User login
Focal Palmoplantar Keratoderma and Gingival Keratosis Caused by a KRT16 Mutation
To the Editor:
Focal palmoplantar keratoderma and gingival keratosis (FPGK)(Online Mendelian Inheritance in Man [OMIM] 148730) is a rare autosomal-dominant syndrome featuring focal, pressure-related, painful palmoplantar keratoderma and gingival hyperkeratosis presenting as leukokeratosis. Focal palmoplantar keratoderma and gingival keratosis was first defined by Gorlin1 in 1976. Since then, only a few cases have been reported, but no causative mutations have been identified.2
Focal pressure-related palmoplantar keratoderma (PPK) and oral hyperkeratosis also are seen in pachyonychia congenita (PC)(OMIM 167200, 615726, 615728, 167210), a rare autosomal-dominant disorder of keratinization characterized by PPK and nail dystrophy. Patients with PC often present with plantar pain; more variable features include oral leukokeratosis, follicular hyperkeratosis, pilosebaceous and epidermal inclusion cysts, hoarseness, hyperhidrosis, and natal teeth. Pachyonychia congenita is caused by mutation in keratin genes KRT6A, KRT6B, KRT16, or KRT17.
Focal palmoplantar keratoderma and gingival keratosis as well as PC are distinct from other forms of PPK with gingival involvement such as
Despite the common features of FPGK and PC, they are considered distinct disorders due to absence of nail changes in FPGK and no prior evidence of a common genetic cause. We present a patient with familial FPGK found by whole exome sequencing to be caused by a mutation in KRT16.

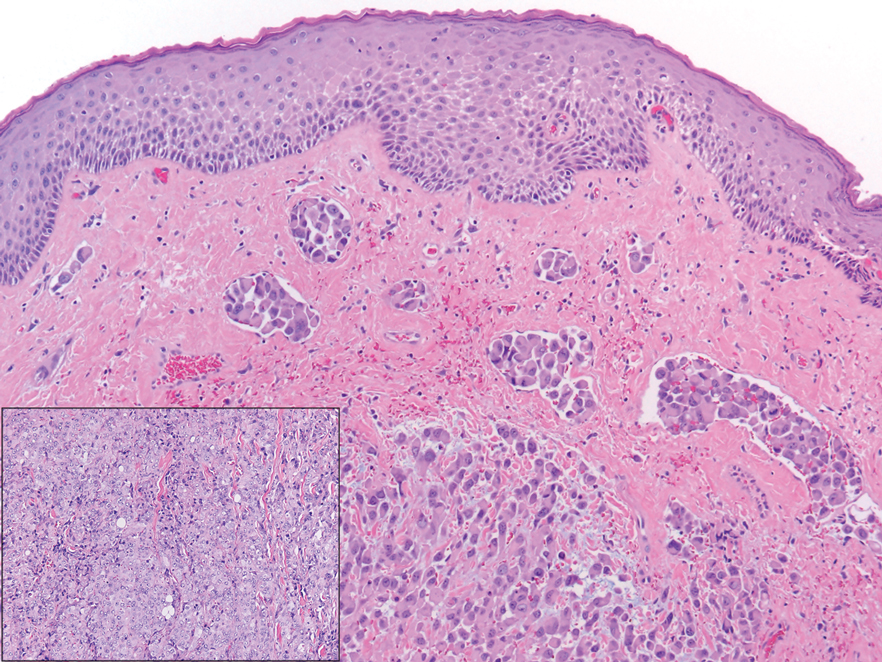
The proband was a 57-year-old man born to unrelated parents (Figure 1). He had no skin problems at birth, and his development was normal. He had painful focal keratoderma since childhood that were most prominent at pressure points on the soles and toes (Figure 2A), in addition to gingival hyperkeratosis and oral leukokeratosis (Figure 2B). He had no associated abnormalities of the skin, hair, or teeth and no nail findings (Figure 2C). He reported that his father and 2 of his 3 sisters were affected with similar symptoms. A punch biopsy of the right fifth toe was consistent with verrucous epidermal hyperplasia with perinuclear keratinization in the spinous layer (Figure 3A). A gingival biopsy showed perinuclear eosinophilic globules and basophilic stranding in the cytoplasm (Figure 3B). His older sister had more severe and painful focal keratoderma of the soles, punctate keratoderma of the palms, gingival hyperkeratosis, and leukokeratosis of the tongue.

Whole exome sequencing of the proband revealed a heterozygous missense mutation in KRT16 (c.380G>A, p.R127H, rs57424749). Sanger sequencing confirmed this mutation and showed that it was heterozygous in both of his affected sisters and absent in his unaffected niece (Figure 1). The patient was treated with topical and systemic retinoids, keratolytics, and mechanical removal to moderate effect, with noted improvement in the appearance and associated pain of the plantar keratoderma.

Phenotypic heterogeneity is common in PC, though PC due to KRT6A mutations demonstrates more severe nail disease with oral lesions, cysts, and follicular hyperkeratosis, while PC caused by KRT16 mutations generally presents with more extensive and painful PPK.4KRT16 mutations affecting p.R127 are frequent causes of PC, and genotype-phenotype correlations have been observed. Individuals with p.R127P mutations exhibit more severe disease with earlier age of onset, more extensive nail involvement and oral leukokeratosis, and greater impact on daily quality of life than in individuals with p.R127C mutations.5 Cases of PC with KRT16 p.R127S and p.R127G mutations also have been observed. The KRT16 c.380G>A, p.R127H mutation we documented has been reported in one kindred with PC who presented with PPK, oral leukokeratosis, toenail thickening, and pilosebaceous and follicular hyperkeratosis.6
Although patients with FPGK lack the thickening of fingernails and/or toenails considered a defining feature of PC, the disorders otherwise are phenotypically similar, suggesting the possibility of common pathogenesis. One linkage study of familial FPGK excluded genetic intervals containing type I and type II keratins but was limited to a single small kindred.2 This study and our data together suggest that, similar to PC, there are multiple genes in which mutations cause FPGK.
Murine Krt16 knockouts show distinct phenotypes depending on the mouse strain in which they are propagated, ranging from perinatal lethality to differences in the severity of oral and PPK lesions.7 These observations provide evidence that additional genetic variants contribute to Krt16 phenotypes in mice and suggest the same could be true for humans.
We propose that some cases of FPGK are due to mutations in KRT16 and thus share a genetic pathogenesis with PC, underscoring the utility of whole exome sequencing in providing genetic diagnoses for disorders that are genetically and clinically heterogeneous. Further biologic investigation of phenotypes caused by KRT16 mutation may reveal respective contributions of additional genetic variation and environmental effects to the variable clinical presentations.
- Gorlin RJ. Focal palmoplantar and marginal gingival hyperkeratosis—a syndrome. Birth Defects Orig Artic Ser. 1976;12:239-242.
- Kolde G, Hennies HC, Bethke G, et al. Focal palmoplantar and gingival keratosis: a distinct palmoplantar ectodermal dysplasia with epidermolytic alterations but lack of mutations in known keratins. J Am Acad Dermatol. 2005;52(3 pt 1):403-409.
- Duchatelet S, Hovnanian A. Olmsted syndrome: clinical, molecular and therapeutic aspects. Orphanet J Rare Dis. 2015;10:33.
- Spaunhurst KM, Hogendorf AM, Smith FJ, et al. Pachyonychia congenita patients with mutations in KRT6A have more extensive disease compared with patients who have mutations in KRT16. Br J Dermatol. 2012;166:875-878.
- Fu T, Leachman SA, Wilson NJ, et al. Genotype-phenotype correlations among pachyonychia congenita patients with K16 mutations. J Invest Dermatol. 2011;131:1025-1028.
- Wilson NJ, O’Toole EA, Milstone LM, et al. The molecular genetic analysis of the expanding pachyonychia congenita case collection. Br J Dermatol. 2014;171:343-355.
- Zieman A, Coulombe PA. The keratin 16 null phenotype is modestly impacted by genetic strain background in mice. Exp Dermatol. 2018;27:672-674.
To the Editor:
Focal palmoplantar keratoderma and gingival keratosis (FPGK)(Online Mendelian Inheritance in Man [OMIM] 148730) is a rare autosomal-dominant syndrome featuring focal, pressure-related, painful palmoplantar keratoderma and gingival hyperkeratosis presenting as leukokeratosis. Focal palmoplantar keratoderma and gingival keratosis was first defined by Gorlin1 in 1976. Since then, only a few cases have been reported, but no causative mutations have been identified.2
Focal pressure-related palmoplantar keratoderma (PPK) and oral hyperkeratosis also are seen in pachyonychia congenita (PC)(OMIM 167200, 615726, 615728, 167210), a rare autosomal-dominant disorder of keratinization characterized by PPK and nail dystrophy. Patients with PC often present with plantar pain; more variable features include oral leukokeratosis, follicular hyperkeratosis, pilosebaceous and epidermal inclusion cysts, hoarseness, hyperhidrosis, and natal teeth. Pachyonychia congenita is caused by mutation in keratin genes KRT6A, KRT6B, KRT16, or KRT17.
Focal palmoplantar keratoderma and gingival keratosis as well as PC are distinct from other forms of PPK with gingival involvement such as
Despite the common features of FPGK and PC, they are considered distinct disorders due to absence of nail changes in FPGK and no prior evidence of a common genetic cause. We present a patient with familial FPGK found by whole exome sequencing to be caused by a mutation in KRT16.

The proband was a 57-year-old man born to unrelated parents (Figure 1). He had no skin problems at birth, and his development was normal. He had painful focal keratoderma since childhood that were most prominent at pressure points on the soles and toes (Figure 2A), in addition to gingival hyperkeratosis and oral leukokeratosis (Figure 2B). He had no associated abnormalities of the skin, hair, or teeth and no nail findings (Figure 2C). He reported that his father and 2 of his 3 sisters were affected with similar symptoms. A punch biopsy of the right fifth toe was consistent with verrucous epidermal hyperplasia with perinuclear keratinization in the spinous layer (Figure 3A). A gingival biopsy showed perinuclear eosinophilic globules and basophilic stranding in the cytoplasm (Figure 3B). His older sister had more severe and painful focal keratoderma of the soles, punctate keratoderma of the palms, gingival hyperkeratosis, and leukokeratosis of the tongue.

Whole exome sequencing of the proband revealed a heterozygous missense mutation in KRT16 (c.380G>A, p.R127H, rs57424749). Sanger sequencing confirmed this mutation and showed that it was heterozygous in both of his affected sisters and absent in his unaffected niece (Figure 1). The patient was treated with topical and systemic retinoids, keratolytics, and mechanical removal to moderate effect, with noted improvement in the appearance and associated pain of the plantar keratoderma.

Phenotypic heterogeneity is common in PC, though PC due to KRT6A mutations demonstrates more severe nail disease with oral lesions, cysts, and follicular hyperkeratosis, while PC caused by KRT16 mutations generally presents with more extensive and painful PPK.4KRT16 mutations affecting p.R127 are frequent causes of PC, and genotype-phenotype correlations have been observed. Individuals with p.R127P mutations exhibit more severe disease with earlier age of onset, more extensive nail involvement and oral leukokeratosis, and greater impact on daily quality of life than in individuals with p.R127C mutations.5 Cases of PC with KRT16 p.R127S and p.R127G mutations also have been observed. The KRT16 c.380G>A, p.R127H mutation we documented has been reported in one kindred with PC who presented with PPK, oral leukokeratosis, toenail thickening, and pilosebaceous and follicular hyperkeratosis.6
Although patients with FPGK lack the thickening of fingernails and/or toenails considered a defining feature of PC, the disorders otherwise are phenotypically similar, suggesting the possibility of common pathogenesis. One linkage study of familial FPGK excluded genetic intervals containing type I and type II keratins but was limited to a single small kindred.2 This study and our data together suggest that, similar to PC, there are multiple genes in which mutations cause FPGK.
Murine Krt16 knockouts show distinct phenotypes depending on the mouse strain in which they are propagated, ranging from perinatal lethality to differences in the severity of oral and PPK lesions.7 These observations provide evidence that additional genetic variants contribute to Krt16 phenotypes in mice and suggest the same could be true for humans.
We propose that some cases of FPGK are due to mutations in KRT16 and thus share a genetic pathogenesis with PC, underscoring the utility of whole exome sequencing in providing genetic diagnoses for disorders that are genetically and clinically heterogeneous. Further biologic investigation of phenotypes caused by KRT16 mutation may reveal respective contributions of additional genetic variation and environmental effects to the variable clinical presentations.
To the Editor:
Focal palmoplantar keratoderma and gingival keratosis (FPGK)(Online Mendelian Inheritance in Man [OMIM] 148730) is a rare autosomal-dominant syndrome featuring focal, pressure-related, painful palmoplantar keratoderma and gingival hyperkeratosis presenting as leukokeratosis. Focal palmoplantar keratoderma and gingival keratosis was first defined by Gorlin1 in 1976. Since then, only a few cases have been reported, but no causative mutations have been identified.2
Focal pressure-related palmoplantar keratoderma (PPK) and oral hyperkeratosis also are seen in pachyonychia congenita (PC)(OMIM 167200, 615726, 615728, 167210), a rare autosomal-dominant disorder of keratinization characterized by PPK and nail dystrophy. Patients with PC often present with plantar pain; more variable features include oral leukokeratosis, follicular hyperkeratosis, pilosebaceous and epidermal inclusion cysts, hoarseness, hyperhidrosis, and natal teeth. Pachyonychia congenita is caused by mutation in keratin genes KRT6A, KRT6B, KRT16, or KRT17.
Focal palmoplantar keratoderma and gingival keratosis as well as PC are distinct from other forms of PPK with gingival involvement such as
Despite the common features of FPGK and PC, they are considered distinct disorders due to absence of nail changes in FPGK and no prior evidence of a common genetic cause. We present a patient with familial FPGK found by whole exome sequencing to be caused by a mutation in KRT16.

The proband was a 57-year-old man born to unrelated parents (Figure 1). He had no skin problems at birth, and his development was normal. He had painful focal keratoderma since childhood that were most prominent at pressure points on the soles and toes (Figure 2A), in addition to gingival hyperkeratosis and oral leukokeratosis (Figure 2B). He had no associated abnormalities of the skin, hair, or teeth and no nail findings (Figure 2C). He reported that his father and 2 of his 3 sisters were affected with similar symptoms. A punch biopsy of the right fifth toe was consistent with verrucous epidermal hyperplasia with perinuclear keratinization in the spinous layer (Figure 3A). A gingival biopsy showed perinuclear eosinophilic globules and basophilic stranding in the cytoplasm (Figure 3B). His older sister had more severe and painful focal keratoderma of the soles, punctate keratoderma of the palms, gingival hyperkeratosis, and leukokeratosis of the tongue.

Whole exome sequencing of the proband revealed a heterozygous missense mutation in KRT16 (c.380G>A, p.R127H, rs57424749). Sanger sequencing confirmed this mutation and showed that it was heterozygous in both of his affected sisters and absent in his unaffected niece (Figure 1). The patient was treated with topical and systemic retinoids, keratolytics, and mechanical removal to moderate effect, with noted improvement in the appearance and associated pain of the plantar keratoderma.

Phenotypic heterogeneity is common in PC, though PC due to KRT6A mutations demonstrates more severe nail disease with oral lesions, cysts, and follicular hyperkeratosis, while PC caused by KRT16 mutations generally presents with more extensive and painful PPK.4KRT16 mutations affecting p.R127 are frequent causes of PC, and genotype-phenotype correlations have been observed. Individuals with p.R127P mutations exhibit more severe disease with earlier age of onset, more extensive nail involvement and oral leukokeratosis, and greater impact on daily quality of life than in individuals with p.R127C mutations.5 Cases of PC with KRT16 p.R127S and p.R127G mutations also have been observed. The KRT16 c.380G>A, p.R127H mutation we documented has been reported in one kindred with PC who presented with PPK, oral leukokeratosis, toenail thickening, and pilosebaceous and follicular hyperkeratosis.6
Although patients with FPGK lack the thickening of fingernails and/or toenails considered a defining feature of PC, the disorders otherwise are phenotypically similar, suggesting the possibility of common pathogenesis. One linkage study of familial FPGK excluded genetic intervals containing type I and type II keratins but was limited to a single small kindred.2 This study and our data together suggest that, similar to PC, there are multiple genes in which mutations cause FPGK.
Murine Krt16 knockouts show distinct phenotypes depending on the mouse strain in which they are propagated, ranging from perinatal lethality to differences in the severity of oral and PPK lesions.7 These observations provide evidence that additional genetic variants contribute to Krt16 phenotypes in mice and suggest the same could be true for humans.
We propose that some cases of FPGK are due to mutations in KRT16 and thus share a genetic pathogenesis with PC, underscoring the utility of whole exome sequencing in providing genetic diagnoses for disorders that are genetically and clinically heterogeneous. Further biologic investigation of phenotypes caused by KRT16 mutation may reveal respective contributions of additional genetic variation and environmental effects to the variable clinical presentations.
- Gorlin RJ. Focal palmoplantar and marginal gingival hyperkeratosis—a syndrome. Birth Defects Orig Artic Ser. 1976;12:239-242.
- Kolde G, Hennies HC, Bethke G, et al. Focal palmoplantar and gingival keratosis: a distinct palmoplantar ectodermal dysplasia with epidermolytic alterations but lack of mutations in known keratins. J Am Acad Dermatol. 2005;52(3 pt 1):403-409.
- Duchatelet S, Hovnanian A. Olmsted syndrome: clinical, molecular and therapeutic aspects. Orphanet J Rare Dis. 2015;10:33.
- Spaunhurst KM, Hogendorf AM, Smith FJ, et al. Pachyonychia congenita patients with mutations in KRT6A have more extensive disease compared with patients who have mutations in KRT16. Br J Dermatol. 2012;166:875-878.
- Fu T, Leachman SA, Wilson NJ, et al. Genotype-phenotype correlations among pachyonychia congenita patients with K16 mutations. J Invest Dermatol. 2011;131:1025-1028.
- Wilson NJ, O’Toole EA, Milstone LM, et al. The molecular genetic analysis of the expanding pachyonychia congenita case collection. Br J Dermatol. 2014;171:343-355.
- Zieman A, Coulombe PA. The keratin 16 null phenotype is modestly impacted by genetic strain background in mice. Exp Dermatol. 2018;27:672-674.
- Gorlin RJ. Focal palmoplantar and marginal gingival hyperkeratosis—a syndrome. Birth Defects Orig Artic Ser. 1976;12:239-242.
- Kolde G, Hennies HC, Bethke G, et al. Focal palmoplantar and gingival keratosis: a distinct palmoplantar ectodermal dysplasia with epidermolytic alterations but lack of mutations in known keratins. J Am Acad Dermatol. 2005;52(3 pt 1):403-409.
- Duchatelet S, Hovnanian A. Olmsted syndrome: clinical, molecular and therapeutic aspects. Orphanet J Rare Dis. 2015;10:33.
- Spaunhurst KM, Hogendorf AM, Smith FJ, et al. Pachyonychia congenita patients with mutations in KRT6A have more extensive disease compared with patients who have mutations in KRT16. Br J Dermatol. 2012;166:875-878.
- Fu T, Leachman SA, Wilson NJ, et al. Genotype-phenotype correlations among pachyonychia congenita patients with K16 mutations. J Invest Dermatol. 2011;131:1025-1028.
- Wilson NJ, O’Toole EA, Milstone LM, et al. The molecular genetic analysis of the expanding pachyonychia congenita case collection. Br J Dermatol. 2014;171:343-355.
- Zieman A, Coulombe PA. The keratin 16 null phenotype is modestly impacted by genetic strain background in mice. Exp Dermatol. 2018;27:672-674.
Practice Points
- Focal palmoplantar keratoderma and gingival keratosis (FPGK) is a rare autosomal-dominant syndrome featuring focal, pressure-related, painful palmoplantar keratoderma (PPK) and gingival hyperkeratosis presenting as leukokeratosis.
- Focal pressure-related PPK and oral hyperkeratosis also are seen in pachyonychia congenita (PC), which is caused by mutations in keratin genes and is distinguished from FPGK by characteristic nail changes.
- A shared causative gene suggests that FPGK should be considered part of the PC spectrum.
Fungated Eroded Plaque on the Arm
The Diagnosis: Cutaneous Blastomycosis
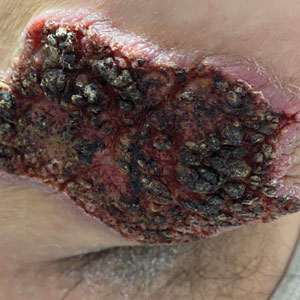
A skin biopsy and fungal cultures confirmed the diagnosis of cutaneous blastomycosis. Grocott- Gomori methenamine-silver staining highlighted fungal organisms with refractile walls and broad-based budding consistent with cutaneous blastomycosis (Figure 1). The histopathologic specimen also demonstrated marked pseudoepitheliomatous hyperplasia (Figure 2A) with neutrophilic microabscesses (Figure 2B). Acid-fast bacillus and Fite staining were negative for bacterial organisms. A fungal culture was positive for Blastomyces dermatitidis. Urine and serum blastomycosis antigen were positive. Although Histoplasma serum antigen also was positive, this likely was from cross-reactivity. Chest radiography was negative for lung involvement, and the patient displayed no neurologic symptoms. He was started on oral itraconazole therapy for the treatment of cutaneous blastomycosis.

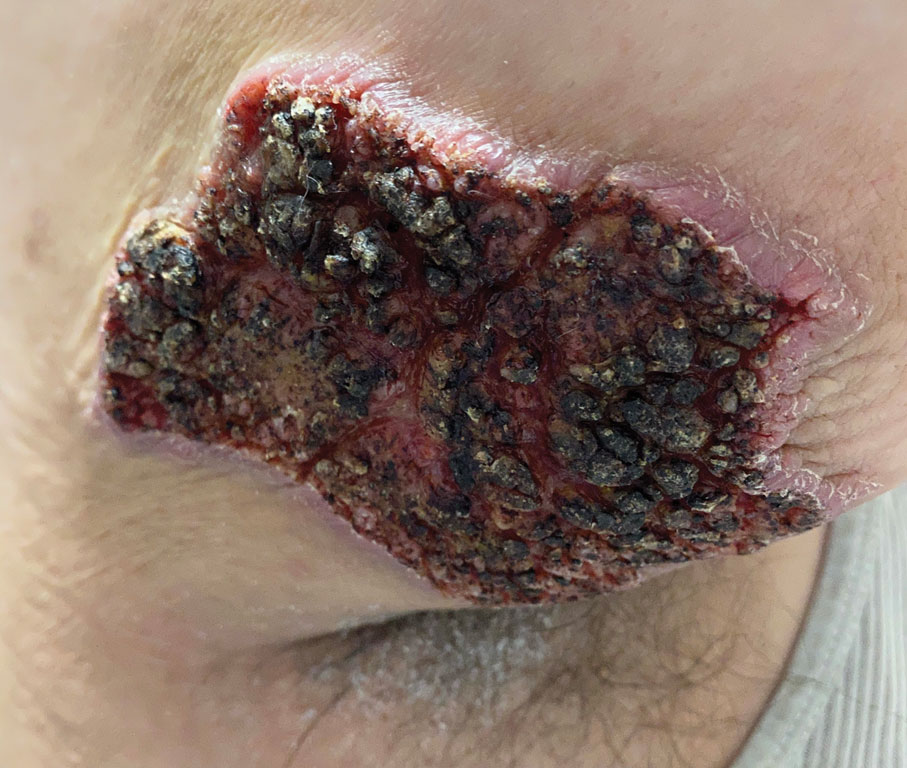
Blastomyces dermatitidis, the causative organism of blastomycosis, is endemic to the Ohio and Mississippi River valleys, Great Lakes region, and southeastern United States. It is a thermally dimorphic fungus found in soils that grows as a mold at 25 °C and yeast at 37 °C. Primary infection of the lungs—blastomycosis pneumonia—often is the only clinical manifestation1; however, subsequent hematogenous dissemination to extrapulmonary sites such as the skin, bones, and genitourinary system can occur. Cutaneous blastomycosis, the most common extrapulmonary manifestation, typically follows pulmonary infection. In rare cases, it can occur from direct inoculation.2,3 Skin lesions can occur anywhere but frequently are found on exposed surfaces of the head, neck, and extremities. Lesions classically present as verrucous crusting plaques with draining microabscesses. Violaceous nodules, ulcers, and pustules also may occur.1

Diagnosis involves obtaining a thorough history of possible environmental exposures such as the patient’s geographic area of residence, occupation, and outdoor activities involving soil or decaying wood. Because blastomycosis can remain latent, remote exposures are relevant. Definitive diagnosis of cutaneous blastomycosis involves skin biopsy of the lesion with fungal culture, but the yeast’s distinctive thick wall and broad-based budding seen with periodic acid–Schiff or Grocott-Gomori methenamine-silver staining provides a rapid presumptive diagnosis.3 Pseudoepitheliomatous hyperplasia and microabscesses also are characteristic features.2 Urine antigen testing for a component of the polysaccharide cell wall has a sensitivity of 93% but a lower specificity of 79% due to cross-reactivity with histoplasmosis.4 Treatment consists of itraconazole for mild to moderate blastomycosis or amphotericin B for those with severe disease or central nervous system involvement or those who are immunosuppressed.1
The differential diagnosis for our patient’s lesion included infectious vs neoplastic etiologies. Histoplasma capsulatum, the dimorphic fungus that causes histoplasmosis, also is endemic to the Ohio and Mississippi River valleys. It is found in soil and droppings of some bats and birds such as chickens and pigeons. Similar to blastomycosis, the primary infection site most commonly is the lungs. It subsequently may disseminate to the skin or less commonly via direct inoculation of injured skin. It can present as papules, plaques, ulcers, purpura, or abscesses. Unlike blastomycosis, tissue biopsy of a cutaneous lesion reveals granuloma formation and distinctive oval, narrow-based budding yeast.5 Atypical mycobacteria are another source of infection to consider. For example, cutaneous Mycobacterium kansasii may present as papules and pustules forming verrucous or granulomatous plaques and ulceration. Histopathologic findings distinguishing mycobacterial infection from blastomycosis include granulomas and acid-fast bacilli in histiocytes.6
Noninfectious etiologies in the differential may include cutaneous squamous cell carcinoma or pemphigus vegetans. Squamous cell carcinoma may present with a broad range of clinical features—papules, plaques, or nodules with smooth, scaly, verrucous, or ulcerative secondary features all are possible presentations.7 Fairskinned individuals, such as our patient, would be at a higher risk in sun-damaged skin. Histologically, cutaneous squamous cell carcinoma is defined as an invasion of the dermis by neoplastic squamous epithelial cells in the form of cords, sheets, individual cells, nodules, or cystic structures.7 Pemphigus vegetans is the rarest variant of a group of autoimmune vesiculobullous diseases known as pemphigus. It can be differentiated from the most common variant—pemphigus vulgaris—by the presence of vegetative plaques in intertriginous areas. However, these verrucous vegetations can be misleading and make clinical diagnosis difficult. Histopathologic findings of hyperkeratosis, pseudoepitheliomatous hyperplasia, papillomatosis, and acantholysis with a suprabasal cleft would confirm the diagnosis.8
In summary, cutaneous blastomycosis classically presents as verrucous crusting plaques, as seen in our patient. It is important to conduct a thorough history for environmental exposures, but definitive diagnosis of cutaneous blastomycosis involves skin biopsy with fungal culture. Treatment depends on the severity of disease and organ involvement. Itraconazole would be appropriate for mild to moderate blastomycosis.
- Miceli A, Krishnamurthy K. Blastomycosis. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK441987/
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Schwartz IS, Kauffman CA. Blastomycosis. Semin Respir Crit Care Med. 2020;41:31-41. doi:10.1055/s-0039-3400281
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am. 2016;30:247-264. doi:10.1016/j.idc.2015.10.002
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348.
- Bhambri S, Bhambri A, Del Rosso JQ. Atypical mycobacterial cutaneous infections. Dermatol Clin. 2009;27:63-73. doi:10.1016/j.det.2008.07.009
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll.2017.06.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK545229/
The Diagnosis: Cutaneous Blastomycosis
A skin biopsy and fungal cultures confirmed the diagnosis of cutaneous blastomycosis. Grocott- Gomori methenamine-silver staining highlighted fungal organisms with refractile walls and broad-based budding consistent with cutaneous blastomycosis (Figure 1). The histopathologic specimen also demonstrated marked pseudoepitheliomatous hyperplasia (Figure 2A) with neutrophilic microabscesses (Figure 2B). Acid-fast bacillus and Fite staining were negative for bacterial organisms. A fungal culture was positive for Blastomyces dermatitidis. Urine and serum blastomycosis antigen were positive. Although Histoplasma serum antigen also was positive, this likely was from cross-reactivity. Chest radiography was negative for lung involvement, and the patient displayed no neurologic symptoms. He was started on oral itraconazole therapy for the treatment of cutaneous blastomycosis.

Blastomyces dermatitidis, the causative organism of blastomycosis, is endemic to the Ohio and Mississippi River valleys, Great Lakes region, and southeastern United States. It is a thermally dimorphic fungus found in soils that grows as a mold at 25 °C and yeast at 37 °C. Primary infection of the lungs—blastomycosis pneumonia—often is the only clinical manifestation1; however, subsequent hematogenous dissemination to extrapulmonary sites such as the skin, bones, and genitourinary system can occur. Cutaneous blastomycosis, the most common extrapulmonary manifestation, typically follows pulmonary infection. In rare cases, it can occur from direct inoculation.2,3 Skin lesions can occur anywhere but frequently are found on exposed surfaces of the head, neck, and extremities. Lesions classically present as verrucous crusting plaques with draining microabscesses. Violaceous nodules, ulcers, and pustules also may occur.1

Diagnosis involves obtaining a thorough history of possible environmental exposures such as the patient’s geographic area of residence, occupation, and outdoor activities involving soil or decaying wood. Because blastomycosis can remain latent, remote exposures are relevant. Definitive diagnosis of cutaneous blastomycosis involves skin biopsy of the lesion with fungal culture, but the yeast’s distinctive thick wall and broad-based budding seen with periodic acid–Schiff or Grocott-Gomori methenamine-silver staining provides a rapid presumptive diagnosis.3 Pseudoepitheliomatous hyperplasia and microabscesses also are characteristic features.2 Urine antigen testing for a component of the polysaccharide cell wall has a sensitivity of 93% but a lower specificity of 79% due to cross-reactivity with histoplasmosis.4 Treatment consists of itraconazole for mild to moderate blastomycosis or amphotericin B for those with severe disease or central nervous system involvement or those who are immunosuppressed.1
The differential diagnosis for our patient’s lesion included infectious vs neoplastic etiologies. Histoplasma capsulatum, the dimorphic fungus that causes histoplasmosis, also is endemic to the Ohio and Mississippi River valleys. It is found in soil and droppings of some bats and birds such as chickens and pigeons. Similar to blastomycosis, the primary infection site most commonly is the lungs. It subsequently may disseminate to the skin or less commonly via direct inoculation of injured skin. It can present as papules, plaques, ulcers, purpura, or abscesses. Unlike blastomycosis, tissue biopsy of a cutaneous lesion reveals granuloma formation and distinctive oval, narrow-based budding yeast.5 Atypical mycobacteria are another source of infection to consider. For example, cutaneous Mycobacterium kansasii may present as papules and pustules forming verrucous or granulomatous plaques and ulceration. Histopathologic findings distinguishing mycobacterial infection from blastomycosis include granulomas and acid-fast bacilli in histiocytes.6
Noninfectious etiologies in the differential may include cutaneous squamous cell carcinoma or pemphigus vegetans. Squamous cell carcinoma may present with a broad range of clinical features—papules, plaques, or nodules with smooth, scaly, verrucous, or ulcerative secondary features all are possible presentations.7 Fairskinned individuals, such as our patient, would be at a higher risk in sun-damaged skin. Histologically, cutaneous squamous cell carcinoma is defined as an invasion of the dermis by neoplastic squamous epithelial cells in the form of cords, sheets, individual cells, nodules, or cystic structures.7 Pemphigus vegetans is the rarest variant of a group of autoimmune vesiculobullous diseases known as pemphigus. It can be differentiated from the most common variant—pemphigus vulgaris—by the presence of vegetative plaques in intertriginous areas. However, these verrucous vegetations can be misleading and make clinical diagnosis difficult. Histopathologic findings of hyperkeratosis, pseudoepitheliomatous hyperplasia, papillomatosis, and acantholysis with a suprabasal cleft would confirm the diagnosis.8
In summary, cutaneous blastomycosis classically presents as verrucous crusting plaques, as seen in our patient. It is important to conduct a thorough history for environmental exposures, but definitive diagnosis of cutaneous blastomycosis involves skin biopsy with fungal culture. Treatment depends on the severity of disease and organ involvement. Itraconazole would be appropriate for mild to moderate blastomycosis.
The Diagnosis: Cutaneous Blastomycosis
A skin biopsy and fungal cultures confirmed the diagnosis of cutaneous blastomycosis. Grocott- Gomori methenamine-silver staining highlighted fungal organisms with refractile walls and broad-based budding consistent with cutaneous blastomycosis (Figure 1). The histopathologic specimen also demonstrated marked pseudoepitheliomatous hyperplasia (Figure 2A) with neutrophilic microabscesses (Figure 2B). Acid-fast bacillus and Fite staining were negative for bacterial organisms. A fungal culture was positive for Blastomyces dermatitidis. Urine and serum blastomycosis antigen were positive. Although Histoplasma serum antigen also was positive, this likely was from cross-reactivity. Chest radiography was negative for lung involvement, and the patient displayed no neurologic symptoms. He was started on oral itraconazole therapy for the treatment of cutaneous blastomycosis.

