User login
Testing lab asks FDA to recall NDMA-tainted metformin
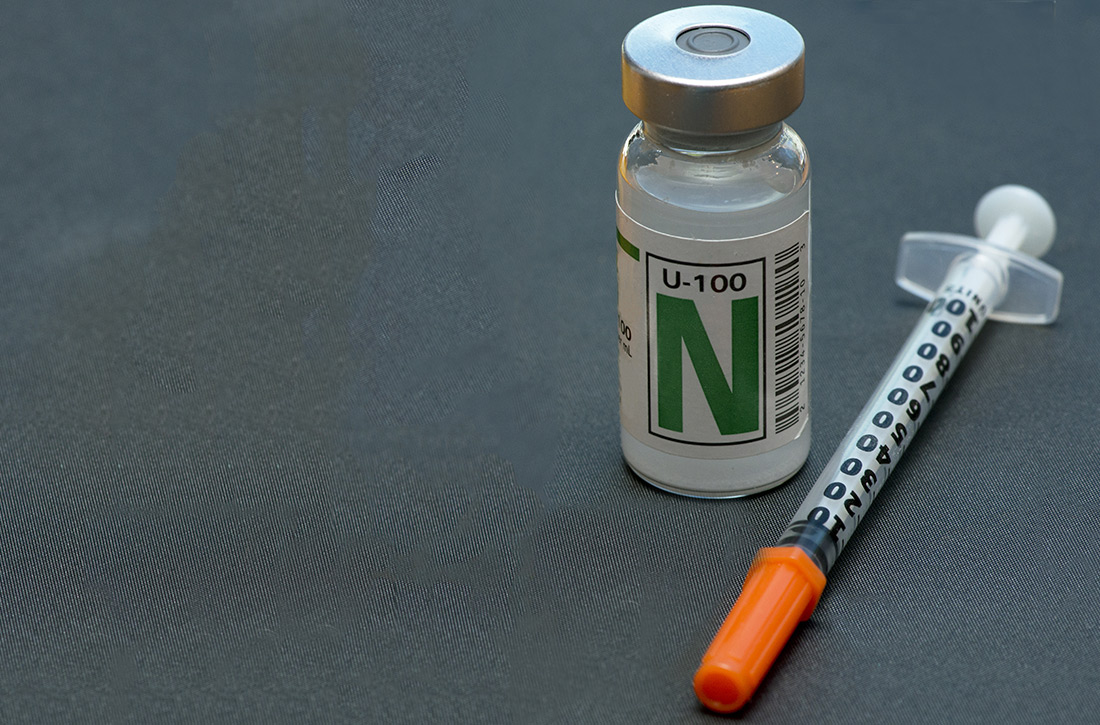
Valisure, an online pharmacy and analytical laboratory based in New Haven, Conn., has asked the U.S. Food and Drug Administration to request recalls of the diabetes drug metformin from 11 companies after its own testing found levels of the probable carcinogen N-nitrosodimethylamine (NDMA) exceeded those recognized by the agency as being safe, according to a petition filed with the FDA.
“The presence of this probable carcinogen in a medication that is taken daily by adults and adolescents for a chronic condition like diabetes, makes [these findings] particularly troubling,” the pharmacy said in the petition. It noted that NDMA is classified by the World Health Organization and the International Agency for Research on Cancer as a Group 2A compound, meaning that it is “probably carcinogenic to humans.”
The Valisure findings are in contrast to those from the FDA’s own testing of metformin from eight companies. In results released Feb. 2, the agency found no NDMA in metformin from seven of the companies, and elevations within the safe limit in metformin from the remaining company. Based on those results, the FDA did not issue a recall.
The FDA declined to comment on the petition, pending a formal written response, a spokesman said.
Valisure said in its petition that one reason it found NDMA at concerning levels, and the FDA did not, could be that the pharmacy’s testing was more thorough than the agency’s because it had used its proprietary analytical technologies in addition to FDA standard assays. The pharmacy said it also cast a wider net, testing samples from 22 companies instead of 8, as the FDA had.
“At least one company that consistently displayed high levels of NDMA in all Valisure-tested batches was a company whose metformin products, to Valisure’s knowledge, were not tested by FDA,” Valisure said in the petition.
The pharmacy tested 38 batches of metformin from 22 companies, and found 16 contaminated batches from 11 companies. Results from one company were confirmed by an independent laboratory in California.
In addition, “there was significant variability from batch to batch, even within a single company, underscoring the importance of batch-level chemical analysis and the necessity of overall increased quality surveillance of medications,” the pharmacy stated in the petition.
David Light, Valisure founder and CEO, said in a press release that the results “indicate that contaminated batches of drugs are scattered and intermixed with clean [batches] throughout the American pharmaceutical supply chain. This strongly suggests that neither the limited testing FDA is able to conduct nor the pharmaceutical companies’ self-reporting of analytical results is sufficient to protect American consumers.”
He explained in an interview that metformin contamination probably comes from poor manufacturing, as NDMA can form during the manufacturing process. Companies are supposed to have processes in place to limit its formation and to check for and remove it before products are shipped. The contaminated batches might have come from China, which makes the bulk of U.S. active pharmaceutical ingredients, he said.
Recently, Apotex and Ranbaxy Pharmaceuticals recalled specific metformin lots in Canada after Canadian health authorities requested NDMA testing. Metformin versions have also been recalled in Singapore. European health authorities are also looking into the issue, according to a Mar. 3 press release from the European Medicines Agency.
Over the past 2 years, NDMA contamination has led to recalls of angiotensin receptor blockers, such as valsartan and losartan, because of NDMA in excess of the daily limit, and ranitidine-containing products such as Zantac. Valisure said it hopes the FDA will avoid the “year-long rolling recalls” that have occurred for angiotensin receptor blockers.
Metformin, which is used to treat type 2 diabetes, is the fourth-most prescribed drug in the United States. About 80 million prescriptions were written for it during 2019, according to the press release.
Valisure launched its investigation after it found NDMA in metformin a client asked it to check.
Valisure, an online pharmacy and analytical laboratory based in New Haven, Conn., has asked the U.S. Food and Drug Administration to request recalls of the diabetes drug metformin from 11 companies after its own testing found levels of the probable carcinogen N-nitrosodimethylamine (NDMA) exceeded those recognized by the agency as being safe, according to a petition filed with the FDA.
“The presence of this probable carcinogen in a medication that is taken daily by adults and adolescents for a chronic condition like diabetes, makes [these findings] particularly troubling,” the pharmacy said in the petition. It noted that NDMA is classified by the World Health Organization and the International Agency for Research on Cancer as a Group 2A compound, meaning that it is “probably carcinogenic to humans.”
The Valisure findings are in contrast to those from the FDA’s own testing of metformin from eight companies. In results released Feb. 2, the agency found no NDMA in metformin from seven of the companies, and elevations within the safe limit in metformin from the remaining company. Based on those results, the FDA did not issue a recall.
The FDA declined to comment on the petition, pending a formal written response, a spokesman said.
Valisure said in its petition that one reason it found NDMA at concerning levels, and the FDA did not, could be that the pharmacy’s testing was more thorough than the agency’s because it had used its proprietary analytical technologies in addition to FDA standard assays. The pharmacy said it also cast a wider net, testing samples from 22 companies instead of 8, as the FDA had.
“At least one company that consistently displayed high levels of NDMA in all Valisure-tested batches was a company whose metformin products, to Valisure’s knowledge, were not tested by FDA,” Valisure said in the petition.
The pharmacy tested 38 batches of metformin from 22 companies, and found 16 contaminated batches from 11 companies. Results from one company were confirmed by an independent laboratory in California.
In addition, “there was significant variability from batch to batch, even within a single company, underscoring the importance of batch-level chemical analysis and the necessity of overall increased quality surveillance of medications,” the pharmacy stated in the petition.
David Light, Valisure founder and CEO, said in a press release that the results “indicate that contaminated batches of drugs are scattered and intermixed with clean [batches] throughout the American pharmaceutical supply chain. This strongly suggests that neither the limited testing FDA is able to conduct nor the pharmaceutical companies’ self-reporting of analytical results is sufficient to protect American consumers.”
He explained in an interview that metformin contamination probably comes from poor manufacturing, as NDMA can form during the manufacturing process. Companies are supposed to have processes in place to limit its formation and to check for and remove it before products are shipped. The contaminated batches might have come from China, which makes the bulk of U.S. active pharmaceutical ingredients, he said.
Recently, Apotex and Ranbaxy Pharmaceuticals recalled specific metformin lots in Canada after Canadian health authorities requested NDMA testing. Metformin versions have also been recalled in Singapore. European health authorities are also looking into the issue, according to a Mar. 3 press release from the European Medicines Agency.
Over the past 2 years, NDMA contamination has led to recalls of angiotensin receptor blockers, such as valsartan and losartan, because of NDMA in excess of the daily limit, and ranitidine-containing products such as Zantac. Valisure said it hopes the FDA will avoid the “year-long rolling recalls” that have occurred for angiotensin receptor blockers.
Metformin, which is used to treat type 2 diabetes, is the fourth-most prescribed drug in the United States. About 80 million prescriptions were written for it during 2019, according to the press release.
Valisure launched its investigation after it found NDMA in metformin a client asked it to check.
Valisure, an online pharmacy and analytical laboratory based in New Haven, Conn., has asked the U.S. Food and Drug Administration to request recalls of the diabetes drug metformin from 11 companies after its own testing found levels of the probable carcinogen N-nitrosodimethylamine (NDMA) exceeded those recognized by the agency as being safe, according to a petition filed with the FDA.
“The presence of this probable carcinogen in a medication that is taken daily by adults and adolescents for a chronic condition like diabetes, makes [these findings] particularly troubling,” the pharmacy said in the petition. It noted that NDMA is classified by the World Health Organization and the International Agency for Research on Cancer as a Group 2A compound, meaning that it is “probably carcinogenic to humans.”
The Valisure findings are in contrast to those from the FDA’s own testing of metformin from eight companies. In results released Feb. 2, the agency found no NDMA in metformin from seven of the companies, and elevations within the safe limit in metformin from the remaining company. Based on those results, the FDA did not issue a recall.
The FDA declined to comment on the petition, pending a formal written response, a spokesman said.
Valisure said in its petition that one reason it found NDMA at concerning levels, and the FDA did not, could be that the pharmacy’s testing was more thorough than the agency’s because it had used its proprietary analytical technologies in addition to FDA standard assays. The pharmacy said it also cast a wider net, testing samples from 22 companies instead of 8, as the FDA had.
“At least one company that consistently displayed high levels of NDMA in all Valisure-tested batches was a company whose metformin products, to Valisure’s knowledge, were not tested by FDA,” Valisure said in the petition.
The pharmacy tested 38 batches of metformin from 22 companies, and found 16 contaminated batches from 11 companies. Results from one company were confirmed by an independent laboratory in California.
In addition, “there was significant variability from batch to batch, even within a single company, underscoring the importance of batch-level chemical analysis and the necessity of overall increased quality surveillance of medications,” the pharmacy stated in the petition.
David Light, Valisure founder and CEO, said in a press release that the results “indicate that contaminated batches of drugs are scattered and intermixed with clean [batches] throughout the American pharmaceutical supply chain. This strongly suggests that neither the limited testing FDA is able to conduct nor the pharmaceutical companies’ self-reporting of analytical results is sufficient to protect American consumers.”
He explained in an interview that metformin contamination probably comes from poor manufacturing, as NDMA can form during the manufacturing process. Companies are supposed to have processes in place to limit its formation and to check for and remove it before products are shipped. The contaminated batches might have come from China, which makes the bulk of U.S. active pharmaceutical ingredients, he said.
Recently, Apotex and Ranbaxy Pharmaceuticals recalled specific metformin lots in Canada after Canadian health authorities requested NDMA testing. Metformin versions have also been recalled in Singapore. European health authorities are also looking into the issue, according to a Mar. 3 press release from the European Medicines Agency.
Over the past 2 years, NDMA contamination has led to recalls of angiotensin receptor blockers, such as valsartan and losartan, because of NDMA in excess of the daily limit, and ranitidine-containing products such as Zantac. Valisure said it hopes the FDA will avoid the “year-long rolling recalls” that have occurred for angiotensin receptor blockers.
Metformin, which is used to treat type 2 diabetes, is the fourth-most prescribed drug in the United States. About 80 million prescriptions were written for it during 2019, according to the press release.
Valisure launched its investigation after it found NDMA in metformin a client asked it to check.
MACE benefits with dapagliflozin improve with disease duration
Treatment with the sodium-glucose transporter 2 inhibitor dapagliflozin reduced the risk for cardiovascular disease or hospitalization for heart failure (CVD/HHF) in patients with diabetes, regardless of the duration of the disease, but had a greater protective benefit against major adverse cardiovascular events (MACE) and renal events in patients with longer disease duration, according to new findings from a post hoc analysis of the DECLARE-TIMI 58 trial.
The positive effect of dapagliflozin in patients with MACE – which includes myocardial infarction (MI), CVD, and ischemic stroke – may have been driven by lower rates of MI and ischemic stroke with the drug, compared with placebo, in patients with longer disease duration, wrote Harpreet S. Bajaj, MD, and colleagues. Their report is in Diabetes, Obesity and Metabolism (2020 Feb 23. doi: 10.1111/dom.14011).
It has been previously reported that the risk for complications in diabetes increases with increasing duration of the disease. Recent studies with SGLT-2 inhibitors have shown that the drugs improve cardiovascular and renal outcomes in diabetes, and they are recommended by the American Diabetes Association as second-line therapy in patients with atherosclerotic cardiovascular disease, chronic kidney disease, or heart failure. The European Society of Cardiology and the European Association for the Study of Diabetes recommend that patients with diabetes patients who have three or more risk factors, or those with a disease duration of more than 20 years, should be deemed very high risk and be considered for early treatment with SGLT2 inhibitors.
“The MACE benefit observed with dapagliflozin in this study in patients with diabetes duration of [more than] 20 years, clearly supports that notion,” the authors wrote.
In DECLARE-TIMI 58, 17,160 patients with type 2 diabetes received dapagliflozin or placebo and were followed for a median of 4.2 years. Of those patients, 22.4% had a disease duration of fewer than 5 years; 27.6%, a duration of 5-10 years; 23.0%, 10-15 years; 14.2%, 10-15 years; and 12.9%, more than 20 years. The median duration of disease was 11 years.
Patients in all the age groups had similar reductions in CVD/HHF, compared with placebo, with hazard ratios of 0.79 (disease duration of 5 or fewer years), 0.86, 0.92, 0.81, and 0.75 (duration of 20 years), respectively (interaction trend P = .760).
Treatment with dapagliflozin reduced the incidence of MACE, but the benefit was more apparent in patients with longer-term disease: HR, 1.08; 1.02; 0.94; 0.92; and 0.67, respectively (interaction trend P = .004). Similar trends were seen with MI (interaction trend P = .019) and ischemic stroke (interaction trend P = .015).
