User login
Data Trends 2023: Depression
1. Moradi Y et al. BMC Psychiatry. 2021;21(1):510. doi:10.1186/s12888-021-03526-2
2. Ziobrowski HN et al. J Affect Disord. 2021;290:227-236. doi:10.1016/j.jad.2021.04.033
3. Szukis H et al. Curr Med Res Opin. 2021;37(8):1393-1401. doi:10.1080/03007995.2021.1918073
4. Levey DF et al. Nat Neurosci. 2021;24(7):954-963. doi:10.1038/s41593-021-00860-2
5. Madore MR et al. J Affect Disord. 2022;297:671-678. doi:10.1016/j.jad.2021.10.025
6. Cheng CM et al. Adv Exp Med Biol. 2021;1305:333-349. doi:10.1007/978-981-33-6044-0_18
1. Moradi Y et al. BMC Psychiatry. 2021;21(1):510. doi:10.1186/s12888-021-03526-2
2. Ziobrowski HN et al. J Affect Disord. 2021;290:227-236. doi:10.1016/j.jad.2021.04.033
3. Szukis H et al. Curr Med Res Opin. 2021;37(8):1393-1401. doi:10.1080/03007995.2021.1918073
4. Levey DF et al. Nat Neurosci. 2021;24(7):954-963. doi:10.1038/s41593-021-00860-2
5. Madore MR et al. J Affect Disord. 2022;297:671-678. doi:10.1016/j.jad.2021.10.025
6. Cheng CM et al. Adv Exp Med Biol. 2021;1305:333-349. doi:10.1007/978-981-33-6044-0_18
1. Moradi Y et al. BMC Psychiatry. 2021;21(1):510. doi:10.1186/s12888-021-03526-2
2. Ziobrowski HN et al. J Affect Disord. 2021;290:227-236. doi:10.1016/j.jad.2021.04.033
3. Szukis H et al. Curr Med Res Opin. 2021;37(8):1393-1401. doi:10.1080/03007995.2021.1918073
4. Levey DF et al. Nat Neurosci. 2021;24(7):954-963. doi:10.1038/s41593-021-00860-2
5. Madore MR et al. J Affect Disord. 2022;297:671-678. doi:10.1016/j.jad.2021.10.025
6. Cheng CM et al. Adv Exp Med Biol. 2021;1305:333-349. doi:10.1007/978-981-33-6044-0_18
Emotional blunting in patients taking antidepressants
When used to treat anxiety or depressive disorders, antidepressants can cause a variety of adverse effects, including emotional blunting. Emotional blunting has been described as emotional numbness, indifference, decreased responsiveness, or numbing. In a study of 669 patients who had been receiving antidepressants (selective serotonin reuptake inhibitors [SSRIs], serotonin-norepinephrine reuptake inhibitors [SNRIs], or other antidepressants), 46% said they had experienced emotional blunting.1 A 2019 study found that approximately one-third of patients with unipolar depression or bipolar depression stopped taking their antidepressant due to emotional blunting.2
Historically, there has been difficulty parsing out emotional blunting (a general decrease of all range of emotions) from anhedonia (a restriction of positive emotions). Additionally, some researchers have questioned if the blunting of emotions is part of depressive symptomatology. In a study of 38 adults, most felt able to differentiate emotional blunting due to antidepressants by considering the resolution of other depressive symptoms and timeline of onset.3
A significant limitation has been how clinicians measure or assess emotional blunting. The Oxford Depression Questionnaire (ODQ), previously known as the Oxford Questionnaire on the Emotional Side-effects of Antidepressants, was created based on a qualitative survey of patients who endorsed emotional blunting.4 A validated scale, the ODQ divides emotional blunting into 4 dimensions:
- general reduction in emotions
- reduction in positive emotions
- emotional detachment from others
- not caring.4
The sections of ODQ focus on exploring specific aspects of patients’ emotional experiences, comparing experiences in the past week to before the development of illness/emotional blunting, and patients’ opinions about antidepressants. Example statements from the ODQ (Table4) may help clinicians better understand and explore emotional blunting with their patients.

There are 2 leading theories behind the mechanism of emotional blunting on antidepressants, both focused on serotonin. The first theory offers that SSRIs alter frontal lobe activity through serotonergic effects. The second theory is focused on the downward effects of serotonin on dopamine in reward pathways.5
Treatment options: Limited evidence
Data on how to address antidepressant-induced emotional blunting are limited and based largely on case reports. One open-label study (N = 143) found that patients experiencing emotional blunting while taking SSRIs and SNRIs who were switched to vortioxetine had a statistically significant decrease in ODQ total score; 50% reported no emotional blunting.6 Options to address emotional blunting include decreasing the antidepressant dose, augmenting with or switching to another agent, or considering other treatments such as neuromodulation.5 Further research is necessary to clarify which intervention is best.
Clinicians will encounter emotional blunting in patients who are taking antidepressants. It is important to recognize and address these symptoms to help improve patients’ adherence and overall quality of life.
1. Goodwin GM, Price J, De Bodinat C, et al. Emotional blunting with antidepressant treatments: a survey among depressed patients. J Affect Disord. 2017;221:31-35.
2. Rosenblat JD, Simon GE, Sachs GS, et al. Treatment effectiveness and tolerability outcomes that are most important to individuals with bipolar and unipolar depression. J Affect Disord. 2019;243:116-120.
3. Price J, Cole V, Goodwin GM. Emotional side-effects of selective serotonin reuptake inhibitors: qualitative study. Br J Psychiatry. 2009;195(3):211-217.
4. Price J, Cole V, Doll H, et al. The Oxford Questionnaire on the Emotional Side-effects of Antidepressants (OQuESA): development, validity, reliability and sensitivity to change. J Affect Disord. 2012;140(1):66-74.
5. Ma H, Cai M, Wang H. Emotional blunting in patients with major depressive disorder: a brief non-systematic review of current research. Front Psychiatry. 2021;12:792960. doi:10.3389/fpsyt.2021.792960
6. Fagiolini A, Florea I, Loft H, et al. Effectiveness of vortioxetine on emotional blunting in patients with major depressive disorder with inadequate response to SSRI/SNRI treatment. J Affect Disord. 2021;283:472-479.
When used to treat anxiety or depressive disorders, antidepressants can cause a variety of adverse effects, including emotional blunting. Emotional blunting has been described as emotional numbness, indifference, decreased responsiveness, or numbing. In a study of 669 patients who had been receiving antidepressants (selective serotonin reuptake inhibitors [SSRIs], serotonin-norepinephrine reuptake inhibitors [SNRIs], or other antidepressants), 46% said they had experienced emotional blunting.1 A 2019 study found that approximately one-third of patients with unipolar depression or bipolar depression stopped taking their antidepressant due to emotional blunting.2
Historically, there has been difficulty parsing out emotional blunting (a general decrease of all range of emotions) from anhedonia (a restriction of positive emotions). Additionally, some researchers have questioned if the blunting of emotions is part of depressive symptomatology. In a study of 38 adults, most felt able to differentiate emotional blunting due to antidepressants by considering the resolution of other depressive symptoms and timeline of onset.3
A significant limitation has been how clinicians measure or assess emotional blunting. The Oxford Depression Questionnaire (ODQ), previously known as the Oxford Questionnaire on the Emotional Side-effects of Antidepressants, was created based on a qualitative survey of patients who endorsed emotional blunting.4 A validated scale, the ODQ divides emotional blunting into 4 dimensions:
- general reduction in emotions
- reduction in positive emotions
- emotional detachment from others
- not caring.4
The sections of ODQ focus on exploring specific aspects of patients’ emotional experiences, comparing experiences in the past week to before the development of illness/emotional blunting, and patients’ opinions about antidepressants. Example statements from the ODQ (Table4) may help clinicians better understand and explore emotional blunting with their patients.

There are 2 leading theories behind the mechanism of emotional blunting on antidepressants, both focused on serotonin. The first theory offers that SSRIs alter frontal lobe activity through serotonergic effects. The second theory is focused on the downward effects of serotonin on dopamine in reward pathways.5
Treatment options: Limited evidence
Data on how to address antidepressant-induced emotional blunting are limited and based largely on case reports. One open-label study (N = 143) found that patients experiencing emotional blunting while taking SSRIs and SNRIs who were switched to vortioxetine had a statistically significant decrease in ODQ total score; 50% reported no emotional blunting.6 Options to address emotional blunting include decreasing the antidepressant dose, augmenting with or switching to another agent, or considering other treatments such as neuromodulation.5 Further research is necessary to clarify which intervention is best.
Clinicians will encounter emotional blunting in patients who are taking antidepressants. It is important to recognize and address these symptoms to help improve patients’ adherence and overall quality of life.
When used to treat anxiety or depressive disorders, antidepressants can cause a variety of adverse effects, including emotional blunting. Emotional blunting has been described as emotional numbness, indifference, decreased responsiveness, or numbing. In a study of 669 patients who had been receiving antidepressants (selective serotonin reuptake inhibitors [SSRIs], serotonin-norepinephrine reuptake inhibitors [SNRIs], or other antidepressants), 46% said they had experienced emotional blunting.1 A 2019 study found that approximately one-third of patients with unipolar depression or bipolar depression stopped taking their antidepressant due to emotional blunting.2
Historically, there has been difficulty parsing out emotional blunting (a general decrease of all range of emotions) from anhedonia (a restriction of positive emotions). Additionally, some researchers have questioned if the blunting of emotions is part of depressive symptomatology. In a study of 38 adults, most felt able to differentiate emotional blunting due to antidepressants by considering the resolution of other depressive symptoms and timeline of onset.3
A significant limitation has been how clinicians measure or assess emotional blunting. The Oxford Depression Questionnaire (ODQ), previously known as the Oxford Questionnaire on the Emotional Side-effects of Antidepressants, was created based on a qualitative survey of patients who endorsed emotional blunting.4 A validated scale, the ODQ divides emotional blunting into 4 dimensions:
- general reduction in emotions
- reduction in positive emotions
- emotional detachment from others
- not caring.4
The sections of ODQ focus on exploring specific aspects of patients’ emotional experiences, comparing experiences in the past week to before the development of illness/emotional blunting, and patients’ opinions about antidepressants. Example statements from the ODQ (Table4) may help clinicians better understand and explore emotional blunting with their patients.

There are 2 leading theories behind the mechanism of emotional blunting on antidepressants, both focused on serotonin. The first theory offers that SSRIs alter frontal lobe activity through serotonergic effects. The second theory is focused on the downward effects of serotonin on dopamine in reward pathways.5
Treatment options: Limited evidence
Data on how to address antidepressant-induced emotional blunting are limited and based largely on case reports. One open-label study (N = 143) found that patients experiencing emotional blunting while taking SSRIs and SNRIs who were switched to vortioxetine had a statistically significant decrease in ODQ total score; 50% reported no emotional blunting.6 Options to address emotional blunting include decreasing the antidepressant dose, augmenting with or switching to another agent, or considering other treatments such as neuromodulation.5 Further research is necessary to clarify which intervention is best.
Clinicians will encounter emotional blunting in patients who are taking antidepressants. It is important to recognize and address these symptoms to help improve patients’ adherence and overall quality of life.
1. Goodwin GM, Price J, De Bodinat C, et al. Emotional blunting with antidepressant treatments: a survey among depressed patients. J Affect Disord. 2017;221:31-35.
2. Rosenblat JD, Simon GE, Sachs GS, et al. Treatment effectiveness and tolerability outcomes that are most important to individuals with bipolar and unipolar depression. J Affect Disord. 2019;243:116-120.
3. Price J, Cole V, Goodwin GM. Emotional side-effects of selective serotonin reuptake inhibitors: qualitative study. Br J Psychiatry. 2009;195(3):211-217.
4. Price J, Cole V, Doll H, et al. The Oxford Questionnaire on the Emotional Side-effects of Antidepressants (OQuESA): development, validity, reliability and sensitivity to change. J Affect Disord. 2012;140(1):66-74.
5. Ma H, Cai M, Wang H. Emotional blunting in patients with major depressive disorder: a brief non-systematic review of current research. Front Psychiatry. 2021;12:792960. doi:10.3389/fpsyt.2021.792960
6. Fagiolini A, Florea I, Loft H, et al. Effectiveness of vortioxetine on emotional blunting in patients with major depressive disorder with inadequate response to SSRI/SNRI treatment. J Affect Disord. 2021;283:472-479.
1. Goodwin GM, Price J, De Bodinat C, et al. Emotional blunting with antidepressant treatments: a survey among depressed patients. J Affect Disord. 2017;221:31-35.
2. Rosenblat JD, Simon GE, Sachs GS, et al. Treatment effectiveness and tolerability outcomes that are most important to individuals with bipolar and unipolar depression. J Affect Disord. 2019;243:116-120.
3. Price J, Cole V, Goodwin GM. Emotional side-effects of selective serotonin reuptake inhibitors: qualitative study. Br J Psychiatry. 2009;195(3):211-217.
4. Price J, Cole V, Doll H, et al. The Oxford Questionnaire on the Emotional Side-effects of Antidepressants (OQuESA): development, validity, reliability and sensitivity to change. J Affect Disord. 2012;140(1):66-74.
5. Ma H, Cai M, Wang H. Emotional blunting in patients with major depressive disorder: a brief non-systematic review of current research. Front Psychiatry. 2021;12:792960. doi:10.3389/fpsyt.2021.792960
6. Fagiolini A, Florea I, Loft H, et al. Effectiveness of vortioxetine on emotional blunting in patients with major depressive disorder with inadequate response to SSRI/SNRI treatment. J Affect Disord. 2021;283:472-479.
Neuropsychiatric aspects of Parkinson’s disease: Practical considerations
Parkinson’s disease (PD) is a neurodegenerative condition diagnosed pathologically by alpha synuclein–containing Lewy bodies and dopaminergic cell loss in the substantia nigra pars compacta of the midbrain. Loss of dopaminergic input to the caudate and putamen disrupts the direct and indirect basal ganglia pathways for motor control and contributes to the motor symptoms of PD.1 According to the Movement Disorder Society criteria, PD is diagnosed clinically by bradykinesia (slowness of movement) plus resting tremor and/or rigidity in the presence of supportive criteria, such as levodopa responsiveness and hyposmia, and in the absence of exclusion criteria and red flags that would suggest atypical parkinsonism or an alternative diagnosis.2
Although the diagnosis and treatment of PD focus heavily on the motor symptoms, nonmotor symptoms can arise decades before the onset of motor symptoms and continue throughout the lifespan. Nonmotor symptoms affect patients from head (ie, cognition and mood) to toe (ie, striatal toe pain) and multiple organ systems in between, including the olfactory, integumentary, cardiovascular, gastrointestinal, genitourinary, and autonomic nervous systems. Thus, it is not surprising that nonmotor symptoms of PD impact health-related quality of life more substantially than motor symptoms.3 A helpful analogy is to consider the motor symptoms of PD as the tip of the iceberg and the nonmotor symptoms as the larger, submerged portions of the iceberg.4
Nonmotor symptoms can negatively impact the treatment of motor symptoms. For example, imagine a patient who is very rigid and dyscoordinated in the arms and legs, which limits their ability to dress and walk. If this patient also suffers from nonmotor symptoms of orthostatic hypotension and psychosis—both of which can be exacerbated by levodopa—dose escalation of levodopa for the rigidity and dyscoordination could be compromised, rendering the patient undertreated and less mobile.
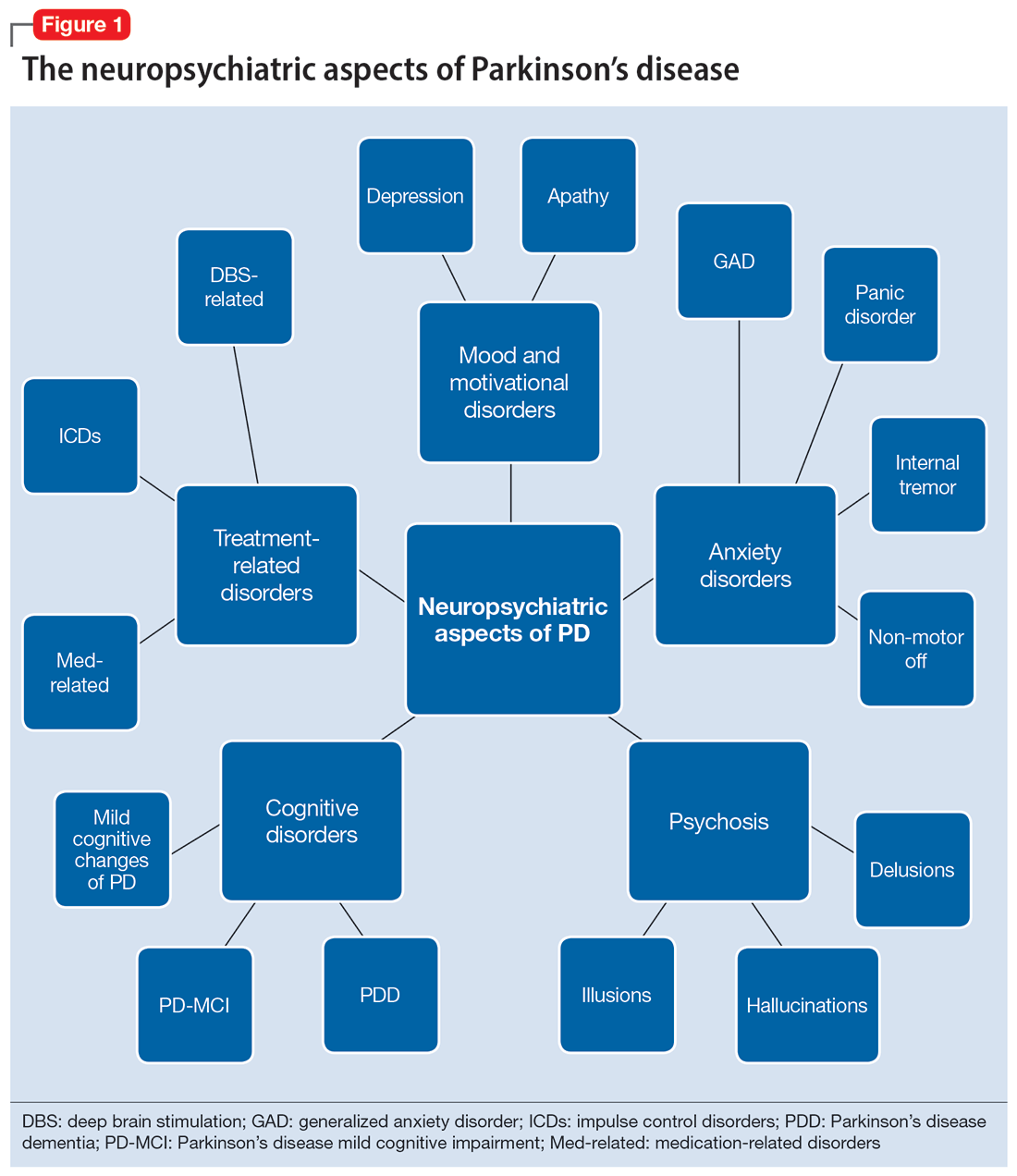
In this review, we focus on identifying and managing nonmotor symptoms of PD that are relevant to psychiatric practice, including mood and motivational disorders, anxiety disorders, psychosis, cognitive disorders, and disorders related to the pharmacologic and surgical treatment of PD (Figure 1).
Mood and motivational disorders
Depression
Depression is a common symptom in PD that can occur in the prodromal period years to decades before the onset of motor symptoms, as well as throughout the disease course.5 The prevalence of depression in PD varies from 3% to 90%, depending on the methods of assessment, clinical setting of assessment, motor symptom severity, and other factors; clinically significant depression likely affects approximately 35% to 38% of patients.5,6 How depression in patients with PD differs from depression in the general population is not entirely understood, but there does seem to be less guilt and suicidal ideation and a substantial component of negative affect, including dysphoria and anxiety.7 Practically speaking, depression is treated similarly in PD and general populations, with a few considerations.
Despite limited randomized controlled trials (RCTs) for efficacy specifically in patients with PD, selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are generally considered first-line treatments. There is also evidence for tricyclic antidepressants (TCAs), but due to potential worsening of orthostatic hypotension and cognition, TCAs may not be a favorable option for certain patients with PD.8,9 All antidepressants have the potential to worsen tremor. Theoretically, SNRIs, with noradrenergic activity, may be less tolerable than SSRIs in patients with PD. However, worsening tremor generally has not been a clinically significant adverse event reported in PD depression clinical trials, although it was seen in 17% of patients receiving paroxetine and 21% of patients receiving venlafaxine compared to 7% of patients receiving placebo.9-11 If tremor worsens, mirtazapine could be considered because it has been reported to cause less tremor than SSRIs or TCAs.12
Among medications for PD, pramipexole, a dopamine agonist, may have a beneficial effect on depression.13 Additionally, some evidence supports rasagiline, a monoamine oxidase type B inhibitor, as an adjunctive medication for depression in PD.14 Nevertheless, antidepressant medications remain the standard pharmacologic treatment for PD depression.
Continue to: In terms of nonpharmacologic options...
In terms of nonpharmacologic options, cognitive-behavioral therapy (CBT) is likely efficacious, exercise (especially yoga) is likely efficacious, and repetitive transcranial magnetic stimulation may be efficacious.15,16 While further high-quality trials are needed, these treatments are low-risk and can be considered, especially for patients who cannot tolerate medications.
Apathy
Apathy—a loss of motivation and goal-directed behavior—can occur in up to 30% of patients during the prodromal period of PD, and in up to 70% of patients throughout the disease course.17 Apathy can coexist with depression, which can make apathy difficult to diagnose.17 Given the time constraints of a clinic visit, a practical approach would be to first screen for depression and cognitive impairment. If there is continued suspicion of apathy, the Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale part I question (“In the past week have you felt indifferent to doing activities or being with people?”) can be used to screen for apathy, and more detailed scales, such as the Apathy Scale (AS) or Lille Apathy Rating Scale (LARS), could be used if indicated.18
There are limited high-quality positive trials of apathy-specific treatments in PD. In an RCT of patients with PD who did not have depression or dementia, rivastigmine improved LARS scores compared to placebo.15 Piribedil, a D2/D3 receptor agonist, improved apathy in patients who underwent subthalamic nucleus deep brain stimulation (STN DBS).15 Exercise such as individualized physical therapy programs, dance, and Nordic walking as well as mindfulness interventions were shown to significantly reduce apathy scale scores.19 SSRIs, SNRIs, and rotigotine showed a trend toward reducing AS scores in RCTs.10,20
Larger, high-quality studies are needed to clarify the treatment of apathy in PD. In the meantime, a reasonable approach is to first treat any comorbid psychiatric or cognitive disorders, since apathy can be associated with these conditions, and to optimize antiparkinsonian medications for motor symptoms, motor fluctuations, and nonmotor fluctuations. Then, the investigational apathy treatments described in this section could be considered on an individual basis.
Anxiety disorders
Anxiety is seen throughout the disease course of PD in approximately 30% to 50% of patients.21 It can manifest as generalized anxiety disorder, panic disorder, and other anxiety disorders. There are no high-quality RCTs of pharmacologic treatments of anxiety specifically in patients with PD, except for a negative safety and tolerability study of buspirone in which one-half of patients experienced worsening motor symptoms.15,22 Thus, the treatment of anxiety in patients with PD is similar to treatments in the general population. SSRIs and SNRIs are typically considered first-line, benzodiazepines are sometimes used with caution (although cognitive adverse effects and fall risk need to be considered), and nonpharmacologic treatments such as mindfulness yoga, exercise, CBT, and psychotherapy can be effective.16,21,23
Continue to: Because there is the lack...
