User login
Pediatric data on novel axillary hyperhidrosis treatment reported
PARIS – Two compelling reasons exist to take excessive sweating in children and adolescents more seriously, Lawrence J. Green, MD, asserted at the annual congress of the European Academy of Dermatology and Venereology.
One is that this is a surprisingly common and embarrassing medical condition that can have a profound adverse developmental impact in young people at a time when they are engaged in forming their self-image.
The other reason to get serious about addressing primary axillary hyperhidrosis in pediatric patients is the recent approval of glycopyrronium tosylate as a topical therapy, Dr. Green, a dermatologist at George Washington University, Washington. The treatment, glycopyrronium pads (Qbrexza), was approved by the Food and Drug Administration for the topical treatment of primary axillary hyperhidrosis in patients aged 9 years and older in June 2018, and will be available in October 2018.
He presented new data from a 44-week, open-label extension of two pivotal 4-week, phase 3, randomized, double-blind, placebo-controlled trials known as ATMOS-1 and ATMOS-2. The new post hoc analysis from the extension study, known as the ARIDO study, provides reassurance that the product remains both safe and durably effective with longterm use.
Dr. Green’s analysis focused on the 44 pediatric participants aged 9-16 years. That’s because even though primary axillary hyperhidrosis affects people of all ages, with an estimated 4.8% prevalence in the U.S. population – 5.3 million people – it is more common in children and adolescents than adults. And it hits them particularly hard.
“Hyperhidrosis is largely underdiagnosed and undertreated, particularly among pediatric patients,” he said. “The impact on quality of life is comparable to or greater than acne, psoriasis, or eczema.”
The glycopyrronium pad is self-applied as a once-daily wipe. Glycopyrronium is an anticholinergic agent, which blocks sweat production by inhibiting the receptors that activate sweat glands.
Dr. Green noted several key findings from the 44-week ARIDO analysis, presented for the first time at the EADV congress.
The median absolute decrease in sweat production in pediatric patients at 44 weeks as measured gravimetrically was 50.3 mg per 5 minutes from a baseline of 150 mg per 5 minutes, comparable with the mean 75 mg reduction from a baseline of 175 mg in the 507-patient older cohort. However, Dr. Green advised not to make too much of this endpoint, as sweat production is notoriously difficult to measure accurately. In addition, an individual’s sweat rate can vary widely depending upon a multitude of factors, including ambient temperature and even what a patient is thinking about. The FDA recognizes this and therefore elevated several validated patient-reported outcomes to the status of coprimary endpoints in the clinical trials.
A positive result on one such patient-reported outcome, the Hyperhidrosis Severity Scale, was achieved in 57% of pediatric patients and 64% of adults at week 44 of open-label therapy. This required at least a 2-grade improvement from baseline, when roughly 60% of youths had a score of 3 and the remainder scored 4 on the 1-4 point scale.
From a mean baseline score of 9.2 on the Children’s Dermatology Life Quality Index, the pediatric group averaged a mean 6.2-point improvement at week 44, while adults experienced a mean 8.7-point improvement on the Dermatology Life Quality Index from a baseline of 11.25.
There was no diminution in treatment efficacy through 44 weeks, Dr. Green noted. Treatment-emergent adverse events consisted largely of transient mild to moderate anticholinergic effects, which seldom led to study discontinuation.
Dilated pupils and blurred vision were more common in children than adults (7.9% and 10.5% vs. 5.1% and 6.4%, respectively). “Why that is I can only speculate. Kids do tend to touch their eyes more often than adults. Pretty much everything else was the same. The adverse events can be worked around by educating people to use the pads appropriately. We saw the anticholinergic side effects more often in the first 4 weeks of the double-blind trials than in the longterm extension because once patients learned how to use the pad and not touch themselves afterwards, the adverse events came down,” he said.
The studies were sponsored by Dermira. Dr. Green has received research funding from and been a consultant to the company.
PARIS – Two compelling reasons exist to take excessive sweating in children and adolescents more seriously, Lawrence J. Green, MD, asserted at the annual congress of the European Academy of Dermatology and Venereology.
One is that this is a surprisingly common and embarrassing medical condition that can have a profound adverse developmental impact in young people at a time when they are engaged in forming their self-image.
The other reason to get serious about addressing primary axillary hyperhidrosis in pediatric patients is the recent approval of glycopyrronium tosylate as a topical therapy, Dr. Green, a dermatologist at George Washington University, Washington. The treatment, glycopyrronium pads (Qbrexza), was approved by the Food and Drug Administration for the topical treatment of primary axillary hyperhidrosis in patients aged 9 years and older in June 2018, and will be available in October 2018.
He presented new data from a 44-week, open-label extension of two pivotal 4-week, phase 3, randomized, double-blind, placebo-controlled trials known as ATMOS-1 and ATMOS-2. The new post hoc analysis from the extension study, known as the ARIDO study, provides reassurance that the product remains both safe and durably effective with longterm use.
Dr. Green’s analysis focused on the 44 pediatric participants aged 9-16 years. That’s because even though primary axillary hyperhidrosis affects people of all ages, with an estimated 4.8% prevalence in the U.S. population – 5.3 million people – it is more common in children and adolescents than adults. And it hits them particularly hard.
“Hyperhidrosis is largely underdiagnosed and undertreated, particularly among pediatric patients,” he said. “The impact on quality of life is comparable to or greater than acne, psoriasis, or eczema.”
The glycopyrronium pad is self-applied as a once-daily wipe. Glycopyrronium is an anticholinergic agent, which blocks sweat production by inhibiting the receptors that activate sweat glands.
Dr. Green noted several key findings from the 44-week ARIDO analysis, presented for the first time at the EADV congress.
The median absolute decrease in sweat production in pediatric patients at 44 weeks as measured gravimetrically was 50.3 mg per 5 minutes from a baseline of 150 mg per 5 minutes, comparable with the mean 75 mg reduction from a baseline of 175 mg in the 507-patient older cohort. However, Dr. Green advised not to make too much of this endpoint, as sweat production is notoriously difficult to measure accurately. In addition, an individual’s sweat rate can vary widely depending upon a multitude of factors, including ambient temperature and even what a patient is thinking about. The FDA recognizes this and therefore elevated several validated patient-reported outcomes to the status of coprimary endpoints in the clinical trials.
A positive result on one such patient-reported outcome, the Hyperhidrosis Severity Scale, was achieved in 57% of pediatric patients and 64% of adults at week 44 of open-label therapy. This required at least a 2-grade improvement from baseline, when roughly 60% of youths had a score of 3 and the remainder scored 4 on the 1-4 point scale.
From a mean baseline score of 9.2 on the Children’s Dermatology Life Quality Index, the pediatric group averaged a mean 6.2-point improvement at week 44, while adults experienced a mean 8.7-point improvement on the Dermatology Life Quality Index from a baseline of 11.25.
There was no diminution in treatment efficacy through 44 weeks, Dr. Green noted. Treatment-emergent adverse events consisted largely of transient mild to moderate anticholinergic effects, which seldom led to study discontinuation.
Dilated pupils and blurred vision were more common in children than adults (7.9% and 10.5% vs. 5.1% and 6.4%, respectively). “Why that is I can only speculate. Kids do tend to touch their eyes more often than adults. Pretty much everything else was the same. The adverse events can be worked around by educating people to use the pads appropriately. We saw the anticholinergic side effects more often in the first 4 weeks of the double-blind trials than in the longterm extension because once patients learned how to use the pad and not touch themselves afterwards, the adverse events came down,” he said.
The studies were sponsored by Dermira. Dr. Green has received research funding from and been a consultant to the company.
PARIS – Two compelling reasons exist to take excessive sweating in children and adolescents more seriously, Lawrence J. Green, MD, asserted at the annual congress of the European Academy of Dermatology and Venereology.
One is that this is a surprisingly common and embarrassing medical condition that can have a profound adverse developmental impact in young people at a time when they are engaged in forming their self-image.
The other reason to get serious about addressing primary axillary hyperhidrosis in pediatric patients is the recent approval of glycopyrronium tosylate as a topical therapy, Dr. Green, a dermatologist at George Washington University, Washington. The treatment, glycopyrronium pads (Qbrexza), was approved by the Food and Drug Administration for the topical treatment of primary axillary hyperhidrosis in patients aged 9 years and older in June 2018, and will be available in October 2018.
He presented new data from a 44-week, open-label extension of two pivotal 4-week, phase 3, randomized, double-blind, placebo-controlled trials known as ATMOS-1 and ATMOS-2. The new post hoc analysis from the extension study, known as the ARIDO study, provides reassurance that the product remains both safe and durably effective with longterm use.
Dr. Green’s analysis focused on the 44 pediatric participants aged 9-16 years. That’s because even though primary axillary hyperhidrosis affects people of all ages, with an estimated 4.8% prevalence in the U.S. population – 5.3 million people – it is more common in children and adolescents than adults. And it hits them particularly hard.
“Hyperhidrosis is largely underdiagnosed and undertreated, particularly among pediatric patients,” he said. “The impact on quality of life is comparable to or greater than acne, psoriasis, or eczema.”
The glycopyrronium pad is self-applied as a once-daily wipe. Glycopyrronium is an anticholinergic agent, which blocks sweat production by inhibiting the receptors that activate sweat glands.
Dr. Green noted several key findings from the 44-week ARIDO analysis, presented for the first time at the EADV congress.
The median absolute decrease in sweat production in pediatric patients at 44 weeks as measured gravimetrically was 50.3 mg per 5 minutes from a baseline of 150 mg per 5 minutes, comparable with the mean 75 mg reduction from a baseline of 175 mg in the 507-patient older cohort. However, Dr. Green advised not to make too much of this endpoint, as sweat production is notoriously difficult to measure accurately. In addition, an individual’s sweat rate can vary widely depending upon a multitude of factors, including ambient temperature and even what a patient is thinking about. The FDA recognizes this and therefore elevated several validated patient-reported outcomes to the status of coprimary endpoints in the clinical trials.
A positive result on one such patient-reported outcome, the Hyperhidrosis Severity Scale, was achieved in 57% of pediatric patients and 64% of adults at week 44 of open-label therapy. This required at least a 2-grade improvement from baseline, when roughly 60% of youths had a score of 3 and the remainder scored 4 on the 1-4 point scale.
From a mean baseline score of 9.2 on the Children’s Dermatology Life Quality Index, the pediatric group averaged a mean 6.2-point improvement at week 44, while adults experienced a mean 8.7-point improvement on the Dermatology Life Quality Index from a baseline of 11.25.
There was no diminution in treatment efficacy through 44 weeks, Dr. Green noted. Treatment-emergent adverse events consisted largely of transient mild to moderate anticholinergic effects, which seldom led to study discontinuation.
Dilated pupils and blurred vision were more common in children than adults (7.9% and 10.5% vs. 5.1% and 6.4%, respectively). “Why that is I can only speculate. Kids do tend to touch their eyes more often than adults. Pretty much everything else was the same. The adverse events can be worked around by educating people to use the pads appropriately. We saw the anticholinergic side effects more often in the first 4 weeks of the double-blind trials than in the longterm extension because once patients learned how to use the pad and not touch themselves afterwards, the adverse events came down,” he said.
The studies were sponsored by Dermira. Dr. Green has received research funding from and been a consultant to the company.
REPORTING FROM THE EADV CONGRESS
Key clinical point: Glycopyrronium tosylate pads address a common and undertreated medical condition in children: primary axillary hyperhidrosis.
Major finding: Mean scores on the Children’s Dermatology Life Quality Index improved by an average of 6.2 points from a baseline of 9.2 in children aged 9-16 years with primary axillary hyperhidrosis treated with once-daily glycopyrronium tosylate pads during an open-label, 44-week study.
Study details: This was a post hoc analysis of 44 patients aged 9-16 years and 507 patients aged 17 years and older who participated in a 44-week, open-label extension study of once-daily glycopyrronium tosylate pads for treatment of primary axillary hyperhidrosis.
Disclosures: The study was sponsored by Dermira. The presenter has received research funding from and been a consultant to the company.
Adjuvant Pembrolizumab Improves Progression-Free Survival in Stage III Melanoma
Study Overview
Objective. To evaluate pembrolizumab as adjuvant therapy for patients with resected, high-risk stage III melanoma.
Design. International randomized phase 3 trial.
Setting and participants. This multicenter international trial enrolled patients who had histologically confirmed cutaneous melanoma with regional lymph node metastasis (stage IIIA, IIIB or IIIC with no in-transit metastases). Patients had to have undergone a complete regional lymphadenectomy within 13 weeks before the start of treatment. Exclusion criteria were: ECOG performance status score > 1, autoimmune disease, current steroid use, and prior systemic therapy for melanoma. All tumor samples from melanoma-positive lymph nodes were required to be sent to the central lab for evaluation of programmed death ligand 1 (PD-L1) expression; PD-L1 positivity was defined as a tumor proportion score (TPS) ≥ 1%.
Intervention. Patients were randomized in a 1:1 fashion and stratified according to stage and geographic region. Local pharmacies were aware of trial-group assignments. Patients received either an intravenous infusion of pembrolizumab 200 mg or placebo every 3 weeks for a total of 18 doses or until disease recurrence or unacceptable toxicity occurred. If recurrence was detected, patients were able to cross over.
Main outcome measures. The primary outcome was recurrence-free survival (RFS) in the intention-to-treat population and in the subgroup of PD-L1–positive patients. Secondary endpoints included distant metastasis–free survival, overall survival (OS), safety, and quality of life.
Results. A total of 1019 patients were recruited from 123 centers in 23 countries: 514 were assigned to the pembrolizumab group and 505 were assigned to the placebo group. In the pembrolizumab group, 70 patients (13.8%) discontinued treatment because of an adverse event; in 66 patients of these patients the event was deemed drug-related. In the placebo group, 11 (2.2%) patients discontinued treatment due to an adverse event. Discontinuation due to disease recurrence was seen in 109 (21%) patients in the pembrolizumab group and 179 (35.7%) patients in the placebo group. The median duration of follow up was 15 months. In the overall intention-to-treat population, the 12-month RFS rate was 75.4% in the pembrolizumab group versus 61% in the placebo group (P < 0.001). At 18 months the RFS rates were 71.4% and 53.2%, respectively. The 18-month incidence of distant metastasis at recurrence was lower in the pembrolizumab group (16.7% vs. 29.7%, hazard ratio [HR] 0.53; 95% confidence interval 0.37 to 0.76). In those who were PD-L1–positive (n = 853), the 12-month RFS rate was 77.1% in the pembrolizumab group versus 62.6% in the placebo group. PD-L1 status had no impact on pembrolizumab efficacy. The benefit of pembrolizumab was noted across all subgroups, and no difference was seen in patients with stage IIIA, IIIB or IIIC disease. The benefit of pembrolizumab was similar in those with macroscopic or microscopic nodal metastasis. BRAF status did not influence RFS between the pembrolizumab and placebo groups.
Adverse events of grade 3 or higher were seen in 14.7% and 3.4% of the pembrolizumab and placebo groups, respectively. Immune-related adverse events of any grade were noted in 37% of patients in the pembrolizumab group. There was 1 pembrolizumab-related death secondary to myositis. Grades 3 or 4 immune-related events in the pembrolizumab group occurred at a low rate, including colitis (2% and 0.2%), hypophysitis (0.6% and 0%), and type 1 diabetes mellitus (1% and 0%).
Conclusion. Adjuvant pembrolizumab for patients with high-risk stage III melanoma significantly improved RFS compared with placebo and should be considered as an option for adjuvant therapy in this patient population.
