User login
Pedunculated gluteal mass
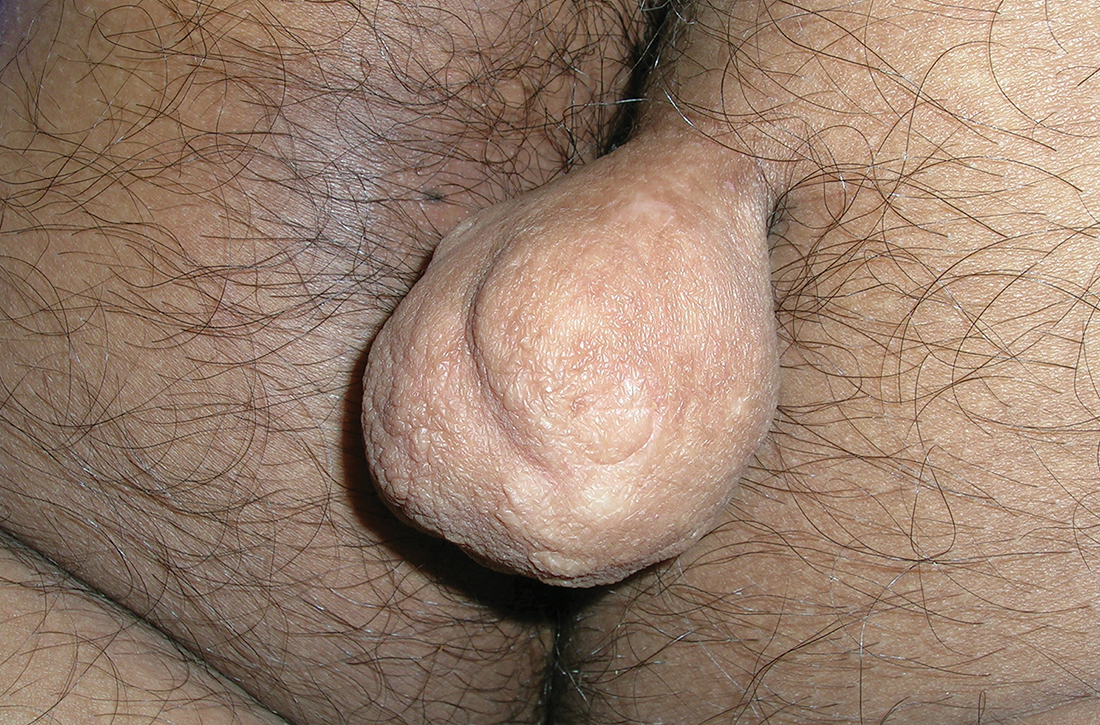
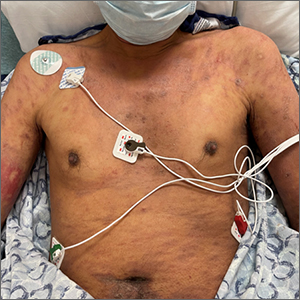
A 30-YEAR-OLD MAN presented for evaluation of a solitary, flesh-colored, pedunculated mass on his right inner gluteal area (FIGURE) that had gradually enlarged over the previous 18 months. The lesion had manifested 4 years prior as a small papule that was stable for many years. It began to grow steadily after the patient compressed the papule forcefully. Activities of daily living, such as sitting, were now uncomfortable, so he sought treatment. He denied pain, pruritis, and bleeding and reported no history of trauma or surgery in the area of the mass.
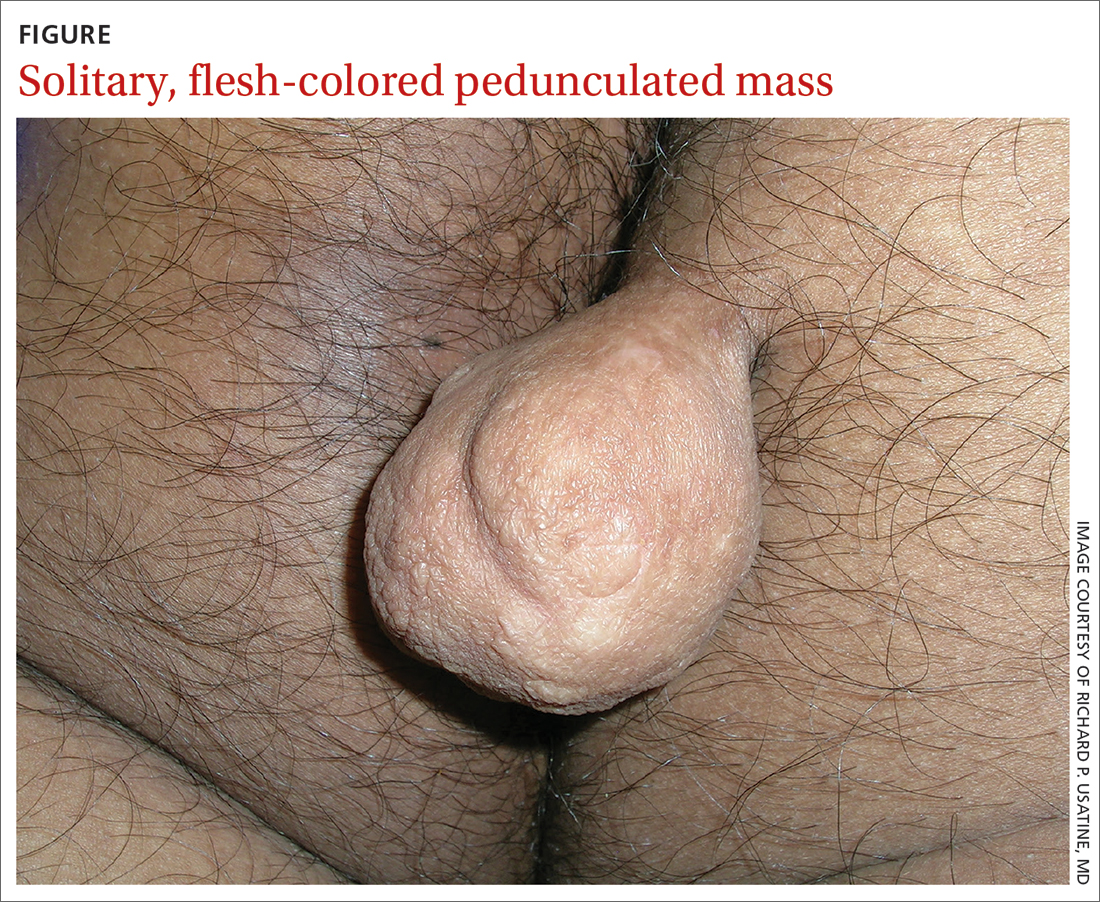
On physical examination, the mass measured 3.5 × 4.5 cm with a 1.2-cm base. It was smooth, soft, nontender, and compressible—but nonfluctuant. There were no signs of ulceration or bleeding. No regional lymphadenopathy was noted. An excisional biopsy was performed.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Fibrolipoma
The biopsy confirmed a diagnosis of fibrolipoma—a rare variant of lipoma composed of a mixture of adipocytes and thick bands of fibrous connective tissues.1 Etiology for fibrolipomas is unknown. Blunt trauma rupture of the fibrous septa that prevent fat migration may result in a proliferation of adipose tissue and thereby enlargement of fibrolipomas and other lipoma variants.2 In this case, the patient’s compression of the original papule likely served as the trauma that led to its enlargement. Malignant change has not been reported with fibrolipomas.
What you’ll see—and on whom. Fibrolipomas typically are flesh-colored, pedunculated, compressible, and relatively asymptomatic.3 They have been reported on the face, neck, back, and pubic areas, among other locations. Size is variable; they can be as small as 1 cm in diameter and as large as 10 cm in diameter.4 However, fibrolipomas can grow to be “giant” if they exceed 10 cm (or 1000 g).2
Men and women are affected equally by fibrolipomas. Prevalence does not differ by race or ethnicity.
The differential include sother lipomas and skin tags
The differential for a mass such as this one includes lipomas, acrochordons (also known as skin tags), and fibrokeratomas.
Lipomas are the most common benign soft-tissue tumors and are composed of adipocytes.5 The fibrolipoma is just one variant of lipoma; others include the myxolipoma, myolipoma, spindle cell lipoma, angiolipoma, osteolipoma, and chondrolipoma.2 Lipomas typically are subcutaneous and located over the scalp, neck, and upper trunk area but can occur anywhere on the body. They are mobile and typically well circumscribed. Lipomas have a broad base with well-demarcated swelling; fibrolipomas are usually pedunculated.
Continue to: Acrochordons ("skin tags")
Acrochordons (“skin tags”) usually contain a peduncle but may be sessile. They range from 1 mm to 1 cm in diameter and typically are located in skin folds, especially in the neck, axillae, and inguinal areas.6 Obesity, older
Fibrokeratomas typically are benign, solitary, fibrous tissue tumors that are found on fingers and seldom are pedunculated. They are flesh-colored and conical or nodular, with a hyperkeratotic collar. Fibrokeratomas are smaller and thicker than fibromas, as well as firm in consistency. They are acquired tumors that have been shown to be related to repetitive trauma.6
Treatment involves surgical excision
The preferred treatment for fibrolipoma is complete surgical excision, although cryotherapy is another option for lesions < 1 cm.4 Without surgical excision, the mass will continue to grow, albeit slowly.
This patient’s mass was excised successfully in its entirety; there were no complications. Follow-up is usually unnecessary.
1. Kim YT, Kim WS, Park YL, et al. A case of fibrolipoma. Korean J Dermatol. 2003;41:939-941.
2. Mazzocchi M, Onesti MG, Pasquini P, et al. Giant fibrolipoma in the leg—a case report. Anticancer Res. 2006;26:3649-3654.
3. Shin SJ. Subcutaneous fibrolipoma on the back. J Craniofac Surg. 2013;24:1051-1053. doi: 10.1097/SCS.0b013e3182802517
4. Suleiman J, Suleman M, Amsi P, et al. Giant pedunculated lipofibroma of the thigh. J Surg Case Rep. 2023;2023(3):rjad153. doi: 10.1093/jscr/rjad153
5. Dai X-M, Li Y-S, Liu H, et al. Giant pedunculated fibrolipoma arising from right facial and cervical region. J Oral and Maxillofac Surg. 2009;67:1323-1326. doi: 10.1016/j.joms.2008.12.037
6. Lee JA, Khodaee M. Enlarging, pedunculated skin lesion. Am Fam Physician. 2012;85:1191-1192.
7. Banik R, Lubach D. Skin tags: localization and frequencies according to sex and age. Dermatologica. 1987;174:180-183. doi: 10.1159/000249169
A 30-YEAR-OLD MAN presented for evaluation of a solitary, flesh-colored, pedunculated mass on his right inner gluteal area (FIGURE) that had gradually enlarged over the previous 18 months. The lesion had manifested 4 years prior as a small papule that was stable for many years. It began to grow steadily after the patient compressed the papule forcefully. Activities of daily living, such as sitting, were now uncomfortable, so he sought treatment. He denied pain, pruritis, and bleeding and reported no history of trauma or surgery in the area of the mass.

On physical examination, the mass measured 3.5 × 4.5 cm with a 1.2-cm base. It was smooth, soft, nontender, and compressible—but nonfluctuant. There were no signs of ulceration or bleeding. No regional lymphadenopathy was noted. An excisional biopsy was performed.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Fibrolipoma
The biopsy confirmed a diagnosis of fibrolipoma—a rare variant of lipoma composed of a mixture of adipocytes and thick bands of fibrous connective tissues.1 Etiology for fibrolipomas is unknown. Blunt trauma rupture of the fibrous septa that prevent fat migration may result in a proliferation of adipose tissue and thereby enlargement of fibrolipomas and other lipoma variants.2 In this case, the patient’s compression of the original papule likely served as the trauma that led to its enlargement. Malignant change has not been reported with fibrolipomas.
What you’ll see—and on whom. Fibrolipomas typically are flesh-colored, pedunculated, compressible, and relatively asymptomatic.3 They have been reported on the face, neck, back, and pubic areas, among other locations. Size is variable; they can be as small as 1 cm in diameter and as large as 10 cm in diameter.4 However, fibrolipomas can grow to be “giant” if they exceed 10 cm (or 1000 g).2
Men and women are affected equally by fibrolipomas. Prevalence does not differ by race or ethnicity.
The differential include sother lipomas and skin tags
The differential for a mass such as this one includes lipomas, acrochordons (also known as skin tags), and fibrokeratomas.
Lipomas are the most common benign soft-tissue tumors and are composed of adipocytes.5 The fibrolipoma is just one variant of lipoma; others include the myxolipoma, myolipoma, spindle cell lipoma, angiolipoma, osteolipoma, and chondrolipoma.2 Lipomas typically are subcutaneous and located over the scalp, neck, and upper trunk area but can occur anywhere on the body. They are mobile and typically well circumscribed. Lipomas have a broad base with well-demarcated swelling; fibrolipomas are usually pedunculated.
Continue to: Acrochordons ("skin tags")
Acrochordons (“skin tags”) usually contain a peduncle but may be sessile. They range from 1 mm to 1 cm in diameter and typically are located in skin folds, especially in the neck, axillae, and inguinal areas.6 Obesity, older
Fibrokeratomas typically are benign, solitary, fibrous tissue tumors that are found on fingers and seldom are pedunculated. They are flesh-colored and conical or nodular, with a hyperkeratotic collar. Fibrokeratomas are smaller and thicker than fibromas, as well as firm in consistency. They are acquired tumors that have been shown to be related to repetitive trauma.6
Treatment involves surgical excision
The preferred treatment for fibrolipoma is complete surgical excision, although cryotherapy is another option for lesions < 1 cm.4 Without surgical excision, the mass will continue to grow, albeit slowly.
This patient’s mass was excised successfully in its entirety; there were no complications. Follow-up is usually unnecessary.
A 30-YEAR-OLD MAN presented for evaluation of a solitary, flesh-colored, pedunculated mass on his right inner gluteal area (FIGURE) that had gradually enlarged over the previous 18 months. The lesion had manifested 4 years prior as a small papule that was stable for many years. It began to grow steadily after the patient compressed the papule forcefully. Activities of daily living, such as sitting, were now uncomfortable, so he sought treatment. He denied pain, pruritis, and bleeding and reported no history of trauma or surgery in the area of the mass.

On physical examination, the mass measured 3.5 × 4.5 cm with a 1.2-cm base. It was smooth, soft, nontender, and compressible—but nonfluctuant. There were no signs of ulceration or bleeding. No regional lymphadenopathy was noted. An excisional biopsy was performed.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Fibrolipoma
The biopsy confirmed a diagnosis of fibrolipoma—a rare variant of lipoma composed of a mixture of adipocytes and thick bands of fibrous connective tissues.1 Etiology for fibrolipomas is unknown. Blunt trauma rupture of the fibrous septa that prevent fat migration may result in a proliferation of adipose tissue and thereby enlargement of fibrolipomas and other lipoma variants.2 In this case, the patient’s compression of the original papule likely served as the trauma that led to its enlargement. Malignant change has not been reported with fibrolipomas.
What you’ll see—and on whom. Fibrolipomas typically are flesh-colored, pedunculated, compressible, and relatively asymptomatic.3 They have been reported on the face, neck, back, and pubic areas, among other locations. Size is variable; they can be as small as 1 cm in diameter and as large as 10 cm in diameter.4 However, fibrolipomas can grow to be “giant” if they exceed 10 cm (or 1000 g).2
Men and women are affected equally by fibrolipomas. Prevalence does not differ by race or ethnicity.
The differential include sother lipomas and skin tags
The differential for a mass such as this one includes lipomas, acrochordons (also known as skin tags), and fibrokeratomas.
Lipomas are the most common benign soft-tissue tumors and are composed of adipocytes.5 The fibrolipoma is just one variant of lipoma; others include the myxolipoma, myolipoma, spindle cell lipoma, angiolipoma, osteolipoma, and chondrolipoma.2 Lipomas typically are subcutaneous and located over the scalp, neck, and upper trunk area but can occur anywhere on the body. They are mobile and typically well circumscribed. Lipomas have a broad base with well-demarcated swelling; fibrolipomas are usually pedunculated.
Continue to: Acrochordons ("skin tags")
Acrochordons (“skin tags”) usually contain a peduncle but may be sessile. They range from 1 mm to 1 cm in diameter and typically are located in skin folds, especially in the neck, axillae, and inguinal areas.6 Obesity, older
Fibrokeratomas typically are benign, solitary, fibrous tissue tumors that are found on fingers and seldom are pedunculated. They are flesh-colored and conical or nodular, with a hyperkeratotic collar. Fibrokeratomas are smaller and thicker than fibromas, as well as firm in consistency. They are acquired tumors that have been shown to be related to repetitive trauma.6
Treatment involves surgical excision
The preferred treatment for fibrolipoma is complete surgical excision, although cryotherapy is another option for lesions < 1 cm.4 Without surgical excision, the mass will continue to grow, albeit slowly.
This patient’s mass was excised successfully in its entirety; there were no complications. Follow-up is usually unnecessary.
1. Kim YT, Kim WS, Park YL, et al. A case of fibrolipoma. Korean J Dermatol. 2003;41:939-941.
2. Mazzocchi M, Onesti MG, Pasquini P, et al. Giant fibrolipoma in the leg—a case report. Anticancer Res. 2006;26:3649-3654.
3. Shin SJ. Subcutaneous fibrolipoma on the back. J Craniofac Surg. 2013;24:1051-1053. doi: 10.1097/SCS.0b013e3182802517
4. Suleiman J, Suleman M, Amsi P, et al. Giant pedunculated lipofibroma of the thigh. J Surg Case Rep. 2023;2023(3):rjad153. doi: 10.1093/jscr/rjad153
5. Dai X-M, Li Y-S, Liu H, et al. Giant pedunculated fibrolipoma arising from right facial and cervical region. J Oral and Maxillofac Surg. 2009;67:1323-1326. doi: 10.1016/j.joms.2008.12.037
6. Lee JA, Khodaee M. Enlarging, pedunculated skin lesion. Am Fam Physician. 2012;85:1191-1192.
7. Banik R, Lubach D. Skin tags: localization and frequencies according to sex and age. Dermatologica. 1987;174:180-183. doi: 10.1159/000249169
1. Kim YT, Kim WS, Park YL, et al. A case of fibrolipoma. Korean J Dermatol. 2003;41:939-941.
2. Mazzocchi M, Onesti MG, Pasquini P, et al. Giant fibrolipoma in the leg—a case report. Anticancer Res. 2006;26:3649-3654.
