User login
Noninvasive fibrosis scores not sensitive in people with fatty liver disease and T2D
Noninvasive fibrosis scores, which are widely used to predict advanced fibrosis in people with nonalcoholic fatty liver disease (NAFLD), do not do a good job of picking up advanced fibrosis in patients with underlying diabetes, according to a new study.
Advanced fibrosis is associated with an increased risk of cirrhosis, end-stage liver disease, and liver failure. Underlying diabetes is a risk factor for both advanced fibrosis and death in patients with NAFLD.
While liver biopsy remains the gold standard for detecting advanced fibrosis, high costs and risks limit its use. Noninvasive scores such as the AST/ALT ratio; AST to platelet ratio index (APRI); fibrosis-4 (FIB-4) index; and NAFLD fibrosis score (NFS) have gained popularity in recent years, as they offer the compelling advantage of using easily and cheaply attained clinical and laboratory measures to assess likelihood of disease.
But their accuracy has come into question, particularly for people with diabetes.
In research published in the Journal of Clinical Gastroenterology, Amandeep Singh, MD, and colleagues at the Cleveland Clinic looked at their center’s records for 1,157 patients with type 2 diabetes (65% women, 88% white, 85% with obesity) who had undergone a liver biopsy for suspected advanced fibrosis between 2000 and 2015. Biopsy results revealed that a third of the cohort (32%) was positive for advanced fibrosis.
The investigators then pulled patients’ laboratory results for AST, ALT, cholesterol, triglycerides, fasting glucose, hemoglobin A1c, bilirubin, albumin, platelet count, alkaline phosphatase, albumin, and lipid levels, all collected within a year of biopsy. After plugging these into the algorithms of four different scoring systems for advanced fibrosis, they compared results with results from the biopsies.
The scores of AST/ALT greater than 1.4, APRI of at least 1.5, NFS greater than 0.676, and FIB-4 index greater than 2.67 had high specificities of 84%, 97%, 70%, and 93%, respectively, but sensitivities of only 27%, 17%, 64%, and 44%. Even when the cutoff measures were tightened, the scoring systems still missed a lot of disease. This suggests, Dr. Singh and colleagues wrote, that “the presence of diabetes could decrease the predictive value of these scores to detect advanced disease in NAFLD patients.” Reliable noninvasive biomarkers are “urgently needed” for this patient population.
In an interview, Dr. Singh advised that clinicians continue to use current noninvasive scores in patients with diabetes – preferably the NFS – “until we have a better scoring system.” If clinicians suspect advanced fibrosis based on lab tests and clinical data, then “liver biopsy should be considered,” he said.
The investigators described among the limitations of their study its retrospective, single-center design, with patients who were mostly white and from one geographic region.
Dr. Singh and colleagues reported no conflicts of interest or outside funding for their study.
SOURCE: Singh A et al. J Clin Gastroenterol. 2020 Mar 11. doi: 10.1097/MCG.0000000000001339.
Noninvasive fibrosis scores, which are widely used to predict advanced fibrosis in people with nonalcoholic fatty liver disease (NAFLD), do not do a good job of picking up advanced fibrosis in patients with underlying diabetes, according to a new study.
Advanced fibrosis is associated with an increased risk of cirrhosis, end-stage liver disease, and liver failure. Underlying diabetes is a risk factor for both advanced fibrosis and death in patients with NAFLD.
While liver biopsy remains the gold standard for detecting advanced fibrosis, high costs and risks limit its use. Noninvasive scores such as the AST/ALT ratio; AST to platelet ratio index (APRI); fibrosis-4 (FIB-4) index; and NAFLD fibrosis score (NFS) have gained popularity in recent years, as they offer the compelling advantage of using easily and cheaply attained clinical and laboratory measures to assess likelihood of disease.
But their accuracy has come into question, particularly for people with diabetes.
In research published in the Journal of Clinical Gastroenterology, Amandeep Singh, MD, and colleagues at the Cleveland Clinic looked at their center’s records for 1,157 patients with type 2 diabetes (65% women, 88% white, 85% with obesity) who had undergone a liver biopsy for suspected advanced fibrosis between 2000 and 2015. Biopsy results revealed that a third of the cohort (32%) was positive for advanced fibrosis.
The investigators then pulled patients’ laboratory results for AST, ALT, cholesterol, triglycerides, fasting glucose, hemoglobin A1c, bilirubin, albumin, platelet count, alkaline phosphatase, albumin, and lipid levels, all collected within a year of biopsy. After plugging these into the algorithms of four different scoring systems for advanced fibrosis, they compared results with results from the biopsies.
The scores of AST/ALT greater than 1.4, APRI of at least 1.5, NFS greater than 0.676, and FIB-4 index greater than 2.67 had high specificities of 84%, 97%, 70%, and 93%, respectively, but sensitivities of only 27%, 17%, 64%, and 44%. Even when the cutoff measures were tightened, the scoring systems still missed a lot of disease. This suggests, Dr. Singh and colleagues wrote, that “the presence of diabetes could decrease the predictive value of these scores to detect advanced disease in NAFLD patients.” Reliable noninvasive biomarkers are “urgently needed” for this patient population.
In an interview, Dr. Singh advised that clinicians continue to use current noninvasive scores in patients with diabetes – preferably the NFS – “until we have a better scoring system.” If clinicians suspect advanced fibrosis based on lab tests and clinical data, then “liver biopsy should be considered,” he said.
The investigators described among the limitations of their study its retrospective, single-center design, with patients who were mostly white and from one geographic region.
Dr. Singh and colleagues reported no conflicts of interest or outside funding for their study.
SOURCE: Singh A et al. J Clin Gastroenterol. 2020 Mar 11. doi: 10.1097/MCG.0000000000001339.
Noninvasive fibrosis scores, which are widely used to predict advanced fibrosis in people with nonalcoholic fatty liver disease (NAFLD), do not do a good job of picking up advanced fibrosis in patients with underlying diabetes, according to a new study.
Advanced fibrosis is associated with an increased risk of cirrhosis, end-stage liver disease, and liver failure. Underlying diabetes is a risk factor for both advanced fibrosis and death in patients with NAFLD.
While liver biopsy remains the gold standard for detecting advanced fibrosis, high costs and risks limit its use. Noninvasive scores such as the AST/ALT ratio; AST to platelet ratio index (APRI); fibrosis-4 (FIB-4) index; and NAFLD fibrosis score (NFS) have gained popularity in recent years, as they offer the compelling advantage of using easily and cheaply attained clinical and laboratory measures to assess likelihood of disease.
But their accuracy has come into question, particularly for people with diabetes.
In research published in the Journal of Clinical Gastroenterology, Amandeep Singh, MD, and colleagues at the Cleveland Clinic looked at their center’s records for 1,157 patients with type 2 diabetes (65% women, 88% white, 85% with obesity) who had undergone a liver biopsy for suspected advanced fibrosis between 2000 and 2015. Biopsy results revealed that a third of the cohort (32%) was positive for advanced fibrosis.
The investigators then pulled patients’ laboratory results for AST, ALT, cholesterol, triglycerides, fasting glucose, hemoglobin A1c, bilirubin, albumin, platelet count, alkaline phosphatase, albumin, and lipid levels, all collected within a year of biopsy. After plugging these into the algorithms of four different scoring systems for advanced fibrosis, they compared results with results from the biopsies.
The scores of AST/ALT greater than 1.4, APRI of at least 1.5, NFS greater than 0.676, and FIB-4 index greater than 2.67 had high specificities of 84%, 97%, 70%, and 93%, respectively, but sensitivities of only 27%, 17%, 64%, and 44%. Even when the cutoff measures were tightened, the scoring systems still missed a lot of disease. This suggests, Dr. Singh and colleagues wrote, that “the presence of diabetes could decrease the predictive value of these scores to detect advanced disease in NAFLD patients.” Reliable noninvasive biomarkers are “urgently needed” for this patient population.
In an interview, Dr. Singh advised that clinicians continue to use current noninvasive scores in patients with diabetes – preferably the NFS – “until we have a better scoring system.” If clinicians suspect advanced fibrosis based on lab tests and clinical data, then “liver biopsy should be considered,” he said.
The investigators described among the limitations of their study its retrospective, single-center design, with patients who were mostly white and from one geographic region.
Dr. Singh and colleagues reported no conflicts of interest or outside funding for their study.
SOURCE: Singh A et al. J Clin Gastroenterol. 2020 Mar 11. doi: 10.1097/MCG.0000000000001339.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Patients with preexisting diabetes benefit less from bariatric surgery
according to a retrospective review of patients receiving both sleeve gastrectomy and gastric bypass.
The difference was particularly pronounced and persistent for patients who had gastric bypass, Yingying Luo, MD, said during a virtual news conference held by the Endocrine Society. The study was slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
“Our findings demonstrated that having bariatric surgery before developing diabetes may result in greater weight loss from the surgery, especially within the first 3 years after surgery and in patients undergoing gastric bypass surgery,” said Dr. Luo.
More than a third of U.S. adults have obesity, and more than half the population is overweight or has obesity, said Dr. Luo, citing data from the Centers for Disease Control and Prevention.
Bariatric surgery not only reduces body weight, but also “can lead to remission of many metabolic disorders, including diabetes, hypertension, and dyslipidemia,” said Dr. Luo, a visiting scholar at the University of Michigan’s division of metabolism, endocrinology, and diabetes. However, until now, it has not been known how diabetes interacts with bariatric surgery to affect weight loss outcomes.
To address that question, Dr. Luo and her colleagues looked at patients in the Michigan Bariatric Surgery Cohort who were at least 18 years old and had a body mass index (BMI) of more than 40 kg/m2, or of more than 35 kg/m2 with comorbidities.
The researchers followed 380 patients who received gastric bypass and 334 who received sleeve gastrectomy for at least 5 years. Over time, sleeve gastrectomy became the predominant type of surgery conducted, noted Dr. Luo.
At baseline, and yearly for 5 years thereafter, the researchers recorded participants’ BMI as well as their lipid levels and other laboratory values. Medication use was also tracked. Patients with a diagnosis of diabetes also had their hemoglobin A1c levels recorded at each visit.
Overall, patients in the sleeve gastrectomy group were more overweight, and those in the gastric bypass group had higher HbA1c and total cholesterol levels. The mean baseline weight for the sleeve gastrectomy recipients was 141.5 kg, compared with 133.5 kg for those receiving gastric bypass (BMI, 49.9 vs. 47.3 kg/m2, respectively; P < .01 for both measures). Mean HbA1c was 6.5% for the gastric bypass group, compared with 6.3% for the sleeve gastrectomy group (P = .03).
At baseline, 149 (39.2%) of the gastric bypass patients had diabetes, compared with 108 (32.3%) of the sleeve gastrectomy patients, but the difference was not statistically significant.
About two-thirds of the full cohort were tracked for at least 5 years, which is still considered “a good follow-up rate in a real-world study,” said Dr. Luo.
Total weight loss was defined as the difference between initial weight and postoperative weight at a given point in time. Excess weight was the difference between initial weight and an individual’s ideal weight, that is, what their weight would have been if they had a BMI of 25 kg/m2.
“The probability of achieving a BMI of less than 30 kg/m2 or excess weight loss of 50% or more was higher in patients who did not have diabetes diagnosis at baseline. We found that the presence of diabetes at baseline substantially impacted the probability of achieving both indicators,” said Dr. Luo. “Individuals without diabetes had a 1.5 times higher chance of achieving a BMI of under 30 kg/m2, and … [they also] had a 1.6 times higher chance of achieving excess body weight loss of 50%, or more.” Both of those differences were statistically significant on univariate analysis (P = .0249 and .0021, respectively).
The researchers conducted further statistical analysis – adjusted for age, gender, surgery type, and baseline weight – to examine whether diabetes still predicted future weight loss after bariatric surgery. After those adjustments, they still found that “the presence of diabetes before surgery is an indicator of future weight loss outcomes,” said Dr. Luo.
The differences in outcomes for those with and without diabetes tended to diminish over time in looking at the cohort as a whole. However, greater BMI reduction for those without diabetes persisted for the full 5 years of follow-up for the gastric bypass recipients. Those trends held when the researchers looked at the proportion of patients whose BMI dropped to below 30 kg/m2, and those who achieved excess weight loss of more than 50%.
Dr. Luo acknowledged that an ideal study would track patients for longer than 5 years and that studies involving more patients would also be useful. Still, she said, “our study opens the door for further research to understand why diabetes diminishes the weight loss effect of bariatric surgery.”
The research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
Dr. Luo reported no outside sources of funding and no conflicts of interest.
SOURCE: Luo Y et al. ENDO 2020, Abstract 590.
according to a retrospective review of patients receiving both sleeve gastrectomy and gastric bypass.
The difference was particularly pronounced and persistent for patients who had gastric bypass, Yingying Luo, MD, said during a virtual news conference held by the Endocrine Society. The study was slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
“Our findings demonstrated that having bariatric surgery before developing diabetes may result in greater weight loss from the surgery, especially within the first 3 years after surgery and in patients undergoing gastric bypass surgery,” said Dr. Luo.
More than a third of U.S. adults have obesity, and more than half the population is overweight or has obesity, said Dr. Luo, citing data from the Centers for Disease Control and Prevention.
Bariatric surgery not only reduces body weight, but also “can lead to remission of many metabolic disorders, including diabetes, hypertension, and dyslipidemia,” said Dr. Luo, a visiting scholar at the University of Michigan’s division of metabolism, endocrinology, and diabetes. However, until now, it has not been known how diabetes interacts with bariatric surgery to affect weight loss outcomes.
To address that question, Dr. Luo and her colleagues looked at patients in the Michigan Bariatric Surgery Cohort who were at least 18 years old and had a body mass index (BMI) of more than 40 kg/m2, or of more than 35 kg/m2 with comorbidities.
The researchers followed 380 patients who received gastric bypass and 334 who received sleeve gastrectomy for at least 5 years. Over time, sleeve gastrectomy became the predominant type of surgery conducted, noted Dr. Luo.
At baseline, and yearly for 5 years thereafter, the researchers recorded participants’ BMI as well as their lipid levels and other laboratory values. Medication use was also tracked. Patients with a diagnosis of diabetes also had their hemoglobin A1c levels recorded at each visit.
Overall, patients in the sleeve gastrectomy group were more overweight, and those in the gastric bypass group had higher HbA1c and total cholesterol levels. The mean baseline weight for the sleeve gastrectomy recipients was 141.5 kg, compared with 133.5 kg for those receiving gastric bypass (BMI, 49.9 vs. 47.3 kg/m2, respectively; P < .01 for both measures). Mean HbA1c was 6.5% for the gastric bypass group, compared with 6.3% for the sleeve gastrectomy group (P = .03).
At baseline, 149 (39.2%) of the gastric bypass patients had diabetes, compared with 108 (32.3%) of the sleeve gastrectomy patients, but the difference was not statistically significant.
About two-thirds of the full cohort were tracked for at least 5 years, which is still considered “a good follow-up rate in a real-world study,” said Dr. Luo.
Total weight loss was defined as the difference between initial weight and postoperative weight at a given point in time. Excess weight was the difference between initial weight and an individual’s ideal weight, that is, what their weight would have been if they had a BMI of 25 kg/m2.
“The probability of achieving a BMI of less than 30 kg/m2 or excess weight loss of 50% or more was higher in patients who did not have diabetes diagnosis at baseline. We found that the presence of diabetes at baseline substantially impacted the probability of achieving both indicators,” said Dr. Luo. “Individuals without diabetes had a 1.5 times higher chance of achieving a BMI of under 30 kg/m2, and … [they also] had a 1.6 times higher chance of achieving excess body weight loss of 50%, or more.” Both of those differences were statistically significant on univariate analysis (P = .0249 and .0021, respectively).
The researchers conducted further statistical analysis – adjusted for age, gender, surgery type, and baseline weight – to examine whether diabetes still predicted future weight loss after bariatric surgery. After those adjustments, they still found that “the presence of diabetes before surgery is an indicator of future weight loss outcomes,” said Dr. Luo.
The differences in outcomes for those with and without diabetes tended to diminish over time in looking at the cohort as a whole. However, greater BMI reduction for those without diabetes persisted for the full 5 years of follow-up for the gastric bypass recipients. Those trends held when the researchers looked at the proportion of patients whose BMI dropped to below 30 kg/m2, and those who achieved excess weight loss of more than 50%.
Dr. Luo acknowledged that an ideal study would track patients for longer than 5 years and that studies involving more patients would also be useful. Still, she said, “our study opens the door for further research to understand why diabetes diminishes the weight loss effect of bariatric surgery.”
The research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
Dr. Luo reported no outside sources of funding and no conflicts of interest.
SOURCE: Luo Y et al. ENDO 2020, Abstract 590.
according to a retrospective review of patients receiving both sleeve gastrectomy and gastric bypass.
The difference was particularly pronounced and persistent for patients who had gastric bypass, Yingying Luo, MD, said during a virtual news conference held by the Endocrine Society. The study was slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
“Our findings demonstrated that having bariatric surgery before developing diabetes may result in greater weight loss from the surgery, especially within the first 3 years after surgery and in patients undergoing gastric bypass surgery,” said Dr. Luo.
More than a third of U.S. adults have obesity, and more than half the population is overweight or has obesity, said Dr. Luo, citing data from the Centers for Disease Control and Prevention.
Bariatric surgery not only reduces body weight, but also “can lead to remission of many metabolic disorders, including diabetes, hypertension, and dyslipidemia,” said Dr. Luo, a visiting scholar at the University of Michigan’s division of metabolism, endocrinology, and diabetes. However, until now, it has not been known how diabetes interacts with bariatric surgery to affect weight loss outcomes.
To address that question, Dr. Luo and her colleagues looked at patients in the Michigan Bariatric Surgery Cohort who were at least 18 years old and had a body mass index (BMI) of more than 40 kg/m2, or of more than 35 kg/m2 with comorbidities.
The researchers followed 380 patients who received gastric bypass and 334 who received sleeve gastrectomy for at least 5 years. Over time, sleeve gastrectomy became the predominant type of surgery conducted, noted Dr. Luo.
At baseline, and yearly for 5 years thereafter, the researchers recorded participants’ BMI as well as their lipid levels and other laboratory values. Medication use was also tracked. Patients with a diagnosis of diabetes also had their hemoglobin A1c levels recorded at each visit.
Overall, patients in the sleeve gastrectomy group were more overweight, and those in the gastric bypass group had higher HbA1c and total cholesterol levels. The mean baseline weight for the sleeve gastrectomy recipients was 141.5 kg, compared with 133.5 kg for those receiving gastric bypass (BMI, 49.9 vs. 47.3 kg/m2, respectively; P < .01 for both measures). Mean HbA1c was 6.5% for the gastric bypass group, compared with 6.3% for the sleeve gastrectomy group (P = .03).
At baseline, 149 (39.2%) of the gastric bypass patients had diabetes, compared with 108 (32.3%) of the sleeve gastrectomy patients, but the difference was not statistically significant.
About two-thirds of the full cohort were tracked for at least 5 years, which is still considered “a good follow-up rate in a real-world study,” said Dr. Luo.
Total weight loss was defined as the difference between initial weight and postoperative weight at a given point in time. Excess weight was the difference between initial weight and an individual’s ideal weight, that is, what their weight would have been if they had a BMI of 25 kg/m2.
“The probability of achieving a BMI of less than 30 kg/m2 or excess weight loss of 50% or more was higher in patients who did not have diabetes diagnosis at baseline. We found that the presence of diabetes at baseline substantially impacted the probability of achieving both indicators,” said Dr. Luo. “Individuals without diabetes had a 1.5 times higher chance of achieving a BMI of under 30 kg/m2, and … [they also] had a 1.6 times higher chance of achieving excess body weight loss of 50%, or more.” Both of those differences were statistically significant on univariate analysis (P = .0249 and .0021, respectively).
