User login
Dapagliflozin Improves Cardiovascular Outcomes in Patients With Heart Failure and Reduced Ejection Fraction
Study Overview
Objective. To evaluate the effects of dapagliflozin in patients with heart failure with reduced ejection fraction in the presence or absence of type 2 diabetes.
Design. Multicenter, international, double-blind, prospective, randomized, controlled trial.
Setting and participants. Adult patients with symptomatic heart failure with an ejection fraction of 40% or less and elevated heart failure biomarkers who were already on appropriate guideline-directed therapies were eligible for the study.
Intervention. A total of 4744 patients were randomly assigned to receive dapagliflozin (10 mg once daily) or placebo, in addition to recommended therapy. Randomization was stratified by the presence or absence of type 2 diabetes.
Main outcome measures. The primary outcome was the composite of a first episode of worsening heart failure (hospitalization or urgent intravenous therapy) or cardiovascular death.
Main results. Median follow-up was 18.2 months; during this time, the primary outcome occurred in 16.3% (386 of 2373) of patients in the dapagliflozin group and in 21.2% (502 of 2371) of patients in the placebo group (hazard ratio [HR], 0.74; 95% confidence interval [CI], 0.65-0.85; P < 0.001). In the dapagliflozin group, 237 patients (10.0%) experienced a first worsening heart failure event, as compared with 326 patients (13.7%) in the placebo group (HR, 0.70; 95% CI, 0.59-0.83). The dapagliflozin group hadlower rates of death from cardiovascular causes (9.6% vs 11.5%; HR, 0.82; 95% CI, 0.69-0.98) and from any causes (11.6% vs 13.9%; HR, 0.83; 95% CI, 0.71-0.97), compared to the placebo group. Findings in patients with diabetes were similar to those in patients without diabetes.
Conclusion. Among patients with heart failure and a reduced ejection fraction, the risk of worsening heart failure or death from cardiovascular causes was lower among those who received dapagliflozin than among those who received placebo, regardless of the presence or absence of diabetes.
Commentary
Inhibitors of sodium-glucose cotransporter 2 (SGLT-2) are a novel class of diabetic medication that decrease renal glucose reabsorption, thereby increasing urinary glucose excretion. In several large clinical trials of these medications for patients with diabetes, which were designed to meet the regulatory requirements for cardiovascular safety in novel diabetic agents, investigators unexpectedly found that SGLT-2 inhibitors were associated with a reduction in cardiovascular events, driven by a reduction in heart failure hospitalizations. The results of EMPA-REG OUTCOME, the first of these trials, showed significantly lower risks of both death from any cause and hospitalization for heart failure in patients treated with empagliflozin.1 This improvement in cardiovascular outcomes was subsequently confirmed as a class effect of SGLT-2 inhibitors in the CANVAS Program (canagliflozin) and DECLARE TIMI 58 (dapagliflozin) trials.2,3
While these trials were designed for patients with type 2 diabetes who had either established cardiovascular disease or multiple risk factors for it, most patients did not have heart failure at baseline. Accordingly, despite a signal toward benefit of SGLT-2 inhibitors in patients with heart failure, the trials were not powered to test the hypothesis that SGLT-2 inhibitors benefit patients with heart failure, regardless of diabetes status. Therefore, McMurray et al designed the DAPA-HF trial to investigate whether SGLT-2 inhibitors can improve cardiovascular outcomes in patients with heart failure with reduced ejection fraction, with or without diabetes. The trial included 4744 patients with heart failure with reduced ejection fraction, who were randomly assigned to dapagliflozin 10 mg once daily or placebo, atop guideline-directed heart failure therapy, with randomization stratified by presence or absence of type 2 diabetes. Investigators found that the composite primary outcome, a first episode of worsening heart failure or cardiovascular death, occurred less frequently in patients in the dapagliflozin group compared to the placebo group (16.3% vs 21.2%; HR, 0.74; 95% CI, 0.65-0.85; P < 0.001). Individual components of the primary outcome and death from any cause were all significantly lower, and heart failure–related quality of life was significantly improved in the dapagliflozin group compared to placebo.
DAPA-HF was the first randomized study to investigate the effect of SGLT-2 inhibitors on patients with heart failure regardless of the presence of diabetes. In addition to the reduction in the above-mentioned primary and secondary endpoints, the study yielded other important findings worth noting. First, the consistent benefit of dapagliflozin on cardiovascular outcomes in patients with and without diabetes suggests that the cardioprotective effect of dapagliflozin is independent of its glucose-lowering effect. Prior studies have proposed alternative mechanisms, such as diuretic function and related hemodynamic actions, effects on myocardial metabolism, ion transporters, fibrosis, adipokines, vascular function, and the preservation of renal function. Future studies are needed to fully understand the likely pleiotropic effects of this class of medication on patients with heart failure. Second, there was no difference in the safety endpoints between the groups, including renal adverse events and major hypoglycemia, implying dapagliflozin is as safe as placebo.
There are a few limitations of this trial. First, as the authors point out, the study included mostly white males—less than 5% of participants were African Americans—and the finding may not be generalizable to all patient populations. Second, although all patients were already treated with guideline-directed heart failure therapy, only 10% of patients were on sacubitril–valsartan, which is more effective than renin–angiotensin system blockade alone at reducing the incidence of hospitalization for heart failure and death from cardiovascular causes. Also, mineralocorticoid receptor blockers were used in only 70% of the population. Finally, since the doses were not provided, whether patients were on the maximal tolerated dose of heart failure therapy prior to enrollment is unclear.
Based on the results of the DAPA-HF trial, the Food and Drug Administration approved dapagliflozin for the treatment of heart failure with reduced ejection fraction on May 5, 2020. This is the first diabetic drug approved for the treatment of heart failure.
Applications for Clinical Practice
SGLT-2 inhibitors represent a fourth class of medication that patients with heart failure with reduced ejection fraction should be initiated on, in addition to beta blocker, ACE inhibitor/angiotensin receptor blocker/neprilysin inhibitor, and mineralocorticoid receptor blocker. SGLT-2 inhibitors may be especially applicable in patients with heart failure with reduced ejection fraction and relative hypotension, as these agents are not associated with a significant blood-pressure-lowering effect, which can often limit our ability to initiate or uptitrate the other main 3 classes of guideline-directed medical therapy.
—Rie Hirai, MD, Fukui Kosei Hospital, Fukui, Japan
—Taishi Hirai, MD, University of Missouri Medical Center, Columbia, MO
—Timothy Fendler, MD, St. Luke’s Mid America Heart Institute, Kansas City, MO
1. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
2. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
3. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
Study Overview
Objective. To evaluate the effects of dapagliflozin in patients with heart failure with reduced ejection fraction in the presence or absence of type 2 diabetes.
Design. Multicenter, international, double-blind, prospective, randomized, controlled trial.
Setting and participants. Adult patients with symptomatic heart failure with an ejection fraction of 40% or less and elevated heart failure biomarkers who were already on appropriate guideline-directed therapies were eligible for the study.
Intervention. A total of 4744 patients were randomly assigned to receive dapagliflozin (10 mg once daily) or placebo, in addition to recommended therapy. Randomization was stratified by the presence or absence of type 2 diabetes.
Main outcome measures. The primary outcome was the composite of a first episode of worsening heart failure (hospitalization or urgent intravenous therapy) or cardiovascular death.
Main results. Median follow-up was 18.2 months; during this time, the primary outcome occurred in 16.3% (386 of 2373) of patients in the dapagliflozin group and in 21.2% (502 of 2371) of patients in the placebo group (hazard ratio [HR], 0.74; 95% confidence interval [CI], 0.65-0.85; P < 0.001). In the dapagliflozin group, 237 patients (10.0%) experienced a first worsening heart failure event, as compared with 326 patients (13.7%) in the placebo group (HR, 0.70; 95% CI, 0.59-0.83). The dapagliflozin group hadlower rates of death from cardiovascular causes (9.6% vs 11.5%; HR, 0.82; 95% CI, 0.69-0.98) and from any causes (11.6% vs 13.9%; HR, 0.83; 95% CI, 0.71-0.97), compared to the placebo group. Findings in patients with diabetes were similar to those in patients without diabetes.
Conclusion. Among patients with heart failure and a reduced ejection fraction, the risk of worsening heart failure or death from cardiovascular causes was lower among those who received dapagliflozin than among those who received placebo, regardless of the presence or absence of diabetes.
Commentary
Inhibitors of sodium-glucose cotransporter 2 (SGLT-2) are a novel class of diabetic medication that decrease renal glucose reabsorption, thereby increasing urinary glucose excretion. In several large clinical trials of these medications for patients with diabetes, which were designed to meet the regulatory requirements for cardiovascular safety in novel diabetic agents, investigators unexpectedly found that SGLT-2 inhibitors were associated with a reduction in cardiovascular events, driven by a reduction in heart failure hospitalizations. The results of EMPA-REG OUTCOME, the first of these trials, showed significantly lower risks of both death from any cause and hospitalization for heart failure in patients treated with empagliflozin.1 This improvement in cardiovascular outcomes was subsequently confirmed as a class effect of SGLT-2 inhibitors in the CANVAS Program (canagliflozin) and DECLARE TIMI 58 (dapagliflozin) trials.2,3
While these trials were designed for patients with type 2 diabetes who had either established cardiovascular disease or multiple risk factors for it, most patients did not have heart failure at baseline. Accordingly, despite a signal toward benefit of SGLT-2 inhibitors in patients with heart failure, the trials were not powered to test the hypothesis that SGLT-2 inhibitors benefit patients with heart failure, regardless of diabetes status. Therefore, McMurray et al designed the DAPA-HF trial to investigate whether SGLT-2 inhibitors can improve cardiovascular outcomes in patients with heart failure with reduced ejection fraction, with or without diabetes. The trial included 4744 patients with heart failure with reduced ejection fraction, who were randomly assigned to dapagliflozin 10 mg once daily or placebo, atop guideline-directed heart failure therapy, with randomization stratified by presence or absence of type 2 diabetes. Investigators found that the composite primary outcome, a first episode of worsening heart failure or cardiovascular death, occurred less frequently in patients in the dapagliflozin group compared to the placebo group (16.3% vs 21.2%; HR, 0.74; 95% CI, 0.65-0.85; P < 0.001). Individual components of the primary outcome and death from any cause were all significantly lower, and heart failure–related quality of life was significantly improved in the dapagliflozin group compared to placebo.
DAPA-HF was the first randomized study to investigate the effect of SGLT-2 inhibitors on patients with heart failure regardless of the presence of diabetes. In addition to the reduction in the above-mentioned primary and secondary endpoints, the study yielded other important findings worth noting. First, the consistent benefit of dapagliflozin on cardiovascular outcomes in patients with and without diabetes suggests that the cardioprotective effect of dapagliflozin is independent of its glucose-lowering effect. Prior studies have proposed alternative mechanisms, such as diuretic function and related hemodynamic actions, effects on myocardial metabolism, ion transporters, fibrosis, adipokines, vascular function, and the preservation of renal function. Future studies are needed to fully understand the likely pleiotropic effects of this class of medication on patients with heart failure. Second, there was no difference in the safety endpoints between the groups, including renal adverse events and major hypoglycemia, implying dapagliflozin is as safe as placebo.
There are a few limitations of this trial. First, as the authors point out, the study included mostly white males—less than 5% of participants were African Americans—and the finding may not be generalizable to all patient populations. Second, although all patients were already treated with guideline-directed heart failure therapy, only 10% of patients were on sacubitril–valsartan, which is more effective than renin–angiotensin system blockade alone at reducing the incidence of hospitalization for heart failure and death from cardiovascular causes. Also, mineralocorticoid receptor blockers were used in only 70% of the population. Finally, since the doses were not provided, whether patients were on the maximal tolerated dose of heart failure therapy prior to enrollment is unclear.
Based on the results of the DAPA-HF trial, the Food and Drug Administration approved dapagliflozin for the treatment of heart failure with reduced ejection fraction on May 5, 2020. This is the first diabetic drug approved for the treatment of heart failure.
Applications for Clinical Practice
SGLT-2 inhibitors represent a fourth class of medication that patients with heart failure with reduced ejection fraction should be initiated on, in addition to beta blocker, ACE inhibitor/angiotensin receptor blocker/neprilysin inhibitor, and mineralocorticoid receptor blocker. SGLT-2 inhibitors may be especially applicable in patients with heart failure with reduced ejection fraction and relative hypotension, as these agents are not associated with a significant blood-pressure-lowering effect, which can often limit our ability to initiate or uptitrate the other main 3 classes of guideline-directed medical therapy.
—Rie Hirai, MD, Fukui Kosei Hospital, Fukui, Japan
—Taishi Hirai, MD, University of Missouri Medical Center, Columbia, MO
—Timothy Fendler, MD, St. Luke’s Mid America Heart Institute, Kansas City, MO
Study Overview
Objective. To evaluate the effects of dapagliflozin in patients with heart failure with reduced ejection fraction in the presence or absence of type 2 diabetes.
Design. Multicenter, international, double-blind, prospective, randomized, controlled trial.
Setting and participants. Adult patients with symptomatic heart failure with an ejection fraction of 40% or less and elevated heart failure biomarkers who were already on appropriate guideline-directed therapies were eligible for the study.
Intervention. A total of 4744 patients were randomly assigned to receive dapagliflozin (10 mg once daily) or placebo, in addition to recommended therapy. Randomization was stratified by the presence or absence of type 2 diabetes.
Main outcome measures. The primary outcome was the composite of a first episode of worsening heart failure (hospitalization or urgent intravenous therapy) or cardiovascular death.
Main results. Median follow-up was 18.2 months; during this time, the primary outcome occurred in 16.3% (386 of 2373) of patients in the dapagliflozin group and in 21.2% (502 of 2371) of patients in the placebo group (hazard ratio [HR], 0.74; 95% confidence interval [CI], 0.65-0.85; P < 0.001). In the dapagliflozin group, 237 patients (10.0%) experienced a first worsening heart failure event, as compared with 326 patients (13.7%) in the placebo group (HR, 0.70; 95% CI, 0.59-0.83). The dapagliflozin group hadlower rates of death from cardiovascular causes (9.6% vs 11.5%; HR, 0.82; 95% CI, 0.69-0.98) and from any causes (11.6% vs 13.9%; HR, 0.83; 95% CI, 0.71-0.97), compared to the placebo group. Findings in patients with diabetes were similar to those in patients without diabetes.
Conclusion. Among patients with heart failure and a reduced ejection fraction, the risk of worsening heart failure or death from cardiovascular causes was lower among those who received dapagliflozin than among those who received placebo, regardless of the presence or absence of diabetes.
Commentary
Inhibitors of sodium-glucose cotransporter 2 (SGLT-2) are a novel class of diabetic medication that decrease renal glucose reabsorption, thereby increasing urinary glucose excretion. In several large clinical trials of these medications for patients with diabetes, which were designed to meet the regulatory requirements for cardiovascular safety in novel diabetic agents, investigators unexpectedly found that SGLT-2 inhibitors were associated with a reduction in cardiovascular events, driven by a reduction in heart failure hospitalizations. The results of EMPA-REG OUTCOME, the first of these trials, showed significantly lower risks of both death from any cause and hospitalization for heart failure in patients treated with empagliflozin.1 This improvement in cardiovascular outcomes was subsequently confirmed as a class effect of SGLT-2 inhibitors in the CANVAS Program (canagliflozin) and DECLARE TIMI 58 (dapagliflozin) trials.2,3
While these trials were designed for patients with type 2 diabetes who had either established cardiovascular disease or multiple risk factors for it, most patients did not have heart failure at baseline. Accordingly, despite a signal toward benefit of SGLT-2 inhibitors in patients with heart failure, the trials were not powered to test the hypothesis that SGLT-2 inhibitors benefit patients with heart failure, regardless of diabetes status. Therefore, McMurray et al designed the DAPA-HF trial to investigate whether SGLT-2 inhibitors can improve cardiovascular outcomes in patients with heart failure with reduced ejection fraction, with or without diabetes. The trial included 4744 patients with heart failure with reduced ejection fraction, who were randomly assigned to dapagliflozin 10 mg once daily or placebo, atop guideline-directed heart failure therapy, with randomization stratified by presence or absence of type 2 diabetes. Investigators found that the composite primary outcome, a first episode of worsening heart failure or cardiovascular death, occurred less frequently in patients in the dapagliflozin group compared to the placebo group (16.3% vs 21.2%; HR, 0.74; 95% CI, 0.65-0.85; P < 0.001). Individual components of the primary outcome and death from any cause were all significantly lower, and heart failure–related quality of life was significantly improved in the dapagliflozin group compared to placebo.
DAPA-HF was the first randomized study to investigate the effect of SGLT-2 inhibitors on patients with heart failure regardless of the presence of diabetes. In addition to the reduction in the above-mentioned primary and secondary endpoints, the study yielded other important findings worth noting. First, the consistent benefit of dapagliflozin on cardiovascular outcomes in patients with and without diabetes suggests that the cardioprotective effect of dapagliflozin is independent of its glucose-lowering effect. Prior studies have proposed alternative mechanisms, such as diuretic function and related hemodynamic actions, effects on myocardial metabolism, ion transporters, fibrosis, adipokines, vascular function, and the preservation of renal function. Future studies are needed to fully understand the likely pleiotropic effects of this class of medication on patients with heart failure. Second, there was no difference in the safety endpoints between the groups, including renal adverse events and major hypoglycemia, implying dapagliflozin is as safe as placebo.
There are a few limitations of this trial. First, as the authors point out, the study included mostly white males—less than 5% of participants were African Americans—and the finding may not be generalizable to all patient populations. Second, although all patients were already treated with guideline-directed heart failure therapy, only 10% of patients were on sacubitril–valsartan, which is more effective than renin–angiotensin system blockade alone at reducing the incidence of hospitalization for heart failure and death from cardiovascular causes. Also, mineralocorticoid receptor blockers were used in only 70% of the population. Finally, since the doses were not provided, whether patients were on the maximal tolerated dose of heart failure therapy prior to enrollment is unclear.
Based on the results of the DAPA-HF trial, the Food and Drug Administration approved dapagliflozin for the treatment of heart failure with reduced ejection fraction on May 5, 2020. This is the first diabetic drug approved for the treatment of heart failure.
Applications for Clinical Practice
SGLT-2 inhibitors represent a fourth class of medication that patients with heart failure with reduced ejection fraction should be initiated on, in addition to beta blocker, ACE inhibitor/angiotensin receptor blocker/neprilysin inhibitor, and mineralocorticoid receptor blocker. SGLT-2 inhibitors may be especially applicable in patients with heart failure with reduced ejection fraction and relative hypotension, as these agents are not associated with a significant blood-pressure-lowering effect, which can often limit our ability to initiate or uptitrate the other main 3 classes of guideline-directed medical therapy.
