User login
How should we monitor for ovarian cancer recurrence?
Several practice-changing developments in the treatment of ovarian cancer were seen in 2019, including the results of the pivotal trial Gynecologic Oncology Group (GOG)-213, which were published in November in the New England Journal of Medicine.1 This trial randomly assigned women with ovarian cancer who had achieved a remission of more than 6 months after primary therapy (“platinum sensitive”) to either a repeat surgical cytoreduction followed by chemotherapy versus chemotherapy alone. It found that the addition of surgery provided no benefit in overall survival, challenging the notion that repeat surgical “debulking” should be routinely considered for the treatment of women with platinum-sensitive ovarian cancer.
The primary treatment of ovarian cancer includes a combination of surgery and chemotherapy, after which the vast majority of patients will experience a complete clinical response, a so-called “remission.” At that time patients enter surveillance care, in which their providers evaluate them, typically every 3 months in the first 2-3 years. These visits are designed to address ongoing toxicities of therapy in addition to evaluation for recurrence. At these visits, it is common for providers to assess tumor markers, such as CA 125 (cancer antigen 125), if they had been elevated at original diagnosis. As a gynecologic oncologist, I can vouch for the fact that patients “sweat” on this lab result the most. No matter how reassuring my physical exams or their symptom profiles are, there is nothing more comforting as a normal, stable CA 125 value in black and white. However, and may, in fact, be harmful.
Providers have drawn tumor markers at surveillance exams under the working premise that abnormal or rising values signal the onset of asymptomatic recurrence, and that earlier treatment will be associated with better responses to salvage therapy. However, this has not been shown to be the case in randomized, controlled trials. In a large European cooperative-group trial, more than 500 patients with a history of completely treated ovarian cancer were randomized to either reinitiation of chemotherapy (salvage therapy) when CA 125 values first doubled or to reinitiation of therapy when they became symptomatic without knowledge of their CA 125 values.2 In this trial the mean survival of both groups was the same (26 months for the early initiation of chemotherapy vs. 27 for late initiation). However, what did differ were the quality of life scores, which were lower for the group who initiated chemotherapy earlier, likely because they received toxic therapies for longer periods of time.
The results of this trial were challenged by those who felt that this study did not evaluate the role that surgery might play. Their argument was that surgery in the recurrent setting would improve the outcomes from chemotherapy for certain patients with long platinum-free intervals (duration of remission since last receiving a platinum-containing drug), oligometastatic disease, and good performance status, just as it had in the primary setting. Retrospective series seemed to confirm this phenomenon, particularly if surgeons were able to achieve a complete resection (no residual measurable disease).3,4 By detecting asymptomatic patients with early elevations in CA 125, they proposed they might identify patients with lower disease burden in whom complete debulking would be more feasible. Whereas, in waiting for symptoms alone, they might “miss the boat,” and discover recurrence when it was too advanced to be completely resected.
The results of the GOG-213 study significantly challenge this line of thought, although with some caveats. Because this new trial showed no survival benefit for women with secondary debulking prior to chemotherapy, one could question whether there is any benefit in screening for asymptomatic, early recurrence. The authors of the study looked in subgroup analyses to attempt to identify groups who might benefit over others, such as women who had complete surgical cytoreduction (no residual disease) but still did not find a benefit to surgery. The trial population as a whole included women who had very favorable prognostic factors, including very long disease-free intervals (median, 20.4 months), and most women had only one or two sites of measurable recurrence. Yet it is remarkable that, in this group of patients who were predisposed to optimal outcomes, no benefit from surgery was observed.
However, it is important to recognize that the equivalent results of single-modality chemotherapy were achieved with the majority of women receiving bevacizumab with their chemotherapy regimen. An additional consideration is that the chemotherapy for platinum-sensitive, recurrent ovarian cancer has changed in recent years as we have learned the benefit of poly (ADP-ribose) polymerase (PARP) inhibitor drugs as maintenance therapy following complete or partial response to chemotherapy.5 It is unclear how the addition of PARP inhibitor maintenance therapy might have influenced the results of GOG-213. Further advancements in targeted therapies and consideration of hyperthermic intraperitoneal chemotherapy at the time of surgery also are being developed, and so, the answer of optimal therapy for platinum-sensitive ovarian cancer is a fluid one and might include a role for surgery for some of these patients.
However, in the meantime, before routinely ordering that tumor marker assessment in the surveillance period, it is important to remember that, if secondary cytoreduction is not beneficial and early initiation of chemotherapy is not helpful either, then these tumor marker results might provide more hindrance than help. Why search for recurrence at an earlier time point with CA 125 elevations if there isn’t a benefit to the patient in doing so? There certainly appears to be worse quality of life in doing so, and most likely also additional cost. Perhaps we should wait for clinical symptoms to confirm recurrence?
In the meantime, we will continue to have discussions with patients after primary therapy regarding how to best monitor them in the surveillance period. We will educate them about the limitations of early initiation of chemotherapy and the potentially limited role for surgery. Hopefully with individualized care and shared decision making, patients can guide us as to how they best be evaluated. While receiving a normal CA 125 result is powerfully reassuring, it is just as powerfully confusing and difficult for a patient to receive an abnormal one followed by a period of “doing nothing,” otherwise known as expectant management, if immediate treatment is not beneficial.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email her at [email protected].
References
1. N Engl J Med. 2019 Nov 14;381(20):1929-39.
2. Lancet. 2010 Oct 2;376(9747):1155-63.
3. Gynecol Oncol. 2009 Jan;112(1):265-74.
4. Br J Cancer. 2011 Sep 27;105(7):890-6.
5. N Engl J Med. 2016 Dec 1;375(22):2154-64.
Several practice-changing developments in the treatment of ovarian cancer were seen in 2019, including the results of the pivotal trial Gynecologic Oncology Group (GOG)-213, which were published in November in the New England Journal of Medicine.1 This trial randomly assigned women with ovarian cancer who had achieved a remission of more than 6 months after primary therapy (“platinum sensitive”) to either a repeat surgical cytoreduction followed by chemotherapy versus chemotherapy alone. It found that the addition of surgery provided no benefit in overall survival, challenging the notion that repeat surgical “debulking” should be routinely considered for the treatment of women with platinum-sensitive ovarian cancer.
The primary treatment of ovarian cancer includes a combination of surgery and chemotherapy, after which the vast majority of patients will experience a complete clinical response, a so-called “remission.” At that time patients enter surveillance care, in which their providers evaluate them, typically every 3 months in the first 2-3 years. These visits are designed to address ongoing toxicities of therapy in addition to evaluation for recurrence. At these visits, it is common for providers to assess tumor markers, such as CA 125 (cancer antigen 125), if they had been elevated at original diagnosis. As a gynecologic oncologist, I can vouch for the fact that patients “sweat” on this lab result the most. No matter how reassuring my physical exams or their symptom profiles are, there is nothing more comforting as a normal, stable CA 125 value in black and white. However, and may, in fact, be harmful.
Providers have drawn tumor markers at surveillance exams under the working premise that abnormal or rising values signal the onset of asymptomatic recurrence, and that earlier treatment will be associated with better responses to salvage therapy. However, this has not been shown to be the case in randomized, controlled trials. In a large European cooperative-group trial, more than 500 patients with a history of completely treated ovarian cancer were randomized to either reinitiation of chemotherapy (salvage therapy) when CA 125 values first doubled or to reinitiation of therapy when they became symptomatic without knowledge of their CA 125 values.2 In this trial the mean survival of both groups was the same (26 months for the early initiation of chemotherapy vs. 27 for late initiation). However, what did differ were the quality of life scores, which were lower for the group who initiated chemotherapy earlier, likely because they received toxic therapies for longer periods of time.
The results of this trial were challenged by those who felt that this study did not evaluate the role that surgery might play. Their argument was that surgery in the recurrent setting would improve the outcomes from chemotherapy for certain patients with long platinum-free intervals (duration of remission since last receiving a platinum-containing drug), oligometastatic disease, and good performance status, just as it had in the primary setting. Retrospective series seemed to confirm this phenomenon, particularly if surgeons were able to achieve a complete resection (no residual measurable disease).3,4 By detecting asymptomatic patients with early elevations in CA 125, they proposed they might identify patients with lower disease burden in whom complete debulking would be more feasible. Whereas, in waiting for symptoms alone, they might “miss the boat,” and discover recurrence when it was too advanced to be completely resected.
The results of the GOG-213 study significantly challenge this line of thought, although with some caveats. Because this new trial showed no survival benefit for women with secondary debulking prior to chemotherapy, one could question whether there is any benefit in screening for asymptomatic, early recurrence. The authors of the study looked in subgroup analyses to attempt to identify groups who might benefit over others, such as women who had complete surgical cytoreduction (no residual disease) but still did not find a benefit to surgery. The trial population as a whole included women who had very favorable prognostic factors, including very long disease-free intervals (median, 20.4 months), and most women had only one or two sites of measurable recurrence. Yet it is remarkable that, in this group of patients who were predisposed to optimal outcomes, no benefit from surgery was observed.
However, it is important to recognize that the equivalent results of single-modality chemotherapy were achieved with the majority of women receiving bevacizumab with their chemotherapy regimen. An additional consideration is that the chemotherapy for platinum-sensitive, recurrent ovarian cancer has changed in recent years as we have learned the benefit of poly (ADP-ribose) polymerase (PARP) inhibitor drugs as maintenance therapy following complete or partial response to chemotherapy.5 It is unclear how the addition of PARP inhibitor maintenance therapy might have influenced the results of GOG-213. Further advancements in targeted therapies and consideration of hyperthermic intraperitoneal chemotherapy at the time of surgery also are being developed, and so, the answer of optimal therapy for platinum-sensitive ovarian cancer is a fluid one and might include a role for surgery for some of these patients.
However, in the meantime, before routinely ordering that tumor marker assessment in the surveillance period, it is important to remember that, if secondary cytoreduction is not beneficial and early initiation of chemotherapy is not helpful either, then these tumor marker results might provide more hindrance than help. Why search for recurrence at an earlier time point with CA 125 elevations if there isn’t a benefit to the patient in doing so? There certainly appears to be worse quality of life in doing so, and most likely also additional cost. Perhaps we should wait for clinical symptoms to confirm recurrence?
In the meantime, we will continue to have discussions with patients after primary therapy regarding how to best monitor them in the surveillance period. We will educate them about the limitations of early initiation of chemotherapy and the potentially limited role for surgery. Hopefully with individualized care and shared decision making, patients can guide us as to how they best be evaluated. While receiving a normal CA 125 result is powerfully reassuring, it is just as powerfully confusing and difficult for a patient to receive an abnormal one followed by a period of “doing nothing,” otherwise known as expectant management, if immediate treatment is not beneficial.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email her at [email protected].
References
1. N Engl J Med. 2019 Nov 14;381(20):1929-39.
2. Lancet. 2010 Oct 2;376(9747):1155-63.
3. Gynecol Oncol. 2009 Jan;112(1):265-74.
4. Br J Cancer. 2011 Sep 27;105(7):890-6.
5. N Engl J Med. 2016 Dec 1;375(22):2154-64.
Several practice-changing developments in the treatment of ovarian cancer were seen in 2019, including the results of the pivotal trial Gynecologic Oncology Group (GOG)-213, which were published in November in the New England Journal of Medicine.1 This trial randomly assigned women with ovarian cancer who had achieved a remission of more than 6 months after primary therapy (“platinum sensitive”) to either a repeat surgical cytoreduction followed by chemotherapy versus chemotherapy alone. It found that the addition of surgery provided no benefit in overall survival, challenging the notion that repeat surgical “debulking” should be routinely considered for the treatment of women with platinum-sensitive ovarian cancer.
The primary treatment of ovarian cancer includes a combination of surgery and chemotherapy, after which the vast majority of patients will experience a complete clinical response, a so-called “remission.” At that time patients enter surveillance care, in which their providers evaluate them, typically every 3 months in the first 2-3 years. These visits are designed to address ongoing toxicities of therapy in addition to evaluation for recurrence. At these visits, it is common for providers to assess tumor markers, such as CA 125 (cancer antigen 125), if they had been elevated at original diagnosis. As a gynecologic oncologist, I can vouch for the fact that patients “sweat” on this lab result the most. No matter how reassuring my physical exams or their symptom profiles are, there is nothing more comforting as a normal, stable CA 125 value in black and white. However, and may, in fact, be harmful.
Providers have drawn tumor markers at surveillance exams under the working premise that abnormal or rising values signal the onset of asymptomatic recurrence, and that earlier treatment will be associated with better responses to salvage therapy. However, this has not been shown to be the case in randomized, controlled trials. In a large European cooperative-group trial, more than 500 patients with a history of completely treated ovarian cancer were randomized to either reinitiation of chemotherapy (salvage therapy) when CA 125 values first doubled or to reinitiation of therapy when they became symptomatic without knowledge of their CA 125 values.2 In this trial the mean survival of both groups was the same (26 months for the early initiation of chemotherapy vs. 27 for late initiation). However, what did differ were the quality of life scores, which were lower for the group who initiated chemotherapy earlier, likely because they received toxic therapies for longer periods of time.
The results of this trial were challenged by those who felt that this study did not evaluate the role that surgery might play. Their argument was that surgery in the recurrent setting would improve the outcomes from chemotherapy for certain patients with long platinum-free intervals (duration of remission since last receiving a platinum-containing drug), oligometastatic disease, and good performance status, just as it had in the primary setting. Retrospective series seemed to confirm this phenomenon, particularly if surgeons were able to achieve a complete resection (no residual measurable disease).3,4 By detecting asymptomatic patients with early elevations in CA 125, they proposed they might identify patients with lower disease burden in whom complete debulking would be more feasible. Whereas, in waiting for symptoms alone, they might “miss the boat,” and discover recurrence when it was too advanced to be completely resected.
The results of the GOG-213 study significantly challenge this line of thought, although with some caveats. Because this new trial showed no survival benefit for women with secondary debulking prior to chemotherapy, one could question whether there is any benefit in screening for asymptomatic, early recurrence. The authors of the study looked in subgroup analyses to attempt to identify groups who might benefit over others, such as women who had complete surgical cytoreduction (no residual disease) but still did not find a benefit to surgery. The trial population as a whole included women who had very favorable prognostic factors, including very long disease-free intervals (median, 20.4 months), and most women had only one or two sites of measurable recurrence. Yet it is remarkable that, in this group of patients who were predisposed to optimal outcomes, no benefit from surgery was observed.
However, it is important to recognize that the equivalent results of single-modality chemotherapy were achieved with the majority of women receiving bevacizumab with their chemotherapy regimen. An additional consideration is that the chemotherapy for platinum-sensitive, recurrent ovarian cancer has changed in recent years as we have learned the benefit of poly (ADP-ribose) polymerase (PARP) inhibitor drugs as maintenance therapy following complete or partial response to chemotherapy.5 It is unclear how the addition of PARP inhibitor maintenance therapy might have influenced the results of GOG-213. Further advancements in targeted therapies and consideration of hyperthermic intraperitoneal chemotherapy at the time of surgery also are being developed, and so, the answer of optimal therapy for platinum-sensitive ovarian cancer is a fluid one and might include a role for surgery for some of these patients.
However, in the meantime, before routinely ordering that tumor marker assessment in the surveillance period, it is important to remember that, if secondary cytoreduction is not beneficial and early initiation of chemotherapy is not helpful either, then these tumor marker results might provide more hindrance than help. Why search for recurrence at an earlier time point with CA 125 elevations if there isn’t a benefit to the patient in doing so? There certainly appears to be worse quality of life in doing so, and most likely also additional cost. Perhaps we should wait for clinical symptoms to confirm recurrence?
In the meantime, we will continue to have discussions with patients after primary therapy regarding how to best monitor them in the surveillance period. We will educate them about the limitations of early initiation of chemotherapy and the potentially limited role for surgery. Hopefully with individualized care and shared decision making, patients can guide us as to how they best be evaluated. While receiving a normal CA 125 result is powerfully reassuring, it is just as powerfully confusing and difficult for a patient to receive an abnormal one followed by a period of “doing nothing,” otherwise known as expectant management, if immediate treatment is not beneficial.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email her at [email protected].
References
1. N Engl J Med. 2019 Nov 14;381(20):1929-39.
2. Lancet. 2010 Oct 2;376(9747):1155-63.
3. Gynecol Oncol. 2009 Jan;112(1):265-74.
4. Br J Cancer. 2011 Sep 27;105(7):890-6.
5. N Engl J Med. 2016 Dec 1;375(22):2154-64.
In recurrent ovarian cancer, secondary surgery does not extend survival
Phase 3 findings ‘call into question’ merits of surgical cytoreduction
Secondary surgical cytoreduction was feasible but did not extend overall survival among women with platinum-sensitive, recurrent ovarian cancer in a prospective, randomized, phase 3 clinical trial, investigators report.
Women who received platinum-based chemotherapy plus surgery had a median overall survival of about 51 months, compared with 64.7 months for women who received platinum-based chemotherapy and no surgery, according to the results of the Gynecologic Oncology Group (GOG)-0213 study, a multicenter, open-label, randomized, phase 3 trial.
These findings “call into question” the merits of surgical cytoreduction, said the authors, led by Robert L. Coleman, MD, of the department of gynecologic oncology and reproductive medicine at the University of Texas M.D. Anderson Cancer Center, Houston.
Specifically, the shorter overall survival in the surgery group vs. no-surgery group emphasizes the “importance of formally assessing the value of the procedure in clinical care,” said Dr. Coleman and coauthors in the report on GOG-0213. The study was published in the New England Journal of Medicine.
Clinical practice guidelines from the National Comprehensive Cancer Network currently cite secondary cytoreduction as an option for treatment of patients who experience a treatment-free interval of at least 6 months after a complete remission achieved on prior chemotherapy, the GOG-0213 investigators wrote.
Beyond GOG-0213, there are several other randomized trials underway in this setting, including DESKTOP III, a multicenter study comparing the efficacy of chemotherapy alone to chemotherapy plus additional tumor debulking surgery in women with recurrent platinum-sensitive ovarian cancer.
wrote Dr. Coleman and colleagues.
The GOG-0213 study, conducted in 67 centers, 65 of which were in the United States, had both a chemotherapy objective and a surgical objective in patients with platinum-sensitive recurrent ovarian cancer, investigators said.
Results of the chemotherapy objective, published in 2017 in Lancet Oncology, indicated that bevacizumab added to standard chemotherapy, followed by maintenance bevacizumab until progression, improved median overall survival.
The more recently reported results focused on 485 women of who 245 were randomized to receive chemotherapy alone. While 240 were randomized to receive cytoreduction prior to chemotherapy, 15 declined surgery, leaving 225 eligible patients (94%).
The adjusted hazard ratio for death was 1.29 (95% confidence interval, 0.97-1.72; P = 0.08) for surgery, compared with no surgery, which translated into median overall survival times of 50.6 months in the surgery arm and 64.7 months in the no-surgery arm, Dr. Coleman and coauthors reported.
However, 30-day morbidity and mortality were low, at 9% (20 patients) and 0.4% (1 patient), and just 4% of cases (8 patients) were aborted, they added.
Quality of life significantly declined right after secondary cytoreduction, although after recovery no significant differences were found between groups, according to the investigators.
Taken together, those findings “did not indicate that surgery plus chemotherapy was superior to chemotherapy alone,” investigators concluded.
However, several factors in GOG-0213, including longer-than-expected survival times and substantial platinum sensitivity among women in the trial, could have diluted an independent surgical effect, they said.
Dr. Coleman reported disclosures related to several pharmaceutical companies, including Agenus, AstraZeneca, Clovis, GamaMabs, Genmab, Janssen, Medivation, Merck, Regeneron, Roche/Genentech, OncoQuest, and Tesaro.
SOURCE: Coleman RL et al. N Engl J Med. 2019;381:1929-39.
Phase 3 findings ‘call into question’ merits of surgical cytoreduction
Phase 3 findings ‘call into question’ merits of surgical cytoreduction
Secondary surgical cytoreduction was feasible but did not extend overall survival among women with platinum-sensitive, recurrent ovarian cancer in a prospective, randomized, phase 3 clinical trial, investigators report.
Women who received platinum-based chemotherapy plus surgery had a median overall survival of about 51 months, compared with 64.7 months for women who received platinum-based chemotherapy and no surgery, according to the results of the Gynecologic Oncology Group (GOG)-0213 study, a multicenter, open-label, randomized, phase 3 trial.
These findings “call into question” the merits of surgical cytoreduction, said the authors, led by Robert L. Coleman, MD, of the department of gynecologic oncology and reproductive medicine at the University of Texas M.D. Anderson Cancer Center, Houston.
Specifically, the shorter overall survival in the surgery group vs. no-surgery group emphasizes the “importance of formally assessing the value of the procedure in clinical care,” said Dr. Coleman and coauthors in the report on GOG-0213. The study was published in the New England Journal of Medicine.
Clinical practice guidelines from the National Comprehensive Cancer Network currently cite secondary cytoreduction as an option for treatment of patients who experience a treatment-free interval of at least 6 months after a complete remission achieved on prior chemotherapy, the GOG-0213 investigators wrote.
Beyond GOG-0213, there are several other randomized trials underway in this setting, including DESKTOP III, a multicenter study comparing the efficacy of chemotherapy alone to chemotherapy plus additional tumor debulking surgery in women with recurrent platinum-sensitive ovarian cancer.
wrote Dr. Coleman and colleagues.
The GOG-0213 study, conducted in 67 centers, 65 of which were in the United States, had both a chemotherapy objective and a surgical objective in patients with platinum-sensitive recurrent ovarian cancer, investigators said.
Results of the chemotherapy objective, published in 2017 in Lancet Oncology, indicated that bevacizumab added to standard chemotherapy, followed by maintenance bevacizumab until progression, improved median overall survival.
The more recently reported results focused on 485 women of who 245 were randomized to receive chemotherapy alone. While 240 were randomized to receive cytoreduction prior to chemotherapy, 15 declined surgery, leaving 225 eligible patients (94%).
The adjusted hazard ratio for death was 1.29 (95% confidence interval, 0.97-1.72; P = 0.08) for surgery, compared with no surgery, which translated into median overall survival times of 50.6 months in the surgery arm and 64.7 months in the no-surgery arm, Dr. Coleman and coauthors reported.
However, 30-day morbidity and mortality were low, at 9% (20 patients) and 0.4% (1 patient), and just 4% of cases (8 patients) were aborted, they added.
Quality of life significantly declined right after secondary cytoreduction, although after recovery no significant differences were found between groups, according to the investigators.
Taken together, those findings “did not indicate that surgery plus chemotherapy was superior to chemotherapy alone,” investigators concluded.
However, several factors in GOG-0213, including longer-than-expected survival times and substantial platinum sensitivity among women in the trial, could have diluted an independent surgical effect, they said.
Dr. Coleman reported disclosures related to several pharmaceutical companies, including Agenus, AstraZeneca, Clovis, GamaMabs, Genmab, Janssen, Medivation, Merck, Regeneron, Roche/Genentech, OncoQuest, and Tesaro.
SOURCE: Coleman RL et al. N Engl J Med. 2019;381:1929-39.
Secondary surgical cytoreduction was feasible but did not extend overall survival among women with platinum-sensitive, recurrent ovarian cancer in a prospective, randomized, phase 3 clinical trial, investigators report.
Women who received platinum-based chemotherapy plus surgery had a median overall survival of about 51 months, compared with 64.7 months for women who received platinum-based chemotherapy and no surgery, according to the results of the Gynecologic Oncology Group (GOG)-0213 study, a multicenter, open-label, randomized, phase 3 trial.
These findings “call into question” the merits of surgical cytoreduction, said the authors, led by Robert L. Coleman, MD, of the department of gynecologic oncology and reproductive medicine at the University of Texas M.D. Anderson Cancer Center, Houston.
Specifically, the shorter overall survival in the surgery group vs. no-surgery group emphasizes the “importance of formally assessing the value of the procedure in clinical care,” said Dr. Coleman and coauthors in the report on GOG-0213. The study was published in the New England Journal of Medicine.
Clinical practice guidelines from the National Comprehensive Cancer Network currently cite secondary cytoreduction as an option for treatment of patients who experience a treatment-free interval of at least 6 months after a complete remission achieved on prior chemotherapy, the GOG-0213 investigators wrote.
Beyond GOG-0213, there are several other randomized trials underway in this setting, including DESKTOP III, a multicenter study comparing the efficacy of chemotherapy alone to chemotherapy plus additional tumor debulking surgery in women with recurrent platinum-sensitive ovarian cancer.
wrote Dr. Coleman and colleagues.
The GOG-0213 study, conducted in 67 centers, 65 of which were in the United States, had both a chemotherapy objective and a surgical objective in patients with platinum-sensitive recurrent ovarian cancer, investigators said.
Results of the chemotherapy objective, published in 2017 in Lancet Oncology, indicated that bevacizumab added to standard chemotherapy, followed by maintenance bevacizumab until progression, improved median overall survival.
The more recently reported results focused on 485 women of who 245 were randomized to receive chemotherapy alone. While 240 were randomized to receive cytoreduction prior to chemotherapy, 15 declined surgery, leaving 225 eligible patients (94%).
The adjusted hazard ratio for death was 1.29 (95% confidence interval, 0.97-1.72; P = 0.08) for surgery, compared with no surgery, which translated into median overall survival times of 50.6 months in the surgery arm and 64.7 months in the no-surgery arm, Dr. Coleman and coauthors reported.
However, 30-day morbidity and mortality were low, at 9% (20 patients) and 0.4% (1 patient), and just 4% of cases (8 patients) were aborted, they added.
Quality of life significantly declined right after secondary cytoreduction, although after recovery no significant differences were found between groups, according to the investigators.
Taken together, those findings “did not indicate that surgery plus chemotherapy was superior to chemotherapy alone,” investigators concluded.
However, several factors in GOG-0213, including longer-than-expected survival times and substantial platinum sensitivity among women in the trial, could have diluted an independent surgical effect, they said.
Dr. Coleman reported disclosures related to several pharmaceutical companies, including Agenus, AstraZeneca, Clovis, GamaMabs, Genmab, Janssen, Medivation, Merck, Regeneron, Roche/Genentech, OncoQuest, and Tesaro.
SOURCE: Coleman RL et al. N Engl J Med. 2019;381:1929-39.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Surgical staging improves cervical cancer outcomes
VANCOUVER – Follow-up oncologic data from the UTERUS-11 trial shows advantages to surgical staging over clinical staging in stage IIB-IVA cervical cancer, with little apparent risk.
Compared with clinical staging using CT, laparoscopic staging led to an improvement in cancer-specific survival, with no delays in treatment or increases in toxicity. It also prompted surgical up-staging and led to treatment changes in 33% of cases. There was no difference in overall survival, but progression-free survival trended towards better outcomes in the surgical-staging group.
The new study presents 5-year follow-up data from patients randomly assigned to surgical (n = 121) or clinical staging (n = 114). The original study, published in 2017 (Oncology. 2017;92[4]:213-20), reported that 33% of surgical-staging patients in the surgical staging were up-staged as a result, compared with 6% who were revealed to have positive paraaortic lymph nodes through a CT-guided core biopsy after suspicious CT results. After a median follow-up of 90 months in both arms, overall survival was similar between the two groups, and progression-free survival trended towards an improvement in the surgical-staging group (P = .088). Cancer-specific survival was better in the surgical-staging arm, compared with clinical staging (P=.028), Audrey Tsunoda, MD, PhD, reported.
Surgical staging didn’t impact the toxicity profile, said Dr. Tsunoda, a surgical oncologist focused in gynecologic cancer surgery who practices at Hospital Erasto Gaertner in Curitiba, Brazil.
The mean time to initiation of chemoradiotherapy following surgery was 14 days (range, 7-21 days) after surgery: 64% had intensity-modulated radiotherapy and 36% had three-dimensional radiotherapy. There were no grade 5 toxicities during chemoradiotherapy and both groups had similar gastrointestinal and genitourinary toxicity profiles. About 97% of the surgical staging procedures were conducted laparoscopically. Two patients had a blood loss of more than 500 cc, and two had a delay to primary chemoradiotherapy (4 days and 5 days). One patient had to be converted to an open approach because of obesity and severe adhesions, and there was no intraoperative mortality.
Previous retrospective studies examining surgical staging in these patients led to confusion and disagreements among guidelines. Surgical staging is clearly associated with increased up-staging, but the oncologic benefit is uncertain. The LiLACS study attempted to address the question with prospective data, but failed to accrue enough patients and was later abandoned. That leaves the UTERUS-11 study, the initial results of which were published in 2017, as the first prospective study to examine the benefit of surgical staging.
The new follow-up results suggest a benefit to surgical staging, but they leave an important question unanswered, according to Lois Ramondetta, MD, professor of gynecologic oncology at the University of Texas MD Anderson Cancer Center, Houston, who served as a discussant at the meeting sponsored by AAGL. “Paraaortic lymph node status does connect to clinical benefit, but the question is really [whether] the removal of the lymph nodes accounts for the benefit, or is the identification of them and the change in treatment plan responsible? [If the latter is the case], a PET scan would have done a better job,” said Dr. Ramondetta. “The question remains unanswered, but I think this was huge progress in trying to answer it. Future studies need to incorporate a PET scan.”
Dr. Tsunoda has received honoraria from AstraZeneca and Roche. Dr. Ramondetta has no relevant financial disclosures.
VANCOUVER – Follow-up oncologic data from the UTERUS-11 trial shows advantages to surgical staging over clinical staging in stage IIB-IVA cervical cancer, with little apparent risk.
Compared with clinical staging using CT, laparoscopic staging led to an improvement in cancer-specific survival, with no delays in treatment or increases in toxicity. It also prompted surgical up-staging and led to treatment changes in 33% of cases. There was no difference in overall survival, but progression-free survival trended towards better outcomes in the surgical-staging group.
The new study presents 5-year follow-up data from patients randomly assigned to surgical (n = 121) or clinical staging (n = 114). The original study, published in 2017 (Oncology. 2017;92[4]:213-20), reported that 33% of surgical-staging patients in the surgical staging were up-staged as a result, compared with 6% who were revealed to have positive paraaortic lymph nodes through a CT-guided core biopsy after suspicious CT results. After a median follow-up of 90 months in both arms, overall survival was similar between the two groups, and progression-free survival trended towards an improvement in the surgical-staging group (P = .088). Cancer-specific survival was better in the surgical-staging arm, compared with clinical staging (P=.028), Audrey Tsunoda, MD, PhD, reported.
Surgical staging didn’t impact the toxicity profile, said Dr. Tsunoda, a surgical oncologist focused in gynecologic cancer surgery who practices at Hospital Erasto Gaertner in Curitiba, Brazil.
The mean time to initiation of chemoradiotherapy following surgery was 14 days (range, 7-21 days) after surgery: 64% had intensity-modulated radiotherapy and 36% had three-dimensional radiotherapy. There were no grade 5 toxicities during chemoradiotherapy and both groups had similar gastrointestinal and genitourinary toxicity profiles. About 97% of the surgical staging procedures were conducted laparoscopically. Two patients had a blood loss of more than 500 cc, and two had a delay to primary chemoradiotherapy (4 days and 5 days). One patient had to be converted to an open approach because of obesity and severe adhesions, and there was no intraoperative mortality.
Previous retrospective studies examining surgical staging in these patients led to confusion and disagreements among guidelines. Surgical staging is clearly associated with increased up-staging, but the oncologic benefit is uncertain. The LiLACS study attempted to address the question with prospective data, but failed to accrue enough patients and was later abandoned. That leaves the UTERUS-11 study, the initial results of which were published in 2017, as the first prospective study to examine the benefit of surgical staging.
The new follow-up results suggest a benefit to surgical staging, but they leave an important question unanswered, according to Lois Ramondetta, MD, professor of gynecologic oncology at the University of Texas MD Anderson Cancer Center, Houston, who served as a discussant at the meeting sponsored by AAGL. “Paraaortic lymph node status does connect to clinical benefit, but the question is really [whether] the removal of the lymph nodes accounts for the benefit, or is the identification of them and the change in treatment plan responsible? [If the latter is the case], a PET scan would have done a better job,” said Dr. Ramondetta. “The question remains unanswered, but I think this was huge progress in trying to answer it. Future studies need to incorporate a PET scan.”
Dr. Tsunoda has received honoraria from AstraZeneca and Roche. Dr. Ramondetta has no relevant financial disclosures.
VANCOUVER – Follow-up oncologic data from the UTERUS-11 trial shows advantages to surgical staging over clinical staging in stage IIB-IVA cervical cancer, with little apparent risk.
Compared with clinical staging using CT, laparoscopic staging led to an improvement in cancer-specific survival, with no delays in treatment or increases in toxicity. It also prompted surgical up-staging and led to treatment changes in 33% of cases. There was no difference in overall survival, but progression-free survival trended towards better outcomes in the surgical-staging group.
The new study presents 5-year follow-up data from patients randomly assigned to surgical (n = 121) or clinical staging (n = 114). The original study, published in 2017 (Oncology. 2017;92[4]:213-20), reported that 33% of surgical-staging patients in the surgical staging were up-staged as a result, compared with 6% who were revealed to have positive paraaortic lymph nodes through a CT-guided core biopsy after suspicious CT results. After a median follow-up of 90 months in both arms, overall survival was similar between the two groups, and progression-free survival trended towards an improvement in the surgical-staging group (P = .088). Cancer-specific survival was better in the surgical-staging arm, compared with clinical staging (P=.028), Audrey Tsunoda, MD, PhD, reported.
Surgical staging didn’t impact the toxicity profile, said Dr. Tsunoda, a surgical oncologist focused in gynecologic cancer surgery who practices at Hospital Erasto Gaertner in Curitiba, Brazil.
The mean time to initiation of chemoradiotherapy following surgery was 14 days (range, 7-21 days) after surgery: 64% had intensity-modulated radiotherapy and 36% had three-dimensional radiotherapy. There were no grade 5 toxicities during chemoradiotherapy and both groups had similar gastrointestinal and genitourinary toxicity profiles. About 97% of the surgical staging procedures were conducted laparoscopically. Two patients had a blood loss of more than 500 cc, and two had a delay to primary chemoradiotherapy (4 days and 5 days). One patient had to be converted to an open approach because of obesity and severe adhesions, and there was no intraoperative mortality.
Previous retrospective studies examining surgical staging in these patients led to confusion and disagreements among guidelines. Surgical staging is clearly associated with increased up-staging, but the oncologic benefit is uncertain. The LiLACS study attempted to address the question with prospective data, but failed to accrue enough patients and was later abandoned. That leaves the UTERUS-11 study, the initial results of which were published in 2017, as the first prospective study to examine the benefit of surgical staging.
The new follow-up results suggest a benefit to surgical staging, but they leave an important question unanswered, according to Lois Ramondetta, MD, professor of gynecologic oncology at the University of Texas MD Anderson Cancer Center, Houston, who served as a discussant at the meeting sponsored by AAGL. “Paraaortic lymph node status does connect to clinical benefit, but the question is really [whether] the removal of the lymph nodes accounts for the benefit, or is the identification of them and the change in treatment plan responsible? [If the latter is the case], a PET scan would have done a better job,” said Dr. Ramondetta. “The question remains unanswered, but I think this was huge progress in trying to answer it. Future studies need to incorporate a PET scan.”
Dr. Tsunoda has received honoraria from AstraZeneca and Roche. Dr. Ramondetta has no relevant financial disclosures.
REPORTING FROM THE AAGL GLOBAL CONGRESS
Sentinel node biopsy safe for women with vulval cancer
Women with vulval cancer who have a negative sentinel node biopsy have a low risk of recurrence and good disease-specific survival outcomes, investigators report.
One of two current standard treatment approaches for early-stage vulval cancer is radical excision of the tumor and inguinofemoral lymph node dissection, wrote Ligita P. Froeding, MD, of Copenhagen University Hospital Rigshospitalet, and coauthors. Their report is in Gynecologic Oncology. However, this procedure is associated with the disabling complication of leg lymphedema, which can significantly affect a woman’s quality of life.
Radical excision with sentinel biopsy is also an option, but the authors said large, population-based studies on the safety of this procedure when performed outside multicenter clinical trials were lacking.
In a prospective, nationwide cohort study, researchers analyzed data from 190 patients with vulval cancer who underwent the sentinel node procedure and had a negative biopsy. Of these, 73 patients had a unilateral procedure and 117 had a bilateral biopsy.
Over a median follow-up of 30 months’ follow-up, 32 patients (16.8%) died – 12 (37.5%) of vulval cancer and 20 (62.5%) from other causes. The 3-year overall survival rate was 84% and disease-specific survival was 93%.
The overall rate of recurrence in these sentinel node–negative women was 12.1% during the follow-up period. Fourteen patients (7.4%) experienced an isolated local vulval recurrence at a median time of 16 months after their primary treatment, and eight of these patients subsequently underwent inguinofemoral lymph node dissection following treatment of the recurrence. Three patients in this group died from vulval cancer, so the 3-year overall survival rate for patients with recurrent disease was 58%.
Four patients developed an isolated groin recurrence at a median of 12 months, and were treated with a combination of inguinofemoral lymph node dissection and chemoradiation. Two then developed a second recurrence.
Histopathological revision of original sentinel node specimens from the four women who experienced groin recurrences revealed that two patients actually had metastases at the time of the sentinel node procedure. In one case there were scattered tumor cells measuring less than 0.1 mm, while in the other there was a metastasis measuring 0.9 mm that was seen in six consecutive slides.
The authors noted that the failure to detect these metastases occurred despite strict adherence to histopathological procedure protocols. They suggested the first misdiagnosis may have been the result of the pathologist’s reluctance to make a histological diagnosis with so few tumor cells present. The second slide was originally screened microscopically by a specially trained medical laboratory technician, before being signed out by a pathologist, which “potentially decreases the pathologist’s diagnostic awareness,” they suggested.
“In conclusion, our study showed that the SN procedure is safe in selected VC patients when the current guidelines are strictly followed, and the procedure is performed in specialized gynecological oncology centers with a high volume of patients.”
No conflicts of interest were declared.
SOURCE: Froeding L et al. Gynecol Oncol 2019 Nov 8. doi: 10.1016/j.ygyno.2019.10.024.
Women with vulval cancer who have a negative sentinel node biopsy have a low risk of recurrence and good disease-specific survival outcomes, investigators report.
One of two current standard treatment approaches for early-stage vulval cancer is radical excision of the tumor and inguinofemoral lymph node dissection, wrote Ligita P. Froeding, MD, of Copenhagen University Hospital Rigshospitalet, and coauthors. Their report is in Gynecologic Oncology. However, this procedure is associated with the disabling complication of leg lymphedema, which can significantly affect a woman’s quality of life.
Radical excision with sentinel biopsy is also an option, but the authors said large, population-based studies on the safety of this procedure when performed outside multicenter clinical trials were lacking.
In a prospective, nationwide cohort study, researchers analyzed data from 190 patients with vulval cancer who underwent the sentinel node procedure and had a negative biopsy. Of these, 73 patients had a unilateral procedure and 117 had a bilateral biopsy.
Over a median follow-up of 30 months’ follow-up, 32 patients (16.8%) died – 12 (37.5%) of vulval cancer and 20 (62.5%) from other causes. The 3-year overall survival rate was 84% and disease-specific survival was 93%.
The overall rate of recurrence in these sentinel node–negative women was 12.1% during the follow-up period. Fourteen patients (7.4%) experienced an isolated local vulval recurrence at a median time of 16 months after their primary treatment, and eight of these patients subsequently underwent inguinofemoral lymph node dissection following treatment of the recurrence. Three patients in this group died from vulval cancer, so the 3-year overall survival rate for patients with recurrent disease was 58%.
Four patients developed an isolated groin recurrence at a median of 12 months, and were treated with a combination of inguinofemoral lymph node dissection and chemoradiation. Two then developed a second recurrence.
Histopathological revision of original sentinel node specimens from the four women who experienced groin recurrences revealed that two patients actually had metastases at the time of the sentinel node procedure. In one case there were scattered tumor cells measuring less than 0.1 mm, while in the other there was a metastasis measuring 0.9 mm that was seen in six consecutive slides.
The authors noted that the failure to detect these metastases occurred despite strict adherence to histopathological procedure protocols. They suggested the first misdiagnosis may have been the result of the pathologist’s reluctance to make a histological diagnosis with so few tumor cells present. The second slide was originally screened microscopically by a specially trained medical laboratory technician, before being signed out by a pathologist, which “potentially decreases the pathologist’s diagnostic awareness,” they suggested.
“In conclusion, our study showed that the SN procedure is safe in selected VC patients when the current guidelines are strictly followed, and the procedure is performed in specialized gynecological oncology centers with a high volume of patients.”
No conflicts of interest were declared.
SOURCE: Froeding L et al. Gynecol Oncol 2019 Nov 8. doi: 10.1016/j.ygyno.2019.10.024.
Women with vulval cancer who have a negative sentinel node biopsy have a low risk of recurrence and good disease-specific survival outcomes, investigators report.
One of two current standard treatment approaches for early-stage vulval cancer is radical excision of the tumor and inguinofemoral lymph node dissection, wrote Ligita P. Froeding, MD, of Copenhagen University Hospital Rigshospitalet, and coauthors. Their report is in Gynecologic Oncology. However, this procedure is associated with the disabling complication of leg lymphedema, which can significantly affect a woman’s quality of life.
Radical excision with sentinel biopsy is also an option, but the authors said large, population-based studies on the safety of this procedure when performed outside multicenter clinical trials were lacking.
In a prospective, nationwide cohort study, researchers analyzed data from 190 patients with vulval cancer who underwent the sentinel node procedure and had a negative biopsy. Of these, 73 patients had a unilateral procedure and 117 had a bilateral biopsy.
Over a median follow-up of 30 months’ follow-up, 32 patients (16.8%) died – 12 (37.5%) of vulval cancer and 20 (62.5%) from other causes. The 3-year overall survival rate was 84% and disease-specific survival was 93%.
The overall rate of recurrence in these sentinel node–negative women was 12.1% during the follow-up period. Fourteen patients (7.4%) experienced an isolated local vulval recurrence at a median time of 16 months after their primary treatment, and eight of these patients subsequently underwent inguinofemoral lymph node dissection following treatment of the recurrence. Three patients in this group died from vulval cancer, so the 3-year overall survival rate for patients with recurrent disease was 58%.
Four patients developed an isolated groin recurrence at a median of 12 months, and were treated with a combination of inguinofemoral lymph node dissection and chemoradiation. Two then developed a second recurrence.
Histopathological revision of original sentinel node specimens from the four women who experienced groin recurrences revealed that two patients actually had metastases at the time of the sentinel node procedure. In one case there were scattered tumor cells measuring less than 0.1 mm, while in the other there was a metastasis measuring 0.9 mm that was seen in six consecutive slides.
The authors noted that the failure to detect these metastases occurred despite strict adherence to histopathological procedure protocols. They suggested the first misdiagnosis may have been the result of the pathologist’s reluctance to make a histological diagnosis with so few tumor cells present. The second slide was originally screened microscopically by a specially trained medical laboratory technician, before being signed out by a pathologist, which “potentially decreases the pathologist’s diagnostic awareness,” they suggested.
“In conclusion, our study showed that the SN procedure is safe in selected VC patients when the current guidelines are strictly followed, and the procedure is performed in specialized gynecological oncology centers with a high volume of patients.”
No conflicts of interest were declared.
SOURCE: Froeding L et al. Gynecol Oncol 2019 Nov 8. doi: 10.1016/j.ygyno.2019.10.024.
FROM GYNECOLOGIC ONCOLOGY
Molecule exhibits activity in heavily pretreated, HER2-positive solid tumors
NATIONAL HARBOR, MD – PRS-343, a 4-1BB/HER2 bispecific molecule, has demonstrated safety and antitumor activity in patients with heavily pretreated, HER2-positive solid tumors, an investigator reported at the annual meeting of the Society for Immunotherapy of Cancer.
In a phase 1 trial of 18 evaluable patients, PRS-343 produced partial responses in 2 patients and enabled 8 patients to maintain stable disease. PRS-343 was considered well tolerated at all doses and schedules tested.
“PRS-343 is a bispecific construct targeting HER2 as well as 4-1BB,” said Geoffrey Y. Ku, MD, of Memorial Sloan Kettering Cancer Center in New York. “The HER2 component of the molecule localizes it into the tumor microenvironment of any HER2-positive cells. If the density of the HER2 protein is high enough, that facilitates cross-linkage of 4-1BB.
“4-1BB is an immune agonist that’s present in activated T cells, and cross-linkage helps to improve T-cell exhaustion and is also critical for T-cell expansion. The idea is that, by localizing 4-1BB activation to the tumor microenvironment, we can avoid some of the systemic toxicities associated with naked 4-1BB antibodies,” Dr. Ku added.
The ongoing, phase 1 trial of PRS-343 (NCT03330561) has enrolled 53 patients with a range of HER2-positive malignancies. To be eligible, patients must have progressed on standard therapy or have a tumor for which no standard therapy is available.
The most common diagnosis among enrolled patients is gastroesophageal cancer (n = 19), followed by breast cancer (n = 14), gynecologic cancers (n = 6), colorectal cancer (n = 5), and other malignancies.
The patients’ median age at baseline was 61 years (range, 29-92 years), and a majority were female (62%). Most patients (79%) had received three or more prior lines of therapy, including anti-HER2 treatments. Breast cancer patients had received a median of four anti-HER2 treatments, and gastric cancer patients had received a median of two.
The patients have been treated with PRS-343 at 11 dose levels, ranging from 0.0005 mg/kg to 8 mg/kg, given every 3 weeks. The highest dose, 8 mg/kg, was also given every 2 weeks.
Treatment-related adverse events (TRAEs) included infusion-related reactions (9%), fatigue (9%), chills (6%), flushing (6%), nausea (6%), diarrhea (6%), vomiting (5%), and noncardiac chest pain (4%).
“This was an extremely well-tolerated drug,” Dr. Ku said. “Out of 111 TRAEs, only a tiny proportion were grade 3, and toxicities mostly clustered around infusion-related reactions, constitutional symptoms, as well as gastrointestinal symptoms.”
Grade 3 TRAEs included infusion-related reactions (2%), fatigue (1%), flushing (3%), and noncardiac chest pain (1%). There were no grade 4-5 TRAEs.
At the data cutoff (Oct. 23, 2019), 18 patients were evaluable for a response at active dose levels (2.5 mg/kg, 5 mg/kg, and 8 mg/kg).
Two patients achieved a partial response, and eight had stable disease. “This translates to a response rate of 11% and a disease control rate of 55%,” Dr. Ku noted.
Both responders received PRS-343 at 8 mg/kg every 2 weeks. One of these patients had stage 4 gastric adenocarcinoma, and one had stage 4 gynecologic carcinoma.
Of the eight patients with stable disease, three received PRS-343 at 8 mg/kg every 2 weeks, two received 8 mg/kg every 3 weeks, one received the 5 mg/kg dose, and two received the 2.5 mg/kg dose.
Dr. Ku noted that the average time on treatment significantly increased in patients who received PRS-343 at 8 mg/kg every 2 weeks. Additionally, both responders and patients with stable disease had a “clear increase” in CD8+ T cells.
“[PRS-343] has demonstrated antitumor activity in heavily pretreated patients across multiple tumor types, and the treatment history, specifically the receipt of prior anti-HER2 therapy, indicates this is a 4-1BB-driven mechanism of action,” Dr. Ku said. “Based on these results, future studies are planned for continued development in defined HER2-positive indications.”
The current study is sponsored by Pieris Pharmaceuticals. Dr. Ku disclosed relationships with Arog Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Merck, Zymeworks, and Pieris Pharmaceuticals.
SOURCE: Ku GY et al. SITC 2019, Abstract O82.
NATIONAL HARBOR, MD – PRS-343, a 4-1BB/HER2 bispecific molecule, has demonstrated safety and antitumor activity in patients with heavily pretreated, HER2-positive solid tumors, an investigator reported at the annual meeting of the Society for Immunotherapy of Cancer.
In a phase 1 trial of 18 evaluable patients, PRS-343 produced partial responses in 2 patients and enabled 8 patients to maintain stable disease. PRS-343 was considered well tolerated at all doses and schedules tested.
“PRS-343 is a bispecific construct targeting HER2 as well as 4-1BB,” said Geoffrey Y. Ku, MD, of Memorial Sloan Kettering Cancer Center in New York. “The HER2 component of the molecule localizes it into the tumor microenvironment of any HER2-positive cells. If the density of the HER2 protein is high enough, that facilitates cross-linkage of 4-1BB.
“4-1BB is an immune agonist that’s present in activated T cells, and cross-linkage helps to improve T-cell exhaustion and is also critical for T-cell expansion. The idea is that, by localizing 4-1BB activation to the tumor microenvironment, we can avoid some of the systemic toxicities associated with naked 4-1BB antibodies,” Dr. Ku added.
The ongoing, phase 1 trial of PRS-343 (NCT03330561) has enrolled 53 patients with a range of HER2-positive malignancies. To be eligible, patients must have progressed on standard therapy or have a tumor for which no standard therapy is available.
The most common diagnosis among enrolled patients is gastroesophageal cancer (n = 19), followed by breast cancer (n = 14), gynecologic cancers (n = 6), colorectal cancer (n = 5), and other malignancies.
The patients’ median age at baseline was 61 years (range, 29-92 years), and a majority were female (62%). Most patients (79%) had received three or more prior lines of therapy, including anti-HER2 treatments. Breast cancer patients had received a median of four anti-HER2 treatments, and gastric cancer patients had received a median of two.
The patients have been treated with PRS-343 at 11 dose levels, ranging from 0.0005 mg/kg to 8 mg/kg, given every 3 weeks. The highest dose, 8 mg/kg, was also given every 2 weeks.
Treatment-related adverse events (TRAEs) included infusion-related reactions (9%), fatigue (9%), chills (6%), flushing (6%), nausea (6%), diarrhea (6%), vomiting (5%), and noncardiac chest pain (4%).
“This was an extremely well-tolerated drug,” Dr. Ku said. “Out of 111 TRAEs, only a tiny proportion were grade 3, and toxicities mostly clustered around infusion-related reactions, constitutional symptoms, as well as gastrointestinal symptoms.”
Grade 3 TRAEs included infusion-related reactions (2%), fatigue (1%), flushing (3%), and noncardiac chest pain (1%). There were no grade 4-5 TRAEs.
At the data cutoff (Oct. 23, 2019), 18 patients were evaluable for a response at active dose levels (2.5 mg/kg, 5 mg/kg, and 8 mg/kg).
Two patients achieved a partial response, and eight had stable disease. “This translates to a response rate of 11% and a disease control rate of 55%,” Dr. Ku noted.
Both responders received PRS-343 at 8 mg/kg every 2 weeks. One of these patients had stage 4 gastric adenocarcinoma, and one had stage 4 gynecologic carcinoma.
Of the eight patients with stable disease, three received PRS-343 at 8 mg/kg every 2 weeks, two received 8 mg/kg every 3 weeks, one received the 5 mg/kg dose, and two received the 2.5 mg/kg dose.
Dr. Ku noted that the average time on treatment significantly increased in patients who received PRS-343 at 8 mg/kg every 2 weeks. Additionally, both responders and patients with stable disease had a “clear increase” in CD8+ T cells.
“[PRS-343] has demonstrated antitumor activity in heavily pretreated patients across multiple tumor types, and the treatment history, specifically the receipt of prior anti-HER2 therapy, indicates this is a 4-1BB-driven mechanism of action,” Dr. Ku said. “Based on these results, future studies are planned for continued development in defined HER2-positive indications.”
The current study is sponsored by Pieris Pharmaceuticals. Dr. Ku disclosed relationships with Arog Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Merck, Zymeworks, and Pieris Pharmaceuticals.
SOURCE: Ku GY et al. SITC 2019, Abstract O82.
NATIONAL HARBOR, MD – PRS-343, a 4-1BB/HER2 bispecific molecule, has demonstrated safety and antitumor activity in patients with heavily pretreated, HER2-positive solid tumors, an investigator reported at the annual meeting of the Society for Immunotherapy of Cancer.
In a phase 1 trial of 18 evaluable patients, PRS-343 produced partial responses in 2 patients and enabled 8 patients to maintain stable disease. PRS-343 was considered well tolerated at all doses and schedules tested.
“PRS-343 is a bispecific construct targeting HER2 as well as 4-1BB,” said Geoffrey Y. Ku, MD, of Memorial Sloan Kettering Cancer Center in New York. “The HER2 component of the molecule localizes it into the tumor microenvironment of any HER2-positive cells. If the density of the HER2 protein is high enough, that facilitates cross-linkage of 4-1BB.
“4-1BB is an immune agonist that’s present in activated T cells, and cross-linkage helps to improve T-cell exhaustion and is also critical for T-cell expansion. The idea is that, by localizing 4-1BB activation to the tumor microenvironment, we can avoid some of the systemic toxicities associated with naked 4-1BB antibodies,” Dr. Ku added.
The ongoing, phase 1 trial of PRS-343 (NCT03330561) has enrolled 53 patients with a range of HER2-positive malignancies. To be eligible, patients must have progressed on standard therapy or have a tumor for which no standard therapy is available.
The most common diagnosis among enrolled patients is gastroesophageal cancer (n = 19), followed by breast cancer (n = 14), gynecologic cancers (n = 6), colorectal cancer (n = 5), and other malignancies.
The patients’ median age at baseline was 61 years (range, 29-92 years), and a majority were female (62%). Most patients (79%) had received three or more prior lines of therapy, including anti-HER2 treatments. Breast cancer patients had received a median of four anti-HER2 treatments, and gastric cancer patients had received a median of two.
The patients have been treated with PRS-343 at 11 dose levels, ranging from 0.0005 mg/kg to 8 mg/kg, given every 3 weeks. The highest dose, 8 mg/kg, was also given every 2 weeks.
Treatment-related adverse events (TRAEs) included infusion-related reactions (9%), fatigue (9%), chills (6%), flushing (6%), nausea (6%), diarrhea (6%), vomiting (5%), and noncardiac chest pain (4%).
“This was an extremely well-tolerated drug,” Dr. Ku said. “Out of 111 TRAEs, only a tiny proportion were grade 3, and toxicities mostly clustered around infusion-related reactions, constitutional symptoms, as well as gastrointestinal symptoms.”
Grade 3 TRAEs included infusion-related reactions (2%), fatigue (1%), flushing (3%), and noncardiac chest pain (1%). There were no grade 4-5 TRAEs.
At the data cutoff (Oct. 23, 2019), 18 patients were evaluable for a response at active dose levels (2.5 mg/kg, 5 mg/kg, and 8 mg/kg).
Two patients achieved a partial response, and eight had stable disease. “This translates to a response rate of 11% and a disease control rate of 55%,” Dr. Ku noted.
Both responders received PRS-343 at 8 mg/kg every 2 weeks. One of these patients had stage 4 gastric adenocarcinoma, and one had stage 4 gynecologic carcinoma.
Of the eight patients with stable disease, three received PRS-343 at 8 mg/kg every 2 weeks, two received 8 mg/kg every 3 weeks, one received the 5 mg/kg dose, and two received the 2.5 mg/kg dose.
Dr. Ku noted that the average time on treatment significantly increased in patients who received PRS-343 at 8 mg/kg every 2 weeks. Additionally, both responders and patients with stable disease had a “clear increase” in CD8+ T cells.
“[PRS-343] has demonstrated antitumor activity in heavily pretreated patients across multiple tumor types, and the treatment history, specifically the receipt of prior anti-HER2 therapy, indicates this is a 4-1BB-driven mechanism of action,” Dr. Ku said. “Based on these results, future studies are planned for continued development in defined HER2-positive indications.”
The current study is sponsored by Pieris Pharmaceuticals. Dr. Ku disclosed relationships with Arog Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Merck, Zymeworks, and Pieris Pharmaceuticals.
SOURCE: Ku GY et al. SITC 2019, Abstract O82.
REPORTING FROM SITC 2019
Does BSO status affect health outcomes for women taking estrogen for menopause?
Do health effects of menopausal estrogen therapy differ between women with bilateral oophorectomy versus those with conserved ovaries? To answer this question a group of investigators performed a subanalysis of the Women’s Health Initiative (WHI) Estrogen-Alone Trial,1 which included 40 clinical centers across the United States. They examined estrogen therapy outcomes by bilateral salpingo-oophorectomy (BSO) status, with additional stratification by 10-year age groups in 9,939 women aged 50 to 79 years with prior hysterectomy and known oophorectomy status. In the WHI trial, women were randomly assigned to conjugated equine estrogens (CEE) 0.625 mg/d or placebo for a median of 7.2 years. Investigators assessed the incidence of coronary heart disease and invasive breast cancer (the trial’s 2 primary end points), all-cause mortality, and a “global index”—these end points plus stroke, pulmonary embolism, colorectal cancer, and hip fracture—during the intervention phase and 18-year cumulative follow-up.
OBG Management caught up with lead author JoAnn E. Manson, MD, DrPH, NCMP, to discuss the study’s results.
OBG Management : How many women undergo BSO with their hysterectomy?
Dr. JoAnn E. Manson, MD, DrPH, NCMP: Of the 425,000 women who undergo hysterectomy in the United States for benign reasons each year,2,3 about 40% of them undergo BSO—so between 150,000 and 200,000 women per year undergo BSO with their hysterectomy.4,5
OBG Management : Although BSO is performed with hysterectomy to minimize patients’ future ovarian cancer risk, does BSO have health risks of its own, and how has estrogen been shown to affect these risks?
Dr. Manson: First, yes, BSO has been associated with health risks, especially when it is performed at a young age, such as before age 45. It has been linked to an increased risk of heart disease, osteoporosis, cognitive decline, and all-cause mortality. According to observational studies, estrogen therapy appears to offset many of these risks, particularly those related to heart disease and osteoporosis (the evidence is less clear on cognitive deficits).5
OBG Management : What did you find in your trial when you randomly assigned women in the age groups of 50 to 79 who underwent hysterectomy with and without BSO to estrogen therapy or placebo?
Dr. Manson: The WHI is the first study to be conducted in a randomized trial setting to analyze the health risks and benefits of estrogen therapy according to whether or not women had their ovaries removed. What we found was that the woman’s age had a strong influence on the effects of estrogen therapy among women who had BSO but only a negligible effect among women who had conserved ovaries. Overall, across the full age range, the effects of estrogen therapy did not differ substantially between women who had a BSO and those who had their ovaries conserved.
However, there were major differences by age group among the women who had BSO. A significant 32% reduction in all-cause mortality emerged during the 18-year follow-up period among the younger women (below age 60) who had BSO when they received estrogen therapy as compared with placebo. By contrast, the women who had conserved ovaries did not have this significant reduction in all-cause mortality, or in most of the other outcomes on estrogen compared with placebo. Overall, the effects of estrogen therapy tended to be relatively neutral in the women with conserved ovaries.
Now, the reduction in all-cause mortality with estrogen therapy was particularly pronounced among women who had BSO before age 45. They had a 40% statistically significant reduction in all-cause mortality with estrogen therapy compared with placebo. Also, among the women with BSO, there was a strong association between the timing of estrogen initiation and the magnitude of reduction in mortality. Women who started the estrogen therapy within 10 years of having the BSO had a 34% significant reduction in all-cause mortality, and those who started estrogen more than 20 years after having their ovaries removed had no reduction in mortality.
Continue to:
OBG Management : Do your data give support to the timing hypothesis?
Dr. Manson: Yes, our findings do support a timing hypothesis that was particularly pronounced for women who underwent BSO. It was the women who had early surgical menopause (before age 45) and those who started the estrogen therapy within 10 years of having their ovaries removed who had the greatest reduction in all-cause mortality and the most favorable benefit-risk profile from hormone therapy. So, the results do lend support to the timing hypothesis.
By contrast, women who had BSO at hysterectomy and began hormone therapy at age 70 or older had net adverse effects from hormone therapy. They posted a 40% increase in the global index—which is a summary measure of adverse effects on cardiovascular disease, cancer, and other major health outcomes. So, the women with BSO who were randomized in the trial at age 70 and older, had unfavorable results from estrogen therapy and an increase in the global index, in contrast to the women who were below age 60 or within 10 years of menopause.
OBG Management : Given your study findings, in which women would you recommend estrogen therapy? And are there groups of women in which you would advise avoiding estrogen therapy?
Dr. Manson: Current guidelines6,7 recommend estrogen therapy for women who have early menopause, particularly an early surgical menopause and BSO prior to the average age at natural menopause. Unless the woman has contraindications to estrogen therapy, the recommendations are to treat with estrogen until the average age of menopause—until about age 50 to 51.
Our study findings provide reassurance that, if a woman continues to have indications for estrogen (vasomotor symptoms, or other indications for estrogen therapy), there is relative safety of continuing estrogen-alone therapy through her 50s, until age 60. For example, a woman who, after the average age of menopause continues to have vasomotor symptoms, or if she has bone health problems, our study would suggest that estrogen therapy would continue to have a favorable benefit-risk profile until at least the age of 60. Decisions would have to be individualized, especially after age 60, with shared decision-making particularly important for those decisions. (Some women, depending on their risk profile, may continue to be candidates for estrogen therapy past age 60.)
So, this study provides reassurance regarding use of estrogen therapy for women in their 50s if they have had BSO. Actually, the women who had conserved ovaries also had relative safety with estrogen therapy until age 60. They just didn’t show the significant benefits for all-cause mortality. Overall, their pattern of health-related benefits and risks was neutral. Thus, if vasomotor symptom management, quality of life benefits, or bone health effects are sought, taking hormone therapy is a quite reasonable choice for these women.
By contrast, women who have had a BSO and are age 70 or older should really avoid initiating estrogen therapy because it would follow a prolonged period of estrogen deficiency, or very low estrogen levels, and these women appeared to have a net adverse effect from initiating hormone therapy (with increases in the global index found).
Continue to:
OBG Management : Did taking estrogen therapy prior to trial enrollment make a difference when it came to study outcomes?
Dr. Manson: We found minimal if any effect in our analyses. In fact, even the women who did not have prior (pre-randomization) use of estrogen therapy tended to do well on estrogen-alone therapy if they were younger than age 60. This was particularly true for the women who had BSO. Even if they had not used estrogen previously, and they were many years past the BSO, they still did well on estrogen therapy if they were below age 60.
1. Manson JE, Aragaki AK, Bassuk SS. Menopausal estrogen-alone therapy and health outcomes in women with and without bilateral oophorectomy: a randomized trial. Ann Intern Med. 2019 September 10. doi:10.7326/M19-0274.
2. Einarsson J. Are hysterectomy volumes in the US really falling? Contemporary OB/GYN. 1 September 2017. www.contemporaryobgyn.net/gynecology/are-hysterectomy-volumes-us-really-falling. November 4, 2019.
3. Temkin SM, Minasian L, Noone AM. The end of the hysterectomy epidemic and endometrial cancer incidence: what are the unintended consequences of declining hysterectomy rates? Front Oncol. 2016;6:89.
4. Doll KM, Dusetzina SB, Robinson W. Trends in inpatient and outpatient hysterectomy and oophorectomy rates among commercially insured women in the United States, 2000-2014. JAMA Surg. 2016;151:876-877.
5. Adelman MR, Sharp HT. Ovarian conservation vs removal at the time of benign hysterectomy. Am J Obstet Gynecol. 2018;218:269-279.
6. ACOG Practice Bulletin No. 141: management of menopausal symptoms [published corrections appear in: Obstet Gynecol. 2016;127(1):166. and Obstet Gynecol. 2018;131(3):604]. Obstet Gynecol. 2014;123:202-216.
7. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
Do health effects of menopausal estrogen therapy differ between women with bilateral oophorectomy versus those with conserved ovaries? To answer this question a group of investigators performed a subanalysis of the Women’s Health Initiative (WHI) Estrogen-Alone Trial,1 which included 40 clinical centers across the United States. They examined estrogen therapy outcomes by bilateral salpingo-oophorectomy (BSO) status, with additional stratification by 10-year age groups in 9,939 women aged 50 to 79 years with prior hysterectomy and known oophorectomy status. In the WHI trial, women were randomly assigned to conjugated equine estrogens (CEE) 0.625 mg/d or placebo for a median of 7.2 years. Investigators assessed the incidence of coronary heart disease and invasive breast cancer (the trial’s 2 primary end points), all-cause mortality, and a “global index”—these end points plus stroke, pulmonary embolism, colorectal cancer, and hip fracture—during the intervention phase and 18-year cumulative follow-up.
OBG Management caught up with lead author JoAnn E. Manson, MD, DrPH, NCMP, to discuss the study’s results.
OBG Management : How many women undergo BSO with their hysterectomy?
Dr. JoAnn E. Manson, MD, DrPH, NCMP: Of the 425,000 women who undergo hysterectomy in the United States for benign reasons each year,2,3 about 40% of them undergo BSO—so between 150,000 and 200,000 women per year undergo BSO with their hysterectomy.4,5
OBG Management : Although BSO is performed with hysterectomy to minimize patients’ future ovarian cancer risk, does BSO have health risks of its own, and how has estrogen been shown to affect these risks?
Dr. Manson: First, yes, BSO has been associated with health risks, especially when it is performed at a young age, such as before age 45. It has been linked to an increased risk of heart disease, osteoporosis, cognitive decline, and all-cause mortality. According to observational studies, estrogen therapy appears to offset many of these risks, particularly those related to heart disease and osteoporosis (the evidence is less clear on cognitive deficits).5
OBG Management : What did you find in your trial when you randomly assigned women in the age groups of 50 to 79 who underwent hysterectomy with and without BSO to estrogen therapy or placebo?
Dr. Manson: The WHI is the first study to be conducted in a randomized trial setting to analyze the health risks and benefits of estrogen therapy according to whether or not women had their ovaries removed. What we found was that the woman’s age had a strong influence on the effects of estrogen therapy among women who had BSO but only a negligible effect among women who had conserved ovaries. Overall, across the full age range, the effects of estrogen therapy did not differ substantially between women who had a BSO and those who had their ovaries conserved.
However, there were major differences by age group among the women who had BSO. A significant 32% reduction in all-cause mortality emerged during the 18-year follow-up period among the younger women (below age 60) who had BSO when they received estrogen therapy as compared with placebo. By contrast, the women who had conserved ovaries did not have this significant reduction in all-cause mortality, or in most of the other outcomes on estrogen compared with placebo. Overall, the effects of estrogen therapy tended to be relatively neutral in the women with conserved ovaries.
Now, the reduction in all-cause mortality with estrogen therapy was particularly pronounced among women who had BSO before age 45. They had a 40% statistically significant reduction in all-cause mortality with estrogen therapy compared with placebo. Also, among the women with BSO, there was a strong association between the timing of estrogen initiation and the magnitude of reduction in mortality. Women who started the estrogen therapy within 10 years of having the BSO had a 34% significant reduction in all-cause mortality, and those who started estrogen more than 20 years after having their ovaries removed had no reduction in mortality.
Continue to:
OBG Management : Do your data give support to the timing hypothesis?
Dr. Manson: Yes, our findings do support a timing hypothesis that was particularly pronounced for women who underwent BSO. It was the women who had early surgical menopause (before age 45) and those who started the estrogen therapy within 10 years of having their ovaries removed who had the greatest reduction in all-cause mortality and the most favorable benefit-risk profile from hormone therapy. So, the results do lend support to the timing hypothesis.
By contrast, women who had BSO at hysterectomy and began hormone therapy at age 70 or older had net adverse effects from hormone therapy. They posted a 40% increase in the global index—which is a summary measure of adverse effects on cardiovascular disease, cancer, and other major health outcomes. So, the women with BSO who were randomized in the trial at age 70 and older, had unfavorable results from estrogen therapy and an increase in the global index, in contrast to the women who were below age 60 or within 10 years of menopause.
OBG Management : Given your study findings, in which women would you recommend estrogen therapy? And are there groups of women in which you would advise avoiding estrogen therapy?
Dr. Manson: Current guidelines6,7 recommend estrogen therapy for women who have early menopause, particularly an early surgical menopause and BSO prior to the average age at natural menopause. Unless the woman has contraindications to estrogen therapy, the recommendations are to treat with estrogen until the average age of menopause—until about age 50 to 51.
Our study findings provide reassurance that, if a woman continues to have indications for estrogen (vasomotor symptoms, or other indications for estrogen therapy), there is relative safety of continuing estrogen-alone therapy through her 50s, until age 60. For example, a woman who, after the average age of menopause continues to have vasomotor symptoms, or if she has bone health problems, our study would suggest that estrogen therapy would continue to have a favorable benefit-risk profile until at least the age of 60. Decisions would have to be individualized, especially after age 60, with shared decision-making particularly important for those decisions. (Some women, depending on their risk profile, may continue to be candidates for estrogen therapy past age 60.)
So, this study provides reassurance regarding use of estrogen therapy for women in their 50s if they have had BSO. Actually, the women who had conserved ovaries also had relative safety with estrogen therapy until age 60. They just didn’t show the significant benefits for all-cause mortality. Overall, their pattern of health-related benefits and risks was neutral. Thus, if vasomotor symptom management, quality of life benefits, or bone health effects are sought, taking hormone therapy is a quite reasonable choice for these women.
By contrast, women who have had a BSO and are age 70 or older should really avoid initiating estrogen therapy because it would follow a prolonged period of estrogen deficiency, or very low estrogen levels, and these women appeared to have a net adverse effect from initiating hormone therapy (with increases in the global index found).
Continue to:
OBG Management : Did taking estrogen therapy prior to trial enrollment make a difference when it came to study outcomes?
Dr. Manson: We found minimal if any effect in our analyses. In fact, even the women who did not have prior (pre-randomization) use of estrogen therapy tended to do well on estrogen-alone therapy if they were younger than age 60. This was particularly true for the women who had BSO. Even if they had not used estrogen previously, and they were many years past the BSO, they still did well on estrogen therapy if they were below age 60.
Do health effects of menopausal estrogen therapy differ between women with bilateral oophorectomy versus those with conserved ovaries? To answer this question a group of investigators performed a subanalysis of the Women’s Health Initiative (WHI) Estrogen-Alone Trial,1 which included 40 clinical centers across the United States. They examined estrogen therapy outcomes by bilateral salpingo-oophorectomy (BSO) status, with additional stratification by 10-year age groups in 9,939 women aged 50 to 79 years with prior hysterectomy and known oophorectomy status. In the WHI trial, women were randomly assigned to conjugated equine estrogens (CEE) 0.625 mg/d or placebo for a median of 7.2 years. Investigators assessed the incidence of coronary heart disease and invasive breast cancer (the trial’s 2 primary end points), all-cause mortality, and a “global index”—these end points plus stroke, pulmonary embolism, colorectal cancer, and hip fracture—during the intervention phase and 18-year cumulative follow-up.
OBG Management caught up with lead author JoAnn E. Manson, MD, DrPH, NCMP, to discuss the study’s results.
OBG Management : How many women undergo BSO with their hysterectomy?
Dr. JoAnn E. Manson, MD, DrPH, NCMP: Of the 425,000 women who undergo hysterectomy in the United States for benign reasons each year,2,3 about 40% of them undergo BSO—so between 150,000 and 200,000 women per year undergo BSO with their hysterectomy.4,5
OBG Management : Although BSO is performed with hysterectomy to minimize patients’ future ovarian cancer risk, does BSO have health risks of its own, and how has estrogen been shown to affect these risks?
Dr. Manson: First, yes, BSO has been associated with health risks, especially when it is performed at a young age, such as before age 45. It has been linked to an increased risk of heart disease, osteoporosis, cognitive decline, and all-cause mortality. According to observational studies, estrogen therapy appears to offset many of these risks, particularly those related to heart disease and osteoporosis (the evidence is less clear on cognitive deficits).5
OBG Management : What did you find in your trial when you randomly assigned women in the age groups of 50 to 79 who underwent hysterectomy with and without BSO to estrogen therapy or placebo?
Dr. Manson: The WHI is the first study to be conducted in a randomized trial setting to analyze the health risks and benefits of estrogen therapy according to whether or not women had their ovaries removed. What we found was that the woman’s age had a strong influence on the effects of estrogen therapy among women who had BSO but only a negligible effect among women who had conserved ovaries. Overall, across the full age range, the effects of estrogen therapy did not differ substantially between women who had a BSO and those who had their ovaries conserved.
However, there were major differences by age group among the women who had BSO. A significant 32% reduction in all-cause mortality emerged during the 18-year follow-up period among the younger women (below age 60) who had BSO when they received estrogen therapy as compared with placebo. By contrast, the women who had conserved ovaries did not have this significant reduction in all-cause mortality, or in most of the other outcomes on estrogen compared with placebo. Overall, the effects of estrogen therapy tended to be relatively neutral in the women with conserved ovaries.
Now, the reduction in all-cause mortality with estrogen therapy was particularly pronounced among women who had BSO before age 45. They had a 40% statistically significant reduction in all-cause mortality with estrogen therapy compared with placebo. Also, among the women with BSO, there was a strong association between the timing of estrogen initiation and the magnitude of reduction in mortality. Women who started the estrogen therapy within 10 years of having the BSO had a 34% significant reduction in all-cause mortality, and those who started estrogen more than 20 years after having their ovaries removed had no reduction in mortality.
Continue to:
OBG Management : Do your data give support to the timing hypothesis?
Dr. Manson: Yes, our findings do support a timing hypothesis that was particularly pronounced for women who underwent BSO. It was the women who had early surgical menopause (before age 45) and those who started the estrogen therapy within 10 years of having their ovaries removed who had the greatest reduction in all-cause mortality and the most favorable benefit-risk profile from hormone therapy. So, the results do lend support to the timing hypothesis.
By contrast, women who had BSO at hysterectomy and began hormone therapy at age 70 or older had net adverse effects from hormone therapy. They posted a 40% increase in the global index—which is a summary measure of adverse effects on cardiovascular disease, cancer, and other major health outcomes. So, the women with BSO who were randomized in the trial at age 70 and older, had unfavorable results from estrogen therapy and an increase in the global index, in contrast to the women who were below age 60 or within 10 years of menopause.
OBG Management : Given your study findings, in which women would you recommend estrogen therapy? And are there groups of women in which you would advise avoiding estrogen therapy?
Dr. Manson: Current guidelines6,7 recommend estrogen therapy for women who have early menopause, particularly an early surgical menopause and BSO prior to the average age at natural menopause. Unless the woman has contraindications to estrogen therapy, the recommendations are to treat with estrogen until the average age of menopause—until about age 50 to 51.
Our study findings provide reassurance that, if a woman continues to have indications for estrogen (vasomotor symptoms, or other indications for estrogen therapy), there is relative safety of continuing estrogen-alone therapy through her 50s, until age 60. For example, a woman who, after the average age of menopause continues to have vasomotor symptoms, or if she has bone health problems, our study would suggest that estrogen therapy would continue to have a favorable benefit-risk profile until at least the age of 60. Decisions would have to be individualized, especially after age 60, with shared decision-making particularly important for those decisions. (Some women, depending on their risk profile, may continue to be candidates for estrogen therapy past age 60.)
So, this study provides reassurance regarding use of estrogen therapy for women in their 50s if they have had BSO. Actually, the women who had conserved ovaries also had relative safety with estrogen therapy until age 60. They just didn’t show the significant benefits for all-cause mortality. Overall, their pattern of health-related benefits and risks was neutral. Thus, if vasomotor symptom management, quality of life benefits, or bone health effects are sought, taking hormone therapy is a quite reasonable choice for these women.
By contrast, women who have had a BSO and are age 70 or older should really avoid initiating estrogen therapy because it would follow a prolonged period of estrogen deficiency, or very low estrogen levels, and these women appeared to have a net adverse effect from initiating hormone therapy (with increases in the global index found).
Continue to:
OBG Management : Did taking estrogen therapy prior to trial enrollment make a difference when it came to study outcomes?
Dr. Manson: We found minimal if any effect in our analyses. In fact, even the women who did not have prior (pre-randomization) use of estrogen therapy tended to do well on estrogen-alone therapy if they were younger than age 60. This was particularly true for the women who had BSO. Even if they had not used estrogen previously, and they were many years past the BSO, they still did well on estrogen therapy if they were below age 60.
1. Manson JE, Aragaki AK, Bassuk SS. Menopausal estrogen-alone therapy and health outcomes in women with and without bilateral oophorectomy: a randomized trial. Ann Intern Med. 2019 September 10. doi:10.7326/M19-0274.
2. Einarsson J. Are hysterectomy volumes in the US really falling? Contemporary OB/GYN. 1 September 2017. www.contemporaryobgyn.net/gynecology/are-hysterectomy-volumes-us-really-falling. November 4, 2019.
3. Temkin SM, Minasian L, Noone AM. The end of the hysterectomy epidemic and endometrial cancer incidence: what are the unintended consequences of declining hysterectomy rates? Front Oncol. 2016;6:89.
4. Doll KM, Dusetzina SB, Robinson W. Trends in inpatient and outpatient hysterectomy and oophorectomy rates among commercially insured women in the United States, 2000-2014. JAMA Surg. 2016;151:876-877.
5. Adelman MR, Sharp HT. Ovarian conservation vs removal at the time of benign hysterectomy. Am J Obstet Gynecol. 2018;218:269-279.
6. ACOG Practice Bulletin No. 141: management of menopausal symptoms [published corrections appear in: Obstet Gynecol. 2016;127(1):166. and Obstet Gynecol. 2018;131(3):604]. Obstet Gynecol. 2014;123:202-216.
7. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
1. Manson JE, Aragaki AK, Bassuk SS. Menopausal estrogen-alone therapy and health outcomes in women with and without bilateral oophorectomy: a randomized trial. Ann Intern Med. 2019 September 10. doi:10.7326/M19-0274.
2. Einarsson J. Are hysterectomy volumes in the US really falling? Contemporary OB/GYN. 1 September 2017. www.contemporaryobgyn.net/gynecology/are-hysterectomy-volumes-us-really-falling. November 4, 2019.
3. Temkin SM, Minasian L, Noone AM. The end of the hysterectomy epidemic and endometrial cancer incidence: what are the unintended consequences of declining hysterectomy rates? Front Oncol. 2016;6:89.
4. Doll KM, Dusetzina SB, Robinson W. Trends in inpatient and outpatient hysterectomy and oophorectomy rates among commercially insured women in the United States, 2000-2014. JAMA Surg. 2016;151:876-877.
5. Adelman MR, Sharp HT. Ovarian conservation vs removal at the time of benign hysterectomy. Am J Obstet Gynecol. 2018;218:269-279.
6. ACOG Practice Bulletin No. 141: management of menopausal symptoms [published corrections appear in: Obstet Gynecol. 2016;127(1):166. and Obstet Gynecol. 2018;131(3):604]. Obstet Gynecol. 2014;123:202-216.
7. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
Disparity in endometrial cancer outcomes: What can we do?
While the incidence of most cancers is falling, endometrial cancer rates continue to rise, in large part because of increasing life expectancy and obesity rates. However, what is even more alarming is the observation that there is a clear disparity in outcomes between black and white women with this disease. But there are things that all health care providers, including nononcologists, can do to help to overcome this disparity.
Black women are nearly twice as likely as non-Hispanic white women to die from the endometrial cancer. The 5-year survival for stage III and IV cancer is 43% for non-Hispanic white women, yet only 25% for black women.1 For a long time, this survival disparity was assumed to be a function of the more aggressive cancer histologies, such as serous, which are more commonly seen in black women. These high-grade cancers are more likely to present in advanced stages and with poorer responses to treatments; however, the predisposition to aggressive cancers tells only part of the story of racial disparities in endometrial cancer and their presentation at later stages. Indeed, fueling the problem are the findings that black women report symptoms less, experience more delays in diagnosis or more frequent deviations from guideline-directed diagnostics, undergo more morbid surgical approaches, receive less surgical staging, are enrolled less in clinical trials, have lower socioeconomic status and lower rates of health insurance, and receive less differential administration of adjuvant therapies, as well as have a background of higher all-cause mortality and comorbidities. While this array of contributing factors may seem overwhelming, it also can be considered a guide for health care providers because most of these factors, unlike histologic cell type, are modifiable, and it is important that we all consider what role we can play in dismantling them.
Black women are less likely to receive guideline-recommended care upon presentation. Research by Kemi M. Doll, MD, from the University of Washington, Seattle, demonstrated that, among women with endometrial cancers, black women were less likely to have documented histories of postmenopausal bleeding within 2 years of the diagnosis, presumably because of factors related to underreporting and inadequate ascertainment by medical professionals of whether or not they had experienced postmenopausal bleeding.2 Additionally, when postmenopausal bleeding was reported by these women, they were less likely to receive the appropriate diagnostic work-up as described by American College of Obstetricians and Gynecologists guidelines, and their bleeding was more likely to be ascribed to nonmalignant pathologies. Her work raises the important question about how black women view the health care profession and their willingness to engage early in good faith that their concerns will be met. These concerns are understandable given the documented different responsiveness of providers to black patients’ symptoms such as pain.3
both of which are considered the standard of care.1,4 Lower rates of minimally invasive surgery expose black women to increased morbidity and are deleterious to quality of life, return to work, and functionality. If surgical staging is omitted, which is more common for these women, clinicians are less able to appropriately prescribe adjuvant therapies which might prevent lethal recurrences from unrecognized advanced cancer or they may overtreat early-stage cancers with adjuvant therapy to make up for gaps in staging information.1,5 However, adjuvant therapy is not a benign intervention, and itself is associated with morbidity.
As mentioned earlier, black women are at a higher risk for developing more aggressive cancer subtypes, and this phenomenon may appear unmodifiable. However, important research is looking at the concept of epigenetics and how modifiable environmental factors may contribute to the development of more aggressive types of cancer through gene expression. Additionally, differences in the gene mutations and gene expression of cancers more frequently acquired by black women may negatively influence how these cancers respond to conventional therapies. In the GOG210 study, which evaluated the outcomes of women with comprehensively staged endometrial cancer, black women demonstrated worse survival from cancer, even though they were more likely to receive chemotherapy.5 One explanation for this finding is that these women’s cancers were less responsive to conventional chemotherapy agents.
This raises a critical issue of disparity in clinical trial inclusion. Black women are underrepresented in clinical trials in the United States. There is a dark history in medical research and minority populations, particularly African American populations, which continues to be remembered and felt. However, not all of this underrepresentation may be from unwillingness to participate: For black women, issues of lack of access to or being considered for clinical trials is also a factor. But without adequate representation in trials of novel agents, we will not know whether they are effective for all populations, and indeed it would appear that we should not assume they are equally effective based on the results to date.
So how can we all individually help to overcome these disparities in endometrial cancer outcomes? To begin with, it is important to acknowledge that black women commonly report negative experiences with reproductive health care. From early in their lives, we must sensitively engage all of our patients and ensure they all feel heard and valued. They should know that their symptoms, including pain or bleeding, are taken and treated seriously. If we can do better with this throughout a woman’s earlier reproductive health care experiences, perhaps later in her life, when she experiences postmenopausal bleeding, she will feel comfortable raising this issue with her health care provider who in turn must take this symptom seriously and expeditiously engage all of the appropriate diagnostic resources. Health care delivery is about more than simply offering the best treatment. We also are responsible for education and shared decision making to ensure that we can deliver the best treatment.
We also can support organizations such as ECANA (Endometrial Cancer Action Network for African Americans) which serves to inform black women in their communities about the threat that endometrial cancer plays and empowers them through education about its symptoms and the need to seek care.
Systematically we must ensure black women have access to the same standards in surgical and nonsurgical management of these cancers. This includes referral of all women with cancer, including minorities, to high-volume centers with oncology specialists and explaining to those who may be reluctant to travel that this is associated with improved outcomes in the short and long term. We also must actively consider our black patients for clinical trials, sensitively educate them about their benefits, and overcome barriers to access. One simple way to do this is to explain that the treatments that we have developed for endometrial cancer have mostly been tested on white women, which may explain in part why they do not work so well for nonwhite women.
The racial disparity in endometrial cancer outcomes cannot entirely be attributed to the passive phenomenon of patient and tumor genetics, particularly with consideration that race is a social construct rather than a biological phenomenon. We can all make a difference through advocacy, access, education, and heightened awareness to combat this inequity and overcome these disparate outcomes.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Gynecol Oncol. 2016 Oct;143(1):98-104.
2. Am J Obstet Gynecol. 2018 Dec;219(6):593.e1-14.
3. J Clin Oncol. 2012 Jun 1;30(16):1980-8.
4. Obstet Gynecol. 2016 Sep;128(3):526-34.
5. Am J Obstet Gynecol. 2018 Nov;219(5):459.e1-11.
While the incidence of most cancers is falling, endometrial cancer rates continue to rise, in large part because of increasing life expectancy and obesity rates. However, what is even more alarming is the observation that there is a clear disparity in outcomes between black and white women with this disease. But there are things that all health care providers, including nononcologists, can do to help to overcome this disparity.
Black women are nearly twice as likely as non-Hispanic white women to die from the endometrial cancer. The 5-year survival for stage III and IV cancer is 43% for non-Hispanic white women, yet only 25% for black women.1 For a long time, this survival disparity was assumed to be a function of the more aggressive cancer histologies, such as serous, which are more commonly seen in black women. These high-grade cancers are more likely to present in advanced stages and with poorer responses to treatments; however, the predisposition to aggressive cancers tells only part of the story of racial disparities in endometrial cancer and their presentation at later stages. Indeed, fueling the problem are the findings that black women report symptoms less, experience more delays in diagnosis or more frequent deviations from guideline-directed diagnostics, undergo more morbid surgical approaches, receive less surgical staging, are enrolled less in clinical trials, have lower socioeconomic status and lower rates of health insurance, and receive less differential administration of adjuvant therapies, as well as have a background of higher all-cause mortality and comorbidities. While this array of contributing factors may seem overwhelming, it also can be considered a guide for health care providers because most of these factors, unlike histologic cell type, are modifiable, and it is important that we all consider what role we can play in dismantling them.
Black women are less likely to receive guideline-recommended care upon presentation. Research by Kemi M. Doll, MD, from the University of Washington, Seattle, demonstrated that, among women with endometrial cancers, black women were less likely to have documented histories of postmenopausal bleeding within 2 years of the diagnosis, presumably because of factors related to underreporting and inadequate ascertainment by medical professionals of whether or not they had experienced postmenopausal bleeding.2 Additionally, when postmenopausal bleeding was reported by these women, they were less likely to receive the appropriate diagnostic work-up as described by American College of Obstetricians and Gynecologists guidelines, and their bleeding was more likely to be ascribed to nonmalignant pathologies. Her work raises the important question about how black women view the health care profession and their willingness to engage early in good faith that their concerns will be met. These concerns are understandable given the documented different responsiveness of providers to black patients’ symptoms such as pain.3
both of which are considered the standard of care.1,4 Lower rates of minimally invasive surgery expose black women to increased morbidity and are deleterious to quality of life, return to work, and functionality. If surgical staging is omitted, which is more common for these women, clinicians are less able to appropriately prescribe adjuvant therapies which might prevent lethal recurrences from unrecognized advanced cancer or they may overtreat early-stage cancers with adjuvant therapy to make up for gaps in staging information.1,5 However, adjuvant therapy is not a benign intervention, and itself is associated with morbidity.
As mentioned earlier, black women are at a higher risk for developing more aggressive cancer subtypes, and this phenomenon may appear unmodifiable. However, important research is looking at the concept of epigenetics and how modifiable environmental factors may contribute to the development of more aggressive types of cancer through gene expression. Additionally, differences in the gene mutations and gene expression of cancers more frequently acquired by black women may negatively influence how these cancers respond to conventional therapies. In the GOG210 study, which evaluated the outcomes of women with comprehensively staged endometrial cancer, black women demonstrated worse survival from cancer, even though they were more likely to receive chemotherapy.5 One explanation for this finding is that these women’s cancers were less responsive to conventional chemotherapy agents.
This raises a critical issue of disparity in clinical trial inclusion. Black women are underrepresented in clinical trials in the United States. There is a dark history in medical research and minority populations, particularly African American populations, which continues to be remembered and felt. However, not all of this underrepresentation may be from unwillingness to participate: For black women, issues of lack of access to or being considered for clinical trials is also a factor. But without adequate representation in trials of novel agents, we will not know whether they are effective for all populations, and indeed it would appear that we should not assume they are equally effective based on the results to date.
So how can we all individually help to overcome these disparities in endometrial cancer outcomes? To begin with, it is important to acknowledge that black women commonly report negative experiences with reproductive health care. From early in their lives, we must sensitively engage all of our patients and ensure they all feel heard and valued. They should know that their symptoms, including pain or bleeding, are taken and treated seriously. If we can do better with this throughout a woman’s earlier reproductive health care experiences, perhaps later in her life, when she experiences postmenopausal bleeding, she will feel comfortable raising this issue with her health care provider who in turn must take this symptom seriously and expeditiously engage all of the appropriate diagnostic resources. Health care delivery is about more than simply offering the best treatment. We also are responsible for education and shared decision making to ensure that we can deliver the best treatment.
We also can support organizations such as ECANA (Endometrial Cancer Action Network for African Americans) which serves to inform black women in their communities about the threat that endometrial cancer plays and empowers them through education about its symptoms and the need to seek care.
Systematically we must ensure black women have access to the same standards in surgical and nonsurgical management of these cancers. This includes referral of all women with cancer, including minorities, to high-volume centers with oncology specialists and explaining to those who may be reluctant to travel that this is associated with improved outcomes in the short and long term. We also must actively consider our black patients for clinical trials, sensitively educate them about their benefits, and overcome barriers to access. One simple way to do this is to explain that the treatments that we have developed for endometrial cancer have mostly been tested on white women, which may explain in part why they do not work so well for nonwhite women.
The racial disparity in endometrial cancer outcomes cannot entirely be attributed to the passive phenomenon of patient and tumor genetics, particularly with consideration that race is a social construct rather than a biological phenomenon. We can all make a difference through advocacy, access, education, and heightened awareness to combat this inequity and overcome these disparate outcomes.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Gynecol Oncol. 2016 Oct;143(1):98-104.
2. Am J Obstet Gynecol. 2018 Dec;219(6):593.e1-14.
3. J Clin Oncol. 2012 Jun 1;30(16):1980-8.
4. Obstet Gynecol. 2016 Sep;128(3):526-34.
5. Am J Obstet Gynecol. 2018 Nov;219(5):459.e1-11.
While the incidence of most cancers is falling, endometrial cancer rates continue to rise, in large part because of increasing life expectancy and obesity rates. However, what is even more alarming is the observation that there is a clear disparity in outcomes between black and white women with this disease. But there are things that all health care providers, including nononcologists, can do to help to overcome this disparity.
Black women are nearly twice as likely as non-Hispanic white women to die from the endometrial cancer. The 5-year survival for stage III and IV cancer is 43% for non-Hispanic white women, yet only 25% for black women.1 For a long time, this survival disparity was assumed to be a function of the more aggressive cancer histologies, such as serous, which are more commonly seen in black women. These high-grade cancers are more likely to present in advanced stages and with poorer responses to treatments; however, the predisposition to aggressive cancers tells only part of the story of racial disparities in endometrial cancer and their presentation at later stages. Indeed, fueling the problem are the findings that black women report symptoms less, experience more delays in diagnosis or more frequent deviations from guideline-directed diagnostics, undergo more morbid surgical approaches, receive less surgical staging, are enrolled less in clinical trials, have lower socioeconomic status and lower rates of health insurance, and receive less differential administration of adjuvant therapies, as well as have a background of higher all-cause mortality and comorbidities. While this array of contributing factors may seem overwhelming, it also can be considered a guide for health care providers because most of these factors, unlike histologic cell type, are modifiable, and it is important that we all consider what role we can play in dismantling them.
Black women are less likely to receive guideline-recommended care upon presentation. Research by Kemi M. Doll, MD, from the University of Washington, Seattle, demonstrated that, among women with endometrial cancers, black women were less likely to have documented histories of postmenopausal bleeding within 2 years of the diagnosis, presumably because of factors related to underreporting and inadequate ascertainment by medical professionals of whether or not they had experienced postmenopausal bleeding.2 Additionally, when postmenopausal bleeding was reported by these women, they were less likely to receive the appropriate diagnostic work-up as described by American College of Obstetricians and Gynecologists guidelines, and their bleeding was more likely to be ascribed to nonmalignant pathologies. Her work raises the important question about how black women view the health care profession and their willingness to engage early in good faith that their concerns will be met. These concerns are understandable given the documented different responsiveness of providers to black patients’ symptoms such as pain.3
both of which are considered the standard of care.1,4 Lower rates of minimally invasive surgery expose black women to increased morbidity and are deleterious to quality of life, return to work, and functionality. If surgical staging is omitted, which is more common for these women, clinicians are less able to appropriately prescribe adjuvant therapies which might prevent lethal recurrences from unrecognized advanced cancer or they may overtreat early-stage cancers with adjuvant therapy to make up for gaps in staging information.1,5 However, adjuvant therapy is not a benign intervention, and itself is associated with morbidity.
As mentioned earlier, black women are at a higher risk for developing more aggressive cancer subtypes, and this phenomenon may appear unmodifiable. However, important research is looking at the concept of epigenetics and how modifiable environmental factors may contribute to the development of more aggressive types of cancer through gene expression. Additionally, differences in the gene mutations and gene expression of cancers more frequently acquired by black women may negatively influence how these cancers respond to conventional therapies. In the GOG210 study, which evaluated the outcomes of women with comprehensively staged endometrial cancer, black women demonstrated worse survival from cancer, even though they were more likely to receive chemotherapy.5 One explanation for this finding is that these women’s cancers were less responsive to conventional chemotherapy agents.
This raises a critical issue of disparity in clinical trial inclusion. Black women are underrepresented in clinical trials in the United States. There is a dark history in medical research and minority populations, particularly African American populations, which continues to be remembered and felt. However, not all of this underrepresentation may be from unwillingness to participate: For black women, issues of lack of access to or being considered for clinical trials is also a factor. But without adequate representation in trials of novel agents, we will not know whether they are effective for all populations, and indeed it would appear that we should not assume they are equally effective based on the results to date.
So how can we all individually help to overcome these disparities in endometrial cancer outcomes? To begin with, it is important to acknowledge that black women commonly report negative experiences with reproductive health care. From early in their lives, we must sensitively engage all of our patients and ensure they all feel heard and valued. They should know that their symptoms, including pain or bleeding, are taken and treated seriously. If we can do better with this throughout a woman’s earlier reproductive health care experiences, perhaps later in her life, when she experiences postmenopausal bleeding, she will feel comfortable raising this issue with her health care provider who in turn must take this symptom seriously and expeditiously engage all of the appropriate diagnostic resources. Health care delivery is about more than simply offering the best treatment. We also are responsible for education and shared decision making to ensure that we can deliver the best treatment.
We also can support organizations such as ECANA (Endometrial Cancer Action Network for African Americans) which serves to inform black women in their communities about the threat that endometrial cancer plays and empowers them through education about its symptoms and the need to seek care.
Systematically we must ensure black women have access to the same standards in surgical and nonsurgical management of these cancers. This includes referral of all women with cancer, including minorities, to high-volume centers with oncology specialists and explaining to those who may be reluctant to travel that this is associated with improved outcomes in the short and long term. We also must actively consider our black patients for clinical trials, sensitively educate them about their benefits, and overcome barriers to access. One simple way to do this is to explain that the treatments that we have developed for endometrial cancer have mostly been tested on white women, which may explain in part why they do not work so well for nonwhite women.
The racial disparity in endometrial cancer outcomes cannot entirely be attributed to the passive phenomenon of patient and tumor genetics, particularly with consideration that race is a social construct rather than a biological phenomenon. We can all make a difference through advocacy, access, education, and heightened awareness to combat this inequity and overcome these disparate outcomes.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Gynecol Oncol. 2016 Oct;143(1):98-104.
2. Am J Obstet Gynecol. 2018 Dec;219(6):593.e1-14.
3. J Clin Oncol. 2012 Jun 1;30(16):1980-8.
4. Obstet Gynecol. 2016 Sep;128(3):526-34.
5. Am J Obstet Gynecol. 2018 Nov;219(5):459.e1-11.
Medical management of abnormal uterine bleeding in reproductive-age women
Case 1 Multiparous woman presents with heavy regular menses
Over the past several years, a 34-year-old woman has noted increasing intensity and duration of menstrual flow, which now persists for 8 days and includes clots “the size of quarters” and soaks a pad within 1 hour. Sometimes she misses or leaves work on her heaviest days of flow. She reports that menstrual cramps prior to and during flow are increasingly bothersome and do not respond adequately to ibuprofen. She intermittently uses condoms for contraception. She does not wish to be pregnant currently; however, she recently entered into a new relationship and may wish to conceive in the future.
On bimanual examination, the uterus appears bulky. Her hemoglobin is 10.9 g/dL with low mean corpuscular volume and a serum ferritin level indicating iron depletion. Pelvic ultrasonography suggests uterine adenomyosis; no fibroids are imaged (FIGURE 1).
You advise the patient to take ferrous sulfate 325 mg every other day. After discussion with the patient regarding different treatment options, she chooses to proceed with placement of a 52-mg levonorgestrel (LNG) intrauterine device (IUD; Mirena or Liletta).
Case 2 Older adolescent presents with irregular bleeding
A 19-year-old patient reports approximately 6 bleeding episodes each year. She reports the duration of her bleeding as variable, and sometimes the bleeding is heavy with small clots passed. She has been previously diagnosed with polycystic ovary syndrome (PCOS). Combination estrogen-progestin oral contraceptives have been prescribed several times in the past, but she always has discontinued them due to nausea. The patient is in a same-sex relationship and does not anticipate being sexually active with a male. She reports having to shave her mustache and chin twice weekly for the past 1 to 2 years.
On physical examination, the patient is obese (body mass index [BMI], 32 kg/m2), facial acne and hirsutism are present, and hair extends from the mons toward the umbilicus. Bimanual examination reveals a normal size, mobile, nontender uterus without obvious adnexal pathology. Pelvic ultrasonography demonstrates a normal-appearing uterus with multiplanar endometrium (consistent with proliferative changes) (FIGURE 2). Ovarian imaging demonstrates ≥12 follicles per image (FIGURE 3).
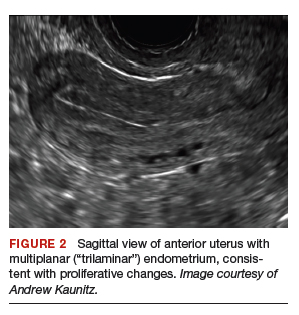

