User login
RECOVERY trial of COVID-19 treatments stops colchicine arm
On the advice of its independent data monitoring committee (DMC), the RECOVERY trial has stopped recruitment to the colchicine arm for lack of efficacy in patients hospitalized with COVID-19.
“The DMC saw no convincing evidence that further recruitment would provide conclusive proof of worthwhile mortality benefit either overall or in any prespecified subgroup,” the British investigators announced on March 5.
“The RECOVERY trial has already identified two anti-inflammatory drugs – dexamethasone and tocilizumab – that improve the chances of survival for patients with severe COVID-19. So, it is disappointing that colchicine, which is widely used to treat gout and other inflammatory conditions, has no effect in these patients,” cochief investigator Martin Landray, MBChB, PhD, said in a statement.
“We do large, randomized trials to establish whether a drug that seems promising in theory has real benefits for patients in practice. Unfortunately, colchicine is not one of those,” said Dr. Landry, University of Oxford (England).
The RECOVERY trial is evaluating a range of potential treatments for COVID-19 at 180 hospitals in the United Kingdom, Indonesia, and Nepal, and was designed with the expectation that drugs would be added or dropped as the evidence changes. Since November 2020, the trial has included an arm comparing colchicine with usual care alone.
As part of a routine meeting March 4, the DMC reviewed data from a preliminary analysis based on 2,178 deaths among 11,162 patients, 94% of whom were being treated with a corticosteroid such as dexamethasone.
The results showed no significant difference in the primary endpoint of 28-day mortality in patients randomized to colchicine versus usual care alone (20% vs. 19%; risk ratio, 1.02; 95% confidence interval, 0.94-1.11; P = .63).
Follow-up is ongoing and final results will be published as soon as possible, the investigators said. Thus far, there has been no convincing evidence of an effect of colchicine on clinical outcomes in hospitalized COVID-19 patients.
Recruitment will continue to all other treatment arms – aspirin, baricitinib, Regeneron’s antibody cocktail, and, in select hospitals, dimethyl fumarate – the investigators said.
Cochief investigator Peter Hornby, MD, PhD, also from the University of Oxford, noted that this has been the largest trial ever of colchicine. “Whilst we are disappointed that the overall result is negative, it is still important information for the future care of patients in the U.K. and worldwide.”
A version of this article first appeared on Medscape.com.
On the advice of its independent data monitoring committee (DMC), the RECOVERY trial has stopped recruitment to the colchicine arm for lack of efficacy in patients hospitalized with COVID-19.
“The DMC saw no convincing evidence that further recruitment would provide conclusive proof of worthwhile mortality benefit either overall or in any prespecified subgroup,” the British investigators announced on March 5.
“The RECOVERY trial has already identified two anti-inflammatory drugs – dexamethasone and tocilizumab – that improve the chances of survival for patients with severe COVID-19. So, it is disappointing that colchicine, which is widely used to treat gout and other inflammatory conditions, has no effect in these patients,” cochief investigator Martin Landray, MBChB, PhD, said in a statement.
“We do large, randomized trials to establish whether a drug that seems promising in theory has real benefits for patients in practice. Unfortunately, colchicine is not one of those,” said Dr. Landry, University of Oxford (England).
The RECOVERY trial is evaluating a range of potential treatments for COVID-19 at 180 hospitals in the United Kingdom, Indonesia, and Nepal, and was designed with the expectation that drugs would be added or dropped as the evidence changes. Since November 2020, the trial has included an arm comparing colchicine with usual care alone.
As part of a routine meeting March 4, the DMC reviewed data from a preliminary analysis based on 2,178 deaths among 11,162 patients, 94% of whom were being treated with a corticosteroid such as dexamethasone.
The results showed no significant difference in the primary endpoint of 28-day mortality in patients randomized to colchicine versus usual care alone (20% vs. 19%; risk ratio, 1.02; 95% confidence interval, 0.94-1.11; P = .63).
Follow-up is ongoing and final results will be published as soon as possible, the investigators said. Thus far, there has been no convincing evidence of an effect of colchicine on clinical outcomes in hospitalized COVID-19 patients.
Recruitment will continue to all other treatment arms – aspirin, baricitinib, Regeneron’s antibody cocktail, and, in select hospitals, dimethyl fumarate – the investigators said.
Cochief investigator Peter Hornby, MD, PhD, also from the University of Oxford, noted that this has been the largest trial ever of colchicine. “Whilst we are disappointed that the overall result is negative, it is still important information for the future care of patients in the U.K. and worldwide.”
A version of this article first appeared on Medscape.com.
On the advice of its independent data monitoring committee (DMC), the RECOVERY trial has stopped recruitment to the colchicine arm for lack of efficacy in patients hospitalized with COVID-19.
“The DMC saw no convincing evidence that further recruitment would provide conclusive proof of worthwhile mortality benefit either overall or in any prespecified subgroup,” the British investigators announced on March 5.
“The RECOVERY trial has already identified two anti-inflammatory drugs – dexamethasone and tocilizumab – that improve the chances of survival for patients with severe COVID-19. So, it is disappointing that colchicine, which is widely used to treat gout and other inflammatory conditions, has no effect in these patients,” cochief investigator Martin Landray, MBChB, PhD, said in a statement.
“We do large, randomized trials to establish whether a drug that seems promising in theory has real benefits for patients in practice. Unfortunately, colchicine is not one of those,” said Dr. Landry, University of Oxford (England).
The RECOVERY trial is evaluating a range of potential treatments for COVID-19 at 180 hospitals in the United Kingdom, Indonesia, and Nepal, and was designed with the expectation that drugs would be added or dropped as the evidence changes. Since November 2020, the trial has included an arm comparing colchicine with usual care alone.
As part of a routine meeting March 4, the DMC reviewed data from a preliminary analysis based on 2,178 deaths among 11,162 patients, 94% of whom were being treated with a corticosteroid such as dexamethasone.
The results showed no significant difference in the primary endpoint of 28-day mortality in patients randomized to colchicine versus usual care alone (20% vs. 19%; risk ratio, 1.02; 95% confidence interval, 0.94-1.11; P = .63).
Follow-up is ongoing and final results will be published as soon as possible, the investigators said. Thus far, there has been no convincing evidence of an effect of colchicine on clinical outcomes in hospitalized COVID-19 patients.
Recruitment will continue to all other treatment arms – aspirin, baricitinib, Regeneron’s antibody cocktail, and, in select hospitals, dimethyl fumarate – the investigators said.
Cochief investigator Peter Hornby, MD, PhD, also from the University of Oxford, noted that this has been the largest trial ever of colchicine. “Whilst we are disappointed that the overall result is negative, it is still important information for the future care of patients in the U.K. and worldwide.”
A version of this article first appeared on Medscape.com.
COVID-19 vaccination recommended for rheumatology patients
People with rheumatic diseases should get vaccinated against SARS-CoV-2 as soon as possible, the American College of Rheumatology (ACR) recommends.
“It may be that people with rheumatic diseases are at increased risk of developing COVID or serious COVID-related complications,” Jonathan Hausmann, MD, assistant professor of medicine at Harvard Medical School, Boston, said in an ACR podcast. “So the need to prevent COVID-19 is incredibly important in this group of patients.”
The guidelines recommend a delay in vaccination only in rare circumstances, such as for patients with very severe illness or who have recently been administered rituximab, Jeffrey R. Curtis, MD, MPH, lead author of the guidelines, said in the podcast.
“Our members have been inundated with questions and concerns from their patients on whether they should receive the vaccine,” ACR President David Karp, MD, PhD, said in a press release.
So the ACR convened a panel of nine rheumatologists, two infectious disease specialists, and two public health experts. Over the course of 8 weeks, the task force reviewed the literature and agreed on recommendations. The organization posted a summary of the guidelines on its website after its board of directors approved it Feb. 8. The paper is pending journal peer review.
Some risks are real
The task force confined its research to the COVID-19 vaccines being offered by Pfizer and Moderna because they are currently the only ones approved by the Food and Drug Administration. It found no reason to distinguish between the two vaccines in its recommendations.
Because little research has directly addressed the question concerning COVID-19 vaccination for patients with rheumatic diseases, the task force extrapolated from data on other vaccinations in people with rheumatic disease and on the COVID-19 vaccinations in other populations.
It analyzed reports that other types of vaccination, such as for influenza, triggered flares of rheumatic conditions. “It is really individual case reports or small cohorts where there may be a somewhat higher incidence of flare, but it’s usually not very large in its magnitude nor duration,” said Dr. Curtis of the University of Alabama at Birmingham.
The task force also considered the possibility that vaccinations could lead to a new autoimmune disorder, such as Guillain-Barré syndrome or Bell palsy. The risk is real, the task force decided, but not significant enough to influence their recommendations.
Likewise, in immunocompromised people, vaccinations with live virus, such as those for shingles, might trigger the infection the vaccination is meant to prevent. But this can’t happen with the Pfizer and Moderna COVID-19 vaccines because they contain messenger RNA instead of live viruses, Dr. Curtis said.
Although it might be optimal to administer the vaccines when rheumatic diseases are quiescent, the urgency of getting vaccinated overrides that consideration, Dr. Curtis said. “By and large, there was a general consensus to not want to delay vaccination until somebody was stable and doing great, because you don’t know how long that’s going to be,” he said.
How well does it work?
One unanswered question is whether the COVID-19 vaccines work as well for patients with rheumatic diseases. The task force was reassured by data showing efficacy across a range of subgroups, including some with immunosenescence, Dr. Curtis said. “But until we have data in rheumatology patients, we’re just not going to know,” he said.
The guidelines specify that some drug regimens be modified when patients are vaccinated.
For patients taking rituximab, vaccination should be delayed, but only for those who are able to maintain safe social distancing to reduce the risk for COVID-19 exposure, Dr. Curtis said. “If somebody has just gotten rituximab recently, it might be more ideal to complete the vaccine series about 2-4 weeks before the next rituximab dose,” he said. “So if you are giving that therapy, say, at 6-month intervals, if you could vaccinate them at around month 5 from the most recent rituximab cycle, that might be more ideal.”
The guidance calls for withholding JAK inhibitors for a week after each vaccine dose is administered.
It calls for holding SQ abatacept 1 week prior and 1 week after the first COVID-19 vaccine dose, with no interruption after the second dose.
For abatacept IV, clinicians should “time vaccine administration so that the first vaccination will occur 4 weeks after abatacept infusion (i.e., the entire dosing interval), and postpone the subsequent abatacept infusion by 1 week (i.e., a 5-week gap in total).” It recommends no medication adjustment for the second vaccine dose.
For cyclophosphamide, the guidance recommends timing administration to occur about a week after each vaccine dose, when feasible.
None of this advice should supersede clinical judgment, Dr. Curtis said.
A version of this article first appeared on Medscape.com.
People with rheumatic diseases should get vaccinated against SARS-CoV-2 as soon as possible, the American College of Rheumatology (ACR) recommends.
“It may be that people with rheumatic diseases are at increased risk of developing COVID or serious COVID-related complications,” Jonathan Hausmann, MD, assistant professor of medicine at Harvard Medical School, Boston, said in an ACR podcast. “So the need to prevent COVID-19 is incredibly important in this group of patients.”
The guidelines recommend a delay in vaccination only in rare circumstances, such as for patients with very severe illness or who have recently been administered rituximab, Jeffrey R. Curtis, MD, MPH, lead author of the guidelines, said in the podcast.
“Our members have been inundated with questions and concerns from their patients on whether they should receive the vaccine,” ACR President David Karp, MD, PhD, said in a press release.
So the ACR convened a panel of nine rheumatologists, two infectious disease specialists, and two public health experts. Over the course of 8 weeks, the task force reviewed the literature and agreed on recommendations. The organization posted a summary of the guidelines on its website after its board of directors approved it Feb. 8. The paper is pending journal peer review.
Some risks are real
The task force confined its research to the COVID-19 vaccines being offered by Pfizer and Moderna because they are currently the only ones approved by the Food and Drug Administration. It found no reason to distinguish between the two vaccines in its recommendations.
Because little research has directly addressed the question concerning COVID-19 vaccination for patients with rheumatic diseases, the task force extrapolated from data on other vaccinations in people with rheumatic disease and on the COVID-19 vaccinations in other populations.
It analyzed reports that other types of vaccination, such as for influenza, triggered flares of rheumatic conditions. “It is really individual case reports or small cohorts where there may be a somewhat higher incidence of flare, but it’s usually not very large in its magnitude nor duration,” said Dr. Curtis of the University of Alabama at Birmingham.
The task force also considered the possibility that vaccinations could lead to a new autoimmune disorder, such as Guillain-Barré syndrome or Bell palsy. The risk is real, the task force decided, but not significant enough to influence their recommendations.
Likewise, in immunocompromised people, vaccinations with live virus, such as those for shingles, might trigger the infection the vaccination is meant to prevent. But this can’t happen with the Pfizer and Moderna COVID-19 vaccines because they contain messenger RNA instead of live viruses, Dr. Curtis said.
Although it might be optimal to administer the vaccines when rheumatic diseases are quiescent, the urgency of getting vaccinated overrides that consideration, Dr. Curtis said. “By and large, there was a general consensus to not want to delay vaccination until somebody was stable and doing great, because you don’t know how long that’s going to be,” he said.
How well does it work?
One unanswered question is whether the COVID-19 vaccines work as well for patients with rheumatic diseases. The task force was reassured by data showing efficacy across a range of subgroups, including some with immunosenescence, Dr. Curtis said. “But until we have data in rheumatology patients, we’re just not going to know,” he said.
The guidelines specify that some drug regimens be modified when patients are vaccinated.
For patients taking rituximab, vaccination should be delayed, but only for those who are able to maintain safe social distancing to reduce the risk for COVID-19 exposure, Dr. Curtis said. “If somebody has just gotten rituximab recently, it might be more ideal to complete the vaccine series about 2-4 weeks before the next rituximab dose,” he said. “So if you are giving that therapy, say, at 6-month intervals, if you could vaccinate them at around month 5 from the most recent rituximab cycle, that might be more ideal.”
The guidance calls for withholding JAK inhibitors for a week after each vaccine dose is administered.
It calls for holding SQ abatacept 1 week prior and 1 week after the first COVID-19 vaccine dose, with no interruption after the second dose.
For abatacept IV, clinicians should “time vaccine administration so that the first vaccination will occur 4 weeks after abatacept infusion (i.e., the entire dosing interval), and postpone the subsequent abatacept infusion by 1 week (i.e., a 5-week gap in total).” It recommends no medication adjustment for the second vaccine dose.
For cyclophosphamide, the guidance recommends timing administration to occur about a week after each vaccine dose, when feasible.
None of this advice should supersede clinical judgment, Dr. Curtis said.
A version of this article first appeared on Medscape.com.
People with rheumatic diseases should get vaccinated against SARS-CoV-2 as soon as possible, the American College of Rheumatology (ACR) recommends.
“It may be that people with rheumatic diseases are at increased risk of developing COVID or serious COVID-related complications,” Jonathan Hausmann, MD, assistant professor of medicine at Harvard Medical School, Boston, said in an ACR podcast. “So the need to prevent COVID-19 is incredibly important in this group of patients.”
The guidelines recommend a delay in vaccination only in rare circumstances, such as for patients with very severe illness or who have recently been administered rituximab, Jeffrey R. Curtis, MD, MPH, lead author of the guidelines, said in the podcast.
“Our members have been inundated with questions and concerns from their patients on whether they should receive the vaccine,” ACR President David Karp, MD, PhD, said in a press release.
So the ACR convened a panel of nine rheumatologists, two infectious disease specialists, and two public health experts. Over the course of 8 weeks, the task force reviewed the literature and agreed on recommendations. The organization posted a summary of the guidelines on its website after its board of directors approved it Feb. 8. The paper is pending journal peer review.
Some risks are real
The task force confined its research to the COVID-19 vaccines being offered by Pfizer and Moderna because they are currently the only ones approved by the Food and Drug Administration. It found no reason to distinguish between the two vaccines in its recommendations.
Because little research has directly addressed the question concerning COVID-19 vaccination for patients with rheumatic diseases, the task force extrapolated from data on other vaccinations in people with rheumatic disease and on the COVID-19 vaccinations in other populations.
It analyzed reports that other types of vaccination, such as for influenza, triggered flares of rheumatic conditions. “It is really individual case reports or small cohorts where there may be a somewhat higher incidence of flare, but it’s usually not very large in its magnitude nor duration,” said Dr. Curtis of the University of Alabama at Birmingham.
The task force also considered the possibility that vaccinations could lead to a new autoimmune disorder, such as Guillain-Barré syndrome or Bell palsy. The risk is real, the task force decided, but not significant enough to influence their recommendations.
Likewise, in immunocompromised people, vaccinations with live virus, such as those for shingles, might trigger the infection the vaccination is meant to prevent. But this can’t happen with the Pfizer and Moderna COVID-19 vaccines because they contain messenger RNA instead of live viruses, Dr. Curtis said.
Although it might be optimal to administer the vaccines when rheumatic diseases are quiescent, the urgency of getting vaccinated overrides that consideration, Dr. Curtis said. “By and large, there was a general consensus to not want to delay vaccination until somebody was stable and doing great, because you don’t know how long that’s going to be,” he said.
How well does it work?
One unanswered question is whether the COVID-19 vaccines work as well for patients with rheumatic diseases. The task force was reassured by data showing efficacy across a range of subgroups, including some with immunosenescence, Dr. Curtis said. “But until we have data in rheumatology patients, we’re just not going to know,” he said.
The guidelines specify that some drug regimens be modified when patients are vaccinated.
For patients taking rituximab, vaccination should be delayed, but only for those who are able to maintain safe social distancing to reduce the risk for COVID-19 exposure, Dr. Curtis said. “If somebody has just gotten rituximab recently, it might be more ideal to complete the vaccine series about 2-4 weeks before the next rituximab dose,” he said. “So if you are giving that therapy, say, at 6-month intervals, if you could vaccinate them at around month 5 from the most recent rituximab cycle, that might be more ideal.”
The guidance calls for withholding JAK inhibitors for a week after each vaccine dose is administered.
It calls for holding SQ abatacept 1 week prior and 1 week after the first COVID-19 vaccine dose, with no interruption after the second dose.
For abatacept IV, clinicians should “time vaccine administration so that the first vaccination will occur 4 weeks after abatacept infusion (i.e., the entire dosing interval), and postpone the subsequent abatacept infusion by 1 week (i.e., a 5-week gap in total).” It recommends no medication adjustment for the second vaccine dose.
For cyclophosphamide, the guidance recommends timing administration to occur about a week after each vaccine dose, when feasible.
None of this advice should supersede clinical judgment, Dr. Curtis said.
A version of this article first appeared on Medscape.com.
