User login
Cediranib may alter DNA repair capacity
Cediranib was found to confer sensitivity to olaparib through downregulation of the homology-directed DNA repair (HDR) pathway in tumor cells, investigators report.
“The objective of this study was to define the effects of cediranib on the HDR pathway of DNA repair,” wrote Alanna R. Kaplan, MD, of Yale University, New Haven, Conn., and colleagues. The report is in Science Translational Medicine.
The researchers explored the effects of combination cediranib and olaparib therapy at the molecular level using various in vitro and in vivo experiments. Tumor growth studies were conducted in a mouse model with sample sizes selected based on prior experience.
“In vitro experiments were performed in biological triplicate unless otherwise stated,” the researchers wrote. “For in vivo experiments, mice were randomly assigned to treatment groups,” they added.
After analysis, the researchers found that cediranib provides sensitivity to olaparib through suppression of the HDR pathway in malignant cells. The downregulation was explained in part by the inducement of hypoxia, which inhibited gene expression of certain factors in the pathway.
“We noted a decrease in the expression of HDR factors BRCA1, BRCA2, and RAD51 in the cediranib-treated groups compared to controls,” the researchers explained.
In addition, the team reported that cediranib alone exhibits direct effects on the HDR pathway outside of mechanisms related to tumor hypoxia.
“This downregulation was seen in mouse tumor xenografts but not in mouse bone marrow, providing a therapeutic window for combining cediranib and olaparib in cancer therapy,” the team wrote.
The researchers acknowledged that a key limitation of the study was the lack of inquiry into the effects of other mutations on the HDR pathway, which could possibly influence the effects of cediranib in tumor cells.
“These findings identify a pathway by which cediranib can alter the DNA repair capacity of cancer cells that has implications for the design of cancer therapies,” the authors concluded.
The study was supported by grant funding from the National Institutes of Health. One of the researchers reported financial affiliations with Trucode Gene Repair, Cybrexa Therapeutics, and Patrys.
SOURCE: Kaplan AR et al. Sci Transl Med. 2019 May 15. doi: 10.1126/scitranslmed.aav4508.
Cediranib was found to confer sensitivity to olaparib through downregulation of the homology-directed DNA repair (HDR) pathway in tumor cells, investigators report.
“The objective of this study was to define the effects of cediranib on the HDR pathway of DNA repair,” wrote Alanna R. Kaplan, MD, of Yale University, New Haven, Conn., and colleagues. The report is in Science Translational Medicine.
The researchers explored the effects of combination cediranib and olaparib therapy at the molecular level using various in vitro and in vivo experiments. Tumor growth studies were conducted in a mouse model with sample sizes selected based on prior experience.
“In vitro experiments were performed in biological triplicate unless otherwise stated,” the researchers wrote. “For in vivo experiments, mice were randomly assigned to treatment groups,” they added.
After analysis, the researchers found that cediranib provides sensitivity to olaparib through suppression of the HDR pathway in malignant cells. The downregulation was explained in part by the inducement of hypoxia, which inhibited gene expression of certain factors in the pathway.
“We noted a decrease in the expression of HDR factors BRCA1, BRCA2, and RAD51 in the cediranib-treated groups compared to controls,” the researchers explained.
In addition, the team reported that cediranib alone exhibits direct effects on the HDR pathway outside of mechanisms related to tumor hypoxia.
“This downregulation was seen in mouse tumor xenografts but not in mouse bone marrow, providing a therapeutic window for combining cediranib and olaparib in cancer therapy,” the team wrote.
The researchers acknowledged that a key limitation of the study was the lack of inquiry into the effects of other mutations on the HDR pathway, which could possibly influence the effects of cediranib in tumor cells.
“These findings identify a pathway by which cediranib can alter the DNA repair capacity of cancer cells that has implications for the design of cancer therapies,” the authors concluded.
The study was supported by grant funding from the National Institutes of Health. One of the researchers reported financial affiliations with Trucode Gene Repair, Cybrexa Therapeutics, and Patrys.
SOURCE: Kaplan AR et al. Sci Transl Med. 2019 May 15. doi: 10.1126/scitranslmed.aav4508.
Cediranib was found to confer sensitivity to olaparib through downregulation of the homology-directed DNA repair (HDR) pathway in tumor cells, investigators report.
