User login
COVID-19 cases highlight longstanding racial disparities in health care
African Americans are overrepresented among patients who have died as a result of the COVID-19 pandemic, but the current crisis puts a spotlight on long-standing racial disparities in health care and health access in the United States, according to David R. Williams, PhD, a professor of public health at the Harvard T.H. Chan School of Public Health in Boston.
Dr. Williams, a social scientist specializing in the link between race and health, is a professor of African and African American Studies and of Sociology at Harvard. He spoke on the topic of racial disparities amid the COVID-19 pandemic in a teleconference sponsored by the Robert Wood Johnson Foundation.
“Many Americans are shocked” by the higher mortality rates among African American COVID-19 patients, said Dr. Williams. However, data from decades of research show that “black people in America live sicker and shorter lives,” he said.
Keys to the increased mortality among African Americans include an increased prevalence of risk factors, increased risk for exposure to the virus because of socioeconomic factors, and less access to health care if they do become ill, he said.
Many minority individuals work outside the home in areas deemed essential during the pandemic, such as transit, delivery, maintenance, cleaning, and in businesses such as grocery stores, although in general “race continues to matter for health at every level of income and education,” Dr. Williams said.
In addition, social distance guidelines are not realistic for many people in high-density, low-income areas, who often live in shared, multigenerational housing, he said.
Data show that individuals with chronic conditions such as diabetes and cardiovascular disease are more likely to die as a result of COVID-19, and minority populations are more likely to develop these conditions at younger ages, Dr. Williams noted. Access to health care also plays a role. Many minority individuals of lower socioeconomic status are less likely to have health insurance, or if they do, may have Medicaid, which is not consistently accepted, he said. Also, some low-income neighborhoods lack convenient access to primary care and thus to screening services, he noted.
Dr. Williams said the COVID-19 pandemic could serve as an opportunity to examine and improve health care services for underserved communities. In the short term, “we need to collect data so we can see patterns” and address pressing needs, he said, but long-term goals should “prioritize investments that would create healthy homes and communities,” he emphasized.
A recent study from the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report cited COVID-NET (the COVID-19 Associated Hospitalization Surveillance Network) as showing that, in their catchment population, “approximately 59% of residents are white, 18% are black, and 14% are Hispanic; however, among 580 hospitalized COVID-19 patients with race/ethnicity data, approximately 45% were white, 33% were black, and 8% were Hispanic, suggesting that black populations might be disproportionately affected by COVID-19,” the researchers said.
“These findings, including the potential impact of both sex and race on COVID-19–associated hospitalization rates, need to be confirmed with additional data,” according to the report.
Collecting racial/ethnic information is not always feasible on the front lines, and many areas still face shortages of ventilators and protective equipment, said Dr. Williams.
“I want to salute the providers on the front lines of this pandemic, many putting their own lives at risk, I want to acknowledge the good that they are doing,” Dr. Williams emphasized. He noted that all of us, himself included, may have conscious or unconscious stereotypes, but the key is to acknowledge the potential for these thoughts and feelings and continue to provide the best care.
Clyde W. Yancy, MD, of Northwestern University in Chicago, expressed similar concerns about disparity in COVID-19 cases in an editorial published on April 15 in JAMA.
“Researchers have emphasized older age, male sex, hypertension, diabetes, obesity, concomitant cardiovascular diseases (including coronary artery disease and heart failure), and myocardial injury as important risk factors associated with worse outcomes,” wrote Dr. Yancy. However, evidence also suggests that “persons who are African American or black are contracting SARS-CoV-2 at higher rates and are more likely to die,” he said.
“Why is this uniquely important to me? I am an academic cardiologist; I study health care disparities; and I am a black man,” he wrote.
“Even though these data are preliminary and further study is warranted, the pattern is irrefutable: Underrepresented minorities are developing COVID-19 infection more frequently and dying disproportionately,” said Dr. Yancy.
Dr. Williams’ and Dr. Yancy’s comments were supported by an analysis of COVID-19 patient data from several areas of the country conducted by the Washington Post. In that analysis, data showed that several counties with a majority black population showed three times the rate of COVID-19 infections and approximately six times as many deaths compared with counties with a majority of white residents.
“The U.S. has needed a trigger to fully address health care disparities; COVID-19 may be that bellwether event,” said Dr. Yancy. “Certainly, within the broad and powerful economic and legislative engines of the US, there is room to definitively address a scourge even worse than COVID-19: health care disparities. It only takes will. It is time to end the refrain,” he said.
Dr. Williams had no financial conflicts to disclose. Dr. Yancy had no financial conflicts to disclose.
SOURCES: Yancy CW. JAMA 2020 Apr 15. doi: 10.1001/jama.2020.6548Garg S et al. MMWR Morb Mortal Wkly Rep 2020 Apr 8;69:458-64.
Thebault R et al. The coronavirus is infecting and killing black Americans at an alarmingly high rate. Washington Post. 2020 Apr 7.
African Americans are overrepresented among patients who have died as a result of the COVID-19 pandemic, but the current crisis puts a spotlight on long-standing racial disparities in health care and health access in the United States, according to David R. Williams, PhD, a professor of public health at the Harvard T.H. Chan School of Public Health in Boston.
Dr. Williams, a social scientist specializing in the link between race and health, is a professor of African and African American Studies and of Sociology at Harvard. He spoke on the topic of racial disparities amid the COVID-19 pandemic in a teleconference sponsored by the Robert Wood Johnson Foundation.
“Many Americans are shocked” by the higher mortality rates among African American COVID-19 patients, said Dr. Williams. However, data from decades of research show that “black people in America live sicker and shorter lives,” he said.
Keys to the increased mortality among African Americans include an increased prevalence of risk factors, increased risk for exposure to the virus because of socioeconomic factors, and less access to health care if they do become ill, he said.
Many minority individuals work outside the home in areas deemed essential during the pandemic, such as transit, delivery, maintenance, cleaning, and in businesses such as grocery stores, although in general “race continues to matter for health at every level of income and education,” Dr. Williams said.
In addition, social distance guidelines are not realistic for many people in high-density, low-income areas, who often live in shared, multigenerational housing, he said.
Data show that individuals with chronic conditions such as diabetes and cardiovascular disease are more likely to die as a result of COVID-19, and minority populations are more likely to develop these conditions at younger ages, Dr. Williams noted. Access to health care also plays a role. Many minority individuals of lower socioeconomic status are less likely to have health insurance, or if they do, may have Medicaid, which is not consistently accepted, he said. Also, some low-income neighborhoods lack convenient access to primary care and thus to screening services, he noted.
Dr. Williams said the COVID-19 pandemic could serve as an opportunity to examine and improve health care services for underserved communities. In the short term, “we need to collect data so we can see patterns” and address pressing needs, he said, but long-term goals should “prioritize investments that would create healthy homes and communities,” he emphasized.
A recent study from the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report cited COVID-NET (the COVID-19 Associated Hospitalization Surveillance Network) as showing that, in their catchment population, “approximately 59% of residents are white, 18% are black, and 14% are Hispanic; however, among 580 hospitalized COVID-19 patients with race/ethnicity data, approximately 45% were white, 33% were black, and 8% were Hispanic, suggesting that black populations might be disproportionately affected by COVID-19,” the researchers said.
“These findings, including the potential impact of both sex and race on COVID-19–associated hospitalization rates, need to be confirmed with additional data,” according to the report.
Collecting racial/ethnic information is not always feasible on the front lines, and many areas still face shortages of ventilators and protective equipment, said Dr. Williams.
“I want to salute the providers on the front lines of this pandemic, many putting their own lives at risk, I want to acknowledge the good that they are doing,” Dr. Williams emphasized. He noted that all of us, himself included, may have conscious or unconscious stereotypes, but the key is to acknowledge the potential for these thoughts and feelings and continue to provide the best care.
Clyde W. Yancy, MD, of Northwestern University in Chicago, expressed similar concerns about disparity in COVID-19 cases in an editorial published on April 15 in JAMA.
“Researchers have emphasized older age, male sex, hypertension, diabetes, obesity, concomitant cardiovascular diseases (including coronary artery disease and heart failure), and myocardial injury as important risk factors associated with worse outcomes,” wrote Dr. Yancy. However, evidence also suggests that “persons who are African American or black are contracting SARS-CoV-2 at higher rates and are more likely to die,” he said.
“Why is this uniquely important to me? I am an academic cardiologist; I study health care disparities; and I am a black man,” he wrote.
“Even though these data are preliminary and further study is warranted, the pattern is irrefutable: Underrepresented minorities are developing COVID-19 infection more frequently and dying disproportionately,” said Dr. Yancy.
Dr. Williams’ and Dr. Yancy’s comments were supported by an analysis of COVID-19 patient data from several areas of the country conducted by the Washington Post. In that analysis, data showed that several counties with a majority black population showed three times the rate of COVID-19 infections and approximately six times as many deaths compared with counties with a majority of white residents.
“The U.S. has needed a trigger to fully address health care disparities; COVID-19 may be that bellwether event,” said Dr. Yancy. “Certainly, within the broad and powerful economic and legislative engines of the US, there is room to definitively address a scourge even worse than COVID-19: health care disparities. It only takes will. It is time to end the refrain,” he said.
Dr. Williams had no financial conflicts to disclose. Dr. Yancy had no financial conflicts to disclose.
SOURCES: Yancy CW. JAMA 2020 Apr 15. doi: 10.1001/jama.2020.6548Garg S et al. MMWR Morb Mortal Wkly Rep 2020 Apr 8;69:458-64.
Thebault R et al. The coronavirus is infecting and killing black Americans at an alarmingly high rate. Washington Post. 2020 Apr 7.
African Americans are overrepresented among patients who have died as a result of the COVID-19 pandemic, but the current crisis puts a spotlight on long-standing racial disparities in health care and health access in the United States, according to David R. Williams, PhD, a professor of public health at the Harvard T.H. Chan School of Public Health in Boston.
Dr. Williams, a social scientist specializing in the link between race and health, is a professor of African and African American Studies and of Sociology at Harvard. He spoke on the topic of racial disparities amid the COVID-19 pandemic in a teleconference sponsored by the Robert Wood Johnson Foundation.
“Many Americans are shocked” by the higher mortality rates among African American COVID-19 patients, said Dr. Williams. However, data from decades of research show that “black people in America live sicker and shorter lives,” he said.
Keys to the increased mortality among African Americans include an increased prevalence of risk factors, increased risk for exposure to the virus because of socioeconomic factors, and less access to health care if they do become ill, he said.
Many minority individuals work outside the home in areas deemed essential during the pandemic, such as transit, delivery, maintenance, cleaning, and in businesses such as grocery stores, although in general “race continues to matter for health at every level of income and education,” Dr. Williams said.
In addition, social distance guidelines are not realistic for many people in high-density, low-income areas, who often live in shared, multigenerational housing, he said.
Data show that individuals with chronic conditions such as diabetes and cardiovascular disease are more likely to die as a result of COVID-19, and minority populations are more likely to develop these conditions at younger ages, Dr. Williams noted. Access to health care also plays a role. Many minority individuals of lower socioeconomic status are less likely to have health insurance, or if they do, may have Medicaid, which is not consistently accepted, he said. Also, some low-income neighborhoods lack convenient access to primary care and thus to screening services, he noted.
Dr. Williams said the COVID-19 pandemic could serve as an opportunity to examine and improve health care services for underserved communities. In the short term, “we need to collect data so we can see patterns” and address pressing needs, he said, but long-term goals should “prioritize investments that would create healthy homes and communities,” he emphasized.
A recent study from the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report cited COVID-NET (the COVID-19 Associated Hospitalization Surveillance Network) as showing that, in their catchment population, “approximately 59% of residents are white, 18% are black, and 14% are Hispanic; however, among 580 hospitalized COVID-19 patients with race/ethnicity data, approximately 45% were white, 33% were black, and 8% were Hispanic, suggesting that black populations might be disproportionately affected by COVID-19,” the researchers said.
“These findings, including the potential impact of both sex and race on COVID-19–associated hospitalization rates, need to be confirmed with additional data,” according to the report.
Collecting racial/ethnic information is not always feasible on the front lines, and many areas still face shortages of ventilators and protective equipment, said Dr. Williams.
“I want to salute the providers on the front lines of this pandemic, many putting their own lives at risk, I want to acknowledge the good that they are doing,” Dr. Williams emphasized. He noted that all of us, himself included, may have conscious or unconscious stereotypes, but the key is to acknowledge the potential for these thoughts and feelings and continue to provide the best care.
Clyde W. Yancy, MD, of Northwestern University in Chicago, expressed similar concerns about disparity in COVID-19 cases in an editorial published on April 15 in JAMA.
“Researchers have emphasized older age, male sex, hypertension, diabetes, obesity, concomitant cardiovascular diseases (including coronary artery disease and heart failure), and myocardial injury as important risk factors associated with worse outcomes,” wrote Dr. Yancy. However, evidence also suggests that “persons who are African American or black are contracting SARS-CoV-2 at higher rates and are more likely to die,” he said.
“Why is this uniquely important to me? I am an academic cardiologist; I study health care disparities; and I am a black man,” he wrote.
“Even though these data are preliminary and further study is warranted, the pattern is irrefutable: Underrepresented minorities are developing COVID-19 infection more frequently and dying disproportionately,” said Dr. Yancy.
Dr. Williams’ and Dr. Yancy’s comments were supported by an analysis of COVID-19 patient data from several areas of the country conducted by the Washington Post. In that analysis, data showed that several counties with a majority black population showed three times the rate of COVID-19 infections and approximately six times as many deaths compared with counties with a majority of white residents.
“The U.S. has needed a trigger to fully address health care disparities; COVID-19 may be that bellwether event,” said Dr. Yancy. “Certainly, within the broad and powerful economic and legislative engines of the US, there is room to definitively address a scourge even worse than COVID-19: health care disparities. It only takes will. It is time to end the refrain,” he said.
Dr. Williams had no financial conflicts to disclose. Dr. Yancy had no financial conflicts to disclose.
SOURCES: Yancy CW. JAMA 2020 Apr 15. doi: 10.1001/jama.2020.6548Garg S et al. MMWR Morb Mortal Wkly Rep 2020 Apr 8;69:458-64.
Thebault R et al. The coronavirus is infecting and killing black Americans at an alarmingly high rate. Washington Post. 2020 Apr 7.
FROM A TELECONFERENCE SPONSORED BY THE ROBERT WOOD JOHNSON FOUNDATION
Metformin use linked to improved surgery outcomes
Patients with type 2 diabetes who take metformin may have lower risk-adjusted mortality and readmission rates after surgery than do those who don’t take metformin, findings from a large retrospective cohort study suggest.
Of 10,088 individuals with diabetes who underwent a major surgery requiring hospital admission between January 1, 2010, and January 1, 2016, a total of 5,962 (59%) had received a prescription for metformin in the 180 days before surgery, and 5,460 of those patients were propensity score–matched to controls who did not receive a metformin prescription.
The study participants had a mean age of 67.7 years and underwent surgery requiring general anesthesia and postoperative admission at any of 15 hospitals in a single Pennsylvania health system. In addition to being prescribed metformin within 180 days before surgery, they also had metformin on their list of active medications at their most recent preoperative encounter before the surgery. The were followed until December 18, 2018.
In all, the 90-day and 5-year mortality hazards were reduced by 28% and 26%, respectively, in the metformin prescription recipients, compared with the propensity score–matched controls (hazard ratios, 0.72 and 0.74, respectively), Katherine M. Reitz, MD, and colleagues at the University of Pittsburgh reported in JAMA Surgery.
The readmission hazard – with mortality as a competing risk – was reduced by 16% at 30 days and 14% at 90 days (sub-HRs, 0.84 and 0.86, respectively), the researchers found.
“Hospital readmissions among those with preoperative metformin prescriptions were observed by postdischarge days 30 and 90 (304 [11%] and 538 [20.1%], respectively), whereas among those without prescriptions, 361 readmissions (13%) occurred by day 30 and 614 (23%) by day 90,” they wrote.
The investigators also noted that inflammation was reduced in patients who received a metformin prescription, compared with those who did not (mean preoperative neutrophil to leukocyte ratio, 4.5 vs. 5.0, respectively).
“In the full cohort, multivariable regression analysis similarly demonstrated that metformin was associated with a reduced hazard for both 90-day and 5-year mortality (adjusted HRs, 0.77 and 0.80, respectively) and for 30-day and 90-day readmission (aHR, 0.83 and 0.86), with mortality as a competing risk,” they added.
The findings support those from previous studies showing a decrease in all-cause mortality among diabetes patients taking metformin, said the researchers, noting that those patients had fewer age-related chronic diseases.
“These associations may reflect the anti-aging properties of metformin against the onset of disease or diabetes-associated complications. This study extends these finding by demonstrating that preoperative metformin prescriptions were associated with a reduction in postoperative mortality and readmission, a surrogate for postoperative complications, and with long-term mortality,” they wrote.
The study was limited by a number of factors, such as the potential for residual confounding inherent in retrospective analyses and a lack of adequate power to evaluate the association between metformin use and outcomes for individual surgical procedures. But the authors added that the findings are of note, because adults with comorbidities, such as diabetes, have less physiological reserve and an increased postoperative mortality and readmission rate. The results, therefore, warrant investigation with a prospective randomized clinical trial, they concluded.
In an accompanying editorial, Elizabeth L. George, MD, and Sherry M. Wren, MD, of Stanford (Calif.) University wrote that the study “demonstrates how variables, besides coexisting medical diseases, can affect surgical outcomes.”
“Metformin now joins beta-blockers, statins, and immunonutrition as preoperative agents associated with improved surgical outcomes,” they wrote, adding that future studies should factor in statin use and whether those and other medications should be continued postoperatively because metformin is often held after surgery owing to concerns about contrast agent interactions, whereas statin continuation is recommended.
Future studies of metformin in this setting should exclude patients who are taking statins, or look at possible interactions between the two agents, they said, adding that they would be “interested in seeing a subanalysis of this data set that excludes patients who were prescribed statins.”
“Those data would further solidify the role of metformin as a possible modifiable perioperative factor,” they wrote.
The study was funded by the University of Pittsburgh Medical Center and by grants from the National Heart, Lung, and Blood Institute and the National Institutes of Health. Dr. Reitz reported having no disclosures. Dr. George and Dr. Wren also reported having no disclosures.
SOURCE: Reitz K et al. JAMA Surg. 2020 Apr 8. doi: 10.1001/jamasurg.2020.0416.
Patients with type 2 diabetes who take metformin may have lower risk-adjusted mortality and readmission rates after surgery than do those who don’t take metformin, findings from a large retrospective cohort study suggest.
Of 10,088 individuals with diabetes who underwent a major surgery requiring hospital admission between January 1, 2010, and January 1, 2016, a total of 5,962 (59%) had received a prescription for metformin in the 180 days before surgery, and 5,460 of those patients were propensity score–matched to controls who did not receive a metformin prescription.
The study participants had a mean age of 67.7 years and underwent surgery requiring general anesthesia and postoperative admission at any of 15 hospitals in a single Pennsylvania health system. In addition to being prescribed metformin within 180 days before surgery, they also had metformin on their list of active medications at their most recent preoperative encounter before the surgery. The were followed until December 18, 2018.
In all, the 90-day and 5-year mortality hazards were reduced by 28% and 26%, respectively, in the metformin prescription recipients, compared with the propensity score–matched controls (hazard ratios, 0.72 and 0.74, respectively), Katherine M. Reitz, MD, and colleagues at the University of Pittsburgh reported in JAMA Surgery.
The readmission hazard – with mortality as a competing risk – was reduced by 16% at 30 days and 14% at 90 days (sub-HRs, 0.84 and 0.86, respectively), the researchers found.
“Hospital readmissions among those with preoperative metformin prescriptions were observed by postdischarge days 30 and 90 (304 [11%] and 538 [20.1%], respectively), whereas among those without prescriptions, 361 readmissions (13%) occurred by day 30 and 614 (23%) by day 90,” they wrote.
The investigators also noted that inflammation was reduced in patients who received a metformin prescription, compared with those who did not (mean preoperative neutrophil to leukocyte ratio, 4.5 vs. 5.0, respectively).
“In the full cohort, multivariable regression analysis similarly demonstrated that metformin was associated with a reduced hazard for both 90-day and 5-year mortality (adjusted HRs, 0.77 and 0.80, respectively) and for 30-day and 90-day readmission (aHR, 0.83 and 0.86), with mortality as a competing risk,” they added.
The findings support those from previous studies showing a decrease in all-cause mortality among diabetes patients taking metformin, said the researchers, noting that those patients had fewer age-related chronic diseases.
“These associations may reflect the anti-aging properties of metformin against the onset of disease or diabetes-associated complications. This study extends these finding by demonstrating that preoperative metformin prescriptions were associated with a reduction in postoperative mortality and readmission, a surrogate for postoperative complications, and with long-term mortality,” they wrote.
The study was limited by a number of factors, such as the potential for residual confounding inherent in retrospective analyses and a lack of adequate power to evaluate the association between metformin use and outcomes for individual surgical procedures. But the authors added that the findings are of note, because adults with comorbidities, such as diabetes, have less physiological reserve and an increased postoperative mortality and readmission rate. The results, therefore, warrant investigation with a prospective randomized clinical trial, they concluded.
In an accompanying editorial, Elizabeth L. George, MD, and Sherry M. Wren, MD, of Stanford (Calif.) University wrote that the study “demonstrates how variables, besides coexisting medical diseases, can affect surgical outcomes.”
“Metformin now joins beta-blockers, statins, and immunonutrition as preoperative agents associated with improved surgical outcomes,” they wrote, adding that future studies should factor in statin use and whether those and other medications should be continued postoperatively because metformin is often held after surgery owing to concerns about contrast agent interactions, whereas statin continuation is recommended.
Future studies of metformin in this setting should exclude patients who are taking statins, or look at possible interactions between the two agents, they said, adding that they would be “interested in seeing a subanalysis of this data set that excludes patients who were prescribed statins.”
“Those data would further solidify the role of metformin as a possible modifiable perioperative factor,” they wrote.
The study was funded by the University of Pittsburgh Medical Center and by grants from the National Heart, Lung, and Blood Institute and the National Institutes of Health. Dr. Reitz reported having no disclosures. Dr. George and Dr. Wren also reported having no disclosures.
SOURCE: Reitz K et al. JAMA Surg. 2020 Apr 8. doi: 10.1001/jamasurg.2020.0416.
Patients with type 2 diabetes who take metformin may have lower risk-adjusted mortality and readmission rates after surgery than do those who don’t take metformin, findings from a large retrospective cohort study suggest.