ColCORONA: More questions than answers for colchicine in COVID-19
Science by press release and preprint has cooled clinician enthusiasm for the use of colchicine in nonhospitalized patients with COVID-19, despite a pressing need for early treatments.
As previously reported by this news organization, a Jan. 22 press release announced that the massive ColCORONA study missed its primary endpoint of hospitalization or death among 4,488 newly diagnosed patients at increased risk for hospitalization.
But it also touted that use of the anti-inflammatory drug significantly reduced the primary endpoint in 4,159 of those patients with polymerase chain reaction–confirmed COVID and led to reductions of 25%, 50%, and 44%, respectively, for hospitalizations, ventilations, and death.
Lead investigator Jean-Claude Tardif, MD, director of the Montreal Heart Institute Research Centre, deemed the findings a “medical breakthrough.”
When the preprint released a few days later, however, newly revealed confidence intervals showed colchicine did not meaningfully reduce the need for mechanical ventilation (odds ratio, 0.50; 95% confidence interval, 0.23-1.07) or death alone (OR, 0.56; 95% CI, 0.19-1.66).
Further, the significant benefit on the primary outcome came at the cost of a fivefold increase in pulmonary embolism (11 vs. 2; P = .01), which was not mentioned in the press release.
“Whether this represents a real phenomenon or simply the play of chance is not known,” Dr. Tardif and colleagues noted later in the preprint.
“I read the preprint on colchicine and I have so many questions,” Aaron E. Glatt, MD, spokesperson for the Infectious Diseases Society of America and chief of infectious diseases, Mount Sinai South Nassau, Hewlett, N.Y., said in an interview. “I’ve been burned too many times with COVID and prefer to see better data.
“People sometimes say if you wait for perfect data, people are going to die,” he said. “Yeah, but we have no idea if people are going to die from getting this drug more than not getting it. That’s what concerns me. How many pulmonary emboli are going to be fatal versus the slight benefit that the study showed?”
The pushback to the non–peer-reviewed data on social media and via emails was so strong that Dr. Tardif posted a nearly 2,000-word letter responding to the many questions at play.
Chief among them was why the trial, originally planned for 6,000 patients, was stopped early by the investigators without consultation with the data safety monitoring board (DSMB).
The explanation in the letter that logistical issues like running the study call center, budget constraints, and a perceived need to quickly communicate the results left some calling foul that the study wasn’t allowed to finish and come to a more definitive conclusion.
“I can be a little bit sympathetic to their cause but at the same time the DSMB should have said no,” said David Boulware, MD, MPH, who led a recent hydroxychloroquine trial in COVID-19. “The problem is we’re sort of left in limbo, where some people kind of believe it and some say it’s not really a thing. So it’s not really moving the needle, as far as guidelines go.”
Indeed, a Twitter poll by cardiologist James Januzzi Jr., MD, captured the uncertainty, with 28% of respondents saying the trial was “neutral,” 58% saying “maybe but meh,” and 14% saying “colchicine for all.”
Another poll cheekily asked whether ColCORONA was the Gamestop/Reddit equivalent of COVID.
“The press release really didn’t help things because it very much oversold the effect. That, I think, poisoned the well,” said Dr. Boulware, professor of medicine in infectious diseases at the University of Minnesota, Minneapolis.
“The question I’m left with is not whether colchicine works, but who does it work in,” he said. “That’s really the fundamental question because it does seem that there are probably high-risk groups in their trial and others where they benefit, whereas other groups don’t benefit. In the subgroup analysis, there was absolutely no beneficial effect in women.”
According to the authors, the number needed to treat to prevent one death or hospitalization was 71 overall, but 29 for patients with diabetes, 31 for those aged 70 years and older, 53 for patients with respiratory disease, and 25 for those with coronary disease or heart failure.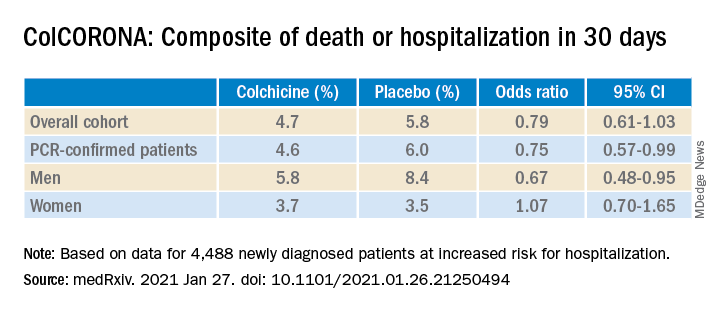
Men are at higher risk overall for poor outcomes. But “the authors didn’t present a multivariable analysis, so it is unclear if another factor, such as a differential prevalence of smoking or cardiovascular risk factors, contributed to the differential benefit,” Rachel Bender Ignacio, MD, MPH, infectious disease specialist, University of Washington, Seattle, said in an interview.
Importantly, in this pragmatic study, duration and severity of symptoms were not reported, observed Dr. Bender Ignacio, who is also a STOP-COVID-2 investigator. “We don’t yet have data as to whether colchicine shortens duration or severity of symptoms or prevents long COVID, so we need more data on that.”
The overall risk for serious adverse events was lower in the colchicine group, but the difference in pulmonary embolism (PE) was striking, she said. This could be caused by a real biologic effect, or it’s possible that persons with shortness of breath and hypoxia, without evident viral pneumonia on chest x-ray after a positive COVID-19 test, were more likely to receive a CT-PE study.
The press release also failed to include information, later noted in the preprint, that the MHI has submitted two patents related to colchicine: “Methods of treating a coronavirus infection using colchicine” and “Early administration of low-dose colchicine after myocardial infarction.”
Reached for clarification, MHI communications adviser Camille Turbide said in an interview that the first patent “simply refers to the novel concept of preventing complications of COVID-19, such as admission to the hospital, with colchicine as tested in the ColCORONA study.”
The second patent, she said, refers to the “novel concept that administering colchicine early after a major adverse cardiovascular event is better than waiting several days,” as supported by the COLCOT study, which Dr. Tardif also led.
The patents are being reviewed by authorities and “Dr. Tardif has waived his rights in these patents and does not stand to benefit financially at all if colchicine becomes used as a treatment for COVID-19,” Ms. Turbide said.
Dr. Tardif did not respond to interview requests for this story. Dr. Glatt said conflicts of interest must be assessed and are “something that is of great concern in any scientific study.”
Cardiologist Steve Nissen, MD, of the Cleveland Clinic said in an interview that, “despite the negative results, the study does suggest that colchicine might have a benefit and should be studied in future trials. These findings are not sufficient evidence to suggest use of the drug in patients infected with COVID-19.”
He noted that adverse effects like diarrhea were expected but that the excess PE was unexpected and needs greater clarification.
“Stopping the trial for administrative reasons is puzzling and undermined the ability of the trial to give a reliable answer,” Dr. Nissen said. “This is a reasonable pilot study that should be viewed as hypothesis generating but inconclusive.”
Several sources said a new trial is unlikely, particularly given the cost and 28 trials already evaluating colchicine. Among these are RECOVERY and COLCOVID, testing whether colchicine can reduce the duration of hospitalization or death in hospitalized patients with COVID-19.
Because there are so many trials ongoing right now, including for antivirals and other immunomodulators, it’s important that, if colchicine comes to routine clinical use, it provides access to treatment for those not able or willing to access clinical trials, rather than impeding clinical trial enrollment, Dr. Bender Ignacio suggested.
“We have already learned the lesson in the pandemic that early adoption of potentially promising therapies can negatively impact our ability to study and develop other promising treatments,” she said.
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the National Heart, Lung, and Blood Institute of the National Institutes of Health; Montreal philanthropist Sophie Desmarais, and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators. Dr. Glatt reported no conflicts of interest. Dr. Boulware reported receiving $18 in food and beverages from Gilead Sciences in 2018.
A version of this article first appeared on Medscape.com.
Science by press release and preprint has cooled clinician enthusiasm for the use of colchicine in nonhospitalized patients with COVID-19, despite a pressing need for early treatments.
As previously reported by this news organization, a Jan. 22 press release announced that the massive ColCORONA study missed its primary endpoint of hospitalization or death among 4,488 newly diagnosed patients at increased risk for hospitalization.
But it also touted that use of the anti-inflammatory drug significantly reduced the primary endpoint in 4,159 of those patients with polymerase chain reaction–confirmed COVID and led to reductions of 25%, 50%, and 44%, respectively, for hospitalizations, ventilations, and death.
Lead investigator Jean-Claude Tardif, MD, director of the Montreal Heart Institute Research Centre, deemed the findings a “medical breakthrough.”
When the preprint released a few days later, however, newly revealed confidence intervals showed colchicine did not meaningfully reduce the need for mechanical ventilation (odds ratio, 0.50; 95% confidence interval, 0.23-1.07) or death alone (OR, 0.56; 95% CI, 0.19-1.66).
Further, the significant benefit on the primary outcome came at the cost of a fivefold increase in pulmonary embolism (11 vs. 2; P = .01), which was not mentioned in the press release.
“Whether this represents a real phenomenon or simply the play of chance is not known,” Dr. Tardif and colleagues noted later in the preprint.
“I read the preprint on colchicine and I have so many questions,” Aaron E. Glatt, MD, spokesperson for the Infectious Diseases Society of America and chief of infectious diseases, Mount Sinai South Nassau, Hewlett, N.Y., said in an interview. “I’ve been burned too many times with COVID and prefer to see better data.
“People sometimes say if you wait for perfect data, people are going to die,” he said. “Yeah, but we have no idea if people are going to die from getting this drug more than not getting it. That’s what concerns me. How many pulmonary emboli are going to be fatal versus the slight benefit that the study showed?”
The pushback to the non–peer-reviewed data on social media and via emails was so strong that Dr. Tardif posted a nearly 2,000-word letter responding to the many questions at play.
Chief among them was why the trial, originally planned for 6,000 patients, was stopped early by the investigators without consultation with the data safety monitoring board (DSMB).
The explanation in the letter that logistical issues like running the study call center, budget constraints, and a perceived need to quickly communicate the results left some calling foul that the study wasn’t allowed to finish and come to a more definitive conclusion.
“I can be a little bit sympathetic to their cause but at the same time the DSMB should have said no,” said David Boulware, MD, MPH, who led a recent hydroxychloroquine trial in COVID-19. “The problem is we’re sort of left in limbo, where some people kind of believe it and some say it’s not really a thing. So it’s not really moving the needle, as far as guidelines go.”
Indeed, a Twitter poll by cardiologist James Januzzi Jr., MD, captured the uncertainty, with 28% of respondents saying the trial was “neutral,” 58% saying “maybe but meh,” and 14% saying “colchicine for all.”
Another poll cheekily asked whether ColCORONA was the Gamestop/Reddit equivalent of COVID.
“The press release really didn’t help things because it very much oversold the effect. That, I think, poisoned the well,” said Dr. Boulware, professor of medicine in infectious diseases at the University of Minnesota, Minneapolis.
“The question I’m left with is not whether colchicine works, but who does it work in,” he said. “That’s really the fundamental question because it does seem that there are probably high-risk groups in their trial and others where they benefit, whereas other groups don’t benefit. In the subgroup analysis, there was absolutely no beneficial effect in women.”
According to the authors, the number needed to treat to prevent one death or hospitalization was 71 overall, but 29 for patients with diabetes, 31 for those aged 70 years and older, 53 for patients with respiratory disease, and 25 for those with coronary disease or heart failure.
Men are at higher risk overall for poor outcomes. But “the authors didn’t present a multivariable analysis, so it is unclear if another factor, such as a differential prevalence of smoking or cardiovascular risk factors, contributed to the differential benefit,” Rachel Bender Ignacio, MD, MPH, infectious disease specialist, University of Washington, Seattle, said in an interview.
Importantly, in this pragmatic study, duration and severity of symptoms were not reported, observed Dr. Bender Ignacio, who is also a STOP-COVID-2 investigator. “We don’t yet have data as to whether colchicine shortens duration or severity of symptoms or prevents long COVID, so we need more data on that.”
The overall risk for serious adverse events was lower in the colchicine group, but the difference in pulmonary embolism (PE) was striking, she said. This could be caused by a real biologic effect, or it’s possible that persons with shortness of breath and hypoxia, without evident viral pneumonia on chest x-ray after a positive COVID-19 test, were more likely to receive a CT-PE study.
The press release also failed to include information, later noted in the preprint, that the MHI has submitted two patents related to colchicine: “Methods of treating a coronavirus infection using colchicine” and “Early administration of low-dose colchicine after myocardial infarction.”
Reached for clarification, MHI communications adviser Camille Turbide said in an interview that the first patent “simply refers to the novel concept of preventing complications of COVID-19, such as admission to the hospital, with colchicine as tested in the ColCORONA study.”
The second patent, she said, refers to the “novel concept that administering colchicine early after a major adverse cardiovascular event is better than waiting several days,” as supported by the COLCOT study, which Dr. Tardif also led.
The patents are being reviewed by authorities and “Dr. Tardif has waived his rights in these patents and does not stand to benefit financially at all if colchicine becomes used as a treatment for COVID-19,” Ms. Turbide said.
Dr. Tardif did not respond to interview requests for this story. Dr. Glatt said conflicts of interest must be assessed and are “something that is of great concern in any scientific study.”
Cardiologist Steve Nissen, MD, of the Cleveland Clinic said in an interview that, “despite the negative results, the study does suggest that colchicine might have a benefit and should be studied in future trials. These findings are not sufficient evidence to suggest use of the drug in patients infected with COVID-19.”
He noted that adverse effects like diarrhea were expected but that the excess PE was unexpected and needs greater clarification.
“Stopping the trial for administrative reasons is puzzling and undermined the ability of the trial to give a reliable answer,” Dr. Nissen said. “This is a reasonable pilot study that should be viewed as hypothesis generating but inconclusive.”
Several sources said a new trial is unlikely, particularly given the cost and 28 trials already evaluating colchicine. Among these are RECOVERY and COLCOVID, testing whether colchicine can reduce the duration of hospitalization or death in hospitalized patients with COVID-19.
Because there are so many trials ongoing right now, including for antivirals and other immunomodulators, it’s important that, if colchicine comes to routine clinical use, it provides access to treatment for those not able or willing to access clinical trials, rather than impeding clinical trial enrollment, Dr. Bender Ignacio suggested.
“We have already learned the lesson in the pandemic that early adoption of potentially promising therapies can negatively impact our ability to study and develop other promising treatments,” she said.
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the National Heart, Lung, and Blood Institute of the National Institutes of Health; Montreal philanthropist Sophie Desmarais, and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators. Dr. Glatt reported no conflicts of interest. Dr. Boulware reported receiving $18 in food and beverages from Gilead Sciences in 2018.
A version of this article first appeared on Medscape.com.
Science by press release and preprint has cooled clinician enthusiasm for the use of colchicine in nonhospitalized patients with COVID-19, despite a pressing need for early treatments.
As previously reported by this news organization, a Jan. 22 press release announced that the massive ColCORONA study missed its primary endpoint of hospitalization or death among 4,488 newly diagnosed patients at increased risk for hospitalization.
But it also touted that use of the anti-inflammatory drug significantly reduced the primary endpoint in 4,159 of those patients with polymerase chain reaction–confirmed COVID and led to reductions of 25%, 50%, and 44%, respectively, for hospitalizations, ventilations, and death.
Lead investigator Jean-Claude Tardif, MD, director of the Montreal Heart Institute Research Centre, deemed the findings a “medical breakthrough.”
When the preprint released a few days later, however, newly revealed confidence intervals showed colchicine did not meaningfully reduce the need for mechanical ventilation (odds ratio, 0.50; 95% confidence interval, 0.23-1.07) or death alone (OR, 0.56; 95% CI, 0.19-1.66).
Further, the significant benefit on the primary outcome came at the cost of a fivefold increase in pulmonary embolism (11 vs. 2; P = .01), which was not mentioned in the press release.
“Whether this represents a real phenomenon or simply the play of chance is not known,” Dr. Tardif and colleagues noted later in the preprint.
“I read the preprint on colchicine and I have so many questions,” Aaron E. Glatt, MD, spokesperson for the Infectious Diseases Society of America and chief of infectious diseases, Mount Sinai South Nassau, Hewlett, N.Y., said in an interview. “I’ve been burned too many times with COVID and prefer to see better data.
“People sometimes say if you wait for perfect data, people are going to die,” he said. “Yeah, but we have no idea if people are going to die from getting this drug more than not getting it. That’s what concerns me. How many pulmonary emboli are going to be fatal versus the slight benefit that the study showed?”
The pushback to the non–peer-reviewed data on social media and via emails was so strong that Dr. Tardif posted a nearly 2,000-word letter responding to the many questions at play.
Chief among them was why the trial, originally planned for 6,000 patients, was stopped early by the investigators without consultation with the data safety monitoring board (DSMB).
The explanation in the letter that logistical issues like running the study call center, budget constraints, and a perceived need to quickly communicate the results left some calling foul that the study wasn’t allowed to finish and come to a more definitive conclusion.
“I can be a little bit sympathetic to their cause but at the same time the DSMB should have said no,” said David Boulware, MD, MPH, who led a recent hydroxychloroquine trial in COVID-19. “The problem is we’re sort of left in limbo, where some people kind of believe it and some say it’s not really a thing. So it’s not really moving the needle, as far as guidelines go.”
Indeed, a Twitter poll by cardiologist James Januzzi Jr., MD, captured the uncertainty, with 28% of respondents saying the trial was “neutral,” 58% saying “maybe but meh,” and 14% saying “colchicine for all.”
Another poll cheekily asked whether ColCORONA was the Gamestop/Reddit equivalent of COVID.
“The press release really didn’t help things because it very much oversold the effect. That, I think, poisoned the well,” said Dr. Boulware, professor of medicine in infectious diseases at the University of Minnesota, Minneapolis.
“The question I’m left with is not whether colchicine works, but who does it work in,” he said. “That’s really the fundamental question because it does seem that there are probably high-risk groups in their trial and others where they benefit, whereas other groups don’t benefit. In the subgroup analysis, there was absolutely no beneficial effect in women.”
According to the authors, the number needed to treat to prevent one death or hospitalization was 71 overall, but 29 for patients with diabetes, 31 for those aged 70 years and older, 53 for patients with respiratory disease, and 25 for those with coronary disease or heart failure.
Men are at higher risk overall for poor outcomes. But “the authors didn’t present a multivariable analysis, so it is unclear if another factor, such as a differential prevalence of smoking or cardiovascular risk factors, contributed to the differential benefit,” Rachel Bender Ignacio, MD, MPH, infectious disease specialist, University of Washington, Seattle, said in an interview.
