User login
COVID-19: Guiding vaccinated patients through to the ‘new normal’
As COVID-related restrictions are lifting and the streets, restaurants, and events are filling back up, we must encourage our patients to take inventory. It is time to help them create posttraumatic growth.
As we help them navigate this part of the pandemic, encourage them to ask what they learned over the last year and how they plan to integrate what they’ve been through to successfully create the “new normal.”
The Biden administration had set a goal of getting at least one shot to 70% of American adults by July 4, and that goal will not be reached. That shortfall, combined with the increase of the highly transmissible Delta variant of SARS-CoV-2 means that we and our patients must not let our guards completely down. At the same time, we can encourage our vaccinated patients to get back to their prepandemic lives – to the extent that they feel comfortable doing so.
Ultimately, this is about respecting physical and emotional boundaries. How do we greet vaccinated people now? Is it okay to shake hands, hug, or kiss to greet a friend or family member – or should we continue to elbow bump – or perhaps wave? Should we confront family members who have opted not to get vaccinated for reasons not related to health? Is it safe to visit with older relatives who are vaccinated? What about children under 12 who are not?
Those who were on the front lines of the pandemic faced unfathomable pain and suffering – and mental and physical exhaustion. And we know that the nightmare is not over. Several areas of the country with large numbers of unvaccinated people could face “very dense outbreaks,” in large part because of the Delta variant.
As we sort through the remaining challenges, I urge us all to reflect. We have been in this together and will emerge together. We know that the closer we were to the trauma, the longer recovery will take.
Ask patients to consider what is most important to resume and what can still wait. Some are eager to jump back into the deep end of the pool; others prefer to continue to wait cautiously. Families need to be on the same page as they assess risks and opportunities going forward, because household spread continues to be at the highest risk. Remind patients that the health of one of us affects the health of all of us.
Urge patients to take time to explore the following questions as they process the pandemic. We can also ask ourselves these same questions and share them with colleagues who are also rebuilding.
- Did you prioritize your family more? How can you continue to spend quality time them as other opportunities emerge?
- Did you have to withdraw from friends/coworkers and family members because of the pandemic? If so, how can you reincorporate them in our lives?
- Did you send more time caring for yourself with exercise and meditation? Can those new habits remain in place as life presents more options? How can you continue to make time for self-care while adding back other responsibilities?
- Did you eat better or worse in quarantine? Can you maintain the positive habits you developed as you venture back to restaurants, parties, and gatherings?
- What habits did you break that you are now better off without?
- What new habits or hobbies did you create that you want to continue?
- What hobbies should you resume that you missed during the last year?
- What new coping skills have you gained?
- Has your alcohol consumption declined or increased during the pandemic?
- Did you neglect/decide to forgo your medical and dental care? How quickly can you safely resume that care?
- How did your value system shift this year?
- Did the people you feel closest to change?
- How can you use this trauma to appreciate life more?
Life might get very busy this summer, so encourage patients to find time to answer these questions. Journaling can be a great way to think through all that we have experienced. Our brains will need to change again to adapt. Many of us have felt sad or anxious for a quite a while, and we want to move toward more positive feelings of safety, happiness, optimism, and joy. This will take effort. After all, we have lost more than 600,000 people to COVID, and much of the world is still in the middle of the pandemic. But this will get much easier as the threat of COVID-19 continues to recede. We must now work toward creating better times ahead.
Dr. Ritvo has almost 30 years’ experience in psychiatry and is currently practicing telemedicine. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018). She has no conflicts of interest.
As COVID-related restrictions are lifting and the streets, restaurants, and events are filling back up, we must encourage our patients to take inventory. It is time to help them create posttraumatic growth.
As we help them navigate this part of the pandemic, encourage them to ask what they learned over the last year and how they plan to integrate what they’ve been through to successfully create the “new normal.”
The Biden administration had set a goal of getting at least one shot to 70% of American adults by July 4, and that goal will not be reached. That shortfall, combined with the increase of the highly transmissible Delta variant of SARS-CoV-2 means that we and our patients must not let our guards completely down. At the same time, we can encourage our vaccinated patients to get back to their prepandemic lives – to the extent that they feel comfortable doing so.
Ultimately, this is about respecting physical and emotional boundaries. How do we greet vaccinated people now? Is it okay to shake hands, hug, or kiss to greet a friend or family member – or should we continue to elbow bump – or perhaps wave? Should we confront family members who have opted not to get vaccinated for reasons not related to health? Is it safe to visit with older relatives who are vaccinated? What about children under 12 who are not?
Those who were on the front lines of the pandemic faced unfathomable pain and suffering – and mental and physical exhaustion. And we know that the nightmare is not over. Several areas of the country with large numbers of unvaccinated people could face “very dense outbreaks,” in large part because of the Delta variant.
As we sort through the remaining challenges, I urge us all to reflect. We have been in this together and will emerge together. We know that the closer we were to the trauma, the longer recovery will take.
Ask patients to consider what is most important to resume and what can still wait. Some are eager to jump back into the deep end of the pool; others prefer to continue to wait cautiously. Families need to be on the same page as they assess risks and opportunities going forward, because household spread continues to be at the highest risk. Remind patients that the health of one of us affects the health of all of us.
Urge patients to take time to explore the following questions as they process the pandemic. We can also ask ourselves these same questions and share them with colleagues who are also rebuilding.
- Did you prioritize your family more? How can you continue to spend quality time them as other opportunities emerge?
- Did you have to withdraw from friends/coworkers and family members because of the pandemic? If so, how can you reincorporate them in our lives?
- Did you send more time caring for yourself with exercise and meditation? Can those new habits remain in place as life presents more options? How can you continue to make time for self-care while adding back other responsibilities?
- Did you eat better or worse in quarantine? Can you maintain the positive habits you developed as you venture back to restaurants, parties, and gatherings?
- What habits did you break that you are now better off without?
- What new habits or hobbies did you create that you want to continue?
- What hobbies should you resume that you missed during the last year?
- What new coping skills have you gained?
- Has your alcohol consumption declined or increased during the pandemic?
- Did you neglect/decide to forgo your medical and dental care? How quickly can you safely resume that care?
- How did your value system shift this year?
- Did the people you feel closest to change?
- How can you use this trauma to appreciate life more?
Life might get very busy this summer, so encourage patients to find time to answer these questions. Journaling can be a great way to think through all that we have experienced. Our brains will need to change again to adapt. Many of us have felt sad or anxious for a quite a while, and we want to move toward more positive feelings of safety, happiness, optimism, and joy. This will take effort. After all, we have lost more than 600,000 people to COVID, and much of the world is still in the middle of the pandemic. But this will get much easier as the threat of COVID-19 continues to recede. We must now work toward creating better times ahead.
Dr. Ritvo has almost 30 years’ experience in psychiatry and is currently practicing telemedicine. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018). She has no conflicts of interest.
As COVID-related restrictions are lifting and the streets, restaurants, and events are filling back up, we must encourage our patients to take inventory. It is time to help them create posttraumatic growth.
As we help them navigate this part of the pandemic, encourage them to ask what they learned over the last year and how they plan to integrate what they’ve been through to successfully create the “new normal.”
The Biden administration had set a goal of getting at least one shot to 70% of American adults by July 4, and that goal will not be reached. That shortfall, combined with the increase of the highly transmissible Delta variant of SARS-CoV-2 means that we and our patients must not let our guards completely down. At the same time, we can encourage our vaccinated patients to get back to their prepandemic lives – to the extent that they feel comfortable doing so.
Ultimately, this is about respecting physical and emotional boundaries. How do we greet vaccinated people now? Is it okay to shake hands, hug, or kiss to greet a friend or family member – or should we continue to elbow bump – or perhaps wave? Should we confront family members who have opted not to get vaccinated for reasons not related to health? Is it safe to visit with older relatives who are vaccinated? What about children under 12 who are not?
Those who were on the front lines of the pandemic faced unfathomable pain and suffering – and mental and physical exhaustion. And we know that the nightmare is not over. Several areas of the country with large numbers of unvaccinated people could face “very dense outbreaks,” in large part because of the Delta variant.
As we sort through the remaining challenges, I urge us all to reflect. We have been in this together and will emerge together. We know that the closer we were to the trauma, the longer recovery will take.
Ask patients to consider what is most important to resume and what can still wait. Some are eager to jump back into the deep end of the pool; others prefer to continue to wait cautiously. Families need to be on the same page as they assess risks and opportunities going forward, because household spread continues to be at the highest risk. Remind patients that the health of one of us affects the health of all of us.
Urge patients to take time to explore the following questions as they process the pandemic. We can also ask ourselves these same questions and share them with colleagues who are also rebuilding.
- Did you prioritize your family more? How can you continue to spend quality time them as other opportunities emerge?
- Did you have to withdraw from friends/coworkers and family members because of the pandemic? If so, how can you reincorporate them in our lives?
- Did you send more time caring for yourself with exercise and meditation? Can those new habits remain in place as life presents more options? How can you continue to make time for self-care while adding back other responsibilities?
- Did you eat better or worse in quarantine? Can you maintain the positive habits you developed as you venture back to restaurants, parties, and gatherings?
- What habits did you break that you are now better off without?
- What new habits or hobbies did you create that you want to continue?
- What hobbies should you resume that you missed during the last year?
- What new coping skills have you gained?
- Has your alcohol consumption declined or increased during the pandemic?
- Did you neglect/decide to forgo your medical and dental care? How quickly can you safely resume that care?
- How did your value system shift this year?
- Did the people you feel closest to change?
- How can you use this trauma to appreciate life more?
Life might get very busy this summer, so encourage patients to find time to answer these questions. Journaling can be a great way to think through all that we have experienced. Our brains will need to change again to adapt. Many of us have felt sad or anxious for a quite a while, and we want to move toward more positive feelings of safety, happiness, optimism, and joy. This will take effort. After all, we have lost more than 600,000 people to COVID, and much of the world is still in the middle of the pandemic. But this will get much easier as the threat of COVID-19 continues to recede. We must now work toward creating better times ahead.
Dr. Ritvo has almost 30 years’ experience in psychiatry and is currently practicing telemedicine. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018). She has no conflicts of interest.
Recommending esketamine? 4 factors to consider
Since receiving FDA approval in March 2019, intranasal esketamine—the S-enantiomer of ketamine—has become a valuable treatment option for adults with treatment-resistant depression (TRD), owing to its limited adverse effects profile, rapid onset, and potential for significant improvement in depressive symptoms. In August 2020, the FDA expanded esketamine’s indication to include treatment of depressive symptoms in adults with acute suicidal ideation or behavior, thus providing psychiatrists with an additional option for improving the care of their most seriously ill patients. In this article, we review 4 factors to consider before recommending esketamine.
1. Confirm that the patient’s depression qualifies as treatment-resistant. A patient is considered to have TRD if they have long-standing depression that meets DSM-5 criteria for major depressive disorder, and have not adequately responded to at least 2 antidepressant trials of adequate dose and duration.
2. Confirm that the patient’s suicidal ideation and/or suicidal behavior does not require acute hospitalization. The time between the onset of suicidal ideation and a suicide attempt typically is short, which highlights the need to intervene quickly in these patients.1 Being able to provide a treatment that works quickly and effectively may be lifesaving. However, to receive esketamine, patients must be enrolled in the Risk Evaluation Mitigation Strategy (REMS) patient registry through a certified treatment center, and prior authorization from insurance generally is required. These steps take time, so patients at high or imminent risk for suicide may initially require psychiatric hospitalization before they are able to begin esketamine treatment. Parsing out whether the suicidal ideation is chronic or acute can help clinicians assess current dangerousness and determine if esketamine treatment might be appropriate. If a patient with chronic suicidal ideation is stable for outpatient treatment with close monitoring, esketamine might provide an effective treatment option for treating both depression and suicidality. Esketamine’s rapid effect may be an integral part of the treatment for a suicidal patient by bridging the gap caused by the delayed onset of action in typical antidepressants.2
3. Identify a local certified treatment center. Use the online database at www.spravato.com/find-a-center to locate a nearby certified esketamine treatment center. Choosing a center that you can collaborate with regularly is important to determine if the treatment is effective, to provide updates on the treatment course, and to consider tailoring of ongoing treatment.
4. Ensure the patient is also treated with an oral antidepressant. Esketamine should be administered in conjunction with an oral antidepressant. As such, patients must be willing and able to tolerate treatment with a medication that can be construed as an antidepressant while undergoing esketamine treatment. A long-term maintenance trial found that patients with TRD who experienced remission or response after esketamine treatment had a delayed relapse of symptoms when they continued esketamine in addition to an oral antidepressant.3
Considering its rapid onset of action and low adverse effects profile with manageable tolerability, esketamine adjunctive to an oral antidepressant is a reasonable option to consider for patients with TRD, including those with suicidality.
1. Deisenhammer EA, Ing CM, Strauss R, et al. The duration of the suicidal process: how much time is left for intervention between consideration and accomplishment of a suicide attempt? J Clin Psychiatry. 2009;70(1):19-24.
2. Canuso CM, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;175(7):620-630.
3. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression. JAMA Psychiatry. 2019;76(9):893-903.
Since receiving FDA approval in March 2019, intranasal esketamine—the S-enantiomer of ketamine—has become a valuable treatment option for adults with treatment-resistant depression (TRD), owing to its limited adverse effects profile, rapid onset, and potential for significant improvement in depressive symptoms. In August 2020, the FDA expanded esketamine’s indication to include treatment of depressive symptoms in adults with acute suicidal ideation or behavior, thus providing psychiatrists with an additional option for improving the care of their most seriously ill patients. In this article, we review 4 factors to consider before recommending esketamine.
1. Confirm that the patient’s depression qualifies as treatment-resistant. A patient is considered to have TRD if they have long-standing depression that meets DSM-5 criteria for major depressive disorder, and have not adequately responded to at least 2 antidepressant trials of adequate dose and duration.
2. Confirm that the patient’s suicidal ideation and/or suicidal behavior does not require acute hospitalization. The time between the onset of suicidal ideation and a suicide attempt typically is short, which highlights the need to intervene quickly in these patients.1 Being able to provide a treatment that works quickly and effectively may be lifesaving. However, to receive esketamine, patients must be enrolled in the Risk Evaluation Mitigation Strategy (REMS) patient registry through a certified treatment center, and prior authorization from insurance generally is required. These steps take time, so patients at high or imminent risk for suicide may initially require psychiatric hospitalization before they are able to begin esketamine treatment. Parsing out whether the suicidal ideation is chronic or acute can help clinicians assess current dangerousness and determine if esketamine treatment might be appropriate. If a patient with chronic suicidal ideation is stable for outpatient treatment with close monitoring, esketamine might provide an effective treatment option for treating both depression and suicidality. Esketamine’s rapid effect may be an integral part of the treatment for a suicidal patient by bridging the gap caused by the delayed onset of action in typical antidepressants.2
3. Identify a local certified treatment center. Use the online database at www.spravato.com/find-a-center to locate a nearby certified esketamine treatment center. Choosing a center that you can collaborate with regularly is important to determine if the treatment is effective, to provide updates on the treatment course, and to consider tailoring of ongoing treatment.