Of 10,088 individuals with diabetes who underwent a major surgery requiring hospital admission between January 1, 2010, and January 1, 2016, a total of 5,962 (59%) had received a prescription for metformin in the 180 days before surgery, and 5,460 of those patients were propensity score–matched to controls who did not receive a metformin prescription.
The study participants had a mean age of 67.7 years and underwent surgery requiring general anesthesia and postoperative admission at any of 15 hospitals in a single Pennsylvania health system. In addition to being prescribed metformin within 180 days before surgery, they also had metformin on their list of active medications at their most recent preoperative encounter before the surgery. The were followed until December 18, 2018.
In all, the 90-day and 5-year mortality hazards were reduced by 28% and 26%, respectively, in the metformin prescription recipients, compared with the propensity score–matched controls (hazard ratios, 0.72 and 0.74, respectively), Katherine M. Reitz, MD, and colleagues at the University of Pittsburgh reported in JAMA Surgery.
The readmission hazard – with mortality as a competing risk – was reduced by 16% at 30 days and 14% at 90 days (sub-HRs, 0.84 and 0.86, respectively), the researchers found.
“Hospital readmissions among those with preoperative metformin prescriptions were observed by postdischarge days 30 and 90 (304 [11%] and 538 [20.1%], respectively), whereas among those without prescriptions, 361 readmissions (13%) occurred by day 30 and 614 (23%) by day 90,” they wrote.
The investigators also noted that inflammation was reduced in patients who received a metformin prescription, compared with those who did not (mean preoperative neutrophil to leukocyte ratio, 4.5 vs. 5.0, respectively).
“In the full cohort, multivariable regression analysis similarly demonstrated that metformin was associated with a reduced hazard for both 90-day and 5-year mortality (adjusted HRs, 0.77 and 0.80, respectively) and for 30-day and 90-day readmission (aHR, 0.83 and 0.86), with mortality as a competing risk,” they added.
The findings support those from previous studies showing a decrease in all-cause mortality among diabetes patients taking metformin, said the researchers, noting that those patients had fewer age-related chronic diseases.
“These associations may reflect the anti-aging properties of metformin against the onset of disease or diabetes-associated complications. This study extends these finding by demonstrating that preoperative metformin prescriptions were associated with a reduction in postoperative mortality and readmission, a surrogate for postoperative complications, and with long-term mortality,” they wrote.
The study was limited by a number of factors, such as the potential for residual confounding inherent in retrospective analyses and a lack of adequate power to evaluate the association between metformin use and outcomes for individual surgical procedures. But the authors added that the findings are of note, because adults with comorbidities, such as diabetes, have less physiological reserve and an increased postoperative mortality and readmission rate. The results, therefore, warrant investigation with a prospective randomized clinical trial, they concluded.
In an accompanying editorial, Elizabeth L. George, MD, and Sherry M. Wren, MD, of Stanford (Calif.) University wrote that the study “demonstrates how variables, besides coexisting medical diseases, can affect surgical outcomes.”
“Metformin now joins beta-blockers, statins, and immunonutrition as preoperative agents associated with improved surgical outcomes,” they wrote, adding that future studies should factor in statin use and whether those and other medications should be continued postoperatively because metformin is often held after surgery owing to concerns about contrast agent interactions, whereas statin continuation is recommended.
Future studies of metformin in this setting should exclude patients who are taking statins, or look at possible interactions between the two agents, they said, adding that they would be “interested in seeing a subanalysis of this data set that excludes patients who were prescribed statins.”
“Those data would further solidify the role of metformin as a possible modifiable perioperative factor,” they wrote.
The study was funded by the University of Pittsburgh Medical Center and by grants from the National Heart, Lung, and Blood Institute and the National Institutes of Health. Dr. Reitz reported having no disclosures. Dr. George and Dr. Wren also reported having no disclosures.
SOURCE: Reitz K et al. JAMA Surg. 2020 Apr 8. doi: 10.1001/jamasurg.2020.0416.
FROM JAMA SURGERY
Inflammatory markers may explain COVID-19, diabetes dynamic
COVID-19 infection in patients with type 2 diabetes is associated with a greater increase in inflammatory and coagulation markers, compared with COVID-19 patients without diabetes, according to preliminary findings from a retrospective analysis of COVID-19 patients in Wuhan, China.
The results, though preliminary, could help explain why patients with diabetes and COVID-19 are at greater risk for more severe disease and death.
The results also suggest that more severe disease in patients with diabetes may be the result of a cytokine storm, in which the patient’s immune system overreacts to the virus and inflicts collateral damage on its own organs, according to Herbert I. Rettinger, MD, a clinical endocrinologist in Orange County, Calif., and member of the editorial advisory board for Clinical Endocrinology News. “Understanding the mechanism might help us understand the best way to treat,” COVID-19 in patients with diabetes, he said in an interview.
Dr. Rettinger, who was not involved in the research, noted that the study included only 24 patients with diabetes. Nevertheless, the finding of heightened inflammatory and coagulation markers was “fascinating.”
“This is the first paper I’ve seen [suggesting] that. I don’t know if we can extrapolate [the findings] to other populations, but if biomarkers are elevated in patients with COVID-19 and diabetes, then it’s something worth looking into, and to be aware of and cautious of. We need to pay attention to this,” he commented.
The study was led by Weina Guo and Desheng Hu at Huazhong University of Science and Technology in Wuhan, China, and published in Diabetes/Metabolism Research and Reviews.
The sample included 174 patients with COVID-19, who were treated consecutively during Feb. 10-29, 2020, at a single center. The researchers first assigned the patients to one of two groups – those with comorbid diabetes and those without. They further excluded all other comorbidities, focusing only on 26 patients with no comorbidities and 24 with only diabetes as a comorbidity, to remove all other comorbidities as possible confounding factors. Patients in the diabetes group were significantly older than those without diabetes (61 vs. 41 years, P < .01). The mortality rate was 16.5% in patients with diabetes and 0% in those without (P = .03).
COVID-19 patients with diabetes alone as a comorbidity had a greater risk for severe pneumonia, as evidenced by a higher mean CT score, compared with those without diabetes and no other comorbidities (P = .04). Patients with diabetes also had higher measures of release of tissue injury–related enzymes and were at higher risk of uncontrolled inflammation and hypercoagulable state. In particular, they had higher levels of interleukin-6 (13.7 vs. 4.1 pg/mL, respectively; P < .01), C-reactive protein (76.4 vs. 7.43 mg/L; P < .01), serum ferritin (764.8 vs. 128.9 ng/mL; P < .01), and D-dimer (1.16 vs. 0.25 mcg/mL; P < .01).
“It’s noteworthy that, for diseases that can induce a cytokine storm, IL-6 is a very good predictor of disease severity and prognosis, and its expression time is longer than other cytokines ([tumor necrosis factor] and IL-1). In addition, a significant rise in serum ferritin indicates the activation of the monocyte-macrophage system, which is a crucial part of inflammatory storm. These results indicate that patients with diabetes are susceptible to form an inflammatory storm, which eventually lead to rapid deterioration of COVID-19,” the authors wrote.
They also cited previous findings suggesting that coronavirus might exacerbate, or even cause, diabetes by seriously damaging islets (Acta Diabetol. 2010;47[3]:193-9). “Since viral infection may cause sharp fluctuation of the blood glucose levels of diabetes patients, which adversely affect the recovery of patients, there is reason to suspect that diabetes combined with SARS-CoV-2 pneumonia may form a vicious circle,” they wrote.
That’s one more reason to carefully monitor diabetes patients, said Dr. Rettinger. “Those patients who are able to make insulin might not be able to do so with the infection, and that may last a while, and they may require insulin. You want to keep a watch on things, and if oral agents are not working well, you want to go to insulin as quickly as you can. Probably diabetics should be way more careful and maybe visit the emergency department at earlier than a nondiabetic would.”
Raghavendra Mirmira, MD, PhD, who conducts translational research on diabetes and insulin production, said that the finding was not a complete surprise to him. “With a lot of diseases, having diabetes as a comorbidity can mean worse outcomes, and that’s certainly true of influenza. It was true for the other COVID-like illnesses, such as SARS and MERS,” Dr. Mirmira, who was not involved in the research, said in an interview.
If the findings hold up in larger numbers of patients and across multiple centers, they have the potential to inform patient management, said Dr. Mirmira, director of the Translational Research Center in the department of medicine at the University of Chicago. That will be especially true as data from long-term follow-up of become available. Elevated values in some biomarkers might dictate a patient be sent straight to the ICU or dictate admission to the hospital rather than being sent home, or it could assist patient selection for some of the new therapies that physicians hope will become available.
“The more information we get [about] total outcome, the more informed we’d be about who would benefit from some of the therapies that are in clinical trials now,” he said. Still, it will be a challenge to prove causation, because patients with diabetes have unique clinical characteristics that could also be the source of the difference.
Dr. Mirmira noted that patients with diabetes only were 20 years older on average than those with no comorbidities. “It’s really hard to know if what you’re looking at for the worse outcomes for people with diabetes is because they were older, and we know that older people tend to do much worse with COVID than younger people.” Ideally, patients would also be matched by age, but there are not enough data to do that yet.
The study was funded by
SOURCE: Guo W et al. Diabetes Metab Res Rev. 2020 Mar 31. doi: 10.1002/dmrr.3319.
COVID-19 infection in patients with type 2 diabetes is associated with a greater increase in inflammatory and coagulation markers, compared with COVID-19 patients without diabetes, according to preliminary findings from a retrospective analysis of COVID-19 patients in Wuhan, China.
The results, though preliminary, could help explain why patients with diabetes and COVID-19 are at greater risk for more severe disease and death.
The results also suggest that more severe disease in patients with diabetes may be the result of a cytokine storm, in which the patient’s immune system overreacts to the virus and inflicts collateral damage on its own organs, according to Herbert I. Rettinger, MD, a clinical endocrinologist in Orange County, Calif., and member of the editorial advisory board for Clinical Endocrinology News. “Understanding the mechanism might help us understand the best way to treat,” COVID-19 in patients with diabetes, he said in an interview.
Dr. Rettinger, who was not involved in the research, noted that the study included only 24 patients with diabetes. Nevertheless, the finding of heightened inflammatory and coagulation markers was “fascinating.”
“This is the first paper I’ve seen [suggesting] that. I don’t know if we can extrapolate [the findings] to other populations, but if biomarkers are elevated in patients with COVID-19 and diabetes, then it’s something worth looking into, and to be aware of and cautious of. We need to pay attention to this,” he commented.
The study was led by Weina Guo and Desheng Hu at Huazhong University of Science and Technology in Wuhan, China, and published in Diabetes/Metabolism Research and Reviews.
The sample included 174 patients with COVID-19, who were treated consecutively during Feb. 10-29, 2020, at a single center. The researchers first assigned the patients to one of two groups – those with comorbid diabetes and those without. They further excluded all other comorbidities, focusing only on 26 patients with no comorbidities and 24 with only diabetes as a comorbidity, to remove all other comorbidities as possible confounding factors. Patients in the diabetes group were significantly older than those without diabetes (61 vs. 41 years, P < .01). The mortality rate was 16.5% in patients with diabetes and 0% in those without (P = .03).
COVID-19 patients with diabetes alone as a comorbidity had a greater risk for severe pneumonia, as evidenced by a higher mean CT score, compared with those without diabetes and no other comorbidities (P = .04). Patients with diabetes also had higher measures of release of tissue injury–related enzymes and were at higher risk of uncontrolled inflammation and hypercoagulable state. In particular, they had higher levels of interleukin-6 (13.7 vs. 4.1 pg/mL, respectively; P < .01), C-reactive protein (76.4 vs. 7.43 mg/L; P < .01), serum ferritin (764.8 vs. 128.9 ng/mL; P < .01), and D-dimer (1.16 vs. 0.25 mcg/mL; P < .01).
“It’s noteworthy that, for diseases that can induce a cytokine storm, IL-6 is a very good predictor of disease severity and prognosis, and its expression time is longer than other cytokines ([tumor necrosis factor] and IL-1). In addition, a significant rise in serum ferritin indicates the activation of the monocyte-macrophage system, which is a crucial part of inflammatory storm. These results indicate that patients with diabetes are susceptible to form an inflammatory storm, which eventually lead to rapid deterioration of COVID-19,” the authors wrote.
They also cited previous findings suggesting that coronavirus might exacerbate, or even cause, diabetes by seriously damaging islets (Acta Diabetol. 2010;47[3]:193-9). “Since viral infection may cause sharp fluctuation of the blood glucose levels of diabetes patients, which adversely affect the recovery of patients, there is reason to suspect that diabetes combined with SARS-CoV-2 pneumonia may form a vicious circle,” they wrote.
That’s one more reason to carefully monitor diabetes patients, said Dr. Rettinger. “Those patients who are able to make insulin might not be able to do so with the infection, and that may last a while, and they may require insulin. You want to keep a watch on things, and if oral agents are not working well, you want to go to insulin as quickly as you can. Probably diabetics should be way more careful and maybe visit the emergency department at earlier than a nondiabetic would.”
Raghavendra Mirmira, MD, PhD, who conducts translational research on diabetes and insulin production, said that the finding was not a complete surprise to him. “With a lot of diseases, having diabetes as a comorbidity can mean worse outcomes, and that’s certainly true of influenza. It was true for the other COVID-like illnesses, such as SARS and MERS,” Dr. Mirmira, who was not involved in the research, said in an interview.
If the findings hold up in larger numbers of patients and across multiple centers, they have the potential to inform patient management, said Dr. Mirmira, director of the Translational Research Center in the department of medicine at the University of Chicago. That will be especially true as data from long-term follow-up of become available. Elevated values in some biomarkers might dictate a patient be sent straight to the ICU or dictate admission to the hospital rather than being sent home, or it could assist patient selection for some of the new therapies that physicians hope will become available.
“The more information we get [about] total outcome, the more informed we’d be about who would benefit from some of the therapies that are in clinical trials now,” he said. Still, it will be a challenge to prove causation, because patients with diabetes have unique clinical characteristics that could also be the source of the difference.
Dr. Mirmira noted that patients with diabetes only were 20 years older on average than those with no comorbidities. “It’s really hard to know if what you’re looking at for the worse outcomes for people with diabetes is because they were older, and we know that older people tend to do much worse with COVID than younger people.” Ideally, patients would also be matched by age, but there are not enough data to do that yet.
The study was funded by
SOURCE: Guo W et al. Diabetes Metab Res Rev. 2020 Mar 31. doi: 10.1002/dmrr.3319.
COVID-19 infection in patients with type 2 diabetes is associated with a greater increase in inflammatory and coagulation markers, compared with COVID-19 patients without diabetes, according to preliminary findings from a retrospective analysis of COVID-19 patients in Wuhan, China.
The results, though preliminary, could help explain why patients with diabetes and COVID-19 are at greater risk for more severe disease and death.
The results also suggest that more severe disease in patients with diabetes may be the result of a cytokine storm, in which the patient’s immune system overreacts to the virus and inflicts collateral damage on its own organs, according to Herbert I. Rettinger, MD, a clinical endocrinologist in Orange County, Calif., and member of the editorial advisory board for Clinical Endocrinology News. “Understanding the mechanism might help us understand the best way to treat,” COVID-19 in patients with diabetes, he said in an interview.
Dr. Rettinger, who was not involved in the research, noted that the study included only 24 patients with diabetes. Nevertheless, the finding of heightened inflammatory and coagulation markers was “fascinating.”
“This is the first paper I’ve seen [suggesting] that. I don’t know if we can extrapolate [the findings] to other populations, but if biomarkers are elevated in patients with COVID-19 and diabetes, then it’s something worth looking into, and to be aware of and cautious of. We need to pay attention to this,” he commented.
The study was led by Weina Guo and Desheng Hu at Huazhong University of Science and Technology in Wuhan, China, and published in Diabetes/Metabolism Research and Reviews.
The sample included 174 patients with COVID-19, who were treated consecutively during Feb. 10-29, 2020, at a single center. The researchers first assigned the patients to one of two groups – those with comorbid diabetes and those without. They further excluded all other comorbidities, focusing only on 26 patients with no comorbidities and 24 with only diabetes as a comorbidity, to remove all other comorbidities as possible confounding factors. Patients in the diabetes group were significantly older than those without diabetes (61 vs. 41 years, P < .01). The mortality rate was 16.5% in patients with diabetes and 0% in those without (P = .03).
COVID-19 patients with diabetes alone as a comorbidity had a greater risk for severe pneumonia, as evidenced by a higher mean CT score, compared with those without diabetes and no other comorbidities (P = .04). Patients with diabetes also had higher measures of release of tissue injury–related enzymes and were at higher risk of uncontrolled inflammation and hypercoagulable state. In particular, they had higher levels of interleukin-6 (13.7 vs. 4.1 pg/mL, respectively; P < .01), C-reactive protein (76.4 vs. 7.43 mg/L; P < .01), serum ferritin (764.8 vs. 128.9 ng/mL; P < .01), and D-dimer (1.16 vs. 0.25 mcg/mL; P < .01).
“It’s noteworthy that, for diseases that can induce a cytokine storm, IL-6 is a very good predictor of disease severity and prognosis, and its expression time is longer than other cytokines ([tumor necrosis factor] and IL-1). In addition, a significant rise in serum ferritin indicates the activation of the monocyte-macrophage system, which is a crucial part of inflammatory storm. These results indicate that patients with diabetes are susceptible to form an inflammatory storm, which eventually lead to rapid deterioration of COVID-19,” the authors wrote.
They also cited previous findings suggesting that coronavirus might exacerbate, or even cause, diabetes by seriously damaging islets (Acta Diabetol. 2010;47[3]:193-9). “Since viral infection may cause sharp fluctuation of the blood glucose levels of diabetes patients, which adversely affect the recovery of patients, there is reason to suspect that diabetes combined with SARS-CoV-2 pneumonia may form a vicious circle,” they wrote.
That’s one more reason to carefully monitor diabetes patients, said Dr. Rettinger. “Those patients who are able to make insulin might not be able to do so with the infection, and that may last a while, and they may require insulin. You want to keep a watch on things, and if oral agents are not working well, you want to go to insulin as quickly as you can. Probably diabetics should be way more careful and maybe visit the emergency department at earlier than a nondiabetic would.”
Raghavendra Mirmira, MD, PhD, who conducts translational research on diabetes and insulin production, said that the finding was not a complete surprise to him. “With a lot of diseases, having diabetes as a comorbidity can mean worse outcomes, and that’s certainly true of influenza. It was true for the other COVID-like illnesses, such as SARS and MERS,” Dr. Mirmira, who was not involved in the research, said in an interview.
If the findings hold up in larger numbers of patients and across multiple centers, they have the potential to inform patient management, said Dr. Mirmira, director of the Translational Research Center in the department of medicine at the University of Chicago. That will be especially true as data from long-term follow-up of become available. Elevated values in some biomarkers might dictate a patient be sent straight to the ICU or dictate admission to the hospital rather than being sent home, or it could assist patient selection for some of the new therapies that physicians hope will become available.
“The more information we get [about] total outcome, the more informed we’d be about who would benefit from some of the therapies that are in clinical trials now,” he said. Still, it will be a challenge to prove causation, because patients with diabetes have unique clinical characteristics that could also be the source of the difference.
Dr. Mirmira noted that patients with diabetes only were 20 years older on average than those with no comorbidities. “It’s really hard to know if what you’re looking at for the worse outcomes for people with diabetes is because they were older, and we know that older people tend to do much worse with COVID than younger people.” Ideally, patients would also be matched by age, but there are not enough data to do that yet.
The study was funded by
SOURCE: Guo W et al. Diabetes Metab Res Rev. 2020 Mar 31. doi: 10.1002/dmrr.3319.
FROM DIABETES/METABOLISM RESEARCH AND REVIEWS
Drone flight launches first-ever insulin drop
After a year of planning, researchers sent a drone flight off the coast of western Ireland to the Aran Islands, delivering insulin and glucagon and retrieving a blood sample from the first patient to receive insulin successfully by autonomous drone delivery.
The nuts and bolts of arranging the drop and retrieval, which occurred in September 2019, were detailed by Spyridoula Maraka, MD, during a virtual news conference held by the Endocrine Society. The study had been slated for presentaion during ENDO 2020, the society's annual meeting, which was canceled because of the COVID-19 pandemic.
“There are multiple medical drone delivery opportunities that could be lifesaving during sentinel events such as hurricanes, earthquakes, and, of course, pandemics like the one we are currently experiencing,” said Dr. Maraka.
Drones, or unmanned aerial vehicles, are popular for recreational use and in some commercial applications – notably photography – but they are largely untapped as a medical resource, said Dr. Maraka, a collaborator on the project and an endocrinologist at the University of Arkansas for Medical Sciences, Little Rock.
Most of the exploration of drones for medical purposes has been in countries with emerging economies, such as Ghana and Rwanda in Africa, where the unmanned vehicles have been used by the U.S. medical product delivery company Zipline since 2016 to deliver blood.
The autonomous drone delivery of insulin originated in Galway, where the project’s lead investigator, Derek O’Keefe, MD, PhD, is an endocrinologist and professor of medical device technology at the National University of Ireland.
In 2017, Ireland was pummeled by Ophelia, a category 3 hurricane, and a year later by Storm Emma, a winter blizzard, said Dr. Maraka. Those extreme weather events trapped patients in their homes, made streets impassable for days on end, and interrupted the delivery of essential medical supplies, including insulin.
Until then, Ireland’s medical management plan had been passive and rested on the assumption that any weather-related interruptions would be relatively brief and not result in large-scale disruption of care and supply delivery for geographically isolated patients, said Dr. Maraka. But the two extreme and disruptive weather events in relatively quick succession prompted a reassessment of emergency medical management plans.
“We realized that [the prevailing plans were] not good enough,” said Dr. Maraka. “Medicine has a track record of practicing for emergencies before they actually happen,” to make sure that necessary resources are available and protocols in place in case of an emergency. The researchers extrapolated this preparedness mindset to medication delivery and realized that drones could be used both for a medication drop and to bring blood or other samples back from patients for testing.
Ireland’s Aran Islands came to mind as a location that was at risk of being cut off from services, but that was reachable by drone from Galway. “We quickly realized that this project would be very challenging, as no one in the developed world had done drone deliveries beyond the visual line of sight,” said Dr. Maraka, adding that flight operations had significant regulatory constraints.