After reviewing various treatment options, you prescribe oral medroxyprogesterone acetate 20 mg (two 10-mg tablets) daily in a continuous fashion. You counsel her that she should not be surprised or concerned if frequent or even continuous bleeding occurs initially, and that she should continue this medication despite the occurrence of such.
About one-third of all women experience abnormal uterine bleeding (AUB) sometime during their lifetime and AUB can impair quality of life.1 Surgical management, including hysterectomy and endometrial ablation, plays an important role in the management of AUB in patients who do not desire future pregnancies. However, many cases of AUB occur in women who may not have completed childbearing or in women who prefer to avoid surgery.2 AUB can be managed effectively medically in most cases.1 Accordingly, in this review, we focus on nonsurgical management of AUB.
Continue to: Because previously used terms, including...
Because previously used terms, including menorrhagia and meno-metrorrhagia, were inconsistently defined and confusing, the International Federation of Gynecology and Obstetrics introduced updated terminology in 2011 to better describe and characterize AUB in nonpregnant women. Heavy menstrual bleeding (HMB) refers to ovulatory (cyclic) bleeding that is more than 8 days’ duration, or sufficiently heavy to impair a woman’s quality of life. HMB is a pattern of AUB distinct from the irregular bleeding pattern typically caused by ovulatory dysfunction (AUB-O).1
Clinical evaluation
Obtain menstrual history. In addition to a medical, surgical, and gynecologic history, a thorough menstrual history should be obtained to further characterize the patient’s bleeding pattern. In contrast to the cyclical or ovulatory bleeding seen with HMB, bleeding associated with inconsistent ovulation (AUB-O) is unpredictable or irregular, and is commonly associated with PCOS. AUB-O is also encountered in recently menarchal girls (secondary to immaturity of the hypothalamic-pituitary-gonadal axis) and in those who are perimenopausal. In addition, medications that can induce hyperprolactinemia (such as certain antipsychotics) can cause AUB-O.
Evaluate for all sources of bleeding. Be sure to evaluate for extrauterine causes of bleeding, including the cervix, vagina, vulva, or the urinary or gastrointestinal tracts for bleeding. Intermenstrual bleeding occurring between normal regular menses may be caused by an endometrial polyp, submucosal fibroid, endometritis, or an IUD. The patient report of postcoital bleeding suggests that cervical disease (cervicitis, polyp, or malignancy) may be present. Uterine leiomyoma or adenomyosis represent common causes of HMB. However, HMB also may be caused by a copper IUD, coagulation disorders (including von Willebrand disease), or use of anticoagulant medications. Hormonal contraceptives also can cause irregular bleeding.
Perform a pelvic examination and measure vital signs. The presence of fever suggests the possible presence of pelvic inflammatory disease (PID), while orthostatic hypotension raises the possibility of hypovolemia. When vaginal speculum examination is performed, a cervical cause of abnormal bleeding may be noted. The presence of fresh or old blood or finding clots in the vaginal vault or at the cervical os are all consistent with AUB. A bimanual examination that reveals an enlarged or lobular uterus suggests leiomyoma or adenomyosis. Cervical or adnexal tenderness is often noted in women with PID, which itself may be associated with endometritis. The presence of hyperandrogenic signs on physical examination (eg, acne, hirsutism, or clitoromegaly) suggests PCOS. The finding of galactorrhea suggests that hyperprolactinemia may be present.
Laboratory assessment
Test for pregnancy, cervical disease, and sexually transmitted infection when appropriate. Pregnancy testing is appropriate for women with AUB aged 55 years or younger. If patients with AUB are not up to date with normal cervical cancer screening results, cervical cytology and/or human papillomavirus testing should be performed. Testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis should be performed in patients:
- younger than 25 years
- when the history indicates new or multiple sexual partners, or
- when vaginal discharge, cervicitis, cervical motion, or adnexal tenderness is present.
Continue to: Obtain a complete blood count and serum ferritin levels...
Obtain a complete blood count and serum ferritin levels. In women presenting with HMB, iron depletion and iron deficiency anemia are common. The finding of leukocytosis raises the possibility of PID or postpartum endometritis. In women with presumptive AUB-O, checking the levels of thyroid-stimulating hormone, free T4, and prolactin should be performed.
Screen for a hemostasis disorder. Women with excessive menstrual bleeding should be clinically screened for an underlying disorder of hemostasis (TABLE 1).3 When a hemostasis disorder is suspected, initial laboratory evaluation includes a partial thromboplastin time, prothrombin time, activated partial thromboplastin time, and fibrinogen. Women who have a positive clinical screen for a possible bleeding disorder or abnormal initial laboratory test results for disorders of hemostasis should undergo further laboratory evaluation, including von Willebrand factor antigen, ristocetin cofactor assay, and factor VIII. Consultation with a hematologist should be considered in these cases.