The researchers conducted further statistical analysis – adjusted for age, gender, surgery type, and baseline weight – to examine whether diabetes still predicted future weight loss after bariatric surgery. After those adjustments, they still found that “the presence of diabetes before surgery is an indicator of future weight loss outcomes,” said Dr. Luo.
The differences in outcomes for those with and without diabetes tended to diminish over time in looking at the cohort as a whole. However, greater BMI reduction for those without diabetes persisted for the full 5 years of follow-up for the gastric bypass recipients. Those trends held when the researchers looked at the proportion of patients whose BMI dropped to below 30 kg/m2, and those who achieved excess weight loss of more than 50%.
Dr. Luo acknowledged that an ideal study would track patients for longer than 5 years and that studies involving more patients would also be useful. Still, she said, “our study opens the door for further research to understand why diabetes diminishes the weight loss effect of bariatric surgery.”
The research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
Dr. Luo reported no outside sources of funding and no conflicts of interest.
SOURCE: Luo Y et al. ENDO 2020, Abstract 590.
FROM ENDO 2020
Dapagliflozin trial in CKD halted because of high efficacy
AstraZeneca has announced that the phase 3 DAPA-CKD trial for dapagliflozin (Farxiga) in patients with chronic kidney disease has been halted early because of overwhelming efficacy of the drug, at the recommendation of an independent data monitoring committee.
DAPA-CKD is an international, multicenter, randomized, double-blinded trial in 4,245 patients with stage 2-4 chronic kidney disease. Patients received either 10 mg of the dapagliflozin once-daily or a placebo. The primary composite endpoint is worsening of renal function, defined as a composite of an estimated glomerular filtration rate decline of at least 50%, onset of end-stage kidney disease, and death from cardiovascular or renal cause.
The decision to stop the trial came after a routine assessment of efficacy and safety that showed dapagliflozin’s benefits significantly earlier than expected. AstraZeneca will initiate closure of the study, and results will be published and submitted for presentation at a forthcoming medical meeting.
Dapagliflozin is a sodium-glucose transporter 2 inhibitor currently indicated for the treatment type 2 diabetes patients with inadequately controlled type 2 diabetes and for reduction of the risk of hospitalization for heart failure. In August 2019, the drug was granted Fast Track status by the Food and Drug Administration for the treatment of chronic kidney disease. In January 2020, the agency also granted Fast Track status for the reduction of risk of cardiovascular death or worsening of heart failure in adult patients, regardless of diabetes status, with heart failure with reduced ejection fraction.
“Chronic kidney disease patients have limited treatment options, particularly those without type-2 diabetes. We are very pleased the data monitoring committee concluded that patients experienced overwhelming benefit. Farxiga has the potential to change the management of chronic kidney disease for patients around the world,” Mene Pangalos, executive vice president of BioPharmaceuticals R&D, said in the press release.
AstraZeneca has announced that the phase 3 DAPA-CKD trial for dapagliflozin (Farxiga) in patients with chronic kidney disease has been halted early because of overwhelming efficacy of the drug, at the recommendation of an independent data monitoring committee.
DAPA-CKD is an international, multicenter, randomized, double-blinded trial in 4,245 patients with stage 2-4 chronic kidney disease. Patients received either 10 mg of the dapagliflozin once-daily or a placebo. The primary composite endpoint is worsening of renal function, defined as a composite of an estimated glomerular filtration rate decline of at least 50%, onset of end-stage kidney disease, and death from cardiovascular or renal cause.
The decision to stop the trial came after a routine assessment of efficacy and safety that showed dapagliflozin’s benefits significantly earlier than expected. AstraZeneca will initiate closure of the study, and results will be published and submitted for presentation at a forthcoming medical meeting.
Dapagliflozin is a sodium-glucose transporter 2 inhibitor currently indicated for the treatment type 2 diabetes patients with inadequately controlled type 2 diabetes and for reduction of the risk of hospitalization for heart failure. In August 2019, the drug was granted Fast Track status by the Food and Drug Administration for the treatment of chronic kidney disease. In January 2020, the agency also granted Fast Track status for the reduction of risk of cardiovascular death or worsening of heart failure in adult patients, regardless of diabetes status, with heart failure with reduced ejection fraction.
“Chronic kidney disease patients have limited treatment options, particularly those without type-2 diabetes. We are very pleased the data monitoring committee concluded that patients experienced overwhelming benefit. Farxiga has the potential to change the management of chronic kidney disease for patients around the world,” Mene Pangalos, executive vice president of BioPharmaceuticals R&D, said in the press release.
AstraZeneca has announced that the phase 3 DAPA-CKD trial for dapagliflozin (Farxiga) in patients with chronic kidney disease has been halted early because of overwhelming efficacy of the drug, at the recommendation of an independent data monitoring committee.
DAPA-CKD is an international, multicenter, randomized, double-blinded trial in 4,245 patients with stage 2-4 chronic kidney disease. Patients received either 10 mg of the dapagliflozin once-daily or a placebo. The primary composite endpoint is worsening of renal function, defined as a composite of an estimated glomerular filtration rate decline of at least 50%, onset of end-stage kidney disease, and death from cardiovascular or renal cause.
The decision to stop the trial came after a routine assessment of efficacy and safety that showed dapagliflozin’s benefits significantly earlier than expected. AstraZeneca will initiate closure of the study, and results will be published and submitted for presentation at a forthcoming medical meeting.
Dapagliflozin is a sodium-glucose transporter 2 inhibitor currently indicated for the treatment type 2 diabetes patients with inadequately controlled type 2 diabetes and for reduction of the risk of hospitalization for heart failure. In August 2019, the drug was granted Fast Track status by the Food and Drug Administration for the treatment of chronic kidney disease. In January 2020, the agency also granted Fast Track status for the reduction of risk of cardiovascular death or worsening of heart failure in adult patients, regardless of diabetes status, with heart failure with reduced ejection fraction.
“Chronic kidney disease patients have limited treatment options, particularly those without type-2 diabetes. We are very pleased the data monitoring committee concluded that patients experienced overwhelming benefit. Farxiga has the potential to change the management of chronic kidney disease for patients around the world,” Mene Pangalos, executive vice president of BioPharmaceuticals R&D, said in the press release.
Factors Associated With Lower-Extremity Amputation in Patients With Diabetic Foot Ulcers
From Northwell Health System, Lake Success, NY.
Abstract
- Objective: To explore factors associated with lower-extremity amputation (LEA) in patients with diabetic foot ulcers using data from the Online Wound Electronic Medical Record Database.
- Design: Retrospective analysis of medical records.
- Setting and participants: Data from 169 individuals with previously diagnosed diabetes mellitus who received wound care for a 6-month period within a span of 2 years was analyzed. A baseline evaluation was obtained and wound(s) were treated, managed, and monitored.
Treatment continued until the patient healed, required an LEA, or phased out of the study, neither healing nor undergoing an amputation. Of the 149 patients who completed the study, 38 had healed ulcers, 14 underwent amputation, and 97 neither healed nor underwent an amputation. All patients were treated under the care of vascular and/or podiatric surgeons. - Measurements: Variables included wound status (healed, amputated, and unhealed/non-amputated); size of wound area; age, gender, race, and ethnicity; white blood cell (WBC) count, hemoglobin A1c (HbA1c), blood glucose, and body mass index (BMI); and presence of osteomyelitis, gangrene, and peripheral vascular disease.
- Results: As compared to the healed and unhealed/non-amputated group, the group of patients who underwent LEA was older and had higher percentages of males, Hispanics, and African Americans; had a higher WBC count, larger wound area, and higher rates of wound infection, osteomyelitis, and neuropathy; and had lower average values of HbA1c, blood glucose, and BMI and a lower rate of peripheral vascular disease.
- Conclusion: The association between HbA1c and LEA highlights a window of relative safety among an at-risk population. By identifying and focusing on factors associated with LEA, health care professionals may be able to decrease the prevalence of LEA in patients with diabetes.
Keywords: diabetic foot ulcer; lower-extremity amputation; risk factors; HbA1c.
An estimated 30.3 million people, or 9.4% of the US population, has diabetes. In 2014, approximately 108,000 amputations were performed on adults with diagnosed diabetes.1 Furthermore, patients with diabetes have a 10-fold increased risk for lower-extremity amputation (LEA), as compared with patients without diabetes.2 The frequency of amputations in the diabetic population is a public health crisis.
Amputation has significant, life-altering consequences. Patients who undergo LEA often face debilitation in their daily activities and must undergo intense rehabilitation to learn basic tasks. Amputations can also impact individuals’ psychological well-being as they come to terms with their altered body and may face challenges in self-perception, confidence, self-esteem, work life, and relationships. In addition, the mortality rate for patients with diabetes 5 years after undergoing LEA is 30%.2 However, public health studies estimate that more than half of LEAs in patients with diabetes are preventable.3
Although studies have explored the relationship between diabetes and LEA, few have sought to identify factors directly correlated with wound care. In the United States, patients with diabetic ulcerations are typically treated in wound care facilities; however, previous studies have concentrated on the conditions that lead to the formation of an ulcer or amputation, viewing amputation and ulcer as 2 separate entities. Our study took into account systemic variables, patient demographics, and specific wound characteristics to explore factors associated with LEA in a high-risk group of patients with diabetes. This study was designed to assess ailments that are prevalent in patients who require a LEA.
Methods
Patients and Setting
A total of 169 patients who were treated at the Comprehensive Wound Healing and Hyperbaric Center (Lake Success, NY), a tertiary facility of the Northwell Health system, participated in this retrospective study. The data for this study were obtained in conjunction with the development of the New York University School of Medicine’s Online Wound Electronic Medical Record to Decrease Limb Amputations in Persons with Diabetes (OWEMR) database. The OWEMR collects individual patient data from satellite locations across the country. Using this database, researchers can analyze similarities and differences between patients who undergo LEA.
This study utilized patient data specific to the Northwell Health facility. All of the patients in our study were enrolled under the criteria of the OWEMR database. In order to be included in the OWEMR database, patients had to be diagnosed with type 1 or type 2 diabetes; have a break in the skin ≥ 0.5 cm2; be 18 years of age or older; and have a measured hemoglobin A1c (HbA1c) value within the past 120 days. Study patients signed an informed consent and committed to being available for follow-up visits to the wound care facility for 6 months after entering the study. Patients were enrolled between 2012 and 2014, and each patient was monitored for a period of 6 months within this time period. Participants were treated with current standards of care using diet, lifestyle, and pharmacologic interventions. This study was approved by the Northwell Health System Institutional Review Board Human Research Protection Program (Manhasset, NY).
Data Collection
On their first visit to the facility, patients were given a physical examination and initial interview regarding their medical history. Clinicians were required to select 1 ulcer that would be examined for the duration of the study. The selection of the ulcer was based on a point system that awarded points for pedal pulses, the ability to be probed to the bone, the location of the ulcer (ie, located on the foot rather than a toe), and the presence of multiple ulcerations. The ulcer with the highest score was selected for the study. If numerous ulcers were evaluated with the same score, the largest and deepest was selected. Wagner classification of the wound was recorded at baseline and taken at each subsequent patient visit. In addition, peripheral sensation was assessed for signs of neuropathy using Semmes-Weinstein monofilament testing.
Once selected, the wound was clinically evaluated, samples for culture were obtained, and blood tests were performed to detect the presence of wound infection. The patient’s blood was drawn for a full laboratory analysis, including white blood cell (WBC) count and measurement of blood glucose and HbA1c levels. Bone biopsy, magnetic resonance imaging, and bone scans were used to detect the presence of osteomyelitis at the discretion of the health care provider. Wounds suspected of infection, underlying osteomyelitis, or gangrene at baseline were excluded. Patients would then return for follow-up visits at least once every 6 weeks, plus or minus 2 weeks, for a maximum of 6 months.
Statistical Analysis
Utilizing SAS version 9.3 (Cary, NC), descriptive statistics (minimum, maximum, mean, median, and SD) were calculated for the following variables: age, WBC count, wound area, HbA1c, blood glucose, and body mass index (BMI). These variables were collected for each patient as per the OWEMR protocol and provided a basis for which to compare patients who underwent amputation and those who did not. Twenty patients were lost to follow-up, and therefore we altered the window of our statistics from 6 months to 3 months to provide the most accurate data, as 6-month follow-up data were limited. The patients were classified into the following categories: healed, amputated, and unhealed/non-amputated. Descriptive statistics were calculated for these 3 groups, analyzing the same variables (age, WBC count, wound area, HbA1c, blood glucose, and BMI). Additional statistical computations were utilized in order to show the prevalence and frequency of our categorical variables: gender, race, ethnicity, osteomyelitis, gangrene, and peripheral vascular disease. The baseline values of WBC count, HbA1c, wound area, and BMI of the 3 groups were analyzed with descriptive statistics for comparison. A multinomial logistic regression was then performed using a 3-level outcome variable: healed, amputated, or unhealed/non-amputated. Each predictor variable was analyzed independently due to the small sample size.
Results
Of the 169 registered patients treated at the Northwell Health facility, all qualified for the OWEMR study and met the study criteria. In the original 169 patients, there were 19 amputations: 6 toe, 6 trans-metatarsal, 6 below knee, and 1 above knee (Table 1). 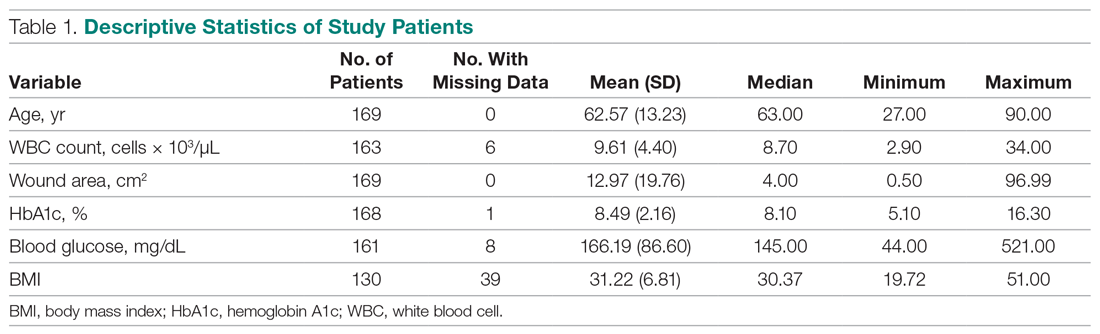
The descriptive statistics of 149 patients grouped into 3 categories (healed, amputated, unhealed/non-amputated) are shown in Table 2.
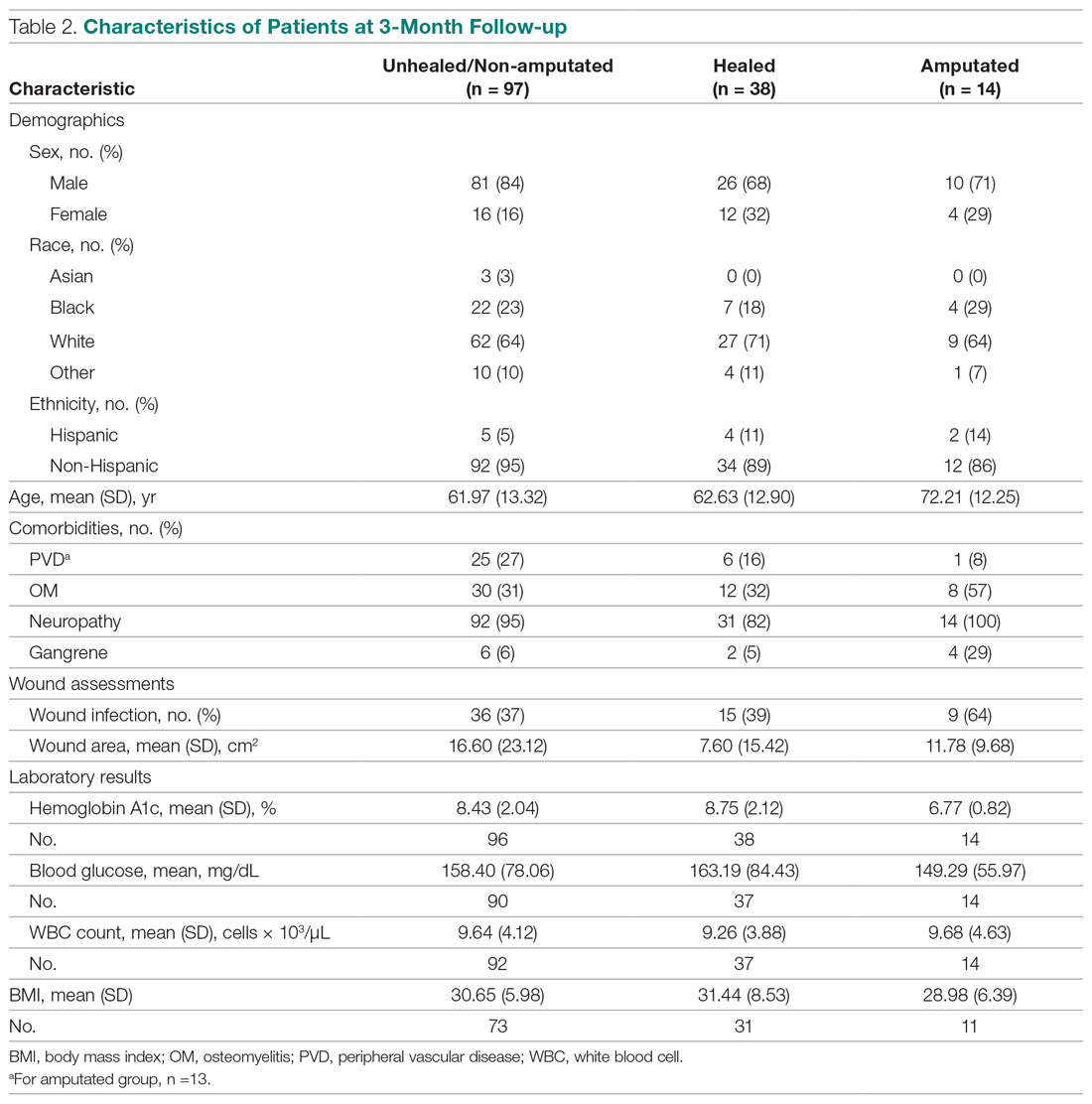
The results of the logistic regression exploring the differences between the amputation and healed groups and the unhealed/non-amputated group are shown in Table 3. The amputation group had a higher mean age and WBC count and greater wound area. Increased age was determined to be a significant predictor of the odds of amputation (P = 0.0089). For each year increase in age, the odds of amputation increased by 6.5% (odds ratio, 1.07 [95% confidence interval {CI}, 1.02-1.12]). Patients in the amputation group were more likely to be male, Hispanic, and African American and to have wound infections and comorbidities (osteomyelitis, neuropathy, and gangrene).
