User login
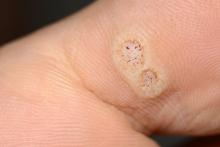
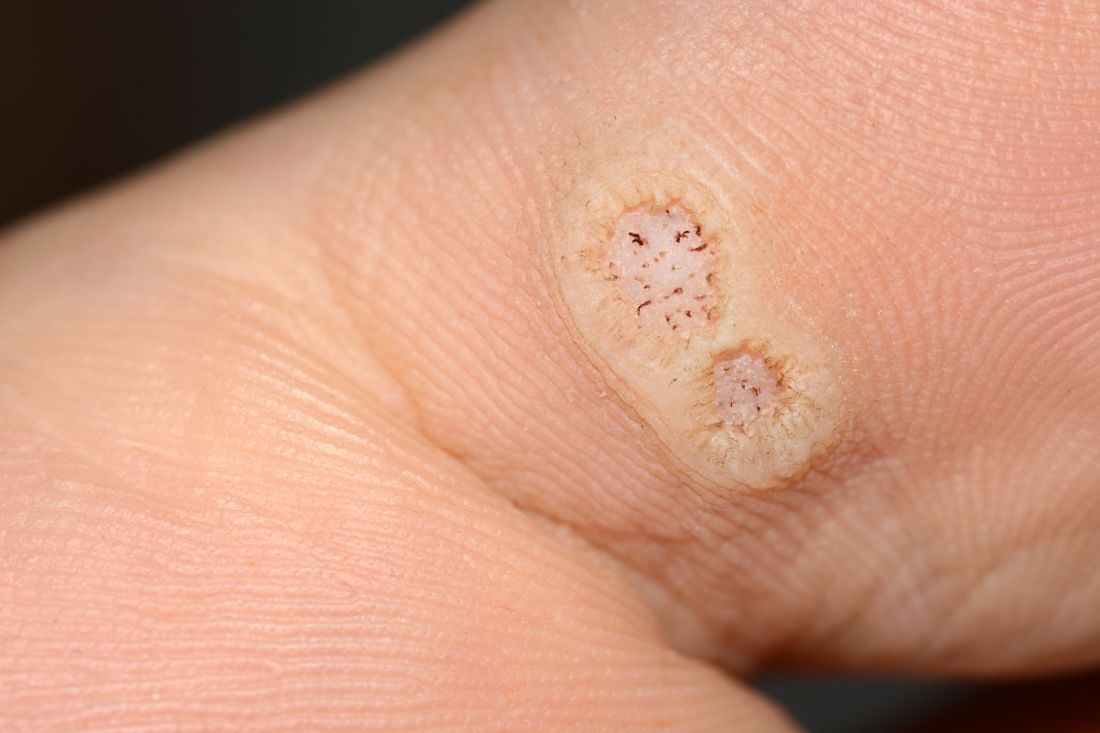
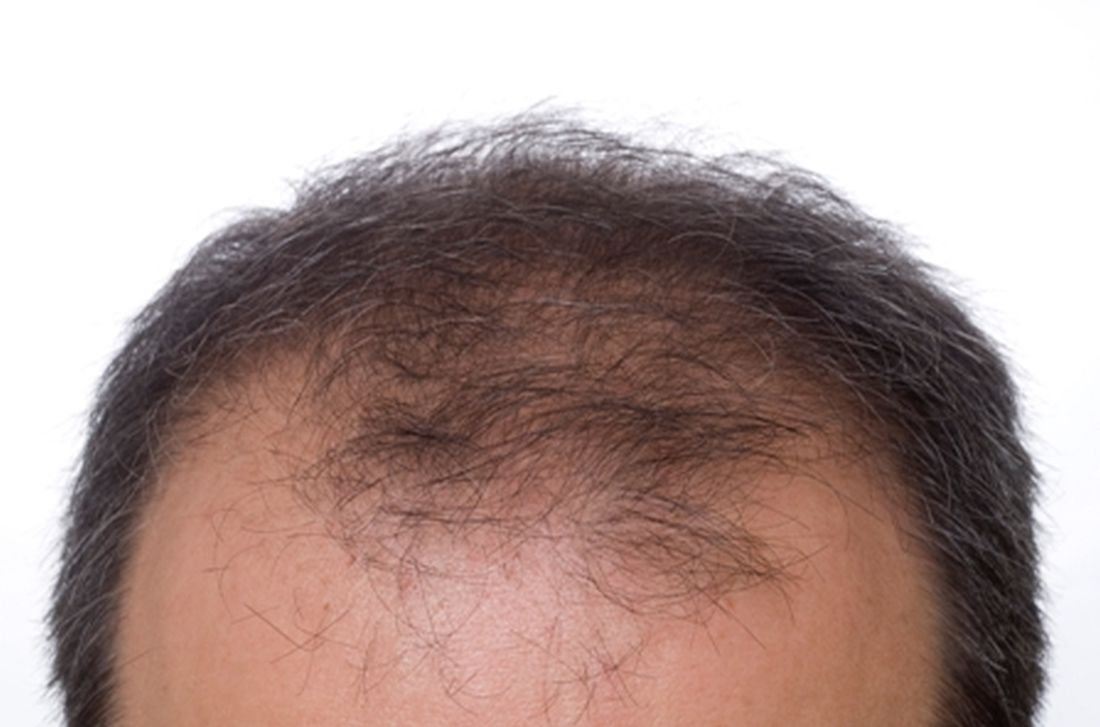
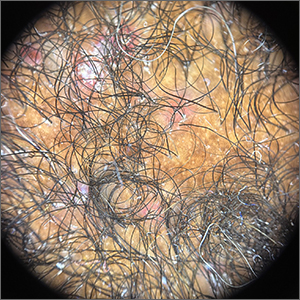
Warts difficult to eradicate in immunocompromised children
.
Only a quarter of patients (24%) who were undergoing active cancer treatment experienced complete resolution of their warts, compared with 63.3% of patients who were not on active treatment.
In addition, warts persisted or worsened in 56.0% of patients receiving active treatment compared with 13.4% of those who were not receiving it.
“These data enable providers treating warts in children with cancer to have an educated discussion regarding the expected clinical progression of warts and the likelihood of response to wart therapy while on and off anti-cancer treatment,” the authors wrote in the study, published in Pediatric Dermatology.
In immunocompromised children, warts are more common than in the general pediatric population, and more resistant to treatment. But as the authors noted, data on the course and prognosis of warts in pediatric patients who are actively receiving anti-cancer therapy compared with patients who have completed treatment are limited.
Tina Ho, MD, PhD, of the department of dermatology, and colleagues from Boston Children’s Hospital, sought to analyze the clinical course of warts treated in this patient population at their institution over a 10-year period. They conducted a retrospective study of 72 children who were treated for cancer between 2011 and 2021, and who had also been treated for warts.
The median age of the cohort was 12 years, and they were followed for a median of 2 years following their diagnosis of warts. Within this group, more than half (55%) had hematologic malignancies, while 27% had a history of bone marrow transplantation.
Of note, the authors pointed out, 54% of the patients had plantar warts, and 60% of patients (38 of 63) with a documented number of warts had more than five at the time of presentation.
The treatment regimens that the children had received varied, with 81% of patients receiving cytotoxic chemotherapy and 23% of patients on targeted therapies that included immunotherapy.
The warts were most commonly treated with cryotherapy and topical salicylic acid; this was the case for those actively receiving oncology treatment or those who had completed their treatment regimens.
Outcomes of wart treatments were available in 25 of the patients undergoing active cancer treatment and in 30 of those who had completed treatment. For children on active oncology treatment, 5 (20%) achieved partial resolution, 6 (24%) achieved complete resolution, and 14 (56%) experienced persistence or worsening of their warts following therapy. Those who had completed treatment had better outcomes: Seven (23.3%) had a partial response, 19 (63.3%) had complete resolution, and 4 (13.4%) had persistence or worsening of warts after treatment of warts.
The authors also pointed out the treatment of warts can be painful, expensive, and time-consuming. “It is thus imperative that the risks and benefits of these treatments are carefully considered before proceeding with treatment,” wrote Dr. Ho and colleagues. “This is especially true in medically complex children with cancer who may be fearful of procedures and spend significant portions of their young lives within the medical system.”
Limitations to the study include its retrospective design and small sample size. Clinical data were not uniformly complete, and follow-up intervals varied among the participants. Also, it was conducted at a single-institution and at a large tertiary center, so the results may not be fully generalizable.
The authors declared no conflict of interest. No outside funding source was listed.
.
Only a quarter of patients (24%) who were undergoing active cancer treatment experienced complete resolution of their warts, compared with 63.3% of patients who were not on active treatment.
In addition, warts persisted or worsened in 56.0% of patients receiving active treatment compared with 13.4% of those who were not receiving it.
“These data enable providers treating warts in children with cancer to have an educated discussion regarding the expected clinical progression of warts and the likelihood of response to wart therapy while on and off anti-cancer treatment,” the authors wrote in the study, published in Pediatric Dermatology.
In immunocompromised children, warts are more common than in the general pediatric population, and more resistant to treatment. But as the authors noted, data on the course and prognosis of warts in pediatric patients who are actively receiving anti-cancer therapy compared with patients who have completed treatment are limited.
Tina Ho, MD, PhD, of the department of dermatology, and colleagues from Boston Children’s Hospital, sought to analyze the clinical course of warts treated in this patient population at their institution over a 10-year period. They conducted a retrospective study of 72 children who were treated for cancer between 2011 and 2021, and who had also been treated for warts.
The median age of the cohort was 12 years, and they were followed for a median of 2 years following their diagnosis of warts. Within this group, more than half (55%) had hematologic malignancies, while 27% had a history of bone marrow transplantation.
Of note, the authors pointed out, 54% of the patients had plantar warts, and 60% of patients (38 of 63) with a documented number of warts had more than five at the time of presentation.
The treatment regimens that the children had received varied, with 81% of patients receiving cytotoxic chemotherapy and 23% of patients on targeted therapies that included immunotherapy.
The warts were most commonly treated with cryotherapy and topical salicylic acid; this was the case for those actively receiving oncology treatment or those who had completed their treatment regimens.
Outcomes of wart treatments were available in 25 of the patients undergoing active cancer treatment and in 30 of those who had completed treatment. For children on active oncology treatment, 5 (20%) achieved partial resolution, 6 (24%) achieved complete resolution, and 14 (56%) experienced persistence or worsening of their warts following therapy. Those who had completed treatment had better outcomes: Seven (23.3%) had a partial response, 19 (63.3%) had complete resolution, and 4 (13.4%) had persistence or worsening of warts after treatment of warts.
The authors also pointed out the treatment of warts can be painful, expensive, and time-consuming. “It is thus imperative that the risks and benefits of these treatments are carefully considered before proceeding with treatment,” wrote Dr. Ho and colleagues. “This is especially true in medically complex children with cancer who may be fearful of procedures and spend significant portions of their young lives within the medical system.”
Limitations to the study include its retrospective design and small sample size. Clinical data were not uniformly complete, and follow-up intervals varied among the participants. Also, it was conducted at a single-institution and at a large tertiary center, so the results may not be fully generalizable.
The authors declared no conflict of interest. No outside funding source was listed.
.
Only a quarter of patients (24%) who were undergoing active cancer treatment experienced complete resolution of their warts, compared with 63.3% of patients who were not on active treatment.
In addition, warts persisted or worsened in 56.0% of patients receiving active treatment compared with 13.4% of those who were not receiving it.
“These data enable providers treating warts in children with cancer to have an educated discussion regarding the expected clinical progression of warts and the likelihood of response to wart therapy while on and off anti-cancer treatment,” the authors wrote in the study, published in Pediatric Dermatology.
In immunocompromised children, warts are more common than in the general pediatric population, and more resistant to treatment. But as the authors noted, data on the course and prognosis of warts in pediatric patients who are actively receiving anti-cancer therapy compared with patients who have completed treatment are limited.
Tina Ho, MD, PhD, of the department of dermatology, and colleagues from Boston Children’s Hospital, sought to analyze the clinical course of warts treated in this patient population at their institution over a 10-year period. They conducted a retrospective study of 72 children who were treated for cancer between 2011 and 2021, and who had also been treated for warts.
The median age of the cohort was 12 years, and they were followed for a median of 2 years following their diagnosis of warts. Within this group, more than half (55%) had hematologic malignancies, while 27% had a history of bone marrow transplantation.
Of note, the authors pointed out, 54% of the patients had plantar warts, and 60% of patients (38 of 63) with a documented number of warts had more than five at the time of presentation.
The treatment regimens that the children had received varied, with 81% of patients receiving cytotoxic chemotherapy and 23% of patients on targeted therapies that included immunotherapy.
The warts were most commonly treated with cryotherapy and topical salicylic acid; this was the case for those actively receiving oncology treatment or those who had completed their treatment regimens.
Outcomes of wart treatments were available in 25 of the patients undergoing active cancer treatment and in 30 of those who had completed treatment. For children on active oncology treatment, 5 (20%) achieved partial resolution, 6 (24%) achieved complete resolution, and 14 (56%) experienced persistence or worsening of their warts following therapy. Those who had completed treatment had better outcomes: Seven (23.3%) had a partial response, 19 (63.3%) had complete resolution, and 4 (13.4%) had persistence or worsening of warts after treatment of warts.
The authors also pointed out the treatment of warts can be painful, expensive, and time-consuming. “It is thus imperative that the risks and benefits of these treatments are carefully considered before proceeding with treatment,” wrote Dr. Ho and colleagues. “This is especially true in medically complex children with cancer who may be fearful of procedures and spend significant portions of their young lives within the medical system.”