The researchers also reported improved benefits in renal-specific outcome with increasing disease duration, with HRs ranging from 0.79 in patients with diabetes duration of fewer than 5 years, to 0.42 in those with a duration of more than 20 years (interaction trend P = .084).
Limitations of the study include the fact that the information about diabetes duration relied on patient reports, and that the original trial was not powered for all subgroup interactions. This authors emphasized that this was a post hoc analysis and as such, should be considered hypothesis generating.
All but two of the authors reported relationships with Astra Zeneca, which funded the study, and other drug companies.
SOURCE: Bajaj HS et al. Diabetes Obes Metab. 2020 Feb 23. doi: 10.1111/dom.14011.
Treatment with the sodium-glucose transporter 2 inhibitor dapagliflozin reduced the risk for cardiovascular disease or hospitalization for heart failure (CVD/HHF) in patients with diabetes, regardless of the duration of the disease, but had a greater protective benefit against major adverse cardiovascular events (MACE) and renal events in patients with longer disease duration, according to new findings from a post hoc analysis of the DECLARE-TIMI 58 trial.
The positive effect of dapagliflozin in patients with MACE – which includes myocardial infarction (MI), CVD, and ischemic stroke – may have been driven by lower rates of MI and ischemic stroke with the drug, compared with placebo, in patients with longer disease duration, wrote Harpreet S. Bajaj, MD, and colleagues. Their report is in Diabetes, Obesity and Metabolism (2020 Feb 23. doi: 10.1111/dom.14011).
It has been previously reported that the risk for complications in diabetes increases with increasing duration of the disease. Recent studies with SGLT-2 inhibitors have shown that the drugs improve cardiovascular and renal outcomes in diabetes, and they are recommended by the American Diabetes Association as second-line therapy in patients with atherosclerotic cardiovascular disease, chronic kidney disease, or heart failure. The European Society of Cardiology and the European Association for the Study of Diabetes recommend that patients with diabetes patients who have three or more risk factors, or those with a disease duration of more than 20 years, should be deemed very high risk and be considered for early treatment with SGLT2 inhibitors.
“The MACE benefit observed with dapagliflozin in this study in patients with diabetes duration of [more than] 20 years, clearly supports that notion,” the authors wrote.
In DECLARE-TIMI 58, 17,160 patients with type 2 diabetes received dapagliflozin or placebo and were followed for a median of 4.2 years. Of those patients, 22.4% had a disease duration of fewer than 5 years; 27.6%, a duration of 5-10 years; 23.0%, 10-15 years; 14.2%, 10-15 years; and 12.9%, more than 20 years. The median duration of disease was 11 years.
Patients in all the age groups had similar reductions in CVD/HHF, compared with placebo, with hazard ratios of 0.79 (disease duration of 5 or fewer years), 0.86, 0.92, 0.81, and 0.75 (duration of 20 years), respectively (interaction trend P = .760).
Treatment with dapagliflozin reduced the incidence of MACE, but the benefit was more apparent in patients with longer-term disease: HR, 1.08; 1.02; 0.94; 0.92; and 0.67, respectively (interaction trend P = .004). Similar trends were seen with MI (interaction trend P = .019) and ischemic stroke (interaction trend P = .015).
The researchers also reported improved benefits in renal-specific outcome with increasing disease duration, with HRs ranging from 0.79 in patients with diabetes duration of fewer than 5 years, to 0.42 in those with a duration of more than 20 years (interaction trend P = .084).
Limitations of the study include the fact that the information about diabetes duration relied on patient reports, and that the original trial was not powered for all subgroup interactions. This authors emphasized that this was a post hoc analysis and as such, should be considered hypothesis generating.
All but two of the authors reported relationships with Astra Zeneca, which funded the study, and other drug companies.
SOURCE: Bajaj HS et al. Diabetes Obes Metab. 2020 Feb 23. doi: 10.1111/dom.14011.
Treatment with the sodium-glucose transporter 2 inhibitor dapagliflozin reduced the risk for cardiovascular disease or hospitalization for heart failure (CVD/HHF) in patients with diabetes, regardless of the duration of the disease, but had a greater protective benefit against major adverse cardiovascular events (MACE) and renal events in patients with longer disease duration, according to new findings from a post hoc analysis of the DECLARE-TIMI 58 trial.
The positive effect of dapagliflozin in patients with MACE – which includes myocardial infarction (MI), CVD, and ischemic stroke – may have been driven by lower rates of MI and ischemic stroke with the drug, compared with placebo, in patients with longer disease duration, wrote Harpreet S. Bajaj, MD, and colleagues. Their report is in Diabetes, Obesity and Metabolism (2020 Feb 23. doi: 10.1111/dom.14011).
It has been previously reported that the risk for complications in diabetes increases with increasing duration of the disease. Recent studies with SGLT-2 inhibitors have shown that the drugs improve cardiovascular and renal outcomes in diabetes, and they are recommended by the American Diabetes Association as second-line therapy in patients with atherosclerotic cardiovascular disease, chronic kidney disease, or heart failure. The European Society of Cardiology and the European Association for the Study of Diabetes recommend that patients with diabetes patients who have three or more risk factors, or those with a disease duration of more than 20 years, should be deemed very high risk and be considered for early treatment with SGLT2 inhibitors.
“The MACE benefit observed with dapagliflozin in this study in patients with diabetes duration of [more than] 20 years, clearly supports that notion,” the authors wrote.
In DECLARE-TIMI 58, 17,160 patients with type 2 diabetes received dapagliflozin or placebo and were followed for a median of 4.2 years. Of those patients, 22.4% had a disease duration of fewer than 5 years; 27.6%, a duration of 5-10 years; 23.0%, 10-15 years; 14.2%, 10-15 years; and 12.9%, more than 20 years. The median duration of disease was 11 years.
Patients in all the age groups had similar reductions in CVD/HHF, compared with placebo, with hazard ratios of 0.79 (disease duration of 5 or fewer years), 0.86, 0.92, 0.81, and 0.75 (duration of 20 years), respectively (interaction trend P = .760).
Treatment with dapagliflozin reduced the incidence of MACE, but the benefit was more apparent in patients with longer-term disease: HR, 1.08; 1.02; 0.94; 0.92; and 0.67, respectively (interaction trend P = .004). Similar trends were seen with MI (interaction trend P = .019) and ischemic stroke (interaction trend P = .015).
The researchers also reported improved benefits in renal-specific outcome with increasing disease duration, with HRs ranging from 0.79 in patients with diabetes duration of fewer than 5 years, to 0.42 in those with a duration of more than 20 years (interaction trend P = .084).
Limitations of the study include the fact that the information about diabetes duration relied on patient reports, and that the original trial was not powered for all subgroup interactions. This authors emphasized that this was a post hoc analysis and as such, should be considered hypothesis generating.
All but two of the authors reported relationships with Astra Zeneca, which funded the study, and other drug companies.
SOURCE: Bajaj HS et al. Diabetes Obes Metab. 2020 Feb 23. doi: 10.1111/dom.14011.
FROM DIABETES, OBESITY AND METABOLISM
Latent diabetes warrants earlier, tighter glycemic control
according a post hoc analysis of a large European database.
However, Ernesto Maddaloni, MD, of Sapienza University of Rome and University of Oxford (England), and colleagues noted that the risk is less than half that in patients with type 2 disease during the first several years after diagnosis but that, after 9 years, the risk curves cross over, and patients with latent autoimmune diabetes of adulthood (LADA) matriculate to a 25% greater risk microvascular complications than do their type 2 counterparts.
The results point to a need for tighter glycemic control in patients with latent autoimmune disease and “might have relevant implications for the understanding of the differential risk of complications between type 2 diabetes and autoimmune diabetes in general,” the researchers wrote online in The Lancet Diabetes & Endocrinology. They emphasized that the study represents the largest population of patients with latent autoimmune diabetes with the longest follow-up in a randomized controlled trial so far.
Diabetic microvascular complications are a major cause of end-stage renal disease and blindness in LADA, therefore, “implementing strict glycemic control from the time of diagnosis could reduce the later risk of microvascular complications in [these patients],” the authors wrote.
The researchers analyzed 30 years of data from the United Kingdom Prospective Diabetes Study, focusing on 564 patients with LADA and 4,464 adults with type 2 diabetes. The primary outcome was first occurrence of renal failure, death from renal disease, blindness in one eye, vitreous hemorrhage, or retinal laser treatment.
With a median follow-up of 17.3 years, 21% of all patients (1,041) developed microvascular complications, of which there were 65 renal events and 976 retinopathy events. Secondary outcomes were nephropathy and retinopathy.
The study measured incidence in 1,000 person-years and found that the incidence for the overall primary composite microvascular outcome was 5.3% for LADA and 10% for type 2 diabetes in the first 9 years after diagnosis (P = .0020), but 13.6% and 9.2%, respectively, after that (P less than .0001). That translated into adjusted hazard ratios of 0.45 for LADA, compared with type 2 diabetes, in the first 9 years (P less than .0001) and 1.25 beyond 9 years (P = .047). The incidence of retinopathy events was 5.3% for LADA and 9.6% for type 2 diabetes up to 9 years (P = .003), and 12.5% and 8.6% thereafter (P = .001). Nephropathy rates were similar in both groups at 1.3% or less.
“The lower risk of microvascular complications during the first years after the diagnosis of latent autoimmune diabetes needs further examination,” Dr. Maddaloni and colleagues wrote.
They cautioned that LADA is often misdiagnosed as a form of type 1 diabetes. “Therefore, latent autoimmune diabetes could be the right bench test for studying differences between autoimmune diabetes and type 2 diabetes, because of fewer disparities in age and disease duration than with the comparison of type 1 diabetes and type 2 diabetes,” they wrote.
In an accompanying editorial, Didac Mauricio, MD, of the Autonomous University of Barcelona, credited Dr. Maddaloni and colleagues with presenting evidence “of major relevance” in an adequately powered study that provided “a robust conclusion” about the risk of microvascular complications in latent autoimmune diabetes.
Dr. Mauricio noted that the study adds to the literature that different subgroups of type 2 diabetes patients exist and highlights the distinct characteristics of latent autoimmune diabetes. In addition, it builds on a previous study by Dr. Maddaloni and coauthors that found cardiovascular disease outcomes did not differ between latent autoimmune and type 2 diabetes (Diabetes Obes Metab. 2019;21:2115-22), he wrote. The research team’s most recent findings “emphasize the need for early identification of latent autoimmune disease,” he stated.
The findings also raise important questions about screening all patients for antibodies upon diagnosis of diabetes, he said. “I firmly believe that it is time to take action,” first, because antibody testing is likely cost-effective and cost-saving because it facilitates better-informed, more timely decisions early in the disease trajectory, and second, it has already been well documented that patients with latent autoimmune diabetes have a higher glycemic burden.
An alternative to early universal screening for antibodies would be to raise awareness, especially among general practitioners, about the importance of timely diagnosis of LADA, Dr. Mauricio added.
The study received funding from the European Foundation for the Study of Diabetes Mentorship Program, supported by AstraZeneca. Dr. Maddaloni disclosed financial relationships with Sanofi, Eli Lilly, Abbott, and AstraZeneca. Another author disclosed financial relationships with Boehringer Ingelheim, Merck, Bayer, AstraZeneca, Novartis, and Novo Nordisk. All the other authors had no relevant financial relationships to disclose. Dr. Mauricio disclosed financial relationships with AstraZeneca, Eli Lilly, Merck Sharp & Dohme, NovoNordisk, Sanofi, Almirall, Boehringer Ingelheim, Eli Lilly, Ferrer, Janssen, Menarini, and URGO.
SOURCE: Maddaloni E et al. Lancet Diabetes Endocrinol. 2020 Feb 4. doi: 0.1016/S2213-8587(20)30003-6.
according a post hoc analysis of a large European database.
However, Ernesto Maddaloni, MD, of Sapienza University of Rome and University of Oxford (England), and colleagues noted that the risk is less than half that in patients with type 2 disease during the first several years after diagnosis but that, after 9 years, the risk curves cross over, and patients with latent autoimmune diabetes of adulthood (LADA) matriculate to a 25% greater risk microvascular complications than do their type 2 counterparts.
The results point to a need for tighter glycemic control in patients with latent autoimmune disease and “might have relevant implications for the understanding of the differential risk of complications between type 2 diabetes and autoimmune diabetes in general,” the researchers wrote online in The Lancet Diabetes & Endocrinology. They emphasized that the study represents the largest population of patients with latent autoimmune diabetes with the longest follow-up in a randomized controlled trial so far.
Diabetic microvascular complications are a major cause of end-stage renal disease and blindness in LADA, therefore, “implementing strict glycemic control from the time of diagnosis could reduce the later risk of microvascular complications in [these patients],” the authors wrote.
The researchers analyzed 30 years of data from the United Kingdom Prospective Diabetes Study, focusing on 564 patients with LADA and 4,464 adults with type 2 diabetes. The primary outcome was first occurrence of renal failure, death from renal disease, blindness in one eye, vitreous hemorrhage, or retinal laser treatment.
With a median follow-up of 17.3 years, 21% of all patients (1,041) developed microvascular complications, of which there were 65 renal events and 976 retinopathy events. Secondary outcomes were nephropathy and retinopathy.
The study measured incidence in 1,000 person-years and found that the incidence for the overall primary composite microvascular outcome was 5.3% for LADA and 10% for type 2 diabetes in the first 9 years after diagnosis (P = .0020), but 13.6% and 9.2%, respectively, after that (P less than .0001). That translated into adjusted hazard ratios of 0.45 for LADA, compared with type 2 diabetes, in the first 9 years (P less than .0001) and 1.25 beyond 9 years (P = .047). The incidence of retinopathy events was 5.3% for LADA and 9.6% for type 2 diabetes up to 9 years (P = .003), and 12.5% and 8.6% thereafter (P = .001). Nephropathy rates were similar in both groups at 1.3% or less.
“The lower risk of microvascular complications during the first years after the diagnosis of latent autoimmune diabetes needs further examination,” Dr. Maddaloni and colleagues wrote.
They cautioned that LADA is often misdiagnosed as a form of type 1 diabetes. “Therefore, latent autoimmune diabetes could be the right bench test for studying differences between autoimmune diabetes and type 2 diabetes, because of fewer disparities in age and disease duration than with the comparison of type 1 diabetes and type 2 diabetes,” they wrote.
In an accompanying editorial, Didac Mauricio, MD, of the Autonomous University of Barcelona, credited Dr. Maddaloni and colleagues with presenting evidence “of major relevance” in an adequately powered study that provided “a robust conclusion” about the risk of microvascular complications in latent autoimmune diabetes.
Dr. Mauricio noted that the study adds to the literature that different subgroups of type 2 diabetes patients exist and highlights the distinct characteristics of latent autoimmune diabetes. In addition, it builds on a previous study by Dr. Maddaloni and coauthors that found cardiovascular disease outcomes did not differ between latent autoimmune and type 2 diabetes (Diabetes Obes Metab. 2019;21:2115-22), he wrote. The research team’s most recent findings “emphasize the need for early identification of latent autoimmune disease,” he stated.