Commentary
Prior to the development of immune checkpoint inhibitors, high-dose interferon alfa was the sole option for adjuvant therapy in high-risk melanoma. Although adjuvant interferon alfa is associated with improvements in disease-free survival [1], it is also associated with significant toxicity, including myelosuppression, neurologic adverse effects, and hepatotoxicity. The development of checkpoint inhibition represents an important advancement in the management of patients with melanoma. In the previously reported EORTC 18071 trial, Eggermont and colleagues demonstrated that adjuvant therapy with the CTLA-4 antibody ipilimumab improved both RFS (41% vs. 30%) and OS (65% vs. 54%) at 5 years in patients with stage III melanoma [2]. In 2017, Weber and colleagues demonstrated superior RFS (70% vs. 60%) and a lower rate of grade 3 or 4 adverse events with adjuvant nivolumab compared to ipilimumab in the CheckMate-238 trial [3].
In the current article, Eggermont and colleagues present the results of the EORTC 1325/KEYNOTE-054 study comparing the use of the PD-1 antibody pembrolizumab to placebo in the adjuvant setting for stage III melanoma. This study demonstrated a 43% reduced risk of recurrence or death favoring the pembrolizumab group (HR 0.57; P < 0.001). The 12-month RFS was 75.4% in the pembrolizumab arm versus 61% in the placebo arm. Treatment-related adverse events of grade 3 or higher occurred more commonly in the pembrolizumab arm (14.7% vs. 3.4%), with approximately 7% of these patients experiencing a grade 3 or higher immune-related adverse event. The results of this study corroborate prior data on the efficacy of PD-1 inhibitors in melanoma. Also, the investigators assessed RFS based on patient’s PD-L1 status (positivity defined as TPS ≥ 1% ) as a co-primary endpoint, and found consistent efficacy regardless of PD-L1 expression, with a hazard ratio of 0.47 in the 116 patients who had no PD-L1 expression.
Although the results of this study demonstrate a significant increase in RFS associated with adjuvant pembrolizumab therapy, an OS benefit has not yet been demonstrated. As noted, the only adjuvant checkpoint inhibitor trial to demonstrate an OS advantage thus far is the EORTC 18071 study of ipilimumab. However, the toxicity profile of adjuvant ipilimumab makes it an unattractive option compared to the PD-1 inhibitors. Which of the PD-1 inhibitors should be the treatment of choice for adjuvant therapy remains unclear, although it is worth noting that only nivolumab was compared to the best alternate therapy, ipilimumab [3]. It is also important to note that EORTC 1325/KEYNOTE-054 included patients with stage IIIA disease (N1a disease with at least 1 micrometastasis > 1 mm) or stage IIIB or IIIC without in-transit metastases, while CheckMate-238 did not include stage IIIA patients. Thus, for stage IIIA patients pembrolizumab remains the only PD-1 inhibitor with randomized data demonstrating a benefit.
Applications for Clinical Practice
The results from the EORTC 1325/KEYNOTE-054 study demonstrate a 43% reduction in the risk of progression or death with the use of adjuvant pembrolizumab in patients with stage III melanoma. As of now, the only checkpoint inhibitor to demonstrate an improvement in OS is ipilimumab, and whether the RFS benefit of both pembrolizumab and nivolumab will translate into an OS benefit is yet to be demonstrated.
—Daniel Isaac, DO, MS
1. Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1996;14:7–17.
2. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med 2016;375:1845–55.
3. Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017;377:1824–35.
Study Overview
Objective. To evaluate pembrolizumab as adjuvant therapy for patients with resected, high-risk stage III melanoma.
Design. International randomized phase 3 trial.
Setting and participants. This multicenter international trial enrolled patients who had histologically confirmed cutaneous melanoma with regional lymph node metastasis (stage IIIA, IIIB or IIIC with no in-transit metastases). Patients had to have undergone a complete regional lymphadenectomy within 13 weeks before the start of treatment. Exclusion criteria were: ECOG performance status score > 1, autoimmune disease, current steroid use, and prior systemic therapy for melanoma. All tumor samples from melanoma-positive lymph nodes were required to be sent to the central lab for evaluation of programmed death ligand 1 (PD-L1) expression; PD-L1 positivity was defined as a tumor proportion score (TPS) ≥ 1%.
Intervention. Patients were randomized in a 1:1 fashion and stratified according to stage and geographic region. Local pharmacies were aware of trial-group assignments. Patients received either an intravenous infusion of pembrolizumab 200 mg or placebo every 3 weeks for a total of 18 doses or until disease recurrence or unacceptable toxicity occurred. If recurrence was detected, patients were able to cross over.
Main outcome measures. The primary outcome was recurrence-free survival (RFS) in the intention-to-treat population and in the subgroup of PD-L1–positive patients. Secondary endpoints included distant metastasis–free survival, overall survival (OS), safety, and quality of life.
Results. A total of 1019 patients were recruited from 123 centers in 23 countries: 514 were assigned to the pembrolizumab group and 505 were assigned to the placebo group. In the pembrolizumab group, 70 patients (13.8%) discontinued treatment because of an adverse event; in 66 patients of these patients the event was deemed drug-related. In the placebo group, 11 (2.2%) patients discontinued treatment due to an adverse event. Discontinuation due to disease recurrence was seen in 109 (21%) patients in the pembrolizumab group and 179 (35.7%) patients in the placebo group. The median duration of follow up was 15 months. In the overall intention-to-treat population, the 12-month RFS rate was 75.4% in the pembrolizumab group versus 61% in the placebo group (P < 0.001). At 18 months the RFS rates were 71.4% and 53.2%, respectively. The 18-month incidence of distant metastasis at recurrence was lower in the pembrolizumab group (16.7% vs. 29.7%, hazard ratio [HR] 0.53; 95% confidence interval 0.37 to 0.76). In those who were PD-L1–positive (n = 853), the 12-month RFS rate was 77.1% in the pembrolizumab group versus 62.6% in the placebo group. PD-L1 status had no impact on pembrolizumab efficacy. The benefit of pembrolizumab was noted across all subgroups, and no difference was seen in patients with stage IIIA, IIIB or IIIC disease. The benefit of pembrolizumab was similar in those with macroscopic or microscopic nodal metastasis. BRAF status did not influence RFS between the pembrolizumab and placebo groups.
Adverse events of grade 3 or higher were seen in 14.7% and 3.4% of the pembrolizumab and placebo groups, respectively. Immune-related adverse events of any grade were noted in 37% of patients in the pembrolizumab group. There was 1 pembrolizumab-related death secondary to myositis. Grades 3 or 4 immune-related events in the pembrolizumab group occurred at a low rate, including colitis (2% and 0.2%), hypophysitis (0.6% and 0%), and type 1 diabetes mellitus (1% and 0%).
Conclusion. Adjuvant pembrolizumab for patients with high-risk stage III melanoma significantly improved RFS compared with placebo and should be considered as an option for adjuvant therapy in this patient population.
Commentary
Prior to the development of immune checkpoint inhibitors, high-dose interferon alfa was the sole option for adjuvant therapy in high-risk melanoma. Although adjuvant interferon alfa is associated with improvements in disease-free survival [1], it is also associated with significant toxicity, including myelosuppression, neurologic adverse effects, and hepatotoxicity. The development of checkpoint inhibition represents an important advancement in the management of patients with melanoma. In the previously reported EORTC 18071 trial, Eggermont and colleagues demonstrated that adjuvant therapy with the CTLA-4 antibody ipilimumab improved both RFS (41% vs. 30%) and OS (65% vs. 54%) at 5 years in patients with stage III melanoma [2]. In 2017, Weber and colleagues demonstrated superior RFS (70% vs. 60%) and a lower rate of grade 3 or 4 adverse events with adjuvant nivolumab compared to ipilimumab in the CheckMate-238 trial [3].
In the current article, Eggermont and colleagues present the results of the EORTC 1325/KEYNOTE-054 study comparing the use of the PD-1 antibody pembrolizumab to placebo in the adjuvant setting for stage III melanoma. This study demonstrated a 43% reduced risk of recurrence or death favoring the pembrolizumab group (HR 0.57; P < 0.001). The 12-month RFS was 75.4% in the pembrolizumab arm versus 61% in the placebo arm. Treatment-related adverse events of grade 3 or higher occurred more commonly in the pembrolizumab arm (14.7% vs. 3.4%), with approximately 7% of these patients experiencing a grade 3 or higher immune-related adverse event. The results of this study corroborate prior data on the efficacy of PD-1 inhibitors in melanoma. Also, the investigators assessed RFS based on patient’s PD-L1 status (positivity defined as TPS ≥ 1% ) as a co-primary endpoint, and found consistent efficacy regardless of PD-L1 expression, with a hazard ratio of 0.47 in the 116 patients who had no PD-L1 expression.
Although the results of this study demonstrate a significant increase in RFS associated with adjuvant pembrolizumab therapy, an OS benefit has not yet been demonstrated. As noted, the only adjuvant checkpoint inhibitor trial to demonstrate an OS advantage thus far is the EORTC 18071 study of ipilimumab. However, the toxicity profile of adjuvant ipilimumab makes it an unattractive option compared to the PD-1 inhibitors. Which of the PD-1 inhibitors should be the treatment of choice for adjuvant therapy remains unclear, although it is worth noting that only nivolumab was compared to the best alternate therapy, ipilimumab [3]. It is also important to note that EORTC 1325/KEYNOTE-054 included patients with stage IIIA disease (N1a disease with at least 1 micrometastasis > 1 mm) or stage IIIB or IIIC without in-transit metastases, while CheckMate-238 did not include stage IIIA patients. Thus, for stage IIIA patients pembrolizumab remains the only PD-1 inhibitor with randomized data demonstrating a benefit.
Applications for Clinical Practice
The results from the EORTC 1325/KEYNOTE-054 study demonstrate a 43% reduction in the risk of progression or death with the use of adjuvant pembrolizumab in patients with stage III melanoma. As of now, the only checkpoint inhibitor to demonstrate an improvement in OS is ipilimumab, and whether the RFS benefit of both pembrolizumab and nivolumab will translate into an OS benefit is yet to be demonstrated.
—Daniel Isaac, DO, MS
Study Overview
Objective. To evaluate pembrolizumab as adjuvant therapy for patients with resected, high-risk stage III melanoma.
Design. International randomized phase 3 trial.
Setting and participants. This multicenter international trial enrolled patients who had histologically confirmed cutaneous melanoma with regional lymph node metastasis (stage IIIA, IIIB or IIIC with no in-transit metastases). Patients had to have undergone a complete regional lymphadenectomy within 13 weeks before the start of treatment. Exclusion criteria were: ECOG performance status score > 1, autoimmune disease, current steroid use, and prior systemic therapy for melanoma. All tumor samples from melanoma-positive lymph nodes were required to be sent to the central lab for evaluation of programmed death ligand 1 (PD-L1) expression; PD-L1 positivity was defined as a tumor proportion score (TPS) ≥ 1%.
Intervention. Patients were randomized in a 1:1 fashion and stratified according to stage and geographic region. Local pharmacies were aware of trial-group assignments. Patients received either an intravenous infusion of pembrolizumab 200 mg or placebo every 3 weeks for a total of 18 doses or until disease recurrence or unacceptable toxicity occurred. If recurrence was detected, patients were able to cross over.
Main outcome measures. The primary outcome was recurrence-free survival (RFS) in the intention-to-treat population and in the subgroup of PD-L1–positive patients. Secondary endpoints included distant metastasis–free survival, overall survival (OS), safety, and quality of life.
Results. A total of 1019 patients were recruited from 123 centers in 23 countries: 514 were assigned to the pembrolizumab group and 505 were assigned to the placebo group. In the pembrolizumab group, 70 patients (13.8%) discontinued treatment because of an adverse event; in 66 patients of these patients the event was deemed drug-related. In the placebo group, 11 (2.2%) patients discontinued treatment due to an adverse event. Discontinuation due to disease recurrence was seen in 109 (21%) patients in the pembrolizumab group and 179 (35.7%) patients in the placebo group. The median duration of follow up was 15 months. In the overall intention-to-treat population, the 12-month RFS rate was 75.4% in the pembrolizumab group versus 61% in the placebo group (P < 0.001). At 18 months the RFS rates were 71.4% and 53.2%, respectively. The 18-month incidence of distant metastasis at recurrence was lower in the pembrolizumab group (16.7% vs. 29.7%, hazard ratio [HR] 0.53; 95% confidence interval 0.37 to 0.76). In those who were PD-L1–positive (n = 853), the 12-month RFS rate was 77.1% in the pembrolizumab group versus 62.6% in the placebo group. PD-L1 status had no impact on pembrolizumab efficacy. The benefit of pembrolizumab was noted across all subgroups, and no difference was seen in patients with stage IIIA, IIIB or IIIC disease. The benefit of pembrolizumab was similar in those with macroscopic or microscopic nodal metastasis. BRAF status did not influence RFS between the pembrolizumab and placebo groups.
Adverse events of grade 3 or higher were seen in 14.7% and 3.4% of the pembrolizumab and placebo groups, respectively. Immune-related adverse events of any grade were noted in 37% of patients in the pembrolizumab group. There was 1 pembrolizumab-related death secondary to myositis. Grades 3 or 4 immune-related events in the pembrolizumab group occurred at a low rate, including colitis (2% and 0.2%), hypophysitis (0.6% and 0%), and type 1 diabetes mellitus (1% and 0%).
Conclusion. Adjuvant pembrolizumab for patients with high-risk stage III melanoma significantly improved RFS compared with placebo and should be considered as an option for adjuvant therapy in this patient population.
Commentary
Prior to the development of immune checkpoint inhibitors, high-dose interferon alfa was the sole option for adjuvant therapy in high-risk melanoma. Although adjuvant interferon alfa is associated with improvements in disease-free survival [1], it is also associated with significant toxicity, including myelosuppression, neurologic adverse effects, and hepatotoxicity. The development of checkpoint inhibition represents an important advancement in the management of patients with melanoma. In the previously reported EORTC 18071 trial, Eggermont and colleagues demonstrated that adjuvant therapy with the CTLA-4 antibody ipilimumab improved both RFS (41% vs. 30%) and OS (65% vs. 54%) at 5 years in patients with stage III melanoma [2]. In 2017, Weber and colleagues demonstrated superior RFS (70% vs. 60%) and a lower rate of grade 3 or 4 adverse events with adjuvant nivolumab compared to ipilimumab in the CheckMate-238 trial [3].
In the current article, Eggermont and colleagues present the results of the EORTC 1325/KEYNOTE-054 study comparing the use of the PD-1 antibody pembrolizumab to placebo in the adjuvant setting for stage III melanoma. This study demonstrated a 43% reduced risk of recurrence or death favoring the pembrolizumab group (HR 0.57; P < 0.001). The 12-month RFS was 75.4% in the pembrolizumab arm versus 61% in the placebo arm. Treatment-related adverse events of grade 3 or higher occurred more commonly in the pembrolizumab arm (14.7% vs. 3.4%), with approximately 7% of these patients experiencing a grade 3 or higher immune-related adverse event. The results of this study corroborate prior data on the efficacy of PD-1 inhibitors in melanoma. Also, the investigators assessed RFS based on patient’s PD-L1 status (positivity defined as TPS ≥ 1% ) as a co-primary endpoint, and found consistent efficacy regardless of PD-L1 expression, with a hazard ratio of 0.47 in the 116 patients who had no PD-L1 expression.