“The objective of this study was to define the effects of cediranib on the HDR pathway of DNA repair,” wrote Alanna R. Kaplan, MD, of Yale University, New Haven, Conn., and colleagues. The report is in Science Translational Medicine.
The researchers explored the effects of combination cediranib and olaparib therapy at the molecular level using various in vitro and in vivo experiments. Tumor growth studies were conducted in a mouse model with sample sizes selected based on prior experience.
“In vitro experiments were performed in biological triplicate unless otherwise stated,” the researchers wrote. “For in vivo experiments, mice were randomly assigned to treatment groups,” they added.
After analysis, the researchers found that cediranib provides sensitivity to olaparib through suppression of the HDR pathway in malignant cells. The downregulation was explained in part by the inducement of hypoxia, which inhibited gene expression of certain factors in the pathway.
“We noted a decrease in the expression of HDR factors BRCA1, BRCA2, and RAD51 in the cediranib-treated groups compared to controls,” the researchers explained.
In addition, the team reported that cediranib alone exhibits direct effects on the HDR pathway outside of mechanisms related to tumor hypoxia.
“This downregulation was seen in mouse tumor xenografts but not in mouse bone marrow, providing a therapeutic window for combining cediranib and olaparib in cancer therapy,” the team wrote.
The researchers acknowledged that a key limitation of the study was the lack of inquiry into the effects of other mutations on the HDR pathway, which could possibly influence the effects of cediranib in tumor cells.
“These findings identify a pathway by which cediranib can alter the DNA repair capacity of cancer cells that has implications for the design of cancer therapies,” the authors concluded.
The study was supported by grant funding from the National Institutes of Health. One of the researchers reported financial affiliations with Trucode Gene Repair, Cybrexa Therapeutics, and Patrys.
SOURCE: Kaplan AR et al. Sci Transl Med. 2019 May 15. doi: 10.1126/scitranslmed.aav4508.
FROM SCIENCE TRANSLATIONAL MEDICINE
HPV vaccine: Is one dose enough?
LJUBLJANA, SLOVENIA – There is good news and bad news about human papillomavirus (HPV) vaccination as a means of preventing cervical cancer.
The bad news is the HPV vaccines are projected to be in short supply, unable to meet global demand until at least 2024. The good news is that – in one study, for 11 years and counting – which would effectively double the existing supply, Aimee R. Kreimer, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
These data come from post hoc analyses of major phase 3 randomized controlled trials of bivalent HPV vaccine in Costa Rica and quadrivalent vaccine in India. However, these secondary analyses aren’t considered rock solid evidence because the subjects who got a single dose weren’t randomized to that strategy, they simply for one reason or another didn’t receive the recommended additional dose or doses.
“I don’t know if these studies are enough, so several studies have been launched over the past couple of years with an eye toward generating the quality of data that would be sufficient to motivate policy change, if in fact one dose is proven to be effective,” said Dr. Kreimer, a senior scientist at the National Cancer Institute in Bethesda, Md.
The first of these formal randomized, controlled trials – a delayed second-dose study in 9- to 11-year-old U.S. boys and girls – is due to be completed next year. Four other trials ongoing in Africa and Costa Rica, all in females, are expected to report findings in 2022-2025.
Dr. Kreimer is first author of a soon-to-be-published 11-year update from the phase 3 Costa Rica HPV Vaccine Trial, which was launched prior to licensure of the GlaxoSmithKline bivalent HPV vaccine. Previous analyses showed that at both 4 and 7 years of follow-up, a single dose of the vaccine was as effective as two or three in preventing infection with HPV types 16 and 18, which are covered by the vaccine.
“Now the research question has transitioned to, ‘Will one dose be sufficiently durable?’ she explained.
The answer from this study is yes. At 11 years since receipt of the bivalent HPV vaccine, there was no difference in terms of prevalent HPV 16/18 infection between the one-, two-, and three-dose groups. To address the issue of possible selection bias in this post hoc nonrandomized comparison, Dr. Kreimer and her coinvestigators looked at rates of infection with HPV 31 and 45, which aren’t covered by the vaccine. The rates were similar regardless of the number of vaccine doses received 11 years earlier, indicating women in all three dosing groups are at similar risk for acquiring HPV infection, thus bolstering the legitimacy of the conclusion that one dose provides effective long-term protection.