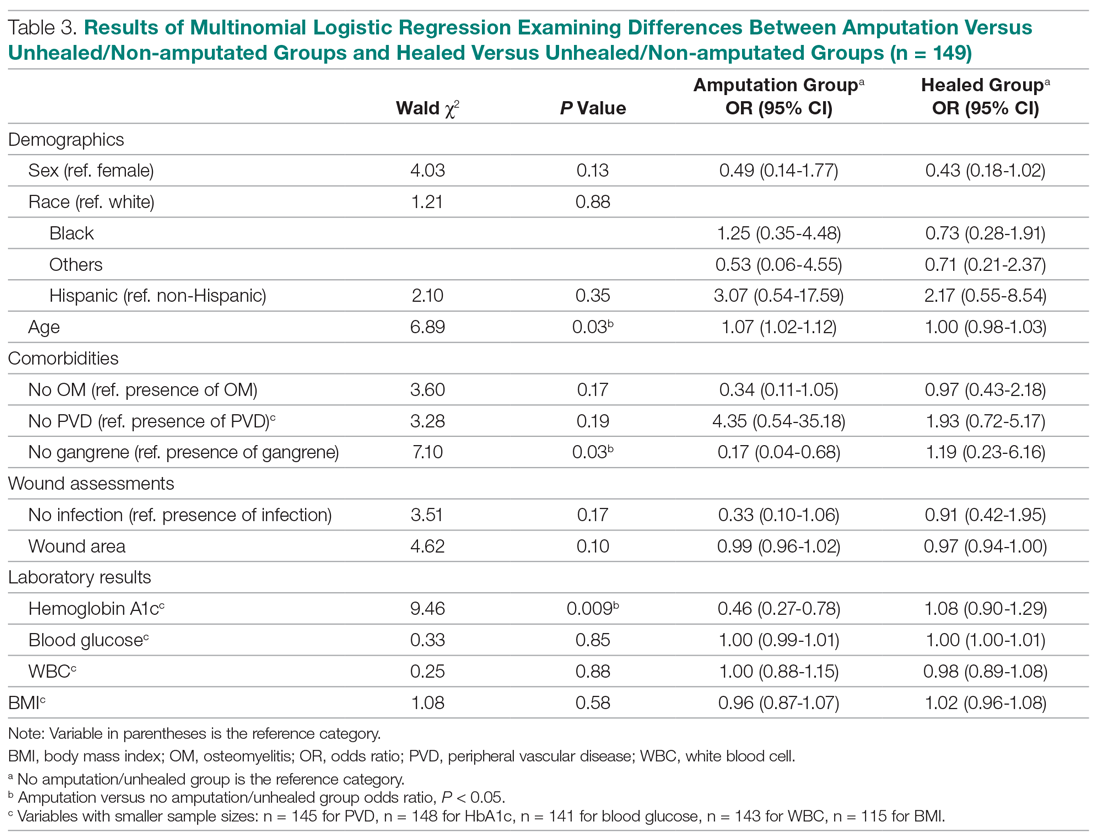
The presence of gangrene was significantly associated with LEA (P = 0.03). Specifically, the odds of patients without gangrene undergoing a LEA were substantially lower compared with their counterparts with gangrene (odds ratio, 0.17; 95% CI, 0.04-0.68; P = 0.0131). However, the presence of gangrene was not associated with the odds of healing compared with the odds of neither healing nor undergoing amputation (P = 0.84; not shown in Table 3).
The amputation group had lower mean values for HbA1c, BMI, and blood glucose levels and a lower rate of peripheral vascular disease. Only the relationship between lower HbA1c and increased odds of amputation versus not healing/non-amputation was found to be statistically significant (95% CI, 0.27-0.78; P = 0.009).
Discussion
This retrospective study was undertaken to evaluate factors associated with LEA in patients with diabetic foot ulcers. Patients with diabetes being treated at a wound care facility often require continuous surgical and metabolic intervention to promote optimal healing: drainage, surgical debridement, irrigation, culturing for infection, and monitoring of blood glucose levels. This treatment requires strict compliance with medical directions and, oftentimes, additional care, such as home-care nursing visits, to maintain a curative environment for the wound. Frequently, wounds on the lower extremity further complicate the healing process by reducing the patient’s mobility and daily life. Due to these factors, many patients progress to LEA. The link between diabetic ulcers and amputation has already been well described in previous studies, with studies showing that history of diabetic foot ulcer significantly predisposes an individual to LEA.4 However, few studies have further investigated demographic factors associated with risk for an amputation. Our study analyzed several categories of patient data taken from a baseline visit. We found that those with highly elevated HbA1c values were less likely to have an amputation than persons with relatively lower levels, a finding that is contrary to previous studies.
Our study’s findings suggest a higher risk for LEA with increased age. The amputation group was, on average, 7 years older than the other 2 groups. A recent study showed that risk for amputation is directly correlated to patient age, as is the mortality rate after undergoing LEA (2.3%; P < 0.05).5 Our study found that with each increase in age of 1 year, the odds of amputation increased by 6.5%. However, recent evidence on LEA risk and aging suggests that age is of less consequence than the duration of diabetes. One study found that the propensity to develop diabetic foot ulcers increases with the duration of diabetes.6 The same study found that prevalence of ulceration was correlated with age, but the relationship between age and LEA was less significant. A follow-up study for LEA could be done to examine the role of disease duration versus age in LEA.
A consensus among previous studies is that men have a higher risk for LEA.5,7 Men comprised the majority in all 3 groups in our study. In addition, the amputation group in our study had the lowest BMI. Higher BMI generally is associated with an increased risk for health complications. However, a past study conducted in Taiwan reported that obese patients with diabetes were less likely to undergo LEA than those within the normal range for BMI.8 Neither study suggests that obesity is a deterrent for LEA, but both studies may suggest that risk of amputation may approach a maximum frequency at a specific BMI range, and then decrease. This unconfirmed “cyclic” relationship should be evaluated further in a larger sample size.
Most patients in our analysis were Caucasian, followed by African American and South Asian. African Americans were the only racial group with an increased frequency in the amputation group. This finding is supported by a previous study that found that the rate of LEA among patients with diabetes in low-income, predominantly African-American neighborhoods was nearly double that in wealthier, predominantly Caucasian areas.9 A potential problem in the comparison between our data with previous studies is that the studies did not analyze patients with our inclusion criteria. All patients with diabetes in previous investigations were grouped by race, but were not necessarily required to have 1 or more ulcers. Multiple ulcers may predispose an individual to a greater risk for amputation.
Multinomial logistic regression did not suggest an association between initial size of a patient’s wound and the risk of amputation. However, the descriptive data suggests a trend. Patients who did not heal or require an amputation had the largest average wound area. This finding is not surprising in that our study followed individuals for only 3 months. Many wounds require a long course of treatment, especially in patients with diabetes, who may have poor vascularization. However, in comparison to the healed patients, the patients who required an amputation had a larger average wound area. A larger wound requires a plentiful vascular supply for the delivery of clotting factors and nutrients to the damaged area. As wound size increases, an individual’s body must transmit an increased quantity of these factors and nutrients for the regeneration of tissue. In addition, wounds that possess a larger surface area require more debridement and present a greater opportunity for infection. This may also foreshadow a longer, more costly course of treatment. Additionally, individuals coping with large ulcerations are burdened by more elaborate and complex wound dressings.
Elevated levels of HbA1c are associated with increased adverse effects of diabetes, including end-stage renal disease, neuropathy, and infection.10 In a previous study, the risk for amputation was 1.2 times higher in patients with elevated HbA1c.11 In contrast, our study suggested the odds of LEA versus not healing/not undergoing amputation decreased as HbA1c increased. As a patient’s HbA1c level increased by a value of 1, their odds for LEA decreased by 54.3%. This finding contradicts prior studies that have found a positive association between HbA1c and LEA risk, including a study where each percentage increase in HbA1c correlated with a 13% to 15% increased risk of LEA.12 The finding that patients who underwent amputation in our study had lower levels of HbA1c and blood glucose cannot be fully explained. The maximum HbA1c value in the amputated group was 7.9%. The average values for healed patients and those who underwent LEA were 8.75% and 6.77%, respectively.
Blood glucose levels were also found to be the lowest in the amputated group in our study (mean, 149.29 mg/dL vs 163.19 mg/dL in the healed group). Similar results were found in a Brazilian study, in which patients who did not require amputation had higher HbA1c levels. This study also found an association between blood glucose levels above 200 mg/dL and amputations.3 These findings provide interesting opportunities for repeat studies, preferably with a larger number of participants.
Our study is limited by the small sample size. The sample population had to be reduced, as many patients were lost to follow-up. Although this paring down of the sample size can introduce bias, we are confident that our study is representative of the demographic of patients treated in our facility. The loss of patients to follow-up in turn caused the window of analysis to be narrowed, as long-term outcome data were not available. A multisite study observing various population samples can better explore the relationship between HbA1c and risk of amputation.
Conclusion
This retrospective study exploring factors associated with LEA was unique in that all our participants had 1 or more diabetic foot ulcerations, and thus already had an extremely high risk for amputation, in contrast to previous studies that followed persons at risk for developing diabetic foot ulcerations. In contrast to several previous studies, we found that the risk for amputation actually decreased as baseline measurements of HbA1c increased. The results of this study offer many opportunities for future investigations, preferably with a larger sample size. By further isolating and scrutinizing specific factors associated with LEA, researchers can help clinicians focus on providing wound care that promotes limb salvage.
Corresponding author: Alisha Oropallo, MD, MS, Northwell Health Comprehensive Wound Care Healing Center and Hyperbarics, 1999 Marcus Avenue, Suite M6, Lake Success, NY 11042; [email protected].
Financial disclosures: Funding for this research was provided by a multi-institutional AHRQ governmental grant.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA: US Department of Health and Human Services; 2017.
2. Uccioli L, Giurato L, Meloni M, et al. Comment on Hoffstad et al. Diabetes, lower-extremity amputation, and death. Diabetes Care. 2015;38:1852-1857.
3. Gamba MA, Gotlieb SLD, Bergamaschi DP, Vianna LAC. Lower extremity amputations in diabetic patients: a case-control study. Rev Saúde Pública. 2004;38:399-404.
4. Martins-Mendes D, Monteiro-Soares M, Boyko EJ, et al. The independent contribution of diabetic foot ulcer on lower extremity amputation and mortality risk. J Diabetes Complications. 2014;28:632-638.
5. Lipsky BA, Weigelt JA, Sun X, et al. Developing and validating a risk score for lower-extremity amputation in patients hospitalized for a diabetic foot infection. Diabetes Care. 2011;34:1695-1700.
6. Al-Rubeaan K, Al Derwish M, Ouizi S, et al. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS One. 2015;10:e0124446.
7. Pickwell K, Siersma V, Kars M, et al. Predictors of lower-extremity amputation in patients with an infected diabetic foot ulcer. Diabetes Care. 2015;38:852-857.
8. Lin C, Hsu BR, Tsai J, et al. Effect of limb preservation status and body mass index on the survival of patients with limb-threatening diabetic foot ulcers. J Diabetes Complications. 2017;31:180-185.
9. Stevens CD, Schriger DL, Raffetto B, et al. Geographic clustering of diabetic lower-extremity amputations in low-income regions of California. Health Aff. 2014;33:1383-1390.
10. Liao L, Li C, Liu C, et al. Extreme levels of HbA1c increase incident ESRD risk in Chinese patients with type 2 diabetes: competing risk analysis in national cohort of Taiwan diabetes study. PLoS One. 2015; 10:e0130828.
11. Miyajima S, Shirai A, Yamamoto S, et al. Risk factors for major limb amputations in diabetic foot gangrene patients. Diabetes Res Clin Pract. 2006;71:272-279.
12. Zhao W, Katzmarzyk PT, Horswell R, et al. HbA1c and lower-extremity amputation risk in low-income patients with diabetes. Diabetes Care. 2013;36:3591-3598.
From Northwell Health System, Lake Success, NY.
Abstract
- Objective: To explore factors associated with lower-extremity amputation (LEA) in patients with diabetic foot ulcers using data from the Online Wound Electronic Medical Record Database.
- Design: Retrospective analysis of medical records.
- Setting and participants: Data from 169 individuals with previously diagnosed diabetes mellitus who received wound care for a 6-month period within a span of 2 years was analyzed. A baseline evaluation was obtained and wound(s) were treated, managed, and monitored.
Treatment continued until the patient healed, required an LEA, or phased out of the study, neither healing nor undergoing an amputation. Of the 149 patients who completed the study, 38 had healed ulcers, 14 underwent amputation, and 97 neither healed nor underwent an amputation. All patients were treated under the care of vascular and/or podiatric surgeons. - Measurements: Variables included wound status (healed, amputated, and unhealed/non-amputated); size of wound area; age, gender, race, and ethnicity; white blood cell (WBC) count, hemoglobin A1c (HbA1c), blood glucose, and body mass index (BMI); and presence of osteomyelitis, gangrene, and peripheral vascular disease.
- Results: As compared to the healed and unhealed/non-amputated group, the group of patients who underwent LEA was older and had higher percentages of males, Hispanics, and African Americans; had a higher WBC count, larger wound area, and higher rates of wound infection, osteomyelitis, and neuropathy; and had lower average values of HbA1c, blood glucose, and BMI and a lower rate of peripheral vascular disease.
- Conclusion: The association between HbA1c and LEA highlights a window of relative safety among an at-risk population. By identifying and focusing on factors associated with LEA, health care professionals may be able to decrease the prevalence of LEA in patients with diabetes.
Keywords: diabetic foot ulcer; lower-extremity amputation; risk factors; HbA1c.
An estimated 30.3 million people, or 9.4% of the US population, has diabetes. In 2014, approximately 108,000 amputations were performed on adults with diagnosed diabetes.1 Furthermore, patients with diabetes have a 10-fold increased risk for lower-extremity amputation (LEA), as compared with patients without diabetes.2 The frequency of amputations in the diabetic population is a public health crisis.
Amputation has significant, life-altering consequences. Patients who undergo LEA often face debilitation in their daily activities and must undergo intense rehabilitation to learn basic tasks. Amputations can also impact individuals’ psychological well-being as they come to terms with their altered body and may face challenges in self-perception, confidence, self-esteem, work life, and relationships. In addition, the mortality rate for patients with diabetes 5 years after undergoing LEA is 30%.2 However, public health studies estimate that more than half of LEAs in patients with diabetes are preventable.3
Although studies have explored the relationship between diabetes and LEA, few have sought to identify factors directly correlated with wound care. In the United States, patients with diabetic ulcerations are typically treated in wound care facilities; however, previous studies have concentrated on the conditions that lead to the formation of an ulcer or amputation, viewing amputation and ulcer as 2 separate entities. Our study took into account systemic variables, patient demographics, and specific wound characteristics to explore factors associated with LEA in a high-risk group of patients with diabetes. This study was designed to assess ailments that are prevalent in patients who require a LEA.
Methods
Patients and Setting
A total of 169 patients who were treated at the Comprehensive Wound Healing and Hyperbaric Center (Lake Success, NY), a tertiary facility of the Northwell Health system, participated in this retrospective study. The data for this study were obtained in conjunction with the development of the New York University School of Medicine’s Online Wound Electronic Medical Record to Decrease Limb Amputations in Persons with Diabetes (OWEMR) database. The OWEMR collects individual patient data from satellite locations across the country. Using this database, researchers can analyze similarities and differences between patients who undergo LEA.
This study utilized patient data specific to the Northwell Health facility. All of the patients in our study were enrolled under the criteria of the OWEMR database. In order to be included in the OWEMR database, patients had to be diagnosed with type 1 or type 2 diabetes; have a break in the skin ≥ 0.5 cm2; be 18 years of age or older; and have a measured hemoglobin A1c (HbA1c) value within the past 120 days. Study patients signed an informed consent and committed to being available for follow-up visits to the wound care facility for 6 months after entering the study. Patients were enrolled between 2012 and 2014, and each patient was monitored for a period of 6 months within this time period. Participants were treated with current standards of care using diet, lifestyle, and pharmacologic interventions. This study was approved by the Northwell Health System Institutional Review Board Human Research Protection Program (Manhasset, NY).
Data Collection
On their first visit to the facility, patients were given a physical examination and initial interview regarding their medical history. Clinicians were required to select 1 ulcer that would be examined for the duration of the study. The selection of the ulcer was based on a point system that awarded points for pedal pulses, the ability to be probed to the bone, the location of the ulcer (ie, located on the foot rather than a toe), and the presence of multiple ulcerations. The ulcer with the highest score was selected for the study. If numerous ulcers were evaluated with the same score, the largest and deepest was selected. Wagner classification of the wound was recorded at baseline and taken at each subsequent patient visit. In addition, peripheral sensation was assessed for signs of neuropathy using Semmes-Weinstein monofilament testing.
Once selected, the wound was clinically evaluated, samples for culture were obtained, and blood tests were performed to detect the presence of wound infection. The patient’s blood was drawn for a full laboratory analysis, including white blood cell (WBC) count and measurement of blood glucose and HbA1c levels. Bone biopsy, magnetic resonance imaging, and bone scans were used to detect the presence of osteomyelitis at the discretion of the health care provider. Wounds suspected of infection, underlying osteomyelitis, or gangrene at baseline were excluded. Patients would then return for follow-up visits at least once every 6 weeks, plus or minus 2 weeks, for a maximum of 6 months.
Statistical Analysis
Utilizing SAS version 9.3 (Cary, NC), descriptive statistics (minimum, maximum, mean, median, and SD) were calculated for the following variables: age, WBC count, wound area, HbA1c, blood glucose, and body mass index (BMI). These variables were collected for each patient as per the OWEMR protocol and provided a basis for which to compare patients who underwent amputation and those who did not. Twenty patients were lost to follow-up, and therefore we altered the window of our statistics from 6 months to 3 months to provide the most accurate data, as 6-month follow-up data were limited. The patients were classified into the following categories: healed, amputated, and unhealed/non-amputated. Descriptive statistics were calculated for these 3 groups, analyzing the same variables (age, WBC count, wound area, HbA1c, blood glucose, and BMI). Additional statistical computations were utilized in order to show the prevalence and frequency of our categorical variables: gender, race, ethnicity, osteomyelitis, gangrene, and peripheral vascular disease. The baseline values of WBC count, HbA1c, wound area, and BMI of the 3 groups were analyzed with descriptive statistics for comparison. A multinomial logistic regression was then performed using a 3-level outcome variable: healed, amputated, or unhealed/non-amputated. Each predictor variable was analyzed independently due to the small sample size.
Results
Of the 169 registered patients treated at the Northwell Health facility, all qualified for the OWEMR study and met the study criteria. In the original 169 patients, there were 19 amputations: 6 toe, 6 trans-metatarsal, 6 below knee, and 1 above knee (Table 1). 
The descriptive statistics of 149 patients grouped into 3 categories (healed, amputated, unhealed/non-amputated) are shown in Table 2.

The results of the logistic regression exploring the differences between the amputation and healed groups and the unhealed/non-amputated group are shown in Table 3. The amputation group had a higher mean age and WBC count and greater wound area. Increased age was determined to be a significant predictor of the odds of amputation (P = 0.0089). For each year increase in age, the odds of amputation increased by 6.5% (odds ratio, 1.07 [95% confidence interval {CI}, 1.02-1.12]). Patients in the amputation group were more likely to be male, Hispanic, and African American and to have wound infections and comorbidities (osteomyelitis, neuropathy, and gangrene).

The presence of gangrene was significantly associated with LEA (P = 0.03). Specifically, the odds of patients without gangrene undergoing a LEA were substantially lower compared with their counterparts with gangrene (odds ratio, 0.17; 95% CI, 0.04-0.68; P = 0.0131). However, the presence of gangrene was not associated with the odds of healing compared with the odds of neither healing nor undergoing amputation (P = 0.84; not shown in Table 3).
The amputation group had lower mean values for HbA1c, BMI, and blood glucose levels and a lower rate of peripheral vascular disease. Only the relationship between lower HbA1c and increased odds of amputation versus not healing/non-amputation was found to be statistically significant (95% CI, 0.27-0.78; P = 0.009).
Discussion
This retrospective study was undertaken to evaluate factors associated with LEA in patients with diabetic foot ulcers. Patients with diabetes being treated at a wound care facility often require continuous surgical and metabolic intervention to promote optimal healing: drainage, surgical debridement, irrigation, culturing for infection, and monitoring of blood glucose levels. This treatment requires strict compliance with medical directions and, oftentimes, additional care, such as home-care nursing visits, to maintain a curative environment for the wound. Frequently, wounds on the lower extremity further complicate the healing process by reducing the patient’s mobility and daily life. Due to these factors, many patients progress to LEA. The link between diabetic ulcers and amputation has already been well described in previous studies, with studies showing that history of diabetic foot ulcer significantly predisposes an individual to LEA.4 However, few studies have further investigated demographic factors associated with risk for an amputation. Our study analyzed several categories of patient data taken from a baseline visit. We found that those with highly elevated HbA1c values were less likely to have an amputation than persons with relatively lower levels, a finding that is contrary to previous studies.