Although the results of this study demonstrate a significant increase in RFS associated with adjuvant pembrolizumab therapy, an OS benefit has not yet been demonstrated. As noted, the only adjuvant checkpoint inhibitor trial to demonstrate an OS advantage thus far is the EORTC 18071 study of ipilimumab. However, the toxicity profile of adjuvant ipilimumab makes it an unattractive option compared to the PD-1 inhibitors. Which of the PD-1 inhibitors should be the treatment of choice for adjuvant therapy remains unclear, although it is worth noting that only nivolumab was compared to the best alternate therapy, ipilimumab [3]. It is also important to note that EORTC 1325/KEYNOTE-054 included patients with stage IIIA disease (N1a disease with at least 1 micrometastasis > 1 mm) or stage IIIB or IIIC without in-transit metastases, while CheckMate-238 did not include stage IIIA patients. Thus, for stage IIIA patients pembrolizumab remains the only PD-1 inhibitor with randomized data demonstrating a benefit.
Applications for Clinical Practice
The results from the EORTC 1325/KEYNOTE-054 study demonstrate a 43% reduction in the risk of progression or death with the use of adjuvant pembrolizumab in patients with stage III melanoma. As of now, the only checkpoint inhibitor to demonstrate an improvement in OS is ipilimumab, and whether the RFS benefit of both pembrolizumab and nivolumab will translate into an OS benefit is yet to be demonstrated.
—Daniel Isaac, DO, MS
1. Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1996;14:7–17.
2. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med 2016;375:1845–55.
3. Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017;377:1824–35.
1. Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1996;14:7–17.
2. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med 2016;375:1845–55.
3. Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017;377:1824–35.
Trifarotene cream for acne meets all endpoints in twin phase 3 trials
PARIS – A novel kinder, gentler topical retinoid met all of its primary and secondary endpoints in two identical phase 3 randomized trials totaling 2,420 patients with moderate acne vulgaris on both the face and trunk.
Trifarotene cream 50 mcg/g selectively targets the gamma retinoic acid receptor. This unique selectivity for just one of the three retinoic acid receptors results in less of the classic retinoid side effects – redness, scaling, dryness, stinging, burning – that can limit the clinical utility of existing retinoids. This was borne out by the high completion rates in trifarotene-treated participants in the two 12-week trials: 88.2% in the PERFECT 1 trial and 92.7% in PERFECT 2, compared with rates of 89.8% and 93.9% in vehicle-treated controls, Jerry K.L. Tan, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
“Most adverse events involved local intolerance occurring at application sites and were mild and transient,” Dr. Tan, a dermatologist at the University of Western Ontario, Windsor, and head of Windsor Clinical Research, reported.
PERFECT 1 and 2 were multicenter, double-blind, randomized, vehicle-controlled, 12-week phase 3 trials. Of note, these were the first-ever large-scale randomized trials to simultaneously evaluate a topical therapy for treatment of both facial and truncal acne. This ambitious goal created some unique challenges, which Dr. Tan described.
Participants, all of whom had moderate acne vulgaris on the face and trunk, ranged in age from 9 to 58 years, with a mean age of 19 years. They were randomized to once-daily application of trifarotene cream 50 mcg/g or its vehicle in the evening.
Primary and secondary outcomes
One major efficacy endpoint on the face was achievement of Investigator Global Assessment success as defined by a score of 0 or 1, meaning clear or almost clear, coupled with at least a 2-grade improvement from baseline to week 12. In PERFECT 1 and 2 this was achieved by 29.7% and 42.8% of trifarotene cream-treated patients, response rates significantly better than the 20% and 25.8% in vehicle-treated controls.
Another endpoint for facial therapy were the absolute reductions from baseline in facial inflammatory and noninflammatory acne lesions. The mean reduction in inflammatory lesion count in trifarotene-treated patients was 19.6% in PERFECT 1 and 24.6% in PERFECT 2, both significantly better than the mean 15.8% and 19.6% decreases in controls. Noninflammatory facial lesion counts dropped by 26.7% and 30.4% with trifarotene, versus 18.9% and 22.3% with vehicle.
The efficacy yardsticks utilized on the trunk were the same as on the face except that Physician Global Assessment was the terminology utilized in lieu of Investigator Global Assessment. Physician Global Assessment success on the trunk was achieved by 35.8% and 41.1% of trifarotene-treated patients in the two trials, as compared with 25.7% and 30.1% of controls.
The mean reductions in truncal inflammatory lesion count obtained with trifarotene cream were 22% and 26.1%, both significantly better than the 18.8% and 20.3% rates with vehicle.
Treatment-emergent adverse events leading to study discontinuation occurred in 1.9% of trifarotene-treated patients in one trial and 1% in the other.
One audience member commented that the vehicle response rates looked too strong for that compound to be inert. Dr. Tan agreed. “The vehicle looks really good. One of the issues with vehicles is that many of them have to contain products to prevent decay, fermentation, and proliferation of yeast and bacteria. So I quite agree: I think many of our vehicles do have active ingredients,” he replied. “If you look at the topical dapsone trials, the vehicles look amazing.”
“The other possibility is that there’s what we call ‘investigator creep,’” the dermatologist continued. “It’s the notion that you have no idea what the patients are getting, but they look like maybe they’re getting better, so you grade it as better.”
Dr. Tan reported serving as an advisor and consultant to, speaker for, and recipient of research grants from Galderma, which sponsored the two phase 3 trials. The company is also developing trifarotene for the treatment of lamellar ichthyosis.
PARIS – A novel kinder, gentler topical retinoid met all of its primary and secondary endpoints in two identical phase 3 randomized trials totaling 2,420 patients with moderate acne vulgaris on both the face and trunk.
Trifarotene cream 50 mcg/g selectively targets the gamma retinoic acid receptor. This unique selectivity for just one of the three retinoic acid receptors results in less of the classic retinoid side effects – redness, scaling, dryness, stinging, burning – that can limit the clinical utility of existing retinoids. This was borne out by the high completion rates in trifarotene-treated participants in the two 12-week trials: 88.2% in the PERFECT 1 trial and 92.7% in PERFECT 2, compared with rates of 89.8% and 93.9% in vehicle-treated controls, Jerry K.L. Tan, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
“Most adverse events involved local intolerance occurring at application sites and were mild and transient,” Dr. Tan, a dermatologist at the University of Western Ontario, Windsor, and head of Windsor Clinical Research, reported.
PERFECT 1 and 2 were multicenter, double-blind, randomized, vehicle-controlled, 12-week phase 3 trials. Of note, these were the first-ever large-scale randomized trials to simultaneously evaluate a topical therapy for treatment of both facial and truncal acne. This ambitious goal created some unique challenges, which Dr. Tan described.
Participants, all of whom had moderate acne vulgaris on the face and trunk, ranged in age from 9 to 58 years, with a mean age of 19 years. They were randomized to once-daily application of trifarotene cream 50 mcg/g or its vehicle in the evening.
Primary and secondary outcomes
One major efficacy endpoint on the face was achievement of Investigator Global Assessment success as defined by a score of 0 or 1, meaning clear or almost clear, coupled with at least a 2-grade improvement from baseline to week 12. In PERFECT 1 and 2 this was achieved by 29.7% and 42.8% of trifarotene cream-treated patients, response rates significantly better than the 20% and 25.8% in vehicle-treated controls.
Another endpoint for facial therapy were the absolute reductions from baseline in facial inflammatory and noninflammatory acne lesions. The mean reduction in inflammatory lesion count in trifarotene-treated patients was 19.6% in PERFECT 1 and 24.6% in PERFECT 2, both significantly better than the mean 15.8% and 19.6% decreases in controls. Noninflammatory facial lesion counts dropped by 26.7% and 30.4% with trifarotene, versus 18.9% and 22.3% with vehicle.
The efficacy yardsticks utilized on the trunk were the same as on the face except that Physician Global Assessment was the terminology utilized in lieu of Investigator Global Assessment. Physician Global Assessment success on the trunk was achieved by 35.8% and 41.1% of trifarotene-treated patients in the two trials, as compared with 25.7% and 30.1% of controls.
The mean reductions in truncal inflammatory lesion count obtained with trifarotene cream were 22% and 26.1%, both significantly better than the 18.8% and 20.3% rates with vehicle.
Treatment-emergent adverse events leading to study discontinuation occurred in 1.9% of trifarotene-treated patients in one trial and 1% in the other.
One audience member commented that the vehicle response rates looked too strong for that compound to be inert. Dr. Tan agreed. “The vehicle looks really good. One of the issues with vehicles is that many of them have to contain products to prevent decay, fermentation, and proliferation of yeast and bacteria. So I quite agree: I think many of our vehicles do have active ingredients,” he replied. “If you look at the topical dapsone trials, the vehicles look amazing.”
“The other possibility is that there’s what we call ‘investigator creep,’” the dermatologist continued. “It’s the notion that you have no idea what the patients are getting, but they look like maybe they’re getting better, so you grade it as better.”
Dr. Tan reported serving as an advisor and consultant to, speaker for, and recipient of research grants from Galderma, which sponsored the two phase 3 trials. The company is also developing trifarotene for the treatment of lamellar ichthyosis.
PARIS – A novel kinder, gentler topical retinoid met all of its primary and secondary endpoints in two identical phase 3 randomized trials totaling 2,420 patients with moderate acne vulgaris on both the face and trunk.
Trifarotene cream 50 mcg/g selectively targets the gamma retinoic acid receptor. This unique selectivity for just one of the three retinoic acid receptors results in less of the classic retinoid side effects – redness, scaling, dryness, stinging, burning – that can limit the clinical utility of existing retinoids. This was borne out by the high completion rates in trifarotene-treated participants in the two 12-week trials: 88.2% in the PERFECT 1 trial and 92.7% in PERFECT 2, compared with rates of 89.8% and 93.9% in vehicle-treated controls, Jerry K.L. Tan, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
“Most adverse events involved local intolerance occurring at application sites and were mild and transient,” Dr. Tan, a dermatologist at the University of Western Ontario, Windsor, and head of Windsor Clinical Research, reported.
PERFECT 1 and 2 were multicenter, double-blind, randomized, vehicle-controlled, 12-week phase 3 trials. Of note, these were the first-ever large-scale randomized trials to simultaneously evaluate a topical therapy for treatment of both facial and truncal acne. This ambitious goal created some unique challenges, which Dr. Tan described.
Participants, all of whom had moderate acne vulgaris on the face and trunk, ranged in age from 9 to 58 years, with a mean age of 19 years. They were randomized to once-daily application of trifarotene cream 50 mcg/g or its vehicle in the evening.
Primary and secondary outcomes
One major efficacy endpoint on the face was achievement of Investigator Global Assessment success as defined by a score of 0 or 1, meaning clear or almost clear, coupled with at least a 2-grade improvement from baseline to week 12. In PERFECT 1 and 2 this was achieved by 29.7% and 42.8% of trifarotene cream-treated patients, response rates significantly better than the 20% and 25.8% in vehicle-treated controls.
Another endpoint for facial therapy were the absolute reductions from baseline in facial inflammatory and noninflammatory acne lesions. The mean reduction in inflammatory lesion count in trifarotene-treated patients was 19.6% in PERFECT 1 and 24.6% in PERFECT 2, both significantly better than the mean 15.8% and 19.6% decreases in controls. Noninflammatory facial lesion counts dropped by 26.7% and 30.4% with trifarotene, versus 18.9% and 22.3% with vehicle.
The efficacy yardsticks utilized on the trunk were the same as on the face except that Physician Global Assessment was the terminology utilized in lieu of Investigator Global Assessment. Physician Global Assessment success on the trunk was achieved by 35.8% and 41.1% of trifarotene-treated patients in the two trials, as compared with 25.7% and 30.1% of controls.
The mean reductions in truncal inflammatory lesion count obtained with trifarotene cream were 22% and 26.1%, both significantly better than the 18.8% and 20.3% rates with vehicle.
Treatment-emergent adverse events leading to study discontinuation occurred in 1.9% of trifarotene-treated patients in one trial and 1% in the other.
One audience member commented that the vehicle response rates looked too strong for that compound to be inert. Dr. Tan agreed. “The vehicle looks really good. One of the issues with vehicles is that many of them have to contain products to prevent decay, fermentation, and proliferation of yeast and bacteria. So I quite agree: I think many of our vehicles do have active ingredients,” he replied. “If you look at the topical dapsone trials, the vehicles look amazing.”
“The other possibility is that there’s what we call ‘investigator creep,’” the dermatologist continued. “It’s the notion that you have no idea what the patients are getting, but they look like maybe they’re getting better, so you grade it as better.”
Dr. Tan reported serving as an advisor and consultant to, speaker for, and recipient of research grants from Galderma, which sponsored the two phase 3 trials. The company is also developing trifarotene for the treatment of lamellar ichthyosis.
REPORTING FROM THE EADV CONGRESS
Key clinical point:
Major finding: Treatment-emergent adverse events leading to study discontinuation occurred in 1.9% of trifarotene cream–treated patients in one trial and 1% in the other.
Study details: PERFECT 1 and PERFECT 2 were identically designed 12-week phase 3 randomized trials including 2,420 patients with moderate facial and truncal acne.
Disclosures: The study presenter reported serving as an advisor, consultant to, speaker for, and recipient of research grants from Galderma, which sponsored the two phase 3 trials.
Brown spot on cheek
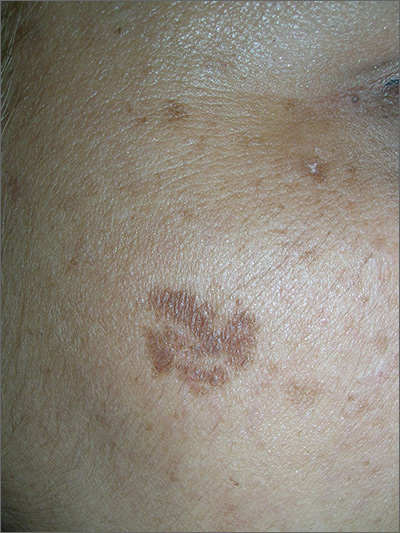
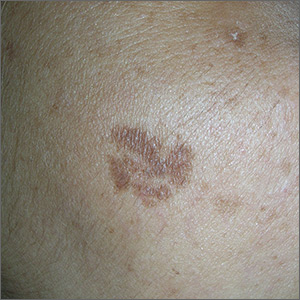
The FP noted that the patient had a dominant brown patch on her right cheek that was larger and darker than the other light brown spots found on her face. Fortunately, he had a dermatoscope and examined the spot closely, finding only features of a benign solar lentigo. There were no suspicious features for melanoma.
The FP gave the patient a choice between a broad shave biopsy that day, or having the lesion monitored (safe with a flat lesion) using photography and dermoscopy.
The patient didn’t want a biopsy on her face and was willing to have the area monitored. The clinical and dermoscopic photographs were taken and stored. The patient was given a follow-up appointment in 4 to 6 months and instructions to avoid the sun as much as possible. She was also told to use sunscreen and hats when out in the sun.
After 5 months, the previous photos were compared with the new photos on a computer screen and there were no changes. Everyone was reassured, and the patient indicated that she was being careful about her sun exposure.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine, R. Lentigo maligna. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:981-984.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP noted that the patient had a dominant brown patch on her right cheek that was larger and darker than the other light brown spots found on her face. Fortunately, he had a dermatoscope and examined the spot closely, finding only features of a benign solar lentigo. There were no suspicious features for melanoma.
The FP gave the patient a choice between a broad shave biopsy that day, or having the lesion monitored (safe with a flat lesion) using photography and dermoscopy.
The patient didn’t want a biopsy on her face and was willing to have the area monitored. The clinical and dermoscopic photographs were taken and stored. The patient was given a follow-up appointment in 4 to 6 months and instructions to avoid the sun as much as possible. She was also told to use sunscreen and hats when out in the sun.