The cross-disciplinary team that was pulled together to run the Diabetes Drone Mission, as the project was dubbed, included physicians and experts from pharmacies and pharmaceutical companies. To address drone operation specifically, a drone manufacturer, a flight operations firm, and a telecommunications company were also engaged. Drone pilots had to be licensed for beyond-the-visual-line-of-sight (BVLOS) operation, and Irish and European aviation regulators were consulted.
It took a full year to pull the pieces together for the inaugural flight. “One of the first challenges we faced was that we wanted to perform a civilian drone flight covering more than 40 kilometers,” said Dr. Maraka, whereas most drones flights are in the range of 1-10 km (0.6-6.2 miles). This long-range BVLOS flight required the drone to send live camera feed for the flight duration, which necessitated uninterrupted 4G wireless connectivity with satellite telecommunications as backup.
The Wingcopter 178 drone that was eventually chosen has a wingspan of 178 cm (about 70 inches) and can reach a top speed of 130 km/hr (about 81 mph) in fixed-wing mode.
“We had to comply with medication-dispensing legislation ... and we had to comply with medication transportation cold-chain legislation,” said Dr. Maraka. In other words, the insulin could not be loaded and delivered without the usual prescribing, dispensing, and chain-of-custody procedures being met.
In the end, the successful proof-of-concept flight saw the drone covering 43.3 km (26.9 miles) in a 32-minute flight to deliver insulin and glucagon and return a blood sample for hemoglobin A1c testing.
Dr. Maraka said she and her collaborators have an active collaboration with United Parcel Service and drone suppliers to expand into regular medical supply deliveries.
Dr. Maraka reported no conflicts of interest.
The report will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
SOURCE: Maraka S et al. ENDO 2020, Abstract OR30-04.
This article was updated on 4/17/2020.
After a year of planning, researchers sent a drone flight off the coast of western Ireland to the Aran Islands, delivering insulin and glucagon and retrieving a blood sample from the first patient to receive insulin successfully by autonomous drone delivery.
The nuts and bolts of arranging the drop and retrieval, which occurred in September 2019, were detailed by Spyridoula Maraka, MD, during a virtual news conference held by the Endocrine Society. The study had been slated for presentaion during ENDO 2020, the society's annual meeting, which was canceled because of the COVID-19 pandemic.
“There are multiple medical drone delivery opportunities that could be lifesaving during sentinel events such as hurricanes, earthquakes, and, of course, pandemics like the one we are currently experiencing,” said Dr. Maraka.
Drones, or unmanned aerial vehicles, are popular for recreational use and in some commercial applications – notably photography – but they are largely untapped as a medical resource, said Dr. Maraka, a collaborator on the project and an endocrinologist at the University of Arkansas for Medical Sciences, Little Rock.
Most of the exploration of drones for medical purposes has been in countries with emerging economies, such as Ghana and Rwanda in Africa, where the unmanned vehicles have been used by the U.S. medical product delivery company Zipline since 2016 to deliver blood.
The autonomous drone delivery of insulin originated in Galway, where the project’s lead investigator, Derek O’Keefe, MD, PhD, is an endocrinologist and professor of medical device technology at the National University of Ireland.
In 2017, Ireland was pummeled by Ophelia, a category 3 hurricane, and a year later by Storm Emma, a winter blizzard, said Dr. Maraka. Those extreme weather events trapped patients in their homes, made streets impassable for days on end, and interrupted the delivery of essential medical supplies, including insulin.
Until then, Ireland’s medical management plan had been passive and rested on the assumption that any weather-related interruptions would be relatively brief and not result in large-scale disruption of care and supply delivery for geographically isolated patients, said Dr. Maraka. But the two extreme and disruptive weather events in relatively quick succession prompted a reassessment of emergency medical management plans.
“We realized that [the prevailing plans were] not good enough,” said Dr. Maraka. “Medicine has a track record of practicing for emergencies before they actually happen,” to make sure that necessary resources are available and protocols in place in case of an emergency. The researchers extrapolated this preparedness mindset to medication delivery and realized that drones could be used both for a medication drop and to bring blood or other samples back from patients for testing.
Ireland’s Aran Islands came to mind as a location that was at risk of being cut off from services, but that was reachable by drone from Galway. “We quickly realized that this project would be very challenging, as no one in the developed world had done drone deliveries beyond the visual line of sight,” said Dr. Maraka, adding that flight operations had significant regulatory constraints.
The cross-disciplinary team that was pulled together to run the Diabetes Drone Mission, as the project was dubbed, included physicians and experts from pharmacies and pharmaceutical companies. To address drone operation specifically, a drone manufacturer, a flight operations firm, and a telecommunications company were also engaged. Drone pilots had to be licensed for beyond-the-visual-line-of-sight (BVLOS) operation, and Irish and European aviation regulators were consulted.
It took a full year to pull the pieces together for the inaugural flight. “One of the first challenges we faced was that we wanted to perform a civilian drone flight covering more than 40 kilometers,” said Dr. Maraka, whereas most drones flights are in the range of 1-10 km (0.6-6.2 miles). This long-range BVLOS flight required the drone to send live camera feed for the flight duration, which necessitated uninterrupted 4G wireless connectivity with satellite telecommunications as backup.
The Wingcopter 178 drone that was eventually chosen has a wingspan of 178 cm (about 70 inches) and can reach a top speed of 130 km/hr (about 81 mph) in fixed-wing mode.
“We had to comply with medication-dispensing legislation ... and we had to comply with medication transportation cold-chain legislation,” said Dr. Maraka. In other words, the insulin could not be loaded and delivered without the usual prescribing, dispensing, and chain-of-custody procedures being met.
In the end, the successful proof-of-concept flight saw the drone covering 43.3 km (26.9 miles) in a 32-minute flight to deliver insulin and glucagon and return a blood sample for hemoglobin A1c testing.
Dr. Maraka said she and her collaborators have an active collaboration with United Parcel Service and drone suppliers to expand into regular medical supply deliveries.
Dr. Maraka reported no conflicts of interest.
The report will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
SOURCE: Maraka S et al. ENDO 2020, Abstract OR30-04.
This article was updated on 4/17/2020.
After a year of planning, researchers sent a drone flight off the coast of western Ireland to the Aran Islands, delivering insulin and glucagon and retrieving a blood sample from the first patient to receive insulin successfully by autonomous drone delivery.
The nuts and bolts of arranging the drop and retrieval, which occurred in September 2019, were detailed by Spyridoula Maraka, MD, during a virtual news conference held by the Endocrine Society. The study had been slated for presentaion during ENDO 2020, the society's annual meeting, which was canceled because of the COVID-19 pandemic.
“There are multiple medical drone delivery opportunities that could be lifesaving during sentinel events such as hurricanes, earthquakes, and, of course, pandemics like the one we are currently experiencing,” said Dr. Maraka.
Drones, or unmanned aerial vehicles, are popular for recreational use and in some commercial applications – notably photography – but they are largely untapped as a medical resource, said Dr. Maraka, a collaborator on the project and an endocrinologist at the University of Arkansas for Medical Sciences, Little Rock.
Most of the exploration of drones for medical purposes has been in countries with emerging economies, such as Ghana and Rwanda in Africa, where the unmanned vehicles have been used by the U.S. medical product delivery company Zipline since 2016 to deliver blood.
The autonomous drone delivery of insulin originated in Galway, where the project’s lead investigator, Derek O’Keefe, MD, PhD, is an endocrinologist and professor of medical device technology at the National University of Ireland.
In 2017, Ireland was pummeled by Ophelia, a category 3 hurricane, and a year later by Storm Emma, a winter blizzard, said Dr. Maraka. Those extreme weather events trapped patients in their homes, made streets impassable for days on end, and interrupted the delivery of essential medical supplies, including insulin.
Until then, Ireland’s medical management plan had been passive and rested on the assumption that any weather-related interruptions would be relatively brief and not result in large-scale disruption of care and supply delivery for geographically isolated patients, said Dr. Maraka. But the two extreme and disruptive weather events in relatively quick succession prompted a reassessment of emergency medical management plans.
“We realized that [the prevailing plans were] not good enough,” said Dr. Maraka. “Medicine has a track record of practicing for emergencies before they actually happen,” to make sure that necessary resources are available and protocols in place in case of an emergency. The researchers extrapolated this preparedness mindset to medication delivery and realized that drones could be used both for a medication drop and to bring blood or other samples back from patients for testing.
Ireland’s Aran Islands came to mind as a location that was at risk of being cut off from services, but that was reachable by drone from Galway. “We quickly realized that this project would be very challenging, as no one in the developed world had done drone deliveries beyond the visual line of sight,” said Dr. Maraka, adding that flight operations had significant regulatory constraints.
The cross-disciplinary team that was pulled together to run the Diabetes Drone Mission, as the project was dubbed, included physicians and experts from pharmacies and pharmaceutical companies. To address drone operation specifically, a drone manufacturer, a flight operations firm, and a telecommunications company were also engaged. Drone pilots had to be licensed for beyond-the-visual-line-of-sight (BVLOS) operation, and Irish and European aviation regulators were consulted.
It took a full year to pull the pieces together for the inaugural flight. “One of the first challenges we faced was that we wanted to perform a civilian drone flight covering more than 40 kilometers,” said Dr. Maraka, whereas most drones flights are in the range of 1-10 km (0.6-6.2 miles). This long-range BVLOS flight required the drone to send live camera feed for the flight duration, which necessitated uninterrupted 4G wireless connectivity with satellite telecommunications as backup.
The Wingcopter 178 drone that was eventually chosen has a wingspan of 178 cm (about 70 inches) and can reach a top speed of 130 km/hr (about 81 mph) in fixed-wing mode.
“We had to comply with medication-dispensing legislation ... and we had to comply with medication transportation cold-chain legislation,” said Dr. Maraka. In other words, the insulin could not be loaded and delivered without the usual prescribing, dispensing, and chain-of-custody procedures being met.
In the end, the successful proof-of-concept flight saw the drone covering 43.3 km (26.9 miles) in a 32-minute flight to deliver insulin and glucagon and return a blood sample for hemoglobin A1c testing.
Dr. Maraka said she and her collaborators have an active collaboration with United Parcel Service and drone suppliers to expand into regular medical supply deliveries.
Dr. Maraka reported no conflicts of interest.
The report will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
SOURCE: Maraka S et al. ENDO 2020, Abstract OR30-04.
This article was updated on 4/17/2020.
FROM ENDO 2020
Use of diabetes technology is lower among black, Hispanic patients
A retrospective study of patients at a minority-serving, safety-net hospital showed low uptake of diabetes technology among black patients with type 1 diabetes, compared with their white counterparts.
The researchers also found lower usage of the technology among Hispanic patients, but the difference, compared with their white counterparts, was not statistically significant after adjustments for language, insurance, age, and income. Patients who identified as “other” also were less likely than white patients to use the technology, which included continuous glucose monitors and continuous subcutaneous insulin infusion devices.
The data differes from other, similar studies of technology use in patients with type 1 diabetes, because the study population, drawn from the Boston University Medical Center, was more diverse than other studies, according to Kamonkiat Wirunsawanya, who is an endocrinology fellow at the medical center. The abstract had been slated for presentation at ENDO 2020, the Endocrine Society's annual meeting, which was canceled because of the COVID-19 pandemic.
Dr. Wirunsawanya and his colleagues are now using questionnaires to try to identify specific patient and physician factors that might explain the differences in technology use.
“Once we know which factors could be a barrier to using the technology, we’ll be able to implement a strategy to increase use in those patients,” Dr. Wirunsawanya said in an interview. The issue could be a two-way street, he noted, because some providers may be uncomfortable using the technology, or may perceive minorities as less adept at using technology.
The study included 227 adult patients who were seen at the medical center between October 2016 and September 2017. The mean age was 39 years, and 59% were men. The mean duration of type 1 diabetes was 21 years, and 30% of the patients were overweight, 22% were obese, 80% spoke English, and 50% were on government insurance. In all, 43% of the patients were white, 25% were black, 15% were Hispanic, 2% were Asian, and 15% identified as other.
Patients who used technology had lower mean levels of hemoglobin A1c, compared with nonusers (8.27% vs. 9.49%, respectively). Those with government health insurance were less likely than those with private insurance to use technology (odds ratio, 0.43; 95% confidential interval, 0.25-0.74).
Overall, 26% of the patients used continuous subcutaneous insulin infusion devices. Of those, 43% were white, 10% black, 14% Hispanic, none were Asian, and 18% identified as other.
In addition, 30% of the patients used continuous glucose monitors; of those, 47% were white, 14% black, 23% Hispanic, 25% Asian, and none identified as other.
After adjustments for insurance and language, the researchers found that black patients were less likely to use technology than were the white patients (OR, 0.25; 95% CI, 0.11-0.53). The same was found for those who identified as other (OR, 0.33; 95% CI, 0.12-0.89). There was no significant differences in technology use between white and Asian patients. After adjustments, the researchers showed that fewer Hispanic patients used technology, compared with their white counterparts, but the difference was not statistically significant.
In a multivariable logistic regression model that adjusted for insurance and language, black patients had lower odds of using technology, compared with white patients (OR, 0.25; 95% CI, 0.11-0.53), as did those identifying as other (OR, 0.33; 95% CI, 0.12-0.89).
In an interview, Anne Peters, MD, director of clinical diabetes programs at the University of Southern California, Los Angeles, said that the study highlights a common problem with introducing technology to underserved populations. “These study [findings are] not at all surprising. It’s something that is a puzzle for those of us who work in the field of diabetes management in patients from underserved communities. Even if you can get access to the technology, even when I get them tools and native Spanish-speaking educators and people who should be able to teach them how to use the technology, the adoption of the technology has been really much less than would be expected,” said Dr. Peters, who is also a professor of medicine at USC and was not involved in the research.
Part of the problem may be lack of contact with health care services, she said. White children with type 1 diabetes are treated from an early age and learn how to manage daily blood sugar levels. They often grow up to embrace technology, even enthusiastically. But in some minority communities, diabetes is viewed as something to be hidden away. That cultural difference is also a frustrating barrier.
“What happens in more affluent groups is that people learn from their peers, and they see that [managing their blood sugar] is possible. One of my big beliefs is that the answer has to come from [the community]. ... We need to get champions, people from the community who use these tools, to encourage others, and that’s hard to do because type 1 diabetes is such a small subset of the people with diabetes who are [black] or Latino,” said Dr. Peters.
Ultimately, the solution will require a shift in messaging by finding a way to help communities look differently at diabetes and its treatment. “There’s something that must come educationally and culturally that I’ve not figured out [yet]. I can get resources and fight for them, but we have to figure out how to make [technology] part of that culture, and I don’t know that we’ve done that,” she said.
Dr. Wirunsawanya reported no financial conflicts of interest. Dr. Peters has been on an advisory panel for Abbott Diabetes Care and has received research funding from Dexcom.
Dr. Wirunsawanya and colleagues’ research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
SOURCE: Wirunsawanya K et al. ENDO 2020, Abstract OR30-03.
This article was updated on 4/17/2020.
A retrospective study of patients at a minority-serving, safety-net hospital showed low uptake of diabetes technology among black patients with type 1 diabetes, compared with their white counterparts.
The researchers also found lower usage of the technology among Hispanic patients, but the difference, compared with their white counterparts, was not statistically significant after adjustments for language, insurance, age, and income. Patients who identified as “other” also were less likely than white patients to use the technology, which included continuous glucose monitors and continuous subcutaneous insulin infusion devices.
The data differes from other, similar studies of technology use in patients with type 1 diabetes, because the study population, drawn from the Boston University Medical Center, was more diverse than other studies, according to Kamonkiat Wirunsawanya, who is an endocrinology fellow at the medical center. The abstract had been slated for presentation at ENDO 2020, the Endocrine Society's annual meeting, which was canceled because of the COVID-19 pandemic.
Dr. Wirunsawanya and his colleagues are now using questionnaires to try to identify specific patient and physician factors that might explain the differences in technology use.
“Once we know which factors could be a barrier to using the technology, we’ll be able to implement a strategy to increase use in those patients,” Dr. Wirunsawanya said in an interview. The issue could be a two-way street, he noted, because some providers may be uncomfortable using the technology, or may perceive minorities as less adept at using technology.
The study included 227 adult patients who were seen at the medical center between October 2016 and September 2017. The mean age was 39 years, and 59% were men. The mean duration of type 1 diabetes was 21 years, and 30% of the patients were overweight, 22% were obese, 80% spoke English, and 50% were on government insurance. In all, 43% of the patients were white, 25% were black, 15% were Hispanic, 2% were Asian, and 15% identified as other.
Patients who used technology had lower mean levels of hemoglobin A1c, compared with nonusers (8.27% vs. 9.49%, respectively). Those with government health insurance were less likely than those with private insurance to use technology (odds ratio, 0.43; 95% confidential interval, 0.25-0.74).
Overall, 26% of the patients used continuous subcutaneous insulin infusion devices. Of those, 43% were white, 10% black, 14% Hispanic, none were Asian, and 18% identified as other.
In addition, 30% of the patients used continuous glucose monitors; of those, 47% were white, 14% black, 23% Hispanic, 25% Asian, and none identified as other.
After adjustments for insurance and language, the researchers found that black patients were less likely to use technology than were the white patients (OR, 0.25; 95% CI, 0.11-0.53). The same was found for those who identified as other (OR, 0.33; 95% CI, 0.12-0.89). There was no significant differences in technology use between white and Asian patients. After adjustments, the researchers showed that fewer Hispanic patients used technology, compared with their white counterparts, but the difference was not statistically significant.
In a multivariable logistic regression model that adjusted for insurance and language, black patients had lower odds of using technology, compared with white patients (OR, 0.25; 95% CI, 0.11-0.53), as did those identifying as other (OR, 0.33; 95% CI, 0.12-0.89).
In an interview, Anne Peters, MD, director of clinical diabetes programs at the University of Southern California, Los Angeles, said that the study highlights a common problem with introducing technology to underserved populations. “These study [findings are] not at all surprising. It’s something that is a puzzle for those of us who work in the field of diabetes management in patients from underserved communities. Even if you can get access to the technology, even when I get them tools and native Spanish-speaking educators and people who should be able to teach them how to use the technology, the adoption of the technology has been really much less than would be expected,” said Dr. Peters, who is also a professor of medicine at USC and was not involved in the research.
Part of the problem may be lack of contact with health care services, she said. White children with type 1 diabetes are treated from an early age and learn how to manage daily blood sugar levels. They often grow up to embrace technology, even enthusiastically. But in some minority communities, diabetes is viewed as something to be hidden away. That cultural difference is also a frustrating barrier.
“What happens in more affluent groups is that people learn from their peers, and they see that [managing their blood sugar] is possible. One of my big beliefs is that the answer has to come from [the community]. ... We need to get champions, people from the community who use these tools, to encourage others, and that’s hard to do because type 1 diabetes is such a small subset of the people with diabetes who are [black] or Latino,” said Dr. Peters.
Ultimately, the solution will require a shift in messaging by finding a way to help communities look differently at diabetes and its treatment. “There’s something that must come educationally and culturally that I’ve not figured out [yet]. I can get resources and fight for them, but we have to figure out how to make [technology] part of that culture, and I don’t know that we’ve done that,” she said.
Dr. Wirunsawanya reported no financial conflicts of interest. Dr. Peters has been on an advisory panel for Abbott Diabetes Care and has received research funding from Dexcom.
Dr. Wirunsawanya and colleagues’ research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
SOURCE: Wirunsawanya K et al. ENDO 2020, Abstract OR30-03.
This article was updated on 4/17/2020.
A retrospective study of patients at a minority-serving, safety-net hospital showed low uptake of diabetes technology among black patients with type 1 diabetes, compared with their white counterparts.
The researchers also found lower usage of the technology among Hispanic patients, but the difference, compared with their white counterparts, was not statistically significant after adjustments for language, insurance, age, and income. Patients who identified as “other” also were less likely than white patients to use the technology, which included continuous glucose monitors and continuous subcutaneous insulin infusion devices.
The data differes from other, similar studies of technology use in patients with type 1 diabetes, because the study population, drawn from the Boston University Medical Center, was more diverse than other studies, according to Kamonkiat Wirunsawanya, who is an endocrinology fellow at the medical center. The abstract had been slated for presentation at ENDO 2020, the Endocrine Society's annual meeting, which was canceled because of the COVID-19 pandemic.
Dr. Wirunsawanya and his colleagues are now using questionnaires to try to identify specific patient and physician factors that might explain the differences in technology use.
“Once we know which factors could be a barrier to using the technology, we’ll be able to implement a strategy to increase use in those patients,” Dr. Wirunsawanya said in an interview. The issue could be a two-way street, he noted, because some providers may be uncomfortable using the technology, or may perceive minorities as less adept at using technology.
The study included 227 adult patients who were seen at the medical center between October 2016 and September 2017. The mean age was 39 years, and 59% were men. The mean duration of type 1 diabetes was 21 years, and 30% of the patients were overweight, 22% were obese, 80% spoke English, and 50% were on government insurance. In all, 43% of the patients were white, 25% were black, 15% were Hispanic, 2% were Asian, and 15% identified as other.
Patients who used technology had lower mean levels of hemoglobin A1c, compared with nonusers (8.27% vs. 9.49%, respectively). Those with government health insurance were less likely than those with private insurance to use technology (odds ratio, 0.43; 95% confidential interval, 0.25-0.74).
Overall, 26% of the patients used continuous subcutaneous insulin infusion devices. Of those, 43% were white, 10% black, 14% Hispanic, none were Asian, and 18% identified as other.
In addition, 30% of the patients used continuous glucose monitors; of those, 47% were white, 14% black, 23% Hispanic, 25% Asian, and none identified as other.
After adjustments for insurance and language, the researchers found that black patients were less likely to use technology than were the white patients (OR, 0.25; 95% CI, 0.11-0.53). The same was found for those who identified as other (OR, 0.33; 95% CI, 0.12-0.89). There was no significant differences in technology use between white and Asian patients. After adjustments, the researchers showed that fewer Hispanic patients used technology, compared with their white counterparts, but the difference was not statistically significant.
In a multivariable logistic regression model that adjusted for insurance and language, black patients had lower odds of using technology, compared with white patients (OR, 0.25; 95% CI, 0.11-0.53), as did those identifying as other (OR, 0.33; 95% CI, 0.12-0.89).
In an interview, Anne Peters, MD, director of clinical diabetes programs at the University of Southern California, Los Angeles, said that the study highlights a common problem with introducing technology to underserved populations. “These study [findings are] not at all surprising. It’s something that is a puzzle for those of us who work in the field of diabetes management in patients from underserved communities. Even if you can get access to the technology, even when I get them tools and native Spanish-speaking educators and people who should be able to teach them how to use the technology, the adoption of the technology has been really much less than would be expected,” said Dr. Peters, who is also a professor of medicine at USC and was not involved in the research.
