User login
Company submits supplemental NDA for topical atopic dermatitis treatment
in adults and children aged 6 years and older.
Roflumilast cream 0.3% (Zoryve) is currently approved by the FDA for the topical treatment of plaque psoriasis, including intertriginous areas, in patients 12 years of age and older. Submission of the sNDA is based on positive results from the Interventional Trial Evaluating Roflumilast Cream for the Treatment of Atopic Dermatitis (INTEGUMENT-1 and INTEGUMENT-2) trials; two identical Phase 3, vehicle-controlled trials in which roflumilast cream 0.15% or vehicle was applied once daily for 4 weeks to individuals 6 years of age and older with mild to moderate AD involving at least 3% body surface area. Roflumilast is a phosphodiesterase-4 (PDE-4) inhibitor.
According to a press release from Arcutis, both studies met the primary endpoint of IGA Success, which was defined as a validated Investigator Global Assessment – Atopic Dermatitis (vIGA-AD) score of ‘clear’ or ‘almost clear’ plus a 2-grade improvement from baseline at week 4. In INTEGUMENT-1 this endpoint was achieved by 32.0% of subjects in the roflumilast cream group vs. 15.2% of those in the vehicle group (P < .0001). In INTEGUMENT-2, this endpoint was achieved by 28.9% of subjects in the roflumilast cream group vs. 12.0% of those in the vehicle group (P < .0001). The most common adverse reactions based on data from the combined trials were headache (2.9%), nausea (1.9%), application-site pain (1.5%), diarrhea (1.5%), and vomiting (1.5%).
in adults and children aged 6 years and older.
Roflumilast cream 0.3% (Zoryve) is currently approved by the FDA for the topical treatment of plaque psoriasis, including intertriginous areas, in patients 12 years of age and older. Submission of the sNDA is based on positive results from the Interventional Trial Evaluating Roflumilast Cream for the Treatment of Atopic Dermatitis (INTEGUMENT-1 and INTEGUMENT-2) trials; two identical Phase 3, vehicle-controlled trials in which roflumilast cream 0.15% or vehicle was applied once daily for 4 weeks to individuals 6 years of age and older with mild to moderate AD involving at least 3% body surface area. Roflumilast is a phosphodiesterase-4 (PDE-4) inhibitor.
According to a press release from Arcutis, both studies met the primary endpoint of IGA Success, which was defined as a validated Investigator Global Assessment – Atopic Dermatitis (vIGA-AD) score of ‘clear’ or ‘almost clear’ plus a 2-grade improvement from baseline at week 4. In INTEGUMENT-1 this endpoint was achieved by 32.0% of subjects in the roflumilast cream group vs. 15.2% of those in the vehicle group (P < .0001). In INTEGUMENT-2, this endpoint was achieved by 28.9% of subjects in the roflumilast cream group vs. 12.0% of those in the vehicle group (P < .0001). The most common adverse reactions based on data from the combined trials were headache (2.9%), nausea (1.9%), application-site pain (1.5%), diarrhea (1.5%), and vomiting (1.5%).
in adults and children aged 6 years and older.
Roflumilast cream 0.3% (Zoryve) is currently approved by the FDA for the topical treatment of plaque psoriasis, including intertriginous areas, in patients 12 years of age and older. Submission of the sNDA is based on positive results from the Interventional Trial Evaluating Roflumilast Cream for the Treatment of Atopic Dermatitis (INTEGUMENT-1 and INTEGUMENT-2) trials; two identical Phase 3, vehicle-controlled trials in which roflumilast cream 0.15% or vehicle was applied once daily for 4 weeks to individuals 6 years of age and older with mild to moderate AD involving at least 3% body surface area. Roflumilast is a phosphodiesterase-4 (PDE-4) inhibitor.
According to a press release from Arcutis, both studies met the primary endpoint of IGA Success, which was defined as a validated Investigator Global Assessment – Atopic Dermatitis (vIGA-AD) score of ‘clear’ or ‘almost clear’ plus a 2-grade improvement from baseline at week 4. In INTEGUMENT-1 this endpoint was achieved by 32.0% of subjects in the roflumilast cream group vs. 15.2% of those in the vehicle group (P < .0001). In INTEGUMENT-2, this endpoint was achieved by 28.9% of subjects in the roflumilast cream group vs. 12.0% of those in the vehicle group (P < .0001). The most common adverse reactions based on data from the combined trials were headache (2.9%), nausea (1.9%), application-site pain (1.5%), diarrhea (1.5%), and vomiting (1.5%).
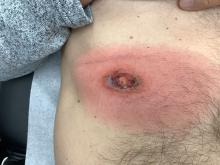
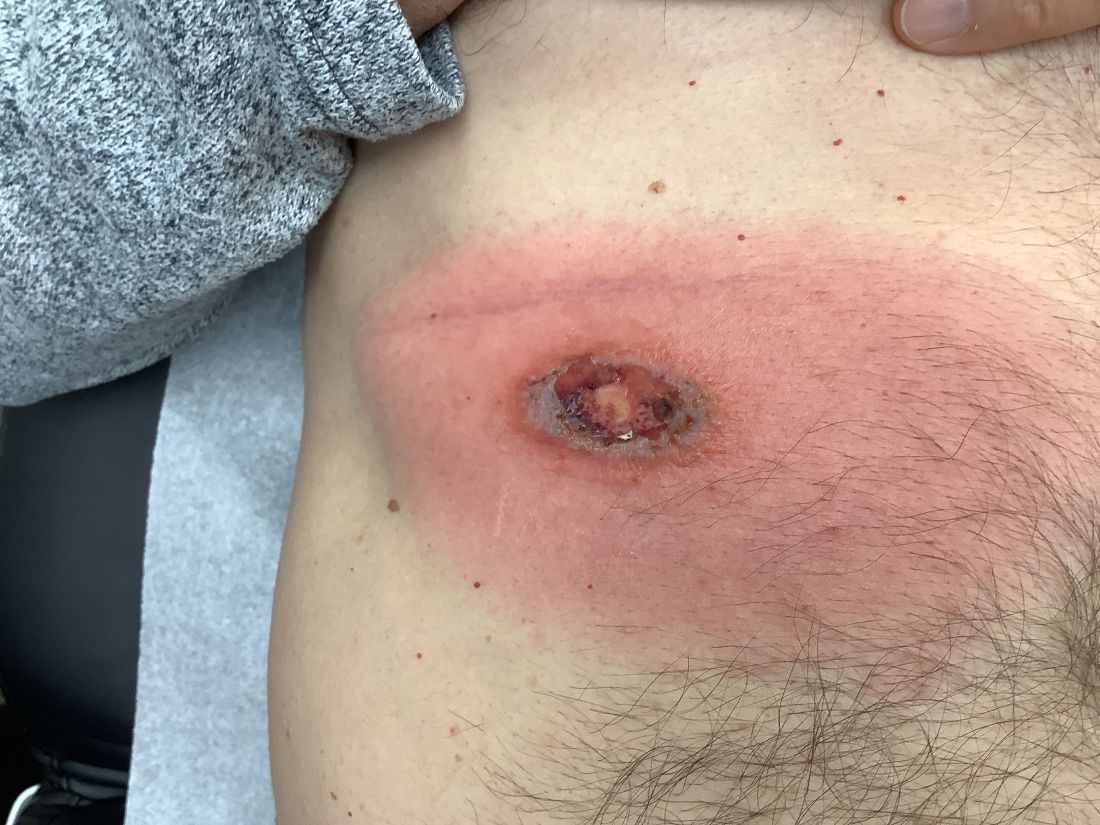
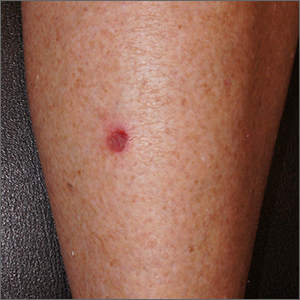
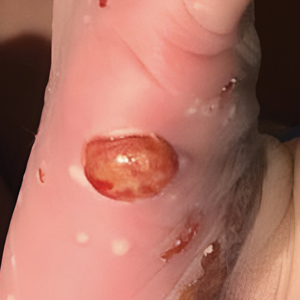
A White male presented with a purulent erythematous edematous plaque with central necrosis and ulceration on his right flank
Lyme disease is the most commonly transmitted tick-borne illness in the United States. This infection is typically transmitted through a bite by the Ixodes tick commonly found in the Midwest, Northeast, and mid-Atlantic regions; however, the geographical distribution continues to expand over time in the United States. Ticks must be attached for 24-48 hours to transmit the pathogen. There are three general stages of the disease: early localized, early disseminated, and late disseminated.
The most common presentation is the early localized disease, which manifests between 3 and 30 days after an infected tick bite. Approximately 70%-80% of cases feature a targetlike lesion that expands centrifugally at the site of the bite. Most commonly, lesions appear on the abdomen, groin, axilla, and popliteal fossa. The diagnosis of ECM requires lesions at least 5 cm in size. Lesions may be asymptomatic, although burning may occur in half of patients. Atypical presentations include bullous, vesicular, hemorrhagic, or necrotic lesions. Up to half of patients may develop multiple ECM lesions. Palms and soles are spared. Differential diagnoses include arthropod reactions, pyoderma gangrenosum, cellulitis, herpes simplex virus and varicella zoster virus, contact dermatitis, or granuloma annulare. The rash is often accompanied by systemic symptoms including fatigue, myalgia, headache, and fever.
The next two stages include early and late disseminated infection. Early disseminated infection often occurs 3-12 weeks after infection and is characterized by muscle pain, dizziness, headache, and cardiac symptoms. CNS involvement occurs in about 20% of patients. Joint involvement may include the knee, ankle, and wrist. If symptoms are only in one joint, septic arthritis is part of the differential diagnosis, so clinical correlation and labs must be considered. Late disseminated infection occurs months or years after initial infection and includes neurologic and rheumatologic symptoms including meningitis, Bell’s palsy, arthritis, and dysesthesia. Knee arthritis is a key feature of this stage. Patients commonly have radicular pain and fibromyalgia-type pain. More severe disease processes include encephalomyelitis, arrhythmias, and heart block.
ECM is often a clinical diagnosis because serologic testing may not be positive during the first 2 weeks of infection. The screening serologic test is the ELISA, and a Western blot confirms the results. Skin histopathology for Lyme disease is often nonspecific and reveals a perivascular infiltrate of histiocytes, plasma cells, and lymphocytes. Silver stain or antibody testing may be used to identify the spirochete. In acrodermatitis chronica atrophicans, late Lyme disease presenting on the distal extremities, lymphocytic and plasma cell infiltrates are present. In borrelial lymphocytoma, a dense dermal lymphocytic infiltrate is present.
The standard for treatment of early localized disease is oral doxycycline in adults. Alternatives may be used if a patient is allergic or for children under 9. Disseminated disease may be treated with IV ceftriaxone and topical steroids are used if ocular symptoms are involved. Early treatment is often curative.
This patient’s antibodies were negative initially, but became positive after 6 weeks. He was treated empirically at the time of his office visit with doxycycline for 1 month.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to [email protected].
References
Carriveau A et al. Nurs Clin North Am. 2019 Jun;54(2):261-75.
Skar GL and Simonsen KA. Lyme Disease. [Updated 2023 May 31]. In: “StatPearls” [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan.
Tiger JB et al. J Am Acad Dermatol. 2014 Oct;71(4):e133-4.
Lyme disease is the most commonly transmitted tick-borne illness in the United States. This infection is typically transmitted through a bite by the Ixodes tick commonly found in the Midwest, Northeast, and mid-Atlantic regions; however, the geographical distribution continues to expand over time in the United States. Ticks must be attached for 24-48 hours to transmit the pathogen. There are three general stages of the disease: early localized, early disseminated, and late disseminated.
The most common presentation is the early localized disease, which manifests between 3 and 30 days after an infected tick bite. Approximately 70%-80% of cases feature a targetlike lesion that expands centrifugally at the site of the bite. Most commonly, lesions appear on the abdomen, groin, axilla, and popliteal fossa. The diagnosis of ECM requires lesions at least 5 cm in size. Lesions may be asymptomatic, although burning may occur in half of patients. Atypical presentations include bullous, vesicular, hemorrhagic, or necrotic lesions. Up to half of patients may develop multiple ECM lesions. Palms and soles are spared. Differential diagnoses include arthropod reactions, pyoderma gangrenosum, cellulitis, herpes simplex virus and varicella zoster virus, contact dermatitis, or granuloma annulare. The rash is often accompanied by systemic symptoms including fatigue, myalgia, headache, and fever.
The next two stages include early and late disseminated infection. Early disseminated infection often occurs 3-12 weeks after infection and is characterized by muscle pain, dizziness, headache, and cardiac symptoms. CNS involvement occurs in about 20% of patients. Joint involvement may include the knee, ankle, and wrist. If symptoms are only in one joint, septic arthritis is part of the differential diagnosis, so clinical correlation and labs must be considered. Late disseminated infection occurs months or years after initial infection and includes neurologic and rheumatologic symptoms including meningitis, Bell’s palsy, arthritis, and dysesthesia. Knee arthritis is a key feature of this stage. Patients commonly have radicular pain and fibromyalgia-type pain. More severe disease processes include encephalomyelitis, arrhythmias, and heart block.
ECM is often a clinical diagnosis because serologic testing may not be positive during the first 2 weeks of infection. The screening serologic test is the ELISA, and a Western blot confirms the results. Skin histopathology for Lyme disease is often nonspecific and reveals a perivascular infiltrate of histiocytes, plasma cells, and lymphocytes. Silver stain or antibody testing may be used to identify the spirochete. In acrodermatitis chronica atrophicans, late Lyme disease presenting on the distal extremities, lymphocytic and plasma cell infiltrates are present. In borrelial lymphocytoma, a dense dermal lymphocytic infiltrate is present.
The standard for treatment of early localized disease is oral doxycycline in adults. Alternatives may be used if a patient is allergic or for children under 9. Disseminated disease may be treated with IV ceftriaxone and topical steroids are used if ocular symptoms are involved. Early treatment is often curative.
This patient’s antibodies were negative initially, but became positive after 6 weeks. He was treated empirically at the time of his office visit with doxycycline for 1 month.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to [email protected].
References
Carriveau A et al. Nurs Clin North Am. 2019 Jun;54(2):261-75.
Skar GL and Simonsen KA. Lyme Disease. [Updated 2023 May 31]. In: “StatPearls” [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan.
Tiger JB et al. J Am Acad Dermatol. 2014 Oct;71(4):e133-4.
Lyme disease is the most commonly transmitted tick-borne illness in the United States. This infection is typically transmitted through a bite by the Ixodes tick commonly found in the Midwest, Northeast, and mid-Atlantic regions; however, the geographical distribution continues to expand over time in the United States. Ticks must be attached for 24-48 hours to transmit the pathogen. There are three general stages of the disease: early localized, early disseminated, and late disseminated.
The most common presentation is the early localized disease, which manifests between 3 and 30 days after an infected tick bite. Approximately 70%-80% of cases feature a targetlike lesion that expands centrifugally at the site of the bite. Most commonly, lesions appear on the abdomen, groin, axilla, and popliteal fossa. The diagnosis of ECM requires lesions at least 5 cm in size. Lesions may be asymptomatic, although burning may occur in half of patients. Atypical presentations include bullous, vesicular, hemorrhagic, or necrotic lesions. Up to half of patients may develop multiple ECM lesions. Palms and soles are spared. Differential diagnoses include arthropod reactions, pyoderma gangrenosum, cellulitis, herpes simplex virus and varicella zoster virus, contact dermatitis, or granuloma annulare. The rash is often accompanied by systemic symptoms including fatigue, myalgia, headache, and fever.
The next two stages include early and late disseminated infection. Early disseminated infection often occurs 3-12 weeks after infection and is characterized by muscle pain, dizziness, headache, and cardiac symptoms. CNS involvement occurs in about 20% of patients. Joint involvement may include the knee, ankle, and wrist. If symptoms are only in one joint, septic arthritis is part of the differential diagnosis, so clinical correlation and labs must be considered. Late disseminated infection occurs months or years after initial infection and includes neurologic and rheumatologic symptoms including meningitis, Bell’s palsy, arthritis, and dysesthesia. Knee arthritis is a key feature of this stage. Patients commonly have radicular pain and fibromyalgia-type pain. More severe disease processes include encephalomyelitis, arrhythmias, and heart block.
ECM is often a clinical diagnosis because serologic testing may not be positive during the first 2 weeks of infection. The screening serologic test is the ELISA, and a Western blot confirms the results. Skin histopathology for Lyme disease is often nonspecific and reveals a perivascular infiltrate of histiocytes, plasma cells, and lymphocytes. Silver stain or antibody testing may be used to identify the spirochete. In acrodermatitis chronica atrophicans, late Lyme disease presenting on the distal extremities, lymphocytic and plasma cell infiltrates are present. In borrelial lymphocytoma, a dense dermal lymphocytic infiltrate is present.
The standard for treatment of early localized disease is oral doxycycline in adults. Alternatives may be used if a patient is allergic or for children under 9. Disseminated disease may be treated with IV ceftriaxone and topical steroids are used if ocular symptoms are involved. Early treatment is often curative.
This patient’s antibodies were negative initially, but became positive after 6 weeks. He was treated empirically at the time of his office visit with doxycycline for 1 month.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to [email protected].
References
Carriveau A et al. Nurs Clin North Am. 2019 Jun;54(2):261-75.
Skar GL and Simonsen KA. Lyme Disease. [Updated 2023 May 31]. In: “StatPearls” [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan.
Tiger JB et al. J Am Acad Dermatol. 2014 Oct;71(4):e133-4.
A worthwhile tool in evaluating worrisome lesions
ABSTRACT
Background: We sought to examine whether electrical impedance spectroscopy (EIS), a diagnostic tool approved by the US Food and Drug Administration for the evaluation of pigmented skin lesions (PSLs), is beneficial to primary care providers (PCPs) by comparing the accuracy of PCPs’ management decisions for PSLs based on visual examination alone with those based on concurrent visual and EIS evaluation.
Methods: Physicians and nurse practitioners (NPs) participated in an anonymous online survey in which they viewed clinical images of PSLs and were asked to make 2 clinical decisions before and after being provided an EIS score that indicated the likelihood that the lesion was a melanoma. They were asked (1) if they would biopsy the lesion/refer the patient out and (2) what they expected the pathology results would show.
Results: Forty-four physicians and 17 NPs participated, making clinical decisions for 1354 presented lesions. Overall, with the addition of EIS to visual inspection of clinical images, the sensitivity of biopsy/referral decisions for melanomas and severely dysplastic nevi (SDN) increased from 69.2% to 90.0% (P < .001), while specificity increased from 44.0% to 72.6% (P < .001). Physicians and NPs, regardless of years of experience, each saw significant improvements in sensitivity, specificity, and diagnostic accuracy with the addition of EIS scores.
Conclusions: The incorporation of EIS data into clinical decision-making by PCPs significantly increased the sensitivity and specificity of biopsy/referral decisions for melanomas and SDN and overall diagnostic accuracy compared with visual inspection alone. The results of this study suggest that diagnostic accuracy for PSLs by PCPs may be improved with adjunctive use of EIS with visual inspection.
Primary care providers (PCPs) are often the first line of defense in detecting skin cancers. For patients with concerning skin lesions, PCPs may choose to perform a biopsy or facilitate access to specialty services (eg, Dermatology). Consequently, PCPs play a critical role in the timely detection of skin cancers, and it is paramount to employ continually improving detection methods, such as the application of technologic advances.1
Differentiating benign nevi from melanoma and severely dysplastic nevi (SDN), both of which warrant excision, poses a unique challenge to clinicians examining pigmented skin lesions (PSLs). PCPs often rely on visual inspection to differentiate benign skin lesions from malignant skin cancers. In some primary care practices, dermoscopy, which involves using a handheld device to evaluate lesions with polarized light and magnification, is used to improve melanoma detection. However, while visual inspection and dermoscopy are valid, effective techniques for the diagnosis of melanocytic lesions, in many instances they still can lead to missed cancers or unnecessary biopsies and specialty referrals. Adjunctive use of dermoscopy with visual inspection has been shown to increase the probability of skin cancer detection, but it fails to achieve a near-100% success rate.2 Furthermore, dermoscopy is heavily user-dependent, requiring significant training and experience for appropriate use.3
Another option is an electrical impedance spectroscopy (EIS) device (Nevisense, Scibase, Stockholm, Sweden), which has been approved by the US Food and Drug Administration (FDA) to assist in the detection of melanoma and differentiation from benign PSLs.4 EIS is a noninvasive, rapidly applied technology designed to accompany the visual examination of melanocytic lesions in office, with or without dermoscopy. Still relatively new, the technology is employed today by many dermatologists, increasing diagnostic accuracy for PSLs.5 The lightweight and portable instrument features a handheld probe, which is held against a lesion to obtain a reading. EIS uses a low-voltage electrode to apply a harmless electrical current to the skin at various frequencies.6 As benign and malignant tissues vary in cell shape, size, and composition, EIS distinguishes differential electrical resistance of the tissue to aid in diagnosis.7
Continue to: EIS provides high-sensitivity...
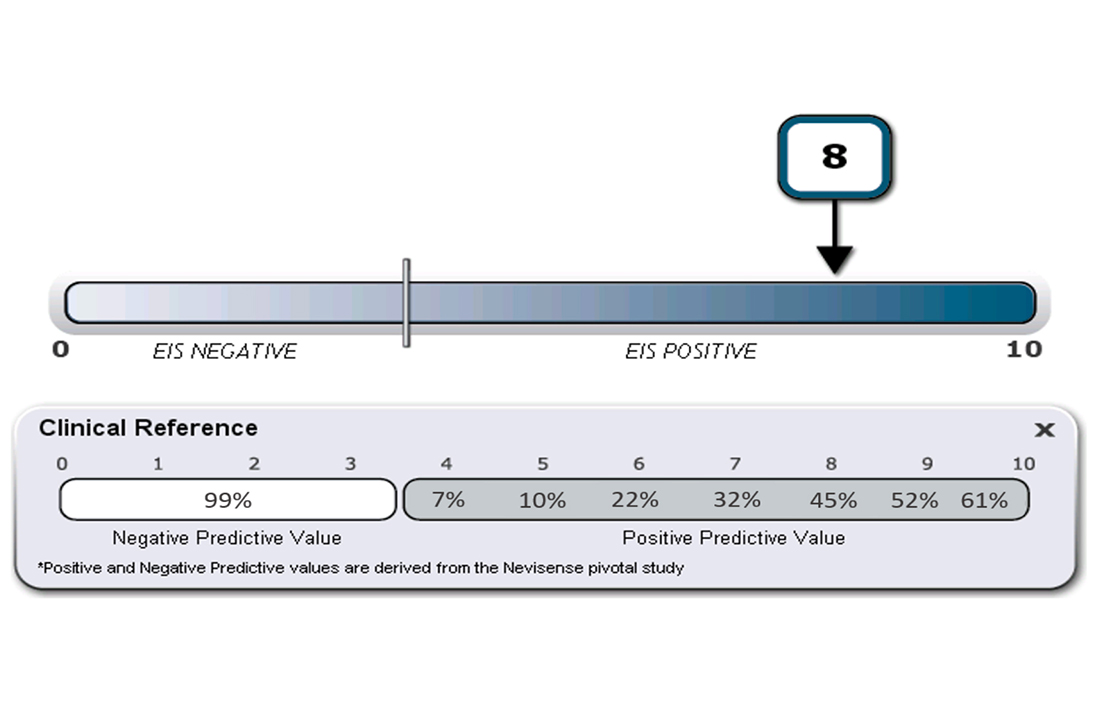
EIS provides high-sensitivity melanoma diagnosis vs histopathologic confirmation from biopsies, with 1 study showing a 96.6% sensitivity rating, detecting 256 of 265 melanomas.4 The EIS device, by measuring differences in electrical resistance between benign and cancerous cells, outputs a simple integer score ranging from 0 to 10 associated with the likelihood of the lesion being a melanoma.8 Based on data from the Nevisense pivotal trial,4 Nevisense reports that scores of 0 to 3 carry a negative predictive value of 99% for melanoma, whereas scores of 4 to 10 signify increasingly greater positive predictive values from 7% to 61%.
We aimed to assess whether EIS may be beneficial to PCPs by comparing the accuracy of clinical decision-making for PSLs based on visual examination alone with that based on concurrent visual and EIS evaluation.
METHODS
A questionnaire was distributed via email to 142 clinicians at clinics affiliated with either of 2 organizations delivering care to the New York City area through a network of community health centers: the Institute for Family Health (IFH) and the Community Healthcare Network (CHN). Of these recipients, 72 were affiliated with IFH across 27 community health centers and 70 were affiliated with CHN across 14 community health centers. Recipients were physicians and nurse practitioners (NPs) practicing at primary health care facilities.
Survey instrument. The first section of the survey instrument (APPENDIX) solicited demographic information and explained how to apply the EIS scores for diagnostic decision-making. The second featured images of 12 randomly selected, histologically confirmed, and EIS-evaluated PSLs from a previously published prospective blinded trial of 2416 lesions.4 The Institutional Review Board of the Icahn School of Medicine at Mount Sinai reviewed and approved the study and survey instrument.
Clinical images of these lesions, comprising 4 melanocytic nevi, 4 dysplastic nevi (including 3 mild-moderately dysplastic and 1 severely dysplastic nevus), and 4 melanomas
Continue to: Analysis
Analysis. A biopsy or referral rating of 4 or 5 was considered a decision to biopsy or refer (ie, a diagnostic decision consistent with melanoma or SDN warranting excision), whereas a selection of 1 to 3 was considered a decision not to biopsy or refer (ie, a diagnostic decision consistent with a benign PSL). The sensitivity and specificity of biopsy/referral decisions for melanomas and SDN, the proportion of missed melanomas and SDN, and the proportion of biopsy/referral decisions for benign lesions were separately determined for visual inspection alone and visual inspection with EIS score. Similarly, diagnostic accuracy was calculated for these clinical scenarios. These metrics were further stratified among different subsets of the respondent population. Differences in sensitivity, specificity, biopsy/referral decision proportions, and diagnostic accuracy were calculated using McNemar’s test for paired proportions.
RESULTS
Sixty-one respondents, comprising 44 physicians and 17 NPs, completed the survey, yielding a response rate of 43% (TABLE 1). In total, 1354 clinical decisions (677 based on visual inspection alone and 677 based on visual inspection plus EIS) were made. A biopsy/referral decision was made after assessing 416 of 677 cases (61%) with visual inspection alone and 360 of 677 cases (53%) when relying on visual inspection plus EIS. None of the respondents reported any prior experience with EIS.
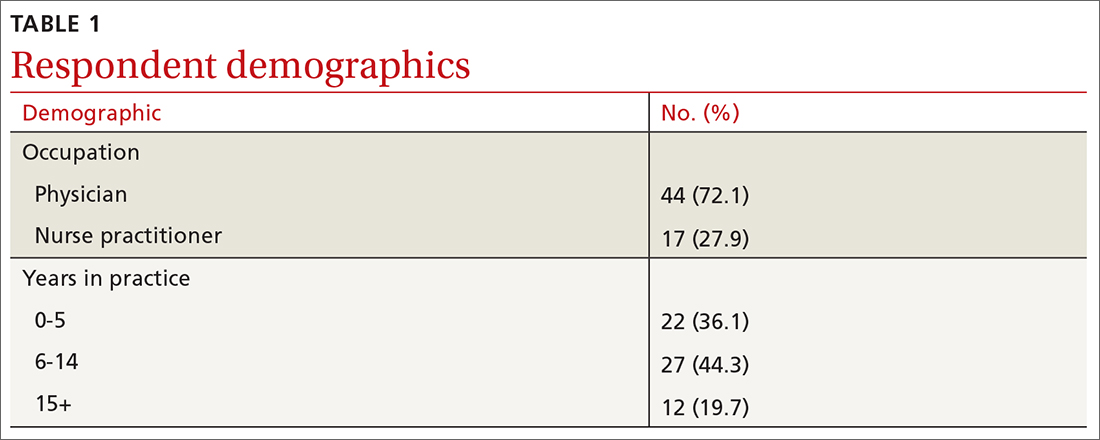
When incorporating EIS scores, respondents’ mean sensitivity for melanomas and SDN increased from 69.2% to 90.0% (P < .001) and specificity from 44.0% to 72.6% (P < .001; TABLE 2). At baseline, physicians demonstrated a sensitivity and specificity of 74.6% and 46.5%, respectively, while NPs demonstrated a sensitivity and specificity of 56.1% and 37.9%, respectively.
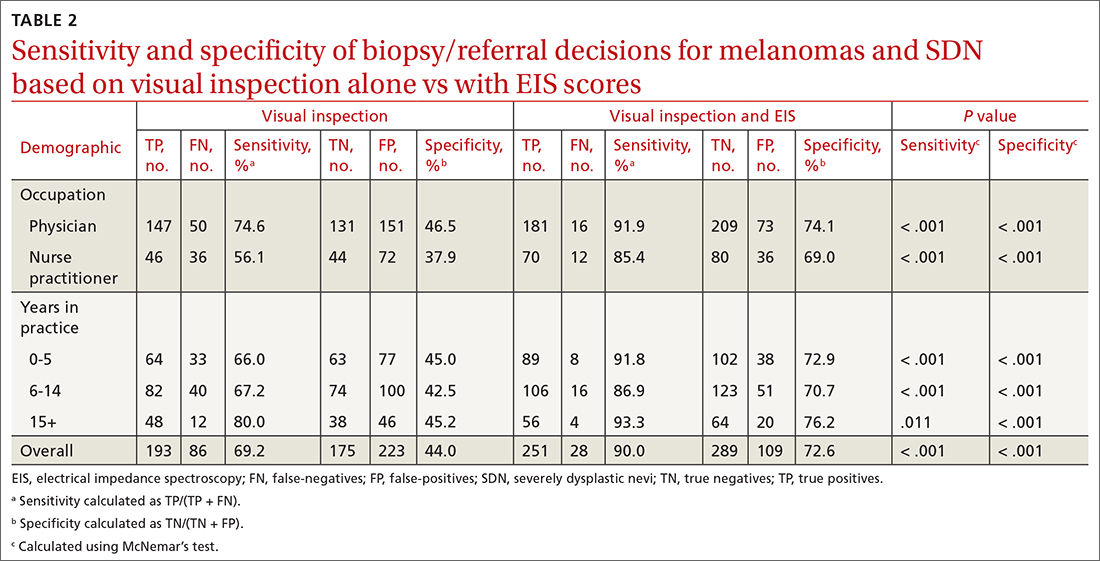
All respondent subgroups stratified by occupation and years of experience saw significant increases in both sensitivity and specificity upon the incorporation of EIS scores, with NPs seeing a greater increase in sensitivity (56.1% vs 85.4%; P < .001) and specificity (37.9% vs 69.0%; P < .001) than physicians (sensitivity: 74.6% vs 91.9%; P < .001; specificity: 46.5% vs 74.1%; P < .001). The only difference in diagnostic performance based on years of experience was a greater pre-EIS sensitivity by clinicians who had been in practice for ≥ 15 years, compared with those in practice for shorter periods (TABLE 2).
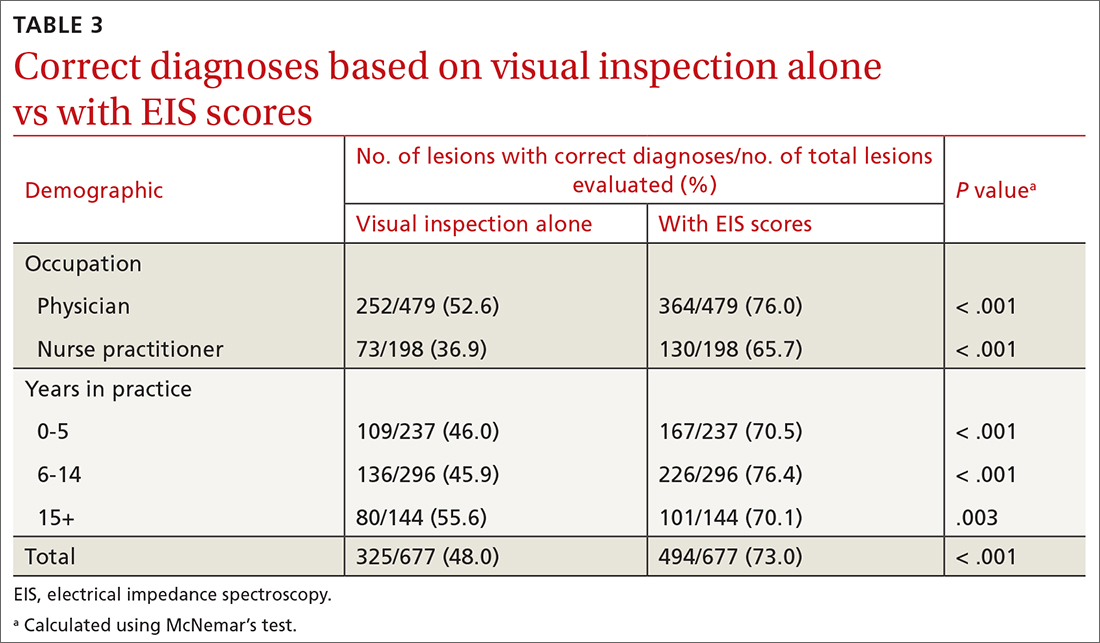
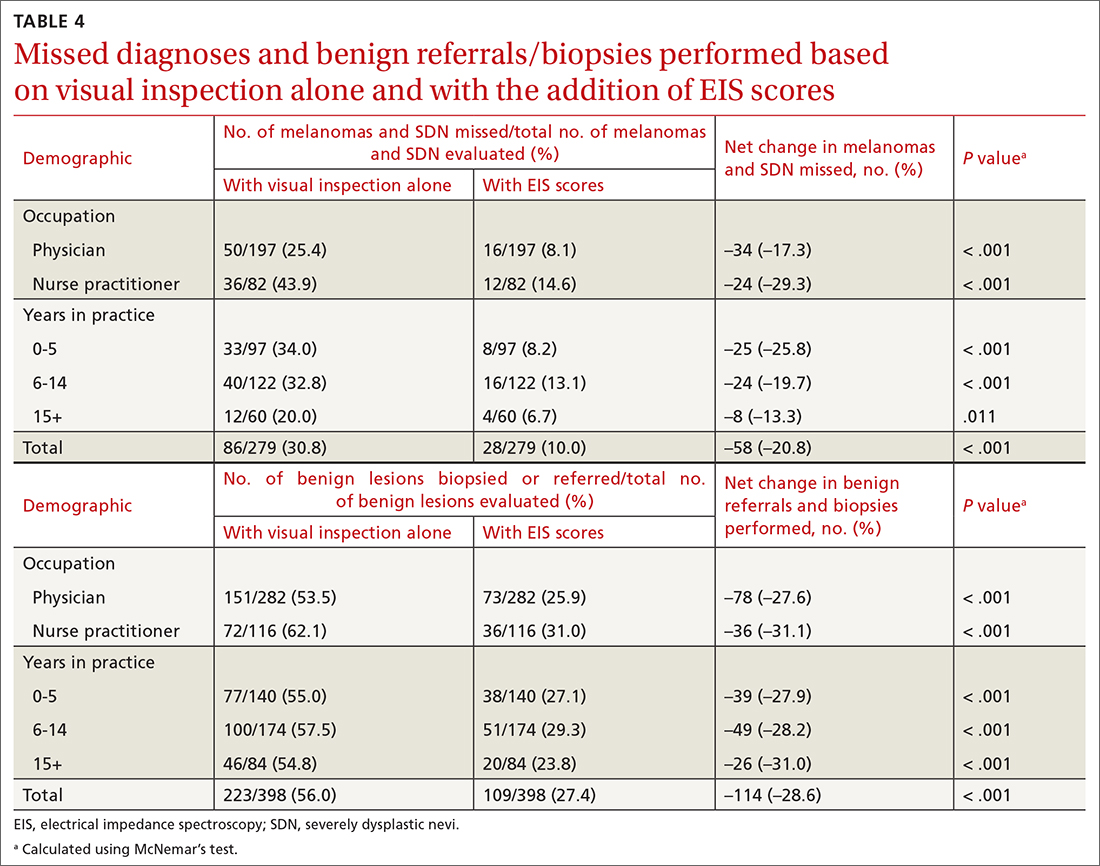
Diagnostic accuracy increased significantly from 48% when based on visual inspection alone to 73% with the addition of EIS scores (P < .001; TABLE 3). Physicians and NPs each significantly increased their diagnostic accuracy upon the incorporation of EIS, with NPs exhibiting the greatest increase (from 36.9% to 65.7%; P < .001). PCPs with 6 to 14 years of experience saw the greatest increase in diagnostic accuracy when adding EIS (45.9% vs 76.4%; P < .001). Overall, the addition of EIS scores resulted in 58 fewer missed melanomas and SDN and 114 fewer benign referrals or biopsies (TABLE 4).
Continue to: DISCUSSION
DISCUSSION
Primary care evaluation plays a significant role in the diagnosis and management of PSLs, ultimately shaping outcomes for patients with melanoma. Improved accuracy of PSL classification could yield greater sensitivity for the diagnosis of melanomas and high-risk melanocytic lesions at earlier stages, while also reducing the number of unnecessary biopsies and referrals—leading to decreased patient morbidity and mortality and reduced health care spending.9
Diagnostic tools are valuable insofar as they can improve accuracy and positively impact clinical management and patient outcomes.10 In this case, increased sensitivity reduced missed melanoma diagnoses, while increased specificity avoided the additional costs and patient toll associated with a biopsy or referral for a benign lesion.
Dermoscopy has been shown to improve the sensitivity and specificity of PSL diagnosis compared with visual inspection alone; however, without substantial training and experience, accuracy with dermoscopy can be no better than examination with the naked eye.3,11,12 The dropout rates are high for training PCPs in its use, given that several months of training may be needed for competent use.13,14 To improve the clinical management of PSLs broadly in primary care, a need exists for easy-to-use adjunctive tools that increase diagnostic accuracy.15
In this study, with only a brief explanation of how to interpret EIS scores, clinicians without any prior experience using EIS demonstrated significantly improved accuracy in deciding appropriate management and classifying melanocytic lesions with the addition of EIS to visual inspection. These improvements, seen in clinicians of varying training and experience, suggest that the learning curve of EIS may not be as steep as that of dermoscopy.
The greater baseline sensitivity, specificity, and diagnostic accuracy of physicians’ clinical decision-making compared with NPs before the incorporation of EIS in the study may be a product of comparatively more extensive medical training. In addition, EIS yielded a greater benefit to NPs than to physicians, with greater increases in sensitivity and specificity noted. This suggests that the use of EIS is particularly advantageous to clinicians who are less proficient in assessing melanocytic lesions. Using visual inspection alone, more experienced respondents made biopsy/referral decisions with greater sensitivity but similar specificity to those with less experience. With the incorporation of EIS scores, the sensitivity and specificity of respondents’ clinical decision-making rose to comparable levels across all experience groups, providing further indication of EIS’s particular value to clinicians who are less proficient in PSL evaluation.
Continue to: This technology holds the potential...
This technology holds the potential to be seamlessly implemented into primary care practice, given that dermatology expertise training is not required to use the EIS device; this could allow for EIS measurement of lesions to be delegated to office staff (eg, nurses, medical assistants).16 Future studies are needed to assess EIS use among PCPs in a real-world setting, where factors such as its application on nonmelanocytic lesions (eg, seborrheic keratoses) and its pairing with patient historical data could produce varying results.
Limitations. While revealing, this study had its limitations. Respondents did not have access to additional pertinent clinical information, such as patients’ histories and risk factors. Clinical decisions in this survey were made based on digital images rather than in vivo examination. This may not represent a real-life evaluation; there is the potential for minimization of the true consequences of a missed melanoma or unnecessary biopsy in the minds of participants, and this does not factor in the operation of the actual EIS device. The Hawthorne effect may also have influenced PCPs’ diagnostic selections. Also, the limited sample size constitutes another limitation.
Of note, in this survey format, respondents rated their inclination to biopsy or refer each lesion from 1 to 5. For statistical analyses, lesions rated 1 to 3 were considered as not biopsied/referred and those rated 4 to 5 as biopsied/referred. The sensitivity and specificity values observed, for both visual examination and concurrent visual and EIS evaluation, are therefore based on this classification system of participants’ provided ratings. It is conceivable that differing sensitivity and specificity values might have been detected if clinicians were instead given a binary choice for referral/biopsy decisions.
CONCLUSIONS
Among PCPs tasked with evaluating melanocytic lesions, the incorporation of EIS data into clinical decision-making in this study significantly increased the sensitivity, specificity, and overall diagnostic accuracy of biopsy or referral decisions for melanomas and SDN compared with visual inspection alone. Overall, the results of this preliminary study suggest that diagnostic accuracy for PSLs by PCPs may be improved with the adjunctive use of EIS with visual inspection. This would ultimately improve patient care and reduce the morbidity and mortality of a melanoma diagnosis.
CORRESPONDENCE
Jonathan Ungar, MD, Kimberly and Eric J. Waldman Department of Dermatology, Icahn School of Medicine at Mount Sinai, 5 East 98th Street, 5th Floor, New York, NY 10029; [email protected]
1. Goetsch NJ, Hoehns JD, Sutherland JE, et al. Assessment of postgraduate skin lesion education among Iowa family physicians. SAGE Open Med. 2017;5:2050312117691392. doi: 10.1177/2050312117691392
2. Dinnes J, Deeks JJ, Chuchu N, et al. Dermoscopy, with and without visual inspection, for diagnosing melanoma in adults. Cochrane Database Syst Rev. 2018;12:CD011902. doi: 10.1002/14651858.CD011902.pub2
3. Jones OT, Jurascheck LC, van Melle MA, et al. Dermoscopy for melanoma detection and triage in primary care: a systematic review. BMJ Open. 2019;9:e027529. doi: 10.1136/bmjopen-2018-027529
4. Malvehy J, Hauschild A, Curiel-Lewandrowski C, et al. Clinical performance of the Nevisense system in cutaneous melanoma detection: an international, multicentre, prospective and blinded clinical trial on efficacy and safety. Br J Dermatol. 2014;171:1099-1107. doi: 10.1111/bjd.13121
5. Svoboda RM, Prado G, Mirsky RS, et al. Assessment of clinician accuracy for diagnosing melanoma on the basis of electrical impedance spectroscopy score plus morphology versus lesion morphology alone. J Am Acad Dermatol. 2019;80:285-287. doi: 10.1016/j.jaad.2018.08.048
6. Mohr P, Birgersson U, Berking C, et al. Electrical impedance spectroscopy as a potential adjunct diagnostic tool for cutaneous melanoma. Skin Res Technol. 2013;19:75-83. doi: 10.1111/srt.12008
7. Rocha L, Menzies SW, Lo S, et al. Analysis of an electrical impedance spectroscopy system in short-term digital dermoscopy imaging of melanocytic lesions. Br J Dermatol. 2017;177:1432-1438. doi: 10.1111/bjd.15595
8. Litchman GH, Teplitz RW, Marson JW, et al. Impact of electrical impedance spectroscopy on dermatologists’ number needed to biopsy metric and biopsy decisions for pigmented skin lesions. J Am Acad Dermatol. 2021;85:976-979. doi: 10.1016/j.jaad.2020.09.011
9. Greenwood-Lee J, Jewett L, Woodhouse L, et al. A categorisation of problems and solutions to improve patient referrals from primary to specialty care. BMC Health Serv Res. 2018;18:1-16. doi: 10.1186/s12913-018-3745-y
10. Bossuyt PM, Reitsma JB, Linnet K, et al. Beyond diagnostic accuracy: the clinical utility of diagnostic tests. Clin Chem. 2012;58:1636-1643. doi: 10.1373/clinchem.2012.182576
11. Argenziano G, Cerroni L, Zalaudek I , et al. Accuracy in melanoma detection: a 10-year multicenter survey. J Am Acad Dermatol. 2012;67:54-59. doi: 10.1016/j.jaad.2011.07.019
12. Menzies SW, Vestergaard ME, Macaskill P, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi: 10.1111/j.1365-2133.2008.08713.x
13. Menzies SW, Emery J, Staples M, et al. Impact of dermoscopy and short-term sequential digital dermoscopy imaging for the management of pigmented lesions in primary care: a sequential intervention trial. Br J Dermatol. 2009;161:1270-1277. doi: 10.1111/j.1365-2133.2009.09374.x
14. Noor O, Nanda A, Rao BK. A dermoscopy survey to assess who is using it and why it is or is not being used. Int J Dermatol. 2009;48:951-952. doi: 10.1111/j.1365-4632.2009.04095.x
15. Weigl BH, Boyle DS, de los Santos T, et al. Simplicity of use: a critical feature for widespread adoption of diagnostic technologies in low-resource settings. Expert Rev Med Devices. 2009;6:461-464. doi: 10.1586/erd.09.31
16. Sarac E, Meiwes A, Eigentler T, et al. Diagnostic accuracy of electrical impedance spectroscopy in non-melanoma skin cancer. Acta Derm Venereol. 2020;100:adv00328. doi: 10.2340/00015555-3689
ABSTRACT
Background: We sought to examine whether electrical impedance spectroscopy (EIS), a diagnostic tool approved by the US Food and Drug Administration for the evaluation of pigmented skin lesions (PSLs), is beneficial to primary care providers (PCPs) by comparing the accuracy of PCPs’ management decisions for PSLs based on visual examination alone with those based on concurrent visual and EIS evaluation.
Methods: Physicians and nurse practitioners (NPs) participated in an anonymous online survey in which they viewed clinical images of PSLs and were asked to make 2 clinical decisions before and after being provided an EIS score that indicated the likelihood that the lesion was a melanoma. They were asked (1) if they would biopsy the lesion/refer the patient out and (2) what they expected the pathology results would show.
Results: Forty-four physicians and 17 NPs participated, making clinical decisions for 1354 presented lesions. Overall, with the addition of EIS to visual inspection of clinical images, the sensitivity of biopsy/referral decisions for melanomas and severely dysplastic nevi (SDN) increased from 69.2% to 90.0% (P < .001), while specificity increased from 44.0% to 72.6% (P < .001). Physicians and NPs, regardless of years of experience, each saw significant improvements in sensitivity, specificity, and diagnostic accuracy with the addition of EIS scores.
Conclusions: The incorporation of EIS data into clinical decision-making by PCPs significantly increased the sensitivity and specificity of biopsy/referral decisions for melanomas and SDN and overall diagnostic accuracy compared with visual inspection alone. The results of this study suggest that diagnostic accuracy for PSLs by PCPs may be improved with adjunctive use of EIS with visual inspection.
Primary care providers (PCPs) are often the first line of defense in detecting skin cancers. For patients with concerning skin lesions, PCPs may choose to perform a biopsy or facilitate access to specialty services (eg, Dermatology). Consequently, PCPs play a critical role in the timely detection of skin cancers, and it is paramount to employ continually improving detection methods, such as the application of technologic advances.1
Differentiating benign nevi from melanoma and severely dysplastic nevi (SDN), both of which warrant excision, poses a unique challenge to clinicians examining pigmented skin lesions (PSLs). PCPs often rely on visual inspection to differentiate benign skin lesions from malignant skin cancers. In some primary care practices, dermoscopy, which involves using a handheld device to evaluate lesions with polarized light and magnification, is used to improve melanoma detection. However, while visual inspection and dermoscopy are valid, effective techniques for the diagnosis of melanocytic lesions, in many instances they still can lead to missed cancers or unnecessary biopsies and specialty referrals. Adjunctive use of dermoscopy with visual inspection has been shown to increase the probability of skin cancer detection, but it fails to achieve a near-100% success rate.2 Furthermore, dermoscopy is heavily user-dependent, requiring significant training and experience for appropriate use.3
Another option is an electrical impedance spectroscopy (EIS) device (Nevisense, Scibase, Stockholm, Sweden), which has been approved by the US Food and Drug Administration (FDA) to assist in the detection of melanoma and differentiation from benign PSLs.4 EIS is a noninvasive, rapidly applied technology designed to accompany the visual examination of melanocytic lesions in office, with or without dermoscopy. Still relatively new, the technology is employed today by many dermatologists, increasing diagnostic accuracy for PSLs.5 The lightweight and portable instrument features a handheld probe, which is held against a lesion to obtain a reading. EIS uses a low-voltage electrode to apply a harmless electrical current to the skin at various frequencies.6 As benign and malignant tissues vary in cell shape, size, and composition, EIS distinguishes differential electrical resistance of the tissue to aid in diagnosis.7
Continue to: EIS provides high-sensitivity...
EIS provides high-sensitivity melanoma diagnosis vs histopathologic confirmation from biopsies, with 1 study showing a 96.6% sensitivity rating, detecting 256 of 265 melanomas.4 The EIS device, by measuring differences in electrical resistance between benign and cancerous cells, outputs a simple integer score ranging from 0 to 10 associated with the likelihood of the lesion being a melanoma.8 Based on data from the Nevisense pivotal trial,4 Nevisense reports that scores of 0 to 3 carry a negative predictive value of 99% for melanoma, whereas scores of 4 to 10 signify increasingly greater positive predictive values from 7% to 61%.
We aimed to assess whether EIS may be beneficial to PCPs by comparing the accuracy of clinical decision-making for PSLs based on visual examination alone with that based on concurrent visual and EIS evaluation.
METHODS
A questionnaire was distributed via email to 142 clinicians at clinics affiliated with either of 2 organizations delivering care to the New York City area through a network of community health centers: the Institute for Family Health (IFH) and the Community Healthcare Network (CHN). Of these recipients, 72 were affiliated with IFH across 27 community health centers and 70 were affiliated with CHN across 14 community health centers. Recipients were physicians and nurse practitioners (NPs) practicing at primary health care facilities.
Survey instrument. The first section of the survey instrument (APPENDIX) solicited demographic information and explained how to apply the EIS scores for diagnostic decision-making. The second featured images of 12 randomly selected, histologically confirmed, and EIS-evaluated PSLs from a previously published prospective blinded trial of 2416 lesions.4 The Institutional Review Board of the Icahn School of Medicine at Mount Sinai reviewed and approved the study and survey instrument.
Clinical images of these lesions, comprising 4 melanocytic nevi, 4 dysplastic nevi (including 3 mild-moderately dysplastic and 1 severely dysplastic nevus), and 4 melanomas
Continue to: Analysis
Analysis. A biopsy or referral rating of 4 or 5 was considered a decision to biopsy or refer (ie, a diagnostic decision consistent with melanoma or SDN warranting excision), whereas a selection of 1 to 3 was considered a decision not to biopsy or refer (ie, a diagnostic decision consistent with a benign PSL). The sensitivity and specificity of biopsy/referral decisions for melanomas and SDN, the proportion of missed melanomas and SDN, and the proportion of biopsy/referral decisions for benign lesions were separately determined for visual inspection alone and visual inspection with EIS score. Similarly, diagnostic accuracy was calculated for these clinical scenarios. These metrics were further stratified among different subsets of the respondent population. Differences in sensitivity, specificity, biopsy/referral decision proportions, and diagnostic accuracy were calculated using McNemar’s test for paired proportions.
RESULTS
Sixty-one respondents, comprising 44 physicians and 17 NPs, completed the survey, yielding a response rate of 43% (TABLE 1). In total, 1354 clinical decisions (677 based on visual inspection alone and 677 based on visual inspection plus EIS) were made. A biopsy/referral decision was made after assessing 416 of 677 cases (61%) with visual inspection alone and 360 of 677 cases (53%) when relying on visual inspection plus EIS. None of the respondents reported any prior experience with EIS.