Because there is the lack of evidence-based treatments for anxiety in PD, we highlight 2 PD-specific anxiety disorders: internal tremor, and nonmotor “off” anxiety.
Internal tremor
Internal tremor is a sense of vibration in the axial and/or appendicular muscles that cannot be seen externally by the patient or examiner. It is not yet fully understood if this phenomenon is sensory, anxiety-related, related to subclinical tremor, or the result of a combination of these factors (ie, sensory awareness of a subclinical tremor that triggers or is worsened by anxiety). There is some evidence for subclinical tremor on electromyography, but internal tremor does not respond to antiparkinsonian medications in 70% of patients.24 More electrophysiological research is needed to clarify this phenomenon. Internal tremor has been associated with anxiety in 64% of patients and often improves with anxiolytic therapies.24
Although poorly understood, internal tremor is a documented phenomenon in 33% to 44% of patients with PD, and in some cases, it may be an initial symptom that motivates a patient to seek medical attention for the first time.24,25 Internal tremor has also been reported in patients with essential tremor and multiple sclerosis.25 Therefore, physicians should be aware of internal tremor because this symptom could herald an underlying neurological disease.
Nonmotor ‘off’ anxiety
Patients with PD are commonly prescribed carbidopa-levodopa, a dopamine precursor, at least 3 times daily. Initially, this medication controls motor symptoms well from 1 dose to the next. However, as the disease progresses, some patients report motor fluctuations in which an individual dose of carbidopa-levodopa may wear off early, take longer than usual to take effect, or not take effect at all. Patients describe these periods as an “off” state in which they do not feel their medications are working. Such motor fluctuations can lead to anxiety and avoidance behaviors, because patients fear being in public at times when the medication does not adequately control their motor symptoms.
In addition to these motor symptom fluctuations and related anxiety, patients can also experience nonmotor symptom fluctuations. A wide variety of nonmotor symptoms, such as mood, cognitive, and behavioral symptoms, have been reported to fluctuate in parallel with motor symptoms.26,27 One study reported fluctuating restlessness in 39% of patients with PD, excessive worry in 17%, shortness of breath in 13%, excessive sweating and fear in 12%, and palpitations in 10%.27 A patient with fluctuating shortness of breath, sweating, and palpitations (for example) may repeatedly present to the emergency department with a negative cardiac workup and eventually be diagnosed with panic disorder, whereas the patient is truly experiencing nonmotor “off” symptoms. Thus, it is important to be aware of nonmotor fluctuations so this diagnosis can be made and the symptoms appropriately treated. The first step in treating nonmotor fluctuations is to optimize the antiparkinsonian regimen to minimize fluctuations. If “off” anxiety symptoms persist, anxiolytic medications can be prescribed.21
Continue to: Psychosis
Psychosis
Psychosis can occur in prodromal and early PD but is most common in advanced PD.28 One study reported that 60% of patients developed hallucinations or delusions after 12 years of follow-up.29 Disease duration, disease severity, dementia, and rapid eye movement sleep behavior disorder are significant risk factors for psychosis in PD.30 Well-formed visual hallucinations are the most common manifestation of psychosis in patients with PD. Auditory hallucinations and delusions are less common. Delusions are usually seen in patients with dementia and are often paranoid delusions, such as of spousal infidelity.30 Sensory hallucinations can occur, but should not be mistaken with formication, a central pain syndrome in PD that can represent a nonmotor “off” symptom that may respond to dopaminergic medication.31 Other more mild psychotic symptoms include illusions or misinterpretation of stimuli, false sense of presence, and passage hallucinations of fleeting figures in the peripheral vision.30
The pathophysiology of PD psychosis is not entirely understood but differs from psychosis in other disorders. It can occur in the absence of antiparkinsonian medication exposure and is thought to be a consequence of the underlying disease process of PD involving neurodegeneration in certain brain regions and aberrant neurotransmission of not only dopamine but also serotonin, acetylcholine, and glutamate.30
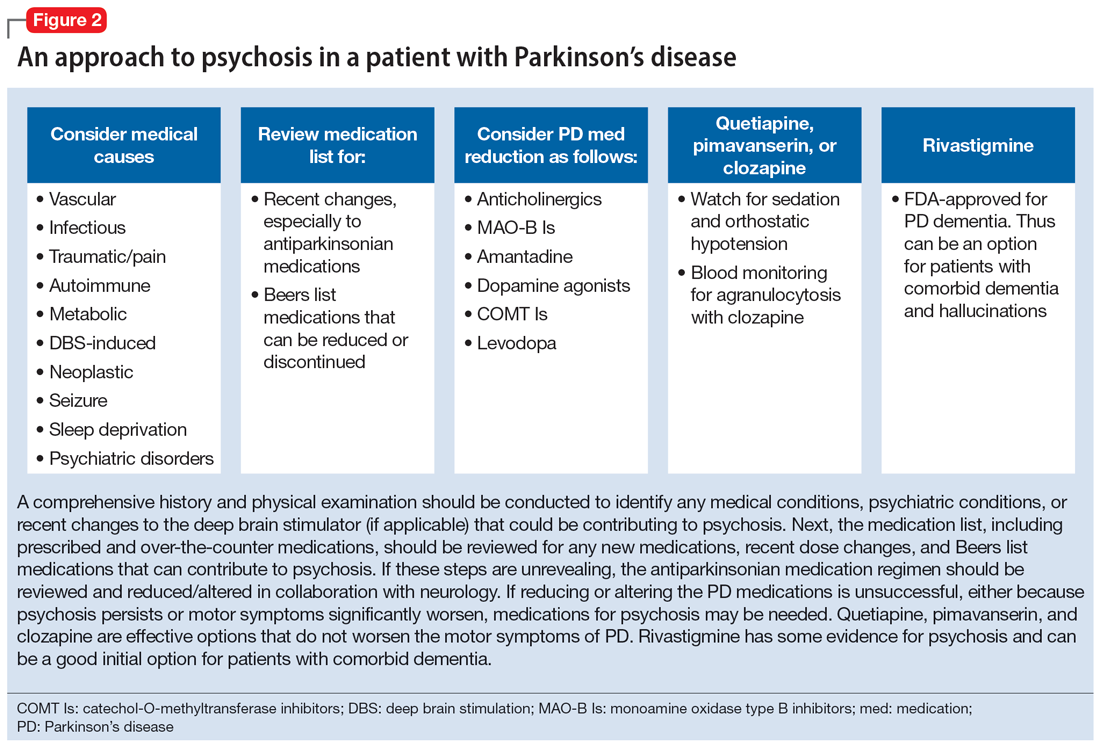
Figure 2 outlines the management of psychosis in PD. After addressing medical and medication-related causes, it is important to determine if the psychotic symptom is sufficiently bothersome to and/or potentially dangerous for the patient to warrant treatment. If treatment is indicated, pimavanserin and clozapine are efficacious for psychosis in PD without worsening motor symptoms, and quetiapine is possibly efficacious with a low risk of worsening motor symptoms.15 Other antipsychotics, such as olanzapine, risperidone, and haloperidol, can substantially worsen motor symptoms.15 Both second-generation antipsychotics and pimavanserin have an FDA black-box warning for a higher risk of all-cause mortality in older patients with dementia; however, because psychosis is associated with early mortality in PD, the risk/benefit ratio should be discussed with the patient and family for shared decision-making.30 If the patient also has dementia, rivastigmine—which is FDA-approved for PD dementia (PDD)—may also improve hallucinations.32
Cognitive disorders
This section focuses on PD mild cognitive impairment (PD-MCI) and PDD. When a patient with PD reports cognitive concerns, the approach outlined in Figure 3 can be used to diagnose the cognitive disorder. A detailed history, medication review, and physical examination can identify any medical or psychiatric conditions that could affect cognition. The American Academy of Neurology recommends screening for depression, obtaining blood levels of vitamin B12 and thyroid-stimulating hormone, and obtaining a CT or MRI of the brain to rule out reversible causes of dementia.33 A validated screening test such as the Montreal Cognitive Assessment, which has higher sensitivity for PD-MCI than the Mini-Mental State Examination, is used to identify and quantify cognitive impairment.34 Neuropsychological testing is the gold standard and can be used to confirm and/or better quantify the degree and domains of cognitive impairment.35 Typically, cognitive deficits in PD affect executive function, attention, and/or visuospatial domains more than memory and language early on, and deficits in visuospatial and language domains have the highest sensitivity for predicting progression to PDD.36
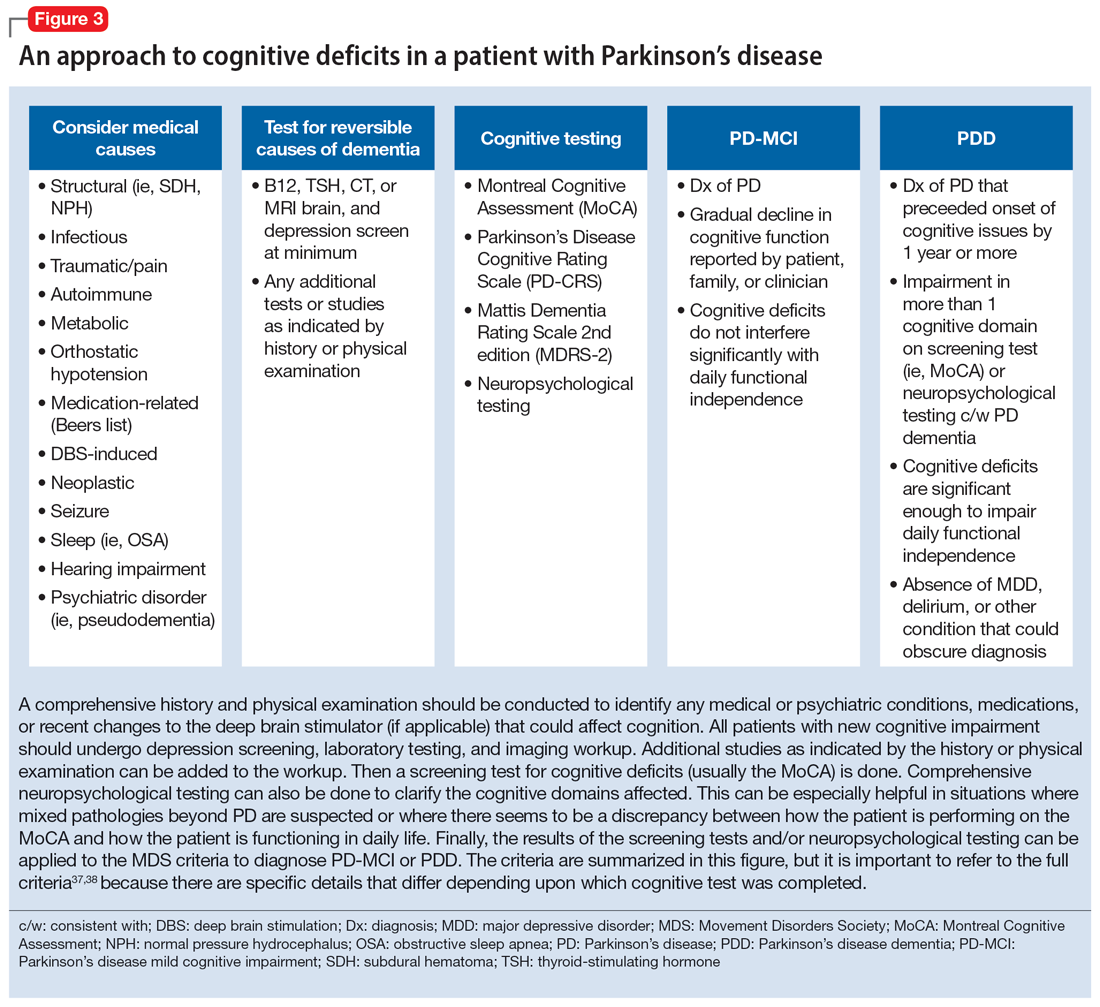
Once reversible causes of dementia are addressed or ruled out and cognitive testing is completed, the Movement Disorder Society (MDS) criteria for PD-MCI and PDD summarized in Figure 3 can be used to diagnose the cognitive disorder.37,38 The MDS criteria for PDD require a diagnosis of PD for ≥1 year prior to the onset of dementia to differentiate PDD from dementia with Lewy bodies (DLB). If the dementia starts within 1 year of the onset of parkinsonism, the diagnosis would be DLB. PDD and DLB are on the spectrum of Lewy body dementia, with the same Lewy body pathology in different temporal and spatial distributions in the brain.38
Continue to: PD-MCI is present in...
PD-MCI is present in approximately 25% of patients.35 PD-MCI does not always progress to dementia but increases the risk of dementia 6-fold. The prevalence of PDD increases with disease duration; it is present in approximately 50% of patients at 10 years and 80% of patients at 20 years of disease.35 Rivastigmine is the only FDA-approved medication to slow progression of PDD. There is insufficient evidence for other acetylcholinesterase inhibitors and memantine.15 Unfortunately, RCTs of pharmacotherapy for PD-MCI have failed to show efficacy. However, exercise, cognitive rehabilitation, and neuromodulation are being studied. In the meantime, addressing modifiable risk factors (such as vascular risk factors and alcohol consumption) and treating comorbid orthostatic hypotension, obstructive sleep apnea, and depression may improve cognition.35,39
Treatment-related disorders
Impulse control disorders
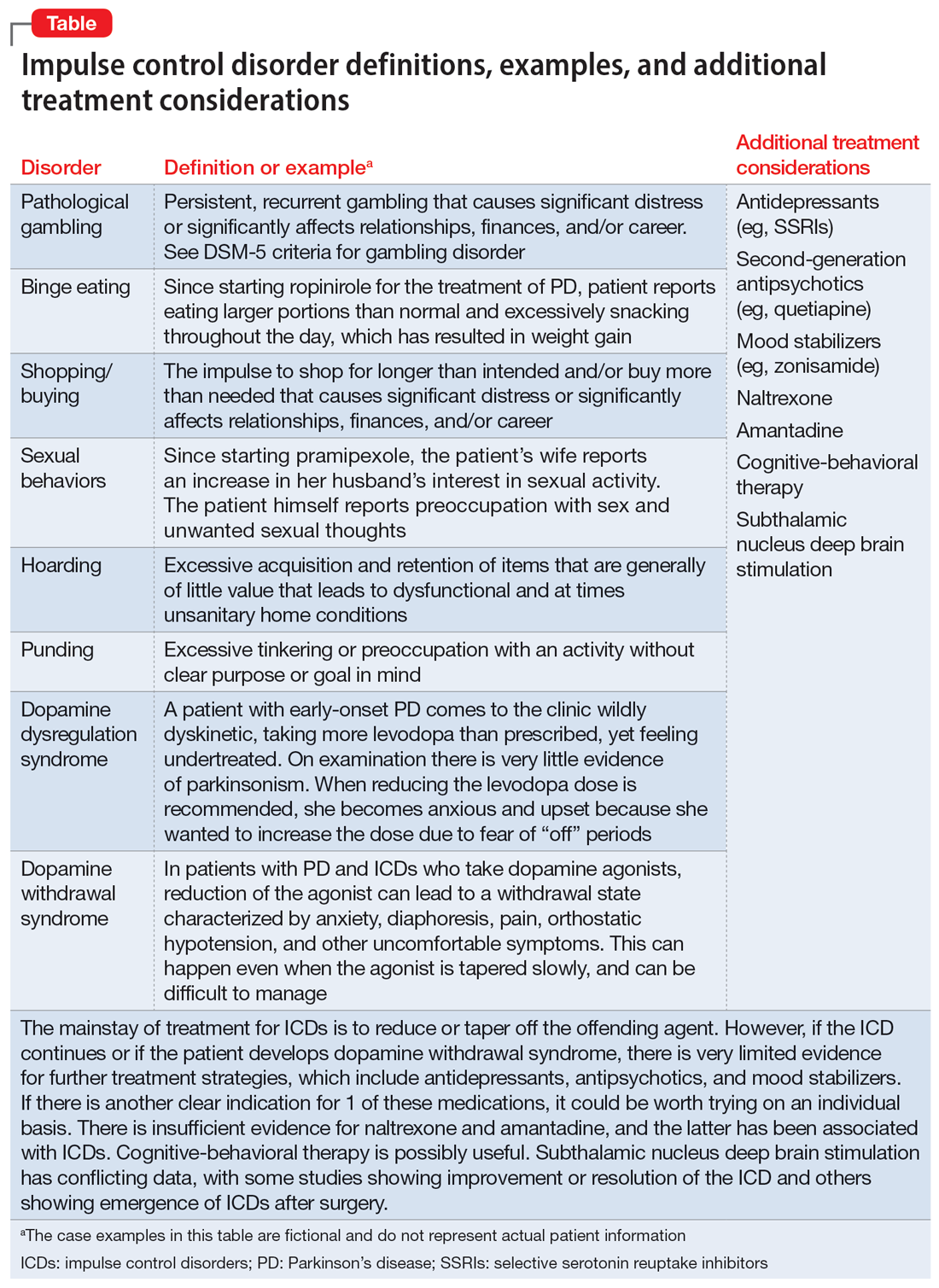
Impulse control disorders (ICDs) are an important medication-related consideration in patients with PD. The ICDs seen in PD include pathological gambling, binge eating, excessive shopping, hypersexual behaviors, and dopamine dysregulation syndrome (Table). These disorders are more common in younger patients with a history of impulsive personality traits and addictive behaviors (eg, history of tobacco or alcohol abuse), and are most strongly associated with dopaminergic therapies, particularly the dopamine agonists.40,41 In the DOMINION study, the odds of ICDs were 2- to 3.5-fold higher in patients taking dopamine agonists.42 This is mainly thought to be due to stimulation of D2/D3 receptors in the mesolimbic system.40 High doses of levodopa, monoamine oxidase inhibitors, and amantadine are also associated with ICDs.40-42
The first step in managing ICDs is diagnosing them, which can be difficult because patients often are not forthcoming about these problems due to embarrassment or failure to recognize that the ICD is related to PD medications. If a family member accompanies the patient at the visit, the patient may not want to disclose the amount of money they spend or the extent to which the behavior is a problem. Thus, a screening questionnaire, such as the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease (QUIP) can be a helpful way for patients to alert the clinician to the issue.41 Education for the patient and family is crucial before the ICD causes significant financial, health, or relationship problems.
The mainstay of treatment is to reduce or taper off the dopamine agonist or other offending agent while monitoring for worsening motor symptoms and dopamine withdrawal syndrome. If this is unsuccessful, there is very limited evidence for further treatment strategies (Table), including antidepressants, antipsychotics, and mood stabilizers.40,43,44 There is insufficient evidence for naltrexone based on an RCT that failed to meet its primary endpoint, although naltrexone did significantly reduce QUIP scores.15,44 There is also insufficient evidence for amantadine, which showed benefit in some studies but was associated with ICDs in the DOMINION study.15,40,42 In terms of nonpharmacologic treatments, CBT is likely efficacious.15,40 There are mixed results for STN DBS. Some studies showed improvement in the ICD, due at least in part to dopaminergic medication reduction postoperatively, but this treatment has also been reported to increase impulsivity.40,45
Deep brain stimulation–related disorders
For patients with PD, the ideal lead location for STN DBS is the dorsolateral aspect of the STN, as this is the motor region of the nucleus. The STN functions in indirect and hyperdirect pathways to put the brake on certain motor programs so only the desired movement can be executed. Its function is clinically demonstrated by patients with STN stroke who develop excessive ballistic movements. Adjacent to the motor region of the STN is a centrally located associative region and a medially located limbic region. Thus, when stimulating the dorsolateral STN, current can spread to those regions as well, and the STN’s ability to put the brake on behavioral and emotional programs can be affected.46 Stimulation of the STN has been associated with mania, euphoria, new-onset ICDs, decreased verbal fluency, and executive dysfunction. Depression, apathy, and anxiety can also occur, but more commonly result from rapid withdrawal of antiparkinsonian medications after DBS surgery.46,47 Therefore, for PD patients with DBS with new or worsening psychiatric or cognitive symptoms, it is important to inquire about any recent programming sessions with neurology as well as recent self-increases in stimulation by the patient using their controller. Collaboration with neurology is important to troubleshoot whether stimulation could be contributing to the patient’s psychiatric or cognitive symptoms.
Continue to: Bottom Line
Bottom Line
Mood, anxiety, psychotic, and cognitive symptoms and disorders are common psychiatric manifestations associated with Parkinson’s disease (PD). In addition, patients with PD may experience impulsive control disorders and other symptoms related to treatments they receive for PD. Careful assessment and collaboration with neurology is crucial to alleviating the effects of these conditions.
Related Resources
- Weintraub D, Aarsland D, Chaudhuri KR, et al. The neuropsychiatry of Parkinson’s disease: advances and challenges. Lancet Neurology. 2022;21(1):89-102. doi:10.1016/S1474-4422(21)00330-6
- Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurologic Clinics. 2020;38(2):269-292. doi:10.1016/j.ncl.2019.12.003
- Castrioto A, Lhommee E, Moro E et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurology. 2014;13(3):287-305. doi:10.1016/ S1474-4422(13)70294-1
Drug Brand Names
Amantadine • Gocovri
Carbidopa-levodopa • Sinemet
Clozapine • Clozaril
Haloperidol • Haldol
Memantine • Namenda
Mirtazapine • Remeron
Naltrexone • Vivitrol
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimavanserin • Nuplazid
Piribedil • Pronoran
Pramipexole • Mirapex
Quetiapine • Seroquel
Rasagiline • Azilect
Risperidone • Risperdal
Rivastigmine • Exelon
Ropinirole • Requip
Rotigotine • Neupro
Venlafaxine • Effexor
Zonisamide • Zonegran
1. Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet Neurology. 2021;397(10291):2284-2303.
2. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Movement Disorders. 2015;30(12):1591-1601.
3. Martinez-Martin P, Rodriguez-Blazquez C, Kurtiz MM, et al. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord. 2011;26(3):399-406.
4. Langston WJ. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59(4):591-596.
5. Cong S, Xiang C, Zhang S, et al. Prevalence and clinical aspects of depression in Parkinson’s disease: a systematic review and meta‑analysis of 129 studies. Neurosci Biobehav Rev. 2022;141:104749. doi:10.1016/j.neubiorev.2022.104749
6. Reijnders JS, Ehrt U, Weber WE, et al. A systematic review of prevalence studies in depression in Parkinson’s disease. Mov Disord. 2008;23(2):183-189.
7. Zahodne LB, Marsiske M, Okun MS, et al. Components of depression in Parkinson disease. J Geriatr Psychiatry Neurol. 2012;25(3):131-137.
8. Skapinakis P, Bakola E, Salanti G, et al. Efficacy and acceptability of selective serotonin reuptake inhibitors for the treatment of depression in Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. BMC Neurology. 2010;10:49. doi:10.1186/1471-2377-10-49
9. Richard IH, McDermott MP, Kurlan R, et al; SAD-PD Study Group. A randomized, double-blind placebo-controlled trial of antidepressants in Parkinson’s disease. Neurology. 2012;78(16):1229-1236.