Part of the problem may be lack of contact with health care services, she said. White children with type 1 diabetes are treated from an early age and learn how to manage daily blood sugar levels. They often grow up to embrace technology, even enthusiastically. But in some minority communities, diabetes is viewed as something to be hidden away. That cultural difference is also a frustrating barrier.
“What happens in more affluent groups is that people learn from their peers, and they see that [managing their blood sugar] is possible. One of my big beliefs is that the answer has to come from [the community]. ... We need to get champions, people from the community who use these tools, to encourage others, and that’s hard to do because type 1 diabetes is such a small subset of the people with diabetes who are [black] or Latino,” said Dr. Peters.
Ultimately, the solution will require a shift in messaging by finding a way to help communities look differently at diabetes and its treatment. “There’s something that must come educationally and culturally that I’ve not figured out [yet]. I can get resources and fight for them, but we have to figure out how to make [technology] part of that culture, and I don’t know that we’ve done that,” she said.
Dr. Wirunsawanya reported no financial conflicts of interest. Dr. Peters has been on an advisory panel for Abbott Diabetes Care and has received research funding from Dexcom.
Dr. Wirunsawanya and colleagues’ research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
SOURCE: Wirunsawanya K et al. ENDO 2020, Abstract OR30-03.
This article was updated on 4/17/2020.
FROM ENDO 2020
Can diabetes be cured?
In his Guest Editorial “How to help patients become successful diabetes self-managers” (J Fam Pract. 2020;69:8-9), Dr. Unger makes several very good points. I especially liked his recommendation to ask patients why they are concerned about having diabetes; this question alone can kick-start the behavior modification process leading to improved diabetes control.
However, I disagree with Dr. Unger’s assertion that “diabetes cannot be cured.” Based on multiple case studies, clinical trials, results from lifestyle intervention programs, and my own experience as a family physician, it is clear that diabetes can be reversed with lifestyle changes designed to counteract the modifiable factors (eg, diet, lack of exercise) that usually cause this condition.
Rather than merely considering the diabetes measure of success to be blood glucose controlled by prescribed medication, it is important to offer a more collaborative approach to patients willing to make lifestyle changes. We can show many—if not most— that they can achieve the goal of blood glucose control without medication.
Allan Olson MD
Diplomate, American Board of Family Medicine and American Board of Lifestyle Medicine
Kewaunee, WI
In his Guest Editorial “How to help patients become successful diabetes self-managers” (J Fam Pract. 2020;69:8-9), Dr. Unger makes several very good points. I especially liked his recommendation to ask patients why they are concerned about having diabetes; this question alone can kick-start the behavior modification process leading to improved diabetes control.
However, I disagree with Dr. Unger’s assertion that “diabetes cannot be cured.” Based on multiple case studies, clinical trials, results from lifestyle intervention programs, and my own experience as a family physician, it is clear that diabetes can be reversed with lifestyle changes designed to counteract the modifiable factors (eg, diet, lack of exercise) that usually cause this condition.
Rather than merely considering the diabetes measure of success to be blood glucose controlled by prescribed medication, it is important to offer a more collaborative approach to patients willing to make lifestyle changes. We can show many—if not most— that they can achieve the goal of blood glucose control without medication.
Allan Olson MD
Diplomate, American Board of Family Medicine and American Board of Lifestyle Medicine
Kewaunee, WI
In his Guest Editorial “How to help patients become successful diabetes self-managers” (J Fam Pract. 2020;69:8-9), Dr. Unger makes several very good points. I especially liked his recommendation to ask patients why they are concerned about having diabetes; this question alone can kick-start the behavior modification process leading to improved diabetes control.
However, I disagree with Dr. Unger’s assertion that “diabetes cannot be cured.” Based on multiple case studies, clinical trials, results from lifestyle intervention programs, and my own experience as a family physician, it is clear that diabetes can be reversed with lifestyle changes designed to counteract the modifiable factors (eg, diet, lack of exercise) that usually cause this condition.
Rather than merely considering the diabetes measure of success to be blood glucose controlled by prescribed medication, it is important to offer a more collaborative approach to patients willing to make lifestyle changes. We can show many—if not most— that they can achieve the goal of blood glucose control without medication.
Allan Olson MD
Diplomate, American Board of Family Medicine and American Board of Lifestyle Medicine
Kewaunee, WI
AHA updates management when CAD and T2DM coincide
Patients with stable coronary artery disease and type 2 diabetes mellitus could benefit from a “plethora of newly available risk-reduction strategies,” but their “adoption into clinical practice has been slow” and inconsistent, prompting an expert panel organized by the American Heart Association to collate the range of treatment recommendations now applicable to this patient population in a scientific statement released on April 13.
“There are a number of things to consider when treating patients with stable coronary artery disease [CAD] and type 2 diabetes mellitus [T2DM], with new medications and trials and data emerging. It’s difficult to keep up with all of the complexities,” which was why the Association’s Councils on Lifestyle and Cardiometabolic Health and on Clinical Cardiology put together a writing group to summarize and prioritize the range of lifestyle, medical, and interventional options that now require consideration and potential use on patients managed in routine practice, explained Suzanne V. Arnold, MD, chair of the writing group, in an interview.
The new scientific statement (Circulation. 2020 Apr 13; doi: 10.1161/CIR.0000000000000766), aimed primarily at cardiologists but also intended to inform primary care physicians, endocrinologists, and all other clinicians who deal with these patients, pulls together “everything someone needs to think about if they care for patients with CAD and T2DM,” said Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and vice chair of the statement-writing panel in an interview. “There is a lot to know,” he added.
The statement covers antithrombotic therapies; blood pressure control, with a discussion of both the appropriate pressure goal and the best drug types used to reach it; lipid management; glycemic control; lifestyle modification; weight management, including the role of bariatric surgery; and approaches to managing stable angina, both medically and with revascularization.
“The goal was to give clinicians a good sense of what new treatments they should consider” for these patients, said Dr. Bhatt, who is also director of interventional cardiovascular programs at Brigham and Women’s Hospital, also in Boston. Because of the tight associations between T2DM and cardiovascular disease in general including CAD, “cardiologists are increasingly involved in managing patients with T2DM,” he noted. The statement gives a comprehensive overview and critical assessment of the management of these patients as of the end of 2019 as a consensus from a panel of 11 experts .
The statement also stressed that “substantial portions of patients with T2DM and CAD, including those after an acute coronary syndrome, do not receive therapies with proven cardiovascular benefit, such as high-intensity statins, dual-antiplatelet therapy, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, and glucose-lowering agents with proven cardiovascular benefits.
“These gaps in care highlight a critical opportunity for cardiovascular specialists to assume a more active role in the collaborative care of patients with T2DM and CAD,” the statement said. This includes “encouraging cardiologists to become more active in the selection of glucose-lowering medications” for these patients because it could “really move the needle,” said Dr. Arnold, a cardiologist with Saint Luke’s Health System in Kansas City, Mo. She was referring specifically to broader reliance on both the SGTL2 (sodium-glucose cotransporter 2) inhibitors and the GLP-1 (glucagonlike peptide-1) receptor agonists as top choices for controlling hyperglycemia. Based on recent evidence drugs in these two classes “could be considered first line for patients with T2DM and CAD, and would likely be preferred over metformin,” Dr. Arnold said in an interview. Although the statement identified the SGLT2 inhibitors as “the first drug class [for glycemic control] to show clear benefits on cardiovascular outcomes,” it does not explicitly label the class first-line and it also skirts that designation for the GLP-1 receptor agonist class, while noting that metformin “remains the drug most frequently recommended as first-line therapy in treatment guidelines.”
“I wouldn’t disagree with someone who said that SGLT2 inhibitors and GLP-1 receptor agonists are first line,” but prescribing patterns also depend on familiarity, cost, and access, noted Dr. Bhatt, which can all be issues with agents from these classes compared with metformin, a widely available generic with decades of use. “Metformin is safe and cheap, so we did not want to discount it,” said Dr. Arnold. Dr. Bhatt recently coauthored an editorial that gave an enthusiastic endorsement to using SGLT2 inhibitors in patients with diabetes (Cell Metab. 2019 Nov 5;30[5]:47-9).
Another notable feature of the statement is the potential it assigns to bariatric surgery as a management tool with documented safety and efficacy for improving cardiovascular risk factors. However, the statement also notes that randomized trials “have thus far been inadequately powered to assess cardiovascular events and mortality, although observational studies have consistently shown cardiovascular risk reduction with such procedures.” The statement continues that despite potential cardiovascular benefits “bariatric surgery remains underused among eligible patients,” and said that surgery performed as Roux-en-Y bypass or sleeve gastrectomy “may be another effective tool for cardiovascular risk reduction in the subset of patients with obesity,” particularly patients with a body mass index of at least 35 kg/m2.
“While the percentage of patients who are optimal for bariatric surgery is not known, the most recent NHANES [National Health and Nutrition Examination Study] study showed that less than 0.5% of eligible patients underwent bariatric surgery,” Dr. Arnold noted. Bariatric surgery is “certainly not a recommendation for everyone, or even a majority of patients, but bariatric surgery should be on our radar,” for patients with CAD and T2DM, she said.
Right now, “few cardiologists think about bariatric surgery,” as a treatment option, but study results have shown that “in carefully selected patients treated by skilled surgeons at high-volume centers, patients will do better with bariatric surgery than with best medical therapy for improvements in multiple risk factors, including glycemic control,” Dr. Bhatt said in the interview. “It’s not first-line treatment, but it’s an option to consider,” he added, while also noting that bariatric surgery is most beneficial to patients relatively early in the course of T2DM, when its been in place for just a few years rather than a couple of decades.
The statement also notably included a “first-line” call out for icosapent ethyl (Vascepa), a novel agent approved in December 2019 for routine use in U.S. patients, including those with CAD and T2DM as long as their blood triglyceride level was at least 150 mg/dL. Dr. Bhatt, who led the REDUCE-IT study that was pivotal for proving the safety and efficacy of icosapent ethyl (N Engl J Med. 2019 Jan 3;380[1]:11-22), estimated that anywhere from 15% to as many as half the patients with CAD and T2DM might have a triglyceride level that would allow them to receive icosapent ethyl. One population-based study in Canada of nearly 200,000 people with atherosclerotic cardiovascular disease found a 25% prevalence of the triglyceride level needed to qualify to receive icosapent ethyl under current labeling, he noted (Eur Heart J. 2020 Jan 1;41[1]:86-94). However, the FDA label does not specify that triglycerides be measured when fasting, and a nonfasting level of about 150 mg/dL will likely appear for patients with fasting levels that fall as low as about 100 mg/dL, Dr. Bhatt said. He hoped that future studies will assess the efficacy of icosapent ethyl in patients with even lower triglyceride levels.
Other sections of the statement also recommend that clinicians: Target long-term dual-antiplatelet therapy to CAD and T2DM patients with additional high-risk markers such as prior MI, younger age, and tobacco use; prescribe a low-dose oral anticoagulant along with an antiplatelet drug such as aspirin for secondary-prevention patients; promote a blood pressure target of less than 140/90 mm Hg for all CAD and T2DM patients and apply a goal of less than 130/80 mm Hg in higher-risk patients such as blacks, Asians, and those with cerebrovascular disease; and reassure patients that “despite a modest increase in blood sugars, the risk-benefit ratio is clearly in favor of administering statins to people with T2DM and CAD.”
Dr. Arnold had no disclosures. Dr. Bhatt has been an adviser to Cardax, Cereno Scientific, Medscape Cardiology, PhaseBio; PLx Pharma, and Regado Biosciences, and he has received research funding from numerous companies including Amarin, the company that markets icosapent ethyl.
Patients with stable coronary artery disease and type 2 diabetes mellitus could benefit from a “plethora of newly available risk-reduction strategies,” but their “adoption into clinical practice has been slow” and inconsistent, prompting an expert panel organized by the American Heart Association to collate the range of treatment recommendations now applicable to this patient population in a scientific statement released on April 13.
“There are a number of things to consider when treating patients with stable coronary artery disease [CAD] and type 2 diabetes mellitus [T2DM], with new medications and trials and data emerging. It’s difficult to keep up with all of the complexities,” which was why the Association’s Councils on Lifestyle and Cardiometabolic Health and on Clinical Cardiology put together a writing group to summarize and prioritize the range of lifestyle, medical, and interventional options that now require consideration and potential use on patients managed in routine practice, explained Suzanne V. Arnold, MD, chair of the writing group, in an interview.
The new scientific statement (Circulation. 2020 Apr 13; doi: 10.1161/CIR.0000000000000766), aimed primarily at cardiologists but also intended to inform primary care physicians, endocrinologists, and all other clinicians who deal with these patients, pulls together “everything someone needs to think about if they care for patients with CAD and T2DM,” said Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and vice chair of the statement-writing panel in an interview. “There is a lot to know,” he added.
The statement covers antithrombotic therapies; blood pressure control, with a discussion of both the appropriate pressure goal and the best drug types used to reach it; lipid management; glycemic control; lifestyle modification; weight management, including the role of bariatric surgery; and approaches to managing stable angina, both medically and with revascularization.
“The goal was to give clinicians a good sense of what new treatments they should consider” for these patients, said Dr. Bhatt, who is also director of interventional cardiovascular programs at Brigham and Women’s Hospital, also in Boston. Because of the tight associations between T2DM and cardiovascular disease in general including CAD, “cardiologists are increasingly involved in managing patients with T2DM,” he noted. The statement gives a comprehensive overview and critical assessment of the management of these patients as of the end of 2019 as a consensus from a panel of 11 experts .
The statement also stressed that “substantial portions of patients with T2DM and CAD, including those after an acute coronary syndrome, do not receive therapies with proven cardiovascular benefit, such as high-intensity statins, dual-antiplatelet therapy, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, and glucose-lowering agents with proven cardiovascular benefits.
“These gaps in care highlight a critical opportunity for cardiovascular specialists to assume a more active role in the collaborative care of patients with T2DM and CAD,” the statement said. This includes “encouraging cardiologists to become more active in the selection of glucose-lowering medications” for these patients because it could “really move the needle,” said Dr. Arnold, a cardiologist with Saint Luke’s Health System in Kansas City, Mo. She was referring specifically to broader reliance on both the SGTL2 (sodium-glucose cotransporter 2) inhibitors and the GLP-1 (glucagonlike peptide-1) receptor agonists as top choices for controlling hyperglycemia. Based on recent evidence drugs in these two classes “could be considered first line for patients with T2DM and CAD, and would likely be preferred over metformin,” Dr. Arnold said in an interview. Although the statement identified the SGLT2 inhibitors as “the first drug class [for glycemic control] to show clear benefits on cardiovascular outcomes,” it does not explicitly label the class first-line and it also skirts that designation for the GLP-1 receptor agonist class, while noting that metformin “remains the drug most frequently recommended as first-line therapy in treatment guidelines.”
“I wouldn’t disagree with someone who said that SGLT2 inhibitors and GLP-1 receptor agonists are first line,” but prescribing patterns also depend on familiarity, cost, and access, noted Dr. Bhatt, which can all be issues with agents from these classes compared with metformin, a widely available generic with decades of use. “Metformin is safe and cheap, so we did not want to discount it,” said Dr. Arnold. Dr. Bhatt recently coauthored an editorial that gave an enthusiastic endorsement to using SGLT2 inhibitors in patients with diabetes (Cell Metab. 2019 Nov 5;30[5]:47-9).
Another notable feature of the statement is the potential it assigns to bariatric surgery as a management tool with documented safety and efficacy for improving cardiovascular risk factors. However, the statement also notes that randomized trials “have thus far been inadequately powered to assess cardiovascular events and mortality, although observational studies have consistently shown cardiovascular risk reduction with such procedures.” The statement continues that despite potential cardiovascular benefits “bariatric surgery remains underused among eligible patients,” and said that surgery performed as Roux-en-Y bypass or sleeve gastrectomy “may be another effective tool for cardiovascular risk reduction in the subset of patients with obesity,” particularly patients with a body mass index of at least 35 kg/m2.
“While the percentage of patients who are optimal for bariatric surgery is not known, the most recent NHANES [National Health and Nutrition Examination Study] study showed that less than 0.5% of eligible patients underwent bariatric surgery,” Dr. Arnold noted. Bariatric surgery is “certainly not a recommendation for everyone, or even a majority of patients, but bariatric surgery should be on our radar,” for patients with CAD and T2DM, she said.
Right now, “few cardiologists think about bariatric surgery,” as a treatment option, but study results have shown that “in carefully selected patients treated by skilled surgeons at high-volume centers, patients will do better with bariatric surgery than with best medical therapy for improvements in multiple risk factors, including glycemic control,” Dr. Bhatt said in the interview. “It’s not first-line treatment, but it’s an option to consider,” he added, while also noting that bariatric surgery is most beneficial to patients relatively early in the course of T2DM, when its been in place for just a few years rather than a couple of decades.
The statement also notably included a “first-line” call out for icosapent ethyl (Vascepa), a novel agent approved in December 2019 for routine use in U.S. patients, including those with CAD and T2DM as long as their blood triglyceride level was at least 150 mg/dL. Dr. Bhatt, who led the REDUCE-IT study that was pivotal for proving the safety and efficacy of icosapent ethyl (N Engl J Med. 2019 Jan 3;380[1]:11-22), estimated that anywhere from 15% to as many as half the patients with CAD and T2DM might have a triglyceride level that would allow them to receive icosapent ethyl. One population-based study in Canada of nearly 200,000 people with atherosclerotic cardiovascular disease found a 25% prevalence of the triglyceride level needed to qualify to receive icosapent ethyl under current labeling, he noted (Eur Heart J. 2020 Jan 1;41[1]:86-94). However, the FDA label does not specify that triglycerides be measured when fasting, and a nonfasting level of about 150 mg/dL will likely appear for patients with fasting levels that fall as low as about 100 mg/dL, Dr. Bhatt said. He hoped that future studies will assess the efficacy of icosapent ethyl in patients with even lower triglyceride levels.
Other sections of the statement also recommend that clinicians: Target long-term dual-antiplatelet therapy to CAD and T2DM patients with additional high-risk markers such as prior MI, younger age, and tobacco use; prescribe a low-dose oral anticoagulant along with an antiplatelet drug such as aspirin for secondary-prevention patients; promote a blood pressure target of less than 140/90 mm Hg for all CAD and T2DM patients and apply a goal of less than 130/80 mm Hg in higher-risk patients such as blacks, Asians, and those with cerebrovascular disease; and reassure patients that “despite a modest increase in blood sugars, the risk-benefit ratio is clearly in favor of administering statins to people with T2DM and CAD.”
Dr. Arnold had no disclosures. Dr. Bhatt has been an adviser to Cardax, Cereno Scientific, Medscape Cardiology, PhaseBio; PLx Pharma, and Regado Biosciences, and he has received research funding from numerous companies including Amarin, the company that markets icosapent ethyl.
Patients with stable coronary artery disease and type 2 diabetes mellitus could benefit from a “plethora of newly available risk-reduction strategies,” but their “adoption into clinical practice has been slow” and inconsistent, prompting an expert panel organized by the American Heart Association to collate the range of treatment recommendations now applicable to this patient population in a scientific statement released on April 13.
“There are a number of things to consider when treating patients with stable coronary artery disease [CAD] and type 2 diabetes mellitus [T2DM], with new medications and trials and data emerging. It’s difficult to keep up with all of the complexities,” which was why the Association’s Councils on Lifestyle and Cardiometabolic Health and on Clinical Cardiology put together a writing group to summarize and prioritize the range of lifestyle, medical, and interventional options that now require consideration and potential use on patients managed in routine practice, explained Suzanne V. Arnold, MD, chair of the writing group, in an interview.
The new scientific statement (Circulation. 2020 Apr 13; doi: 10.1161/CIR.0000000000000766), aimed primarily at cardiologists but also intended to inform primary care physicians, endocrinologists, and all other clinicians who deal with these patients, pulls together “everything someone needs to think about if they care for patients with CAD and T2DM,” said Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and vice chair of the statement-writing panel in an interview. “There is a lot to know,” he added.
The statement covers antithrombotic therapies; blood pressure control, with a discussion of both the appropriate pressure goal and the best drug types used to reach it; lipid management; glycemic control; lifestyle modification; weight management, including the role of bariatric surgery; and approaches to managing stable angina, both medically and with revascularization.
“The goal was to give clinicians a good sense of what new treatments they should consider” for these patients, said Dr. Bhatt, who is also director of interventional cardiovascular programs at Brigham and Women’s Hospital, also in Boston. Because of the tight associations between T2DM and cardiovascular disease in general including CAD, “cardiologists are increasingly involved in managing patients with T2DM,” he noted. The statement gives a comprehensive overview and critical assessment of the management of these patients as of the end of 2019 as a consensus from a panel of 11 experts .
The statement also stressed that “substantial portions of patients with T2DM and CAD, including those after an acute coronary syndrome, do not receive therapies with proven cardiovascular benefit, such as high-intensity statins, dual-antiplatelet therapy, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, and glucose-lowering agents with proven cardiovascular benefits.
“These gaps in care highlight a critical opportunity for cardiovascular specialists to assume a more active role in the collaborative care of patients with T2DM and CAD,” the statement said. This includes “encouraging cardiologists to become more active in the selection of glucose-lowering medications” for these patients because it could “really move the needle,” said Dr. Arnold, a cardiologist with Saint Luke’s Health System in Kansas City, Mo. She was referring specifically to broader reliance on both the SGTL2 (sodium-glucose cotransporter 2) inhibitors and the GLP-1 (glucagonlike peptide-1) receptor agonists as top choices for controlling hyperglycemia. Based on recent evidence drugs in these two classes “could be considered first line for patients with T2DM and CAD, and would likely be preferred over metformin,” Dr. Arnold said in an interview. Although the statement identified the SGLT2 inhibitors as “the first drug class [for glycemic control] to show clear benefits on cardiovascular outcomes,” it does not explicitly label the class first-line and it also skirts that designation for the GLP-1 receptor agonist class, while noting that metformin “remains the drug most frequently recommended as first-line therapy in treatment guidelines.”
“I wouldn’t disagree with someone who said that SGLT2 inhibitors and GLP-1 receptor agonists are first line,” but prescribing patterns also depend on familiarity, cost, and access, noted Dr. Bhatt, which can all be issues with agents from these classes compared with metformin, a widely available generic with decades of use. “Metformin is safe and cheap, so we did not want to discount it,” said Dr. Arnold. Dr. Bhatt recently coauthored an editorial that gave an enthusiastic endorsement to using SGLT2 inhibitors in patients with diabetes (Cell Metab. 2019 Nov 5;30[5]:47-9).