When incorporating EIS scores, respondents’ mean sensitivity for melanomas and SDN increased from 69.2% to 90.0% (P < .001) and specificity from 44.0% to 72.6% (P < .001; TABLE 2). At baseline, physicians demonstrated a sensitivity and specificity of 74.6% and 46.5%, respectively, while NPs demonstrated a sensitivity and specificity of 56.1% and 37.9%, respectively.

All respondent subgroups stratified by occupation and years of experience saw significant increases in both sensitivity and specificity upon the incorporation of EIS scores, with NPs seeing a greater increase in sensitivity (56.1% vs 85.4%; P < .001) and specificity (37.9% vs 69.0%; P < .001) than physicians (sensitivity: 74.6% vs 91.9%; P < .001; specificity: 46.5% vs 74.1%; P < .001). The only difference in diagnostic performance based on years of experience was a greater pre-EIS sensitivity by clinicians who had been in practice for ≥ 15 years, compared with those in practice for shorter periods (TABLE 2).

Diagnostic accuracy increased significantly from 48% when based on visual inspection alone to 73% with the addition of EIS scores (P < .001; TABLE 3). Physicians and NPs each significantly increased their diagnostic accuracy upon the incorporation of EIS, with NPs exhibiting the greatest increase (from 36.9% to 65.7%; P < .001). PCPs with 6 to 14 years of experience saw the greatest increase in diagnostic accuracy when adding EIS (45.9% vs 76.4%; P < .001). Overall, the addition of EIS scores resulted in 58 fewer missed melanomas and SDN and 114 fewer benign referrals or biopsies (TABLE 4).

Continue to: DISCUSSION
DISCUSSION
Primary care evaluation plays a significant role in the diagnosis and management of PSLs, ultimately shaping outcomes for patients with melanoma. Improved accuracy of PSL classification could yield greater sensitivity for the diagnosis of melanomas and high-risk melanocytic lesions at earlier stages, while also reducing the number of unnecessary biopsies and referrals—leading to decreased patient morbidity and mortality and reduced health care spending.9
Diagnostic tools are valuable insofar as they can improve accuracy and positively impact clinical management and patient outcomes.10 In this case, increased sensitivity reduced missed melanoma diagnoses, while increased specificity avoided the additional costs and patient toll associated with a biopsy or referral for a benign lesion.
Dermoscopy has been shown to improve the sensitivity and specificity of PSL diagnosis compared with visual inspection alone; however, without substantial training and experience, accuracy with dermoscopy can be no better than examination with the naked eye.3,11,12 The dropout rates are high for training PCPs in its use, given that several months of training may be needed for competent use.13,14 To improve the clinical management of PSLs broadly in primary care, a need exists for easy-to-use adjunctive tools that increase diagnostic accuracy.15
In this study, with only a brief explanation of how to interpret EIS scores, clinicians without any prior experience using EIS demonstrated significantly improved accuracy in deciding appropriate management and classifying melanocytic lesions with the addition of EIS to visual inspection. These improvements, seen in clinicians of varying training and experience, suggest that the learning curve of EIS may not be as steep as that of dermoscopy.
The greater baseline sensitivity, specificity, and diagnostic accuracy of physicians’ clinical decision-making compared with NPs before the incorporation of EIS in the study may be a product of comparatively more extensive medical training. In addition, EIS yielded a greater benefit to NPs than to physicians, with greater increases in sensitivity and specificity noted. This suggests that the use of EIS is particularly advantageous to clinicians who are less proficient in assessing melanocytic lesions. Using visual inspection alone, more experienced respondents made biopsy/referral decisions with greater sensitivity but similar specificity to those with less experience. With the incorporation of EIS scores, the sensitivity and specificity of respondents’ clinical decision-making rose to comparable levels across all experience groups, providing further indication of EIS’s particular value to clinicians who are less proficient in PSL evaluation.
Continue to: This technology holds the potential...
This technology holds the potential to be seamlessly implemented into primary care practice, given that dermatology expertise training is not required to use the EIS device; this could allow for EIS measurement of lesions to be delegated to office staff (eg, nurses, medical assistants).16 Future studies are needed to assess EIS use among PCPs in a real-world setting, where factors such as its application on nonmelanocytic lesions (eg, seborrheic keratoses) and its pairing with patient historical data could produce varying results.
Limitations. While revealing, this study had its limitations. Respondents did not have access to additional pertinent clinical information, such as patients’ histories and risk factors. Clinical decisions in this survey were made based on digital images rather than in vivo examination. This may not represent a real-life evaluation; there is the potential for minimization of the true consequences of a missed melanoma or unnecessary biopsy in the minds of participants, and this does not factor in the operation of the actual EIS device. The Hawthorne effect may also have influenced PCPs’ diagnostic selections. Also, the limited sample size constitutes another limitation.
Of note, in this survey format, respondents rated their inclination to biopsy or refer each lesion from 1 to 5. For statistical analyses, lesions rated 1 to 3 were considered as not biopsied/referred and those rated 4 to 5 as biopsied/referred. The sensitivity and specificity values observed, for both visual examination and concurrent visual and EIS evaluation, are therefore based on this classification system of participants’ provided ratings. It is conceivable that differing sensitivity and specificity values might have been detected if clinicians were instead given a binary choice for referral/biopsy decisions.
CONCLUSIONS
Among PCPs tasked with evaluating melanocytic lesions, the incorporation of EIS data into clinical decision-making in this study significantly increased the sensitivity, specificity, and overall diagnostic accuracy of biopsy or referral decisions for melanomas and SDN compared with visual inspection alone. Overall, the results of this preliminary study suggest that diagnostic accuracy for PSLs by PCPs may be improved with the adjunctive use of EIS with visual inspection. This would ultimately improve patient care and reduce the morbidity and mortality of a melanoma diagnosis.
CORRESPONDENCE
Jonathan Ungar, MD, Kimberly and Eric J. Waldman Department of Dermatology, Icahn School of Medicine at Mount Sinai, 5 East 98th Street, 5th Floor, New York, NY 10029; [email protected]
ABSTRACT
Background: We sought to examine whether electrical impedance spectroscopy (EIS), a diagnostic tool approved by the US Food and Drug Administration for the evaluation of pigmented skin lesions (PSLs), is beneficial to primary care providers (PCPs) by comparing the accuracy of PCPs’ management decisions for PSLs based on visual examination alone with those based on concurrent visual and EIS evaluation.
Methods: Physicians and nurse practitioners (NPs) participated in an anonymous online survey in which they viewed clinical images of PSLs and were asked to make 2 clinical decisions before and after being provided an EIS score that indicated the likelihood that the lesion was a melanoma. They were asked (1) if they would biopsy the lesion/refer the patient out and (2) what they expected the pathology results would show.
Results: Forty-four physicians and 17 NPs participated, making clinical decisions for 1354 presented lesions. Overall, with the addition of EIS to visual inspection of clinical images, the sensitivity of biopsy/referral decisions for melanomas and severely dysplastic nevi (SDN) increased from 69.2% to 90.0% (P < .001), while specificity increased from 44.0% to 72.6% (P < .001). Physicians and NPs, regardless of years of experience, each saw significant improvements in sensitivity, specificity, and diagnostic accuracy with the addition of EIS scores.
Conclusions: The incorporation of EIS data into clinical decision-making by PCPs significantly increased the sensitivity and specificity of biopsy/referral decisions for melanomas and SDN and overall diagnostic accuracy compared with visual inspection alone. The results of this study suggest that diagnostic accuracy for PSLs by PCPs may be improved with adjunctive use of EIS with visual inspection.
Primary care providers (PCPs) are often the first line of defense in detecting skin cancers. For patients with concerning skin lesions, PCPs may choose to perform a biopsy or facilitate access to specialty services (eg, Dermatology). Consequently, PCPs play a critical role in the timely detection of skin cancers, and it is paramount to employ continually improving detection methods, such as the application of technologic advances.1
Differentiating benign nevi from melanoma and severely dysplastic nevi (SDN), both of which warrant excision, poses a unique challenge to clinicians examining pigmented skin lesions (PSLs). PCPs often rely on visual inspection to differentiate benign skin lesions from malignant skin cancers. In some primary care practices, dermoscopy, which involves using a handheld device to evaluate lesions with polarized light and magnification, is used to improve melanoma detection. However, while visual inspection and dermoscopy are valid, effective techniques for the diagnosis of melanocytic lesions, in many instances they still can lead to missed cancers or unnecessary biopsies and specialty referrals. Adjunctive use of dermoscopy with visual inspection has been shown to increase the probability of skin cancer detection, but it fails to achieve a near-100% success rate.2 Furthermore, dermoscopy is heavily user-dependent, requiring significant training and experience for appropriate use.3
Another option is an electrical impedance spectroscopy (EIS) device (Nevisense, Scibase, Stockholm, Sweden), which has been approved by the US Food and Drug Administration (FDA) to assist in the detection of melanoma and differentiation from benign PSLs.4 EIS is a noninvasive, rapidly applied technology designed to accompany the visual examination of melanocytic lesions in office, with or without dermoscopy. Still relatively new, the technology is employed today by many dermatologists, increasing diagnostic accuracy for PSLs.5 The lightweight and portable instrument features a handheld probe, which is held against a lesion to obtain a reading. EIS uses a low-voltage electrode to apply a harmless electrical current to the skin at various frequencies.6 As benign and malignant tissues vary in cell shape, size, and composition, EIS distinguishes differential electrical resistance of the tissue to aid in diagnosis.7
Continue to: EIS provides high-sensitivity...
EIS provides high-sensitivity melanoma diagnosis vs histopathologic confirmation from biopsies, with 1 study showing a 96.6% sensitivity rating, detecting 256 of 265 melanomas.4 The EIS device, by measuring differences in electrical resistance between benign and cancerous cells, outputs a simple integer score ranging from 0 to 10 associated with the likelihood of the lesion being a melanoma.8 Based on data from the Nevisense pivotal trial,4 Nevisense reports that scores of 0 to 3 carry a negative predictive value of 99% for melanoma, whereas scores of 4 to 10 signify increasingly greater positive predictive values from 7% to 61%.
We aimed to assess whether EIS may be beneficial to PCPs by comparing the accuracy of clinical decision-making for PSLs based on visual examination alone with that based on concurrent visual and EIS evaluation.
METHODS
A questionnaire was distributed via email to 142 clinicians at clinics affiliated with either of 2 organizations delivering care to the New York City area through a network of community health centers: the Institute for Family Health (IFH) and the Community Healthcare Network (CHN). Of these recipients, 72 were affiliated with IFH across 27 community health centers and 70 were affiliated with CHN across 14 community health centers. Recipients were physicians and nurse practitioners (NPs) practicing at primary health care facilities.
Survey instrument. The first section of the survey instrument (APPENDIX) solicited demographic information and explained how to apply the EIS scores for diagnostic decision-making. The second featured images of 12 randomly selected, histologically confirmed, and EIS-evaluated PSLs from a previously published prospective blinded trial of 2416 lesions.4 The Institutional Review Board of the Icahn School of Medicine at Mount Sinai reviewed and approved the study and survey instrument.
Clinical images of these lesions, comprising 4 melanocytic nevi, 4 dysplastic nevi (including 3 mild-moderately dysplastic and 1 severely dysplastic nevus), and 4 melanomas
Continue to: Analysis
Analysis. A biopsy or referral rating of 4 or 5 was considered a decision to biopsy or refer (ie, a diagnostic decision consistent with melanoma or SDN warranting excision), whereas a selection of 1 to 3 was considered a decision not to biopsy or refer (ie, a diagnostic decision consistent with a benign PSL). The sensitivity and specificity of biopsy/referral decisions for melanomas and SDN, the proportion of missed melanomas and SDN, and the proportion of biopsy/referral decisions for benign lesions were separately determined for visual inspection alone and visual inspection with EIS score. Similarly, diagnostic accuracy was calculated for these clinical scenarios. These metrics were further stratified among different subsets of the respondent population. Differences in sensitivity, specificity, biopsy/referral decision proportions, and diagnostic accuracy were calculated using McNemar’s test for paired proportions.
RESULTS
Sixty-one respondents, comprising 44 physicians and 17 NPs, completed the survey, yielding a response rate of 43% (TABLE 1). In total, 1354 clinical decisions (677 based on visual inspection alone and 677 based on visual inspection plus EIS) were made. A biopsy/referral decision was made after assessing 416 of 677 cases (61%) with visual inspection alone and 360 of 677 cases (53%) when relying on visual inspection plus EIS. None of the respondents reported any prior experience with EIS.

When incorporating EIS scores, respondents’ mean sensitivity for melanomas and SDN increased from 69.2% to 90.0% (P < .001) and specificity from 44.0% to 72.6% (P < .001; TABLE 2). At baseline, physicians demonstrated a sensitivity and specificity of 74.6% and 46.5%, respectively, while NPs demonstrated a sensitivity and specificity of 56.1% and 37.9%, respectively.

All respondent subgroups stratified by occupation and years of experience saw significant increases in both sensitivity and specificity upon the incorporation of EIS scores, with NPs seeing a greater increase in sensitivity (56.1% vs 85.4%; P < .001) and specificity (37.9% vs 69.0%; P < .001) than physicians (sensitivity: 74.6% vs 91.9%; P < .001; specificity: 46.5% vs 74.1%; P < .001). The only difference in diagnostic performance based on years of experience was a greater pre-EIS sensitivity by clinicians who had been in practice for ≥ 15 years, compared with those in practice for shorter periods (TABLE 2).

Diagnostic accuracy increased significantly from 48% when based on visual inspection alone to 73% with the addition of EIS scores (P < .001; TABLE 3). Physicians and NPs each significantly increased their diagnostic accuracy upon the incorporation of EIS, with NPs exhibiting the greatest increase (from 36.9% to 65.7%; P < .001). PCPs with 6 to 14 years of experience saw the greatest increase in diagnostic accuracy when adding EIS (45.9% vs 76.4%; P < .001). Overall, the addition of EIS scores resulted in 58 fewer missed melanomas and SDN and 114 fewer benign referrals or biopsies (TABLE 4).