Limitations to the study include its retrospective design and small sample size. Clinical data were not uniformly complete, and follow-up intervals varied among the participants. Also, it was conducted at a single-institution and at a large tertiary center, so the results may not be fully generalizable.
The authors declared no conflict of interest. No outside funding source was listed.
FROM PEDIATRIC DERMATOLOGY
Prognostic factors of SCCs in organ transplant recipients worse compared with general population
, results from a dual cohort study demonstrated.
The findings build on previous research and underscore the need for early diagnosis and aggressive surveillance in this patient population, corresponding author Adele C. Green, MBBS, PhD, professor and senior scientist at the QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia, and colleagues wrote in the study, which was published online in JAMA Dermatology. “Squamous cell carcinomas (SCCs) of the skin develop up to 77 times more frequently in immunosuppressed organ transplant recipients (OTRs) than the general population,” they wrote. “Because SCCs cause substantially more morbidity and death in the former, they are postulated to be innately more aggressive than in immunocompetent patients, but OTRs’ higher SCC mortality may simply reflect greater SCC tumor burdens per patient.”
In what is believed to be the first study of its kind, Dr. Green and colleagues drew data from two cohort studies to evaluate five key clinicopathologic indicators of poor SCC outcomes in organ transplant recipients, and in those from the general population in Queensland, Australia: cephalic location, perineural invasion, invasion to/beyond subcutaneous fat, poor differentiation, and tumor size greater than 20 mm. The study population included organ transplant recipients at high risk of skin cancer, who were enrolled in the Skin Tumours in Allograft Recipients (STAR) study, and those from a population-based cohort, the QSkin Sun and Health Study. STAR consisted of lung transplant recipients and kidney and liver transplant recipients at high risk of skin cancer who were recruited from tertiary centers and diagnosed with histopathologically confirmed SCC from 2012 to 2015. QSkin consisted of individuals from Queensland’s general adult population diagnosed with SCCs from 2012 to 2015.
SCC cases in QSkin were ascertained through Australia’s universal health insurance agency and linked with histopathology records. Next, the researchers performed data analysis from both cohort studies to determine the prevalence ratio (PR) of head/neck location, perineural invasion, tumor invasion to/beyond subcutaneous fat, poor cellular differentiation, and tumor diameter greater than 20 mm among SCCs among organ transplant recipients compared with the general population.
After combining the two studies, the researchers compared 741 SCCs excised from 191 organ transplant recipients and 2,558 SCCs excised from 1,507 individuals in the general population. Their median ages were similar (62.7 and 63.7 years, respectively) and most were male (78% and 63.4%, respectively).
As for site of involvement, SCCs developed most often on the head and neck in the transplant recipients (38.6%) and on the arms and hands in the general population (35.2%). After adjustment for age and sex, perineural invasion of SCCs was more than twice as common in transplant recipients than among cases in the general population, as was invasion to/beyond subcutaneous fat (PR of 2.37 for both associations).
In other findings, compared with SCCs in the general population, poorly vs. well-differentiated SCCs were more than threefold more common in transplant recipients (PR, 3.45), while the prevalence of tumors greater than 20 mm vs. 20 mm or smaller was moderately higher in transplant recipients (PR, 1.52).
“These findings are considered generalizable, confirming that OTRs’ poorer SCC outcomes are associated with not only their sheer numbers of SCC tumors, but also with a strong shift toward more invasive, less differentiated, and larger SCC tumors, in agreement with previous findings,” the researchers wrote. “This shift is likely associated with decreased immunosurveillance resulting from immunosuppressive therapy (since carcinogenesis decelerates with therapy cessation) interacting with effects of high UV radiation exposure.”
They acknowledged certain limitations of their analysis, chiefly the lack of central review of SCCs to ensure standard assessment of histopathologic features “including caliber of nerves with perineural invasion and cell differentiation; such a review would not have been feasible logistically.”
The study was supported by grants from the National Health and Medical Research Council of Australia. The researchers reported having no disclosures related to the submitted work.
, results from a dual cohort study demonstrated.
The findings build on previous research and underscore the need for early diagnosis and aggressive surveillance in this patient population, corresponding author Adele C. Green, MBBS, PhD, professor and senior scientist at the QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia, and colleagues wrote in the study, which was published online in JAMA Dermatology. “Squamous cell carcinomas (SCCs) of the skin develop up to 77 times more frequently in immunosuppressed organ transplant recipients (OTRs) than the general population,” they wrote. “Because SCCs cause substantially more morbidity and death in the former, they are postulated to be innately more aggressive than in immunocompetent patients, but OTRs’ higher SCC mortality may simply reflect greater SCC tumor burdens per patient.”
In what is believed to be the first study of its kind, Dr. Green and colleagues drew data from two cohort studies to evaluate five key clinicopathologic indicators of poor SCC outcomes in organ transplant recipients, and in those from the general population in Queensland, Australia: cephalic location, perineural invasion, invasion to/beyond subcutaneous fat, poor differentiation, and tumor size greater than 20 mm. The study population included organ transplant recipients at high risk of skin cancer, who were enrolled in the Skin Tumours in Allograft Recipients (STAR) study, and those from a population-based cohort, the QSkin Sun and Health Study. STAR consisted of lung transplant recipients and kidney and liver transplant recipients at high risk of skin cancer who were recruited from tertiary centers and diagnosed with histopathologically confirmed SCC from 2012 to 2015. QSkin consisted of individuals from Queensland’s general adult population diagnosed with SCCs from 2012 to 2015.
SCC cases in QSkin were ascertained through Australia’s universal health insurance agency and linked with histopathology records. Next, the researchers performed data analysis from both cohort studies to determine the prevalence ratio (PR) of head/neck location, perineural invasion, tumor invasion to/beyond subcutaneous fat, poor cellular differentiation, and tumor diameter greater than 20 mm among SCCs among organ transplant recipients compared with the general population.
After combining the two studies, the researchers compared 741 SCCs excised from 191 organ transplant recipients and 2,558 SCCs excised from 1,507 individuals in the general population. Their median ages were similar (62.7 and 63.7 years, respectively) and most were male (78% and 63.4%, respectively).
As for site of involvement, SCCs developed most often on the head and neck in the transplant recipients (38.6%) and on the arms and hands in the general population (35.2%). After adjustment for age and sex, perineural invasion of SCCs was more than twice as common in transplant recipients than among cases in the general population, as was invasion to/beyond subcutaneous fat (PR of 2.37 for both associations).
In other findings, compared with SCCs in the general population, poorly vs. well-differentiated SCCs were more than threefold more common in transplant recipients (PR, 3.45), while the prevalence of tumors greater than 20 mm vs. 20 mm or smaller was moderately higher in transplant recipients (PR, 1.52).
“These findings are considered generalizable, confirming that OTRs’ poorer SCC outcomes are associated with not only their sheer numbers of SCC tumors, but also with a strong shift toward more invasive, less differentiated, and larger SCC tumors, in agreement with previous findings,” the researchers wrote. “This shift is likely associated with decreased immunosurveillance resulting from immunosuppressive therapy (since carcinogenesis decelerates with therapy cessation) interacting with effects of high UV radiation exposure.”
They acknowledged certain limitations of their analysis, chiefly the lack of central review of SCCs to ensure standard assessment of histopathologic features “including caliber of nerves with perineural invasion and cell differentiation; such a review would not have been feasible logistically.”
The study was supported by grants from the National Health and Medical Research Council of Australia. The researchers reported having no disclosures related to the submitted work.
, results from a dual cohort study demonstrated.
The findings build on previous research and underscore the need for early diagnosis and aggressive surveillance in this patient population, corresponding author Adele C. Green, MBBS, PhD, professor and senior scientist at the QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia, and colleagues wrote in the study, which was published online in JAMA Dermatology. “Squamous cell carcinomas (SCCs) of the skin develop up to 77 times more frequently in immunosuppressed organ transplant recipients (OTRs) than the general population,” they wrote. “Because SCCs cause substantially more morbidity and death in the former, they are postulated to be innately more aggressive than in immunocompetent patients, but OTRs’ higher SCC mortality may simply reflect greater SCC tumor burdens per patient.”
In what is believed to be the first study of its kind, Dr. Green and colleagues drew data from two cohort studies to evaluate five key clinicopathologic indicators of poor SCC outcomes in organ transplant recipients, and in those from the general population in Queensland, Australia: cephalic location, perineural invasion, invasion to/beyond subcutaneous fat, poor differentiation, and tumor size greater than 20 mm. The study population included organ transplant recipients at high risk of skin cancer, who were enrolled in the Skin Tumours in Allograft Recipients (STAR) study, and those from a population-based cohort, the QSkin Sun and Health Study. STAR consisted of lung transplant recipients and kidney and liver transplant recipients at high risk of skin cancer who were recruited from tertiary centers and diagnosed with histopathologically confirmed SCC from 2012 to 2015. QSkin consisted of individuals from Queensland’s general adult population diagnosed with SCCs from 2012 to 2015.
SCC cases in QSkin were ascertained through Australia’s universal health insurance agency and linked with histopathology records. Next, the researchers performed data analysis from both cohort studies to determine the prevalence ratio (PR) of head/neck location, perineural invasion, tumor invasion to/beyond subcutaneous fat, poor cellular differentiation, and tumor diameter greater than 20 mm among SCCs among organ transplant recipients compared with the general population.
After combining the two studies, the researchers compared 741 SCCs excised from 191 organ transplant recipients and 2,558 SCCs excised from 1,507 individuals in the general population. Their median ages were similar (62.7 and 63.7 years, respectively) and most were male (78% and 63.4%, respectively).
As for site of involvement, SCCs developed most often on the head and neck in the transplant recipients (38.6%) and on the arms and hands in the general population (35.2%). After adjustment for age and sex, perineural invasion of SCCs was more than twice as common in transplant recipients than among cases in the general population, as was invasion to/beyond subcutaneous fat (PR of 2.37 for both associations).
In other findings, compared with SCCs in the general population, poorly vs. well-differentiated SCCs were more than threefold more common in transplant recipients (PR, 3.45), while the prevalence of tumors greater than 20 mm vs. 20 mm or smaller was moderately higher in transplant recipients (PR, 1.52).
“These findings are considered generalizable, confirming that OTRs’ poorer SCC outcomes are associated with not only their sheer numbers of SCC tumors, but also with a strong shift toward more invasive, less differentiated, and larger SCC tumors, in agreement with previous findings,” the researchers wrote. “This shift is likely associated with decreased immunosurveillance resulting from immunosuppressive therapy (since carcinogenesis decelerates with therapy cessation) interacting with effects of high UV radiation exposure.”
They acknowledged certain limitations of their analysis, chiefly the lack of central review of SCCs to ensure standard assessment of histopathologic features “including caliber of nerves with perineural invasion and cell differentiation; such a review would not have been feasible logistically.”
The study was supported by grants from the National Health and Medical Research Council of Australia. The researchers reported having no disclosures related to the submitted work.
FROM JAMA DERMATOLOGY
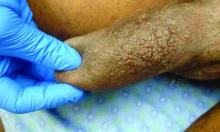
A 63-year-old male presented for evaluation of worsening genital lesions and associated swelling
.1 Clinically, ENV presents as verrucous, hyperkeratotic, cobblestone-like patches, plaques, and nodules with associated nonpitting edema of the affected body area.1 Secondary bacterial infections are common and often worsen the clinical course. The etiology of ENV involves chronic lymphatic obstruction and venous insufficiency, with additional risk factors including obesity, chronic lymphedema, bacterial infection, surgery or trauma, neoplasia, radiation, congestive heart failure, or scleroderma.2,3 While most commonly presenting on the lower extremities, cases have been reported involving the abdomen, sacrum, ears, buttocks, and penoscrotal area.1,2
Regardless of location, the pathogenesis of ENV remains the same. Chronic lymphatic obstruction results in accumulation and lymphostasis of protein-rich dermal fluid, which subsequently precipitates fibroblast proliferation and activation, suppression of the local immune response and development of recurrent lymphangitis, chronic inflammation, and potential secondary bacterial infection.2,4
There is no standard of care for the treatment and management of ENV and recurrence is common. Interventions often involve those used for chronic lymphedema – including leg elevation, compression stockings or devices, skin hygiene, and lymphatic pumping.2,3 Medical management with topical and oral retinoids has been reported, as well as emphasis on weight loss and infection control.1,4 Surgical intervention is often reserved for refractory cases that fail to respond to more conservative management, or severe presentations resulting in extensive functional and aesthetic impairment. Less commonly reported treatment modalities include lymphaticovenular anastomosis and ablative carbon dioxide laser use, although this latter intervention demonstrated minimal improvement in this patient.5,6
Penoscrotal ENV is a rare form of ENV affecting the genital region of males, often resulting in significant disfigurement, functional impairment, and psychosocial distress. Penoscrotal elephantiasis can be idiopathic, due to filarial infections, scleroinflammatory stricture of the urethra, Chlamydia trachomatis infection, and lymphostasis secondary to chronic inflammatory conditions such as streptococcal infections, radiotherapy, surgery, chronic venous stasis, or Kaposi sarcoma.7
In addition, hidradenitis suppurativa (HS) has been documented multiple times in the literature in association with the development of ENV, detailing lymphatic scarring secondary to chronic inguinal HS as the main pathogenic factor.8,9
Surgery is the mainstay of treatment for penoscrotal ENV, which not only improves functionality and cosmesis, but also aids in prevention of rare malignant sequelae, such as lymphangiosarcoma.10 Such interventions can involve lymphangioplasty to aid in lymphatic drainage or excision of the mass and subcutaneous tissue with full-thickness skin grafting for reconstruction.7 Collaboration between urology, plastic surgery, and dermatology is often essential to obtain adequate care with satisfactory outcomes and minimal recurrence for patients with this uncommon condition.