—Rie Hirai, MD, Fukui Kosei Hospital, Fukui, Japan
—Taishi Hirai, MD, University of Missouri Medical Center, Columbia, MO
—Timothy Fendler, MD, St. Luke’s Mid America Heart Institute, Kansas City, MO
1. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
2. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
3. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
1. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
2. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
3. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
Part 5: Screening for “Opathies” in Diabetes Patients
Previously, we discussed monitoring for chronic kidney disease in patients with diabetes. In this final part of our series, we’ll discuss screening to prevent impairment to the patient’s mobility and sight.
CASE CONTINUED
Mr. W is appreciative of your efforts to improve his health, but he fears his quality of life with diabetes will suffer. Because his father experienced impaired sight and limited mobility during the final years of his life, Mr. W is concerned he will endure similar complications from his diabetes. What can you do to help safeguard his abilities for sight and mobility?
Detecting peripheral neuropathy
Evaluation of Mr. W’s feet is an appropriate first step in the right direction. Peripheral neuropathy—one of the most common complications in diabetes—occurs in up to 50% of patients with diabetes, and about 50% of peripheral neuropathies may be asymptomatic.40 It is the most significant risk factor for foot ulceration, which in turn is the leading cause of amputation in patients with diabetes.40 Therefore, early identification of peripheral neuropathy is important because it provides an opportunity for patient education on preventive practices and prompts podiatric care.
Screening for peripheral neuropathy should include a detailed history of the risk factors and a thorough physical exam, including pinprick sensation (small sensory fiber function), vibration perception (large sensory fiber function), and 10-g monofilament testing.7,8,40 Clinicians should screen their patients within 5 years of the diagnosis of type 1 diabetes and at the time of diagnosis of type 2 diabetes, subsequently scheduling at least annual screening with a full foot exam.7,8
Further assessment to identify risk factors for diabetic foot wounds should include evaluation for foot deformities and vascular disease.7,8 Findings that indicate vascular disease should prompt ankle-brachial index testing.7,8
Patients are considered at high-risk for peripheral neuropathy if they have sensory impairment, a history of podiatric complications, or foot deformities, or if they actively smoke.8 Such patients should have a thorough foot exam during each visit with their primary care provider, and referral to a foot care specialist would be appropriate.8 High-risk individuals would benefit from close surveillance to prevent complications, and specialized footwear may be helpful.8
How to Screen for Diabetic Retinopathy
Also high on the list of Mr. W’s priorities is maintaining his eyesight. All patients with diabetes require adequate screening for diabetic retinopathy, which is a contributing factor in the progression to blindness.41 Referral to an optometrist or ophthalmologist for a dilated fundoscopic eye exam is recommended for patients within 5 years of a diagnosis of type 1 diabetes and for patients with type 2 diabetes at the time of diagnosis.2,7,8 Prompt referral is need for patients with macular edema, severe nonproliferative diabetic retinopathy, or proliferative diabetic retinopathy. The ADA considers the use of retinal photography in detecting diabetic retinopathy an appropriate component of the fundoscopic exam because it has high sensitivity, specificity, and inter- and intra-examination agreement.8,41,42
Continue to: For patients with...
For patients with poorly controlled diabetes or known diabetic retinopathy, dilated retinal examinations should be scheduled on at least an annual basis.2 For those with well-controlled diabetes and no signs of retinopathy, repeat screening no less frequently than every 2 years may be appropriate.2 This allows prompt diagnosis and treatment of a potentially sight-limiting disease before irreversible damage is caused.
In Conclusion: Empowering Patients with Diabetes
The more Mr. W knows about how to maintain his health, the more control he has over his future with diabetes. Providing patients with knowledge of the risks and empowering them through evidence-based methods is invaluable. DSMES programs help achieve this goal and should be considered at multiple stages in the patient’s disease course, including at the time of initial diagnosis, annually, and when complications or transitions in treatment occur.2,9 Involving patients in their own medical care and management helps them to advocate for their well-being. The patient as a fellow collaborator in treatment can help the clinician design a successful management plan that increases the likelihood of better outcomes for patients such as Mr. W.
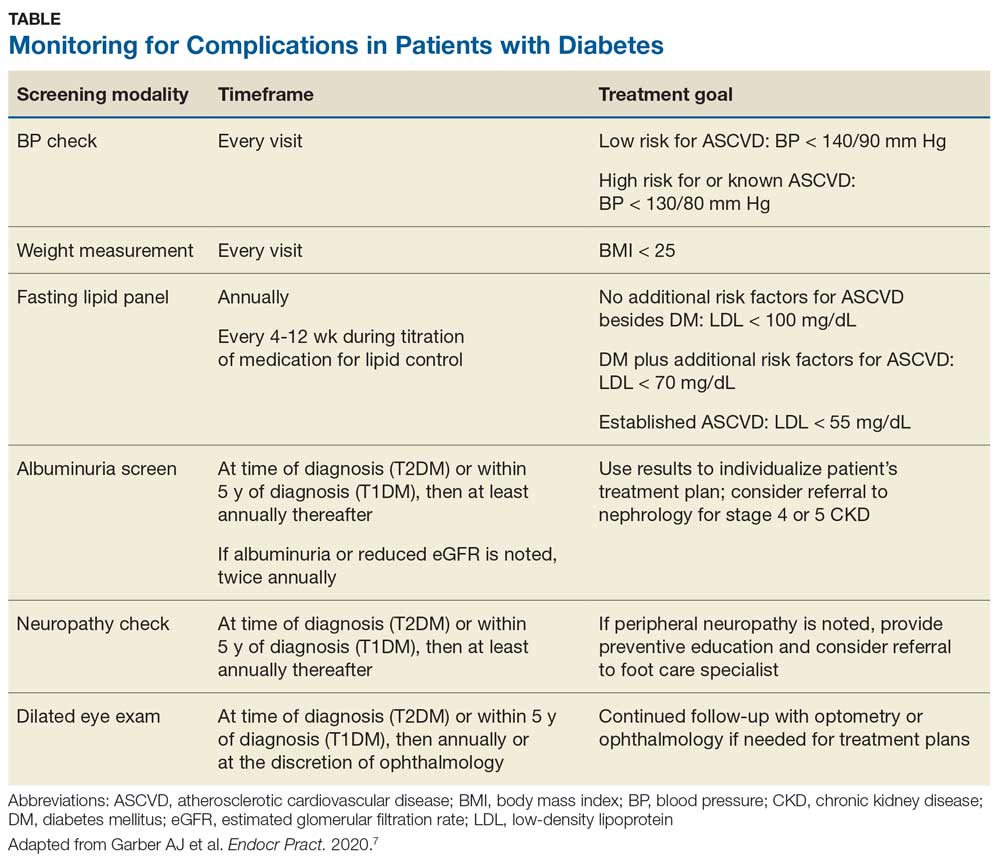
To review the important areas of prevention of and screening for complications in patients with diabetes, see the Table. Additional guidance can be found in the ADA and AACE recommendations.2,8
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
Previously, we discussed monitoring for chronic kidney disease in patients with diabetes. In this final part of our series, we’ll discuss screening to prevent impairment to the patient’s mobility and sight.
CASE CONTINUED
Mr. W is appreciative of your efforts to improve his health, but he fears his quality of life with diabetes will suffer. Because his father experienced impaired sight and limited mobility during the final years of his life, Mr. W is concerned he will endure similar complications from his diabetes. What can you do to help safeguard his abilities for sight and mobility?
Detecting peripheral neuropathy
Evaluation of Mr. W’s feet is an appropriate first step in the right direction. Peripheral neuropathy—one of the most common complications in diabetes—occurs in up to 50% of patients with diabetes, and about 50% of peripheral neuropathies may be asymptomatic.40 It is the most significant risk factor for foot ulceration, which in turn is the leading cause of amputation in patients with diabetes.40 Therefore, early identification of peripheral neuropathy is important because it provides an opportunity for patient education on preventive practices and prompts podiatric care.
Screening for peripheral neuropathy should include a detailed history of the risk factors and a thorough physical exam, including pinprick sensation (small sensory fiber function), vibration perception (large sensory fiber function), and 10-g monofilament testing.7,8,40 Clinicians should screen their patients within 5 years of the diagnosis of type 1 diabetes and at the time of diagnosis of type 2 diabetes, subsequently scheduling at least annual screening with a full foot exam.7,8
Further assessment to identify risk factors for diabetic foot wounds should include evaluation for foot deformities and vascular disease.7,8 Findings that indicate vascular disease should prompt ankle-brachial index testing.7,8
Patients are considered at high-risk for peripheral neuropathy if they have sensory impairment, a history of podiatric complications, or foot deformities, or if they actively smoke.8 Such patients should have a thorough foot exam during each visit with their primary care provider, and referral to a foot care specialist would be appropriate.8 High-risk individuals would benefit from close surveillance to prevent complications, and specialized footwear may be helpful.8
How to Screen for Diabetic Retinopathy
Also high on the list of Mr. W’s priorities is maintaining his eyesight. All patients with diabetes require adequate screening for diabetic retinopathy, which is a contributing factor in the progression to blindness.41 Referral to an optometrist or ophthalmologist for a dilated fundoscopic eye exam is recommended for patients within 5 years of a diagnosis of type 1 diabetes and for patients with type 2 diabetes at the time of diagnosis.2,7,8 Prompt referral is need for patients with macular edema, severe nonproliferative diabetic retinopathy, or proliferative diabetic retinopathy. The ADA considers the use of retinal photography in detecting diabetic retinopathy an appropriate component of the fundoscopic exam because it has high sensitivity, specificity, and inter- and intra-examination agreement.8,41,42
Continue to: For patients with...
For patients with poorly controlled diabetes or known diabetic retinopathy, dilated retinal examinations should be scheduled on at least an annual basis.2 For those with well-controlled diabetes and no signs of retinopathy, repeat screening no less frequently than every 2 years may be appropriate.2 This allows prompt diagnosis and treatment of a potentially sight-limiting disease before irreversible damage is caused.
In Conclusion: Empowering Patients with Diabetes
The more Mr. W knows about how to maintain his health, the more control he has over his future with diabetes. Providing patients with knowledge of the risks and empowering them through evidence-based methods is invaluable. DSMES programs help achieve this goal and should be considered at multiple stages in the patient’s disease course, including at the time of initial diagnosis, annually, and when complications or transitions in treatment occur.2,9 Involving patients in their own medical care and management helps them to advocate for their well-being. The patient as a fellow collaborator in treatment can help the clinician design a successful management plan that increases the likelihood of better outcomes for patients such as Mr. W.
To review the important areas of prevention of and screening for complications in patients with diabetes, see the Table. Additional guidance can be found in the ADA and AACE recommendations.2,8

Previously, we discussed monitoring for chronic kidney disease in patients with diabetes. In this final part of our series, we’ll discuss screening to prevent impairment to the patient’s mobility and sight.
CASE CONTINUED
Mr. W is appreciative of your efforts to improve his health, but he fears his quality of life with diabetes will suffer. Because his father experienced impaired sight and limited mobility during the final years of his life, Mr. W is concerned he will endure similar complications from his diabetes. What can you do to help safeguard his abilities for sight and mobility?
Detecting peripheral neuropathy
Evaluation of Mr. W’s feet is an appropriate first step in the right direction. Peripheral neuropathy—one of the most common complications in diabetes—occurs in up to 50% of patients with diabetes, and about 50% of peripheral neuropathies may be asymptomatic.40 It is the most significant risk factor for foot ulceration, which in turn is the leading cause of amputation in patients with diabetes.40 Therefore, early identification of peripheral neuropathy is important because it provides an opportunity for patient education on preventive practices and prompts podiatric care.
Screening for peripheral neuropathy should include a detailed history of the risk factors and a thorough physical exam, including pinprick sensation (small sensory fiber function), vibration perception (large sensory fiber function), and 10-g monofilament testing.7,8,40 Clinicians should screen their patients within 5 years of the diagnosis of type 1 diabetes and at the time of diagnosis of type 2 diabetes, subsequently scheduling at least annual screening with a full foot exam.7,8
Further assessment to identify risk factors for diabetic foot wounds should include evaluation for foot deformities and vascular disease.7,8 Findings that indicate vascular disease should prompt ankle-brachial index testing.7,8
Patients are considered at high-risk for peripheral neuropathy if they have sensory impairment, a history of podiatric complications, or foot deformities, or if they actively smoke.8 Such patients should have a thorough foot exam during each visit with their primary care provider, and referral to a foot care specialist would be appropriate.8 High-risk individuals would benefit from close surveillance to prevent complications, and specialized footwear may be helpful.8
How to Screen for Diabetic Retinopathy
Also high on the list of Mr. W’s priorities is maintaining his eyesight. All patients with diabetes require adequate screening for diabetic retinopathy, which is a contributing factor in the progression to blindness.41 Referral to an optometrist or ophthalmologist for a dilated fundoscopic eye exam is recommended for patients within 5 years of a diagnosis of type 1 diabetes and for patients with type 2 diabetes at the time of diagnosis.2,7,8 Prompt referral is need for patients with macular edema, severe nonproliferative diabetic retinopathy, or proliferative diabetic retinopathy. The ADA considers the use of retinal photography in detecting diabetic retinopathy an appropriate component of the fundoscopic exam because it has high sensitivity, specificity, and inter- and intra-examination agreement.8,41,42
Continue to: For patients with...
For patients with poorly controlled diabetes or known diabetic retinopathy, dilated retinal examinations should be scheduled on at least an annual basis.2 For those with well-controlled diabetes and no signs of retinopathy, repeat screening no less frequently than every 2 years may be appropriate.2 This allows prompt diagnosis and treatment of a potentially sight-limiting disease before irreversible damage is caused.
In Conclusion: Empowering Patients with Diabetes
The more Mr. W knows about how to maintain his health, the more control he has over his future with diabetes. Providing patients with knowledge of the risks and empowering them through evidence-based methods is invaluable. DSMES programs help achieve this goal and should be considered at multiple stages in the patient’s disease course, including at the time of initial diagnosis, annually, and when complications or transitions in treatment occur.2,9 Involving patients in their own medical care and management helps them to advocate for their well-being. The patient as a fellow collaborator in treatment can help the clinician design a successful management plan that increases the likelihood of better outcomes for patients such as Mr. W.
To review the important areas of prevention of and screening for complications in patients with diabetes, see the Table. Additional guidance can be found in the ADA and AACE recommendations.2,8

1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
Cleaner data confirm severe COVID-19 link to diabetes, hypertension
Further refinement of data from patients hospitalized worldwide for COVID-19 disease showed a 12% prevalence rate of patients with diabetes in this population and a 17% prevalence rate for hypertension.
These are lower rates than previously reported for COVID-19 patients with either of these two comorbidities, yet the findings still document important epidemiologic links between diabetes, hypertension, and COVID-19, said the study’s authors.
A meta-analysis of data from 15,794 patients hospitalized because of COVID-19 disease that was drawn from 65 carefully curated reports published from December 1, 2019, to April 6, 2020, also showed that, among the hospitalized COVID-19 patients with diabetes (either type 1 or type 2), the rate of patients who required ICU admission was 96% higher than among those without diabetes and mortality was 2.78-fold higher, both statistically significant differences.
The rate of ICU admissions among those hospitalized with COVID-19 who also had hypertension was 2.95-fold above those without hypertension, and mortality was 2.39-fold higher, also statistically significant differences, reported a team of researchers in the recently published report.
The new meta-analysis was notable for the extra effort investigators employed to eliminate duplicated patients from their database of COVID-19 patients included in various published reports, a potential source of bias that likely introduced errors into prior meta-analyses that used similar data. “We found an overwhelming proportion of studies at high risk of data repetition,” the report said. Virtually all of the included studies were retrospective case studies, nearly two-thirds had data from a single center, and 71% of the studies included only patients in China.
“We developed a method to identify reports that had a high risk for repetitions” of included patients, said Fady Hannah-Shmouni, MD, a senior author of the study. “We also used methods to minimize bias, we excluded certain patients populations, and we applied a uniform definition of COVID-19 disease severity,” specifically patients who died or needed ICU admission, because the definitions used originally by many of the reports were very heterogeneous, said Dr. Hannah-Shmouni, principal investigator for Endocrine, Genetics, and Hypertension at the National Institute of Child Health and Human Development.
Despite the effort to eliminate case duplications, the analysis remains subject to additional confounders, in part because of a lack of comprehensive patient information on factors such as smoking, body mass index, socioeconomic status, and the specific type of diabetes or hypertension a patient had. “Even with these limitations, we were able to show that the prevalence of hypertension and diabetes is elevated in patients with COVID-19, that patients with diabetes have increased risk for both death and ICU admissions, and that there is the potential for reverse causality in the reporting of hypertension as a risk factor for COVID-19,” Dr. Hannah-Shmouni said in an interview. “We believe the explosion of data that associated hypertension and COVID-19 may be partially the result of reverse causality.”
One possible example of this reverse causality is the overlap between hypertension and age as potential risk factors for COVID-19 disease or increased infection severity. People “older than 80 frequently develop severe disease if infected with the novel coronavirus, and 80% of people older than 80 have hypertension, so it’s not surprising that hypertension is highly prevalent among hospitalized COVID-19 patients,” but this “does not imply a causal relationship between hypertension and severe COVID-19; the risk of hypertension probably depends on older age,” noted Ernesto L. Schiffrin, MD, a coauthor of the study, as well as professor of medicine at McGill University and director of the Hypertension and Vascular Research Unit at the Lady Davis Institute for Medical Research, both in Montreal. “My current opinion, on the basis of the totality of data, is that hypertension does not worsen [COVID-19] outcomes, but patients who are elderly, obese, diabetic, or immunocompromised are susceptible to more severe COVID-19 and worse outcomes,” said Dr. Schiffrin in an interview.
The new findings show “there is certainly an interplay between the virus, diabetes, and hypertension and other risk factors,” and while still limited by biases, the new findings “get closer” to correctly estimating the COVID-19 risks associated with these comorbidities,” Dr. Hannah-Shmouni said.
The connections identified between COVID-19, diabetes, and hypertension mean that patients with these chronic diseases should receive education about their COVID-19 risks and should have adequate access to the drugs and supplies they need to control blood pressure and hyperglycemia. Patients with diabetes also need to be current on vaccinations to reduce their risk for pneumonia. And recognition of the heightened COVID-19 risk for people with these comorbidities is important among people who work in relevant government agencies, health care workers, and patient advocacy groups, he added.
The study received no commercial funding. Dr. Hannah-Shmouni and Dr. Schiffrin had no disclosures.
SOURCE: Barrera FJ et al. J Endocn Soc. 2020 July 21. doi: 10.1210/jendso/bvaa102.
Further refinement of data from patients hospitalized worldwide for COVID-19 disease showed a 12% prevalence rate of patients with diabetes in this population and a 17% prevalence rate for hypertension.