3. Shin SJ. Subcutaneous fibrolipoma on the back. J Craniofac Surg. 2013;24:1051-1053. doi: 10.1097/SCS.0b013e3182802517
4. Suleiman J, Suleman M, Amsi P, et al. Giant pedunculated lipofibroma of the thigh. J Surg Case Rep. 2023;2023(3):rjad153. doi: 10.1093/jscr/rjad153
5. Dai X-M, Li Y-S, Liu H, et al. Giant pedunculated fibrolipoma arising from right facial and cervical region. J Oral and Maxillofac Surg. 2009;67:1323-1326. doi: 10.1016/j.joms.2008.12.037
6. Lee JA, Khodaee M. Enlarging, pedunculated skin lesion. Am Fam Physician. 2012;85:1191-1192.
7. Banik R, Lubach D. Skin tags: localization and frequencies according to sex and age. Dermatologica. 1987;174:180-183. doi: 10.1159/000249169
Systemic lupus erythematosus
THE COMPARISON
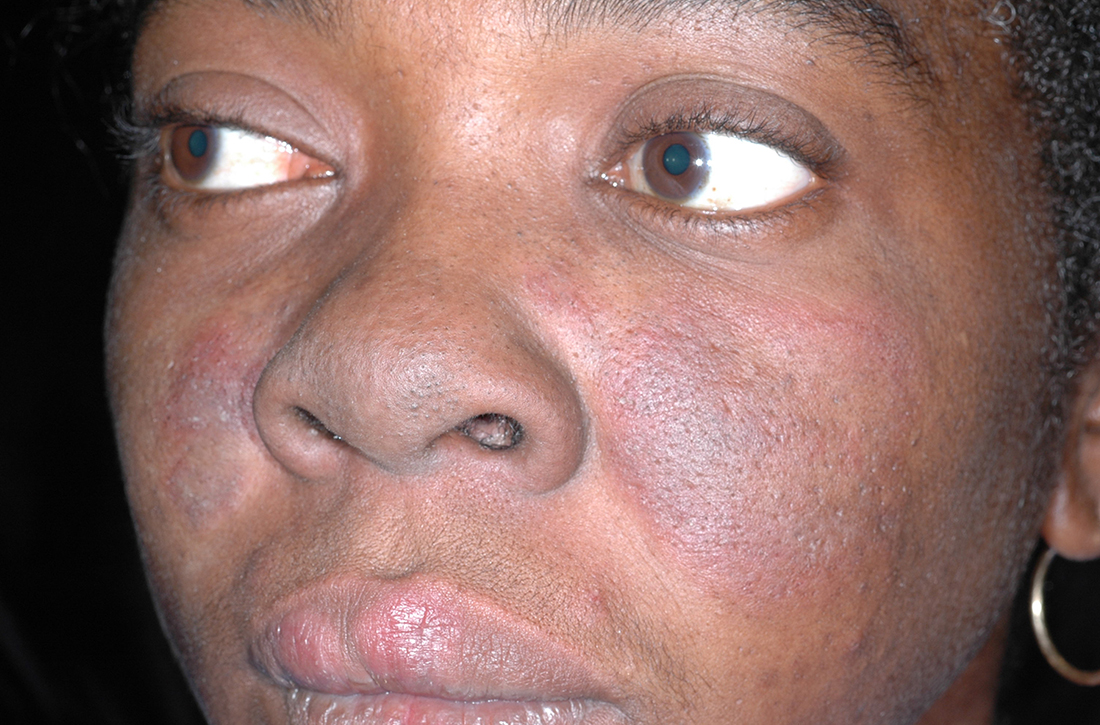
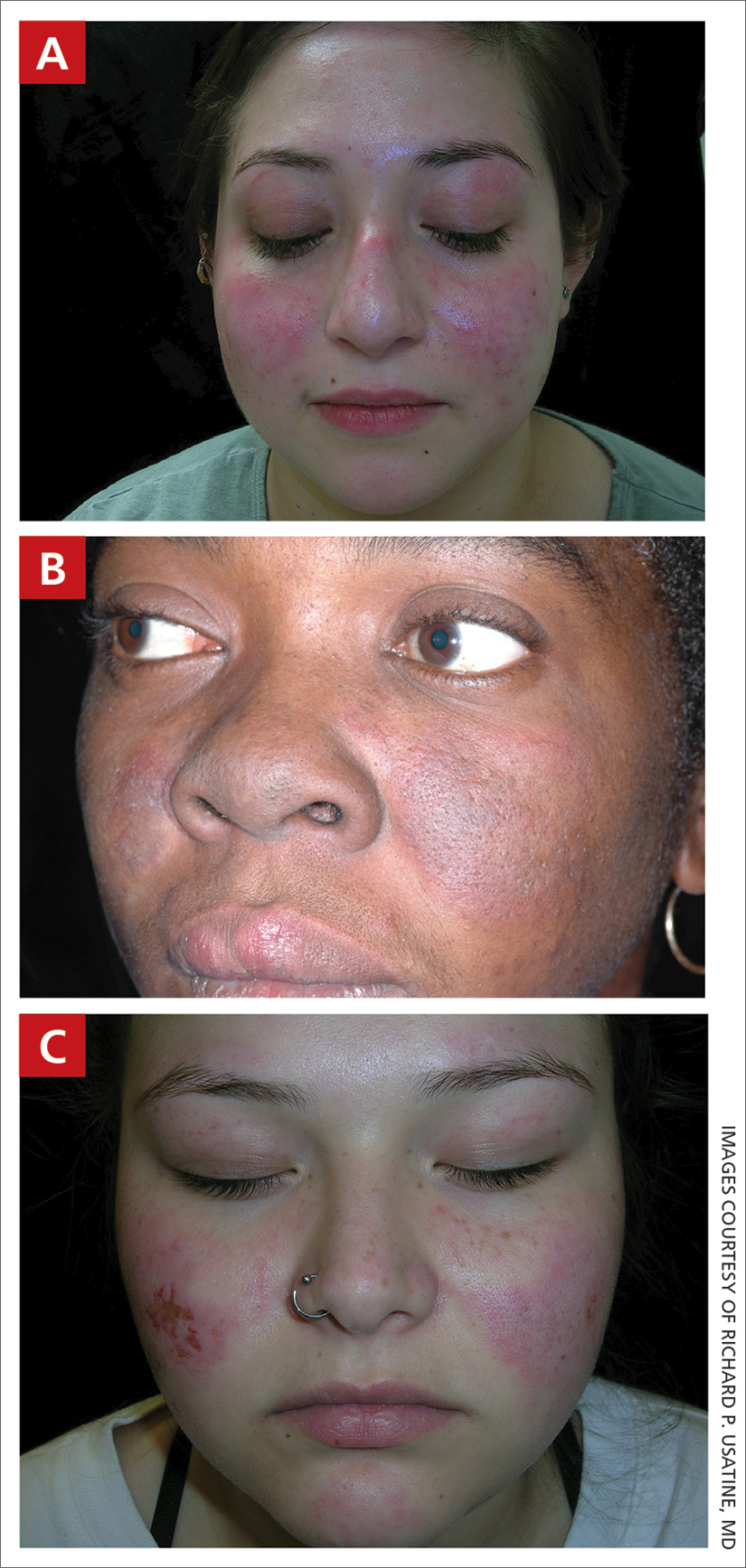
A A 23-year-old White woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose and eyelids but spares the nasolabial folds.
B A Black woman with malar erythema and hyperpigmentation from acute cutaneous lupus erythematosus. The nasolabial folds are spared.
C A 19-year-old Latina woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose, chin, and eyelids but spares the nasolabial folds. Cutaneous erosions are present on the right cheek as part of the lupus flare.
Systemic lupus erythematosus (SLE) is a chronic autoimmune condition that affects the kidneys, lungs, brain, and heart, although it is not limited to these organs. Dermatologists and primary care physicians play a critical role in the early identification of SLE (particularly in those with skin of color), as the standardized mortality rate is 2.6-fold higher in patients with SLE compared to the general population.1 The clinical manifestations of SLE vary.
Epidemiology
A meta-analysis of data from the Centers for Disease Control and Prevention National Lupus Registry network including 5417 patients revealed a prevalence of 72.8 cases per 100,000 person-years.2 The prevalence was higher in females than males and highest among females identifying as Black. White and Asian/ Pacific Islander females had the lowest prevalence. The American Indian (indigenous)/Alaska Native–identifying population had the highest race-specific SLE estimates among both females and males compared to other racial/ethnic groups.2
Key clinical features in people with darker skin tones
The diagnosis of SLE is based on clinical and immunologic criteria from the European League Against Rheumatism/American College of Rheumatology.3,4 An antinuclear antibody titer of 1:80 or higher at least once is required for the diagnosis of SLE, as long as there is not another more likely diagnosis. If it is present, 22 additive weighted classification criteria are considered; each criterion is assigned points, ranging from 2 to 10. Patients with at least 1 clinical criterion and 10 or more points are classified as having SLE. If more than 1 of the criteria are met in a domain, then the one with the highest numerical value is counted.3,4
Aringer et al3,4 outline the criteria and numerical points to make the diagnosis of SLE. The mucocutaneous component of the SLE diagnostic criteria3,4 includes nonscarring alopecia, oral ulcers, subacute cutaneous or discoid lupus erythematosus,5 and acute cutaneous lupus erythematosus, with acute cutaneous lupus erythematosus being the highest-weighted criterion in that domain. The other clinical domains are constitutional, hematologic, neuropsychiatric, serosal, musculoskeletal, renal, antiphospholipid antibodies, complement proteins, and SLE-specific antibodies.3,4
The malar (“butterfly”) rash of SLE characteristically includes erythema that spares the nasolabial folds but affects the nasal bridge and cheeks.6 The rash occasionally may be pruritic and painful, lasting days to weeks. Photosensitivity occurs, resulting in rashes or even an overall worsening of SLE symptoms. In those with darker skin tones, erythema may appear violaceous or may not be as readily appreciated.6
Worth noting
- Patients with skin of color are at an increased risk for postinflammatory hypopigmentation and hyperpigmentation (pigment alteration), hypertrophic scars, and keloids.7,8
- The mortality rate for those with SLE is high despite early recognition and treatment when compared to the general population.1,9
Health disparity highlight
Those at greatest risk for death from SLE in the United States are those of African descent, Hispanic individuals, men, and those with low socioeconomic status,9 which likely is primarily driven by social determinants of health instead of genetic patterns. Income level, educational attainment, insurance status, and environmental factors10 have farreaching effects, negatively impacting quality of life and even mortality.
1. Lee YH, Choi SJ, Ji JD, et al. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus. 2016;25:727-734.
2. Izmirly PM, Parton H, Wang L, et al. Prevalence of systemic lupus erythematosus in the United States: estimates from a meta-analysis of the Centers for Disease Control and Prevention National Lupus Registries. Arthritis Rheumatol. 2021;73:991-996. doi: 10.1002/art.41632
3. Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71:1400-1412. doi: 10.1002/art.40930
4. Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78:1151-1159.
5. Heath CR, Usatine RP. Discoid lupus. Cutis. 2022;109:172-173.
6. Firestein GS, Budd RC, Harris ED Jr, et al, eds. Kelley’s Textbook of Rheumatology. 8th ed. Saunders Elsevier; 2008.
7. Nozile W, Adgerson CH, Cohen GF. Cutaneous lupus erythematosus in skin of color. J Drugs Dermatol. 2015;14:343-349.
8. Cardinali F, Kovacs D, Picardo M. Mechanisms underlying postinflammatory hyperpigmentation: lessons for solar. Ann Dermatol Venereol. 2012;139(suppl 4):S148-S152.
9. Ocampo-Piraquive V, Nieto-Aristizábal I, Cañas CA, et al. Mortality in systemic lupus erythematosus: causes, predictors and interventions. Expert Rev Clin Immunol. 2018;14:1043-1053. doi: 10.1080/17446 66X.2018.1538789
10. Carter EE, Barr SG, Clarke AE. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol. 2016;12:605-620. doi: 10.1038/nrrheum.2016.137
THE COMPARISON
A A 23-year-old White woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose and eyelids but spares the nasolabial folds.
B A Black woman with malar erythema and hyperpigmentation from acute cutaneous lupus erythematosus. The nasolabial folds are spared.
C A 19-year-old Latina woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose, chin, and eyelids but spares the nasolabial folds. Cutaneous erosions are present on the right cheek as part of the lupus flare.

Systemic lupus erythematosus (SLE) is a chronic autoimmune condition that affects the kidneys, lungs, brain, and heart, although it is not limited to these organs. Dermatologists and primary care physicians play a critical role in the early identification of SLE (particularly in those with skin of color), as the standardized mortality rate is 2.6-fold higher in patients with SLE compared to the general population.1 The clinical manifestations of SLE vary.
Epidemiology
A meta-analysis of data from the Centers for Disease Control and Prevention National Lupus Registry network including 5417 patients revealed a prevalence of 72.8 cases per 100,000 person-years.2 The prevalence was higher in females than males and highest among females identifying as Black. White and Asian/ Pacific Islander females had the lowest prevalence. The American Indian (indigenous)/Alaska Native–identifying population had the highest race-specific SLE estimates among both females and males compared to other racial/ethnic groups.2
Key clinical features in people with darker skin tones
The diagnosis of SLE is based on clinical and immunologic criteria from the European League Against Rheumatism/American College of Rheumatology.3,4 An antinuclear antibody titer of 1:80 or higher at least once is required for the diagnosis of SLE, as long as there is not another more likely diagnosis. If it is present, 22 additive weighted classification criteria are considered; each criterion is assigned points, ranging from 2 to 10. Patients with at least 1 clinical criterion and 10 or more points are classified as having SLE. If more than 1 of the criteria are met in a domain, then the one with the highest numerical value is counted.3,4
Aringer et al3,4 outline the criteria and numerical points to make the diagnosis of SLE. The mucocutaneous component of the SLE diagnostic criteria3,4 includes nonscarring alopecia, oral ulcers, subacute cutaneous or discoid lupus erythematosus,5 and acute cutaneous lupus erythematosus, with acute cutaneous lupus erythematosus being the highest-weighted criterion in that domain. The other clinical domains are constitutional, hematologic, neuropsychiatric, serosal, musculoskeletal, renal, antiphospholipid antibodies, complement proteins, and SLE-specific antibodies.3,4
The malar (“butterfly”) rash of SLE characteristically includes erythema that spares the nasolabial folds but affects the nasal bridge and cheeks.6 The rash occasionally may be pruritic and painful, lasting days to weeks. Photosensitivity occurs, resulting in rashes or even an overall worsening of SLE symptoms. In those with darker skin tones, erythema may appear violaceous or may not be as readily appreciated.6
Worth noting
- Patients with skin of color are at an increased risk for postinflammatory hypopigmentation and hyperpigmentation (pigment alteration), hypertrophic scars, and keloids.7,8
- The mortality rate for those with SLE is high despite early recognition and treatment when compared to the general population.1,9
Health disparity highlight
Those at greatest risk for death from SLE in the United States are those of African descent, Hispanic individuals, men, and those with low socioeconomic status,9 which likely is primarily driven by social determinants of health instead of genetic patterns. Income level, educational attainment, insurance status, and environmental factors10 have farreaching effects, negatively impacting quality of life and even mortality.
THE COMPARISON
A A 23-year-old White woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose and eyelids but spares the nasolabial folds.
B A Black woman with malar erythema and hyperpigmentation from acute cutaneous lupus erythematosus. The nasolabial folds are spared.
C A 19-year-old Latina woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose, chin, and eyelids but spares the nasolabial folds. Cutaneous erosions are present on the right cheek as part of the lupus flare.

Systemic lupus erythematosus (SLE) is a chronic autoimmune condition that affects the kidneys, lungs, brain, and heart, although it is not limited to these organs. Dermatologists and primary care physicians play a critical role in the early identification of SLE (particularly in those with skin of color), as the standardized mortality rate is 2.6-fold higher in patients with SLE compared to the general population.1 The clinical manifestations of SLE vary.
Epidemiology
A meta-analysis of data from the Centers for Disease Control and Prevention National Lupus Registry network including 5417 patients revealed a prevalence of 72.8 cases per 100,000 person-years.2 The prevalence was higher in females than males and highest among females identifying as Black. White and Asian/ Pacific Islander females had the lowest prevalence. The American Indian (indigenous)/Alaska Native–identifying population had the highest race-specific SLE estimates among both females and males compared to other racial/ethnic groups.2
Key clinical features in people with darker skin tones
The diagnosis of SLE is based on clinical and immunologic criteria from the European League Against Rheumatism/American College of Rheumatology.3,4 An antinuclear antibody titer of 1:80 or higher at least once is required for the diagnosis of SLE, as long as there is not another more likely diagnosis. If it is present, 22 additive weighted classification criteria are considered; each criterion is assigned points, ranging from 2 to 10. Patients with at least 1 clinical criterion and 10 or more points are classified as having SLE. If more than 1 of the criteria are met in a domain, then the one with the highest numerical value is counted.3,4
Aringer et al3,4 outline the criteria and numerical points to make the diagnosis of SLE. The mucocutaneous component of the SLE diagnostic criteria3,4 includes nonscarring alopecia, oral ulcers, subacute cutaneous or discoid lupus erythematosus,5 and acute cutaneous lupus erythematosus, with acute cutaneous lupus erythematosus being the highest-weighted criterion in that domain. The other clinical domains are constitutional, hematologic, neuropsychiatric, serosal, musculoskeletal, renal, antiphospholipid antibodies, complement proteins, and SLE-specific antibodies.3,4
The malar (“butterfly”) rash of SLE characteristically includes erythema that spares the nasolabial folds but affects the nasal bridge and cheeks.6 The rash occasionally may be pruritic and painful, lasting days to weeks. Photosensitivity occurs, resulting in rashes or even an overall worsening of SLE symptoms. In those with darker skin tones, erythema may appear violaceous or may not be as readily appreciated.6
Worth noting
- Patients with skin of color are at an increased risk for postinflammatory hypopigmentation and hyperpigmentation (pigment alteration), hypertrophic scars, and keloids.7,8
- The mortality rate for those with SLE is high despite early recognition and treatment when compared to the general population.1,9
Health disparity highlight
Those at greatest risk for death from SLE in the United States are those of African descent, Hispanic individuals, men, and those with low socioeconomic status,9 which likely is primarily driven by social determinants of health instead of genetic patterns. Income level, educational attainment, insurance status, and environmental factors10 have farreaching effects, negatively impacting quality of life and even mortality.
1. Lee YH, Choi SJ, Ji JD, et al. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus. 2016;25:727-734.
2. Izmirly PM, Parton H, Wang L, et al. Prevalence of systemic lupus erythematosus in the United States: estimates from a meta-analysis of the Centers for Disease Control and Prevention National Lupus Registries. Arthritis Rheumatol. 2021;73:991-996. doi: 10.1002/art.41632
3. Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71:1400-1412. doi: 10.1002/art.40930
4. Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78:1151-1159.
5. Heath CR, Usatine RP. Discoid lupus. Cutis. 2022;109:172-173.
6. Firestein GS, Budd RC, Harris ED Jr, et al, eds. Kelley’s Textbook of Rheumatology. 8th ed. Saunders Elsevier; 2008.
7. Nozile W, Adgerson CH, Cohen GF. Cutaneous lupus erythematosus in skin of color. J Drugs Dermatol. 2015;14:343-349.
8. Cardinali F, Kovacs D, Picardo M. Mechanisms underlying postinflammatory hyperpigmentation: lessons for solar. Ann Dermatol Venereol. 2012;139(suppl 4):S148-S152.
9. Ocampo-Piraquive V, Nieto-Aristizábal I, Cañas CA, et al. Mortality in systemic lupus erythematosus: causes, predictors and interventions. Expert Rev Clin Immunol. 2018;14:1043-1053. doi: 10.1080/17446 66X.2018.1538789
10. Carter EE, Barr SG, Clarke AE. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol. 2016;12:605-620. doi: 10.1038/nrrheum.2016.137
1. Lee YH, Choi SJ, Ji JD, et al. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus. 2016;25:727-734.
2. Izmirly PM, Parton H, Wang L, et al. Prevalence of systemic lupus erythematosus in the United States: estimates from a meta-analysis of the Centers for Disease Control and Prevention National Lupus Registries. Arthritis Rheumatol. 2021;73:991-996. doi: 10.1002/art.41632
3. Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71:1400-1412. doi: 10.1002/art.40930
4. Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78:1151-1159.
5. Heath CR, Usatine RP. Discoid lupus. Cutis. 2022;109:172-173.
6. Firestein GS, Budd RC, Harris ED Jr, et al, eds. Kelley’s Textbook of Rheumatology. 8th ed. Saunders Elsevier; 2008.
7. Nozile W, Adgerson CH, Cohen GF. Cutaneous lupus erythematosus in skin of color. J Drugs Dermatol. 2015;14:343-349.
8. Cardinali F, Kovacs D, Picardo M. Mechanisms underlying postinflammatory hyperpigmentation: lessons for solar. Ann Dermatol Venereol. 2012;139(suppl 4):S148-S152.
