User login
Deployed Airbag Causes Bullous Reaction Following a Motor Vehicle Accident
Airbags are lifesaving during motor vehicle accidents (MVAs), but their deployment has been associated with skin issues such as irritant dermatitis1; lacerations2; abrasions3; and thermal, friction, and chemical burns.4-6 Ocular issues such as alkaline chemical keratitis7 and ocular alkali injuries8 also have been described.
Airbag deployment is triggered by rapid deceleration and impact, which ignite a sodium azide cartridge, causing the woven nylon bag to inflate with hydrocarbon gases.8 This leads to release of sodium hydroxide, sodium bicarbonate, and metallic oxides in an aerosolized form. If a tear in the meshwork of the airbag occurs, exposure to an even larger amount of powder containing caustic alkali chemicals can occur.8
We describe a patient who developed a bullous reaction to airbag contents after he was involved in an MVA in which the airbag deployed.
Case Report
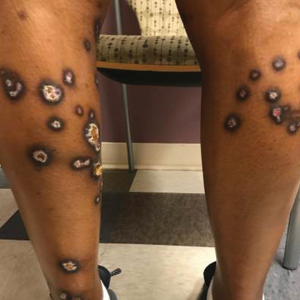
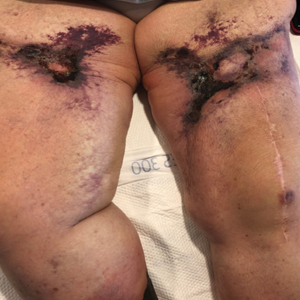
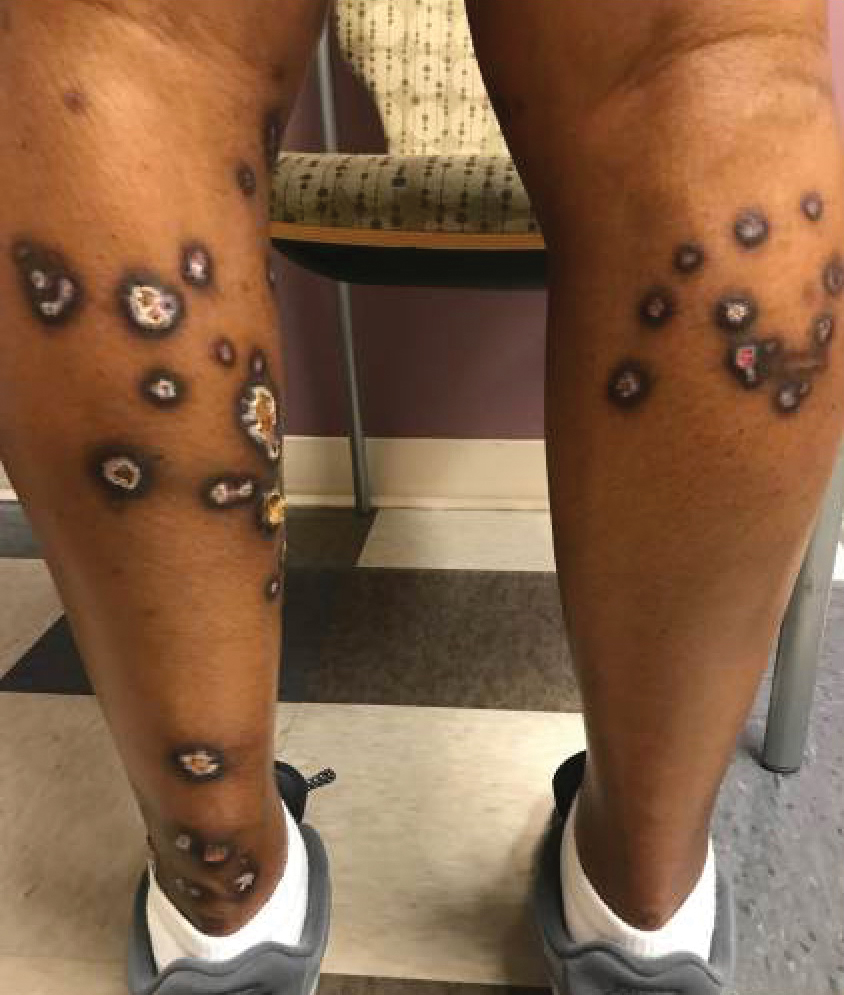
A 35-year-old man with a history of type 2 diabetes mellitus and chronic hepatitis B presented to the dermatology clinic for an evaluation of new-onset blisters. The rash occurred 1 day after the patient was involved in an MVA in which he was exposed to the airbag’s contents after it burst. He had been evaluated twice in the emergency department for the skin eruption before being referred to dermatology. He noted the lesions were pruritic and painful. Prior treatments included silver sulfadiazine cream 1% and clobetasol cream 0.05%, though he discontinued using the latter because of burning with application. Physical examination revealed tense vesicles and bullae on an erythematous base on the right lower flank, forearms, and legs, with the exception of the lower right leg where a cast had been from a prior injury (Figure 1).

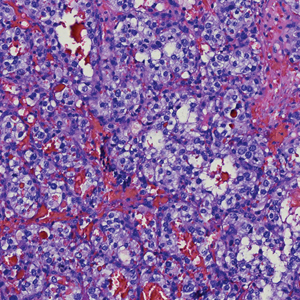
Two punch biopsies of the left arm were performed and sent for hematoxylin and eosin staining and direct immunofluorescence to rule out bullous diseases, such as bullous pemphigoid, linear IgA, and bullous lupus. Hematoxylin and eosin staining revealed extensive spongiosis with blister formation and a dense perivascular infiltrate in the superficial and mid dermis composed of lymphocytes with numerous scattered eosinophils (Figures 2 and 3). Direct immunofluorescence studies were negative. Treatment with oral prednisone and oral antihistamines was initiated.
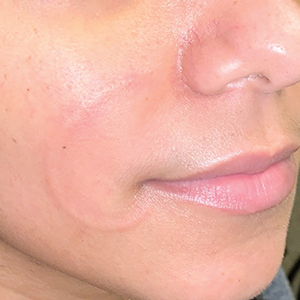
At 10-day follow-up, the patient had a few residual bullae; most lesions were demonstrating various stages of healing (Figure 4). The patient’s cast had been removed, and there were no lesions in this previously covered area. At 6-week follow-up he had continued healing of the bullae and erosions as well as postinflammatory hyperpigmentation (Figure 5).

Comment
With the advent of airbags for safety purposes, these potentially lifesaving devices also have been known to cause injury.9 In 1998, the most commonly reported airbag injuries were ocular injuries.10 Cutaneous manifestations of airbag injury are less well known.11
Two cases of airbag deployment with skin blistering have been reported in the literature based on a PubMed search of articles indexed for MEDLINE using the terms airbag blistering or airbag bullae12,13; however, the blistering was described in the context of a burn. One case of the effects of airbag deployment residue highlights a patient arriving to the emergency department with erythema and blisters on the hands within 48 hours of airbag deployment in an MVA, and the treatment was standard burn therapy.12 Another case report described a patient with a second-degree burn with a 12-cm blister occurring on the radial side of the hand and distal forearm following an MVA and airbag deployment, which was treated conservatively.13 Cases of thermal burns, chemical burns, and irritant contact dermatitis after airbag deployment have been described in the literature.4-6,11,12,14,15 Our patient’s distal right lower leg was covered with a cast for osteomyelitis, and no blisters had developed in this area. It is likely that the transfer of airbag contents occurred during the process of unbuckling his seatbelt, which could explain the bullae that developed on the right flank. Per the Centers for Disease Control and Prevention, individuals should quickly remove clothing and wash their body with large amounts of soap and water following exposure to sodium azide.16
In 1989, the Federal Motor Vehicle Safety Standard No. 208 (occupant crash protection) became effective, stating all cars must have vehicle crash protection.12 Prior to 1993, it was reported that there had been no associated chemical injuries with airbag deployment. Subsequently, a 6-month retrospective study in 1993 showed that dermal injuries were found in connection with the presence of sodium hydroxide in automobile airbags.12 By 2004, it was known that airbags could cause chemical and thermal burns in addition to traumatic injuries from deployment.1 Since 2007, all motor vehicles have been required to have advanced airbags, which are engineered to sense the presence of passengers and determine if the airbag will deploy, and if so, how much to deploy to minimize airbag-related injury.3
The brand of car that our patient drove during the MVA is one with known airbag recalls due to safety defects; however, the year and actual model of the vehicle are not known, so specific information about the airbag in question is not available. It has been noted that some defective airbag inflators that were exposed to excess moisture during the manufacturing process could explode during deployment, causing shrapnel and airbag rupture, which has been linked to nearly 300 injuries worldwide.17
Conclusion
It is evident that the use of airbag devices reduces morbidity and mortality due to MVAs.9 It also had been reported that up to 96% of airbag-related injuries are relatively minor, which many would argue justifies their use.18 Furthermore, it has been reported that 99.8% of skin injuries following airbag deployment are minor.19 In the United States, it is mandated that every vehicle have functional airbags installed.8
This case highlights the potential for substantial airbag-induced skin reactions, specifically a bullous reaction, following airbag deployment. The persistent pruritus and lasting postinflammatory hyperpigmentation seen in this case were certainly worrisome for our patient. We also present this case to remind dermatology providers of possible treatment approaches to these skin reactions. Immediate cleansing of the affected areas of skin may help avoid such reactions.
- Corazza M, Trincone S, Zampino MR, et al. Air bags and the skin. Skinmed. 2004;3:256-258.
- Corazza M, Trincone S, Virgili A. Effects of airbag deployment: lesions, epidemiology, and management. Am J Clin Dermatol. 2004;5:295-300.
- Kuska TC. Air bag safety: an update. J Emerg Nurs. 2016;42:438-441.
- Ulrich D, Noah EM, Fuchs P, et al. Burn injuries caused by air bag deployment. Burns. 2001;27:196-199.
- Erpenbeck SP, Roy E, Ziembicki JA, et al. A systematic review on airbag-induced burns. J Burn Care Res. 2021;42:481-487.
- Skibba KEH, Cleveland CN, Bell DE. Airbag burns: an unfortunate consequence of motor vehicle safety. J Burn Care Res. 2021;42:71-73.
- Smally AJ, Binzer A, Dolin S, et al. Alkaline chemical keratitis: eye injury from airbags. Ann Emerg Med. 1992;21:1400-1402.
- Barnes SS, Wong W Jr, Affeldt JC. A case of severe airbag related ocular alkali injury. Hawaii J Med Public Health. 2012;71:229-231.
- Wallis LA, Greaves I. Injuries associated with airbag deployment. Emerg Med J. 2002;19:490-493.
- Mohamed AA, Banerjee A. Patterns of injury associated with automobile airbag use. Postgrad Med J. 1998;74:455-458.
- Foley E, Helm TN. Air bag injury and the dermatologist. Cutis. 2000;66:251-252.
- Swanson-Biearman B, Mrvos R, Dean BS, et al. Air bags: lifesaving with toxic potential? Am J Emerg Med. 1993;11:38-39.
- Roth T, Meredith P. Traumatic lesions caused by the “air-bag” system [in French]. Z Unfallchir Versicherungsmed. 1993;86:189-193.
- Wu JJ, Sanchez-Palacios C, Brieva J, et al. A case of air bag dermatitis. Arch Dermatol. 2002;138:1383-1384.
- Vitello W, Kim M, Johnson RM, et al. Full-thickness burn to the hand from an automobile airbag. J Burn Care Rehabil. 1999;20:212-215.
- Centers for Disease Control and Prevention. Facts about sodium azide. Updated April 4, 2018. Accessed May 15, 2022. https://emergency.cdc.gov/agent/sodiumazide/basics/facts.asp
- Shepardson D. Honda to recall 1.2 million vehicles in North America to replace Takata airbags. March 12, 2019. Accessed March 22, 2022. https://www.reuters.com/article/us-honda-takata-recall/honda-to-recall-1-2-million-vehicles-in-north-america-to-replace-takata-airbags-idUSKBN1QT1C9
- Gabauer DJ, Gabler HC. The effects of airbags and seatbelts on occupant injury in longitudinal barrier crashes. J Safety Res. 2010;41:9-15.
- Rath AL, Jernigan MV, Stitzel JD, et al. The effects of depowered airbags on skin injuries in frontal automobile crashes. Plast Reconstr Surg. 2005;115:428-435.
Airbags are lifesaving during motor vehicle accidents (MVAs), but their deployment has been associated with skin issues such as irritant dermatitis1; lacerations2; abrasions3; and thermal, friction, and chemical burns.4-6 Ocular issues such as alkaline chemical keratitis7 and ocular alkali injuries8 also have been described.
Airbag deployment is triggered by rapid deceleration and impact, which ignite a sodium azide cartridge, causing the woven nylon bag to inflate with hydrocarbon gases.8 This leads to release of sodium hydroxide, sodium bicarbonate, and metallic oxides in an aerosolized form. If a tear in the meshwork of the airbag occurs, exposure to an even larger amount of powder containing caustic alkali chemicals can occur.8
We describe a patient who developed a bullous reaction to airbag contents after he was involved in an MVA in which the airbag deployed.
Case Report
A 35-year-old man with a history of type 2 diabetes mellitus and chronic hepatitis B presented to the dermatology clinic for an evaluation of new-onset blisters. The rash occurred 1 day after the patient was involved in an MVA in which he was exposed to the airbag’s contents after it burst. He had been evaluated twice in the emergency department for the skin eruption before being referred to dermatology. He noted the lesions were pruritic and painful. Prior treatments included silver sulfadiazine cream 1% and clobetasol cream 0.05%, though he discontinued using the latter because of burning with application. Physical examination revealed tense vesicles and bullae on an erythematous base on the right lower flank, forearms, and legs, with the exception of the lower right leg where a cast had been from a prior injury (Figure 1).

Two punch biopsies of the left arm were performed and sent for hematoxylin and eosin staining and direct immunofluorescence to rule out bullous diseases, such as bullous pemphigoid, linear IgA, and bullous lupus. Hematoxylin and eosin staining revealed extensive spongiosis with blister formation and a dense perivascular infiltrate in the superficial and mid dermis composed of lymphocytes with numerous scattered eosinophils (Figures 2 and 3). Direct immunofluorescence studies were negative. Treatment with oral prednisone and oral antihistamines was initiated.


At 10-day follow-up, the patient had a few residual bullae; most lesions were demonstrating various stages of healing (Figure 4). The patient’s cast had been removed, and there were no lesions in this previously covered area. At 6-week follow-up he had continued healing of the bullae and erosions as well as postinflammatory hyperpigmentation (Figure 5).


Comment
With the advent of airbags for safety purposes, these potentially lifesaving devices also have been known to cause injury.9 In 1998, the most commonly reported airbag injuries were ocular injuries.10 Cutaneous manifestations of airbag injury are less well known.11
Two cases of airbag deployment with skin blistering have been reported in the literature based on a PubMed search of articles indexed for MEDLINE using the terms airbag blistering or airbag bullae12,13; however, the blistering was described in the context of a burn. One case of the effects of airbag deployment residue highlights a patient arriving to the emergency department with erythema and blisters on the hands within 48 hours of airbag deployment in an MVA, and the treatment was standard burn therapy.12 Another case report described a patient with a second-degree burn with a 12-cm blister occurring on the radial side of the hand and distal forearm following an MVA and airbag deployment, which was treated conservatively.13 Cases of thermal burns, chemical burns, and irritant contact dermatitis after airbag deployment have been described in the literature.4-6,11,12,14,15 Our patient’s distal right lower leg was covered with a cast for osteomyelitis, and no blisters had developed in this area. It is likely that the transfer of airbag contents occurred during the process of unbuckling his seatbelt, which could explain the bullae that developed on the right flank. Per the Centers for Disease Control and Prevention, individuals should quickly remove clothing and wash their body with large amounts of soap and water following exposure to sodium azide.16
In 1989, the Federal Motor Vehicle Safety Standard No. 208 (occupant crash protection) became effective, stating all cars must have vehicle crash protection.12 Prior to 1993, it was reported that there had been no associated chemical injuries with airbag deployment. Subsequently, a 6-month retrospective study in 1993 showed that dermal injuries were found in connection with the presence of sodium hydroxide in automobile airbags.12 By 2004, it was known that airbags could cause chemical and thermal burns in addition to traumatic injuries from deployment.1 Since 2007, all motor vehicles have been required to have advanced airbags, which are engineered to sense the presence of passengers and determine if the airbag will deploy, and if so, how much to deploy to minimize airbag-related injury.3
The brand of car that our patient drove during the MVA is one with known airbag recalls due to safety defects; however, the year and actual model of the vehicle are not known, so specific information about the airbag in question is not available. It has been noted that some defective airbag inflators that were exposed to excess moisture during the manufacturing process could explode during deployment, causing shrapnel and airbag rupture, which has been linked to nearly 300 injuries worldwide.17
Conclusion
It is evident that the use of airbag devices reduces morbidity and mortality due to MVAs.9 It also had been reported that up to 96% of airbag-related injuries are relatively minor, which many would argue justifies their use.18 Furthermore, it has been reported that 99.8% of skin injuries following airbag deployment are minor.19 In the United States, it is mandated that every vehicle have functional airbags installed.8
This case highlights the potential for substantial airbag-induced skin reactions, specifically a bullous reaction, following airbag deployment. The persistent pruritus and lasting postinflammatory hyperpigmentation seen in this case were certainly worrisome for our patient. We also present this case to remind dermatology providers of possible treatment approaches to these skin reactions. Immediate cleansing of the affected areas of skin may help avoid such reactions.
Airbags are lifesaving during motor vehicle accidents (MVAs), but their deployment has been associated with skin issues such as irritant dermatitis1; lacerations2; abrasions3; and thermal, friction, and chemical burns.4-6 Ocular issues such as alkaline chemical keratitis7 and ocular alkali injuries8 also have been described.
Airbag deployment is triggered by rapid deceleration and impact, which ignite a sodium azide cartridge, causing the woven nylon bag to inflate with hydrocarbon gases.8 This leads to release of sodium hydroxide, sodium bicarbonate, and metallic oxides in an aerosolized form. If a tear in the meshwork of the airbag occurs, exposure to an even larger amount of powder containing caustic alkali chemicals can occur.8
We describe a patient who developed a bullous reaction to airbag contents after he was involved in an MVA in which the airbag deployed.
Case Report
A 35-year-old man with a history of type 2 diabetes mellitus and chronic hepatitis B presented to the dermatology clinic for an evaluation of new-onset blisters. The rash occurred 1 day after the patient was involved in an MVA in which he was exposed to the airbag’s contents after it burst. He had been evaluated twice in the emergency department for the skin eruption before being referred to dermatology. He noted the lesions were pruritic and painful. Prior treatments included silver sulfadiazine cream 1% and clobetasol cream 0.05%, though he discontinued using the latter because of burning with application. Physical examination revealed tense vesicles and bullae on an erythematous base on the right lower flank, forearms, and legs, with the exception of the lower right leg where a cast had been from a prior injury (Figure 1).

Two punch biopsies of the left arm were performed and sent for hematoxylin and eosin staining and direct immunofluorescence to rule out bullous diseases, such as bullous pemphigoid, linear IgA, and bullous lupus. Hematoxylin and eosin staining revealed extensive spongiosis with blister formation and a dense perivascular infiltrate in the superficial and mid dermis composed of lymphocytes with numerous scattered eosinophils (Figures 2 and 3). Direct immunofluorescence studies were negative. Treatment with oral prednisone and oral antihistamines was initiated.


At 10-day follow-up, the patient had a few residual bullae; most lesions were demonstrating various stages of healing (Figure 4). The patient’s cast had been removed, and there were no lesions in this previously covered area. At 6-week follow-up he had continued healing of the bullae and erosions as well as postinflammatory hyperpigmentation (Figure 5).


Comment
With the advent of airbags for safety purposes, these potentially lifesaving devices also have been known to cause injury.9 In 1998, the most commonly reported airbag injuries were ocular injuries.10 Cutaneous manifestations of airbag injury are less well known.11
Two cases of airbag deployment with skin blistering have been reported in the literature based on a PubMed search of articles indexed for MEDLINE using the terms airbag blistering or airbag bullae12,13; however, the blistering was described in the context of a burn. One case of the effects of airbag deployment residue highlights a patient arriving to the emergency department with erythema and blisters on the hands within 48 hours of airbag deployment in an MVA, and the treatment was standard burn therapy.12 Another case report described a patient with a second-degree burn with a 12-cm blister occurring on the radial side of the hand and distal forearm following an MVA and airbag deployment, which was treated conservatively.13 Cases of thermal burns, chemical burns, and irritant contact dermatitis after airbag deployment have been described in the literature.4-6,11,12,14,15 Our patient’s distal right lower leg was covered with a cast for osteomyelitis, and no blisters had developed in this area. It is likely that the transfer of airbag contents occurred during the process of unbuckling his seatbelt, which could explain the bullae that developed on the right flank. Per the Centers for Disease Control and Prevention, individuals should quickly remove clothing and wash their body with large amounts of soap and water following exposure to sodium azide.16
In 1989, the Federal Motor Vehicle Safety Standard No. 208 (occupant crash protection) became effective, stating all cars must have vehicle crash protection.12 Prior to 1993, it was reported that there had been no associated chemical injuries with airbag deployment. Subsequently, a 6-month retrospective study in 1993 showed that dermal injuries were found in connection with the presence of sodium hydroxide in automobile airbags.12 By 2004, it was known that airbags could cause chemical and thermal burns in addition to traumatic injuries from deployment.1 Since 2007, all motor vehicles have been required to have advanced airbags, which are engineered to sense the presence of passengers and determine if the airbag will deploy, and if so, how much to deploy to minimize airbag-related injury.3
The brand of car that our patient drove during the MVA is one with known airbag recalls due to safety defects; however, the year and actual model of the vehicle are not known, so specific information about the airbag in question is not available. It has been noted that some defective airbag inflators that were exposed to excess moisture during the manufacturing process could explode during deployment, causing shrapnel and airbag rupture, which has been linked to nearly 300 injuries worldwide.17
Conclusion
It is evident that the use of airbag devices reduces morbidity and mortality due to MVAs.9 It also had been reported that up to 96% of airbag-related injuries are relatively minor, which many would argue justifies their use.18 Furthermore, it has been reported that 99.8% of skin injuries following airbag deployment are minor.19 In the United States, it is mandated that every vehicle have functional airbags installed.8
This case highlights the potential for substantial airbag-induced skin reactions, specifically a bullous reaction, following airbag deployment. The persistent pruritus and lasting postinflammatory hyperpigmentation seen in this case were certainly worrisome for our patient. We also present this case to remind dermatology providers of possible treatment approaches to these skin reactions. Immediate cleansing of the affected areas of skin may help avoid such reactions.
- Corazza M, Trincone S, Zampino MR, et al. Air bags and the skin. Skinmed. 2004;3:256-258.
- Corazza M, Trincone S, Virgili A. Effects of airbag deployment: lesions, epidemiology, and management. Am J Clin Dermatol. 2004;5:295-300.
- Kuska TC. Air bag safety: an update. J Emerg Nurs. 2016;42:438-441.
- Ulrich D, Noah EM, Fuchs P, et al. Burn injuries caused by air bag deployment. Burns. 2001;27:196-199.
- Erpenbeck SP, Roy E, Ziembicki JA, et al. A systematic review on airbag-induced burns. J Burn Care Res. 2021;42:481-487.
- Skibba KEH, Cleveland CN, Bell DE. Airbag burns: an unfortunate consequence of motor vehicle safety. J Burn Care Res. 2021;42:71-73.
- Smally AJ, Binzer A, Dolin S, et al. Alkaline chemical keratitis: eye injury from airbags. Ann Emerg Med. 1992;21:1400-1402.
- Barnes SS, Wong W Jr, Affeldt JC. A case of severe airbag related ocular alkali injury. Hawaii J Med Public Health. 2012;71:229-231.
- Wallis LA, Greaves I. Injuries associated with airbag deployment. Emerg Med J. 2002;19:490-493.
- Mohamed AA, Banerjee A. Patterns of injury associated with automobile airbag use. Postgrad Med J. 1998;74:455-458.
- Foley E, Helm TN. Air bag injury and the dermatologist. Cutis. 2000;66:251-252.
- Swanson-Biearman B, Mrvos R, Dean BS, et al. Air bags: lifesaving with toxic potential? Am J Emerg Med. 1993;11:38-39.
- Roth T, Meredith P. Traumatic lesions caused by the “air-bag” system [in French]. Z Unfallchir Versicherungsmed. 1993;86:189-193.
- Wu JJ, Sanchez-Palacios C, Brieva J, et al. A case of air bag dermatitis. Arch Dermatol. 2002;138:1383-1384.
- Vitello W, Kim M, Johnson RM, et al. Full-thickness burn to the hand from an automobile airbag. J Burn Care Rehabil. 1999;20:212-215.
- Centers for Disease Control and Prevention. Facts about sodium azide. Updated April 4, 2018. Accessed May 15, 2022. https://emergency.cdc.gov/agent/sodiumazide/basics/facts.asp
- Shepardson D. Honda to recall 1.2 million vehicles in North America to replace Takata airbags. March 12, 2019. Accessed March 22, 2022. https://www.reuters.com/article/us-honda-takata-recall/honda-to-recall-1-2-million-vehicles-in-north-america-to-replace-takata-airbags-idUSKBN1QT1C9
- Gabauer DJ, Gabler HC. The effects of airbags and seatbelts on occupant injury in longitudinal barrier crashes. J Safety Res. 2010;41:9-15.
- Rath AL, Jernigan MV, Stitzel JD, et al. The effects of depowered airbags on skin injuries in frontal automobile crashes. Plast Reconstr Surg. 2005;115:428-435.
- Corazza M, Trincone S, Zampino MR, et al. Air bags and the skin. Skinmed. 2004;3:256-258.
- Corazza M, Trincone S, Virgili A. Effects of airbag deployment: lesions, epidemiology, and management. Am J Clin Dermatol. 2004;5:295-300.
- Kuska TC. Air bag safety: an update. J Emerg Nurs. 2016;42:438-441.
- Ulrich D, Noah EM, Fuchs P, et al. Burn injuries caused by air bag deployment. Burns. 2001;27:196-199.
- Erpenbeck SP, Roy E, Ziembicki JA, et al. A systematic review on airbag-induced burns. J Burn Care Res. 2021;42:481-487.
- Skibba KEH, Cleveland CN, Bell DE. Airbag burns: an unfortunate consequence of motor vehicle safety. J Burn Care Res. 2021;42:71-73.
- Smally AJ, Binzer A, Dolin S, et al. Alkaline chemical keratitis: eye injury from airbags. Ann Emerg Med. 1992;21:1400-1402.
- Barnes SS, Wong W Jr, Affeldt JC. A case of severe airbag related ocular alkali injury. Hawaii J Med Public Health. 2012;71:229-231.
- Wallis LA, Greaves I. Injuries associated with airbag deployment. Emerg Med J. 2002;19:490-493.
- Mohamed AA, Banerjee A. Patterns of injury associated with automobile airbag use. Postgrad Med J. 1998;74:455-458.
- Foley E, Helm TN. Air bag injury and the dermatologist. Cutis. 2000;66:251-252.
- Swanson-Biearman B, Mrvos R, Dean BS, et al. Air bags: lifesaving with toxic potential? Am J Emerg Med. 1993;11:38-39.
- Roth T, Meredith P. Traumatic lesions caused by the “air-bag” system [in French]. Z Unfallchir Versicherungsmed. 1993;86:189-193.
- Wu JJ, Sanchez-Palacios C, Brieva J, et al. A case of air bag dermatitis. Arch Dermatol. 2002;138:1383-1384.
- Vitello W, Kim M, Johnson RM, et al. Full-thickness burn to the hand from an automobile airbag. J Burn Care Rehabil. 1999;20:212-215.
- Centers for Disease Control and Prevention. Facts about sodium azide. Updated April 4, 2018. Accessed May 15, 2022. https://emergency.cdc.gov/agent/sodiumazide/basics/facts.asp
- Shepardson D. Honda to recall 1.2 million vehicles in North America to replace Takata airbags. March 12, 2019. Accessed March 22, 2022. https://www.reuters.com/article/us-honda-takata-recall/honda-to-recall-1-2-million-vehicles-in-north-america-to-replace-takata-airbags-idUSKBN1QT1C9
- Gabauer DJ, Gabler HC. The effects of airbags and seatbelts on occupant injury in longitudinal barrier crashes. J Safety Res. 2010;41:9-15.
- Rath AL, Jernigan MV, Stitzel JD, et al. The effects of depowered airbags on skin injuries in frontal automobile crashes. Plast Reconstr Surg. 2005;115:428-435.
Practice Points
- This case highlights the potential for a bullous reaction following airbag deployment.
- After airbag deployment, it is important to immediately cleanse the affected areas of skin with soap and water.
Vascular Plaque in a Pregnant Patient With a History of Breast Cancer
The Diagnosis: Tufted Angioma
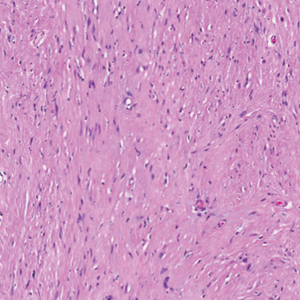
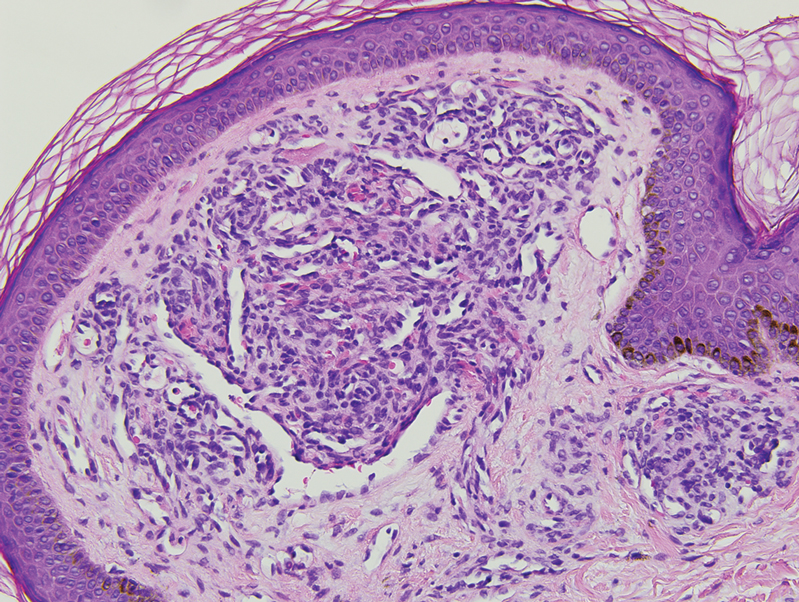
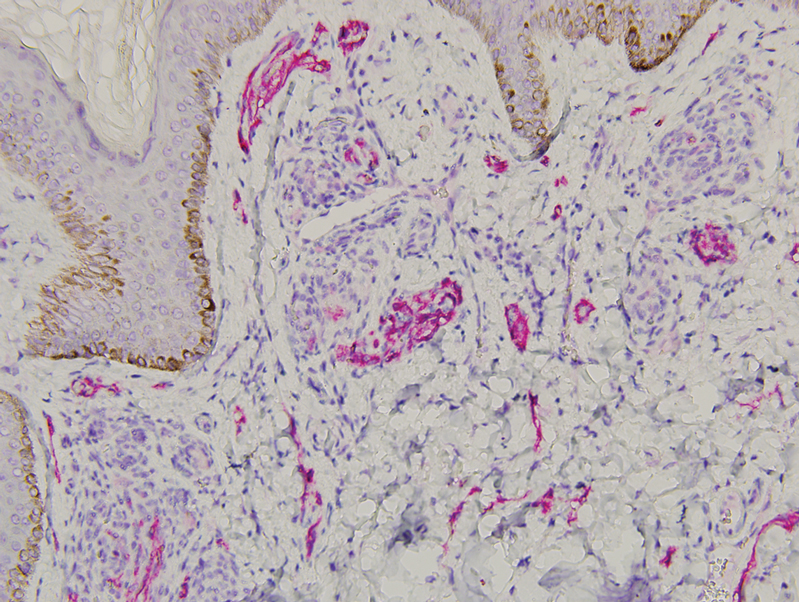
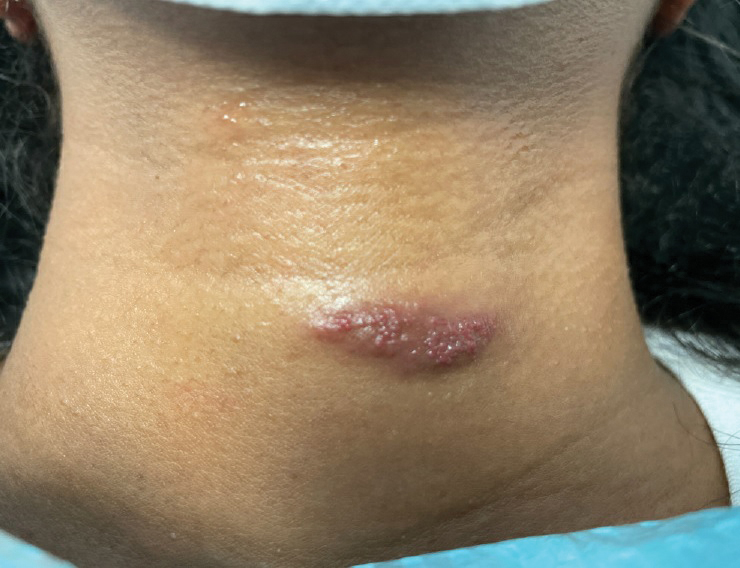
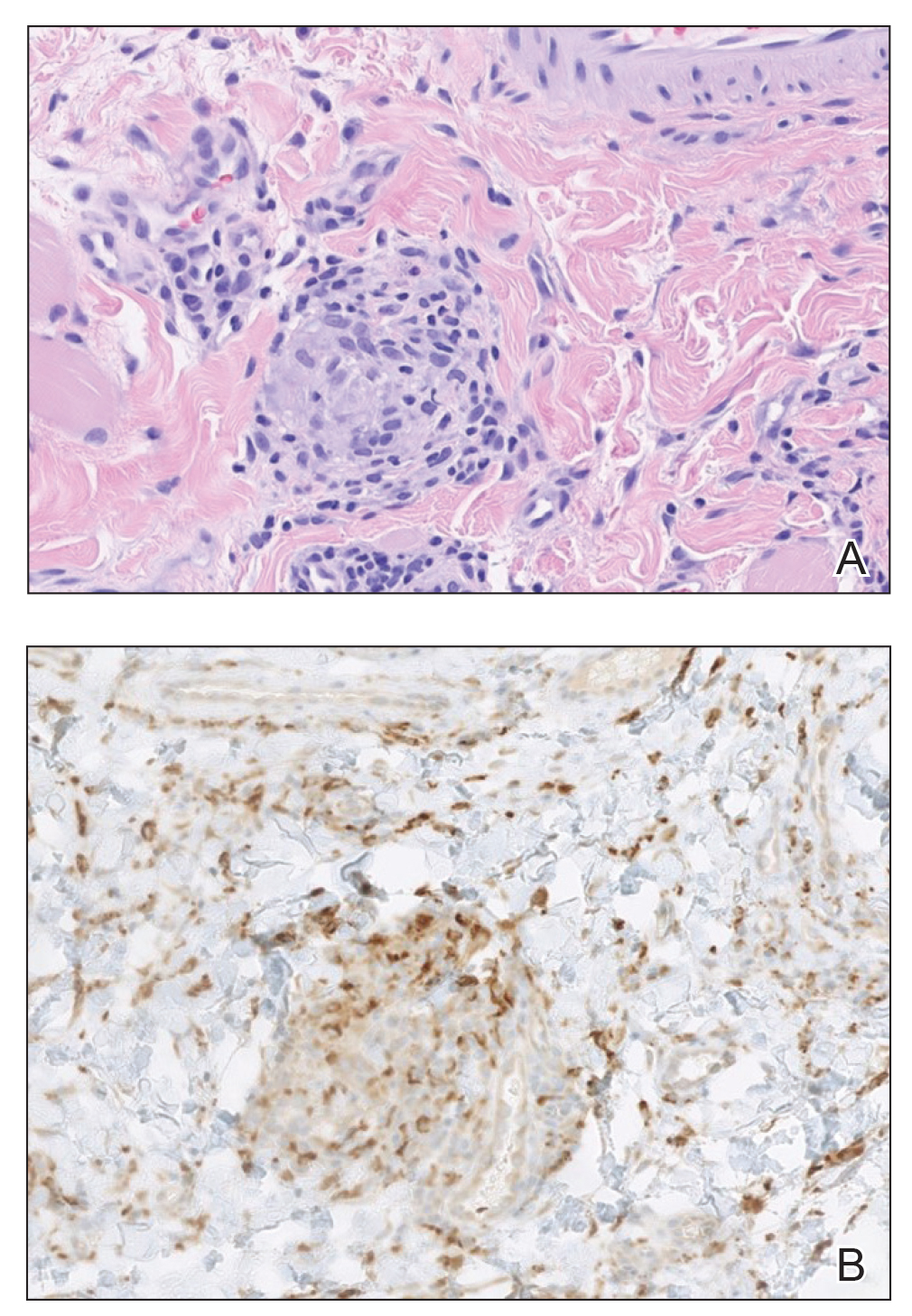
Histopathology revealed discrete lobules of closely packed capillaries with bland endothelial cells throughout the upper and lower dermis (Figure 1). The surrounding crescentlike vessels and lymphatics stained with D2-40 (Figure 2). These histologic findings were consistent with tufted angioma, and the patient elected for observation.
Tufted angiomas are benign vascular lesions named for the tufted appearance of capillaries on histology.1 They commonly present in children, with a lower incidence in adults and rare cases in pregnancy.2 Tufted angiomas typically present as solitary, slowly expanding, erythematous macules, plaques, or nodules on the neck or trunk ranging in size from less than 1 to 10 cm.2-4 They can be histologically distinguished from other vascular tumors, including aggressive malignant neoplasms.1
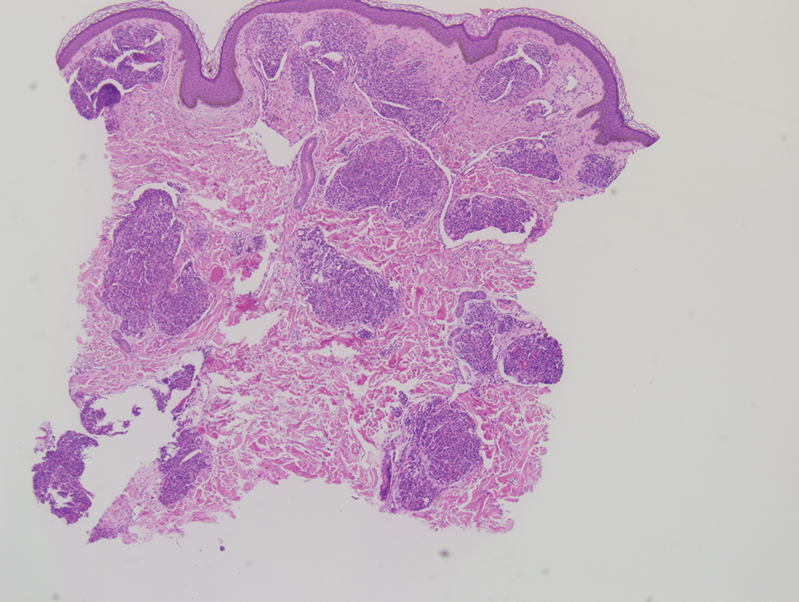
Tufted angiomas are identified by characteristic “cannon ball tufts” of capillaries in the dermis and subcutis at low power.3,5 Distinct cellular lobules may be found bulging into thin-walled vascular channels at the margins of the lobules in the dermis and subcutis (Figure 3).4 The lobules are formed by cells with spindle-shaped nuclei.6 Some mitotic figures may be present, but no cellular atypia is seen.2 The capillaries at the periphery appear as dilated semilunar vessels.4 Dilated lymphatics, which stain with D2-40, can be found at the periphery of the tufted capillaries and throughout the remaining dermis.3,4
Tufted angiomas may arise independently in adults but also have been associated with conditions such as pregnancy. Omori et al7 identified an acquired tufted angioma in pregnancy that was positive for estrogen and progesterone receptors. Reports of tufted angiomas in pregnancy vary; some are multiple lesions, some regress postpartum, and some undergo successful surgical treatment.3,5
Vascular lesions such as tufted angiomas specifically may appear in pregnancy due to a high-volume state with vasodilation and increased vascular proliferation. Although tumor angiogenesis has been linked to specific growth factors and cytokines, it has been hypothesized that the systemic hormones of pregnancy such as human chorionic gonadotropin, estradiol, and progesterone also shift the body to a more angiogenic state.8 In a study of cutaneous changes in pregnant women (N=905), 41% developed a vascular skin change, including spider veins, varicosities, hemangiomas, and granulomas.9 The most common vascular tumor in pregnancy is pyogenic granuloma. Pyogenic granulomas are small, solitary, friable papules that commonly are found on the hands, forearms, face, or in the mouth; histologically they demonstrate dilated capillaries in lobular structures accompanied by larger thick-walled vessels.3,10,11
Tufted angiomas may mimic a variety of other conditions. Epithelioid hemangioma, considered by some to be on the same morphologic spectrum as angiolymphoid hyperplasia with eosinophilia, classically occurs in young adults on the head and in the neck region. It histologically demonstrates a lobular appearance at low power; however, these lobules are made up of vessels with histiocytoid to epithelioid endothelial cells surrounded by a prominent inflammatory infiltrate consisting of lymphocytes and eosinophils.12
Kaposi sarcoma may appear on the neck but most often presents as macules and patches on the extremities that may form nodules with a rubbery consistency. In tufted angiomas, the cellular nodules with dilated channels at the margins bear a resemblance to Kaposi sarcoma or kaposiform hemangioendothelioma; however, in tufted angiomas the lobules are composed of bland spindle cells and slitlike vessels at the periphery.3,13,14 Tufted angiomas are negative for human herpesvirus 8 and typically do not have an associated inflammatory infiltrate with plasma cells.11,15
Moreover, it is important to differentiate tufted angioma from a cutaneous manifestation of an underlying malignancy, which has been described previously in cases of breast cancer.16,17 Our case illustrates a rare vascular tumor arising in the novel context of a pregnant patient with breast cancer. Distinguishing tufted angioma from other benign or malignant vascular tumors is necessary to avoid inappropriate therapeutic interventions.
- Jones EW, Orkin M. Tufted angioma (angioblastoma). a benign progressive angioma, not to be confused with Kaposi’s sarcoma or low-grade angiosarcoma. J Am Acad Dermatol. 1989;20(2 pt 1):214-225.
- Lee B, Chiu M, Soriano T, et al. Adult-onset tufted angioma: a case report and review of the literature. Cutis. 2006;78:341-345.
- Kim YK, Kim HJ, Lee KG. Acquired tufted angioma associated with pregnancy. Clin Exp Dermatol. 1992;17:458-459.
- Feito-Rodriguez M, Sanchez-Orta A, De Lucas R, et al. Congenital tufted angioma: a multicenter retrospective study of 30 cases. Pediatr Dermatol. 2018;35:808-816.
- Pietroletti R, Leardi S, Simi M. Perianal acquired tufted angioma associated with pregnancy: case report. Tech Coloproctol. 2002;6:117-119.
- Osio A, Fraitag S, Hadj-Rabia S, et al. Clinical spectrum of tufted angiomas in childhood: a report of 13 cases and a review of the literature. Arch Dermatol. 2010;146:758-763.
- Omori M, Bito T, Nishigori C. Acquired tufted angioma in pregnancy showing expression of estrogen and progesterone receptors. Eur J Dermatol. 2013;23:898-899.
- Boeldt DS, Bird IM. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J Endocrinol. 2017;232:R27-R44.
- Fernandes LB, Amaral W. Clinical study of skin changes in low and high risk pregnant women. An Bras Dermatol. 2015;90:822-826.
- Walker JL, Wang AR, Kroumpouzos G, et al. Cutaneous tumors in pregnancy. Clin Dermatol. 2016;34:359-367.
- Sarwal P, Lapumnuaypol K. Pyogenic granuloma. In: StatPearls. StatPearls Publishing; 2021.
- Ortins-Pina A, Llamas-Velasco M, Turpin S, et al. FOSB immunoreactivity in endothelia of epithelioid hemangioma (angiolymphoid hyperplasia with eosinophilia). J Cutan Pathol. 2018;45:395-402.
- Arai E, Kuramochi A, Tsuchida T, et al. Usefulness of D2-40 immunohistochemistry for differentiation between kaposiform hemangioendothelioma and tufted angioma. J Cutan Pathol. 2006;33:492-497.
- Grassi S, Carugno A, Vignini M, et al. Adult-onset tufted angiomas associated with an arteriovenous malformation in a renal transplant recipient: case report and review of the literature. Am J Dermatopathol. 2015;37:162-165.
- Lyons LL, North PE, Mac-Moune Lai F, et al. Kaposiform hemangioendothelioma: a study of 33 cases emphasizing its pathologic, immunophenotypic, and biologic uniqueness from juvenile hemangioma. Am J Surg Pathol. 2004;28:559-568.
- Putra HP, Djawad K, Nurdin AR. Cutaneous lesions as the first manifestation of breast cancer: a rare case. Pan Afr Med J. 2020;37:383.
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98.
The Diagnosis: Tufted Angioma
Histopathology revealed discrete lobules of closely packed capillaries with bland endothelial cells throughout the upper and lower dermis (Figure 1). The surrounding crescentlike vessels and lymphatics stained with D2-40 (Figure 2). These histologic findings were consistent with tufted angioma, and the patient elected for observation.

