User login
Does metformin reduce risk for death in COVID-19?
Accumulating observational data suggest that metformin use in patients with type 2 diabetes might reduce the risk for death from COVID-19, but the randomized trials needed to prove this are unlikely to be carried out, according to experts.
The latest results, which are not yet peer reviewed, were published online July 31. The study was conducted by Andrew B. Crouse, PhD, of the Hugh Kaul Precision Medicine Institute, University of Alabama at Birmingham, and colleagues.
The researchers found that among more than 600 patients with diabetes and COVID-19, use of metformin was associated with a nearly 70% reduction in mortality after adjustment for multiple confounders.
Data from four previous studies that also show a reduction in mortality among metformin users compared to nonusers were summarized in a “mini review” by André J. Scheen, MD, PhD, published Aug. 1 in Diabetes and Metabolism.
Dr. Scheen, of the division of diabetes, nutrition, and metabolic disorders and the division of clinical pharmacology at Liège (Belgium) University, discussed possible mechanisms behind this observation.
“Because metformin exerts various effects beyond its glucose-lowering action, among which are anti-inflammatory effects, it may be speculated that this biguanide might positively influence the prognosis of patients with [type 2 diabetes] hospitalized for COVID-19,” he said.
“However, given the potential confounders inherently found in observational studies, caution is required before drawing any firm conclusions in the absence of randomized controlled trials,” Dr. Scheen wrote.
Indeed, when asked to comment, endocrinologist Kasia Lipska, MD, of Yale University, New Haven, Conn., said in an interview: “Metformin users tend to do better in many different settings with respect to many different outcomes. To me, it is still unclear whether metformin is truly a miracle drug or whether it is simply used more often among people who are healthier and who do not have contraindications to its use.”
She added, “I don’t think we have enough data to suggest metformin use for COVID-19 mitigation at this point.”
Alabama authors say confounding effects ‘unlikely’
In the retrospective analysis of electronic health records from their institution, Dr. Crouse and colleagues reviewed data from 604 patients who were confirmed to have tested positive for COVID-19 between Feb. 25 and June 22, 2020. Of those individuals, 40% had diabetes.
Death occurred in 11% (n = 67); the odds ratio (OR) for death among those with, vs. without, diabetes was 3.62 (P < .0001).
Individuals with diabetes accounted for >60% of all deaths. In multiple logistic regression, age 50-70 vs. <50, male sex, and diabetes emerged as independent predictors of death.
Of the 42 patients with diabetes who died, 8 (19%) had used metformin, and 34 (81%) had not*, a significant difference (OR, 0.38; P = .0221). Insulin use, on the other hand, had no effect on mortality (P = .5728).
“In fact, with 11% [being] the mortality of metformin users, [this] was comparable to that of the general COVID-19-positive population and dramatically lower than the 23% mortality observed in subjects with diabetes and not on metformin,” the authors said.
The survival benefit observed with metformin remained after exclusion of patients with classic metformin contraindications, such as chronic kidney disease and heart failure (OR, 0.17; P = .0231).
“This makes any potential confounding effects from skewing metformin users toward healthier subjects without these additional comorbidities very unlikely,” Dr. Crouse and colleagues contended.
After further analysis that controlled for other covariates (age, sex, obesity status, and hypertension), age, sex, and metformin use remained independent predictors of mortality.
For metformin, the odds ratio was 0.33 (P = .0210).
But, Dr. Lipska pointed out, “Observational studies can take into account confounders that are measured. However, unmeasured confounders may still affect the conclusions of these studies ... Propensity score matching to account for the likelihood of use of metformin could be used to better account for differences between metformin users and nonusers.”
If metformin does reduce COVID-19 deaths, multiple mechanisms likely
In his article, Dr. Scheen noted that several mechanisms have been proposed for the possible beneficial effect of metformin on COVID-19 outcomes, including direct improvements in glucose control, body weight, and insulin resistance; reduction in inflammation; inhibition of virus penetration via phosphorylation of ACE2; inhibition of an immune hyperactivation pathway; and neutrophil reduction. All remain theoretical, he emphasized.
He noted that some authors have raised concerns about possible harms from the use of metformin by patients with type 2 diabetes who are hospitalized for COVID-19, particularly because of the potential risk for lactic acidosis in cases of multiple organ failure.
In totality, four studies suggest 25% death reduction with metformin
Taken together, the four observational studies that Dr. Scheen reviewed showed that metformin had a positive effect, with an overall 25% reduction in death (P < .00001), albeit with relatively high heterogeneity (I² = 61%).
The largest of these, from the United States, included 6,256 patients hospitalized with COVID-19 and involved propensity matching. A significant reduction in mortality with metformin use was seen in women but not men (odds ratio, 0.759).
The French Coronavirus-SARS-CoV-2 and Diabetes Outcomes (CORONADO) study of 1,317 patients with diabetes and confirmed COVID-19 who were admitted to 53 French hospitals also showed a significant survival benefit for metformin, although the study wasn’t designed to address that issue.
In that study, the odds ratio for death on day 7 in prior metformin users compared to nonusers was 0.59. This finding lost significance but remained a trend after full adjustments (0.80).
Two smaller observational studies produced similar trends toward survival benefit with metformin.
Nonetheless, Dr. Scheen cautioned: “Firm conclusions about the impact of metformin therapy can only be drawn from double-blind randomized controlled trials (RCTs), and such trials are almost impossible in the context of COVID-19.”
He added: “Because metformin is out of patent and very inexpensive, no pharmaceutical company is likely to be interested in planning a study to demonstrate the benefits of metformin on COVID-19-related clinical outcomes.”
Dr. Lipska agreed: “RCTs are unlikely to be conducted to settle these issues. In their absence, metformin use should be based on its safety and effectiveness profile.”
Dr. Scheen concluded, however, that “there are at least no negative safety indications, so there is no reason to stop metformin therapy during COVID-19 infection except in cases of severe gastrointestinal symptoms, hypoxia and/or multiple organ failure.”
Dr. Lipska has received grants from the National Institutes of Health and works under contract for the Centers for Medicare & Medicaid Services to develop publicly reported quality measures. Dr. Scheen has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
*A previous version reversed these two outcomes in error.
Accumulating observational data suggest that metformin use in patients with type 2 diabetes might reduce the risk for death from COVID-19, but the randomized trials needed to prove this are unlikely to be carried out, according to experts.
The latest results, which are not yet peer reviewed, were published online July 31. The study was conducted by Andrew B. Crouse, PhD, of the Hugh Kaul Precision Medicine Institute, University of Alabama at Birmingham, and colleagues.
The researchers found that among more than 600 patients with diabetes and COVID-19, use of metformin was associated with a nearly 70% reduction in mortality after adjustment for multiple confounders.
Data from four previous studies that also show a reduction in mortality among metformin users compared to nonusers were summarized in a “mini review” by André J. Scheen, MD, PhD, published Aug. 1 in Diabetes and Metabolism.
Dr. Scheen, of the division of diabetes, nutrition, and metabolic disorders and the division of clinical pharmacology at Liège (Belgium) University, discussed possible mechanisms behind this observation.
“Because metformin exerts various effects beyond its glucose-lowering action, among which are anti-inflammatory effects, it may be speculated that this biguanide might positively influence the prognosis of patients with [type 2 diabetes] hospitalized for COVID-19,” he said.
“However, given the potential confounders inherently found in observational studies, caution is required before drawing any firm conclusions in the absence of randomized controlled trials,” Dr. Scheen wrote.
Indeed, when asked to comment, endocrinologist Kasia Lipska, MD, of Yale University, New Haven, Conn., said in an interview: “Metformin users tend to do better in many different settings with respect to many different outcomes. To me, it is still unclear whether metformin is truly a miracle drug or whether it is simply used more often among people who are healthier and who do not have contraindications to its use.”
She added, “I don’t think we have enough data to suggest metformin use for COVID-19 mitigation at this point.”
Alabama authors say confounding effects ‘unlikely’
In the retrospective analysis of electronic health records from their institution, Dr. Crouse and colleagues reviewed data from 604 patients who were confirmed to have tested positive for COVID-19 between Feb. 25 and June 22, 2020. Of those individuals, 40% had diabetes.
Death occurred in 11% (n = 67); the odds ratio (OR) for death among those with, vs. without, diabetes was 3.62 (P < .0001).
Individuals with diabetes accounted for >60% of all deaths. In multiple logistic regression, age 50-70 vs. <50, male sex, and diabetes emerged as independent predictors of death.
Of the 42 patients with diabetes who died, 8 (19%) had used metformin, and 34 (81%) had not*, a significant difference (OR, 0.38; P = .0221). Insulin use, on the other hand, had no effect on mortality (P = .5728).
“In fact, with 11% [being] the mortality of metformin users, [this] was comparable to that of the general COVID-19-positive population and dramatically lower than the 23% mortality observed in subjects with diabetes and not on metformin,” the authors said.
The survival benefit observed with metformin remained after exclusion of patients with classic metformin contraindications, such as chronic kidney disease and heart failure (OR, 0.17; P = .0231).
“This makes any potential confounding effects from skewing metformin users toward healthier subjects without these additional comorbidities very unlikely,” Dr. Crouse and colleagues contended.
After further analysis that controlled for other covariates (age, sex, obesity status, and hypertension), age, sex, and metformin use remained independent predictors of mortality.
For metformin, the odds ratio was 0.33 (P = .0210).
But, Dr. Lipska pointed out, “Observational studies can take into account confounders that are measured. However, unmeasured confounders may still affect the conclusions of these studies ... Propensity score matching to account for the likelihood of use of metformin could be used to better account for differences between metformin users and nonusers.”
If metformin does reduce COVID-19 deaths, multiple mechanisms likely
In his article, Dr. Scheen noted that several mechanisms have been proposed for the possible beneficial effect of metformin on COVID-19 outcomes, including direct improvements in glucose control, body weight, and insulin resistance; reduction in inflammation; inhibition of virus penetration via phosphorylation of ACE2; inhibition of an immune hyperactivation pathway; and neutrophil reduction. All remain theoretical, he emphasized.
He noted that some authors have raised concerns about possible harms from the use of metformin by patients with type 2 diabetes who are hospitalized for COVID-19, particularly because of the potential risk for lactic acidosis in cases of multiple organ failure.
In totality, four studies suggest 25% death reduction with metformin
Taken together, the four observational studies that Dr. Scheen reviewed showed that metformin had a positive effect, with an overall 25% reduction in death (P < .00001), albeit with relatively high heterogeneity (I² = 61%).
The largest of these, from the United States, included 6,256 patients hospitalized with COVID-19 and involved propensity matching. A significant reduction in mortality with metformin use was seen in women but not men (odds ratio, 0.759).
The French Coronavirus-SARS-CoV-2 and Diabetes Outcomes (CORONADO) study of 1,317 patients with diabetes and confirmed COVID-19 who were admitted to 53 French hospitals also showed a significant survival benefit for metformin, although the study wasn’t designed to address that issue.
In that study, the odds ratio for death on day 7 in prior metformin users compared to nonusers was 0.59. This finding lost significance but remained a trend after full adjustments (0.80).
Two smaller observational studies produced similar trends toward survival benefit with metformin.
Nonetheless, Dr. Scheen cautioned: “Firm conclusions about the impact of metformin therapy can only be drawn from double-blind randomized controlled trials (RCTs), and such trials are almost impossible in the context of COVID-19.”
He added: “Because metformin is out of patent and very inexpensive, no pharmaceutical company is likely to be interested in planning a study to demonstrate the benefits of metformin on COVID-19-related clinical outcomes.”
Dr. Lipska agreed: “RCTs are unlikely to be conducted to settle these issues. In their absence, metformin use should be based on its safety and effectiveness profile.”
Dr. Scheen concluded, however, that “there are at least no negative safety indications, so there is no reason to stop metformin therapy during COVID-19 infection except in cases of severe gastrointestinal symptoms, hypoxia and/or multiple organ failure.”
Dr. Lipska has received grants from the National Institutes of Health and works under contract for the Centers for Medicare & Medicaid Services to develop publicly reported quality measures. Dr. Scheen has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
*A previous version reversed these two outcomes in error.
Accumulating observational data suggest that metformin use in patients with type 2 diabetes might reduce the risk for death from COVID-19, but the randomized trials needed to prove this are unlikely to be carried out, according to experts.
The latest results, which are not yet peer reviewed, were published online July 31. The study was conducted by Andrew B. Crouse, PhD, of the Hugh Kaul Precision Medicine Institute, University of Alabama at Birmingham, and colleagues.
The researchers found that among more than 600 patients with diabetes and COVID-19, use of metformin was associated with a nearly 70% reduction in mortality after adjustment for multiple confounders.
Data from four previous studies that also show a reduction in mortality among metformin users compared to nonusers were summarized in a “mini review” by André J. Scheen, MD, PhD, published Aug. 1 in Diabetes and Metabolism.
Dr. Scheen, of the division of diabetes, nutrition, and metabolic disorders and the division of clinical pharmacology at Liège (Belgium) University, discussed possible mechanisms behind this observation.
“Because metformin exerts various effects beyond its glucose-lowering action, among which are anti-inflammatory effects, it may be speculated that this biguanide might positively influence the prognosis of patients with [type 2 diabetes] hospitalized for COVID-19,” he said.
“However, given the potential confounders inherently found in observational studies, caution is required before drawing any firm conclusions in the absence of randomized controlled trials,” Dr. Scheen wrote.
Indeed, when asked to comment, endocrinologist Kasia Lipska, MD, of Yale University, New Haven, Conn., said in an interview: “Metformin users tend to do better in many different settings with respect to many different outcomes. To me, it is still unclear whether metformin is truly a miracle drug or whether it is simply used more often among people who are healthier and who do not have contraindications to its use.”
She added, “I don’t think we have enough data to suggest metformin use for COVID-19 mitigation at this point.”
Alabama authors say confounding effects ‘unlikely’
In the retrospective analysis of electronic health records from their institution, Dr. Crouse and colleagues reviewed data from 604 patients who were confirmed to have tested positive for COVID-19 between Feb. 25 and June 22, 2020. Of those individuals, 40% had diabetes.
Death occurred in 11% (n = 67); the odds ratio (OR) for death among those with, vs. without, diabetes was 3.62 (P < .0001).
Individuals with diabetes accounted for >60% of all deaths. In multiple logistic regression, age 50-70 vs. <50, male sex, and diabetes emerged as independent predictors of death.
Of the 42 patients with diabetes who died, 8 (19%) had used metformin, and 34 (81%) had not*, a significant difference (OR, 0.38; P = .0221). Insulin use, on the other hand, had no effect on mortality (P = .5728).
“In fact, with 11% [being] the mortality of metformin users, [this] was comparable to that of the general COVID-19-positive population and dramatically lower than the 23% mortality observed in subjects with diabetes and not on metformin,” the authors said.
The survival benefit observed with metformin remained after exclusion of patients with classic metformin contraindications, such as chronic kidney disease and heart failure (OR, 0.17; P = .0231).
“This makes any potential confounding effects from skewing metformin users toward healthier subjects without these additional comorbidities very unlikely,” Dr. Crouse and colleagues contended.
After further analysis that controlled for other covariates (age, sex, obesity status, and hypertension), age, sex, and metformin use remained independent predictors of mortality.
For metformin, the odds ratio was 0.33 (P = .0210).
But, Dr. Lipska pointed out, “Observational studies can take into account confounders that are measured. However, unmeasured confounders may still affect the conclusions of these studies ... Propensity score matching to account for the likelihood of use of metformin could be used to better account for differences between metformin users and nonusers.”
If metformin does reduce COVID-19 deaths, multiple mechanisms likely
In his article, Dr. Scheen noted that several mechanisms have been proposed for the possible beneficial effect of metformin on COVID-19 outcomes, including direct improvements in glucose control, body weight, and insulin resistance; reduction in inflammation; inhibition of virus penetration via phosphorylation of ACE2; inhibition of an immune hyperactivation pathway; and neutrophil reduction. All remain theoretical, he emphasized.
He noted that some authors have raised concerns about possible harms from the use of metformin by patients with type 2 diabetes who are hospitalized for COVID-19, particularly because of the potential risk for lactic acidosis in cases of multiple organ failure.
In totality, four studies suggest 25% death reduction with metformin
Taken together, the four observational studies that Dr. Scheen reviewed showed that metformin had a positive effect, with an overall 25% reduction in death (P < .00001), albeit with relatively high heterogeneity (I² = 61%).
The largest of these, from the United States, included 6,256 patients hospitalized with COVID-19 and involved propensity matching. A significant reduction in mortality with metformin use was seen in women but not men (odds ratio, 0.759).
The French Coronavirus-SARS-CoV-2 and Diabetes Outcomes (CORONADO) study of 1,317 patients with diabetes and confirmed COVID-19 who were admitted to 53 French hospitals also showed a significant survival benefit for metformin, although the study wasn’t designed to address that issue.
In that study, the odds ratio for death on day 7 in prior metformin users compared to nonusers was 0.59. This finding lost significance but remained a trend after full adjustments (0.80).
Two smaller observational studies produced similar trends toward survival benefit with metformin.
Nonetheless, Dr. Scheen cautioned: “Firm conclusions about the impact of metformin therapy can only be drawn from double-blind randomized controlled trials (RCTs), and such trials are almost impossible in the context of COVID-19.”
He added: “Because metformin is out of patent and very inexpensive, no pharmaceutical company is likely to be interested in planning a study to demonstrate the benefits of metformin on COVID-19-related clinical outcomes.”
Dr. Lipska agreed: “RCTs are unlikely to be conducted to settle these issues. In their absence, metformin use should be based on its safety and effectiveness profile.”
Dr. Scheen concluded, however, that “there are at least no negative safety indications, so there is no reason to stop metformin therapy during COVID-19 infection except in cases of severe gastrointestinal symptoms, hypoxia and/or multiple organ failure.”
Dr. Lipska has received grants from the National Institutes of Health and works under contract for the Centers for Medicare & Medicaid Services to develop publicly reported quality measures. Dr. Scheen has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
*A previous version reversed these two outcomes in error.
RA patients show decreased risk for new-onset type 2 diabetes
Patients with RA were at lower risk for developing incident type 2 diabetes mellitus (T2DM) in comparison with patients with hypertension, psoriatic arthritis (PsA), or osteoarthritis, as well as the general population without RA in a retrospective cohort study of a large, nationwide, commercial health insurance claims database.
This result goes against what the study researchers from the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, initially hypothesized: The “risk of incident T2DM in RA patients would be similar to or less than PsA and [hypertension] patients, but higher, compared to general non-RA and OA patients.”
Prior epidemiologic studies of the relationship between RA and incident diabetes have yielded inconclusive results suggesting a small increase or no increase in risk of T2DM in patients with RA, possibly because of differences in the risk of T2DM in comparison groups used by previous studies to calculate relative risk, first author Yinzhu Jin and colleagues noted in their report published in Arthritis Care & Research.
After mining a nationwide U.S. commercial health insurance claims database, the Optum Clinformatics Data Mart, for claims data from Jan. 1, 2005, to Dec. 31, 2017, the researchers matched a total of 108,568 patients in RA, general population non-RA, hypertension, and OA cohorts based on age, sex, and index date (the date of disease-specific medication dispensing). Overall, 77% of those patients were female and had a mean age of nearly 56 years, whereas 48% of patients with PsA were female and their mean age was nearly 49 years. (PsA patients were not matched because of smaller numbers.)
During a median follow-up period of 1.4-1.8 years across the comparison groups, the crude incidence rate for diabetes per 1,000 person-years in the cohorts was 7.0 for RA, 7.4 for general non-RA, 12.3 for hypertension, 7.8 for OA, and 9.9 for PsA. The hazard ratios and 95% confidence interval for risk of diabetes in patients with RA – after adjustment for more than 40 baseline covariates that included demographics, comorbidities, medication use, and health care utilization – was 0.72 (0.66-0.78) in comparison withh the general non-RA cohort, 0.65 (0.60-0.71) in comparison with the hypertension cohort, 0.75 (0.69-0.81) in comparison with the OA cohort, and 0.76 (0.67-0.86) in comparison with the PsA cohort. These values correspond to RA patients having a 24%-35% lower risk of incident diabetes versus the comparison groups, the researchers noted. They observed results consistent to these when they conducted a sensitivity analysis using a 1-year lag time from the index date before starting follow-up.
The lower risk of T2DM in patients with RA in comparison with patients in the non-RA cohort “may be, in part, due to the effect of biologic DMARD [disease-modifying antirheumatic drug] treatment in RA which likely modifies the risk of DM,” the researchers wrote. “Both the increasing use of biologic DMARDs for RA in the U.S. over the last decade and our cohort entry criteria for the RA cohort (i.e., at least one dispensing of a DMARD) may explain the finding of the lower risk of DM in RA.”
The results found with the other three cohorts did not surprise the researchers. The reduced risk of diabetes among RA patients versus those with OA jibes with “higher rates of obesity and other comorbidities in patients with OA” as well as findings from a recent study that found a higher incidence rate of diabetes in OA, compared with RA. Ms. Jin and colleagues also acknowledged it is well known that “hypertension and PsA are associated with metabolic dysregulation and increase the risk of diabetes.”