4. Ensure the patient is also treated with an oral antidepressant. Esketamine should be administered in conjunction with an oral antidepressant. As such, patients must be willing and able to tolerate treatment with a medication that can be construed as an antidepressant while undergoing esketamine treatment. A long-term maintenance trial found that patients with TRD who experienced remission or response after esketamine treatment had a delayed relapse of symptoms when they continued esketamine in addition to an oral antidepressant.3
Considering its rapid onset of action and low adverse effects profile with manageable tolerability, esketamine adjunctive to an oral antidepressant is a reasonable option to consider for patients with TRD, including those with suicidality.
Since receiving FDA approval in March 2019, intranasal esketamine—the S-enantiomer of ketamine—has become a valuable treatment option for adults with treatment-resistant depression (TRD), owing to its limited adverse effects profile, rapid onset, and potential for significant improvement in depressive symptoms. In August 2020, the FDA expanded esketamine’s indication to include treatment of depressive symptoms in adults with acute suicidal ideation or behavior, thus providing psychiatrists with an additional option for improving the care of their most seriously ill patients. In this article, we review 4 factors to consider before recommending esketamine.
1. Confirm that the patient’s depression qualifies as treatment-resistant. A patient is considered to have TRD if they have long-standing depression that meets DSM-5 criteria for major depressive disorder, and have not adequately responded to at least 2 antidepressant trials of adequate dose and duration.
2. Confirm that the patient’s suicidal ideation and/or suicidal behavior does not require acute hospitalization. The time between the onset of suicidal ideation and a suicide attempt typically is short, which highlights the need to intervene quickly in these patients.1 Being able to provide a treatment that works quickly and effectively may be lifesaving. However, to receive esketamine, patients must be enrolled in the Risk Evaluation Mitigation Strategy (REMS) patient registry through a certified treatment center, and prior authorization from insurance generally is required. These steps take time, so patients at high or imminent risk for suicide may initially require psychiatric hospitalization before they are able to begin esketamine treatment. Parsing out whether the suicidal ideation is chronic or acute can help clinicians assess current dangerousness and determine if esketamine treatment might be appropriate. If a patient with chronic suicidal ideation is stable for outpatient treatment with close monitoring, esketamine might provide an effective treatment option for treating both depression and suicidality. Esketamine’s rapid effect may be an integral part of the treatment for a suicidal patient by bridging the gap caused by the delayed onset of action in typical antidepressants.2
3. Identify a local certified treatment center. Use the online database at www.spravato.com/find-a-center to locate a nearby certified esketamine treatment center. Choosing a center that you can collaborate with regularly is important to determine if the treatment is effective, to provide updates on the treatment course, and to consider tailoring of ongoing treatment.
4. Ensure the patient is also treated with an oral antidepressant. Esketamine should be administered in conjunction with an oral antidepressant. As such, patients must be willing and able to tolerate treatment with a medication that can be construed as an antidepressant while undergoing esketamine treatment. A long-term maintenance trial found that patients with TRD who experienced remission or response after esketamine treatment had a delayed relapse of symptoms when they continued esketamine in addition to an oral antidepressant.3
Considering its rapid onset of action and low adverse effects profile with manageable tolerability, esketamine adjunctive to an oral antidepressant is a reasonable option to consider for patients with TRD, including those with suicidality.
1. Deisenhammer EA, Ing CM, Strauss R, et al. The duration of the suicidal process: how much time is left for intervention between consideration and accomplishment of a suicide attempt? J Clin Psychiatry. 2009;70(1):19-24.
2. Canuso CM, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;175(7):620-630.
3. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression. JAMA Psychiatry. 2019;76(9):893-903.
1. Deisenhammer EA, Ing CM, Strauss R, et al. The duration of the suicidal process: how much time is left for intervention between consideration and accomplishment of a suicide attempt? J Clin Psychiatry. 2009;70(1):19-24.
2. Canuso CM, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;175(7):620-630.
3. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression. JAMA Psychiatry. 2019;76(9):893-903.
Patients’ sexual problems: Be proactive, make discussions routine
If the goal of a clinical encounter is to identify issues that adversely affect health, well-being, and life satisfaction, open-ended questions on sexual problems are essential, according to an expert who provided tips during a session presented by Current Psychiatry and the American Academy of Clinical Psychiatrists about how to begin a productive dialogue.
For identifying and treating the obstacles to sexual health, “the onus is on the provider,” said Anita H. Clayton, MD, chair of psychiatry and neurobehavioral sciences at the University of Virginia, Charlottesville.
In a poll published more than 20 years ago, 91% of men and 84% of women reported that a satisfying sex life is important, while 90% agreed that sexual difficulties cause emotional problems, said Dr. Clayton, who sees no reason to think that those percentages have changed. Yet, patients are traditionally reluctant to raise their concerns about sexual issues to a physician.
In the same poll, about 50% of the respondents characterized themselves as “very concerned” that a clinician would simply dismiss a sexual complaint or that there would be no treatment. Of the other respondents, 40% were somewhat concerned. Dr. Clayton assumes that those numbers are still valid and that they provide the rationale for asking routinely about sexual health, she said at the virtual meeting, presented by MedscapeLive.
Raising sexual health issues
“The clinician has to initiate the discussion and make it part of the routine examination,” said Dr. Clayton, also a professor of obstetrics and gynecology at the university. She indicated that unresolved sexual issues are a common and important but treatable problem, whether the underlying issue has a medical or psychological origin.
Yet, language is critical. Many physicians might have no difficulty discussing sexual problems, but patients often do. Dr. Clayton recommended developing strategies that might it easy if not seamless to elicit information about sexual health in the context of inquiring about other clinical issues.
“Use bridging statements,” Dr. Clayton suggested.
Bridging statements allow an easy transition into a discussion of sexual function from another clinical issue, Dr. Clayton said. As examples, she suggested moving to questions about sex from inquiries about conditions, such as diabetes, or medications, such as antidepressants, that are known to have an impact on sexual dysfunction.
Avoid yes-no questions.
To prompt a dialogue, Dr. Clayton advised against using yes-no questions that allow the patient to quickly dismiss the topic with a negative response. She tries to frame a question that requires a complete thought. In an inquiry addressed to a patient with diabetes, for example, she might first inform the patient that sexual issues are common with this disorder and then ask what types of sexual issues the patient is experiencing.
Once the topic is raised, a checklist approach is appropriate. Patients might be more or less willing to talk any one of the range of issues that influence sexual health, ranging from issues of desire and arousal to discomfort or pain. The door should be opened to a discussion of specific sexual organ function, such as ability to achieve an erection or adequate lubrication.
“Do not assume the patient is heterosexual,” Dr. Clayton cautioned.
It is reasonable and appropriate to bring up sexual health during the intake history. A discussion of sexual health can be initiated by simply posing the question: “Are you sexually active?” Importantly, Dr. Clayton strongly recommended a follow-up question when adults reply that they are not sexually active.
In the ELIXIR study, which evaluated sexual function in patients with depression, more than twice as many patients reported impairments when asked by the physician than who volunteered this information spontaneously, according to Dr. Clayton, citing a study that found sexual issues in more than 70% of the 4,557 participants.
Prioritize choice of language.
Once sexual impairments are uncovered, clinicians will need to determine how to intervene, but Dr. Clayton recommended using clear and frank language to define the problem even if the language is tailored to the patient’s comfort level. Patients should be encouraged to recognize that there are solutions for most problems, but clinicians should recognize and respect cultural issues in directing patients toward solutions.
Dr. Clayton is not alone in recommending that patients be asked routinely about sexual health. Margot Savoy, MD, MPH, chair of family and community medicine, Temple University, Philadelphia, has also advocated for a proactive approach.
“Patients deserve whole-patient care that includes sexual health,” said Dr. Savoy, who was coauthor of a recent article that also outlined techniques for eliciting a sexual history.
She suggested that the need to inquire should not be considered age specific.
“Asking patients about their sexual history and concerns is a critical part of routine primary care across the lifespan,” she said.
“We also need to intentionally create a safe environment where it is as normal to talk about sexual questions or concerns as it is about how to care for a cold or manage a backache,” she added.
MedscapeLive and this news organization are owned by the same company. Dr. Clayton disclosed financial relationships with Acadia, Alkermes, Allergan, AMAG, Astellas, Fabre-Kramer, Janssen, Ovoca Bio, PureTech Health, Relmada, S1 Biopharma, Safe Therapeutics, Takeda, and WCG MedAd-vante-Prophase. Dr. Savoy reported no conflicts of interest.
If the goal of a clinical encounter is to identify issues that adversely affect health, well-being, and life satisfaction, open-ended questions on sexual problems are essential, according to an expert who provided tips during a session presented by Current Psychiatry and the American Academy of Clinical Psychiatrists about how to begin a productive dialogue.
For identifying and treating the obstacles to sexual health, “the onus is on the provider,” said Anita H. Clayton, MD, chair of psychiatry and neurobehavioral sciences at the University of Virginia, Charlottesville.
In a poll published more than 20 years ago, 91% of men and 84% of women reported that a satisfying sex life is important, while 90% agreed that sexual difficulties cause emotional problems, said Dr. Clayton, who sees no reason to think that those percentages have changed. Yet, patients are traditionally reluctant to raise their concerns about sexual issues to a physician.
In the same poll, about 50% of the respondents characterized themselves as “very concerned” that a clinician would simply dismiss a sexual complaint or that there would be no treatment. Of the other respondents, 40% were somewhat concerned. Dr. Clayton assumes that those numbers are still valid and that they provide the rationale for asking routinely about sexual health, she said at the virtual meeting, presented by MedscapeLive.
Raising sexual health issues
“The clinician has to initiate the discussion and make it part of the routine examination,” said Dr. Clayton, also a professor of obstetrics and gynecology at the university. She indicated that unresolved sexual issues are a common and important but treatable problem, whether the underlying issue has a medical or psychological origin.
Yet, language is critical. Many physicians might have no difficulty discussing sexual problems, but patients often do. Dr. Clayton recommended developing strategies that might it easy if not seamless to elicit information about sexual health in the context of inquiring about other clinical issues.
“Use bridging statements,” Dr. Clayton suggested.
Bridging statements allow an easy transition into a discussion of sexual function from another clinical issue, Dr. Clayton said. As examples, she suggested moving to questions about sex from inquiries about conditions, such as diabetes, or medications, such as antidepressants, that are known to have an impact on sexual dysfunction.
Avoid yes-no questions.
To prompt a dialogue, Dr. Clayton advised against using yes-no questions that allow the patient to quickly dismiss the topic with a negative response. She tries to frame a question that requires a complete thought. In an inquiry addressed to a patient with diabetes, for example, she might first inform the patient that sexual issues are common with this disorder and then ask what types of sexual issues the patient is experiencing.
Once the topic is raised, a checklist approach is appropriate. Patients might be more or less willing to talk any one of the range of issues that influence sexual health, ranging from issues of desire and arousal to discomfort or pain. The door should be opened to a discussion of specific sexual organ function, such as ability to achieve an erection or adequate lubrication.
“Do not assume the patient is heterosexual,” Dr. Clayton cautioned.
It is reasonable and appropriate to bring up sexual health during the intake history. A discussion of sexual health can be initiated by simply posing the question: “Are you sexually active?” Importantly, Dr. Clayton strongly recommended a follow-up question when adults reply that they are not sexually active.
In the ELIXIR study, which evaluated sexual function in patients with depression, more than twice as many patients reported impairments when asked by the physician than who volunteered this information spontaneously, according to Dr. Clayton, citing a study that found sexual issues in more than 70% of the 4,557 participants.
Prioritize choice of language.
Once sexual impairments are uncovered, clinicians will need to determine how to intervene, but Dr. Clayton recommended using clear and frank language to define the problem even if the language is tailored to the patient’s comfort level. Patients should be encouraged to recognize that there are solutions for most problems, but clinicians should recognize and respect cultural issues in directing patients toward solutions.
Dr. Clayton is not alone in recommending that patients be asked routinely about sexual health. Margot Savoy, MD, MPH, chair of family and community medicine, Temple University, Philadelphia, has also advocated for a proactive approach.
“Patients deserve whole-patient care that includes sexual health,” said Dr. Savoy, who was coauthor of a recent article that also outlined techniques for eliciting a sexual history.
She suggested that the need to inquire should not be considered age specific.
“Asking patients about their sexual history and concerns is a critical part of routine primary care across the lifespan,” she said.
“We also need to intentionally create a safe environment where it is as normal to talk about sexual questions or concerns as it is about how to care for a cold or manage a backache,” she added.
MedscapeLive and this news organization are owned by the same company. Dr. Clayton disclosed financial relationships with Acadia, Alkermes, Allergan, AMAG, Astellas, Fabre-Kramer, Janssen, Ovoca Bio, PureTech Health, Relmada, S1 Biopharma, Safe Therapeutics, Takeda, and WCG MedAd-vante-Prophase. Dr. Savoy reported no conflicts of interest.
If the goal of a clinical encounter is to identify issues that adversely affect health, well-being, and life satisfaction, open-ended questions on sexual problems are essential, according to an expert who provided tips during a session presented by Current Psychiatry and the American Academy of Clinical Psychiatrists about how to begin a productive dialogue.
For identifying and treating the obstacles to sexual health, “the onus is on the provider,” said Anita H. Clayton, MD, chair of psychiatry and neurobehavioral sciences at the University of Virginia, Charlottesville.
In a poll published more than 20 years ago, 91% of men and 84% of women reported that a satisfying sex life is important, while 90% agreed that sexual difficulties cause emotional problems, said Dr. Clayton, who sees no reason to think that those percentages have changed. Yet, patients are traditionally reluctant to raise their concerns about sexual issues to a physician.
In the same poll, about 50% of the respondents characterized themselves as “very concerned” that a clinician would simply dismiss a sexual complaint or that there would be no treatment. Of the other respondents, 40% were somewhat concerned. Dr. Clayton assumes that those numbers are still valid and that they provide the rationale for asking routinely about sexual health, she said at the virtual meeting, presented by MedscapeLive.
Raising sexual health issues
“The clinician has to initiate the discussion and make it part of the routine examination,” said Dr. Clayton, also a professor of obstetrics and gynecology at the university. She indicated that unresolved sexual issues are a common and important but treatable problem, whether the underlying issue has a medical or psychological origin.
Yet, language is critical. Many physicians might have no difficulty discussing sexual problems, but patients often do. Dr. Clayton recommended developing strategies that might it easy if not seamless to elicit information about sexual health in the context of inquiring about other clinical issues.
“Use bridging statements,” Dr. Clayton suggested.
Bridging statements allow an easy transition into a discussion of sexual function from another clinical issue, Dr. Clayton said. As examples, she suggested moving to questions about sex from inquiries about conditions, such as diabetes, or medications, such as antidepressants, that are known to have an impact on sexual dysfunction.
Avoid yes-no questions.
To prompt a dialogue, Dr. Clayton advised against using yes-no questions that allow the patient to quickly dismiss the topic with a negative response. She tries to frame a question that requires a complete thought. In an inquiry addressed to a patient with diabetes, for example, she might first inform the patient that sexual issues are common with this disorder and then ask what types of sexual issues the patient is experiencing.
Once the topic is raised, a checklist approach is appropriate. Patients might be more or less willing to talk any one of the range of issues that influence sexual health, ranging from issues of desire and arousal to discomfort or pain. The door should be opened to a discussion of specific sexual organ function, such as ability to achieve an erection or adequate lubrication.