Tufted angiomas are benign vascular lesions named for the tufted appearance of capillaries on histology.1 They commonly present in children, with a lower incidence in adults and rare cases in pregnancy.2 Tufted angiomas typically present as solitary, slowly expanding, erythematous macules, plaques, or nodules on the neck or trunk ranging in size from less than 1 to 10 cm.2-4 They can be histologically distinguished from other vascular tumors, including aggressive malignant neoplasms.1

Tufted angiomas are identified by characteristic “cannon ball tufts” of capillaries in the dermis and subcutis at low power.3,5 Distinct cellular lobules may be found bulging into thin-walled vascular channels at the margins of the lobules in the dermis and subcutis (Figure 3).4 The lobules are formed by cells with spindle-shaped nuclei.6 Some mitotic figures may be present, but no cellular atypia is seen.2 The capillaries at the periphery appear as dilated semilunar vessels.4 Dilated lymphatics, which stain with D2-40, can be found at the periphery of the tufted capillaries and throughout the remaining dermis.3,4

Tufted angiomas may arise independently in adults but also have been associated with conditions such as pregnancy. Omori et al7 identified an acquired tufted angioma in pregnancy that was positive for estrogen and progesterone receptors. Reports of tufted angiomas in pregnancy vary; some are multiple lesions, some regress postpartum, and some undergo successful surgical treatment.3,5
Vascular lesions such as tufted angiomas specifically may appear in pregnancy due to a high-volume state with vasodilation and increased vascular proliferation. Although tumor angiogenesis has been linked to specific growth factors and cytokines, it has been hypothesized that the systemic hormones of pregnancy such as human chorionic gonadotropin, estradiol, and progesterone also shift the body to a more angiogenic state.8 In a study of cutaneous changes in pregnant women (N=905), 41% developed a vascular skin change, including spider veins, varicosities, hemangiomas, and granulomas.9 The most common vascular tumor in pregnancy is pyogenic granuloma. Pyogenic granulomas are small, solitary, friable papules that commonly are found on the hands, forearms, face, or in the mouth; histologically they demonstrate dilated capillaries in lobular structures accompanied by larger thick-walled vessels.3,10,11
Tufted angiomas may mimic a variety of other conditions. Epithelioid hemangioma, considered by some to be on the same morphologic spectrum as angiolymphoid hyperplasia with eosinophilia, classically occurs in young adults on the head and in the neck region. It histologically demonstrates a lobular appearance at low power; however, these lobules are made up of vessels with histiocytoid to epithelioid endothelial cells surrounded by a prominent inflammatory infiltrate consisting of lymphocytes and eosinophils.12
Kaposi sarcoma may appear on the neck but most often presents as macules and patches on the extremities that may form nodules with a rubbery consistency. In tufted angiomas, the cellular nodules with dilated channels at the margins bear a resemblance to Kaposi sarcoma or kaposiform hemangioendothelioma; however, in tufted angiomas the lobules are composed of bland spindle cells and slitlike vessels at the periphery.3,13,14 Tufted angiomas are negative for human herpesvirus 8 and typically do not have an associated inflammatory infiltrate with plasma cells.11,15
Moreover, it is important to differentiate tufted angioma from a cutaneous manifestation of an underlying malignancy, which has been described previously in cases of breast cancer.16,17 Our case illustrates a rare vascular tumor arising in the novel context of a pregnant patient with breast cancer. Distinguishing tufted angioma from other benign or malignant vascular tumors is necessary to avoid inappropriate therapeutic interventions.
The Diagnosis: Tufted Angioma
Histopathology revealed discrete lobules of closely packed capillaries with bland endothelial cells throughout the upper and lower dermis (Figure 1). The surrounding crescentlike vessels and lymphatics stained with D2-40 (Figure 2). These histologic findings were consistent with tufted angioma, and the patient elected for observation.

Tufted angiomas are benign vascular lesions named for the tufted appearance of capillaries on histology.1 They commonly present in children, with a lower incidence in adults and rare cases in pregnancy.2 Tufted angiomas typically present as solitary, slowly expanding, erythematous macules, plaques, or nodules on the neck or trunk ranging in size from less than 1 to 10 cm.2-4 They can be histologically distinguished from other vascular tumors, including aggressive malignant neoplasms.1

Tufted angiomas are identified by characteristic “cannon ball tufts” of capillaries in the dermis and subcutis at low power.3,5 Distinct cellular lobules may be found bulging into thin-walled vascular channels at the margins of the lobules in the dermis and subcutis (Figure 3).4 The lobules are formed by cells with spindle-shaped nuclei.6 Some mitotic figures may be present, but no cellular atypia is seen.2 The capillaries at the periphery appear as dilated semilunar vessels.4 Dilated lymphatics, which stain with D2-40, can be found at the periphery of the tufted capillaries and throughout the remaining dermis.3,4

Tufted angiomas may arise independently in adults but also have been associated with conditions such as pregnancy. Omori et al7 identified an acquired tufted angioma in pregnancy that was positive for estrogen and progesterone receptors. Reports of tufted angiomas in pregnancy vary; some are multiple lesions, some regress postpartum, and some undergo successful surgical treatment.3,5
Vascular lesions such as tufted angiomas specifically may appear in pregnancy due to a high-volume state with vasodilation and increased vascular proliferation. Although tumor angiogenesis has been linked to specific growth factors and cytokines, it has been hypothesized that the systemic hormones of pregnancy such as human chorionic gonadotropin, estradiol, and progesterone also shift the body to a more angiogenic state.8 In a study of cutaneous changes in pregnant women (N=905), 41% developed a vascular skin change, including spider veins, varicosities, hemangiomas, and granulomas.9 The most common vascular tumor in pregnancy is pyogenic granuloma. Pyogenic granulomas are small, solitary, friable papules that commonly are found on the hands, forearms, face, or in the mouth; histologically they demonstrate dilated capillaries in lobular structures accompanied by larger thick-walled vessels.3,10,11
Tufted angiomas may mimic a variety of other conditions. Epithelioid hemangioma, considered by some to be on the same morphologic spectrum as angiolymphoid hyperplasia with eosinophilia, classically occurs in young adults on the head and in the neck region. It histologically demonstrates a lobular appearance at low power; however, these lobules are made up of vessels with histiocytoid to epithelioid endothelial cells surrounded by a prominent inflammatory infiltrate consisting of lymphocytes and eosinophils.12
Kaposi sarcoma may appear on the neck but most often presents as macules and patches on the extremities that may form nodules with a rubbery consistency. In tufted angiomas, the cellular nodules with dilated channels at the margins bear a resemblance to Kaposi sarcoma or kaposiform hemangioendothelioma; however, in tufted angiomas the lobules are composed of bland spindle cells and slitlike vessels at the periphery.3,13,14 Tufted angiomas are negative for human herpesvirus 8 and typically do not have an associated inflammatory infiltrate with plasma cells.11,15
Moreover, it is important to differentiate tufted angioma from a cutaneous manifestation of an underlying malignancy, which has been described previously in cases of breast cancer.16,17 Our case illustrates a rare vascular tumor arising in the novel context of a pregnant patient with breast cancer. Distinguishing tufted angioma from other benign or malignant vascular tumors is necessary to avoid inappropriate therapeutic interventions.
- Jones EW, Orkin M. Tufted angioma (angioblastoma). a benign progressive angioma, not to be confused with Kaposi’s sarcoma or low-grade angiosarcoma. J Am Acad Dermatol. 1989;20(2 pt 1):214-225.
- Lee B, Chiu M, Soriano T, et al. Adult-onset tufted angioma: a case report and review of the literature. Cutis. 2006;78:341-345.
- Kim YK, Kim HJ, Lee KG. Acquired tufted angioma associated with pregnancy. Clin Exp Dermatol. 1992;17:458-459.
- Feito-Rodriguez M, Sanchez-Orta A, De Lucas R, et al. Congenital tufted angioma: a multicenter retrospective study of 30 cases. Pediatr Dermatol. 2018;35:808-816.
- Pietroletti R, Leardi S, Simi M. Perianal acquired tufted angioma associated with pregnancy: case report. Tech Coloproctol. 2002;6:117-119.
- Osio A, Fraitag S, Hadj-Rabia S, et al. Clinical spectrum of tufted angiomas in childhood: a report of 13 cases and a review of the literature. Arch Dermatol. 2010;146:758-763.
- Omori M, Bito T, Nishigori C. Acquired tufted angioma in pregnancy showing expression of estrogen and progesterone receptors. Eur J Dermatol. 2013;23:898-899.
- Boeldt DS, Bird IM. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J Endocrinol. 2017;232:R27-R44.
- Fernandes LB, Amaral W. Clinical study of skin changes in low and high risk pregnant women. An Bras Dermatol. 2015;90:822-826.
- Walker JL, Wang AR, Kroumpouzos G, et al. Cutaneous tumors in pregnancy. Clin Dermatol. 2016;34:359-367.
- Sarwal P, Lapumnuaypol K. Pyogenic granuloma. In: StatPearls. StatPearls Publishing; 2021.
- Ortins-Pina A, Llamas-Velasco M, Turpin S, et al. FOSB immunoreactivity in endothelia of epithelioid hemangioma (angiolymphoid hyperplasia with eosinophilia). J Cutan Pathol. 2018;45:395-402.
- Arai E, Kuramochi A, Tsuchida T, et al. Usefulness of D2-40 immunohistochemistry for differentiation between kaposiform hemangioendothelioma and tufted angioma. J Cutan Pathol. 2006;33:492-497.
- Grassi S, Carugno A, Vignini M, et al. Adult-onset tufted angiomas associated with an arteriovenous malformation in a renal transplant recipient: case report and review of the literature. Am J Dermatopathol. 2015;37:162-165.
- Lyons LL, North PE, Mac-Moune Lai F, et al. Kaposiform hemangioendothelioma: a study of 33 cases emphasizing its pathologic, immunophenotypic, and biologic uniqueness from juvenile hemangioma. Am J Surg Pathol. 2004;28:559-568.
- Putra HP, Djawad K, Nurdin AR. Cutaneous lesions as the first manifestation of breast cancer: a rare case. Pan Afr Med J. 2020;37:383.
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98.
- Jones EW, Orkin M. Tufted angioma (angioblastoma). a benign progressive angioma, not to be confused with Kaposi’s sarcoma or low-grade angiosarcoma. J Am Acad Dermatol. 1989;20(2 pt 1):214-225.
- Lee B, Chiu M, Soriano T, et al. Adult-onset tufted angioma: a case report and review of the literature. Cutis. 2006;78:341-345.
- Kim YK, Kim HJ, Lee KG. Acquired tufted angioma associated with pregnancy. Clin Exp Dermatol. 1992;17:458-459.
- Feito-Rodriguez M, Sanchez-Orta A, De Lucas R, et al. Congenital tufted angioma: a multicenter retrospective study of 30 cases. Pediatr Dermatol. 2018;35:808-816.
- Pietroletti R, Leardi S, Simi M. Perianal acquired tufted angioma associated with pregnancy: case report. Tech Coloproctol. 2002;6:117-119.
- Osio A, Fraitag S, Hadj-Rabia S, et al. Clinical spectrum of tufted angiomas in childhood: a report of 13 cases and a review of the literature. Arch Dermatol. 2010;146:758-763.
- Omori M, Bito T, Nishigori C. Acquired tufted angioma in pregnancy showing expression of estrogen and progesterone receptors. Eur J Dermatol. 2013;23:898-899.
- Boeldt DS, Bird IM. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J Endocrinol. 2017;232:R27-R44.
- Fernandes LB, Amaral W. Clinical study of skin changes in low and high risk pregnant women. An Bras Dermatol. 2015;90:822-826.
- Walker JL, Wang AR, Kroumpouzos G, et al. Cutaneous tumors in pregnancy. Clin Dermatol. 2016;34:359-367.
- Sarwal P, Lapumnuaypol K. Pyogenic granuloma. In: StatPearls. StatPearls Publishing; 2021.
- Ortins-Pina A, Llamas-Velasco M, Turpin S, et al. FOSB immunoreactivity in endothelia of epithelioid hemangioma (angiolymphoid hyperplasia with eosinophilia). J Cutan Pathol. 2018;45:395-402.
- Arai E, Kuramochi A, Tsuchida T, et al. Usefulness of D2-40 immunohistochemistry for differentiation between kaposiform hemangioendothelioma and tufted angioma. J Cutan Pathol. 2006;33:492-497.
- Grassi S, Carugno A, Vignini M, et al. Adult-onset tufted angiomas associated with an arteriovenous malformation in a renal transplant recipient: case report and review of the literature. Am J Dermatopathol. 2015;37:162-165.
- Lyons LL, North PE, Mac-Moune Lai F, et al. Kaposiform hemangioendothelioma: a study of 33 cases emphasizing its pathologic, immunophenotypic, and biologic uniqueness from juvenile hemangioma. Am J Surg Pathol. 2004;28:559-568.
- Putra HP, Djawad K, Nurdin AR. Cutaneous lesions as the first manifestation of breast cancer: a rare case. Pan Afr Med J. 2020;37:383.
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98.
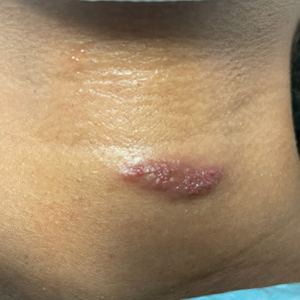
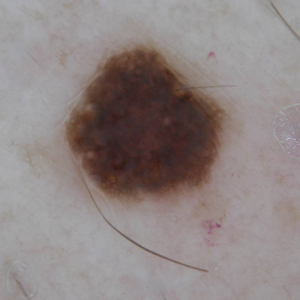
A 31-year-old woman at 34 weeks’ gestation presented with skin discoloration of the anterior neck of 7 months’ duration. Her pregnancy had been complicated by a diagnosis of invasive papillary carcinoma of the breast with unilateral complete mastectomy and negative sentinel lymph node biopsy in the first trimester. The lesion was tender, darkening, and rapidly enlarging. Physical examination demonstrated a linear, violaceous, vascular, and indurated plaque with microvesiculation that was 3.5 cm in width. She had no history of blistering sunburns, frequent UV exposure, or skin cancer.
Persistent Lip Swelling
The Diagnosis: Granulomatous Cheilitis
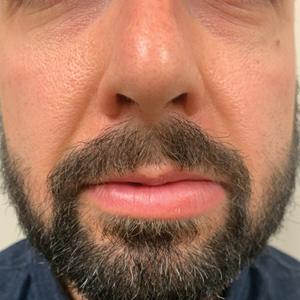
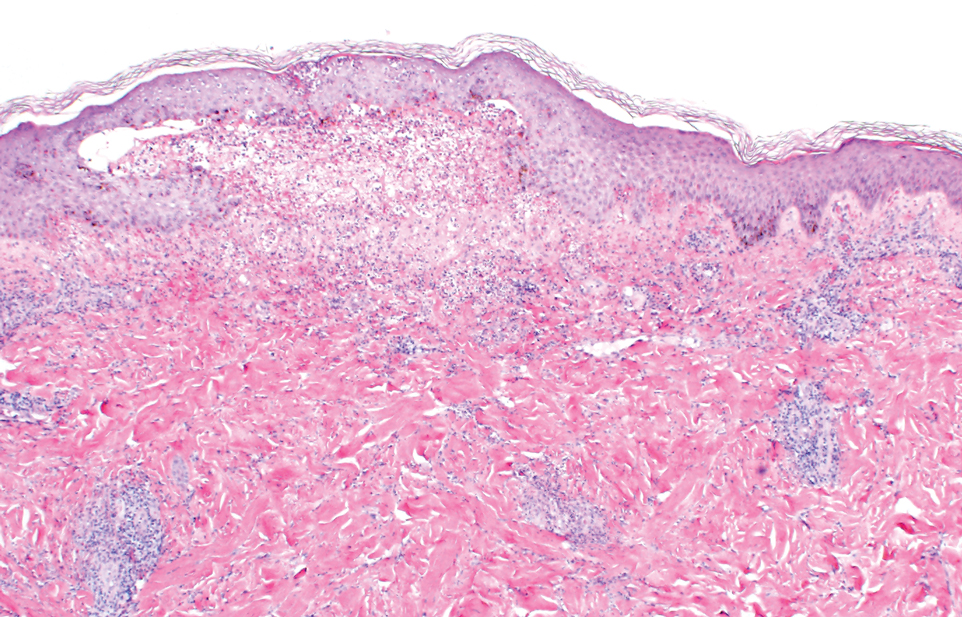
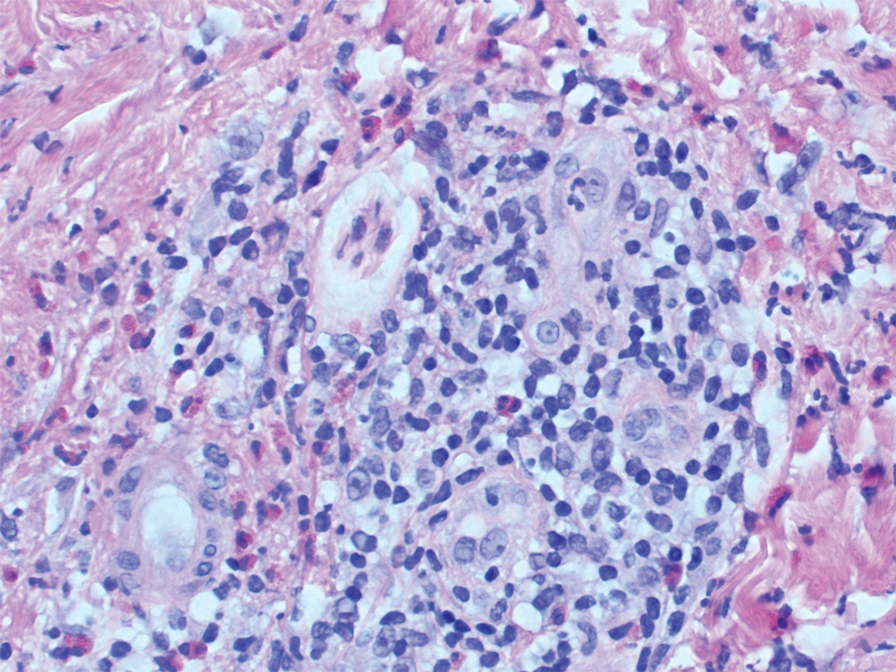
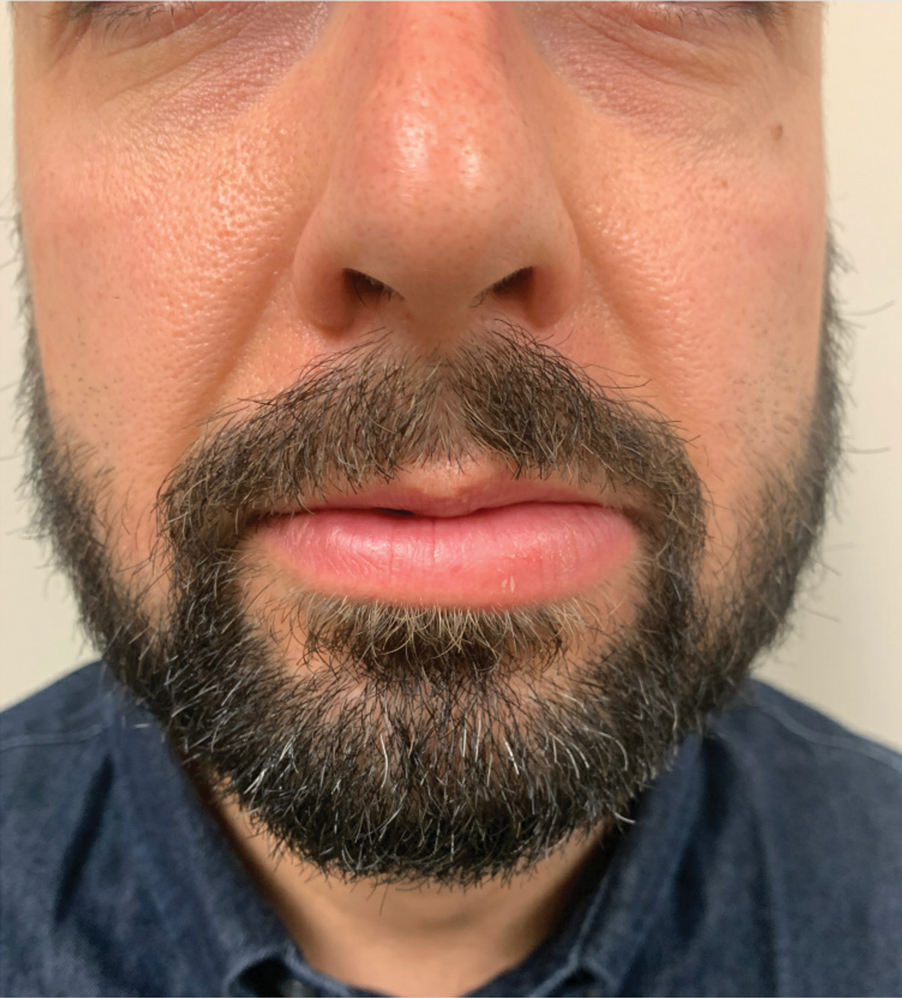
A punch biopsy of the lip revealed a noncaseating microgranuloma in the submucosa with modest submucosal vascular ectasia and perivascular lymphoplasmacytic infiltrates (Figure). Comprehensive metabolic panel, complete blood cell count, angiotensinconverting enzyme (ACE) levels, and inflammatory markers (ie, erythrocyte sedimentation rate, C-reactive protein) all were within reference range. A serum environmental allergen test was negative except for ragweed. Levels of complements—C1 esterase inhibitor (C1-INH) antigen and function, C1q, C3, and C4—and antinuclear antibodies all were normal. Chest radiography was unremarkable. In lieu of a colonoscopy, a fecal calprotectin obtained by gastroenterology was normal. Given the clinical presentation and histopathologic findings, a diagnosis of granulomatous cheilitis (GC) was made.
Granulomatous cheilitis (also known as Miescher cheilitis) is an idiopathic condition characterized by recurrent or persistent swelling of one or both lips. Granulomatous cheilitis usually is an isolated finding but can occur in the setting of Melkersson-Rosenthal syndrome, which refers to a triad of orofacial swelling, facial paralysis, and fissured tongue. Orofacial granulomatosis is a unifying term for any orofacial swelling associated with histologic findings of noncaseating granulomas without evidence of a systemic disease.
Granulomatous cheilitis is a rare disease that most commonly occurs in young adults without any sex predilection.1 The etiology still is unknown, but genetic predisposition, idiopathic influx of inflammatory cells, sensitivity to food or dental materials, and infections have been implicated.2 Granulomatous cheilitis initially presents as soft, nonerythematous, nontender swelling affecting one or both lips. The first episode usually resolves in hours or days, but the frequency and duration of the attacks may increase until the swelling becomes persistent and indurated.3 Granulomatous cheilitis often is a diagnosis of exclusion. A tissue biopsy may show noncaseating epithelioid and multinucleated giant cells with associated lymphedema and fibrosis4; however, histologic findings may be nonspecific, especially early in the disease course, and may be indistinguishable from those of other granulomatous diseases such as sarcoidosis and Crohn disease (CD).5
Lip swelling may be an oral manifestation of CD. Compared with GC, however, CD more commonly is associated with ulcerations, buccal sulcus involvement, abnormalities in complete blood cell count such as anemia and thrombocytosis, and elevated C-reactive protein and erythrocyte sedimentation rate. Although infrequent, GC may coincide with or precede the onset of CD.6 Thus, a detailed gastrointestinal history and appropriate laboratory tests are needed to rule out undiagnosed CD. Nevertheless, performing a routine colonoscopy in the absence of gastrointestinal symptoms is debated.7,8
Sarcoidosis is a systemic granulomatous disease that can have oral involvement in the form of edema, nodules, or ulcers. Oral sarcoidosis usually occurs in patients with chronic multisystemic sarcoidosis and likely is accompanied by pulmonary manifestations such as hilar adenopathy and infiltrates on chest radiography, which are found in more than 90% of patients with sarcoidosis.9,10 A diagnosis of sarcoidosis is additionally supported by other organ involvement such as the joints, skin, or eyes, as well as elevated ACE and calcium levels.
Foreign bodies are another source of granulomatous inflammation and may present with nonspecific findings of swelling, masses, erythema, pain, or ulceration in oral tissues.11 Foreign body reactions to dental materials, retained sutures, and cosmetic fillers have been reported.12-14 In many cases, the foreign material is evident on biopsy.
Angioedema may mimic GC and should be excluded before more extensive testing is done, as it can result in life-threatening respiratory compromise. Numerous etiologies of angioedema have been identified including allergens, acquired or hereditary C1-INH deficiency, nonsteroidal anti-inflammatory drugs, ACE inhibitors, autoimmune disorders, and chronic infections.15 Patients with angioedema may have abnormalities in C4 and C1-INH levels or report certain medication use, allergen exposure, or family history of unexplained recurrent swellings or gastrointestinal symptoms.
There currently is no established treatment of GC due to the unclear etiology and unpredictable clinical course that can lead to spontaneous remissions or frequent recurrences. Corticosteroids administered systemically, intralesionally, or topically have been the mainstay treatment of GC.2 In particular, intralesional injections have been reported as effective in reducing swelling and preventing recurrences in several studies.16,17 Numerous other treatments have been reported in the literature with inconsistent outcomes, including antibiotics such as minocycline, metronidazole, and roxithromycin; clofazimine; thalidomide; immunomodulators such as tumor necrosis factor inhibitors and methotrexate; fumaric acid esters; and cheiloplasty in severe cases.16 Our patient showed near-complete resolution of the lip swelling after a single intralesional injection of 0.5 cc of triamcinolone acetonide 5 mg/mL. The patient has since received 5 additional maintenance injections of 0.1 to 0.2 cc of triamcinolone acetonide 2.5 to 5 mg/mL spaced 2 to 4 months apart with excellent control of the lip swelling, which the patient feels has resolved. We anticipate that repeated injections and monitoring of recurrences may be required for long-term remission.
- McCartan BE, Healy CM, McCreary CE, et al. Characteristics of patients with orofacial granulomatosis. Oral Dis. 2011;17:696-704.
- Grave B, McCullough M, Wiesenfeld D. Orofacial granulomatosis—a 20-year review. Oral Dis. 2009;15:46-51.
- Critchlow WA, Chang D. Cheilitis granulomatosa: a review. Head Neck Pathol. 2014;8:209-213.
- Wiesenfeld D, Ferguson MM, Mitchell DN, et al. Oro-facial granulomatosis—a clinical and pathological analysis. Q J Med. 1985;54:101-113.
- Rogers RS 3rd. Melkersson-Rosenthal syndrome and orofacial granulomatosis. Dermatol Clin. 1996;14:371-379.
- Campbell H, Escudier M, Patel P, et al. Distinguishing orofacial granulomatosis from Crohn’s disease: two separate disease entities? Inflamm Bowel Dis. 2011;17:2109-2115.
- Plauth M, Jenss H, Meyle J. Oral manifestations of Crohn’s disease. an analysis of 79 cases. J Clin Gastroenterol. 1991;13:29-37.
- Van der Waal RI, Schulten EA, van der Meij EH, et al. Cheilitis granulomatosa: overview of 13 patients with long-term follow-up— results of management. Int J Dermatol. 2002;41:225-229.
- Bouaziz A, Le Scanff J, Chapelon-Abric C, et al. Oral involvement in sarcoidosis: report of 12 cases. QJM. 2012;105:755-767.
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736-755.
- Alawi F. An update on granulomatous diseases of the oral tissues. Dent Clin North Am. 2013;57:657-671.
- Stewart CM, Watson RE. Experimental oral foreign body reactions. commonly employed dental materials. Oral Surg Oral Med Oral Pathol. 1990;69:713-719.
- Selvig KA, Biagiotti GR, Leknes KN, et al. Oral tissue reactions to suture materials. Int J Periodontics Restorative Dent. 1998;18:474-487.
- Jham BC, Nikitakis NG, Scheper MA, et al. Granulomatous foreignbody reaction involving oral and perioral tissues after injection of biomaterials: a series of 7 cases and review of the literature. J Oral Maxillofac Surg. 2009;67:280-285.
- Zingale LC, Beltrami L, Zanichelli A, et al. Angioedema without urticaria: a large clinical survey. CMAJ. 2006;175:1065-1070.
- Banks T, Gada S. A comprehensive review of current treatments for granulomatous cheilitis. Br J Dermatol. 2012;166:934-937.
- Fedele S, Fung PP, Bamashmous N, et al. Long-term effectiveness of intralesional triamcinolone acetonide therapy in orofacial granulomatosis: an observational cohort study. Br J Dermatol. 2014;170:794-801.
The Diagnosis: Granulomatous Cheilitis
A punch biopsy of the lip revealed a noncaseating microgranuloma in the submucosa with modest submucosal vascular ectasia and perivascular lymphoplasmacytic infiltrates (Figure). Comprehensive metabolic panel, complete blood cell count, angiotensinconverting enzyme (ACE) levels, and inflammatory markers (ie, erythrocyte sedimentation rate, C-reactive protein) all were within reference range. A serum environmental allergen test was negative except for ragweed. Levels of complements—C1 esterase inhibitor (C1-INH) antigen and function, C1q, C3, and C4—and antinuclear antibodies all were normal. Chest radiography was unremarkable. In lieu of a colonoscopy, a fecal calprotectin obtained by gastroenterology was normal. Given the clinical presentation and histopathologic findings, a diagnosis of granulomatous cheilitis (GC) was made.