Blastomyces dermatitidis, the causative organism of blastomycosis, is endemic to the Ohio and Mississippi River valleys, Great Lakes region, and southeastern United States. It is a thermally dimorphic fungus found in soils that grows as a mold at 25 °C and yeast at 37 °C. Primary infection of the lungs—blastomycosis pneumonia—often is the only clinical manifestation1; however, subsequent hematogenous dissemination to extrapulmonary sites such as the skin, bones, and genitourinary system can occur. Cutaneous blastomycosis, the most common extrapulmonary manifestation, typically follows pulmonary infection. In rare cases, it can occur from direct inoculation.2,3 Skin lesions can occur anywhere but frequently are found on exposed surfaces of the head, neck, and extremities. Lesions classically present as verrucous crusting plaques with draining microabscesses. Violaceous nodules, ulcers, and pustules also may occur.1

Diagnosis involves obtaining a thorough history of possible environmental exposures such as the patient’s geographic area of residence, occupation, and outdoor activities involving soil or decaying wood. Because blastomycosis can remain latent, remote exposures are relevant. Definitive diagnosis of cutaneous blastomycosis involves skin biopsy of the lesion with fungal culture, but the yeast’s distinctive thick wall and broad-based budding seen with periodic acid–Schiff or Grocott-Gomori methenamine-silver staining provides a rapid presumptive diagnosis.3 Pseudoepitheliomatous hyperplasia and microabscesses also are characteristic features.2 Urine antigen testing for a component of the polysaccharide cell wall has a sensitivity of 93% but a lower specificity of 79% due to cross-reactivity with histoplasmosis.4 Treatment consists of itraconazole for mild to moderate blastomycosis or amphotericin B for those with severe disease or central nervous system involvement or those who are immunosuppressed.1
The differential diagnosis for our patient’s lesion included infectious vs neoplastic etiologies. Histoplasma capsulatum, the dimorphic fungus that causes histoplasmosis, also is endemic to the Ohio and Mississippi River valleys. It is found in soil and droppings of some bats and birds such as chickens and pigeons. Similar to blastomycosis, the primary infection site most commonly is the lungs. It subsequently may disseminate to the skin or less commonly via direct inoculation of injured skin. It can present as papules, plaques, ulcers, purpura, or abscesses. Unlike blastomycosis, tissue biopsy of a cutaneous lesion reveals granuloma formation and distinctive oval, narrow-based budding yeast.5 Atypical mycobacteria are another source of infection to consider. For example, cutaneous Mycobacterium kansasii may present as papules and pustules forming verrucous or granulomatous plaques and ulceration. Histopathologic findings distinguishing mycobacterial infection from blastomycosis include granulomas and acid-fast bacilli in histiocytes.6
Noninfectious etiologies in the differential may include cutaneous squamous cell carcinoma or pemphigus vegetans. Squamous cell carcinoma may present with a broad range of clinical features—papules, plaques, or nodules with smooth, scaly, verrucous, or ulcerative secondary features all are possible presentations.7 Fairskinned individuals, such as our patient, would be at a higher risk in sun-damaged skin. Histologically, cutaneous squamous cell carcinoma is defined as an invasion of the dermis by neoplastic squamous epithelial cells in the form of cords, sheets, individual cells, nodules, or cystic structures.7 Pemphigus vegetans is the rarest variant of a group of autoimmune vesiculobullous diseases known as pemphigus. It can be differentiated from the most common variant—pemphigus vulgaris—by the presence of vegetative plaques in intertriginous areas. However, these verrucous vegetations can be misleading and make clinical diagnosis difficult. Histopathologic findings of hyperkeratosis, pseudoepitheliomatous hyperplasia, papillomatosis, and acantholysis with a suprabasal cleft would confirm the diagnosis.8
In summary, cutaneous blastomycosis classically presents as verrucous crusting plaques, as seen in our patient. It is important to conduct a thorough history for environmental exposures, but definitive diagnosis of cutaneous blastomycosis involves skin biopsy with fungal culture. Treatment depends on the severity of disease and organ involvement. Itraconazole would be appropriate for mild to moderate blastomycosis.
- Miceli A, Krishnamurthy K. Blastomycosis. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK441987/
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Schwartz IS, Kauffman CA. Blastomycosis. Semin Respir Crit Care Med. 2020;41:31-41. doi:10.1055/s-0039-3400281
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am. 2016;30:247-264. doi:10.1016/j.idc.2015.10.002
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348.
- Bhambri S, Bhambri A, Del Rosso JQ. Atypical mycobacterial cutaneous infections. Dermatol Clin. 2009;27:63-73. doi:10.1016/j.det.2008.07.009
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll.2017.06.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK545229/
- Miceli A, Krishnamurthy K. Blastomycosis. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK441987/
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Schwartz IS, Kauffman CA. Blastomycosis. Semin Respir Crit Care Med. 2020;41:31-41. doi:10.1055/s-0039-3400281
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am. 2016;30:247-264. doi:10.1016/j.idc.2015.10.002
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348.
- Bhambri S, Bhambri A, Del Rosso JQ. Atypical mycobacterial cutaneous infections. Dermatol Clin. 2009;27:63-73. doi:10.1016/j.det.2008.07.009
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll.2017.06.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK545229/
A 39-year-old man from Ohio presented with a tender, 10×6-cm, fungated, eroded plaque on the right medial upper arm that developed over the last 4 years. He initially noticed a firm lump under the right arm 4 years prior that was diagnosed as possible cellulitis at an outside clinic and treated with trimethoprim-sulfamethoxazole. The lesion then began to erode and became a chronic nonhealing wound. Approximately 1 year prior to the current presentation, the patient recalled unloading a truckload of soil around the same time the wound began to enlarge in diameter and depth. He denied any prior or current respiratory or systemic symptoms including fevers, chills, or weight loss.
Erythematous Papules on the Ears
The Diagnosis: Borrelial Lymphocytoma (Lymphocytoma Cutis)
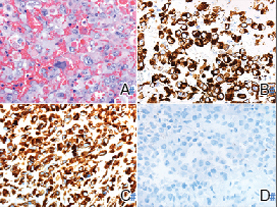
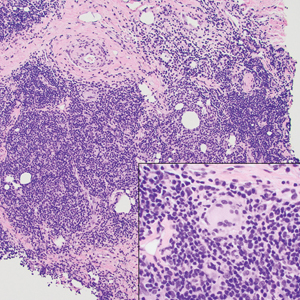
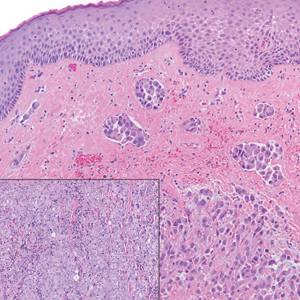
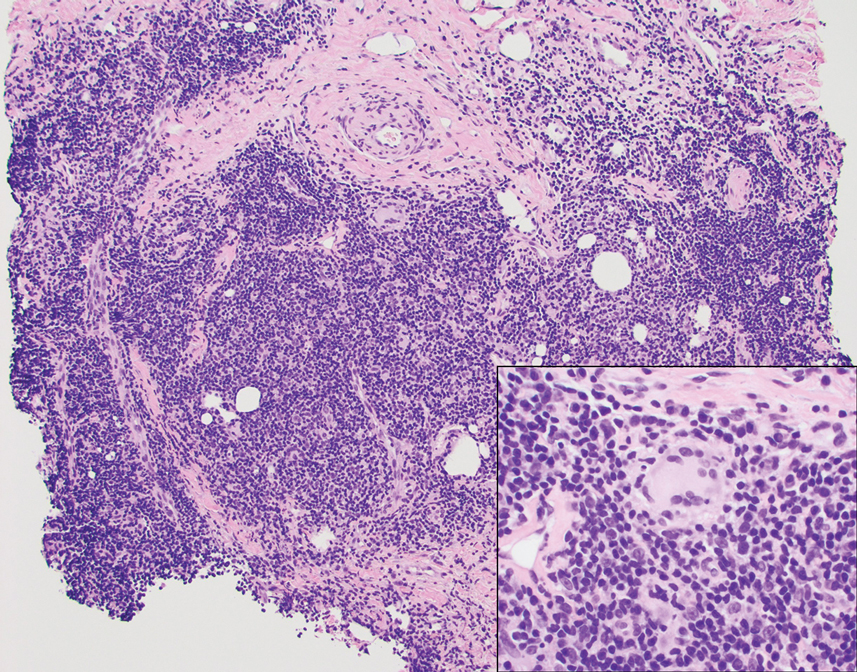
A punch biopsy revealed an atypical lobular lymphoid infiltrate within the dermis and subcutaneous tissue with a mixed composition of CD3+ T cells and CD20+ B cells (quiz image, bottom). Immunohistochemical studies revealed a normal CD4:CD8 ratio with preservation of CD5 and CD7. CD30 was largely negative. CD21 failed to detect follicular dendritic cell networks, and κ/λ light chain staining confirmed a preserved ratio of polytypic plasma cells. There was limited staining with B-cell lymphoma (Bcl-2 and Bcl-6). Polymerase chain reaction studies for both T- and B-cell receptors were negative (polyclonal).
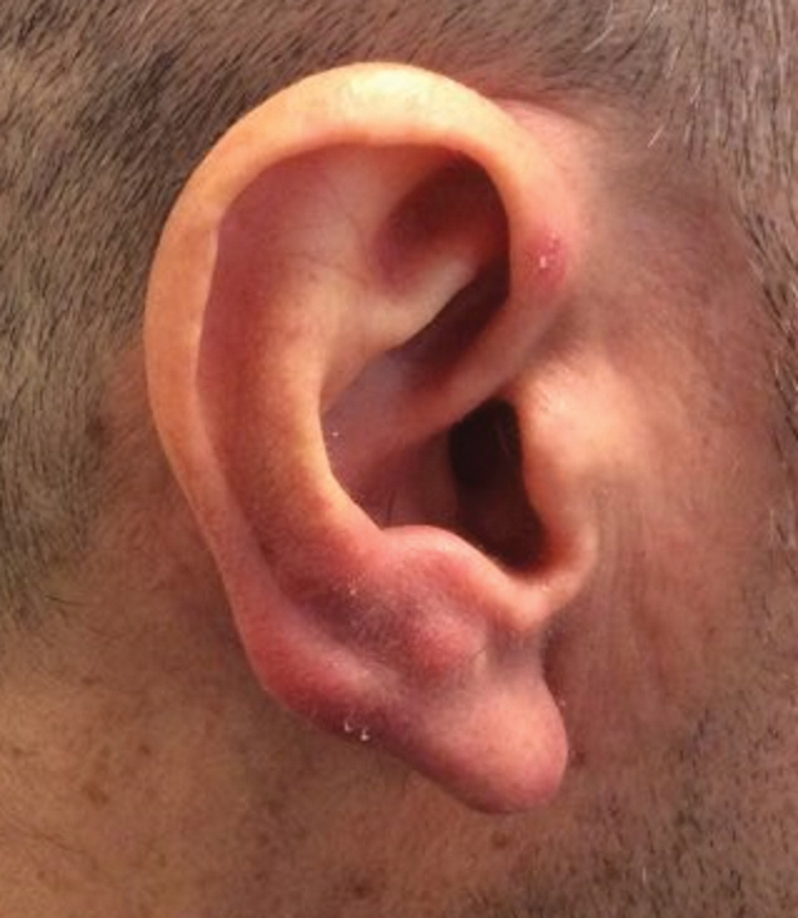
Lyme disease is the most frequently reported vectorborne infectious disease in the United States, and borrelial lymphocytoma (BL) is a rare clinical sequela. Borrelial lymphocytoma is a variant of lymphocytoma cutis (also known as benign reactive lymphoid hyperplasia), which is an inflammatory lesion that can mimic malignant lymphoma clinically and histologically. Lymphocytoma cutis is considered the prototypical example of cutaneous B-cell pseudolymphoma.1 Due to suspicion for lymphocytoma cutis based on the histologic findings and characteristic location of the lesions in our patient, Lyme serologies were ordered and were positive for IgM antibodies against p23, p39, and p41 antigens in high titers. Our patient was treated with doxycycline 100 mg twice daily for 3 weeks with complete resolution of the lesions at 3-month follow-up.
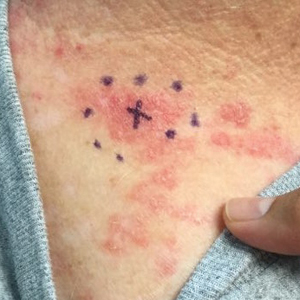
Clinically, BL appears as erythematous papules, plaques, or nodules commonly on the lobules of the ears (quiz image, top). Most cases of lymphocytoma cutis are idiopathic but may be triggered by identifiable associated etiologies including Borrelia burgdorferi, Leishmania donovani, molluscum contagiosum, herpes zoster virus, vaccinations, tattoos, insect bites, and drugs. The main differential diagnosis of lymphocytoma cutis is cutaneous B-cell lymphoma. Pseudolymphoma of the skin can mimic nearly all immunohistochemical staining patterns of true B-cell lymphomas.2
Primary cutaneous follicle center lymphoma frequently occurs on the head and neck. This true lymphoma of the skin can demonstrate prominent follicle centers with centrocytes and fragmented germinal centers (Figure 1) or show a diffuse pattern.3 Most cases show conspicuous Bcl-6 staining, and IgH gene rearrangements can detect a clonal B-cell population in more than 50% of cases.4
Diffuse large B-cell lymphoma can occur as a primary cutaneous malignancy or as a manifestation of systemic disease.4 When arising in the skin, lesions tend to affect the extremities, and the disease is classified as diffuse large B-cell lymphoma, leg type. Histologically, sheets of large atypical lymphocytes with numerous mitoses are seen (Figure 2). These cells stain positively with Bcl-2 and frequently demonstrate Bcl-6 and MUM-1, none of which were seen in our case.4 Lymphomatoid papulosis (LyP) tends to present with relapsing erythematous papules. Patients occasionally develop LyP in association with mycosis fungoides or other lymphomas. Both LyP and primary cutaneous anaplastic large cell lymphoma demonstrate conspicuous CD30+ large cells that can be multinucleated or resemble the Reed-Sternberg cells seen in Hodgkin lymphoma (Figure 3).4 Arthropod bite reactions are common but may be confused with lymphomas and pseudolymphomas. The perivascular lymphocytic infiltrate seen in arthropod bite reactions may be dense and usually is associated with numerous eosinophils (Figure 4). Occasional plasma cells also can be seen, and if the infiltrate closely adheres to vascular structures, a diagnosis of erythema chronicum migrans also can be considered. Patients with chronic lymphocytic leukemia/lymphoma may demonstrate exaggerated or persistent arthropod bite reactions, and atypical lymphocytes can be detected admixed with the otherwise reactive infiltrate.4
Borrelia burgdorferi is primarily endemic to North America and Europe. It is a spirochete bacterium spread by the Ixodes tick that was first recognized as the etiologic agent in 1975 in Old Lyme, Connecticut, where it received its name.5 Most reported cases of Lyme disease occur in the northeastern United States, which correlates with this case given our patient’s place of residence.6 Borrelial lymphocytoma cutis occurs in areas endemic for the Ixodes tick in Europe and North America.7 When describing the genotyping of Borrelia seen in BL, the strain B burgdorferi previously was grouped with Borrelia afzelii and Borrelia garinii.2 In the contemporary literature, however, B burgdorferi is referred to as sensu stricto when specifically talking about the strain B burgdorferi, and the term sensu lato is used when referencing the combination of strains (B burgdorferi, B afzelii, B garinii).
A 2016 study by Maraspin et al8 comprising 144 patients diagnosed with BL showed that the lesions mainly were located on the breast (106 patients [73.6%]) and the earlobe (27 patients [18.8%]), with the remaining cases occurring elsewhere on the body (11 patients [7.6%]). The Borrelia strains isolated from the BL lesions included B afzelii, Borrelia bissettii, and B garinii, with B afzelii being the most commonly identified (84.6% [11/13]).8
Borrelial lymphocytoma usually is categorized as a form of early disseminated Lyme disease and is treated as such. The treatment of choice for early disseminated Lyme disease is doxycycline 100 mg twice daily for 14 to 21 days. Ceftriaxone and azithromycin are reasonable treatment options for patients who have tetracycline allergies or who are pregnant.9
In conclusion, the presentation of red papules or nodules on the ears should prompt clinical suspicion of Lyme disease, particularly in endemic areas. Differentiating pseudolymphomas from true lymphomas and other reactive conditions can be challenging.
- Mitteldorf C, Kempf W. Cutaneous pseudolymphoma. Surg Pathol Clin. 2017;10:455-476. doi:10.1016/j.path.2017.01.002
- Colli C, Leinweber B, Müllegger R, et al. Borrelia burgdorferiassociated lymphocytoma cutis: clinicopathologic, immunophenotypic, and molecular study of 106 cases. J Cutan Pathol. 2004;31:232-240. doi:10.1111/j.0303-6987.2003.00167.x
- Wehbe AM, Neppalli V, Syrbu S, et al. Diffuse follicle centre lymphoma presents with high frequency of extranodal disease. J Clin Oncol. 2008;26(15 suppl):19511. doi:10.1200/jco.2008.26.15_suppl.19511
- Patterson JW, Hosler GA. Cutaneous infiltrates—lymphomatous and leukemic. In: Patterson JW, ed. Weedon’s Skin Pathology. 4th ed. Elsevier; 2016:1171-1217.
- Cardenas-de la Garza JA, De la Cruz-Valadez E, Ocampo -Candiani J, et al. Clinical spectrum of Lyme disease. Eur J Clin Microbiol Infect Dis. 2019;38:201-208. doi:10.1007/s10096-018-3417-1
- Shapiro ED, Gerber MA. Lyme disease. Clin Infect Dis. 2000;31:533-542. doi:10.1086/313982
- Kandhari R, Kandhari S, Jain S. Borrelial lymphocytoma cutis: a diagnostic dilemma. Indian J Dermatol. 2014;59:595-597. doi:10.4103/0019-5154.143530
- Maraspin V, Nahtigal Klevišar M, Ružic´-Sabljic´ E, et al. Borrelial lymphocytoma in adult patients. Clin Infect Dis. 2016;63:914-921. doi:10.1093/cid/ciw417
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006; 43:1089-1134. doi:10.1086/508667
The Diagnosis: Borrelial Lymphocytoma (Lymphocytoma Cutis)
A punch biopsy revealed an atypical lobular lymphoid infiltrate within the dermis and subcutaneous tissue with a mixed composition of CD3+ T cells and CD20+ B cells (quiz image, bottom). Immunohistochemical studies revealed a normal CD4:CD8 ratio with preservation of CD5 and CD7. CD30 was largely negative. CD21 failed to detect follicular dendritic cell networks, and κ/λ light chain staining confirmed a preserved ratio of polytypic plasma cells. There was limited staining with B-cell lymphoma (Bcl-2 and Bcl-6). Polymerase chain reaction studies for both T- and B-cell receptors were negative (polyclonal).
Lyme disease is the most frequently reported vectorborne infectious disease in the United States, and borrelial lymphocytoma (BL) is a rare clinical sequela. Borrelial lymphocytoma is a variant of lymphocytoma cutis (also known as benign reactive lymphoid hyperplasia), which is an inflammatory lesion that can mimic malignant lymphoma clinically and histologically. Lymphocytoma cutis is considered the prototypical example of cutaneous B-cell pseudolymphoma.1 Due to suspicion for lymphocytoma cutis based on the histologic findings and characteristic location of the lesions in our patient, Lyme serologies were ordered and were positive for IgM antibodies against p23, p39, and p41 antigens in high titers. Our patient was treated with doxycycline 100 mg twice daily for 3 weeks with complete resolution of the lesions at 3-month follow-up.
Clinically, BL appears as erythematous papules, plaques, or nodules commonly on the lobules of the ears (quiz image, top). Most cases of lymphocytoma cutis are idiopathic but may be triggered by identifiable associated etiologies including Borrelia burgdorferi, Leishmania donovani, molluscum contagiosum, herpes zoster virus, vaccinations, tattoos, insect bites, and drugs. The main differential diagnosis of lymphocytoma cutis is cutaneous B-cell lymphoma. Pseudolymphoma of the skin can mimic nearly all immunohistochemical staining patterns of true B-cell lymphomas.2
Primary cutaneous follicle center lymphoma frequently occurs on the head and neck. This true lymphoma of the skin can demonstrate prominent follicle centers with centrocytes and fragmented germinal centers (Figure 1) or show a diffuse pattern.3 Most cases show conspicuous Bcl-6 staining, and IgH gene rearrangements can detect a clonal B-cell population in more than 50% of cases.4
Diffuse large B-cell lymphoma can occur as a primary cutaneous malignancy or as a manifestation of systemic disease.4 When arising in the skin, lesions tend to affect the extremities, and the disease is classified as diffuse large B-cell lymphoma, leg type. Histologically, sheets of large atypical lymphocytes with numerous mitoses are seen (Figure 2). These cells stain positively with Bcl-2 and frequently demonstrate Bcl-6 and MUM-1, none of which were seen in our case.4 Lymphomatoid papulosis (LyP) tends to present with relapsing erythematous papules. Patients occasionally develop LyP in association with mycosis fungoides or other lymphomas. Both LyP and primary cutaneous anaplastic large cell lymphoma demonstrate conspicuous CD30+ large cells that can be multinucleated or resemble the Reed-Sternberg cells seen in Hodgkin lymphoma (Figure 3).4 Arthropod bite reactions are common but may be confused with lymphomas and pseudolymphomas. The perivascular lymphocytic infiltrate seen in arthropod bite reactions may be dense and usually is associated with numerous eosinophils (Figure 4). Occasional plasma cells also can be seen, and if the infiltrate closely adheres to vascular structures, a diagnosis of erythema chronicum migrans also can be considered. Patients with chronic lymphocytic leukemia/lymphoma may demonstrate exaggerated or persistent arthropod bite reactions, and atypical lymphocytes can be detected admixed with the otherwise reactive infiltrate.4
Borrelia burgdorferi is primarily endemic to North America and Europe. It is a spirochete bacterium spread by the Ixodes tick that was first recognized as the etiologic agent in 1975 in Old Lyme, Connecticut, where it received its name.5 Most reported cases of Lyme disease occur in the northeastern United States, which correlates with this case given our patient’s place of residence.6 Borrelial lymphocytoma cutis occurs in areas endemic for the Ixodes tick in Europe and North America.7 When describing the genotyping of Borrelia seen in BL, the strain B burgdorferi previously was grouped with Borrelia afzelii and Borrelia garinii.2 In the contemporary literature, however, B burgdorferi is referred to as sensu stricto when specifically talking about the strain B burgdorferi, and the term sensu lato is used when referencing the combination of strains (B burgdorferi, B afzelii, B garinii).
A 2016 study by Maraspin et al8 comprising 144 patients diagnosed with BL showed that the lesions mainly were located on the breast (106 patients [73.6%]) and the earlobe (27 patients [18.8%]), with the remaining cases occurring elsewhere on the body (11 patients [7.6%]). The Borrelia strains isolated from the BL lesions included B afzelii, Borrelia bissettii, and B garinii, with B afzelii being the most commonly identified (84.6% [11/13]).8
Borrelial lymphocytoma usually is categorized as a form of early disseminated Lyme disease and is treated as such. The treatment of choice for early disseminated Lyme disease is doxycycline 100 mg twice daily for 14 to 21 days. Ceftriaxone and azithromycin are reasonable treatment options for patients who have tetracycline allergies or who are pregnant.9
In conclusion, the presentation of red papules or nodules on the ears should prompt clinical suspicion of Lyme disease, particularly in endemic areas. Differentiating pseudolymphomas from true lymphomas and other reactive conditions can be challenging.
The Diagnosis: Borrelial Lymphocytoma (Lymphocytoma Cutis)
A punch biopsy revealed an atypical lobular lymphoid infiltrate within the dermis and subcutaneous tissue with a mixed composition of CD3+ T cells and CD20+ B cells (quiz image, bottom). Immunohistochemical studies revealed a normal CD4:CD8 ratio with preservation of CD5 and CD7. CD30 was largely negative. CD21 failed to detect follicular dendritic cell networks, and κ/λ light chain staining confirmed a preserved ratio of polytypic plasma cells. There was limited staining with B-cell lymphoma (Bcl-2 and Bcl-6). Polymerase chain reaction studies for both T- and B-cell receptors were negative (polyclonal).
Lyme disease is the most frequently reported vectorborne infectious disease in the United States, and borrelial lymphocytoma (BL) is a rare clinical sequela. Borrelial lymphocytoma is a variant of lymphocytoma cutis (also known as benign reactive lymphoid hyperplasia), which is an inflammatory lesion that can mimic malignant lymphoma clinically and histologically. Lymphocytoma cutis is considered the prototypical example of cutaneous B-cell pseudolymphoma.1 Due to suspicion for lymphocytoma cutis based on the histologic findings and characteristic location of the lesions in our patient, Lyme serologies were ordered and were positive for IgM antibodies against p23, p39, and p41 antigens in high titers. Our patient was treated with doxycycline 100 mg twice daily for 3 weeks with complete resolution of the lesions at 3-month follow-up.
Clinically, BL appears as erythematous papules, plaques, or nodules commonly on the lobules of the ears (quiz image, top). Most cases of lymphocytoma cutis are idiopathic but may be triggered by identifiable associated etiologies including Borrelia burgdorferi, Leishmania donovani, molluscum contagiosum, herpes zoster virus, vaccinations, tattoos, insect bites, and drugs. The main differential diagnosis of lymphocytoma cutis is cutaneous B-cell lymphoma. Pseudolymphoma of the skin can mimic nearly all immunohistochemical staining patterns of true B-cell lymphomas.2
Primary cutaneous follicle center lymphoma frequently occurs on the head and neck. This true lymphoma of the skin can demonstrate prominent follicle centers with centrocytes and fragmented germinal centers (Figure 1) or show a diffuse pattern.3 Most cases show conspicuous Bcl-6 staining, and IgH gene rearrangements can detect a clonal B-cell population in more than 50% of cases.4
Diffuse large B-cell lymphoma can occur as a primary cutaneous malignancy or as a manifestation of systemic disease.4 When arising in the skin, lesions tend to affect the extremities, and the disease is classified as diffuse large B-cell lymphoma, leg type. Histologically, sheets of large atypical lymphocytes with numerous mitoses are seen (Figure 2). These cells stain positively with Bcl-2 and frequently demonstrate Bcl-6 and MUM-1, none of which were seen in our case.4 Lymphomatoid papulosis (LyP) tends to present with relapsing erythematous papules. Patients occasionally develop LyP in association with mycosis fungoides or other lymphomas. Both LyP and primary cutaneous anaplastic large cell lymphoma demonstrate conspicuous CD30+ large cells that can be multinucleated or resemble the Reed-Sternberg cells seen in Hodgkin lymphoma (Figure 3).4 Arthropod bite reactions are common but may be confused with lymphomas and pseudolymphomas. The perivascular lymphocytic infiltrate seen in arthropod bite reactions may be dense and usually is associated with numerous eosinophils (Figure 4). Occasional plasma cells also can be seen, and if the infiltrate closely adheres to vascular structures, a diagnosis of erythema chronicum migrans also can be considered. Patients with chronic lymphocytic leukemia/lymphoma may demonstrate exaggerated or persistent arthropod bite reactions, and atypical lymphocytes can be detected admixed with the otherwise reactive infiltrate.4
Borrelia burgdorferi is primarily endemic to North America and Europe. It is a spirochete bacterium spread by the Ixodes tick that was first recognized as the etiologic agent in 1975 in Old Lyme, Connecticut, where it received its name.5 Most reported cases of Lyme disease occur in the northeastern United States, which correlates with this case given our patient’s place of residence.6 Borrelial lymphocytoma cutis occurs in areas endemic for the Ixodes tick in Europe and North America.7 When describing the genotyping of Borrelia seen in BL, the strain B burgdorferi previously was grouped with Borrelia afzelii and Borrelia garinii.2 In the contemporary literature, however, B burgdorferi is referred to as sensu stricto when specifically talking about the strain B burgdorferi, and the term sensu lato is used when referencing the combination of strains (B burgdorferi, B afzelii, B garinii).
A 2016 study by Maraspin et al8 comprising 144 patients diagnosed with BL showed that the lesions mainly were located on the breast (106 patients [73.6%]) and the earlobe (27 patients [18.8%]), with the remaining cases occurring elsewhere on the body (11 patients [7.6%]). The Borrelia strains isolated from the BL lesions included B afzelii, Borrelia bissettii, and B garinii, with B afzelii being the most commonly identified (84.6% [11/13]).8
Borrelial lymphocytoma usually is categorized as a form of early disseminated Lyme disease and is treated as such. The treatment of choice for early disseminated Lyme disease is doxycycline 100 mg twice daily for 14 to 21 days. Ceftriaxone and azithromycin are reasonable treatment options for patients who have tetracycline allergies or who are pregnant.9
In conclusion, the presentation of red papules or nodules on the ears should prompt clinical suspicion of Lyme disease, particularly in endemic areas. Differentiating pseudolymphomas from true lymphomas and other reactive conditions can be challenging.
- Mitteldorf C, Kempf W. Cutaneous pseudolymphoma. Surg Pathol Clin. 2017;10:455-476. doi:10.1016/j.path.2017.01.002
- Colli C, Leinweber B, Müllegger R, et al. Borrelia burgdorferiassociated lymphocytoma cutis: clinicopathologic, immunophenotypic, and molecular study of 106 cases. J Cutan Pathol. 2004;31:232-240. doi:10.1111/j.0303-6987.2003.00167.x
- Wehbe AM, Neppalli V, Syrbu S, et al. Diffuse follicle centre lymphoma presents with high frequency of extranodal disease. J Clin Oncol. 2008;26(15 suppl):19511. doi:10.1200/jco.2008.26.15_suppl.19511
- Patterson JW, Hosler GA. Cutaneous infiltrates—lymphomatous and leukemic. In: Patterson JW, ed. Weedon’s Skin Pathology. 4th ed. Elsevier; 2016:1171-1217.
- Cardenas-de la Garza JA, De la Cruz-Valadez E, Ocampo -Candiani J, et al. Clinical spectrum of Lyme disease. Eur J Clin Microbiol Infect Dis. 2019;38:201-208. doi:10.1007/s10096-018-3417-1
- Shapiro ED, Gerber MA. Lyme disease. Clin Infect Dis. 2000;31:533-542. doi:10.1086/313982
- Kandhari R, Kandhari S, Jain S. Borrelial lymphocytoma cutis: a diagnostic dilemma. Indian J Dermatol. 2014;59:595-597. doi:10.4103/0019-5154.143530
- Maraspin V, Nahtigal Klevišar M, Ružic´-Sabljic´ E, et al. Borrelial lymphocytoma in adult patients. Clin Infect Dis. 2016;63:914-921. doi:10.1093/cid/ciw417
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006; 43:1089-1134. doi:10.1086/508667
- Mitteldorf C, Kempf W. Cutaneous pseudolymphoma. Surg Pathol Clin. 2017;10:455-476. doi:10.1016/j.path.2017.01.002
- Colli C, Leinweber B, Müllegger R, et al. Borrelia burgdorferiassociated lymphocytoma cutis: clinicopathologic, immunophenotypic, and molecular study of 106 cases. J Cutan Pathol. 2004;31:232-240. doi:10.1111/j.0303-6987.2003.00167.x
- Wehbe AM, Neppalli V, Syrbu S, et al. Diffuse follicle centre lymphoma presents with high frequency of extranodal disease. J Clin Oncol. 2008;26(15 suppl):19511. doi:10.1200/jco.2008.26.15_suppl.19511
- Patterson JW, Hosler GA. Cutaneous infiltrates—lymphomatous and leukemic. In: Patterson JW, ed. Weedon’s Skin Pathology. 4th ed. Elsevier; 2016:1171-1217.
- Cardenas-de la Garza JA, De la Cruz-Valadez E, Ocampo -Candiani J, et al. Clinical spectrum of Lyme disease. Eur J Clin Microbiol Infect Dis. 2019;38:201-208. doi:10.1007/s10096-018-3417-1
- Shapiro ED, Gerber MA. Lyme disease. Clin Infect Dis. 2000;31:533-542. doi:10.1086/313982
- Kandhari R, Kandhari S, Jain S. Borrelial lymphocytoma cutis: a diagnostic dilemma. Indian J Dermatol. 2014;59:595-597. doi:10.4103/0019-5154.143530
- Maraspin V, Nahtigal Klevišar M, Ružic´-Sabljic´ E, et al. Borrelial lymphocytoma in adult patients. Clin Infect Dis. 2016;63:914-921. doi:10.1093/cid/ciw417
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006; 43:1089-1134. doi:10.1086/508667
A 53-year-old man with a history of atopic dermatitis presented with pain and redness of the lobules of both ears of 9 months’ duration. He had no known allergies and took no medications. He lived in suburban Virginia and had not recently traveled outside of the region. Physical examination revealed tender erythematous and edematous nodules on the lobules of both ears (top). There was no evidence of arthritis or neurologic deficits. A punch biopsy was performed (bottom).
Acute Generalized Exanthematous Pustulosis Induced by the Second-Generation Antipsychotic Cariprazine
To the Editor:
A 57-year-old woman presented to an outpatient clinic with severe pruritus and burning of the skin as well as subjective fevers and chills. She had been discharged from a psychiatric hospital for attempted suicide 1 day prior. There were no recent changes in the medication regimen, which consisted of linaclotide, fluoxetine, lorazepam, and gabapentin. While admitted, the patient was started on the atypical antipsychotic cariprazine. Within 24 hours of the first dose, she developed severe facial erythema that progressed to diffuse erythema over more than 60% of the body surface area. The attending psychiatrist promptly discontinued cariprazine. During the next 24 hours, there were no reports of fever, leukocytosis, or signs of systemic organ involvement. Given the patient’s mental and medical stability, she was discharged with instructions to follow up with the outpatient dermatology clinic.
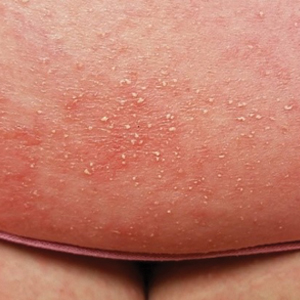
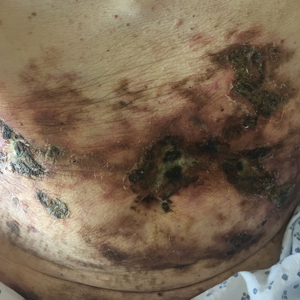
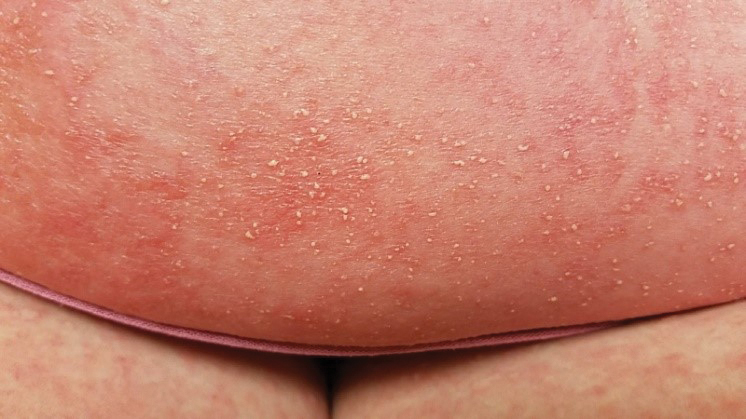
At the current presentation, physical examination revealed innumerable 1- to 4-mm pustules coalescing to lakes of pus on an erythematous base over more than 60% of the body surface area (Figure 1). The mucous membranes were clear of lesions, the Nikolsky sign was negative, and the patient’s temperature was 99.6 °F in the office. Complete blood cell count and complete metabolic panel results were within reference range.
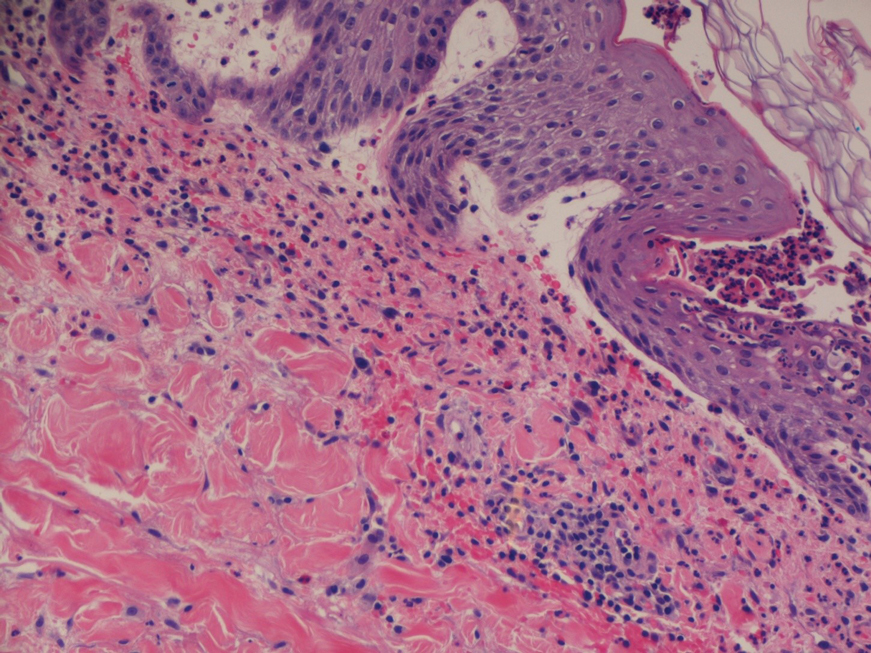
A 4-mm abdominal punch biopsy showed subcorneal neutrophilic pustules, papillary dermal edema, and superficial dermal lymphohistiocytic inflammation with numerous neutrophils, eosinophils, and extravasated red blood cells, consistent with acute generalized exanthematous pustulosis (AGEP)(Figure 2). The patient was started on wet wraps with triamcinolone cream 0.1%.
Two days later, physical examination revealed the erythema noted on initial examination had notably decreased, and the patient no longer reported burning or pruritus. One week after initial presentation to the clinic, the patient’s rash had resolved, and only a few small areas of desquamation remained.
Acute generalized exanthematous pustulosis is a severe cutaneous adverse reaction characterized by the development of numerous nonfollicular sterile pustules on an edematous and erythematous base. In almost 90% of reported cases, the cause is related to use of antibiotics, antifungals, antimalarials, or diltiazem (a calcium channel blocker). This rare cutaneous reaction occurs in 1 to 5 patients per million per year1; it carries a 1% to 2% mortality rate with proper supportive treatment.
The clinical symptoms of AGEP typically present 24 to 48 hours after drug initiation with the rapid development of dozens to thousands of 1- to 4-mm pustules, typically localized to the flexor surfaces and face. In the setting of AGEP, acute onset of fever and leukocytosis typically occur at the time of the cutaneous eruption. These features were absent in this patient. The eruption usually starts on the face and then migrates to the trunk and extremities, sparing the palms and soles. Systemic involvement most commonly presents as hepatic, renal, or pulmonary insufficiency, which has been seen in 20% of cases.2
The immunologic response associated with the reaction has been studied in vitro. Drug-specific CD8 T cells use perforin/granzyme B and Fas ligand mechanisms to induce apoptosis of the keratinocytes within the epidermis, leading to vesicle formation.3 During the very first stages of formation, vesicles mainly comprise CD8 T cells and keratinocytes. These cells then begin producing CXC-18, a potent neutrophil chemokine, leading to extensive chemotaxis of neutrophils into vesicles, which then rapidly transform to pustules.3 This rapid transformation leads to the lakes of pustules, a description often associated with AGEP.
Treatment of AGEP is mainly supportive and consists of discontinuing use of the causative agent. Topical corticosteroids can be used during the pustular phase for symptom management. There is no evidence that systemic steroids reduce the duration of the disease.2 Other supportive measures such as application of wet wraps can be used to provide comfort.
Cutaneous adverse drug reactions commonly are associated with psychiatric pharmacotherapy, but first-and second-generation antipsychotics rarely are associated with these types of reactions. In this patient, the causative agent of the AGEP was cariprazine, an atypical antipsychotic that had no reported association with AGEP or cutaneous adverse drug reactions prior to this presentation.
- Fernando SL. Acute generalised exanthematous pustulosis. Australas J Dermatol. 2012;53:87-92.
- Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci. 2016;17:1214.
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2015;73:843-848.
To the Editor:
A 57-year-old woman presented to an outpatient clinic with severe pruritus and burning of the skin as well as subjective fevers and chills. She had been discharged from a psychiatric hospital for attempted suicide 1 day prior. There were no recent changes in the medication regimen, which consisted of linaclotide, fluoxetine, lorazepam, and gabapentin. While admitted, the patient was started on the atypical antipsychotic cariprazine. Within 24 hours of the first dose, she developed severe facial erythema that progressed to diffuse erythema over more than 60% of the body surface area. The attending psychiatrist promptly discontinued cariprazine. During the next 24 hours, there were no reports of fever, leukocytosis, or signs of systemic organ involvement. Given the patient’s mental and medical stability, she was discharged with instructions to follow up with the outpatient dermatology clinic.
At the current presentation, physical examination revealed innumerable 1- to 4-mm pustules coalescing to lakes of pus on an erythematous base over more than 60% of the body surface area (Figure 1). The mucous membranes were clear of lesions, the Nikolsky sign was negative, and the patient’s temperature was 99.6 °F in the office. Complete blood cell count and complete metabolic panel results were within reference range.