Intriguingly, HPV serum antibody levels in the single-dose group have remained stable for 11 years at a level that’s only about one-quarter of that associated with three doses of the vaccine, albeit an order of magnitude greater than the level induced by natural immunity.
“This really challenges the dogma of the HPV vaccine,” according to Dr. Kreimer. “It suggests that inferior [HPV] antibodies do not necessarily mean inferior protection.”
The explanation for this phenomenon appears to be that HPV subunit vaccine mimics the shell of authentic virions so well that the immune system sees it as dangerous and mounts long-term antibody production. Also, cervical infection by HPV is a relatively slow process, allowing time for vaccine-induced antibodies to interrupt it, she said.
In contrast to the encouraging findings from this post hoc analysis and another from a phase 3 trial of quadrivalent vaccine in India, numerous phase 4 vaccine effectiveness monitoring studies have shown markedly lower vaccine effectiveness for one dose of HPV vaccine. Dr. Kreimer cautioned that this is a flawed conclusion attributable to a methodologic artifact whereby the investigators have lumped together single-dose recipients who were 17 years old or more at the time with those who were younger.
“The problem is that many people who are aged 17-18 years already have HPV infection, so when they are vaccinated it shows up as a vaccine failure. That’s not correct. These are prophylactic HPV vaccines. They’re not meant to help clear an infection,” she noted.
Stepping back, Dr. Kreimer observed that cervical cancer “is really a story of inequality.” Indeed, 90% of cervical cancers occur in low-income countries, where HPV vaccination uptake remains very low even more than a decade after licensure. When modelers project out in the future, they estimate that at current HPV vaccination levels in Sub-Saharan Africa, which has the highest cervical cancer rates in the world, it would take more than 100 years to achieve the World Health Organization goal of eliminating the malignancy.
Asked by an audience member how low a single-dose vaccine effectiveness level she considers acceptable to help reach the goal of eliminating cervical cancer in developing countries, Dr. Kreimer cautioned against the tendency to let ‘perfect’ become the enemy of ‘good.’
“I’ll remind everyone that, in this moment, very few of the target girls in the lower– and upper-lower–income countries are getting any vaccination. So I don’t think it’s a question of whether we should be going from two to one dose, I think it’s really a question of, for those who are at zero doses, how do we get them one dose? And with the HPV vaccine, we’ve even seen suggestions of herd immunity if we have 50% uptake,” she replied.
Dr. Kreimer reported having no financial conflicts regarding her presentation.
LJUBLJANA, SLOVENIA – There is good news and bad news about human papillomavirus (HPV) vaccination as a means of preventing cervical cancer.
The bad news is the HPV vaccines are projected to be in short supply, unable to meet global demand until at least 2024. The good news is that – in one study, for 11 years and counting – which would effectively double the existing supply, Aimee R. Kreimer, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
These data come from post hoc analyses of major phase 3 randomized controlled trials of bivalent HPV vaccine in Costa Rica and quadrivalent vaccine in India. However, these secondary analyses aren’t considered rock solid evidence because the subjects who got a single dose weren’t randomized to that strategy, they simply for one reason or another didn’t receive the recommended additional dose or doses.
“I don’t know if these studies are enough, so several studies have been launched over the past couple of years with an eye toward generating the quality of data that would be sufficient to motivate policy change, if in fact one dose is proven to be effective,” said Dr. Kreimer, a senior scientist at the National Cancer Institute in Bethesda, Md.
The first of these formal randomized, controlled trials – a delayed second-dose study in 9- to 11-year-old U.S. boys and girls – is due to be completed next year. Four other trials ongoing in Africa and Costa Rica, all in females, are expected to report findings in 2022-2025.
Dr. Kreimer is first author of a soon-to-be-published 11-year update from the phase 3 Costa Rica HPV Vaccine Trial, which was launched prior to licensure of the GlaxoSmithKline bivalent HPV vaccine. Previous analyses showed that at both 4 and 7 years of follow-up, a single dose of the vaccine was as effective as two or three in preventing infection with HPV types 16 and 18, which are covered by the vaccine.
“Now the research question has transitioned to, ‘Will one dose be sufficiently durable?’ she explained.