Importantly, in this pragmatic study, duration and severity of symptoms were not reported, observed Dr. Bender Ignacio, who is also a STOP-COVID-2 investigator. “We don’t yet have data as to whether colchicine shortens duration or severity of symptoms or prevents long COVID, so we need more data on that.”
The overall risk for serious adverse events was lower in the colchicine group, but the difference in pulmonary embolism (PE) was striking, she said. This could be caused by a real biologic effect, or it’s possible that persons with shortness of breath and hypoxia, without evident viral pneumonia on chest x-ray after a positive COVID-19 test, were more likely to receive a CT-PE study.
The press release also failed to include information, later noted in the preprint, that the MHI has submitted two patents related to colchicine: “Methods of treating a coronavirus infection using colchicine” and “Early administration of low-dose colchicine after myocardial infarction.”
Reached for clarification, MHI communications adviser Camille Turbide said in an interview that the first patent “simply refers to the novel concept of preventing complications of COVID-19, such as admission to the hospital, with colchicine as tested in the ColCORONA study.”
The second patent, she said, refers to the “novel concept that administering colchicine early after a major adverse cardiovascular event is better than waiting several days,” as supported by the COLCOT study, which Dr. Tardif also led.
The patents are being reviewed by authorities and “Dr. Tardif has waived his rights in these patents and does not stand to benefit financially at all if colchicine becomes used as a treatment for COVID-19,” Ms. Turbide said.
Dr. Tardif did not respond to interview requests for this story. Dr. Glatt said conflicts of interest must be assessed and are “something that is of great concern in any scientific study.”
Cardiologist Steve Nissen, MD, of the Cleveland Clinic said in an interview that, “despite the negative results, the study does suggest that colchicine might have a benefit and should be studied in future trials. These findings are not sufficient evidence to suggest use of the drug in patients infected with COVID-19.”
He noted that adverse effects like diarrhea were expected but that the excess PE was unexpected and needs greater clarification.
“Stopping the trial for administrative reasons is puzzling and undermined the ability of the trial to give a reliable answer,” Dr. Nissen said. “This is a reasonable pilot study that should be viewed as hypothesis generating but inconclusive.”
Several sources said a new trial is unlikely, particularly given the cost and 28 trials already evaluating colchicine. Among these are RECOVERY and COLCOVID, testing whether colchicine can reduce the duration of hospitalization or death in hospitalized patients with COVID-19.
Because there are so many trials ongoing right now, including for antivirals and other immunomodulators, it’s important that, if colchicine comes to routine clinical use, it provides access to treatment for those not able or willing to access clinical trials, rather than impeding clinical trial enrollment, Dr. Bender Ignacio suggested.
“We have already learned the lesson in the pandemic that early adoption of potentially promising therapies can negatively impact our ability to study and develop other promising treatments,” she said.
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the National Heart, Lung, and Blood Institute of the National Institutes of Health; Montreal philanthropist Sophie Desmarais, and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators. Dr. Glatt reported no conflicts of interest. Dr. Boulware reported receiving $18 in food and beverages from Gilead Sciences in 2018.
A version of this article first appeared on Medscape.com.
Rheumatologic disease activity an important influencer of COVID-19 death risk
People with rheumatic and musculoskeletal diseases (RMDs) who contract the SARS-CoV-2 virus appear more likely to die from COVID-19 if their rheumatologic condition is not being well controlled at the time of their infection.
New data from the COVID-19 Global Rheumatology Alliance (GRA) physician registry reported in Annals of the Rheumatic Diseases have found that the odds of dying from COVID-19 were 87% higher in individuals recorded as having moderate to high disease activity versus those reported to be in remission or having low disease activity.
“I think this really highlights the importance of continuing to appropriately, and actively, treat our patients, and the importance of controlling their disease,” Pedro Machado, MD, PhD, said in an interview. Dr. Machado, an associate professor in rheumatology and muscle diseases at University College London and a consultant rheumatologist at several U.K. hospitals, has been involved in the GRA physician registry from the start, and sits on the GRA steering committee.
Alongside higher disease activity, several other important factors were found to be associated with increased odds of dying from COVID-19 – older age, male gender, and the presence of one or more comorbidities, such as hypertension combined with cardiovascular disease or chronic lung disease.
These demographic and disease-based factors have been linked to an increased risk for COVID-19–related hospitalization before, both in people with RMDs and in the general population, but the latest GRA physician registry data now take that a step further, and link them also to an increased risk for death, together with several other factors more specific to RMDs.
Logging COVID-19 rheumatologic cases
Since the start of the global pandemic, the potential effects that SARS-CoV-2 infection might have on people with RMDs in particular has concerned the rheumatology community. The main worries being that, either because of the underlying RMD itself or to its treatment, there may be immunoregulatory deficits or other risk factors that would make individuals more susceptible to not only infection but also to developing more severe COVID-19 than the general population.
These concerns led to the rapid formation of the GRA and the COVID-19 GRA physician registry in March 2020 to collect and analyze data on adults with rheumatic disease and confirmed or presumptive COVID-19. Entries into the registry are made by or under the direction of rheumatologists, and this is a voluntary process.
“This population cannot ever be entirely representative of the population of patients with rheumatic diseases,” Dr. Machado acknowledged. There will be selection and other biases that affect the reported data. That said, it’s the largest database of reported COVID-19 cases in adult rheumatology patients across the world, with more than 9,000 cases so far included from multiple registries, including those based in Europe and North and South America. Data from one of these – the French RMD cohort – have also recently been published in Annals of the Rheumatic Diseases, showing much the same findings but on a national level.
Hospitalization was the focus of a previous report because “you need large sample sizes” to look at endpoints that occur less frequently. When the first analysis was done, there were around 600 cases from 40 countries in the registry with sufficient data that could be used. Now, with a greater number of recorded cases, factors influencing the risk for death could be examined.
Death rate and risk factors found
Data on 3,729 COVID-19 cases in people with RMDs were included in the current analysis, all recorded in the first few months of the registry being open and up until July 1, 2020. In all, 390 (10.5%) of people died. While this is “clearly higher” than reported in the general population in most countries, the analysis was not designed to calculate a precise estimate.
“It should not be taken as an estimate of the overall death rate among patients with rheumatic diseases and COVID-19,” Dr. Machado and coauthors have been keen to point out.
“Age is always the biggest risk factor,” Dr. Machado explained. “There’s always a gradient: the older the patient, the worse the outcome.”
Indeed, there was a threefold increased risk for death among those aged 66-75 years versus those who were 65 years or younger (odds ratio, 3.00), and a sixfold increased risk for patients older than 75, compared with the younger age group (OR, 6.18).
Having both hypertension and cardiovascular disease was associated with an OR of 1.89, and coexisting chronic lung disease also significantly increased the chances of dying from COVID-19 (OR, 1.68).
Being of male sex was associated with a 46% increased risk for death from COVID-19 versus being of female sex.
The risk for COVID-19 death also rose with the use of corticosteroids. Compared with no steroid use, there was a 69% increased risk for with death at doses of 10 mg or more prednisolone equivalent per day.
“The finding about moderate to high doses of steroids being associated with a worse outcome is consistent with the first report; it was the same for hospitalization,” Dr. Machado observed.
The general consensus on steroid use in the COVID-19 setting is that they should be continued as needed, but at the lowest possible dose, as outlined in provisional recommendations set out by the recently renamed European Alliance of Associations for Rheumatology.
The GRA physician registry findings provide further support for this, suggesting that disease control should be optimized with disease-modifying antirheumatic drugs, ideally without increasing the dose of steroids.
Surprise over sulfasalazine risk
“Taking all medications into account – such as methotrexate, leflunomide, hydroxychloroquine, [tumor necrosis factor] blockers, interleukin-6 blockers, and [Janus kinase] inhibitors – it is quite reassuring because we did not see an association with worse outcome with those drugs overall,” Dr. Machado said.
However, treatment with rituximab (OR, 4.0), sulfasalazine (OR, 3.6), and immunosuppressive agents such as azathioprine, cyclophosphamide, cyclosporine, mycophenolate, or tacrolimus (OR, 2.2), were associated with higher odds of dying from COVID-19 when compared with treatment with methotrexate alone.
The findings for rituximab and immunosuppressant use were perhaps not unexpected, but the possible association between sulfasalazine and COVID-19 death was “a bit intriguing,” Dr. Machado observed. “Sulfasalazine is believed to have low immunosuppressive effect.”
This warrants further investigation, but there are likely a range of confounding factors at play. One could be that people considered to be at higher risk may have been more often prescribed sulfasalazine because it was thought to be less immunosuppressive. Another might be because people taking sulfasalazine were more likely to be smokers, and they were also not advised to protect themselves from exposure to the virus (shielding) during the first wave of the pandemic, at least not in the United Kingdom.
Rituximab caution and vaccination
“Rituximab is a concern,” Dr. Machado acknowledged. “It is a concern that rheumatologists are now aware of and they are addressing, but then it’s a concern for a very specific subgroup of patients.”
While rheumatologists are, and will continue to prescribe it, there will be even more careful consideration over when, in whom, and how to use it during, and possibly even after, the pandemic.
“COVID is here to stay, it will become endemic, and it’s going to be part of our lives like the flu virus is,” Dr. Machado predicted.
Then there is the issue on vaccinating people against COVID-19, should those on rituximab still receive it? The answer is a yes, but, as with other vaccinations it’s all about the timing of when the vaccination is given.
Societies such as the British Society for Rheumatology have already begun to include guidance on this, recommending one of the available COVID-19 vaccines is given at least a month before the next or first dose of rituximab is due. As rituximab is given every few months, with doses sometimes spaced as much as 9 months or even a year apart, this should not be too much of a problem, but it is “better to have the vaccine first,” Dr. Machado said.
Has COVID-19 care improved in RMDs?
In separate research published in The Lancet Rheumatology, April Jorge, MD, of Massachusetts General Hospital and Harvard Medical School, both in Boston, and associates found that the risks of severe COVID-19 outcomes have improved over time, although they still “remain substantial.”
Dr. Jorge and colleagues looked at temporal trends in COVID-19 outcomes in patients with RMDs over the course of the first 6 months of the pandemic in 2020, using data from a large, multicenter, electronic health record network (TriNetX).
They formed two patient cohorts – a late (diagnosed from April 20 to July 20) and an early (diagnosed from January 20 to April 20) cohort – to see if outcomes had improved and discovered lower relative risks among patients in the late cohort for hospitalization (0.67), admission to the ICU (0.56), mechanical ventilation (0.39), acute kidney injury (0.66), renal replacement (0.53), and death (0.39).
“These results are encouraging,” but it’s difficult to match these different populations of patients, Dr. Machado said. “There are always factors that you cannot match for” and were not included in the U.S. analysis.
While there are important caveats in how the analysis was performed and thus in interpreting these data, they do “suggest that one of the reasons why outcomes have improved is because we have become better at treating these patients,” Dr. Machado added.
“Our treatment has improved, and our capacity to treat the complications has improved. We understand better how the disease behaves – we know that they can have thromboembolic complications that we can manage, and we are now able to manage ventilation issues better.”
Moreover, Dr. Machado said that, not only were clinicians more aware of what they should or should not do, there were treatments that were being used routinely or in some cases based on recent clinical trial results. “I think we are indeed treating these patients better.”
The COVID-19 GRA physician registry is financially supported by the American College of Rheumatology and EULAR. Dr. Machado had no relevant conflicts of interest.
People with rheumatic and musculoskeletal diseases (RMDs) who contract the SARS-CoV-2 virus appear more likely to die from COVID-19 if their rheumatologic condition is not being well controlled at the time of their infection.
New data from the COVID-19 Global Rheumatology Alliance (GRA) physician registry reported in Annals of the Rheumatic Diseases have found that the odds of dying from COVID-19 were 87% higher in individuals recorded as having moderate to high disease activity versus those reported to be in remission or having low disease activity.
“I think this really highlights the importance of continuing to appropriately, and actively, treat our patients, and the importance of controlling their disease,” Pedro Machado, MD, PhD, said in an interview. Dr. Machado, an associate professor in rheumatology and muscle diseases at University College London and a consultant rheumatologist at several U.K. hospitals, has been involved in the GRA physician registry from the start, and sits on the GRA steering committee.
Alongside higher disease activity, several other important factors were found to be associated with increased odds of dying from COVID-19 – older age, male gender, and the presence of one or more comorbidities, such as hypertension combined with cardiovascular disease or chronic lung disease.
These demographic and disease-based factors have been linked to an increased risk for COVID-19–related hospitalization before, both in people with RMDs and in the general population, but the latest GRA physician registry data now take that a step further, and link them also to an increased risk for death, together with several other factors more specific to RMDs.
Logging COVID-19 rheumatologic cases
Since the start of the global pandemic, the potential effects that SARS-CoV-2 infection might have on people with RMDs in particular has concerned the rheumatology community. The main worries being that, either because of the underlying RMD itself or to its treatment, there may be immunoregulatory deficits or other risk factors that would make individuals more susceptible to not only infection but also to developing more severe COVID-19 than the general population.
These concerns led to the rapid formation of the GRA and the COVID-19 GRA physician registry in March 2020 to collect and analyze data on adults with rheumatic disease and confirmed or presumptive COVID-19. Entries into the registry are made by or under the direction of rheumatologists, and this is a voluntary process.
“This population cannot ever be entirely representative of the population of patients with rheumatic diseases,” Dr. Machado acknowledged. There will be selection and other biases that affect the reported data. That said, it’s the largest database of reported COVID-19 cases in adult rheumatology patients across the world, with more than 9,000 cases so far included from multiple registries, including those based in Europe and North and South America. Data from one of these – the French RMD cohort – have also recently been published in Annals of the Rheumatic Diseases, showing much the same findings but on a national level.
Hospitalization was the focus of a previous report because “you need large sample sizes” to look at endpoints that occur less frequently. When the first analysis was done, there were around 600 cases from 40 countries in the registry with sufficient data that could be used. Now, with a greater number of recorded cases, factors influencing the risk for death could be examined.
Death rate and risk factors found
Data on 3,729 COVID-19 cases in people with RMDs were included in the current analysis, all recorded in the first few months of the registry being open and up until July 1, 2020. In all, 390 (10.5%) of people died. While this is “clearly higher” than reported in the general population in most countries, the analysis was not designed to calculate a precise estimate.
“It should not be taken as an estimate of the overall death rate among patients with rheumatic diseases and COVID-19,” Dr. Machado and coauthors have been keen to point out.
“Age is always the biggest risk factor,” Dr. Machado explained. “There’s always a gradient: the older the patient, the worse the outcome.”
Indeed, there was a threefold increased risk for death among those aged 66-75 years versus those who were 65 years or younger (odds ratio, 3.00), and a sixfold increased risk for patients older than 75, compared with the younger age group (OR, 6.18).
Having both hypertension and cardiovascular disease was associated with an OR of 1.89, and coexisting chronic lung disease also significantly increased the chances of dying from COVID-19 (OR, 1.68).
Being of male sex was associated with a 46% increased risk for death from COVID-19 versus being of female sex.
The risk for COVID-19 death also rose with the use of corticosteroids. Compared with no steroid use, there was a 69% increased risk for with death at doses of 10 mg or more prednisolone equivalent per day.
“The finding about moderate to high doses of steroids being associated with a worse outcome is consistent with the first report; it was the same for hospitalization,” Dr. Machado observed.
The general consensus on steroid use in the COVID-19 setting is that they should be continued as needed, but at the lowest possible dose, as outlined in provisional recommendations set out by the recently renamed European Alliance of Associations for Rheumatology.
The GRA physician registry findings provide further support for this, suggesting that disease control should be optimized with disease-modifying antirheumatic drugs, ideally without increasing the dose of steroids.
Surprise over sulfasalazine risk
“Taking all medications into account – such as methotrexate, leflunomide, hydroxychloroquine, [tumor necrosis factor] blockers, interleukin-6 blockers, and [Janus kinase] inhibitors – it is quite reassuring because we did not see an association with worse outcome with those drugs overall,” Dr. Machado said.
However, treatment with rituximab (OR, 4.0), sulfasalazine (OR, 3.6), and immunosuppressive agents such as azathioprine, cyclophosphamide, cyclosporine, mycophenolate, or tacrolimus (OR, 2.2), were associated with higher odds of dying from COVID-19 when compared with treatment with methotrexate alone.
The findings for rituximab and immunosuppressant use were perhaps not unexpected, but the possible association between sulfasalazine and COVID-19 death was “a bit intriguing,” Dr. Machado observed. “Sulfasalazine is believed to have low immunosuppressive effect.”
This warrants further investigation, but there are likely a range of confounding factors at play. One could be that people considered to be at higher risk may have been more often prescribed sulfasalazine because it was thought to be less immunosuppressive. Another might be because people taking sulfasalazine were more likely to be smokers, and they were also not advised to protect themselves from exposure to the virus (shielding) during the first wave of the pandemic, at least not in the United Kingdom.
Rituximab caution and vaccination
“Rituximab is a concern,” Dr. Machado acknowledged. “It is a concern that rheumatologists are now aware of and they are addressing, but then it’s a concern for a very specific subgroup of patients.”
While rheumatologists are, and will continue to prescribe it, there will be even more careful consideration over when, in whom, and how to use it during, and possibly even after, the pandemic.
“COVID is here to stay, it will become endemic, and it’s going to be part of our lives like the flu virus is,” Dr. Machado predicted.
Then there is the issue on vaccinating people against COVID-19, should those on rituximab still receive it? The answer is a yes, but, as with other vaccinations it’s all about the timing of when the vaccination is given.
Societies such as the British Society for Rheumatology have already begun to include guidance on this, recommending one of the available COVID-19 vaccines is given at least a month before the next or first dose of rituximab is due. As rituximab is given every few months, with doses sometimes spaced as much as 9 months or even a year apart, this should not be too much of a problem, but it is “better to have the vaccine first,” Dr. Machado said.
Has COVID-19 care improved in RMDs?
In separate research published in The Lancet Rheumatology, April Jorge, MD, of Massachusetts General Hospital and Harvard Medical School, both in Boston, and associates found that the risks of severe COVID-19 outcomes have improved over time, although they still “remain substantial.”
Dr. Jorge and colleagues looked at temporal trends in COVID-19 outcomes in patients with RMDs over the course of the first 6 months of the pandemic in 2020, using data from a large, multicenter, electronic health record network (TriNetX).
They formed two patient cohorts – a late (diagnosed from April 20 to July 20) and an early (diagnosed from January 20 to April 20) cohort – to see if outcomes had improved and discovered lower relative risks among patients in the late cohort for hospitalization (0.67), admission to the ICU (0.56), mechanical ventilation (0.39), acute kidney injury (0.66), renal replacement (0.53), and death (0.39).
“These results are encouraging,” but it’s difficult to match these different populations of patients, Dr. Machado said. “There are always factors that you cannot match for” and were not included in the U.S. analysis.