This case and photo were submitted by Marlee Hill, a medical student at the University of Oklahoma, Oklahoma City; and Michael Franzetti, MD, and Jeffrey McBride, MD, department of dermatology, University of Oklahoma Health Sciences Center. The column was edited by Donna Bilu Martin, MD.
Dr. Donna Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to d[email protected].
References
1. Hadian Y et al. Dermatol Online J. 2019 Dec 15;25(12):13030/qt6rn1s8ff.
2. Judge N and Kilic A. J Dermatol Case Rep. 2016 Nov 13;10(2):32-4.
3. Dean SM et al. J Am Acad Dermatol. 2011 Jun;64(6):1104-10.
4. Sisto K and Khachemoune A. Am J Clin Dermatol. 2008;9(3):141-6.
5. Motegi S et al. Dermatology. 2007;215(2):147-51.
6. Robinson CG et al. J Cutan Med Surg. 2018;22(6):611-3.
7. Koualla S et al. Ann Chir Plast Esthet. 2023 Apr 10;S0294-1260(23)00035-3.
8. Lelonek E et al. Acta Derm Venereol. 2021 Feb 11;101(2):adv00389.
9. Good LM et al. J Am Acad Dermatol. 2011 May;64(5):993-4.
10. Cerri A et al. Eur J Dermatol. 1998 Oct-Nov;8(7):511-4.
.1 Clinically, ENV presents as verrucous, hyperkeratotic, cobblestone-like patches, plaques, and nodules with associated nonpitting edema of the affected body area.1 Secondary bacterial infections are common and often worsen the clinical course. The etiology of ENV involves chronic lymphatic obstruction and venous insufficiency, with additional risk factors including obesity, chronic lymphedema, bacterial infection, surgery or trauma, neoplasia, radiation, congestive heart failure, or scleroderma.2,3 While most commonly presenting on the lower extremities, cases have been reported involving the abdomen, sacrum, ears, buttocks, and penoscrotal area.1,2
Regardless of location, the pathogenesis of ENV remains the same. Chronic lymphatic obstruction results in accumulation and lymphostasis of protein-rich dermal fluid, which subsequently precipitates fibroblast proliferation and activation, suppression of the local immune response and development of recurrent lymphangitis, chronic inflammation, and potential secondary bacterial infection.2,4
There is no standard of care for the treatment and management of ENV and recurrence is common. Interventions often involve those used for chronic lymphedema – including leg elevation, compression stockings or devices, skin hygiene, and lymphatic pumping.2,3 Medical management with topical and oral retinoids has been reported, as well as emphasis on weight loss and infection control.1,4 Surgical intervention is often reserved for refractory cases that fail to respond to more conservative management, or severe presentations resulting in extensive functional and aesthetic impairment. Less commonly reported treatment modalities include lymphaticovenular anastomosis and ablative carbon dioxide laser use, although this latter intervention demonstrated minimal improvement in this patient.5,6
Penoscrotal ENV is a rare form of ENV affecting the genital region of males, often resulting in significant disfigurement, functional impairment, and psychosocial distress. Penoscrotal elephantiasis can be idiopathic, due to filarial infections, scleroinflammatory stricture of the urethra, Chlamydia trachomatis infection, and lymphostasis secondary to chronic inflammatory conditions such as streptococcal infections, radiotherapy, surgery, chronic venous stasis, or Kaposi sarcoma.7
In addition, hidradenitis suppurativa (HS) has been documented multiple times in the literature in association with the development of ENV, detailing lymphatic scarring secondary to chronic inguinal HS as the main pathogenic factor.8,9
Surgery is the mainstay of treatment for penoscrotal ENV, which not only improves functionality and cosmesis, but also aids in prevention of rare malignant sequelae, such as lymphangiosarcoma.10 Such interventions can involve lymphangioplasty to aid in lymphatic drainage or excision of the mass and subcutaneous tissue with full-thickness skin grafting for reconstruction.7 Collaboration between urology, plastic surgery, and dermatology is often essential to obtain adequate care with satisfactory outcomes and minimal recurrence for patients with this uncommon condition.
This case and photo were submitted by Marlee Hill, a medical student at the University of Oklahoma, Oklahoma City; and Michael Franzetti, MD, and Jeffrey McBride, MD, department of dermatology, University of Oklahoma Health Sciences Center. The column was edited by Donna Bilu Martin, MD.
Dr. Donna Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to d[email protected].
References
1. Hadian Y et al. Dermatol Online J. 2019 Dec 15;25(12):13030/qt6rn1s8ff.
2. Judge N and Kilic A. J Dermatol Case Rep. 2016 Nov 13;10(2):32-4.
3. Dean SM et al. J Am Acad Dermatol. 2011 Jun;64(6):1104-10.
4. Sisto K and Khachemoune A. Am J Clin Dermatol. 2008;9(3):141-6.
5. Motegi S et al. Dermatology. 2007;215(2):147-51.
6. Robinson CG et al. J Cutan Med Surg. 2018;22(6):611-3.
7. Koualla S et al. Ann Chir Plast Esthet. 2023 Apr 10;S0294-1260(23)00035-3.
8. Lelonek E et al. Acta Derm Venereol. 2021 Feb 11;101(2):adv00389.
9. Good LM et al. J Am Acad Dermatol. 2011 May;64(5):993-4.
10. Cerri A et al. Eur J Dermatol. 1998 Oct-Nov;8(7):511-4.
.1 Clinically, ENV presents as verrucous, hyperkeratotic, cobblestone-like patches, plaques, and nodules with associated nonpitting edema of the affected body area.1 Secondary bacterial infections are common and often worsen the clinical course. The etiology of ENV involves chronic lymphatic obstruction and venous insufficiency, with additional risk factors including obesity, chronic lymphedema, bacterial infection, surgery or trauma, neoplasia, radiation, congestive heart failure, or scleroderma.2,3 While most commonly presenting on the lower extremities, cases have been reported involving the abdomen, sacrum, ears, buttocks, and penoscrotal area.1,2
Regardless of location, the pathogenesis of ENV remains the same. Chronic lymphatic obstruction results in accumulation and lymphostasis of protein-rich dermal fluid, which subsequently precipitates fibroblast proliferation and activation, suppression of the local immune response and development of recurrent lymphangitis, chronic inflammation, and potential secondary bacterial infection.2,4
There is no standard of care for the treatment and management of ENV and recurrence is common. Interventions often involve those used for chronic lymphedema – including leg elevation, compression stockings or devices, skin hygiene, and lymphatic pumping.2,3 Medical management with topical and oral retinoids has been reported, as well as emphasis on weight loss and infection control.1,4 Surgical intervention is often reserved for refractory cases that fail to respond to more conservative management, or severe presentations resulting in extensive functional and aesthetic impairment. Less commonly reported treatment modalities include lymphaticovenular anastomosis and ablative carbon dioxide laser use, although this latter intervention demonstrated minimal improvement in this patient.5,6
Penoscrotal ENV is a rare form of ENV affecting the genital region of males, often resulting in significant disfigurement, functional impairment, and psychosocial distress. Penoscrotal elephantiasis can be idiopathic, due to filarial infections, scleroinflammatory stricture of the urethra, Chlamydia trachomatis infection, and lymphostasis secondary to chronic inflammatory conditions such as streptococcal infections, radiotherapy, surgery, chronic venous stasis, or Kaposi sarcoma.7
In addition, hidradenitis suppurativa (HS) has been documented multiple times in the literature in association with the development of ENV, detailing lymphatic scarring secondary to chronic inguinal HS as the main pathogenic factor.8,9
Surgery is the mainstay of treatment for penoscrotal ENV, which not only improves functionality and cosmesis, but also aids in prevention of rare malignant sequelae, such as lymphangiosarcoma.10 Such interventions can involve lymphangioplasty to aid in lymphatic drainage or excision of the mass and subcutaneous tissue with full-thickness skin grafting for reconstruction.7 Collaboration between urology, plastic surgery, and dermatology is often essential to obtain adequate care with satisfactory outcomes and minimal recurrence for patients with this uncommon condition.
This case and photo were submitted by Marlee Hill, a medical student at the University of Oklahoma, Oklahoma City; and Michael Franzetti, MD, and Jeffrey McBride, MD, department of dermatology, University of Oklahoma Health Sciences Center. The column was edited by Donna Bilu Martin, MD.
Dr. Donna Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to d[email protected].
References
1. Hadian Y et al. Dermatol Online J. 2019 Dec 15;25(12):13030/qt6rn1s8ff.
2. Judge N and Kilic A. J Dermatol Case Rep. 2016 Nov 13;10(2):32-4.
3. Dean SM et al. J Am Acad Dermatol. 2011 Jun;64(6):1104-10.
4. Sisto K and Khachemoune A. Am J Clin Dermatol. 2008;9(3):141-6.
5. Motegi S et al. Dermatology. 2007;215(2):147-51.
6. Robinson CG et al. J Cutan Med Surg. 2018;22(6):611-3.
7. Koualla S et al. Ann Chir Plast Esthet. 2023 Apr 10;S0294-1260(23)00035-3.
8. Lelonek E et al. Acta Derm Venereol. 2021 Feb 11;101(2):adv00389.
9. Good LM et al. J Am Acad Dermatol. 2011 May;64(5):993-4.
10. Cerri A et al. Eur J Dermatol. 1998 Oct-Nov;8(7):511-4.
FDA warns of tattoo ink tied to dangerous infections
The Food and Drug Administration draft guidance released recently on possible contamination of tattoo ink was not concerning Whitney Donohue, 34, owner of Forget Me Not Tattoo in Billings, Mont.
“I get our ink directly through the manufacturer – not at a store or through Amazon or eBay,” she said. “You never know if it’s going to be repackaged.”
Tattoo artists themselves, she said, regulate the quality of ink they use.
Still, the threat is real, said Bruce Brod, MD, a clinical professor of dermatology at the University of Pennsylvania Health System. “I’ve seen several different infections from tattooing, and they are from organisms that tend to contaminate things in damp, liquid-type environments.”
, dermatologists said.
“Tattooing involves puncturing the epidermis about 100 times per second with needles and depositing ink 1.5 to 2 millimeters below the surface of the skin, deep into the dermis,” the guidance states. “Contaminated tattoo ink can cause infections and serious injuries. Because these inks are injected, pathogens or other harmful substances in these inks can travel from the injection site through the blood and lymphatic systems to other parts of the body.”
The guidance comes as body art continues to get more popular. According to a 2019 poll, 30% of Americans had at least one tattoo – up from 21% in 2012. Forty percent of people 18-34 and 36% of those ages 35-54 had at least one tattoo. And though they are commonplace, tattoos come with medical risks that should be known beforehand, doctors said.
Commonly reported symptoms of tattoo ink–associated infections include rashes, blisters, painful nodules, and severe abscesses. One of the most common bacteria found in contaminated tattoo ink is nontuberculous mycobacteria, which is related to the bacteria that causes tuberculosis and can be found in soil and water.
The guidance lists several unsanitary manufacturing conditions that may lead to ink contamination, including:
- Preparing or packing of tattoo inks in facilities that are hard to sanitize, such as carpeted areas
- Ink or ink components left uncovered, especially near open air ducts
- Unsanitary mixing of tattoo inks, including with unclean utensils or containers
- Lack of appropriate attire by staff, failure to use hairnets, lab coats, aprons, gowns, masks, or gloves
“Infections will often spread along the drainage channels in the skin and create squiggly, uneven lines of big red, lumpy nodules,” Dr. Brod said.
Between 2003 and 2023, there were 18 recalls of tattoo inks that were contaminated with various microorganisms, according to the FDA. In May 2019, the FDA issued a safety alert advising consumers, tattoo artists, and retailers to avoid using or selling certain tattoo inks contaminated with microorganisms.
Reputable ink manufacturers use a process called gamma radiation, which refers to electromagnetic radiation of high frequencies to kill microorganisms in the ink and its packaging.
Most of the trustworthy, high-quality ink manufacturers are well-known among tattoo artists, Ms. Donohue said.
While she has seen customers with sensitive skin have allergic reactions, she has not seen someone come back with an infection in her 9 years working in the tattoo industry.
Because tattoo ink is considered a cosmetic product, there is not much regulatory oversight involved, which means the sterility and quality of ingredients vary, said Teo Soleymani, MD, an assistant clinical professor of dermatology and dermatological surgery at the UCLA David Geffen School of Medicine.
“Cosmeceuticals aren’t regulated by the FDA like prescription medication,” he said. “What we’ve seen many times is inadvertent contamination during the application process or contamination while the inks are being made.”
In years past, unclean needles spreading hepatitis and HIV were more of a concern, but those rates have dropped significantly, Dr. Soleymani said.
The infections that have increased are from rare bacteria that exist in stagnant water. And they are injected into a part of the body that allows them to evade the immune system, he said: shallow enough that there aren’t many associated blood vessels, but not still below the layer of skin that gets sloughed off every 28 days.
Sometimes, antibiotics alone won’t cut it, and the tattoo will require surgical removal.
“The aesthetic you were going for has to be not only removed, but you’re left with a surgical scar,” Dr. Soleymani said. “Tattoos can be beautiful, but they can come with unwanted visitors that can cause months of misery.”
A version of this article first appeared on WebMD.com.
The Food and Drug Administration draft guidance released recently on possible contamination of tattoo ink was not concerning Whitney Donohue, 34, owner of Forget Me Not Tattoo in Billings, Mont.