Perform endometrial biopsy when indicated
After excluding pregnancy, endometrial biopsy (through pipelle biospy or brush sampling; FIGURE 4) should be performed in women with AUB who are at increased risk for endometrial neoplasia. The prevalence of endometrial neoplasia is substantially higher among women ≥45 years of age4 and among patients with AUB who are also obese (BMI, ≥30 kg/m2).5 In addition, AUB patients with unopposed estrogen exposure (presumed anovulation/PCOS), as well as those with persistent AUB or failed medical management, should undergo endometrial biopsy.6
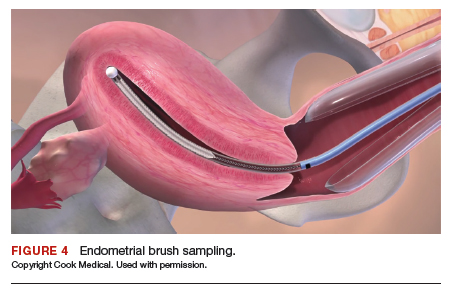
Utilize transvaginal ultrasonography
Transvaginal ultrasonography is often useful in the evaluation of patients with AUB, as it may identify uterine fibroids or adenomyosis, suggest intracavitary pathology (such as an endometrial polyp or submucosal fibroid), or raise the possibility of PCOS. In virginal patients or those in whom vaginal ultrasound is not appropriate, abdominal pelvic ultrasonography represents appropriate imaging. If unenhanced ultrasound suggests endometrial polyps or fibroids within the endometrial cavity, an office-based saline infusion sonogram (sonohysterogram) (FIGURE 5) or hysteroscopy should be performed. Targeted endometrial sampling and biopsy of intracavitary pathology can be performed at the time of hysteroscopy.
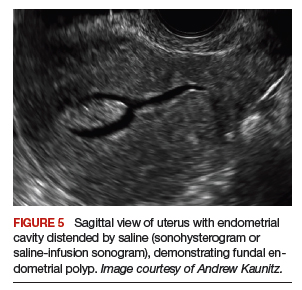
Treatment
When HMB impairs quality of life, is bothersome to the patient, or results in anemia, treatment is appropriate. Although bleeding episodes in women with AUB-O may be infrequent (as with Case 2), treatment prevents heavy or prolonged bleeding episodes as well as endometrial neoplasia that may otherwise occur in anovulatory women.
Many women with AUB can be managed medically. However, treatment choices will vary with respect to the patient’s desire for future fertility, medical comorbidities, personal preferences, and financial barriers. While many women may prefer outpatient medical management (TABLE 2),7-14 others might desire surgical therapy, including endometrial ablation or hysterectomy.