While there are important caveats in how the analysis was performed and thus in interpreting these data, they do “suggest that one of the reasons why outcomes have improved is because we have become better at treating these patients,” Dr. Machado added.
“Our treatment has improved, and our capacity to treat the complications has improved. We understand better how the disease behaves – we know that they can have thromboembolic complications that we can manage, and we are now able to manage ventilation issues better.”
Moreover, Dr. Machado said that, not only were clinicians more aware of what they should or should not do, there were treatments that were being used routinely or in some cases based on recent clinical trial results. “I think we are indeed treating these patients better.”
The COVID-19 GRA physician registry is financially supported by the American College of Rheumatology and EULAR. Dr. Machado had no relevant conflicts of interest.
People with rheumatic and musculoskeletal diseases (RMDs) who contract the SARS-CoV-2 virus appear more likely to die from COVID-19 if their rheumatologic condition is not being well controlled at the time of their infection.
New data from the COVID-19 Global Rheumatology Alliance (GRA) physician registry reported in Annals of the Rheumatic Diseases have found that the odds of dying from COVID-19 were 87% higher in individuals recorded as having moderate to high disease activity versus those reported to be in remission or having low disease activity.
“I think this really highlights the importance of continuing to appropriately, and actively, treat our patients, and the importance of controlling their disease,” Pedro Machado, MD, PhD, said in an interview. Dr. Machado, an associate professor in rheumatology and muscle diseases at University College London and a consultant rheumatologist at several U.K. hospitals, has been involved in the GRA physician registry from the start, and sits on the GRA steering committee.
Alongside higher disease activity, several other important factors were found to be associated with increased odds of dying from COVID-19 – older age, male gender, and the presence of one or more comorbidities, such as hypertension combined with cardiovascular disease or chronic lung disease.
These demographic and disease-based factors have been linked to an increased risk for COVID-19–related hospitalization before, both in people with RMDs and in the general population, but the latest GRA physician registry data now take that a step further, and link them also to an increased risk for death, together with several other factors more specific to RMDs.
Logging COVID-19 rheumatologic cases
Since the start of the global pandemic, the potential effects that SARS-CoV-2 infection might have on people with RMDs in particular has concerned the rheumatology community. The main worries being that, either because of the underlying RMD itself or to its treatment, there may be immunoregulatory deficits or other risk factors that would make individuals more susceptible to not only infection but also to developing more severe COVID-19 than the general population.
These concerns led to the rapid formation of the GRA and the COVID-19 GRA physician registry in March 2020 to collect and analyze data on adults with rheumatic disease and confirmed or presumptive COVID-19. Entries into the registry are made by or under the direction of rheumatologists, and this is a voluntary process.
“This population cannot ever be entirely representative of the population of patients with rheumatic diseases,” Dr. Machado acknowledged. There will be selection and other biases that affect the reported data. That said, it’s the largest database of reported COVID-19 cases in adult rheumatology patients across the world, with more than 9,000 cases so far included from multiple registries, including those based in Europe and North and South America. Data from one of these – the French RMD cohort – have also recently been published in Annals of the Rheumatic Diseases, showing much the same findings but on a national level.
Hospitalization was the focus of a previous report because “you need large sample sizes” to look at endpoints that occur less frequently. When the first analysis was done, there were around 600 cases from 40 countries in the registry with sufficient data that could be used. Now, with a greater number of recorded cases, factors influencing the risk for death could be examined.
Death rate and risk factors found
Data on 3,729 COVID-19 cases in people with RMDs were included in the current analysis, all recorded in the first few months of the registry being open and up until July 1, 2020. In all, 390 (10.5%) of people died. While this is “clearly higher” than reported in the general population in most countries, the analysis was not designed to calculate a precise estimate.
“It should not be taken as an estimate of the overall death rate among patients with rheumatic diseases and COVID-19,” Dr. Machado and coauthors have been keen to point out.
“Age is always the biggest risk factor,” Dr. Machado explained. “There’s always a gradient: the older the patient, the worse the outcome.”
Indeed, there was a threefold increased risk for death among those aged 66-75 years versus those who were 65 years or younger (odds ratio, 3.00), and a sixfold increased risk for patients older than 75, compared with the younger age group (OR, 6.18).
Having both hypertension and cardiovascular disease was associated with an OR of 1.89, and coexisting chronic lung disease also significantly increased the chances of dying from COVID-19 (OR, 1.68).
Being of male sex was associated with a 46% increased risk for death from COVID-19 versus being of female sex.
The risk for COVID-19 death also rose with the use of corticosteroids. Compared with no steroid use, there was a 69% increased risk for with death at doses of 10 mg or more prednisolone equivalent per day.
“The finding about moderate to high doses of steroids being associated with a worse outcome is consistent with the first report; it was the same for hospitalization,” Dr. Machado observed.
The general consensus on steroid use in the COVID-19 setting is that they should be continued as needed, but at the lowest possible dose, as outlined in provisional recommendations set out by the recently renamed European Alliance of Associations for Rheumatology.
The GRA physician registry findings provide further support for this, suggesting that disease control should be optimized with disease-modifying antirheumatic drugs, ideally without increasing the dose of steroids.
Surprise over sulfasalazine risk
“Taking all medications into account – such as methotrexate, leflunomide, hydroxychloroquine, [tumor necrosis factor] blockers, interleukin-6 blockers, and [Janus kinase] inhibitors – it is quite reassuring because we did not see an association with worse outcome with those drugs overall,” Dr. Machado said.
However, treatment with rituximab (OR, 4.0), sulfasalazine (OR, 3.6), and immunosuppressive agents such as azathioprine, cyclophosphamide, cyclosporine, mycophenolate, or tacrolimus (OR, 2.2), were associated with higher odds of dying from COVID-19 when compared with treatment with methotrexate alone.
The findings for rituximab and immunosuppressant use were perhaps not unexpected, but the possible association between sulfasalazine and COVID-19 death was “a bit intriguing,” Dr. Machado observed. “Sulfasalazine is believed to have low immunosuppressive effect.”
This warrants further investigation, but there are likely a range of confounding factors at play. One could be that people considered to be at higher risk may have been more often prescribed sulfasalazine because it was thought to be less immunosuppressive. Another might be because people taking sulfasalazine were more likely to be smokers, and they were also not advised to protect themselves from exposure to the virus (shielding) during the first wave of the pandemic, at least not in the United Kingdom.
Rituximab caution and vaccination
“Rituximab is a concern,” Dr. Machado acknowledged. “It is a concern that rheumatologists are now aware of and they are addressing, but then it’s a concern for a very specific subgroup of patients.”
While rheumatologists are, and will continue to prescribe it, there will be even more careful consideration over when, in whom, and how to use it during, and possibly even after, the pandemic.
“COVID is here to stay, it will become endemic, and it’s going to be part of our lives like the flu virus is,” Dr. Machado predicted.
Then there is the issue on vaccinating people against COVID-19, should those on rituximab still receive it? The answer is a yes, but, as with other vaccinations it’s all about the timing of when the vaccination is given.
Societies such as the British Society for Rheumatology have already begun to include guidance on this, recommending one of the available COVID-19 vaccines is given at least a month before the next or first dose of rituximab is due. As rituximab is given every few months, with doses sometimes spaced as much as 9 months or even a year apart, this should not be too much of a problem, but it is “better to have the vaccine first,” Dr. Machado said.
Has COVID-19 care improved in RMDs?
In separate research published in The Lancet Rheumatology, April Jorge, MD, of Massachusetts General Hospital and Harvard Medical School, both in Boston, and associates found that the risks of severe COVID-19 outcomes have improved over time, although they still “remain substantial.”
Dr. Jorge and colleagues looked at temporal trends in COVID-19 outcomes in patients with RMDs over the course of the first 6 months of the pandemic in 2020, using data from a large, multicenter, electronic health record network (TriNetX).
They formed two patient cohorts – a late (diagnosed from April 20 to July 20) and an early (diagnosed from January 20 to April 20) cohort – to see if outcomes had improved and discovered lower relative risks among patients in the late cohort for hospitalization (0.67), admission to the ICU (0.56), mechanical ventilation (0.39), acute kidney injury (0.66), renal replacement (0.53), and death (0.39).
“These results are encouraging,” but it’s difficult to match these different populations of patients, Dr. Machado said. “There are always factors that you cannot match for” and were not included in the U.S. analysis.
While there are important caveats in how the analysis was performed and thus in interpreting these data, they do “suggest that one of the reasons why outcomes have improved is because we have become better at treating these patients,” Dr. Machado added.
“Our treatment has improved, and our capacity to treat the complications has improved. We understand better how the disease behaves – we know that they can have thromboembolic complications that we can manage, and we are now able to manage ventilation issues better.”
Moreover, Dr. Machado said that, not only were clinicians more aware of what they should or should not do, there were treatments that were being used routinely or in some cases based on recent clinical trial results. “I think we are indeed treating these patients better.”
The COVID-19 GRA physician registry is financially supported by the American College of Rheumatology and EULAR. Dr. Machado had no relevant conflicts of interest.
FROM ANNALS OF THE RHEUMATIC DISEASES
COVID-19: Another study links colchicine to better results
The gout drug colchicine appears to lower the severity of COVID-19, a small new Brazilian study finds, adding to evidence that the familiar medication holds promise as a treatment for hospitalized patients.
Patients who received colchicine in this randomized, double-blinded, placebo-controlled clinical trial presented better evolution in terms of the need for supplemental oxygen and the length of hospitalisation. ... Colchicine was safe and well tolerated,” the study authors wrote in RMD Open. However, deaths were rare in the trial, they added, and it is impossible to “evaluate the capacity of colchicine to avoid admission to ICU and reduce mortality.”
The oral anti-inflammatory colchicine, widely used as treatment in rheumatic disease, was first approved in the United States 60 years ago. Researchers began to explore its potential as a COVID-19 treatment in the early months of the pandemic.
On Jan. 25, an international team of researchers reported in a press release – but not yet a published paper – that the drug seemed to reduce hospitalizations, mechanical ventilation, and deaths in the ColCORONA trial. Earlier, a much-smaller, randomized, open-label, Greek trial linked the drug to reduced time to clinical deterioration and hospital stay.
The Brazilian authors of the new study, led by Maria Isabel Lopes of the University of São Paulo’s Ribeirão Preto Medical School, randomly assigned 75 hospitalized patients with moderate to severe COVID-19 to colchicine or placebo. A total of 72 subjects completed the April-August 2020 trial: 36 received colchicine (typically 0.5 mg three times for 5 days, then 0.5 mg twice daily for 5 days; doses were adjusted in low-weight patients and those with chronic kidney disease). The other 36 received the placebo.
(In the United States, 0.6-mg tablets of generic colchicine cost as little as $1.90 each with free coupons, according to goodrx.com.)
The median age in the groups was similar (55 years); and the placebo group had more women (61% vs. 47% in the colchicine group, P = .34). All 72 patients received the same COVID-19 treatment at the time of the trial: azithromycin, hydroxychloroquine, and unfractionated heparin. Most patients, about two-thirds in both groups, also received methylprednisolone because they needed higher amounts of supplemental oxygen.
Patients in the colchicine group needed supplemental oxygen for less time: Their median time of need was 4.0 days (interquartile range [IQR], 2.0-6.0) vs. 6.5 days (IQR, 4.0-9.0) for the placebo group (P < .001). The median time for hospitalization was also lower at 7.0 days (IQR, 5.0–9.0) for the colchicine group vs. 9.0 (IQR, 7.0–12.0) for the placebo group (log rank test, 10.6; P = .001).
The researchers also reported the percentage of patients who needed supplemental oxygen at day 2 as 67% with colchicine vs. 86% with placebo, and at day 7 as 9% vs. 42% (log rank test, 10.6; P = .001). Two patients in the placebo group died, both from ventilator-associated pneumonia.
As for side effects, new or worsened diarrhea was reported more often in the colchicine group (17% vs. 6% with placebo), but the difference was not statistically significant (P = .26), and diarrhea was controlled via medication.
The researchers reported that limitations include the exclusion criteria and their inability to link colchicine to rates of ICU admissions and death.
The drug appears to help patients with COVID-19, the study authors wrote, by “inhibiting inflammasome, reducing neutrophil migration and activation, or preventing endothelial damage.”
A “well-conceived and well-designed” study
In an interview, NYU Langone Health rheumatologist Michael H. Pillinger, MD – an investigator with the ColCORONA trial – praised the Brazilian study. It “appears well-conceived and well-designed, and was enrolled at a rate that was greater than the sample size that was estimated to be needed based on power analysis,” he said.
The Brazilian study is small, he noted. (In contrast, the ColCORONA trial had 4,488 outpatient participants.) “This study differs from ColCORONA in several ways – the most important being that it is a study of inpatients with moderate to severe COVID (really mostly moderate),” he added. “ColCORONA is looking at a target audience that is much larger – outpatients with mild to moderate COVID with risk factors for hospitalization. Both questions are really important and certainly not mutually exclusive, since our care remains inadequate in both venues. This study also adds value in that several other studies have been conducted in hospital patients with enrollment criteria relatively similar to this one, and all showed benefit, but those were open-label or retrospective, and this is blinded and placebo-controlled.”
Using colchicine in patients with COVID-19
Should physicians turn to colchicine in patients with COVID-19? “I would rather that it still be used in the context of research until formal recommendations can be made by bodies like the NIH and CDC,” Dr. Pillinger said. “But certainly, there may be times when physicians feel compelled to treat patients off label.”
He cautioned, however, that colchicine should never be used with some other drugs. Its interaction with the antibiotic clarithromycin can be fatal, he noted. And, he said, the drug must be monitored in general since it can cause rare, severe problems.
“Overall, colchicine probably works on the overabundant inflammatory response to COVID, and it may be that it can be combined with other drugs that affect viral replication or promote immunity – e.g. vaccines,” Dr. Pillinger said. “So far, it seems as if there is no safety problem with combining colchicine with other approaches, but this has not been studied in a rigorous manner.”
Moving forward, he said, the drug’s very low price outside of the United States “could provide resource-poor countries with a way to help keep patients out of precious hospital beds – or help them go home sooner once admitted.” For now, however, “we need a large-scale inpatient study, and one is currently going on in Great Britain. We also need validation of the outpatient ColCORONA study, and studies to look at whether colchicine can work in conjunction with other strategies.”
The study was funded by grants from the São Paulo Research Foundation, Brazilian National Council for Scientific and Technological Development, and CAPES Foundation. No disclosures are reported. Dr. Pillinger reports serving as an investigator for the ColCORONA trial and receiving a unrelated investigator-initiated grant from Hikma, a colchicine manufacturer.
The gout drug colchicine appears to lower the severity of COVID-19, a small new Brazilian study finds, adding to evidence that the familiar medication holds promise as a treatment for hospitalized patients.
Patients who received colchicine in this randomized, double-blinded, placebo-controlled clinical trial presented better evolution in terms of the need for supplemental oxygen and the length of hospitalisation. ... Colchicine was safe and well tolerated,” the study authors wrote in RMD Open. However, deaths were rare in the trial, they added, and it is impossible to “evaluate the capacity of colchicine to avoid admission to ICU and reduce mortality.”
The oral anti-inflammatory colchicine, widely used as treatment in rheumatic disease, was first approved in the United States 60 years ago. Researchers began to explore its potential as a COVID-19 treatment in the early months of the pandemic.
On Jan. 25, an international team of researchers reported in a press release – but not yet a published paper – that the drug seemed to reduce hospitalizations, mechanical ventilation, and deaths in the ColCORONA trial. Earlier, a much-smaller, randomized, open-label, Greek trial linked the drug to reduced time to clinical deterioration and hospital stay.
The Brazilian authors of the new study, led by Maria Isabel Lopes of the University of São Paulo’s Ribeirão Preto Medical School, randomly assigned 75 hospitalized patients with moderate to severe COVID-19 to colchicine or placebo. A total of 72 subjects completed the April-August 2020 trial: 36 received colchicine (typically 0.5 mg three times for 5 days, then 0.5 mg twice daily for 5 days; doses were adjusted in low-weight patients and those with chronic kidney disease). The other 36 received the placebo.
(In the United States, 0.6-mg tablets of generic colchicine cost as little as $1.90 each with free coupons, according to goodrx.com.)
The median age in the groups was similar (55 years); and the placebo group had more women (61% vs. 47% in the colchicine group, P = .34). All 72 patients received the same COVID-19 treatment at the time of the trial: azithromycin, hydroxychloroquine, and unfractionated heparin. Most patients, about two-thirds in both groups, also received methylprednisolone because they needed higher amounts of supplemental oxygen.
Patients in the colchicine group needed supplemental oxygen for less time: Their median time of need was 4.0 days (interquartile range [IQR], 2.0-6.0) vs. 6.5 days (IQR, 4.0-9.0) for the placebo group (P < .001). The median time for hospitalization was also lower at 7.0 days (IQR, 5.0–9.0) for the colchicine group vs. 9.0 (IQR, 7.0–12.0) for the placebo group (log rank test, 10.6; P = .001).
The researchers also reported the percentage of patients who needed supplemental oxygen at day 2 as 67% with colchicine vs. 86% with placebo, and at day 7 as 9% vs. 42% (log rank test, 10.6; P = .001). Two patients in the placebo group died, both from ventilator-associated pneumonia.
As for side effects, new or worsened diarrhea was reported more often in the colchicine group (17% vs. 6% with placebo), but the difference was not statistically significant (P = .26), and diarrhea was controlled via medication.
The researchers reported that limitations include the exclusion criteria and their inability to link colchicine to rates of ICU admissions and death.
The drug appears to help patients with COVID-19, the study authors wrote, by “inhibiting inflammasome, reducing neutrophil migration and activation, or preventing endothelial damage.”
A “well-conceived and well-designed” study
In an interview, NYU Langone Health rheumatologist Michael H. Pillinger, MD – an investigator with the ColCORONA trial – praised the Brazilian study. It “appears well-conceived and well-designed, and was enrolled at a rate that was greater than the sample size that was estimated to be needed based on power analysis,” he said.
The Brazilian study is small, he noted. (In contrast, the ColCORONA trial had 4,488 outpatient participants.) “This study differs from ColCORONA in several ways – the most important being that it is a study of inpatients with moderate to severe COVID (really mostly moderate),” he added. “ColCORONA is looking at a target audience that is much larger – outpatients with mild to moderate COVID with risk factors for hospitalization. Both questions are really important and certainly not mutually exclusive, since our care remains inadequate in both venues. This study also adds value in that several other studies have been conducted in hospital patients with enrollment criteria relatively similar to this one, and all showed benefit, but those were open-label or retrospective, and this is blinded and placebo-controlled.”