The answer from this study is yes. At 11 years since receipt of the bivalent HPV vaccine, there was no difference in terms of prevalent HPV 16/18 infection between the one-, two-, and three-dose groups. To address the issue of possible selection bias in this post hoc nonrandomized comparison, Dr. Kreimer and her coinvestigators looked at rates of infection with HPV 31 and 45, which aren’t covered by the vaccine. The rates were similar regardless of the number of vaccine doses received 11 years earlier, indicating women in all three dosing groups are at similar risk for acquiring HPV infection, thus bolstering the legitimacy of the conclusion that one dose provides effective long-term protection.
Intriguingly, HPV serum antibody levels in the single-dose group have remained stable for 11 years at a level that’s only about one-quarter of that associated with three doses of the vaccine, albeit an order of magnitude greater than the level induced by natural immunity.
“This really challenges the dogma of the HPV vaccine,” according to Dr. Kreimer. “It suggests that inferior [HPV] antibodies do not necessarily mean inferior protection.”
The explanation for this phenomenon appears to be that HPV subunit vaccine mimics the shell of authentic virions so well that the immune system sees it as dangerous and mounts long-term antibody production. Also, cervical infection by HPV is a relatively slow process, allowing time for vaccine-induced antibodies to interrupt it, she said.
In contrast to the encouraging findings from this post hoc analysis and another from a phase 3 trial of quadrivalent vaccine in India, numerous phase 4 vaccine effectiveness monitoring studies have shown markedly lower vaccine effectiveness for one dose of HPV vaccine. Dr. Kreimer cautioned that this is a flawed conclusion attributable to a methodologic artifact whereby the investigators have lumped together single-dose recipients who were 17 years old or more at the time with those who were younger.
“The problem is that many people who are aged 17-18 years already have HPV infection, so when they are vaccinated it shows up as a vaccine failure. That’s not correct. These are prophylactic HPV vaccines. They’re not meant to help clear an infection,” she noted.
Stepping back, Dr. Kreimer observed that cervical cancer “is really a story of inequality.” Indeed, 90% of cervical cancers occur in low-income countries, where HPV vaccination uptake remains very low even more than a decade after licensure. When modelers project out in the future, they estimate that at current HPV vaccination levels in Sub-Saharan Africa, which has the highest cervical cancer rates in the world, it would take more than 100 years to achieve the World Health Organization goal of eliminating the malignancy.
Asked by an audience member how low a single-dose vaccine effectiveness level she considers acceptable to help reach the goal of eliminating cervical cancer in developing countries, Dr. Kreimer cautioned against the tendency to let ‘perfect’ become the enemy of ‘good.’
“I’ll remind everyone that, in this moment, very few of the target girls in the lower– and upper-lower–income countries are getting any vaccination. So I don’t think it’s a question of whether we should be going from two to one dose, I think it’s really a question of, for those who are at zero doses, how do we get them one dose? And with the HPV vaccine, we’ve even seen suggestions of herd immunity if we have 50% uptake,” she replied.
Dr. Kreimer reported having no financial conflicts regarding her presentation.
LJUBLJANA, SLOVENIA – There is good news and bad news about human papillomavirus (HPV) vaccination as a means of preventing cervical cancer.
The bad news is the HPV vaccines are projected to be in short supply, unable to meet global demand until at least 2024. The good news is that – in one study, for 11 years and counting – which would effectively double the existing supply, Aimee R. Kreimer, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
These data come from post hoc analyses of major phase 3 randomized controlled trials of bivalent HPV vaccine in Costa Rica and quadrivalent vaccine in India. However, these secondary analyses aren’t considered rock solid evidence because the subjects who got a single dose weren’t randomized to that strategy, they simply for one reason or another didn’t receive the recommended additional dose or doses.
“I don’t know if these studies are enough, so several studies have been launched over the past couple of years with an eye toward generating the quality of data that would be sufficient to motivate policy change, if in fact one dose is proven to be effective,” said Dr. Kreimer, a senior scientist at the National Cancer Institute in Bethesda, Md.
The first of these formal randomized, controlled trials – a delayed second-dose study in 9- to 11-year-old U.S. boys and girls – is due to be completed next year. Four other trials ongoing in Africa and Costa Rica, all in females, are expected to report findings in 2022-2025.