A 4-mm abdominal punch biopsy showed subcorneal neutrophilic pustules, papillary dermal edema, and superficial dermal lymphohistiocytic inflammation with numerous neutrophils, eosinophils, and extravasated red blood cells, consistent with acute generalized exanthematous pustulosis (AGEP)(Figure 2). The patient was started on wet wraps with triamcinolone cream 0.1%.

Two days later, physical examination revealed the erythema noted on initial examination had notably decreased, and the patient no longer reported burning or pruritus. One week after initial presentation to the clinic, the patient’s rash had resolved, and only a few small areas of desquamation remained.
Acute generalized exanthematous pustulosis is a severe cutaneous adverse reaction characterized by the development of numerous nonfollicular sterile pustules on an edematous and erythematous base. In almost 90% of reported cases, the cause is related to use of antibiotics, antifungals, antimalarials, or diltiazem (a calcium channel blocker). This rare cutaneous reaction occurs in 1 to 5 patients per million per year1; it carries a 1% to 2% mortality rate with proper supportive treatment.
The clinical symptoms of AGEP typically present 24 to 48 hours after drug initiation with the rapid development of dozens to thousands of 1- to 4-mm pustules, typically localized to the flexor surfaces and face. In the setting of AGEP, acute onset of fever and leukocytosis typically occur at the time of the cutaneous eruption. These features were absent in this patient. The eruption usually starts on the face and then migrates to the trunk and extremities, sparing the palms and soles. Systemic involvement most commonly presents as hepatic, renal, or pulmonary insufficiency, which has been seen in 20% of cases.2
The immunologic response associated with the reaction has been studied in vitro. Drug-specific CD8 T cells use perforin/granzyme B and Fas ligand mechanisms to induce apoptosis of the keratinocytes within the epidermis, leading to vesicle formation.3 During the very first stages of formation, vesicles mainly comprise CD8 T cells and keratinocytes. These cells then begin producing CXC-18, a potent neutrophil chemokine, leading to extensive chemotaxis of neutrophils into vesicles, which then rapidly transform to pustules.3 This rapid transformation leads to the lakes of pustules, a description often associated with AGEP.
Treatment of AGEP is mainly supportive and consists of discontinuing use of the causative agent. Topical corticosteroids can be used during the pustular phase for symptom management. There is no evidence that systemic steroids reduce the duration of the disease.2 Other supportive measures such as application of wet wraps can be used to provide comfort.
Cutaneous adverse drug reactions commonly are associated with psychiatric pharmacotherapy, but first-and second-generation antipsychotics rarely are associated with these types of reactions. In this patient, the causative agent of the AGEP was cariprazine, an atypical antipsychotic that had no reported association with AGEP or cutaneous adverse drug reactions prior to this presentation.
To the Editor:
A 57-year-old woman presented to an outpatient clinic with severe pruritus and burning of the skin as well as subjective fevers and chills. She had been discharged from a psychiatric hospital for attempted suicide 1 day prior. There were no recent changes in the medication regimen, which consisted of linaclotide, fluoxetine, lorazepam, and gabapentin. While admitted, the patient was started on the atypical antipsychotic cariprazine. Within 24 hours of the first dose, she developed severe facial erythema that progressed to diffuse erythema over more than 60% of the body surface area. The attending psychiatrist promptly discontinued cariprazine. During the next 24 hours, there were no reports of fever, leukocytosis, or signs of systemic organ involvement. Given the patient’s mental and medical stability, she was discharged with instructions to follow up with the outpatient dermatology clinic.
At the current presentation, physical examination revealed innumerable 1- to 4-mm pustules coalescing to lakes of pus on an erythematous base over more than 60% of the body surface area (Figure 1). The mucous membranes were clear of lesions, the Nikolsky sign was negative, and the patient’s temperature was 99.6 °F in the office. Complete blood cell count and complete metabolic panel results were within reference range.

A 4-mm abdominal punch biopsy showed subcorneal neutrophilic pustules, papillary dermal edema, and superficial dermal lymphohistiocytic inflammation with numerous neutrophils, eosinophils, and extravasated red blood cells, consistent with acute generalized exanthematous pustulosis (AGEP)(Figure 2). The patient was started on wet wraps with triamcinolone cream 0.1%.

Two days later, physical examination revealed the erythema noted on initial examination had notably decreased, and the patient no longer reported burning or pruritus. One week after initial presentation to the clinic, the patient’s rash had resolved, and only a few small areas of desquamation remained.
Acute generalized exanthematous pustulosis is a severe cutaneous adverse reaction characterized by the development of numerous nonfollicular sterile pustules on an edematous and erythematous base. In almost 90% of reported cases, the cause is related to use of antibiotics, antifungals, antimalarials, or diltiazem (a calcium channel blocker). This rare cutaneous reaction occurs in 1 to 5 patients per million per year1; it carries a 1% to 2% mortality rate with proper supportive treatment.
The clinical symptoms of AGEP typically present 24 to 48 hours after drug initiation with the rapid development of dozens to thousands of 1- to 4-mm pustules, typically localized to the flexor surfaces and face. In the setting of AGEP, acute onset of fever and leukocytosis typically occur at the time of the cutaneous eruption. These features were absent in this patient. The eruption usually starts on the face and then migrates to the trunk and extremities, sparing the palms and soles. Systemic involvement most commonly presents as hepatic, renal, or pulmonary insufficiency, which has been seen in 20% of cases.2
The immunologic response associated with the reaction has been studied in vitro. Drug-specific CD8 T cells use perforin/granzyme B and Fas ligand mechanisms to induce apoptosis of the keratinocytes within the epidermis, leading to vesicle formation.3 During the very first stages of formation, vesicles mainly comprise CD8 T cells and keratinocytes. These cells then begin producing CXC-18, a potent neutrophil chemokine, leading to extensive chemotaxis of neutrophils into vesicles, which then rapidly transform to pustules.3 This rapid transformation leads to the lakes of pustules, a description often associated with AGEP.
Treatment of AGEP is mainly supportive and consists of discontinuing use of the causative agent. Topical corticosteroids can be used during the pustular phase for symptom management. There is no evidence that systemic steroids reduce the duration of the disease.2 Other supportive measures such as application of wet wraps can be used to provide comfort.
Cutaneous adverse drug reactions commonly are associated with psychiatric pharmacotherapy, but first-and second-generation antipsychotics rarely are associated with these types of reactions. In this patient, the causative agent of the AGEP was cariprazine, an atypical antipsychotic that had no reported association with AGEP or cutaneous adverse drug reactions prior to this presentation.
- Fernando SL. Acute generalised exanthematous pustulosis. Australas J Dermatol. 2012;53:87-92.
- Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci. 2016;17:1214.
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2015;73:843-848.
- Fernando SL. Acute generalised exanthematous pustulosis. Australas J Dermatol. 2012;53:87-92.
- Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci. 2016;17:1214.
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2015;73:843-848.
Practice Points
- The second-generation antipsychotic cariprazine has been shown to be a potential causative agent in acute generalized exanthematous pustulosis (AGEP).
- Treatment of AGEP is mainly supportive and consists of discontinuation of the causative agent as well as symptom control using cold compresses and topical corticosteroids.
Itchy Vesicular Rash
The Diagnosis: Tinea Corporis Bullosa
At the time of presentation, a potassium hydroxide (KOH) preparation, fungal culture, and punch biopsy of the right ventral wrist was performed. The KOH preparation was positive for fungal hyphae characteristic of dermatophyte infections. Histologically, the biopsy showed intraepidermal and subepidermal blisters with neutrophil- and lymphocyte-rich contents (Figure 1). Fungal hyphae and spores were present within the stratum corneum and superficial epidermis (Figure 2), and fungal cultures grew Microsporum canis. The extent of the rash (upper and lower extremities, chest, and back), positive fungal culture, and KOH preparation all supported the diagnosis of tinea corporis bullosa, which was confirmed with biopsy. Oral prednisone use was discouraged and triamcinolone ointment was discontinued given that inappropriate treatment with steroids in the setting of fungal infection suppresses an inflammatory response and alters clinical appearance, obviating the persistent underlying infection.
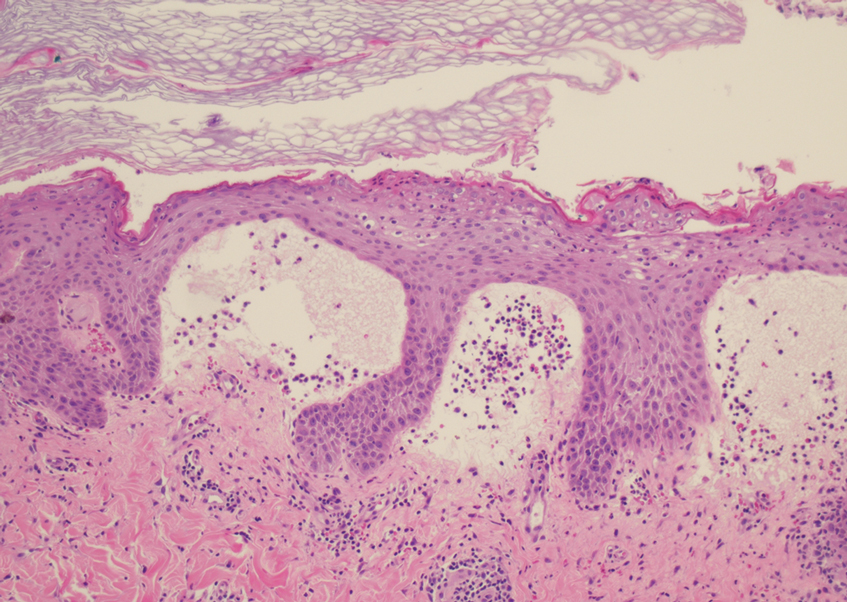
Tinea corporis bullosa is a rare superficial dermatophyte fungal infection that often is acquired by close personto- person contact or contact with domestic animals. The infection begins as a circular pruritic plaque, generally with raised borders, which may be erythematous or hyperpigmented. By definition, tinea corporis occurs in sites other than the face, feet, hands, or groin area. Bullae formation is thought to be secondary to a delayed hypersensitivity reaction provoked by the presence of a dermatophyte antigen.1
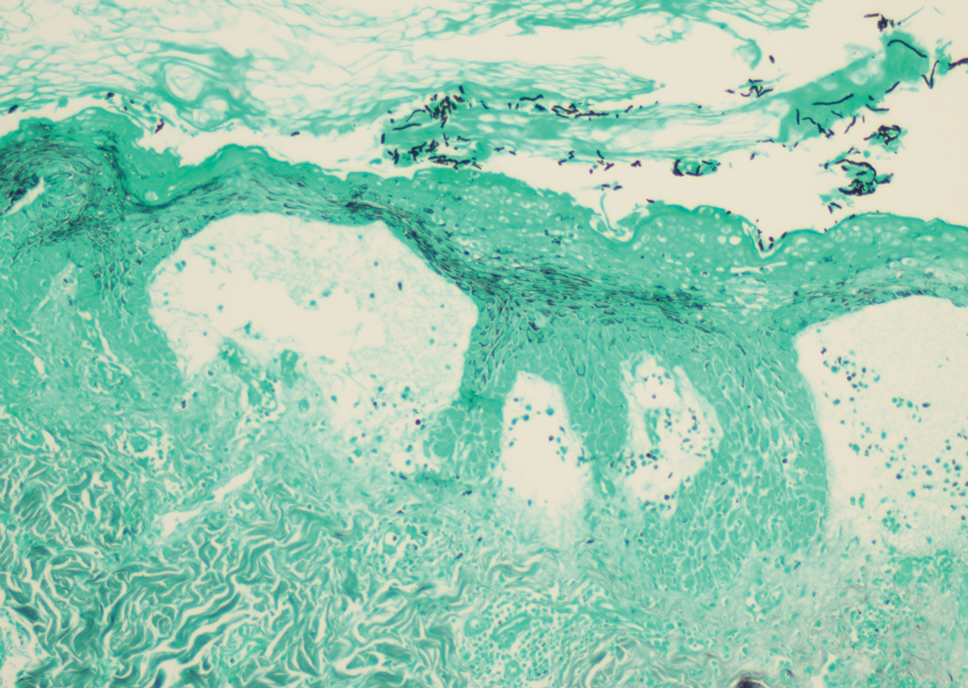
Linear IgA bullous dermatosis is an immunemediated disease characterized by IgA deposition at the dermoepidermal junction. Linear IgA bullous dermatosis classically presents as widespread tense vesicles in an arciform or annular pattern. Mucosal involvement is common and typically presents with erosions, ulcerations, and scarring.2 Given the absence of mucosal involvement in our patient and a positive KOH preparation, linear IgA bullous dermatosis was an unlikely diagnosis.
Benign inoculation lymphoreticulosis, more commonly known as cat scratch disease (CSD), is a Bartonella henselae infection that results from a cat scratch or bite. Cat scratch disease can present as localized cutaneous and nodal involvement (lymphadenopathy) near the site of inoculation, or it may present as disseminated disease. Cutaneous lesions generally progress through vesicular, erythematous, and papular phases. Regional lymphadenopathy proximal to the inoculation site is the hallmark of CSD.3 Given the absence of lymphadenopathy in our patient as well as the sporadic distribution of lesions, CSD was an unlikely diagnosis.
Dermatitis herpetiformis (DH) is an autoimmune disorder with cutaneous manifestations of gluten sensitivity. Dermatitis herpetiformis presents as extremely pruritic papules and vesicles arranged in groups on areas such as the elbows, dorsal aspects of the forearms, knees, scalp, back, and buttocks. Most patients with DH have celiac disease or small bowel disease related to gluten sensitivity.4 Given our patient’s acute presentation in adulthood and lack of gluten sensitivity, DH was an unlikely diagnosis.
Bullous fixed drug reaction is a cutaneous eruption that typically presents in the setting of exposure to an offending drug/agent. Drug reactions can have various cutaneous presentations, with the most common being pigmented macules that progress into plaques.5 Given the isolated nature of our patient’s episode and apparent lack of association with medication, bullous fixed drug reaction was an unlikely diagnosis.
Tinea corporis bullosa is a rare clinical variant of tinea corporis that has only been reported in patients with a history of contact with different animals. There are many causative organisms related to tinea corporis; Trichophyton rubrum is the most common etiology of tinea corporis, while tinea corporis due to close contact with domesticated animals often is caused by M canis.6 The immunoinhibitory properties of the mannans in the fungal cell wall allow the organisms to adhere to the skin prior to invasion. Cutaneous invasion into dead cornified layers of the skin is credited to the proteases, subtilisinlike proteases (subtilases), and keratinases produced by the fungus.1 There are many different clinical presentations of tinea corporis due to the variability of causative organisms. An annular (ring-shaped) lesion with a central plaque and advancing border is the most typical presentation. Tinea corporis bullosa is characterized by the presence of bullae or vesicles in the borders of the scaly plaque. Rupture of the bullae subsequently leads to erosions and crusts over the plaque.
The diagnosis of tinea corporis bullosa often is clinical if the lesion is typical and can be confirmed using KOH preparation and fungal culture. Once the diagnosis is confirmed, topical antifungals are the standard treatment approach for localized superficial tinea corporis. Systemic antifungal treatment can be initiated if the lesion is extensive, recurrent, chronic, or unresponsive to topical treatment.1 Given our patient’s characteristic presentation, she was managed with an over-the-counter topical antifungal (terbinafine). The patient’s lesions dramatically improved, rendering oral therapy unnecessary. At 1-month follow-up, the rash had nearly resolved.
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review [published online July 20, 2020]. Drugs Context. doi:10.7573/dic.2020-5-6
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Lamps LW, Scott MA. Cat-scratch disease: historic, clinical, and pathologic perspectives. Pathology Patterns Reviews. 2004;121(suppl):S71-S80.
- Caproni M, Antiga E, Melani L, et al. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009;23:633-638.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ziemer M, Seyfarth F, Elsner P, et al. Atypical manifestations of tinea corporis. Mycoses. 2007;50:31-35.
The Diagnosis: Tinea Corporis Bullosa
At the time of presentation, a potassium hydroxide (KOH) preparation, fungal culture, and punch biopsy of the right ventral wrist was performed. The KOH preparation was positive for fungal hyphae characteristic of dermatophyte infections. Histologically, the biopsy showed intraepidermal and subepidermal blisters with neutrophil- and lymphocyte-rich contents (Figure 1). Fungal hyphae and spores were present within the stratum corneum and superficial epidermis (Figure 2), and fungal cultures grew Microsporum canis. The extent of the rash (upper and lower extremities, chest, and back), positive fungal culture, and KOH preparation all supported the diagnosis of tinea corporis bullosa, which was confirmed with biopsy. Oral prednisone use was discouraged and triamcinolone ointment was discontinued given that inappropriate treatment with steroids in the setting of fungal infection suppresses an inflammatory response and alters clinical appearance, obviating the persistent underlying infection.

Tinea corporis bullosa is a rare superficial dermatophyte fungal infection that often is acquired by close personto- person contact or contact with domestic animals. The infection begins as a circular pruritic plaque, generally with raised borders, which may be erythematous or hyperpigmented. By definition, tinea corporis occurs in sites other than the face, feet, hands, or groin area. Bullae formation is thought to be secondary to a delayed hypersensitivity reaction provoked by the presence of a dermatophyte antigen.1

Linear IgA bullous dermatosis is an immunemediated disease characterized by IgA deposition at the dermoepidermal junction. Linear IgA bullous dermatosis classically presents as widespread tense vesicles in an arciform or annular pattern. Mucosal involvement is common and typically presents with erosions, ulcerations, and scarring.2 Given the absence of mucosal involvement in our patient and a positive KOH preparation, linear IgA bullous dermatosis was an unlikely diagnosis.
Benign inoculation lymphoreticulosis, more commonly known as cat scratch disease (CSD), is a Bartonella henselae infection that results from a cat scratch or bite. Cat scratch disease can present as localized cutaneous and nodal involvement (lymphadenopathy) near the site of inoculation, or it may present as disseminated disease. Cutaneous lesions generally progress through vesicular, erythematous, and papular phases. Regional lymphadenopathy proximal to the inoculation site is the hallmark of CSD.3 Given the absence of lymphadenopathy in our patient as well as the sporadic distribution of lesions, CSD was an unlikely diagnosis.
Dermatitis herpetiformis (DH) is an autoimmune disorder with cutaneous manifestations of gluten sensitivity. Dermatitis herpetiformis presents as extremely pruritic papules and vesicles arranged in groups on areas such as the elbows, dorsal aspects of the forearms, knees, scalp, back, and buttocks. Most patients with DH have celiac disease or small bowel disease related to gluten sensitivity.4 Given our patient’s acute presentation in adulthood and lack of gluten sensitivity, DH was an unlikely diagnosis.
Bullous fixed drug reaction is a cutaneous eruption that typically presents in the setting of exposure to an offending drug/agent. Drug reactions can have various cutaneous presentations, with the most common being pigmented macules that progress into plaques.5 Given the isolated nature of our patient’s episode and apparent lack of association with medication, bullous fixed drug reaction was an unlikely diagnosis.
Tinea corporis bullosa is a rare clinical variant of tinea corporis that has only been reported in patients with a history of contact with different animals. There are many causative organisms related to tinea corporis; Trichophyton rubrum is the most common etiology of tinea corporis, while tinea corporis due to close contact with domesticated animals often is caused by M canis.6 The immunoinhibitory properties of the mannans in the fungal cell wall allow the organisms to adhere to the skin prior to invasion. Cutaneous invasion into dead cornified layers of the skin is credited to the proteases, subtilisinlike proteases (subtilases), and keratinases produced by the fungus.1 There are many different clinical presentations of tinea corporis due to the variability of causative organisms. An annular (ring-shaped) lesion with a central plaque and advancing border is the most typical presentation. Tinea corporis bullosa is characterized by the presence of bullae or vesicles in the borders of the scaly plaque. Rupture of the bullae subsequently leads to erosions and crusts over the plaque.
The diagnosis of tinea corporis bullosa often is clinical if the lesion is typical and can be confirmed using KOH preparation and fungal culture. Once the diagnosis is confirmed, topical antifungals are the standard treatment approach for localized superficial tinea corporis. Systemic antifungal treatment can be initiated if the lesion is extensive, recurrent, chronic, or unresponsive to topical treatment.1 Given our patient’s characteristic presentation, she was managed with an over-the-counter topical antifungal (terbinafine). The patient’s lesions dramatically improved, rendering oral therapy unnecessary. At 1-month follow-up, the rash had nearly resolved.
The Diagnosis: Tinea Corporis Bullosa
At the time of presentation, a potassium hydroxide (KOH) preparation, fungal culture, and punch biopsy of the right ventral wrist was performed. The KOH preparation was positive for fungal hyphae characteristic of dermatophyte infections. Histologically, the biopsy showed intraepidermal and subepidermal blisters with neutrophil- and lymphocyte-rich contents (Figure 1). Fungal hyphae and spores were present within the stratum corneum and superficial epidermis (Figure 2), and fungal cultures grew Microsporum canis. The extent of the rash (upper and lower extremities, chest, and back), positive fungal culture, and KOH preparation all supported the diagnosis of tinea corporis bullosa, which was confirmed with biopsy. Oral prednisone use was discouraged and triamcinolone ointment was discontinued given that inappropriate treatment with steroids in the setting of fungal infection suppresses an inflammatory response and alters clinical appearance, obviating the persistent underlying infection.