Our study’s findings suggest a higher risk for LEA with increased age. The amputation group was, on average, 7 years older than the other 2 groups. A recent study showed that risk for amputation is directly correlated to patient age, as is the mortality rate after undergoing LEA (2.3%; P < 0.05).5 Our study found that with each increase in age of 1 year, the odds of amputation increased by 6.5%. However, recent evidence on LEA risk and aging suggests that age is of less consequence than the duration of diabetes. One study found that the propensity to develop diabetic foot ulcers increases with the duration of diabetes.6 The same study found that prevalence of ulceration was correlated with age, but the relationship between age and LEA was less significant. A follow-up study for LEA could be done to examine the role of disease duration versus age in LEA.
A consensus among previous studies is that men have a higher risk for LEA.5,7 Men comprised the majority in all 3 groups in our study. In addition, the amputation group in our study had the lowest BMI. Higher BMI generally is associated with an increased risk for health complications. However, a past study conducted in Taiwan reported that obese patients with diabetes were less likely to undergo LEA than those within the normal range for BMI.8 Neither study suggests that obesity is a deterrent for LEA, but both studies may suggest that risk of amputation may approach a maximum frequency at a specific BMI range, and then decrease. This unconfirmed “cyclic” relationship should be evaluated further in a larger sample size.
Most patients in our analysis were Caucasian, followed by African American and South Asian. African Americans were the only racial group with an increased frequency in the amputation group. This finding is supported by a previous study that found that the rate of LEA among patients with diabetes in low-income, predominantly African-American neighborhoods was nearly double that in wealthier, predominantly Caucasian areas.9 A potential problem in the comparison between our data with previous studies is that the studies did not analyze patients with our inclusion criteria. All patients with diabetes in previous investigations were grouped by race, but were not necessarily required to have 1 or more ulcers. Multiple ulcers may predispose an individual to a greater risk for amputation.
Multinomial logistic regression did not suggest an association between initial size of a patient’s wound and the risk of amputation. However, the descriptive data suggests a trend. Patients who did not heal or require an amputation had the largest average wound area. This finding is not surprising in that our study followed individuals for only 3 months. Many wounds require a long course of treatment, especially in patients with diabetes, who may have poor vascularization. However, in comparison to the healed patients, the patients who required an amputation had a larger average wound area. A larger wound requires a plentiful vascular supply for the delivery of clotting factors and nutrients to the damaged area. As wound size increases, an individual’s body must transmit an increased quantity of these factors and nutrients for the regeneration of tissue. In addition, wounds that possess a larger surface area require more debridement and present a greater opportunity for infection. This may also foreshadow a longer, more costly course of treatment. Additionally, individuals coping with large ulcerations are burdened by more elaborate and complex wound dressings.
Elevated levels of HbA1c are associated with increased adverse effects of diabetes, including end-stage renal disease, neuropathy, and infection.10 In a previous study, the risk for amputation was 1.2 times higher in patients with elevated HbA1c.11 In contrast, our study suggested the odds of LEA versus not healing/not undergoing amputation decreased as HbA1c increased. As a patient’s HbA1c level increased by a value of 1, their odds for LEA decreased by 54.3%. This finding contradicts prior studies that have found a positive association between HbA1c and LEA risk, including a study where each percentage increase in HbA1c correlated with a 13% to 15% increased risk of LEA.12 The finding that patients who underwent amputation in our study had lower levels of HbA1c and blood glucose cannot be fully explained. The maximum HbA1c value in the amputated group was 7.9%. The average values for healed patients and those who underwent LEA were 8.75% and 6.77%, respectively.
Blood glucose levels were also found to be the lowest in the amputated group in our study (mean, 149.29 mg/dL vs 163.19 mg/dL in the healed group). Similar results were found in a Brazilian study, in which patients who did not require amputation had higher HbA1c levels. This study also found an association between blood glucose levels above 200 mg/dL and amputations.3 These findings provide interesting opportunities for repeat studies, preferably with a larger number of participants.
Our study is limited by the small sample size. The sample population had to be reduced, as many patients were lost to follow-up. Although this paring down of the sample size can introduce bias, we are confident that our study is representative of the demographic of patients treated in our facility. The loss of patients to follow-up in turn caused the window of analysis to be narrowed, as long-term outcome data were not available. A multisite study observing various population samples can better explore the relationship between HbA1c and risk of amputation.
Conclusion
This retrospective study exploring factors associated with LEA was unique in that all our participants had 1 or more diabetic foot ulcerations, and thus already had an extremely high risk for amputation, in contrast to previous studies that followed persons at risk for developing diabetic foot ulcerations. In contrast to several previous studies, we found that the risk for amputation actually decreased as baseline measurements of HbA1c increased. The results of this study offer many opportunities for future investigations, preferably with a larger sample size. By further isolating and scrutinizing specific factors associated with LEA, researchers can help clinicians focus on providing wound care that promotes limb salvage.
Corresponding author: Alisha Oropallo, MD, MS, Northwell Health Comprehensive Wound Care Healing Center and Hyperbarics, 1999 Marcus Avenue, Suite M6, Lake Success, NY 11042; [email protected].
Financial disclosures: Funding for this research was provided by a multi-institutional AHRQ governmental grant.
From Northwell Health System, Lake Success, NY.
Abstract
- Objective: To explore factors associated with lower-extremity amputation (LEA) in patients with diabetic foot ulcers using data from the Online Wound Electronic Medical Record Database.
- Design: Retrospective analysis of medical records.
- Setting and participants: Data from 169 individuals with previously diagnosed diabetes mellitus who received wound care for a 6-month period within a span of 2 years was analyzed. A baseline evaluation was obtained and wound(s) were treated, managed, and monitored.
Treatment continued until the patient healed, required an LEA, or phased out of the study, neither healing nor undergoing an amputation. Of the 149 patients who completed the study, 38 had healed ulcers, 14 underwent amputation, and 97 neither healed nor underwent an amputation. All patients were treated under the care of vascular and/or podiatric surgeons. - Measurements: Variables included wound status (healed, amputated, and unhealed/non-amputated); size of wound area; age, gender, race, and ethnicity; white blood cell (WBC) count, hemoglobin A1c (HbA1c), blood glucose, and body mass index (BMI); and presence of osteomyelitis, gangrene, and peripheral vascular disease.
- Results: As compared to the healed and unhealed/non-amputated group, the group of patients who underwent LEA was older and had higher percentages of males, Hispanics, and African Americans; had a higher WBC count, larger wound area, and higher rates of wound infection, osteomyelitis, and neuropathy; and had lower average values of HbA1c, blood glucose, and BMI and a lower rate of peripheral vascular disease.
- Conclusion: The association between HbA1c and LEA highlights a window of relative safety among an at-risk population. By identifying and focusing on factors associated with LEA, health care professionals may be able to decrease the prevalence of LEA in patients with diabetes.
Keywords: diabetic foot ulcer; lower-extremity amputation; risk factors; HbA1c.
An estimated 30.3 million people, or 9.4% of the US population, has diabetes. In 2014, approximately 108,000 amputations were performed on adults with diagnosed diabetes.1 Furthermore, patients with diabetes have a 10-fold increased risk for lower-extremity amputation (LEA), as compared with patients without diabetes.2 The frequency of amputations in the diabetic population is a public health crisis.
Amputation has significant, life-altering consequences. Patients who undergo LEA often face debilitation in their daily activities and must undergo intense rehabilitation to learn basic tasks. Amputations can also impact individuals’ psychological well-being as they come to terms with their altered body and may face challenges in self-perception, confidence, self-esteem, work life, and relationships. In addition, the mortality rate for patients with diabetes 5 years after undergoing LEA is 30%.2 However, public health studies estimate that more than half of LEAs in patients with diabetes are preventable.3
Although studies have explored the relationship between diabetes and LEA, few have sought to identify factors directly correlated with wound care. In the United States, patients with diabetic ulcerations are typically treated in wound care facilities; however, previous studies have concentrated on the conditions that lead to the formation of an ulcer or amputation, viewing amputation and ulcer as 2 separate entities. Our study took into account systemic variables, patient demographics, and specific wound characteristics to explore factors associated with LEA in a high-risk group of patients with diabetes. This study was designed to assess ailments that are prevalent in patients who require a LEA.
Methods
Patients and Setting
A total of 169 patients who were treated at the Comprehensive Wound Healing and Hyperbaric Center (Lake Success, NY), a tertiary facility of the Northwell Health system, participated in this retrospective study. The data for this study were obtained in conjunction with the development of the New York University School of Medicine’s Online Wound Electronic Medical Record to Decrease Limb Amputations in Persons with Diabetes (OWEMR) database. The OWEMR collects individual patient data from satellite locations across the country. Using this database, researchers can analyze similarities and differences between patients who undergo LEA.
This study utilized patient data specific to the Northwell Health facility. All of the patients in our study were enrolled under the criteria of the OWEMR database. In order to be included in the OWEMR database, patients had to be diagnosed with type 1 or type 2 diabetes; have a break in the skin ≥ 0.5 cm2; be 18 years of age or older; and have a measured hemoglobin A1c (HbA1c) value within the past 120 days. Study patients signed an informed consent and committed to being available for follow-up visits to the wound care facility for 6 months after entering the study. Patients were enrolled between 2012 and 2014, and each patient was monitored for a period of 6 months within this time period. Participants were treated with current standards of care using diet, lifestyle, and pharmacologic interventions. This study was approved by the Northwell Health System Institutional Review Board Human Research Protection Program (Manhasset, NY).
Data Collection
On their first visit to the facility, patients were given a physical examination and initial interview regarding their medical history. Clinicians were required to select 1 ulcer that would be examined for the duration of the study. The selection of the ulcer was based on a point system that awarded points for pedal pulses, the ability to be probed to the bone, the location of the ulcer (ie, located on the foot rather than a toe), and the presence of multiple ulcerations. The ulcer with the highest score was selected for the study. If numerous ulcers were evaluated with the same score, the largest and deepest was selected. Wagner classification of the wound was recorded at baseline and taken at each subsequent patient visit. In addition, peripheral sensation was assessed for signs of neuropathy using Semmes-Weinstein monofilament testing.
Once selected, the wound was clinically evaluated, samples for culture were obtained, and blood tests were performed to detect the presence of wound infection. The patient’s blood was drawn for a full laboratory analysis, including white blood cell (WBC) count and measurement of blood glucose and HbA1c levels. Bone biopsy, magnetic resonance imaging, and bone scans were used to detect the presence of osteomyelitis at the discretion of the health care provider. Wounds suspected of infection, underlying osteomyelitis, or gangrene at baseline were excluded. Patients would then return for follow-up visits at least once every 6 weeks, plus or minus 2 weeks, for a maximum of 6 months.
Statistical Analysis
Utilizing SAS version 9.3 (Cary, NC), descriptive statistics (minimum, maximum, mean, median, and SD) were calculated for the following variables: age, WBC count, wound area, HbA1c, blood glucose, and body mass index (BMI). These variables were collected for each patient as per the OWEMR protocol and provided a basis for which to compare patients who underwent amputation and those who did not. Twenty patients were lost to follow-up, and therefore we altered the window of our statistics from 6 months to 3 months to provide the most accurate data, as 6-month follow-up data were limited. The patients were classified into the following categories: healed, amputated, and unhealed/non-amputated. Descriptive statistics were calculated for these 3 groups, analyzing the same variables (age, WBC count, wound area, HbA1c, blood glucose, and BMI). Additional statistical computations were utilized in order to show the prevalence and frequency of our categorical variables: gender, race, ethnicity, osteomyelitis, gangrene, and peripheral vascular disease. The baseline values of WBC count, HbA1c, wound area, and BMI of the 3 groups were analyzed with descriptive statistics for comparison. A multinomial logistic regression was then performed using a 3-level outcome variable: healed, amputated, or unhealed/non-amputated. Each predictor variable was analyzed independently due to the small sample size.
Results
Of the 169 registered patients treated at the Northwell Health facility, all qualified for the OWEMR study and met the study criteria. In the original 169 patients, there were 19 amputations: 6 toe, 6 trans-metatarsal, 6 below knee, and 1 above knee (Table 1). 
The descriptive statistics of 149 patients grouped into 3 categories (healed, amputated, unhealed/non-amputated) are shown in Table 2.

The results of the logistic regression exploring the differences between the amputation and healed groups and the unhealed/non-amputated group are shown in Table 3. The amputation group had a higher mean age and WBC count and greater wound area. Increased age was determined to be a significant predictor of the odds of amputation (P = 0.0089). For each year increase in age, the odds of amputation increased by 6.5% (odds ratio, 1.07 [95% confidence interval {CI}, 1.02-1.12]). Patients in the amputation group were more likely to be male, Hispanic, and African American and to have wound infections and comorbidities (osteomyelitis, neuropathy, and gangrene).

The presence of gangrene was significantly associated with LEA (P = 0.03). Specifically, the odds of patients without gangrene undergoing a LEA were substantially lower compared with their counterparts with gangrene (odds ratio, 0.17; 95% CI, 0.04-0.68; P = 0.0131). However, the presence of gangrene was not associated with the odds of healing compared with the odds of neither healing nor undergoing amputation (P = 0.84; not shown in Table 3).
The amputation group had lower mean values for HbA1c, BMI, and blood glucose levels and a lower rate of peripheral vascular disease. Only the relationship between lower HbA1c and increased odds of amputation versus not healing/non-amputation was found to be statistically significant (95% CI, 0.27-0.78; P = 0.009).
Discussion
This retrospective study was undertaken to evaluate factors associated with LEA in patients with diabetic foot ulcers. Patients with diabetes being treated at a wound care facility often require continuous surgical and metabolic intervention to promote optimal healing: drainage, surgical debridement, irrigation, culturing for infection, and monitoring of blood glucose levels. This treatment requires strict compliance with medical directions and, oftentimes, additional care, such as home-care nursing visits, to maintain a curative environment for the wound. Frequently, wounds on the lower extremity further complicate the healing process by reducing the patient’s mobility and daily life. Due to these factors, many patients progress to LEA. The link between diabetic ulcers and amputation has already been well described in previous studies, with studies showing that history of diabetic foot ulcer significantly predisposes an individual to LEA.4 However, few studies have further investigated demographic factors associated with risk for an amputation. Our study analyzed several categories of patient data taken from a baseline visit. We found that those with highly elevated HbA1c values were less likely to have an amputation than persons with relatively lower levels, a finding that is contrary to previous studies.
Our study’s findings suggest a higher risk for LEA with increased age. The amputation group was, on average, 7 years older than the other 2 groups. A recent study showed that risk for amputation is directly correlated to patient age, as is the mortality rate after undergoing LEA (2.3%; P < 0.05).5 Our study found that with each increase in age of 1 year, the odds of amputation increased by 6.5%. However, recent evidence on LEA risk and aging suggests that age is of less consequence than the duration of diabetes. One study found that the propensity to develop diabetic foot ulcers increases with the duration of diabetes.6 The same study found that prevalence of ulceration was correlated with age, but the relationship between age and LEA was less significant. A follow-up study for LEA could be done to examine the role of disease duration versus age in LEA.
A consensus among previous studies is that men have a higher risk for LEA.5,7 Men comprised the majority in all 3 groups in our study. In addition, the amputation group in our study had the lowest BMI. Higher BMI generally is associated with an increased risk for health complications. However, a past study conducted in Taiwan reported that obese patients with diabetes were less likely to undergo LEA than those within the normal range for BMI.8 Neither study suggests that obesity is a deterrent for LEA, but both studies may suggest that risk of amputation may approach a maximum frequency at a specific BMI range, and then decrease. This unconfirmed “cyclic” relationship should be evaluated further in a larger sample size.
Most patients in our analysis were Caucasian, followed by African American and South Asian. African Americans were the only racial group with an increased frequency in the amputation group. This finding is supported by a previous study that found that the rate of LEA among patients with diabetes in low-income, predominantly African-American neighborhoods was nearly double that in wealthier, predominantly Caucasian areas.9 A potential problem in the comparison between our data with previous studies is that the studies did not analyze patients with our inclusion criteria. All patients with diabetes in previous investigations were grouped by race, but were not necessarily required to have 1 or more ulcers. Multiple ulcers may predispose an individual to a greater risk for amputation.
Multinomial logistic regression did not suggest an association between initial size of a patient’s wound and the risk of amputation. However, the descriptive data suggests a trend. Patients who did not heal or require an amputation had the largest average wound area. This finding is not surprising in that our study followed individuals for only 3 months. Many wounds require a long course of treatment, especially in patients with diabetes, who may have poor vascularization. However, in comparison to the healed patients, the patients who required an amputation had a larger average wound area. A larger wound requires a plentiful vascular supply for the delivery of clotting factors and nutrients to the damaged area. As wound size increases, an individual’s body must transmit an increased quantity of these factors and nutrients for the regeneration of tissue. In addition, wounds that possess a larger surface area require more debridement and present a greater opportunity for infection. This may also foreshadow a longer, more costly course of treatment. Additionally, individuals coping with large ulcerations are burdened by more elaborate and complex wound dressings.
Elevated levels of HbA1c are associated with increased adverse effects of diabetes, including end-stage renal disease, neuropathy, and infection.10 In a previous study, the risk for amputation was 1.2 times higher in patients with elevated HbA1c.11 In contrast, our study suggested the odds of LEA versus not healing/not undergoing amputation decreased as HbA1c increased. As a patient’s HbA1c level increased by a value of 1, their odds for LEA decreased by 54.3%. This finding contradicts prior studies that have found a positive association between HbA1c and LEA risk, including a study where each percentage increase in HbA1c correlated with a 13% to 15% increased risk of LEA.12 The finding that patients who underwent amputation in our study had lower levels of HbA1c and blood glucose cannot be fully explained. The maximum HbA1c value in the amputated group was 7.9%. The average values for healed patients and those who underwent LEA were 8.75% and 6.77%, respectively.
Blood glucose levels were also found to be the lowest in the amputated group in our study (mean, 149.29 mg/dL vs 163.19 mg/dL in the healed group). Similar results were found in a Brazilian study, in which patients who did not require amputation had higher HbA1c levels. This study also found an association between blood glucose levels above 200 mg/dL and amputations.3 These findings provide interesting opportunities for repeat studies, preferably with a larger number of participants.
Our study is limited by the small sample size. The sample population had to be reduced, as many patients were lost to follow-up. Although this paring down of the sample size can introduce bias, we are confident that our study is representative of the demographic of patients treated in our facility. The loss of patients to follow-up in turn caused the window of analysis to be narrowed, as long-term outcome data were not available. A multisite study observing various population samples can better explore the relationship between HbA1c and risk of amputation.
Conclusion
This retrospective study exploring factors associated with LEA was unique in that all our participants had 1 or more diabetic foot ulcerations, and thus already had an extremely high risk for amputation, in contrast to previous studies that followed persons at risk for developing diabetic foot ulcerations. In contrast to several previous studies, we found that the risk for amputation actually decreased as baseline measurements of HbA1c increased. The results of this study offer many opportunities for future investigations, preferably with a larger sample size. By further isolating and scrutinizing specific factors associated with LEA, researchers can help clinicians focus on providing wound care that promotes limb salvage.
Corresponding author: Alisha Oropallo, MD, MS, Northwell Health Comprehensive Wound Care Healing Center and Hyperbarics, 1999 Marcus Avenue, Suite M6, Lake Success, NY 11042; [email protected].
Financial disclosures: Funding for this research was provided by a multi-institutional AHRQ governmental grant.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA: US Department of Health and Human Services; 2017.