9. Ocampo-Piraquive V, Nieto-Aristizábal I, Cañas CA, et al. Mortality in systemic lupus erythematosus: causes, predictors and interventions. Expert Rev Clin Immunol. 2018;14:1043-1053. doi: 10.1080/17446 66X.2018.1538789
10. Carter EE, Barr SG, Clarke AE. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol. 2016;12:605-620. doi: 10.1038/nrrheum.2016.137
Advising patients on AD treatment options: Expert pearls
WASHINGTON –
The question was top of mind for experts who shared their advice during a “Tips and Tricks” session at the Revolutionizing Atopic Dermatitis meeting. Dupilumab dosing and dupilumab-associated facial redness and ocular disease, self-image issues, topical regimen adherence, and the quantification of disease were among the other topics raised by the experts.
Here are some of their practice pearls.
Treatment decisions, safety concerns
Deciding on a treatment is “kind of confusing ... particularly in the last year ... and it will only get more complicated,” said Raj J. Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago. “We’re all about some version of shared decision-making, but if all else is equal, sometimes it pays to explicitly ask the patient, what do you want to do?”
Questions about how long a systemic treatment should be tried, both initially and in the long run, are common. “I think that oftentimes we all get antsy about making changes when we’re not getting to the endpoint we want to. And at least in my real-world experience, late responders are a real thing. Sometimes 3-4 months ... isn’t enough,” he said.
Trial extension data show that patients who were nonresponders for various endpoints at 16 weeks are “captured continuously as you go further and further out,” Dr. Chovatiya said. Regarding the long term, “realistically, there’s no perfect time to call it quits.”
Addressing fears about Janus kinase inhibitors can be challenging, he said. “When you’ve identified the right patient and labs are done ... have them take the medication in front of you and hang out,” he advised. “It may sound ridiculous, but for the extremely anxious person it can be a big stress reducer for everyone involved.”
Regarding treatment fears more generally, “asking patients ‘what is the biggest risk of not treating your disease?’ sometimes gets people thinking,” Dr. Chovatiya said.
For parents of children with AD, said Robert Sidbury, MD, MPH, risks of not treating can become apparent once treatments are started and benefits are realized. “It’s so easy to focus on the risks of any treatment because they’re right there in black and white, and the risks of not treating are not always as apparent, even though – or maybe because – they live with them every day.”
When treatment is underway, “they see [how] everyone sleeps better, how school performance gets better, how concentration gets better,” said Dr. Sidbury, professor in the department of pediatrics at the University of Washington. Seattle, and chief of dermatology at Seattle Children’s Hospital.
“Always contextualize,” he advised. “As dermatologists, we’re savvy with navigating boxed warnings.”
David Rosmarin, MD, chair of dermatology, at Indiana University, Indianapolis, and formerly vice chair for research and education at Tufts Medical Center, Boston, said he addresses questions about the length of systemic treatment by advising patients: “Why don’t we start taking [the medication] for 3 months and then we’ll take it from there.”
In some pediatric cases, Dr. Rosmarin said, having the child express “what their AD means to them – how it affects them,” and then acknowledging and validating what the child says, is helpful to parents who are concerned about systemic treatments.
Dupilumab in the real world
Some patients on dupilumab do not have a complete response with dosing every 2 weeks and may benefit from more frequent dosing, said Dr. Rosmarin.
“We know from the SOLO-1 and SOLO-2 studies that dupilumab weekly dosing was evaluated. It was only the every-other-week dosing that was approved, and we can see why – in terms of the changes in EASI [Eczema Area and Severity Index] score they’re close to overlapping,” he said.
In real life, however, “some patients benefit from different dosing. It’s important to realize that. I think we all have some patients who may dose more frequently and some who may dose less frequently,” Dr. Rosmarin said.
For a patient who “gets absolutely no response from dupilumab after 3-4 months, I’d switch them to something else. But for those who are partial responders, particularly those who tell me they’re getting itchy before their next dose, they’re the ones who benefit most from doubling the dose to dupilumab weekly,” he said.
For patients who experience dupilumab-associated head and neck dermatitis, itraconazole may help, Dr. Rosmarin added. “We’re using 200 mg daily for 2 weeks and weekly thereafter, and it helps some of our patients.” The average self-reported improvement was 52% for patients with dupilumab-associated facial redness treated with itraconazole in a retrospective medical record review that he and his colleagues published in 2022.
Dr. Rosmarin pointed to a multicenter prospective cohort study also published in 2022 showing that baseline/pretreatment levels of Malassezia-specific IgE were associated with the development of dupilumab-associated head and neck dermatitis. The median levels of Malassezia-specific IgE were 32 kUL–1 versus 2.3 kUL–1 in patients who experienced dupilumab-associated facial redness, compared with those who did not.
He said that, while there “may be multiple reasons” for dupilumab-associated head and neck dermatitis and that “plenty of patients” who don’t have Malassezia-specific IgE develop head and neck dermatitis, “this could be one cause.”
Itraconazole has been shown in his practice to be superior to fluconazole, likely because it has greater anti-inflammatory effects and provides better coverage of Malassezia because it is more lipophilic, said Dr. Rosmarin, who does not test for Malassezia-specific IgE before trying itraconazole.
For dupilumab-associated ocular surface disease, Elaine C. Siegfried, MD, offered her first-line suggestions: warm compresses (such as a microwaved bean bag), bland ocular lubricant (such as preservative-free artificial tears), oral hydration, and if needed and accessible, the prescription ophthalmic solution lifitegrast.
“It’s become an issue – what the dermatologist can do first line,” said Dr. Siegfried, professor of pediatrics and dermatology at Saint Louis University and director of the division of pediatric dermatology at Cardinal Glennon Children’s Hospital, St. Louis.
“If these don’t work, then I’ll identify an ophthalmologist who’s knowledgeable about Dupixent-related ocular surface disease,” she said. Selection is “important because they’re not all knowledgeable ... corneal specialists typically have the most knowledge.”
Topical adherence with diffuse xerosis and mild-moderate AD
For patients with diffuse xerosis and mild-moderate AD, especially those who are older and having difficulty with topical regimens, Anna De Benedetto, MD, said she tries to enhance adherence by simplifying the regimen. She asks patients to buy a pound jar of base cream (ceramide base) – “whatever emollient they like” – and mixes into it a high-potency steroid solution. They’re instructed to apply the combined cream once daily for 1-2 weeks, and then three times a week alternating with a nonmedicated cream.
“This way they’re using one [cream] to target the immune system and the skin barrier,” said Dr. De Benedetto, associate professor of dermatology and director of the dermatology clinical trial unit at the University of Rochester (N.Y.) Medical Center.
‘Wet wrap’ pajamas; self-image for children, teens
Dermatologist Melinda Gooderham, MSc, MD, assistant professor at Queen’s University, Kingston, Ont., and medical director at the SKiN Centre for Dermatology, said that, for widespread and troublesome AD, she advises patients or parents to wet a thin cotton pajama top and bottom and spin it in the dryer “so it’s almost dry but still moist.” Dry, looser pajamas or a light track suit can then be worn over the damp pajamas. “I usually tell [patients] to buy one size up,” she said.
Body dysmorphia is common with skin disease, and its incidence is six times higher in people with eczema than those without the disease, said Dr. Siegfried. “I’ve found that, for patients subjected to AD for a long time,” this is still an issue, “even when you clear their skin.”
For children, teens and their families, the nonprofit organization Made a Masterpiece can be valuable, Dr. Siegfried said. It offers resources from parents, children, psychologists, dermatologists, and others to help manage the emotional, social and spiritual aspects of living with a skin condition.
To use or not to use BSA; environmental counseling
“I think [assessing] body surface area [BSA] is very important in pediatrics and for adolescents [especially in those with moderate to severe disease] because it quantifies the disease for the family,” said Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics and vice chair of the department of dermatology at the University of California, San Diego.
“Families live with the disease, but quantification really matters” for understanding the extent and impact of the disease and for motivating families to treat, said Dr. Eichenfield.
(When the disease is markedly diminished in follow-up, knowing the BSA then “helps families to register the improvement and gives positive reinforcement,” Dr. Eichenfeld said after the meeting.)
Young patients can participate, he noted at the meeting. “When I do telemedicine visits, kids can tell me how many hands of eczema they have.”
Dr. Eichenfield also said that he now routinely counsels on the environmental impacts on eczema. For example, “I explain to people that we’re probably going to have a bad wildfire season in California, and it’s the kind of environmental perturbation that may impact some eczema patients,” he said, noting the 2021 study documenting an association of wildfire air pollution from the 2018 California Camp fire with an increase in dermatology clinic visits for AD and itch in San Francisco.
“It helps to keep an eye out for that, and also to be aware of some of the environmental changes,” he said.
Dr. Chovatiya reported ties with AbbVie, Eli Lilly, Incyte, Leo Pharma, Pfizer, Regeneron, and Sanofi, among others. Dr. Sidbury reported ties with Regeneron, UCB, Pfizer, Leo Pharma, and Lilly, among others. Dr. Rosmarin reported ties with AbbVie, Incyte, Lilly, Pfizer, Regeneron, and Sanofi, among others. Dr. Siegfried reported ties with Regeneron, Sanofi Genzyme, AbbVie, Incyte, Leo, and Pfizer, among others. Dr. De Benedetto reported ties with Incyte, Pfizer, AbbVie, and Sanofi Advent, among others. Dr. Gooderham reported ties with AbbVie, Eli Lilly, Incyte, Leo Pharma, Pfizer, Regeneron, and Sanofi Genzyme, among others. Dr. Eichenfield disclosed ties with AbbVie, Eli Lilly, Incyte, Leo Pharma, Pfizer, Regeneron, and Sanofi, among others.
WASHINGTON –
The question was top of mind for experts who shared their advice during a “Tips and Tricks” session at the Revolutionizing Atopic Dermatitis meeting. Dupilumab dosing and dupilumab-associated facial redness and ocular disease, self-image issues, topical regimen adherence, and the quantification of disease were among the other topics raised by the experts.
Here are some of their practice pearls.
Treatment decisions, safety concerns
Deciding on a treatment is “kind of confusing ... particularly in the last year ... and it will only get more complicated,” said Raj J. Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago. “We’re all about some version of shared decision-making, but if all else is equal, sometimes it pays to explicitly ask the patient, what do you want to do?”
Questions about how long a systemic treatment should be tried, both initially and in the long run, are common. “I think that oftentimes we all get antsy about making changes when we’re not getting to the endpoint we want to. And at least in my real-world experience, late responders are a real thing. Sometimes 3-4 months ... isn’t enough,” he said.
Trial extension data show that patients who were nonresponders for various endpoints at 16 weeks are “captured continuously as you go further and further out,” Dr. Chovatiya said. Regarding the long term, “realistically, there’s no perfect time to call it quits.”
Addressing fears about Janus kinase inhibitors can be challenging, he said. “When you’ve identified the right patient and labs are done ... have them take the medication in front of you and hang out,” he advised. “It may sound ridiculous, but for the extremely anxious person it can be a big stress reducer for everyone involved.”
Regarding treatment fears more generally, “asking patients ‘what is the biggest risk of not treating your disease?’ sometimes gets people thinking,” Dr. Chovatiya said.
For parents of children with AD, said Robert Sidbury, MD, MPH, risks of not treating can become apparent once treatments are started and benefits are realized. “It’s so easy to focus on the risks of any treatment because they’re right there in black and white, and the risks of not treating are not always as apparent, even though – or maybe because – they live with them every day.”
When treatment is underway, “they see [how] everyone sleeps better, how school performance gets better, how concentration gets better,” said Dr. Sidbury, professor in the department of pediatrics at the University of Washington. Seattle, and chief of dermatology at Seattle Children’s Hospital.
“Always contextualize,” he advised. “As dermatologists, we’re savvy with navigating boxed warnings.”
David Rosmarin, MD, chair of dermatology, at Indiana University, Indianapolis, and formerly vice chair for research and education at Tufts Medical Center, Boston, said he addresses questions about the length of systemic treatment by advising patients: “Why don’t we start taking [the medication] for 3 months and then we’ll take it from there.”
In some pediatric cases, Dr. Rosmarin said, having the child express “what their AD means to them – how it affects them,” and then acknowledging and validating what the child says, is helpful to parents who are concerned about systemic treatments.
Dupilumab in the real world
Some patients on dupilumab do not have a complete response with dosing every 2 weeks and may benefit from more frequent dosing, said Dr. Rosmarin.
“We know from the SOLO-1 and SOLO-2 studies that dupilumab weekly dosing was evaluated. It was only the every-other-week dosing that was approved, and we can see why – in terms of the changes in EASI [Eczema Area and Severity Index] score they’re close to overlapping,” he said.
In real life, however, “some patients benefit from different dosing. It’s important to realize that. I think we all have some patients who may dose more frequently and some who may dose less frequently,” Dr. Rosmarin said.
For a patient who “gets absolutely no response from dupilumab after 3-4 months, I’d switch them to something else. But for those who are partial responders, particularly those who tell me they’re getting itchy before their next dose, they’re the ones who benefit most from doubling the dose to dupilumab weekly,” he said.
For patients who experience dupilumab-associated head and neck dermatitis, itraconazole may help, Dr. Rosmarin added. “We’re using 200 mg daily for 2 weeks and weekly thereafter, and it helps some of our patients.” The average self-reported improvement was 52% for patients with dupilumab-associated facial redness treated with itraconazole in a retrospective medical record review that he and his colleagues published in 2022.
Dr. Rosmarin pointed to a multicenter prospective cohort study also published in 2022 showing that baseline/pretreatment levels of Malassezia-specific IgE were associated with the development of dupilumab-associated head and neck dermatitis. The median levels of Malassezia-specific IgE were 32 kUL–1 versus 2.3 kUL–1 in patients who experienced dupilumab-associated facial redness, compared with those who did not.
He said that, while there “may be multiple reasons” for dupilumab-associated head and neck dermatitis and that “plenty of patients” who don’t have Malassezia-specific IgE develop head and neck dermatitis, “this could be one cause.”
Itraconazole has been shown in his practice to be superior to fluconazole, likely because it has greater anti-inflammatory effects and provides better coverage of Malassezia because it is more lipophilic, said Dr. Rosmarin, who does not test for Malassezia-specific IgE before trying itraconazole.
For dupilumab-associated ocular surface disease, Elaine C. Siegfried, MD, offered her first-line suggestions: warm compresses (such as a microwaved bean bag), bland ocular lubricant (such as preservative-free artificial tears), oral hydration, and if needed and accessible, the prescription ophthalmic solution lifitegrast.
“It’s become an issue – what the dermatologist can do first line,” said Dr. Siegfried, professor of pediatrics and dermatology at Saint Louis University and director of the division of pediatric dermatology at Cardinal Glennon Children’s Hospital, St. Louis.
“If these don’t work, then I’ll identify an ophthalmologist who’s knowledgeable about Dupixent-related ocular surface disease,” she said. Selection is “important because they’re not all knowledgeable ... corneal specialists typically have the most knowledge.”
Topical adherence with diffuse xerosis and mild-moderate AD
For patients with diffuse xerosis and mild-moderate AD, especially those who are older and having difficulty with topical regimens, Anna De Benedetto, MD, said she tries to enhance adherence by simplifying the regimen. She asks patients to buy a pound jar of base cream (ceramide base) – “whatever emollient they like” – and mixes into it a high-potency steroid solution. They’re instructed to apply the combined cream once daily for 1-2 weeks, and then three times a week alternating with a nonmedicated cream.
“This way they’re using one [cream] to target the immune system and the skin barrier,” said Dr. De Benedetto, associate professor of dermatology and director of the dermatology clinical trial unit at the University of Rochester (N.Y.) Medical Center.
‘Wet wrap’ pajamas; self-image for children, teens
Dermatologist Melinda Gooderham, MSc, MD, assistant professor at Queen’s University, Kingston, Ont., and medical director at the SKiN Centre for Dermatology, said that, for widespread and troublesome AD, she advises patients or parents to wet a thin cotton pajama top and bottom and spin it in the dryer “so it’s almost dry but still moist.” Dry, looser pajamas or a light track suit can then be worn over the damp pajamas. “I usually tell [patients] to buy one size up,” she said.
Body dysmorphia is common with skin disease, and its incidence is six times higher in people with eczema than those without the disease, said Dr. Siegfried. “I’ve found that, for patients subjected to AD for a long time,” this is still an issue, “even when you clear their skin.”
For children, teens and their families, the nonprofit organization Made a Masterpiece can be valuable, Dr. Siegfried said. It offers resources from parents, children, psychologists, dermatologists, and others to help manage the emotional, social and spiritual aspects of living with a skin condition.
To use or not to use BSA; environmental counseling
“I think [assessing] body surface area [BSA] is very important in pediatrics and for adolescents [especially in those with moderate to severe disease] because it quantifies the disease for the family,” said Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics and vice chair of the department of dermatology at the University of California, San Diego.
“Families live with the disease, but quantification really matters” for understanding the extent and impact of the disease and for motivating families to treat, said Dr. Eichenfield.
(When the disease is markedly diminished in follow-up, knowing the BSA then “helps families to register the improvement and gives positive reinforcement,” Dr. Eichenfeld said after the meeting.)
Young patients can participate, he noted at the meeting. “When I do telemedicine visits, kids can tell me how many hands of eczema they have.”
Dr. Eichenfield also said that he now routinely counsels on the environmental impacts on eczema. For example, “I explain to people that we’re probably going to have a bad wildfire season in California, and it’s the kind of environmental perturbation that may impact some eczema patients,” he said, noting the 2021 study documenting an association of wildfire air pollution from the 2018 California Camp fire with an increase in dermatology clinic visits for AD and itch in San Francisco.
“It helps to keep an eye out for that, and also to be aware of some of the environmental changes,” he said.
Dr. Chovatiya reported ties with AbbVie, Eli Lilly, Incyte, Leo Pharma, Pfizer, Regeneron, and Sanofi, among others. Dr. Sidbury reported ties with Regeneron, UCB, Pfizer, Leo Pharma, and Lilly, among others. Dr. Rosmarin reported ties with AbbVie, Incyte, Lilly, Pfizer, Regeneron, and Sanofi, among others. Dr. Siegfried reported ties with Regeneron, Sanofi Genzyme, AbbVie, Incyte, Leo, and Pfizer, among others. Dr. De Benedetto reported ties with Incyte, Pfizer, AbbVie, and Sanofi Advent, among others. Dr. Gooderham reported ties with AbbVie, Eli Lilly, Incyte, Leo Pharma, Pfizer, Regeneron, and Sanofi Genzyme, among others. Dr. Eichenfield disclosed ties with AbbVie, Eli Lilly, Incyte, Leo Pharma, Pfizer, Regeneron, and Sanofi, among others.
WASHINGTON –
The question was top of mind for experts who shared their advice during a “Tips and Tricks” session at the Revolutionizing Atopic Dermatitis meeting. Dupilumab dosing and dupilumab-associated facial redness and ocular disease, self-image issues, topical regimen adherence, and the quantification of disease were among the other topics raised by the experts.
Here are some of their practice pearls.
Treatment decisions, safety concerns
Deciding on a treatment is “kind of confusing ... particularly in the last year ... and it will only get more complicated,” said Raj J. Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago. “We’re all about some version of shared decision-making, but if all else is equal, sometimes it pays to explicitly ask the patient, what do you want to do?”
Questions about how long a systemic treatment should be tried, both initially and in the long run, are common. “I think that oftentimes we all get antsy about making changes when we’re not getting to the endpoint we want to. And at least in my real-world experience, late responders are a real thing. Sometimes 3-4 months ... isn’t enough,” he said.