“Do not assume the patient is heterosexual,” Dr. Clayton cautioned.
It is reasonable and appropriate to bring up sexual health during the intake history. A discussion of sexual health can be initiated by simply posing the question: “Are you sexually active?” Importantly, Dr. Clayton strongly recommended a follow-up question when adults reply that they are not sexually active.
In the ELIXIR study, which evaluated sexual function in patients with depression, more than twice as many patients reported impairments when asked by the physician than who volunteered this information spontaneously, according to Dr. Clayton, citing a study that found sexual issues in more than 70% of the 4,557 participants.
Prioritize choice of language.
Once sexual impairments are uncovered, clinicians will need to determine how to intervene, but Dr. Clayton recommended using clear and frank language to define the problem even if the language is tailored to the patient’s comfort level. Patients should be encouraged to recognize that there are solutions for most problems, but clinicians should recognize and respect cultural issues in directing patients toward solutions.
Dr. Clayton is not alone in recommending that patients be asked routinely about sexual health. Margot Savoy, MD, MPH, chair of family and community medicine, Temple University, Philadelphia, has also advocated for a proactive approach.
“Patients deserve whole-patient care that includes sexual health,” said Dr. Savoy, who was coauthor of a recent article that also outlined techniques for eliciting a sexual history.
She suggested that the need to inquire should not be considered age specific.
“Asking patients about their sexual history and concerns is a critical part of routine primary care across the lifespan,” she said.
“We also need to intentionally create a safe environment where it is as normal to talk about sexual questions or concerns as it is about how to care for a cold or manage a backache,” she added.
MedscapeLive and this news organization are owned by the same company. Dr. Clayton disclosed financial relationships with Acadia, Alkermes, Allergan, AMAG, Astellas, Fabre-Kramer, Janssen, Ovoca Bio, PureTech Health, Relmada, S1 Biopharma, Safe Therapeutics, Takeda, and WCG MedAd-vante-Prophase. Dr. Savoy reported no conflicts of interest.
FROM CP/AACP PSYCHIATRY UPDATE
Cannabis use tied to increased risk for suicidal thoughts, actions
Young adults who use cannabis – either sporadically, daily, or those who have cannabis use disorder – have a significantly increased risk for suicidal thoughts and actions, according to U.S. national drug survey data.
The risks appear greater for women than men and remained regardless of whether the individual was depressed.
“We cannot establish that cannabis use caused increased suicidality,” Nora Volkow, MD, director, National Institute on Drug Abuse (NIDA), told this news organization.
“However, it is likely that these two factors influence one another bidirectionally, meaning people with suicidal thinking might be more vulnerable to cannabis use to self-medicate their distress, and cannabis use may trigger negative moods and suicidal thinking in some people,” said Dr. Volkow.
“It is also possible that these factors are not causally linked to one another at all but rather reflect the common and related risk factors underlying both suicidality and substance use. For instance, one’s genetics may put them at a higher risk for both suicide and for using marijuana,” she added.
The study was published online June 22 in JAMA Network Open.
Marked increase in use
Cannabis use among U.S. adults has increased markedly over the past 10 years, with a parallel increase in suicidality. However, the links between cannabis use and suicidality among young adults are poorly understood.
NIDA researchers sought to fill this gap. They examined data on 281,650 young men and women aged 18 to 34 years who participated in National Surveys on Drug Use and Health from 2008 to 2019.
Status regarding past-year cannabis use was categorized as past-year daily or near-daily use (greater than or equal to 300 days), non-daily use, and no cannabis use.
Although suicidality was associated with cannabis use, even young adults who did not use cannabis on a daily basis were more likely to have suicidal thoughts or actions than those who did not use the drug at all, the researchers found.
Among young adults without a major depressive episode, about 3% of those who did not use cannabis had suicidal ideation, compared with about 7% of non-daily cannabis users, about 9% of daily cannabis users, and 14% of those with a cannabis use disorder.
Among young adults with depression, the corresponding percentages were 35%, 44%, 53%, and 50%.
Similar trends existed for the associations between the different levels of cannabis use and suicide plan or attempt.
Women at greatest risk
Gender differences also emerged. than men with the same levels of cannabis use.
Among those without a major depressive episode, the prevalence of suicidal ideation for those with versus without a cannabis use disorder was around 14% versus 4.0% among women and 10% versus 3.0% among men.
Among young adults with both cannabis use disorder and major depressive episode, the prevalence of past-year suicide plan was 52% higher for women (24%) than for men (16%).
“Suicide is a leading cause of death among young adults in the United States, and the findings of this study offer important information that may help us reduce this risk,” lead author and NIDA researcher Beth Han, MD, PhD, MPH, said in a news release.
“Depression and cannabis use disorder are treatable conditions, and cannabis use can be modified. Through better understanding the associations of different risk factors for suicidality, we hope to offer new targets for prevention and intervention in individuals that we know may be at high risk. These findings also underscore the importance of tailoring interventions in a way that takes sex and gender into account,” said Dr. Han.
“Additional research is needed to better understand these complex associations, especially given the great burden of suicide on young adults,” said Dr. Volkow.
Gender difference ‘striking’
Commenting on the findings for this news organization, Charles B. Nemeroff, MD, PhD, professor and chair, department of psychiatry and behavioral sciences, Dell Medical School, University of Texas at Austin, said this study is “clearly of great interest; of course correlation and causality are completely distinct entities, and this study is all about correlation.
“This does not, of course, mean that cannabis use causes suicide but suggests that in individuals who use cannabis, suicidality in the broadest sense is increased in prevalence rate,” said Dr. Nemeroff, who serves as principal investigator of the Texas Child Trauma Network.
Dr. Nemeroff said “the most striking finding” was the larger effect in women than men – “striking because suicide is, in almost all cultures, higher in prevalence in men versus women.”
Dr. Nemeroff said he’d like to know more about other potential contributing factors, “which would include a history of child abuse and neglect, a major vulnerability factor for suicidality, comorbid alcohol and other substance abuse, [and] comorbid psychiatric diagnosis such as posttraumatic stress disorder.”
The study was sponsored by NIDA, of the National Institutes of Health. Dr. Volkow, Dr. Han, and Dr. Nemeroff have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Young adults who use cannabis – either sporadically, daily, or those who have cannabis use disorder – have a significantly increased risk for suicidal thoughts and actions, according to U.S. national drug survey data.
The risks appear greater for women than men and remained regardless of whether the individual was depressed.
“We cannot establish that cannabis use caused increased suicidality,” Nora Volkow, MD, director, National Institute on Drug Abuse (NIDA), told this news organization.
“However, it is likely that these two factors influence one another bidirectionally, meaning people with suicidal thinking might be more vulnerable to cannabis use to self-medicate their distress, and cannabis use may trigger negative moods and suicidal thinking in some people,” said Dr. Volkow.
“It is also possible that these factors are not causally linked to one another at all but rather reflect the common and related risk factors underlying both suicidality and substance use. For instance, one’s genetics may put them at a higher risk for both suicide and for using marijuana,” she added.
The study was published online June 22 in JAMA Network Open.
Marked increase in use
Cannabis use among U.S. adults has increased markedly over the past 10 years, with a parallel increase in suicidality. However, the links between cannabis use and suicidality among young adults are poorly understood.
NIDA researchers sought to fill this gap. They examined data on 281,650 young men and women aged 18 to 34 years who participated in National Surveys on Drug Use and Health from 2008 to 2019.
Status regarding past-year cannabis use was categorized as past-year daily or near-daily use (greater than or equal to 300 days), non-daily use, and no cannabis use.
Although suicidality was associated with cannabis use, even young adults who did not use cannabis on a daily basis were more likely to have suicidal thoughts or actions than those who did not use the drug at all, the researchers found.
Among young adults without a major depressive episode, about 3% of those who did not use cannabis had suicidal ideation, compared with about 7% of non-daily cannabis users, about 9% of daily cannabis users, and 14% of those with a cannabis use disorder.
Among young adults with depression, the corresponding percentages were 35%, 44%, 53%, and 50%.
Similar trends existed for the associations between the different levels of cannabis use and suicide plan or attempt.
Women at greatest risk
Gender differences also emerged. than men with the same levels of cannabis use.
Among those without a major depressive episode, the prevalence of suicidal ideation for those with versus without a cannabis use disorder was around 14% versus 4.0% among women and 10% versus 3.0% among men.
Among young adults with both cannabis use disorder and major depressive episode, the prevalence of past-year suicide plan was 52% higher for women (24%) than for men (16%).
“Suicide is a leading cause of death among young adults in the United States, and the findings of this study offer important information that may help us reduce this risk,” lead author and NIDA researcher Beth Han, MD, PhD, MPH, said in a news release.
“Depression and cannabis use disorder are treatable conditions, and cannabis use can be modified. Through better understanding the associations of different risk factors for suicidality, we hope to offer new targets for prevention and intervention in individuals that we know may be at high risk. These findings also underscore the importance of tailoring interventions in a way that takes sex and gender into account,” said Dr. Han.
“Additional research is needed to better understand these complex associations, especially given the great burden of suicide on young adults,” said Dr. Volkow.
Gender difference ‘striking’
Commenting on the findings for this news organization, Charles B. Nemeroff, MD, PhD, professor and chair, department of psychiatry and behavioral sciences, Dell Medical School, University of Texas at Austin, said this study is “clearly of great interest; of course correlation and causality are completely distinct entities, and this study is all about correlation.
“This does not, of course, mean that cannabis use causes suicide but suggests that in individuals who use cannabis, suicidality in the broadest sense is increased in prevalence rate,” said Dr. Nemeroff, who serves as principal investigator of the Texas Child Trauma Network.
Dr. Nemeroff said “the most striking finding” was the larger effect in women than men – “striking because suicide is, in almost all cultures, higher in prevalence in men versus women.”
Dr. Nemeroff said he’d like to know more about other potential contributing factors, “which would include a history of child abuse and neglect, a major vulnerability factor for suicidality, comorbid alcohol and other substance abuse, [and] comorbid psychiatric diagnosis such as posttraumatic stress disorder.”
The study was sponsored by NIDA, of the National Institutes of Health. Dr. Volkow, Dr. Han, and Dr. Nemeroff have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Young adults who use cannabis – either sporadically, daily, or those who have cannabis use disorder – have a significantly increased risk for suicidal thoughts and actions, according to U.S. national drug survey data.
The risks appear greater for women than men and remained regardless of whether the individual was depressed.
“We cannot establish that cannabis use caused increased suicidality,” Nora Volkow, MD, director, National Institute on Drug Abuse (NIDA), told this news organization.
“However, it is likely that these two factors influence one another bidirectionally, meaning people with suicidal thinking might be more vulnerable to cannabis use to self-medicate their distress, and cannabis use may trigger negative moods and suicidal thinking in some people,” said Dr. Volkow.
“It is also possible that these factors are not causally linked to one another at all but rather reflect the common and related risk factors underlying both suicidality and substance use. For instance, one’s genetics may put them at a higher risk for both suicide and for using marijuana,” she added.
The study was published online June 22 in JAMA Network Open.
Marked increase in use
Cannabis use among U.S. adults has increased markedly over the past 10 years, with a parallel increase in suicidality. However, the links between cannabis use and suicidality among young adults are poorly understood.
NIDA researchers sought to fill this gap. They examined data on 281,650 young men and women aged 18 to 34 years who participated in National Surveys on Drug Use and Health from 2008 to 2019.
Status regarding past-year cannabis use was categorized as past-year daily or near-daily use (greater than or equal to 300 days), non-daily use, and no cannabis use.
Although suicidality was associated with cannabis use, even young adults who did not use cannabis on a daily basis were more likely to have suicidal thoughts or actions than those who did not use the drug at all, the researchers found.
Among young adults without a major depressive episode, about 3% of those who did not use cannabis had suicidal ideation, compared with about 7% of non-daily cannabis users, about 9% of daily cannabis users, and 14% of those with a cannabis use disorder.
Among young adults with depression, the corresponding percentages were 35%, 44%, 53%, and 50%.
Similar trends existed for the associations between the different levels of cannabis use and suicide plan or attempt.
Women at greatest risk
Gender differences also emerged. than men with the same levels of cannabis use.
Among those without a major depressive episode, the prevalence of suicidal ideation for those with versus without a cannabis use disorder was around 14% versus 4.0% among women and 10% versus 3.0% among men.
Among young adults with both cannabis use disorder and major depressive episode, the prevalence of past-year suicide plan was 52% higher for women (24%) than for men (16%).
“Suicide is a leading cause of death among young adults in the United States, and the findings of this study offer important information that may help us reduce this risk,” lead author and NIDA researcher Beth Han, MD, PhD, MPH, said in a news release.
“Depression and cannabis use disorder are treatable conditions, and cannabis use can be modified. Through better understanding the associations of different risk factors for suicidality, we hope to offer new targets for prevention and intervention in individuals that we know may be at high risk. These findings also underscore the importance of tailoring interventions in a way that takes sex and gender into account,” said Dr. Han.
“Additional research is needed to better understand these complex associations, especially given the great burden of suicide on young adults,” said Dr. Volkow.
Gender difference ‘striking’
Commenting on the findings for this news organization, Charles B. Nemeroff, MD, PhD, professor and chair, department of psychiatry and behavioral sciences, Dell Medical School, University of Texas at Austin, said this study is “clearly of great interest; of course correlation and causality are completely distinct entities, and this study is all about correlation.
“This does not, of course, mean that cannabis use causes suicide but suggests that in individuals who use cannabis, suicidality in the broadest sense is increased in prevalence rate,” said Dr. Nemeroff, who serves as principal investigator of the Texas Child Trauma Network.
Dr. Nemeroff said “the most striking finding” was the larger effect in women than men – “striking because suicide is, in almost all cultures, higher in prevalence in men versus women.”
Dr. Nemeroff said he’d like to know more about other potential contributing factors, “which would include a history of child abuse and neglect, a major vulnerability factor for suicidality, comorbid alcohol and other substance abuse, [and] comorbid psychiatric diagnosis such as posttraumatic stress disorder.”
The study was sponsored by NIDA, of the National Institutes of Health. Dr. Volkow, Dr. Han, and Dr. Nemeroff have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dose-dependent effect of ‘internet addiction’ and sleep problems
More evidence suggests the severity of internet addiction (IA) is directly related to the severity of sleep problems in youth.
Results from a study of more than 4,000 adolescent students show IA severity was linked to less sleep and to daytime sleepiness. In addition, boys aged 12-14 years who were addicted to computer games versus social media networking were the most affected.
Sleep issues could be “easily detectable manifestations of pathological internet addiction,” investigator Sergey Tereshchenko, PhD, Scientific Research Institute for Medical Problems of the North, Krasnoyask State Medical University, Russia, told this news organization.
These sleep problems require attention and correction, Dr. Tereshchenko added.
The findings were presented at the virtual Congress of the European Academy of Neurology 2021.
New phenomenon
IA is a relatively new psychological phenomenon and is most prevalent in “socially vulnerable groups,” such as adolescents, Dr. Tereshchenko said.
He cited numerous studies that have “convincingly demonstrated” IA is comorbid with a broad range of psychopathologic conditions, including depression, anxiety, and attention deficit hyperactivity disorder.
There is also growing evidence, including from systematic reviews in 2014 and 2019, that IA affects a wide range of sleep parameters.