Granulomatous cheilitis (also known as Miescher cheilitis) is an idiopathic condition characterized by recurrent or persistent swelling of one or both lips. Granulomatous cheilitis usually is an isolated finding but can occur in the setting of Melkersson-Rosenthal syndrome, which refers to a triad of orofacial swelling, facial paralysis, and fissured tongue. Orofacial granulomatosis is a unifying term for any orofacial swelling associated with histologic findings of noncaseating granulomas without evidence of a systemic disease.
Granulomatous cheilitis is a rare disease that most commonly occurs in young adults without any sex predilection.1 The etiology still is unknown, but genetic predisposition, idiopathic influx of inflammatory cells, sensitivity to food or dental materials, and infections have been implicated.2 Granulomatous cheilitis initially presents as soft, nonerythematous, nontender swelling affecting one or both lips. The first episode usually resolves in hours or days, but the frequency and duration of the attacks may increase until the swelling becomes persistent and indurated.3 Granulomatous cheilitis often is a diagnosis of exclusion. A tissue biopsy may show noncaseating epithelioid and multinucleated giant cells with associated lymphedema and fibrosis4; however, histologic findings may be nonspecific, especially early in the disease course, and may be indistinguishable from those of other granulomatous diseases such as sarcoidosis and Crohn disease (CD).5
Lip swelling may be an oral manifestation of CD. Compared with GC, however, CD more commonly is associated with ulcerations, buccal sulcus involvement, abnormalities in complete blood cell count such as anemia and thrombocytosis, and elevated C-reactive protein and erythrocyte sedimentation rate. Although infrequent, GC may coincide with or precede the onset of CD.6 Thus, a detailed gastrointestinal history and appropriate laboratory tests are needed to rule out undiagnosed CD. Nevertheless, performing a routine colonoscopy in the absence of gastrointestinal symptoms is debated.7,8
Sarcoidosis is a systemic granulomatous disease that can have oral involvement in the form of edema, nodules, or ulcers. Oral sarcoidosis usually occurs in patients with chronic multisystemic sarcoidosis and likely is accompanied by pulmonary manifestations such as hilar adenopathy and infiltrates on chest radiography, which are found in more than 90% of patients with sarcoidosis.9,10 A diagnosis of sarcoidosis is additionally supported by other organ involvement such as the joints, skin, or eyes, as well as elevated ACE and calcium levels.
Foreign bodies are another source of granulomatous inflammation and may present with nonspecific findings of swelling, masses, erythema, pain, or ulceration in oral tissues.11 Foreign body reactions to dental materials, retained sutures, and cosmetic fillers have been reported.12-14 In many cases, the foreign material is evident on biopsy.
Angioedema may mimic GC and should be excluded before more extensive testing is done, as it can result in life-threatening respiratory compromise. Numerous etiologies of angioedema have been identified including allergens, acquired or hereditary C1-INH deficiency, nonsteroidal anti-inflammatory drugs, ACE inhibitors, autoimmune disorders, and chronic infections.15 Patients with angioedema may have abnormalities in C4 and C1-INH levels or report certain medication use, allergen exposure, or family history of unexplained recurrent swellings or gastrointestinal symptoms.
There currently is no established treatment of GC due to the unclear etiology and unpredictable clinical course that can lead to spontaneous remissions or frequent recurrences. Corticosteroids administered systemically, intralesionally, or topically have been the mainstay treatment of GC.2 In particular, intralesional injections have been reported as effective in reducing swelling and preventing recurrences in several studies.16,17 Numerous other treatments have been reported in the literature with inconsistent outcomes, including antibiotics such as minocycline, metronidazole, and roxithromycin; clofazimine; thalidomide; immunomodulators such as tumor necrosis factor inhibitors and methotrexate; fumaric acid esters; and cheiloplasty in severe cases.16 Our patient showed near-complete resolution of the lip swelling after a single intralesional injection of 0.5 cc of triamcinolone acetonide 5 mg/mL. The patient has since received 5 additional maintenance injections of 0.1 to 0.2 cc of triamcinolone acetonide 2.5 to 5 mg/mL spaced 2 to 4 months apart with excellent control of the lip swelling, which the patient feels has resolved. We anticipate that repeated injections and monitoring of recurrences may be required for long-term remission.
The Diagnosis: Granulomatous Cheilitis
A punch biopsy of the lip revealed a noncaseating microgranuloma in the submucosa with modest submucosal vascular ectasia and perivascular lymphoplasmacytic infiltrates (Figure). Comprehensive metabolic panel, complete blood cell count, angiotensinconverting enzyme (ACE) levels, and inflammatory markers (ie, erythrocyte sedimentation rate, C-reactive protein) all were within reference range. A serum environmental allergen test was negative except for ragweed. Levels of complements—C1 esterase inhibitor (C1-INH) antigen and function, C1q, C3, and C4—and antinuclear antibodies all were normal. Chest radiography was unremarkable. In lieu of a colonoscopy, a fecal calprotectin obtained by gastroenterology was normal. Given the clinical presentation and histopathologic findings, a diagnosis of granulomatous cheilitis (GC) was made.

Granulomatous cheilitis (also known as Miescher cheilitis) is an idiopathic condition characterized by recurrent or persistent swelling of one or both lips. Granulomatous cheilitis usually is an isolated finding but can occur in the setting of Melkersson-Rosenthal syndrome, which refers to a triad of orofacial swelling, facial paralysis, and fissured tongue. Orofacial granulomatosis is a unifying term for any orofacial swelling associated with histologic findings of noncaseating granulomas without evidence of a systemic disease.
Granulomatous cheilitis is a rare disease that most commonly occurs in young adults without any sex predilection.1 The etiology still is unknown, but genetic predisposition, idiopathic influx of inflammatory cells, sensitivity to food or dental materials, and infections have been implicated.2 Granulomatous cheilitis initially presents as soft, nonerythematous, nontender swelling affecting one or both lips. The first episode usually resolves in hours or days, but the frequency and duration of the attacks may increase until the swelling becomes persistent and indurated.3 Granulomatous cheilitis often is a diagnosis of exclusion. A tissue biopsy may show noncaseating epithelioid and multinucleated giant cells with associated lymphedema and fibrosis4; however, histologic findings may be nonspecific, especially early in the disease course, and may be indistinguishable from those of other granulomatous diseases such as sarcoidosis and Crohn disease (CD).5
Lip swelling may be an oral manifestation of CD. Compared with GC, however, CD more commonly is associated with ulcerations, buccal sulcus involvement, abnormalities in complete blood cell count such as anemia and thrombocytosis, and elevated C-reactive protein and erythrocyte sedimentation rate. Although infrequent, GC may coincide with or precede the onset of CD.6 Thus, a detailed gastrointestinal history and appropriate laboratory tests are needed to rule out undiagnosed CD. Nevertheless, performing a routine colonoscopy in the absence of gastrointestinal symptoms is debated.7,8
Sarcoidosis is a systemic granulomatous disease that can have oral involvement in the form of edema, nodules, or ulcers. Oral sarcoidosis usually occurs in patients with chronic multisystemic sarcoidosis and likely is accompanied by pulmonary manifestations such as hilar adenopathy and infiltrates on chest radiography, which are found in more than 90% of patients with sarcoidosis.9,10 A diagnosis of sarcoidosis is additionally supported by other organ involvement such as the joints, skin, or eyes, as well as elevated ACE and calcium levels.
Foreign bodies are another source of granulomatous inflammation and may present with nonspecific findings of swelling, masses, erythema, pain, or ulceration in oral tissues.11 Foreign body reactions to dental materials, retained sutures, and cosmetic fillers have been reported.12-14 In many cases, the foreign material is evident on biopsy.
Angioedema may mimic GC and should be excluded before more extensive testing is done, as it can result in life-threatening respiratory compromise. Numerous etiologies of angioedema have been identified including allergens, acquired or hereditary C1-INH deficiency, nonsteroidal anti-inflammatory drugs, ACE inhibitors, autoimmune disorders, and chronic infections.15 Patients with angioedema may have abnormalities in C4 and C1-INH levels or report certain medication use, allergen exposure, or family history of unexplained recurrent swellings or gastrointestinal symptoms.
There currently is no established treatment of GC due to the unclear etiology and unpredictable clinical course that can lead to spontaneous remissions or frequent recurrences. Corticosteroids administered systemically, intralesionally, or topically have been the mainstay treatment of GC.2 In particular, intralesional injections have been reported as effective in reducing swelling and preventing recurrences in several studies.16,17 Numerous other treatments have been reported in the literature with inconsistent outcomes, including antibiotics such as minocycline, metronidazole, and roxithromycin; clofazimine; thalidomide; immunomodulators such as tumor necrosis factor inhibitors and methotrexate; fumaric acid esters; and cheiloplasty in severe cases.16 Our patient showed near-complete resolution of the lip swelling after a single intralesional injection of 0.5 cc of triamcinolone acetonide 5 mg/mL. The patient has since received 5 additional maintenance injections of 0.1 to 0.2 cc of triamcinolone acetonide 2.5 to 5 mg/mL spaced 2 to 4 months apart with excellent control of the lip swelling, which the patient feels has resolved. We anticipate that repeated injections and monitoring of recurrences may be required for long-term remission.
- McCartan BE, Healy CM, McCreary CE, et al. Characteristics of patients with orofacial granulomatosis. Oral Dis. 2011;17:696-704.
- Grave B, McCullough M, Wiesenfeld D. Orofacial granulomatosis—a 20-year review. Oral Dis. 2009;15:46-51.
- Critchlow WA, Chang D. Cheilitis granulomatosa: a review. Head Neck Pathol. 2014;8:209-213.
- Wiesenfeld D, Ferguson MM, Mitchell DN, et al. Oro-facial granulomatosis—a clinical and pathological analysis. Q J Med. 1985;54:101-113.
- Rogers RS 3rd. Melkersson-Rosenthal syndrome and orofacial granulomatosis. Dermatol Clin. 1996;14:371-379.
- Campbell H, Escudier M, Patel P, et al. Distinguishing orofacial granulomatosis from Crohn’s disease: two separate disease entities? Inflamm Bowel Dis. 2011;17:2109-2115.
- Plauth M, Jenss H, Meyle J. Oral manifestations of Crohn’s disease. an analysis of 79 cases. J Clin Gastroenterol. 1991;13:29-37.
- Van der Waal RI, Schulten EA, van der Meij EH, et al. Cheilitis granulomatosa: overview of 13 patients with long-term follow-up— results of management. Int J Dermatol. 2002;41:225-229.
- Bouaziz A, Le Scanff J, Chapelon-Abric C, et al. Oral involvement in sarcoidosis: report of 12 cases. QJM. 2012;105:755-767.
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736-755.
- Alawi F. An update on granulomatous diseases of the oral tissues. Dent Clin North Am. 2013;57:657-671.
- Stewart CM, Watson RE. Experimental oral foreign body reactions. commonly employed dental materials. Oral Surg Oral Med Oral Pathol. 1990;69:713-719.
- Selvig KA, Biagiotti GR, Leknes KN, et al. Oral tissue reactions to suture materials. Int J Periodontics Restorative Dent. 1998;18:474-487.
- Jham BC, Nikitakis NG, Scheper MA, et al. Granulomatous foreignbody reaction involving oral and perioral tissues after injection of biomaterials: a series of 7 cases and review of the literature. J Oral Maxillofac Surg. 2009;67:280-285.
- Zingale LC, Beltrami L, Zanichelli A, et al. Angioedema without urticaria: a large clinical survey. CMAJ. 2006;175:1065-1070.
- Banks T, Gada S. A comprehensive review of current treatments for granulomatous cheilitis. Br J Dermatol. 2012;166:934-937.
- Fedele S, Fung PP, Bamashmous N, et al. Long-term effectiveness of intralesional triamcinolone acetonide therapy in orofacial granulomatosis: an observational cohort study. Br J Dermatol. 2014;170:794-801.
- McCartan BE, Healy CM, McCreary CE, et al. Characteristics of patients with orofacial granulomatosis. Oral Dis. 2011;17:696-704.
- Grave B, McCullough M, Wiesenfeld D. Orofacial granulomatosis—a 20-year review. Oral Dis. 2009;15:46-51.
- Critchlow WA, Chang D. Cheilitis granulomatosa: a review. Head Neck Pathol. 2014;8:209-213.
- Wiesenfeld D, Ferguson MM, Mitchell DN, et al. Oro-facial granulomatosis—a clinical and pathological analysis. Q J Med. 1985;54:101-113.
- Rogers RS 3rd. Melkersson-Rosenthal syndrome and orofacial granulomatosis. Dermatol Clin. 1996;14:371-379.
- Campbell H, Escudier M, Patel P, et al. Distinguishing orofacial granulomatosis from Crohn’s disease: two separate disease entities? Inflamm Bowel Dis. 2011;17:2109-2115.
- Plauth M, Jenss H, Meyle J. Oral manifestations of Crohn’s disease. an analysis of 79 cases. J Clin Gastroenterol. 1991;13:29-37.
- Van der Waal RI, Schulten EA, van der Meij EH, et al. Cheilitis granulomatosa: overview of 13 patients with long-term follow-up— results of management. Int J Dermatol. 2002;41:225-229.
- Bouaziz A, Le Scanff J, Chapelon-Abric C, et al. Oral involvement in sarcoidosis: report of 12 cases. QJM. 2012;105:755-767.
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736-755.
- Alawi F. An update on granulomatous diseases of the oral tissues. Dent Clin North Am. 2013;57:657-671.
- Stewart CM, Watson RE. Experimental oral foreign body reactions. commonly employed dental materials. Oral Surg Oral Med Oral Pathol. 1990;69:713-719.
- Selvig KA, Biagiotti GR, Leknes KN, et al. Oral tissue reactions to suture materials. Int J Periodontics Restorative Dent. 1998;18:474-487.
- Jham BC, Nikitakis NG, Scheper MA, et al. Granulomatous foreignbody reaction involving oral and perioral tissues after injection of biomaterials: a series of 7 cases and review of the literature. J Oral Maxillofac Surg. 2009;67:280-285.
- Zingale LC, Beltrami L, Zanichelli A, et al. Angioedema without urticaria: a large clinical survey. CMAJ. 2006;175:1065-1070.
- Banks T, Gada S. A comprehensive review of current treatments for granulomatous cheilitis. Br J Dermatol. 2012;166:934-937.
- Fedele S, Fung PP, Bamashmous N, et al. Long-term effectiveness of intralesional triamcinolone acetonide therapy in orofacial granulomatosis: an observational cohort study. Br J Dermatol. 2014;170:794-801.
A 36-year-old man with allergic rhinitis presented with lower lip swelling of several months’ duration. The swelling was persistent and predominantly on the left side of the lower lip but occasionally spread to the entire lower lip. The episodes of increased swelling would last for several days and were not associated with any apparent triggers. He denied any pain, pruritus, or dryness. He noted more drooling from the affected side but denied any associated breathing difficulty or throat discomfort. Treatment with an oral antihistamine provided no relief. He denied any recent nonsteroidal anti-inflammatory drug or angiotensinconverting enzyme inhibitor use. His family history was notable for lupus in his maternal grandmother and maternal aunt. He denied any personal or family history of inflammatory bowel disease or recent gastrointestinal tract symptoms. Physical examination revealed nontender edema in the left side of the lower lip with no surface changes. No warmth or erythema were noted. The tongue and the rest of the oral cavity were unremarkable.
Aquatic Antagonists: Marine Rashes (Seabather’s Eruption and Diver’s Dermatitis)
Background and Clinical Presentation
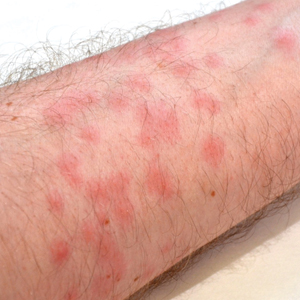
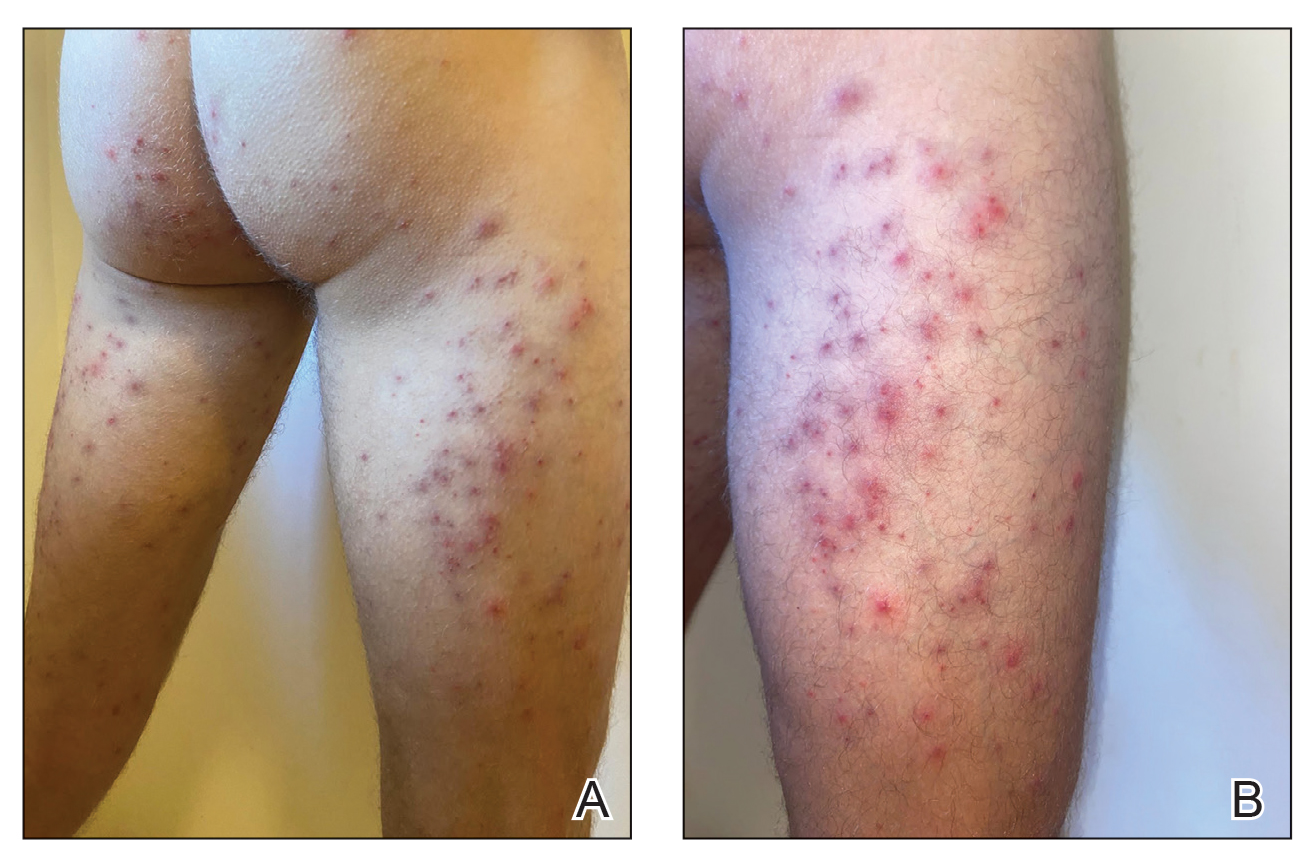
Seabather’s Eruption—Seabather’s eruption is a type I and IV hypersensitivity reaction caused by nematocysts of larval-stage thimble jellyfish (Linuche unguiculata), sea anemones (eg, Edwardsiella lineata), and larval cnidarians.1Linuche unguiculata commonly is found along the southeast coast of the United States and in the Caribbean, the Gulf of Mexico, and the coasts of Florida; less commonly, it has been reported along the coasts of Brazil and Papua New Guinea. Edwardsiella lineata more commonly is seen along the East Coast of the United States.2 Seabather’s eruption presents as numerous scattered, pruritic, red macules and papules (measuring 1 mm to 1.5 cm in size) distributed in areas covered by skin folds, wet clothing, or hair following exposure to marine water (Figure 1). This maculopapular rash generally appears shortly after exiting the water and can last up to several weeks in some cases.3 The cause for this delayed presentation is that the marine organisms become entrapped between the skin of the human contact and another object (eg, swimwear) but do not release their preformed antivenom until they are exposed to air after removal from the water, at which point the organisms die and cell lysis results in injection of the venom.
Diver’s Dermatitis—Diver’s dermatitis (also referred to as “swimmer’s itch”) is a type I and IV hypersensitivity reaction caused by schistosome cercariae released by aquatic snails.4 There are several different cercarial species known to be capable of causing diver dermatitis, but the most commonly implicated genera are Trichobilharzia and Gigantobilharzia. These parasites most commonly are found in freshwater lakes but also occur in oceans, particularly in brackish areas adjacent to freshwater access. Factors associated with increased concentrations of these parasites include shallow, slow-moving water and prolonged onshore wind causing accumulation near the shoreline. It also is thought that the snail host will shed greater concentrations of the parasitic worm in the morning hours and after prolonged exposure to sunlight.4 These flatworm trematodes have a 2-host life cycle. The snails function as intermediate hosts for the parasites before they enter their final host, which are birds. Humans only function as incidental and nonviable hosts for these worms. The parasites gain access to the human body by burrowing into exposed skin. Because the parasite is unable to survive on human hosts, it dies shortly after penetrating the skin, which leads to an intense inflammatory response causing symptoms of pruritus within hours of exposure (Figure 2). The initial eruption progresses over a few days into a diffuse, maculopapular, pruritic rash, similar to that seen in seabather’s eruption. This rash then regresses completely in 1 to 3 weeks. Subsequent exposure to the same parasite is associated with increased severity of future rashes, likely due to antibody-mediated sensitization.4
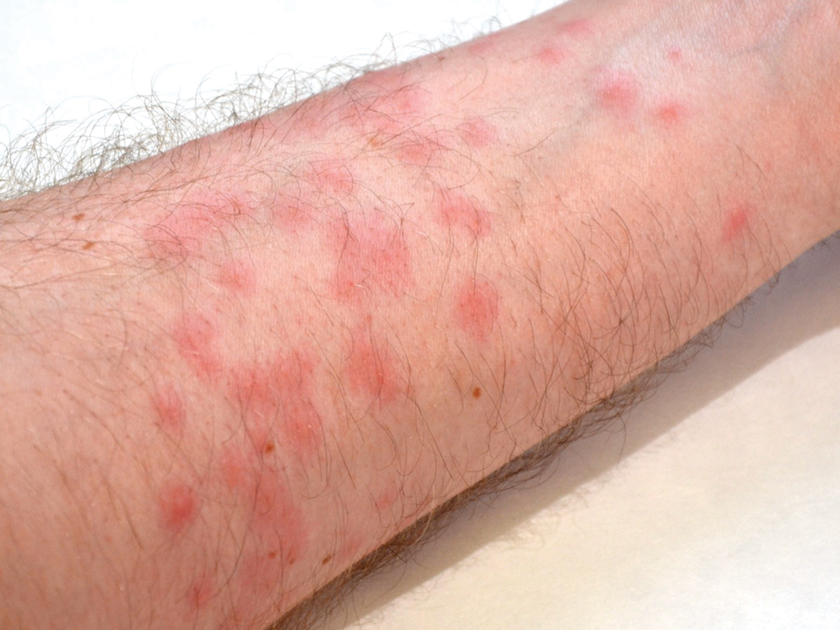
Diagnosis—Marine-derived dermatoses from various sources can present very similarly; thus, it is difficult to discern the specific etiology behind the clinical presentation. No commonly utilized imaging modalities can differentiate between seabather’s eruption and diver’s dermatitis, but eliciting a thorough patient history often can aid in differentiation of the cause of the eruption. For example, lesions located only on nonexposed areas of the skin increases the likelihood of seabather’s eruption due to nematocysts being trapped between clothing and the skin. In contrast, diver’s dermatitis generally appears on areas of the skin that were directly exposed to water and uncovered by clothing.5 Patient reports of a lack of symptoms until shortly after exiting the water further support a diagnosis of seabather’s eruption, as this delayed presentation of symptoms is caused by lysis of the culprit organisms following removal from the marine environment. The cell lysis is responsible for the widespread injection of preformed venom via the numerous nematocysts trapped between clothing and the patient’s body.1
Treatment
For both conditions, the symptoms are treated with hydrocortisone or other topical steroid solutions in conjunction with oral hydroxyzine. Alternative treatments include calamine lotion with 1% menthol and nonsteroidal anti-inflammatory drugs. Taking baths with oatmeal, Epsom salts, or baking soda also may alleviate some of the pruritic symptoms.2
Prevention
The ability to diagnose the precise cause of these similar marine rashes can bring peace of mind to both patients and physicians regardless of their similar management strategies. Severe contact dermatitis of unknown etiology can be disconcerting for patients. Additionally, documenting the causes of marine rashes in particular geographic locations can be beneficial for establishing which organisms are most likely to affect visitors to those areas. This type of data collection can be utilized to develop preventative recommendations, such as deciding when to avoid the water. Education of the public can be done with the use of informational posters located near popular swimming areas and online public service announcements. Informing the general public about the dangers of entering the ocean, especially during certain times of the year when nematocyst-equipped sea creatures are in abundance, could serve to prevent numerous cases of seabather’s eruption. Likewise, advising against immersion in shallow, slow-moving water during the morning hours or after prolonged sun exposure in trematode-endemic areas could prevent numerous cases of diver’s dermatitis. Basic information on what to expect if afflicted by a marine rash also may reduce the number of emergency department visits for these conditions, thus providing economic benefit for patients and for hospitals since patients would better know how to acutely treat these rashes and lessen the patient load at hospital emergency departments. If individuals can assure themselves of the self-limited nature of these types of dermatoses, they may be less inclined to seek medical consultation.
Final Thoughts
As the climate continues to change, the incidence of marine rashes such as seabather’s eruption and diver’s dermatitis is expected to increase due to warmer surface temperatures causing more frequent and earlier blooms of L unguiculata and E lineata. Cases of diver’s dermatitis also could increase due to a longer season of more frequent human exposure from an increase in warmer temperatures. The projected uptick in incidences of these marine rashes makes understanding these pathologies even more pertinent for physicians.6 Increasing our understanding of the different types of marine rashes and their causes will help guide future recommendations for the general public when visiting the ocean.
Future research may wish to investigate unique ways in which to prevent contact between these organisms and humans. Past research on mice indicated that topical application of DEET (N,N-diethyl-meta-toluamide) prior to trematode exposure prevented penetration of the skin by parasitic worms.7 Future studies are needed to examine the effectiveness of this preventative technique on humans. For now, dermatologists may counsel our ocean-going patients on preventative behaviors as well as provide reassurance and symptomatic relief when they present to our clinics with marine rashes.
- Parrish DO. Seabather’s eruption or diver’s dermatitis? JAMA. 1993;270:2300-2301. doi:10.1001/jama.1993.03510190054021
- Tomchik RS, Russell MT, Szmant AM, et al. Clinical perspectives on seabather’s eruption, also known as ‘sea lice’. JAMA. 1993;269:1669-1672. doi:10.1001/jama.1993.03500130083037
- Bonamonte D, Filoni A, Verni P, et al. Dermatitis caused by algae and Bryozoans. In: Bonamonte D, Angelini G, eds. Aquatic Dermatology: Biotic, Chemical, and Physical Agents. Springer; 2016:127-137.
- Tracz ES, Al-Jubury A, Buchmann K, et al. Outbreak of swimmer’s itch in Denmark. Acta Derm Venereol. 2019;99:1116-1120. doi:10.2340/00015555-3309
- Freudenthal AR, Joseph PR. Seabather’s eruption. N Engl J Med. 1993;329:542-544. doi:10.1056/NEJM199308193290805
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. JAAD. 2016;76:140-147. doi:10.1016/j.jaad.2016.08.014
- Salafsky B, Ramaswamy K, He YX, et al. Development and evaluation of LIPODEET, a new long-acting formulation of N, N-diethyl-m-toluamide (DEET) for the prevention of schistosomiasis. Am J Trop Med Hyg. 1999;61:743-750. doi:10.4269/ajtmh.1999.61.743
Background and Clinical Presentation
Seabather’s Eruption—Seabather’s eruption is a type I and IV hypersensitivity reaction caused by nematocysts of larval-stage thimble jellyfish (Linuche unguiculata), sea anemones (eg, Edwardsiella lineata), and larval cnidarians.1Linuche unguiculata commonly is found along the southeast coast of the United States and in the Caribbean, the Gulf of Mexico, and the coasts of Florida; less commonly, it has been reported along the coasts of Brazil and Papua New Guinea. Edwardsiella lineata more commonly is seen along the East Coast of the United States.2 Seabather’s eruption presents as numerous scattered, pruritic, red macules and papules (measuring 1 mm to 1.5 cm in size) distributed in areas covered by skin folds, wet clothing, or hair following exposure to marine water (Figure 1). This maculopapular rash generally appears shortly after exiting the water and can last up to several weeks in some cases.3 The cause for this delayed presentation is that the marine organisms become entrapped between the skin of the human contact and another object (eg, swimwear) but do not release their preformed antivenom until they are exposed to air after removal from the water, at which point the organisms die and cell lysis results in injection of the venom.