The researchers defined patients with RA as having at least twoinpatient or outpatient ICD-9 or ICD-10 diagnosis codes of RA, separated by 7-365 days and having at least one dispensing for DMARDs within 1 year from the first RA diagnosis date, and defined the primary outcome of incident T2DM as at least one inpatient or outpatient diagnosis of T2DM plus at least one dispensing of an antidiabetic drug. They set the general non-RA cohort by selecting patients with any inpatient or outpatient diagnosis codes and a dispensing of any medications, and the hypertension, PsA, and OA comparator groups as having at least two inpatient or outpatient disease-specific ICD-9/ICD-10 codes separated by 7-365 days and at least one dispensing of disease-specific medication within 1 year from the first diagnosis date. They excluded patients with RA, PsA, or psoriasis diagnosis or disease-specific medication dispensing any time prior to or on the index date (the date of disease-specific medication dispensing).
The researchers recognized that the conclusions that can be drawn from the study are limited by the “potential misclassification of cohorts and covariates” because they “mainly used diagnosis codes and pharmacy dispensing records in claims data,” and some “important covariates such as baseline obesity are likely underreported and not adequately captured in claims data.” The level of covariate misclassification also may have been different across the study cohorts on “unmeasured covariates such as body mass index, diet, and physical activity, as well as disease specific measures,” thus introducing residual confounding. They also could not “examine potential difference in the risk of T2DM in untreated or undertreated RA patients” because “RA and all the non-RA comparator cohorts were required to use a disease-specific drug,” they wrote.
“While systemic inflammation in RA is thought to increase the risk of [cardiovascular disease] and cardiovascular risk factors such as DM, our findings suggest having RA itself does not confer an increased risk of DM. Future study should determine whether untreated RA or undertreated RA is associated with a greater risk of developing DM,” the researchers concluded.
The study was supported by a research grant from Bristol-Myers Squibb, which “played no role in the study design, data analysis or interpretation of data or presentation of results,” the researchers said. The company was “given the opportunity to make nonbinding comments on a draft of the manuscript, but the authors retained the right of publication and to determine the final wording.” One author reported receiving research grants from Brigham and Women’s Hospital from Pfizer, AbbVie, Bristol-Myers Squibb, and Roche for unrelated topics.
SOURCE: Jin Y et al. Arthritis Care Res. 2020 Aug 4. doi: 10.1002/acr.24343.
Patients with RA were at lower risk for developing incident type 2 diabetes mellitus (T2DM) in comparison with patients with hypertension, psoriatic arthritis (PsA), or osteoarthritis, as well as the general population without RA in a retrospective cohort study of a large, nationwide, commercial health insurance claims database.
This result goes against what the study researchers from the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, initially hypothesized: The “risk of incident T2DM in RA patients would be similar to or less than PsA and [hypertension] patients, but higher, compared to general non-RA and OA patients.”
Prior epidemiologic studies of the relationship between RA and incident diabetes have yielded inconclusive results suggesting a small increase or no increase in risk of T2DM in patients with RA, possibly because of differences in the risk of T2DM in comparison groups used by previous studies to calculate relative risk, first author Yinzhu Jin and colleagues noted in their report published in Arthritis Care & Research.
After mining a nationwide U.S. commercial health insurance claims database, the Optum Clinformatics Data Mart, for claims data from Jan. 1, 2005, to Dec. 31, 2017, the researchers matched a total of 108,568 patients in RA, general population non-RA, hypertension, and OA cohorts based on age, sex, and index date (the date of disease-specific medication dispensing). Overall, 77% of those patients were female and had a mean age of nearly 56 years, whereas 48% of patients with PsA were female and their mean age was nearly 49 years. (PsA patients were not matched because of smaller numbers.)
During a median follow-up period of 1.4-1.8 years across the comparison groups, the crude incidence rate for diabetes per 1,000 person-years in the cohorts was 7.0 for RA, 7.4 for general non-RA, 12.3 for hypertension, 7.8 for OA, and 9.9 for PsA. The hazard ratios and 95% confidence interval for risk of diabetes in patients with RA – after adjustment for more than 40 baseline covariates that included demographics, comorbidities, medication use, and health care utilization – was 0.72 (0.66-0.78) in comparison withh the general non-RA cohort, 0.65 (0.60-0.71) in comparison with the hypertension cohort, 0.75 (0.69-0.81) in comparison with the OA cohort, and 0.76 (0.67-0.86) in comparison with the PsA cohort. These values correspond to RA patients having a 24%-35% lower risk of incident diabetes versus the comparison groups, the researchers noted. They observed results consistent to these when they conducted a sensitivity analysis using a 1-year lag time from the index date before starting follow-up.
The lower risk of T2DM in patients with RA in comparison with patients in the non-RA cohort “may be, in part, due to the effect of biologic DMARD [disease-modifying antirheumatic drug] treatment in RA which likely modifies the risk of DM,” the researchers wrote. “Both the increasing use of biologic DMARDs for RA in the U.S. over the last decade and our cohort entry criteria for the RA cohort (i.e., at least one dispensing of a DMARD) may explain the finding of the lower risk of DM in RA.”
The results found with the other three cohorts did not surprise the researchers. The reduced risk of diabetes among RA patients versus those with OA jibes with “higher rates of obesity and other comorbidities in patients with OA” as well as findings from a recent study that found a higher incidence rate of diabetes in OA, compared with RA. Ms. Jin and colleagues also acknowledged it is well known that “hypertension and PsA are associated with metabolic dysregulation and increase the risk of diabetes.”
The researchers defined patients with RA as having at least twoinpatient or outpatient ICD-9 or ICD-10 diagnosis codes of RA, separated by 7-365 days and having at least one dispensing for DMARDs within 1 year from the first RA diagnosis date, and defined the primary outcome of incident T2DM as at least one inpatient or outpatient diagnosis of T2DM plus at least one dispensing of an antidiabetic drug. They set the general non-RA cohort by selecting patients with any inpatient or outpatient diagnosis codes and a dispensing of any medications, and the hypertension, PsA, and OA comparator groups as having at least two inpatient or outpatient disease-specific ICD-9/ICD-10 codes separated by 7-365 days and at least one dispensing of disease-specific medication within 1 year from the first diagnosis date. They excluded patients with RA, PsA, or psoriasis diagnosis or disease-specific medication dispensing any time prior to or on the index date (the date of disease-specific medication dispensing).
The researchers recognized that the conclusions that can be drawn from the study are limited by the “potential misclassification of cohorts and covariates” because they “mainly used diagnosis codes and pharmacy dispensing records in claims data,” and some “important covariates such as baseline obesity are likely underreported and not adequately captured in claims data.” The level of covariate misclassification also may have been different across the study cohorts on “unmeasured covariates such as body mass index, diet, and physical activity, as well as disease specific measures,” thus introducing residual confounding. They also could not “examine potential difference in the risk of T2DM in untreated or undertreated RA patients” because “RA and all the non-RA comparator cohorts were required to use a disease-specific drug,” they wrote.
“While systemic inflammation in RA is thought to increase the risk of [cardiovascular disease] and cardiovascular risk factors such as DM, our findings suggest having RA itself does not confer an increased risk of DM. Future study should determine whether untreated RA or undertreated RA is associated with a greater risk of developing DM,” the researchers concluded.
The study was supported by a research grant from Bristol-Myers Squibb, which “played no role in the study design, data analysis or interpretation of data or presentation of results,” the researchers said. The company was “given the opportunity to make nonbinding comments on a draft of the manuscript, but the authors retained the right of publication and to determine the final wording.” One author reported receiving research grants from Brigham and Women’s Hospital from Pfizer, AbbVie, Bristol-Myers Squibb, and Roche for unrelated topics.
SOURCE: Jin Y et al. Arthritis Care Res. 2020 Aug 4. doi: 10.1002/acr.24343.
Patients with RA were at lower risk for developing incident type 2 diabetes mellitus (T2DM) in comparison with patients with hypertension, psoriatic arthritis (PsA), or osteoarthritis, as well as the general population without RA in a retrospective cohort study of a large, nationwide, commercial health insurance claims database.
This result goes against what the study researchers from the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, initially hypothesized: The “risk of incident T2DM in RA patients would be similar to or less than PsA and [hypertension] patients, but higher, compared to general non-RA and OA patients.”
Prior epidemiologic studies of the relationship between RA and incident diabetes have yielded inconclusive results suggesting a small increase or no increase in risk of T2DM in patients with RA, possibly because of differences in the risk of T2DM in comparison groups used by previous studies to calculate relative risk, first author Yinzhu Jin and colleagues noted in their report published in Arthritis Care & Research.
After mining a nationwide U.S. commercial health insurance claims database, the Optum Clinformatics Data Mart, for claims data from Jan. 1, 2005, to Dec. 31, 2017, the researchers matched a total of 108,568 patients in RA, general population non-RA, hypertension, and OA cohorts based on age, sex, and index date (the date of disease-specific medication dispensing). Overall, 77% of those patients were female and had a mean age of nearly 56 years, whereas 48% of patients with PsA were female and their mean age was nearly 49 years. (PsA patients were not matched because of smaller numbers.)
During a median follow-up period of 1.4-1.8 years across the comparison groups, the crude incidence rate for diabetes per 1,000 person-years in the cohorts was 7.0 for RA, 7.4 for general non-RA, 12.3 for hypertension, 7.8 for OA, and 9.9 for PsA. The hazard ratios and 95% confidence interval for risk of diabetes in patients with RA – after adjustment for more than 40 baseline covariates that included demographics, comorbidities, medication use, and health care utilization – was 0.72 (0.66-0.78) in comparison withh the general non-RA cohort, 0.65 (0.60-0.71) in comparison with the hypertension cohort, 0.75 (0.69-0.81) in comparison with the OA cohort, and 0.76 (0.67-0.86) in comparison with the PsA cohort. These values correspond to RA patients having a 24%-35% lower risk of incident diabetes versus the comparison groups, the researchers noted. They observed results consistent to these when they conducted a sensitivity analysis using a 1-year lag time from the index date before starting follow-up.
The lower risk of T2DM in patients with RA in comparison with patients in the non-RA cohort “may be, in part, due to the effect of biologic DMARD [disease-modifying antirheumatic drug] treatment in RA which likely modifies the risk of DM,” the researchers wrote. “Both the increasing use of biologic DMARDs for RA in the U.S. over the last decade and our cohort entry criteria for the RA cohort (i.e., at least one dispensing of a DMARD) may explain the finding of the lower risk of DM in RA.”
The results found with the other three cohorts did not surprise the researchers. The reduced risk of diabetes among RA patients versus those with OA jibes with “higher rates of obesity and other comorbidities in patients with OA” as well as findings from a recent study that found a higher incidence rate of diabetes in OA, compared with RA. Ms. Jin and colleagues also acknowledged it is well known that “hypertension and PsA are associated with metabolic dysregulation and increase the risk of diabetes.”
The researchers defined patients with RA as having at least twoinpatient or outpatient ICD-9 or ICD-10 diagnosis codes of RA, separated by 7-365 days and having at least one dispensing for DMARDs within 1 year from the first RA diagnosis date, and defined the primary outcome of incident T2DM as at least one inpatient or outpatient diagnosis of T2DM plus at least one dispensing of an antidiabetic drug. They set the general non-RA cohort by selecting patients with any inpatient or outpatient diagnosis codes and a dispensing of any medications, and the hypertension, PsA, and OA comparator groups as having at least two inpatient or outpatient disease-specific ICD-9/ICD-10 codes separated by 7-365 days and at least one dispensing of disease-specific medication within 1 year from the first diagnosis date. They excluded patients with RA, PsA, or psoriasis diagnosis or disease-specific medication dispensing any time prior to or on the index date (the date of disease-specific medication dispensing).
The researchers recognized that the conclusions that can be drawn from the study are limited by the “potential misclassification of cohorts and covariates” because they “mainly used diagnosis codes and pharmacy dispensing records in claims data,” and some “important covariates such as baseline obesity are likely underreported and not adequately captured in claims data.” The level of covariate misclassification also may have been different across the study cohorts on “unmeasured covariates such as body mass index, diet, and physical activity, as well as disease specific measures,” thus introducing residual confounding. They also could not “examine potential difference in the risk of T2DM in untreated or undertreated RA patients” because “RA and all the non-RA comparator cohorts were required to use a disease-specific drug,” they wrote.
“While systemic inflammation in RA is thought to increase the risk of [cardiovascular disease] and cardiovascular risk factors such as DM, our findings suggest having RA itself does not confer an increased risk of DM. Future study should determine whether untreated RA or undertreated RA is associated with a greater risk of developing DM,” the researchers concluded.
The study was supported by a research grant from Bristol-Myers Squibb, which “played no role in the study design, data analysis or interpretation of data or presentation of results,” the researchers said. The company was “given the opportunity to make nonbinding comments on a draft of the manuscript, but the authors retained the right of publication and to determine the final wording.” One author reported receiving research grants from Brigham and Women’s Hospital from Pfizer, AbbVie, Bristol-Myers Squibb, and Roche for unrelated topics.
SOURCE: Jin Y et al. Arthritis Care Res. 2020 Aug 4. doi: 10.1002/acr.24343.
FROM ARTHRITIS CARE & RESEARCH
Screening criteria for diabetes in youth won’t capture all at high risk
and therefore “may miss high-risk youth who should be targeted for diabetes prevention,” according to the investigators of a cross-sectional analysis of youth in the 1999-2016 National Health and Nutrition Examination Survey (NHANES) database.
Regardless of whether or not youth meet screening eligibility, they say, hemoglobin A1c appears to be a “specific and useful test” for detecting high-risk youth.
Those with prediabetic levels of A1c or fasting plasma glucose (FPG) – A1c especially – had a high burden of other cardiometabolic risk factors that could benefit from lifestyle interventions to prevent diabetes and cardiovascular risk in adulthood, wrote Amelia S. Wallace and coinvestigators at the Johns Hopkins Bloomberg School of Public Health, Baltimore. The report is in Pediatrics.Their epidemiologic study had two aims: To assess the performance of the American Diabetes Association guidelines for screening in youth, and to evaluate how well various clinical definitions of diabetes and prediabetes identify U.S. youth at high cardiometabolic risk.
The 2018 ADA guidelines recommend screening for type 2 diabetes and prediabetes in all asymptomatic youth ages 10 years and older who are overweight or obese and who have at least one risk factor for diabetes: nonwhite race, family history of type 2 diabetes, maternal gestational diabetes, or signs of insulin resistance or conditions associated with insulin resistance (Diabetes Care. 2018:41[suppl 1:S13-S37]).
Approximately one-quarter of U.S. youth were found to be eligible for screening under the current ADA criteria, but there were few cases of confirmed diabetes (A1c greater than or equal to 6.5% and fasting plasma glucose greater than or equal to 126 mg/dL) that had gone undiagnosed (less than 0.5%), said Ms. Wallace and her associates.
Considering all hyperglycemia (undiagnosed diabetes or prediabetes) in the NHANES youth population, the sensitivity and specificity of the ADA criteria for detecting A1c-defined hyperglycemia (greater than or equal to 5.7%) were 56% and 76%, respectively, and the sensitivity and specificity for detecting FBG-defined hyperglycemia (greater than or equal to 100 mg/dL) were 36% and 77%.
The prevalence of any hyperglycemia was higher in youth who met ADA screening criteria than in those who didn’t, but there were also “a substantial number of youth with hyperglycemia in the non–screening eligible population,” they wrote. “In fact, the absolute number of youth with elevated FPG was larger in the non–screening eligible population, and the majority (88.5%) of these youth were of normal weight.”
Across all youth (irrespective of screening eligibility), both FPG and A1c-defined hyperglycemia effectively identified children and adolescents who had a high burden of cardiometabolic risk (obesity, metabolic syndrome, and hypercholesterolemia). Using a confirmatory definition of elevations in both FPG and A1c “provided the highest discrimination for cardiometabolic risk,” Ms. Wallace and her associates said.
But in comparing the single tests, risk factor associations with hyperglycemia were consistently stronger with A1c-defined hyperglycemia (odds ratios of 2.6-4.1) than FBG-defined hyperglycemia (ORs of 1.5-3.0). A1c-defined hyperglycemia “identifies a smaller, but higher-risk, population than FPG-defined hyperglycemia,” they said.
In an accompanying commentary, Tamara S. Hannon, MD, MS, of the division of pediatric endocrinology and diabetology at Indiana University in Indianapolis, said that more effective algorithms to determine who should have laboratory testing “could be useful.” Still, “for youth with obesity and multiple risk factors for developing type 2 diabetes, the principal challenge is how to effectively prevent or delay this disease for them and future generations.”
Pediatricians, she said, should screen for prediabetes and type 2 diabetes “according to professional recommendations with simple clinical tests, such as A1c. Screening and education about prediabetes alone can lead to better rates of follow-up for obesity,” she noted (Pediatrics. August 2020. doi: 10.1542/peds.2020-010272).
Sheela N. Magge, MD, MSCE, who directs the division of pediatric endocrinology and diabetes at John Hopkins University, Baltimore, and was asked to comment on the study, similarly said that the findings should not discourage use of the ADA guidelines.
While the guidelines may not have optimal sensitivity and specificity, “neither HbA1c nor fasting glucose are perfect screening tools for prediabetes and likely give us different mechanistic information,” she said. (The ADA guidelines also allow the use of a 2-hour oral glucose tolerance test, but this is not often used by pediatricians, she noted.)
The measurements are “only tools used to identify children who have prediabetes and are therefore at increased risk for type 2 diabetes,” said Dr. Magge, the Lawson Wilkins Endowed Chair of Pediatric Endocrinology at the university. “These children then need to be managed and followed to try to prevent worsening glycemia.”
Both she and Dr. Hannon stressed that youth with type 2 diabetes have more rapidly progressive disease compared with adults.
Microvascular complications are seen even at diagnosis, Dr. Magge said, and “youth may face serious complications such as cardiovascular disease decades earlier than previous generations.”
Dr. Hannon also noted in her commentary that oral diabetes medications often fail in youth with type 2 diabetes, leading to insulin therapy early on.
The prevalence of youth-onset type 2 diabetes has increased because of rising rates of pediatric overweight and obesity, Dr. Magge emphasized. In her experience, the diabetes risk factors that guide the ADA’s screening approach “are so common in overweight and obese youth that they all have at least one.”
The NHANES data did not contain information on all the variables that make up the current diabetes screening criteria in youth; there was no explicit information on history of maternal gestational diabetes and family history of type 2 diabetes, for instance, or the presence of acanthosis nigricans or polycystic ovarian syndrome – conditions associated with insulin resistance. The investigators said it’s likely, therefore, that the study underestimated the number of U.S. youth who would be eligible for diabetes screening.
And, as Dr. Magge said, “it is difficult to determine which risk factors [in the ADA guidelines] were less predictive.”
The NHANES analysis covered 14,119 youth in the 1999-2016 NHANES surveys, which consisted of interviews and standardized physical exams, including laboratory tests, in home and at a mobile examination center. Analyses involving any fasting lab tests were limited to a random subsample of participants aged 12-19 years without diagnosed diabetes who were asked to fast the night before; 6,225 youth properly followed instructions and were included in this subsample.
The surveys are conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention. The study authors and the editorial author indicated that they have no relevant financial disclosures or conflicts of interest. Dr. Magge also said she has no relevant disclosures.
SOURCE: Wallace AS et al. Pediatrics. August 2020. doi: 10.1542/peds.2020-0265.
and therefore “may miss high-risk youth who should be targeted for diabetes prevention,” according to the investigators of a cross-sectional analysis of youth in the 1999-2016 National Health and Nutrition Examination Survey (NHANES) database.
Regardless of whether or not youth meet screening eligibility, they say, hemoglobin A1c appears to be a “specific and useful test” for detecting high-risk youth.
Those with prediabetic levels of A1c or fasting plasma glucose (FPG) – A1c especially – had a high burden of other cardiometabolic risk factors that could benefit from lifestyle interventions to prevent diabetes and cardiovascular risk in adulthood, wrote Amelia S. Wallace and coinvestigators at the Johns Hopkins Bloomberg School of Public Health, Baltimore. The report is in Pediatrics.Their epidemiologic study had two aims: To assess the performance of the American Diabetes Association guidelines for screening in youth, and to evaluate how well various clinical definitions of diabetes and prediabetes identify U.S. youth at high cardiometabolic risk.
The 2018 ADA guidelines recommend screening for type 2 diabetes and prediabetes in all asymptomatic youth ages 10 years and older who are overweight or obese and who have at least one risk factor for diabetes: nonwhite race, family history of type 2 diabetes, maternal gestational diabetes, or signs of insulin resistance or conditions associated with insulin resistance (Diabetes Care. 2018:41[suppl 1:S13-S37]).
Approximately one-quarter of U.S. youth were found to be eligible for screening under the current ADA criteria, but there were few cases of confirmed diabetes (A1c greater than or equal to 6.5% and fasting plasma glucose greater than or equal to 126 mg/dL) that had gone undiagnosed (less than 0.5%), said Ms. Wallace and her associates.
Considering all hyperglycemia (undiagnosed diabetes or prediabetes) in the NHANES youth population, the sensitivity and specificity of the ADA criteria for detecting A1c-defined hyperglycemia (greater than or equal to 5.7%) were 56% and 76%, respectively, and the sensitivity and specificity for detecting FBG-defined hyperglycemia (greater than or equal to 100 mg/dL) were 36% and 77%.
The prevalence of any hyperglycemia was higher in youth who met ADA screening criteria than in those who didn’t, but there were also “a substantial number of youth with hyperglycemia in the non–screening eligible population,” they wrote. “In fact, the absolute number of youth with elevated FPG was larger in the non–screening eligible population, and the majority (88.5%) of these youth were of normal weight.”