Oral contraceptives
Combination estrogen-progestin oral contraceptives represent appropriate initial therapy for many women in the reproductive-age group with AUB, whether women have HMB or AUB-O. However, contraceptive doses of estrogen are not appropriate for some women with risk factors for cardiovascular disease, including those who smoke cigarettes and are age ≥35 years or those who have hypertension (TABLE 3).15,16
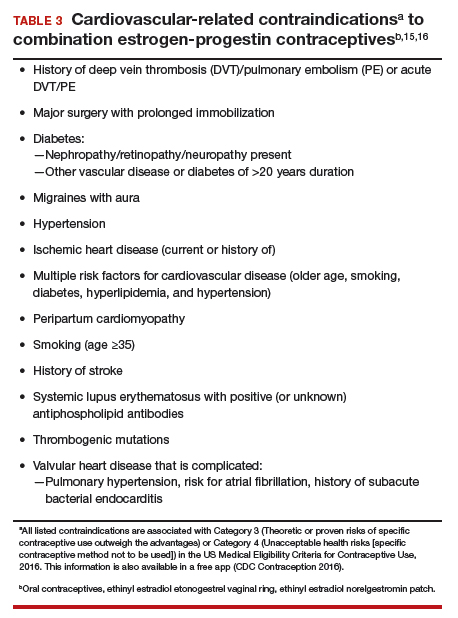
Continue to: Menopausal dosages of HT...
Menopausal dosages of HT
If use of contraceptive doses of estrogen is not appropriate, continuous off-label use of menopausal combination formulations (physiologic dosage) of hormonal therapy (HT; ie, lower doses of estrogen than contraceptives) may be effective in reducing or eliminating AUB. Options for menopausal combination formulations include generic ethinyl estradiol 5 µg/norethindrone acetate 1 mg or estradiol 1 mg/norethindrone acetate 0.5 mg.7 High-dose oral progestin therapy (norethindrone acetate 5 mg tablet once daily or medroxyprogesterone acetate 10 mg tablets 1–3 times daily) also can be used when combination contraceptives are contraindicated and may be more effective than lower-dose combination formulations.
Package labeling, as well as some guidelines, indicate that oral progestins used to treat AUB should be taken cyclically.8 However, continuous daily use is easier for many patients and may be more effective in reducing bleeding. Accordingly, we counsel patients with AUB who are using progestins and who do not wish to conceive to take these medications continuously. High-dose oral progestin therapy may cause bloating, dysphoria, and increased appetite/weight gain. Women initiating hormonal management (including the progestin IUDs detailed below) for AUB should be counseled that irregular or even continuous light bleeding/spotting is common initially, but this bleeding pattern typically decreases with continued use.
IUDs
The LNG 52 mg IUD (Mirena or Liletta) effectively treats HMB, reducing bleeding in a manner comparable to that of endometrial ablation.9,10 The Mirena IUD is approved for treatment of HMB in women desiring intrauterine contraception. In contrast to oral medications, use of progestin IUDs does not involve daily administration and may represent an attractive option for women with HMB who would like to avoid surgery or preserve fertility. With ongoing use, continuous oral or intrauterine hormonal management may result in amenorrhea in some women with AUB.
When the LNG 52 mg IUD is used to treat HMB, the menstrual suppression impact may begin to attenuate after approximately 4 years of use; in this setting, replacing the IUD often restores effective menstrual suppression.11 The LNG 52 mg IUD effectively suppresses menses in women with coagulation disorders; if menstrual suppression with the progestin IUD is not adequate in this setting, it may be appropriate to add an oral combination estrogen-progestin contraceptive or high-dose oral progestin.11,12
NSAIDs and tranexamic acid
Off-label use of nonsteroidal anti-inflammatory drugs (naproxen 500–1,000 mg daily for 5 days beginning at the onset of menstrual flow or tranexamic acid two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can suppress HMB and is useful for women who prefer to avoid or have contraindications to hormonal treatments.13,14 Unfortunately, these agents are not as effective as hormonal management in treating AUB.
Iron supplementation is often needed
Iron depletion commonly results from HMB, often resulting in iron deficiency anemia. When iron depletion (readily identified by checking a serum ferritin level) or iron deficiency anemia is identified, iron supplementation should be recommended. Every-other-day administration of iron supplements maximizes iron absorption while minimizing the adverse effects of unabsorbed iron, such as nausea. Sixty mg of elemental iron (ferrous sulfate 325 mg) administered every other day represents an inexpensive and effective treatment for iron deficiency/anemia.17 In patients who cannot tolerate oral iron supplementation or for those in whom oral therapy is not appropriate or effective, newer intravenous iron formulations are safe and effective.18
Continue to: Case 1 Follow-up...
Case 1 Follow-up
The patient noted marked improvement in her menstrual cramps following LNG-containing IUD placement. Although she also reported that she no longer experienced heavy menstrual flow or cramps, she was bothered by frequent, unpredictable light bleeding/spotting. You prescribed norethindrone acetate (NETA) 5-mg tablet orally once daily, to be used in addition to her IUD. After using the IUD with concomitant NETA for 2 months’ duration, she noted that her bleeding/spotting almost completely resolved; however, she did report feeling irritable with use of the progestin tablets. She subsequently stopped the NETA tablets and, after 6 months of additional follow-up, reported only minimal spotting and no cramps.
At this later follow-up visit, you noted that her hemoglobin level increased to 12.6 g/dL, and the ferritin level no longer indicated iron depletion. After the IUD had been in place for 4 years, she reported that she was beginning to experience frequent light bleeding again. A follow-up vaginal sonogram noted a well-positioned IUD, there was no suggestion of intracavitary pathology, and adenomyosis continued to be imaged. She underwent IUD removal and placement of a new LNG 52 mg IUD. This resulted in marked reduction in her bleeding.
Case 2 Follow-up
Two weeks after beginning continuous oral progestin therapy, the patient called reporting frequent irregular bleeding. She was reassured that this was not unexpected and encouraged to continue oral progestin therapy. During a 3-month follow-up visit, the patient noted little, if any, bleeding over the previous 2 months and was pleased with this result. She continued to note acne and hirsutism and asked about the possibility of adding spironolactone to her oral progestin regimen.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynecol Obstet. 2018;143:393-408.
- Kaunitz AM. Abnormal uterine bleeding in reproductive-age women. JAMA. 2019;321:2126-2127.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Uterine Cancer. http://seer.cancer.gov/statfacts/html/corp.html. Accessed October 10, 2019.
- Wise MR, Gill P, Lensen S, et al. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am J Obstet Gynecol. 2016;215:598.e1-598.e8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- The North American Menopause Society. Menopause Practice–A Clinician’s Guide. 5th ed. NAMS: Mayfield Heights, OH; 2014.
- National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. https://www.nice.org.uk/guidance/ng88. Accessed October 10, 2019.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625-632.
- Kaunitz AM, Meredith S, Inki P, et al. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113:1104-1116.
- Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72:193-215.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8.
- Ylikorkala O, Pekonen F. Naproxen reduces idiopathic but not fibromyoma-induced menorrhagia. Obstet Gynecol. 1986;68:10-12.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:865-875.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1–103.
- ACOG Practice Bulletin no. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524–e533.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
Case 1 Multiparous woman presents with heavy regular menses
Over the past several years, a 34-year-old woman has noted increasing intensity and duration of menstrual flow, which now persists for 8 days and includes clots “the size of quarters” and soaks a pad within 1 hour. Sometimes she misses or leaves work on her heaviest days of flow. She reports that menstrual cramps prior to and during flow are increasingly bothersome and do not respond adequately to ibuprofen. She intermittently uses condoms for contraception. She does not wish to be pregnant currently; however, she recently entered into a new relationship and may wish to conceive in the future.
On bimanual examination, the uterus appears bulky. Her hemoglobin is 10.9 g/dL with low mean corpuscular volume and a serum ferritin level indicating iron depletion. Pelvic ultrasonography suggests uterine adenomyosis; no fibroids are imaged (FIGURE 1).
You advise the patient to take ferrous sulfate 325 mg every other day. After discussion with the patient regarding different treatment options, she chooses to proceed with placement of a 52-mg levonorgestrel (LNG) intrauterine device (IUD; Mirena or Liletta).
Case 2 Older adolescent presents with irregular bleeding
A 19-year-old patient reports approximately 6 bleeding episodes each year. She reports the duration of her bleeding as variable, and sometimes the bleeding is heavy with small clots passed. She has been previously diagnosed with polycystic ovary syndrome (PCOS). Combination estrogen-progestin oral contraceptives have been prescribed several times in the past, but she always has discontinued them due to nausea. The patient is in a same-sex relationship and does not anticipate being sexually active with a male. She reports having to shave her mustache and chin twice weekly for the past 1 to 2 years.
On physical examination, the patient is obese (body mass index [BMI], 32 kg/m2), facial acne and hirsutism are present, and hair extends from the mons toward the umbilicus. Bimanual examination reveals a normal size, mobile, nontender uterus without obvious adnexal pathology. Pelvic ultrasonography demonstrates a normal-appearing uterus with multiplanar endometrium (consistent with proliferative changes) (FIGURE 2). Ovarian imaging demonstrates ≥12 follicles per image (FIGURE 3).