However, most studies in adolescents have used only one psychometric tool to assess addiction, revealing only the “general IA pattern” and not the type of IA, Dr. Tereshchenko noted.
Adolescents may not be addicted to the internet itself but to certain behaviors like gaming or social networking, he said.
The “undoubted advantage” of his team’s research is the use of more than one tool, making it possible to “verify the predominant content of the addiction,” he added.
The investigators previously assessed general prevalence of IA in adolescents in Siberia and found about 6.8% of participants displayed pathological IA behavior – and that gaming addiction is more common in boys whereas addiction to social networking is more common in girls.
This prevalence rate is lower than in the Philippines (21.1%), Hong Kong (16.4%), Malaysia (14.1%), China (11%), and South Korea (9.7%), but slightly higher than in Japan (6.2%).
IA prevalence among adolescents in Europe ranges from 1% to 11%, with an average of 4.4%, said Dr. Tereshchenko.
Siberian students’ sleep
The current study included 4,344 students aged 12-18 years (average age, about 15 years) from 10 public schools in three large cities of Central Siberia (Krasnoyarsk, Abakan, and Kyzyl). There were slightly more girls than boys in the study sample.
Participants completed the Russian language version of the Chen Internet Addiction Scale (CIAS), which covers five symptomatic criteria for addictive behavior: withdrawal symptoms, signs of tolerance, compulsive use, psychological or physical problems, and difficulty managing time.
In this questionnaire, respondents rate several statements regarding the effect of internet use, each on a 4-point Likert scale: not at all (1 point), a little bit (2 points), moderately (3 points) and extremely (4). The total score ranges from 26 to 104.
A CIAS score of 26-42 indicates adaptive internet use, 43-64 indicates maladaptive internet use, and 65 and above indicates pathological internet use (PIU), which was classified as “internet-addicted.”
The researchers also used the nine-item Social Media Disorder Scale, as well as the Pittsburgh Sleep Quality Index to assess nighttime sleep.
Among other questions, teens were asked how long it usually took them to fall asleep and when they typically went to bed and woke up on school nights.
For daytime sleepiness, investigators used the targeted Pediatric Daytime Sleepiness Scale questionnaire, making them among the few research groups to use this psychometric instrument, Dr. Tereshchenko noted.
After parental consent was given, students completed the tests at the end of the day’s lessons. Total test time was about 45 minutes.
Sleep disturbance
Initial study results showed that compared with the other groups, adolescents with PIU tended to go to bed later, wake up later, take longer to fall asleep, sleep less at night, have more nighttime awakenings, and have more daytime sleepiness.
Sleep quality was the most impaired in boys aged 12-14 years who are addicted to internet computer games.
“In this group, 5 of the 6 sleep assessment parameters we studied were changed,” Dr. Tereshchenko reported.
Decreased total nighttime sleep was more common in older adolescents.
On average, boys and girls aged 15-18 years got less than the recommended 8 hours of sleep per night. Boys with IA got only about 6.4 hours per night and girls with IA got about 6.6 hours.
Interestingly, IA is generally more prevalent among teen girls than boys in Russia, which is not the case in Europe and North America, Dr. Tereshchenko noted.
Mechanisms linking IA and sleep disorders are not clear, but the relationship is probably multifactorial and perhaps interrelated, creating something of a “vicious circle,” he said.
“Sleep disturbances, which reflect psychosocial problems, depression, and anxiety-phobic disorders, can precede and contribute to IA. On the other hand, sleep disturbances such as insomnia can lead to increased use of the internet in the evening and at night, further exacerbating the problem,” said Dr. Tereshchenko.
Research is lacking on useful treatments for youth with IA, but these kids would likely benefit from behavioral therapy approaches, he added.
No escape?
Commenting on the study for this news organization, Maurice M. Ohayon, MD, DSc, PhD, director of the Stanford Sleep Epidemiology Research Center, Stanford University, California, said the topic of youth IA is “very important.”
Previous research in this field has shown a major impact from IA not only on sleep but also on mood – with irritability, depression, and even thoughts of suicide being possible red flags, said Dr. Ohayon, who was not involved in the current study.
Interestingly, his own research has also found that young teenage boys are most at risk for gaming addiction.
Although internet gaming has some positive effects, such as fostering leadership skills and relationships, it has become increasingly violent and isolating, with more adult professional gamers preying on younger players, Dr. Ohayon said.
“The major problem is that it’s putting children in a virtual world from which it’s difficult to escape,” he added.
Dr. Ohayon also noted concern about future developmental effects in kids who play video games for hours on end without coming out of their bedroom and with no physical contact with fellow players.
Parents should intervene before this situation occurs and limit the time their children spend on the gaming console, he said.
A version of this article first appeared on Medscape.com.
More evidence suggests the severity of internet addiction (IA) is directly related to the severity of sleep problems in youth.
Results from a study of more than 4,000 adolescent students show IA severity was linked to less sleep and to daytime sleepiness. In addition, boys aged 12-14 years who were addicted to computer games versus social media networking were the most affected.
Sleep issues could be “easily detectable manifestations of pathological internet addiction,” investigator Sergey Tereshchenko, PhD, Scientific Research Institute for Medical Problems of the North, Krasnoyask State Medical University, Russia, told this news organization.
These sleep problems require attention and correction, Dr. Tereshchenko added.
The findings were presented at the virtual Congress of the European Academy of Neurology 2021.
New phenomenon
IA is a relatively new psychological phenomenon and is most prevalent in “socially vulnerable groups,” such as adolescents, Dr. Tereshchenko said.
He cited numerous studies that have “convincingly demonstrated” IA is comorbid with a broad range of psychopathologic conditions, including depression, anxiety, and attention deficit hyperactivity disorder.
There is also growing evidence, including from systematic reviews in 2014 and 2019, that IA affects a wide range of sleep parameters.
However, most studies in adolescents have used only one psychometric tool to assess addiction, revealing only the “general IA pattern” and not the type of IA, Dr. Tereshchenko noted.
Adolescents may not be addicted to the internet itself but to certain behaviors like gaming or social networking, he said.
The “undoubted advantage” of his team’s research is the use of more than one tool, making it possible to “verify the predominant content of the addiction,” he added.
The investigators previously assessed general prevalence of IA in adolescents in Siberia and found about 6.8% of participants displayed pathological IA behavior – and that gaming addiction is more common in boys whereas addiction to social networking is more common in girls.
This prevalence rate is lower than in the Philippines (21.1%), Hong Kong (16.4%), Malaysia (14.1%), China (11%), and South Korea (9.7%), but slightly higher than in Japan (6.2%).
IA prevalence among adolescents in Europe ranges from 1% to 11%, with an average of 4.4%, said Dr. Tereshchenko.
Siberian students’ sleep
The current study included 4,344 students aged 12-18 years (average age, about 15 years) from 10 public schools in three large cities of Central Siberia (Krasnoyarsk, Abakan, and Kyzyl). There were slightly more girls than boys in the study sample.
Participants completed the Russian language version of the Chen Internet Addiction Scale (CIAS), which covers five symptomatic criteria for addictive behavior: withdrawal symptoms, signs of tolerance, compulsive use, psychological or physical problems, and difficulty managing time.
In this questionnaire, respondents rate several statements regarding the effect of internet use, each on a 4-point Likert scale: not at all (1 point), a little bit (2 points), moderately (3 points) and extremely (4). The total score ranges from 26 to 104.
A CIAS score of 26-42 indicates adaptive internet use, 43-64 indicates maladaptive internet use, and 65 and above indicates pathological internet use (PIU), which was classified as “internet-addicted.”
The researchers also used the nine-item Social Media Disorder Scale, as well as the Pittsburgh Sleep Quality Index to assess nighttime sleep.
Among other questions, teens were asked how long it usually took them to fall asleep and when they typically went to bed and woke up on school nights.
For daytime sleepiness, investigators used the targeted Pediatric Daytime Sleepiness Scale questionnaire, making them among the few research groups to use this psychometric instrument, Dr. Tereshchenko noted.
After parental consent was given, students completed the tests at the end of the day’s lessons. Total test time was about 45 minutes.
Sleep disturbance
Initial study results showed that compared with the other groups, adolescents with PIU tended to go to bed later, wake up later, take longer to fall asleep, sleep less at night, have more nighttime awakenings, and have more daytime sleepiness.
Sleep quality was the most impaired in boys aged 12-14 years who are addicted to internet computer games.
“In this group, 5 of the 6 sleep assessment parameters we studied were changed,” Dr. Tereshchenko reported.
Decreased total nighttime sleep was more common in older adolescents.
On average, boys and girls aged 15-18 years got less than the recommended 8 hours of sleep per night. Boys with IA got only about 6.4 hours per night and girls with IA got about 6.6 hours.
Interestingly, IA is generally more prevalent among teen girls than boys in Russia, which is not the case in Europe and North America, Dr. Tereshchenko noted.
Mechanisms linking IA and sleep disorders are not clear, but the relationship is probably multifactorial and perhaps interrelated, creating something of a “vicious circle,” he said.
“Sleep disturbances, which reflect psychosocial problems, depression, and anxiety-phobic disorders, can precede and contribute to IA. On the other hand, sleep disturbances such as insomnia can lead to increased use of the internet in the evening and at night, further exacerbating the problem,” said Dr. Tereshchenko.
Research is lacking on useful treatments for youth with IA, but these kids would likely benefit from behavioral therapy approaches, he added.
No escape?
Commenting on the study for this news organization, Maurice M. Ohayon, MD, DSc, PhD, director of the Stanford Sleep Epidemiology Research Center, Stanford University, California, said the topic of youth IA is “very important.”
Previous research in this field has shown a major impact from IA not only on sleep but also on mood – with irritability, depression, and even thoughts of suicide being possible red flags, said Dr. Ohayon, who was not involved in the current study.
Interestingly, his own research has also found that young teenage boys are most at risk for gaming addiction.
Although internet gaming has some positive effects, such as fostering leadership skills and relationships, it has become increasingly violent and isolating, with more adult professional gamers preying on younger players, Dr. Ohayon said.
“The major problem is that it’s putting children in a virtual world from which it’s difficult to escape,” he added.
Dr. Ohayon also noted concern about future developmental effects in kids who play video games for hours on end without coming out of their bedroom and with no physical contact with fellow players.
Parents should intervene before this situation occurs and limit the time their children spend on the gaming console, he said.
A version of this article first appeared on Medscape.com.
More evidence suggests the severity of internet addiction (IA) is directly related to the severity of sleep problems in youth.
Results from a study of more than 4,000 adolescent students show IA severity was linked to less sleep and to daytime sleepiness. In addition, boys aged 12-14 years who were addicted to computer games versus social media networking were the most affected.
Sleep issues could be “easily detectable manifestations of pathological internet addiction,” investigator Sergey Tereshchenko, PhD, Scientific Research Institute for Medical Problems of the North, Krasnoyask State Medical University, Russia, told this news organization.
These sleep problems require attention and correction, Dr. Tereshchenko added.
The findings were presented at the virtual Congress of the European Academy of Neurology 2021.
New phenomenon
IA is a relatively new psychological phenomenon and is most prevalent in “socially vulnerable groups,” such as adolescents, Dr. Tereshchenko said.
He cited numerous studies that have “convincingly demonstrated” IA is comorbid with a broad range of psychopathologic conditions, including depression, anxiety, and attention deficit hyperactivity disorder.
There is also growing evidence, including from systematic reviews in 2014 and 2019, that IA affects a wide range of sleep parameters.
However, most studies in adolescents have used only one psychometric tool to assess addiction, revealing only the “general IA pattern” and not the type of IA, Dr. Tereshchenko noted.
Adolescents may not be addicted to the internet itself but to certain behaviors like gaming or social networking, he said.
The “undoubted advantage” of his team’s research is the use of more than one tool, making it possible to “verify the predominant content of the addiction,” he added.
The investigators previously assessed general prevalence of IA in adolescents in Siberia and found about 6.8% of participants displayed pathological IA behavior – and that gaming addiction is more common in boys whereas addiction to social networking is more common in girls.
This prevalence rate is lower than in the Philippines (21.1%), Hong Kong (16.4%), Malaysia (14.1%), China (11%), and South Korea (9.7%), but slightly higher than in Japan (6.2%).
IA prevalence among adolescents in Europe ranges from 1% to 11%, with an average of 4.4%, said Dr. Tereshchenko.
Siberian students’ sleep
The current study included 4,344 students aged 12-18 years (average age, about 15 years) from 10 public schools in three large cities of Central Siberia (Krasnoyarsk, Abakan, and Kyzyl). There were slightly more girls than boys in the study sample.
Participants completed the Russian language version of the Chen Internet Addiction Scale (CIAS), which covers five symptomatic criteria for addictive behavior: withdrawal symptoms, signs of tolerance, compulsive use, psychological or physical problems, and difficulty managing time.
In this questionnaire, respondents rate several statements regarding the effect of internet use, each on a 4-point Likert scale: not at all (1 point), a little bit (2 points), moderately (3 points) and extremely (4). The total score ranges from 26 to 104.
A CIAS score of 26-42 indicates adaptive internet use, 43-64 indicates maladaptive internet use, and 65 and above indicates pathological internet use (PIU), which was classified as “internet-addicted.”
The researchers also used the nine-item Social Media Disorder Scale, as well as the Pittsburgh Sleep Quality Index to assess nighttime sleep.
Among other questions, teens were asked how long it usually took them to fall asleep and when they typically went to bed and woke up on school nights.
For daytime sleepiness, investigators used the targeted Pediatric Daytime Sleepiness Scale questionnaire, making them among the few research groups to use this psychometric instrument, Dr. Tereshchenko noted.
After parental consent was given, students completed the tests at the end of the day’s lessons. Total test time was about 45 minutes.
Sleep disturbance
Initial study results showed that compared with the other groups, adolescents with PIU tended to go to bed later, wake up later, take longer to fall asleep, sleep less at night, have more nighttime awakenings, and have more daytime sleepiness.
Sleep quality was the most impaired in boys aged 12-14 years who are addicted to internet computer games.
“In this group, 5 of the 6 sleep assessment parameters we studied were changed,” Dr. Tereshchenko reported.
Decreased total nighttime sleep was more common in older adolescents.
On average, boys and girls aged 15-18 years got less than the recommended 8 hours of sleep per night. Boys with IA got only about 6.4 hours per night and girls with IA got about 6.6 hours.
Interestingly, IA is generally more prevalent among teen girls than boys in Russia, which is not the case in Europe and North America, Dr. Tereshchenko noted.
Mechanisms linking IA and sleep disorders are not clear, but the relationship is probably multifactorial and perhaps interrelated, creating something of a “vicious circle,” he said.
“Sleep disturbances, which reflect psychosocial problems, depression, and anxiety-phobic disorders, can precede and contribute to IA. On the other hand, sleep disturbances such as insomnia can lead to increased use of the internet in the evening and at night, further exacerbating the problem,” said Dr. Tereshchenko.
Research is lacking on useful treatments for youth with IA, but these kids would likely benefit from behavioral therapy approaches, he added.
No escape?
Commenting on the study for this news organization, Maurice M. Ohayon, MD, DSc, PhD, director of the Stanford Sleep Epidemiology Research Center, Stanford University, California, said the topic of youth IA is “very important.”
