User login
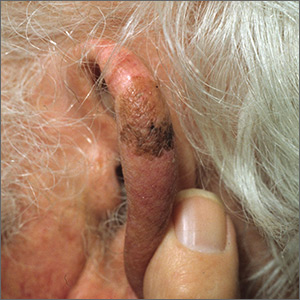
Brown spot on ear
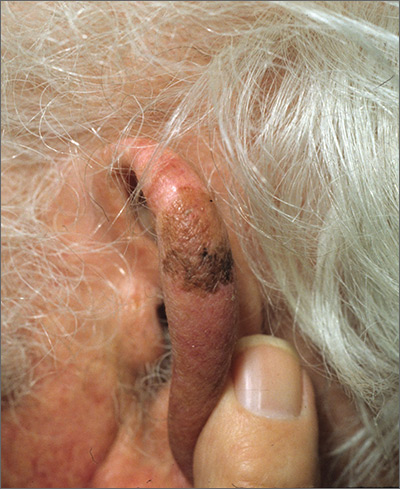
The FP explained to the patient that this could be a skin cancer—specifically, a melanoma.
The FP performed a broad shave biopsy, being careful not to cut into the cartilage. (See the Watch & Learn video on “Shave biopsy.”) The FP did his best to include most of the pigmented area involved, but the convex surface made it difficult to biopsy the whole lesion. He was especially careful to include the darker area because it looked most atypical. The diagnosis came back as lentigo maligna.
The patient was referred for Mohs surgery for complete excision and repair. (Mohs surgery is recommended to spare tissue and maximize cure.) After complete excision, the patient learned that the melanoma was not invasive, but in situ. This suggested a very good prognosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine, R. Lentigo maligna. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:981-984.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP explained to the patient that this could be a skin cancer—specifically, a melanoma.
The FP performed a broad shave biopsy, being careful not to cut into the cartilage. (See the Watch & Learn video on “Shave biopsy.”) The FP did his best to include most of the pigmented area involved, but the convex surface made it difficult to biopsy the whole lesion. He was especially careful to include the darker area because it looked most atypical. The diagnosis came back as lentigo maligna.
The patient was referred for Mohs surgery for complete excision and repair. (Mohs surgery is recommended to spare tissue and maximize cure.) After complete excision, the patient learned that the melanoma was not invasive, but in situ. This suggested a very good prognosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine, R. Lentigo maligna. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:981-984.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP explained to the patient that this could be a skin cancer—specifically, a melanoma.
The FP performed a broad shave biopsy, being careful not to cut into the cartilage. (See the Watch & Learn video on “Shave biopsy.”) The FP did his best to include most of the pigmented area involved, but the convex surface made it difficult to biopsy the whole lesion. He was especially careful to include the darker area because it looked most atypical. The diagnosis came back as lentigo maligna.
The patient was referred for Mohs surgery for complete excision and repair. (Mohs surgery is recommended to spare tissue and maximize cure.) After complete excision, the patient learned that the melanoma was not invasive, but in situ. This suggested a very good prognosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine, R. Lentigo maligna. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:981-984.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
October 2018
Allergic contact dermatitis (ACD) can affect individuals regardless of age, race, or sex, but ACD accounts for 20% of all contact dermatitis reactions. ACD results in an inflammatory reaction in those who have been previously sensitized to an allergen. This type of delayed hypersensitivity reaction is known as cell-mediated hypersensitivity. Generally, no reaction is elicited upon the first exposure to the allergen. In fact, it may take years of exposure to allergens for someone to develop an allergic contact dermatitis.
Once sensitized, epidermal antigen-presenting cells (APCs) called Langerhans cells process the allergen and present it in a complex on the surface of the cell to a CD4+ T cell. Subsequently, inflammatory cytokines and mediators are released, resulting in an allergic cutaneous (eczematous) reaction. Lesions may appear to be vesicular or bullous. Occasionally, a generalized eruption may occur. With repeated exposure, reactions may be acute or chronic.
Common causes of allergic contact dermatitis include toxicodendron plants (poison ivy, oak, and sumac; cashew nut tree; and mango), metals (nickel and gold), topical antibiotics (neomycin and bacitracin), fragrance and Balsam of Peru, deodorant, preservatives (formaldehyde), and rubber (elastic and gloves).
Patch testing is the standard means of detecting which allergen is causing the sensitization in an individual. The Thin-Layer Rapid Use Epicutaneous (TRUE) test or individually prepared aluminum (Finn) chambers containing the most common allergens are applied to the patient’s upper back. The patches are removed after 48 hours and read, and then reevaluated at day 4 or 5. Positive reactions appear as eczematous or vesicular papules or plaques.
Treatment includes avoidance of the allergens. Topical corticosteroid creams are helpful. For severe or generalized reactions, oral prednisone may be used. It is important to note that patient may be allergic to topical steroids. Patch testing can be performed to elucidate such allergens.
In contrast, 80% of contact dermatitis reactions are irritant, not allergic. Irritant contact dermatitis results is a local inflammatory reaction in people who have come into contact with a substance. Previous sensitization is not required. The reaction usually occurs immediately after exposure. Common causes include alkalis (detergents, soaps), acids (often found as an industrial work exposure), metals, solvents (occupational dermatitis), hydrocarbons, and chlorinated compounds.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
Allergic contact dermatitis (ACD) can affect individuals regardless of age, race, or sex, but ACD accounts for 20% of all contact dermatitis reactions. ACD results in an inflammatory reaction in those who have been previously sensitized to an allergen. This type of delayed hypersensitivity reaction is known as cell-mediated hypersensitivity. Generally, no reaction is elicited upon the first exposure to the allergen. In fact, it may take years of exposure to allergens for someone to develop an allergic contact dermatitis.
Once sensitized, epidermal antigen-presenting cells (APCs) called Langerhans cells process the allergen and present it in a complex on the surface of the cell to a CD4+ T cell. Subsequently, inflammatory cytokines and mediators are released, resulting in an allergic cutaneous (eczematous) reaction. Lesions may appear to be vesicular or bullous. Occasionally, a generalized eruption may occur. With repeated exposure, reactions may be acute or chronic.
Common causes of allergic contact dermatitis include toxicodendron plants (poison ivy, oak, and sumac; cashew nut tree; and mango), metals (nickel and gold), topical antibiotics (neomycin and bacitracin), fragrance and Balsam of Peru, deodorant, preservatives (formaldehyde), and rubber (elastic and gloves).
Patch testing is the standard means of detecting which allergen is causing the sensitization in an individual. The Thin-Layer Rapid Use Epicutaneous (TRUE) test or individually prepared aluminum (Finn) chambers containing the most common allergens are applied to the patient’s upper back. The patches are removed after 48 hours and read, and then reevaluated at day 4 or 5. Positive reactions appear as eczematous or vesicular papules or plaques.
Treatment includes avoidance of the allergens. Topical corticosteroid creams are helpful. For severe or generalized reactions, oral prednisone may be used. It is important to note that patient may be allergic to topical steroids. Patch testing can be performed to elucidate such allergens.
In contrast, 80% of contact dermatitis reactions are irritant, not allergic. Irritant contact dermatitis results is a local inflammatory reaction in people who have come into contact with a substance. Previous sensitization is not required. The reaction usually occurs immediately after exposure. Common causes include alkalis (detergents, soaps), acids (often found as an industrial work exposure), metals, solvents (occupational dermatitis), hydrocarbons, and chlorinated compounds.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
Allergic contact dermatitis (ACD) can affect individuals regardless of age, race, or sex, but ACD accounts for 20% of all contact dermatitis reactions. ACD results in an inflammatory reaction in those who have been previously sensitized to an allergen. This type of delayed hypersensitivity reaction is known as cell-mediated hypersensitivity. Generally, no reaction is elicited upon the first exposure to the allergen. In fact, it may take years of exposure to allergens for someone to develop an allergic contact dermatitis.
Once sensitized, epidermal antigen-presenting cells (APCs) called Langerhans cells process the allergen and present it in a complex on the surface of the cell to a CD4+ T cell. Subsequently, inflammatory cytokines and mediators are released, resulting in an allergic cutaneous (eczematous) reaction. Lesions may appear to be vesicular or bullous. Occasionally, a generalized eruption may occur. With repeated exposure, reactions may be acute or chronic.
Common causes of allergic contact dermatitis include toxicodendron plants (poison ivy, oak, and sumac; cashew nut tree; and mango), metals (nickel and gold), topical antibiotics (neomycin and bacitracin), fragrance and Balsam of Peru, deodorant, preservatives (formaldehyde), and rubber (elastic and gloves).
Patch testing is the standard means of detecting which allergen is causing the sensitization in an individual. The Thin-Layer Rapid Use Epicutaneous (TRUE) test or individually prepared aluminum (Finn) chambers containing the most common allergens are applied to the patient’s upper back. The patches are removed after 48 hours and read, and then reevaluated at day 4 or 5. Positive reactions appear as eczematous or vesicular papules or plaques.
Treatment includes avoidance of the allergens. Topical corticosteroid creams are helpful. For severe or generalized reactions, oral prednisone may be used. It is important to note that patient may be allergic to topical steroids. Patch testing can be performed to elucidate such allergens.
In contrast, 80% of contact dermatitis reactions are irritant, not allergic. Irritant contact dermatitis results is a local inflammatory reaction in people who have come into contact with a substance. Previous sensitization is not required. The reaction usually occurs immediately after exposure. Common causes include alkalis (detergents, soaps), acids (often found as an industrial work exposure), metals, solvents (occupational dermatitis), hydrocarbons, and chlorinated compounds.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
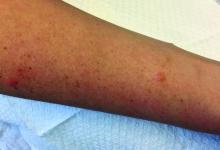
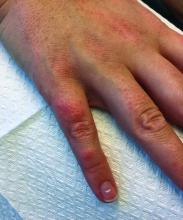
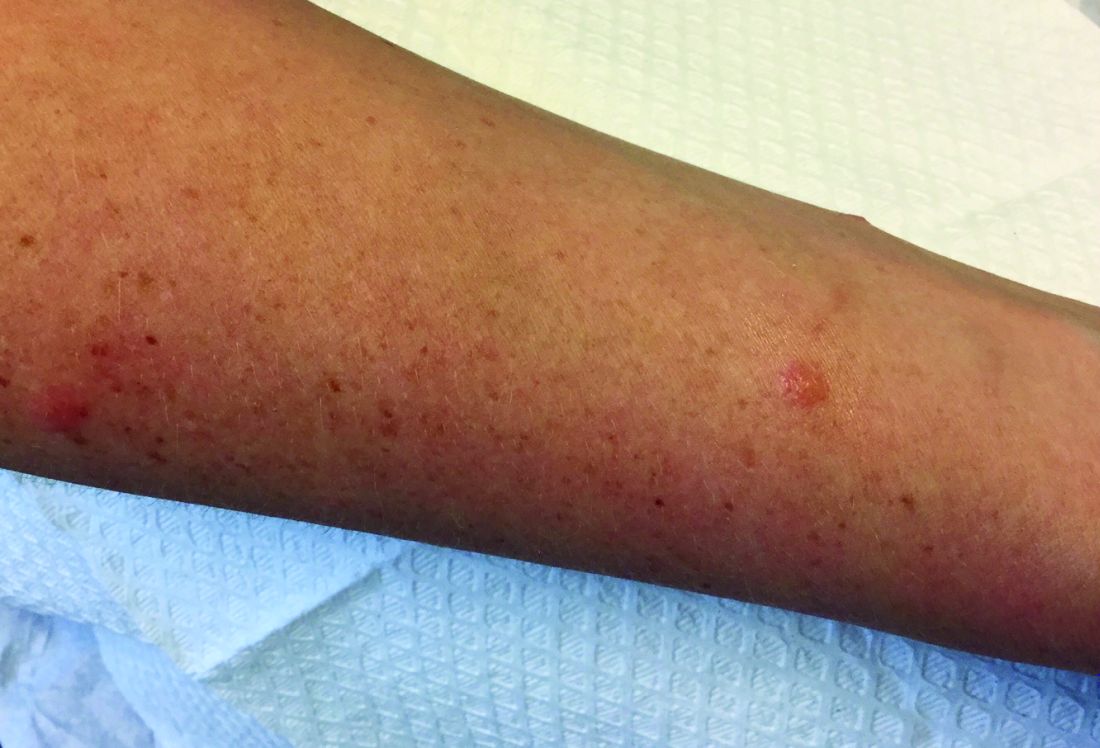
A 30-year-old female presented with 2 days of intensely pruritic erythematous papules and vesicles on her bilateral arms and hands. The lesions began appearing 1 day after a camping trip. Her neck, chest, and upper back were clear.