Another notable feature of the statement is the potential it assigns to bariatric surgery as a management tool with documented safety and efficacy for improving cardiovascular risk factors. However, the statement also notes that randomized trials “have thus far been inadequately powered to assess cardiovascular events and mortality, although observational studies have consistently shown cardiovascular risk reduction with such procedures.” The statement continues that despite potential cardiovascular benefits “bariatric surgery remains underused among eligible patients,” and said that surgery performed as Roux-en-Y bypass or sleeve gastrectomy “may be another effective tool for cardiovascular risk reduction in the subset of patients with obesity,” particularly patients with a body mass index of at least 35 kg/m2.
“While the percentage of patients who are optimal for bariatric surgery is not known, the most recent NHANES [National Health and Nutrition Examination Study] study showed that less than 0.5% of eligible patients underwent bariatric surgery,” Dr. Arnold noted. Bariatric surgery is “certainly not a recommendation for everyone, or even a majority of patients, but bariatric surgery should be on our radar,” for patients with CAD and T2DM, she said.
Right now, “few cardiologists think about bariatric surgery,” as a treatment option, but study results have shown that “in carefully selected patients treated by skilled surgeons at high-volume centers, patients will do better with bariatric surgery than with best medical therapy for improvements in multiple risk factors, including glycemic control,” Dr. Bhatt said in the interview. “It’s not first-line treatment, but it’s an option to consider,” he added, while also noting that bariatric surgery is most beneficial to patients relatively early in the course of T2DM, when its been in place for just a few years rather than a couple of decades.
The statement also notably included a “first-line” call out for icosapent ethyl (Vascepa), a novel agent approved in December 2019 for routine use in U.S. patients, including those with CAD and T2DM as long as their blood triglyceride level was at least 150 mg/dL. Dr. Bhatt, who led the REDUCE-IT study that was pivotal for proving the safety and efficacy of icosapent ethyl (N Engl J Med. 2019 Jan 3;380[1]:11-22), estimated that anywhere from 15% to as many as half the patients with CAD and T2DM might have a triglyceride level that would allow them to receive icosapent ethyl. One population-based study in Canada of nearly 200,000 people with atherosclerotic cardiovascular disease found a 25% prevalence of the triglyceride level needed to qualify to receive icosapent ethyl under current labeling, he noted (Eur Heart J. 2020 Jan 1;41[1]:86-94). However, the FDA label does not specify that triglycerides be measured when fasting, and a nonfasting level of about 150 mg/dL will likely appear for patients with fasting levels that fall as low as about 100 mg/dL, Dr. Bhatt said. He hoped that future studies will assess the efficacy of icosapent ethyl in patients with even lower triglyceride levels.
Other sections of the statement also recommend that clinicians: Target long-term dual-antiplatelet therapy to CAD and T2DM patients with additional high-risk markers such as prior MI, younger age, and tobacco use; prescribe a low-dose oral anticoagulant along with an antiplatelet drug such as aspirin for secondary-prevention patients; promote a blood pressure target of less than 140/90 mm Hg for all CAD and T2DM patients and apply a goal of less than 130/80 mm Hg in higher-risk patients such as blacks, Asians, and those with cerebrovascular disease; and reassure patients that “despite a modest increase in blood sugars, the risk-benefit ratio is clearly in favor of administering statins to people with T2DM and CAD.”
Dr. Arnold had no disclosures. Dr. Bhatt has been an adviser to Cardax, Cereno Scientific, Medscape Cardiology, PhaseBio; PLx Pharma, and Regado Biosciences, and he has received research funding from numerous companies including Amarin, the company that markets icosapent ethyl.
FROM CIRCULATION
Diabetic retinopathy: The FP’s role in preserving vision
As of 2015, an estimated 30.2 million adults in the United States—12.2% of the population— had diabetes mellitus (DM). During that year, approximately 1.5 million new cases (6.7 cases for every 1000 people) were diagnosed in adults (≥ 18 years of age).1
As the number of people with DM increases, so will the number of cases of diabetic retinopathy, the main cause of new cases of blindness in adults in the United States2 and the leading cause of blindness among US working-age (20 to 74 years) adults.3 It is estimated that 4.1 million Americans have diabetic retinopathy3; it is projected that prevalence will reach 6 million this year.4
Blindness related to DM costs the United States approximately $500 million each year,5 including health care utilization: physician office visits, diagnostic testing, medication and other treatments, and hospitalization.6 Impairment of vision also results in social isolation, dependence on others to perform daily functions, and a decline in physical activity.
Several professional organizations, including the American Diabetes Association and the American Academy of Ophthalmology, have developed practice guidelines for diabetic retinopathy screening. Guidelines notwithstanding, only about 55% of people with DM in the United States receive the recommended dilated eye examination at established intervals.2,3 In addition to screening by an ophthalmologist or optometrist, adherence to clinical guidelines for risk assessment, prevention, and early referral helps reduce the incidence and severity of retinopathy.5
This article describes how to assess the risk of diabetic retinopathy in your patients, details the crucial role that you, the primary care physician, can play in prevention, and emphasizes the importance of referral to an eye specialist for screening, evaluation, treatment (when indicated), and follow-up.
Pathophysiology and classification
Diabetic retinopathy, the result of progressive blood vessel damage to the retina, has 2 major forms: nonproliferative and proliferative. Those forms are distinguished by the absence or presence of new growth of blood vessels (retinal neovascularization).3,7 To improve communication and coordination among physicians who care for patients with DM worldwide, the International Clinical Diabetic Retinopathy Disease Severity Scale for diabetic retinopathy was developed,8-10 comprising 5 levels of severity that are based on findings on dilated ophthalmoscopy (Table 18-10):
- Level 1. No apparent retinopathy. Funduscopic abnormalities are absent.
- Level 2. Mild nonproliferative diabetic retinopathy (NPDR). Only a few microaneurysms are seen.
- Level 3: Moderate NPDR. Characterized by microaneurysms and by intraretinal hemorrhage and venous beading, but less severe than what is seen in Level 4.
- Level 4. Severe NPDR. More than 20 intraretinal hemorrhages in each quadrant of the retina, definite venous beading in > 2 quadrants, intraretinal microvascular abnormalities in > 1 quadrant, or any combination of these findings.
- Level 5. Proliferative diabetic retinopathy. Characterized by neovascularization of the disc, retina, iris, or angle; vitreous hemorrhage; retinal detachment; or any combination of these findings. Further classified as “mild,” “moderate,” or “severe” if macular edema is present; severity is dependent on the distance of thickening and exudates from the center of the macula.9
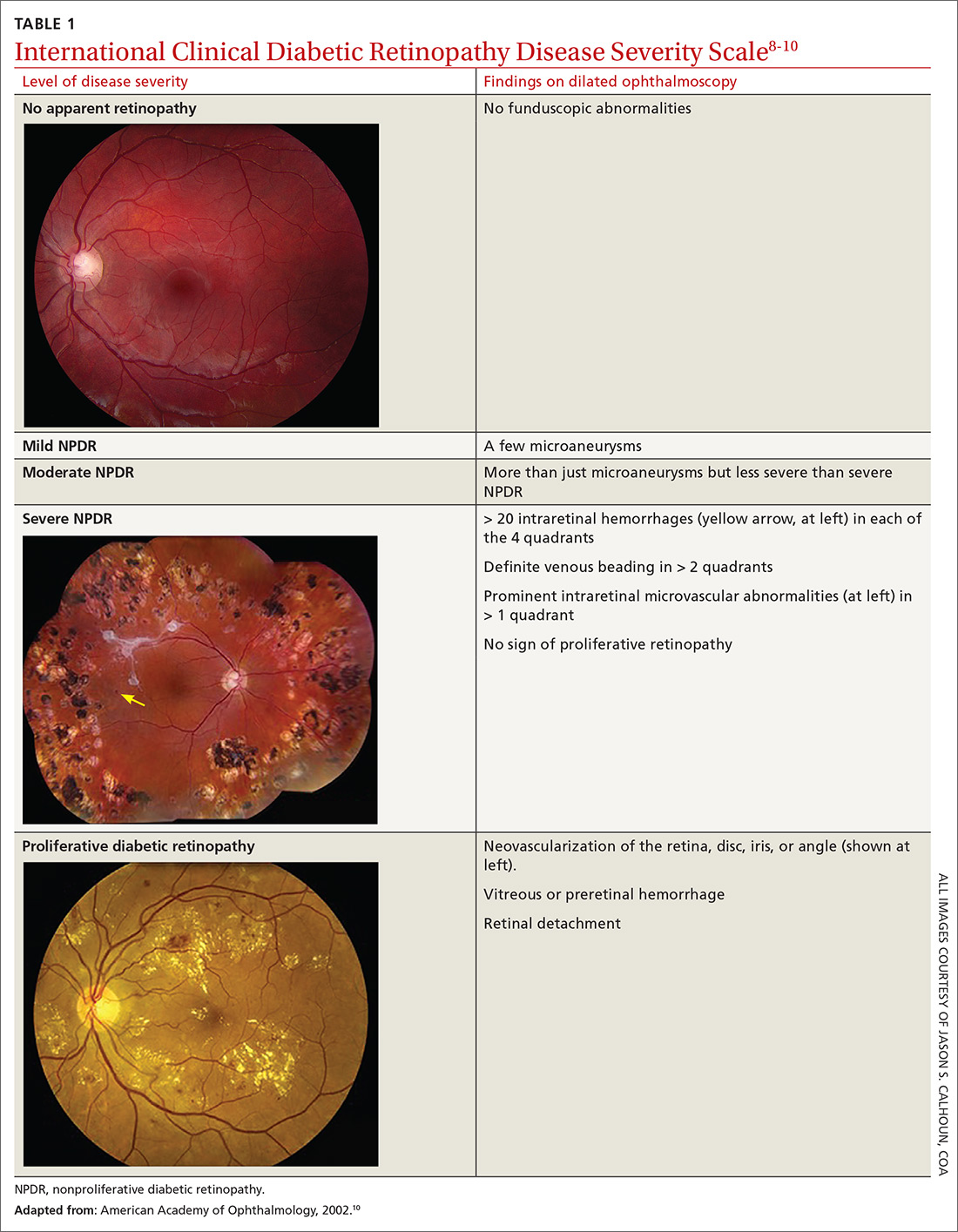
Be attentive to risk factors
There are several risk factors for diabetic retinopathy, including duration of disease, type 1 DM, male gender, black race (non-Hispanic), elevated hemoglobin A1C(HbA1C) level, elevated systolic and diastolic blood pressure (BP), and insulin therapy. 4,5,11,12
Continue to: Time since diagnosis
Time since diagnosis. The Wisconsin Epidemiologic Study of Diabetic Retinopathy found that the prevalence of diabetic retinopathy varied from 28.8% in people who had DM for < 5 years to 77.8% in people who had DM for ≥ 15 years. The rate of proliferative diabetic retinopathy was 2% in people who had DM for < 5 years and 15.5% in those who had DM for ≥ 15 years.11
Demographic variables. The prevalence of diabetic retinopathy is higher in men, non-Hispanic blacks (38.8%), and people with type 1 DM.4,5,11-13 The Veterans Affairs Diabetes Trial found a higher prevalence of moderate-to-severe diabetic retinopathy in Hispanics (36%) and African Americans (29%) than in non-Hispanic whites (22%).14
Among people with DM who have diabetic retinopathy, systolic and diastolic BP and the HbA1C level tend to be higher. They are more likely to use insulin to control disease.4,5,13 In a recent cross-sectional analysis, the prevalence of vision-threatening retinopathy was higher among people ≥ 65 years of age (1%; 95% confidence interval [CI], 0.7%-1.5%) than among people 40 to 64 years of age (0.4%; 95% CI, 0.3%-0.7%) (P = .009).5
Does pregnancy exacerbate retinopathy? Controversy surrounds the role of pregnancy in the development and progression of diabetic retinopathy. The Diabetes Control and Complications Trial found a short-term increase in the level of retinopathy during pregnancy that persisted into the first postpartum year. A 1.63-fold greater risk of any deterioration of retinopathy was observed in women who received intensive DM treatment from before to during pregnancy (P < .05); pregnant women who received conventional treatment had a 2.48-fold greater risk than nonpregnant women with DM who received conventional treatment (P < .001).
Deterioration of retinopathy during pregnancy had no long-term consequences, however, regardless of type of treatment.15 More importantly, in most cases, changes in the level of retinopathy revert to the pre-pregnancy level after 1 year or longer, and pregnancy does not appear to affect long-term progression of retinopathy.15
Continue to: Proven primary prevention strategies
Proven primary prevention strategies
Glycemic control. Optimal glycemic control is an essential component of prevention of diabetic retinopathy. From 1983 to 1993, the Diabetes Control and Complications Trial randomized 1441 patients with type 1 DM to receive intensive therapy (median HbA1C level, 7.2%) or conventional therapy (median HbA1C level, 9.1%). During a mean of 6 years of follow-up, intensive therapy reduced the adjusted mean risk of retinopathy by 76% (95% CI, 62%-85%).16,17 A 2007 systematic review of 44 studies of the treatment of diabetic retinopathy found that strict glycemic control was beneficial in reducing the incidence and progression of retinopathy.17
The American Diabetes Association’s Standards of Medical Care in Diabetes—2019 Abridged for Primary Care Providers recommends that most nonpregnant adults maintain an HbA1Clevel < 7%. For patients with a history of hypoglycemia, limited life expectancy, advanced microvascular or macrovascular disease, other significant comorbid conditions, or longstanding DM in which it is difficult to achieve the optimal goal, a higher HbA1clevel (< 8%) might be appropriate.18
Control of BP. Strict control of BP is a major modifier of the incidence and progression of diabetic retinopathy.17,19 In the United Kingdom Prospective Diabetes Study, 1148 patients with type 2 DM and a mean BP of 160/94 mm Hg at the onset of the study were randomly assigned to either (1) a “tight” blood pressure group (< 150/85 mm Hg) or (2) a “less-tight” group (< 180/105 mm Hg). The primary therapy for controlling BP was captopril or atenolol. After 9 years of follow-up, the tight-control group had a 34% mean reduction in risk in the percentage of patients with deterioration of retinopathy (99% CI, 11%-50%; P = .0004) and a 47% reduction in risk (99% CI, 7%-70%; P = .004) of deterioration in visual acuity.20
Most patients with DM and hypertension should be treated to maintain a BP < 140/90 mm Hg. Although there is insufficient evidence to recommend a specific antihypertensive agent for preventing diabetic retinopathy, therapy should include agents from drug classes that have a demonstrated reduction in cardiovascular events in patients with DM. These include angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, thiazide diuretics, and dihydropyridine calcium channel blockers.18
Lipid management. The benefit of targeted therapy for lowering lipids for the prevention of diabetic retinopathy is not well established.17 In the Collaborative Atorvastatin Diabetes Study, 2838 patients with type 2 DM were randomized to atorvastatin (10 mg) or placebo; microvascular endpoint analysis demonstrated that patients taking atorvastatin needed less laser therapy (P = .14); however, progression of diabetic retinopathy was not reduced.21 Similarly, in the Action to Control Cardiovascular Risk in Diabetes Eye Study, slowing of progression to retinopathy was observed in patients with type 2 DM who were treated with fenofibrate (ie, progression in 6.5%, compared with progression in 10.2% of untreated subjects [odds ratio = 0.60 (95% CI, 0.42-0.87); P = .0056]).22
Continue to: Despite limited data on...
Despite limited data on the impact of lipid-lowering agents on patients with diabetic retinopathy, those with type 2 DM (especially) and those who have, or are at risk of, atherosclerotic cardiovascular disease should receive statin therapy.18
Aspirin therapy. Aspirin has not been found to be beneficial for slowing progression of diabetic retinopathy. However, aspirin did not cause further deterioration of disease, specifically in patients with vitreous hemorrhages4; patients with diabetic retinopathy who require aspirin therapy for other medical reasons can therefore continue to take it without increasing the risk of damage to the retina.4,18
When should you refer patients for screening?
Screening for diabetic retinopathy is important because affected patients can be asymptomatic but have significant disease. Early detection also helps determine which patients need treatment when it is most beneficial: early in its course.4
Type 1 DM. Retinopathy can become apparent as early as 6 or 7 years after the onset of disease, and is rare in children prior to puberty.4,11 As a result, patients with type 1 DM should first be screened with a comprehensive eye examination by an ophthalmologist or optometrist within 5 years of DM onset.4,18
Type 2 DM. Because of the insidious onset of type 2 DM, patients who are given a diagnosis of DM after 30 years of age might already have high-risk features of retinopathy.9 In patients with type 2 DM, therefore, initial screening for diabetic retinopathy should begin at the time of diagnosis and include a comprehensive eye examination by an ophthalmologist or optometrist.4,18,23
Continue to: Components of the exam
Components of the exam. Initial evaluation by the ophthalmologist or optometrist should include a detailed history and comprehensive eye exam with pupil dilation. Table 24 lists elements of the initial physical exam, which should assess for features that often lead to visual impairment. These features include macular edema, retinal hemorrhage, venous beading, neovascularization, and vitreous hemorrhage.4
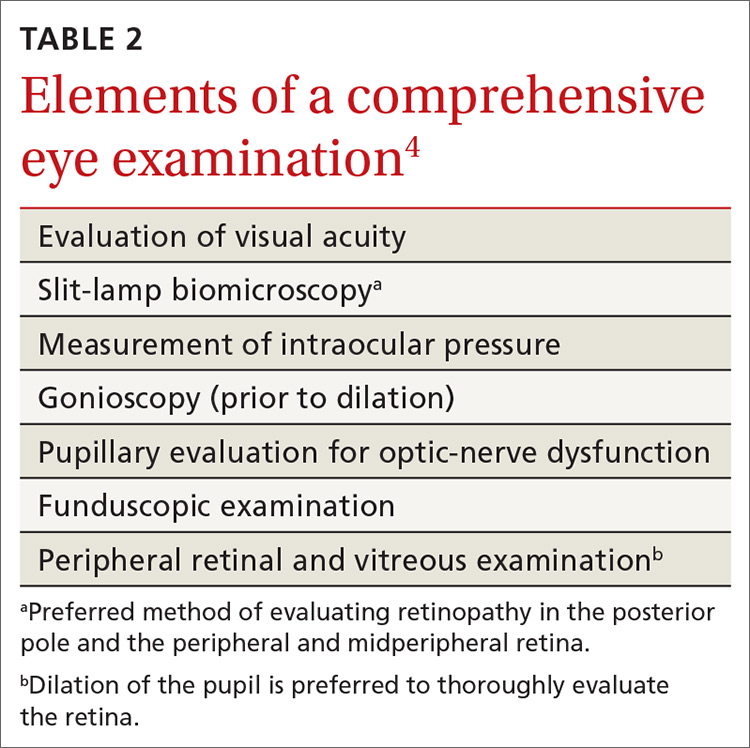
Frequency of follow-up. The interval between subsequent examinations should be individualized, based on the findings of the initial assessment. Consider that:
- Screening should occur every 1 or 2 years in patients without evidence of retinopathy and with adequate glycemic control.4,18,23
- Screening every 1 or 2 years appears to be cost-effective in patients who have had 1 or more normal eye exams.
- A 3-year screening interval does not appear to present a risk in well-controlled patients with type 2 DM.24
- Women with type 1 or type 2 DM who are planning pregnancy or who are pregnant should have an eye exam prior to pregnancy or early in the first trimester.4,18,23 They should then be monitored each trimester and at the end of the first postpartum year, depending on the severity of retinopathy.18
Alternative screening modalities
Seven-field stereoscopic fundus photography is an alternative screening tool that compares favorably to ophthalmoscopy when performed by an experienced ophthalmologist, optometrist, or ophthalmologic technician.25 Nonmydriatic digital stereoscopic retinal imaging has been shown to be a cost-effective method of screening patients for diabetic retinopathy.26 In a study that compared digital imaging with dilated funduscopic examination, investigators reported that, of 311 eyes evaluated, there was agreement between the methods in 86% of cases. Disagreement was mostly related to the greater frequency of finding mild-to-moderate NPDR when using digital imaging.27
Screening in primary care
Programs that use telemedicine-based fundus photography to screen for diabetic retinopathy during primary care visits, followed by remote interpretation by an ophthalmologist, have been shown to increase the rate of retinal screening by offering an option other than direct referral to an ophthalmologist or optometrist.28 However, telemedicine-based retinal photography can be successful as a screening tool for retinopathy only if timely referral to an eye specialist is arranged when indicated by findings.18
SIDEBAR
Key points in the progression of diabetic retinopathy care
Duration of diabetes, poor glycemic control, and uncontrolled hypertension are major risk factors for diabetic retinopathy.
To reduce the risk of diabetic retinopathy, patients with diabetes mellitus should:
- sustain good glycemic control (hemoglobin A1C level, < 7%)
- maintain blood pressure < 140/90 mm Hg
- undergo periodic routine screening eye examination.
Early detection of diabetic retinopathy by dilated eye examination or fundus photography can lead to early therapeutic intervention, which can reduce the risk of visual impairment and vision loss.
Treatment is based on severity of disease and can include anti-vascular-endothelial growth factor therapy, photocoagulation, or surgery.
What therapy will your referred patients receive?
Patients found to have signs of diabetic retinopathy should be referred to an ophthalmologist who is knowledgeable and experienced in the management of diabetic retinopathy. Care will be managed according to the severity of the patient’s diabetic retinopathy.
Continue to: Patients with mild-to-moderate NPDR but without macular edema
Patients with mild-to-moderate NPDR but without macular edema. Treatment is generally not recommended. Patients should be reevaluated every 6 to 12 months because they have an increased risk of progression.5
Patients with mild-to-moderate NPDR and clinically significant macular edema (CSME). It is important for the eye specialist to assess for edema at the center of the macula because the risk of vision loss and need for treatment is greater when the center is involved. Vascular–endothelial growth factor (VEGF) is an important mediator of neovascularization and macular edema in diabetic retinopathy. For patients with center-involving CSME, intravitreous injection of an anti-VEGF agent provides significant benefit and is first-line treatment in these cases.4,29
The Early Treatment for Diabetic Retinopathy Study evaluated the efficacy of focal photocoagulation, a painless laser therapy, for CSME and demonstrated that this modality reduces the risk of moderate visual loss; increases the likelihood of improvement in vision; and decreases the frequency of persistent macular edema.30 Focal photocoagulation has been found effective in both non-center-involving CSME and center-involving CSME.5
Patients with severe NPDR. The recommendation is to initiate full panretinal photocoagulation prior to progression to proliferative diabetic retinopathy PDR. Researchers noted a 50% reduction in vision loss and vitrectomy when patients with type 2 DM were treated with panretinal photocoagulation early, compared with those in whom treatment was deferred until PDR developed.4,31 The role of anti-VEGF treatment of severe NPDR is under investigation.4
Patients with high-risk and severe PDR. Panretinal photocoagulation is the recommended treatment for patients with high-risk and severe PDR, and usually induces regression of retinal neovascularization. In patients with CSME and high-risk PDR, the combination of anti-VEGF therapy and panretinal photocoagulation should be considered. Vitrectomy should be considered for patients who have failed panretinal photocoagulation or are not amenable to photocoagulation.4
CORRESPONDENCE
Bryan Farford, DO, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL 32224; [email protected].