10. Takahashi M, Tabu H, Ozaki A, et al. Antidepressants for depression, apathy, and gait instability in Parkinson’s disease: a multicenter randomized study. Intern Med. 2019;58(3):361-368.
11. Bonuccelli U, Mecco G, Fabrini G, et al. A non-comparative assessment of tolerability and efficacy of duloxetine in the treatment of depressed patients with Parkinson’s disease. Expert Opin Pharmacother. 2012;13(16):2269-2280.
12. Wantanabe N, Omorio IM, Nakagawa A, et al; MANGA (Meta-Analysis of New Generation Antidepressants) Study Group. Safety reporting and adverse-event profile of mirtazapine described in randomized controlled trials in comparison with other classes of antidepressants in the acute-phase treatment of adults with depression. CNS Drugs. 2010;24(1):35-53.
13. Barone P, Scarzella L, Marconi R, et al; Depression/Parkinson Italian Study Group. Pramipexole versus sertraline in the treatment of depression in Parkinson’s disease: a national multicenter parallel-group randomized study. J Neurol. 2006;253(5):601-607.
14. Smith KM, Eyal E, Weintraub D, et al; ADAGIO Investigators. Combined rasagiline and anti-depressant use in Parkinson’s disease in the ADAGIO study: effects on non-motor symptoms and tolerability. JAMA Neurology. 2015;72(1):88-95.
15. Seppi K, Chaudhuri R, Coelho M, et al; the collaborators of the Parkinson’s Disease Update on Non-Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence-Based Medicine Committee. Update on treatments for nonmotor symptoms of Parkinson’s disease--an evidence-based medicine review. Mov Disord. 2019;34(2):180-198.
16. Kwok JYY, Kwan JCY, Auyeung M, et al. Effects of mindfulness yoga vs stretching and resistance training exercises on anxiety and depression for people with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2019;76(7):755-763.
17. De Waele S, Cras P, Crosiers D. Apathy in Parkinson’s disease: defining the Park apathy subtype. Brain Sci. 2022;12(7):923.
18. Mele B, Van S, Holroyd-Leduc J, et al. Diagnosis, treatment and management of apathy in Parkinson’s disease: a scoping review. BMJ Open. 2020;10(9):037632. doi:10.1136/bmjopen-2020-037632
19. Mele B, Ismail Z, Goodarzi Z, et al. Non-pharmacological interventions to treat apathy in Parkinson’s disease: a realist review. Clin Park Relat Disord. 2021;4:100096. doi:10.1016/j.prdoa.2021.100096
20. Chung SJ, Asgharnejad M, Bauer L, et al. Evaluation of rotigotine transdermal patch for the treatment of depressive symptoms in patients with Parkinson’s disease. Expert Opin Pharmacother. 2016;(17)11:1453-1461.
21. Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurol Clin. 2020;38(2):269-292.
22. Schneider RB, Auinger P, Tarolli CG, et al. A trial of buspirone for anxiety in Parkinson’s disease: safety and tolerability. Parkinsonism Relat Disord. 2020;81:69-74.
23. Moonen AJH, Mulders AEP, Defebvre L, et al. Cognitive behavioral therapy for anxiety in Parkinson’s disease: a randomized controlled trial. Mov Disord. 2021;36(11):2539-2548.
24. Shulman LM, Singer C, Bean JA, et al. Internal tremor in patient with Parkinson’s disease. Mov Disord. 1996;11(1):3-7.
25. Cochrane GD, Rizvi S, Abrantes A, et al. Internal tremor in Parkinson’s disease, multiple sclerosis, and essential tremor. Parkinsonism Relat Disord. 2015;21(10):1145-1147.
26. Del Prete E, Schmitt E, Meoni S, et al. Do neuropsychiatric fluctuations temporally match motor fluctuations in Parkinson’s disease? Neurol Sci. 2022;43(6):3641-3647.
27. Kleiner G, Fernandez HH, Chou KL, et al. Non-motor fluctuations in Parkinson’s disease: validation of the non-motor fluctuation assessment questionnaire. Mov Disord. 2021;36(6):1392-1400.
28. Pachi I, Maraki MI, Giagkou N, et al. Late life psychotic features in prodromal Parkinson’s disease. Parkinsonism Relat Disord. 2021;86:67-73.
29. Forsaa EB, Larsen JP, Wentzel-Larsen T, et al. A 12-year population-based study of psychosis in Parkinson’s disease. Arch Neurol. 2010;67(8):996-1001.
30. Chang A, Fox SH. Psychosis in Parkinson’s disease: epidemiology, pathophysiology, and management. Drugs. 2016;76(11):1093-1118.
31. Kasunich A, Kilbane C, Wiggins R. Movement disorders moment: pain and palliative care in movement disorders. Practical Neurology. 2021;20(4):63-67.
32. Burn D, Emre M, McKeith I, et al. Effects of rivastigmine in patients with and without visual hallucinations in dementia associated with Parkinson’s disease. Mov Disord. 2006;21(11):1899-1907.
33. Tripathi M, Vibha D. Reversible dementias. Indian J Psychiatry. 2009; 51 Suppl 1(Suppl 1): S52-S55.
34. Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75(19):1717-1725.
35. Goldman J, Sieg, E. Cognitive impairment and dementia in Parkinson disease. Clin Geriatr Med. 2020;36(2):365-377.
36. Gonzalez-Latapi P, Bayram E, Litvan I, et al. Cognitive impairment in Parkinson’s disease: epidemiology, clinical profile, protective and risk factors. Behav Sci (Basel). 2021;11(5):74.
37. Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force Guidelines. Mov Disord. 2012;27(3):349-356.
38. Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22(16):2314-2324.
39. Aarsland D, Batzu L, Halliday GM, et al. Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers. 2021;7(1):47. doi:10.1038/s41572-021-00280-3
40. Weintraub D, Claassen DO. Impulse control and related disorders in Parkinson’s disease. Int Rev Neurobiol. 2017;133:679-717.
41. Vilas D, Pont-Sunyer C, Tolosa E. Impulse control disorders in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18 Suppl 1:S80-S84.
42. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589-595.
43. Faouzi J, Corvol JC, Mariani LL. Impulse control disorders and related behaviors in Parkinson’s disease: risk factors, clinical and genetic aspects, and management. Curr Opin Neurol. 2021;34(4):547-555.
44. Samuel M, Rodriguez-Oroz M, Antonini A, et al. Impulse control disorders in Parkinson’s disease: management, controversies, and potential approaches. Mov Disord. 2015;30(2):150-159.
45. Frank MJ, Samanta J, Moustafa AA, et al. Hold your horses: impulsivity, deep brain stimulation and medication in Parkinsonism. Science. 2007;318(5854):1309-1312.
46. Jahanshahi M, Obeso I, Baunez C, et al. Parkinson’s disease, the subthalamic nucleus, inhibition, and impulsivity. Mov Disord. 2015;30(2):128-140.
47. Castrioto A, Lhommée E, Moro E, et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurol. 2014;13(3):287-305.
Parkinson’s disease (PD) is a neurodegenerative condition diagnosed pathologically by alpha synuclein–containing Lewy bodies and dopaminergic cell loss in the substantia nigra pars compacta of the midbrain. Loss of dopaminergic input to the caudate and putamen disrupts the direct and indirect basal ganglia pathways for motor control and contributes to the motor symptoms of PD.1 According to the Movement Disorder Society criteria, PD is diagnosed clinically by bradykinesia (slowness of movement) plus resting tremor and/or rigidity in the presence of supportive criteria, such as levodopa responsiveness and hyposmia, and in the absence of exclusion criteria and red flags that would suggest atypical parkinsonism or an alternative diagnosis.2
Although the diagnosis and treatment of PD focus heavily on the motor symptoms, nonmotor symptoms can arise decades before the onset of motor symptoms and continue throughout the lifespan. Nonmotor symptoms affect patients from head (ie, cognition and mood) to toe (ie, striatal toe pain) and multiple organ systems in between, including the olfactory, integumentary, cardiovascular, gastrointestinal, genitourinary, and autonomic nervous systems. Thus, it is not surprising that nonmotor symptoms of PD impact health-related quality of life more substantially than motor symptoms.3 A helpful analogy is to consider the motor symptoms of PD as the tip of the iceberg and the nonmotor symptoms as the larger, submerged portions of the iceberg.4
Nonmotor symptoms can negatively impact the treatment of motor symptoms. For example, imagine a patient who is very rigid and dyscoordinated in the arms and legs, which limits their ability to dress and walk. If this patient also suffers from nonmotor symptoms of orthostatic hypotension and psychosis—both of which can be exacerbated by levodopa—dose escalation of levodopa for the rigidity and dyscoordination could be compromised, rendering the patient undertreated and less mobile.
In this review, we focus on identifying and managing nonmotor symptoms of PD that are relevant to psychiatric practice, including mood and motivational disorders, anxiety disorders, psychosis, cognitive disorders, and disorders related to the pharmacologic and surgical treatment of PD (Figure 1).

Mood and motivational disorders
Depression
Depression is a common symptom in PD that can occur in the prodromal period years to decades before the onset of motor symptoms, as well as throughout the disease course.5 The prevalence of depression in PD varies from 3% to 90%, depending on the methods of assessment, clinical setting of assessment, motor symptom severity, and other factors; clinically significant depression likely affects approximately 35% to 38% of patients.5,6 How depression in patients with PD differs from depression in the general population is not entirely understood, but there does seem to be less guilt and suicidal ideation and a substantial component of negative affect, including dysphoria and anxiety.7 Practically speaking, depression is treated similarly in PD and general populations, with a few considerations.
Despite limited randomized controlled trials (RCTs) for efficacy specifically in patients with PD, selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are generally considered first-line treatments. There is also evidence for tricyclic antidepressants (TCAs), but due to potential worsening of orthostatic hypotension and cognition, TCAs may not be a favorable option for certain patients with PD.8,9 All antidepressants have the potential to worsen tremor. Theoretically, SNRIs, with noradrenergic activity, may be less tolerable than SSRIs in patients with PD. However, worsening tremor generally has not been a clinically significant adverse event reported in PD depression clinical trials, although it was seen in 17% of patients receiving paroxetine and 21% of patients receiving venlafaxine compared to 7% of patients receiving placebo.9-11 If tremor worsens, mirtazapine could be considered because it has been reported to cause less tremor than SSRIs or TCAs.12
Among medications for PD, pramipexole, a dopamine agonist, may have a beneficial effect on depression.13 Additionally, some evidence supports rasagiline, a monoamine oxidase type B inhibitor, as an adjunctive medication for depression in PD.14 Nevertheless, antidepressant medications remain the standard pharmacologic treatment for PD depression.
Continue to: In terms of nonpharmacologic options...
In terms of nonpharmacologic options, cognitive-behavioral therapy (CBT) is likely efficacious, exercise (especially yoga) is likely efficacious, and repetitive transcranial magnetic stimulation may be efficacious.15,16 While further high-quality trials are needed, these treatments are low-risk and can be considered, especially for patients who cannot tolerate medications.
Apathy
Apathy—a loss of motivation and goal-directed behavior—can occur in up to 30% of patients during the prodromal period of PD, and in up to 70% of patients throughout the disease course.17 Apathy can coexist with depression, which can make apathy difficult to diagnose.17 Given the time constraints of a clinic visit, a practical approach would be to first screen for depression and cognitive impairment. If there is continued suspicion of apathy, the Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale part I question (“In the past week have you felt indifferent to doing activities or being with people?”) can be used to screen for apathy, and more detailed scales, such as the Apathy Scale (AS) or Lille Apathy Rating Scale (LARS), could be used if indicated.18
There are limited high-quality positive trials of apathy-specific treatments in PD. In an RCT of patients with PD who did not have depression or dementia, rivastigmine improved LARS scores compared to placebo.15 Piribedil, a D2/D3 receptor agonist, improved apathy in patients who underwent subthalamic nucleus deep brain stimulation (STN DBS).15 Exercise such as individualized physical therapy programs, dance, and Nordic walking as well as mindfulness interventions were shown to significantly reduce apathy scale scores.19 SSRIs, SNRIs, and rotigotine showed a trend toward reducing AS scores in RCTs.10,20
Larger, high-quality studies are needed to clarify the treatment of apathy in PD. In the meantime, a reasonable approach is to first treat any comorbid psychiatric or cognitive disorders, since apathy can be associated with these conditions, and to optimize antiparkinsonian medications for motor symptoms, motor fluctuations, and nonmotor fluctuations. Then, the investigational apathy treatments described in this section could be considered on an individual basis.
Anxiety disorders
Anxiety is seen throughout the disease course of PD in approximately 30% to 50% of patients.21 It can manifest as generalized anxiety disorder, panic disorder, and other anxiety disorders. There are no high-quality RCTs of pharmacologic treatments of anxiety specifically in patients with PD, except for a negative safety and tolerability study of buspirone in which one-half of patients experienced worsening motor symptoms.15,22 Thus, the treatment of anxiety in patients with PD is similar to treatments in the general population. SSRIs and SNRIs are typically considered first-line, benzodiazepines are sometimes used with caution (although cognitive adverse effects and fall risk need to be considered), and nonpharmacologic treatments such as mindfulness yoga, exercise, CBT, and psychotherapy can be effective.16,21,23
Continue to: Because there is the lack...
Because there is the lack of evidence-based treatments for anxiety in PD, we highlight 2 PD-specific anxiety disorders: internal tremor, and nonmotor “off” anxiety.
Internal tremor
Internal tremor is a sense of vibration in the axial and/or appendicular muscles that cannot be seen externally by the patient or examiner. It is not yet fully understood if this phenomenon is sensory, anxiety-related, related to subclinical tremor, or the result of a combination of these factors (ie, sensory awareness of a subclinical tremor that triggers or is worsened by anxiety). There is some evidence for subclinical tremor on electromyography, but internal tremor does not respond to antiparkinsonian medications in 70% of patients.24 More electrophysiological research is needed to clarify this phenomenon. Internal tremor has been associated with anxiety in 64% of patients and often improves with anxiolytic therapies.24
Although poorly understood, internal tremor is a documented phenomenon in 33% to 44% of patients with PD, and in some cases, it may be an initial symptom that motivates a patient to seek medical attention for the first time.24,25 Internal tremor has also been reported in patients with essential tremor and multiple sclerosis.25 Therefore, physicians should be aware of internal tremor because this symptom could herald an underlying neurological disease.
Nonmotor ‘off’ anxiety
Patients with PD are commonly prescribed carbidopa-levodopa, a dopamine precursor, at least 3 times daily. Initially, this medication controls motor symptoms well from 1 dose to the next. However, as the disease progresses, some patients report motor fluctuations in which an individual dose of carbidopa-levodopa may wear off early, take longer than usual to take effect, or not take effect at all. Patients describe these periods as an “off” state in which they do not feel their medications are working. Such motor fluctuations can lead to anxiety and avoidance behaviors, because patients fear being in public at times when the medication does not adequately control their motor symptoms.
In addition to these motor symptom fluctuations and related anxiety, patients can also experience nonmotor symptom fluctuations. A wide variety of nonmotor symptoms, such as mood, cognitive, and behavioral symptoms, have been reported to fluctuate in parallel with motor symptoms.26,27 One study reported fluctuating restlessness in 39% of patients with PD, excessive worry in 17%, shortness of breath in 13%, excessive sweating and fear in 12%, and palpitations in 10%.27 A patient with fluctuating shortness of breath, sweating, and palpitations (for example) may repeatedly present to the emergency department with a negative cardiac workup and eventually be diagnosed with panic disorder, whereas the patient is truly experiencing nonmotor “off” symptoms. Thus, it is important to be aware of nonmotor fluctuations so this diagnosis can be made and the symptoms appropriately treated. The first step in treating nonmotor fluctuations is to optimize the antiparkinsonian regimen to minimize fluctuations. If “off” anxiety symptoms persist, anxiolytic medications can be prescribed.21
Continue to: Psychosis
Psychosis
Psychosis can occur in prodromal and early PD but is most common in advanced PD.28 One study reported that 60% of patients developed hallucinations or delusions after 12 years of follow-up.29 Disease duration, disease severity, dementia, and rapid eye movement sleep behavior disorder are significant risk factors for psychosis in PD.30 Well-formed visual hallucinations are the most common manifestation of psychosis in patients with PD. Auditory hallucinations and delusions are less common. Delusions are usually seen in patients with dementia and are often paranoid delusions, such as of spousal infidelity.30 Sensory hallucinations can occur, but should not be mistaken with formication, a central pain syndrome in PD that can represent a nonmotor “off” symptom that may respond to dopaminergic medication.31 Other more mild psychotic symptoms include illusions or misinterpretation of stimuli, false sense of presence, and passage hallucinations of fleeting figures in the peripheral vision.30
The pathophysiology of PD psychosis is not entirely understood but differs from psychosis in other disorders. It can occur in the absence of antiparkinsonian medication exposure and is thought to be a consequence of the underlying disease process of PD involving neurodegeneration in certain brain regions and aberrant neurotransmission of not only dopamine but also serotonin, acetylcholine, and glutamate.30
Figure 2 outlines the management of psychosis in PD. After addressing medical and medication-related causes, it is important to determine if the psychotic symptom is sufficiently bothersome to and/or potentially dangerous for the patient to warrant treatment. If treatment is indicated, pimavanserin and clozapine are efficacious for psychosis in PD without worsening motor symptoms, and quetiapine is possibly efficacious with a low risk of worsening motor symptoms.15 Other antipsychotics, such as olanzapine, risperidone, and haloperidol, can substantially worsen motor symptoms.15 Both second-generation antipsychotics and pimavanserin have an FDA black-box warning for a higher risk of all-cause mortality in older patients with dementia; however, because psychosis is associated with early mortality in PD, the risk/benefit ratio should be discussed with the patient and family for shared decision-making.30 If the patient also has dementia, rivastigmine—which is FDA-approved for PD dementia (PDD)—may also improve hallucinations.32

Cognitive disorders
This section focuses on PD mild cognitive impairment (PD-MCI) and PDD. When a patient with PD reports cognitive concerns, the approach outlined in Figure 3 can be used to diagnose the cognitive disorder. A detailed history, medication review, and physical examination can identify any medical or psychiatric conditions that could affect cognition. The American Academy of Neurology recommends screening for depression, obtaining blood levels of vitamin B12 and thyroid-stimulating hormone, and obtaining a CT or MRI of the brain to rule out reversible causes of dementia.33 A validated screening test such as the Montreal Cognitive Assessment, which has higher sensitivity for PD-MCI than the Mini-Mental State Examination, is used to identify and quantify cognitive impairment.34 Neuropsychological testing is the gold standard and can be used to confirm and/or better quantify the degree and domains of cognitive impairment.35 Typically, cognitive deficits in PD affect executive function, attention, and/or visuospatial domains more than memory and language early on, and deficits in visuospatial and language domains have the highest sensitivity for predicting progression to PDD.36

Once reversible causes of dementia are addressed or ruled out and cognitive testing is completed, the Movement Disorder Society (MDS) criteria for PD-MCI and PDD summarized in Figure 3 can be used to diagnose the cognitive disorder.37,38 The MDS criteria for PDD require a diagnosis of PD for ≥1 year prior to the onset of dementia to differentiate PDD from dementia with Lewy bodies (DLB). If the dementia starts within 1 year of the onset of parkinsonism, the diagnosis would be DLB. PDD and DLB are on the spectrum of Lewy body dementia, with the same Lewy body pathology in different temporal and spatial distributions in the brain.38
Continue to: PD-MCI is present in...
PD-MCI is present in approximately 25% of patients.35 PD-MCI does not always progress to dementia but increases the risk of dementia 6-fold. The prevalence of PDD increases with disease duration; it is present in approximately 50% of patients at 10 years and 80% of patients at 20 years of disease.35 Rivastigmine is the only FDA-approved medication to slow progression of PDD. There is insufficient evidence for other acetylcholinesterase inhibitors and memantine.15 Unfortunately, RCTs of pharmacotherapy for PD-MCI have failed to show efficacy. However, exercise, cognitive rehabilitation, and neuromodulation are being studied. In the meantime, addressing modifiable risk factors (such as vascular risk factors and alcohol consumption) and treating comorbid orthostatic hypotension, obstructive sleep apnea, and depression may improve cognition.35,39
Treatment-related disorders
Impulse control disorders
Impulse control disorders (ICDs) are an important medication-related consideration in patients with PD. The ICDs seen in PD include pathological gambling, binge eating, excessive shopping, hypersexual behaviors, and dopamine dysregulation syndrome (Table). These disorders are more common in younger patients with a history of impulsive personality traits and addictive behaviors (eg, history of tobacco or alcohol abuse), and are most strongly associated with dopaminergic therapies, particularly the dopamine agonists.40,41 In the DOMINION study, the odds of ICDs were 2- to 3.5-fold higher in patients taking dopamine agonists.42 This is mainly thought to be due to stimulation of D2/D3 receptors in the mesolimbic system.40 High doses of levodopa, monoamine oxidase inhibitors, and amantadine are also associated with ICDs.40-42

The first step in managing ICDs is diagnosing them, which can be difficult because patients often are not forthcoming about these problems due to embarrassment or failure to recognize that the ICD is related to PD medications. If a family member accompanies the patient at the visit, the patient may not want to disclose the amount of money they spend or the extent to which the behavior is a problem. Thus, a screening questionnaire, such as the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease (QUIP) can be a helpful way for patients to alert the clinician to the issue.41 Education for the patient and family is crucial before the ICD causes significant financial, health, or relationship problems.
The mainstay of treatment is to reduce or taper off the dopamine agonist or other offending agent while monitoring for worsening motor symptoms and dopamine withdrawal syndrome. If this is unsuccessful, there is very limited evidence for further treatment strategies (Table), including antidepressants, antipsychotics, and mood stabilizers.40,43,44 There is insufficient evidence for naltrexone based on an RCT that failed to meet its primary endpoint, although naltrexone did significantly reduce QUIP scores.15,44 There is also insufficient evidence for amantadine, which showed benefit in some studies but was associated with ICDs in the DOMINION study.15,40,42 In terms of nonpharmacologic treatments, CBT is likely efficacious.15,40 There are mixed results for STN DBS. Some studies showed improvement in the ICD, due at least in part to dopaminergic medication reduction postoperatively, but this treatment has also been reported to increase impulsivity.40,45
Deep brain stimulation–related disorders
For patients with PD, the ideal lead location for STN DBS is the dorsolateral aspect of the STN, as this is the motor region of the nucleus. The STN functions in indirect and hyperdirect pathways to put the brake on certain motor programs so only the desired movement can be executed. Its function is clinically demonstrated by patients with STN stroke who develop excessive ballistic movements. Adjacent to the motor region of the STN is a centrally located associative region and a medially located limbic region. Thus, when stimulating the dorsolateral STN, current can spread to those regions as well, and the STN’s ability to put the brake on behavioral and emotional programs can be affected.46 Stimulation of the STN has been associated with mania, euphoria, new-onset ICDs, decreased verbal fluency, and executive dysfunction. Depression, apathy, and anxiety can also occur, but more commonly result from rapid withdrawal of antiparkinsonian medications after DBS surgery.46,47 Therefore, for PD patients with DBS with new or worsening psychiatric or cognitive symptoms, it is important to inquire about any recent programming sessions with neurology as well as recent self-increases in stimulation by the patient using their controller. Collaboration with neurology is important to troubleshoot whether stimulation could be contributing to the patient’s psychiatric or cognitive symptoms.