These are lower rates than previously reported for COVID-19 patients with either of these two comorbidities, yet the findings still document important epidemiologic links between diabetes, hypertension, and COVID-19, said the study’s authors.
A meta-analysis of data from 15,794 patients hospitalized because of COVID-19 disease that was drawn from 65 carefully curated reports published from December 1, 2019, to April 6, 2020, also showed that, among the hospitalized COVID-19 patients with diabetes (either type 1 or type 2), the rate of patients who required ICU admission was 96% higher than among those without diabetes and mortality was 2.78-fold higher, both statistically significant differences.
The rate of ICU admissions among those hospitalized with COVID-19 who also had hypertension was 2.95-fold above those without hypertension, and mortality was 2.39-fold higher, also statistically significant differences, reported a team of researchers in the recently published report.
The new meta-analysis was notable for the extra effort investigators employed to eliminate duplicated patients from their database of COVID-19 patients included in various published reports, a potential source of bias that likely introduced errors into prior meta-analyses that used similar data. “We found an overwhelming proportion of studies at high risk of data repetition,” the report said. Virtually all of the included studies were retrospective case studies, nearly two-thirds had data from a single center, and 71% of the studies included only patients in China.
“We developed a method to identify reports that had a high risk for repetitions” of included patients, said Fady Hannah-Shmouni, MD, a senior author of the study. “We also used methods to minimize bias, we excluded certain patients populations, and we applied a uniform definition of COVID-19 disease severity,” specifically patients who died or needed ICU admission, because the definitions used originally by many of the reports were very heterogeneous, said Dr. Hannah-Shmouni, principal investigator for Endocrine, Genetics, and Hypertension at the National Institute of Child Health and Human Development.
Despite the effort to eliminate case duplications, the analysis remains subject to additional confounders, in part because of a lack of comprehensive patient information on factors such as smoking, body mass index, socioeconomic status, and the specific type of diabetes or hypertension a patient had. “Even with these limitations, we were able to show that the prevalence of hypertension and diabetes is elevated in patients with COVID-19, that patients with diabetes have increased risk for both death and ICU admissions, and that there is the potential for reverse causality in the reporting of hypertension as a risk factor for COVID-19,” Dr. Hannah-Shmouni said in an interview. “We believe the explosion of data that associated hypertension and COVID-19 may be partially the result of reverse causality.”
One possible example of this reverse causality is the overlap between hypertension and age as potential risk factors for COVID-19 disease or increased infection severity. People “older than 80 frequently develop severe disease if infected with the novel coronavirus, and 80% of people older than 80 have hypertension, so it’s not surprising that hypertension is highly prevalent among hospitalized COVID-19 patients,” but this “does not imply a causal relationship between hypertension and severe COVID-19; the risk of hypertension probably depends on older age,” noted Ernesto L. Schiffrin, MD, a coauthor of the study, as well as professor of medicine at McGill University and director of the Hypertension and Vascular Research Unit at the Lady Davis Institute for Medical Research, both in Montreal. “My current opinion, on the basis of the totality of data, is that hypertension does not worsen [COVID-19] outcomes, but patients who are elderly, obese, diabetic, or immunocompromised are susceptible to more severe COVID-19 and worse outcomes,” said Dr. Schiffrin in an interview.
The new findings show “there is certainly an interplay between the virus, diabetes, and hypertension and other risk factors,” and while still limited by biases, the new findings “get closer” to correctly estimating the COVID-19 risks associated with these comorbidities,” Dr. Hannah-Shmouni said.
The connections identified between COVID-19, diabetes, and hypertension mean that patients with these chronic diseases should receive education about their COVID-19 risks and should have adequate access to the drugs and supplies they need to control blood pressure and hyperglycemia. Patients with diabetes also need to be current on vaccinations to reduce their risk for pneumonia. And recognition of the heightened COVID-19 risk for people with these comorbidities is important among people who work in relevant government agencies, health care workers, and patient advocacy groups, he added.
The study received no commercial funding. Dr. Hannah-Shmouni and Dr. Schiffrin had no disclosures.
SOURCE: Barrera FJ et al. J Endocn Soc. 2020 July 21. doi: 10.1210/jendso/bvaa102.
Further refinement of data from patients hospitalized worldwide for COVID-19 disease showed a 12% prevalence rate of patients with diabetes in this population and a 17% prevalence rate for hypertension.
These are lower rates than previously reported for COVID-19 patients with either of these two comorbidities, yet the findings still document important epidemiologic links between diabetes, hypertension, and COVID-19, said the study’s authors.
A meta-analysis of data from 15,794 patients hospitalized because of COVID-19 disease that was drawn from 65 carefully curated reports published from December 1, 2019, to April 6, 2020, also showed that, among the hospitalized COVID-19 patients with diabetes (either type 1 or type 2), the rate of patients who required ICU admission was 96% higher than among those without diabetes and mortality was 2.78-fold higher, both statistically significant differences.
The rate of ICU admissions among those hospitalized with COVID-19 who also had hypertension was 2.95-fold above those without hypertension, and mortality was 2.39-fold higher, also statistically significant differences, reported a team of researchers in the recently published report.
The new meta-analysis was notable for the extra effort investigators employed to eliminate duplicated patients from their database of COVID-19 patients included in various published reports, a potential source of bias that likely introduced errors into prior meta-analyses that used similar data. “We found an overwhelming proportion of studies at high risk of data repetition,” the report said. Virtually all of the included studies were retrospective case studies, nearly two-thirds had data from a single center, and 71% of the studies included only patients in China.
“We developed a method to identify reports that had a high risk for repetitions” of included patients, said Fady Hannah-Shmouni, MD, a senior author of the study. “We also used methods to minimize bias, we excluded certain patients populations, and we applied a uniform definition of COVID-19 disease severity,” specifically patients who died or needed ICU admission, because the definitions used originally by many of the reports were very heterogeneous, said Dr. Hannah-Shmouni, principal investigator for Endocrine, Genetics, and Hypertension at the National Institute of Child Health and Human Development.
Despite the effort to eliminate case duplications, the analysis remains subject to additional confounders, in part because of a lack of comprehensive patient information on factors such as smoking, body mass index, socioeconomic status, and the specific type of diabetes or hypertension a patient had. “Even with these limitations, we were able to show that the prevalence of hypertension and diabetes is elevated in patients with COVID-19, that patients with diabetes have increased risk for both death and ICU admissions, and that there is the potential for reverse causality in the reporting of hypertension as a risk factor for COVID-19,” Dr. Hannah-Shmouni said in an interview. “We believe the explosion of data that associated hypertension and COVID-19 may be partially the result of reverse causality.”
One possible example of this reverse causality is the overlap between hypertension and age as potential risk factors for COVID-19 disease or increased infection severity. People “older than 80 frequently develop severe disease if infected with the novel coronavirus, and 80% of people older than 80 have hypertension, so it’s not surprising that hypertension is highly prevalent among hospitalized COVID-19 patients,” but this “does not imply a causal relationship between hypertension and severe COVID-19; the risk of hypertension probably depends on older age,” noted Ernesto L. Schiffrin, MD, a coauthor of the study, as well as professor of medicine at McGill University and director of the Hypertension and Vascular Research Unit at the Lady Davis Institute for Medical Research, both in Montreal. “My current opinion, on the basis of the totality of data, is that hypertension does not worsen [COVID-19] outcomes, but patients who are elderly, obese, diabetic, or immunocompromised are susceptible to more severe COVID-19 and worse outcomes,” said Dr. Schiffrin in an interview.
The new findings show “there is certainly an interplay between the virus, diabetes, and hypertension and other risk factors,” and while still limited by biases, the new findings “get closer” to correctly estimating the COVID-19 risks associated with these comorbidities,” Dr. Hannah-Shmouni said.
The connections identified between COVID-19, diabetes, and hypertension mean that patients with these chronic diseases should receive education about their COVID-19 risks and should have adequate access to the drugs and supplies they need to control blood pressure and hyperglycemia. Patients with diabetes also need to be current on vaccinations to reduce their risk for pneumonia. And recognition of the heightened COVID-19 risk for people with these comorbidities is important among people who work in relevant government agencies, health care workers, and patient advocacy groups, he added.
The study received no commercial funding. Dr. Hannah-Shmouni and Dr. Schiffrin had no disclosures.
SOURCE: Barrera FJ et al. J Endocn Soc. 2020 July 21. doi: 10.1210/jendso/bvaa102.
FROM JOURNAL OF THE ENDOCRINE SOCIETY
Part 4: Monitoring for CKD in Diabetes Patients
Previously, we discussed assessment and treatment for dyslipidemia in patients with diabetes. Now we’ll explore how to monitor for kidney disease in this population.
CASE CONTINUED
Mr. W’s basic metabolic panel includes an estimated glomerular filtration rate (eGFR) of 55 ml/min/1.73 m2 (reference range, > 60 ml/min/1.73 m2). In the absence of any other markers of kidney disease, you obtain a spot urinary albumin-to-creatinine ratio (UACR). The UACR results show a ratio of 64 mg/g, confirming stage 3 chronic kidney disease (CKD).
Monitoring for Chronic Kidney Disease
CKD is characterized by persistent albuminuria, low eGFR, and manifestations of kidney damage, and it increases cardiovascular risk.2 According to the ADA, clinicians should obtain a UACR and eGFR at least annually in patients who have had type 1 diabetes for at least 5 years and in all patients with type 2 diabetes.2 Monitoring is needed twice a year for those who begin to show signs of albuminuria or a reduced eGFR. This helps define the presence or stage of CKD and allows for further treatment planning.
Notably, patients with an eGFR < 30 ml/min/1.73m2, an unclear cause of kidney disease, or signs of rapidly progressive disease (eg, decline in GFR category plus ≥ 25% decline in eGFR from baseline) should be seen by nephrology for further evaluation and treatment recommendations.2,36
Diabetes medications for kidney health. Sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagon-like peptide 1 (GLP-1) receptor agonists may be good candidates to promote kidney health in patients such as Mr. W. Recent trials show that SGLT2 inhibitors reduce the risk for progressive diabetic kidney disease, and the ADA recommends these medications for patients with CKD.2,16,36 GLP-1 receptor agonists also may be associated with a lower rate of development and progression of diabetic kidney disease, but this effect appears to be less robust.7,15,16 ADA guidelines recommend SGLT2 inhibitors for patients whose eGFR is adequate.37
ADA and AACE guidelines offer specific treatment recommendations on the use of SGLT2 inhibitors and GLP-1 receptor agonists in the management of diabetes.10,37 Note that neither SGLT2 inhibitors nor GLP-1 agonists are strictly under the purview of endocrinologists. Rather, multiple guidelines state that they can be utilized safely by a variety of practitioners.6,38,39
In the concluding part of this series, we will explore how to screen for peripheral neuropathy and diabetic retinopathy—identification of which can improve the patient’s quality of life.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
Previously, we discussed assessment and treatment for dyslipidemia in patients with diabetes. Now we’ll explore how to monitor for kidney disease in this population.
CASE CONTINUED
Mr. W’s basic metabolic panel includes an estimated glomerular filtration rate (eGFR) of 55 ml/min/1.73 m2 (reference range, > 60 ml/min/1.73 m2). In the absence of any other markers of kidney disease, you obtain a spot urinary albumin-to-creatinine ratio (UACR). The UACR results show a ratio of 64 mg/g, confirming stage 3 chronic kidney disease (CKD).
Monitoring for Chronic Kidney Disease
CKD is characterized by persistent albuminuria, low eGFR, and manifestations of kidney damage, and it increases cardiovascular risk.2 According to the ADA, clinicians should obtain a UACR and eGFR at least annually in patients who have had type 1 diabetes for at least 5 years and in all patients with type 2 diabetes.2 Monitoring is needed twice a year for those who begin to show signs of albuminuria or a reduced eGFR. This helps define the presence or stage of CKD and allows for further treatment planning.
Notably, patients with an eGFR < 30 ml/min/1.73m2, an unclear cause of kidney disease, or signs of rapidly progressive disease (eg, decline in GFR category plus ≥ 25% decline in eGFR from baseline) should be seen by nephrology for further evaluation and treatment recommendations.2,36
Diabetes medications for kidney health. Sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagon-like peptide 1 (GLP-1) receptor agonists may be good candidates to promote kidney health in patients such as Mr. W. Recent trials show that SGLT2 inhibitors reduce the risk for progressive diabetic kidney disease, and the ADA recommends these medications for patients with CKD.2,16,36 GLP-1 receptor agonists also may be associated with a lower rate of development and progression of diabetic kidney disease, but this effect appears to be less robust.7,15,16 ADA guidelines recommend SGLT2 inhibitors for patients whose eGFR is adequate.37
ADA and AACE guidelines offer specific treatment recommendations on the use of SGLT2 inhibitors and GLP-1 receptor agonists in the management of diabetes.10,37 Note that neither SGLT2 inhibitors nor GLP-1 agonists are strictly under the purview of endocrinologists. Rather, multiple guidelines state that they can be utilized safely by a variety of practitioners.6,38,39
In the concluding part of this series, we will explore how to screen for peripheral neuropathy and diabetic retinopathy—identification of which can improve the patient’s quality of life.
Previously, we discussed assessment and treatment for dyslipidemia in patients with diabetes. Now we’ll explore how to monitor for kidney disease in this population.
CASE CONTINUED
Mr. W’s basic metabolic panel includes an estimated glomerular filtration rate (eGFR) of 55 ml/min/1.73 m2 (reference range, > 60 ml/min/1.73 m2). In the absence of any other markers of kidney disease, you obtain a spot urinary albumin-to-creatinine ratio (UACR). The UACR results show a ratio of 64 mg/g, confirming stage 3 chronic kidney disease (CKD).
Monitoring for Chronic Kidney Disease
CKD is characterized by persistent albuminuria, low eGFR, and manifestations of kidney damage, and it increases cardiovascular risk.2 According to the ADA, clinicians should obtain a UACR and eGFR at least annually in patients who have had type 1 diabetes for at least 5 years and in all patients with type 2 diabetes.2 Monitoring is needed twice a year for those who begin to show signs of albuminuria or a reduced eGFR. This helps define the presence or stage of CKD and allows for further treatment planning.
Notably, patients with an eGFR < 30 ml/min/1.73m2, an unclear cause of kidney disease, or signs of rapidly progressive disease (eg, decline in GFR category plus ≥ 25% decline in eGFR from baseline) should be seen by nephrology for further evaluation and treatment recommendations.2,36
Diabetes medications for kidney health. Sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagon-like peptide 1 (GLP-1) receptor agonists may be good candidates to promote kidney health in patients such as Mr. W. Recent trials show that SGLT2 inhibitors reduce the risk for progressive diabetic kidney disease, and the ADA recommends these medications for patients with CKD.2,16,36 GLP-1 receptor agonists also may be associated with a lower rate of development and progression of diabetic kidney disease, but this effect appears to be less robust.7,15,16 ADA guidelines recommend SGLT2 inhibitors for patients whose eGFR is adequate.37
ADA and AACE guidelines offer specific treatment recommendations on the use of SGLT2 inhibitors and GLP-1 receptor agonists in the management of diabetes.10,37 Note that neither SGLT2 inhibitors nor GLP-1 agonists are strictly under the purview of endocrinologists. Rather, multiple guidelines state that they can be utilized safely by a variety of practitioners.6,38,39
In the concluding part of this series, we will explore how to screen for peripheral neuropathy and diabetic retinopathy—identification of which can improve the patient’s quality of life.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
SGLT2 inhibitors, developed for T2D, now ‘belong to cardiologists and nephrologists’
It’s passé to think of the sodium-glucose cotransporter 2 (SGLT2) inhibitor drugs as agents that primarily treat hyperglycemia because their major clinical role has rapidly morphed into treating or preventing heart failure and chronic kidney disease.
This change suddenly thrust primary responsibility for prescribing these drug into the hands of cardiologists and nephrologists, though endocrinologists, diabetologists, and primary care physicians remain in the prescribing mix, experts agreed at the virtual annual scientific sessions of the American Diabetes Association.
“Glucose lowering plays little or no role in the cardiorenal protection from drugs in the sodium-glucose cotransporter 2 inhibitor class,” said David Z. Cherney, MD, a nephrologist and professor of medicine at the University of Toronto.
The SGLT2 inhibitor drugs “belong to cardiologists and nephrologists,” declared endocrinologist Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of The Metabolic Institute of America in Tarzana, Calif.
But therein lies a problem. “Cardiologists and nephrologists often say that they don’t want to start SGLT2 inhibitors because they do not want to interfere with the glucose reducing medications a patient takes,” Dr. Cherney added.
“Cardiologists are absolutely afraid to prescribe SGLT2 inhibitors,” claimed John J.V. McMurray MD, a professor of medical cardiology at the University of Glasgow. “Cardiologists need to talk with diabetologists about the importance of treating heart failure” in patients with type 2 diabetes (T2D), and diabetologists “need to help cardiologists understand how to use these and other effective glucose-lowering drugs that reduce cardiovascular disease risk,” said Dr. McMurray during the ADA sessions.
“I don’t think any medical specialty owns this drug class,” said Silvio E. Inzucchi, MD, professor of medicine at Yale University, New Haven, Conn., and director of the Yale Medicine Diabetes Center. “No permission is needed” from an endocrinologist for another specialist to prescribe an SGLT2 inhibitor to patients with T2D or to appropriate patients without diabetes, he maintained.
The need for greater involvement by cardiologists in prescribing SGLT2 inhibitors to patients with T2D was underscored in findings recently reported by Dr. Inzucchi and associates. They analyzed the physician encounters that patients with T2D had with cardiologists and endocrinologists during 2017 at two U.S. health systems: one centered around clinicians affiliated with Yale Medicine and Yale University, and a second with clinicians drawn from the staffs of the Saint Luke’s Health System, including Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
During 2017, the two systems has outpatient encounters with 109,747 patients with T2D, who averaged 67 years of age and were roughly evenly split between women and men: 43% had prevalent cardiovascular disease, including 30% with coronary artery disease and 15% with heart failure. These patients had more than 110,000 physician visits, and the number of these consultations with a cardiologist was double the number with an endocrinologist, Dr. Inzucchi and associates recently reported (Cardiovasc Endocrinol Metab. 2020 Jun;9[2]:56-9).
Among the 30% of T2D patients with prevalent cardiovascular disease, the consultation rate with a cardiologist was four times greater than with an endocrinologist; among the 15% with heart failure, a visit with a cardiologist was nearly seven times more common that with an endocrinologist.
“Based on these data, cardiovascular specialists encouraging the use of these medications, or, if comfortable, actually prescribing these medications, would likely significantly hasten the adoption of evidence-based glucose-lowering therapies in those patients most apt to benefit from them,” concluded the study’s authors.