2. Uccioli L, Giurato L, Meloni M, et al. Comment on Hoffstad et al. Diabetes, lower-extremity amputation, and death. Diabetes Care. 2015;38:1852-1857.
3. Gamba MA, Gotlieb SLD, Bergamaschi DP, Vianna LAC. Lower extremity amputations in diabetic patients: a case-control study. Rev Saúde Pública. 2004;38:399-404.
4. Martins-Mendes D, Monteiro-Soares M, Boyko EJ, et al. The independent contribution of diabetic foot ulcer on lower extremity amputation and mortality risk. J Diabetes Complications. 2014;28:632-638.
5. Lipsky BA, Weigelt JA, Sun X, et al. Developing and validating a risk score for lower-extremity amputation in patients hospitalized for a diabetic foot infection. Diabetes Care. 2011;34:1695-1700.
6. Al-Rubeaan K, Al Derwish M, Ouizi S, et al. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS One. 2015;10:e0124446.
7. Pickwell K, Siersma V, Kars M, et al. Predictors of lower-extremity amputation in patients with an infected diabetic foot ulcer. Diabetes Care. 2015;38:852-857.
8. Lin C, Hsu BR, Tsai J, et al. Effect of limb preservation status and body mass index on the survival of patients with limb-threatening diabetic foot ulcers. J Diabetes Complications. 2017;31:180-185.
9. Stevens CD, Schriger DL, Raffetto B, et al. Geographic clustering of diabetic lower-extremity amputations in low-income regions of California. Health Aff. 2014;33:1383-1390.
10. Liao L, Li C, Liu C, et al. Extreme levels of HbA1c increase incident ESRD risk in Chinese patients with type 2 diabetes: competing risk analysis in national cohort of Taiwan diabetes study. PLoS One. 2015; 10:e0130828.
11. Miyajima S, Shirai A, Yamamoto S, et al. Risk factors for major limb amputations in diabetic foot gangrene patients. Diabetes Res Clin Pract. 2006;71:272-279.
12. Zhao W, Katzmarzyk PT, Horswell R, et al. HbA1c and lower-extremity amputation risk in low-income patients with diabetes. Diabetes Care. 2013;36:3591-3598.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA: US Department of Health and Human Services; 2017.
2. Uccioli L, Giurato L, Meloni M, et al. Comment on Hoffstad et al. Diabetes, lower-extremity amputation, and death. Diabetes Care. 2015;38:1852-1857.
3. Gamba MA, Gotlieb SLD, Bergamaschi DP, Vianna LAC. Lower extremity amputations in diabetic patients: a case-control study. Rev Saúde Pública. 2004;38:399-404.
4. Martins-Mendes D, Monteiro-Soares M, Boyko EJ, et al. The independent contribution of diabetic foot ulcer on lower extremity amputation and mortality risk. J Diabetes Complications. 2014;28:632-638.
5. Lipsky BA, Weigelt JA, Sun X, et al. Developing and validating a risk score for lower-extremity amputation in patients hospitalized for a diabetic foot infection. Diabetes Care. 2011;34:1695-1700.
6. Al-Rubeaan K, Al Derwish M, Ouizi S, et al. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS One. 2015;10:e0124446.
7. Pickwell K, Siersma V, Kars M, et al. Predictors of lower-extremity amputation in patients with an infected diabetic foot ulcer. Diabetes Care. 2015;38:852-857.
8. Lin C, Hsu BR, Tsai J, et al. Effect of limb preservation status and body mass index on the survival of patients with limb-threatening diabetic foot ulcers. J Diabetes Complications. 2017;31:180-185.
9. Stevens CD, Schriger DL, Raffetto B, et al. Geographic clustering of diabetic lower-extremity amputations in low-income regions of California. Health Aff. 2014;33:1383-1390.
10. Liao L, Li C, Liu C, et al. Extreme levels of HbA1c increase incident ESRD risk in Chinese patients with type 2 diabetes: competing risk analysis in national cohort of Taiwan diabetes study. PLoS One. 2015; 10:e0130828.
11. Miyajima S, Shirai A, Yamamoto S, et al. Risk factors for major limb amputations in diabetic foot gangrene patients. Diabetes Res Clin Pract. 2006;71:272-279.
12. Zhao W, Katzmarzyk PT, Horswell R, et al. HbA1c and lower-extremity amputation risk in low-income patients with diabetes. Diabetes Care. 2013;36:3591-3598.
Larger absolute rivaroxaban benefit in diabetes: COMPASS
In the COMPASS trial of patients with stable coronary or peripheral artery disease (PAD), the combination of aspirin plus rivaroxaban, 2.5 mg twice daily, provided a larger absolute benefit on cardiovascular endpoints — including a threefold greater reduction in all-cause mortality — in patients with diabetes compared with the overall population.
The results of the diabetes subset of the COMPASS trial were presented by Deepak Bhatt, MD, Brigham and Women’s Hospital Heart & Vascular Center, Boston, Massachusetts, on March 28 at the “virtual” American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). They were also simultaneously published online in Circulation.
“Use of dual pathway inhibition with low-dose rivaroxaban plus aspirin is particularly attractive in high-risk patients such as those with diabetes,” Bhatt concluded.
The COMPASS trial was first reported in 2017 and showed a new low dose of rivaroxaban (2.5-mg twice-daily; Xarelto, Bayer/Janssen Pharmaceuticals) plus aspirin, 100 mg once daily, was associated with a reduction in ischemic events and mortality and a superior net clinical benefit, balancing ischemic benefit with severe bleeding, compared with aspirin alone for secondary prevention in patients with stable atherosclerotic vascular disease.
But clinicians have been slow to prescribe rivaroxaban in this new and very large population.
“It’s been more than 2 years now since main COMPASS results, and there isn’t a sense that this therapy has really caught on,” chair of the current ACC session at which the diabetes subgroup results were presented, Hadley Wilson, MD, Sanger Heart and Vascular Institute, Charlotte, North Carolina, commented:
He asked Bhatt whether the diabetes subgroup may be “the tipping point that will make people aware of rivaroxaban and then that may trickle down to other patients.”
Bhatt said that he hoped that would be the case. “We as a steering committee of this trial could say the results were positive so rivaroxaban should now be used in everyone with stable coronary or peripheral arterial disease, but that is impractical and as you out point out it hasn’t happened,” he replied.
“In PAD/vascular medicine we have embraced this new therapy. In the broader cardiology world there are a lot of patients with stable coronary arterial disease at high ischemic risk who could take rivaroxaban, but its use is bound to be limited by it being a branded drug and the fact that there is a bleeding risk,” Bhatt explained.
“I think we need to identify patients with the highest ischemic risk and focus drugs such as these with a financial cost and a bleeding risk on those who most likely will derive the greatest absolute reduction in risk,” he said. “The PAD subgroup is one group where this is the case, and now we have shown the diabetes subgroup is another. And there is no incremental bleeding risk in this group over the whole population, so they get a much greater benefit without a greater risk. I hope this helps get rivaroxaban at the new lower dose used much more often.”
A total of 18,278 patients were randomly assigned to the combination of rivaroxaban and aspirin or aspirin alone in the COMPASS trial. Of these, 6922 had diabetes mellitus at baseline and 11,356 did not have diabetes.
Results from the current analysis show a consistent and similar relative risk reduction for benefit of rivaroxaban plus aspirin vs placebo plus aspirin in patients both with and without diabetes for the primary efficacy endpoint, a composite of cardiovascular death, myocardial infarction (MI), or stroke, with a hazard ratio of 0.74 for patients with diabetes and 0.77 for those without diabetes, the researchers report.
Because of the higher baseline risk in the diabetes subgroup, these patients had numerically larger absolute risk reductions with rivaroxaban than those without diabetes for the primary efficacy endpoint at 3 years (2.3% vs 1.4%) and for all-cause mortality (1.9% vs 0.6%).
These results translate into a number needed to treat (NNT) with rivaroxaban for 3 years to prevent one CV death, MI, or stroke of 44 for the diabetes group vs 73 for the nondiabetes group; the NNT to prevent one all-cause death was 54 for the diabetes group vs 167 for the nondiabetes group, the authors write.
Because the bleeding hazards were similar among patients with and without diabetes, the absolute net clinical benefit (MI, stroke, cardiovascular death, or bleeding leading to death or symptomatic bleeding into a critical organ) for rivaroxaban was “particularly favorable” in the diabetes group (2.7% fewer events in the diabetes group vs 1.0% fewer events in the nondiabetes group), they add.
Panelist at the ACC Featured Clinical Research session at which these results were presented, Jennifer Robinson, MD, University of Iowa College of Public Health, Iowa City, asked Bhatt how clinicians were supposed to decide which of the many new agents now becoming available for patients with stable coronary artery disease to prescribe first.
“We often forget about rivaroxaban when we’re thinking about what to add next for our secondary prevention patients,” she said. “You also led the REDUCE-IT trial showing benefit of icosapent ethyl, icosapent ethyl icosapent ethyl icosapent ethyl and there is also ezetimibe, PCSK9 inhibitors and SGLT2 inhibitors. For your patients with coronary disease who are already on a high dose statin which one of these would you add next?”
“That is what physicians need to ponder all the time,” Bhatt replied. “And when a patient has several risk factors that are not well controlled, I guess it’s all important. I go through a checklist with my patients and try and figure what they’re not on that could further reduce their risk.”
“In the COMPASS trial there was an overall positive result with rivaroxaban in the whole population. And now we have shown that patients with diabetes had an even greater absolute risk reduction. That pattern has also been seen with other classes of agents including the statins, PCSK9 inhibitors, and icosapent ethyl,” Bhatt noted.
“In patients with diabetes, I will usually target whatever is standing out most at that time. If their glycemic control is completely out of whack, then that is what I would focus on first, and these days often with a SGLT2 inhibitor or GLP-1 agonist. If the LDL was out of control, I would add ezetimibe or a PCSK9 inhibitor. If the triglycerides were high, I would add icosapent ethyl. If multiple things were out of control, I would usually focus on the number most out of kilter first and try not to forget about everything else.”
But Bhatt noted that the challenge with rivaroxaban is that there is no test of thrombosis risk that would prompt the physician to take action. “Basically, the doctor just has to remember to do it. In that regard I would consider whether patients are at low bleeding risk and are they still at high ischemic risk despite controlling other risk factors and, if so, then I would add this low dose of rivaroxaban.”
Another panel member, Sekar Kathiresan, MD, asked Bhatt whether he recommended using available scores to assess the bleeding/thrombosis risk/benefits of adding an antithrombotic.
Bhatt replied: “That’s a terrific question. I guess the right answer is that we should be doing that, but in reality I have to concede that I don’t use these scores. They have shown appropriate C statistics in populations, but they are not fantastic in individual patients.”
“I have to confess that I use the eyeball test. There is nothing as good at predicting future bleeding as past bleeding. So if a patient has had bleeding problems on aspirin alone I wouldn’t add rivaroxaban. But if a patient hasn’t bled before, especially if they had some experience of dual antiplatelet therapy, then they would be good candidates for a low vascular dose of rivaroxaban,” he said.
“It is not as easy as with other drugs as there is always a bleeding trade-off with an antithrombotic. There is no such thing as a free lunch. So patients need careful assessment when considering prescribing rivaroxaban and regular reassessment over time to check if they have had any bleeding,” he added.
The COMPASS study was funded by Bayer. Bhatt reports honoraria from Bayer via the Population Health Research Institute for his role on the COMPASS trial and other research funding from Bayer to the Brigham & Women’s Hospital.
American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). Abstract 20-LB-20544-ACC. Presented March 28, 2020.
Circulation. Published online March 28, 2020. Full text.
This article first appeared on Medscape.com.
In the COMPASS trial of patients with stable coronary or peripheral artery disease (PAD), the combination of aspirin plus rivaroxaban, 2.5 mg twice daily, provided a larger absolute benefit on cardiovascular endpoints — including a threefold greater reduction in all-cause mortality — in patients with diabetes compared with the overall population.
The results of the diabetes subset of the COMPASS trial were presented by Deepak Bhatt, MD, Brigham and Women’s Hospital Heart & Vascular Center, Boston, Massachusetts, on March 28 at the “virtual” American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). They were also simultaneously published online in Circulation.
“Use of dual pathway inhibition with low-dose rivaroxaban plus aspirin is particularly attractive in high-risk patients such as those with diabetes,” Bhatt concluded.
The COMPASS trial was first reported in 2017 and showed a new low dose of rivaroxaban (2.5-mg twice-daily; Xarelto, Bayer/Janssen Pharmaceuticals) plus aspirin, 100 mg once daily, was associated with a reduction in ischemic events and mortality and a superior net clinical benefit, balancing ischemic benefit with severe bleeding, compared with aspirin alone for secondary prevention in patients with stable atherosclerotic vascular disease.
But clinicians have been slow to prescribe rivaroxaban in this new and very large population.
“It’s been more than 2 years now since main COMPASS results, and there isn’t a sense that this therapy has really caught on,” chair of the current ACC session at which the diabetes subgroup results were presented, Hadley Wilson, MD, Sanger Heart and Vascular Institute, Charlotte, North Carolina, commented:
He asked Bhatt whether the diabetes subgroup may be “the tipping point that will make people aware of rivaroxaban and then that may trickle down to other patients.”
Bhatt said that he hoped that would be the case. “We as a steering committee of this trial could say the results were positive so rivaroxaban should now be used in everyone with stable coronary or peripheral arterial disease, but that is impractical and as you out point out it hasn’t happened,” he replied.
“In PAD/vascular medicine we have embraced this new therapy. In the broader cardiology world there are a lot of patients with stable coronary arterial disease at high ischemic risk who could take rivaroxaban, but its use is bound to be limited by it being a branded drug and the fact that there is a bleeding risk,” Bhatt explained.
“I think we need to identify patients with the highest ischemic risk and focus drugs such as these with a financial cost and a bleeding risk on those who most likely will derive the greatest absolute reduction in risk,” he said. “The PAD subgroup is one group where this is the case, and now we have shown the diabetes subgroup is another. And there is no incremental bleeding risk in this group over the whole population, so they get a much greater benefit without a greater risk. I hope this helps get rivaroxaban at the new lower dose used much more often.”
A total of 18,278 patients were randomly assigned to the combination of rivaroxaban and aspirin or aspirin alone in the COMPASS trial. Of these, 6922 had diabetes mellitus at baseline and 11,356 did not have diabetes.
Results from the current analysis show a consistent and similar relative risk reduction for benefit of rivaroxaban plus aspirin vs placebo plus aspirin in patients both with and without diabetes for the primary efficacy endpoint, a composite of cardiovascular death, myocardial infarction (MI), or stroke, with a hazard ratio of 0.74 for patients with diabetes and 0.77 for those without diabetes, the researchers report.
Because of the higher baseline risk in the diabetes subgroup, these patients had numerically larger absolute risk reductions with rivaroxaban than those without diabetes for the primary efficacy endpoint at 3 years (2.3% vs 1.4%) and for all-cause mortality (1.9% vs 0.6%).
These results translate into a number needed to treat (NNT) with rivaroxaban for 3 years to prevent one CV death, MI, or stroke of 44 for the diabetes group vs 73 for the nondiabetes group; the NNT to prevent one all-cause death was 54 for the diabetes group vs 167 for the nondiabetes group, the authors write.
Because the bleeding hazards were similar among patients with and without diabetes, the absolute net clinical benefit (MI, stroke, cardiovascular death, or bleeding leading to death or symptomatic bleeding into a critical organ) for rivaroxaban was “particularly favorable” in the diabetes group (2.7% fewer events in the diabetes group vs 1.0% fewer events in the nondiabetes group), they add.
Panelist at the ACC Featured Clinical Research session at which these results were presented, Jennifer Robinson, MD, University of Iowa College of Public Health, Iowa City, asked Bhatt how clinicians were supposed to decide which of the many new agents now becoming available for patients with stable coronary artery disease to prescribe first.
“We often forget about rivaroxaban when we’re thinking about what to add next for our secondary prevention patients,” she said. “You also led the REDUCE-IT trial showing benefit of icosapent ethyl, icosapent ethyl icosapent ethyl icosapent ethyl and there is also ezetimibe, PCSK9 inhibitors and SGLT2 inhibitors. For your patients with coronary disease who are already on a high dose statin which one of these would you add next?”
“That is what physicians need to ponder all the time,” Bhatt replied. “And when a patient has several risk factors that are not well controlled, I guess it’s all important. I go through a checklist with my patients and try and figure what they’re not on that could further reduce their risk.”
“In the COMPASS trial there was an overall positive result with rivaroxaban in the whole population. And now we have shown that patients with diabetes had an even greater absolute risk reduction. That pattern has also been seen with other classes of agents including the statins, PCSK9 inhibitors, and icosapent ethyl,” Bhatt noted.
“In patients with diabetes, I will usually target whatever is standing out most at that time. If their glycemic control is completely out of whack, then that is what I would focus on first, and these days often with a SGLT2 inhibitor or GLP-1 agonist. If the LDL was out of control, I would add ezetimibe or a PCSK9 inhibitor. If the triglycerides were high, I would add icosapent ethyl. If multiple things were out of control, I would usually focus on the number most out of kilter first and try not to forget about everything else.”
But Bhatt noted that the challenge with rivaroxaban is that there is no test of thrombosis risk that would prompt the physician to take action. “Basically, the doctor just has to remember to do it. In that regard I would consider whether patients are at low bleeding risk and are they still at high ischemic risk despite controlling other risk factors and, if so, then I would add this low dose of rivaroxaban.”
Another panel member, Sekar Kathiresan, MD, asked Bhatt whether he recommended using available scores to assess the bleeding/thrombosis risk/benefits of adding an antithrombotic.
Bhatt replied: “That’s a terrific question. I guess the right answer is that we should be doing that, but in reality I have to concede that I don’t use these scores. They have shown appropriate C statistics in populations, but they are not fantastic in individual patients.”
“I have to confess that I use the eyeball test. There is nothing as good at predicting future bleeding as past bleeding. So if a patient has had bleeding problems on aspirin alone I wouldn’t add rivaroxaban. But if a patient hasn’t bled before, especially if they had some experience of dual antiplatelet therapy, then they would be good candidates for a low vascular dose of rivaroxaban,” he said.
“It is not as easy as with other drugs as there is always a bleeding trade-off with an antithrombotic. There is no such thing as a free lunch. So patients need careful assessment when considering prescribing rivaroxaban and regular reassessment over time to check if they have had any bleeding,” he added.
The COMPASS study was funded by Bayer. Bhatt reports honoraria from Bayer via the Population Health Research Institute for his role on the COMPASS trial and other research funding from Bayer to the Brigham & Women’s Hospital.
American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). Abstract 20-LB-20544-ACC. Presented March 28, 2020.
Circulation. Published online March 28, 2020. Full text.
This article first appeared on Medscape.com.
In the COMPASS trial of patients with stable coronary or peripheral artery disease (PAD), the combination of aspirin plus rivaroxaban, 2.5 mg twice daily, provided a larger absolute benefit on cardiovascular endpoints — including a threefold greater reduction in all-cause mortality — in patients with diabetes compared with the overall population.
The results of the diabetes subset of the COMPASS trial were presented by Deepak Bhatt, MD, Brigham and Women’s Hospital Heart & Vascular Center, Boston, Massachusetts, on March 28 at the “virtual” American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). They were also simultaneously published online in Circulation.
“Use of dual pathway inhibition with low-dose rivaroxaban plus aspirin is particularly attractive in high-risk patients such as those with diabetes,” Bhatt concluded.
The COMPASS trial was first reported in 2017 and showed a new low dose of rivaroxaban (2.5-mg twice-daily; Xarelto, Bayer/Janssen Pharmaceuticals) plus aspirin, 100 mg once daily, was associated with a reduction in ischemic events and mortality and a superior net clinical benefit, balancing ischemic benefit with severe bleeding, compared with aspirin alone for secondary prevention in patients with stable atherosclerotic vascular disease.
