User login
Lipid-lowering bempedoic acid does not hasten or worsen diabetes
In an analysis of four phase 3 trials, the oral lipid-lowering drug bempedoic acid (Nexletol; Esperion) did not worsen glycemic control or increase the incidence of type 2 diabetes.
As previously reported, this first-in-class drug, which acts by inhibiting ATP-citrate lyase, was approved by the Food and Drug Administration in February 2020.
Lawrence A. Leiter MD, from the University of Toronto, delivered the findings of this latest analysis in an oral presentation at the virtual American Diabetes Association 80th Scientific Sessions.
“The current study is important as it shows overall consistent efficacy and safety regardless of glycemic status and no increase in new-onset diabetes,” Dr. Leiter said in an interview.
There is interest in how lipid-lowering drugs might affect glycemia because “meta-analyses have shown about a 10% increased risk of new-onset diabetes in statin users, although the absolute increased risk is 1 extra case per 255 treated patients [in whom one would expect 5.4 cardiovascular events to be prevented by the statin],” he noted.
In a comment, John R. Guyton, MD, from Duke University Medical Center, Durham, N.C., agreed that the new study demonstrates that “patients with diabetes and prediabetes respond to bempedoic acid with LDL cholesterol lowering that is similar to that in patients with normal glucose tolerance.”
Although “statins have a slight effect of worsening glucose tolerance and a modest effect of increasing cases of new-onset diabetes,” the current research shows that “bempedoic acid appears to be free of these effects,” said Dr. Guyton, who discussed this drug in another symposium at the meeting where he also discussed how the agent will “fit” into prescribing patterns.
How do patients with diabetes, prediabetes fare?
“Current guidelines support aggressive LDL cholesterol lowering in patients with diabetes, given the increased risk of cardiovascular morbidity and mortality,” said Dr. Leiter.
Bempedoic acid was approved as an adjunct to diet and maximally tolerated statin therapy to treat adults with atherosclerotic cardiovascular disease (ASCVD) and/or heterozygous familial hypercholesterolemia (HeFH) who require additional lowering of LDL cholesterol, although its effect on cardiovascular morbidity and mortality has not been determined, the prescribing information states.
However, it has been unknown how bempedoic acid affects LDL cholesterol or hemoglobin A1c levels in patients with diabetes, prediabetes, or normoglycemia.
To examine this, the researchers pooled data from four phase 3 trials in 3623 patients with ASCVD or HeFH who had been randomized 2:1 to bempedoic acid 180 mg/day or placebo for 12 or 24 weeks (if they were statin intolerant) or 52 weeks (if they were also on statins).
In the pooled sample, about half the patients had prediabetes (52%), and the rest had diabetes (31%) or normoglycemia (17%). Overall, 75%-84% of patients had a history of ASCVD.
Mean LDL cholesterol levels were higher in patients with normoglycemia (119 mg/dL) or prediabetes (115 mg/dL) than in patients with diabetes (110 mg/dL).
The primary outcome was percent change in LDL cholesterol from baseline to week 12.
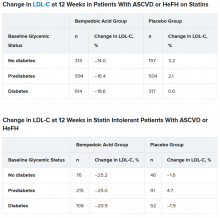
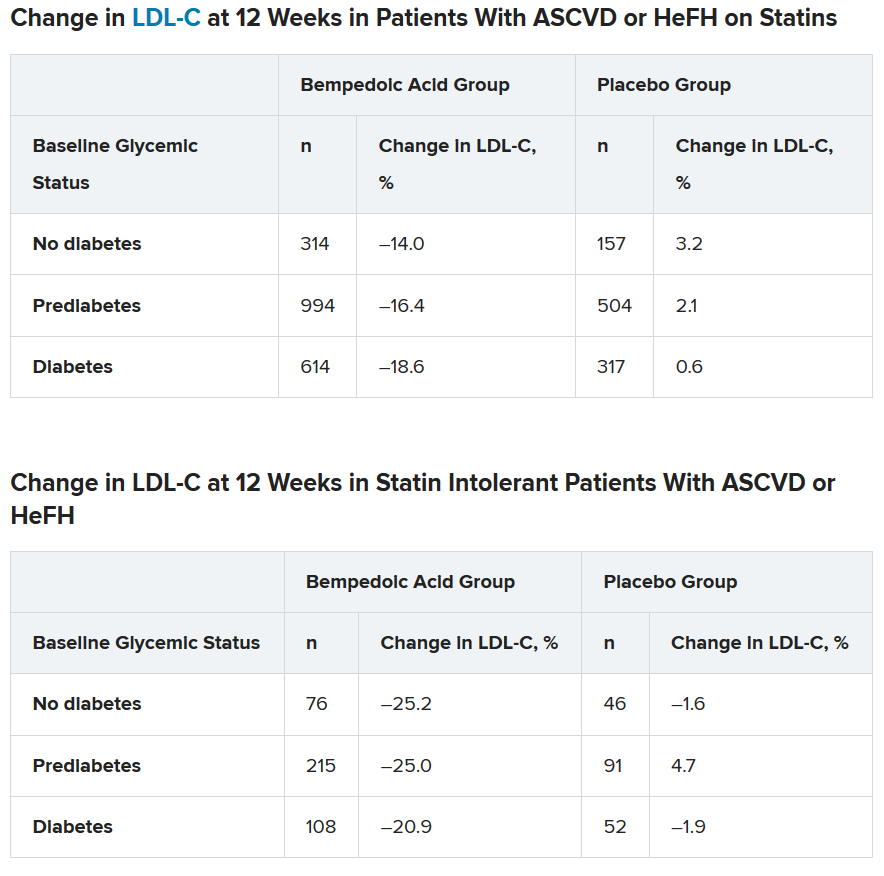
In the two types of patients (all with ASCVD or HeFH) – those on statins and those with statin intolerance – LDL cholesterol at 12 weeks was significantly lower in patients who received bempedoic acid, compared with placebo, regardless of whether they had no diabetes, prediabetes, or diabetes (all P < .001).
Similarly, patients who received bempedoic acid also had significant reductions in total cholesterol, non–HDL cholesterol, apolipoprotein B, and high-sensitivity C-reactive protein (hsCRP) at 12 weeks, compared with patients who received placebo (all P < .01).
The safety profile of bempedoic acid was similar to placebo and did not vary by glycemic status.
“Of course, with any lipid-lowering therapy, there’s lots of interest in changes in glycemic parameters,” said Dr. Leiter. “A1c did not increase. In fact, it was significantly lower in patients with prediabetes and diabetes on bempedoic acid versus placebo.”
In addition, “statin trials have shown small increases in body weight. We did not observe this,” he reported.
Where does bempedoic acid ‘fit?’
“Bempedoic acid will be a useful add-on to any patient who requires additional LDL cholesterol lowering,” according to Dr. Leiter. “It will typically be used as an add-on to statins, but will also be very useful in the statin-intolerant patient, especially when used in combination with ezetimib.”
The fixed-dose combination of bempedoic acid plus ezetimibe (Nexlizet; Esperion), was also approved in the United States in February, just days after bempedoic acid as a solo agent was cleared for marketing.
“Bempedoic acid would not be chosen in preference to a statin, ezetimibe, or PCSK9 inhibitor,” Dr. Guyton said. Rather, “its chief use will be in patients with statin intolerance and either FH or ASCVD when LDL-cholesterol is poorly controlled despite maximum tolerated lipid-lowering therapy.”
According to Dr. Guyton, “use of bempedoic acid should be undertaken only when provider-patient discussion acknowledges that it has not been shown to reduce cardiovascular events, although preliminary evidence from genetic analysis [Mendelian randomization study] suggests that it will,” as previously reported.
The CLEAR Outcomes cardiovascular outcomes trial of bempedoic acid completed enrollment in August 2019, involving 14,032 patients with hypercholesterolemia and high CVD risk according to a company statement.
The study was funded by Esperion. Dr. Leiter has reported being on advisory panels for Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, HLS Therapeutics, Janssen, Merck, Novo Nordisk, Sanofi, and Servier, receiving research support from Amgen, AstraZeneca, Kowa Pharmaceuticals, and the Medicines Company, and being on speakers bureaus for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, HLS Therapeutics, Janssen, Medscape, Merck, Novo Nordisk, Sanofi, and Servier. Disclosures for the other authors are listed with the abstract. Dr. Guyton has reported being a consultant for Amarin and receiving research support form Regeneron.
A version of this article originally appeared on Medscape.com.
In an analysis of four phase 3 trials, the oral lipid-lowering drug bempedoic acid (Nexletol; Esperion) did not worsen glycemic control or increase the incidence of type 2 diabetes.
As previously reported, this first-in-class drug, which acts by inhibiting ATP-citrate lyase, was approved by the Food and Drug Administration in February 2020.
Lawrence A. Leiter MD, from the University of Toronto, delivered the findings of this latest analysis in an oral presentation at the virtual American Diabetes Association 80th Scientific Sessions.
“The current study is important as it shows overall consistent efficacy and safety regardless of glycemic status and no increase in new-onset diabetes,” Dr. Leiter said in an interview.
There is interest in how lipid-lowering drugs might affect glycemia because “meta-analyses have shown about a 10% increased risk of new-onset diabetes in statin users, although the absolute increased risk is 1 extra case per 255 treated patients [in whom one would expect 5.4 cardiovascular events to be prevented by the statin],” he noted.
In a comment, John R. Guyton, MD, from Duke University Medical Center, Durham, N.C., agreed that the new study demonstrates that “patients with diabetes and prediabetes respond to bempedoic acid with LDL cholesterol lowering that is similar to that in patients with normal glucose tolerance.”
Although “statins have a slight effect of worsening glucose tolerance and a modest effect of increasing cases of new-onset diabetes,” the current research shows that “bempedoic acid appears to be free of these effects,” said Dr. Guyton, who discussed this drug in another symposium at the meeting where he also discussed how the agent will “fit” into prescribing patterns.
How do patients with diabetes, prediabetes fare?
“Current guidelines support aggressive LDL cholesterol lowering in patients with diabetes, given the increased risk of cardiovascular morbidity and mortality,” said Dr. Leiter.
Bempedoic acid was approved as an adjunct to diet and maximally tolerated statin therapy to treat adults with atherosclerotic cardiovascular disease (ASCVD) and/or heterozygous familial hypercholesterolemia (HeFH) who require additional lowering of LDL cholesterol, although its effect on cardiovascular morbidity and mortality has not been determined, the prescribing information states.
However, it has been unknown how bempedoic acid affects LDL cholesterol or hemoglobin A1c levels in patients with diabetes, prediabetes, or normoglycemia.
To examine this, the researchers pooled data from four phase 3 trials in 3623 patients with ASCVD or HeFH who had been randomized 2:1 to bempedoic acid 180 mg/day or placebo for 12 or 24 weeks (if they were statin intolerant) or 52 weeks (if they were also on statins).
In the pooled sample, about half the patients had prediabetes (52%), and the rest had diabetes (31%) or normoglycemia (17%). Overall, 75%-84% of patients had a history of ASCVD.
Mean LDL cholesterol levels were higher in patients with normoglycemia (119 mg/dL) or prediabetes (115 mg/dL) than in patients with diabetes (110 mg/dL).
The primary outcome was percent change in LDL cholesterol from baseline to week 12.
In the two types of patients (all with ASCVD or HeFH) – those on statins and those with statin intolerance – LDL cholesterol at 12 weeks was significantly lower in patients who received bempedoic acid, compared with placebo, regardless of whether they had no diabetes, prediabetes, or diabetes (all P < .001).
Similarly, patients who received bempedoic acid also had significant reductions in total cholesterol, non–HDL cholesterol, apolipoprotein B, and high-sensitivity C-reactive protein (hsCRP) at 12 weeks, compared with patients who received placebo (all P < .01).
The safety profile of bempedoic acid was similar to placebo and did not vary by glycemic status.
“Of course, with any lipid-lowering therapy, there’s lots of interest in changes in glycemic parameters,” said Dr. Leiter. “A1c did not increase. In fact, it was significantly lower in patients with prediabetes and diabetes on bempedoic acid versus placebo.”
In addition, “statin trials have shown small increases in body weight. We did not observe this,” he reported.
Where does bempedoic acid ‘fit?’
“Bempedoic acid will be a useful add-on to any patient who requires additional LDL cholesterol lowering,” according to Dr. Leiter. “It will typically be used as an add-on to statins, but will also be very useful in the statin-intolerant patient, especially when used in combination with ezetimib.”
The fixed-dose combination of bempedoic acid plus ezetimibe (Nexlizet; Esperion), was also approved in the United States in February, just days after bempedoic acid as a solo agent was cleared for marketing.
“Bempedoic acid would not be chosen in preference to a statin, ezetimibe, or PCSK9 inhibitor,” Dr. Guyton said. Rather, “its chief use will be in patients with statin intolerance and either FH or ASCVD when LDL-cholesterol is poorly controlled despite maximum tolerated lipid-lowering therapy.”
According to Dr. Guyton, “use of bempedoic acid should be undertaken only when provider-patient discussion acknowledges that it has not been shown to reduce cardiovascular events, although preliminary evidence from genetic analysis [Mendelian randomization study] suggests that it will,” as previously reported.
The CLEAR Outcomes cardiovascular outcomes trial of bempedoic acid completed enrollment in August 2019, involving 14,032 patients with hypercholesterolemia and high CVD risk according to a company statement.
The study was funded by Esperion. Dr. Leiter has reported being on advisory panels for Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, HLS Therapeutics, Janssen, Merck, Novo Nordisk, Sanofi, and Servier, receiving research support from Amgen, AstraZeneca, Kowa Pharmaceuticals, and the Medicines Company, and being on speakers bureaus for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, HLS Therapeutics, Janssen, Medscape, Merck, Novo Nordisk, Sanofi, and Servier. Disclosures for the other authors are listed with the abstract. Dr. Guyton has reported being a consultant for Amarin and receiving research support form Regeneron.
A version of this article originally appeared on Medscape.com.
In an analysis of four phase 3 trials, the oral lipid-lowering drug bempedoic acid (Nexletol; Esperion) did not worsen glycemic control or increase the incidence of type 2 diabetes.
As previously reported, this first-in-class drug, which acts by inhibiting ATP-citrate lyase, was approved by the Food and Drug Administration in February 2020.
Lawrence A. Leiter MD, from the University of Toronto, delivered the findings of this latest analysis in an oral presentation at the virtual American Diabetes Association 80th Scientific Sessions.
“The current study is important as it shows overall consistent efficacy and safety regardless of glycemic status and no increase in new-onset diabetes,” Dr. Leiter said in an interview.
There is interest in how lipid-lowering drugs might affect glycemia because “meta-analyses have shown about a 10% increased risk of new-onset diabetes in statin users, although the absolute increased risk is 1 extra case per 255 treated patients [in whom one would expect 5.4 cardiovascular events to be prevented by the statin],” he noted.
In a comment, John R. Guyton, MD, from Duke University Medical Center, Durham, N.C., agreed that the new study demonstrates that “patients with diabetes and prediabetes respond to bempedoic acid with LDL cholesterol lowering that is similar to that in patients with normal glucose tolerance.”
Although “statins have a slight effect of worsening glucose tolerance and a modest effect of increasing cases of new-onset diabetes,” the current research shows that “bempedoic acid appears to be free of these effects,” said Dr. Guyton, who discussed this drug in another symposium at the meeting where he also discussed how the agent will “fit” into prescribing patterns.
How do patients with diabetes, prediabetes fare?
“Current guidelines support aggressive LDL cholesterol lowering in patients with diabetes, given the increased risk of cardiovascular morbidity and mortality,” said Dr. Leiter.
Bempedoic acid was approved as an adjunct to diet and maximally tolerated statin therapy to treat adults with atherosclerotic cardiovascular disease (ASCVD) and/or heterozygous familial hypercholesterolemia (HeFH) who require additional lowering of LDL cholesterol, although its effect on cardiovascular morbidity and mortality has not been determined, the prescribing information states.
However, it has been unknown how bempedoic acid affects LDL cholesterol or hemoglobin A1c levels in patients with diabetes, prediabetes, or normoglycemia.
To examine this, the researchers pooled data from four phase 3 trials in 3623 patients with ASCVD or HeFH who had been randomized 2:1 to bempedoic acid 180 mg/day or placebo for 12 or 24 weeks (if they were statin intolerant) or 52 weeks (if they were also on statins).
In the pooled sample, about half the patients had prediabetes (52%), and the rest had diabetes (31%) or normoglycemia (17%). Overall, 75%-84% of patients had a history of ASCVD.
Mean LDL cholesterol levels were higher in patients with normoglycemia (119 mg/dL) or prediabetes (115 mg/dL) than in patients with diabetes (110 mg/dL).
The primary outcome was percent change in LDL cholesterol from baseline to week 12.
In the two types of patients (all with ASCVD or HeFH) – those on statins and those with statin intolerance – LDL cholesterol at 12 weeks was significantly lower in patients who received bempedoic acid, compared with placebo, regardless of whether they had no diabetes, prediabetes, or diabetes (all P < .001).
Similarly, patients who received bempedoic acid also had significant reductions in total cholesterol, non–HDL cholesterol, apolipoprotein B, and high-sensitivity C-reactive protein (hsCRP) at 12 weeks, compared with patients who received placebo (all P < .01).
The safety profile of bempedoic acid was similar to placebo and did not vary by glycemic status.
“Of course, with any lipid-lowering therapy, there’s lots of interest in changes in glycemic parameters,” said Dr. Leiter. “A1c did not increase. In fact, it was significantly lower in patients with prediabetes and diabetes on bempedoic acid versus placebo.”
In addition, “statin trials have shown small increases in body weight. We did not observe this,” he reported.
Where does bempedoic acid ‘fit?’
“Bempedoic acid will be a useful add-on to any patient who requires additional LDL cholesterol lowering,” according to Dr. Leiter. “It will typically be used as an add-on to statins, but will also be very useful in the statin-intolerant patient, especially when used in combination with ezetimib.”
The fixed-dose combination of bempedoic acid plus ezetimibe (Nexlizet; Esperion), was also approved in the United States in February, just days after bempedoic acid as a solo agent was cleared for marketing.
“Bempedoic acid would not be chosen in preference to a statin, ezetimibe, or PCSK9 inhibitor,” Dr. Guyton said. Rather, “its chief use will be in patients with statin intolerance and either FH or ASCVD when LDL-cholesterol is poorly controlled despite maximum tolerated lipid-lowering therapy.”
According to Dr. Guyton, “use of bempedoic acid should be undertaken only when provider-patient discussion acknowledges that it has not been shown to reduce cardiovascular events, although preliminary evidence from genetic analysis [Mendelian randomization study] suggests that it will,” as previously reported.
The CLEAR Outcomes cardiovascular outcomes trial of bempedoic acid completed enrollment in August 2019, involving 14,032 patients with hypercholesterolemia and high CVD risk according to a company statement.