Eye Can’t See a Thing
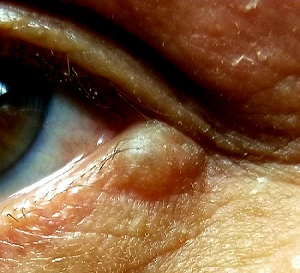
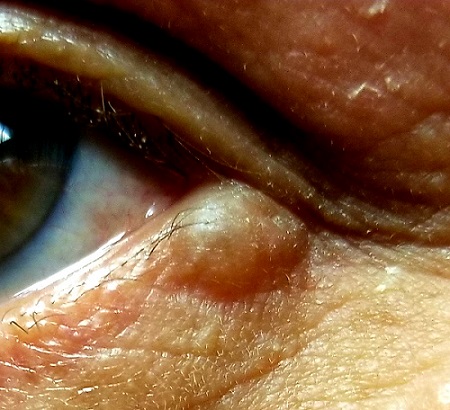
About 10 years ago, this 63-year-old man noticed a lesion on his eyelid. It didn’t bother him, so he ignored it—until recently, when it reached a size sufficient to interfere with his vision. This development, and subsequent commentary from friends concerned by its proximity to his eye and fears of cancer, disturbed him enough to seek evaluation.
He first consulted an ophthalmologist, who provided a diagnosis that the patient promptly forgot. However, he was also advised to see a dermatologist or plastic surgeon for further evaluation, since the lesion does not affect the eye itself. The patient wants the lesion removed but seeks a dermatology referral first.
He denies pain, discomfort, or trauma to the affected area.
EXAMINATION
A translucent, round, 7-mm cystic lesion is located on the left lateral lower eyelid just below the margin, resembling a bleb. No redness is seen in the area. Palpation confirms the soft, cystic nature of the lesion.
Examination of the other eye and the rest of the patient’s facial skin reveals no abnormalities.
What is the diagnosis?
DISCUSSION
This is a typical presentation of an apocrine hidrocystoma (AH), a benign lesion of uncertain etiology. The eyelid is rich in apocrine, eccrine, and sebaceous glands, all of which can transform into cysts via traumatic plugging.
AH is also known as cystadenoma, Moll gland cyst, or sudoriferous cyst. It is an entity distinct from chalazions (a granulomatous reaction to sebaceous glands in the eyelid) and lacrimal duct cysts. The differential includes basal cell carcinoma, intradermal nevus, and eccrine cyst.
In my experience, merely incising and draining the cyst is useless in the long run; while this does reduce swelling, it also invites recurrence. Therefore, removal by saucerization and cauterization of the base is the best treatment option.
TAKE-HOME LEARNING POINTS
- Apocrine hidrocystomas (AHs) are benign cysts derived from plugged apocrine sweat glands, which are found in numerous areas around the body, including the eyes.
- AHs are also known as cystadenomas, Moll gland cysts, or sudoriferous cysts.
- Though AHs are often found near the eye, they are not technically an eye problem—but they do have potential to obstruct the visual field.
- Removal is usually by saucerization, with cautery of the base for hemostasis and prevention of recurrence.
About 10 years ago, this 63-year-old man noticed a lesion on his eyelid. It didn’t bother him, so he ignored it—until recently, when it reached a size sufficient to interfere with his vision. This development, and subsequent commentary from friends concerned by its proximity to his eye and fears of cancer, disturbed him enough to seek evaluation.
He first consulted an ophthalmologist, who provided a diagnosis that the patient promptly forgot. However, he was also advised to see a dermatologist or plastic surgeon for further evaluation, since the lesion does not affect the eye itself. The patient wants the lesion removed but seeks a dermatology referral first.
He denies pain, discomfort, or trauma to the affected area.
EXAMINATION
A translucent, round, 7-mm cystic lesion is located on the left lateral lower eyelid just below the margin, resembling a bleb. No redness is seen in the area. Palpation confirms the soft, cystic nature of the lesion.

Examination of the other eye and the rest of the patient’s facial skin reveals no abnormalities.
What is the diagnosis?
DISCUSSION
This is a typical presentation of an apocrine hidrocystoma (AH), a benign lesion of uncertain etiology. The eyelid is rich in apocrine, eccrine, and sebaceous glands, all of which can transform into cysts via traumatic plugging.
AH is also known as cystadenoma, Moll gland cyst, or sudoriferous cyst. It is an entity distinct from chalazions (a granulomatous reaction to sebaceous glands in the eyelid) and lacrimal duct cysts. The differential includes basal cell carcinoma, intradermal nevus, and eccrine cyst.
In my experience, merely incising and draining the cyst is useless in the long run; while this does reduce swelling, it also invites recurrence. Therefore, removal by saucerization and cauterization of the base is the best treatment option.
TAKE-HOME LEARNING POINTS
- Apocrine hidrocystomas (AHs) are benign cysts derived from plugged apocrine sweat glands, which are found in numerous areas around the body, including the eyes.
- AHs are also known as cystadenomas, Moll gland cysts, or sudoriferous cysts.
- Though AHs are often found near the eye, they are not technically an eye problem—but they do have potential to obstruct the visual field.
- Removal is usually by saucerization, with cautery of the base for hemostasis and prevention of recurrence.
About 10 years ago, this 63-year-old man noticed a lesion on his eyelid. It didn’t bother him, so he ignored it—until recently, when it reached a size sufficient to interfere with his vision. This development, and subsequent commentary from friends concerned by its proximity to his eye and fears of cancer, disturbed him enough to seek evaluation.
He first consulted an ophthalmologist, who provided a diagnosis that the patient promptly forgot. However, he was also advised to see a dermatologist or plastic surgeon for further evaluation, since the lesion does not affect the eye itself. The patient wants the lesion removed but seeks a dermatology referral first.
He denies pain, discomfort, or trauma to the affected area.
EXAMINATION
A translucent, round, 7-mm cystic lesion is located on the left lateral lower eyelid just below the margin, resembling a bleb. No redness is seen in the area. Palpation confirms the soft, cystic nature of the lesion.

Examination of the other eye and the rest of the patient’s facial skin reveals no abnormalities.
What is the diagnosis?
DISCUSSION
This is a typical presentation of an apocrine hidrocystoma (AH), a benign lesion of uncertain etiology. The eyelid is rich in apocrine, eccrine, and sebaceous glands, all of which can transform into cysts via traumatic plugging.
AH is also known as cystadenoma, Moll gland cyst, or sudoriferous cyst. It is an entity distinct from chalazions (a granulomatous reaction to sebaceous glands in the eyelid) and lacrimal duct cysts. The differential includes basal cell carcinoma, intradermal nevus, and eccrine cyst.
In my experience, merely incising and draining the cyst is useless in the long run; while this does reduce swelling, it also invites recurrence. Therefore, removal by saucerization and cauterization of the base is the best treatment option.
TAKE-HOME LEARNING POINTS
- Apocrine hidrocystomas (AHs) are benign cysts derived from plugged apocrine sweat glands, which are found in numerous areas around the body, including the eyes.
- AHs are also known as cystadenomas, Moll gland cysts, or sudoriferous cysts.
- Though AHs are often found near the eye, they are not technically an eye problem—but they do have potential to obstruct the visual field.
- Removal is usually by saucerization, with cautery of the base for hemostasis and prevention of recurrence.
Cutaneous lesions? Consider C. diphtheriae in those with foreign travel
ATLANTA – Seven cases of imported Corynebacterium diphtheriae in Minnesota highlight the importance of maintaining suspicion that cutaneous lesions in individuals with recent travel to endemic countries might be associated with C. diphtheriae infection.
The cases also underscore the importance of referring C. diphtheriae isolates to state health departments for confirmatory testing, Jayne Griffith, of the Minnesota Department of Health, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“C. diphtheriae infections was not clinically suspected in any of these case-patients. All infections were initially identified solely by [matrix-assisted laser desorption/ionization time-of-flight spectrometry] testing performed at the clinical institutions,” the investigators wrote. “Confirmation and further toxigenicity testing allowed for prompt case investigation and public health response, preventing disease spread.”
Infections caused by toxigenic C. diphtheriae are rare in the United States because of widespread vaccination, but remain endemic in countries with suboptimal vaccine coverage. For this reason, infection is a concern for unvaccinated individuals traveling to diphtheria-endemic countries as well as for those who have contact with people from these areas. The investigators noted that “infections are primarily respiratory or cutaneous; respiratory infections can be life-threatening and cutaneous wounds may serve as a reservoir from which bacteria can be transmitted to susceptible contacts.”
The Minnesota cases involved patients who presented with cutaneous ulcers between 2014 and 2017. The Minnesota Department of Health confirmed C. diphtheriae status by culture after the initial identification at private institutions or providers using matrix-assisted laser desorption/ionization time-of-flight spectrometry. Isolates were sent to the Centers for Disease Control and Prevention Pertussis and Diphtheria Laboratory for biotyping and confirmation of toxigenicity.
The CDC confirmed that isolates from two patients were toxigenic C. diphtheriae biotype mitis. The remaining cases were nontoxigenic diphtheria, including C. diphtheriae mitis (three case-patients, including one who also had Staphylococcus aureus, and another who also had methicillin-resistant S. aureus) and two case-patients with C. diphtheriae belfanti.
The patients with toxigenic infection included a 35-year-old woman who developed an abdominal boil after spending months in Somalia, and a 48-year-old man who cut his leg while in Ethiopia.
The patients with nontoxigenic infection included four foreign-born individuals ranging in age from 7 to 66 years and one 24-year-old man from the United States. One of the foreign-born individuals had immigrated from a diphtheria-endemic country 3 months prior to his diagnosis, but none of the remaining four had traveled outside the United States in the 6 months prior to infection onset. One, however, lived in a home with family members who traveled frequently to eastern Africa. The vaccination status of these patients was unknown.
Contact tracing was conducted and prophylactic antibiotics were recommended as appropriate. Vaccination was recommended when a case-patient and/or contact was inadequately immunized.
Both patients with toxigenic infection experienced wound healing after appropriate antibiotic therapy.
Ms. Griffith reported having no disclosures.
ATLANTA – Seven cases of imported Corynebacterium diphtheriae in Minnesota highlight the importance of maintaining suspicion that cutaneous lesions in individuals with recent travel to endemic countries might be associated with C. diphtheriae infection.
The cases also underscore the importance of referring C. diphtheriae isolates to state health departments for confirmatory testing, Jayne Griffith, of the Minnesota Department of Health, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“C. diphtheriae infections was not clinically suspected in any of these case-patients. All infections were initially identified solely by [matrix-assisted laser desorption/ionization time-of-flight spectrometry] testing performed at the clinical institutions,” the investigators wrote. “Confirmation and further toxigenicity testing allowed for prompt case investigation and public health response, preventing disease spread.”
Infections caused by toxigenic C. diphtheriae are rare in the United States because of widespread vaccination, but remain endemic in countries with suboptimal vaccine coverage. For this reason, infection is a concern for unvaccinated individuals traveling to diphtheria-endemic countries as well as for those who have contact with people from these areas. The investigators noted that “infections are primarily respiratory or cutaneous; respiratory infections can be life-threatening and cutaneous wounds may serve as a reservoir from which bacteria can be transmitted to susceptible contacts.”
The Minnesota cases involved patients who presented with cutaneous ulcers between 2014 and 2017. The Minnesota Department of Health confirmed C. diphtheriae status by culture after the initial identification at private institutions or providers using matrix-assisted laser desorption/ionization time-of-flight spectrometry. Isolates were sent to the Centers for Disease Control and Prevention Pertussis and Diphtheria Laboratory for biotyping and confirmation of toxigenicity.
The CDC confirmed that isolates from two patients were toxigenic C. diphtheriae biotype mitis. The remaining cases were nontoxigenic diphtheria, including C. diphtheriae mitis (three case-patients, including one who also had Staphylococcus aureus, and another who also had methicillin-resistant S. aureus) and two case-patients with C. diphtheriae belfanti.
The patients with toxigenic infection included a 35-year-old woman who developed an abdominal boil after spending months in Somalia, and a 48-year-old man who cut his leg while in Ethiopia.
The patients with nontoxigenic infection included four foreign-born individuals ranging in age from 7 to 66 years and one 24-year-old man from the United States. One of the foreign-born individuals had immigrated from a diphtheria-endemic country 3 months prior to his diagnosis, but none of the remaining four had traveled outside the United States in the 6 months prior to infection onset. One, however, lived in a home with family members who traveled frequently to eastern Africa. The vaccination status of these patients was unknown.
Contact tracing was conducted and prophylactic antibiotics were recommended as appropriate. Vaccination was recommended when a case-patient and/or contact was inadequately immunized.
Both patients with toxigenic infection experienced wound healing after appropriate antibiotic therapy.
Ms. Griffith reported having no disclosures.
