quality.
- Light therapy reduced agitation (MD, −3.97; P < .00001), depression (MD, −2.55; P < .00001), and caregiver burden (MD, −3.57; P < .00001).
- Light therapy also had a significant advantage over usual care in reducing the severity of psychobehavioral symptoms as assessed by the Neuropsychiatric Inventory (MD, −3.07; P < .00001).
- Light therapy had no statistically significant effect on improving cognitive function as measured by the Mini-Mental State Examination.
IN PRACTICE:
“These findings, combined with its low side-effects, suggest the role of light therapy as a promising treatment for AD. Although light therapy has fewer side effects than pharmacological treatment, adverse behavioral outcomes in patients due to bright light exposure should be considered,” the authors wrote.
SOURCE:
The study by Lili Zang and colleagues from Weifang Medical University School of Nursing, Shandong Province, China, was published online on December 6, 2023, in PLOS One.
LIMITATIONS:
The types and degrees of dementia in the included studies were inconsistent, potentially affecting the outcome indicators. Some articles did not clearly describe their randomization and allocation concealment methods, indicating possible bias in these studies.
DISCLOSURES:
The study was supported by the Natural Science Foundation of Shandong Province, China. The authors declared no competing interests.
Megan Brooks has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
Light therapy leads to significant improvement in several sleep measures and helps alleviate depression and agitation in patients with Alzheimer’s disease (AD), a meta-analysis of 15 high-quality trials shows.
METHODOLOGY:
- This meta-analysis included 15 randomized controlled trials involving 598 patients with mild to moderate AD.
- The included trials were written in English, published between 2005 and 2022, and performed in seven countries. A fixed-effects model was used for data analysis.
TAKEAWAY:
- Light therapy significantly improved sleep efficiency (mean difference [MD], −2.42; P < .00001), increased interdaily stability (MD, −0.04; P < .00001), and reduced intradaily variability (MD, −0.04; P < .00001), indicating better sleep quality.
- Light therapy reduced agitation (MD, −3.97; P < .00001), depression (MD, −2.55; P < .00001), and caregiver burden (MD, −3.57; P < .00001).
- Light therapy also had a significant advantage over usual care in reducing the severity of psychobehavioral symptoms as assessed by the Neuropsychiatric Inventory (MD, −3.07; P < .00001).
- Light therapy had no statistically significant effect on improving cognitive function as measured by the Mini-Mental State Examination.
IN PRACTICE:
“These findings, combined with its low side-effects, suggest the role of light therapy as a promising treatment for AD. Although light therapy has fewer side effects than pharmacological treatment, adverse behavioral outcomes in patients due to bright light exposure should be considered,” the authors wrote.
SOURCE:
The study by Lili Zang and colleagues from Weifang Medical University School of Nursing, Shandong Province, China, was published online on December 6, 2023, in PLOS One.
LIMITATIONS:
The types and degrees of dementia in the included studies were inconsistent, potentially affecting the outcome indicators. Some articles did not clearly describe their randomization and allocation concealment methods, indicating possible bias in these studies.
DISCLOSURES:
The study was supported by the Natural Science Foundation of Shandong Province, China. The authors declared no competing interests.
Megan Brooks has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
Light therapy leads to significant improvement in several sleep measures and helps alleviate depression and agitation in patients with Alzheimer’s disease (AD), a meta-analysis of 15 high-quality trials shows.
METHODOLOGY:
- This meta-analysis included 15 randomized controlled trials involving 598 patients with mild to moderate AD.
- The included trials were written in English, published between 2005 and 2022, and performed in seven countries. A fixed-effects model was used for data analysis.
TAKEAWAY:
- Light therapy significantly improved sleep efficiency (mean difference [MD], −2.42; P < .00001), increased interdaily stability (MD, −0.04; P < .00001), and reduced intradaily variability (MD, −0.04; P < .00001), indicating better sleep quality.
- Light therapy reduced agitation (MD, −3.97; P < .00001), depression (MD, −2.55; P < .00001), and caregiver burden (MD, −3.57; P < .00001).
- Light therapy also had a significant advantage over usual care in reducing the severity of psychobehavioral symptoms as assessed by the Neuropsychiatric Inventory (MD, −3.07; P < .00001).
- Light therapy had no statistically significant effect on improving cognitive function as measured by the Mini-Mental State Examination.
IN PRACTICE:
“These findings, combined with its low side-effects, suggest the role of light therapy as a promising treatment for AD. Although light therapy has fewer side effects than pharmacological treatment, adverse behavioral outcomes in patients due to bright light exposure should be considered,” the authors wrote.
SOURCE:
The study by Lili Zang and colleagues from Weifang Medical University School of Nursing, Shandong Province, China, was published online on December 6, 2023, in PLOS One.
LIMITATIONS:
The types and degrees of dementia in the included studies were inconsistent, potentially affecting the outcome indicators. Some articles did not clearly describe their randomization and allocation concealment methods, indicating possible bias in these studies.
DISCLOSURES:
The study was supported by the Natural Science Foundation of Shandong Province, China. The authors declared no competing interests.
Megan Brooks has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Depression, constipation, UTIs early signs of MS?
However, these prodromal symptoms are also more likely to occur in people with two other autoimmune diseases — lupus and Crohn’s disease — and therefore, will not help earlier diagnosis, study investigator, Céline Louapre, professor of neurology, Sorbonne University and Paris Brain Institute, Paris, France, said in an interview.
“On the other hand, in certain patients who may be at particular risk of developing MS, such as in certain familial forms or in patients with incidental inflammatory lesions discovered on MRI, the presence of these symptoms could suggest an already active process, prior to the first typical symptoms of the disease,” she noted.
Retracing MS Origins
The case-control study, published online in Neurology, included 20,174 people with newly diagnosed MS who were matched to 54,790 without MS, as well as 30,477 with Crohn’s disease and 7337 with lupus.
Using International Classification of Diseases, 10th revision (ICD-10) codes in electronic health records, the researchers assessed the associations between 113 diseases and symptoms in the 5 years before and after an MS diagnosis.
Twelve ICD-10 codes were significantly positively associated with the risk for MS compared with controls without MS.
After considering ICD-10 codes suggestive of neurologic symptoms as the first diagnosis of MS, the following five ICD-10 codes remained significantly associated with MS:
- Depression (odds ratio [OR], 1.22; 95% CI, 1.11-1.34)
- Sexual dysfunction (OR, 1.47; 95% CI, 1.11-1.95)
- Constipation (OR, 1.5; 95% CI, 1.27-1.78)
- Cystitis (OR, 1.21; 95% CI, 1.05-1.39)
- UTIs of unspecified site (OR, 1.38; 95% CI, 1.18-1.61)
However, none of these conditions was selectively associated with MS in comparison with both lupus and Crohn’s disease. All five ICD-10 codes identified were still associated with MS during the 5 years after diagnosis.
“The importance of investigating prodromal signs in MS is that it allows us to retrace the origins of the disease,” said Dr. Louapre.
“The main contribution of the data on prodromes in MS is to clarify that the disease and its mechanisms are frequently underway well before the first typical neurological symptoms, and that the causes of MS are probably present many years before diagnosis,” she added.
A limitation of the study was that data were not available for other factors that could influence people’s risk of developing MS, such as education level, ethnicity, body mass index, socioeconomic status, or genetic information.
It also remains unclear whether the conditions linked to MS are risk factors for the disease or nonspecific early MS symptoms.
Preventing Disease Evolution
In a linked editorial, Ruth Ann Marrie, MD, PhD, with the University of Manitoba, Manitoba, Canada, and Raffaele Palladino, MD, PhD, with the University of Naples Federico II, Naples, Italy, note these findings highlight the challenges of accurately identifying the prodromal stage of a specific disease.
“Commonalities of prodromal features are recognized across neurodegenerative diseases; this is also true for immune-mediated diseases, and it is not surprising, given shared etiologic factors and pathobiological mechanisms,” they point out.
“This suggests that we should be trying to link prodromal features to specific underlying pathobiological changes rather than specific diseases. This approach would require use of different study designs, including broad, deeply phenotyped cohorts, but would allow us to develop and test interventions targeted at those mechanisms, and could ultimately achieve the goal of preventing disease evolution,” they add.
The study was supported by the French National Research Agency. Dr. Louapre has received consulting or travel fees from Biogen, Novartis, Roche, Sanofi, Teva, and Merck Serono, unrelated to this study. Dr. Marrie is a coinvestigator on studies receiving funding from Biogen Idec and Roche Canada; receives research funding from CIHR, Research Manitoba, Multiple Sclerosis Society of Canada, Multiple Sclerosis Scientific Foundation, Crohn’s and Colitis Canada, National Multiple Sclerosis Society, CMSC, the Arthritis Society and the US Department of Defense; and serves on the editorial board of Neurology. Dr. Palladino has taken part in advisory boards/consultancy for MSD and Sanofi and has received support from the UK MS Society.
A version of this article appeared on Medscape.com.
However, these prodromal symptoms are also more likely to occur in people with two other autoimmune diseases — lupus and Crohn’s disease — and therefore, will not help earlier diagnosis, study investigator, Céline Louapre, professor of neurology, Sorbonne University and Paris Brain Institute, Paris, France, said in an interview.
“On the other hand, in certain patients who may be at particular risk of developing MS, such as in certain familial forms or in patients with incidental inflammatory lesions discovered on MRI, the presence of these symptoms could suggest an already active process, prior to the first typical symptoms of the disease,” she noted.
Retracing MS Origins
The case-control study, published online in Neurology, included 20,174 people with newly diagnosed MS who were matched to 54,790 without MS, as well as 30,477 with Crohn’s disease and 7337 with lupus.
Using International Classification of Diseases, 10th revision (ICD-10) codes in electronic health records, the researchers assessed the associations between 113 diseases and symptoms in the 5 years before and after an MS diagnosis.
Twelve ICD-10 codes were significantly positively associated with the risk for MS compared with controls without MS.
After considering ICD-10 codes suggestive of neurologic symptoms as the first diagnosis of MS, the following five ICD-10 codes remained significantly associated with MS:
- Depression (odds ratio [OR], 1.22; 95% CI, 1.11-1.34)
- Sexual dysfunction (OR, 1.47; 95% CI, 1.11-1.95)
- Constipation (OR, 1.5; 95% CI, 1.27-1.78)
- Cystitis (OR, 1.21; 95% CI, 1.05-1.39)
- UTIs of unspecified site (OR, 1.38; 95% CI, 1.18-1.61)
However, none of these conditions was selectively associated with MS in comparison with both lupus and Crohn’s disease. All five ICD-10 codes identified were still associated with MS during the 5 years after diagnosis.
“The importance of investigating prodromal signs in MS is that it allows us to retrace the origins of the disease,” said Dr. Louapre.
“The main contribution of the data on prodromes in MS is to clarify that the disease and its mechanisms are frequently underway well before the first typical neurological symptoms, and that the causes of MS are probably present many years before diagnosis,” she added.
A limitation of the study was that data were not available for other factors that could influence people’s risk of developing MS, such as education level, ethnicity, body mass index, socioeconomic status, or genetic information.
It also remains unclear whether the conditions linked to MS are risk factors for the disease or nonspecific early MS symptoms.
Preventing Disease Evolution
In a linked editorial, Ruth Ann Marrie, MD, PhD, with the University of Manitoba, Manitoba, Canada, and Raffaele Palladino, MD, PhD, with the University of Naples Federico II, Naples, Italy, note these findings highlight the challenges of accurately identifying the prodromal stage of a specific disease.
“Commonalities of prodromal features are recognized across neurodegenerative diseases; this is also true for immune-mediated diseases, and it is not surprising, given shared etiologic factors and pathobiological mechanisms,” they point out.
“This suggests that we should be trying to link prodromal features to specific underlying pathobiological changes rather than specific diseases. This approach would require use of different study designs, including broad, deeply phenotyped cohorts, but would allow us to develop and test interventions targeted at those mechanisms, and could ultimately achieve the goal of preventing disease evolution,” they add.
The study was supported by the French National Research Agency. Dr. Louapre has received consulting or travel fees from Biogen, Novartis, Roche, Sanofi, Teva, and Merck Serono, unrelated to this study. Dr. Marrie is a coinvestigator on studies receiving funding from Biogen Idec and Roche Canada; receives research funding from CIHR, Research Manitoba, Multiple Sclerosis Society of Canada, Multiple Sclerosis Scientific Foundation, Crohn’s and Colitis Canada, National Multiple Sclerosis Society, CMSC, the Arthritis Society and the US Department of Defense; and serves on the editorial board of Neurology. Dr. Palladino has taken part in advisory boards/consultancy for MSD and Sanofi and has received support from the UK MS Society.
A version of this article appeared on Medscape.com.
However, these prodromal symptoms are also more likely to occur in people with two other autoimmune diseases — lupus and Crohn’s disease — and therefore, will not help earlier diagnosis, study investigator, Céline Louapre, professor of neurology, Sorbonne University and Paris Brain Institute, Paris, France, said in an interview.
“On the other hand, in certain patients who may be at particular risk of developing MS, such as in certain familial forms or in patients with incidental inflammatory lesions discovered on MRI, the presence of these symptoms could suggest an already active process, prior to the first typical symptoms of the disease,” she noted.
Retracing MS Origins
The case-control study, published online in Neurology, included 20,174 people with newly diagnosed MS who were matched to 54,790 without MS, as well as 30,477 with Crohn’s disease and 7337 with lupus.
Using International Classification of Diseases, 10th revision (ICD-10) codes in electronic health records, the researchers assessed the associations between 113 diseases and symptoms in the 5 years before and after an MS diagnosis.
Twelve ICD-10 codes were significantly positively associated with the risk for MS compared with controls without MS.
After considering ICD-10 codes suggestive of neurologic symptoms as the first diagnosis of MS, the following five ICD-10 codes remained significantly associated with MS:
- Depression (odds ratio [OR], 1.22; 95% CI, 1.11-1.34)
- Sexual dysfunction (OR, 1.47; 95% CI, 1.11-1.95)
- Constipation (OR, 1.5; 95% CI, 1.27-1.78)
- Cystitis (OR, 1.21; 95% CI, 1.05-1.39)
- UTIs of unspecified site (OR, 1.38; 95% CI, 1.18-1.61)
However, none of these conditions was selectively associated with MS in comparison with both lupus and Crohn’s disease. All five ICD-10 codes identified were still associated with MS during the 5 years after diagnosis.
“The importance of investigating prodromal signs in MS is that it allows us to retrace the origins of the disease,” said Dr. Louapre.
“The main contribution of the data on prodromes in MS is to clarify that the disease and its mechanisms are frequently underway well before the first typical neurological symptoms, and that the causes of MS are probably present many years before diagnosis,” she added.
A limitation of the study was that data were not available for other factors that could influence people’s risk of developing MS, such as education level, ethnicity, body mass index, socioeconomic status, or genetic information.
It also remains unclear whether the conditions linked to MS are risk factors for the disease or nonspecific early MS symptoms.
Preventing Disease Evolution
In a linked editorial, Ruth Ann Marrie, MD, PhD, with the University of Manitoba, Manitoba, Canada, and Raffaele Palladino, MD, PhD, with the University of Naples Federico II, Naples, Italy, note these findings highlight the challenges of accurately identifying the prodromal stage of a specific disease.
“Commonalities of prodromal features are recognized across neurodegenerative diseases; this is also true for immune-mediated diseases, and it is not surprising, given shared etiologic factors and pathobiological mechanisms,” they point out.
“This suggests that we should be trying to link prodromal features to specific underlying pathobiological changes rather than specific diseases. This approach would require use of different study designs, including broad, deeply phenotyped cohorts, but would allow us to develop and test interventions targeted at those mechanisms, and could ultimately achieve the goal of preventing disease evolution,” they add.
The study was supported by the French National Research Agency. Dr. Louapre has received consulting or travel fees from Biogen, Novartis, Roche, Sanofi, Teva, and Merck Serono, unrelated to this study. Dr. Marrie is a coinvestigator on studies receiving funding from Biogen Idec and Roche Canada; receives research funding from CIHR, Research Manitoba, Multiple Sclerosis Society of Canada, Multiple Sclerosis Scientific Foundation, Crohn’s and Colitis Canada, National Multiple Sclerosis Society, CMSC, the Arthritis Society and the US Department of Defense; and serves on the editorial board of Neurology. Dr. Palladino has taken part in advisory boards/consultancy for MSD and Sanofi and has received support from the UK MS Society.
A version of this article appeared on Medscape.com.
FROM NEUROLOGY
Toward a better framework for postmarketing reproductive safety surveillance of medications
For the last 30 years, the Center for Women’s Mental Health at Massachusetts General Hospital (MGH) has had as part of its mission, the conveying of accurate information about the reproductive safety of psychiatric medications. There has been a spectrum of medicines developed across psychiatric indications over the last several decades, and many studies over those decades have attempted to delineate the reproductive safety of these agents.
With the development of new antidepressants and second-generation antipsychotics has come an appreciation of the utility of these agents across a wide range of psychiatric disease states and psychiatric symptoms. More and more data demonstrate the efficacy of these medicines for mood and anxiety disorders; these agents are also used for a broad array of symptoms from insomnia, irritability, and symptoms of posttraumatic stress disorder (PTSD) just as examples — even absent formal approval by the US Food and Drug Administration (FDA) for these specific indications. With the growing use of medicines, including new antidepressants like selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors, and second-generation atypical antipsychotics, there has been a greater interest and appreciation of the need to provide women with the best information about reproductive safety of these medicines as well.
When I began working in reproductive psychiatry, the FDA was using the pregnancy labeling categories introduced in 1979. The categories were simple, but also oversimplified in terms of incompletely conveying information about reproductive safety. For instance, category labels of B and C under the old labeling system could be nebulous, containing sparse information (in the case of category B) or animal data and some conflicting human data (in the case of category C) that may not have translated into relevant or easily interpretable safety information for patients and clinicians.
It was on that basis the current Pregnancy and Lactation Labeling (PLLR) Final Rule was published in 2014, which was a shift from categorical labeling to more descriptive labeling, including updated actual information on the package insert about available reproductive safety data, animal data, and data on lactation.
Even following the publication of the PLLR, there has still been an acknowledgment in the field that our assessment tools for postmarketing reproductive safety surveillance are incomplete. A recent 2-day FDA workshop hosted by the Duke-Margolis Center for Health Policy on optimizing the use of postapproval pregnancy safety studies sought to discuss the many questions that still surround this issue. Based on presentations at this workshop, a framework emerged for the future of assessing the reproductive safety of medications, which included an effort to develop the most effective model using tools such as pregnancy registries and harnessing “big data,” whether through electronic health records or large administrative databases from public and private insurers. Together, these various sources of information can provide signals of potential concern, prompting the need for a more rigorous look at the reproductive safety of a medication, or provide reassurance if data fail to indicate the absence of a signal of risk.
FDA’s new commitments under the latest reauthorization of the Prescription Drug User Fee Act (PDUFA VII) include pregnancy-specific postmarketing safety requirements as well as the creation of a framework for how data from pregnancy-specific postmarketing studies can be used. The agency is also conducting demonstration projects, including one for assessing the performance of pregnancy registries for the potential to detect safety signals for medications early in pregnancy. FDA is expanding its Sentinel Initiative to help accomplish these aims, and is implementing an Active Risk Identification and Analysis (ARIA) system to conduct active safety surveillance of medications used during pregnancy.