Tinea corporis bullosa is a rare superficial dermatophyte fungal infection that often is acquired by close personto- person contact or contact with domestic animals. The infection begins as a circular pruritic plaque, generally with raised borders, which may be erythematous or hyperpigmented. By definition, tinea corporis occurs in sites other than the face, feet, hands, or groin area. Bullae formation is thought to be secondary to a delayed hypersensitivity reaction provoked by the presence of a dermatophyte antigen.1

Linear IgA bullous dermatosis is an immunemediated disease characterized by IgA deposition at the dermoepidermal junction. Linear IgA bullous dermatosis classically presents as widespread tense vesicles in an arciform or annular pattern. Mucosal involvement is common and typically presents with erosions, ulcerations, and scarring.2 Given the absence of mucosal involvement in our patient and a positive KOH preparation, linear IgA bullous dermatosis was an unlikely diagnosis.
Benign inoculation lymphoreticulosis, more commonly known as cat scratch disease (CSD), is a Bartonella henselae infection that results from a cat scratch or bite. Cat scratch disease can present as localized cutaneous and nodal involvement (lymphadenopathy) near the site of inoculation, or it may present as disseminated disease. Cutaneous lesions generally progress through vesicular, erythematous, and papular phases. Regional lymphadenopathy proximal to the inoculation site is the hallmark of CSD.3 Given the absence of lymphadenopathy in our patient as well as the sporadic distribution of lesions, CSD was an unlikely diagnosis.
Dermatitis herpetiformis (DH) is an autoimmune disorder with cutaneous manifestations of gluten sensitivity. Dermatitis herpetiformis presents as extremely pruritic papules and vesicles arranged in groups on areas such as the elbows, dorsal aspects of the forearms, knees, scalp, back, and buttocks. Most patients with DH have celiac disease or small bowel disease related to gluten sensitivity.4 Given our patient’s acute presentation in adulthood and lack of gluten sensitivity, DH was an unlikely diagnosis.
Bullous fixed drug reaction is a cutaneous eruption that typically presents in the setting of exposure to an offending drug/agent. Drug reactions can have various cutaneous presentations, with the most common being pigmented macules that progress into plaques.5 Given the isolated nature of our patient’s episode and apparent lack of association with medication, bullous fixed drug reaction was an unlikely diagnosis.
Tinea corporis bullosa is a rare clinical variant of tinea corporis that has only been reported in patients with a history of contact with different animals. There are many causative organisms related to tinea corporis; Trichophyton rubrum is the most common etiology of tinea corporis, while tinea corporis due to close contact with domesticated animals often is caused by M canis.6 The immunoinhibitory properties of the mannans in the fungal cell wall allow the organisms to adhere to the skin prior to invasion. Cutaneous invasion into dead cornified layers of the skin is credited to the proteases, subtilisinlike proteases (subtilases), and keratinases produced by the fungus.1 There are many different clinical presentations of tinea corporis due to the variability of causative organisms. An annular (ring-shaped) lesion with a central plaque and advancing border is the most typical presentation. Tinea corporis bullosa is characterized by the presence of bullae or vesicles in the borders of the scaly plaque. Rupture of the bullae subsequently leads to erosions and crusts over the plaque.
The diagnosis of tinea corporis bullosa often is clinical if the lesion is typical and can be confirmed using KOH preparation and fungal culture. Once the diagnosis is confirmed, topical antifungals are the standard treatment approach for localized superficial tinea corporis. Systemic antifungal treatment can be initiated if the lesion is extensive, recurrent, chronic, or unresponsive to topical treatment.1 Given our patient’s characteristic presentation, she was managed with an over-the-counter topical antifungal (terbinafine). The patient’s lesions dramatically improved, rendering oral therapy unnecessary. At 1-month follow-up, the rash had nearly resolved.
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review [published online July 20, 2020]. Drugs Context. doi:10.7573/dic.2020-5-6
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Lamps LW, Scott MA. Cat-scratch disease: historic, clinical, and pathologic perspectives. Pathology Patterns Reviews. 2004;121(suppl):S71-S80.
- Caproni M, Antiga E, Melani L, et al. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009;23:633-638.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ziemer M, Seyfarth F, Elsner P, et al. Atypical manifestations of tinea corporis. Mycoses. 2007;50:31-35.
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review [published online July 20, 2020]. Drugs Context. doi:10.7573/dic.2020-5-6
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Lamps LW, Scott MA. Cat-scratch disease: historic, clinical, and pathologic perspectives. Pathology Patterns Reviews. 2004;121(suppl):S71-S80.
- Caproni M, Antiga E, Melani L, et al. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009;23:633-638.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ziemer M, Seyfarth F, Elsner P, et al. Atypical manifestations of tinea corporis. Mycoses. 2007;50:31-35.
A 38-year-old woman presented with a rash of 5 days’ duration that initially appeared on the wrists after playing with her kitten, with subsequent involvement of the chest, back, abdomen, and upper and lower extremities. Physical examination revealed multiple annular plaques with raised erythematous borders, rare peripheral vesicles, and superficial central scaling. Extreme pruritus accompanied the plaques, both of which developed after playing with her kitten. The patient noted that all lesions on the upper extremities evolved in areas subject to deep puncture while more superficially excoriated areas were unaffected. She denied any other prior skin conditions and had received a 5-day course of azithromycin without improvement prior to presentation; triamcinolone ointment 0.1% had provided only temporary relief. Primary care providers prescribed a short course of oral prednisone; however, she did not start it prior to presentation.
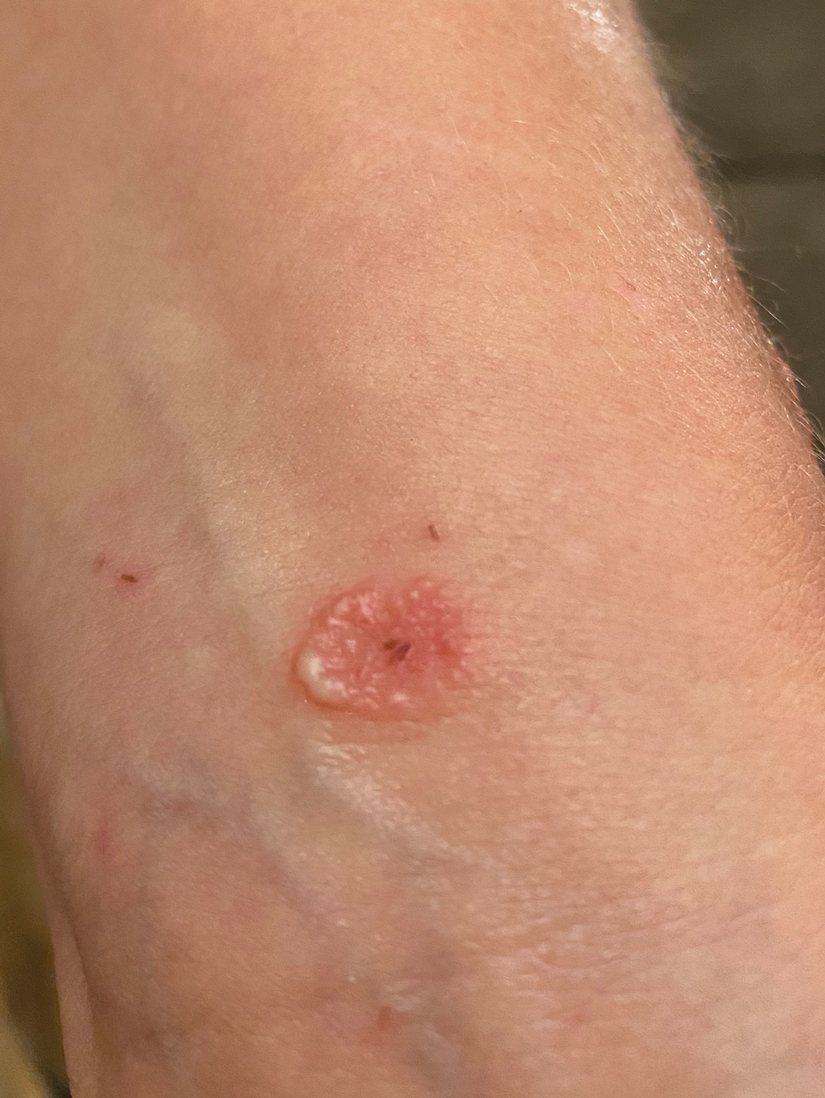
Rapidly Evolving Papulonodular Eruption in the Axilla
The Diagnosis: Lymphomatoid Papulosis
At the time of the initial visit, a punch biopsy was performed on the posterior shoulder girdle. Histopathology revealed mild epidermal spongiosis and acanthosis with associated parakeratosis and a dermal lymphocytic infiltrate with extravasated erythrocytes consistent with pityriasis rosea (Figure 1). Two weeks after the biopsy, the patient returned for suture removal and to discuss the biopsy results. The patient reported more evolving lesions despite completing the prescribed course of dicloxacillin. At this time, physical examination revealed the persistence of several reddishbrown papules along with new nodular lesions on the arms and thighs, some with central ulceration and crusting (Figure 2). A second biopsy of a nodular lesion on the right distal forearm was performed at this visit along with a superficial tissue culture, which was negative for bacterial or fungal elements. The biopsy revealed an atypical CD30+ lymphoid proliferation (Figure 3). These cells were strongly PD-L1 positive and also positive for CD3, CD4, and granzyme-B. Ki67 showed a high proliferation rate, and T-cell gene rearrangement studies were positive. Given these histologic findings and the clinical context of rapidly evolving skin lesions from small papules to nodular skin tumors, a diagnosis of lymphomatoid papulosis (LyP) was established.
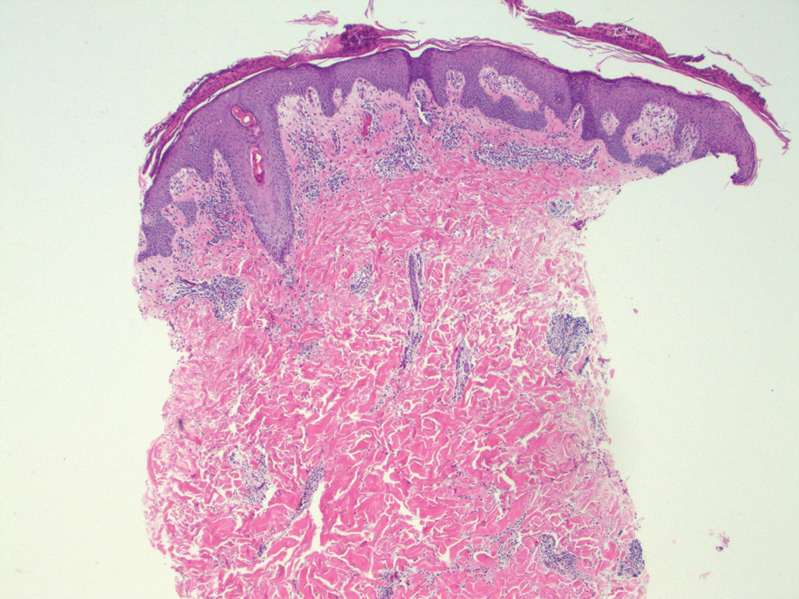
Because of the notable pathologic discordance between the 2 biopsy specimens, re-evaluation of the initial specimen was requested. The initial biopsy was subsequently found to be CD30+ with an identical peak on gene rearrangement studies as the second biopsy, further validating the diagnosis of LyP (Figure 4). Our patient was offered low-dose methotrexate therapy but declined the treatment plan, as the skin lesions had begun to resolve.
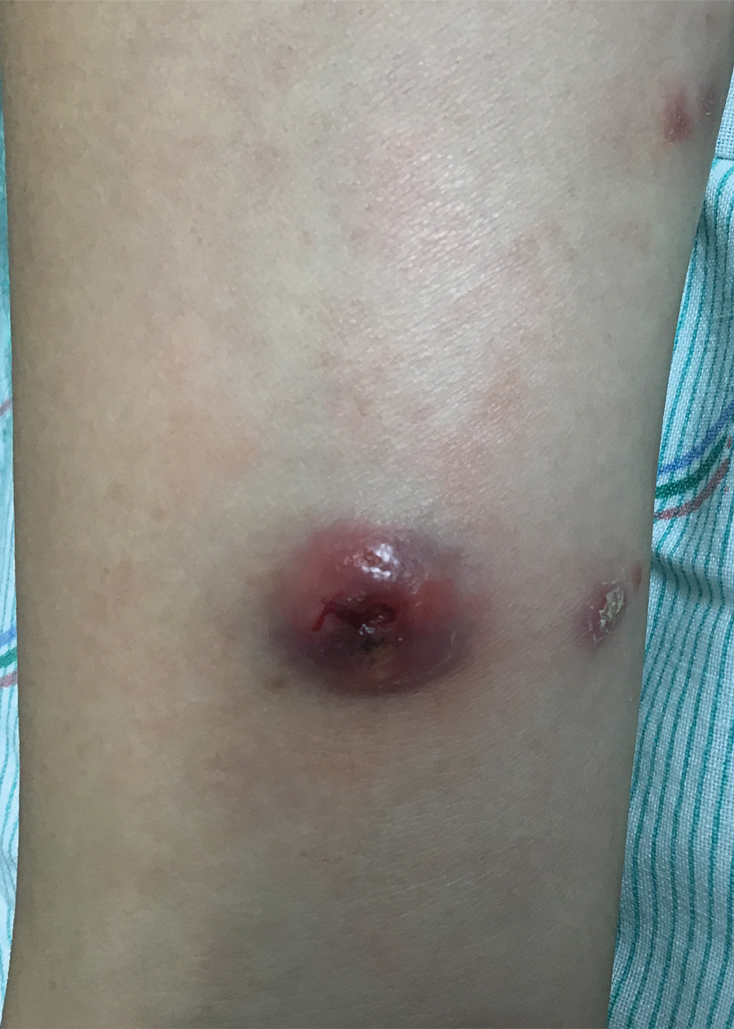
Lymphomatoid papulosis is a chronic CD30+ lymphoproliferative disorder with a characteristic recurrent and self-remitting disease course.1,2 Although it typically has a benign clinical course, it is histologically malignant and considered a low-grade variant of cutaneous T-cell lymphoma. 2,3 The classic clinical presentation of LyP involves the presence of reddish-brown papules and nodules typically measuring less than 2.0 cm, which may show evidence of central ulceration, hemorrhage, necrosis, and/or crust formation.1-5 It is characteristic that a patient may present with these skin lesions in different stages of evolution and that biopsies of these lesions may reflect different histologic features depending on the age of the lesion, making a definitive diagnosis more difficult to obtain if not clinically correlated.1,2 Any part of the body may be involved; however, there appears to be a predilection for the trunk and extremities in most cases.1-3,5 The skin eruptions usually are asymptomatic, but pruritus is a commonly associated concern.1,2,4,5
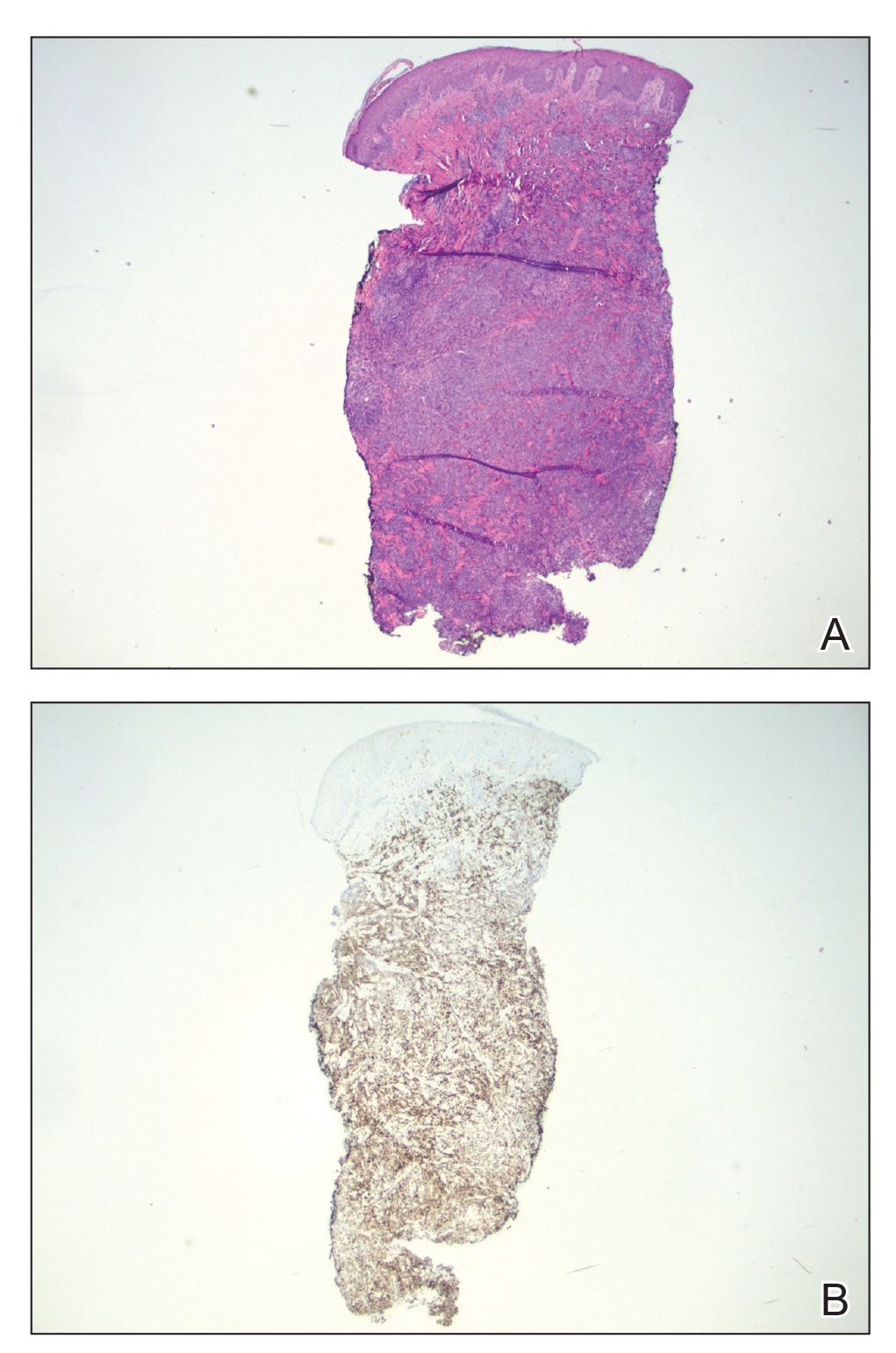
Lymphomatoid papulosis can have a localized, clustered, or generalized distribution pattern and typically will spontaneously regress without treatment within 3 to 12 weeks of symptom onset.2,3 Lymphomatoid papulosis has a slight male predominance with a male to female ratio of 1.5:1. It occurs most commonly between 35 and 45 years of age, though it can present at any age. The overall duration of the disease can range from months to decades.2,3 Lymphomatoid papulosis makes up approximately 15% of all cutaneous T-cell lymphomas.2,3 Although the overall prognosis is excellent, patients with LyP are at an increased risk of developing cutaneous or systemic lymphoma, most commonly mycosis fungoides, anaplastic large cell lymphoma, or Hodgkin lymphoma.1-3 This increased lifelong risk is the reason that patients with LyP must be followed long-term every 6 to 12 months for surveillance of emerging malignancy.1,2,6
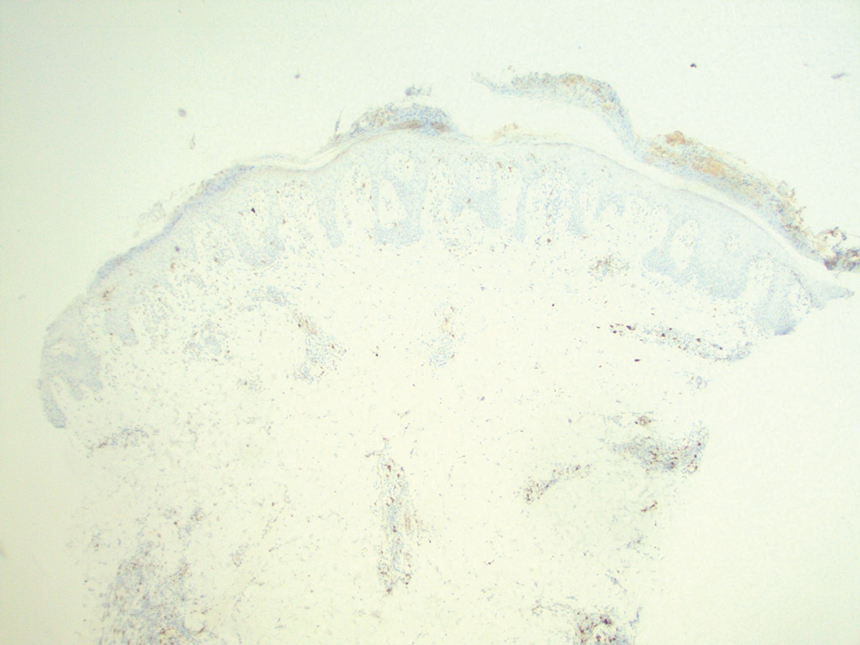
The pathogenesis of LyP remains unknown. Some have hypothesized a possible viral trigger; however, there is insufficient data to support this theory.2,6 A diagnostic hallmark of LyP is its CD30 positivity, which is a known marker for T-cell activation.6 The spontaneous regression of skin lesions that is characteristic of LyP is believed to involve the interactions between CD30 and its ligand (CD30L), which may contribute to apoptosis of neoplastic T cells.2,3,6 With regards to the possible mechanisms contributing to tumor progression in LyP, a mutation in the transforming growth factor β receptor gene on CD30+ tumor cells within LyP lesions may allow for these cells to evade growth regulation and progress to lymphoma.2,6 A large percentage of LyP biopsy specimens show evidence of T-cell receptor gene monoclonal rearrangement, which can aid in establishing a diagnosis.1,2
The histologic features of LyP can vary greatly depending on the age of the lesion sampled.1,2 Histologic subtypes of LyP have been established, with type A being the most common (approximately 75% of cases), displaying a wedge-shaped infiltrate of scattered or clustered, large, atypical CD30+ T cells.1,2 Types B through E vary in histologic features, with the exception that all subtypes contain a CD30+ lymphocytic infiltrate.2,3
Treatment of LyP depends on the symptom/disease burden that the patient is experiencing. For patients with a limited number of nonscarring skin lesions in areas that are not cosmetically sensitive, observation is recommended. 1-3 For symptomatic patients with an extensive number of lesions, particularly those that may be scarring and/or in cosmetically sensitive areas, low-dose oral methotrexate therapy is considered first-line treatment.1-4 A methotrexate dose of 5 to 20 mg weekly can be effective in reducing the number and severity of lesions, with duration of treatment depending on clinical response.1,2 For patients who have contraindications to or who cannot tolerate oral methotrexate, phototherapy using psoralen plus UVA twice weekly for 6 to 8 weeks is another treatment option.1,2 Topical corticosteroids also can be used in children or for patients experiencing substantial pruritus.1,2,4 Oral or topical retinoids, topical carmustine or mechlorethamine, and brentuximab (an anti-CD30 monoclonal antibody) are all alternative therapies that have shown some beneficial effects.1,2 In the event that any of the skin lesions do not spontaneously regress within a 3- to 12-week time frame, surgical excision or radiotherapy can be performed on those lesions.2
Primary cutaneous anaplastic large cell lymphoma (C-ALCL) is another CD30+ lymphoproliferative disorder with overlapping clinical and histopathological features of LyP. Recurrent crops of multiple lesions favor a diagnosis of LyP, whereas solitary lesions favor C-ALCL; however, multifocal C-ALCL cases may occur.2 Mycosis fungoides is the most common type of cutaneous T-cell lymphoma that characteristically presents in a patch, plaque, tumor progression. Although mycosis fungoides eventually may transform into a CD30+ lymphoma, our patient did not display the characteristic clinical progression to suggest this diagnosis. Pityriasis lichenoides et varioliformis acuta and pityriasis lichenoides chronica also fall into the spectrum of clonal T-cell cutaneous disorders that more commonly affect the pediatric population. Pityriasis lichenoides et varioliformis acuta has a marked CD8+ lymphocyte infiltrate, whereas pityriasis lichenoides chronica has more CD4+ lymphocytes. These disorders typically do not stain positive for CD30.2
All patients with a diagnosis of LyP should maintain lifelong, regular, 6- to 12-month follow-up visits to monitor disease status and screen for any evidence of developing malignancy.1,2,6 A thorough review of clinical history, complete skin examination, and physical examination with a particular focus on detection of lymphadenopathy and hepatosplenomegaly should be included at every followup visit.1 Systemic symptoms such as fever, night sweats, or weight loss are not typical features of LyP; therefore, patients who begin to develop these symptoms should be promptly evaluated for systemic lymphoma.1
- Kadin ME. Lymphomatoid papulosis. UpToDate website. Accessed June 4, 2022. https://www.uptodate.com/contents/lymphomatoid-papulosis
- Willemze R. Cutaneous T-cell lymphoma. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 2. 4th ed. Elsevier Saunders; 2017:2141-2143.
- Wiznia LE, Cohen JM, Beasley JM, et al. Lymphomatoid papulosis. Dermatol Online J. 2018;24:13030/qt4xt046c9.
- Wieser I, Oh CW, Talpur R, et al. Lymphomatoid papulosis: treatment response and associated lymphomas in a study of 180 patients. J Am Acad Dermatol. 2016;74:59-67. doi:10.1016/j.jaad.2015.09.013
- Wolff K, Johnson RA, Saavedra AP, et al. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017.
- Kunishige JH, McDonald H, Alvarez G, et al. Lymphomatoid papulosis and associated lymphomas: a retrospective case series of 84 patients. Clin Exp Dermatol. 2009;34:576-581. doi:10.1111 /j.1365-2230.2008.03024.x
The Diagnosis: Lymphomatoid Papulosis
At the time of the initial visit, a punch biopsy was performed on the posterior shoulder girdle. Histopathology revealed mild epidermal spongiosis and acanthosis with associated parakeratosis and a dermal lymphocytic infiltrate with extravasated erythrocytes consistent with pityriasis rosea (Figure 1). Two weeks after the biopsy, the patient returned for suture removal and to discuss the biopsy results. The patient reported more evolving lesions despite completing the prescribed course of dicloxacillin. At this time, physical examination revealed the persistence of several reddishbrown papules along with new nodular lesions on the arms and thighs, some with central ulceration and crusting (Figure 2). A second biopsy of a nodular lesion on the right distal forearm was performed at this visit along with a superficial tissue culture, which was negative for bacterial or fungal elements. The biopsy revealed an atypical CD30+ lymphoid proliferation (Figure 3). These cells were strongly PD-L1 positive and also positive for CD3, CD4, and granzyme-B. Ki67 showed a high proliferation rate, and T-cell gene rearrangement studies were positive. Given these histologic findings and the clinical context of rapidly evolving skin lesions from small papules to nodular skin tumors, a diagnosis of lymphomatoid papulosis (LyP) was established.

Because of the notable pathologic discordance between the 2 biopsy specimens, re-evaluation of the initial specimen was requested. The initial biopsy was subsequently found to be CD30+ with an identical peak on gene rearrangement studies as the second biopsy, further validating the diagnosis of LyP (Figure 4). Our patient was offered low-dose methotrexate therapy but declined the treatment plan, as the skin lesions had begun to resolve.

Lymphomatoid papulosis is a chronic CD30+ lymphoproliferative disorder with a characteristic recurrent and self-remitting disease course.1,2 Although it typically has a benign clinical course, it is histologically malignant and considered a low-grade variant of cutaneous T-cell lymphoma. 2,3 The classic clinical presentation of LyP involves the presence of reddish-brown papules and nodules typically measuring less than 2.0 cm, which may show evidence of central ulceration, hemorrhage, necrosis, and/or crust formation.1-5 It is characteristic that a patient may present with these skin lesions in different stages of evolution and that biopsies of these lesions may reflect different histologic features depending on the age of the lesion, making a definitive diagnosis more difficult to obtain if not clinically correlated.1,2 Any part of the body may be involved; however, there appears to be a predilection for the trunk and extremities in most cases.1-3,5 The skin eruptions usually are asymptomatic, but pruritus is a commonly associated concern.1,2,4,5

Lymphomatoid papulosis can have a localized, clustered, or generalized distribution pattern and typically will spontaneously regress without treatment within 3 to 12 weeks of symptom onset.2,3 Lymphomatoid papulosis has a slight male predominance with a male to female ratio of 1.5:1. It occurs most commonly between 35 and 45 years of age, though it can present at any age. The overall duration of the disease can range from months to decades.2,3 Lymphomatoid papulosis makes up approximately 15% of all cutaneous T-cell lymphomas.2,3 Although the overall prognosis is excellent, patients with LyP are at an increased risk of developing cutaneous or systemic lymphoma, most commonly mycosis fungoides, anaplastic large cell lymphoma, or Hodgkin lymphoma.1-3 This increased lifelong risk is the reason that patients with LyP must be followed long-term every 6 to 12 months for surveillance of emerging malignancy.1,2,6

The pathogenesis of LyP remains unknown. Some have hypothesized a possible viral trigger; however, there is insufficient data to support this theory.2,6 A diagnostic hallmark of LyP is its CD30 positivity, which is a known marker for T-cell activation.6 The spontaneous regression of skin lesions that is characteristic of LyP is believed to involve the interactions between CD30 and its ligand (CD30L), which may contribute to apoptosis of neoplastic T cells.2,3,6 With regards to the possible mechanisms contributing to tumor progression in LyP, a mutation in the transforming growth factor β receptor gene on CD30+ tumor cells within LyP lesions may allow for these cells to evade growth regulation and progress to lymphoma.2,6 A large percentage of LyP biopsy specimens show evidence of T-cell receptor gene monoclonal rearrangement, which can aid in establishing a diagnosis.1,2
The histologic features of LyP can vary greatly depending on the age of the lesion sampled.1,2 Histologic subtypes of LyP have been established, with type A being the most common (approximately 75% of cases), displaying a wedge-shaped infiltrate of scattered or clustered, large, atypical CD30+ T cells.1,2 Types B through E vary in histologic features, with the exception that all subtypes contain a CD30+ lymphocytic infiltrate.2,3
Treatment of LyP depends on the symptom/disease burden that the patient is experiencing. For patients with a limited number of nonscarring skin lesions in areas that are not cosmetically sensitive, observation is recommended. 1-3 For symptomatic patients with an extensive number of lesions, particularly those that may be scarring and/or in cosmetically sensitive areas, low-dose oral methotrexate therapy is considered first-line treatment.1-4 A methotrexate dose of 5 to 20 mg weekly can be effective in reducing the number and severity of lesions, with duration of treatment depending on clinical response.1,2 For patients who have contraindications to or who cannot tolerate oral methotrexate, phototherapy using psoralen plus UVA twice weekly for 6 to 8 weeks is another treatment option.1,2 Topical corticosteroids also can be used in children or for patients experiencing substantial pruritus.1,2,4 Oral or topical retinoids, topical carmustine or mechlorethamine, and brentuximab (an anti-CD30 monoclonal antibody) are all alternative therapies that have shown some beneficial effects.1,2 In the event that any of the skin lesions do not spontaneously regress within a 3- to 12-week time frame, surgical excision or radiotherapy can be performed on those lesions.2
Primary cutaneous anaplastic large cell lymphoma (C-ALCL) is another CD30+ lymphoproliferative disorder with overlapping clinical and histopathological features of LyP. Recurrent crops of multiple lesions favor a diagnosis of LyP, whereas solitary lesions favor C-ALCL; however, multifocal C-ALCL cases may occur.2 Mycosis fungoides is the most common type of cutaneous T-cell lymphoma that characteristically presents in a patch, plaque, tumor progression. Although mycosis fungoides eventually may transform into a CD30+ lymphoma, our patient did not display the characteristic clinical progression to suggest this diagnosis. Pityriasis lichenoides et varioliformis acuta and pityriasis lichenoides chronica also fall into the spectrum of clonal T-cell cutaneous disorders that more commonly affect the pediatric population. Pityriasis lichenoides et varioliformis acuta has a marked CD8+ lymphocyte infiltrate, whereas pityriasis lichenoides chronica has more CD4+ lymphocytes. These disorders typically do not stain positive for CD30.2
All patients with a diagnosis of LyP should maintain lifelong, regular, 6- to 12-month follow-up visits to monitor disease status and screen for any evidence of developing malignancy.1,2,6 A thorough review of clinical history, complete skin examination, and physical examination with a particular focus on detection of lymphadenopathy and hepatosplenomegaly should be included at every followup visit.1 Systemic symptoms such as fever, night sweats, or weight loss are not typical features of LyP; therefore, patients who begin to develop these symptoms should be promptly evaluated for systemic lymphoma.1
The Diagnosis: Lymphomatoid Papulosis
At the time of the initial visit, a punch biopsy was performed on the posterior shoulder girdle. Histopathology revealed mild epidermal spongiosis and acanthosis with associated parakeratosis and a dermal lymphocytic infiltrate with extravasated erythrocytes consistent with pityriasis rosea (Figure 1). Two weeks after the biopsy, the patient returned for suture removal and to discuss the biopsy results. The patient reported more evolving lesions despite completing the prescribed course of dicloxacillin. At this time, physical examination revealed the persistence of several reddishbrown papules along with new nodular lesions on the arms and thighs, some with central ulceration and crusting (Figure 2). A second biopsy of a nodular lesion on the right distal forearm was performed at this visit along with a superficial tissue culture, which was negative for bacterial or fungal elements. The biopsy revealed an atypical CD30+ lymphoid proliferation (Figure 3). These cells were strongly PD-L1 positive and also positive for CD3, CD4, and granzyme-B. Ki67 showed a high proliferation rate, and T-cell gene rearrangement studies were positive. Given these histologic findings and the clinical context of rapidly evolving skin lesions from small papules to nodular skin tumors, a diagnosis of lymphomatoid papulosis (LyP) was established.