ATLANTA – Seven cases of imported Corynebacterium diphtheriae in Minnesota highlight the importance of maintaining suspicion that cutaneous lesions in individuals with recent travel to endemic countries might be associated with C. diphtheriae infection.
The cases also underscore the importance of referring C. diphtheriae isolates to state health departments for confirmatory testing, Jayne Griffith, of the Minnesota Department of Health, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“C. diphtheriae infections was not clinically suspected in any of these case-patients. All infections were initially identified solely by [matrix-assisted laser desorption/ionization time-of-flight spectrometry] testing performed at the clinical institutions,” the investigators wrote. “Confirmation and further toxigenicity testing allowed for prompt case investigation and public health response, preventing disease spread.”
Infections caused by toxigenic C. diphtheriae are rare in the United States because of widespread vaccination, but remain endemic in countries with suboptimal vaccine coverage. For this reason, infection is a concern for unvaccinated individuals traveling to diphtheria-endemic countries as well as for those who have contact with people from these areas. The investigators noted that “infections are primarily respiratory or cutaneous; respiratory infections can be life-threatening and cutaneous wounds may serve as a reservoir from which bacteria can be transmitted to susceptible contacts.”
The Minnesota cases involved patients who presented with cutaneous ulcers between 2014 and 2017. The Minnesota Department of Health confirmed C. diphtheriae status by culture after the initial identification at private institutions or providers using matrix-assisted laser desorption/ionization time-of-flight spectrometry. Isolates were sent to the Centers for Disease Control and Prevention Pertussis and Diphtheria Laboratory for biotyping and confirmation of toxigenicity.
The CDC confirmed that isolates from two patients were toxigenic C. diphtheriae biotype mitis. The remaining cases were nontoxigenic diphtheria, including C. diphtheriae mitis (three case-patients, including one who also had Staphylococcus aureus, and another who also had methicillin-resistant S. aureus) and two case-patients with C. diphtheriae belfanti.
The patients with toxigenic infection included a 35-year-old woman who developed an abdominal boil after spending months in Somalia, and a 48-year-old man who cut his leg while in Ethiopia.
The patients with nontoxigenic infection included four foreign-born individuals ranging in age from 7 to 66 years and one 24-year-old man from the United States. One of the foreign-born individuals had immigrated from a diphtheria-endemic country 3 months prior to his diagnosis, but none of the remaining four had traveled outside the United States in the 6 months prior to infection onset. One, however, lived in a home with family members who traveled frequently to eastern Africa. The vaccination status of these patients was unknown.
Contact tracing was conducted and prophylactic antibiotics were recommended as appropriate. Vaccination was recommended when a case-patient and/or contact was inadequately immunized.
Both patients with toxigenic infection experienced wound healing after appropriate antibiotic therapy.
Ms. Griffith reported having no disclosures.
REPORTING FROM ICEID 2018
Key clinical point: Corynebacterium diphtheriae should be considered in individuals who present with cutaneous lesions after traveling to diphtheria-endemic countries.
Major finding: Refer suspect C. diphtheriae isolates to state health departments.
Study details: A review of seven C. diphtheriae cases.
Disclosures: Ms. Griffith reported having no disclosures.
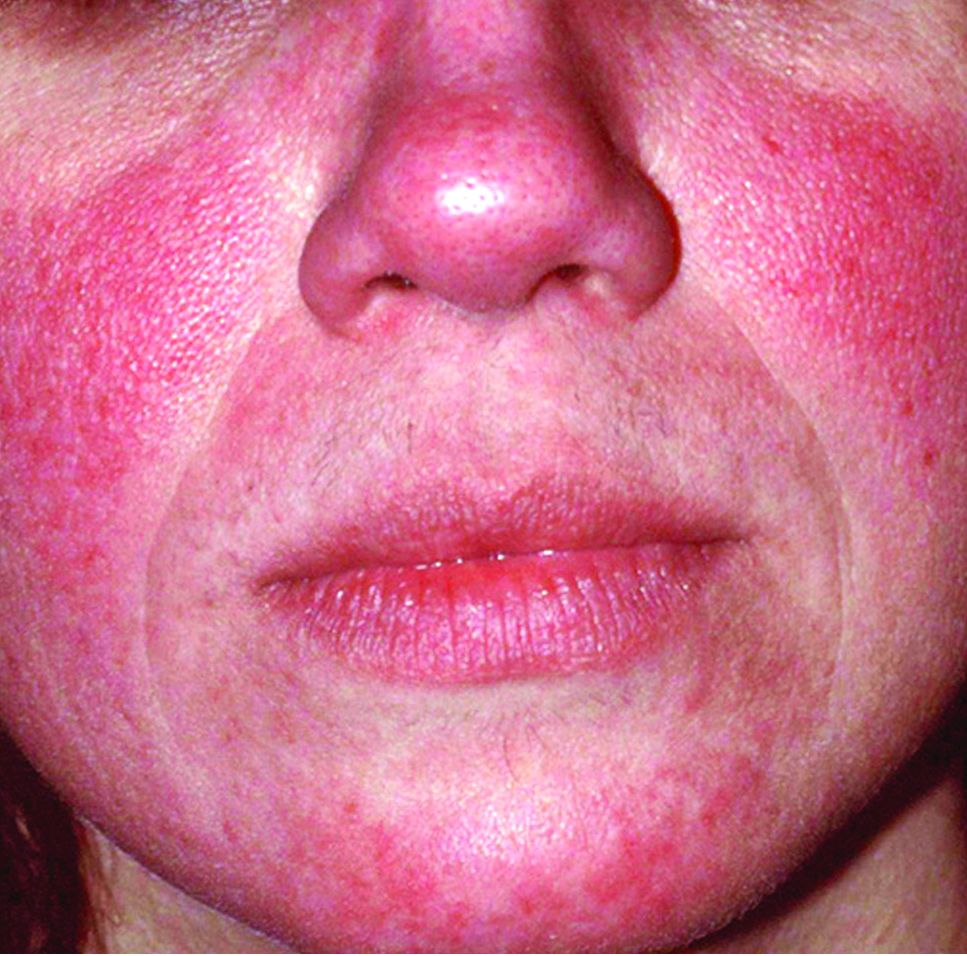
Global incidence of rosacea estimated to be over 5%
The global incidence of rosacea among adults is approximately 5%, based on data from a meta-analysis and systematic review.
The National Rosacea Society Expert Committee recently updated its phenotype-based classification system, but the global prevalence and incidence of rosacea remain unknown, wrote Lise Gether, MD, of the University of Copenhagen, Denmark, and her colleagues.
In a review published in the British Journal of Dermatology, the researchers examined the prevalence of rosacea among dermatology patients and in the general population worldwide by analyzing 32 studies including 41 populations and 26,538,319 individuals. The study populations comprised 22 from Europe, 9 from North America, 4 from Asia, 3 from South America, and 3 from Africa. Of the 32 studies, 18 included the general population and 14 included only dermatology patients.
Overall, the pooled proportion of individuals with rosacea was 5.46% in the general population and 2.39% among dermatology patients. Of note, the pooled proportions varied when the studies were grouped by diagnostic method: self-diagnosis (9.7%), physician diagnosis (5.5%), and health care database estimate (1.05%).
Rosacea prevalence by age was highest among individuals aged 45-60 years, but “based on the available data, it was not possible to make useful stratified estimates,” the researchers said.
The researchers estimated rosacea prevalence by gender using data from the 10 studies that reported numbers from both genders. Based on a population of 5,601,642 women and 3,529,559 men, the pooled proportions were similar; 5.4% for women and 3.9% for men.
“The methods used to study the prevalence of rosacea are of great importance as misclassification may be a concern,” the researchers noted. Individuals with mild to moderate rosacea might not seek medical treatment, which might contribute to the low prevalence from health care database estimates. Conversely, the high prevalence with self-reports might suggest a lack of specificity in the questionnaires.
There were no external funding sources. Dr. Gether had no relevant financial disclosures. Coauthors disclosed relationships with companies including Galderma, Pfizer, Eli Lilly, Novartis, and Janssen.
SOURCE: Gether L et al. Br J Dermatol. 2018. doi: 10.1111/bjd.16481
The global incidence of rosacea among adults is approximately 5%, based on data from a meta-analysis and systematic review.
The National Rosacea Society Expert Committee recently updated its phenotype-based classification system, but the global prevalence and incidence of rosacea remain unknown, wrote Lise Gether, MD, of the University of Copenhagen, Denmark, and her colleagues.
In a review published in the British Journal of Dermatology, the researchers examined the prevalence of rosacea among dermatology patients and in the general population worldwide by analyzing 32 studies including 41 populations and 26,538,319 individuals. The study populations comprised 22 from Europe, 9 from North America, 4 from Asia, 3 from South America, and 3 from Africa. Of the 32 studies, 18 included the general population and 14 included only dermatology patients.
Overall, the pooled proportion of individuals with rosacea was 5.46% in the general population and 2.39% among dermatology patients. Of note, the pooled proportions varied when the studies were grouped by diagnostic method: self-diagnosis (9.7%), physician diagnosis (5.5%), and health care database estimate (1.05%).
Rosacea prevalence by age was highest among individuals aged 45-60 years, but “based on the available data, it was not possible to make useful stratified estimates,” the researchers said.
The researchers estimated rosacea prevalence by gender using data from the 10 studies that reported numbers from both genders. Based on a population of 5,601,642 women and 3,529,559 men, the pooled proportions were similar; 5.4% for women and 3.9% for men.
“The methods used to study the prevalence of rosacea are of great importance as misclassification may be a concern,” the researchers noted. Individuals with mild to moderate rosacea might not seek medical treatment, which might contribute to the low prevalence from health care database estimates. Conversely, the high prevalence with self-reports might suggest a lack of specificity in the questionnaires.
There were no external funding sources. Dr. Gether had no relevant financial disclosures. Coauthors disclosed relationships with companies including Galderma, Pfizer, Eli Lilly, Novartis, and Janssen.
SOURCE: Gether L et al. Br J Dermatol. 2018. doi: 10.1111/bjd.16481
The global incidence of rosacea among adults is approximately 5%, based on data from a meta-analysis and systematic review.
The National Rosacea Society Expert Committee recently updated its phenotype-based classification system, but the global prevalence and incidence of rosacea remain unknown, wrote Lise Gether, MD, of the University of Copenhagen, Denmark, and her colleagues.
In a review published in the British Journal of Dermatology, the researchers examined the prevalence of rosacea among dermatology patients and in the general population worldwide by analyzing 32 studies including 41 populations and 26,538,319 individuals. The study populations comprised 22 from Europe, 9 from North America, 4 from Asia, 3 from South America, and 3 from Africa. Of the 32 studies, 18 included the general population and 14 included only dermatology patients.
Overall, the pooled proportion of individuals with rosacea was 5.46% in the general population and 2.39% among dermatology patients. Of note, the pooled proportions varied when the studies were grouped by diagnostic method: self-diagnosis (9.7%), physician diagnosis (5.5%), and health care database estimate (1.05%).
Rosacea prevalence by age was highest among individuals aged 45-60 years, but “based on the available data, it was not possible to make useful stratified estimates,” the researchers said.
The researchers estimated rosacea prevalence by gender using data from the 10 studies that reported numbers from both genders. Based on a population of 5,601,642 women and 3,529,559 men, the pooled proportions were similar; 5.4% for women and 3.9% for men.
“The methods used to study the prevalence of rosacea are of great importance as misclassification may be a concern,” the researchers noted. Individuals with mild to moderate rosacea might not seek medical treatment, which might contribute to the low prevalence from health care database estimates. Conversely, the high prevalence with self-reports might suggest a lack of specificity in the questionnaires.
There were no external funding sources. Dr. Gether had no relevant financial disclosures. Coauthors disclosed relationships with companies including Galderma, Pfizer, Eli Lilly, Novartis, and Janssen.
SOURCE: Gether L et al. Br J Dermatol. 2018. doi: 10.1111/bjd.16481
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: Rosacea occurs worldwide in both men and women, but diagnosis remains inconsistent.
Major finding:
Study details: A meta-analysis of 32 studies and 26,538,319 individuals.
Disclosures: There were no external funding sources. Dr. Gether had no relevant financial disclosures. Coauthors disclosed relationships with companies including Galderma, Pfizer, Eli Lilly, Novartis, and Janssen.
Source: Gether L et al. Br J Dermatol. 2018. doi: 10.1111/bjd.16481.
Certain skin conditions signal potential overgrowth disorder
LAKE TAHOE, CALIF. – and during human development, Leslie G. Biesecker, MD said at the annual meeting of the Society for Pediatric Dermatology.