After 5 months, the previous photos were compared with the new photos on a computer screen and there were no changes. Everyone was reassured, and the patient indicated that she was being careful about her sun exposure.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine, R. Lentigo maligna. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:981-984.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP noted that the patient had a dominant brown patch on her right cheek that was larger and darker than the other light brown spots found on her face. Fortunately, he had a dermatoscope and examined the spot closely, finding only features of a benign solar lentigo. There were no suspicious features for melanoma.
The FP gave the patient a choice between a broad shave biopsy that day, or having the lesion monitored (safe with a flat lesion) using photography and dermoscopy.
The patient didn’t want a biopsy on her face and was willing to have the area monitored. The clinical and dermoscopic photographs were taken and stored. The patient was given a follow-up appointment in 4 to 6 months and instructions to avoid the sun as much as possible. She was also told to use sunscreen and hats when out in the sun.
After 5 months, the previous photos were compared with the new photos on a computer screen and there were no changes. Everyone was reassured, and the patient indicated that she was being careful about her sun exposure.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine, R. Lentigo maligna. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:981-984.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
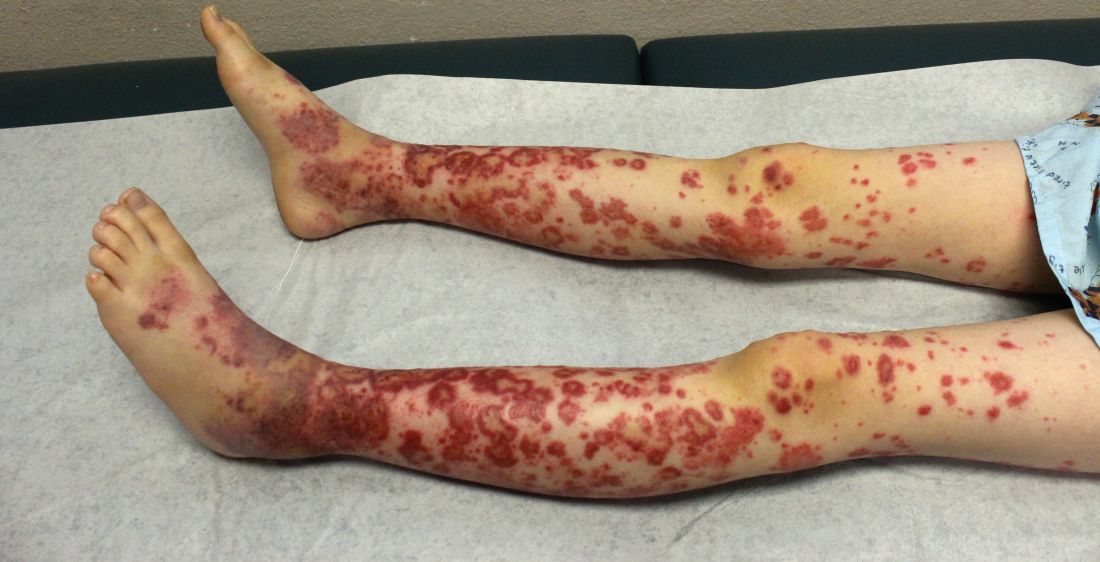
IgA vasculitis may be more common in adults than assumed
LAS VEGAS – IgA vasculitis has a reputation as an illness of childhood, but rheumatologist Alexandra Villa-Forte, MD, MPH, cautioned colleagues that it can strike adults, too, often in a much more severe form. And, she warned, it’s likely not as rare as physicians assume.
“I believe it’s more common in adults than reported. There’s a huge problem with establishing the right way to make the diagnosis in adults, which is why it is missed,” said Dr. Villa-Forte of the Cleveland Clinic, who spoke at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
But even if IgA vasculitis (IV) is diagnosed correctly in adults, there are many questions about how to move forward, she said. “Treatment in adults remains a problem since we don’t have data.”
IV is also known as Henoch-Schönlein purpura, or HSP, and “spring fever” because it often appears in children in the spring following an upper respiratory infection.
The condition causes vasculitis, the swelling of small blood vessels in organs such as the skin, joints, kidneys, and intestines. Leaking blood vessels can cause skin rashes known as purpura.
The estimated ranges of disease are high, with the annual incidence in children estimated at 3-26 per 100,000 and in adults at 0.1-1.8 per 100,000. Dr. Villa-Forte noted that the male-to-female ratio is 1.5, and she said the condition is less common in African Americans.
“In children, this is a disease that is frequently self-limited. Most of them don’t need treatment, and most of them do not relapse,” Dr. Villa-Forte said. “In adults, it’s more resistant to treatment, frequently chronic, and frequently relapsing over the years.”
There are other differences in IV between children and adults. “In children, there is a clear seasonal pattern of disease that is not seen in adults,” she said, and it’s linked to preceding infections.
“In adults, there are multiple causes, and most of the time they’re not identified,” she said.
As for diagnosis, she suggests looking at clinical presentation and whether tissue biopsy shows cutaneous leukocytoclastic vasculitis with IgA deposits. She cautioned that increased serum IgA is seen in about 50% of adult patients, making it an unreliable indicator.
Prognosis is much better for children than adults. According to a 2014 study, 80% of children completely recover, compared with 40% of adults. Persistent hematuria or proteinuria occurs in 30% of children and 60% of adults, respectively, while chronic renal failure occurs in 2% of children and 10% of adults (J Korean Med Sci. 2014 Feb;29[2]:198-203).
It’s possible that the latter number may be higher, with as many as 30% of adults developing chronic kidney disease (CKD), Dr. Villa-Forte said.
An estimated 97% of nephritis develops within the first 6 months of disease onset in adults, she said, “and in adults, the active renal disease can persist for over 20 years.”
Guidelines suggest that patients be monitored for CKD for 6 months after disease onset, but Dr. Villa-Forte said it’s better to monitor them for 12 months.
What about treatment? “The major challenge in adults is the real absence between correlation between initial presentation and long-term renal outcome,” she said. “That makes for a difficult choice in terms of treatment selection.”
Fever and cutaneous lesions above the waist may predict renal involvement, she said, although that isn’t confirmed, and increasing proteinuria is a probable factor predicting progression/complications.
According to Dr. Villa-Forte, the value of early treatment hasn’t been proved. Due to the risk of kidney problems, she said, “we don’t feel like we can just watch patients – that we need to do something. But there’s not good data supporting that.”
Various protocols involving steroids, early plasmapheresis, rituximab (Rituxan), and other drug regimens lack evidence, she said, although use of steroids in early nephritis may be beneficial in adults.
Dr. Villa-Forte had no relevant disclosures.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – IgA vasculitis has a reputation as an illness of childhood, but rheumatologist Alexandra Villa-Forte, MD, MPH, cautioned colleagues that it can strike adults, too, often in a much more severe form. And, she warned, it’s likely not as rare as physicians assume.
“I believe it’s more common in adults than reported. There’s a huge problem with establishing the right way to make the diagnosis in adults, which is why it is missed,” said Dr. Villa-Forte of the Cleveland Clinic, who spoke at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
But even if IgA vasculitis (IV) is diagnosed correctly in adults, there are many questions about how to move forward, she said. “Treatment in adults remains a problem since we don’t have data.”
IV is also known as Henoch-Schönlein purpura, or HSP, and “spring fever” because it often appears in children in the spring following an upper respiratory infection.
The condition causes vasculitis, the swelling of small blood vessels in organs such as the skin, joints, kidneys, and intestines. Leaking blood vessels can cause skin rashes known as purpura.
The estimated ranges of disease are high, with the annual incidence in children estimated at 3-26 per 100,000 and in adults at 0.1-1.8 per 100,000. Dr. Villa-Forte noted that the male-to-female ratio is 1.5, and she said the condition is less common in African Americans.
“In children, this is a disease that is frequently self-limited. Most of them don’t need treatment, and most of them do not relapse,” Dr. Villa-Forte said. “In adults, it’s more resistant to treatment, frequently chronic, and frequently relapsing over the years.”
There are other differences in IV between children and adults. “In children, there is a clear seasonal pattern of disease that is not seen in adults,” she said, and it’s linked to preceding infections.
“In adults, there are multiple causes, and most of the time they’re not identified,” she said.
As for diagnosis, she suggests looking at clinical presentation and whether tissue biopsy shows cutaneous leukocytoclastic vasculitis with IgA deposits. She cautioned that increased serum IgA is seen in about 50% of adult patients, making it an unreliable indicator.
Prognosis is much better for children than adults. According to a 2014 study, 80% of children completely recover, compared with 40% of adults. Persistent hematuria or proteinuria occurs in 30% of children and 60% of adults, respectively, while chronic renal failure occurs in 2% of children and 10% of adults (J Korean Med Sci. 2014 Feb;29[2]:198-203).
It’s possible that the latter number may be higher, with as many as 30% of adults developing chronic kidney disease (CKD), Dr. Villa-Forte said.
An estimated 97% of nephritis develops within the first 6 months of disease onset in adults, she said, “and in adults, the active renal disease can persist for over 20 years.”
Guidelines suggest that patients be monitored for CKD for 6 months after disease onset, but Dr. Villa-Forte said it’s better to monitor them for 12 months.
What about treatment? “The major challenge in adults is the real absence between correlation between initial presentation and long-term renal outcome,” she said. “That makes for a difficult choice in terms of treatment selection.”
Fever and cutaneous lesions above the waist may predict renal involvement, she said, although that isn’t confirmed, and increasing proteinuria is a probable factor predicting progression/complications.
According to Dr. Villa-Forte, the value of early treatment hasn’t been proved. Due to the risk of kidney problems, she said, “we don’t feel like we can just watch patients – that we need to do something. But there’s not good data supporting that.”
Various protocols involving steroids, early plasmapheresis, rituximab (Rituxan), and other drug regimens lack evidence, she said, although use of steroids in early nephritis may be beneficial in adults.
Dr. Villa-Forte had no relevant disclosures.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – IgA vasculitis has a reputation as an illness of childhood, but rheumatologist Alexandra Villa-Forte, MD, MPH, cautioned colleagues that it can strike adults, too, often in a much more severe form. And, she warned, it’s likely not as rare as physicians assume.
“I believe it’s more common in adults than reported. There’s a huge problem with establishing the right way to make the diagnosis in adults, which is why it is missed,” said Dr. Villa-Forte of the Cleveland Clinic, who spoke at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
But even if IgA vasculitis (IV) is diagnosed correctly in adults, there are many questions about how to move forward, she said. “Treatment in adults remains a problem since we don’t have data.”
IV is also known as Henoch-Schönlein purpura, or HSP, and “spring fever” because it often appears in children in the spring following an upper respiratory infection.
The condition causes vasculitis, the swelling of small blood vessels in organs such as the skin, joints, kidneys, and intestines. Leaking blood vessels can cause skin rashes known as purpura.
The estimated ranges of disease are high, with the annual incidence in children estimated at 3-26 per 100,000 and in adults at 0.1-1.8 per 100,000. Dr. Villa-Forte noted that the male-to-female ratio is 1.5, and she said the condition is less common in African Americans.
“In children, this is a disease that is frequently self-limited. Most of them don’t need treatment, and most of them do not relapse,” Dr. Villa-Forte said. “In adults, it’s more resistant to treatment, frequently chronic, and frequently relapsing over the years.”
There are other differences in IV between children and adults. “In children, there is a clear seasonal pattern of disease that is not seen in adults,” she said, and it’s linked to preceding infections.
“In adults, there are multiple causes, and most of the time they’re not identified,” she said.
As for diagnosis, she suggests looking at clinical presentation and whether tissue biopsy shows cutaneous leukocytoclastic vasculitis with IgA deposits. She cautioned that increased serum IgA is seen in about 50% of adult patients, making it an unreliable indicator.
Prognosis is much better for children than adults. According to a 2014 study, 80% of children completely recover, compared with 40% of adults. Persistent hematuria or proteinuria occurs in 30% of children and 60% of adults, respectively, while chronic renal failure occurs in 2% of children and 10% of adults (J Korean Med Sci. 2014 Feb;29[2]:198-203).
It’s possible that the latter number may be higher, with as many as 30% of adults developing chronic kidney disease (CKD), Dr. Villa-Forte said.
An estimated 97% of nephritis develops within the first 6 months of disease onset in adults, she said, “and in adults, the active renal disease can persist for over 20 years.”
Guidelines suggest that patients be monitored for CKD for 6 months after disease onset, but Dr. Villa-Forte said it’s better to monitor them for 12 months.
What about treatment? “The major challenge in adults is the real absence between correlation between initial presentation and long-term renal outcome,” she said. “That makes for a difficult choice in terms of treatment selection.”
Fever and cutaneous lesions above the waist may predict renal involvement, she said, although that isn’t confirmed, and increasing proteinuria is a probable factor predicting progression/complications.
According to Dr. Villa-Forte, the value of early treatment hasn’t been proved. Due to the risk of kidney problems, she said, “we don’t feel like we can just watch patients – that we need to do something. But there’s not good data supporting that.”
Various protocols involving steroids, early plasmapheresis, rituximab (Rituxan), and other drug regimens lack evidence, she said, although use of steroids in early nephritis may be beneficial in adults.
Dr. Villa-Forte had no relevant disclosures.
Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM THE ANNUAL PERSPECTIVES IN RHEUMATIC DISEASES
Novel oral agent shows unprecedented efficacy in psoriasis
PARIS – A novel in a phase 2 clinical trial including 267 adults with moderate to severe disease, James G. Krueger, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“I would say the clinical response here is almost dead-on as a copy for ustekinumab, which is an [injectable interleukin] IL-23/IL-12 blocker. And we’re only at 12 weeks here; some of the curves look like they’re on a trajectory to go up further in terms of improvement. So I’m getting a performance with an oral drug that is just so much better than the approved alternatives that we have,” said Dr. Krueger, head of the laboratory of investigative dermatology and professor in clinical investigation at Rockefeller University in New York.
Oral apremilast (Otezla), for example, can’t touch those PASI 75 response rates in patients with moderate to severe psoriasis. Indeed, many psoriasis experts favor reserving apremilast for patients with moderate disease.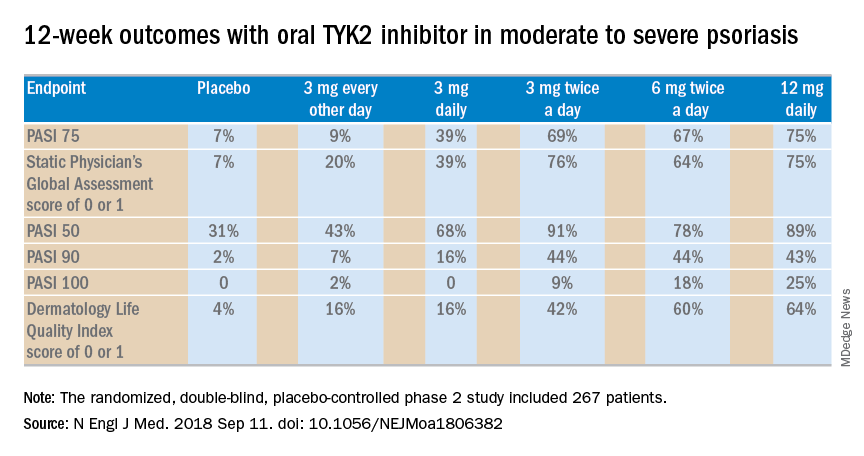
The 12-week, double-blind, placebo-controlled study was conducted at 82 sites in the United States and seven other countries. In this dose-ranging study, participants were randomized to the oral selective tyrosine kinase 2 (TYK2) inhibitor, known for the time being as BMS-986165, at 3 mg every other day, 3 mg daily, 3 mg twice a day, 6 mg twice a day, 12 mg daily, or to placebo.