Pregnancy registries have now been available for decades, and some have been more successful than others across different classes of medicines, with the most rigorous registries including prospective follow-up of women across pregnancies and careful documentation of malformations (at best with original source data and with a blinded dysmorphologist). Still, with all of its rigor, even the best-intentioned efforts with respect to pregnancy registries have limitations. As I mentioned in my testimony during the public comment portion of the workshop, the sheer volume of pregnancy data from administrative databases we now have access to is attractive, but the quality of these data needs to be good enough to ascertain a signal of risk if they are to be used as a basis for reproductive safety determination.
The flip side of using data from large administrative databases is using carefully collected data from pregnancy registries. With a pregnancy registry, accrual of a substantial number of participants can also take a considerable period of time, and initial risk estimates of outcomes can have typically large confidence intervals, which can make it difficult to discern whether a drug is safe for women of reproductive age.
Another key issue is a lack of participation from manufacturers with respect to commitment to collection of high-quality reproductive safety data. History has shown that many medication manufacturers, unless required to have a dedicated registry as part of a postmarketing requirement or commitment, will invest sparse resources to track data on safety of fetal drug exposure. Participation is typically voluntary and varies from company to company unless, as noted previously, there is a postmarketing requirement or commitment tied to the approval of a medication. Just as a recent concrete example, the manufacturer of a new medication recently approved by the FDA for the treatment of postpartum depression (which will include presumably sexually active women well into the first postpartum year) has no plan to support the collection of reproductive safety data on this new medication because it is not required to, based on current FDA guidelines and the absence of a postmarketing requirement to do so.
Looking ahead
While the PLLR was a huge step forward in the field from the old pregnancy category system that could misinform women contemplating pregnancy, it also sets the stage for the next iteration of a system that allows us to generate information more quickly about the reproductive safety of medications. In psychiatry, as many as 10% of women use SSRIs during pregnancy. With drugs like atypical antipsychotics being used across disease states — in schizophrenia, bipolar disorder, depression, anxiety, insomnia, and PTSD — and where new classes of medicine are becoming available, like with ketamine or steroids, we need to have a system by which we can more quickly ascertain reproductive safety information. This information informs treatment decisions during a critical life event of deciding to try to become pregnant or during an actual pregnancy.
In my mind, it is reassuring when a registry has even as few as 50-60 cases of fetal exposure without an increase in the risk for malformation, because it can mean we are not seeing a repeat of the past with medications like thalidomide and sodium valproate. However, patients and clinicians are starved for better data. Risk assessment is also different from clinician to clinician and patient to patient. We want to empower patients to make decisions that work for them based on more rapidly accumulating information and help inform their decisions.
To come out on the “other side” of the PLLR, , which can be confusing when study results frequently conflict. I believe we have an obligation today to do this better, because the areas of reproductive toxicology and pharmacovigilance are growing incredibly quickly, and clinicians and patients are seeing these volumes of data being published without the ability to integrate that information in a systematic way.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at [email protected].
For the last 30 years, the Center for Women’s Mental Health at Massachusetts General Hospital (MGH) has had as part of its mission, the conveying of accurate information about the reproductive safety of psychiatric medications. There has been a spectrum of medicines developed across psychiatric indications over the last several decades, and many studies over those decades have attempted to delineate the reproductive safety of these agents.
With the development of new antidepressants and second-generation antipsychotics has come an appreciation of the utility of these agents across a wide range of psychiatric disease states and psychiatric symptoms. More and more data demonstrate the efficacy of these medicines for mood and anxiety disorders; these agents are also used for a broad array of symptoms from insomnia, irritability, and symptoms of posttraumatic stress disorder (PTSD) just as examples — even absent formal approval by the US Food and Drug Administration (FDA) for these specific indications. With the growing use of medicines, including new antidepressants like selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors, and second-generation atypical antipsychotics, there has been a greater interest and appreciation of the need to provide women with the best information about reproductive safety of these medicines as well.
When I began working in reproductive psychiatry, the FDA was using the pregnancy labeling categories introduced in 1979. The categories were simple, but also oversimplified in terms of incompletely conveying information about reproductive safety. For instance, category labels of B and C under the old labeling system could be nebulous, containing sparse information (in the case of category B) or animal data and some conflicting human data (in the case of category C) that may not have translated into relevant or easily interpretable safety information for patients and clinicians.
It was on that basis the current Pregnancy and Lactation Labeling (PLLR) Final Rule was published in 2014, which was a shift from categorical labeling to more descriptive labeling, including updated actual information on the package insert about available reproductive safety data, animal data, and data on lactation.
Even following the publication of the PLLR, there has still been an acknowledgment in the field that our assessment tools for postmarketing reproductive safety surveillance are incomplete. A recent 2-day FDA workshop hosted by the Duke-Margolis Center for Health Policy on optimizing the use of postapproval pregnancy safety studies sought to discuss the many questions that still surround this issue. Based on presentations at this workshop, a framework emerged for the future of assessing the reproductive safety of medications, which included an effort to develop the most effective model using tools such as pregnancy registries and harnessing “big data,” whether through electronic health records or large administrative databases from public and private insurers. Together, these various sources of information can provide signals of potential concern, prompting the need for a more rigorous look at the reproductive safety of a medication, or provide reassurance if data fail to indicate the absence of a signal of risk.
FDA’s new commitments under the latest reauthorization of the Prescription Drug User Fee Act (PDUFA VII) include pregnancy-specific postmarketing safety requirements as well as the creation of a framework for how data from pregnancy-specific postmarketing studies can be used. The agency is also conducting demonstration projects, including one for assessing the performance of pregnancy registries for the potential to detect safety signals for medications early in pregnancy. FDA is expanding its Sentinel Initiative to help accomplish these aims, and is implementing an Active Risk Identification and Analysis (ARIA) system to conduct active safety surveillance of medications used during pregnancy.
Pregnancy registries have now been available for decades, and some have been more successful than others across different classes of medicines, with the most rigorous registries including prospective follow-up of women across pregnancies and careful documentation of malformations (at best with original source data and with a blinded dysmorphologist). Still, with all of its rigor, even the best-intentioned efforts with respect to pregnancy registries have limitations. As I mentioned in my testimony during the public comment portion of the workshop, the sheer volume of pregnancy data from administrative databases we now have access to is attractive, but the quality of these data needs to be good enough to ascertain a signal of risk if they are to be used as a basis for reproductive safety determination.
The flip side of using data from large administrative databases is using carefully collected data from pregnancy registries. With a pregnancy registry, accrual of a substantial number of participants can also take a considerable period of time, and initial risk estimates of outcomes can have typically large confidence intervals, which can make it difficult to discern whether a drug is safe for women of reproductive age.
Another key issue is a lack of participation from manufacturers with respect to commitment to collection of high-quality reproductive safety data. History has shown that many medication manufacturers, unless required to have a dedicated registry as part of a postmarketing requirement or commitment, will invest sparse resources to track data on safety of fetal drug exposure. Participation is typically voluntary and varies from company to company unless, as noted previously, there is a postmarketing requirement or commitment tied to the approval of a medication. Just as a recent concrete example, the manufacturer of a new medication recently approved by the FDA for the treatment of postpartum depression (which will include presumably sexually active women well into the first postpartum year) has no plan to support the collection of reproductive safety data on this new medication because it is not required to, based on current FDA guidelines and the absence of a postmarketing requirement to do so.
Looking ahead
While the PLLR was a huge step forward in the field from the old pregnancy category system that could misinform women contemplating pregnancy, it also sets the stage for the next iteration of a system that allows us to generate information more quickly about the reproductive safety of medications. In psychiatry, as many as 10% of women use SSRIs during pregnancy. With drugs like atypical antipsychotics being used across disease states — in schizophrenia, bipolar disorder, depression, anxiety, insomnia, and PTSD — and where new classes of medicine are becoming available, like with ketamine or steroids, we need to have a system by which we can more quickly ascertain reproductive safety information. This information informs treatment decisions during a critical life event of deciding to try to become pregnant or during an actual pregnancy.
In my mind, it is reassuring when a registry has even as few as 50-60 cases of fetal exposure without an increase in the risk for malformation, because it can mean we are not seeing a repeat of the past with medications like thalidomide and sodium valproate. However, patients and clinicians are starved for better data. Risk assessment is also different from clinician to clinician and patient to patient. We want to empower patients to make decisions that work for them based on more rapidly accumulating information and help inform their decisions.
To come out on the “other side” of the PLLR, , which can be confusing when study results frequently conflict. I believe we have an obligation today to do this better, because the areas of reproductive toxicology and pharmacovigilance are growing incredibly quickly, and clinicians and patients are seeing these volumes of data being published without the ability to integrate that information in a systematic way.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at [email protected].
For the last 30 years, the Center for Women’s Mental Health at Massachusetts General Hospital (MGH) has had as part of its mission, the conveying of accurate information about the reproductive safety of psychiatric medications. There has been a spectrum of medicines developed across psychiatric indications over the last several decades, and many studies over those decades have attempted to delineate the reproductive safety of these agents.
With the development of new antidepressants and second-generation antipsychotics has come an appreciation of the utility of these agents across a wide range of psychiatric disease states and psychiatric symptoms. More and more data demonstrate the efficacy of these medicines for mood and anxiety disorders; these agents are also used for a broad array of symptoms from insomnia, irritability, and symptoms of posttraumatic stress disorder (PTSD) just as examples — even absent formal approval by the US Food and Drug Administration (FDA) for these specific indications. With the growing use of medicines, including new antidepressants like selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors, and second-generation atypical antipsychotics, there has been a greater interest and appreciation of the need to provide women with the best information about reproductive safety of these medicines as well.
When I began working in reproductive psychiatry, the FDA was using the pregnancy labeling categories introduced in 1979. The categories were simple, but also oversimplified in terms of incompletely conveying information about reproductive safety. For instance, category labels of B and C under the old labeling system could be nebulous, containing sparse information (in the case of category B) or animal data and some conflicting human data (in the case of category C) that may not have translated into relevant or easily interpretable safety information for patients and clinicians.
It was on that basis the current Pregnancy and Lactation Labeling (PLLR) Final Rule was published in 2014, which was a shift from categorical labeling to more descriptive labeling, including updated actual information on the package insert about available reproductive safety data, animal data, and data on lactation.
Even following the publication of the PLLR, there has still been an acknowledgment in the field that our assessment tools for postmarketing reproductive safety surveillance are incomplete. A recent 2-day FDA workshop hosted by the Duke-Margolis Center for Health Policy on optimizing the use of postapproval pregnancy safety studies sought to discuss the many questions that still surround this issue. Based on presentations at this workshop, a framework emerged for the future of assessing the reproductive safety of medications, which included an effort to develop the most effective model using tools such as pregnancy registries and harnessing “big data,” whether through electronic health records or large administrative databases from public and private insurers. Together, these various sources of information can provide signals of potential concern, prompting the need for a more rigorous look at the reproductive safety of a medication, or provide reassurance if data fail to indicate the absence of a signal of risk.
FDA’s new commitments under the latest reauthorization of the Prescription Drug User Fee Act (PDUFA VII) include pregnancy-specific postmarketing safety requirements as well as the creation of a framework for how data from pregnancy-specific postmarketing studies can be used. The agency is also conducting demonstration projects, including one for assessing the performance of pregnancy registries for the potential to detect safety signals for medications early in pregnancy. FDA is expanding its Sentinel Initiative to help accomplish these aims, and is implementing an Active Risk Identification and Analysis (ARIA) system to conduct active safety surveillance of medications used during pregnancy.
Pregnancy registries have now been available for decades, and some have been more successful than others across different classes of medicines, with the most rigorous registries including prospective follow-up of women across pregnancies and careful documentation of malformations (at best with original source data and with a blinded dysmorphologist). Still, with all of its rigor, even the best-intentioned efforts with respect to pregnancy registries have limitations. As I mentioned in my testimony during the public comment portion of the workshop, the sheer volume of pregnancy data from administrative databases we now have access to is attractive, but the quality of these data needs to be good enough to ascertain a signal of risk if they are to be used as a basis for reproductive safety determination.
The flip side of using data from large administrative databases is using carefully collected data from pregnancy registries. With a pregnancy registry, accrual of a substantial number of participants can also take a considerable period of time, and initial risk estimates of outcomes can have typically large confidence intervals, which can make it difficult to discern whether a drug is safe for women of reproductive age.
Another key issue is a lack of participation from manufacturers with respect to commitment to collection of high-quality reproductive safety data. History has shown that many medication manufacturers, unless required to have a dedicated registry as part of a postmarketing requirement or commitment, will invest sparse resources to track data on safety of fetal drug exposure. Participation is typically voluntary and varies from company to company unless, as noted previously, there is a postmarketing requirement or commitment tied to the approval of a medication. Just as a recent concrete example, the manufacturer of a new medication recently approved by the FDA for the treatment of postpartum depression (which will include presumably sexually active women well into the first postpartum year) has no plan to support the collection of reproductive safety data on this new medication because it is not required to, based on current FDA guidelines and the absence of a postmarketing requirement to do so.
Looking ahead
While the PLLR was a huge step forward in the field from the old pregnancy category system that could misinform women contemplating pregnancy, it also sets the stage for the next iteration of a system that allows us to generate information more quickly about the reproductive safety of medications. In psychiatry, as many as 10% of women use SSRIs during pregnancy. With drugs like atypical antipsychotics being used across disease states — in schizophrenia, bipolar disorder, depression, anxiety, insomnia, and PTSD — and where new classes of medicine are becoming available, like with ketamine or steroids, we need to have a system by which we can more quickly ascertain reproductive safety information. This information informs treatment decisions during a critical life event of deciding to try to become pregnant or during an actual pregnancy.
In my mind, it is reassuring when a registry has even as few as 50-60 cases of fetal exposure without an increase in the risk for malformation, because it can mean we are not seeing a repeat of the past with medications like thalidomide and sodium valproate. However, patients and clinicians are starved for better data. Risk assessment is also different from clinician to clinician and patient to patient. We want to empower patients to make decisions that work for them based on more rapidly accumulating information and help inform their decisions.
To come out on the “other side” of the PLLR, , which can be confusing when study results frequently conflict. I believe we have an obligation today to do this better, because the areas of reproductive toxicology and pharmacovigilance are growing incredibly quickly, and clinicians and patients are seeing these volumes of data being published without the ability to integrate that information in a systematic way.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at [email protected].
1 in 3 women have lasting health problems after giving birth: Study
Those problems include pain during sexual intercourse (35%), low back pain (32%), urinary incontinence (8% to 31%), anxiety (9% to 24%), anal incontinence (19%), depression (11% to 17%), fear of childbirth (6% to 15%), perineal pain (11%), and secondary infertility (11%).
Other problems included pelvic organ prolapse, posttraumatic stress disorder, thyroid dysfunction, mastitis, HIV seroconversion (when the body begins to produce detectable levels of HIV antibodies), nerve injury, and psychosis.
The study says most women see a doctor 6 to 12 weeks after birth and then rarely talk to doctors about these nagging health problems. Many of the problems don’t show up until 6 or more weeks after birth.
“To comprehensively address these conditions, broader and more comprehensive health service opportunities are needed, which should extend beyond 6 weeks postpartum and embrace multidisciplinary models of care,” the study says. “This approach can ensure that these conditions are promptly identified and given the attention that they deserve.”
The study is part of a series organized by the United Nation’s Special Program on Human Reproduction, the World Health Organization, and the U.S. Agency for International Development. The authors said most of the data came from high-income nations. There was little data from low-income and middle-income countries except for postpartum depression, anxiety, and psychosis.
“Many postpartum conditions cause considerable suffering in women’s daily life long after birth, both emotionally and physically, and yet they are largely underappreciated, underrecognized, and underreported,” Pascale Allotey, MD, director of Sexual and Reproductive Health and Research at WHO, said in a statement.
“Throughout their lives, and beyond motherhood, women need access to a range of services from health-care providers who listen to their concerns and meet their needs — so they not only survive childbirth but can enjoy good health and quality of life.”
A version of this article appeared on WebMD.com.
Those problems include pain during sexual intercourse (35%), low back pain (32%), urinary incontinence (8% to 31%), anxiety (9% to 24%), anal incontinence (19%), depression (11% to 17%), fear of childbirth (6% to 15%), perineal pain (11%), and secondary infertility (11%).
Other problems included pelvic organ prolapse, posttraumatic stress disorder, thyroid dysfunction, mastitis, HIV seroconversion (when the body begins to produce detectable levels of HIV antibodies), nerve injury, and psychosis.
The study says most women see a doctor 6 to 12 weeks after birth and then rarely talk to doctors about these nagging health problems. Many of the problems don’t show up until 6 or more weeks after birth.
“To comprehensively address these conditions, broader and more comprehensive health service opportunities are needed, which should extend beyond 6 weeks postpartum and embrace multidisciplinary models of care,” the study says. “This approach can ensure that these conditions are promptly identified and given the attention that they deserve.”
The study is part of a series organized by the United Nation’s Special Program on Human Reproduction, the World Health Organization, and the U.S. Agency for International Development. The authors said most of the data came from high-income nations. There was little data from low-income and middle-income countries except for postpartum depression, anxiety, and psychosis.
“Many postpartum conditions cause considerable suffering in women’s daily life long after birth, both emotionally and physically, and yet they are largely underappreciated, underrecognized, and underreported,” Pascale Allotey, MD, director of Sexual and Reproductive Health and Research at WHO, said in a statement.
“Throughout their lives, and beyond motherhood, women need access to a range of services from health-care providers who listen to their concerns and meet their needs — so they not only survive childbirth but can enjoy good health and quality of life.”
A version of this article appeared on WebMD.com.
Those problems include pain during sexual intercourse (35%), low back pain (32%), urinary incontinence (8% to 31%), anxiety (9% to 24%), anal incontinence (19%), depression (11% to 17%), fear of childbirth (6% to 15%), perineal pain (11%), and secondary infertility (11%).
Other problems included pelvic organ prolapse, posttraumatic stress disorder, thyroid dysfunction, mastitis, HIV seroconversion (when the body begins to produce detectable levels of HIV antibodies), nerve injury, and psychosis.
The study says most women see a doctor 6 to 12 weeks after birth and then rarely talk to doctors about these nagging health problems. Many of the problems don’t show up until 6 or more weeks after birth.
“To comprehensively address these conditions, broader and more comprehensive health service opportunities are needed, which should extend beyond 6 weeks postpartum and embrace multidisciplinary models of care,” the study says. “This approach can ensure that these conditions are promptly identified and given the attention that they deserve.”
The study is part of a series organized by the United Nation’s Special Program on Human Reproduction, the World Health Organization, and the U.S. Agency for International Development. The authors said most of the data came from high-income nations. There was little data from low-income and middle-income countries except for postpartum depression, anxiety, and psychosis.
“Many postpartum conditions cause considerable suffering in women’s daily life long after birth, both emotionally and physically, and yet they are largely underappreciated, underrecognized, and underreported,” Pascale Allotey, MD, director of Sexual and Reproductive Health and Research at WHO, said in a statement.
“Throughout their lives, and beyond motherhood, women need access to a range of services from health-care providers who listen to their concerns and meet their needs — so they not only survive childbirth but can enjoy good health and quality of life.”
A version of this article appeared on WebMD.com.