Continue to: Bottom Line
Bottom Line
Mood, anxiety, psychotic, and cognitive symptoms and disorders are common psychiatric manifestations associated with Parkinson’s disease (PD). In addition, patients with PD may experience impulsive control disorders and other symptoms related to treatments they receive for PD. Careful assessment and collaboration with neurology is crucial to alleviating the effects of these conditions.
Related Resources
- Weintraub D, Aarsland D, Chaudhuri KR, et al. The neuropsychiatry of Parkinson’s disease: advances and challenges. Lancet Neurology. 2022;21(1):89-102. doi:10.1016/S1474-4422(21)00330-6
- Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurologic Clinics. 2020;38(2):269-292. doi:10.1016/j.ncl.2019.12.003
- Castrioto A, Lhommee E, Moro E et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurology. 2014;13(3):287-305. doi:10.1016/ S1474-4422(13)70294-1
Drug Brand Names
Amantadine • Gocovri
Carbidopa-levodopa • Sinemet
Clozapine • Clozaril
Haloperidol • Haldol
Memantine • Namenda
Mirtazapine • Remeron
Naltrexone • Vivitrol
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimavanserin • Nuplazid
Piribedil • Pronoran
Pramipexole • Mirapex
Quetiapine • Seroquel
Rasagiline • Azilect
Risperidone • Risperdal
Rivastigmine • Exelon
Ropinirole • Requip
Rotigotine • Neupro
Venlafaxine • Effexor
Zonisamide • Zonegran
Parkinson’s disease (PD) is a neurodegenerative condition diagnosed pathologically by alpha synuclein–containing Lewy bodies and dopaminergic cell loss in the substantia nigra pars compacta of the midbrain. Loss of dopaminergic input to the caudate and putamen disrupts the direct and indirect basal ganglia pathways for motor control and contributes to the motor symptoms of PD.1 According to the Movement Disorder Society criteria, PD is diagnosed clinically by bradykinesia (slowness of movement) plus resting tremor and/or rigidity in the presence of supportive criteria, such as levodopa responsiveness and hyposmia, and in the absence of exclusion criteria and red flags that would suggest atypical parkinsonism or an alternative diagnosis.2
Although the diagnosis and treatment of PD focus heavily on the motor symptoms, nonmotor symptoms can arise decades before the onset of motor symptoms and continue throughout the lifespan. Nonmotor symptoms affect patients from head (ie, cognition and mood) to toe (ie, striatal toe pain) and multiple organ systems in between, including the olfactory, integumentary, cardiovascular, gastrointestinal, genitourinary, and autonomic nervous systems. Thus, it is not surprising that nonmotor symptoms of PD impact health-related quality of life more substantially than motor symptoms.3 A helpful analogy is to consider the motor symptoms of PD as the tip of the iceberg and the nonmotor symptoms as the larger, submerged portions of the iceberg.4
Nonmotor symptoms can negatively impact the treatment of motor symptoms. For example, imagine a patient who is very rigid and dyscoordinated in the arms and legs, which limits their ability to dress and walk. If this patient also suffers from nonmotor symptoms of orthostatic hypotension and psychosis—both of which can be exacerbated by levodopa—dose escalation of levodopa for the rigidity and dyscoordination could be compromised, rendering the patient undertreated and less mobile.
In this review, we focus on identifying and managing nonmotor symptoms of PD that are relevant to psychiatric practice, including mood and motivational disorders, anxiety disorders, psychosis, cognitive disorders, and disorders related to the pharmacologic and surgical treatment of PD (Figure 1).

Mood and motivational disorders
Depression
Depression is a common symptom in PD that can occur in the prodromal period years to decades before the onset of motor symptoms, as well as throughout the disease course.5 The prevalence of depression in PD varies from 3% to 90%, depending on the methods of assessment, clinical setting of assessment, motor symptom severity, and other factors; clinically significant depression likely affects approximately 35% to 38% of patients.5,6 How depression in patients with PD differs from depression in the general population is not entirely understood, but there does seem to be less guilt and suicidal ideation and a substantial component of negative affect, including dysphoria and anxiety.7 Practically speaking, depression is treated similarly in PD and general populations, with a few considerations.
Despite limited randomized controlled trials (RCTs) for efficacy specifically in patients with PD, selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are generally considered first-line treatments. There is also evidence for tricyclic antidepressants (TCAs), but due to potential worsening of orthostatic hypotension and cognition, TCAs may not be a favorable option for certain patients with PD.8,9 All antidepressants have the potential to worsen tremor. Theoretically, SNRIs, with noradrenergic activity, may be less tolerable than SSRIs in patients with PD. However, worsening tremor generally has not been a clinically significant adverse event reported in PD depression clinical trials, although it was seen in 17% of patients receiving paroxetine and 21% of patients receiving venlafaxine compared to 7% of patients receiving placebo.9-11 If tremor worsens, mirtazapine could be considered because it has been reported to cause less tremor than SSRIs or TCAs.12
Among medications for PD, pramipexole, a dopamine agonist, may have a beneficial effect on depression.13 Additionally, some evidence supports rasagiline, a monoamine oxidase type B inhibitor, as an adjunctive medication for depression in PD.14 Nevertheless, antidepressant medications remain the standard pharmacologic treatment for PD depression.
Continue to: In terms of nonpharmacologic options...
In terms of nonpharmacologic options, cognitive-behavioral therapy (CBT) is likely efficacious, exercise (especially yoga) is likely efficacious, and repetitive transcranial magnetic stimulation may be efficacious.15,16 While further high-quality trials are needed, these treatments are low-risk and can be considered, especially for patients who cannot tolerate medications.
Apathy
Apathy—a loss of motivation and goal-directed behavior—can occur in up to 30% of patients during the prodromal period of PD, and in up to 70% of patients throughout the disease course.17 Apathy can coexist with depression, which can make apathy difficult to diagnose.17 Given the time constraints of a clinic visit, a practical approach would be to first screen for depression and cognitive impairment. If there is continued suspicion of apathy, the Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale part I question (“In the past week have you felt indifferent to doing activities or being with people?”) can be used to screen for apathy, and more detailed scales, such as the Apathy Scale (AS) or Lille Apathy Rating Scale (LARS), could be used if indicated.18
There are limited high-quality positive trials of apathy-specific treatments in PD. In an RCT of patients with PD who did not have depression or dementia, rivastigmine improved LARS scores compared to placebo.15 Piribedil, a D2/D3 receptor agonist, improved apathy in patients who underwent subthalamic nucleus deep brain stimulation (STN DBS).15 Exercise such as individualized physical therapy programs, dance, and Nordic walking as well as mindfulness interventions were shown to significantly reduce apathy scale scores.19 SSRIs, SNRIs, and rotigotine showed a trend toward reducing AS scores in RCTs.10,20
Larger, high-quality studies are needed to clarify the treatment of apathy in PD. In the meantime, a reasonable approach is to first treat any comorbid psychiatric or cognitive disorders, since apathy can be associated with these conditions, and to optimize antiparkinsonian medications for motor symptoms, motor fluctuations, and nonmotor fluctuations. Then, the investigational apathy treatments described in this section could be considered on an individual basis.
Anxiety disorders
Anxiety is seen throughout the disease course of PD in approximately 30% to 50% of patients.21 It can manifest as generalized anxiety disorder, panic disorder, and other anxiety disorders. There are no high-quality RCTs of pharmacologic treatments of anxiety specifically in patients with PD, except for a negative safety and tolerability study of buspirone in which one-half of patients experienced worsening motor symptoms.15,22 Thus, the treatment of anxiety in patients with PD is similar to treatments in the general population. SSRIs and SNRIs are typically considered first-line, benzodiazepines are sometimes used with caution (although cognitive adverse effects and fall risk need to be considered), and nonpharmacologic treatments such as mindfulness yoga, exercise, CBT, and psychotherapy can be effective.16,21,23
Continue to: Because there is the lack...
Because there is the lack of evidence-based treatments for anxiety in PD, we highlight 2 PD-specific anxiety disorders: internal tremor, and nonmotor “off” anxiety.
Internal tremor
Internal tremor is a sense of vibration in the axial and/or appendicular muscles that cannot be seen externally by the patient or examiner. It is not yet fully understood if this phenomenon is sensory, anxiety-related, related to subclinical tremor, or the result of a combination of these factors (ie, sensory awareness of a subclinical tremor that triggers or is worsened by anxiety). There is some evidence for subclinical tremor on electromyography, but internal tremor does not respond to antiparkinsonian medications in 70% of patients.24 More electrophysiological research is needed to clarify this phenomenon. Internal tremor has been associated with anxiety in 64% of patients and often improves with anxiolytic therapies.24
Although poorly understood, internal tremor is a documented phenomenon in 33% to 44% of patients with PD, and in some cases, it may be an initial symptom that motivates a patient to seek medical attention for the first time.24,25 Internal tremor has also been reported in patients with essential tremor and multiple sclerosis.25 Therefore, physicians should be aware of internal tremor because this symptom could herald an underlying neurological disease.
Nonmotor ‘off’ anxiety
Patients with PD are commonly prescribed carbidopa-levodopa, a dopamine precursor, at least 3 times daily. Initially, this medication controls motor symptoms well from 1 dose to the next. However, as the disease progresses, some patients report motor fluctuations in which an individual dose of carbidopa-levodopa may wear off early, take longer than usual to take effect, or not take effect at all. Patients describe these periods as an “off” state in which they do not feel their medications are working. Such motor fluctuations can lead to anxiety and avoidance behaviors, because patients fear being in public at times when the medication does not adequately control their motor symptoms.
In addition to these motor symptom fluctuations and related anxiety, patients can also experience nonmotor symptom fluctuations. A wide variety of nonmotor symptoms, such as mood, cognitive, and behavioral symptoms, have been reported to fluctuate in parallel with motor symptoms.26,27 One study reported fluctuating restlessness in 39% of patients with PD, excessive worry in 17%, shortness of breath in 13%, excessive sweating and fear in 12%, and palpitations in 10%.27 A patient with fluctuating shortness of breath, sweating, and palpitations (for example) may repeatedly present to the emergency department with a negative cardiac workup and eventually be diagnosed with panic disorder, whereas the patient is truly experiencing nonmotor “off” symptoms. Thus, it is important to be aware of nonmotor fluctuations so this diagnosis can be made and the symptoms appropriately treated. The first step in treating nonmotor fluctuations is to optimize the antiparkinsonian regimen to minimize fluctuations. If “off” anxiety symptoms persist, anxiolytic medications can be prescribed.21
Continue to: Psychosis
Psychosis
Psychosis can occur in prodromal and early PD but is most common in advanced PD.28 One study reported that 60% of patients developed hallucinations or delusions after 12 years of follow-up.29 Disease duration, disease severity, dementia, and rapid eye movement sleep behavior disorder are significant risk factors for psychosis in PD.30 Well-formed visual hallucinations are the most common manifestation of psychosis in patients with PD. Auditory hallucinations and delusions are less common. Delusions are usually seen in patients with dementia and are often paranoid delusions, such as of spousal infidelity.30 Sensory hallucinations can occur, but should not be mistaken with formication, a central pain syndrome in PD that can represent a nonmotor “off” symptom that may respond to dopaminergic medication.31 Other more mild psychotic symptoms include illusions or misinterpretation of stimuli, false sense of presence, and passage hallucinations of fleeting figures in the peripheral vision.30
The pathophysiology of PD psychosis is not entirely understood but differs from psychosis in other disorders. It can occur in the absence of antiparkinsonian medication exposure and is thought to be a consequence of the underlying disease process of PD involving neurodegeneration in certain brain regions and aberrant neurotransmission of not only dopamine but also serotonin, acetylcholine, and glutamate.30
Figure 2 outlines the management of psychosis in PD. After addressing medical and medication-related causes, it is important to determine if the psychotic symptom is sufficiently bothersome to and/or potentially dangerous for the patient to warrant treatment. If treatment is indicated, pimavanserin and clozapine are efficacious for psychosis in PD without worsening motor symptoms, and quetiapine is possibly efficacious with a low risk of worsening motor symptoms.15 Other antipsychotics, such as olanzapine, risperidone, and haloperidol, can substantially worsen motor symptoms.15 Both second-generation antipsychotics and pimavanserin have an FDA black-box warning for a higher risk of all-cause mortality in older patients with dementia; however, because psychosis is associated with early mortality in PD, the risk/benefit ratio should be discussed with the patient and family for shared decision-making.30 If the patient also has dementia, rivastigmine—which is FDA-approved for PD dementia (PDD)—may also improve hallucinations.32

Cognitive disorders
This section focuses on PD mild cognitive impairment (PD-MCI) and PDD. When a patient with PD reports cognitive concerns, the approach outlined in Figure 3 can be used to diagnose the cognitive disorder. A detailed history, medication review, and physical examination can identify any medical or psychiatric conditions that could affect cognition. The American Academy of Neurology recommends screening for depression, obtaining blood levels of vitamin B12 and thyroid-stimulating hormone, and obtaining a CT or MRI of the brain to rule out reversible causes of dementia.33 A validated screening test such as the Montreal Cognitive Assessment, which has higher sensitivity for PD-MCI than the Mini-Mental State Examination, is used to identify and quantify cognitive impairment.34 Neuropsychological testing is the gold standard and can be used to confirm and/or better quantify the degree and domains of cognitive impairment.35 Typically, cognitive deficits in PD affect executive function, attention, and/or visuospatial domains more than memory and language early on, and deficits in visuospatial and language domains have the highest sensitivity for predicting progression to PDD.36

Once reversible causes of dementia are addressed or ruled out and cognitive testing is completed, the Movement Disorder Society (MDS) criteria for PD-MCI and PDD summarized in Figure 3 can be used to diagnose the cognitive disorder.37,38 The MDS criteria for PDD require a diagnosis of PD for ≥1 year prior to the onset of dementia to differentiate PDD from dementia with Lewy bodies (DLB). If the dementia starts within 1 year of the onset of parkinsonism, the diagnosis would be DLB. PDD and DLB are on the spectrum of Lewy body dementia, with the same Lewy body pathology in different temporal and spatial distributions in the brain.38
Continue to: PD-MCI is present in...
PD-MCI is present in approximately 25% of patients.35 PD-MCI does not always progress to dementia but increases the risk of dementia 6-fold. The prevalence of PDD increases with disease duration; it is present in approximately 50% of patients at 10 years and 80% of patients at 20 years of disease.35 Rivastigmine is the only FDA-approved medication to slow progression of PDD. There is insufficient evidence for other acetylcholinesterase inhibitors and memantine.15 Unfortunately, RCTs of pharmacotherapy for PD-MCI have failed to show efficacy. However, exercise, cognitive rehabilitation, and neuromodulation are being studied. In the meantime, addressing modifiable risk factors (such as vascular risk factors and alcohol consumption) and treating comorbid orthostatic hypotension, obstructive sleep apnea, and depression may improve cognition.35,39
Treatment-related disorders
Impulse control disorders
Impulse control disorders (ICDs) are an important medication-related consideration in patients with PD. The ICDs seen in PD include pathological gambling, binge eating, excessive shopping, hypersexual behaviors, and dopamine dysregulation syndrome (Table). These disorders are more common in younger patients with a history of impulsive personality traits and addictive behaviors (eg, history of tobacco or alcohol abuse), and are most strongly associated with dopaminergic therapies, particularly the dopamine agonists.40,41 In the DOMINION study, the odds of ICDs were 2- to 3.5-fold higher in patients taking dopamine agonists.42 This is mainly thought to be due to stimulation of D2/D3 receptors in the mesolimbic system.40 High doses of levodopa, monoamine oxidase inhibitors, and amantadine are also associated with ICDs.40-42

The first step in managing ICDs is diagnosing them, which can be difficult because patients often are not forthcoming about these problems due to embarrassment or failure to recognize that the ICD is related to PD medications. If a family member accompanies the patient at the visit, the patient may not want to disclose the amount of money they spend or the extent to which the behavior is a problem. Thus, a screening questionnaire, such as the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease (QUIP) can be a helpful way for patients to alert the clinician to the issue.41 Education for the patient and family is crucial before the ICD causes significant financial, health, or relationship problems.
The mainstay of treatment is to reduce or taper off the dopamine agonist or other offending agent while monitoring for worsening motor symptoms and dopamine withdrawal syndrome. If this is unsuccessful, there is very limited evidence for further treatment strategies (Table), including antidepressants, antipsychotics, and mood stabilizers.40,43,44 There is insufficient evidence for naltrexone based on an RCT that failed to meet its primary endpoint, although naltrexone did significantly reduce QUIP scores.15,44 There is also insufficient evidence for amantadine, which showed benefit in some studies but was associated with ICDs in the DOMINION study.15,40,42 In terms of nonpharmacologic treatments, CBT is likely efficacious.15,40 There are mixed results for STN DBS. Some studies showed improvement in the ICD, due at least in part to dopaminergic medication reduction postoperatively, but this treatment has also been reported to increase impulsivity.40,45
Deep brain stimulation–related disorders
For patients with PD, the ideal lead location for STN DBS is the dorsolateral aspect of the STN, as this is the motor region of the nucleus. The STN functions in indirect and hyperdirect pathways to put the brake on certain motor programs so only the desired movement can be executed. Its function is clinically demonstrated by patients with STN stroke who develop excessive ballistic movements. Adjacent to the motor region of the STN is a centrally located associative region and a medially located limbic region. Thus, when stimulating the dorsolateral STN, current can spread to those regions as well, and the STN’s ability to put the brake on behavioral and emotional programs can be affected.46 Stimulation of the STN has been associated with mania, euphoria, new-onset ICDs, decreased verbal fluency, and executive dysfunction. Depression, apathy, and anxiety can also occur, but more commonly result from rapid withdrawal of antiparkinsonian medications after DBS surgery.46,47 Therefore, for PD patients with DBS with new or worsening psychiatric or cognitive symptoms, it is important to inquire about any recent programming sessions with neurology as well as recent self-increases in stimulation by the patient using their controller. Collaboration with neurology is important to troubleshoot whether stimulation could be contributing to the patient’s psychiatric or cognitive symptoms.
Continue to: Bottom Line
Bottom Line
Mood, anxiety, psychotic, and cognitive symptoms and disorders are common psychiatric manifestations associated with Parkinson’s disease (PD). In addition, patients with PD may experience impulsive control disorders and other symptoms related to treatments they receive for PD. Careful assessment and collaboration with neurology is crucial to alleviating the effects of these conditions.
Related Resources
- Weintraub D, Aarsland D, Chaudhuri KR, et al. The neuropsychiatry of Parkinson’s disease: advances and challenges. Lancet Neurology. 2022;21(1):89-102. doi:10.1016/S1474-4422(21)00330-6
- Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurologic Clinics. 2020;38(2):269-292. doi:10.1016/j.ncl.2019.12.003
- Castrioto A, Lhommee E, Moro E et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurology. 2014;13(3):287-305. doi:10.1016/ S1474-4422(13)70294-1
Drug Brand Names
Amantadine • Gocovri
Carbidopa-levodopa • Sinemet
Clozapine • Clozaril
Haloperidol • Haldol
Memantine • Namenda
Mirtazapine • Remeron
Naltrexone • Vivitrol
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimavanserin • Nuplazid
Piribedil • Pronoran
Pramipexole • Mirapex
Quetiapine • Seroquel
Rasagiline • Azilect
Risperidone • Risperdal
Rivastigmine • Exelon
Ropinirole • Requip
Rotigotine • Neupro
Venlafaxine • Effexor
Zonisamide • Zonegran
1. Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet Neurology. 2021;397(10291):2284-2303.
2. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Movement Disorders. 2015;30(12):1591-1601.
3. Martinez-Martin P, Rodriguez-Blazquez C, Kurtiz MM, et al. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord. 2011;26(3):399-406.
4. Langston WJ. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59(4):591-596.
5. Cong S, Xiang C, Zhang S, et al. Prevalence and clinical aspects of depression in Parkinson’s disease: a systematic review and meta‑analysis of 129 studies. Neurosci Biobehav Rev. 2022;141:104749. doi:10.1016/j.neubiorev.2022.104749
6. Reijnders JS, Ehrt U, Weber WE, et al. A systematic review of prevalence studies in depression in Parkinson’s disease. Mov Disord. 2008;23(2):183-189.
7. Zahodne LB, Marsiske M, Okun MS, et al. Components of depression in Parkinson disease. J Geriatr Psychiatry Neurol. 2012;25(3):131-137.
8. Skapinakis P, Bakola E, Salanti G, et al. Efficacy and acceptability of selective serotonin reuptake inhibitors for the treatment of depression in Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. BMC Neurology. 2010;10:49. doi:10.1186/1471-2377-10-49
9. Richard IH, McDermott MP, Kurlan R, et al; SAD-PD Study Group. A randomized, double-blind placebo-controlled trial of antidepressants in Parkinson’s disease. Neurology. 2012;78(16):1229-1236.
10. Takahashi M, Tabu H, Ozaki A, et al. Antidepressants for depression, apathy, and gait instability in Parkinson’s disease: a multicenter randomized study. Intern Med. 2019;58(3):361-368.
11. Bonuccelli U, Mecco G, Fabrini G, et al. A non-comparative assessment of tolerability and efficacy of duloxetine in the treatment of depressed patients with Parkinson’s disease. Expert Opin Pharmacother. 2012;13(16):2269-2280.
12. Wantanabe N, Omorio IM, Nakagawa A, et al; MANGA (Meta-Analysis of New Generation Antidepressants) Study Group. Safety reporting and adverse-event profile of mirtazapine described in randomized controlled trials in comparison with other classes of antidepressants in the acute-phase treatment of adults with depression. CNS Drugs. 2010;24(1):35-53.
13. Barone P, Scarzella L, Marconi R, et al; Depression/Parkinson Italian Study Group. Pramipexole versus sertraline in the treatment of depression in Parkinson’s disease: a national multicenter parallel-group randomized study. J Neurol. 2006;253(5):601-607.
14. Smith KM, Eyal E, Weintraub D, et al; ADAGIO Investigators. Combined rasagiline and anti-depressant use in Parkinson’s disease in the ADAGIO study: effects on non-motor symptoms and tolerability. JAMA Neurology. 2015;72(1):88-95.
15. Seppi K, Chaudhuri R, Coelho M, et al; the collaborators of the Parkinson’s Disease Update on Non-Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence-Based Medicine Committee. Update on treatments for nonmotor symptoms of Parkinson’s disease--an evidence-based medicine review. Mov Disord. 2019;34(2):180-198.
16. Kwok JYY, Kwan JCY, Auyeung M, et al. Effects of mindfulness yoga vs stretching and resistance training exercises on anxiety and depression for people with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2019;76(7):755-763.
17. De Waele S, Cras P, Crosiers D. Apathy in Parkinson’s disease: defining the Park apathy subtype. Brain Sci. 2022;12(7):923.
18. Mele B, Van S, Holroyd-Leduc J, et al. Diagnosis, treatment and management of apathy in Parkinson’s disease: a scoping review. BMJ Open. 2020;10(9):037632. doi:10.1136/bmjopen-2020-037632
19. Mele B, Ismail Z, Goodarzi Z, et al. Non-pharmacological interventions to treat apathy in Parkinson’s disease: a realist review. Clin Park Relat Disord. 2021;4:100096. doi:10.1016/j.prdoa.2021.100096
20. Chung SJ, Asgharnejad M, Bauer L, et al. Evaluation of rotigotine transdermal patch for the treatment of depressive symptoms in patients with Parkinson’s disease. Expert Opin Pharmacother. 2016;(17)11:1453-1461.
21. Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurol Clin. 2020;38(2):269-292.
22. Schneider RB, Auinger P, Tarolli CG, et al. A trial of buspirone for anxiety in Parkinson’s disease: safety and tolerability. Parkinsonism Relat Disord. 2020;81:69-74.
23. Moonen AJH, Mulders AEP, Defebvre L, et al. Cognitive behavioral therapy for anxiety in Parkinson’s disease: a randomized controlled trial. Mov Disord. 2021;36(11):2539-2548.
24. Shulman LM, Singer C, Bean JA, et al. Internal tremor in patient with Parkinson’s disease. Mov Disord. 1996;11(1):3-7.
25. Cochrane GD, Rizvi S, Abrantes A, et al. Internal tremor in Parkinson’s disease, multiple sclerosis, and essential tremor. Parkinsonism Relat Disord. 2015;21(10):1145-1147.
26. Del Prete E, Schmitt E, Meoni S, et al. Do neuropsychiatric fluctuations temporally match motor fluctuations in Parkinson’s disease? Neurol Sci. 2022;43(6):3641-3647.
27. Kleiner G, Fernandez HH, Chou KL, et al. Non-motor fluctuations in Parkinson’s disease: validation of the non-motor fluctuation assessment questionnaire. Mov Disord. 2021;36(6):1392-1400.
28. Pachi I, Maraki MI, Giagkou N, et al. Late life psychotic features in prodromal Parkinson’s disease. Parkinsonism Relat Disord. 2021;86:67-73.
29. Forsaa EB, Larsen JP, Wentzel-Larsen T, et al. A 12-year population-based study of psychosis in Parkinson’s disease. Arch Neurol. 2010;67(8):996-1001.
30. Chang A, Fox SH. Psychosis in Parkinson’s disease: epidemiology, pathophysiology, and management. Drugs. 2016;76(11):1093-1118.
31. Kasunich A, Kilbane C, Wiggins R. Movement disorders moment: pain and palliative care in movement disorders. Practical Neurology. 2021;20(4):63-67.
32. Burn D, Emre M, McKeith I, et al. Effects of rivastigmine in patients with and without visual hallucinations in dementia associated with Parkinson’s disease. Mov Disord. 2006;21(11):1899-1907.
33. Tripathi M, Vibha D. Reversible dementias. Indian J Psychiatry. 2009; 51 Suppl 1(Suppl 1): S52-S55.
34. Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75(19):1717-1725.
35. Goldman J, Sieg, E. Cognitive impairment and dementia in Parkinson disease. Clin Geriatr Med. 2020;36(2):365-377.
36. Gonzalez-Latapi P, Bayram E, Litvan I, et al. Cognitive impairment in Parkinson’s disease: epidemiology, clinical profile, protective and risk factors. Behav Sci (Basel). 2021;11(5):74.
37. Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force Guidelines. Mov Disord. 2012;27(3):349-356.
38. Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22(16):2314-2324.
39. Aarsland D, Batzu L, Halliday GM, et al. Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers. 2021;7(1):47. doi:10.1038/s41572-021-00280-3
40. Weintraub D, Claassen DO. Impulse control and related disorders in Parkinson’s disease. Int Rev Neurobiol. 2017;133:679-717.
41. Vilas D, Pont-Sunyer C, Tolosa E. Impulse control disorders in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18 Suppl 1:S80-S84.
42. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589-595.
43. Faouzi J, Corvol JC, Mariani LL. Impulse control disorders and related behaviors in Parkinson’s disease: risk factors, clinical and genetic aspects, and management. Curr Opin Neurol. 2021;34(4):547-555.
44. Samuel M, Rodriguez-Oroz M, Antonini A, et al. Impulse control disorders in Parkinson’s disease: management, controversies, and potential approaches. Mov Disord. 2015;30(2):150-159.
45. Frank MJ, Samanta J, Moustafa AA, et al. Hold your horses: impulsivity, deep brain stimulation and medication in Parkinsonism. Science. 2007;318(5854):1309-1312.
46. Jahanshahi M, Obeso I, Baunez C, et al. Parkinson’s disease, the subthalamic nucleus, inhibition, and impulsivity. Mov Disord. 2015;30(2):128-140.
47. Castrioto A, Lhommée E, Moro E, et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurol. 2014;13(3):287-305.
1. Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet Neurology. 2021;397(10291):2284-2303.
2. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Movement Disorders. 2015;30(12):1591-1601.
3. Martinez-Martin P, Rodriguez-Blazquez C, Kurtiz MM, et al. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord. 2011;26(3):399-406.
4. Langston WJ. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59(4):591-596.
5. Cong S, Xiang C, Zhang S, et al. Prevalence and clinical aspects of depression in Parkinson’s disease: a systematic review and meta‑analysis of 129 studies. Neurosci Biobehav Rev. 2022;141:104749. doi:10.1016/j.neubiorev.2022.104749
6. Reijnders JS, Ehrt U, Weber WE, et al. A systematic review of prevalence studies in depression in Parkinson’s disease. Mov Disord. 2008;23(2):183-189.
7. Zahodne LB, Marsiske M, Okun MS, et al. Components of depression in Parkinson disease. J Geriatr Psychiatry Neurol. 2012;25(3):131-137.
8. Skapinakis P, Bakola E, Salanti G, et al. Efficacy and acceptability of selective serotonin reuptake inhibitors for the treatment of depression in Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. BMC Neurology. 2010;10:49. doi:10.1186/1471-2377-10-49
9. Richard IH, McDermott MP, Kurlan R, et al; SAD-PD Study Group. A randomized, double-blind placebo-controlled trial of antidepressants in Parkinson’s disease. Neurology. 2012;78(16):1229-1236.
10. Takahashi M, Tabu H, Ozaki A, et al. Antidepressants for depression, apathy, and gait instability in Parkinson’s disease: a multicenter randomized study. Intern Med. 2019;58(3):361-368.
11. Bonuccelli U, Mecco G, Fabrini G, et al. A non-comparative assessment of tolerability and efficacy of duloxetine in the treatment of depressed patients with Parkinson’s disease. Expert Opin Pharmacother. 2012;13(16):2269-2280.
12. Wantanabe N, Omorio IM, Nakagawa A, et al; MANGA (Meta-Analysis of New Generation Antidepressants) Study Group. Safety reporting and adverse-event profile of mirtazapine described in randomized controlled trials in comparison with other classes of antidepressants in the acute-phase treatment of adults with depression. CNS Drugs. 2010;24(1):35-53.
13. Barone P, Scarzella L, Marconi R, et al; Depression/Parkinson Italian Study Group. Pramipexole versus sertraline in the treatment of depression in Parkinson’s disease: a national multicenter parallel-group randomized study. J Neurol. 2006;253(5):601-607.
14. Smith KM, Eyal E, Weintraub D, et al; ADAGIO Investigators. Combined rasagiline and anti-depressant use in Parkinson’s disease in the ADAGIO study: effects on non-motor symptoms and tolerability. JAMA Neurology. 2015;72(1):88-95.
15. Seppi K, Chaudhuri R, Coelho M, et al; the collaborators of the Parkinson’s Disease Update on Non-Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence-Based Medicine Committee. Update on treatments for nonmotor symptoms of Parkinson’s disease--an evidence-based medicine review. Mov Disord. 2019;34(2):180-198.
16. Kwok JYY, Kwan JCY, Auyeung M, et al. Effects of mindfulness yoga vs stretching and resistance training exercises on anxiety and depression for people with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2019;76(7):755-763.
17. De Waele S, Cras P, Crosiers D. Apathy in Parkinson’s disease: defining the Park apathy subtype. Brain Sci. 2022;12(7):923.
18. Mele B, Van S, Holroyd-Leduc J, et al. Diagnosis, treatment and management of apathy in Parkinson’s disease: a scoping review. BMJ Open. 2020;10(9):037632. doi:10.1136/bmjopen-2020-037632
19. Mele B, Ismail Z, Goodarzi Z, et al. Non-pharmacological interventions to treat apathy in Parkinson’s disease: a realist review. Clin Park Relat Disord. 2021;4:100096. doi:10.1016/j.prdoa.2021.100096
20. Chung SJ, Asgharnejad M, Bauer L, et al. Evaluation of rotigotine transdermal patch for the treatment of depressive symptoms in patients with Parkinson’s disease. Expert Opin Pharmacother. 2016;(17)11:1453-1461.
21. Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurol Clin. 2020;38(2):269-292.
22. Schneider RB, Auinger P, Tarolli CG, et al. A trial of buspirone for anxiety in Parkinson’s disease: safety and tolerability. Parkinsonism Relat Disord. 2020;81:69-74.
23. Moonen AJH, Mulders AEP, Defebvre L, et al. Cognitive behavioral therapy for anxiety in Parkinson’s disease: a randomized controlled trial. Mov Disord. 2021;36(11):2539-2548.
24. Shulman LM, Singer C, Bean JA, et al. Internal tremor in patient with Parkinson’s disease. Mov Disord. 1996;11(1):3-7.
25. Cochrane GD, Rizvi S, Abrantes A, et al. Internal tremor in Parkinson’s disease, multiple sclerosis, and essential tremor. Parkinsonism Relat Disord. 2015;21(10):1145-1147.
26. Del Prete E, Schmitt E, Meoni S, et al. Do neuropsychiatric fluctuations temporally match motor fluctuations in Parkinson’s disease? Neurol Sci. 2022;43(6):3641-3647.
27. Kleiner G, Fernandez HH, Chou KL, et al. Non-motor fluctuations in Parkinson’s disease: validation of the non-motor fluctuation assessment questionnaire. Mov Disord. 2021;36(6):1392-1400.
28. Pachi I, Maraki MI, Giagkou N, et al. Late life psychotic features in prodromal Parkinson’s disease. Parkinsonism Relat Disord. 2021;86:67-73.
29. Forsaa EB, Larsen JP, Wentzel-Larsen T, et al. A 12-year population-based study of psychosis in Parkinson’s disease. Arch Neurol. 2010;67(8):996-1001.
30. Chang A, Fox SH. Psychosis in Parkinson’s disease: epidemiology, pathophysiology, and management. Drugs. 2016;76(11):1093-1118.
31. Kasunich A, Kilbane C, Wiggins R. Movement disorders moment: pain and palliative care in movement disorders. Practical Neurology. 2021;20(4):63-67.
32. Burn D, Emre M, McKeith I, et al. Effects of rivastigmine in patients with and without visual hallucinations in dementia associated with Parkinson’s disease. Mov Disord. 2006;21(11):1899-1907.
33. Tripathi M, Vibha D. Reversible dementias. Indian J Psychiatry. 2009; 51 Suppl 1(Suppl 1): S52-S55.
34. Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75(19):1717-1725.
35. Goldman J, Sieg, E. Cognitive impairment and dementia in Parkinson disease. Clin Geriatr Med. 2020;36(2):365-377.
36. Gonzalez-Latapi P, Bayram E, Litvan I, et al. Cognitive impairment in Parkinson’s disease: epidemiology, clinical profile, protective and risk factors. Behav Sci (Basel). 2021;11(5):74.
37. Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force Guidelines. Mov Disord. 2012;27(3):349-356.
38. Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22(16):2314-2324.
39. Aarsland D, Batzu L, Halliday GM, et al. Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers. 2021;7(1):47. doi:10.1038/s41572-021-00280-3
40. Weintraub D, Claassen DO. Impulse control and related disorders in Parkinson’s disease. Int Rev Neurobiol. 2017;133:679-717.
41. Vilas D, Pont-Sunyer C, Tolosa E. Impulse control disorders in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18 Suppl 1:S80-S84.
42. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589-595.
43. Faouzi J, Corvol JC, Mariani LL. Impulse control disorders and related behaviors in Parkinson’s disease: risk factors, clinical and genetic aspects, and management. Curr Opin Neurol. 2021;34(4):547-555.
44. Samuel M, Rodriguez-Oroz M, Antonini A, et al. Impulse control disorders in Parkinson’s disease: management, controversies, and potential approaches. Mov Disord. 2015;30(2):150-159.
45. Frank MJ, Samanta J, Moustafa AA, et al. Hold your horses: impulsivity, deep brain stimulation and medication in Parkinsonism. Science. 2007;318(5854):1309-1312.
46. Jahanshahi M, Obeso I, Baunez C, et al. Parkinson’s disease, the subthalamic nucleus, inhibition, and impulsivity. Mov Disord. 2015;30(2):128-140.
47. Castrioto A, Lhommée E, Moro E, et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurol. 2014;13(3):287-305.
Overburdened: Health care workers more likely to die by suicide
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
If you run into a health care provider these days and ask, “How are you doing?” you’re likely to get a response like this one: “You know, hanging in there.” You smile and move on. But it may be time to go a step further. If you ask that next question – “No, really, how are you doing?” Well, you might need to carve out some time.
It’s been a rough few years for those of us in the health care professions. Our lives, dominated by COVID-related concerns at home, were equally dominated by COVID concerns at work. On the job, there were fewer and fewer of us around as exploitation and COVID-related stressors led doctors, nurses, and others to leave the profession entirely or take early retirement. Even now, I’m not sure we’ve recovered. Staffing in the hospitals is still a huge problem, and the persistence of impersonal meetings via teleconference – which not only prevent any sort of human connection but, audaciously, run from one into another without a break – robs us of even the subtle joy of walking from one hallway to another for 5 minutes of reflection before sitting down to view the next hastily cobbled together PowerPoint.
I’m speaking in generalities, of course.
I’m talking about how bad things are now because, in truth, they’ve never been great. And that may be why health care workers – people with jobs focused on serving others – are nevertheless at substantially increased risk for suicide.
Analyses through the years have shown that physicians tend to have higher rates of death from suicide than the general population. There are reasons for this that may not entirely be because of work-related stress. Doctors’ suicide attempts are more often lethal – we know what is likely to work, after all.
And, according to this paper in JAMA, it is those people who may be suffering most of all.
The study is a nationally representative sample based on the 2008 American Community Survey. Records were linked to the National Death Index through 2019.
Survey respondents were classified into five categories of health care worker, as you can see here. And 1,666,000 non–health care workers served as the control group.
Let’s take a look at the numbers.
I’m showing you age- and sex-standardized rates of death from suicide, starting with non–health care workers. In this study, physicians have similar rates of death from suicide to the general population. Nurses have higher rates, but health care support workers – nurses’ aides, home health aides – have rates nearly twice that of the general population.
Only social and behavioral health workers had rates lower than those in the general population, perhaps because they know how to access life-saving resources.
Of course, these groups differ in a lot of ways – education and income, for example. But even after adjustment for these factors as well as for sex, race, and marital status, the results persist. The only group with even a trend toward lower suicide rates are social and behavioral health workers.
There has been much hand-wringing about rates of physician suicide in the past. It is still a very real problem. But this paper finally highlights that there is a lot more to the health care profession than physicians. It’s time we acknowledge and support the people in our profession who seem to be suffering more than any of us: the aides, the techs, the support staff – the overworked and underpaid who have to deal with all the stresses that physicians like me face and then some.
There’s more to suicide risk than just your job; I know that. Family matters. Relationships matter. Medical and psychiatric illnesses matter. But to ignore this problem when it is right here, in our own house so to speak, can’t continue.
Might I suggest we start by asking someone in our profession – whether doctor, nurse, aide, or tech – how they are doing. How they are really doing. And when we are done listening, we use what we hear to advocate for real change.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
If you run into a health care provider these days and ask, “How are you doing?” you’re likely to get a response like this one: “You know, hanging in there.” You smile and move on. But it may be time to go a step further. If you ask that next question – “No, really, how are you doing?” Well, you might need to carve out some time.
It’s been a rough few years for those of us in the health care professions. Our lives, dominated by COVID-related concerns at home, were equally dominated by COVID concerns at work. On the job, there were fewer and fewer of us around as exploitation and COVID-related stressors led doctors, nurses, and others to leave the profession entirely or take early retirement. Even now, I’m not sure we’ve recovered. Staffing in the hospitals is still a huge problem, and the persistence of impersonal meetings via teleconference – which not only prevent any sort of human connection but, audaciously, run from one into another without a break – robs us of even the subtle joy of walking from one hallway to another for 5 minutes of reflection before sitting down to view the next hastily cobbled together PowerPoint.
I’m speaking in generalities, of course.
I’m talking about how bad things are now because, in truth, they’ve never been great. And that may be why health care workers – people with jobs focused on serving others – are nevertheless at substantially increased risk for suicide.
Analyses through the years have shown that physicians tend to have higher rates of death from suicide than the general population. There are reasons for this that may not entirely be because of work-related stress. Doctors’ suicide attempts are more often lethal – we know what is likely to work, after all.
And, according to this paper in JAMA, it is those people who may be suffering most of all.
The study is a nationally representative sample based on the 2008 American Community Survey. Records were linked to the National Death Index through 2019.
Survey respondents were classified into five categories of health care worker, as you can see here. And 1,666,000 non–health care workers served as the control group.
Let’s take a look at the numbers.
I’m showing you age- and sex-standardized rates of death from suicide, starting with non–health care workers. In this study, physicians have similar rates of death from suicide to the general population. Nurses have higher rates, but health care support workers – nurses’ aides, home health aides – have rates nearly twice that of the general population.
Only social and behavioral health workers had rates lower than those in the general population, perhaps because they know how to access life-saving resources.
Of course, these groups differ in a lot of ways – education and income, for example. But even after adjustment for these factors as well as for sex, race, and marital status, the results persist. The only group with even a trend toward lower suicide rates are social and behavioral health workers.
There has been much hand-wringing about rates of physician suicide in the past. It is still a very real problem. But this paper finally highlights that there is a lot more to the health care profession than physicians. It’s time we acknowledge and support the people in our profession who seem to be suffering more than any of us: the aides, the techs, the support staff – the overworked and underpaid who have to deal with all the stresses that physicians like me face and then some.
There’s more to suicide risk than just your job; I know that. Family matters. Relationships matter. Medical and psychiatric illnesses matter. But to ignore this problem when it is right here, in our own house so to speak, can’t continue.
Might I suggest we start by asking someone in our profession – whether doctor, nurse, aide, or tech – how they are doing. How they are really doing. And when we are done listening, we use what we hear to advocate for real change.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
If you run into a health care provider these days and ask, “How are you doing?” you’re likely to get a response like this one: “You know, hanging in there.” You smile and move on. But it may be time to go a step further. If you ask that next question – “No, really, how are you doing?” Well, you might need to carve out some time.
It’s been a rough few years for those of us in the health care professions. Our lives, dominated by COVID-related concerns at home, were equally dominated by COVID concerns at work. On the job, there were fewer and fewer of us around as exploitation and COVID-related stressors led doctors, nurses, and others to leave the profession entirely or take early retirement. Even now, I’m not sure we’ve recovered. Staffing in the hospitals is still a huge problem, and the persistence of impersonal meetings via teleconference – which not only prevent any sort of human connection but, audaciously, run from one into another without a break – robs us of even the subtle joy of walking from one hallway to another for 5 minutes of reflection before sitting down to view the next hastily cobbled together PowerPoint.
I’m speaking in generalities, of course.
I’m talking about how bad things are now because, in truth, they’ve never been great. And that may be why health care workers – people with jobs focused on serving others – are nevertheless at substantially increased risk for suicide.
Analyses through the years have shown that physicians tend to have higher rates of death from suicide than the general population. There are reasons for this that may not entirely be because of work-related stress. Doctors’ suicide attempts are more often lethal – we know what is likely to work, after all.
And, according to this paper in JAMA, it is those people who may be suffering most of all.
The study is a nationally representative sample based on the 2008 American Community Survey. Records were linked to the National Death Index through 2019.
Survey respondents were classified into five categories of health care worker, as you can see here. And 1,666,000 non–health care workers served as the control group.
Let’s take a look at the numbers.
I’m showing you age- and sex-standardized rates of death from suicide, starting with non–health care workers. In this study, physicians have similar rates of death from suicide to the general population. Nurses have higher rates, but health care support workers – nurses’ aides, home health aides – have rates nearly twice that of the general population.
Only social and behavioral health workers had rates lower than those in the general population, perhaps because they know how to access life-saving resources.
Of course, these groups differ in a lot of ways – education and income, for example. But even after adjustment for these factors as well as for sex, race, and marital status, the results persist. The only group with even a trend toward lower suicide rates are social and behavioral health workers.
There has been much hand-wringing about rates of physician suicide in the past. It is still a very real problem. But this paper finally highlights that there is a lot more to the health care profession than physicians. It’s time we acknowledge and support the people in our profession who seem to be suffering more than any of us: the aides, the techs, the support staff – the overworked and underpaid who have to deal with all the stresses that physicians like me face and then some.
There’s more to suicide risk than just your job; I know that. Family matters. Relationships matter. Medical and psychiatric illnesses matter. But to ignore this problem when it is right here, in our own house so to speak, can’t continue.
Might I suggest we start by asking someone in our profession – whether doctor, nurse, aide, or tech – how they are doing. How they are really doing. And when we are done listening, we use what we hear to advocate for real change.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Artificial sweeteners in processed foods tied to increased depression risk
new data from the Nurses Health Study II (NHS II) suggest.
Nurses who consumed more than eight servings daily had about a 50% higher risk of developing depression than nurses who consumed four or fewer servings daily.
However, in a secondary analysis, in which the researchers tried to tease out specific foods that may be associated with increased risk, only artificial sweeteners and artificially sweetened beverages were associated with an increased risk of depression.
“Animal studies have shown that artificial sweeteners may trigger the transmission of particular signaling molecules in the brain that are important for mood,” study investigator Andrew T. Chan, MD, MPH, of the clinical and translational epidemiology unit at Massachusetts General Hospital, Boston, said in an interview.
“Given this potential association between ultraprocessed food and multiple adverse health conditions, wherever possible individuals may wish to limit their intake of such foods. This may be a lifestyle change that could have important benefits, particularly for those who struggle with mental health,” Dr. Chan said.
The study was published online in JAMA Network Open.
Multiple potential mechanisms
The findings are based on 31,712 mostly non-Hispanic White women who were free of depression at baseline. The mean age of the patients at baseline was 52 years. As part of the NHS II, the women provided information on diet every 4 years using validated food frequency questionnaires.
Compared with women with low UPF intake, those with high UPF intake had greater body mass index (BMI). In addition, they were apt to smoke and have diabetes, hypertension, and dyslipidemia, and they were less apt to exercise regularly.
During the study period, there were 2,122 incident cases of depression, as determined using a strict definition that required self-reported clinician-diagnosed depression and regular antidepressant use. There were 4,840 incident cases, as determined using a broad definition that required clinical diagnosis and/or antidepressant use.
Compared with women in the lowest quintile of UPF consumption (fewer than four daily servings), those in the highest quintile (more than 8.8 daily servings) had an increased risk of depression.
This was noted for both the strict depression definition (hazard ratio, 1.49; 95% confidence interval, 1.26-1.76; P < .001) and the broad one (HR, 1.34; 95% CI, 1.20-1.50; P < .001).
“Models were not materially altered after inclusion of potential confounders. We did not observe differential associations in subgroups defined by age, BMI, physical activity, or smoking,” the researchers reported.