Continue to: DISCUSSION
DISCUSSION
Primary care evaluation plays a significant role in the diagnosis and management of PSLs, ultimately shaping outcomes for patients with melanoma. Improved accuracy of PSL classification could yield greater sensitivity for the diagnosis of melanomas and high-risk melanocytic lesions at earlier stages, while also reducing the number of unnecessary biopsies and referrals—leading to decreased patient morbidity and mortality and reduced health care spending.9
Diagnostic tools are valuable insofar as they can improve accuracy and positively impact clinical management and patient outcomes.10 In this case, increased sensitivity reduced missed melanoma diagnoses, while increased specificity avoided the additional costs and patient toll associated with a biopsy or referral for a benign lesion.
Dermoscopy has been shown to improve the sensitivity and specificity of PSL diagnosis compared with visual inspection alone; however, without substantial training and experience, accuracy with dermoscopy can be no better than examination with the naked eye.3,11,12 The dropout rates are high for training PCPs in its use, given that several months of training may be needed for competent use.13,14 To improve the clinical management of PSLs broadly in primary care, a need exists for easy-to-use adjunctive tools that increase diagnostic accuracy.15
In this study, with only a brief explanation of how to interpret EIS scores, clinicians without any prior experience using EIS demonstrated significantly improved accuracy in deciding appropriate management and classifying melanocytic lesions with the addition of EIS to visual inspection. These improvements, seen in clinicians of varying training and experience, suggest that the learning curve of EIS may not be as steep as that of dermoscopy.
The greater baseline sensitivity, specificity, and diagnostic accuracy of physicians’ clinical decision-making compared with NPs before the incorporation of EIS in the study may be a product of comparatively more extensive medical training. In addition, EIS yielded a greater benefit to NPs than to physicians, with greater increases in sensitivity and specificity noted. This suggests that the use of EIS is particularly advantageous to clinicians who are less proficient in assessing melanocytic lesions. Using visual inspection alone, more experienced respondents made biopsy/referral decisions with greater sensitivity but similar specificity to those with less experience. With the incorporation of EIS scores, the sensitivity and specificity of respondents’ clinical decision-making rose to comparable levels across all experience groups, providing further indication of EIS’s particular value to clinicians who are less proficient in PSL evaluation.
Continue to: This technology holds the potential...
This technology holds the potential to be seamlessly implemented into primary care practice, given that dermatology expertise training is not required to use the EIS device; this could allow for EIS measurement of lesions to be delegated to office staff (eg, nurses, medical assistants).16 Future studies are needed to assess EIS use among PCPs in a real-world setting, where factors such as its application on nonmelanocytic lesions (eg, seborrheic keratoses) and its pairing with patient historical data could produce varying results.
Limitations. While revealing, this study had its limitations. Respondents did not have access to additional pertinent clinical information, such as patients’ histories and risk factors. Clinical decisions in this survey were made based on digital images rather than in vivo examination. This may not represent a real-life evaluation; there is the potential for minimization of the true consequences of a missed melanoma or unnecessary biopsy in the minds of participants, and this does not factor in the operation of the actual EIS device. The Hawthorne effect may also have influenced PCPs’ diagnostic selections. Also, the limited sample size constitutes another limitation.
Of note, in this survey format, respondents rated their inclination to biopsy or refer each lesion from 1 to 5. For statistical analyses, lesions rated 1 to 3 were considered as not biopsied/referred and those rated 4 to 5 as biopsied/referred. The sensitivity and specificity values observed, for both visual examination and concurrent visual and EIS evaluation, are therefore based on this classification system of participants’ provided ratings. It is conceivable that differing sensitivity and specificity values might have been detected if clinicians were instead given a binary choice for referral/biopsy decisions.
CONCLUSIONS
Among PCPs tasked with evaluating melanocytic lesions, the incorporation of EIS data into clinical decision-making in this study significantly increased the sensitivity, specificity, and overall diagnostic accuracy of biopsy or referral decisions for melanomas and SDN compared with visual inspection alone. Overall, the results of this preliminary study suggest that diagnostic accuracy for PSLs by PCPs may be improved with the adjunctive use of EIS with visual inspection. This would ultimately improve patient care and reduce the morbidity and mortality of a melanoma diagnosis.
CORRESPONDENCE
Jonathan Ungar, MD, Kimberly and Eric J. Waldman Department of Dermatology, Icahn School of Medicine at Mount Sinai, 5 East 98th Street, 5th Floor, New York, NY 10029; [email protected]
1. Goetsch NJ, Hoehns JD, Sutherland JE, et al. Assessment of postgraduate skin lesion education among Iowa family physicians. SAGE Open Med. 2017;5:2050312117691392. doi: 10.1177/2050312117691392
2. Dinnes J, Deeks JJ, Chuchu N, et al. Dermoscopy, with and without visual inspection, for diagnosing melanoma in adults. Cochrane Database Syst Rev. 2018;12:CD011902. doi: 10.1002/14651858.CD011902.pub2
3. Jones OT, Jurascheck LC, van Melle MA, et al. Dermoscopy for melanoma detection and triage in primary care: a systematic review. BMJ Open. 2019;9:e027529. doi: 10.1136/bmjopen-2018-027529
4. Malvehy J, Hauschild A, Curiel-Lewandrowski C, et al. Clinical performance of the Nevisense system in cutaneous melanoma detection: an international, multicentre, prospective and blinded clinical trial on efficacy and safety. Br J Dermatol. 2014;171:1099-1107. doi: 10.1111/bjd.13121
5. Svoboda RM, Prado G, Mirsky RS, et al. Assessment of clinician accuracy for diagnosing melanoma on the basis of electrical impedance spectroscopy score plus morphology versus lesion morphology alone. J Am Acad Dermatol. 2019;80:285-287. doi: 10.1016/j.jaad.2018.08.048
6. Mohr P, Birgersson U, Berking C, et al. Electrical impedance spectroscopy as a potential adjunct diagnostic tool for cutaneous melanoma. Skin Res Technol. 2013;19:75-83. doi: 10.1111/srt.12008
7. Rocha L, Menzies SW, Lo S, et al. Analysis of an electrical impedance spectroscopy system in short-term digital dermoscopy imaging of melanocytic lesions. Br J Dermatol. 2017;177:1432-1438. doi: 10.1111/bjd.15595
8. Litchman GH, Teplitz RW, Marson JW, et al. Impact of electrical impedance spectroscopy on dermatologists’ number needed to biopsy metric and biopsy decisions for pigmented skin lesions. J Am Acad Dermatol. 2021;85:976-979. doi: 10.1016/j.jaad.2020.09.011
9. Greenwood-Lee J, Jewett L, Woodhouse L, et al. A categorisation of problems and solutions to improve patient referrals from primary to specialty care. BMC Health Serv Res. 2018;18:1-16. doi: 10.1186/s12913-018-3745-y
10. Bossuyt PM, Reitsma JB, Linnet K, et al. Beyond diagnostic accuracy: the clinical utility of diagnostic tests. Clin Chem. 2012;58:1636-1643. doi: 10.1373/clinchem.2012.182576
11. Argenziano G, Cerroni L, Zalaudek I , et al. Accuracy in melanoma detection: a 10-year multicenter survey. J Am Acad Dermatol. 2012;67:54-59. doi: 10.1016/j.jaad.2011.07.019
12. Menzies SW, Vestergaard ME, Macaskill P, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi: 10.1111/j.1365-2133.2008.08713.x
13. Menzies SW, Emery J, Staples M, et al. Impact of dermoscopy and short-term sequential digital dermoscopy imaging for the management of pigmented lesions in primary care: a sequential intervention trial. Br J Dermatol. 2009;161:1270-1277. doi: 10.1111/j.1365-2133.2009.09374.x
14. Noor O, Nanda A, Rao BK. A dermoscopy survey to assess who is using it and why it is or is not being used. Int J Dermatol. 2009;48:951-952. doi: 10.1111/j.1365-4632.2009.04095.x
15. Weigl BH, Boyle DS, de los Santos T, et al. Simplicity of use: a critical feature for widespread adoption of diagnostic technologies in low-resource settings. Expert Rev Med Devices. 2009;6:461-464. doi: 10.1586/erd.09.31
16. Sarac E, Meiwes A, Eigentler T, et al. Diagnostic accuracy of electrical impedance spectroscopy in non-melanoma skin cancer. Acta Derm Venereol. 2020;100:adv00328. doi: 10.2340/00015555-3689
1. Goetsch NJ, Hoehns JD, Sutherland JE, et al. Assessment of postgraduate skin lesion education among Iowa family physicians. SAGE Open Med. 2017;5:2050312117691392. doi: 10.1177/2050312117691392
2. Dinnes J, Deeks JJ, Chuchu N, et al. Dermoscopy, with and without visual inspection, for diagnosing melanoma in adults. Cochrane Database Syst Rev. 2018;12:CD011902. doi: 10.1002/14651858.CD011902.pub2
3. Jones OT, Jurascheck LC, van Melle MA, et al. Dermoscopy for melanoma detection and triage in primary care: a systematic review. BMJ Open. 2019;9:e027529. doi: 10.1136/bmjopen-2018-027529
4. Malvehy J, Hauschild A, Curiel-Lewandrowski C, et al. Clinical performance of the Nevisense system in cutaneous melanoma detection: an international, multicentre, prospective and blinded clinical trial on efficacy and safety. Br J Dermatol. 2014;171:1099-1107. doi: 10.1111/bjd.13121
5. Svoboda RM, Prado G, Mirsky RS, et al. Assessment of clinician accuracy for diagnosing melanoma on the basis of electrical impedance spectroscopy score plus morphology versus lesion morphology alone. J Am Acad Dermatol. 2019;80:285-287. doi: 10.1016/j.jaad.2018.08.048
6. Mohr P, Birgersson U, Berking C, et al. Electrical impedance spectroscopy as a potential adjunct diagnostic tool for cutaneous melanoma. Skin Res Technol. 2013;19:75-83. doi: 10.1111/srt.12008
7. Rocha L, Menzies SW, Lo S, et al. Analysis of an electrical impedance spectroscopy system in short-term digital dermoscopy imaging of melanocytic lesions. Br J Dermatol. 2017;177:1432-1438. doi: 10.1111/bjd.15595
8. Litchman GH, Teplitz RW, Marson JW, et al. Impact of electrical impedance spectroscopy on dermatologists’ number needed to biopsy metric and biopsy decisions for pigmented skin lesions. J Am Acad Dermatol. 2021;85:976-979. doi: 10.1016/j.jaad.2020.09.011
9. Greenwood-Lee J, Jewett L, Woodhouse L, et al. A categorisation of problems and solutions to improve patient referrals from primary to specialty care. BMC Health Serv Res. 2018;18:1-16. doi: 10.1186/s12913-018-3745-y
10. Bossuyt PM, Reitsma JB, Linnet K, et al. Beyond diagnostic accuracy: the clinical utility of diagnostic tests. Clin Chem. 2012;58:1636-1643. doi: 10.1373/clinchem.2012.182576
11. Argenziano G, Cerroni L, Zalaudek I , et al. Accuracy in melanoma detection: a 10-year multicenter survey. J Am Acad Dermatol. 2012;67:54-59. doi: 10.1016/j.jaad.2011.07.019
12. Menzies SW, Vestergaard ME, Macaskill P, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi: 10.1111/j.1365-2133.2008.08713.x
13. Menzies SW, Emery J, Staples M, et al. Impact of dermoscopy and short-term sequential digital dermoscopy imaging for the management of pigmented lesions in primary care: a sequential intervention trial. Br J Dermatol. 2009;161:1270-1277. doi: 10.1111/j.1365-2133.2009.09374.x
14. Noor O, Nanda A, Rao BK. A dermoscopy survey to assess who is using it and why it is or is not being used. Int J Dermatol. 2009;48:951-952. doi: 10.1111/j.1365-4632.2009.04095.x
15. Weigl BH, Boyle DS, de los Santos T, et al. Simplicity of use: a critical feature for widespread adoption of diagnostic technologies in low-resource settings. Expert Rev Med Devices. 2009;6:461-464. doi: 10.1586/erd.09.31
16. Sarac E, Meiwes A, Eigentler T, et al. Diagnostic accuracy of electrical impedance spectroscopy in non-melanoma skin cancer. Acta Derm Venereol. 2020;100:adv00328. doi: 10.2340/00015555-3689
Persistent ‘postherpetic neuralgia’ and well-demarcated plaque
A 75-YEAR-OLD MAN presented to the dermatology clinic for evaluation of localized, persistent burning pain and discomfort attributed to shingles and postherpetic neuralgia. He had received a diagnosis of shingles on his left upper back about 3 years prior to this presentation.
In the ensuing years, the patient had been evaluated and treated by his primary care physician, a pain management team, and a neurologist. These clinicians treated the symptoms as postherpetic neuralgia, with no consensus explanation for the skin findings. The patient reported that his symptoms were unresponsive to trials of gabapentin 800 mg tid, duloxetine 60 mg PO qd, and acetaminophen 1 to 3 g/d PO. He also had undergone several rounds of acupuncture, thoracic and cervical spine steroid injections, and epidurals, without resolution of symptoms. The patient believed the only treatment that helped was a lidocaine 4% patch, which he had used nearly every day for the previous 3 years.
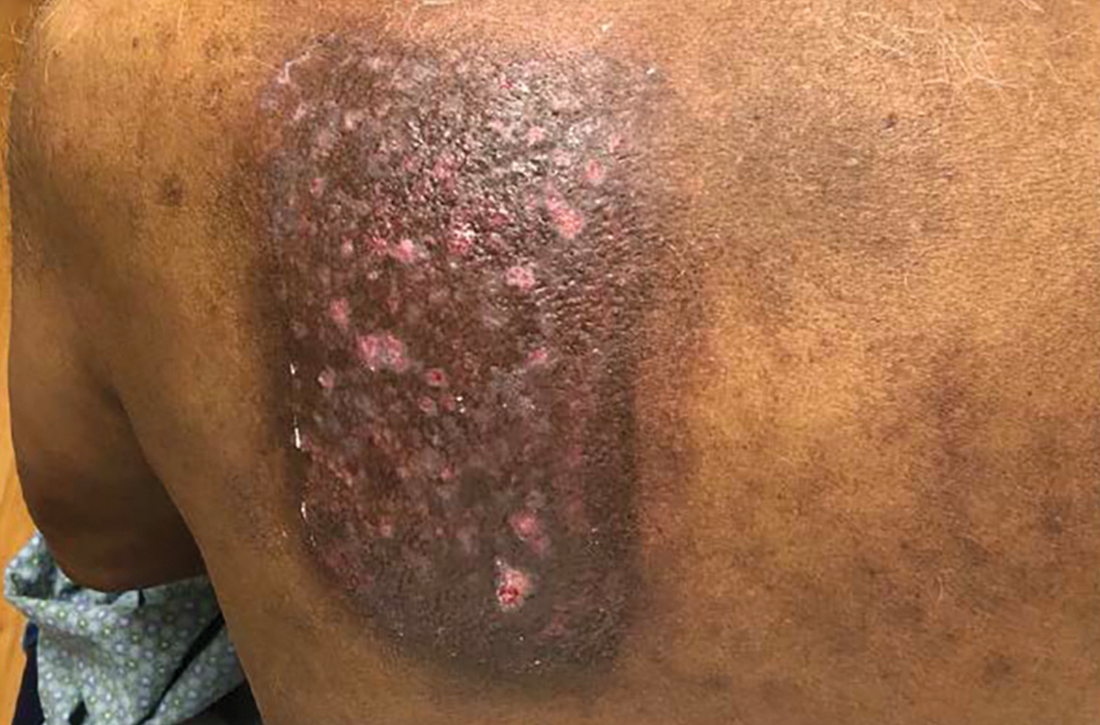
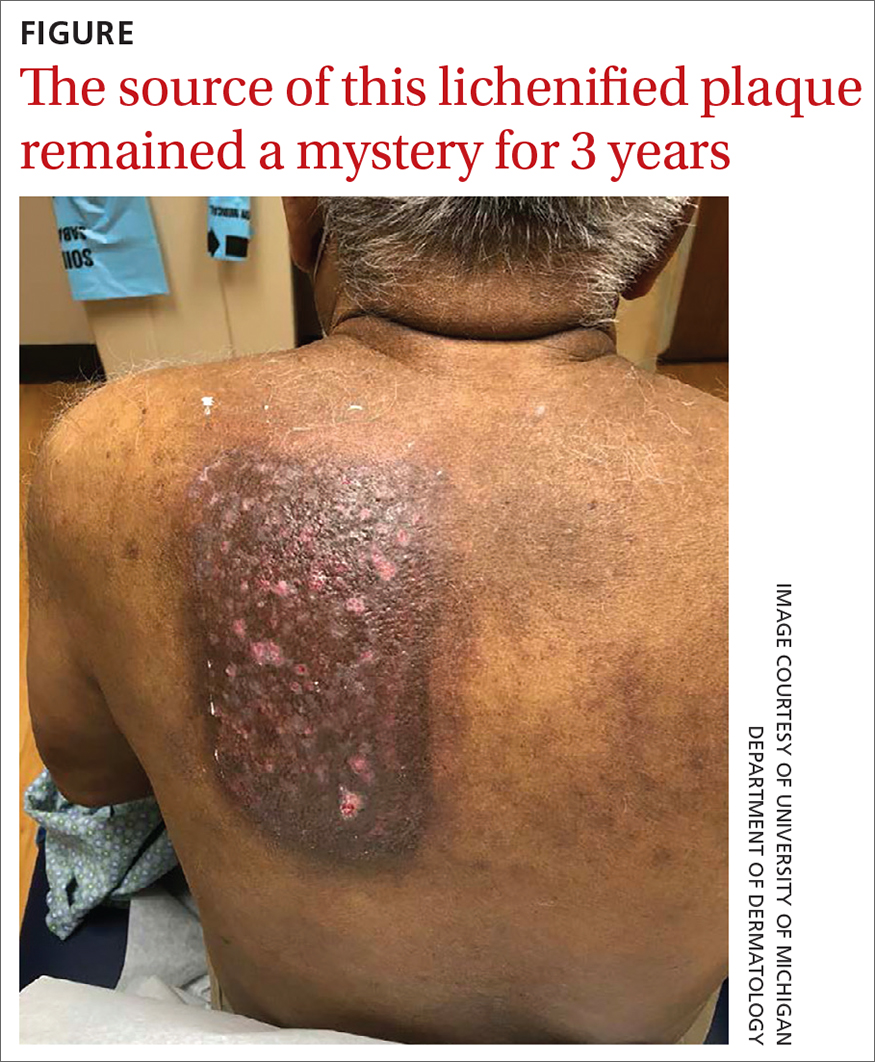
Physical exam by the dermatologist revealed a lidocaine patch applied to the patient’s left upper back. Upon its removal, skin examination showed a well-demarcated, erythematous, hyperpigmented, lichenified plaque with excoriations and erosions where the patch had been (FIGURE).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Contact dermatitis
The patient’s history and skin exam provided enough information to diagnose contact dermatitis. The pruritus, burning, and pain the patient had experienced were due to continuous application of the lidocaine patch to the area rather than postherpetic neuralgia.
There are 2 types of contact dermatitis: irritant and allergic. Irritant contact dermatitis is an inflammatory reaction caused directly by a substance, while allergic contact dermatitis is a delayed hypersensitivity reaction to specific allergens.1 While data to elucidate the incidence and prevalence of allergic contact dermatitis are unknown, common causes include latex, dyes, oils, resins, and compounds in textiles, rubber, cosmetics, and other products used in daily life.1
Allergic contact dermatitis due to lidocaine is becoming more prevalent with increased use and availability of over-the-counter products.2 A retrospective chart review of 1819 patch-tested patients from the University of British Columbia Contact Dermatitis Clinic showed a significant proportion of patients (2.4%) were found to have
The differential varies by area affected
The differential diagnosis for contact dermatitis varies by area affected and the distribution of rash. Atopic dermatitis, lichen planus, and psoriasis are a few dermatologic conditions to consider in the differential diagnosis. They can look similar to contact dermatitis, but the patient’s history can help to discern the most likely diagnosis.1
Atopic dermatitis is a complex dysfunction of the skin barrier and immune factors that often begins in childhood and persists in some patients throughout their lifetime. Atopic dermatitis is associated with other forms of atopy including asthma, allergic rhinitis, and food and contact allergies. Atopic dermatitis in the absence of contact allergies may manifest with chronic, diffuse, scaly patches with poorly defined borders. The patches appear in a symmetrical distribution and favor the flexural surfaces, such as the antecubital fossa, wrists, and neck.
Continue to: Lichen planus
Lichen planus most often manifests in the fourth through sixth decade of life as flat-topped itchy pink-to-purple polygonal papules to plaques. Lesions range from 2 to 10 mm and favor the volar wrists, shins, and lower back, although they may be widespread. Oral lesions manifesting as ulcers or white lacy patches in the buccal mucosa are common and may be a clue to the diagnosis. Unlike more generalized contact dermatitis, lichen planus lesions are discrete.
Psoriasis manifests as well-demarcated scaly plaques distributed symmetrically over extensor surfaces. The plaques commonly are found on the elbows, knees, and scalp. When psoriasis manifests in a very limited form (as just a single plaque or limited number of plaques), it can be hard to confidently exclude other etiologies. In these circumstances, look for psoriasis signs in more unique locations (eg, pitting in the nails or plaques on the scalp or in the gluteal cleft). Adding those findings to an otherwise solitary plaque significantly adds to diagnostic certainty.
Diagnosis entails getting the shape of things
Diagnosis is based on history of exposure to irritating or allergic substances, as well as a clinical exam. Skin examination of contact dermatitis can vary based on how long it has been present: Acute manifestations include erythema, oozing, scale, vesicles, and bullae, while chronic contact dermatitis tends to demonstrate lichenification and scale.1
Distinctive findings. The most distinctive physical exam findings in patients with contact dermatitis are often shape and distribution of the rash, which reflect points of contact with the offending agent. This clue helped to elucidate the diagnosis in our patient: his rash was perfectly demarcated within the precise area where the patch was applied daily.
Irritant vs allergic. Patch testing can be performed to differentiate irritant vs allergic contact dermatitis.1 Irritant contact dermatitis usually is apparent when removing a patch and will resolve over a day, whereas allergic contact dermatitis forms over time and the skin rash is most prominent several days after the patch has been removed.1
Continue to: Treatment
Treatment: First, stop the offense
Treatment of both variants of contact dermatitis includes avoidance of the causative substance and symptomatic treatment with topical steroids, antihistamines, and possibly oral steroids depending on the severity.1
For our patient, a viral swab was taken and submitted for varicella zoster virus polymerase chain reaction testing to rule out persistent herpes zoster infection; the result was negative. The patient was counseled to discontinue use of the lidocaine patch.
Given the severity and protracted duration of the patient’s symptoms, he also was started on high-potency topical steroids (clobetasol 0.05% ointment to be applied twice daily under occlusion for 2 months), a 4-week prednisone taper (60 mg × 1 week, 40 mg × 1 week, 20 mg × 1 week, 10 mg × 1 week, then stop), and hydroxyzine (25 mg nightly as needed for pruritus). The patient’s rash and symptoms improved dramatically within the first few doses of prednisone and completely cleared by Week 4 of the prednisone taper. At his follow-up appointment 1 month after completing the prednisone taper, he stated that the pain on his back had resolved
1. Li Y, Li L. Contact dermatitis: classifications and management. Clin Rev Allergy Immunol. 2021;61:245-281. doi: 10.1007/s12016-021-08875-0
2. Cline AE, Turrentine JE. Compounded topical analgesics for chronic pain. Dermatitis. 2016;27:263-271. doi: 10.1097/DER.0000000000000216
3. To D, Kossintseva I, de Gannes G. Lidocaine contact allergy is becoming more prevalent. Dermatol Surg. 2014;40:1367-1372. doi: 10.1097/DSS.0000000000000190
A 75-YEAR-OLD MAN presented to the dermatology clinic for evaluation of localized, persistent burning pain and discomfort attributed to shingles and postherpetic neuralgia. He had received a diagnosis of shingles on his left upper back about 3 years prior to this presentation.
In the ensuing years, the patient had been evaluated and treated by his primary care physician, a pain management team, and a neurologist. These clinicians treated the symptoms as postherpetic neuralgia, with no consensus explanation for the skin findings. The patient reported that his symptoms were unresponsive to trials of gabapentin 800 mg tid, duloxetine 60 mg PO qd, and acetaminophen 1 to 3 g/d PO. He also had undergone several rounds of acupuncture, thoracic and cervical spine steroid injections, and epidurals, without resolution of symptoms. The patient believed the only treatment that helped was a lidocaine 4% patch, which he had used nearly every day for the previous 3 years.
Physical exam by the dermatologist revealed a lidocaine patch applied to the patient’s left upper back. Upon its removal, skin examination showed a well-demarcated, erythematous, hyperpigmented, lichenified plaque with excoriations and erosions where the patch had been (FIGURE).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Contact dermatitis
The patient’s history and skin exam provided enough information to diagnose contact dermatitis. The pruritus, burning, and pain the patient had experienced were due to continuous application of the lidocaine patch to the area rather than postherpetic neuralgia.
There are 2 types of contact dermatitis: irritant and allergic. Irritant contact dermatitis is an inflammatory reaction caused directly by a substance, while allergic contact dermatitis is a delayed hypersensitivity reaction to specific allergens.1 While data to elucidate the incidence and prevalence of allergic contact dermatitis are unknown, common causes include latex, dyes, oils, resins, and compounds in textiles, rubber, cosmetics, and other products used in daily life.1
Allergic contact dermatitis due to lidocaine is becoming more prevalent with increased use and availability of over-the-counter products.2 A retrospective chart review of 1819 patch-tested patients from the University of British Columbia Contact Dermatitis Clinic showed a significant proportion of patients (2.4%) were found to have
The differential varies by area affected
The differential diagnosis for contact dermatitis varies by area affected and the distribution of rash. Atopic dermatitis, lichen planus, and psoriasis are a few dermatologic conditions to consider in the differential diagnosis. They can look similar to contact dermatitis, but the patient’s history can help to discern the most likely diagnosis.1
Atopic dermatitis is a complex dysfunction of the skin barrier and immune factors that often begins in childhood and persists in some patients throughout their lifetime. Atopic dermatitis is associated with other forms of atopy including asthma, allergic rhinitis, and food and contact allergies. Atopic dermatitis in the absence of contact allergies may manifest with chronic, diffuse, scaly patches with poorly defined borders. The patches appear in a symmetrical distribution and favor the flexural surfaces, such as the antecubital fossa, wrists, and neck.
Continue to: Lichen planus
Lichen planus most often manifests in the fourth through sixth decade of life as flat-topped itchy pink-to-purple polygonal papules to plaques. Lesions range from 2 to 10 mm and favor the volar wrists, shins, and lower back, although they may be widespread. Oral lesions manifesting as ulcers or white lacy patches in the buccal mucosa are common and may be a clue to the diagnosis. Unlike more generalized contact dermatitis, lichen planus lesions are discrete.
Psoriasis manifests as well-demarcated scaly plaques distributed symmetrically over extensor surfaces. The plaques commonly are found on the elbows, knees, and scalp. When psoriasis manifests in a very limited form (as just a single plaque or limited number of plaques), it can be hard to confidently exclude other etiologies. In these circumstances, look for psoriasis signs in more unique locations (eg, pitting in the nails or plaques on the scalp or in the gluteal cleft). Adding those findings to an otherwise solitary plaque significantly adds to diagnostic certainty.
Diagnosis entails getting the shape of things
Diagnosis is based on history of exposure to irritating or allergic substances, as well as a clinical exam. Skin examination of contact dermatitis can vary based on how long it has been present: Acute manifestations include erythema, oozing, scale, vesicles, and bullae, while chronic contact dermatitis tends to demonstrate lichenification and scale.1
Distinctive findings. The most distinctive physical exam findings in patients with contact dermatitis are often shape and distribution of the rash, which reflect points of contact with the offending agent. This clue helped to elucidate the diagnosis in our patient: his rash was perfectly demarcated within the precise area where the patch was applied daily.
Irritant vs allergic. Patch testing can be performed to differentiate irritant vs allergic contact dermatitis.1 Irritant contact dermatitis usually is apparent when removing a patch and will resolve over a day, whereas allergic contact dermatitis forms over time and the skin rash is most prominent several days after the patch has been removed.1
Continue to: Treatment
Treatment: First, stop the offense
Treatment of both variants of contact dermatitis includes avoidance of the causative substance and symptomatic treatment with topical steroids, antihistamines, and possibly oral steroids depending on the severity.1
For our patient, a viral swab was taken and submitted for varicella zoster virus polymerase chain reaction testing to rule out persistent herpes zoster infection; the result was negative. The patient was counseled to discontinue use of the lidocaine patch.
Given the severity and protracted duration of the patient’s symptoms, he also was started on high-potency topical steroids (clobetasol 0.05% ointment to be applied twice daily under occlusion for 2 months), a 4-week prednisone taper (60 mg × 1 week, 40 mg × 1 week, 20 mg × 1 week, 10 mg × 1 week, then stop), and hydroxyzine (25 mg nightly as needed for pruritus). The patient’s rash and symptoms improved dramatically within the first few doses of prednisone and completely cleared by Week 4 of the prednisone taper. At his follow-up appointment 1 month after completing the prednisone taper, he stated that the pain on his back had resolved
A 75-YEAR-OLD MAN presented to the dermatology clinic for evaluation of localized, persistent burning pain and discomfort attributed to shingles and postherpetic neuralgia. He had received a diagnosis of shingles on his left upper back about 3 years prior to this presentation.
In the ensuing years, the patient had been evaluated and treated by his primary care physician, a pain management team, and a neurologist. These clinicians treated the symptoms as postherpetic neuralgia, with no consensus explanation for the skin findings. The patient reported that his symptoms were unresponsive to trials of gabapentin 800 mg tid, duloxetine 60 mg PO qd, and acetaminophen 1 to 3 g/d PO. He also had undergone several rounds of acupuncture, thoracic and cervical spine steroid injections, and epidurals, without resolution of symptoms. The patient believed the only treatment that helped was a lidocaine 4% patch, which he had used nearly every day for the previous 3 years.
Physical exam by the dermatologist revealed a lidocaine patch applied to the patient’s left upper back. Upon its removal, skin examination showed a well-demarcated, erythematous, hyperpigmented, lichenified plaque with excoriations and erosions where the patch had been (FIGURE).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Contact dermatitis
The patient’s history and skin exam provided enough information to diagnose contact dermatitis. The pruritus, burning, and pain the patient had experienced were due to continuous application of the lidocaine patch to the area rather than postherpetic neuralgia.
There are 2 types of contact dermatitis: irritant and allergic. Irritant contact dermatitis is an inflammatory reaction caused directly by a substance, while allergic contact dermatitis is a delayed hypersensitivity reaction to specific allergens.1 While data to elucidate the incidence and prevalence of allergic contact dermatitis are unknown, common causes include latex, dyes, oils, resins, and compounds in textiles, rubber, cosmetics, and other products used in daily life.1
Allergic contact dermatitis due to lidocaine is becoming more prevalent with increased use and availability of over-the-counter products.2 A retrospective chart review of 1819 patch-tested patients from the University of British Columbia Contact Dermatitis Clinic showed a significant proportion of patients (2.4%) were found to have
The differential varies by area affected
The differential diagnosis for contact dermatitis varies by area affected and the distribution of rash. Atopic dermatitis, lichen planus, and psoriasis are a few dermatologic conditions to consider in the differential diagnosis. They can look similar to contact dermatitis, but the patient’s history can help to discern the most likely diagnosis.1
Atopic dermatitis is a complex dysfunction of the skin barrier and immune factors that often begins in childhood and persists in some patients throughout their lifetime. Atopic dermatitis is associated with other forms of atopy including asthma, allergic rhinitis, and food and contact allergies. Atopic dermatitis in the absence of contact allergies may manifest with chronic, diffuse, scaly patches with poorly defined borders. The patches appear in a symmetrical distribution and favor the flexural surfaces, such as the antecubital fossa, wrists, and neck.
Continue to: Lichen planus
Lichen planus most often manifests in the fourth through sixth decade of life as flat-topped itchy pink-to-purple polygonal papules to plaques. Lesions range from 2 to 10 mm and favor the volar wrists, shins, and lower back, although they may be widespread. Oral lesions manifesting as ulcers or white lacy patches in the buccal mucosa are common and may be a clue to the diagnosis. Unlike more generalized contact dermatitis, lichen planus lesions are discrete.
Psoriasis manifests as well-demarcated scaly plaques distributed symmetrically over extensor surfaces. The plaques commonly are found on the elbows, knees, and scalp. When psoriasis manifests in a very limited form (as just a single plaque or limited number of plaques), it can be hard to confidently exclude other etiologies. In these circumstances, look for psoriasis signs in more unique locations (eg, pitting in the nails or plaques on the scalp or in the gluteal cleft). Adding those findings to an otherwise solitary plaque significantly adds to diagnostic certainty.
Diagnosis entails getting the shape of things
Diagnosis is based on history of exposure to irritating or allergic substances, as well as a clinical exam. Skin examination of contact dermatitis can vary based on how long it has been present: Acute manifestations include erythema, oozing, scale, vesicles, and bullae, while chronic contact dermatitis tends to demonstrate lichenification and scale.1
Distinctive findings. The most distinctive physical exam findings in patients with contact dermatitis are often shape and distribution of the rash, which reflect points of contact with the offending agent. This clue helped to elucidate the diagnosis in our patient: his rash was perfectly demarcated within the precise area where the patch was applied daily.
Irritant vs allergic. Patch testing can be performed to differentiate irritant vs allergic contact dermatitis.1 Irritant contact dermatitis usually is apparent when removing a patch and will resolve over a day, whereas allergic contact dermatitis forms over time and the skin rash is most prominent several days after the patch has been removed.1
Continue to: Treatment
Treatment: First, stop the offense
Treatment of both variants of contact dermatitis includes avoidance of the causative substance and symptomatic treatment with topical steroids, antihistamines, and possibly oral steroids depending on the severity.1
For our patient, a viral swab was taken and submitted for varicella zoster virus polymerase chain reaction testing to rule out persistent herpes zoster infection; the result was negative. The patient was counseled to discontinue use of the lidocaine patch.
Given the severity and protracted duration of the patient’s symptoms, he also was started on high-potency topical steroids (clobetasol 0.05% ointment to be applied twice daily under occlusion for 2 months), a 4-week prednisone taper (60 mg × 1 week, 40 mg × 1 week, 20 mg × 1 week, 10 mg × 1 week, then stop), and hydroxyzine (25 mg nightly as needed for pruritus). The patient’s rash and symptoms improved dramatically within the first few doses of prednisone and completely cleared by Week 4 of the prednisone taper. At his follow-up appointment 1 month after completing the prednisone taper, he stated that the pain on his back had resolved
1. Li Y, Li L. Contact dermatitis: classifications and management. Clin Rev Allergy Immunol. 2021;61:245-281. doi: 10.1007/s12016-021-08875-0
2. Cline AE, Turrentine JE. Compounded topical analgesics for chronic pain. Dermatitis. 2016;27:263-271. doi: 10.1097/DER.0000000000000216
3. To D, Kossintseva I, de Gannes G. Lidocaine contact allergy is becoming more prevalent. Dermatol Surg. 2014;40:1367-1372. doi: 10.1097/DSS.0000000000000190
1. Li Y, Li L. Contact dermatitis: classifications and management. Clin Rev Allergy Immunol. 2021;61:245-281. doi: 10.1007/s12016-021-08875-0
2. Cline AE, Turrentine JE. Compounded topical analgesics for chronic pain. Dermatitis. 2016;27:263-271. doi: 10.1097/DER.0000000000000216
3. To D, Kossintseva I, de Gannes G. Lidocaine contact allergy is becoming more prevalent. Dermatol Surg. 2014;40:1367-1372. doi: 10.1097/DSS.0000000000000190
Clinical Impact of UV Mutational Signatures in Veterans With Cancer
PURPOSE
Assess the clinical impact (CI) of UV-related DNA damage signatures (UVsig) in Veterans with cancer of unknown primary (CUP) and cancer of extracutaneous origin (CEO).
BACKGROUND
UVsig have been reported in CUP and CEO (i.e. head and neck cancer and lung cancer). The presence of UVsig suggests a cutaneous origin and potential misclassification of CEO using conventional histopathologic evaluation. Literature on the association of UVsig in pan-cancer genomics is limited.
METHODS
This is a retrospective study of Veterans who underwent comprehensive genomic profiling with FoundationOne CDx during 2/1/2019 to 9/30/2022 through the VA National Precision Oncology Program. The outcome was the CI of UVsig (high, medium, and low) determined by blinded chart reviews: (1) high: UVsig leading to change in diagnoses (CID) and a different first-line therapy (FLT) would have been offered; (2) medium: UVsig leading to CID, but appropriate FLT offered; (3) low: diagnoses modified by clinicians and treated as cutaneous cancers. NCCN Guidelines were referenced for FLT.
DATA ANALYSIS
Descriptive statistics and chi-square tests were utilized to evaluate the UVsig CI.
RESULTS
Among 5,565 cases with 10 or more assessable alterations for UVsig analysis, 650 (11.7%) were positive for UVsig. CUP and CEO cohorts each had 41 cases analyzed. In the CUP cases, 20 (48.8%), 9 (21.9%), and 12 (29.3%) were categorized as having high, medium, and low CI, respectively; and in the CEO cases, it was 22 (53.7%), 15 (36.6%), and 4 (9.8%). There was no difference statistically between the CUP and CEO groups on the percentage distribution of CI (p=0.06). Among the 42 out of 82 cases having high CI, 37 (88.1%) received cytotoxic chemotherapy without any indication, and 5 (11.9%) were not offered immunotherapy (IO) as FLT. More than half of the 82 cases had high CI; more than 90% of the CEO cases had high and medium CI.
IMPLICATIONS
UVsig serves as a useful biomarker for cancers with cutaneous origin. About 1% of the 5,565 cases analyzed had high UVsig CI. Knowledge of UVsig could lead to omission of chemotherapy (hence avoiding toxicities) or addition of IO (for potential benefits).
PURPOSE
Assess the clinical impact (CI) of UV-related DNA damage signatures (UVsig) in Veterans with cancer of unknown primary (CUP) and cancer of extracutaneous origin (CEO).
BACKGROUND
UVsig have been reported in CUP and CEO (i.e. head and neck cancer and lung cancer). The presence of UVsig suggests a cutaneous origin and potential misclassification of CEO using conventional histopathologic evaluation. Literature on the association of UVsig in pan-cancer genomics is limited.
METHODS
This is a retrospective study of Veterans who underwent comprehensive genomic profiling with FoundationOne CDx during 2/1/2019 to 9/30/2022 through the VA National Precision Oncology Program. The outcome was the CI of UVsig (high, medium, and low) determined by blinded chart reviews: (1) high: UVsig leading to change in diagnoses (CID) and a different first-line therapy (FLT) would have been offered; (2) medium: UVsig leading to CID, but appropriate FLT offered; (3) low: diagnoses modified by clinicians and treated as cutaneous cancers. NCCN Guidelines were referenced for FLT.
DATA ANALYSIS
Descriptive statistics and chi-square tests were utilized to evaluate the UVsig CI.
RESULTS
Among 5,565 cases with 10 or more assessable alterations for UVsig analysis, 650 (11.7%) were positive for UVsig. CUP and CEO cohorts each had 41 cases analyzed. In the CUP cases, 20 (48.8%), 9 (21.9%), and 12 (29.3%) were categorized as having high, medium, and low CI, respectively; and in the CEO cases, it was 22 (53.7%), 15 (36.6%), and 4 (9.8%). There was no difference statistically between the CUP and CEO groups on the percentage distribution of CI (p=0.06). Among the 42 out of 82 cases having high CI, 37 (88.1%) received cytotoxic chemotherapy without any indication, and 5 (11.9%) were not offered immunotherapy (IO) as FLT. More than half of the 82 cases had high CI; more than 90% of the CEO cases had high and medium CI.
IMPLICATIONS
UVsig serves as a useful biomarker for cancers with cutaneous origin. About 1% of the 5,565 cases analyzed had high UVsig CI. Knowledge of UVsig could lead to omission of chemotherapy (hence avoiding toxicities) or addition of IO (for potential benefits).
PURPOSE
Assess the clinical impact (CI) of UV-related DNA damage signatures (UVsig) in Veterans with cancer of unknown primary (CUP) and cancer of extracutaneous origin (CEO).
BACKGROUND
UVsig have been reported in CUP and CEO (i.e. head and neck cancer and lung cancer). The presence of UVsig suggests a cutaneous origin and potential misclassification of CEO using conventional histopathologic evaluation. Literature on the association of UVsig in pan-cancer genomics is limited.
METHODS
This is a retrospective study of Veterans who underwent comprehensive genomic profiling with FoundationOne CDx during 2/1/2019 to 9/30/2022 through the VA National Precision Oncology Program. The outcome was the CI of UVsig (high, medium, and low) determined by blinded chart reviews: (1) high: UVsig leading to change in diagnoses (CID) and a different first-line therapy (FLT) would have been offered; (2) medium: UVsig leading to CID, but appropriate FLT offered; (3) low: diagnoses modified by clinicians and treated as cutaneous cancers. NCCN Guidelines were referenced for FLT.
DATA ANALYSIS
Descriptive statistics and chi-square tests were utilized to evaluate the UVsig CI.
RESULTS
Among 5,565 cases with 10 or more assessable alterations for UVsig analysis, 650 (11.7%) were positive for UVsig. CUP and CEO cohorts each had 41 cases analyzed. In the CUP cases, 20 (48.8%), 9 (21.9%), and 12 (29.3%) were categorized as having high, medium, and low CI, respectively; and in the CEO cases, it was 22 (53.7%), 15 (36.6%), and 4 (9.8%). There was no difference statistically between the CUP and CEO groups on the percentage distribution of CI (p=0.06). Among the 42 out of 82 cases having high CI, 37 (88.1%) received cytotoxic chemotherapy without any indication, and 5 (11.9%) were not offered immunotherapy (IO) as FLT. More than half of the 82 cases had high CI; more than 90% of the CEO cases had high and medium CI.
IMPLICATIONS
UVsig serves as a useful biomarker for cancers with cutaneous origin. About 1% of the 5,565 cases analyzed had high UVsig CI. Knowledge of UVsig could lead to omission of chemotherapy (hence avoiding toxicities) or addition of IO (for potential benefits).
What is the diagnosis?
Answer: A
Pityriasis alba is a common benign skin disorder that presents as hypopigmented skin most noticeable in darker skin types. It presents as whitish or mildly erythematous patches, commonly on the face, though it can appear on the trunk and extremities as well. It is estimated that about 1% of the general population is affected and may be more common after months with more extended sun exposure.
While a specific cause has not been identified, it is thought to represent post-inflammatory hypopigmentation, and is thought by many experts to be more common in atopic individuals; it is considered a minor clinical criterion for atopic dermatitis. The name relates to its appearance at times being scaly (pityriasis) and its whitish coloration (alba) and may represent a non-specific dermatitis.
It occurs predominantly in children and adolescents, and a slight male predominance has been noted. Even though this condition is not seasonal, the lesions become more obvious in the spring and summer because of sun exposure and darkening of the surrounding normal skin.