FROM THE LANCET GLOBAL HEALTH
Breastfeeding by patients with serious mental illness: An ethical approach
Difficult ethical situations can arise when treating perinatal women who have serious mental illness (SMI). Clinicians must consider ethical issues related to administering antipsychotic medications, the safety of breastfeeding, and concerns for child welfare. They need to carefully weigh the risks and benefits of each decision when treating perinatal women who have SMI. Ethical guidelines can help clinicians best support families in these situations.
In this article, we describe 2 cases of women with psychotic disorders who requested to breastfeed after delivering their child during an inpatient psychiatric hospitalization. The course of their hospitalizations illustrated common ethical questions and facilitated the creation of a framework to assist with complex decision-making regarding breastfeeding on inpatient psychiatric units.
CASE 1
Ms. C, age 41, is multigravida with a psychiatric history of chronic, severe schizoaffective disorder and lives in supportive housing. When Ms. C presents to the hospital in search of a rape kit, clinicians discover she is 22 weeks pregnant but has not received any prenatal care. Psychiatry is consulted because she is found to be intermittently agitated and endorses grandiose delusions. Ms. C requires involuntary hospitalization for decompensated psychosis because she refuses prenatal and psychiatric care. Because it has reassuring reproductive safety data,1 olanzapine 5 mg/d is started. However, Ms. C experiences minimal improvement from a maximum dose of 20 mg/d. After 13 weeks on the psychiatry unit, she is transferred to obstetrics service for preeclampsia with severe features. Ms. C requires an urgent cesarean delivery at 37 weeks. Her baby boy is transferred to the neonatal intensive care unit (NICU) for transient tachypnea. After delivery and in consultation with psychiatry, the pediatrics team calls Child Protective Services (CPS) due to concern for neglect driven by Ms. C’s psychiatric condition. Ms. C visits the child with medical unit staff supervision in the NICU without consulting with the psychiatry service or CPS. On postpartum Day 2, Ms. C is transferred back to psychiatry for persistent psychosis.
On postpartum Day 3, Ms. C starts to produce breastmilk and requests to breastfeed. At this time, the multidisciplinary team determines she is not able to visit her child in the NICU due to psychiatric instability. No plan is developed to facilitate hand expression or pumping of breastmilk while Ms. C is on the psychiatric unit. The clinical teams discuss whether the benefits of breastfeeding and/or pumping breastmilk would outweigh the risks. CPS determines that Ms. C is unable to retain custody and places the child in kinship foster care while awaiting clinical improvement from her.
CASE 2
Ms. S, age 32, has a history of schizophrenia. She lives with her husband and parents. She is pregnant for the first time and has been receiving consistent prenatal care. Ms. S is brought to the hospital by her husband for bizarre behavior and paranoia after self-discontinuing risperidone 2 mg twice daily due to concern about the medication’s influence on her pregnancy. An ultrasound confirms she is 37 weeks pregnant. Psychiatry is consulted because Ms. S is internally preoccupied, delusional, and endorses auditory hallucinations. She requires involuntary hospitalization for decompensated psychosis. During admission, Ms. S experiences improvement of her psychiatric symptoms while receiving risperidone 2 mg twice daily, which she takes consistently after receiving extensive psychoeducation regarding its safety profile during pregnancy and lactation.
After 2 weeks on the psychiatry unit, Ms. S’s care team transfers her to the obstetrics service with one-to-one supervision. At 39 weeks gestation, she has a vaginal delivery without complications. Because there are no concerns about infant harm, obstetrics, pediatrics, and psychiatry coordinate care so the baby can room in with Ms. S, her husband, and a staff supervisor to facilitate bonding. Ms. S starts to lactate, wishes to breastfeed, and meets with lactation, pediatric, obstetric, and psychiatric specialists to discuss the risks and benefits of breastfeeding and pumping breastmilk. She pursues direct breastfeeding until the baby is discharged home with the husband at postpartum Day 2. CPS is not called because there are no concerns for parental abuse or neglect at the infant’s discharge.
On postpartum Day 2, the obstetrics service transfers Ms. S back to the psychiatric unit for further treatment of her paranoia. She wishes to pump breastmilk while hospitalized, so the treatment team supplies a breast pump, facilitates the storage of breastmilk, and coordinates supervision during pumping to reduce the ligature risk. Ms. S’s husband visits daily to transport the milk and feed the infant breastmilk and formula to meet its nutritional needs. Ms. S maintains psychiatric stability while breast pumping, and the team helps transition her to breastfeeding during visitation with her husband and infant until she is discharged home at 2 weeks postpartum.
Continue to: Approaching care with a relational ethics framework
Approaching care with a relational ethics framework
A relational ethics framework was constructed to evaluate whether to support breastfeeding for both patients during their psychiatric hospitalizations. A relational ethics perspective is defined as “a moral responsibility within a context of human relations” [that] “recognizes the human interdependency and reciprocity within which personal autonomy is embedded.”2 This framework values connectedness and commonality between various and even conflicting parties. In the setting of a clinician-patient relationship, health care decisions are made with consideration of the patient’s traditional beliefs, values, and principles rather than the application of impartial moral principles. For these complex cases, this framework was chosen to determine the safest possible outcome for both mother and child.
Risks/benefits of breastfeeding by patients who have SMI
There are several methods of breastfeeding, including direct breastfeeding and other ways of expressing breastmilk such as pumping or hand expression.3 Unlike other forms of feeding using breastmilk, direct breastfeeding has been extensively studied, has well-established medical and psychological benefits for newborns and mothers, and enhances long-term bonding.4 Compared with their counterparts who do not breastfeed, mothers who breastfeed have lower rates of unintended pregnancy, cardiovascular disease, postpartum bleeding, osteoporosis, and breast and ovarian cancer.5 Among its key psychological benefits, breastfeeding is associated with an increase in maternal self-efficacy and, in some research, has been shown to be associated with a decreased risk of postpartum depression and stress. Additionally, breastfed infants experience lower rates of childhood infection and obesity, and improved nutrition, cognitive development, and immune function.6 The American Academy of Pediatrics recognizes these benefits and recommends that women exclusively breastfeed for 6 months postpartum and continue to breastfeed for 2 years or beyond if mutually desired by the mother and child.7 Absolute contraindications to breastfeeding must be ruled out (eg, infant classic galactosemia; maternal use of illicit substances such as cocaine, opioids, or phencyclidine; maternal HIV infection, etc).
The risks of breastfeeding by patients who have SMI must also be considered. In severe situations, the infant can be exposed to a mother’s agitation secondary to psychosis.8,9 The transmission of antipsychotic medication through breastmilk and associated adverse effects (eg, sedation, poor feeding, and extrapyramidal symptoms) are also potential risks and varies among different antipsychotic medications.1,10 Therefore, when prescribing an antipsychotic for a patient with SMI who breastfeeds, it is crucial to consider the medication’s safety profile as well as other factors, such as the relative infant dose (the weight-adjusted [ie, mg/kg] percentage of the maternal dosage ingested by a fully breastfed infant) and the molecular characteristics of the medication.10-12 Neonates should be routinely monitored for adverse effects, medication toxicity, and withdrawal symptoms, and care should be coordinated with the infant’s pediatrician. Certain antipsychotic medications, such as aripiprazole, may impact breastmilk production through the dopamine agonist’s interference of the prolactin reflex and anticholinergic properties.11,13 For a patient with SMI, perhaps the most significant risk involves the time and resources needed for breastfeeding, which can interfere with sleep and psychiatric treatment and possibly further exacerbate psychiatric symptoms.14-16 Additionally, breastfeeding difficulties or disruption can increase the risk of psychiatric symptoms and psychological distress.17 In Ms. C’s case, there was a delay in the baby latching as well as multiple medical and psychiatric factors that hindered the milk-ejection reflex to properly initiate; both of these factors rendered breastfeeding particularly difficult while Ms. C was on the inpatient psychiatry unit.17 In comparison, Ms. S was able to bond with her infant shortly after delivery, which facilitated the milk-ejection reflex and lactation.
Patients who wish to directly breastfeed but struggle to do so while tending to their acute psychiatric condition can benefit from expression of breastmilk that can be provided to the infant or discarded to facilitate breastfeeding in the future.18 While expression of breastmilk may not be as advantageous for infant health as direct breastfeeding due to the potential changes in breastmilk composition from collecting, storing, and heating, this option can be more protective than formula feeding and facilitate future breastfeeding.19 In these clinical scenarios, it is standard care to provide a hospital-grade breast pump to the patient, much like a continuous positive airway pressure machine is provided to patients with obstructive sleep apnea.20 However, there is often considerable difficulty obtaining proper breastfeeding equipment and a lack of services devoted to perinatal care in general inpatient settings. Barriers to direct breastfeeding and pumping of breastmilk are highlighted in the Table.21
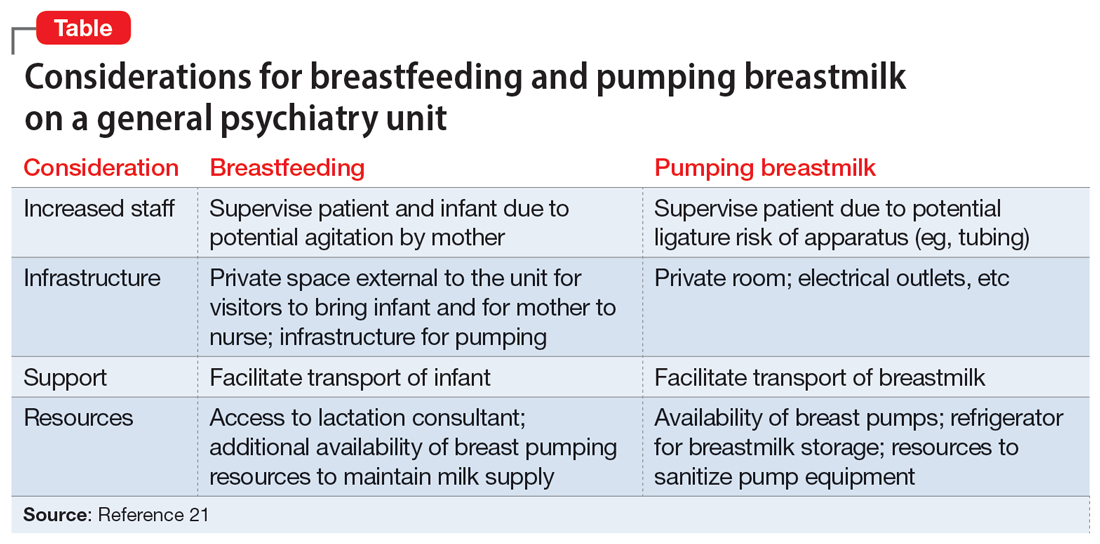
Limitations on breastfeeding on an inpatient unit
The limitations in care and restrictions placed on breastfeeding are more optimally addressed in a mother and baby unit (MBU). MBUs are specialized inpatient psychiatric units designed for mothers experiencing severe perinatal psychiatric difficulties. Unlike general psychiatric units, MBUs allow for joint, full-time admission of mothers and their infants. These units also include multidisciplinary staff who specialize in treating perinatal mental health issues as well as infant care and child development.22 Admission into an MBU is considered best practice for new mothers requiring treatment, particularly in the United Kingdom, Australia, and France, as it is well-recognized that the separation of mother and baby can be psychologically harmful.23 In the UK, most patients admitted to an MBU showed significant improvement of their psychiatric symptoms and reported overall high satisfaction with care.24,25 Patients who experience postpartum psychosis prefer MBUs over general psychiatric units because the latter often lack specialized perinatal support, appropriate visitor arrangements, and adequate time with their infant.26-28
Continue to: The resistance to adopting MBUs in the United States...
The resistance to adopting MBUs in the United States has posed significant barriers in care for perinatal patients and has been attributed to financial barriers, medicolegal risk, staffing, and safety concerns.29 Though currently there are no MBUs in the US, other specialized units have been created. A partial day hospitalization program created in 2000 in Rhode Island for mothers and infants revolutionized the psychiatric care experience for new mothers.30 Since then, other institutions have significantly expanded their services to include perinatal psychiatry inpatient units, yet unlike MBUs, these units typically do not provide overnight rooming-in with infants.31 They have the necessary resources and facilities to accommodate the mother’s needs and maximize positive mother-infant interaction, while actively integrating the infant into the mother’s treatment. Breast pumping is treated as a necessary medical procedure and patients can easily access hospital-grade breast pumps with staff supervision. At one such perinatal psychiatric inpatient unit, high rates of treatment satisfaction and significant improvements in symptoms of depression, anxiety, active suicidal ideation, and overall functioning were observed at discharge.32 Therefore, it is crucial to incorporate strategies in general psychiatry units to improve perinatal care, acknowledging that most patients will not have access to these specialized units.21
A framework to approaching the relational ethics decisions
An interdisciplinary team used a relational ethics perspective to carefully analyze the risks and benefits of these complex cases. In Figure 1, we propose a framework for the relational ethics decisions of breastfeeding on general inpatient psychiatric units. In creating this framework, we considered principles of autonomy, beneficence, and nonmaleficence, along with the medical and logistical barriers to breastfeeding.
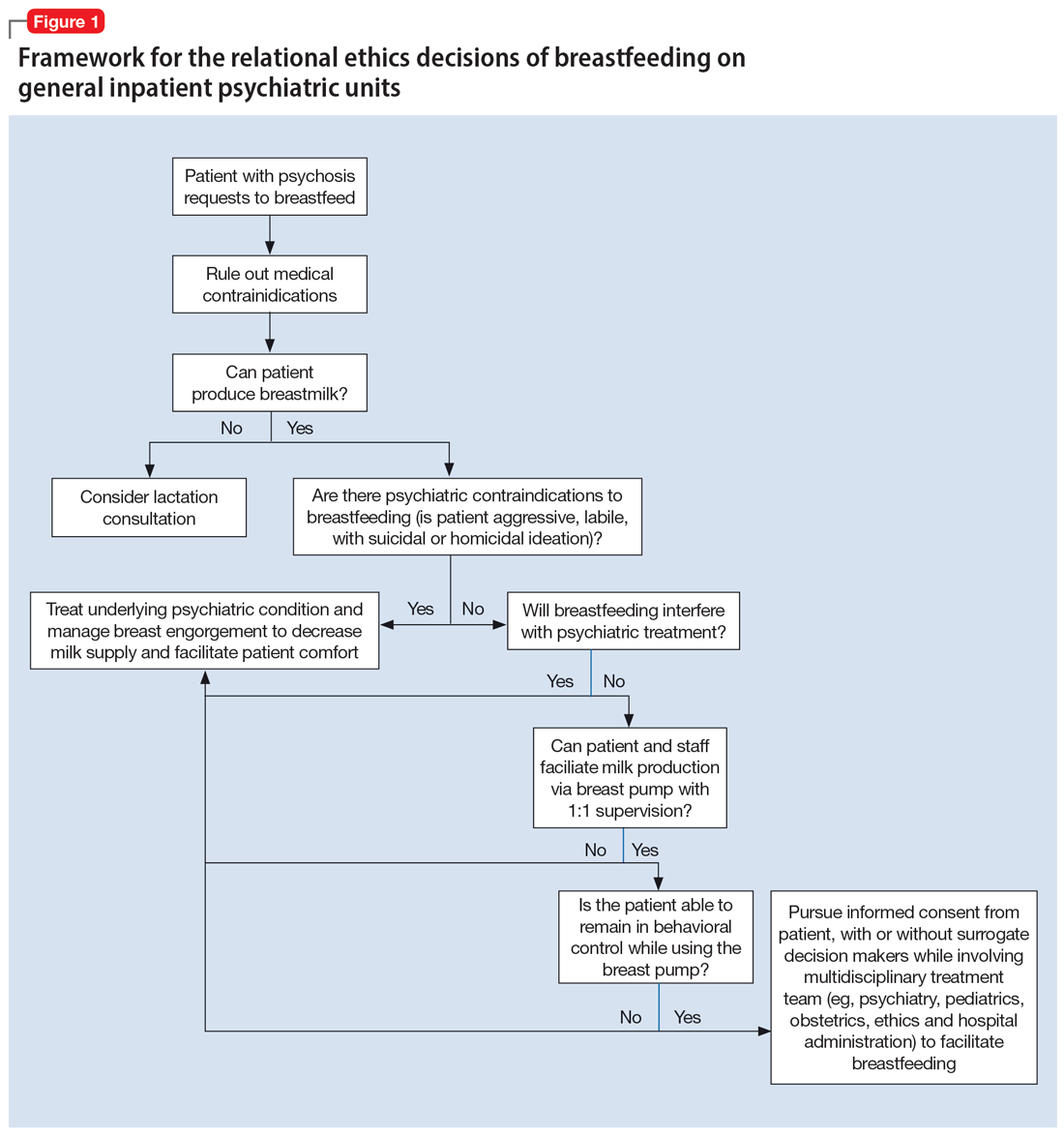
In Ms. C’s case, the team determined that the risks—which included disrupting the mother’s psychiatric treatment, exposing her to psychological harm due to increasing attachment before remanding the child to CPS custody, and risks to the child due to potential unpredictable agitation driven by the treatment-refractory psychosis of the mother as well as that of other psychiatric patients—outweighed the benefits of breastfeeding. We instead recommended breast pumping as an alternative once Ms. C’s psychiatric stability improved. We presented Ms. C with the option of breast pumping on postpartum Day 5. During a 1-day period in which she showed improved behavioral control, she was counseled on the risks and benefits of breastfeeding and exclusive pumping and was notified that the team would help her with the necessary resources, including consultation with a lactation specialist and breast pump. Despite lactation consultant support, Ms. C had low milk production and difficulty with hand expression, which was very discouraging to her. She produced 1 ounce of milk that was shared with the newborn while in the NICU. Because Ms. C’s psychiatric symptoms continued to be severe, with lability and aggression, and because pumping was triggering distress, the multidisciplinary team determined the best course of care would be to focus on her psychiatric recovery rather than on pumping breastmilk. To reduce milk production and minimize discomfort secondary to breast engorgement, the lactation consultant recommended cold compresses, pain management, and compression of breasts. Ultimately, the mother-infant dyad was unable to reap the benefits of breastfeeding (via pumping or direct breastfeeding) due to the mother’s underlying psychiatric illness, although the staffing, psychosocial support, and logistical limitations contributed to this outcome.33
In Ms. S’s case, the treatment team determined that there were no medical or psychiatric contraindications to breastfeeding, and she was counseled on the risks and benefits of direct breastfeeding and pumping. The treatment team determined it was safe for Ms. S to directly breastfeed as there were no concerns for infant harm postdelivery with constant supervision while on the obstetrics floor. The patient opted to directly breastfeed, which was successful with the guidance of a lactation specialist. When she was transferred to the psychiatric unit on postpartum Day 2, her child was discharged home with the husband. The patient was then encouraged to pump while the psychiatrists monitored her symptoms closely and facilitated increased staff and resources. Transportation of breastmilk was made possible by the family, and on postpartum Day 5, as the patient maintained psychiatric stability, the team discussed with Ms. S and her husband the prospect of direct breastfeeding. The treatment team arranged for separate visitation hours to minimize the possibility of exposing the infant to aggression from other patients on the unit and advocated with hospital leadership to approve of infant visitation on the unit.