Because of the notable pathologic discordance between the 2 biopsy specimens, re-evaluation of the initial specimen was requested. The initial biopsy was subsequently found to be CD30+ with an identical peak on gene rearrangement studies as the second biopsy, further validating the diagnosis of LyP (Figure 4). Our patient was offered low-dose methotrexate therapy but declined the treatment plan, as the skin lesions had begun to resolve.

Lymphomatoid papulosis is a chronic CD30+ lymphoproliferative disorder with a characteristic recurrent and self-remitting disease course.1,2 Although it typically has a benign clinical course, it is histologically malignant and considered a low-grade variant of cutaneous T-cell lymphoma. 2,3 The classic clinical presentation of LyP involves the presence of reddish-brown papules and nodules typically measuring less than 2.0 cm, which may show evidence of central ulceration, hemorrhage, necrosis, and/or crust formation.1-5 It is characteristic that a patient may present with these skin lesions in different stages of evolution and that biopsies of these lesions may reflect different histologic features depending on the age of the lesion, making a definitive diagnosis more difficult to obtain if not clinically correlated.1,2 Any part of the body may be involved; however, there appears to be a predilection for the trunk and extremities in most cases.1-3,5 The skin eruptions usually are asymptomatic, but pruritus is a commonly associated concern.1,2,4,5

Lymphomatoid papulosis can have a localized, clustered, or generalized distribution pattern and typically will spontaneously regress without treatment within 3 to 12 weeks of symptom onset.2,3 Lymphomatoid papulosis has a slight male predominance with a male to female ratio of 1.5:1. It occurs most commonly between 35 and 45 years of age, though it can present at any age. The overall duration of the disease can range from months to decades.2,3 Lymphomatoid papulosis makes up approximately 15% of all cutaneous T-cell lymphomas.2,3 Although the overall prognosis is excellent, patients with LyP are at an increased risk of developing cutaneous or systemic lymphoma, most commonly mycosis fungoides, anaplastic large cell lymphoma, or Hodgkin lymphoma.1-3 This increased lifelong risk is the reason that patients with LyP must be followed long-term every 6 to 12 months for surveillance of emerging malignancy.1,2,6

The pathogenesis of LyP remains unknown. Some have hypothesized a possible viral trigger; however, there is insufficient data to support this theory.2,6 A diagnostic hallmark of LyP is its CD30 positivity, which is a known marker for T-cell activation.6 The spontaneous regression of skin lesions that is characteristic of LyP is believed to involve the interactions between CD30 and its ligand (CD30L), which may contribute to apoptosis of neoplastic T cells.2,3,6 With regards to the possible mechanisms contributing to tumor progression in LyP, a mutation in the transforming growth factor β receptor gene on CD30+ tumor cells within LyP lesions may allow for these cells to evade growth regulation and progress to lymphoma.2,6 A large percentage of LyP biopsy specimens show evidence of T-cell receptor gene monoclonal rearrangement, which can aid in establishing a diagnosis.1,2
The histologic features of LyP can vary greatly depending on the age of the lesion sampled.1,2 Histologic subtypes of LyP have been established, with type A being the most common (approximately 75% of cases), displaying a wedge-shaped infiltrate of scattered or clustered, large, atypical CD30+ T cells.1,2 Types B through E vary in histologic features, with the exception that all subtypes contain a CD30+ lymphocytic infiltrate.2,3
Treatment of LyP depends on the symptom/disease burden that the patient is experiencing. For patients with a limited number of nonscarring skin lesions in areas that are not cosmetically sensitive, observation is recommended. 1-3 For symptomatic patients with an extensive number of lesions, particularly those that may be scarring and/or in cosmetically sensitive areas, low-dose oral methotrexate therapy is considered first-line treatment.1-4 A methotrexate dose of 5 to 20 mg weekly can be effective in reducing the number and severity of lesions, with duration of treatment depending on clinical response.1,2 For patients who have contraindications to or who cannot tolerate oral methotrexate, phototherapy using psoralen plus UVA twice weekly for 6 to 8 weeks is another treatment option.1,2 Topical corticosteroids also can be used in children or for patients experiencing substantial pruritus.1,2,4 Oral or topical retinoids, topical carmustine or mechlorethamine, and brentuximab (an anti-CD30 monoclonal antibody) are all alternative therapies that have shown some beneficial effects.1,2 In the event that any of the skin lesions do not spontaneously regress within a 3- to 12-week time frame, surgical excision or radiotherapy can be performed on those lesions.2
Primary cutaneous anaplastic large cell lymphoma (C-ALCL) is another CD30+ lymphoproliferative disorder with overlapping clinical and histopathological features of LyP. Recurrent crops of multiple lesions favor a diagnosis of LyP, whereas solitary lesions favor C-ALCL; however, multifocal C-ALCL cases may occur.2 Mycosis fungoides is the most common type of cutaneous T-cell lymphoma that characteristically presents in a patch, plaque, tumor progression. Although mycosis fungoides eventually may transform into a CD30+ lymphoma, our patient did not display the characteristic clinical progression to suggest this diagnosis. Pityriasis lichenoides et varioliformis acuta and pityriasis lichenoides chronica also fall into the spectrum of clonal T-cell cutaneous disorders that more commonly affect the pediatric population. Pityriasis lichenoides et varioliformis acuta has a marked CD8+ lymphocyte infiltrate, whereas pityriasis lichenoides chronica has more CD4+ lymphocytes. These disorders typically do not stain positive for CD30.2
All patients with a diagnosis of LyP should maintain lifelong, regular, 6- to 12-month follow-up visits to monitor disease status and screen for any evidence of developing malignancy.1,2,6 A thorough review of clinical history, complete skin examination, and physical examination with a particular focus on detection of lymphadenopathy and hepatosplenomegaly should be included at every followup visit.1 Systemic symptoms such as fever, night sweats, or weight loss are not typical features of LyP; therefore, patients who begin to develop these symptoms should be promptly evaluated for systemic lymphoma.1
- Kadin ME. Lymphomatoid papulosis. UpToDate website. Accessed June 4, 2022. https://www.uptodate.com/contents/lymphomatoid-papulosis
- Willemze R. Cutaneous T-cell lymphoma. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 2. 4th ed. Elsevier Saunders; 2017:2141-2143.
- Wiznia LE, Cohen JM, Beasley JM, et al. Lymphomatoid papulosis. Dermatol Online J. 2018;24:13030/qt4xt046c9.
- Wieser I, Oh CW, Talpur R, et al. Lymphomatoid papulosis: treatment response and associated lymphomas in a study of 180 patients. J Am Acad Dermatol. 2016;74:59-67. doi:10.1016/j.jaad.2015.09.013
- Wolff K, Johnson RA, Saavedra AP, et al. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017.
- Kunishige JH, McDonald H, Alvarez G, et al. Lymphomatoid papulosis and associated lymphomas: a retrospective case series of 84 patients. Clin Exp Dermatol. 2009;34:576-581. doi:10.1111 /j.1365-2230.2008.03024.x
- Kadin ME. Lymphomatoid papulosis. UpToDate website. Accessed June 4, 2022. https://www.uptodate.com/contents/lymphomatoid-papulosis
- Willemze R. Cutaneous T-cell lymphoma. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 2. 4th ed. Elsevier Saunders; 2017:2141-2143.
- Wiznia LE, Cohen JM, Beasley JM, et al. Lymphomatoid papulosis. Dermatol Online J. 2018;24:13030/qt4xt046c9.
- Wieser I, Oh CW, Talpur R, et al. Lymphomatoid papulosis: treatment response and associated lymphomas in a study of 180 patients. J Am Acad Dermatol. 2016;74:59-67. doi:10.1016/j.jaad.2015.09.013
- Wolff K, Johnson RA, Saavedra AP, et al. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017.
- Kunishige JH, McDonald H, Alvarez G, et al. Lymphomatoid papulosis and associated lymphomas: a retrospective case series of 84 patients. Clin Exp Dermatol. 2009;34:576-581. doi:10.1111 /j.1365-2230.2008.03024.x
A 37-year-old woman presented to our dermatology clinic with a pruritic erythematous eruption involving the trunk, axillae, and proximal extremities of 10 days’ duration. Her medical history was notable only for eczema, and she denied taking any medications. Physical examination revealed scattered erythematous papules and crusts involving the trunk bilaterally and the extremities. We initially made a clinical diagnosis of bullous impetigo, and the patient was prescribed mupirocin ointment and dicloxacillin. At 1-week follow-up, the patient reported persistent skin lesions that were evolving despite therapy. Physical examination at this visit revealed an evolving eruption of multiple reddish-brown scaly papules involving the axillae, arms, forearms, and thighs, as depicted here.
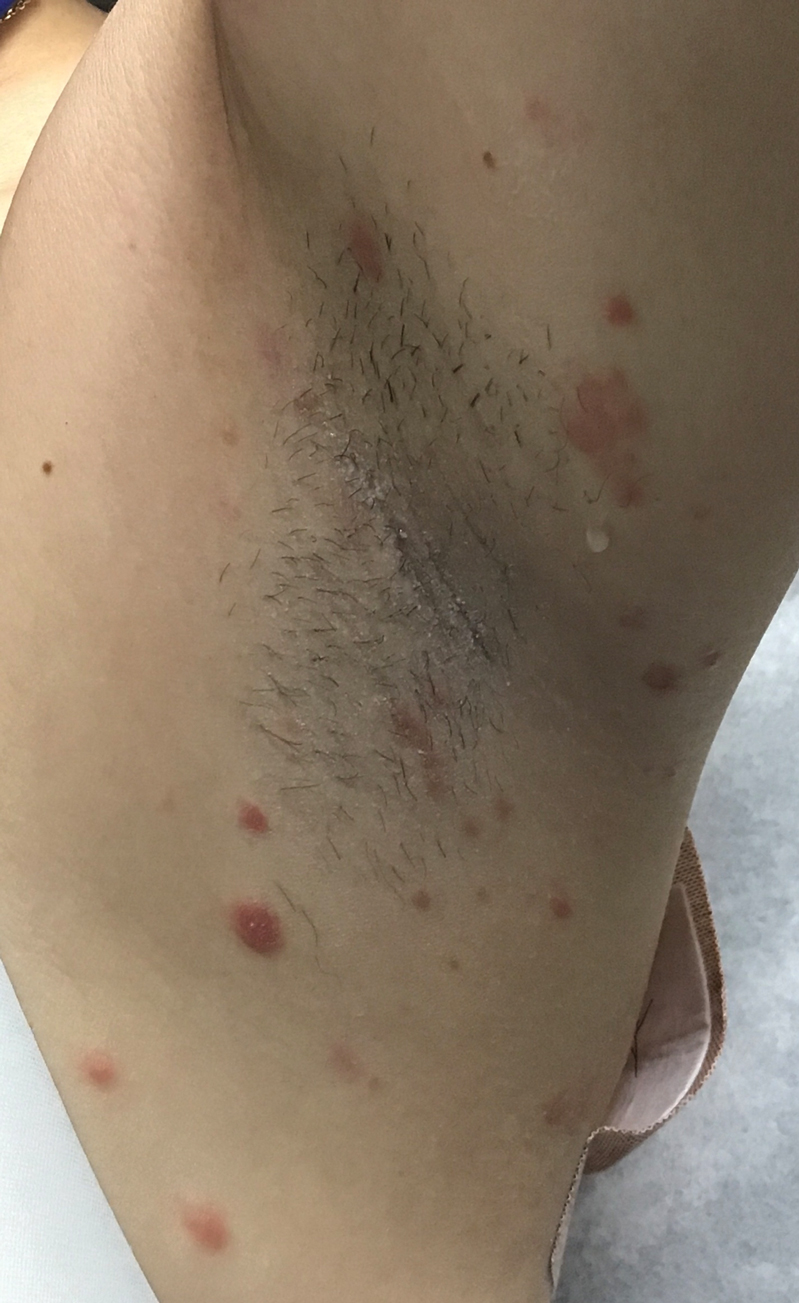
Chronic Retiform Purpura of the Abdomen and Thighs: A Fatal Case of Intravascular Large Cell Lymphoma
To the Editor:
Intravascular large cell lymphoma (ILCL) is a rare B-cell lymphoma that is defined by the presence of large neoplastic B cells in the lumen of blood vessels.1 At least 3 variants of ILCL have been described based on case reports and a small case series: classic, cutaneous, and hemophagocytic. The classic variant presents in elderly patients as nonspecific constitutional symptoms (fever or pain, or less frequently weight loss) or as signs of multiorgan failure (most commonly of the central nervous system). Skin involvement, which is present in nearly half of these patients, can take on multiple morphologies, including retiform purpura, ulcerated nodules, or pseudocellulitis. The cutaneous variant typically presents in middle-aged women with normal hematologic studies. Systemic involvement is less common in this variant of disease than the classic variant, which may partly explain why overall survival is superior in this variant. The hemophagocytic variant manifests as intravascular lymphoma accompanied by hemophagocytic syndrome (fever, hepatosplenomegaly, thrombocytopenia, and bone marrow involvement).
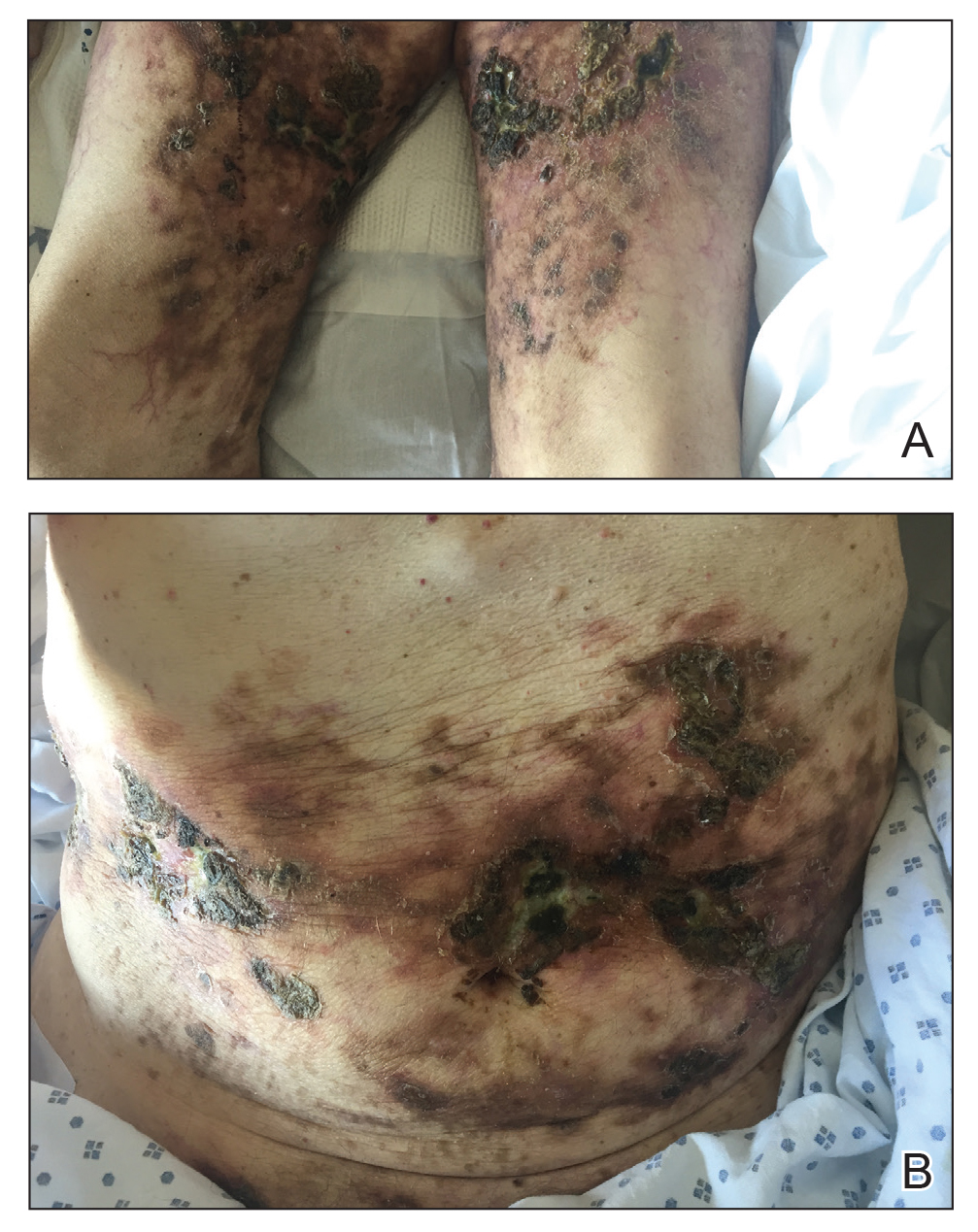
A 69-year-old man presented to the emergency department for failure to thrive and nonhealing wounds of 1 year’s duration. His medical history was notable for poorly controlled diabetes mellitus, progressive multifocal ischemic and hemorrhagic cerebral infarcts, and bilateral deep venous thromboses. Physical examination revealed large purpuric to brown plaques in a retiform configuration with central necrotic eschars on the thighs and abdomen (Figure 1). There was no palpable lymphadenopathy. Laboratory tests revealed normocytic anemia with a hemoglobin level of 10.5 g/dL (reference range, 12–18 g/dL), elevated lactate dehydrogenase level of 525 U/L (reference range, 118–242 U/L), elevated erythrocyte sedimentation rate of 73 mm/h (reference range, <20 mm/h), antinuclear antibody (ANA) titer of 1:2560 (reference range, <1:80), and polyclonal hypergammaglobulinemia. The patient’s white blood cell and platelet counts, creatinine level, and liver function tests were within reference range. Cryoglobulins, coagulation studies, and cardiolipin antibodies were negative. Chest and abdominal imaging also were negative. An incisional skin biopsy and skin punch biopsy showed thrombotic coagulopathy and dilated vessels. A bone marrow biopsy revealed a hypercellular marrow but no plasma cell neoplasm. A repeat incisional skin biopsy demonstrated large CD20+ and CD45+ atypical lymphocytes within the small capillaries of the deep dermis and subcutaneous fat (Figure 2), which confirmed ILCL. Too deconditioned to tolerate chemotherapy, the patient opted for palliative care and died 18 months after initial presentation.
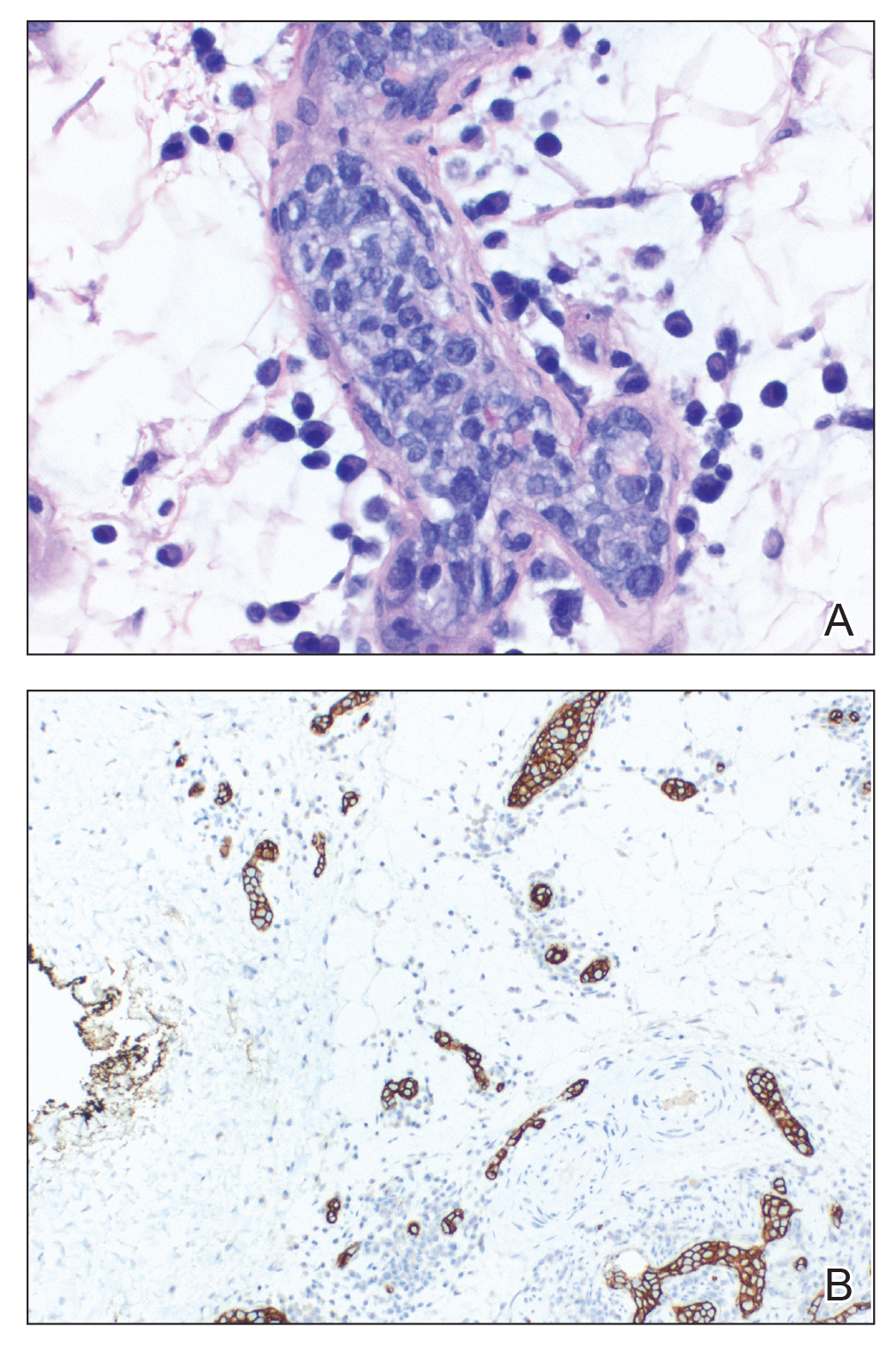
The diagnosis of ILCL often is delayed for several reasons.2 Patients can present with a variety of signs and symptoms related to small vessel occlusion that can be misattributed to other conditions.3,4 In our case, the patient’s recurrent infarcts were thought to be due to his poorly controlled diabetes mellitus, which was diagnosed a few weeks prior, and a positive ANA, even though the workup for antiphospholipid syndrome was negative. Interestingly, a positive ANA (without signs or symptoms of lupus or other autoimmune conditions) has been reported in patients with lymphoma.3 A positive antineutrophil cytoplasmic antibody level (without symptoms or other signs of vasculitis) has been reported in patients with ILCL.4,5 Therefore, distractors are common.
Multiple incisional skin biopsies in the absence of clinical findings (ie, random skin biopsy) are moderately sensitive (77.8%) for the diagnosis of ILCL.2 In a study by Matsue et al,2 111 suspected cases of ILCL underwent 3 incisional biopsies of fat-containing areas of the skin, such as the thigh, abdomen, and upper arm. Intravascular large cell lymphoma was confirmed in 26 cases. Seven additional cases were diagnosed as ILCL, 2 by additional skin biopsies (1 by a second round and 1 by a third round) and 5 by internal organ biopsy (4 bone marrow and 1 adrenal gland). The remaining cases ultimately were found to be a diagnostic mimicker of ILCL, including non-ILCL.2 Although random skin biopsies are reasonably sensitive for ILCL, multiple biopsies are needed, and in some cases, sampling of an internal organ may be required to establish the diagnosis of ILCL.
The prognosis of ILCL is poor; the 3-year overall survival rate for classic and cutaneous variants is 22% and 56%, respectively.6 Anthracycline-based chemotherapy, such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), is considered first-line treatment, and the addition of rituximab to the CHOP regimen may improve remission rates and survival.7
- Ponzoni M, Campo E, Nakamura S. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks [published online August 15, 2018]. Blood. 2018;132:1561-1567. doi:10.1182/blood-2017-04-737445
- Matsue K, Abe Y, Kitadate A, et al. Sensitivity and specificity of incisional random skin biopsy for diagnosis of intravascular large B-cell lymphoma. Blood. 2019;133:1257-1259.
- Altintas A, Cil T, Pasa S, et al. Clinical significance of elevated antinuclear antibody test in patients with Hodgkin’s and non-Hodgkin’s lymphoma. Minerva Med. 2008;99:7-14.
- Shinkawa Y, Hatachi S, Yagita M. Intravascular large B-cell lymphoma with a high titer of proteinase-3-anti-neutrophil cytoplasmic antibody mimicking granulomatosis with polyangiitis. Mod Rheumatol. 2019;29:195-197.
- Sugiyama A, Kobayashi M, Daizo A, et al. Diffuse cerebral vasoconstriction in a intravascular lymphoma patient with a high serum MPO-ANCA level. Intern Med. 2017;56:1715-1718.
- Ferreri AJ, Campo E, Seymour JF, et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant.’ Br J Haematol. 2004;127:173-183.
- Ferreri AJM, Dognini GP, Bairey O, et al; International Extranodal Lyphoma Study Group. The addition of rituximab to anthracycline-based chemotherapy significantly improves outcome in ‘Western’ patients with intravascular large B-cell lymphoma [published online August 10, 2008]. Br J Haematol. 2008;143:253-257. doi:10.1111/j.1365-2141.2008.07338.x
To the Editor:
Intravascular large cell lymphoma (ILCL) is a rare B-cell lymphoma that is defined by the presence of large neoplastic B cells in the lumen of blood vessels.1 At least 3 variants of ILCL have been described based on case reports and a small case series: classic, cutaneous, and hemophagocytic. The classic variant presents in elderly patients as nonspecific constitutional symptoms (fever or pain, or less frequently weight loss) or as signs of multiorgan failure (most commonly of the central nervous system). Skin involvement, which is present in nearly half of these patients, can take on multiple morphologies, including retiform purpura, ulcerated nodules, or pseudocellulitis. The cutaneous variant typically presents in middle-aged women with normal hematologic studies. Systemic involvement is less common in this variant of disease than the classic variant, which may partly explain why overall survival is superior in this variant. The hemophagocytic variant manifests as intravascular lymphoma accompanied by hemophagocytic syndrome (fever, hepatosplenomegaly, thrombocytopenia, and bone marrow involvement).

A 69-year-old man presented to the emergency department for failure to thrive and nonhealing wounds of 1 year’s duration. His medical history was notable for poorly controlled diabetes mellitus, progressive multifocal ischemic and hemorrhagic cerebral infarcts, and bilateral deep venous thromboses. Physical examination revealed large purpuric to brown plaques in a retiform configuration with central necrotic eschars on the thighs and abdomen (Figure 1). There was no palpable lymphadenopathy. Laboratory tests revealed normocytic anemia with a hemoglobin level of 10.5 g/dL (reference range, 12–18 g/dL), elevated lactate dehydrogenase level of 525 U/L (reference range, 118–242 U/L), elevated erythrocyte sedimentation rate of 73 mm/h (reference range, <20 mm/h), antinuclear antibody (ANA) titer of 1:2560 (reference range, <1:80), and polyclonal hypergammaglobulinemia. The patient’s white blood cell and platelet counts, creatinine level, and liver function tests were within reference range. Cryoglobulins, coagulation studies, and cardiolipin antibodies were negative. Chest and abdominal imaging also were negative. An incisional skin biopsy and skin punch biopsy showed thrombotic coagulopathy and dilated vessels. A bone marrow biopsy revealed a hypercellular marrow but no plasma cell neoplasm. A repeat incisional skin biopsy demonstrated large CD20+ and CD45+ atypical lymphocytes within the small capillaries of the deep dermis and subcutaneous fat (Figure 2), which confirmed ILCL. Too deconditioned to tolerate chemotherapy, the patient opted for palliative care and died 18 months after initial presentation.

The diagnosis of ILCL often is delayed for several reasons.2 Patients can present with a variety of signs and symptoms related to small vessel occlusion that can be misattributed to other conditions.3,4 In our case, the patient’s recurrent infarcts were thought to be due to his poorly controlled diabetes mellitus, which was diagnosed a few weeks prior, and a positive ANA, even though the workup for antiphospholipid syndrome was negative. Interestingly, a positive ANA (without signs or symptoms of lupus or other autoimmune conditions) has been reported in patients with lymphoma.3 A positive antineutrophil cytoplasmic antibody level (without symptoms or other signs of vasculitis) has been reported in patients with ILCL.4,5 Therefore, distractors are common.
Multiple incisional skin biopsies in the absence of clinical findings (ie, random skin biopsy) are moderately sensitive (77.8%) for the diagnosis of ILCL.2 In a study by Matsue et al,2 111 suspected cases of ILCL underwent 3 incisional biopsies of fat-containing areas of the skin, such as the thigh, abdomen, and upper arm. Intravascular large cell lymphoma was confirmed in 26 cases. Seven additional cases were diagnosed as ILCL, 2 by additional skin biopsies (1 by a second round and 1 by a third round) and 5 by internal organ biopsy (4 bone marrow and 1 adrenal gland). The remaining cases ultimately were found to be a diagnostic mimicker of ILCL, including non-ILCL.2 Although random skin biopsies are reasonably sensitive for ILCL, multiple biopsies are needed, and in some cases, sampling of an internal organ may be required to establish the diagnosis of ILCL.
The prognosis of ILCL is poor; the 3-year overall survival rate for classic and cutaneous variants is 22% and 56%, respectively.6 Anthracycline-based chemotherapy, such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), is considered first-line treatment, and the addition of rituximab to the CHOP regimen may improve remission rates and survival.7
To the Editor:
Intravascular large cell lymphoma (ILCL) is a rare B-cell lymphoma that is defined by the presence of large neoplastic B cells in the lumen of blood vessels.1 At least 3 variants of ILCL have been described based on case reports and a small case series: classic, cutaneous, and hemophagocytic. The classic variant presents in elderly patients as nonspecific constitutional symptoms (fever or pain, or less frequently weight loss) or as signs of multiorgan failure (most commonly of the central nervous system). Skin involvement, which is present in nearly half of these patients, can take on multiple morphologies, including retiform purpura, ulcerated nodules, or pseudocellulitis. The cutaneous variant typically presents in middle-aged women with normal hematologic studies. Systemic involvement is less common in this variant of disease than the classic variant, which may partly explain why overall survival is superior in this variant. The hemophagocytic variant manifests as intravascular lymphoma accompanied by hemophagocytic syndrome (fever, hepatosplenomegaly, thrombocytopenia, and bone marrow involvement).