Dr. Cherney has been a consultant to or has received honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Mitsubishi Tanabe Pharma, and Sanofi. Dr. Handelsman has been a consultant to or speaker on behalf of Amarin, Amgen, Applied Therapeutic, AstraZeneca, Boehringer Ingelheim, Esperion, Gilead, Janssen, Merck, Merck-Pfizer, Novo Nordisk, Regeneron, and Sanofi. Dr. McMurray’s employer, the University of Glasgow, received payments from AstraZeneca for his involvement in trials involving dapagliflozin. Dr. Inzucchi has been a consultant to or helped run trials for Abbott, AstraZeneca, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics.
It’s passé to think of the sodium-glucose cotransporter 2 (SGLT2) inhibitor drugs as agents that primarily treat hyperglycemia because their major clinical role has rapidly morphed into treating or preventing heart failure and chronic kidney disease.
This change suddenly thrust primary responsibility for prescribing these drug into the hands of cardiologists and nephrologists, though endocrinologists, diabetologists, and primary care physicians remain in the prescribing mix, experts agreed at the virtual annual scientific sessions of the American Diabetes Association.
“Glucose lowering plays little or no role in the cardiorenal protection from drugs in the sodium-glucose cotransporter 2 inhibitor class,” said David Z. Cherney, MD, a nephrologist and professor of medicine at the University of Toronto.
The SGLT2 inhibitor drugs “belong to cardiologists and nephrologists,” declared endocrinologist Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of The Metabolic Institute of America in Tarzana, Calif.
But therein lies a problem. “Cardiologists and nephrologists often say that they don’t want to start SGLT2 inhibitors because they do not want to interfere with the glucose reducing medications a patient takes,” Dr. Cherney added.
“Cardiologists are absolutely afraid to prescribe SGLT2 inhibitors,” claimed John J.V. McMurray MD, a professor of medical cardiology at the University of Glasgow. “Cardiologists need to talk with diabetologists about the importance of treating heart failure” in patients with type 2 diabetes (T2D), and diabetologists “need to help cardiologists understand how to use these and other effective glucose-lowering drugs that reduce cardiovascular disease risk,” said Dr. McMurray during the ADA sessions.
“I don’t think any medical specialty owns this drug class,” said Silvio E. Inzucchi, MD, professor of medicine at Yale University, New Haven, Conn., and director of the Yale Medicine Diabetes Center. “No permission is needed” from an endocrinologist for another specialist to prescribe an SGLT2 inhibitor to patients with T2D or to appropriate patients without diabetes, he maintained.
The need for greater involvement by cardiologists in prescribing SGLT2 inhibitors to patients with T2D was underscored in findings recently reported by Dr. Inzucchi and associates. They analyzed the physician encounters that patients with T2D had with cardiologists and endocrinologists during 2017 at two U.S. health systems: one centered around clinicians affiliated with Yale Medicine and Yale University, and a second with clinicians drawn from the staffs of the Saint Luke’s Health System, including Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
During 2017, the two systems has outpatient encounters with 109,747 patients with T2D, who averaged 67 years of age and were roughly evenly split between women and men: 43% had prevalent cardiovascular disease, including 30% with coronary artery disease and 15% with heart failure. These patients had more than 110,000 physician visits, and the number of these consultations with a cardiologist was double the number with an endocrinologist, Dr. Inzucchi and associates recently reported (Cardiovasc Endocrinol Metab. 2020 Jun;9[2]:56-9).
Among the 30% of T2D patients with prevalent cardiovascular disease, the consultation rate with a cardiologist was four times greater than with an endocrinologist; among the 15% with heart failure, a visit with a cardiologist was nearly seven times more common that with an endocrinologist.
“Based on these data, cardiovascular specialists encouraging the use of these medications, or, if comfortable, actually prescribing these medications, would likely significantly hasten the adoption of evidence-based glucose-lowering therapies in those patients most apt to benefit from them,” concluded the study’s authors.
Dr. Cherney has been a consultant to or has received honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Mitsubishi Tanabe Pharma, and Sanofi. Dr. Handelsman has been a consultant to or speaker on behalf of Amarin, Amgen, Applied Therapeutic, AstraZeneca, Boehringer Ingelheim, Esperion, Gilead, Janssen, Merck, Merck-Pfizer, Novo Nordisk, Regeneron, and Sanofi. Dr. McMurray’s employer, the University of Glasgow, received payments from AstraZeneca for his involvement in trials involving dapagliflozin. Dr. Inzucchi has been a consultant to or helped run trials for Abbott, AstraZeneca, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics.
It’s passé to think of the sodium-glucose cotransporter 2 (SGLT2) inhibitor drugs as agents that primarily treat hyperglycemia because their major clinical role has rapidly morphed into treating or preventing heart failure and chronic kidney disease.
This change suddenly thrust primary responsibility for prescribing these drug into the hands of cardiologists and nephrologists, though endocrinologists, diabetologists, and primary care physicians remain in the prescribing mix, experts agreed at the virtual annual scientific sessions of the American Diabetes Association.
“Glucose lowering plays little or no role in the cardiorenal protection from drugs in the sodium-glucose cotransporter 2 inhibitor class,” said David Z. Cherney, MD, a nephrologist and professor of medicine at the University of Toronto.
The SGLT2 inhibitor drugs “belong to cardiologists and nephrologists,” declared endocrinologist Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of The Metabolic Institute of America in Tarzana, Calif.
But therein lies a problem. “Cardiologists and nephrologists often say that they don’t want to start SGLT2 inhibitors because they do not want to interfere with the glucose reducing medications a patient takes,” Dr. Cherney added.
“Cardiologists are absolutely afraid to prescribe SGLT2 inhibitors,” claimed John J.V. McMurray MD, a professor of medical cardiology at the University of Glasgow. “Cardiologists need to talk with diabetologists about the importance of treating heart failure” in patients with type 2 diabetes (T2D), and diabetologists “need to help cardiologists understand how to use these and other effective glucose-lowering drugs that reduce cardiovascular disease risk,” said Dr. McMurray during the ADA sessions.
“I don’t think any medical specialty owns this drug class,” said Silvio E. Inzucchi, MD, professor of medicine at Yale University, New Haven, Conn., and director of the Yale Medicine Diabetes Center. “No permission is needed” from an endocrinologist for another specialist to prescribe an SGLT2 inhibitor to patients with T2D or to appropriate patients without diabetes, he maintained.
The need for greater involvement by cardiologists in prescribing SGLT2 inhibitors to patients with T2D was underscored in findings recently reported by Dr. Inzucchi and associates. They analyzed the physician encounters that patients with T2D had with cardiologists and endocrinologists during 2017 at two U.S. health systems: one centered around clinicians affiliated with Yale Medicine and Yale University, and a second with clinicians drawn from the staffs of the Saint Luke’s Health System, including Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
During 2017, the two systems has outpatient encounters with 109,747 patients with T2D, who averaged 67 years of age and were roughly evenly split between women and men: 43% had prevalent cardiovascular disease, including 30% with coronary artery disease and 15% with heart failure. These patients had more than 110,000 physician visits, and the number of these consultations with a cardiologist was double the number with an endocrinologist, Dr. Inzucchi and associates recently reported (Cardiovasc Endocrinol Metab. 2020 Jun;9[2]:56-9).
Among the 30% of T2D patients with prevalent cardiovascular disease, the consultation rate with a cardiologist was four times greater than with an endocrinologist; among the 15% with heart failure, a visit with a cardiologist was nearly seven times more common that with an endocrinologist.
“Based on these data, cardiovascular specialists encouraging the use of these medications, or, if comfortable, actually prescribing these medications, would likely significantly hasten the adoption of evidence-based glucose-lowering therapies in those patients most apt to benefit from them,” concluded the study’s authors.
Dr. Cherney has been a consultant to or has received honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Mitsubishi Tanabe Pharma, and Sanofi. Dr. Handelsman has been a consultant to or speaker on behalf of Amarin, Amgen, Applied Therapeutic, AstraZeneca, Boehringer Ingelheim, Esperion, Gilead, Janssen, Merck, Merck-Pfizer, Novo Nordisk, Regeneron, and Sanofi. Dr. McMurray’s employer, the University of Glasgow, received payments from AstraZeneca for his involvement in trials involving dapagliflozin. Dr. Inzucchi has been a consultant to or helped run trials for Abbott, AstraZeneca, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics.
FROM ADA 2020
Guidance addresses elders with diabetes during COVID-19
Two experts in geriatric diabetes are offering some contemporary practical recommendations for diabetes management in older adults during the COVID-19 pandemic.
The viewpoint, entitled, “Caring for Older Adults With Diabetes During the COVID-19 Pandemic,” was published online in JAMA Internal Medicine by Medha N. Munshi, MD, director of the geriatrics program at the Joslin Diabetes Center, Boston, and Sarah L. Sy, MD, a geriatrician in the same program.
Adults aged 70 years and older with comorbidities such as diabetes are among those at highest risk for adverse outcomes and mortality due to COVID-19.
At the same time, those who don’t have the illness face major challenges in avoiding it, including disruptions in normal activities and barriers to receiving health care.
Although telemedicine has become much more widely adopted in diabetes management since the pandemic began, older adults may not be as tech savvy, may not have computer or Internet access, and/or may have cognitive dysfunction that precludes its use.
“These unprecedented times pose a great challenge to this heterogeneous population with varying levels of complexity, frailty, and multimorbidity,” Munshi and Sy point out, noting that “clinicians can lessen the load by guiding, reassuring, and supporting them through this pandemic time.”
Because the pandemic could last for several months longer, the authors offer the following advice for clinicians who care for older adults with diabetes.
- Accessibility to health care: When possible, use telemedicine, diabetes care apps, or platforms to obtain data from glucose meters, continuous glucose monitors, and/or pumps. When use of technology isn’t possible, schedule telephone appointments and have the patient or caregiver read the glucose values.
- Multicomplexity and geriatric syndromes: Identify high-risk patients, such as those with or recurrent , and prioritize patient goals. If appropriate, simplify the diabetes treatment plan and reinforce with repeated education and instructions. Glucose goals may need to be liberalized. Advise patients to stay hydrated to minimize the risk of dehydration and falls. Take steps to avoid hypoglycemia, reduce polypharmacy, and consolidate medication doses.
- Burden of diabetes self-care: Bloodwork for can be delayed by a few months. Patients with can decrease the frequency of blood glucose checks if their glucose levels are generally within acceptable range. Encourage patients to eat healthily with regular meals rather than optimizing the diet for glucose levels, and adjust medications for any changes in diet. Advise safe options for physical activity such as walking inside the home or walking in place for 10 minutes, three times per day, and incorporating strength training, such as with resistance bands. Online exercise programs are another option.
- Psychological stress: Check in with patients and encourage them to stay as connected as possible using technology (phone, video chat, text message), letters, or cards with family, friends, and/or religious communities. Screen for , using either the Geriatric Depression Scale or Patient Health Questionnaire-2, and refer to mental health colleagues if appropriate. Speak or email with caregivers to assess the patient’s mental health state and offer local support resources, if needed.
- Medication and equipment issues: Refill 90-day prescriptions and equipment, and request mail or home (contactless) delivery. Patients should also have backups in case of equipment failures, such as syringes and long-acting insulin in case of pump failure, and test strips/meter for continuous glucose monitor problems.
Munshi and Sy conclude: “Many of the recommendations presented in this article are practical and will continue to be relevant after COVID-19. When this is all over, patients will remember how we made them feel, and how we kept them safe and healthy at home.”
Munshi is a consultant for Sanofi and Lilly. Sy has reported no relevant financial relationships.
This article first appeared on Medscape.com.
Two experts in geriatric diabetes are offering some contemporary practical recommendations for diabetes management in older adults during the COVID-19 pandemic.
The viewpoint, entitled, “Caring for Older Adults With Diabetes During the COVID-19 Pandemic,” was published online in JAMA Internal Medicine by Medha N. Munshi, MD, director of the geriatrics program at the Joslin Diabetes Center, Boston, and Sarah L. Sy, MD, a geriatrician in the same program.
Adults aged 70 years and older with comorbidities such as diabetes are among those at highest risk for adverse outcomes and mortality due to COVID-19.
At the same time, those who don’t have the illness face major challenges in avoiding it, including disruptions in normal activities and barriers to receiving health care.
Although telemedicine has become much more widely adopted in diabetes management since the pandemic began, older adults may not be as tech savvy, may not have computer or Internet access, and/or may have cognitive dysfunction that precludes its use.
“These unprecedented times pose a great challenge to this heterogeneous population with varying levels of complexity, frailty, and multimorbidity,” Munshi and Sy point out, noting that “clinicians can lessen the load by guiding, reassuring, and supporting them through this pandemic time.”
Because the pandemic could last for several months longer, the authors offer the following advice for clinicians who care for older adults with diabetes.
- Accessibility to health care: When possible, use telemedicine, diabetes care apps, or platforms to obtain data from glucose meters, continuous glucose monitors, and/or pumps. When use of technology isn’t possible, schedule telephone appointments and have the patient or caregiver read the glucose values.
- Multicomplexity and geriatric syndromes: Identify high-risk patients, such as those with or recurrent , and prioritize patient goals. If appropriate, simplify the diabetes treatment plan and reinforce with repeated education and instructions. Glucose goals may need to be liberalized. Advise patients to stay hydrated to minimize the risk of dehydration and falls. Take steps to avoid hypoglycemia, reduce polypharmacy, and consolidate medication doses.
- Burden of diabetes self-care: Bloodwork for can be delayed by a few months. Patients with can decrease the frequency of blood glucose checks if their glucose levels are generally within acceptable range. Encourage patients to eat healthily with regular meals rather than optimizing the diet for glucose levels, and adjust medications for any changes in diet. Advise safe options for physical activity such as walking inside the home or walking in place for 10 minutes, three times per day, and incorporating strength training, such as with resistance bands. Online exercise programs are another option.
- Psychological stress: Check in with patients and encourage them to stay as connected as possible using technology (phone, video chat, text message), letters, or cards with family, friends, and/or religious communities. Screen for , using either the Geriatric Depression Scale or Patient Health Questionnaire-2, and refer to mental health colleagues if appropriate. Speak or email with caregivers to assess the patient’s mental health state and offer local support resources, if needed.
- Medication and equipment issues: Refill 90-day prescriptions and equipment, and request mail or home (contactless) delivery. Patients should also have backups in case of equipment failures, such as syringes and long-acting insulin in case of pump failure, and test strips/meter for continuous glucose monitor problems.
Munshi and Sy conclude: “Many of the recommendations presented in this article are practical and will continue to be relevant after COVID-19. When this is all over, patients will remember how we made them feel, and how we kept them safe and healthy at home.”
Munshi is a consultant for Sanofi and Lilly. Sy has reported no relevant financial relationships.
This article first appeared on Medscape.com.
Two experts in geriatric diabetes are offering some contemporary practical recommendations for diabetes management in older adults during the COVID-19 pandemic.
The viewpoint, entitled, “Caring for Older Adults With Diabetes During the COVID-19 Pandemic,” was published online in JAMA Internal Medicine by Medha N. Munshi, MD, director of the geriatrics program at the Joslin Diabetes Center, Boston, and Sarah L. Sy, MD, a geriatrician in the same program.
Adults aged 70 years and older with comorbidities such as diabetes are among those at highest risk for adverse outcomes and mortality due to COVID-19.
At the same time, those who don’t have the illness face major challenges in avoiding it, including disruptions in normal activities and barriers to receiving health care.
Although telemedicine has become much more widely adopted in diabetes management since the pandemic began, older adults may not be as tech savvy, may not have computer or Internet access, and/or may have cognitive dysfunction that precludes its use.
“These unprecedented times pose a great challenge to this heterogeneous population with varying levels of complexity, frailty, and multimorbidity,” Munshi and Sy point out, noting that “clinicians can lessen the load by guiding, reassuring, and supporting them through this pandemic time.”
Because the pandemic could last for several months longer, the authors offer the following advice for clinicians who care for older adults with diabetes.
- Accessibility to health care: When possible, use telemedicine, diabetes care apps, or platforms to obtain data from glucose meters, continuous glucose monitors, and/or pumps. When use of technology isn’t possible, schedule telephone appointments and have the patient or caregiver read the glucose values.
- Multicomplexity and geriatric syndromes: Identify high-risk patients, such as those with or recurrent , and prioritize patient goals. If appropriate, simplify the diabetes treatment plan and reinforce with repeated education and instructions. Glucose goals may need to be liberalized. Advise patients to stay hydrated to minimize the risk of dehydration and falls. Take steps to avoid hypoglycemia, reduce polypharmacy, and consolidate medication doses.
- Burden of diabetes self-care: Bloodwork for can be delayed by a few months. Patients with can decrease the frequency of blood glucose checks if their glucose levels are generally within acceptable range. Encourage patients to eat healthily with regular meals rather than optimizing the diet for glucose levels, and adjust medications for any changes in diet. Advise safe options for physical activity such as walking inside the home or walking in place for 10 minutes, three times per day, and incorporating strength training, such as with resistance bands. Online exercise programs are another option.
- Psychological stress: Check in with patients and encourage them to stay as connected as possible using technology (phone, video chat, text message), letters, or cards with family, friends, and/or religious communities. Screen for , using either the Geriatric Depression Scale or Patient Health Questionnaire-2, and refer to mental health colleagues if appropriate. Speak or email with caregivers to assess the patient’s mental health state and offer local support resources, if needed.
- Medication and equipment issues: Refill 90-day prescriptions and equipment, and request mail or home (contactless) delivery. Patients should also have backups in case of equipment failures, such as syringes and long-acting insulin in case of pump failure, and test strips/meter for continuous glucose monitor problems.
Munshi and Sy conclude: “Many of the recommendations presented in this article are practical and will continue to be relevant after COVID-19. When this is all over, patients will remember how we made them feel, and how we kept them safe and healthy at home.”
Munshi is a consultant for Sanofi and Lilly. Sy has reported no relevant financial relationships.
This article first appeared on Medscape.com.
Novel program cuts weight retention after gestational diabetes
An online, lifestyle-based weight loss initiative known as the Balance After Baby (BAB) program is effective at reducing weight retention a year after birth among women with recent gestational diabetes.
Specifically, results of the study were positive in women of most ethnicities, bar those of a small group of Hispanic origin.
Jacinda Nicklas, MD, from the University of Colorado at Denver, Aurora, presented findings of the BAB trial during the virtual annual scientific sessions of the American Diabetes Association. She was coprincipal investigator alongside Ellen Seely, MD, from Brigham and Women’s Hospital, Boston.
“Looking at the entire population of women on the BAB program, there was a trend in weight loss from 6 weeks postpartum to 12 months (P = .09), and significantly less postpartum weight retention at 12 months (P = .04),” Dr. Nicklas said.