In secondary analyses, they classified UPF into their components, including ultraprocessed grain foods, sweet snacks, ready-to-eat meals, fats, sauces, ultraprocessed dairy products, savory snacks, processed meat, beverages, and artificial sweeteners.
Comparing the highest with the lowest quintiles, only high intake of artificially sweetened beverages (HR, 1.37; 95% CI, 1.19-1.57; P < .001) and artificial sweeteners (HR, 1.26; 95% CI, 1.10-1.43; P < .001) was associated with greater risk of depression and after multivariable regression.
In an exploratory analysis, women who reduced their UPF intake by at least three servings per day were at lower risk of depression (strict definition: HR, 0.84; 95% CI, 0.71-0.99), compared with those with relatively stable intake in each 4-year period.
“Ultraprocessed foods have been associated with several different health outcomes which may reflect an effect on common pathways that underlie chronic conditions,” said Dr. Chan.
For example, UPF intake has been associated with chronic inflammation, which in turns leads to multiple potential adverse health effects, including depression, he explained.
There is also a link between UPF and disruption of the gut microbiome.
“This is an important potential mechanism linking ultraprocessed food to depression since there is emerging evidence that microbes in the gut have been linked with mood through their role in metabolizing and producing proteins that have activity in the brain,” Dr. Chan said.
Association, not causation
Several experts weighed in on the study results in a statement from the U.K. nonprofit organization, Science Media Centre.
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading (England), cautioned that the study only offers information on association – not causation.
“It is very possible that people with depression change their diet and might decide to consume foods that are easier to prepare – which would often be foods considered to be ultraprocessed,” Dr. Kuhnle said.
What’s most interesting is that the association between UPF intake and depression was driven by a single factor – artificial sweeteners.
“This supports one of the main criticisms of the UPF concept, that it combines a wide range of different foods and thereby makes it difficult to identify underlying causes,” Dr. Kuhnle added.
“There are currently no data that link artificial sweetener use to mental health, despite most of them having been available for some time. It is also important to note that there are a wide range of different artificial sweeteners that are metabolized very differently and that there might be reverse causality,” Dr. Kuhnle commented.
Paul Keedwell, MBChB, PhD, consultant psychiatrist and fellow of the Royal College of Psychiatrists, said this is an “interesting and important finding, but one that raises more questions. At this stage, we cannot say how big an effect diet has on depression risk compared to other risk factors, like family history of depression, stress levels, and having a supportive social network.”
Dr. Keedwell noted that the investigators carefully excluded the possibility that the effect is mediated by obesity or lack of exercise.
“However, an important consideration is that a diet based on ready meals and artificially sweetened drinks might indicate a hectic lifestyle or one with shift work. In other words, a fast-food diet could be an indirect marker of chronic stress. Prolonged stress probably remains the main risk factor for depression,” Dr. Keedwell said.
Keith Frayn, PhD, professor emeritus of human metabolism, University of Oxford (England), noted that the relationship between artificial sweeteners and depression “stands out clearly” even after adjusting for multiple confounding factors, including BMI, smoking, and exercise.
“This adds to growing concerns about artificial sweeteners and cardiometabolic health. The link with depression needs confirmation and further research to suggest how it might be brought about,” Dr. Frayn cautioned.
The NHS II was funded by a grant from the National Cancer Institute. Dr. Chan reported receiving grants from Bayer and Zoe and personal fees from Boehringer Ingelheim, Pfizer, and Freenome outside this work. Dr. Keedwell and Dr. Kuhnle disclosed no relevant financial relationships. Dr. Frayn is an author of books on nutrition and metabolism.
A version of this article first appeared on Medscape.com.
new data from the Nurses Health Study II (NHS II) suggest.
Nurses who consumed more than eight servings daily had about a 50% higher risk of developing depression than nurses who consumed four or fewer servings daily.
However, in a secondary analysis, in which the researchers tried to tease out specific foods that may be associated with increased risk, only artificial sweeteners and artificially sweetened beverages were associated with an increased risk of depression.
“Animal studies have shown that artificial sweeteners may trigger the transmission of particular signaling molecules in the brain that are important for mood,” study investigator Andrew T. Chan, MD, MPH, of the clinical and translational epidemiology unit at Massachusetts General Hospital, Boston, said in an interview.
“Given this potential association between ultraprocessed food and multiple adverse health conditions, wherever possible individuals may wish to limit their intake of such foods. This may be a lifestyle change that could have important benefits, particularly for those who struggle with mental health,” Dr. Chan said.
The study was published online in JAMA Network Open.
Multiple potential mechanisms
The findings are based on 31,712 mostly non-Hispanic White women who were free of depression at baseline. The mean age of the patients at baseline was 52 years. As part of the NHS II, the women provided information on diet every 4 years using validated food frequency questionnaires.
Compared with women with low UPF intake, those with high UPF intake had greater body mass index (BMI). In addition, they were apt to smoke and have diabetes, hypertension, and dyslipidemia, and they were less apt to exercise regularly.
During the study period, there were 2,122 incident cases of depression, as determined using a strict definition that required self-reported clinician-diagnosed depression and regular antidepressant use. There were 4,840 incident cases, as determined using a broad definition that required clinical diagnosis and/or antidepressant use.
Compared with women in the lowest quintile of UPF consumption (fewer than four daily servings), those in the highest quintile (more than 8.8 daily servings) had an increased risk of depression.
This was noted for both the strict depression definition (hazard ratio, 1.49; 95% confidence interval, 1.26-1.76; P < .001) and the broad one (HR, 1.34; 95% CI, 1.20-1.50; P < .001).
“Models were not materially altered after inclusion of potential confounders. We did not observe differential associations in subgroups defined by age, BMI, physical activity, or smoking,” the researchers reported.
In secondary analyses, they classified UPF into their components, including ultraprocessed grain foods, sweet snacks, ready-to-eat meals, fats, sauces, ultraprocessed dairy products, savory snacks, processed meat, beverages, and artificial sweeteners.
Comparing the highest with the lowest quintiles, only high intake of artificially sweetened beverages (HR, 1.37; 95% CI, 1.19-1.57; P < .001) and artificial sweeteners (HR, 1.26; 95% CI, 1.10-1.43; P < .001) was associated with greater risk of depression and after multivariable regression.
In an exploratory analysis, women who reduced their UPF intake by at least three servings per day were at lower risk of depression (strict definition: HR, 0.84; 95% CI, 0.71-0.99), compared with those with relatively stable intake in each 4-year period.
“Ultraprocessed foods have been associated with several different health outcomes which may reflect an effect on common pathways that underlie chronic conditions,” said Dr. Chan.
For example, UPF intake has been associated with chronic inflammation, which in turns leads to multiple potential adverse health effects, including depression, he explained.
There is also a link between UPF and disruption of the gut microbiome.
“This is an important potential mechanism linking ultraprocessed food to depression since there is emerging evidence that microbes in the gut have been linked with mood through their role in metabolizing and producing proteins that have activity in the brain,” Dr. Chan said.
Association, not causation
Several experts weighed in on the study results in a statement from the U.K. nonprofit organization, Science Media Centre.
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading (England), cautioned that the study only offers information on association – not causation.
“It is very possible that people with depression change their diet and might decide to consume foods that are easier to prepare – which would often be foods considered to be ultraprocessed,” Dr. Kuhnle said.
What’s most interesting is that the association between UPF intake and depression was driven by a single factor – artificial sweeteners.
“This supports one of the main criticisms of the UPF concept, that it combines a wide range of different foods and thereby makes it difficult to identify underlying causes,” Dr. Kuhnle added.
“There are currently no data that link artificial sweetener use to mental health, despite most of them having been available for some time. It is also important to note that there are a wide range of different artificial sweeteners that are metabolized very differently and that there might be reverse causality,” Dr. Kuhnle commented.
Paul Keedwell, MBChB, PhD, consultant psychiatrist and fellow of the Royal College of Psychiatrists, said this is an “interesting and important finding, but one that raises more questions. At this stage, we cannot say how big an effect diet has on depression risk compared to other risk factors, like family history of depression, stress levels, and having a supportive social network.”
Dr. Keedwell noted that the investigators carefully excluded the possibility that the effect is mediated by obesity or lack of exercise.
“However, an important consideration is that a diet based on ready meals and artificially sweetened drinks might indicate a hectic lifestyle or one with shift work. In other words, a fast-food diet could be an indirect marker of chronic stress. Prolonged stress probably remains the main risk factor for depression,” Dr. Keedwell said.
Keith Frayn, PhD, professor emeritus of human metabolism, University of Oxford (England), noted that the relationship between artificial sweeteners and depression “stands out clearly” even after adjusting for multiple confounding factors, including BMI, smoking, and exercise.
“This adds to growing concerns about artificial sweeteners and cardiometabolic health. The link with depression needs confirmation and further research to suggest how it might be brought about,” Dr. Frayn cautioned.
The NHS II was funded by a grant from the National Cancer Institute. Dr. Chan reported receiving grants from Bayer and Zoe and personal fees from Boehringer Ingelheim, Pfizer, and Freenome outside this work. Dr. Keedwell and Dr. Kuhnle disclosed no relevant financial relationships. Dr. Frayn is an author of books on nutrition and metabolism.
A version of this article first appeared on Medscape.com.
new data from the Nurses Health Study II (NHS II) suggest.
Nurses who consumed more than eight servings daily had about a 50% higher risk of developing depression than nurses who consumed four or fewer servings daily.
However, in a secondary analysis, in which the researchers tried to tease out specific foods that may be associated with increased risk, only artificial sweeteners and artificially sweetened beverages were associated with an increased risk of depression.
“Animal studies have shown that artificial sweeteners may trigger the transmission of particular signaling molecules in the brain that are important for mood,” study investigator Andrew T. Chan, MD, MPH, of the clinical and translational epidemiology unit at Massachusetts General Hospital, Boston, said in an interview.
“Given this potential association between ultraprocessed food and multiple adverse health conditions, wherever possible individuals may wish to limit their intake of such foods. This may be a lifestyle change that could have important benefits, particularly for those who struggle with mental health,” Dr. Chan said.
The study was published online in JAMA Network Open.
Multiple potential mechanisms
The findings are based on 31,712 mostly non-Hispanic White women who were free of depression at baseline. The mean age of the patients at baseline was 52 years. As part of the NHS II, the women provided information on diet every 4 years using validated food frequency questionnaires.
Compared with women with low UPF intake, those with high UPF intake had greater body mass index (BMI). In addition, they were apt to smoke and have diabetes, hypertension, and dyslipidemia, and they were less apt to exercise regularly.
During the study period, there were 2,122 incident cases of depression, as determined using a strict definition that required self-reported clinician-diagnosed depression and regular antidepressant use. There were 4,840 incident cases, as determined using a broad definition that required clinical diagnosis and/or antidepressant use.
Compared with women in the lowest quintile of UPF consumption (fewer than four daily servings), those in the highest quintile (more than 8.8 daily servings) had an increased risk of depression.
This was noted for both the strict depression definition (hazard ratio, 1.49; 95% confidence interval, 1.26-1.76; P < .001) and the broad one (HR, 1.34; 95% CI, 1.20-1.50; P < .001).
“Models were not materially altered after inclusion of potential confounders. We did not observe differential associations in subgroups defined by age, BMI, physical activity, or smoking,” the researchers reported.
In secondary analyses, they classified UPF into their components, including ultraprocessed grain foods, sweet snacks, ready-to-eat meals, fats, sauces, ultraprocessed dairy products, savory snacks, processed meat, beverages, and artificial sweeteners.
Comparing the highest with the lowest quintiles, only high intake of artificially sweetened beverages (HR, 1.37; 95% CI, 1.19-1.57; P < .001) and artificial sweeteners (HR, 1.26; 95% CI, 1.10-1.43; P < .001) was associated with greater risk of depression and after multivariable regression.
In an exploratory analysis, women who reduced their UPF intake by at least three servings per day were at lower risk of depression (strict definition: HR, 0.84; 95% CI, 0.71-0.99), compared with those with relatively stable intake in each 4-year period.
“Ultraprocessed foods have been associated with several different health outcomes which may reflect an effect on common pathways that underlie chronic conditions,” said Dr. Chan.
For example, UPF intake has been associated with chronic inflammation, which in turns leads to multiple potential adverse health effects, including depression, he explained.
There is also a link between UPF and disruption of the gut microbiome.
“This is an important potential mechanism linking ultraprocessed food to depression since there is emerging evidence that microbes in the gut have been linked with mood through their role in metabolizing and producing proteins that have activity in the brain,” Dr. Chan said.
Association, not causation
Several experts weighed in on the study results in a statement from the U.K. nonprofit organization, Science Media Centre.
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading (England), cautioned that the study only offers information on association – not causation.
“It is very possible that people with depression change their diet and might decide to consume foods that are easier to prepare – which would often be foods considered to be ultraprocessed,” Dr. Kuhnle said.
What’s most interesting is that the association between UPF intake and depression was driven by a single factor – artificial sweeteners.
“This supports one of the main criticisms of the UPF concept, that it combines a wide range of different foods and thereby makes it difficult to identify underlying causes,” Dr. Kuhnle added.
“There are currently no data that link artificial sweetener use to mental health, despite most of them having been available for some time. It is also important to note that there are a wide range of different artificial sweeteners that are metabolized very differently and that there might be reverse causality,” Dr. Kuhnle commented.
Paul Keedwell, MBChB, PhD, consultant psychiatrist and fellow of the Royal College of Psychiatrists, said this is an “interesting and important finding, but one that raises more questions. At this stage, we cannot say how big an effect diet has on depression risk compared to other risk factors, like family history of depression, stress levels, and having a supportive social network.”
Dr. Keedwell noted that the investigators carefully excluded the possibility that the effect is mediated by obesity or lack of exercise.
“However, an important consideration is that a diet based on ready meals and artificially sweetened drinks might indicate a hectic lifestyle or one with shift work. In other words, a fast-food diet could be an indirect marker of chronic stress. Prolonged stress probably remains the main risk factor for depression,” Dr. Keedwell said.
Keith Frayn, PhD, professor emeritus of human metabolism, University of Oxford (England), noted that the relationship between artificial sweeteners and depression “stands out clearly” even after adjusting for multiple confounding factors, including BMI, smoking, and exercise.
“This adds to growing concerns about artificial sweeteners and cardiometabolic health. The link with depression needs confirmation and further research to suggest how it might be brought about,” Dr. Frayn cautioned.
The NHS II was funded by a grant from the National Cancer Institute. Dr. Chan reported receiving grants from Bayer and Zoe and personal fees from Boehringer Ingelheim, Pfizer, and Freenome outside this work. Dr. Keedwell and Dr. Kuhnle disclosed no relevant financial relationships. Dr. Frayn is an author of books on nutrition and metabolism.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Online CBT aids remission of anxiety, depression in students
, according to a study published in JAMA Psychiatry . The intervention was developed by researchers from the United States, Mexico, and Colombia and studied in undergraduate university students.
The research included 1,319 students with anxiety and depression. The students were randomly assigned to three groups that received either remote (internet-based) cognitive behavioral therapy guided by a therapist, self-guided cognitive behavioral therapy (without support from a therapist), or standard treatment provided by the health care services within their community (the control condition).
Students who received guided cognitive behavioral therapy had higher combined rates of remission of these disorders (51.8%) than students who received self-guided therapy (37.8%) or conventional therapy (40%). These differences were not significant for remission of anxiety, however.
Guided cognitive behavioral therapy was associated with the highest probability of remission of anxiety and depression in 91.7% of students, the highest probability of remission of anxiety in all students, and the highest probability of remission of depression in 71.5% of participants.
The results of this analysis could be used to improve psychological care by optimizing how different treatment methods are assigned, especially in mental health institutions where available technical and human resources are limited, according to the investigators.
“We started designing this study before COVID-19 with the idea of optimizing care for these mental health problems,” said study author Corina Benjet Miner, PhD, an epidemiological and psychosocial researcher at the Ramón de la Fuente National Institute of Psychiatry, Mexico City. “We wanted to find additional strategies to achieve better care. The pandemic helped us because, even though this has been undergoing research for many years, internet-delivered interventions were not as well accepted. But during the pandemic, there weren’t any other options.”
Given the high prevalence of mental disorders before and after the pandemic, no health care system in the world would be able to provide in-person care to each patient with depression or anxiety, said Dr. Benjet Miner. “So, the idea is to look for other cost-effective strategies that can ramp up our interventions and reach a greater number of people without negatively impacting the quality of care,” she explained.
“I believe that [the precision model] is an excellent proposal that can save financial resources and avoid transfers,” said Juana Olvera Méndez, PhD, research professor working with the cognitive behavioral approach at the Iztacala Faculty of Higher Studies (FESI) of the National Autonomous University of Mexico, Mexico City. “It also makes it possible to provide patients with immediate care, in contrast to when someone has to go in for [in-person] therapy, which will depend a lot on how the therapist approaches the situation.”
Students from seven universities in Colombia and Mexico were included in the study. They were aged 18 years or older and had a score of 10 or greater on the self-administered Generalized Anxiety Disorder scale-7 test, or had depression with scores of 10 or greater on the nine-item Patient Health Questionnaire, which is also self-administered.
The study’s exclusion criteria included a history of bipolar disorder, nonaffective psychosis, or suicidal ideation with suicide attempts. The investigators used 284 prescription predictors to anticipate the differential response to antianxiety and antidepression therapy.
By grouping these predictors into 11 conceptual categories (such as demographic characteristics, COVID-19–linked stressors, or mental disorder comorbidities) and using machine learning algorithms, the investigators were able to predict in an individualized manner the probability of remission for participants in each of the groups.
“For depression, we found that 28.5% of patients could experience better or equivalent effects from the self-guided program (in comparison to the guided program). Once you have this program, it doesn’t cost anything, so there could be a massive number of people who could benefit from a cost-free therapy,” said Dr. Benjet Miner.
While numerous studies in precision medicine have tried to determine the most appropriate treatment for each patient, “they don’t have the high number of predictors that we used in this research, and I feel like this gives us a significant edge,” she added.
She also explained that they found no differences in user satisfaction between the guided and unguided version of the therapy, so now they must discover why the guided version works better. One notable point is that patients accessed (online) the guided program twice as many times as those who used the self-guided version, but the number of times used is not enough to explain the better outcomes.
“We believe that patients develop some sort of connection with the guides, who are not providing therapy but only making recommendations in brief interactions with patients once a week. It has something to do with that connection, in addition to the longer time spent interacting with the platform, which provides better results with the guided version,” stated Dr. Benjet Miner.
One of the main limitations of this study is that, though it compares three treatment methods, the third one (standard care) is not homogeneous, because each of the seven universities from which the students were selected has different resources for this purpose. “Some universities, like the National Autonomous University of Mexico, have very formal services, with teams of psychologists and psychiatrists, while others don’t have this type of service, or they cover additional aspects, like vocational counseling. So, it’s very difficult to determine exactly what kind of care patients are receiving, because it’s not homogeneous,” she said.
As many as nine assessments using psychometric tests are sometimes required before the intervention can be evaluated, said Dr. Méndez. “This study doesn’t go into too much detail in that area, focusing rather on treatment. So, it would be important to know the diagnoses of the users, who may be experiencing different degrees of depression or anxiety. It would be worth asking what happens if a user requires psychiatric treatment or support.”
Dr. Méndez, who provides psychological therapy in person and online at the Student Support and Counselling Center at FESI, pointed out that it would be important to provide close follow-up on these results to see whether they are sustained in the short and long terms. In her opinion, this model could be presented to other users requiring treatment for anxiety or depression, provided that they can use information and communication technologies.
This precision model, which can also be supported on mobile phones or tablets, could be transferred to primary care facilities or vulnerable populations in rural areas, said Dr. Benjet Miner. “The idea is to reach a point where these algorithms become accurate enough and have a really strong predictive power so that clinicians can use them. The goal is always to find the best treatment at the lowest cost, so that it’s sustainable,” she concluded.
This study was funded by grant number R01MH120648 from the National Institute of Mental Health and the Fogarty International Center. Dr. Benjet Miner reports no relevant financial relationships; the declarations of the remaining authors can be found at the publication’s website.
This article was translated from Medscape’s Spanish Edition and a version first appeared on Medscape.com.
, according to a study published in JAMA Psychiatry . The intervention was developed by researchers from the United States, Mexico, and Colombia and studied in undergraduate university students.
The research included 1,319 students with anxiety and depression. The students were randomly assigned to three groups that received either remote (internet-based) cognitive behavioral therapy guided by a therapist, self-guided cognitive behavioral therapy (without support from a therapist), or standard treatment provided by the health care services within their community (the control condition).
Students who received guided cognitive behavioral therapy had higher combined rates of remission of these disorders (51.8%) than students who received self-guided therapy (37.8%) or conventional therapy (40%). These differences were not significant for remission of anxiety, however.
Guided cognitive behavioral therapy was associated with the highest probability of remission of anxiety and depression in 91.7% of students, the highest probability of remission of anxiety in all students, and the highest probability of remission of depression in 71.5% of participants.
The results of this analysis could be used to improve psychological care by optimizing how different treatment methods are assigned, especially in mental health institutions where available technical and human resources are limited, according to the investigators.
“We started designing this study before COVID-19 with the idea of optimizing care for these mental health problems,” said study author Corina Benjet Miner, PhD, an epidemiological and psychosocial researcher at the Ramón de la Fuente National Institute of Psychiatry, Mexico City. “We wanted to find additional strategies to achieve better care. The pandemic helped us because, even though this has been undergoing research for many years, internet-delivered interventions were not as well accepted. But during the pandemic, there weren’t any other options.”
Given the high prevalence of mental disorders before and after the pandemic, no health care system in the world would be able to provide in-person care to each patient with depression or anxiety, said Dr. Benjet Miner. “So, the idea is to look for other cost-effective strategies that can ramp up our interventions and reach a greater number of people without negatively impacting the quality of care,” she explained.
“I believe that [the precision model] is an excellent proposal that can save financial resources and avoid transfers,” said Juana Olvera Méndez, PhD, research professor working with the cognitive behavioral approach at the Iztacala Faculty of Higher Studies (FESI) of the National Autonomous University of Mexico, Mexico City. “It also makes it possible to provide patients with immediate care, in contrast to when someone has to go in for [in-person] therapy, which will depend a lot on how the therapist approaches the situation.”
Students from seven universities in Colombia and Mexico were included in the study. They were aged 18 years or older and had a score of 10 or greater on the self-administered Generalized Anxiety Disorder scale-7 test, or had depression with scores of 10 or greater on the nine-item Patient Health Questionnaire, which is also self-administered.
The study’s exclusion criteria included a history of bipolar disorder, nonaffective psychosis, or suicidal ideation with suicide attempts. The investigators used 284 prescription predictors to anticipate the differential response to antianxiety and antidepression therapy.
By grouping these predictors into 11 conceptual categories (such as demographic characteristics, COVID-19–linked stressors, or mental disorder comorbidities) and using machine learning algorithms, the investigators were able to predict in an individualized manner the probability of remission for participants in each of the groups.
“For depression, we found that 28.5% of patients could experience better or equivalent effects from the self-guided program (in comparison to the guided program). Once you have this program, it doesn’t cost anything, so there could be a massive number of people who could benefit from a cost-free therapy,” said Dr. Benjet Miner.
While numerous studies in precision medicine have tried to determine the most appropriate treatment for each patient, “they don’t have the high number of predictors that we used in this research, and I feel like this gives us a significant edge,” she added.