Diver’s Dermatitis—Diver’s dermatitis (also referred to as “swimmer’s itch”) is a type I and IV hypersensitivity reaction caused by schistosome cercariae released by aquatic snails.4 There are several different cercarial species known to be capable of causing diver dermatitis, but the most commonly implicated genera are Trichobilharzia and Gigantobilharzia. These parasites most commonly are found in freshwater lakes but also occur in oceans, particularly in brackish areas adjacent to freshwater access. Factors associated with increased concentrations of these parasites include shallow, slow-moving water and prolonged onshore wind causing accumulation near the shoreline. It also is thought that the snail host will shed greater concentrations of the parasitic worm in the morning hours and after prolonged exposure to sunlight.4 These flatworm trematodes have a 2-host life cycle. The snails function as intermediate hosts for the parasites before they enter their final host, which are birds. Humans only function as incidental and nonviable hosts for these worms. The parasites gain access to the human body by burrowing into exposed skin. Because the parasite is unable to survive on human hosts, it dies shortly after penetrating the skin, which leads to an intense inflammatory response causing symptoms of pruritus within hours of exposure (Figure 2). The initial eruption progresses over a few days into a diffuse, maculopapular, pruritic rash, similar to that seen in seabather’s eruption. This rash then regresses completely in 1 to 3 weeks. Subsequent exposure to the same parasite is associated with increased severity of future rashes, likely due to antibody-mediated sensitization.4

Diagnosis—Marine-derived dermatoses from various sources can present very similarly; thus, it is difficult to discern the specific etiology behind the clinical presentation. No commonly utilized imaging modalities can differentiate between seabather’s eruption and diver’s dermatitis, but eliciting a thorough patient history often can aid in differentiation of the cause of the eruption. For example, lesions located only on nonexposed areas of the skin increases the likelihood of seabather’s eruption due to nematocysts being trapped between clothing and the skin. In contrast, diver’s dermatitis generally appears on areas of the skin that were directly exposed to water and uncovered by clothing.5 Patient reports of a lack of symptoms until shortly after exiting the water further support a diagnosis of seabather’s eruption, as this delayed presentation of symptoms is caused by lysis of the culprit organisms following removal from the marine environment. The cell lysis is responsible for the widespread injection of preformed venom via the numerous nematocysts trapped between clothing and the patient’s body.1
Treatment
For both conditions, the symptoms are treated with hydrocortisone or other topical steroid solutions in conjunction with oral hydroxyzine. Alternative treatments include calamine lotion with 1% menthol and nonsteroidal anti-inflammatory drugs. Taking baths with oatmeal, Epsom salts, or baking soda also may alleviate some of the pruritic symptoms.2
Prevention
The ability to diagnose the precise cause of these similar marine rashes can bring peace of mind to both patients and physicians regardless of their similar management strategies. Severe contact dermatitis of unknown etiology can be disconcerting for patients. Additionally, documenting the causes of marine rashes in particular geographic locations can be beneficial for establishing which organisms are most likely to affect visitors to those areas. This type of data collection can be utilized to develop preventative recommendations, such as deciding when to avoid the water. Education of the public can be done with the use of informational posters located near popular swimming areas and online public service announcements. Informing the general public about the dangers of entering the ocean, especially during certain times of the year when nematocyst-equipped sea creatures are in abundance, could serve to prevent numerous cases of seabather’s eruption. Likewise, advising against immersion in shallow, slow-moving water during the morning hours or after prolonged sun exposure in trematode-endemic areas could prevent numerous cases of diver’s dermatitis. Basic information on what to expect if afflicted by a marine rash also may reduce the number of emergency department visits for these conditions, thus providing economic benefit for patients and for hospitals since patients would better know how to acutely treat these rashes and lessen the patient load at hospital emergency departments. If individuals can assure themselves of the self-limited nature of these types of dermatoses, they may be less inclined to seek medical consultation.
Final Thoughts
As the climate continues to change, the incidence of marine rashes such as seabather’s eruption and diver’s dermatitis is expected to increase due to warmer surface temperatures causing more frequent and earlier blooms of L unguiculata and E lineata. Cases of diver’s dermatitis also could increase due to a longer season of more frequent human exposure from an increase in warmer temperatures. The projected uptick in incidences of these marine rashes makes understanding these pathologies even more pertinent for physicians.6 Increasing our understanding of the different types of marine rashes and their causes will help guide future recommendations for the general public when visiting the ocean.
Future research may wish to investigate unique ways in which to prevent contact between these organisms and humans. Past research on mice indicated that topical application of DEET (N,N-diethyl-meta-toluamide) prior to trematode exposure prevented penetration of the skin by parasitic worms.7 Future studies are needed to examine the effectiveness of this preventative technique on humans. For now, dermatologists may counsel our ocean-going patients on preventative behaviors as well as provide reassurance and symptomatic relief when they present to our clinics with marine rashes.
Background and Clinical Presentation
Seabather’s Eruption—Seabather’s eruption is a type I and IV hypersensitivity reaction caused by nematocysts of larval-stage thimble jellyfish (Linuche unguiculata), sea anemones (eg, Edwardsiella lineata), and larval cnidarians.1Linuche unguiculata commonly is found along the southeast coast of the United States and in the Caribbean, the Gulf of Mexico, and the coasts of Florida; less commonly, it has been reported along the coasts of Brazil and Papua New Guinea. Edwardsiella lineata more commonly is seen along the East Coast of the United States.2 Seabather’s eruption presents as numerous scattered, pruritic, red macules and papules (measuring 1 mm to 1.5 cm in size) distributed in areas covered by skin folds, wet clothing, or hair following exposure to marine water (Figure 1). This maculopapular rash generally appears shortly after exiting the water and can last up to several weeks in some cases.3 The cause for this delayed presentation is that the marine organisms become entrapped between the skin of the human contact and another object (eg, swimwear) but do not release their preformed antivenom until they are exposed to air after removal from the water, at which point the organisms die and cell lysis results in injection of the venom.

Diver’s Dermatitis—Diver’s dermatitis (also referred to as “swimmer’s itch”) is a type I and IV hypersensitivity reaction caused by schistosome cercariae released by aquatic snails.4 There are several different cercarial species known to be capable of causing diver dermatitis, but the most commonly implicated genera are Trichobilharzia and Gigantobilharzia. These parasites most commonly are found in freshwater lakes but also occur in oceans, particularly in brackish areas adjacent to freshwater access. Factors associated with increased concentrations of these parasites include shallow, slow-moving water and prolonged onshore wind causing accumulation near the shoreline. It also is thought that the snail host will shed greater concentrations of the parasitic worm in the morning hours and after prolonged exposure to sunlight.4 These flatworm trematodes have a 2-host life cycle. The snails function as intermediate hosts for the parasites before they enter their final host, which are birds. Humans only function as incidental and nonviable hosts for these worms. The parasites gain access to the human body by burrowing into exposed skin. Because the parasite is unable to survive on human hosts, it dies shortly after penetrating the skin, which leads to an intense inflammatory response causing symptoms of pruritus within hours of exposure (Figure 2). The initial eruption progresses over a few days into a diffuse, maculopapular, pruritic rash, similar to that seen in seabather’s eruption. This rash then regresses completely in 1 to 3 weeks. Subsequent exposure to the same parasite is associated with increased severity of future rashes, likely due to antibody-mediated sensitization.4

Diagnosis—Marine-derived dermatoses from various sources can present very similarly; thus, it is difficult to discern the specific etiology behind the clinical presentation. No commonly utilized imaging modalities can differentiate between seabather’s eruption and diver’s dermatitis, but eliciting a thorough patient history often can aid in differentiation of the cause of the eruption. For example, lesions located only on nonexposed areas of the skin increases the likelihood of seabather’s eruption due to nematocysts being trapped between clothing and the skin. In contrast, diver’s dermatitis generally appears on areas of the skin that were directly exposed to water and uncovered by clothing.5 Patient reports of a lack of symptoms until shortly after exiting the water further support a diagnosis of seabather’s eruption, as this delayed presentation of symptoms is caused by lysis of the culprit organisms following removal from the marine environment. The cell lysis is responsible for the widespread injection of preformed venom via the numerous nematocysts trapped between clothing and the patient’s body.1
Treatment
For both conditions, the symptoms are treated with hydrocortisone or other topical steroid solutions in conjunction with oral hydroxyzine. Alternative treatments include calamine lotion with 1% menthol and nonsteroidal anti-inflammatory drugs. Taking baths with oatmeal, Epsom salts, or baking soda also may alleviate some of the pruritic symptoms.2
Prevention
The ability to diagnose the precise cause of these similar marine rashes can bring peace of mind to both patients and physicians regardless of their similar management strategies. Severe contact dermatitis of unknown etiology can be disconcerting for patients. Additionally, documenting the causes of marine rashes in particular geographic locations can be beneficial for establishing which organisms are most likely to affect visitors to those areas. This type of data collection can be utilized to develop preventative recommendations, such as deciding when to avoid the water. Education of the public can be done with the use of informational posters located near popular swimming areas and online public service announcements. Informing the general public about the dangers of entering the ocean, especially during certain times of the year when nematocyst-equipped sea creatures are in abundance, could serve to prevent numerous cases of seabather’s eruption. Likewise, advising against immersion in shallow, slow-moving water during the morning hours or after prolonged sun exposure in trematode-endemic areas could prevent numerous cases of diver’s dermatitis. Basic information on what to expect if afflicted by a marine rash also may reduce the number of emergency department visits for these conditions, thus providing economic benefit for patients and for hospitals since patients would better know how to acutely treat these rashes and lessen the patient load at hospital emergency departments. If individuals can assure themselves of the self-limited nature of these types of dermatoses, they may be less inclined to seek medical consultation.
Final Thoughts
As the climate continues to change, the incidence of marine rashes such as seabather’s eruption and diver’s dermatitis is expected to increase due to warmer surface temperatures causing more frequent and earlier blooms of L unguiculata and E lineata. Cases of diver’s dermatitis also could increase due to a longer season of more frequent human exposure from an increase in warmer temperatures. The projected uptick in incidences of these marine rashes makes understanding these pathologies even more pertinent for physicians.6 Increasing our understanding of the different types of marine rashes and their causes will help guide future recommendations for the general public when visiting the ocean.
Future research may wish to investigate unique ways in which to prevent contact between these organisms and humans. Past research on mice indicated that topical application of DEET (N,N-diethyl-meta-toluamide) prior to trematode exposure prevented penetration of the skin by parasitic worms.7 Future studies are needed to examine the effectiveness of this preventative technique on humans. For now, dermatologists may counsel our ocean-going patients on preventative behaviors as well as provide reassurance and symptomatic relief when they present to our clinics with marine rashes.
- Parrish DO. Seabather’s eruption or diver’s dermatitis? JAMA. 1993;270:2300-2301. doi:10.1001/jama.1993.03510190054021
- Tomchik RS, Russell MT, Szmant AM, et al. Clinical perspectives on seabather’s eruption, also known as ‘sea lice’. JAMA. 1993;269:1669-1672. doi:10.1001/jama.1993.03500130083037
- Bonamonte D, Filoni A, Verni P, et al. Dermatitis caused by algae and Bryozoans. In: Bonamonte D, Angelini G, eds. Aquatic Dermatology: Biotic, Chemical, and Physical Agents. Springer; 2016:127-137.
- Tracz ES, Al-Jubury A, Buchmann K, et al. Outbreak of swimmer’s itch in Denmark. Acta Derm Venereol. 2019;99:1116-1120. doi:10.2340/00015555-3309
- Freudenthal AR, Joseph PR. Seabather’s eruption. N Engl J Med. 1993;329:542-544. doi:10.1056/NEJM199308193290805
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. JAAD. 2016;76:140-147. doi:10.1016/j.jaad.2016.08.014
- Salafsky B, Ramaswamy K, He YX, et al. Development and evaluation of LIPODEET, a new long-acting formulation of N, N-diethyl-m-toluamide (DEET) for the prevention of schistosomiasis. Am J Trop Med Hyg. 1999;61:743-750. doi:10.4269/ajtmh.1999.61.743
- Parrish DO. Seabather’s eruption or diver’s dermatitis? JAMA. 1993;270:2300-2301. doi:10.1001/jama.1993.03510190054021
- Tomchik RS, Russell MT, Szmant AM, et al. Clinical perspectives on seabather’s eruption, also known as ‘sea lice’. JAMA. 1993;269:1669-1672. doi:10.1001/jama.1993.03500130083037
- Bonamonte D, Filoni A, Verni P, et al. Dermatitis caused by algae and Bryozoans. In: Bonamonte D, Angelini G, eds. Aquatic Dermatology: Biotic, Chemical, and Physical Agents. Springer; 2016:127-137.
- Tracz ES, Al-Jubury A, Buchmann K, et al. Outbreak of swimmer’s itch in Denmark. Acta Derm Venereol. 2019;99:1116-1120. doi:10.2340/00015555-3309
- Freudenthal AR, Joseph PR. Seabather’s eruption. N Engl J Med. 1993;329:542-544. doi:10.1056/NEJM199308193290805
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. JAAD. 2016;76:140-147. doi:10.1016/j.jaad.2016.08.014
- Salafsky B, Ramaswamy K, He YX, et al. Development and evaluation of LIPODEET, a new long-acting formulation of N, N-diethyl-m-toluamide (DEET) for the prevention of schistosomiasis. Am J Trop Med Hyg. 1999;61:743-750. doi:10.4269/ajtmh.1999.61.743
Practice Points
- Seabather’s eruption and diver’s dermatitis have similar clinical presentations but differ in the ways that organisms come in contact with the skin.
- No commonly utilized imaging modality can differentiate between seabather’s eruption and diver’s dermatitis, but eliciting a thorough history often can aid in differentiating these marine rashes.
- Physicians should understand the pathologies of common marine rashes due to a projected uptick in the number of cases related to climate change.
Bleeding Nodule on the Lip
The Diagnosis: Metastatic Clear Cell Renal Cell Carcinoma
Renal cell carcinoma (RCC) is a common genitourinary system malignancy with incidence peaking between 50 and 70 years of age and a male predominance.1 The clear cell variant is the most common subtype of RCC, accounting for 70% to 75% of all cases. It is known to be a highly aggressive malignancy that frequently metastasizes to the lungs, lymphatics, bones, liver, and brain.2,3 Approximately 20% to 50% of patients with RCC eventually will develop metastasis after nephrectomy.4 Survival with metastatic RCC to any site typically is in the range of 10 to 22 months.5,6 Cutaneous metastases of RCC rarely have been reported in the literature (3%–6% of cases7) and most commonly are found on the scalp, followed by the chest or abdomen. 8 Cutaneous metastases generally are regarded as a late manifestation of the disease with a very poor prognosis. 9 It is unusual to identify cutaneous RCC metastasis without known RCC or other symptoms consistent with advanced RCC, such as hematuria or abdominal/flank pain. Renal cell carcinoma accounts for an estimated 6% to 7% of all cutaneous metastatic lesions.10 Cutaneous metastatic lesions of RCC often are solitary and grow rapidly, with the clinical appearance of an erythematous or violaceous, nodular, highly vascular, and often hemorrhagic growth.9,11,12
Following the histologic diagnosis of metastatic clear cell RCC, our patient was referred to medical oncology for further workup. Magnetic resonance imaging and a positron emission tomography scan demonstrated widespread disease with a 7-cm left renal mass, liver and lung metastases, and bilateral mediastinal lymphadenopathy. The patient was started on combination immunotherapy as a palliative treatment given the widespread disease.
Histologically, clear cell RCC is characterized by lipid and glycogen-rich cells with ample cytoplasm and a well-developed vascular network, which often is thin walled with a chicken wire–like architecture. Metastatic clear cell RCC tumor cells may form glandular, acinar, or papillary structures with variable lymphocytic inflammatory infiltrates and abundant capillary formation. Immunohistochemically, the tumor cells should demonstrate positivity for paired box gene 8, PAX8, and RCC marker antigen.13 Vimentin and carcinoembryonic antigen may be utilized to distinguish from hidradenoma as carcinoembryonic antigen will be positive in hidradenoma and vimentin will be negative.14 Renal cell carcinoma also has a common molecular signature of von Hippel-Lindau tumor suppressor gene inactivation as well as upregulation of hypoxia inducible factor and vascular endothelial growth factor.15
Balloon cell nevi often clinically present in young patients as bicolored nevi that sometimes are polypoid or verrucous in appearance with central yellow globules surrounded by a peripheral reticular pattern on dermoscopy. Histologically, balloon cell nevi are characterized by large cells with small, round, centrally located basophilic nuclei and clear foamy cytoplasm (Figure 1), which are thought to be formed by progressive vacuolization of melanocytes due to the enlargement and disintegration of melanosomes. This ballooning change reflects an seen in malignant melanoma, in which case nuclear pleomorphism, atypia, and increased mitotic activity also are observed. The prominent vascular network characteristic of RCC typically is not present.16
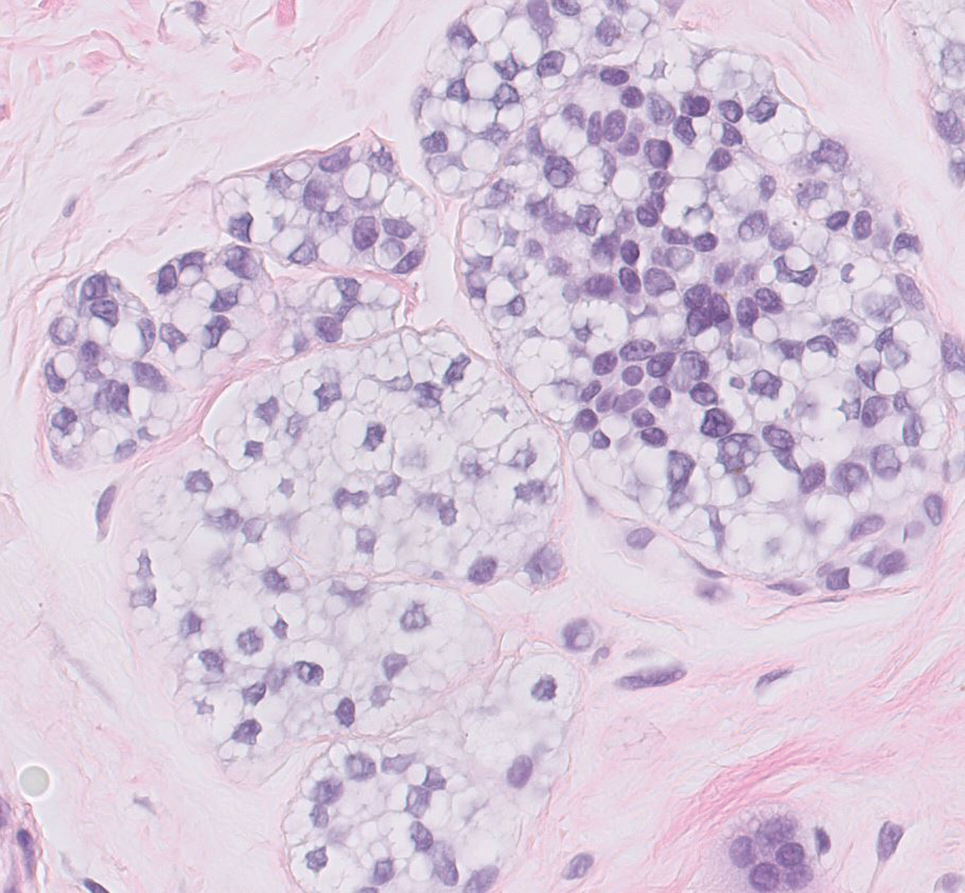
Clear cell hidradenomas are benign skin appendage tumors that often present as small, firm, solitary dermal nodules that may extend into the subcutaneous fat. They have a predilection for the head, face, and arms and demonstrate 2 predominant cell types, including a polyhedral cell with a rounded nucleus and slightly basophilic cytoplasm as well as a round cell with clear cytoplasm and bland nuclei (Figure 2). The latter cell type is less common, representing the predominant cell type in less than one-third of hidradenomas, and can present a diagnostic quandary based on histologic similarity to other clear cell neoplasms. The clear cells contain glycogen but no lipid. Ductlike structures often are present, and the intervening stroma varies from delicate vascularized cords of fibrous tissue to dense hyalinized collagen. Immunohistochemistry may be required for definitive diagnosis, and clear cell hidradenomas should react with monoclonal antibodies that label both eccrine and apocrine secretory elements, such as cytokeratins 6/18, 7, and 8/18.17
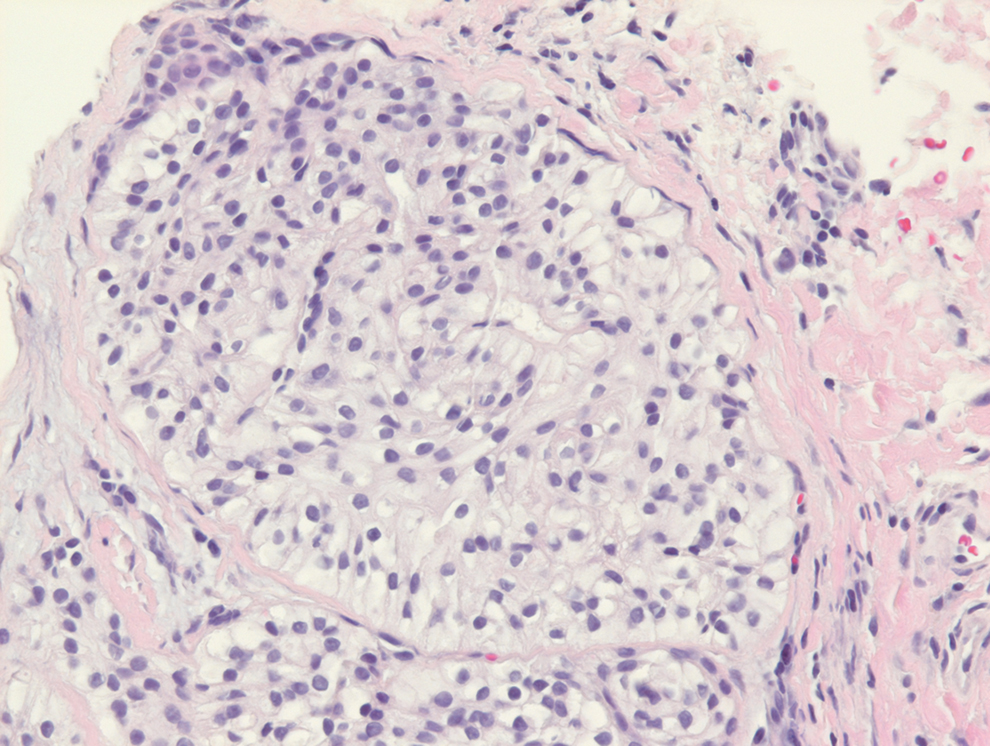
Pyogenic granulomas (also referred to as lobular capillary hemangiomas) are common and present clinically as rapidly growing, polypoid, red masses surrounded by a thickened epidermis that often are found on the fingers or lips. This entity is benign and often regresses spontaneously. Histologically, pyogenic granulomas are characterized by a lobular pattern of vascular proliferation associated with edema and inflammation resembling granulation tissue, with acanthosis and hyperkeratosis at the edges of the lesion (Figure 3).18
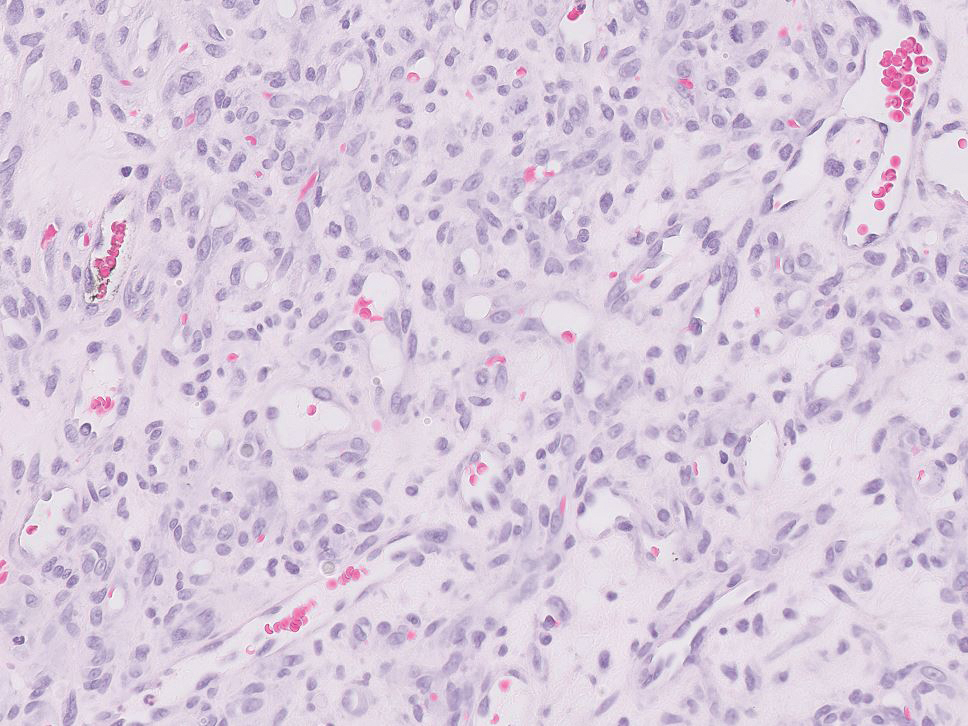
Sebaceous carcinoma is a locally aggressive malignant neoplasm arising from the cells of the sebaceous glands and occurring most commonly in the periorbital area. This neoplasm most often affects older adults, with a mean age at diagnosis of 63 to 77 years. It commonly presents as a solitary nodule with yellowish discoloration and madarosis, which is a key distinguishing feature to differentiate this entity from a chalazion or hordeolum. Histologically, sebaceous carcinoma is a dermal-based infiltrative, nodular tumor with varying degrees of clear cell changes—well-differentiated tumors show more clear cell change as compared to more poorly differentiated variants—along with basaloid or squamous features and abundant mitotic activity (Figure 4), which may be useful in distinguishing it from the other entities in the clear cell neoplasm differential.19-22
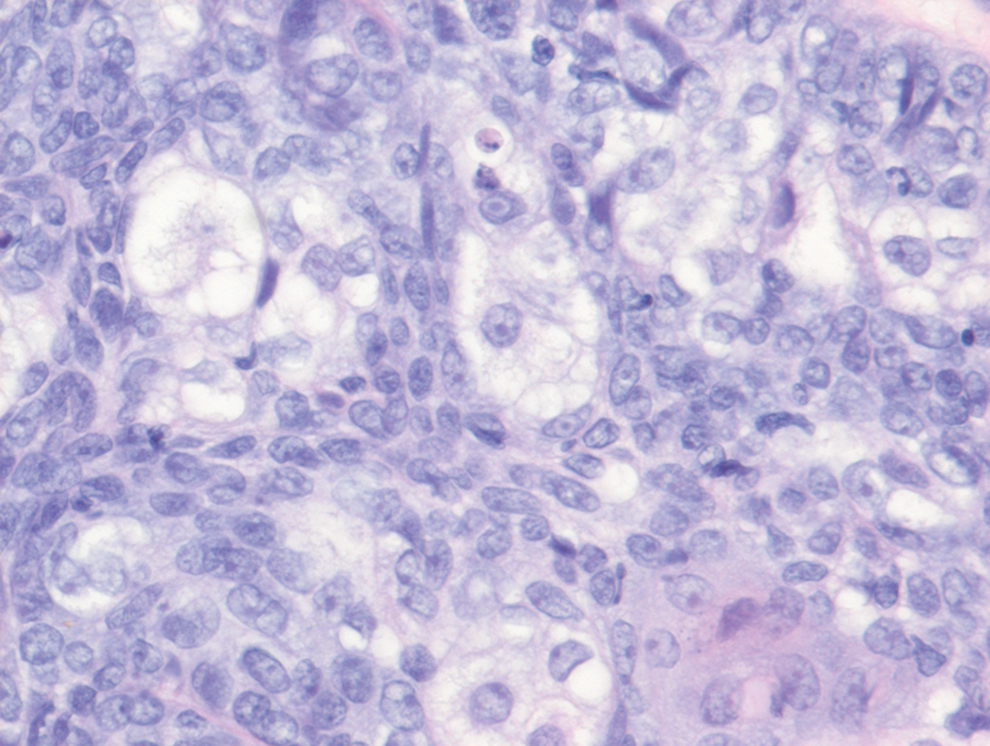
- Alves de Paula T, Lopes da Silva P, Sueth Berriel LG. Renal cell carcinoma with cutaneous metastasis: case report. J Bras Nefrol. 2010;32:213-215.
- Amaadour L, Atreche L, Azegrar M, et al. Cutaneous metastasis of renal cell carcinoma: a case report. J Cancer Ther. 2017;8:603-607.
- Weiss L, Harlos JP, Torhorst J, et al. Metastatic patterns of renal carcinoma: an analysis of 687 necropsies. J Cancer Res Clin Oncol. 1988;114:605-612.
- Flamigan RC, Campbell SC, Clark JI, et al. Metastatic renal cell carcinoma. Curr Treat Options Oncol. 2003;4:385-390.
- Motzer RJ, Bacik J, Schwarz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:453-463.
- Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor–targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5694-5799.
- Smyth LG, Rowan GC, David MQ. Renal cell carcinoma presenting as an ominous metachronous scalp metastasis. Can Urol Assoc J. 2010;4:E64-E66.
- Dorairajan LN, Hemal AK, Aron M, et al. Cutaneous metastases in renal cell carcinoma. Urol Int. 1999;63:164-167.
- Koga S, Tsuda S, Nishikido M, et al. Renal cell carcinoma metastatic to the skin. Anticancer Res. 2000;20:1939-1940.
- Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a metaanalysis of the data. South Med J. 2003;96:164-167.
- Amano Y, Ohni S, Ishige T, et al. A case of cutaneous metastasis from a clear cell renal cell carcinoma with an eosinophilic cell component to the submandibular region. J Nihon Univ Med Assoc. 2015;74:73-77.
- Arrabal-Polo MA, Arias-Santiago SA, Aneiros-Fernandez J, et al. Cutaneous metastases in renal cell carcinoma: a case report. Cases J. 2009;2:7948.
- Sangoi AR, Karamchandani J, Kim J, et al. The use of immunohistochemistry in the diagnosis of metastatic clear cell renal cell carcinoma: a review of PAX-8, PAX-2, hKIM-1, RCCma, and CD10. Adv Anat Pathol. 2010;17:377-393.
- Velez MJ, Thomas CL, Stratton J, et al. The utility of using immunohistochemistry in the differentiation of metastatic, cutaneous clear cell renal cell carcinoma and clear cell hidradenoma. J Cutan Pathol. 2017;44:612-615.
- Nezami BG, MacLennan G. Clear cell. PathologyOutlines website. Published April 20, 2021. Updated March 2, 2022. Accessed April 22, 2022. https://www.pathologyoutlines.com/topic/kidneytumormalignantrccclear.html
- Dhaille F, Courville P, Joly P, et al. Balloon cell nevus: histologic and dermoscopic features. J Am Acad Dermatol. 2015;72:E55-E56.
- Volmar KE, Cummings TJ, Wang WH, et al. Clear cell hidradenoma: a mimic of metastatic clear cell tumors. Arch Pathol Lab Med. 2005;129:E113-E116.
- Hale CS. Capillary/pyogenic granuloma. Pathology Outlines website. Published August 1, 2012. Updated March 10, 2022. Accessed April 20, 2022. https://www.pathologyoutlines.com/topic/skintumornonmelanocyticpyogenicgranuloma.html
- Zada S, Lee BA. Sebaceous carcinoma. Pathology Outlines website. Published August 11, 2021. Accessed April 20, 2022. https://www.pathologyoutlines.com/topic/skintumornonmelanocyticsebaceouscarcinoma.html
- Kahana A, Pribila, JT, Nelson CC, et al. Sebaceous cell carcinoma. In: Levin LA, Albert DM, eds. Ocular Disease: Mechanisms and Management. Elsevier; 2010:396-407.
- Wick MR. Cutaneous tumors and pseudotumors of the head and neck. In: Gnepp DR, ed. Diagnostic Surgical Pathology of the Head and Neck. 2nd ed. Saunders Elsevier; 2009:975-1068.
- Cassarino DS, Dadras SS, Lindberg MR, et al. Sebaceous carcinoma. In: Cassarino DS, Dadras SS, Lindberg MR, et al, eds. Diagnostic Pathology: Neoplastic Dermatopathology. 2nd ed. Elsevier; 2017:174-179.
The Diagnosis: Metastatic Clear Cell Renal Cell Carcinoma
Renal cell carcinoma (RCC) is a common genitourinary system malignancy with incidence peaking between 50 and 70 years of age and a male predominance.1 The clear cell variant is the most common subtype of RCC, accounting for 70% to 75% of all cases. It is known to be a highly aggressive malignancy that frequently metastasizes to the lungs, lymphatics, bones, liver, and brain.2,3 Approximately 20% to 50% of patients with RCC eventually will develop metastasis after nephrectomy.4 Survival with metastatic RCC to any site typically is in the range of 10 to 22 months.5,6 Cutaneous metastases of RCC rarely have been reported in the literature (3%–6% of cases7) and most commonly are found on the scalp, followed by the chest or abdomen. 8 Cutaneous metastases generally are regarded as a late manifestation of the disease with a very poor prognosis. 9 It is unusual to identify cutaneous RCC metastasis without known RCC or other symptoms consistent with advanced RCC, such as hematuria or abdominal/flank pain. Renal cell carcinoma accounts for an estimated 6% to 7% of all cutaneous metastatic lesions.10 Cutaneous metastatic lesions of RCC often are solitary and grow rapidly, with the clinical appearance of an erythematous or violaceous, nodular, highly vascular, and often hemorrhagic growth.9,11,12
Following the histologic diagnosis of metastatic clear cell RCC, our patient was referred to medical oncology for further workup. Magnetic resonance imaging and a positron emission tomography scan demonstrated widespread disease with a 7-cm left renal mass, liver and lung metastases, and bilateral mediastinal lymphadenopathy. The patient was started on combination immunotherapy as a palliative treatment given the widespread disease.
Histologically, clear cell RCC is characterized by lipid and glycogen-rich cells with ample cytoplasm and a well-developed vascular network, which often is thin walled with a chicken wire–like architecture. Metastatic clear cell RCC tumor cells may form glandular, acinar, or papillary structures with variable lymphocytic inflammatory infiltrates and abundant capillary formation. Immunohistochemically, the tumor cells should demonstrate positivity for paired box gene 8, PAX8, and RCC marker antigen.13 Vimentin and carcinoembryonic antigen may be utilized to distinguish from hidradenoma as carcinoembryonic antigen will be positive in hidradenoma and vimentin will be negative.14 Renal cell carcinoma also has a common molecular signature of von Hippel-Lindau tumor suppressor gene inactivation as well as upregulation of hypoxia inducible factor and vascular endothelial growth factor.15
Balloon cell nevi often clinically present in young patients as bicolored nevi that sometimes are polypoid or verrucous in appearance with central yellow globules surrounded by a peripheral reticular pattern on dermoscopy. Histologically, balloon cell nevi are characterized by large cells with small, round, centrally located basophilic nuclei and clear foamy cytoplasm (Figure 1), which are thought to be formed by progressive vacuolization of melanocytes due to the enlargement and disintegration of melanosomes. This ballooning change reflects an seen in malignant melanoma, in which case nuclear pleomorphism, atypia, and increased mitotic activity also are observed. The prominent vascular network characteristic of RCC typically is not present.16