The study was funded by Esperion. Dr. Leiter has reported being on advisory panels for Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, HLS Therapeutics, Janssen, Merck, Novo Nordisk, Sanofi, and Servier, receiving research support from Amgen, AstraZeneca, Kowa Pharmaceuticals, and the Medicines Company, and being on speakers bureaus for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, HLS Therapeutics, Janssen, Medscape, Merck, Novo Nordisk, Sanofi, and Servier. Disclosures for the other authors are listed with the abstract. Dr. Guyton has reported being a consultant for Amarin and receiving research support form Regeneron.
A version of this article originally appeared on Medscape.com.
Lyumjev ultra-rapid-acting insulin gets FDA nod
The US Food and Drug Administration has approved rapid-acting insulin lispro-aabc injection 100 and 200 units/mL (Lyumjev, Eli Lilly) for the treatment of adults with type 1 and type 2 diabetes.
The product is a novel formulation of insulin lispro developed to speed absorption of insulin into the bloodstream. It will be available in two strengths: U-100 (100 units/mL) and U-200 (200 units/mL). The Lyumjev U-200 prefilled pen contains twice as much insulin per 1 mL as standard (U-100) insulin.
Approval was based on data from two phase 3 randomized, active-controlled, treat-to-target studies comparing lispro-aabc with insulin lispro injection 100 units/mL (Humalog, Lilly) in people with type 1 diabetes (PRONTO-T1D) and type 2 diabetes (PRONTO-T2D).
In both studies, noninferiority in A1c reduction was demonstrated when the two insulins were dosed at mealtime, but lispro-aabc showed superior blood glucose reduction at 1-hour and 2-hours post-meal compared with lispro.
Lyumjev is approved only in the United States for use as part of a multiple daily injection regimen, not for use in insulin pumps. Lilly intends to submit for this latter indication later in 2020.
Lyumjev will compete with Novo Nordisk’s fast-acting insulin aspart injection 100 units/mL (Fiasp).
Fiasp had a big head start: It was approved for use in adults in the United States in September 2017, for use in insulin pumps in October 2019, and for use in children with diabetes in January 2020.
However, in a poster presented at the American Diabetes Association 79th Scientific Sessions in 2019, lispro-aabb demonstrated faster insulin absorption than lispro, insulin aspart (Novolog/Novorapid, Novo Nordisk), or Fiasp.
Early half-maximal drug concentration was reached at 13 minutes with lispro-aabb, compared with 19 minutes with faster aspart and 25-27 minutes with the two conventional insulins (P < .05 for lispro-aabb vs other insulins).
Insulin lispro-aabc was approved in the European Union and Japan in March 2020.
Lilly is currently working to make Lyumjev available to adults with diabetes in the United States as quickly as possible and says it will be included in the Lilly Insulin Value Program, allowing anyone with commercial insurance and those without insurance to fill their monthly prescription of Lyumjev for $35.
The list price of Lyumjev will be the same as the list price for Humalog, it adds.
This article first appeared on Medscape.com.
The US Food and Drug Administration has approved rapid-acting insulin lispro-aabc injection 100 and 200 units/mL (Lyumjev, Eli Lilly) for the treatment of adults with type 1 and type 2 diabetes.
The product is a novel formulation of insulin lispro developed to speed absorption of insulin into the bloodstream. It will be available in two strengths: U-100 (100 units/mL) and U-200 (200 units/mL). The Lyumjev U-200 prefilled pen contains twice as much insulin per 1 mL as standard (U-100) insulin.
Approval was based on data from two phase 3 randomized, active-controlled, treat-to-target studies comparing lispro-aabc with insulin lispro injection 100 units/mL (Humalog, Lilly) in people with type 1 diabetes (PRONTO-T1D) and type 2 diabetes (PRONTO-T2D).
In both studies, noninferiority in A1c reduction was demonstrated when the two insulins were dosed at mealtime, but lispro-aabc showed superior blood glucose reduction at 1-hour and 2-hours post-meal compared with lispro.
Lyumjev is approved only in the United States for use as part of a multiple daily injection regimen, not for use in insulin pumps. Lilly intends to submit for this latter indication later in 2020.
Lyumjev will compete with Novo Nordisk’s fast-acting insulin aspart injection 100 units/mL (Fiasp).
Fiasp had a big head start: It was approved for use in adults in the United States in September 2017, for use in insulin pumps in October 2019, and for use in children with diabetes in January 2020.
However, in a poster presented at the American Diabetes Association 79th Scientific Sessions in 2019, lispro-aabb demonstrated faster insulin absorption than lispro, insulin aspart (Novolog/Novorapid, Novo Nordisk), or Fiasp.
Early half-maximal drug concentration was reached at 13 minutes with lispro-aabb, compared with 19 minutes with faster aspart and 25-27 minutes with the two conventional insulins (P < .05 for lispro-aabb vs other insulins).
Insulin lispro-aabc was approved in the European Union and Japan in March 2020.
Lilly is currently working to make Lyumjev available to adults with diabetes in the United States as quickly as possible and says it will be included in the Lilly Insulin Value Program, allowing anyone with commercial insurance and those without insurance to fill their monthly prescription of Lyumjev for $35.
The list price of Lyumjev will be the same as the list price for Humalog, it adds.
This article first appeared on Medscape.com.
The US Food and Drug Administration has approved rapid-acting insulin lispro-aabc injection 100 and 200 units/mL (Lyumjev, Eli Lilly) for the treatment of adults with type 1 and type 2 diabetes.
The product is a novel formulation of insulin lispro developed to speed absorption of insulin into the bloodstream. It will be available in two strengths: U-100 (100 units/mL) and U-200 (200 units/mL). The Lyumjev U-200 prefilled pen contains twice as much insulin per 1 mL as standard (U-100) insulin.
Approval was based on data from two phase 3 randomized, active-controlled, treat-to-target studies comparing lispro-aabc with insulin lispro injection 100 units/mL (Humalog, Lilly) in people with type 1 diabetes (PRONTO-T1D) and type 2 diabetes (PRONTO-T2D).
In both studies, noninferiority in A1c reduction was demonstrated when the two insulins were dosed at mealtime, but lispro-aabc showed superior blood glucose reduction at 1-hour and 2-hours post-meal compared with lispro.
Lyumjev is approved only in the United States for use as part of a multiple daily injection regimen, not for use in insulin pumps. Lilly intends to submit for this latter indication later in 2020.
Lyumjev will compete with Novo Nordisk’s fast-acting insulin aspart injection 100 units/mL (Fiasp).
Fiasp had a big head start: It was approved for use in adults in the United States in September 2017, for use in insulin pumps in October 2019, and for use in children with diabetes in January 2020.
However, in a poster presented at the American Diabetes Association 79th Scientific Sessions in 2019, lispro-aabb demonstrated faster insulin absorption than lispro, insulin aspart (Novolog/Novorapid, Novo Nordisk), or Fiasp.
Early half-maximal drug concentration was reached at 13 minutes with lispro-aabb, compared with 19 minutes with faster aspart and 25-27 minutes with the two conventional insulins (P < .05 for lispro-aabb vs other insulins).
Insulin lispro-aabc was approved in the European Union and Japan in March 2020.
Lilly is currently working to make Lyumjev available to adults with diabetes in the United States as quickly as possible and says it will be included in the Lilly Insulin Value Program, allowing anyone with commercial insurance and those without insurance to fill their monthly prescription of Lyumjev for $35.
The list price of Lyumjev will be the same as the list price for Humalog, it adds.
This article first appeared on Medscape.com.
Where does dexamethasone fit in with diabetic ketoacidosis in COVID-19?
A new article in the Journal of Clinical Endocrinology & Metabolism (JCEM) addresses unique concerns and considerations regarding diabetic ketoacidosis (DKA) in the setting of COVID-19.
Corresponding author Marie E. McDonnell, MD, director of the diabetes program at Brigham and Women’s Hospital, Boston, Massachusetts, discussed the recommendations with Medscape Medical News and also spoke about the news this week that the corticosteroid dexamethasone reduced death rates in severely ill patients with COVID-19.
The full JCEM article, by lead author Nadine E. Palermo, DO, Division of Endocrinology, Diabetes, and Hypertension, also at Brigham and Women’s Hospital, covers DKA diagnosis and triage, and emphasizes that usual hospital protocols for DKA management may need to be adjusted during COVID-19 to help preserve personal protective equipment and ICU beds.
“Hospitals and clinicians need to be able to quickly identify and manage DKA in COVID patients to save lives. This involves determining the options for management, including when less intensive subcutaneous insulin is indicated, and understanding how to guide patients on avoiding this serious complication,” McDonnell said in an Endocrine Society statement.
What about dexamethasone for severe COVID-19 in diabetes?
The new article briefly touches on the fact that upward adjustments to intensive intravenous insulin therapy for DKA may be necessary in patients with COVID-19 who are receiving concomitant corticosteroids or vasopressors.
But it was written prior to the June 16 announcement of the “RECOVERY” trial results with dexamethasone. The UK National Health Service immediately approved the drug’s use in the COVID-19 setting, despite the fact that there has been no published article on the findings yet.
McDonnell told Medscape Medical News that she would need to see formal results to better understand exactly which patients were studied and which ones benefited.
“The peer review will be critical. It looks as if it only benefits people who need respiratory support, but I want to understand that in much more detail,” she said. “If they all had acute respiratory distress syndrome [ARDS],” that’s different.
“There are already some data supporting steroid use in ARDS,” she noted, but added that not all of it suggests benefit.
She pointed to one of several studies now showing that diabetes, and hyperglycemia among people without a prior diabetes diagnosis, are both strong predictors of mortality in hospitalized patients with COVID-19.
“There was a very clear relationship between hyperglycemia and outcomes. We really shouldn’t put people at risk until we have clear data,” she said.
If, once the data are reviewed and appropriate dexamethasone becomes an established treatment for severe COVID-19, hyperglycemia would be a concern among all patients, not just those with previously diagnosed diabetes, she noted.
“We know a good number of people with prediabetes develop hyperglycemia when put on steroids. They can push people over the edge. We’re not going to miss anybody, but treating steroid-induced hyperglycemia is really hard,” McDonnell explained.
She also recommended 2014 guidance from Diabetes UK and the Association of British Clinical Diabetologists, which addresses management of inpatient steroid-induced DKA in patients with and without pre-existing diabetes.
Another major concern, she said, is “patients trying to get dexamethasone when they start to get sick” because this is not the right population to use this agent.
“We worry about people who do not need this drug. If they have diabetes, they put themselves at risk of hyperglycemia, which then increases the risk of severe COVID-19. And then they’re also putting themselves at risk of DKA. It would just be bad medicine,” she said.
Managing DKA in the face of COVID-19: Flexibility is key
In the JCEM article, Palermo and colleagues emphasize that the usual hospital protocols for DKA management may need to be adjusted during COVID-19 in the interest of reducing transmission risk and preserving scare resources.
They provide evidence for alternative treatment strategies, such as the use of subcutaneous rather than intravenous insulin when appropriate.
“We wanted to outline when exactly you should consider nonintensive management strategies for DKA,” McDonnell further explained to Medscape Medical News.
“That would include those with mild or some with moderate DKA. ... The idea is to remind our colleagues about that because hospitals tend to operate on a protocol-driven algorithmic methodology, they can forget to step off the usual care pathway even if evidence supports that,” she said.
But on the other hand, she also said that, in some very complex or severely ill patients with COVID-19, classical intravenous insulin therapy makes the most sense even if their DKA is mild.
The outpatient setting: Prevention and preparation
The new article also addresses several concerns regarding DKA prevention in the outpatient setting.
As with other guidelines, it includes a reminder that patients with diabetes should be advised to discontinue sodium-glucose cotransporter 2 (SGLT2) inhibitors if they become ill with COVID-19, especially if they’re not eating or drinking normally, because they raise the risk for DKA.
Also, for patients with type 1 diabetes, particularly those with a history of repeated DKA, “this is the time to make sure we reach out to patients to refill their insulin prescriptions and address issues related to cost and other access difficulties,” McDonnell said.
The authors also emphasize that insulin starts and education should not be postponed during the pandemic. “Patients identified as meeting criteria to start insulin should be referred for urgent education, either in person or, whenever possible and practical, via video teleconferencing,” they urge.
McDonnell has reported receiving research funding from Novo Nordisk. The other two authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
A new article in the Journal of Clinical Endocrinology & Metabolism (JCEM) addresses unique concerns and considerations regarding diabetic ketoacidosis (DKA) in the setting of COVID-19.
Corresponding author Marie E. McDonnell, MD, director of the diabetes program at Brigham and Women’s Hospital, Boston, Massachusetts, discussed the recommendations with Medscape Medical News and also spoke about the news this week that the corticosteroid dexamethasone reduced death rates in severely ill patients with COVID-19.
The full JCEM article, by lead author Nadine E. Palermo, DO, Division of Endocrinology, Diabetes, and Hypertension, also at Brigham and Women’s Hospital, covers DKA diagnosis and triage, and emphasizes that usual hospital protocols for DKA management may need to be adjusted during COVID-19 to help preserve personal protective equipment and ICU beds.
“Hospitals and clinicians need to be able to quickly identify and manage DKA in COVID patients to save lives. This involves determining the options for management, including when less intensive subcutaneous insulin is indicated, and understanding how to guide patients on avoiding this serious complication,” McDonnell said in an Endocrine Society statement.
What about dexamethasone for severe COVID-19 in diabetes?
The new article briefly touches on the fact that upward adjustments to intensive intravenous insulin therapy for DKA may be necessary in patients with COVID-19 who are receiving concomitant corticosteroids or vasopressors.
But it was written prior to the June 16 announcement of the “RECOVERY” trial results with dexamethasone. The UK National Health Service immediately approved the drug’s use in the COVID-19 setting, despite the fact that there has been no published article on the findings yet.
McDonnell told Medscape Medical News that she would need to see formal results to better understand exactly which patients were studied and which ones benefited.
“The peer review will be critical. It looks as if it only benefits people who need respiratory support, but I want to understand that in much more detail,” she said. “If they all had acute respiratory distress syndrome [ARDS],” that’s different.
“There are already some data supporting steroid use in ARDS,” she noted, but added that not all of it suggests benefit.
She pointed to one of several studies now showing that diabetes, and hyperglycemia among people without a prior diabetes diagnosis, are both strong predictors of mortality in hospitalized patients with COVID-19.
“There was a very clear relationship between hyperglycemia and outcomes. We really shouldn’t put people at risk until we have clear data,” she said.
If, once the data are reviewed and appropriate dexamethasone becomes an established treatment for severe COVID-19, hyperglycemia would be a concern among all patients, not just those with previously diagnosed diabetes, she noted.
“We know a good number of people with prediabetes develop hyperglycemia when put on steroids. They can push people over the edge. We’re not going to miss anybody, but treating steroid-induced hyperglycemia is really hard,” McDonnell explained.
She also recommended 2014 guidance from Diabetes UK and the Association of British Clinical Diabetologists, which addresses management of inpatient steroid-induced DKA in patients with and without pre-existing diabetes.
Another major concern, she said, is “patients trying to get dexamethasone when they start to get sick” because this is not the right population to use this agent.
“We worry about people who do not need this drug. If they have diabetes, they put themselves at risk of hyperglycemia, which then increases the risk of severe COVID-19. And then they’re also putting themselves at risk of DKA. It would just be bad medicine,” she said.
Managing DKA in the face of COVID-19: Flexibility is key
In the JCEM article, Palermo and colleagues emphasize that the usual hospital protocols for DKA management may need to be adjusted during COVID-19 in the interest of reducing transmission risk and preserving scare resources.
They provide evidence for alternative treatment strategies, such as the use of subcutaneous rather than intravenous insulin when appropriate.
“We wanted to outline when exactly you should consider nonintensive management strategies for DKA,” McDonnell further explained to Medscape Medical News.
“That would include those with mild or some with moderate DKA. ... The idea is to remind our colleagues about that because hospitals tend to operate on a protocol-driven algorithmic methodology, they can forget to step off the usual care pathway even if evidence supports that,” she said.
But on the other hand, she also said that, in some very complex or severely ill patients with COVID-19, classical intravenous insulin therapy makes the most sense even if their DKA is mild.
The outpatient setting: Prevention and preparation
The new article also addresses several concerns regarding DKA prevention in the outpatient setting.
As with other guidelines, it includes a reminder that patients with diabetes should be advised to discontinue sodium-glucose cotransporter 2 (SGLT2) inhibitors if they become ill with COVID-19, especially if they’re not eating or drinking normally, because they raise the risk for DKA.
Also, for patients with type 1 diabetes, particularly those with a history of repeated DKA, “this is the time to make sure we reach out to patients to refill their insulin prescriptions and address issues related to cost and other access difficulties,” McDonnell said.
The authors also emphasize that insulin starts and education should not be postponed during the pandemic. “Patients identified as meeting criteria to start insulin should be referred for urgent education, either in person or, whenever possible and practical, via video teleconferencing,” they urge.
McDonnell has reported receiving research funding from Novo Nordisk. The other two authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
A new article in the Journal of Clinical Endocrinology & Metabolism (JCEM) addresses unique concerns and considerations regarding diabetic ketoacidosis (DKA) in the setting of COVID-19.
Corresponding author Marie E. McDonnell, MD, director of the diabetes program at Brigham and Women’s Hospital, Boston, Massachusetts, discussed the recommendations with Medscape Medical News and also spoke about the news this week that the corticosteroid dexamethasone reduced death rates in severely ill patients with COVID-19.
The full JCEM article, by lead author Nadine E. Palermo, DO, Division of Endocrinology, Diabetes, and Hypertension, also at Brigham and Women’s Hospital, covers DKA diagnosis and triage, and emphasizes that usual hospital protocols for DKA management may need to be adjusted during COVID-19 to help preserve personal protective equipment and ICU beds.
“Hospitals and clinicians need to be able to quickly identify and manage DKA in COVID patients to save lives. This involves determining the options for management, including when less intensive subcutaneous insulin is indicated, and understanding how to guide patients on avoiding this serious complication,” McDonnell said in an Endocrine Society statement.
What about dexamethasone for severe COVID-19 in diabetes?
The new article briefly touches on the fact that upward adjustments to intensive intravenous insulin therapy for DKA may be necessary in patients with COVID-19 who are receiving concomitant corticosteroids or vasopressors.
But it was written prior to the June 16 announcement of the “RECOVERY” trial results with dexamethasone. The UK National Health Service immediately approved the drug’s use in the COVID-19 setting, despite the fact that there has been no published article on the findings yet.
McDonnell told Medscape Medical News that she would need to see formal results to better understand exactly which patients were studied and which ones benefited.
“The peer review will be critical. It looks as if it only benefits people who need respiratory support, but I want to understand that in much more detail,” she said. “If they all had acute respiratory distress syndrome [ARDS],” that’s different.
“There are already some data supporting steroid use in ARDS,” she noted, but added that not all of it suggests benefit.
She pointed to one of several studies now showing that diabetes, and hyperglycemia among people without a prior diabetes diagnosis, are both strong predictors of mortality in hospitalized patients with COVID-19.
“There was a very clear relationship between hyperglycemia and outcomes. We really shouldn’t put people at risk until we have clear data,” she said.
If, once the data are reviewed and appropriate dexamethasone becomes an established treatment for severe COVID-19, hyperglycemia would be a concern among all patients, not just those with previously diagnosed diabetes, she noted.