Previous research in this field has shown a major impact from IA not only on sleep but also on mood – with irritability, depression, and even thoughts of suicide being possible red flags, said Dr. Ohayon, who was not involved in the current study.
Interestingly, his own research has also found that young teenage boys are most at risk for gaming addiction.
Although internet gaming has some positive effects, such as fostering leadership skills and relationships, it has become increasingly violent and isolating, with more adult professional gamers preying on younger players, Dr. Ohayon said.
“The major problem is that it’s putting children in a virtual world from which it’s difficult to escape,” he added.
Dr. Ohayon also noted concern about future developmental effects in kids who play video games for hours on end without coming out of their bedroom and with no physical contact with fellow players.
Parents should intervene before this situation occurs and limit the time their children spend on the gaming console, he said.
A version of this article first appeared on Medscape.com.
Key driver of fish oil’s antidepressant effects revealed
A key molecular mechanism underpinning the anti-inflammatory, antidepressant, and neuroprotective effects of omega-3 fatty acids has been identified. In findings that could lead to the development of new treatments for depression, the research provides the “first evidence” that hippocampal neurons are able to produce two key lipid metabolites of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) – lipoxygenase and cytochrome P450, lead investigator Alessandra Borsini, PhD, told this news organization.
This is how EPA and DHA exert their anti-inflammatory and neurogenic properties in vitro, as well as antidepressant properties in patients with depression, said Dr. Borsini, from King’s College London.
“Indeed, we found evidence for a correlation between increased levels of these metabolites and a decrease in severity of depressive symptoms in patients with major depressive disorder,” Dr. Borsini said.
The study was published online June 16 in Molecular Psychiatry.
‘Depression in a dish’
Despite the known role of inflammation in depression, there remains a lack of data showing anti-inflammatory strategies that are effective, safe for everyday use, and with a clear mechanism of action, the researchers note.
Dr. Borsini and colleagues tested the theory that when EPA and DHA are metabolized, some of their metabolites, or lipid mediators, can protect the brain from the harmful effects of inflammation. They used a validated “depression in a dish” in vitro human hippocampal cell model to test their theory.
They found that treating human hippocampal cells with EPA or DHA before exposing them to cytokines prevented increased cell death and decreased neurogenesis. Both these impacts had been previously observed in cells exposed to cytokines alone.
They confirmed that these effects were mediated by the formation of several key lipid mediators produced by EPA and DHA – namely hydroxyeicosapentaenoic acid, hydroxydocosahexaenoic acid, epoxyeicosatetraenoic acid (EpETE), and epoxydocosapentaenoic acid (EpDPA).
It’s the first time these lipid mediators were detected in human hippocampal neurons, the researchers say.
They also found that treating the neurons with an enzyme inhibitor increased the availability of two of these metabolites (EpETE and EpDPA), suggesting a possible way by which future treatments could be optimized.
The findings were replicated in 22 patients with major depression given either EPA (3 g/day) or DHA (1.4 g/day) for 12 weeks. In both groups, EPA or DHA treatment was associated with an increase in their respective metabolites and significant improvement in depressive symptoms.
The average reduction in symptom scores was 64% and 71% in the EPA and DHA groups, respectively, and there was some evidence that higher levels of the same metabolites correlated with less severe depressive symptoms.
“For some time we have known that omega-3 [polyunsaturated fatty acid (PUFA)] can induce antidepressant and anti-inflammatory effects, but, without further understanding of how this happens in the human brain, it has been difficult to develop treatments,” Dr. Borsini said in a news release.
“ which can inform the development of potential new treatments for depression using omega-3 PUFA,” Dr. Borsini added.
“We need to be cautious when interpreting data generated from the correlation between levels of metabolites and depressive symptoms as findings require further validation in a bigger sample of patients,” Dr. Borsini said.
“It is important to highlight that our research has not shown that by simply increasing omega-3 fatty acids in our diets or through taking nutritional supplements we can reduce inflammation or depression,” study author Carmine Pariante, MD, PhD, from King’s College London, said in the news release.
“The mechanisms behind the associations between depression and omega-3 PUFA are complicated and require further research and clinical trials to fully understand how they work and inform future therapeutic approaches,” Dr. Pariante said.
No clinical implications
Weighing in on this research in a Science Media Centre statement, Kevin McConway, emeritus professor of applied statistics, The Open University, Milton Keynes, United Kingdom, said, “The point of the study was to throw some light on the mechanisms in the body by which omega-3 fatty acids might work to reduce inflammation or depression.”
“The research mostly involved cells in laboratory dishes, but it also involved treating a small sample of patients with major depression by giving them supplements of one or other of the two omega-3 acids under investigation for 12 weeks,” he noted.
“The researchers found that the patients’ average scores on a standard set of questions, used to diagnose and measure depression, improved over that 12-week period, for each of the two fatty acids.
While depression symptoms improved over 12 weeks with omega-3 fatty acid treatment, “depression symptoms change over time anyway, for many reasons,” and depressive symptoms might have improved over 12 weeks even if the patients had not been given the omega-3 acids, Dr. McConway said.
“We just can’t tell since every patient got omega-3 fatty acids. So these results can hint that omega-3 fatty acids might help in depression, but it comes nowhere near showing that this is the case with a reasonable degree of certainty,” he cautioned.
“Indeed the researchers did not carry out this part of their study to see whether the omega-3 supplements help with depression – they did it to see whether the biochemical changes that they had seen in cell cultures in the lab might also occur in human bodies,” he noted.
This research was funded in part by grants to the investigators from the U.K. Medical Research Council, the European Commission Horizon 2020, and the National Institute for Health Research (NIHR), Maudsley Biomedical Research Centre (BRC) at South London and Maudsley NHS Foundation Trust and King’s College London. Dr. Borsini has received research funding from Johnson & Johnson for research on depression and inflammation. Dr. McConway is a trustee of the Science Media Centre and a member of its advisory committee.
A version of this article first appeared on Medscape.com.
A key molecular mechanism underpinning the anti-inflammatory, antidepressant, and neuroprotective effects of omega-3 fatty acids has been identified. In findings that could lead to the development of new treatments for depression, the research provides the “first evidence” that hippocampal neurons are able to produce two key lipid metabolites of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) – lipoxygenase and cytochrome P450, lead investigator Alessandra Borsini, PhD, told this news organization.
This is how EPA and DHA exert their anti-inflammatory and neurogenic properties in vitro, as well as antidepressant properties in patients with depression, said Dr. Borsini, from King’s College London.
“Indeed, we found evidence for a correlation between increased levels of these metabolites and a decrease in severity of depressive symptoms in patients with major depressive disorder,” Dr. Borsini said.
The study was published online June 16 in Molecular Psychiatry.
‘Depression in a dish’
Despite the known role of inflammation in depression, there remains a lack of data showing anti-inflammatory strategies that are effective, safe for everyday use, and with a clear mechanism of action, the researchers note.
Dr. Borsini and colleagues tested the theory that when EPA and DHA are metabolized, some of their metabolites, or lipid mediators, can protect the brain from the harmful effects of inflammation. They used a validated “depression in a dish” in vitro human hippocampal cell model to test their theory.
They found that treating human hippocampal cells with EPA or DHA before exposing them to cytokines prevented increased cell death and decreased neurogenesis. Both these impacts had been previously observed in cells exposed to cytokines alone.
They confirmed that these effects were mediated by the formation of several key lipid mediators produced by EPA and DHA – namely hydroxyeicosapentaenoic acid, hydroxydocosahexaenoic acid, epoxyeicosatetraenoic acid (EpETE), and epoxydocosapentaenoic acid (EpDPA).
It’s the first time these lipid mediators were detected in human hippocampal neurons, the researchers say.
They also found that treating the neurons with an enzyme inhibitor increased the availability of two of these metabolites (EpETE and EpDPA), suggesting a possible way by which future treatments could be optimized.
The findings were replicated in 22 patients with major depression given either EPA (3 g/day) or DHA (1.4 g/day) for 12 weeks. In both groups, EPA or DHA treatment was associated with an increase in their respective metabolites and significant improvement in depressive symptoms.
The average reduction in symptom scores was 64% and 71% in the EPA and DHA groups, respectively, and there was some evidence that higher levels of the same metabolites correlated with less severe depressive symptoms.
“For some time we have known that omega-3 [polyunsaturated fatty acid (PUFA)] can induce antidepressant and anti-inflammatory effects, but, without further understanding of how this happens in the human brain, it has been difficult to develop treatments,” Dr. Borsini said in a news release.
“ which can inform the development of potential new treatments for depression using omega-3 PUFA,” Dr. Borsini added.
“We need to be cautious when interpreting data generated from the correlation between levels of metabolites and depressive symptoms as findings require further validation in a bigger sample of patients,” Dr. Borsini said.
“It is important to highlight that our research has not shown that by simply increasing omega-3 fatty acids in our diets or through taking nutritional supplements we can reduce inflammation or depression,” study author Carmine Pariante, MD, PhD, from King’s College London, said in the news release.
“The mechanisms behind the associations between depression and omega-3 PUFA are complicated and require further research and clinical trials to fully understand how they work and inform future therapeutic approaches,” Dr. Pariante said.
No clinical implications
Weighing in on this research in a Science Media Centre statement, Kevin McConway, emeritus professor of applied statistics, The Open University, Milton Keynes, United Kingdom, said, “The point of the study was to throw some light on the mechanisms in the body by which omega-3 fatty acids might work to reduce inflammation or depression.”
“The research mostly involved cells in laboratory dishes, but it also involved treating a small sample of patients with major depression by giving them supplements of one or other of the two omega-3 acids under investigation for 12 weeks,” he noted.
“The researchers found that the patients’ average scores on a standard set of questions, used to diagnose and measure depression, improved over that 12-week period, for each of the two fatty acids.
While depression symptoms improved over 12 weeks with omega-3 fatty acid treatment, “depression symptoms change over time anyway, for many reasons,” and depressive symptoms might have improved over 12 weeks even if the patients had not been given the omega-3 acids, Dr. McConway said.
“We just can’t tell since every patient got omega-3 fatty acids. So these results can hint that omega-3 fatty acids might help in depression, but it comes nowhere near showing that this is the case with a reasonable degree of certainty,” he cautioned.
“Indeed the researchers did not carry out this part of their study to see whether the omega-3 supplements help with depression – they did it to see whether the biochemical changes that they had seen in cell cultures in the lab might also occur in human bodies,” he noted.
This research was funded in part by grants to the investigators from the U.K. Medical Research Council, the European Commission Horizon 2020, and the National Institute for Health Research (NIHR), Maudsley Biomedical Research Centre (BRC) at South London and Maudsley NHS Foundation Trust and King’s College London. Dr. Borsini has received research funding from Johnson & Johnson for research on depression and inflammation. Dr. McConway is a trustee of the Science Media Centre and a member of its advisory committee.
A version of this article first appeared on Medscape.com.
A key molecular mechanism underpinning the anti-inflammatory, antidepressant, and neuroprotective effects of omega-3 fatty acids has been identified. In findings that could lead to the development of new treatments for depression, the research provides the “first evidence” that hippocampal neurons are able to produce two key lipid metabolites of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) – lipoxygenase and cytochrome P450, lead investigator Alessandra Borsini, PhD, told this news organization.
This is how EPA and DHA exert their anti-inflammatory and neurogenic properties in vitro, as well as antidepressant properties in patients with depression, said Dr. Borsini, from King’s College London.
“Indeed, we found evidence for a correlation between increased levels of these metabolites and a decrease in severity of depressive symptoms in patients with major depressive disorder,” Dr. Borsini said.
The study was published online June 16 in Molecular Psychiatry.
‘Depression in a dish’
Despite the known role of inflammation in depression, there remains a lack of data showing anti-inflammatory strategies that are effective, safe for everyday use, and with a clear mechanism of action, the researchers note.
Dr. Borsini and colleagues tested the theory that when EPA and DHA are metabolized, some of their metabolites, or lipid mediators, can protect the brain from the harmful effects of inflammation. They used a validated “depression in a dish” in vitro human hippocampal cell model to test their theory.
They found that treating human hippocampal cells with EPA or DHA before exposing them to cytokines prevented increased cell death and decreased neurogenesis. Both these impacts had been previously observed in cells exposed to cytokines alone.
They confirmed that these effects were mediated by the formation of several key lipid mediators produced by EPA and DHA – namely hydroxyeicosapentaenoic acid, hydroxydocosahexaenoic acid, epoxyeicosatetraenoic acid (EpETE), and epoxydocosapentaenoic acid (EpDPA).
It’s the first time these lipid mediators were detected in human hippocampal neurons, the researchers say.
They also found that treating the neurons with an enzyme inhibitor increased the availability of two of these metabolites (EpETE and EpDPA), suggesting a possible way by which future treatments could be optimized.
The findings were replicated in 22 patients with major depression given either EPA (3 g/day) or DHA (1.4 g/day) for 12 weeks. In both groups, EPA or DHA treatment was associated with an increase in their respective metabolites and significant improvement in depressive symptoms.
The average reduction in symptom scores was 64% and 71% in the EPA and DHA groups, respectively, and there was some evidence that higher levels of the same metabolites correlated with less severe depressive symptoms.
“For some time we have known that omega-3 [polyunsaturated fatty acid (PUFA)] can induce antidepressant and anti-inflammatory effects, but, without further understanding of how this happens in the human brain, it has been difficult to develop treatments,” Dr. Borsini said in a news release.
“ which can inform the development of potential new treatments for depression using omega-3 PUFA,” Dr. Borsini added.
“We need to be cautious when interpreting data generated from the correlation between levels of metabolites and depressive symptoms as findings require further validation in a bigger sample of patients,” Dr. Borsini said.
“It is important to highlight that our research has not shown that by simply increasing omega-3 fatty acids in our diets or through taking nutritional supplements we can reduce inflammation or depression,” study author Carmine Pariante, MD, PhD, from King’s College London, said in the news release.
“The mechanisms behind the associations between depression and omega-3 PUFA are complicated and require further research and clinical trials to fully understand how they work and inform future therapeutic approaches,” Dr. Pariante said.
No clinical implications
Weighing in on this research in a Science Media Centre statement, Kevin McConway, emeritus professor of applied statistics, The Open University, Milton Keynes, United Kingdom, said, “The point of the study was to throw some light on the mechanisms in the body by which omega-3 fatty acids might work to reduce inflammation or depression.”
“The research mostly involved cells in laboratory dishes, but it also involved treating a small sample of patients with major depression by giving them supplements of one or other of the two omega-3 acids under investigation for 12 weeks,” he noted.
“The researchers found that the patients’ average scores on a standard set of questions, used to diagnose and measure depression, improved over that 12-week period, for each of the two fatty acids.
While depression symptoms improved over 12 weeks with omega-3 fatty acid treatment, “depression symptoms change over time anyway, for many reasons,” and depressive symptoms might have improved over 12 weeks even if the patients had not been given the omega-3 acids, Dr. McConway said.
“We just can’t tell since every patient got omega-3 fatty acids. So these results can hint that omega-3 fatty acids might help in depression, but it comes nowhere near showing that this is the case with a reasonable degree of certainty,” he cautioned.
“Indeed the researchers did not carry out this part of their study to see whether the omega-3 supplements help with depression – they did it to see whether the biochemical changes that they had seen in cell cultures in the lab might also occur in human bodies,” he noted.