The findings also raise important questions about screening all patients for antibodies upon diagnosis of diabetes, he said. “I firmly believe that it is time to take action,” first, because antibody testing is likely cost-effective and cost-saving because it facilitates better-informed, more timely decisions early in the disease trajectory, and second, it has already been well documented that patients with latent autoimmune diabetes have a higher glycemic burden.
An alternative to early universal screening for antibodies would be to raise awareness, especially among general practitioners, about the importance of timely diagnosis of LADA, Dr. Mauricio added.
The study received funding from the European Foundation for the Study of Diabetes Mentorship Program, supported by AstraZeneca. Dr. Maddaloni disclosed financial relationships with Sanofi, Eli Lilly, Abbott, and AstraZeneca. Another author disclosed financial relationships with Boehringer Ingelheim, Merck, Bayer, AstraZeneca, Novartis, and Novo Nordisk. All the other authors had no relevant financial relationships to disclose. Dr. Mauricio disclosed financial relationships with AstraZeneca, Eli Lilly, Merck Sharp & Dohme, NovoNordisk, Sanofi, Almirall, Boehringer Ingelheim, Eli Lilly, Ferrer, Janssen, Menarini, and URGO.
SOURCE: Maddaloni E et al. Lancet Diabetes Endocrinol. 2020 Feb 4. doi: 0.1016/S2213-8587(20)30003-6.
according a post hoc analysis of a large European database.
However, Ernesto Maddaloni, MD, of Sapienza University of Rome and University of Oxford (England), and colleagues noted that the risk is less than half that in patients with type 2 disease during the first several years after diagnosis but that, after 9 years, the risk curves cross over, and patients with latent autoimmune diabetes of adulthood (LADA) matriculate to a 25% greater risk microvascular complications than do their type 2 counterparts.
The results point to a need for tighter glycemic control in patients with latent autoimmune disease and “might have relevant implications for the understanding of the differential risk of complications between type 2 diabetes and autoimmune diabetes in general,” the researchers wrote online in The Lancet Diabetes & Endocrinology. They emphasized that the study represents the largest population of patients with latent autoimmune diabetes with the longest follow-up in a randomized controlled trial so far.
Diabetic microvascular complications are a major cause of end-stage renal disease and blindness in LADA, therefore, “implementing strict glycemic control from the time of diagnosis could reduce the later risk of microvascular complications in [these patients],” the authors wrote.
The researchers analyzed 30 years of data from the United Kingdom Prospective Diabetes Study, focusing on 564 patients with LADA and 4,464 adults with type 2 diabetes. The primary outcome was first occurrence of renal failure, death from renal disease, blindness in one eye, vitreous hemorrhage, or retinal laser treatment.
With a median follow-up of 17.3 years, 21% of all patients (1,041) developed microvascular complications, of which there were 65 renal events and 976 retinopathy events. Secondary outcomes were nephropathy and retinopathy.
The study measured incidence in 1,000 person-years and found that the incidence for the overall primary composite microvascular outcome was 5.3% for LADA and 10% for type 2 diabetes in the first 9 years after diagnosis (P = .0020), but 13.6% and 9.2%, respectively, after that (P less than .0001). That translated into adjusted hazard ratios of 0.45 for LADA, compared with type 2 diabetes, in the first 9 years (P less than .0001) and 1.25 beyond 9 years (P = .047). The incidence of retinopathy events was 5.3% for LADA and 9.6% for type 2 diabetes up to 9 years (P = .003), and 12.5% and 8.6% thereafter (P = .001). Nephropathy rates were similar in both groups at 1.3% or less.
“The lower risk of microvascular complications during the first years after the diagnosis of latent autoimmune diabetes needs further examination,” Dr. Maddaloni and colleagues wrote.
They cautioned that LADA is often misdiagnosed as a form of type 1 diabetes. “Therefore, latent autoimmune diabetes could be the right bench test for studying differences between autoimmune diabetes and type 2 diabetes, because of fewer disparities in age and disease duration than with the comparison of type 1 diabetes and type 2 diabetes,” they wrote.
In an accompanying editorial, Didac Mauricio, MD, of the Autonomous University of Barcelona, credited Dr. Maddaloni and colleagues with presenting evidence “of major relevance” in an adequately powered study that provided “a robust conclusion” about the risk of microvascular complications in latent autoimmune diabetes.
Dr. Mauricio noted that the study adds to the literature that different subgroups of type 2 diabetes patients exist and highlights the distinct characteristics of latent autoimmune diabetes. In addition, it builds on a previous study by Dr. Maddaloni and coauthors that found cardiovascular disease outcomes did not differ between latent autoimmune and type 2 diabetes (Diabetes Obes Metab. 2019;21:2115-22), he wrote. The research team’s most recent findings “emphasize the need for early identification of latent autoimmune disease,” he stated.
The findings also raise important questions about screening all patients for antibodies upon diagnosis of diabetes, he said. “I firmly believe that it is time to take action,” first, because antibody testing is likely cost-effective and cost-saving because it facilitates better-informed, more timely decisions early in the disease trajectory, and second, it has already been well documented that patients with latent autoimmune diabetes have a higher glycemic burden.
An alternative to early universal screening for antibodies would be to raise awareness, especially among general practitioners, about the importance of timely diagnosis of LADA, Dr. Mauricio added.
The study received funding from the European Foundation for the Study of Diabetes Mentorship Program, supported by AstraZeneca. Dr. Maddaloni disclosed financial relationships with Sanofi, Eli Lilly, Abbott, and AstraZeneca. Another author disclosed financial relationships with Boehringer Ingelheim, Merck, Bayer, AstraZeneca, Novartis, and Novo Nordisk. All the other authors had no relevant financial relationships to disclose. Dr. Mauricio disclosed financial relationships with AstraZeneca, Eli Lilly, Merck Sharp & Dohme, NovoNordisk, Sanofi, Almirall, Boehringer Ingelheim, Eli Lilly, Ferrer, Janssen, Menarini, and URGO.
SOURCE: Maddaloni E et al. Lancet Diabetes Endocrinol. 2020 Feb 4. doi: 0.1016/S2213-8587(20)30003-6.
FROM THE LANCET DIABETES & ENDOCRINOLOGY
NPH insulin: It remains a good option
ILLUSTRATIVE CASE
Blanche is a 54-year-old overweight woman who has had type 2 diabetes mellitus (T2DM) for 5 years. She has been optimized on both metformin (1000 mg bid) and exenatide (2 mg weekly). While taking these medications, her hemoglobin A1C (HbA1C) has dropped from 11.2 to 8.4, and her body mass index (BMI) has declined from 35 to 31. However, she is still not at goal. You decide to start her on long-acting basal insulin. She has limited income, and she currently spends $75/month for her metformin, exenatide, atorvastatin, and lisinopril. What insulin do you prescribe?
The Centers for Disease Control and Prevention (CDC) reported that the prevalence of diabetes in the United States was 9.4% (30.3 million people) in 2015.2 Among those affected, approximately 95.8% had T2DM.2 The same report estimated that 1.5 million new cases of diabetes (6.7 per 1000 persons) were diagnosed annually among US adults ≥ 18 years of age, and that about $7900 of annual medical expenses for patients diagnosed with diabetes was directly attributable to diabetes.2
In the United States, neutral protamine Hagedorn (NPH) insulin was the most commonly used intermediate- to long-acting insulin until the introduction of the long-acting insulin analogs (insulin glargine in 2000 and insulin detemir in 2005).3 Despite being considerably more expensive than NPH insulin, long-acting insulin analogs had captured more than 80% of the total long-acting insulin market by 2010.4 The market share for NPH insulin dropped from 81.9% in 2001 to 16.2% in 2010.4
While the newer insulin analogs are significantly more expensive than NPH insulin, with higher corresponding out-of-pocket costs to patients, researchers have had a difficult time demonstrating greater effectiveness or any definitive differences in any long-term outcomes between NPH and the insulin analogs. A 2007 Cochrane review comparing NPH insulin to both glargine and detemir showed little difference in metabolic control (as measured by HbA1C) or in the rate of severe hypoglycemia. However, the rates of symptomatic, overall, and nocturnal hypoglycemia were statistically lower with the insulin analogs.5
A 2015 retrospective observational study from the Veterans Health Administration (N = 142,940) covering a 10-year period from 2000 to 2010 found no consistent differences in long-term health outcomes when comparing the use of long-acting insulin analogs to that of NPH insulin.3,6
STUDY SUMMARY
Study compares performance of basal insulin analogs to that of NPH
This retrospective, observational study included 25,489 adult patients with T2DM who were enrolled in Kaiser Permanente of Northern California, had full medical and prescription coverage, and initiated basal insulin therapy with either NPH or an insulin analog between 2006 and 2015.
The primary outcome was the time from basal insulin therapy initiation to a hypoglycemia-related emergency department (ED) visit or hospital admission. The secondary outcome was the change in HbA1C level within 1 year of initiation of basal insulin therapy.
Continue to: Per 1000 person-years...
Per 1000 person-years, there was no significant difference in hypoglycemia-related ED visits or hospital admissions between the analog and NPH groups (11.9 events vs 8.8 events, respectively; between-group difference, 3.1 events; 95% confidence interval [CI], –1.5 to 7.7). HbA1C reduction was statistically greater with NPH, but most likely not clinically significant between insulin analogs and NPH (1.26 vs 1.48 percentage points; between group difference, –0.22%; 95% CI, –0.09% to –0.37%).
WHAT’S NEW?
No clinically relevant differences between insulin analogs and NPH
This study revealed that there is no clinically relevant difference in HbA1C levels and no difference in patient-focused outcomes of hypoglycemia-related ED visits or hospital admissions between NPH insulin and the more expensive insulin analogs. This makes a strong case for a different approach to initial basal insulin therapy for patients with T2DM who need insulin for glucose control.
CAVEATS
Demographics and less severe hypoglycemia might be at issue
This retrospective, observational study has broad demographics (but moderate under-representation of African-Americans), minimal patient health care disparities, and good access to medications. But generalizability outside of an integrated health delivery system may be limited. The study design also is subject to confounding, as not all potential impacts on the results can be corrected for or controlled in an observational study. Also, less profound hypoglycemia that did not require an ED visit or hospital admission was not captured.
CHALLENGES TO IMPLEMENTATION
Convenience and marketing factors may hinder change
Insulin analogs may have a number of convenience and marketing factors that may make it hard for providers and systems to change and use more NPH. However, the easy-to-use insulin analog pens are matched in availability and convenience by the much less advertised NPH insulin pens produced by at least 3 major pharmaceutical companies. In addition, while the overall cost for the insulin analogs continues to be 2 to 3 times that of non-human NPH insulin, insurance often covers up to, or more than, 80% of the cost of the insulin analogs, making the difference in the patient’s copay between the 2 not as severe. For example, patients may pay $30 to $40 per month for insulin analogs vs $10 to $25 per month for cheaper versions of NPH.7,8
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Lipska KJ, Parker MM, Moffet HH, et al. Association of initiation of basal insulin analogs vs neutral protamine Hagedorn insulin with hypoglycemia-related emergency department visits or hospital admissions and with glycemic control in patients with type 2 diabetes. JAMA. 2018;320:53-62.
2. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed January 15, 2020.
3. Prentice JC, Conlin PR, Gellad WF, et al. Long-term outcomes of analogue insulin compared with NPH for patients with type 2 diabetes mellitus. Am J Manag Care. 2015;21:e235-e243.
4. Turner LW, Nartey D, Stafford RS, et al. Ambulatory treatment of type 2 diabetes in the U.S., 1997-2012. Diabetes Care. 2014;37:985-992.
5. Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2007;(2):CD005613.
6. Chamberlain JJ, Herman WH, Leal S, et al. Pharmacologic therapy for type 2 diabetes: synopsis of the 2017 American Diabetes Association standards of medical care in diabetes. Ann Intern Med. 2017;166:572-578.
7. GoodRx.com. Insulins. www.goodrx.com/insulins. Accessed January 20, 2020. 8. Cefalu WT, Dawes DE, Gavlak G, et al. Insulin access and affordability working group: conclusions and recommendations. Diabetes Care. 2018;41:1299-1311.
ILLUSTRATIVE CASE
Blanche is a 54-year-old overweight woman who has had type 2 diabetes mellitus (T2DM) for 5 years. She has been optimized on both metformin (1000 mg bid) and exenatide (2 mg weekly). While taking these medications, her hemoglobin A1C (HbA1C) has dropped from 11.2 to 8.4, and her body mass index (BMI) has declined from 35 to 31. However, she is still not at goal. You decide to start her on long-acting basal insulin. She has limited income, and she currently spends $75/month for her metformin, exenatide, atorvastatin, and lisinopril. What insulin do you prescribe?
The Centers for Disease Control and Prevention (CDC) reported that the prevalence of diabetes in the United States was 9.4% (30.3 million people) in 2015.2 Among those affected, approximately 95.8% had T2DM.2 The same report estimated that 1.5 million new cases of diabetes (6.7 per 1000 persons) were diagnosed annually among US adults ≥ 18 years of age, and that about $7900 of annual medical expenses for patients diagnosed with diabetes was directly attributable to diabetes.2
In the United States, neutral protamine Hagedorn (NPH) insulin was the most commonly used intermediate- to long-acting insulin until the introduction of the long-acting insulin analogs (insulin glargine in 2000 and insulin detemir in 2005).3 Despite being considerably more expensive than NPH insulin, long-acting insulin analogs had captured more than 80% of the total long-acting insulin market by 2010.4 The market share for NPH insulin dropped from 81.9% in 2001 to 16.2% in 2010.4
While the newer insulin analogs are significantly more expensive than NPH insulin, with higher corresponding out-of-pocket costs to patients, researchers have had a difficult time demonstrating greater effectiveness or any definitive differences in any long-term outcomes between NPH and the insulin analogs. A 2007 Cochrane review comparing NPH insulin to both glargine and detemir showed little difference in metabolic control (as measured by HbA1C) or in the rate of severe hypoglycemia. However, the rates of symptomatic, overall, and nocturnal hypoglycemia were statistically lower with the insulin analogs.5
A 2015 retrospective observational study from the Veterans Health Administration (N = 142,940) covering a 10-year period from 2000 to 2010 found no consistent differences in long-term health outcomes when comparing the use of long-acting insulin analogs to that of NPH insulin.3,6
STUDY SUMMARY
Study compares performance of basal insulin analogs to that of NPH
This retrospective, observational study included 25,489 adult patients with T2DM who were enrolled in Kaiser Permanente of Northern California, had full medical and prescription coverage, and initiated basal insulin therapy with either NPH or an insulin analog between 2006 and 2015.
The primary outcome was the time from basal insulin therapy initiation to a hypoglycemia-related emergency department (ED) visit or hospital admission. The secondary outcome was the change in HbA1C level within 1 year of initiation of basal insulin therapy.
Continue to: Per 1000 person-years...