“I get our ink directly through the manufacturer – not at a store or through Amazon or eBay,” she said. “You never know if it’s going to be repackaged.”
Tattoo artists themselves, she said, regulate the quality of ink they use.
Still, the threat is real, said Bruce Brod, MD, a clinical professor of dermatology at the University of Pennsylvania Health System. “I’ve seen several different infections from tattooing, and they are from organisms that tend to contaminate things in damp, liquid-type environments.”
, dermatologists said.
“Tattooing involves puncturing the epidermis about 100 times per second with needles and depositing ink 1.5 to 2 millimeters below the surface of the skin, deep into the dermis,” the guidance states. “Contaminated tattoo ink can cause infections and serious injuries. Because these inks are injected, pathogens or other harmful substances in these inks can travel from the injection site through the blood and lymphatic systems to other parts of the body.”
The guidance comes as body art continues to get more popular. According to a 2019 poll, 30% of Americans had at least one tattoo – up from 21% in 2012. Forty percent of people 18-34 and 36% of those ages 35-54 had at least one tattoo. And though they are commonplace, tattoos come with medical risks that should be known beforehand, doctors said.
Commonly reported symptoms of tattoo ink–associated infections include rashes, blisters, painful nodules, and severe abscesses. One of the most common bacteria found in contaminated tattoo ink is nontuberculous mycobacteria, which is related to the bacteria that causes tuberculosis and can be found in soil and water.
The guidance lists several unsanitary manufacturing conditions that may lead to ink contamination, including:
- Preparing or packing of tattoo inks in facilities that are hard to sanitize, such as carpeted areas
- Ink or ink components left uncovered, especially near open air ducts
- Unsanitary mixing of tattoo inks, including with unclean utensils or containers
- Lack of appropriate attire by staff, failure to use hairnets, lab coats, aprons, gowns, masks, or gloves
“Infections will often spread along the drainage channels in the skin and create squiggly, uneven lines of big red, lumpy nodules,” Dr. Brod said.
Between 2003 and 2023, there were 18 recalls of tattoo inks that were contaminated with various microorganisms, according to the FDA. In May 2019, the FDA issued a safety alert advising consumers, tattoo artists, and retailers to avoid using or selling certain tattoo inks contaminated with microorganisms.
Reputable ink manufacturers use a process called gamma radiation, which refers to electromagnetic radiation of high frequencies to kill microorganisms in the ink and its packaging.
Most of the trustworthy, high-quality ink manufacturers are well-known among tattoo artists, Ms. Donohue said.
While she has seen customers with sensitive skin have allergic reactions, she has not seen someone come back with an infection in her 9 years working in the tattoo industry.
Because tattoo ink is considered a cosmetic product, there is not much regulatory oversight involved, which means the sterility and quality of ingredients vary, said Teo Soleymani, MD, an assistant clinical professor of dermatology and dermatological surgery at the UCLA David Geffen School of Medicine.
“Cosmeceuticals aren’t regulated by the FDA like prescription medication,” he said. “What we’ve seen many times is inadvertent contamination during the application process or contamination while the inks are being made.”
In years past, unclean needles spreading hepatitis and HIV were more of a concern, but those rates have dropped significantly, Dr. Soleymani said.
The infections that have increased are from rare bacteria that exist in stagnant water. And they are injected into a part of the body that allows them to evade the immune system, he said: shallow enough that there aren’t many associated blood vessels, but not still below the layer of skin that gets sloughed off every 28 days.
Sometimes, antibiotics alone won’t cut it, and the tattoo will require surgical removal.
“The aesthetic you were going for has to be not only removed, but you’re left with a surgical scar,” Dr. Soleymani said. “Tattoos can be beautiful, but they can come with unwanted visitors that can cause months of misery.”
A version of this article first appeared on WebMD.com.
The Food and Drug Administration draft guidance released recently on possible contamination of tattoo ink was not concerning Whitney Donohue, 34, owner of Forget Me Not Tattoo in Billings, Mont.
“I get our ink directly through the manufacturer – not at a store or through Amazon or eBay,” she said. “You never know if it’s going to be repackaged.”
Tattoo artists themselves, she said, regulate the quality of ink they use.
Still, the threat is real, said Bruce Brod, MD, a clinical professor of dermatology at the University of Pennsylvania Health System. “I’ve seen several different infections from tattooing, and they are from organisms that tend to contaminate things in damp, liquid-type environments.”
, dermatologists said.
“Tattooing involves puncturing the epidermis about 100 times per second with needles and depositing ink 1.5 to 2 millimeters below the surface of the skin, deep into the dermis,” the guidance states. “Contaminated tattoo ink can cause infections and serious injuries. Because these inks are injected, pathogens or other harmful substances in these inks can travel from the injection site through the blood and lymphatic systems to other parts of the body.”
The guidance comes as body art continues to get more popular. According to a 2019 poll, 30% of Americans had at least one tattoo – up from 21% in 2012. Forty percent of people 18-34 and 36% of those ages 35-54 had at least one tattoo. And though they are commonplace, tattoos come with medical risks that should be known beforehand, doctors said.
Commonly reported symptoms of tattoo ink–associated infections include rashes, blisters, painful nodules, and severe abscesses. One of the most common bacteria found in contaminated tattoo ink is nontuberculous mycobacteria, which is related to the bacteria that causes tuberculosis and can be found in soil and water.
The guidance lists several unsanitary manufacturing conditions that may lead to ink contamination, including:
- Preparing or packing of tattoo inks in facilities that are hard to sanitize, such as carpeted areas
- Ink or ink components left uncovered, especially near open air ducts
- Unsanitary mixing of tattoo inks, including with unclean utensils or containers
- Lack of appropriate attire by staff, failure to use hairnets, lab coats, aprons, gowns, masks, or gloves
“Infections will often spread along the drainage channels in the skin and create squiggly, uneven lines of big red, lumpy nodules,” Dr. Brod said.
Between 2003 and 2023, there were 18 recalls of tattoo inks that were contaminated with various microorganisms, according to the FDA. In May 2019, the FDA issued a safety alert advising consumers, tattoo artists, and retailers to avoid using or selling certain tattoo inks contaminated with microorganisms.
Reputable ink manufacturers use a process called gamma radiation, which refers to electromagnetic radiation of high frequencies to kill microorganisms in the ink and its packaging.
Most of the trustworthy, high-quality ink manufacturers are well-known among tattoo artists, Ms. Donohue said.
While she has seen customers with sensitive skin have allergic reactions, she has not seen someone come back with an infection in her 9 years working in the tattoo industry.
Because tattoo ink is considered a cosmetic product, there is not much regulatory oversight involved, which means the sterility and quality of ingredients vary, said Teo Soleymani, MD, an assistant clinical professor of dermatology and dermatological surgery at the UCLA David Geffen School of Medicine.
“Cosmeceuticals aren’t regulated by the FDA like prescription medication,” he said. “What we’ve seen many times is inadvertent contamination during the application process or contamination while the inks are being made.”
In years past, unclean needles spreading hepatitis and HIV were more of a concern, but those rates have dropped significantly, Dr. Soleymani said.
The infections that have increased are from rare bacteria that exist in stagnant water. And they are injected into a part of the body that allows them to evade the immune system, he said: shallow enough that there aren’t many associated blood vessels, but not still below the layer of skin that gets sloughed off every 28 days.
Sometimes, antibiotics alone won’t cut it, and the tattoo will require surgical removal.
“The aesthetic you were going for has to be not only removed, but you’re left with a surgical scar,” Dr. Soleymani said. “Tattoos can be beautiful, but they can come with unwanted visitors that can cause months of misery.”
A version of this article first appeared on WebMD.com.
After Yusimry’s steep discount, little clarity on future adalimumab biosimilar pricing
Adalimumab, sold under the brand name Humira, enjoyed a long run as one of the world’s best-selling medicines. But its 20-year, competition-free period has ended, and despite its best efforts to delay their arrival, drug manufacturer AbbVie now faces increasing competition from biosimilars entering the marketplace.
But one biosimilar about to be launched may be something of a game changer. Coherus BioSciences has announced plans to market its biosimilar Yusimry (adalimumab-aqvh) at a cost of $995 for two autoinjectors. This represents an approximate 85% discount over Humira’s sale list price of $6922.
This price, however, is slated to plunge even further as Coherus has also revealed that it will work with the Mark Cuban Cost Plus Drug Company (MCCPDC) to offer an even lower price. When Yusimry launches in July, it will sell for about $579 for two autoinjectors, making it the lowest-priced adalimumab biosimilar on the market.
“Coherus and Cost Plus Drug Company share a common mission, to increase access to high-quality medicine for patients at an affordable price,” said Dennis Lanfear, MBA, president, CEO and chairman of Coherus. “Mark Cuban and his team offer innovative solutions to health care problems, and Coherus is also a highly innovative company focused on unmet patient needs.”
He noted that, with adalimumab biosimilar pricing, this translates to a low list price approach. “We are pleased that Yusimry will be a part of that, as the first biologic they carry,” Mr. Lanfear said.
MCCPDC prices are based on the cost of ingredients and manufacturing plus 15% margin, a $3 pharmacy dispensing fee, and a $5 shipping fee. The company has expanded its inventory from 100 generics to more than 350 medications since it launched in January 2022. While MCCPDC is primarily directed to people who are paying cash for drugs, it does take insurance from select plans. And even for people who are covered by other insurers, the cost of drugs from Mr. Cuban’s company may be less than their out-of-pocket costs if they did go through their payer.
The low pricing of Yusimry is welcome, said Marcus Snow, MD, an assistant professor in the division of rheumatology at the University of Nebraska Medical Center, Omaha, but he pointed out that it is still a very expensive drug. “For patients who can’t afford Humira due to poor insurance coverage and high out-of-pocket costs, it is a welcome option. But it’s also unclear how many patients who lack adequate health insurance coverage can afford to pay $579 a month out of their own pockets.”
The biosimilars are coming
By early December 2022, the Food and Drug Administration had approved seven Humira biosimilars, and Amgen launched the first biosimilar to come on the market, Amjevita, soon afterward. By July 2023, half a dozen more are expected to enter the marketplace, said Steven Horvitz, managing director of EMC Analytics Group, a pharmaceutical research firm.
Mr. Horvitz agrees that the system is out of control, but it is unclear how much of an effect the low price tag on the Coherus product will have. “Some insurers may say, ‘we want the lowest price, and we don’t care about rebates,’ and will go with it,” he said. “PBMs [pharmacy benefit managers] are all about economics, so we have to see how many of their major clients will ask for the lowest price.”
Amgen has more or less followed the status quo on pricing for its biosimilar, but with a twist. It›s being offered at two different prices: $85,494 a year, which is only a 5% discount from Humira’s list price, or at $40,497 a year, a 55% discount. However, to date, the lower price has generally not been granted favorable formulary placement by PBMs. The plans that adopt the higher-priced biosimilar will get bigger rebates, but patients with coinsurance and deductibles will pay more out of pocket.
It is yet unknown how the pricing on Yusimry will affect the biosimilars ready to launch. “Will it give them pause for thought or not make any difference?” Mr. Horvitz said. “The companies do not reveal their pricing before the fact, so we have to wait and see.”
Large PBMs have not jumped at the opportunity to offer the Coherus biosimilar, but SmithRx, which bills itself as “next-generation pharmacy benefits management,” announced that it will offer Yusimry to its members at a discount of more than 90%.
“Unlike traditional PBMs, SmithRx prioritizes transparency and up-front cost savings. Humira is often an employer’s top drug expense so offering a low-cost alternative will have significant impact,” Jake Frenz, CEO and founder of SmithRx, said in a statement. “We’re excited to work with Cost Plus Drugs to bring this biosimilar to our members – and significantly reduce costs for them and their employers.”
A version of this article first appeared on Medscape.com.
Adalimumab, sold under the brand name Humira, enjoyed a long run as one of the world’s best-selling medicines. But its 20-year, competition-free period has ended, and despite its best efforts to delay their arrival, drug manufacturer AbbVie now faces increasing competition from biosimilars entering the marketplace.
But one biosimilar about to be launched may be something of a game changer. Coherus BioSciences has announced plans to market its biosimilar Yusimry (adalimumab-aqvh) at a cost of $995 for two autoinjectors. This represents an approximate 85% discount over Humira’s sale list price of $6922.
This price, however, is slated to plunge even further as Coherus has also revealed that it will work with the Mark Cuban Cost Plus Drug Company (MCCPDC) to offer an even lower price. When Yusimry launches in July, it will sell for about $579 for two autoinjectors, making it the lowest-priced adalimumab biosimilar on the market.
“Coherus and Cost Plus Drug Company share a common mission, to increase access to high-quality medicine for patients at an affordable price,” said Dennis Lanfear, MBA, president, CEO and chairman of Coherus. “Mark Cuban and his team offer innovative solutions to health care problems, and Coherus is also a highly innovative company focused on unmet patient needs.”
He noted that, with adalimumab biosimilar pricing, this translates to a low list price approach. “We are pleased that Yusimry will be a part of that, as the first biologic they carry,” Mr. Lanfear said.
MCCPDC prices are based on the cost of ingredients and manufacturing plus 15% margin, a $3 pharmacy dispensing fee, and a $5 shipping fee. The company has expanded its inventory from 100 generics to more than 350 medications since it launched in January 2022. While MCCPDC is primarily directed to people who are paying cash for drugs, it does take insurance from select plans. And even for people who are covered by other insurers, the cost of drugs from Mr. Cuban’s company may be less than their out-of-pocket costs if they did go through their payer.