This research was funded in part by grants to the investigators from the U.K. Medical Research Council, the European Commission Horizon 2020, and the National Institute for Health Research (NIHR), Maudsley Biomedical Research Centre (BRC) at South London and Maudsley NHS Foundation Trust and King’s College London. Dr. Borsini has received research funding from Johnson & Johnson for research on depression and inflammation. Dr. McConway is a trustee of the Science Media Centre and a member of its advisory committee.
A version of this article first appeared on Medscape.com.
Risk to infant may warrant drug treatment for postpartum depression
If moderate to severe postpartum depression poses a risk to child development, that argues in favor of pharmacologic therapy, according to a detailed risk-benefit assessment presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“It is important to consider that there are potential risks of antidepressant drugs, but there are also potential risks from not providing effective treatment,” reported Neha Hudepohl, MD, at the virtual meeting, presented by MedscapeLive.
On a website maintained by the Centers for Disease Control and Prevention, the question of whether antidepressants pose a risk to breast-feeding children is answered with a “maybe.” Although many of these drugs can be detected in breast milk, according to the CDC, “most have little or no effect on milk supply or on infant well-being.”
This is enough uncertainty that antidepressants are not first-line intervention when postpartum depression is mild, said Dr. Hudepohl, director of women’s mental health at Prisma Health Upstate, Greenville, S.C. However, she noted that the American College of Obstetricians and Gynecologists is among the organizations that recommend drug treatment if symptoms are moderate to severe.
“Depression in the mother affects interactions in feeding practices, sleep routines and patterns, and safety practices,” said Dr. Hudepohl, citing published studies supporting each of these consequences.
For the child, there is some degree of uncertainty about risk from untreated maternal depression as well as from breast mild exposure to antidepressants. Conclusive statements are not offered by ACOG and others.
“Some but not all studies have shown an impact of either antenatal or postnatal depression on speech recognition in infancy of native versus nonnative languages, IQ, and cognitive development, and reduction in left frontal brain electrical activity associated with impaired positive emotions,” Dr. Hudepohl reported.
Sifting through published data, Dr. Hudepohl cited studies associating persistent postpartum depression with a more than fourfold risk of behavioral problems at the age of 3.5 years, lower grades in mathematics at age 16, and a higher prevalence of depression at age 18. Among children who had depressed mothers in infancy, there have also been studies showing a higher reactivity to stressors and higher baseline cortisol levels.
“The good news is that Dr. Hudepohl said. In fact, she cited evidence of a correlation between improvement in maternal symptoms and a reduction in the complications in children, such as behavioral problems.
Postpartum depression, which can develop anytime in the first 12 months after childbirth, is not uncommon, occurring in approximately 15% of women, according to Dr. Hudepohl. Risk factors include personal or family history of depression, anxiety or depression during pregnancy, and a prior history of postpartum depression.
Postpartum depression increases the risk of maternal suicide by about 70-fold, Dr. Hudepohl reported. She noted that the peaks in suicide attempts in the 1st and 12th month after delivery. Adverse infant outcomes are not a predictor of increase risk of attempts, but fetal or infant death are.
According to one study, about 40% of mothers with postpartum depression have intrusive thoughts that involve harming their child. About 15% fear being alone with their infant. Behaviors such as decreased playfulness, less talking, or other interactions with the child, and inconsistent response to the child are all likely to contribute to impaired maternal-child bonding, Dr. Hudepohl reported.
For women who discontinued antidepressants for pregnancy but have now developed significant postpartum depression, Dr. Hudepohl recommended using “what has worked in the past.” She considered monotherapy preferable if possible, but severe symptoms warrant more aggressive intervention. Dr. Hudepohl pointed out that the risks of antidepressants taken by the breast-feeding mother to the infant remain unclear despite multiple studies attempting to establish and quantify risk.
“Antidepressants are the most researched medication in pregnancy,” she said.
Conversely, the risks of untreated symptoms to the mother are significant, and the potential risks to the infant and family – if variable – are not insignificant.
Overall, “nonpharmacologic treatment is preferred first line for mild symptoms,” Dr. Hudepohl, but she and others consider a risk-benefit ratio growing increasingly in favor of drug therapy when this approach is the best option for bringing moderate to severe symptoms under control.
Whether depression arises during pregnancy or in the postpartum period, “psychotherapy is generally considered first-line treatment,” agreed Nancy Byatt, DO, MS, MBA, professor of psychiatry and of obstetrics and gynecology at the University of Massachusetts, Worcester.
Dr. Byatt, who has published frequently on this topic, further agreed that risks to the mother and the child increase with uncontrolled depression in the postpartum period. With symptoms of greater intensity, the uncertain risks of medication are outweighed by substantial potential benefits.
“When a pregnant or postpartum individual has moderate to severe illness, treatment with medication is typically recommended, because the benefits are thought to outweigh the risks,” she said, echoing a consensus opinion among experts and organized medicine.
MedscapeLive and this news organization are owned by the same parent company. Dr. Hudepohl and Dr. Byatt reported no potential financial conflicts of interest.
If moderate to severe postpartum depression poses a risk to child development, that argues in favor of pharmacologic therapy, according to a detailed risk-benefit assessment presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“It is important to consider that there are potential risks of antidepressant drugs, but there are also potential risks from not providing effective treatment,” reported Neha Hudepohl, MD, at the virtual meeting, presented by MedscapeLive.
On a website maintained by the Centers for Disease Control and Prevention, the question of whether antidepressants pose a risk to breast-feeding children is answered with a “maybe.” Although many of these drugs can be detected in breast milk, according to the CDC, “most have little or no effect on milk supply or on infant well-being.”
This is enough uncertainty that antidepressants are not first-line intervention when postpartum depression is mild, said Dr. Hudepohl, director of women’s mental health at Prisma Health Upstate, Greenville, S.C. However, she noted that the American College of Obstetricians and Gynecologists is among the organizations that recommend drug treatment if symptoms are moderate to severe.
“Depression in the mother affects interactions in feeding practices, sleep routines and patterns, and safety practices,” said Dr. Hudepohl, citing published studies supporting each of these consequences.
For the child, there is some degree of uncertainty about risk from untreated maternal depression as well as from breast mild exposure to antidepressants. Conclusive statements are not offered by ACOG and others.
“Some but not all studies have shown an impact of either antenatal or postnatal depression on speech recognition in infancy of native versus nonnative languages, IQ, and cognitive development, and reduction in left frontal brain electrical activity associated with impaired positive emotions,” Dr. Hudepohl reported.
Sifting through published data, Dr. Hudepohl cited studies associating persistent postpartum depression with a more than fourfold risk of behavioral problems at the age of 3.5 years, lower grades in mathematics at age 16, and a higher prevalence of depression at age 18. Among children who had depressed mothers in infancy, there have also been studies showing a higher reactivity to stressors and higher baseline cortisol levels.
“The good news is that Dr. Hudepohl said. In fact, she cited evidence of a correlation between improvement in maternal symptoms and a reduction in the complications in children, such as behavioral problems.
Postpartum depression, which can develop anytime in the first 12 months after childbirth, is not uncommon, occurring in approximately 15% of women, according to Dr. Hudepohl. Risk factors include personal or family history of depression, anxiety or depression during pregnancy, and a prior history of postpartum depression.
Postpartum depression increases the risk of maternal suicide by about 70-fold, Dr. Hudepohl reported. She noted that the peaks in suicide attempts in the 1st and 12th month after delivery. Adverse infant outcomes are not a predictor of increase risk of attempts, but fetal or infant death are.
According to one study, about 40% of mothers with postpartum depression have intrusive thoughts that involve harming their child. About 15% fear being alone with their infant. Behaviors such as decreased playfulness, less talking, or other interactions with the child, and inconsistent response to the child are all likely to contribute to impaired maternal-child bonding, Dr. Hudepohl reported.
For women who discontinued antidepressants for pregnancy but have now developed significant postpartum depression, Dr. Hudepohl recommended using “what has worked in the past.” She considered monotherapy preferable if possible, but severe symptoms warrant more aggressive intervention. Dr. Hudepohl pointed out that the risks of antidepressants taken by the breast-feeding mother to the infant remain unclear despite multiple studies attempting to establish and quantify risk.
“Antidepressants are the most researched medication in pregnancy,” she said.
Conversely, the risks of untreated symptoms to the mother are significant, and the potential risks to the infant and family – if variable – are not insignificant.
Overall, “nonpharmacologic treatment is preferred first line for mild symptoms,” Dr. Hudepohl, but she and others consider a risk-benefit ratio growing increasingly in favor of drug therapy when this approach is the best option for bringing moderate to severe symptoms under control.
Whether depression arises during pregnancy or in the postpartum period, “psychotherapy is generally considered first-line treatment,” agreed Nancy Byatt, DO, MS, MBA, professor of psychiatry and of obstetrics and gynecology at the University of Massachusetts, Worcester.
Dr. Byatt, who has published frequently on this topic, further agreed that risks to the mother and the child increase with uncontrolled depression in the postpartum period. With symptoms of greater intensity, the uncertain risks of medication are outweighed by substantial potential benefits.
“When a pregnant or postpartum individual has moderate to severe illness, treatment with medication is typically recommended, because the benefits are thought to outweigh the risks,” she said, echoing a consensus opinion among experts and organized medicine.
MedscapeLive and this news organization are owned by the same parent company. Dr. Hudepohl and Dr. Byatt reported no potential financial conflicts of interest.
If moderate to severe postpartum depression poses a risk to child development, that argues in favor of pharmacologic therapy, according to a detailed risk-benefit assessment presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“It is important to consider that there are potential risks of antidepressant drugs, but there are also potential risks from not providing effective treatment,” reported Neha Hudepohl, MD, at the virtual meeting, presented by MedscapeLive.
On a website maintained by the Centers for Disease Control and Prevention, the question of whether antidepressants pose a risk to breast-feeding children is answered with a “maybe.” Although many of these drugs can be detected in breast milk, according to the CDC, “most have little or no effect on milk supply or on infant well-being.”
This is enough uncertainty that antidepressants are not first-line intervention when postpartum depression is mild, said Dr. Hudepohl, director of women’s mental health at Prisma Health Upstate, Greenville, S.C. However, she noted that the American College of Obstetricians and Gynecologists is among the organizations that recommend drug treatment if symptoms are moderate to severe.
“Depression in the mother affects interactions in feeding practices, sleep routines and patterns, and safety practices,” said Dr. Hudepohl, citing published studies supporting each of these consequences.
For the child, there is some degree of uncertainty about risk from untreated maternal depression as well as from breast mild exposure to antidepressants. Conclusive statements are not offered by ACOG and others.
“Some but not all studies have shown an impact of either antenatal or postnatal depression on speech recognition in infancy of native versus nonnative languages, IQ, and cognitive development, and reduction in left frontal brain electrical activity associated with impaired positive emotions,” Dr. Hudepohl reported.
Sifting through published data, Dr. Hudepohl cited studies associating persistent postpartum depression with a more than fourfold risk of behavioral problems at the age of 3.5 years, lower grades in mathematics at age 16, and a higher prevalence of depression at age 18. Among children who had depressed mothers in infancy, there have also been studies showing a higher reactivity to stressors and higher baseline cortisol levels.
“The good news is that Dr. Hudepohl said. In fact, she cited evidence of a correlation between improvement in maternal symptoms and a reduction in the complications in children, such as behavioral problems.
Postpartum depression, which can develop anytime in the first 12 months after childbirth, is not uncommon, occurring in approximately 15% of women, according to Dr. Hudepohl. Risk factors include personal or family history of depression, anxiety or depression during pregnancy, and a prior history of postpartum depression.
Postpartum depression increases the risk of maternal suicide by about 70-fold, Dr. Hudepohl reported. She noted that the peaks in suicide attempts in the 1st and 12th month after delivery. Adverse infant outcomes are not a predictor of increase risk of attempts, but fetal or infant death are.
According to one study, about 40% of mothers with postpartum depression have intrusive thoughts that involve harming their child. About 15% fear being alone with their infant. Behaviors such as decreased playfulness, less talking, or other interactions with the child, and inconsistent response to the child are all likely to contribute to impaired maternal-child bonding, Dr. Hudepohl reported.
For women who discontinued antidepressants for pregnancy but have now developed significant postpartum depression, Dr. Hudepohl recommended using “what has worked in the past.” She considered monotherapy preferable if possible, but severe symptoms warrant more aggressive intervention. Dr. Hudepohl pointed out that the risks of antidepressants taken by the breast-feeding mother to the infant remain unclear despite multiple studies attempting to establish and quantify risk.
“Antidepressants are the most researched medication in pregnancy,” she said.
Conversely, the risks of untreated symptoms to the mother are significant, and the potential risks to the infant and family – if variable – are not insignificant.
Overall, “nonpharmacologic treatment is preferred first line for mild symptoms,” Dr. Hudepohl, but she and others consider a risk-benefit ratio growing increasingly in favor of drug therapy when this approach is the best option for bringing moderate to severe symptoms under control.
Whether depression arises during pregnancy or in the postpartum period, “psychotherapy is generally considered first-line treatment,” agreed Nancy Byatt, DO, MS, MBA, professor of psychiatry and of obstetrics and gynecology at the University of Massachusetts, Worcester.
Dr. Byatt, who has published frequently on this topic, further agreed that risks to the mother and the child increase with uncontrolled depression in the postpartum period. With symptoms of greater intensity, the uncertain risks of medication are outweighed by substantial potential benefits.
“When a pregnant or postpartum individual has moderate to severe illness, treatment with medication is typically recommended, because the benefits are thought to outweigh the risks,” she said, echoing a consensus opinion among experts and organized medicine.
MedscapeLive and this news organization are owned by the same parent company. Dr. Hudepohl and Dr. Byatt reported no potential financial conflicts of interest.
FROM CP/AACP PSYCHIATRY UPDATE
Depression remains common among dystonia patients
About one-third of individuals with adult-onset idiopathic dystonia experience major depression or dysthymia, data from a meta-analysis of 54 studies show.
Adult-onset idiopathic dystonia (AOID) is the third-most common movement disorder after essential tremor and Parkinson’s disease, and data show that depression and anxiety are the largest contributors to reduced quality of life in these patients, wrote Alex Medina Escobar, MD, of the University of Calgary (Alta.), and colleagues. However, “the pathogenic mechanisms of depression and anxiety in AOID remain unclear” and might involve a combination of biologic factors, as well as social stigma.
In the meta-analysis, published in Neuroscience and Biobehavioral Reviews, the researchers examined the point prevalence of supraclinical threshold depressive symptoms/depressive disorders in AOID using 54 studies. The resulting study population included 12,635 patients: 6,977 with cervical dystonia, 732 with cranial dystonia, 4,504 with mixed forms, 303 with laryngeal dystonia, and 119 with upper-limb dystonia. The studies were published between 1988 and 2020, and included patients from 21 countries in 52 single-center studies and 2 multicenter studies.
Overall, the pooled prevalence of either supraclinical threshold depressive symptoms or depressive disorders was 31.5% for cervical dystonia, 29.2 % for cranial dystonia, and 33.6 % for clinical samples with mixed forms of AOID.