“We know a good number of people with prediabetes develop hyperglycemia when put on steroids. They can push people over the edge. We’re not going to miss anybody, but treating steroid-induced hyperglycemia is really hard,” McDonnell explained.
She also recommended 2014 guidance from Diabetes UK and the Association of British Clinical Diabetologists, which addresses management of inpatient steroid-induced DKA in patients with and without pre-existing diabetes.
Another major concern, she said, is “patients trying to get dexamethasone when they start to get sick” because this is not the right population to use this agent.
“We worry about people who do not need this drug. If they have diabetes, they put themselves at risk of hyperglycemia, which then increases the risk of severe COVID-19. And then they’re also putting themselves at risk of DKA. It would just be bad medicine,” she said.
Managing DKA in the face of COVID-19: Flexibility is key
In the JCEM article, Palermo and colleagues emphasize that the usual hospital protocols for DKA management may need to be adjusted during COVID-19 in the interest of reducing transmission risk and preserving scare resources.
They provide evidence for alternative treatment strategies, such as the use of subcutaneous rather than intravenous insulin when appropriate.
“We wanted to outline when exactly you should consider nonintensive management strategies for DKA,” McDonnell further explained to Medscape Medical News.
“That would include those with mild or some with moderate DKA. ... The idea is to remind our colleagues about that because hospitals tend to operate on a protocol-driven algorithmic methodology, they can forget to step off the usual care pathway even if evidence supports that,” she said.
But on the other hand, she also said that, in some very complex or severely ill patients with COVID-19, classical intravenous insulin therapy makes the most sense even if their DKA is mild.
The outpatient setting: Prevention and preparation
The new article also addresses several concerns regarding DKA prevention in the outpatient setting.
As with other guidelines, it includes a reminder that patients with diabetes should be advised to discontinue sodium-glucose cotransporter 2 (SGLT2) inhibitors if they become ill with COVID-19, especially if they’re not eating or drinking normally, because they raise the risk for DKA.
Also, for patients with type 1 diabetes, particularly those with a history of repeated DKA, “this is the time to make sure we reach out to patients to refill their insulin prescriptions and address issues related to cost and other access difficulties,” McDonnell said.
The authors also emphasize that insulin starts and education should not be postponed during the pandemic. “Patients identified as meeting criteria to start insulin should be referred for urgent education, either in person or, whenever possible and practical, via video teleconferencing,” they urge.
McDonnell has reported receiving research funding from Novo Nordisk. The other two authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
Dapagliflozin’s T2D renal protection extends to ‘fast decline’ of eGFR
Treatment of patients with type 2 diabetes with the SGLT2 inhibitor dapagliflozin led to a significant drop in the occurrence of ‘fast decline’ of renal function in more than 15,000 patients enrolled in the drug’s main cardiovascular outcome trial, another example of the potent renal protective effects of agents from this drug class.
Among patients with type 2 diabetes enrolled in the DECLARE-TIMI 58 trial, the incidence of a fast decline in renal function, defined as a drop in estimated glomerular filtration rate (eGFR) of at least 3 mL/min per 1.73 m2, was 27% among patients treated with dapagliflozin and 37% in control patients who received placebo, a statistically significant difference for this post-hoc analysis, Itamar Raz, MD, said at the virtual annual scientific sessions of the American Diabetes Association.
This finding, which adds to a long list of other renal function parameters reported to have been improved by treatment with sodium-glucose cotransporter 2 (SGLT2) inhibitors, “emphasizes the value of SGLT2 inhibitors as an important component of both prevention and treatment of chronic kidney disease among patients with type 2 diabetes,” said Dr. Raz, a diabetes researcher and professor of medicine at Hadassah University Hospital in Jerusalem.
The primary, prespecified renal outcomes in DECLARE-TIMI 58 were a cardiorenal composite outcome of sustained decline of at least 40% in eGFR to less than 60 mL/min per 1.73 m2, end-stage renal disease (defined as dialysis for at least 90 days, kidney transplantation, or confirmed sustained eGFR of less than 15 mL/min per 1.73 m2), or death from renal or cardiovascular causes; and a second prespecified renal-specific composite outcome that was the same except for excluding death from cardiovascular causes. The results showed that the cardiorenal outcome dropped by a statistically significant 24% with dapagliflozin treatment relative to control patients, and the renal-specific outcome fell by a statistically significant 47% with dapagliflozin relative to control patients (Lancet Diab Endocrinol. 2019 Aug 1;7[8];606-17).
The new findings on the incidence of fast decline in renal function help to further flesh out the scope of renal benefit exerted by SGLT2 inhibitors like dapagliflozin in patients with type 2 diabetes, said experts. Fast decline is a relatively recently devised measure of a high-risk, precipitous loss of renal function that has been defined as a drop of either 3 or 5 mL/min per 1.73 m2 per year (Kidney Int. 2017 Jun;91[6]:1300-11); for this analysis Dr. Raz and his associates used the less stringent definition.
Finding and treating ‘fast decliners’
The new report from Dr. Raz “confirms the original [renal] findings and looks to expand them to a particularly high risk group: the fast decliners,” commented Robert A. Gabbay, MD, chief science & medical officer of the ADA. “In some ways, the group of patients that we need to find a better treatment for most are those whose GFR declines quickly. We don’t always know who they are until after the fact, and studies have been looking for markers that might prospectively identify them,” he said in an interview.
The new analysis showed that dapagliflozin “was effective in this subgroup of patients. Furthermore, it didn’t matter if they had significant baseline disease or not. Even people with normal kidney function [at baseline] who were still fast decliners fared better with the drug than without it. This suggests that, if it can be confirmed in a prospective study, dapagliflozin might be effective very early in the course of treatment if we can identify who will be the fast decliners.”
Dr. Raz and his associates had the data necessary to calculate the rates of eGFR decline during the full follow-up period for 15,012 of the 17,160 patients enrolled in DECLARE-TIMI 58, and they found that 4,788 (32%) were fast decliners and 10,224 had a slower rate of renal deterioration. The average annual decline in eGFR during the period from 6 months after study entry through 4 years was 6.3 mL/min per 1.73 m2 per year (median of 5.1 mL/min per 1.73 m2 per year) among the fast decliners, and zero (median of 0.6 mL/min per 1.73 m2 per year) among the other patients.
Overcoming dapagliflozin’s initial eGFR reduction
The researchers focused on the 6-month to 4-year period of treatment as more representative of the impact of dapagliflozin because the SGLT2 inhibitors have an established pattern of triggering an initial, moderate decline in eGFR over roughly the first 6 months on the drug, which is similar to what happens to patients who start treatment with an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker.
“Some patients get as much as a 10% decline in eGFR” when SGLT2 inhibitor treatment starts, but “patients do better over time even with this initial hit,” the same way they do on drugs that act on the renin-angiotensin system, explained Silvio E. Inzucchi, MD, an endocrinologist and professor of medicine at Yale University in New Haven who has extensively studied the SGLT2 inhibitors.
The analyses reported by Dr. Raz showed that the protection against fast decline during the 6-month to 4-year period with dapagliflozin treatment was consistent across a range of patient subgroups regardless of age, duration of their type 2 diabetes, their baseline level of hyperglycemia, and their baseline eGFR. Nearly half the patients enrolled in DECLARE-TIMI 58 had an eGFR at baseline of at least 91 mL/min per 1.73 m2 and in this subgroup the incidence of fast decliners was 23% with dapagliflozin and 31% on placebo. Among the 45% of patients who began with an eGFR of 60-90 mL/min per 1.73 m2 the fast-decliner incidence was 32% and 43% when on or off dapagliflozin. Among the 7% of patients who entered with an eGFR below 60 mL/min per 1.73 m2, the fast-decliner incidence was 25% on dapagliflozin and 36% among controls. All the between-group differences were statistically significant.
The incidence of fast decliners was also lower with dapagliflozin treatment when the analysis included the entire first 4 years on treatment, including the first 6 months when SGLT2s usually spikes a loss of renal function. For the entire 4-year period, fast decline occurred among 34% of patients on dapagliflozin and in 37% of control patients, a statistically significant difference.
The mechanisms behind the consistent renal-protective effects of the SGLT2 inhibitors remain unclear right now, but likely seem related to the “perfect” diuretic action the drugs produce, said Dr. Inzucchi. “They’re not as hugely effective as diuretics, but they’re gentler.” While the SGLT2 inhibitors cause a modest amount of fluid loss ”for some reason they don’t activate the compensatory mechanisms that prevent further reductions in plasma volume,” a property that manifests as little or no change in catecholamines or renin-angiotensin activity, which sets this diuretic action apart from what happens with conventional diuretic drugs, he said in an interview.
In DECLARE-TIMI 58 treatment with dapagliflozin met its primary safety outcome of noninferiority to placebo with respect to major adverse cardiovascular events. The results failed to show statistically significant superiority for one of the primary efficacy endpoints, the rate of major adverse coronary events, but they did show significantly better performance for the second primary efficacy outcome of the rate of cardiovascular death or hospitalization for heart failure, which occurred in 4.9% of patients treated with dapagliflozin and in 5.8% of the control patients during a median follow-up of 4.2 years (N Engl J Med. 2019 Jan 24;380[4]:347-57).
DECLARE-TIMI 58 was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Raz has been an advisor to and speaker on behalf of AstraZeneca as well as several other companies. Dr. Gabbay had no relevant disclosures. Dr. Inzucchi has been a consultant to AstraZeneca, and also to Abbott, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics.
SOURCE: Raz I et al. ADA 2020, Abstract 303-OR.
Treatment of patients with type 2 diabetes with the SGLT2 inhibitor dapagliflozin led to a significant drop in the occurrence of ‘fast decline’ of renal function in more than 15,000 patients enrolled in the drug’s main cardiovascular outcome trial, another example of the potent renal protective effects of agents from this drug class.
Among patients with type 2 diabetes enrolled in the DECLARE-TIMI 58 trial, the incidence of a fast decline in renal function, defined as a drop in estimated glomerular filtration rate (eGFR) of at least 3 mL/min per 1.73 m2, was 27% among patients treated with dapagliflozin and 37% in control patients who received placebo, a statistically significant difference for this post-hoc analysis, Itamar Raz, MD, said at the virtual annual scientific sessions of the American Diabetes Association.
This finding, which adds to a long list of other renal function parameters reported to have been improved by treatment with sodium-glucose cotransporter 2 (SGLT2) inhibitors, “emphasizes the value of SGLT2 inhibitors as an important component of both prevention and treatment of chronic kidney disease among patients with type 2 diabetes,” said Dr. Raz, a diabetes researcher and professor of medicine at Hadassah University Hospital in Jerusalem.
The primary, prespecified renal outcomes in DECLARE-TIMI 58 were a cardiorenal composite outcome of sustained decline of at least 40% in eGFR to less than 60 mL/min per 1.73 m2, end-stage renal disease (defined as dialysis for at least 90 days, kidney transplantation, or confirmed sustained eGFR of less than 15 mL/min per 1.73 m2), or death from renal or cardiovascular causes; and a second prespecified renal-specific composite outcome that was the same except for excluding death from cardiovascular causes. The results showed that the cardiorenal outcome dropped by a statistically significant 24% with dapagliflozin treatment relative to control patients, and the renal-specific outcome fell by a statistically significant 47% with dapagliflozin relative to control patients (Lancet Diab Endocrinol. 2019 Aug 1;7[8];606-17).
The new findings on the incidence of fast decline in renal function help to further flesh out the scope of renal benefit exerted by SGLT2 inhibitors like dapagliflozin in patients with type 2 diabetes, said experts. Fast decline is a relatively recently devised measure of a high-risk, precipitous loss of renal function that has been defined as a drop of either 3 or 5 mL/min per 1.73 m2 per year (Kidney Int. 2017 Jun;91[6]:1300-11); for this analysis Dr. Raz and his associates used the less stringent definition.
Finding and treating ‘fast decliners’
The new report from Dr. Raz “confirms the original [renal] findings and looks to expand them to a particularly high risk group: the fast decliners,” commented Robert A. Gabbay, MD, chief science & medical officer of the ADA. “In some ways, the group of patients that we need to find a better treatment for most are those whose GFR declines quickly. We don’t always know who they are until after the fact, and studies have been looking for markers that might prospectively identify them,” he said in an interview.
The new analysis showed that dapagliflozin “was effective in this subgroup of patients. Furthermore, it didn’t matter if they had significant baseline disease or not. Even people with normal kidney function [at baseline] who were still fast decliners fared better with the drug than without it. This suggests that, if it can be confirmed in a prospective study, dapagliflozin might be effective very early in the course of treatment if we can identify who will be the fast decliners.”
Dr. Raz and his associates had the data necessary to calculate the rates of eGFR decline during the full follow-up period for 15,012 of the 17,160 patients enrolled in DECLARE-TIMI 58, and they found that 4,788 (32%) were fast decliners and 10,224 had a slower rate of renal deterioration. The average annual decline in eGFR during the period from 6 months after study entry through 4 years was 6.3 mL/min per 1.73 m2 per year (median of 5.1 mL/min per 1.73 m2 per year) among the fast decliners, and zero (median of 0.6 mL/min per 1.73 m2 per year) among the other patients.
Overcoming dapagliflozin’s initial eGFR reduction
The researchers focused on the 6-month to 4-year period of treatment as more representative of the impact of dapagliflozin because the SGLT2 inhibitors have an established pattern of triggering an initial, moderate decline in eGFR over roughly the first 6 months on the drug, which is similar to what happens to patients who start treatment with an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker.
“Some patients get as much as a 10% decline in eGFR” when SGLT2 inhibitor treatment starts, but “patients do better over time even with this initial hit,” the same way they do on drugs that act on the renin-angiotensin system, explained Silvio E. Inzucchi, MD, an endocrinologist and professor of medicine at Yale University in New Haven who has extensively studied the SGLT2 inhibitors.
The analyses reported by Dr. Raz showed that the protection against fast decline during the 6-month to 4-year period with dapagliflozin treatment was consistent across a range of patient subgroups regardless of age, duration of their type 2 diabetes, their baseline level of hyperglycemia, and their baseline eGFR. Nearly half the patients enrolled in DECLARE-TIMI 58 had an eGFR at baseline of at least 91 mL/min per 1.73 m2 and in this subgroup the incidence of fast decliners was 23% with dapagliflozin and 31% on placebo. Among the 45% of patients who began with an eGFR of 60-90 mL/min per 1.73 m2 the fast-decliner incidence was 32% and 43% when on or off dapagliflozin. Among the 7% of patients who entered with an eGFR below 60 mL/min per 1.73 m2, the fast-decliner incidence was 25% on dapagliflozin and 36% among controls. All the between-group differences were statistically significant.
The incidence of fast decliners was also lower with dapagliflozin treatment when the analysis included the entire first 4 years on treatment, including the first 6 months when SGLT2s usually spikes a loss of renal function. For the entire 4-year period, fast decline occurred among 34% of patients on dapagliflozin and in 37% of control patients, a statistically significant difference.
The mechanisms behind the consistent renal-protective effects of the SGLT2 inhibitors remain unclear right now, but likely seem related to the “perfect” diuretic action the drugs produce, said Dr. Inzucchi. “They’re not as hugely effective as diuretics, but they’re gentler.” While the SGLT2 inhibitors cause a modest amount of fluid loss ”for some reason they don’t activate the compensatory mechanisms that prevent further reductions in plasma volume,” a property that manifests as little or no change in catecholamines or renin-angiotensin activity, which sets this diuretic action apart from what happens with conventional diuretic drugs, he said in an interview.
In DECLARE-TIMI 58 treatment with dapagliflozin met its primary safety outcome of noninferiority to placebo with respect to major adverse cardiovascular events. The results failed to show statistically significant superiority for one of the primary efficacy endpoints, the rate of major adverse coronary events, but they did show significantly better performance for the second primary efficacy outcome of the rate of cardiovascular death or hospitalization for heart failure, which occurred in 4.9% of patients treated with dapagliflozin and in 5.8% of the control patients during a median follow-up of 4.2 years (N Engl J Med. 2019 Jan 24;380[4]:347-57).
DECLARE-TIMI 58 was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Raz has been an advisor to and speaker on behalf of AstraZeneca as well as several other companies. Dr. Gabbay had no relevant disclosures. Dr. Inzucchi has been a consultant to AstraZeneca, and also to Abbott, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics.
SOURCE: Raz I et al. ADA 2020, Abstract 303-OR.
Treatment of patients with type 2 diabetes with the SGLT2 inhibitor dapagliflozin led to a significant drop in the occurrence of ‘fast decline’ of renal function in more than 15,000 patients enrolled in the drug’s main cardiovascular outcome trial, another example of the potent renal protective effects of agents from this drug class.
Among patients with type 2 diabetes enrolled in the DECLARE-TIMI 58 trial, the incidence of a fast decline in renal function, defined as a drop in estimated glomerular filtration rate (eGFR) of at least 3 mL/min per 1.73 m2, was 27% among patients treated with dapagliflozin and 37% in control patients who received placebo, a statistically significant difference for this post-hoc analysis, Itamar Raz, MD, said at the virtual annual scientific sessions of the American Diabetes Association.
This finding, which adds to a long list of other renal function parameters reported to have been improved by treatment with sodium-glucose cotransporter 2 (SGLT2) inhibitors, “emphasizes the value of SGLT2 inhibitors as an important component of both prevention and treatment of chronic kidney disease among patients with type 2 diabetes,” said Dr. Raz, a diabetes researcher and professor of medicine at Hadassah University Hospital in Jerusalem.
The primary, prespecified renal outcomes in DECLARE-TIMI 58 were a cardiorenal composite outcome of sustained decline of at least 40% in eGFR to less than 60 mL/min per 1.73 m2, end-stage renal disease (defined as dialysis for at least 90 days, kidney transplantation, or confirmed sustained eGFR of less than 15 mL/min per 1.73 m2), or death from renal or cardiovascular causes; and a second prespecified renal-specific composite outcome that was the same except for excluding death from cardiovascular causes. The results showed that the cardiorenal outcome dropped by a statistically significant 24% with dapagliflozin treatment relative to control patients, and the renal-specific outcome fell by a statistically significant 47% with dapagliflozin relative to control patients (Lancet Diab Endocrinol. 2019 Aug 1;7[8];606-17).
The new findings on the incidence of fast decline in renal function help to further flesh out the scope of renal benefit exerted by SGLT2 inhibitors like dapagliflozin in patients with type 2 diabetes, said experts. Fast decline is a relatively recently devised measure of a high-risk, precipitous loss of renal function that has been defined as a drop of either 3 or 5 mL/min per 1.73 m2 per year (Kidney Int. 2017 Jun;91[6]:1300-11); for this analysis Dr. Raz and his associates used the less stringent definition.
Finding and treating ‘fast decliners’
The new report from Dr. Raz “confirms the original [renal] findings and looks to expand them to a particularly high risk group: the fast decliners,” commented Robert A. Gabbay, MD, chief science & medical officer of the ADA. “In some ways, the group of patients that we need to find a better treatment for most are those whose GFR declines quickly. We don’t always know who they are until after the fact, and studies have been looking for markers that might prospectively identify them,” he said in an interview.