Impact of involvement of Child Protective Services
The involvement of CPS also added complexity to Ms. C’s case. Without proper legal guidance, mothers with psychosis who lose custody can find it difficult to navigate the legal system and maintain contact with their children.34 As the prevalence of custody loss in mothers with psychosis is high (approximately 50% according to research published in the last 10 years), effective interventions to reunite the mother and child must be promoted (Figure 2).35-39 Ultimately, the goal of psychiatric hospitalization for perinatal women who have SMI is psychiatric stabilization. The preemptive involvement of psychiatry is crucial because it can allow for early postpartum planning and can provide an opportunity to address feeding options and custody concerns with the patient, social supports and services, and various medical teams. In Ms. C’s case, she visited her baby in the NICU on postpartum Day 2 without consultation with psychiatry or CPS, which posed risks to the patient, infant, and staff. It is vital that various clinicians collaborate with each other and the patient, working towards the goal of optimizing the patient’s mental health to allow for parenting rights in the future and maximizing a sustainable attachment between the parent and child. In Ms. S’s case, the husband was able to facilitate caring for the baby while the mother was hospitalized and played an integral role in the feeding process via pumped breastmilk and transport of the infant for direct breastfeeding.
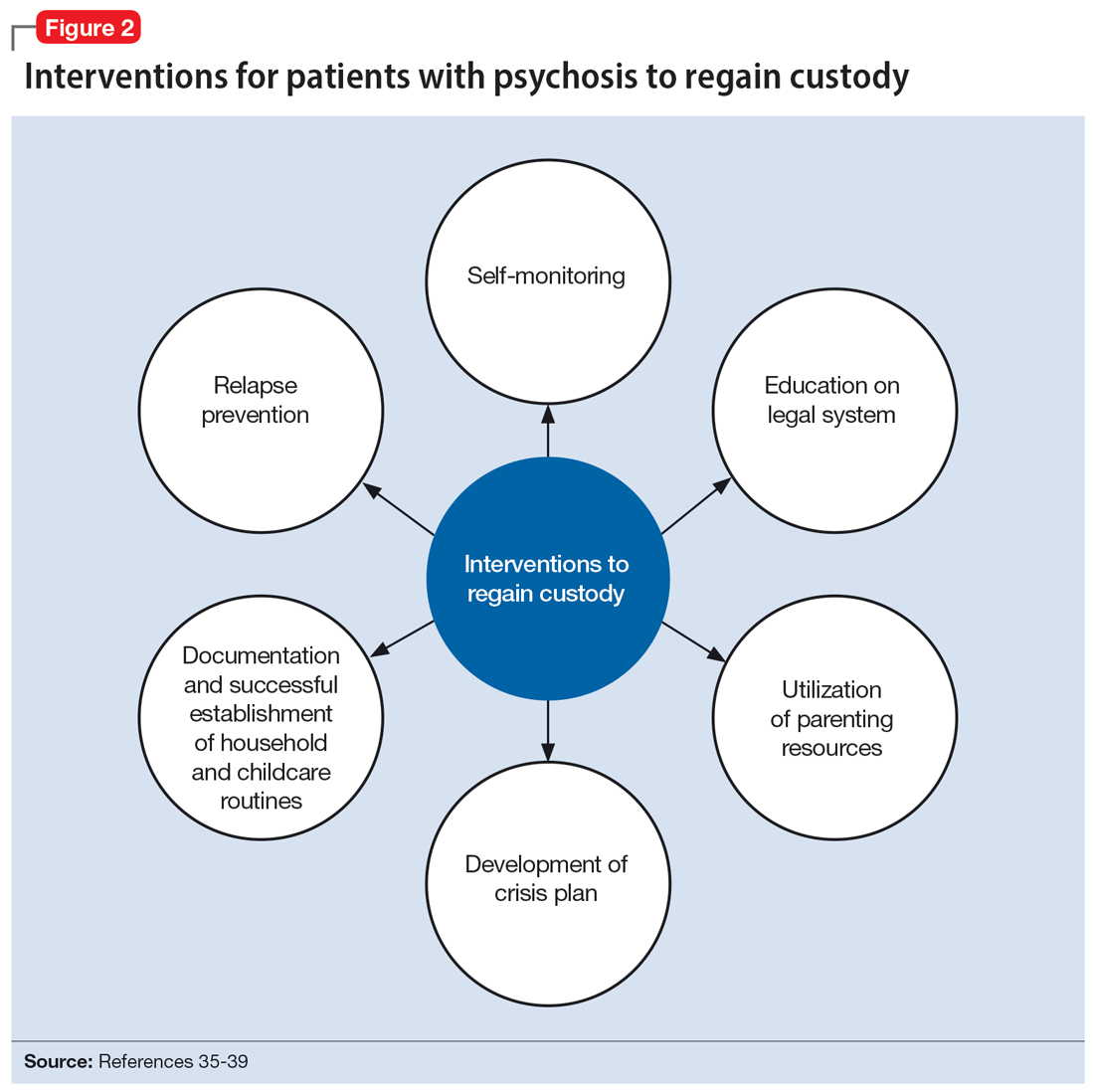
Continue to: The differences in these 2 cases...
The differences in these 2 cases show the extreme importance of social support to benefit both the mother and child, and the need for more comprehensive social services for women who do not have a social safety net.
Bottom Line
These complex cases highlight an ethical decision-making approach to breastfeeding in perinatal women who have serious mental illness. Collaborative care and shared decision-making, which highlight the interests of the mother and baby, are crucial when assessing the risks and benefits of breastfeeding and pumping breastmilk. Our relational ethics framework can be used to better evaluate and implement breastfeeding options on general psychiatric units.
Related Resources
- Tillman B, Sloan N, Westmoreland P. How COVID-19 affects peripartum women’s mental health. Current Psychiatry. 2021;20(6):18-22. doi:10.12788/cp.0129
- Koch J, Preinitz J. Antidepressants for patients who are breastfeeding: what to consider. Current Psychiatry. 2023;22(5):20-23,48. doi:10.12788/cp.0355
Drug Brand Names
Aripiprazole • Abilify
Olanzapine • Zyprexa
Risperidone • Risperdal
1. Brunner E, Falk DM, Jones M, et al. Olanzapine in pregnancy and breastfeeding: a review of data from global safety surveillance. BMC Pharmacol Toxicol. 2013;14:38. doi:10.1186/2050-6511-14-38
2. Seeman MV. Relational ethics: when mothers suffer from psychosis. Arch Womens Ment Health. 2004;7(3):201-210. doi:10.1007/s00737-004-0054-8
3. Motee A, Jeewon R. Importance of exclusive breastfeeding and complementary feeding among infants. Curr Res Nutr Food Sci. 2014;2(2). doi:10.12944/CRNFSJ.2.2.02
4. Committee Opinion No. 570: breastfeeding in underserved women: increasing initiation and continuation of breastfeeding. Obstet Gynecol. 2013;122(2 Pt 1):423-427. doi:10.1097/01.AOG.0000433008.93971.6a
5. Sibolboro Mezzacappa E, Endicott J. Parity mediates the association between infant feeding method and maternal depressive symptoms in the postpartum. Arch Womens Ment Health. 2007;10(6):259-266. doi:10.1007/s00737-007-0207-7
6. Kramer MS, Chalmers B, Hodnett ED, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285(4):413-420. doi:10.1001/jama.285.4.413
7. American Academy of Pediatrics. American Academy of Pediatrics calls for more support for breastfeeding mothers within updated policy recommendations. June 27, 2022. Accessed October 4, 2022. https://www.aap.org/en/news-room/news-releases/aap/2022/american-academy-of-pediatrics-calls-for-more-support-for-breastfeeding-mothers-within-updated-policy-recommendations
8. Hipwell AE, Kumar R. Maternal psychopathology and prediction of outcome based on mother-infant interaction ratings (BMIS). Br J Psychiatry. 1996;169(5):655-661. doi:10.1192/bjp.169.5.655
9. Chandra PS, Bhargavaraman RP, Raghunandan VN, et al. Delusions related to infant and their association with mother-infant interactions in postpartum psychotic disorders. Arch Womens Ment Health. 2006;9(5):285-288. doi:10.1007/s00737-006-0147-7
10. Klinger G, Stahl B, Fusar-Poli P, et al. Antipsychotic drugs and breastfeeding. Pediatr Endocrinol Rev. 2013;10(3):308-317.
11. Uguz F. A new safety scoring system for the use of psychotropic drugs during lactation. Am J Ther. 2021;28(1):e118-e126. doi:10.1097/MJT.0000000000000909
12. Hale TW, Krutsch K. Hale’s Medications & Mothers’ Milk, 2023: A Manual of Lactational Pharmacology. 20th ed. Springer Publishing Company; 2023.
13. Komaroff A. Aripiprazole and lactation failure: the importance of shared decision making. A case report. Case Rep Womens Health. 2021;30:e00308. doi:10.1016/j.crwh.2021.e00308
14. Dennis CL, McQueen K. Does maternal postpartum depressive symptomatology influence infant feeding outcomes? Acta Pediatr. 2007;96(4):590-594. doi:10.1111/j.1651-2227.2007.00184.x
15. Chaput KH, Nettel-Aguirre A, Musto R, et al. Breastfeeding difficulties and supports and risk of postpartum depression in a cohort of women who have given birth in Calgary: a prospective cohort study. CMAJ Open. 2016;4(1):E103-E109. doi:10.9778/cmajo.20150009
16. Dias CC, Figueiredo B. Breastfeeding and depression: a systematic review of the literature. J Affect Disord. 2015;171:142-154. doi:10.1016/j.jad.2014.09.022
17. Brown A, Rance J, Bennett P. Understanding the relationship between breastfeeding and postnatal depression: the role of pain and physical difficulties. J Adv Nurs. 2016;72(2):273-282. doi:10.1111/jan.12832
18. Rosenbaum KA. Exclusive breastmilk pumping: a concept analysis. Nurs Forum. 2022;57(5):946-953. doi:10.1111/nuf.12766
19. Boone KM, Geraghty SR, Keim SA. Feeding at the breast and expressed milk feeding: associations with otitis media and diarrhea in infants. J Pediatr. 2016;174:118-125. doi:10.1016/j.jpeds.2016.04.006
20. Epstein LJ, Kristo D, Strollo PJ Jr, et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263-276.
21. Caan MP, Sreshta NE, Okwerekwu JA, et al. Clinical and legal considerations regarding breastfeeding on psychiatric units. J Am Acad Psychiatry Law. 2022;50(2):200-207. doi:10.29158/JAAPL.210086-21
22. Glangeaud-Freudenthal NMC, Rainelli C, Cazas O, et al. Inpatient mother and baby psychiatric units (MBUs) and day cares. In: Sutter-Dallay AL, Glangeaud-Freudenthal NC, Guedeney A, et al, eds. Joint Care of Parents and Infants in Perinatal Psychiatry. Springer, Cham; 2016:147-164. doi:10.1007/978-3-319-21557-0_10
23. Dembosky A. A humane approach to caring for new mothers in psychiatric crisis. Health Aff (Millwood). 2021;40(10):1528-1533. doi:10.1377/hlthaff.2021.01288
24. Connellan K, Bartholomaeus C, Due C, et al. A systematic review of research on psychiatric mother-baby units. Arch Womens Ment Health. 2017;20(3):373-388. doi:10.1007/s00737-017-0718-9
25. Griffiths J, Lever Taylor B, Morant N, et al. A qualitative comparison of experiences of specialist mother and baby units versus general psychiatric wards. BMC Psychiatry. 2019;19(1):401. doi:10.1186/s12888-019-2389-8
26. Heron J, Gilbert N, Dolman C, et al. Information and support needs during recovery from postpartum psychosis. Arch Womens Ment Health. 2012;15(3):155-165. doi:10.1007/s00737-012-0267-1
27. Robertson E, Lyons A. Living with puerperal psychosis: a qualitative analysis. Psychol Psychother. 2003;76(Pt 4):411-431. doi:10.1348/147608303770584755
28. Mental Welfare Commission for Scotland. Perinatal Themed Visit Report: Keeping Mothers and Babies in Mind. Mental Welfare Commission for Scotland; 2016.
29. Wisner KL, Jennings KD, Conley B. Clinical dilemmas due to the lack of inpatient mother-baby units. Int J Psychiatry Med. 1996;26(4):479-493. doi:10.2190/NFJK-A4V7-CXUU-AM89
30. Battle CL, Howard MM. A mother-baby psychiatric day hospital: history, rationale, and why perinatal mental health is important for obstetric medicine. Obstet Med. 2014;7(2):66-70. doi:10.1177/1753495X13514402
31. Bullard ES, Meltzer-Brody S, Rubinow DR. The need for comprehensive psychiatric perinatal care-the University of North Carolina at Chapel Hill, Department of Psychiatry, Center for Women’s Mood Disorders launches the first dedicated inpatient program in the United States. Am J Obstet Gynecol. 2009;201(5):e10-e11. doi:10.1016/j.ajog.2009.05.004
32. Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health. 2014;17(2):107-113. doi:10.1007/s00737-013-0390-7
33. Alvarez-Toro V. Gender-specific care for women in psychiatric units. J Am Acad Psychiatry Law. 2022;JAAPL.220015-21. doi:10.29158/JAAPL.220015-21
34. Diaz-Caneja A, Johnson S. The views and experiences of severely mentally ill mothers--a qualitative study. Soc Psychiatry Psychiatr Epidemiol. 2004;39(6):472-482. doi:10.1007/s00127-004-0772-2
35. Gewurtz R, Krupa T, Eastabrook S, et al. Prevalence and characteristics of parenting among people served by assertive community treatment. Psychiatr Rehabil J. 2004;28(1):63-65. doi:10.2975/28.2004.63.65
36. Howard LM, Kumar R, Thornicroft G. Psychosocial characteristics and needs of mothers with psychotic disorders. Br J Psychiatry. 2001;178:427-432. doi:10.1192/bjp.178.5.427
37. Hollingsworth LD. Child custody loss among women with persistent severe mental illness. Social Work Research. 2004;28(4):199-209. doi:10.1093/swr/28.4.199
38. Dipple H, Smith S, Andrews H, et al. The experience of motherhood in women with severe and enduring mental illness. Soc Psychiatry Psychiatr Epidemiolf. 2002;37(7):336-340. doi:10.1007/s00127-002-0559-2
39. Seeman MV. Intervention to prevent child custody loss in mothers with schizophrenia. Schizophr Res Treatment. 2012;2012:796763. doi:10.1155/2012/796763
Difficult ethical situations can arise when treating perinatal women who have serious mental illness (SMI). Clinicians must consider ethical issues related to administering antipsychotic medications, the safety of breastfeeding, and concerns for child welfare. They need to carefully weigh the risks and benefits of each decision when treating perinatal women who have SMI. Ethical guidelines can help clinicians best support families in these situations.
In this article, we describe 2 cases of women with psychotic disorders who requested to breastfeed after delivering their child during an inpatient psychiatric hospitalization. The course of their hospitalizations illustrated common ethical questions and facilitated the creation of a framework to assist with complex decision-making regarding breastfeeding on inpatient psychiatric units.
CASE 1
Ms. C, age 41, is multigravida with a psychiatric history of chronic, severe schizoaffective disorder and lives in supportive housing. When Ms. C presents to the hospital in search of a rape kit, clinicians discover she is 22 weeks pregnant but has not received any prenatal care. Psychiatry is consulted because she is found to be intermittently agitated and endorses grandiose delusions. Ms. C requires involuntary hospitalization for decompensated psychosis because she refuses prenatal and psychiatric care. Because it has reassuring reproductive safety data,1 olanzapine 5 mg/d is started. However, Ms. C experiences minimal improvement from a maximum dose of 20 mg/d. After 13 weeks on the psychiatry unit, she is transferred to obstetrics service for preeclampsia with severe features. Ms. C requires an urgent cesarean delivery at 37 weeks. Her baby boy is transferred to the neonatal intensive care unit (NICU) for transient tachypnea. After delivery and in consultation with psychiatry, the pediatrics team calls Child Protective Services (CPS) due to concern for neglect driven by Ms. C’s psychiatric condition. Ms. C visits the child with medical unit staff supervision in the NICU without consulting with the psychiatry service or CPS. On postpartum Day 2, Ms. C is transferred back to psychiatry for persistent psychosis.
On postpartum Day 3, Ms. C starts to produce breastmilk and requests to breastfeed. At this time, the multidisciplinary team determines she is not able to visit her child in the NICU due to psychiatric instability. No plan is developed to facilitate hand expression or pumping of breastmilk while Ms. C is on the psychiatric unit. The clinical teams discuss whether the benefits of breastfeeding and/or pumping breastmilk would outweigh the risks. CPS determines that Ms. C is unable to retain custody and places the child in kinship foster care while awaiting clinical improvement from her.
CASE 2
Ms. S, age 32, has a history of schizophrenia. She lives with her husband and parents. She is pregnant for the first time and has been receiving consistent prenatal care. Ms. S is brought to the hospital by her husband for bizarre behavior and paranoia after self-discontinuing risperidone 2 mg twice daily due to concern about the medication’s influence on her pregnancy. An ultrasound confirms she is 37 weeks pregnant. Psychiatry is consulted because Ms. S is internally preoccupied, delusional, and endorses auditory hallucinations. She requires involuntary hospitalization for decompensated psychosis. During admission, Ms. S experiences improvement of her psychiatric symptoms while receiving risperidone 2 mg twice daily, which she takes consistently after receiving extensive psychoeducation regarding its safety profile during pregnancy and lactation.
After 2 weeks on the psychiatry unit, Ms. S’s care team transfers her to the obstetrics service with one-to-one supervision. At 39 weeks gestation, she has a vaginal delivery without complications. Because there are no concerns about infant harm, obstetrics, pediatrics, and psychiatry coordinate care so the baby can room in with Ms. S, her husband, and a staff supervisor to facilitate bonding. Ms. S starts to lactate, wishes to breastfeed, and meets with lactation, pediatric, obstetric, and psychiatric specialists to discuss the risks and benefits of breastfeeding and pumping breastmilk. She pursues direct breastfeeding until the baby is discharged home with the husband at postpartum Day 2. CPS is not called because there are no concerns for parental abuse or neglect at the infant’s discharge.
On postpartum Day 2, the obstetrics service transfers Ms. S back to the psychiatric unit for further treatment of her paranoia. She wishes to pump breastmilk while hospitalized, so the treatment team supplies a breast pump, facilitates the storage of breastmilk, and coordinates supervision during pumping to reduce the ligature risk. Ms. S’s husband visits daily to transport the milk and feed the infant breastmilk and formula to meet its nutritional needs. Ms. S maintains psychiatric stability while breast pumping, and the team helps transition her to breastfeeding during visitation with her husband and infant until she is discharged home at 2 weeks postpartum.
Continue to: Approaching care with a relational ethics framework
Approaching care with a relational ethics framework
A relational ethics framework was constructed to evaluate whether to support breastfeeding for both patients during their psychiatric hospitalizations. A relational ethics perspective is defined as “a moral responsibility within a context of human relations” [that] “recognizes the human interdependency and reciprocity within which personal autonomy is embedded.”2 This framework values connectedness and commonality between various and even conflicting parties. In the setting of a clinician-patient relationship, health care decisions are made with consideration of the patient’s traditional beliefs, values, and principles rather than the application of impartial moral principles. For these complex cases, this framework was chosen to determine the safest possible outcome for both mother and child.