After reviewing various treatment options, you prescribe oral medroxyprogesterone acetate 20 mg (two 10-mg tablets) daily in a continuous fashion. You counsel her that she should not be surprised or concerned if frequent or even continuous bleeding occurs initially, and that she should continue this medication despite the occurrence of such.
About one-third of all women experience abnormal uterine bleeding (AUB) sometime during their lifetime and AUB can impair quality of life.1 Surgical management, including hysterectomy and endometrial ablation, plays an important role in the management of AUB in patients who do not desire future pregnancies. However, many cases of AUB occur in women who may not have completed childbearing or in women who prefer to avoid surgery.2 AUB can be managed effectively medically in most cases.1 Accordingly, in this review, we focus on nonsurgical management of AUB.
Continue to: Because previously used terms, including...
Because previously used terms, including menorrhagia and meno-metrorrhagia, were inconsistently defined and confusing, the International Federation of Gynecology and Obstetrics introduced updated terminology in 2011 to better describe and characterize AUB in nonpregnant women. Heavy menstrual bleeding (HMB) refers to ovulatory (cyclic) bleeding that is more than 8 days’ duration, or sufficiently heavy to impair a woman’s quality of life. HMB is a pattern of AUB distinct from the irregular bleeding pattern typically caused by ovulatory dysfunction (AUB-O).1
Clinical evaluation
Obtain menstrual history. In addition to a medical, surgical, and gynecologic history, a thorough menstrual history should be obtained to further characterize the patient’s bleeding pattern. In contrast to the cyclical or ovulatory bleeding seen with HMB, bleeding associated with inconsistent ovulation (AUB-O) is unpredictable or irregular, and is commonly associated with PCOS. AUB-O is also encountered in recently menarchal girls (secondary to immaturity of the hypothalamic-pituitary-gonadal axis) and in those who are perimenopausal. In addition, medications that can induce hyperprolactinemia (such as certain antipsychotics) can cause AUB-O.
Evaluate for all sources of bleeding. Be sure to evaluate for extrauterine causes of bleeding, including the cervix, vagina, vulva, or the urinary or gastrointestinal tracts for bleeding. Intermenstrual bleeding occurring between normal regular menses may be caused by an endometrial polyp, submucosal fibroid, endometritis, or an IUD. The patient report of postcoital bleeding suggests that cervical disease (cervicitis, polyp, or malignancy) may be present. Uterine leiomyoma or adenomyosis represent common causes of HMB. However, HMB also may be caused by a copper IUD, coagulation disorders (including von Willebrand disease), or use of anticoagulant medications. Hormonal contraceptives also can cause irregular bleeding.
Perform a pelvic examination and measure vital signs. The presence of fever suggests the possible presence of pelvic inflammatory disease (PID), while orthostatic hypotension raises the possibility of hypovolemia. When vaginal speculum examination is performed, a cervical cause of abnormal bleeding may be noted. The presence of fresh or old blood or finding clots in the vaginal vault or at the cervical os are all consistent with AUB. A bimanual examination that reveals an enlarged or lobular uterus suggests leiomyoma or adenomyosis. Cervical or adnexal tenderness is often noted in women with PID, which itself may be associated with endometritis. The presence of hyperandrogenic signs on physical examination (eg, acne, hirsutism, or clitoromegaly) suggests PCOS. The finding of galactorrhea suggests that hyperprolactinemia may be present.
Laboratory assessment
Test for pregnancy, cervical disease, and sexually transmitted infection when appropriate. Pregnancy testing is appropriate for women with AUB aged 55 years or younger. If patients with AUB are not up to date with normal cervical cancer screening results, cervical cytology and/or human papillomavirus testing should be performed. Testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis should be performed in patients:
- younger than 25 years
- when the history indicates new or multiple sexual partners, or
- when vaginal discharge, cervicitis, cervical motion, or adnexal tenderness is present.
Continue to: Obtain a complete blood count and serum ferritin levels...
Obtain a complete blood count and serum ferritin levels. In women presenting with HMB, iron depletion and iron deficiency anemia are common. The finding of leukocytosis raises the possibility of PID or postpartum endometritis. In women with presumptive AUB-O, checking the levels of thyroid-stimulating hormone, free T4, and prolactin should be performed.
Screen for a hemostasis disorder. Women with excessive menstrual bleeding should be clinically screened for an underlying disorder of hemostasis (TABLE 1).3 When a hemostasis disorder is suspected, initial laboratory evaluation includes a partial thromboplastin time, prothrombin time, activated partial thromboplastin time, and fibrinogen. Women who have a positive clinical screen for a possible bleeding disorder or abnormal initial laboratory test results for disorders of hemostasis should undergo further laboratory evaluation, including von Willebrand factor antigen, ristocetin cofactor assay, and factor VIII. Consultation with a hematologist should be considered in these cases.