Using colchicine in patients with COVID-19
Should physicians turn to colchicine in patients with COVID-19? “I would rather that it still be used in the context of research until formal recommendations can be made by bodies like the NIH and CDC,” Dr. Pillinger said. “But certainly, there may be times when physicians feel compelled to treat patients off label.”
He cautioned, however, that colchicine should never be used with some other drugs. Its interaction with the antibiotic clarithromycin can be fatal, he noted. And, he said, the drug must be monitored in general since it can cause rare, severe problems.
“Overall, colchicine probably works on the overabundant inflammatory response to COVID, and it may be that it can be combined with other drugs that affect viral replication or promote immunity – e.g. vaccines,” Dr. Pillinger said. “So far, it seems as if there is no safety problem with combining colchicine with other approaches, but this has not been studied in a rigorous manner.”
Moving forward, he said, the drug’s very low price outside of the United States “could provide resource-poor countries with a way to help keep patients out of precious hospital beds – or help them go home sooner once admitted.” For now, however, “we need a large-scale inpatient study, and one is currently going on in Great Britain. We also need validation of the outpatient ColCORONA study, and studies to look at whether colchicine can work in conjunction with other strategies.”
The study was funded by grants from the São Paulo Research Foundation, Brazilian National Council for Scientific and Technological Development, and CAPES Foundation. No disclosures are reported. Dr. Pillinger reports serving as an investigator for the ColCORONA trial and receiving a unrelated investigator-initiated grant from Hikma, a colchicine manufacturer.
The gout drug colchicine appears to lower the severity of COVID-19, a small new Brazilian study finds, adding to evidence that the familiar medication holds promise as a treatment for hospitalized patients.
Patients who received colchicine in this randomized, double-blinded, placebo-controlled clinical trial presented better evolution in terms of the need for supplemental oxygen and the length of hospitalisation. ... Colchicine was safe and well tolerated,” the study authors wrote in RMD Open. However, deaths were rare in the trial, they added, and it is impossible to “evaluate the capacity of colchicine to avoid admission to ICU and reduce mortality.”
The oral anti-inflammatory colchicine, widely used as treatment in rheumatic disease, was first approved in the United States 60 years ago. Researchers began to explore its potential as a COVID-19 treatment in the early months of the pandemic.
On Jan. 25, an international team of researchers reported in a press release – but not yet a published paper – that the drug seemed to reduce hospitalizations, mechanical ventilation, and deaths in the ColCORONA trial. Earlier, a much-smaller, randomized, open-label, Greek trial linked the drug to reduced time to clinical deterioration and hospital stay.
The Brazilian authors of the new study, led by Maria Isabel Lopes of the University of São Paulo’s Ribeirão Preto Medical School, randomly assigned 75 hospitalized patients with moderate to severe COVID-19 to colchicine or placebo. A total of 72 subjects completed the April-August 2020 trial: 36 received colchicine (typically 0.5 mg three times for 5 days, then 0.5 mg twice daily for 5 days; doses were adjusted in low-weight patients and those with chronic kidney disease). The other 36 received the placebo.
(In the United States, 0.6-mg tablets of generic colchicine cost as little as $1.90 each with free coupons, according to goodrx.com.)
The median age in the groups was similar (55 years); and the placebo group had more women (61% vs. 47% in the colchicine group, P = .34). All 72 patients received the same COVID-19 treatment at the time of the trial: azithromycin, hydroxychloroquine, and unfractionated heparin. Most patients, about two-thirds in both groups, also received methylprednisolone because they needed higher amounts of supplemental oxygen.
Patients in the colchicine group needed supplemental oxygen for less time: Their median time of need was 4.0 days (interquartile range [IQR], 2.0-6.0) vs. 6.5 days (IQR, 4.0-9.0) for the placebo group (P < .001). The median time for hospitalization was also lower at 7.0 days (IQR, 5.0–9.0) for the colchicine group vs. 9.0 (IQR, 7.0–12.0) for the placebo group (log rank test, 10.6; P = .001).
The researchers also reported the percentage of patients who needed supplemental oxygen at day 2 as 67% with colchicine vs. 86% with placebo, and at day 7 as 9% vs. 42% (log rank test, 10.6; P = .001). Two patients in the placebo group died, both from ventilator-associated pneumonia.
As for side effects, new or worsened diarrhea was reported more often in the colchicine group (17% vs. 6% with placebo), but the difference was not statistically significant (P = .26), and diarrhea was controlled via medication.
The researchers reported that limitations include the exclusion criteria and their inability to link colchicine to rates of ICU admissions and death.
The drug appears to help patients with COVID-19, the study authors wrote, by “inhibiting inflammasome, reducing neutrophil migration and activation, or preventing endothelial damage.”
A “well-conceived and well-designed” study
In an interview, NYU Langone Health rheumatologist Michael H. Pillinger, MD – an investigator with the ColCORONA trial – praised the Brazilian study. It “appears well-conceived and well-designed, and was enrolled at a rate that was greater than the sample size that was estimated to be needed based on power analysis,” he said.
The Brazilian study is small, he noted. (In contrast, the ColCORONA trial had 4,488 outpatient participants.) “This study differs from ColCORONA in several ways – the most important being that it is a study of inpatients with moderate to severe COVID (really mostly moderate),” he added. “ColCORONA is looking at a target audience that is much larger – outpatients with mild to moderate COVID with risk factors for hospitalization. Both questions are really important and certainly not mutually exclusive, since our care remains inadequate in both venues. This study also adds value in that several other studies have been conducted in hospital patients with enrollment criteria relatively similar to this one, and all showed benefit, but those were open-label or retrospective, and this is blinded and placebo-controlled.”
Using colchicine in patients with COVID-19
Should physicians turn to colchicine in patients with COVID-19? “I would rather that it still be used in the context of research until formal recommendations can be made by bodies like the NIH and CDC,” Dr. Pillinger said. “But certainly, there may be times when physicians feel compelled to treat patients off label.”
He cautioned, however, that colchicine should never be used with some other drugs. Its interaction with the antibiotic clarithromycin can be fatal, he noted. And, he said, the drug must be monitored in general since it can cause rare, severe problems.
“Overall, colchicine probably works on the overabundant inflammatory response to COVID, and it may be that it can be combined with other drugs that affect viral replication or promote immunity – e.g. vaccines,” Dr. Pillinger said. “So far, it seems as if there is no safety problem with combining colchicine with other approaches, but this has not been studied in a rigorous manner.”
Moving forward, he said, the drug’s very low price outside of the United States “could provide resource-poor countries with a way to help keep patients out of precious hospital beds – or help them go home sooner once admitted.” For now, however, “we need a large-scale inpatient study, and one is currently going on in Great Britain. We also need validation of the outpatient ColCORONA study, and studies to look at whether colchicine can work in conjunction with other strategies.”
The study was funded by grants from the São Paulo Research Foundation, Brazilian National Council for Scientific and Technological Development, and CAPES Foundation. No disclosures are reported. Dr. Pillinger reports serving as an investigator for the ColCORONA trial and receiving a unrelated investigator-initiated grant from Hikma, a colchicine manufacturer.
FROM RMD OPEN
ColCORONA: Colchicine reduces complications in outpatient COVID-19
The oral, anti-inflammatory drug colchicine can prevent complications and hospitalizations in nonhospitalized patients newly diagnosed with COVID-19, according to a press release from the ColCORONA trial investigators.
After 1 month of therapy, there was a 21% risk reduction in the primary composite endpoint of death or hospitalizations that missed statistical significance, compared with placebo among 4,488 outpatients enrolled in the global, phase 3 trial.
After excluding 329 patients without a confirmatory polymerase chain reaction test, however, the use of colchicine was reported to significantly reduce hospitalizations by 25%, the need for mechanical ventilation by 50%, and deaths by 44%.
“We believe that this is a medical breakthrough. There’s no approved therapy to prevent complications of COVID-19 in outpatients, to prevent them from reaching the hospital,” lead investigator Jean-Claude Tardif, MD, from the Montreal Heart Institute, said in an interview.
“I know that several countries will be reviewing the data very rapidly and that Greece approved it today,” he said. “So this is providing hope for patients.”
Having been burned by hydroxychloroquine and other treatments brought forth without peer review, the response to the announcement was tempered by a desire for more details.
Asked for comment, Steven E. Nissen, MD, of the Cleveland Clinic Foundation, was cautious. “The press release about the trial is vague and lacks details such as hazard ratios, confidence intervals, and P values,” he said in an interview.
“It is impossible to evaluate the results of this trial without these details. It is also uncertain how rigorously data were collected,” he added. “We’ll need to see the manuscript to adequately interpret the results.”
The evidence in the press release is hard to interpret, but early intervention with anti-inflammatory therapy has considerable biologic appeal in COVID, said Paul Ridker, MD, MPH, who led the pivotal CANTOS trial of the anti-inflammatory drug canakinumab in the post-MI setting, and is also chair of the ACTIV-4B trial currently investigating anticoagulants and antithrombotics in outpatient COVID-19.
“Colchicine is both inexpensive and generally well tolerated, and the apparent benefits so far reported are substantial,” Dr. Ridker, from Brigham and Women’s Hospital in Boston, said in an interview. “We are eager to see the full data as rapidly as possible.”
The commonly used gout and rheumatic disease agent costs about 26 cents in Canada and between $4 and $6 in the United States. As previously reported, it reduced the time to clinical deterioration and hospital stay but not mortality in the 105-patient Greek Study in the Effects of Colchicine in COVID-19 Complications Prevention (GRECCO-19) study.
Dr. Tardif said he’s looking forward to having the data in the public domain and that they acted swiftly because the evidence was “clinically persuasive” and “the health system is congested now.”
“We received the results Friday, Jan. 22 at 5 p.m., an hour later we were in meetings with our data safety monitoring board [DSMB], 2 hours later we issued a press release, and a day later we’re submitting a full manuscript to a major scientific journal, so I don’t know if anyone has done this at this speed,” he said. “So we are actually very proud of what we did.”
ColCORONA was designed to enroll 6,000 outpatients, at least 40 years of age, who were diagnosed with COVID-19 infection within the previous 24 hours, and had a least one high-risk criterion, including age at least 70 years, body mass index of at least 30 kg/m2, diabetes mellitus, uncontrolled hypertension, known respiratory disease, heart failure or coronary disease, fever of at least 38.4° C within the last 48 hours, dyspnea at presentation, bicytopenia, pancytopenia, or the combination of high neutrophil count and low lymphocyte count.
Participants were randomly assigned to receive either placebo or colchicine 0.5 mg twice daily for 3 days and then once daily for another 27 days.
The number needed to prevent one COVID-19 complication is about 60 patients, Dr. Tardif said.
Colchicine was well tolerated and resulted in fewer serious adverse events than with placebo, he said. Diarrhea occurred more often with colchicine, but there was no increase in pneumonia. Caution should be used, however, in treating patients with severe renal disease.
Dr. Tardif said he would not prescribe colchicine to an 18-year-old COVID outpatient who doesn’t have any concomitant diseases, but would for those meeting the study protocol.
“As long as a patient appears to me to be at risk of a complication, I would prescribe it, without a doubt,” he said. “I can tell you that when we held the meeting with the DSMB Friday evening, I actually put each member on the spot and asked them: ‘If it were you – not even treating a patient, but if you had COVID today, would you take it based on the data you’ve seen?’ and all of the DSMB members said they would.
“So we’ll have that debate in the public domain when the paper is out, but I believe most physicians will use it to treat their patients.”
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the U.S. National Heart, Lung, and Blood Institute; Montreal philanthropist Sophie Desmarais; and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators.
A version of this article first appeared on Medscape.com.
The oral, anti-inflammatory drug colchicine can prevent complications and hospitalizations in nonhospitalized patients newly diagnosed with COVID-19, according to a press release from the ColCORONA trial investigators.
After 1 month of therapy, there was a 21% risk reduction in the primary composite endpoint of death or hospitalizations that missed statistical significance, compared with placebo among 4,488 outpatients enrolled in the global, phase 3 trial.
After excluding 329 patients without a confirmatory polymerase chain reaction test, however, the use of colchicine was reported to significantly reduce hospitalizations by 25%, the need for mechanical ventilation by 50%, and deaths by 44%.
“We believe that this is a medical breakthrough. There’s no approved therapy to prevent complications of COVID-19 in outpatients, to prevent them from reaching the hospital,” lead investigator Jean-Claude Tardif, MD, from the Montreal Heart Institute, said in an interview.
“I know that several countries will be reviewing the data very rapidly and that Greece approved it today,” he said. “So this is providing hope for patients.”
Having been burned by hydroxychloroquine and other treatments brought forth without peer review, the response to the announcement was tempered by a desire for more details.
Asked for comment, Steven E. Nissen, MD, of the Cleveland Clinic Foundation, was cautious. “The press release about the trial is vague and lacks details such as hazard ratios, confidence intervals, and P values,” he said in an interview.
“It is impossible to evaluate the results of this trial without these details. It is also uncertain how rigorously data were collected,” he added. “We’ll need to see the manuscript to adequately interpret the results.”
The evidence in the press release is hard to interpret, but early intervention with anti-inflammatory therapy has considerable biologic appeal in COVID, said Paul Ridker, MD, MPH, who led the pivotal CANTOS trial of the anti-inflammatory drug canakinumab in the post-MI setting, and is also chair of the ACTIV-4B trial currently investigating anticoagulants and antithrombotics in outpatient COVID-19.
“Colchicine is both inexpensive and generally well tolerated, and the apparent benefits so far reported are substantial,” Dr. Ridker, from Brigham and Women’s Hospital in Boston, said in an interview. “We are eager to see the full data as rapidly as possible.”
The commonly used gout and rheumatic disease agent costs about 26 cents in Canada and between $4 and $6 in the United States. As previously reported, it reduced the time to clinical deterioration and hospital stay but not mortality in the 105-patient Greek Study in the Effects of Colchicine in COVID-19 Complications Prevention (GRECCO-19) study.
Dr. Tardif said he’s looking forward to having the data in the public domain and that they acted swiftly because the evidence was “clinically persuasive” and “the health system is congested now.”
“We received the results Friday, Jan. 22 at 5 p.m., an hour later we were in meetings with our data safety monitoring board [DSMB], 2 hours later we issued a press release, and a day later we’re submitting a full manuscript to a major scientific journal, so I don’t know if anyone has done this at this speed,” he said. “So we are actually very proud of what we did.”
ColCORONA was designed to enroll 6,000 outpatients, at least 40 years of age, who were diagnosed with COVID-19 infection within the previous 24 hours, and had a least one high-risk criterion, including age at least 70 years, body mass index of at least 30 kg/m2, diabetes mellitus, uncontrolled hypertension, known respiratory disease, heart failure or coronary disease, fever of at least 38.4° C within the last 48 hours, dyspnea at presentation, bicytopenia, pancytopenia, or the combination of high neutrophil count and low lymphocyte count.
Participants were randomly assigned to receive either placebo or colchicine 0.5 mg twice daily for 3 days and then once daily for another 27 days.
The number needed to prevent one COVID-19 complication is about 60 patients, Dr. Tardif said.
Colchicine was well tolerated and resulted in fewer serious adverse events than with placebo, he said. Diarrhea occurred more often with colchicine, but there was no increase in pneumonia. Caution should be used, however, in treating patients with severe renal disease.
Dr. Tardif said he would not prescribe colchicine to an 18-year-old COVID outpatient who doesn’t have any concomitant diseases, but would for those meeting the study protocol.
“As long as a patient appears to me to be at risk of a complication, I would prescribe it, without a doubt,” he said. “I can tell you that when we held the meeting with the DSMB Friday evening, I actually put each member on the spot and asked them: ‘If it were you – not even treating a patient, but if you had COVID today, would you take it based on the data you’ve seen?’ and all of the DSMB members said they would.
“So we’ll have that debate in the public domain when the paper is out, but I believe most physicians will use it to treat their patients.”
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the U.S. National Heart, Lung, and Blood Institute; Montreal philanthropist Sophie Desmarais; and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators.
A version of this article first appeared on Medscape.com.
The oral, anti-inflammatory drug colchicine can prevent complications and hospitalizations in nonhospitalized patients newly diagnosed with COVID-19, according to a press release from the ColCORONA trial investigators.
After 1 month of therapy, there was a 21% risk reduction in the primary composite endpoint of death or hospitalizations that missed statistical significance, compared with placebo among 4,488 outpatients enrolled in the global, phase 3 trial.
After excluding 329 patients without a confirmatory polymerase chain reaction test, however, the use of colchicine was reported to significantly reduce hospitalizations by 25%, the need for mechanical ventilation by 50%, and deaths by 44%.
“We believe that this is a medical breakthrough. There’s no approved therapy to prevent complications of COVID-19 in outpatients, to prevent them from reaching the hospital,” lead investigator Jean-Claude Tardif, MD, from the Montreal Heart Institute, said in an interview.
“I know that several countries will be reviewing the data very rapidly and that Greece approved it today,” he said. “So this is providing hope for patients.”
Having been burned by hydroxychloroquine and other treatments brought forth without peer review, the response to the announcement was tempered by a desire for more details.
Asked for comment, Steven E. Nissen, MD, of the Cleveland Clinic Foundation, was cautious. “The press release about the trial is vague and lacks details such as hazard ratios, confidence intervals, and P values,” he said in an interview.
“It is impossible to evaluate the results of this trial without these details. It is also uncertain how rigorously data were collected,” he added. “We’ll need to see the manuscript to adequately interpret the results.”
The evidence in the press release is hard to interpret, but early intervention with anti-inflammatory therapy has considerable biologic appeal in COVID, said Paul Ridker, MD, MPH, who led the pivotal CANTOS trial of the anti-inflammatory drug canakinumab in the post-MI setting, and is also chair of the ACTIV-4B trial currently investigating anticoagulants and antithrombotics in outpatient COVID-19.
“Colchicine is both inexpensive and generally well tolerated, and the apparent benefits so far reported are substantial,” Dr. Ridker, from Brigham and Women’s Hospital in Boston, said in an interview. “We are eager to see the full data as rapidly as possible.”
The commonly used gout and rheumatic disease agent costs about 26 cents in Canada and between $4 and $6 in the United States. As previously reported, it reduced the time to clinical deterioration and hospital stay but not mortality in the 105-patient Greek Study in the Effects of Colchicine in COVID-19 Complications Prevention (GRECCO-19) study.
Dr. Tardif said he’s looking forward to having the data in the public domain and that they acted swiftly because the evidence was “clinically persuasive” and “the health system is congested now.”
“We received the results Friday, Jan. 22 at 5 p.m., an hour later we were in meetings with our data safety monitoring board [DSMB], 2 hours later we issued a press release, and a day later we’re submitting a full manuscript to a major scientific journal, so I don’t know if anyone has done this at this speed,” he said. “So we are actually very proud of what we did.”