The low pricing of Yusimry is welcome, said Marcus Snow, MD, an assistant professor in the division of rheumatology at the University of Nebraska Medical Center, Omaha, but he pointed out that it is still a very expensive drug. “For patients who can’t afford Humira due to poor insurance coverage and high out-of-pocket costs, it is a welcome option. But it’s also unclear how many patients who lack adequate health insurance coverage can afford to pay $579 a month out of their own pockets.”
The biosimilars are coming
By early December 2022, the Food and Drug Administration had approved seven Humira biosimilars, and Amgen launched the first biosimilar to come on the market, Amjevita, soon afterward. By July 2023, half a dozen more are expected to enter the marketplace, said Steven Horvitz, managing director of EMC Analytics Group, a pharmaceutical research firm.
Mr. Horvitz agrees that the system is out of control, but it is unclear how much of an effect the low price tag on the Coherus product will have. “Some insurers may say, ‘we want the lowest price, and we don’t care about rebates,’ and will go with it,” he said. “PBMs [pharmacy benefit managers] are all about economics, so we have to see how many of their major clients will ask for the lowest price.”
Amgen has more or less followed the status quo on pricing for its biosimilar, but with a twist. It›s being offered at two different prices: $85,494 a year, which is only a 5% discount from Humira’s list price, or at $40,497 a year, a 55% discount. However, to date, the lower price has generally not been granted favorable formulary placement by PBMs. The plans that adopt the higher-priced biosimilar will get bigger rebates, but patients with coinsurance and deductibles will pay more out of pocket.
It is yet unknown how the pricing on Yusimry will affect the biosimilars ready to launch. “Will it give them pause for thought or not make any difference?” Mr. Horvitz said. “The companies do not reveal their pricing before the fact, so we have to wait and see.”
Large PBMs have not jumped at the opportunity to offer the Coherus biosimilar, but SmithRx, which bills itself as “next-generation pharmacy benefits management,” announced that it will offer Yusimry to its members at a discount of more than 90%.
“Unlike traditional PBMs, SmithRx prioritizes transparency and up-front cost savings. Humira is often an employer’s top drug expense so offering a low-cost alternative will have significant impact,” Jake Frenz, CEO and founder of SmithRx, said in a statement. “We’re excited to work with Cost Plus Drugs to bring this biosimilar to our members – and significantly reduce costs for them and their employers.”
A version of this article first appeared on Medscape.com.
Adalimumab, sold under the brand name Humira, enjoyed a long run as one of the world’s best-selling medicines. But its 20-year, competition-free period has ended, and despite its best efforts to delay their arrival, drug manufacturer AbbVie now faces increasing competition from biosimilars entering the marketplace.
But one biosimilar about to be launched may be something of a game changer. Coherus BioSciences has announced plans to market its biosimilar Yusimry (adalimumab-aqvh) at a cost of $995 for two autoinjectors. This represents an approximate 85% discount over Humira’s sale list price of $6922.
This price, however, is slated to plunge even further as Coherus has also revealed that it will work with the Mark Cuban Cost Plus Drug Company (MCCPDC) to offer an even lower price. When Yusimry launches in July, it will sell for about $579 for two autoinjectors, making it the lowest-priced adalimumab biosimilar on the market.
“Coherus and Cost Plus Drug Company share a common mission, to increase access to high-quality medicine for patients at an affordable price,” said Dennis Lanfear, MBA, president, CEO and chairman of Coherus. “Mark Cuban and his team offer innovative solutions to health care problems, and Coherus is also a highly innovative company focused on unmet patient needs.”
He noted that, with adalimumab biosimilar pricing, this translates to a low list price approach. “We are pleased that Yusimry will be a part of that, as the first biologic they carry,” Mr. Lanfear said.
MCCPDC prices are based on the cost of ingredients and manufacturing plus 15% margin, a $3 pharmacy dispensing fee, and a $5 shipping fee. The company has expanded its inventory from 100 generics to more than 350 medications since it launched in January 2022. While MCCPDC is primarily directed to people who are paying cash for drugs, it does take insurance from select plans. And even for people who are covered by other insurers, the cost of drugs from Mr. Cuban’s company may be less than their out-of-pocket costs if they did go through their payer.
The low pricing of Yusimry is welcome, said Marcus Snow, MD, an assistant professor in the division of rheumatology at the University of Nebraska Medical Center, Omaha, but he pointed out that it is still a very expensive drug. “For patients who can’t afford Humira due to poor insurance coverage and high out-of-pocket costs, it is a welcome option. But it’s also unclear how many patients who lack adequate health insurance coverage can afford to pay $579 a month out of their own pockets.”
The biosimilars are coming
By early December 2022, the Food and Drug Administration had approved seven Humira biosimilars, and Amgen launched the first biosimilar to come on the market, Amjevita, soon afterward. By July 2023, half a dozen more are expected to enter the marketplace, said Steven Horvitz, managing director of EMC Analytics Group, a pharmaceutical research firm.
Mr. Horvitz agrees that the system is out of control, but it is unclear how much of an effect the low price tag on the Coherus product will have. “Some insurers may say, ‘we want the lowest price, and we don’t care about rebates,’ and will go with it,” he said. “PBMs [pharmacy benefit managers] are all about economics, so we have to see how many of their major clients will ask for the lowest price.”
Amgen has more or less followed the status quo on pricing for its biosimilar, but with a twist. It›s being offered at two different prices: $85,494 a year, which is only a 5% discount from Humira’s list price, or at $40,497 a year, a 55% discount. However, to date, the lower price has generally not been granted favorable formulary placement by PBMs. The plans that adopt the higher-priced biosimilar will get bigger rebates, but patients with coinsurance and deductibles will pay more out of pocket.
It is yet unknown how the pricing on Yusimry will affect the biosimilars ready to launch. “Will it give them pause for thought or not make any difference?” Mr. Horvitz said. “The companies do not reveal their pricing before the fact, so we have to wait and see.”
Large PBMs have not jumped at the opportunity to offer the Coherus biosimilar, but SmithRx, which bills itself as “next-generation pharmacy benefits management,” announced that it will offer Yusimry to its members at a discount of more than 90%.
“Unlike traditional PBMs, SmithRx prioritizes transparency and up-front cost savings. Humira is often an employer’s top drug expense so offering a low-cost alternative will have significant impact,” Jake Frenz, CEO and founder of SmithRx, said in a statement. “We’re excited to work with Cost Plus Drugs to bring this biosimilar to our members – and significantly reduce costs for them and their employers.”
A version of this article first appeared on Medscape.com.
Experts share their sun protection tips for children
“I basically say, ‘sun protection means clothing, shade, [considering the] time of day of exposure, and sunscreen if you are going to be otherwise exposed,’ ” Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady’s Children’s Hospital, San Diego, said during a panel discussion about sunscreen use at the Hawaii Dermatology Seminar provided by MedscapeLIVE! He recommends photoprotective gear such as rash guards for surfers and other water sport enthusiasts. When patients ask him if they should use sunscreen, he often replies with a question of his own.
“Do you brush your teeth?” he’ll ask.
“Yes, I do.”
“Well, you should put sunscreen on every day.”
Another panelist, Adelaide A. Hebert, MD, professor of dermatology and pediatrics and chief of pediatric dermatology at the University of Texas, Houston, said that she advises new parents to start sun protection efforts early. “Most sunscreens are not approved for use in children under the age of 6 months because testing has not been done in this age group, but I do recommend protective clothing. I also recommend wrap-around sunglasses, which offer 5% more protection from the sun than regular sunglasses.”
In her opinion, stick sunscreens are “a good add-on,” especially for under the eyes and the backs of the hands, but she is not a fan of spray sunscreens, which can leave large areas of skin unprotected if not applied properly.
Fellow panelist Jennifer Huang, MD, a pediatric dermatologist at Boston Children’s Hospital, who has a special interest in taking care of dermatologic conditions of children with cancer, generally recommends mineral-based sunscreens. “There is data to suggest that nonmineral sunscreens are less safe than mineral sunscreens for humans, and mineral sunscreens are considered to be better for the environment,” Dr. Huang said. “Plus, there are more elegant versions of mineral sunscreens that don’t make your skin pasty white.” However, for patients with darker skin tones, “it can be hard to apply a pasty white sunscreen, so I lean on some recommendations for tinted sunscreens, too, so there are options. I specifically recommend sunscreens that have iron oxides in them so that it can block physical rays and help with the cosmetic appearance.”
Moise Levy, MD, professor of internal medicine and pediatrics at the University of Texas at Austin, said that his approach to imparting sunscreen advice to children and their parents involves a mix of spoken information, printed information, and sunscreen samples for children to try in the office, in the presence of a parent. To help patients choose among different samples, be they ointments, gels, or lotions, he will often ask the child: “‘What do you like the feel of better?’ If the child says, ‘I like this one,’ I make sure the parent hears that,” Dr. Levy said.
Next, Dr. Eichenfield, who moderated the discussion, asked his fellow panelists how they would counsel someone who comes to their practice for evaluation of moles and has a family history of nonmelanoma skin cancer. “I think this is one of the easier counseling sessions, because there are enough kids who are asked about the moles on their skin when they’re at school,” Dr. Hebert said. “I think they’re very ready to wear sun protective clothing and I certainly don’t want any sun exposure that would pose an increased risk for their child.”
In addition to routine sun protection, Dr. Huang recommends annual mole checks for children who have a first-degree relative with a history of malignant melanoma. Other high-risk groups that should undergo annual skin exams include anyone who has received high doses of radiation, bone marrow transplants, prolonged use of voriconazole, or prolonged systemic immunosuppression. Without a known genetic predisposition syndrome, a family history of nonmelanoma skin cancer would not raise concern for melanoma in an otherwise healthy child.
Dr. Eichenfield added that freckling used to be the secondary risk factor for melanoma, “but it’s flipped over to a primary risk factor. A history of immunosuppression or prior cancer is a major risk factor in childhood and teenage years.”
Dr. Eichenfield disclosed that he is a consultant or adviser for numerous pharmaceutical companies. He has also received research funding from AbbVie, Bausch & Lomb, Galderma Laboratories, and Pfizer. Dr. Hebert disclosed that she is a consultant or adviser for AbbVie, Almirall, Amryt Pharma, Arcutis Biotherapeutics, Beiersdorf, Dermavant Sciences, Galderma Laboratories, L’Oreal, Novan, Ortho Dermatologics, Pfizer, and Verrica. Dr. Levy disclosed that he is consultant or adviser for Abeona, Castle Creek, Dusa Pharma, Krystal Bio, Novan, Regeneron, and Sanofi Genzyme. Dr. Huang disclosed that she is an adviser for EllaOla.
MedscapeLive! and this news organization are owned by the same parent company.
“I basically say, ‘sun protection means clothing, shade, [considering the] time of day of exposure, and sunscreen if you are going to be otherwise exposed,’ ” Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady’s Children’s Hospital, San Diego, said during a panel discussion about sunscreen use at the Hawaii Dermatology Seminar provided by MedscapeLIVE! He recommends photoprotective gear such as rash guards for surfers and other water sport enthusiasts. When patients ask him if they should use sunscreen, he often replies with a question of his own.
“Do you brush your teeth?” he’ll ask.
“Yes, I do.”
“Well, you should put sunscreen on every day.”
Another panelist, Adelaide A. Hebert, MD, professor of dermatology and pediatrics and chief of pediatric dermatology at the University of Texas, Houston, said that she advises new parents to start sun protection efforts early. “Most sunscreens are not approved for use in children under the age of 6 months because testing has not been done in this age group, but I do recommend protective clothing. I also recommend wrap-around sunglasses, which offer 5% more protection from the sun than regular sunglasses.”
In her opinion, stick sunscreens are “a good add-on,” especially for under the eyes and the backs of the hands, but she is not a fan of spray sunscreens, which can leave large areas of skin unprotected if not applied properly.
Fellow panelist Jennifer Huang, MD, a pediatric dermatologist at Boston Children’s Hospital, who has a special interest in taking care of dermatologic conditions of children with cancer, generally recommends mineral-based sunscreens. “There is data to suggest that nonmineral sunscreens are less safe than mineral sunscreens for humans, and mineral sunscreens are considered to be better for the environment,” Dr. Huang said. “Plus, there are more elegant versions of mineral sunscreens that don’t make your skin pasty white.” However, for patients with darker skin tones, “it can be hard to apply a pasty white sunscreen, so I lean on some recommendations for tinted sunscreens, too, so there are options. I specifically recommend sunscreens that have iron oxides in them so that it can block physical rays and help with the cosmetic appearance.”
Moise Levy, MD, professor of internal medicine and pediatrics at the University of Texas at Austin, said that his approach to imparting sunscreen advice to children and their parents involves a mix of spoken information, printed information, and sunscreen samples for children to try in the office, in the presence of a parent. To help patients choose among different samples, be they ointments, gels, or lotions, he will often ask the child: “‘What do you like the feel of better?’ If the child says, ‘I like this one,’ I make sure the parent hears that,” Dr. Levy said.
Next, Dr. Eichenfield, who moderated the discussion, asked his fellow panelists how they would counsel someone who comes to their practice for evaluation of moles and has a family history of nonmelanoma skin cancer. “I think this is one of the easier counseling sessions, because there are enough kids who are asked about the moles on their skin when they’re at school,” Dr. Hebert said. “I think they’re very ready to wear sun protective clothing and I certainly don’t want any sun exposure that would pose an increased risk for their child.”