Clear cell hidradenomas are benign skin appendage tumors that often present as small, firm, solitary dermal nodules that may extend into the subcutaneous fat. They have a predilection for the head, face, and arms and demonstrate 2 predominant cell types, including a polyhedral cell with a rounded nucleus and slightly basophilic cytoplasm as well as a round cell with clear cytoplasm and bland nuclei (Figure 2). The latter cell type is less common, representing the predominant cell type in less than one-third of hidradenomas, and can present a diagnostic quandary based on histologic similarity to other clear cell neoplasms. The clear cells contain glycogen but no lipid. Ductlike structures often are present, and the intervening stroma varies from delicate vascularized cords of fibrous tissue to dense hyalinized collagen. Immunohistochemistry may be required for definitive diagnosis, and clear cell hidradenomas should react with monoclonal antibodies that label both eccrine and apocrine secretory elements, such as cytokeratins 6/18, 7, and 8/18.17

Pyogenic granulomas (also referred to as lobular capillary hemangiomas) are common and present clinically as rapidly growing, polypoid, red masses surrounded by a thickened epidermis that often are found on the fingers or lips. This entity is benign and often regresses spontaneously. Histologically, pyogenic granulomas are characterized by a lobular pattern of vascular proliferation associated with edema and inflammation resembling granulation tissue, with acanthosis and hyperkeratosis at the edges of the lesion (Figure 3).18

Sebaceous carcinoma is a locally aggressive malignant neoplasm arising from the cells of the sebaceous glands and occurring most commonly in the periorbital area. This neoplasm most often affects older adults, with a mean age at diagnosis of 63 to 77 years. It commonly presents as a solitary nodule with yellowish discoloration and madarosis, which is a key distinguishing feature to differentiate this entity from a chalazion or hordeolum. Histologically, sebaceous carcinoma is a dermal-based infiltrative, nodular tumor with varying degrees of clear cell changes—well-differentiated tumors show more clear cell change as compared to more poorly differentiated variants—along with basaloid or squamous features and abundant mitotic activity (Figure 4), which may be useful in distinguishing it from the other entities in the clear cell neoplasm differential.19-22

The Diagnosis: Metastatic Clear Cell Renal Cell Carcinoma
Renal cell carcinoma (RCC) is a common genitourinary system malignancy with incidence peaking between 50 and 70 years of age and a male predominance.1 The clear cell variant is the most common subtype of RCC, accounting for 70% to 75% of all cases. It is known to be a highly aggressive malignancy that frequently metastasizes to the lungs, lymphatics, bones, liver, and brain.2,3 Approximately 20% to 50% of patients with RCC eventually will develop metastasis after nephrectomy.4 Survival with metastatic RCC to any site typically is in the range of 10 to 22 months.5,6 Cutaneous metastases of RCC rarely have been reported in the literature (3%–6% of cases7) and most commonly are found on the scalp, followed by the chest or abdomen. 8 Cutaneous metastases generally are regarded as a late manifestation of the disease with a very poor prognosis. 9 It is unusual to identify cutaneous RCC metastasis without known RCC or other symptoms consistent with advanced RCC, such as hematuria or abdominal/flank pain. Renal cell carcinoma accounts for an estimated 6% to 7% of all cutaneous metastatic lesions.10 Cutaneous metastatic lesions of RCC often are solitary and grow rapidly, with the clinical appearance of an erythematous or violaceous, nodular, highly vascular, and often hemorrhagic growth.9,11,12
Following the histologic diagnosis of metastatic clear cell RCC, our patient was referred to medical oncology for further workup. Magnetic resonance imaging and a positron emission tomography scan demonstrated widespread disease with a 7-cm left renal mass, liver and lung metastases, and bilateral mediastinal lymphadenopathy. The patient was started on combination immunotherapy as a palliative treatment given the widespread disease.
Histologically, clear cell RCC is characterized by lipid and glycogen-rich cells with ample cytoplasm and a well-developed vascular network, which often is thin walled with a chicken wire–like architecture. Metastatic clear cell RCC tumor cells may form glandular, acinar, or papillary structures with variable lymphocytic inflammatory infiltrates and abundant capillary formation. Immunohistochemically, the tumor cells should demonstrate positivity for paired box gene 8, PAX8, and RCC marker antigen.13 Vimentin and carcinoembryonic antigen may be utilized to distinguish from hidradenoma as carcinoembryonic antigen will be positive in hidradenoma and vimentin will be negative.14 Renal cell carcinoma also has a common molecular signature of von Hippel-Lindau tumor suppressor gene inactivation as well as upregulation of hypoxia inducible factor and vascular endothelial growth factor.15
Balloon cell nevi often clinically present in young patients as bicolored nevi that sometimes are polypoid or verrucous in appearance with central yellow globules surrounded by a peripheral reticular pattern on dermoscopy. Histologically, balloon cell nevi are characterized by large cells with small, round, centrally located basophilic nuclei and clear foamy cytoplasm (Figure 1), which are thought to be formed by progressive vacuolization of melanocytes due to the enlargement and disintegration of melanosomes. This ballooning change reflects an seen in malignant melanoma, in which case nuclear pleomorphism, atypia, and increased mitotic activity also are observed. The prominent vascular network characteristic of RCC typically is not present.16

Clear cell hidradenomas are benign skin appendage tumors that often present as small, firm, solitary dermal nodules that may extend into the subcutaneous fat. They have a predilection for the head, face, and arms and demonstrate 2 predominant cell types, including a polyhedral cell with a rounded nucleus and slightly basophilic cytoplasm as well as a round cell with clear cytoplasm and bland nuclei (Figure 2). The latter cell type is less common, representing the predominant cell type in less than one-third of hidradenomas, and can present a diagnostic quandary based on histologic similarity to other clear cell neoplasms. The clear cells contain glycogen but no lipid. Ductlike structures often are present, and the intervening stroma varies from delicate vascularized cords of fibrous tissue to dense hyalinized collagen. Immunohistochemistry may be required for definitive diagnosis, and clear cell hidradenomas should react with monoclonal antibodies that label both eccrine and apocrine secretory elements, such as cytokeratins 6/18, 7, and 8/18.17

Pyogenic granulomas (also referred to as lobular capillary hemangiomas) are common and present clinically as rapidly growing, polypoid, red masses surrounded by a thickened epidermis that often are found on the fingers or lips. This entity is benign and often regresses spontaneously. Histologically, pyogenic granulomas are characterized by a lobular pattern of vascular proliferation associated with edema and inflammation resembling granulation tissue, with acanthosis and hyperkeratosis at the edges of the lesion (Figure 3).18

Sebaceous carcinoma is a locally aggressive malignant neoplasm arising from the cells of the sebaceous glands and occurring most commonly in the periorbital area. This neoplasm most often affects older adults, with a mean age at diagnosis of 63 to 77 years. It commonly presents as a solitary nodule with yellowish discoloration and madarosis, which is a key distinguishing feature to differentiate this entity from a chalazion or hordeolum. Histologically, sebaceous carcinoma is a dermal-based infiltrative, nodular tumor with varying degrees of clear cell changes—well-differentiated tumors show more clear cell change as compared to more poorly differentiated variants—along with basaloid or squamous features and abundant mitotic activity (Figure 4), which may be useful in distinguishing it from the other entities in the clear cell neoplasm differential.19-22

- Alves de Paula T, Lopes da Silva P, Sueth Berriel LG. Renal cell carcinoma with cutaneous metastasis: case report. J Bras Nefrol. 2010;32:213-215.
- Amaadour L, Atreche L, Azegrar M, et al. Cutaneous metastasis of renal cell carcinoma: a case report. J Cancer Ther. 2017;8:603-607.
- Weiss L, Harlos JP, Torhorst J, et al. Metastatic patterns of renal carcinoma: an analysis of 687 necropsies. J Cancer Res Clin Oncol. 1988;114:605-612.
- Flamigan RC, Campbell SC, Clark JI, et al. Metastatic renal cell carcinoma. Curr Treat Options Oncol. 2003;4:385-390.
- Motzer RJ, Bacik J, Schwarz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:453-463.
- Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor–targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5694-5799.
- Smyth LG, Rowan GC, David MQ. Renal cell carcinoma presenting as an ominous metachronous scalp metastasis. Can Urol Assoc J. 2010;4:E64-E66.
- Dorairajan LN, Hemal AK, Aron M, et al. Cutaneous metastases in renal cell carcinoma. Urol Int. 1999;63:164-167.
- Koga S, Tsuda S, Nishikido M, et al. Renal cell carcinoma metastatic to the skin. Anticancer Res. 2000;20:1939-1940.
- Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a metaanalysis of the data. South Med J. 2003;96:164-167.
- Amano Y, Ohni S, Ishige T, et al. A case of cutaneous metastasis from a clear cell renal cell carcinoma with an eosinophilic cell component to the submandibular region. J Nihon Univ Med Assoc. 2015;74:73-77.
- Arrabal-Polo MA, Arias-Santiago SA, Aneiros-Fernandez J, et al. Cutaneous metastases in renal cell carcinoma: a case report. Cases J. 2009;2:7948.
- Sangoi AR, Karamchandani J, Kim J, et al. The use of immunohistochemistry in the diagnosis of metastatic clear cell renal cell carcinoma: a review of PAX-8, PAX-2, hKIM-1, RCCma, and CD10. Adv Anat Pathol. 2010;17:377-393.
- Velez MJ, Thomas CL, Stratton J, et al. The utility of using immunohistochemistry in the differentiation of metastatic, cutaneous clear cell renal cell carcinoma and clear cell hidradenoma. J Cutan Pathol. 2017;44:612-615.
- Nezami BG, MacLennan G. Clear cell. PathologyOutlines website. Published April 20, 2021. Updated March 2, 2022. Accessed April 22, 2022. https://www.pathologyoutlines.com/topic/kidneytumormalignantrccclear.html
- Dhaille F, Courville P, Joly P, et al. Balloon cell nevus: histologic and dermoscopic features. J Am Acad Dermatol. 2015;72:E55-E56.
- Volmar KE, Cummings TJ, Wang WH, et al. Clear cell hidradenoma: a mimic of metastatic clear cell tumors. Arch Pathol Lab Med. 2005;129:E113-E116.
- Hale CS. Capillary/pyogenic granuloma. Pathology Outlines website. Published August 1, 2012. Updated March 10, 2022. Accessed April 20, 2022. https://www.pathologyoutlines.com/topic/skintumornonmelanocyticpyogenicgranuloma.html
- Zada S, Lee BA. Sebaceous carcinoma. Pathology Outlines website. Published August 11, 2021. Accessed April 20, 2022. https://www.pathologyoutlines.com/topic/skintumornonmelanocyticsebaceouscarcinoma.html
- Kahana A, Pribila, JT, Nelson CC, et al. Sebaceous cell carcinoma. In: Levin LA, Albert DM, eds. Ocular Disease: Mechanisms and Management. Elsevier; 2010:396-407.
- Wick MR. Cutaneous tumors and pseudotumors of the head and neck. In: Gnepp DR, ed. Diagnostic Surgical Pathology of the Head and Neck. 2nd ed. Saunders Elsevier; 2009:975-1068.
- Cassarino DS, Dadras SS, Lindberg MR, et al. Sebaceous carcinoma. In: Cassarino DS, Dadras SS, Lindberg MR, et al, eds. Diagnostic Pathology: Neoplastic Dermatopathology. 2nd ed. Elsevier; 2017:174-179.
- Alves de Paula T, Lopes da Silva P, Sueth Berriel LG. Renal cell carcinoma with cutaneous metastasis: case report. J Bras Nefrol. 2010;32:213-215.
- Amaadour L, Atreche L, Azegrar M, et al. Cutaneous metastasis of renal cell carcinoma: a case report. J Cancer Ther. 2017;8:603-607.
- Weiss L, Harlos JP, Torhorst J, et al. Metastatic patterns of renal carcinoma: an analysis of 687 necropsies. J Cancer Res Clin Oncol. 1988;114:605-612.
- Flamigan RC, Campbell SC, Clark JI, et al. Metastatic renal cell carcinoma. Curr Treat Options Oncol. 2003;4:385-390.
- Motzer RJ, Bacik J, Schwarz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:453-463.
- Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor–targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5694-5799.
- Smyth LG, Rowan GC, David MQ. Renal cell carcinoma presenting as an ominous metachronous scalp metastasis. Can Urol Assoc J. 2010;4:E64-E66.
- Dorairajan LN, Hemal AK, Aron M, et al. Cutaneous metastases in renal cell carcinoma. Urol Int. 1999;63:164-167.
- Koga S, Tsuda S, Nishikido M, et al. Renal cell carcinoma metastatic to the skin. Anticancer Res. 2000;20:1939-1940.
- Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a metaanalysis of the data. South Med J. 2003;96:164-167.
- Amano Y, Ohni S, Ishige T, et al. A case of cutaneous metastasis from a clear cell renal cell carcinoma with an eosinophilic cell component to the submandibular region. J Nihon Univ Med Assoc. 2015;74:73-77.
- Arrabal-Polo MA, Arias-Santiago SA, Aneiros-Fernandez J, et al. Cutaneous metastases in renal cell carcinoma: a case report. Cases J. 2009;2:7948.
- Sangoi AR, Karamchandani J, Kim J, et al. The use of immunohistochemistry in the diagnosis of metastatic clear cell renal cell carcinoma: a review of PAX-8, PAX-2, hKIM-1, RCCma, and CD10. Adv Anat Pathol. 2010;17:377-393.
- Velez MJ, Thomas CL, Stratton J, et al. The utility of using immunohistochemistry in the differentiation of metastatic, cutaneous clear cell renal cell carcinoma and clear cell hidradenoma. J Cutan Pathol. 2017;44:612-615.
- Nezami BG, MacLennan G. Clear cell. PathologyOutlines website. Published April 20, 2021. Updated March 2, 2022. Accessed April 22, 2022. https://www.pathologyoutlines.com/topic/kidneytumormalignantrccclear.html
- Dhaille F, Courville P, Joly P, et al. Balloon cell nevus: histologic and dermoscopic features. J Am Acad Dermatol. 2015;72:E55-E56.
- Volmar KE, Cummings TJ, Wang WH, et al. Clear cell hidradenoma: a mimic of metastatic clear cell tumors. Arch Pathol Lab Med. 2005;129:E113-E116.
- Hale CS. Capillary/pyogenic granuloma. Pathology Outlines website. Published August 1, 2012. Updated March 10, 2022. Accessed April 20, 2022. https://www.pathologyoutlines.com/topic/skintumornonmelanocyticpyogenicgranuloma.html
- Zada S, Lee BA. Sebaceous carcinoma. Pathology Outlines website. Published August 11, 2021. Accessed April 20, 2022. https://www.pathologyoutlines.com/topic/skintumornonmelanocyticsebaceouscarcinoma.html
- Kahana A, Pribila, JT, Nelson CC, et al. Sebaceous cell carcinoma. In: Levin LA, Albert DM, eds. Ocular Disease: Mechanisms and Management. Elsevier; 2010:396-407.
- Wick MR. Cutaneous tumors and pseudotumors of the head and neck. In: Gnepp DR, ed. Diagnostic Surgical Pathology of the Head and Neck. 2nd ed. Saunders Elsevier; 2009:975-1068.
- Cassarino DS, Dadras SS, Lindberg MR, et al. Sebaceous carcinoma. In: Cassarino DS, Dadras SS, Lindberg MR, et al, eds. Diagnostic Pathology: Neoplastic Dermatopathology. 2nd ed. Elsevier; 2017:174-179.
A 71-year-old man with no notable medical history presented with a bleeding nodule on the right lower cutaneous lip of 9 weeks’ duration. The patient denied any systemic symptoms. A shave biopsy was performed.
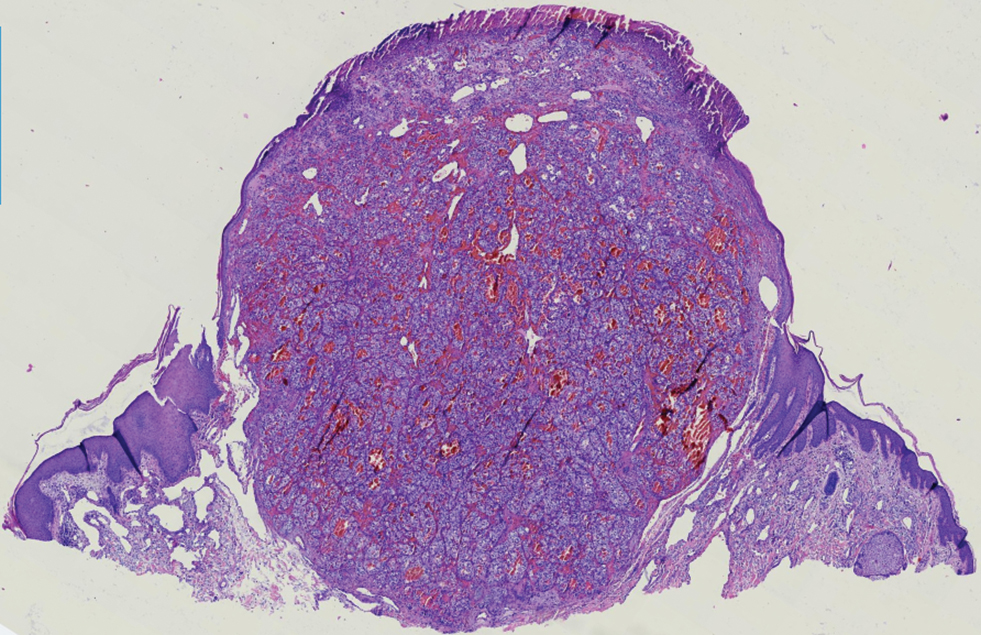
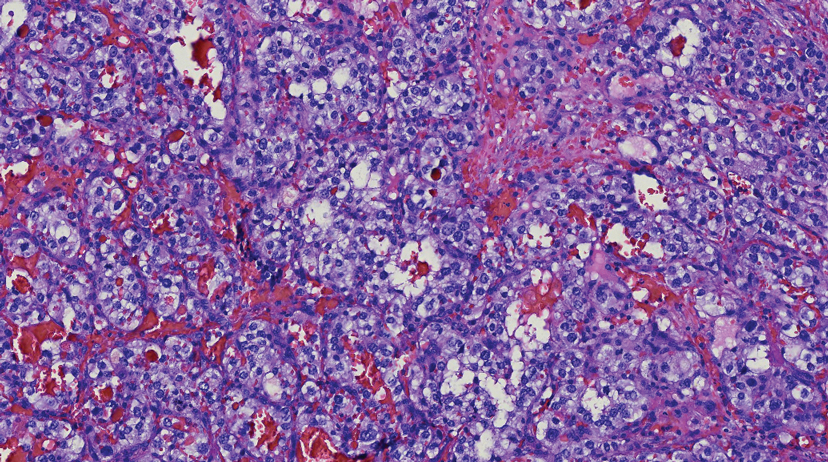
Recurrent Arciform Plaque on the Face
The Diagnosis: Lupus Erythematosus Tumidus
Histopathologic evaluation of a punch biopsy revealed focal dermal mucin deposition and CD123+ discrete clusters of plasmacytoid dendritic cells without interface changes (Figure 1), favoring a diagnosis of lupus erythematosus tumidus (LET) in our patient. There was no clinical improvement in symptoms when she previously was treated with topical antifungals or class III corticosteroid creams. Tacrolimus ointment 0.1% twice daily for 1 month did not result in substantial improvement in the appearance of the plaque, and it spontaneously resolved after 2 to 3 months. She declined treatment with hydroxychloroquine.
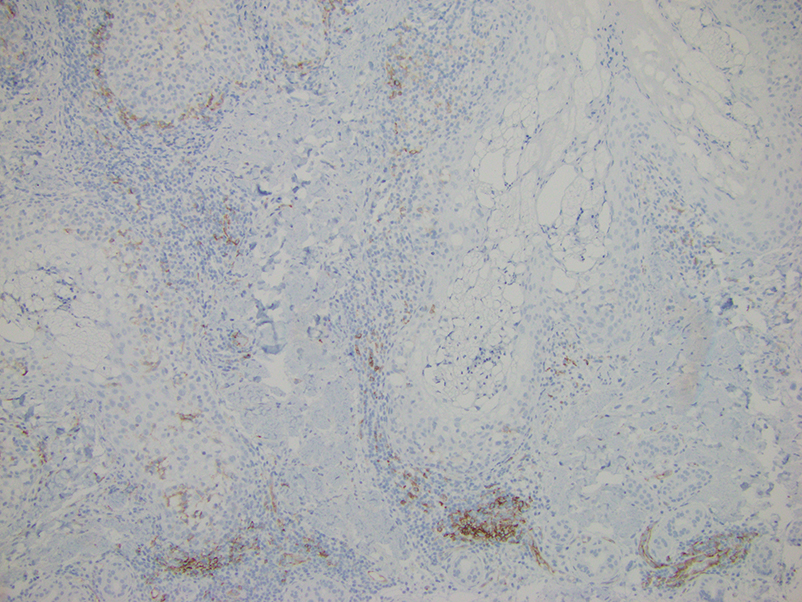
Lupus erythematosus tumidus is an uncommon subtype of chronic cutaneous lupus erythematosus with no distinct etiology. It is clinically characterized by edematous, urticarial, single or multiple plaques with a smooth surface affecting sun-exposed areas that can last for months to years.1 In contrast to other variations of chronic cutaneous lupus such as discoid lupus erythematosus, LET lesions lack surface papulosquamous features such as scaling, atrophy, and follicular plugging.1-4 Based solely on histologic findings, LET may be indistinguishable from reticular erythematous mucinosis and Jessner lymphocytic infiltration of the skin (JLIS) due to a similar lack of epidermal involvement and presence of a perivascular lymphocytic infiltrate (Figure 2).
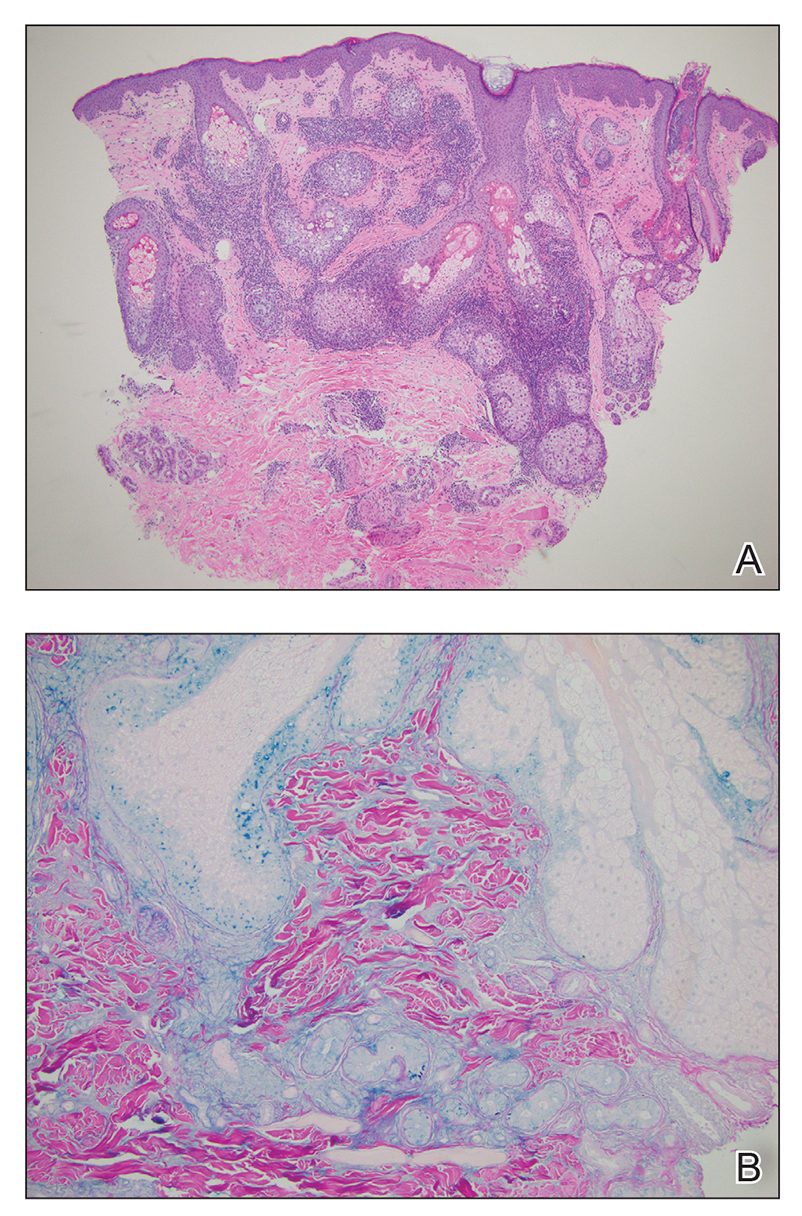
The average age at disease onset is 36 years, nearly the same as that described in discoid lupus erythematosus.4 Lupus erythematosus tumidus has a favorable prognosis and commonly presents without other autoimmune signs, serologic abnormalities, or gender preference, with concomitant systemic lupus erythematosus sometimes reported.5
The absence of clinical and histological epidermal involvement are the most important clues to aid in the diagnosis. It has been postulated that JLIS could be an early cutaneous manifestation of LET.6 The differential diagnosis also may include erythema annulare centrifugum, granuloma annulare, and urticarial vasculitis. Lesions typically respond well to photoprotection, topical corticosteroids, and/or antimalarials. The addition of tacrolimus ointment 0.1% may result in complete regression without recurrence.7
Erythema annulare centrifugum is a reactive erythema that classically begins as a pink papule that gradually enlarges to form an annular erythematous plaque with a fine trailing scale that may recur.8 The histopathology of erythema annulare centrifugum shares features seen in LET, making the diagnosis difficult; however, secondary changes to the epidermis (eg, spongiosis, hyperkeratosis) may be seen. This condition has been associated with lymphoproliferative malignancies.8
Reticular erythematous mucinosis is clinically distinguished from LET, as it presents as reticular, rather than arciform, erythematous macules, papules, or plaques that may be asymptomatic or pruritic.9 Histopathology typically shows more superficial mucin deposition than in LET as well as superficial to mid-dermal perivascular and periadnexal lymphocytic infiltrates. Reticular erythematous mucinosis more frequently is reported in women in their 30s and 40s and has been associated with UV exposure and hormonal triggers, such as oral contraceptive medications and pregnancy.9
Granuloma annulare typically presents as asymptomatic, erythematous, annular plaques or papules in young women.10 There are several histologic subtypes that show focal collagen degeneration, inflammation with palisaded or interstitial histiocytes, and mucin deposition, regardless of clinical presentation. Granuloma annulare has been associated with systemic diseases including type 2 diabetes mellitus and thyroid disease. Localized granuloma annulare most commonly presents on the dorsal aspects of the hands or feet.10
We present a case of LET on the face. Although histologically similar to other dermatoses, LET often lacks epidermal involvement and presents on sun-exposed areas of the body. Jessner lymphocytic infiltration of the skin also should be considered in the differential, as there is an overlap of clinical and histopathological features; JLIS lacks mucin deposits.6 This case reinforces the importance of correlating clinical with histopathologic findings. Our patient was treated with tacrolimus ointment 0.1%, and the plaque eventually resolved in 2 to 3 months without recurrence. This condition should be included in the differential diagnosis of recurring annular plaques on sunexposed areas, particularly in middle-aged adults, even in the absence of systemic involvement.
- Liu E, Daze RP, Moon S. Tumid lupus erythematosus: a rare and distinctive variant of cutaneous lupus erythematosus masquerading as urticarial vasculitis. Cureus. 2020;12:E8305. doi:10.7759/cureus.8305
- Saleh D, Crane JS. Tumid lupus erythematosus. In: StatPearls. StatPearls Publishing; 2020.
- Verma P, Sharma S, Yadav P, et al. Tumid lupus erythematosus: an intriguing dermatopathological connotation treated successfully with topical tacrolimus and hydroxychloroquine combination. Indian J Dermatol. 2014;59:210. doi:10.4103/0019-5154.127716
- Kuhn A, Bein D, Bonsmann G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009;8:441-448. doi:10.1016/j. autrev.2008.12.010
- Jatwani K, Chugh K, Osholowu OS, et al. Tumid lupus erythematosus and systemic lupus erythematosus: a report on their rare coexistence. Cureus. 2020;12:E7545. doi:10.7759/cureus.7545
- Tomasini D, Mentzel T, Hantschke M, et al. Plasmacytoid dendritic cells: an overview of their presence and distribution in different inflammatory skin diseases, with special emphasis on Jessner’s lymphocytic infiltrate of the skin and cutaneous lupus erythematosus. J Cutan Pathol. 2010;37:1132-1139. doi:10.1111/j.1600-0560.2010.01587.x
- Patsinakidis N, Kautz O, Gibbs BF, et al. Lupus erythematosus tumidus: clinical perspectives. Clin Cosmet Investig Dermatol. 2019;12:707-719. doi:10.2147/CCID.S166723
- Mu EW, Sanchez M, Mir A, et al. Paraneoplastic erythema annulare centrifugum eruption (PEACE). Dermatol Online J. 2015;21:13030/ qt6053h29n.
- Ocanha-Xavier JP, Cola-Senra CO, Xavier-Junior JCC. Reticular erythematous mucinosis: literature review and case report of a 24-year-old patient with systemic erythematosus lupus. Lupus. 2021;30:325-335. doi:10.1177/0961203320965702 10. Keimig EL. Granuloma annulare. Dermatol Clin. 2015;33:315-329. doi:10.1016/j.det.2015.03.001
The Diagnosis: Lupus Erythematosus Tumidus
Histopathologic evaluation of a punch biopsy revealed focal dermal mucin deposition and CD123+ discrete clusters of plasmacytoid dendritic cells without interface changes (Figure 1), favoring a diagnosis of lupus erythematosus tumidus (LET) in our patient. There was no clinical improvement in symptoms when she previously was treated with topical antifungals or class III corticosteroid creams. Tacrolimus ointment 0.1% twice daily for 1 month did not result in substantial improvement in the appearance of the plaque, and it spontaneously resolved after 2 to 3 months. She declined treatment with hydroxychloroquine.