Risks/benefits of breastfeeding by patients who have SMI
There are several methods of breastfeeding, including direct breastfeeding and other ways of expressing breastmilk such as pumping or hand expression.3 Unlike other forms of feeding using breastmilk, direct breastfeeding has been extensively studied, has well-established medical and psychological benefits for newborns and mothers, and enhances long-term bonding.4 Compared with their counterparts who do not breastfeed, mothers who breastfeed have lower rates of unintended pregnancy, cardiovascular disease, postpartum bleeding, osteoporosis, and breast and ovarian cancer.5 Among its key psychological benefits, breastfeeding is associated with an increase in maternal self-efficacy and, in some research, has been shown to be associated with a decreased risk of postpartum depression and stress. Additionally, breastfed infants experience lower rates of childhood infection and obesity, and improved nutrition, cognitive development, and immune function.6 The American Academy of Pediatrics recognizes these benefits and recommends that women exclusively breastfeed for 6 months postpartum and continue to breastfeed for 2 years or beyond if mutually desired by the mother and child.7 Absolute contraindications to breastfeeding must be ruled out (eg, infant classic galactosemia; maternal use of illicit substances such as cocaine, opioids, or phencyclidine; maternal HIV infection, etc).
The risks of breastfeeding by patients who have SMI must also be considered. In severe situations, the infant can be exposed to a mother’s agitation secondary to psychosis.8,9 The transmission of antipsychotic medication through breastmilk and associated adverse effects (eg, sedation, poor feeding, and extrapyramidal symptoms) are also potential risks and varies among different antipsychotic medications.1,10 Therefore, when prescribing an antipsychotic for a patient with SMI who breastfeeds, it is crucial to consider the medication’s safety profile as well as other factors, such as the relative infant dose (the weight-adjusted [ie, mg/kg] percentage of the maternal dosage ingested by a fully breastfed infant) and the molecular characteristics of the medication.10-12 Neonates should be routinely monitored for adverse effects, medication toxicity, and withdrawal symptoms, and care should be coordinated with the infant’s pediatrician. Certain antipsychotic medications, such as aripiprazole, may impact breastmilk production through the dopamine agonist’s interference of the prolactin reflex and anticholinergic properties.11,13 For a patient with SMI, perhaps the most significant risk involves the time and resources needed for breastfeeding, which can interfere with sleep and psychiatric treatment and possibly further exacerbate psychiatric symptoms.14-16 Additionally, breastfeeding difficulties or disruption can increase the risk of psychiatric symptoms and psychological distress.17 In Ms. C’s case, there was a delay in the baby latching as well as multiple medical and psychiatric factors that hindered the milk-ejection reflex to properly initiate; both of these factors rendered breastfeeding particularly difficult while Ms. C was on the inpatient psychiatry unit.17 In comparison, Ms. S was able to bond with her infant shortly after delivery, which facilitated the milk-ejection reflex and lactation.
Patients who wish to directly breastfeed but struggle to do so while tending to their acute psychiatric condition can benefit from expression of breastmilk that can be provided to the infant or discarded to facilitate breastfeeding in the future.18 While expression of breastmilk may not be as advantageous for infant health as direct breastfeeding due to the potential changes in breastmilk composition from collecting, storing, and heating, this option can be more protective than formula feeding and facilitate future breastfeeding.19 In these clinical scenarios, it is standard care to provide a hospital-grade breast pump to the patient, much like a continuous positive airway pressure machine is provided to patients with obstructive sleep apnea.20 However, there is often considerable difficulty obtaining proper breastfeeding equipment and a lack of services devoted to perinatal care in general inpatient settings. Barriers to direct breastfeeding and pumping of breastmilk are highlighted in the Table.21

Limitations on breastfeeding on an inpatient unit
The limitations in care and restrictions placed on breastfeeding are more optimally addressed in a mother and baby unit (MBU). MBUs are specialized inpatient psychiatric units designed for mothers experiencing severe perinatal psychiatric difficulties. Unlike general psychiatric units, MBUs allow for joint, full-time admission of mothers and their infants. These units also include multidisciplinary staff who specialize in treating perinatal mental health issues as well as infant care and child development.22 Admission into an MBU is considered best practice for new mothers requiring treatment, particularly in the United Kingdom, Australia, and France, as it is well-recognized that the separation of mother and baby can be psychologically harmful.23 In the UK, most patients admitted to an MBU showed significant improvement of their psychiatric symptoms and reported overall high satisfaction with care.24,25 Patients who experience postpartum psychosis prefer MBUs over general psychiatric units because the latter often lack specialized perinatal support, appropriate visitor arrangements, and adequate time with their infant.26-28
Continue to: The resistance to adopting MBUs in the United States...
The resistance to adopting MBUs in the United States has posed significant barriers in care for perinatal patients and has been attributed to financial barriers, medicolegal risk, staffing, and safety concerns.29 Though currently there are no MBUs in the US, other specialized units have been created. A partial day hospitalization program created in 2000 in Rhode Island for mothers and infants revolutionized the psychiatric care experience for new mothers.30 Since then, other institutions have significantly expanded their services to include perinatal psychiatry inpatient units, yet unlike MBUs, these units typically do not provide overnight rooming-in with infants.31 They have the necessary resources and facilities to accommodate the mother’s needs and maximize positive mother-infant interaction, while actively integrating the infant into the mother’s treatment. Breast pumping is treated as a necessary medical procedure and patients can easily access hospital-grade breast pumps with staff supervision. At one such perinatal psychiatric inpatient unit, high rates of treatment satisfaction and significant improvements in symptoms of depression, anxiety, active suicidal ideation, and overall functioning were observed at discharge.32 Therefore, it is crucial to incorporate strategies in general psychiatry units to improve perinatal care, acknowledging that most patients will not have access to these specialized units.21
A framework to approaching the relational ethics decisions
An interdisciplinary team used a relational ethics perspective to carefully analyze the risks and benefits of these complex cases. In Figure 1, we propose a framework for the relational ethics decisions of breastfeeding on general inpatient psychiatric units. In creating this framework, we considered principles of autonomy, beneficence, and nonmaleficence, along with the medical and logistical barriers to breastfeeding.

In Ms. C’s case, the team determined that the risks—which included disrupting the mother’s psychiatric treatment, exposing her to psychological harm due to increasing attachment before remanding the child to CPS custody, and risks to the child due to potential unpredictable agitation driven by the treatment-refractory psychosis of the mother as well as that of other psychiatric patients—outweighed the benefits of breastfeeding. We instead recommended breast pumping as an alternative once Ms. C’s psychiatric stability improved. We presented Ms. C with the option of breast pumping on postpartum Day 5. During a 1-day period in which she showed improved behavioral control, she was counseled on the risks and benefits of breastfeeding and exclusive pumping and was notified that the team would help her with the necessary resources, including consultation with a lactation specialist and breast pump. Despite lactation consultant support, Ms. C had low milk production and difficulty with hand expression, which was very discouraging to her. She produced 1 ounce of milk that was shared with the newborn while in the NICU. Because Ms. C’s psychiatric symptoms continued to be severe, with lability and aggression, and because pumping was triggering distress, the multidisciplinary team determined the best course of care would be to focus on her psychiatric recovery rather than on pumping breastmilk. To reduce milk production and minimize discomfort secondary to breast engorgement, the lactation consultant recommended cold compresses, pain management, and compression of breasts. Ultimately, the mother-infant dyad was unable to reap the benefits of breastfeeding (via pumping or direct breastfeeding) due to the mother’s underlying psychiatric illness, although the staffing, psychosocial support, and logistical limitations contributed to this outcome.33
In Ms. S’s case, the treatment team determined that there were no medical or psychiatric contraindications to breastfeeding, and she was counseled on the risks and benefits of direct breastfeeding and pumping. The treatment team determined it was safe for Ms. S to directly breastfeed as there were no concerns for infant harm postdelivery with constant supervision while on the obstetrics floor. The patient opted to directly breastfeed, which was successful with the guidance of a lactation specialist. When she was transferred to the psychiatric unit on postpartum Day 2, her child was discharged home with the husband. The patient was then encouraged to pump while the psychiatrists monitored her symptoms closely and facilitated increased staff and resources. Transportation of breastmilk was made possible by the family, and on postpartum Day 5, as the patient maintained psychiatric stability, the team discussed with Ms. S and her husband the prospect of direct breastfeeding. The treatment team arranged for separate visitation hours to minimize the possibility of exposing the infant to aggression from other patients on the unit and advocated with hospital leadership to approve of infant visitation on the unit.
Impact of involvement of Child Protective Services
The involvement of CPS also added complexity to Ms. C’s case. Without proper legal guidance, mothers with psychosis who lose custody can find it difficult to navigate the legal system and maintain contact with their children.34 As the prevalence of custody loss in mothers with psychosis is high (approximately 50% according to research published in the last 10 years), effective interventions to reunite the mother and child must be promoted (Figure 2).35-39 Ultimately, the goal of psychiatric hospitalization for perinatal women who have SMI is psychiatric stabilization. The preemptive involvement of psychiatry is crucial because it can allow for early postpartum planning and can provide an opportunity to address feeding options and custody concerns with the patient, social supports and services, and various medical teams. In Ms. C’s case, she visited her baby in the NICU on postpartum Day 2 without consultation with psychiatry or CPS, which posed risks to the patient, infant, and staff. It is vital that various clinicians collaborate with each other and the patient, working towards the goal of optimizing the patient’s mental health to allow for parenting rights in the future and maximizing a sustainable attachment between the parent and child. In Ms. S’s case, the husband was able to facilitate caring for the baby while the mother was hospitalized and played an integral role in the feeding process via pumped breastmilk and transport of the infant for direct breastfeeding.

Continue to: The differences in these 2 cases...
The differences in these 2 cases show the extreme importance of social support to benefit both the mother and child, and the need for more comprehensive social services for women who do not have a social safety net.
Bottom Line
These complex cases highlight an ethical decision-making approach to breastfeeding in perinatal women who have serious mental illness. Collaborative care and shared decision-making, which highlight the interests of the mother and baby, are crucial when assessing the risks and benefits of breastfeeding and pumping breastmilk. Our relational ethics framework can be used to better evaluate and implement breastfeeding options on general psychiatric units.
Related Resources
- Tillman B, Sloan N, Westmoreland P. How COVID-19 affects peripartum women’s mental health. Current Psychiatry. 2021;20(6):18-22. doi:10.12788/cp.0129
- Koch J, Preinitz J. Antidepressants for patients who are breastfeeding: what to consider. Current Psychiatry. 2023;22(5):20-23,48. doi:10.12788/cp.0355
Drug Brand Names
Aripiprazole • Abilify
Olanzapine • Zyprexa
Risperidone • Risperdal
Difficult ethical situations can arise when treating perinatal women who have serious mental illness (SMI). Clinicians must consider ethical issues related to administering antipsychotic medications, the safety of breastfeeding, and concerns for child welfare. They need to carefully weigh the risks and benefits of each decision when treating perinatal women who have SMI. Ethical guidelines can help clinicians best support families in these situations.
In this article, we describe 2 cases of women with psychotic disorders who requested to breastfeed after delivering their child during an inpatient psychiatric hospitalization. The course of their hospitalizations illustrated common ethical questions and facilitated the creation of a framework to assist with complex decision-making regarding breastfeeding on inpatient psychiatric units.
CASE 1
Ms. C, age 41, is multigravida with a psychiatric history of chronic, severe schizoaffective disorder and lives in supportive housing. When Ms. C presents to the hospital in search of a rape kit, clinicians discover she is 22 weeks pregnant but has not received any prenatal care. Psychiatry is consulted because she is found to be intermittently agitated and endorses grandiose delusions. Ms. C requires involuntary hospitalization for decompensated psychosis because she refuses prenatal and psychiatric care. Because it has reassuring reproductive safety data,1 olanzapine 5 mg/d is started. However, Ms. C experiences minimal improvement from a maximum dose of 20 mg/d. After 13 weeks on the psychiatry unit, she is transferred to obstetrics service for preeclampsia with severe features. Ms. C requires an urgent cesarean delivery at 37 weeks. Her baby boy is transferred to the neonatal intensive care unit (NICU) for transient tachypnea. After delivery and in consultation with psychiatry, the pediatrics team calls Child Protective Services (CPS) due to concern for neglect driven by Ms. C’s psychiatric condition. Ms. C visits the child with medical unit staff supervision in the NICU without consulting with the psychiatry service or CPS. On postpartum Day 2, Ms. C is transferred back to psychiatry for persistent psychosis.
On postpartum Day 3, Ms. C starts to produce breastmilk and requests to breastfeed. At this time, the multidisciplinary team determines she is not able to visit her child in the NICU due to psychiatric instability. No plan is developed to facilitate hand expression or pumping of breastmilk while Ms. C is on the psychiatric unit. The clinical teams discuss whether the benefits of breastfeeding and/or pumping breastmilk would outweigh the risks. CPS determines that Ms. C is unable to retain custody and places the child in kinship foster care while awaiting clinical improvement from her.
CASE 2
Ms. S, age 32, has a history of schizophrenia. She lives with her husband and parents. She is pregnant for the first time and has been receiving consistent prenatal care. Ms. S is brought to the hospital by her husband for bizarre behavior and paranoia after self-discontinuing risperidone 2 mg twice daily due to concern about the medication’s influence on her pregnancy. An ultrasound confirms she is 37 weeks pregnant. Psychiatry is consulted because Ms. S is internally preoccupied, delusional, and endorses auditory hallucinations. She requires involuntary hospitalization for decompensated psychosis. During admission, Ms. S experiences improvement of her psychiatric symptoms while receiving risperidone 2 mg twice daily, which she takes consistently after receiving extensive psychoeducation regarding its safety profile during pregnancy and lactation.
After 2 weeks on the psychiatry unit, Ms. S’s care team transfers her to the obstetrics service with one-to-one supervision. At 39 weeks gestation, she has a vaginal delivery without complications. Because there are no concerns about infant harm, obstetrics, pediatrics, and psychiatry coordinate care so the baby can room in with Ms. S, her husband, and a staff supervisor to facilitate bonding. Ms. S starts to lactate, wishes to breastfeed, and meets with lactation, pediatric, obstetric, and psychiatric specialists to discuss the risks and benefits of breastfeeding and pumping breastmilk. She pursues direct breastfeeding until the baby is discharged home with the husband at postpartum Day 2. CPS is not called because there are no concerns for parental abuse or neglect at the infant’s discharge.
On postpartum Day 2, the obstetrics service transfers Ms. S back to the psychiatric unit for further treatment of her paranoia. She wishes to pump breastmilk while hospitalized, so the treatment team supplies a breast pump, facilitates the storage of breastmilk, and coordinates supervision during pumping to reduce the ligature risk. Ms. S’s husband visits daily to transport the milk and feed the infant breastmilk and formula to meet its nutritional needs. Ms. S maintains psychiatric stability while breast pumping, and the team helps transition her to breastfeeding during visitation with her husband and infant until she is discharged home at 2 weeks postpartum.
Continue to: Approaching care with a relational ethics framework
Approaching care with a relational ethics framework
A relational ethics framework was constructed to evaluate whether to support breastfeeding for both patients during their psychiatric hospitalizations. A relational ethics perspective is defined as “a moral responsibility within a context of human relations” [that] “recognizes the human interdependency and reciprocity within which personal autonomy is embedded.”2 This framework values connectedness and commonality between various and even conflicting parties. In the setting of a clinician-patient relationship, health care decisions are made with consideration of the patient’s traditional beliefs, values, and principles rather than the application of impartial moral principles. For these complex cases, this framework was chosen to determine the safest possible outcome for both mother and child.
Risks/benefits of breastfeeding by patients who have SMI
There are several methods of breastfeeding, including direct breastfeeding and other ways of expressing breastmilk such as pumping or hand expression.3 Unlike other forms of feeding using breastmilk, direct breastfeeding has been extensively studied, has well-established medical and psychological benefits for newborns and mothers, and enhances long-term bonding.4 Compared with their counterparts who do not breastfeed, mothers who breastfeed have lower rates of unintended pregnancy, cardiovascular disease, postpartum bleeding, osteoporosis, and breast and ovarian cancer.5 Among its key psychological benefits, breastfeeding is associated with an increase in maternal self-efficacy and, in some research, has been shown to be associated with a decreased risk of postpartum depression and stress. Additionally, breastfed infants experience lower rates of childhood infection and obesity, and improved nutrition, cognitive development, and immune function.6 The American Academy of Pediatrics recognizes these benefits and recommends that women exclusively breastfeed for 6 months postpartum and continue to breastfeed for 2 years or beyond if mutually desired by the mother and child.7 Absolute contraindications to breastfeeding must be ruled out (eg, infant classic galactosemia; maternal use of illicit substances such as cocaine, opioids, or phencyclidine; maternal HIV infection, etc).
The risks of breastfeeding by patients who have SMI must also be considered. In severe situations, the infant can be exposed to a mother’s agitation secondary to psychosis.8,9 The transmission of antipsychotic medication through breastmilk and associated adverse effects (eg, sedation, poor feeding, and extrapyramidal symptoms) are also potential risks and varies among different antipsychotic medications.1,10 Therefore, when prescribing an antipsychotic for a patient with SMI who breastfeeds, it is crucial to consider the medication’s safety profile as well as other factors, such as the relative infant dose (the weight-adjusted [ie, mg/kg] percentage of the maternal dosage ingested by a fully breastfed infant) and the molecular characteristics of the medication.10-12 Neonates should be routinely monitored for adverse effects, medication toxicity, and withdrawal symptoms, and care should be coordinated with the infant’s pediatrician. Certain antipsychotic medications, such as aripiprazole, may impact breastmilk production through the dopamine agonist’s interference of the prolactin reflex and anticholinergic properties.11,13 For a patient with SMI, perhaps the most significant risk involves the time and resources needed for breastfeeding, which can interfere with sleep and psychiatric treatment and possibly further exacerbate psychiatric symptoms.14-16 Additionally, breastfeeding difficulties or disruption can increase the risk of psychiatric symptoms and psychological distress.17 In Ms. C’s case, there was a delay in the baby latching as well as multiple medical and psychiatric factors that hindered the milk-ejection reflex to properly initiate; both of these factors rendered breastfeeding particularly difficult while Ms. C was on the inpatient psychiatry unit.17 In comparison, Ms. S was able to bond with her infant shortly after delivery, which facilitated the milk-ejection reflex and lactation.
Patients who wish to directly breastfeed but struggle to do so while tending to their acute psychiatric condition can benefit from expression of breastmilk that can be provided to the infant or discarded to facilitate breastfeeding in the future.18 While expression of breastmilk may not be as advantageous for infant health as direct breastfeeding due to the potential changes in breastmilk composition from collecting, storing, and heating, this option can be more protective than formula feeding and facilitate future breastfeeding.19 In these clinical scenarios, it is standard care to provide a hospital-grade breast pump to the patient, much like a continuous positive airway pressure machine is provided to patients with obstructive sleep apnea.20 However, there is often considerable difficulty obtaining proper breastfeeding equipment and a lack of services devoted to perinatal care in general inpatient settings. Barriers to direct breastfeeding and pumping of breastmilk are highlighted in the Table.21

Limitations on breastfeeding on an inpatient unit
The limitations in care and restrictions placed on breastfeeding are more optimally addressed in a mother and baby unit (MBU). MBUs are specialized inpatient psychiatric units designed for mothers experiencing severe perinatal psychiatric difficulties. Unlike general psychiatric units, MBUs allow for joint, full-time admission of mothers and their infants. These units also include multidisciplinary staff who specialize in treating perinatal mental health issues as well as infant care and child development.22 Admission into an MBU is considered best practice for new mothers requiring treatment, particularly in the United Kingdom, Australia, and France, as it is well-recognized that the separation of mother and baby can be psychologically harmful.23 In the UK, most patients admitted to an MBU showed significant improvement of their psychiatric symptoms and reported overall high satisfaction with care.24,25 Patients who experience postpartum psychosis prefer MBUs over general psychiatric units because the latter often lack specialized perinatal support, appropriate visitor arrangements, and adequate time with their infant.26-28
Continue to: The resistance to adopting MBUs in the United States...