Trial extension data show that patients who were nonresponders for various endpoints at 16 weeks are “captured continuously as you go further and further out,” Dr. Chovatiya said. Regarding the long term, “realistically, there’s no perfect time to call it quits.”
Addressing fears about Janus kinase inhibitors can be challenging, he said. “When you’ve identified the right patient and labs are done ... have them take the medication in front of you and hang out,” he advised. “It may sound ridiculous, but for the extremely anxious person it can be a big stress reducer for everyone involved.”
Regarding treatment fears more generally, “asking patients ‘what is the biggest risk of not treating your disease?’ sometimes gets people thinking,” Dr. Chovatiya said.
For parents of children with AD, said Robert Sidbury, MD, MPH, risks of not treating can become apparent once treatments are started and benefits are realized. “It’s so easy to focus on the risks of any treatment because they’re right there in black and white, and the risks of not treating are not always as apparent, even though – or maybe because – they live with them every day.”
When treatment is underway, “they see [how] everyone sleeps better, how school performance gets better, how concentration gets better,” said Dr. Sidbury, professor in the department of pediatrics at the University of Washington. Seattle, and chief of dermatology at Seattle Children’s Hospital.
“Always contextualize,” he advised. “As dermatologists, we’re savvy with navigating boxed warnings.”
David Rosmarin, MD, chair of dermatology, at Indiana University, Indianapolis, and formerly vice chair for research and education at Tufts Medical Center, Boston, said he addresses questions about the length of systemic treatment by advising patients: “Why don’t we start taking [the medication] for 3 months and then we’ll take it from there.”
In some pediatric cases, Dr. Rosmarin said, having the child express “what their AD means to them – how it affects them,” and then acknowledging and validating what the child says, is helpful to parents who are concerned about systemic treatments.
Dupilumab in the real world
Some patients on dupilumab do not have a complete response with dosing every 2 weeks and may benefit from more frequent dosing, said Dr. Rosmarin.
“We know from the SOLO-1 and SOLO-2 studies that dupilumab weekly dosing was evaluated. It was only the every-other-week dosing that was approved, and we can see why – in terms of the changes in EASI [Eczema Area and Severity Index] score they’re close to overlapping,” he said.
In real life, however, “some patients benefit from different dosing. It’s important to realize that. I think we all have some patients who may dose more frequently and some who may dose less frequently,” Dr. Rosmarin said.
For a patient who “gets absolutely no response from dupilumab after 3-4 months, I’d switch them to something else. But for those who are partial responders, particularly those who tell me they’re getting itchy before their next dose, they’re the ones who benefit most from doubling the dose to dupilumab weekly,” he said.
For patients who experience dupilumab-associated head and neck dermatitis, itraconazole may help, Dr. Rosmarin added. “We’re using 200 mg daily for 2 weeks and weekly thereafter, and it helps some of our patients.” The average self-reported improvement was 52% for patients with dupilumab-associated facial redness treated with itraconazole in a retrospective medical record review that he and his colleagues published in 2022.
Dr. Rosmarin pointed to a multicenter prospective cohort study also published in 2022 showing that baseline/pretreatment levels of Malassezia-specific IgE were associated with the development of dupilumab-associated head and neck dermatitis. The median levels of Malassezia-specific IgE were 32 kUL–1 versus 2.3 kUL–1 in patients who experienced dupilumab-associated facial redness, compared with those who did not.
He said that, while there “may be multiple reasons” for dupilumab-associated head and neck dermatitis and that “plenty of patients” who don’t have Malassezia-specific IgE develop head and neck dermatitis, “this could be one cause.”
Itraconazole has been shown in his practice to be superior to fluconazole, likely because it has greater anti-inflammatory effects and provides better coverage of Malassezia because it is more lipophilic, said Dr. Rosmarin, who does not test for Malassezia-specific IgE before trying itraconazole.
For dupilumab-associated ocular surface disease, Elaine C. Siegfried, MD, offered her first-line suggestions: warm compresses (such as a microwaved bean bag), bland ocular lubricant (such as preservative-free artificial tears), oral hydration, and if needed and accessible, the prescription ophthalmic solution lifitegrast.
“It’s become an issue – what the dermatologist can do first line,” said Dr. Siegfried, professor of pediatrics and dermatology at Saint Louis University and director of the division of pediatric dermatology at Cardinal Glennon Children’s Hospital, St. Louis.
“If these don’t work, then I’ll identify an ophthalmologist who’s knowledgeable about Dupixent-related ocular surface disease,” she said. Selection is “important because they’re not all knowledgeable ... corneal specialists typically have the most knowledge.”
Topical adherence with diffuse xerosis and mild-moderate AD
For patients with diffuse xerosis and mild-moderate AD, especially those who are older and having difficulty with topical regimens, Anna De Benedetto, MD, said she tries to enhance adherence by simplifying the regimen. She asks patients to buy a pound jar of base cream (ceramide base) – “whatever emollient they like” – and mixes into it a high-potency steroid solution. They’re instructed to apply the combined cream once daily for 1-2 weeks, and then three times a week alternating with a nonmedicated cream.
“This way they’re using one [cream] to target the immune system and the skin barrier,” said Dr. De Benedetto, associate professor of dermatology and director of the dermatology clinical trial unit at the University of Rochester (N.Y.) Medical Center.
‘Wet wrap’ pajamas; self-image for children, teens
Dermatologist Melinda Gooderham, MSc, MD, assistant professor at Queen’s University, Kingston, Ont., and medical director at the SKiN Centre for Dermatology, said that, for widespread and troublesome AD, she advises patients or parents to wet a thin cotton pajama top and bottom and spin it in the dryer “so it’s almost dry but still moist.” Dry, looser pajamas or a light track suit can then be worn over the damp pajamas. “I usually tell [patients] to buy one size up,” she said.
Body dysmorphia is common with skin disease, and its incidence is six times higher in people with eczema than those without the disease, said Dr. Siegfried. “I’ve found that, for patients subjected to AD for a long time,” this is still an issue, “even when you clear their skin.”
For children, teens and their families, the nonprofit organization Made a Masterpiece can be valuable, Dr. Siegfried said. It offers resources from parents, children, psychologists, dermatologists, and others to help manage the emotional, social and spiritual aspects of living with a skin condition.
To use or not to use BSA; environmental counseling
“I think [assessing] body surface area [BSA] is very important in pediatrics and for adolescents [especially in those with moderate to severe disease] because it quantifies the disease for the family,” said Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics and vice chair of the department of dermatology at the University of California, San Diego.
“Families live with the disease, but quantification really matters” for understanding the extent and impact of the disease and for motivating families to treat, said Dr. Eichenfield.
(When the disease is markedly diminished in follow-up, knowing the BSA then “helps families to register the improvement and gives positive reinforcement,” Dr. Eichenfeld said after the meeting.)
Young patients can participate, he noted at the meeting. “When I do telemedicine visits, kids can tell me how many hands of eczema they have.”
Dr. Eichenfield also said that he now routinely counsels on the environmental impacts on eczema. For example, “I explain to people that we’re probably going to have a bad wildfire season in California, and it’s the kind of environmental perturbation that may impact some eczema patients,” he said, noting the 2021 study documenting an association of wildfire air pollution from the 2018 California Camp fire with an increase in dermatology clinic visits for AD and itch in San Francisco.
“It helps to keep an eye out for that, and also to be aware of some of the environmental changes,” he said.
Dr. Chovatiya reported ties with AbbVie, Eli Lilly, Incyte, Leo Pharma, Pfizer, Regeneron, and Sanofi, among others. Dr. Sidbury reported ties with Regeneron, UCB, Pfizer, Leo Pharma, and Lilly, among others. Dr. Rosmarin reported ties with AbbVie, Incyte, Lilly, Pfizer, Regeneron, and Sanofi, among others. Dr. Siegfried reported ties with Regeneron, Sanofi Genzyme, AbbVie, Incyte, Leo, and Pfizer, among others. Dr. De Benedetto reported ties with Incyte, Pfizer, AbbVie, and Sanofi Advent, among others. Dr. Gooderham reported ties with AbbVie, Eli Lilly, Incyte, Leo Pharma, Pfizer, Regeneron, and Sanofi Genzyme, among others. Dr. Eichenfield disclosed ties with AbbVie, Eli Lilly, Incyte, Leo Pharma, Pfizer, Regeneron, and Sanofi, among others.
AT RAD 2023
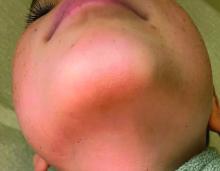
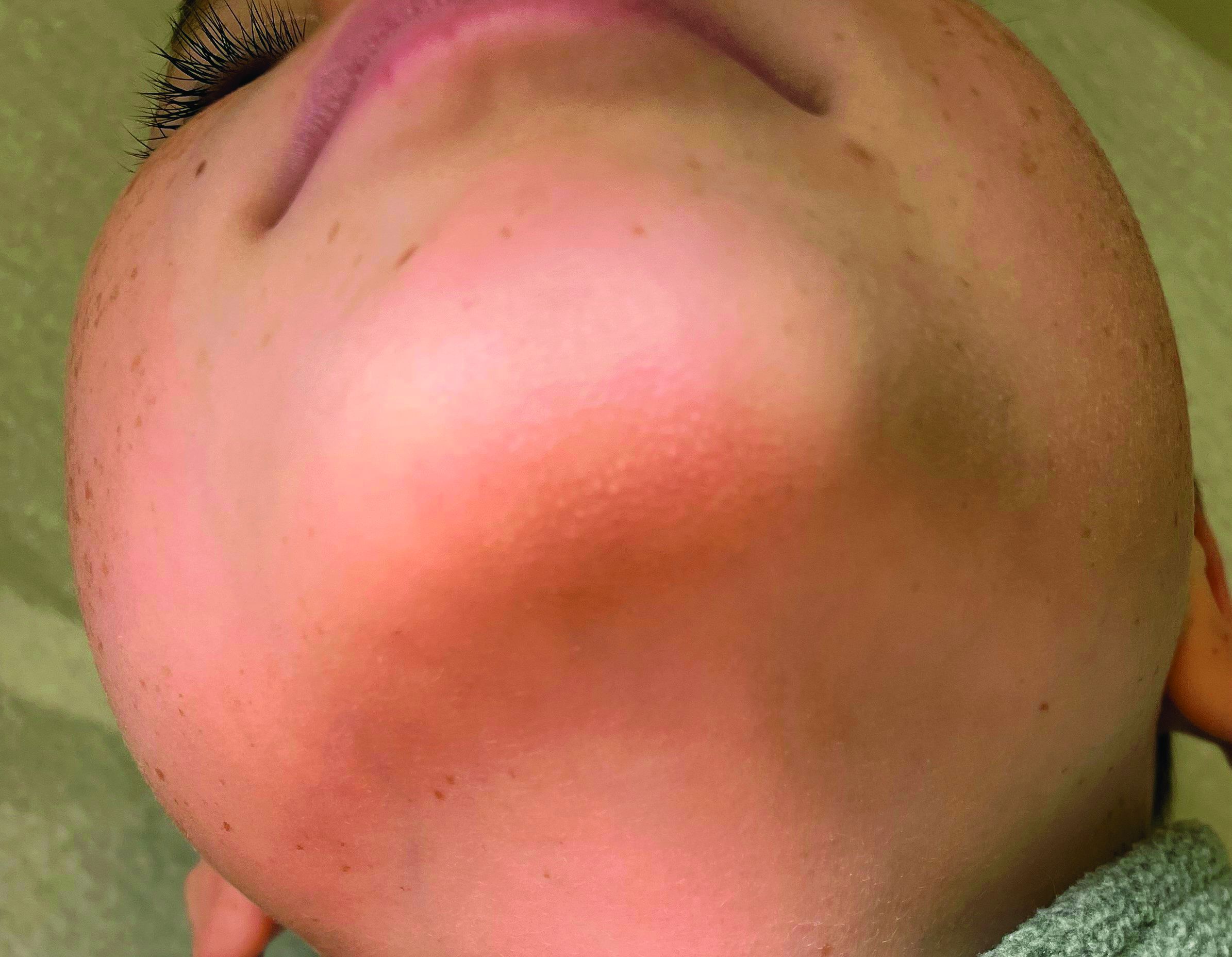
A 7-year-old male has a bumpy rash on the chin for several months
Given the presentation and the unique location of the lesions he was diagnosed with follicular keratosis of the chin (FKC).
This is a rare and poorly understood condition that can be present in older children and young teenagers. In the cases reported by Kanzaki et al.1 were two boys who presented with the condition; it was thought to be associated with rubbing of the chin with their hands when watching TV or reading. The author described improvement with habit change. This condition is usually described in boys, and some cases presented in brothers,2 suggesting a genetic predisposition. Some reports lack a history of rubbing or trauma to the area.
Histopathologic evaluation of the lesions demonstrates dilated hair follicles containing keratotic basophilic material without any signs of inflammation.
The lesions can be confused with keratosis pilaris (KP). Keratosis pilaris can be described in association with atopic dermatitis and ichthyosis, which were not present in our patient. The lesions usually present on the sides of the cheeks and lateral region of the arms and legs. Compared with follicular keratosis, KP lesions usually present with associated perifollicular erythema. Our patient did not present with lesions on the cheeks or the sides of the arms or legs. Milia can present on the chin of children, usually if there is history of rubbing or trauma, or on a scar. Milia are micro keratin cysts, usually seen in areas of the face. Lichen spinulous is described as rough small follicular papules that present in oval or circular patches that can grow up to 5 cm and spread rapidly. They usually present on the extensor surfaces of the extremities, neck, abdomen, and knees. These lesions are thought to be secondary to infections, have been associated with atopy, and have been seen in patients with atopic dermatitis. There is a probable genetic predisposition. The lesions are usually treated with gentle soaps and moisturizer containing keratolytics like urea or salicylic acid, and in some cases topical retinoids can also be tried. Follicular mucinosis can also present similarly to keratosis follicularis. The lesions present as scaly plaques or as grouped skin color papules on the face, scalp, or the neck that can also be associated with hair loss. Sometimes a biopsy needs to be done to be able to distinguish it from follicular keratosis. There is an increase of mucin around hair follicles and sebaceous glands with associated inflammation and degeneration of the follicular structures. In patients with primary follicular mucinosis the lesions can resolve spontaneously in a couple of years. Lesions can be treated with topical corticosteroids, oral antibiotics like macrolides or tetracyclines, dapsone, and phototherapy.
KFC can be treated with vitamin D analogues. It is usually unresponsive to corticosteroids, keratolytic lotions, and retinoids. Our patient was prescribed calcipotriene.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego
References
1. Kanzaki T et al. J Am Acad Dermatol. 1992;26(1):134-5.
2. Buechner AA et al. JAMA Dermatol. 2018 Jan 1;154(1):111-2.
Given the presentation and the unique location of the lesions he was diagnosed with follicular keratosis of the chin (FKC).
This is a rare and poorly understood condition that can be present in older children and young teenagers. In the cases reported by Kanzaki et al.1 were two boys who presented with the condition; it was thought to be associated with rubbing of the chin with their hands when watching TV or reading. The author described improvement with habit change. This condition is usually described in boys, and some cases presented in brothers,2 suggesting a genetic predisposition. Some reports lack a history of rubbing or trauma to the area.
Histopathologic evaluation of the lesions demonstrates dilated hair follicles containing keratotic basophilic material without any signs of inflammation.
The lesions can be confused with keratosis pilaris (KP). Keratosis pilaris can be described in association with atopic dermatitis and ichthyosis, which were not present in our patient. The lesions usually present on the sides of the cheeks and lateral region of the arms and legs. Compared with follicular keratosis, KP lesions usually present with associated perifollicular erythema. Our patient did not present with lesions on the cheeks or the sides of the arms or legs. Milia can present on the chin of children, usually if there is history of rubbing or trauma, or on a scar. Milia are micro keratin cysts, usually seen in areas of the face. Lichen spinulous is described as rough small follicular papules that present in oval or circular patches that can grow up to 5 cm and spread rapidly. They usually present on the extensor surfaces of the extremities, neck, abdomen, and knees. These lesions are thought to be secondary to infections, have been associated with atopy, and have been seen in patients with atopic dermatitis. There is a probable genetic predisposition. The lesions are usually treated with gentle soaps and moisturizer containing keratolytics like urea or salicylic acid, and in some cases topical retinoids can also be tried. Follicular mucinosis can also present similarly to keratosis follicularis. The lesions present as scaly plaques or as grouped skin color papules on the face, scalp, or the neck that can also be associated with hair loss. Sometimes a biopsy needs to be done to be able to distinguish it from follicular keratosis. There is an increase of mucin around hair follicles and sebaceous glands with associated inflammation and degeneration of the follicular structures. In patients with primary follicular mucinosis the lesions can resolve spontaneously in a couple of years. Lesions can be treated with topical corticosteroids, oral antibiotics like macrolides or tetracyclines, dapsone, and phototherapy.
KFC can be treated with vitamin D analogues. It is usually unresponsive to corticosteroids, keratolytic lotions, and retinoids. Our patient was prescribed calcipotriene.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego
References
1. Kanzaki T et al. J Am Acad Dermatol. 1992;26(1):134-5.
2. Buechner AA et al. JAMA Dermatol. 2018 Jan 1;154(1):111-2.
Given the presentation and the unique location of the lesions he was diagnosed with follicular keratosis of the chin (FKC).
This is a rare and poorly understood condition that can be present in older children and young teenagers. In the cases reported by Kanzaki et al.1 were two boys who presented with the condition; it was thought to be associated with rubbing of the chin with their hands when watching TV or reading. The author described improvement with habit change. This condition is usually described in boys, and some cases presented in brothers,2 suggesting a genetic predisposition. Some reports lack a history of rubbing or trauma to the area.
Histopathologic evaluation of the lesions demonstrates dilated hair follicles containing keratotic basophilic material without any signs of inflammation.
The lesions can be confused with keratosis pilaris (KP). Keratosis pilaris can be described in association with atopic dermatitis and ichthyosis, which were not present in our patient. The lesions usually present on the sides of the cheeks and lateral region of the arms and legs. Compared with follicular keratosis, KP lesions usually present with associated perifollicular erythema. Our patient did not present with lesions on the cheeks or the sides of the arms or legs. Milia can present on the chin of children, usually if there is history of rubbing or trauma, or on a scar. Milia are micro keratin cysts, usually seen in areas of the face. Lichen spinulous is described as rough small follicular papules that present in oval or circular patches that can grow up to 5 cm and spread rapidly. They usually present on the extensor surfaces of the extremities, neck, abdomen, and knees. These lesions are thought to be secondary to infections, have been associated with atopy, and have been seen in patients with atopic dermatitis. There is a probable genetic predisposition. The lesions are usually treated with gentle soaps and moisturizer containing keratolytics like urea or salicylic acid, and in some cases topical retinoids can also be tried. Follicular mucinosis can also present similarly to keratosis follicularis. The lesions present as scaly plaques or as grouped skin color papules on the face, scalp, or the neck that can also be associated with hair loss. Sometimes a biopsy needs to be done to be able to distinguish it from follicular keratosis. There is an increase of mucin around hair follicles and sebaceous glands with associated inflammation and degeneration of the follicular structures. In patients with primary follicular mucinosis the lesions can resolve spontaneously in a couple of years. Lesions can be treated with topical corticosteroids, oral antibiotics like macrolides or tetracyclines, dapsone, and phototherapy.
KFC can be treated with vitamin D analogues. It is usually unresponsive to corticosteroids, keratolytic lotions, and retinoids. Our patient was prescribed calcipotriene.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego
References
1. Kanzaki T et al. J Am Acad Dermatol. 1992;26(1):134-5.
2. Buechner AA et al. JAMA Dermatol. 2018 Jan 1;154(1):111-2.
He is a healthy child with no past medical history. He is not taking any medications.