ColCORONA was designed to enroll 6,000 outpatients, at least 40 years of age, who were diagnosed with COVID-19 infection within the previous 24 hours, and had a least one high-risk criterion, including age at least 70 years, body mass index of at least 30 kg/m2, diabetes mellitus, uncontrolled hypertension, known respiratory disease, heart failure or coronary disease, fever of at least 38.4° C within the last 48 hours, dyspnea at presentation, bicytopenia, pancytopenia, or the combination of high neutrophil count and low lymphocyte count.
Participants were randomly assigned to receive either placebo or colchicine 0.5 mg twice daily for 3 days and then once daily for another 27 days.
The number needed to prevent one COVID-19 complication is about 60 patients, Dr. Tardif said.
Colchicine was well tolerated and resulted in fewer serious adverse events than with placebo, he said. Diarrhea occurred more often with colchicine, but there was no increase in pneumonia. Caution should be used, however, in treating patients with severe renal disease.
Dr. Tardif said he would not prescribe colchicine to an 18-year-old COVID outpatient who doesn’t have any concomitant diseases, but would for those meeting the study protocol.
“As long as a patient appears to me to be at risk of a complication, I would prescribe it, without a doubt,” he said. “I can tell you that when we held the meeting with the DSMB Friday evening, I actually put each member on the spot and asked them: ‘If it were you – not even treating a patient, but if you had COVID today, would you take it based on the data you’ve seen?’ and all of the DSMB members said they would.
“So we’ll have that debate in the public domain when the paper is out, but I believe most physicians will use it to treat their patients.”
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the U.S. National Heart, Lung, and Blood Institute; Montreal philanthropist Sophie Desmarais; and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators.
A version of this article first appeared on Medscape.com.
New findings add to questions about existence of gouty nephropathy
Is gouty nephropathy real? It’s a question that has been posed often in rheumatology over the last several decades.
A new study found 36% of patients with untreated gout at a medical center in Vietnam have diffuse hyperechoic renal medulla as seen on ultrasound, which could indicate the presence of microcrystalline nephropathy. However, the results, published in Kidney International, may raise more questions than answers about the existence of gouty nephropathy and its relation to chronic kidney disease (CKD).
In their study, Thomas Bardin, MD, of the department of rheumatology at Lariboisière Hospital in Paris and colleagues evaluated 502 consecutive patients from Vien Gut Medical Center in Ho Chi Minh City, Vietnam, using B-mode renal ultrasound. The patients were mostly men with a median age of 46 years, body mass index of 25 kg/m2, estimated disease duration of 4 years, and uricemia of 423.2 micromol/L (7.11 mg/dL). Patients had a median estimated glomerular filtration rate (eGFR) of 78 mL/min per 1.73 m2. There was a history of hypertension in 112 patients (22.3%), type 2 diabetes in 58 patients (11.5%), renal lithiasis in 28 patients (5.6%), and coronary heart disease in 5 patients (1%).
While 39% of patients had previously used allopurinol for “a generally short period,” patients were not on urate-lowering therapy at the time of the study. Clinical tophi were present in 279 patients (55.6%), urate arthropathies in 154 patients (30.7%), and 43 patients (10.4%) used steroids daily.
B-mode renal ultrasound showed 181 patients (36%; 95% confidence interval, 32%-40%) had “hyperechoic pattern of Malpighi pyramids compared with the adjacent cortex,” which was “associated with twinkling artifacts” visible on color Doppler ultrasound. There was a significant association between renal medulla hyperechogenicity and patient age, disease duration, use of steroids, clinical tophi, and urate arthropathy (P less than .0001 for all). A significant association was also seen between renal medulla hyperechogenicity and decreased eGFR (P < .0001), proteinuria (P = .0006), leukocyturia (P = .0008), hypertension (P = .0008), hyperuricemia (P = .002), and coronary heart disease (P = .006).
In a multivariate analysis, there was a significant association between renal medulla hyperechogenicity and clinical tophi (odds ratio, 7.27; 95% CI, 3.68–15.19; P < .0001), urate arthropathy (OR, 3.46; 95% CI, 1.99–6.09; P < .0001), estimated gout duration (OR, 2.13; 95% CI, 1.55–2.96; P < .0001), double contour thickness (OR, 1.45; 95% CI, 1.06–1.97; P < .02), and eGFR (OR, 0.30; 95% CI, 0.09–0.89; P < .034).
“The finding was observed mainly in tophaceous gout, which involved a large proportion of our patients who had received very little treatment with urate-lowering drugs and was associated with moderately impaired renal function and urinary features compatible with tubulointerstitial nephritis,” Dr. Bardin and colleagues wrote in the study. The researchers also found “similar features” in 4 of 10 French patients at the Paris Necker Hospital in Paris, and noted that similar findings have been reported in Japan and Korea, which they said may mean hyperechoic medulla “is not unique to Vietnamese patients.”
Relation to CKD still unclear
In a related editorial, Federica Piani, MD, and Richard J. Johnson, MD, of the division of renal diseases and hypertension at the University of Colorado at Denver, Aurora, explained that gout was considered by some clinicians to be a cause of CKD in a time before urate-lowering therapies, because as many as 25% of patients with gout went on to experience kidney failure and about half experienced lower kidney function.
The association between gout and CKD was thought to be attributable to “frequent deposition of urate crystals in the tubular lumens and interstitium in the outer medulla of these patients,” but the concept was later challenged because “the crystals were generally found focally and did not readily explain the kidney damage.”
But even as interest in rheumatology moved away from the concept of gouty nephropathy to how serum uric acid impacts CKD, “the possibility that urate crystal deposition in the kidney could also be contributing to the kidney injury was never ruled out,” according to Dr. Piani and Dr. Johnson.
Kidney biopsies can sometimes miss urate crystals because the crystals dissolve if alcohol fixation is not used and because the biopsy site is often in the renal cortex, the authors noted. Recent research has identified that dual-energy CT scans can distinguish between calcium deposits and urate crystals better than ultrasound, and previous research from Thomas Bardin, MD, and colleagues in two patients noted a correlation between dual-energy CT scan findings of urate crystals and hyperechoic medulla findings on renal ultrasound.
The results by Dr. Bardin and associates, they said, “have reawakened the entity of urate microcrystalline nephropathy as a possible cause of CKD.”
Robert Terkeltaub, MD, professor of medicine at the University of California, San Diego, and section chief of Rheumatology at the San Diego VA Medical Center, said in an interview that he also believes the findings by Dr. Bardin and associates are real. He cited a study by Isabelle Ayoub, MD, and colleagues in Clinical Nephrology from 2016 that evaluated kidney biopsies in Germany and found medullary tophi were more likely to be present in patients with CKD than without.
“Chronic gouty nephropathy did not disappear. It still exists,” said Dr. Terkeltaub, who was not involved in the study by Dr. Bardin and colleagues.
The prospect that, if “you look hard enough, you’re going to see urate crystals and a pattern that’s attributed in the renal medulla” in patients with untreated gout is “very provocative, and interesting, and clinically relevant, and merits more investigation,” noted Dr. Terkeltaub, who is also president of the Gout, Hyperuricemia and Crystal-Associated Disease Network.
If verified, the results have important implications for patients with gout and its relationship to CKD, Dr. Terkeltaub said, but they raise “more questions than answers.
“I think it’s a really good wake-up call to start looking, doing good detective work here, and looking especially in people who have gout as opposed to just people with chronic kidney disease,” he said.
The authors reported no relevant conflicts of interest. Dr. Johnson, who coauthored an accompanying editorial, reported having equity in XORTX Therapeutics, serving as a consultant for Horizon Pharma, and having equity in Colorado Research Partners LLC. Dr. Terkeltaub reported receiving a research grant from AstraZeneca in the field of hyperuricemia and consultancies with AstraZeneca, Horizon, Sobi, Selecta Biosciences.
Is gouty nephropathy real? It’s a question that has been posed often in rheumatology over the last several decades.
A new study found 36% of patients with untreated gout at a medical center in Vietnam have diffuse hyperechoic renal medulla as seen on ultrasound, which could indicate the presence of microcrystalline nephropathy. However, the results, published in Kidney International, may raise more questions than answers about the existence of gouty nephropathy and its relation to chronic kidney disease (CKD).
In their study, Thomas Bardin, MD, of the department of rheumatology at Lariboisière Hospital in Paris and colleagues evaluated 502 consecutive patients from Vien Gut Medical Center in Ho Chi Minh City, Vietnam, using B-mode renal ultrasound. The patients were mostly men with a median age of 46 years, body mass index of 25 kg/m2, estimated disease duration of 4 years, and uricemia of 423.2 micromol/L (7.11 mg/dL). Patients had a median estimated glomerular filtration rate (eGFR) of 78 mL/min per 1.73 m2. There was a history of hypertension in 112 patients (22.3%), type 2 diabetes in 58 patients (11.5%), renal lithiasis in 28 patients (5.6%), and coronary heart disease in 5 patients (1%).
While 39% of patients had previously used allopurinol for “a generally short period,” patients were not on urate-lowering therapy at the time of the study. Clinical tophi were present in 279 patients (55.6%), urate arthropathies in 154 patients (30.7%), and 43 patients (10.4%) used steroids daily.
B-mode renal ultrasound showed 181 patients (36%; 95% confidence interval, 32%-40%) had “hyperechoic pattern of Malpighi pyramids compared with the adjacent cortex,” which was “associated with twinkling artifacts” visible on color Doppler ultrasound. There was a significant association between renal medulla hyperechogenicity and patient age, disease duration, use of steroids, clinical tophi, and urate arthropathy (P less than .0001 for all). A significant association was also seen between renal medulla hyperechogenicity and decreased eGFR (P < .0001), proteinuria (P = .0006), leukocyturia (P = .0008), hypertension (P = .0008), hyperuricemia (P = .002), and coronary heart disease (P = .006).
In a multivariate analysis, there was a significant association between renal medulla hyperechogenicity and clinical tophi (odds ratio, 7.27; 95% CI, 3.68–15.19; P < .0001), urate arthropathy (OR, 3.46; 95% CI, 1.99–6.09; P < .0001), estimated gout duration (OR, 2.13; 95% CI, 1.55–2.96; P < .0001), double contour thickness (OR, 1.45; 95% CI, 1.06–1.97; P < .02), and eGFR (OR, 0.30; 95% CI, 0.09–0.89; P < .034).
“The finding was observed mainly in tophaceous gout, which involved a large proportion of our patients who had received very little treatment with urate-lowering drugs and was associated with moderately impaired renal function and urinary features compatible with tubulointerstitial nephritis,” Dr. Bardin and colleagues wrote in the study. The researchers also found “similar features” in 4 of 10 French patients at the Paris Necker Hospital in Paris, and noted that similar findings have been reported in Japan and Korea, which they said may mean hyperechoic medulla “is not unique to Vietnamese patients.”
Relation to CKD still unclear
In a related editorial, Federica Piani, MD, and Richard J. Johnson, MD, of the division of renal diseases and hypertension at the University of Colorado at Denver, Aurora, explained that gout was considered by some clinicians to be a cause of CKD in a time before urate-lowering therapies, because as many as 25% of patients with gout went on to experience kidney failure and about half experienced lower kidney function.
The association between gout and CKD was thought to be attributable to “frequent deposition of urate crystals in the tubular lumens and interstitium in the outer medulla of these patients,” but the concept was later challenged because “the crystals were generally found focally and did not readily explain the kidney damage.”
But even as interest in rheumatology moved away from the concept of gouty nephropathy to how serum uric acid impacts CKD, “the possibility that urate crystal deposition in the kidney could also be contributing to the kidney injury was never ruled out,” according to Dr. Piani and Dr. Johnson.
Kidney biopsies can sometimes miss urate crystals because the crystals dissolve if alcohol fixation is not used and because the biopsy site is often in the renal cortex, the authors noted. Recent research has identified that dual-energy CT scans can distinguish between calcium deposits and urate crystals better than ultrasound, and previous research from Thomas Bardin, MD, and colleagues in two patients noted a correlation between dual-energy CT scan findings of urate crystals and hyperechoic medulla findings on renal ultrasound.
The results by Dr. Bardin and associates, they said, “have reawakened the entity of urate microcrystalline nephropathy as a possible cause of CKD.”
Robert Terkeltaub, MD, professor of medicine at the University of California, San Diego, and section chief of Rheumatology at the San Diego VA Medical Center, said in an interview that he also believes the findings by Dr. Bardin and associates are real. He cited a study by Isabelle Ayoub, MD, and colleagues in Clinical Nephrology from 2016 that evaluated kidney biopsies in Germany and found medullary tophi were more likely to be present in patients with CKD than without.
“Chronic gouty nephropathy did not disappear. It still exists,” said Dr. Terkeltaub, who was not involved in the study by Dr. Bardin and colleagues.
The prospect that, if “you look hard enough, you’re going to see urate crystals and a pattern that’s attributed in the renal medulla” in patients with untreated gout is “very provocative, and interesting, and clinically relevant, and merits more investigation,” noted Dr. Terkeltaub, who is also president of the Gout, Hyperuricemia and Crystal-Associated Disease Network.
If verified, the results have important implications for patients with gout and its relationship to CKD, Dr. Terkeltaub said, but they raise “more questions than answers.
“I think it’s a really good wake-up call to start looking, doing good detective work here, and looking especially in people who have gout as opposed to just people with chronic kidney disease,” he said.
The authors reported no relevant conflicts of interest. Dr. Johnson, who coauthored an accompanying editorial, reported having equity in XORTX Therapeutics, serving as a consultant for Horizon Pharma, and having equity in Colorado Research Partners LLC. Dr. Terkeltaub reported receiving a research grant from AstraZeneca in the field of hyperuricemia and consultancies with AstraZeneca, Horizon, Sobi, Selecta Biosciences.
Is gouty nephropathy real? It’s a question that has been posed often in rheumatology over the last several decades.
A new study found 36% of patients with untreated gout at a medical center in Vietnam have diffuse hyperechoic renal medulla as seen on ultrasound, which could indicate the presence of microcrystalline nephropathy. However, the results, published in Kidney International, may raise more questions than answers about the existence of gouty nephropathy and its relation to chronic kidney disease (CKD).
In their study, Thomas Bardin, MD, of the department of rheumatology at Lariboisière Hospital in Paris and colleagues evaluated 502 consecutive patients from Vien Gut Medical Center in Ho Chi Minh City, Vietnam, using B-mode renal ultrasound. The patients were mostly men with a median age of 46 years, body mass index of 25 kg/m2, estimated disease duration of 4 years, and uricemia of 423.2 micromol/L (7.11 mg/dL). Patients had a median estimated glomerular filtration rate (eGFR) of 78 mL/min per 1.73 m2. There was a history of hypertension in 112 patients (22.3%), type 2 diabetes in 58 patients (11.5%), renal lithiasis in 28 patients (5.6%), and coronary heart disease in 5 patients (1%).
While 39% of patients had previously used allopurinol for “a generally short period,” patients were not on urate-lowering therapy at the time of the study. Clinical tophi were present in 279 patients (55.6%), urate arthropathies in 154 patients (30.7%), and 43 patients (10.4%) used steroids daily.
B-mode renal ultrasound showed 181 patients (36%; 95% confidence interval, 32%-40%) had “hyperechoic pattern of Malpighi pyramids compared with the adjacent cortex,” which was “associated with twinkling artifacts” visible on color Doppler ultrasound. There was a significant association between renal medulla hyperechogenicity and patient age, disease duration, use of steroids, clinical tophi, and urate arthropathy (P less than .0001 for all). A significant association was also seen between renal medulla hyperechogenicity and decreased eGFR (P < .0001), proteinuria (P = .0006), leukocyturia (P = .0008), hypertension (P = .0008), hyperuricemia (P = .002), and coronary heart disease (P = .006).
In a multivariate analysis, there was a significant association between renal medulla hyperechogenicity and clinical tophi (odds ratio, 7.27; 95% CI, 3.68–15.19; P < .0001), urate arthropathy (OR, 3.46; 95% CI, 1.99–6.09; P < .0001), estimated gout duration (OR, 2.13; 95% CI, 1.55–2.96; P < .0001), double contour thickness (OR, 1.45; 95% CI, 1.06–1.97; P < .02), and eGFR (OR, 0.30; 95% CI, 0.09–0.89; P < .034).
“The finding was observed mainly in tophaceous gout, which involved a large proportion of our patients who had received very little treatment with urate-lowering drugs and was associated with moderately impaired renal function and urinary features compatible with tubulointerstitial nephritis,” Dr. Bardin and colleagues wrote in the study. The researchers also found “similar features” in 4 of 10 French patients at the Paris Necker Hospital in Paris, and noted that similar findings have been reported in Japan and Korea, which they said may mean hyperechoic medulla “is not unique to Vietnamese patients.”
Relation to CKD still unclear
In a related editorial, Federica Piani, MD, and Richard J. Johnson, MD, of the division of renal diseases and hypertension at the University of Colorado at Denver, Aurora, explained that gout was considered by some clinicians to be a cause of CKD in a time before urate-lowering therapies, because as many as 25% of patients with gout went on to experience kidney failure and about half experienced lower kidney function.
The association between gout and CKD was thought to be attributable to “frequent deposition of urate crystals in the tubular lumens and interstitium in the outer medulla of these patients,” but the concept was later challenged because “the crystals were generally found focally and did not readily explain the kidney damage.”
But even as interest in rheumatology moved away from the concept of gouty nephropathy to how serum uric acid impacts CKD, “the possibility that urate crystal deposition in the kidney could also be contributing to the kidney injury was never ruled out,” according to Dr. Piani and Dr. Johnson.
Kidney biopsies can sometimes miss urate crystals because the crystals dissolve if alcohol fixation is not used and because the biopsy site is often in the renal cortex, the authors noted. Recent research has identified that dual-energy CT scans can distinguish between calcium deposits and urate crystals better than ultrasound, and previous research from Thomas Bardin, MD, and colleagues in two patients noted a correlation between dual-energy CT scan findings of urate crystals and hyperechoic medulla findings on renal ultrasound.
The results by Dr. Bardin and associates, they said, “have reawakened the entity of urate microcrystalline nephropathy as a possible cause of CKD.”
Robert Terkeltaub, MD, professor of medicine at the University of California, San Diego, and section chief of Rheumatology at the San Diego VA Medical Center, said in an interview that he also believes the findings by Dr. Bardin and associates are real. He cited a study by Isabelle Ayoub, MD, and colleagues in Clinical Nephrology from 2016 that evaluated kidney biopsies in Germany and found medullary tophi were more likely to be present in patients with CKD than without.
“Chronic gouty nephropathy did not disappear. It still exists,” said Dr. Terkeltaub, who was not involved in the study by Dr. Bardin and colleagues.