Physical examination reveals multiple round or oval shaped hypopigmented poorly defined macules, patches, or thin plaques. Mild scaling may be present. The number of lesions is variable. The most common presentation is asymptomatic, although some patients report mild pruritus. Two infrequent variants have been reported. Pigmented pityriasis is mostly reported in patients with darker skin in South Africa and the Middle East and presents with hyperpigmented bluish patches surrounded by a hypopigmented ring. Extensive pityriasis alba is another uncommon variant, characterized by widespread symmetrical lesions distributed predominantly on the trunk. Seborrheic dermatitis presents as a mild form of dandruff, often with asymptomatic or mildly itchy scalp with scaling, though involvement of the face can be seen around the eyebrows, glabella, and nasolabial areas.
Less common conditions in the differential diagnosis include other inflammatory conditions (contact dermatitis, psoriasis), genodermatoses (such as ash-leaf macules of tuberous sclerosis), infectious diseases (leprosy, and tinea corporis or faciei) and nevoid conditions (such as nevus anemicus). Leprosy is tremendously rare in children in the United States and can present as sharply demarcated usually elevated plaques often with diminished sensation. Hypopigmentation secondary to topical medications or skin procedures should also be considered. When encountering chronic, refractory, or extensive cases, an alarm for pityriasis lichenoides chronica and cutaneous lymphoma (hypopigmented mycosis fungoides) might be considered.
Pityriasis alba is a self-limited condition with a good prognosis and expected complete resolution, most commonly within 1 year. Patients and their parents should be educated regarding the benign and self-limited nature of pityriasis alba. Affected areas should be sun-protected to avoid worsening of the cosmetic appearance and prevent sunburn in the hypopigmented areas. The frequent use of emollients is the mainstay of treatment. Some topical treatments may reduce erythema and pruritus and accelerate repigmentation. Low-potency topical steroids, such as 1% hydrocortisone, are an alternative treatment, especially when itchiness is present. Topical calcineurin inhibitors such as 0.1% tacrolimus or 1% pimecrolimus have also been reported to be effective, as well as topical vitamin D derivatives (calcitriol and calcipotriol).
Suggested reading
1. Treat: Abdel-Wahab HM and Ragaie MH. Pityriasis alba: Toward an effective treatment. J Dermatolog Treat. 2022 Jun;33(4):2285-9. doi: 10.1080/09546634.2021.1959014. Epub 2021 Aug 1.
2. PEARLS: Givler DN et al. Pityriasis alba. 2023 Feb 19. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
3. Choi SH et al. Pityriasis alba in pediatric patients with skin of color. J Drugs Dermatol. 2023 Apr 1;22(4):417-8. doi: 10.36849/JDD.7221.
4. Gawai SR et al. Association of pityriasis alba with atopic dermatitis: A cross-sectional study. Indian J Dermatol. 2021 Sep-Oct;66(5):567-8. doi: 10.4103/ijd.ijd_936_20.
Dr. Guelfand is a visiting dermatology resident in the division of pediatric and adolescent dermatology, University of California, San Diego. Dr. Vuong is a clinical fellow in the division of pediatric and adolescent dermatology, University of California, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and distinguished professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. No author has any relevant financial disclosures.
Answer: A
Pityriasis alba is a common benign skin disorder that presents as hypopigmented skin most noticeable in darker skin types. It presents as whitish or mildly erythematous patches, commonly on the face, though it can appear on the trunk and extremities as well. It is estimated that about 1% of the general population is affected and may be more common after months with more extended sun exposure.
While a specific cause has not been identified, it is thought to represent post-inflammatory hypopigmentation, and is thought by many experts to be more common in atopic individuals; it is considered a minor clinical criterion for atopic dermatitis. The name relates to its appearance at times being scaly (pityriasis) and its whitish coloration (alba) and may represent a non-specific dermatitis.
It occurs predominantly in children and adolescents, and a slight male predominance has been noted. Even though this condition is not seasonal, the lesions become more obvious in the spring and summer because of sun exposure and darkening of the surrounding normal skin.
Physical examination reveals multiple round or oval shaped hypopigmented poorly defined macules, patches, or thin plaques. Mild scaling may be present. The number of lesions is variable. The most common presentation is asymptomatic, although some patients report mild pruritus. Two infrequent variants have been reported. Pigmented pityriasis is mostly reported in patients with darker skin in South Africa and the Middle East and presents with hyperpigmented bluish patches surrounded by a hypopigmented ring. Extensive pityriasis alba is another uncommon variant, characterized by widespread symmetrical lesions distributed predominantly on the trunk. Seborrheic dermatitis presents as a mild form of dandruff, often with asymptomatic or mildly itchy scalp with scaling, though involvement of the face can be seen around the eyebrows, glabella, and nasolabial areas.
Less common conditions in the differential diagnosis include other inflammatory conditions (contact dermatitis, psoriasis), genodermatoses (such as ash-leaf macules of tuberous sclerosis), infectious diseases (leprosy, and tinea corporis or faciei) and nevoid conditions (such as nevus anemicus). Leprosy is tremendously rare in children in the United States and can present as sharply demarcated usually elevated plaques often with diminished sensation. Hypopigmentation secondary to topical medications or skin procedures should also be considered. When encountering chronic, refractory, or extensive cases, an alarm for pityriasis lichenoides chronica and cutaneous lymphoma (hypopigmented mycosis fungoides) might be considered.
Pityriasis alba is a self-limited condition with a good prognosis and expected complete resolution, most commonly within 1 year. Patients and their parents should be educated regarding the benign and self-limited nature of pityriasis alba. Affected areas should be sun-protected to avoid worsening of the cosmetic appearance and prevent sunburn in the hypopigmented areas. The frequent use of emollients is the mainstay of treatment. Some topical treatments may reduce erythema and pruritus and accelerate repigmentation. Low-potency topical steroids, such as 1% hydrocortisone, are an alternative treatment, especially when itchiness is present. Topical calcineurin inhibitors such as 0.1% tacrolimus or 1% pimecrolimus have also been reported to be effective, as well as topical vitamin D derivatives (calcitriol and calcipotriol).
Suggested reading
1. Treat: Abdel-Wahab HM and Ragaie MH. Pityriasis alba: Toward an effective treatment. J Dermatolog Treat. 2022 Jun;33(4):2285-9. doi: 10.1080/09546634.2021.1959014. Epub 2021 Aug 1.
2. PEARLS: Givler DN et al. Pityriasis alba. 2023 Feb 19. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
3. Choi SH et al. Pityriasis alba in pediatric patients with skin of color. J Drugs Dermatol. 2023 Apr 1;22(4):417-8. doi: 10.36849/JDD.7221.
4. Gawai SR et al. Association of pityriasis alba with atopic dermatitis: A cross-sectional study. Indian J Dermatol. 2021 Sep-Oct;66(5):567-8. doi: 10.4103/ijd.ijd_936_20.
Dr. Guelfand is a visiting dermatology resident in the division of pediatric and adolescent dermatology, University of California, San Diego. Dr. Vuong is a clinical fellow in the division of pediatric and adolescent dermatology, University of California, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and distinguished professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. No author has any relevant financial disclosures.
Answer: A
Pityriasis alba is a common benign skin disorder that presents as hypopigmented skin most noticeable in darker skin types. It presents as whitish or mildly erythematous patches, commonly on the face, though it can appear on the trunk and extremities as well. It is estimated that about 1% of the general population is affected and may be more common after months with more extended sun exposure.
While a specific cause has not been identified, it is thought to represent post-inflammatory hypopigmentation, and is thought by many experts to be more common in atopic individuals; it is considered a minor clinical criterion for atopic dermatitis. The name relates to its appearance at times being scaly (pityriasis) and its whitish coloration (alba) and may represent a non-specific dermatitis.
It occurs predominantly in children and adolescents, and a slight male predominance has been noted. Even though this condition is not seasonal, the lesions become more obvious in the spring and summer because of sun exposure and darkening of the surrounding normal skin.
Physical examination reveals multiple round or oval shaped hypopigmented poorly defined macules, patches, or thin plaques. Mild scaling may be present. The number of lesions is variable. The most common presentation is asymptomatic, although some patients report mild pruritus. Two infrequent variants have been reported. Pigmented pityriasis is mostly reported in patients with darker skin in South Africa and the Middle East and presents with hyperpigmented bluish patches surrounded by a hypopigmented ring. Extensive pityriasis alba is another uncommon variant, characterized by widespread symmetrical lesions distributed predominantly on the trunk. Seborrheic dermatitis presents as a mild form of dandruff, often with asymptomatic or mildly itchy scalp with scaling, though involvement of the face can be seen around the eyebrows, glabella, and nasolabial areas.
Less common conditions in the differential diagnosis include other inflammatory conditions (contact dermatitis, psoriasis), genodermatoses (such as ash-leaf macules of tuberous sclerosis), infectious diseases (leprosy, and tinea corporis or faciei) and nevoid conditions (such as nevus anemicus). Leprosy is tremendously rare in children in the United States and can present as sharply demarcated usually elevated plaques often with diminished sensation. Hypopigmentation secondary to topical medications or skin procedures should also be considered. When encountering chronic, refractory, or extensive cases, an alarm for pityriasis lichenoides chronica and cutaneous lymphoma (hypopigmented mycosis fungoides) might be considered.
Pityriasis alba is a self-limited condition with a good prognosis and expected complete resolution, most commonly within 1 year. Patients and their parents should be educated regarding the benign and self-limited nature of pityriasis alba. Affected areas should be sun-protected to avoid worsening of the cosmetic appearance and prevent sunburn in the hypopigmented areas. The frequent use of emollients is the mainstay of treatment. Some topical treatments may reduce erythema and pruritus and accelerate repigmentation. Low-potency topical steroids, such as 1% hydrocortisone, are an alternative treatment, especially when itchiness is present. Topical calcineurin inhibitors such as 0.1% tacrolimus or 1% pimecrolimus have also been reported to be effective, as well as topical vitamin D derivatives (calcitriol and calcipotriol).
Suggested reading
1. Treat: Abdel-Wahab HM and Ragaie MH. Pityriasis alba: Toward an effective treatment. J Dermatolog Treat. 2022 Jun;33(4):2285-9. doi: 10.1080/09546634.2021.1959014. Epub 2021 Aug 1.
2. PEARLS: Givler DN et al. Pityriasis alba. 2023 Feb 19. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
3. Choi SH et al. Pityriasis alba in pediatric patients with skin of color. J Drugs Dermatol. 2023 Apr 1;22(4):417-8. doi: 10.36849/JDD.7221.
4. Gawai SR et al. Association of pityriasis alba with atopic dermatitis: A cross-sectional study. Indian J Dermatol. 2021 Sep-Oct;66(5):567-8. doi: 10.4103/ijd.ijd_936_20.
Dr. Guelfand is a visiting dermatology resident in the division of pediatric and adolescent dermatology, University of California, San Diego. Dr. Vuong is a clinical fellow in the division of pediatric and adolescent dermatology, University of California, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and distinguished professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. No author has any relevant financial disclosures.
The lesions were asymptomatic, and the review of systems was otherwise negative.
Physical examination revealed multiple poorly defined thin hypopigmented patches with a bilateral distribution, mostly on the cheeks.
The patches had focal superficial nonadherent thin white scales and were mildly rough to the touch. The rest of the physical exam was unremarkable, including no active eczematous lesions on the trunk or extremities.
New AI-enhanced bandages poised to transform wound treatment
You cut yourself. You put on a bandage. In a week or so, your wound heals.
Most people take this routine for granted. But for the more than 8.2 million Americans who have chronic wounds, it’s not so simple.
Traumatic injuries, post-surgical complications, advanced age, and chronic illnesses like diabetes and vascular disease can all disrupt the delicate healing process, leading to wounds that last months or years.
Left untreated, about 30% led to amputation. And recent studies show the risk of dying from a chronic wound complication within 5 years rivals that of most cancers.
Yet until recently, medical technology had not kept up with what experts say is a snowballing threat to public health.
“Wound care – even with all of the billions of products that are sold – still exists on kind of a medieval level,” said Geoffrey Gurtner, MD, chair of the department of surgery and professor of biomedical engineering at the University of Arizona College of Medicine. “We’re still putting on poultices and salves ... and when it comes to diagnosing infection, it’s really an art. I think we can do better.”
Old-school bandage meets AI
Dr. Gurtner is among dozens of clinicians and researchers reimagining the humble bandage, combining cutting-edge materials science with artificial intelligence and patient data to develop “smart bandages” that do far more than shield a wound.
Someday soon, these paper-thin bandages embedded with miniaturized electronics could monitor the healing process in real time, alerting the patient – or a doctor – when things go wrong. With the press of a smartphone button, that bandage could deliver medicine to fight an infection or an electrical pulse to stimulate healing.
Some “closed-loop” designs need no prompting, instead monitoring the wound and automatically giving it what it needs.
Others in development could halt a battlefield wound from hemorrhaging or kick-start healing in a blast wound, preventing longer-term disability.
The same technologies could – if the price is right – speed up healing and reduce scarring in minor cuts and scrapes, too, said Dr. Gurtner.
And unlike many cutting-edge medical innovations, these next-generation bandages could be made relatively cheaply and benefit some of the most vulnerable populations, including older adults, people with low incomes, and those in developing countries.
They could also save the health care system money, as the U.S. spends more than $28 billion annually treating chronic wounds.
“This is a condition that many patients find shameful and embarrassing, so there hasn’t been a lot of advocacy,” said Dr. Gurtner, outgoing board president of the Wound Healing Society. “It’s a relatively ignored problem afflicting an underserved population that has a huge cost. It’s a perfect storm.”
How wounds heal, or don’t
Wound healing is one of the most complex processes of the human body.
First platelets rush to the injury, prompting blood to clot. Then immune cells emit compounds called inflammatory cytokines, helping to fight off pathogens and keep infection at bay. Other compounds, including nitric oxide, spark the growth of new blood vessels and collagen to rebuild skin and connective tissue. As inflammation slows and stops, the flesh continues to reform.
But some conditions can stall the process, often in the inflammatory stage.
In people with diabetes, high glucose levels and poor circulation tend to sabotage the process. And people with nerve damage from spinal cord injuries, diabetes, or other ailments may not be able to feel it when a wound is getting worse or reinjured.
“We end up with patients going months with open wounds that are festering and infected,” said Roslyn Rivkah Isseroff, MD, professor of dermatology at the University of California Davis and head of the VA Northern California Health Care System’s wound healing clinic. “The patients are upset with the smell. These open ulcers put the patient at risk for systemic infection, like sepsis.” It can impact mental health, draining the patient’s ability to care for their wound.
“We see them once a week and send them home and say change your dressing every day, and they say, ‘I can barely move. I can’t do this,’ ” said Dr. Isseroff.
Checking for infection means removing bandages and culturing the wound. That can be painful, and results take time.
A lot can happen to a wound in a week.
“Sometimes, they come back and it’s a disaster, and they have to be admitted to the ER or even get an amputation,” Dr. Gurtner said.
People who are housing insecure or lack access to health care are even more vulnerable to complications.
“If you had the ability to say ‘there is something bad happening,’ you could do a lot to prevent this cascade and downward spiral.”
Bandages 2.0
In 2019, the Defense Advanced Research Projects Agency, the research arm of the Department of Defense, launched the Bioelectronics for Tissue Regeneration program to encourage scientists to develop a “closed-loop” bandage capable of both monitoring and hastening healing.
Tens of millions in funding has kick-started a flood of innovation since.
“It’s kind of a race to the finish,” said Marco Rolandi, PhD, associate professor of electrical and computer engineering at the University of California Santa Cruz and the principal investigator for a team including engineers, medical doctors, and computer scientists from UC Santa Cruz, UC Davis, and Tufts. “I’ve been amazed and impressed at all the work coming out.”
His team’s goal is to cut healing time in half by using (a) real-time monitoring of how a wound is healing – using indicators like temperature, pH level, oxygen, moisture, glucose, electrical activity, and certain proteins, and (b) appropriate stimulation.
“Every wound is different, so there is no one solution,” said Dr. Isseroff, the team’s clinical lead. “The idea is that it will be able to sense different parameters unique to the wound, use AI to figure out what stage it is in, and provide the right stimulus to kick it out of that stalled stage.”
The team has developed a proof-of-concept prototype: a bandage embedded with a tiny camera that takes pictures and transmits them to a computer algorithm to assess the wound’s progress. Miniaturized battery-powered actuators, or motors, automatically deliver medication.
Phase I trials in rodents went well, Dr. Rolandi said. The team is now testing the bandage on pigs.
Across the globe, other promising developments are underway.
In a scientific paper published in May, researchers at the University of Glasgow described a new “low-cost, environmentally friendly” bandage embedded with light-emitting diodes that use ultraviolet light to kill bacteria – no antibiotics needed. The fabric is stitched with a slim, flexible coil that powers the lights without a battery using wireless power transfer. In lab studies, it eradicated gram-negative bacteria (some of the nastiest bugs) in 6 hours.
Also in May, in the journal Bioactive Materials, a Penn State team detailed a bandage with medicine-injecting microneedles that can halt bleeding immediately after injury. In lab and animal tests, it reduced clotting time from 11.5 minutes to 1.3 minutes and bleeding by 90%.
“With hemorrhaging injuries, it is often the loss of blood – not the injury itself – that causes death,” said study author Amir Sheikhi, PhD, assistant professor of chemical and biomedical engineering at Penn State. “Those 10 minutes could be the difference between life and death.”
Another smart bandage, developed at Northwestern University, Chicago, harmlessly dissolves – electrodes and all – into the body after it is no longer needed, eliminating what can be a painful removal.
Guillermo Ameer, DSc, a study author reporting on the technology in Science Advances, hopes it could be made cheaply and used in developing countries.
“We’d like to create something that you could use in your home, even in a very remote village,” said Dr. Ameer, professor of biomedical engineering at Northwestern.
Timeline for clinical use
These are early days for the smart bandage, scientists say. Most studies have been in rodents and more work is needed to develop human-scale bandages, reduce cost, solve long-term data storage, and ensure material adheres well without irritating the skin.
But Dr. Gurtner is hopeful that some iteration could be used in clinical practice within a few years.
In May, he and colleagues at Stanford (Calif.) University published a paper in Nature Biotechnology describing their smart bandage. It includes a microcontroller unit, a radio antenna, biosensors, and an electrical stimulator all affixed to a rubbery, skin-like polymer (or hydrogel) about the thickness of a single coat of latex paint.
The bandage senses changes in temperature and electrical conductivity as the wound heals, and it gives electrical stimulation to accelerate that healing.
Animals treated with the bandage healed 25% faster, with 50% less scarring.
Electrical currents are already used for wound healing in clinical practice, Dr. Gurtner said. Because the stimulus is already approved and the cost to make the bandage could be low (as little as $10 to $50), he believes it could be ushered through the approval processes relatively quickly.
“Is this the ultimate embodiment of all the bells and whistles that are possible in a smart bandage? No. Not yet,” he said. “But we think it will help people. And right now, that’s good enough.”
A version of this article appeared on WebMD.com.
You cut yourself. You put on a bandage. In a week or so, your wound heals.
Most people take this routine for granted. But for the more than 8.2 million Americans who have chronic wounds, it’s not so simple.
Traumatic injuries, post-surgical complications, advanced age, and chronic illnesses like diabetes and vascular disease can all disrupt the delicate healing process, leading to wounds that last months or years.
Left untreated, about 30% led to amputation. And recent studies show the risk of dying from a chronic wound complication within 5 years rivals that of most cancers.
Yet until recently, medical technology had not kept up with what experts say is a snowballing threat to public health.
“Wound care – even with all of the billions of products that are sold – still exists on kind of a medieval level,” said Geoffrey Gurtner, MD, chair of the department of surgery and professor of biomedical engineering at the University of Arizona College of Medicine. “We’re still putting on poultices and salves ... and when it comes to diagnosing infection, it’s really an art. I think we can do better.”
Old-school bandage meets AI
Dr. Gurtner is among dozens of clinicians and researchers reimagining the humble bandage, combining cutting-edge materials science with artificial intelligence and patient data to develop “smart bandages” that do far more than shield a wound.
Someday soon, these paper-thin bandages embedded with miniaturized electronics could monitor the healing process in real time, alerting the patient – or a doctor – when things go wrong. With the press of a smartphone button, that bandage could deliver medicine to fight an infection or an electrical pulse to stimulate healing.
Some “closed-loop” designs need no prompting, instead monitoring the wound and automatically giving it what it needs.
Others in development could halt a battlefield wound from hemorrhaging or kick-start healing in a blast wound, preventing longer-term disability.
The same technologies could – if the price is right – speed up healing and reduce scarring in minor cuts and scrapes, too, said Dr. Gurtner.
And unlike many cutting-edge medical innovations, these next-generation bandages could be made relatively cheaply and benefit some of the most vulnerable populations, including older adults, people with low incomes, and those in developing countries.
They could also save the health care system money, as the U.S. spends more than $28 billion annually treating chronic wounds.
“This is a condition that many patients find shameful and embarrassing, so there hasn’t been a lot of advocacy,” said Dr. Gurtner, outgoing board president of the Wound Healing Society. “It’s a relatively ignored problem afflicting an underserved population that has a huge cost. It’s a perfect storm.”
How wounds heal, or don’t
Wound healing is one of the most complex processes of the human body.
First platelets rush to the injury, prompting blood to clot. Then immune cells emit compounds called inflammatory cytokines, helping to fight off pathogens and keep infection at bay. Other compounds, including nitric oxide, spark the growth of new blood vessels and collagen to rebuild skin and connective tissue. As inflammation slows and stops, the flesh continues to reform.
But some conditions can stall the process, often in the inflammatory stage.
In people with diabetes, high glucose levels and poor circulation tend to sabotage the process. And people with nerve damage from spinal cord injuries, diabetes, or other ailments may not be able to feel it when a wound is getting worse or reinjured.
“We end up with patients going months with open wounds that are festering and infected,” said Roslyn Rivkah Isseroff, MD, professor of dermatology at the University of California Davis and head of the VA Northern California Health Care System’s wound healing clinic. “The patients are upset with the smell. These open ulcers put the patient at risk for systemic infection, like sepsis.” It can impact mental health, draining the patient’s ability to care for their wound.
“We see them once a week and send them home and say change your dressing every day, and they say, ‘I can barely move. I can’t do this,’ ” said Dr. Isseroff.
Checking for infection means removing bandages and culturing the wound. That can be painful, and results take time.
A lot can happen to a wound in a week.
“Sometimes, they come back and it’s a disaster, and they have to be admitted to the ER or even get an amputation,” Dr. Gurtner said.
People who are housing insecure or lack access to health care are even more vulnerable to complications.
“If you had the ability to say ‘there is something bad happening,’ you could do a lot to prevent this cascade and downward spiral.”
Bandages 2.0
In 2019, the Defense Advanced Research Projects Agency, the research arm of the Department of Defense, launched the Bioelectronics for Tissue Regeneration program to encourage scientists to develop a “closed-loop” bandage capable of both monitoring and hastening healing.
Tens of millions in funding has kick-started a flood of innovation since.
“It’s kind of a race to the finish,” said Marco Rolandi, PhD, associate professor of electrical and computer engineering at the University of California Santa Cruz and the principal investigator for a team including engineers, medical doctors, and computer scientists from UC Santa Cruz, UC Davis, and Tufts. “I’ve been amazed and impressed at all the work coming out.”
His team’s goal is to cut healing time in half by using (a) real-time monitoring of how a wound is healing – using indicators like temperature, pH level, oxygen, moisture, glucose, electrical activity, and certain proteins, and (b) appropriate stimulation.
“Every wound is different, so there is no one solution,” said Dr. Isseroff, the team’s clinical lead. “The idea is that it will be able to sense different parameters unique to the wound, use AI to figure out what stage it is in, and provide the right stimulus to kick it out of that stalled stage.”
The team has developed a proof-of-concept prototype: a bandage embedded with a tiny camera that takes pictures and transmits them to a computer algorithm to assess the wound’s progress. Miniaturized battery-powered actuators, or motors, automatically deliver medication.
Phase I trials in rodents went well, Dr. Rolandi said. The team is now testing the bandage on pigs.
Across the globe, other promising developments are underway.
In a scientific paper published in May, researchers at the University of Glasgow described a new “low-cost, environmentally friendly” bandage embedded with light-emitting diodes that use ultraviolet light to kill bacteria – no antibiotics needed. The fabric is stitched with a slim, flexible coil that powers the lights without a battery using wireless power transfer. In lab studies, it eradicated gram-negative bacteria (some of the nastiest bugs) in 6 hours.
Also in May, in the journal Bioactive Materials, a Penn State team detailed a bandage with medicine-injecting microneedles that can halt bleeding immediately after injury. In lab and animal tests, it reduced clotting time from 11.5 minutes to 1.3 minutes and bleeding by 90%.
“With hemorrhaging injuries, it is often the loss of blood – not the injury itself – that causes death,” said study author Amir Sheikhi, PhD, assistant professor of chemical and biomedical engineering at Penn State. “Those 10 minutes could be the difference between life and death.”
Another smart bandage, developed at Northwestern University, Chicago, harmlessly dissolves – electrodes and all – into the body after it is no longer needed, eliminating what can be a painful removal.
Guillermo Ameer, DSc, a study author reporting on the technology in Science Advances, hopes it could be made cheaply and used in developing countries.
“We’d like to create something that you could use in your home, even in a very remote village,” said Dr. Ameer, professor of biomedical engineering at Northwestern.
Timeline for clinical use
These are early days for the smart bandage, scientists say. Most studies have been in rodents and more work is needed to develop human-scale bandages, reduce cost, solve long-term data storage, and ensure material adheres well without irritating the skin.
But Dr. Gurtner is hopeful that some iteration could be used in clinical practice within a few years.
In May, he and colleagues at Stanford (Calif.) University published a paper in Nature Biotechnology describing their smart bandage. It includes a microcontroller unit, a radio antenna, biosensors, and an electrical stimulator all affixed to a rubbery, skin-like polymer (or hydrogel) about the thickness of a single coat of latex paint.
The bandage senses changes in temperature and electrical conductivity as the wound heals, and it gives electrical stimulation to accelerate that healing.
Animals treated with the bandage healed 25% faster, with 50% less scarring.
Electrical currents are already used for wound healing in clinical practice, Dr. Gurtner said. Because the stimulus is already approved and the cost to make the bandage could be low (as little as $10 to $50), he believes it could be ushered through the approval processes relatively quickly.
“Is this the ultimate embodiment of all the bells and whistles that are possible in a smart bandage? No. Not yet,” he said. “But we think it will help people. And right now, that’s good enough.”
A version of this article appeared on WebMD.com.
You cut yourself. You put on a bandage. In a week or so, your wound heals.
Most people take this routine for granted. But for the more than 8.2 million Americans who have chronic wounds, it’s not so simple.
Traumatic injuries, post-surgical complications, advanced age, and chronic illnesses like diabetes and vascular disease can all disrupt the delicate healing process, leading to wounds that last months or years.
Left untreated, about 30% led to amputation. And recent studies show the risk of dying from a chronic wound complication within 5 years rivals that of most cancers.
Yet until recently, medical technology had not kept up with what experts say is a snowballing threat to public health.
“Wound care – even with all of the billions of products that are sold – still exists on kind of a medieval level,” said Geoffrey Gurtner, MD, chair of the department of surgery and professor of biomedical engineering at the University of Arizona College of Medicine. “We’re still putting on poultices and salves ... and when it comes to diagnosing infection, it’s really an art. I think we can do better.”
Old-school bandage meets AI
Dr. Gurtner is among dozens of clinicians and researchers reimagining the humble bandage, combining cutting-edge materials science with artificial intelligence and patient data to develop “smart bandages” that do far more than shield a wound.
Someday soon, these paper-thin bandages embedded with miniaturized electronics could monitor the healing process in real time, alerting the patient – or a doctor – when things go wrong. With the press of a smartphone button, that bandage could deliver medicine to fight an infection or an electrical pulse to stimulate healing.
Some “closed-loop” designs need no prompting, instead monitoring the wound and automatically giving it what it needs.
Others in development could halt a battlefield wound from hemorrhaging or kick-start healing in a blast wound, preventing longer-term disability.
The same technologies could – if the price is right – speed up healing and reduce scarring in minor cuts and scrapes, too, said Dr. Gurtner.
And unlike many cutting-edge medical innovations, these next-generation bandages could be made relatively cheaply and benefit some of the most vulnerable populations, including older adults, people with low incomes, and those in developing countries.
They could also save the health care system money, as the U.S. spends more than $28 billion annually treating chronic wounds.
“This is a condition that many patients find shameful and embarrassing, so there hasn’t been a lot of advocacy,” said Dr. Gurtner, outgoing board president of the Wound Healing Society. “It’s a relatively ignored problem afflicting an underserved population that has a huge cost. It’s a perfect storm.”
How wounds heal, or don’t
Wound healing is one of the most complex processes of the human body.
First platelets rush to the injury, prompting blood to clot. Then immune cells emit compounds called inflammatory cytokines, helping to fight off pathogens and keep infection at bay. Other compounds, including nitric oxide, spark the growth of new blood vessels and collagen to rebuild skin and connective tissue. As inflammation slows and stops, the flesh continues to reform.
But some conditions can stall the process, often in the inflammatory stage.
In people with diabetes, high glucose levels and poor circulation tend to sabotage the process. And people with nerve damage from spinal cord injuries, diabetes, or other ailments may not be able to feel it when a wound is getting worse or reinjured.
“We end up with patients going months with open wounds that are festering and infected,” said Roslyn Rivkah Isseroff, MD, professor of dermatology at the University of California Davis and head of the VA Northern California Health Care System’s wound healing clinic. “The patients are upset with the smell. These open ulcers put the patient at risk for systemic infection, like sepsis.” It can impact mental health, draining the patient’s ability to care for their wound.
“We see them once a week and send them home and say change your dressing every day, and they say, ‘I can barely move. I can’t do this,’ ” said Dr. Isseroff.
Checking for infection means removing bandages and culturing the wound. That can be painful, and results take time.
A lot can happen to a wound in a week.
“Sometimes, they come back and it’s a disaster, and they have to be admitted to the ER or even get an amputation,” Dr. Gurtner said.
People who are housing insecure or lack access to health care are even more vulnerable to complications.
“If you had the ability to say ‘there is something bad happening,’ you could do a lot to prevent this cascade and downward spiral.”
Bandages 2.0
In 2019, the Defense Advanced Research Projects Agency, the research arm of the Department of Defense, launched the Bioelectronics for Tissue Regeneration program to encourage scientists to develop a “closed-loop” bandage capable of both monitoring and hastening healing.
Tens of millions in funding has kick-started a flood of innovation since.
“It’s kind of a race to the finish,” said Marco Rolandi, PhD, associate professor of electrical and computer engineering at the University of California Santa Cruz and the principal investigator for a team including engineers, medical doctors, and computer scientists from UC Santa Cruz, UC Davis, and Tufts. “I’ve been amazed and impressed at all the work coming out.”
His team’s goal is to cut healing time in half by using (a) real-time monitoring of how a wound is healing – using indicators like temperature, pH level, oxygen, moisture, glucose, electrical activity, and certain proteins, and (b) appropriate stimulation.
“Every wound is different, so there is no one solution,” said Dr. Isseroff, the team’s clinical lead. “The idea is that it will be able to sense different parameters unique to the wound, use AI to figure out what stage it is in, and provide the right stimulus to kick it out of that stalled stage.”
The team has developed a proof-of-concept prototype: a bandage embedded with a tiny camera that takes pictures and transmits them to a computer algorithm to assess the wound’s progress. Miniaturized battery-powered actuators, or motors, automatically deliver medication.
Phase I trials in rodents went well, Dr. Rolandi said. The team is now testing the bandage on pigs.
Across the globe, other promising developments are underway.
In a scientific paper published in May, researchers at the University of Glasgow described a new “low-cost, environmentally friendly” bandage embedded with light-emitting diodes that use ultraviolet light to kill bacteria – no antibiotics needed. The fabric is stitched with a slim, flexible coil that powers the lights without a battery using wireless power transfer. In lab studies, it eradicated gram-negative bacteria (some of the nastiest bugs) in 6 hours.
Also in May, in the journal Bioactive Materials, a Penn State team detailed a bandage with medicine-injecting microneedles that can halt bleeding immediately after injury. In lab and animal tests, it reduced clotting time from 11.5 minutes to 1.3 minutes and bleeding by 90%.
“With hemorrhaging injuries, it is often the loss of blood – not the injury itself – that causes death,” said study author Amir Sheikhi, PhD, assistant professor of chemical and biomedical engineering at Penn State. “Those 10 minutes could be the difference between life and death.”
Another smart bandage, developed at Northwestern University, Chicago, harmlessly dissolves – electrodes and all – into the body after it is no longer needed, eliminating what can be a painful removal.
Guillermo Ameer, DSc, a study author reporting on the technology in Science Advances, hopes it could be made cheaply and used in developing countries.
“We’d like to create something that you could use in your home, even in a very remote village,” said Dr. Ameer, professor of biomedical engineering at Northwestern.
Timeline for clinical use
These are early days for the smart bandage, scientists say. Most studies have been in rodents and more work is needed to develop human-scale bandages, reduce cost, solve long-term data storage, and ensure material adheres well without irritating the skin.
But Dr. Gurtner is hopeful that some iteration could be used in clinical practice within a few years.
In May, he and colleagues at Stanford (Calif.) University published a paper in Nature Biotechnology describing their smart bandage. It includes a microcontroller unit, a radio antenna, biosensors, and an electrical stimulator all affixed to a rubbery, skin-like polymer (or hydrogel) about the thickness of a single coat of latex paint.
The bandage senses changes in temperature and electrical conductivity as the wound heals, and it gives electrical stimulation to accelerate that healing.
Animals treated with the bandage healed 25% faster, with 50% less scarring.
Electrical currents are already used for wound healing in clinical practice, Dr. Gurtner said. Because the stimulus is already approved and the cost to make the bandage could be low (as little as $10 to $50), he believes it could be ushered through the approval processes relatively quickly.
“Is this the ultimate embodiment of all the bells and whistles that are possible in a smart bandage? No. Not yet,” he said. “But we think it will help people. And right now, that’s good enough.”
A version of this article appeared on WebMD.com.
Small persistent leg wound