On physical exam he has follicular hyperkeratotic papules on the chin. No lesions on the axilla or thighs.
How does psoriasis affect fertility and birth outcomes?
in a U.K. cohort study.
Those are key findings from what is believed to be one of the largest studies to investigate fertility and obstetric outcomes in patients with psoriasis.
“Studies that have examined fertility and pregnancy outcomes in women with psoriasis have reported conflicting findings,” lead author Teng-Chou Chen, PhD, of the Centre for Pharmacoepidemiology and Drug Safety at the University of Manchester (England), and colleagues from the Global Psoriasis Atlas wrote in the study, published in JAMA Dermatology. Most of the studies were small, with under 100 women, “and are thus likely underpowered to detect a difference in pregnancy outcomes. The majority of those studies used disease registry data or lacked a matched comparison group and hence were unable to estimate the association of fertility and adverse pregnancy outcomes in women with psoriasis when compared with the general population.”
To determine fertility rates and birth outcomes in female patients with psoriasis, compared with age- and practice-matched patients without psoriasis, the researchers evaluated EHR data from a large U.K. primary care database, the Clinical Practice Research Datalink GOLD, from 1998 to 2019. They limited the analysis to patients aged 15-44 years and used relevant codes from clinical consultations to identify those with psoriasis. Then, for each patient with psoriasis, the researchers selected five comparators without psoriasis from the same primary care practice and matched for year of birth.
Both sets of patients were followed from the index date to age 45 years, death, transfer out of practice, last date of data collection, or end of the study period (Dec. 31, 2019), whichever came first. Pregnancy records were extracted for both sets of patients, and birth outcomes were categorized as pregnancy loss, live birth, stillbirth, and preterm birth. Adverse pregnancy outcomes were also collected. Finally, Dr. Chen and colleagues used a negative binomial model to examine the association between psoriasis and the fertility rate, and they applied logistic regression to compare the association between psoriasis and obstetric outcomes.
The analysis included 63,681 patients with psoriasis and 318,405 comparators whose median age on the index date was 30 years and who were followed for a median of 4.1 years. Among patients with psoriasis, 5.1% met criteria for moderate to severe disease in the follow-up period. The researchers observed that, compared with their age- and practice-matched counterparts, patients with psoriasis were more likely to be current smokers, alcohol drinkers, or overweight on the index date. They were also more often diagnosed with diabetes, hypertension, inflammatory bowel disease, thyroid disorders, and respiratory diseases such as asthma and chronic obstructive pulmonary disease.
Fertility, birth outcomes
When they looked at fertility outcomes, the researchers found that, compared with their matched peers without psoriasis, those with psoriasis had higher rates of fertility (risk ratio, 1.30; 95% confidence interval, 1.27-1.33; P < .001). But after the researchers stratified patients based on psoriasis severity, those with moderate to severe disease had significantly lower rates of fertility (RR, 0.75; 95% CI, 0.69-0.83; P < .001), compared those who did not have psoriasis.
As for adverse birth outcomes, compared with their matched comparators, pregnancies in patients with psoriasis were less likely to end in a live birth (odds ratio, 0.91; 95% CI, 0.88-0.93; P < .001). They also had a higher risk of pregnancy loss (OR, 1.06; 95% CI, 1.03-1.10; P < .001), most during the first trimester, at a gestation period of under 91 days.
In addition to psoriasis, patients younger than age 20 (OR, 2.04; 95% CI, 1.94-2.15; P < .011) and those aged between 20 and 24 years (OR, 1.35; 95% CI, 1.31-1.40; P < .001) had a higher risk of pregnancy loss, compared with those aged between 25 and 34 years.
However, no increases in the risks of antenatal hemorrhage, preeclampsia, or gestational diabetes were observed in patients with psoriasis, and no statistically significant differences in the odds of stillbirth and preterm birth were found between patients with psoriasis and matched comparators who did not have psoriasis.
“The mechanism to link the higher risk of pregnancy loss in patients with psoriasis is not clear, but there might be potential explanations,” the researchers wrote. “Psoriasis is characterized by the increased activity of [interleukin]-17, IL-23, and tumor necrosis factor–alpha. Those proinflammatory cytokines may negatively affect the placenta and cause impaired fetal growth.”
They recommended that further studies “evaluate the effects of better management of psoriasis and close monitoring during pregnancy on pregnancy loss.” In particular, “patients with psoriasis were more likely to have comorbidities that may be related to poor pregnancy outcomes, and hence increased emphasis of managing comorbidities as part of the routine management plan is also warranted.”
Asked to comment on the study, Alexa B. Kimball, MD, MPH, who has been involved with research on this topic, said that she and other investigators had observed some years ago that fertility rates for women with moderate to severe psoriasis might be lower than expected.
This trend was observed in some psoriasis registries, some pregnancy registries, and in clinical practice, Dr. Kimball, professor of dermatology at Harvard Medical School, Boston, said in an interview. “This study clearly demonstrates that lower fertility rates in the moderate to severe psoriasis population occurs and compels further exploration of the reason why.” The reasons could be biologic, she continued, including difficulty conceiving or an increased risk of miscarriage, sociobehavioral issues, or a combination.
“Behavioral examples could include that some women with moderate to severe psoriasis can flare during pregnancy, which might affect their choice” to become pregnant, Dr. Kimball said. “Stigma may also play a role in how women with moderate to severe psoriasis form relationships. Now that there are much better treatments for moderate to severe psoriasis and better knowledge about managing psoriasis during pregnancy, it will also be important to explore whether these trends change over time.”
The study was funded by the International League of Dermatological Societies on behalf of the Global Psoriasis Atlas. Two of the study authors reported receiving consulting fees and grant support from many pharmaceutical companies. Dr. Kimball disclosed that she serves or has served on several Organization of Teratology Information Specialists advisory board pregnancy registries, is a consultant and investigator for Abbvie, Janssen, Lilly, Bristol-Myers Squibb, Moonlake, UCB, and Amgen; has fellowship funding from Janssen; and serves on the board of Almirall.
in a U.K. cohort study.
Those are key findings from what is believed to be one of the largest studies to investigate fertility and obstetric outcomes in patients with psoriasis.
“Studies that have examined fertility and pregnancy outcomes in women with psoriasis have reported conflicting findings,” lead author Teng-Chou Chen, PhD, of the Centre for Pharmacoepidemiology and Drug Safety at the University of Manchester (England), and colleagues from the Global Psoriasis Atlas wrote in the study, published in JAMA Dermatology. Most of the studies were small, with under 100 women, “and are thus likely underpowered to detect a difference in pregnancy outcomes. The majority of those studies used disease registry data or lacked a matched comparison group and hence were unable to estimate the association of fertility and adverse pregnancy outcomes in women with psoriasis when compared with the general population.”
To determine fertility rates and birth outcomes in female patients with psoriasis, compared with age- and practice-matched patients without psoriasis, the researchers evaluated EHR data from a large U.K. primary care database, the Clinical Practice Research Datalink GOLD, from 1998 to 2019. They limited the analysis to patients aged 15-44 years and used relevant codes from clinical consultations to identify those with psoriasis. Then, for each patient with psoriasis, the researchers selected five comparators without psoriasis from the same primary care practice and matched for year of birth.
Both sets of patients were followed from the index date to age 45 years, death, transfer out of practice, last date of data collection, or end of the study period (Dec. 31, 2019), whichever came first. Pregnancy records were extracted for both sets of patients, and birth outcomes were categorized as pregnancy loss, live birth, stillbirth, and preterm birth. Adverse pregnancy outcomes were also collected. Finally, Dr. Chen and colleagues used a negative binomial model to examine the association between psoriasis and the fertility rate, and they applied logistic regression to compare the association between psoriasis and obstetric outcomes.
The analysis included 63,681 patients with psoriasis and 318,405 comparators whose median age on the index date was 30 years and who were followed for a median of 4.1 years. Among patients with psoriasis, 5.1% met criteria for moderate to severe disease in the follow-up period. The researchers observed that, compared with their age- and practice-matched counterparts, patients with psoriasis were more likely to be current smokers, alcohol drinkers, or overweight on the index date. They were also more often diagnosed with diabetes, hypertension, inflammatory bowel disease, thyroid disorders, and respiratory diseases such as asthma and chronic obstructive pulmonary disease.
Fertility, birth outcomes
When they looked at fertility outcomes, the researchers found that, compared with their matched peers without psoriasis, those with psoriasis had higher rates of fertility (risk ratio, 1.30; 95% confidence interval, 1.27-1.33; P < .001). But after the researchers stratified patients based on psoriasis severity, those with moderate to severe disease had significantly lower rates of fertility (RR, 0.75; 95% CI, 0.69-0.83; P < .001), compared those who did not have psoriasis.
As for adverse birth outcomes, compared with their matched comparators, pregnancies in patients with psoriasis were less likely to end in a live birth (odds ratio, 0.91; 95% CI, 0.88-0.93; P < .001). They also had a higher risk of pregnancy loss (OR, 1.06; 95% CI, 1.03-1.10; P < .001), most during the first trimester, at a gestation period of under 91 days.
In addition to psoriasis, patients younger than age 20 (OR, 2.04; 95% CI, 1.94-2.15; P < .011) and those aged between 20 and 24 years (OR, 1.35; 95% CI, 1.31-1.40; P < .001) had a higher risk of pregnancy loss, compared with those aged between 25 and 34 years.
However, no increases in the risks of antenatal hemorrhage, preeclampsia, or gestational diabetes were observed in patients with psoriasis, and no statistically significant differences in the odds of stillbirth and preterm birth were found between patients with psoriasis and matched comparators who did not have psoriasis.
“The mechanism to link the higher risk of pregnancy loss in patients with psoriasis is not clear, but there might be potential explanations,” the researchers wrote. “Psoriasis is characterized by the increased activity of [interleukin]-17, IL-23, and tumor necrosis factor–alpha. Those proinflammatory cytokines may negatively affect the placenta and cause impaired fetal growth.”
They recommended that further studies “evaluate the effects of better management of psoriasis and close monitoring during pregnancy on pregnancy loss.” In particular, “patients with psoriasis were more likely to have comorbidities that may be related to poor pregnancy outcomes, and hence increased emphasis of managing comorbidities as part of the routine management plan is also warranted.”
Asked to comment on the study, Alexa B. Kimball, MD, MPH, who has been involved with research on this topic, said that she and other investigators had observed some years ago that fertility rates for women with moderate to severe psoriasis might be lower than expected.
This trend was observed in some psoriasis registries, some pregnancy registries, and in clinical practice, Dr. Kimball, professor of dermatology at Harvard Medical School, Boston, said in an interview. “This study clearly demonstrates that lower fertility rates in the moderate to severe psoriasis population occurs and compels further exploration of the reason why.” The reasons could be biologic, she continued, including difficulty conceiving or an increased risk of miscarriage, sociobehavioral issues, or a combination.
“Behavioral examples could include that some women with moderate to severe psoriasis can flare during pregnancy, which might affect their choice” to become pregnant, Dr. Kimball said. “Stigma may also play a role in how women with moderate to severe psoriasis form relationships. Now that there are much better treatments for moderate to severe psoriasis and better knowledge about managing psoriasis during pregnancy, it will also be important to explore whether these trends change over time.”
The study was funded by the International League of Dermatological Societies on behalf of the Global Psoriasis Atlas. Two of the study authors reported receiving consulting fees and grant support from many pharmaceutical companies. Dr. Kimball disclosed that she serves or has served on several Organization of Teratology Information Specialists advisory board pregnancy registries, is a consultant and investigator for Abbvie, Janssen, Lilly, Bristol-Myers Squibb, Moonlake, UCB, and Amgen; has fellowship funding from Janssen; and serves on the board of Almirall.
in a U.K. cohort study.
Those are key findings from what is believed to be one of the largest studies to investigate fertility and obstetric outcomes in patients with psoriasis.
“Studies that have examined fertility and pregnancy outcomes in women with psoriasis have reported conflicting findings,” lead author Teng-Chou Chen, PhD, of the Centre for Pharmacoepidemiology and Drug Safety at the University of Manchester (England), and colleagues from the Global Psoriasis Atlas wrote in the study, published in JAMA Dermatology. Most of the studies were small, with under 100 women, “and are thus likely underpowered to detect a difference in pregnancy outcomes. The majority of those studies used disease registry data or lacked a matched comparison group and hence were unable to estimate the association of fertility and adverse pregnancy outcomes in women with psoriasis when compared with the general population.”
To determine fertility rates and birth outcomes in female patients with psoriasis, compared with age- and practice-matched patients without psoriasis, the researchers evaluated EHR data from a large U.K. primary care database, the Clinical Practice Research Datalink GOLD, from 1998 to 2019. They limited the analysis to patients aged 15-44 years and used relevant codes from clinical consultations to identify those with psoriasis. Then, for each patient with psoriasis, the researchers selected five comparators without psoriasis from the same primary care practice and matched for year of birth.
Both sets of patients were followed from the index date to age 45 years, death, transfer out of practice, last date of data collection, or end of the study period (Dec. 31, 2019), whichever came first. Pregnancy records were extracted for both sets of patients, and birth outcomes were categorized as pregnancy loss, live birth, stillbirth, and preterm birth. Adverse pregnancy outcomes were also collected. Finally, Dr. Chen and colleagues used a negative binomial model to examine the association between psoriasis and the fertility rate, and they applied logistic regression to compare the association between psoriasis and obstetric outcomes.
The analysis included 63,681 patients with psoriasis and 318,405 comparators whose median age on the index date was 30 years and who were followed for a median of 4.1 years. Among patients with psoriasis, 5.1% met criteria for moderate to severe disease in the follow-up period. The researchers observed that, compared with their age- and practice-matched counterparts, patients with psoriasis were more likely to be current smokers, alcohol drinkers, or overweight on the index date. They were also more often diagnosed with diabetes, hypertension, inflammatory bowel disease, thyroid disorders, and respiratory diseases such as asthma and chronic obstructive pulmonary disease.
Fertility, birth outcomes
When they looked at fertility outcomes, the researchers found that, compared with their matched peers without psoriasis, those with psoriasis had higher rates of fertility (risk ratio, 1.30; 95% confidence interval, 1.27-1.33; P < .001). But after the researchers stratified patients based on psoriasis severity, those with moderate to severe disease had significantly lower rates of fertility (RR, 0.75; 95% CI, 0.69-0.83; P < .001), compared those who did not have psoriasis.
As for adverse birth outcomes, compared with their matched comparators, pregnancies in patients with psoriasis were less likely to end in a live birth (odds ratio, 0.91; 95% CI, 0.88-0.93; P < .001). They also had a higher risk of pregnancy loss (OR, 1.06; 95% CI, 1.03-1.10; P < .001), most during the first trimester, at a gestation period of under 91 days.
In addition to psoriasis, patients younger than age 20 (OR, 2.04; 95% CI, 1.94-2.15; P < .011) and those aged between 20 and 24 years (OR, 1.35; 95% CI, 1.31-1.40; P < .001) had a higher risk of pregnancy loss, compared with those aged between 25 and 34 years.
However, no increases in the risks of antenatal hemorrhage, preeclampsia, or gestational diabetes were observed in patients with psoriasis, and no statistically significant differences in the odds of stillbirth and preterm birth were found between patients with psoriasis and matched comparators who did not have psoriasis.
“The mechanism to link the higher risk of pregnancy loss in patients with psoriasis is not clear, but there might be potential explanations,” the researchers wrote. “Psoriasis is characterized by the increased activity of [interleukin]-17, IL-23, and tumor necrosis factor–alpha. Those proinflammatory cytokines may negatively affect the placenta and cause impaired fetal growth.”
They recommended that further studies “evaluate the effects of better management of psoriasis and close monitoring during pregnancy on pregnancy loss.” In particular, “patients with psoriasis were more likely to have comorbidities that may be related to poor pregnancy outcomes, and hence increased emphasis of managing comorbidities as part of the routine management plan is also warranted.”
Asked to comment on the study, Alexa B. Kimball, MD, MPH, who has been involved with research on this topic, said that she and other investigators had observed some years ago that fertility rates for women with moderate to severe psoriasis might be lower than expected.
This trend was observed in some psoriasis registries, some pregnancy registries, and in clinical practice, Dr. Kimball, professor of dermatology at Harvard Medical School, Boston, said in an interview. “This study clearly demonstrates that lower fertility rates in the moderate to severe psoriasis population occurs and compels further exploration of the reason why.” The reasons could be biologic, she continued, including difficulty conceiving or an increased risk of miscarriage, sociobehavioral issues, or a combination.
“Behavioral examples could include that some women with moderate to severe psoriasis can flare during pregnancy, which might affect their choice” to become pregnant, Dr. Kimball said. “Stigma may also play a role in how women with moderate to severe psoriasis form relationships. Now that there are much better treatments for moderate to severe psoriasis and better knowledge about managing psoriasis during pregnancy, it will also be important to explore whether these trends change over time.”
The study was funded by the International League of Dermatological Societies on behalf of the Global Psoriasis Atlas. Two of the study authors reported receiving consulting fees and grant support from many pharmaceutical companies. Dr. Kimball disclosed that she serves or has served on several Organization of Teratology Information Specialists advisory board pregnancy registries, is a consultant and investigator for Abbvie, Janssen, Lilly, Bristol-Myers Squibb, Moonlake, UCB, and Amgen; has fellowship funding from Janssen; and serves on the board of Almirall.
FROM JAMA DERMATOLOGY
Halting active inflammation key in treating PIH
CHICAGO –
Dr. Desai, clinical assistant professor in the department of dermatology at the University of Texas Southwestern Medical Center, Dallas, spoke at the Pigmentary Disorders Exchange Symposium, provided by MedscapeLive!
Like all dermatologists, he said at the meeting, he sees lots of acne cases. However, PIH is often the presenting reason for the visit in his practice, which focuses predominantly on skin of color.
“Most of my patients come in not even worried about the acne,” he said. “They come in wanting me to fix the dark spots.”
Inflammation persists
Dermatologists, Dr. Desai said, should educate patients with active PIH resulting from acne or other diseases that even though the condition has been labeled post- inflammatory hyperpigmentation, the inflammation continues to be a problem.
He said, while patients may think PIH is “just scars,” the inflammation is still active and the condition needs to be treated from a skin-lightening perspective but, more importantly, with a focus on halting the inflammation. “If you were to biopsy the areas of hyperpigmentation, you would find a high density of active inflammatory behaviors still present in the skin,” he said.
When treating patients, it’s critical to first treat the underlying skin condition aggressively, he said. “Things like topical retinoids and azelaic acid mechanistically actually make a lot more sense for PIH than even hydroquinone, in some cases, because these therapies are actually anti-inflammatory for many of the diseases we treat.”
Dr. Desai noted that, in patients with darker skin tones, even diseases like seborrheic dermatitis and plaque psoriasis can result in PIH, while in patients with lighter skin tones, the same diseases may leave some residual postinflammatory erythema.
“I think it’s very important, particularly when you’re treating a darker skin–toned patient, to arrest the erythema early on to prevent that further worsening of hyperpigmentation,” he said.
Biopsies important
In cases of PIH, determining the best treatment requires finding out where the pigment is and how deep it is, Dr. Desai said.
He noted dermatologists are often worried about doing biopsies, particularly in patients with darker skin, because of the risk of scarring and keloid formation for those more prone to keloids. The preference is also for a therapeutic effect without using invasive procedures.
“But particularly with PIH, in patients who have been therapeutically challenging, I don’t hesitate to do very small biopsies – 2- and 3-mm punch biopsies – particularly if they are from the head and neck area.”