Perform endometrial biopsy when indicated
After excluding pregnancy, endometrial biopsy (through pipelle biospy or brush sampling; FIGURE 4) should be performed in women with AUB who are at increased risk for endometrial neoplasia. The prevalence of endometrial neoplasia is substantially higher among women ≥45 years of age4 and among patients with AUB who are also obese (BMI, ≥30 kg/m2).5 In addition, AUB patients with unopposed estrogen exposure (presumed anovulation/PCOS), as well as those with persistent AUB or failed medical management, should undergo endometrial biopsy.6

Utilize transvaginal ultrasonography
Transvaginal ultrasonography is often useful in the evaluation of patients with AUB, as it may identify uterine fibroids or adenomyosis, suggest intracavitary pathology (such as an endometrial polyp or submucosal fibroid), or raise the possibility of PCOS. In virginal patients or those in whom vaginal ultrasound is not appropriate, abdominal pelvic ultrasonography represents appropriate imaging. If unenhanced ultrasound suggests endometrial polyps or fibroids within the endometrial cavity, an office-based saline infusion sonogram (sonohysterogram) (FIGURE 5) or hysteroscopy should be performed. Targeted endometrial sampling and biopsy of intracavitary pathology can be performed at the time of hysteroscopy.

Treatment
When HMB impairs quality of life, is bothersome to the patient, or results in anemia, treatment is appropriate. Although bleeding episodes in women with AUB-O may be infrequent (as with Case 2), treatment prevents heavy or prolonged bleeding episodes as well as endometrial neoplasia that may otherwise occur in anovulatory women.
Many women with AUB can be managed medically. However, treatment choices will vary with respect to the patient’s desire for future fertility, medical comorbidities, personal preferences, and financial barriers. While many women may prefer outpatient medical management (TABLE 2),7-14 others might desire surgical therapy, including endometrial ablation or hysterectomy.

Oral contraceptives
Combination estrogen-progestin oral contraceptives represent appropriate initial therapy for many women in the reproductive-age group with AUB, whether women have HMB or AUB-O. However, contraceptive doses of estrogen are not appropriate for some women with risk factors for cardiovascular disease, including those who smoke cigarettes and are age ≥35 years or those who have hypertension (TABLE 3).15,16

Continue to: Menopausal dosages of HT...
Menopausal dosages of HT
If use of contraceptive doses of estrogen is not appropriate, continuous off-label use of menopausal combination formulations (physiologic dosage) of hormonal therapy (HT; ie, lower doses of estrogen than contraceptives) may be effective in reducing or eliminating AUB. Options for menopausal combination formulations include generic ethinyl estradiol 5 µg/norethindrone acetate 1 mg or estradiol 1 mg/norethindrone acetate 0.5 mg.7 High-dose oral progestin therapy (norethindrone acetate 5 mg tablet once daily or medroxyprogesterone acetate 10 mg tablets 1–3 times daily) also can be used when combination contraceptives are contraindicated and may be more effective than lower-dose combination formulations.
Package labeling, as well as some guidelines, indicate that oral progestins used to treat AUB should be taken cyclically.8 However, continuous daily use is easier for many patients and may be more effective in reducing bleeding. Accordingly, we counsel patients with AUB who are using progestins and who do not wish to conceive to take these medications continuously. High-dose oral progestin therapy may cause bloating, dysphoria, and increased appetite/weight gain. Women initiating hormonal management (including the progestin IUDs detailed below) for AUB should be counseled that irregular or even continuous light bleeding/spotting is common initially, but this bleeding pattern typically decreases with continued use.
IUDs
The LNG 52 mg IUD (Mirena or Liletta) effectively treats HMB, reducing bleeding in a manner comparable to that of endometrial ablation.9,10 The Mirena IUD is approved for treatment of HMB in women desiring intrauterine contraception. In contrast to oral medications, use of progestin IUDs does not involve daily administration and may represent an attractive option for women with HMB who would like to avoid surgery or preserve fertility. With ongoing use, continuous oral or intrauterine hormonal management may result in amenorrhea in some women with AUB.
When the LNG 52 mg IUD is used to treat HMB, the menstrual suppression impact may begin to attenuate after approximately 4 years of use; in this setting, replacing the IUD often restores effective menstrual suppression.11 The LNG 52 mg IUD effectively suppresses menses in women with coagulation disorders; if menstrual suppression with the progestin IUD is not adequate in this setting, it may be appropriate to add an oral combination estrogen-progestin contraceptive or high-dose oral progestin.11,12
NSAIDs and tranexamic acid
Off-label use of nonsteroidal anti-inflammatory drugs (naproxen 500–1,000 mg daily for 5 days beginning at the onset of menstrual flow or tranexamic acid two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can suppress HMB and is useful for women who prefer to avoid or have contraindications to hormonal treatments.13,14 Unfortunately, these agents are not as effective as hormonal management in treating AUB.
Iron supplementation is often needed
Iron depletion commonly results from HMB, often resulting in iron deficiency anemia. When iron depletion (readily identified by checking a serum ferritin level) or iron deficiency anemia is identified, iron supplementation should be recommended. Every-other-day administration of iron supplements maximizes iron absorption while minimizing the adverse effects of unabsorbed iron, such as nausea. Sixty mg of elemental iron (ferrous sulfate 325 mg) administered every other day represents an inexpensive and effective treatment for iron deficiency/anemia.17 In patients who cannot tolerate oral iron supplementation or for those in whom oral therapy is not appropriate or effective, newer intravenous iron formulations are safe and effective.18
Continue to: Case 1 Follow-up...
Case 1 Follow-up
The patient noted marked improvement in her menstrual cramps following LNG-containing IUD placement. Although she also reported that she no longer experienced heavy menstrual flow or cramps, she was bothered by frequent, unpredictable light bleeding/spotting. You prescribed norethindrone acetate (NETA) 5-mg tablet orally once daily, to be used in addition to her IUD. After using the IUD with concomitant NETA for 2 months’ duration, she noted that her bleeding/spotting almost completely resolved; however, she did report feeling irritable with use of the progestin tablets. She subsequently stopped the NETA tablets and, after 6 months of additional follow-up, reported only minimal spotting and no cramps.
At this later follow-up visit, you noted that her hemoglobin level increased to 12.6 g/dL, and the ferritin level no longer indicated iron depletion. After the IUD had been in place for 4 years, she reported that she was beginning to experience frequent light bleeding again. A follow-up vaginal sonogram noted a well-positioned IUD, there was no suggestion of intracavitary pathology, and adenomyosis continued to be imaged. She underwent IUD removal and placement of a new LNG 52 mg IUD. This resulted in marked reduction in her bleeding.
Case 2 Follow-up
Two weeks after beginning continuous oral progestin therapy, the patient called reporting frequent irregular bleeding. She was reassured that this was not unexpected and encouraged to continue oral progestin therapy. During a 3-month follow-up visit, the patient noted little, if any, bleeding over the previous 2 months and was pleased with this result. She continued to note acne and hirsutism and asked about the possibility of adding spironolactone to her oral progestin regimen.
Case 1 Multiparous woman presents with heavy regular menses
Over the past several years, a 34-year-old woman has noted increasing intensity and duration of menstrual flow, which now persists for 8 days and includes clots “the size of quarters” and soaks a pad within 1 hour. Sometimes she misses or leaves work on her heaviest days of flow. She reports that menstrual cramps prior to and during flow are increasingly bothersome and do not respond adequately to ibuprofen. She intermittently uses condoms for contraception. She does not wish to be pregnant currently; however, she recently entered into a new relationship and may wish to conceive in the future.
On bimanual examination, the uterus appears bulky. Her hemoglobin is 10.9 g/dL with low mean corpuscular volume and a serum ferritin level indicating iron depletion. Pelvic ultrasonography suggests uterine adenomyosis; no fibroids are imaged (FIGURE 1).
You advise the patient to take ferrous sulfate 325 mg every other day. After discussion with the patient regarding different treatment options, she chooses to proceed with placement of a 52-mg levonorgestrel (LNG) intrauterine device (IUD; Mirena or Liletta).
Case 2 Older adolescent presents with irregular bleeding
A 19-year-old patient reports approximately 6 bleeding episodes each year. She reports the duration of her bleeding as variable, and sometimes the bleeding is heavy with small clots passed. She has been previously diagnosed with polycystic ovary syndrome (PCOS). Combination estrogen-progestin oral contraceptives have been prescribed several times in the past, but she always has discontinued them due to nausea. The patient is in a same-sex relationship and does not anticipate being sexually active with a male. She reports having to shave her mustache and chin twice weekly for the past 1 to 2 years.
On physical examination, the patient is obese (body mass index [BMI], 32 kg/m2), facial acne and hirsutism are present, and hair extends from the mons toward the umbilicus. Bimanual examination reveals a normal size, mobile, nontender uterus without obvious adnexal pathology. Pelvic ultrasonography demonstrates a normal-appearing uterus with multiplanar endometrium (consistent with proliferative changes) (FIGURE 2). Ovarian imaging demonstrates ≥12 follicles per image (FIGURE 3).