“Through this effect on postpartum weight retention, the BAB program has potential to delay or prevent development of type 2 diabetes in women with recent gestational diabetes, while the web-based, remote nature of the program is scalable and very relevant in current times,” she added. “However, the lack of efficacy in Hispanic women means it needs to be modified to be successful in this ethnic group.”
Frank Qian, MD, who also presented during the same session, said the BAB program has potential as a viable way of preventing both future pregnancy complications and the progression to overt type 2 diabetes in this high-risk population.
“Large-scale epidemiologic studies show us that weight gain from pregnancy is a major risk factor for long-term cardiometabolic risk, particularly for women with a history of gestational diabetes,” he observed. “In turn, it is critical to implement lifestyle interventions that can help women get as close to the weight they were before pregnancy as possible and keep that weight off.”
Postpartum weight retention a modifiable risk factor for type 2 diabetes
Current evidence shows that a large proportion of women who develop gestational diabetes go on to develop type 2 diabetes within 10 years and that women with a history of gestational diabetes are more likely to retain or gain weight postpartum.
Dr. Nicklas also pointed out that obesity and weight gain are the strongest modifiable risk factors for type 2 diabetes.
“We know from the Diabetes Prevention Program [DPP] that an intensive lifestyle program in women who had had gestational diabetes led to a 53% reduction in type 2 diabetes,” Dr. Nicklas noted.
However, she added there were barriers to adhering to the intensive DPP program – which required 16 one-on-one meetings in the first 24 weeks – including travel, as some participants lived quite remotely, or family responsibilities. Consequently, Dr. Nicklas and colleagues developed the BAB pilot trial, which involved web-based delivery with remote coaching.
The trial involved women with a history of gestational diabetes who were, on average, 7 weeks postpartum. The key outcome was weight at 12 months, compared with both 6-week postpartum weight and prepregnancy weight.
Based on encouraging results in the pilot trial – in which the intervention group showed significant weight loss from 6-week postpartum weight and in 12-month weight retention – a larger, two-site trial was initiated, the BAB Intervention randomized, controlled trial.
Outcome measures were the same as for the pilot study. The 181 participants were aged 18-45 years, had recent gestational diabetes, and had a mean prepregnancy body mass index of approximately 29 kg/m2. Around half were college educated, and 28% were from lower income households. Overall, 48% were white, 22% Asian, 17% African American, and 13% were of other ethnicities, with just over a third being Hispanic.
The initial study visit was at 6 weeks postpartum. Women were randomized to the behavioral intervention website plus a lifestyle coach group or to a control group that consisted of a website plus knowledge links.
The intervention website required women to complete some DPP-derived and bonus modules, and also featured action plans, tracked weight and steps, and had a direct link to contact their lifestyle coach. Follow-up visits were held at 6 and 12 months and A1c, waist circumference, and height/weight were measured. A total of 86% eligible women completed the 6- and 12-month visits.
Why didn’t the BAB program work in Hispanic women?
“The overall result showed that weight change from 6 weeks postpartum to 12 months revealed a slight gain in the control group of 1.3 pounds and a loss in the intervention group of 1.8 pounds, resulting in a between-group difference of 3.1 pounds [P = .09],” reported Dr. Nicklas. Adjustment for gestational weight gain and breastfeeding had no substantial effect.
When 12-month weight retention versus prepregnancy weight was assessed, the former was halved in participants in the BAB program.
The control group gained a mean of 10.1 pounds, and those in the intervention group gained a mean of 5.3 pounds, equivalent to a difference of 4.8 pounds (P = .04).
A prespecified analysis was conducted of 120 non-Hispanic women. At 12 months, weight retention, compared with prepregnancy weight showed an increase of 9 pounds in the control group versus 1.8 pounds in the intervention group (P = .01).
By comparison, in the small group of Hispanic women only, weight retention at 12 months compared to prepregnancy weight showed a 12.7-pound increase and a 13.3-pound increase in the control and intervention groups respectively, reported Dr. Nicklas.
Addressing the key question of why the BAB program was ineffective in Hispanic women, Dr. Nicklas said, “The literature tells us that low income Hispanic women are twice as likely to experience postpartum weight retention compared to white non-Hispanic women. But we also know that low-income Hispanic women generally engage less with interventions, and there is a higher acceptance of overweight among this ethnic group.”
The researchers hope to follow the women from their trial to determine who progresses to type 2 diabetes.
“Hispanic women are a high-risk population for gestational diabetes and type 2 diabetes, and we plan to identify the best options to help Hispanic women with a history of gestational diabetes prevent type 2 diabetes,” Dr. Nicklas said in an interview.
Dr. Qian also remarked on the differences observed in the weight loss outcomes for non-Hispanic versus Hispanic women, noting that it highlights the importance of studying lifestyle interventions in diverse populations. “Environmental and cultural factors that may differ across different racial or ethnic groups could impact the effectiveness of such interventions.
Dr. Nicklas and Dr. Qian have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
An online, lifestyle-based weight loss initiative known as the Balance After Baby (BAB) program is effective at reducing weight retention a year after birth among women with recent gestational diabetes.
Specifically, results of the study were positive in women of most ethnicities, bar those of a small group of Hispanic origin.
Jacinda Nicklas, MD, from the University of Colorado at Denver, Aurora, presented findings of the BAB trial during the virtual annual scientific sessions of the American Diabetes Association. She was coprincipal investigator alongside Ellen Seely, MD, from Brigham and Women’s Hospital, Boston.
“Looking at the entire population of women on the BAB program, there was a trend in weight loss from 6 weeks postpartum to 12 months (P = .09), and significantly less postpartum weight retention at 12 months (P = .04),” Dr. Nicklas said.
“Through this effect on postpartum weight retention, the BAB program has potential to delay or prevent development of type 2 diabetes in women with recent gestational diabetes, while the web-based, remote nature of the program is scalable and very relevant in current times,” she added. “However, the lack of efficacy in Hispanic women means it needs to be modified to be successful in this ethnic group.”
Frank Qian, MD, who also presented during the same session, said the BAB program has potential as a viable way of preventing both future pregnancy complications and the progression to overt type 2 diabetes in this high-risk population.
“Large-scale epidemiologic studies show us that weight gain from pregnancy is a major risk factor for long-term cardiometabolic risk, particularly for women with a history of gestational diabetes,” he observed. “In turn, it is critical to implement lifestyle interventions that can help women get as close to the weight they were before pregnancy as possible and keep that weight off.”
Postpartum weight retention a modifiable risk factor for type 2 diabetes
Current evidence shows that a large proportion of women who develop gestational diabetes go on to develop type 2 diabetes within 10 years and that women with a history of gestational diabetes are more likely to retain or gain weight postpartum.
Dr. Nicklas also pointed out that obesity and weight gain are the strongest modifiable risk factors for type 2 diabetes.
“We know from the Diabetes Prevention Program [DPP] that an intensive lifestyle program in women who had had gestational diabetes led to a 53% reduction in type 2 diabetes,” Dr. Nicklas noted.
However, she added there were barriers to adhering to the intensive DPP program – which required 16 one-on-one meetings in the first 24 weeks – including travel, as some participants lived quite remotely, or family responsibilities. Consequently, Dr. Nicklas and colleagues developed the BAB pilot trial, which involved web-based delivery with remote coaching.
The trial involved women with a history of gestational diabetes who were, on average, 7 weeks postpartum. The key outcome was weight at 12 months, compared with both 6-week postpartum weight and prepregnancy weight.
Based on encouraging results in the pilot trial – in which the intervention group showed significant weight loss from 6-week postpartum weight and in 12-month weight retention – a larger, two-site trial was initiated, the BAB Intervention randomized, controlled trial.
Outcome measures were the same as for the pilot study. The 181 participants were aged 18-45 years, had recent gestational diabetes, and had a mean prepregnancy body mass index of approximately 29 kg/m2. Around half were college educated, and 28% were from lower income households. Overall, 48% were white, 22% Asian, 17% African American, and 13% were of other ethnicities, with just over a third being Hispanic.
The initial study visit was at 6 weeks postpartum. Women were randomized to the behavioral intervention website plus a lifestyle coach group or to a control group that consisted of a website plus knowledge links.
The intervention website required women to complete some DPP-derived and bonus modules, and also featured action plans, tracked weight and steps, and had a direct link to contact their lifestyle coach. Follow-up visits were held at 6 and 12 months and A1c, waist circumference, and height/weight were measured. A total of 86% eligible women completed the 6- and 12-month visits.
Why didn’t the BAB program work in Hispanic women?
“The overall result showed that weight change from 6 weeks postpartum to 12 months revealed a slight gain in the control group of 1.3 pounds and a loss in the intervention group of 1.8 pounds, resulting in a between-group difference of 3.1 pounds [P = .09],” reported Dr. Nicklas. Adjustment for gestational weight gain and breastfeeding had no substantial effect.
When 12-month weight retention versus prepregnancy weight was assessed, the former was halved in participants in the BAB program.
The control group gained a mean of 10.1 pounds, and those in the intervention group gained a mean of 5.3 pounds, equivalent to a difference of 4.8 pounds (P = .04).
A prespecified analysis was conducted of 120 non-Hispanic women. At 12 months, weight retention, compared with prepregnancy weight showed an increase of 9 pounds in the control group versus 1.8 pounds in the intervention group (P = .01).
By comparison, in the small group of Hispanic women only, weight retention at 12 months compared to prepregnancy weight showed a 12.7-pound increase and a 13.3-pound increase in the control and intervention groups respectively, reported Dr. Nicklas.
Addressing the key question of why the BAB program was ineffective in Hispanic women, Dr. Nicklas said, “The literature tells us that low income Hispanic women are twice as likely to experience postpartum weight retention compared to white non-Hispanic women. But we also know that low-income Hispanic women generally engage less with interventions, and there is a higher acceptance of overweight among this ethnic group.”
The researchers hope to follow the women from their trial to determine who progresses to type 2 diabetes.
“Hispanic women are a high-risk population for gestational diabetes and type 2 diabetes, and we plan to identify the best options to help Hispanic women with a history of gestational diabetes prevent type 2 diabetes,” Dr. Nicklas said in an interview.
Dr. Qian also remarked on the differences observed in the weight loss outcomes for non-Hispanic versus Hispanic women, noting that it highlights the importance of studying lifestyle interventions in diverse populations. “Environmental and cultural factors that may differ across different racial or ethnic groups could impact the effectiveness of such interventions.
Dr. Nicklas and Dr. Qian have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
An online, lifestyle-based weight loss initiative known as the Balance After Baby (BAB) program is effective at reducing weight retention a year after birth among women with recent gestational diabetes.
Specifically, results of the study were positive in women of most ethnicities, bar those of a small group of Hispanic origin.
Jacinda Nicklas, MD, from the University of Colorado at Denver, Aurora, presented findings of the BAB trial during the virtual annual scientific sessions of the American Diabetes Association. She was coprincipal investigator alongside Ellen Seely, MD, from Brigham and Women’s Hospital, Boston.
“Looking at the entire population of women on the BAB program, there was a trend in weight loss from 6 weeks postpartum to 12 months (P = .09), and significantly less postpartum weight retention at 12 months (P = .04),” Dr. Nicklas said.
“Through this effect on postpartum weight retention, the BAB program has potential to delay or prevent development of type 2 diabetes in women with recent gestational diabetes, while the web-based, remote nature of the program is scalable and very relevant in current times,” she added. “However, the lack of efficacy in Hispanic women means it needs to be modified to be successful in this ethnic group.”
Frank Qian, MD, who also presented during the same session, said the BAB program has potential as a viable way of preventing both future pregnancy complications and the progression to overt type 2 diabetes in this high-risk population.
“Large-scale epidemiologic studies show us that weight gain from pregnancy is a major risk factor for long-term cardiometabolic risk, particularly for women with a history of gestational diabetes,” he observed. “In turn, it is critical to implement lifestyle interventions that can help women get as close to the weight they were before pregnancy as possible and keep that weight off.”
Postpartum weight retention a modifiable risk factor for type 2 diabetes
Current evidence shows that a large proportion of women who develop gestational diabetes go on to develop type 2 diabetes within 10 years and that women with a history of gestational diabetes are more likely to retain or gain weight postpartum.
Dr. Nicklas also pointed out that obesity and weight gain are the strongest modifiable risk factors for type 2 diabetes.
“We know from the Diabetes Prevention Program [DPP] that an intensive lifestyle program in women who had had gestational diabetes led to a 53% reduction in type 2 diabetes,” Dr. Nicklas noted.
However, she added there were barriers to adhering to the intensive DPP program – which required 16 one-on-one meetings in the first 24 weeks – including travel, as some participants lived quite remotely, or family responsibilities. Consequently, Dr. Nicklas and colleagues developed the BAB pilot trial, which involved web-based delivery with remote coaching.
The trial involved women with a history of gestational diabetes who were, on average, 7 weeks postpartum. The key outcome was weight at 12 months, compared with both 6-week postpartum weight and prepregnancy weight.
Based on encouraging results in the pilot trial – in which the intervention group showed significant weight loss from 6-week postpartum weight and in 12-month weight retention – a larger, two-site trial was initiated, the BAB Intervention randomized, controlled trial.
Outcome measures were the same as for the pilot study. The 181 participants were aged 18-45 years, had recent gestational diabetes, and had a mean prepregnancy body mass index of approximately 29 kg/m2. Around half were college educated, and 28% were from lower income households. Overall, 48% were white, 22% Asian, 17% African American, and 13% were of other ethnicities, with just over a third being Hispanic.
The initial study visit was at 6 weeks postpartum. Women were randomized to the behavioral intervention website plus a lifestyle coach group or to a control group that consisted of a website plus knowledge links.
The intervention website required women to complete some DPP-derived and bonus modules, and also featured action plans, tracked weight and steps, and had a direct link to contact their lifestyle coach. Follow-up visits were held at 6 and 12 months and A1c, waist circumference, and height/weight were measured. A total of 86% eligible women completed the 6- and 12-month visits.
Why didn’t the BAB program work in Hispanic women?
“The overall result showed that weight change from 6 weeks postpartum to 12 months revealed a slight gain in the control group of 1.3 pounds and a loss in the intervention group of 1.8 pounds, resulting in a between-group difference of 3.1 pounds [P = .09],” reported Dr. Nicklas. Adjustment for gestational weight gain and breastfeeding had no substantial effect.
When 12-month weight retention versus prepregnancy weight was assessed, the former was halved in participants in the BAB program.
The control group gained a mean of 10.1 pounds, and those in the intervention group gained a mean of 5.3 pounds, equivalent to a difference of 4.8 pounds (P = .04).
A prespecified analysis was conducted of 120 non-Hispanic women. At 12 months, weight retention, compared with prepregnancy weight showed an increase of 9 pounds in the control group versus 1.8 pounds in the intervention group (P = .01).
By comparison, in the small group of Hispanic women only, weight retention at 12 months compared to prepregnancy weight showed a 12.7-pound increase and a 13.3-pound increase in the control and intervention groups respectively, reported Dr. Nicklas.
Addressing the key question of why the BAB program was ineffective in Hispanic women, Dr. Nicklas said, “The literature tells us that low income Hispanic women are twice as likely to experience postpartum weight retention compared to white non-Hispanic women. But we also know that low-income Hispanic women generally engage less with interventions, and there is a higher acceptance of overweight among this ethnic group.”
The researchers hope to follow the women from their trial to determine who progresses to type 2 diabetes.
“Hispanic women are a high-risk population for gestational diabetes and type 2 diabetes, and we plan to identify the best options to help Hispanic women with a history of gestational diabetes prevent type 2 diabetes,” Dr. Nicklas said in an interview.
Dr. Qian also remarked on the differences observed in the weight loss outcomes for non-Hispanic versus Hispanic women, noting that it highlights the importance of studying lifestyle interventions in diverse populations. “Environmental and cultural factors that may differ across different racial or ethnic groups could impact the effectiveness of such interventions.
Dr. Nicklas and Dr. Qian have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ADA 2020
Good for profits, good for patients: A new form of medical visits
Ten patients smiled and waved out on the computer monitor, as Jacob Mirsky, MD, greeted each one, asked them to introduce themselves, and inquired as to how each was doing with their stress reduction tactics.
The attendees of the online session had been patients at in-person group visits at the Massachusetts General Hospital Revere HealthCare Center. But those in-person group sessions, known as shared medical appointments (SMAs), were shut down when COVID-19 arrived.
“Our group patients have been missing the sessions,” said Dr. Mirsky, a general internist who codirects the center’s group visit program. The online sessions, called virtual SMAs (V-SMAs), work well with COVID-19 social distancing.
In the group sessions, Dr. Mirsky reads a standardized message that addresses privacy concerns during the session. For the next 60-90 minutes, “we ask them to talk about what has gone well for them and what they are struggling with,” he said. “Then I answer their questions using materials in a PowerPoint to address key points, such as reducing salt for high blood pressure or interpreting blood sugar levels for diabetes.
“I try to end group sessions with one area of focus,” Dr. Mirsky said. “In the stress reduction group, this could be meditation. In the diabetes group, it could be a discussion on weight loss.” Then the program’s health coach goes over some key concepts on behavior change and invites participants to contact her after the session.
“The nice thing is that these virtual sessions are fully reimbursable by all of our insurers in Massachusetts,” Dr. Mirsky said. Through evaluation and management (E/M) codes, each patient in a group visit is paid the same as a patient in an individual visit with the same level of complexity.
Dr. Mirsky writes a note in the chart about each patient who was in the group session. “This includes information about the specific patient, such as the history and physical, and information about the group meeting,” he said. In the next few months, the center plans to put its other group sessions online – on blood pressure, obesity, diabetes, and insomnia.
Attracting doctors who hadn’t done groups before
said Marianne Sumego, MD, director of the Cleveland Clinic’s SMA program, which began 21 years ago.
In this era of COVID-19, group visits have either switched to V-SMAs or halted. However, the COVID-19 crisis has given group visits a second wind. Some doctors who never used SMAs before are now trying out this new mode of patient engagement,
Many of the 100 doctors using SMAs at the Cleveland Clinic have switched over to V-SMAs for now, and the new mode is also attracting colleagues who are new to SMAs, she said.
“When doctors started using telemedicine, virtual group visits started making sense to them,” Dr. Sumego said. “This is a time of a great deal of experimentation in practice design.”
Indeed, V-SMAs have eliminated some problems that had discouraged doctors from trying SMAs, said Amy Wheeler, MD, a general internist who founded the Revere SMA program and codirects it with Dr. Mirsky.
V-SMAs eliminate the need for a large space to hold sessions and reduce the number of staff needed to run sessions, Dr. Wheeler said. “Virtual group visits can actually be easier to use than in-person group visits.”
Dr. Sumego believes small practices in particular will take up V-SMAs because they are easier to run than regular SMAs. “Necessity drives change,” she said. “Across the country everyone is looking at the virtual group model.”