Per 1000 person-years, there was no significant difference in hypoglycemia-related ED visits or hospital admissions between the analog and NPH groups (11.9 events vs 8.8 events, respectively; between-group difference, 3.1 events; 95% confidence interval [CI], –1.5 to 7.7). HbA1C reduction was statistically greater with NPH, but most likely not clinically significant between insulin analogs and NPH (1.26 vs 1.48 percentage points; between group difference, –0.22%; 95% CI, –0.09% to –0.37%).
WHAT’S NEW?
No clinically relevant differences between insulin analogs and NPH
This study revealed that there is no clinically relevant difference in HbA1C levels and no difference in patient-focused outcomes of hypoglycemia-related ED visits or hospital admissions between NPH insulin and the more expensive insulin analogs. This makes a strong case for a different approach to initial basal insulin therapy for patients with T2DM who need insulin for glucose control.
CAVEATS
Demographics and less severe hypoglycemia might be at issue
This retrospective, observational study has broad demographics (but moderate under-representation of African-Americans), minimal patient health care disparities, and good access to medications. But generalizability outside of an integrated health delivery system may be limited. The study design also is subject to confounding, as not all potential impacts on the results can be corrected for or controlled in an observational study. Also, less profound hypoglycemia that did not require an ED visit or hospital admission was not captured.
CHALLENGES TO IMPLEMENTATION
Convenience and marketing factors may hinder change
Insulin analogs may have a number of convenience and marketing factors that may make it hard for providers and systems to change and use more NPH. However, the easy-to-use insulin analog pens are matched in availability and convenience by the much less advertised NPH insulin pens produced by at least 3 major pharmaceutical companies. In addition, while the overall cost for the insulin analogs continues to be 2 to 3 times that of non-human NPH insulin, insurance often covers up to, or more than, 80% of the cost of the insulin analogs, making the difference in the patient’s copay between the 2 not as severe. For example, patients may pay $30 to $40 per month for insulin analogs vs $10 to $25 per month for cheaper versions of NPH.7,8
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
Blanche is a 54-year-old overweight woman who has had type 2 diabetes mellitus (T2DM) for 5 years. She has been optimized on both metformin (1000 mg bid) and exenatide (2 mg weekly). While taking these medications, her hemoglobin A1C (HbA1C) has dropped from 11.2 to 8.4, and her body mass index (BMI) has declined from 35 to 31. However, she is still not at goal. You decide to start her on long-acting basal insulin. She has limited income, and she currently spends $75/month for her metformin, exenatide, atorvastatin, and lisinopril. What insulin do you prescribe?
The Centers for Disease Control and Prevention (CDC) reported that the prevalence of diabetes in the United States was 9.4% (30.3 million people) in 2015.2 Among those affected, approximately 95.8% had T2DM.2 The same report estimated that 1.5 million new cases of diabetes (6.7 per 1000 persons) were diagnosed annually among US adults ≥ 18 years of age, and that about $7900 of annual medical expenses for patients diagnosed with diabetes was directly attributable to diabetes.2
In the United States, neutral protamine Hagedorn (NPH) insulin was the most commonly used intermediate- to long-acting insulin until the introduction of the long-acting insulin analogs (insulin glargine in 2000 and insulin detemir in 2005).3 Despite being considerably more expensive than NPH insulin, long-acting insulin analogs had captured more than 80% of the total long-acting insulin market by 2010.4 The market share for NPH insulin dropped from 81.9% in 2001 to 16.2% in 2010.4
While the newer insulin analogs are significantly more expensive than NPH insulin, with higher corresponding out-of-pocket costs to patients, researchers have had a difficult time demonstrating greater effectiveness or any definitive differences in any long-term outcomes between NPH and the insulin analogs. A 2007 Cochrane review comparing NPH insulin to both glargine and detemir showed little difference in metabolic control (as measured by HbA1C) or in the rate of severe hypoglycemia. However, the rates of symptomatic, overall, and nocturnal hypoglycemia were statistically lower with the insulin analogs.5
A 2015 retrospective observational study from the Veterans Health Administration (N = 142,940) covering a 10-year period from 2000 to 2010 found no consistent differences in long-term health outcomes when comparing the use of long-acting insulin analogs to that of NPH insulin.3,6
STUDY SUMMARY
Study compares performance of basal insulin analogs to that of NPH
This retrospective, observational study included 25,489 adult patients with T2DM who were enrolled in Kaiser Permanente of Northern California, had full medical and prescription coverage, and initiated basal insulin therapy with either NPH or an insulin analog between 2006 and 2015.
The primary outcome was the time from basal insulin therapy initiation to a hypoglycemia-related emergency department (ED) visit or hospital admission. The secondary outcome was the change in HbA1C level within 1 year of initiation of basal insulin therapy.
Continue to: Per 1000 person-years...
Per 1000 person-years, there was no significant difference in hypoglycemia-related ED visits or hospital admissions between the analog and NPH groups (11.9 events vs 8.8 events, respectively; between-group difference, 3.1 events; 95% confidence interval [CI], –1.5 to 7.7). HbA1C reduction was statistically greater with NPH, but most likely not clinically significant between insulin analogs and NPH (1.26 vs 1.48 percentage points; between group difference, –0.22%; 95% CI, –0.09% to –0.37%).
WHAT’S NEW?
No clinically relevant differences between insulin analogs and NPH
This study revealed that there is no clinically relevant difference in HbA1C levels and no difference in patient-focused outcomes of hypoglycemia-related ED visits or hospital admissions between NPH insulin and the more expensive insulin analogs. This makes a strong case for a different approach to initial basal insulin therapy for patients with T2DM who need insulin for glucose control.
CAVEATS
Demographics and less severe hypoglycemia might be at issue
This retrospective, observational study has broad demographics (but moderate under-representation of African-Americans), minimal patient health care disparities, and good access to medications. But generalizability outside of an integrated health delivery system may be limited. The study design also is subject to confounding, as not all potential impacts on the results can be corrected for or controlled in an observational study. Also, less profound hypoglycemia that did not require an ED visit or hospital admission was not captured.
CHALLENGES TO IMPLEMENTATION
Convenience and marketing factors may hinder change
Insulin analogs may have a number of convenience and marketing factors that may make it hard for providers and systems to change and use more NPH. However, the easy-to-use insulin analog pens are matched in availability and convenience by the much less advertised NPH insulin pens produced by at least 3 major pharmaceutical companies. In addition, while the overall cost for the insulin analogs continues to be 2 to 3 times that of non-human NPH insulin, insurance often covers up to, or more than, 80% of the cost of the insulin analogs, making the difference in the patient’s copay between the 2 not as severe. For example, patients may pay $30 to $40 per month for insulin analogs vs $10 to $25 per month for cheaper versions of NPH.7,8
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Lipska KJ, Parker MM, Moffet HH, et al. Association of initiation of basal insulin analogs vs neutral protamine Hagedorn insulin with hypoglycemia-related emergency department visits or hospital admissions and with glycemic control in patients with type 2 diabetes. JAMA. 2018;320:53-62.
2. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed January 15, 2020.
3. Prentice JC, Conlin PR, Gellad WF, et al. Long-term outcomes of analogue insulin compared with NPH for patients with type 2 diabetes mellitus. Am J Manag Care. 2015;21:e235-e243.
4. Turner LW, Nartey D, Stafford RS, et al. Ambulatory treatment of type 2 diabetes in the U.S., 1997-2012. Diabetes Care. 2014;37:985-992.
5. Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2007;(2):CD005613.
6. Chamberlain JJ, Herman WH, Leal S, et al. Pharmacologic therapy for type 2 diabetes: synopsis of the 2017 American Diabetes Association standards of medical care in diabetes. Ann Intern Med. 2017;166:572-578.
7. GoodRx.com. Insulins. www.goodrx.com/insulins. Accessed January 20, 2020. 8. Cefalu WT, Dawes DE, Gavlak G, et al. Insulin access and affordability working group: conclusions and recommendations. Diabetes Care. 2018;41:1299-1311.
1. Lipska KJ, Parker MM, Moffet HH, et al. Association of initiation of basal insulin analogs vs neutral protamine Hagedorn insulin with hypoglycemia-related emergency department visits or hospital admissions and with glycemic control in patients with type 2 diabetes. JAMA. 2018;320:53-62.
2. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed January 15, 2020.
3. Prentice JC, Conlin PR, Gellad WF, et al. Long-term outcomes of analogue insulin compared with NPH for patients with type 2 diabetes mellitus. Am J Manag Care. 2015;21:e235-e243.
4. Turner LW, Nartey D, Stafford RS, et al. Ambulatory treatment of type 2 diabetes in the U.S., 1997-2012. Diabetes Care. 2014;37:985-992.
5. Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2007;(2):CD005613.
6. Chamberlain JJ, Herman WH, Leal S, et al. Pharmacologic therapy for type 2 diabetes: synopsis of the 2017 American Diabetes Association standards of medical care in diabetes. Ann Intern Med. 2017;166:572-578.
7. GoodRx.com. Insulins. www.goodrx.com/insulins. Accessed January 20, 2020. 8. Cefalu WT, Dawes DE, Gavlak G, et al. Insulin access and affordability working group: conclusions and recommendations. Diabetes Care. 2018;41:1299-1311.
PRACTICE CHANGER
Consider NPH insulin for patients who require initiation of long-acting insulin therapy because it is as safe as, and more cost-effective than, basal insulin analogs.
STRENGTH OF RECOMMENDATION
B: Based on a single, large, retrospective, observational study.
Lipska KJ, Parker MM, Moffet HH, et al. Association of initiation of basal insulin analogs vs neutral protamine Hagedorn insulin with hypoglycemia-related emergency department visits or hospital admissions and with glycemic control in patients with type 2 diabetes. JAMA. 2018;320:53-62.1
Glucocorticoid use linked to mortality in RA with diabetes
Glucocorticoid use is associated with greater mortality and cardiovascular risk in RA patients, and the associated risk is greater still in patients with RA and comorbid diabetes. The findings come from a new retrospective analysis derived from U.K. primary care records.
Although patients with diabetes actually had a lower relative risk for mortality than the nondiabetes cohort, they had a greater mortality difference because of a greater baseline risk. Ultimately, glucocorticoid (GC) use was associated with an additional 44.9 deaths per 1,000 person-years in the diabetes group, compared with 34.4 per 1,000 person-years in the RA-only group.
The study, led by Ruth Costello and William Dixon, MBBS, PhD of the University of Manchester (England), was published in BMC Rheumatology.
The findings aren’t particularly surprising, given that steroid use and diabetes have associated cardiovascular risks, and physicians generally try to reduce or eliminate their use. “There’s a group [of physicians] saying that we don’t need to use steroids at all in rheumatoid arthritis, except maybe for [a] short time at diagnosis to bridge to other therapies, or during flares,” Gordon Starkebaum, MD, professor emeritus of rheumatology at the University of Washington, Seattle, said in an interview. He recounted a session at last year’s annual meeting of the American College of Rheumatology that advocated for only injectable steroid use during flare-ups. “That was provocative,” Dr. Starkebaum said.
“It’s a retrospective study, so it has some limitations, but it provides good insight, and some substantiation to what we already think,” added Brett Smith, DO, a rheumatologist practicing in Knoxville, Tenn.
Dr. Smith suggested that the study further underscores the need to follow treat-to-target protocols in RA. He emphasized that lifetime exposure to steroids is likely the greatest concern, and that steady accumulating doses are a sign of trouble. “If you need that much steroids, you need to go up on your medication – your methotrexate, or sulfasalazine, or your biologic,” said Dr. Smith. “At least 50% of people will need a biologic to [achieve] disease control, and if you get them on it, they’re going to have better disease control, compliance is typically better, and they’re going to have less steroid exposure.”
Dr. Smith also noted that comorbid diabetes shouldn’t affect treat-to-target strategies. In fact, in such patients “you should probably be following it more tightly to reduce the cardiovascular outcomes,” he said.
The retrospective analysis included 9,085 patients with RA and with or without type 2 diabetes, with a mean follow-up of 5.2 years. They were recruited to the study between 1998 and 2011. Among patients with comorbid diabetes, those exposed to GC had a mortality of 67.4 per 1,000 person-years, compared with 22.5 among those not exposed to GC. Among those with RA alone, mortality was 44.6 versus 10.2 with and without GC exposure, respectively. Those with diabetes had a lower risk ratio for mortality (2.99 vs. 4.37), but a higher mortality difference (44.9 vs. 34.4 per 1,000 person-years).
“The increased absolute hazard for all-cause mortality indicates the greater public health impact of people with RA using GCs if they have [diabetes],” the researchers wrote. “Rheumatologists should consider [diabetes] status when prescribing GCs to patients with RA given this potential impact of GC therapy on glucose control and mortality.”
The study was limited by a lack of information on GC dose and cumulative exposure. Given its retrospective nature, the study could have been affected by confounding by indication, as well as unknown confounders.
The study was funded by the Centre for Epidemiology Versus Arthritis and the National Institute for Health Research Biomedical Research Centre. Dr. Starkebaum has no relevant financial disclosures. Dr. Smith is on the speaker’s bureau for AbbVie and serves on the company’s advisory board. He is also on the advisory boards of Regeneron and Sanofi Genzyme.
SOURCE: Costello R et al. BMC Rheumatol. 2020 Feb 19. doi: 10.1186/s41927-019-0105-4.
Glucocorticoid use is associated with greater mortality and cardiovascular risk in RA patients, and the associated risk is greater still in patients with RA and comorbid diabetes. The findings come from a new retrospective analysis derived from U.K. primary care records.
Although patients with diabetes actually had a lower relative risk for mortality than the nondiabetes cohort, they had a greater mortality difference because of a greater baseline risk. Ultimately, glucocorticoid (GC) use was associated with an additional 44.9 deaths per 1,000 person-years in the diabetes group, compared with 34.4 per 1,000 person-years in the RA-only group.
The study, led by Ruth Costello and William Dixon, MBBS, PhD of the University of Manchester (England), was published in BMC Rheumatology.
The findings aren’t particularly surprising, given that steroid use and diabetes have associated cardiovascular risks, and physicians generally try to reduce or eliminate their use. “There’s a group [of physicians] saying that we don’t need to use steroids at all in rheumatoid arthritis, except maybe for [a] short time at diagnosis to bridge to other therapies, or during flares,” Gordon Starkebaum, MD, professor emeritus of rheumatology at the University of Washington, Seattle, said in an interview. He recounted a session at last year’s annual meeting of the American College of Rheumatology that advocated for only injectable steroid use during flare-ups. “That was provocative,” Dr. Starkebaum said.
“It’s a retrospective study, so it has some limitations, but it provides good insight, and some substantiation to what we already think,” added Brett Smith, DO, a rheumatologist practicing in Knoxville, Tenn.
Dr. Smith suggested that the study further underscores the need to follow treat-to-target protocols in RA. He emphasized that lifetime exposure to steroids is likely the greatest concern, and that steady accumulating doses are a sign of trouble. “If you need that much steroids, you need to go up on your medication – your methotrexate, or sulfasalazine, or your biologic,” said Dr. Smith. “At least 50% of people will need a biologic to [achieve] disease control, and if you get them on it, they’re going to have better disease control, compliance is typically better, and they’re going to have less steroid exposure.”