But clinicians have been slow to prescribe rivaroxaban in this new and very large population.
“It’s been more than 2 years now since main COMPASS results, and there isn’t a sense that this therapy has really caught on,” chair of the current ACC session at which the diabetes subgroup results were presented, Hadley Wilson, MD, Sanger Heart and Vascular Institute, Charlotte, North Carolina, commented:
He asked Bhatt whether the diabetes subgroup may be “the tipping point that will make people aware of rivaroxaban and then that may trickle down to other patients.”
Bhatt said that he hoped that would be the case. “We as a steering committee of this trial could say the results were positive so rivaroxaban should now be used in everyone with stable coronary or peripheral arterial disease, but that is impractical and as you out point out it hasn’t happened,” he replied.
“In PAD/vascular medicine we have embraced this new therapy. In the broader cardiology world there are a lot of patients with stable coronary arterial disease at high ischemic risk who could take rivaroxaban, but its use is bound to be limited by it being a branded drug and the fact that there is a bleeding risk,” Bhatt explained.
“I think we need to identify patients with the highest ischemic risk and focus drugs such as these with a financial cost and a bleeding risk on those who most likely will derive the greatest absolute reduction in risk,” he said. “The PAD subgroup is one group where this is the case, and now we have shown the diabetes subgroup is another. And there is no incremental bleeding risk in this group over the whole population, so they get a much greater benefit without a greater risk. I hope this helps get rivaroxaban at the new lower dose used much more often.”
A total of 18,278 patients were randomly assigned to the combination of rivaroxaban and aspirin or aspirin alone in the COMPASS trial. Of these, 6922 had diabetes mellitus at baseline and 11,356 did not have diabetes.
Results from the current analysis show a consistent and similar relative risk reduction for benefit of rivaroxaban plus aspirin vs placebo plus aspirin in patients both with and without diabetes for the primary efficacy endpoint, a composite of cardiovascular death, myocardial infarction (MI), or stroke, with a hazard ratio of 0.74 for patients with diabetes and 0.77 for those without diabetes, the researchers report.
Because of the higher baseline risk in the diabetes subgroup, these patients had numerically larger absolute risk reductions with rivaroxaban than those without diabetes for the primary efficacy endpoint at 3 years (2.3% vs 1.4%) and for all-cause mortality (1.9% vs 0.6%).
These results translate into a number needed to treat (NNT) with rivaroxaban for 3 years to prevent one CV death, MI, or stroke of 44 for the diabetes group vs 73 for the nondiabetes group; the NNT to prevent one all-cause death was 54 for the diabetes group vs 167 for the nondiabetes group, the authors write.
Because the bleeding hazards were similar among patients with and without diabetes, the absolute net clinical benefit (MI, stroke, cardiovascular death, or bleeding leading to death or symptomatic bleeding into a critical organ) for rivaroxaban was “particularly favorable” in the diabetes group (2.7% fewer events in the diabetes group vs 1.0% fewer events in the nondiabetes group), they add.
Panelist at the ACC Featured Clinical Research session at which these results were presented, Jennifer Robinson, MD, University of Iowa College of Public Health, Iowa City, asked Bhatt how clinicians were supposed to decide which of the many new agents now becoming available for patients with stable coronary artery disease to prescribe first.
“We often forget about rivaroxaban when we’re thinking about what to add next for our secondary prevention patients,” she said. “You also led the REDUCE-IT trial showing benefit of icosapent ethyl, icosapent ethyl icosapent ethyl icosapent ethyl and there is also ezetimibe, PCSK9 inhibitors and SGLT2 inhibitors. For your patients with coronary disease who are already on a high dose statin which one of these would you add next?”
“That is what physicians need to ponder all the time,” Bhatt replied. “And when a patient has several risk factors that are not well controlled, I guess it’s all important. I go through a checklist with my patients and try and figure what they’re not on that could further reduce their risk.”
“In the COMPASS trial there was an overall positive result with rivaroxaban in the whole population. And now we have shown that patients with diabetes had an even greater absolute risk reduction. That pattern has also been seen with other classes of agents including the statins, PCSK9 inhibitors, and icosapent ethyl,” Bhatt noted.
“In patients with diabetes, I will usually target whatever is standing out most at that time. If their glycemic control is completely out of whack, then that is what I would focus on first, and these days often with a SGLT2 inhibitor or GLP-1 agonist. If the LDL was out of control, I would add ezetimibe or a PCSK9 inhibitor. If the triglycerides were high, I would add icosapent ethyl. If multiple things were out of control, I would usually focus on the number most out of kilter first and try not to forget about everything else.”
But Bhatt noted that the challenge with rivaroxaban is that there is no test of thrombosis risk that would prompt the physician to take action. “Basically, the doctor just has to remember to do it. In that regard I would consider whether patients are at low bleeding risk and are they still at high ischemic risk despite controlling other risk factors and, if so, then I would add this low dose of rivaroxaban.”
Another panel member, Sekar Kathiresan, MD, asked Bhatt whether he recommended using available scores to assess the bleeding/thrombosis risk/benefits of adding an antithrombotic.
Bhatt replied: “That’s a terrific question. I guess the right answer is that we should be doing that, but in reality I have to concede that I don’t use these scores. They have shown appropriate C statistics in populations, but they are not fantastic in individual patients.”
“I have to confess that I use the eyeball test. There is nothing as good at predicting future bleeding as past bleeding. So if a patient has had bleeding problems on aspirin alone I wouldn’t add rivaroxaban. But if a patient hasn’t bled before, especially if they had some experience of dual antiplatelet therapy, then they would be good candidates for a low vascular dose of rivaroxaban,” he said.
“It is not as easy as with other drugs as there is always a bleeding trade-off with an antithrombotic. There is no such thing as a free lunch. So patients need careful assessment when considering prescribing rivaroxaban and regular reassessment over time to check if they have had any bleeding,” he added.
The COMPASS study was funded by Bayer. Bhatt reports honoraria from Bayer via the Population Health Research Institute for his role on the COMPASS trial and other research funding from Bayer to the Brigham & Women’s Hospital.
American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). Abstract 20-LB-20544-ACC. Presented March 28, 2020.
Circulation. Published online March 28, 2020. Full text.
This article first appeared on Medscape.com.
Keep calm: Under 25s with diabetes aren't being hospitalized for COVID-19
Reports from pediatric endocrinologists in COVID-19 hot spots globally indicate that children, adolescents, and young adults with diabetes have so far not shown a different disease pattern with the virus compared to children and younger people who do not have diabetes.
Indeed, to date [as of March 24]” according to a new statement from the International Society for Pediatric and Adolescent Diabetes (ISPAD), which currently has about 1,300 members around the globe and has instituted a discussion forum about the topic of treating children with both diabetes and COVID-19.
“We find these reports [from colleagues around the world], though anecdotal, to be reassuring,” it notes. However, there are real worries regarding other potentially dangerous effects. ISPAD has expressed concern, for example, that the COVID-19 pandemic will prevent youngsters with existing diabetes who are having diabetic emergencies from seeking hospital care.
Chinese physicians have reported to ISPAD a number of cases of delayed hospital admissions for diabetic ketoacidosis (DKA) in children with known type 1 diabetes because hospital services were closed for non–COVID-19 care.
Andrea Scaramuzza, MD, a pediatric endocrinologist at Ospedale Maggiore di Cremona, Italy, has similarly reported multiple cases of patients presenting to emergency services there with severe DKA.
“These experiences reinforce the importance of continued attentiveness to standard diabetes care to avoid the need for hospitalization and emergency or urgent care visits,” says ISPAD, under the strapline: “Keep calm and mind your diabetes care.”
But it nevertheless stresses that these resources should be used “if needed.”
Worries that new-onset diabetes will be missed during COVID-19
Dr. Scaramuzza said in an interview that there also are concerns regarding delays in diagnoses of new cases of type 1 diabetes “due to the fear families have to go to the emergency department because of COVID-19.”
Indeed, in Italy, a few patients have arrived with very serious DKA, he said. Dr. Scaramuzza noted a colleague from Naples, Dario Iafusco, MD, and colleagues have made a video to keep awareness high regarding new-onset diabetes.
“This coronavirus pandemic can be defeated if you stay at home, but if you know of a child who has excessive thirst, frequent urination, or who starts vomiting,” seek health care advice immediately. “This child could have [type 1] diabetes. Prevent severe DKA, or worse, death,” Dr. Iafusco of the Regional Centre of Paediatric Diabetology G.Stoppoloni Via S. Andrea delle Dame, Naples, said in the video.
Physicians from China have similar observations, reporting to ISPAD several cases of delayed admissions of newly diagnosed type 1 diabetes because hospital services were closed for non–COVID-19 care.
Keep calm and mind your diabetes care; physicians use telemedicine
Meanwhile, last week ISPAD issued guidance for young people with diabetes and their carers about what to do if COVID-19 infection is suspected.
Most advice is the same as for the general public because reports of COVID-19 infection suggest it is much less severe in children and adolescents, and the summary currently serves “as reassurance that youth with diabetes are not more affected by COVID-19 than peers,” it adds.
“Our approach to treating a child with diabetes would be to follow the ISPAD sick-day guidelines, which provide generalized diabetes management in any flu-like illness. We wouldn’t do anything very different right now,” one of the authors, Jamie Wood, MD, associate professor of clinical pediatrics at Case Western Reserve University, Cleveland, said in an interview.
“Any illness makes diabetes more difficult to manage and can increase the risk of DKA,” she emphasized.
“We would reinforce frequent monitoring of blood glucose and ketone levels, to never stop insulin – in fact, when most people are ill, the body is stressed and requires more insulin – and to stay hydrated and treat the underlying symptoms.”
And make sure to “treat the fever,” she stressed. “When patients with type 1 diabetes get fever, they have a tendency to make more ketones, so we recommend aggressive control of fever.”
ISPAD recommends young people aim to keep blood glucose levels between 4 and 10 mmol/L (72-180 mg/dL) and blood ketones below 0.6 mmol/L (10.8 mg/dL) during illness and to never stop insulin.
Guidance is provided on when to seek urgent specialist advice with possible referral to emergency care, for example, in cases in which the patient has DKA symptoms, such as persistent and/or worsened fruity breath odor or vomiting.
Dr. Scaramuzza said in an interview that, in Italy, he and his colleagues have increased their use of telemedicine to keep monitoring their patients with diabetes even from a distance and that it was working very well.
“Technology – such as downloading [records from] insulin pumps, continuous glucose monitoring systems, and the possibility to use Skype or other platforms – really helps,” he noted.
“There has been a rapid increase in telehealth as a way to continue to care for youth with diabetes and decrease risk for infection,” said ISPAD.
“Communication between patients, families, and health care teams is vitally important. Methods to do so that avoid visits to clinics or hospitals can provide needed diabetes advice and reduce risk for COVID-19 transmission.”
A version of this article originally appeared on Medscape.com.
Reports from pediatric endocrinologists in COVID-19 hot spots globally indicate that children, adolescents, and young adults with diabetes have so far not shown a different disease pattern with the virus compared to children and younger people who do not have diabetes.
Indeed, to date [as of March 24]” according to a new statement from the International Society for Pediatric and Adolescent Diabetes (ISPAD), which currently has about 1,300 members around the globe and has instituted a discussion forum about the topic of treating children with both diabetes and COVID-19.
“We find these reports [from colleagues around the world], though anecdotal, to be reassuring,” it notes. However, there are real worries regarding other potentially dangerous effects. ISPAD has expressed concern, for example, that the COVID-19 pandemic will prevent youngsters with existing diabetes who are having diabetic emergencies from seeking hospital care.
Chinese physicians have reported to ISPAD a number of cases of delayed hospital admissions for diabetic ketoacidosis (DKA) in children with known type 1 diabetes because hospital services were closed for non–COVID-19 care.
Andrea Scaramuzza, MD, a pediatric endocrinologist at Ospedale Maggiore di Cremona, Italy, has similarly reported multiple cases of patients presenting to emergency services there with severe DKA.
“These experiences reinforce the importance of continued attentiveness to standard diabetes care to avoid the need for hospitalization and emergency or urgent care visits,” says ISPAD, under the strapline: “Keep calm and mind your diabetes care.”
But it nevertheless stresses that these resources should be used “if needed.”
Worries that new-onset diabetes will be missed during COVID-19
Dr. Scaramuzza said in an interview that there also are concerns regarding delays in diagnoses of new cases of type 1 diabetes “due to the fear families have to go to the emergency department because of COVID-19.”
Indeed, in Italy, a few patients have arrived with very serious DKA, he said. Dr. Scaramuzza noted a colleague from Naples, Dario Iafusco, MD, and colleagues have made a video to keep awareness high regarding new-onset diabetes.
“This coronavirus pandemic can be defeated if you stay at home, but if you know of a child who has excessive thirst, frequent urination, or who starts vomiting,” seek health care advice immediately. “This child could have [type 1] diabetes. Prevent severe DKA, or worse, death,” Dr. Iafusco of the Regional Centre of Paediatric Diabetology G.Stoppoloni Via S. Andrea delle Dame, Naples, said in the video.
Physicians from China have similar observations, reporting to ISPAD several cases of delayed admissions of newly diagnosed type 1 diabetes because hospital services were closed for non–COVID-19 care.
Keep calm and mind your diabetes care; physicians use telemedicine
Meanwhile, last week ISPAD issued guidance for young people with diabetes and their carers about what to do if COVID-19 infection is suspected.
Most advice is the same as for the general public because reports of COVID-19 infection suggest it is much less severe in children and adolescents, and the summary currently serves “as reassurance that youth with diabetes are not more affected by COVID-19 than peers,” it adds.
“Our approach to treating a child with diabetes would be to follow the ISPAD sick-day guidelines, which provide generalized diabetes management in any flu-like illness. We wouldn’t do anything very different right now,” one of the authors, Jamie Wood, MD, associate professor of clinical pediatrics at Case Western Reserve University, Cleveland, said in an interview.
“Any illness makes diabetes more difficult to manage and can increase the risk of DKA,” she emphasized.
“We would reinforce frequent monitoring of blood glucose and ketone levels, to never stop insulin – in fact, when most people are ill, the body is stressed and requires more insulin – and to stay hydrated and treat the underlying symptoms.”
And make sure to “treat the fever,” she stressed. “When patients with type 1 diabetes get fever, they have a tendency to make more ketones, so we recommend aggressive control of fever.”
ISPAD recommends young people aim to keep blood glucose levels between 4 and 10 mmol/L (72-180 mg/dL) and blood ketones below 0.6 mmol/L (10.8 mg/dL) during illness and to never stop insulin.
Guidance is provided on when to seek urgent specialist advice with possible referral to emergency care, for example, in cases in which the patient has DKA symptoms, such as persistent and/or worsened fruity breath odor or vomiting.
Dr. Scaramuzza said in an interview that, in Italy, he and his colleagues have increased their use of telemedicine to keep monitoring their patients with diabetes even from a distance and that it was working very well.
“Technology – such as downloading [records from] insulin pumps, continuous glucose monitoring systems, and the possibility to use Skype or other platforms – really helps,” he noted.
“There has been a rapid increase in telehealth as a way to continue to care for youth with diabetes and decrease risk for infection,” said ISPAD.
“Communication between patients, families, and health care teams is vitally important. Methods to do so that avoid visits to clinics or hospitals can provide needed diabetes advice and reduce risk for COVID-19 transmission.”
A version of this article originally appeared on Medscape.com.
Reports from pediatric endocrinologists in COVID-19 hot spots globally indicate that children, adolescents, and young adults with diabetes have so far not shown a different disease pattern with the virus compared to children and younger people who do not have diabetes.
Indeed, to date [as of March 24]” according to a new statement from the International Society for Pediatric and Adolescent Diabetes (ISPAD), which currently has about 1,300 members around the globe and has instituted a discussion forum about the topic of treating children with both diabetes and COVID-19.
“We find these reports [from colleagues around the world], though anecdotal, to be reassuring,” it notes. However, there are real worries regarding other potentially dangerous effects. ISPAD has expressed concern, for example, that the COVID-19 pandemic will prevent youngsters with existing diabetes who are having diabetic emergencies from seeking hospital care.
Chinese physicians have reported to ISPAD a number of cases of delayed hospital admissions for diabetic ketoacidosis (DKA) in children with known type 1 diabetes because hospital services were closed for non–COVID-19 care.
Andrea Scaramuzza, MD, a pediatric endocrinologist at Ospedale Maggiore di Cremona, Italy, has similarly reported multiple cases of patients presenting to emergency services there with severe DKA.
“These experiences reinforce the importance of continued attentiveness to standard diabetes care to avoid the need for hospitalization and emergency or urgent care visits,” says ISPAD, under the strapline: “Keep calm and mind your diabetes care.”
But it nevertheless stresses that these resources should be used “if needed.”
Worries that new-onset diabetes will be missed during COVID-19
Dr. Scaramuzza said in an interview that there also are concerns regarding delays in diagnoses of new cases of type 1 diabetes “due to the fear families have to go to the emergency department because of COVID-19.”
Indeed, in Italy, a few patients have arrived with very serious DKA, he said. Dr. Scaramuzza noted a colleague from Naples, Dario Iafusco, MD, and colleagues have made a video to keep awareness high regarding new-onset diabetes.
“This coronavirus pandemic can be defeated if you stay at home, but if you know of a child who has excessive thirst, frequent urination, or who starts vomiting,” seek health care advice immediately. “This child could have [type 1] diabetes. Prevent severe DKA, or worse, death,” Dr. Iafusco of the Regional Centre of Paediatric Diabetology G.Stoppoloni Via S. Andrea delle Dame, Naples, said in the video.
Physicians from China have similar observations, reporting to ISPAD several cases of delayed admissions of newly diagnosed type 1 diabetes because hospital services were closed for non–COVID-19 care.
Keep calm and mind your diabetes care; physicians use telemedicine
Meanwhile, last week ISPAD issued guidance for young people with diabetes and their carers about what to do if COVID-19 infection is suspected.
Most advice is the same as for the general public because reports of COVID-19 infection suggest it is much less severe in children and adolescents, and the summary currently serves “as reassurance that youth with diabetes are not more affected by COVID-19 than peers,” it adds.
“Our approach to treating a child with diabetes would be to follow the ISPAD sick-day guidelines, which provide generalized diabetes management in any flu-like illness. We wouldn’t do anything very different right now,” one of the authors, Jamie Wood, MD, associate professor of clinical pediatrics at Case Western Reserve University, Cleveland, said in an interview.
“Any illness makes diabetes more difficult to manage and can increase the risk of DKA,” she emphasized.
“We would reinforce frequent monitoring of blood glucose and ketone levels, to never stop insulin – in fact, when most people are ill, the body is stressed and requires more insulin – and to stay hydrated and treat the underlying symptoms.”
And make sure to “treat the fever,” she stressed. “When patients with type 1 diabetes get fever, they have a tendency to make more ketones, so we recommend aggressive control of fever.”
ISPAD recommends young people aim to keep blood glucose levels between 4 and 10 mmol/L (72-180 mg/dL) and blood ketones below 0.6 mmol/L (10.8 mg/dL) during illness and to never stop insulin.
Guidance is provided on when to seek urgent specialist advice with possible referral to emergency care, for example, in cases in which the patient has DKA symptoms, such as persistent and/or worsened fruity breath odor or vomiting.
Dr. Scaramuzza said in an interview that, in Italy, he and his colleagues have increased their use of telemedicine to keep monitoring their patients with diabetes even from a distance and that it was working very well.
“Technology – such as downloading [records from] insulin pumps, continuous glucose monitoring systems, and the possibility to use Skype or other platforms – really helps,” he noted.