The primary outcome was a 75% or greater reduction from baseline in Psoriasis Area and Severity Index score (PASI 75) at week 12. The TYK2 inhibitor outperformed placebo in dose-dependent fashion starting at the 3 mg/day dose. The PASI 75 rate was 7% with placebo, 9% with 3 mg of BMS-986165 every other day, 39% with 3 mg daily, 69% with 3 mg BID, 67% with 6 mg BID, and 75% with 12 mg/day. All secondary endpoints followed suit.
A striking finding in the phase 2 study was that when the drug was stopped for a month at the end of the 12-week treatment period, for the most part, the PASI 75 response and other clinical benefits were retained.
“I would contrast this to experiments that I have personally done with cyclosporine, where I have cleared people with cyclosporine, stopped it, and a month later every single patient has rip-roaring disease back. So I think this TYK2 inhibitor has some different performance features than just blocking a downstream T-cell transduction molecule,” observed the dermatologist, who is credited as the discoverer of the importance of the T cell in psoriasis pathogenesis.
The strong multidimensional evidence of clinical efficacy in the phase 2 study was supported mechanistically by analysis of skin biopsies obtained on study days 1, 15, and 85. The laboratory studies showed that the oral drug improved molecular, cellular, and clinical biomarkers associated with treatment efficacy. For example, at doses of 3 mg twice a day or higher, the TYK2 inhibitor reduced expression of IL-19 and IL-36A, which are key drivers of keratinocyte activation and epidermal hyperplasia. The drug also markedly decreased expression of genes in the Th17 pathway and essentially normalized expression of the proinflammatory genes beta defensin and S100A9.
In contrast to the Janus kinase (JAK) 1/3 and JAK 2 inhibitors in development for treatment of psoriasis, which paint with a much broader brush, the TYK2 inhibitor is highly selective for IL-23, IL-12, and interferon alpha.
“Previous studies have shown pan-JAK inhibition can be very effective in remitting psoriasis. The problem is that if one inhibits JAK1 and JAK3, one blocks the transduction of effector cytokines that are essentially there for protective immunity. That could lead to undesirable levels of immunosuppression,” Dr. Krueger explained.
The most important cytokine in the pathogenesis of psoriasis is clearly IL-23, he continued. In cell-based assays, the TYK2 inhibitor has been shown to be 100 times more selective in inhibiting IL-23 , IL-12, and interferon-alpha than JAK 1/3 inhibitors and 3,000 times more selective than JAK 2 inhibitors. This high degree of selectivity makes for fewer off-target effects and for a favorable safety profile.
“There were no major safety signals that would lead you to be concerned,” Dr. Krueger said. Indeed, based upon the encouraging safety and efficacy demonstrated this phase 2 study, a phase 3 program known as POETYK-PSO is underway (POETYK-PSO-1 and POETYK-PSO-2).
The phase 2 clinical trial results were published online in conjunction with the EADV congress.
The TYK2 inhibitor is being developed by Bristol-Myers Squibb. Dr. Krueger reported receiving personal fees as well as research grants paid directly to Rockefeller University from that pharmaceutical company and numerous others.
Source: Papp K et al. N Engl J Med. 2018 Sep 11. doi: 10.1056/NEJMoa1806382.
PARIS – A novel in a phase 2 clinical trial including 267 adults with moderate to severe disease, James G. Krueger, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“I would say the clinical response here is almost dead-on as a copy for ustekinumab, which is an [injectable interleukin] IL-23/IL-12 blocker. And we’re only at 12 weeks here; some of the curves look like they’re on a trajectory to go up further in terms of improvement. So I’m getting a performance with an oral drug that is just so much better than the approved alternatives that we have,” said Dr. Krueger, head of the laboratory of investigative dermatology and professor in clinical investigation at Rockefeller University in New York.
Oral apremilast (Otezla), for example, can’t touch those PASI 75 response rates in patients with moderate to severe psoriasis. Indeed, many psoriasis experts favor reserving apremilast for patients with moderate disease.
The 12-week, double-blind, placebo-controlled study was conducted at 82 sites in the United States and seven other countries. In this dose-ranging study, participants were randomized to the oral selective tyrosine kinase 2 (TYK2) inhibitor, known for the time being as BMS-986165, at 3 mg every other day, 3 mg daily, 3 mg twice a day, 6 mg twice a day, 12 mg daily, or to placebo.
The primary outcome was a 75% or greater reduction from baseline in Psoriasis Area and Severity Index score (PASI 75) at week 12. The TYK2 inhibitor outperformed placebo in dose-dependent fashion starting at the 3 mg/day dose. The PASI 75 rate was 7% with placebo, 9% with 3 mg of BMS-986165 every other day, 39% with 3 mg daily, 69% with 3 mg BID, 67% with 6 mg BID, and 75% with 12 mg/day. All secondary endpoints followed suit.
A striking finding in the phase 2 study was that when the drug was stopped for a month at the end of the 12-week treatment period, for the most part, the PASI 75 response and other clinical benefits were retained.
“I would contrast this to experiments that I have personally done with cyclosporine, where I have cleared people with cyclosporine, stopped it, and a month later every single patient has rip-roaring disease back. So I think this TYK2 inhibitor has some different performance features than just blocking a downstream T-cell transduction molecule,” observed the dermatologist, who is credited as the discoverer of the importance of the T cell in psoriasis pathogenesis.
The strong multidimensional evidence of clinical efficacy in the phase 2 study was supported mechanistically by analysis of skin biopsies obtained on study days 1, 15, and 85. The laboratory studies showed that the oral drug improved molecular, cellular, and clinical biomarkers associated with treatment efficacy. For example, at doses of 3 mg twice a day or higher, the TYK2 inhibitor reduced expression of IL-19 and IL-36A, which are key drivers of keratinocyte activation and epidermal hyperplasia. The drug also markedly decreased expression of genes in the Th17 pathway and essentially normalized expression of the proinflammatory genes beta defensin and S100A9.
In contrast to the Janus kinase (JAK) 1/3 and JAK 2 inhibitors in development for treatment of psoriasis, which paint with a much broader brush, the TYK2 inhibitor is highly selective for IL-23, IL-12, and interferon alpha.
“Previous studies have shown pan-JAK inhibition can be very effective in remitting psoriasis. The problem is that if one inhibits JAK1 and JAK3, one blocks the transduction of effector cytokines that are essentially there for protective immunity. That could lead to undesirable levels of immunosuppression,” Dr. Krueger explained.
The most important cytokine in the pathogenesis of psoriasis is clearly IL-23, he continued. In cell-based assays, the TYK2 inhibitor has been shown to be 100 times more selective in inhibiting IL-23 , IL-12, and interferon-alpha than JAK 1/3 inhibitors and 3,000 times more selective than JAK 2 inhibitors. This high degree of selectivity makes for fewer off-target effects and for a favorable safety profile.
“There were no major safety signals that would lead you to be concerned,” Dr. Krueger said. Indeed, based upon the encouraging safety and efficacy demonstrated this phase 2 study, a phase 3 program known as POETYK-PSO is underway (POETYK-PSO-1 and POETYK-PSO-2).
The phase 2 clinical trial results were published online in conjunction with the EADV congress.
The TYK2 inhibitor is being developed by Bristol-Myers Squibb. Dr. Krueger reported receiving personal fees as well as research grants paid directly to Rockefeller University from that pharmaceutical company and numerous others.
Source: Papp K et al. N Engl J Med. 2018 Sep 11. doi: 10.1056/NEJMoa1806382.
PARIS – A novel in a phase 2 clinical trial including 267 adults with moderate to severe disease, James G. Krueger, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“I would say the clinical response here is almost dead-on as a copy for ustekinumab, which is an [injectable interleukin] IL-23/IL-12 blocker. And we’re only at 12 weeks here; some of the curves look like they’re on a trajectory to go up further in terms of improvement. So I’m getting a performance with an oral drug that is just so much better than the approved alternatives that we have,” said Dr. Krueger, head of the laboratory of investigative dermatology and professor in clinical investigation at Rockefeller University in New York.
Oral apremilast (Otezla), for example, can’t touch those PASI 75 response rates in patients with moderate to severe psoriasis. Indeed, many psoriasis experts favor reserving apremilast for patients with moderate disease.
The 12-week, double-blind, placebo-controlled study was conducted at 82 sites in the United States and seven other countries. In this dose-ranging study, participants were randomized to the oral selective tyrosine kinase 2 (TYK2) inhibitor, known for the time being as BMS-986165, at 3 mg every other day, 3 mg daily, 3 mg twice a day, 6 mg twice a day, 12 mg daily, or to placebo.
The primary outcome was a 75% or greater reduction from baseline in Psoriasis Area and Severity Index score (PASI 75) at week 12. The TYK2 inhibitor outperformed placebo in dose-dependent fashion starting at the 3 mg/day dose. The PASI 75 rate was 7% with placebo, 9% with 3 mg of BMS-986165 every other day, 39% with 3 mg daily, 69% with 3 mg BID, 67% with 6 mg BID, and 75% with 12 mg/day. All secondary endpoints followed suit.
A striking finding in the phase 2 study was that when the drug was stopped for a month at the end of the 12-week treatment period, for the most part, the PASI 75 response and other clinical benefits were retained.
“I would contrast this to experiments that I have personally done with cyclosporine, where I have cleared people with cyclosporine, stopped it, and a month later every single patient has rip-roaring disease back. So I think this TYK2 inhibitor has some different performance features than just blocking a downstream T-cell transduction molecule,” observed the dermatologist, who is credited as the discoverer of the importance of the T cell in psoriasis pathogenesis.
The strong multidimensional evidence of clinical efficacy in the phase 2 study was supported mechanistically by analysis of skin biopsies obtained on study days 1, 15, and 85. The laboratory studies showed that the oral drug improved molecular, cellular, and clinical biomarkers associated with treatment efficacy. For example, at doses of 3 mg twice a day or higher, the TYK2 inhibitor reduced expression of IL-19 and IL-36A, which are key drivers of keratinocyte activation and epidermal hyperplasia. The drug also markedly decreased expression of genes in the Th17 pathway and essentially normalized expression of the proinflammatory genes beta defensin and S100A9.
In contrast to the Janus kinase (JAK) 1/3 and JAK 2 inhibitors in development for treatment of psoriasis, which paint with a much broader brush, the TYK2 inhibitor is highly selective for IL-23, IL-12, and interferon alpha.
“Previous studies have shown pan-JAK inhibition can be very effective in remitting psoriasis. The problem is that if one inhibits JAK1 and JAK3, one blocks the transduction of effector cytokines that are essentially there for protective immunity. That could lead to undesirable levels of immunosuppression,” Dr. Krueger explained.
The most important cytokine in the pathogenesis of psoriasis is clearly IL-23, he continued. In cell-based assays, the TYK2 inhibitor has been shown to be 100 times more selective in inhibiting IL-23 , IL-12, and interferon-alpha than JAK 1/3 inhibitors and 3,000 times more selective than JAK 2 inhibitors. This high degree of selectivity makes for fewer off-target effects and for a favorable safety profile.
“There were no major safety signals that would lead you to be concerned,” Dr. Krueger said. Indeed, based upon the encouraging safety and efficacy demonstrated this phase 2 study, a phase 3 program known as POETYK-PSO is underway (POETYK-PSO-1 and POETYK-PSO-2).
The phase 2 clinical trial results were published online in conjunction with the EADV congress.
The TYK2 inhibitor is being developed by Bristol-Myers Squibb. Dr. Krueger reported receiving personal fees as well as research grants paid directly to Rockefeller University from that pharmaceutical company and numerous others.
Source: Papp K et al. N Engl J Med. 2018 Sep 11. doi: 10.1056/NEJMoa1806382.
REPORTING FROM THE EADV CONGRESS
Key clinical point: A novel selective tyrosine kinase 2 inhibitor achieves response rates previously unheard of in oral therapy for moderate to severe psoriasis.
Major finding: At the top dose of oral BMS-986165 studied to date, the PASI 75 rate at 12 weeks was 75%.
Study details: This eight-country, randomized, double-blind, placebo-controlled phase 2 study included 267 patients with moderate to severe psoriasis.
Disclosures: The study was sponsored by Bristol-Myers Squibb. The presenter reported receiving personal fees and institutional research grants from that pharmaceutical company and numerous others.
Source: Papp K et al. N Engl J Med. 2018 Sep 11. doi: 10.1056/NEJMoa1806382.
Elevated type 2 diabetes risk seen in PsA patients
Patients with incident psoriatic arthritis are at a significantly increased risk of developing type 2 diabetes when compared against patients with psoriasis alone and with the general population, according to recent research published in Rheumatology.
Rachel Charlton, PhD, of the department of pharmacy and pharmacology at the University of Bath (England), and her colleagues performed an analysis of 6,783 incident cases of psoriatic arthritis (PsA) from the U.K. Clinical Practice Research Datalink who were diagnosed during 1998-2014. Patients were between 18 years and 89 years old with a median age of 49 years at PsA diagnosis.
In the study, the researchers randomly matched PsA cases at a 1:4 ratio to either a cohort of general population patients with no PsA, psoriasis, or inflammatory arthritis or a cohort of patients with psoriasis but no PsA or inflammatory arthritis. Patients were followed from match to the point where they either no longer met inclusion criteria for the cohort or received a diagnosis of type 2 diabetes, cerebrovascular disease (CVD), ischemic heart disease (IHD), or peripheral vascular disease (PVD) with a mean follow-up duration of approximately 5.5 years across all patient groups.
Patients in the PsA group had a significantly higher incidence of type 2 diabetes, compared with the general population (adjusted relative risk, 1.40; 95% confidence interval, 1.15-1.70; P = .0007) and psoriasis groups (adjusted RR, 1.53; 95% CI, 1.19-1.97; P = .0009). In the PsA group, risk of CVD (adjusted RR, 1.24; 95% CI, 0.99-1.56; P = .06), IHD (adjusted RR, 1.27; 95% CI, 1.05-1.54; P = .02), and PVD (adjusted RR, 1.40; 95% CI, 1.02-1.92; P = .04) were significantly higher than in the general population but not when compared with the psoriasis group. The overall risk of cardiovascular disease (including CVD, IHD, and PVD) for the PsA group was significantly higher (adjusted RR, 1.29; 95% CI, 1.12-1.48; P = .0005), compared with the general population.
“These results support the proposal in existing clinical guidelines that, in order to reduce cardiovascular risk in patients with PsA, it is important to treat inflammatory disease as well as to screen and treat traditional risk factors early in the disease course,” Ms. Charlton and her colleagues wrote in their study.
This study was funded by a grant from the National Institute for Health Research in the United Kingdom. The authors reported no relevant conflicts of interest.
SOURCE: Charlton RA et al. Rheumatology. 2018 Sep 6. doi: 10.1093/rheumatology/key286.
Patients with incident psoriatic arthritis are at a significantly increased risk of developing type 2 diabetes when compared against patients with psoriasis alone and with the general population, according to recent research published in Rheumatology.
Rachel Charlton, PhD, of the department of pharmacy and pharmacology at the University of Bath (England), and her colleagues performed an analysis of 6,783 incident cases of psoriatic arthritis (PsA) from the U.K. Clinical Practice Research Datalink who were diagnosed during 1998-2014. Patients were between 18 years and 89 years old with a median age of 49 years at PsA diagnosis.
In the study, the researchers randomly matched PsA cases at a 1:4 ratio to either a cohort of general population patients with no PsA, psoriasis, or inflammatory arthritis or a cohort of patients with psoriasis but no PsA or inflammatory arthritis. Patients were followed from match to the point where they either no longer met inclusion criteria for the cohort or received a diagnosis of type 2 diabetes, cerebrovascular disease (CVD), ischemic heart disease (IHD), or peripheral vascular disease (PVD) with a mean follow-up duration of approximately 5.5 years across all patient groups.