Across all youth (irrespective of screening eligibility), both FPG and A1c-defined hyperglycemia effectively identified children and adolescents who had a high burden of cardiometabolic risk (obesity, metabolic syndrome, and hypercholesterolemia). Using a confirmatory definition of elevations in both FPG and A1c “provided the highest discrimination for cardiometabolic risk,” Ms. Wallace and her associates said.
But in comparing the single tests, risk factor associations with hyperglycemia were consistently stronger with A1c-defined hyperglycemia (odds ratios of 2.6-4.1) than FBG-defined hyperglycemia (ORs of 1.5-3.0). A1c-defined hyperglycemia “identifies a smaller, but higher-risk, population than FPG-defined hyperglycemia,” they said.
In an accompanying commentary, Tamara S. Hannon, MD, MS, of the division of pediatric endocrinology and diabetology at Indiana University in Indianapolis, said that more effective algorithms to determine who should have laboratory testing “could be useful.” Still, “for youth with obesity and multiple risk factors for developing type 2 diabetes, the principal challenge is how to effectively prevent or delay this disease for them and future generations.”
Pediatricians, she said, should screen for prediabetes and type 2 diabetes “according to professional recommendations with simple clinical tests, such as A1c. Screening and education about prediabetes alone can lead to better rates of follow-up for obesity,” she noted (Pediatrics. August 2020. doi: 10.1542/peds.2020-010272).
Sheela N. Magge, MD, MSCE, who directs the division of pediatric endocrinology and diabetes at John Hopkins University, Baltimore, and was asked to comment on the study, similarly said that the findings should not discourage use of the ADA guidelines.
While the guidelines may not have optimal sensitivity and specificity, “neither HbA1c nor fasting glucose are perfect screening tools for prediabetes and likely give us different mechanistic information,” she said. (The ADA guidelines also allow the use of a 2-hour oral glucose tolerance test, but this is not often used by pediatricians, she noted.)
The measurements are “only tools used to identify children who have prediabetes and are therefore at increased risk for type 2 diabetes,” said Dr. Magge, the Lawson Wilkins Endowed Chair of Pediatric Endocrinology at the university. “These children then need to be managed and followed to try to prevent worsening glycemia.”
Both she and Dr. Hannon stressed that youth with type 2 diabetes have more rapidly progressive disease compared with adults.
Microvascular complications are seen even at diagnosis, Dr. Magge said, and “youth may face serious complications such as cardiovascular disease decades earlier than previous generations.”
Dr. Hannon also noted in her commentary that oral diabetes medications often fail in youth with type 2 diabetes, leading to insulin therapy early on.
The prevalence of youth-onset type 2 diabetes has increased because of rising rates of pediatric overweight and obesity, Dr. Magge emphasized. In her experience, the diabetes risk factors that guide the ADA’s screening approach “are so common in overweight and obese youth that they all have at least one.”
The NHANES data did not contain information on all the variables that make up the current diabetes screening criteria in youth; there was no explicit information on history of maternal gestational diabetes and family history of type 2 diabetes, for instance, or the presence of acanthosis nigricans or polycystic ovarian syndrome – conditions associated with insulin resistance. The investigators said it’s likely, therefore, that the study underestimated the number of U.S. youth who would be eligible for diabetes screening.
And, as Dr. Magge said, “it is difficult to determine which risk factors [in the ADA guidelines] were less predictive.”
The NHANES analysis covered 14,119 youth in the 1999-2016 NHANES surveys, which consisted of interviews and standardized physical exams, including laboratory tests, in home and at a mobile examination center. Analyses involving any fasting lab tests were limited to a random subsample of participants aged 12-19 years without diagnosed diabetes who were asked to fast the night before; 6,225 youth properly followed instructions and were included in this subsample.
The surveys are conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention. The study authors and the editorial author indicated that they have no relevant financial disclosures or conflicts of interest. Dr. Magge also said she has no relevant disclosures.
SOURCE: Wallace AS et al. Pediatrics. August 2020. doi: 10.1542/peds.2020-0265.
and therefore “may miss high-risk youth who should be targeted for diabetes prevention,” according to the investigators of a cross-sectional analysis of youth in the 1999-2016 National Health and Nutrition Examination Survey (NHANES) database.
Regardless of whether or not youth meet screening eligibility, they say, hemoglobin A1c appears to be a “specific and useful test” for detecting high-risk youth.
Those with prediabetic levels of A1c or fasting plasma glucose (FPG) – A1c especially – had a high burden of other cardiometabolic risk factors that could benefit from lifestyle interventions to prevent diabetes and cardiovascular risk in adulthood, wrote Amelia S. Wallace and coinvestigators at the Johns Hopkins Bloomberg School of Public Health, Baltimore. The report is in Pediatrics.Their epidemiologic study had two aims: To assess the performance of the American Diabetes Association guidelines for screening in youth, and to evaluate how well various clinical definitions of diabetes and prediabetes identify U.S. youth at high cardiometabolic risk.
The 2018 ADA guidelines recommend screening for type 2 diabetes and prediabetes in all asymptomatic youth ages 10 years and older who are overweight or obese and who have at least one risk factor for diabetes: nonwhite race, family history of type 2 diabetes, maternal gestational diabetes, or signs of insulin resistance or conditions associated with insulin resistance (Diabetes Care. 2018:41[suppl 1:S13-S37]).
Approximately one-quarter of U.S. youth were found to be eligible for screening under the current ADA criteria, but there were few cases of confirmed diabetes (A1c greater than or equal to 6.5% and fasting plasma glucose greater than or equal to 126 mg/dL) that had gone undiagnosed (less than 0.5%), said Ms. Wallace and her associates.
Considering all hyperglycemia (undiagnosed diabetes or prediabetes) in the NHANES youth population, the sensitivity and specificity of the ADA criteria for detecting A1c-defined hyperglycemia (greater than or equal to 5.7%) were 56% and 76%, respectively, and the sensitivity and specificity for detecting FBG-defined hyperglycemia (greater than or equal to 100 mg/dL) were 36% and 77%.
The prevalence of any hyperglycemia was higher in youth who met ADA screening criteria than in those who didn’t, but there were also “a substantial number of youth with hyperglycemia in the non–screening eligible population,” they wrote. “In fact, the absolute number of youth with elevated FPG was larger in the non–screening eligible population, and the majority (88.5%) of these youth were of normal weight.”
Across all youth (irrespective of screening eligibility), both FPG and A1c-defined hyperglycemia effectively identified children and adolescents who had a high burden of cardiometabolic risk (obesity, metabolic syndrome, and hypercholesterolemia). Using a confirmatory definition of elevations in both FPG and A1c “provided the highest discrimination for cardiometabolic risk,” Ms. Wallace and her associates said.
But in comparing the single tests, risk factor associations with hyperglycemia were consistently stronger with A1c-defined hyperglycemia (odds ratios of 2.6-4.1) than FBG-defined hyperglycemia (ORs of 1.5-3.0). A1c-defined hyperglycemia “identifies a smaller, but higher-risk, population than FPG-defined hyperglycemia,” they said.
In an accompanying commentary, Tamara S. Hannon, MD, MS, of the division of pediatric endocrinology and diabetology at Indiana University in Indianapolis, said that more effective algorithms to determine who should have laboratory testing “could be useful.” Still, “for youth with obesity and multiple risk factors for developing type 2 diabetes, the principal challenge is how to effectively prevent or delay this disease for them and future generations.”
Pediatricians, she said, should screen for prediabetes and type 2 diabetes “according to professional recommendations with simple clinical tests, such as A1c. Screening and education about prediabetes alone can lead to better rates of follow-up for obesity,” she noted (Pediatrics. August 2020. doi: 10.1542/peds.2020-010272).
Sheela N. Magge, MD, MSCE, who directs the division of pediatric endocrinology and diabetes at John Hopkins University, Baltimore, and was asked to comment on the study, similarly said that the findings should not discourage use of the ADA guidelines.
While the guidelines may not have optimal sensitivity and specificity, “neither HbA1c nor fasting glucose are perfect screening tools for prediabetes and likely give us different mechanistic information,” she said. (The ADA guidelines also allow the use of a 2-hour oral glucose tolerance test, but this is not often used by pediatricians, she noted.)
The measurements are “only tools used to identify children who have prediabetes and are therefore at increased risk for type 2 diabetes,” said Dr. Magge, the Lawson Wilkins Endowed Chair of Pediatric Endocrinology at the university. “These children then need to be managed and followed to try to prevent worsening glycemia.”
Both she and Dr. Hannon stressed that youth with type 2 diabetes have more rapidly progressive disease compared with adults.
Microvascular complications are seen even at diagnosis, Dr. Magge said, and “youth may face serious complications such as cardiovascular disease decades earlier than previous generations.”
Dr. Hannon also noted in her commentary that oral diabetes medications often fail in youth with type 2 diabetes, leading to insulin therapy early on.
The prevalence of youth-onset type 2 diabetes has increased because of rising rates of pediatric overweight and obesity, Dr. Magge emphasized. In her experience, the diabetes risk factors that guide the ADA’s screening approach “are so common in overweight and obese youth that they all have at least one.”
The NHANES data did not contain information on all the variables that make up the current diabetes screening criteria in youth; there was no explicit information on history of maternal gestational diabetes and family history of type 2 diabetes, for instance, or the presence of acanthosis nigricans or polycystic ovarian syndrome – conditions associated with insulin resistance. The investigators said it’s likely, therefore, that the study underestimated the number of U.S. youth who would be eligible for diabetes screening.
And, as Dr. Magge said, “it is difficult to determine which risk factors [in the ADA guidelines] were less predictive.”
The NHANES analysis covered 14,119 youth in the 1999-2016 NHANES surveys, which consisted of interviews and standardized physical exams, including laboratory tests, in home and at a mobile examination center. Analyses involving any fasting lab tests were limited to a random subsample of participants aged 12-19 years without diagnosed diabetes who were asked to fast the night before; 6,225 youth properly followed instructions and were included in this subsample.
The surveys are conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention. The study authors and the editorial author indicated that they have no relevant financial disclosures or conflicts of interest. Dr. Magge also said she has no relevant disclosures.
SOURCE: Wallace AS et al. Pediatrics. August 2020. doi: 10.1542/peds.2020-0265.
FROM PEDIATRICS
Guidance covers glycemia in dexamethasone-treated COVID-19 patients
New guidance from the U.K. National Diabetes COVID-19 Response Group addresses glucose management in patients with COVID-19 who are receiving dexamethasone therapy.
Although there are already guidelines that address inpatient management of steroid-induced hyperglycemia, the authors of the new document wrote that this new expert opinion paper was needed “given the ‘triple insult’ of dexamethasone-induced–impaired glucose metabolism, COVID-19–induced insulin resistance, and COVID-19–impaired insulin production.”
RECOVERY trial spurs response
The document, which is the latest in a series from the Association of British Clinical Diabetologists, was published online Aug. 2 in Diabetic Medicine. The group is chaired by Gerry Rayman, MD, consultant physician at the diabetes centre and diabetes research unit, East Suffolk (England) and North East NHS Foundation Trust.
The guidance was developed in response to the recent “breakthrough” Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial, which showed that dexamethasone reduced deaths in patients with COVID-19 on ventilators or receiving oxygen therapy. The advice is not intended for critical care units but can be adapted for that use.
The dose used in RECOVERY – 6 mg daily for 10 days – is 400%-500% greater than the therapeutic glucocorticoid replacement dose. High glucocorticoid doses can exacerbate hyperglycemia in people with established diabetes, unmask undiagnosed diabetes, precipitate hyperglycemia or new-onset diabetes, and can also cause hyperglycemic hyperosmolar state (HHS), the authors explained.
They recommended a target glucose of 6.0-10.0 mmol/L (108-180 mg/dL), although they say up to 12 mmol/L (216 mg/dL) is “acceptable.” They then gave advice on frequency of monitoring for people with and without known diabetes, exclusion of diabetic ketoacidosis and HHS, correction of initial hyperglycemia and maintenance of glycemic control using subcutaneous insulin, and prevention of hypoglycemia at the end of dexamethasone therapy (day 10) with insulin down-titration, discharge, and follow-up.
The detailed insulin guidance covers dose escalation for both insulin-treated and insulin-naive patients. A table suggests increasing correction doses of rapid-acting insulin based on prior total daily dose or weight.
Use of once- or twice-daily NPH insulin is recommended for patients whose glucose has risen above 12 mmol/L, in some cases with the addition of a long-acting analog. A second chart gives dose adjustments for those insulins. Additional guidance addresses patients on insulin pumps.
Guidance useful for U.S. physicians
Francisco Pasquel, MD, assistant professor of medicine in the division of endocrinology at Emory University, Atlanta, said in an interview that he believes the guidance is “acceptable” for worldwide use, and that “it’s coherent and consistent with what we typically do.”
However, Dr. Pasquel, who founded COVID-in-Diabetes, an online repository of published guidance and shared experience – to which this new document has now been added – did take issue with one piece of advice. The guidance says that patients already taking premixed insulin formulations can continue using them while increasing the dose by 20%-40%. Given the risk of hypoglycemia associated with those formulations, Dr. Pasquel said he would switch those patients to NPH during the time that they’re on dexamethasone.
He also noted that the rapid-acting insulin dose range of 2-10 units provided in the first table, for correction of initial hyperglycemia, are more conservative than those used at his hospital, where correction doses of up to 14-16 units are sometimes necessary.
But Dr. Pasquel praised the group’s overall efforts since the pandemic began, noting that “they’re very organized and constantly updating their recommendations. They have a unified system in the [National Health Service], so it’s easier to standardize. They have a unique [electronic health record] which is far superior to what we do from a public health perspective.”
Dr. Rayman reported no relevant financial relationships. Dr. Pasquel reported receiving research funding from Dexcom, Merck, and the National Institutes of Health, and consulting for AstraZeneca, Eli Lilly, Merck, and Boehringer Ingelheim.
A version of this article originally appeared on Medscape.com.
New guidance from the U.K. National Diabetes COVID-19 Response Group addresses glucose management in patients with COVID-19 who are receiving dexamethasone therapy.
Although there are already guidelines that address inpatient management of steroid-induced hyperglycemia, the authors of the new document wrote that this new expert opinion paper was needed “given the ‘triple insult’ of dexamethasone-induced–impaired glucose metabolism, COVID-19–induced insulin resistance, and COVID-19–impaired insulin production.”
RECOVERY trial spurs response
The document, which is the latest in a series from the Association of British Clinical Diabetologists, was published online Aug. 2 in Diabetic Medicine. The group is chaired by Gerry Rayman, MD, consultant physician at the diabetes centre and diabetes research unit, East Suffolk (England) and North East NHS Foundation Trust.
The guidance was developed in response to the recent “breakthrough” Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial, which showed that dexamethasone reduced deaths in patients with COVID-19 on ventilators or receiving oxygen therapy. The advice is not intended for critical care units but can be adapted for that use.
The dose used in RECOVERY – 6 mg daily for 10 days – is 400%-500% greater than the therapeutic glucocorticoid replacement dose. High glucocorticoid doses can exacerbate hyperglycemia in people with established diabetes, unmask undiagnosed diabetes, precipitate hyperglycemia or new-onset diabetes, and can also cause hyperglycemic hyperosmolar state (HHS), the authors explained.
They recommended a target glucose of 6.0-10.0 mmol/L (108-180 mg/dL), although they say up to 12 mmol/L (216 mg/dL) is “acceptable.” They then gave advice on frequency of monitoring for people with and without known diabetes, exclusion of diabetic ketoacidosis and HHS, correction of initial hyperglycemia and maintenance of glycemic control using subcutaneous insulin, and prevention of hypoglycemia at the end of dexamethasone therapy (day 10) with insulin down-titration, discharge, and follow-up.
The detailed insulin guidance covers dose escalation for both insulin-treated and insulin-naive patients. A table suggests increasing correction doses of rapid-acting insulin based on prior total daily dose or weight.
Use of once- or twice-daily NPH insulin is recommended for patients whose glucose has risen above 12 mmol/L, in some cases with the addition of a long-acting analog. A second chart gives dose adjustments for those insulins. Additional guidance addresses patients on insulin pumps.
Guidance useful for U.S. physicians
Francisco Pasquel, MD, assistant professor of medicine in the division of endocrinology at Emory University, Atlanta, said in an interview that he believes the guidance is “acceptable” for worldwide use, and that “it’s coherent and consistent with what we typically do.”
However, Dr. Pasquel, who founded COVID-in-Diabetes, an online repository of published guidance and shared experience – to which this new document has now been added – did take issue with one piece of advice. The guidance says that patients already taking premixed insulin formulations can continue using them while increasing the dose by 20%-40%. Given the risk of hypoglycemia associated with those formulations, Dr. Pasquel said he would switch those patients to NPH during the time that they’re on dexamethasone.
He also noted that the rapid-acting insulin dose range of 2-10 units provided in the first table, for correction of initial hyperglycemia, are more conservative than those used at his hospital, where correction doses of up to 14-16 units are sometimes necessary.
But Dr. Pasquel praised the group’s overall efforts since the pandemic began, noting that “they’re very organized and constantly updating their recommendations. They have a unified system in the [National Health Service], so it’s easier to standardize. They have a unique [electronic health record] which is far superior to what we do from a public health perspective.”
Dr. Rayman reported no relevant financial relationships. Dr. Pasquel reported receiving research funding from Dexcom, Merck, and the National Institutes of Health, and consulting for AstraZeneca, Eli Lilly, Merck, and Boehringer Ingelheim.
A version of this article originally appeared on Medscape.com.
New guidance from the U.K. National Diabetes COVID-19 Response Group addresses glucose management in patients with COVID-19 who are receiving dexamethasone therapy.
Although there are already guidelines that address inpatient management of steroid-induced hyperglycemia, the authors of the new document wrote that this new expert opinion paper was needed “given the ‘triple insult’ of dexamethasone-induced–impaired glucose metabolism, COVID-19–induced insulin resistance, and COVID-19–impaired insulin production.”
RECOVERY trial spurs response
The document, which is the latest in a series from the Association of British Clinical Diabetologists, was published online Aug. 2 in Diabetic Medicine. The group is chaired by Gerry Rayman, MD, consultant physician at the diabetes centre and diabetes research unit, East Suffolk (England) and North East NHS Foundation Trust.
The guidance was developed in response to the recent “breakthrough” Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial, which showed that dexamethasone reduced deaths in patients with COVID-19 on ventilators or receiving oxygen therapy. The advice is not intended for critical care units but can be adapted for that use.
The dose used in RECOVERY – 6 mg daily for 10 days – is 400%-500% greater than the therapeutic glucocorticoid replacement dose. High glucocorticoid doses can exacerbate hyperglycemia in people with established diabetes, unmask undiagnosed diabetes, precipitate hyperglycemia or new-onset diabetes, and can also cause hyperglycemic hyperosmolar state (HHS), the authors explained.
They recommended a target glucose of 6.0-10.0 mmol/L (108-180 mg/dL), although they say up to 12 mmol/L (216 mg/dL) is “acceptable.” They then gave advice on frequency of monitoring for people with and without known diabetes, exclusion of diabetic ketoacidosis and HHS, correction of initial hyperglycemia and maintenance of glycemic control using subcutaneous insulin, and prevention of hypoglycemia at the end of dexamethasone therapy (day 10) with insulin down-titration, discharge, and follow-up.
The detailed insulin guidance covers dose escalation for both insulin-treated and insulin-naive patients. A table suggests increasing correction doses of rapid-acting insulin based on prior total daily dose or weight.
Use of once- or twice-daily NPH insulin is recommended for patients whose glucose has risen above 12 mmol/L, in some cases with the addition of a long-acting analog. A second chart gives dose adjustments for those insulins. Additional guidance addresses patients on insulin pumps.
Guidance useful for U.S. physicians
Francisco Pasquel, MD, assistant professor of medicine in the division of endocrinology at Emory University, Atlanta, said in an interview that he believes the guidance is “acceptable” for worldwide use, and that “it’s coherent and consistent with what we typically do.”
However, Dr. Pasquel, who founded COVID-in-Diabetes, an online repository of published guidance and shared experience – to which this new document has now been added – did take issue with one piece of advice. The guidance says that patients already taking premixed insulin formulations can continue using them while increasing the dose by 20%-40%. Given the risk of hypoglycemia associated with those formulations, Dr. Pasquel said he would switch those patients to NPH during the time that they’re on dexamethasone.
He also noted that the rapid-acting insulin dose range of 2-10 units provided in the first table, for correction of initial hyperglycemia, are more conservative than those used at his hospital, where correction doses of up to 14-16 units are sometimes necessary.
But Dr. Pasquel praised the group’s overall efforts since the pandemic began, noting that “they’re very organized and constantly updating their recommendations. They have a unified system in the [National Health Service], so it’s easier to standardize. They have a unique [electronic health record] which is far superior to what we do from a public health perspective.”
Dr. Rayman reported no relevant financial relationships. Dr. Pasquel reported receiving research funding from Dexcom, Merck, and the National Institutes of Health, and consulting for AstraZeneca, Eli Lilly, Merck, and Boehringer Ingelheim.
A version of this article originally appeared on Medscape.com.