Dr. Smith also noted that comorbid diabetes shouldn’t affect treat-to-target strategies. In fact, in such patients “you should probably be following it more tightly to reduce the cardiovascular outcomes,” he said.
The retrospective analysis included 9,085 patients with RA and with or without type 2 diabetes, with a mean follow-up of 5.2 years. They were recruited to the study between 1998 and 2011. Among patients with comorbid diabetes, those exposed to GC had a mortality of 67.4 per 1,000 person-years, compared with 22.5 among those not exposed to GC. Among those with RA alone, mortality was 44.6 versus 10.2 with and without GC exposure, respectively. Those with diabetes had a lower risk ratio for mortality (2.99 vs. 4.37), but a higher mortality difference (44.9 vs. 34.4 per 1,000 person-years).
“The increased absolute hazard for all-cause mortality indicates the greater public health impact of people with RA using GCs if they have [diabetes],” the researchers wrote. “Rheumatologists should consider [diabetes] status when prescribing GCs to patients with RA given this potential impact of GC therapy on glucose control and mortality.”
The study was limited by a lack of information on GC dose and cumulative exposure. Given its retrospective nature, the study could have been affected by confounding by indication, as well as unknown confounders.
The study was funded by the Centre for Epidemiology Versus Arthritis and the National Institute for Health Research Biomedical Research Centre. Dr. Starkebaum has no relevant financial disclosures. Dr. Smith is on the speaker’s bureau for AbbVie and serves on the company’s advisory board. He is also on the advisory boards of Regeneron and Sanofi Genzyme.
SOURCE: Costello R et al. BMC Rheumatol. 2020 Feb 19. doi: 10.1186/s41927-019-0105-4.
Glucocorticoid use is associated with greater mortality and cardiovascular risk in RA patients, and the associated risk is greater still in patients with RA and comorbid diabetes. The findings come from a new retrospective analysis derived from U.K. primary care records.
Although patients with diabetes actually had a lower relative risk for mortality than the nondiabetes cohort, they had a greater mortality difference because of a greater baseline risk. Ultimately, glucocorticoid (GC) use was associated with an additional 44.9 deaths per 1,000 person-years in the diabetes group, compared with 34.4 per 1,000 person-years in the RA-only group.
The study, led by Ruth Costello and William Dixon, MBBS, PhD of the University of Manchester (England), was published in BMC Rheumatology.
The findings aren’t particularly surprising, given that steroid use and diabetes have associated cardiovascular risks, and physicians generally try to reduce or eliminate their use. “There’s a group [of physicians] saying that we don’t need to use steroids at all in rheumatoid arthritis, except maybe for [a] short time at diagnosis to bridge to other therapies, or during flares,” Gordon Starkebaum, MD, professor emeritus of rheumatology at the University of Washington, Seattle, said in an interview. He recounted a session at last year’s annual meeting of the American College of Rheumatology that advocated for only injectable steroid use during flare-ups. “That was provocative,” Dr. Starkebaum said.
“It’s a retrospective study, so it has some limitations, but it provides good insight, and some substantiation to what we already think,” added Brett Smith, DO, a rheumatologist practicing in Knoxville, Tenn.
Dr. Smith suggested that the study further underscores the need to follow treat-to-target protocols in RA. He emphasized that lifetime exposure to steroids is likely the greatest concern, and that steady accumulating doses are a sign of trouble. “If you need that much steroids, you need to go up on your medication – your methotrexate, or sulfasalazine, or your biologic,” said Dr. Smith. “At least 50% of people will need a biologic to [achieve] disease control, and if you get them on it, they’re going to have better disease control, compliance is typically better, and they’re going to have less steroid exposure.”
Dr. Smith also noted that comorbid diabetes shouldn’t affect treat-to-target strategies. In fact, in such patients “you should probably be following it more tightly to reduce the cardiovascular outcomes,” he said.
The retrospective analysis included 9,085 patients with RA and with or without type 2 diabetes, with a mean follow-up of 5.2 years. They were recruited to the study between 1998 and 2011. Among patients with comorbid diabetes, those exposed to GC had a mortality of 67.4 per 1,000 person-years, compared with 22.5 among those not exposed to GC. Among those with RA alone, mortality was 44.6 versus 10.2 with and without GC exposure, respectively. Those with diabetes had a lower risk ratio for mortality (2.99 vs. 4.37), but a higher mortality difference (44.9 vs. 34.4 per 1,000 person-years).
“The increased absolute hazard for all-cause mortality indicates the greater public health impact of people with RA using GCs if they have [diabetes],” the researchers wrote. “Rheumatologists should consider [diabetes] status when prescribing GCs to patients with RA given this potential impact of GC therapy on glucose control and mortality.”
The study was limited by a lack of information on GC dose and cumulative exposure. Given its retrospective nature, the study could have been affected by confounding by indication, as well as unknown confounders.
The study was funded by the Centre for Epidemiology Versus Arthritis and the National Institute for Health Research Biomedical Research Centre. Dr. Starkebaum has no relevant financial disclosures. Dr. Smith is on the speaker’s bureau for AbbVie and serves on the company’s advisory board. He is also on the advisory boards of Regeneron and Sanofi Genzyme.
SOURCE: Costello R et al. BMC Rheumatol. 2020 Feb 19. doi: 10.1186/s41927-019-0105-4.
REPORTING FROM BMC RHEUMATOLOGY
In gestational diabetes, early postpartum glucose testing is a winner
GRAPEVINE, TEX. – Early postpartum glucose tolerance testing for women with gestational diabetes resulted in a 99% adherence rate, with similar sensitivity and specificity as the currently recommended 4- to 12-week postpartum testing schedule.
“Two-day postpartum glucose tolerance testing has similar diagnostic utility as the 4- to 12-week postpartum glucose tolerance test to identify impaired glucose metabolism and diabetes at 1 year postpartum,” said Erika Werner, MD, speaking at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Overall, 29% of women studied had impaired glucose metabolism at 2 days postpartum, as did 25% in the 4- to 12-weeks postpartum window. At 1 year, that figure was 35%. The number of women meeting diagnostic criteria for diabetes held steady at 4% for all three time points.
The findings warrant “consideration for the 2-day postpartum glucose tolerance test (GTT) as the initial postpartum test for women who have gestational diabetes, with repeat testing at 1 year,” said Dr. Werner, a maternal-fetal medicine physician at Brown University, Providence, R.I.
Glucose testing for women with gestational diabetes mellitus (GDM) is recommended at 4-12 weeks postpartum by both the American Diabetes Association and the American College of Obstetricians and Gynecologists.
Testing can allow detection and treatment of impaired glucose metabolism, seen in 15%-40% of women with a history of GDM. Up to 1 in 20 women with GDM will receive a postpartum diagnosis of type 2 diabetes.
However, fewer than one in five women will actually have postpartum glucose testing, representing a large missed opportunity, said Dr. Werner.
Several factors likely contribute to those screening failures, she added. In addition to the potential for public insurance to lapse at 6 weeks postpartum, the logistical realities and time demands of parenting a newborn are themselves a significant barrier.
“What if we changed the timing?” and shifted glucose testing to the early postpartum days, before hospital discharge, asked Dr. Werner. Several pilot studies had already compared glucose screening in the first few days postpartum with the routine schedule, finding good correlation between the early and routine GTT schedule.
Importantly, the earlier studies achieved an adherence rate of more than 90% for early GTT. By contrast, fewer than half of the participants in the usual-care arms actually returned for postpartum GTT in the 4- to 12-week postpartum window, even under the optimized conditions associated with a medical study.
The single-center prospective cohort study conducted by Dr. Werner and collaborators enrolled 300 women with GDM. Women agreed to participate in glucose tolerance testing as inpatients, at 2 days postpartum, in addition to receiving a GTT between 4 and 12 weeks postpartum, and additional screening that included a glycosylated hemoglobin (HbA1c) test at 1 year postpartum.
The investigators obtained postpartum day 2 GTTs for all but four of the patients. A total of 201 patients returned in the 4- to 12-week postpartum window, and 168 of those participants returned for HbA1c testing at 1 year. Of the 95 patients who didn’t come back for the 4- to 12-week test, 33 did return at 1 year for HbA1c testing.
Dr. Werner and her coinvestigators included adult women who spoke either fluent Spanish or English and had GDM diagnosed by the Carpenter-Coustan criteria, or by having a blood glucose level of 200 mg/dL or more in a 1-hour glucose challenge test.
The early GTT results weren’t shared with patients or their health care providers. For outpatient visits, participants were offered financial incentives and received multiple reminder phone calls and the offer of free transportation.
For the purposes of the study, impaired glucose metabolism was defined as fasting blood glucose of 100 mg/dL or greater, a 2-hour GTT blood glucose level of 140 mg/dL or greater, or HbA1c of 5.7% or greater.
Participants were diagnosed with diabetes if they had a fasting blood glucose of 126 mg/dL or greater, a 2-hour GTT blood glucose level of 200 mg/dL or greater, or HbA1c of 6.5% or greater.
Dr. Werner and colleagues conducted two analyses of their results. In the first, they included only women in both arms who had complete data. In the second analysis, they looked at all women who had data for the 1-year postpartum mark, assuming that interval GTTs were negative for women who were missing these values.
The statistical analysis showed that, for women with complete data, both early and later postpartum GTTs were similar in predicting impaired glucose metabolism at 1 year postpartum (areas under the receiver operating curve [AUC], 0.63 and 0.60, respectively).
For identifying diabetes at 1 year, both early and late testing had high negative predictive value (98% and 99%, respectively), but the later testing strategy had higher sensitivity and specificity, yielding an AUC of 0.83, compared with 0.65 for early testing.
Turning to the second analysis that included all women who had 1-year postpartum HbA1c values, negative predictive values for diabetes were similarly high (98%) for both the early and late testing strategies. For identifying impaired glucose metabolism at 1 year in this group, both the positive and negative predictive value of the early and late strategies were similar.
Patients were about 32 years old at baseline, with a mean body mass index of 31.7 kg/m2. More than half of patients (52.3%) had private insurance, and 22% had GDM in a pregnancy prior to the index pregnancy. Black patients made up about 9% of the study population; 54% of participants were white, and 23% Hispanic. About one-third of patients were nulliparous, and two-thirds had education beyond high school.
During their pregnancies, about 44% of patients managed GDM by diet alone, 40% required insulin, with an additional 1% also requiring an oral agent. The remainder required oral agents alone. Patients delivered at a mean 38.3 weeks gestation, with about 40% receiving cesarean deliveries.
Some of the study’s strengths included its prospective nature, the diverse population recruited, and the fact that participants and providers were both blinded to the 2-day GTT results. Although more than half of participants completed the study – besting the previous pilots – 44% of patients still had incomplete data, noted Dr. Werner.
The American Diabetes Association sponsored the study. Dr. Werner reported no other conflicts of interest.
SOURCE: Werner E et al. SMFM 2020. Abstract 72.
GRAPEVINE, TEX. – Early postpartum glucose tolerance testing for women with gestational diabetes resulted in a 99% adherence rate, with similar sensitivity and specificity as the currently recommended 4- to 12-week postpartum testing schedule.
“Two-day postpartum glucose tolerance testing has similar diagnostic utility as the 4- to 12-week postpartum glucose tolerance test to identify impaired glucose metabolism and diabetes at 1 year postpartum,” said Erika Werner, MD, speaking at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Overall, 29% of women studied had impaired glucose metabolism at 2 days postpartum, as did 25% in the 4- to 12-weeks postpartum window. At 1 year, that figure was 35%. The number of women meeting diagnostic criteria for diabetes held steady at 4% for all three time points.
The findings warrant “consideration for the 2-day postpartum glucose tolerance test (GTT) as the initial postpartum test for women who have gestational diabetes, with repeat testing at 1 year,” said Dr. Werner, a maternal-fetal medicine physician at Brown University, Providence, R.I.
Glucose testing for women with gestational diabetes mellitus (GDM) is recommended at 4-12 weeks postpartum by both the American Diabetes Association and the American College of Obstetricians and Gynecologists.
Testing can allow detection and treatment of impaired glucose metabolism, seen in 15%-40% of women with a history of GDM. Up to 1 in 20 women with GDM will receive a postpartum diagnosis of type 2 diabetes.
However, fewer than one in five women will actually have postpartum glucose testing, representing a large missed opportunity, said Dr. Werner.
Several factors likely contribute to those screening failures, she added. In addition to the potential for public insurance to lapse at 6 weeks postpartum, the logistical realities and time demands of parenting a newborn are themselves a significant barrier.
“What if we changed the timing?” and shifted glucose testing to the early postpartum days, before hospital discharge, asked Dr. Werner. Several pilot studies had already compared glucose screening in the first few days postpartum with the routine schedule, finding good correlation between the early and routine GTT schedule.
Importantly, the earlier studies achieved an adherence rate of more than 90% for early GTT. By contrast, fewer than half of the participants in the usual-care arms actually returned for postpartum GTT in the 4- to 12-week postpartum window, even under the optimized conditions associated with a medical study.
The single-center prospective cohort study conducted by Dr. Werner and collaborators enrolled 300 women with GDM. Women agreed to participate in glucose tolerance testing as inpatients, at 2 days postpartum, in addition to receiving a GTT between 4 and 12 weeks postpartum, and additional screening that included a glycosylated hemoglobin (HbA1c) test at 1 year postpartum.
The investigators obtained postpartum day 2 GTTs for all but four of the patients. A total of 201 patients returned in the 4- to 12-week postpartum window, and 168 of those participants returned for HbA1c testing at 1 year. Of the 95 patients who didn’t come back for the 4- to 12-week test, 33 did return at 1 year for HbA1c testing.
Dr. Werner and her coinvestigators included adult women who spoke either fluent Spanish or English and had GDM diagnosed by the Carpenter-Coustan criteria, or by having a blood glucose level of 200 mg/dL or more in a 1-hour glucose challenge test.
The early GTT results weren’t shared with patients or their health care providers. For outpatient visits, participants were offered financial incentives and received multiple reminder phone calls and the offer of free transportation.
For the purposes of the study, impaired glucose metabolism was defined as fasting blood glucose of 100 mg/dL or greater, a 2-hour GTT blood glucose level of 140 mg/dL or greater, or HbA1c of 5.7% or greater.
Participants were diagnosed with diabetes if they had a fasting blood glucose of 126 mg/dL or greater, a 2-hour GTT blood glucose level of 200 mg/dL or greater, or HbA1c of 6.5% or greater.
Dr. Werner and colleagues conducted two analyses of their results. In the first, they included only women in both arms who had complete data. In the second analysis, they looked at all women who had data for the 1-year postpartum mark, assuming that interval GTTs were negative for women who were missing these values.
The statistical analysis showed that, for women with complete data, both early and later postpartum GTTs were similar in predicting impaired glucose metabolism at 1 year postpartum (areas under the receiver operating curve [AUC], 0.63 and 0.60, respectively).