A 69-year-old man presented to the emergency department for failure to thrive and nonhealing wounds of 1 year’s duration. His medical history was notable for poorly controlled diabetes mellitus, progressive multifocal ischemic and hemorrhagic cerebral infarcts, and bilateral deep venous thromboses. Physical examination revealed large purpuric to brown plaques in a retiform configuration with central necrotic eschars on the thighs and abdomen (Figure 1). There was no palpable lymphadenopathy. Laboratory tests revealed normocytic anemia with a hemoglobin level of 10.5 g/dL (reference range, 12–18 g/dL), elevated lactate dehydrogenase level of 525 U/L (reference range, 118–242 U/L), elevated erythrocyte sedimentation rate of 73 mm/h (reference range, <20 mm/h), antinuclear antibody (ANA) titer of 1:2560 (reference range, <1:80), and polyclonal hypergammaglobulinemia. The patient’s white blood cell and platelet counts, creatinine level, and liver function tests were within reference range. Cryoglobulins, coagulation studies, and cardiolipin antibodies were negative. Chest and abdominal imaging also were negative. An incisional skin biopsy and skin punch biopsy showed thrombotic coagulopathy and dilated vessels. A bone marrow biopsy revealed a hypercellular marrow but no plasma cell neoplasm. A repeat incisional skin biopsy demonstrated large CD20+ and CD45+ atypical lymphocytes within the small capillaries of the deep dermis and subcutaneous fat (Figure 2), which confirmed ILCL. Too deconditioned to tolerate chemotherapy, the patient opted for palliative care and died 18 months after initial presentation.

The diagnosis of ILCL often is delayed for several reasons.2 Patients can present with a variety of signs and symptoms related to small vessel occlusion that can be misattributed to other conditions.3,4 In our case, the patient’s recurrent infarcts were thought to be due to his poorly controlled diabetes mellitus, which was diagnosed a few weeks prior, and a positive ANA, even though the workup for antiphospholipid syndrome was negative. Interestingly, a positive ANA (without signs or symptoms of lupus or other autoimmune conditions) has been reported in patients with lymphoma.3 A positive antineutrophil cytoplasmic antibody level (without symptoms or other signs of vasculitis) has been reported in patients with ILCL.4,5 Therefore, distractors are common.
Multiple incisional skin biopsies in the absence of clinical findings (ie, random skin biopsy) are moderately sensitive (77.8%) for the diagnosis of ILCL.2 In a study by Matsue et al,2 111 suspected cases of ILCL underwent 3 incisional biopsies of fat-containing areas of the skin, such as the thigh, abdomen, and upper arm. Intravascular large cell lymphoma was confirmed in 26 cases. Seven additional cases were diagnosed as ILCL, 2 by additional skin biopsies (1 by a second round and 1 by a third round) and 5 by internal organ biopsy (4 bone marrow and 1 adrenal gland). The remaining cases ultimately were found to be a diagnostic mimicker of ILCL, including non-ILCL.2 Although random skin biopsies are reasonably sensitive for ILCL, multiple biopsies are needed, and in some cases, sampling of an internal organ may be required to establish the diagnosis of ILCL.
The prognosis of ILCL is poor; the 3-year overall survival rate for classic and cutaneous variants is 22% and 56%, respectively.6 Anthracycline-based chemotherapy, such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), is considered first-line treatment, and the addition of rituximab to the CHOP regimen may improve remission rates and survival.7
- Ponzoni M, Campo E, Nakamura S. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks [published online August 15, 2018]. Blood. 2018;132:1561-1567. doi:10.1182/blood-2017-04-737445
- Matsue K, Abe Y, Kitadate A, et al. Sensitivity and specificity of incisional random skin biopsy for diagnosis of intravascular large B-cell lymphoma. Blood. 2019;133:1257-1259.
- Altintas A, Cil T, Pasa S, et al. Clinical significance of elevated antinuclear antibody test in patients with Hodgkin’s and non-Hodgkin’s lymphoma. Minerva Med. 2008;99:7-14.
- Shinkawa Y, Hatachi S, Yagita M. Intravascular large B-cell lymphoma with a high titer of proteinase-3-anti-neutrophil cytoplasmic antibody mimicking granulomatosis with polyangiitis. Mod Rheumatol. 2019;29:195-197.
- Sugiyama A, Kobayashi M, Daizo A, et al. Diffuse cerebral vasoconstriction in a intravascular lymphoma patient with a high serum MPO-ANCA level. Intern Med. 2017;56:1715-1718.
- Ferreri AJ, Campo E, Seymour JF, et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant.’ Br J Haematol. 2004;127:173-183.
- Ferreri AJM, Dognini GP, Bairey O, et al; International Extranodal Lyphoma Study Group. The addition of rituximab to anthracycline-based chemotherapy significantly improves outcome in ‘Western’ patients with intravascular large B-cell lymphoma [published online August 10, 2008]. Br J Haematol. 2008;143:253-257. doi:10.1111/j.1365-2141.2008.07338.x
- Ponzoni M, Campo E, Nakamura S. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks [published online August 15, 2018]. Blood. 2018;132:1561-1567. doi:10.1182/blood-2017-04-737445
- Matsue K, Abe Y, Kitadate A, et al. Sensitivity and specificity of incisional random skin biopsy for diagnosis of intravascular large B-cell lymphoma. Blood. 2019;133:1257-1259.
- Altintas A, Cil T, Pasa S, et al. Clinical significance of elevated antinuclear antibody test in patients with Hodgkin’s and non-Hodgkin’s lymphoma. Minerva Med. 2008;99:7-14.
- Shinkawa Y, Hatachi S, Yagita M. Intravascular large B-cell lymphoma with a high titer of proteinase-3-anti-neutrophil cytoplasmic antibody mimicking granulomatosis with polyangiitis. Mod Rheumatol. 2019;29:195-197.
- Sugiyama A, Kobayashi M, Daizo A, et al. Diffuse cerebral vasoconstriction in a intravascular lymphoma patient with a high serum MPO-ANCA level. Intern Med. 2017;56:1715-1718.
- Ferreri AJ, Campo E, Seymour JF, et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant.’ Br J Haematol. 2004;127:173-183.
- Ferreri AJM, Dognini GP, Bairey O, et al; International Extranodal Lyphoma Study Group. The addition of rituximab to anthracycline-based chemotherapy significantly improves outcome in ‘Western’ patients with intravascular large B-cell lymphoma [published online August 10, 2008]. Br J Haematol. 2008;143:253-257. doi:10.1111/j.1365-2141.2008.07338.x
Practice Points
- Intravascular large cell lymphoma (ILCL) is a life-threatening malignancy that can present with retiform purpura and other symptoms of vascular occlusion.
- The diagnosis of ILCL can be challenging because of the presence of distractors, and multiple biopsies may be required to establish pathology.
Tumor Necrosis Factor α Inhibitor–Induced Lupuslike Syndrome in a Patient Prescribed Certolizumab Pegol
To the Editor:
Tumor necrosis factor α (TNF-α) inhibitor–induced lupuslike syndrome (TAILS) is a newly described entity that refers to the onset of subacute cutaneous lupus erythematosus (SCLE) during drug therapy with TNF-α antagonists. The condition is unique because it is thought to occur via a separate pathophysiologic mechanism than all other agents implicated in the development of drug-induced lupus erythematosus (DILE). Infliximab and etanercept are the 2 most common TNF-α antagonists associated with TAILS. Although rare, adalimumab, golimumab, and certolizumab pegol have been reported to induce this state of autoimmunity. We report an uncommon presentation of TAILS in a patient taking certolizumab pegol with a brief discussion of the pathogenesis underlying TAILS.
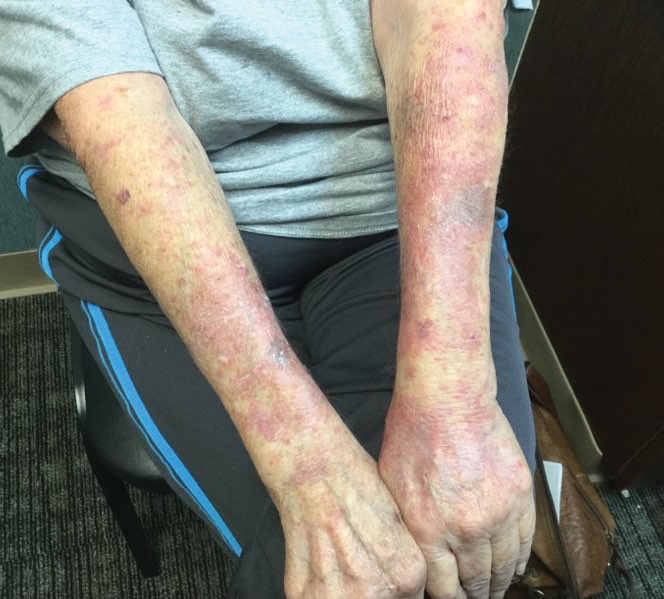
A 71-year-old woman presented to the dermatology clinic with a rash located on the arms, face, and trunk that she reported as having been present for months. She had a medical history of rheumatoid arthritis and currently was receiving certolizumab pegol injections. Physical examination revealed erythematous patches and plaques with overlying scaling and evidence of atrophic scarring on sun-exposed areas of the body. The lesions predominantly were in a symmetrical distribution across the extensor surfaces of both outer arms as well as the posterior superior thoracic region extending anteriorly along the bilateral supraclavicular area (Figures 1 and 2). A 4-mm punch biopsy was obtained and sent for histologic analysis, along with a sample of the patient’s serum for antinuclear antibody (ANA) testing.
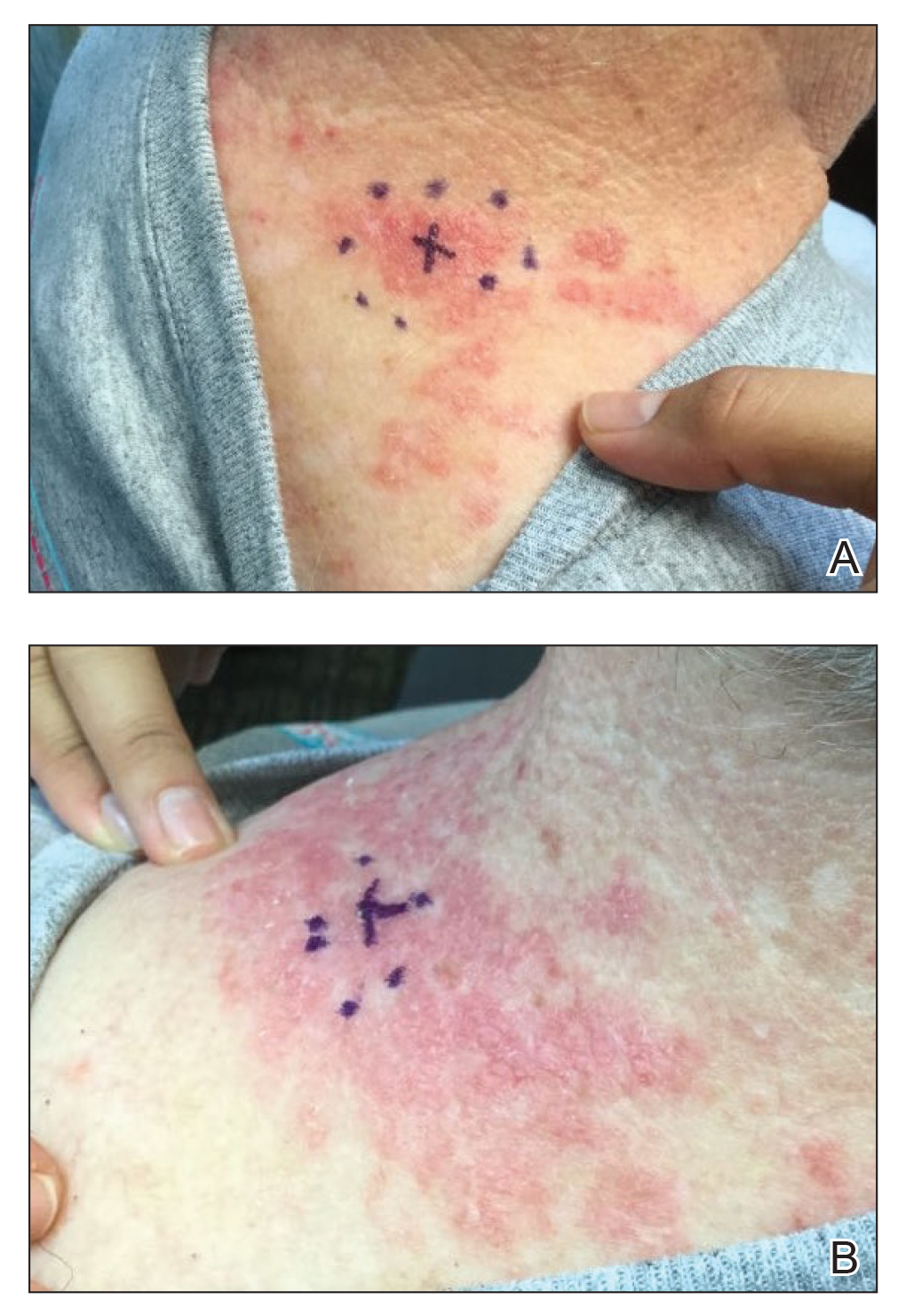
Hematoxylin and eosin–stained tissue sections of the right superior thoracic lesions revealed epidermal atrophy, hyperkeratosis, and vacuolar alteration of the basal layer with apoptosis, consistent with a lichenoid tissue reaction. In addition, both superficial and deep perivascular and periadnexal lymphocytic infiltrates were observed as well as increased dermal mucin. Serologic testing was performed with a comprehensive ANA panel of the patient’s serum (Table). Of note, there was a speckled ANA pattern (1:1280), with elevated anti–double-stranded DNA (anti-dsDNA) and anti–Sjögren syndrome–related antigen A (anti-SSA)(also called anti-Ro antibodies) levels. The patient’s rheumatologist was consulted; certolizumab pegol was removed from the current drug regimen and switched to a daily regimen of hydroxychloroquine and prednisone. Seven weeks after discontinuation of certolizumab pegol, the patient was symptom free and without any cutaneous involvement. Based on the histologic analysis, presence of anti-SSA (Ro) autoantibodies, and the resolution of symptoms following withdrawal of anti–TNF-α therapy, a diagnosis of TAILS was made.

Subacute cutaneous lupus erythematosus, the most common subset of DILE, typically presents with annular polycyclic or papulosquamous skin eruptions on the legs; patients often test positive for anti-SSA/Ro and/or anti–Sjögren syndrome–related antigen B (also called anti-La) antibodies. Pharmaceutical agents linked to the development of SCLE are calcium channel blockers, angiotensin-converting enzyme inhibitors, thiazide diuretics, terbinafine, the chemotherapeutic agent gemcitabine, and TNF-α antagonists.1,2 Tumor necrosis factor α antagonists are biologic agents that commonly are used in the management of systemic inflammatory diseases such as ulcerative colitis, Crohn disease, seronegative spondyloarthropathies, and rheumatoid arthritis. Among this family of therapeutics includes adalimumab (humanized monoclonal antibody), infliximab (chimeric monoclonal TNF-α antagonist), etanercept (soluble receptor fusion protein), certolizumab pegol (Fab fraction of a human IgG monoclonal antibody), and golimumab (humanized monoclonal antibody).
Tumor necrosis factor α inhibitor–induced lupuslike syndrome most commonly occurs in women in the fifth decade of life, and it is seen more often in those using infliximab or entanercept.3 Although reports do exist, TAILS rarely complicates treatment with adalimumab, golimumab, or certolizumab.4,5 Due to the lack of reports, there are no diagnostic criteria nor an acceptable theory regarding the pathogenesis. In one study in France, the estimated incidence was thought to be 0.19% for infliximab and 0.18% for etanercept.6 Tumor necrosis factor α inhibitor–induced lupuslike syndrome is unique in that it is thought to occur by a different mechanism than that of other known offending agents in the development of DILE. Molecular mimicry, direct cytotoxicity, altered T-cell gene expression, and disruption of central immune tolerance have all been hypothesized to cause drug-induced systemic lupus erythematosus, SCLE, and chronic cutaneous lupus erythematosus. Tumor necrosis factor α inhibitors, are postulated to cause the induction of SCLE via an independent route separate from not only other drugs that cause SCLE but also all forms of DILE as a whole, making it a distinctive player within the realm of agents known to cause a lupuslike syndrome. The following hypotheses may explain this occurrence:
1. Increased humoral autoimmunity: Under normal circumstances, TNF-α activation leads to upregulation in the production of cytotoxic CD8+ T lymphocytes. The upregulation of CD8+ T lymphocytes concurrently leads to a simultaneous suppression of B lymphocytes. Inhibiting the effects of TNF-α on the other hand promotes cytotoxic T-lymphocyte suppression, leading to an increased synthesis of B cells and subsequently a state of increased humoral autoimmunity.7
2. Infection: The immunosuppressive effects of TNF-α inhibitors are well known, and the propensity to develop microbial infections, such as tuberculosis, is markedly increased on the use of these agents. Infections brought on by TNF-α inhibitor usage are hypothesized to induce a widespread activation of polyclonal B lymphocytes, eventually leading to the formation of antibodies against these polyclonal B lymphocytes and subsequently SCLE.8
3. Helper T cell (TH2) response: The inhibition of TH1 CD4+ lymphocytes by TNF-α inversely leads to an increased production of TH2 CD4+ lymphocytes. This increase in the levels of circulating TH2 CD4+ lymphocytes brought on by the action of anti–TNF-α agents is thought to promote the development of SCLE.9,10
4. Apoptosis theory: Molecules of TNF-α inhibitors are capable of binding to TNF-α receptors on the cell surface. In doing so, cellular apoptosis is triggered, resulting in the release of nucleosomal autoantigens from the apoptotic cells. In susceptible individuals, autoantibodies then begin to form against the nucleosomal autoantigens, leading to an autoimmune reaction that is characterized by SCLE.11,12
Major histone compatibility (MHC) antigen testing performed by Sontheimer et al12 established the presence of the HLA class I, HLA-B8, and/or HLA-DR3 haplotypes in patients with SCLE.13,14 Furthermore, there is a well-known association between the antinuclear profile of known SCLE patients and the presence of anti-SSA (Ro) antibodies.13 Therefore, we propose that in susceptible individuals, such as those with the HLA class I, HLA-B8, or HLA-DR3 haplotypes, the initiation of a TNF-α inhibitor causes cellular apoptosis with the subsequent release of nucleosomal and cytoplasmic components (namely that of the Ro autoantigens), inducing a state of autoimmunity. An ensuing immunogenic response is then initiated in predisposed individuals for which anti-SSA (Ro) autoantibodies are produced against these previously mentioned autoantigens.
Drug-induced SCLE is most common in females (71%), with a median age of 58 years. The most common site of cutaneous manifestations is the legs.15 Although our patient was in the eighth decade of life with predominant cutaneous involvement of the upper extremity, the erythematous plaques with a symmetric, annular, polycyclic appearance in photosensitive regions raised a heightened suspicion for lupus erythematosus. Histology classically involves an interface dermatitis with vacuolar or hydropic change and lymphocytic infiltrates,16 consistent with the analysis of tissue sections from our patient. Moreover, the speckled ANA profile with positive anti-dsDNA and anti-SSA (Ro) antibodies in the absence of a negative rheumatoid factor and anticyclic citrullinated peptide antibodies strongly favored the diagnosis of SCLE over alternative diagnoses.2
The supraclavicular rash in our patient raises clinical suspicion for the shawl sign of dermatomyositis, which also is associated with musculoskeletal pain and photosensitivity. In addition, skin biopsy revealed vacuolar alteration of the basement membrane zoneand dermal mucin in both lupus erythematosus and dermatomyositis; therefore, skin biopsy is of little use in distinguishing the 2 conditions, and antibody testing must be performed. Although anti-SSA (Ro) antibodies commonly are associated with SCLE, there are reports involving positivity for the extractable nuclear antigen in cases of dermatomyositis.17 Based on our patient’s current drug regimen, including that of a known offending agent for SCLE, a presumptive diagnosis of TAILS was made. Following withdrawal of certolizumab pegol injections and subsequent resolution of the skin lesions, our patient was given a definitive diagnosis of TAILS based on clinical and pathological assessments.
The clinical diagnosis of TAILS should be made according to the triad of at least 1 serologic and 1 nonserologic American College of Rheumatology criteria, such as anti-SSA (Ro) antibodies and a photosensitive rash, respectively, as well as a relationship between the onset of symptoms and TNF-α inhibitor therapy.18 Both the definitive diagnosis and the treatment of TAILS can be made via withdrawal of the TNF-α inhibitor, which was true in our case whereby chronologically the onset of use with a TNF-α inhibitor was associated with disease onset. Furthermore, withdrawal led to complete improvement of all signs and symptoms, collectively supporting a diagnosis of TAILS. Notably, switching to a different TNF-α inhibitor has been shown to be safe and effective.19
- Marzano AV, Vezzoli P, Crosti C. Drug-induced lupus: an update on its dermatological aspects. Lupus. 2009;18:935-940.
- Wiznia LE, Subtil A, Choi JN. Subacute cutaneous lupus erythematosus induced by chemotherapy: gemcitabine as a causative agent. JAMA Dermatol. 2013;149:1071-1075.
- Williams VL, Cohen PR. TNF alpha antagonist-induced lupus-like syndrome: report and review of the literature with implications for treatment with alternative TNF alpha antagonists. Int J Dermatol. 2011;50:619-625.
- Pasut G. Pegylation of biological molecules and potential benefits: pharmacological properties of certolizumab pegol. Bio Drugs. 2014;28(suppl 1):15-23.
- Mudduluru BM, Shah S, Shamah S. et al. TNF-alpha antagonist induced lupus on three different agents. Postgrad Med. 2017;129:304-306.
- De Bandt M. Anti-TNF-alpha-induced lupus. Arthritis Res Ther. 2019;21:235.
- Costa MF, Said NR, Zimmermann B. Drug-induced lupus due to anti-tumor necrosis factor alfa agents. Semin Arthritis Rheum. 2008;37:381-387.
- Caramaschi P, Biasi D, Colombatti M. Anti-TNF alpha therapy in rheumatoid arthritis and autoimmunity. Rheumatol Int. 2006;26:209-214.
- Yung RL, Quddus J, Chrisp CE, et al. Mechanism of drug-induced lupus. I. cloned Th2 cells modified with DNA methylation inhibitors in vitro cause autoimmunity in vivo. J Immunol. 1995;154:3025-3035.
- Yung R, Powers D, Johnson K, et al. Mechanisms of drug-induced lupus. II. T cells overexpressing lymphocyte function-associated antigen 1 become autoreactive and cause a lupuslike disease in syngeneic mice. J Clin Invest. 1996;97:2866-2871.
- Sontheimer RD, Stastny P, Gilliam JN. Human histocompatibility antigen associations in subacute cutaneous lupus erythematosus. J Clin Invest. 1981;67:312-316.
- Sontheimer RD, Maddison PJ, Reichlin M, et al. Serologic and HLA associations in subacute cutaneous lupus erythematosus, a clinical subset of lupus erythematosus. Ann Intern Med. 1982;97:664-671.
- Lee LA, Roberts CM, Frank MB, et al. The autoantibody response to Ro/SSA in cutaneous lupus erythematosus. Arch Dermatol. 1994;130:1262-1268.
- Deutscher SL, Harley JB, Keene JD. Molecular analysis of the 60-kDa human Ro ribonucleoprotein. Proc Natl Acad Sci. 1988;85:9479-9483.
- DalleVedove C, Simon JC, Girolomoni G. Drug-induced lupus erythematosus with emphasis on skin manifestations and the role of anti-TNFα agents [article in German]. J Dtsch Dermatol Ges. 2012;10:889-897.
- Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27:391-404.
- Schulte-Pelkum J, Fritzler M, Mahler M. Latest update on the Ro/SS-A autoantibody system. Autoimmun Rev. 2009;8:632-637.
- De Bandt M, Sibilia J, Le Loët X, et al. Systemic lupus erythematosus induced by anti-tumour necrosis factor alpha therapy: a French national survey. Arthritis Res Ther. 2005;7:R545-R551.
- Lupu A, Tieranu C, Constantinescu CL, et al. TNFα inhibitor induced lupus-like syndrome (TAILS) in a patient with IBD. Current Health Sci J. 2014;40:285-288.
To the Editor:
Tumor necrosis factor α (TNF-α) inhibitor–induced lupuslike syndrome (TAILS) is a newly described entity that refers to the onset of subacute cutaneous lupus erythematosus (SCLE) during drug therapy with TNF-α antagonists. The condition is unique because it is thought to occur via a separate pathophysiologic mechanism than all other agents implicated in the development of drug-induced lupus erythematosus (DILE). Infliximab and etanercept are the 2 most common TNF-α antagonists associated with TAILS. Although rare, adalimumab, golimumab, and certolizumab pegol have been reported to induce this state of autoimmunity. We report an uncommon presentation of TAILS in a patient taking certolizumab pegol with a brief discussion of the pathogenesis underlying TAILS.

A 71-year-old woman presented to the dermatology clinic with a rash located on the arms, face, and trunk that she reported as having been present for months. She had a medical history of rheumatoid arthritis and currently was receiving certolizumab pegol injections. Physical examination revealed erythematous patches and plaques with overlying scaling and evidence of atrophic scarring on sun-exposed areas of the body. The lesions predominantly were in a symmetrical distribution across the extensor surfaces of both outer arms as well as the posterior superior thoracic region extending anteriorly along the bilateral supraclavicular area (Figures 1 and 2). A 4-mm punch biopsy was obtained and sent for histologic analysis, along with a sample of the patient’s serum for antinuclear antibody (ANA) testing.

Hematoxylin and eosin–stained tissue sections of the right superior thoracic lesions revealed epidermal atrophy, hyperkeratosis, and vacuolar alteration of the basal layer with apoptosis, consistent with a lichenoid tissue reaction. In addition, both superficial and deep perivascular and periadnexal lymphocytic infiltrates were observed as well as increased dermal mucin. Serologic testing was performed with a comprehensive ANA panel of the patient’s serum (Table). Of note, there was a speckled ANA pattern (1:1280), with elevated anti–double-stranded DNA (anti-dsDNA) and anti–Sjögren syndrome–related antigen A (anti-SSA)(also called anti-Ro antibodies) levels. The patient’s rheumatologist was consulted; certolizumab pegol was removed from the current drug regimen and switched to a daily regimen of hydroxychloroquine and prednisone. Seven weeks after discontinuation of certolizumab pegol, the patient was symptom free and without any cutaneous involvement. Based on the histologic analysis, presence of anti-SSA (Ro) autoantibodies, and the resolution of symptoms following withdrawal of anti–TNF-α therapy, a diagnosis of TAILS was made.

Subacute cutaneous lupus erythematosus, the most common subset of DILE, typically presents with annular polycyclic or papulosquamous skin eruptions on the legs; patients often test positive for anti-SSA/Ro and/or anti–Sjögren syndrome–related antigen B (also called anti-La) antibodies. Pharmaceutical agents linked to the development of SCLE are calcium channel blockers, angiotensin-converting enzyme inhibitors, thiazide diuretics, terbinafine, the chemotherapeutic agent gemcitabine, and TNF-α antagonists.1,2 Tumor necrosis factor α antagonists are biologic agents that commonly are used in the management of systemic inflammatory diseases such as ulcerative colitis, Crohn disease, seronegative spondyloarthropathies, and rheumatoid arthritis. Among this family of therapeutics includes adalimumab (humanized monoclonal antibody), infliximab (chimeric monoclonal TNF-α antagonist), etanercept (soluble receptor fusion protein), certolizumab pegol (Fab fraction of a human IgG monoclonal antibody), and golimumab (humanized monoclonal antibody).
Tumor necrosis factor α inhibitor–induced lupuslike syndrome most commonly occurs in women in the fifth decade of life, and it is seen more often in those using infliximab or entanercept.3 Although reports do exist, TAILS rarely complicates treatment with adalimumab, golimumab, or certolizumab.4,5 Due to the lack of reports, there are no diagnostic criteria nor an acceptable theory regarding the pathogenesis. In one study in France, the estimated incidence was thought to be 0.19% for infliximab and 0.18% for etanercept.6 Tumor necrosis factor α inhibitor–induced lupuslike syndrome is unique in that it is thought to occur by a different mechanism than that of other known offending agents in the development of DILE. Molecular mimicry, direct cytotoxicity, altered T-cell gene expression, and disruption of central immune tolerance have all been hypothesized to cause drug-induced systemic lupus erythematosus, SCLE, and chronic cutaneous lupus erythematosus. Tumor necrosis factor α inhibitors, are postulated to cause the induction of SCLE via an independent route separate from not only other drugs that cause SCLE but also all forms of DILE as a whole, making it a distinctive player within the realm of agents known to cause a lupuslike syndrome. The following hypotheses may explain this occurrence:
1. Increased humoral autoimmunity: Under normal circumstances, TNF-α activation leads to upregulation in the production of cytotoxic CD8+ T lymphocytes. The upregulation of CD8+ T lymphocytes concurrently leads to a simultaneous suppression of B lymphocytes. Inhibiting the effects of TNF-α on the other hand promotes cytotoxic T-lymphocyte suppression, leading to an increased synthesis of B cells and subsequently a state of increased humoral autoimmunity.7
2. Infection: The immunosuppressive effects of TNF-α inhibitors are well known, and the propensity to develop microbial infections, such as tuberculosis, is markedly increased on the use of these agents. Infections brought on by TNF-α inhibitor usage are hypothesized to induce a widespread activation of polyclonal B lymphocytes, eventually leading to the formation of antibodies against these polyclonal B lymphocytes and subsequently SCLE.8
3. Helper T cell (TH2) response: The inhibition of TH1 CD4+ lymphocytes by TNF-α inversely leads to an increased production of TH2 CD4+ lymphocytes. This increase in the levels of circulating TH2 CD4+ lymphocytes brought on by the action of anti–TNF-α agents is thought to promote the development of SCLE.9,10
4. Apoptosis theory: Molecules of TNF-α inhibitors are capable of binding to TNF-α receptors on the cell surface. In doing so, cellular apoptosis is triggered, resulting in the release of nucleosomal autoantigens from the apoptotic cells. In susceptible individuals, autoantibodies then begin to form against the nucleosomal autoantigens, leading to an autoimmune reaction that is characterized by SCLE.11,12
Major histone compatibility (MHC) antigen testing performed by Sontheimer et al12 established the presence of the HLA class I, HLA-B8, and/or HLA-DR3 haplotypes in patients with SCLE.13,14 Furthermore, there is a well-known association between the antinuclear profile of known SCLE patients and the presence of anti-SSA (Ro) antibodies.13 Therefore, we propose that in susceptible individuals, such as those with the HLA class I, HLA-B8, or HLA-DR3 haplotypes, the initiation of a TNF-α inhibitor causes cellular apoptosis with the subsequent release of nucleosomal and cytoplasmic components (namely that of the Ro autoantigens), inducing a state of autoimmunity. An ensuing immunogenic response is then initiated in predisposed individuals for which anti-SSA (Ro) autoantibodies are produced against these previously mentioned autoantigens.
Drug-induced SCLE is most common in females (71%), with a median age of 58 years. The most common site of cutaneous manifestations is the legs.15 Although our patient was in the eighth decade of life with predominant cutaneous involvement of the upper extremity, the erythematous plaques with a symmetric, annular, polycyclic appearance in photosensitive regions raised a heightened suspicion for lupus erythematosus. Histology classically involves an interface dermatitis with vacuolar or hydropic change and lymphocytic infiltrates,16 consistent with the analysis of tissue sections from our patient. Moreover, the speckled ANA profile with positive anti-dsDNA and anti-SSA (Ro) antibodies in the absence of a negative rheumatoid factor and anticyclic citrullinated peptide antibodies strongly favored the diagnosis of SCLE over alternative diagnoses.2
The supraclavicular rash in our patient raises clinical suspicion for the shawl sign of dermatomyositis, which also is associated with musculoskeletal pain and photosensitivity. In addition, skin biopsy revealed vacuolar alteration of the basement membrane zoneand dermal mucin in both lupus erythematosus and dermatomyositis; therefore, skin biopsy is of little use in distinguishing the 2 conditions, and antibody testing must be performed. Although anti-SSA (Ro) antibodies commonly are associated with SCLE, there are reports involving positivity for the extractable nuclear antigen in cases of dermatomyositis.17 Based on our patient’s current drug regimen, including that of a known offending agent for SCLE, a presumptive diagnosis of TAILS was made. Following withdrawal of certolizumab pegol injections and subsequent resolution of the skin lesions, our patient was given a definitive diagnosis of TAILS based on clinical and pathological assessments.
The clinical diagnosis of TAILS should be made according to the triad of at least 1 serologic and 1 nonserologic American College of Rheumatology criteria, such as anti-SSA (Ro) antibodies and a photosensitive rash, respectively, as well as a relationship between the onset of symptoms and TNF-α inhibitor therapy.18 Both the definitive diagnosis and the treatment of TAILS can be made via withdrawal of the TNF-α inhibitor, which was true in our case whereby chronologically the onset of use with a TNF-α inhibitor was associated with disease onset. Furthermore, withdrawal led to complete improvement of all signs and symptoms, collectively supporting a diagnosis of TAILS. Notably, switching to a different TNF-α inhibitor has been shown to be safe and effective.19
To the Editor:
Tumor necrosis factor α (TNF-α) inhibitor–induced lupuslike syndrome (TAILS) is a newly described entity that refers to the onset of subacute cutaneous lupus erythematosus (SCLE) during drug therapy with TNF-α antagonists. The condition is unique because it is thought to occur via a separate pathophysiologic mechanism than all other agents implicated in the development of drug-induced lupus erythematosus (DILE). Infliximab and etanercept are the 2 most common TNF-α antagonists associated with TAILS. Although rare, adalimumab, golimumab, and certolizumab pegol have been reported to induce this state of autoimmunity. We report an uncommon presentation of TAILS in a patient taking certolizumab pegol with a brief discussion of the pathogenesis underlying TAILS.