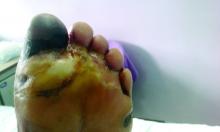
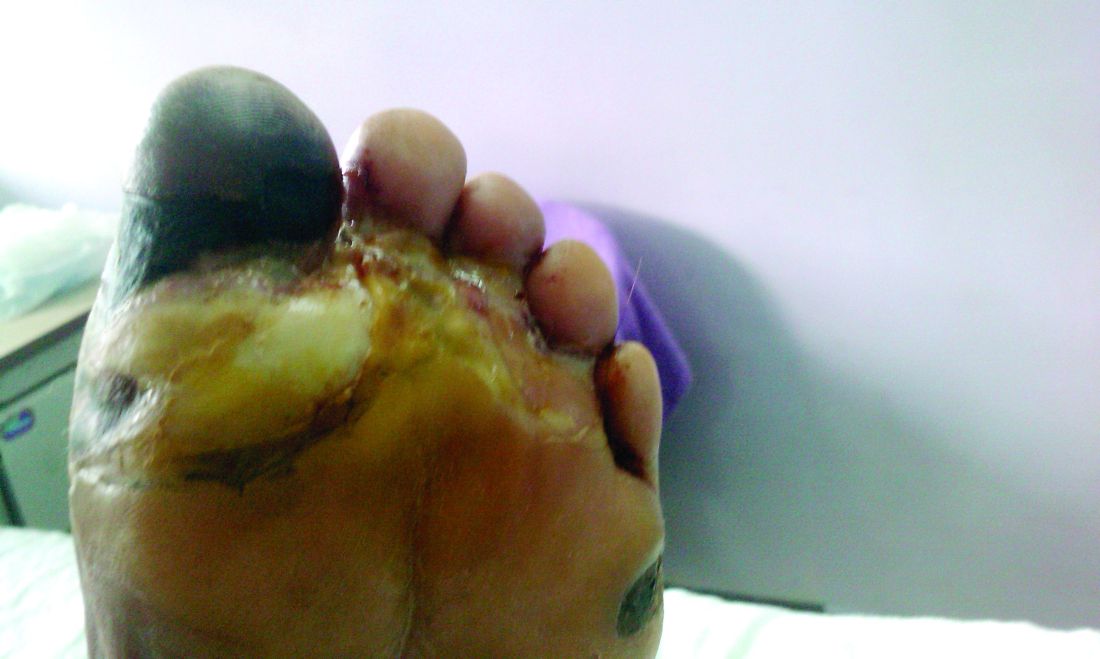
Diabetic amputations soared amid Italian pandemic lockdown
Amid a mandatory national lockdown, the rates of amputations skyrocketed at a hospital far from the hardest-hit region as many patients developed gangrene.
The findings offer critical lessons for the United States, said wound care specialist William H. Tettelbach, MD, of Western Peaks Specialty Hospital near Salt Lake City. “It’s become more obvious that outpatient wound care is a critical care need for the community because of the risk of ignoring these chronic wounds and letting them remain open. We cannot let these services be closed down like some were when the pandemic started.”
The study, led by Paola Caruso, MD, of the University of Campania Luigi Vanvitelli in Naples, appeared in Diabetes Care.
The researchers launched the study to understand how patients with diabetes and DFU fared during the height of the pandemic in Italy, where tens of thousands of people died, mainly in the northern region of the country. They focused on patients in the southern region who were admitted to the division of endocrinology and metabolic diseases at the Teaching Hospital at the University of Campania Luigi Vanvitelli.
The study compared 25 patients who were admitted from March 9 to May 18, 2020, with 38 patients who were admitted from a longer period between January and May 2019. The demographics of the groups are similar, with average ages in the early 60s and more men than women (21:4, respectively, in 2020 and 23:15, respectively, in 2019.)
The results reveal high numbers of emergent and serious cases in 2020. Compared with 2019, fewer were outpatients (16% vs. 45%, P = .028) and more were emergency patients (76% vs. 26%, P < .001).
Clinically, gangrene was much more common in the 2020 group, compared with the 2019 group (64% vs. 29%, P = .009), as was amputation (60% vs. 18%, P = .001).
The researchers determined that amputation was more than three times more likely in the 2020 versus the 2019 group (relative risk, 3.26; 95% confidence interval, 1.55-6.84) even though the 2019 period was longer. After adjustment for gender, the heightened risk in 2020 was 2.50 (95% CI, 1.18-5.29).
There was no statistically significant increase in the risk of revascularization.
“The COVID-19 lockdown may have had a detrimental impact on amputation risk because of the sudden interruption of DFU care and lower-limb preservation pathways, resulting in delayed diagnosis and treatment,” the researchers wrote. “DFU is often characterized by progressive clinical course, which can rapidly lead patients to critical worsening of their ulcers.”
They added that “the higher risk of amputation observed during COVID-19 lockdown confirms the need for proper and timely management of DFU patients to prevent dramatic outcomes responsible for a reduction of quality of life and increased morbidity and mortality.”
The study authors didn’t discuss why more patients seemed to have stayed home and not gotten proper care. It’s not clear if they were scared to get treatment or couldn’t obtain it because of the national shutdown.
Both have been factors affecting diabetic foot care in the United States during the pandemic, said Dr. Tettelbach. He called the study “timely and pertinent,” and said it highlights how wound care is “a critical need” that must remain available even when other medical services such as elective surgeries are shut down.
Infection-control protocols such as allowing patients to wait for appointments in their cars instead of waiting rooms will alleviate the fears of certain patients about seeking in-person care during the pandemic, he said. But some patients will be afraid to come in no matter what, he said, and home health may be the best solution for their care.
Several of the study authors reported various disclosures. Dr. Tettelbach reported no relevant disclosures.
SOURCE: Caruso P et al. Diabetes Care. 2020 Jul 23. doi:10.2337/dc20-1347.
Amid a mandatory national lockdown, the rates of amputations skyrocketed at a hospital far from the hardest-hit region as many patients developed gangrene.
The findings offer critical lessons for the United States, said wound care specialist William H. Tettelbach, MD, of Western Peaks Specialty Hospital near Salt Lake City. “It’s become more obvious that outpatient wound care is a critical care need for the community because of the risk of ignoring these chronic wounds and letting them remain open. We cannot let these services be closed down like some were when the pandemic started.”
The study, led by Paola Caruso, MD, of the University of Campania Luigi Vanvitelli in Naples, appeared in Diabetes Care.
The researchers launched the study to understand how patients with diabetes and DFU fared during the height of the pandemic in Italy, where tens of thousands of people died, mainly in the northern region of the country. They focused on patients in the southern region who were admitted to the division of endocrinology and metabolic diseases at the Teaching Hospital at the University of Campania Luigi Vanvitelli.
The study compared 25 patients who were admitted from March 9 to May 18, 2020, with 38 patients who were admitted from a longer period between January and May 2019. The demographics of the groups are similar, with average ages in the early 60s and more men than women (21:4, respectively, in 2020 and 23:15, respectively, in 2019.)
The results reveal high numbers of emergent and serious cases in 2020. Compared with 2019, fewer were outpatients (16% vs. 45%, P = .028) and more were emergency patients (76% vs. 26%, P < .001).
Clinically, gangrene was much more common in the 2020 group, compared with the 2019 group (64% vs. 29%, P = .009), as was amputation (60% vs. 18%, P = .001).
The researchers determined that amputation was more than three times more likely in the 2020 versus the 2019 group (relative risk, 3.26; 95% confidence interval, 1.55-6.84) even though the 2019 period was longer. After adjustment for gender, the heightened risk in 2020 was 2.50 (95% CI, 1.18-5.29).
There was no statistically significant increase in the risk of revascularization.
“The COVID-19 lockdown may have had a detrimental impact on amputation risk because of the sudden interruption of DFU care and lower-limb preservation pathways, resulting in delayed diagnosis and treatment,” the researchers wrote. “DFU is often characterized by progressive clinical course, which can rapidly lead patients to critical worsening of their ulcers.”
They added that “the higher risk of amputation observed during COVID-19 lockdown confirms the need for proper and timely management of DFU patients to prevent dramatic outcomes responsible for a reduction of quality of life and increased morbidity and mortality.”
The study authors didn’t discuss why more patients seemed to have stayed home and not gotten proper care. It’s not clear if they were scared to get treatment or couldn’t obtain it because of the national shutdown.
Both have been factors affecting diabetic foot care in the United States during the pandemic, said Dr. Tettelbach. He called the study “timely and pertinent,” and said it highlights how wound care is “a critical need” that must remain available even when other medical services such as elective surgeries are shut down.
Infection-control protocols such as allowing patients to wait for appointments in their cars instead of waiting rooms will alleviate the fears of certain patients about seeking in-person care during the pandemic, he said. But some patients will be afraid to come in no matter what, he said, and home health may be the best solution for their care.
Several of the study authors reported various disclosures. Dr. Tettelbach reported no relevant disclosures.
SOURCE: Caruso P et al. Diabetes Care. 2020 Jul 23. doi:10.2337/dc20-1347.
Amid a mandatory national lockdown, the rates of amputations skyrocketed at a hospital far from the hardest-hit region as many patients developed gangrene.
The findings offer critical lessons for the United States, said wound care specialist William H. Tettelbach, MD, of Western Peaks Specialty Hospital near Salt Lake City. “It’s become more obvious that outpatient wound care is a critical care need for the community because of the risk of ignoring these chronic wounds and letting them remain open. We cannot let these services be closed down like some were when the pandemic started.”
The study, led by Paola Caruso, MD, of the University of Campania Luigi Vanvitelli in Naples, appeared in Diabetes Care.
The researchers launched the study to understand how patients with diabetes and DFU fared during the height of the pandemic in Italy, where tens of thousands of people died, mainly in the northern region of the country. They focused on patients in the southern region who were admitted to the division of endocrinology and metabolic diseases at the Teaching Hospital at the University of Campania Luigi Vanvitelli.
The study compared 25 patients who were admitted from March 9 to May 18, 2020, with 38 patients who were admitted from a longer period between January and May 2019. The demographics of the groups are similar, with average ages in the early 60s and more men than women (21:4, respectively, in 2020 and 23:15, respectively, in 2019.)
The results reveal high numbers of emergent and serious cases in 2020. Compared with 2019, fewer were outpatients (16% vs. 45%, P = .028) and more were emergency patients (76% vs. 26%, P < .001).
Clinically, gangrene was much more common in the 2020 group, compared with the 2019 group (64% vs. 29%, P = .009), as was amputation (60% vs. 18%, P = .001).
The researchers determined that amputation was more than three times more likely in the 2020 versus the 2019 group (relative risk, 3.26; 95% confidence interval, 1.55-6.84) even though the 2019 period was longer. After adjustment for gender, the heightened risk in 2020 was 2.50 (95% CI, 1.18-5.29).
There was no statistically significant increase in the risk of revascularization.
“The COVID-19 lockdown may have had a detrimental impact on amputation risk because of the sudden interruption of DFU care and lower-limb preservation pathways, resulting in delayed diagnosis and treatment,” the researchers wrote. “DFU is often characterized by progressive clinical course, which can rapidly lead patients to critical worsening of their ulcers.”
They added that “the higher risk of amputation observed during COVID-19 lockdown confirms the need for proper and timely management of DFU patients to prevent dramatic outcomes responsible for a reduction of quality of life and increased morbidity and mortality.”
The study authors didn’t discuss why more patients seemed to have stayed home and not gotten proper care. It’s not clear if they were scared to get treatment or couldn’t obtain it because of the national shutdown.
Both have been factors affecting diabetic foot care in the United States during the pandemic, said Dr. Tettelbach. He called the study “timely and pertinent,” and said it highlights how wound care is “a critical need” that must remain available even when other medical services such as elective surgeries are shut down.
Infection-control protocols such as allowing patients to wait for appointments in their cars instead of waiting rooms will alleviate the fears of certain patients about seeking in-person care during the pandemic, he said. But some patients will be afraid to come in no matter what, he said, and home health may be the best solution for their care.
Several of the study authors reported various disclosures. Dr. Tettelbach reported no relevant disclosures.
SOURCE: Caruso P et al. Diabetes Care. 2020 Jul 23. doi:10.2337/dc20-1347.
FROM DIABETES CARE
Novel probiotic shows promise in treating type 2 diabetes
A novel probiotic product (Pendulum Glucose Control) containing gut bacteria strains that are deficient in people with type 2 diabetes modestly improves blood glucose levels, new research suggests.
The findings were published in BMJ Open Diabetes Research & Care by Fanny Perraudeau, PhD, and colleagues, all employees of Pendulum Therapeutics.
The product, classified as a medical food, is currently available for purchase on the company’s website without a prescription.
It contains the oligosaccharide-consuming Akkermansia muciniphila and Bifidobacterium infantis, the butyrate producers Anaerobutyricum hallii, Clostridium beijerinckii, and Clostridium butyricum, along with the “prebiotic” dietary fiber inulin.
In the 12-week trial of people with type 2 diabetes who were already taking metformin, with or without a sulfonylurea, 23 were randomized to the product and 26 received placebo capsules.
Participants in the active-treatment arm had significantly reduced glucose levels after a 3-hour standard meal-tolerance test, by 36.1 mg/dL (P = .05), and average A1c reduction of 0.6 percentage points (P = .054), compared with those taking placebo.
There were no major safety or tolerability issues, only transient gastrointestinal symptoms (nausea, diarrhea) lasting 3-5 days. No changes were seen in body weight, insulin sensitivity, or fasting blood glucose.
Asked to comment on the findings, Nanette I. Steinle, MD, an endocrinologist with expertise in nutrition who was not involved in the research, said that “to me it looks like the research was designed well and they didn’t overstate the results. ... I would say, for folks with mild to modest blood glucose elevations, it could be helpful to augment a healthy lifestyle.”
However, the product is not cheap, so cost could be a limiting factor for some patients, said Dr. Steinle, who is associate professor of medicine at the University of Maryland, Baltimore, and chief of the endocrine section, Maryland Veterans Affairs Health Care System.
Lead author Orville Kolterman, MD, chief medical officer at Pendulum, said in an interview that the formulation’s specificity distinguishes it from most commercially available probiotics.
“The ones sold in stores are reconfigurations of food probiotics, which are primarily aerobic organisms, whereas the abnormalities in the microbiome associated with type 2 diabetes reside in anaerobic organisms, which are more difficult to manufacture,” he explained.
The fiber component, inulin, is important as well, he said. “This product may make the dietary management of type 2 diabetes more effective, in that you need both the fiber and the microbes to ferment the fiber and produce short-chain fatty acids that appear to be very important for many reasons.”
The blood glucose-lowering effect is related in part to the three organisms’ production of butyrate, which binds to epithelial cells in the gut to secrete glucagonlike peptide–1, leading to inhibition of glucagon secretion among other actions.
And Akkermansia muciniphila protects the gut epithelium and has shown some evidence of improving insulin sensitivity and other beneficial metabolic effects in humans.
Dr. Kolterman, who was with Amylin Pharmaceuticals prior to moving to Pendulum, commented: “After doing this for 30 years or so, I’ve come to the strong appreciation that whenever you can do something to move back toward what Mother Nature set up, you’re doing a good thing.”
Clinically, Dr. Kolterman said, “I think perhaps the ideal place to try this would be shortly after diagnosis of type 2 diabetes, before patients go on to pharmacologic therapy.”
However, for practical reasons the study was done in patients who were already taking metformin, he said. “The results we have are that it’s beneficial above and beyond metformin, since [these] patients weren’t controlled with metformin.”
He also noted that it might benefit patients who can’t tolerate metformin or who have prediabetes; there’s an ongoing investigator-initiated study of the latter.
Dr. Steinle also endorsed the possibility that the product may benefit people with prediabetes. “I would suspect this could be very helpful to augment attempts to prevent diabetes. ... The group with prediabetes is huge.”
However, she cautioned, “if the blood glucose is over 200 [mg/dL], I wouldn’t think a probiotic would get them where they need to go.”
Overall, she pointed out that targeting the microbiome is a very active and potentially important field of medical research, and that it has received support from the National Institutes of Health. “I think we’re in the early stages of understanding how what grows in us, and on us, impacts our health and how we may be able to use these organisms to our benefit. I would expect we’ll see more of these probiotics being marketed in various forms.”
Dr. Kolterman is an employee of Pendulum. Dr. Steinle has reported receiving funding from the NIH, and she is conducting a study funded by Kowa through the VA.
A version of this article originally appeared on Medscape.com.
A novel probiotic product (Pendulum Glucose Control) containing gut bacteria strains that are deficient in people with type 2 diabetes modestly improves blood glucose levels, new research suggests.
The findings were published in BMJ Open Diabetes Research & Care by Fanny Perraudeau, PhD, and colleagues, all employees of Pendulum Therapeutics.
The product, classified as a medical food, is currently available for purchase on the company’s website without a prescription.
It contains the oligosaccharide-consuming Akkermansia muciniphila and Bifidobacterium infantis, the butyrate producers Anaerobutyricum hallii, Clostridium beijerinckii, and Clostridium butyricum, along with the “prebiotic” dietary fiber inulin.
In the 12-week trial of people with type 2 diabetes who were already taking metformin, with or without a sulfonylurea, 23 were randomized to the product and 26 received placebo capsules.
Participants in the active-treatment arm had significantly reduced glucose levels after a 3-hour standard meal-tolerance test, by 36.1 mg/dL (P = .05), and average A1c reduction of 0.6 percentage points (P = .054), compared with those taking placebo.
There were no major safety or tolerability issues, only transient gastrointestinal symptoms (nausea, diarrhea) lasting 3-5 days. No changes were seen in body weight, insulin sensitivity, or fasting blood glucose.
Asked to comment on the findings, Nanette I. Steinle, MD, an endocrinologist with expertise in nutrition who was not involved in the research, said that “to me it looks like the research was designed well and they didn’t overstate the results. ... I would say, for folks with mild to modest blood glucose elevations, it could be helpful to augment a healthy lifestyle.”
However, the product is not cheap, so cost could be a limiting factor for some patients, said Dr. Steinle, who is associate professor of medicine at the University of Maryland, Baltimore, and chief of the endocrine section, Maryland Veterans Affairs Health Care System.
Lead author Orville Kolterman, MD, chief medical officer at Pendulum, said in an interview that the formulation’s specificity distinguishes it from most commercially available probiotics.
“The ones sold in stores are reconfigurations of food probiotics, which are primarily aerobic organisms, whereas the abnormalities in the microbiome associated with type 2 diabetes reside in anaerobic organisms, which are more difficult to manufacture,” he explained.
The fiber component, inulin, is important as well, he said. “This product may make the dietary management of type 2 diabetes more effective, in that you need both the fiber and the microbes to ferment the fiber and produce short-chain fatty acids that appear to be very important for many reasons.”
The blood glucose-lowering effect is related in part to the three organisms’ production of butyrate, which binds to epithelial cells in the gut to secrete glucagonlike peptide–1, leading to inhibition of glucagon secretion among other actions.
And Akkermansia muciniphila protects the gut epithelium and has shown some evidence of improving insulin sensitivity and other beneficial metabolic effects in humans.
Dr. Kolterman, who was with Amylin Pharmaceuticals prior to moving to Pendulum, commented: “After doing this for 30 years or so, I’ve come to the strong appreciation that whenever you can do something to move back toward what Mother Nature set up, you’re doing a good thing.”
Clinically, Dr. Kolterman said, “I think perhaps the ideal place to try this would be shortly after diagnosis of type 2 diabetes, before patients go on to pharmacologic therapy.”
However, for practical reasons the study was done in patients who were already taking metformin, he said. “The results we have are that it’s beneficial above and beyond metformin, since [these] patients weren’t controlled with metformin.”
He also noted that it might benefit patients who can’t tolerate metformin or who have prediabetes; there’s an ongoing investigator-initiated study of the latter.
Dr. Steinle also endorsed the possibility that the product may benefit people with prediabetes. “I would suspect this could be very helpful to augment attempts to prevent diabetes. ... The group with prediabetes is huge.”
However, she cautioned, “if the blood glucose is over 200 [mg/dL], I wouldn’t think a probiotic would get them where they need to go.”
Overall, she pointed out that targeting the microbiome is a very active and potentially important field of medical research, and that it has received support from the National Institutes of Health. “I think we’re in the early stages of understanding how what grows in us, and on us, impacts our health and how we may be able to use these organisms to our benefit. I would expect we’ll see more of these probiotics being marketed in various forms.”
Dr. Kolterman is an employee of Pendulum. Dr. Steinle has reported receiving funding from the NIH, and she is conducting a study funded by Kowa through the VA.
A version of this article originally appeared on Medscape.com.
A novel probiotic product (Pendulum Glucose Control) containing gut bacteria strains that are deficient in people with type 2 diabetes modestly improves blood glucose levels, new research suggests.
The findings were published in BMJ Open Diabetes Research & Care by Fanny Perraudeau, PhD, and colleagues, all employees of Pendulum Therapeutics.
The product, classified as a medical food, is currently available for purchase on the company’s website without a prescription.
It contains the oligosaccharide-consuming Akkermansia muciniphila and Bifidobacterium infantis, the butyrate producers Anaerobutyricum hallii, Clostridium beijerinckii, and Clostridium butyricum, along with the “prebiotic” dietary fiber inulin.
In the 12-week trial of people with type 2 diabetes who were already taking metformin, with or without a sulfonylurea, 23 were randomized to the product and 26 received placebo capsules.
Participants in the active-treatment arm had significantly reduced glucose levels after a 3-hour standard meal-tolerance test, by 36.1 mg/dL (P = .05), and average A1c reduction of 0.6 percentage points (P = .054), compared with those taking placebo.
There were no major safety or tolerability issues, only transient gastrointestinal symptoms (nausea, diarrhea) lasting 3-5 days. No changes were seen in body weight, insulin sensitivity, or fasting blood glucose.
Asked to comment on the findings, Nanette I. Steinle, MD, an endocrinologist with expertise in nutrition who was not involved in the research, said that “to me it looks like the research was designed well and they didn’t overstate the results. ... I would say, for folks with mild to modest blood glucose elevations, it could be helpful to augment a healthy lifestyle.”