1. National Diabetes Statistic Report 2020: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 20, 2020.
2. Fitch K, Weisman T, Engel T, et al. Longitudinal commercial claims-based cost analysis of diabetic retinopathy screening patterns. Am Health Drug Benefits. 2015;8:300-308.
3. Centers for Disease Control and Prevention. Common eye disorders. September 29, 2015. www.cdc.gov/visionhealth/basics/ced/index.html. Accessed March 20, 2020.
4. American Academy of Ophthalmology PPP Retina/Vitreous Committee, Hoskins Center for Quality Eye Care. Diabetic Retinopathy PPP 2019. San Francisco, CA: American Academy of Ophthalmology. October 2019. https://www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp. Accessed March 20, 2020.
5. Zhang X, Saaddine JB, Chou C-F, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304:649-656.
6. Stewart MW. Socioeconomic cost of diabetic retinopathy and therapy. In: Diabetic Retinopathy. Singapore: Adis; 2017:257-268.
7. Tarr JM, Kaul K, Chopra M, et al. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560.
8. Wilkinson CP, Ferris FL 3rd, Klein RE, et al. Proposed International Clinical Diabetic Retinopathy and Diabetic Macular Edema Disease Severity Scales. Ophthalmology. 2003;110:1677-1682.
9. Wu L, Fernandez-Loaiza P, Sauma J, et al. Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes. 2013;4:290-294.
10. American Academy of Ophthalmology. International Clinical Diabetic Retinopathy Disease Severity Scale detailed table. October 2002. http://www.icoph.org/downloads/Diabetic-Retinopathy-Detail.pdf. Accessed March 20, 2020.
11. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Ophthalmol. 1984;102:527-532.
12. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ten-year incidence and progression of diabetic retinopathy. Arch Ophthalmol. 1994;112:1217-1228.
13. Klein R, Knudtson MD, Lee KE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXII. The twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115:1859-1868.
14. Emanuele N, Sacks J, Klein R, et al. Ethnicity, race, and baseline retinopathy correlates in the Veterans Affairs Diabetes Trial. Diabetes Care. 2005;28:1954-1958.
15. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Diabetes Care. 2000;23:1084-1091.
16. , , The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
17. Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007;298:902-916.
18. American Diabetes Association. Standards of Medical Care in Diabetes—2019 abridged for primary care providers. Clin Diabetes. 2019;37:11-34.
19. Do DV, Wang X, Vedula SS, et al. Blood pressure control for diabetic retinopathy. Cochrane Database Syst Rev. 2015;(1):CD006127.
20. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703-713.
21. Colhoun HM, Betteridge DJ, Durrington PN; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685-696.
22. Chew EY, Davis MD, Danis RP, et al. Action to Control Cardiovascular Risk in Diabetes Eye Study Research Group. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology. 2014;121:2443-2451.
23. Fong DS, Aiello L, Gardner TW, et al American Diabetes Association. Retinopathy in diabetes. Diabetes Care. 2004;27(suppl 1):S84-S87.
24. 11. Microvascular complications and foot care: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S124-S138.
25. Moss SE, Klein R, Kessler SD, et al. Comparison between ophthalmoscopy and fundus photography in determining severity of diabetic retinopathy. Ophthalmology. 1985;92:62-67.
26. Kirkizlar E, Serban N, Sisson JA, et al. Evaluation of telemedicine for screening of diabetic Retinopathy in the Veterans Health Administration. Ophthalmology. 2013;120:2604-2610.
27. Ahmed J, Ward TP, Bursell S-E, et al. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Diabetes Care. 2006;29:2205-2209.
28. Taylor CR, Merin LM, Salunga AM, et al. Improving diabetic retinopathy screening ratios using telemedicine-based digital retinal imaging technology: the Vine Hill study. Diabetes Care. 2007;30:574.
29. , , , Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193-1203.
30. Early photocoagulation for diabetic retinopathy. ETDRS Report Number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 suppl):766-785.
31. Ferris F. Early photocoagulation in patients with either type I or type II diabetes. Trans Am Ophthalmol Soc. 1996;94:505-537.
As of 2015, an estimated 30.2 million adults in the United States—12.2% of the population— had diabetes mellitus (DM). During that year, approximately 1.5 million new cases (6.7 cases for every 1000 people) were diagnosed in adults (≥ 18 years of age).1
As the number of people with DM increases, so will the number of cases of diabetic retinopathy, the main cause of new cases of blindness in adults in the United States2 and the leading cause of blindness among US working-age (20 to 74 years) adults.3 It is estimated that 4.1 million Americans have diabetic retinopathy3; it is projected that prevalence will reach 6 million this year.4
Blindness related to DM costs the United States approximately $500 million each year,5 including health care utilization: physician office visits, diagnostic testing, medication and other treatments, and hospitalization.6 Impairment of vision also results in social isolation, dependence on others to perform daily functions, and a decline in physical activity.
Several professional organizations, including the American Diabetes Association and the American Academy of Ophthalmology, have developed practice guidelines for diabetic retinopathy screening. Guidelines notwithstanding, only about 55% of people with DM in the United States receive the recommended dilated eye examination at established intervals.2,3 In addition to screening by an ophthalmologist or optometrist, adherence to clinical guidelines for risk assessment, prevention, and early referral helps reduce the incidence and severity of retinopathy.5
This article describes how to assess the risk of diabetic retinopathy in your patients, details the crucial role that you, the primary care physician, can play in prevention, and emphasizes the importance of referral to an eye specialist for screening, evaluation, treatment (when indicated), and follow-up.
Pathophysiology and classification
Diabetic retinopathy, the result of progressive blood vessel damage to the retina, has 2 major forms: nonproliferative and proliferative. Those forms are distinguished by the absence or presence of new growth of blood vessels (retinal neovascularization).3,7 To improve communication and coordination among physicians who care for patients with DM worldwide, the International Clinical Diabetic Retinopathy Disease Severity Scale for diabetic retinopathy was developed,8-10 comprising 5 levels of severity that are based on findings on dilated ophthalmoscopy (Table 18-10):
- Level 1. No apparent retinopathy. Funduscopic abnormalities are absent.
- Level 2. Mild nonproliferative diabetic retinopathy (NPDR). Only a few microaneurysms are seen.
- Level 3: Moderate NPDR. Characterized by microaneurysms and by intraretinal hemorrhage and venous beading, but less severe than what is seen in Level 4.
- Level 4. Severe NPDR. More than 20 intraretinal hemorrhages in each quadrant of the retina, definite venous beading in > 2 quadrants, intraretinal microvascular abnormalities in > 1 quadrant, or any combination of these findings.
- Level 5. Proliferative diabetic retinopathy. Characterized by neovascularization of the disc, retina, iris, or angle; vitreous hemorrhage; retinal detachment; or any combination of these findings. Further classified as “mild,” “moderate,” or “severe” if macular edema is present; severity is dependent on the distance of thickening and exudates from the center of the macula.9

Be attentive to risk factors
There are several risk factors for diabetic retinopathy, including duration of disease, type 1 DM, male gender, black race (non-Hispanic), elevated hemoglobin A1C(HbA1C) level, elevated systolic and diastolic blood pressure (BP), and insulin therapy. 4,5,11,12
Continue to: Time since diagnosis
Time since diagnosis. The Wisconsin Epidemiologic Study of Diabetic Retinopathy found that the prevalence of diabetic retinopathy varied from 28.8% in people who had DM for < 5 years to 77.8% in people who had DM for ≥ 15 years. The rate of proliferative diabetic retinopathy was 2% in people who had DM for < 5 years and 15.5% in those who had DM for ≥ 15 years.11
Demographic variables. The prevalence of diabetic retinopathy is higher in men, non-Hispanic blacks (38.8%), and people with type 1 DM.4,5,11-13 The Veterans Affairs Diabetes Trial found a higher prevalence of moderate-to-severe diabetic retinopathy in Hispanics (36%) and African Americans (29%) than in non-Hispanic whites (22%).14
Among people with DM who have diabetic retinopathy, systolic and diastolic BP and the HbA1C level tend to be higher. They are more likely to use insulin to control disease.4,5,13 In a recent cross-sectional analysis, the prevalence of vision-threatening retinopathy was higher among people ≥ 65 years of age (1%; 95% confidence interval [CI], 0.7%-1.5%) than among people 40 to 64 years of age (0.4%; 95% CI, 0.3%-0.7%) (P = .009).5
Does pregnancy exacerbate retinopathy? Controversy surrounds the role of pregnancy in the development and progression of diabetic retinopathy. The Diabetes Control and Complications Trial found a short-term increase in the level of retinopathy during pregnancy that persisted into the first postpartum year. A 1.63-fold greater risk of any deterioration of retinopathy was observed in women who received intensive DM treatment from before to during pregnancy (P < .05); pregnant women who received conventional treatment had a 2.48-fold greater risk than nonpregnant women with DM who received conventional treatment (P < .001).
Deterioration of retinopathy during pregnancy had no long-term consequences, however, regardless of type of treatment.15 More importantly, in most cases, changes in the level of retinopathy revert to the pre-pregnancy level after 1 year or longer, and pregnancy does not appear to affect long-term progression of retinopathy.15
Continue to: Proven primary prevention strategies
Proven primary prevention strategies
Glycemic control. Optimal glycemic control is an essential component of prevention of diabetic retinopathy. From 1983 to 1993, the Diabetes Control and Complications Trial randomized 1441 patients with type 1 DM to receive intensive therapy (median HbA1C level, 7.2%) or conventional therapy (median HbA1C level, 9.1%). During a mean of 6 years of follow-up, intensive therapy reduced the adjusted mean risk of retinopathy by 76% (95% CI, 62%-85%).16,17 A 2007 systematic review of 44 studies of the treatment of diabetic retinopathy found that strict glycemic control was beneficial in reducing the incidence and progression of retinopathy.17
The American Diabetes Association’s Standards of Medical Care in Diabetes—2019 Abridged for Primary Care Providers recommends that most nonpregnant adults maintain an HbA1Clevel < 7%. For patients with a history of hypoglycemia, limited life expectancy, advanced microvascular or macrovascular disease, other significant comorbid conditions, or longstanding DM in which it is difficult to achieve the optimal goal, a higher HbA1clevel (< 8%) might be appropriate.18
Control of BP. Strict control of BP is a major modifier of the incidence and progression of diabetic retinopathy.17,19 In the United Kingdom Prospective Diabetes Study, 1148 patients with type 2 DM and a mean BP of 160/94 mm Hg at the onset of the study were randomly assigned to either (1) a “tight” blood pressure group (< 150/85 mm Hg) or (2) a “less-tight” group (< 180/105 mm Hg). The primary therapy for controlling BP was captopril or atenolol. After 9 years of follow-up, the tight-control group had a 34% mean reduction in risk in the percentage of patients with deterioration of retinopathy (99% CI, 11%-50%; P = .0004) and a 47% reduction in risk (99% CI, 7%-70%; P = .004) of deterioration in visual acuity.20
Most patients with DM and hypertension should be treated to maintain a BP < 140/90 mm Hg. Although there is insufficient evidence to recommend a specific antihypertensive agent for preventing diabetic retinopathy, therapy should include agents from drug classes that have a demonstrated reduction in cardiovascular events in patients with DM. These include angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, thiazide diuretics, and dihydropyridine calcium channel blockers.18
Lipid management. The benefit of targeted therapy for lowering lipids for the prevention of diabetic retinopathy is not well established.17 In the Collaborative Atorvastatin Diabetes Study, 2838 patients with type 2 DM were randomized to atorvastatin (10 mg) or placebo; microvascular endpoint analysis demonstrated that patients taking atorvastatin needed less laser therapy (P = .14); however, progression of diabetic retinopathy was not reduced.21 Similarly, in the Action to Control Cardiovascular Risk in Diabetes Eye Study, slowing of progression to retinopathy was observed in patients with type 2 DM who were treated with fenofibrate (ie, progression in 6.5%, compared with progression in 10.2% of untreated subjects [odds ratio = 0.60 (95% CI, 0.42-0.87); P = .0056]).22
Continue to: Despite limited data on...
Despite limited data on the impact of lipid-lowering agents on patients with diabetic retinopathy, those with type 2 DM (especially) and those who have, or are at risk of, atherosclerotic cardiovascular disease should receive statin therapy.18
Aspirin therapy. Aspirin has not been found to be beneficial for slowing progression of diabetic retinopathy. However, aspirin did not cause further deterioration of disease, specifically in patients with vitreous hemorrhages4; patients with diabetic retinopathy who require aspirin therapy for other medical reasons can therefore continue to take it without increasing the risk of damage to the retina.4,18
When should you refer patients for screening?
Screening for diabetic retinopathy is important because affected patients can be asymptomatic but have significant disease. Early detection also helps determine which patients need treatment when it is most beneficial: early in its course.4
Type 1 DM. Retinopathy can become apparent as early as 6 or 7 years after the onset of disease, and is rare in children prior to puberty.4,11 As a result, patients with type 1 DM should first be screened with a comprehensive eye examination by an ophthalmologist or optometrist within 5 years of DM onset.4,18
Type 2 DM. Because of the insidious onset of type 2 DM, patients who are given a diagnosis of DM after 30 years of age might already have high-risk features of retinopathy.9 In patients with type 2 DM, therefore, initial screening for diabetic retinopathy should begin at the time of diagnosis and include a comprehensive eye examination by an ophthalmologist or optometrist.4,18,23
Continue to: Components of the exam
Components of the exam. Initial evaluation by the ophthalmologist or optometrist should include a detailed history and comprehensive eye exam with pupil dilation. Table 24 lists elements of the initial physical exam, which should assess for features that often lead to visual impairment. These features include macular edema, retinal hemorrhage, venous beading, neovascularization, and vitreous hemorrhage.4

Frequency of follow-up. The interval between subsequent examinations should be individualized, based on the findings of the initial assessment. Consider that:
- Screening should occur every 1 or 2 years in patients without evidence of retinopathy and with adequate glycemic control.4,18,23
- Screening every 1 or 2 years appears to be cost-effective in patients who have had 1 or more normal eye exams.
- A 3-year screening interval does not appear to present a risk in well-controlled patients with type 2 DM.24
- Women with type 1 or type 2 DM who are planning pregnancy or who are pregnant should have an eye exam prior to pregnancy or early in the first trimester.4,18,23 They should then be monitored each trimester and at the end of the first postpartum year, depending on the severity of retinopathy.18
Alternative screening modalities
Seven-field stereoscopic fundus photography is an alternative screening tool that compares favorably to ophthalmoscopy when performed by an experienced ophthalmologist, optometrist, or ophthalmologic technician.25 Nonmydriatic digital stereoscopic retinal imaging has been shown to be a cost-effective method of screening patients for diabetic retinopathy.26 In a study that compared digital imaging with dilated funduscopic examination, investigators reported that, of 311 eyes evaluated, there was agreement between the methods in 86% of cases. Disagreement was mostly related to the greater frequency of finding mild-to-moderate NPDR when using digital imaging.27
Screening in primary care
Programs that use telemedicine-based fundus photography to screen for diabetic retinopathy during primary care visits, followed by remote interpretation by an ophthalmologist, have been shown to increase the rate of retinal screening by offering an option other than direct referral to an ophthalmologist or optometrist.28 However, telemedicine-based retinal photography can be successful as a screening tool for retinopathy only if timely referral to an eye specialist is arranged when indicated by findings.18
SIDEBAR
Key points in the progression of diabetic retinopathy care
Duration of diabetes, poor glycemic control, and uncontrolled hypertension are major risk factors for diabetic retinopathy.
To reduce the risk of diabetic retinopathy, patients with diabetes mellitus should:
- sustain good glycemic control (hemoglobin A1C level, < 7%)
- maintain blood pressure < 140/90 mm Hg
- undergo periodic routine screening eye examination.
Early detection of diabetic retinopathy by dilated eye examination or fundus photography can lead to early therapeutic intervention, which can reduce the risk of visual impairment and vision loss.
Treatment is based on severity of disease and can include anti-vascular-endothelial growth factor therapy, photocoagulation, or surgery.
What therapy will your referred patients receive?
Patients found to have signs of diabetic retinopathy should be referred to an ophthalmologist who is knowledgeable and experienced in the management of diabetic retinopathy. Care will be managed according to the severity of the patient’s diabetic retinopathy.
Continue to: Patients with mild-to-moderate NPDR but without macular edema
Patients with mild-to-moderate NPDR but without macular edema. Treatment is generally not recommended. Patients should be reevaluated every 6 to 12 months because they have an increased risk of progression.5
Patients with mild-to-moderate NPDR and clinically significant macular edema (CSME). It is important for the eye specialist to assess for edema at the center of the macula because the risk of vision loss and need for treatment is greater when the center is involved. Vascular–endothelial growth factor (VEGF) is an important mediator of neovascularization and macular edema in diabetic retinopathy. For patients with center-involving CSME, intravitreous injection of an anti-VEGF agent provides significant benefit and is first-line treatment in these cases.4,29
The Early Treatment for Diabetic Retinopathy Study evaluated the efficacy of focal photocoagulation, a painless laser therapy, for CSME and demonstrated that this modality reduces the risk of moderate visual loss; increases the likelihood of improvement in vision; and decreases the frequency of persistent macular edema.30 Focal photocoagulation has been found effective in both non-center-involving CSME and center-involving CSME.5
Patients with severe NPDR. The recommendation is to initiate full panretinal photocoagulation prior to progression to proliferative diabetic retinopathy PDR. Researchers noted a 50% reduction in vision loss and vitrectomy when patients with type 2 DM were treated with panretinal photocoagulation early, compared with those in whom treatment was deferred until PDR developed.4,31 The role of anti-VEGF treatment of severe NPDR is under investigation.4
Patients with high-risk and severe PDR. Panretinal photocoagulation is the recommended treatment for patients with high-risk and severe PDR, and usually induces regression of retinal neovascularization. In patients with CSME and high-risk PDR, the combination of anti-VEGF therapy and panretinal photocoagulation should be considered. Vitrectomy should be considered for patients who have failed panretinal photocoagulation or are not amenable to photocoagulation.4
CORRESPONDENCE
Bryan Farford, DO, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL 32224; [email protected].
As of 2015, an estimated 30.2 million adults in the United States—12.2% of the population— had diabetes mellitus (DM). During that year, approximately 1.5 million new cases (6.7 cases for every 1000 people) were diagnosed in adults (≥ 18 years of age).1
As the number of people with DM increases, so will the number of cases of diabetic retinopathy, the main cause of new cases of blindness in adults in the United States2 and the leading cause of blindness among US working-age (20 to 74 years) adults.3 It is estimated that 4.1 million Americans have diabetic retinopathy3; it is projected that prevalence will reach 6 million this year.4
Blindness related to DM costs the United States approximately $500 million each year,5 including health care utilization: physician office visits, diagnostic testing, medication and other treatments, and hospitalization.6 Impairment of vision also results in social isolation, dependence on others to perform daily functions, and a decline in physical activity.
Several professional organizations, including the American Diabetes Association and the American Academy of Ophthalmology, have developed practice guidelines for diabetic retinopathy screening. Guidelines notwithstanding, only about 55% of people with DM in the United States receive the recommended dilated eye examination at established intervals.2,3 In addition to screening by an ophthalmologist or optometrist, adherence to clinical guidelines for risk assessment, prevention, and early referral helps reduce the incidence and severity of retinopathy.5
This article describes how to assess the risk of diabetic retinopathy in your patients, details the crucial role that you, the primary care physician, can play in prevention, and emphasizes the importance of referral to an eye specialist for screening, evaluation, treatment (when indicated), and follow-up.
Pathophysiology and classification
Diabetic retinopathy, the result of progressive blood vessel damage to the retina, has 2 major forms: nonproliferative and proliferative. Those forms are distinguished by the absence or presence of new growth of blood vessels (retinal neovascularization).3,7 To improve communication and coordination among physicians who care for patients with DM worldwide, the International Clinical Diabetic Retinopathy Disease Severity Scale for diabetic retinopathy was developed,8-10 comprising 5 levels of severity that are based on findings on dilated ophthalmoscopy (Table 18-10):
- Level 1. No apparent retinopathy. Funduscopic abnormalities are absent.
- Level 2. Mild nonproliferative diabetic retinopathy (NPDR). Only a few microaneurysms are seen.
- Level 3: Moderate NPDR. Characterized by microaneurysms and by intraretinal hemorrhage and venous beading, but less severe than what is seen in Level 4.
- Level 4. Severe NPDR. More than 20 intraretinal hemorrhages in each quadrant of the retina, definite venous beading in > 2 quadrants, intraretinal microvascular abnormalities in > 1 quadrant, or any combination of these findings.