For identifying diabetes at 1 year, both early and late testing had high negative predictive value (98% and 99%, respectively), but the later testing strategy had higher sensitivity and specificity, yielding an AUC of 0.83, compared with 0.65 for early testing.
Turning to the second analysis that included all women who had 1-year postpartum HbA1c values, negative predictive values for diabetes were similarly high (98%) for both the early and late testing strategies. For identifying impaired glucose metabolism at 1 year in this group, both the positive and negative predictive value of the early and late strategies were similar.
Patients were about 32 years old at baseline, with a mean body mass index of 31.7 kg/m2. More than half of patients (52.3%) had private insurance, and 22% had GDM in a pregnancy prior to the index pregnancy. Black patients made up about 9% of the study population; 54% of participants were white, and 23% Hispanic. About one-third of patients were nulliparous, and two-thirds had education beyond high school.
During their pregnancies, about 44% of patients managed GDM by diet alone, 40% required insulin, with an additional 1% also requiring an oral agent. The remainder required oral agents alone. Patients delivered at a mean 38.3 weeks gestation, with about 40% receiving cesarean deliveries.
Some of the study’s strengths included its prospective nature, the diverse population recruited, and the fact that participants and providers were both blinded to the 2-day GTT results. Although more than half of participants completed the study – besting the previous pilots – 44% of patients still had incomplete data, noted Dr. Werner.
The American Diabetes Association sponsored the study. Dr. Werner reported no other conflicts of interest.
SOURCE: Werner E et al. SMFM 2020. Abstract 72.
GRAPEVINE, TEX. – Early postpartum glucose tolerance testing for women with gestational diabetes resulted in a 99% adherence rate, with similar sensitivity and specificity as the currently recommended 4- to 12-week postpartum testing schedule.
“Two-day postpartum glucose tolerance testing has similar diagnostic utility as the 4- to 12-week postpartum glucose tolerance test to identify impaired glucose metabolism and diabetes at 1 year postpartum,” said Erika Werner, MD, speaking at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Overall, 29% of women studied had impaired glucose metabolism at 2 days postpartum, as did 25% in the 4- to 12-weeks postpartum window. At 1 year, that figure was 35%. The number of women meeting diagnostic criteria for diabetes held steady at 4% for all three time points.
The findings warrant “consideration for the 2-day postpartum glucose tolerance test (GTT) as the initial postpartum test for women who have gestational diabetes, with repeat testing at 1 year,” said Dr. Werner, a maternal-fetal medicine physician at Brown University, Providence, R.I.
Glucose testing for women with gestational diabetes mellitus (GDM) is recommended at 4-12 weeks postpartum by both the American Diabetes Association and the American College of Obstetricians and Gynecologists.
Testing can allow detection and treatment of impaired glucose metabolism, seen in 15%-40% of women with a history of GDM. Up to 1 in 20 women with GDM will receive a postpartum diagnosis of type 2 diabetes.
However, fewer than one in five women will actually have postpartum glucose testing, representing a large missed opportunity, said Dr. Werner.
Several factors likely contribute to those screening failures, she added. In addition to the potential for public insurance to lapse at 6 weeks postpartum, the logistical realities and time demands of parenting a newborn are themselves a significant barrier.
“What if we changed the timing?” and shifted glucose testing to the early postpartum days, before hospital discharge, asked Dr. Werner. Several pilot studies had already compared glucose screening in the first few days postpartum with the routine schedule, finding good correlation between the early and routine GTT schedule.
Importantly, the earlier studies achieved an adherence rate of more than 90% for early GTT. By contrast, fewer than half of the participants in the usual-care arms actually returned for postpartum GTT in the 4- to 12-week postpartum window, even under the optimized conditions associated with a medical study.
The single-center prospective cohort study conducted by Dr. Werner and collaborators enrolled 300 women with GDM. Women agreed to participate in glucose tolerance testing as inpatients, at 2 days postpartum, in addition to receiving a GTT between 4 and 12 weeks postpartum, and additional screening that included a glycosylated hemoglobin (HbA1c) test at 1 year postpartum.
The investigators obtained postpartum day 2 GTTs for all but four of the patients. A total of 201 patients returned in the 4- to 12-week postpartum window, and 168 of those participants returned for HbA1c testing at 1 year. Of the 95 patients who didn’t come back for the 4- to 12-week test, 33 did return at 1 year for HbA1c testing.
Dr. Werner and her coinvestigators included adult women who spoke either fluent Spanish or English and had GDM diagnosed by the Carpenter-Coustan criteria, or by having a blood glucose level of 200 mg/dL or more in a 1-hour glucose challenge test.
The early GTT results weren’t shared with patients or their health care providers. For outpatient visits, participants were offered financial incentives and received multiple reminder phone calls and the offer of free transportation.
For the purposes of the study, impaired glucose metabolism was defined as fasting blood glucose of 100 mg/dL or greater, a 2-hour GTT blood glucose level of 140 mg/dL or greater, or HbA1c of 5.7% or greater.
Participants were diagnosed with diabetes if they had a fasting blood glucose of 126 mg/dL or greater, a 2-hour GTT blood glucose level of 200 mg/dL or greater, or HbA1c of 6.5% or greater.
Dr. Werner and colleagues conducted two analyses of their results. In the first, they included only women in both arms who had complete data. In the second analysis, they looked at all women who had data for the 1-year postpartum mark, assuming that interval GTTs were negative for women who were missing these values.
The statistical analysis showed that, for women with complete data, both early and later postpartum GTTs were similar in predicting impaired glucose metabolism at 1 year postpartum (areas under the receiver operating curve [AUC], 0.63 and 0.60, respectively).
For identifying diabetes at 1 year, both early and late testing had high negative predictive value (98% and 99%, respectively), but the later testing strategy had higher sensitivity and specificity, yielding an AUC of 0.83, compared with 0.65 for early testing.
Turning to the second analysis that included all women who had 1-year postpartum HbA1c values, negative predictive values for diabetes were similarly high (98%) for both the early and late testing strategies. For identifying impaired glucose metabolism at 1 year in this group, both the positive and negative predictive value of the early and late strategies were similar.
Patients were about 32 years old at baseline, with a mean body mass index of 31.7 kg/m2. More than half of patients (52.3%) had private insurance, and 22% had GDM in a pregnancy prior to the index pregnancy. Black patients made up about 9% of the study population; 54% of participants were white, and 23% Hispanic. About one-third of patients were nulliparous, and two-thirds had education beyond high school.
During their pregnancies, about 44% of patients managed GDM by diet alone, 40% required insulin, with an additional 1% also requiring an oral agent. The remainder required oral agents alone. Patients delivered at a mean 38.3 weeks gestation, with about 40% receiving cesarean deliveries.
Some of the study’s strengths included its prospective nature, the diverse population recruited, and the fact that participants and providers were both blinded to the 2-day GTT results. Although more than half of participants completed the study – besting the previous pilots – 44% of patients still had incomplete data, noted Dr. Werner.
The American Diabetes Association sponsored the study. Dr. Werner reported no other conflicts of interest.
SOURCE: Werner E et al. SMFM 2020. Abstract 72.
REPORTING FROM THE PREGNANCY MEETING
Dulaglutide OK for primary, secondary CV risk reduction in U.S.
The US Food and Drug Administration (FDA) has additionally approved dulaglutide (Trulicity) for reducing the risk of major adverse cardiovascular events (MACE) in adults with type 2 diabetes with and without established cardiovascular disease (CVD) or multiple CV risk factors, the company has announced.
Dulaglutide is a once-weekly injectable glucagonlike peptide-1 (GLP-1) receptor agonist first approved in the United States in 2014 for the treatment of type 2 diabetes.
It is now the first and only type 2 diabetes medicine approved to reduce the risk of CV events for both primary and secondary prevention populations. The European Medicines Agency approved a similar indication for dulaglutide last fall.
The new US indication is based on results of the CV outcomes trial for dulaglutide, known as REWIND, which was the longest-running CV outcomes trial in the GLP-1 agonist class.
Chair of the REWIND study, Hertzel Gerstein, MD, professor of medicine at McMaster University and Hamilton Health Sciences, Ontario, Canada, said in a Lilly statement that the trial included a “broad population of people living with type 2 diabetes, reflective of those in the general population. We therefore assessed the effect of Trulicity in people with established CVD as well as those with multiple CV risk factors.”
“Globally, over 415 million people have type 2 diabetes, which is itself a CV risk factor. However, only about one third have established CVD, which is why this new indication, and the supporting evidence, is important for the millions of people in the United States living with diabetes,” he added.
Other GLP-1 agonists have been granted approvals for additional reduction of CV events in patients with type 2 diabetes, but only for secondary prevention.
Most recently the FDA expanded the indication for once-weekly semaglutide to include reducing the risk for MACE, including CV death, nonfatal myocardial infarction, or nonfatal stroke, in adults with type 2 diabetes who have established CVD.
Additional approval based on REWIND trial
The REWIND trial included primarily people with type 2 diabetes without established CVD. The full study results were presented at the 2019 American Diabetes Association Scientific Sessions.
REWIND showed a significant reduction in risk of MACE – a composite endpoint of nonfatal myocardial infarction, nonfatal stroke, or CV death – which occurred in 12.0% of patients in the dulaglutide group, compared with 13.4% of patients in the placebo group, for a risk reduction of 0.88 (95% confidence interval, 0.79-0.99; P = .026), which was consistent across subgroups.
All three components of the MACE primary endpoint showed a reduction with dulaglutide, compared with placebo, including CV death (hazard ratio, 0.91; 95% CI, 0.78-1.06) and nonfatal MI (HR, 0.96; 95% CI, 0.79-1.16), with the strongest and only significant effect seen in nonfatal stroke (HR, 0.76; 95% CI, 0.61-0.95).
No difference was seen between groups in hospital admissions for heart failure.
Dulaglutide was also found to modestly reduce weight by around 1.5 kg (P = .0001) and systolic blood pressure by 1.7 mm Hg (P = .0001).
The safety profile of dulaglutide in REWIND was consistent with other members of the GLP-1 agonist class, with gastrointestinal events being the most common adverse event leading to discontinuation.
Sherry Martin, MD, Lilly’s vice president, medical affairs, noted in the company statement: “For the first time, health care providers can prescribe a diabetes medicine proven to significantly reduce the risk of experiencing a CV event for people with type 2 diabetes with and without established CVD.”
“Trulicity can help people achieve their A1C goals and protect them from experiencing a CV event with a once-weekly, easy-to-use treatment option,” added Martin.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has additionally approved dulaglutide (Trulicity) for reducing the risk of major adverse cardiovascular events (MACE) in adults with type 2 diabetes with and without established cardiovascular disease (CVD) or multiple CV risk factors, the company has announced.
Dulaglutide is a once-weekly injectable glucagonlike peptide-1 (GLP-1) receptor agonist first approved in the United States in 2014 for the treatment of type 2 diabetes.
It is now the first and only type 2 diabetes medicine approved to reduce the risk of CV events for both primary and secondary prevention populations. The European Medicines Agency approved a similar indication for dulaglutide last fall.
The new US indication is based on results of the CV outcomes trial for dulaglutide, known as REWIND, which was the longest-running CV outcomes trial in the GLP-1 agonist class.
Chair of the REWIND study, Hertzel Gerstein, MD, professor of medicine at McMaster University and Hamilton Health Sciences, Ontario, Canada, said in a Lilly statement that the trial included a “broad population of people living with type 2 diabetes, reflective of those in the general population. We therefore assessed the effect of Trulicity in people with established CVD as well as those with multiple CV risk factors.”
“Globally, over 415 million people have type 2 diabetes, which is itself a CV risk factor. However, only about one third have established CVD, which is why this new indication, and the supporting evidence, is important for the millions of people in the United States living with diabetes,” he added.
Other GLP-1 agonists have been granted approvals for additional reduction of CV events in patients with type 2 diabetes, but only for secondary prevention.
Most recently the FDA expanded the indication for once-weekly semaglutide to include reducing the risk for MACE, including CV death, nonfatal myocardial infarction, or nonfatal stroke, in adults with type 2 diabetes who have established CVD.
Additional approval based on REWIND trial
The REWIND trial included primarily people with type 2 diabetes without established CVD. The full study results were presented at the 2019 American Diabetes Association Scientific Sessions.
REWIND showed a significant reduction in risk of MACE – a composite endpoint of nonfatal myocardial infarction, nonfatal stroke, or CV death – which occurred in 12.0% of patients in the dulaglutide group, compared with 13.4% of patients in the placebo group, for a risk reduction of 0.88 (95% confidence interval, 0.79-0.99; P = .026), which was consistent across subgroups.
All three components of the MACE primary endpoint showed a reduction with dulaglutide, compared with placebo, including CV death (hazard ratio, 0.91; 95% CI, 0.78-1.06) and nonfatal MI (HR, 0.96; 95% CI, 0.79-1.16), with the strongest and only significant effect seen in nonfatal stroke (HR, 0.76; 95% CI, 0.61-0.95).
No difference was seen between groups in hospital admissions for heart failure.
Dulaglutide was also found to modestly reduce weight by around 1.5 kg (P = .0001) and systolic blood pressure by 1.7 mm Hg (P = .0001).
The safety profile of dulaglutide in REWIND was consistent with other members of the GLP-1 agonist class, with gastrointestinal events being the most common adverse event leading to discontinuation.
Sherry Martin, MD, Lilly’s vice president, medical affairs, noted in the company statement: “For the first time, health care providers can prescribe a diabetes medicine proven to significantly reduce the risk of experiencing a CV event for people with type 2 diabetes with and without established CVD.”
“Trulicity can help people achieve their A1C goals and protect them from experiencing a CV event with a once-weekly, easy-to-use treatment option,” added Martin.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has additionally approved dulaglutide (Trulicity) for reducing the risk of major adverse cardiovascular events (MACE) in adults with type 2 diabetes with and without established cardiovascular disease (CVD) or multiple CV risk factors, the company has announced.
Dulaglutide is a once-weekly injectable glucagonlike peptide-1 (GLP-1) receptor agonist first approved in the United States in 2014 for the treatment of type 2 diabetes.
It is now the first and only type 2 diabetes medicine approved to reduce the risk of CV events for both primary and secondary prevention populations. The European Medicines Agency approved a similar indication for dulaglutide last fall.
The new US indication is based on results of the CV outcomes trial for dulaglutide, known as REWIND, which was the longest-running CV outcomes trial in the GLP-1 agonist class.
Chair of the REWIND study, Hertzel Gerstein, MD, professor of medicine at McMaster University and Hamilton Health Sciences, Ontario, Canada, said in a Lilly statement that the trial included a “broad population of people living with type 2 diabetes, reflective of those in the general population. We therefore assessed the effect of Trulicity in people with established CVD as well as those with multiple CV risk factors.”
“Globally, over 415 million people have type 2 diabetes, which is itself a CV risk factor. However, only about one third have established CVD, which is why this new indication, and the supporting evidence, is important for the millions of people in the United States living with diabetes,” he added.
Other GLP-1 agonists have been granted approvals for additional reduction of CV events in patients with type 2 diabetes, but only for secondary prevention.