Lupus erythematosus tumidus is an uncommon subtype of chronic cutaneous lupus erythematosus with no distinct etiology. It is clinically characterized by edematous, urticarial, single or multiple plaques with a smooth surface affecting sun-exposed areas that can last for months to years.1 In contrast to other variations of chronic cutaneous lupus such as discoid lupus erythematosus, LET lesions lack surface papulosquamous features such as scaling, atrophy, and follicular plugging.1-4 Based solely on histologic findings, LET may be indistinguishable from reticular erythematous mucinosis and Jessner lymphocytic infiltration of the skin (JLIS) due to a similar lack of epidermal involvement and presence of a perivascular lymphocytic infiltrate (Figure 2).

The average age at disease onset is 36 years, nearly the same as that described in discoid lupus erythematosus.4 Lupus erythematosus tumidus has a favorable prognosis and commonly presents without other autoimmune signs, serologic abnormalities, or gender preference, with concomitant systemic lupus erythematosus sometimes reported.5
The absence of clinical and histological epidermal involvement are the most important clues to aid in the diagnosis. It has been postulated that JLIS could be an early cutaneous manifestation of LET.6 The differential diagnosis also may include erythema annulare centrifugum, granuloma annulare, and urticarial vasculitis. Lesions typically respond well to photoprotection, topical corticosteroids, and/or antimalarials. The addition of tacrolimus ointment 0.1% may result in complete regression without recurrence.7
Erythema annulare centrifugum is a reactive erythema that classically begins as a pink papule that gradually enlarges to form an annular erythematous plaque with a fine trailing scale that may recur.8 The histopathology of erythema annulare centrifugum shares features seen in LET, making the diagnosis difficult; however, secondary changes to the epidermis (eg, spongiosis, hyperkeratosis) may be seen. This condition has been associated with lymphoproliferative malignancies.8
Reticular erythematous mucinosis is clinically distinguished from LET, as it presents as reticular, rather than arciform, erythematous macules, papules, or plaques that may be asymptomatic or pruritic.9 Histopathology typically shows more superficial mucin deposition than in LET as well as superficial to mid-dermal perivascular and periadnexal lymphocytic infiltrates. Reticular erythematous mucinosis more frequently is reported in women in their 30s and 40s and has been associated with UV exposure and hormonal triggers, such as oral contraceptive medications and pregnancy.9
Granuloma annulare typically presents as asymptomatic, erythematous, annular plaques or papules in young women.10 There are several histologic subtypes that show focal collagen degeneration, inflammation with palisaded or interstitial histiocytes, and mucin deposition, regardless of clinical presentation. Granuloma annulare has been associated with systemic diseases including type 2 diabetes mellitus and thyroid disease. Localized granuloma annulare most commonly presents on the dorsal aspects of the hands or feet.10
We present a case of LET on the face. Although histologically similar to other dermatoses, LET often lacks epidermal involvement and presents on sun-exposed areas of the body. Jessner lymphocytic infiltration of the skin also should be considered in the differential, as there is an overlap of clinical and histopathological features; JLIS lacks mucin deposits.6 This case reinforces the importance of correlating clinical with histopathologic findings. Our patient was treated with tacrolimus ointment 0.1%, and the plaque eventually resolved in 2 to 3 months without recurrence. This condition should be included in the differential diagnosis of recurring annular plaques on sunexposed areas, particularly in middle-aged adults, even in the absence of systemic involvement.
The Diagnosis: Lupus Erythematosus Tumidus
Histopathologic evaluation of a punch biopsy revealed focal dermal mucin deposition and CD123+ discrete clusters of plasmacytoid dendritic cells without interface changes (Figure 1), favoring a diagnosis of lupus erythematosus tumidus (LET) in our patient. There was no clinical improvement in symptoms when she previously was treated with topical antifungals or class III corticosteroid creams. Tacrolimus ointment 0.1% twice daily for 1 month did not result in substantial improvement in the appearance of the plaque, and it spontaneously resolved after 2 to 3 months. She declined treatment with hydroxychloroquine.

Lupus erythematosus tumidus is an uncommon subtype of chronic cutaneous lupus erythematosus with no distinct etiology. It is clinically characterized by edematous, urticarial, single or multiple plaques with a smooth surface affecting sun-exposed areas that can last for months to years.1 In contrast to other variations of chronic cutaneous lupus such as discoid lupus erythematosus, LET lesions lack surface papulosquamous features such as scaling, atrophy, and follicular plugging.1-4 Based solely on histologic findings, LET may be indistinguishable from reticular erythematous mucinosis and Jessner lymphocytic infiltration of the skin (JLIS) due to a similar lack of epidermal involvement and presence of a perivascular lymphocytic infiltrate (Figure 2).

The average age at disease onset is 36 years, nearly the same as that described in discoid lupus erythematosus.4 Lupus erythematosus tumidus has a favorable prognosis and commonly presents without other autoimmune signs, serologic abnormalities, or gender preference, with concomitant systemic lupus erythematosus sometimes reported.5
The absence of clinical and histological epidermal involvement are the most important clues to aid in the diagnosis. It has been postulated that JLIS could be an early cutaneous manifestation of LET.6 The differential diagnosis also may include erythema annulare centrifugum, granuloma annulare, and urticarial vasculitis. Lesions typically respond well to photoprotection, topical corticosteroids, and/or antimalarials. The addition of tacrolimus ointment 0.1% may result in complete regression without recurrence.7
Erythema annulare centrifugum is a reactive erythema that classically begins as a pink papule that gradually enlarges to form an annular erythematous plaque with a fine trailing scale that may recur.8 The histopathology of erythema annulare centrifugum shares features seen in LET, making the diagnosis difficult; however, secondary changes to the epidermis (eg, spongiosis, hyperkeratosis) may be seen. This condition has been associated with lymphoproliferative malignancies.8
Reticular erythematous mucinosis is clinically distinguished from LET, as it presents as reticular, rather than arciform, erythematous macules, papules, or plaques that may be asymptomatic or pruritic.9 Histopathology typically shows more superficial mucin deposition than in LET as well as superficial to mid-dermal perivascular and periadnexal lymphocytic infiltrates. Reticular erythematous mucinosis more frequently is reported in women in their 30s and 40s and has been associated with UV exposure and hormonal triggers, such as oral contraceptive medications and pregnancy.9
Granuloma annulare typically presents as asymptomatic, erythematous, annular plaques or papules in young women.10 There are several histologic subtypes that show focal collagen degeneration, inflammation with palisaded or interstitial histiocytes, and mucin deposition, regardless of clinical presentation. Granuloma annulare has been associated with systemic diseases including type 2 diabetes mellitus and thyroid disease. Localized granuloma annulare most commonly presents on the dorsal aspects of the hands or feet.10
We present a case of LET on the face. Although histologically similar to other dermatoses, LET often lacks epidermal involvement and presents on sun-exposed areas of the body. Jessner lymphocytic infiltration of the skin also should be considered in the differential, as there is an overlap of clinical and histopathological features; JLIS lacks mucin deposits.6 This case reinforces the importance of correlating clinical with histopathologic findings. Our patient was treated with tacrolimus ointment 0.1%, and the plaque eventually resolved in 2 to 3 months without recurrence. This condition should be included in the differential diagnosis of recurring annular plaques on sunexposed areas, particularly in middle-aged adults, even in the absence of systemic involvement.
- Liu E, Daze RP, Moon S. Tumid lupus erythematosus: a rare and distinctive variant of cutaneous lupus erythematosus masquerading as urticarial vasculitis. Cureus. 2020;12:E8305. doi:10.7759/cureus.8305
- Saleh D, Crane JS. Tumid lupus erythematosus. In: StatPearls. StatPearls Publishing; 2020.
- Verma P, Sharma S, Yadav P, et al. Tumid lupus erythematosus: an intriguing dermatopathological connotation treated successfully with topical tacrolimus and hydroxychloroquine combination. Indian J Dermatol. 2014;59:210. doi:10.4103/0019-5154.127716
- Kuhn A, Bein D, Bonsmann G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009;8:441-448. doi:10.1016/j. autrev.2008.12.010
- Jatwani K, Chugh K, Osholowu OS, et al. Tumid lupus erythematosus and systemic lupus erythematosus: a report on their rare coexistence. Cureus. 2020;12:E7545. doi:10.7759/cureus.7545
- Tomasini D, Mentzel T, Hantschke M, et al. Plasmacytoid dendritic cells: an overview of their presence and distribution in different inflammatory skin diseases, with special emphasis on Jessner’s lymphocytic infiltrate of the skin and cutaneous lupus erythematosus. J Cutan Pathol. 2010;37:1132-1139. doi:10.1111/j.1600-0560.2010.01587.x
- Patsinakidis N, Kautz O, Gibbs BF, et al. Lupus erythematosus tumidus: clinical perspectives. Clin Cosmet Investig Dermatol. 2019;12:707-719. doi:10.2147/CCID.S166723
- Mu EW, Sanchez M, Mir A, et al. Paraneoplastic erythema annulare centrifugum eruption (PEACE). Dermatol Online J. 2015;21:13030/ qt6053h29n.
- Ocanha-Xavier JP, Cola-Senra CO, Xavier-Junior JCC. Reticular erythematous mucinosis: literature review and case report of a 24-year-old patient with systemic erythematosus lupus. Lupus. 2021;30:325-335. doi:10.1177/0961203320965702 10. Keimig EL. Granuloma annulare. Dermatol Clin. 2015;33:315-329. doi:10.1016/j.det.2015.03.001
- Liu E, Daze RP, Moon S. Tumid lupus erythematosus: a rare and distinctive variant of cutaneous lupus erythematosus masquerading as urticarial vasculitis. Cureus. 2020;12:E8305. doi:10.7759/cureus.8305
- Saleh D, Crane JS. Tumid lupus erythematosus. In: StatPearls. StatPearls Publishing; 2020.
- Verma P, Sharma S, Yadav P, et al. Tumid lupus erythematosus: an intriguing dermatopathological connotation treated successfully with topical tacrolimus and hydroxychloroquine combination. Indian J Dermatol. 2014;59:210. doi:10.4103/0019-5154.127716
- Kuhn A, Bein D, Bonsmann G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009;8:441-448. doi:10.1016/j. autrev.2008.12.010
- Jatwani K, Chugh K, Osholowu OS, et al. Tumid lupus erythematosus and systemic lupus erythematosus: a report on their rare coexistence. Cureus. 2020;12:E7545. doi:10.7759/cureus.7545
- Tomasini D, Mentzel T, Hantschke M, et al. Plasmacytoid dendritic cells: an overview of their presence and distribution in different inflammatory skin diseases, with special emphasis on Jessner’s lymphocytic infiltrate of the skin and cutaneous lupus erythematosus. J Cutan Pathol. 2010;37:1132-1139. doi:10.1111/j.1600-0560.2010.01587.x
- Patsinakidis N, Kautz O, Gibbs BF, et al. Lupus erythematosus tumidus: clinical perspectives. Clin Cosmet Investig Dermatol. 2019;12:707-719. doi:10.2147/CCID.S166723
- Mu EW, Sanchez M, Mir A, et al. Paraneoplastic erythema annulare centrifugum eruption (PEACE). Dermatol Online J. 2015;21:13030/ qt6053h29n.
- Ocanha-Xavier JP, Cola-Senra CO, Xavier-Junior JCC. Reticular erythematous mucinosis: literature review and case report of a 24-year-old patient with systemic erythematosus lupus. Lupus. 2021;30:325-335. doi:10.1177/0961203320965702 10. Keimig EL. Granuloma annulare. Dermatol Clin. 2015;33:315-329. doi:10.1016/j.det.2015.03.001
An otherwise healthy 31-year-old woman presented with a gradual growth of a semiannular, arciform, mildly pruritic plaque around the mouth of 10 years’ duration that recurred biannually, persisted for a few months, and spontaneously remitted without residual scarring. She denied joint pain, muscle aches, sores in the mouth, personal or family history of autoimmune diseases, or other remarkable review of systems. Physical examination revealed a welldefined, edematous, smooth, arciform plaque on the face with no mucous membrane involvement. Laboratory evaluation, including complete blood cell count, comprehensive metabolic panel, and antinuclear antibody titer, was unremarkable. A punch biopsy was obtained.

A 64-year-old woman presents with a history of asymptomatic erythematous grouped papules on the right breast
. Recurrences may occur. Rarely, lymph nodes, the gastrointestinal system, lung, bone and bone marrow may be involved as extracutaneous sites.
Primary cutaneous B-cell lymphomas account for approximately 25% of all cutaneous lymphomas. Clinically, patients present with either solitary or multiple papules or plaques, typically on the upper extremities or trunk.
Histopathology is vital for the correct diagnosis. In this patient, the histologic report was written as follows: “The findings are those of a well-differentiated but atypical diffuse mixed small lymphocytic infiltrate representing a mixture of T-cells and B-cells. The minor component of the infiltrate is of T-cell lineage, whereby the cells do not show any phenotypic abnormalities. The background cell population is interpreted as reactive. However, the dominant cell population is in fact of B-cell lineage. It is extensively highlighted by CD20. Only a minor component of the B cell infiltrate appeared to be in the context of representing germinal centers as characterized by small foci of centrocytic and centroblastic infiltration highlighted by BCL6 and CD10. The overwhelming B-cell component is a non–germinal center small B cell that does demonstrate BCL2 positivity and significant immunoreactivity for CD23. This small lymphocytic infiltrate obscures the germinal centers. There are only a few plasma cells; they do not show light chain restriction.”
The pathologist remarked that “this type of morphology of a diffuse small B-cell lymphocytic infiltrate that is without any evidence of light chain restriction amidst plasma cells, whereby the B cell component is dominant over the T-cell component would in fact be consistent with a unique variant of marginal zone lymphoma derived from a naive mantle zone.”
PCMZL has an excellent prognosis. When limited to the skin, local radiation or excision are effective treatments. Intravenous rituximab has been used to treat multifocal PCMZL. This patient was found to have no extracutaneous involvement and was treated with radiation.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Virmani P et al. JAAD Case Rep. 2017 Jun 14;3(4):269-72.
Magro CM and Olson LC. Ann Diagn Pathol. 2018 Jun;34:116-21.
. Recurrences may occur. Rarely, lymph nodes, the gastrointestinal system, lung, bone and bone marrow may be involved as extracutaneous sites.
Primary cutaneous B-cell lymphomas account for approximately 25% of all cutaneous lymphomas. Clinically, patients present with either solitary or multiple papules or plaques, typically on the upper extremities or trunk.
Histopathology is vital for the correct diagnosis. In this patient, the histologic report was written as follows: “The findings are those of a well-differentiated but atypical diffuse mixed small lymphocytic infiltrate representing a mixture of T-cells and B-cells. The minor component of the infiltrate is of T-cell lineage, whereby the cells do not show any phenotypic abnormalities. The background cell population is interpreted as reactive. However, the dominant cell population is in fact of B-cell lineage. It is extensively highlighted by CD20. Only a minor component of the B cell infiltrate appeared to be in the context of representing germinal centers as characterized by small foci of centrocytic and centroblastic infiltration highlighted by BCL6 and CD10. The overwhelming B-cell component is a non–germinal center small B cell that does demonstrate BCL2 positivity and significant immunoreactivity for CD23. This small lymphocytic infiltrate obscures the germinal centers. There are only a few plasma cells; they do not show light chain restriction.”
The pathologist remarked that “this type of morphology of a diffuse small B-cell lymphocytic infiltrate that is without any evidence of light chain restriction amidst plasma cells, whereby the B cell component is dominant over the T-cell component would in fact be consistent with a unique variant of marginal zone lymphoma derived from a naive mantle zone.”
PCMZL has an excellent prognosis. When limited to the skin, local radiation or excision are effective treatments. Intravenous rituximab has been used to treat multifocal PCMZL. This patient was found to have no extracutaneous involvement and was treated with radiation.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Virmani P et al. JAAD Case Rep. 2017 Jun 14;3(4):269-72.
Magro CM and Olson LC. Ann Diagn Pathol. 2018 Jun;34:116-21.
. Recurrences may occur. Rarely, lymph nodes, the gastrointestinal system, lung, bone and bone marrow may be involved as extracutaneous sites.
Primary cutaneous B-cell lymphomas account for approximately 25% of all cutaneous lymphomas. Clinically, patients present with either solitary or multiple papules or plaques, typically on the upper extremities or trunk.
Histopathology is vital for the correct diagnosis. In this patient, the histologic report was written as follows: “The findings are those of a well-differentiated but atypical diffuse mixed small lymphocytic infiltrate representing a mixture of T-cells and B-cells. The minor component of the infiltrate is of T-cell lineage, whereby the cells do not show any phenotypic abnormalities. The background cell population is interpreted as reactive. However, the dominant cell population is in fact of B-cell lineage. It is extensively highlighted by CD20. Only a minor component of the B cell infiltrate appeared to be in the context of representing germinal centers as characterized by small foci of centrocytic and centroblastic infiltration highlighted by BCL6 and CD10. The overwhelming B-cell component is a non–germinal center small B cell that does demonstrate BCL2 positivity and significant immunoreactivity for CD23. This small lymphocytic infiltrate obscures the germinal centers. There are only a few plasma cells; they do not show light chain restriction.”
The pathologist remarked that “this type of morphology of a diffuse small B-cell lymphocytic infiltrate that is without any evidence of light chain restriction amidst plasma cells, whereby the B cell component is dominant over the T-cell component would in fact be consistent with a unique variant of marginal zone lymphoma derived from a naive mantle zone.”
PCMZL has an excellent prognosis. When limited to the skin, local radiation or excision are effective treatments. Intravenous rituximab has been used to treat multifocal PCMZL. This patient was found to have no extracutaneous involvement and was treated with radiation.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Virmani P et al. JAAD Case Rep. 2017 Jun 14;3(4):269-72.
Magro CM and Olson LC. Ann Diagn Pathol. 2018 Jun;34:116-21.
Retiform Purpura on the Legs
The Diagnosis: Calciphylaxis
Histopathology revealed epidermal and dermal necrosis, a perivascular neutrophilic infiltrate, and scattered microcalcifications within small- and medium-sized subcutaneous vessels, consistent with a diagnosis of calciphylaxis (Figure). Calciphylaxis (also known as calcific uremic arteriolopathy) is a rare, severe, and often fatal vasculopathy that predominately occurs in patients with end-stage renal failure.1 The pathogenesis of calciphylaxis remains poorly understood; however, it generally is thought that an imbalance in calcium homeostasis in susceptible hosts results in the precipitation of calcium phosphate within vessel walls leading to endothelial damage with subsequent thrombotic vasculopathy and ischemic tissue damage. Acquired and congenital hypercoagulable states have been implicated in the pathogenesis of calciphylaxis.2
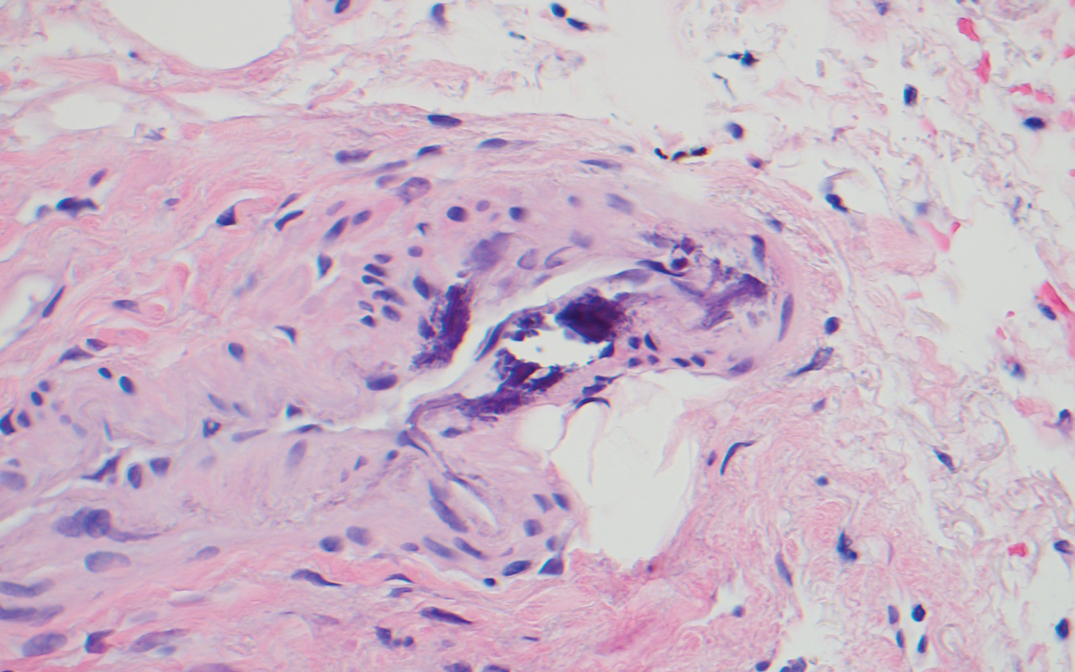
Treatment of calciphylaxis is directed at normalizing abnormal calcium metabolism; removing possible exacerbating agents, such as warfarin, systemic corticosteroids, calcium, and iron; and transitioning patients with end-stage renal disease to hemodialysis, if not already initiated. The treatment approach is multifaceted, and numerous therapies usually are attempted simultaneously. Vitamin K supplementation, low-calcium dialysate, non–calcium carbonate phosphate binders, cinacalcet, becaplermin, bisphosphonates, hyperbaric oxygen, and intravenous sodium thiosulfate all have been utilized with some success. Currently, intravenous sodium thiosulfate is the mainstay therapy for the treatment of calciphylaxis.2 Although the mechanism of sodium thiosulfate is not entirely understood, it is known to have anticalcification, vasodilatory, and antioxidant properties.
Retiform purpura clinically is characterized by reticulated, branching, purpuric skin lesions. It occurs following vascular insult by way of vessel lumen occlusion (thrombotic vasculopathy) and less frequently by vessel wall inflammation (vasculitis). The differential diagnosis for retiform purpura includes various causes of microvascular occlusion, including hypercoagulable states and type I cryoglobulinemia, calciphylaxis, infections, autoimmune vasculitic conditions, and embolic causes.3
Cutaneous disease in individuals with antiphospholipid antibodies may present similarly with retiform purpura in the form of necrotizing livedo reticularis, leg ulcers, or widespread cutaneous necrosis. Histopathologic findings include vascular thrombi with partial or complete obstruction of the small- to medium-sized arteries at the dermoepidermal junction, often in the absence of an inflammatory infiltrate.4 True vasculitis is not typical of antiphospholipid syndrome.
Medium vessel vasculitides, such as polyarteritis nodosa, clinically present with livedo reticularis, subcutaneous nodules, and tissue necrosis. Dermatopathologic evaluation of a medium-sized vessel vasculitis would demonstrate a neutrophilic vasculitis involving vessels within the deep dermis and septa of subcutaneous fat.5 Tissue sampling should be deep and wide enough to visualize the pathology, as shallow biopsies may show intraluminal thrombi of the superficial dermal plexus only, while a narrow specimen may result in falsenegative findings due to the focal nature of vessel involvement in conditions such as polyarteritis nodosa.
Type I cryoglobulinemia often is a manifestation of plasma cell dyscrasia and commonly presents with Raynaud phenomenon, livedo reticularis, and acrocyanosis of helices6 ; pathology demonstrates vessel occlusion and erythrocyte extravasation. In contrast, types II and III, also known as mixed cryoglobulinemia, are associated with hepatitis C and autoimmune connective tissue disease. They clinically present as purpuric plaques and nodules that have a propensity to vesiculate and ulcerate.7 Histopathologically, features of leukocytoclastic vasculitis are seen, and direct immunofluorescence demonstrates perivascular granular deposits consisting predominantly of IgM and C3 in the papillary dermis.8
Warfarin therapy, particularly in high initial doses, can induce lesions of cutaneous necrosis, which clinically may resemble the appearance of calciphylaxis. Warfarininduced skin necrosis typically occurs 3 to 5 days after the initiation of therapy and is the result of a temporary prothrombotic state.9 The half-life of antithrombotic protein C is shorter than vitamin K–dependent prothrombotic factors II, X, and IX. Early in warfarin treatment, an acquired state of reduced protein C level exists, which can lead to vessel thrombosis and subsequent cutaneous necrosis. Treatment of warfarin-induced skin necrosis involves cessation of warfarin, supplementation with vitamin K to reverse the effects of warfarin, and the initiation of heparin or low-molecular-weight heparin.9
- Hayashi M. Calciphylaxis: diagnosis and clinical features. Clin Exp Nephrol. 2013;17:498-503.
- Strazzula L, Nigwekar SU, Steele D, et al. Intralesional sodium thiosulfate for the treatment of calciphylaxis. JAMA Dermatol. 2013;149:946-949.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Llamas-Velasco M, Alegría V, Santos-Briz Á, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662.
- Daoud MS, Hutton KP, Gibson LE. Cutaneous periarteritis nodosa: a clinicopathologic study of 79 cases. Br J Dermatol. 1997; 136:706-713.
- Fraser Gibson J, Leventhal JS, King B. Purpuric lesions on acral sites. type I cryoglobulinemia associated with multiple myeloma. JAMA Dermatol. 2015;151:659-660.
- Pakula AS, Garden JM, Roth SI. Mixed cryoglobulinemia and hepatitis C virus infection. J Am Acad Dermatol. 1994;30:143.
- Daoud MS, el-Azhary RA, Gibson LE, et al. Chronic hepatitis C, cryoglobulinemia, and cutaneous necrotizing vasculitis. clinical, pathologic, and immunopathologic study of twelve patients. J Am Acad Dermatol. 1996;34:219-223.
- Nazarian RM, Van Cott EM, Zembowicz A, et al. Warfarin-induced skin necrosis. J Am Acad Dermatol. 2009;61:325-332.
The Diagnosis: Calciphylaxis
Histopathology revealed epidermal and dermal necrosis, a perivascular neutrophilic infiltrate, and scattered microcalcifications within small- and medium-sized subcutaneous vessels, consistent with a diagnosis of calciphylaxis (Figure). Calciphylaxis (also known as calcific uremic arteriolopathy) is a rare, severe, and often fatal vasculopathy that predominately occurs in patients with end-stage renal failure.1 The pathogenesis of calciphylaxis remains poorly understood; however, it generally is thought that an imbalance in calcium homeostasis in susceptible hosts results in the precipitation of calcium phosphate within vessel walls leading to endothelial damage with subsequent thrombotic vasculopathy and ischemic tissue damage. Acquired and congenital hypercoagulable states have been implicated in the pathogenesis of calciphylaxis.2

Treatment of calciphylaxis is directed at normalizing abnormal calcium metabolism; removing possible exacerbating agents, such as warfarin, systemic corticosteroids, calcium, and iron; and transitioning patients with end-stage renal disease to hemodialysis, if not already initiated. The treatment approach is multifaceted, and numerous therapies usually are attempted simultaneously. Vitamin K supplementation, low-calcium dialysate, non–calcium carbonate phosphate binders, cinacalcet, becaplermin, bisphosphonates, hyperbaric oxygen, and intravenous sodium thiosulfate all have been utilized with some success. Currently, intravenous sodium thiosulfate is the mainstay therapy for the treatment of calciphylaxis.2 Although the mechanism of sodium thiosulfate is not entirely understood, it is known to have anticalcification, vasodilatory, and antioxidant properties.
Retiform purpura clinically is characterized by reticulated, branching, purpuric skin lesions. It occurs following vascular insult by way of vessel lumen occlusion (thrombotic vasculopathy) and less frequently by vessel wall inflammation (vasculitis). The differential diagnosis for retiform purpura includes various causes of microvascular occlusion, including hypercoagulable states and type I cryoglobulinemia, calciphylaxis, infections, autoimmune vasculitic conditions, and embolic causes.3
Cutaneous disease in individuals with antiphospholipid antibodies may present similarly with retiform purpura in the form of necrotizing livedo reticularis, leg ulcers, or widespread cutaneous necrosis. Histopathologic findings include vascular thrombi with partial or complete obstruction of the small- to medium-sized arteries at the dermoepidermal junction, often in the absence of an inflammatory infiltrate.4 True vasculitis is not typical of antiphospholipid syndrome.
Medium vessel vasculitides, such as polyarteritis nodosa, clinically present with livedo reticularis, subcutaneous nodules, and tissue necrosis. Dermatopathologic evaluation of a medium-sized vessel vasculitis would demonstrate a neutrophilic vasculitis involving vessels within the deep dermis and septa of subcutaneous fat.5 Tissue sampling should be deep and wide enough to visualize the pathology, as shallow biopsies may show intraluminal thrombi of the superficial dermal plexus only, while a narrow specimen may result in falsenegative findings due to the focal nature of vessel involvement in conditions such as polyarteritis nodosa.
Type I cryoglobulinemia often is a manifestation of plasma cell dyscrasia and commonly presents with Raynaud phenomenon, livedo reticularis, and acrocyanosis of helices6 ; pathology demonstrates vessel occlusion and erythrocyte extravasation. In contrast, types II and III, also known as mixed cryoglobulinemia, are associated with hepatitis C and autoimmune connective tissue disease. They clinically present as purpuric plaques and nodules that have a propensity to vesiculate and ulcerate.7 Histopathologically, features of leukocytoclastic vasculitis are seen, and direct immunofluorescence demonstrates perivascular granular deposits consisting predominantly of IgM and C3 in the papillary dermis.8
Warfarin therapy, particularly in high initial doses, can induce lesions of cutaneous necrosis, which clinically may resemble the appearance of calciphylaxis. Warfarininduced skin necrosis typically occurs 3 to 5 days after the initiation of therapy and is the result of a temporary prothrombotic state.9 The half-life of antithrombotic protein C is shorter than vitamin K–dependent prothrombotic factors II, X, and IX. Early in warfarin treatment, an acquired state of reduced protein C level exists, which can lead to vessel thrombosis and subsequent cutaneous necrosis. Treatment of warfarin-induced skin necrosis involves cessation of warfarin, supplementation with vitamin K to reverse the effects of warfarin, and the initiation of heparin or low-molecular-weight heparin.9
The Diagnosis: Calciphylaxis
Histopathology revealed epidermal and dermal necrosis, a perivascular neutrophilic infiltrate, and scattered microcalcifications within small- and medium-sized subcutaneous vessels, consistent with a diagnosis of calciphylaxis (Figure). Calciphylaxis (also known as calcific uremic arteriolopathy) is a rare, severe, and often fatal vasculopathy that predominately occurs in patients with end-stage renal failure.1 The pathogenesis of calciphylaxis remains poorly understood; however, it generally is thought that an imbalance in calcium homeostasis in susceptible hosts results in the precipitation of calcium phosphate within vessel walls leading to endothelial damage with subsequent thrombotic vasculopathy and ischemic tissue damage. Acquired and congenital hypercoagulable states have been implicated in the pathogenesis of calciphylaxis.2