The prospect that, if “you look hard enough, you’re going to see urate crystals and a pattern that’s attributed in the renal medulla” in patients with untreated gout is “very provocative, and interesting, and clinically relevant, and merits more investigation,” noted Dr. Terkeltaub, who is also president of the Gout, Hyperuricemia and Crystal-Associated Disease Network.
If verified, the results have important implications for patients with gout and its relationship to CKD, Dr. Terkeltaub said, but they raise “more questions than answers.
“I think it’s a really good wake-up call to start looking, doing good detective work here, and looking especially in people who have gout as opposed to just people with chronic kidney disease,” he said.
The authors reported no relevant conflicts of interest. Dr. Johnson, who coauthored an accompanying editorial, reported having equity in XORTX Therapeutics, serving as a consultant for Horizon Pharma, and having equity in Colorado Research Partners LLC. Dr. Terkeltaub reported receiving a research grant from AstraZeneca in the field of hyperuricemia and consultancies with AstraZeneca, Horizon, Sobi, Selecta Biosciences.
FROM KIDNEY INTERNATIONAL
EULAR recommendations define strategies to improve adherence in RMDs
Clinicians who care for patients with rheumatic and musculoskeletal diseases (RMDs) can now refer to a new set of strategies and points to consider from a European League Against Rheumatism (EULAR) task force in building a patient-centered approach to improve adherence to treatments.
Nonadherence to treatments is concerning given that 30%-80% of patients who have RMDs are thought to not follow a recommended treatment plan according to their physicians’ instructions, according to first author Valentin Ritschl of the Medical University of Vienna and colleagues.
“The problem of poor adherence is addressed in some EULAR recommendations/points to consider on the management of specific health conditions or on the role of professionals,” Mr. Ritschl said in an interview. “However, all these recommendations focus on limited aspects of nonadherence and do not cover the multifaceted nature of this phenomenon.”
Mr. Ritschl and colleagues conducted an extensive systematic literature review, the results of which they presented to a task force consisting of a panel of international experts hailing from 12 different countries. The task force included rheumatologists and other health professionals in rheumatology, as well as patient representatives.
The collaboration resulted in investigators crafting a definition of adherence in addition to drafting four overarching principles and nine points to consider, which were published Dec. 18 in Annals of the Rheumatic Diseases.
They defined adherence as “the extent to which a person’s behavior corresponds with the agreed prescription, of pharmacological or nonpharmacological treatments, by a health care provider.”
The four overarching principles emphasize the following concepts: that adherence affects outcomes in people who have RMDs; the importance of shared decision-making, with the understanding that the adherence describes the patient’s behavior “following an agreed prescription”; that numerous factors can affect adherence; and the notion of adherence being a dynamic process that, consequently, requires continuous evaluation.
Among the nine points to consider, Mr. Ritschl and coauthors encouraged all health care providers involved in caring for RMD patients to assume responsibility for promoting adherence. Practitioners should also strive to create an ongoing, open dialogue to discuss adherence, especially in cases in which the patient’s RMD is not well controlled. The patient-centered recommendations include taking into account the patient’s goals and preferences because these greatly contribute to the patient’s ability to adhere to any medication regimen. Another arm of that exploration also requires the medical professional to evaluate any circumstances that could bear a negative effect on the patient’s adherence – whether it be medication access issues related to cost or availability, or functional challenges such as memory, motivation, or complexity of the medication regimen.
Mr. Ritschl believed the task force’s recommendations will add value and help improve overall outcomes in RMD population management.
“Until today, there are no recommendations or points to consider developed in order to support our patients to be adherent to the agreed treatment plan,” he said. “In our project/initiative, we therefore developed for the first time points to consider to detect, assess, and manage nonadherence in people with RMDs.”
Additionally, the recommendations offer some strategic insights to help improve clinical trials because the deleterious effects of nonadherence also affect study results.
Looking ahead, Mr. Ritschl said randomized, controlled trials are necessary to test strategies that might improve adherence. He strongly emphasized the importance of designing future research studies that are heavily patient centered and effective for shared decision-making.
The project was funded by EULAR. Mr. Ritschl reported having no disclosures, but many of his coauthors reported financial relationships with pharmaceutical companies.
SOURCE: Ritschl V et al. Ann Rheum Dis. 2020 Dec 18. doi: 10.1136/annrheumdis-2020-218986.
Clinicians who care for patients with rheumatic and musculoskeletal diseases (RMDs) can now refer to a new set of strategies and points to consider from a European League Against Rheumatism (EULAR) task force in building a patient-centered approach to improve adherence to treatments.
Nonadherence to treatments is concerning given that 30%-80% of patients who have RMDs are thought to not follow a recommended treatment plan according to their physicians’ instructions, according to first author Valentin Ritschl of the Medical University of Vienna and colleagues.
“The problem of poor adherence is addressed in some EULAR recommendations/points to consider on the management of specific health conditions or on the role of professionals,” Mr. Ritschl said in an interview. “However, all these recommendations focus on limited aspects of nonadherence and do not cover the multifaceted nature of this phenomenon.”
Mr. Ritschl and colleagues conducted an extensive systematic literature review, the results of which they presented to a task force consisting of a panel of international experts hailing from 12 different countries. The task force included rheumatologists and other health professionals in rheumatology, as well as patient representatives.
The collaboration resulted in investigators crafting a definition of adherence in addition to drafting four overarching principles and nine points to consider, which were published Dec. 18 in Annals of the Rheumatic Diseases.
They defined adherence as “the extent to which a person’s behavior corresponds with the agreed prescription, of pharmacological or nonpharmacological treatments, by a health care provider.”
The four overarching principles emphasize the following concepts: that adherence affects outcomes in people who have RMDs; the importance of shared decision-making, with the understanding that the adherence describes the patient’s behavior “following an agreed prescription”; that numerous factors can affect adherence; and the notion of adherence being a dynamic process that, consequently, requires continuous evaluation.
Among the nine points to consider, Mr. Ritschl and coauthors encouraged all health care providers involved in caring for RMD patients to assume responsibility for promoting adherence. Practitioners should also strive to create an ongoing, open dialogue to discuss adherence, especially in cases in which the patient’s RMD is not well controlled. The patient-centered recommendations include taking into account the patient’s goals and preferences because these greatly contribute to the patient’s ability to adhere to any medication regimen. Another arm of that exploration also requires the medical professional to evaluate any circumstances that could bear a negative effect on the patient’s adherence – whether it be medication access issues related to cost or availability, or functional challenges such as memory, motivation, or complexity of the medication regimen.
Mr. Ritschl believed the task force’s recommendations will add value and help improve overall outcomes in RMD population management.
“Until today, there are no recommendations or points to consider developed in order to support our patients to be adherent to the agreed treatment plan,” he said. “In our project/initiative, we therefore developed for the first time points to consider to detect, assess, and manage nonadherence in people with RMDs.”
Additionally, the recommendations offer some strategic insights to help improve clinical trials because the deleterious effects of nonadherence also affect study results.
Looking ahead, Mr. Ritschl said randomized, controlled trials are necessary to test strategies that might improve adherence. He strongly emphasized the importance of designing future research studies that are heavily patient centered and effective for shared decision-making.
The project was funded by EULAR. Mr. Ritschl reported having no disclosures, but many of his coauthors reported financial relationships with pharmaceutical companies.
SOURCE: Ritschl V et al. Ann Rheum Dis. 2020 Dec 18. doi: 10.1136/annrheumdis-2020-218986.
Clinicians who care for patients with rheumatic and musculoskeletal diseases (RMDs) can now refer to a new set of strategies and points to consider from a European League Against Rheumatism (EULAR) task force in building a patient-centered approach to improve adherence to treatments.
Nonadherence to treatments is concerning given that 30%-80% of patients who have RMDs are thought to not follow a recommended treatment plan according to their physicians’ instructions, according to first author Valentin Ritschl of the Medical University of Vienna and colleagues.
“The problem of poor adherence is addressed in some EULAR recommendations/points to consider on the management of specific health conditions or on the role of professionals,” Mr. Ritschl said in an interview. “However, all these recommendations focus on limited aspects of nonadherence and do not cover the multifaceted nature of this phenomenon.”
Mr. Ritschl and colleagues conducted an extensive systematic literature review, the results of which they presented to a task force consisting of a panel of international experts hailing from 12 different countries. The task force included rheumatologists and other health professionals in rheumatology, as well as patient representatives.
The collaboration resulted in investigators crafting a definition of adherence in addition to drafting four overarching principles and nine points to consider, which were published Dec. 18 in Annals of the Rheumatic Diseases.
They defined adherence as “the extent to which a person’s behavior corresponds with the agreed prescription, of pharmacological or nonpharmacological treatments, by a health care provider.”
The four overarching principles emphasize the following concepts: that adherence affects outcomes in people who have RMDs; the importance of shared decision-making, with the understanding that the adherence describes the patient’s behavior “following an agreed prescription”; that numerous factors can affect adherence; and the notion of adherence being a dynamic process that, consequently, requires continuous evaluation.
Among the nine points to consider, Mr. Ritschl and coauthors encouraged all health care providers involved in caring for RMD patients to assume responsibility for promoting adherence. Practitioners should also strive to create an ongoing, open dialogue to discuss adherence, especially in cases in which the patient’s RMD is not well controlled. The patient-centered recommendations include taking into account the patient’s goals and preferences because these greatly contribute to the patient’s ability to adhere to any medication regimen. Another arm of that exploration also requires the medical professional to evaluate any circumstances that could bear a negative effect on the patient’s adherence – whether it be medication access issues related to cost or availability, or functional challenges such as memory, motivation, or complexity of the medication regimen.
Mr. Ritschl believed the task force’s recommendations will add value and help improve overall outcomes in RMD population management.
“Until today, there are no recommendations or points to consider developed in order to support our patients to be adherent to the agreed treatment plan,” he said. “In our project/initiative, we therefore developed for the first time points to consider to detect, assess, and manage nonadherence in people with RMDs.”
Additionally, the recommendations offer some strategic insights to help improve clinical trials because the deleterious effects of nonadherence also affect study results.
Looking ahead, Mr. Ritschl said randomized, controlled trials are necessary to test strategies that might improve adherence. He strongly emphasized the importance of designing future research studies that are heavily patient centered and effective for shared decision-making.
The project was funded by EULAR. Mr. Ritschl reported having no disclosures, but many of his coauthors reported financial relationships with pharmaceutical companies.
SOURCE: Ritschl V et al. Ann Rheum Dis. 2020 Dec 18. doi: 10.1136/annrheumdis-2020-218986.
FROM ANNALS OF THE RHEUMATIC DISEASES
Weight loss may be paramount lifestyle change in preventing gout
A recent analysis of the incidence of gout in men, published in JAMA Network Open, offers new insights on the role of lifestyle changes in preventing gout, particularly the importance of obesity and its modification.
Prior gout research, although it addressed lifestyle issues, had not quantified the impact of obesity on incident gout cases, noted first author Natalie McCormick, PhD, and colleagues at Harvard Medical School and Massachusetts General Hospital in Boston. “To date the proportion of actual gout itself that could potentially be prevented by modifying such risk factors remains unknown.” To address that lack of data, they set out to estimate the proportion of avoidable incident gout in a large database in the Health Professionals Follow-up Study, initially of some 51,529 male health professionals who have completed a biannual personal health questionnaire since 1986. The follow-up rate for completing these questionnaires exceeds 90%.
For their analysis, the researchers tracked 44,654 of these men, with an average age of 54 at the 1986 baseline and no history of gout, through the year 2012. They looked at four lifestyle risk factors attributed to gout: body mass index; alcohol intake; adherence to a Dietary Approach to Stop Hypertension (DASH)-style diet, which recommends less red meat and sweetened beverages and more fruits, vegetables, and low-fat dairy products; and the absence of diuretic drugs, which are used to treat blood pressure or heart failure, in order to observe and compare their effects on new reports of gout. Over the subsequent 26 years, nearly 4% of the men developed gout, the most common inflammatory arthritis. Obese men had 2.65 times greater risk for developing gout than did those with a normal body mass index.
If one addressed all four risk factors – modifying obesity, having no alcohol intake, not taking diuretic drugs, and following a DASH-style, lower-fat diet – 77% of new gout cases would disappear, the study’s corresponding author, Hyon K. Choi, MD, DrPH, of the division of rheumatology, allergy, and immunology at Massachusetts General Hospital, said in an interview. “But we learned that if you don’t include modifying obesity as a targetable risk factor, none of the other factors alone reaches significance. We can’t make firm conclusions about cause and effect, but modifying obesity seems to be a prerequisite to preventing gout through lifestyle. It’s a very interesting finding that needs to be confirmed in further research,” he said.
Of course, identifying the importance of lifestyle risk factors is not the same as actually achieving modifications of those factors. Changing lifestyle is difficult, Dr. Choi acknowledged. “But there’s not much potential for achieving the goal if the clinician doesn’t understand the target. Now we know obesity has a lot to do with gout. We can see it as a public health issue, especially since gout increases risks for comorbidities and mortality. All of these risk factors deserve intervention by the physician.”
A worldwide gout epidemic
Currently, there is a kind of worldwide gout epidemic linked to obesity, Dr. Choi said. The disease burden of gout is increasing worldwide. “This may be more of an issue for family practice or primary care physicians, who see 80%-90% of gout cases, rather than for rheumatologists, who are more likely to see advanced cases in need of drug therapy. But we would say: Don’t lose sight of the lifestyle risk factors, which are interrelated. This is not only the responsibility of one doctor or the other.”
The new findings should give practicing rheumatologists more confidence in addressing lifestyle issues, particularly weight loss, with their patients, said Angelo Gaffo, MD, section chief of rheumatology at the Birmingham VA Medical Center and associate professor of medicine in the division of rheumatology at the University of Alabama at Birmingham.
“Our patients with gout are interested in what they can do in their lives that might help with their gout. In the past, we’ve had generic advice about changing their diet. But in general, the evidence for the impact of dietary changes has not been strong.”
Doctors can now recommend a DASH-style diet, allowing room for moderate consumption of red meat, so long as patients are working on their weight loss – and showing results. “Now we have the information to give advice that’s more evidence-based,” Dr. Gaffo said. “You can ask the question whether this study is applicable to patients who already have gout. It doesn’t directly address them. But it mainly builds on the narrative that weight loss is important.”
Other studies have also looked at how weight loss led to serum urate reduction. This study adds to a growing body of literature emphasizing that the most important lifestyle factor relative to gout risk is weight gain, and the simplest, most effective intervention is counseling patients about weight loss, he said.
This research was supported by grants from the National Institutes of Health. Dr. Choi reported receiving research support from Ironwood and Horizon and consulting fees from Ironwood, Selecta, Horizon, Takeda, Kowa, and Vaxart. No other relevant financial disclosures were reported.
SOURCE: McCormick N et al. JAMA Netw Open. 2020;3(11):e2027421. doi: 10.1001/jamanetworkopen.2020.27421.
A recent analysis of the incidence of gout in men, published in JAMA Network Open, offers new insights on the role of lifestyle changes in preventing gout, particularly the importance of obesity and its modification.
Prior gout research, although it addressed lifestyle issues, had not quantified the impact of obesity on incident gout cases, noted first author Natalie McCormick, PhD, and colleagues at Harvard Medical School and Massachusetts General Hospital in Boston. “To date the proportion of actual gout itself that could potentially be prevented by modifying such risk factors remains unknown.” To address that lack of data, they set out to estimate the proportion of avoidable incident gout in a large database in the Health Professionals Follow-up Study, initially of some 51,529 male health professionals who have completed a biannual personal health questionnaire since 1986. The follow-up rate for completing these questionnaires exceeds 90%.
For their analysis, the researchers tracked 44,654 of these men, with an average age of 54 at the 1986 baseline and no history of gout, through the year 2012. They looked at four lifestyle risk factors attributed to gout: body mass index; alcohol intake; adherence to a Dietary Approach to Stop Hypertension (DASH)-style diet, which recommends less red meat and sweetened beverages and more fruits, vegetables, and low-fat dairy products; and the absence of diuretic drugs, which are used to treat blood pressure or heart failure, in order to observe and compare their effects on new reports of gout. Over the subsequent 26 years, nearly 4% of the men developed gout, the most common inflammatory arthritis. Obese men had 2.65 times greater risk for developing gout than did those with a normal body mass index.
If one addressed all four risk factors – modifying obesity, having no alcohol intake, not taking diuretic drugs, and following a DASH-style, lower-fat diet – 77% of new gout cases would disappear, the study’s corresponding author, Hyon K. Choi, MD, DrPH, of the division of rheumatology, allergy, and immunology at Massachusetts General Hospital, said in an interview. “But we learned that if you don’t include modifying obesity as a targetable risk factor, none of the other factors alone reaches significance. We can’t make firm conclusions about cause and effect, but modifying obesity seems to be a prerequisite to preventing gout through lifestyle. It’s a very interesting finding that needs to be confirmed in further research,” he said.
Of course, identifying the importance of lifestyle risk factors is not the same as actually achieving modifications of those factors. Changing lifestyle is difficult, Dr. Choi acknowledged. “But there’s not much potential for achieving the goal if the clinician doesn’t understand the target. Now we know obesity has a lot to do with gout. We can see it as a public health issue, especially since gout increases risks for comorbidities and mortality. All of these risk factors deserve intervention by the physician.”
A worldwide gout epidemic
Currently, there is a kind of worldwide gout epidemic linked to obesity, Dr. Choi said. The disease burden of gout is increasing worldwide. “This may be more of an issue for family practice or primary care physicians, who see 80%-90% of gout cases, rather than for rheumatologists, who are more likely to see advanced cases in need of drug therapy. But we would say: Don’t lose sight of the lifestyle risk factors, which are interrelated. This is not only the responsibility of one doctor or the other.”
The new findings should give practicing rheumatologists more confidence in addressing lifestyle issues, particularly weight loss, with their patients, said Angelo Gaffo, MD, section chief of rheumatology at the Birmingham VA Medical Center and associate professor of medicine in the division of rheumatology at the University of Alabama at Birmingham.
“Our patients with gout are interested in what they can do in their lives that might help with their gout. In the past, we’ve had generic advice about changing their diet. But in general, the evidence for the impact of dietary changes has not been strong.”
Doctors can now recommend a DASH-style diet, allowing room for moderate consumption of red meat, so long as patients are working on their weight loss – and showing results. “Now we have the information to give advice that’s more evidence-based,” Dr. Gaffo said. “You can ask the question whether this study is applicable to patients who already have gout. It doesn’t directly address them. But it mainly builds on the narrative that weight loss is important.”
Other studies have also looked at how weight loss led to serum urate reduction. This study adds to a growing body of literature emphasizing that the most important lifestyle factor relative to gout risk is weight gain, and the simplest, most effective intervention is counseling patients about weight loss, he said.