After reviewing various treatment options, you prescribe oral medroxyprogesterone acetate 20 mg (two 10-mg tablets) daily in a continuous fashion. You counsel her that she should not be surprised or concerned if frequent or even continuous bleeding occurs initially, and that she should continue this medication despite the occurrence of such.
About one-third of all women experience abnormal uterine bleeding (AUB) sometime during their lifetime and AUB can impair quality of life.1 Surgical management, including hysterectomy and endometrial ablation, plays an important role in the management of AUB in patients who do not desire future pregnancies. However, many cases of AUB occur in women who may not have completed childbearing or in women who prefer to avoid surgery.2 AUB can be managed effectively medically in most cases.1 Accordingly, in this review, we focus on nonsurgical management of AUB.
Continue to: Because previously used terms, including...
Because previously used terms, including menorrhagia and meno-metrorrhagia, were inconsistently defined and confusing, the International Federation of Gynecology and Obstetrics introduced updated terminology in 2011 to better describe and characterize AUB in nonpregnant women. Heavy menstrual bleeding (HMB) refers to ovulatory (cyclic) bleeding that is more than 8 days’ duration, or sufficiently heavy to impair a woman’s quality of life. HMB is a pattern of AUB distinct from the irregular bleeding pattern typically caused by ovulatory dysfunction (AUB-O).1
Clinical evaluation
Obtain menstrual history. In addition to a medical, surgical, and gynecologic history, a thorough menstrual history should be obtained to further characterize the patient’s bleeding pattern. In contrast to the cyclical or ovulatory bleeding seen with HMB, bleeding associated with inconsistent ovulation (AUB-O) is unpredictable or irregular, and is commonly associated with PCOS. AUB-O is also encountered in recently menarchal girls (secondary to immaturity of the hypothalamic-pituitary-gonadal axis) and in those who are perimenopausal. In addition, medications that can induce hyperprolactinemia (such as certain antipsychotics) can cause AUB-O.
Evaluate for all sources of bleeding. Be sure to evaluate for extrauterine causes of bleeding, including the cervix, vagina, vulva, or the urinary or gastrointestinal tracts for bleeding. Intermenstrual bleeding occurring between normal regular menses may be caused by an endometrial polyp, submucosal fibroid, endometritis, or an IUD. The patient report of postcoital bleeding suggests that cervical disease (cervicitis, polyp, or malignancy) may be present. Uterine leiomyoma or adenomyosis represent common causes of HMB. However, HMB also may be caused by a copper IUD, coagulation disorders (including von Willebrand disease), or use of anticoagulant medications. Hormonal contraceptives also can cause irregular bleeding.
Perform a pelvic examination and measure vital signs. The presence of fever suggests the possible presence of pelvic inflammatory disease (PID), while orthostatic hypotension raises the possibility of hypovolemia. When vaginal speculum examination is performed, a cervical cause of abnormal bleeding may be noted. The presence of fresh or old blood or finding clots in the vaginal vault or at the cervical os are all consistent with AUB. A bimanual examination that reveals an enlarged or lobular uterus suggests leiomyoma or adenomyosis. Cervical or adnexal tenderness is often noted in women with PID, which itself may be associated with endometritis. The presence of hyperandrogenic signs on physical examination (eg, acne, hirsutism, or clitoromegaly) suggests PCOS. The finding of galactorrhea suggests that hyperprolactinemia may be present.
Laboratory assessment
Test for pregnancy, cervical disease, and sexually transmitted infection when appropriate. Pregnancy testing is appropriate for women with AUB aged 55 years or younger. If patients with AUB are not up to date with normal cervical cancer screening results, cervical cytology and/or human papillomavirus testing should be performed. Testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis should be performed in patients:
- younger than 25 years
- when the history indicates new or multiple sexual partners, or
- when vaginal discharge, cervicitis, cervical motion, or adnexal tenderness is present.
Continue to: Obtain a complete blood count and serum ferritin levels...
Obtain a complete blood count and serum ferritin levels. In women presenting with HMB, iron depletion and iron deficiency anemia are common. The finding of leukocytosis raises the possibility of PID or postpartum endometritis. In women with presumptive AUB-O, checking the levels of thyroid-stimulating hormone, free T4, and prolactin should be performed.
Screen for a hemostasis disorder. Women with excessive menstrual bleeding should be clinically screened for an underlying disorder of hemostasis (TABLE 1).3 When a hemostasis disorder is suspected, initial laboratory evaluation includes a partial thromboplastin time, prothrombin time, activated partial thromboplastin time, and fibrinogen. Women who have a positive clinical screen for a possible bleeding disorder or abnormal initial laboratory test results for disorders of hemostasis should undergo further laboratory evaluation, including von Willebrand factor antigen, ristocetin cofactor assay, and factor VIII. Consultation with a hematologist should be considered in these cases.

Perform endometrial biopsy when indicated
After excluding pregnancy, endometrial biopsy (through pipelle biospy or brush sampling; FIGURE 4) should be performed in women with AUB who are at increased risk for endometrial neoplasia. The prevalence of endometrial neoplasia is substantially higher among women ≥45 years of age4 and among patients with AUB who are also obese (BMI, ≥30 kg/m2).5 In addition, AUB patients with unopposed estrogen exposure (presumed anovulation/PCOS), as well as those with persistent AUB or failed medical management, should undergo endometrial biopsy.6

Utilize transvaginal ultrasonography
Transvaginal ultrasonography is often useful in the evaluation of patients with AUB, as it may identify uterine fibroids or adenomyosis, suggest intracavitary pathology (such as an endometrial polyp or submucosal fibroid), or raise the possibility of PCOS. In virginal patients or those in whom vaginal ultrasound is not appropriate, abdominal pelvic ultrasonography represents appropriate imaging. If unenhanced ultrasound suggests endometrial polyps or fibroids within the endometrial cavity, an office-based saline infusion sonogram (sonohysterogram) (FIGURE 5) or hysteroscopy should be performed. Targeted endometrial sampling and biopsy of intracavitary pathology can be performed at the time of hysteroscopy.

Treatment
When HMB impairs quality of life, is bothersome to the patient, or results in anemia, treatment is appropriate. Although bleeding episodes in women with AUB-O may be infrequent (as with Case 2), treatment prevents heavy or prolonged bleeding episodes as well as endometrial neoplasia that may otherwise occur in anovulatory women.
Many women with AUB can be managed medically. However, treatment choices will vary with respect to the patient’s desire for future fertility, medical comorbidities, personal preferences, and financial barriers. While many women may prefer outpatient medical management (TABLE 2),7-14 others might desire surgical therapy, including endometrial ablation or hysterectomy.

Oral contraceptives
Combination estrogen-progestin oral contraceptives represent appropriate initial therapy for many women in the reproductive-age group with AUB, whether women have HMB or AUB-O. However, contraceptive doses of estrogen are not appropriate for some women with risk factors for cardiovascular disease, including those who smoke cigarettes and are age ≥35 years or those who have hypertension (TABLE 3).15,16

Continue to: Menopausal dosages of HT...
Menopausal dosages of HT
If use of contraceptive doses of estrogen is not appropriate, continuous off-label use of menopausal combination formulations (physiologic dosage) of hormonal therapy (HT; ie, lower doses of estrogen than contraceptives) may be effective in reducing or eliminating AUB. Options for menopausal combination formulations include generic ethinyl estradiol 5 µg/norethindrone acetate 1 mg or estradiol 1 mg/norethindrone acetate 0.5 mg.7 High-dose oral progestin therapy (norethindrone acetate 5 mg tablet once daily or medroxyprogesterone acetate 10 mg tablets 1–3 times daily) also can be used when combination contraceptives are contraindicated and may be more effective than lower-dose combination formulations.
Package labeling, as well as some guidelines, indicate that oral progestins used to treat AUB should be taken cyclically.8 However, continuous daily use is easier for many patients and may be more effective in reducing bleeding. Accordingly, we counsel patients with AUB who are using progestins and who do not wish to conceive to take these medications continuously. High-dose oral progestin therapy may cause bloating, dysphoria, and increased appetite/weight gain. Women initiating hormonal management (including the progestin IUDs detailed below) for AUB should be counseled that irregular or even continuous light bleeding/spotting is common initially, but this bleeding pattern typically decreases with continued use.
IUDs
The LNG 52 mg IUD (Mirena or Liletta) effectively treats HMB, reducing bleeding in a manner comparable to that of endometrial ablation.9,10 The Mirena IUD is approved for treatment of HMB in women desiring intrauterine contraception. In contrast to oral medications, use of progestin IUDs does not involve daily administration and may represent an attractive option for women with HMB who would like to avoid surgery or preserve fertility. With ongoing use, continuous oral or intrauterine hormonal management may result in amenorrhea in some women with AUB.
When the LNG 52 mg IUD is used to treat HMB, the menstrual suppression impact may begin to attenuate after approximately 4 years of use; in this setting, replacing the IUD often restores effective menstrual suppression.11 The LNG 52 mg IUD effectively suppresses menses in women with coagulation disorders; if menstrual suppression with the progestin IUD is not adequate in this setting, it may be appropriate to add an oral combination estrogen-progestin contraceptive or high-dose oral progestin.11,12
NSAIDs and tranexamic acid
Off-label use of nonsteroidal anti-inflammatory drugs (naproxen 500–1,000 mg daily for 5 days beginning at the onset of menstrual flow or tranexamic acid two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can suppress HMB and is useful for women who prefer to avoid or have contraindications to hormonal treatments.13,14 Unfortunately, these agents are not as effective as hormonal management in treating AUB.
Iron supplementation is often needed
Iron depletion commonly results from HMB, often resulting in iron deficiency anemia. When iron depletion (readily identified by checking a serum ferritin level) or iron deficiency anemia is identified, iron supplementation should be recommended. Every-other-day administration of iron supplements maximizes iron absorption while minimizing the adverse effects of unabsorbed iron, such as nausea. Sixty mg of elemental iron (ferrous sulfate 325 mg) administered every other day represents an inexpensive and effective treatment for iron deficiency/anemia.17 In patients who cannot tolerate oral iron supplementation or for those in whom oral therapy is not appropriate or effective, newer intravenous iron formulations are safe and effective.18
Continue to: Case 1 Follow-up...
Case 1 Follow-up
The patient noted marked improvement in her menstrual cramps following LNG-containing IUD placement. Although she also reported that she no longer experienced heavy menstrual flow or cramps, she was bothered by frequent, unpredictable light bleeding/spotting. You prescribed norethindrone acetate (NETA) 5-mg tablet orally once daily, to be used in addition to her IUD. After using the IUD with concomitant NETA for 2 months’ duration, she noted that her bleeding/spotting almost completely resolved; however, she did report feeling irritable with use of the progestin tablets. She subsequently stopped the NETA tablets and, after 6 months of additional follow-up, reported only minimal spotting and no cramps.
At this later follow-up visit, you noted that her hemoglobin level increased to 12.6 g/dL, and the ferritin level no longer indicated iron depletion. After the IUD had been in place for 4 years, she reported that she was beginning to experience frequent light bleeding again. A follow-up vaginal sonogram noted a well-positioned IUD, there was no suggestion of intracavitary pathology, and adenomyosis continued to be imaged. She underwent IUD removal and placement of a new LNG 52 mg IUD. This resulted in marked reduction in her bleeding.
Case 2 Follow-up
Two weeks after beginning continuous oral progestin therapy, the patient called reporting frequent irregular bleeding. She was reassured that this was not unexpected and encouraged to continue oral progestin therapy. During a 3-month follow-up visit, the patient noted little, if any, bleeding over the previous 2 months and was pleased with this result. She continued to note acne and hirsutism and asked about the possibility of adding spironolactone to her oral progestin regimen.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynecol Obstet. 2018;143:393-408.
- Kaunitz AM. Abnormal uterine bleeding in reproductive-age women. JAMA. 2019;321:2126-2127.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Uterine Cancer. http://seer.cancer.gov/statfacts/html/corp.html. Accessed October 10, 2019.
- Wise MR, Gill P, Lensen S, et al. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am J Obstet Gynecol. 2016;215:598.e1-598.e8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- The North American Menopause Society. Menopause Practice–A Clinician’s Guide. 5th ed. NAMS: Mayfield Heights, OH; 2014.
- National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. https://www.nice.org.uk/guidance/ng88. Accessed October 10, 2019.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625-632.
- Kaunitz AM, Meredith S, Inki P, et al. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113:1104-1116.
- Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72:193-215.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8.
- Ylikorkala O, Pekonen F. Naproxen reduces idiopathic but not fibromyoma-induced menorrhagia. Obstet Gynecol. 1986;68:10-12.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:865-875.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1–103.
- ACOG Practice Bulletin no. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524–e533.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynecol Obstet. 2018;143:393-408.
- Kaunitz AM. Abnormal uterine bleeding in reproductive-age women. JAMA. 2019;321:2126-2127.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Uterine Cancer. http://seer.cancer.gov/statfacts/html/corp.html. Accessed October 10, 2019.
- Wise MR, Gill P, Lensen S, et al. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am J Obstet Gynecol. 2016;215:598.e1-598.e8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- The North American Menopause Society. Menopause Practice–A Clinician’s Guide. 5th ed. NAMS: Mayfield Heights, OH; 2014.
- National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. https://www.nice.org.uk/guidance/ng88. Accessed October 10, 2019.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625-632.
- Kaunitz AM, Meredith S, Inki P, et al. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113:1104-1116.
- Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72:193-215.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8.
- Ylikorkala O, Pekonen F. Naproxen reduces idiopathic but not fibromyoma-induced menorrhagia. Obstet Gynecol. 1986;68:10-12.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:865-875.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1–103.
- ACOG Practice Bulletin no. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524–e533.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
USPSTF recommendations on risk assessment, genetic counseling, and genetic testing for BRCA-related cancer
Breast cancer screening recommendations have evolved over the past decade. BRCA1/2 genes are tumor-suppressor genes. Mutations in these genes place women at an increased risk for developing breast, ovarian, fallopian tube, and peritoneal cancer. Detection of BRCA1/2 mutations through genetic screening can provide patients with more information about their cancer risk and can lead to discussion of prophylactic therapies. This includes increased screening frequency, medical therapy, and surgical interventions.
New USPSTF recommendations address who is at an increased risk for BRCA1/2 mutations. They recommend using screening tools focusing on family history that primary care physicians can utilize to determine who should be referred for genetic counseling to discuss the risks and benefits of genetic screening. The following are the task force’s two primary recommendations:
The USPSTF recommends that primary care clinicians assess women with a personal or family history of breast, ovarian, tubal, or peritoneal cancer or who have an ancestry associated with BRCA1/2 gene mutations with an appropriate brief familial risk assessment tool. Women with a positive result on the risk assessment tool should receive genetic counseling and, if indicated after counseling, genetic testing. (B recommendation)
The USPSTF recommends against routine risk assessment, genetic counseling, or genetic testing for women whose personal or family history or ancestry is not associated with potentially harmful BRCA1/2 gene mutations. (D recommendation)
Breast cancer is the second leading cause of cancer and cancer death for women in the United States. Ovarian cancer ranks fifth in cancer deaths for women in the U.S. By age 70, women with BRCA1/2 mutations have a 45%-65% cumulative lifetime risk of developing breast cancer.
Mutations in BRCA1, specifically, are associated with a 39% lifetime risk for ovarian, fallopian tube, and peritoneal cancer. In contrast, mutations in BRCA2 are associated with a 10%-17% lifetime risk.
The USPSTF also underscores the increased prevalence of BRCA1/2 mutations in the Ashkenazi Jewish population. Three out of seven familial risk assessment tools inquire about Jewish ancestry. This is because the Ashkenazi Jewish population have a higher prevalence of three founder mutations in the BRCA1/2 gene. A member of this population has a 1 in 40 chance of carrying one of these three mutations, whereas the general population has a 1 in 300 chance.
The USPSTF recommends a multistep process of screening. The first step is taking a family history of cancer. For women who have a family history of breast, ovarian, tubal, or peritoneal cancer or a personal history of these cancers, a brief familial risk assessment tool should be used to determine the need for referral for in-depth genetic counseling to determine the need for genetic testing.
It is important to recognize that the validated tools recommended by the USPSTF are specific for genetic risk assessment. General breast cancer risk assessment tools, including the National Cancer Institute Breast Cancer Risk Assessment Tool, which is based on the Gail model, are not recommended.
The sensitivity of the tools recommended by the USPSTF range between 77% and 100%. A number of tools are given as an option with no one tool being better than the other. Perhaps the easiest to implement of the validated tools recommended is the Pedigree Assessment Tool. For this tool, points are assigned for every family member with breast or ovarian cancer as indicated in the table below.

A positive result on a screening tool will lead primary care physicians to appropriately refer patients for genetic counseling. Genetic testing for BRCA1/2 mutations should be limited to those individuals whose personal and/or family history reflects an increased risk for gene mutations after detailed genetic assessment and counseling. The results of the genetic screening should assist a patient in their decision making.
Prophylactic treatment for BRCA1/2 mutation carriers are outside the scope of this recommendation. However the guidelines briefly review risk-reducing therapies including screening, medical, and surgical options. Medical therapy for patients who have BRCA1/2 mutations include the use of tamoxifen, raloxifene, and aromatase inhibitors. Surgical interventions include bilateral mastectomy and salpingo-oopherectomy.
Screening options include earlier and more frequent mammograms and breast MRIs. Screening is largely based on family history and the USPSTF acknowledges their uncertainty when screening women with an unknown family history. Male breast cancer, pancreatic cancer, prostate cancer, and melanoma are also associated with BRCA1/2 mutations. They are not included in this recommendation.
The bottom line
USPSTF recommended that primary care physicians should use familial risk assessment tools to screen women for BRCA1/2 mutations. This includes women with a personal and/or family history of breast, ovarian, tubal, or peritoneal cancer or women with a family history of BRCA1/2 gene mutations. Patients who test positive through one of the suggested screening tools should be referred for genetic counseling. This could lead to genetic testing and subsequent prophylactic therapies and/or increased screenings if the patient so desires. It is of importance to note the USPSTF recommends against routine screening of BRCA1/2 gene mutations for women who do not meet the above requirements.
Reference
USPSTF Recommendation: Assessment, counseling, and testing for BRCA-related cancer. JAMA. 2019;322(7):652-65. doi: 10.1001/jama.2019.10987.
Dr. Style is a second-year resident in the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
Breast cancer screening recommendations have evolved over the past decade. BRCA1/2 genes are tumor-suppressor genes. Mutations in these genes place women at an increased risk for developing breast, ovarian, fallopian tube, and peritoneal cancer. Detection of BRCA1/2 mutations through genetic screening can provide patients with more information about their cancer risk and can lead to discussion of prophylactic therapies. This includes increased screening frequency, medical therapy, and surgical interventions.
New USPSTF recommendations address who is at an increased risk for BRCA1/2 mutations. They recommend using screening tools focusing on family history that primary care physicians can utilize to determine who should be referred for genetic counseling to discuss the risks and benefits of genetic screening. The following are the task force’s two primary recommendations:
The USPSTF recommends that primary care clinicians assess women with a personal or family history of breast, ovarian, tubal, or peritoneal cancer or who have an ancestry associated with BRCA1/2 gene mutations with an appropriate brief familial risk assessment tool. Women with a positive result on the risk assessment tool should receive genetic counseling and, if indicated after counseling, genetic testing. (B recommendation)
The USPSTF recommends against routine risk assessment, genetic counseling, or genetic testing for women whose personal or family history or ancestry is not associated with potentially harmful BRCA1/2 gene mutations. (D recommendation)
Breast cancer is the second leading cause of cancer and cancer death for women in the United States. Ovarian cancer ranks fifth in cancer deaths for women in the U.S. By age 70, women with BRCA1/2 mutations have a 45%-65% cumulative lifetime risk of developing breast cancer.
Mutations in BRCA1, specifically, are associated with a 39% lifetime risk for ovarian, fallopian tube, and peritoneal cancer. In contrast, mutations in BRCA2 are associated with a 10%-17% lifetime risk.
The USPSTF also underscores the increased prevalence of BRCA1/2 mutations in the Ashkenazi Jewish population. Three out of seven familial risk assessment tools inquire about Jewish ancestry. This is because the Ashkenazi Jewish population have a higher prevalence of three founder mutations in the BRCA1/2 gene. A member of this population has a 1 in 40 chance of carrying one of these three mutations, whereas the general population has a 1 in 300 chance.
The USPSTF recommends a multistep process of screening. The first step is taking a family history of cancer. For women who have a family history of breast, ovarian, tubal, or peritoneal cancer or a personal history of these cancers, a brief familial risk assessment tool should be used to determine the need for referral for in-depth genetic counseling to determine the need for genetic testing.
It is important to recognize that the validated tools recommended by the USPSTF are specific for genetic risk assessment. General breast cancer risk assessment tools, including the National Cancer Institute Breast Cancer Risk Assessment Tool, which is based on the Gail model, are not recommended.
The sensitivity of the tools recommended by the USPSTF range between 77% and 100%. A number of tools are given as an option with no one tool being better than the other. Perhaps the easiest to implement of the validated tools recommended is the Pedigree Assessment Tool. For this tool, points are assigned for every family member with breast or ovarian cancer as indicated in the table below.