Treatment of calciphylaxis is directed at normalizing abnormal calcium metabolism; removing possible exacerbating agents, such as warfarin, systemic corticosteroids, calcium, and iron; and transitioning patients with end-stage renal disease to hemodialysis, if not already initiated. The treatment approach is multifaceted, and numerous therapies usually are attempted simultaneously. Vitamin K supplementation, low-calcium dialysate, non–calcium carbonate phosphate binders, cinacalcet, becaplermin, bisphosphonates, hyperbaric oxygen, and intravenous sodium thiosulfate all have been utilized with some success. Currently, intravenous sodium thiosulfate is the mainstay therapy for the treatment of calciphylaxis.2 Although the mechanism of sodium thiosulfate is not entirely understood, it is known to have anticalcification, vasodilatory, and antioxidant properties.
Retiform purpura clinically is characterized by reticulated, branching, purpuric skin lesions. It occurs following vascular insult by way of vessel lumen occlusion (thrombotic vasculopathy) and less frequently by vessel wall inflammation (vasculitis). The differential diagnosis for retiform purpura includes various causes of microvascular occlusion, including hypercoagulable states and type I cryoglobulinemia, calciphylaxis, infections, autoimmune vasculitic conditions, and embolic causes.3
Cutaneous disease in individuals with antiphospholipid antibodies may present similarly with retiform purpura in the form of necrotizing livedo reticularis, leg ulcers, or widespread cutaneous necrosis. Histopathologic findings include vascular thrombi with partial or complete obstruction of the small- to medium-sized arteries at the dermoepidermal junction, often in the absence of an inflammatory infiltrate.4 True vasculitis is not typical of antiphospholipid syndrome.
Medium vessel vasculitides, such as polyarteritis nodosa, clinically present with livedo reticularis, subcutaneous nodules, and tissue necrosis. Dermatopathologic evaluation of a medium-sized vessel vasculitis would demonstrate a neutrophilic vasculitis involving vessels within the deep dermis and septa of subcutaneous fat.5 Tissue sampling should be deep and wide enough to visualize the pathology, as shallow biopsies may show intraluminal thrombi of the superficial dermal plexus only, while a narrow specimen may result in falsenegative findings due to the focal nature of vessel involvement in conditions such as polyarteritis nodosa.
Type I cryoglobulinemia often is a manifestation of plasma cell dyscrasia and commonly presents with Raynaud phenomenon, livedo reticularis, and acrocyanosis of helices6 ; pathology demonstrates vessel occlusion and erythrocyte extravasation. In contrast, types II and III, also known as mixed cryoglobulinemia, are associated with hepatitis C and autoimmune connective tissue disease. They clinically present as purpuric plaques and nodules that have a propensity to vesiculate and ulcerate.7 Histopathologically, features of leukocytoclastic vasculitis are seen, and direct immunofluorescence demonstrates perivascular granular deposits consisting predominantly of IgM and C3 in the papillary dermis.8
Warfarin therapy, particularly in high initial doses, can induce lesions of cutaneous necrosis, which clinically may resemble the appearance of calciphylaxis. Warfarininduced skin necrosis typically occurs 3 to 5 days after the initiation of therapy and is the result of a temporary prothrombotic state.9 The half-life of antithrombotic protein C is shorter than vitamin K–dependent prothrombotic factors II, X, and IX. Early in warfarin treatment, an acquired state of reduced protein C level exists, which can lead to vessel thrombosis and subsequent cutaneous necrosis. Treatment of warfarin-induced skin necrosis involves cessation of warfarin, supplementation with vitamin K to reverse the effects of warfarin, and the initiation of heparin or low-molecular-weight heparin.9
- Hayashi M. Calciphylaxis: diagnosis and clinical features. Clin Exp Nephrol. 2013;17:498-503.
- Strazzula L, Nigwekar SU, Steele D, et al. Intralesional sodium thiosulfate for the treatment of calciphylaxis. JAMA Dermatol. 2013;149:946-949.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Llamas-Velasco M, Alegría V, Santos-Briz Á, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662.
- Daoud MS, Hutton KP, Gibson LE. Cutaneous periarteritis nodosa: a clinicopathologic study of 79 cases. Br J Dermatol. 1997; 136:706-713.
- Fraser Gibson J, Leventhal JS, King B. Purpuric lesions on acral sites. type I cryoglobulinemia associated with multiple myeloma. JAMA Dermatol. 2015;151:659-660.
- Pakula AS, Garden JM, Roth SI. Mixed cryoglobulinemia and hepatitis C virus infection. J Am Acad Dermatol. 1994;30:143.
- Daoud MS, el-Azhary RA, Gibson LE, et al. Chronic hepatitis C, cryoglobulinemia, and cutaneous necrotizing vasculitis. clinical, pathologic, and immunopathologic study of twelve patients. J Am Acad Dermatol. 1996;34:219-223.
- Nazarian RM, Van Cott EM, Zembowicz A, et al. Warfarin-induced skin necrosis. J Am Acad Dermatol. 2009;61:325-332.
- Hayashi M. Calciphylaxis: diagnosis and clinical features. Clin Exp Nephrol. 2013;17:498-503.
- Strazzula L, Nigwekar SU, Steele D, et al. Intralesional sodium thiosulfate for the treatment of calciphylaxis. JAMA Dermatol. 2013;149:946-949.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Llamas-Velasco M, Alegría V, Santos-Briz Á, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662.
- Daoud MS, Hutton KP, Gibson LE. Cutaneous periarteritis nodosa: a clinicopathologic study of 79 cases. Br J Dermatol. 1997; 136:706-713.
- Fraser Gibson J, Leventhal JS, King B. Purpuric lesions on acral sites. type I cryoglobulinemia associated with multiple myeloma. JAMA Dermatol. 2015;151:659-660.
- Pakula AS, Garden JM, Roth SI. Mixed cryoglobulinemia and hepatitis C virus infection. J Am Acad Dermatol. 1994;30:143.
- Daoud MS, el-Azhary RA, Gibson LE, et al. Chronic hepatitis C, cryoglobulinemia, and cutaneous necrotizing vasculitis. clinical, pathologic, and immunopathologic study of twelve patients. J Am Acad Dermatol. 1996;34:219-223.
- Nazarian RM, Van Cott EM, Zembowicz A, et al. Warfarin-induced skin necrosis. J Am Acad Dermatol. 2009;61:325-332.
A 70-year-old woman with a medical history of Takayasu arteritis, end-stage renal disease on peritoneal dialysis, coronary artery disease, hypertension, hypothyroidism, and anemia of chronic disease presented to the emergency department with enlarging painful stellate eschars of the legs with associated edema of 3 weeks’ duration. She denied a history of similar-appearing skin lesions. She initially thought the lesions were burns secondary to frequent hot showers for relief of uremic pruritus. For the treatment of these suspected burns prior to hospitalization, she had been applying over-the-counter antibiotic ointments to the affected areas and had completed a 2-week course of oral cephalexin without notable improvement. Physical examination revealed retiform purpura of the legs with large stellate eschars overlying the anteromedial thighs and right medial calf. Computed tomography angiogram of the abdomen and pelvis demonstrated diffuse calcifications of the aortic wall and its associated branches that were most pronounced in the legs without evidence of vessel wall thickening. Punch biopsies were performed, and nephrology, rheumatology, and wound care services were consulted.
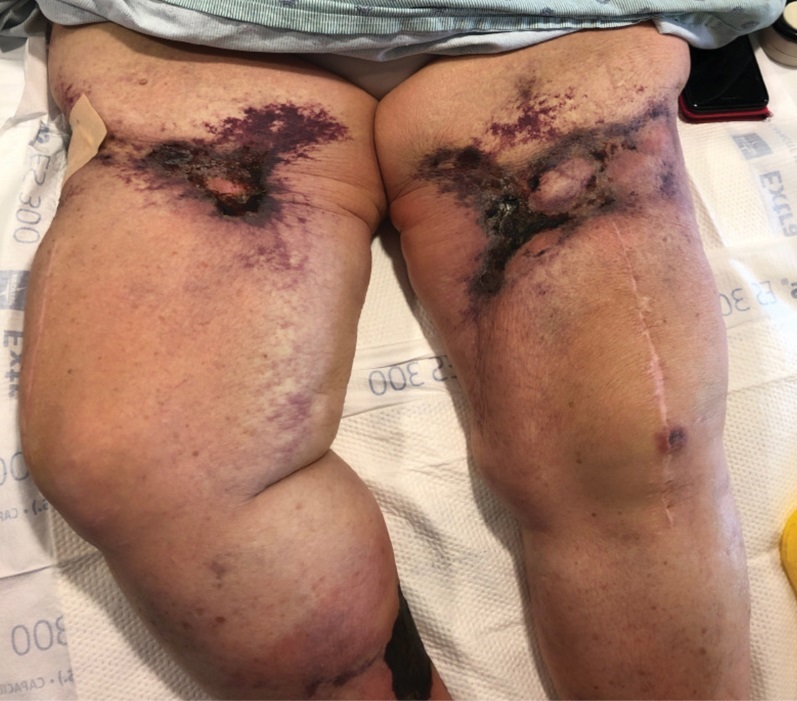
Reflectance Confocal Microscopy Findings in a Small-Diameter Invasive Melanoma
Melanomas have been designated as small melanomas or micromelanomas according to their long-axis diameter (<6 mm and ≤3 mm, respectively).1-3 Because small-diameter melanomas also have the potential to metastasize, particularly if nodular, early diagnosis can be highly rewarding. Deep melanomas with small diameters may have the same potential for metastasis as large-diameter melanomas. In this context, dermoscopy, digital dermoscopic monitoring, and total-body photography are useful in clinical practice. However, these techniques are of limited utility for small, dermoscopic feature–poor melanomas. Conversely, less than 10% of changing lesions, which are spotted via digital dermoscopic surveillance, turn out to be melanomas; therefore, simply removing all changing lesions may result in many unnecessary excisions of benign lesions.4
In vivo reflectance confocal microscopy (RCM) is an advanced technique that allows recognition of the architectural and cellular details of pigmented lesions. Reflectance confocal microscopy has the potential to reduce the rate of unnecessary excisions and to diminish the risk for missing a melanoma.5-7 In meta-analyses, RCM sensitivity was reported as 90% to 93% and specificity was reported as 78% to 82% in detecting melanoma.8,9
We describe a case that highlights the potential role of RCM in the diagnosis of small-diameter melanomas.
Case Report
A 57-year-old man with Fitzpatrick skin type III presented to the dermato-oncology unit for evaluation of multiple nevi. He was otherwise healthy and denied a history of skin cancer. Total-body skin examination with dermoscopy was performed, and several mildly atypical lesions were identified. We decided to perform digital dermoscopic monitoring. The patient’s 6-month monitoring appointment had been scheduled, but he did not arrive for the follow-up visit until 10 months after the initial examination. A lesion on the left arm, which initially was 1.5 mm in diameter, had enlarged. It was now a dark brown–gray papule with a 2.5-mm diameter (Figure 1). Dermoscopy revealed grayish globules/dots at the center of the lesion, reticular gray-blue areas, and few milialike cysts; at the periphery, a narrow rim of brownish delicate pigment network also was seen (Figure 2). The clinical and dermoscopic differential diagnosis was either an atypical nevus or an early melanoma. For a more precise diagnosis before excision, the lesion was evaluated with RCM, which takes 10 to 15 minutes to perform.
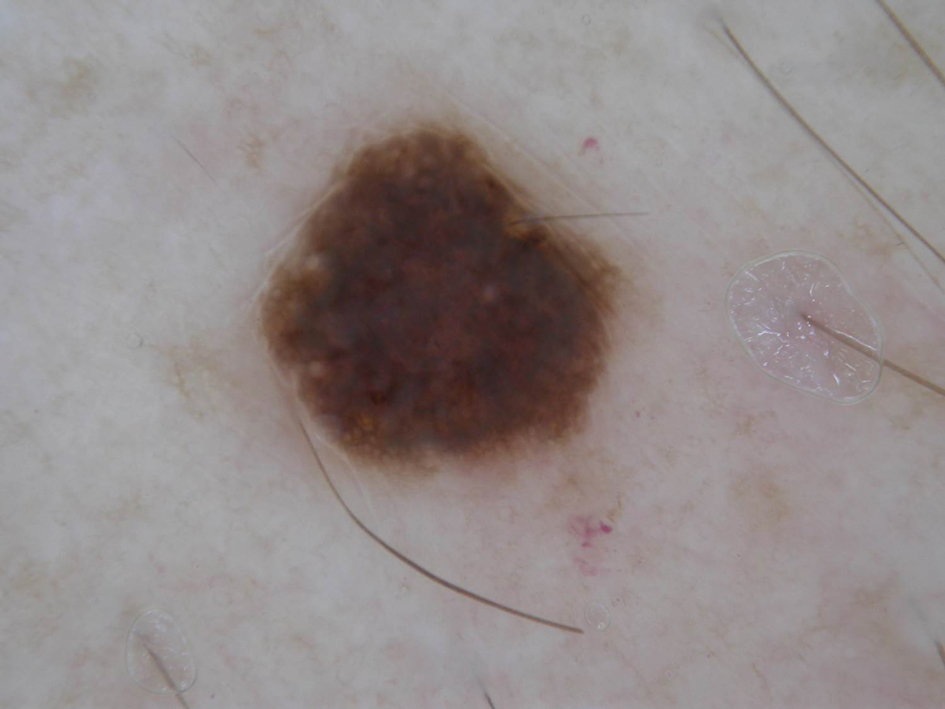
Under RCM at the epidermis level, there was a cobblestone pattern that showed a focus with mild disarrangement and few small, roundish, nucleated cells (Figure 3). A mosaic image, akin to low-magnification microscopy that enables overview of the entire lesion, at the level of the dermoepidermal junction (DEJ) showed an overall irregular meshwork pattern. Higher-magnification optical sections showed marked and diffuse (extending >10% of lesion area) architectural disorder with confluent junctional nests that were irregular to bizarre in shape and uneven in size and spacing as well as edged and nonedged papillae. At the superficial dermal level, atypical bright nucleated cells (>5 cells/mm2) were observed (Figure 4). Bright dots and/or plump bright cells within papillae also were observed. These RCM findings were highly suggestive for melanoma.
Histopathology showed an asymmetric, junctional, lentiginous, and nested proliferation of atypical epithelioid melanocytes, with few melanocytes in a pagetoid spread. There were small nests of atypical epithelioid melanocytes at the superficial dermis extending to a depth of 0.3 mm. The atypical epithelioid melanocytes displayed angulated hyperchromatic nuclei with conspicuous nucleoli and dusty brown cytoplasm. There was notable inflammation and pigment incontinence at the dermis. There was no evidence of ulceration or mitosis at the dermal component. The diagnosis of a pT1a malignant melanoma was reported (Figure 5).
Comment
A small but enlarging dark gray papule with reticular gray-blue areas under dermoscopy in a 57-year-old man is obviously suspicious for melanoma. In daily practice, this type of small-diameter melanoma is difficult to diagnose with high confidence. We balance our aim to diagnose melanomas early with the need to reduce unnecessary excisions. Reflectance confocal microscopy may allow the clinician to arrive at the correct diagnosis and management decision with confidence before excision of the lesion.
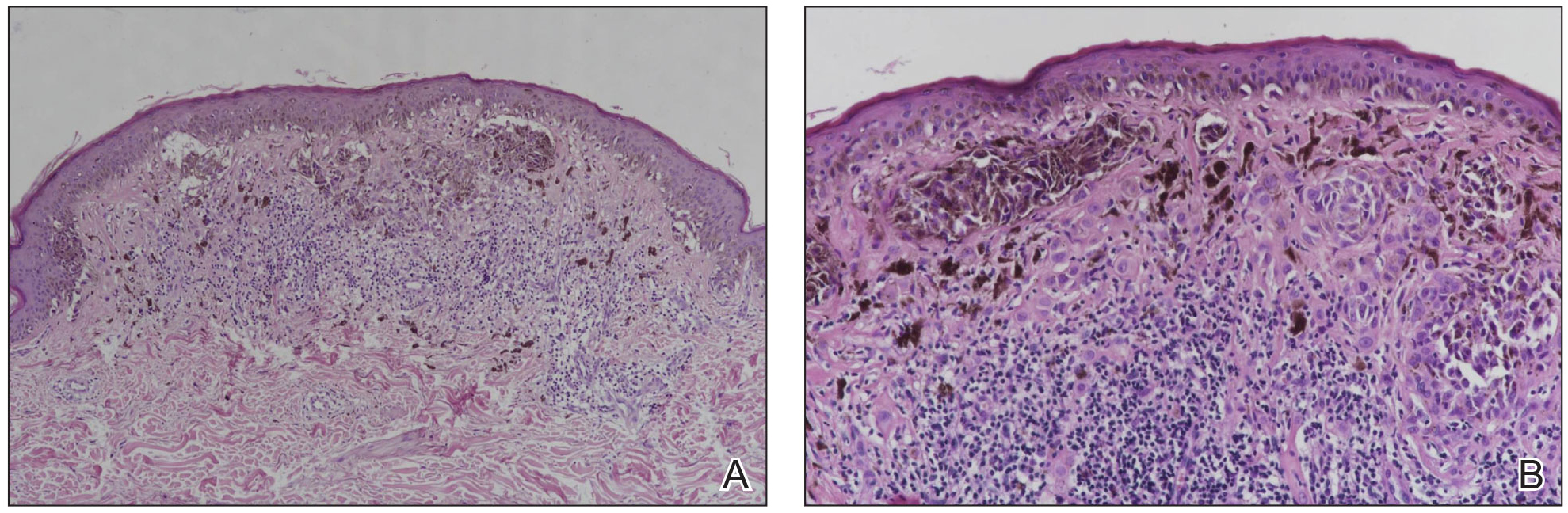
The distinction of a small-diameter melanoma from a nevus via RCM relies on evaluation of the architectural and cellular features. Findings on RCM in small-diameter melanomas have been scarcely reported in the literature; Pupelli et al10 evaluated small melanomas with a diameter of 2 to 5 mm. Among these small-diameter melanomas, the RCM features suggestive for melanomas were the presence of cytologic atypia with cellular pleomorphism, architectural disorder with irregular nests, at least 5 pagetoid cells/mm2, dendrites or tangled lines (ie, short fine lines with no visible nucleus interlacing with the adjacent keratinocytes) within the epidermis, and atypical roundish cells at the DEJ.10
The distinction between an atypical nevus and a small-diameter melanoma using RCM occasionally may be challenging.11 Pellacani et al12 reported an algorithm to distinguish melanoma from atypical nevi. According to this algorithm, when at least 1 of the architectural atypia features (irregular junctional nests, short interconnections between junctional nests, and nonhomogeneous cellularity within junctional nests) and at least 1 of the cytologic atypia features (round pagetoid cells or atypical cells at the DEJ) are observed simultaneously, the lesion is diagnosed as a dysplastic nevus or a melanoma in the first step. In the second step, the RCM diagnosis of melanoma requires at least 1 of 3 parameters: roundish pagetoid cells encompassing at least 50% of the lesional area at the spinous layer, atypical cells involving at least 50% of the lesional area at the DEJ level, and nonedged papillae involving at least 10% of the lesional area.12 Accordingly, our case corresponded with these RCM criteria for a melanoma, given that there were irregular junctional nests, atypical cells at the DEJ, and nonedged papillae involving at least 10% of the lesion.
The current limitations of RCM are the high cost of the device (approximately $58,125–$139,400 for different models), the amount of time needed to train staff in RCM units (seminars, congresses, and special courses organized by the International Confocal Working Group), and the amount of time needed for evaluation of individual lesions (15–20 minutes). However, RCM can be valuable in the clinical diagnosis of difficult lesions, as seen in our case.
Conclusion
Our case highlights the benefit of RCM in allowing the confident diagnosis and correct management of a small-diameter melanoma that turned out to be a melanoma with 0.3-mm Breslow thickness. Even so, histopathologic evaluation remains the gold standard for the diagnosis of melanoma.
- Bergman R, Katz I, Lichtig C, et al. Malignant melanomas with histologic diameters less than 6 mm. J Am Acad Dermatol. 1992;26:462-466.
- Bono A, Tolomio E, Trincone S, et al. Micro-melanoma detection: a clinical study on 206 consecutive cases of pigmented skin lesions with a diameter < or = 3 mm. Br J Dermatol. 2006;155:570-573.
- Bono A, Bartoli C, Baldi M, et al. Micro-melanoma detection. a clinical study on 22 cases of melanoma with a diameter equal to or less than 3 mm. Tumori. 2004;90:128-131.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol. 2014;171:1044-1051.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Ferrari B, Pupelli G, Farnetani F, et al. Dermoscopic difficult lesions: an objective evaluation of reflectance confocal microscopy impact for accurate diagnosis. J Eur Acad Dermatol Venereol. 2015;29:1135-1140.
- Dinnes J, Deeks JJ, Saleh D, et al. Reflectance confocal microscopy for diagnosing cutaneous melanoma in adults. Cochrane Database Syst Rev. 2018;12:CD013190.
- Xiong YQ, Ma SJ, Mo Y, et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627-1635.
- Pupelli G, Longo C, Veneziano L, et al. Small-diameter melanocytic lesions: morphological analysis by means of in vivo confocal microscopy. Br J Dermatol. 2013;168:1027-1033.
- Carrera C, Marghoob AA. Discriminating nevi from melanomas: clues and pitfalls. Dermatol Clin. 2016;34:395-409.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
Melanomas have been designated as small melanomas or micromelanomas according to their long-axis diameter (<6 mm and ≤3 mm, respectively).1-3 Because small-diameter melanomas also have the potential to metastasize, particularly if nodular, early diagnosis can be highly rewarding. Deep melanomas with small diameters may have the same potential for metastasis as large-diameter melanomas. In this context, dermoscopy, digital dermoscopic monitoring, and total-body photography are useful in clinical practice. However, these techniques are of limited utility for small, dermoscopic feature–poor melanomas. Conversely, less than 10% of changing lesions, which are spotted via digital dermoscopic surveillance, turn out to be melanomas; therefore, simply removing all changing lesions may result in many unnecessary excisions of benign lesions.4
In vivo reflectance confocal microscopy (RCM) is an advanced technique that allows recognition of the architectural and cellular details of pigmented lesions. Reflectance confocal microscopy has the potential to reduce the rate of unnecessary excisions and to diminish the risk for missing a melanoma.5-7 In meta-analyses, RCM sensitivity was reported as 90% to 93% and specificity was reported as 78% to 82% in detecting melanoma.8,9
We describe a case that highlights the potential role of RCM in the diagnosis of small-diameter melanomas.

Case Report
A 57-year-old man with Fitzpatrick skin type III presented to the dermato-oncology unit for evaluation of multiple nevi. He was otherwise healthy and denied a history of skin cancer. Total-body skin examination with dermoscopy was performed, and several mildly atypical lesions were identified. We decided to perform digital dermoscopic monitoring. The patient’s 6-month monitoring appointment had been scheduled, but he did not arrive for the follow-up visit until 10 months after the initial examination. A lesion on the left arm, which initially was 1.5 mm in diameter, had enlarged. It was now a dark brown–gray papule with a 2.5-mm diameter (Figure 1). Dermoscopy revealed grayish globules/dots at the center of the lesion, reticular gray-blue areas, and few milialike cysts; at the periphery, a narrow rim of brownish delicate pigment network also was seen (Figure 2). The clinical and dermoscopic differential diagnosis was either an atypical nevus or an early melanoma. For a more precise diagnosis before excision, the lesion was evaluated with RCM, which takes 10 to 15 minutes to perform.

Under RCM at the epidermis level, there was a cobblestone pattern that showed a focus with mild disarrangement and few small, roundish, nucleated cells (Figure 3). A mosaic image, akin to low-magnification microscopy that enables overview of the entire lesion, at the level of the dermoepidermal junction (DEJ) showed an overall irregular meshwork pattern. Higher-magnification optical sections showed marked and diffuse (extending >10% of lesion area) architectural disorder with confluent junctional nests that were irregular to bizarre in shape and uneven in size and spacing as well as edged and nonedged papillae. At the superficial dermal level, atypical bright nucleated cells (>5 cells/mm2) were observed (Figure 4). Bright dots and/or plump bright cells within papillae also were observed. These RCM findings were highly suggestive for melanoma.

Histopathology showed an asymmetric, junctional, lentiginous, and nested proliferation of atypical epithelioid melanocytes, with few melanocytes in a pagetoid spread. There were small nests of atypical epithelioid melanocytes at the superficial dermis extending to a depth of 0.3 mm. The atypical epithelioid melanocytes displayed angulated hyperchromatic nuclei with conspicuous nucleoli and dusty brown cytoplasm. There was notable inflammation and pigment incontinence at the dermis. There was no evidence of ulceration or mitosis at the dermal component. The diagnosis of a pT1a malignant melanoma was reported (Figure 5).

Comment
A small but enlarging dark gray papule with reticular gray-blue areas under dermoscopy in a 57-year-old man is obviously suspicious for melanoma. In daily practice, this type of small-diameter melanoma is difficult to diagnose with high confidence. We balance our aim to diagnose melanomas early with the need to reduce unnecessary excisions. Reflectance confocal microscopy may allow the clinician to arrive at the correct diagnosis and management decision with confidence before excision of the lesion.

The distinction of a small-diameter melanoma from a nevus via RCM relies on evaluation of the architectural and cellular features. Findings on RCM in small-diameter melanomas have been scarcely reported in the literature; Pupelli et al10 evaluated small melanomas with a diameter of 2 to 5 mm. Among these small-diameter melanomas, the RCM features suggestive for melanomas were the presence of cytologic atypia with cellular pleomorphism, architectural disorder with irregular nests, at least 5 pagetoid cells/mm2, dendrites or tangled lines (ie, short fine lines with no visible nucleus interlacing with the adjacent keratinocytes) within the epidermis, and atypical roundish cells at the DEJ.10
The distinction between an atypical nevus and a small-diameter melanoma using RCM occasionally may be challenging.11 Pellacani et al12 reported an algorithm to distinguish melanoma from atypical nevi. According to this algorithm, when at least 1 of the architectural atypia features (irregular junctional nests, short interconnections between junctional nests, and nonhomogeneous cellularity within junctional nests) and at least 1 of the cytologic atypia features (round pagetoid cells or atypical cells at the DEJ) are observed simultaneously, the lesion is diagnosed as a dysplastic nevus or a melanoma in the first step. In the second step, the RCM diagnosis of melanoma requires at least 1 of 3 parameters: roundish pagetoid cells encompassing at least 50% of the lesional area at the spinous layer, atypical cells involving at least 50% of the lesional area at the DEJ level, and nonedged papillae involving at least 10% of the lesional area.12 Accordingly, our case corresponded with these RCM criteria for a melanoma, given that there were irregular junctional nests, atypical cells at the DEJ, and nonedged papillae involving at least 10% of the lesion.
The current limitations of RCM are the high cost of the device (approximately $58,125–$139,400 for different models), the amount of time needed to train staff in RCM units (seminars, congresses, and special courses organized by the International Confocal Working Group), and the amount of time needed for evaluation of individual lesions (15–20 minutes). However, RCM can be valuable in the clinical diagnosis of difficult lesions, as seen in our case.
Conclusion
Our case highlights the benefit of RCM in allowing the confident diagnosis and correct management of a small-diameter melanoma that turned out to be a melanoma with 0.3-mm Breslow thickness. Even so, histopathologic evaluation remains the gold standard for the diagnosis of melanoma.
Melanomas have been designated as small melanomas or micromelanomas according to their long-axis diameter (<6 mm and ≤3 mm, respectively).1-3 Because small-diameter melanomas also have the potential to metastasize, particularly if nodular, early diagnosis can be highly rewarding. Deep melanomas with small diameters may have the same potential for metastasis as large-diameter melanomas. In this context, dermoscopy, digital dermoscopic monitoring, and total-body photography are useful in clinical practice. However, these techniques are of limited utility for small, dermoscopic feature–poor melanomas. Conversely, less than 10% of changing lesions, which are spotted via digital dermoscopic surveillance, turn out to be melanomas; therefore, simply removing all changing lesions may result in many unnecessary excisions of benign lesions.4
In vivo reflectance confocal microscopy (RCM) is an advanced technique that allows recognition of the architectural and cellular details of pigmented lesions. Reflectance confocal microscopy has the potential to reduce the rate of unnecessary excisions and to diminish the risk for missing a melanoma.5-7 In meta-analyses, RCM sensitivity was reported as 90% to 93% and specificity was reported as 78% to 82% in detecting melanoma.8,9
We describe a case that highlights the potential role of RCM in the diagnosis of small-diameter melanomas.

Case Report
A 57-year-old man with Fitzpatrick skin type III presented to the dermato-oncology unit for evaluation of multiple nevi. He was otherwise healthy and denied a history of skin cancer. Total-body skin examination with dermoscopy was performed, and several mildly atypical lesions were identified. We decided to perform digital dermoscopic monitoring. The patient’s 6-month monitoring appointment had been scheduled, but he did not arrive for the follow-up visit until 10 months after the initial examination. A lesion on the left arm, which initially was 1.5 mm in diameter, had enlarged. It was now a dark brown–gray papule with a 2.5-mm diameter (Figure 1). Dermoscopy revealed grayish globules/dots at the center of the lesion, reticular gray-blue areas, and few milialike cysts; at the periphery, a narrow rim of brownish delicate pigment network also was seen (Figure 2). The clinical and dermoscopic differential diagnosis was either an atypical nevus or an early melanoma. For a more precise diagnosis before excision, the lesion was evaluated with RCM, which takes 10 to 15 minutes to perform.

Under RCM at the epidermis level, there was a cobblestone pattern that showed a focus with mild disarrangement and few small, roundish, nucleated cells (Figure 3). A mosaic image, akin to low-magnification microscopy that enables overview of the entire lesion, at the level of the dermoepidermal junction (DEJ) showed an overall irregular meshwork pattern. Higher-magnification optical sections showed marked and diffuse (extending >10% of lesion area) architectural disorder with confluent junctional nests that were irregular to bizarre in shape and uneven in size and spacing as well as edged and nonedged papillae. At the superficial dermal level, atypical bright nucleated cells (>5 cells/mm2) were observed (Figure 4). Bright dots and/or plump bright cells within papillae also were observed. These RCM findings were highly suggestive for melanoma.

Histopathology showed an asymmetric, junctional, lentiginous, and nested proliferation of atypical epithelioid melanocytes, with few melanocytes in a pagetoid spread. There were small nests of atypical epithelioid melanocytes at the superficial dermis extending to a depth of 0.3 mm. The atypical epithelioid melanocytes displayed angulated hyperchromatic nuclei with conspicuous nucleoli and dusty brown cytoplasm. There was notable inflammation and pigment incontinence at the dermis. There was no evidence of ulceration or mitosis at the dermal component. The diagnosis of a pT1a malignant melanoma was reported (Figure 5).

Comment
A small but enlarging dark gray papule with reticular gray-blue areas under dermoscopy in a 57-year-old man is obviously suspicious for melanoma. In daily practice, this type of small-diameter melanoma is difficult to diagnose with high confidence. We balance our aim to diagnose melanomas early with the need to reduce unnecessary excisions. Reflectance confocal microscopy may allow the clinician to arrive at the correct diagnosis and management decision with confidence before excision of the lesion.