In addition to routine sun protection, Dr. Huang recommends annual mole checks for children who have a first-degree relative with a history of malignant melanoma. Other high-risk groups that should undergo annual skin exams include anyone who has received high doses of radiation, bone marrow transplants, prolonged use of voriconazole, or prolonged systemic immunosuppression. Without a known genetic predisposition syndrome, a family history of nonmelanoma skin cancer would not raise concern for melanoma in an otherwise healthy child.
Dr. Eichenfield added that freckling used to be the secondary risk factor for melanoma, “but it’s flipped over to a primary risk factor. A history of immunosuppression or prior cancer is a major risk factor in childhood and teenage years.”
Dr. Eichenfield disclosed that he is a consultant or adviser for numerous pharmaceutical companies. He has also received research funding from AbbVie, Bausch & Lomb, Galderma Laboratories, and Pfizer. Dr. Hebert disclosed that she is a consultant or adviser for AbbVie, Almirall, Amryt Pharma, Arcutis Biotherapeutics, Beiersdorf, Dermavant Sciences, Galderma Laboratories, L’Oreal, Novan, Ortho Dermatologics, Pfizer, and Verrica. Dr. Levy disclosed that he is consultant or adviser for Abeona, Castle Creek, Dusa Pharma, Krystal Bio, Novan, Regeneron, and Sanofi Genzyme. Dr. Huang disclosed that she is an adviser for EllaOla.
MedscapeLive! and this news organization are owned by the same parent company.
“I basically say, ‘sun protection means clothing, shade, [considering the] time of day of exposure, and sunscreen if you are going to be otherwise exposed,’ ” Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady’s Children’s Hospital, San Diego, said during a panel discussion about sunscreen use at the Hawaii Dermatology Seminar provided by MedscapeLIVE! He recommends photoprotective gear such as rash guards for surfers and other water sport enthusiasts. When patients ask him if they should use sunscreen, he often replies with a question of his own.
“Do you brush your teeth?” he’ll ask.
“Yes, I do.”
“Well, you should put sunscreen on every day.”
Another panelist, Adelaide A. Hebert, MD, professor of dermatology and pediatrics and chief of pediatric dermatology at the University of Texas, Houston, said that she advises new parents to start sun protection efforts early. “Most sunscreens are not approved for use in children under the age of 6 months because testing has not been done in this age group, but I do recommend protective clothing. I also recommend wrap-around sunglasses, which offer 5% more protection from the sun than regular sunglasses.”
In her opinion, stick sunscreens are “a good add-on,” especially for under the eyes and the backs of the hands, but she is not a fan of spray sunscreens, which can leave large areas of skin unprotected if not applied properly.
Fellow panelist Jennifer Huang, MD, a pediatric dermatologist at Boston Children’s Hospital, who has a special interest in taking care of dermatologic conditions of children with cancer, generally recommends mineral-based sunscreens. “There is data to suggest that nonmineral sunscreens are less safe than mineral sunscreens for humans, and mineral sunscreens are considered to be better for the environment,” Dr. Huang said. “Plus, there are more elegant versions of mineral sunscreens that don’t make your skin pasty white.” However, for patients with darker skin tones, “it can be hard to apply a pasty white sunscreen, so I lean on some recommendations for tinted sunscreens, too, so there are options. I specifically recommend sunscreens that have iron oxides in them so that it can block physical rays and help with the cosmetic appearance.”
Moise Levy, MD, professor of internal medicine and pediatrics at the University of Texas at Austin, said that his approach to imparting sunscreen advice to children and their parents involves a mix of spoken information, printed information, and sunscreen samples for children to try in the office, in the presence of a parent. To help patients choose among different samples, be they ointments, gels, or lotions, he will often ask the child: “‘What do you like the feel of better?’ If the child says, ‘I like this one,’ I make sure the parent hears that,” Dr. Levy said.
Next, Dr. Eichenfield, who moderated the discussion, asked his fellow panelists how they would counsel someone who comes to their practice for evaluation of moles and has a family history of nonmelanoma skin cancer. “I think this is one of the easier counseling sessions, because there are enough kids who are asked about the moles on their skin when they’re at school,” Dr. Hebert said. “I think they’re very ready to wear sun protective clothing and I certainly don’t want any sun exposure that would pose an increased risk for their child.”
In addition to routine sun protection, Dr. Huang recommends annual mole checks for children who have a first-degree relative with a history of malignant melanoma. Other high-risk groups that should undergo annual skin exams include anyone who has received high doses of radiation, bone marrow transplants, prolonged use of voriconazole, or prolonged systemic immunosuppression. Without a known genetic predisposition syndrome, a family history of nonmelanoma skin cancer would not raise concern for melanoma in an otherwise healthy child.
Dr. Eichenfield added that freckling used to be the secondary risk factor for melanoma, “but it’s flipped over to a primary risk factor. A history of immunosuppression or prior cancer is a major risk factor in childhood and teenage years.”
Dr. Eichenfield disclosed that he is a consultant or adviser for numerous pharmaceutical companies. He has also received research funding from AbbVie, Bausch & Lomb, Galderma Laboratories, and Pfizer. Dr. Hebert disclosed that she is a consultant or adviser for AbbVie, Almirall, Amryt Pharma, Arcutis Biotherapeutics, Beiersdorf, Dermavant Sciences, Galderma Laboratories, L’Oreal, Novan, Ortho Dermatologics, Pfizer, and Verrica. Dr. Levy disclosed that he is consultant or adviser for Abeona, Castle Creek, Dusa Pharma, Krystal Bio, Novan, Regeneron, and Sanofi Genzyme. Dr. Huang disclosed that she is an adviser for EllaOla.
MedscapeLive! and this news organization are owned by the same parent company.
FROM THE MEDSCAPELIVE! HAWAII DERMATOLOGY SEMINAR
Low-dose oral minoxidil for hair loss soars after NYT article
.
The weekly rate of first-time low-dose oral minoxidil (LDOM) prescriptions per 10,000 outpatient encounters was “significantly higher 8 weeks after vs. 8 weeks before article publication,” at 0.9 prescriptions, compared with 0.5 per 10,000, wrote the authors of the research letter, published in JAMA Network Open. There was no similar bump for first-time finasteride or hypertension prescriptions, wrote the authors, from Harvard Medical School and Massachusetts General Hospital, Boston, and Truveta, a company that provides EHR data from U.S. health care systems.
The New York Times article noted that LDOM was relatively unknown to patients and doctors – and not approved by the Food and Drug Administration for treating hair loss – but that it was inexpensive, safe, and very effective for many individuals. “The article did not report new research findings or large-scale randomized evidence,” wrote the authors of the JAMA study.
Rodney Sinclair, MD, professor of dermatology at the University of Melbourne, who conducted the original research on LDOM and hair loss and was quoted in the Times story, told this news organization that “the sharp uplift after the New York Times article was on the back of a gradual increase.” He added that “the momentum for minoxidil prescriptions is increasing,” so much so that it has led to a global shortage of LDOM. The drug appears to still be widely available in the United States, however. It is not on the ASHP shortages list.
“There has been growing momentum for minoxidil use since I first presented our data about 6 years ago,” Dr. Sinclair said. He noted that 2022 International Society of Hair Restoration Surgery survey data found that 26% of treating physicians always or often prescribed off-label oral minoxidil, up from 10% in 2019 and 0% in 2017, while another 20% said they prescribed it sometimes.
The authors of the new study looked at prescriptions for patients at eight health care systems before and after the Times article was published in August 2022. They calculated the rate of first-time oral minoxidil prescriptions for 2.5 mg and 5 mg tablets, excluding 10 mg tablets, which are prescribed for hypertension.
Among those receiving first-time prescriptions, 2,846 received them in the 7 months before the article and 3,695 in the 5 months after publication. Men (43.6% after vs. 37.7% before publication) and White individuals (68.6% after vs. 60.8% before publication) accounted for a higher proportion of prescriptions after the article was published. There was a 2.4-fold increase in first-time prescriptions among men, and a 1.7-fold increase among females, while people with comorbidities accounted for a smaller proportion after the publication.
“Socioeconomic factors, such as access to health care and education and income levels, may be associated with individuals seeking low-dose oral minoxidil after article publication,” wrote the authors.
In an interview, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said that he was not surprised to see an uptick in prescriptions after the Times article.
He and his colleagues were curious as to whether the article might have prompted newfound interest in LDOM. They experienced an uptick at George Washington, which Dr. Friedman thought could have been because he was quoted in the Times story. He and colleagues conducted a national survey of dermatologists asking if more patients had called, emailed, or come in to the office asking about LDOM after the article’s publication. “Over 85% said yes,” Dr. Friedman said in the interview. He and his coauthors also found a huge increase in Google searches for terms such as hair loss, alopecia, and minoxidil in the weeks after the article, he said.
The results are expected to published soon in the Journal of Drugs in Dermatology.
“I think a lot of people know about [LDOM] and it’s certainly has gained a lot more attention and acceptance in recent years,” said Dr. Friedman, but he added that “there’s no question” that the Times article increased interest.
That is not necessarily a bad thing, he said. “With one article, education on a common disease was disseminated worldwide in a way that no one doctor can do,” he said. The article was truthful, evidence-based, and included expert dermatologists, he noted.
“It probably got people who never thought twice about their hair thinning to actually think that there’s hope,” he said, adding that it also likely prompted them to seek care, and, more importantly, “to seek care from the person who should be taking care of this, which is the dermatologist.”
However, the article might also inspire some people to think LDOM can help when it can’t, or they might insist on a prescription when another medication is more appropriate, said Dr. Friedman.
Both he and Dr. Sinclair expect demand for LDOM to continue increasing.
“Word of mouth will drive the next wave of prescriptions,” said Dr. Sinclair. “We are continuing to do work to improve safety, to understand its mechanism of action, and identify ways to improve equity of access to treatment for men and women who are concerned about their hair loss and motivated to treat it,” he said.
Dr. Sinclair and Dr. Friedman report no relevant financial relationships.
.
The weekly rate of first-time low-dose oral minoxidil (LDOM) prescriptions per 10,000 outpatient encounters was “significantly higher 8 weeks after vs. 8 weeks before article publication,” at 0.9 prescriptions, compared with 0.5 per 10,000, wrote the authors of the research letter, published in JAMA Network Open. There was no similar bump for first-time finasteride or hypertension prescriptions, wrote the authors, from Harvard Medical School and Massachusetts General Hospital, Boston, and Truveta, a company that provides EHR data from U.S. health care systems.
The New York Times article noted that LDOM was relatively unknown to patients and doctors – and not approved by the Food and Drug Administration for treating hair loss – but that it was inexpensive, safe, and very effective for many individuals. “The article did not report new research findings or large-scale randomized evidence,” wrote the authors of the JAMA study.
Rodney Sinclair, MD, professor of dermatology at the University of Melbourne, who conducted the original research on LDOM and hair loss and was quoted in the Times story, told this news organization that “the sharp uplift after the New York Times article was on the back of a gradual increase.” He added that “the momentum for minoxidil prescriptions is increasing,” so much so that it has led to a global shortage of LDOM. The drug appears to still be widely available in the United States, however. It is not on the ASHP shortages list.
“There has been growing momentum for minoxidil use since I first presented our data about 6 years ago,” Dr. Sinclair said. He noted that 2022 International Society of Hair Restoration Surgery survey data found that 26% of treating physicians always or often prescribed off-label oral minoxidil, up from 10% in 2019 and 0% in 2017, while another 20% said they prescribed it sometimes.
The authors of the new study looked at prescriptions for patients at eight health care systems before and after the Times article was published in August 2022. They calculated the rate of first-time oral minoxidil prescriptions for 2.5 mg and 5 mg tablets, excluding 10 mg tablets, which are prescribed for hypertension.
Among those receiving first-time prescriptions, 2,846 received them in the 7 months before the article and 3,695 in the 5 months after publication. Men (43.6% after vs. 37.7% before publication) and White individuals (68.6% after vs. 60.8% before publication) accounted for a higher proportion of prescriptions after the article was published. There was a 2.4-fold increase in first-time prescriptions among men, and a 1.7-fold increase among females, while people with comorbidities accounted for a smaller proportion after the publication.
“Socioeconomic factors, such as access to health care and education and income levels, may be associated with individuals seeking low-dose oral minoxidil after article publication,” wrote the authors.
In an interview, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said that he was not surprised to see an uptick in prescriptions after the Times article.
He and his colleagues were curious as to whether the article might have prompted newfound interest in LDOM. They experienced an uptick at George Washington, which Dr. Friedman thought could have been because he was quoted in the Times story. He and colleagues conducted a national survey of dermatologists asking if more patients had called, emailed, or come in to the office asking about LDOM after the article’s publication. “Over 85% said yes,” Dr. Friedman said in the interview. He and his coauthors also found a huge increase in Google searches for terms such as hair loss, alopecia, and minoxidil in the weeks after the article, he said.
The results are expected to published soon in the Journal of Drugs in Dermatology.
“I think a lot of people know about [LDOM] and it’s certainly has gained a lot more attention and acceptance in recent years,” said Dr. Friedman, but he added that “there’s no question” that the Times article increased interest.
That is not necessarily a bad thing, he said. “With one article, education on a common disease was disseminated worldwide in a way that no one doctor can do,” he said. The article was truthful, evidence-based, and included expert dermatologists, he noted.
“It probably got people who never thought twice about their hair thinning to actually think that there’s hope,” he said, adding that it also likely prompted them to seek care, and, more importantly, “to seek care from the person who should be taking care of this, which is the dermatologist.”
However, the article might also inspire some people to think LDOM can help when it can’t, or they might insist on a prescription when another medication is more appropriate, said Dr. Friedman.
Both he and Dr. Sinclair expect demand for LDOM to continue increasing.