“There has been a rapid increase in telehealth as a way to continue to care for youth with diabetes and decrease risk for infection,” said ISPAD.
“Communication between patients, families, and health care teams is vitally important. Methods to do so that avoid visits to clinics or hospitals can provide needed diabetes advice and reduce risk for COVID-19 transmission.”
A version of this article originally appeared on Medscape.com.
Low fitness level linked to higher risk of heart failure in diabetes
PHOENIX – Lower baseline fitness and greater decline in fitness over time are independently associated with a higher risk of heart failure in patients with diabetes, results from a large analysis showed.
“Diabetes is an important risk factor for the development of heart failure, and the diagnosis of diabetes in newly diagnosed cases of heart failure has been increasing,” Ambarish Pandey, MD, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting. “Type 2 diabetes is associated with increased burden of traditional risk factors such as hypertension, kidney dysfunction, and dyslipidemia – each of which in turn increase the risk of both atherothrombotic disease as well as heart failure.”
Recent data from the Swedish National Diabetes Register have shown that optimal management of these risk factors in patients with type 2 diabetes can actually mitigate the risk of atherosclerotic events such as acute MI, but the risk of heart failure does not significantly lower with optimal management of these traditional cardiovascular risk factors (N Engl J Med. 2018;379:633-44). “These findings highlight that novel approaches that go beyond just managing traditional cardiovascular risk factors are needed for prevention of heart failure in patients with type 2 diabetes,” said Dr. Pandey, of the division of cardiology at the University of Texas Southwestern Medical Center, Dallas. “Our group has demonstrated that physical inactivity and low levels of fitness are associated with a higher risk of heart failure. We have also shown that the protective effect of physical activity against heart failure risk is stronger against heart failure with preserved ejection fraction, which is a subtype of heart failure that is increasing in prevalence and has no effective therapies.”
Dr. Pandey and his colleagues set out to test the research hypothesis that fitness decline and increases in body mass index over time are significantly associated with a higher risk of heart failure. To do this, they drew from the LookAHEAD Trial, a multicenter analysis of 5,145 overweight or obese patients with type 2 diabetes who were randomized to an intensive lifestyle intervention or to usual care. The intervention consisted of a caloric intake goal of 1,200 to 1,800 kcal per day and engaging in at least 175 minutes per week of physical activity. Participants were stratified into one of three fitness group levels: low, moderate, and high, from 5 metabolic equivalents (METs) in the lowest fitness tertile to 9 METs in the highest fitness tertile. The primary outcome of the trial was adverse cardiovascular events. The intervention was implemented for almost 10 years, and patients were followed for up to 12 years from baseline.
The heart failure outcomes were not systematically adjudicated in the primary LookAHEAD trial, so Dr. Pandey and colleagues conducted an ancillary study of all incident hospitalizations in the study and followed them for 2 additional years. Overall, the researchers identified 257 incident heart failure events. The cumulative incidence of heart failure for the usual care versus the intensive lifestyle intervention arm was not statistically different (an event rate of 4.53 vs. 4.32 per 1,000 person-years, respectively; hazard ratio, 0.96). “This demonstrated that the intensive lifestyle intervention in the LookAHEAD trial did not significantly modify the risk of heart failure,” Dr. Pandey said.
However, an adjusted analysis revealed that the risk of heart failure was 39% lower in the moderately fit group and 62% lower in the high fit group, compared with the low-fitness group. Among heart failure subtypes, the risk of heart failure with preserved ejection fraction (HFpEF) was 40% lower in the moderately fit group and 77% lower in the high-fitness group. On the other hand, baseline level of fitness level was not associated with risk of heart failure reduced ejection fraction (HFrEF) after the researchers adjusted for cardiovascular risk factors.
Next, Dr. Pandey and his colleagues used Cox modeling to examine the association of baseline and longitudinal changes in fitness and BMI with risk of heart failure. For change in fitness and BMI analysis, they used the 4-year follow-up data in 3,092 participants who underwent repeat fitness testing and had available data on BMI. They excluded patients who developed heart failure within the first 4 years of the study.
The mean age of the ancillary study population was about 60 years, and there was a lower proportion of women in the high fitness tertile (41%). The researchers observed a graded, inverse association between higher fitness levels and lower risk of heart failure such that increasing fitness from baseline was associated with a substantial decrease in the risk of heart failure. Specifically, a 10% decline in fitness over the 4 years of follow-up was associated with a 11% increase in the overall risk of heart failure (HR, 1.11). “This was largely consistent with the two heart failure subtypes,” he said. Similarly, a 10% increase in BMI over the 4 years of follow-up was associated with a 25% increase in the overall risk of heart failure (HR 1.25). On the other hand, a 10% decrease BMI was associated with a 20% decrease in the risk of heart failure (HR .80). This was also largely consistent for both heart failure subtypes. According to co-lead investigator Kershaw Patel, MD, “these findings suggest that therapies targeting large and sustained improvements in fitness and weight loss may modify the risk of heart failure among patients with diabetes.”
“Lower fitness at baseline was more strongly associated with the risk of HFpEF vs. HFrEF, and greater weight loss over follow-up is associated with a lower risk of heart failure independent of changes in other risk factors,” Dr. Pandey concluded at the meeting, which was sponsored by the American Heart Association.
In an interview, session moderator Joshua J. Joseph, MD, said that it remains unclear what type of setting is ideal for carrying out cardiorespiratory fitness in this patient population. “What is the supervision needed for that to occur?” asked Dr. Joseph, of The Ohio State University, Columbus. “Can patients do this on their own, or do they need guidance? What is the best approach? That’s the question we all have to answer individually in our own communities.”
Dr. Pandey reported having no disclosures.
SOURCE: Pandey A. Epi/Lifestyle 2020, Abstract 16.
PHOENIX – Lower baseline fitness and greater decline in fitness over time are independently associated with a higher risk of heart failure in patients with diabetes, results from a large analysis showed.
“Diabetes is an important risk factor for the development of heart failure, and the diagnosis of diabetes in newly diagnosed cases of heart failure has been increasing,” Ambarish Pandey, MD, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting. “Type 2 diabetes is associated with increased burden of traditional risk factors such as hypertension, kidney dysfunction, and dyslipidemia – each of which in turn increase the risk of both atherothrombotic disease as well as heart failure.”
Recent data from the Swedish National Diabetes Register have shown that optimal management of these risk factors in patients with type 2 diabetes can actually mitigate the risk of atherosclerotic events such as acute MI, but the risk of heart failure does not significantly lower with optimal management of these traditional cardiovascular risk factors (N Engl J Med. 2018;379:633-44). “These findings highlight that novel approaches that go beyond just managing traditional cardiovascular risk factors are needed for prevention of heart failure in patients with type 2 diabetes,” said Dr. Pandey, of the division of cardiology at the University of Texas Southwestern Medical Center, Dallas. “Our group has demonstrated that physical inactivity and low levels of fitness are associated with a higher risk of heart failure. We have also shown that the protective effect of physical activity against heart failure risk is stronger against heart failure with preserved ejection fraction, which is a subtype of heart failure that is increasing in prevalence and has no effective therapies.”
Dr. Pandey and his colleagues set out to test the research hypothesis that fitness decline and increases in body mass index over time are significantly associated with a higher risk of heart failure. To do this, they drew from the LookAHEAD Trial, a multicenter analysis of 5,145 overweight or obese patients with type 2 diabetes who were randomized to an intensive lifestyle intervention or to usual care. The intervention consisted of a caloric intake goal of 1,200 to 1,800 kcal per day and engaging in at least 175 minutes per week of physical activity. Participants were stratified into one of three fitness group levels: low, moderate, and high, from 5 metabolic equivalents (METs) in the lowest fitness tertile to 9 METs in the highest fitness tertile. The primary outcome of the trial was adverse cardiovascular events. The intervention was implemented for almost 10 years, and patients were followed for up to 12 years from baseline.
The heart failure outcomes were not systematically adjudicated in the primary LookAHEAD trial, so Dr. Pandey and colleagues conducted an ancillary study of all incident hospitalizations in the study and followed them for 2 additional years. Overall, the researchers identified 257 incident heart failure events. The cumulative incidence of heart failure for the usual care versus the intensive lifestyle intervention arm was not statistically different (an event rate of 4.53 vs. 4.32 per 1,000 person-years, respectively; hazard ratio, 0.96). “This demonstrated that the intensive lifestyle intervention in the LookAHEAD trial did not significantly modify the risk of heart failure,” Dr. Pandey said.
However, an adjusted analysis revealed that the risk of heart failure was 39% lower in the moderately fit group and 62% lower in the high fit group, compared with the low-fitness group. Among heart failure subtypes, the risk of heart failure with preserved ejection fraction (HFpEF) was 40% lower in the moderately fit group and 77% lower in the high-fitness group. On the other hand, baseline level of fitness level was not associated with risk of heart failure reduced ejection fraction (HFrEF) after the researchers adjusted for cardiovascular risk factors.
Next, Dr. Pandey and his colleagues used Cox modeling to examine the association of baseline and longitudinal changes in fitness and BMI with risk of heart failure. For change in fitness and BMI analysis, they used the 4-year follow-up data in 3,092 participants who underwent repeat fitness testing and had available data on BMI. They excluded patients who developed heart failure within the first 4 years of the study.
The mean age of the ancillary study population was about 60 years, and there was a lower proportion of women in the high fitness tertile (41%). The researchers observed a graded, inverse association between higher fitness levels and lower risk of heart failure such that increasing fitness from baseline was associated with a substantial decrease in the risk of heart failure. Specifically, a 10% decline in fitness over the 4 years of follow-up was associated with a 11% increase in the overall risk of heart failure (HR, 1.11). “This was largely consistent with the two heart failure subtypes,” he said. Similarly, a 10% increase in BMI over the 4 years of follow-up was associated with a 25% increase in the overall risk of heart failure (HR 1.25). On the other hand, a 10% decrease BMI was associated with a 20% decrease in the risk of heart failure (HR .80). This was also largely consistent for both heart failure subtypes. According to co-lead investigator Kershaw Patel, MD, “these findings suggest that therapies targeting large and sustained improvements in fitness and weight loss may modify the risk of heart failure among patients with diabetes.”
“Lower fitness at baseline was more strongly associated with the risk of HFpEF vs. HFrEF, and greater weight loss over follow-up is associated with a lower risk of heart failure independent of changes in other risk factors,” Dr. Pandey concluded at the meeting, which was sponsored by the American Heart Association.
In an interview, session moderator Joshua J. Joseph, MD, said that it remains unclear what type of setting is ideal for carrying out cardiorespiratory fitness in this patient population. “What is the supervision needed for that to occur?” asked Dr. Joseph, of The Ohio State University, Columbus. “Can patients do this on their own, or do they need guidance? What is the best approach? That’s the question we all have to answer individually in our own communities.”
Dr. Pandey reported having no disclosures.
SOURCE: Pandey A. Epi/Lifestyle 2020, Abstract 16.
PHOENIX – Lower baseline fitness and greater decline in fitness over time are independently associated with a higher risk of heart failure in patients with diabetes, results from a large analysis showed.
“Diabetes is an important risk factor for the development of heart failure, and the diagnosis of diabetes in newly diagnosed cases of heart failure has been increasing,” Ambarish Pandey, MD, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting. “Type 2 diabetes is associated with increased burden of traditional risk factors such as hypertension, kidney dysfunction, and dyslipidemia – each of which in turn increase the risk of both atherothrombotic disease as well as heart failure.”
Recent data from the Swedish National Diabetes Register have shown that optimal management of these risk factors in patients with type 2 diabetes can actually mitigate the risk of atherosclerotic events such as acute MI, but the risk of heart failure does not significantly lower with optimal management of these traditional cardiovascular risk factors (N Engl J Med. 2018;379:633-44). “These findings highlight that novel approaches that go beyond just managing traditional cardiovascular risk factors are needed for prevention of heart failure in patients with type 2 diabetes,” said Dr. Pandey, of the division of cardiology at the University of Texas Southwestern Medical Center, Dallas. “Our group has demonstrated that physical inactivity and low levels of fitness are associated with a higher risk of heart failure. We have also shown that the protective effect of physical activity against heart failure risk is stronger against heart failure with preserved ejection fraction, which is a subtype of heart failure that is increasing in prevalence and has no effective therapies.”
Dr. Pandey and his colleagues set out to test the research hypothesis that fitness decline and increases in body mass index over time are significantly associated with a higher risk of heart failure. To do this, they drew from the LookAHEAD Trial, a multicenter analysis of 5,145 overweight or obese patients with type 2 diabetes who were randomized to an intensive lifestyle intervention or to usual care. The intervention consisted of a caloric intake goal of 1,200 to 1,800 kcal per day and engaging in at least 175 minutes per week of physical activity. Participants were stratified into one of three fitness group levels: low, moderate, and high, from 5 metabolic equivalents (METs) in the lowest fitness tertile to 9 METs in the highest fitness tertile. The primary outcome of the trial was adverse cardiovascular events. The intervention was implemented for almost 10 years, and patients were followed for up to 12 years from baseline.
The heart failure outcomes were not systematically adjudicated in the primary LookAHEAD trial, so Dr. Pandey and colleagues conducted an ancillary study of all incident hospitalizations in the study and followed them for 2 additional years. Overall, the researchers identified 257 incident heart failure events. The cumulative incidence of heart failure for the usual care versus the intensive lifestyle intervention arm was not statistically different (an event rate of 4.53 vs. 4.32 per 1,000 person-years, respectively; hazard ratio, 0.96). “This demonstrated that the intensive lifestyle intervention in the LookAHEAD trial did not significantly modify the risk of heart failure,” Dr. Pandey said.
However, an adjusted analysis revealed that the risk of heart failure was 39% lower in the moderately fit group and 62% lower in the high fit group, compared with the low-fitness group. Among heart failure subtypes, the risk of heart failure with preserved ejection fraction (HFpEF) was 40% lower in the moderately fit group and 77% lower in the high-fitness group. On the other hand, baseline level of fitness level was not associated with risk of heart failure reduced ejection fraction (HFrEF) after the researchers adjusted for cardiovascular risk factors.
Next, Dr. Pandey and his colleagues used Cox modeling to examine the association of baseline and longitudinal changes in fitness and BMI with risk of heart failure. For change in fitness and BMI analysis, they used the 4-year follow-up data in 3,092 participants who underwent repeat fitness testing and had available data on BMI. They excluded patients who developed heart failure within the first 4 years of the study.
The mean age of the ancillary study population was about 60 years, and there was a lower proportion of women in the high fitness tertile (41%). The researchers observed a graded, inverse association between higher fitness levels and lower risk of heart failure such that increasing fitness from baseline was associated with a substantial decrease in the risk of heart failure. Specifically, a 10% decline in fitness over the 4 years of follow-up was associated with a 11% increase in the overall risk of heart failure (HR, 1.11). “This was largely consistent with the two heart failure subtypes,” he said. Similarly, a 10% increase in BMI over the 4 years of follow-up was associated with a 25% increase in the overall risk of heart failure (HR 1.25). On the other hand, a 10% decrease BMI was associated with a 20% decrease in the risk of heart failure (HR .80). This was also largely consistent for both heart failure subtypes. According to co-lead investigator Kershaw Patel, MD, “these findings suggest that therapies targeting large and sustained improvements in fitness and weight loss may modify the risk of heart failure among patients with diabetes.”
“Lower fitness at baseline was more strongly associated with the risk of HFpEF vs. HFrEF, and greater weight loss over follow-up is associated with a lower risk of heart failure independent of changes in other risk factors,” Dr. Pandey concluded at the meeting, which was sponsored by the American Heart Association.
In an interview, session moderator Joshua J. Joseph, MD, said that it remains unclear what type of setting is ideal for carrying out cardiorespiratory fitness in this patient population. “What is the supervision needed for that to occur?” asked Dr. Joseph, of The Ohio State University, Columbus. “Can patients do this on their own, or do they need guidance? What is the best approach? That’s the question we all have to answer individually in our own communities.”
Dr. Pandey reported having no disclosures.
SOURCE: Pandey A. Epi/Lifestyle 2020, Abstract 16.
REPORTING FROM EPI/LIFESTYLE 2020
Cardiovascular risk varies between black ethnic subgroups
PHOENIX, ARIZ. – Cardiovascular disease risk factors differ significantly between three black ethnic subgroups in the United States, compared with whites, results from a large, long-term cross-sectional study show.
“Race alone does not account for health disparities in CVD risk factors,” lead author Diana Baptiste, DNP, RN, CNE, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting. “We must consider the environmental, psychosocial, and social factors that may play a larger role in CVD risk among these populations.”
Dr. Baptiste, of the Johns Hopkins University School of Nursing Center for Cardiovascular and Chronic Care in Baltimore, noted that blacks bear a disproportionately greater burden of CVD than that of any other racial group. “Blacks living in the U.S. are not monolithic and include different ethnic subgroups: African Americans, Afro-Caribbeans, defined as black persons who are born in the Caribbean islands, and African immigrants, defined as black persons who are born in Africa,” she said. “It is unclear how Afro-Caribbeans and African immigrants compare to African Americans and whites with regard to CVD risk factors.”
To examine trends in CVD risk factors among the three black ethnic subgroups compared with whites, she and her colleagues performed a cross-sectional analysis of 452,997 adults who participated in the 2010-2018 National Health Interview Survey (NHIS). Of these, 82% were white and 18% were black. Among blacks, 89% were African Americans, 6% were Afro-Caribbeans, and 5% were African immigrants. Outcomes of interest were four self-reported CVD risk factors: hypertension, diabetes, overweight/obesity, and smoking. The researchers used generalized linear models with Poisson distribution to calculate predictive probabilities of CVD risk factors, adjusted for age and sex.
Dr. Baptiste reported that African immigrants represented the youngest subgroup, with an average age of 41 years, compared with an average age of 50 among whites. They were also less likely to have health insurance (76%), compared with Afro-Caribbeans (81%), African Americans (83%), and whites (91%; P < .001). Disparities were observed in the proportion of individuals living below the poverty level. This was led by African Americans (24%), followed by African immigrants (22%), Afro-Caribbeans (18%), and whites (9%).
African immigrants were most likely to be college educated (36%), compared with whites (32%), Afro-Caribbeans (23%), and African Americans (17%; P =.001). In addition, only 33% of African Americans were married, compared with more than 50% of participants in the other ethnic groups.
African Americans had the highest prevalence of hypertension over the time period (from 44% in 2010 to 42% in 2018), while African immigrants had the lowest (from 19% to 17%). African Americans also had the highest prevalence of diabetes over the time period (from 14% to 15%), while African immigrants had the lowest (from 9% to 7%). The prevalence of overweight and obesity was highest among African Americans (from 74% to 76%), while African immigrants had the lowest (63% to 60%). Finally, smoking prevalence was highest in whites and African Americans compared with African immigrants and Afro-Caribbeans, but the prevalence decreased significantly between 2010 and 2018 (P for trend < .001).
In an interview, one of the meeting session’s moderators, Sherry-Ann Brown, MD, PhD, said that the study’s findings underscore the importance of heterogeneity when counseling patients about CVD risk factors. “Everybody comes from a different cultural background,” said Dr. Brown, a cardiologist and physician scientist at Mayo Clinic, Rochester, Minn. “Cultural backgrounds have an impact on when people eat, how they eat, who they eat with, when they exercise, and whether obesity is valued or not. It’s important to recognize that those cultural underpinnings can contribute to heterogeneity. Other factors – whether they are psychosocial or socioeconomic or environmental – also contribute.”
Strengths of the study, Dr. Baptiste said, included the use of a large, nationally representative dataset. Limitations included its cross-sectional design and the National Health Interview Survey’s reliance on self-reported data. “There were also small sample sizes for African immigrants and Afro-Caribbeans,” she said.