However, the product is not cheap, so cost could be a limiting factor for some patients, said Dr. Steinle, who is associate professor of medicine at the University of Maryland, Baltimore, and chief of the endocrine section, Maryland Veterans Affairs Health Care System.
Lead author Orville Kolterman, MD, chief medical officer at Pendulum, said in an interview that the formulation’s specificity distinguishes it from most commercially available probiotics.
“The ones sold in stores are reconfigurations of food probiotics, which are primarily aerobic organisms, whereas the abnormalities in the microbiome associated with type 2 diabetes reside in anaerobic organisms, which are more difficult to manufacture,” he explained.
The fiber component, inulin, is important as well, he said. “This product may make the dietary management of type 2 diabetes more effective, in that you need both the fiber and the microbes to ferment the fiber and produce short-chain fatty acids that appear to be very important for many reasons.”
The blood glucose-lowering effect is related in part to the three organisms’ production of butyrate, which binds to epithelial cells in the gut to secrete glucagonlike peptide–1, leading to inhibition of glucagon secretion among other actions.
And Akkermansia muciniphila protects the gut epithelium and has shown some evidence of improving insulin sensitivity and other beneficial metabolic effects in humans.
Dr. Kolterman, who was with Amylin Pharmaceuticals prior to moving to Pendulum, commented: “After doing this for 30 years or so, I’ve come to the strong appreciation that whenever you can do something to move back toward what Mother Nature set up, you’re doing a good thing.”
Clinically, Dr. Kolterman said, “I think perhaps the ideal place to try this would be shortly after diagnosis of type 2 diabetes, before patients go on to pharmacologic therapy.”
However, for practical reasons the study was done in patients who were already taking metformin, he said. “The results we have are that it’s beneficial above and beyond metformin, since [these] patients weren’t controlled with metformin.”
He also noted that it might benefit patients who can’t tolerate metformin or who have prediabetes; there’s an ongoing investigator-initiated study of the latter.
Dr. Steinle also endorsed the possibility that the product may benefit people with prediabetes. “I would suspect this could be very helpful to augment attempts to prevent diabetes. ... The group with prediabetes is huge.”
However, she cautioned, “if the blood glucose is over 200 [mg/dL], I wouldn’t think a probiotic would get them where they need to go.”
Overall, she pointed out that targeting the microbiome is a very active and potentially important field of medical research, and that it has received support from the National Institutes of Health. “I think we’re in the early stages of understanding how what grows in us, and on us, impacts our health and how we may be able to use these organisms to our benefit. I would expect we’ll see more of these probiotics being marketed in various forms.”
Dr. Kolterman is an employee of Pendulum. Dr. Steinle has reported receiving funding from the NIH, and she is conducting a study funded by Kowa through the VA.
A version of this article originally appeared on Medscape.com.
SGLT2 inhibitors have a breakout year
The benefits from sodium-glucose cotransporter 2 inhibitor drugs proven during the past year for cutting heart failure hospitalization rates substantially in patients with heart failure with reduced ejection fraction and slowing progression of chronic kidney disease, all regardless of diabetes status, have thrust this drug class into the top tier of agents for potentially treating millions of patients with cardiac or renal disease.
The sodium-glucose cotransporter 2 (SGLT2) inhibitors, first licensed for U.S. marketing in 2013 purely for glycemic control, have, during the 5 years since the first cardiovascular outcome trial results for the class came out, shown benefits in a range of patients reminiscent of what’s been established for ACE inhibitors and angiotensin receptor blockers (ARBs).
The wide-reaching benefits of SGLT2 inhibitors have recently become even more relevant by showing clinically meaningful effects in patients without type 2 diabetes (T2D). And in an uncanny coincidence, the SGLT2 inhibitors appear to act in complementary harmony with the ACE inhibitors and ARBs for preserving heart and renal function. These properties have made the SGLT2 inhibitors especially attractive as a new weapon for controlling the ascendant disorder of cardiorenal syndrome.
“SGLT2 inhibitors have a relatively greater impact on cardiovascular outcomes, compared with ACE inhibitors and ARBs, but the effects [of the two classes] are synergistic and ideally patients receive both,” said Peter McCullough, MD, a specialist in treating cardiorenal syndrome and other cardiovascular and renal disorders at Baylor, Scott, and White Heart and Vascular Hospital in Dallas. The SGLT2 inhibitors are among the drugs best suited to both treating and preventing cardiorenal syndrome by targeting both ends of the disorder, said Dr. McCullough, who chaired an American Heart Association panel that last year issued a scientific statement on cardiorenal syndrome (Circulation. 2019 Apr 16;139[16]:e840-78).
Although data on the SGLT2 inhibitors “are evolving,” the drug class is “going in the direction” of being “reasonably compared” with the ACE inhibitors and ARBs, said Javed Butler, MD, professor and chair of medicine at the University of Mississippi Medical Center, Jackson. “There are certainly complementary benefits that we see for both cardiovascular and renal outcomes.”
“We’ll think more and more about the SGLT2 inhibitors like renin-angiotensin system [RAS] inhibitors,” said David Z. Cherney, MD, referring to the drug class that includes ACE inhibitors and ARBs. “We should start to approach SGLT2 inhibitors like RAS inhibitors, with pleiotropic effects that go beyond glucose,” said Dr. Cherney, a nephrologist and professor of medicine at the University of Toronto, during the virtual annual scientific sessions of the American Diabetes Association in June 2020.
Working together in the nephron
One of the clearest complementary interactions between the SGLT2 inhibitors and the RAS inhibitors is their ability to reduce intraglomerular pressure, a key mechanism that slows nephron loss and progression of chronic kidney disease. SGLT2 inhibitors reduce sodium absorption in the proximal tubule that causes, through tubuloglomerular feedback, afferent arteriole constriction that lowers intraglomerular pressure, while the RAS inhibitors inhibit efferent arteriole constriction mediated by angiotensin II, also cutting intraglomerular pressure. Together, “they almost work in tandem,” explained Janani Rangaswami, MD, a nephrologist at Einstein Medical Center in Philadelphia, vice chair of the Kidney Council of the AHA, and first author of the 2019 cardiorenal syndrome AHA statement.
“Many had worried that if we target both the afferent and efferent arterioles simultaneously, it might increase the risk for acute kidney injury. What has been reassuring in both the recent data from the DAPA-HF trial and in recent meta-analysis was no evidence of increased risk for acute kidney injury with use of the SGLT2 inhibitor,” Dr. Rangaswami said in an interview. For example, a recent report on more than 39,000 Canadian patients with T2D who were at least 66 years old and newly begun on either an SGLT2 inhibitor or a different oral diabetes drug (a dipeptidyl peptidase–4 inhibitor), found a statistically significant 21% lower rate of acute kidney injury during the first 90 days on treatment with an SGLT2 inhibitor in a propensity score–matched analysis (CMAJ. 2020 Apr 6;192: e351-60).
Much of the concern about possible acute kidney injury stemmed from a property that the SGLT2 inhibitors share with RAS inhibitors: They cause an initial, reversible decline in glomerular filtration rate (GFR), followed by longer-term nephron preservation, a pattern attributable to reduced intraglomerular pressure. The question early on was: “ ‘Does this harm the kidney?’ But what we’ve seen is that patients do better over time, even with this initial hit. Whenever you offload the glomerulus you cut barotrauma and protect renal function,” explained Silvio E. Inzucchi, MD, professor of medicine at Yale University, New Haven, Conn., and director of the Yale Medicine Diabetes Center.
Dr. Inzucchi cautioned, however, that a small number of patients starting treatment with an SGLT2 inhibitor may have their GFR drop too sharply, especially if their GFR was low to start with. “You need to be careful, especially at the lower end of the GFR range. I recheck renal function after 1 month” after a patient starts an SGLT2. Patients whose level falls too low may need to discontinue. He added that it’s hard to set a uniform threshold for alarm, and instead assess patients on a case-by-case basis, but “you need some threshold in mind, where you will stop” treatment.
A smarter diuretic
One of the most intriguing renal effects of SGLT2 inhibitors is their diuretic action. During a talk at the virtual ADA scientific sessions, cardiologist Jeffrey Testani, MD, called them “smart” diuretics, because their effect on diuresis is relatively modest but comes without the neurohormonal price paid when patients take conventional loop diuretics.
”Loop diuretics are particularly bad,” causing neurohormonal activation that includes norepinephrine, renin, and vasopressin, said Dr. Testani, director of heart failure research at Yale. They also fail to produce a meaningful drop in blood volume despite causing substantial natriuresis.
In contrast, SGLT2 inhibitors cause “moderate” natriuresis while producing a significant cut in blood volume. “The body seems content with this lower plasma volume without activating catecholamines or renin, and that’s how the SGLT2 inhibitors differ from other diuretics,” said Dr. Inzucchi.
The class also maintains serum levels of potassium and magnesium, produces significant improvements in serum uric acid levels, and avoids the electrolyte abnormalities, volume depletion, and acute kidney injury that can occur with conventional distal diuretics, Dr. Testani said.
In short, the SGLT2 inhibitors “are safe and easy-to-use diuretics,” which allows them to fill a “huge unmet need for patients with heart failure.” As evidence accumulates for the benefits of the drug class in patients with heart failure and renal disease, “uptake will be extensive,” Dr. Testani predicted, driven in part by how easy it is to add the class to existing cardiorenal drug regimens.
Other standard therapies for patients with heart failure with reduced ejection fraction (HFrEF) risk electrolyte abnormalities, renal dysfunction, significantly lower blood pressure, often make patients feel worse, and involve a slow and laborious titration process, Dr. Testani noted. The SGLT2 inhibitor agents avoid these issues, a property that has played out in quality of life assessments of patients with HFrEF who received a drug from this class.
Outcomes met in trial after trial
In the DAPA-HF trial, with 4,443 patients with HFrEF and divided roughly equally between those with or without T2D, treatment with dapagliflozin (Farxiga) linked with significant improvements in health status and quality of life measured by the Kansas City Cardiomyopathy Questionnaire (Circulation. 2020 Jan 14;141[2]:90-9). “Not all treatments for HFrEF improve symptoms,” but in this study the SGTL2 inhibitor dapagliflozin did, boosting the Kansas City Cardiomyopathy Questionnaire score by about the same magnitude as treatment with a cardiac resynchronization device in patients with HFrEF, said Mikhail N. Kosiborod, MD, director of Cardiometabolic Research at Saint Luke’s Mid America Heart Institute in Kansas City, Mo., speaking at the virtual ADA scientific sessions.
Two more recent renal observations have further solidified the growing role of these drugs for kidney protection. Results from the CREDENCE trial that looked at canagliflozin (Invokana) treatment in 4,401 patients with T2D and albuminuria and chronic kidney disease showed canagliflozin treatment cut the primary, composite renal endpoint by a statistically significant 30%, compared with placebo (N Engl J Med. 2019 Jun 13;380[24]:2295-306). The study stopped earlier than planned because of how effective canagliflozin appeared.
“Never before has a renal protection clinical trial stopped for overwhelming efficacy,” noted nephrologist Katherine R. Tuttle, MD, executive director for research at Providence Health Care in Spokane, Wash. “It’s very exciting to have a treatment that works on both the heart and kidney, given their interrelationship,” she said during the ADA sessions. Dr. Tuttle called the cardiorenal effects from the SGLT2 inhibitors “amazing.”
Just as the DAPA-HF trial’s primary outcome showed the ability of at least one drug from the class, dapagliflozin, to improve outcomes in HFrEF patients without T2D, topline results recently reported from the DAPA-CDK trial showed for the first time renal protection by an SGLT2 inhibitor in patients with chronic kidney disease but no T2D, in a study with about 4,300 patients.
Although detailed results from DAPA-CKD are not yet available, so far the outcomes seem consistent with the CREDENCE findings, and the cumulative renal findings for the class show the SGLT2 inhibitors have “potential for a profound impact on the patients we see in every nephrology clinic, and with dual cardiorenal disease,” said Dr. Rangaswami. The class is now established as “standard of care for patients with chronic kidney disease. The CREDENCE results made that clear.”
The DAPA-CKD findings in patients with chronic kidney disease regardless of their diabetes status “are very important. We really have not had any advances in this space for some time, and chronic kidney disease patients have very poor outcomes, both cardiovascular and renal,” commented Dr. Butler. The advantage from using this drug class in these patients “is huge.”
The DAPA-CKD findings are a “major advance,” agreed Dr. McCullough.
SGLT2 inhibitor use needs to grow
Experts lament that although the evidence favoring the class has been very bullish, prescribing uptake has been slow, perhaps partly explained by the retail U.S. cost for most of these agents, generally about $17/day.
Cost is, unfortunately, an issue right now for these drugs, said Dr. Butler. Generic formulations are imminent, “but we cannot accept waiting. Providing this therapy when insurance coverage is available,” is essential.
The FDA has already granted tentative approval to some generic formulations, although resolution of patent issues can delay generics actually reaching the market. “Generic dapagliflozin will have a major impact; the marketplace for these drugs will shift very quickly,” predicted Dr. McCullough.
But price may not be the sole barrier, cautioned Dr. Rangaswami. “I don’t think it’s just a cost issue. Several factors explain the slow uptake,” of the SGLT2 inhibitors. “The biggest barrier is that this is a new drug class, and understanding how to use the class is not yet where it needs to be in the physician community.” One of the biggest problems is that the SGLT2 inhibitors are still primarily regarded as drugs to treat hyperglycemia.
Physicians who treat patients with heart or renal disease “need to wrap their head around the idea that a drug with antihyperglycemic effects is now in their practice territory, and something they need to prescribe,” she noted. Currently “there is a reluctance to prescribe these drugs given the perception that they are antihyperglycemic agents, and usually get deferred to primary care physicians or endocrinologists. This results in huge missed opportunities by cardiologists and nephrologists in initiating these agents that have major cardiorenal risk reduction effects.”
The key role that cardiologists need to play in prescribing the SGLT2 inhibitors was brought home in a recent study of two representative U.S. health systems that showed patients with T2D were far more likely to see a cardiologist than an endocrinologist (Cardiovasc Endocrinol Metab. 2020 Jun;9[2]:56-9).
“The SGLT2 inhibitors are definitely a game-changing drug class,” summed up Dr. Rangaswami. “We’re going to see a lot of use in patients with heart and kidney disease.”
Dr. Cherney has been a consultant to or has received honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Mitsubishi Tanabe Pharma, and Sanofi. Dr. Butler has had financial relationships with numerous pharmaceutical companies. Dr. McCullough and Dr. Rangaswami had no disclosures. Dr. Inzucchi has been a consultant to or helped run trials for Abbott, AstraZeneca, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics. Dr. Testani has been a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, cardionomic, FIRE1 Magenta Med, Novartis, Reprieve, Sanofi, and W.L. Gore. Dr. Kosiborod has been a consultant to or led trials for Amarin, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Glytec, Janssen, Eli Lilly, Merck, Novartis, Novo Nordisk, Sanofi, and Vifor. Dr. Tuttle has been a consultant to AstraZeneca, Boehringer Ingelheim, Gilead, Goldfinch Bio, Eli Lilly, and Novo Nordisk.
The benefits from sodium-glucose cotransporter 2 inhibitor drugs proven during the past year for cutting heart failure hospitalization rates substantially in patients with heart failure with reduced ejection fraction and slowing progression of chronic kidney disease, all regardless of diabetes status, have thrust this drug class into the top tier of agents for potentially treating millions of patients with cardiac or renal disease.
The sodium-glucose cotransporter 2 (SGLT2) inhibitors, first licensed for U.S. marketing in 2013 purely for glycemic control, have, during the 5 years since the first cardiovascular outcome trial results for the class came out, shown benefits in a range of patients reminiscent of what’s been established for ACE inhibitors and angiotensin receptor blockers (ARBs).
The wide-reaching benefits of SGLT2 inhibitors have recently become even more relevant by showing clinically meaningful effects in patients without type 2 diabetes (T2D). And in an uncanny coincidence, the SGLT2 inhibitors appear to act in complementary harmony with the ACE inhibitors and ARBs for preserving heart and renal function. These properties have made the SGLT2 inhibitors especially attractive as a new weapon for controlling the ascendant disorder of cardiorenal syndrome.
“SGLT2 inhibitors have a relatively greater impact on cardiovascular outcomes, compared with ACE inhibitors and ARBs, but the effects [of the two classes] are synergistic and ideally patients receive both,” said Peter McCullough, MD, a specialist in treating cardiorenal syndrome and other cardiovascular and renal disorders at Baylor, Scott, and White Heart and Vascular Hospital in Dallas. The SGLT2 inhibitors are among the drugs best suited to both treating and preventing cardiorenal syndrome by targeting both ends of the disorder, said Dr. McCullough, who chaired an American Heart Association panel that last year issued a scientific statement on cardiorenal syndrome (Circulation. 2019 Apr 16;139[16]:e840-78).
Although data on the SGLT2 inhibitors “are evolving,” the drug class is “going in the direction” of being “reasonably compared” with the ACE inhibitors and ARBs, said Javed Butler, MD, professor and chair of medicine at the University of Mississippi Medical Center, Jackson. “There are certainly complementary benefits that we see for both cardiovascular and renal outcomes.”
“We’ll think more and more about the SGLT2 inhibitors like renin-angiotensin system [RAS] inhibitors,” said David Z. Cherney, MD, referring to the drug class that includes ACE inhibitors and ARBs. “We should start to approach SGLT2 inhibitors like RAS inhibitors, with pleiotropic effects that go beyond glucose,” said Dr. Cherney, a nephrologist and professor of medicine at the University of Toronto, during the virtual annual scientific sessions of the American Diabetes Association in June 2020.
Working together in the nephron
One of the clearest complementary interactions between the SGLT2 inhibitors and the RAS inhibitors is their ability to reduce intraglomerular pressure, a key mechanism that slows nephron loss and progression of chronic kidney disease. SGLT2 inhibitors reduce sodium absorption in the proximal tubule that causes, through tubuloglomerular feedback, afferent arteriole constriction that lowers intraglomerular pressure, while the RAS inhibitors inhibit efferent arteriole constriction mediated by angiotensin II, also cutting intraglomerular pressure. Together, “they almost work in tandem,” explained Janani Rangaswami, MD, a nephrologist at Einstein Medical Center in Philadelphia, vice chair of the Kidney Council of the AHA, and first author of the 2019 cardiorenal syndrome AHA statement.
“Many had worried that if we target both the afferent and efferent arterioles simultaneously, it might increase the risk for acute kidney injury. What has been reassuring in both the recent data from the DAPA-HF trial and in recent meta-analysis was no evidence of increased risk for acute kidney injury with use of the SGLT2 inhibitor,” Dr. Rangaswami said in an interview. For example, a recent report on more than 39,000 Canadian patients with T2D who were at least 66 years old and newly begun on either an SGLT2 inhibitor or a different oral diabetes drug (a dipeptidyl peptidase–4 inhibitor), found a statistically significant 21% lower rate of acute kidney injury during the first 90 days on treatment with an SGLT2 inhibitor in a propensity score–matched analysis (CMAJ. 2020 Apr 6;192: e351-60).
Much of the concern about possible acute kidney injury stemmed from a property that the SGLT2 inhibitors share with RAS inhibitors: They cause an initial, reversible decline in glomerular filtration rate (GFR), followed by longer-term nephron preservation, a pattern attributable to reduced intraglomerular pressure. The question early on was: “ ‘Does this harm the kidney?’ But what we’ve seen is that patients do better over time, even with this initial hit. Whenever you offload the glomerulus you cut barotrauma and protect renal function,” explained Silvio E. Inzucchi, MD, professor of medicine at Yale University, New Haven, Conn., and director of the Yale Medicine Diabetes Center.
Dr. Inzucchi cautioned, however, that a small number of patients starting treatment with an SGLT2 inhibitor may have their GFR drop too sharply, especially if their GFR was low to start with. “You need to be careful, especially at the lower end of the GFR range. I recheck renal function after 1 month” after a patient starts an SGLT2. Patients whose level falls too low may need to discontinue. He added that it’s hard to set a uniform threshold for alarm, and instead assess patients on a case-by-case basis, but “you need some threshold in mind, where you will stop” treatment.
A smarter diuretic
One of the most intriguing renal effects of SGLT2 inhibitors is their diuretic action. During a talk at the virtual ADA scientific sessions, cardiologist Jeffrey Testani, MD, called them “smart” diuretics, because their effect on diuresis is relatively modest but comes without the neurohormonal price paid when patients take conventional loop diuretics.
”Loop diuretics are particularly bad,” causing neurohormonal activation that includes norepinephrine, renin, and vasopressin, said Dr. Testani, director of heart failure research at Yale. They also fail to produce a meaningful drop in blood volume despite causing substantial natriuresis.
In contrast, SGLT2 inhibitors cause “moderate” natriuresis while producing a significant cut in blood volume. “The body seems content with this lower plasma volume without activating catecholamines or renin, and that’s how the SGLT2 inhibitors differ from other diuretics,” said Dr. Inzucchi.
The class also maintains serum levels of potassium and magnesium, produces significant improvements in serum uric acid levels, and avoids the electrolyte abnormalities, volume depletion, and acute kidney injury that can occur with conventional distal diuretics, Dr. Testani said.
In short, the SGLT2 inhibitors “are safe and easy-to-use diuretics,” which allows them to fill a “huge unmet need for patients with heart failure.” As evidence accumulates for the benefits of the drug class in patients with heart failure and renal disease, “uptake will be extensive,” Dr. Testani predicted, driven in part by how easy it is to add the class to existing cardiorenal drug regimens.