“Word of mouth will drive the next wave of prescriptions,” said Dr. Sinclair. “We are continuing to do work to improve safety, to understand its mechanism of action, and identify ways to improve equity of access to treatment for men and women who are concerned about their hair loss and motivated to treat it,” he said.
Dr. Sinclair and Dr. Friedman report no relevant financial relationships.
.
The weekly rate of first-time low-dose oral minoxidil (LDOM) prescriptions per 10,000 outpatient encounters was “significantly higher 8 weeks after vs. 8 weeks before article publication,” at 0.9 prescriptions, compared with 0.5 per 10,000, wrote the authors of the research letter, published in JAMA Network Open. There was no similar bump for first-time finasteride or hypertension prescriptions, wrote the authors, from Harvard Medical School and Massachusetts General Hospital, Boston, and Truveta, a company that provides EHR data from U.S. health care systems.
The New York Times article noted that LDOM was relatively unknown to patients and doctors – and not approved by the Food and Drug Administration for treating hair loss – but that it was inexpensive, safe, and very effective for many individuals. “The article did not report new research findings or large-scale randomized evidence,” wrote the authors of the JAMA study.
Rodney Sinclair, MD, professor of dermatology at the University of Melbourne, who conducted the original research on LDOM and hair loss and was quoted in the Times story, told this news organization that “the sharp uplift after the New York Times article was on the back of a gradual increase.” He added that “the momentum for minoxidil prescriptions is increasing,” so much so that it has led to a global shortage of LDOM. The drug appears to still be widely available in the United States, however. It is not on the ASHP shortages list.
“There has been growing momentum for minoxidil use since I first presented our data about 6 years ago,” Dr. Sinclair said. He noted that 2022 International Society of Hair Restoration Surgery survey data found that 26% of treating physicians always or often prescribed off-label oral minoxidil, up from 10% in 2019 and 0% in 2017, while another 20% said they prescribed it sometimes.
The authors of the new study looked at prescriptions for patients at eight health care systems before and after the Times article was published in August 2022. They calculated the rate of first-time oral minoxidil prescriptions for 2.5 mg and 5 mg tablets, excluding 10 mg tablets, which are prescribed for hypertension.
Among those receiving first-time prescriptions, 2,846 received them in the 7 months before the article and 3,695 in the 5 months after publication. Men (43.6% after vs. 37.7% before publication) and White individuals (68.6% after vs. 60.8% before publication) accounted for a higher proportion of prescriptions after the article was published. There was a 2.4-fold increase in first-time prescriptions among men, and a 1.7-fold increase among females, while people with comorbidities accounted for a smaller proportion after the publication.
“Socioeconomic factors, such as access to health care and education and income levels, may be associated with individuals seeking low-dose oral minoxidil after article publication,” wrote the authors.
In an interview, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said that he was not surprised to see an uptick in prescriptions after the Times article.
He and his colleagues were curious as to whether the article might have prompted newfound interest in LDOM. They experienced an uptick at George Washington, which Dr. Friedman thought could have been because he was quoted in the Times story. He and colleagues conducted a national survey of dermatologists asking if more patients had called, emailed, or come in to the office asking about LDOM after the article’s publication. “Over 85% said yes,” Dr. Friedman said in the interview. He and his coauthors also found a huge increase in Google searches for terms such as hair loss, alopecia, and minoxidil in the weeks after the article, he said.
The results are expected to published soon in the Journal of Drugs in Dermatology.
“I think a lot of people know about [LDOM] and it’s certainly has gained a lot more attention and acceptance in recent years,” said Dr. Friedman, but he added that “there’s no question” that the Times article increased interest.
That is not necessarily a bad thing, he said. “With one article, education on a common disease was disseminated worldwide in a way that no one doctor can do,” he said. The article was truthful, evidence-based, and included expert dermatologists, he noted.
“It probably got people who never thought twice about their hair thinning to actually think that there’s hope,” he said, adding that it also likely prompted them to seek care, and, more importantly, “to seek care from the person who should be taking care of this, which is the dermatologist.”
However, the article might also inspire some people to think LDOM can help when it can’t, or they might insist on a prescription when another medication is more appropriate, said Dr. Friedman.
Both he and Dr. Sinclair expect demand for LDOM to continue increasing.
“Word of mouth will drive the next wave of prescriptions,” said Dr. Sinclair. “We are continuing to do work to improve safety, to understand its mechanism of action, and identify ways to improve equity of access to treatment for men and women who are concerned about their hair loss and motivated to treat it,” he said.
Dr. Sinclair and Dr. Friedman report no relevant financial relationships.
FROM JAMA NETWORK OPEN
Report eyes complications from microwave energy devices for hyperhidrosis
database showed.
While microwave energy devices (MEDs) are used to treat hyperhidrosis, the largest MED clinical trial included only 101 patients, Samantha Jo Albucker and Shari Lipner, MD, PhD, wrote in a research letter reporting the results.
For the study, published online in the Journal of the American Academy of Dermatology, Ms. Albucker, a student at Tulane University, New Orleans, and Dr. Lipner, associate professor of clinical dermatology at Weill Cornell Medicine, New York, searched the MAUDE database between Feb. 28, 2013, and Dec. 29, 2022, for adverse events (AEs) involving MEDs for hyperhidrosis treatment. Of the 502 medical device reports identified over the study period, the axilla was the most frequent injury site in 50.4% of cases. The three most common complications were infections (45.4%); neurological symptoms including neuropathy, nerve damage, and numbness (21.7%); and burns/ulcerations/erosions (19.1%).
In other findings, 2.4% of patients required hospitalization, most often because of infection (83.3%), followed by burn and coma (8.3% each). The average symptom onset was 2 months postprocedure, and the most common treatment was antibiotics in 62.2% of cases, followed by incision and drainage/aspiration in 21.7% of cases.
A codiagnosis of hidradenitis suppurativa (HS) was reported in 5.4% of all medical device reports. The researchers noted that in a published randomized clinical trial of eight HS patients undergoing MED treatment to assess the effect on HS symptoms, the treatment showed no clinical advantage. In addition, they referred to two case reports describing new-onset HS after MED treatment for hyperhidrosis.
“Therefore, we recommend questioning patients about HS history and examining for HS clinical findings before performing MED for hyperhidrosis,” they wrote, adding that the data, “taken together, suggests that avoidance of MED treatment of hyperhidrosis in HS patients is prudent and alternative treatments may be prescribed.”
The researchers acknowledged certain limitations of their analysis, including uncompleted medical device reports, patient reporting, and unverified causes of adverse events. “Large multicenter studies are needed to corroborate our results,” they concluded.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, said that primary idiopathic hyperhidrosis is a common medical condition that is often overlooked as a legitimate concern, and causes a quality-of-life burden. “Even with the striking numbers in the millions, there are limited treatment options available for axillary let alone other forms of primary hyperhidrosis,” said Dr. Friedman, who was not involved with the study.
“Therefore, for the short treatment list we have, it is important to have some predictive power with respect to clinical impact to provide realistic expectations as well as potential adverse events to ensure best practices and meaningful patient guidance. In this research letter, our colleagues highlight complications that can ensue from microwave therapy for hyperhidrosis and the frequency of said adverse events. Knowing these data is half the battle, and I for one would not have assumed infection was number one on the list of adverse events.”
Ms. Albucker had no relevant conflicts of interest to disclose. Dr. Lipner disclosed that she has served as a consultant for Ortho Dermatologics, Hoth Therapeutics, BelleTorus Corporation, and Moberg Pharmaceuticals.
Dr. Friedman disclosed that he is a consultant and/or advisory board member for Medscape/SanovaWorks, Oakstone Institute, L’Oréal, La Roche Posay, Galderma, Aveeno, Ortho Dermatologic, Microcures, Pfizer, Novartis, Lilly, Hoth Therapeutics, Zylo Therapeutics, BMS, Vial, Janssen, Novocure, Dermavant, Regeneron/Sanofi, and Incyte. He has also received grants from Pfizer, the Dermatology Foundation, Lilly, Janssen, Incyte, and Galderma.
database showed.
While microwave energy devices (MEDs) are used to treat hyperhidrosis, the largest MED clinical trial included only 101 patients, Samantha Jo Albucker and Shari Lipner, MD, PhD, wrote in a research letter reporting the results.
For the study, published online in the Journal of the American Academy of Dermatology, Ms. Albucker, a student at Tulane University, New Orleans, and Dr. Lipner, associate professor of clinical dermatology at Weill Cornell Medicine, New York, searched the MAUDE database between Feb. 28, 2013, and Dec. 29, 2022, for adverse events (AEs) involving MEDs for hyperhidrosis treatment. Of the 502 medical device reports identified over the study period, the axilla was the most frequent injury site in 50.4% of cases. The three most common complications were infections (45.4%); neurological symptoms including neuropathy, nerve damage, and numbness (21.7%); and burns/ulcerations/erosions (19.1%).
In other findings, 2.4% of patients required hospitalization, most often because of infection (83.3%), followed by burn and coma (8.3% each). The average symptom onset was 2 months postprocedure, and the most common treatment was antibiotics in 62.2% of cases, followed by incision and drainage/aspiration in 21.7% of cases.
A codiagnosis of hidradenitis suppurativa (HS) was reported in 5.4% of all medical device reports. The researchers noted that in a published randomized clinical trial of eight HS patients undergoing MED treatment to assess the effect on HS symptoms, the treatment showed no clinical advantage. In addition, they referred to two case reports describing new-onset HS after MED treatment for hyperhidrosis.
“Therefore, we recommend questioning patients about HS history and examining for HS clinical findings before performing MED for hyperhidrosis,” they wrote, adding that the data, “taken together, suggests that avoidance of MED treatment of hyperhidrosis in HS patients is prudent and alternative treatments may be prescribed.”
The researchers acknowledged certain limitations of their analysis, including uncompleted medical device reports, patient reporting, and unverified causes of adverse events. “Large multicenter studies are needed to corroborate our results,” they concluded.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, said that primary idiopathic hyperhidrosis is a common medical condition that is often overlooked as a legitimate concern, and causes a quality-of-life burden. “Even with the striking numbers in the millions, there are limited treatment options available for axillary let alone other forms of primary hyperhidrosis,” said Dr. Friedman, who was not involved with the study.
“Therefore, for the short treatment list we have, it is important to have some predictive power with respect to clinical impact to provide realistic expectations as well as potential adverse events to ensure best practices and meaningful patient guidance. In this research letter, our colleagues highlight complications that can ensue from microwave therapy for hyperhidrosis and the frequency of said adverse events. Knowing these data is half the battle, and I for one would not have assumed infection was number one on the list of adverse events.”
Ms. Albucker had no relevant conflicts of interest to disclose. Dr. Lipner disclosed that she has served as a consultant for Ortho Dermatologics, Hoth Therapeutics, BelleTorus Corporation, and Moberg Pharmaceuticals.
Dr. Friedman disclosed that he is a consultant and/or advisory board member for Medscape/SanovaWorks, Oakstone Institute, L’Oréal, La Roche Posay, Galderma, Aveeno, Ortho Dermatologic, Microcures, Pfizer, Novartis, Lilly, Hoth Therapeutics, Zylo Therapeutics, BMS, Vial, Janssen, Novocure, Dermavant, Regeneron/Sanofi, and Incyte. He has also received grants from Pfizer, the Dermatology Foundation, Lilly, Janssen, Incyte, and Galderma.
database showed.
While microwave energy devices (MEDs) are used to treat hyperhidrosis, the largest MED clinical trial included only 101 patients, Samantha Jo Albucker and Shari Lipner, MD, PhD, wrote in a research letter reporting the results.
For the study, published online in the Journal of the American Academy of Dermatology, Ms. Albucker, a student at Tulane University, New Orleans, and Dr. Lipner, associate professor of clinical dermatology at Weill Cornell Medicine, New York, searched the MAUDE database between Feb. 28, 2013, and Dec. 29, 2022, for adverse events (AEs) involving MEDs for hyperhidrosis treatment. Of the 502 medical device reports identified over the study period, the axilla was the most frequent injury site in 50.4% of cases. The three most common complications were infections (45.4%); neurological symptoms including neuropathy, nerve damage, and numbness (21.7%); and burns/ulcerations/erosions (19.1%).
In other findings, 2.4% of patients required hospitalization, most often because of infection (83.3%), followed by burn and coma (8.3% each). The average symptom onset was 2 months postprocedure, and the most common treatment was antibiotics in 62.2% of cases, followed by incision and drainage/aspiration in 21.7% of cases.
A codiagnosis of hidradenitis suppurativa (HS) was reported in 5.4% of all medical device reports. The researchers noted that in a published randomized clinical trial of eight HS patients undergoing MED treatment to assess the effect on HS symptoms, the treatment showed no clinical advantage. In addition, they referred to two case reports describing new-onset HS after MED treatment for hyperhidrosis.
“Therefore, we recommend questioning patients about HS history and examining for HS clinical findings before performing MED for hyperhidrosis,” they wrote, adding that the data, “taken together, suggests that avoidance of MED treatment of hyperhidrosis in HS patients is prudent and alternative treatments may be prescribed.”
The researchers acknowledged certain limitations of their analysis, including uncompleted medical device reports, patient reporting, and unverified causes of adverse events. “Large multicenter studies are needed to corroborate our results,” they concluded.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, said that primary idiopathic hyperhidrosis is a common medical condition that is often overlooked as a legitimate concern, and causes a quality-of-life burden. “Even with the striking numbers in the millions, there are limited treatment options available for axillary let alone other forms of primary hyperhidrosis,” said Dr. Friedman, who was not involved with the study.