The new analysis showed that dapagliflozin “was effective in this subgroup of patients. Furthermore, it didn’t matter if they had significant baseline disease or not. Even people with normal kidney function [at baseline] who were still fast decliners fared better with the drug than without it. This suggests that, if it can be confirmed in a prospective study, dapagliflozin might be effective very early in the course of treatment if we can identify who will be the fast decliners.”
Dr. Raz and his associates had the data necessary to calculate the rates of eGFR decline during the full follow-up period for 15,012 of the 17,160 patients enrolled in DECLARE-TIMI 58, and they found that 4,788 (32%) were fast decliners and 10,224 had a slower rate of renal deterioration. The average annual decline in eGFR during the period from 6 months after study entry through 4 years was 6.3 mL/min per 1.73 m2 per year (median of 5.1 mL/min per 1.73 m2 per year) among the fast decliners, and zero (median of 0.6 mL/min per 1.73 m2 per year) among the other patients.
Overcoming dapagliflozin’s initial eGFR reduction
The researchers focused on the 6-month to 4-year period of treatment as more representative of the impact of dapagliflozin because the SGLT2 inhibitors have an established pattern of triggering an initial, moderate decline in eGFR over roughly the first 6 months on the drug, which is similar to what happens to patients who start treatment with an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker.
“Some patients get as much as a 10% decline in eGFR” when SGLT2 inhibitor treatment starts, but “patients do better over time even with this initial hit,” the same way they do on drugs that act on the renin-angiotensin system, explained Silvio E. Inzucchi, MD, an endocrinologist and professor of medicine at Yale University in New Haven who has extensively studied the SGLT2 inhibitors.
The analyses reported by Dr. Raz showed that the protection against fast decline during the 6-month to 4-year period with dapagliflozin treatment was consistent across a range of patient subgroups regardless of age, duration of their type 2 diabetes, their baseline level of hyperglycemia, and their baseline eGFR. Nearly half the patients enrolled in DECLARE-TIMI 58 had an eGFR at baseline of at least 91 mL/min per 1.73 m2 and in this subgroup the incidence of fast decliners was 23% with dapagliflozin and 31% on placebo. Among the 45% of patients who began with an eGFR of 60-90 mL/min per 1.73 m2 the fast-decliner incidence was 32% and 43% when on or off dapagliflozin. Among the 7% of patients who entered with an eGFR below 60 mL/min per 1.73 m2, the fast-decliner incidence was 25% on dapagliflozin and 36% among controls. All the between-group differences were statistically significant.
The incidence of fast decliners was also lower with dapagliflozin treatment when the analysis included the entire first 4 years on treatment, including the first 6 months when SGLT2s usually spikes a loss of renal function. For the entire 4-year period, fast decline occurred among 34% of patients on dapagliflozin and in 37% of control patients, a statistically significant difference.
The mechanisms behind the consistent renal-protective effects of the SGLT2 inhibitors remain unclear right now, but likely seem related to the “perfect” diuretic action the drugs produce, said Dr. Inzucchi. “They’re not as hugely effective as diuretics, but they’re gentler.” While the SGLT2 inhibitors cause a modest amount of fluid loss ”for some reason they don’t activate the compensatory mechanisms that prevent further reductions in plasma volume,” a property that manifests as little or no change in catecholamines or renin-angiotensin activity, which sets this diuretic action apart from what happens with conventional diuretic drugs, he said in an interview.
In DECLARE-TIMI 58 treatment with dapagliflozin met its primary safety outcome of noninferiority to placebo with respect to major adverse cardiovascular events. The results failed to show statistically significant superiority for one of the primary efficacy endpoints, the rate of major adverse coronary events, but they did show significantly better performance for the second primary efficacy outcome of the rate of cardiovascular death or hospitalization for heart failure, which occurred in 4.9% of patients treated with dapagliflozin and in 5.8% of the control patients during a median follow-up of 4.2 years (N Engl J Med. 2019 Jan 24;380[4]:347-57).
DECLARE-TIMI 58 was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Raz has been an advisor to and speaker on behalf of AstraZeneca as well as several other companies. Dr. Gabbay had no relevant disclosures. Dr. Inzucchi has been a consultant to AstraZeneca, and also to Abbott, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics.
SOURCE: Raz I et al. ADA 2020, Abstract 303-OR.
FROM ADA 2020
VERTIS-CV: Ertugliflozin’s CV outcomes trial confirms SGLT2i benefits
The cardiovascular outcome trial results for a fourth sodium-glucose cotransporter 2 (SGLT2) inhibitor, ertugliflozin, were most notable for their consistency with the four prior, similar trials run on the three other drugs from this class on the U.S. market, canagliflozin, dapagliflozin, and empagliflozin, further solidifying the important role this drug class has recently taken on for patients with type 2 diabetes.
But the ertugliflozin results, which showed statistically significant superiority to placebo for just one endpoint, hospitalization for heart failure, made it unclear whether clinicians will regard ertugliflozin as the top agent from this class to prescribe.
“Our big takeaway is that the findings are consistent with what’s been seen in the other studies” of cardiovascular and renal outcomes in the EMPA-REG OUTCOME study of empagliflozin (N Engl J Med. 2015 Nov 26;373[22]:2117-28 ), the CANVAS (N Engl J Med. 2017 Aug 17;377[7]:644-57) and CREDENCE (N Engl J Med. 2019 June 13;380[24]:2295-306 ) studies of canagliflozin, and the DECLARE-TIMI 58 trial with dapagliflozin (N Engl J Med. 2019 Jan 24;380[4]:347-57), Christopher P. Cannon, MD, said at the virtual annual scientific sessions of the American Diabetes Association.
The cardiovascular outcome trials (CVOTs), mandated in 2008 by Food and Drug Administration guidance for type 2 diabetes drugs that is now in the process of undergoing an update, have had the main goal of proving safety, and the primary endpoint of the new ertugliflozin trial, VERTIS-CV, was noninferiority to placebo when used on top of standard type 2 diabetes medications for the combined endpoint of cardiovascular death, nonfatal MI, or nonfatal stroke.
Key findings
Both of the tested dosages of ertugliflozin, 5 mg and 15 mg daily, met this endpoint, with event rates over a median 3.0 years of follow-up that ran very close to the placebo rate, clearly proving noninferiority. But the results showed no suggestion of superiority in a study that randomized 5,499 patients to either of the ertugliflozin regimens and 2,747 to placebo, reported Dr. Cannon, a cardiologist and professor of medicine at Harvard Medical School, Boston.
The primary outcome also showed similar event rates for each component of the composite endpoint, and subgroup analysis showed consistent results from ertugliflozin, compared with placebo, regardless of study-cohort subdivision by demographic, clinical, or treatment factors.
The trial design called for a hierarchical sequence of secondary-outcome superiority analyses, starting with the impact of ertugliflozin on cardiovascular death or heart failure hospitalization, and for this outcome ertugliflozin showed a point estimate of a 12% relative risk reduction, compared with placebo-treated patients, but this difference was not statistically significant. This meant that all subsequent superiority analyses in this trial could only be hypothesis generating and not definitive.
This negated the statistical validity of the only statistically significant treatment difference between ertugliflozin and placebo seen in VERTIS-CV, for the outcome of hospitalization for heart failure, where ertugliflozin treatment cut this outcome by 30%, compared with placebo patients. The rate of cardiovascular death alone, as well as a renal composite endpoint each showed no statistically significant benefit of ertugliflozin, compared with placebo, although the renal endpoint came close, with ertugliflozin reducing the combined rate of renal death, need for dialysis, need for renal transplant, or a doubling of serum creatinine from baseline by 19%, compared with placebo (P = .08).
How results compare with prior CVOTs
In some ways, these results seemed to contrast with outcomes from the CVOTs for the other SGLT2 inhibitors, which all showed at least two statistically significant benefits for major endpoints when compared with placebo.
As summarized in a new meta-analysis of all the CVOTs by Darren K. McGuire, MD, a cardiologist and professor of medicine at the University of Texas, Dallas, both empagliflozin and canagliflozin showed statistically significant superiority compared with placebo for their trial’s primary, combined major cardiovascular adverse event endpoint, but dapagliflozin and ertugliflozin did not. Empagliflozin was the sole SGLT2 inhibitor to show a statistically significant cut in cardiovascular deaths, compared with placebo.
The primary, composite renal efficacy endpoints used in these trials were hardest to compare because they differed from study to study, but unlike ertugliflozin, all the other three drugs in the class showed a statistically significant improvement, compared with placebo, for their respective renal outcomes. On the other hand, the pattern of estimated glomerular filtration rates measured at multiple times during the various trials showed a high level of consistency across the CVOTs.
The greatest consistently among the major endpoints across the trials was for heart failure hospitalization. All four agents showed statistically significant improvements, compared with placebo, and all four had roughly equal magnitudes of effect, a cut in event rates by about one-third.
“The greatest magnitude of benefit is for reductions in heart failure hospitalizations and for renal outcomes,” with the heart failure outcomes the “most consistent” across the studies and the renal outcomes “largely consistent,” concluded Dr. McGuire. All together, the five CVOTs for these four SGLT2 inhibitors involved more than 46,000 patients.
“A lot of data suggest these are all class effects,” that are roughly similar across all four of these SGLT2 inhibitors, commented Mark E. Cooper, MBBS, a professor and head of the department of diabetes at Monash University, Melbourne, and designated discussant for the study.
There was “clear homogeneity” between the VERTIS-CV results for hospitalization for heart failure and the other CVOTs, he noted. “I think there is a difference” in the cardiovascular death outcomes, specifically the sole statistically significant, 38% relative risk reduction with empagliflozin that stood out from the other CVOTs, but this difference is “totally unexplained,” added Dr. Cooper. “To really determine differences you’d need head-to-head studies that are unlikely to happen.”
The results of new SGLT2 inhibitor meta-analysis appeared to also “support contemporary society recommendations to prioritize the use of SGLT2 inhibitors independent of glucose-control considerations in patients with type 2 diabetes with or at high risk for cardiovascular and renal complications,” said Dr. McGuire.
“The guidelines have it right. Now it’s on us to implement these treatments to appropriate patients,” concluded Dr. Cannon.
Study details
VERTIS-CV (Cardiovascular Outcomes Following Ertugliflozin Treatment in Type 2 Diabetes Mellitus Participants With Vascular Disease) enrolled and followed patients with type 2 diabetes and established atherosclerotic cardiovascular disease at 531 centers in 34 countries during December 2013–December 2019. Other effects from ertugliflozin recorded during the trial were consistent with prior studies of the drug, which is already FDA approved for glycemic control: Compared with placebo, ertugliflozin treatment reduced hemoglobin A1c by an average of 0.5% after 1 year, cut average body weight by about 2.5 kg after 1 year with additional modest weight loss, during subsequent years on the drug, and reduced systolic blood pressure by about 3 mm Hg after 1 year.
The drug’s safety profile was generally reassuring and consistent with prior studies of this drug and others in the class, with overall no increase in total adverse events or serious adverse events, compared with placebo, and modestly increased rates of urinary tract and mycotic genital infections.
VERTIS-CV was sponsored by Merck and Pfizer, the companies that market ertugliflozin (Steglatro). Dr. Cannon has received research funding and fees from Merck and Pfizer and from several other companies. Dr. McGuire has received honoraria from Merck, has been a consultant to Pfizer, and has had similar relationships with several other companies. Dr. Cooper has been an advisor to and received honoraria from Merck. He has also received honoraria from or been an adviser to AstraZeneca, Boehringer Ingelheim, Lilly, MundiPharma, Novartis, Reata, and Servier, and he has received research funding from Boehringer Ingelheim and Novo Nordisk.
The cardiovascular outcome trial results for a fourth sodium-glucose cotransporter 2 (SGLT2) inhibitor, ertugliflozin, were most notable for their consistency with the four prior, similar trials run on the three other drugs from this class on the U.S. market, canagliflozin, dapagliflozin, and empagliflozin, further solidifying the important role this drug class has recently taken on for patients with type 2 diabetes.
But the ertugliflozin results, which showed statistically significant superiority to placebo for just one endpoint, hospitalization for heart failure, made it unclear whether clinicians will regard ertugliflozin as the top agent from this class to prescribe.
“Our big takeaway is that the findings are consistent with what’s been seen in the other studies” of cardiovascular and renal outcomes in the EMPA-REG OUTCOME study of empagliflozin (N Engl J Med. 2015 Nov 26;373[22]:2117-28 ), the CANVAS (N Engl J Med. 2017 Aug 17;377[7]:644-57) and CREDENCE (N Engl J Med. 2019 June 13;380[24]:2295-306 ) studies of canagliflozin, and the DECLARE-TIMI 58 trial with dapagliflozin (N Engl J Med. 2019 Jan 24;380[4]:347-57), Christopher P. Cannon, MD, said at the virtual annual scientific sessions of the American Diabetes Association.
The cardiovascular outcome trials (CVOTs), mandated in 2008 by Food and Drug Administration guidance for type 2 diabetes drugs that is now in the process of undergoing an update, have had the main goal of proving safety, and the primary endpoint of the new ertugliflozin trial, VERTIS-CV, was noninferiority to placebo when used on top of standard type 2 diabetes medications for the combined endpoint of cardiovascular death, nonfatal MI, or nonfatal stroke.
Key findings
Both of the tested dosages of ertugliflozin, 5 mg and 15 mg daily, met this endpoint, with event rates over a median 3.0 years of follow-up that ran very close to the placebo rate, clearly proving noninferiority. But the results showed no suggestion of superiority in a study that randomized 5,499 patients to either of the ertugliflozin regimens and 2,747 to placebo, reported Dr. Cannon, a cardiologist and professor of medicine at Harvard Medical School, Boston.
The primary outcome also showed similar event rates for each component of the composite endpoint, and subgroup analysis showed consistent results from ertugliflozin, compared with placebo, regardless of study-cohort subdivision by demographic, clinical, or treatment factors.
The trial design called for a hierarchical sequence of secondary-outcome superiority analyses, starting with the impact of ertugliflozin on cardiovascular death or heart failure hospitalization, and for this outcome ertugliflozin showed a point estimate of a 12% relative risk reduction, compared with placebo-treated patients, but this difference was not statistically significant. This meant that all subsequent superiority analyses in this trial could only be hypothesis generating and not definitive.
This negated the statistical validity of the only statistically significant treatment difference between ertugliflozin and placebo seen in VERTIS-CV, for the outcome of hospitalization for heart failure, where ertugliflozin treatment cut this outcome by 30%, compared with placebo patients. The rate of cardiovascular death alone, as well as a renal composite endpoint each showed no statistically significant benefit of ertugliflozin, compared with placebo, although the renal endpoint came close, with ertugliflozin reducing the combined rate of renal death, need for dialysis, need for renal transplant, or a doubling of serum creatinine from baseline by 19%, compared with placebo (P = .08).
How results compare with prior CVOTs
In some ways, these results seemed to contrast with outcomes from the CVOTs for the other SGLT2 inhibitors, which all showed at least two statistically significant benefits for major endpoints when compared with placebo.
As summarized in a new meta-analysis of all the CVOTs by Darren K. McGuire, MD, a cardiologist and professor of medicine at the University of Texas, Dallas, both empagliflozin and canagliflozin showed statistically significant superiority compared with placebo for their trial’s primary, combined major cardiovascular adverse event endpoint, but dapagliflozin and ertugliflozin did not. Empagliflozin was the sole SGLT2 inhibitor to show a statistically significant cut in cardiovascular deaths, compared with placebo.
The primary, composite renal efficacy endpoints used in these trials were hardest to compare because they differed from study to study, but unlike ertugliflozin, all the other three drugs in the class showed a statistically significant improvement, compared with placebo, for their respective renal outcomes. On the other hand, the pattern of estimated glomerular filtration rates measured at multiple times during the various trials showed a high level of consistency across the CVOTs.
The greatest consistently among the major endpoints across the trials was for heart failure hospitalization. All four agents showed statistically significant improvements, compared with placebo, and all four had roughly equal magnitudes of effect, a cut in event rates by about one-third.
“The greatest magnitude of benefit is for reductions in heart failure hospitalizations and for renal outcomes,” with the heart failure outcomes the “most consistent” across the studies and the renal outcomes “largely consistent,” concluded Dr. McGuire. All together, the five CVOTs for these four SGLT2 inhibitors involved more than 46,000 patients.
“A lot of data suggest these are all class effects,” that are roughly similar across all four of these SGLT2 inhibitors, commented Mark E. Cooper, MBBS, a professor and head of the department of diabetes at Monash University, Melbourne, and designated discussant for the study.
There was “clear homogeneity” between the VERTIS-CV results for hospitalization for heart failure and the other CVOTs, he noted. “I think there is a difference” in the cardiovascular death outcomes, specifically the sole statistically significant, 38% relative risk reduction with empagliflozin that stood out from the other CVOTs, but this difference is “totally unexplained,” added Dr. Cooper. “To really determine differences you’d need head-to-head studies that are unlikely to happen.”
The results of new SGLT2 inhibitor meta-analysis appeared to also “support contemporary society recommendations to prioritize the use of SGLT2 inhibitors independent of glucose-control considerations in patients with type 2 diabetes with or at high risk for cardiovascular and renal complications,” said Dr. McGuire.
“The guidelines have it right. Now it’s on us to implement these treatments to appropriate patients,” concluded Dr. Cannon.
Study details
VERTIS-CV (Cardiovascular Outcomes Following Ertugliflozin Treatment in Type 2 Diabetes Mellitus Participants With Vascular Disease) enrolled and followed patients with type 2 diabetes and established atherosclerotic cardiovascular disease at 531 centers in 34 countries during December 2013–December 2019. Other effects from ertugliflozin recorded during the trial were consistent with prior studies of the drug, which is already FDA approved for glycemic control: Compared with placebo, ertugliflozin treatment reduced hemoglobin A1c by an average of 0.5% after 1 year, cut average body weight by about 2.5 kg after 1 year with additional modest weight loss, during subsequent years on the drug, and reduced systolic blood pressure by about 3 mm Hg after 1 year.
The drug’s safety profile was generally reassuring and consistent with prior studies of this drug and others in the class, with overall no increase in total adverse events or serious adverse events, compared with placebo, and modestly increased rates of urinary tract and mycotic genital infections.
VERTIS-CV was sponsored by Merck and Pfizer, the companies that market ertugliflozin (Steglatro). Dr. Cannon has received research funding and fees from Merck and Pfizer and from several other companies. Dr. McGuire has received honoraria from Merck, has been a consultant to Pfizer, and has had similar relationships with several other companies. Dr. Cooper has been an advisor to and received honoraria from Merck. He has also received honoraria from or been an adviser to AstraZeneca, Boehringer Ingelheim, Lilly, MundiPharma, Novartis, Reata, and Servier, and he has received research funding from Boehringer Ingelheim and Novo Nordisk.
The cardiovascular outcome trial results for a fourth sodium-glucose cotransporter 2 (SGLT2) inhibitor, ertugliflozin, were most notable for their consistency with the four prior, similar trials run on the three other drugs from this class on the U.S. market, canagliflozin, dapagliflozin, and empagliflozin, further solidifying the important role this drug class has recently taken on for patients with type 2 diabetes.