The distinction of a small-diameter melanoma from a nevus via RCM relies on evaluation of the architectural and cellular features. Findings on RCM in small-diameter melanomas have been scarcely reported in the literature; Pupelli et al10 evaluated small melanomas with a diameter of 2 to 5 mm. Among these small-diameter melanomas, the RCM features suggestive for melanomas were the presence of cytologic atypia with cellular pleomorphism, architectural disorder with irregular nests, at least 5 pagetoid cells/mm2, dendrites or tangled lines (ie, short fine lines with no visible nucleus interlacing with the adjacent keratinocytes) within the epidermis, and atypical roundish cells at the DEJ.10
The distinction between an atypical nevus and a small-diameter melanoma using RCM occasionally may be challenging.11 Pellacani et al12 reported an algorithm to distinguish melanoma from atypical nevi. According to this algorithm, when at least 1 of the architectural atypia features (irregular junctional nests, short interconnections between junctional nests, and nonhomogeneous cellularity within junctional nests) and at least 1 of the cytologic atypia features (round pagetoid cells or atypical cells at the DEJ) are observed simultaneously, the lesion is diagnosed as a dysplastic nevus or a melanoma in the first step. In the second step, the RCM diagnosis of melanoma requires at least 1 of 3 parameters: roundish pagetoid cells encompassing at least 50% of the lesional area at the spinous layer, atypical cells involving at least 50% of the lesional area at the DEJ level, and nonedged papillae involving at least 10% of the lesional area.12 Accordingly, our case corresponded with these RCM criteria for a melanoma, given that there were irregular junctional nests, atypical cells at the DEJ, and nonedged papillae involving at least 10% of the lesion.
The current limitations of RCM are the high cost of the device (approximately $58,125–$139,400 for different models), the amount of time needed to train staff in RCM units (seminars, congresses, and special courses organized by the International Confocal Working Group), and the amount of time needed for evaluation of individual lesions (15–20 minutes). However, RCM can be valuable in the clinical diagnosis of difficult lesions, as seen in our case.
Conclusion
Our case highlights the benefit of RCM in allowing the confident diagnosis and correct management of a small-diameter melanoma that turned out to be a melanoma with 0.3-mm Breslow thickness. Even so, histopathologic evaluation remains the gold standard for the diagnosis of melanoma.
- Bergman R, Katz I, Lichtig C, et al. Malignant melanomas with histologic diameters less than 6 mm. J Am Acad Dermatol. 1992;26:462-466.
- Bono A, Tolomio E, Trincone S, et al. Micro-melanoma detection: a clinical study on 206 consecutive cases of pigmented skin lesions with a diameter < or = 3 mm. Br J Dermatol. 2006;155:570-573.
- Bono A, Bartoli C, Baldi M, et al. Micro-melanoma detection. a clinical study on 22 cases of melanoma with a diameter equal to or less than 3 mm. Tumori. 2004;90:128-131.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol. 2014;171:1044-1051.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Ferrari B, Pupelli G, Farnetani F, et al. Dermoscopic difficult lesions: an objective evaluation of reflectance confocal microscopy impact for accurate diagnosis. J Eur Acad Dermatol Venereol. 2015;29:1135-1140.
- Dinnes J, Deeks JJ, Saleh D, et al. Reflectance confocal microscopy for diagnosing cutaneous melanoma in adults. Cochrane Database Syst Rev. 2018;12:CD013190.
- Xiong YQ, Ma SJ, Mo Y, et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627-1635.
- Pupelli G, Longo C, Veneziano L, et al. Small-diameter melanocytic lesions: morphological analysis by means of in vivo confocal microscopy. Br J Dermatol. 2013;168:1027-1033.
- Carrera C, Marghoob AA. Discriminating nevi from melanomas: clues and pitfalls. Dermatol Clin. 2016;34:395-409.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
- Bergman R, Katz I, Lichtig C, et al. Malignant melanomas with histologic diameters less than 6 mm. J Am Acad Dermatol. 1992;26:462-466.
- Bono A, Tolomio E, Trincone S, et al. Micro-melanoma detection: a clinical study on 206 consecutive cases of pigmented skin lesions with a diameter < or = 3 mm. Br J Dermatol. 2006;155:570-573.
- Bono A, Bartoli C, Baldi M, et al. Micro-melanoma detection. a clinical study on 22 cases of melanoma with a diameter equal to or less than 3 mm. Tumori. 2004;90:128-131.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol. 2014;171:1044-1051.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Ferrari B, Pupelli G, Farnetani F, et al. Dermoscopic difficult lesions: an objective evaluation of reflectance confocal microscopy impact for accurate diagnosis. J Eur Acad Dermatol Venereol. 2015;29:1135-1140.
- Dinnes J, Deeks JJ, Saleh D, et al. Reflectance confocal microscopy for diagnosing cutaneous melanoma in adults. Cochrane Database Syst Rev. 2018;12:CD013190.
- Xiong YQ, Ma SJ, Mo Y, et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627-1635.
- Pupelli G, Longo C, Veneziano L, et al. Small-diameter melanocytic lesions: morphological analysis by means of in vivo confocal microscopy. Br J Dermatol. 2013;168:1027-1033.
- Carrera C, Marghoob AA. Discriminating nevi from melanomas: clues and pitfalls. Dermatol Clin. 2016;34:395-409.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
Practice Points
- Melanomas with a long-axis diameter smaller than 6 mm are considered small melanomas, and those with diameters of 3 mm and smaller are considered micromelanomas; both are difficult to detect.
- Digital dermoscopic monitoring and reflectance confocal microscopy are important tools in detecting small melanomas.
Harlequin Syndrome: Discovery of an Ancient Schwannoma
To the Editor:
A 52-year-old man who was otherwise healthy and a long-distance runner presented with the sudden onset of diminished sweating on the left side of the body of 6 weeks’ duration. While training for a marathon, he reported that he perspired only on the right side of the body during runs of 12 to 15 miles; he observed a lack of sweating on the left side of the face, left side of the trunk, left arm, and left leg. This absence of sweating was accompanied by intense flushing on the right side of the face and trunk.
The patient did not take any medications. He reported no history of trauma and exhibited no neurologic deficits. A chest radiograph was negative. Thyroid function testing and a comprehensive metabolic panel were normal. Contrast-enhanced computed tomography of the chest and abdomen revealed a 4.3-cm soft-tissue mass in the left superior mediastinum that was superior to the aortic arch, posterior to the left subclavian artery in proximity to the sympathetic chain, and lateral to the trachea. The patient was diagnosed with Harlequin syndrome (HS).
Open thoracotomy was performed to remove the lesion. Analysis of the mass showed cystic areas, areas of hemorrhage (Figure 1A), and alternating zones of compact Antoni A spindle cells admixed with areas of less orderly Antoni B spindle cells within a hypocellular stroma (Figure 1B). Individual cells were characterized by eosinophilic cytoplasm and tapered nuclei. The mass appeared to be completely encapsulated. No mitotic figures were seen on multiple slides. The cells stained diffusely positive for S-100 proteins. At 6-month follow-up, the patient reported that he did not notice any return of normal sweating on the left side. However, the right-sided flushing had resolved.
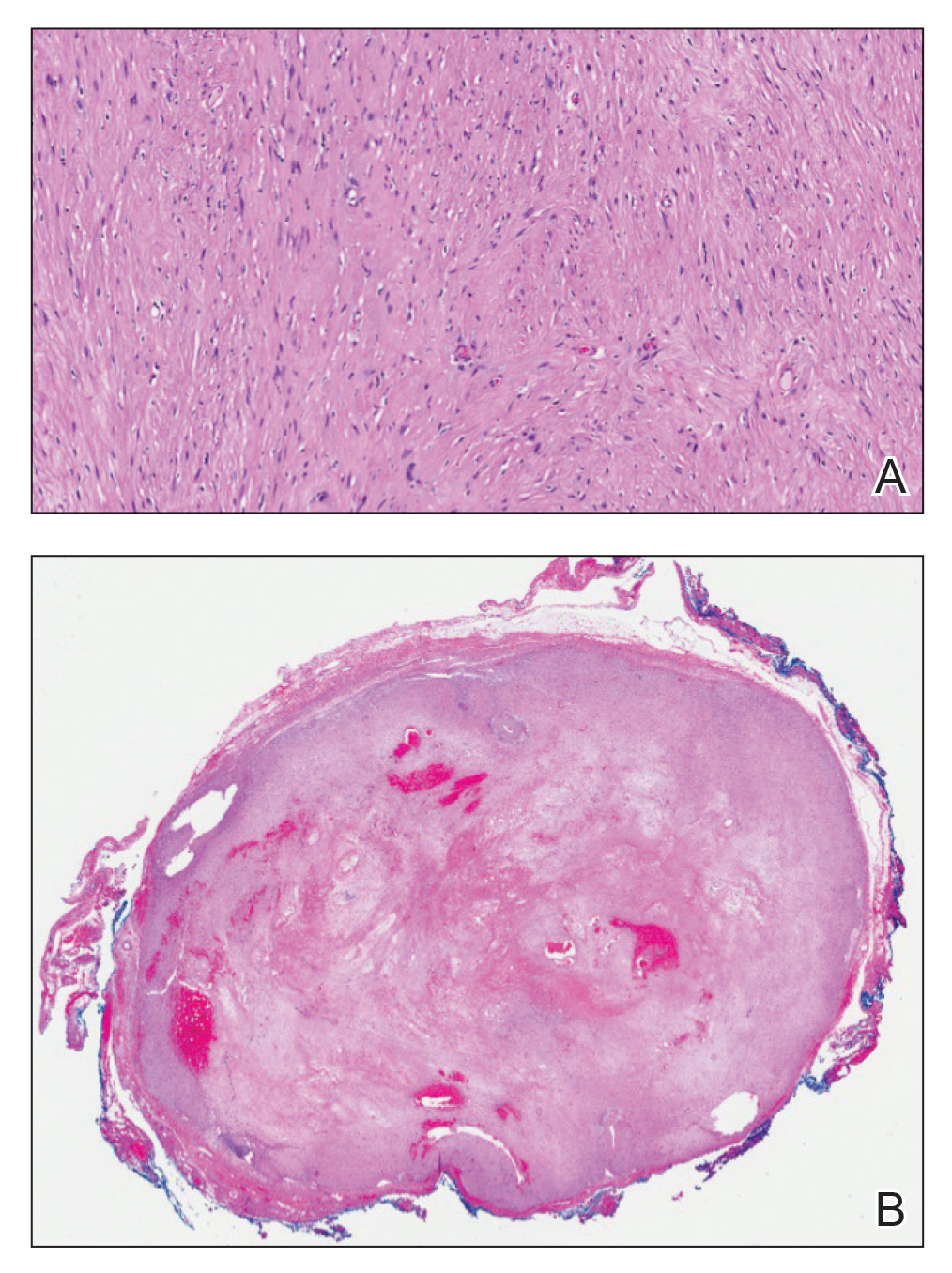
Harlequin syndrome (also called the Harlequin sign) is a rare disorder of the sympathetic nervous system and should not be confused with lethal harlequin-type ichthyosis, an autosomal-recessive congenital disorder in which the affected newborn’s skin is hard and thickened over most of the body.1 Harlequin syndrome usually is characterized by unilateral flushing and sweating that can affect the face, trunk, and extremities.2 Physical stimuli, such as exercising (as in our patient), high body temperature, and the consumption of spicy or pungent food, or an emotional response can unmask or exacerbate symptoms of HS. The syndrome also can present with cluster headache.3 Harlequin syndrome is more common in females (66% of cases).4 Originally, the side of the face marked by increased sweating and flushing was perceived to be the pathologic side; now it is recognized that the anhidrotic side is affected by the causative pathology. The side of the face characterized by flushing might gradually darken as it compensates for lack of thermal regulation on the other side.2,5
Usually, HS is an idiopathic condition associated with localized failure of upper thoracic sympathetic chain ganglia.5 A theory is that HS is part of a spectrum of autoimmune autonomic ganglionopathy.6 Typically, the syndrome is asymptomatic at rest, but testing can reveal an underlying sympathetic lesion.7 Structural lesions have been reported as a cause of the syndrome,6 similar to our patient.
Disrupted thermoregulatory vasodilation in HS is caused by an ipsilateral lesion of the sympathetic vasodilator neurons that innervate the face. Hemifacial anhidrosis also occurs because sudomotor neurons travel within the same pathways as vasodilator neurons.4
Our patient had a posterior mediastinal ancient schwannoma to the left of the subclavian artery, lateral to the trachea, with ipsilateral anhidrosis of the forehead, cheek, chin, and torso. In the medical literature, the forehead, cheek, and chin are described as being affected in HS when the lesion is located under the bifurcation of the carotid artery.3,5 Most of the sudomotor and vasomotor fibers that innervate the face leave the spinal cord through ventral roots T2-T34 (symptomatic areas are described in Figure 2), which correlates with the hypothesis that HS results from a deficit originating in the third thoracic nerve that is caused by a peripheral lesion affecting sympathetic outflow through the third thoracic root.2 The location of our patient’s lesion supports this claim.
Harlequin syndrome can present simultaneously with ipsilateral Horner, Adie, and Ross syndromes.8 There are varying clinical presentations of Horner syndrome. Some patients with HS show autonomic ocular signs, such as miosis and ptosis, exhibiting Horner syndrome as an additional feature.5 Adie syndrome is characterized by tonic pupils with hyporeflexia and is unilateral in most cases. Ross syndrome is similar to Adie syndrome—including tonic pupils with hyporeflexia—in addition to a finding of segmental anhidrosis; it is bilateral in most cases.4
In some cases, Horner syndrome and HS originate from unilateral pharmaceutical sympathetic denervation (ie, as a consequence of paravertebral spread of local anesthetic to ipsilateral stellate ganglion).9 Facial nonflushing areas in HS typically are identical with anhidrotic areas10; Horner syndrome often is ipsilateral to the affected sympathetic region.11
Our patient exhibited secondary HS from a tumor effect; however, an underlying tumor or infarct is absent in many cases. In primary (idiopathic) cases of HS, treatment is not recommended because the syndrome is benign.10,11
If symptoms of HS cause notable social embarrassment, contralateral sympathectomy can be considered.5,12 Repeated stellate ganglion block with a local anesthetic could be a less invasive treatment option.13 When considered on a case-by-case-basis, botulinum toxin type A has been effective as a treatment of compensatory hyperhidrosis on the unaffected side.14
In cases of secondary HS, surgical removal of the lesion may alleviate symptoms, though thoracotomy in our patient to remove the schwannoma did not alleviate anhidrosis. The Table lists treatment options for primary and secondary HS.4,5,11
- Harlequin ichthyosis. MedlinePlus. National Library of Medicine [Internet]. Updated January 7, 2022. Accessed April 5, 2022. https://ghr.nlm.nih.gov/condition/harlequin-ichthyosis
- Lance JW, Drummond PD, Gandevia SC, et al. Harlequin syndrome: the sudden onset of unilateral flushing and sweating. J Neurol Neurosurg Psych. 1988;51:635-642. doi:10.1136/jnnp.51.5.635
- Lehman K, Kumar N, Vu Q, et al. Harlequin syndrome in cluster headache. Headache. 2016;56:1053-1054. doi:10.1111/head.12852
- Willaert WIM, Scheltinga MRM, Steenhuisen SF, et al. Harlequin syndrome: two new cases and a management proposal. Acta Neurol Belg. 2009;109:214-220.
- Duddy ME, Baker MR. Images in clinical medicine. Harlequin’s darker side. N Engl J Med. 2007;357:E22. doi:10.1056/NEJMicm067851
- Karam C. Harlequin syndrome in a patient with putative autoimmune autonomic ganglionopathy. Auton Neurosci. 2016;194:58-59. doi:10.1016/j.autneu.2015.12.004
- Wasner G, Maag R, Ludwig J, et al. Harlequin syndrome—one face of many etiologies. Nat Clin Pract Neurol. 2005;1:54-59. doi:10.1038/ncpneuro0040
- Guilloton L, Demarquay G, Quesnel L, et al. Dysautonomic syndrome of the face with Harlequin sign and syndrome: three new cases and a review of the literature. Rev Neurol (Paris). 2013;169:884-891. doi:10.1016/j.neurol.2013.01.628
- Burlacu CL, Buggy DJ. Coexisting Harlequin and Horner syndromes after high thoracic paravertebral anaesthesia. Br J Anaesth. 2005;95:822-824. doi:10.1093/bja/aei258
- Morrison DA, Bibby K, Woodruff G. The “Harlequin” sign and congenital Horner’s syndrome. J Neurol Neurosurg Psych. 1997;62:626-628. doi:10.1136/jnnp.62.6.626
- Bremner F, Smith S. Pupillographic findings in 39 consecutive cases of Harlequin syndrome. J Neuroophthalmol. 2008;28:171-177. doi:10.1097/WNO.0b013e318183c885
- Kaur S, Aggarwal P, Jindal N, et al. Harlequin syndrome: a mask of rare dysautonomic syndromes. Dermatol Online J. 2015;21:13030/qt3q39d7mz.
- Reddy H, Fatah S, Gulve A, et al. Novel management of Harlequin syndrome with stellate ganglion block. Br J Dermatol. 2013;169:954-956. doi:10.1111/bjd.12561
- ManhRKJV, Spitz M, Vasconcellos LF. Botulinum toxin for treatment of Harlequin syndrome. Parkinsonism Relat Disord. 2016;23:112-113. doi:10.1016/j.parkreldis.2015.11.030
To the Editor:
A 52-year-old man who was otherwise healthy and a long-distance runner presented with the sudden onset of diminished sweating on the left side of the body of 6 weeks’ duration. While training for a marathon, he reported that he perspired only on the right side of the body during runs of 12 to 15 miles; he observed a lack of sweating on the left side of the face, left side of the trunk, left arm, and left leg. This absence of sweating was accompanied by intense flushing on the right side of the face and trunk.
The patient did not take any medications. He reported no history of trauma and exhibited no neurologic deficits. A chest radiograph was negative. Thyroid function testing and a comprehensive metabolic panel were normal. Contrast-enhanced computed tomography of the chest and abdomen revealed a 4.3-cm soft-tissue mass in the left superior mediastinum that was superior to the aortic arch, posterior to the left subclavian artery in proximity to the sympathetic chain, and lateral to the trachea. The patient was diagnosed with Harlequin syndrome (HS).
Open thoracotomy was performed to remove the lesion. Analysis of the mass showed cystic areas, areas of hemorrhage (Figure 1A), and alternating zones of compact Antoni A spindle cells admixed with areas of less orderly Antoni B spindle cells within a hypocellular stroma (Figure 1B). Individual cells were characterized by eosinophilic cytoplasm and tapered nuclei. The mass appeared to be completely encapsulated. No mitotic figures were seen on multiple slides. The cells stained diffusely positive for S-100 proteins. At 6-month follow-up, the patient reported that he did not notice any return of normal sweating on the left side. However, the right-sided flushing had resolved.

Harlequin syndrome (also called the Harlequin sign) is a rare disorder of the sympathetic nervous system and should not be confused with lethal harlequin-type ichthyosis, an autosomal-recessive congenital disorder in which the affected newborn’s skin is hard and thickened over most of the body.1 Harlequin syndrome usually is characterized by unilateral flushing and sweating that can affect the face, trunk, and extremities.2 Physical stimuli, such as exercising (as in our patient), high body temperature, and the consumption of spicy or pungent food, or an emotional response can unmask or exacerbate symptoms of HS. The syndrome also can present with cluster headache.3 Harlequin syndrome is more common in females (66% of cases).4 Originally, the side of the face marked by increased sweating and flushing was perceived to be the pathologic side; now it is recognized that the anhidrotic side is affected by the causative pathology. The side of the face characterized by flushing might gradually darken as it compensates for lack of thermal regulation on the other side.2,5
Usually, HS is an idiopathic condition associated with localized failure of upper thoracic sympathetic chain ganglia.5 A theory is that HS is part of a spectrum of autoimmune autonomic ganglionopathy.6 Typically, the syndrome is asymptomatic at rest, but testing can reveal an underlying sympathetic lesion.7 Structural lesions have been reported as a cause of the syndrome,6 similar to our patient.
Disrupted thermoregulatory vasodilation in HS is caused by an ipsilateral lesion of the sympathetic vasodilator neurons that innervate the face. Hemifacial anhidrosis also occurs because sudomotor neurons travel within the same pathways as vasodilator neurons.4
Our patient had a posterior mediastinal ancient schwannoma to the left of the subclavian artery, lateral to the trachea, with ipsilateral anhidrosis of the forehead, cheek, chin, and torso. In the medical literature, the forehead, cheek, and chin are described as being affected in HS when the lesion is located under the bifurcation of the carotid artery.3,5 Most of the sudomotor and vasomotor fibers that innervate the face leave the spinal cord through ventral roots T2-T34 (symptomatic areas are described in Figure 2), which correlates with the hypothesis that HS results from a deficit originating in the third thoracic nerve that is caused by a peripheral lesion affecting sympathetic outflow through the third thoracic root.2 The location of our patient’s lesion supports this claim.

Harlequin syndrome can present simultaneously with ipsilateral Horner, Adie, and Ross syndromes.8 There are varying clinical presentations of Horner syndrome. Some patients with HS show autonomic ocular signs, such as miosis and ptosis, exhibiting Horner syndrome as an additional feature.5 Adie syndrome is characterized by tonic pupils with hyporeflexia and is unilateral in most cases. Ross syndrome is similar to Adie syndrome—including tonic pupils with hyporeflexia—in addition to a finding of segmental anhidrosis; it is bilateral in most cases.4
In some cases, Horner syndrome and HS originate from unilateral pharmaceutical sympathetic denervation (ie, as a consequence of paravertebral spread of local anesthetic to ipsilateral stellate ganglion).9 Facial nonflushing areas in HS typically are identical with anhidrotic areas10; Horner syndrome often is ipsilateral to the affected sympathetic region.11
Our patient exhibited secondary HS from a tumor effect; however, an underlying tumor or infarct is absent in many cases. In primary (idiopathic) cases of HS, treatment is not recommended because the syndrome is benign.10,11
If symptoms of HS cause notable social embarrassment, contralateral sympathectomy can be considered.5,12 Repeated stellate ganglion block with a local anesthetic could be a less invasive treatment option.13 When considered on a case-by-case-basis, botulinum toxin type A has been effective as a treatment of compensatory hyperhidrosis on the unaffected side.14
In cases of secondary HS, surgical removal of the lesion may alleviate symptoms, though thoracotomy in our patient to remove the schwannoma did not alleviate anhidrosis. The Table lists treatment options for primary and secondary HS.4,5,11

To the Editor:
A 52-year-old man who was otherwise healthy and a long-distance runner presented with the sudden onset of diminished sweating on the left side of the body of 6 weeks’ duration. While training for a marathon, he reported that he perspired only on the right side of the body during runs of 12 to 15 miles; he observed a lack of sweating on the left side of the face, left side of the trunk, left arm, and left leg. This absence of sweating was accompanied by intense flushing on the right side of the face and trunk.
The patient did not take any medications. He reported no history of trauma and exhibited no neurologic deficits. A chest radiograph was negative. Thyroid function testing and a comprehensive metabolic panel were normal. Contrast-enhanced computed tomography of the chest and abdomen revealed a 4.3-cm soft-tissue mass in the left superior mediastinum that was superior to the aortic arch, posterior to the left subclavian artery in proximity to the sympathetic chain, and lateral to the trachea. The patient was diagnosed with Harlequin syndrome (HS).
Open thoracotomy was performed to remove the lesion. Analysis of the mass showed cystic areas, areas of hemorrhage (Figure 1A), and alternating zones of compact Antoni A spindle cells admixed with areas of less orderly Antoni B spindle cells within a hypocellular stroma (Figure 1B). Individual cells were characterized by eosinophilic cytoplasm and tapered nuclei. The mass appeared to be completely encapsulated. No mitotic figures were seen on multiple slides. The cells stained diffusely positive for S-100 proteins. At 6-month follow-up, the patient reported that he did not notice any return of normal sweating on the left side. However, the right-sided flushing had resolved.

Harlequin syndrome (also called the Harlequin sign) is a rare disorder of the sympathetic nervous system and should not be confused with lethal harlequin-type ichthyosis, an autosomal-recessive congenital disorder in which the affected newborn’s skin is hard and thickened over most of the body.1 Harlequin syndrome usually is characterized by unilateral flushing and sweating that can affect the face, trunk, and extremities.2 Physical stimuli, such as exercising (as in our patient), high body temperature, and the consumption of spicy or pungent food, or an emotional response can unmask or exacerbate symptoms of HS. The syndrome also can present with cluster headache.3 Harlequin syndrome is more common in females (66% of cases).4 Originally, the side of the face marked by increased sweating and flushing was perceived to be the pathologic side; now it is recognized that the anhidrotic side is affected by the causative pathology. The side of the face characterized by flushing might gradually darken as it compensates for lack of thermal regulation on the other side.2,5
Usually, HS is an idiopathic condition associated with localized failure of upper thoracic sympathetic chain ganglia.5 A theory is that HS is part of a spectrum of autoimmune autonomic ganglionopathy.6 Typically, the syndrome is asymptomatic at rest, but testing can reveal an underlying sympathetic lesion.7 Structural lesions have been reported as a cause of the syndrome,6 similar to our patient.
Disrupted thermoregulatory vasodilation in HS is caused by an ipsilateral lesion of the sympathetic vasodilator neurons that innervate the face. Hemifacial anhidrosis also occurs because sudomotor neurons travel within the same pathways as vasodilator neurons.4
Our patient had a posterior mediastinal ancient schwannoma to the left of the subclavian artery, lateral to the trachea, with ipsilateral anhidrosis of the forehead, cheek, chin, and torso. In the medical literature, the forehead, cheek, and chin are described as being affected in HS when the lesion is located under the bifurcation of the carotid artery.3,5 Most of the sudomotor and vasomotor fibers that innervate the face leave the spinal cord through ventral roots T2-T34 (symptomatic areas are described in Figure 2), which correlates with the hypothesis that HS results from a deficit originating in the third thoracic nerve that is caused by a peripheral lesion affecting sympathetic outflow through the third thoracic root.2 The location of our patient’s lesion supports this claim.

Harlequin syndrome can present simultaneously with ipsilateral Horner, Adie, and Ross syndromes.8 There are varying clinical presentations of Horner syndrome. Some patients with HS show autonomic ocular signs, such as miosis and ptosis, exhibiting Horner syndrome as an additional feature.5 Adie syndrome is characterized by tonic pupils with hyporeflexia and is unilateral in most cases. Ross syndrome is similar to Adie syndrome—including tonic pupils with hyporeflexia—in addition to a finding of segmental anhidrosis; it is bilateral in most cases.4
In some cases, Horner syndrome and HS originate from unilateral pharmaceutical sympathetic denervation (ie, as a consequence of paravertebral spread of local anesthetic to ipsilateral stellate ganglion).9 Facial nonflushing areas in HS typically are identical with anhidrotic areas10; Horner syndrome often is ipsilateral to the affected sympathetic region.11
Our patient exhibited secondary HS from a tumor effect; however, an underlying tumor or infarct is absent in many cases. In primary (idiopathic) cases of HS, treatment is not recommended because the syndrome is benign.10,11
If symptoms of HS cause notable social embarrassment, contralateral sympathectomy can be considered.5,12 Repeated stellate ganglion block with a local anesthetic could be a less invasive treatment option.13 When considered on a case-by-case-basis, botulinum toxin type A has been effective as a treatment of compensatory hyperhidrosis on the unaffected side.14
In cases of secondary HS, surgical removal of the lesion may alleviate symptoms, though thoracotomy in our patient to remove the schwannoma did not alleviate anhidrosis. The Table lists treatment options for primary and secondary HS.4,5,11

- Harlequin ichthyosis. MedlinePlus. National Library of Medicine [Internet]. Updated January 7, 2022. Accessed April 5, 2022. https://ghr.nlm.nih.gov/condition/harlequin-ichthyosis
- Lance JW, Drummond PD, Gandevia SC, et al. Harlequin syndrome: the sudden onset of unilateral flushing and sweating. J Neurol Neurosurg Psych. 1988;51:635-642. doi:10.1136/jnnp.51.5.635
- Lehman K, Kumar N, Vu Q, et al. Harlequin syndrome in cluster headache. Headache. 2016;56:1053-1054. doi:10.1111/head.12852
- Willaert WIM, Scheltinga MRM, Steenhuisen SF, et al. Harlequin syndrome: two new cases and a management proposal. Acta Neurol Belg. 2009;109:214-220.
- Duddy ME, Baker MR. Images in clinical medicine. Harlequin’s darker side. N Engl J Med. 2007;357:E22. doi:10.1056/NEJMicm067851
- Karam C. Harlequin syndrome in a patient with putative autoimmune autonomic ganglionopathy. Auton Neurosci. 2016;194:58-59. doi:10.1016/j.autneu.2015.12.004
- Wasner G, Maag R, Ludwig J, et al. Harlequin syndrome—one face of many etiologies. Nat Clin Pract Neurol. 2005;1:54-59. doi:10.1038/ncpneuro0040
- Guilloton L, Demarquay G, Quesnel L, et al. Dysautonomic syndrome of the face with Harlequin sign and syndrome: three new cases and a review of the literature. Rev Neurol (Paris). 2013;169:884-891. doi:10.1016/j.neurol.2013.01.628
- Burlacu CL, Buggy DJ. Coexisting Harlequin and Horner syndromes after high thoracic paravertebral anaesthesia. Br J Anaesth. 2005;95:822-824. doi:10.1093/bja/aei258
- Morrison DA, Bibby K, Woodruff G. The “Harlequin” sign and congenital Horner’s syndrome. J Neurol Neurosurg Psych. 1997;62:626-628. doi:10.1136/jnnp.62.6.626
- Bremner F, Smith S. Pupillographic findings in 39 consecutive cases of Harlequin syndrome. J Neuroophthalmol. 2008;28:171-177. doi:10.1097/WNO.0b013e318183c885
- Kaur S, Aggarwal P, Jindal N, et al. Harlequin syndrome: a mask of rare dysautonomic syndromes. Dermatol Online J. 2015;21:13030/qt3q39d7mz.
- Reddy H, Fatah S, Gulve A, et al. Novel management of Harlequin syndrome with stellate ganglion block. Br J Dermatol. 2013;169:954-956. doi:10.1111/bjd.12561
- ManhRKJV, Spitz M, Vasconcellos LF. Botulinum toxin for treatment of Harlequin syndrome. Parkinsonism Relat Disord. 2016;23:112-113. doi:10.1016/j.parkreldis.2015.11.030
- Harlequin ichthyosis. MedlinePlus. National Library of Medicine [Internet]. Updated January 7, 2022. Accessed April 5, 2022. https://ghr.nlm.nih.gov/condition/harlequin-ichthyosis
- Lance JW, Drummond PD, Gandevia SC, et al. Harlequin syndrome: the sudden onset of unilateral flushing and sweating. J Neurol Neurosurg Psych. 1988;51:635-642. doi:10.1136/jnnp.51.5.635
- Lehman K, Kumar N, Vu Q, et al. Harlequin syndrome in cluster headache. Headache. 2016;56:1053-1054. doi:10.1111/head.12852
- Willaert WIM, Scheltinga MRM, Steenhuisen SF, et al. Harlequin syndrome: two new cases and a management proposal. Acta Neurol Belg. 2009;109:214-220.
- Duddy ME, Baker MR. Images in clinical medicine. Harlequin’s darker side. N Engl J Med. 2007;357:E22. doi:10.1056/NEJMicm067851
- Karam C. Harlequin syndrome in a patient with putative autoimmune autonomic ganglionopathy. Auton Neurosci. 2016;194:58-59. doi:10.1016/j.autneu.2015.12.004
- Wasner G, Maag R, Ludwig J, et al. Harlequin syndrome—one face of many etiologies. Nat Clin Pract Neurol. 2005;1:54-59. doi:10.1038/ncpneuro0040
- Guilloton L, Demarquay G, Quesnel L, et al. Dysautonomic syndrome of the face with Harlequin sign and syndrome: three new cases and a review of the literature. Rev Neurol (Paris). 2013;169:884-891. doi:10.1016/j.neurol.2013.01.628
- Burlacu CL, Buggy DJ. Coexisting Harlequin and Horner syndromes after high thoracic paravertebral anaesthesia. Br J Anaesth. 2005;95:822-824. doi:10.1093/bja/aei258
- Morrison DA, Bibby K, Woodruff G. The “Harlequin” sign and congenital Horner’s syndrome. J Neurol Neurosurg Psych. 1997;62:626-628. doi:10.1136/jnnp.62.6.626
- Bremner F, Smith S. Pupillographic findings in 39 consecutive cases of Harlequin syndrome. J Neuroophthalmol. 2008;28:171-177. doi:10.1097/WNO.0b013e318183c885
- Kaur S, Aggarwal P, Jindal N, et al. Harlequin syndrome: a mask of rare dysautonomic syndromes. Dermatol Online J. 2015;21:13030/qt3q39d7mz.
- Reddy H, Fatah S, Gulve A, et al. Novel management of Harlequin syndrome with stellate ganglion block. Br J Dermatol. 2013;169:954-956. doi:10.1111/bjd.12561
- ManhRKJV, Spitz M, Vasconcellos LF. Botulinum toxin for treatment of Harlequin syndrome. Parkinsonism Relat Disord. 2016;23:112-113. doi:10.1016/j.parkreldis.2015.11.030
Practice Points
- Harlequin syndrome is a rare disorder of the sympathetic nervous system that is characterized by unilateral flushing and sweating that can affect the face, trunk, and extremities.
- Secondary causes can be from schwannomas in the cervical chain ganglion.