She also explained that they found no differences in user satisfaction between the guided and unguided version of the therapy, so now they must discover why the guided version works better. One notable point is that patients accessed (online) the guided program twice as many times as those who used the self-guided version, but the number of times used is not enough to explain the better outcomes.
“We believe that patients develop some sort of connection with the guides, who are not providing therapy but only making recommendations in brief interactions with patients once a week. It has something to do with that connection, in addition to the longer time spent interacting with the platform, which provides better results with the guided version,” stated Dr. Benjet Miner.
One of the main limitations of this study is that, though it compares three treatment methods, the third one (standard care) is not homogeneous, because each of the seven universities from which the students were selected has different resources for this purpose. “Some universities, like the National Autonomous University of Mexico, have very formal services, with teams of psychologists and psychiatrists, while others don’t have this type of service, or they cover additional aspects, like vocational counseling. So, it’s very difficult to determine exactly what kind of care patients are receiving, because it’s not homogeneous,” she said.
As many as nine assessments using psychometric tests are sometimes required before the intervention can be evaluated, said Dr. Méndez. “This study doesn’t go into too much detail in that area, focusing rather on treatment. So, it would be important to know the diagnoses of the users, who may be experiencing different degrees of depression or anxiety. It would be worth asking what happens if a user requires psychiatric treatment or support.”
Dr. Méndez, who provides psychological therapy in person and online at the Student Support and Counselling Center at FESI, pointed out that it would be important to provide close follow-up on these results to see whether they are sustained in the short and long terms. In her opinion, this model could be presented to other users requiring treatment for anxiety or depression, provided that they can use information and communication technologies.
This precision model, which can also be supported on mobile phones or tablets, could be transferred to primary care facilities or vulnerable populations in rural areas, said Dr. Benjet Miner. “The idea is to reach a point where these algorithms become accurate enough and have a really strong predictive power so that clinicians can use them. The goal is always to find the best treatment at the lowest cost, so that it’s sustainable,” she concluded.
This study was funded by grant number R01MH120648 from the National Institute of Mental Health and the Fogarty International Center. Dr. Benjet Miner reports no relevant financial relationships; the declarations of the remaining authors can be found at the publication’s website.
This article was translated from Medscape’s Spanish Edition and a version first appeared on Medscape.com.
, according to a study published in JAMA Psychiatry . The intervention was developed by researchers from the United States, Mexico, and Colombia and studied in undergraduate university students.
The research included 1,319 students with anxiety and depression. The students were randomly assigned to three groups that received either remote (internet-based) cognitive behavioral therapy guided by a therapist, self-guided cognitive behavioral therapy (without support from a therapist), or standard treatment provided by the health care services within their community (the control condition).
Students who received guided cognitive behavioral therapy had higher combined rates of remission of these disorders (51.8%) than students who received self-guided therapy (37.8%) or conventional therapy (40%). These differences were not significant for remission of anxiety, however.
Guided cognitive behavioral therapy was associated with the highest probability of remission of anxiety and depression in 91.7% of students, the highest probability of remission of anxiety in all students, and the highest probability of remission of depression in 71.5% of participants.
The results of this analysis could be used to improve psychological care by optimizing how different treatment methods are assigned, especially in mental health institutions where available technical and human resources are limited, according to the investigators.
“We started designing this study before COVID-19 with the idea of optimizing care for these mental health problems,” said study author Corina Benjet Miner, PhD, an epidemiological and psychosocial researcher at the Ramón de la Fuente National Institute of Psychiatry, Mexico City. “We wanted to find additional strategies to achieve better care. The pandemic helped us because, even though this has been undergoing research for many years, internet-delivered interventions were not as well accepted. But during the pandemic, there weren’t any other options.”
Given the high prevalence of mental disorders before and after the pandemic, no health care system in the world would be able to provide in-person care to each patient with depression or anxiety, said Dr. Benjet Miner. “So, the idea is to look for other cost-effective strategies that can ramp up our interventions and reach a greater number of people without negatively impacting the quality of care,” she explained.
“I believe that [the precision model] is an excellent proposal that can save financial resources and avoid transfers,” said Juana Olvera Méndez, PhD, research professor working with the cognitive behavioral approach at the Iztacala Faculty of Higher Studies (FESI) of the National Autonomous University of Mexico, Mexico City. “It also makes it possible to provide patients with immediate care, in contrast to when someone has to go in for [in-person] therapy, which will depend a lot on how the therapist approaches the situation.”
Students from seven universities in Colombia and Mexico were included in the study. They were aged 18 years or older and had a score of 10 or greater on the self-administered Generalized Anxiety Disorder scale-7 test, or had depression with scores of 10 or greater on the nine-item Patient Health Questionnaire, which is also self-administered.
The study’s exclusion criteria included a history of bipolar disorder, nonaffective psychosis, or suicidal ideation with suicide attempts. The investigators used 284 prescription predictors to anticipate the differential response to antianxiety and antidepression therapy.
By grouping these predictors into 11 conceptual categories (such as demographic characteristics, COVID-19–linked stressors, or mental disorder comorbidities) and using machine learning algorithms, the investigators were able to predict in an individualized manner the probability of remission for participants in each of the groups.
“For depression, we found that 28.5% of patients could experience better or equivalent effects from the self-guided program (in comparison to the guided program). Once you have this program, it doesn’t cost anything, so there could be a massive number of people who could benefit from a cost-free therapy,” said Dr. Benjet Miner.
While numerous studies in precision medicine have tried to determine the most appropriate treatment for each patient, “they don’t have the high number of predictors that we used in this research, and I feel like this gives us a significant edge,” she added.
She also explained that they found no differences in user satisfaction between the guided and unguided version of the therapy, so now they must discover why the guided version works better. One notable point is that patients accessed (online) the guided program twice as many times as those who used the self-guided version, but the number of times used is not enough to explain the better outcomes.
“We believe that patients develop some sort of connection with the guides, who are not providing therapy but only making recommendations in brief interactions with patients once a week. It has something to do with that connection, in addition to the longer time spent interacting with the platform, which provides better results with the guided version,” stated Dr. Benjet Miner.
One of the main limitations of this study is that, though it compares three treatment methods, the third one (standard care) is not homogeneous, because each of the seven universities from which the students were selected has different resources for this purpose. “Some universities, like the National Autonomous University of Mexico, have very formal services, with teams of psychologists and psychiatrists, while others don’t have this type of service, or they cover additional aspects, like vocational counseling. So, it’s very difficult to determine exactly what kind of care patients are receiving, because it’s not homogeneous,” she said.
As many as nine assessments using psychometric tests are sometimes required before the intervention can be evaluated, said Dr. Méndez. “This study doesn’t go into too much detail in that area, focusing rather on treatment. So, it would be important to know the diagnoses of the users, who may be experiencing different degrees of depression or anxiety. It would be worth asking what happens if a user requires psychiatric treatment or support.”
Dr. Méndez, who provides psychological therapy in person and online at the Student Support and Counselling Center at FESI, pointed out that it would be important to provide close follow-up on these results to see whether they are sustained in the short and long terms. In her opinion, this model could be presented to other users requiring treatment for anxiety or depression, provided that they can use information and communication technologies.
This precision model, which can also be supported on mobile phones or tablets, could be transferred to primary care facilities or vulnerable populations in rural areas, said Dr. Benjet Miner. “The idea is to reach a point where these algorithms become accurate enough and have a really strong predictive power so that clinicians can use them. The goal is always to find the best treatment at the lowest cost, so that it’s sustainable,” she concluded.
This study was funded by grant number R01MH120648 from the National Institute of Mental Health and the Fogarty International Center. Dr. Benjet Miner reports no relevant financial relationships; the declarations of the remaining authors can be found at the publication’s website.
This article was translated from Medscape’s Spanish Edition and a version first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
U.S. counties hit hard by a lack of psychiatric care
TOPLINE:
, new research shows.
METHODOLOGY:
- In the United States, there is a severe lack of psychiatrists and access to mental health care. In 2019, 21.3 million U.S. residents were without broadband access. These patients were forced either to use telephone consultation or to not use telehealth services at all, although use of telehealth during COVID-19 somewhat improved access to psychiatric care.
- For the study, researchers gathered sociodemographic and other county-level information from the American Community Survey. They also used data on the psychiatrist workforce from the Health Resources and Services Administration (HRSA) Area Health Resources Files.
- Information on broadband Internet coverage came from the Federal Communications Commission, and measures of mental health outcomes were from the Centers for Disease Control and Prevention.
TAKEAWAY:
- The study identified 596 counties (19% of all U.S. counties) that were without psychiatrists and in which there was inadequate broadband coverage. The population represented 10.5 million residents.
- Compared with other counties, those with lack of coverage were more likely to be rural (adjusted odds ratio, 3.05; 95% confidence interval, 2.41-3.84), to have higher unemployment (aOR, 1.12; 95% CI, 1.02-1.24), and to have higher uninsurance rates (aOR, 1.03; 95% CI, 1.00-1.06). In those counties, there were also fewer residents with a bachelor’s degree (aOR, 0.92; 95% CI, 0.90-0.94) and fewer Hispanics (aOR 0.98; 95% CI, 0.97-0.99), although those counties were not designated by the HRSA as having a psychiatrist shortage. That designation brings additional funding for the recruitment of clinicians.
- After adjustment for sociodemographic factors, counties without psychiatrists and broadband had significantly higher rates of adult depression, frequent mental distress, drug overdose mortality, and completed suicide, compared with other counties.
- Further analysis showed that the adjusted difference remained statistically significant for drug overdose mortality per 100,000 (9.2; 95% CI, 8.0-10.5, vs. 5.2; 95% CI, 4.9-5.6; P < .001) and completed suicide (10.6; 95% CI, 8.9-12.3, vs. 7.6; 95% CI, 7.0-8.2; P < .001), but not for the other two measures.
IN PRACTICE:
“Our finding suggests that lacking access to virtual and in-person psychiatric care continues to be a key factor associated with adverse outcomes,” the investigators write. They note that federal and state-level investments in broadband and the psychiatric workforce are needed.
SOURCE:
The study was conducted by Tarun Ramesh, BS, department of population medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, and colleagues. It was published online as a research letter in JAMA Network Open.
LIMITATIONS:
The investigators did not consider whether recent legislation, including the Consolidated Appropriations Act of 2021 and the American Rescue Plan, which expanded psychiatry residency slots and broadband infrastructure, reduces adverse outcomes, something the authors say future research should examine.
DISCLOSURES:
The study received support from the National Institutes of Health, including the National Institute on Minority Health and Health Disparities and the National Institute of Mental Health. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
, new research shows.
METHODOLOGY:
- In the United States, there is a severe lack of psychiatrists and access to mental health care. In 2019, 21.3 million U.S. residents were without broadband access. These patients were forced either to use telephone consultation or to not use telehealth services at all, although use of telehealth during COVID-19 somewhat improved access to psychiatric care.
- For the study, researchers gathered sociodemographic and other county-level information from the American Community Survey. They also used data on the psychiatrist workforce from the Health Resources and Services Administration (HRSA) Area Health Resources Files.
- Information on broadband Internet coverage came from the Federal Communications Commission, and measures of mental health outcomes were from the Centers for Disease Control and Prevention.
TAKEAWAY:
- The study identified 596 counties (19% of all U.S. counties) that were without psychiatrists and in which there was inadequate broadband coverage. The population represented 10.5 million residents.
- Compared with other counties, those with lack of coverage were more likely to be rural (adjusted odds ratio, 3.05; 95% confidence interval, 2.41-3.84), to have higher unemployment (aOR, 1.12; 95% CI, 1.02-1.24), and to have higher uninsurance rates (aOR, 1.03; 95% CI, 1.00-1.06). In those counties, there were also fewer residents with a bachelor’s degree (aOR, 0.92; 95% CI, 0.90-0.94) and fewer Hispanics (aOR 0.98; 95% CI, 0.97-0.99), although those counties were not designated by the HRSA as having a psychiatrist shortage. That designation brings additional funding for the recruitment of clinicians.
- After adjustment for sociodemographic factors, counties without psychiatrists and broadband had significantly higher rates of adult depression, frequent mental distress, drug overdose mortality, and completed suicide, compared with other counties.
- Further analysis showed that the adjusted difference remained statistically significant for drug overdose mortality per 100,000 (9.2; 95% CI, 8.0-10.5, vs. 5.2; 95% CI, 4.9-5.6; P < .001) and completed suicide (10.6; 95% CI, 8.9-12.3, vs. 7.6; 95% CI, 7.0-8.2; P < .001), but not for the other two measures.
IN PRACTICE:
“Our finding suggests that lacking access to virtual and in-person psychiatric care continues to be a key factor associated with adverse outcomes,” the investigators write. They note that federal and state-level investments in broadband and the psychiatric workforce are needed.
SOURCE:
The study was conducted by Tarun Ramesh, BS, department of population medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, and colleagues. It was published online as a research letter in JAMA Network Open.
LIMITATIONS:
The investigators did not consider whether recent legislation, including the Consolidated Appropriations Act of 2021 and the American Rescue Plan, which expanded psychiatry residency slots and broadband infrastructure, reduces adverse outcomes, something the authors say future research should examine.
DISCLOSURES:
The study received support from the National Institutes of Health, including the National Institute on Minority Health and Health Disparities and the National Institute of Mental Health. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
, new research shows.
METHODOLOGY:
- In the United States, there is a severe lack of psychiatrists and access to mental health care. In 2019, 21.3 million U.S. residents were without broadband access. These patients were forced either to use telephone consultation or to not use telehealth services at all, although use of telehealth during COVID-19 somewhat improved access to psychiatric care.
- For the study, researchers gathered sociodemographic and other county-level information from the American Community Survey. They also used data on the psychiatrist workforce from the Health Resources and Services Administration (HRSA) Area Health Resources Files.
- Information on broadband Internet coverage came from the Federal Communications Commission, and measures of mental health outcomes were from the Centers for Disease Control and Prevention.
TAKEAWAY:
- The study identified 596 counties (19% of all U.S. counties) that were without psychiatrists and in which there was inadequate broadband coverage. The population represented 10.5 million residents.
- Compared with other counties, those with lack of coverage were more likely to be rural (adjusted odds ratio, 3.05; 95% confidence interval, 2.41-3.84), to have higher unemployment (aOR, 1.12; 95% CI, 1.02-1.24), and to have higher uninsurance rates (aOR, 1.03; 95% CI, 1.00-1.06). In those counties, there were also fewer residents with a bachelor’s degree (aOR, 0.92; 95% CI, 0.90-0.94) and fewer Hispanics (aOR 0.98; 95% CI, 0.97-0.99), although those counties were not designated by the HRSA as having a psychiatrist shortage. That designation brings additional funding for the recruitment of clinicians.
- After adjustment for sociodemographic factors, counties without psychiatrists and broadband had significantly higher rates of adult depression, frequent mental distress, drug overdose mortality, and completed suicide, compared with other counties.
- Further analysis showed that the adjusted difference remained statistically significant for drug overdose mortality per 100,000 (9.2; 95% CI, 8.0-10.5, vs. 5.2; 95% CI, 4.9-5.6; P < .001) and completed suicide (10.6; 95% CI, 8.9-12.3, vs. 7.6; 95% CI, 7.0-8.2; P < .001), but not for the other two measures.
IN PRACTICE:
“Our finding suggests that lacking access to virtual and in-person psychiatric care continues to be a key factor associated with adverse outcomes,” the investigators write. They note that federal and state-level investments in broadband and the psychiatric workforce are needed.
SOURCE:
The study was conducted by Tarun Ramesh, BS, department of population medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, and colleagues. It was published online as a research letter in JAMA Network Open.
LIMITATIONS:
The investigators did not consider whether recent legislation, including the Consolidated Appropriations Act of 2021 and the American Rescue Plan, which expanded psychiatry residency slots and broadband infrastructure, reduces adverse outcomes, something the authors say future research should examine.
DISCLOSURES:
The study received support from the National Institutes of Health, including the National Institute on Minority Health and Health Disparities and the National Institute of Mental Health. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sexual dysfunction common in schizophrenia
TOPLINE:
, results of a systematic review and meta-analysis show.
METHODOLOGY:
- Data on sexual dysfunction prevalence in people with schizophrenia should be updated because the only meta-analysis on this topic was published over 10 years ago, and factors that could explain the heterogeneity of sexual dysfunctions in schizophrenia also need reexamining.
- After carrying out a literature search for observational studies reporting prevalence of sexual dysfunction in outpatients receiving treatment for schizophrenia or schizoaffective disorder, researchers included 72 studies with 21,076 patients from 33 countries published between 1979 and 2021 in their review.
- They determined pooled estimates of sexual dysfunction prevalence in men and women and of each specific dysfunction.
TAKEAWAY:
- Pooled estimates for global prevalence were: 56.4% for sexual dysfunctions (95% confidence interval, 50.5-62.2), 40.6% for loss of libido (95% CI, 30.7-51.4), 28.0% for orgasm dysfunction (95% CI, 18.4-40.2), and 6.1% for genital pain (95% CI, 2.8-12.7), with study design, sociodemographic data, and other factors associated with the high heterogeneity of sexual dysfunctions.
- In men, estimates were: 55.7% for sexual dysfunction (95% CI, 48.1-63.1), 44.0% for erectile dysfunction (95% CI, 33.5-55.2), and 38.6% ejaculation dysfunction (95% CI, 26.8-51.8).
- In women, estimates were: 60.0% for sexual dysfunction (95% CI, 48.0-70.8), 25.1% for amenorrhea (95% CI, 17.3-35.0), and 7.7% for galactorrhea (95% CI, 3.7-15.3).
- Studies with the highest proportion of antidepressant prescriptions reported lower rates of sexual dysfunctions.
IN PRACTICE:
The review shows that sexual dysfunction is “extremely frequent” in schizophrenia and uncovers “important evidence” suggesting that better screening and treatment of depression “may be an effective strategy to improve sexual health in patients with schizophrenia,” write the authors.
SOURCE:
The study was carried out by Théo Korchia, MD, Assistance Publique-Hopitaux de Marseille, Aix-Marseille University, CEReSS: Health Service Research and Quality of Life Center, France, and colleagues. It was published online in JAMA Psychiatry.
LIMITATIONS:
Most factors known to increase sexual dysfunction, including hypertension, diabetes, obesity, smoking, and sleep disorders, were poorly explored in the included studies. Results may not be extrapolated to continents such as Africa and Polynesia because they were underrepresented in the review. The presence of publication bias in the meta-analysis can’t be entirely ruled out. Heterogeneity or methodological differences may have contributed to the observed results.
DISCLOSURES:
The authors have no relevant conflict of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
, results of a systematic review and meta-analysis show.
METHODOLOGY:
- Data on sexual dysfunction prevalence in people with schizophrenia should be updated because the only meta-analysis on this topic was published over 10 years ago, and factors that could explain the heterogeneity of sexual dysfunctions in schizophrenia also need reexamining.
- After carrying out a literature search for observational studies reporting prevalence of sexual dysfunction in outpatients receiving treatment for schizophrenia or schizoaffective disorder, researchers included 72 studies with 21,076 patients from 33 countries published between 1979 and 2021 in their review.
- They determined pooled estimates of sexual dysfunction prevalence in men and women and of each specific dysfunction.
TAKEAWAY:
- Pooled estimates for global prevalence were: 56.4% for sexual dysfunctions (95% confidence interval, 50.5-62.2), 40.6% for loss of libido (95% CI, 30.7-51.4), 28.0% for orgasm dysfunction (95% CI, 18.4-40.2), and 6.1% for genital pain (95% CI, 2.8-12.7), with study design, sociodemographic data, and other factors associated with the high heterogeneity of sexual dysfunctions.
- In men, estimates were: 55.7% for sexual dysfunction (95% CI, 48.1-63.1), 44.0% for erectile dysfunction (95% CI, 33.5-55.2), and 38.6% ejaculation dysfunction (95% CI, 26.8-51.8).
- In women, estimates were: 60.0% for sexual dysfunction (95% CI, 48.0-70.8), 25.1% for amenorrhea (95% CI, 17.3-35.0), and 7.7% for galactorrhea (95% CI, 3.7-15.3).
- Studies with the highest proportion of antidepressant prescriptions reported lower rates of sexual dysfunctions.
IN PRACTICE:
The review shows that sexual dysfunction is “extremely frequent” in schizophrenia and uncovers “important evidence” suggesting that better screening and treatment of depression “may be an effective strategy to improve sexual health in patients with schizophrenia,” write the authors.
SOURCE:
The study was carried out by Théo Korchia, MD, Assistance Publique-Hopitaux de Marseille, Aix-Marseille University, CEReSS: Health Service Research and Quality of Life Center, France, and colleagues. It was published online in JAMA Psychiatry.
LIMITATIONS:
Most factors known to increase sexual dysfunction, including hypertension, diabetes, obesity, smoking, and sleep disorders, were poorly explored in the included studies. Results may not be extrapolated to continents such as Africa and Polynesia because they were underrepresented in the review. The presence of publication bias in the meta-analysis can’t be entirely ruled out. Heterogeneity or methodological differences may have contributed to the observed results.
DISCLOSURES:
The authors have no relevant conflict of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
, results of a systematic review and meta-analysis show.
METHODOLOGY:
- Data on sexual dysfunction prevalence in people with schizophrenia should be updated because the only meta-analysis on this topic was published over 10 years ago, and factors that could explain the heterogeneity of sexual dysfunctions in schizophrenia also need reexamining.
- After carrying out a literature search for observational studies reporting prevalence of sexual dysfunction in outpatients receiving treatment for schizophrenia or schizoaffective disorder, researchers included 72 studies with 21,076 patients from 33 countries published between 1979 and 2021 in their review.
- They determined pooled estimates of sexual dysfunction prevalence in men and women and of each specific dysfunction.
TAKEAWAY:
- Pooled estimates for global prevalence were: 56.4% for sexual dysfunctions (95% confidence interval, 50.5-62.2), 40.6% for loss of libido (95% CI, 30.7-51.4), 28.0% for orgasm dysfunction (95% CI, 18.4-40.2), and 6.1% for genital pain (95% CI, 2.8-12.7), with study design, sociodemographic data, and other factors associated with the high heterogeneity of sexual dysfunctions.
- In men, estimates were: 55.7% for sexual dysfunction (95% CI, 48.1-63.1), 44.0% for erectile dysfunction (95% CI, 33.5-55.2), and 38.6% ejaculation dysfunction (95% CI, 26.8-51.8).
- In women, estimates were: 60.0% for sexual dysfunction (95% CI, 48.0-70.8), 25.1% for amenorrhea (95% CI, 17.3-35.0), and 7.7% for galactorrhea (95% CI, 3.7-15.3).
- Studies with the highest proportion of antidepressant prescriptions reported lower rates of sexual dysfunctions.
IN PRACTICE:
The review shows that sexual dysfunction is “extremely frequent” in schizophrenia and uncovers “important evidence” suggesting that better screening and treatment of depression “may be an effective strategy to improve sexual health in patients with schizophrenia,” write the authors.
SOURCE:
The study was carried out by Théo Korchia, MD, Assistance Publique-Hopitaux de Marseille, Aix-Marseille University, CEReSS: Health Service Research and Quality of Life Center, France, and colleagues. It was published online in JAMA Psychiatry.