But the ertugliflozin results, which showed statistically significant superiority to placebo for just one endpoint, hospitalization for heart failure, made it unclear whether clinicians will regard ertugliflozin as the top agent from this class to prescribe.
“Our big takeaway is that the findings are consistent with what’s been seen in the other studies” of cardiovascular and renal outcomes in the EMPA-REG OUTCOME study of empagliflozin (N Engl J Med. 2015 Nov 26;373[22]:2117-28 ), the CANVAS (N Engl J Med. 2017 Aug 17;377[7]:644-57) and CREDENCE (N Engl J Med. 2019 June 13;380[24]:2295-306 ) studies of canagliflozin, and the DECLARE-TIMI 58 trial with dapagliflozin (N Engl J Med. 2019 Jan 24;380[4]:347-57), Christopher P. Cannon, MD, said at the virtual annual scientific sessions of the American Diabetes Association.
The cardiovascular outcome trials (CVOTs), mandated in 2008 by Food and Drug Administration guidance for type 2 diabetes drugs that is now in the process of undergoing an update, have had the main goal of proving safety, and the primary endpoint of the new ertugliflozin trial, VERTIS-CV, was noninferiority to placebo when used on top of standard type 2 diabetes medications for the combined endpoint of cardiovascular death, nonfatal MI, or nonfatal stroke.
Key findings
Both of the tested dosages of ertugliflozin, 5 mg and 15 mg daily, met this endpoint, with event rates over a median 3.0 years of follow-up that ran very close to the placebo rate, clearly proving noninferiority. But the results showed no suggestion of superiority in a study that randomized 5,499 patients to either of the ertugliflozin regimens and 2,747 to placebo, reported Dr. Cannon, a cardiologist and professor of medicine at Harvard Medical School, Boston.
The primary outcome also showed similar event rates for each component of the composite endpoint, and subgroup analysis showed consistent results from ertugliflozin, compared with placebo, regardless of study-cohort subdivision by demographic, clinical, or treatment factors.
The trial design called for a hierarchical sequence of secondary-outcome superiority analyses, starting with the impact of ertugliflozin on cardiovascular death or heart failure hospitalization, and for this outcome ertugliflozin showed a point estimate of a 12% relative risk reduction, compared with placebo-treated patients, but this difference was not statistically significant. This meant that all subsequent superiority analyses in this trial could only be hypothesis generating and not definitive.
This negated the statistical validity of the only statistically significant treatment difference between ertugliflozin and placebo seen in VERTIS-CV, for the outcome of hospitalization for heart failure, where ertugliflozin treatment cut this outcome by 30%, compared with placebo patients. The rate of cardiovascular death alone, as well as a renal composite endpoint each showed no statistically significant benefit of ertugliflozin, compared with placebo, although the renal endpoint came close, with ertugliflozin reducing the combined rate of renal death, need for dialysis, need for renal transplant, or a doubling of serum creatinine from baseline by 19%, compared with placebo (P = .08).
How results compare with prior CVOTs
In some ways, these results seemed to contrast with outcomes from the CVOTs for the other SGLT2 inhibitors, which all showed at least two statistically significant benefits for major endpoints when compared with placebo.
As summarized in a new meta-analysis of all the CVOTs by Darren K. McGuire, MD, a cardiologist and professor of medicine at the University of Texas, Dallas, both empagliflozin and canagliflozin showed statistically significant superiority compared with placebo for their trial’s primary, combined major cardiovascular adverse event endpoint, but dapagliflozin and ertugliflozin did not. Empagliflozin was the sole SGLT2 inhibitor to show a statistically significant cut in cardiovascular deaths, compared with placebo.
The primary, composite renal efficacy endpoints used in these trials were hardest to compare because they differed from study to study, but unlike ertugliflozin, all the other three drugs in the class showed a statistically significant improvement, compared with placebo, for their respective renal outcomes. On the other hand, the pattern of estimated glomerular filtration rates measured at multiple times during the various trials showed a high level of consistency across the CVOTs.
The greatest consistently among the major endpoints across the trials was for heart failure hospitalization. All four agents showed statistically significant improvements, compared with placebo, and all four had roughly equal magnitudes of effect, a cut in event rates by about one-third.
“The greatest magnitude of benefit is for reductions in heart failure hospitalizations and for renal outcomes,” with the heart failure outcomes the “most consistent” across the studies and the renal outcomes “largely consistent,” concluded Dr. McGuire. All together, the five CVOTs for these four SGLT2 inhibitors involved more than 46,000 patients.
“A lot of data suggest these are all class effects,” that are roughly similar across all four of these SGLT2 inhibitors, commented Mark E. Cooper, MBBS, a professor and head of the department of diabetes at Monash University, Melbourne, and designated discussant for the study.
There was “clear homogeneity” between the VERTIS-CV results for hospitalization for heart failure and the other CVOTs, he noted. “I think there is a difference” in the cardiovascular death outcomes, specifically the sole statistically significant, 38% relative risk reduction with empagliflozin that stood out from the other CVOTs, but this difference is “totally unexplained,” added Dr. Cooper. “To really determine differences you’d need head-to-head studies that are unlikely to happen.”
The results of new SGLT2 inhibitor meta-analysis appeared to also “support contemporary society recommendations to prioritize the use of SGLT2 inhibitors independent of glucose-control considerations in patients with type 2 diabetes with or at high risk for cardiovascular and renal complications,” said Dr. McGuire.
“The guidelines have it right. Now it’s on us to implement these treatments to appropriate patients,” concluded Dr. Cannon.
Study details
VERTIS-CV (Cardiovascular Outcomes Following Ertugliflozin Treatment in Type 2 Diabetes Mellitus Participants With Vascular Disease) enrolled and followed patients with type 2 diabetes and established atherosclerotic cardiovascular disease at 531 centers in 34 countries during December 2013–December 2019. Other effects from ertugliflozin recorded during the trial were consistent with prior studies of the drug, which is already FDA approved for glycemic control: Compared with placebo, ertugliflozin treatment reduced hemoglobin A1c by an average of 0.5% after 1 year, cut average body weight by about 2.5 kg after 1 year with additional modest weight loss, during subsequent years on the drug, and reduced systolic blood pressure by about 3 mm Hg after 1 year.
The drug’s safety profile was generally reassuring and consistent with prior studies of this drug and others in the class, with overall no increase in total adverse events or serious adverse events, compared with placebo, and modestly increased rates of urinary tract and mycotic genital infections.
VERTIS-CV was sponsored by Merck and Pfizer, the companies that market ertugliflozin (Steglatro). Dr. Cannon has received research funding and fees from Merck and Pfizer and from several other companies. Dr. McGuire has received honoraria from Merck, has been a consultant to Pfizer, and has had similar relationships with several other companies. Dr. Cooper has been an advisor to and received honoraria from Merck. He has also received honoraria from or been an adviser to AstraZeneca, Boehringer Ingelheim, Lilly, MundiPharma, Novartis, Reata, and Servier, and he has received research funding from Boehringer Ingelheim and Novo Nordisk.
FROM ADA 2020
Frequent hypoglycemic episodes raise cardiac event risk
Frequent hypoglycemic episodes were linked to a raised incidence of cardiovascular events in adults with type 2 diabetes in a recent retrospective study, suggesting certain hypoglycemia-associated diabetes drugs should be avoided, an investigator said.
Patients who had more than five hypoglycemic episodes per year had a 61% greater risk of cardiovascular (CV) events, compared with patients with less frequent episodes, according to results of the study.
Although there were fewer strokes among younger patients, the overall increase in cardiovascular event risk held up regardless of age group, according to investigator Aman Rajpal, MD, of Louis Stokes Veterans Affairs Medical Center and Case Western Reserve University, both in Cleveland.
On the basis of these and earlier studies tying hypoglycemia to CV risk, health care providers need to “pay close attention” to low blood sugar and personalize glycemic control targets for each patient based on risk of hypoglycemia, Dr. Rajpal said in a presentation of the study at the virtual annual scientific sessions of the American Diabetes Association.
“Also, this suggests that avoidance of drugs associated with increased risk of hypoglycemia – namely insulin, sulfonylureas, or others – is essential to avoid and minimize the risk of cardiovascular events in this patient population with type 2 diabetes,” said Dr. Rajpal. “Let us remember part of our Hippocratic oath: ‘Above all, do no harm.’ ”
Tailoring treatment to mitigate risk
Mark Schutta, MD, medical director of Penn Rodebaugh Diabetes Center in Philadelphia, said that results of this study suggest a need to carefully select medical therapy for each individual patient with diabetes in order to mitigate CV risk.
“It’s really about tailoring their drugs to their personal situation,” Dr. Schutta said in an interview.
Although newer diabetes drug classes are associated with low to no risk of hypoglycemia, Dr. Schutta said that there is still a place for drugs such as sulfonylureas in certain situations.
Among sulfonylureas, glyburide comes with a much higher incidence of hypoglycemia, compared with glipizide and glimepiride, according to Dr. Schutta. “I think there’s a role for both drugs, but you have to be very careful, and you have to get the data from your patients.”
Hypoglycemia frequency and outcomes
Speculation that hypoglycemia could be linked to adverse CV outcomes was sparked years ago by trials such as ADVANCE. Severe hypoglycemia in that study was associated with a 168% increased risk of death from a CV cause (N Engl J Med. 2010 Oct 7;363:1410-8).
At the time, ADVANCE investigators said they were unable to find evidence that multiple severe hypoglycemia episodes conferred a greater risk of CV events versus a single hypoglycemia episode, though they added that few patients had recurrent events.
“In other words, the association between the number of hypoglycemia events, and adverse CV outcomes is still unclear,” said Dr. Rajpal in his virtual ADA presentation.
Potential elevated risks with more than five episodes
To evaluate the association between frequent hypoglycemic episodes (i.e., more than five per year, compared with one to five episodes) and CV events, Dr. Rajpal and colleagues evaluated outcomes data for 4.9 million adults with type 2 diabetes found in a large commercial database including information on patients in 27 U.S. health care networks.
Database records indicated that about 182,000 patients, or nearly 4%, had episodes of hyperglycemia, which Dr. Rajpal said was presumed to mean a plasma glucose level of less than 70 mg/dL.
Characteristics of the patients with more than five hypoglycemic episodes were similar to those with one to five episodes, although they were more likely to be 65 years or older, and were “slightly more likely” to be on insulin, which could possibly precipitate more hypoglycemic episodes in that group, Dr. Rajpal said.
Key findings
In the main analysis, Dr. Rajpal said, risk of CV events was significantly increased in those with more than five hypoglycemic episodes, compared with those with one to five episodes, with an odds ratio of 1.61 (95% confidence interval, 1.56-1.66). The incidence of cardiovascular events was 33.1% in those with more than five episodes and 23.5% in those with one to five episodes, according to the data presented.
Risks were also significantly increased specifically for cardiac arrhythmias, cerebrovascular accidents, and MI, Dr. Rajpal said, with ORs of 1.65 (95% CI, 1.9-1.71), 1.38 (95% CI, 1.22-1.56), and 1.43 (95% CI, 1.36-1.50), respectively.
Because individuals in the group with more than five hypoglycemic episodes were more likely to be elderly, Dr. Rajpal said that he and coinvestigators decided to perform an age-specific stratified analysis.
Although cerebral vascular incidence was low in younger patients, risk of CV events overall was nevertheless significantly elevated for those aged 65 years or older, 45-64 years, and 18-44 years, with ORs of 1.69 (95% CI, 1.61-1.7), 1.58 (95% CI, 1.48-1.69), and 1.62 (95% CI, 1.33-1.97).
“The results were still valid in stratified analysis based on different age groups,” Dr. Rajpal said.
Dr. Rajpal and coauthors reported that he had no conflicts of interest related to the research.
SOURCE: Rajpal A et al. ADA 2020, Abstract 161-OR.
Frequent hypoglycemic episodes were linked to a raised incidence of cardiovascular events in adults with type 2 diabetes in a recent retrospective study, suggesting certain hypoglycemia-associated diabetes drugs should be avoided, an investigator said.
Patients who had more than five hypoglycemic episodes per year had a 61% greater risk of cardiovascular (CV) events, compared with patients with less frequent episodes, according to results of the study.
Although there were fewer strokes among younger patients, the overall increase in cardiovascular event risk held up regardless of age group, according to investigator Aman Rajpal, MD, of Louis Stokes Veterans Affairs Medical Center and Case Western Reserve University, both in Cleveland.
On the basis of these and earlier studies tying hypoglycemia to CV risk, health care providers need to “pay close attention” to low blood sugar and personalize glycemic control targets for each patient based on risk of hypoglycemia, Dr. Rajpal said in a presentation of the study at the virtual annual scientific sessions of the American Diabetes Association.
“Also, this suggests that avoidance of drugs associated with increased risk of hypoglycemia – namely insulin, sulfonylureas, or others – is essential to avoid and minimize the risk of cardiovascular events in this patient population with type 2 diabetes,” said Dr. Rajpal. “Let us remember part of our Hippocratic oath: ‘Above all, do no harm.’ ”
Tailoring treatment to mitigate risk
Mark Schutta, MD, medical director of Penn Rodebaugh Diabetes Center in Philadelphia, said that results of this study suggest a need to carefully select medical therapy for each individual patient with diabetes in order to mitigate CV risk.
“It’s really about tailoring their drugs to their personal situation,” Dr. Schutta said in an interview.
Although newer diabetes drug classes are associated with low to no risk of hypoglycemia, Dr. Schutta said that there is still a place for drugs such as sulfonylureas in certain situations.
Among sulfonylureas, glyburide comes with a much higher incidence of hypoglycemia, compared with glipizide and glimepiride, according to Dr. Schutta. “I think there’s a role for both drugs, but you have to be very careful, and you have to get the data from your patients.”
Hypoglycemia frequency and outcomes
Speculation that hypoglycemia could be linked to adverse CV outcomes was sparked years ago by trials such as ADVANCE. Severe hypoglycemia in that study was associated with a 168% increased risk of death from a CV cause (N Engl J Med. 2010 Oct 7;363:1410-8).
At the time, ADVANCE investigators said they were unable to find evidence that multiple severe hypoglycemia episodes conferred a greater risk of CV events versus a single hypoglycemia episode, though they added that few patients had recurrent events.
“In other words, the association between the number of hypoglycemia events, and adverse CV outcomes is still unclear,” said Dr. Rajpal in his virtual ADA presentation.
Potential elevated risks with more than five episodes
To evaluate the association between frequent hypoglycemic episodes (i.e., more than five per year, compared with one to five episodes) and CV events, Dr. Rajpal and colleagues evaluated outcomes data for 4.9 million adults with type 2 diabetes found in a large commercial database including information on patients in 27 U.S. health care networks.
Database records indicated that about 182,000 patients, or nearly 4%, had episodes of hyperglycemia, which Dr. Rajpal said was presumed to mean a plasma glucose level of less than 70 mg/dL.
Characteristics of the patients with more than five hypoglycemic episodes were similar to those with one to five episodes, although they were more likely to be 65 years or older, and were “slightly more likely” to be on insulin, which could possibly precipitate more hypoglycemic episodes in that group, Dr. Rajpal said.
Key findings
In the main analysis, Dr. Rajpal said, risk of CV events was significantly increased in those with more than five hypoglycemic episodes, compared with those with one to five episodes, with an odds ratio of 1.61 (95% confidence interval, 1.56-1.66). The incidence of cardiovascular events was 33.1% in those with more than five episodes and 23.5% in those with one to five episodes, according to the data presented.
Risks were also significantly increased specifically for cardiac arrhythmias, cerebrovascular accidents, and MI, Dr. Rajpal said, with ORs of 1.65 (95% CI, 1.9-1.71), 1.38 (95% CI, 1.22-1.56), and 1.43 (95% CI, 1.36-1.50), respectively.
Because individuals in the group with more than five hypoglycemic episodes were more likely to be elderly, Dr. Rajpal said that he and coinvestigators decided to perform an age-specific stratified analysis.
Although cerebral vascular incidence was low in younger patients, risk of CV events overall was nevertheless significantly elevated for those aged 65 years or older, 45-64 years, and 18-44 years, with ORs of 1.69 (95% CI, 1.61-1.7), 1.58 (95% CI, 1.48-1.69), and 1.62 (95% CI, 1.33-1.97).
“The results were still valid in stratified analysis based on different age groups,” Dr. Rajpal said.
Dr. Rajpal and coauthors reported that he had no conflicts of interest related to the research.
SOURCE: Rajpal A et al. ADA 2020, Abstract 161-OR.
Frequent hypoglycemic episodes were linked to a raised incidence of cardiovascular events in adults with type 2 diabetes in a recent retrospective study, suggesting certain hypoglycemia-associated diabetes drugs should be avoided, an investigator said.
Patients who had more than five hypoglycemic episodes per year had a 61% greater risk of cardiovascular (CV) events, compared with patients with less frequent episodes, according to results of the study.
Although there were fewer strokes among younger patients, the overall increase in cardiovascular event risk held up regardless of age group, according to investigator Aman Rajpal, MD, of Louis Stokes Veterans Affairs Medical Center and Case Western Reserve University, both in Cleveland.
On the basis of these and earlier studies tying hypoglycemia to CV risk, health care providers need to “pay close attention” to low blood sugar and personalize glycemic control targets for each patient based on risk of hypoglycemia, Dr. Rajpal said in a presentation of the study at the virtual annual scientific sessions of the American Diabetes Association.
“Also, this suggests that avoidance of drugs associated with increased risk of hypoglycemia – namely insulin, sulfonylureas, or others – is essential to avoid and minimize the risk of cardiovascular events in this patient population with type 2 diabetes,” said Dr. Rajpal. “Let us remember part of our Hippocratic oath: ‘Above all, do no harm.’ ”
Tailoring treatment to mitigate risk
Mark Schutta, MD, medical director of Penn Rodebaugh Diabetes Center in Philadelphia, said that results of this study suggest a need to carefully select medical therapy for each individual patient with diabetes in order to mitigate CV risk.
“It’s really about tailoring their drugs to their personal situation,” Dr. Schutta said in an interview.
Although newer diabetes drug classes are associated with low to no risk of hypoglycemia, Dr. Schutta said that there is still a place for drugs such as sulfonylureas in certain situations.
Among sulfonylureas, glyburide comes with a much higher incidence of hypoglycemia, compared with glipizide and glimepiride, according to Dr. Schutta. “I think there’s a role for both drugs, but you have to be very careful, and you have to get the data from your patients.”
Hypoglycemia frequency and outcomes
Speculation that hypoglycemia could be linked to adverse CV outcomes was sparked years ago by trials such as ADVANCE. Severe hypoglycemia in that study was associated with a 168% increased risk of death from a CV cause (N Engl J Med. 2010 Oct 7;363:1410-8).
At the time, ADVANCE investigators said they were unable to find evidence that multiple severe hypoglycemia episodes conferred a greater risk of CV events versus a single hypoglycemia episode, though they added that few patients had recurrent events.
“In other words, the association between the number of hypoglycemia events, and adverse CV outcomes is still unclear,” said Dr. Rajpal in his virtual ADA presentation.