A 71-year-old woman presented to the dermatology clinic with a rash located on the arms, face, and trunk that she reported as having been present for months. She had a medical history of rheumatoid arthritis and currently was receiving certolizumab pegol injections. Physical examination revealed erythematous patches and plaques with overlying scaling and evidence of atrophic scarring on sun-exposed areas of the body. The lesions predominantly were in a symmetrical distribution across the extensor surfaces of both outer arms as well as the posterior superior thoracic region extending anteriorly along the bilateral supraclavicular area (Figures 1 and 2). A 4-mm punch biopsy was obtained and sent for histologic analysis, along with a sample of the patient’s serum for antinuclear antibody (ANA) testing.

Hematoxylin and eosin–stained tissue sections of the right superior thoracic lesions revealed epidermal atrophy, hyperkeratosis, and vacuolar alteration of the basal layer with apoptosis, consistent with a lichenoid tissue reaction. In addition, both superficial and deep perivascular and periadnexal lymphocytic infiltrates were observed as well as increased dermal mucin. Serologic testing was performed with a comprehensive ANA panel of the patient’s serum (Table). Of note, there was a speckled ANA pattern (1:1280), with elevated anti–double-stranded DNA (anti-dsDNA) and anti–Sjögren syndrome–related antigen A (anti-SSA)(also called anti-Ro antibodies) levels. The patient’s rheumatologist was consulted; certolizumab pegol was removed from the current drug regimen and switched to a daily regimen of hydroxychloroquine and prednisone. Seven weeks after discontinuation of certolizumab pegol, the patient was symptom free and without any cutaneous involvement. Based on the histologic analysis, presence of anti-SSA (Ro) autoantibodies, and the resolution of symptoms following withdrawal of anti–TNF-α therapy, a diagnosis of TAILS was made.

Subacute cutaneous lupus erythematosus, the most common subset of DILE, typically presents with annular polycyclic or papulosquamous skin eruptions on the legs; patients often test positive for anti-SSA/Ro and/or anti–Sjögren syndrome–related antigen B (also called anti-La) antibodies. Pharmaceutical agents linked to the development of SCLE are calcium channel blockers, angiotensin-converting enzyme inhibitors, thiazide diuretics, terbinafine, the chemotherapeutic agent gemcitabine, and TNF-α antagonists.1,2 Tumor necrosis factor α antagonists are biologic agents that commonly are used in the management of systemic inflammatory diseases such as ulcerative colitis, Crohn disease, seronegative spondyloarthropathies, and rheumatoid arthritis. Among this family of therapeutics includes adalimumab (humanized monoclonal antibody), infliximab (chimeric monoclonal TNF-α antagonist), etanercept (soluble receptor fusion protein), certolizumab pegol (Fab fraction of a human IgG monoclonal antibody), and golimumab (humanized monoclonal antibody).
Tumor necrosis factor α inhibitor–induced lupuslike syndrome most commonly occurs in women in the fifth decade of life, and it is seen more often in those using infliximab or entanercept.3 Although reports do exist, TAILS rarely complicates treatment with adalimumab, golimumab, or certolizumab.4,5 Due to the lack of reports, there are no diagnostic criteria nor an acceptable theory regarding the pathogenesis. In one study in France, the estimated incidence was thought to be 0.19% for infliximab and 0.18% for etanercept.6 Tumor necrosis factor α inhibitor–induced lupuslike syndrome is unique in that it is thought to occur by a different mechanism than that of other known offending agents in the development of DILE. Molecular mimicry, direct cytotoxicity, altered T-cell gene expression, and disruption of central immune tolerance have all been hypothesized to cause drug-induced systemic lupus erythematosus, SCLE, and chronic cutaneous lupus erythematosus. Tumor necrosis factor α inhibitors, are postulated to cause the induction of SCLE via an independent route separate from not only other drugs that cause SCLE but also all forms of DILE as a whole, making it a distinctive player within the realm of agents known to cause a lupuslike syndrome. The following hypotheses may explain this occurrence:
1. Increased humoral autoimmunity: Under normal circumstances, TNF-α activation leads to upregulation in the production of cytotoxic CD8+ T lymphocytes. The upregulation of CD8+ T lymphocytes concurrently leads to a simultaneous suppression of B lymphocytes. Inhibiting the effects of TNF-α on the other hand promotes cytotoxic T-lymphocyte suppression, leading to an increased synthesis of B cells and subsequently a state of increased humoral autoimmunity.7
2. Infection: The immunosuppressive effects of TNF-α inhibitors are well known, and the propensity to develop microbial infections, such as tuberculosis, is markedly increased on the use of these agents. Infections brought on by TNF-α inhibitor usage are hypothesized to induce a widespread activation of polyclonal B lymphocytes, eventually leading to the formation of antibodies against these polyclonal B lymphocytes and subsequently SCLE.8
3. Helper T cell (TH2) response: The inhibition of TH1 CD4+ lymphocytes by TNF-α inversely leads to an increased production of TH2 CD4+ lymphocytes. This increase in the levels of circulating TH2 CD4+ lymphocytes brought on by the action of anti–TNF-α agents is thought to promote the development of SCLE.9,10
4. Apoptosis theory: Molecules of TNF-α inhibitors are capable of binding to TNF-α receptors on the cell surface. In doing so, cellular apoptosis is triggered, resulting in the release of nucleosomal autoantigens from the apoptotic cells. In susceptible individuals, autoantibodies then begin to form against the nucleosomal autoantigens, leading to an autoimmune reaction that is characterized by SCLE.11,12
Major histone compatibility (MHC) antigen testing performed by Sontheimer et al12 established the presence of the HLA class I, HLA-B8, and/or HLA-DR3 haplotypes in patients with SCLE.13,14 Furthermore, there is a well-known association between the antinuclear profile of known SCLE patients and the presence of anti-SSA (Ro) antibodies.13 Therefore, we propose that in susceptible individuals, such as those with the HLA class I, HLA-B8, or HLA-DR3 haplotypes, the initiation of a TNF-α inhibitor causes cellular apoptosis with the subsequent release of nucleosomal and cytoplasmic components (namely that of the Ro autoantigens), inducing a state of autoimmunity. An ensuing immunogenic response is then initiated in predisposed individuals for which anti-SSA (Ro) autoantibodies are produced against these previously mentioned autoantigens.
Drug-induced SCLE is most common in females (71%), with a median age of 58 years. The most common site of cutaneous manifestations is the legs.15 Although our patient was in the eighth decade of life with predominant cutaneous involvement of the upper extremity, the erythematous plaques with a symmetric, annular, polycyclic appearance in photosensitive regions raised a heightened suspicion for lupus erythematosus. Histology classically involves an interface dermatitis with vacuolar or hydropic change and lymphocytic infiltrates,16 consistent with the analysis of tissue sections from our patient. Moreover, the speckled ANA profile with positive anti-dsDNA and anti-SSA (Ro) antibodies in the absence of a negative rheumatoid factor and anticyclic citrullinated peptide antibodies strongly favored the diagnosis of SCLE over alternative diagnoses.2
The supraclavicular rash in our patient raises clinical suspicion for the shawl sign of dermatomyositis, which also is associated with musculoskeletal pain and photosensitivity. In addition, skin biopsy revealed vacuolar alteration of the basement membrane zoneand dermal mucin in both lupus erythematosus and dermatomyositis; therefore, skin biopsy is of little use in distinguishing the 2 conditions, and antibody testing must be performed. Although anti-SSA (Ro) antibodies commonly are associated with SCLE, there are reports involving positivity for the extractable nuclear antigen in cases of dermatomyositis.17 Based on our patient’s current drug regimen, including that of a known offending agent for SCLE, a presumptive diagnosis of TAILS was made. Following withdrawal of certolizumab pegol injections and subsequent resolution of the skin lesions, our patient was given a definitive diagnosis of TAILS based on clinical and pathological assessments.
The clinical diagnosis of TAILS should be made according to the triad of at least 1 serologic and 1 nonserologic American College of Rheumatology criteria, such as anti-SSA (Ro) antibodies and a photosensitive rash, respectively, as well as a relationship between the onset of symptoms and TNF-α inhibitor therapy.18 Both the definitive diagnosis and the treatment of TAILS can be made via withdrawal of the TNF-α inhibitor, which was true in our case whereby chronologically the onset of use with a TNF-α inhibitor was associated with disease onset. Furthermore, withdrawal led to complete improvement of all signs and symptoms, collectively supporting a diagnosis of TAILS. Notably, switching to a different TNF-α inhibitor has been shown to be safe and effective.19
- Marzano AV, Vezzoli P, Crosti C. Drug-induced lupus: an update on its dermatological aspects. Lupus. 2009;18:935-940.
- Wiznia LE, Subtil A, Choi JN. Subacute cutaneous lupus erythematosus induced by chemotherapy: gemcitabine as a causative agent. JAMA Dermatol. 2013;149:1071-1075.
- Williams VL, Cohen PR. TNF alpha antagonist-induced lupus-like syndrome: report and review of the literature with implications for treatment with alternative TNF alpha antagonists. Int J Dermatol. 2011;50:619-625.
- Pasut G. Pegylation of biological molecules and potential benefits: pharmacological properties of certolizumab pegol. Bio Drugs. 2014;28(suppl 1):15-23.
- Mudduluru BM, Shah S, Shamah S. et al. TNF-alpha antagonist induced lupus on three different agents. Postgrad Med. 2017;129:304-306.
- De Bandt M. Anti-TNF-alpha-induced lupus. Arthritis Res Ther. 2019;21:235.
- Costa MF, Said NR, Zimmermann B. Drug-induced lupus due to anti-tumor necrosis factor alfa agents. Semin Arthritis Rheum. 2008;37:381-387.
- Caramaschi P, Biasi D, Colombatti M. Anti-TNF alpha therapy in rheumatoid arthritis and autoimmunity. Rheumatol Int. 2006;26:209-214.
- Yung RL, Quddus J, Chrisp CE, et al. Mechanism of drug-induced lupus. I. cloned Th2 cells modified with DNA methylation inhibitors in vitro cause autoimmunity in vivo. J Immunol. 1995;154:3025-3035.
- Yung R, Powers D, Johnson K, et al. Mechanisms of drug-induced lupus. II. T cells overexpressing lymphocyte function-associated antigen 1 become autoreactive and cause a lupuslike disease in syngeneic mice. J Clin Invest. 1996;97:2866-2871.
- Sontheimer RD, Stastny P, Gilliam JN. Human histocompatibility antigen associations in subacute cutaneous lupus erythematosus. J Clin Invest. 1981;67:312-316.
- Sontheimer RD, Maddison PJ, Reichlin M, et al. Serologic and HLA associations in subacute cutaneous lupus erythematosus, a clinical subset of lupus erythematosus. Ann Intern Med. 1982;97:664-671.
- Lee LA, Roberts CM, Frank MB, et al. The autoantibody response to Ro/SSA in cutaneous lupus erythematosus. Arch Dermatol. 1994;130:1262-1268.
- Deutscher SL, Harley JB, Keene JD. Molecular analysis of the 60-kDa human Ro ribonucleoprotein. Proc Natl Acad Sci. 1988;85:9479-9483.
- DalleVedove C, Simon JC, Girolomoni G. Drug-induced lupus erythematosus with emphasis on skin manifestations and the role of anti-TNFα agents [article in German]. J Dtsch Dermatol Ges. 2012;10:889-897.
- Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27:391-404.
- Schulte-Pelkum J, Fritzler M, Mahler M. Latest update on the Ro/SS-A autoantibody system. Autoimmun Rev. 2009;8:632-637.
- De Bandt M, Sibilia J, Le Loët X, et al. Systemic lupus erythematosus induced by anti-tumour necrosis factor alpha therapy: a French national survey. Arthritis Res Ther. 2005;7:R545-R551.
- Lupu A, Tieranu C, Constantinescu CL, et al. TNFα inhibitor induced lupus-like syndrome (TAILS) in a patient with IBD. Current Health Sci J. 2014;40:285-288.
- Marzano AV, Vezzoli P, Crosti C. Drug-induced lupus: an update on its dermatological aspects. Lupus. 2009;18:935-940.
- Wiznia LE, Subtil A, Choi JN. Subacute cutaneous lupus erythematosus induced by chemotherapy: gemcitabine as a causative agent. JAMA Dermatol. 2013;149:1071-1075.
- Williams VL, Cohen PR. TNF alpha antagonist-induced lupus-like syndrome: report and review of the literature with implications for treatment with alternative TNF alpha antagonists. Int J Dermatol. 2011;50:619-625.
- Pasut G. Pegylation of biological molecules and potential benefits: pharmacological properties of certolizumab pegol. Bio Drugs. 2014;28(suppl 1):15-23.
- Mudduluru BM, Shah S, Shamah S. et al. TNF-alpha antagonist induced lupus on three different agents. Postgrad Med. 2017;129:304-306.
- De Bandt M. Anti-TNF-alpha-induced lupus. Arthritis Res Ther. 2019;21:235.
- Costa MF, Said NR, Zimmermann B. Drug-induced lupus due to anti-tumor necrosis factor alfa agents. Semin Arthritis Rheum. 2008;37:381-387.
- Caramaschi P, Biasi D, Colombatti M. Anti-TNF alpha therapy in rheumatoid arthritis and autoimmunity. Rheumatol Int. 2006;26:209-214.
- Yung RL, Quddus J, Chrisp CE, et al. Mechanism of drug-induced lupus. I. cloned Th2 cells modified with DNA methylation inhibitors in vitro cause autoimmunity in vivo. J Immunol. 1995;154:3025-3035.
- Yung R, Powers D, Johnson K, et al. Mechanisms of drug-induced lupus. II. T cells overexpressing lymphocyte function-associated antigen 1 become autoreactive and cause a lupuslike disease in syngeneic mice. J Clin Invest. 1996;97:2866-2871.
- Sontheimer RD, Stastny P, Gilliam JN. Human histocompatibility antigen associations in subacute cutaneous lupus erythematosus. J Clin Invest. 1981;67:312-316.
- Sontheimer RD, Maddison PJ, Reichlin M, et al. Serologic and HLA associations in subacute cutaneous lupus erythematosus, a clinical subset of lupus erythematosus. Ann Intern Med. 1982;97:664-671.
- Lee LA, Roberts CM, Frank MB, et al. The autoantibody response to Ro/SSA in cutaneous lupus erythematosus. Arch Dermatol. 1994;130:1262-1268.
- Deutscher SL, Harley JB, Keene JD. Molecular analysis of the 60-kDa human Ro ribonucleoprotein. Proc Natl Acad Sci. 1988;85:9479-9483.
- DalleVedove C, Simon JC, Girolomoni G. Drug-induced lupus erythematosus with emphasis on skin manifestations and the role of anti-TNFα agents [article in German]. J Dtsch Dermatol Ges. 2012;10:889-897.
- Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27:391-404.
- Schulte-Pelkum J, Fritzler M, Mahler M. Latest update on the Ro/SS-A autoantibody system. Autoimmun Rev. 2009;8:632-637.
- De Bandt M, Sibilia J, Le Loët X, et al. Systemic lupus erythematosus induced by anti-tumour necrosis factor alpha therapy: a French national survey. Arthritis Res Ther. 2005;7:R545-R551.
- Lupu A, Tieranu C, Constantinescu CL, et al. TNFα inhibitor induced lupus-like syndrome (TAILS) in a patient with IBD. Current Health Sci J. 2014;40:285-288.
Practice Points
- Tumor necrosis factor α (TNF-α) inhibitor–induced lupuslike syndrome (TAILS) is a form of drug-induced lupus specific to patients on anti–TNF-α therapy.
- The underlying mechanism of disease development is unique compared to other types of drug-induced lupus.
- TAILS most commonly is associated with the use of infliximab and etanercept but also has been reported with adalimumab, golimumab, and certolizumab pegol.
Postherpetic Pink, Smooth, Annular Convalescing Plaques
The Diagnosis: Granuloma Annulare
A biopsy of a lesion on the right flank demonstrated granulomatous inflammation and interstitial mucin (Figure), characteristic of granuloma annulare (GA).1,2 Granuloma annulare is a relatively common skin disorder with an unknown etiology. It typically presents as smooth, annular, erythematous plaques.1 The most common variants of GA are localized, generalized, and subcutaneous. Our case demonstrated Wolf isotopic response, an unrelated skin disease that forms at the same location as a previously healed skin lesion.2 It is important to be aware of this phenomenon so that it is not confused with a recurrence of herpes zoster virus (HZV).
Although relatively infrequent, GA is the most common isotopic response following HZV infections.3-5 Other postherpetic isotopic eruptions include cutaneous malignancies, lichen planus, sarcoidosis, morphea, reactive perforating collagenosis, psoriasis, and infections, among others.3,5,6 The time between HZV infection and GA can be variable, ranging from a few weeks to many years apart.3
Oftentimes GA will spontaneously resolve within 2 years; however, recurrence is common.7-9 There currently are no standard treatment guidelines. The most promising treatment options include intralesional or topical glucocorticoids for localized GA as well as phototherapy or hydroxychloroquine for widespread disease.8,10

Annular elastolytic giant cell granuloma (also called actinic granuloma) is a rare idiopathic inflammatory skin disease. It is characterized by erythematous annular papules or plaques mainly found on sun-exposed skin, such as the backs of the hands, forearms, or face.11,12 Therefore, based on the distribution of our patient’s lesions, annular elastolytic giant cell granuloma was an unlikely diagnosis. Furthermore, it is not a known postherpetic isotopic reaction. Annular elastolytic giant cell granuloma can appear histologically similar to GA. Differentiating histologic features include a nonpalisading granuloma as well as the absence of mucin and necrobiosis.12
Annular lichen planus is a long-recognized but uncommon clinical variant of lichen planus that typically presents as pruritic, purple, annular plaques on the penis, scrotum, or intertriginous areas.13 The violaceous coloring is more characteristic of lichen planus. Histology is helpful in differentiating from GA.
Nummular eczema presents as scattered, welldefined, pruritic, erythematous, coin-shaped, coin-sized plaques in patients with diffusely dry skin.14 The scaling and serous crusting as well as more prominent pruritus help distinguish it from GA. The appearance of nummular eczema is quite characteristic; therefore, a biopsy typically is unnecessary for diagnosis. However, a potassium hydroxide wet mount examination of a skin scraping should be performed if tinea corporis also is suspected.
Superficial erythema annulare centrifugum classically presents as an annular or arciform pruritic lesion with an advancing outer erythematous edge with an inner rim of scale that most commonly occurs on the lower extremities. 15 The presence of pruritus and trailing scale helps distinguish this lesion from GA. Histologically, there are epidermal changes of hyperplasia, spongiosis, and parakeratosis, as well as lymphohistiocytic infiltrate surrounding the superficial dermal vessels.16
We report this case to highlight GA as the most common postherpetic isotopic response. It should be on the differential diagnosis when a patient presents with erythematous, smooth, annular plaques occurring in the distribution of a resolved case of HZV.
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465.
- . Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Kapoor R, Piris A, Saavedra AP, et al. Wolf isotopic response manifesting as postherpetic granuloma annulare: a case series. Arch Pathol Lab Med. 2013;137:255-258.
- Ezra N, Ahdout J, Haley JC, et al. Granuloma annulare in a zoster scar of a patient with multiple myeloma. Cutis. 2011;87:240-244.
- Noh TW, Park SH, Kang YS, et al. Morphea developing at the site of healed herpes zoster. Ann Dermatol. 2011;23:242-245.
- Ruocco V, Ruocco E, Ghersetich I, et al. Isotopic response after herpesvirus infection: an update. J Am Acad Dermatol. 2002;46:90-94.
- Sparrow G, Abell E. Granuloma annulare and necrobiosis lipoidica treated by jet injector. Br J Dermatol. 1975;93:85-89.
- Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479.
- Thornsberry LA, English JC. Etiology, diagnosis, and therapeutic management of granuloma annulare: an update. Am J Clin Dermatol. 2013;14:279-290.
- Rubin CB, Rosenbach M. Granuloma annulare: a retrospective series of 133 patients. Cutis. 2019;103:102-106.
- Stein JA, Fangman B, Strober B. Actinic granuloma. Dermatol Online J. 2007;13:19.
- Mistry AM, Patel R, Mistry M, et al. Annular elastolytic giant cell granuloma. Cureus. 2020;12:E11456.
- Reich HL, Nguyen JT, James WD. Annular lichen planus: a case series of 20 patients. J Am Acad Dermatol. 2004;50:595-599.
- Leung AKC, Lam JM, Leong KF, et al. Nummular eczema: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:146-155.
- Weyers W, Diaz-Cascajo C, Weyers I. Erythema annulare centrifugum: results of a clinicopathologic study of 73 patients. Am J Dermatopathol. 2003;25:451-462.
- Coronel-Pérez IM, Morillo-Andújar M. Erythema annulare centrifugum responding to natural ultraviolet light [in Spanish]. Actas Dermosifiliogr. 2010;101:177-178.
The Diagnosis: Granuloma Annulare
A biopsy of a lesion on the right flank demonstrated granulomatous inflammation and interstitial mucin (Figure), characteristic of granuloma annulare (GA).1,2 Granuloma annulare is a relatively common skin disorder with an unknown etiology. It typically presents as smooth, annular, erythematous plaques.1 The most common variants of GA are localized, generalized, and subcutaneous. Our case demonstrated Wolf isotopic response, an unrelated skin disease that forms at the same location as a previously healed skin lesion.2 It is important to be aware of this phenomenon so that it is not confused with a recurrence of herpes zoster virus (HZV).
Although relatively infrequent, GA is the most common isotopic response following HZV infections.3-5 Other postherpetic isotopic eruptions include cutaneous malignancies, lichen planus, sarcoidosis, morphea, reactive perforating collagenosis, psoriasis, and infections, among others.3,5,6 The time between HZV infection and GA can be variable, ranging from a few weeks to many years apart.3
Oftentimes GA will spontaneously resolve within 2 years; however, recurrence is common.7-9 There currently are no standard treatment guidelines. The most promising treatment options include intralesional or topical glucocorticoids for localized GA as well as phototherapy or hydroxychloroquine for widespread disease.8,10

Annular elastolytic giant cell granuloma (also called actinic granuloma) is a rare idiopathic inflammatory skin disease. It is characterized by erythematous annular papules or plaques mainly found on sun-exposed skin, such as the backs of the hands, forearms, or face.11,12 Therefore, based on the distribution of our patient’s lesions, annular elastolytic giant cell granuloma was an unlikely diagnosis. Furthermore, it is not a known postherpetic isotopic reaction. Annular elastolytic giant cell granuloma can appear histologically similar to GA. Differentiating histologic features include a nonpalisading granuloma as well as the absence of mucin and necrobiosis.12
Annular lichen planus is a long-recognized but uncommon clinical variant of lichen planus that typically presents as pruritic, purple, annular plaques on the penis, scrotum, or intertriginous areas.13 The violaceous coloring is more characteristic of lichen planus. Histology is helpful in differentiating from GA.
Nummular eczema presents as scattered, welldefined, pruritic, erythematous, coin-shaped, coin-sized plaques in patients with diffusely dry skin.14 The scaling and serous crusting as well as more prominent pruritus help distinguish it from GA. The appearance of nummular eczema is quite characteristic; therefore, a biopsy typically is unnecessary for diagnosis. However, a potassium hydroxide wet mount examination of a skin scraping should be performed if tinea corporis also is suspected.
Superficial erythema annulare centrifugum classically presents as an annular or arciform pruritic lesion with an advancing outer erythematous edge with an inner rim of scale that most commonly occurs on the lower extremities. 15 The presence of pruritus and trailing scale helps distinguish this lesion from GA. Histologically, there are epidermal changes of hyperplasia, spongiosis, and parakeratosis, as well as lymphohistiocytic infiltrate surrounding the superficial dermal vessels.16
We report this case to highlight GA as the most common postherpetic isotopic response. It should be on the differential diagnosis when a patient presents with erythematous, smooth, annular plaques occurring in the distribution of a resolved case of HZV.
The Diagnosis: Granuloma Annulare
A biopsy of a lesion on the right flank demonstrated granulomatous inflammation and interstitial mucin (Figure), characteristic of granuloma annulare (GA).1,2 Granuloma annulare is a relatively common skin disorder with an unknown etiology. It typically presents as smooth, annular, erythematous plaques.1 The most common variants of GA are localized, generalized, and subcutaneous. Our case demonstrated Wolf isotopic response, an unrelated skin disease that forms at the same location as a previously healed skin lesion.2 It is important to be aware of this phenomenon so that it is not confused with a recurrence of herpes zoster virus (HZV).
Although relatively infrequent, GA is the most common isotopic response following HZV infections.3-5 Other postherpetic isotopic eruptions include cutaneous malignancies, lichen planus, sarcoidosis, morphea, reactive perforating collagenosis, psoriasis, and infections, among others.3,5,6 The time between HZV infection and GA can be variable, ranging from a few weeks to many years apart.3
Oftentimes GA will spontaneously resolve within 2 years; however, recurrence is common.7-9 There currently are no standard treatment guidelines. The most promising treatment options include intralesional or topical glucocorticoids for localized GA as well as phototherapy or hydroxychloroquine for widespread disease.8,10

Annular elastolytic giant cell granuloma (also called actinic granuloma) is a rare idiopathic inflammatory skin disease. It is characterized by erythematous annular papules or plaques mainly found on sun-exposed skin, such as the backs of the hands, forearms, or face.11,12 Therefore, based on the distribution of our patient’s lesions, annular elastolytic giant cell granuloma was an unlikely diagnosis. Furthermore, it is not a known postherpetic isotopic reaction. Annular elastolytic giant cell granuloma can appear histologically similar to GA. Differentiating histologic features include a nonpalisading granuloma as well as the absence of mucin and necrobiosis.12
Annular lichen planus is a long-recognized but uncommon clinical variant of lichen planus that typically presents as pruritic, purple, annular plaques on the penis, scrotum, or intertriginous areas.13 The violaceous coloring is more characteristic of lichen planus. Histology is helpful in differentiating from GA.
Nummular eczema presents as scattered, welldefined, pruritic, erythematous, coin-shaped, coin-sized plaques in patients with diffusely dry skin.14 The scaling and serous crusting as well as more prominent pruritus help distinguish it from GA. The appearance of nummular eczema is quite characteristic; therefore, a biopsy typically is unnecessary for diagnosis. However, a potassium hydroxide wet mount examination of a skin scraping should be performed if tinea corporis also is suspected.
Superficial erythema annulare centrifugum classically presents as an annular or arciform pruritic lesion with an advancing outer erythematous edge with an inner rim of scale that most commonly occurs on the lower extremities. 15 The presence of pruritus and trailing scale helps distinguish this lesion from GA. Histologically, there are epidermal changes of hyperplasia, spongiosis, and parakeratosis, as well as lymphohistiocytic infiltrate surrounding the superficial dermal vessels.16
We report this case to highlight GA as the most common postherpetic isotopic response. It should be on the differential diagnosis when a patient presents with erythematous, smooth, annular plaques occurring in the distribution of a resolved case of HZV.
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465.
- . Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Kapoor R, Piris A, Saavedra AP, et al. Wolf isotopic response manifesting as postherpetic granuloma annulare: a case series. Arch Pathol Lab Med. 2013;137:255-258.
- Ezra N, Ahdout J, Haley JC, et al. Granuloma annulare in a zoster scar of a patient with multiple myeloma. Cutis. 2011;87:240-244.
- Noh TW, Park SH, Kang YS, et al. Morphea developing at the site of healed herpes zoster. Ann Dermatol. 2011;23:242-245.
- Ruocco V, Ruocco E, Ghersetich I, et al. Isotopic response after herpesvirus infection: an update. J Am Acad Dermatol. 2002;46:90-94.
- Sparrow G, Abell E. Granuloma annulare and necrobiosis lipoidica treated by jet injector. Br J Dermatol. 1975;93:85-89.
- Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479.
- Thornsberry LA, English JC. Etiology, diagnosis, and therapeutic management of granuloma annulare: an update. Am J Clin Dermatol. 2013;14:279-290.
- Rubin CB, Rosenbach M. Granuloma annulare: a retrospective series of 133 patients. Cutis. 2019;103:102-106.
- Stein JA, Fangman B, Strober B. Actinic granuloma. Dermatol Online J. 2007;13:19.
- Mistry AM, Patel R, Mistry M, et al. Annular elastolytic giant cell granuloma. Cureus. 2020;12:E11456.
- Reich HL, Nguyen JT, James WD. Annular lichen planus: a case series of 20 patients. J Am Acad Dermatol. 2004;50:595-599.
- Leung AKC, Lam JM, Leong KF, et al. Nummular eczema: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:146-155.
- Weyers W, Diaz-Cascajo C, Weyers I. Erythema annulare centrifugum: results of a clinicopathologic study of 73 patients. Am J Dermatopathol. 2003;25:451-462.
- Coronel-Pérez IM, Morillo-Andújar M. Erythema annulare centrifugum responding to natural ultraviolet light [in Spanish]. Actas Dermosifiliogr. 2010;101:177-178.
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465.
- . Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Kapoor R, Piris A, Saavedra AP, et al. Wolf isotopic response manifesting as postherpetic granuloma annulare: a case series. Arch Pathol Lab Med. 2013;137:255-258.
- Ezra N, Ahdout J, Haley JC, et al. Granuloma annulare in a zoster scar of a patient with multiple myeloma. Cutis. 2011;87:240-244.
- Noh TW, Park SH, Kang YS, et al. Morphea developing at the site of healed herpes zoster. Ann Dermatol. 2011;23:242-245.
- Ruocco V, Ruocco E, Ghersetich I, et al. Isotopic response after herpesvirus infection: an update. J Am Acad Dermatol. 2002;46:90-94.
- Sparrow G, Abell E. Granuloma annulare and necrobiosis lipoidica treated by jet injector. Br J Dermatol. 1975;93:85-89.
- Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479.
- Thornsberry LA, English JC. Etiology, diagnosis, and therapeutic management of granuloma annulare: an update. Am J Clin Dermatol. 2013;14:279-290.
- Rubin CB, Rosenbach M. Granuloma annulare: a retrospective series of 133 patients. Cutis. 2019;103:102-106.
- Stein JA, Fangman B, Strober B. Actinic granuloma. Dermatol Online J. 2007;13:19.
- Mistry AM, Patel R, Mistry M, et al. Annular elastolytic giant cell granuloma. Cureus. 2020;12:E11456.
- Reich HL, Nguyen JT, James WD. Annular lichen planus: a case series of 20 patients. J Am Acad Dermatol. 2004;50:595-599.
- Leung AKC, Lam JM, Leong KF, et al. Nummular eczema: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:146-155.
- Weyers W, Diaz-Cascajo C, Weyers I. Erythema annulare centrifugum: results of a clinicopathologic study of 73 patients. Am J Dermatopathol. 2003;25:451-462.
- Coronel-Pérez IM, Morillo-Andújar M. Erythema annulare centrifugum responding to natural ultraviolet light [in Spanish]. Actas Dermosifiliogr. 2010;101:177-178.
An 82-year-old man presented with painful, pink, smooth, annular convalescing plaques on the right back, flank, and abdomen in a zosteriform distribution involving the T10/11 dermatome. He had a history of hypertension and type 2 diabetes mellitus, and 12 months prior to presentation he had an outbreak of herpes zoster virus in the same distribution that was treated with valacyclovir 1000 mg 3 times daily for 7 days. Over the following month he noticed a resolution of blisters and crusting as they morphed into the current lesions.