A leg ulcer may have many causes, including venous stasis, trauma, vasculitis, infection, or (as in this case) squamous cell carcinoma in situ (SCCis), aka Bowen’s Disease.
SCC and SCCis are common skin cancers that occur less frequently than basal cell carcinomas (BCCs).1 SCCis is normally scaly and hyperkeratotic, but it can manifest in rare cases as a chronic ulcer. Fair skin, long history of sun damage, and immunosuppression are significant risk factors for both SCCis and SCC.
While history and other clinical features may help narrow the diagnosis, a wound that does not heal despite treatments should be biopsied. Shave and punch biopsies are both excellent ways to diagnose an SCCis that has a classic appearance. However, ulcers and blisters can be caused by inflammatory processes (as in pyoderma gangrenosum or a fixed drug eruption) with characteristic findings deeper in the dermis; these lesions are better assessed with a punch biopsy.
In this case, a 4-mm punch biopsy was performed at the tissue edge and showed atypical keratinocytes limited to the epidermis. These atypical keratinocytes are associated with vesicle formation and ulcer, consistent with SCCis.
SCCis transforms into invasive disease in 3% to 5% of cases.2 Surgical treatment includes fusiform excision and electrodessication and curettage, both with cure rates that often exceed 90%.2,3 Nonsurgical options include topical 5-fluorouracil (67%-92% effective), topical imiquimod (75%-93%), and photodynamic therapy (52%-98%).4
Treatment choices depend on patient preference and provider capabilities. With surgical options there is the risk of bleeding and the need to care for a healing wound. Nonsurgical treatments can last longer and require topical treatment regimens and medications.
This patient opted for a fusiform excision and linear closure. She will continue to undergo serial skin evaluations twice a year for at least 2 years.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, Maine.
1. Lukowiak TM, Aizman L, Perz A, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to cutaneous squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156:1192-1198. doi:10.1001/jamadermatol.2020.2571
2. Morton CA, Birnie AJ, Eedy DJ. British Association of Dermatologists’ guidelines for the management of squamous cell carcinoma in situ (Bowen's disease). Br J Dermatol. 2014;170:245-246. doi: 10.1111/bjd.12766
3. Veverka KK, Stratman EJ. Electrodesiccation and curettage for squamous cell carcinoma in situ: the effect of anatomic location on local recurrence. Dermatol Surg. 2023;49:821-824. doi: 10.1097/DSS.0000000000003855
4. Algarin, YA, Jambusaria-Pahlajani A. Ruiz E, et al. Advances in topical treatments of cutaneous malignancies. Am J Clin Dermatol. 2023;24:69-80. doi: 10.1007/s40257-022-00731-x