Group visits can help your bottom line
Medicare and many private payers cover group visits. In most cases, they tend to pay the same rate as for an individual office visit. As with telehealth, Medicare and many other payers are temporarily reimbursing for virtual visits at the same rate as for real visits.
Not all payers have a stated policy about covering SMAs, and physicians have to ask. The Centers for Medicare & Medicaid Services, for example, has not published any coding rules on SMAs. But in response to a query by the American Academy of Family Physicians, CMS said it would allow use of CPT codes for E/M services for individual patients.
Blue Cross Blue Shield of North Carolina is one of the few payers with a clearly stated policy on its website. Like Medicare, the insurer accepts E/M codes, and it requires that patients’ attendance must be voluntary; they must be established patients; and the visit must be specific to a disease or condition, although several conditions are allowed.
Dr. Mirsky said his group uses the same E/M level – 99213 – for all of his SMA patients. “Since a regular primary care visit is usually billed at a level 3 or 4, depending on how many topics are covered, we chose level 3 for groups, because the group session deals with just one topic.”
One challenge for billing for SMAs is that most health insurers require patients to provide a copay for each visit, which can discourage patients in groups that meet frequently, says Wayne Dysinger, MD, founder of Lifestyle Medical Solutions, a two-physician primary care practice in Riverside, Calif.
But Dr. Dysinger, who has been using SMAs for 5 years, usually doesn’t have to worry about copays because much of his work is capitated and doesn’t require a copay.
Also, some of Dr. Dysinger’s SMA patients are in direct primary care, in which the patients pay an $18 monthly membership fee. Other practices may charge a flat out-of-pocket fee.
How group visits operate
SMAs are based on the observation that patients with the same condition generally ask their doctor the same questions, and rather than repeat the answers each time, why not provide them to a group?
Dr. Wheeler said trying to be more efficient with her time was the primary reason she became interested in SMAs a dozen years ago. “I was trying to squeeze the advice patients needed into a normal patient visit, and it wasn’t working. When I tried to tell them everything they needed to know, I’d run behind for the rest of my day’s visits.”
She found she was continually repeating the same conversation with patients, but these talks weren’t detailed enough to be effective. “When my weight loss patients came back for the next appointment, they had not made the recommended changes in lifestyle. I started to realize how complicated weight loss was.” So Dr. Wheeler founded the SMA program at the Revere Center.
Doctors enjoy the patient interaction
Some doctors who use SMAs talk about how connected they feel with their patients. “For me, the group sessions are the most gratifying part of the week,” Dr. Dysinger says. “I like to see the patients interacting with me and with each other, and watch their health behavior change over time.”
“These groups have a great deal of energy,” he said. “They have a kind of vulnerability that is very raw, very human. People make commitments to meet goals. Will they meet them or not?”
Dr. Dysinger’s enthusiasm has been echoed by other doctors. In a study of older patients, physicians who used SMAs were more satisfied with care than physicians who relied on standard one-to-one interactions. In another study, the researchers surmised that, in SMAs, doctors learn from their patients how they can better meet their needs.
Dr. Dysinger thinks SMAs are widely applicable in primary care. He estimates that 80%-85% of appointments at a primary care practice involve chronic diseases, and this type of patient is a good fit for group visits. SMAs typically treat patients with diabetes, asthma, arthritis, and obesity.
Dr. Sumego said SMAs are used for specialty care at Cleveland Clinic, such as to help patients before and after bariatric surgery. SMAs have also been used to treat patients with ulcerative colitis, multiple sclerosis, cancer, HIV, menopause, insomnia, and stress, according to one report.
Dr. Dysinger, who runs a small practice, organizes his group sessions somewhat differently. He doesn’t organize his groups around conditions like diabetes, but instead his groups focus on four “pillars” of lifestyle medicine: nourishment, movement, resilience (involving sleep and stress), and connectedness.
Why patients like group visits
Feeling part of a whole is a major draw for many patients. “Patients seem to like committing to something bigger than just themselves,” Dr. Wheeler said. “They enjoy the sense of community that groups have, the joy of supporting one another.”
“It’s feeling that you’re not alone,” Dr. Mirsky said. “When a patient struggling with diabetes hears how hard it is for another patient, it validates their experience and gives them someone to connect with. There is a positive peer pressure.”
Many programs, including Dr. Wheeler’s and Dr. Mirsky’s in Boston, allow patients to drop in and out of sessions, rather than attending one course all the way through. But even under this format, Dr. Wheeler said that patients often tend to stick together. “At the end of a session, one patient asks another: ‘Which session do you want to go to next?’ ” she said.
Patients also learn from each other in SMAs. Patients exchange experiences and share advice they may not have had the chance to get during an individual visit.
The group dynamic can make it easier for some patients to reveal sensitive information, said Dr. Dysinger. “In these groups, people feel free to talk about their bowel movements, or about having to deal with the influence of a parent on their lives,” Dr. Dysinger said. “The sessions can have the feel of an [Alcoholics Anonymous] meeting, but they’re firmly grounded in medicine.”
Potential downsides of virtual group visits
SMAs and VSMAs may not work for every practice. Some small practices may not have enough patients to organize a group visit around a particular condition – even a common one like diabetes. In a presentation before the Society of General Internal Medicine, a physician from the Medical University of South Carolina, Charleston, warned that it may be difficult for a practice to fill diabetes group visits every year.
Additionally, some patients don’t want to talk about personal matters in a group. “They may not want to reveal certain things about themselves,” Dr. Mirsky said. “So I tell the group that if there is anything that anyone wants to talk about in private, I’m available.”
Another drawback of SMAs is that more experienced patients may have to slog through information they already know, which is a particular problem when patients can drop in and out of sessions. Dr. Mirsky noted that “what often ends up happening is that the experienced participant helps the newcomer.”
Finally, confidentially is a big concern in a group session. “In a one-on-one visit, you can go into details about the patient’s health, and even bring up an entry in the chart,” Dr. Wheeler said. “But in a group visit, you can’t raise any personal details about a patient unless the patient brings it up first.”
SMA patients sign confidentiality agreements in which they agree not to talk about other patients outside the session. Ensuring confidentiality becomes more complicated in virtual group visits, because someone located in the room near a participant could overhear the conversation. For this reason, patients in V-SMAs are advised to use headphones or, at a minimum, close the door to the room they are in.
To address privacy concerns, Zoom encrypts its data, but some privacy breeches have been reported, and a U.S. senator has been looking into Zoom’s privacy vulnerabilities.
Transferring groups to virtual groups
It took the COVID-19 crisis for most doctors to take up virtual SMAs. Dr. Sumego said that the Cleveland Clinic started virtual SMAs more than a year ago, but most other groups operating SMAs were apparently not providing them virtually before COVID-19 started.
Dr. Dysinger said he tried virtual SMAs in 2017 but dropped them because the technology – using Zoom – was challenging at the time, and his staff and most patients were resistant. “Only three to five people were attending the virtual sessions, and the meetings took place in the evening, which was hard on the staff.”
“When COVID-19 first appeared, our initial response was to try to keep the in-person group and add social distancing to it, but that wasn’t workable, so very quickly we shifted to Zoom meetings,” Dr. Dysinger said. “We had experience with Zoom already, and the Zoom technology had improved and was easier to use. COVID-19 forced it all forward.”
Are V-SMAs effective? While there have been many studies showing the effectiveness of in-person SMAs, there have been very few on V-SMAs. One 2018 study of obesity patients found that those attending in-person SMAs lost somewhat more weight than those in V-SMAs.
As with telemedicine, some patients have trouble with the technology of V-SMAs. Dr. Dysinger said 5%-10% of his SMA patients don’t make the switch over to V-SMAs – mainly because of problems in adapting to the technology – but the rest are happy. “We’re averaging 10 people per meeting, and as many as 20.”
Getting comfortable with group visits
Dealing with group visits takes a very different mindset than what doctors normally have, Dr. Wheeler said. “It took me 6-8 months to feel comfortable enough with group sessions to do them myself,” she recalled. “This was a very different way to practice, compared to the one-on-one care I was trained to give patients. Others may find the transition easier, though.
“Doctors are used to being in control of the patient visit, but the exchange in a group visit is more fluid,” Dr. Wheeler said. “Patients offer their own opinions, and this sends the discussion off on a tangent that is often quite useful. As doctors, we have to learn when to let these tangents continue, and know when the discussion might have to be brought back to the theme at hand. Often it’s better not to intercede.”
Do doctors need training to conduct SMAs? Patients in group visits reported worse communication with physicians than those in individual visits, according to a 2014 study. The authors surmised that the doctors needed to learn how to talk to groups and suggested that they get some training.
The potential staying power of V-SMAs post COVID?
Once the COVID-19 crisis is over, Medicare is scheduled to no longer provide the same level of reimbursement for virtual sessions as for real sessions. Dr. Mirsky anticipates a great deal of resistance to this change from thousands of physicians and patients who have become comfortable with telehealth, including virtual SMAs.
Dr. Dysinger thinks V-SMAs will continue. “When COVID-19 clears and we can go back to in-person groups, we expect to keep some virtual groups. People have already come to accept and value virtual groups.”
Dr. Wheeler sees virtual groups playing an essential role post COVID-19, when practices have to get back up to speed. “Virtual group visits could make it easier to deal with a large backlog of patients who couldn’t be seen up until now,” she said. “And virtual groups will be the only way to see patients who are still reluctant to meet in a group.”
A version of this article originally appeared on Medscape.com.
Ten patients smiled and waved out on the computer monitor, as Jacob Mirsky, MD, greeted each one, asked them to introduce themselves, and inquired as to how each was doing with their stress reduction tactics.
The attendees of the online session had been patients at in-person group visits at the Massachusetts General Hospital Revere HealthCare Center. But those in-person group sessions, known as shared medical appointments (SMAs), were shut down when COVID-19 arrived.
“Our group patients have been missing the sessions,” said Dr. Mirsky, a general internist who codirects the center’s group visit program. The online sessions, called virtual SMAs (V-SMAs), work well with COVID-19 social distancing.
In the group sessions, Dr. Mirsky reads a standardized message that addresses privacy concerns during the session. For the next 60-90 minutes, “we ask them to talk about what has gone well for them and what they are struggling with,” he said. “Then I answer their questions using materials in a PowerPoint to address key points, such as reducing salt for high blood pressure or interpreting blood sugar levels for diabetes.
“I try to end group sessions with one area of focus,” Dr. Mirsky said. “In the stress reduction group, this could be meditation. In the diabetes group, it could be a discussion on weight loss.” Then the program’s health coach goes over some key concepts on behavior change and invites participants to contact her after the session.
“The nice thing is that these virtual sessions are fully reimbursable by all of our insurers in Massachusetts,” Dr. Mirsky said. Through evaluation and management (E/M) codes, each patient in a group visit is paid the same as a patient in an individual visit with the same level of complexity.
Dr. Mirsky writes a note in the chart about each patient who was in the group session. “This includes information about the specific patient, such as the history and physical, and information about the group meeting,” he said. In the next few months, the center plans to put its other group sessions online – on blood pressure, obesity, diabetes, and insomnia.
Attracting doctors who hadn’t done groups before
said Marianne Sumego, MD, director of the Cleveland Clinic’s SMA program, which began 21 years ago.
In this era of COVID-19, group visits have either switched to V-SMAs or halted. However, the COVID-19 crisis has given group visits a second wind. Some doctors who never used SMAs before are now trying out this new mode of patient engagement,
Many of the 100 doctors using SMAs at the Cleveland Clinic have switched over to V-SMAs for now, and the new mode is also attracting colleagues who are new to SMAs, she said.
“When doctors started using telemedicine, virtual group visits started making sense to them,” Dr. Sumego said. “This is a time of a great deal of experimentation in practice design.”
Indeed, V-SMAs have eliminated some problems that had discouraged doctors from trying SMAs, said Amy Wheeler, MD, a general internist who founded the Revere SMA program and codirects it with Dr. Mirsky.
V-SMAs eliminate the need for a large space to hold sessions and reduce the number of staff needed to run sessions, Dr. Wheeler said. “Virtual group visits can actually be easier to use than in-person group visits.”
Dr. Sumego believes small practices in particular will take up V-SMAs because they are easier to run than regular SMAs. “Necessity drives change,” she said. “Across the country everyone is looking at the virtual group model.”
Group visits can help your bottom line
Medicare and many private payers cover group visits. In most cases, they tend to pay the same rate as for an individual office visit. As with telehealth, Medicare and many other payers are temporarily reimbursing for virtual visits at the same rate as for real visits.
Not all payers have a stated policy about covering SMAs, and physicians have to ask. The Centers for Medicare & Medicaid Services, for example, has not published any coding rules on SMAs. But in response to a query by the American Academy of Family Physicians, CMS said it would allow use of CPT codes for E/M services for individual patients.
Blue Cross Blue Shield of North Carolina is one of the few payers with a clearly stated policy on its website. Like Medicare, the insurer accepts E/M codes, and it requires that patients’ attendance must be voluntary; they must be established patients; and the visit must be specific to a disease or condition, although several conditions are allowed.
Dr. Mirsky said his group uses the same E/M level – 99213 – for all of his SMA patients. “Since a regular primary care visit is usually billed at a level 3 or 4, depending on how many topics are covered, we chose level 3 for groups, because the group session deals with just one topic.”
One challenge for billing for SMAs is that most health insurers require patients to provide a copay for each visit, which can discourage patients in groups that meet frequently, says Wayne Dysinger, MD, founder of Lifestyle Medical Solutions, a two-physician primary care practice in Riverside, Calif.
But Dr. Dysinger, who has been using SMAs for 5 years, usually doesn’t have to worry about copays because much of his work is capitated and doesn’t require a copay.
Also, some of Dr. Dysinger’s SMA patients are in direct primary care, in which the patients pay an $18 monthly membership fee. Other practices may charge a flat out-of-pocket fee.
How group visits operate
SMAs are based on the observation that patients with the same condition generally ask their doctor the same questions, and rather than repeat the answers each time, why not provide them to a group?
Dr. Wheeler said trying to be more efficient with her time was the primary reason she became interested in SMAs a dozen years ago. “I was trying to squeeze the advice patients needed into a normal patient visit, and it wasn’t working. When I tried to tell them everything they needed to know, I’d run behind for the rest of my day’s visits.”
She found she was continually repeating the same conversation with patients, but these talks weren’t detailed enough to be effective. “When my weight loss patients came back for the next appointment, they had not made the recommended changes in lifestyle. I started to realize how complicated weight loss was.” So Dr. Wheeler founded the SMA program at the Revere Center.
Doctors enjoy the patient interaction
Some doctors who use SMAs talk about how connected they feel with their patients. “For me, the group sessions are the most gratifying part of the week,” Dr. Dysinger says. “I like to see the patients interacting with me and with each other, and watch their health behavior change over time.”
“These groups have a great deal of energy,” he said. “They have a kind of vulnerability that is very raw, very human. People make commitments to meet goals. Will they meet them or not?”
Dr. Dysinger’s enthusiasm has been echoed by other doctors. In a study of older patients, physicians who used SMAs were more satisfied with care than physicians who relied on standard one-to-one interactions. In another study, the researchers surmised that, in SMAs, doctors learn from their patients how they can better meet their needs.
Dr. Dysinger thinks SMAs are widely applicable in primary care. He estimates that 80%-85% of appointments at a primary care practice involve chronic diseases, and this type of patient is a good fit for group visits. SMAs typically treat patients with diabetes, asthma, arthritis, and obesity.
Dr. Sumego said SMAs are used for specialty care at Cleveland Clinic, such as to help patients before and after bariatric surgery. SMAs have also been used to treat patients with ulcerative colitis, multiple sclerosis, cancer, HIV, menopause, insomnia, and stress, according to one report.
Dr. Dysinger, who runs a small practice, organizes his group sessions somewhat differently. He doesn’t organize his groups around conditions like diabetes, but instead his groups focus on four “pillars” of lifestyle medicine: nourishment, movement, resilience (involving sleep and stress), and connectedness.
Why patients like group visits
Feeling part of a whole is a major draw for many patients. “Patients seem to like committing to something bigger than just themselves,” Dr. Wheeler said. “They enjoy the sense of community that groups have, the joy of supporting one another.”
“It’s feeling that you’re not alone,” Dr. Mirsky said. “When a patient struggling with diabetes hears how hard it is for another patient, it validates their experience and gives them someone to connect with. There is a positive peer pressure.”
Many programs, including Dr. Wheeler’s and Dr. Mirsky’s in Boston, allow patients to drop in and out of sessions, rather than attending one course all the way through. But even under this format, Dr. Wheeler said that patients often tend to stick together. “At the end of a session, one patient asks another: ‘Which session do you want to go to next?’ ” she said.
Patients also learn from each other in SMAs. Patients exchange experiences and share advice they may not have had the chance to get during an individual visit.
The group dynamic can make it easier for some patients to reveal sensitive information, said Dr. Dysinger. “In these groups, people feel free to talk about their bowel movements, or about having to deal with the influence of a parent on their lives,” Dr. Dysinger said. “The sessions can have the feel of an [Alcoholics Anonymous] meeting, but they’re firmly grounded in medicine.”
Potential downsides of virtual group visits
SMAs and VSMAs may not work for every practice. Some small practices may not have enough patients to organize a group visit around a particular condition – even a common one like diabetes. In a presentation before the Society of General Internal Medicine, a physician from the Medical University of South Carolina, Charleston, warned that it may be difficult for a practice to fill diabetes group visits every year.
Additionally, some patients don’t want to talk about personal matters in a group. “They may not want to reveal certain things about themselves,” Dr. Mirsky said. “So I tell the group that if there is anything that anyone wants to talk about in private, I’m available.”
Another drawback of SMAs is that more experienced patients may have to slog through information they already know, which is a particular problem when patients can drop in and out of sessions. Dr. Mirsky noted that “what often ends up happening is that the experienced participant helps the newcomer.”
Finally, confidentially is a big concern in a group session. “In a one-on-one visit, you can go into details about the patient’s health, and even bring up an entry in the chart,” Dr. Wheeler said. “But in a group visit, you can’t raise any personal details about a patient unless the patient brings it up first.”
SMA patients sign confidentiality agreements in which they agree not to talk about other patients outside the session. Ensuring confidentiality becomes more complicated in virtual group visits, because someone located in the room near a participant could overhear the conversation. For this reason, patients in V-SMAs are advised to use headphones or, at a minimum, close the door to the room they are in.
To address privacy concerns, Zoom encrypts its data, but some privacy breeches have been reported, and a U.S. senator has been looking into Zoom’s privacy vulnerabilities.