Patients in the PsA group had a significantly higher incidence of type 2 diabetes, compared with the general population (adjusted relative risk, 1.40; 95% confidence interval, 1.15-1.70; P = .0007) and psoriasis groups (adjusted RR, 1.53; 95% CI, 1.19-1.97; P = .0009). In the PsA group, risk of CVD (adjusted RR, 1.24; 95% CI, 0.99-1.56; P = .06), IHD (adjusted RR, 1.27; 95% CI, 1.05-1.54; P = .02), and PVD (adjusted RR, 1.40; 95% CI, 1.02-1.92; P = .04) were significantly higher than in the general population but not when compared with the psoriasis group. The overall risk of cardiovascular disease (including CVD, IHD, and PVD) for the PsA group was significantly higher (adjusted RR, 1.29; 95% CI, 1.12-1.48; P = .0005), compared with the general population.
“These results support the proposal in existing clinical guidelines that, in order to reduce cardiovascular risk in patients with PsA, it is important to treat inflammatory disease as well as to screen and treat traditional risk factors early in the disease course,” Ms. Charlton and her colleagues wrote in their study.
This study was funded by a grant from the National Institute for Health Research in the United Kingdom. The authors reported no relevant conflicts of interest.
SOURCE: Charlton RA et al. Rheumatology. 2018 Sep 6. doi: 10.1093/rheumatology/key286.
Patients with incident psoriatic arthritis are at a significantly increased risk of developing type 2 diabetes when compared against patients with psoriasis alone and with the general population, according to recent research published in Rheumatology.
Rachel Charlton, PhD, of the department of pharmacy and pharmacology at the University of Bath (England), and her colleagues performed an analysis of 6,783 incident cases of psoriatic arthritis (PsA) from the U.K. Clinical Practice Research Datalink who were diagnosed during 1998-2014. Patients were between 18 years and 89 years old with a median age of 49 years at PsA diagnosis.
In the study, the researchers randomly matched PsA cases at a 1:4 ratio to either a cohort of general population patients with no PsA, psoriasis, or inflammatory arthritis or a cohort of patients with psoriasis but no PsA or inflammatory arthritis. Patients were followed from match to the point where they either no longer met inclusion criteria for the cohort or received a diagnosis of type 2 diabetes, cerebrovascular disease (CVD), ischemic heart disease (IHD), or peripheral vascular disease (PVD) with a mean follow-up duration of approximately 5.5 years across all patient groups.
Patients in the PsA group had a significantly higher incidence of type 2 diabetes, compared with the general population (adjusted relative risk, 1.40; 95% confidence interval, 1.15-1.70; P = .0007) and psoriasis groups (adjusted RR, 1.53; 95% CI, 1.19-1.97; P = .0009). In the PsA group, risk of CVD (adjusted RR, 1.24; 95% CI, 0.99-1.56; P = .06), IHD (adjusted RR, 1.27; 95% CI, 1.05-1.54; P = .02), and PVD (adjusted RR, 1.40; 95% CI, 1.02-1.92; P = .04) were significantly higher than in the general population but not when compared with the psoriasis group. The overall risk of cardiovascular disease (including CVD, IHD, and PVD) for the PsA group was significantly higher (adjusted RR, 1.29; 95% CI, 1.12-1.48; P = .0005), compared with the general population.
“These results support the proposal in existing clinical guidelines that, in order to reduce cardiovascular risk in patients with PsA, it is important to treat inflammatory disease as well as to screen and treat traditional risk factors early in the disease course,” Ms. Charlton and her colleagues wrote in their study.
This study was funded by a grant from the National Institute for Health Research in the United Kingdom. The authors reported no relevant conflicts of interest.
SOURCE: Charlton RA et al. Rheumatology. 2018 Sep 6. doi: 10.1093/rheumatology/key286.
FROM RHEUMATOLOGY
Key clinical point: It is important to treat inflammatory disease as well as to screen and treat traditional cardiovascular risk factors early in the course of PsA.
Major finding: (adjusted RR = 1.53) and a general population control group (adjusted RR = 1.40).
Study details: An analysis of 6,783 patients with psoriatic arthritis in the U.K. Clinical Practice Research Datalink who were diagnosed between 1998 and 2014.
Disclosures: This study was funded by a grant from the National Institute for Health Research in the United Kingdom. The authors reported no relevant conflicts of interest.
Source: Charlton RA et al. Rheumatology. 2018 Sep 6. doi: 10.1093/rheumatology/key286.
Pruritus linked to wide variety of cancers
A wide variety of hematologic, dermatologic, and solid organ malignancies are associated with pruritus, a large, single-center, retrospective study suggests.
Blacks with pruritus had a higher odds ratio of hematologic malignancies, among others, while whites had higher likelihood of liver, gastrointestinal, respiratory and gynecologic cancers, results of the study show.
The results by race help address a gap in the literature, according to Shawn G. Kwatra, MD, of Johns Hopkins University, Baltimore, and his coinvestigators.
“Little is known about the association between pruritus and malignancy among different ethnic groups,” Dr. Kwatra and his coauthors wrote in the Journal of the American Academy of Dermatology.
The study shows a stronger association with more types of malignancies than has been reported previously, according to the investigators.
“The main difference is that prior studies focused on diagnosis of malignancy after the onset of pruritus, while our study includes malignancies diagnosed on or after pruritus onset,” they wrote.
Retrospective data for the study, which came from the Johns Hopkins Health System, included 16,925 patients aged 18 years or older who presented with itching or pruritus between April 4, 2013 and Dec. 31, 2017.
Of those 16,925 patients, 2,903 were also diagnosed with a concomitant malignancy during that time period. Compared with patients with no itching diagnosis during that time period, the pruritus patients more likely to have a concomitant malignancy, with an OR of 5.76 (95% confidence interval, 5.53-6.00), Dr. Kwatra and his colleagues found.
Malignancies most strongly associated with pruritus included those of the skin, liver, gallbladder and biliary tract, and hematopoietic system.
Among hematologic malignancies, pruritus was most strongly linked to myeloid leukemia and primary cutaneous lymphoma, while among skin cancers, squamous cell carcinoma was most strongly linked.
Whites had higher odds of any malignancy versus blacks, according to investigators, with ORs of 6.12 (95% CI, 5.81-6.46) and 5.61 (95% CI, 5.21-6.04), respectively.
Blacks with pruritus had higher ORs for hematologic and soft tissue malignancies including those of the muscle, fat, and peripheral nerve, investigators said, while whites had higher ORs for skin and liver malignancies.
The investigators also looked at the prevalence of skin eruptions in patients with pruritus and malignancy. “Eruption is variable by malignancy type and points to differing underlying mechanisms of pruritus,” they reported.
The highest rates of skin eruption were in patients with myeloid leukemia at 66%, followed by bone cancers at 58%, lymphocytic leukemia at 57%, multiple myeloma at 53%, and bronchus at 53%. The lowest rates of skin eruption were in patients with gallbladder and biliary tract, colon, pancreas, and liver malignancies.
Dr. Kwatra reported that he is an advisory board member for Menlo Therapeutics and Trevi Therapeutics.
SOURCE: Kwatra SG et al. J Am Acad Dermatol. 2018 Sep 11. doi: 10.1016/j.jaad.2018.08.044.
A wide variety of hematologic, dermatologic, and solid organ malignancies are associated with pruritus, a large, single-center, retrospective study suggests.
Blacks with pruritus had a higher odds ratio of hematologic malignancies, among others, while whites had higher likelihood of liver, gastrointestinal, respiratory and gynecologic cancers, results of the study show.
The results by race help address a gap in the literature, according to Shawn G. Kwatra, MD, of Johns Hopkins University, Baltimore, and his coinvestigators.
“Little is known about the association between pruritus and malignancy among different ethnic groups,” Dr. Kwatra and his coauthors wrote in the Journal of the American Academy of Dermatology.
The study shows a stronger association with more types of malignancies than has been reported previously, according to the investigators.
“The main difference is that prior studies focused on diagnosis of malignancy after the onset of pruritus, while our study includes malignancies diagnosed on or after pruritus onset,” they wrote.
Retrospective data for the study, which came from the Johns Hopkins Health System, included 16,925 patients aged 18 years or older who presented with itching or pruritus between April 4, 2013 and Dec. 31, 2017.
Of those 16,925 patients, 2,903 were also diagnosed with a concomitant malignancy during that time period. Compared with patients with no itching diagnosis during that time period, the pruritus patients more likely to have a concomitant malignancy, with an OR of 5.76 (95% confidence interval, 5.53-6.00), Dr. Kwatra and his colleagues found.
Malignancies most strongly associated with pruritus included those of the skin, liver, gallbladder and biliary tract, and hematopoietic system.
Among hematologic malignancies, pruritus was most strongly linked to myeloid leukemia and primary cutaneous lymphoma, while among skin cancers, squamous cell carcinoma was most strongly linked.
Whites had higher odds of any malignancy versus blacks, according to investigators, with ORs of 6.12 (95% CI, 5.81-6.46) and 5.61 (95% CI, 5.21-6.04), respectively.
Blacks with pruritus had higher ORs for hematologic and soft tissue malignancies including those of the muscle, fat, and peripheral nerve, investigators said, while whites had higher ORs for skin and liver malignancies.
The investigators also looked at the prevalence of skin eruptions in patients with pruritus and malignancy. “Eruption is variable by malignancy type and points to differing underlying mechanisms of pruritus,” they reported.
The highest rates of skin eruption were in patients with myeloid leukemia at 66%, followed by bone cancers at 58%, lymphocytic leukemia at 57%, multiple myeloma at 53%, and bronchus at 53%. The lowest rates of skin eruption were in patients with gallbladder and biliary tract, colon, pancreas, and liver malignancies.
Dr. Kwatra reported that he is an advisory board member for Menlo Therapeutics and Trevi Therapeutics.
SOURCE: Kwatra SG et al. J Am Acad Dermatol. 2018 Sep 11. doi: 10.1016/j.jaad.2018.08.044.
A wide variety of hematologic, dermatologic, and solid organ malignancies are associated with pruritus, a large, single-center, retrospective study suggests.
Blacks with pruritus had a higher odds ratio of hematologic malignancies, among others, while whites had higher likelihood of liver, gastrointestinal, respiratory and gynecologic cancers, results of the study show.
The results by race help address a gap in the literature, according to Shawn G. Kwatra, MD, of Johns Hopkins University, Baltimore, and his coinvestigators.
“Little is known about the association between pruritus and malignancy among different ethnic groups,” Dr. Kwatra and his coauthors wrote in the Journal of the American Academy of Dermatology.
The study shows a stronger association with more types of malignancies than has been reported previously, according to the investigators.
“The main difference is that prior studies focused on diagnosis of malignancy after the onset of pruritus, while our study includes malignancies diagnosed on or after pruritus onset,” they wrote.
Retrospective data for the study, which came from the Johns Hopkins Health System, included 16,925 patients aged 18 years or older who presented with itching or pruritus between April 4, 2013 and Dec. 31, 2017.
Of those 16,925 patients, 2,903 were also diagnosed with a concomitant malignancy during that time period. Compared with patients with no itching diagnosis during that time period, the pruritus patients more likely to have a concomitant malignancy, with an OR of 5.76 (95% confidence interval, 5.53-6.00), Dr. Kwatra and his colleagues found.
Malignancies most strongly associated with pruritus included those of the skin, liver, gallbladder and biliary tract, and hematopoietic system.
Among hematologic malignancies, pruritus was most strongly linked to myeloid leukemia and primary cutaneous lymphoma, while among skin cancers, squamous cell carcinoma was most strongly linked.
Whites had higher odds of any malignancy versus blacks, according to investigators, with ORs of 6.12 (95% CI, 5.81-6.46) and 5.61 (95% CI, 5.21-6.04), respectively.
Blacks with pruritus had higher ORs for hematologic and soft tissue malignancies including those of the muscle, fat, and peripheral nerve, investigators said, while whites had higher ORs for skin and liver malignancies.
The investigators also looked at the prevalence of skin eruptions in patients with pruritus and malignancy. “Eruption is variable by malignancy type and points to differing underlying mechanisms of pruritus,” they reported.
The highest rates of skin eruption were in patients with myeloid leukemia at 66%, followed by bone cancers at 58%, lymphocytic leukemia at 57%, multiple myeloma at 53%, and bronchus at 53%. The lowest rates of skin eruption were in patients with gallbladder and biliary tract, colon, pancreas, and liver malignancies.
Dr. Kwatra reported that he is an advisory board member for Menlo Therapeutics and Trevi Therapeutics.
SOURCE: Kwatra SG et al. J Am Acad Dermatol. 2018 Sep 11. doi: 10.1016/j.jaad.2018.08.044.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point:
Major finding: Blacks with pruritus had higher odds ratios for hematologic and soft tissue malignancies, while whites had higher ORs for skin and liver malignancies.
Study details: A retrospective study of 16,925 adults with itching or pruritus seen at a tertiary care center.
Disclosures: Dr. Kwatra reported serving as an advisory board member for Menlo Therapeutics and Trevi Therapeutics.
Source: Kwatra SG et al. J Am Acad Dermatol. 2018 Sep 11. doi: 10.1016/j.jaad.2018.08.044.
Noninvasive fat removal devices continue to gain popularity
SAN DIEGO – Noninvasive fat removal, such as laser treatment and cryolipolysis, is here to stay.
That’s what Mathew M. Avram, MD, JD, told attendees at the annual Masters of Aesthetics Symposium.
“It does not compare to liposuction, but many patients prefer these devices because there’s less downtime,” he said. “They don’t want pain. They don’t want time away from work.”
In the decade or so since the inception of the noninvasive body contouring field, noninvasive and minimally invasive devices have become far more popular than traditional liposuction, said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital in Boston. “It’s not even close. Among American Society for Dermatologic Surgery [ASDS] members, for every 1 liposuction, there are over 10 noninvasive body sculpting treatments.”
There were 408,000 body-sculpting procedures performed in 2017. More than half of those (208,000) were cryolipolysis, followed by use of radiofrequency (89,000), deoxycholic acid (46,000), laser lipolysis (25,000), “other” procedures (25,000), and tumescent liposuction (15,000), according to data from the ASDS.
“Each treatment has improved in efficacy with time,” Dr. Avram said.
Devices currently cleared by the Food and Drug Administration for the noninvasive removal of fat include ultrasound, lasers, low-level laser therapy, cryolipolysis, and radiofrequency. One of the most common is low-level laser therapy.
“There are several different devices on the market, but there’s no good histology to confirm mechanism of action, so you question what the efficacy is,” said Dr. Avram, who is also the immediate past president of the American Society for Laser Medicine and Surgery.
SculpSure
In the traditional laser domain, the one most commonly used for fat removal is SculpSure, an FDA-cleared hyperthermic 1,060-nm diode laser. It features four flat, nonsuction applicators, a contact cooling system, and it enables the user to treat more than one area in a 25-minute session. There is minimal absorption by melanin.
“You shouldn’t treat over tattoos, however, because if there’s black ink, the tattoo will absorb the wavelength,” Dr. Avram said. “It’s safe in all skin types and there are a variety of different configurations you can use to treat the desired area.”
Initially there is a 4-minute warm-up cycle to achieve the target temperature. Over the next 21 minutes, 1,060-nm energy is delivered via proprietary modulation, alternating between heat and cooling.
“There is definitely some pain with this device,” he said. “Whether or not it’s a relevant endpoint is not known at this point. But typically, if you’re destroying fat with heat there should be some pain. It’s relieved by a period of cooling. A submental fat treatment is now available. I have not personally used it, but it’s the same technology.”