LIMITATIONS:
Most factors known to increase sexual dysfunction, including hypertension, diabetes, obesity, smoking, and sleep disorders, were poorly explored in the included studies. Results may not be extrapolated to continents such as Africa and Polynesia because they were underrepresented in the review. The presence of publication bias in the meta-analysis can’t be entirely ruled out. Heterogeneity or methodological differences may have contributed to the observed results.
DISCLOSURES:
The authors have no relevant conflict of interest.
A version of this article first appeared on Medscape.com.
In utero SSRI exposure tied to lower brain volume in kids
However, the investigators, led by Henning Tiemeier, MD, PhD, professor of social and behavioral sciences at Harvard School of Public Health in Boston, note that the findings should be interpreted cautiously because the size of the study population who received brain MRI was relatively small.
Dr. Tiemeier said in an interview that the associations detected were small and could not show causality between prenatal SSRI use and a decrease in gray and white matter across certain areas of the brain.
“Women who are pregnant and on maintenance therapy should consult their therapist if preventive therapy is still needed and if there are alternatives. This choice must be carefully considered, and women should be carefully advised,” he said.
The study was published online in JAMA Psychiatry.
An important decision
The investigators note that the decision to prescribe antidepressants, particularly SSRIs, during pregnancy is challenging. Though SSRI use during pregnancy is generally considered safe, some previous research suggests an association with negative outcomes in offspring, including adverse effects on neurodevelopment.
However, the researchers also note that it’s possible that pregnant women who use SSRIs may have other factors, including more severe depressive symptoms, which may be independently associated with adverse outcomes in offspring.
To investigate the link between intrauterine SSRI exposure and brain development, the researchers conducted a prospective, population-based study that included 3,198 pregnant individuals with an expected delivery date between April 2002 and January 2006. Study participants were divided into five groups: 41 who used SSRIs during pregnancy, 257 who did not use the medications but had depressive symptoms during pregnancy, 77 who used SSRIs prenatally, 74 who developed depressive symptoms after giving birth, and 2,749 controls with no SSRI use or depressive symptoms. Participants had a mean age of 31 years, and all identified as women.
Of those who took SSRIs during pregnancy, 20 used them during the first trimester only, and 21 used them the first or in one or two additional trimesters. The SSRIs used included paroxetine, fluoxetine, sertraline, fluvoxamine, and citalopram.
Offspring of the women enrolled in the study received MRIs at three different times between the ages 7 and 15 years.
The 41 children born to the women who took SSRIs prenatally had 80 scans in total, the 257 with mothers who did not use SSRIs yet had depressive symptoms while pregnant had 477 MRIs, the 77 children born to the mothers who took SSRIs before pregnancy had 126 MRIs, the 74 born to mothers with postnatal depression only had 128 MRIs, and the 2,749 children born to the mothers with no SSRI use or depression had 4,813 MRIs.
The study’s primary outcome was brain morphometry in offspring including global and cortical brain volumes, measured by three MRI assessments from ages 7 to 15 years.
Reduced brain volume
Compared with children with no in utero SSRI exposure, those who were exposed had reduced gray and white matter volume that persisted up to 15 years of age (P = .006), particularly in the corticolimbic circuit.
Investigators observed a “persistent association between prenatal SSRI exposure and less cortical volumes across the 10-year follow-up period, including in the superior frontal cortex, medial orbitofrontal cortex, parahippocampal gyrus, rostral anterior cingulate cortex, and posterior cingulate.”
Investigators noted that prenatal SSRI exposure was consistently associated with 5%-10% lower brain volume in the frontal, cingulate, and temporal cortex throughout the age range studied.
In a couple of areas of the brain, however, the brain volume gradually increased back to levels seen in non-SSRI exposed children. For instance, smaller amygdala volumes had increased by age 15 years, so children who were exposed to SSRIs were not any different from control children.
Among the group of women with postnatal depression using an SSRI before or during pregnancy who had depressive symptoms post natally, neonates had a reduced fusiform gyrus (P = .002)
Dr. Tiemeier could not speculate on the effects of the volume differences on children’s development, although the parts of the brain found to be reduced are primarily responsible for emotion regulation.
Investigators noted there was limited ability to investigate trimester-specific outcomes of SSRI use and assess associations with specific SSRIs due to low prevalence of SSRI use.
In addition, research on the long-term behavioral and psychological outcomes associated with demonstrated brain changes is needed, investigators noted.
Clinical significance ‘unclear’
In an accompanying editorial, Ardesheer Talati, PhD, Columbia University, New York, noted that though the research enhances understanding of how brain development through adolescence may be associated with SSRI exposure, “the clinical significance was unclear, especially as key limbic regions, including the amygdala, normalized over time.”
If future evidence links brain anomalies to adverse youth outcomes, Dr. Talati writes, this will need to be “calibrated into the risk-benefit profile.” Until then, he said, the findings must not be overinterpreted “to either promote or discourage antidepressant medication use during the critical period of pregnancy.”
The study was funded by the Netherlands Organization for Scientific Research, European Union’s Horizon Research and Innovation Program, the Netherlands Organization for Health Research and Development, the Sophia Foundation for Neuroimaging, and the European Union’s Horizon Research and Innovation 5 Program. Dr. Talati reported receiving grants from the National Institutes of Health outside of the submitted work.
A version of this article first appeared on Medscape.com.
However, the investigators, led by Henning Tiemeier, MD, PhD, professor of social and behavioral sciences at Harvard School of Public Health in Boston, note that the findings should be interpreted cautiously because the size of the study population who received brain MRI was relatively small.
Dr. Tiemeier said in an interview that the associations detected were small and could not show causality between prenatal SSRI use and a decrease in gray and white matter across certain areas of the brain.
“Women who are pregnant and on maintenance therapy should consult their therapist if preventive therapy is still needed and if there are alternatives. This choice must be carefully considered, and women should be carefully advised,” he said.
The study was published online in JAMA Psychiatry.
An important decision
The investigators note that the decision to prescribe antidepressants, particularly SSRIs, during pregnancy is challenging. Though SSRI use during pregnancy is generally considered safe, some previous research suggests an association with negative outcomes in offspring, including adverse effects on neurodevelopment.
However, the researchers also note that it’s possible that pregnant women who use SSRIs may have other factors, including more severe depressive symptoms, which may be independently associated with adverse outcomes in offspring.
To investigate the link between intrauterine SSRI exposure and brain development, the researchers conducted a prospective, population-based study that included 3,198 pregnant individuals with an expected delivery date between April 2002 and January 2006. Study participants were divided into five groups: 41 who used SSRIs during pregnancy, 257 who did not use the medications but had depressive symptoms during pregnancy, 77 who used SSRIs prenatally, 74 who developed depressive symptoms after giving birth, and 2,749 controls with no SSRI use or depressive symptoms. Participants had a mean age of 31 years, and all identified as women.
Of those who took SSRIs during pregnancy, 20 used them during the first trimester only, and 21 used them the first or in one or two additional trimesters. The SSRIs used included paroxetine, fluoxetine, sertraline, fluvoxamine, and citalopram.
Offspring of the women enrolled in the study received MRIs at three different times between the ages 7 and 15 years.
The 41 children born to the women who took SSRIs prenatally had 80 scans in total, the 257 with mothers who did not use SSRIs yet had depressive symptoms while pregnant had 477 MRIs, the 77 children born to the mothers who took SSRIs before pregnancy had 126 MRIs, the 74 born to mothers with postnatal depression only had 128 MRIs, and the 2,749 children born to the mothers with no SSRI use or depression had 4,813 MRIs.
The study’s primary outcome was brain morphometry in offspring including global and cortical brain volumes, measured by three MRI assessments from ages 7 to 15 years.
Reduced brain volume
Compared with children with no in utero SSRI exposure, those who were exposed had reduced gray and white matter volume that persisted up to 15 years of age (P = .006), particularly in the corticolimbic circuit.
Investigators observed a “persistent association between prenatal SSRI exposure and less cortical volumes across the 10-year follow-up period, including in the superior frontal cortex, medial orbitofrontal cortex, parahippocampal gyrus, rostral anterior cingulate cortex, and posterior cingulate.”
Investigators noted that prenatal SSRI exposure was consistently associated with 5%-10% lower brain volume in the frontal, cingulate, and temporal cortex throughout the age range studied.
In a couple of areas of the brain, however, the brain volume gradually increased back to levels seen in non-SSRI exposed children. For instance, smaller amygdala volumes had increased by age 15 years, so children who were exposed to SSRIs were not any different from control children.
Among the group of women with postnatal depression using an SSRI before or during pregnancy who had depressive symptoms post natally, neonates had a reduced fusiform gyrus (P = .002)
Dr. Tiemeier could not speculate on the effects of the volume differences on children’s development, although the parts of the brain found to be reduced are primarily responsible for emotion regulation.
Investigators noted there was limited ability to investigate trimester-specific outcomes of SSRI use and assess associations with specific SSRIs due to low prevalence of SSRI use.
In addition, research on the long-term behavioral and psychological outcomes associated with demonstrated brain changes is needed, investigators noted.
Clinical significance ‘unclear’
In an accompanying editorial, Ardesheer Talati, PhD, Columbia University, New York, noted that though the research enhances understanding of how brain development through adolescence may be associated with SSRI exposure, “the clinical significance was unclear, especially as key limbic regions, including the amygdala, normalized over time.”
If future evidence links brain anomalies to adverse youth outcomes, Dr. Talati writes, this will need to be “calibrated into the risk-benefit profile.” Until then, he said, the findings must not be overinterpreted “to either promote or discourage antidepressant medication use during the critical period of pregnancy.”
The study was funded by the Netherlands Organization for Scientific Research, European Union’s Horizon Research and Innovation Program, the Netherlands Organization for Health Research and Development, the Sophia Foundation for Neuroimaging, and the European Union’s Horizon Research and Innovation 5 Program. Dr. Talati reported receiving grants from the National Institutes of Health outside of the submitted work.
A version of this article first appeared on Medscape.com.
However, the investigators, led by Henning Tiemeier, MD, PhD, professor of social and behavioral sciences at Harvard School of Public Health in Boston, note that the findings should be interpreted cautiously because the size of the study population who received brain MRI was relatively small.
Dr. Tiemeier said in an interview that the associations detected were small and could not show causality between prenatal SSRI use and a decrease in gray and white matter across certain areas of the brain.
“Women who are pregnant and on maintenance therapy should consult their therapist if preventive therapy is still needed and if there are alternatives. This choice must be carefully considered, and women should be carefully advised,” he said.
The study was published online in JAMA Psychiatry.
An important decision
The investigators note that the decision to prescribe antidepressants, particularly SSRIs, during pregnancy is challenging. Though SSRI use during pregnancy is generally considered safe, some previous research suggests an association with negative outcomes in offspring, including adverse effects on neurodevelopment.
However, the researchers also note that it’s possible that pregnant women who use SSRIs may have other factors, including more severe depressive symptoms, which may be independently associated with adverse outcomes in offspring.
To investigate the link between intrauterine SSRI exposure and brain development, the researchers conducted a prospective, population-based study that included 3,198 pregnant individuals with an expected delivery date between April 2002 and January 2006. Study participants were divided into five groups: 41 who used SSRIs during pregnancy, 257 who did not use the medications but had depressive symptoms during pregnancy, 77 who used SSRIs prenatally, 74 who developed depressive symptoms after giving birth, and 2,749 controls with no SSRI use or depressive symptoms. Participants had a mean age of 31 years, and all identified as women.
Of those who took SSRIs during pregnancy, 20 used them during the first trimester only, and 21 used them the first or in one or two additional trimesters. The SSRIs used included paroxetine, fluoxetine, sertraline, fluvoxamine, and citalopram.
Offspring of the women enrolled in the study received MRIs at three different times between the ages 7 and 15 years.
The 41 children born to the women who took SSRIs prenatally had 80 scans in total, the 257 with mothers who did not use SSRIs yet had depressive symptoms while pregnant had 477 MRIs, the 77 children born to the mothers who took SSRIs before pregnancy had 126 MRIs, the 74 born to mothers with postnatal depression only had 128 MRIs, and the 2,749 children born to the mothers with no SSRI use or depression had 4,813 MRIs.
The study’s primary outcome was brain morphometry in offspring including global and cortical brain volumes, measured by three MRI assessments from ages 7 to 15 years.
Reduced brain volume
Compared with children with no in utero SSRI exposure, those who were exposed had reduced gray and white matter volume that persisted up to 15 years of age (P = .006), particularly in the corticolimbic circuit.
Investigators observed a “persistent association between prenatal SSRI exposure and less cortical volumes across the 10-year follow-up period, including in the superior frontal cortex, medial orbitofrontal cortex, parahippocampal gyrus, rostral anterior cingulate cortex, and posterior cingulate.”
Investigators noted that prenatal SSRI exposure was consistently associated with 5%-10% lower brain volume in the frontal, cingulate, and temporal cortex throughout the age range studied.
In a couple of areas of the brain, however, the brain volume gradually increased back to levels seen in non-SSRI exposed children. For instance, smaller amygdala volumes had increased by age 15 years, so children who were exposed to SSRIs were not any different from control children.
Among the group of women with postnatal depression using an SSRI before or during pregnancy who had depressive symptoms post natally, neonates had a reduced fusiform gyrus (P = .002)
Dr. Tiemeier could not speculate on the effects of the volume differences on children’s development, although the parts of the brain found to be reduced are primarily responsible for emotion regulation.
Investigators noted there was limited ability to investigate trimester-specific outcomes of SSRI use and assess associations with specific SSRIs due to low prevalence of SSRI use.
In addition, research on the long-term behavioral and psychological outcomes associated with demonstrated brain changes is needed, investigators noted.
Clinical significance ‘unclear’
In an accompanying editorial, Ardesheer Talati, PhD, Columbia University, New York, noted that though the research enhances understanding of how brain development through adolescence may be associated with SSRI exposure, “the clinical significance was unclear, especially as key limbic regions, including the amygdala, normalized over time.”
If future evidence links brain anomalies to adverse youth outcomes, Dr. Talati writes, this will need to be “calibrated into the risk-benefit profile.” Until then, he said, the findings must not be overinterpreted “to either promote or discourage antidepressant medication use during the critical period of pregnancy.”
The study was funded by the Netherlands Organization for Scientific Research, European Union’s Horizon Research and Innovation Program, the Netherlands Organization for Health Research and Development, the Sophia Foundation for Neuroimaging, and the European Union’s Horizon Research and Innovation 5 Program. Dr. Talati reported receiving grants from the National Institutes of Health outside of the submitted work.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Social media use may promote depression in pregnancy
Depressive symptoms among pregnant women have risen in recent years, but the potential impact of social media use on depression in pregnancy has not been well studied, wrote Lotte Muskens, a PhD candidate at Tilburg (the Netherlands) University and colleagues.
In a study published in the Journal of Affective Disorders, the researchers surveyed 697 pregnant women aged 19-42 years who were part of a larger longitudinal prospective study (the Brabant Study) in the Netherlands. The mean age of the participants was 31 years; 96% were employed, 99% had a partner, and 71% had a bachelor’s degree or higher. Depressive symptoms were assessed at 12, 20, and 28 weeks of pregnancy using the Dutch version of the 10-item Edinburgh Depression Scale (EDS).
The researchers categorized the participants into trajectories of depressive symptoms during pregnancy, with 489 identified as low stable (mean EDS scores 2.8-3.0), 183 as intermediate stable (mean EDS scores 8.4-8.8), and 25 as high stable (mean EDS scores 15.1-16.9).
Problematic SMU was identified using the six-item Bergen Social Media Addiction Scale (BSMAS) at 12 weeks of pregnancy; scores ranged from 6 to 30, with higher scores representing more problematic SMU.
The mean BSMAS scores were 9.0, 10.7, and 12.6 for the low-stable, intermediate-stable, and high-stable depression groups, respectively.
Data on social media use (SMU) were collected at 12 weeks of pregnancy. Social media was defined as common platforms including Facebook, Instagram, LinkedIn, Pinterest, Twitter, and YouTube.
SMU was defined in terms of intensity, measured by time and frequency. Time was measured by asking participants to list how many hours per day they used social media on a scale of 1 (no use of social media) to 9 (7 or more hours per day). Frequency was measured by asking how often participants visited the various social media platforms, on a scale of 1 (no use of social media) to 7 (five or more visits per day). Overall, the participants averaged 1.6 hours per day and 19.5 visits per week on SMU.
Increased time and frequency of SMU was significantly associated with increased odds of being in the high-stable group, compared with the low-stable group in an adjusted analysis (odds ratios, 1.51 and 1.05, respectively; P = .017 and P = .019, respectively).
In addition, problematic SMU (as defined by higher BSMAS scores) remained significantly associated with increased odds of belonging to the intermediate-stable or high-stable classes in an adjusted analysis (odds ratios, 1.17 and 1.31; P < .001 for both).
“While our results suggest that SMU can have negative consequences for pregnant women’s mental wellbeing, it is important to note that SMU during pregnancy may also be helpful for some pregnant women,” as many women, especially first-time mothers, find information and support through social media, the researchers wrote in their discussion.
The findings were limited by several factors, including the variation in group sizes for depressive symptoms, reliance on self-reports, and the collection of data during the COVID-19 pandemic, which may have affected the results, the researchers noted.
However, the results were strengthened by the large sample size and longitudinal design that allowed measurement of trajectories. More research is needed to determine causal relationships, but the data indicate an association between higher levels of depression during pregnancy and more intense and problematic SMU use, and health care providers should discuss SMU in addition to other risk factors for depression in pregnant women, the researchers concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Depressive symptoms among pregnant women have risen in recent years, but the potential impact of social media use on depression in pregnancy has not been well studied, wrote Lotte Muskens, a PhD candidate at Tilburg (the Netherlands) University and colleagues.
In a study published in the Journal of Affective Disorders, the researchers surveyed 697 pregnant women aged 19-42 years who were part of a larger longitudinal prospective study (the Brabant Study) in the Netherlands. The mean age of the participants was 31 years; 96% were employed, 99% had a partner, and 71% had a bachelor’s degree or higher. Depressive symptoms were assessed at 12, 20, and 28 weeks of pregnancy using the Dutch version of the 10-item Edinburgh Depression Scale (EDS).
The researchers categorized the participants into trajectories of depressive symptoms during pregnancy, with 489 identified as low stable (mean EDS scores 2.8-3.0), 183 as intermediate stable (mean EDS scores 8.4-8.8), and 25 as high stable (mean EDS scores 15.1-16.9).
Problematic SMU was identified using the six-item Bergen Social Media Addiction Scale (BSMAS) at 12 weeks of pregnancy; scores ranged from 6 to 30, with higher scores representing more problematic SMU.
The mean BSMAS scores were 9.0, 10.7, and 12.6 for the low-stable, intermediate-stable, and high-stable depression groups, respectively.
Data on social media use (SMU) were collected at 12 weeks of pregnancy. Social media was defined as common platforms including Facebook, Instagram, LinkedIn, Pinterest, Twitter, and YouTube.
SMU was defined in terms of intensity, measured by time and frequency. Time was measured by asking participants to list how many hours per day they used social media on a scale of 1 (no use of social media) to 9 (7 or more hours per day). Frequency was measured by asking how often participants visited the various social media platforms, on a scale of 1 (no use of social media) to 7 (five or more visits per day). Overall, the participants averaged 1.6 hours per day and 19.5 visits per week on SMU.
Increased time and frequency of SMU was significantly associated with increased odds of being in the high-stable group, compared with the low-stable group in an adjusted analysis (odds ratios, 1.51 and 1.05, respectively; P = .017 and P = .019, respectively).
In addition, problematic SMU (as defined by higher BSMAS scores) remained significantly associated with increased odds of belonging to the intermediate-stable or high-stable classes in an adjusted analysis (odds ratios, 1.17 and 1.31; P < .001 for both).
“While our results suggest that SMU can have negative consequences for pregnant women’s mental wellbeing, it is important to note that SMU during pregnancy may also be helpful for some pregnant women,” as many women, especially first-time mothers, find information and support through social media, the researchers wrote in their discussion.
The findings were limited by several factors, including the variation in group sizes for depressive symptoms, reliance on self-reports, and the collection of data during the COVID-19 pandemic, which may have affected the results, the researchers noted.
However, the results were strengthened by the large sample size and longitudinal design that allowed measurement of trajectories. More research is needed to determine causal relationships, but the data indicate an association between higher levels of depression during pregnancy and more intense and problematic SMU use, and health care providers should discuss SMU in addition to other risk factors for depression in pregnant women, the researchers concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Depressive symptoms among pregnant women have risen in recent years, but the potential impact of social media use on depression in pregnancy has not been well studied, wrote Lotte Muskens, a PhD candidate at Tilburg (the Netherlands) University and colleagues.
In a study published in the Journal of Affective Disorders, the researchers surveyed 697 pregnant women aged 19-42 years who were part of a larger longitudinal prospective study (the Brabant Study) in the Netherlands. The mean age of the participants was 31 years; 96% were employed, 99% had a partner, and 71% had a bachelor’s degree or higher. Depressive symptoms were assessed at 12, 20, and 28 weeks of pregnancy using the Dutch version of the 10-item Edinburgh Depression Scale (EDS).
The researchers categorized the participants into trajectories of depressive symptoms during pregnancy, with 489 identified as low stable (mean EDS scores 2.8-3.0), 183 as intermediate stable (mean EDS scores 8.4-8.8), and 25 as high stable (mean EDS scores 15.1-16.9).
Problematic SMU was identified using the six-item Bergen Social Media Addiction Scale (BSMAS) at 12 weeks of pregnancy; scores ranged from 6 to 30, with higher scores representing more problematic SMU.
The mean BSMAS scores were 9.0, 10.7, and 12.6 for the low-stable, intermediate-stable, and high-stable depression groups, respectively.
Data on social media use (SMU) were collected at 12 weeks of pregnancy. Social media was defined as common platforms including Facebook, Instagram, LinkedIn, Pinterest, Twitter, and YouTube.
SMU was defined in terms of intensity, measured by time and frequency. Time was measured by asking participants to list how many hours per day they used social media on a scale of 1 (no use of social media) to 9 (7 or more hours per day). Frequency was measured by asking how often participants visited the various social media platforms, on a scale of 1 (no use of social media) to 7 (five or more visits per day). Overall, the participants averaged 1.6 hours per day and 19.5 visits per week on SMU.
Increased time and frequency of SMU was significantly associated with increased odds of being in the high-stable group, compared with the low-stable group in an adjusted analysis (odds ratios, 1.51 and 1.05, respectively; P = .017 and P = .019, respectively).
In addition, problematic SMU (as defined by higher BSMAS scores) remained significantly associated with increased odds of belonging to the intermediate-stable or high-stable classes in an adjusted analysis (odds ratios, 1.17 and 1.31; P < .001 for both).
“While our results suggest that SMU can have negative consequences for pregnant women’s mental wellbeing, it is important to note that SMU during pregnancy may also be helpful for some pregnant women,” as many women, especially first-time mothers, find information and support through social media, the researchers wrote in their discussion.
The findings were limited by several factors, including the variation in group sizes for depressive symptoms, reliance on self-reports, and the collection of data during the COVID-19 pandemic, which may have affected the results, the researchers noted.
However, the results were strengthened by the large sample size and longitudinal design that allowed measurement of trajectories. More research is needed to determine causal relationships, but the data indicate an association between higher levels of depression during pregnancy and more intense and problematic SMU use, and health care providers should discuss SMU in addition to other risk factors for depression in pregnant women, the researchers concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF AFFECTIVE DISORDERS