He suggests doing biopsies on part of the ear, lower jaw line, or the neck area, as these areas tend to heal nicely. “You don’t have to be so concerned about the scarring if you counsel appropriately,” he said.
The biopsy can be valuable in determining whether a very expensive treatment will reach the intended target.
Topical retinoids play an important role as anti-inflammatories for PIH, Dr. Desai said.
He gave an example of a patient with Fitzpatrick skin type IV or V with chronic acne and extensive PIH. “Are you going to effectively tell that patient to apply 4% hydroquinone triple-combination compound across 30 different areas of PIH on their face? The answer is that’s really not very efficient or effective.”
That’s why therapies, such as retinoids, that target the pathogenesis of PIH, particularly the inflammatory component, are important, he added.
Psychological burden
PIH comes with significant stigma and loss of quality of life loss that can last many years.
During another presentation at the meeting, Susan C. Taylor, MD, professor and vice chair of diversity, equity and inclusion in the department of dermatology, at the University of Pennsylvania, Philadelphia, pointed out that in a 2016 study of 324 patients in seven Asian countries, acne-related PIH lasted longer than 1 year in 65.2% of patients and 5 years or longer in 22.3%, significantly affecting their quality of life.
Dr. Desai added that, in a paper recently published in the British Journal of Dermatology, on the impact of postacne hyperpigmentation in patients, the authors pointed out that the reported prevalence of PIH in patients with acne ranges between 45.5% and 87.2%, depending on skin phototype, and that in most cases, PIH takes more than a year to fade.
“Studies have demonstrated that patients with acne and resulting scarring often face stigmatization, leading to quality of life impairment, social withdrawal and body image disorders, which can further contribute to higher risk for depression and social anxiety,” the paper’s authors wrote.
Dr. Desai reported no financial disclosures relevant to his talk.
CHICAGO –
Dr. Desai, clinical assistant professor in the department of dermatology at the University of Texas Southwestern Medical Center, Dallas, spoke at the Pigmentary Disorders Exchange Symposium, provided by MedscapeLive!
Like all dermatologists, he said at the meeting, he sees lots of acne cases. However, PIH is often the presenting reason for the visit in his practice, which focuses predominantly on skin of color.
“Most of my patients come in not even worried about the acne,” he said. “They come in wanting me to fix the dark spots.”
Inflammation persists
Dermatologists, Dr. Desai said, should educate patients with active PIH resulting from acne or other diseases that even though the condition has been labeled post- inflammatory hyperpigmentation, the inflammation continues to be a problem.
He said, while patients may think PIH is “just scars,” the inflammation is still active and the condition needs to be treated from a skin-lightening perspective but, more importantly, with a focus on halting the inflammation. “If you were to biopsy the areas of hyperpigmentation, you would find a high density of active inflammatory behaviors still present in the skin,” he said.
When treating patients, it’s critical to first treat the underlying skin condition aggressively, he said. “Things like topical retinoids and azelaic acid mechanistically actually make a lot more sense for PIH than even hydroquinone, in some cases, because these therapies are actually anti-inflammatory for many of the diseases we treat.”
Dr. Desai noted that, in patients with darker skin tones, even diseases like seborrheic dermatitis and plaque psoriasis can result in PIH, while in patients with lighter skin tones, the same diseases may leave some residual postinflammatory erythema.
“I think it’s very important, particularly when you’re treating a darker skin–toned patient, to arrest the erythema early on to prevent that further worsening of hyperpigmentation,” he said.
Biopsies important
In cases of PIH, determining the best treatment requires finding out where the pigment is and how deep it is, Dr. Desai said.
He noted dermatologists are often worried about doing biopsies, particularly in patients with darker skin, because of the risk of scarring and keloid formation for those more prone to keloids. The preference is also for a therapeutic effect without using invasive procedures.
“But particularly with PIH, in patients who have been therapeutically challenging, I don’t hesitate to do very small biopsies – 2- and 3-mm punch biopsies – particularly if they are from the head and neck area.”
He suggests doing biopsies on part of the ear, lower jaw line, or the neck area, as these areas tend to heal nicely. “You don’t have to be so concerned about the scarring if you counsel appropriately,” he said.
The biopsy can be valuable in determining whether a very expensive treatment will reach the intended target.
Topical retinoids play an important role as anti-inflammatories for PIH, Dr. Desai said.
He gave an example of a patient with Fitzpatrick skin type IV or V with chronic acne and extensive PIH. “Are you going to effectively tell that patient to apply 4% hydroquinone triple-combination compound across 30 different areas of PIH on their face? The answer is that’s really not very efficient or effective.”
That’s why therapies, such as retinoids, that target the pathogenesis of PIH, particularly the inflammatory component, are important, he added.
Psychological burden
PIH comes with significant stigma and loss of quality of life loss that can last many years.
During another presentation at the meeting, Susan C. Taylor, MD, professor and vice chair of diversity, equity and inclusion in the department of dermatology, at the University of Pennsylvania, Philadelphia, pointed out that in a 2016 study of 324 patients in seven Asian countries, acne-related PIH lasted longer than 1 year in 65.2% of patients and 5 years or longer in 22.3%, significantly affecting their quality of life.
Dr. Desai added that, in a paper recently published in the British Journal of Dermatology, on the impact of postacne hyperpigmentation in patients, the authors pointed out that the reported prevalence of PIH in patients with acne ranges between 45.5% and 87.2%, depending on skin phototype, and that in most cases, PIH takes more than a year to fade.
“Studies have demonstrated that patients with acne and resulting scarring often face stigmatization, leading to quality of life impairment, social withdrawal and body image disorders, which can further contribute to higher risk for depression and social anxiety,” the paper’s authors wrote.
Dr. Desai reported no financial disclosures relevant to his talk.
CHICAGO –
Dr. Desai, clinical assistant professor in the department of dermatology at the University of Texas Southwestern Medical Center, Dallas, spoke at the Pigmentary Disorders Exchange Symposium, provided by MedscapeLive!
Like all dermatologists, he said at the meeting, he sees lots of acne cases. However, PIH is often the presenting reason for the visit in his practice, which focuses predominantly on skin of color.
“Most of my patients come in not even worried about the acne,” he said. “They come in wanting me to fix the dark spots.”
Inflammation persists
Dermatologists, Dr. Desai said, should educate patients with active PIH resulting from acne or other diseases that even though the condition has been labeled post- inflammatory hyperpigmentation, the inflammation continues to be a problem.
He said, while patients may think PIH is “just scars,” the inflammation is still active and the condition needs to be treated from a skin-lightening perspective but, more importantly, with a focus on halting the inflammation. “If you were to biopsy the areas of hyperpigmentation, you would find a high density of active inflammatory behaviors still present in the skin,” he said.
When treating patients, it’s critical to first treat the underlying skin condition aggressively, he said. “Things like topical retinoids and azelaic acid mechanistically actually make a lot more sense for PIH than even hydroquinone, in some cases, because these therapies are actually anti-inflammatory for many of the diseases we treat.”
Dr. Desai noted that, in patients with darker skin tones, even diseases like seborrheic dermatitis and plaque psoriasis can result in PIH, while in patients with lighter skin tones, the same diseases may leave some residual postinflammatory erythema.
“I think it’s very important, particularly when you’re treating a darker skin–toned patient, to arrest the erythema early on to prevent that further worsening of hyperpigmentation,” he said.
Biopsies important
In cases of PIH, determining the best treatment requires finding out where the pigment is and how deep it is, Dr. Desai said.
He noted dermatologists are often worried about doing biopsies, particularly in patients with darker skin, because of the risk of scarring and keloid formation for those more prone to keloids. The preference is also for a therapeutic effect without using invasive procedures.
“But particularly with PIH, in patients who have been therapeutically challenging, I don’t hesitate to do very small biopsies – 2- and 3-mm punch biopsies – particularly if they are from the head and neck area.”
He suggests doing biopsies on part of the ear, lower jaw line, or the neck area, as these areas tend to heal nicely. “You don’t have to be so concerned about the scarring if you counsel appropriately,” he said.
The biopsy can be valuable in determining whether a very expensive treatment will reach the intended target.
Topical retinoids play an important role as anti-inflammatories for PIH, Dr. Desai said.
He gave an example of a patient with Fitzpatrick skin type IV or V with chronic acne and extensive PIH. “Are you going to effectively tell that patient to apply 4% hydroquinone triple-combination compound across 30 different areas of PIH on their face? The answer is that’s really not very efficient or effective.”
That’s why therapies, such as retinoids, that target the pathogenesis of PIH, particularly the inflammatory component, are important, he added.
Psychological burden
PIH comes with significant stigma and loss of quality of life loss that can last many years.
During another presentation at the meeting, Susan C. Taylor, MD, professor and vice chair of diversity, equity and inclusion in the department of dermatology, at the University of Pennsylvania, Philadelphia, pointed out that in a 2016 study of 324 patients in seven Asian countries, acne-related PIH lasted longer than 1 year in 65.2% of patients and 5 years or longer in 22.3%, significantly affecting their quality of life.
Dr. Desai added that, in a paper recently published in the British Journal of Dermatology, on the impact of postacne hyperpigmentation in patients, the authors pointed out that the reported prevalence of PIH in patients with acne ranges between 45.5% and 87.2%, depending on skin phototype, and that in most cases, PIH takes more than a year to fade.
“Studies have demonstrated that patients with acne and resulting scarring often face stigmatization, leading to quality of life impairment, social withdrawal and body image disorders, which can further contribute to higher risk for depression and social anxiety,” the paper’s authors wrote.
Dr. Desai reported no financial disclosures relevant to his talk.
AT THE MEDSCAPELIVE! PIGMENTARY DISORDERS SYMPOSIUM
Macular dermal hyperpigmentation: Treatment tips from an expert
CHICAGO – based on cases she has treated in her practice.
Heather Woolery-Lloyd, MD, director of the skin of color division in the dermatology department at University of Miami, provided three general pointers.
- When in doubt, biopsy.
- For inflammatory disorders, always treat the inflammation in addition to the hyperpigmentation.
- Avoid long-term hydroquinone use in these patients.
Dr. Woolery-Lloyd also reviewed examples of what she has found successful in treating her patients with these conditions.
Lichen planus pigmentosus (LPP)
“It’s one of the hardest things that we treat,” said Dr. Woolery-Lloyd, who often sees cases of LPP in patients in their 30s, 40s, and 50s.
Lesions first appear as small, ill-defined oval-to-round macules, which later become confluent and form large areas of pigmentation. In different patients, the pigment on the face and neck, and sometimes on the forearms can be slate gray or brownish black.
In 2013, dermatologist N.C. Dlova, MD, at the University of KwaZulu‐Natal, Durban, South Africa, reported a link between frontal fibrosing alopecia and LPP in the British Journal of Dermatology. “I definitely see this connection in my practice,” said Dr. Woolery-Lloyd, noting that “both conditions often result in the loss of both eyebrows.”
She recommends always using a topical anti-inflammatory that is safe for the face. One combination she uses is azelaic acid 20% plus hydrocortisone 2.5%.
“We do use a lot of azelaic acid in my practice because it’s affordable,” she said, at the meeting, provided by MedscapeLive! She added that the hardest area to treat in women is around the chin.
Two other conditions, ashy dermatosis and erythema dyschromicum perstans (EDP), are similar. Ashy dermatosis mimics LPP but occurs more prominently on the trunk and extremities. EDP often has a preceding ring of erythema.
Dr. Woolery-Lloyd said the term EDP is often used to cover both EDP and ashy dermatosis in North America because “ashy” can have a negative connotation.
She noted there is no consensus on effective therapy for LPP, ashy dermatosis, or EDP.
A review of the literature on EDP, which included 16 studies on treatment outcomes, found the following:
- Narrow-band ultraviolet B and tacrolimus were effective treatments with minimal side effects.
- Clofazimine was effective, but had side effects, which, ironically, included pigmentary changes.
- Griseofulvin, isotretinoin, and dapsone were comparatively ineffective as lesions recurred after discontinuation.
- Lasers were largely ineffective and can also result in postinflammatory hyperpigmentation and fibrosis.
Ochronosis
Dr. Woolery-Lloyd said she may see one to two patients a year with ochronosis, which is characterized by paradoxical darkening of the skin with long-term hydroquinone use. It usually starts with redness followed by blue-black patches on the face where hydroquinone is applied. In severe cases, blue-black papules and nodules can occur.
“When I give a patient hydroquinone, I always say: ‘I don’t want to see any redness,’” Dr. Woolery-Lloyd said. “If you have any redness, please stop because ochronosis is typically preceded by this redness.”
But, she noted, “people will come in actively using hydroquinone, will have the dark brown or deep black papules or macules on their face, and then this background of redness because they are so inflamed.”
She said that ochronosis can occur in any skin type, not just in patients with darker skin tones. Dr. Woolery-Lloyd advised: “Do not hesitate to biopsy the face if ochronosis is suspected. I always biopsy ochronosis.”
There are two reasons for doing so, she explained. It can help with the diagnosis but it will also provide the patient with an incentive to stop using hydroquinone. “People who are using hydroquinone are addicted to it. They love it. They don’t want to stop. They keep using it despite the fact that their face is getting darker.” When they see a biopsy report, they may be convinced to stop.
Dr. Woolery-Lloyd said she does a 2-mm punch biopsy in the crow’s feet area because there’s almost always ochronosis in that area and it does not leave an obvious scar.
Eventually, she said, if the person stops using hydroquinone, it will clear up, “but it will take years.” Again, here she has had success with her “special formula” of azelaic acid 20% plus hydrocortisone 2.5%
“Don’t tell patients there’s no treatment. That’s the take-home,” she said.
Drug-induced facial hyperpigmentation
“I see this all the time in my African American patients,” Dr. Woolery-Lloyd said. The condition usually is characterized by dark brown hyperpigmentation on the face.
In this situation, the first question to ask is whether the patient is taking medication for hypertension, and the second question is whether it is “HCTZ.” It’s important to use the abbreviation for hydrochlorothiazide – the most common cause of drug-induced facial hyperpigmentation – because that’s what a patient sees on the bottle.
If they are taking HCTZ or another blood pressure medication associated with photosensitivity, they need to switch to a nonphotosensitizing antihypertensive agent (there are several options) and they should start treatment with a topical anti-inflammatory, Dr. Woolery-Lloyd said. Then, she suggests introducing hydrocortisone 2.5% cream and a hydroquinone-free skin brightener (azelaic acid, for example).
Importantly, with any of these conditions, Dr Woolery-Lloyd said, dermatologists should talk with patients about realistic expectations. “It takes a long time for dermal pigment to clear,” she emphasized.
Dr. Woolery-Lloyd has been a speaker for Ortho Dermatologics, L’Oreal, and EPI; has done research for Pfizer, Galderma, Allergan, Arcutis, Vyne, Merz, and Eirion; and has been on advisory boards for L’Oreal, Allergan, Ortho Dermatologics, Pfizer, and Merz.
CHICAGO – based on cases she has treated in her practice.
Heather Woolery-Lloyd, MD, director of the skin of color division in the dermatology department at University of Miami, provided three general pointers.
- When in doubt, biopsy.
- For inflammatory disorders, always treat the inflammation in addition to the hyperpigmentation.
- Avoid long-term hydroquinone use in these patients.
Dr. Woolery-Lloyd also reviewed examples of what she has found successful in treating her patients with these conditions.
Lichen planus pigmentosus (LPP)
“It’s one of the hardest things that we treat,” said Dr. Woolery-Lloyd, who often sees cases of LPP in patients in their 30s, 40s, and 50s.
Lesions first appear as small, ill-defined oval-to-round macules, which later become confluent and form large areas of pigmentation. In different patients, the pigment on the face and neck, and sometimes on the forearms can be slate gray or brownish black.
In 2013, dermatologist N.C. Dlova, MD, at the University of KwaZulu‐Natal, Durban, South Africa, reported a link between frontal fibrosing alopecia and LPP in the British Journal of Dermatology. “I definitely see this connection in my practice,” said Dr. Woolery-Lloyd, noting that “both conditions often result in the loss of both eyebrows.”
She recommends always using a topical anti-inflammatory that is safe for the face. One combination she uses is azelaic acid 20% plus hydrocortisone 2.5%.
“We do use a lot of azelaic acid in my practice because it’s affordable,” she said, at the meeting, provided by MedscapeLive! She added that the hardest area to treat in women is around the chin.
Two other conditions, ashy dermatosis and erythema dyschromicum perstans (EDP), are similar. Ashy dermatosis mimics LPP but occurs more prominently on the trunk and extremities. EDP often has a preceding ring of erythema.
Dr. Woolery-Lloyd said the term EDP is often used to cover both EDP and ashy dermatosis in North America because “ashy” can have a negative connotation.
She noted there is no consensus on effective therapy for LPP, ashy dermatosis, or EDP.
A review of the literature on EDP, which included 16 studies on treatment outcomes, found the following:
- Narrow-band ultraviolet B and tacrolimus were effective treatments with minimal side effects.
- Clofazimine was effective, but had side effects, which, ironically, included pigmentary changes.
- Griseofulvin, isotretinoin, and dapsone were comparatively ineffective as lesions recurred after discontinuation.
- Lasers were largely ineffective and can also result in postinflammatory hyperpigmentation and fibrosis.
Ochronosis
Dr. Woolery-Lloyd said she may see one to two patients a year with ochronosis, which is characterized by paradoxical darkening of the skin with long-term hydroquinone use. It usually starts with redness followed by blue-black patches on the face where hydroquinone is applied. In severe cases, blue-black papules and nodules can occur.
“When I give a patient hydroquinone, I always say: ‘I don’t want to see any redness,’” Dr. Woolery-Lloyd said. “If you have any redness, please stop because ochronosis is typically preceded by this redness.”
But, she noted, “people will come in actively using hydroquinone, will have the dark brown or deep black papules or macules on their face, and then this background of redness because they are so inflamed.”
She said that ochronosis can occur in any skin type, not just in patients with darker skin tones. Dr. Woolery-Lloyd advised: “Do not hesitate to biopsy the face if ochronosis is suspected. I always biopsy ochronosis.”
There are two reasons for doing so, she explained. It can help with the diagnosis but it will also provide the patient with an incentive to stop using hydroquinone. “People who are using hydroquinone are addicted to it. They love it. They don’t want to stop. They keep using it despite the fact that their face is getting darker.” When they see a biopsy report, they may be convinced to stop.
Dr. Woolery-Lloyd said she does a 2-mm punch biopsy in the crow’s feet area because there’s almost always ochronosis in that area and it does not leave an obvious scar.
Eventually, she said, if the person stops using hydroquinone, it will clear up, “but it will take years.” Again, here she has had success with her “special formula” of azelaic acid 20% plus hydrocortisone 2.5%
“Don’t tell patients there’s no treatment. That’s the take-home,” she said.
Drug-induced facial hyperpigmentation
“I see this all the time in my African American patients,” Dr. Woolery-Lloyd said. The condition usually is characterized by dark brown hyperpigmentation on the face.
In this situation, the first question to ask is whether the patient is taking medication for hypertension, and the second question is whether it is “HCTZ.” It’s important to use the abbreviation for hydrochlorothiazide – the most common cause of drug-induced facial hyperpigmentation – because that’s what a patient sees on the bottle.
If they are taking HCTZ or another blood pressure medication associated with photosensitivity, they need to switch to a nonphotosensitizing antihypertensive agent (there are several options) and they should start treatment with a topical anti-inflammatory, Dr. Woolery-Lloyd said. Then, she suggests introducing hydrocortisone 2.5% cream and a hydroquinone-free skin brightener (azelaic acid, for example).
Importantly, with any of these conditions, Dr Woolery-Lloyd said, dermatologists should talk with patients about realistic expectations. “It takes a long time for dermal pigment to clear,” she emphasized.