Dr. Biesecker, senior investigator and head of the clinical genomics section of the National Human Genome Research Institute’s Medical Genomics and Metabolic Genetics Branch, discussed mosaicism and a number of overgrowth syndromes that he and his associates have been studying that have clinical relevance for pediatric dermatologists. He noted that mosaicism can affect any tissue, anywhere, in any pattern. “If an affected cell cannot survive gametogenesis, fertilization, or survive early development, this generates Happle-type mosaicism,” explained Dr. Biesecker, who is trained in pediatrics and in clinical and molecular genetics.
“This is characterized by patchy manifestations, and no parent-to-child transmission or recurrence. You must always be careful here, though, because Mother Nature does what she wants to. Mosaic mutations can happen more than once, but it’s a very unlikely outcome. Happle-type mosaicism is also characterized by discordant monozygotic twins,” he noted.
The prototype for Happle-type mosaicism is Proteus syndrome, formerly known as Elephant Man disease, which is caused by a mutation in the AKT1 gene. Patients with Proteus syndrome undergo severe, relentless overgrowth, and about 25% of them die during childhood. “If you see one of these patients, you have a serious clinical problem on your hands,” he said. “There is enormous individual variability, but it is ultra rare.”
Dermatologic lesions that are characteristic of Proteus syndrome include cerebriform connective tissue nevus, which typically presents on the hands and feet. “A wide range of vascular malformations have also been associated with this, even patients with arteriovenous malformations,” Dr. Biesecker said. “They are a serious problem.” Linear verrucous epidermal nevus is another characteristic lesion of Proteus syndrome. It can present in a number of ways and in various body sites. “The natural history of these lesions is important,” he commented. “Over time, are they stable, or do they spread and expand over time? These lesions do not ever spontaneously regress. This does enable molecular diagnosis, but don’t bother sampling their blood, because it will be negative. You have to have a biopsy sample.”
Overgrowth syndromes that do not meet criteria for Proteus syndrome fall into a category known as PIK3CA-related overgrowth spectrum, which Dr. Biesecker characterized as “a bunch of clinical designations all caused by the same underlying somatic mutation in a gene called PIK3CA. There is an enormous variability in these patients, ranging from those who have profound overgrowth, including malformations, truncal overgrowth, and vascular malformations, and digital overgrowth in all sorts of patterns. We designate this as PIK3CA-related overgrowth spectrum (PROS), because we can’t clinically separate these things from one another.”
These conditions include what used to be called CLOVES syndrome (congenital lipomatous overgrowth, vascular malformations, epidermal nevi, and scoliosis/skeletal/spinal anomalies), facial infiltrating lipomatosis, and megalencephaly-capillary malformation syndrome. PROS is about 100 times more common than Proteus syndrome. “There are no rational boundaries to distinguish these entities,” Dr. Biesecker said. “They are rationalized under a combined clinical-molecular PROS framework, meaning that the molecular diagnosis is absolutely key to correctly diagnosing these patients.”
In this way, mosaicism challenges the traditional concept of diagnosing overgrowth disorders. “What we thought were separate disorders are in fact many manifestations of a single disorder,” he continued. “When I was doing my genetics training, we were taught that it would turn out that there was one gene for every disease, and one disease for every gene. That is completely wrong; it’s much more complicated than that. Mosaicism is also important for us as biologists, because it gives us a window into biology we otherwise would not see. Without a mosaicism, Proteus syndrome cannot exist biologically. So if I want to understand that gene product, I have to study patients who are mosaics. Mosaicism can happen in any tissue, whether it’s visible or not.”
Dr. Biesecker, who has been elected to serve as president of the American Society of Human Genetics for 2019, noted that most of the gene mutations that cause overgrowth disorders are the same ones implicated in cancer. “It makes sense, because cancer is a disorder of uncontrolled proliferation and differentiation,” he said. “These overgrowth disorders are similar but less severe manifestations of the same problem. It turns out that these mosaic patients are single gene model systems of cancer biology.” Therefore, when a drug company develops an anti-cancer drug, he continued, it also can be useful for PROS or Proteus syndrome. It’s much easier to inhibit a protein that’s overactive than it is to replace the activity of a gene that has lost its function.
But in PROS and Proteus, “we have very different treatment objectives than oncologists do,” he said. “Our goal is to reduce the signaling caused by these mutations; we do not want to kill the cells. Some of my patients with these disorders have pretty close to 50% of cells in their body carrying these mutations. If I were thinking like an oncologist, the oncologist wants to kill cancer cells; that’s their objective. If I were to kill all of the mutant cells in my patients, I’m certain that would kill them.”
One promising development is the investigational oral agent ARQ 092, which is an inhibitor of AKT1. Dr. Biesecker and his colleagues at the NIH have been working to figure out what dosing should be used in humans based on mouse data, lab data, and data from cancer patients. They started with about one-twelvth the dose that oncologists use. After treating the first patient with overgrowth syndrome, on day 15 that person’s AKT1 level dropped to about 20% of normal, while on day 75 it moved to around 60% of normal. “We are right in that zone where we want to drive the activity of that protein to about half of what it should be,” Dr. Biesecker said. He and his colleagues also have observed regression of lesions in a patient with cerebriform connective tissue nevus who was treated with ARQ 092. “We’ve never seen this before.”
Dr. Biesecker disclosed that he is a member of the Illumina medical ethics board. He has received royalties from Genentech and in-kind research support from ArQule and Pfizer.
[email protected]
LAKE TAHOE, CALIF. – and during human development, Leslie G. Biesecker, MD said at the annual meeting of the Society for Pediatric Dermatology.
Dr. Biesecker, senior investigator and head of the clinical genomics section of the National Human Genome Research Institute’s Medical Genomics and Metabolic Genetics Branch, discussed mosaicism and a number of overgrowth syndromes that he and his associates have been studying that have clinical relevance for pediatric dermatologists. He noted that mosaicism can affect any tissue, anywhere, in any pattern. “If an affected cell cannot survive gametogenesis, fertilization, or survive early development, this generates Happle-type mosaicism,” explained Dr. Biesecker, who is trained in pediatrics and in clinical and molecular genetics.
“This is characterized by patchy manifestations, and no parent-to-child transmission or recurrence. You must always be careful here, though, because Mother Nature does what she wants to. Mosaic mutations can happen more than once, but it’s a very unlikely outcome. Happle-type mosaicism is also characterized by discordant monozygotic twins,” he noted.
The prototype for Happle-type mosaicism is Proteus syndrome, formerly known as Elephant Man disease, which is caused by a mutation in the AKT1 gene. Patients with Proteus syndrome undergo severe, relentless overgrowth, and about 25% of them die during childhood. “If you see one of these patients, you have a serious clinical problem on your hands,” he said. “There is enormous individual variability, but it is ultra rare.”
Dermatologic lesions that are characteristic of Proteus syndrome include cerebriform connective tissue nevus, which typically presents on the hands and feet. “A wide range of vascular malformations have also been associated with this, even patients with arteriovenous malformations,” Dr. Biesecker said. “They are a serious problem.” Linear verrucous epidermal nevus is another characteristic lesion of Proteus syndrome. It can present in a number of ways and in various body sites. “The natural history of these lesions is important,” he commented. “Over time, are they stable, or do they spread and expand over time? These lesions do not ever spontaneously regress. This does enable molecular diagnosis, but don’t bother sampling their blood, because it will be negative. You have to have a biopsy sample.”
Overgrowth syndromes that do not meet criteria for Proteus syndrome fall into a category known as PIK3CA-related overgrowth spectrum, which Dr. Biesecker characterized as “a bunch of clinical designations all caused by the same underlying somatic mutation in a gene called PIK3CA. There is an enormous variability in these patients, ranging from those who have profound overgrowth, including malformations, truncal overgrowth, and vascular malformations, and digital overgrowth in all sorts of patterns. We designate this as PIK3CA-related overgrowth spectrum (PROS), because we can’t clinically separate these things from one another.”
These conditions include what used to be called CLOVES syndrome (congenital lipomatous overgrowth, vascular malformations, epidermal nevi, and scoliosis/skeletal/spinal anomalies), facial infiltrating lipomatosis, and megalencephaly-capillary malformation syndrome. PROS is about 100 times more common than Proteus syndrome. “There are no rational boundaries to distinguish these entities,” Dr. Biesecker said. “They are rationalized under a combined clinical-molecular PROS framework, meaning that the molecular diagnosis is absolutely key to correctly diagnosing these patients.”
In this way, mosaicism challenges the traditional concept of diagnosing overgrowth disorders. “What we thought were separate disorders are in fact many manifestations of a single disorder,” he continued. “When I was doing my genetics training, we were taught that it would turn out that there was one gene for every disease, and one disease for every gene. That is completely wrong; it’s much more complicated than that. Mosaicism is also important for us as biologists, because it gives us a window into biology we otherwise would not see. Without a mosaicism, Proteus syndrome cannot exist biologically. So if I want to understand that gene product, I have to study patients who are mosaics. Mosaicism can happen in any tissue, whether it’s visible or not.”
Dr. Biesecker, who has been elected to serve as president of the American Society of Human Genetics for 2019, noted that most of the gene mutations that cause overgrowth disorders are the same ones implicated in cancer. “It makes sense, because cancer is a disorder of uncontrolled proliferation and differentiation,” he said. “These overgrowth disorders are similar but less severe manifestations of the same problem. It turns out that these mosaic patients are single gene model systems of cancer biology.” Therefore, when a drug company develops an anti-cancer drug, he continued, it also can be useful for PROS or Proteus syndrome. It’s much easier to inhibit a protein that’s overactive than it is to replace the activity of a gene that has lost its function.
But in PROS and Proteus, “we have very different treatment objectives than oncologists do,” he said. “Our goal is to reduce the signaling caused by these mutations; we do not want to kill the cells. Some of my patients with these disorders have pretty close to 50% of cells in their body carrying these mutations. If I were thinking like an oncologist, the oncologist wants to kill cancer cells; that’s their objective. If I were to kill all of the mutant cells in my patients, I’m certain that would kill them.”
One promising development is the investigational oral agent ARQ 092, which is an inhibitor of AKT1. Dr. Biesecker and his colleagues at the NIH have been working to figure out what dosing should be used in humans based on mouse data, lab data, and data from cancer patients. They started with about one-twelvth the dose that oncologists use. After treating the first patient with overgrowth syndrome, on day 15 that person’s AKT1 level dropped to about 20% of normal, while on day 75 it moved to around 60% of normal. “We are right in that zone where we want to drive the activity of that protein to about half of what it should be,” Dr. Biesecker said. He and his colleagues also have observed regression of lesions in a patient with cerebriform connective tissue nevus who was treated with ARQ 092. “We’ve never seen this before.”
Dr. Biesecker disclosed that he is a member of the Illumina medical ethics board. He has received royalties from Genentech and in-kind research support from ArQule and Pfizer.
[email protected]
LAKE TAHOE, CALIF. – and during human development, Leslie G. Biesecker, MD said at the annual meeting of the Society for Pediatric Dermatology.
Dr. Biesecker, senior investigator and head of the clinical genomics section of the National Human Genome Research Institute’s Medical Genomics and Metabolic Genetics Branch, discussed mosaicism and a number of overgrowth syndromes that he and his associates have been studying that have clinical relevance for pediatric dermatologists. He noted that mosaicism can affect any tissue, anywhere, in any pattern. “If an affected cell cannot survive gametogenesis, fertilization, or survive early development, this generates Happle-type mosaicism,” explained Dr. Biesecker, who is trained in pediatrics and in clinical and molecular genetics.
“This is characterized by patchy manifestations, and no parent-to-child transmission or recurrence. You must always be careful here, though, because Mother Nature does what she wants to. Mosaic mutations can happen more than once, but it’s a very unlikely outcome. Happle-type mosaicism is also characterized by discordant monozygotic twins,” he noted.
The prototype for Happle-type mosaicism is Proteus syndrome, formerly known as Elephant Man disease, which is caused by a mutation in the AKT1 gene. Patients with Proteus syndrome undergo severe, relentless overgrowth, and about 25% of them die during childhood. “If you see one of these patients, you have a serious clinical problem on your hands,” he said. “There is enormous individual variability, but it is ultra rare.”
Dermatologic lesions that are characteristic of Proteus syndrome include cerebriform connective tissue nevus, which typically presents on the hands and feet. “A wide range of vascular malformations have also been associated with this, even patients with arteriovenous malformations,” Dr. Biesecker said. “They are a serious problem.” Linear verrucous epidermal nevus is another characteristic lesion of Proteus syndrome. It can present in a number of ways and in various body sites. “The natural history of these lesions is important,” he commented. “Over time, are they stable, or do they spread and expand over time? These lesions do not ever spontaneously regress. This does enable molecular diagnosis, but don’t bother sampling their blood, because it will be negative. You have to have a biopsy sample.”