“Therefore, for the short treatment list we have, it is important to have some predictive power with respect to clinical impact to provide realistic expectations as well as potential adverse events to ensure best practices and meaningful patient guidance. In this research letter, our colleagues highlight complications that can ensue from microwave therapy for hyperhidrosis and the frequency of said adverse events. Knowing these data is half the battle, and I for one would not have assumed infection was number one on the list of adverse events.”
Ms. Albucker had no relevant conflicts of interest to disclose. Dr. Lipner disclosed that she has served as a consultant for Ortho Dermatologics, Hoth Therapeutics, BelleTorus Corporation, and Moberg Pharmaceuticals.
Dr. Friedman disclosed that he is a consultant and/or advisory board member for Medscape/SanovaWorks, Oakstone Institute, L’Oréal, La Roche Posay, Galderma, Aveeno, Ortho Dermatologic, Microcures, Pfizer, Novartis, Lilly, Hoth Therapeutics, Zylo Therapeutics, BMS, Vial, Janssen, Novocure, Dermavant, Regeneron/Sanofi, and Incyte. He has also received grants from Pfizer, the Dermatology Foundation, Lilly, Janssen, Incyte, and Galderma.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Crusted scalp rash

Dermoscopy showed not only the erythema, inflammation, and crusting visible during the initial examination, but it also revealed that each lesion had a hair growing through it. This pointed to a diagnosis of superficial folliculitis of the scalp.
The physician ruled out tinea capitis, acne keloidalis nuchae, and scarring alopecia based on the dermoscopic exam. There were no broken hairs that one would expect with tinea capitis. Also, there was no polytrichia (multiple hairs pushed into a single follicular opening due to scarring of the skin) that would be expected with acne keloidalis nuchae and scarring alopecias.
There are multiple types of scalp folliculitis. This patient had superficial folliculitis, in which pustules develop at the ostium of the hair follicles. Deep folliculitis is more severe and includes furuncles and carbuncles.1
Folliculitis is usually caused by a bacterial infection and, less commonly, fungal infection. In addition to superficial and deep folliculitis, inflammation with scarring of the follicles occurs with folliculitis decalvans, which is one of the scarring alopecias.1
Mild cases of superficial bacterial folliculitis are treated with topical antibiotics (eg, topical clindamycin 1% applied twice daily). Depending on the severity, oral antibiotics including doxycycline 100 mg twice daily for 7 days or trimethoprim sulfamethoxazole 160 mg/800 mg (double strength) twice daily for 7 days may be used. There is also a chronic nonscarring form of scalp folliculitis that often responds initially to antibiotics but then recurs. This has been treated with longer courses of oral antibiotics and, if the lesions don’t respond or continue to recur, with low-dose isotretinoin.2
Due to the amount of scalp involvement, crusting, and inflammation seen on this patient’s scalp, he was treated with trimethoprim sulfamethoxazole 160 mg/800 mg twice daily for 7 days. After 1 week, he reported that he was doing much better and that the lesions had nearly resolved. He was told to return for reevaluation if the lesions did not completely resolve.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Lugović-Mihić L, Barisić F, Bulat V, et al. Differential diagnosis of the scalp hair folliculitis. Acta Clin Croat. 2011;50:395-402.
2. Romero-Maté A, Arias-Palomo D, Hernández-Núñez A, et al. Chronic nonscarring scalp folliculitis: retrospective case series study of 34 cases. J Am Acad Dermatol. 2019;81:1023-1024. doi: 10.1016/j.jaad.2019.02.065

Dermoscopy showed not only the erythema, inflammation, and crusting visible during the initial examination, but it also revealed that each lesion had a hair growing through it. This pointed to a diagnosis of superficial folliculitis of the scalp.
The physician ruled out tinea capitis, acne keloidalis nuchae, and scarring alopecia based on the dermoscopic exam. There were no broken hairs that one would expect with tinea capitis. Also, there was no polytrichia (multiple hairs pushed into a single follicular opening due to scarring of the skin) that would be expected with acne keloidalis nuchae and scarring alopecias.
There are multiple types of scalp folliculitis. This patient had superficial folliculitis, in which pustules develop at the ostium of the hair follicles. Deep folliculitis is more severe and includes furuncles and carbuncles.1
Folliculitis is usually caused by a bacterial infection and, less commonly, fungal infection. In addition to superficial and deep folliculitis, inflammation with scarring of the follicles occurs with folliculitis decalvans, which is one of the scarring alopecias.1
Mild cases of superficial bacterial folliculitis are treated with topical antibiotics (eg, topical clindamycin 1% applied twice daily). Depending on the severity, oral antibiotics including doxycycline 100 mg twice daily for 7 days or trimethoprim sulfamethoxazole 160 mg/800 mg (double strength) twice daily for 7 days may be used. There is also a chronic nonscarring form of scalp folliculitis that often responds initially to antibiotics but then recurs. This has been treated with longer courses of oral antibiotics and, if the lesions don’t respond or continue to recur, with low-dose isotretinoin.2
Due to the amount of scalp involvement, crusting, and inflammation seen on this patient’s scalp, he was treated with trimethoprim sulfamethoxazole 160 mg/800 mg twice daily for 7 days. After 1 week, he reported that he was doing much better and that the lesions had nearly resolved. He was told to return for reevaluation if the lesions did not completely resolve.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

Dermoscopy showed not only the erythema, inflammation, and crusting visible during the initial examination, but it also revealed that each lesion had a hair growing through it. This pointed to a diagnosis of superficial folliculitis of the scalp.
The physician ruled out tinea capitis, acne keloidalis nuchae, and scarring alopecia based on the dermoscopic exam. There were no broken hairs that one would expect with tinea capitis. Also, there was no polytrichia (multiple hairs pushed into a single follicular opening due to scarring of the skin) that would be expected with acne keloidalis nuchae and scarring alopecias.
There are multiple types of scalp folliculitis. This patient had superficial folliculitis, in which pustules develop at the ostium of the hair follicles. Deep folliculitis is more severe and includes furuncles and carbuncles.1
Folliculitis is usually caused by a bacterial infection and, less commonly, fungal infection. In addition to superficial and deep folliculitis, inflammation with scarring of the follicles occurs with folliculitis decalvans, which is one of the scarring alopecias.1
Mild cases of superficial bacterial folliculitis are treated with topical antibiotics (eg, topical clindamycin 1% applied twice daily). Depending on the severity, oral antibiotics including doxycycline 100 mg twice daily for 7 days or trimethoprim sulfamethoxazole 160 mg/800 mg (double strength) twice daily for 7 days may be used. There is also a chronic nonscarring form of scalp folliculitis that often responds initially to antibiotics but then recurs. This has been treated with longer courses of oral antibiotics and, if the lesions don’t respond or continue to recur, with low-dose isotretinoin.2
Due to the amount of scalp involvement, crusting, and inflammation seen on this patient’s scalp, he was treated with trimethoprim sulfamethoxazole 160 mg/800 mg twice daily for 7 days. After 1 week, he reported that he was doing much better and that the lesions had nearly resolved. He was told to return for reevaluation if the lesions did not completely resolve.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Lugović-Mihić L, Barisić F, Bulat V, et al. Differential diagnosis of the scalp hair folliculitis. Acta Clin Croat. 2011;50:395-402.
2. Romero-Maté A, Arias-Palomo D, Hernández-Núñez A, et al. Chronic nonscarring scalp folliculitis: retrospective case series study of 34 cases. J Am Acad Dermatol. 2019;81:1023-1024. doi: 10.1016/j.jaad.2019.02.065
1. Lugović-Mihić L, Barisić F, Bulat V, et al. Differential diagnosis of the scalp hair folliculitis. Acta Clin Croat. 2011;50:395-402.
2. Romero-Maté A, Arias-Palomo D, Hernández-Núñez A, et al. Chronic nonscarring scalp folliculitis: retrospective case series study of 34 cases. J Am Acad Dermatol. 2019;81:1023-1024. doi: 10.1016/j.jaad.2019.02.065
Persistent scaling rash
The clinical pattern of a scaly herald patch predating the eruption of multiple scaly macules is the hallmark of pityriasis rosea (PR). This patient’s severe itching is also classic for PR.
PR’s etiology is believed to be a reactivation of infection from human herpes viruses 6 and 7.1 Prodromal viral symptoms of malaise, sore throat, myalgias, and fever are common.2 Along with the prodromal symptoms, there is often a several-centimeter herald patch that occurs on the trunk. It is often confused with eczema or tinea due to its erythema and scale. (Secondary syphilis is also in the differential.) Sometimes PR can be differentiated by the scale pattern being a collarette instead of diffuse. The diagnosis becomes clearer 1 to 2 weeks later with the onset of multiple small scaly macules across the trunk following the Langer’s skin lines. The course is self-limited but takes several weeks to months to resolve.
If severe, PR may be treated with acyclovir 800 mg orally 5 times daily for 5 days; this is the same regimen for treating varicella zoster (shingles).1,2 Estimated recurrence rates are 4% to 24%.1,3
At age 49 years, this woman was older than the average patient with PR, as the usual age range is 10 to 35 years.1 Her physician advised her that the outbreak might recur. She was also given a prescription for oral hydroxyzine 25 mg to be taken at bedtime if the itching was interfering with her sleep. Her physician told her to return for reevaluation if the rash did not resolve in 3 months. She did not return for reevaluation.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Drago F, Ciccarese G, Parodi A. Commentary on: "pityriasis rosea recurrence is much higher than previously known: a prospective study." Acta Derm Venereol. 2019;99:1053-1054. doi: 10.2340/00015555-3265
2. Villalon-Gomez JM. Pityriasis rosea: diagnosis and treatment. Am Fam Physician. 2018;97:38-44.
3. Yüksel M. Pityriasis rosea recurrence is much higher than previously known: a prospective study. Acta Derm Venereol. 2019;99:664-667. doi: 10.2340/00015555-3169

The clinical pattern of a scaly herald patch predating the eruption of multiple scaly macules is the hallmark of pityriasis rosea (PR). This patient’s severe itching is also classic for PR.
PR’s etiology is believed to be a reactivation of infection from human herpes viruses 6 and 7.1 Prodromal viral symptoms of malaise, sore throat, myalgias, and fever are common.2 Along with the prodromal symptoms, there is often a several-centimeter herald patch that occurs on the trunk. It is often confused with eczema or tinea due to its erythema and scale. (Secondary syphilis is also in the differential.) Sometimes PR can be differentiated by the scale pattern being a collarette instead of diffuse. The diagnosis becomes clearer 1 to 2 weeks later with the onset of multiple small scaly macules across the trunk following the Langer’s skin lines. The course is self-limited but takes several weeks to months to resolve.
If severe, PR may be treated with acyclovir 800 mg orally 5 times daily for 5 days; this is the same regimen for treating varicella zoster (shingles).1,2 Estimated recurrence rates are 4% to 24%.1,3
At age 49 years, this woman was older than the average patient with PR, as the usual age range is 10 to 35 years.1 Her physician advised her that the outbreak might recur. She was also given a prescription for oral hydroxyzine 25 mg to be taken at bedtime if the itching was interfering with her sleep. Her physician told her to return for reevaluation if the rash did not resolve in 3 months. She did not return for reevaluation.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

The clinical pattern of a scaly herald patch predating the eruption of multiple scaly macules is the hallmark of pityriasis rosea (PR). This patient’s severe itching is also classic for PR.
PR’s etiology is believed to be a reactivation of infection from human herpes viruses 6 and 7.1 Prodromal viral symptoms of malaise, sore throat, myalgias, and fever are common.2 Along with the prodromal symptoms, there is often a several-centimeter herald patch that occurs on the trunk. It is often confused with eczema or tinea due to its erythema and scale. (Secondary syphilis is also in the differential.) Sometimes PR can be differentiated by the scale pattern being a collarette instead of diffuse. The diagnosis becomes clearer 1 to 2 weeks later with the onset of multiple small scaly macules across the trunk following the Langer’s skin lines. The course is self-limited but takes several weeks to months to resolve.
If severe, PR may be treated with acyclovir 800 mg orally 5 times daily for 5 days; this is the same regimen for treating varicella zoster (shingles).1,2 Estimated recurrence rates are 4% to 24%.1,3
At age 49 years, this woman was older than the average patient with PR, as the usual age range is 10 to 35 years.1 Her physician advised her that the outbreak might recur. She was also given a prescription for oral hydroxyzine 25 mg to be taken at bedtime if the itching was interfering with her sleep. Her physician told her to return for reevaluation if the rash did not resolve in 3 months. She did not return for reevaluation.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Drago F, Ciccarese G, Parodi A. Commentary on: "pityriasis rosea recurrence is much higher than previously known: a prospective study." Acta Derm Venereol. 2019;99:1053-1054. doi: 10.2340/00015555-3265
2. Villalon-Gomez JM. Pityriasis rosea: diagnosis and treatment. Am Fam Physician. 2018;97:38-44.
3. Yüksel M. Pityriasis rosea recurrence is much higher than previously known: a prospective study. Acta Derm Venereol. 2019;99:664-667. doi: 10.2340/00015555-3169
1. Drago F, Ciccarese G, Parodi A. Commentary on: "pityriasis rosea recurrence is much higher than previously known: a prospective study." Acta Derm Venereol. 2019;99:1053-1054. doi: 10.2340/00015555-3265
2. Villalon-Gomez JM. Pityriasis rosea: diagnosis and treatment. Am Fam Physician. 2018;97:38-44.
3. Yüksel M. Pityriasis rosea recurrence is much higher than previously known: a prospective study. Acta Derm Venereol. 2019;99:664-667. doi: 10.2340/00015555-3169