Among patients with cervical dystonia, major depressive disorder was more prevalent than dysthymia, but among patients with cranial dystonia, dysthymia was more prevalent. Among patients with mixed forms, the prevalence of major depressive disorder was higher than dysthymia. Heterogeneity varied among the studies but was higher in studies that used rating scales.
Treatment of patients with AOID does not take into account the impact of depression on quality of life, Dr. Escobar and colleagues reported.
“ Such model appears to be inefficient to guarantee resources to address these comorbidities within secondary or tertiary care, or through shared care pathways engaging both primary and hospital-based care.” They also said the use of antidepressants and cognitive-behavioral therapy as a way to target negative body concept or social stigma among these patients are “underexplored and underutilized.”
The study findings were limited by several factors, including the inclusion only of studies published in English. In addition, most of the studies were conducted at movement disorders clinics, which may have yielded a patient population with more severe AOID. Further limitations included the inability to perform subgroup analysis based on demographic and clinical factors, and the insufficient number of studies for meta-analysis of laryngeal and hand dystonia, Dr. Escobar and colleagues added.
However, the results represent the first pooled estimate of depression prevalence in AOID and confirm a high prevalence across different clinical forms, the researchers said. The heterogeneity across studies highlights the need for standardized screening for depression and improved diagnosis of mood disorders in AOID.
“The meta-analytic estimates provided here will be highly useful for the planning of future mechanistic and interventional studies, as well as for the redefinition of current models of care,” they concluded.
The study received no outside funding. Dr. Escobar and colleagues had no disclosures.
About one-third of individuals with adult-onset idiopathic dystonia experience major depression or dysthymia, data from a meta-analysis of 54 studies show.
Adult-onset idiopathic dystonia (AOID) is the third-most common movement disorder after essential tremor and Parkinson’s disease, and data show that depression and anxiety are the largest contributors to reduced quality of life in these patients, wrote Alex Medina Escobar, MD, of the University of Calgary (Alta.), and colleagues. However, “the pathogenic mechanisms of depression and anxiety in AOID remain unclear” and might involve a combination of biologic factors, as well as social stigma.
In the meta-analysis, published in Neuroscience and Biobehavioral Reviews, the researchers examined the point prevalence of supraclinical threshold depressive symptoms/depressive disorders in AOID using 54 studies. The resulting study population included 12,635 patients: 6,977 with cervical dystonia, 732 with cranial dystonia, 4,504 with mixed forms, 303 with laryngeal dystonia, and 119 with upper-limb dystonia. The studies were published between 1988 and 2020, and included patients from 21 countries in 52 single-center studies and 2 multicenter studies.
Overall, the pooled prevalence of either supraclinical threshold depressive symptoms or depressive disorders was 31.5% for cervical dystonia, 29.2 % for cranial dystonia, and 33.6 % for clinical samples with mixed forms of AOID.
Among patients with cervical dystonia, major depressive disorder was more prevalent than dysthymia, but among patients with cranial dystonia, dysthymia was more prevalent. Among patients with mixed forms, the prevalence of major depressive disorder was higher than dysthymia. Heterogeneity varied among the studies but was higher in studies that used rating scales.
Treatment of patients with AOID does not take into account the impact of depression on quality of life, Dr. Escobar and colleagues reported.
“ Such model appears to be inefficient to guarantee resources to address these comorbidities within secondary or tertiary care, or through shared care pathways engaging both primary and hospital-based care.” They also said the use of antidepressants and cognitive-behavioral therapy as a way to target negative body concept or social stigma among these patients are “underexplored and underutilized.”
The study findings were limited by several factors, including the inclusion only of studies published in English. In addition, most of the studies were conducted at movement disorders clinics, which may have yielded a patient population with more severe AOID. Further limitations included the inability to perform subgroup analysis based on demographic and clinical factors, and the insufficient number of studies for meta-analysis of laryngeal and hand dystonia, Dr. Escobar and colleagues added.
However, the results represent the first pooled estimate of depression prevalence in AOID and confirm a high prevalence across different clinical forms, the researchers said. The heterogeneity across studies highlights the need for standardized screening for depression and improved diagnosis of mood disorders in AOID.
“The meta-analytic estimates provided here will be highly useful for the planning of future mechanistic and interventional studies, as well as for the redefinition of current models of care,” they concluded.
The study received no outside funding. Dr. Escobar and colleagues had no disclosures.
About one-third of individuals with adult-onset idiopathic dystonia experience major depression or dysthymia, data from a meta-analysis of 54 studies show.
Adult-onset idiopathic dystonia (AOID) is the third-most common movement disorder after essential tremor and Parkinson’s disease, and data show that depression and anxiety are the largest contributors to reduced quality of life in these patients, wrote Alex Medina Escobar, MD, of the University of Calgary (Alta.), and colleagues. However, “the pathogenic mechanisms of depression and anxiety in AOID remain unclear” and might involve a combination of biologic factors, as well as social stigma.
In the meta-analysis, published in Neuroscience and Biobehavioral Reviews, the researchers examined the point prevalence of supraclinical threshold depressive symptoms/depressive disorders in AOID using 54 studies. The resulting study population included 12,635 patients: 6,977 with cervical dystonia, 732 with cranial dystonia, 4,504 with mixed forms, 303 with laryngeal dystonia, and 119 with upper-limb dystonia. The studies were published between 1988 and 2020, and included patients from 21 countries in 52 single-center studies and 2 multicenter studies.
Overall, the pooled prevalence of either supraclinical threshold depressive symptoms or depressive disorders was 31.5% for cervical dystonia, 29.2 % for cranial dystonia, and 33.6 % for clinical samples with mixed forms of AOID.
Among patients with cervical dystonia, major depressive disorder was more prevalent than dysthymia, but among patients with cranial dystonia, dysthymia was more prevalent. Among patients with mixed forms, the prevalence of major depressive disorder was higher than dysthymia. Heterogeneity varied among the studies but was higher in studies that used rating scales.
Treatment of patients with AOID does not take into account the impact of depression on quality of life, Dr. Escobar and colleagues reported.
“ Such model appears to be inefficient to guarantee resources to address these comorbidities within secondary or tertiary care, or through shared care pathways engaging both primary and hospital-based care.” They also said the use of antidepressants and cognitive-behavioral therapy as a way to target negative body concept or social stigma among these patients are “underexplored and underutilized.”
The study findings were limited by several factors, including the inclusion only of studies published in English. In addition, most of the studies were conducted at movement disorders clinics, which may have yielded a patient population with more severe AOID. Further limitations included the inability to perform subgroup analysis based on demographic and clinical factors, and the insufficient number of studies for meta-analysis of laryngeal and hand dystonia, Dr. Escobar and colleagues added.
However, the results represent the first pooled estimate of depression prevalence in AOID and confirm a high prevalence across different clinical forms, the researchers said. The heterogeneity across studies highlights the need for standardized screening for depression and improved diagnosis of mood disorders in AOID.
“The meta-analytic estimates provided here will be highly useful for the planning of future mechanistic and interventional studies, as well as for the redefinition of current models of care,” they concluded.
The study received no outside funding. Dr. Escobar and colleagues had no disclosures.
FROM NEUROSCIENCE AND BIOBEHAVIORAL REVIEWS
High rates of work-related trauma, PTSD in intern physicians
Work-related posttraumatic stress disorder is three times higher in interns than the general population, new research shows.
Investigators assessed PTSD in more than 1,100 physicians at the end of their internship year and found that a little over half reported work-related trauma exposure, and of these, 20% screened positive for PTSD.
Overall, 10% of participants screened positive for PTSD by the end of the internship year, compared with a 12-month PTSD prevalence of 3.6% in the general population.
“Work-related trauma exposure and PTSD are common and underdiscussed phenomena among intern physicians,” lead author Mary Vance, MD, assistant professor of psychiatry, Uniformed Services University of the Health Sciences, Bethesda, Md., said in an interview.
“I urge medical educators and policy makers to include this topic in their discussions about physician well-being and to implement effective interventions to mitigate the impact of work-related trauma and PTSD among physician trainees,” she said.
The study was published online June 8 in JAMA Network Open.
Burnout, depression, suicide
“Burnout, depression, and suicide are increasingly recognized as occupational mental health hazards among health care professionals, including physicians,” Dr. Vance said.
“However, in my professional experience as a physician and educator, despite observing anecdotal evidence among my peers and trainees that this is also an issue,” she added.
This gap prompted her “to investigate rates of work-related trauma exposure and PTSD among physicians.”
The researchers sent emails to 4,350 individuals during academic year 2018-2019, 2 months prior to starting internships. Of these, 2,129 agreed to participate and 1,134 (58.6% female, 61.6% non-Hispanic White; mean age, 27.52) completed the study.
Prior to beginning internship, participants completed a baseline survey that assessed demographic characteristics as well as medical education and psychological and psychosocial factors.
Participants completed follow-up surveys sent by email at 3, 6, 9, and 12 months of the internship year. The surveys assessed stressful life events, concern over perceived medical errors in the past 3 months, and number of hours worked over the past week.
At month 12, current PTSD and symptoms of depression and anxiety were also assessed using the Primary Care PTSD Screen for DSM-5, the 9-item Patient Health Questionnaire, and the Generalized Anxiety Disorder 7-item scale, respectively.
Participants were asked to self-report whether they ever had an episode of depression and to complete the Risky Families Questionnaire to assess if they had experienced childhood abuse, neglect, and family conflict. Additionally, they completed an 11-item scale developed specifically for the study regarding recent stressful events.
‘Crucible’ year
A total of 56.4% of respondents reported work-related trauma exposure, and among these, 19.0% screened positive for PTSD. One-tenth (10.8%) of the entire sample screened positive for PTSD by the end of internship year, which is three times higher than the 12-month prevalence of PTSD in the general population (3.6%), the authors noted.
Trauma exposure differed by specialty, ranging from 43.1% in anesthesiology to 72.4% in emergency medicine. Of the respondents in internal medicine, surgery, and medicine/pediatrics, 56.6%, 63.3%, and 71%, respectively, reported work-related trauma exposure.
Work-related PTSD also differed by specialty, ranging from 7.5% in ob.gyn. to 30.0% in pediatrics. Of respondents in internal medicine and family practice, 23.9% and 25.9%, respectively, reported work-related PTSD.
Dr. Vance called the intern year “a crucible, during which newly minted doctors receive intensive on-the-job training at the front lines of patient care [and] work long hours in rapidly shifting environments, often caring for critically ill patients.”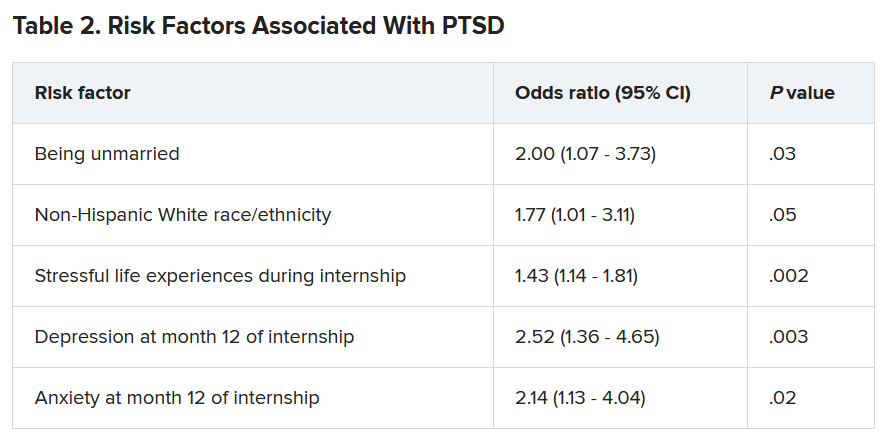
Work-related trauma exposure “is more likely to occur during this high-stress internship year than during the same year in the general population,” she said.
She noted that the “issue of workplace trauma and PTSD among health care workers became even more salient during the height of COVID,” adding that she expects it “to remain a pressure issue for healthcare workers in the post-COVID era.”
Call to action
Commenting on the study David A. Marcus, MD, chair, GME Physician Well-Being Committee, Northwell Health, New Hyde Park, N.Y., noted the study’s “relatively low response rate” is a “significant limitation” of the study.
An additional limitation is the lack of a baseline PTSD assessment, said Dr. Marcus, an assistant professor at Hofstra University, Hempstead, N.Y., who was not involved in the research.
Nevertheless, the “overall prevalence [of work-related PTSD] should serve as a call to action for physician leaders and for leaders in academic medicine,” he said.
Additionally, the study “reminds us that trauma-informed care should be an essential part of mental health support services provided to trainees and to physicians in general,” Dr. Marcus stated.
Also commenting on the study, Lotte N. Dyrbye, MD, professor of medicine and medical education, Mayo Clinic, Rochester, Minn., agreed.
“Organizational strategies should include system-level interventions to reduce the risk of frightening, horrible, or traumatic events from occurring in the workplace in the first place, as well as faculty development efforts to upskill teaching faculty in their ability to support trainees when such events do occur,” she said.
These approaches “should coincide with organizational efforts to support individual trainees by providing adequate time off after traumatic events, ensuring trainees can access affordable mental healthcare, and reducing other barriers to appropriate help-seeking, such as stigma, and efforts to build a culture of well-being,” suggested Dr. Dyrbye, who is codirector of the Mayo Clinic Program on Physician Wellbeing and was not involved in the study.
The study was supported by grants from the Blue Cross Blue Shield Foundation of Michigan and National Institutes of Health. Dr. Vance and coauthors, Dr. Marcus, and Dr. Dyrbye reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Work-related posttraumatic stress disorder is three times higher in interns than the general population, new research shows.
Investigators assessed PTSD in more than 1,100 physicians at the end of their internship year and found that a little over half reported work-related trauma exposure, and of these, 20% screened positive for PTSD.
Overall, 10% of participants screened positive for PTSD by the end of the internship year, compared with a 12-month PTSD prevalence of 3.6% in the general population.
“Work-related trauma exposure and PTSD are common and underdiscussed phenomena among intern physicians,” lead author Mary Vance, MD, assistant professor of psychiatry, Uniformed Services University of the Health Sciences, Bethesda, Md., said in an interview.
“I urge medical educators and policy makers to include this topic in their discussions about physician well-being and to implement effective interventions to mitigate the impact of work-related trauma and PTSD among physician trainees,” she said.
The study was published online June 8 in JAMA Network Open.
Burnout, depression, suicide
“Burnout, depression, and suicide are increasingly recognized as occupational mental health hazards among health care professionals, including physicians,” Dr. Vance said.
“However, in my professional experience as a physician and educator, despite observing anecdotal evidence among my peers and trainees that this is also an issue,” she added.
This gap prompted her “to investigate rates of work-related trauma exposure and PTSD among physicians.”
The researchers sent emails to 4,350 individuals during academic year 2018-2019, 2 months prior to starting internships. Of these, 2,129 agreed to participate and 1,134 (58.6% female, 61.6% non-Hispanic White; mean age, 27.52) completed the study.
Prior to beginning internship, participants completed a baseline survey that assessed demographic characteristics as well as medical education and psychological and psychosocial factors.
Participants completed follow-up surveys sent by email at 3, 6, 9, and 12 months of the internship year. The surveys assessed stressful life events, concern over perceived medical errors in the past 3 months, and number of hours worked over the past week.