Transferring groups to virtual groups
It took the COVID-19 crisis for most doctors to take up virtual SMAs. Dr. Sumego said that the Cleveland Clinic started virtual SMAs more than a year ago, but most other groups operating SMAs were apparently not providing them virtually before COVID-19 started.
Dr. Dysinger said he tried virtual SMAs in 2017 but dropped them because the technology – using Zoom – was challenging at the time, and his staff and most patients were resistant. “Only three to five people were attending the virtual sessions, and the meetings took place in the evening, which was hard on the staff.”
“When COVID-19 first appeared, our initial response was to try to keep the in-person group and add social distancing to it, but that wasn’t workable, so very quickly we shifted to Zoom meetings,” Dr. Dysinger said. “We had experience with Zoom already, and the Zoom technology had improved and was easier to use. COVID-19 forced it all forward.”
Are V-SMAs effective? While there have been many studies showing the effectiveness of in-person SMAs, there have been very few on V-SMAs. One 2018 study of obesity patients found that those attending in-person SMAs lost somewhat more weight than those in V-SMAs.
As with telemedicine, some patients have trouble with the technology of V-SMAs. Dr. Dysinger said 5%-10% of his SMA patients don’t make the switch over to V-SMAs – mainly because of problems in adapting to the technology – but the rest are happy. “We’re averaging 10 people per meeting, and as many as 20.”
Getting comfortable with group visits
Dealing with group visits takes a very different mindset than what doctors normally have, Dr. Wheeler said. “It took me 6-8 months to feel comfortable enough with group sessions to do them myself,” she recalled. “This was a very different way to practice, compared to the one-on-one care I was trained to give patients. Others may find the transition easier, though.
“Doctors are used to being in control of the patient visit, but the exchange in a group visit is more fluid,” Dr. Wheeler said. “Patients offer their own opinions, and this sends the discussion off on a tangent that is often quite useful. As doctors, we have to learn when to let these tangents continue, and know when the discussion might have to be brought back to the theme at hand. Often it’s better not to intercede.”
Do doctors need training to conduct SMAs? Patients in group visits reported worse communication with physicians than those in individual visits, according to a 2014 study. The authors surmised that the doctors needed to learn how to talk to groups and suggested that they get some training.
The potential staying power of V-SMAs post COVID?
Once the COVID-19 crisis is over, Medicare is scheduled to no longer provide the same level of reimbursement for virtual sessions as for real sessions. Dr. Mirsky anticipates a great deal of resistance to this change from thousands of physicians and patients who have become comfortable with telehealth, including virtual SMAs.
Dr. Dysinger thinks V-SMAs will continue. “When COVID-19 clears and we can go back to in-person groups, we expect to keep some virtual groups. People have already come to accept and value virtual groups.”
Dr. Wheeler sees virtual groups playing an essential role post COVID-19, when practices have to get back up to speed. “Virtual group visits could make it easier to deal with a large backlog of patients who couldn’t be seen up until now,” she said. “And virtual groups will be the only way to see patients who are still reluctant to meet in a group.”
A version of this article originally appeared on Medscape.com.
Ten patients smiled and waved out on the computer monitor, as Jacob Mirsky, MD, greeted each one, asked them to introduce themselves, and inquired as to how each was doing with their stress reduction tactics.
The attendees of the online session had been patients at in-person group visits at the Massachusetts General Hospital Revere HealthCare Center. But those in-person group sessions, known as shared medical appointments (SMAs), were shut down when COVID-19 arrived.
“Our group patients have been missing the sessions,” said Dr. Mirsky, a general internist who codirects the center’s group visit program. The online sessions, called virtual SMAs (V-SMAs), work well with COVID-19 social distancing.
In the group sessions, Dr. Mirsky reads a standardized message that addresses privacy concerns during the session. For the next 60-90 minutes, “we ask them to talk about what has gone well for them and what they are struggling with,” he said. “Then I answer their questions using materials in a PowerPoint to address key points, such as reducing salt for high blood pressure or interpreting blood sugar levels for diabetes.
“I try to end group sessions with one area of focus,” Dr. Mirsky said. “In the stress reduction group, this could be meditation. In the diabetes group, it could be a discussion on weight loss.” Then the program’s health coach goes over some key concepts on behavior change and invites participants to contact her after the session.
“The nice thing is that these virtual sessions are fully reimbursable by all of our insurers in Massachusetts,” Dr. Mirsky said. Through evaluation and management (E/M) codes, each patient in a group visit is paid the same as a patient in an individual visit with the same level of complexity.
Dr. Mirsky writes a note in the chart about each patient who was in the group session. “This includes information about the specific patient, such as the history and physical, and information about the group meeting,” he said. In the next few months, the center plans to put its other group sessions online – on blood pressure, obesity, diabetes, and insomnia.
Attracting doctors who hadn’t done groups before
said Marianne Sumego, MD, director of the Cleveland Clinic’s SMA program, which began 21 years ago.
In this era of COVID-19, group visits have either switched to V-SMAs or halted. However, the COVID-19 crisis has given group visits a second wind. Some doctors who never used SMAs before are now trying out this new mode of patient engagement,
Many of the 100 doctors using SMAs at the Cleveland Clinic have switched over to V-SMAs for now, and the new mode is also attracting colleagues who are new to SMAs, she said.
“When doctors started using telemedicine, virtual group visits started making sense to them,” Dr. Sumego said. “This is a time of a great deal of experimentation in practice design.”
Indeed, V-SMAs have eliminated some problems that had discouraged doctors from trying SMAs, said Amy Wheeler, MD, a general internist who founded the Revere SMA program and codirects it with Dr. Mirsky.
V-SMAs eliminate the need for a large space to hold sessions and reduce the number of staff needed to run sessions, Dr. Wheeler said. “Virtual group visits can actually be easier to use than in-person group visits.”
Dr. Sumego believes small practices in particular will take up V-SMAs because they are easier to run than regular SMAs. “Necessity drives change,” she said. “Across the country everyone is looking at the virtual group model.”
Group visits can help your bottom line
Medicare and many private payers cover group visits. In most cases, they tend to pay the same rate as for an individual office visit. As with telehealth, Medicare and many other payers are temporarily reimbursing for virtual visits at the same rate as for real visits.
Not all payers have a stated policy about covering SMAs, and physicians have to ask. The Centers for Medicare & Medicaid Services, for example, has not published any coding rules on SMAs. But in response to a query by the American Academy of Family Physicians, CMS said it would allow use of CPT codes for E/M services for individual patients.
Blue Cross Blue Shield of North Carolina is one of the few payers with a clearly stated policy on its website. Like Medicare, the insurer accepts E/M codes, and it requires that patients’ attendance must be voluntary; they must be established patients; and the visit must be specific to a disease or condition, although several conditions are allowed.
Dr. Mirsky said his group uses the same E/M level – 99213 – for all of his SMA patients. “Since a regular primary care visit is usually billed at a level 3 or 4, depending on how many topics are covered, we chose level 3 for groups, because the group session deals with just one topic.”
One challenge for billing for SMAs is that most health insurers require patients to provide a copay for each visit, which can discourage patients in groups that meet frequently, says Wayne Dysinger, MD, founder of Lifestyle Medical Solutions, a two-physician primary care practice in Riverside, Calif.
But Dr. Dysinger, who has been using SMAs for 5 years, usually doesn’t have to worry about copays because much of his work is capitated and doesn’t require a copay.
Also, some of Dr. Dysinger’s SMA patients are in direct primary care, in which the patients pay an $18 monthly membership fee. Other practices may charge a flat out-of-pocket fee.
How group visits operate
SMAs are based on the observation that patients with the same condition generally ask their doctor the same questions, and rather than repeat the answers each time, why not provide them to a group?
Dr. Wheeler said trying to be more efficient with her time was the primary reason she became interested in SMAs a dozen years ago. “I was trying to squeeze the advice patients needed into a normal patient visit, and it wasn’t working. When I tried to tell them everything they needed to know, I’d run behind for the rest of my day’s visits.”
She found she was continually repeating the same conversation with patients, but these talks weren’t detailed enough to be effective. “When my weight loss patients came back for the next appointment, they had not made the recommended changes in lifestyle. I started to realize how complicated weight loss was.” So Dr. Wheeler founded the SMA program at the Revere Center.
Doctors enjoy the patient interaction
Some doctors who use SMAs talk about how connected they feel with their patients. “For me, the group sessions are the most gratifying part of the week,” Dr. Dysinger says. “I like to see the patients interacting with me and with each other, and watch their health behavior change over time.”
“These groups have a great deal of energy,” he said. “They have a kind of vulnerability that is very raw, very human. People make commitments to meet goals. Will they meet them or not?”
Dr. Dysinger’s enthusiasm has been echoed by other doctors. In a study of older patients, physicians who used SMAs were more satisfied with care than physicians who relied on standard one-to-one interactions. In another study, the researchers surmised that, in SMAs, doctors learn from their patients how they can better meet their needs.
Dr. Dysinger thinks SMAs are widely applicable in primary care. He estimates that 80%-85% of appointments at a primary care practice involve chronic diseases, and this type of patient is a good fit for group visits. SMAs typically treat patients with diabetes, asthma, arthritis, and obesity.
Dr. Sumego said SMAs are used for specialty care at Cleveland Clinic, such as to help patients before and after bariatric surgery. SMAs have also been used to treat patients with ulcerative colitis, multiple sclerosis, cancer, HIV, menopause, insomnia, and stress, according to one report.
Dr. Dysinger, who runs a small practice, organizes his group sessions somewhat differently. He doesn’t organize his groups around conditions like diabetes, but instead his groups focus on four “pillars” of lifestyle medicine: nourishment, movement, resilience (involving sleep and stress), and connectedness.
Why patients like group visits
Feeling part of a whole is a major draw for many patients. “Patients seem to like committing to something bigger than just themselves,” Dr. Wheeler said. “They enjoy the sense of community that groups have, the joy of supporting one another.”
“It’s feeling that you’re not alone,” Dr. Mirsky said. “When a patient struggling with diabetes hears how hard it is for another patient, it validates their experience and gives them someone to connect with. There is a positive peer pressure.”
Many programs, including Dr. Wheeler’s and Dr. Mirsky’s in Boston, allow patients to drop in and out of sessions, rather than attending one course all the way through. But even under this format, Dr. Wheeler said that patients often tend to stick together. “At the end of a session, one patient asks another: ‘Which session do you want to go to next?’ ” she said.
Patients also learn from each other in SMAs. Patients exchange experiences and share advice they may not have had the chance to get during an individual visit.
The group dynamic can make it easier for some patients to reveal sensitive information, said Dr. Dysinger. “In these groups, people feel free to talk about their bowel movements, or about having to deal with the influence of a parent on their lives,” Dr. Dysinger said. “The sessions can have the feel of an [Alcoholics Anonymous] meeting, but they’re firmly grounded in medicine.”
Potential downsides of virtual group visits
SMAs and VSMAs may not work for every practice. Some small practices may not have enough patients to organize a group visit around a particular condition – even a common one like diabetes. In a presentation before the Society of General Internal Medicine, a physician from the Medical University of South Carolina, Charleston, warned that it may be difficult for a practice to fill diabetes group visits every year.
Additionally, some patients don’t want to talk about personal matters in a group. “They may not want to reveal certain things about themselves,” Dr. Mirsky said. “So I tell the group that if there is anything that anyone wants to talk about in private, I’m available.”
Another drawback of SMAs is that more experienced patients may have to slog through information they already know, which is a particular problem when patients can drop in and out of sessions. Dr. Mirsky noted that “what often ends up happening is that the experienced participant helps the newcomer.”
Finally, confidentially is a big concern in a group session. “In a one-on-one visit, you can go into details about the patient’s health, and even bring up an entry in the chart,” Dr. Wheeler said. “But in a group visit, you can’t raise any personal details about a patient unless the patient brings it up first.”
SMA patients sign confidentiality agreements in which they agree not to talk about other patients outside the session. Ensuring confidentiality becomes more complicated in virtual group visits, because someone located in the room near a participant could overhear the conversation. For this reason, patients in V-SMAs are advised to use headphones or, at a minimum, close the door to the room they are in.
To address privacy concerns, Zoom encrypts its data, but some privacy breeches have been reported, and a U.S. senator has been looking into Zoom’s privacy vulnerabilities.
Transferring groups to virtual groups
It took the COVID-19 crisis for most doctors to take up virtual SMAs. Dr. Sumego said that the Cleveland Clinic started virtual SMAs more than a year ago, but most other groups operating SMAs were apparently not providing them virtually before COVID-19 started.
Dr. Dysinger said he tried virtual SMAs in 2017 but dropped them because the technology – using Zoom – was challenging at the time, and his staff and most patients were resistant. “Only three to five people were attending the virtual sessions, and the meetings took place in the evening, which was hard on the staff.”
“When COVID-19 first appeared, our initial response was to try to keep the in-person group and add social distancing to it, but that wasn’t workable, so very quickly we shifted to Zoom meetings,” Dr. Dysinger said. “We had experience with Zoom already, and the Zoom technology had improved and was easier to use. COVID-19 forced it all forward.”
Are V-SMAs effective? While there have been many studies showing the effectiveness of in-person SMAs, there have been very few on V-SMAs. One 2018 study of obesity patients found that those attending in-person SMAs lost somewhat more weight than those in V-SMAs.
As with telemedicine, some patients have trouble with the technology of V-SMAs. Dr. Dysinger said 5%-10% of his SMA patients don’t make the switch over to V-SMAs – mainly because of problems in adapting to the technology – but the rest are happy. “We’re averaging 10 people per meeting, and as many as 20.”
Getting comfortable with group visits
Dealing with group visits takes a very different mindset than what doctors normally have, Dr. Wheeler said. “It took me 6-8 months to feel comfortable enough with group sessions to do them myself,” she recalled. “This was a very different way to practice, compared to the one-on-one care I was trained to give patients. Others may find the transition easier, though.
“Doctors are used to being in control of the patient visit, but the exchange in a group visit is more fluid,” Dr. Wheeler said. “Patients offer their own opinions, and this sends the discussion off on a tangent that is often quite useful. As doctors, we have to learn when to let these tangents continue, and know when the discussion might have to be brought back to the theme at hand. Often it’s better not to intercede.”
Do doctors need training to conduct SMAs? Patients in group visits reported worse communication with physicians than those in individual visits, according to a 2014 study. The authors surmised that the doctors needed to learn how to talk to groups and suggested that they get some training.
The potential staying power of V-SMAs post COVID?
Once the COVID-19 crisis is over, Medicare is scheduled to no longer provide the same level of reimbursement for virtual sessions as for real sessions. Dr. Mirsky anticipates a great deal of resistance to this change from thousands of physicians and patients who have become comfortable with telehealth, including virtual SMAs.
Dr. Dysinger thinks V-SMAs will continue. “When COVID-19 clears and we can go back to in-person groups, we expect to keep some virtual groups. People have already come to accept and value virtual groups.”
Dr. Wheeler sees virtual groups playing an essential role post COVID-19, when practices have to get back up to speed. “Virtual group visits could make it easier to deal with a large backlog of patients who couldn’t be seen up until now,” she said. “And virtual groups will be the only way to see patients who are still reluctant to meet in a group.”
A version of this article originally appeared on Medscape.com.
Part 3: Lipid Management in Diabetes Patients
Previously, we explored blood pressure control in a patient with diabetes. Now, we’ll discuss the value of a fasting lipid panel and treatment for dyslipidemia in this population.
CASE CONTINUED
Mr. W completed a fasting lipid panel, which revealed the following: triglycerides, 145 mg/dL; high-density lipoprotein (HDL) level, 32 mg/dL; and low-density lipoprotein (LDL) level, 108 mg/dL. He is currently receiving low-dose statin therapy. Based on these results, Mr. W fits the criteria for dyslipidemia.
Dyslipidemia
Dyslipidemia marked by elevated LDL levels—as observed in Mr. W—is a well-known contributing factor to development of cardiovascular disease in patients with diabetes. Elevated triglycerides and low HDL levels also are often noted in these patients. Patients with diabetes are particularly vulnerable to atherosclerosis due to a combination of pro-inflammatory factors and hyperglycemic effects. Both the ADA and the AACE agree that lipid management, including fasting lipid panels and appropriate treatment, is of paramount importance in patients with diabetes.7,8
Fasting Lipid Panels
The AACE recommends administering at least annual fasting lipid panels in all adults with diabetes, and LDL goal levels should be based on the cardiovascular risk of the patient.7 For patients with
- established ASCVD, the LDL goal is < 55 mg/dL
- risk factors for ASCVD (eg, hypertension, tobacco use, family history of ASCVD) in addition to diabetes, the LDL goal is < 70 mg/dL
- no risk factors, the LDL goal is < 100 mg/dL.7
Statin Therapy
Research indicates that statins reduce the risk for cardiovascular events and are recommended as first-line treatment for dyslipidemia.2,7 Statin therapy is recommended for patients with LDL levels above goal without contraindications.10 Higher-dose statins have been shown to help improve cardiovascular outcomes, and most—if not all—guidelines recommend up-titration of these medications as tolerated by the patient. 7,8,29 After initiation of statin therapy, clinicians should continue to monitor lipid levels every 4 to 12 weeks after a change in lipid therapy and then schedule monitoring annually.2
Unfortunately, a recent large-scale retrospective study of the medical records of 125,464 patients with type 2 diabetes showed that although 99% of the patients were at high risk for or already had ASCVD, only 63% were receiving the recommended statin therapy.30 Therefore, all patients with diabetes at risk for ASCVD require evaluation to determine the need for statins.
Additional treatments. If the patient’s levels remain above goal, strong consideration should be given to additional therapies. Ezetimibe has been shown to have some benefit in reducing LDL levels and cardiovascular risk.31 PCSK9 inhibitors are a newer treatment for cardiovascular disease and are particularly beneficial for patients with known ASCVD. The FOURIER and ODYSSEY trials demonstrated that PCSK9 inhibitors had relative risk reductions of 48% to 53% for major ASCVD events and showed that these medications help reduce LDL levels and, most importantly, cardiovascular risk.32,33
Continue to: Recommendations for other lipid components
Recommendations for other lipid components—non–HDL-C, apolipoprotein B, or LDL-P—are very specific and consideration may be given for referral to an endocrinologist or lipidologist for evaluation and treatment.7,8 Evidence on reducing cardiovascular risk with therapies for decreasing triglyceride levels is limited. Recently though, icosapent ethyl received FDA approval as an adjunct to maximally tolerated statin therapy to reduce the risk for cardiovascular events in patients with elevated triglyceride levels (≥ 150 mg/dL).34,35 ADA guidelines recommend icosapent ethyl for patients with diabetes, 1 additional cardiovascular risk factor, and triglyceride levels between 135 and 499 mg/dL.2
In Part 4, I’ll explore how clinicians can best monitor for chronic kidney disease in patients with diabetes. We’ll also discuss the medications used for improving kidney health in these patients.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
Previously, we explored blood pressure control in a patient with diabetes. Now, we’ll discuss the value of a fasting lipid panel and treatment for dyslipidemia in this population.