Dr. Woolery-Lloyd has been a speaker for Ortho Dermatologics, L’Oreal, and EPI; has done research for Pfizer, Galderma, Allergan, Arcutis, Vyne, Merz, and Eirion; and has been on advisory boards for L’Oreal, Allergan, Ortho Dermatologics, Pfizer, and Merz.
CHICAGO – based on cases she has treated in her practice.
Heather Woolery-Lloyd, MD, director of the skin of color division in the dermatology department at University of Miami, provided three general pointers.
- When in doubt, biopsy.
- For inflammatory disorders, always treat the inflammation in addition to the hyperpigmentation.
- Avoid long-term hydroquinone use in these patients.
Dr. Woolery-Lloyd also reviewed examples of what she has found successful in treating her patients with these conditions.
Lichen planus pigmentosus (LPP)
“It’s one of the hardest things that we treat,” said Dr. Woolery-Lloyd, who often sees cases of LPP in patients in their 30s, 40s, and 50s.
Lesions first appear as small, ill-defined oval-to-round macules, which later become confluent and form large areas of pigmentation. In different patients, the pigment on the face and neck, and sometimes on the forearms can be slate gray or brownish black.
In 2013, dermatologist N.C. Dlova, MD, at the University of KwaZulu‐Natal, Durban, South Africa, reported a link between frontal fibrosing alopecia and LPP in the British Journal of Dermatology. “I definitely see this connection in my practice,” said Dr. Woolery-Lloyd, noting that “both conditions often result in the loss of both eyebrows.”
She recommends always using a topical anti-inflammatory that is safe for the face. One combination she uses is azelaic acid 20% plus hydrocortisone 2.5%.
“We do use a lot of azelaic acid in my practice because it’s affordable,” she said, at the meeting, provided by MedscapeLive! She added that the hardest area to treat in women is around the chin.
Two other conditions, ashy dermatosis and erythema dyschromicum perstans (EDP), are similar. Ashy dermatosis mimics LPP but occurs more prominently on the trunk and extremities. EDP often has a preceding ring of erythema.
Dr. Woolery-Lloyd said the term EDP is often used to cover both EDP and ashy dermatosis in North America because “ashy” can have a negative connotation.
She noted there is no consensus on effective therapy for LPP, ashy dermatosis, or EDP.
A review of the literature on EDP, which included 16 studies on treatment outcomes, found the following:
- Narrow-band ultraviolet B and tacrolimus were effective treatments with minimal side effects.
- Clofazimine was effective, but had side effects, which, ironically, included pigmentary changes.
- Griseofulvin, isotretinoin, and dapsone were comparatively ineffective as lesions recurred after discontinuation.
- Lasers were largely ineffective and can also result in postinflammatory hyperpigmentation and fibrosis.
Ochronosis
Dr. Woolery-Lloyd said she may see one to two patients a year with ochronosis, which is characterized by paradoxical darkening of the skin with long-term hydroquinone use. It usually starts with redness followed by blue-black patches on the face where hydroquinone is applied. In severe cases, blue-black papules and nodules can occur.
“When I give a patient hydroquinone, I always say: ‘I don’t want to see any redness,’” Dr. Woolery-Lloyd said. “If you have any redness, please stop because ochronosis is typically preceded by this redness.”
But, she noted, “people will come in actively using hydroquinone, will have the dark brown or deep black papules or macules on their face, and then this background of redness because they are so inflamed.”
She said that ochronosis can occur in any skin type, not just in patients with darker skin tones. Dr. Woolery-Lloyd advised: “Do not hesitate to biopsy the face if ochronosis is suspected. I always biopsy ochronosis.”
There are two reasons for doing so, she explained. It can help with the diagnosis but it will also provide the patient with an incentive to stop using hydroquinone. “People who are using hydroquinone are addicted to it. They love it. They don’t want to stop. They keep using it despite the fact that their face is getting darker.” When they see a biopsy report, they may be convinced to stop.
Dr. Woolery-Lloyd said she does a 2-mm punch biopsy in the crow’s feet area because there’s almost always ochronosis in that area and it does not leave an obvious scar.
Eventually, she said, if the person stops using hydroquinone, it will clear up, “but it will take years.” Again, here she has had success with her “special formula” of azelaic acid 20% plus hydrocortisone 2.5%
“Don’t tell patients there’s no treatment. That’s the take-home,” she said.
Drug-induced facial hyperpigmentation
“I see this all the time in my African American patients,” Dr. Woolery-Lloyd said. The condition usually is characterized by dark brown hyperpigmentation on the face.
In this situation, the first question to ask is whether the patient is taking medication for hypertension, and the second question is whether it is “HCTZ.” It’s important to use the abbreviation for hydrochlorothiazide – the most common cause of drug-induced facial hyperpigmentation – because that’s what a patient sees on the bottle.
If they are taking HCTZ or another blood pressure medication associated with photosensitivity, they need to switch to a nonphotosensitizing antihypertensive agent (there are several options) and they should start treatment with a topical anti-inflammatory, Dr. Woolery-Lloyd said. Then, she suggests introducing hydrocortisone 2.5% cream and a hydroquinone-free skin brightener (azelaic acid, for example).
Importantly, with any of these conditions, Dr Woolery-Lloyd said, dermatologists should talk with patients about realistic expectations. “It takes a long time for dermal pigment to clear,” she emphasized.
Dr. Woolery-Lloyd has been a speaker for Ortho Dermatologics, L’Oreal, and EPI; has done research for Pfizer, Galderma, Allergan, Arcutis, Vyne, Merz, and Eirion; and has been on advisory boards for L’Oreal, Allergan, Ortho Dermatologics, Pfizer, and Merz.
AT THE MEDSCAPELIVE! PIGMENTARY DISORDERS SYMPOSIUM
Tips, contraindications for superficial chemical peels reviewed
CHICAGO – Heather Woolery-Lloyd, MD, says she’s generally “risk averse,” but when it comes to superficial chemical peels, she’s in her comfort zone.
Superficial peeling is “one of the most common cosmetic procedures that I do,” Dr. Woolery-Lloyd, director of the skin of color division in the dermatology department at the University of Miami, said at the Pigmentary Disorders Exchange Symposium.
In her practice, .
Contraindications are an active bacterial infection, open wounds, and active herpes simplex virus. “If someone looks like they even have a remnant of a cold sore, I tell them to come back,” she said.
Setting expectations for patients is critical, Dr. Woolery-Lloyd said, as a series of superficial peels is needed before the desired results are evident.
The peel she uses most is salicylic acid, a beta-hydroxy acid, at a strength of 20%-30%. “It’s very effective on our acne patients,” she said at the meeting, provided by MedscapeLIVE! “If you’re just starting with peels, I think this is a very safe one. You don’t have to time it, and you don’t have to neutralize it,” and at lower concentrations, is “very safe.”
Dr. Woolery-Lloyd provided these other tips during her presentation:
- Even superficial peels can be uncomfortable, she noted, so she keeps a fan nearby to use when needed to help with discomfort.
- Find the peel you’re comfortable with, master that peel, and don’t jump from peel to peel. Get familiar with the side effects and how to predict results.
- Stop retinoids up to 7 days before a peel. Consider placing the patient on hydroquinone before the chemical peel to decrease the risk of hyperpigmentation.
- Before the procedure, prep the skin with acetone or alcohol. Applying petrolatum helps protect around the eyes, alar crease, and other sensitive areas, “or anywhere you’re concerned about the depth of the peel.”
- Application with rough gauze helps avoid the waste that comes with makeup sponges soaking up the product. It also helps add exfoliation.
- Have everything ready before starting the procedure, including (depending on the peel), a neutralizer or soapless cleanser. Although peels are generally safe, you want to be able to remove one quickly, if needed, without having to leave the room.
- Start with the lowest concentration (salicylic acid or glycolic acid) then titrate up. Ask patients about any reactions they experienced with the previous peel before making the decision on the next concentration.
- For a peel to treat hyperpigmentation, she recommends one peel about every 4 weeks for a series of 5-6 peels.
- After a peel, the patient should use a mineral sunscreen; chemical sunscreens will sting.
Know your comfort zone
Conference chair Pearl Grimes, MD, director of The Vitiligo & Pigmentation Institute of Southern California in Los Angeles, said superficial peels are best for dermatologists new to peeling until they gain comfort with experience.
Superficial and medium-depth peels work well for mild to moderate photoaging, she said at the meeting.
“We know that in darker skin we have more intrinsic aging rather than photoaging. We have more textural changes, hyperpigmentation,” Dr. Grimes said.
For Fitzpatrick skin types I-III, she said, “you can do superficial, medium, and deep peels.” For darker skin types, “I typically stay in the superficial, medium range.”
She said that she uses retinoids to exfoliate before a superficial peel but added, “you’ve got to stop them early because retinoids can make a superficial peel a medium-depth peel.”
Taking photos is important before any procedure, she said, as is spending time with patients clarifying their outcome expectations.
“I love peeling,” Dr. Grimes said. “And it’s cost effective. If you don’t want to spend a ton of money, it’s amazing what you can achieve with chemical peeling.”
When asked by a member of the audience whether they avoid superficial peels in women who are pregnant or breastfeeding, both Dr. Woolery-Lloyd and Dr. Grimes said they do avoid them in those patients.
Dr. Grimes said she tells her patients, especially in the first trimester, “I am the most conservative woman on the planet. I do nothing during the first trimester.”
Dr. Woolery-Lloyd has been a speaker for Ortho Dermatologics, Loreal and EPI, and has done research for Pfizer, Galderma, Allergan, Arcutis, Vyne, Merz, and Eirion. She has been on advisory boards for Loreal, Allergan, Ortho Dermatologics, Pfize,r and Merz. Dr. Grimes reports grant/research Support from Clinuvel Pharmaceuticals, Incyte, Johnson & Johnson, LASEROPTEK, L’Oréal USA, Pfizer, Procter & Gamble, skinbetter science, and Versicolor Technologies, and is on the speakers bureau/receives honoraria for non-CME for Incyte and Procter & Gamble; and is a consultant or is on the advisory board for L’Oréal USA and Procter & Gamble. She has stock options in Versicolor Technologies.
CHICAGO – Heather Woolery-Lloyd, MD, says she’s generally “risk averse,” but when it comes to superficial chemical peels, she’s in her comfort zone.
Superficial peeling is “one of the most common cosmetic procedures that I do,” Dr. Woolery-Lloyd, director of the skin of color division in the dermatology department at the University of Miami, said at the Pigmentary Disorders Exchange Symposium.
In her practice, .
Contraindications are an active bacterial infection, open wounds, and active herpes simplex virus. “If someone looks like they even have a remnant of a cold sore, I tell them to come back,” she said.
Setting expectations for patients is critical, Dr. Woolery-Lloyd said, as a series of superficial peels is needed before the desired results are evident.
The peel she uses most is salicylic acid, a beta-hydroxy acid, at a strength of 20%-30%. “It’s very effective on our acne patients,” she said at the meeting, provided by MedscapeLIVE! “If you’re just starting with peels, I think this is a very safe one. You don’t have to time it, and you don’t have to neutralize it,” and at lower concentrations, is “very safe.”
Dr. Woolery-Lloyd provided these other tips during her presentation:
- Even superficial peels can be uncomfortable, she noted, so she keeps a fan nearby to use when needed to help with discomfort.
- Find the peel you’re comfortable with, master that peel, and don’t jump from peel to peel. Get familiar with the side effects and how to predict results.
- Stop retinoids up to 7 days before a peel. Consider placing the patient on hydroquinone before the chemical peel to decrease the risk of hyperpigmentation.
- Before the procedure, prep the skin with acetone or alcohol. Applying petrolatum helps protect around the eyes, alar crease, and other sensitive areas, “or anywhere you’re concerned about the depth of the peel.”
- Application with rough gauze helps avoid the waste that comes with makeup sponges soaking up the product. It also helps add exfoliation.
- Have everything ready before starting the procedure, including (depending on the peel), a neutralizer or soapless cleanser. Although peels are generally safe, you want to be able to remove one quickly, if needed, without having to leave the room.
- Start with the lowest concentration (salicylic acid or glycolic acid) then titrate up. Ask patients about any reactions they experienced with the previous peel before making the decision on the next concentration.
- For a peel to treat hyperpigmentation, she recommends one peel about every 4 weeks for a series of 5-6 peels.
- After a peel, the patient should use a mineral sunscreen; chemical sunscreens will sting.
Know your comfort zone
Conference chair Pearl Grimes, MD, director of The Vitiligo & Pigmentation Institute of Southern California in Los Angeles, said superficial peels are best for dermatologists new to peeling until they gain comfort with experience.
Superficial and medium-depth peels work well for mild to moderate photoaging, she said at the meeting.
“We know that in darker skin we have more intrinsic aging rather than photoaging. We have more textural changes, hyperpigmentation,” Dr. Grimes said.
For Fitzpatrick skin types I-III, she said, “you can do superficial, medium, and deep peels.” For darker skin types, “I typically stay in the superficial, medium range.”
She said that she uses retinoids to exfoliate before a superficial peel but added, “you’ve got to stop them early because retinoids can make a superficial peel a medium-depth peel.”
Taking photos is important before any procedure, she said, as is spending time with patients clarifying their outcome expectations.
“I love peeling,” Dr. Grimes said. “And it’s cost effective. If you don’t want to spend a ton of money, it’s amazing what you can achieve with chemical peeling.”
When asked by a member of the audience whether they avoid superficial peels in women who are pregnant or breastfeeding, both Dr. Woolery-Lloyd and Dr. Grimes said they do avoid them in those patients.
Dr. Grimes said she tells her patients, especially in the first trimester, “I am the most conservative woman on the planet. I do nothing during the first trimester.”
Dr. Woolery-Lloyd has been a speaker for Ortho Dermatologics, Loreal and EPI, and has done research for Pfizer, Galderma, Allergan, Arcutis, Vyne, Merz, and Eirion. She has been on advisory boards for Loreal, Allergan, Ortho Dermatologics, Pfize,r and Merz. Dr. Grimes reports grant/research Support from Clinuvel Pharmaceuticals, Incyte, Johnson & Johnson, LASEROPTEK, L’Oréal USA, Pfizer, Procter & Gamble, skinbetter science, and Versicolor Technologies, and is on the speakers bureau/receives honoraria for non-CME for Incyte and Procter & Gamble; and is a consultant or is on the advisory board for L’Oréal USA and Procter & Gamble. She has stock options in Versicolor Technologies.
CHICAGO – Heather Woolery-Lloyd, MD, says she’s generally “risk averse,” but when it comes to superficial chemical peels, she’s in her comfort zone.
Superficial peeling is “one of the most common cosmetic procedures that I do,” Dr. Woolery-Lloyd, director of the skin of color division in the dermatology department at the University of Miami, said at the Pigmentary Disorders Exchange Symposium.
In her practice, .
Contraindications are an active bacterial infection, open wounds, and active herpes simplex virus. “If someone looks like they even have a remnant of a cold sore, I tell them to come back,” she said.
Setting expectations for patients is critical, Dr. Woolery-Lloyd said, as a series of superficial peels is needed before the desired results are evident.
The peel she uses most is salicylic acid, a beta-hydroxy acid, at a strength of 20%-30%. “It’s very effective on our acne patients,” she said at the meeting, provided by MedscapeLIVE! “If you’re just starting with peels, I think this is a very safe one. You don’t have to time it, and you don’t have to neutralize it,” and at lower concentrations, is “very safe.”
Dr. Woolery-Lloyd provided these other tips during her presentation:
- Even superficial peels can be uncomfortable, she noted, so she keeps a fan nearby to use when needed to help with discomfort.
- Find the peel you’re comfortable with, master that peel, and don’t jump from peel to peel. Get familiar with the side effects and how to predict results.
- Stop retinoids up to 7 days before a peel. Consider placing the patient on hydroquinone before the chemical peel to decrease the risk of hyperpigmentation.
- Before the procedure, prep the skin with acetone or alcohol. Applying petrolatum helps protect around the eyes, alar crease, and other sensitive areas, “or anywhere you’re concerned about the depth of the peel.”
- Application with rough gauze helps avoid the waste that comes with makeup sponges soaking up the product. It also helps add exfoliation.
- Have everything ready before starting the procedure, including (depending on the peel), a neutralizer or soapless cleanser. Although peels are generally safe, you want to be able to remove one quickly, if needed, without having to leave the room.
- Start with the lowest concentration (salicylic acid or glycolic acid) then titrate up. Ask patients about any reactions they experienced with the previous peel before making the decision on the next concentration.
- For a peel to treat hyperpigmentation, she recommends one peel about every 4 weeks for a series of 5-6 peels.
- After a peel, the patient should use a mineral sunscreen; chemical sunscreens will sting.
Know your comfort zone
Conference chair Pearl Grimes, MD, director of The Vitiligo & Pigmentation Institute of Southern California in Los Angeles, said superficial peels are best for dermatologists new to peeling until they gain comfort with experience.
Superficial and medium-depth peels work well for mild to moderate photoaging, she said at the meeting.
“We know that in darker skin we have more intrinsic aging rather than photoaging. We have more textural changes, hyperpigmentation,” Dr. Grimes said.
For Fitzpatrick skin types I-III, she said, “you can do superficial, medium, and deep peels.” For darker skin types, “I typically stay in the superficial, medium range.”
She said that she uses retinoids to exfoliate before a superficial peel but added, “you’ve got to stop them early because retinoids can make a superficial peel a medium-depth peel.”
Taking photos is important before any procedure, she said, as is spending time with patients clarifying their outcome expectations.
“I love peeling,” Dr. Grimes said. “And it’s cost effective. If you don’t want to spend a ton of money, it’s amazing what you can achieve with chemical peeling.”
When asked by a member of the audience whether they avoid superficial peels in women who are pregnant or breastfeeding, both Dr. Woolery-Lloyd and Dr. Grimes said they do avoid them in those patients.
Dr. Grimes said she tells her patients, especially in the first trimester, “I am the most conservative woman on the planet. I do nothing during the first trimester.”
Dr. Woolery-Lloyd has been a speaker for Ortho Dermatologics, Loreal and EPI, and has done research for Pfizer, Galderma, Allergan, Arcutis, Vyne, Merz, and Eirion. She has been on advisory boards for Loreal, Allergan, Ortho Dermatologics, Pfize,r and Merz. Dr. Grimes reports grant/research Support from Clinuvel Pharmaceuticals, Incyte, Johnson & Johnson, LASEROPTEK, L’Oréal USA, Pfizer, Procter & Gamble, skinbetter science, and Versicolor Technologies, and is on the speakers bureau/receives honoraria for non-CME for Incyte and Procter & Gamble; and is a consultant or is on the advisory board for L’Oréal USA and Procter & Gamble. She has stock options in Versicolor Technologies.
AT THE MEDSCAPE LIVE! PIGMENTARY DISORDERS SYMPOSIUM
Acute diffuse rash on trunk
This patient’s diffusely erythematous and scaly rash, in association with recent antibiotic use, was a classic presentation of a drug eruption. Drug eruptions are adverse cutaneous reactions to various medications; they frequently involve antibiotics and anti-epileptics. They can manifest in a multitude of ways with different morphologies. Medication history and timing to onset of symptoms are paramount in making the diagnosis.
Classic reactions include those that are morbilliform (erythematous macules and papules), lichenoid (violaceous and hyperpigmented papules), exfoliative/erythrodermic, and/or urticarial.1 Petechiae and palpable purpura may also manifest.1 Severe reactions, while less common, must always be considered, given their significant morbidity and mortality. These include2:
- Stevens-Johnson syndrome/toxic epidermal necrolysis with diffuse erythema and areas of denuded, necrotic epidermis,
- Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, and
- Acute, generalized, exanthematous pustulosis (AGEP) consisting of confluent, nonfollicular pustules.