A leg ulcer may have many causes, including venous stasis, trauma, vasculitis, infection, or (as in this case) squamous cell carcinoma in situ (SCCis), aka Bowen’s Disease.
SCC and SCCis are common skin cancers that occur less frequently than basal cell carcinomas (BCCs).1 SCCis is normally scaly and hyperkeratotic, but it can manifest in rare cases as a chronic ulcer. Fair skin, long history of sun damage, and immunosuppression are significant risk factors for both SCCis and SCC.
While history and other clinical features may help narrow the diagnosis, a wound that does not heal despite treatments should be biopsied. Shave and punch biopsies are both excellent ways to diagnose an SCCis that has a classic appearance. However, ulcers and blisters can be caused by inflammatory processes (as in pyoderma gangrenosum or a fixed drug eruption) with characteristic findings deeper in the dermis; these lesions are better assessed with a punch biopsy.
In this case, a 4-mm punch biopsy was performed at the tissue edge and showed atypical keratinocytes limited to the epidermis. These atypical keratinocytes are associated with vesicle formation and ulcer, consistent with SCCis.
SCCis transforms into invasive disease in 3% to 5% of cases.2 Surgical treatment includes fusiform excision and electrodessication and curettage, both with cure rates that often exceed 90%.2,3 Nonsurgical options include topical 5-fluorouracil (67%-92% effective), topical imiquimod (75%-93%), and photodynamic therapy (52%-98%).4
Treatment choices depend on patient preference and provider capabilities. With surgical options there is the risk of bleeding and the need to care for a healing wound. Nonsurgical treatments can last longer and require topical treatment regimens and medications.
This patient opted for a fusiform excision and linear closure. She will continue to undergo serial skin evaluations twice a year for at least 2 years.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, Maine.