Other standard therapies for patients with heart failure with reduced ejection fraction (HFrEF) risk electrolyte abnormalities, renal dysfunction, significantly lower blood pressure, often make patients feel worse, and involve a slow and laborious titration process, Dr. Testani noted. The SGLT2 inhibitor agents avoid these issues, a property that has played out in quality of life assessments of patients with HFrEF who received a drug from this class.
Outcomes met in trial after trial
In the DAPA-HF trial, with 4,443 patients with HFrEF and divided roughly equally between those with or without T2D, treatment with dapagliflozin (Farxiga) linked with significant improvements in health status and quality of life measured by the Kansas City Cardiomyopathy Questionnaire (Circulation. 2020 Jan 14;141[2]:90-9). “Not all treatments for HFrEF improve symptoms,” but in this study the SGTL2 inhibitor dapagliflozin did, boosting the Kansas City Cardiomyopathy Questionnaire score by about the same magnitude as treatment with a cardiac resynchronization device in patients with HFrEF, said Mikhail N. Kosiborod, MD, director of Cardiometabolic Research at Saint Luke’s Mid America Heart Institute in Kansas City, Mo., speaking at the virtual ADA scientific sessions.
Two more recent renal observations have further solidified the growing role of these drugs for kidney protection. Results from the CREDENCE trial that looked at canagliflozin (Invokana) treatment in 4,401 patients with T2D and albuminuria and chronic kidney disease showed canagliflozin treatment cut the primary, composite renal endpoint by a statistically significant 30%, compared with placebo (N Engl J Med. 2019 Jun 13;380[24]:2295-306). The study stopped earlier than planned because of how effective canagliflozin appeared.
“Never before has a renal protection clinical trial stopped for overwhelming efficacy,” noted nephrologist Katherine R. Tuttle, MD, executive director for research at Providence Health Care in Spokane, Wash. “It’s very exciting to have a treatment that works on both the heart and kidney, given their interrelationship,” she said during the ADA sessions. Dr. Tuttle called the cardiorenal effects from the SGLT2 inhibitors “amazing.”
Just as the DAPA-HF trial’s primary outcome showed the ability of at least one drug from the class, dapagliflozin, to improve outcomes in HFrEF patients without T2D, topline results recently reported from the DAPA-CDK trial showed for the first time renal protection by an SGLT2 inhibitor in patients with chronic kidney disease but no T2D, in a study with about 4,300 patients.
Although detailed results from DAPA-CKD are not yet available, so far the outcomes seem consistent with the CREDENCE findings, and the cumulative renal findings for the class show the SGLT2 inhibitors have “potential for a profound impact on the patients we see in every nephrology clinic, and with dual cardiorenal disease,” said Dr. Rangaswami. The class is now established as “standard of care for patients with chronic kidney disease. The CREDENCE results made that clear.”
The DAPA-CKD findings in patients with chronic kidney disease regardless of their diabetes status “are very important. We really have not had any advances in this space for some time, and chronic kidney disease patients have very poor outcomes, both cardiovascular and renal,” commented Dr. Butler. The advantage from using this drug class in these patients “is huge.”
The DAPA-CKD findings are a “major advance,” agreed Dr. McCullough.
SGLT2 inhibitor use needs to grow
Experts lament that although the evidence favoring the class has been very bullish, prescribing uptake has been slow, perhaps partly explained by the retail U.S. cost for most of these agents, generally about $17/day.
Cost is, unfortunately, an issue right now for these drugs, said Dr. Butler. Generic formulations are imminent, “but we cannot accept waiting. Providing this therapy when insurance coverage is available,” is essential.
The FDA has already granted tentative approval to some generic formulations, although resolution of patent issues can delay generics actually reaching the market. “Generic dapagliflozin will have a major impact; the marketplace for these drugs will shift very quickly,” predicted Dr. McCullough.
But price may not be the sole barrier, cautioned Dr. Rangaswami. “I don’t think it’s just a cost issue. Several factors explain the slow uptake,” of the SGLT2 inhibitors. “The biggest barrier is that this is a new drug class, and understanding how to use the class is not yet where it needs to be in the physician community.” One of the biggest problems is that the SGLT2 inhibitors are still primarily regarded as drugs to treat hyperglycemia.
Physicians who treat patients with heart or renal disease “need to wrap their head around the idea that a drug with antihyperglycemic effects is now in their practice territory, and something they need to prescribe,” she noted. Currently “there is a reluctance to prescribe these drugs given the perception that they are antihyperglycemic agents, and usually get deferred to primary care physicians or endocrinologists. This results in huge missed opportunities by cardiologists and nephrologists in initiating these agents that have major cardiorenal risk reduction effects.”
The key role that cardiologists need to play in prescribing the SGLT2 inhibitors was brought home in a recent study of two representative U.S. health systems that showed patients with T2D were far more likely to see a cardiologist than an endocrinologist (Cardiovasc Endocrinol Metab. 2020 Jun;9[2]:56-9).
“The SGLT2 inhibitors are definitely a game-changing drug class,” summed up Dr. Rangaswami. “We’re going to see a lot of use in patients with heart and kidney disease.”
Dr. Cherney has been a consultant to or has received honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Mitsubishi Tanabe Pharma, and Sanofi. Dr. Butler has had financial relationships with numerous pharmaceutical companies. Dr. McCullough and Dr. Rangaswami had no disclosures. Dr. Inzucchi has been a consultant to or helped run trials for Abbott, AstraZeneca, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics. Dr. Testani has been a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, cardionomic, FIRE1 Magenta Med, Novartis, Reprieve, Sanofi, and W.L. Gore. Dr. Kosiborod has been a consultant to or led trials for Amarin, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Glytec, Janssen, Eli Lilly, Merck, Novartis, Novo Nordisk, Sanofi, and Vifor. Dr. Tuttle has been a consultant to AstraZeneca, Boehringer Ingelheim, Gilead, Goldfinch Bio, Eli Lilly, and Novo Nordisk.
The benefits from sodium-glucose cotransporter 2 inhibitor drugs proven during the past year for cutting heart failure hospitalization rates substantially in patients with heart failure with reduced ejection fraction and slowing progression of chronic kidney disease, all regardless of diabetes status, have thrust this drug class into the top tier of agents for potentially treating millions of patients with cardiac or renal disease.
The sodium-glucose cotransporter 2 (SGLT2) inhibitors, first licensed for U.S. marketing in 2013 purely for glycemic control, have, during the 5 years since the first cardiovascular outcome trial results for the class came out, shown benefits in a range of patients reminiscent of what’s been established for ACE inhibitors and angiotensin receptor blockers (ARBs).
The wide-reaching benefits of SGLT2 inhibitors have recently become even more relevant by showing clinically meaningful effects in patients without type 2 diabetes (T2D). And in an uncanny coincidence, the SGLT2 inhibitors appear to act in complementary harmony with the ACE inhibitors and ARBs for preserving heart and renal function. These properties have made the SGLT2 inhibitors especially attractive as a new weapon for controlling the ascendant disorder of cardiorenal syndrome.
“SGLT2 inhibitors have a relatively greater impact on cardiovascular outcomes, compared with ACE inhibitors and ARBs, but the effects [of the two classes] are synergistic and ideally patients receive both,” said Peter McCullough, MD, a specialist in treating cardiorenal syndrome and other cardiovascular and renal disorders at Baylor, Scott, and White Heart and Vascular Hospital in Dallas. The SGLT2 inhibitors are among the drugs best suited to both treating and preventing cardiorenal syndrome by targeting both ends of the disorder, said Dr. McCullough, who chaired an American Heart Association panel that last year issued a scientific statement on cardiorenal syndrome (Circulation. 2019 Apr 16;139[16]:e840-78).
Although data on the SGLT2 inhibitors “are evolving,” the drug class is “going in the direction” of being “reasonably compared” with the ACE inhibitors and ARBs, said Javed Butler, MD, professor and chair of medicine at the University of Mississippi Medical Center, Jackson. “There are certainly complementary benefits that we see for both cardiovascular and renal outcomes.”
“We’ll think more and more about the SGLT2 inhibitors like renin-angiotensin system [RAS] inhibitors,” said David Z. Cherney, MD, referring to the drug class that includes ACE inhibitors and ARBs. “We should start to approach SGLT2 inhibitors like RAS inhibitors, with pleiotropic effects that go beyond glucose,” said Dr. Cherney, a nephrologist and professor of medicine at the University of Toronto, during the virtual annual scientific sessions of the American Diabetes Association in June 2020.
Working together in the nephron
One of the clearest complementary interactions between the SGLT2 inhibitors and the RAS inhibitors is their ability to reduce intraglomerular pressure, a key mechanism that slows nephron loss and progression of chronic kidney disease. SGLT2 inhibitors reduce sodium absorption in the proximal tubule that causes, through tubuloglomerular feedback, afferent arteriole constriction that lowers intraglomerular pressure, while the RAS inhibitors inhibit efferent arteriole constriction mediated by angiotensin II, also cutting intraglomerular pressure. Together, “they almost work in tandem,” explained Janani Rangaswami, MD, a nephrologist at Einstein Medical Center in Philadelphia, vice chair of the Kidney Council of the AHA, and first author of the 2019 cardiorenal syndrome AHA statement.
“Many had worried that if we target both the afferent and efferent arterioles simultaneously, it might increase the risk for acute kidney injury. What has been reassuring in both the recent data from the DAPA-HF trial and in recent meta-analysis was no evidence of increased risk for acute kidney injury with use of the SGLT2 inhibitor,” Dr. Rangaswami said in an interview. For example, a recent report on more than 39,000 Canadian patients with T2D who were at least 66 years old and newly begun on either an SGLT2 inhibitor or a different oral diabetes drug (a dipeptidyl peptidase–4 inhibitor), found a statistically significant 21% lower rate of acute kidney injury during the first 90 days on treatment with an SGLT2 inhibitor in a propensity score–matched analysis (CMAJ. 2020 Apr 6;192: e351-60).
Much of the concern about possible acute kidney injury stemmed from a property that the SGLT2 inhibitors share with RAS inhibitors: They cause an initial, reversible decline in glomerular filtration rate (GFR), followed by longer-term nephron preservation, a pattern attributable to reduced intraglomerular pressure. The question early on was: “ ‘Does this harm the kidney?’ But what we’ve seen is that patients do better over time, even with this initial hit. Whenever you offload the glomerulus you cut barotrauma and protect renal function,” explained Silvio E. Inzucchi, MD, professor of medicine at Yale University, New Haven, Conn., and director of the Yale Medicine Diabetes Center.
Dr. Inzucchi cautioned, however, that a small number of patients starting treatment with an SGLT2 inhibitor may have their GFR drop too sharply, especially if their GFR was low to start with. “You need to be careful, especially at the lower end of the GFR range. I recheck renal function after 1 month” after a patient starts an SGLT2. Patients whose level falls too low may need to discontinue. He added that it’s hard to set a uniform threshold for alarm, and instead assess patients on a case-by-case basis, but “you need some threshold in mind, where you will stop” treatment.
A smarter diuretic
One of the most intriguing renal effects of SGLT2 inhibitors is their diuretic action. During a talk at the virtual ADA scientific sessions, cardiologist Jeffrey Testani, MD, called them “smart” diuretics, because their effect on diuresis is relatively modest but comes without the neurohormonal price paid when patients take conventional loop diuretics.
”Loop diuretics are particularly bad,” causing neurohormonal activation that includes norepinephrine, renin, and vasopressin, said Dr. Testani, director of heart failure research at Yale. They also fail to produce a meaningful drop in blood volume despite causing substantial natriuresis.
In contrast, SGLT2 inhibitors cause “moderate” natriuresis while producing a significant cut in blood volume. “The body seems content with this lower plasma volume without activating catecholamines or renin, and that’s how the SGLT2 inhibitors differ from other diuretics,” said Dr. Inzucchi.
The class also maintains serum levels of potassium and magnesium, produces significant improvements in serum uric acid levels, and avoids the electrolyte abnormalities, volume depletion, and acute kidney injury that can occur with conventional distal diuretics, Dr. Testani said.
In short, the SGLT2 inhibitors “are safe and easy-to-use diuretics,” which allows them to fill a “huge unmet need for patients with heart failure.” As evidence accumulates for the benefits of the drug class in patients with heart failure and renal disease, “uptake will be extensive,” Dr. Testani predicted, driven in part by how easy it is to add the class to existing cardiorenal drug regimens.
Other standard therapies for patients with heart failure with reduced ejection fraction (HFrEF) risk electrolyte abnormalities, renal dysfunction, significantly lower blood pressure, often make patients feel worse, and involve a slow and laborious titration process, Dr. Testani noted. The SGLT2 inhibitor agents avoid these issues, a property that has played out in quality of life assessments of patients with HFrEF who received a drug from this class.
Outcomes met in trial after trial
In the DAPA-HF trial, with 4,443 patients with HFrEF and divided roughly equally between those with or without T2D, treatment with dapagliflozin (Farxiga) linked with significant improvements in health status and quality of life measured by the Kansas City Cardiomyopathy Questionnaire (Circulation. 2020 Jan 14;141[2]:90-9). “Not all treatments for HFrEF improve symptoms,” but in this study the SGTL2 inhibitor dapagliflozin did, boosting the Kansas City Cardiomyopathy Questionnaire score by about the same magnitude as treatment with a cardiac resynchronization device in patients with HFrEF, said Mikhail N. Kosiborod, MD, director of Cardiometabolic Research at Saint Luke’s Mid America Heart Institute in Kansas City, Mo., speaking at the virtual ADA scientific sessions.
Two more recent renal observations have further solidified the growing role of these drugs for kidney protection. Results from the CREDENCE trial that looked at canagliflozin (Invokana) treatment in 4,401 patients with T2D and albuminuria and chronic kidney disease showed canagliflozin treatment cut the primary, composite renal endpoint by a statistically significant 30%, compared with placebo (N Engl J Med. 2019 Jun 13;380[24]:2295-306). The study stopped earlier than planned because of how effective canagliflozin appeared.
“Never before has a renal protection clinical trial stopped for overwhelming efficacy,” noted nephrologist Katherine R. Tuttle, MD, executive director for research at Providence Health Care in Spokane, Wash. “It’s very exciting to have a treatment that works on both the heart and kidney, given their interrelationship,” she said during the ADA sessions. Dr. Tuttle called the cardiorenal effects from the SGLT2 inhibitors “amazing.”
Just as the DAPA-HF trial’s primary outcome showed the ability of at least one drug from the class, dapagliflozin, to improve outcomes in HFrEF patients without T2D, topline results recently reported from the DAPA-CDK trial showed for the first time renal protection by an SGLT2 inhibitor in patients with chronic kidney disease but no T2D, in a study with about 4,300 patients.
Although detailed results from DAPA-CKD are not yet available, so far the outcomes seem consistent with the CREDENCE findings, and the cumulative renal findings for the class show the SGLT2 inhibitors have “potential for a profound impact on the patients we see in every nephrology clinic, and with dual cardiorenal disease,” said Dr. Rangaswami. The class is now established as “standard of care for patients with chronic kidney disease. The CREDENCE results made that clear.”
The DAPA-CKD findings in patients with chronic kidney disease regardless of their diabetes status “are very important. We really have not had any advances in this space for some time, and chronic kidney disease patients have very poor outcomes, both cardiovascular and renal,” commented Dr. Butler. The advantage from using this drug class in these patients “is huge.”
The DAPA-CKD findings are a “major advance,” agreed Dr. McCullough.
SGLT2 inhibitor use needs to grow
Experts lament that although the evidence favoring the class has been very bullish, prescribing uptake has been slow, perhaps partly explained by the retail U.S. cost for most of these agents, generally about $17/day.
Cost is, unfortunately, an issue right now for these drugs, said Dr. Butler. Generic formulations are imminent, “but we cannot accept waiting. Providing this therapy when insurance coverage is available,” is essential.
The FDA has already granted tentative approval to some generic formulations, although resolution of patent issues can delay generics actually reaching the market. “Generic dapagliflozin will have a major impact; the marketplace for these drugs will shift very quickly,” predicted Dr. McCullough.
But price may not be the sole barrier, cautioned Dr. Rangaswami. “I don’t think it’s just a cost issue. Several factors explain the slow uptake,” of the SGLT2 inhibitors. “The biggest barrier is that this is a new drug class, and understanding how to use the class is not yet where it needs to be in the physician community.” One of the biggest problems is that the SGLT2 inhibitors are still primarily regarded as drugs to treat hyperglycemia.
Physicians who treat patients with heart or renal disease “need to wrap their head around the idea that a drug with antihyperglycemic effects is now in their practice territory, and something they need to prescribe,” she noted. Currently “there is a reluctance to prescribe these drugs given the perception that they are antihyperglycemic agents, and usually get deferred to primary care physicians or endocrinologists. This results in huge missed opportunities by cardiologists and nephrologists in initiating these agents that have major cardiorenal risk reduction effects.”
The key role that cardiologists need to play in prescribing the SGLT2 inhibitors was brought home in a recent study of two representative U.S. health systems that showed patients with T2D were far more likely to see a cardiologist than an endocrinologist (Cardiovasc Endocrinol Metab. 2020 Jun;9[2]:56-9).
“The SGLT2 inhibitors are definitely a game-changing drug class,” summed up Dr. Rangaswami. “We’re going to see a lot of use in patients with heart and kidney disease.”
Dr. Cherney has been a consultant to or has received honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Mitsubishi Tanabe Pharma, and Sanofi. Dr. Butler has had financial relationships with numerous pharmaceutical companies. Dr. McCullough and Dr. Rangaswami had no disclosures. Dr. Inzucchi has been a consultant to or helped run trials for Abbott, AstraZeneca, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics. Dr. Testani has been a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, cardionomic, FIRE1 Magenta Med, Novartis, Reprieve, Sanofi, and W.L. Gore. Dr. Kosiborod has been a consultant to or led trials for Amarin, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Glytec, Janssen, Eli Lilly, Merck, Novartis, Novo Nordisk, Sanofi, and Vifor. Dr. Tuttle has been a consultant to AstraZeneca, Boehringer Ingelheim, Gilead, Goldfinch Bio, Eli Lilly, and Novo Nordisk.
Unexpected rosuvastatin-canagliflozin adverse effect reported
A 76-year-old woman presented recently to a Toronto-area hospital with acute onset muscle pain, limb weakness, difficulty walking, and rhabdomyolysis associated with a sharp spike in her plasma level of rosuvastatin – a drug she had been on uneventfully for more than 5 years, within days of starting for the first time treatment with the SGLT2 inhibitor canagliflozin (Invokana).
The patient’s Canadian clinicians stopped her treatment with both rosuvastatin and canagliflozin, administered intravenous crystalloid fluids, and within days her pain subsided and her limb weakness gradually improved, allowing her discharge 10 days later while she was ambulating with a walker.
“To our knowledge this is the first published report of a drug interaction between rosuvastatin and canagliflozin,” wrote the authors of the case report (Ann Intern Med. 2020 Aug 3. doi: 10.7326/L20-0549). They cited the importance of the observation given the widespread use today of rosuvastatin for lowering low density lipoprotein cholesterol and exerting pleiotropic effects; and canagliflozin for its modest effects for reducing hyperglycemia, as well as its important role in reducing adverse cardiovascular outcomes, slowing progression of chronic kidney disease, and having a mild but important diuretic effect. “We encourage clinicians to remain vigilant for features of myotoxicity when canagliflozin and rosuvastatin are coprescribed,” they wrote, avoiding discussion of whether this may represent class or drug-specific effects.
“It’s reasonable to be mindful of this risk, but this is not a reason to not use rosuvastatin and canagliflozin in a patient,” nor for the time being to avoid any other combination of a statin and SGLT2 (sodium-glucose cotransporter 2) inhibitor, said David Juurlink, MD, head of the division of clinical pharmacology and toxicology at Sunnybrook Health Sciences Centre in Toronto and lead author of the report. “Few drug interactions have absolute contraindications. The admonition is just to be careful. It’s premature to say they shouldn’t be used together,” he said in an interview.
“We don’t know how much of an outlier this patient is. But it would be important to tell patients” on this or a similar combination to alert their clinicians if they start to have muscle aches, which should be a “red flag” to stop the statin, the SGLT2 inhibitor, or both until the situation can be fully assessed, Dr. Juurlink advised.
Sky high rosuvastatin levels
The linchpin of the observed adverse effects appeared to be a startlingly high elevation of the patient’s plasma rosuvastatin level when she was hospitalized 15 days after starting canagliflozin and 12 days after the onset of her thigh pain and weakness. Testing showed a plasma rosuvastatin concentration of 176 ng/mL, “more than 15-fold higher than the mean value expected” in patients taking 40 mg rosuvastatin daily, the maximum labeled dosage for the drug and what the affected patient had been taking without prior incident for more than 5 years. The patient’s canagliflozin dosage was 100 mg/day, the standard starting dosage according to the drug’s label.
The report’s authors noted that genetic assessment of the patient, a woman originally from the Philippines who was “high functioning,” and diagnosed with type 2 diabetes, showed she was heterozygous for a polymorphism, c.421C>A, which is linked with increased rosuvastatin plasma levels in the plasma. They also cited a report that canagliflozin can interact with proteins involved in hepatic drug uptake.