A general principle in the management of drug eruptions is the discontinuation of the offending drug (if known) as soon as possible. If the agent is not known, it is important to discontinue all drugs that are not deemed as essential, particularly medications that are often associated with reactions, such as antibiotics and anti-epileptics. Additionally, evaluation of the oral mucosa, eyes, and genitourinary tract is helpful to diagnose Stevens-Johnson syndrome, if indicated by symptoms or history.
Wound care with cleansing and covering of denuded skin with emollients and wet dressings should be performed. Infections are common complications in these patients due to the increased inflammation, fissuring, and excoriations that accompany the rash, with sepsis from staphylococcal bacteria being the most concerning complication of infection. Additionally, the compromised skin barrier may lead to heat loss and hypothermia, a compensatory hypermetabolism with hyperthermia, and electrolyte imbalances from insensible water losses.2
Most mild eruptions can be treated with topical corticosteroids and antihistamines. However, in severe eruptions, systemic corticosteroids, or referral for immunosuppressive and anticytokine therapies, also should be considered.1
This patient was treated with both a short course of systemic corticosteroids (prednisone 40 mg/d for 5 days, then tapered over 15 days) and topical steroids (triamcinolone 0.1% ointment bid) for symptomatic care. He also was started on an antihistamine (cetirizine 10 mg bid) for itching. Doxycycline and Augmentin were added to his allergy list. At a 1-week follow up, the patient had near resolution of his rash.
Images courtesy of Jose L. Cortez, MD. Text courtesy of Jose L. Cortez, MD, Department of Dermatology, University of New Mexico School of Medicine, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Riedl MA, Casillas AM. Adverse drug reactions: types and treatment options. Am Fam Physician. 2003;68:1781-1790.
2. Zhang J, Lei Z, Xu C, et al. Current perspectives on severe drug eruption. Clin Rev Allergy Immunol. 2021;61:282-298. doi: 10.1007/s12016-021-08859-0

This patient’s diffusely erythematous and scaly rash, in association with recent antibiotic use, was a classic presentation of a drug eruption. Drug eruptions are adverse cutaneous reactions to various medications; they frequently involve antibiotics and anti-epileptics. They can manifest in a multitude of ways with different morphologies. Medication history and timing to onset of symptoms are paramount in making the diagnosis.
Classic reactions include those that are morbilliform (erythematous macules and papules), lichenoid (violaceous and hyperpigmented papules), exfoliative/erythrodermic, and/or urticarial.1 Petechiae and palpable purpura may also manifest.1 Severe reactions, while less common, must always be considered, given their significant morbidity and mortality. These include2:
- Stevens-Johnson syndrome/toxic epidermal necrolysis with diffuse erythema and areas of denuded, necrotic epidermis,
- Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, and
- Acute, generalized, exanthematous pustulosis (AGEP) consisting of confluent, nonfollicular pustules.
A general principle in the management of drug eruptions is the discontinuation of the offending drug (if known) as soon as possible. If the agent is not known, it is important to discontinue all drugs that are not deemed as essential, particularly medications that are often associated with reactions, such as antibiotics and anti-epileptics. Additionally, evaluation of the oral mucosa, eyes, and genitourinary tract is helpful to diagnose Stevens-Johnson syndrome, if indicated by symptoms or history.
Wound care with cleansing and covering of denuded skin with emollients and wet dressings should be performed. Infections are common complications in these patients due to the increased inflammation, fissuring, and excoriations that accompany the rash, with sepsis from staphylococcal bacteria being the most concerning complication of infection. Additionally, the compromised skin barrier may lead to heat loss and hypothermia, a compensatory hypermetabolism with hyperthermia, and electrolyte imbalances from insensible water losses.2
Most mild eruptions can be treated with topical corticosteroids and antihistamines. However, in severe eruptions, systemic corticosteroids, or referral for immunosuppressive and anticytokine therapies, also should be considered.1
This patient was treated with both a short course of systemic corticosteroids (prednisone 40 mg/d for 5 days, then tapered over 15 days) and topical steroids (triamcinolone 0.1% ointment bid) for symptomatic care. He also was started on an antihistamine (cetirizine 10 mg bid) for itching. Doxycycline and Augmentin were added to his allergy list. At a 1-week follow up, the patient had near resolution of his rash.
Images courtesy of Jose L. Cortez, MD. Text courtesy of Jose L. Cortez, MD, Department of Dermatology, University of New Mexico School of Medicine, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

This patient’s diffusely erythematous and scaly rash, in association with recent antibiotic use, was a classic presentation of a drug eruption. Drug eruptions are adverse cutaneous reactions to various medications; they frequently involve antibiotics and anti-epileptics. They can manifest in a multitude of ways with different morphologies. Medication history and timing to onset of symptoms are paramount in making the diagnosis.
Classic reactions include those that are morbilliform (erythematous macules and papules), lichenoid (violaceous and hyperpigmented papules), exfoliative/erythrodermic, and/or urticarial.1 Petechiae and palpable purpura may also manifest.1 Severe reactions, while less common, must always be considered, given their significant morbidity and mortality. These include2:
- Stevens-Johnson syndrome/toxic epidermal necrolysis with diffuse erythema and areas of denuded, necrotic epidermis,
- Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, and
- Acute, generalized, exanthematous pustulosis (AGEP) consisting of confluent, nonfollicular pustules.
A general principle in the management of drug eruptions is the discontinuation of the offending drug (if known) as soon as possible. If the agent is not known, it is important to discontinue all drugs that are not deemed as essential, particularly medications that are often associated with reactions, such as antibiotics and anti-epileptics. Additionally, evaluation of the oral mucosa, eyes, and genitourinary tract is helpful to diagnose Stevens-Johnson syndrome, if indicated by symptoms or history.
Wound care with cleansing and covering of denuded skin with emollients and wet dressings should be performed. Infections are common complications in these patients due to the increased inflammation, fissuring, and excoriations that accompany the rash, with sepsis from staphylococcal bacteria being the most concerning complication of infection. Additionally, the compromised skin barrier may lead to heat loss and hypothermia, a compensatory hypermetabolism with hyperthermia, and electrolyte imbalances from insensible water losses.2
Most mild eruptions can be treated with topical corticosteroids and antihistamines. However, in severe eruptions, systemic corticosteroids, or referral for immunosuppressive and anticytokine therapies, also should be considered.1
This patient was treated with both a short course of systemic corticosteroids (prednisone 40 mg/d for 5 days, then tapered over 15 days) and topical steroids (triamcinolone 0.1% ointment bid) for symptomatic care. He also was started on an antihistamine (cetirizine 10 mg bid) for itching. Doxycycline and Augmentin were added to his allergy list. At a 1-week follow up, the patient had near resolution of his rash.
Images courtesy of Jose L. Cortez, MD. Text courtesy of Jose L. Cortez, MD, Department of Dermatology, University of New Mexico School of Medicine, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Riedl MA, Casillas AM. Adverse drug reactions: types and treatment options. Am Fam Physician. 2003;68:1781-1790.
2. Zhang J, Lei Z, Xu C, et al. Current perspectives on severe drug eruption. Clin Rev Allergy Immunol. 2021;61:282-298. doi: 10.1007/s12016-021-08859-0
1. Riedl MA, Casillas AM. Adverse drug reactions: types and treatment options. Am Fam Physician. 2003;68:1781-1790.
2. Zhang J, Lei Z, Xu C, et al. Current perspectives on severe drug eruption. Clin Rev Allergy Immunol. 2021;61:282-298. doi: 10.1007/s12016-021-08859-0
Cell activity in psoriasis may predict disease severity and provide clues to comorbidities
The activity and clustering of certain cell types may distinguish mild and severe forms of psoriasis, with severe disease altering the cellular and metabolic composition of distal unaffected skin sites, according to a new analysis using single-cell transcriptomic technology.
On the surface, psoriasis severity is identified based on the visible lesions, Rochelle L. Castillo, MD, of the division of rheumatology and the NYU Psoriatic Arthritis Center, NYU Langone Health, New York, and colleagues wrote in their study, published in Science Immunology. Although cellular and molecular features of inflammatory skin diseases such as psoriasis have been characterized, activity at the tissue level and its systemic impact has not been explored.
“Our initial goal was to find measurable molecular signals that could tell us who is more likely to develop severe psoriasis, as well as who is at higher risk of developing related disorders that often accompany psoriasis, such as arthritis and cardiovascular disease,” study co–senior investigator Jose Scher, MD, director of the Psoriatic Arthritis Center and the Judith and Stewart Colton Center for Autoimmunity at NYU Langone Health, said in a press release accompanying the publication of the findings. “Having found signals with potential systemic consequences, we are now working to understand how skin inflammation can lead to widespread disease affecting other organs,”
In the study, the researchers used spatial transcriptomics, a technique that positions tissue sections onto genetic arrays to determine gene expression by cell type and histological location, helping to create a broad image-based map of where certain cell types are located in tissues and with what other cells they are communicating. They characterized the cell activity of skin samples from 11 men and women with mild to severe psoriasis/psoriatic arthritis, and three healthy adults who did not have psoriasis. They defined the cellular composition of 25 healthy skin biopsies and matched skin biopsies from psoriatic lesional and nonlesional skin, and identified 17 distinct clusters of cells, which they grouped into epidermal, dermis, pilosebaceous, and adipose categories.
The researchers found that cell activity associated with inflammation, as shown by clusters of fibroblasts and dermal macrophages, was more common in the upper layers of the skin in samples from patients with more severe psoriasis, compared with healthy control samples.
They also examined patterns of immune activity at the cellular level and found significant patterns around the upper follicle, around the perifollicular dermis, and within the hair follicle, where immune cells were enriched in healthy skin. Other cells enriched in these upper layer areas in healthy skin included dendritic cells, innate lymphoid cells, T helper cells, T cytotoxic cells, and myeloid cells.
Clusters of fibroblasts and macrophages, which are associated with inflammation, were clustered in psoriatic lesional skin, which also showed more inflammation at the dermal and suprabasal epidermal levels. B lymphocytes also were more prevalent in lesional skin.
The researchers then analyzed the skin samples according to disease severity; mild psoriasis was defined as a Psoriasis Area and Severity Index score less than 12; moderate to severe disease was defined as a PASI score of 12 or higher. The macrophage, fibroblast, and lymphatic endothelium–associated clusters distinguished mild and moderate to severe endotypes.
The pathology of moderate to severe psoriasis in lesional and nonlesional skin showed the extensive effects of psoriasis-related inflammation. Although nonlesional mild disease was clustered with healthy skin, in cases of moderate to severe disease, nonlesional and lesional groups were clustered together. This effect was segregated according to disease severity, independent of the presence of joint disease, and “was particularly evident in distal, nonlesional samples,” the researchers wrote.
The researchers also found evidence of increased gene activity in more than three dozen molecular pathways associated with metabolism and lipid levels in areas of lesional and nonlesional skin, Dr. Scher said.
The findings were limited by several factors including the small sample size and the limits of spatial transcriptomics technology resolution, the researchers wrote. “As this technology evolves, platforms with higher density, and by extension, resolution, of spatially barcoded beads will provide more granularity about cellular microenvironments in healthy and diseased states.”
The study was supported by the National Institutes of Health, the National Psoriasis Foundation, the NYU Colton Center for Autoimmunity, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis, the Beatrice Snyder Foundation, The Riley Family Foundation, the Rheumatology Research Foundation, and the NY Stem Cell Foundation. Dr. Castillo had no financial conflicts to disclose. Dr. Scher has served as a consultant for Janssen, Abbvie, Novartis, Pfizer, Sanofi, UCB, and Bristol-Myers Squibb, and has received research funding from Janssen and Pfizer.
The activity and clustering of certain cell types may distinguish mild and severe forms of psoriasis, with severe disease altering the cellular and metabolic composition of distal unaffected skin sites, according to a new analysis using single-cell transcriptomic technology.
On the surface, psoriasis severity is identified based on the visible lesions, Rochelle L. Castillo, MD, of the division of rheumatology and the NYU Psoriatic Arthritis Center, NYU Langone Health, New York, and colleagues wrote in their study, published in Science Immunology. Although cellular and molecular features of inflammatory skin diseases such as psoriasis have been characterized, activity at the tissue level and its systemic impact has not been explored.
“Our initial goal was to find measurable molecular signals that could tell us who is more likely to develop severe psoriasis, as well as who is at higher risk of developing related disorders that often accompany psoriasis, such as arthritis and cardiovascular disease,” study co–senior investigator Jose Scher, MD, director of the Psoriatic Arthritis Center and the Judith and Stewart Colton Center for Autoimmunity at NYU Langone Health, said in a press release accompanying the publication of the findings. “Having found signals with potential systemic consequences, we are now working to understand how skin inflammation can lead to widespread disease affecting other organs,”
In the study, the researchers used spatial transcriptomics, a technique that positions tissue sections onto genetic arrays to determine gene expression by cell type and histological location, helping to create a broad image-based map of where certain cell types are located in tissues and with what other cells they are communicating. They characterized the cell activity of skin samples from 11 men and women with mild to severe psoriasis/psoriatic arthritis, and three healthy adults who did not have psoriasis. They defined the cellular composition of 25 healthy skin biopsies and matched skin biopsies from psoriatic lesional and nonlesional skin, and identified 17 distinct clusters of cells, which they grouped into epidermal, dermis, pilosebaceous, and adipose categories.
The researchers found that cell activity associated with inflammation, as shown by clusters of fibroblasts and dermal macrophages, was more common in the upper layers of the skin in samples from patients with more severe psoriasis, compared with healthy control samples.
They also examined patterns of immune activity at the cellular level and found significant patterns around the upper follicle, around the perifollicular dermis, and within the hair follicle, where immune cells were enriched in healthy skin. Other cells enriched in these upper layer areas in healthy skin included dendritic cells, innate lymphoid cells, T helper cells, T cytotoxic cells, and myeloid cells.
Clusters of fibroblasts and macrophages, which are associated with inflammation, were clustered in psoriatic lesional skin, which also showed more inflammation at the dermal and suprabasal epidermal levels. B lymphocytes also were more prevalent in lesional skin.
The researchers then analyzed the skin samples according to disease severity; mild psoriasis was defined as a Psoriasis Area and Severity Index score less than 12; moderate to severe disease was defined as a PASI score of 12 or higher. The macrophage, fibroblast, and lymphatic endothelium–associated clusters distinguished mild and moderate to severe endotypes.
The pathology of moderate to severe psoriasis in lesional and nonlesional skin showed the extensive effects of psoriasis-related inflammation. Although nonlesional mild disease was clustered with healthy skin, in cases of moderate to severe disease, nonlesional and lesional groups were clustered together. This effect was segregated according to disease severity, independent of the presence of joint disease, and “was particularly evident in distal, nonlesional samples,” the researchers wrote.
The researchers also found evidence of increased gene activity in more than three dozen molecular pathways associated with metabolism and lipid levels in areas of lesional and nonlesional skin, Dr. Scher said.
The findings were limited by several factors including the small sample size and the limits of spatial transcriptomics technology resolution, the researchers wrote. “As this technology evolves, platforms with higher density, and by extension, resolution, of spatially barcoded beads will provide more granularity about cellular microenvironments in healthy and diseased states.”
The study was supported by the National Institutes of Health, the National Psoriasis Foundation, the NYU Colton Center for Autoimmunity, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis, the Beatrice Snyder Foundation, The Riley Family Foundation, the Rheumatology Research Foundation, and the NY Stem Cell Foundation. Dr. Castillo had no financial conflicts to disclose. Dr. Scher has served as a consultant for Janssen, Abbvie, Novartis, Pfizer, Sanofi, UCB, and Bristol-Myers Squibb, and has received research funding from Janssen and Pfizer.
The activity and clustering of certain cell types may distinguish mild and severe forms of psoriasis, with severe disease altering the cellular and metabolic composition of distal unaffected skin sites, according to a new analysis using single-cell transcriptomic technology.
On the surface, psoriasis severity is identified based on the visible lesions, Rochelle L. Castillo, MD, of the division of rheumatology and the NYU Psoriatic Arthritis Center, NYU Langone Health, New York, and colleagues wrote in their study, published in Science Immunology. Although cellular and molecular features of inflammatory skin diseases such as psoriasis have been characterized, activity at the tissue level and its systemic impact has not been explored.
“Our initial goal was to find measurable molecular signals that could tell us who is more likely to develop severe psoriasis, as well as who is at higher risk of developing related disorders that often accompany psoriasis, such as arthritis and cardiovascular disease,” study co–senior investigator Jose Scher, MD, director of the Psoriatic Arthritis Center and the Judith and Stewart Colton Center for Autoimmunity at NYU Langone Health, said in a press release accompanying the publication of the findings. “Having found signals with potential systemic consequences, we are now working to understand how skin inflammation can lead to widespread disease affecting other organs,”
In the study, the researchers used spatial transcriptomics, a technique that positions tissue sections onto genetic arrays to determine gene expression by cell type and histological location, helping to create a broad image-based map of where certain cell types are located in tissues and with what other cells they are communicating. They characterized the cell activity of skin samples from 11 men and women with mild to severe psoriasis/psoriatic arthritis, and three healthy adults who did not have psoriasis. They defined the cellular composition of 25 healthy skin biopsies and matched skin biopsies from psoriatic lesional and nonlesional skin, and identified 17 distinct clusters of cells, which they grouped into epidermal, dermis, pilosebaceous, and adipose categories.
The researchers found that cell activity associated with inflammation, as shown by clusters of fibroblasts and dermal macrophages, was more common in the upper layers of the skin in samples from patients with more severe psoriasis, compared with healthy control samples.
They also examined patterns of immune activity at the cellular level and found significant patterns around the upper follicle, around the perifollicular dermis, and within the hair follicle, where immune cells were enriched in healthy skin. Other cells enriched in these upper layer areas in healthy skin included dendritic cells, innate lymphoid cells, T helper cells, T cytotoxic cells, and myeloid cells.
Clusters of fibroblasts and macrophages, which are associated with inflammation, were clustered in psoriatic lesional skin, which also showed more inflammation at the dermal and suprabasal epidermal levels. B lymphocytes also were more prevalent in lesional skin.
The researchers then analyzed the skin samples according to disease severity; mild psoriasis was defined as a Psoriasis Area and Severity Index score less than 12; moderate to severe disease was defined as a PASI score of 12 or higher. The macrophage, fibroblast, and lymphatic endothelium–associated clusters distinguished mild and moderate to severe endotypes.
The pathology of moderate to severe psoriasis in lesional and nonlesional skin showed the extensive effects of psoriasis-related inflammation. Although nonlesional mild disease was clustered with healthy skin, in cases of moderate to severe disease, nonlesional and lesional groups were clustered together. This effect was segregated according to disease severity, independent of the presence of joint disease, and “was particularly evident in distal, nonlesional samples,” the researchers wrote.
The researchers also found evidence of increased gene activity in more than three dozen molecular pathways associated with metabolism and lipid levels in areas of lesional and nonlesional skin, Dr. Scher said.
The findings were limited by several factors including the small sample size and the limits of spatial transcriptomics technology resolution, the researchers wrote. “As this technology evolves, platforms with higher density, and by extension, resolution, of spatially barcoded beads will provide more granularity about cellular microenvironments in healthy and diseased states.”
The study was supported by the National Institutes of Health, the National Psoriasis Foundation, the NYU Colton Center for Autoimmunity, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis, the Beatrice Snyder Foundation, The Riley Family Foundation, the Rheumatology Research Foundation, and the NY Stem Cell Foundation. Dr. Castillo had no financial conflicts to disclose. Dr. Scher has served as a consultant for Janssen, Abbvie, Novartis, Pfizer, Sanofi, UCB, and Bristol-Myers Squibb, and has received research funding from Janssen and Pfizer.
FROM SCIENCE IMMUNOLOGY