Overgrowth syndromes that do not meet criteria for Proteus syndrome fall into a category known as PIK3CA-related overgrowth spectrum, which Dr. Biesecker characterized as “a bunch of clinical designations all caused by the same underlying somatic mutation in a gene called PIK3CA. There is an enormous variability in these patients, ranging from those who have profound overgrowth, including malformations, truncal overgrowth, and vascular malformations, and digital overgrowth in all sorts of patterns. We designate this as PIK3CA-related overgrowth spectrum (PROS), because we can’t clinically separate these things from one another.”
These conditions include what used to be called CLOVES syndrome (congenital lipomatous overgrowth, vascular malformations, epidermal nevi, and scoliosis/skeletal/spinal anomalies), facial infiltrating lipomatosis, and megalencephaly-capillary malformation syndrome. PROS is about 100 times more common than Proteus syndrome. “There are no rational boundaries to distinguish these entities,” Dr. Biesecker said. “They are rationalized under a combined clinical-molecular PROS framework, meaning that the molecular diagnosis is absolutely key to correctly diagnosing these patients.”
In this way, mosaicism challenges the traditional concept of diagnosing overgrowth disorders. “What we thought were separate disorders are in fact many manifestations of a single disorder,” he continued. “When I was doing my genetics training, we were taught that it would turn out that there was one gene for every disease, and one disease for every gene. That is completely wrong; it’s much more complicated than that. Mosaicism is also important for us as biologists, because it gives us a window into biology we otherwise would not see. Without a mosaicism, Proteus syndrome cannot exist biologically. So if I want to understand that gene product, I have to study patients who are mosaics. Mosaicism can happen in any tissue, whether it’s visible or not.”
Dr. Biesecker, who has been elected to serve as president of the American Society of Human Genetics for 2019, noted that most of the gene mutations that cause overgrowth disorders are the same ones implicated in cancer. “It makes sense, because cancer is a disorder of uncontrolled proliferation and differentiation,” he said. “These overgrowth disorders are similar but less severe manifestations of the same problem. It turns out that these mosaic patients are single gene model systems of cancer biology.” Therefore, when a drug company develops an anti-cancer drug, he continued, it also can be useful for PROS or Proteus syndrome. It’s much easier to inhibit a protein that’s overactive than it is to replace the activity of a gene that has lost its function.
But in PROS and Proteus, “we have very different treatment objectives than oncologists do,” he said. “Our goal is to reduce the signaling caused by these mutations; we do not want to kill the cells. Some of my patients with these disorders have pretty close to 50% of cells in their body carrying these mutations. If I were thinking like an oncologist, the oncologist wants to kill cancer cells; that’s their objective. If I were to kill all of the mutant cells in my patients, I’m certain that would kill them.”
One promising development is the investigational oral agent ARQ 092, which is an inhibitor of AKT1. Dr. Biesecker and his colleagues at the NIH have been working to figure out what dosing should be used in humans based on mouse data, lab data, and data from cancer patients. They started with about one-twelvth the dose that oncologists use. After treating the first patient with overgrowth syndrome, on day 15 that person’s AKT1 level dropped to about 20% of normal, while on day 75 it moved to around 60% of normal. “We are right in that zone where we want to drive the activity of that protein to about half of what it should be,” Dr. Biesecker said. He and his colleagues also have observed regression of lesions in a patient with cerebriform connective tissue nevus who was treated with ARQ 092. “We’ve never seen this before.”
Dr. Biesecker disclosed that he is a member of the Illumina medical ethics board. He has received royalties from Genentech and in-kind research support from ArQule and Pfizer.
[email protected]
EXPERT ANALYSIS FROM THE SPD ANNUAL MEETING
JAK inhibitors emerge as promising alopecia treatment
LAKE TAHOE, CALIF. – After Brett King, MD, PhD, and his wife and collaborator, Brittany G. Craiglow, MD, published an index case of oral tofacitinib reversing alopecia universalis in a 25-year-old male patient back in 2014 (J Invest Dermatol. 2014;134:2988-90), they received hundreds of e-mails and phone calls from clinicians and patients.
“We also received quite a bit of media attention from around the world,” Dr. King recalled at the annual meeting of the Society for Pediatric Dermatology.
After all, alopecia areata and its variants affect 1%-2% of the population and have a marked impact on health-related quality of life, with high rates of concomitant generalized anxiety disorder and major depressive disorder. “The health-related quality of life is similar to that of atopic dermatitis and psoriasis, and there are no reliably effective therapies, especially for severe disease,” he said. “”
Currently available Janus kinase (JAK) inhibitors include tofacitinib (Xeljanz), ruxolitinib (Jakafi), and baricitinib (Olumiant). “These medicines are not [Food and Drug Administration] approved for alopecia areata, though tofacitinib was recently approved for psoriatic arthritis, and so we have formal entry of this medicine into dermatology for the first time,” said Dr. King, who is a dermatologist at Yale University, New Haven, Conn.
Potential adverse effects of JAKs include nasopharyngitis, headache, diarrhea, elevated cholesterol, uncommonly herpes zoster, cytopenias, transaminitis, and rarely non-melanoma skin cancer, solid organ malignancy and lymphoma, and GI perforation. Tofacitinib has an FDA black box warning regarding serious infections and malignancies, and baricitinib has these plus an additional warning about thrombosis.
In an open label, two-center trial that followed the index patient report, Dr. King and his associates enrolled 66 patients aged 19-35 years who had greater than 50% scalp hair loss for at least 6 months to receive tofacitinib 5 mg twice daily for 3 months (JCI Insight. 2016; 1[15]:e89776). A primary outcome of interest was regrowth of hair as measured by the percent change in Severity of Alopecia Tool (SALT) score. A SALT score of 100 indicates baldness, while a score of zero indicates no hair loss. Following 3 months of treatment, 32% of patients had a greater than 50% change in their SALT score, 32% had a change in the range of 5%-50%, while 36% had a change that was less than 5%.
“One of the interesting findings was that long duration of current episode of complete scalp hair loss was a negative predictor of treatment response, especially for those who have had hair loss greater than 10 years,” Dr. King said. Adverse events were “pretty bland,” with the most common being upper respiratory infection (17%), headache (8%), abdominal pain (8%), and acne (8%). Weight gain occurred in 1.5% of patients.
Next, Dr. King and colleagues reviewed the records of 90 patients aged 18 years or older who were treated with tofacitinib for at least 4 months (J Am Acad Dermatol. 2017;76[1]:22-8). Patients had greater than 40% scalp hair loss, and the tofacitinib dose was up to 10 mg per day at the discretion of the physician. “About 43% of patients were treated with tofacitinib 5 mg” twice daily, Dr. King said. “Other patients had higher doses or the addition of prednisone for three doses to see if that would help.”
After treatment, 20% of patients had a greater than 90% change in their SALT score (complete scalp hair regrowth), while 38.4% had a change that ranged from 51%-90%. At the same time, 18% had a change in their SALT score that ranged from 6%-50%, while 23% had a change that was 5% or less. As observed in the earlier trial, researchers saw a negative correlation between duration of current episode of hair loss and latest percent change in SALT score.
“We believe that you have to catch people before they get to more than 10 years of complete scalp hair loss,” Dr. King said. “This is important for the pediatric age group. I just saw somebody who’s 13, and they’ve been bald for 8 years. You might make the argument that this person deserves treatment, at least for a period of time long enough to regrow their hair in order to reset the clock.”
The most common adverse events were acne and weight gain.
In a separate analysis, Dr. King, Dr. Craiglow, and Lucy Y. Liu, evaluated the use of tofacitinib for at least 2 months in 13 alopecia areata patients aged 12-17 years (J Am Acad Dermatol. 2017;76[1]:29-32). They limited the analysis to those who had greater than 20% scalp hair loss, alopecia totalis, or alopecia universalis that was stable or worsening for 6 months or longer. Of the 13 patients, 9 (69%) were responders. Of the four non-responders, one had a very long duration of baldness. The percent change in SALT score was 93% overall, including 100% in the responder group over a median of 5 months and just 1% in the non-responder group over a median of 4 months. “This does not work every time,” Dr. King said.
While some preliminary studies of topical JAK inhibitors for alopecia areata show promise, it remains unclear if this approach will translate in a clinically meaningful way, he said. Clinical trials are currently under way.
Dr. King disclosed that he has received honoraria or consulting fees from Aclaris Therapeutics, Celgene, Concert Pharmaceuticals, Eli Lilly, Pfizer, Regeneron Pharmaceuticals, and Dermavant Sciences. He has also received funding support from The Ranjini and Ajay Poddar Resource Fund for Dermatologic Diseases Research.
[email protected]
LAKE TAHOE, CALIF. – After Brett King, MD, PhD, and his wife and collaborator, Brittany G. Craiglow, MD, published an index case of oral tofacitinib reversing alopecia universalis in a 25-year-old male patient back in 2014 (J Invest Dermatol. 2014;134:2988-90), they received hundreds of e-mails and phone calls from clinicians and patients.
“We also received quite a bit of media attention from around the world,” Dr. King recalled at the annual meeting of the Society for Pediatric Dermatology.
After all, alopecia areata and its variants affect 1%-2% of the population and have a marked impact on health-related quality of life, with high rates of concomitant generalized anxiety disorder and major depressive disorder. “The health-related quality of life is similar to that of atopic dermatitis and psoriasis, and there are no reliably effective therapies, especially for severe disease,” he said. “”
Currently available Janus kinase (JAK) inhibitors include tofacitinib (Xeljanz), ruxolitinib (Jakafi), and baricitinib (Olumiant). “These medicines are not [Food and Drug Administration] approved for alopecia areata, though tofacitinib was recently approved for psoriatic arthritis, and so we have formal entry of this medicine into dermatology for the first time,” said Dr. King, who is a dermatologist at Yale University, New Haven, Conn.
Potential adverse effects of JAKs include nasopharyngitis, headache, diarrhea, elevated cholesterol, uncommonly herpes zoster, cytopenias, transaminitis, and rarely non-melanoma skin cancer, solid organ malignancy and lymphoma, and GI perforation. Tofacitinib has an FDA black box warning regarding serious infections and malignancies, and baricitinib has these plus an additional warning about thrombosis.
In an open label, two-center trial that followed the index patient report, Dr. King and his associates enrolled 66 patients aged 19-35 years who had greater than 50% scalp hair loss for at least 6 months to receive tofacitinib 5 mg twice daily for 3 months (JCI Insight. 2016; 1[15]:e89776). A primary outcome of interest was regrowth of hair as measured by the percent change in Severity of Alopecia Tool (SALT) score. A SALT score of 100 indicates baldness, while a score of zero indicates no hair loss. Following 3 months of treatment, 32% of patients had a greater than 50% change in their SALT score, 32% had a change in the range of 5%-50%, while 36% had a change that was less than 5%.
“One of the interesting findings was that long duration of current episode of complete scalp hair loss was a negative predictor of treatment response, especially for those who have had hair loss greater than 10 years,” Dr. King said. Adverse events were “pretty bland,” with the most common being upper respiratory infection (17%), headache (8%), abdominal pain (8%), and acne (8%). Weight gain occurred in 1.5% of patients.
Next, Dr. King and colleagues reviewed the records of 90 patients aged 18 years or older who were treated with tofacitinib for at least 4 months (J Am Acad Dermatol. 2017;76[1]:22-8). Patients had greater than 40% scalp hair loss, and the tofacitinib dose was up to 10 mg per day at the discretion of the physician. “About 43% of patients were treated with tofacitinib 5 mg” twice daily, Dr. King said. “Other patients had higher doses or the addition of prednisone for three doses to see if that would help.”
After treatment, 20% of patients had a greater than 90% change in their SALT score (complete scalp hair regrowth), while 38.4% had a change that ranged from 51%-90%. At the same time, 18% had a change in their SALT score that ranged from 6%-50%, while 23% had a change that was 5% or less. As observed in the earlier trial, researchers saw a negative correlation between duration of current episode of hair loss and latest percent change in SALT score.
“We believe that you have to catch people before they get to more than 10 years of complete scalp hair loss,” Dr. King said. “This is important for the pediatric age group. I just saw somebody who’s 13, and they’ve been bald for 8 years. You might make the argument that this person deserves treatment, at least for a period of time long enough to regrow their hair in order to reset the clock.”
The most common adverse events were acne and weight gain.
In a separate analysis, Dr. King, Dr. Craiglow, and Lucy Y. Liu, evaluated the use of tofacitinib for at least 2 months in 13 alopecia areata patients aged 12-17 years (J Am Acad Dermatol. 2017;76[1]:29-32). They limited the analysis to those who had greater than 20% scalp hair loss, alopecia totalis, or alopecia universalis that was stable or worsening for 6 months or longer. Of the 13 patients, 9 (69%) were responders. Of the four non-responders, one had a very long duration of baldness. The percent change in SALT score was 93% overall, including 100% in the responder group over a median of 5 months and just 1% in the non-responder group over a median of 4 months. “This does not work every time,” Dr. King said.