Painless Vulvar Nodule
The Diagnosis: Proximal-Type Epithelioid Sarcoma
Proximal-type epithelioid sarcoma (PES) is a rare high-grade sarcoma of uncertain histogenesis that may present with a benign clinical appearance. Proximal-type epithelioid sarcoma peaks at 20 to 40 years of age and has a slight male predominance. This tumor exhibits aggressive behavior with both local recurrence and metastasis.1 The average overall survival is poor; however, tumor size less than 5 cm and complete excision with tumor-free margin status improves the prognosis.2 Proximal-type epithelioid sarcoma should not be confused with distal-type epithelioid sarcoma, which has a better prognosis and occurs in younger patients.1 Treatment of PES is wide excision, and chemotherapy trials with tazemetostat are ongoing.3
The deceptively banal clinical appearance of PES may delay appropriate diagnosis and treatment. Proximal-type epithelioid sarcoma often grows in sheets (quiz image [top] inset) and loose nests1 but also may take on a more corded appearance mimicking myoepithelial carcinoma. The cells themselves are plump dyscohesive epithelioid cells (quiz image [top]) with large nucleoli and eosinophilic cytoplasm or hyaline globules1 (quiz image [bottom]), but cells also may be focally spindled. Myxoid stroma, hemorrhage, and necrosis often are prominent features. Epithelioid sarcomas characteristically demonstrate positive immunostaining for both epithelial and mesenchymal markers (pan-cytokeratin and vimentin),1 with the majority having loss of expression of integrase interactor 1 (INI-1).2 Histology in this case was positive for cytokeratin monoclonal antibodies CAM5.2 and OSCAR, epithelial membrane antigen, and vimentin; it showed loss of INI-1 staining (quiz image [bottom]). Negative stains included S-100, p63, cytokeratins 7 and 20, CD34, CD31, ERG, glial fibrillary acidic protein, transducin-like enhancer of split 1, CD117, myogenin, synaptophysin, chromogranin, CD10, inhibin, CD99, and estrogen receptor.
The differential diagnosis of PES includes poorly differentiated squamous cell carcinoma (Figure 1 [inset]), melanoma, myoepithelial carcinoma, and epithelioid angiosarcoma. Primary squamous cell carcinoma of the vulva presents as an endophytic or exophytic mass with raised borders. Vulvar cancer is uncommon among gynecologic malignancies, with squamous cell carcinoma being the most commonly encountered.4 Vulvar intraepithelial neoplasia (VIN) is increasing in incidence, while the occurrence of invasive squamous cell carcinoma remains stable.5 Human papillomavirus–related VIN (usual-type VIN) is less likely to progress to squamous cell carcinoma than differentiated VIN (d-VIN), a dysplasia that is unrelated to human papillomavirus that frequently harbors p53 mutations.4 The presence of histologic epidermal involvement can help distinguish squamous cell carcinoma from PES (Figure 1). As opposed to PES, metastatic squamous cell carcinoma is characterized by intercellular bridges and often at least focal keratinization (Figure 1). Squamous cell carcinoma demonstrates positivity with p63 and p40 immunohistochemical stains, while PES rarely stains for either.6
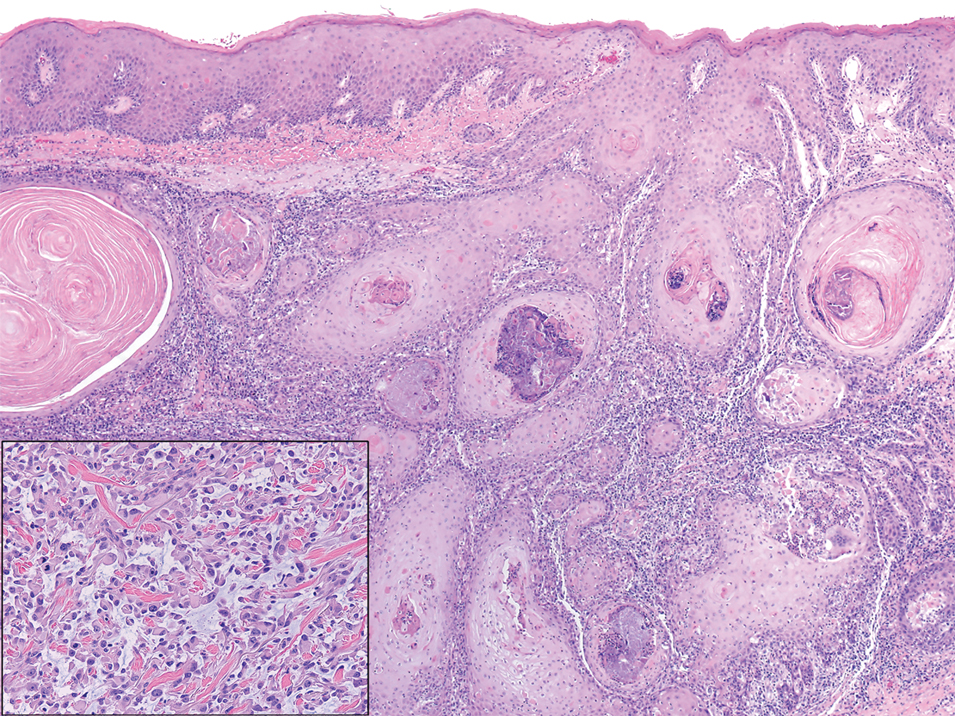
Melanoma is the second most common vulvar malignancy. Vulvar melanoma tends to occur in women of advanced age but has been reported in girls as young as 10 years old.7 There is some evidence that patients with lichen sclerosus may be at an increased risk for the development of vulvar melanoma.8 Compared to PES, primary vulvar melanoma usually demonstrates epidermal involvement as well as clinical findings of a pigmented lesion (Figure 2). A notable minority of vulvar melanomas are amelanotic.9 Melanoma may be distinguished from PES with a panel of melanocytic markers—human melanoma black 45, Melan-A, SRY-box transcription factor 10, S-100, and microphthalmia transcription factor—that rarely are expressed in the latter. Both PES and rhabdoid melanoma have eosinophilic and tinctorial cytoplasmic inclusions.10 Melanin pigment and more cohesive nests are helpful clues that may point to melanoma when present.
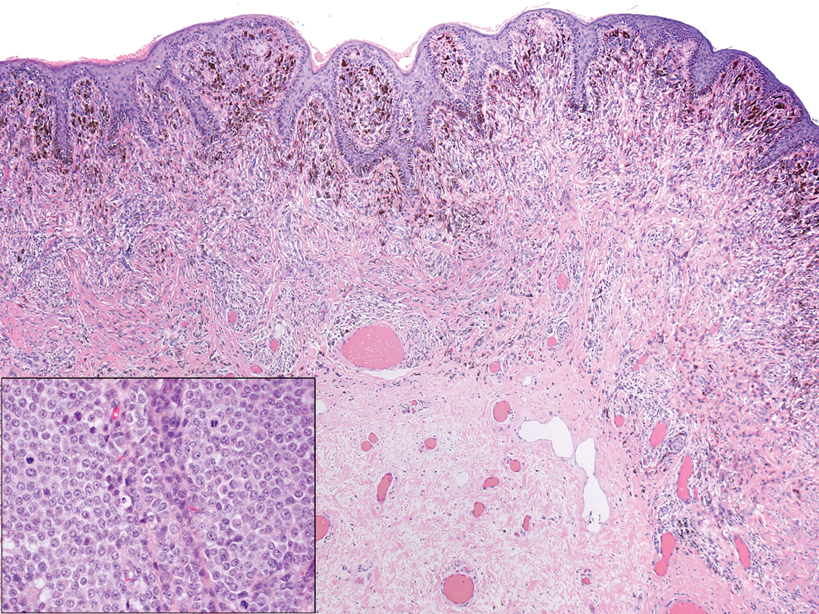
Myoepithelial carcinoma of the vulva is rare.11 Myoepithelial carcinoma of soft tissue is more aggressive than its benign counterpart, with up to a 50% metastasis rate.12 The presence of prominent corded or trabecular growth in a myxoid or hyaline background may point to the diagnosis (Figure 3). Similar to PES, myoepithelial carcinoma may lose expression of nuclear INI-1, while myoepithelial carcinoma is more likely to express S-100 and glial fibrillary acidic protein.13 Rearrangements of EWS RNA binding protein 1, EWSR1, have been found in half of myoepithelial neoplasms.12
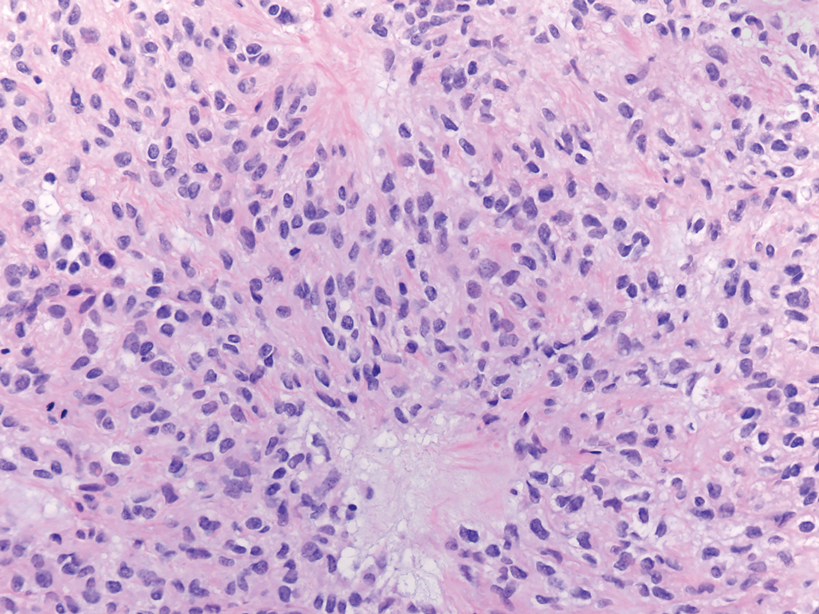
Angiosarcomas represent 5% of cutaneous sarcomas and rarely have been reported in the vulva, primarily occurring in the setting of long-standing lymphedema and radiation.14 Angiosarcoma more often occurs on the head and neck, breasts, or extremities. Additional risk factors for the development of angiosarcoma include toxin exposure (eg, polyvinyl chloride, thorium dioxide, arsenic), anabolic steroids, and filariasis, as well as genetic disorders (eg, neurofibromatosis type 1, BRCA gene mutations, Maffucci syndrome).15 Epithelioid angiosarcoma is an infiltrative tumor composed of irregular anastomosing vascular channels with extravasated erythrocytes (Figure 4). Solid growth and necrosis may be present in more aggressive tumors. The cells themselves are pleomorphic endothelial cells with vesicular chromatin and prominent nucleoli. Epithelioid angiosarcoma may resemble carcinoma and have focal keratin expression. However, the characteristic eosinophilic cytoplasm seen in PES should not be identified in epithelioid angiosarcoma. Unlike PES, epithelioid angiosarcoma is positive for CD31 and has retained expression for INI-1. Both angiosarcoma and proximal-type epithelioid sarcoma may express vascular markers CD34 and FLI-116; thus an expanded panel of immunohistochemical studies may be of utility.
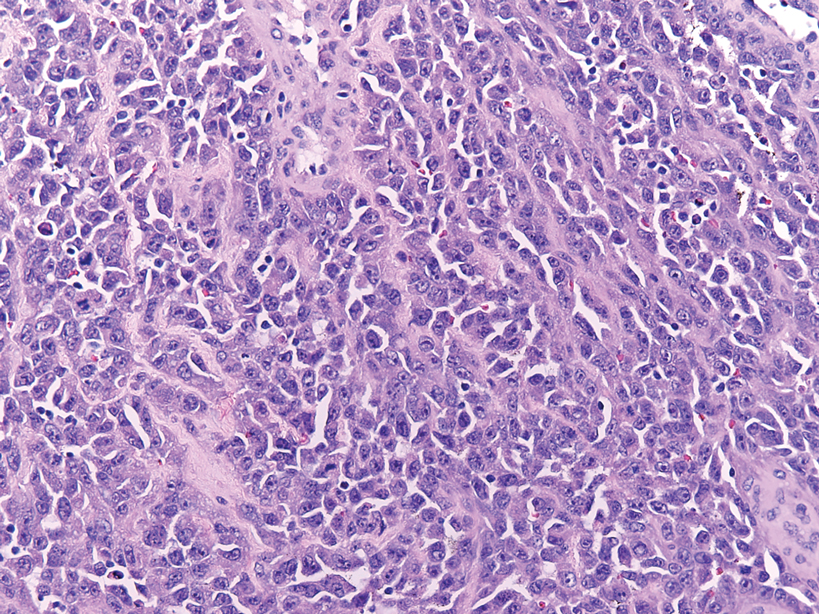
- Guillou L, Wadden C, Coindre JM, et al. “Proximal-type” epithelioid sarcoma, a distinctive aggressive neoplasm showing rhabdoid features. clinicopathologic, immunohistochemical, and ultrastructural study of a series. Am J Surg Pathol. 1997;21:130-146.
- Hasegawa T, Matsuno Y, Shimoda T, et al. Proximal-type epithelioid sarcoma: a clinicopathological study of 20 cases. Mod Pathol. 2001;14:655-663.
- Czarnecka AM, Sobczuk P, Kostrzanowski M, et al. Epithelioid sarcoma—from genetics to clinical practice. Cancers. 2020:12:2112.
- Hoang LH, Park KJ, Soslow RA, et al. Squamous precursor lesions of the vulva: current classification and diagnostic challenges. Pathology. 2016;48:291-302.
- Allbritton J. Vulvar neoplasms, benign and malignant. Obstet Gynecol Clin North Am. 2017;44:339-352.
- Laskin WB, Miettinen M. Epithelioid sarcoma: new insights based on an extended immunohistochemical analysis. Arch Pathol Lab Med. 2003;127:1161-1168.
- Boer FL, Eikelder MLGT, Kapitejn EH, et al. Vulvar malignant melanoma: pathogenesis, clinical behavior and management: review of the literature. Cancer Treat Rev. 2019;73:91-103.
- Hieta N, Rintala SKM, Soderlund J, et al. Association of vulvar melanoma with lichen sclerosus. Acta Derm Venereol. 2019;99:339-340.
- Edwards L. Pigmented vulvar lesions. Dermatol Ther. 2010;23:449-457.
- Patterson JW, Hosler GA, Prenshaw KL, eds. Weedon's Skin Pathology. Elsevier Limited; 2021.
- Kyriazi MA, Carvounis EE, Kitsou M, et al. Myoepithelial carcinoma of the vulva mimicking Bartholin gland abscess in a pregnant woman: case report and review of literature. Int J Gynecol Pathol. 2010:29:501-504.
- Jo VY, Fletcher CD. Myoepithelial neoplasma of soft tissue: an updated review of the clinicopathological, immunophenotypic, and genetic features. Head Neck Pathol. 2015;9:32-38.
- Rekhi B, Sable M, Jambhekar NA. Histopathological, immunohistochemical and molecular spectrum of myoepithelial tumours of soft tissues. Virchows Arch. 2012;461:687-697.
- Yost S, Bradish J, Grossheim L, et al. Epithelioid angiosarcoma of the vulva: a case report. Gynecol Oncol Rep. 2017;21:91-93.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Stockman DL, Hornick JL, Deavers MT, et al. ERG and FLI1 protein expression in epithelioid sarcoma. Mod Pathol. 2014;27:496-501.
The Diagnosis: Proximal-Type Epithelioid Sarcoma
Proximal-type epithelioid sarcoma (PES) is a rare high-grade sarcoma of uncertain histogenesis that may present with a benign clinical appearance. Proximal-type epithelioid sarcoma peaks at 20 to 40 years of age and has a slight male predominance. This tumor exhibits aggressive behavior with both local recurrence and metastasis.1 The average overall survival is poor; however, tumor size less than 5 cm and complete excision with tumor-free margin status improves the prognosis.2 Proximal-type epithelioid sarcoma should not be confused with distal-type epithelioid sarcoma, which has a better prognosis and occurs in younger patients.1 Treatment of PES is wide excision, and chemotherapy trials with tazemetostat are ongoing.3
The deceptively banal clinical appearance of PES may delay appropriate diagnosis and treatment. Proximal-type epithelioid sarcoma often grows in sheets (quiz image [top] inset) and loose nests1 but also may take on a more corded appearance mimicking myoepithelial carcinoma. The cells themselves are plump dyscohesive epithelioid cells (quiz image [top]) with large nucleoli and eosinophilic cytoplasm or hyaline globules1 (quiz image [bottom]), but cells also may be focally spindled. Myxoid stroma, hemorrhage, and necrosis often are prominent features. Epithelioid sarcomas characteristically demonstrate positive immunostaining for both epithelial and mesenchymal markers (pan-cytokeratin and vimentin),1 with the majority having loss of expression of integrase interactor 1 (INI-1).2 Histology in this case was positive for cytokeratin monoclonal antibodies CAM5.2 and OSCAR, epithelial membrane antigen, and vimentin; it showed loss of INI-1 staining (quiz image [bottom]). Negative stains included S-100, p63, cytokeratins 7 and 20, CD34, CD31, ERG, glial fibrillary acidic protein, transducin-like enhancer of split 1, CD117, myogenin, synaptophysin, chromogranin, CD10, inhibin, CD99, and estrogen receptor.
The differential diagnosis of PES includes poorly differentiated squamous cell carcinoma (Figure 1 [inset]), melanoma, myoepithelial carcinoma, and epithelioid angiosarcoma. Primary squamous cell carcinoma of the vulva presents as an endophytic or exophytic mass with raised borders. Vulvar cancer is uncommon among gynecologic malignancies, with squamous cell carcinoma being the most commonly encountered.4 Vulvar intraepithelial neoplasia (VIN) is increasing in incidence, while the occurrence of invasive squamous cell carcinoma remains stable.5 Human papillomavirus–related VIN (usual-type VIN) is less likely to progress to squamous cell carcinoma than differentiated VIN (d-VIN), a dysplasia that is unrelated to human papillomavirus that frequently harbors p53 mutations.4 The presence of histologic epidermal involvement can help distinguish squamous cell carcinoma from PES (Figure 1). As opposed to PES, metastatic squamous cell carcinoma is characterized by intercellular bridges and often at least focal keratinization (Figure 1). Squamous cell carcinoma demonstrates positivity with p63 and p40 immunohistochemical stains, while PES rarely stains for either.6

Melanoma is the second most common vulvar malignancy. Vulvar melanoma tends to occur in women of advanced age but has been reported in girls as young as 10 years old.7 There is some evidence that patients with lichen sclerosus may be at an increased risk for the development of vulvar melanoma.8 Compared to PES, primary vulvar melanoma usually demonstrates epidermal involvement as well as clinical findings of a pigmented lesion (Figure 2). A notable minority of vulvar melanomas are amelanotic.9 Melanoma may be distinguished from PES with a panel of melanocytic markers—human melanoma black 45, Melan-A, SRY-box transcription factor 10, S-100, and microphthalmia transcription factor—that rarely are expressed in the latter. Both PES and rhabdoid melanoma have eosinophilic and tinctorial cytoplasmic inclusions.10 Melanin pigment and more cohesive nests are helpful clues that may point to melanoma when present.

Myoepithelial carcinoma of the vulva is rare.11 Myoepithelial carcinoma of soft tissue is more aggressive than its benign counterpart, with up to a 50% metastasis rate.12 The presence of prominent corded or trabecular growth in a myxoid or hyaline background may point to the diagnosis (Figure 3). Similar to PES, myoepithelial carcinoma may lose expression of nuclear INI-1, while myoepithelial carcinoma is more likely to express S-100 and glial fibrillary acidic protein.13 Rearrangements of EWS RNA binding protein 1, EWSR1, have been found in half of myoepithelial neoplasms.12

Angiosarcomas represent 5% of cutaneous sarcomas and rarely have been reported in the vulva, primarily occurring in the setting of long-standing lymphedema and radiation.14 Angiosarcoma more often occurs on the head and neck, breasts, or extremities. Additional risk factors for the development of angiosarcoma include toxin exposure (eg, polyvinyl chloride, thorium dioxide, arsenic), anabolic steroids, and filariasis, as well as genetic disorders (eg, neurofibromatosis type 1, BRCA gene mutations, Maffucci syndrome).15 Epithelioid angiosarcoma is an infiltrative tumor composed of irregular anastomosing vascular channels with extravasated erythrocytes (Figure 4). Solid growth and necrosis may be present in more aggressive tumors. The cells themselves are pleomorphic endothelial cells with vesicular chromatin and prominent nucleoli. Epithelioid angiosarcoma may resemble carcinoma and have focal keratin expression. However, the characteristic eosinophilic cytoplasm seen in PES should not be identified in epithelioid angiosarcoma. Unlike PES, epithelioid angiosarcoma is positive for CD31 and has retained expression for INI-1. Both angiosarcoma and proximal-type epithelioid sarcoma may express vascular markers CD34 and FLI-116; thus an expanded panel of immunohistochemical studies may be of utility.

The Diagnosis: Proximal-Type Epithelioid Sarcoma
Proximal-type epithelioid sarcoma (PES) is a rare high-grade sarcoma of uncertain histogenesis that may present with a benign clinical appearance. Proximal-type epithelioid sarcoma peaks at 20 to 40 years of age and has a slight male predominance. This tumor exhibits aggressive behavior with both local recurrence and metastasis.1 The average overall survival is poor; however, tumor size less than 5 cm and complete excision with tumor-free margin status improves the prognosis.2 Proximal-type epithelioid sarcoma should not be confused with distal-type epithelioid sarcoma, which has a better prognosis and occurs in younger patients.1 Treatment of PES is wide excision, and chemotherapy trials with tazemetostat are ongoing.3
The deceptively banal clinical appearance of PES may delay appropriate diagnosis and treatment. Proximal-type epithelioid sarcoma often grows in sheets (quiz image [top] inset) and loose nests1 but also may take on a more corded appearance mimicking myoepithelial carcinoma. The cells themselves are plump dyscohesive epithelioid cells (quiz image [top]) with large nucleoli and eosinophilic cytoplasm or hyaline globules1 (quiz image [bottom]), but cells also may be focally spindled. Myxoid stroma, hemorrhage, and necrosis often are prominent features. Epithelioid sarcomas characteristically demonstrate positive immunostaining for both epithelial and mesenchymal markers (pan-cytokeratin and vimentin),1 with the majority having loss of expression of integrase interactor 1 (INI-1).2 Histology in this case was positive for cytokeratin monoclonal antibodies CAM5.2 and OSCAR, epithelial membrane antigen, and vimentin; it showed loss of INI-1 staining (quiz image [bottom]). Negative stains included S-100, p63, cytokeratins 7 and 20, CD34, CD31, ERG, glial fibrillary acidic protein, transducin-like enhancer of split 1, CD117, myogenin, synaptophysin, chromogranin, CD10, inhibin, CD99, and estrogen receptor.
The differential diagnosis of PES includes poorly differentiated squamous cell carcinoma (Figure 1 [inset]), melanoma, myoepithelial carcinoma, and epithelioid angiosarcoma. Primary squamous cell carcinoma of the vulva presents as an endophytic or exophytic mass with raised borders. Vulvar cancer is uncommon among gynecologic malignancies, with squamous cell carcinoma being the most commonly encountered.4 Vulvar intraepithelial neoplasia (VIN) is increasing in incidence, while the occurrence of invasive squamous cell carcinoma remains stable.5 Human papillomavirus–related VIN (usual-type VIN) is less likely to progress to squamous cell carcinoma than differentiated VIN (d-VIN), a dysplasia that is unrelated to human papillomavirus that frequently harbors p53 mutations.4 The presence of histologic epidermal involvement can help distinguish squamous cell carcinoma from PES (Figure 1). As opposed to PES, metastatic squamous cell carcinoma is characterized by intercellular bridges and often at least focal keratinization (Figure 1). Squamous cell carcinoma demonstrates positivity with p63 and p40 immunohistochemical stains, while PES rarely stains for either.6

Melanoma is the second most common vulvar malignancy. Vulvar melanoma tends to occur in women of advanced age but has been reported in girls as young as 10 years old.7 There is some evidence that patients with lichen sclerosus may be at an increased risk for the development of vulvar melanoma.8 Compared to PES, primary vulvar melanoma usually demonstrates epidermal involvement as well as clinical findings of a pigmented lesion (Figure 2). A notable minority of vulvar melanomas are amelanotic.9 Melanoma may be distinguished from PES with a panel of melanocytic markers—human melanoma black 45, Melan-A, SRY-box transcription factor 10, S-100, and microphthalmia transcription factor—that rarely are expressed in the latter. Both PES and rhabdoid melanoma have eosinophilic and tinctorial cytoplasmic inclusions.10 Melanin pigment and more cohesive nests are helpful clues that may point to melanoma when present.

Myoepithelial carcinoma of the vulva is rare.11 Myoepithelial carcinoma of soft tissue is more aggressive than its benign counterpart, with up to a 50% metastasis rate.12 The presence of prominent corded or trabecular growth in a myxoid or hyaline background may point to the diagnosis (Figure 3). Similar to PES, myoepithelial carcinoma may lose expression of nuclear INI-1, while myoepithelial carcinoma is more likely to express S-100 and glial fibrillary acidic protein.13 Rearrangements of EWS RNA binding protein 1, EWSR1, have been found in half of myoepithelial neoplasms.12

Angiosarcomas represent 5% of cutaneous sarcomas and rarely have been reported in the vulva, primarily occurring in the setting of long-standing lymphedema and radiation.14 Angiosarcoma more often occurs on the head and neck, breasts, or extremities. Additional risk factors for the development of angiosarcoma include toxin exposure (eg, polyvinyl chloride, thorium dioxide, arsenic), anabolic steroids, and filariasis, as well as genetic disorders (eg, neurofibromatosis type 1, BRCA gene mutations, Maffucci syndrome).15 Epithelioid angiosarcoma is an infiltrative tumor composed of irregular anastomosing vascular channels with extravasated erythrocytes (Figure 4). Solid growth and necrosis may be present in more aggressive tumors. The cells themselves are pleomorphic endothelial cells with vesicular chromatin and prominent nucleoli. Epithelioid angiosarcoma may resemble carcinoma and have focal keratin expression. However, the characteristic eosinophilic cytoplasm seen in PES should not be identified in epithelioid angiosarcoma. Unlike PES, epithelioid angiosarcoma is positive for CD31 and has retained expression for INI-1. Both angiosarcoma and proximal-type epithelioid sarcoma may express vascular markers CD34 and FLI-116; thus an expanded panel of immunohistochemical studies may be of utility.

- Guillou L, Wadden C, Coindre JM, et al. “Proximal-type” epithelioid sarcoma, a distinctive aggressive neoplasm showing rhabdoid features. clinicopathologic, immunohistochemical, and ultrastructural study of a series. Am J Surg Pathol. 1997;21:130-146.
- Hasegawa T, Matsuno Y, Shimoda T, et al. Proximal-type epithelioid sarcoma: a clinicopathological study of 20 cases. Mod Pathol. 2001;14:655-663.
- Czarnecka AM, Sobczuk P, Kostrzanowski M, et al. Epithelioid sarcoma—from genetics to clinical practice. Cancers. 2020:12:2112.
- Hoang LH, Park KJ, Soslow RA, et al. Squamous precursor lesions of the vulva: current classification and diagnostic challenges. Pathology. 2016;48:291-302.
- Allbritton J. Vulvar neoplasms, benign and malignant. Obstet Gynecol Clin North Am. 2017;44:339-352.
- Laskin WB, Miettinen M. Epithelioid sarcoma: new insights based on an extended immunohistochemical analysis. Arch Pathol Lab Med. 2003;127:1161-1168.
- Boer FL, Eikelder MLGT, Kapitejn EH, et al. Vulvar malignant melanoma: pathogenesis, clinical behavior and management: review of the literature. Cancer Treat Rev. 2019;73:91-103.
- Hieta N, Rintala SKM, Soderlund J, et al. Association of vulvar melanoma with lichen sclerosus. Acta Derm Venereol. 2019;99:339-340.
- Edwards L. Pigmented vulvar lesions. Dermatol Ther. 2010;23:449-457.
- Patterson JW, Hosler GA, Prenshaw KL, eds. Weedon's Skin Pathology. Elsevier Limited; 2021.
- Kyriazi MA, Carvounis EE, Kitsou M, et al. Myoepithelial carcinoma of the vulva mimicking Bartholin gland abscess in a pregnant woman: case report and review of literature. Int J Gynecol Pathol. 2010:29:501-504.
- Jo VY, Fletcher CD. Myoepithelial neoplasma of soft tissue: an updated review of the clinicopathological, immunophenotypic, and genetic features. Head Neck Pathol. 2015;9:32-38.
- Rekhi B, Sable M, Jambhekar NA. Histopathological, immunohistochemical and molecular spectrum of myoepithelial tumours of soft tissues. Virchows Arch. 2012;461:687-697.
- Yost S, Bradish J, Grossheim L, et al. Epithelioid angiosarcoma of the vulva: a case report. Gynecol Oncol Rep. 2017;21:91-93.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Stockman DL, Hornick JL, Deavers MT, et al. ERG and FLI1 protein expression in epithelioid sarcoma. Mod Pathol. 2014;27:496-501.
- Guillou L, Wadden C, Coindre JM, et al. “Proximal-type” epithelioid sarcoma, a distinctive aggressive neoplasm showing rhabdoid features. clinicopathologic, immunohistochemical, and ultrastructural study of a series. Am J Surg Pathol. 1997;21:130-146.
- Hasegawa T, Matsuno Y, Shimoda T, et al. Proximal-type epithelioid sarcoma: a clinicopathological study of 20 cases. Mod Pathol. 2001;14:655-663.
- Czarnecka AM, Sobczuk P, Kostrzanowski M, et al. Epithelioid sarcoma—from genetics to clinical practice. Cancers. 2020:12:2112.
- Hoang LH, Park KJ, Soslow RA, et al. Squamous precursor lesions of the vulva: current classification and diagnostic challenges. Pathology. 2016;48:291-302.
- Allbritton J. Vulvar neoplasms, benign and malignant. Obstet Gynecol Clin North Am. 2017;44:339-352.
- Laskin WB, Miettinen M. Epithelioid sarcoma: new insights based on an extended immunohistochemical analysis. Arch Pathol Lab Med. 2003;127:1161-1168.
- Boer FL, Eikelder MLGT, Kapitejn EH, et al. Vulvar malignant melanoma: pathogenesis, clinical behavior and management: review of the literature. Cancer Treat Rev. 2019;73:91-103.
- Hieta N, Rintala SKM, Soderlund J, et al. Association of vulvar melanoma with lichen sclerosus. Acta Derm Venereol. 2019;99:339-340.
- Edwards L. Pigmented vulvar lesions. Dermatol Ther. 2010;23:449-457.
- Patterson JW, Hosler GA, Prenshaw KL, eds. Weedon's Skin Pathology. Elsevier Limited; 2021.
- Kyriazi MA, Carvounis EE, Kitsou M, et al. Myoepithelial carcinoma of the vulva mimicking Bartholin gland abscess in a pregnant woman: case report and review of literature. Int J Gynecol Pathol. 2010:29:501-504.
- Jo VY, Fletcher CD. Myoepithelial neoplasma of soft tissue: an updated review of the clinicopathological, immunophenotypic, and genetic features. Head Neck Pathol. 2015;9:32-38.
- Rekhi B, Sable M, Jambhekar NA. Histopathological, immunohistochemical and molecular spectrum of myoepithelial tumours of soft tissues. Virchows Arch. 2012;461:687-697.
- Yost S, Bradish J, Grossheim L, et al. Epithelioid angiosarcoma of the vulva: a case report. Gynecol Oncol Rep. 2017;21:91-93.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Stockman DL, Hornick JL, Deavers MT, et al. ERG and FLI1 protein expression in epithelioid sarcoma. Mod Pathol. 2014;27:496-501.
A 45-year-old woman with no notable medical history presented with a small nodule in the left pubic region lateral to the left labia majora. The lesion grew to 8 cm over the course of several months, and she underwent a simple excision for what clinically appeared to be a cyst.