Potential elevated risks with more than five episodes
To evaluate the association between frequent hypoglycemic episodes (i.e., more than five per year, compared with one to five episodes) and CV events, Dr. Rajpal and colleagues evaluated outcomes data for 4.9 million adults with type 2 diabetes found in a large commercial database including information on patients in 27 U.S. health care networks.
Database records indicated that about 182,000 patients, or nearly 4%, had episodes of hyperglycemia, which Dr. Rajpal said was presumed to mean a plasma glucose level of less than 70 mg/dL.
Characteristics of the patients with more than five hypoglycemic episodes were similar to those with one to five episodes, although they were more likely to be 65 years or older, and were “slightly more likely” to be on insulin, which could possibly precipitate more hypoglycemic episodes in that group, Dr. Rajpal said.
Key findings
In the main analysis, Dr. Rajpal said, risk of CV events was significantly increased in those with more than five hypoglycemic episodes, compared with those with one to five episodes, with an odds ratio of 1.61 (95% confidence interval, 1.56-1.66). The incidence of cardiovascular events was 33.1% in those with more than five episodes and 23.5% in those with one to five episodes, according to the data presented.
Risks were also significantly increased specifically for cardiac arrhythmias, cerebrovascular accidents, and MI, Dr. Rajpal said, with ORs of 1.65 (95% CI, 1.9-1.71), 1.38 (95% CI, 1.22-1.56), and 1.43 (95% CI, 1.36-1.50), respectively.
Because individuals in the group with more than five hypoglycemic episodes were more likely to be elderly, Dr. Rajpal said that he and coinvestigators decided to perform an age-specific stratified analysis.
Although cerebral vascular incidence was low in younger patients, risk of CV events overall was nevertheless significantly elevated for those aged 65 years or older, 45-64 years, and 18-44 years, with ORs of 1.69 (95% CI, 1.61-1.7), 1.58 (95% CI, 1.48-1.69), and 1.62 (95% CI, 1.33-1.97).
“The results were still valid in stratified analysis based on different age groups,” Dr. Rajpal said.
Dr. Rajpal and coauthors reported that he had no conflicts of interest related to the research.
SOURCE: Rajpal A et al. ADA 2020, Abstract 161-OR.
FROM ADA 2020
Novel insulin shows early promise for once-weekly treatment
Julio Rosenstock, MD, of the University of Texas, Dallas, presented the data from the phase 2 pivotal study of icodec on June 14 during the virtual American Diabetes Association 80th Scientific Sessions.
Insulin icodec binds to albumin to create a circulating depot with a 196-hour half-life. A once-weekly injection is designed to cover an individual’s basal insulin requirements for a full week with steady insulin release. Because of its concentrated formulation, its injection volume is equivalent to that of daily glargine U100.
“Many people with type 2 diabetes are reluctant to start on insulin therapy due to the need for daily injections. ... I’m truly excited about the potential of such innovative treatments which could reduce the number of basal insulin injections for my patients with diabetes,” Dr. Rosenstock commented in a Novo Nordisk statement.
During his presentation, he added that the product “has the potential to be a major player in the management of type 2 diabetes if eventually approved.”
Charles M. Alexander, MD, an endocrinologist and managing director of Alexander Associates, Gwynedd Valley, Pa., said that “it’s a phase 2 study. Obviously we need to see the phase 3 data, but it’s very encouraging.”
Dr. Alexander, who was global medical director for diabetes in medical affairs at Merck from 2008 to 2015, observed that “the theory is that you have better adherence to once-weekly, compared to daily [dosing], but when you actually do the studies it’s very difficult to prove that.
“I think the big advantage is that the company can develop a coformulation of [the glucagonlike peptide–1 receptor agonist] semaglutide and icodec in the same pen or vial. ... There is a convenience factor of once weekly over daily.”
In fact, he noted, Novo Nordisk is already in phase 1 trials with that product, called icosema.
“Potential to be transformational”
The phase 2, randomized, double-blind, double-dummy, parallel-group, treat-to-target trial included 247 insulin-naive patients with type 2 diabetes with hemoglobin A1c levels of 7.0%-9.5% despite taking metformin, with about half also taking a dipeptidyl peptidase–4 inhibitor.
They were randomized to weekly insulin icodec plus daily placebo (n = 125) or daily insulin glargine U100 plus weekly placebo (n = 122). All participants took seven injections per week with a vial and syringe plus one injection per week with a pen injector. Doses were titrated up or down to achieve blood glucose levels 70-108 mg/dL, with glargine dose adjustments of 2 or 4 units and icodec units of 14 or 28 units.
Participants were a mean age of 59.6 years, had a diabetes duration of 9.7 years, and 56.3% were men. Baseline A1c was 8.0% overall and fasting blood glucose was 181 mg/dL, and both were similar between the two groups.
The primary endpoint, change in A1c from baseline to week 26, dropped 1.33 percentage points with icodec and 1.15 percentage points with glargine, which was not significantly different (P = .08). Estimated mean A1c levels were 6.7% for icodec and 6.9% for glargine.
The icodec result, Dr. Rosenstock said, “is a very impressive final A1c.”
The proportions of patients achieving A1c <7% by week 26 for icodec versus glargine were 72% versus 68%, and for A1c ≤6.5% were 49% and 39%, respectively. Those differences weren’t statistically significant because of lack of power, Dr. Rosenstock observed.
Fasting plasma glucose levels were nearly identical at 26 weeks, with drops of 58 mg/dL with icodec and 54 mg/dL with glargine (P = .34).
However, there was a significant difference in favor of icodec in the 9-point self-monitoring of blood glucose profile, with a difference in mean change from baseline to week 26 of –7.9 mg/dL (P = .01).
Lower postbreakfast and postlunch glucose peaks at 90 minutes accounted for most of the difference, Dr. Rosenstock noted.
Total insulin doses during the last 2 weeks of treatment with icodec versus glargine were 229 versus 284 units/week (P = .01); those translate to approximate daily doses of 33 versus 41 units/day, respectively.
Both groups gained a small amount of weight, 1.5 kg with icodec and 1.6 kg with glargine by week 26 (P = .88).
Hypoglycemia was more common with icodec than glargine, including mild (53.6% vs. 37.7%), moderate or clinically significant (16.0% vs. 9.8%), and severe (1 [0.8%] vs. 0 participants). Corresponding event rates were 508.9 versus 210.8 per 100 patient-years (mild hypoglycemia), 52.5 versus 45.6 per 100 patient-years (moderate or clinically significant), and 1.4 versus 0 per 100 patient-years (severe) for icodec versus glargine.
The difference between the two groups in moderate or clinically significant hypoglycemia wasn’t statistically significant (P = .85), and the duration of hypoglycemia wasn’t longer with icodec, compared with glargine, despite its longer duration of action, Dr. Rosenstock emphasized.
Rates of other adverse events were similar between groups.
“Based on the robustness of these data, further evidence on the role of weekly basal insulin icodec will be pursued in a comprehensive phase 3 clinical development program,” Dr. Rosenstock explained. If those data confirm the phase 2 results, “I believe personally that a weekly basal insulin has the potential to be transformational in the management of people with type 2 diabetes needing insulin therapy.”
Dr. Rosenstock has reported receiving research support from, being on advisory boards for, and/or receiving consulting honoraria from Merck, Pfizer, Sanofi, Novo Nordisk, Eli Lilly, GlaxoSmithKline, AstraZeneca, Janssen, Genentech, Oramed, Boehringer Ingelheim, Applied Therapeutics, and Intarcia. Dr. Alexander has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Julio Rosenstock, MD, of the University of Texas, Dallas, presented the data from the phase 2 pivotal study of icodec on June 14 during the virtual American Diabetes Association 80th Scientific Sessions.
Insulin icodec binds to albumin to create a circulating depot with a 196-hour half-life. A once-weekly injection is designed to cover an individual’s basal insulin requirements for a full week with steady insulin release. Because of its concentrated formulation, its injection volume is equivalent to that of daily glargine U100.
“Many people with type 2 diabetes are reluctant to start on insulin therapy due to the need for daily injections. ... I’m truly excited about the potential of such innovative treatments which could reduce the number of basal insulin injections for my patients with diabetes,” Dr. Rosenstock commented in a Novo Nordisk statement.
During his presentation, he added that the product “has the potential to be a major player in the management of type 2 diabetes if eventually approved.”
Charles M. Alexander, MD, an endocrinologist and managing director of Alexander Associates, Gwynedd Valley, Pa., said that “it’s a phase 2 study. Obviously we need to see the phase 3 data, but it’s very encouraging.”
Dr. Alexander, who was global medical director for diabetes in medical affairs at Merck from 2008 to 2015, observed that “the theory is that you have better adherence to once-weekly, compared to daily [dosing], but when you actually do the studies it’s very difficult to prove that.
“I think the big advantage is that the company can develop a coformulation of [the glucagonlike peptide–1 receptor agonist] semaglutide and icodec in the same pen or vial. ... There is a convenience factor of once weekly over daily.”
In fact, he noted, Novo Nordisk is already in phase 1 trials with that product, called icosema.
“Potential to be transformational”
The phase 2, randomized, double-blind, double-dummy, parallel-group, treat-to-target trial included 247 insulin-naive patients with type 2 diabetes with hemoglobin A1c levels of 7.0%-9.5% despite taking metformin, with about half also taking a dipeptidyl peptidase–4 inhibitor.
They were randomized to weekly insulin icodec plus daily placebo (n = 125) or daily insulin glargine U100 plus weekly placebo (n = 122). All participants took seven injections per week with a vial and syringe plus one injection per week with a pen injector. Doses were titrated up or down to achieve blood glucose levels 70-108 mg/dL, with glargine dose adjustments of 2 or 4 units and icodec units of 14 or 28 units.
Participants were a mean age of 59.6 years, had a diabetes duration of 9.7 years, and 56.3% were men. Baseline A1c was 8.0% overall and fasting blood glucose was 181 mg/dL, and both were similar between the two groups.
The primary endpoint, change in A1c from baseline to week 26, dropped 1.33 percentage points with icodec and 1.15 percentage points with glargine, which was not significantly different (P = .08). Estimated mean A1c levels were 6.7% for icodec and 6.9% for glargine.
The icodec result, Dr. Rosenstock said, “is a very impressive final A1c.”
The proportions of patients achieving A1c <7% by week 26 for icodec versus glargine were 72% versus 68%, and for A1c ≤6.5% were 49% and 39%, respectively. Those differences weren’t statistically significant because of lack of power, Dr. Rosenstock observed.
Fasting plasma glucose levels were nearly identical at 26 weeks, with drops of 58 mg/dL with icodec and 54 mg/dL with glargine (P = .34).
However, there was a significant difference in favor of icodec in the 9-point self-monitoring of blood glucose profile, with a difference in mean change from baseline to week 26 of –7.9 mg/dL (P = .01).
Lower postbreakfast and postlunch glucose peaks at 90 minutes accounted for most of the difference, Dr. Rosenstock noted.
Total insulin doses during the last 2 weeks of treatment with icodec versus glargine were 229 versus 284 units/week (P = .01); those translate to approximate daily doses of 33 versus 41 units/day, respectively.
Both groups gained a small amount of weight, 1.5 kg with icodec and 1.6 kg with glargine by week 26 (P = .88).
Hypoglycemia was more common with icodec than glargine, including mild (53.6% vs. 37.7%), moderate or clinically significant (16.0% vs. 9.8%), and severe (1 [0.8%] vs. 0 participants). Corresponding event rates were 508.9 versus 210.8 per 100 patient-years (mild hypoglycemia), 52.5 versus 45.6 per 100 patient-years (moderate or clinically significant), and 1.4 versus 0 per 100 patient-years (severe) for icodec versus glargine.
The difference between the two groups in moderate or clinically significant hypoglycemia wasn’t statistically significant (P = .85), and the duration of hypoglycemia wasn’t longer with icodec, compared with glargine, despite its longer duration of action, Dr. Rosenstock emphasized.
Rates of other adverse events were similar between groups.
“Based on the robustness of these data, further evidence on the role of weekly basal insulin icodec will be pursued in a comprehensive phase 3 clinical development program,” Dr. Rosenstock explained. If those data confirm the phase 2 results, “I believe personally that a weekly basal insulin has the potential to be transformational in the management of people with type 2 diabetes needing insulin therapy.”
Dr. Rosenstock has reported receiving research support from, being on advisory boards for, and/or receiving consulting honoraria from Merck, Pfizer, Sanofi, Novo Nordisk, Eli Lilly, GlaxoSmithKline, AstraZeneca, Janssen, Genentech, Oramed, Boehringer Ingelheim, Applied Therapeutics, and Intarcia. Dr. Alexander has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Julio Rosenstock, MD, of the University of Texas, Dallas, presented the data from the phase 2 pivotal study of icodec on June 14 during the virtual American Diabetes Association 80th Scientific Sessions.
Insulin icodec binds to albumin to create a circulating depot with a 196-hour half-life. A once-weekly injection is designed to cover an individual’s basal insulin requirements for a full week with steady insulin release. Because of its concentrated formulation, its injection volume is equivalent to that of daily glargine U100.
“Many people with type 2 diabetes are reluctant to start on insulin therapy due to the need for daily injections. ... I’m truly excited about the potential of such innovative treatments which could reduce the number of basal insulin injections for my patients with diabetes,” Dr. Rosenstock commented in a Novo Nordisk statement.
During his presentation, he added that the product “has the potential to be a major player in the management of type 2 diabetes if eventually approved.”
Charles M. Alexander, MD, an endocrinologist and managing director of Alexander Associates, Gwynedd Valley, Pa., said that “it’s a phase 2 study. Obviously we need to see the phase 3 data, but it’s very encouraging.”
Dr. Alexander, who was global medical director for diabetes in medical affairs at Merck from 2008 to 2015, observed that “the theory is that you have better adherence to once-weekly, compared to daily [dosing], but when you actually do the studies it’s very difficult to prove that.
“I think the big advantage is that the company can develop a coformulation of [the glucagonlike peptide–1 receptor agonist] semaglutide and icodec in the same pen or vial. ... There is a convenience factor of once weekly over daily.”
In fact, he noted, Novo Nordisk is already in phase 1 trials with that product, called icosema.
“Potential to be transformational”
The phase 2, randomized, double-blind, double-dummy, parallel-group, treat-to-target trial included 247 insulin-naive patients with type 2 diabetes with hemoglobin A1c levels of 7.0%-9.5% despite taking metformin, with about half also taking a dipeptidyl peptidase–4 inhibitor.
They were randomized to weekly insulin icodec plus daily placebo (n = 125) or daily insulin glargine U100 plus weekly placebo (n = 122). All participants took seven injections per week with a vial and syringe plus one injection per week with a pen injector. Doses were titrated up or down to achieve blood glucose levels 70-108 mg/dL, with glargine dose adjustments of 2 or 4 units and icodec units of 14 or 28 units.
Participants were a mean age of 59.6 years, had a diabetes duration of 9.7 years, and 56.3% were men. Baseline A1c was 8.0% overall and fasting blood glucose was 181 mg/dL, and both were similar between the two groups.
The primary endpoint, change in A1c from baseline to week 26, dropped 1.33 percentage points with icodec and 1.15 percentage points with glargine, which was not significantly different (P = .08). Estimated mean A1c levels were 6.7% for icodec and 6.9% for glargine.
The icodec result, Dr. Rosenstock said, “is a very impressive final A1c.”
The proportions of patients achieving A1c <7% by week 26 for icodec versus glargine were 72% versus 68%, and for A1c ≤6.5% were 49% and 39%, respectively. Those differences weren’t statistically significant because of lack of power, Dr. Rosenstock observed.
Fasting plasma glucose levels were nearly identical at 26 weeks, with drops of 58 mg/dL with icodec and 54 mg/dL with glargine (P = .34).
However, there was a significant difference in favor of icodec in the 9-point self-monitoring of blood glucose profile, with a difference in mean change from baseline to week 26 of –7.9 mg/dL (P = .01).
Lower postbreakfast and postlunch glucose peaks at 90 minutes accounted for most of the difference, Dr. Rosenstock noted.
Total insulin doses during the last 2 weeks of treatment with icodec versus glargine were 229 versus 284 units/week (P = .01); those translate to approximate daily doses of 33 versus 41 units/day, respectively.
Both groups gained a small amount of weight, 1.5 kg with icodec and 1.6 kg with glargine by week 26 (P = .88).
Hypoglycemia was more common with icodec than glargine, including mild (53.6% vs. 37.7%), moderate or clinically significant (16.0% vs. 9.8%), and severe (1 [0.8%] vs. 0 participants). Corresponding event rates were 508.9 versus 210.8 per 100 patient-years (mild hypoglycemia), 52.5 versus 45.6 per 100 patient-years (moderate or clinically significant), and 1.4 versus 0 per 100 patient-years (severe) for icodec versus glargine.
The difference between the two groups in moderate or clinically significant hypoglycemia wasn’t statistically significant (P = .85), and the duration of hypoglycemia wasn’t longer with icodec, compared with glargine, despite its longer duration of action, Dr. Rosenstock emphasized.
Rates of other adverse events were similar between groups.
“Based on the robustness of these data, further evidence on the role of weekly basal insulin icodec will be pursued in a comprehensive phase 3 clinical development program,” Dr. Rosenstock explained. If those data confirm the phase 2 results, “I believe personally that a weekly basal insulin has the potential to be transformational in the management of people with type 2 diabetes needing insulin therapy.”
Dr. Rosenstock has reported receiving research support from, being on advisory boards for, and/or receiving consulting honoraria from Merck, Pfizer, Sanofi, Novo Nordisk, Eli Lilly, GlaxoSmithKline, AstraZeneca, Janssen, Genentech, Oramed, Boehringer Ingelheim, Applied Therapeutics, and Intarcia. Dr. Alexander has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
CAC scoring pinpoints stenoses in asymptomatic diabetes patients
For diabetes patients with no cardiovascular symptoms despite certain risk factors, incorporating coronary calcium scoring into a silent myocardial ischemia screening algorithm may be an effective and cost-conscious strategy that avoids missed coronary stenoses suitable for revascularization, results of a recent study suggest.
Zero patients in need of revascularization were missed in a risk stratification model in which screening for silent myocardial ischemia (SMI) was done only for patients with peripheral artery disease, severe nephropathy, or a high coronary artery calcium (CAC) score, according to investigator Paul Valensi, MD.
In practical terms, that means stress myocardial scintigraphy to detect SMI could be reserved for patients with evidence of target organ damage or a CAC score of 100 or higher, according to Dr. Valensi, head of the department of endocrinology, diabetology, and nutrition at Jean Verdier Hospital in Bondy, France.
“The strategy appears to be a good compromise, and the most cost effective strategy,” Dr. Valensi said in a presentation of the results at the virtual annual scientific sessions of the American Diabetes Association.
Utility of CAC scoring in diabetes
This algorithm proposed by Dr. Valenti and colleagues is a “reasonable” approach to guide risk stratification in asymptomatic diabetes patients, said Matthew J. Budoff, MD, professor of medicine and director of cardiac CT at Harbor-UCLA Medical Center in Torrance, Calif.