CoolSculpting
Another popular technology is cryolipolysis (CoolSculpting), which was developed at Massachusetts General Hospital by R. Rox Anderson, MD, and Dieter Manstein, MD, PhD.
It’s FDA cleared for noninvasive fat removal and there have been more than 6 million cryolipolysis treatments performed around the world. The purported mechanism of action is selective crystallization of lipids and fat cells at temperatures below freezing. An inflammatory process results in fat reduction over 2-4 months.
“When it first started, cryolipolysis was designed to treat local areas like love handles in males,” Dr. Avram said. “Over time, applicators have been designed to treat different areas, most recently, one for the posterior upper arms and above the knees. There is now a larger, faster CoolSculpting applicator which results in a 35-minute treatment. It’s a little larger, there’s a little less pain, a lower temperature, and it’s a little bit more effective. This has been helpful in our practice in terms of getting more treatment cycles in a visit.”
Postprocedurally, massage may improve clinical results by mobilizing lipid crystals created from treatment.
Extracorporeal shock wave therapy
Another modality to consider is extracorporeal shock wave therapy, which is the application of mechanically generated external sound waves. “It’s not the same as ultrasound or focused ultrasound, and it’s FDA cleared for the treatment of cellulite,” Dr. Avram said.
EmSculpt
A newer innovation, known as High Intensity Focused Electromagnetic (HIFEM) technology (EmSculpt), induces 20,000 forced muscle contractions per session, which leads to supramaximal contractions that can’t be achieved through normal voluntary muscle action.
“The idea is that you’re getting hypertrophy of the muscle to get volumetric growth,” he said. “There’s believed to be a cascaded apoptotic effect, inducing apoptosis and fat disruption.”
Dr. Avram added that HIFEM is nonionizing, nonradiating, nonthermal, and it does not affect sensory nerves. “It’s designed to only stimulate motor neurons. Time will tell in terms of what the ultimate results are with that device.”
truSculpt
Some clinicians are using monopolar radiofrequency with truSculpt, the proprietary delivery of deep radiofrequency energy. This device increases fat temperature between 6 and 10 degrees Celsius with dual frequency at 1 and 2 MHz.
Published data show that 45 degrees Celsius sustained for 3 minutes resulted in a 60% loss of adipose tissue viability (Lasers Surg Med. 2009;41:745-50; Lasers Surg Med. 2010;42:361-70).
“The heat delivery induces cell apoptosis, leading to the removal of those cells by natural healing processes,” said Dr. Avram, who added that he has not used the device. “We need more clinical data to assess this as well.”
Selective photothermolysis
Another technology being evaluated for fat removal is selective photothermolysis, a concept developed at Massachusetts General Hospital in 1983. It extends the theory of selective photothermolysis to target the lipids that make up subcutaneous fat.
“The theory is that you must select a wavelength well absorbed by the target chromophore with a pulse duration shorter than the target’s thermal relaxation time,” Dr. Avram explained. “This produces selective, localized heating with focal destruction of the target with minimal damage to the surrounding tissue. It requires a deeply penetrating wavelength.”
Lipids are a tempting target “because they heat quickly and easily and they do not lose their heat easily to surrounding structures. You want to target fat selectively and confine thermal damage effectively,” he said.
Nearly 10 years ago, Dr. Avram and his associates evaluated the effects of noninvasive 1,210-nm laser exposure on the adipose tissue of 24 patients with skin types 1-5 (Laser Surg Med. 2009;41:401-7).
“The laser pulses were painful, which limited the efficacy,” he said.
The contact cooling device failed in some subjects, and two patients had bulla, but no scarring. Histologic evidence of laser-induced fat damage was observed in 89% of test sites at 4-7 weeks, but dermal damage was also seen.
“This was the first study to show histologic evidence of laser-induced damage to subcutaneous fat,” Dr. Avram said.
Development of selective photothermolysis technology fell off the wayside after the Great Recession of 2008, but it is still being evaluated at Massachusetts General Hospital and other centers.
To optimize the technology, Dr. Avram said that longer pulse durations may target larger volumes of fat. “Cooling is essential to protect the epidermis, as well as to control pain.”
Injectables
Injectables provide a new, minimally invasive means to achieve noninvasive fat removal, Dr. Avram noted.
“Many injectables of questionable efficacy and safety had been available internationally for years,” he said, but none had FDA clearance until 2015, when the agency gave ATX-101 (Kybella) the nod.
ATX-101 is a nonanimal-derived formulation of deoxycholic acid that causes preferential adipocytolysis. Data from a phase 3 trial presented at the 2014 American Society of Plastic Surgery and the American Society of Aesthetic Plastic Surgery meetings showed a statistically significant reduction in submental fat among subjects who received ATX-101, compared with placebo. It requires an average of 2-4 treatments.
“In my experience, it tends not to require that many, but based on MRI, as well as clinician and patient-reported outcomes, there are significant improvements in the visual impact of chin fat,” Dr. Avram said.
Most adverse events are mild to moderate in severity, primarily bruising, pain, and a sensation of numbness to the anesthesia. “They decrease in incidence and severity over successive treatments, and they infrequently lead to discontinuation of treatment,” he said.
For submental fat, clinicians can combine cryolipolysis and deoxycholic acid. “Here, the idea is to assess the amount of fat targeted for treatment,” Dr. Avram said. “If the fat fills the cryolipolysis cup, use cryolipolysis alone. If the fat does not fill the cup, inject deoxycholic acid for a more targeted treatment. If there is residual fat after cryolipolysis, consider treating more focally with deoxycholic acid.”
Both treatments can produce temporary marginal mandibular nerve injury. “It’s not common, but that’s something to include in your consent forms,” he said.
Dr. Avram reported that he has received consulting fees from Allergan, Merz Pharma, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis Biosystems, Invasix, and Zalea.
[email protected]
SAN DIEGO – Noninvasive fat removal, such as laser treatment and cryolipolysis, is here to stay.
That’s what Mathew M. Avram, MD, JD, told attendees at the annual Masters of Aesthetics Symposium.
“It does not compare to liposuction, but many patients prefer these devices because there’s less downtime,” he said. “They don’t want pain. They don’t want time away from work.”
In the decade or so since the inception of the noninvasive body contouring field, noninvasive and minimally invasive devices have become far more popular than traditional liposuction, said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital in Boston. “It’s not even close. Among American Society for Dermatologic Surgery [ASDS] members, for every 1 liposuction, there are over 10 noninvasive body sculpting treatments.”
There were 408,000 body-sculpting procedures performed in 2017. More than half of those (208,000) were cryolipolysis, followed by use of radiofrequency (89,000), deoxycholic acid (46,000), laser lipolysis (25,000), “other” procedures (25,000), and tumescent liposuction (15,000), according to data from the ASDS.
“Each treatment has improved in efficacy with time,” Dr. Avram said.
Devices currently cleared by the Food and Drug Administration for the noninvasive removal of fat include ultrasound, lasers, low-level laser therapy, cryolipolysis, and radiofrequency. One of the most common is low-level laser therapy.
“There are several different devices on the market, but there’s no good histology to confirm mechanism of action, so you question what the efficacy is,” said Dr. Avram, who is also the immediate past president of the American Society for Laser Medicine and Surgery.
SculpSure
In the traditional laser domain, the one most commonly used for fat removal is SculpSure, an FDA-cleared hyperthermic 1,060-nm diode laser. It features four flat, nonsuction applicators, a contact cooling system, and it enables the user to treat more than one area in a 25-minute session. There is minimal absorption by melanin.
“You shouldn’t treat over tattoos, however, because if there’s black ink, the tattoo will absorb the wavelength,” Dr. Avram said. “It’s safe in all skin types and there are a variety of different configurations you can use to treat the desired area.”
Initially there is a 4-minute warm-up cycle to achieve the target temperature. Over the next 21 minutes, 1,060-nm energy is delivered via proprietary modulation, alternating between heat and cooling.
“There is definitely some pain with this device,” he said. “Whether or not it’s a relevant endpoint is not known at this point. But typically, if you’re destroying fat with heat there should be some pain. It’s relieved by a period of cooling. A submental fat treatment is now available. I have not personally used it, but it’s the same technology.”
CoolSculpting
Another popular technology is cryolipolysis (CoolSculpting), which was developed at Massachusetts General Hospital by R. Rox Anderson, MD, and Dieter Manstein, MD, PhD.
It’s FDA cleared for noninvasive fat removal and there have been more than 6 million cryolipolysis treatments performed around the world. The purported mechanism of action is selective crystallization of lipids and fat cells at temperatures below freezing. An inflammatory process results in fat reduction over 2-4 months.
“When it first started, cryolipolysis was designed to treat local areas like love handles in males,” Dr. Avram said. “Over time, applicators have been designed to treat different areas, most recently, one for the posterior upper arms and above the knees. There is now a larger, faster CoolSculpting applicator which results in a 35-minute treatment. It’s a little larger, there’s a little less pain, a lower temperature, and it’s a little bit more effective. This has been helpful in our practice in terms of getting more treatment cycles in a visit.”
Postprocedurally, massage may improve clinical results by mobilizing lipid crystals created from treatment.
Extracorporeal shock wave therapy
Another modality to consider is extracorporeal shock wave therapy, which is the application of mechanically generated external sound waves. “It’s not the same as ultrasound or focused ultrasound, and it’s FDA cleared for the treatment of cellulite,” Dr. Avram said.
EmSculpt
A newer innovation, known as High Intensity Focused Electromagnetic (HIFEM) technology (EmSculpt), induces 20,000 forced muscle contractions per session, which leads to supramaximal contractions that can’t be achieved through normal voluntary muscle action.
“The idea is that you’re getting hypertrophy of the muscle to get volumetric growth,” he said. “There’s believed to be a cascaded apoptotic effect, inducing apoptosis and fat disruption.”
Dr. Avram added that HIFEM is nonionizing, nonradiating, nonthermal, and it does not affect sensory nerves. “It’s designed to only stimulate motor neurons. Time will tell in terms of what the ultimate results are with that device.”
truSculpt
Some clinicians are using monopolar radiofrequency with truSculpt, the proprietary delivery of deep radiofrequency energy. This device increases fat temperature between 6 and 10 degrees Celsius with dual frequency at 1 and 2 MHz.
Published data show that 45 degrees Celsius sustained for 3 minutes resulted in a 60% loss of adipose tissue viability (Lasers Surg Med. 2009;41:745-50; Lasers Surg Med. 2010;42:361-70).
“The heat delivery induces cell apoptosis, leading to the removal of those cells by natural healing processes,” said Dr. Avram, who added that he has not used the device. “We need more clinical data to assess this as well.”
Selective photothermolysis
Another technology being evaluated for fat removal is selective photothermolysis, a concept developed at Massachusetts General Hospital in 1983. It extends the theory of selective photothermolysis to target the lipids that make up subcutaneous fat.
“The theory is that you must select a wavelength well absorbed by the target chromophore with a pulse duration shorter than the target’s thermal relaxation time,” Dr. Avram explained. “This produces selective, localized heating with focal destruction of the target with minimal damage to the surrounding tissue. It requires a deeply penetrating wavelength.”
Lipids are a tempting target “because they heat quickly and easily and they do not lose their heat easily to surrounding structures. You want to target fat selectively and confine thermal damage effectively,” he said.
Nearly 10 years ago, Dr. Avram and his associates evaluated the effects of noninvasive 1,210-nm laser exposure on the adipose tissue of 24 patients with skin types 1-5 (Laser Surg Med. 2009;41:401-7).
“The laser pulses were painful, which limited the efficacy,” he said.
The contact cooling device failed in some subjects, and two patients had bulla, but no scarring. Histologic evidence of laser-induced fat damage was observed in 89% of test sites at 4-7 weeks, but dermal damage was also seen.
“This was the first study to show histologic evidence of laser-induced damage to subcutaneous fat,” Dr. Avram said.
Development of selective photothermolysis technology fell off the wayside after the Great Recession of 2008, but it is still being evaluated at Massachusetts General Hospital and other centers.
To optimize the technology, Dr. Avram said that longer pulse durations may target larger volumes of fat. “Cooling is essential to protect the epidermis, as well as to control pain.”
Injectables
Injectables provide a new, minimally invasive means to achieve noninvasive fat removal, Dr. Avram noted.
“Many injectables of questionable efficacy and safety had been available internationally for years,” he said, but none had FDA clearance until 2015, when the agency gave ATX-101 (Kybella) the nod.
ATX-101 is a nonanimal-derived formulation of deoxycholic acid that causes preferential adipocytolysis. Data from a phase 3 trial presented at the 2014 American Society of Plastic Surgery and the American Society of Aesthetic Plastic Surgery meetings showed a statistically significant reduction in submental fat among subjects who received ATX-101, compared with placebo. It requires an average of 2-4 treatments.
“In my experience, it tends not to require that many, but based on MRI, as well as clinician and patient-reported outcomes, there are significant improvements in the visual impact of chin fat,” Dr. Avram said.
Most adverse events are mild to moderate in severity, primarily bruising, pain, and a sensation of numbness to the anesthesia. “They decrease in incidence and severity over successive treatments, and they infrequently lead to discontinuation of treatment,” he said.
For submental fat, clinicians can combine cryolipolysis and deoxycholic acid. “Here, the idea is to assess the amount of fat targeted for treatment,” Dr. Avram said. “If the fat fills the cryolipolysis cup, use cryolipolysis alone. If the fat does not fill the cup, inject deoxycholic acid for a more targeted treatment. If there is residual fat after cryolipolysis, consider treating more focally with deoxycholic acid.”
Both treatments can produce temporary marginal mandibular nerve injury. “It’s not common, but that’s something to include in your consent forms,” he said.
Dr. Avram reported that he has received consulting fees from Allergan, Merz Pharma, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis Biosystems, Invasix, and Zalea.
[email protected]
SAN DIEGO – Noninvasive fat removal, such as laser treatment and cryolipolysis, is here to stay.
That’s what Mathew M. Avram, MD, JD, told attendees at the annual Masters of Aesthetics Symposium.
“It does not compare to liposuction, but many patients prefer these devices because there’s less downtime,” he said. “They don’t want pain. They don’t want time away from work.”
In the decade or so since the inception of the noninvasive body contouring field, noninvasive and minimally invasive devices have become far more popular than traditional liposuction, said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital in Boston. “It’s not even close. Among American Society for Dermatologic Surgery [ASDS] members, for every 1 liposuction, there are over 10 noninvasive body sculpting treatments.”
There were 408,000 body-sculpting procedures performed in 2017. More than half of those (208,000) were cryolipolysis, followed by use of radiofrequency (89,000), deoxycholic acid (46,000), laser lipolysis (25,000), “other” procedures (25,000), and tumescent liposuction (15,000), according to data from the ASDS.
“Each treatment has improved in efficacy with time,” Dr. Avram said.
Devices currently cleared by the Food and Drug Administration for the noninvasive removal of fat include ultrasound, lasers, low-level laser therapy, cryolipolysis, and radiofrequency. One of the most common is low-level laser therapy.
“There are several different devices on the market, but there’s no good histology to confirm mechanism of action, so you question what the efficacy is,” said Dr. Avram, who is also the immediate past president of the American Society for Laser Medicine and Surgery.
SculpSure
In the traditional laser domain, the one most commonly used for fat removal is SculpSure, an FDA-cleared hyperthermic 1,060-nm diode laser. It features four flat, nonsuction applicators, a contact cooling system, and it enables the user to treat more than one area in a 25-minute session. There is minimal absorption by melanin.
“You shouldn’t treat over tattoos, however, because if there’s black ink, the tattoo will absorb the wavelength,” Dr. Avram said. “It’s safe in all skin types and there are a variety of different configurations you can use to treat the desired area.”