The resistance to adopting MBUs in the United States has posed significant barriers in care for perinatal patients and has been attributed to financial barriers, medicolegal risk, staffing, and safety concerns.29 Though currently there are no MBUs in the US, other specialized units have been created. A partial day hospitalization program created in 2000 in Rhode Island for mothers and infants revolutionized the psychiatric care experience for new mothers.30 Since then, other institutions have significantly expanded their services to include perinatal psychiatry inpatient units, yet unlike MBUs, these units typically do not provide overnight rooming-in with infants.31 They have the necessary resources and facilities to accommodate the mother’s needs and maximize positive mother-infant interaction, while actively integrating the infant into the mother’s treatment. Breast pumping is treated as a necessary medical procedure and patients can easily access hospital-grade breast pumps with staff supervision. At one such perinatal psychiatric inpatient unit, high rates of treatment satisfaction and significant improvements in symptoms of depression, anxiety, active suicidal ideation, and overall functioning were observed at discharge.32 Therefore, it is crucial to incorporate strategies in general psychiatry units to improve perinatal care, acknowledging that most patients will not have access to these specialized units.21
A framework to approaching the relational ethics decisions
An interdisciplinary team used a relational ethics perspective to carefully analyze the risks and benefits of these complex cases. In Figure 1, we propose a framework for the relational ethics decisions of breastfeeding on general inpatient psychiatric units. In creating this framework, we considered principles of autonomy, beneficence, and nonmaleficence, along with the medical and logistical barriers to breastfeeding.

In Ms. C’s case, the team determined that the risks—which included disrupting the mother’s psychiatric treatment, exposing her to psychological harm due to increasing attachment before remanding the child to CPS custody, and risks to the child due to potential unpredictable agitation driven by the treatment-refractory psychosis of the mother as well as that of other psychiatric patients—outweighed the benefits of breastfeeding. We instead recommended breast pumping as an alternative once Ms. C’s psychiatric stability improved. We presented Ms. C with the option of breast pumping on postpartum Day 5. During a 1-day period in which she showed improved behavioral control, she was counseled on the risks and benefits of breastfeeding and exclusive pumping and was notified that the team would help her with the necessary resources, including consultation with a lactation specialist and breast pump. Despite lactation consultant support, Ms. C had low milk production and difficulty with hand expression, which was very discouraging to her. She produced 1 ounce of milk that was shared with the newborn while in the NICU. Because Ms. C’s psychiatric symptoms continued to be severe, with lability and aggression, and because pumping was triggering distress, the multidisciplinary team determined the best course of care would be to focus on her psychiatric recovery rather than on pumping breastmilk. To reduce milk production and minimize discomfort secondary to breast engorgement, the lactation consultant recommended cold compresses, pain management, and compression of breasts. Ultimately, the mother-infant dyad was unable to reap the benefits of breastfeeding (via pumping or direct breastfeeding) due to the mother’s underlying psychiatric illness, although the staffing, psychosocial support, and logistical limitations contributed to this outcome.33
In Ms. S’s case, the treatment team determined that there were no medical or psychiatric contraindications to breastfeeding, and she was counseled on the risks and benefits of direct breastfeeding and pumping. The treatment team determined it was safe for Ms. S to directly breastfeed as there were no concerns for infant harm postdelivery with constant supervision while on the obstetrics floor. The patient opted to directly breastfeed, which was successful with the guidance of a lactation specialist. When she was transferred to the psychiatric unit on postpartum Day 2, her child was discharged home with the husband. The patient was then encouraged to pump while the psychiatrists monitored her symptoms closely and facilitated increased staff and resources. Transportation of breastmilk was made possible by the family, and on postpartum Day 5, as the patient maintained psychiatric stability, the team discussed with Ms. S and her husband the prospect of direct breastfeeding. The treatment team arranged for separate visitation hours to minimize the possibility of exposing the infant to aggression from other patients on the unit and advocated with hospital leadership to approve of infant visitation on the unit.
Impact of involvement of Child Protective Services
The involvement of CPS also added complexity to Ms. C’s case. Without proper legal guidance, mothers with psychosis who lose custody can find it difficult to navigate the legal system and maintain contact with their children.34 As the prevalence of custody loss in mothers with psychosis is high (approximately 50% according to research published in the last 10 years), effective interventions to reunite the mother and child must be promoted (Figure 2).35-39 Ultimately, the goal of psychiatric hospitalization for perinatal women who have SMI is psychiatric stabilization. The preemptive involvement of psychiatry is crucial because it can allow for early postpartum planning and can provide an opportunity to address feeding options and custody concerns with the patient, social supports and services, and various medical teams. In Ms. C’s case, she visited her baby in the NICU on postpartum Day 2 without consultation with psychiatry or CPS, which posed risks to the patient, infant, and staff. It is vital that various clinicians collaborate with each other and the patient, working towards the goal of optimizing the patient’s mental health to allow for parenting rights in the future and maximizing a sustainable attachment between the parent and child. In Ms. S’s case, the husband was able to facilitate caring for the baby while the mother was hospitalized and played an integral role in the feeding process via pumped breastmilk and transport of the infant for direct breastfeeding.

Continue to: The differences in these 2 cases...
The differences in these 2 cases show the extreme importance of social support to benefit both the mother and child, and the need for more comprehensive social services for women who do not have a social safety net.
Bottom Line
These complex cases highlight an ethical decision-making approach to breastfeeding in perinatal women who have serious mental illness. Collaborative care and shared decision-making, which highlight the interests of the mother and baby, are crucial when assessing the risks and benefits of breastfeeding and pumping breastmilk. Our relational ethics framework can be used to better evaluate and implement breastfeeding options on general psychiatric units.
Related Resources
- Tillman B, Sloan N, Westmoreland P. How COVID-19 affects peripartum women’s mental health. Current Psychiatry. 2021;20(6):18-22. doi:10.12788/cp.0129
- Koch J, Preinitz J. Antidepressants for patients who are breastfeeding: what to consider. Current Psychiatry. 2023;22(5):20-23,48. doi:10.12788/cp.0355
Drug Brand Names
Aripiprazole • Abilify
Olanzapine • Zyprexa
Risperidone • Risperdal
1. Brunner E, Falk DM, Jones M, et al. Olanzapine in pregnancy and breastfeeding: a review of data from global safety surveillance. BMC Pharmacol Toxicol. 2013;14:38. doi:10.1186/2050-6511-14-38
2. Seeman MV. Relational ethics: when mothers suffer from psychosis. Arch Womens Ment Health. 2004;7(3):201-210. doi:10.1007/s00737-004-0054-8
3. Motee A, Jeewon R. Importance of exclusive breastfeeding and complementary feeding among infants. Curr Res Nutr Food Sci. 2014;2(2). doi:10.12944/CRNFSJ.2.2.02
4. Committee Opinion No. 570: breastfeeding in underserved women: increasing initiation and continuation of breastfeeding. Obstet Gynecol. 2013;122(2 Pt 1):423-427. doi:10.1097/01.AOG.0000433008.93971.6a
5. Sibolboro Mezzacappa E, Endicott J. Parity mediates the association between infant feeding method and maternal depressive symptoms in the postpartum. Arch Womens Ment Health. 2007;10(6):259-266. doi:10.1007/s00737-007-0207-7
6. Kramer MS, Chalmers B, Hodnett ED, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285(4):413-420. doi:10.1001/jama.285.4.413
7. American Academy of Pediatrics. American Academy of Pediatrics calls for more support for breastfeeding mothers within updated policy recommendations. June 27, 2022. Accessed October 4, 2022. https://www.aap.org/en/news-room/news-releases/aap/2022/american-academy-of-pediatrics-calls-for-more-support-for-breastfeeding-mothers-within-updated-policy-recommendations
8. Hipwell AE, Kumar R. Maternal psychopathology and prediction of outcome based on mother-infant interaction ratings (BMIS). Br J Psychiatry. 1996;169(5):655-661. doi:10.1192/bjp.169.5.655
9. Chandra PS, Bhargavaraman RP, Raghunandan VN, et al. Delusions related to infant and their association with mother-infant interactions in postpartum psychotic disorders. Arch Womens Ment Health. 2006;9(5):285-288. doi:10.1007/s00737-006-0147-7
10. Klinger G, Stahl B, Fusar-Poli P, et al. Antipsychotic drugs and breastfeeding. Pediatr Endocrinol Rev. 2013;10(3):308-317.
11. Uguz F. A new safety scoring system for the use of psychotropic drugs during lactation. Am J Ther. 2021;28(1):e118-e126. doi:10.1097/MJT.0000000000000909
12. Hale TW, Krutsch K. Hale’s Medications & Mothers’ Milk, 2023: A Manual of Lactational Pharmacology. 20th ed. Springer Publishing Company; 2023.
13. Komaroff A. Aripiprazole and lactation failure: the importance of shared decision making. A case report. Case Rep Womens Health. 2021;30:e00308. doi:10.1016/j.crwh.2021.e00308
14. Dennis CL, McQueen K. Does maternal postpartum depressive symptomatology influence infant feeding outcomes? Acta Pediatr. 2007;96(4):590-594. doi:10.1111/j.1651-2227.2007.00184.x
15. Chaput KH, Nettel-Aguirre A, Musto R, et al. Breastfeeding difficulties and supports and risk of postpartum depression in a cohort of women who have given birth in Calgary: a prospective cohort study. CMAJ Open. 2016;4(1):E103-E109. doi:10.9778/cmajo.20150009
16. Dias CC, Figueiredo B. Breastfeeding and depression: a systematic review of the literature. J Affect Disord. 2015;171:142-154. doi:10.1016/j.jad.2014.09.022
17. Brown A, Rance J, Bennett P. Understanding the relationship between breastfeeding and postnatal depression: the role of pain and physical difficulties. J Adv Nurs. 2016;72(2):273-282. doi:10.1111/jan.12832
18. Rosenbaum KA. Exclusive breastmilk pumping: a concept analysis. Nurs Forum. 2022;57(5):946-953. doi:10.1111/nuf.12766
19. Boone KM, Geraghty SR, Keim SA. Feeding at the breast and expressed milk feeding: associations with otitis media and diarrhea in infants. J Pediatr. 2016;174:118-125. doi:10.1016/j.jpeds.2016.04.006
20. Epstein LJ, Kristo D, Strollo PJ Jr, et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263-276.
21. Caan MP, Sreshta NE, Okwerekwu JA, et al. Clinical and legal considerations regarding breastfeeding on psychiatric units. J Am Acad Psychiatry Law. 2022;50(2):200-207. doi:10.29158/JAAPL.210086-21
22. Glangeaud-Freudenthal NMC, Rainelli C, Cazas O, et al. Inpatient mother and baby psychiatric units (MBUs) and day cares. In: Sutter-Dallay AL, Glangeaud-Freudenthal NC, Guedeney A, et al, eds. Joint Care of Parents and Infants in Perinatal Psychiatry. Springer, Cham; 2016:147-164. doi:10.1007/978-3-319-21557-0_10
23. Dembosky A. A humane approach to caring for new mothers in psychiatric crisis. Health Aff (Millwood). 2021;40(10):1528-1533. doi:10.1377/hlthaff.2021.01288
24. Connellan K, Bartholomaeus C, Due C, et al. A systematic review of research on psychiatric mother-baby units. Arch Womens Ment Health. 2017;20(3):373-388. doi:10.1007/s00737-017-0718-9
25. Griffiths J, Lever Taylor B, Morant N, et al. A qualitative comparison of experiences of specialist mother and baby units versus general psychiatric wards. BMC Psychiatry. 2019;19(1):401. doi:10.1186/s12888-019-2389-8
26. Heron J, Gilbert N, Dolman C, et al. Information and support needs during recovery from postpartum psychosis. Arch Womens Ment Health. 2012;15(3):155-165. doi:10.1007/s00737-012-0267-1
27. Robertson E, Lyons A. Living with puerperal psychosis: a qualitative analysis. Psychol Psychother. 2003;76(Pt 4):411-431. doi:10.1348/147608303770584755
28. Mental Welfare Commission for Scotland. Perinatal Themed Visit Report: Keeping Mothers and Babies in Mind. Mental Welfare Commission for Scotland; 2016.
29. Wisner KL, Jennings KD, Conley B. Clinical dilemmas due to the lack of inpatient mother-baby units. Int J Psychiatry Med. 1996;26(4):479-493. doi:10.2190/NFJK-A4V7-CXUU-AM89
30. Battle CL, Howard MM. A mother-baby psychiatric day hospital: history, rationale, and why perinatal mental health is important for obstetric medicine. Obstet Med. 2014;7(2):66-70. doi:10.1177/1753495X13514402
31. Bullard ES, Meltzer-Brody S, Rubinow DR. The need for comprehensive psychiatric perinatal care-the University of North Carolina at Chapel Hill, Department of Psychiatry, Center for Women’s Mood Disorders launches the first dedicated inpatient program in the United States. Am J Obstet Gynecol. 2009;201(5):e10-e11. doi:10.1016/j.ajog.2009.05.004
32. Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health. 2014;17(2):107-113. doi:10.1007/s00737-013-0390-7
33. Alvarez-Toro V. Gender-specific care for women in psychiatric units. J Am Acad Psychiatry Law. 2022;JAAPL.220015-21. doi:10.29158/JAAPL.220015-21
34. Diaz-Caneja A, Johnson S. The views and experiences of severely mentally ill mothers--a qualitative study. Soc Psychiatry Psychiatr Epidemiol. 2004;39(6):472-482. doi:10.1007/s00127-004-0772-2
35. Gewurtz R, Krupa T, Eastabrook S, et al. Prevalence and characteristics of parenting among people served by assertive community treatment. Psychiatr Rehabil J. 2004;28(1):63-65. doi:10.2975/28.2004.63.65
36. Howard LM, Kumar R, Thornicroft G. Psychosocial characteristics and needs of mothers with psychotic disorders. Br J Psychiatry. 2001;178:427-432. doi:10.1192/bjp.178.5.427
37. Hollingsworth LD. Child custody loss among women with persistent severe mental illness. Social Work Research. 2004;28(4):199-209. doi:10.1093/swr/28.4.199
38. Dipple H, Smith S, Andrews H, et al. The experience of motherhood in women with severe and enduring mental illness. Soc Psychiatry Psychiatr Epidemiolf. 2002;37(7):336-340. doi:10.1007/s00127-002-0559-2
39. Seeman MV. Intervention to prevent child custody loss in mothers with schizophrenia. Schizophr Res Treatment. 2012;2012:796763. doi:10.1155/2012/796763
1. Brunner E, Falk DM, Jones M, et al. Olanzapine in pregnancy and breastfeeding: a review of data from global safety surveillance. BMC Pharmacol Toxicol. 2013;14:38. doi:10.1186/2050-6511-14-38
2. Seeman MV. Relational ethics: when mothers suffer from psychosis. Arch Womens Ment Health. 2004;7(3):201-210. doi:10.1007/s00737-004-0054-8
3. Motee A, Jeewon R. Importance of exclusive breastfeeding and complementary feeding among infants. Curr Res Nutr Food Sci. 2014;2(2). doi:10.12944/CRNFSJ.2.2.02
4. Committee Opinion No. 570: breastfeeding in underserved women: increasing initiation and continuation of breastfeeding. Obstet Gynecol. 2013;122(2 Pt 1):423-427. doi:10.1097/01.AOG.0000433008.93971.6a
5. Sibolboro Mezzacappa E, Endicott J. Parity mediates the association between infant feeding method and maternal depressive symptoms in the postpartum. Arch Womens Ment Health. 2007;10(6):259-266. doi:10.1007/s00737-007-0207-7
6. Kramer MS, Chalmers B, Hodnett ED, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285(4):413-420. doi:10.1001/jama.285.4.413
7. American Academy of Pediatrics. American Academy of Pediatrics calls for more support for breastfeeding mothers within updated policy recommendations. June 27, 2022. Accessed October 4, 2022. https://www.aap.org/en/news-room/news-releases/aap/2022/american-academy-of-pediatrics-calls-for-more-support-for-breastfeeding-mothers-within-updated-policy-recommendations
8. Hipwell AE, Kumar R. Maternal psychopathology and prediction of outcome based on mother-infant interaction ratings (BMIS). Br J Psychiatry. 1996;169(5):655-661. doi:10.1192/bjp.169.5.655
9. Chandra PS, Bhargavaraman RP, Raghunandan VN, et al. Delusions related to infant and their association with mother-infant interactions in postpartum psychotic disorders. Arch Womens Ment Health. 2006;9(5):285-288. doi:10.1007/s00737-006-0147-7
10. Klinger G, Stahl B, Fusar-Poli P, et al. Antipsychotic drugs and breastfeeding. Pediatr Endocrinol Rev. 2013;10(3):308-317.
11. Uguz F. A new safety scoring system for the use of psychotropic drugs during lactation. Am J Ther. 2021;28(1):e118-e126. doi:10.1097/MJT.0000000000000909
12. Hale TW, Krutsch K. Hale’s Medications & Mothers’ Milk, 2023: A Manual of Lactational Pharmacology. 20th ed. Springer Publishing Company; 2023.
13. Komaroff A. Aripiprazole and lactation failure: the importance of shared decision making. A case report. Case Rep Womens Health. 2021;30:e00308. doi:10.1016/j.crwh.2021.e00308
14. Dennis CL, McQueen K. Does maternal postpartum depressive symptomatology influence infant feeding outcomes? Acta Pediatr. 2007;96(4):590-594. doi:10.1111/j.1651-2227.2007.00184.x
15. Chaput KH, Nettel-Aguirre A, Musto R, et al. Breastfeeding difficulties and supports and risk of postpartum depression in a cohort of women who have given birth in Calgary: a prospective cohort study. CMAJ Open. 2016;4(1):E103-E109. doi:10.9778/cmajo.20150009
16. Dias CC, Figueiredo B. Breastfeeding and depression: a systematic review of the literature. J Affect Disord. 2015;171:142-154. doi:10.1016/j.jad.2014.09.022
17. Brown A, Rance J, Bennett P. Understanding the relationship between breastfeeding and postnatal depression: the role of pain and physical difficulties. J Adv Nurs. 2016;72(2):273-282. doi:10.1111/jan.12832
18. Rosenbaum KA. Exclusive breastmilk pumping: a concept analysis. Nurs Forum. 2022;57(5):946-953. doi:10.1111/nuf.12766
19. Boone KM, Geraghty SR, Keim SA. Feeding at the breast and expressed milk feeding: associations with otitis media and diarrhea in infants. J Pediatr. 2016;174:118-125. doi:10.1016/j.jpeds.2016.04.006
20. Epstein LJ, Kristo D, Strollo PJ Jr, et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263-276.
21. Caan MP, Sreshta NE, Okwerekwu JA, et al. Clinical and legal considerations regarding breastfeeding on psychiatric units. J Am Acad Psychiatry Law. 2022;50(2):200-207. doi:10.29158/JAAPL.210086-21
22. Glangeaud-Freudenthal NMC, Rainelli C, Cazas O, et al. Inpatient mother and baby psychiatric units (MBUs) and day cares. In: Sutter-Dallay AL, Glangeaud-Freudenthal NC, Guedeney A, et al, eds. Joint Care of Parents and Infants in Perinatal Psychiatry. Springer, Cham; 2016:147-164. doi:10.1007/978-3-319-21557-0_10
23. Dembosky A. A humane approach to caring for new mothers in psychiatric crisis. Health Aff (Millwood). 2021;40(10):1528-1533. doi:10.1377/hlthaff.2021.01288
24. Connellan K, Bartholomaeus C, Due C, et al. A systematic review of research on psychiatric mother-baby units. Arch Womens Ment Health. 2017;20(3):373-388. doi:10.1007/s00737-017-0718-9
25. Griffiths J, Lever Taylor B, Morant N, et al. A qualitative comparison of experiences of specialist mother and baby units versus general psychiatric wards. BMC Psychiatry. 2019;19(1):401. doi:10.1186/s12888-019-2389-8
26. Heron J, Gilbert N, Dolman C, et al. Information and support needs during recovery from postpartum psychosis. Arch Womens Ment Health. 2012;15(3):155-165. doi:10.1007/s00737-012-0267-1
27. Robertson E, Lyons A. Living with puerperal psychosis: a qualitative analysis. Psychol Psychother. 2003;76(Pt 4):411-431. doi:10.1348/147608303770584755
28. Mental Welfare Commission for Scotland. Perinatal Themed Visit Report: Keeping Mothers and Babies in Mind. Mental Welfare Commission for Scotland; 2016.