A leg ulcer may have many causes, including venous stasis, trauma, vasculitis, infection, or (as in this case) squamous cell carcinoma in situ (SCCis), aka Bowen’s Disease.
SCC and SCCis are common skin cancers that occur less frequently than basal cell carcinomas (BCCs).1 SCCis is normally scaly and hyperkeratotic, but it can manifest in rare cases as a chronic ulcer. Fair skin, long history of sun damage, and immunosuppression are significant risk factors for both SCCis and SCC.
While history and other clinical features may help narrow the diagnosis, a wound that does not heal despite treatments should be biopsied. Shave and punch biopsies are both excellent ways to diagnose an SCCis that has a classic appearance. However, ulcers and blisters can be caused by inflammatory processes (as in pyoderma gangrenosum or a fixed drug eruption) with characteristic findings deeper in the dermis; these lesions are better assessed with a punch biopsy.
In this case, a 4-mm punch biopsy was performed at the tissue edge and showed atypical keratinocytes limited to the epidermis. These atypical keratinocytes are associated with vesicle formation and ulcer, consistent with SCCis.
SCCis transforms into invasive disease in 3% to 5% of cases.2 Surgical treatment includes fusiform excision and electrodessication and curettage, both with cure rates that often exceed 90%.2,3 Nonsurgical options include topical 5-fluorouracil (67%-92% effective), topical imiquimod (75%-93%), and photodynamic therapy (52%-98%).4
Treatment choices depend on patient preference and provider capabilities. With surgical options there is the risk of bleeding and the need to care for a healing wound. Nonsurgical treatments can last longer and require topical treatment regimens and medications.
This patient opted for a fusiform excision and linear closure. She will continue to undergo serial skin evaluations twice a year for at least 2 years.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, Maine.
1. Lukowiak TM, Aizman L, Perz A, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to cutaneous squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156:1192-1198. doi:10.1001/jamadermatol.2020.2571
2. Morton CA, Birnie AJ, Eedy DJ. British Association of Dermatologists’ guidelines for the management of squamous cell carcinoma in situ (Bowen's disease). Br J Dermatol. 2014;170:245-246. doi: 10.1111/bjd.12766
3. Veverka KK, Stratman EJ. Electrodesiccation and curettage for squamous cell carcinoma in situ: the effect of anatomic location on local recurrence. Dermatol Surg. 2023;49:821-824. doi: 10.1097/DSS.0000000000003855
4. Algarin, YA, Jambusaria-Pahlajani A. Ruiz E, et al. Advances in topical treatments of cutaneous malignancies. Am J Clin Dermatol. 2023;24:69-80. doi: 10.1007/s40257-022-00731-x
1. Lukowiak TM, Aizman L, Perz A, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to cutaneous squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156:1192-1198. doi:10.1001/jamadermatol.2020.2571
2. Morton CA, Birnie AJ, Eedy DJ. British Association of Dermatologists’ guidelines for the management of squamous cell carcinoma in situ (Bowen's disease). Br J Dermatol. 2014;170:245-246. doi: 10.1111/bjd.12766
3. Veverka KK, Stratman EJ. Electrodesiccation and curettage for squamous cell carcinoma in situ: the effect of anatomic location on local recurrence. Dermatol Surg. 2023;49:821-824. doi: 10.1097/DSS.0000000000003855
4. Algarin, YA, Jambusaria-Pahlajani A. Ruiz E, et al. Advances in topical treatments of cutaneous malignancies. Am J Clin Dermatol. 2023;24:69-80. doi: 10.1007/s40257-022-00731-x
Disseminated Papules and Nodules on the Skin and Oral Mucosa in an Infant
The Diagnosis: Congenital Cutaneous Langerhans Cell Histiocytosis
Although the infectious workup was positive for herpes simplex virus type 1 and cytomegalovirus antibodies, serologies for the rest of the TORCH (toxoplasmosis, other agents [syphilis, hepatitis B virus], rubella, cytomegalovirus) group of infections, as well as other bacterial, fungal, and viral infections, were negative. A skin biopsy from the right fifth toe showed a dense infiltrate of CD1a+ histiocytic cells with folded or kidney-shaped nuclei mixed with eosinophils, which was consistent with Langerhans cell histiocytosis (LCH) (Figure 1). Skin lesions were treated with hydrocortisone cream 2.5% and progressively faded over a few weeks.

Langerhans cell histiocytosis is a rare disorder with a variable clinical presentation depending on the sites affected and the extent of involvement. It can involve multiple organ systems, most commonly the skeletal system and the skin. Organ involvement is characterized by histiocyte infiltration. Acute disseminated multisystem disease most commonly is seen in children younger than 3 years.1
Congenital cutaneous LCH presents with variable skin lesions ranging from papules to vesicles, pustules, and ulcers, with onset at birth or in the neonatal period. Various morphologic traits of skin lesions have been described; the most common presentation is multiple red to yellow-brown, crusted papules with accompanying hemorrhage or erosion.1 Other cases have described an eczematous, seborrheic, diffuse eruption or erosive intertrigo. One case of a child with a solitary necrotic nodule on the scalp has been reported.2
Our patient presented with disseminated, nonblanching, purple to dark red papules and nodules of the skin and oral mucosa, as well as nail dystrophy (Figure 2). However, LCH in a neonate can mimic other causes of congenital papulonodular eruptions. Red-brown papules and nodules with or without crusting in a newborn can be mistaken for erythema toxicum neonatorum, transient neonatal pustular melanosis, congenital leukemia cutis, neonatal erythropoiesis, disseminated neonatal hemangiomatosis, infantile acropustulosis, or congenital TORCH infections such as rubella or syphilis. When LCH presents as vesicles or eroded papules or nodules in a newborn, the differential diagnosis includes incontinentia pigmenti and hereditary epidermolysis bullosa.
Langerhans cell histiocytosis may even present with a classic blueberry muffin rash that can lead clinicians to consider cutaneous metastasis from various hematologic malignancies or the more common TORCH infections. Several diagnostic tests can be performed to clarify the diagnosis, including bacterial and viral cultures and stains, serology, immunohistochemistry, flow cytometry, bone marrow aspiration, or skin biopsy.3 Langerhans cell histiocytosis is diagnosed with a combination of histology, immunohistochemistry, and clinical presentation; however, a skin biopsy is crucial. Tissue should be taken from the most easily accessible yet representative lesion. The characteristic appearance of LCH lesions is described as a dense infiltrate of histiocytic cells mixed with numerous eosinophils in the dermis.1 Histiocytes usually have folded nuclei and eosinophilic cytoplasm or kidney-shaped nuclei with prominent nucleoli. Positive CD1a and/or CD207 (Langerin) staining of the cells is required for definitive diagnosis.4 After diagnosis, it is important to obtain baseline laboratory and radiographic studies to determine the extent of systemic involvement.
Treatment of congenital LCH is tailored to the extent of organ involvement. The dermatologic manifestations resolve without medications in many cases. However, true self-resolving LCH can only be diagnosed retrospectively after a full evaluation for other sites of disease. Disseminated disease can be life-threatening and requires more active management. In cases of skin-limited disease, therapies include topical steroids, nitrogen mustard, or imiquimod; surgical resection of isolated lesions; phototherapy; or systemic therapies such as methotrexate, 6-mercaptopurine, vinblastine/vincristine, cladribine, and/or cytarabine. Symptomatic patients initially are treated with methotrexate and 6-mercaptopurine.5 Asymptomatic infants with skin-limited involvement can be managed with topical treatments.
Our patient had skin-limited disease. Abdominal ultrasonography, skeletal survey, and magnetic resonance imaging of the brain revealed no abnormalities. The patient’s family was advised to monitor him for reoccurrence of the skin lesions and to continue close follow-up with hematology and dermatology. Although congenital LCH often is self-resolving, extensive skin involvement increases the risk for internal organ involvement for several years.6 These patients require long-term follow-up for potential musculoskeletal, ophthalmologic, endocrine, hepatic, and/or pulmonary disease.
- Pan Y, Zeng X, Ge J, et al. Congenital self-healing Langerhans cell histiocytosis: clinical and pathological characteristics. Int J Clin Exp Pathol. 2019;12:2275-2278.
- Morren MA, Vanden Broecke K, Vangeebergen L, et al. Diverse cutaneous presentations of Langerhans cell histiocytosis in children: a retrospective cohort study. Pediatr Blood Cancer. 2016;63:486-492. doi:10.1002/pbc.25834
- Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: diagnosis, differential diagnosis, treatment, sequelae, and standardized follow-up. J Am Acad Dermatol. 2018;78:1047-1056. doi:10.1016/j.jaad.2017.05.060
- Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184. doi:10.1002/pbc.24367
- Allen CE, Ladisch S, McClain KL. How I treat Langerhans cell histiocytosis. Blood. 2015;126:26-35. doi:10.1182/blood-2014-12-569301
- Jezierska M, Stefanowicz J, Romanowicz G, et al. Langerhans cell histiocytosis in children—a disease with many faces. recent advances in pathogenesis, diagnostic examinations and treatment. Postepy Dermatol Alergol. 2018;35:6-17. doi:10.5114/pdia.2017.67095
The Diagnosis: Congenital Cutaneous Langerhans Cell Histiocytosis
Although the infectious workup was positive for herpes simplex virus type 1 and cytomegalovirus antibodies, serologies for the rest of the TORCH (toxoplasmosis, other agents [syphilis, hepatitis B virus], rubella, cytomegalovirus) group of infections, as well as other bacterial, fungal, and viral infections, were negative. A skin biopsy from the right fifth toe showed a dense infiltrate of CD1a+ histiocytic cells with folded or kidney-shaped nuclei mixed with eosinophils, which was consistent with Langerhans cell histiocytosis (LCH) (Figure 1). Skin lesions were treated with hydrocortisone cream 2.5% and progressively faded over a few weeks.

Langerhans cell histiocytosis is a rare disorder with a variable clinical presentation depending on the sites affected and the extent of involvement. It can involve multiple organ systems, most commonly the skeletal system and the skin. Organ involvement is characterized by histiocyte infiltration. Acute disseminated multisystem disease most commonly is seen in children younger than 3 years.1
Congenital cutaneous LCH presents with variable skin lesions ranging from papules to vesicles, pustules, and ulcers, with onset at birth or in the neonatal period. Various morphologic traits of skin lesions have been described; the most common presentation is multiple red to yellow-brown, crusted papules with accompanying hemorrhage or erosion.1 Other cases have described an eczematous, seborrheic, diffuse eruption or erosive intertrigo. One case of a child with a solitary necrotic nodule on the scalp has been reported.2
Our patient presented with disseminated, nonblanching, purple to dark red papules and nodules of the skin and oral mucosa, as well as nail dystrophy (Figure 2). However, LCH in a neonate can mimic other causes of congenital papulonodular eruptions. Red-brown papules and nodules with or without crusting in a newborn can be mistaken for erythema toxicum neonatorum, transient neonatal pustular melanosis, congenital leukemia cutis, neonatal erythropoiesis, disseminated neonatal hemangiomatosis, infantile acropustulosis, or congenital TORCH infections such as rubella or syphilis. When LCH presents as vesicles or eroded papules or nodules in a newborn, the differential diagnosis includes incontinentia pigmenti and hereditary epidermolysis bullosa.

Langerhans cell histiocytosis may even present with a classic blueberry muffin rash that can lead clinicians to consider cutaneous metastasis from various hematologic malignancies or the more common TORCH infections. Several diagnostic tests can be performed to clarify the diagnosis, including bacterial and viral cultures and stains, serology, immunohistochemistry, flow cytometry, bone marrow aspiration, or skin biopsy.3 Langerhans cell histiocytosis is diagnosed with a combination of histology, immunohistochemistry, and clinical presentation; however, a skin biopsy is crucial. Tissue should be taken from the most easily accessible yet representative lesion. The characteristic appearance of LCH lesions is described as a dense infiltrate of histiocytic cells mixed with numerous eosinophils in the dermis.1 Histiocytes usually have folded nuclei and eosinophilic cytoplasm or kidney-shaped nuclei with prominent nucleoli. Positive CD1a and/or CD207 (Langerin) staining of the cells is required for definitive diagnosis.4 After diagnosis, it is important to obtain baseline laboratory and radiographic studies to determine the extent of systemic involvement.
Treatment of congenital LCH is tailored to the extent of organ involvement. The dermatologic manifestations resolve without medications in many cases. However, true self-resolving LCH can only be diagnosed retrospectively after a full evaluation for other sites of disease. Disseminated disease can be life-threatening and requires more active management. In cases of skin-limited disease, therapies include topical steroids, nitrogen mustard, or imiquimod; surgical resection of isolated lesions; phototherapy; or systemic therapies such as methotrexate, 6-mercaptopurine, vinblastine/vincristine, cladribine, and/or cytarabine. Symptomatic patients initially are treated with methotrexate and 6-mercaptopurine.5 Asymptomatic infants with skin-limited involvement can be managed with topical treatments.
Our patient had skin-limited disease. Abdominal ultrasonography, skeletal survey, and magnetic resonance imaging of the brain revealed no abnormalities. The patient’s family was advised to monitor him for reoccurrence of the skin lesions and to continue close follow-up with hematology and dermatology. Although congenital LCH often is self-resolving, extensive skin involvement increases the risk for internal organ involvement for several years.6 These patients require long-term follow-up for potential musculoskeletal, ophthalmologic, endocrine, hepatic, and/or pulmonary disease.
The Diagnosis: Congenital Cutaneous Langerhans Cell Histiocytosis
Although the infectious workup was positive for herpes simplex virus type 1 and cytomegalovirus antibodies, serologies for the rest of the TORCH (toxoplasmosis, other agents [syphilis, hepatitis B virus], rubella, cytomegalovirus) group of infections, as well as other bacterial, fungal, and viral infections, were negative. A skin biopsy from the right fifth toe showed a dense infiltrate of CD1a+ histiocytic cells with folded or kidney-shaped nuclei mixed with eosinophils, which was consistent with Langerhans cell histiocytosis (LCH) (Figure 1). Skin lesions were treated with hydrocortisone cream 2.5% and progressively faded over a few weeks.

Langerhans cell histiocytosis is a rare disorder with a variable clinical presentation depending on the sites affected and the extent of involvement. It can involve multiple organ systems, most commonly the skeletal system and the skin. Organ involvement is characterized by histiocyte infiltration. Acute disseminated multisystem disease most commonly is seen in children younger than 3 years.1
Congenital cutaneous LCH presents with variable skin lesions ranging from papules to vesicles, pustules, and ulcers, with onset at birth or in the neonatal period. Various morphologic traits of skin lesions have been described; the most common presentation is multiple red to yellow-brown, crusted papules with accompanying hemorrhage or erosion.1 Other cases have described an eczematous, seborrheic, diffuse eruption or erosive intertrigo. One case of a child with a solitary necrotic nodule on the scalp has been reported.2
Our patient presented with disseminated, nonblanching, purple to dark red papules and nodules of the skin and oral mucosa, as well as nail dystrophy (Figure 2). However, LCH in a neonate can mimic other causes of congenital papulonodular eruptions. Red-brown papules and nodules with or without crusting in a newborn can be mistaken for erythema toxicum neonatorum, transient neonatal pustular melanosis, congenital leukemia cutis, neonatal erythropoiesis, disseminated neonatal hemangiomatosis, infantile acropustulosis, or congenital TORCH infections such as rubella or syphilis. When LCH presents as vesicles or eroded papules or nodules in a newborn, the differential diagnosis includes incontinentia pigmenti and hereditary epidermolysis bullosa.