Initially there is a 4-minute warm-up cycle to achieve the target temperature. Over the next 21 minutes, 1,060-nm energy is delivered via proprietary modulation, alternating between heat and cooling.
“There is definitely some pain with this device,” he said. “Whether or not it’s a relevant endpoint is not known at this point. But typically, if you’re destroying fat with heat there should be some pain. It’s relieved by a period of cooling. A submental fat treatment is now available. I have not personally used it, but it’s the same technology.”
CoolSculpting
Another popular technology is cryolipolysis (CoolSculpting), which was developed at Massachusetts General Hospital by R. Rox Anderson, MD, and Dieter Manstein, MD, PhD.
It’s FDA cleared for noninvasive fat removal and there have been more than 6 million cryolipolysis treatments performed around the world. The purported mechanism of action is selective crystallization of lipids and fat cells at temperatures below freezing. An inflammatory process results in fat reduction over 2-4 months.
“When it first started, cryolipolysis was designed to treat local areas like love handles in males,” Dr. Avram said. “Over time, applicators have been designed to treat different areas, most recently, one for the posterior upper arms and above the knees. There is now a larger, faster CoolSculpting applicator which results in a 35-minute treatment. It’s a little larger, there’s a little less pain, a lower temperature, and it’s a little bit more effective. This has been helpful in our practice in terms of getting more treatment cycles in a visit.”
Postprocedurally, massage may improve clinical results by mobilizing lipid crystals created from treatment.
Extracorporeal shock wave therapy
Another modality to consider is extracorporeal shock wave therapy, which is the application of mechanically generated external sound waves. “It’s not the same as ultrasound or focused ultrasound, and it’s FDA cleared for the treatment of cellulite,” Dr. Avram said.
EmSculpt
A newer innovation, known as High Intensity Focused Electromagnetic (HIFEM) technology (EmSculpt), induces 20,000 forced muscle contractions per session, which leads to supramaximal contractions that can’t be achieved through normal voluntary muscle action.
“The idea is that you’re getting hypertrophy of the muscle to get volumetric growth,” he said. “There’s believed to be a cascaded apoptotic effect, inducing apoptosis and fat disruption.”
Dr. Avram added that HIFEM is nonionizing, nonradiating, nonthermal, and it does not affect sensory nerves. “It’s designed to only stimulate motor neurons. Time will tell in terms of what the ultimate results are with that device.”
truSculpt
Some clinicians are using monopolar radiofrequency with truSculpt, the proprietary delivery of deep radiofrequency energy. This device increases fat temperature between 6 and 10 degrees Celsius with dual frequency at 1 and 2 MHz.
Published data show that 45 degrees Celsius sustained for 3 minutes resulted in a 60% loss of adipose tissue viability (Lasers Surg Med. 2009;41:745-50; Lasers Surg Med. 2010;42:361-70).
“The heat delivery induces cell apoptosis, leading to the removal of those cells by natural healing processes,” said Dr. Avram, who added that he has not used the device. “We need more clinical data to assess this as well.”
Selective photothermolysis
Another technology being evaluated for fat removal is selective photothermolysis, a concept developed at Massachusetts General Hospital in 1983. It extends the theory of selective photothermolysis to target the lipids that make up subcutaneous fat.
“The theory is that you must select a wavelength well absorbed by the target chromophore with a pulse duration shorter than the target’s thermal relaxation time,” Dr. Avram explained. “This produces selective, localized heating with focal destruction of the target with minimal damage to the surrounding tissue. It requires a deeply penetrating wavelength.”
Lipids are a tempting target “because they heat quickly and easily and they do not lose their heat easily to surrounding structures. You want to target fat selectively and confine thermal damage effectively,” he said.
Nearly 10 years ago, Dr. Avram and his associates evaluated the effects of noninvasive 1,210-nm laser exposure on the adipose tissue of 24 patients with skin types 1-5 (Laser Surg Med. 2009;41:401-7).
“The laser pulses were painful, which limited the efficacy,” he said.
The contact cooling device failed in some subjects, and two patients had bulla, but no scarring. Histologic evidence of laser-induced fat damage was observed in 89% of test sites at 4-7 weeks, but dermal damage was also seen.
“This was the first study to show histologic evidence of laser-induced damage to subcutaneous fat,” Dr. Avram said.
Development of selective photothermolysis technology fell off the wayside after the Great Recession of 2008, but it is still being evaluated at Massachusetts General Hospital and other centers.
To optimize the technology, Dr. Avram said that longer pulse durations may target larger volumes of fat. “Cooling is essential to protect the epidermis, as well as to control pain.”
Injectables
Injectables provide a new, minimally invasive means to achieve noninvasive fat removal, Dr. Avram noted.
“Many injectables of questionable efficacy and safety had been available internationally for years,” he said, but none had FDA clearance until 2015, when the agency gave ATX-101 (Kybella) the nod.
ATX-101 is a nonanimal-derived formulation of deoxycholic acid that causes preferential adipocytolysis. Data from a phase 3 trial presented at the 2014 American Society of Plastic Surgery and the American Society of Aesthetic Plastic Surgery meetings showed a statistically significant reduction in submental fat among subjects who received ATX-101, compared with placebo. It requires an average of 2-4 treatments.
“In my experience, it tends not to require that many, but based on MRI, as well as clinician and patient-reported outcomes, there are significant improvements in the visual impact of chin fat,” Dr. Avram said.
Most adverse events are mild to moderate in severity, primarily bruising, pain, and a sensation of numbness to the anesthesia. “They decrease in incidence and severity over successive treatments, and they infrequently lead to discontinuation of treatment,” he said.
For submental fat, clinicians can combine cryolipolysis and deoxycholic acid. “Here, the idea is to assess the amount of fat targeted for treatment,” Dr. Avram said. “If the fat fills the cryolipolysis cup, use cryolipolysis alone. If the fat does not fill the cup, inject deoxycholic acid for a more targeted treatment. If there is residual fat after cryolipolysis, consider treating more focally with deoxycholic acid.”
Both treatments can produce temporary marginal mandibular nerve injury. “It’s not common, but that’s something to include in your consent forms,” he said.
Dr. Avram reported that he has received consulting fees from Allergan, Merz Pharma, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis Biosystems, Invasix, and Zalea.
[email protected]
EXPERT ANALYSIS FROM MOAS 2018
Pantothenic acid enhances doxycycline’s antiacne effects
PARIS – in a randomized, double-blind, placebo-controlled trial, Maria A. Santos, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The beauty of this treatment strategy is that it enables acne patients to obtain the therapeutic benefits of oral antibiotic therapy while minimizing exposure in accord with the current push to curb the growing problem of antibiotic resistance. Indeed, adjunctive oral pantothenic acid (also known as vitamin B5) appears to offer a solution to the high rate of clinical deterioration that often follows antibiotic discontinuation, observed Dr. Santos, a dermatologist at University of Santo Tomas Hospital in Manila.
“The use of pantothenic acid as an adjunct may enhance acne therapy and possibly prolong control despite antibiotic discontinuation,” she said.
Dr. Santos reported on 40 patients aged 16-45 years with moderate to severe acne who were randomized to 2 g/day of pantothenic acid or placebo for 16 weeks on top of 6 weeks of oral doxycycline at 100 mg once daily, plus ongoing topical adapalene and benzoyl peroxide gel.
After 8 weeks – that is, 2 weeks after everyone completed 6 weeks on doxycycline – the mean reduction in noninflammatory and total lesion counts was virtually identical in the two groups. For example, there was a 57.7% decrease in total lesion count compared with baseline in the pantothenic acid group and a 55% reduction in placebo-treated controls. Thereafter, however, the response curves diverged. Backsliding occurred in the control group such that their mean reduction in total lesions was 48.4% at 14 weeks and 40.4% at 16 weeks, compared with baseline. In contrast, patients on daily pantothenic acid had a 78.7% reduction in total lesion count at 14 weeks and an 80% decrease from baseline at 16 weeks.
Similarly, at 16 weeks, the mean reduction in noninflammatory lesions was 49% in the pantothenic acid group versus 19.2% in controls, compared with 34%-36% reductions at 10 weeks, even though all patients remained on topical adapalene and benzoyl peroxide throughout.
Mean reduction in inflammatory lesions followed the same trend; however, the numeric advantage in the pantothenic acid group didn’t achieve statistical significance.
Median Dermatology Life Quality Index scores improved over the course of the study in both groups, although significantly more patients in the pantothenic acid group achieved at least a 4-point improvement over baseline, which is considered the minimum clinically important difference. Moreover, while modified Global Severity Scores and subjective self-assessment scores improved in both groups, from week 12 onwards, the improvements were significantly greater in the pantothenic acid group.
Receiving adjunctive pantothenic acid for 10 weeks was safe. Adverse events were the same in both study arms, consisting chiefly of mild erythema, scaling, dryness, and nausea during the initial weeks on doxycycline.
Pantothenic acid is a water-soluble essential nutrient. Dr. Santos said that while the precise mechanism of the benefit seen in this randomized trial isn’t known, other investigators have generated evidence suggestive of enhanced epidermal barrier function through normalization of keratinocyte proliferation and differentiation in acne patients, coupled with antioxidant and anti-inflammatory effects.
Dr. Santos reported having no financial conflicts regarding her study, conducted free of commercial support.
PARIS – in a randomized, double-blind, placebo-controlled trial, Maria A. Santos, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The beauty of this treatment strategy is that it enables acne patients to obtain the therapeutic benefits of oral antibiotic therapy while minimizing exposure in accord with the current push to curb the growing problem of antibiotic resistance. Indeed, adjunctive oral pantothenic acid (also known as vitamin B5) appears to offer a solution to the high rate of clinical deterioration that often follows antibiotic discontinuation, observed Dr. Santos, a dermatologist at University of Santo Tomas Hospital in Manila.
“The use of pantothenic acid as an adjunct may enhance acne therapy and possibly prolong control despite antibiotic discontinuation,” she said.
Dr. Santos reported on 40 patients aged 16-45 years with moderate to severe acne who were randomized to 2 g/day of pantothenic acid or placebo for 16 weeks on top of 6 weeks of oral doxycycline at 100 mg once daily, plus ongoing topical adapalene and benzoyl peroxide gel.
After 8 weeks – that is, 2 weeks after everyone completed 6 weeks on doxycycline – the mean reduction in noninflammatory and total lesion counts was virtually identical in the two groups. For example, there was a 57.7% decrease in total lesion count compared with baseline in the pantothenic acid group and a 55% reduction in placebo-treated controls. Thereafter, however, the response curves diverged. Backsliding occurred in the control group such that their mean reduction in total lesions was 48.4% at 14 weeks and 40.4% at 16 weeks, compared with baseline. In contrast, patients on daily pantothenic acid had a 78.7% reduction in total lesion count at 14 weeks and an 80% decrease from baseline at 16 weeks.
Similarly, at 16 weeks, the mean reduction in noninflammatory lesions was 49% in the pantothenic acid group versus 19.2% in controls, compared with 34%-36% reductions at 10 weeks, even though all patients remained on topical adapalene and benzoyl peroxide throughout.
Mean reduction in inflammatory lesions followed the same trend; however, the numeric advantage in the pantothenic acid group didn’t achieve statistical significance.
Median Dermatology Life Quality Index scores improved over the course of the study in both groups, although significantly more patients in the pantothenic acid group achieved at least a 4-point improvement over baseline, which is considered the minimum clinically important difference. Moreover, while modified Global Severity Scores and subjective self-assessment scores improved in both groups, from week 12 onwards, the improvements were significantly greater in the pantothenic acid group.
Receiving adjunctive pantothenic acid for 10 weeks was safe. Adverse events were the same in both study arms, consisting chiefly of mild erythema, scaling, dryness, and nausea during the initial weeks on doxycycline.
Pantothenic acid is a water-soluble essential nutrient. Dr. Santos said that while the precise mechanism of the benefit seen in this randomized trial isn’t known, other investigators have generated evidence suggestive of enhanced epidermal barrier function through normalization of keratinocyte proliferation and differentiation in acne patients, coupled with antioxidant and anti-inflammatory effects.
Dr. Santos reported having no financial conflicts regarding her study, conducted free of commercial support.
PARIS – in a randomized, double-blind, placebo-controlled trial, Maria A. Santos, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The beauty of this treatment strategy is that it enables acne patients to obtain the therapeutic benefits of oral antibiotic therapy while minimizing exposure in accord with the current push to curb the growing problem of antibiotic resistance. Indeed, adjunctive oral pantothenic acid (also known as vitamin B5) appears to offer a solution to the high rate of clinical deterioration that often follows antibiotic discontinuation, observed Dr. Santos, a dermatologist at University of Santo Tomas Hospital in Manila.
“The use of pantothenic acid as an adjunct may enhance acne therapy and possibly prolong control despite antibiotic discontinuation,” she said.
Dr. Santos reported on 40 patients aged 16-45 years with moderate to severe acne who were randomized to 2 g/day of pantothenic acid or placebo for 16 weeks on top of 6 weeks of oral doxycycline at 100 mg once daily, plus ongoing topical adapalene and benzoyl peroxide gel.
After 8 weeks – that is, 2 weeks after everyone completed 6 weeks on doxycycline – the mean reduction in noninflammatory and total lesion counts was virtually identical in the two groups. For example, there was a 57.7% decrease in total lesion count compared with baseline in the pantothenic acid group and a 55% reduction in placebo-treated controls. Thereafter, however, the response curves diverged. Backsliding occurred in the control group such that their mean reduction in total lesions was 48.4% at 14 weeks and 40.4% at 16 weeks, compared with baseline. In contrast, patients on daily pantothenic acid had a 78.7% reduction in total lesion count at 14 weeks and an 80% decrease from baseline at 16 weeks.
Similarly, at 16 weeks, the mean reduction in noninflammatory lesions was 49% in the pantothenic acid group versus 19.2% in controls, compared with 34%-36% reductions at 10 weeks, even though all patients remained on topical adapalene and benzoyl peroxide throughout.
Mean reduction in inflammatory lesions followed the same trend; however, the numeric advantage in the pantothenic acid group didn’t achieve statistical significance.
Median Dermatology Life Quality Index scores improved over the course of the study in both groups, although significantly more patients in the pantothenic acid group achieved at least a 4-point improvement over baseline, which is considered the minimum clinically important difference. Moreover, while modified Global Severity Scores and subjective self-assessment scores improved in both groups, from week 12 onwards, the improvements were significantly greater in the pantothenic acid group.
Receiving adjunctive pantothenic acid for 10 weeks was safe. Adverse events were the same in both study arms, consisting chiefly of mild erythema, scaling, dryness, and nausea during the initial weeks on doxycycline.
Pantothenic acid is a water-soluble essential nutrient. Dr. Santos said that while the precise mechanism of the benefit seen in this randomized trial isn’t known, other investigators have generated evidence suggestive of enhanced epidermal barrier function through normalization of keratinocyte proliferation and differentiation in acne patients, coupled with antioxidant and anti-inflammatory effects.
Dr. Santos reported having no financial conflicts regarding her study, conducted free of commercial support.
REPORTING FROM THE EADV CONGRESS
Key clinical point: Oral pantothenic acid at 2 g/day appears to be a safe and effective adjunct to oral doxycycline for moderate to severe acne.
Major finding: Acne patients on oral pantothenic acid had a mean 80% reduction in total lesion count 10 weeks after completing 6 weeks on oral doxycycline, a rate twice that of placebo-treated controls.
Study details: This 16-week, prospective, randomized, double-blind, placebo-controlled study included 40 patients with moderate to severe acne.
Disclosures: Dr. Santos reported having no financial conflicts regarding her study, conducted free of commercial support.