29. Wisner KL, Jennings KD, Conley B. Clinical dilemmas due to the lack of inpatient mother-baby units. Int J Psychiatry Med. 1996;26(4):479-493. doi:10.2190/NFJK-A4V7-CXUU-AM89
30. Battle CL, Howard MM. A mother-baby psychiatric day hospital: history, rationale, and why perinatal mental health is important for obstetric medicine. Obstet Med. 2014;7(2):66-70. doi:10.1177/1753495X13514402
31. Bullard ES, Meltzer-Brody S, Rubinow DR. The need for comprehensive psychiatric perinatal care-the University of North Carolina at Chapel Hill, Department of Psychiatry, Center for Women’s Mood Disorders launches the first dedicated inpatient program in the United States. Am J Obstet Gynecol. 2009;201(5):e10-e11. doi:10.1016/j.ajog.2009.05.004
32. Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health. 2014;17(2):107-113. doi:10.1007/s00737-013-0390-7
33. Alvarez-Toro V. Gender-specific care for women in psychiatric units. J Am Acad Psychiatry Law. 2022;JAAPL.220015-21. doi:10.29158/JAAPL.220015-21
34. Diaz-Caneja A, Johnson S. The views and experiences of severely mentally ill mothers--a qualitative study. Soc Psychiatry Psychiatr Epidemiol. 2004;39(6):472-482. doi:10.1007/s00127-004-0772-2
35. Gewurtz R, Krupa T, Eastabrook S, et al. Prevalence and characteristics of parenting among people served by assertive community treatment. Psychiatr Rehabil J. 2004;28(1):63-65. doi:10.2975/28.2004.63.65
36. Howard LM, Kumar R, Thornicroft G. Psychosocial characteristics and needs of mothers with psychotic disorders. Br J Psychiatry. 2001;178:427-432. doi:10.1192/bjp.178.5.427
37. Hollingsworth LD. Child custody loss among women with persistent severe mental illness. Social Work Research. 2004;28(4):199-209. doi:10.1093/swr/28.4.199
38. Dipple H, Smith S, Andrews H, et al. The experience of motherhood in women with severe and enduring mental illness. Soc Psychiatry Psychiatr Epidemiolf. 2002;37(7):336-340. doi:10.1007/s00127-002-0559-2
39. Seeman MV. Intervention to prevent child custody loss in mothers with schizophrenia. Schizophr Res Treatment. 2012;2012:796763. doi:10.1155/2012/796763
Excessive TV-watching tied to elevated risk for dementia, Parkinson’s disease, and depression
TOPLINE:
whereas a limited amount of daily computer use that is not work-related is linked to a lower risk for dementia.
METHODOLOGY:
- Investigators analyzed data on 473,184 people aged 39-72 years from the UK Biobank who were enrolled from 2006 to 2010 and followed until a diagnosis of dementia, PD, depression, death, or study end (2018 for Wales residents; 2021 for residents of England and Scotland).
- Participants reported on the number of hours they spent outside of work exercising, watching television, and using the computer.
- MRI was conducted to determine participants’ brain volume.
TAKEAWAY:
- During the study, 6096 people developed dementia, 3000 developed PD, 23,600 developed depression, 1200 developed dementia and depression, and 486 developed PD and depression.
- Compared with those who watched TV for under 1 hour per day, those who reported watching 4 or more hours per day had a 28% higher risk for dementia (adjusted hazard ratio [aHR], 1.28; 95% CI, 1.17-1.39), a 35% higher risk for depression, (aHR, 1.35; 95% CI, 1.29-1.40) and a 16% greater risk for PD (aHR, 1.16; 95% CI, 1.03-1.29).
- However, moderate computer use outside of work seemed somewhat protective. Participants who used the computer for 30-60 minutes per day had lower risks for dementia (aHR, 0.68; 95% CI, 0.64-0.72), PD, (aHR, 0.86; 95% CI, 0.79-0.93), and depression (aHR, 0.85; 95% CI, 0.83-0.88) compared with those who reported the lowest levels of computer usage.
- Replacing 30 minutes per day of computer time with an equal amount of structured exercise was associated with decreased risk for dementia (aHR, 0.74; 95% CI, 0.85-0.95) and PD (aHR, 0.84; 95% CI, 0.78-0.90).
IN PRACTICE:
The association between extended periods of TV use and higher risk for PD and dementia could be explained by a lack of activity, the authors note. They add that sedentary behavior is, “associated with biomarkers of low-grade inflammation and changes in inflammation markers that could initiate and or worsen neuroinflammation and contribute to neurodegeneration.”
SOURCE:
Hanzhang Wu, PhD, of Tianjin University of Traditional Medicine in Tianjin, China, led the study, which was published online in the International Journal of Behavioral Nutrition and Physical Activity.
LIMITATIONS:
Screen behaviors were assessed using self-report measures, which is subject to recall bias. Also, there may have been variables confounding the findings for which investigators did not account.
DISCLOSURES:
The study was funded by the National Natural Science Foundation of China, the Tianjin Major Public Health Science and Technology Project, the National Health Commission of China, the Food Science and Technology Foundation of Chinese Institute of Food Science and Technology, the China Cohort Consortium, and the Chinese Nutrition Society Nutrition Research Foundation–DSM Research Fund, China. There were no disclosures reported.
Eve Bender has no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
whereas a limited amount of daily computer use that is not work-related is linked to a lower risk for dementia.
METHODOLOGY:
- Investigators analyzed data on 473,184 people aged 39-72 years from the UK Biobank who were enrolled from 2006 to 2010 and followed until a diagnosis of dementia, PD, depression, death, or study end (2018 for Wales residents; 2021 for residents of England and Scotland).
- Participants reported on the number of hours they spent outside of work exercising, watching television, and using the computer.
- MRI was conducted to determine participants’ brain volume.
TAKEAWAY:
- During the study, 6096 people developed dementia, 3000 developed PD, 23,600 developed depression, 1200 developed dementia and depression, and 486 developed PD and depression.
- Compared with those who watched TV for under 1 hour per day, those who reported watching 4 or more hours per day had a 28% higher risk for dementia (adjusted hazard ratio [aHR], 1.28; 95% CI, 1.17-1.39), a 35% higher risk for depression, (aHR, 1.35; 95% CI, 1.29-1.40) and a 16% greater risk for PD (aHR, 1.16; 95% CI, 1.03-1.29).
- However, moderate computer use outside of work seemed somewhat protective. Participants who used the computer for 30-60 minutes per day had lower risks for dementia (aHR, 0.68; 95% CI, 0.64-0.72), PD, (aHR, 0.86; 95% CI, 0.79-0.93), and depression (aHR, 0.85; 95% CI, 0.83-0.88) compared with those who reported the lowest levels of computer usage.
- Replacing 30 minutes per day of computer time with an equal amount of structured exercise was associated with decreased risk for dementia (aHR, 0.74; 95% CI, 0.85-0.95) and PD (aHR, 0.84; 95% CI, 0.78-0.90).
IN PRACTICE:
The association between extended periods of TV use and higher risk for PD and dementia could be explained by a lack of activity, the authors note. They add that sedentary behavior is, “associated with biomarkers of low-grade inflammation and changes in inflammation markers that could initiate and or worsen neuroinflammation and contribute to neurodegeneration.”
SOURCE:
Hanzhang Wu, PhD, of Tianjin University of Traditional Medicine in Tianjin, China, led the study, which was published online in the International Journal of Behavioral Nutrition and Physical Activity.
LIMITATIONS:
Screen behaviors were assessed using self-report measures, which is subject to recall bias. Also, there may have been variables confounding the findings for which investigators did not account.
DISCLOSURES:
The study was funded by the National Natural Science Foundation of China, the Tianjin Major Public Health Science and Technology Project, the National Health Commission of China, the Food Science and Technology Foundation of Chinese Institute of Food Science and Technology, the China Cohort Consortium, and the Chinese Nutrition Society Nutrition Research Foundation–DSM Research Fund, China. There were no disclosures reported.
Eve Bender has no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
whereas a limited amount of daily computer use that is not work-related is linked to a lower risk for dementia.
METHODOLOGY:
- Investigators analyzed data on 473,184 people aged 39-72 years from the UK Biobank who were enrolled from 2006 to 2010 and followed until a diagnosis of dementia, PD, depression, death, or study end (2018 for Wales residents; 2021 for residents of England and Scotland).
- Participants reported on the number of hours they spent outside of work exercising, watching television, and using the computer.
- MRI was conducted to determine participants’ brain volume.
TAKEAWAY:
- During the study, 6096 people developed dementia, 3000 developed PD, 23,600 developed depression, 1200 developed dementia and depression, and 486 developed PD and depression.
- Compared with those who watched TV for under 1 hour per day, those who reported watching 4 or more hours per day had a 28% higher risk for dementia (adjusted hazard ratio [aHR], 1.28; 95% CI, 1.17-1.39), a 35% higher risk for depression, (aHR, 1.35; 95% CI, 1.29-1.40) and a 16% greater risk for PD (aHR, 1.16; 95% CI, 1.03-1.29).
- However, moderate computer use outside of work seemed somewhat protective. Participants who used the computer for 30-60 minutes per day had lower risks for dementia (aHR, 0.68; 95% CI, 0.64-0.72), PD, (aHR, 0.86; 95% CI, 0.79-0.93), and depression (aHR, 0.85; 95% CI, 0.83-0.88) compared with those who reported the lowest levels of computer usage.
- Replacing 30 minutes per day of computer time with an equal amount of structured exercise was associated with decreased risk for dementia (aHR, 0.74; 95% CI, 0.85-0.95) and PD (aHR, 0.84; 95% CI, 0.78-0.90).
IN PRACTICE:
The association between extended periods of TV use and higher risk for PD and dementia could be explained by a lack of activity, the authors note. They add that sedentary behavior is, “associated with biomarkers of low-grade inflammation and changes in inflammation markers that could initiate and or worsen neuroinflammation and contribute to neurodegeneration.”
SOURCE:
Hanzhang Wu, PhD, of Tianjin University of Traditional Medicine in Tianjin, China, led the study, which was published online in the International Journal of Behavioral Nutrition and Physical Activity.
LIMITATIONS:
Screen behaviors were assessed using self-report measures, which is subject to recall bias. Also, there may have been variables confounding the findings for which investigators did not account.
DISCLOSURES:
The study was funded by the National Natural Science Foundation of China, the Tianjin Major Public Health Science and Technology Project, the National Health Commission of China, the Food Science and Technology Foundation of Chinese Institute of Food Science and Technology, the China Cohort Consortium, and the Chinese Nutrition Society Nutrition Research Foundation–DSM Research Fund, China. There were no disclosures reported.
Eve Bender has no relevant financial relationships.
A version of this article appeared on Medscape.com.
MDMA therapy for loneliness? Researchers say it could work
Some call the drug “ecstasy” or “molly.” Researchers are calling it a potential tool to help treat loneliness.
As public health experts sound the alarm on a rising loneliness epidemic in the United States and across the globe,
In the latest study, MDMA “led to a robust increase in feelings of connection” among people socializing in a controlled setting. Participants were dosed with either MDMA or a placebo and asked to chat with a stranger. Afterward, those who took MDMA said their companion was more responsive and attentive, and that they had plenty in common. The drug also “increased participants’ ratings of liking their partners, feeling connected and finding the conversation enjoyable and meaningful.”
The study was small — just 18 participants — but its results “have implications for MDMA-assisted therapy,” the authors wrote. “This feeling of connectedness could help patients feel safe and trusting, thereby facilitating deeper emotional exploration.”
MDMA “really does seem to make people want to interact more with other people,” says Harriet de Wit, PhD, a neuropharmacologist at the University of Chicago and one of the study’s authors. The results echo those of earlier research using psychedelics like LSD or psilocybin.
It’s important to note that any intervention involving MDMA or psychedelics would be a drug-assisted therapy — that is, used in conjunction with the appropriate therapy and in a therapeutic setting. MDMA-assisted therapy has already drawn popular and scientific attention, as it recently cleared clinical trials for treating posttraumatic stress disorder (PTSD) and may be nearing approval by the US Food and Drug Administration (FDA).
According to Friederike Holze, PhD, psychopharmacologist at the University of Basel, in Switzerland, “there could be a place” for MDMA and psychedelics in treating chronic loneliness, but only under professional supervision.
There would have to be clear guidelines too, says Joshua Woolley, MD, PhD, a psychiatrist at the University of California, San Francisco.
MDMA and psychedelics “induce this plastic state, a state where people can change. They feel open, they feel like things are possible,” Dr. Woolley says. Then, with therapy, “you can help them change.”
Loneliness Can Impact Our Health
On top of the mental health ramifications, the physiologic effects of loneliness could have grave consequences over time. In observational studies, loneliness has been linked to higher risks for cancer and heart disease, and shorter lifespan. One third of Americans over 45 say they are chronically lonely.
Chronic loneliness changes how we think and behave, research shows. It makes us fear contact with others and see them in a more negative light, as more threatening and less trustworthy. Lonely people prefer to stand farther apart from strangers and avoid touch.
This is where MDMA-assisted therapies could potentially help, by easing these defensive tendencies, according to Dr. Woolley.
MDMA, Psychedelics, and Social Behavior
MDMA, or 3,4-methylenedioxymethamphetamine, is a hybrid between a stimulant and a psychedelic. In Dr. de Wit’s earlier experiments, volunteers given MDMA engaged more in communal activities, chatting, and playing games. They used more positive words during social encounters than those who had received a placebo. And after MDMA, people felt less rejected if they were slighted in Cyberball — a virtual ball-tossing game commonly used to measure the effects of social exclusion.
MDMA has been shown to reduce people’s response to other’s negative emotions, diminishing activation of the amygdala (the brain’s fear center) while looking at pictures of angry faces.
This could be helpful. “If you perceive a person’s natural expression as being a little bit angry, if that disappears, then you might be more inclined to interact,” de Wit says.
However, there may be downsides, too. If a drug makes people more trusting and willing to connect, they could be taken advantage of. This is why, Dr. Woolley says, “psychedelics have been used in cults.”
MDMA may also make the experience of touch more pleasant. In a series of experiments in 2019, researchers gently stroked volunteers ’ arms with a goat-hair brush, mimicking the comforting gestures one may receive from a loved one. At the same time, the scientists monitored the volunteers’ facial muscles. People on MDMA perceived gentle touch as more pleasant than those on placebo, and their smile muscles activated more.
MDMA and psychedelics boost social behaviors in animals, too — suggesting that their effects on relationships have a biological basis. Rats on MDMA are more likely to lie next to each other, and mice become more resilient to social stress. Even octopuses become more outgoing after a dose of MDMA, choosing to spend more time with other octopuses instead of a new toy. Classic psychedelics show similar effects — LSD, for example, makes mice more social.
Psychedelics can induce a sense of a “dissolution of the self-other boundary,” Dr. Woolley says. People who take them often say it’s “helped them feel more connected to themselves and other people.” LSD, first synthesized in 1938, may help increase empathy in some people.
Psilocybin, a compound found in over 200 species of mushrooms and used for centuries in Mesoamerican rituals, also seems to boost empathy, with effects persisting for at least seven days. In Cyberball, the online ball-throwing game, people who took psilocybin felt less socially rejected, an outcome reflected in their brain activation patterns in one study — the areas responsible for social-pain processing appeared to dim after a dose.
Making It Legal and Putting It to Use
In 2020, Oregon became the first state to establish a regulatory framework for psilocybin for therapeutic use, and Colorado followed suit in 2022. Such therapeutic applications of psilocybin could help fight loneliness as well, Dr. Woolley believes, because a “ common symptom of depression is that people feel socially withdrawn and lack motivation, ” he says. As mentioned above, MDMA-assisted therapy is also nearing FDA approval for PTSD.
What remain unclear are the exact mechanisms at play.
“MDMA releases oxytocin, and it does that through serotonin receptors,” Dr. de Wit says. Serotonin activates 5-HT1A receptors in the hypothalamus, releasing oxytocin into the bloodstream. In Dr. de Wit’s recent experiments, the more people felt connected after taking MDMA, the more oxytocin was found circulating in their bodies. (Another drug, methamphetamine, also upped the levels of oxytocin but did not increase feelings of connectedness.)
“It’s likely that both something in the serotonin system independent of oxytocin, and oxytocin itself, contribute,” Dr. de Wit says. Dopamine, a neurotransmitter responsible for motivation, appears to increase as well.
The empathy-boosting effects of LSD also seem to be at least partly driven by oxytocin, experiments published in 2021 revealed. Studies in mice, meanwhile, suggest that glutamate, a chemical messenger in the brain, may be behind some of LSD’s prosocial effects.
Scientists are fairly certain which receptors these drugs bind to and which neurotransmitters they affect. “How that gets translated into these higher-order things like empathy and feeling connected to the world, we don’t totally understand,” Dr. Woolley says.
Challenges and the Future
Although MDMA and psychedelics are largely considered safe when taken in a legal, medically controlled setting, there is reason to be cautious.
“They have relatively low impact on the body, like heart rate increase or blood pressure increase. But they might leave some disturbing psychological effects,” says Dr. Holze. Scientists routinely screen experiment volunteers for their risk for psychiatric disorders.
Although risk for addiction is low with both MDMA and psychedelics, there is always some risk for misuse. MDMA “ can produce feelings of well-being, and then people might use it repeatedly, ” Dr. de Wit says. “ That doesn ’ t seem to be a problem for really a lot of people, but it could easily happen. ”
Still, possibilities remain for MDMA in the fight against loneliness.
“[People] feel open, they feel like things are possible, they feel like they’re unstuck,” Dr. Woolley says. “You can harness that in psychotherapy.”
A version of this article appeared on Medscape.com.