CASE CONTINUED
Mr. W completed a fasting lipid panel, which revealed the following: triglycerides, 145 mg/dL; high-density lipoprotein (HDL) level, 32 mg/dL; and low-density lipoprotein (LDL) level, 108 mg/dL. He is currently receiving low-dose statin therapy. Based on these results, Mr. W fits the criteria for dyslipidemia.
Dyslipidemia
Dyslipidemia marked by elevated LDL levels—as observed in Mr. W—is a well-known contributing factor to development of cardiovascular disease in patients with diabetes. Elevated triglycerides and low HDL levels also are often noted in these patients. Patients with diabetes are particularly vulnerable to atherosclerosis due to a combination of pro-inflammatory factors and hyperglycemic effects. Both the ADA and the AACE agree that lipid management, including fasting lipid panels and appropriate treatment, is of paramount importance in patients with diabetes.7,8
Fasting Lipid Panels
The AACE recommends administering at least annual fasting lipid panels in all adults with diabetes, and LDL goal levels should be based on the cardiovascular risk of the patient.7 For patients with
- established ASCVD, the LDL goal is < 55 mg/dL
- risk factors for ASCVD (eg, hypertension, tobacco use, family history of ASCVD) in addition to diabetes, the LDL goal is < 70 mg/dL
- no risk factors, the LDL goal is < 100 mg/dL.7
Statin Therapy
Research indicates that statins reduce the risk for cardiovascular events and are recommended as first-line treatment for dyslipidemia.2,7 Statin therapy is recommended for patients with LDL levels above goal without contraindications.10 Higher-dose statins have been shown to help improve cardiovascular outcomes, and most—if not all—guidelines recommend up-titration of these medications as tolerated by the patient. 7,8,29 After initiation of statin therapy, clinicians should continue to monitor lipid levels every 4 to 12 weeks after a change in lipid therapy and then schedule monitoring annually.2
Unfortunately, a recent large-scale retrospective study of the medical records of 125,464 patients with type 2 diabetes showed that although 99% of the patients were at high risk for or already had ASCVD, only 63% were receiving the recommended statin therapy.30 Therefore, all patients with diabetes at risk for ASCVD require evaluation to determine the need for statins.
Additional treatments. If the patient’s levels remain above goal, strong consideration should be given to additional therapies. Ezetimibe has been shown to have some benefit in reducing LDL levels and cardiovascular risk.31 PCSK9 inhibitors are a newer treatment for cardiovascular disease and are particularly beneficial for patients with known ASCVD. The FOURIER and ODYSSEY trials demonstrated that PCSK9 inhibitors had relative risk reductions of 48% to 53% for major ASCVD events and showed that these medications help reduce LDL levels and, most importantly, cardiovascular risk.32,33
Continue to: Recommendations for other lipid components
Recommendations for other lipid components—non–HDL-C, apolipoprotein B, or LDL-P—are very specific and consideration may be given for referral to an endocrinologist or lipidologist for evaluation and treatment.7,8 Evidence on reducing cardiovascular risk with therapies for decreasing triglyceride levels is limited. Recently though, icosapent ethyl received FDA approval as an adjunct to maximally tolerated statin therapy to reduce the risk for cardiovascular events in patients with elevated triglyceride levels (≥ 150 mg/dL).34,35 ADA guidelines recommend icosapent ethyl for patients with diabetes, 1 additional cardiovascular risk factor, and triglyceride levels between 135 and 499 mg/dL.2
In Part 4, I’ll explore how clinicians can best monitor for chronic kidney disease in patients with diabetes. We’ll also discuss the medications used for improving kidney health in these patients.
Previously, we explored blood pressure control in a patient with diabetes. Now, we’ll discuss the value of a fasting lipid panel and treatment for dyslipidemia in this population.
CASE CONTINUED
Mr. W completed a fasting lipid panel, which revealed the following: triglycerides, 145 mg/dL; high-density lipoprotein (HDL) level, 32 mg/dL; and low-density lipoprotein (LDL) level, 108 mg/dL. He is currently receiving low-dose statin therapy. Based on these results, Mr. W fits the criteria for dyslipidemia.
Dyslipidemia
Dyslipidemia marked by elevated LDL levels—as observed in Mr. W—is a well-known contributing factor to development of cardiovascular disease in patients with diabetes. Elevated triglycerides and low HDL levels also are often noted in these patients. Patients with diabetes are particularly vulnerable to atherosclerosis due to a combination of pro-inflammatory factors and hyperglycemic effects. Both the ADA and the AACE agree that lipid management, including fasting lipid panels and appropriate treatment, is of paramount importance in patients with diabetes.7,8
Fasting Lipid Panels
The AACE recommends administering at least annual fasting lipid panels in all adults with diabetes, and LDL goal levels should be based on the cardiovascular risk of the patient.7 For patients with
- established ASCVD, the LDL goal is < 55 mg/dL
- risk factors for ASCVD (eg, hypertension, tobacco use, family history of ASCVD) in addition to diabetes, the LDL goal is < 70 mg/dL
- no risk factors, the LDL goal is < 100 mg/dL.7
Statin Therapy
Research indicates that statins reduce the risk for cardiovascular events and are recommended as first-line treatment for dyslipidemia.2,7 Statin therapy is recommended for patients with LDL levels above goal without contraindications.10 Higher-dose statins have been shown to help improve cardiovascular outcomes, and most—if not all—guidelines recommend up-titration of these medications as tolerated by the patient. 7,8,29 After initiation of statin therapy, clinicians should continue to monitor lipid levels every 4 to 12 weeks after a change in lipid therapy and then schedule monitoring annually.2
Unfortunately, a recent large-scale retrospective study of the medical records of 125,464 patients with type 2 diabetes showed that although 99% of the patients were at high risk for or already had ASCVD, only 63% were receiving the recommended statin therapy.30 Therefore, all patients with diabetes at risk for ASCVD require evaluation to determine the need for statins.
Additional treatments. If the patient’s levels remain above goal, strong consideration should be given to additional therapies. Ezetimibe has been shown to have some benefit in reducing LDL levels and cardiovascular risk.31 PCSK9 inhibitors are a newer treatment for cardiovascular disease and are particularly beneficial for patients with known ASCVD. The FOURIER and ODYSSEY trials demonstrated that PCSK9 inhibitors had relative risk reductions of 48% to 53% for major ASCVD events and showed that these medications help reduce LDL levels and, most importantly, cardiovascular risk.32,33
Continue to: Recommendations for other lipid components
Recommendations for other lipid components—non–HDL-C, apolipoprotein B, or LDL-P—are very specific and consideration may be given for referral to an endocrinologist or lipidologist for evaluation and treatment.7,8 Evidence on reducing cardiovascular risk with therapies for decreasing triglyceride levels is limited. Recently though, icosapent ethyl received FDA approval as an adjunct to maximally tolerated statin therapy to reduce the risk for cardiovascular events in patients with elevated triglyceride levels (≥ 150 mg/dL).34,35 ADA guidelines recommend icosapent ethyl for patients with diabetes, 1 additional cardiovascular risk factor, and triglyceride levels between 135 and 499 mg/dL.2
In Part 4, I’ll explore how clinicians can best monitor for chronic kidney disease in patients with diabetes. We’ll also discuss the medications used for improving kidney health in these patients.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
Hyperglycemia predicts COVID-19 death even without diabetes
new research indicates.
The findings, from a retrospective analysis of 605 patients with COVID-19 seen at two hospitals in Wuhan, China, were published online July 10 in Diabetologia by Sufei Wang, of the department of respiratory and critical care medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, and colleagues.
Several previous studies have demonstrated a link between hyperglycemia and worse outcomes in COVID-19, and at least one diabetes diagnosis, but this is the first to focus specifically on that group of patients.
Wang and colleagues found that a fasting blood glucose of 7.0 mmol/L (126 mg/dL) or greater on admission – present in 45.6% of those without a prior diabetes diagnosis – was an independent predictor of 28-day mortality.
Although A1c data weren’t analyzed, the population is believed to include both individuals with preexisting but undiagnosed diabetes and those without diabetes who have acute stress hyperglycemia.
“Glycemic testing and control should be recommended for all COVID-19 patients even if they do not have preexisting diabetes, as most COVID-19 patients are prone to glucose metabolic disorders,” they emphasized.
“Addressing elevated fasting blood glucose at an early stage can help clinicians better manage the condition and lower the mortality risk of COVID-19 patients,” Wang and colleagues noted.
Hyperglycemia predicts COVID-19 death, complications
The study involved consecutive patients with COVID-19 and definitive 28-day outcome and fasting blood glucose measurement on admission to two Wuhan-area hospitals between Jan. 24 to Feb. 10, 2020. A total of 605 patients did not have a previous diabetes diagnosis. They were a median age of 59 years and 53.2% were men.
Just over half, 54.4%, had a fasting blood glucose below 6.1 mmol/L (110.0 mg/dL). The rest had dysglycemia: 16.5% had a fasting blood glucose of 6.1-6.9 mmol/L (110-125 mg/dL), considered the prediabetes range, and 29.1% had a fasting blood glucose of 7 mmol/L (126 mg/dL) or above, the cutoff for diabetes.
“These results indicate that our study included both undiagnosed diabetic patients and nondiabetic patients with hyperglycemia caused by an acute blood glucose disorder,” the authors noted.
Over 28 days of hospitalization, 18.8% (114) of the patients died and 39.2% developed one or more in-hospital complications.
The authors used the CRB-65 score, which assigns 1 point for each of four indicators – confusion, respiratory rate >30 breaths/min, systolic blood pressure ≤90 mm Hg or diastolic blood pressure ≤60 mm Hg, and age ≥65 years – to assess pneumonia severity.
Just over half, 55.2%, had a CRB-65 score of 0, 43.1% had a score of 1-2, and 1.7% had a score of 3-4.
In multivariable analysis, significant independent predictors of 28-day mortality were age (hazard ratio, 1.02), male sex (HR, 1.75), CRB-65 score 1-2 (HR, 2.68), CRB-65 score 3-4 (HR, 5.25), and fasting blood glucose ≥7.0 mmol/L (HR, 2.30).
Compared with patients with normal glucose (<6.1 mmol/L), 28-day mortality was twice as high (HR, 2.06) for those with a fasting blood glucose of 6.1-6.9 mmol/L and more than threefold higher for ≥7.0 mmol/L (HR, 3.54).
Pneumonia severity also predicted 28-day mortality, with hazard ratios of 4.35 and 13.80 for patients with CRB-65 scores of 1-2 and 3-4, respectively, compared with 0.
Inhospital complications, including acute respiratory distress syndrome or acute cardiac, kidney, or liver injury or cerebrovascular accident, occurred in 14.2%, 7.9%, and 17.0% of those in the lowest to highest fasting blood glucose groups.
Complications were more than twice as common in patients with a fasting blood glucose of 6.1-6.9 mmol/L (HR, 2.61) and four times more common (HR, 3.99) among those with a fasting blood glucose ≥7.0 mmol/L, compared with those with normoglycemia.
The study was supported by the National Natural Science Foundation of China and Major Projects of the National Science and Technology. The authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
new research indicates.
The findings, from a retrospective analysis of 605 patients with COVID-19 seen at two hospitals in Wuhan, China, were published online July 10 in Diabetologia by Sufei Wang, of the department of respiratory and critical care medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, and colleagues.
Several previous studies have demonstrated a link between hyperglycemia and worse outcomes in COVID-19, and at least one diabetes diagnosis, but this is the first to focus specifically on that group of patients.
Wang and colleagues found that a fasting blood glucose of 7.0 mmol/L (126 mg/dL) or greater on admission – present in 45.6% of those without a prior diabetes diagnosis – was an independent predictor of 28-day mortality.
Although A1c data weren’t analyzed, the population is believed to include both individuals with preexisting but undiagnosed diabetes and those without diabetes who have acute stress hyperglycemia.
“Glycemic testing and control should be recommended for all COVID-19 patients even if they do not have preexisting diabetes, as most COVID-19 patients are prone to glucose metabolic disorders,” they emphasized.
“Addressing elevated fasting blood glucose at an early stage can help clinicians better manage the condition and lower the mortality risk of COVID-19 patients,” Wang and colleagues noted.
Hyperglycemia predicts COVID-19 death, complications
The study involved consecutive patients with COVID-19 and definitive 28-day outcome and fasting blood glucose measurement on admission to two Wuhan-area hospitals between Jan. 24 to Feb. 10, 2020. A total of 605 patients did not have a previous diabetes diagnosis. They were a median age of 59 years and 53.2% were men.
Just over half, 54.4%, had a fasting blood glucose below 6.1 mmol/L (110.0 mg/dL). The rest had dysglycemia: 16.5% had a fasting blood glucose of 6.1-6.9 mmol/L (110-125 mg/dL), considered the prediabetes range, and 29.1% had a fasting blood glucose of 7 mmol/L (126 mg/dL) or above, the cutoff for diabetes.
“These results indicate that our study included both undiagnosed diabetic patients and nondiabetic patients with hyperglycemia caused by an acute blood glucose disorder,” the authors noted.
Over 28 days of hospitalization, 18.8% (114) of the patients died and 39.2% developed one or more in-hospital complications.
The authors used the CRB-65 score, which assigns 1 point for each of four indicators – confusion, respiratory rate >30 breaths/min, systolic blood pressure ≤90 mm Hg or diastolic blood pressure ≤60 mm Hg, and age ≥65 years – to assess pneumonia severity.
Just over half, 55.2%, had a CRB-65 score of 0, 43.1% had a score of 1-2, and 1.7% had a score of 3-4.
In multivariable analysis, significant independent predictors of 28-day mortality were age (hazard ratio, 1.02), male sex (HR, 1.75), CRB-65 score 1-2 (HR, 2.68), CRB-65 score 3-4 (HR, 5.25), and fasting blood glucose ≥7.0 mmol/L (HR, 2.30).
Compared with patients with normal glucose (<6.1 mmol/L), 28-day mortality was twice as high (HR, 2.06) for those with a fasting blood glucose of 6.1-6.9 mmol/L and more than threefold higher for ≥7.0 mmol/L (HR, 3.54).
Pneumonia severity also predicted 28-day mortality, with hazard ratios of 4.35 and 13.80 for patients with CRB-65 scores of 1-2 and 3-4, respectively, compared with 0.
Inhospital complications, including acute respiratory distress syndrome or acute cardiac, kidney, or liver injury or cerebrovascular accident, occurred in 14.2%, 7.9%, and 17.0% of those in the lowest to highest fasting blood glucose groups.
Complications were more than twice as common in patients with a fasting blood glucose of 6.1-6.9 mmol/L (HR, 2.61) and four times more common (HR, 3.99) among those with a fasting blood glucose ≥7.0 mmol/L, compared with those with normoglycemia.
The study was supported by the National Natural Science Foundation of China and Major Projects of the National Science and Technology. The authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
new research indicates.
The findings, from a retrospective analysis of 605 patients with COVID-19 seen at two hospitals in Wuhan, China, were published online July 10 in Diabetologia by Sufei Wang, of the department of respiratory and critical care medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, and colleagues.
Several previous studies have demonstrated a link between hyperglycemia and worse outcomes in COVID-19, and at least one diabetes diagnosis, but this is the first to focus specifically on that group of patients.
Wang and colleagues found that a fasting blood glucose of 7.0 mmol/L (126 mg/dL) or greater on admission – present in 45.6% of those without a prior diabetes diagnosis – was an independent predictor of 28-day mortality.
Although A1c data weren’t analyzed, the population is believed to include both individuals with preexisting but undiagnosed diabetes and those without diabetes who have acute stress hyperglycemia.
“Glycemic testing and control should be recommended for all COVID-19 patients even if they do not have preexisting diabetes, as most COVID-19 patients are prone to glucose metabolic disorders,” they emphasized.
“Addressing elevated fasting blood glucose at an early stage can help clinicians better manage the condition and lower the mortality risk of COVID-19 patients,” Wang and colleagues noted.
Hyperglycemia predicts COVID-19 death, complications
The study involved consecutive patients with COVID-19 and definitive 28-day outcome and fasting blood glucose measurement on admission to two Wuhan-area hospitals between Jan. 24 to Feb. 10, 2020. A total of 605 patients did not have a previous diabetes diagnosis. They were a median age of 59 years and 53.2% were men.
Just over half, 54.4%, had a fasting blood glucose below 6.1 mmol/L (110.0 mg/dL). The rest had dysglycemia: 16.5% had a fasting blood glucose of 6.1-6.9 mmol/L (110-125 mg/dL), considered the prediabetes range, and 29.1% had a fasting blood glucose of 7 mmol/L (126 mg/dL) or above, the cutoff for diabetes.
“These results indicate that our study included both undiagnosed diabetic patients and nondiabetic patients with hyperglycemia caused by an acute blood glucose disorder,” the authors noted.
Over 28 days of hospitalization, 18.8% (114) of the patients died and 39.2% developed one or more in-hospital complications.
The authors used the CRB-65 score, which assigns 1 point for each of four indicators – confusion, respiratory rate >30 breaths/min, systolic blood pressure ≤90 mm Hg or diastolic blood pressure ≤60 mm Hg, and age ≥65 years – to assess pneumonia severity.
Just over half, 55.2%, had a CRB-65 score of 0, 43.1% had a score of 1-2, and 1.7% had a score of 3-4.
In multivariable analysis, significant independent predictors of 28-day mortality were age (hazard ratio, 1.02), male sex (HR, 1.75), CRB-65 score 1-2 (HR, 2.68), CRB-65 score 3-4 (HR, 5.25), and fasting blood glucose ≥7.0 mmol/L (HR, 2.30).
Compared with patients with normal glucose (<6.1 mmol/L), 28-day mortality was twice as high (HR, 2.06) for those with a fasting blood glucose of 6.1-6.9 mmol/L and more than threefold higher for ≥7.0 mmol/L (HR, 3.54).
Pneumonia severity also predicted 28-day mortality, with hazard ratios of 4.35 and 13.80 for patients with CRB-65 scores of 1-2 and 3-4, respectively, compared with 0.
Inhospital complications, including acute respiratory distress syndrome or acute cardiac, kidney, or liver injury or cerebrovascular accident, occurred in 14.2%, 7.9%, and 17.0% of those in the lowest to highest fasting blood glucose groups.
Complications were more than twice as common in patients with a fasting blood glucose of 6.1-6.9 mmol/L (HR, 2.61) and four times more common (HR, 3.99) among those with a fasting blood glucose ≥7.0 mmol/L, compared with those with normoglycemia.
The study was supported by the National Natural Science Foundation of China and Major Projects of the National Science and Technology. The authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.