- Level 5. Proliferative diabetic retinopathy. Characterized by neovascularization of the disc, retina, iris, or angle; vitreous hemorrhage; retinal detachment; or any combination of these findings. Further classified as “mild,” “moderate,” or “severe” if macular edema is present; severity is dependent on the distance of thickening and exudates from the center of the macula.9

Be attentive to risk factors
There are several risk factors for diabetic retinopathy, including duration of disease, type 1 DM, male gender, black race (non-Hispanic), elevated hemoglobin A1C(HbA1C) level, elevated systolic and diastolic blood pressure (BP), and insulin therapy. 4,5,11,12
Continue to: Time since diagnosis
Time since diagnosis. The Wisconsin Epidemiologic Study of Diabetic Retinopathy found that the prevalence of diabetic retinopathy varied from 28.8% in people who had DM for < 5 years to 77.8% in people who had DM for ≥ 15 years. The rate of proliferative diabetic retinopathy was 2% in people who had DM for < 5 years and 15.5% in those who had DM for ≥ 15 years.11
Demographic variables. The prevalence of diabetic retinopathy is higher in men, non-Hispanic blacks (38.8%), and people with type 1 DM.4,5,11-13 The Veterans Affairs Diabetes Trial found a higher prevalence of moderate-to-severe diabetic retinopathy in Hispanics (36%) and African Americans (29%) than in non-Hispanic whites (22%).14
Among people with DM who have diabetic retinopathy, systolic and diastolic BP and the HbA1C level tend to be higher. They are more likely to use insulin to control disease.4,5,13 In a recent cross-sectional analysis, the prevalence of vision-threatening retinopathy was higher among people ≥ 65 years of age (1%; 95% confidence interval [CI], 0.7%-1.5%) than among people 40 to 64 years of age (0.4%; 95% CI, 0.3%-0.7%) (P = .009).5
Does pregnancy exacerbate retinopathy? Controversy surrounds the role of pregnancy in the development and progression of diabetic retinopathy. The Diabetes Control and Complications Trial found a short-term increase in the level of retinopathy during pregnancy that persisted into the first postpartum year. A 1.63-fold greater risk of any deterioration of retinopathy was observed in women who received intensive DM treatment from before to during pregnancy (P < .05); pregnant women who received conventional treatment had a 2.48-fold greater risk than nonpregnant women with DM who received conventional treatment (P < .001).
Deterioration of retinopathy during pregnancy had no long-term consequences, however, regardless of type of treatment.15 More importantly, in most cases, changes in the level of retinopathy revert to the pre-pregnancy level after 1 year or longer, and pregnancy does not appear to affect long-term progression of retinopathy.15
Continue to: Proven primary prevention strategies
Proven primary prevention strategies
Glycemic control. Optimal glycemic control is an essential component of prevention of diabetic retinopathy. From 1983 to 1993, the Diabetes Control and Complications Trial randomized 1441 patients with type 1 DM to receive intensive therapy (median HbA1C level, 7.2%) or conventional therapy (median HbA1C level, 9.1%). During a mean of 6 years of follow-up, intensive therapy reduced the adjusted mean risk of retinopathy by 76% (95% CI, 62%-85%).16,17 A 2007 systematic review of 44 studies of the treatment of diabetic retinopathy found that strict glycemic control was beneficial in reducing the incidence and progression of retinopathy.17
The American Diabetes Association’s Standards of Medical Care in Diabetes—2019 Abridged for Primary Care Providers recommends that most nonpregnant adults maintain an HbA1Clevel < 7%. For patients with a history of hypoglycemia, limited life expectancy, advanced microvascular or macrovascular disease, other significant comorbid conditions, or longstanding DM in which it is difficult to achieve the optimal goal, a higher HbA1clevel (< 8%) might be appropriate.18
Control of BP. Strict control of BP is a major modifier of the incidence and progression of diabetic retinopathy.17,19 In the United Kingdom Prospective Diabetes Study, 1148 patients with type 2 DM and a mean BP of 160/94 mm Hg at the onset of the study were randomly assigned to either (1) a “tight” blood pressure group (< 150/85 mm Hg) or (2) a “less-tight” group (< 180/105 mm Hg). The primary therapy for controlling BP was captopril or atenolol. After 9 years of follow-up, the tight-control group had a 34% mean reduction in risk in the percentage of patients with deterioration of retinopathy (99% CI, 11%-50%; P = .0004) and a 47% reduction in risk (99% CI, 7%-70%; P = .004) of deterioration in visual acuity.20
Most patients with DM and hypertension should be treated to maintain a BP < 140/90 mm Hg. Although there is insufficient evidence to recommend a specific antihypertensive agent for preventing diabetic retinopathy, therapy should include agents from drug classes that have a demonstrated reduction in cardiovascular events in patients with DM. These include angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, thiazide diuretics, and dihydropyridine calcium channel blockers.18
Lipid management. The benefit of targeted therapy for lowering lipids for the prevention of diabetic retinopathy is not well established.17 In the Collaborative Atorvastatin Diabetes Study, 2838 patients with type 2 DM were randomized to atorvastatin (10 mg) or placebo; microvascular endpoint analysis demonstrated that patients taking atorvastatin needed less laser therapy (P = .14); however, progression of diabetic retinopathy was not reduced.21 Similarly, in the Action to Control Cardiovascular Risk in Diabetes Eye Study, slowing of progression to retinopathy was observed in patients with type 2 DM who were treated with fenofibrate (ie, progression in 6.5%, compared with progression in 10.2% of untreated subjects [odds ratio = 0.60 (95% CI, 0.42-0.87); P = .0056]).22
Continue to: Despite limited data on...
Despite limited data on the impact of lipid-lowering agents on patients with diabetic retinopathy, those with type 2 DM (especially) and those who have, or are at risk of, atherosclerotic cardiovascular disease should receive statin therapy.18
Aspirin therapy. Aspirin has not been found to be beneficial for slowing progression of diabetic retinopathy. However, aspirin did not cause further deterioration of disease, specifically in patients with vitreous hemorrhages4; patients with diabetic retinopathy who require aspirin therapy for other medical reasons can therefore continue to take it without increasing the risk of damage to the retina.4,18
When should you refer patients for screening?
Screening for diabetic retinopathy is important because affected patients can be asymptomatic but have significant disease. Early detection also helps determine which patients need treatment when it is most beneficial: early in its course.4
Type 1 DM. Retinopathy can become apparent as early as 6 or 7 years after the onset of disease, and is rare in children prior to puberty.4,11 As a result, patients with type 1 DM should first be screened with a comprehensive eye examination by an ophthalmologist or optometrist within 5 years of DM onset.4,18
Type 2 DM. Because of the insidious onset of type 2 DM, patients who are given a diagnosis of DM after 30 years of age might already have high-risk features of retinopathy.9 In patients with type 2 DM, therefore, initial screening for diabetic retinopathy should begin at the time of diagnosis and include a comprehensive eye examination by an ophthalmologist or optometrist.4,18,23
Continue to: Components of the exam
Components of the exam. Initial evaluation by the ophthalmologist or optometrist should include a detailed history and comprehensive eye exam with pupil dilation. Table 24 lists elements of the initial physical exam, which should assess for features that often lead to visual impairment. These features include macular edema, retinal hemorrhage, venous beading, neovascularization, and vitreous hemorrhage.4

Frequency of follow-up. The interval between subsequent examinations should be individualized, based on the findings of the initial assessment. Consider that:
- Screening should occur every 1 or 2 years in patients without evidence of retinopathy and with adequate glycemic control.4,18,23
- Screening every 1 or 2 years appears to be cost-effective in patients who have had 1 or more normal eye exams.
- A 3-year screening interval does not appear to present a risk in well-controlled patients with type 2 DM.24
- Women with type 1 or type 2 DM who are planning pregnancy or who are pregnant should have an eye exam prior to pregnancy or early in the first trimester.4,18,23 They should then be monitored each trimester and at the end of the first postpartum year, depending on the severity of retinopathy.18
Alternative screening modalities
Seven-field stereoscopic fundus photography is an alternative screening tool that compares favorably to ophthalmoscopy when performed by an experienced ophthalmologist, optometrist, or ophthalmologic technician.25 Nonmydriatic digital stereoscopic retinal imaging has been shown to be a cost-effective method of screening patients for diabetic retinopathy.26 In a study that compared digital imaging with dilated funduscopic examination, investigators reported that, of 311 eyes evaluated, there was agreement between the methods in 86% of cases. Disagreement was mostly related to the greater frequency of finding mild-to-moderate NPDR when using digital imaging.27
Screening in primary care
Programs that use telemedicine-based fundus photography to screen for diabetic retinopathy during primary care visits, followed by remote interpretation by an ophthalmologist, have been shown to increase the rate of retinal screening by offering an option other than direct referral to an ophthalmologist or optometrist.28 However, telemedicine-based retinal photography can be successful as a screening tool for retinopathy only if timely referral to an eye specialist is arranged when indicated by findings.18
SIDEBAR
Key points in the progression of diabetic retinopathy care
Duration of diabetes, poor glycemic control, and uncontrolled hypertension are major risk factors for diabetic retinopathy.
To reduce the risk of diabetic retinopathy, patients with diabetes mellitus should:
- sustain good glycemic control (hemoglobin A1C level, < 7%)
- maintain blood pressure < 140/90 mm Hg
- undergo periodic routine screening eye examination.
Early detection of diabetic retinopathy by dilated eye examination or fundus photography can lead to early therapeutic intervention, which can reduce the risk of visual impairment and vision loss.
Treatment is based on severity of disease and can include anti-vascular-endothelial growth factor therapy, photocoagulation, or surgery.
What therapy will your referred patients receive?
Patients found to have signs of diabetic retinopathy should be referred to an ophthalmologist who is knowledgeable and experienced in the management of diabetic retinopathy. Care will be managed according to the severity of the patient’s diabetic retinopathy.
Continue to: Patients with mild-to-moderate NPDR but without macular edema
Patients with mild-to-moderate NPDR but without macular edema. Treatment is generally not recommended. Patients should be reevaluated every 6 to 12 months because they have an increased risk of progression.5
Patients with mild-to-moderate NPDR and clinically significant macular edema (CSME). It is important for the eye specialist to assess for edema at the center of the macula because the risk of vision loss and need for treatment is greater when the center is involved. Vascular–endothelial growth factor (VEGF) is an important mediator of neovascularization and macular edema in diabetic retinopathy. For patients with center-involving CSME, intravitreous injection of an anti-VEGF agent provides significant benefit and is first-line treatment in these cases.4,29
The Early Treatment for Diabetic Retinopathy Study evaluated the efficacy of focal photocoagulation, a painless laser therapy, for CSME and demonstrated that this modality reduces the risk of moderate visual loss; increases the likelihood of improvement in vision; and decreases the frequency of persistent macular edema.30 Focal photocoagulation has been found effective in both non-center-involving CSME and center-involving CSME.5
Patients with severe NPDR. The recommendation is to initiate full panretinal photocoagulation prior to progression to proliferative diabetic retinopathy PDR. Researchers noted a 50% reduction in vision loss and vitrectomy when patients with type 2 DM were treated with panretinal photocoagulation early, compared with those in whom treatment was deferred until PDR developed.4,31 The role of anti-VEGF treatment of severe NPDR is under investigation.4
Patients with high-risk and severe PDR. Panretinal photocoagulation is the recommended treatment for patients with high-risk and severe PDR, and usually induces regression of retinal neovascularization. In patients with CSME and high-risk PDR, the combination of anti-VEGF therapy and panretinal photocoagulation should be considered. Vitrectomy should be considered for patients who have failed panretinal photocoagulation or are not amenable to photocoagulation.4
CORRESPONDENCE
Bryan Farford, DO, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL 32224; [email protected].
1. National Diabetes Statistic Report 2020: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 20, 2020.
2. Fitch K, Weisman T, Engel T, et al. Longitudinal commercial claims-based cost analysis of diabetic retinopathy screening patterns. Am Health Drug Benefits. 2015;8:300-308.
3. Centers for Disease Control and Prevention. Common eye disorders. September 29, 2015. www.cdc.gov/visionhealth/basics/ced/index.html. Accessed March 20, 2020.
4. American Academy of Ophthalmology PPP Retina/Vitreous Committee, Hoskins Center for Quality Eye Care. Diabetic Retinopathy PPP 2019. San Francisco, CA: American Academy of Ophthalmology. October 2019. https://www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp. Accessed March 20, 2020.
5. Zhang X, Saaddine JB, Chou C-F, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304:649-656.
6. Stewart MW. Socioeconomic cost of diabetic retinopathy and therapy. In: Diabetic Retinopathy. Singapore: Adis; 2017:257-268.
7. Tarr JM, Kaul K, Chopra M, et al. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560.
8. Wilkinson CP, Ferris FL 3rd, Klein RE, et al. Proposed International Clinical Diabetic Retinopathy and Diabetic Macular Edema Disease Severity Scales. Ophthalmology. 2003;110:1677-1682.
9. Wu L, Fernandez-Loaiza P, Sauma J, et al. Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes. 2013;4:290-294.
10. American Academy of Ophthalmology. International Clinical Diabetic Retinopathy Disease Severity Scale detailed table. October 2002. http://www.icoph.org/downloads/Diabetic-Retinopathy-Detail.pdf. Accessed March 20, 2020.
11. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Ophthalmol. 1984;102:527-532.
12. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ten-year incidence and progression of diabetic retinopathy. Arch Ophthalmol. 1994;112:1217-1228.
13. Klein R, Knudtson MD, Lee KE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXII. The twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115:1859-1868.
14. Emanuele N, Sacks J, Klein R, et al. Ethnicity, race, and baseline retinopathy correlates in the Veterans Affairs Diabetes Trial. Diabetes Care. 2005;28:1954-1958.
15. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Diabetes Care. 2000;23:1084-1091.
16. , , The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
17. Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007;298:902-916.
18. American Diabetes Association. Standards of Medical Care in Diabetes—2019 abridged for primary care providers. Clin Diabetes. 2019;37:11-34.
19. Do DV, Wang X, Vedula SS, et al. Blood pressure control for diabetic retinopathy. Cochrane Database Syst Rev. 2015;(1):CD006127.
20. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703-713.
21. Colhoun HM, Betteridge DJ, Durrington PN; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685-696.
22. Chew EY, Davis MD, Danis RP, et al. Action to Control Cardiovascular Risk in Diabetes Eye Study Research Group. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology. 2014;121:2443-2451.
23. Fong DS, Aiello L, Gardner TW, et al American Diabetes Association. Retinopathy in diabetes. Diabetes Care. 2004;27(suppl 1):S84-S87.
24. 11. Microvascular complications and foot care: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S124-S138.
25. Moss SE, Klein R, Kessler SD, et al. Comparison between ophthalmoscopy and fundus photography in determining severity of diabetic retinopathy. Ophthalmology. 1985;92:62-67.
26. Kirkizlar E, Serban N, Sisson JA, et al. Evaluation of telemedicine for screening of diabetic Retinopathy in the Veterans Health Administration. Ophthalmology. 2013;120:2604-2610.
27. Ahmed J, Ward TP, Bursell S-E, et al. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Diabetes Care. 2006;29:2205-2209.
28. Taylor CR, Merin LM, Salunga AM, et al. Improving diabetic retinopathy screening ratios using telemedicine-based digital retinal imaging technology: the Vine Hill study. Diabetes Care. 2007;30:574.
29. , , , Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193-1203.
30. Early photocoagulation for diabetic retinopathy. ETDRS Report Number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 suppl):766-785.
31. Ferris F. Early photocoagulation in patients with either type I or type II diabetes. Trans Am Ophthalmol Soc. 1996;94:505-537.
1. National Diabetes Statistic Report 2020: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 20, 2020.
2. Fitch K, Weisman T, Engel T, et al. Longitudinal commercial claims-based cost analysis of diabetic retinopathy screening patterns. Am Health Drug Benefits. 2015;8:300-308.
3. Centers for Disease Control and Prevention. Common eye disorders. September 29, 2015. www.cdc.gov/visionhealth/basics/ced/index.html. Accessed March 20, 2020.
4. American Academy of Ophthalmology PPP Retina/Vitreous Committee, Hoskins Center for Quality Eye Care. Diabetic Retinopathy PPP 2019. San Francisco, CA: American Academy of Ophthalmology. October 2019. https://www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp. Accessed March 20, 2020.
5. Zhang X, Saaddine JB, Chou C-F, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304:649-656.
6. Stewart MW. Socioeconomic cost of diabetic retinopathy and therapy. In: Diabetic Retinopathy. Singapore: Adis; 2017:257-268.
7. Tarr JM, Kaul K, Chopra M, et al. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560.
8. Wilkinson CP, Ferris FL 3rd, Klein RE, et al. Proposed International Clinical Diabetic Retinopathy and Diabetic Macular Edema Disease Severity Scales. Ophthalmology. 2003;110:1677-1682.
9. Wu L, Fernandez-Loaiza P, Sauma J, et al. Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes. 2013;4:290-294.
10. American Academy of Ophthalmology. International Clinical Diabetic Retinopathy Disease Severity Scale detailed table. October 2002. http://www.icoph.org/downloads/Diabetic-Retinopathy-Detail.pdf. Accessed March 20, 2020.
11. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Ophthalmol. 1984;102:527-532.
12. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ten-year incidence and progression of diabetic retinopathy. Arch Ophthalmol. 1994;112:1217-1228.
13. Klein R, Knudtson MD, Lee KE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXII. The twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115:1859-1868.
14. Emanuele N, Sacks J, Klein R, et al. Ethnicity, race, and baseline retinopathy correlates in the Veterans Affairs Diabetes Trial. Diabetes Care. 2005;28:1954-1958.
15. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Diabetes Care. 2000;23:1084-1091.
16. , , The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
17. Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007;298:902-916.
18. American Diabetes Association. Standards of Medical Care in Diabetes—2019 abridged for primary care providers. Clin Diabetes. 2019;37:11-34.
19. Do DV, Wang X, Vedula SS, et al. Blood pressure control for diabetic retinopathy. Cochrane Database Syst Rev. 2015;(1):CD006127.
20. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703-713.
21. Colhoun HM, Betteridge DJ, Durrington PN; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685-696.
22. Chew EY, Davis MD, Danis RP, et al. Action to Control Cardiovascular Risk in Diabetes Eye Study Research Group. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology. 2014;121:2443-2451.
23. Fong DS, Aiello L, Gardner TW, et al American Diabetes Association. Retinopathy in diabetes. Diabetes Care. 2004;27(suppl 1):S84-S87.
24. 11. Microvascular complications and foot care: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S124-S138.
25. Moss SE, Klein R, Kessler SD, et al. Comparison between ophthalmoscopy and fundus photography in determining severity of diabetic retinopathy. Ophthalmology. 1985;92:62-67.
26. Kirkizlar E, Serban N, Sisson JA, et al. Evaluation of telemedicine for screening of diabetic Retinopathy in the Veterans Health Administration. Ophthalmology. 2013;120:2604-2610.
27. Ahmed J, Ward TP, Bursell S-E, et al. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Diabetes Care. 2006;29:2205-2209.
28. Taylor CR, Merin LM, Salunga AM, et al. Improving diabetic retinopathy screening ratios using telemedicine-based digital retinal imaging technology: the Vine Hill study. Diabetes Care. 2007;30:574.
29. , , , Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193-1203.
30. Early photocoagulation for diabetic retinopathy. ETDRS Report Number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 suppl):766-785.
31. Ferris F. Early photocoagulation in patients with either type I or type II diabetes. Trans Am Ophthalmol Soc. 1996;94:505-537.
PRACTICE RECOMMENDATIONS
› Refer patients with type 1 diabetes mellitus (DM) to an ophthalmologist or optometrist for a dilated and comprehensive eye examination within 5 years of disease onset. B
› Refer patients with type 2 DM to an ophthalmologist or optometrist for an initial dilated and comprehensive eye examination at time of diagnosis. B
› Control blood pressure—ideally, < 140/90 mm Hg—in patients with DM to reduce the risk of diabetic retinopathy. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Loss of tear glands linked to suboptimal diabetes control
Loss of meibomian glands in the eye, which contribute to producing tears, appears to be associated with high rates of dry eye in individuals with diabetes and may serve as a biomarker for suboptimal glycemic control, new research suggests.
Gloria Wu, MD, an ophthalmologist at the University of California, San Francisco, presented the findings from a small study using infrared imaging of the eyelids in 120 patients with dry eye during a virtual press briefing held March 30, originally scheduled for the ENDO 2020 meeting.
The meibomian glands are the vertical striations that line the margins of the lower eyelids. They produce the lipid that combines with aqueous fluid from the lacrimal gland to create the tear film. Absence of meibomian glands can lead to dry eyes, eye pain, discomfort, and blurred vision.
Dry eye affects about 7% of the U.S. population, compared with 57% of people with type 1 diabetes and 70% with type 2 diabetes. Two proposed mechanisms for the phenomenon in diabetes are microischemia and inflammation, Dr. Wu said.
In her study, loss of meibomian glands was far more common among the 60 participants with dry eye and diabetes than among the 60 participants with dry eye but without diabetes, and the amount of gland loss was directly linked to A1c level.
The findings suggest that
Dr. Wu noted many newer smartphones, including the Samsung Galaxy 10S and iPhone 10, Xs, and 11, have infrared cameras that could help characterize dry eye in patients with diabetes.
“In the future, we hope patients can use [smartphones] and flip their own eyelids and take a picture. We hope that in rural health clinics and community health centers we can use this device that people have ... When people complain of dry eye and they have diabetes we can consider [closer diabetes monitoring],” said Dr. Wu.
Asked to comment, endocrinologist David C. Lieb, MD, said in an interview: “It’s important for providers who care for people with diabetes to know that diabetes is associated with a high incidence of meibomian gland dysfunction leading to dry eye. That’s another reason people with diabetes need to make sure they see their eye care specialist on a regular basis.
“When I ask patients if they’ve seen their eye specialist I may add dry eye to my list of questions rather than just asking them when was the last time they went,” added Dr. Lieb, associate professor of medicine at Eastern Virginia Medical School in Norfolk.
“I may ask them if they have symptoms of dry eye, and if they do, it’s something they need to talk about with that individual.”
Gland disappearance correlated with glycemic control
Dr. Wu and colleagues retrospectively reviewed electronic health records for 120 patients diagnosed with dry eye: 60 patients with and 60 patients without type 2 diabetes.
Those with diabetes were a mean age of 65 years, and were split evenly between men and women. The controls were younger, averaging 54 years, and comprised 37 men and 23 women.
Researchers performed infrared imaging (820 nm) of the lid; percentage loss of meibomian glands was calculated for each eye, then averaged per patient.
They found that 51.5% of patients in the diabetes group had lost meibomian glands, compared with just 11.3% of controls, a highly significant difference (P = .0001).
When A1c was also assessed, only 4 of 60 participants with A1c ≤ 5.9% lost ≥ 25% of the glands, compared with 55 of 60 participants with A1c ≥ 6.0%.
And specifically among those with diabetes, 35 of 37 with A1c > 6.6% lost > 40% of the glands, compared with just 12 of 23 participants with A1c < 6.5% (all P < .0001).
“In patients with dry eye and diabetes, loss of meibomian glands is associated with elevated A1c ... [and] may suggest a need for ... further monitoring of the patient’s diabetic condition,” the researchers noted.
Asked whether the glands could re-grow with improved glycemic control, Dr. Wu said she has not looked at that in people with diabetes, but in some patients who receive intensive treatment for dry eye with artificial tears or cyclosporine, the glands do grow back after about 6 months.