At month 12, current PTSD and symptoms of depression and anxiety were also assessed using the Primary Care PTSD Screen for DSM-5, the 9-item Patient Health Questionnaire, and the Generalized Anxiety Disorder 7-item scale, respectively.
Participants were asked to self-report whether they ever had an episode of depression and to complete the Risky Families Questionnaire to assess if they had experienced childhood abuse, neglect, and family conflict. Additionally, they completed an 11-item scale developed specifically for the study regarding recent stressful events.
‘Crucible’ year
A total of 56.4% of respondents reported work-related trauma exposure, and among these, 19.0% screened positive for PTSD. One-tenth (10.8%) of the entire sample screened positive for PTSD by the end of internship year, which is three times higher than the 12-month prevalence of PTSD in the general population (3.6%), the authors noted.
Trauma exposure differed by specialty, ranging from 43.1% in anesthesiology to 72.4% in emergency medicine. Of the respondents in internal medicine, surgery, and medicine/pediatrics, 56.6%, 63.3%, and 71%, respectively, reported work-related trauma exposure.
Work-related PTSD also differed by specialty, ranging from 7.5% in ob.gyn. to 30.0% in pediatrics. Of respondents in internal medicine and family practice, 23.9% and 25.9%, respectively, reported work-related PTSD.
Dr. Vance called the intern year “a crucible, during which newly minted doctors receive intensive on-the-job training at the front lines of patient care [and] work long hours in rapidly shifting environments, often caring for critically ill patients.”
Work-related trauma exposure “is more likely to occur during this high-stress internship year than during the same year in the general population,” she said.
She noted that the “issue of workplace trauma and PTSD among health care workers became even more salient during the height of COVID,” adding that she expects it “to remain a pressure issue for healthcare workers in the post-COVID era.”
Call to action
Commenting on the study David A. Marcus, MD, chair, GME Physician Well-Being Committee, Northwell Health, New Hyde Park, N.Y., noted the study’s “relatively low response rate” is a “significant limitation” of the study.
An additional limitation is the lack of a baseline PTSD assessment, said Dr. Marcus, an assistant professor at Hofstra University, Hempstead, N.Y., who was not involved in the research.
Nevertheless, the “overall prevalence [of work-related PTSD] should serve as a call to action for physician leaders and for leaders in academic medicine,” he said.
Additionally, the study “reminds us that trauma-informed care should be an essential part of mental health support services provided to trainees and to physicians in general,” Dr. Marcus stated.
Also commenting on the study, Lotte N. Dyrbye, MD, professor of medicine and medical education, Mayo Clinic, Rochester, Minn., agreed.
“Organizational strategies should include system-level interventions to reduce the risk of frightening, horrible, or traumatic events from occurring in the workplace in the first place, as well as faculty development efforts to upskill teaching faculty in their ability to support trainees when such events do occur,” she said.
These approaches “should coincide with organizational efforts to support individual trainees by providing adequate time off after traumatic events, ensuring trainees can access affordable mental healthcare, and reducing other barriers to appropriate help-seeking, such as stigma, and efforts to build a culture of well-being,” suggested Dr. Dyrbye, who is codirector of the Mayo Clinic Program on Physician Wellbeing and was not involved in the study.
The study was supported by grants from the Blue Cross Blue Shield Foundation of Michigan and National Institutes of Health. Dr. Vance and coauthors, Dr. Marcus, and Dr. Dyrbye reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Work-related posttraumatic stress disorder is three times higher in interns than the general population, new research shows.
Investigators assessed PTSD in more than 1,100 physicians at the end of their internship year and found that a little over half reported work-related trauma exposure, and of these, 20% screened positive for PTSD.
Overall, 10% of participants screened positive for PTSD by the end of the internship year, compared with a 12-month PTSD prevalence of 3.6% in the general population.
“Work-related trauma exposure and PTSD are common and underdiscussed phenomena among intern physicians,” lead author Mary Vance, MD, assistant professor of psychiatry, Uniformed Services University of the Health Sciences, Bethesda, Md., said in an interview.
“I urge medical educators and policy makers to include this topic in their discussions about physician well-being and to implement effective interventions to mitigate the impact of work-related trauma and PTSD among physician trainees,” she said.
The study was published online June 8 in JAMA Network Open.
Burnout, depression, suicide
“Burnout, depression, and suicide are increasingly recognized as occupational mental health hazards among health care professionals, including physicians,” Dr. Vance said.
“However, in my professional experience as a physician and educator, despite observing anecdotal evidence among my peers and trainees that this is also an issue,” she added.
This gap prompted her “to investigate rates of work-related trauma exposure and PTSD among physicians.”
The researchers sent emails to 4,350 individuals during academic year 2018-2019, 2 months prior to starting internships. Of these, 2,129 agreed to participate and 1,134 (58.6% female, 61.6% non-Hispanic White; mean age, 27.52) completed the study.
Prior to beginning internship, participants completed a baseline survey that assessed demographic characteristics as well as medical education and psychological and psychosocial factors.
Participants completed follow-up surveys sent by email at 3, 6, 9, and 12 months of the internship year. The surveys assessed stressful life events, concern over perceived medical errors in the past 3 months, and number of hours worked over the past week.
At month 12, current PTSD and symptoms of depression and anxiety were also assessed using the Primary Care PTSD Screen for DSM-5, the 9-item Patient Health Questionnaire, and the Generalized Anxiety Disorder 7-item scale, respectively.
Participants were asked to self-report whether they ever had an episode of depression and to complete the Risky Families Questionnaire to assess if they had experienced childhood abuse, neglect, and family conflict. Additionally, they completed an 11-item scale developed specifically for the study regarding recent stressful events.
‘Crucible’ year
A total of 56.4% of respondents reported work-related trauma exposure, and among these, 19.0% screened positive for PTSD. One-tenth (10.8%) of the entire sample screened positive for PTSD by the end of internship year, which is three times higher than the 12-month prevalence of PTSD in the general population (3.6%), the authors noted.
Trauma exposure differed by specialty, ranging from 43.1% in anesthesiology to 72.4% in emergency medicine. Of the respondents in internal medicine, surgery, and medicine/pediatrics, 56.6%, 63.3%, and 71%, respectively, reported work-related trauma exposure.
Work-related PTSD also differed by specialty, ranging from 7.5% in ob.gyn. to 30.0% in pediatrics. Of respondents in internal medicine and family practice, 23.9% and 25.9%, respectively, reported work-related PTSD.
Dr. Vance called the intern year “a crucible, during which newly minted doctors receive intensive on-the-job training at the front lines of patient care [and] work long hours in rapidly shifting environments, often caring for critically ill patients.”
Work-related trauma exposure “is more likely to occur during this high-stress internship year than during the same year in the general population,” she said.
She noted that the “issue of workplace trauma and PTSD among health care workers became even more salient during the height of COVID,” adding that she expects it “to remain a pressure issue for healthcare workers in the post-COVID era.”
Call to action
Commenting on the study David A. Marcus, MD, chair, GME Physician Well-Being Committee, Northwell Health, New Hyde Park, N.Y., noted the study’s “relatively low response rate” is a “significant limitation” of the study.
An additional limitation is the lack of a baseline PTSD assessment, said Dr. Marcus, an assistant professor at Hofstra University, Hempstead, N.Y., who was not involved in the research.
Nevertheless, the “overall prevalence [of work-related PTSD] should serve as a call to action for physician leaders and for leaders in academic medicine,” he said.
Additionally, the study “reminds us that trauma-informed care should be an essential part of mental health support services provided to trainees and to physicians in general,” Dr. Marcus stated.
Also commenting on the study, Lotte N. Dyrbye, MD, professor of medicine and medical education, Mayo Clinic, Rochester, Minn., agreed.
“Organizational strategies should include system-level interventions to reduce the risk of frightening, horrible, or traumatic events from occurring in the workplace in the first place, as well as faculty development efforts to upskill teaching faculty in their ability to support trainees when such events do occur,” she said.
These approaches “should coincide with organizational efforts to support individual trainees by providing adequate time off after traumatic events, ensuring trainees can access affordable mental healthcare, and reducing other barriers to appropriate help-seeking, such as stigma, and efforts to build a culture of well-being,” suggested Dr. Dyrbye, who is codirector of the Mayo Clinic Program on Physician Wellbeing and was not involved in the study.
The study was supported by grants from the Blue Cross Blue Shield Foundation of Michigan and National Institutes of Health. Dr. Vance and coauthors, Dr. Marcus, and Dr. Dyrbye reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cortical surface changes tied to risk for movement disorders in schizophrenia
Schizophrenia patients with parkinsonism show distinctive patterns of cortical surface markers, compared with schizophrenia patients without parkinsonism and healthy controls, results of a multimodal magnetic resonance imaging study suggest.
Sensorimotor abnormalities are common in schizophrenia patients, however, “the neurobiological mechanisms underlying parkinsonism in [schizophrenia], which in treated samples represents the unity of interplay between spontaneous and antipsychotic drug-exacerbated movement disorder, are poorly understood,” wrote Robert Christian Wolf, MD, of Heidelberg (Germany) University, and colleagues.
In a study published in Schizophrenia Research (2021 May;231:54-60), the investigators examined brain imaging findings from 20 healthy controls, 38 schizophrenia patients with parkinsonism (SZ-P), and 35 schizophrenia patients without parkinsonism (SZ-nonP). Dr. Wolf and colleagues examined three cortical surface markers: cortical thickness, complexity of cortical folding, and sulcus depth.
Compared with SZ-nonP patients, the SZ-P patients showed significantly increased complexity of cortical folding in the left supplementary motor cortex (SMC) and significantly decreased left postcentral sulcus (PCS) depth. In addition, left SMC activity was higher in both SZ-P and SZ-nonP patient groups, compared with controls.
In a regression analysis, the researchers examined relationships between parkinsonism severity and brain structure. They found that parkinsonism severity was negatively associated with left middle frontal complexity of cortical folding and left anterior cingulate cortex cortical thickness.
“Overall, the data support the notion that cortical features of distinct neurodevelopmental origin, particularly cortical folding indices such as [complexity of cortical folding] and sulcus depth, contribute to the pathogenesis of parkinsonism in SZ,” the researchers wrote.
The study findings were limited by several factors, including the cross-sectional design, the potential limitations of the Simpson-Angus Scale in characterizing parkinsonism, the inability to record lifetime antibiotics exposure in the patient population, and the inability to identify changes in brain stem nuclei, the researchers noted. However, the results were strengthened by the well-matched study groups and use of multimodal MRI, they said.
Consequently, “,” and suggest a link between abnormal neurodevelopmental processes and an increased risk for movement disorders in schizophrenia, they concluded.
The study was funded by the German Research Foundation and the German Federal Ministry of Education and Research. Dr. Wolf and colleagues disclosed no conflicts.
Schizophrenia patients with parkinsonism show distinctive patterns of cortical surface markers, compared with schizophrenia patients without parkinsonism and healthy controls, results of a multimodal magnetic resonance imaging study suggest.
Sensorimotor abnormalities are common in schizophrenia patients, however, “the neurobiological mechanisms underlying parkinsonism in [schizophrenia], which in treated samples represents the unity of interplay between spontaneous and antipsychotic drug-exacerbated movement disorder, are poorly understood,” wrote Robert Christian Wolf, MD, of Heidelberg (Germany) University, and colleagues.
In a study published in Schizophrenia Research (2021 May;231:54-60), the investigators examined brain imaging findings from 20 healthy controls, 38 schizophrenia patients with parkinsonism (SZ-P), and 35 schizophrenia patients without parkinsonism (SZ-nonP). Dr. Wolf and colleagues examined three cortical surface markers: cortical thickness, complexity of cortical folding, and sulcus depth.
Compared with SZ-nonP patients, the SZ-P patients showed significantly increased complexity of cortical folding in the left supplementary motor cortex (SMC) and significantly decreased left postcentral sulcus (PCS) depth. In addition, left SMC activity was higher in both SZ-P and SZ-nonP patient groups, compared with controls.
In a regression analysis, the researchers examined relationships between parkinsonism severity and brain structure. They found that parkinsonism severity was negatively associated with left middle frontal complexity of cortical folding and left anterior cingulate cortex cortical thickness.
“Overall, the data support the notion that cortical features of distinct neurodevelopmental origin, particularly cortical folding indices such as [complexity of cortical folding] and sulcus depth, contribute to the pathogenesis of parkinsonism in SZ,” the researchers wrote.
The study findings were limited by several factors, including the cross-sectional design, the potential limitations of the Simpson-Angus Scale in characterizing parkinsonism, the inability to record lifetime antibiotics exposure in the patient population, and the inability to identify changes in brain stem nuclei, the researchers noted. However, the results were strengthened by the well-matched study groups and use of multimodal MRI, they said.
Consequently, “,” and suggest a link between abnormal neurodevelopmental processes and an increased risk for movement disorders in schizophrenia, they concluded.
The study was funded by the German Research Foundation and the German Federal Ministry of Education and Research. Dr. Wolf and colleagues disclosed no conflicts.
Schizophrenia patients with parkinsonism show distinctive patterns of cortical surface markers, compared with schizophrenia patients without parkinsonism and healthy controls, results of a multimodal magnetic resonance imaging study suggest.
Sensorimotor abnormalities are common in schizophrenia patients, however, “the neurobiological mechanisms underlying parkinsonism in [schizophrenia], which in treated samples represents the unity of interplay between spontaneous and antipsychotic drug-exacerbated movement disorder, are poorly understood,” wrote Robert Christian Wolf, MD, of Heidelberg (Germany) University, and colleagues.
In a study published in Schizophrenia Research (2021 May;231:54-60), the investigators examined brain imaging findings from 20 healthy controls, 38 schizophrenia patients with parkinsonism (SZ-P), and 35 schizophrenia patients without parkinsonism (SZ-nonP). Dr. Wolf and colleagues examined three cortical surface markers: cortical thickness, complexity of cortical folding, and sulcus depth.
Compared with SZ-nonP patients, the SZ-P patients showed significantly increased complexity of cortical folding in the left supplementary motor cortex (SMC) and significantly decreased left postcentral sulcus (PCS) depth. In addition, left SMC activity was higher in both SZ-P and SZ-nonP patient groups, compared with controls.
In a regression analysis, the researchers examined relationships between parkinsonism severity and brain structure. They found that parkinsonism severity was negatively associated with left middle frontal complexity of cortical folding and left anterior cingulate cortex cortical thickness.
“Overall, the data support the notion that cortical features of distinct neurodevelopmental origin, particularly cortical folding indices such as [complexity of cortical folding] and sulcus depth, contribute to the pathogenesis of parkinsonism in SZ,” the researchers wrote.
The study findings were limited by several factors, including the cross-sectional design, the potential limitations of the Simpson-Angus Scale in characterizing parkinsonism, the inability to record lifetime antibiotics exposure in the patient population, and the inability to identify changes in brain stem nuclei, the researchers noted. However, the results were strengthened by the well-matched study groups and use of multimodal MRI, they said.
Consequently, “,” and suggest a link between abnormal neurodevelopmental processes and an increased risk for movement disorders in schizophrenia, they concluded.
The study was funded by the German Research Foundation and the German Federal Ministry of Education and Research. Dr. Wolf and colleagues disclosed no conflicts.
FROM SCHIZOPHRENIA RESEARCH