While some preliminary studies of topical JAK inhibitors for alopecia areata show promise, it remains unclear if this approach will translate in a clinically meaningful way, he said. Clinical trials are currently under way.
Dr. King disclosed that he has received honoraria or consulting fees from Aclaris Therapeutics, Celgene, Concert Pharmaceuticals, Eli Lilly, Pfizer, Regeneron Pharmaceuticals, and Dermavant Sciences. He has also received funding support from The Ranjini and Ajay Poddar Resource Fund for Dermatologic Diseases Research.
[email protected]
LAKE TAHOE, CALIF. – After Brett King, MD, PhD, and his wife and collaborator, Brittany G. Craiglow, MD, published an index case of oral tofacitinib reversing alopecia universalis in a 25-year-old male patient back in 2014 (J Invest Dermatol. 2014;134:2988-90), they received hundreds of e-mails and phone calls from clinicians and patients.
“We also received quite a bit of media attention from around the world,” Dr. King recalled at the annual meeting of the Society for Pediatric Dermatology.
After all, alopecia areata and its variants affect 1%-2% of the population and have a marked impact on health-related quality of life, with high rates of concomitant generalized anxiety disorder and major depressive disorder. “The health-related quality of life is similar to that of atopic dermatitis and psoriasis, and there are no reliably effective therapies, especially for severe disease,” he said. “”
Currently available Janus kinase (JAK) inhibitors include tofacitinib (Xeljanz), ruxolitinib (Jakafi), and baricitinib (Olumiant). “These medicines are not [Food and Drug Administration] approved for alopecia areata, though tofacitinib was recently approved for psoriatic arthritis, and so we have formal entry of this medicine into dermatology for the first time,” said Dr. King, who is a dermatologist at Yale University, New Haven, Conn.
Potential adverse effects of JAKs include nasopharyngitis, headache, diarrhea, elevated cholesterol, uncommonly herpes zoster, cytopenias, transaminitis, and rarely non-melanoma skin cancer, solid organ malignancy and lymphoma, and GI perforation. Tofacitinib has an FDA black box warning regarding serious infections and malignancies, and baricitinib has these plus an additional warning about thrombosis.
In an open label, two-center trial that followed the index patient report, Dr. King and his associates enrolled 66 patients aged 19-35 years who had greater than 50% scalp hair loss for at least 6 months to receive tofacitinib 5 mg twice daily for 3 months (JCI Insight. 2016; 1[15]:e89776). A primary outcome of interest was regrowth of hair as measured by the percent change in Severity of Alopecia Tool (SALT) score. A SALT score of 100 indicates baldness, while a score of zero indicates no hair loss. Following 3 months of treatment, 32% of patients had a greater than 50% change in their SALT score, 32% had a change in the range of 5%-50%, while 36% had a change that was less than 5%.
“One of the interesting findings was that long duration of current episode of complete scalp hair loss was a negative predictor of treatment response, especially for those who have had hair loss greater than 10 years,” Dr. King said. Adverse events were “pretty bland,” with the most common being upper respiratory infection (17%), headache (8%), abdominal pain (8%), and acne (8%). Weight gain occurred in 1.5% of patients.
Next, Dr. King and colleagues reviewed the records of 90 patients aged 18 years or older who were treated with tofacitinib for at least 4 months (J Am Acad Dermatol. 2017;76[1]:22-8). Patients had greater than 40% scalp hair loss, and the tofacitinib dose was up to 10 mg per day at the discretion of the physician. “About 43% of patients were treated with tofacitinib 5 mg” twice daily, Dr. King said. “Other patients had higher doses or the addition of prednisone for three doses to see if that would help.”
After treatment, 20% of patients had a greater than 90% change in their SALT score (complete scalp hair regrowth), while 38.4% had a change that ranged from 51%-90%. At the same time, 18% had a change in their SALT score that ranged from 6%-50%, while 23% had a change that was 5% or less. As observed in the earlier trial, researchers saw a negative correlation between duration of current episode of hair loss and latest percent change in SALT score.
“We believe that you have to catch people before they get to more than 10 years of complete scalp hair loss,” Dr. King said. “This is important for the pediatric age group. I just saw somebody who’s 13, and they’ve been bald for 8 years. You might make the argument that this person deserves treatment, at least for a period of time long enough to regrow their hair in order to reset the clock.”
The most common adverse events were acne and weight gain.
In a separate analysis, Dr. King, Dr. Craiglow, and Lucy Y. Liu, evaluated the use of tofacitinib for at least 2 months in 13 alopecia areata patients aged 12-17 years (J Am Acad Dermatol. 2017;76[1]:29-32). They limited the analysis to those who had greater than 20% scalp hair loss, alopecia totalis, or alopecia universalis that was stable or worsening for 6 months or longer. Of the 13 patients, 9 (69%) were responders. Of the four non-responders, one had a very long duration of baldness. The percent change in SALT score was 93% overall, including 100% in the responder group over a median of 5 months and just 1% in the non-responder group over a median of 4 months. “This does not work every time,” Dr. King said.
While some preliminary studies of topical JAK inhibitors for alopecia areata show promise, it remains unclear if this approach will translate in a clinically meaningful way, he said. Clinical trials are currently under way.
Dr. King disclosed that he has received honoraria or consulting fees from Aclaris Therapeutics, Celgene, Concert Pharmaceuticals, Eli Lilly, Pfizer, Regeneron Pharmaceuticals, and Dermavant Sciences. He has also received funding support from The Ranjini and Ajay Poddar Resource Fund for Dermatologic Diseases Research.
[email protected]
EXPERT ANALYSIS FROM THE SPD ANNUAL MEETING
Poor gut health tied to increased systemic disease risk
LAKE TAHOE, CALIF. – according to Mark A. Underwood, MD.
“Antibiotic exposure changes the composition of the intestinal microbiota,” he said at the annual meeting of the Society for Pediatric Dermatology. “That clearly causes both antibiotic-associated diarrhea and Clostridium difficile colitis. The bigger question is, is it possible that intestinal dysbiosis is related to a whole bunch of other systemic diseases? In other words, does an insult during a critical window of development cause changes in the intestinal microbiota that can lead to systemic diseases in the brain, the lung, the liver, or the immune system?”
According Dr. Underwood, a pediatrician who is chief of the division of neonatology at the University of California, Davis, the prevalence of dysbiosis is increasing worldwide, particularly in developed countries, where Bifidobacteria are decreasing and Enterobacteriaceae are increasing. “Those changes are associated with gut permeability and alterations in both local and systemic inflammation, and the risk for a number of diseases, including atopic dermatitis,” he said. Key reasons for the increasing prevalence of dysbiosis, he continued, include the use of antibiotics, cesarean section delivery, formula feeding, changes in hygiene that alter the intestinal biota, the high-fat, high-sugar Western diet, and a loss of vertical and horizontal transmission over generations.
In an effort to evaluate the association between early childhood antibiotic use with allergic diseases in later childhood, Japanese researchers followed 1,200 infants to the age of 5 years (Ann Allergy Asthma Immunol. 2017;119:54-8). They found that antibiotic exposure within the first 2 years of life was associated with an increased risk of asthma (adjusted odds ratio 1.72), allergic rhinitis (adjusted OR 1.65), and atopic dermatitis (adjusted OR 1.40). In a more recent, smaller prospective study, 436 Dutch infants were followed to 1 year of age (Pediatr Allergy Immunol. 2018;29[2]:151-8). The researchers found that antibiotic exposure within the first week of life was associated with allergic sensitization (adjusted OR 3.26), colic (adjusted OR 1.66), and wheezing (adjusted OR 1.56).
In a study of 44 term infants with a family history of allergy, changes in the fecal microbiota, especially colonization with Ruminococcus gnavus, preceded the onset of allergic symptoms (Gastroenterol. 2018;154[1]:154-67). The same researchers observed similar findings in an animal model.
One potential mechanism by which intestinal dysbiosis causes systemic disease is stimulation of toll-like receptor 4 (TLR4), “which is a receptor on a variety of mucosal cells that senses the presence of a microbial pathogen-associated patterns, particularly those of Gram-negative Enterobacteriaceae,” Dr. Underwood explained. Other potential mechanisms include increased intestinal permeability, an increase in the pH and decreases in short-chain fatty acids within the gut lumen, and a loss of intraluminal hypoxia. “Think of the colon as an anaerobic chamber,” he said. “The colon lumen should be very low in oxygen. It should be dominated by obligate anaerobes.”
Efforts to prevent or treat dysbiosis-related diseases include the use of probiotics and fecal transplantation. A recent Cochrane review of 8,672 patients found “moderate certainty evidence” that probiotics are effective for preventing C. difficile-associated diarrhea. The analysis included 31 randomized, controlled trials of adults who were treated either with a probiotic or with a placebo. When pooled, the risk ratio was 0.40, which represented a significant protection. In addition, a summary of 7 randomized controlled trials and 30 case series suggests that fecal microbial transplantation is superior to vancomycin for adults with recurrent C. difficile colitis (relative risk 0.23) (Aliment Pharmacol Ther. 2017;46[5]:470-93).
To date, the effect of giving probiotics to pregnant women who have a family history of allergy is less clear. One pooled analysis of such studies put the overall risk ratio at 0.74 (Mil Med. 2014;179[6]:580-92). “While I think the jury’s still out on how to best prevent atopic dermatitis in these families, it looks like there is some potential benefit in treating these moms during pregnancy with probiotics and treating the infant during the first few months of life,” Dr. Underwood said. The most effective were mixtures including one or more Lactobacillus species or L. rhamnosus, mixtures including one or more Bifidobacterium species, or B. lactis by itself. The use of probiotics also has been found to prevent necrotizing enterocolitis, sepsis, and death in premature infants (Semin Pediatr Surg. 2018;27[1]:39-46).
Dr. Underwood disclosed that he has received honoraria from Abbott and that he was a member of the scientific review board for Avexegen. He also chaired the data safety and monitoring board for Infant Bacterial Therapeutics and has received support from Evolve BioSystems to perform a clinical trial.
[email protected]
LAKE TAHOE, CALIF. – according to Mark A. Underwood, MD.
“Antibiotic exposure changes the composition of the intestinal microbiota,” he said at the annual meeting of the Society for Pediatric Dermatology. “That clearly causes both antibiotic-associated diarrhea and Clostridium difficile colitis. The bigger question is, is it possible that intestinal dysbiosis is related to a whole bunch of other systemic diseases? In other words, does an insult during a critical window of development cause changes in the intestinal microbiota that can lead to systemic diseases in the brain, the lung, the liver, or the immune system?”
According Dr. Underwood, a pediatrician who is chief of the division of neonatology at the University of California, Davis, the prevalence of dysbiosis is increasing worldwide, particularly in developed countries, where Bifidobacteria are decreasing and Enterobacteriaceae are increasing. “Those changes are associated with gut permeability and alterations in both local and systemic inflammation, and the risk for a number of diseases, including atopic dermatitis,” he said. Key reasons for the increasing prevalence of dysbiosis, he continued, include the use of antibiotics, cesarean section delivery, formula feeding, changes in hygiene that alter the intestinal biota, the high-fat, high-sugar Western diet, and a loss of vertical and horizontal transmission over generations.
In an effort to evaluate the association between early childhood antibiotic use with allergic diseases in later childhood, Japanese researchers followed 1,200 infants to the age of 5 years (Ann Allergy Asthma Immunol. 2017;119:54-8). They found that antibiotic exposure within the first 2 years of life was associated with an increased risk of asthma (adjusted odds ratio 1.72), allergic rhinitis (adjusted OR 1.65), and atopic dermatitis (adjusted OR 1.40). In a more recent, smaller prospective study, 436 Dutch infants were followed to 1 year of age (Pediatr Allergy Immunol. 2018;29[2]:151-8). The researchers found that antibiotic exposure within the first week of life was associated with allergic sensitization (adjusted OR 3.26), colic (adjusted OR 1.66), and wheezing (adjusted OR 1.56).
In a study of 44 term infants with a family history of allergy, changes in the fecal microbiota, especially colonization with Ruminococcus gnavus, preceded the onset of allergic symptoms (Gastroenterol. 2018;154[1]:154-67). The same researchers observed similar findings in an animal model.
One potential mechanism by which intestinal dysbiosis causes systemic disease is stimulation of toll-like receptor 4 (TLR4), “which is a receptor on a variety of mucosal cells that senses the presence of a microbial pathogen-associated patterns, particularly those of Gram-negative Enterobacteriaceae,” Dr. Underwood explained. Other potential mechanisms include increased intestinal permeability, an increase in the pH and decreases in short-chain fatty acids within the gut lumen, and a loss of intraluminal hypoxia. “Think of the colon as an anaerobic chamber,” he said. “The colon lumen should be very low in oxygen. It should be dominated by obligate anaerobes.”