Most recently the FDA expanded the indication for once-weekly semaglutide to include reducing the risk for MACE, including CV death, nonfatal myocardial infarction, or nonfatal stroke, in adults with type 2 diabetes who have established CVD.
Additional approval based on REWIND trial
The REWIND trial included primarily people with type 2 diabetes without established CVD. The full study results were presented at the 2019 American Diabetes Association Scientific Sessions.
REWIND showed a significant reduction in risk of MACE – a composite endpoint of nonfatal myocardial infarction, nonfatal stroke, or CV death – which occurred in 12.0% of patients in the dulaglutide group, compared with 13.4% of patients in the placebo group, for a risk reduction of 0.88 (95% confidence interval, 0.79-0.99; P = .026), which was consistent across subgroups.
All three components of the MACE primary endpoint showed a reduction with dulaglutide, compared with placebo, including CV death (hazard ratio, 0.91; 95% CI, 0.78-1.06) and nonfatal MI (HR, 0.96; 95% CI, 0.79-1.16), with the strongest and only significant effect seen in nonfatal stroke (HR, 0.76; 95% CI, 0.61-0.95).
No difference was seen between groups in hospital admissions for heart failure.
Dulaglutide was also found to modestly reduce weight by around 1.5 kg (P = .0001) and systolic blood pressure by 1.7 mm Hg (P = .0001).
The safety profile of dulaglutide in REWIND was consistent with other members of the GLP-1 agonist class, with gastrointestinal events being the most common adverse event leading to discontinuation.
Sherry Martin, MD, Lilly’s vice president, medical affairs, noted in the company statement: “For the first time, health care providers can prescribe a diabetes medicine proven to significantly reduce the risk of experiencing a CV event for people with type 2 diabetes with and without established CVD.”
“Trulicity can help people achieve their A1C goals and protect them from experiencing a CV event with a once-weekly, easy-to-use treatment option,” added Martin.
This article first appeared on Medscape.com.
Genetic risk score may flag post-GDM incidence of type 2 disease
Women who had gestational diabetes mellitus had an increased risk for later type 2 diabetes if they carried certain genetic risk factors for the disease, according to a new analysis in BMJ Open Diabetes Research & Care of data from two independent populations.
A higher genetic risk score (GRS) had a modest association with developing type 2 diabetes, but a healthier diet may mitigate this risk, as Mengying Li, PhD, and her colleagues found for participants in the Nurses’ Health Study and members of the Danish National Birth Cohort who developed gestational diabetes mellitus (GDM).
Of 1,884 white women with a history of GDM in the Nurses’ Health Study II (NHSII), 446 (23.7%) went on to develop type 2 diabetes, and of the 550 women who had GDM in the Danish National Birth Cohort (DNBC), 155 (28.2%) developed the disease. The researchers calculated a GRS for type 2 diabetes for the full cohort. Genome-wide association studies completed in European populations were used to identify 59 single-nucleotide polymorphisms (SNPs) associated with the disease.
Dr. Li, an epidemiologist and postdoctoral researcher at the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Bethesda, Md., and her coauthors found that women whose GRS was in the highest quartile had a relative risk of 1.19 for type 2 diabetes. The relative risks for the three lower quartiles were 1.25, 0.97, and 1.00, respectively (P value for trend = .02). For each increase of five risk alleles in the GRS, NHSII participants had a 7% increased risk for type 2 diabetes, and DNBC participants saw a 9% increased risk.
Comparing these findings with other studies looking at genetic risk and type 2 diabetes in the general population, Dr. Li and her coauthors noted that the increase in relative risk for type 2 disease with increase in GRS was actually slightly weaker in the GDM cohort they studied. “The smaller effect size among women with GDM likely reflects an already higher baseline genetic risk for [type 2 diabetes] than the general population, as we have demonstrated,” they explained.
Though 11 individual SNPs had a significant individual association with the risk for type 2 diabetes initially, that association disappeared after correction for a false-discovery rate. Dr. Li and her coinvestigators conducted a sensitivity analysis that included only 42 SNPs that were later definitively associated with type 2 disease and they saw essentially unchanged results.
The researchers also investigated how dietary quality affected the GRS–type 2 diabetes association by dichotomizing self-reported diet quality in both cohorts into healthier diet quality and less healthy diet quality. They found a tighter association between GRS and type 2 diabetes for women with diet quality below the median, whereas women with higher diet quality did not have such a strong association between GRS and type 2 disease. The researchers wrote that there was “suggestive evidence that a healthful diet might mitigate the excessive risk of T2D [type 2 diabetes] related to greater genetic susceptibility, which supports public health efforts of encouraging a healthful diet” for diabetes prevention in this high-risk population.
Patients in the NHSII were followed for a mean 21.3 years, and those in the DNBC were followed for a mean 12.7 years. Mean age at index pregnancy was 30.5 years for the NHSII cohort and 31.7 for the DNBC cohort. In the NHSII cohort, just 8.4% of participants reported smoking before pregnancy, compared with 26.4% of those in the DNBC cohort. The NHSII cohort participants, wrote Dr. Li and her coauthors, “were also less likely to have a family history of diabetes, less likely to smoke, and be leaner than women in the DNBC.”
Dr. Li and her coauthors noted that, “despite being the largest genetic study by far on [type 2 diabetes] among women with GDM, our study may not be sufficiently powered to examine the associations of individual T2D SNPs in relation to the risk of developing T2D.” Another limitation was that for the Danish cohort, information about diet was drawn from a one-time questionnaire administered between 9 and 16 years after the index pregnancy, so full data about dietary quality over time was not available. Also of note is that the study included only white participants, limiting generalizability to women of color. The authors called for expanding this research into more racially diverse populations.
The study was supported by the National Institutes of Health. The authors reported that they had no conflicts of interest.
SOURCE: Li M et al. BMJ Open Diab Res Care. 2020 Feb 13. doi: 10.1136/bmjdrc-2019-000850.
Women who had gestational diabetes mellitus had an increased risk for later type 2 diabetes if they carried certain genetic risk factors for the disease, according to a new analysis in BMJ Open Diabetes Research & Care of data from two independent populations.
A higher genetic risk score (GRS) had a modest association with developing type 2 diabetes, but a healthier diet may mitigate this risk, as Mengying Li, PhD, and her colleagues found for participants in the Nurses’ Health Study and members of the Danish National Birth Cohort who developed gestational diabetes mellitus (GDM).
Of 1,884 white women with a history of GDM in the Nurses’ Health Study II (NHSII), 446 (23.7%) went on to develop type 2 diabetes, and of the 550 women who had GDM in the Danish National Birth Cohort (DNBC), 155 (28.2%) developed the disease. The researchers calculated a GRS for type 2 diabetes for the full cohort. Genome-wide association studies completed in European populations were used to identify 59 single-nucleotide polymorphisms (SNPs) associated with the disease.
Dr. Li, an epidemiologist and postdoctoral researcher at the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Bethesda, Md., and her coauthors found that women whose GRS was in the highest quartile had a relative risk of 1.19 for type 2 diabetes. The relative risks for the three lower quartiles were 1.25, 0.97, and 1.00, respectively (P value for trend = .02). For each increase of five risk alleles in the GRS, NHSII participants had a 7% increased risk for type 2 diabetes, and DNBC participants saw a 9% increased risk.
Comparing these findings with other studies looking at genetic risk and type 2 diabetes in the general population, Dr. Li and her coauthors noted that the increase in relative risk for type 2 disease with increase in GRS was actually slightly weaker in the GDM cohort they studied. “The smaller effect size among women with GDM likely reflects an already higher baseline genetic risk for [type 2 diabetes] than the general population, as we have demonstrated,” they explained.
Though 11 individual SNPs had a significant individual association with the risk for type 2 diabetes initially, that association disappeared after correction for a false-discovery rate. Dr. Li and her coinvestigators conducted a sensitivity analysis that included only 42 SNPs that were later definitively associated with type 2 disease and they saw essentially unchanged results.
The researchers also investigated how dietary quality affected the GRS–type 2 diabetes association by dichotomizing self-reported diet quality in both cohorts into healthier diet quality and less healthy diet quality. They found a tighter association between GRS and type 2 diabetes for women with diet quality below the median, whereas women with higher diet quality did not have such a strong association between GRS and type 2 disease. The researchers wrote that there was “suggestive evidence that a healthful diet might mitigate the excessive risk of T2D [type 2 diabetes] related to greater genetic susceptibility, which supports public health efforts of encouraging a healthful diet” for diabetes prevention in this high-risk population.
Patients in the NHSII were followed for a mean 21.3 years, and those in the DNBC were followed for a mean 12.7 years. Mean age at index pregnancy was 30.5 years for the NHSII cohort and 31.7 for the DNBC cohort. In the NHSII cohort, just 8.4% of participants reported smoking before pregnancy, compared with 26.4% of those in the DNBC cohort. The NHSII cohort participants, wrote Dr. Li and her coauthors, “were also less likely to have a family history of diabetes, less likely to smoke, and be leaner than women in the DNBC.”
Dr. Li and her coauthors noted that, “despite being the largest genetic study by far on [type 2 diabetes] among women with GDM, our study may not be sufficiently powered to examine the associations of individual T2D SNPs in relation to the risk of developing T2D.” Another limitation was that for the Danish cohort, information about diet was drawn from a one-time questionnaire administered between 9 and 16 years after the index pregnancy, so full data about dietary quality over time was not available. Also of note is that the study included only white participants, limiting generalizability to women of color. The authors called for expanding this research into more racially diverse populations.
The study was supported by the National Institutes of Health. The authors reported that they had no conflicts of interest.
SOURCE: Li M et al. BMJ Open Diab Res Care. 2020 Feb 13. doi: 10.1136/bmjdrc-2019-000850.
Women who had gestational diabetes mellitus had an increased risk for later type 2 diabetes if they carried certain genetic risk factors for the disease, according to a new analysis in BMJ Open Diabetes Research & Care of data from two independent populations.
A higher genetic risk score (GRS) had a modest association with developing type 2 diabetes, but a healthier diet may mitigate this risk, as Mengying Li, PhD, and her colleagues found for participants in the Nurses’ Health Study and members of the Danish National Birth Cohort who developed gestational diabetes mellitus (GDM).
Of 1,884 white women with a history of GDM in the Nurses’ Health Study II (NHSII), 446 (23.7%) went on to develop type 2 diabetes, and of the 550 women who had GDM in the Danish National Birth Cohort (DNBC), 155 (28.2%) developed the disease. The researchers calculated a GRS for type 2 diabetes for the full cohort. Genome-wide association studies completed in European populations were used to identify 59 single-nucleotide polymorphisms (SNPs) associated with the disease.
Dr. Li, an epidemiologist and postdoctoral researcher at the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Bethesda, Md., and her coauthors found that women whose GRS was in the highest quartile had a relative risk of 1.19 for type 2 diabetes. The relative risks for the three lower quartiles were 1.25, 0.97, and 1.00, respectively (P value for trend = .02). For each increase of five risk alleles in the GRS, NHSII participants had a 7% increased risk for type 2 diabetes, and DNBC participants saw a 9% increased risk.
Comparing these findings with other studies looking at genetic risk and type 2 diabetes in the general population, Dr. Li and her coauthors noted that the increase in relative risk for type 2 disease with increase in GRS was actually slightly weaker in the GDM cohort they studied. “The smaller effect size among women with GDM likely reflects an already higher baseline genetic risk for [type 2 diabetes] than the general population, as we have demonstrated,” they explained.
Though 11 individual SNPs had a significant individual association with the risk for type 2 diabetes initially, that association disappeared after correction for a false-discovery rate. Dr. Li and her coinvestigators conducted a sensitivity analysis that included only 42 SNPs that were later definitively associated with type 2 disease and they saw essentially unchanged results.
The researchers also investigated how dietary quality affected the GRS–type 2 diabetes association by dichotomizing self-reported diet quality in both cohorts into healthier diet quality and less healthy diet quality. They found a tighter association between GRS and type 2 diabetes for women with diet quality below the median, whereas women with higher diet quality did not have such a strong association between GRS and type 2 disease. The researchers wrote that there was “suggestive evidence that a healthful diet might mitigate the excessive risk of T2D [type 2 diabetes] related to greater genetic susceptibility, which supports public health efforts of encouraging a healthful diet” for diabetes prevention in this high-risk population.
Patients in the NHSII were followed for a mean 21.3 years, and those in the DNBC were followed for a mean 12.7 years. Mean age at index pregnancy was 30.5 years for the NHSII cohort and 31.7 for the DNBC cohort. In the NHSII cohort, just 8.4% of participants reported smoking before pregnancy, compared with 26.4% of those in the DNBC cohort. The NHSII cohort participants, wrote Dr. Li and her coauthors, “were also less likely to have a family history of diabetes, less likely to smoke, and be leaner than women in the DNBC.”
Dr. Li and her coauthors noted that, “despite being the largest genetic study by far on [type 2 diabetes] among women with GDM, our study may not be sufficiently powered to examine the associations of individual T2D SNPs in relation to the risk of developing T2D.” Another limitation was that for the Danish cohort, information about diet was drawn from a one-time questionnaire administered between 9 and 16 years after the index pregnancy, so full data about dietary quality over time was not available. Also of note is that the study included only white participants, limiting generalizability to women of color. The authors called for expanding this research into more racially diverse populations.
The study was supported by the National Institutes of Health. The authors reported that they had no conflicts of interest.
SOURCE: Li M et al. BMJ Open Diab Res Care. 2020 Feb 13. doi: 10.1136/bmjdrc-2019-000850.
FROM BMJ OPEN DIABETES RESEARCH & CARE
FDA opens the door to biosimilar insulin
The Food and Drug Administration published Feb. 21 in the Federal Register a final rule that transitions insulin and other products from regulation as a drug to a biologic. This will provide manufacturers access to the biosimilars approval pathway and is expected to bring more competition to the insulin market. The move comes as insulin manufacturers continue to get increased scrutiny over the significantly increased pricing of their products in recent years.
The transition was required under a provision of the Biologics Price Competition and Innovation Act of 2009.
The move is expected to have no impact on the distribution of insulin and other products affected by the transition.
“In general, prescribers should continue to prescribe and order insulin and other biological products the same way they did before the transition,” the FDA said in an FAQ on the transition for physicians and other health care workers. “In general, pharmacists should continue to dispense and counsel about insulin and other biological products the same way they did before the transition. Prescribers and pharmacists should ensure their patients understand there are no changes to the product and they should continue to use the product the same way as before the transition.”
Other products affected by the transition include human growth hormone (somatropin), pancrelipase, chorionic gonadotropin, follitropin alfa, and menotropins. Information on all the transitioning products will move from the Orange Book (which lists FDA-approved drug products with therapeutic equivalent evaluations) to the Purple Book (which lists FDA-licensed biological products with reference product exclusivity data and biosimilar/interchangeability evaluations).
The FDA in the FAQ reiterated its commitment to reviewing any applications for these transition products within 12 months of submission.