“Calcium scoring could certainly help you identify those patients (at increased risk) as a first-line test, because if their calcium score is zero, their chance of having obstructive disease is probably either zero or very close to zero,” Dr. Budoff said in an interview.
Using CAC scores to assess cardiovascular risk in asymptomatic adults with diabetes was supported by 2010 guidelines from the American College of Cardiology and the American Heart Association, Dr. Budoff said, while 2019 guidelines from the European Society of Cardiology (ESC) describe CAC score combined with CT as a potential risk modifier in the evaluation of certain asymptomatic patients with diabetes.
“We are starting to see that we might be able to understand diabetes better and the cardiovascular implications by understanding how much plaque (patients) have at the time that we see them,” Dr. Budoff said in a presentation on use of CAC scans he gave earlier at the virtual ADA meeting.
In the interview, Dr. Budoff also noted that CAC scores may be particularly useful for guiding use of statins, PCSK9 (proprotein convertase subtilisin kexin 9) inhibitors, or other treatments in patients with diabetes: “There are a lot of therapies that we can apply, if we knew somebody was at higher risk, that would potentially help them avoid a heart attack, stroke, or cardiovascular death,” he said.
CAC scoring and coronary artery stenoses
Although about 20% of patients with type 2 diabetes have SMI, screening for it is “debated,” according to Dr. Valensi.
The recent ESC guidelines state that while routine screening for coronary artery disease in asymptomatic diabetics is not recommended, stress testing or coronary angiography “may be indicated” in asymptomatic diabetics in the very-high cardiovascular risk category.
That position is based on a lack of benefit seen with a broad screening strategy, the guidelines say, possibly due in part to low event rates in randomized controlled trials that have studied the approach.
Using CAC scoring could change the equation by helping to identify a greater proportion of type 2 diabetics with SMI, according to Dr. Valensi.
“The role of the CAC score in the strategy of detection of SMI needs to be defined, and this role may depend on the a priori cardiovascular risk,” he said.
Dr. Valensi and colleagues accordingly tested several different approaches to selecting asymptomatic diabetic patients for SMI screening to see how they would perform in finding patients with coronary stenoses eligible for revascularization.
Their study included 416 diabetes patients with diabetes at very high cardiovascular risk but with no cardiac history or symptoms. A total of 40 patients (9.6%) had SMI, including 15 patients in which coronary stenoses were found; of those, 11 (73.5%) underwent a revascularization procedure.
They found that, by performing myocardial scintigraphy only in those patients with peripheral artery disease or severe nephropathy, they would have missed 6 patients with coronary stenosis suitable for revascularization among the 275 patients who did not meet those target organ damage criteria.
By contrast, zero patients would have been missed by performing myocardial scintigraphy in patients who either met those target organ damage criteria, or who had an elevated CAC score.
“We suggest screening for SMI, using stress myocardial CT scanning and coronary stenosis screening, only the patients with peripheral artery disease or severe nephropathy or with a high CAC score over 100 Agatston units,” said Dr. Valensi.
Dr. Valensi reported disclosures related to Merck Sharp Dohme, Novo Nordisk, Pierre Fabre, Eli Lilly, Bristol-Myers Squibb, AstraZeneca, Daiichi-Sankyo, and others. Coauthors provided no disclosures related to the research. Dr. Budoff reported that he has served as a paid consultant to GE.
SOURCE: Berkane N et al. ADA 2020. Abstract 8-OR.
For diabetes patients with no cardiovascular symptoms despite certain risk factors, incorporating coronary calcium scoring into a silent myocardial ischemia screening algorithm may be an effective and cost-conscious strategy that avoids missed coronary stenoses suitable for revascularization, results of a recent study suggest.
Zero patients in need of revascularization were missed in a risk stratification model in which screening for silent myocardial ischemia (SMI) was done only for patients with peripheral artery disease, severe nephropathy, or a high coronary artery calcium (CAC) score, according to investigator Paul Valensi, MD.
In practical terms, that means stress myocardial scintigraphy to detect SMI could be reserved for patients with evidence of target organ damage or a CAC score of 100 or higher, according to Dr. Valensi, head of the department of endocrinology, diabetology, and nutrition at Jean Verdier Hospital in Bondy, France.
“The strategy appears to be a good compromise, and the most cost effective strategy,” Dr. Valensi said in a presentation of the results at the virtual annual scientific sessions of the American Diabetes Association.
Utility of CAC scoring in diabetes
This algorithm proposed by Dr. Valenti and colleagues is a “reasonable” approach to guide risk stratification in asymptomatic diabetes patients, said Matthew J. Budoff, MD, professor of medicine and director of cardiac CT at Harbor-UCLA Medical Center in Torrance, Calif.
“Calcium scoring could certainly help you identify those patients (at increased risk) as a first-line test, because if their calcium score is zero, their chance of having obstructive disease is probably either zero or very close to zero,” Dr. Budoff said in an interview.
Using CAC scores to assess cardiovascular risk in asymptomatic adults with diabetes was supported by 2010 guidelines from the American College of Cardiology and the American Heart Association, Dr. Budoff said, while 2019 guidelines from the European Society of Cardiology (ESC) describe CAC score combined with CT as a potential risk modifier in the evaluation of certain asymptomatic patients with diabetes.
“We are starting to see that we might be able to understand diabetes better and the cardiovascular implications by understanding how much plaque (patients) have at the time that we see them,” Dr. Budoff said in a presentation on use of CAC scans he gave earlier at the virtual ADA meeting.
In the interview, Dr. Budoff also noted that CAC scores may be particularly useful for guiding use of statins, PCSK9 (proprotein convertase subtilisin kexin 9) inhibitors, or other treatments in patients with diabetes: “There are a lot of therapies that we can apply, if we knew somebody was at higher risk, that would potentially help them avoid a heart attack, stroke, or cardiovascular death,” he said.
CAC scoring and coronary artery stenoses
Although about 20% of patients with type 2 diabetes have SMI, screening for it is “debated,” according to Dr. Valensi.
The recent ESC guidelines state that while routine screening for coronary artery disease in asymptomatic diabetics is not recommended, stress testing or coronary angiography “may be indicated” in asymptomatic diabetics in the very-high cardiovascular risk category.
That position is based on a lack of benefit seen with a broad screening strategy, the guidelines say, possibly due in part to low event rates in randomized controlled trials that have studied the approach.
Using CAC scoring could change the equation by helping to identify a greater proportion of type 2 diabetics with SMI, according to Dr. Valensi.
“The role of the CAC score in the strategy of detection of SMI needs to be defined, and this role may depend on the a priori cardiovascular risk,” he said.
Dr. Valensi and colleagues accordingly tested several different approaches to selecting asymptomatic diabetic patients for SMI screening to see how they would perform in finding patients with coronary stenoses eligible for revascularization.
Their study included 416 diabetes patients with diabetes at very high cardiovascular risk but with no cardiac history or symptoms. A total of 40 patients (9.6%) had SMI, including 15 patients in which coronary stenoses were found; of those, 11 (73.5%) underwent a revascularization procedure.
They found that, by performing myocardial scintigraphy only in those patients with peripheral artery disease or severe nephropathy, they would have missed 6 patients with coronary stenosis suitable for revascularization among the 275 patients who did not meet those target organ damage criteria.
By contrast, zero patients would have been missed by performing myocardial scintigraphy in patients who either met those target organ damage criteria, or who had an elevated CAC score.
“We suggest screening for SMI, using stress myocardial CT scanning and coronary stenosis screening, only the patients with peripheral artery disease or severe nephropathy or with a high CAC score over 100 Agatston units,” said Dr. Valensi.
Dr. Valensi reported disclosures related to Merck Sharp Dohme, Novo Nordisk, Pierre Fabre, Eli Lilly, Bristol-Myers Squibb, AstraZeneca, Daiichi-Sankyo, and others. Coauthors provided no disclosures related to the research. Dr. Budoff reported that he has served as a paid consultant to GE.
SOURCE: Berkane N et al. ADA 2020. Abstract 8-OR.
For diabetes patients with no cardiovascular symptoms despite certain risk factors, incorporating coronary calcium scoring into a silent myocardial ischemia screening algorithm may be an effective and cost-conscious strategy that avoids missed coronary stenoses suitable for revascularization, results of a recent study suggest.
Zero patients in need of revascularization were missed in a risk stratification model in which screening for silent myocardial ischemia (SMI) was done only for patients with peripheral artery disease, severe nephropathy, or a high coronary artery calcium (CAC) score, according to investigator Paul Valensi, MD.
In practical terms, that means stress myocardial scintigraphy to detect SMI could be reserved for patients with evidence of target organ damage or a CAC score of 100 or higher, according to Dr. Valensi, head of the department of endocrinology, diabetology, and nutrition at Jean Verdier Hospital in Bondy, France.
“The strategy appears to be a good compromise, and the most cost effective strategy,” Dr. Valensi said in a presentation of the results at the virtual annual scientific sessions of the American Diabetes Association.
Utility of CAC scoring in diabetes
This algorithm proposed by Dr. Valenti and colleagues is a “reasonable” approach to guide risk stratification in asymptomatic diabetes patients, said Matthew J. Budoff, MD, professor of medicine and director of cardiac CT at Harbor-UCLA Medical Center in Torrance, Calif.
“Calcium scoring could certainly help you identify those patients (at increased risk) as a first-line test, because if their calcium score is zero, their chance of having obstructive disease is probably either zero or very close to zero,” Dr. Budoff said in an interview.
Using CAC scores to assess cardiovascular risk in asymptomatic adults with diabetes was supported by 2010 guidelines from the American College of Cardiology and the American Heart Association, Dr. Budoff said, while 2019 guidelines from the European Society of Cardiology (ESC) describe CAC score combined with CT as a potential risk modifier in the evaluation of certain asymptomatic patients with diabetes.
“We are starting to see that we might be able to understand diabetes better and the cardiovascular implications by understanding how much plaque (patients) have at the time that we see them,” Dr. Budoff said in a presentation on use of CAC scans he gave earlier at the virtual ADA meeting.
In the interview, Dr. Budoff also noted that CAC scores may be particularly useful for guiding use of statins, PCSK9 (proprotein convertase subtilisin kexin 9) inhibitors, or other treatments in patients with diabetes: “There are a lot of therapies that we can apply, if we knew somebody was at higher risk, that would potentially help them avoid a heart attack, stroke, or cardiovascular death,” he said.
CAC scoring and coronary artery stenoses
Although about 20% of patients with type 2 diabetes have SMI, screening for it is “debated,” according to Dr. Valensi.
The recent ESC guidelines state that while routine screening for coronary artery disease in asymptomatic diabetics is not recommended, stress testing or coronary angiography “may be indicated” in asymptomatic diabetics in the very-high cardiovascular risk category.
That position is based on a lack of benefit seen with a broad screening strategy, the guidelines say, possibly due in part to low event rates in randomized controlled trials that have studied the approach.
Using CAC scoring could change the equation by helping to identify a greater proportion of type 2 diabetics with SMI, according to Dr. Valensi.
“The role of the CAC score in the strategy of detection of SMI needs to be defined, and this role may depend on the a priori cardiovascular risk,” he said.
Dr. Valensi and colleagues accordingly tested several different approaches to selecting asymptomatic diabetic patients for SMI screening to see how they would perform in finding patients with coronary stenoses eligible for revascularization.
Their study included 416 diabetes patients with diabetes at very high cardiovascular risk but with no cardiac history or symptoms. A total of 40 patients (9.6%) had SMI, including 15 patients in which coronary stenoses were found; of those, 11 (73.5%) underwent a revascularization procedure.
They found that, by performing myocardial scintigraphy only in those patients with peripheral artery disease or severe nephropathy, they would have missed 6 patients with coronary stenosis suitable for revascularization among the 275 patients who did not meet those target organ damage criteria.
By contrast, zero patients would have been missed by performing myocardial scintigraphy in patients who either met those target organ damage criteria, or who had an elevated CAC score.
“We suggest screening for SMI, using stress myocardial CT scanning and coronary stenosis screening, only the patients with peripheral artery disease or severe nephropathy or with a high CAC score over 100 Agatston units,” said Dr. Valensi.
Dr. Valensi reported disclosures related to Merck Sharp Dohme, Novo Nordisk, Pierre Fabre, Eli Lilly, Bristol-Myers Squibb, AstraZeneca, Daiichi-Sankyo, and others. Coauthors provided no disclosures related to the research. Dr. Budoff reported that he has served as a paid consultant to GE.
SOURCE: Berkane N et al. ADA 2020. Abstract 8-OR.
FROM ADA 2020
DAPA-HF: Dapagliflozin slows T2D onset in heart failure patients
Dapagliflozin treatment of patients with heart failure but without diabetes in the DAPA-HF trial led to a one-third cut in the relative incidence of new-onset diabetes over a median follow-up of 18 months in a prespecified analysis from the multicenter trial that included 2,605 heart failure patients without diabetes at baseline.
The findings represented the first evidence that a drug from dapagliflozin’s class, the sodium-glucose cotransporter 2 (SGLT2) inhibitors, could prevent or slow the onset of type 2 diabetes. It represents “an additional benefit” that dapagliflozin (Farxiga) offers to patients with heart failure with reduced ejection fraction (HFrEF) like those enrolled in the DAPA-HF trial, Silvio E. Inzucchi, MD, said at the virtual annual scientific sessions of the American Diabetes Association. DAPA-HF had previously proved that treatment with this drug significantly reduced the study’s primary endpoint of cardiovascular death or heart failure worsening.
During 18 months of follow-up, 7.1% of patients in the placebo arm developed type 2 diabetes, compared with 4.9% in those who received dapagliflozin, a 2.2% absolute difference and a 32% relative risk reduction that was statistically significant for this prespecified but “exploratory” endpoint, reported Dr. Inzucchi, an endocrinologist and professor of medicine at Yale University, New Haven, Conn.
For this analysis, a hemoglobin A1c level of at least 6.5% measured in two consecutive assessments was the criterion for diagnosing incident diabetes. The 2,605 enrolled patients without diabetes in the DAPA-HF trial represented 55% of the entire trial cohort of 4,744 patients with HFrEF.
The 32% relative risk reduction for incident diabetes was primarily relevant to enrolled patients with prediabetes at entry, who constituted 67% of the enrolled cohort based on the usual definition of prediabetes, an A1c of 5.7%-6.4%.
Among all 157 (6%) of the DAPA-HF patients who developed diabetes during the trial, 150 (96%) occurred in patients with prediabetes by the usual definition; 136 of the incident cases (87%) had prediabetes by a more stringent criterion of an A1c of 6.0%-6.4%.
To put the preventive efficacy of dapagliflozin into more context, Dr. Inzucchi cited the 31% relative protection rate exerted by metformin in the Diabetes Prevention Program study (N Engl J Med. 2002 Feb 7;346[6]:393-403).
The findings showed that “dapagliflozin is the first medication demonstrated to reduce both incident type 2 diabetes and mortality in a single trial,” as well as the first agent from the SGLT2 inhibitor class to show a diabetes prevention effect, Dr. Inzucchi noted. Patients with both heart failure and diabetes are known to have a substantially increased mortality risk, compared with patients with just one of these diseases, and the potent risk posed by the confluence of both was confirmed in the results Dr. Inzucchi reported.
The 157 HFrEF patients in the trial who developed diabetes had a statistically significant 70% increased incidence of all-cause mortality during the trial’s follow-up, compared with similar HFrEF patients who remained free from a diabetes diagnosis, and they also had a significant 77% relative increase in their incidence of cardiovascular death. This analysis failed to show that incident diabetes had a significant impact on hospitalizations for heart failure coupled with cardiovascular death, another endpoint of the trial.
“This is a tremendously important analysis. We recognize that diabetes is an important factor that can forecast heart failure risk, even over relatively short follow-up. A drug that targets both diseases can be quite beneficial,” commented Muthiah Vaduganathan, MD, a cardiologist at Brigham and Women’s Hospital in Boston.
The impact of dapagliflozin on average A1c levels during the DAPA-HF trial was minimal, reducing levels by an average of 0.04% among those who entered with prediabetes and by 0.05% among the other patients. This suggests that the mechanisms by which dapagliflozin reduced incident diabetes was by routes that did not involve simply reducing hyperglycemia, and the observed decrease in incident diabetes was not apparently caused by “masking” of hyperglycemia by dapagliflozin, said Dr. Inzucchi.
One possibility is that dapagliflozin, which also improved quality of life and reduced hospitalizations in the DAPA-HF trial, led to improved function and mobility among patients that had beneficial effects on their insulin sensitivity, Dr. Vaduganathan speculated in an interview.
The new finding of dapagliflozin’s benefit “is great news,” commented Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of the Metabolic Institute of America in Tarzana, Calif. “It’s an impressive and important result, and another reason to use dapagliflozin in patients with HFrEF, a group of patients whom you want to prevent from having worse outcomes” by developing diabetes.
The DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) trial enrolled HFrEF patients at 410 centers in 20 countries during February 2017–August 2018. The study’s primary endpoint was the composite incidence of cardiovascular death or worsening heart failure, which occurred in 16.3% of patients randomized to receive dapagliflozin and in 21.2% of control patients on standard care but on placebo instead of the study drug, a statistically significant relative risk reduction of 26% (N Engl J Med. 2019 Nov 21;381[21]:1995-2008). In the 2,605-patient subgroup without type 2 diabetes at baseline the primary endpoint fell by a statistically significant 27% with dapagliflozin treatment, the first time an SGLT2 inhibitor drug was shown effective for reducing this endpoint in patients with HFrEF but without diabetes. DAPA-HF did not enroll any patients with type 1 diabetes.
DAPA-HF was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Inzucchi has been a consultant to AstraZeneca and to Abbott, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics. Dr. Vaduganathan has been an adviser to AstraZeneca and to Amgen, Baxter, Bayer, Boehringer Ingelheim, Cytokinetics, and Relypsa. Dr. Handelsman has been a consultant to several drug companies including AstraZeneca.
SOURCE: Inzucchi SE et al. ADA 2020, abstract 271-OR.
Dapagliflozin treatment of patients with heart failure but without diabetes in the DAPA-HF trial led to a one-third cut in the relative incidence of new-onset diabetes over a median follow-up of 18 months in a prespecified analysis from the multicenter trial that included 2,605 heart failure patients without diabetes at baseline.
The findings represented the first evidence that a drug from dapagliflozin’s class, the sodium-glucose cotransporter 2 (SGLT2) inhibitors, could prevent or slow the onset of type 2 diabetes. It represents “an additional benefit” that dapagliflozin (Farxiga) offers to patients with heart failure with reduced ejection fraction (HFrEF) like those enrolled in the DAPA-HF trial, Silvio E. Inzucchi, MD, said at the virtual annual scientific sessions of the American Diabetes Association. DAPA-HF had previously proved that treatment with this drug significantly reduced the study’s primary endpoint of cardiovascular death or heart failure worsening.
During 18 months of follow-up, 7.1% of patients in the placebo arm developed type 2 diabetes, compared with 4.9% in those who received dapagliflozin, a 2.2% absolute difference and a 32% relative risk reduction that was statistically significant for this prespecified but “exploratory” endpoint, reported Dr. Inzucchi, an endocrinologist and professor of medicine at Yale University, New Haven, Conn.