Dr. Lieb said he found the smartphone diagnostic idea “fascinating, especially in an area where you might not be able to easily measure an A1c. Most people have access to point-of-care A1c testing but not everybody can make it to a doctor’s office.”
And, he added, “anything that’s noninvasive has some potential benefit.”
Dr. Wu and Dr. Lieb have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Loss of meibomian glands in the eye, which contribute to producing tears, appears to be associated with high rates of dry eye in individuals with diabetes and may serve as a biomarker for suboptimal glycemic control, new research suggests.
Gloria Wu, MD, an ophthalmologist at the University of California, San Francisco, presented the findings from a small study using infrared imaging of the eyelids in 120 patients with dry eye during a virtual press briefing held March 30, originally scheduled for the ENDO 2020 meeting.
The meibomian glands are the vertical striations that line the margins of the lower eyelids. They produce the lipid that combines with aqueous fluid from the lacrimal gland to create the tear film. Absence of meibomian glands can lead to dry eyes, eye pain, discomfort, and blurred vision.
Dry eye affects about 7% of the U.S. population, compared with 57% of people with type 1 diabetes and 70% with type 2 diabetes. Two proposed mechanisms for the phenomenon in diabetes are microischemia and inflammation, Dr. Wu said.
In her study, loss of meibomian glands was far more common among the 60 participants with dry eye and diabetes than among the 60 participants with dry eye but without diabetes, and the amount of gland loss was directly linked to A1c level.
The findings suggest that
Dr. Wu noted many newer smartphones, including the Samsung Galaxy 10S and iPhone 10, Xs, and 11, have infrared cameras that could help characterize dry eye in patients with diabetes.
“In the future, we hope patients can use [smartphones] and flip their own eyelids and take a picture. We hope that in rural health clinics and community health centers we can use this device that people have ... When people complain of dry eye and they have diabetes we can consider [closer diabetes monitoring],” said Dr. Wu.
Asked to comment, endocrinologist David C. Lieb, MD, said in an interview: “It’s important for providers who care for people with diabetes to know that diabetes is associated with a high incidence of meibomian gland dysfunction leading to dry eye. That’s another reason people with diabetes need to make sure they see their eye care specialist on a regular basis.
“When I ask patients if they’ve seen their eye specialist I may add dry eye to my list of questions rather than just asking them when was the last time they went,” added Dr. Lieb, associate professor of medicine at Eastern Virginia Medical School in Norfolk.
“I may ask them if they have symptoms of dry eye, and if they do, it’s something they need to talk about with that individual.”
Gland disappearance correlated with glycemic control
Dr. Wu and colleagues retrospectively reviewed electronic health records for 120 patients diagnosed with dry eye: 60 patients with and 60 patients without type 2 diabetes.
Those with diabetes were a mean age of 65 years, and were split evenly between men and women. The controls were younger, averaging 54 years, and comprised 37 men and 23 women.
Researchers performed infrared imaging (820 nm) of the lid; percentage loss of meibomian glands was calculated for each eye, then averaged per patient.
They found that 51.5% of patients in the diabetes group had lost meibomian glands, compared with just 11.3% of controls, a highly significant difference (P = .0001).
When A1c was also assessed, only 4 of 60 participants with A1c ≤ 5.9% lost ≥ 25% of the glands, compared with 55 of 60 participants with A1c ≥ 6.0%.
And specifically among those with diabetes, 35 of 37 with A1c > 6.6% lost > 40% of the glands, compared with just 12 of 23 participants with A1c < 6.5% (all P < .0001).
“In patients with dry eye and diabetes, loss of meibomian glands is associated with elevated A1c ... [and] may suggest a need for ... further monitoring of the patient’s diabetic condition,” the researchers noted.
Asked whether the glands could re-grow with improved glycemic control, Dr. Wu said she has not looked at that in people with diabetes, but in some patients who receive intensive treatment for dry eye with artificial tears or cyclosporine, the glands do grow back after about 6 months.
Dr. Lieb said he found the smartphone diagnostic idea “fascinating, especially in an area where you might not be able to easily measure an A1c. Most people have access to point-of-care A1c testing but not everybody can make it to a doctor’s office.”
And, he added, “anything that’s noninvasive has some potential benefit.”
Dr. Wu and Dr. Lieb have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Loss of meibomian glands in the eye, which contribute to producing tears, appears to be associated with high rates of dry eye in individuals with diabetes and may serve as a biomarker for suboptimal glycemic control, new research suggests.
Gloria Wu, MD, an ophthalmologist at the University of California, San Francisco, presented the findings from a small study using infrared imaging of the eyelids in 120 patients with dry eye during a virtual press briefing held March 30, originally scheduled for the ENDO 2020 meeting.
The meibomian glands are the vertical striations that line the margins of the lower eyelids. They produce the lipid that combines with aqueous fluid from the lacrimal gland to create the tear film. Absence of meibomian glands can lead to dry eyes, eye pain, discomfort, and blurred vision.
Dry eye affects about 7% of the U.S. population, compared with 57% of people with type 1 diabetes and 70% with type 2 diabetes. Two proposed mechanisms for the phenomenon in diabetes are microischemia and inflammation, Dr. Wu said.
In her study, loss of meibomian glands was far more common among the 60 participants with dry eye and diabetes than among the 60 participants with dry eye but without diabetes, and the amount of gland loss was directly linked to A1c level.
The findings suggest that
Dr. Wu noted many newer smartphones, including the Samsung Galaxy 10S and iPhone 10, Xs, and 11, have infrared cameras that could help characterize dry eye in patients with diabetes.
“In the future, we hope patients can use [smartphones] and flip their own eyelids and take a picture. We hope that in rural health clinics and community health centers we can use this device that people have ... When people complain of dry eye and they have diabetes we can consider [closer diabetes monitoring],” said Dr. Wu.
Asked to comment, endocrinologist David C. Lieb, MD, said in an interview: “It’s important for providers who care for people with diabetes to know that diabetes is associated with a high incidence of meibomian gland dysfunction leading to dry eye. That’s another reason people with diabetes need to make sure they see their eye care specialist on a regular basis.
“When I ask patients if they’ve seen their eye specialist I may add dry eye to my list of questions rather than just asking them when was the last time they went,” added Dr. Lieb, associate professor of medicine at Eastern Virginia Medical School in Norfolk.
“I may ask them if they have symptoms of dry eye, and if they do, it’s something they need to talk about with that individual.”
Gland disappearance correlated with glycemic control
Dr. Wu and colleagues retrospectively reviewed electronic health records for 120 patients diagnosed with dry eye: 60 patients with and 60 patients without type 2 diabetes.
Those with diabetes were a mean age of 65 years, and were split evenly between men and women. The controls were younger, averaging 54 years, and comprised 37 men and 23 women.
Researchers performed infrared imaging (820 nm) of the lid; percentage loss of meibomian glands was calculated for each eye, then averaged per patient.
They found that 51.5% of patients in the diabetes group had lost meibomian glands, compared with just 11.3% of controls, a highly significant difference (P = .0001).
When A1c was also assessed, only 4 of 60 participants with A1c ≤ 5.9% lost ≥ 25% of the glands, compared with 55 of 60 participants with A1c ≥ 6.0%.
And specifically among those with diabetes, 35 of 37 with A1c > 6.6% lost > 40% of the glands, compared with just 12 of 23 participants with A1c < 6.5% (all P < .0001).
“In patients with dry eye and diabetes, loss of meibomian glands is associated with elevated A1c ... [and] may suggest a need for ... further monitoring of the patient’s diabetic condition,” the researchers noted.
Asked whether the glands could re-grow with improved glycemic control, Dr. Wu said she has not looked at that in people with diabetes, but in some patients who receive intensive treatment for dry eye with artificial tears or cyclosporine, the glands do grow back after about 6 months.
Dr. Lieb said he found the smartphone diagnostic idea “fascinating, especially in an area where you might not be able to easily measure an A1c. Most people have access to point-of-care A1c testing but not everybody can make it to a doctor’s office.”
And, he added, “anything that’s noninvasive has some potential benefit.”
Dr. Wu and Dr. Lieb have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ENDO 2020
Enhanced team-based CVD care found to benefit diabetes patients
PHOENIX, ARIZ. – Diabetes patients in China who were enrolled in a team-based care intervention with clinical decision support systems significantly reduced their hemoglobin A1c, systolic blood pressure, and LDL cholesterol over 18 months, compared with those who received team-based care alone.
The finding comes from the Diabetes Complication Control in Community Clinics (D4C), a cluster randomized trial conducted in 38 community health centers in Xiamen, China.
“Diabetes has become a major public health challenge worldwide, especially in low- and middle-income countries where populations are large and growing and health care resources are limited,” Jiang He, MD, PhD, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting.
According to Dr. He, chair and professor of epidemiology at Tulane School of Public Health and Tropical Medicine, New Orleans, the prevalence of diabetes has increased rapidly in recent decades in China, from 2.5% in 1994 to 11.6% in 2010. “It was estimated that 114 million Chinese adults had diabetes in 2010,” he said. “Hyperglycemia, high blood pressure, and elevated LDL cholesterol are major risk factors for cardiovascular disease and premature death. The majority of patients with diabetes have multiple uncontrolled CVD risk factors due to suboptimal care. Diabetes and its complications further strain an already overburdened and overwhelmed health care system, especially tertiary care facilities, in China. On the other hand, community health centers are underutilized.”
In D4C, Dr. He and colleagues set out to evaluate changes in CVD risk factors among patients with diabetes after implementing a team-based care model at community health centers in Xiamen, China. They compared the effectiveness of team-based care with clinical decision support systems versus team-based care alone on CVD risk factor control among patients with diabetes at these community health centers.
The study population consisted of 10,942 patients aged 50 years and older with uncontrolled diabetes and at least one of the following three additional CVD risk factors: systolic BP of at least 140 mm Hg and/or diastolic BP of at least 90 mm Hg; LDL cholesterol of at least 100 mg/dL, or clinical atherosclerotic cardiovascular disease (ASCVD). At the intervention clinics, team-based care was delivered by a team of primary care physicians, nurses, and diabetes specialists. The researchers trained the primary care physicians and nurses, and a clinical decision support system was integrated with guideline-based treatment algorithms for controlling glycemia, blood pressure, and lipids.
At the enhanced care control clinics, team-based care was delivered by a team of primary care physicians, nurses, and diabetes specialists. The city health commission trained the primary care physicians and nurses. The intervention lasted for 18 months in both groups.
Dr. He, the D4C study chair, reported findings from 10,942 patients: 5,394 in the intervention group and 5,548 in the enhanced care group. The mean baseline age was similar between the intervention group and the enhanced care group (a mean of 63 years), as was body mass index (a mean of 24.9 kg/m2), hemoglobin A1c (a mean of 8.8 vs. 8.7%, respectively), LDL cholesterol (121.2 vs. 121.1 mg/dL), systolic blood pressure (136.6 vs. 136.9 mm Hg), and diastolic blood pressure (79.7 vs. 79.8 mm Hg).
The researchers found patients in both groups experienced significant reductions in HbA1c, LDL cholesterol, and BP over the 18-month follow-up, but those in the intervention group fared better in all measures. Specifically, the mean change in HbA1c from baseline was –.85% in the intervention group, compared with –.66% in the enhanced care group, while the change in LDL was –19 mg/dL, compared with –12.8 mg/dL, respectively; the change in systolic blood pressure was –8.9 mm Hg vs. –7.7 mm Hg, and the change in 10-year ASCVD risk was .57% vs. .28% (P < .0001 for all associations).
The researchers also observed that the proportions of controlled HbA1c, LDL, and blood pressure at 18 months were higher in the intervention group, compared with the enhanced care group. Specifically, 38% of patients in the intervention group achieved glycemic control, compared with 35% of those in the enhanced care group (P =. 0006), while 48% vs. 39%, respectively, achieved control of LDL cholesterol (P < .0001), and 78% vs. 75% achieved control of blood pressure (P = .0009). In addition, 15% vs. 12% achieved control of all three risk factors at 18 months (P < .0001).
“Implementing team-based care with a clinical decision support system is an effective and sustainable strategy for diabetes control in primary care settings,” Dr. He said at the meeting, which was sponsored by the American Heart Association. “This implementation strategy could be scaled up within primary care settings in China and other low- to middle-income countries to improve CVD risk factor control in patients with diabetes.”
In an interview, session moderator Joshua J. Joseph, MD, of Ohio State University, Columbus, pointed out that since only 12%-15% of study participants achieved control of all three CVD risk factors, “that leaves a great opportunity for [figuring out] how to we get the other 88% or 85% of patients to target levels. That’s going to be important as we think about cardiovascular disease prevention in type 2 diabetes. The more we can use team-based care along with clinical decision support tools, the more we will continue to improve the lives of patients.”
The study was supported by the Xiamen City Health Commission. Dr. He reported having no financial disclosures.
SOURCE: He J et al. EPI/LIFESTYLE 2020, session 7A, abstract 17.
PHOENIX, ARIZ. – Diabetes patients in China who were enrolled in a team-based care intervention with clinical decision support systems significantly reduced their hemoglobin A1c, systolic blood pressure, and LDL cholesterol over 18 months, compared with those who received team-based care alone.
The finding comes from the Diabetes Complication Control in Community Clinics (D4C), a cluster randomized trial conducted in 38 community health centers in Xiamen, China.
“Diabetes has become a major public health challenge worldwide, especially in low- and middle-income countries where populations are large and growing and health care resources are limited,” Jiang He, MD, PhD, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting.
According to Dr. He, chair and professor of epidemiology at Tulane School of Public Health and Tropical Medicine, New Orleans, the prevalence of diabetes has increased rapidly in recent decades in China, from 2.5% in 1994 to 11.6% in 2010. “It was estimated that 114 million Chinese adults had diabetes in 2010,” he said. “Hyperglycemia, high blood pressure, and elevated LDL cholesterol are major risk factors for cardiovascular disease and premature death. The majority of patients with diabetes have multiple uncontrolled CVD risk factors due to suboptimal care. Diabetes and its complications further strain an already overburdened and overwhelmed health care system, especially tertiary care facilities, in China. On the other hand, community health centers are underutilized.”
In D4C, Dr. He and colleagues set out to evaluate changes in CVD risk factors among patients with diabetes after implementing a team-based care model at community health centers in Xiamen, China. They compared the effectiveness of team-based care with clinical decision support systems versus team-based care alone on CVD risk factor control among patients with diabetes at these community health centers.
The study population consisted of 10,942 patients aged 50 years and older with uncontrolled diabetes and at least one of the following three additional CVD risk factors: systolic BP of at least 140 mm Hg and/or diastolic BP of at least 90 mm Hg; LDL cholesterol of at least 100 mg/dL, or clinical atherosclerotic cardiovascular disease (ASCVD). At the intervention clinics, team-based care was delivered by a team of primary care physicians, nurses, and diabetes specialists. The researchers trained the primary care physicians and nurses, and a clinical decision support system was integrated with guideline-based treatment algorithms for controlling glycemia, blood pressure, and lipids.
At the enhanced care control clinics, team-based care was delivered by a team of primary care physicians, nurses, and diabetes specialists. The city health commission trained the primary care physicians and nurses. The intervention lasted for 18 months in both groups.
Dr. He, the D4C study chair, reported findings from 10,942 patients: 5,394 in the intervention group and 5,548 in the enhanced care group. The mean baseline age was similar between the intervention group and the enhanced care group (a mean of 63 years), as was body mass index (a mean of 24.9 kg/m2), hemoglobin A1c (a mean of 8.8 vs. 8.7%, respectively), LDL cholesterol (121.2 vs. 121.1 mg/dL), systolic blood pressure (136.6 vs. 136.9 mm Hg), and diastolic blood pressure (79.7 vs. 79.8 mm Hg).
The researchers found patients in both groups experienced significant reductions in HbA1c, LDL cholesterol, and BP over the 18-month follow-up, but those in the intervention group fared better in all measures. Specifically, the mean change in HbA1c from baseline was –.85% in the intervention group, compared with –.66% in the enhanced care group, while the change in LDL was –19 mg/dL, compared with –12.8 mg/dL, respectively; the change in systolic blood pressure was –8.9 mm Hg vs. –7.7 mm Hg, and the change in 10-year ASCVD risk was .57% vs. .28% (P < .0001 for all associations).
The researchers also observed that the proportions of controlled HbA1c, LDL, and blood pressure at 18 months were higher in the intervention group, compared with the enhanced care group. Specifically, 38% of patients in the intervention group achieved glycemic control, compared with 35% of those in the enhanced care group (P =. 0006), while 48% vs. 39%, respectively, achieved control of LDL cholesterol (P < .0001), and 78% vs. 75% achieved control of blood pressure (P = .0009). In addition, 15% vs. 12% achieved control of all three risk factors at 18 months (P < .0001).
“Implementing team-based care with a clinical decision support system is an effective and sustainable strategy for diabetes control in primary care settings,” Dr. He said at the meeting, which was sponsored by the American Heart Association. “This implementation strategy could be scaled up within primary care settings in China and other low- to middle-income countries to improve CVD risk factor control in patients with diabetes.”
In an interview, session moderator Joshua J. Joseph, MD, of Ohio State University, Columbus, pointed out that since only 12%-15% of study participants achieved control of all three CVD risk factors, “that leaves a great opportunity for [figuring out] how to we get the other 88% or 85% of patients to target levels. That’s going to be important as we think about cardiovascular disease prevention in type 2 diabetes. The more we can use team-based care along with clinical decision support tools, the more we will continue to improve the lives of patients.”
The study was supported by the Xiamen City Health Commission. Dr. He reported having no financial disclosures.
SOURCE: He J et al. EPI/LIFESTYLE 2020, session 7A, abstract 17.
PHOENIX, ARIZ. – Diabetes patients in China who were enrolled in a team-based care intervention with clinical decision support systems significantly reduced their hemoglobin A1c, systolic blood pressure, and LDL cholesterol over 18 months, compared with those who received team-based care alone.
The finding comes from the Diabetes Complication Control in Community Clinics (D4C), a cluster randomized trial conducted in 38 community health centers in Xiamen, China.
“Diabetes has become a major public health challenge worldwide, especially in low- and middle-income countries where populations are large and growing and health care resources are limited,” Jiang He, MD, PhD, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting.
According to Dr. He, chair and professor of epidemiology at Tulane School of Public Health and Tropical Medicine, New Orleans, the prevalence of diabetes has increased rapidly in recent decades in China, from 2.5% in 1994 to 11.6% in 2010. “It was estimated that 114 million Chinese adults had diabetes in 2010,” he said. “Hyperglycemia, high blood pressure, and elevated LDL cholesterol are major risk factors for cardiovascular disease and premature death. The majority of patients with diabetes have multiple uncontrolled CVD risk factors due to suboptimal care. Diabetes and its complications further strain an already overburdened and overwhelmed health care system, especially tertiary care facilities, in China. On the other hand, community health centers are underutilized.”
In D4C, Dr. He and colleagues set out to evaluate changes in CVD risk factors among patients with diabetes after implementing a team-based care model at community health centers in Xiamen, China. They compared the effectiveness of team-based care with clinical decision support systems versus team-based care alone on CVD risk factor control among patients with diabetes at these community health centers.
The study population consisted of 10,942 patients aged 50 years and older with uncontrolled diabetes and at least one of the following three additional CVD risk factors: systolic BP of at least 140 mm Hg and/or diastolic BP of at least 90 mm Hg; LDL cholesterol of at least 100 mg/dL, or clinical atherosclerotic cardiovascular disease (ASCVD). At the intervention clinics, team-based care was delivered by a team of primary care physicians, nurses, and diabetes specialists. The researchers trained the primary care physicians and nurses, and a clinical decision support system was integrated with guideline-based treatment algorithms for controlling glycemia, blood pressure, and lipids.
At the enhanced care control clinics, team-based care was delivered by a team of primary care physicians, nurses, and diabetes specialists. The city health commission trained the primary care physicians and nurses. The intervention lasted for 18 months in both groups.
Dr. He, the D4C study chair, reported findings from 10,942 patients: 5,394 in the intervention group and 5,548 in the enhanced care group. The mean baseline age was similar between the intervention group and the enhanced care group (a mean of 63 years), as was body mass index (a mean of 24.9 kg/m2), hemoglobin A1c (a mean of 8.8 vs. 8.7%, respectively), LDL cholesterol (121.2 vs. 121.1 mg/dL), systolic blood pressure (136.6 vs. 136.9 mm Hg), and diastolic blood pressure (79.7 vs. 79.8 mm Hg).
The researchers found patients in both groups experienced significant reductions in HbA1c, LDL cholesterol, and BP over the 18-month follow-up, but those in the intervention group fared better in all measures. Specifically, the mean change in HbA1c from baseline was –.85% in the intervention group, compared with –.66% in the enhanced care group, while the change in LDL was –19 mg/dL, compared with –12.8 mg/dL, respectively; the change in systolic blood pressure was –8.9 mm Hg vs. –7.7 mm Hg, and the change in 10-year ASCVD risk was .57% vs. .28% (P < .0001 for all associations).
The researchers also observed that the proportions of controlled HbA1c, LDL, and blood pressure at 18 months were higher in the intervention group, compared with the enhanced care group. Specifically, 38% of patients in the intervention group achieved glycemic control, compared with 35% of those in the enhanced care group (P =. 0006), while 48% vs. 39%, respectively, achieved control of LDL cholesterol (P < .0001), and 78% vs. 75% achieved control of blood pressure (P = .0009). In addition, 15% vs. 12% achieved control of all three risk factors at 18 months (P < .0001).
“Implementing team-based care with a clinical decision support system is an effective and sustainable strategy for diabetes control in primary care settings,” Dr. He said at the meeting, which was sponsored by the American Heart Association. “This implementation strategy could be scaled up within primary care settings in China and other low- to middle-income countries to improve CVD risk factor control in patients with diabetes.”
In an interview, session moderator Joshua J. Joseph, MD, of Ohio State University, Columbus, pointed out that since only 12%-15% of study participants achieved control of all three CVD risk factors, “that leaves a great opportunity for [figuring out] how to we get the other 88% or 85% of patients to target levels. That’s going to be important as we think about cardiovascular disease prevention in type 2 diabetes. The more we can use team-based care along with clinical decision support tools, the more we will continue to improve the lives of patients.”
The study was supported by the Xiamen City Health Commission. Dr. He reported having no financial disclosures.
SOURCE: He J et al. EPI/LIFESTYLE 2020, session 7A, abstract 17.
REPORTING FROM EPI/LIFESTYLE 2020