Some call the drug “ecstasy” or “molly.” Researchers are calling it a potential tool to help treat loneliness.
As public health experts sound the alarm on a rising loneliness epidemic in the United States and across the globe,
In the latest study, MDMA “led to a robust increase in feelings of connection” among people socializing in a controlled setting. Participants were dosed with either MDMA or a placebo and asked to chat with a stranger. Afterward, those who took MDMA said their companion was more responsive and attentive, and that they had plenty in common. The drug also “increased participants’ ratings of liking their partners, feeling connected and finding the conversation enjoyable and meaningful.”
The study was small — just 18 participants — but its results “have implications for MDMA-assisted therapy,” the authors wrote. “This feeling of connectedness could help patients feel safe and trusting, thereby facilitating deeper emotional exploration.”
MDMA “really does seem to make people want to interact more with other people,” says Harriet de Wit, PhD, a neuropharmacologist at the University of Chicago and one of the study’s authors. The results echo those of earlier research using psychedelics like LSD or psilocybin.
It’s important to note that any intervention involving MDMA or psychedelics would be a drug-assisted therapy — that is, used in conjunction with the appropriate therapy and in a therapeutic setting. MDMA-assisted therapy has already drawn popular and scientific attention, as it recently cleared clinical trials for treating posttraumatic stress disorder (PTSD) and may be nearing approval by the US Food and Drug Administration (FDA).
According to Friederike Holze, PhD, psychopharmacologist at the University of Basel, in Switzerland, “there could be a place” for MDMA and psychedelics in treating chronic loneliness, but only under professional supervision.
There would have to be clear guidelines too, says Joshua Woolley, MD, PhD, a psychiatrist at the University of California, San Francisco.
MDMA and psychedelics “induce this plastic state, a state where people can change. They feel open, they feel like things are possible,” Dr. Woolley says. Then, with therapy, “you can help them change.”
Loneliness Can Impact Our Health
On top of the mental health ramifications, the physiologic effects of loneliness could have grave consequences over time. In observational studies, loneliness has been linked to higher risks for cancer and heart disease, and shorter lifespan. One third of Americans over 45 say they are chronically lonely.
Chronic loneliness changes how we think and behave, research shows. It makes us fear contact with others and see them in a more negative light, as more threatening and less trustworthy. Lonely people prefer to stand farther apart from strangers and avoid touch.
This is where MDMA-assisted therapies could potentially help, by easing these defensive tendencies, according to Dr. Woolley.
MDMA, Psychedelics, and Social Behavior
MDMA, or 3,4-methylenedioxymethamphetamine, is a hybrid between a stimulant and a psychedelic. In Dr. de Wit’s earlier experiments, volunteers given MDMA engaged more in communal activities, chatting, and playing games. They used more positive words during social encounters than those who had received a placebo. And after MDMA, people felt less rejected if they were slighted in Cyberball — a virtual ball-tossing game commonly used to measure the effects of social exclusion.
MDMA has been shown to reduce people’s response to other’s negative emotions, diminishing activation of the amygdala (the brain’s fear center) while looking at pictures of angry faces.
This could be helpful. “If you perceive a person’s natural expression as being a little bit angry, if that disappears, then you might be more inclined to interact,” de Wit says.
However, there may be downsides, too. If a drug makes people more trusting and willing to connect, they could be taken advantage of. This is why, Dr. Woolley says, “psychedelics have been used in cults.”
MDMA may also make the experience of touch more pleasant. In a series of experiments in 2019, researchers gently stroked volunteers ’ arms with a goat-hair brush, mimicking the comforting gestures one may receive from a loved one. At the same time, the scientists monitored the volunteers’ facial muscles. People on MDMA perceived gentle touch as more pleasant than those on placebo, and their smile muscles activated more.
MDMA and psychedelics boost social behaviors in animals, too — suggesting that their effects on relationships have a biological basis. Rats on MDMA are more likely to lie next to each other, and mice become more resilient to social stress. Even octopuses become more outgoing after a dose of MDMA, choosing to spend more time with other octopuses instead of a new toy. Classic psychedelics show similar effects — LSD, for example, makes mice more social.
Psychedelics can induce a sense of a “dissolution of the self-other boundary,” Dr. Woolley says. People who take them often say it’s “helped them feel more connected to themselves and other people.” LSD, first synthesized in 1938, may help increase empathy in some people.
Psilocybin, a compound found in over 200 species of mushrooms and used for centuries in Mesoamerican rituals, also seems to boost empathy, with effects persisting for at least seven days. In Cyberball, the online ball-throwing game, people who took psilocybin felt less socially rejected, an outcome reflected in their brain activation patterns in one study — the areas responsible for social-pain processing appeared to dim after a dose.
Making It Legal and Putting It to Use
In 2020, Oregon became the first state to establish a regulatory framework for psilocybin for therapeutic use, and Colorado followed suit in 2022. Such therapeutic applications of psilocybin could help fight loneliness as well, Dr. Woolley believes, because a “ common symptom of depression is that people feel socially withdrawn and lack motivation, ” he says. As mentioned above, MDMA-assisted therapy is also nearing FDA approval for PTSD.
What remain unclear are the exact mechanisms at play.
“MDMA releases oxytocin, and it does that through serotonin receptors,” Dr. de Wit says. Serotonin activates 5-HT1A receptors in the hypothalamus, releasing oxytocin into the bloodstream. In Dr. de Wit’s recent experiments, the more people felt connected after taking MDMA, the more oxytocin was found circulating in their bodies. (Another drug, methamphetamine, also upped the levels of oxytocin but did not increase feelings of connectedness.)
“It’s likely that both something in the serotonin system independent of oxytocin, and oxytocin itself, contribute,” Dr. de Wit says. Dopamine, a neurotransmitter responsible for motivation, appears to increase as well.
The empathy-boosting effects of LSD also seem to be at least partly driven by oxytocin, experiments published in 2021 revealed. Studies in mice, meanwhile, suggest that glutamate, a chemical messenger in the brain, may be behind some of LSD’s prosocial effects.
Scientists are fairly certain which receptors these drugs bind to and which neurotransmitters they affect. “How that gets translated into these higher-order things like empathy and feeling connected to the world, we don’t totally understand,” Dr. Woolley says.
Challenges and the Future
Although MDMA and psychedelics are largely considered safe when taken in a legal, medically controlled setting, there is reason to be cautious.
“They have relatively low impact on the body, like heart rate increase or blood pressure increase. But they might leave some disturbing psychological effects,” says Dr. Holze. Scientists routinely screen experiment volunteers for their risk for psychiatric disorders.
Although risk for addiction is low with both MDMA and psychedelics, there is always some risk for misuse. MDMA “ can produce feelings of well-being, and then people might use it repeatedly, ” Dr. de Wit says. “ That doesn ’ t seem to be a problem for really a lot of people, but it could easily happen. ”
Still, possibilities remain for MDMA in the fight against loneliness.
“[People] feel open, they feel like things are possible, they feel like they’re unstuck,” Dr. Woolley says. “You can harness that in psychotherapy.”
A version of this article appeared on Medscape.com.
Some call the drug “ecstasy” or “molly.” Researchers are calling it a potential tool to help treat loneliness.
As public health experts sound the alarm on a rising loneliness epidemic in the United States and across the globe,
In the latest study, MDMA “led to a robust increase in feelings of connection” among people socializing in a controlled setting. Participants were dosed with either MDMA or a placebo and asked to chat with a stranger. Afterward, those who took MDMA said their companion was more responsive and attentive, and that they had plenty in common. The drug also “increased participants’ ratings of liking their partners, feeling connected and finding the conversation enjoyable and meaningful.”
The study was small — just 18 participants — but its results “have implications for MDMA-assisted therapy,” the authors wrote. “This feeling of connectedness could help patients feel safe and trusting, thereby facilitating deeper emotional exploration.”
MDMA “really does seem to make people want to interact more with other people,” says Harriet de Wit, PhD, a neuropharmacologist at the University of Chicago and one of the study’s authors. The results echo those of earlier research using psychedelics like LSD or psilocybin.
It’s important to note that any intervention involving MDMA or psychedelics would be a drug-assisted therapy — that is, used in conjunction with the appropriate therapy and in a therapeutic setting. MDMA-assisted therapy has already drawn popular and scientific attention, as it recently cleared clinical trials for treating posttraumatic stress disorder (PTSD) and may be nearing approval by the US Food and Drug Administration (FDA).
According to Friederike Holze, PhD, psychopharmacologist at the University of Basel, in Switzerland, “there could be a place” for MDMA and psychedelics in treating chronic loneliness, but only under professional supervision.
There would have to be clear guidelines too, says Joshua Woolley, MD, PhD, a psychiatrist at the University of California, San Francisco.
MDMA and psychedelics “induce this plastic state, a state where people can change. They feel open, they feel like things are possible,” Dr. Woolley says. Then, with therapy, “you can help them change.”
Loneliness Can Impact Our Health
On top of the mental health ramifications, the physiologic effects of loneliness could have grave consequences over time. In observational studies, loneliness has been linked to higher risks for cancer and heart disease, and shorter lifespan. One third of Americans over 45 say they are chronically lonely.
Chronic loneliness changes how we think and behave, research shows. It makes us fear contact with others and see them in a more negative light, as more threatening and less trustworthy. Lonely people prefer to stand farther apart from strangers and avoid touch.
This is where MDMA-assisted therapies could potentially help, by easing these defensive tendencies, according to Dr. Woolley.
MDMA, Psychedelics, and Social Behavior
MDMA, or 3,4-methylenedioxymethamphetamine, is a hybrid between a stimulant and a psychedelic. In Dr. de Wit’s earlier experiments, volunteers given MDMA engaged more in communal activities, chatting, and playing games. They used more positive words during social encounters than those who had received a placebo. And after MDMA, people felt less rejected if they were slighted in Cyberball — a virtual ball-tossing game commonly used to measure the effects of social exclusion.
MDMA has been shown to reduce people’s response to other’s negative emotions, diminishing activation of the amygdala (the brain’s fear center) while looking at pictures of angry faces.
This could be helpful. “If you perceive a person’s natural expression as being a little bit angry, if that disappears, then you might be more inclined to interact,” de Wit says.
However, there may be downsides, too. If a drug makes people more trusting and willing to connect, they could be taken advantage of. This is why, Dr. Woolley says, “psychedelics have been used in cults.”
MDMA may also make the experience of touch more pleasant. In a series of experiments in 2019, researchers gently stroked volunteers ’ arms with a goat-hair brush, mimicking the comforting gestures one may receive from a loved one. At the same time, the scientists monitored the volunteers’ facial muscles. People on MDMA perceived gentle touch as more pleasant than those on placebo, and their smile muscles activated more.
MDMA and psychedelics boost social behaviors in animals, too — suggesting that their effects on relationships have a biological basis. Rats on MDMA are more likely to lie next to each other, and mice become more resilient to social stress. Even octopuses become more outgoing after a dose of MDMA, choosing to spend more time with other octopuses instead of a new toy. Classic psychedelics show similar effects — LSD, for example, makes mice more social.
Psychedelics can induce a sense of a “dissolution of the self-other boundary,” Dr. Woolley says. People who take them often say it’s “helped them feel more connected to themselves and other people.” LSD, first synthesized in 1938, may help increase empathy in some people.
Psilocybin, a compound found in over 200 species of mushrooms and used for centuries in Mesoamerican rituals, also seems to boost empathy, with effects persisting for at least seven days. In Cyberball, the online ball-throwing game, people who took psilocybin felt less socially rejected, an outcome reflected in their brain activation patterns in one study — the areas responsible for social-pain processing appeared to dim after a dose.
Making It Legal and Putting It to Use
In 2020, Oregon became the first state to establish a regulatory framework for psilocybin for therapeutic use, and Colorado followed suit in 2022. Such therapeutic applications of psilocybin could help fight loneliness as well, Dr. Woolley believes, because a “ common symptom of depression is that people feel socially withdrawn and lack motivation, ” he says. As mentioned above, MDMA-assisted therapy is also nearing FDA approval for PTSD.
What remain unclear are the exact mechanisms at play.
“MDMA releases oxytocin, and it does that through serotonin receptors,” Dr. de Wit says. Serotonin activates 5-HT1A receptors in the hypothalamus, releasing oxytocin into the bloodstream. In Dr. de Wit’s recent experiments, the more people felt connected after taking MDMA, the more oxytocin was found circulating in their bodies. (Another drug, methamphetamine, also upped the levels of oxytocin but did not increase feelings of connectedness.)
“It’s likely that both something in the serotonin system independent of oxytocin, and oxytocin itself, contribute,” Dr. de Wit says. Dopamine, a neurotransmitter responsible for motivation, appears to increase as well.
The empathy-boosting effects of LSD also seem to be at least partly driven by oxytocin, experiments published in 2021 revealed. Studies in mice, meanwhile, suggest that glutamate, a chemical messenger in the brain, may be behind some of LSD’s prosocial effects.
Scientists are fairly certain which receptors these drugs bind to and which neurotransmitters they affect. “How that gets translated into these higher-order things like empathy and feeling connected to the world, we don’t totally understand,” Dr. Woolley says.
Challenges and the Future
Although MDMA and psychedelics are largely considered safe when taken in a legal, medically controlled setting, there is reason to be cautious.
“They have relatively low impact on the body, like heart rate increase or blood pressure increase. But they might leave some disturbing psychological effects,” says Dr. Holze. Scientists routinely screen experiment volunteers for their risk for psychiatric disorders.
Although risk for addiction is low with both MDMA and psychedelics, there is always some risk for misuse. MDMA “ can produce feelings of well-being, and then people might use it repeatedly, ” Dr. de Wit says. “ That doesn ’ t seem to be a problem for really a lot of people, but it could easily happen. ”
Still, possibilities remain for MDMA in the fight against loneliness.
“[People] feel open, they feel like things are possible, they feel like they’re unstuck,” Dr. Woolley says. “You can harness that in psychotherapy.”
A version of this article appeared on Medscape.com.
Higher blood levels of oleic acid tied to depression
TOPLINE:
National survey data reveal an association between higher serum oleic acid levels and depression in adults, new research shows.
METHODOLOGY:
Oleic acid is the most abundant fatty acid in plasma and has been associated with multiple neurologic diseases. However, the relationship between oleic acid and depression is unclear.
This cross-sectional analyzed data on 4459 adults from the 2011-2014 National Health and Nutrition Examination Survey (NHANES).
Multivariable logistic regression models adjusting for demographics, health and lifestyle factors quantified the association between oleic acid levels and depression. Depression was assessed using the Patient Health Questionnaire-9 (PHQ-9) with depression defined as a score ≥ 10.
TAKEAWAY:
Serum oleic acid levels were positively associated with depression before and after multivariable adjustment.
After adjusting for all covariates, for every 1 mmol/L increase in serum oleic acid levels, the prevalence of depression increased by 40% (adjusted odds ratio [aOR], 1.40; 95% CI, 1.03-1.90).
Adults in the top quartile of oleic acid (≥ 2.51 mmol/L) had a greater than twofold higher likelihood of depression (aOR, 2.22; 95% CI, 1.04-4.73) compared with peers in the lowest quartile (≤ 1.54 mmol/L).
IN PRACTICE:
“A better understanding of the role of oleic acid in depression may lead to new preventive and therapeutic methods. Thus, carefully designed prospective studies are necessary to explore the positive effects of changing serum oleic acid levels through diet, medicine, or other measures on depression,” the authors write.
SOURCE:
The study, with first author Jiahui Yin of Shandong University of Traditional Chinese Medicine in Jinan, China, was published online on November 16, 2023 in BMC Psychiatry .
LIMITATIONS:
The cross-sectional study can’t prove causality. The findings may not apply to clinically diagnosed major depressive disorder.
DISCLOSURES:
The study had no specific funding. The authors report no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
National survey data reveal an association between higher serum oleic acid levels and depression in adults, new research shows.
METHODOLOGY:
Oleic acid is the most abundant fatty acid in plasma and has been associated with multiple neurologic diseases. However, the relationship between oleic acid and depression is unclear.
This cross-sectional analyzed data on 4459 adults from the 2011-2014 National Health and Nutrition Examination Survey (NHANES).
Multivariable logistic regression models adjusting for demographics, health and lifestyle factors quantified the association between oleic acid levels and depression. Depression was assessed using the Patient Health Questionnaire-9 (PHQ-9) with depression defined as a score ≥ 10.
TAKEAWAY:
Serum oleic acid levels were positively associated with depression before and after multivariable adjustment.
After adjusting for all covariates, for every 1 mmol/L increase in serum oleic acid levels, the prevalence of depression increased by 40% (adjusted odds ratio [aOR], 1.40; 95% CI, 1.03-1.90).
Adults in the top quartile of oleic acid (≥ 2.51 mmol/L) had a greater than twofold higher likelihood of depression (aOR, 2.22; 95% CI, 1.04-4.73) compared with peers in the lowest quartile (≤ 1.54 mmol/L).
IN PRACTICE:
“A better understanding of the role of oleic acid in depression may lead to new preventive and therapeutic methods. Thus, carefully designed prospective studies are necessary to explore the positive effects of changing serum oleic acid levels through diet, medicine, or other measures on depression,” the authors write.
SOURCE:
The study, with first author Jiahui Yin of Shandong University of Traditional Chinese Medicine in Jinan, China, was published online on November 16, 2023 in BMC Psychiatry .
LIMITATIONS:
The cross-sectional study can’t prove causality. The findings may not apply to clinically diagnosed major depressive disorder.
DISCLOSURES:
The study had no specific funding. The authors report no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
National survey data reveal an association between higher serum oleic acid levels and depression in adults, new research shows.
METHODOLOGY:
Oleic acid is the most abundant fatty acid in plasma and has been associated with multiple neurologic diseases. However, the relationship between oleic acid and depression is unclear.
This cross-sectional analyzed data on 4459 adults from the 2011-2014 National Health and Nutrition Examination Survey (NHANES).
Multivariable logistic regression models adjusting for demographics, health and lifestyle factors quantified the association between oleic acid levels and depression. Depression was assessed using the Patient Health Questionnaire-9 (PHQ-9) with depression defined as a score ≥ 10.
TAKEAWAY:
Serum oleic acid levels were positively associated with depression before and after multivariable adjustment.
After adjusting for all covariates, for every 1 mmol/L increase in serum oleic acid levels, the prevalence of depression increased by 40% (adjusted odds ratio [aOR], 1.40; 95% CI, 1.03-1.90).
Adults in the top quartile of oleic acid (≥ 2.51 mmol/L) had a greater than twofold higher likelihood of depression (aOR, 2.22; 95% CI, 1.04-4.73) compared with peers in the lowest quartile (≤ 1.54 mmol/L).
IN PRACTICE:
“A better understanding of the role of oleic acid in depression may lead to new preventive and therapeutic methods. Thus, carefully designed prospective studies are necessary to explore the positive effects of changing serum oleic acid levels through diet, medicine, or other measures on depression,” the authors write.
SOURCE:
The study, with first author Jiahui Yin of Shandong University of Traditional Chinese Medicine in Jinan, China, was published online on November 16, 2023 in BMC Psychiatry .
LIMITATIONS:
The cross-sectional study can’t prove causality. The findings may not apply to clinically diagnosed major depressive disorder.
DISCLOSURES:
The study had no specific funding. The authors report no conflicts of interest.
A version of this article first appeared on Medscape.com.