For this analysis, a hemoglobin A1c level of at least 6.5% measured in two consecutive assessments was the criterion for diagnosing incident diabetes. The 2,605 enrolled patients without diabetes in the DAPA-HF trial represented 55% of the entire trial cohort of 4,744 patients with HFrEF.
The 32% relative risk reduction for incident diabetes was primarily relevant to enrolled patients with prediabetes at entry, who constituted 67% of the enrolled cohort based on the usual definition of prediabetes, an A1c of 5.7%-6.4%.
Among all 157 (6%) of the DAPA-HF patients who developed diabetes during the trial, 150 (96%) occurred in patients with prediabetes by the usual definition; 136 of the incident cases (87%) had prediabetes by a more stringent criterion of an A1c of 6.0%-6.4%.
To put the preventive efficacy of dapagliflozin into more context, Dr. Inzucchi cited the 31% relative protection rate exerted by metformin in the Diabetes Prevention Program study (N Engl J Med. 2002 Feb 7;346[6]:393-403).
The findings showed that “dapagliflozin is the first medication demonstrated to reduce both incident type 2 diabetes and mortality in a single trial,” as well as the first agent from the SGLT2 inhibitor class to show a diabetes prevention effect, Dr. Inzucchi noted. Patients with both heart failure and diabetes are known to have a substantially increased mortality risk, compared with patients with just one of these diseases, and the potent risk posed by the confluence of both was confirmed in the results Dr. Inzucchi reported.
The 157 HFrEF patients in the trial who developed diabetes had a statistically significant 70% increased incidence of all-cause mortality during the trial’s follow-up, compared with similar HFrEF patients who remained free from a diabetes diagnosis, and they also had a significant 77% relative increase in their incidence of cardiovascular death. This analysis failed to show that incident diabetes had a significant impact on hospitalizations for heart failure coupled with cardiovascular death, another endpoint of the trial.
“This is a tremendously important analysis. We recognize that diabetes is an important factor that can forecast heart failure risk, even over relatively short follow-up. A drug that targets both diseases can be quite beneficial,” commented Muthiah Vaduganathan, MD, a cardiologist at Brigham and Women’s Hospital in Boston.
The impact of dapagliflozin on average A1c levels during the DAPA-HF trial was minimal, reducing levels by an average of 0.04% among those who entered with prediabetes and by 0.05% among the other patients. This suggests that the mechanisms by which dapagliflozin reduced incident diabetes was by routes that did not involve simply reducing hyperglycemia, and the observed decrease in incident diabetes was not apparently caused by “masking” of hyperglycemia by dapagliflozin, said Dr. Inzucchi.
One possibility is that dapagliflozin, which also improved quality of life and reduced hospitalizations in the DAPA-HF trial, led to improved function and mobility among patients that had beneficial effects on their insulin sensitivity, Dr. Vaduganathan speculated in an interview.
The new finding of dapagliflozin’s benefit “is great news,” commented Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of the Metabolic Institute of America in Tarzana, Calif. “It’s an impressive and important result, and another reason to use dapagliflozin in patients with HFrEF, a group of patients whom you want to prevent from having worse outcomes” by developing diabetes.
The DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) trial enrolled HFrEF patients at 410 centers in 20 countries during February 2017–August 2018. The study’s primary endpoint was the composite incidence of cardiovascular death or worsening heart failure, which occurred in 16.3% of patients randomized to receive dapagliflozin and in 21.2% of control patients on standard care but on placebo instead of the study drug, a statistically significant relative risk reduction of 26% (N Engl J Med. 2019 Nov 21;381[21]:1995-2008). In the 2,605-patient subgroup without type 2 diabetes at baseline the primary endpoint fell by a statistically significant 27% with dapagliflozin treatment, the first time an SGLT2 inhibitor drug was shown effective for reducing this endpoint in patients with HFrEF but without diabetes. DAPA-HF did not enroll any patients with type 1 diabetes.
DAPA-HF was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Inzucchi has been a consultant to AstraZeneca and to Abbott, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics. Dr. Vaduganathan has been an adviser to AstraZeneca and to Amgen, Baxter, Bayer, Boehringer Ingelheim, Cytokinetics, and Relypsa. Dr. Handelsman has been a consultant to several drug companies including AstraZeneca.
SOURCE: Inzucchi SE et al. ADA 2020, abstract 271-OR.
Dapagliflozin treatment of patients with heart failure but without diabetes in the DAPA-HF trial led to a one-third cut in the relative incidence of new-onset diabetes over a median follow-up of 18 months in a prespecified analysis from the multicenter trial that included 2,605 heart failure patients without diabetes at baseline.
The findings represented the first evidence that a drug from dapagliflozin’s class, the sodium-glucose cotransporter 2 (SGLT2) inhibitors, could prevent or slow the onset of type 2 diabetes. It represents “an additional benefit” that dapagliflozin (Farxiga) offers to patients with heart failure with reduced ejection fraction (HFrEF) like those enrolled in the DAPA-HF trial, Silvio E. Inzucchi, MD, said at the virtual annual scientific sessions of the American Diabetes Association. DAPA-HF had previously proved that treatment with this drug significantly reduced the study’s primary endpoint of cardiovascular death or heart failure worsening.
During 18 months of follow-up, 7.1% of patients in the placebo arm developed type 2 diabetes, compared with 4.9% in those who received dapagliflozin, a 2.2% absolute difference and a 32% relative risk reduction that was statistically significant for this prespecified but “exploratory” endpoint, reported Dr. Inzucchi, an endocrinologist and professor of medicine at Yale University, New Haven, Conn.
For this analysis, a hemoglobin A1c level of at least 6.5% measured in two consecutive assessments was the criterion for diagnosing incident diabetes. The 2,605 enrolled patients without diabetes in the DAPA-HF trial represented 55% of the entire trial cohort of 4,744 patients with HFrEF.
The 32% relative risk reduction for incident diabetes was primarily relevant to enrolled patients with prediabetes at entry, who constituted 67% of the enrolled cohort based on the usual definition of prediabetes, an A1c of 5.7%-6.4%.
Among all 157 (6%) of the DAPA-HF patients who developed diabetes during the trial, 150 (96%) occurred in patients with prediabetes by the usual definition; 136 of the incident cases (87%) had prediabetes by a more stringent criterion of an A1c of 6.0%-6.4%.
To put the preventive efficacy of dapagliflozin into more context, Dr. Inzucchi cited the 31% relative protection rate exerted by metformin in the Diabetes Prevention Program study (N Engl J Med. 2002 Feb 7;346[6]:393-403).
The findings showed that “dapagliflozin is the first medication demonstrated to reduce both incident type 2 diabetes and mortality in a single trial,” as well as the first agent from the SGLT2 inhibitor class to show a diabetes prevention effect, Dr. Inzucchi noted. Patients with both heart failure and diabetes are known to have a substantially increased mortality risk, compared with patients with just one of these diseases, and the potent risk posed by the confluence of both was confirmed in the results Dr. Inzucchi reported.
The 157 HFrEF patients in the trial who developed diabetes had a statistically significant 70% increased incidence of all-cause mortality during the trial’s follow-up, compared with similar HFrEF patients who remained free from a diabetes diagnosis, and they also had a significant 77% relative increase in their incidence of cardiovascular death. This analysis failed to show that incident diabetes had a significant impact on hospitalizations for heart failure coupled with cardiovascular death, another endpoint of the trial.
“This is a tremendously important analysis. We recognize that diabetes is an important factor that can forecast heart failure risk, even over relatively short follow-up. A drug that targets both diseases can be quite beneficial,” commented Muthiah Vaduganathan, MD, a cardiologist at Brigham and Women’s Hospital in Boston.
The impact of dapagliflozin on average A1c levels during the DAPA-HF trial was minimal, reducing levels by an average of 0.04% among those who entered with prediabetes and by 0.05% among the other patients. This suggests that the mechanisms by which dapagliflozin reduced incident diabetes was by routes that did not involve simply reducing hyperglycemia, and the observed decrease in incident diabetes was not apparently caused by “masking” of hyperglycemia by dapagliflozin, said Dr. Inzucchi.
One possibility is that dapagliflozin, which also improved quality of life and reduced hospitalizations in the DAPA-HF trial, led to improved function and mobility among patients that had beneficial effects on their insulin sensitivity, Dr. Vaduganathan speculated in an interview.
The new finding of dapagliflozin’s benefit “is great news,” commented Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of the Metabolic Institute of America in Tarzana, Calif. “It’s an impressive and important result, and another reason to use dapagliflozin in patients with HFrEF, a group of patients whom you want to prevent from having worse outcomes” by developing diabetes.
The DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) trial enrolled HFrEF patients at 410 centers in 20 countries during February 2017–August 2018. The study’s primary endpoint was the composite incidence of cardiovascular death or worsening heart failure, which occurred in 16.3% of patients randomized to receive dapagliflozin and in 21.2% of control patients on standard care but on placebo instead of the study drug, a statistically significant relative risk reduction of 26% (N Engl J Med. 2019 Nov 21;381[21]:1995-2008). In the 2,605-patient subgroup without type 2 diabetes at baseline the primary endpoint fell by a statistically significant 27% with dapagliflozin treatment, the first time an SGLT2 inhibitor drug was shown effective for reducing this endpoint in patients with HFrEF but without diabetes. DAPA-HF did not enroll any patients with type 1 diabetes.
DAPA-HF was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Inzucchi has been a consultant to AstraZeneca and to Abbott, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics. Dr. Vaduganathan has been an adviser to AstraZeneca and to Amgen, Baxter, Bayer, Boehringer Ingelheim, Cytokinetics, and Relypsa. Dr. Handelsman has been a consultant to several drug companies including AstraZeneca.
SOURCE: Inzucchi SE et al. ADA 2020, abstract 271-OR.
FROM ADA 2020
Daily Recap: FDA revokes emergency use of hydroxychloroquine; Hardest hit specialties ranked in financial report
Here are the stories our MDedge editors across specialties think you need to know about today:
It’s official: COVID-19 is bad for your health care business
For the months of March and April 2020, use of medical professional services dropped by 65% and 68%, respectively, compared with last year, and estimated revenue fell by 45% and 48%, FAIR Health, a nonprofit organization that manages a database of 31 billion claim records, said in a new report.
Of the seven specialties included in the study, oral surgery was hit the hardest, followed by gastroenterology, cardiology, orthopedics, dermatology, adult primary care, and pediatric primary care, FAIR Health said.
“Even when medical practices have continued to function via telehealth, many have experienced lower reimbursements for telehealth visits than for in-person visits and more time educating patients on how to use the technology,” according to the report. Read more.
FDA revokes emergency use of hydroxychloroquine
The FDA revoked its decision from March 28 allowing use of hydroxychloroquine and chloroquine to treat people hospitalized with COVID-19 under an emergency use authorization (EUA).
"Based on its ongoing analysis of the EUA and emerging scientific data, the FDA determined that chloroquine and hydroxychloroquine are unlikely to be effective in treating COVID-19," the agency announced in a June 15 statement.
"In light of ongoing serious cardiac adverse events and other potential serious side effects, the known and potential benefits of chloroquine and hydroxychloroquine no longer outweigh the known and potential risks for the authorized use," noted the FDA. Read more.
Secondary infections common in COVID-19, implications unclear
Secondary respiratory infections appear to be highly prevalent among patients with severe COVID-19, but at this point, most physicians aren’t sure what to make of this understudied phenomenon.
“We really do not understand the implications of secondary infections on outcomes in COVID-19 patients,” David L. Bowton, MD, FCCP, said in an interview. “In most early reports the incidence of secondary infections was much higher in patients dying from COVID-19, compared to survivors, but it isn’t clear whether this indicates that the secondary infection itself led to excess mortality or was more a marker of the severity of the COVID-19 infection."
An early retrospective cohort study including 191 COVID-19 patients in Wuhan, China found that of the 54 who died in hospital, half had secondary bacterial lung infections (Lancet. 2020 Mar 28;395[10229]:1054-62). That comes as no surprise to U.S. physicians, who learned in training that many deaths during the so-called Spanish influenza epidemic were actually caused by secondary pneumonia involving Staphylococcus aureus, commented Daniel L. Ouellette, MD, FCCP. Read more.
Automated insulin delivery system ‘getting better and better’
Medtronic’s next-generation automated insulin delivery system offers significant improvements over the currently available model, particularly in young people with type 1 diabetes, new data suggest.
Data from three trials of such systems using Medtronic’s advanced hybrid closed-loop (AHCL) algorithm (trade name SmartGuard) were presented during the virtual American Diabetes Association (ADA) 80th Scientific Sessions.
Taken together, the data from the three trials showed that the AHCL-based system improved glycemic time-in-range with no increased risk for hypoglycemia, including in children and teenagers, with high patient-reported satisfaction.
“None of these devices is perfect, but they are a substantial improvement over what we’ve had ... They might make the quality of [patient] lives better. That’s really underappreciated,” session moderator Timothy S. Bailey, MD, commented. Read more.
Access more top news from the ADA virtual meeting.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
It’s official: COVID-19 is bad for your health care business
For the months of March and April 2020, use of medical professional services dropped by 65% and 68%, respectively, compared with last year, and estimated revenue fell by 45% and 48%, FAIR Health, a nonprofit organization that manages a database of 31 billion claim records, said in a new report.
Of the seven specialties included in the study, oral surgery was hit the hardest, followed by gastroenterology, cardiology, orthopedics, dermatology, adult primary care, and pediatric primary care, FAIR Health said.
“Even when medical practices have continued to function via telehealth, many have experienced lower reimbursements for telehealth visits than for in-person visits and more time educating patients on how to use the technology,” according to the report. Read more.
FDA revokes emergency use of hydroxychloroquine
The FDA revoked its decision from March 28 allowing use of hydroxychloroquine and chloroquine to treat people hospitalized with COVID-19 under an emergency use authorization (EUA).
"Based on its ongoing analysis of the EUA and emerging scientific data, the FDA determined that chloroquine and hydroxychloroquine are unlikely to be effective in treating COVID-19," the agency announced in a June 15 statement.
"In light of ongoing serious cardiac adverse events and other potential serious side effects, the known and potential benefits of chloroquine and hydroxychloroquine no longer outweigh the known and potential risks for the authorized use," noted the FDA. Read more.
Secondary infections common in COVID-19, implications unclear
Secondary respiratory infections appear to be highly prevalent among patients with severe COVID-19, but at this point, most physicians aren’t sure what to make of this understudied phenomenon.
“We really do not understand the implications of secondary infections on outcomes in COVID-19 patients,” David L. Bowton, MD, FCCP, said in an interview. “In most early reports the incidence of secondary infections was much higher in patients dying from COVID-19, compared to survivors, but it isn’t clear whether this indicates that the secondary infection itself led to excess mortality or was more a marker of the severity of the COVID-19 infection."
An early retrospective cohort study including 191 COVID-19 patients in Wuhan, China found that of the 54 who died in hospital, half had secondary bacterial lung infections (Lancet. 2020 Mar 28;395[10229]:1054-62). That comes as no surprise to U.S. physicians, who learned in training that many deaths during the so-called Spanish influenza epidemic were actually caused by secondary pneumonia involving Staphylococcus aureus, commented Daniel L. Ouellette, MD, FCCP. Read more.
Automated insulin delivery system ‘getting better and better’
Medtronic’s next-generation automated insulin delivery system offers significant improvements over the currently available model, particularly in young people with type 1 diabetes, new data suggest.
Data from three trials of such systems using Medtronic’s advanced hybrid closed-loop (AHCL) algorithm (trade name SmartGuard) were presented during the virtual American Diabetes Association (ADA) 80th Scientific Sessions.
Taken together, the data from the three trials showed that the AHCL-based system improved glycemic time-in-range with no increased risk for hypoglycemia, including in children and teenagers, with high patient-reported satisfaction.
“None of these devices is perfect, but they are a substantial improvement over what we’ve had ... They might make the quality of [patient] lives better. That’s really underappreciated,” session moderator Timothy S. Bailey, MD, commented. Read more.
Access more top news from the ADA virtual meeting.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
It’s official: COVID-19 is bad for your health care business
For the months of March and April 2020, use of medical professional services dropped by 65% and 68%, respectively, compared with last year, and estimated revenue fell by 45% and 48%, FAIR Health, a nonprofit organization that manages a database of 31 billion claim records, said in a new report.
Of the seven specialties included in the study, oral surgery was hit the hardest, followed by gastroenterology, cardiology, orthopedics, dermatology, adult primary care, and pediatric primary care, FAIR Health said.
“Even when medical practices have continued to function via telehealth, many have experienced lower reimbursements for telehealth visits than for in-person visits and more time educating patients on how to use the technology,” according to the report. Read more.
FDA revokes emergency use of hydroxychloroquine
The FDA revoked its decision from March 28 allowing use of hydroxychloroquine and chloroquine to treat people hospitalized with COVID-19 under an emergency use authorization (EUA).
"Based on its ongoing analysis of the EUA and emerging scientific data, the FDA determined that chloroquine and hydroxychloroquine are unlikely to be effective in treating COVID-19," the agency announced in a June 15 statement.
"In light of ongoing serious cardiac adverse events and other potential serious side effects, the known and potential benefits of chloroquine and hydroxychloroquine no longer outweigh the known and potential risks for the authorized use," noted the FDA. Read more.
Secondary infections common in COVID-19, implications unclear
Secondary respiratory infections appear to be highly prevalent among patients with severe COVID-19, but at this point, most physicians aren’t sure what to make of this understudied phenomenon.
“We really do not understand the implications of secondary infections on outcomes in COVID-19 patients,” David L. Bowton, MD, FCCP, said in an interview. “In most early reports the incidence of secondary infections was much higher in patients dying from COVID-19, compared to survivors, but it isn’t clear whether this indicates that the secondary infection itself led to excess mortality or was more a marker of the severity of the COVID-19 infection."
An early retrospective cohort study including 191 COVID-19 patients in Wuhan, China found that of the 54 who died in hospital, half had secondary bacterial lung infections (Lancet. 2020 Mar 28;395[10229]:1054-62). That comes as no surprise to U.S. physicians, who learned in training that many deaths during the so-called Spanish influenza epidemic were actually caused by secondary pneumonia involving Staphylococcus aureus, commented Daniel L. Ouellette, MD, FCCP. Read more.
Automated insulin delivery system ‘getting better and better’
Medtronic’s next-generation automated insulin delivery system offers significant improvements over the currently available model, particularly in young people with type 1 diabetes, new data suggest.
Data from three trials of such systems using Medtronic’s advanced hybrid closed-loop (AHCL) algorithm (trade name SmartGuard) were presented during the virtual American Diabetes Association (ADA) 80th Scientific Sessions.
Taken together, the data from the three trials showed that the AHCL-based system improved glycemic time-in-range with no increased risk for hypoglycemia, including in children and teenagers, with high patient-reported satisfaction.
“None of these devices is perfect, but they are a substantial improvement over what we’ve had ... They might make the quality of [patient] lives better. That’s really underappreciated,” session moderator Timothy S. Bailey, MD, commented. Read more.
Access more top news from the ADA virtual meeting.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.