“We speculate that, in our patient, the addition of canagliflozin enhanced intestinal rosuvastatin absorption, inhibited its hepatocellular uptake, and impaired its excretion into bile canaliculi and the proximal tubule, resulting in rosuvastatin accumulation and leading to hepatotoxicity and myotoxicity,” the clinicians wrote in their report.
“There is little doubt this was a drug interaction, but it does not apply uniformly to everyone.” The severity of the interaction would depend on the dosages, the comorbidities a patient has, and their genetic profile, Dr. Juurlink said.
Concern and skepticism
Other clinicians who regularly prescribe these drugs expressed concern about the observation as well as skepticism about the prevalence of patients who could potentially experience similar effects.
“We don’t know how common are these genetic abnormalities. If this is extremely rare, then it doesn’t have many clinical implications, but if a large portion of the population has this [genetic] abnormality, it’s something we’d need to pay attention to,” Steven E. Nissen, MD, chair of cardiovascular medicine at the Cleveland Clinic Foundation, said in an interview. “It will be important to know the prevalence” of the genetic polymorphism carried by the reported patient, said Dr. Nissen, who has done research on lipid-lowering medications and drug safety.
“This could be important, or a very rare one-off. I can’t say which,” based on what’s currently known, he said. “There are many unanswered questions that make it hard to know how important this will be. It requires further investigation. There is a lot of uncertainty.”
Dr. Nissen particularly endorsed studies that approach this issue by looking at the prevalence rates of the implicated genetic polymorphism rather than pharmacovigilance studies that make epidemiologic assessments of adverse-effect prevalence. Studies that look for adverse-effect associations in large patient populations are “sloppy, and unless the interaction is incredibly intense they are not very sensitive,” he said.
But Dr. Juurlink, a pharmacoepidemiologist whose specialty includes studies of this sort, said that they could be useful if carefully designed. He suggested, for example, comparing in large patient databases the observed incidence of rhabdomyolysis among patients on an SGLT2 inhibitor and also on rosuvastatin with those on pravastatin, a statin with a different metabolic profile. Another approach to further examining the observation would be dosage studies with rosuvastatin and canagliflozin in healthy volunteers, he said.
Dr. Nissen noted that rosuvastatin is a key agent from the statin class because it’s the “most effective” for lowering low density lipoprotein cholesterol. “Rosuvastatin is a go-to drug,” he declared. On the other hand, canagliflozin is “a little less used” than other drugs in the SGLT2 inhibitor class, specifically dapagliflozin (Farxiga) and empagliflozin (Jardiance), he said.
One in a million?
“This was a freak accident. I don’t find it at all concerning. It was definitely one in a million,” Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of The Metabolic Institute of America in Tarzana, Calif., said in an interview. “None of us have seen it” in either the several cardiovascular outcome trials now run on multiple drugs in the SGLT2 inhibitor class that included many patients also taking a statin, or in routine practice, he said. Dr. Handelsman noted that in his practice he had never seen a similar case despite treating “hundreds if not thousands of patients” with type 2 diabetes, virtually all of whom were on a statin and were also treated with an SGLT2 inhibitor, including many with canagliflozin.
Dr. Handelsman cited the notably low estimated glomerular filtration rate in the reported patient, who was described as having a serum creatinine level of 150 mcmol/L (1.7 mg/dL) prior to canagliflozin treatment that then rose to 194 mcmol/L (2.19 mg/dL) at the time of hospitalization, which corresponds to estimated glomerular filtration rates of 29-31 and 21-23 mL/min per 1.73 m2, respectively, depending on the calculator used, rates that were possibly below the labeled minimum rate of 30 mL/min per 1.73 m2 for patients starting canagliflozin treatment. The case report cited the patient as having stage 3B chronic kidney disease, which corresponds to a eGFR of 30-44* mL/min per 1.73 m2.
“I think the patient had acute kidney injury” on starting canagliflozin “that may have affected the [rosuvastatin] metabolism,” Dr. Handelsman suggested. “She had severe kidney dysfunction to start with that fell further with SGLT2 inhibitor treatment,” a well described and usually transient effect of starting drugs in this class because of changes the SGLT2 inhibitors cause in renal blood flow. He noted that the patient had not been receiving an angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker, which may have contributed to her acute problems with fluid balance. Most similar patients with type 2 diabetes, cardiovascular disease risk, and chronic kidney disease would be on stable treatment with a drug that inhibits the renin-angiotensin system before starting an SGLT2 inhibitor, and not already having a RAS inhibitor on board before starting canagliflozin may have somehow contributed to the observed adverse effects, Dr. Handelsman said.
Dr. Juurlink was skeptical that the kidneys played a major role. “An abrupt change in renal function can influence statin clearance, but this was a 15-fold increase. You can’t explain such a dramatic increase by a transient reduction in renal function,” he said.
Dr. Juurlink and coauthors had no disclosures. Dr. Nissen had no relevant disclosures. Dr. Handelsman has been a consultant to companies that market drugs in the SGLT2 inhibitor class.
SOURCE: Brailovski E et al. Ann Intern Med. 2020 Aug 3. doi: 10.7326/L20-0549.
*Correction: This value was missing from the original article.
A 76-year-old woman presented recently to a Toronto-area hospital with acute onset muscle pain, limb weakness, difficulty walking, and rhabdomyolysis associated with a sharp spike in her plasma level of rosuvastatin – a drug she had been on uneventfully for more than 5 years, within days of starting for the first time treatment with the SGLT2 inhibitor canagliflozin (Invokana).
The patient’s Canadian clinicians stopped her treatment with both rosuvastatin and canagliflozin, administered intravenous crystalloid fluids, and within days her pain subsided and her limb weakness gradually improved, allowing her discharge 10 days later while she was ambulating with a walker.
“To our knowledge this is the first published report of a drug interaction between rosuvastatin and canagliflozin,” wrote the authors of the case report (Ann Intern Med. 2020 Aug 3. doi: 10.7326/L20-0549). They cited the importance of the observation given the widespread use today of rosuvastatin for lowering low density lipoprotein cholesterol and exerting pleiotropic effects; and canagliflozin for its modest effects for reducing hyperglycemia, as well as its important role in reducing adverse cardiovascular outcomes, slowing progression of chronic kidney disease, and having a mild but important diuretic effect. “We encourage clinicians to remain vigilant for features of myotoxicity when canagliflozin and rosuvastatin are coprescribed,” they wrote, avoiding discussion of whether this may represent class or drug-specific effects.
“It’s reasonable to be mindful of this risk, but this is not a reason to not use rosuvastatin and canagliflozin in a patient,” nor for the time being to avoid any other combination of a statin and SGLT2 (sodium-glucose cotransporter 2) inhibitor, said David Juurlink, MD, head of the division of clinical pharmacology and toxicology at Sunnybrook Health Sciences Centre in Toronto and lead author of the report. “Few drug interactions have absolute contraindications. The admonition is just to be careful. It’s premature to say they shouldn’t be used together,” he said in an interview.
“We don’t know how much of an outlier this patient is. But it would be important to tell patients” on this or a similar combination to alert their clinicians if they start to have muscle aches, which should be a “red flag” to stop the statin, the SGLT2 inhibitor, or both until the situation can be fully assessed, Dr. Juurlink advised.
Sky high rosuvastatin levels
The linchpin of the observed adverse effects appeared to be a startlingly high elevation of the patient’s plasma rosuvastatin level when she was hospitalized 15 days after starting canagliflozin and 12 days after the onset of her thigh pain and weakness. Testing showed a plasma rosuvastatin concentration of 176 ng/mL, “more than 15-fold higher than the mean value expected” in patients taking 40 mg rosuvastatin daily, the maximum labeled dosage for the drug and what the affected patient had been taking without prior incident for more than 5 years. The patient’s canagliflozin dosage was 100 mg/day, the standard starting dosage according to the drug’s label.
The report’s authors noted that genetic assessment of the patient, a woman originally from the Philippines who was “high functioning,” and diagnosed with type 2 diabetes, showed she was heterozygous for a polymorphism, c.421C>A, which is linked with increased rosuvastatin plasma levels in the plasma. They also cited a report that canagliflozin can interact with proteins involved in hepatic drug uptake.
“We speculate that, in our patient, the addition of canagliflozin enhanced intestinal rosuvastatin absorption, inhibited its hepatocellular uptake, and impaired its excretion into bile canaliculi and the proximal tubule, resulting in rosuvastatin accumulation and leading to hepatotoxicity and myotoxicity,” the clinicians wrote in their report.
“There is little doubt this was a drug interaction, but it does not apply uniformly to everyone.” The severity of the interaction would depend on the dosages, the comorbidities a patient has, and their genetic profile, Dr. Juurlink said.
Concern and skepticism
Other clinicians who regularly prescribe these drugs expressed concern about the observation as well as skepticism about the prevalence of patients who could potentially experience similar effects.
“We don’t know how common are these genetic abnormalities. If this is extremely rare, then it doesn’t have many clinical implications, but if a large portion of the population has this [genetic] abnormality, it’s something we’d need to pay attention to,” Steven E. Nissen, MD, chair of cardiovascular medicine at the Cleveland Clinic Foundation, said in an interview. “It will be important to know the prevalence” of the genetic polymorphism carried by the reported patient, said Dr. Nissen, who has done research on lipid-lowering medications and drug safety.
“This could be important, or a very rare one-off. I can’t say which,” based on what’s currently known, he said. “There are many unanswered questions that make it hard to know how important this will be. It requires further investigation. There is a lot of uncertainty.”
Dr. Nissen particularly endorsed studies that approach this issue by looking at the prevalence rates of the implicated genetic polymorphism rather than pharmacovigilance studies that make epidemiologic assessments of adverse-effect prevalence. Studies that look for adverse-effect associations in large patient populations are “sloppy, and unless the interaction is incredibly intense they are not very sensitive,” he said.
But Dr. Juurlink, a pharmacoepidemiologist whose specialty includes studies of this sort, said that they could be useful if carefully designed. He suggested, for example, comparing in large patient databases the observed incidence of rhabdomyolysis among patients on an SGLT2 inhibitor and also on rosuvastatin with those on pravastatin, a statin with a different metabolic profile. Another approach to further examining the observation would be dosage studies with rosuvastatin and canagliflozin in healthy volunteers, he said.
Dr. Nissen noted that rosuvastatin is a key agent from the statin class because it’s the “most effective” for lowering low density lipoprotein cholesterol. “Rosuvastatin is a go-to drug,” he declared. On the other hand, canagliflozin is “a little less used” than other drugs in the SGLT2 inhibitor class, specifically dapagliflozin (Farxiga) and empagliflozin (Jardiance), he said.
One in a million?
“This was a freak accident. I don’t find it at all concerning. It was definitely one in a million,” Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of The Metabolic Institute of America in Tarzana, Calif., said in an interview. “None of us have seen it” in either the several cardiovascular outcome trials now run on multiple drugs in the SGLT2 inhibitor class that included many patients also taking a statin, or in routine practice, he said. Dr. Handelsman noted that in his practice he had never seen a similar case despite treating “hundreds if not thousands of patients” with type 2 diabetes, virtually all of whom were on a statin and were also treated with an SGLT2 inhibitor, including many with canagliflozin.
Dr. Handelsman cited the notably low estimated glomerular filtration rate in the reported patient, who was described as having a serum creatinine level of 150 mcmol/L (1.7 mg/dL) prior to canagliflozin treatment that then rose to 194 mcmol/L (2.19 mg/dL) at the time of hospitalization, which corresponds to estimated glomerular filtration rates of 29-31 and 21-23 mL/min per 1.73 m2, respectively, depending on the calculator used, rates that were possibly below the labeled minimum rate of 30 mL/min per 1.73 m2 for patients starting canagliflozin treatment. The case report cited the patient as having stage 3B chronic kidney disease, which corresponds to a eGFR of 30-44* mL/min per 1.73 m2.
“I think the patient had acute kidney injury” on starting canagliflozin “that may have affected the [rosuvastatin] metabolism,” Dr. Handelsman suggested. “She had severe kidney dysfunction to start with that fell further with SGLT2 inhibitor treatment,” a well described and usually transient effect of starting drugs in this class because of changes the SGLT2 inhibitors cause in renal blood flow. He noted that the patient had not been receiving an angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker, which may have contributed to her acute problems with fluid balance. Most similar patients with type 2 diabetes, cardiovascular disease risk, and chronic kidney disease would be on stable treatment with a drug that inhibits the renin-angiotensin system before starting an SGLT2 inhibitor, and not already having a RAS inhibitor on board before starting canagliflozin may have somehow contributed to the observed adverse effects, Dr. Handelsman said.
Dr. Juurlink was skeptical that the kidneys played a major role. “An abrupt change in renal function can influence statin clearance, but this was a 15-fold increase. You can’t explain such a dramatic increase by a transient reduction in renal function,” he said.
Dr. Juurlink and coauthors had no disclosures. Dr. Nissen had no relevant disclosures. Dr. Handelsman has been a consultant to companies that market drugs in the SGLT2 inhibitor class.
SOURCE: Brailovski E et al. Ann Intern Med. 2020 Aug 3. doi: 10.7326/L20-0549.
*Correction: This value was missing from the original article.
A 76-year-old woman presented recently to a Toronto-area hospital with acute onset muscle pain, limb weakness, difficulty walking, and rhabdomyolysis associated with a sharp spike in her plasma level of rosuvastatin – a drug she had been on uneventfully for more than 5 years, within days of starting for the first time treatment with the SGLT2 inhibitor canagliflozin (Invokana).
The patient’s Canadian clinicians stopped her treatment with both rosuvastatin and canagliflozin, administered intravenous crystalloid fluids, and within days her pain subsided and her limb weakness gradually improved, allowing her discharge 10 days later while she was ambulating with a walker.
“To our knowledge this is the first published report of a drug interaction between rosuvastatin and canagliflozin,” wrote the authors of the case report (Ann Intern Med. 2020 Aug 3. doi: 10.7326/L20-0549). They cited the importance of the observation given the widespread use today of rosuvastatin for lowering low density lipoprotein cholesterol and exerting pleiotropic effects; and canagliflozin for its modest effects for reducing hyperglycemia, as well as its important role in reducing adverse cardiovascular outcomes, slowing progression of chronic kidney disease, and having a mild but important diuretic effect. “We encourage clinicians to remain vigilant for features of myotoxicity when canagliflozin and rosuvastatin are coprescribed,” they wrote, avoiding discussion of whether this may represent class or drug-specific effects.
“It’s reasonable to be mindful of this risk, but this is not a reason to not use rosuvastatin and canagliflozin in a patient,” nor for the time being to avoid any other combination of a statin and SGLT2 (sodium-glucose cotransporter 2) inhibitor, said David Juurlink, MD, head of the division of clinical pharmacology and toxicology at Sunnybrook Health Sciences Centre in Toronto and lead author of the report. “Few drug interactions have absolute contraindications. The admonition is just to be careful. It’s premature to say they shouldn’t be used together,” he said in an interview.
“We don’t know how much of an outlier this patient is. But it would be important to tell patients” on this or a similar combination to alert their clinicians if they start to have muscle aches, which should be a “red flag” to stop the statin, the SGLT2 inhibitor, or both until the situation can be fully assessed, Dr. Juurlink advised.
Sky high rosuvastatin levels
The linchpin of the observed adverse effects appeared to be a startlingly high elevation of the patient’s plasma rosuvastatin level when she was hospitalized 15 days after starting canagliflozin and 12 days after the onset of her thigh pain and weakness. Testing showed a plasma rosuvastatin concentration of 176 ng/mL, “more than 15-fold higher than the mean value expected” in patients taking 40 mg rosuvastatin daily, the maximum labeled dosage for the drug and what the affected patient had been taking without prior incident for more than 5 years. The patient’s canagliflozin dosage was 100 mg/day, the standard starting dosage according to the drug’s label.
The report’s authors noted that genetic assessment of the patient, a woman originally from the Philippines who was “high functioning,” and diagnosed with type 2 diabetes, showed she was heterozygous for a polymorphism, c.421C>A, which is linked with increased rosuvastatin plasma levels in the plasma. They also cited a report that canagliflozin can interact with proteins involved in hepatic drug uptake.
“We speculate that, in our patient, the addition of canagliflozin enhanced intestinal rosuvastatin absorption, inhibited its hepatocellular uptake, and impaired its excretion into bile canaliculi and the proximal tubule, resulting in rosuvastatin accumulation and leading to hepatotoxicity and myotoxicity,” the clinicians wrote in their report.
“There is little doubt this was a drug interaction, but it does not apply uniformly to everyone.” The severity of the interaction would depend on the dosages, the comorbidities a patient has, and their genetic profile, Dr. Juurlink said.
Concern and skepticism
Other clinicians who regularly prescribe these drugs expressed concern about the observation as well as skepticism about the prevalence of patients who could potentially experience similar effects.
“We don’t know how common are these genetic abnormalities. If this is extremely rare, then it doesn’t have many clinical implications, but if a large portion of the population has this [genetic] abnormality, it’s something we’d need to pay attention to,” Steven E. Nissen, MD, chair of cardiovascular medicine at the Cleveland Clinic Foundation, said in an interview. “It will be important to know the prevalence” of the genetic polymorphism carried by the reported patient, said Dr. Nissen, who has done research on lipid-lowering medications and drug safety.
“This could be important, or a very rare one-off. I can’t say which,” based on what’s currently known, he said. “There are many unanswered questions that make it hard to know how important this will be. It requires further investigation. There is a lot of uncertainty.”
Dr. Nissen particularly endorsed studies that approach this issue by looking at the prevalence rates of the implicated genetic polymorphism rather than pharmacovigilance studies that make epidemiologic assessments of adverse-effect prevalence. Studies that look for adverse-effect associations in large patient populations are “sloppy, and unless the interaction is incredibly intense they are not very sensitive,” he said.
But Dr. Juurlink, a pharmacoepidemiologist whose specialty includes studies of this sort, said that they could be useful if carefully designed. He suggested, for example, comparing in large patient databases the observed incidence of rhabdomyolysis among patients on an SGLT2 inhibitor and also on rosuvastatin with those on pravastatin, a statin with a different metabolic profile. Another approach to further examining the observation would be dosage studies with rosuvastatin and canagliflozin in healthy volunteers, he said.
Dr. Nissen noted that rosuvastatin is a key agent from the statin class because it’s the “most effective” for lowering low density lipoprotein cholesterol. “Rosuvastatin is a go-to drug,” he declared. On the other hand, canagliflozin is “a little less used” than other drugs in the SGLT2 inhibitor class, specifically dapagliflozin (Farxiga) and empagliflozin (Jardiance), he said.
One in a million?
“This was a freak accident. I don’t find it at all concerning. It was definitely one in a million,” Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of The Metabolic Institute of America in Tarzana, Calif., said in an interview. “None of us have seen it” in either the several cardiovascular outcome trials now run on multiple drugs in the SGLT2 inhibitor class that included many patients also taking a statin, or in routine practice, he said. Dr. Handelsman noted that in his practice he had never seen a similar case despite treating “hundreds if not thousands of patients” with type 2 diabetes, virtually all of whom were on a statin and were also treated with an SGLT2 inhibitor, including many with canagliflozin.
Dr. Handelsman cited the notably low estimated glomerular filtration rate in the reported patient, who was described as having a serum creatinine level of 150 mcmol/L (1.7 mg/dL) prior to canagliflozin treatment that then rose to 194 mcmol/L (2.19 mg/dL) at the time of hospitalization, which corresponds to estimated glomerular filtration rates of 29-31 and 21-23 mL/min per 1.73 m2, respectively, depending on the calculator used, rates that were possibly below the labeled minimum rate of 30 mL/min per 1.73 m2 for patients starting canagliflozin treatment. The case report cited the patient as having stage 3B chronic kidney disease, which corresponds to a eGFR of 30-44* mL/min per 1.73 m2.
“I think the patient had acute kidney injury” on starting canagliflozin “that may have affected the [rosuvastatin] metabolism,” Dr. Handelsman suggested. “She had severe kidney dysfunction to start with that fell further with SGLT2 inhibitor treatment,” a well described and usually transient effect of starting drugs in this class because of changes the SGLT2 inhibitors cause in renal blood flow. He noted that the patient had not been receiving an angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker, which may have contributed to her acute problems with fluid balance. Most similar patients with type 2 diabetes, cardiovascular disease risk, and chronic kidney disease would be on stable treatment with a drug that inhibits the renin-angiotensin system before starting an SGLT2 inhibitor, and not already having a RAS inhibitor on board before starting canagliflozin may have somehow contributed to the observed adverse effects, Dr. Handelsman said.
Dr. Juurlink was skeptical that the kidneys played a major role. “An abrupt change in renal function can influence statin clearance, but this was a 15-fold increase. You can’t explain such a dramatic increase by a transient reduction in renal function,” he said.
Dr. Juurlink and coauthors had no disclosures. Dr. Nissen had no relevant disclosures. Dr. Handelsman has been a consultant to companies that market drugs in the SGLT2 inhibitor class.
SOURCE: Brailovski E et al. Ann Intern Med. 2020 Aug 3. doi: 10.7326/L20-0549.
*Correction: This value was missing from the original article.
FROM ANNALS OF INTERNAL MEDICINE
COVID-19 taking financial toll on people in U.S. with diabetes
The COVID-19 pandemic is taking a particularly severe financial toll on people with diabetes, new research from the United States suggests.
Results from a national online survey of 5,000 people with diabetes conducted between June 26 and July 1, 2020, were posted July 29 on the American Diabetes Association website.
The survey, conducted by the diabetes research company dQ&A in association with the ADA, revealed that Americans with diabetes are experiencing extreme financial pressures, leading to medication and supply rationing.