This research was supported by grants from the National Institutes of Health. Dr. Choi reported receiving research support from Ironwood and Horizon and consulting fees from Ironwood, Selecta, Horizon, Takeda, Kowa, and Vaxart. No other relevant financial disclosures were reported.
SOURCE: McCormick N et al. JAMA Netw Open. 2020;3(11):e2027421. doi: 10.1001/jamanetworkopen.2020.27421.
A recent analysis of the incidence of gout in men, published in JAMA Network Open, offers new insights on the role of lifestyle changes in preventing gout, particularly the importance of obesity and its modification.
Prior gout research, although it addressed lifestyle issues, had not quantified the impact of obesity on incident gout cases, noted first author Natalie McCormick, PhD, and colleagues at Harvard Medical School and Massachusetts General Hospital in Boston. “To date the proportion of actual gout itself that could potentially be prevented by modifying such risk factors remains unknown.” To address that lack of data, they set out to estimate the proportion of avoidable incident gout in a large database in the Health Professionals Follow-up Study, initially of some 51,529 male health professionals who have completed a biannual personal health questionnaire since 1986. The follow-up rate for completing these questionnaires exceeds 90%.
For their analysis, the researchers tracked 44,654 of these men, with an average age of 54 at the 1986 baseline and no history of gout, through the year 2012. They looked at four lifestyle risk factors attributed to gout: body mass index; alcohol intake; adherence to a Dietary Approach to Stop Hypertension (DASH)-style diet, which recommends less red meat and sweetened beverages and more fruits, vegetables, and low-fat dairy products; and the absence of diuretic drugs, which are used to treat blood pressure or heart failure, in order to observe and compare their effects on new reports of gout. Over the subsequent 26 years, nearly 4% of the men developed gout, the most common inflammatory arthritis. Obese men had 2.65 times greater risk for developing gout than did those with a normal body mass index.
If one addressed all four risk factors – modifying obesity, having no alcohol intake, not taking diuretic drugs, and following a DASH-style, lower-fat diet – 77% of new gout cases would disappear, the study’s corresponding author, Hyon K. Choi, MD, DrPH, of the division of rheumatology, allergy, and immunology at Massachusetts General Hospital, said in an interview. “But we learned that if you don’t include modifying obesity as a targetable risk factor, none of the other factors alone reaches significance. We can’t make firm conclusions about cause and effect, but modifying obesity seems to be a prerequisite to preventing gout through lifestyle. It’s a very interesting finding that needs to be confirmed in further research,” he said.
Of course, identifying the importance of lifestyle risk factors is not the same as actually achieving modifications of those factors. Changing lifestyle is difficult, Dr. Choi acknowledged. “But there’s not much potential for achieving the goal if the clinician doesn’t understand the target. Now we know obesity has a lot to do with gout. We can see it as a public health issue, especially since gout increases risks for comorbidities and mortality. All of these risk factors deserve intervention by the physician.”
A worldwide gout epidemic
Currently, there is a kind of worldwide gout epidemic linked to obesity, Dr. Choi said. The disease burden of gout is increasing worldwide. “This may be more of an issue for family practice or primary care physicians, who see 80%-90% of gout cases, rather than for rheumatologists, who are more likely to see advanced cases in need of drug therapy. But we would say: Don’t lose sight of the lifestyle risk factors, which are interrelated. This is not only the responsibility of one doctor or the other.”
The new findings should give practicing rheumatologists more confidence in addressing lifestyle issues, particularly weight loss, with their patients, said Angelo Gaffo, MD, section chief of rheumatology at the Birmingham VA Medical Center and associate professor of medicine in the division of rheumatology at the University of Alabama at Birmingham.
“Our patients with gout are interested in what they can do in their lives that might help with their gout. In the past, we’ve had generic advice about changing their diet. But in general, the evidence for the impact of dietary changes has not been strong.”
Doctors can now recommend a DASH-style diet, allowing room for moderate consumption of red meat, so long as patients are working on their weight loss – and showing results. “Now we have the information to give advice that’s more evidence-based,” Dr. Gaffo said. “You can ask the question whether this study is applicable to patients who already have gout. It doesn’t directly address them. But it mainly builds on the narrative that weight loss is important.”
Other studies have also looked at how weight loss led to serum urate reduction. This study adds to a growing body of literature emphasizing that the most important lifestyle factor relative to gout risk is weight gain, and the simplest, most effective intervention is counseling patients about weight loss, he said.
This research was supported by grants from the National Institutes of Health. Dr. Choi reported receiving research support from Ironwood and Horizon and consulting fees from Ironwood, Selecta, Horizon, Takeda, Kowa, and Vaxart. No other relevant financial disclosures were reported.
SOURCE: McCormick N et al. JAMA Netw Open. 2020;3(11):e2027421. doi: 10.1001/jamanetworkopen.2020.27421.
FROM JAMA NETWORK OPEN
Colchicine a case study for what’s wrong with U.S. drug pricing
Public spending on colchicine has grown exponentially over the past decade despite generics suggesting an uphill slog for patients seeking access to long-term therapy for gout or cardiac conditions.
Medicaid spending on single-ingredient colchicine jumped 2,833%, from $1.1 million in 2008 to $32.2 million in 2017, new findings show. Medicaid expansion likely played a role in the increase, but 58% was due to price hikes alone.
The centuries-old drug sold for pennies in the United States before increasing 50-fold to about $5 per pill in 2009 after the first FDA-approved colchicine product, Colcrys, was granted 3 years’ market exclusivity for the treatment of acute gout based on a 1-week trial.
If prices had remained at pre-Colcrys levels, Medicaid spending in 2017 would have totaled just $2.1 million rather than $32.2 million according to the analysis, published online Nov. 30 in JAMA Internal Medicine (doi: 10.1001/jamainternmed.2020.5017).
The study was motivated by difficulties gout patients have in accessing colchicine, but also last year’s COLCOT trial, which reported fewer ischemic cardiovascular events in patients receiving colchicine after MI, observed Natalie McCormick, PhD, of Massachusetts General Hospital and Harvard Medical School, both in Boston.
“They were suggesting it could be a cost-effective way for secondary prevention and it is fairly inexpensive in most countries, but not the U.S.,” she said in an interview. “So there’s really a potential to increase public spending if more and more patients are then taking colchicine for prevention of cardiovascular events and the prices don’t change.”
The current pandemic could potentially further increase demand. Results initially slated for September are expected this month from the COLCORONA trial, which is testing whether the anti-inflammatory agent can prevent hospitalizations, lung complications, and death when given early in the course of COVID-19.
University of Oxford (England) researchers also announced last week that colchicine is being added to the massive RECOVERY trial, which is studying treatments for hospitalized COVID-19 patients.
Notably, the Canadian-based COLCOT trial did not use Colcrys, but rather a colchicine product that costs just $0.26 a pill in Canada, roughly the price of most generics available worldwide.
Authorized generics typically drive down drug prices when competing with independent generics, but this competition is missing in the United States, where Colcrys holds patents until 2029, Dr. McCormick and colleagues noted. More than a half-dozen independent generics have FDA approval to date, but only authorized generics with price points set by the brand-name companies are available to treat acute gout, pericarditis, and potentially millions with MI.
“One of the key takeaways is this difference between the brand names and the authorized generics and the independents,” she said. “The authorized [generics] have really not saved money. The list prices were just slightly lower and patients can also have more difficulty in getting those covered.”
For this analysis, the investigators used Medicaid and Medicare data to examine prices for all available forms of colchicine from 2008 to 2017, including unregulated/unapproved colchicine (2008-2010), generic combination probenecid-colchicine (2008-2017), Colcrys (2009-2017), brand-name single-ingredient colchicine Mitigare (approved in late 2014 but not marketed until 2015), and their authorized generics (2015-2017). Medicare trends from 2012 to 2017 were analyzed separately because pre-Colcrys Medicare data were not available.
Based on the results, combined spending on Medicare and Medicaid claims for single-ingredient colchicine exceeded $340 million in 2017.
Inflation- and rebate-adjusted Medicaid unit prices rose from $0.24 a pill in 2008, when unapproved formulations were still available, to $4.20 a pill in 2011 (Colcrys only), and peaked at $4.66 a pill in 2015 (Colcrys plus authorized generics).
Prescribing of lower-priced probenecid-colchicine ($0.66/pill in 2017) remained stable throughout. Medicaid rebate-adjusted prices in 2017 were $3.99/pill for all single-ingredient colchicine products, $5.13/pill for Colcrys, $4.49/pill for Mitigare, and $3.88/pill for authorized generics.
Medicare rebate-adjusted 2017 per-pill prices were $5.81 for all single-ingredient colchicine products, $6.78 for Colcrys, $5.68 for Mitigare, $5.16 for authorized generics, and $0.70 for probenecid-colchicine.
“Authorized generics have still driven high spending,” Dr. McCormick said. “We really need to encourage more competition in order to improve access.”
In an accompanying commentary, B. Joseph Guglielmo, PharmD, University of California, San Francisco, pointed out that the estimated median research and development cost to bring a drug to market is between $985 million and $1,335 million, which inevitably translates into a high selling price for the drug. Such investment and its resultant cost, however, should be associated with potential worth to society.
“Only a fraction of an investment was required for Colcrys, a product that has provided no increased value and an unnecessary, long-term cost burden to the health care system,” he wrote. “The current study findings illustrate that we can never allow such an egregious case to take place again.”
Dr. McCormick reported grants from Canadian Institutes of Health Research during the conduct of the study. Dr. Guglielmo reported having no relevant conflicts of interest.
This article first appeared on Medscape.com.
Public spending on colchicine has grown exponentially over the past decade despite generics suggesting an uphill slog for patients seeking access to long-term therapy for gout or cardiac conditions.
Medicaid spending on single-ingredient colchicine jumped 2,833%, from $1.1 million in 2008 to $32.2 million in 2017, new findings show. Medicaid expansion likely played a role in the increase, but 58% was due to price hikes alone.
The centuries-old drug sold for pennies in the United States before increasing 50-fold to about $5 per pill in 2009 after the first FDA-approved colchicine product, Colcrys, was granted 3 years’ market exclusivity for the treatment of acute gout based on a 1-week trial.
If prices had remained at pre-Colcrys levels, Medicaid spending in 2017 would have totaled just $2.1 million rather than $32.2 million according to the analysis, published online Nov. 30 in JAMA Internal Medicine (doi: 10.1001/jamainternmed.2020.5017).
The study was motivated by difficulties gout patients have in accessing colchicine, but also last year’s COLCOT trial, which reported fewer ischemic cardiovascular events in patients receiving colchicine after MI, observed Natalie McCormick, PhD, of Massachusetts General Hospital and Harvard Medical School, both in Boston.
“They were suggesting it could be a cost-effective way for secondary prevention and it is fairly inexpensive in most countries, but not the U.S.,” she said in an interview. “So there’s really a potential to increase public spending if more and more patients are then taking colchicine for prevention of cardiovascular events and the prices don’t change.”
The current pandemic could potentially further increase demand. Results initially slated for September are expected this month from the COLCORONA trial, which is testing whether the anti-inflammatory agent can prevent hospitalizations, lung complications, and death when given early in the course of COVID-19.
University of Oxford (England) researchers also announced last week that colchicine is being added to the massive RECOVERY trial, which is studying treatments for hospitalized COVID-19 patients.
Notably, the Canadian-based COLCOT trial did not use Colcrys, but rather a colchicine product that costs just $0.26 a pill in Canada, roughly the price of most generics available worldwide.
Authorized generics typically drive down drug prices when competing with independent generics, but this competition is missing in the United States, where Colcrys holds patents until 2029, Dr. McCormick and colleagues noted. More than a half-dozen independent generics have FDA approval to date, but only authorized generics with price points set by the brand-name companies are available to treat acute gout, pericarditis, and potentially millions with MI.
“One of the key takeaways is this difference between the brand names and the authorized generics and the independents,” she said. “The authorized [generics] have really not saved money. The list prices were just slightly lower and patients can also have more difficulty in getting those covered.”
For this analysis, the investigators used Medicaid and Medicare data to examine prices for all available forms of colchicine from 2008 to 2017, including unregulated/unapproved colchicine (2008-2010), generic combination probenecid-colchicine (2008-2017), Colcrys (2009-2017), brand-name single-ingredient colchicine Mitigare (approved in late 2014 but not marketed until 2015), and their authorized generics (2015-2017). Medicare trends from 2012 to 2017 were analyzed separately because pre-Colcrys Medicare data were not available.
Based on the results, combined spending on Medicare and Medicaid claims for single-ingredient colchicine exceeded $340 million in 2017.
Inflation- and rebate-adjusted Medicaid unit prices rose from $0.24 a pill in 2008, when unapproved formulations were still available, to $4.20 a pill in 2011 (Colcrys only), and peaked at $4.66 a pill in 2015 (Colcrys plus authorized generics).
Prescribing of lower-priced probenecid-colchicine ($0.66/pill in 2017) remained stable throughout. Medicaid rebate-adjusted prices in 2017 were $3.99/pill for all single-ingredient colchicine products, $5.13/pill for Colcrys, $4.49/pill for Mitigare, and $3.88/pill for authorized generics.
Medicare rebate-adjusted 2017 per-pill prices were $5.81 for all single-ingredient colchicine products, $6.78 for Colcrys, $5.68 for Mitigare, $5.16 for authorized generics, and $0.70 for probenecid-colchicine.
“Authorized generics have still driven high spending,” Dr. McCormick said. “We really need to encourage more competition in order to improve access.”
In an accompanying commentary, B. Joseph Guglielmo, PharmD, University of California, San Francisco, pointed out that the estimated median research and development cost to bring a drug to market is between $985 million and $1,335 million, which inevitably translates into a high selling price for the drug. Such investment and its resultant cost, however, should be associated with potential worth to society.
“Only a fraction of an investment was required for Colcrys, a product that has provided no increased value and an unnecessary, long-term cost burden to the health care system,” he wrote. “The current study findings illustrate that we can never allow such an egregious case to take place again.”
Dr. McCormick reported grants from Canadian Institutes of Health Research during the conduct of the study. Dr. Guglielmo reported having no relevant conflicts of interest.
This article first appeared on Medscape.com.
Public spending on colchicine has grown exponentially over the past decade despite generics suggesting an uphill slog for patients seeking access to long-term therapy for gout or cardiac conditions.
Medicaid spending on single-ingredient colchicine jumped 2,833%, from $1.1 million in 2008 to $32.2 million in 2017, new findings show. Medicaid expansion likely played a role in the increase, but 58% was due to price hikes alone.
The centuries-old drug sold for pennies in the United States before increasing 50-fold to about $5 per pill in 2009 after the first FDA-approved colchicine product, Colcrys, was granted 3 years’ market exclusivity for the treatment of acute gout based on a 1-week trial.
If prices had remained at pre-Colcrys levels, Medicaid spending in 2017 would have totaled just $2.1 million rather than $32.2 million according to the analysis, published online Nov. 30 in JAMA Internal Medicine (doi: 10.1001/jamainternmed.2020.5017).
The study was motivated by difficulties gout patients have in accessing colchicine, but also last year’s COLCOT trial, which reported fewer ischemic cardiovascular events in patients receiving colchicine after MI, observed Natalie McCormick, PhD, of Massachusetts General Hospital and Harvard Medical School, both in Boston.
“They were suggesting it could be a cost-effective way for secondary prevention and it is fairly inexpensive in most countries, but not the U.S.,” she said in an interview. “So there’s really a potential to increase public spending if more and more patients are then taking colchicine for prevention of cardiovascular events and the prices don’t change.”
The current pandemic could potentially further increase demand. Results initially slated for September are expected this month from the COLCORONA trial, which is testing whether the anti-inflammatory agent can prevent hospitalizations, lung complications, and death when given early in the course of COVID-19.
University of Oxford (England) researchers also announced last week that colchicine is being added to the massive RECOVERY trial, which is studying treatments for hospitalized COVID-19 patients.
Notably, the Canadian-based COLCOT trial did not use Colcrys, but rather a colchicine product that costs just $0.26 a pill in Canada, roughly the price of most generics available worldwide.
Authorized generics typically drive down drug prices when competing with independent generics, but this competition is missing in the United States, where Colcrys holds patents until 2029, Dr. McCormick and colleagues noted. More than a half-dozen independent generics have FDA approval to date, but only authorized generics with price points set by the brand-name companies are available to treat acute gout, pericarditis, and potentially millions with MI.
“One of the key takeaways is this difference between the brand names and the authorized generics and the independents,” she said. “The authorized [generics] have really not saved money. The list prices were just slightly lower and patients can also have more difficulty in getting those covered.”
For this analysis, the investigators used Medicaid and Medicare data to examine prices for all available forms of colchicine from 2008 to 2017, including unregulated/unapproved colchicine (2008-2010), generic combination probenecid-colchicine (2008-2017), Colcrys (2009-2017), brand-name single-ingredient colchicine Mitigare (approved in late 2014 but not marketed until 2015), and their authorized generics (2015-2017). Medicare trends from 2012 to 2017 were analyzed separately because pre-Colcrys Medicare data were not available.
Based on the results, combined spending on Medicare and Medicaid claims for single-ingredient colchicine exceeded $340 million in 2017.
Inflation- and rebate-adjusted Medicaid unit prices rose from $0.24 a pill in 2008, when unapproved formulations were still available, to $4.20 a pill in 2011 (Colcrys only), and peaked at $4.66 a pill in 2015 (Colcrys plus authorized generics).
Prescribing of lower-priced probenecid-colchicine ($0.66/pill in 2017) remained stable throughout. Medicaid rebate-adjusted prices in 2017 were $3.99/pill for all single-ingredient colchicine products, $5.13/pill for Colcrys, $4.49/pill for Mitigare, and $3.88/pill for authorized generics.
Medicare rebate-adjusted 2017 per-pill prices were $5.81 for all single-ingredient colchicine products, $6.78 for Colcrys, $5.68 for Mitigare, $5.16 for authorized generics, and $0.70 for probenecid-colchicine.
“Authorized generics have still driven high spending,” Dr. McCormick said. “We really need to encourage more competition in order to improve access.”
In an accompanying commentary, B. Joseph Guglielmo, PharmD, University of California, San Francisco, pointed out that the estimated median research and development cost to bring a drug to market is between $985 million and $1,335 million, which inevitably translates into a high selling price for the drug. Such investment and its resultant cost, however, should be associated with potential worth to society.
“Only a fraction of an investment was required for Colcrys, a product that has provided no increased value and an unnecessary, long-term cost burden to the health care system,” he wrote. “The current study findings illustrate that we can never allow such an egregious case to take place again.”
Dr. McCormick reported grants from Canadian Institutes of Health Research during the conduct of the study. Dr. Guglielmo reported having no relevant conflicts of interest.
This article first appeared on Medscape.com.