The study was supported by Johns Hopkins University School of Nursing Center for Cardiovascular and Chronic Care. Dr. Baptiste reported having no financial disclosures.
The meeting was sponsored by the American Heart Association.
SOURCE: Baptiste D et al. EPI/Lifestyle 2020, Session 4, Abstract 8.
PHOENIX, ARIZ. – Cardiovascular disease risk factors differ significantly between three black ethnic subgroups in the United States, compared with whites, results from a large, long-term cross-sectional study show.
“Race alone does not account for health disparities in CVD risk factors,” lead author Diana Baptiste, DNP, RN, CNE, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting. “We must consider the environmental, psychosocial, and social factors that may play a larger role in CVD risk among these populations.”
Dr. Baptiste, of the Johns Hopkins University School of Nursing Center for Cardiovascular and Chronic Care in Baltimore, noted that blacks bear a disproportionately greater burden of CVD than that of any other racial group. “Blacks living in the U.S. are not monolithic and include different ethnic subgroups: African Americans, Afro-Caribbeans, defined as black persons who are born in the Caribbean islands, and African immigrants, defined as black persons who are born in Africa,” she said. “It is unclear how Afro-Caribbeans and African immigrants compare to African Americans and whites with regard to CVD risk factors.”
To examine trends in CVD risk factors among the three black ethnic subgroups compared with whites, she and her colleagues performed a cross-sectional analysis of 452,997 adults who participated in the 2010-2018 National Health Interview Survey (NHIS). Of these, 82% were white and 18% were black. Among blacks, 89% were African Americans, 6% were Afro-Caribbeans, and 5% were African immigrants. Outcomes of interest were four self-reported CVD risk factors: hypertension, diabetes, overweight/obesity, and smoking. The researchers used generalized linear models with Poisson distribution to calculate predictive probabilities of CVD risk factors, adjusted for age and sex.
Dr. Baptiste reported that African immigrants represented the youngest subgroup, with an average age of 41 years, compared with an average age of 50 among whites. They were also less likely to have health insurance (76%), compared with Afro-Caribbeans (81%), African Americans (83%), and whites (91%; P < .001). Disparities were observed in the proportion of individuals living below the poverty level. This was led by African Americans (24%), followed by African immigrants (22%), Afro-Caribbeans (18%), and whites (9%).
African immigrants were most likely to be college educated (36%), compared with whites (32%), Afro-Caribbeans (23%), and African Americans (17%; P =.001). In addition, only 33% of African Americans were married, compared with more than 50% of participants in the other ethnic groups.
African Americans had the highest prevalence of hypertension over the time period (from 44% in 2010 to 42% in 2018), while African immigrants had the lowest (from 19% to 17%). African Americans also had the highest prevalence of diabetes over the time period (from 14% to 15%), while African immigrants had the lowest (from 9% to 7%). The prevalence of overweight and obesity was highest among African Americans (from 74% to 76%), while African immigrants had the lowest (63% to 60%). Finally, smoking prevalence was highest in whites and African Americans compared with African immigrants and Afro-Caribbeans, but the prevalence decreased significantly between 2010 and 2018 (P for trend < .001).
In an interview, one of the meeting session’s moderators, Sherry-Ann Brown, MD, PhD, said that the study’s findings underscore the importance of heterogeneity when counseling patients about CVD risk factors. “Everybody comes from a different cultural background,” said Dr. Brown, a cardiologist and physician scientist at Mayo Clinic, Rochester, Minn. “Cultural backgrounds have an impact on when people eat, how they eat, who they eat with, when they exercise, and whether obesity is valued or not. It’s important to recognize that those cultural underpinnings can contribute to heterogeneity. Other factors – whether they are psychosocial or socioeconomic or environmental – also contribute.”
Strengths of the study, Dr. Baptiste said, included the use of a large, nationally representative dataset. Limitations included its cross-sectional design and the National Health Interview Survey’s reliance on self-reported data. “There were also small sample sizes for African immigrants and Afro-Caribbeans,” she said.
The study was supported by Johns Hopkins University School of Nursing Center for Cardiovascular and Chronic Care. Dr. Baptiste reported having no financial disclosures.
The meeting was sponsored by the American Heart Association.
SOURCE: Baptiste D et al. EPI/Lifestyle 2020, Session 4, Abstract 8.
PHOENIX, ARIZ. – Cardiovascular disease risk factors differ significantly between three black ethnic subgroups in the United States, compared with whites, results from a large, long-term cross-sectional study show.
“Race alone does not account for health disparities in CVD risk factors,” lead author Diana Baptiste, DNP, RN, CNE, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting. “We must consider the environmental, psychosocial, and social factors that may play a larger role in CVD risk among these populations.”
Dr. Baptiste, of the Johns Hopkins University School of Nursing Center for Cardiovascular and Chronic Care in Baltimore, noted that blacks bear a disproportionately greater burden of CVD than that of any other racial group. “Blacks living in the U.S. are not monolithic and include different ethnic subgroups: African Americans, Afro-Caribbeans, defined as black persons who are born in the Caribbean islands, and African immigrants, defined as black persons who are born in Africa,” she said. “It is unclear how Afro-Caribbeans and African immigrants compare to African Americans and whites with regard to CVD risk factors.”
To examine trends in CVD risk factors among the three black ethnic subgroups compared with whites, she and her colleagues performed a cross-sectional analysis of 452,997 adults who participated in the 2010-2018 National Health Interview Survey (NHIS). Of these, 82% were white and 18% were black. Among blacks, 89% were African Americans, 6% were Afro-Caribbeans, and 5% were African immigrants. Outcomes of interest were four self-reported CVD risk factors: hypertension, diabetes, overweight/obesity, and smoking. The researchers used generalized linear models with Poisson distribution to calculate predictive probabilities of CVD risk factors, adjusted for age and sex.
Dr. Baptiste reported that African immigrants represented the youngest subgroup, with an average age of 41 years, compared with an average age of 50 among whites. They were also less likely to have health insurance (76%), compared with Afro-Caribbeans (81%), African Americans (83%), and whites (91%; P < .001). Disparities were observed in the proportion of individuals living below the poverty level. This was led by African Americans (24%), followed by African immigrants (22%), Afro-Caribbeans (18%), and whites (9%).
African immigrants were most likely to be college educated (36%), compared with whites (32%), Afro-Caribbeans (23%), and African Americans (17%; P =.001). In addition, only 33% of African Americans were married, compared with more than 50% of participants in the other ethnic groups.
African Americans had the highest prevalence of hypertension over the time period (from 44% in 2010 to 42% in 2018), while African immigrants had the lowest (from 19% to 17%). African Americans also had the highest prevalence of diabetes over the time period (from 14% to 15%), while African immigrants had the lowest (from 9% to 7%). The prevalence of overweight and obesity was highest among African Americans (from 74% to 76%), while African immigrants had the lowest (63% to 60%). Finally, smoking prevalence was highest in whites and African Americans compared with African immigrants and Afro-Caribbeans, but the prevalence decreased significantly between 2010 and 2018 (P for trend < .001).
In an interview, one of the meeting session’s moderators, Sherry-Ann Brown, MD, PhD, said that the study’s findings underscore the importance of heterogeneity when counseling patients about CVD risk factors. “Everybody comes from a different cultural background,” said Dr. Brown, a cardiologist and physician scientist at Mayo Clinic, Rochester, Minn. “Cultural backgrounds have an impact on when people eat, how they eat, who they eat with, when they exercise, and whether obesity is valued or not. It’s important to recognize that those cultural underpinnings can contribute to heterogeneity. Other factors – whether they are psychosocial or socioeconomic or environmental – also contribute.”
Strengths of the study, Dr. Baptiste said, included the use of a large, nationally representative dataset. Limitations included its cross-sectional design and the National Health Interview Survey’s reliance on self-reported data. “There were also small sample sizes for African immigrants and Afro-Caribbeans,” she said.
The study was supported by Johns Hopkins University School of Nursing Center for Cardiovascular and Chronic Care. Dr. Baptiste reported having no financial disclosures.
The meeting was sponsored by the American Heart Association.
SOURCE: Baptiste D et al. EPI/Lifestyle 2020, Session 4, Abstract 8.
REPORTING FROM EPI/LIFESTYLE 2020
Study identifies two distinct type 1 diabetes ‘endotypes’
Two histologically distinct “endotypes” of type 1 diabetes, T1DE1 and T1DE2, have been identified in children based on their age at diagnosis
The findings were published online March 15 in Diabetologia by Pia Leete, PhD, of the Institute of Biomedical and Clinical Science, University of Exeter Medical School, UK, and colleagues.
The results suggest that the immune attack is far more aggressive and the islets more inflamed in the younger-onset group (T1DE1) and less intense in the older-onset group (T1DE2), the authors explain.
“We’re extremely excited to find evidence that type 1 diabetes is two separate conditions: T1DE1 and T1DE2. The significance of this could be enormous in helping us to understand what causes the illness and in unlocking avenues to prevent future generations of children from getting type 1 diabetes,” said senior author Noel G. Morgan, PhD, also of the University of Exeter, in a statement.
Morgan added that the discovery “might also lead to new treatments if we can find ways to reactivate dormant insulin-producing cells in the older age group. This would be a significant step towards the holy grail to find a cure for some people.”
Endotypes can inform immune interventions
The study involved an immunohistological analysis of proinsulin and insulin distribution in the islets of pancreas samples recovered from 19 youth who died soon after (<2 years) onset of type 1 diabetes and from 13 with onset more than 5 years prior to harvesting. Those results were compared with C-peptide and proinsulin measurements in 171 living individuals with type 1 diabetes of longer than 5 years duration.
The Exeter team has previously reported that the immune cell profiles in the inflamed islets of children younger than 7 years of age soon after the diagnosis of type 1 diabetes seem to be distinctly different for those in children aged 13 and older at diagnosis. The younger group at diagnosis (termed “T1DE1”) retained a lower proportion of insulin-containing islets than did the older-onset group (“T1DE2”).
Those aged 7-12 at diagnosis could belong to either group, but there was no continuum. Rather, they appeared to align distinctly with one or the other “endotype,” Leete and colleagues say.
In the new analysis, proinsulin processing was aberrant to a much greater degree among children diagnosed with type 1 diabetes prior to age 7 years than among those diagnosed after age 12 years, with the profiles of proinsulin processing correlating with the previously defined immune cell profiles.
For those aged 7-12, the proinsulin distribution in islets directly correlated with their immune phenotypes, either T1DE1 or T1DE2.
And among the living patients, circulating proinsulin:C-peptide ratios were elevated in the <7-year onset group compared with the ≥13-year group, even 5 years after diagnosis.
“Together, these data imply that, when considered alongside age at diagnosis, measurement of the ratio of proinsulin to C-peptide may represent a convenient biomarker to distinguish the endotypes defined here,” Leete and colleagues say.
The two-endotype proposal isn’t meant to suggest that “a simple dichotomy will ultimately be sufficient to account for the entire heterogeneity seen in people developing type 1 diabetes,” the authors stress. Rather, additional endotypes will likely be defined as more variables are considered.
They write, “Recognition of such differences should inform the design of future immunotherapeutic interventions designed to arrest disease progression.”
The research was sponsored by Diabetes UK and JDRF.
This article first appeared on Medscape.com.
Two histologically distinct “endotypes” of type 1 diabetes, T1DE1 and T1DE2, have been identified in children based on their age at diagnosis
The findings were published online March 15 in Diabetologia by Pia Leete, PhD, of the Institute of Biomedical and Clinical Science, University of Exeter Medical School, UK, and colleagues.
The results suggest that the immune attack is far more aggressive and the islets more inflamed in the younger-onset group (T1DE1) and less intense in the older-onset group (T1DE2), the authors explain.
“We’re extremely excited to find evidence that type 1 diabetes is two separate conditions: T1DE1 and T1DE2. The significance of this could be enormous in helping us to understand what causes the illness and in unlocking avenues to prevent future generations of children from getting type 1 diabetes,” said senior author Noel G. Morgan, PhD, also of the University of Exeter, in a statement.
Morgan added that the discovery “might also lead to new treatments if we can find ways to reactivate dormant insulin-producing cells in the older age group. This would be a significant step towards the holy grail to find a cure for some people.”
Endotypes can inform immune interventions
The study involved an immunohistological analysis of proinsulin and insulin distribution in the islets of pancreas samples recovered from 19 youth who died soon after (<2 years) onset of type 1 diabetes and from 13 with onset more than 5 years prior to harvesting. Those results were compared with C-peptide and proinsulin measurements in 171 living individuals with type 1 diabetes of longer than 5 years duration.
The Exeter team has previously reported that the immune cell profiles in the inflamed islets of children younger than 7 years of age soon after the diagnosis of type 1 diabetes seem to be distinctly different for those in children aged 13 and older at diagnosis. The younger group at diagnosis (termed “T1DE1”) retained a lower proportion of insulin-containing islets than did the older-onset group (“T1DE2”).
Those aged 7-12 at diagnosis could belong to either group, but there was no continuum. Rather, they appeared to align distinctly with one or the other “endotype,” Leete and colleagues say.
In the new analysis, proinsulin processing was aberrant to a much greater degree among children diagnosed with type 1 diabetes prior to age 7 years than among those diagnosed after age 12 years, with the profiles of proinsulin processing correlating with the previously defined immune cell profiles.
For those aged 7-12, the proinsulin distribution in islets directly correlated with their immune phenotypes, either T1DE1 or T1DE2.
And among the living patients, circulating proinsulin:C-peptide ratios were elevated in the <7-year onset group compared with the ≥13-year group, even 5 years after diagnosis.
“Together, these data imply that, when considered alongside age at diagnosis, measurement of the ratio of proinsulin to C-peptide may represent a convenient biomarker to distinguish the endotypes defined here,” Leete and colleagues say.
The two-endotype proposal isn’t meant to suggest that “a simple dichotomy will ultimately be sufficient to account for the entire heterogeneity seen in people developing type 1 diabetes,” the authors stress. Rather, additional endotypes will likely be defined as more variables are considered.
They write, “Recognition of such differences should inform the design of future immunotherapeutic interventions designed to arrest disease progression.”
The research was sponsored by Diabetes UK and JDRF.
This article first appeared on Medscape.com.
Two histologically distinct “endotypes” of type 1 diabetes, T1DE1 and T1DE2, have been identified in children based on their age at diagnosis
The findings were published online March 15 in Diabetologia by Pia Leete, PhD, of the Institute of Biomedical and Clinical Science, University of Exeter Medical School, UK, and colleagues.
The results suggest that the immune attack is far more aggressive and the islets more inflamed in the younger-onset group (T1DE1) and less intense in the older-onset group (T1DE2), the authors explain.
“We’re extremely excited to find evidence that type 1 diabetes is two separate conditions: T1DE1 and T1DE2. The significance of this could be enormous in helping us to understand what causes the illness and in unlocking avenues to prevent future generations of children from getting type 1 diabetes,” said senior author Noel G. Morgan, PhD, also of the University of Exeter, in a statement.
Morgan added that the discovery “might also lead to new treatments if we can find ways to reactivate dormant insulin-producing cells in the older age group. This would be a significant step towards the holy grail to find a cure for some people.”
Endotypes can inform immune interventions
The study involved an immunohistological analysis of proinsulin and insulin distribution in the islets of pancreas samples recovered from 19 youth who died soon after (<2 years) onset of type 1 diabetes and from 13 with onset more than 5 years prior to harvesting. Those results were compared with C-peptide and proinsulin measurements in 171 living individuals with type 1 diabetes of longer than 5 years duration.
The Exeter team has previously reported that the immune cell profiles in the inflamed islets of children younger than 7 years of age soon after the diagnosis of type 1 diabetes seem to be distinctly different for those in children aged 13 and older at diagnosis. The younger group at diagnosis (termed “T1DE1”) retained a lower proportion of insulin-containing islets than did the older-onset group (“T1DE2”).
Those aged 7-12 at diagnosis could belong to either group, but there was no continuum. Rather, they appeared to align distinctly with one or the other “endotype,” Leete and colleagues say.
In the new analysis, proinsulin processing was aberrant to a much greater degree among children diagnosed with type 1 diabetes prior to age 7 years than among those diagnosed after age 12 years, with the profiles of proinsulin processing correlating with the previously defined immune cell profiles.
For those aged 7-12, the proinsulin distribution in islets directly correlated with their immune phenotypes, either T1DE1 or T1DE2.
And among the living patients, circulating proinsulin:C-peptide ratios were elevated in the <7-year onset group compared with the ≥13-year group, even 5 years after diagnosis.
“Together, these data imply that, when considered alongside age at diagnosis, measurement of the ratio of proinsulin to C-peptide may represent a convenient biomarker to distinguish the endotypes defined here,” Leete and colleagues say.
The two-endotype proposal isn’t meant to suggest that “a simple dichotomy will ultimately be sufficient to account for the entire heterogeneity seen in people developing type 1 diabetes,” the authors stress. Rather, additional endotypes will likely be defined as more variables are considered.
They write, “Recognition of such differences should inform the design of future immunotherapeutic interventions designed to arrest disease progression.”
The research was sponsored by Diabetes UK and JDRF.
This article first appeared on Medscape.com.
FDA advises stopping SGLT2 inhibitor treatment prior to surgery
The new changes affect canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin, and were made because surgery may put patients being treated with SGLT2 inhibitors at a higher risk of ketoacidosis. Canagliflozin, dapagliflozin, and empagliflozin should be discontinued 3 days before scheduled surgery, and ertugliflozin should be stopped at least 4 days before, the agency noted in a press release. Blood glucose should be monitored after drug discontinuation and appropriately managed before surgery.
“The SGLT2 inhibitor may be restarted once the patient’s oral intake is back to baseline and any other risk factors for ketoacidosis are resolved,” the agency added.
SGLT2 inhibitors lower blood sugar by causing the kidney to remove sugar from the body through urine. Side effects for the drugs vary, but include urinary tract infections and genital mycotic infection. Patients with severe renal impairment or end-stage renal disease, who are on dialysis treatment, or who have a known hypersensitivity to the medication should not take SGLT2 inhibitors, the FDA said.
The new changes affect canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin, and were made because surgery may put patients being treated with SGLT2 inhibitors at a higher risk of ketoacidosis. Canagliflozin, dapagliflozin, and empagliflozin should be discontinued 3 days before scheduled surgery, and ertugliflozin should be stopped at least 4 days before, the agency noted in a press release. Blood glucose should be monitored after drug discontinuation and appropriately managed before surgery.
“The SGLT2 inhibitor may be restarted once the patient’s oral intake is back to baseline and any other risk factors for ketoacidosis are resolved,” the agency added.
SGLT2 inhibitors lower blood sugar by causing the kidney to remove sugar from the body through urine. Side effects for the drugs vary, but include urinary tract infections and genital mycotic infection. Patients with severe renal impairment or end-stage renal disease, who are on dialysis treatment, or who have a known hypersensitivity to the medication should not take SGLT2 inhibitors, the FDA said.
The new changes affect canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin, and were made because surgery may put patients being treated with SGLT2 inhibitors at a higher risk of ketoacidosis. Canagliflozin, dapagliflozin, and empagliflozin should be discontinued 3 days before scheduled surgery, and ertugliflozin should be stopped at least 4 days before, the agency noted in a press release. Blood glucose should be monitored after drug discontinuation and appropriately managed before surgery.
“The SGLT2 inhibitor may be restarted once the patient’s oral intake is back to baseline and any other risk factors for ketoacidosis are resolved,” the agency added.
SGLT2 inhibitors lower blood sugar by causing the kidney to remove sugar from the body through urine. Side effects for the drugs vary, but include urinary tract infections and genital mycotic infection. Patients with severe renal impairment or end-stage renal disease, who are on dialysis treatment, or who have a known hypersensitivity to the medication should not take SGLT2 inhibitors, the FDA said.