A high proportion of respondents had either lost income or are working in jobs that place them at risk for catching the novel coronavirus.
“These new numbers show the urgency needed to adopt measures to protect and assist the millions of people with diabetes who are suffering through this pandemic,” Tracey D. Brown, CEO of the ADA, said in a statement.
She called for states to extend health care coverage to people who have lost their jobs, for the eradication of insulin copays during the pandemic, and for increased COVID-19 testing capacity in high-risk communities.
“If these actions aren’t taken immediately, we will continue to see devastating impacts and outcomes for millions of vulnerable Americans,” Ms. Brown stressed.
COVID-19 has worsened financial pressures for people with diabetes
In the survey, 24% of respondents reported having used savings, loans, or stimulus check money to pay for diabetes care in the past 3 months. Among those who have lost income, half are using savings or stimulus money.
A quarter of respondents said they have been self-rationing supplies to cut costs.
Extrapolating to the entire U.S. population with diabetes, dQ&A estimated that roughly 650,000 are skipping insulin doses or taking less than prescribed, and 3 million are skipping blood glucose tests.
In June, the unemployment rate for people with diabetes was 18%, higher than the national rate of 12%.
Also higher is the proportion of those working prior to the pandemic who have since lost income: 33%, compared with 29% for the general population.
Among those who are self-employed, 7 in 10 of those with diabetes have lost some or all of their income.
Many with diabetes who are employed are vulnerable to exposure
Of those who remain employed, half said they can’t work from home.
Of those, 60% work in essential industries, with 22% in health care. A large majority, 90%, reported lack of social distancing at work and nearly a third work in places that don’t require masks.
“People with diabetes are helping to provide the services we all depend on during this pandemic, even as it puts their own well-being at risk,” the report said.
It concluded that “these numbers represent a conservative estimate of the pandemic’s impact. They are generated from an ongoing online study of the diabetes population amongst people who have opted in to participate.”
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic is taking a particularly severe financial toll on people with diabetes, new research from the United States suggests.
Results from a national online survey of 5,000 people with diabetes conducted between June 26 and July 1, 2020, were posted July 29 on the American Diabetes Association website.
The survey, conducted by the diabetes research company dQ&A in association with the ADA, revealed that Americans with diabetes are experiencing extreme financial pressures, leading to medication and supply rationing.
A high proportion of respondents had either lost income or are working in jobs that place them at risk for catching the novel coronavirus.
“These new numbers show the urgency needed to adopt measures to protect and assist the millions of people with diabetes who are suffering through this pandemic,” Tracey D. Brown, CEO of the ADA, said in a statement.
She called for states to extend health care coverage to people who have lost their jobs, for the eradication of insulin copays during the pandemic, and for increased COVID-19 testing capacity in high-risk communities.
“If these actions aren’t taken immediately, we will continue to see devastating impacts and outcomes for millions of vulnerable Americans,” Ms. Brown stressed.
COVID-19 has worsened financial pressures for people with diabetes
In the survey, 24% of respondents reported having used savings, loans, or stimulus check money to pay for diabetes care in the past 3 months. Among those who have lost income, half are using savings or stimulus money.
A quarter of respondents said they have been self-rationing supplies to cut costs.
Extrapolating to the entire U.S. population with diabetes, dQ&A estimated that roughly 650,000 are skipping insulin doses or taking less than prescribed, and 3 million are skipping blood glucose tests.
In June, the unemployment rate for people with diabetes was 18%, higher than the national rate of 12%.
Also higher is the proportion of those working prior to the pandemic who have since lost income: 33%, compared with 29% for the general population.
Among those who are self-employed, 7 in 10 of those with diabetes have lost some or all of their income.
Many with diabetes who are employed are vulnerable to exposure
Of those who remain employed, half said they can’t work from home.
Of those, 60% work in essential industries, with 22% in health care. A large majority, 90%, reported lack of social distancing at work and nearly a third work in places that don’t require masks.
“People with diabetes are helping to provide the services we all depend on during this pandemic, even as it puts their own well-being at risk,” the report said.
It concluded that “these numbers represent a conservative estimate of the pandemic’s impact. They are generated from an ongoing online study of the diabetes population amongst people who have opted in to participate.”
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic is taking a particularly severe financial toll on people with diabetes, new research from the United States suggests.
Results from a national online survey of 5,000 people with diabetes conducted between June 26 and July 1, 2020, were posted July 29 on the American Diabetes Association website.
The survey, conducted by the diabetes research company dQ&A in association with the ADA, revealed that Americans with diabetes are experiencing extreme financial pressures, leading to medication and supply rationing.
A high proportion of respondents had either lost income or are working in jobs that place them at risk for catching the novel coronavirus.
“These new numbers show the urgency needed to adopt measures to protect and assist the millions of people with diabetes who are suffering through this pandemic,” Tracey D. Brown, CEO of the ADA, said in a statement.
She called for states to extend health care coverage to people who have lost their jobs, for the eradication of insulin copays during the pandemic, and for increased COVID-19 testing capacity in high-risk communities.
“If these actions aren’t taken immediately, we will continue to see devastating impacts and outcomes for millions of vulnerable Americans,” Ms. Brown stressed.
COVID-19 has worsened financial pressures for people with diabetes
In the survey, 24% of respondents reported having used savings, loans, or stimulus check money to pay for diabetes care in the past 3 months. Among those who have lost income, half are using savings or stimulus money.
A quarter of respondents said they have been self-rationing supplies to cut costs.
Extrapolating to the entire U.S. population with diabetes, dQ&A estimated that roughly 650,000 are skipping insulin doses or taking less than prescribed, and 3 million are skipping blood glucose tests.
In June, the unemployment rate for people with diabetes was 18%, higher than the national rate of 12%.
Also higher is the proportion of those working prior to the pandemic who have since lost income: 33%, compared with 29% for the general population.
Among those who are self-employed, 7 in 10 of those with diabetes have lost some or all of their income.
Many with diabetes who are employed are vulnerable to exposure
Of those who remain employed, half said they can’t work from home.
Of those, 60% work in essential industries, with 22% in health care. A large majority, 90%, reported lack of social distancing at work and nearly a third work in places that don’t require masks.
“People with diabetes are helping to provide the services we all depend on during this pandemic, even as it puts their own well-being at risk,” the report said.
It concluded that “these numbers represent a conservative estimate of the pandemic’s impact. They are generated from an ongoing online study of the diabetes population amongst people who have opted in to participate.”
A version of this article originally appeared on Medscape.com.
Real-world data show SGLT2 inhibitors for diabetes triple DKA risk
according to a new large database analysis.
The findings, which include data on the use of three different SGLT2 inhibitors in Canada and the United Kingdom and suggest a class effect, were published online July 27 in Annals of Internal Medicine by Antonios Douros, MD, PhD, of McGill University and the Centre for Clinical Epidemiology, Lady Davis Institute, Montreal, and colleagues.
“Our results provide robust evidence that SGLT2 inhibitors are associated with an increased risk for DKA. Of note, increased risks were observed in all molecule-specific analyses, with canagliflozin [Invokana, Janssen] showing the highest effect estimate,” they noted.
And because the beneficial effects of SGLT2 inhibitors in the prevention of cardiovascular and renal disease will probably increase their uptake in the coming years, “Physicians should be aware of DKA as a potential adverse effect,” Dr. Douros and colleagues wrote.
Analysis “generally confirms what has already been published”
Asked for comment, Simeon I. Taylor, MD, PhD, professor of medicine at the University of Maryland, Baltimore, said that the study “generally confirms what has already been published” on the topic. He noted that overall “the risk of SGLT2 inhibitor–induced ketoacidosis is quite low in type 2 diabetes, perhaps on the order of 1 episode per 1000 patient-years.”
However, Dr. Taylor cautioned: “Published evidence suggests that the risk of DKA is increased if patients are unable to eat,” such as when hospitalized patients are not permitted to eat.
“In that setting, it is probably prudent to discontinue an SGLT2 inhibitor. Also, it may be prudent not to prescribe SGLT2 inhibitors to patients with a history of DKA,” he added.
Dr. Taylor also advised: “Although not necessarily supported by this publication, I think that caution should be exercised in prescribing SGLT2 inhibitors to insulin-dependent type 2 diabetes patients. ... Some late-stage type 2 diabetes patients may have severe insulin deficiency, and their physiology may resemble that of a type 1 diabetes patient.”
Dr. Taylor has previously advised against using SGLT2 inhibitors altogether in patients with type 1 diabetes.
Increased DKA risk seen across all SGLT2 inhibitors
The study involved electronic health care databases from seven Canadian provinces and the United Kingdom, from which 208,757 new users of SGLT2 inhibitors were propensity-matched 1:1 to new dipeptidyl peptidase-4 (DPP-4) inhibitor users.
Of those taking an SGLT2 inhibitor, 42.3% took canagliflozin, 30.7% dapagliflozin (Farxiga/Forxiga, AstraZeneca), and 27.0% empagliflozin (Jardiance, Boehringer Ingelheim).
Over a mean 0.9-year follow-up, 521 patients were hospitalized with DKA, for an overall incidence rate of 1.41 per 1,000 person-years.
The rate with SGLT2 inhibitors, 2.03 per 1,000 person-years, was nearly three times that seen with DPP-4 inhibitors, at 0.75 per 1,000 person-years, a significant difference (hazard ratio, 2.85).
By individual SGLT2 inhibitor, the hazard ratios compared with DPP-4 inhibitors were 1.86 for dapagliflozin, 2.52 for empagliflozin, and 3.58 for canagliflozin, all statistically significant. Stratification by age, sex, and incident versus prevalent user did not change the association between SGLT2 inhibitors and DKA.
Asked about the higher rate for canagliflozin, Dr. Taylor commented: “It is hard to know whether there are real and reproducible differences in the risks of DKA among the various SGLT2 inhibitors. The differences are not huge and the populations are not well matched.”
But, he noted, “If canagliflozin triggers more glucosuria, it is not surprising that it would also induce more ketosis and possibly ketoacidosis.”
He also noted that the threefold relative increase in DKA with canagliflozin versus comparators is consistent with Janssen’s data, published in 2015.
“It is, of course, reassuring that both [randomized clinical trials] and epidemiology produce similar estimates of the risk of drug-induced adverse events. Interestingly, the incidence of DKA is approximately threefold higher in the Canadian [data] as compared to Janssen’s clinical trials.”
Dr. Taylor also pointed out that, in the Janssen studies, the risk of canagliflozin-induced DKA appeared to be higher among patients with anti-islet antibodies, which suggests that some may have actually had autoimmune (type 1) diabetes. “So the overall risk of SGLT2 inhibitor-induced DKA may depend at least in part on the mix of patients.”
In the current study, individuals who never used insulin had a greater relative increase in risk of DKA with SGLT2 inhibitors, compared with DPP-4 inhibitors, than did those who did use insulin (hazard ratios, 3.96 vs. 2.24, both compared with DPP-4 inhibitors). However, just among those taking SGLT2 inhibitors, the absolute risk for DKA was higher for those with prior insulin use (3.52 vs. 1.43 per 1,000 person-years).
The results of sensitivity analyses were consistent with those of the primary analysis.
The study was funded by the Canadian Institutes of Health Research and supported by ICES. Dr. Douros has reported receiving a salary support award from Fonds de recherche du Quebec – sante. Dr. Taylor was previously employed at Bristol-Myers Squibb. He is currently a consultant for Ionis Pharmaceuticals and has reported receiving research support provided to the University of Maryland School of Medicine by Regeneron.
A version of this article originally appeared on Medscape.com.
according to a new large database analysis.
The findings, which include data on the use of three different SGLT2 inhibitors in Canada and the United Kingdom and suggest a class effect, were published online July 27 in Annals of Internal Medicine by Antonios Douros, MD, PhD, of McGill University and the Centre for Clinical Epidemiology, Lady Davis Institute, Montreal, and colleagues.
“Our results provide robust evidence that SGLT2 inhibitors are associated with an increased risk for DKA. Of note, increased risks were observed in all molecule-specific analyses, with canagliflozin [Invokana, Janssen] showing the highest effect estimate,” they noted.
And because the beneficial effects of SGLT2 inhibitors in the prevention of cardiovascular and renal disease will probably increase their uptake in the coming years, “Physicians should be aware of DKA as a potential adverse effect,” Dr. Douros and colleagues wrote.
Analysis “generally confirms what has already been published”
Asked for comment, Simeon I. Taylor, MD, PhD, professor of medicine at the University of Maryland, Baltimore, said that the study “generally confirms what has already been published” on the topic. He noted that overall “the risk of SGLT2 inhibitor–induced ketoacidosis is quite low in type 2 diabetes, perhaps on the order of 1 episode per 1000 patient-years.”
However, Dr. Taylor cautioned: “Published evidence suggests that the risk of DKA is increased if patients are unable to eat,” such as when hospitalized patients are not permitted to eat.
“In that setting, it is probably prudent to discontinue an SGLT2 inhibitor. Also, it may be prudent not to prescribe SGLT2 inhibitors to patients with a history of DKA,” he added.
Dr. Taylor also advised: “Although not necessarily supported by this publication, I think that caution should be exercised in prescribing SGLT2 inhibitors to insulin-dependent type 2 diabetes patients. ... Some late-stage type 2 diabetes patients may have severe insulin deficiency, and their physiology may resemble that of a type 1 diabetes patient.”
Dr. Taylor has previously advised against using SGLT2 inhibitors altogether in patients with type 1 diabetes.
Increased DKA risk seen across all SGLT2 inhibitors
The study involved electronic health care databases from seven Canadian provinces and the United Kingdom, from which 208,757 new users of SGLT2 inhibitors were propensity-matched 1:1 to new dipeptidyl peptidase-4 (DPP-4) inhibitor users.
Of those taking an SGLT2 inhibitor, 42.3% took canagliflozin, 30.7% dapagliflozin (Farxiga/Forxiga, AstraZeneca), and 27.0% empagliflozin (Jardiance, Boehringer Ingelheim).
Over a mean 0.9-year follow-up, 521 patients were hospitalized with DKA, for an overall incidence rate of 1.41 per 1,000 person-years.
The rate with SGLT2 inhibitors, 2.03 per 1,000 person-years, was nearly three times that seen with DPP-4 inhibitors, at 0.75 per 1,000 person-years, a significant difference (hazard ratio, 2.85).
By individual SGLT2 inhibitor, the hazard ratios compared with DPP-4 inhibitors were 1.86 for dapagliflozin, 2.52 for empagliflozin, and 3.58 for canagliflozin, all statistically significant. Stratification by age, sex, and incident versus prevalent user did not change the association between SGLT2 inhibitors and DKA.
Asked about the higher rate for canagliflozin, Dr. Taylor commented: “It is hard to know whether there are real and reproducible differences in the risks of DKA among the various SGLT2 inhibitors. The differences are not huge and the populations are not well matched.”
But, he noted, “If canagliflozin triggers more glucosuria, it is not surprising that it would also induce more ketosis and possibly ketoacidosis.”
He also noted that the threefold relative increase in DKA with canagliflozin versus comparators is consistent with Janssen’s data, published in 2015.
“It is, of course, reassuring that both [randomized clinical trials] and epidemiology produce similar estimates of the risk of drug-induced adverse events. Interestingly, the incidence of DKA is approximately threefold higher in the Canadian [data] as compared to Janssen’s clinical trials.”
Dr. Taylor also pointed out that, in the Janssen studies, the risk of canagliflozin-induced DKA appeared to be higher among patients with anti-islet antibodies, which suggests that some may have actually had autoimmune (type 1) diabetes. “So the overall risk of SGLT2 inhibitor-induced DKA may depend at least in part on the mix of patients.”
In the current study, individuals who never used insulin had a greater relative increase in risk of DKA with SGLT2 inhibitors, compared with DPP-4 inhibitors, than did those who did use insulin (hazard ratios, 3.96 vs. 2.24, both compared with DPP-4 inhibitors). However, just among those taking SGLT2 inhibitors, the absolute risk for DKA was higher for those with prior insulin use (3.52 vs. 1.43 per 1,000 person-years).
The results of sensitivity analyses were consistent with those of the primary analysis.
The study was funded by the Canadian Institutes of Health Research and supported by ICES. Dr. Douros has reported receiving a salary support award from Fonds de recherche du Quebec – sante. Dr. Taylor was previously employed at Bristol-Myers Squibb. He is currently a consultant for Ionis Pharmaceuticals and has reported receiving research support provided to the University of Maryland School of Medicine by Regeneron.
A version of this article originally appeared on Medscape.com.
according to a new large database analysis.
The findings, which include data on the use of three different SGLT2 inhibitors in Canada and the United Kingdom and suggest a class effect, were published online July 27 in Annals of Internal Medicine by Antonios Douros, MD, PhD, of McGill University and the Centre for Clinical Epidemiology, Lady Davis Institute, Montreal, and colleagues.
“Our results provide robust evidence that SGLT2 inhibitors are associated with an increased risk for DKA. Of note, increased risks were observed in all molecule-specific analyses, with canagliflozin [Invokana, Janssen] showing the highest effect estimate,” they noted.
And because the beneficial effects of SGLT2 inhibitors in the prevention of cardiovascular and renal disease will probably increase their uptake in the coming years, “Physicians should be aware of DKA as a potential adverse effect,” Dr. Douros and colleagues wrote.
Analysis “generally confirms what has already been published”
Asked for comment, Simeon I. Taylor, MD, PhD, professor of medicine at the University of Maryland, Baltimore, said that the study “generally confirms what has already been published” on the topic. He noted that overall “the risk of SGLT2 inhibitor–induced ketoacidosis is quite low in type 2 diabetes, perhaps on the order of 1 episode per 1000 patient-years.”
However, Dr. Taylor cautioned: “Published evidence suggests that the risk of DKA is increased if patients are unable to eat,” such as when hospitalized patients are not permitted to eat.
“In that setting, it is probably prudent to discontinue an SGLT2 inhibitor. Also, it may be prudent not to prescribe SGLT2 inhibitors to patients with a history of DKA,” he added.
Dr. Taylor also advised: “Although not necessarily supported by this publication, I think that caution should be exercised in prescribing SGLT2 inhibitors to insulin-dependent type 2 diabetes patients. ... Some late-stage type 2 diabetes patients may have severe insulin deficiency, and their physiology may resemble that of a type 1 diabetes patient.”
Dr. Taylor has previously advised against using SGLT2 inhibitors altogether in patients with type 1 diabetes.
Increased DKA risk seen across all SGLT2 inhibitors
The study involved electronic health care databases from seven Canadian provinces and the United Kingdom, from which 208,757 new users of SGLT2 inhibitors were propensity-matched 1:1 to new dipeptidyl peptidase-4 (DPP-4) inhibitor users.
Of those taking an SGLT2 inhibitor, 42.3% took canagliflozin, 30.7% dapagliflozin (Farxiga/Forxiga, AstraZeneca), and 27.0% empagliflozin (Jardiance, Boehringer Ingelheim).
Over a mean 0.9-year follow-up, 521 patients were hospitalized with DKA, for an overall incidence rate of 1.41 per 1,000 person-years.
The rate with SGLT2 inhibitors, 2.03 per 1,000 person-years, was nearly three times that seen with DPP-4 inhibitors, at 0.75 per 1,000 person-years, a significant difference (hazard ratio, 2.85).
By individual SGLT2 inhibitor, the hazard ratios compared with DPP-4 inhibitors were 1.86 for dapagliflozin, 2.52 for empagliflozin, and 3.58 for canagliflozin, all statistically significant. Stratification by age, sex, and incident versus prevalent user did not change the association between SGLT2 inhibitors and DKA.
Asked about the higher rate for canagliflozin, Dr. Taylor commented: “It is hard to know whether there are real and reproducible differences in the risks of DKA among the various SGLT2 inhibitors. The differences are not huge and the populations are not well matched.”
But, he noted, “If canagliflozin triggers more glucosuria, it is not surprising that it would also induce more ketosis and possibly ketoacidosis.”
He also noted that the threefold relative increase in DKA with canagliflozin versus comparators is consistent with Janssen’s data, published in 2015.
“It is, of course, reassuring that both [randomized clinical trials] and epidemiology produce similar estimates of the risk of drug-induced adverse events. Interestingly, the incidence of DKA is approximately threefold higher in the Canadian [data] as compared to Janssen’s clinical trials.”
Dr. Taylor also pointed out that, in the Janssen studies, the risk of canagliflozin-induced DKA appeared to be higher among patients with anti-islet antibodies, which suggests that some may have actually had autoimmune (type 1) diabetes. “So the overall risk of SGLT2 inhibitor-induced DKA may depend at least in part on the mix of patients.”
In the current study, individuals who never used insulin had a greater relative increase in risk of DKA with SGLT2 inhibitors, compared with DPP-4 inhibitors, than did those who did use insulin (hazard ratios, 3.96 vs. 2.24, both compared with DPP-4 inhibitors). However, just among those taking SGLT2 inhibitors, the absolute risk for DKA was higher for those with prior insulin use (3.52 vs. 1.43 per 1,000 person-years).
The results of sensitivity analyses were consistent with those of the primary analysis.
The study was funded by the Canadian Institutes of Health Research and supported by ICES. Dr. Douros has reported receiving a salary support award from Fonds de recherche du Quebec – sante. Dr. Taylor was previously employed at Bristol-Myers Squibb. He is currently a consultant for Ionis Pharmaceuticals and has reported receiving research support provided to the University of Maryland School of Medicine by Regeneron.
A version of this article originally appeared on Medscape.com.