Efforts to prevent or treat dysbiosis-related diseases include the use of probiotics and fecal transplantation. A recent Cochrane review of 8,672 patients found “moderate certainty evidence” that probiotics are effective for preventing C. difficile-associated diarrhea. The analysis included 31 randomized, controlled trials of adults who were treated either with a probiotic or with a placebo. When pooled, the risk ratio was 0.40, which represented a significant protection. In addition, a summary of 7 randomized controlled trials and 30 case series suggests that fecal microbial transplantation is superior to vancomycin for adults with recurrent C. difficile colitis (relative risk 0.23) (Aliment Pharmacol Ther. 2017;46[5]:470-93).
To date, the effect of giving probiotics to pregnant women who have a family history of allergy is less clear. One pooled analysis of such studies put the overall risk ratio at 0.74 (Mil Med. 2014;179[6]:580-92). “While I think the jury’s still out on how to best prevent atopic dermatitis in these families, it looks like there is some potential benefit in treating these moms during pregnancy with probiotics and treating the infant during the first few months of life,” Dr. Underwood said. The most effective were mixtures including one or more Lactobacillus species or L. rhamnosus, mixtures including one or more Bifidobacterium species, or B. lactis by itself. The use of probiotics also has been found to prevent necrotizing enterocolitis, sepsis, and death in premature infants (Semin Pediatr Surg. 2018;27[1]:39-46).
Dr. Underwood disclosed that he has received honoraria from Abbott and that he was a member of the scientific review board for Avexegen. He also chaired the data safety and monitoring board for Infant Bacterial Therapeutics and has received support from Evolve BioSystems to perform a clinical trial.
[email protected]
LAKE TAHOE, CALIF. – according to Mark A. Underwood, MD.
“Antibiotic exposure changes the composition of the intestinal microbiota,” he said at the annual meeting of the Society for Pediatric Dermatology. “That clearly causes both antibiotic-associated diarrhea and Clostridium difficile colitis. The bigger question is, is it possible that intestinal dysbiosis is related to a whole bunch of other systemic diseases? In other words, does an insult during a critical window of development cause changes in the intestinal microbiota that can lead to systemic diseases in the brain, the lung, the liver, or the immune system?”
According Dr. Underwood, a pediatrician who is chief of the division of neonatology at the University of California, Davis, the prevalence of dysbiosis is increasing worldwide, particularly in developed countries, where Bifidobacteria are decreasing and Enterobacteriaceae are increasing. “Those changes are associated with gut permeability and alterations in both local and systemic inflammation, and the risk for a number of diseases, including atopic dermatitis,” he said. Key reasons for the increasing prevalence of dysbiosis, he continued, include the use of antibiotics, cesarean section delivery, formula feeding, changes in hygiene that alter the intestinal biota, the high-fat, high-sugar Western diet, and a loss of vertical and horizontal transmission over generations.
In an effort to evaluate the association between early childhood antibiotic use with allergic diseases in later childhood, Japanese researchers followed 1,200 infants to the age of 5 years (Ann Allergy Asthma Immunol. 2017;119:54-8). They found that antibiotic exposure within the first 2 years of life was associated with an increased risk of asthma (adjusted odds ratio 1.72), allergic rhinitis (adjusted OR 1.65), and atopic dermatitis (adjusted OR 1.40). In a more recent, smaller prospective study, 436 Dutch infants were followed to 1 year of age (Pediatr Allergy Immunol. 2018;29[2]:151-8). The researchers found that antibiotic exposure within the first week of life was associated with allergic sensitization (adjusted OR 3.26), colic (adjusted OR 1.66), and wheezing (adjusted OR 1.56).
In a study of 44 term infants with a family history of allergy, changes in the fecal microbiota, especially colonization with Ruminococcus gnavus, preceded the onset of allergic symptoms (Gastroenterol. 2018;154[1]:154-67). The same researchers observed similar findings in an animal model.
One potential mechanism by which intestinal dysbiosis causes systemic disease is stimulation of toll-like receptor 4 (TLR4), “which is a receptor on a variety of mucosal cells that senses the presence of a microbial pathogen-associated patterns, particularly those of Gram-negative Enterobacteriaceae,” Dr. Underwood explained. Other potential mechanisms include increased intestinal permeability, an increase in the pH and decreases in short-chain fatty acids within the gut lumen, and a loss of intraluminal hypoxia. “Think of the colon as an anaerobic chamber,” he said. “The colon lumen should be very low in oxygen. It should be dominated by obligate anaerobes.”
Efforts to prevent or treat dysbiosis-related diseases include the use of probiotics and fecal transplantation. A recent Cochrane review of 8,672 patients found “moderate certainty evidence” that probiotics are effective for preventing C. difficile-associated diarrhea. The analysis included 31 randomized, controlled trials of adults who were treated either with a probiotic or with a placebo. When pooled, the risk ratio was 0.40, which represented a significant protection. In addition, a summary of 7 randomized controlled trials and 30 case series suggests that fecal microbial transplantation is superior to vancomycin for adults with recurrent C. difficile colitis (relative risk 0.23) (Aliment Pharmacol Ther. 2017;46[5]:470-93).
To date, the effect of giving probiotics to pregnant women who have a family history of allergy is less clear. One pooled analysis of such studies put the overall risk ratio at 0.74 (Mil Med. 2014;179[6]:580-92). “While I think the jury’s still out on how to best prevent atopic dermatitis in these families, it looks like there is some potential benefit in treating these moms during pregnancy with probiotics and treating the infant during the first few months of life,” Dr. Underwood said. The most effective were mixtures including one or more Lactobacillus species or L. rhamnosus, mixtures including one or more Bifidobacterium species, or B. lactis by itself. The use of probiotics also has been found to prevent necrotizing enterocolitis, sepsis, and death in premature infants (Semin Pediatr Surg. 2018;27[1]:39-46).
Dr. Underwood disclosed that he has received honoraria from Abbott and that he was a member of the scientific review board for Avexegen. He also chaired the data safety and monitoring board for Infant Bacterial Therapeutics and has received support from Evolve BioSystems to perform a clinical trial.
[email protected]
EXPERT ANALYSIS FROM THE SPD ANNUAL MEETING
So it’s pediatric onychomycosis. Now what?
CHICAGO – Though research shows that nail fungus occurs in just 0.3% of pediatric patients in the United States, that’s not what Sheila Friedlander, MD, is seeing in her southern California practice, where it’s not uncommon to see children whose nails, toe nails in particular, have fungal involvement.
said Dr. Friedlander during a nail-focused session at the annual summer meeting of the American Academy of Dermatology. Dr. Friedlander, professor of dermatology and pediatrics at the University of California San Diego and Rady Children’s Hospital, said that she suspects that more participation in organized sports at a young age may be contributing to the increase, with occlusive sports footwear replacing bare feet or sandals for more hours of the day, presenting more opportunities for toenail trauma in sports such as soccer.
When making the clinical call about a nail problem, bear in mind that the younger the child, the less likely a nail problem is fungal, Dr. Friedlander noted. “Little children are much less likely than older children to have nail fungus. Pediatric nails are thinner, and they are faster growing, with better blood supply to the matrix.”
And if frank onychomadesis is observed, think about the time of year, and ask about recent fevers and rashes, because coxsackievirus may be the culprit. “Be not afraid, and look everywhere if the nail is confusing to you,” she said. In all ages, the diagnosis is primarily clinical, “but I culture them, I ‘PAS’ [periodic acid-Schiff stain] them, too. If you do both, you’ll increase your yield,” Dr. Friedlander said, adding, “the beauty of PAS is you can use it to give your families an answer very soon.”
Once you’ve established that fungus is to blame for a nail problem, there’s a conundrum: There are no Food and Drug Administration-approved therapies, either topical or systemic, for pediatric onychomycosis, Dr. Friedlander said. She, along with coauthors and first author Aditya Gupta, MD, of Mediprobe Research, London, Ontario, Canada, recently published an article reviewing the safety and efficacy of antifungal agents in this age group (Pediatr Dermatol. 2018 Jun 26. doi: 10.1111/pde.13561).
Reviewing information available in the United States and Canada, Dr. Friedlander and her coauthors came up with three topical and four oral options for children, along with recommendations for dosage and duration.
In response to an audience question about the use of topical antifungal treatment for nail involvement, Dr. Friedlander responded, “I think topicals would be great for kids, but it’s for kids where there is no nail matrix involvement. Also, cost is a problem. Nobody will cover it. But some families are willing to do this to avoid systemic therapy,” and if the family budget can accommodate a topical choice, it’s a logical option, she said, noting that partial reimbursement via a coupon system is available from some pharmaceutical companies.
Where appropriate, ciclopirox 8%, efinaconazole 10%, and tavaborole 5% can each be considered. Dr. Friedlander cited one study she coauthored, which reported that 70% of pediatric participants with nonmatrix onychomycosis saw effective treatment, with a 71% mycological cure rate (P = .03), after 32 weeks of treatment with ciclopirox lacquer versus vehicle (Pediatr Dermatol. 2013 May-Jun;30[3]:316-22).
Systemic therapies – which, when studied, have been given at tinea capitis doses – could include griseofulvin, terbinafine, itraconazole, and fluconazole.
In terms of oral options, Dr. Friedlander said, griseofulvin has some practical limitations. While prolonged treatment is required in any case, terbinafine may produce results in about 3 months, whereas griseofulvin may require up to 9 months of therapy. “I always try to use terbinafine … griseofulvin takes a year and a day,” she said.
She also shared some tips to improve pediatric adherence with oral antifungals: “You can tell parents to crush terbinafine tablets and mix in peanut butter or applesauce to improve adherence. Griseofulvin can be flavored by the pharmacy, but volumes are big with griseofulvin, so it’s a challenge to get kids to take it all,” she said.
Dr. Friedlander reported that she had no relevant financial disclosures.
[email protected]
CHICAGO – Though research shows that nail fungus occurs in just 0.3% of pediatric patients in the United States, that’s not what Sheila Friedlander, MD, is seeing in her southern California practice, where it’s not uncommon to see children whose nails, toe nails in particular, have fungal involvement.
said Dr. Friedlander during a nail-focused session at the annual summer meeting of the American Academy of Dermatology. Dr. Friedlander, professor of dermatology and pediatrics at the University of California San Diego and Rady Children’s Hospital, said that she suspects that more participation in organized sports at a young age may be contributing to the increase, with occlusive sports footwear replacing bare feet or sandals for more hours of the day, presenting more opportunities for toenail trauma in sports such as soccer.
When making the clinical call about a nail problem, bear in mind that the younger the child, the less likely a nail problem is fungal, Dr. Friedlander noted. “Little children are much less likely than older children to have nail fungus. Pediatric nails are thinner, and they are faster growing, with better blood supply to the matrix.”
And if frank onychomadesis is observed, think about the time of year, and ask about recent fevers and rashes, because coxsackievirus may be the culprit. “Be not afraid, and look everywhere if the nail is confusing to you,” she said. In all ages, the diagnosis is primarily clinical, “but I culture them, I ‘PAS’ [periodic acid-Schiff stain] them, too. If you do both, you’ll increase your yield,” Dr. Friedlander said, adding, “the beauty of PAS is you can use it to give your families an answer very soon.”
Once you’ve established that fungus is to blame for a nail problem, there’s a conundrum: There are no Food and Drug Administration-approved therapies, either topical or systemic, for pediatric onychomycosis, Dr. Friedlander said. She, along with coauthors and first author Aditya Gupta, MD, of Mediprobe Research, London, Ontario, Canada, recently published an article reviewing the safety and efficacy of antifungal agents in this age group (Pediatr Dermatol. 2018 Jun 26. doi: 10.1111/pde.13561).
Reviewing information available in the United States and Canada, Dr. Friedlander and her coauthors came up with three topical and four oral options for children, along with recommendations for dosage and duration.
In response to an audience question about the use of topical antifungal treatment for nail involvement, Dr. Friedlander responded, “I think topicals would be great for kids, but it’s for kids where there is no nail matrix involvement. Also, cost is a problem. Nobody will cover it. But some families are willing to do this to avoid systemic therapy,” and if the family budget can accommodate a topical choice, it’s a logical option, she said, noting that partial reimbursement via a coupon system is available from some pharmaceutical companies.
Where appropriate, ciclopirox 8%, efinaconazole 10%, and tavaborole 5% can each be considered. Dr. Friedlander cited one study she coauthored, which reported that 70% of pediatric participants with nonmatrix onychomycosis saw effective treatment, with a 71% mycological cure rate (P = .03), after 32 weeks of treatment with ciclopirox lacquer versus vehicle (Pediatr Dermatol. 2013 May-Jun;30[3]:316-22).
Systemic therapies – which, when studied, have been given at tinea capitis doses – could include griseofulvin, terbinafine, itraconazole, and fluconazole.