A positive result on a screening tool will lead primary care physicians to appropriately refer patients for genetic counseling. Genetic testing for BRCA1/2 mutations should be limited to those individuals whose personal and/or family history reflects an increased risk for gene mutations after detailed genetic assessment and counseling. The results of the genetic screening should assist a patient in their decision making.
Prophylactic treatment for BRCA1/2 mutation carriers are outside the scope of this recommendation. However the guidelines briefly review risk-reducing therapies including screening, medical, and surgical options. Medical therapy for patients who have BRCA1/2 mutations include the use of tamoxifen, raloxifene, and aromatase inhibitors. Surgical interventions include bilateral mastectomy and salpingo-oopherectomy.
Screening options include earlier and more frequent mammograms and breast MRIs. Screening is largely based on family history and the USPSTF acknowledges their uncertainty when screening women with an unknown family history. Male breast cancer, pancreatic cancer, prostate cancer, and melanoma are also associated with BRCA1/2 mutations. They are not included in this recommendation.
The bottom line
USPSTF recommended that primary care physicians should use familial risk assessment tools to screen women for BRCA1/2 mutations. This includes women with a personal and/or family history of breast, ovarian, tubal, or peritoneal cancer or women with a family history of BRCA1/2 gene mutations. Patients who test positive through one of the suggested screening tools should be referred for genetic counseling. This could lead to genetic testing and subsequent prophylactic therapies and/or increased screenings if the patient so desires. It is of importance to note the USPSTF recommends against routine screening of BRCA1/2 gene mutations for women who do not meet the above requirements.
Reference
USPSTF Recommendation: Assessment, counseling, and testing for BRCA-related cancer. JAMA. 2019;322(7):652-65. doi: 10.1001/jama.2019.10987.
Dr. Style is a second-year resident in the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
Breast cancer screening recommendations have evolved over the past decade. BRCA1/2 genes are tumor-suppressor genes. Mutations in these genes place women at an increased risk for developing breast, ovarian, fallopian tube, and peritoneal cancer. Detection of BRCA1/2 mutations through genetic screening can provide patients with more information about their cancer risk and can lead to discussion of prophylactic therapies. This includes increased screening frequency, medical therapy, and surgical interventions.
New USPSTF recommendations address who is at an increased risk for BRCA1/2 mutations. They recommend using screening tools focusing on family history that primary care physicians can utilize to determine who should be referred for genetic counseling to discuss the risks and benefits of genetic screening. The following are the task force’s two primary recommendations:
The USPSTF recommends that primary care clinicians assess women with a personal or family history of breast, ovarian, tubal, or peritoneal cancer or who have an ancestry associated with BRCA1/2 gene mutations with an appropriate brief familial risk assessment tool. Women with a positive result on the risk assessment tool should receive genetic counseling and, if indicated after counseling, genetic testing. (B recommendation)
The USPSTF recommends against routine risk assessment, genetic counseling, or genetic testing for women whose personal or family history or ancestry is not associated with potentially harmful BRCA1/2 gene mutations. (D recommendation)
Breast cancer is the second leading cause of cancer and cancer death for women in the United States. Ovarian cancer ranks fifth in cancer deaths for women in the U.S. By age 70, women with BRCA1/2 mutations have a 45%-65% cumulative lifetime risk of developing breast cancer.
Mutations in BRCA1, specifically, are associated with a 39% lifetime risk for ovarian, fallopian tube, and peritoneal cancer. In contrast, mutations in BRCA2 are associated with a 10%-17% lifetime risk.
The USPSTF also underscores the increased prevalence of BRCA1/2 mutations in the Ashkenazi Jewish population. Three out of seven familial risk assessment tools inquire about Jewish ancestry. This is because the Ashkenazi Jewish population have a higher prevalence of three founder mutations in the BRCA1/2 gene. A member of this population has a 1 in 40 chance of carrying one of these three mutations, whereas the general population has a 1 in 300 chance.
The USPSTF recommends a multistep process of screening. The first step is taking a family history of cancer. For women who have a family history of breast, ovarian, tubal, or peritoneal cancer or a personal history of these cancers, a brief familial risk assessment tool should be used to determine the need for referral for in-depth genetic counseling to determine the need for genetic testing.
It is important to recognize that the validated tools recommended by the USPSTF are specific for genetic risk assessment. General breast cancer risk assessment tools, including the National Cancer Institute Breast Cancer Risk Assessment Tool, which is based on the Gail model, are not recommended.
The sensitivity of the tools recommended by the USPSTF range between 77% and 100%. A number of tools are given as an option with no one tool being better than the other. Perhaps the easiest to implement of the validated tools recommended is the Pedigree Assessment Tool. For this tool, points are assigned for every family member with breast or ovarian cancer as indicated in the table below.

A positive result on a screening tool will lead primary care physicians to appropriately refer patients for genetic counseling. Genetic testing for BRCA1/2 mutations should be limited to those individuals whose personal and/or family history reflects an increased risk for gene mutations after detailed genetic assessment and counseling. The results of the genetic screening should assist a patient in their decision making.
Prophylactic treatment for BRCA1/2 mutation carriers are outside the scope of this recommendation. However the guidelines briefly review risk-reducing therapies including screening, medical, and surgical options. Medical therapy for patients who have BRCA1/2 mutations include the use of tamoxifen, raloxifene, and aromatase inhibitors. Surgical interventions include bilateral mastectomy and salpingo-oopherectomy.
Screening options include earlier and more frequent mammograms and breast MRIs. Screening is largely based on family history and the USPSTF acknowledges their uncertainty when screening women with an unknown family history. Male breast cancer, pancreatic cancer, prostate cancer, and melanoma are also associated with BRCA1/2 mutations. They are not included in this recommendation.
The bottom line
USPSTF recommended that primary care physicians should use familial risk assessment tools to screen women for BRCA1/2 mutations. This includes women with a personal and/or family history of breast, ovarian, tubal, or peritoneal cancer or women with a family history of BRCA1/2 gene mutations. Patients who test positive through one of the suggested screening tools should be referred for genetic counseling. This could lead to genetic testing and subsequent prophylactic therapies and/or increased screenings if the patient so desires. It is of importance to note the USPSTF recommends against routine screening of BRCA1/2 gene mutations for women who do not meet the above requirements.
Reference
USPSTF Recommendation: Assessment, counseling, and testing for BRCA-related cancer. JAMA. 2019;322(7):652-65. doi: 10.1001/jama.2019.10987.
Dr. Style is a second-year resident in the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
Is it time to expand the use of PARP inhibitors?
In this edition of “How I will treat my next patient,” I review two recent presentations at the European Society of Medical Oncology Congress regarding the expanded use of poly (adenosine diphosphate-ribose) polymerase inhibitors (PARPi) in patients with advanced solid tumors, potentially broadening the indications for this important class of agents.
Metastatic CRPC
Perhaps 25% of prostate cancer patients have loss-of-function mutations – BRCA1, BRCA2, and ATM – or alterations in homologous recombinant repair (HRR) genes. In the PROfound trial, men with metastatic castration-resistant prostate cancer (mCRPC) who had progressed on either abiraterone or enzalutamide and who had DNA-repair mutations were randomized to either olaparib (300 mg b.i.d.) or treatment of physician’s choice (TPC) with either abiraterone or enzalutamide plus prednisone (Hussain M et al. ESMO 2019, Abstract LBA-12).
Two cohorts were enrolled. Cohort A included 245 men with BRCA1, BRCA2, or ATM mutations, and cohort B included 142 men with other alterations (BARD1, BIRP1, CDK12, CHEK1, CHEK2, FANCL, PALB2, PPP2R2A, RAD15B, RAD15C, RAD15D, or RAD54L). After disease progression, patients could cross over to receive PARPi, which more than 80% of patients eventually did.
Median radiographic progression-free survival (PFS) in cohort A was 7.39 months with PARPi, compared with 3.55 months with TPC, for a hazard ratio for progression on PARPi of 0.34 (P less than .0001). A significant benefit was seen for PARPi in the overall population (both cohorts), with a median radiographic PFS of 5.82 months va. 3.52 months, respectively (HR, 0.49; P less than .0001).
Among patients in cohort A, the objective response rate (ORR) was 33.3% with PARPi, compared with 2.3% for TPC, resulting in an odds ratio for ORR of 20.86 (P less than .0001).
PARPi demonstrated a longer time to pain progression in cohort A, with the median not reached, compared with 9.92 months with TPC (HR, 0.44; P = .0192). Perhaps because of the high proportion of TPC patients who eventually received PARPi, no statistically significant differences in overall survival have yet been seen.
What this means in practice
During my fellowship, a mentor taught that “because quality of life is generally better before progression than afterwards, PFS is a worthy endpoint in its own right.” For that reason, although I would have liked to see the data for cohort B alone, it appears worthwhile for physicians to make every effort to obtain PARPi. The difference in ORR, pain progression, and PFS at 12 months is clinically dramatic.
Of equal significance, however, is that PROfound is the first positive phase 3 biomarker-selected study evaluating a targeted treatment in patients with mCRPC. For prostate cancer – as for breast, ovarian, pancreatic, and several other cancers – the molecular biology and genetic background of our patients dictates the other tumors for which they and their family members are at risk, and expands the treatment armamentarium for them.
For those clinicians who needed to be convinced that “precision medicine” for prostate cancer patients was worthwhile, the PROfound trial should have a profound impact.
Advanced ovarian cancer
The randomized, double-blind, placebo-controlled, phase 3 PAOLA-1/ENGOT-ov25 trial studied patients with stage III-IV ovarian, fallopian tube, or primary peritoneal cancer who had surgery, platinum-taxane chemotherapy, and at least 3 months of bevacizumab. Patients were randomized to maintenance treatment with an additional 12 months of bevacizumab plus 24 months of PARPi with olaparib or placebo. Germline BRCA mutations were not required (Ray-Coquard I et al. ESMO 2019, Abstract LBA2).
As reported at ESMO, adding PARPi to bevacizumab maintenance provided a clinically meaningful PFS benefit of 22.1 months, in comparison with 16.6 months for bevacizumab alone. The difference was statistically significant.
For patients with tumor BRCA mutations (tBRCAm), PFS was 37.2 months with olaparib vs. 21.7 months for placebo (HR, 0.31). The PFS benefit was even more impressive for homologous recombination deficient (HRD)–positive patients, inclusive of those with tBRCAm (PFS 37.2 months for PARPi vs. 17.7 months for placebo; and in the 152 HRD-positive patients without tBRCAm, (median PFS 28.1 months vs. 16.6 months; HR, 0.43).
The improved PFS in patients with tBRCAm is similar to that reported in the SOLO1 trial of olaparib monotherapy vs. chemotherapy in newly diagnosed advanced ovarian cancer (N Engl J Med. 2018; 379:2495-2505), but the PFS in the control arm was longer in PAOLA-1 than in SOLO1, perhaps because of the use of bevacizumab in PAOLA-1. PARPi did not affect tolerance to bevacizumab.
In PAOLA-1, the HRD-positive patients who lacked tBRCAm and, by extension, lacked germline BRCA mutations – a new population of patients – was identified who benefited substantially from maintenance PARPi in the first-line setting.
What this means in practice
PAOLA-1 demonstrates that PARPi can improve outcomes in first-line treatment – and in patients beyond those with germline BRCA mutations. As a result, PAOLA-1 potentially changes the standard of care for initial treatment of the respectable fraction of patients with previously untreated, advanced müllerian cancers who have either tBRCAm or HRD positive tumors.
Importantly, PAOLA-1 is one of many published trials that stimulates the discussion of cost vs. value for combinations of biologics. The incremental benefit from the second biologic (in this case PARPi) is almost never completely additive or supra-additive to the benefit associated with the first biologic (in this case, bevacizumab). In that regard, despite the fact that PARPi showed a PFS benefit in the intent-to-treat population overall, precisely defining the patient population that has the greatest benefit will facilitate the goal of getting the treatments of greatest “value for cost” to our patients in the most responsible way.
Additional research will hopefully define the relative contribution of bevacizumab to PARPi in patients who benefited so dramatically from PARPi in PAOLA-1.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I will treat my next patient,” I review two recent presentations at the European Society of Medical Oncology Congress regarding the expanded use of poly (adenosine diphosphate-ribose) polymerase inhibitors (PARPi) in patients with advanced solid tumors, potentially broadening the indications for this important class of agents.
Metastatic CRPC
Perhaps 25% of prostate cancer patients have loss-of-function mutations – BRCA1, BRCA2, and ATM – or alterations in homologous recombinant repair (HRR) genes. In the PROfound trial, men with metastatic castration-resistant prostate cancer (mCRPC) who had progressed on either abiraterone or enzalutamide and who had DNA-repair mutations were randomized to either olaparib (300 mg b.i.d.) or treatment of physician’s choice (TPC) with either abiraterone or enzalutamide plus prednisone (Hussain M et al. ESMO 2019, Abstract LBA-12).
Two cohorts were enrolled. Cohort A included 245 men with BRCA1, BRCA2, or ATM mutations, and cohort B included 142 men with other alterations (BARD1, BIRP1, CDK12, CHEK1, CHEK2, FANCL, PALB2, PPP2R2A, RAD15B, RAD15C, RAD15D, or RAD54L). After disease progression, patients could cross over to receive PARPi, which more than 80% of patients eventually did.
Median radiographic progression-free survival (PFS) in cohort A was 7.39 months with PARPi, compared with 3.55 months with TPC, for a hazard ratio for progression on PARPi of 0.34 (P less than .0001). A significant benefit was seen for PARPi in the overall population (both cohorts), with a median radiographic PFS of 5.82 months va. 3.52 months, respectively (HR, 0.49; P less than .0001).
Among patients in cohort A, the objective response rate (ORR) was 33.3% with PARPi, compared with 2.3% for TPC, resulting in an odds ratio for ORR of 20.86 (P less than .0001).
PARPi demonstrated a longer time to pain progression in cohort A, with the median not reached, compared with 9.92 months with TPC (HR, 0.44; P = .0192). Perhaps because of the high proportion of TPC patients who eventually received PARPi, no statistically significant differences in overall survival have yet been seen.
What this means in practice
During my fellowship, a mentor taught that “because quality of life is generally better before progression than afterwards, PFS is a worthy endpoint in its own right.” For that reason, although I would have liked to see the data for cohort B alone, it appears worthwhile for physicians to make every effort to obtain PARPi. The difference in ORR, pain progression, and PFS at 12 months is clinically dramatic.
Of equal significance, however, is that PROfound is the first positive phase 3 biomarker-selected study evaluating a targeted treatment in patients with mCRPC. For prostate cancer – as for breast, ovarian, pancreatic, and several other cancers – the molecular biology and genetic background of our patients dictates the other tumors for which they and their family members are at risk, and expands the treatment armamentarium for them.
For those clinicians who needed to be convinced that “precision medicine” for prostate cancer patients was worthwhile, the PROfound trial should have a profound impact.
Advanced ovarian cancer
The randomized, double-blind, placebo-controlled, phase 3 PAOLA-1/ENGOT-ov25 trial studied patients with stage III-IV ovarian, fallopian tube, or primary peritoneal cancer who had surgery, platinum-taxane chemotherapy, and at least 3 months of bevacizumab. Patients were randomized to maintenance treatment with an additional 12 months of bevacizumab plus 24 months of PARPi with olaparib or placebo. Germline BRCA mutations were not required (Ray-Coquard I et al. ESMO 2019, Abstract LBA2).
As reported at ESMO, adding PARPi to bevacizumab maintenance provided a clinically meaningful PFS benefit of 22.1 months, in comparison with 16.6 months for bevacizumab alone. The difference was statistically significant.
For patients with tumor BRCA mutations (tBRCAm), PFS was 37.2 months with olaparib vs. 21.7 months for placebo (HR, 0.31). The PFS benefit was even more impressive for homologous recombination deficient (HRD)–positive patients, inclusive of those with tBRCAm (PFS 37.2 months for PARPi vs. 17.7 months for placebo; and in the 152 HRD-positive patients without tBRCAm, (median PFS 28.1 months vs. 16.6 months; HR, 0.43).
The improved PFS in patients with tBRCAm is similar to that reported in the SOLO1 trial of olaparib monotherapy vs. chemotherapy in newly diagnosed advanced ovarian cancer (N Engl J Med. 2018; 379:2495-2505), but the PFS in the control arm was longer in PAOLA-1 than in SOLO1, perhaps because of the use of bevacizumab in PAOLA-1. PARPi did not affect tolerance to bevacizumab.
In PAOLA-1, the HRD-positive patients who lacked tBRCAm and, by extension, lacked germline BRCA mutations – a new population of patients – was identified who benefited substantially from maintenance PARPi in the first-line setting.
What this means in practice
PAOLA-1 demonstrates that PARPi can improve outcomes in first-line treatment – and in patients beyond those with germline BRCA mutations. As a result, PAOLA-1 potentially changes the standard of care for initial treatment of the respectable fraction of patients with previously untreated, advanced müllerian cancers who have either tBRCAm or HRD positive tumors.
Importantly, PAOLA-1 is one of many published trials that stimulates the discussion of cost vs. value for combinations of biologics. The incremental benefit from the second biologic (in this case PARPi) is almost never completely additive or supra-additive to the benefit associated with the first biologic (in this case, bevacizumab). In that regard, despite the fact that PARPi showed a PFS benefit in the intent-to-treat population overall, precisely defining the patient population that has the greatest benefit will facilitate the goal of getting the treatments of greatest “value for cost” to our patients in the most responsible way.
Additional research will hopefully define the relative contribution of bevacizumab to PARPi in patients who benefited so dramatically from PARPi in PAOLA-1.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I will treat my next patient,” I review two recent presentations at the European Society of Medical Oncology Congress regarding the expanded use of poly (adenosine diphosphate-ribose) polymerase inhibitors (PARPi) in patients with advanced solid tumors, potentially broadening the indications for this important class of agents.
Metastatic CRPC
Perhaps 25% of prostate cancer patients have loss-of-function mutations – BRCA1, BRCA2, and ATM – or alterations in homologous recombinant repair (HRR) genes. In the PROfound trial, men with metastatic castration-resistant prostate cancer (mCRPC) who had progressed on either abiraterone or enzalutamide and who had DNA-repair mutations were randomized to either olaparib (300 mg b.i.d.) or treatment of physician’s choice (TPC) with either abiraterone or enzalutamide plus prednisone (Hussain M et al. ESMO 2019, Abstract LBA-12).
Two cohorts were enrolled. Cohort A included 245 men with BRCA1, BRCA2, or ATM mutations, and cohort B included 142 men with other alterations (BARD1, BIRP1, CDK12, CHEK1, CHEK2, FANCL, PALB2, PPP2R2A, RAD15B, RAD15C, RAD15D, or RAD54L). After disease progression, patients could cross over to receive PARPi, which more than 80% of patients eventually did.
Median radiographic progression-free survival (PFS) in cohort A was 7.39 months with PARPi, compared with 3.55 months with TPC, for a hazard ratio for progression on PARPi of 0.34 (P less than .0001). A significant benefit was seen for PARPi in the overall population (both cohorts), with a median radiographic PFS of 5.82 months va. 3.52 months, respectively (HR, 0.49; P less than .0001).
Among patients in cohort A, the objective response rate (ORR) was 33.3% with PARPi, compared with 2.3% for TPC, resulting in an odds ratio for ORR of 20.86 (P less than .0001).
PARPi demonstrated a longer time to pain progression in cohort A, with the median not reached, compared with 9.92 months with TPC (HR, 0.44; P = .0192). Perhaps because of the high proportion of TPC patients who eventually received PARPi, no statistically significant differences in overall survival have yet been seen.
What this means in practice
During my fellowship, a mentor taught that “because quality of life is generally better before progression than afterwards, PFS is a worthy endpoint in its own right.” For that reason, although I would have liked to see the data for cohort B alone, it appears worthwhile for physicians to make every effort to obtain PARPi. The difference in ORR, pain progression, and PFS at 12 months is clinically dramatic.
Of equal significance, however, is that PROfound is the first positive phase 3 biomarker-selected study evaluating a targeted treatment in patients with mCRPC. For prostate cancer – as for breast, ovarian, pancreatic, and several other cancers – the molecular biology and genetic background of our patients dictates the other tumors for which they and their family members are at risk, and expands the treatment armamentarium for them.
For those clinicians who needed to be convinced that “precision medicine” for prostate cancer patients was worthwhile, the PROfound trial should have a profound impact.
Advanced ovarian cancer
The randomized, double-blind, placebo-controlled, phase 3 PAOLA-1/ENGOT-ov25 trial studied patients with stage III-IV ovarian, fallopian tube, or primary peritoneal cancer who had surgery, platinum-taxane chemotherapy, and at least 3 months of bevacizumab. Patients were randomized to maintenance treatment with an additional 12 months of bevacizumab plus 24 months of PARPi with olaparib or placebo. Germline BRCA mutations were not required (Ray-Coquard I et al. ESMO 2019, Abstract LBA2).
As reported at ESMO, adding PARPi to bevacizumab maintenance provided a clinically meaningful PFS benefit of 22.1 months, in comparison with 16.6 months for bevacizumab alone. The difference was statistically significant.
For patients with tumor BRCA mutations (tBRCAm), PFS was 37.2 months with olaparib vs. 21.7 months for placebo (HR, 0.31). The PFS benefit was even more impressive for homologous recombination deficient (HRD)–positive patients, inclusive of those with tBRCAm (PFS 37.2 months for PARPi vs. 17.7 months for placebo; and in the 152 HRD-positive patients without tBRCAm, (median PFS 28.1 months vs. 16.6 months; HR, 0.43).
The improved PFS in patients with tBRCAm is similar to that reported in the SOLO1 trial of olaparib monotherapy vs. chemotherapy in newly diagnosed advanced ovarian cancer (N Engl J Med. 2018; 379:2495-2505), but the PFS in the control arm was longer in PAOLA-1 than in SOLO1, perhaps because of the use of bevacizumab in PAOLA-1. PARPi did not affect tolerance to bevacizumab.
In PAOLA-1, the HRD-positive patients who lacked tBRCAm and, by extension, lacked germline BRCA mutations – a new population of patients – was identified who benefited substantially from maintenance PARPi in the first-line setting.
What this means in practice
PAOLA-1 demonstrates that PARPi can improve outcomes in first-line treatment – and in patients beyond those with germline BRCA mutations. As a result, PAOLA-1 potentially changes the standard of care for initial treatment of the respectable fraction of patients with previously untreated, advanced müllerian cancers who have either tBRCAm or HRD positive tumors.
Importantly, PAOLA-1 is one of many published trials that stimulates the discussion of cost vs. value for combinations of biologics. The incremental benefit from the second biologic (in this case PARPi) is almost never completely additive or supra-additive to the benefit associated with the first biologic (in this case, bevacizumab). In that regard, despite the fact that PARPi showed a PFS benefit in the intent-to-treat population overall, precisely defining the patient population that has the greatest benefit will facilitate the goal of getting the treatments of greatest “value for cost” to our patients in the most responsible way.
Additional research will hopefully define the relative contribution of bevacizumab to PARPi in patients who benefited so dramatically from PARPi in PAOLA-1.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.