The Food and Drug Administration published Feb. 21 in the Federal Register a final rule that transitions insulin and other products from regulation as a drug to a biologic. This will provide manufacturers access to the biosimilars approval pathway and is expected to bring more competition to the insulin market. The move comes as insulin manufacturers continue to get increased scrutiny over the significantly increased pricing of their products in recent years.
The transition was required under a provision of the Biologics Price Competition and Innovation Act of 2009.
The move is expected to have no impact on the distribution of insulin and other products affected by the transition.
“In general, prescribers should continue to prescribe and order insulin and other biological products the same way they did before the transition,” the FDA said in an FAQ on the transition for physicians and other health care workers. “In general, pharmacists should continue to dispense and counsel about insulin and other biological products the same way they did before the transition. Prescribers and pharmacists should ensure their patients understand there are no changes to the product and they should continue to use the product the same way as before the transition.”
Other products affected by the transition include human growth hormone (somatropin), pancrelipase, chorionic gonadotropin, follitropin alfa, and menotropins. Information on all the transitioning products will move from the Orange Book (which lists FDA-approved drug products with therapeutic equivalent evaluations) to the Purple Book (which lists FDA-licensed biological products with reference product exclusivity data and biosimilar/interchangeability evaluations).
The FDA in the FAQ reiterated its commitment to reviewing any applications for these transition products within 12 months of submission.
The Food and Drug Administration published Feb. 21 in the Federal Register a final rule that transitions insulin and other products from regulation as a drug to a biologic. This will provide manufacturers access to the biosimilars approval pathway and is expected to bring more competition to the insulin market. The move comes as insulin manufacturers continue to get increased scrutiny over the significantly increased pricing of their products in recent years.
The transition was required under a provision of the Biologics Price Competition and Innovation Act of 2009.
The move is expected to have no impact on the distribution of insulin and other products affected by the transition.
“In general, prescribers should continue to prescribe and order insulin and other biological products the same way they did before the transition,” the FDA said in an FAQ on the transition for physicians and other health care workers. “In general, pharmacists should continue to dispense and counsel about insulin and other biological products the same way they did before the transition. Prescribers and pharmacists should ensure their patients understand there are no changes to the product and they should continue to use the product the same way as before the transition.”
Other products affected by the transition include human growth hormone (somatropin), pancrelipase, chorionic gonadotropin, follitropin alfa, and menotropins. Information on all the transitioning products will move from the Orange Book (which lists FDA-approved drug products with therapeutic equivalent evaluations) to the Purple Book (which lists FDA-licensed biological products with reference product exclusivity data and biosimilar/interchangeability evaluations).
The FDA in the FAQ reiterated its commitment to reviewing any applications for these transition products within 12 months of submission.
Stroke risk tied to diabetic retinopathy may not be modifiable
LOS ANGELES – Evidence continues to mount that diabetic retinopathy predicts elevated risk for stroke.
In a new study with nearly 3,000 people, those with diabetic retinopathy were 60% more likely than others with diabetes to develop an incident stroke over time. Investigators also found that addressing glucose, lipids, and blood pressure levels did not mitigate this risk in this secondary analysis of the ACCORD Eye Study.
“We are not surprised with the finding that diabetic retinopathy increases the risk of stroke — as diabetic retinopathy is common microvascular disease that is an established risk factor for cardiovascular disease,” lead author Ka-Ho Wong, BS, MBA, said in an interview.
However, “we were surprised that none of the trial interventions mitigated this risk, in particular the intensive blood pressure reduction, because hypertension is the most important cause of microvascular disease,” he said. Mr. Wong is clinical research coordinator and lab manager of the de Havenon Lab at the University of Utah Health Hospitals and Clinics in Salt Lake City.
The study findings were released Feb. 12, 2020, in advance of formal presentation at the International Stroke Conference sponsored by the American Heart Association.
Common predictor of vascular disease
Diabetic retinopathy is the most common complication of diabetes mellitus, affecting up to 50% of people living with type 1 and type 2 diabetes. In addition, previous research suggests that macrovascular diabetes complications, including stroke, could share a common or synergistic pathway.
This small vessel damage in the eye also has been linked to an increased risk of adverse cardiac events, including heart failure, as previously reported by Medscape Medical News.
To find out more, Mr. Wong and colleagues analyzed 2,828 participants in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. They compared the stroke risk between 874 people with diabetic retinopathy and another 1,954 diabetics without this complication. The average age was 62 years and 62% were men.
Diabetic neuropathy at baseline was diagnosed using the Early Treatment Diabetic Retinopathy Study Severity Scale using seven-field stereoscopic fundus photographs.
A total of 117 participants experienced a stroke during a mean follow-up of 5.4 years.
The investigators found that diabetic retinopathy was more common among patients who had a stroke (41%) versus 31% of those without a stroke (P = .016). The link between diabetic retinopathy and stroke remained in an analysis adjusted for multiple factors, including baseline age, gender, race, total cholesterol, A1c, smoking, and more. Risk remained elevated, with a hazard ratio of 1.60 (95% confidence interval, 1.10-2.32; P = .015).
Regarding the potential for modifying this risk, the association was unaffected among participants randomly assigned to the ACCORD glucose intervention (P = .305), lipid intervention (P = .546), or blood pressure intervention (P = .422).
The study was a secondary analysis, so information on stroke type and location were unavailable.
The big picture
“Diabetic retinopathy is associated with an increased risk of stroke, which suggests that the microvascular pathology inherent to diabetic retinopathy has larger cardiovascular implications,” the researchers noted.
Despite these findings, the researchers suggest that patients with diabetic retinopathy receive aggressive medical management to try to reduce their stroke risk.
“It’s important for everyone with diabetes to maintain good blood glucose control, and those with established diabetic retinopathy should pay particular attention to meeting all the stroke prevention guidelines that are established by the American Stroke Association,” said Mr. Wong.
“Patients with established diabetic retinopathy should pay particular attention to meeting all stroke prevention guidelines established by the [American Heart Association],” he added.
Mr. Wong and colleagues would like to expand on these findings. Pending grant application and funding support, they propose conducting a prospective, observational trial in stroke patients with baseline diabetic retinopathy. One aim would be to identify the most common mechanisms leading to stroke in this population, “which would have important implications for prevention efforts,” he said.
Consistent Findings
“The results of the study showing that having diabetic retinopathy is also associated with an increase in stroke really isn’t surprising. There have been other studies, population-based studies, done in the past, that have found a similar relationship,” Larry B. Goldstein, MD, said in a video commentary on the findings.
“The results are actually quite consistent with several other studies that have evaluated the same relationship,” added Dr. Goldstein, who is chair of the department of neurology and codirector of the Kentucky Neuroscience Institute, University of Kentucky HealthCare, Lexington.
Mr. Wong and Dr. Goldstein have disclosed no relevant financial relationships. The NIH’s National Institute of Neurological Disorders and Stroke funded the study.
This article first appeared on Medscape.com.
LOS ANGELES – Evidence continues to mount that diabetic retinopathy predicts elevated risk for stroke.
In a new study with nearly 3,000 people, those with diabetic retinopathy were 60% more likely than others with diabetes to develop an incident stroke over time. Investigators also found that addressing glucose, lipids, and blood pressure levels did not mitigate this risk in this secondary analysis of the ACCORD Eye Study.
“We are not surprised with the finding that diabetic retinopathy increases the risk of stroke — as diabetic retinopathy is common microvascular disease that is an established risk factor for cardiovascular disease,” lead author Ka-Ho Wong, BS, MBA, said in an interview.
However, “we were surprised that none of the trial interventions mitigated this risk, in particular the intensive blood pressure reduction, because hypertension is the most important cause of microvascular disease,” he said. Mr. Wong is clinical research coordinator and lab manager of the de Havenon Lab at the University of Utah Health Hospitals and Clinics in Salt Lake City.
The study findings were released Feb. 12, 2020, in advance of formal presentation at the International Stroke Conference sponsored by the American Heart Association.
Common predictor of vascular disease
Diabetic retinopathy is the most common complication of diabetes mellitus, affecting up to 50% of people living with type 1 and type 2 diabetes. In addition, previous research suggests that macrovascular diabetes complications, including stroke, could share a common or synergistic pathway.
This small vessel damage in the eye also has been linked to an increased risk of adverse cardiac events, including heart failure, as previously reported by Medscape Medical News.
To find out more, Mr. Wong and colleagues analyzed 2,828 participants in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. They compared the stroke risk between 874 people with diabetic retinopathy and another 1,954 diabetics without this complication. The average age was 62 years and 62% were men.
Diabetic neuropathy at baseline was diagnosed using the Early Treatment Diabetic Retinopathy Study Severity Scale using seven-field stereoscopic fundus photographs.
A total of 117 participants experienced a stroke during a mean follow-up of 5.4 years.
The investigators found that diabetic retinopathy was more common among patients who had a stroke (41%) versus 31% of those without a stroke (P = .016). The link between diabetic retinopathy and stroke remained in an analysis adjusted for multiple factors, including baseline age, gender, race, total cholesterol, A1c, smoking, and more. Risk remained elevated, with a hazard ratio of 1.60 (95% confidence interval, 1.10-2.32; P = .015).
Regarding the potential for modifying this risk, the association was unaffected among participants randomly assigned to the ACCORD glucose intervention (P = .305), lipid intervention (P = .546), or blood pressure intervention (P = .422).
The study was a secondary analysis, so information on stroke type and location were unavailable.
The big picture
“Diabetic retinopathy is associated with an increased risk of stroke, which suggests that the microvascular pathology inherent to diabetic retinopathy has larger cardiovascular implications,” the researchers noted.
Despite these findings, the researchers suggest that patients with diabetic retinopathy receive aggressive medical management to try to reduce their stroke risk.
“It’s important for everyone with diabetes to maintain good blood glucose control, and those with established diabetic retinopathy should pay particular attention to meeting all the stroke prevention guidelines that are established by the American Stroke Association,” said Mr. Wong.
“Patients with established diabetic retinopathy should pay particular attention to meeting all stroke prevention guidelines established by the [American Heart Association],” he added.
Mr. Wong and colleagues would like to expand on these findings. Pending grant application and funding support, they propose conducting a prospective, observational trial in stroke patients with baseline diabetic retinopathy. One aim would be to identify the most common mechanisms leading to stroke in this population, “which would have important implications for prevention efforts,” he said.
Consistent Findings
“The results of the study showing that having diabetic retinopathy is also associated with an increase in stroke really isn’t surprising. There have been other studies, population-based studies, done in the past, that have found a similar relationship,” Larry B. Goldstein, MD, said in a video commentary on the findings.
“The results are actually quite consistent with several other studies that have evaluated the same relationship,” added Dr. Goldstein, who is chair of the department of neurology and codirector of the Kentucky Neuroscience Institute, University of Kentucky HealthCare, Lexington.
Mr. Wong and Dr. Goldstein have disclosed no relevant financial relationships. The NIH’s National Institute of Neurological Disorders and Stroke funded the study.
This article first appeared on Medscape.com.
LOS ANGELES – Evidence continues to mount that diabetic retinopathy predicts elevated risk for stroke.
In a new study with nearly 3,000 people, those with diabetic retinopathy were 60% more likely than others with diabetes to develop an incident stroke over time. Investigators also found that addressing glucose, lipids, and blood pressure levels did not mitigate this risk in this secondary analysis of the ACCORD Eye Study.
“We are not surprised with the finding that diabetic retinopathy increases the risk of stroke — as diabetic retinopathy is common microvascular disease that is an established risk factor for cardiovascular disease,” lead author Ka-Ho Wong, BS, MBA, said in an interview.
However, “we were surprised that none of the trial interventions mitigated this risk, in particular the intensive blood pressure reduction, because hypertension is the most important cause of microvascular disease,” he said. Mr. Wong is clinical research coordinator and lab manager of the de Havenon Lab at the University of Utah Health Hospitals and Clinics in Salt Lake City.
The study findings were released Feb. 12, 2020, in advance of formal presentation at the International Stroke Conference sponsored by the American Heart Association.
Common predictor of vascular disease
Diabetic retinopathy is the most common complication of diabetes mellitus, affecting up to 50% of people living with type 1 and type 2 diabetes. In addition, previous research suggests that macrovascular diabetes complications, including stroke, could share a common or synergistic pathway.
This small vessel damage in the eye also has been linked to an increased risk of adverse cardiac events, including heart failure, as previously reported by Medscape Medical News.
To find out more, Mr. Wong and colleagues analyzed 2,828 participants in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. They compared the stroke risk between 874 people with diabetic retinopathy and another 1,954 diabetics without this complication. The average age was 62 years and 62% were men.
Diabetic neuropathy at baseline was diagnosed using the Early Treatment Diabetic Retinopathy Study Severity Scale using seven-field stereoscopic fundus photographs.
A total of 117 participants experienced a stroke during a mean follow-up of 5.4 years.
The investigators found that diabetic retinopathy was more common among patients who had a stroke (41%) versus 31% of those without a stroke (P = .016). The link between diabetic retinopathy and stroke remained in an analysis adjusted for multiple factors, including baseline age, gender, race, total cholesterol, A1c, smoking, and more. Risk remained elevated, with a hazard ratio of 1.60 (95% confidence interval, 1.10-2.32; P = .015).
Regarding the potential for modifying this risk, the association was unaffected among participants randomly assigned to the ACCORD glucose intervention (P = .305), lipid intervention (P = .546), or blood pressure intervention (P = .422).
The study was a secondary analysis, so information on stroke type and location were unavailable.
The big picture
“Diabetic retinopathy is associated with an increased risk of stroke, which suggests that the microvascular pathology inherent to diabetic retinopathy has larger cardiovascular implications,” the researchers noted.
Despite these findings, the researchers suggest that patients with diabetic retinopathy receive aggressive medical management to try to reduce their stroke risk.
“It’s important for everyone with diabetes to maintain good blood glucose control, and those with established diabetic retinopathy should pay particular attention to meeting all the stroke prevention guidelines that are established by the American Stroke Association,” said Mr. Wong.
“Patients with established diabetic retinopathy should pay particular attention to meeting all stroke prevention guidelines established by the [American Heart Association],” he added.
Mr. Wong and colleagues would like to expand on these findings. Pending grant application and funding support, they propose conducting a prospective, observational trial in stroke patients with baseline diabetic retinopathy. One aim would be to identify the most common mechanisms leading to stroke in this population, “which would have important implications for prevention efforts,” he said.
Consistent Findings
“The results of the study showing that having diabetic retinopathy is also associated with an increase in stroke really isn’t surprising. There have been other studies, population-based studies, done in the past, that have found a similar relationship,” Larry B. Goldstein, MD, said in a video commentary on the findings.
“The results are actually quite consistent with several other studies that have evaluated the same relationship,” added Dr. Goldstein, who is chair of the department of neurology and codirector of the Kentucky Neuroscience Institute, University of Kentucky HealthCare, Lexington.
Mr. Wong and Dr. Goldstein have disclosed no relevant financial relationships. The NIH’s National Institute of Neurological Disorders and Stroke funded the study.
This article first appeared on Medscape.com.
REPORTING FROM ISC 2020