In terms of oral options, Dr. Friedlander said, griseofulvin has some practical limitations. While prolonged treatment is required in any case, terbinafine may produce results in about 3 months, whereas griseofulvin may require up to 9 months of therapy. “I always try to use terbinafine … griseofulvin takes a year and a day,” she said.
She also shared some tips to improve pediatric adherence with oral antifungals: “You can tell parents to crush terbinafine tablets and mix in peanut butter or applesauce to improve adherence. Griseofulvin can be flavored by the pharmacy, but volumes are big with griseofulvin, so it’s a challenge to get kids to take it all,” she said.
Dr. Friedlander reported that she had no relevant financial disclosures.
[email protected]
CHICAGO – Though research shows that nail fungus occurs in just 0.3% of pediatric patients in the United States, that’s not what Sheila Friedlander, MD, is seeing in her southern California practice, where it’s not uncommon to see children whose nails, toe nails in particular, have fungal involvement.
said Dr. Friedlander during a nail-focused session at the annual summer meeting of the American Academy of Dermatology. Dr. Friedlander, professor of dermatology and pediatrics at the University of California San Diego and Rady Children’s Hospital, said that she suspects that more participation in organized sports at a young age may be contributing to the increase, with occlusive sports footwear replacing bare feet or sandals for more hours of the day, presenting more opportunities for toenail trauma in sports such as soccer.
When making the clinical call about a nail problem, bear in mind that the younger the child, the less likely a nail problem is fungal, Dr. Friedlander noted. “Little children are much less likely than older children to have nail fungus. Pediatric nails are thinner, and they are faster growing, with better blood supply to the matrix.”
And if frank onychomadesis is observed, think about the time of year, and ask about recent fevers and rashes, because coxsackievirus may be the culprit. “Be not afraid, and look everywhere if the nail is confusing to you,” she said. In all ages, the diagnosis is primarily clinical, “but I culture them, I ‘PAS’ [periodic acid-Schiff stain] them, too. If you do both, you’ll increase your yield,” Dr. Friedlander said, adding, “the beauty of PAS is you can use it to give your families an answer very soon.”
Once you’ve established that fungus is to blame for a nail problem, there’s a conundrum: There are no Food and Drug Administration-approved therapies, either topical or systemic, for pediatric onychomycosis, Dr. Friedlander said. She, along with coauthors and first author Aditya Gupta, MD, of Mediprobe Research, London, Ontario, Canada, recently published an article reviewing the safety and efficacy of antifungal agents in this age group (Pediatr Dermatol. 2018 Jun 26. doi: 10.1111/pde.13561).
Reviewing information available in the United States and Canada, Dr. Friedlander and her coauthors came up with three topical and four oral options for children, along with recommendations for dosage and duration.
In response to an audience question about the use of topical antifungal treatment for nail involvement, Dr. Friedlander responded, “I think topicals would be great for kids, but it’s for kids where there is no nail matrix involvement. Also, cost is a problem. Nobody will cover it. But some families are willing to do this to avoid systemic therapy,” and if the family budget can accommodate a topical choice, it’s a logical option, she said, noting that partial reimbursement via a coupon system is available from some pharmaceutical companies.
Where appropriate, ciclopirox 8%, efinaconazole 10%, and tavaborole 5% can each be considered. Dr. Friedlander cited one study she coauthored, which reported that 70% of pediatric participants with nonmatrix onychomycosis saw effective treatment, with a 71% mycological cure rate (P = .03), after 32 weeks of treatment with ciclopirox lacquer versus vehicle (Pediatr Dermatol. 2013 May-Jun;30[3]:316-22).
Systemic therapies – which, when studied, have been given at tinea capitis doses – could include griseofulvin, terbinafine, itraconazole, and fluconazole.
In terms of oral options, Dr. Friedlander said, griseofulvin has some practical limitations. While prolonged treatment is required in any case, terbinafine may produce results in about 3 months, whereas griseofulvin may require up to 9 months of therapy. “I always try to use terbinafine … griseofulvin takes a year and a day,” she said.
She also shared some tips to improve pediatric adherence with oral antifungals: “You can tell parents to crush terbinafine tablets and mix in peanut butter or applesauce to improve adherence. Griseofulvin can be flavored by the pharmacy, but volumes are big with griseofulvin, so it’s a challenge to get kids to take it all,” she said.
Dr. Friedlander reported that she had no relevant financial disclosures.
[email protected]
EXPERT ANALYSIS FROM SUMMER AAD 2018
Extended propranolol use boosts success in high-risk infantile hemangioma
Extending oral propranolol treatment up to 12 months of age increased the success rate for high-risk infantile hemangioma, according to results published in Pediatrics.
Previous studies of oral propranol for infantile hemangiomas (IH) have revealed its efficacy, although there is no consensus on the optimal treatment duration. Nonetheless, treatment up to 12 months of age has been proposed if patients don’t respond after 6 months. Infants with high-risk hemangiomas, however, have been excluded from previous studies, authors of the current study explained.
In an open-label study of patients aged 35-150 days the success rate of oral propranolol was 47% after 6 months of treatment. The rate increased to 76% after the initial treatment period, reported Eulalia Baselga, MD, of the department of dermatology at Hospital de la Santa Creu i Sant Pau in Barcelona, and coauthors.
Investigators studied 45 patients from 10 hospitals in Spain and Poland between June 2015 and February 2017. The patients had high-risk IH in the proliferative phase. High-risk hemangiomas were defined as those that were life threatening, at risk for functional impact, disfiguring, or ulcerated nonresponsive to standard wound care measures.
Oral propranolol was administered twice daily at a dosage of 3 mg/kg per day. During the initial treatment period (ITP), patients received propranolol for a minimum of 6 months, and if treatment was not successful, it continued until success or up to 12 months of age.
Patients who achieved success in the initial phase were managed for 3 months with no treatment, and if there was rebound growth, treatment was restarted for up to 6 months at the provider’s discretion.
Treatment was considered a success if the target hemangioma resolved and there was no functional impact. The IH was considered resolved if it disappeared, even if there were minimal telangiectasias, erythema, skin thickening, soft tissue swelling, or the presence of sequelae.
Treatment success was achieved by 21 (47%) patients after 6 months and by 34 (76%) patients by the end of the ITP. Functional impact was determined using the Hemangioma Severity and Hemangioma Dynamic Complication scales. Adverse events, reported by 80% of patients, were resolved by the end of the study and included respiratory syncytial virus bronchiolitis, ulcerated hemangioma, pneumonia and respiratory failure, inguinal hernia, upper respiratory tract infection, dehydration, bronchitis, choking, and thermal burn. Although no patients experienced adverse events that resulted in discontinuation of treatment, 35 events led to temporary discontinuation, primarily due to respiratory events, the authors reported.
The results indicate that “oral propranolol is effective in treating high-risk IH with a favorable safety profile,” the authors concluded.
The study was funded by the Institut de Recherche Pierre Fabre Several authors were employed by or had other relationships with Pierre Fabre. The other authors had no conflicts of interest.
SOURCE: Baselga E et al. Pediatrics. 2018. doi: 10.1542/peds.2017-3866.
Extending oral propranolol treatment up to 12 months of age increased the success rate for high-risk infantile hemangioma, according to results published in Pediatrics.
Previous studies of oral propranol for infantile hemangiomas (IH) have revealed its efficacy, although there is no consensus on the optimal treatment duration. Nonetheless, treatment up to 12 months of age has been proposed if patients don’t respond after 6 months. Infants with high-risk hemangiomas, however, have been excluded from previous studies, authors of the current study explained.
In an open-label study of patients aged 35-150 days the success rate of oral propranolol was 47% after 6 months of treatment. The rate increased to 76% after the initial treatment period, reported Eulalia Baselga, MD, of the department of dermatology at Hospital de la Santa Creu i Sant Pau in Barcelona, and coauthors.
Investigators studied 45 patients from 10 hospitals in Spain and Poland between June 2015 and February 2017. The patients had high-risk IH in the proliferative phase. High-risk hemangiomas were defined as those that were life threatening, at risk for functional impact, disfiguring, or ulcerated nonresponsive to standard wound care measures.
Oral propranolol was administered twice daily at a dosage of 3 mg/kg per day. During the initial treatment period (ITP), patients received propranolol for a minimum of 6 months, and if treatment was not successful, it continued until success or up to 12 months of age.
Patients who achieved success in the initial phase were managed for 3 months with no treatment, and if there was rebound growth, treatment was restarted for up to 6 months at the provider’s discretion.
Treatment was considered a success if the target hemangioma resolved and there was no functional impact. The IH was considered resolved if it disappeared, even if there were minimal telangiectasias, erythema, skin thickening, soft tissue swelling, or the presence of sequelae.
Treatment success was achieved by 21 (47%) patients after 6 months and by 34 (76%) patients by the end of the ITP. Functional impact was determined using the Hemangioma Severity and Hemangioma Dynamic Complication scales. Adverse events, reported by 80% of patients, were resolved by the end of the study and included respiratory syncytial virus bronchiolitis, ulcerated hemangioma, pneumonia and respiratory failure, inguinal hernia, upper respiratory tract infection, dehydration, bronchitis, choking, and thermal burn. Although no patients experienced adverse events that resulted in discontinuation of treatment, 35 events led to temporary discontinuation, primarily due to respiratory events, the authors reported.
The results indicate that “oral propranolol is effective in treating high-risk IH with a favorable safety profile,” the authors concluded.
The study was funded by the Institut de Recherche Pierre Fabre Several authors were employed by or had other relationships with Pierre Fabre. The other authors had no conflicts of interest.
SOURCE: Baselga E et al. Pediatrics. 2018. doi: 10.1542/peds.2017-3866.
Extending oral propranolol treatment up to 12 months of age increased the success rate for high-risk infantile hemangioma, according to results published in Pediatrics.
Previous studies of oral propranol for infantile hemangiomas (IH) have revealed its efficacy, although there is no consensus on the optimal treatment duration. Nonetheless, treatment up to 12 months of age has been proposed if patients don’t respond after 6 months. Infants with high-risk hemangiomas, however, have been excluded from previous studies, authors of the current study explained.
In an open-label study of patients aged 35-150 days the success rate of oral propranolol was 47% after 6 months of treatment. The rate increased to 76% after the initial treatment period, reported Eulalia Baselga, MD, of the department of dermatology at Hospital de la Santa Creu i Sant Pau in Barcelona, and coauthors.
Investigators studied 45 patients from 10 hospitals in Spain and Poland between June 2015 and February 2017. The patients had high-risk IH in the proliferative phase. High-risk hemangiomas were defined as those that were life threatening, at risk for functional impact, disfiguring, or ulcerated nonresponsive to standard wound care measures.
Oral propranolol was administered twice daily at a dosage of 3 mg/kg per day. During the initial treatment period (ITP), patients received propranolol for a minimum of 6 months, and if treatment was not successful, it continued until success or up to 12 months of age.
Patients who achieved success in the initial phase were managed for 3 months with no treatment, and if there was rebound growth, treatment was restarted for up to 6 months at the provider’s discretion.
Treatment was considered a success if the target hemangioma resolved and there was no functional impact. The IH was considered resolved if it disappeared, even if there were minimal telangiectasias, erythema, skin thickening, soft tissue swelling, or the presence of sequelae.
Treatment success was achieved by 21 (47%) patients after 6 months and by 34 (76%) patients by the end of the ITP. Functional impact was determined using the Hemangioma Severity and Hemangioma Dynamic Complication scales. Adverse events, reported by 80% of patients, were resolved by the end of the study and included respiratory syncytial virus bronchiolitis, ulcerated hemangioma, pneumonia and respiratory failure, inguinal hernia, upper respiratory tract infection, dehydration, bronchitis, choking, and thermal burn. Although no patients experienced adverse events that resulted in discontinuation of treatment, 35 events led to temporary discontinuation, primarily due to respiratory events, the authors reported.
The results indicate that “oral propranolol is effective in treating high-risk IH with a favorable safety profile,” the authors concluded.
The study was funded by the Institut de Recherche Pierre Fabre Several authors were employed by or had other relationships with Pierre Fabre. The other authors had no conflicts of interest.
SOURCE: Baselga E et al. Pediatrics. 2018. doi: 10.1542/peds.2017-3866.
FROM PEDIATRICS
Key clinical point:
Major finding: After 6 months of treatment, the success rate was 47%, and it rose to 76% at the end of the treatment period.
Study details: A phase 3 study of 45 patients aged 35-150 days with high-risk IH.
Disclosures: The study was funded by the Institut de Recherche Pierre Fabre Several authors were employed by or had other relationships with Pierre Fabre. The other authors had no conflicts of interest.
Source: Baselga E et al. Pediatrics. 2018. doi: 10.1542/peds.2017-3866