Langerhans cell histiocytosis may even present with a classic blueberry muffin rash that can lead clinicians to consider cutaneous metastasis from various hematologic malignancies or the more common TORCH infections. Several diagnostic tests can be performed to clarify the diagnosis, including bacterial and viral cultures and stains, serology, immunohistochemistry, flow cytometry, bone marrow aspiration, or skin biopsy.3 Langerhans cell histiocytosis is diagnosed with a combination of histology, immunohistochemistry, and clinical presentation; however, a skin biopsy is crucial. Tissue should be taken from the most easily accessible yet representative lesion. The characteristic appearance of LCH lesions is described as a dense infiltrate of histiocytic cells mixed with numerous eosinophils in the dermis.1 Histiocytes usually have folded nuclei and eosinophilic cytoplasm or kidney-shaped nuclei with prominent nucleoli. Positive CD1a and/or CD207 (Langerin) staining of the cells is required for definitive diagnosis.4 After diagnosis, it is important to obtain baseline laboratory and radiographic studies to determine the extent of systemic involvement.
Treatment of congenital LCH is tailored to the extent of organ involvement. The dermatologic manifestations resolve without medications in many cases. However, true self-resolving LCH can only be diagnosed retrospectively after a full evaluation for other sites of disease. Disseminated disease can be life-threatening and requires more active management. In cases of skin-limited disease, therapies include topical steroids, nitrogen mustard, or imiquimod; surgical resection of isolated lesions; phototherapy; or systemic therapies such as methotrexate, 6-mercaptopurine, vinblastine/vincristine, cladribine, and/or cytarabine. Symptomatic patients initially are treated with methotrexate and 6-mercaptopurine.5 Asymptomatic infants with skin-limited involvement can be managed with topical treatments.
Our patient had skin-limited disease. Abdominal ultrasonography, skeletal survey, and magnetic resonance imaging of the brain revealed no abnormalities. The patient’s family was advised to monitor him for reoccurrence of the skin lesions and to continue close follow-up with hematology and dermatology. Although congenital LCH often is self-resolving, extensive skin involvement increases the risk for internal organ involvement for several years.6 These patients require long-term follow-up for potential musculoskeletal, ophthalmologic, endocrine, hepatic, and/or pulmonary disease.
- Pan Y, Zeng X, Ge J, et al. Congenital self-healing Langerhans cell histiocytosis: clinical and pathological characteristics. Int J Clin Exp Pathol. 2019;12:2275-2278.
- Morren MA, Vanden Broecke K, Vangeebergen L, et al. Diverse cutaneous presentations of Langerhans cell histiocytosis in children: a retrospective cohort study. Pediatr Blood Cancer. 2016;63:486-492. doi:10.1002/pbc.25834
- Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: diagnosis, differential diagnosis, treatment, sequelae, and standardized follow-up. J Am Acad Dermatol. 2018;78:1047-1056. doi:10.1016/j.jaad.2017.05.060
- Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184. doi:10.1002/pbc.24367
- Allen CE, Ladisch S, McClain KL. How I treat Langerhans cell histiocytosis. Blood. 2015;126:26-35. doi:10.1182/blood-2014-12-569301
- Jezierska M, Stefanowicz J, Romanowicz G, et al. Langerhans cell histiocytosis in children—a disease with many faces. recent advances in pathogenesis, diagnostic examinations and treatment. Postepy Dermatol Alergol. 2018;35:6-17. doi:10.5114/pdia.2017.67095
- Pan Y, Zeng X, Ge J, et al. Congenital self-healing Langerhans cell histiocytosis: clinical and pathological characteristics. Int J Clin Exp Pathol. 2019;12:2275-2278.
- Morren MA, Vanden Broecke K, Vangeebergen L, et al. Diverse cutaneous presentations of Langerhans cell histiocytosis in children: a retrospective cohort study. Pediatr Blood Cancer. 2016;63:486-492. doi:10.1002/pbc.25834
- Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: diagnosis, differential diagnosis, treatment, sequelae, and standardized follow-up. J Am Acad Dermatol. 2018;78:1047-1056. doi:10.1016/j.jaad.2017.05.060
- Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184. doi:10.1002/pbc.24367
- Allen CE, Ladisch S, McClain KL. How I treat Langerhans cell histiocytosis. Blood. 2015;126:26-35. doi:10.1182/blood-2014-12-569301
- Jezierska M, Stefanowicz J, Romanowicz G, et al. Langerhans cell histiocytosis in children—a disease with many faces. recent advances in pathogenesis, diagnostic examinations and treatment. Postepy Dermatol Alergol. 2018;35:6-17. doi:10.5114/pdia.2017.67095
A 38-week-old infant boy presented at birth with disseminated, nonblanching, purple to dark red papules and nodules on the skin and oral mucosa. He was born spontaneously after an uncomplicated pregnancy. The mother experienced an episode of oral herpes simplex virus during pregnancy. The infant was otherwise healthy. Laboratory tests including a complete blood cell count and routine serum biochemical analyses were within reference range; however, an infectious workup was positive for herpes simplex virus type 1 and cytomegalovirus antibodies. Ophthalmologic and auditory screenings were normal.
Ruxolitinib for vitiligo: Experts share experiences from first year
.
The Food and Drug Administration approved the cream formulation of ruxolitinib (Opzelura), a JAK inhibitor, for repigmentation of nonsegmental vitiligo in July 2022 for people aged 12 years and older.
Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, said that he likes to use ruxolitinib cream in combination with other treatments.
“In the real world with vitiligo patients, we’re oftentimes doing combinatorial therapy anyway. So phototherapy, specifically, narrow-band UVB, is something that we have a lot of clinical evidence for over the years, and it’s a modality that can combine with topical steroids and topical calcineurin inhibitors.”
He said trials to study combinations will yield better guidance on optimal use of ruxolitinib cream. “In general, vitiligo patients can really benefit from phototherapy,” he said in an interview. (Labeling recommends against combination with other JAK inhibitors, biologics, or potent immunosuppressants, such as azathioprine or cyclosporine.)
This first year has shown that ruxolitinib is an effective option, but counseling patients to expect slow improvement is important so that patients stick with it, he noted.
Documenting what treatments patients with vitiligo have used before is important, he said, as is counseling patients that ruxolitinib is approved only for use on up to 10% of a person’s body surface area. (Product labeling recommends that a thin layer be applied twice a day to affected areas up to 10% of body surface area.)
Ruxolitinib has brought a “louder voice” to vitiligo and has opened up options for patients with the disease, Dr. Chovatiya said. “Having the ability to topically treat people who have very extensive disease really gives us a lot more flexibility than we have had before.”
Good experiences with payers at safety-net hospital
Candrice R. Heath, MD, assistant professor of dermatology at Temple University, Philadelphia, said that real-world experience with topical ruxolitinib will be more evident after its been on the market for 18-24 months.
Dr. Heath said she, too, encourages use of narrow-band UVB phototherapy in conjunction with the treatment.
From an insurance reimbursement standpoint, she said that she is glad that there have been fewer hurdles in getting ruxolitinib to patients than she has experienced with other medications.
In her safety-net hospital, she told this news organization, she sees patients with many types of insurance, but most have Medicaid. “So, I’m always expecting the step therapies, denials, pushbacks, etc.,” she said. But the path has been smoother for ruxolitinib coverage, she noted.
Her colleagues are committed to documenting everything the patient has tried, she added, and that helps with prior authorization.
Dr. Heath said that pointing out to insurers that ruxolitinib is the only approved treatment for repigmentation helps facilitate coverage.
“The science is advancing, and I’m happy to be practicing during a time when we actually have something approved for vitiligo,” she said. But she pointed out that phototherapy often is not covered for vitiligo, “which is horrible, when it is readily approved for psoriasis and atopic dermatitis.”
To document progress, Dr. Heath said that she always takes photographs of her patients with vitiligo because “the pictures remind us how far we have come.”
Data spotlight success in adolescents
Data from two trials give a clinical picture of the drug’s safety and efficacy in younger patients.
Adolescents had particularly good results in the first year with ruxolitinib, according to pooled phase 3 data from TRuE-V1 and TRuE-V2, this news organization reported.
The findings, presented at the 25th World Congress of Dermatology in Singapore, indicate that more than half of the participants achieved at least a 50% improvement from baseline in the total Vitiligo Area Scoring Index (T-VASI50) at 52 weeks.
The percentages of young patients aged 12-17 years taking twice-daily ruxolitinib who achieved T-VASI 50 at weeks 12, 24, and 52 were 11.5%, 26.9%, and 57.7%, respectively. The corresponding percentages for all in the study population were 10.7%, 22.7%, and 44.4%, respectively.
At the meeting, the presenter, Julien Seneschal, MD, PhD, professor of dermatology and head of the vitiligo and pigmentary disorders clinic at the University of Bordeaux, France, said, “This suggests that younger patients can respond better to the treatment.” He noted, however, that there were few adolescents in the studies.
New excitement in the field
Daniel Gutierrez, MD, assistant professor of dermatology at New York University, said the treatment has brought new excitement to the field.
“Patients with vitiligo are very motivated to treat their disease,” he said, because it typically is on the face and other highly visual areas, which can affect their overall perception of self.
Previously, he noted in an interview, the only FDA-approved treatment was monobenzone, but that was for depigmentation rather than repigmentation.
Otherwise, treatments were being used off label, and patients were receiving compounded formulations that often weren’t covered by insurance and often had shorter shelf life.
He said that he still occasionally gets denials from payers who consider vitiligo a cosmetic condition.
“I’ve had more luck with insurance, at least in the New York State area.” He added that sometimes payers require use of a topical calcineurin inhibitor for about 12 weeks before they will cover ruxolitinib.
Dr. Gutierrez also recommends using phototherapy with topical ruxolitinib “because they work on slightly different pathways.”
When he starts patients on a new therapy such as ruxolitinib, he asks them to come back in 3 months, and often by then, progress is evident. Facial areas show the most response, he said, while hands and feet are less likely to show significant improvement.
He said that it’s important for physicians and patients to know that improvements can take weeks or months to be noticeable. “I tell patients not to give up,” he added.
Showing the patients pictures from the current appointment and comparing them with pictures from previous appointments can help them better understand their progress, he said.
Lead investigator adds observations
David Rosmarin, MD, chair of the department of dermatology at Indiana University, Indianapolis, was the lead investigator of the pivotal TruE-V1 and TruE-V2 trials for vitiligo. In that role, he has been treating vitiligo patients with topical ruxolitinib since 2015.
In an interview, he said that many patients “don’t hit their optimal results at 3 months, 6 months, even the year mark. With continued use, many can see continued benefit.”
Other patients, he said, don’t respond within the first 6 months but with continued use may eventually respond, he said.
“Unfortunately, we have no way of knowing, based on clinical characteristics or baseline demographics, whether a patient will be a delayed responder or not or an early responder,” Dr. Rosmarin added.
He provided several observations about people who have stopped taking the medication.
“When people stop,” he said, “some maintain their response, but some start to depigment again. Again, we have no way of predicting who will be in which category.”
He said that once patients have hit their desired response, he usually advises them to taper down to maybe twice a week or to stop treatment, but if they see any recurrence, they should start reusing the medicine.
“We have some patients who have gone 6 or 7 years now before they had a recurrence, but others may start to depigment again in 2 to 3 months,” Dr. Rosmarin said.
As for phototherapy, he said, the combination with topical ruxolitinib is being studied.
“We think the combination is synergistic and better than either alone, but we’re still waiting for data to prove that,” he said.
In his practice, he offers patients the option either to use just ruxolitinib cream or the combination early on. Many patients, because of convenience, say they’ll first try the cream to see if that works.
“The challenge with light [therapy] is that it can be very inconvenient,” he said. Patients have to live close to a phototherapy unit to receive therapy 2-3 times a week or have a phototherapy product in their home.
Next in the pipeline
Experts say the progress doesn’t stop with ruxolitinib cream. Current trials of several medications show there’s more to come for patients with vitiligo.
Dr. Chovatiya said that next up may be oral ritlecitinib (Litfulo), a JAK inhibitor that was approved for severe alopecia areata in June for people aged 12 years and older. Phase 2 results have been published for its use with vitiligo.
“This would be an oral medication that may be able to help people with much more extensive disease as far as vitiligo goes,” he said, adding that he expects approval for a vitiligo indication within a few years.
He pointed out that longer-term safety data will be available because it is already on the market for alopecia.
Upadacitinib (Rinvoq), an oral JAK inhibitor, is approved for atopic dermatitis but is being studied for vitiligo as well, he noted. “I’m very excited to see what that holds for patients as well,” Dr. Chovatiya said.
Dr. Gutierrez said that he is excited about oral JAK inhibitors but sees potential in finding new ways to transplant melanocytes into areas where there are none.
The pigmentation field has seen new energy since last year’s approval, he said, particularly among people of color.
“We have new options for vitiligo that were lacking compared with other conditions, such as atopic dermatitis and psoriasis,” he said. “Hopefully, there will be more promising breakthroughs.”
Dr. Rosmarin is the chief investigator for the pivotal trials that led to FDA approval of ruxolitinib. He disclosed ties with AbbVie, Abcuro, AltruBio, Amgen, Arena, Boehringer Ingelheim, Bristol-Meyers Squibb, Celgene, Concert, CSL Behring, Dermavant, Dermira, Galderma, Incyte, Janssen, Kyowa Kirin, Lilly, Merck, Novartis, Pfizer, Regeneron, Revolo Biotherapeutics, Sanofi, Sun Pharmaceuticals, UCB, and Viela Bio. Dr. Chovatiya disclosed ties with AbbVie, Arcutis, Arena, Argenx, Beiersdorf, Bristol-Myers Squibb, Dermavant, Eli Lilly, EPI Health, Incyte, LEO Pharma, L’Oréal, National Eczema Association, Pfizer, Regeneron, Sanofi, and UCB. Dr. Heath and Dr. Gutierrez report no relevant financial relationships.
A version of this article appeared on Medscape.com.
.
The Food and Drug Administration approved the cream formulation of ruxolitinib (Opzelura), a JAK inhibitor, for repigmentation of nonsegmental vitiligo in July 2022 for people aged 12 years and older.
Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, said that he likes to use ruxolitinib cream in combination with other treatments.
“In the real world with vitiligo patients, we’re oftentimes doing combinatorial therapy anyway. So phototherapy, specifically, narrow-band UVB, is something that we have a lot of clinical evidence for over the years, and it’s a modality that can combine with topical steroids and topical calcineurin inhibitors.”
He said trials to study combinations will yield better guidance on optimal use of ruxolitinib cream. “In general, vitiligo patients can really benefit from phototherapy,” he said in an interview. (Labeling recommends against combination with other JAK inhibitors, biologics, or potent immunosuppressants, such as azathioprine or cyclosporine.)
This first year has shown that ruxolitinib is an effective option, but counseling patients to expect slow improvement is important so that patients stick with it, he noted.
Documenting what treatments patients with vitiligo have used before is important, he said, as is counseling patients that ruxolitinib is approved only for use on up to 10% of a person’s body surface area. (Product labeling recommends that a thin layer be applied twice a day to affected areas up to 10% of body surface area.)
Ruxolitinib has brought a “louder voice” to vitiligo and has opened up options for patients with the disease, Dr. Chovatiya said. “Having the ability to topically treat people who have very extensive disease really gives us a lot more flexibility than we have had before.”
Good experiences with payers at safety-net hospital
Candrice R. Heath, MD, assistant professor of dermatology at Temple University, Philadelphia, said that real-world experience with topical ruxolitinib will be more evident after its been on the market for 18-24 months.
Dr. Heath said she, too, encourages use of narrow-band UVB phototherapy in conjunction with the treatment.
From an insurance reimbursement standpoint, she said that she is glad that there have been fewer hurdles in getting ruxolitinib to patients than she has experienced with other medications.
In her safety-net hospital, she told this news organization, she sees patients with many types of insurance, but most have Medicaid. “So, I’m always expecting the step therapies, denials, pushbacks, etc.,” she said. But the path has been smoother for ruxolitinib coverage, she noted.
Her colleagues are committed to documenting everything the patient has tried, she added, and that helps with prior authorization.
Dr. Heath said that pointing out to insurers that ruxolitinib is the only approved treatment for repigmentation helps facilitate coverage.
“The science is advancing, and I’m happy to be practicing during a time when we actually have something approved for vitiligo,” she said. But she pointed out that phototherapy often is not covered for vitiligo, “which is horrible, when it is readily approved for psoriasis and atopic dermatitis.”
To document progress, Dr. Heath said that she always takes photographs of her patients with vitiligo because “the pictures remind us how far we have come.”
Data spotlight success in adolescents
Data from two trials give a clinical picture of the drug’s safety and efficacy in younger patients.
Adolescents had particularly good results in the first year with ruxolitinib, according to pooled phase 3 data from TRuE-V1 and TRuE-V2, this news organization reported.
The findings, presented at the 25th World Congress of Dermatology in Singapore, indicate that more than half of the participants achieved at least a 50% improvement from baseline in the total Vitiligo Area Scoring Index (T-VASI50) at 52 weeks.
The percentages of young patients aged 12-17 years taking twice-daily ruxolitinib who achieved T-VASI 50 at weeks 12, 24, and 52 were 11.5%, 26.9%, and 57.7%, respectively. The corresponding percentages for all in the study population were 10.7%, 22.7%, and 44.4%, respectively.
At the meeting, the presenter, Julien Seneschal, MD, PhD, professor of dermatology and head of the vitiligo and pigmentary disorders clinic at the University of Bordeaux, France, said, “This suggests that younger patients can respond better to the treatment.” He noted, however, that there were few adolescents in the studies.
New excitement in the field
Daniel Gutierrez, MD, assistant professor of dermatology at New York University, said the treatment has brought new excitement to the field.
“Patients with vitiligo are very motivated to treat their disease,” he said, because it typically is on the face and other highly visual areas, which can affect their overall perception of self.
Previously, he noted in an interview, the only FDA-approved treatment was monobenzone, but that was for depigmentation rather than repigmentation.
Otherwise, treatments were being used off label, and patients were receiving compounded formulations that often weren’t covered by insurance and often had shorter shelf life.
He said that he still occasionally gets denials from payers who consider vitiligo a cosmetic condition.
“I’ve had more luck with insurance, at least in the New York State area.” He added that sometimes payers require use of a topical calcineurin inhibitor for about 12 weeks before they will cover ruxolitinib.
Dr. Gutierrez also recommends using phototherapy with topical ruxolitinib “because they work on slightly different pathways.”
When he starts patients on a new therapy such as ruxolitinib, he asks them to come back in 3 months, and often by then, progress is evident. Facial areas show the most response, he said, while hands and feet are less likely to show significant improvement.
He said that it’s important for physicians and patients to know that improvements can take weeks or months to be noticeable. “I tell patients not to give up,” he added.
Showing the patients pictures from the current appointment and comparing them with pictures from previous appointments can help them better understand their progress, he said.
Lead investigator adds observations
David Rosmarin, MD, chair of the department of dermatology at Indiana University, Indianapolis, was the lead investigator of the pivotal TruE-V1 and TruE-V2 trials for vitiligo. In that role, he has been treating vitiligo patients with topical ruxolitinib since 2015.
In an interview, he said that many patients “don’t hit their optimal results at 3 months, 6 months, even the year mark. With continued use, many can see continued benefit.”
Other patients, he said, don’t respond within the first 6 months but with continued use may eventually respond, he said.
“Unfortunately, we have no way of knowing, based on clinical characteristics or baseline demographics, whether a patient will be a delayed responder or not or an early responder,” Dr. Rosmarin added.
He provided several observations about people who have stopped taking the medication.
“When people stop,” he said, “some maintain their response, but some start to depigment again. Again, we have no way of predicting who will be in which category.”
He said that once patients have hit their desired response, he usually advises them to taper down to maybe twice a week or to stop treatment, but if they see any recurrence, they should start reusing the medicine.
“We have some patients who have gone 6 or 7 years now before they had a recurrence, but others may start to depigment again in 2 to 3 months,” Dr. Rosmarin said.
As for phototherapy, he said, the combination with topical ruxolitinib is being studied.
“We think the combination is synergistic and better than either alone, but we’re still waiting for data to prove that,” he said.
In his practice, he offers patients the option either to use just ruxolitinib cream or the combination early on. Many patients, because of convenience, say they’ll first try the cream to see if that works.
“The challenge with light [therapy] is that it can be very inconvenient,” he said. Patients have to live close to a phototherapy unit to receive therapy 2-3 times a week or have a phototherapy product in their home.
Next in the pipeline
Experts say the progress doesn’t stop with ruxolitinib cream. Current trials of several medications show there’s more to come for patients with vitiligo.
Dr. Chovatiya said that next up may be oral ritlecitinib (Litfulo), a JAK inhibitor that was approved for severe alopecia areata in June for people aged 12 years and older. Phase 2 results have been published for its use with vitiligo.
“This would be an oral medication that may be able to help people with much more extensive disease as far as vitiligo goes,” he said, adding that he expects approval for a vitiligo indication within a few years.
He pointed out that longer-term safety data will be available because it is already on the market for alopecia.
Upadacitinib (Rinvoq), an oral JAK inhibitor, is approved for atopic dermatitis but is being studied for vitiligo as well, he noted. “I’m very excited to see what that holds for patients as well,” Dr. Chovatiya said.
Dr. Gutierrez said that he is excited about oral JAK inhibitors but sees potential in finding new ways to transplant melanocytes into areas where there are none.
The pigmentation field has seen new energy since last year’s approval, he said, particularly among people of color.
“We have new options for vitiligo that were lacking compared with other conditions, such as atopic dermatitis and psoriasis,” he said. “Hopefully, there will be more promising breakthroughs.”
Dr. Rosmarin is the chief investigator for the pivotal trials that led to FDA approval of ruxolitinib. He disclosed ties with AbbVie, Abcuro, AltruBio, Amgen, Arena, Boehringer Ingelheim, Bristol-Meyers Squibb, Celgene, Concert, CSL Behring, Dermavant, Dermira, Galderma, Incyte, Janssen, Kyowa Kirin, Lilly, Merck, Novartis, Pfizer, Regeneron, Revolo Biotherapeutics, Sanofi, Sun Pharmaceuticals, UCB, and Viela Bio. Dr. Chovatiya disclosed ties with AbbVie, Arcutis, Arena, Argenx, Beiersdorf, Bristol-Myers Squibb, Dermavant, Eli Lilly, EPI Health, Incyte, LEO Pharma, L’Oréal, National Eczema Association, Pfizer, Regeneron, Sanofi, and UCB. Dr. Heath and Dr. Gutierrez report no relevant financial relationships.
A version of this article appeared on Medscape.com.
.
The Food and Drug Administration approved the cream formulation of ruxolitinib (Opzelura), a JAK inhibitor, for repigmentation of nonsegmental vitiligo in July 2022 for people aged 12 years and older.
Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, said that he likes to use ruxolitinib cream in combination with other treatments.
“In the real world with vitiligo patients, we’re oftentimes doing combinatorial therapy anyway. So phototherapy, specifically, narrow-band UVB, is something that we have a lot of clinical evidence for over the years, and it’s a modality that can combine with topical steroids and topical calcineurin inhibitors.”
He said trials to study combinations will yield better guidance on optimal use of ruxolitinib cream. “In general, vitiligo patients can really benefit from phototherapy,” he said in an interview. (Labeling recommends against combination with other JAK inhibitors, biologics, or potent immunosuppressants, such as azathioprine or cyclosporine.)
This first year has shown that ruxolitinib is an effective option, but counseling patients to expect slow improvement is important so that patients stick with it, he noted.
Documenting what treatments patients with vitiligo have used before is important, he said, as is counseling patients that ruxolitinib is approved only for use on up to 10% of a person’s body surface area. (Product labeling recommends that a thin layer be applied twice a day to affected areas up to 10% of body surface area.)
Ruxolitinib has brought a “louder voice” to vitiligo and has opened up options for patients with the disease, Dr. Chovatiya said. “Having the ability to topically treat people who have very extensive disease really gives us a lot more flexibility than we have had before.”
Good experiences with payers at safety-net hospital
Candrice R. Heath, MD, assistant professor of dermatology at Temple University, Philadelphia, said that real-world experience with topical ruxolitinib will be more evident after its been on the market for 18-24 months.
Dr. Heath said she, too, encourages use of narrow-band UVB phototherapy in conjunction with the treatment.
From an insurance reimbursement standpoint, she said that she is glad that there have been fewer hurdles in getting ruxolitinib to patients than she has experienced with other medications.
In her safety-net hospital, she told this news organization, she sees patients with many types of insurance, but most have Medicaid. “So, I’m always expecting the step therapies, denials, pushbacks, etc.,” she said. But the path has been smoother for ruxolitinib coverage, she noted.
Her colleagues are committed to documenting everything the patient has tried, she added, and that helps with prior authorization.
Dr. Heath said that pointing out to insurers that ruxolitinib is the only approved treatment for repigmentation helps facilitate coverage.
“The science is advancing, and I’m happy to be practicing during a time when we actually have something approved for vitiligo,” she said. But she pointed out that phototherapy often is not covered for vitiligo, “which is horrible, when it is readily approved for psoriasis and atopic dermatitis.”
To document progress, Dr. Heath said that she always takes photographs of her patients with vitiligo because “the pictures remind us how far we have come.”
Data spotlight success in adolescents
Data from two trials give a clinical picture of the drug’s safety and efficacy in younger patients.
Adolescents had particularly good results in the first year with ruxolitinib, according to pooled phase 3 data from TRuE-V1 and TRuE-V2, this news organization reported.
The findings, presented at the 25th World Congress of Dermatology in Singapore, indicate that more than half of the participants achieved at least a 50% improvement from baseline in the total Vitiligo Area Scoring Index (T-VASI50) at 52 weeks.
The percentages of young patients aged 12-17 years taking twice-daily ruxolitinib who achieved T-VASI 50 at weeks 12, 24, and 52 were 11.5%, 26.9%, and 57.7%, respectively. The corresponding percentages for all in the study population were 10.7%, 22.7%, and 44.4%, respectively.
At the meeting, the presenter, Julien Seneschal, MD, PhD, professor of dermatology and head of the vitiligo and pigmentary disorders clinic at the University of Bordeaux, France, said, “This suggests that younger patients can respond better to the treatment.” He noted, however, that there were few adolescents in the studies.
New excitement in the field
Daniel Gutierrez, MD, assistant professor of dermatology at New York University, said the treatment has brought new excitement to the field.
“Patients with vitiligo are very motivated to treat their disease,” he said, because it typically is on the face and other highly visual areas, which can affect their overall perception of self.
Previously, he noted in an interview, the only FDA-approved treatment was monobenzone, but that was for depigmentation rather than repigmentation.
Otherwise, treatments were being used off label, and patients were receiving compounded formulations that often weren’t covered by insurance and often had shorter shelf life.
He said that he still occasionally gets denials from payers who consider vitiligo a cosmetic condition.
“I’ve had more luck with insurance, at least in the New York State area.” He added that sometimes payers require use of a topical calcineurin inhibitor for about 12 weeks before they will cover ruxolitinib.
Dr. Gutierrez also recommends using phototherapy with topical ruxolitinib “because they work on slightly different pathways.”
When he starts patients on a new therapy such as ruxolitinib, he asks them to come back in 3 months, and often by then, progress is evident. Facial areas show the most response, he said, while hands and feet are less likely to show significant improvement.
He said that it’s important for physicians and patients to know that improvements can take weeks or months to be noticeable. “I tell patients not to give up,” he added.
Showing the patients pictures from the current appointment and comparing them with pictures from previous appointments can help them better understand their progress, he said.
Lead investigator adds observations
David Rosmarin, MD, chair of the department of dermatology at Indiana University, Indianapolis, was the lead investigator of the pivotal TruE-V1 and TruE-V2 trials for vitiligo. In that role, he has been treating vitiligo patients with topical ruxolitinib since 2015.
In an interview, he said that many patients “don’t hit their optimal results at 3 months, 6 months, even the year mark. With continued use, many can see continued benefit.”
Other patients, he said, don’t respond within the first 6 months but with continued use may eventually respond, he said.
“Unfortunately, we have no way of knowing, based on clinical characteristics or baseline demographics, whether a patient will be a delayed responder or not or an early responder,” Dr. Rosmarin added.
He provided several observations about people who have stopped taking the medication.
“When people stop,” he said, “some maintain their response, but some start to depigment again. Again, we have no way of predicting who will be in which category.”
He said that once patients have hit their desired response, he usually advises them to taper down to maybe twice a week or to stop treatment, but if they see any recurrence, they should start reusing the medicine.
“We have some patients who have gone 6 or 7 years now before they had a recurrence, but others may start to depigment again in 2 to 3 months,” Dr. Rosmarin said.
As for phototherapy, he said, the combination with topical ruxolitinib is being studied.
“We think the combination is synergistic and better than either alone, but we’re still waiting for data to prove that,” he said.
In his practice, he offers patients the option either to use just ruxolitinib cream or the combination early on. Many patients, because of convenience, say they’ll first try the cream to see if that works.
“The challenge with light [therapy] is that it can be very inconvenient,” he said. Patients have to live close to a phototherapy unit to receive therapy 2-3 times a week or have a phototherapy product in their home.
Next in the pipeline
Experts say the progress doesn’t stop with ruxolitinib cream. Current trials of several medications show there’s more to come for patients with vitiligo.
Dr. Chovatiya said that next up may be oral ritlecitinib (Litfulo), a JAK inhibitor that was approved for severe alopecia areata in June for people aged 12 years and older. Phase 2 results have been published for its use with vitiligo.
“This would be an oral medication that may be able to help people with much more extensive disease as far as vitiligo goes,” he said, adding that he expects approval for a vitiligo indication within a few years.
He pointed out that longer-term safety data will be available because it is already on the market for alopecia.
Upadacitinib (Rinvoq), an oral JAK inhibitor, is approved for atopic dermatitis but is being studied for vitiligo as well, he noted. “I’m very excited to see what that holds for patients as well,” Dr. Chovatiya said.
Dr. Gutierrez said that he is excited about oral JAK inhibitors but sees potential in finding new ways to transplant melanocytes into areas where there are none.
The pigmentation field has seen new energy since last year’s approval, he said, particularly among people of color.
“We have new options for vitiligo that were lacking compared with other conditions, such as atopic dermatitis and psoriasis,” he said. “Hopefully, there will be more promising breakthroughs.”
Dr. Rosmarin is the chief investigator for the pivotal trials that led to FDA approval of ruxolitinib. He disclosed ties with AbbVie, Abcuro, AltruBio, Amgen, Arena, Boehringer Ingelheim, Bristol-Meyers Squibb, Celgene, Concert, CSL Behring, Dermavant, Dermira, Galderma, Incyte, Janssen, Kyowa Kirin, Lilly, Merck, Novartis, Pfizer, Regeneron, Revolo Biotherapeutics, Sanofi, Sun Pharmaceuticals, UCB, and Viela Bio. Dr. Chovatiya disclosed ties with AbbVie, Arcutis, Arena, Argenx, Beiersdorf, Bristol-Myers Squibb, Dermavant, Eli Lilly, EPI Health, Incyte, LEO Pharma, L’Oréal, National Eczema Association, Pfizer, Regeneron, Sanofi, and UCB. Dr. Heath and Dr. Gutierrez report no relevant financial relationships.
A version of this article appeared on Medscape.com.