Dr. Kreimer is first author of a soon-to-be-published 11-year update from the phase 3 Costa Rica HPV Vaccine Trial, which was launched prior to licensure of the GlaxoSmithKline bivalent HPV vaccine. Previous analyses showed that at both 4 and 7 years of follow-up, a single dose of the vaccine was as effective as two or three in preventing infection with HPV types 16 and 18, which are covered by the vaccine.
“Now the research question has transitioned to, ‘Will one dose be sufficiently durable?’ she explained.
The answer from this study is yes. At 11 years since receipt of the bivalent HPV vaccine, there was no difference in terms of prevalent HPV 16/18 infection between the one-, two-, and three-dose groups. To address the issue of possible selection bias in this post hoc nonrandomized comparison, Dr. Kreimer and her coinvestigators looked at rates of infection with HPV 31 and 45, which aren’t covered by the vaccine. The rates were similar regardless of the number of vaccine doses received 11 years earlier, indicating women in all three dosing groups are at similar risk for acquiring HPV infection, thus bolstering the legitimacy of the conclusion that one dose provides effective long-term protection.
Intriguingly, HPV serum antibody levels in the single-dose group have remained stable for 11 years at a level that’s only about one-quarter of that associated with three doses of the vaccine, albeit an order of magnitude greater than the level induced by natural immunity.
“This really challenges the dogma of the HPV vaccine,” according to Dr. Kreimer. “It suggests that inferior [HPV] antibodies do not necessarily mean inferior protection.”
The explanation for this phenomenon appears to be that HPV subunit vaccine mimics the shell of authentic virions so well that the immune system sees it as dangerous and mounts long-term antibody production. Also, cervical infection by HPV is a relatively slow process, allowing time for vaccine-induced antibodies to interrupt it, she said.
In contrast to the encouraging findings from this post hoc analysis and another from a phase 3 trial of quadrivalent vaccine in India, numerous phase 4 vaccine effectiveness monitoring studies have shown markedly lower vaccine effectiveness for one dose of HPV vaccine. Dr. Kreimer cautioned that this is a flawed conclusion attributable to a methodologic artifact whereby the investigators have lumped together single-dose recipients who were 17 years old or more at the time with those who were younger.
“The problem is that many people who are aged 17-18 years already have HPV infection, so when they are vaccinated it shows up as a vaccine failure. That’s not correct. These are prophylactic HPV vaccines. They’re not meant to help clear an infection,” she noted.
Stepping back, Dr. Kreimer observed that cervical cancer “is really a story of inequality.” Indeed, 90% of cervical cancers occur in low-income countries, where HPV vaccination uptake remains very low even more than a decade after licensure. When modelers project out in the future, they estimate that at current HPV vaccination levels in Sub-Saharan Africa, which has the highest cervical cancer rates in the world, it would take more than 100 years to achieve the World Health Organization goal of eliminating the malignancy.
Asked by an audience member how low a single-dose vaccine effectiveness level she considers acceptable to help reach the goal of eliminating cervical cancer in developing countries, Dr. Kreimer cautioned against the tendency to let ‘perfect’ become the enemy of ‘good.’
“I’ll remind everyone that, in this moment, very few of the target girls in the lower– and upper-lower–income countries are getting any vaccination. So I don’t think it’s a question of whether we should be going from two to one dose, I think it’s really a question of, for those who are at zero doses, how do we get them one dose? And with the HPV vaccine, we’ve even seen suggestions of herd immunity if we have 50% uptake,” she replied.
Dr. Kreimer reported having no financial conflicts regarding her presentation.
EXPERT ANALYSIS FROM ESPID 2019
Targeted sequencing panel IDs Lynch syndrome in women with/at risk for endometrial cancer
NASHVILLE, TENN. – , according to findings in a prospective patient cohort.
The findings, which also suggest that the incidence of Lynch syndrome among endometrial cancer patients is higher than previously recognized, have “immediate and major implications for the individual patient with endometrial cancer ... and implications for related family members,” Maria Mercedes M. Padron, MD, reported during an e-poster session at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Of 71 patients included in the study, 67 were undergoing endometrial cancer treatment and 7 (3 among those undergoing endometrial cancer treatment and 4 who did not have endometrial cancer) were known to have Lynch syndrome.
Of the 67 undergoing treatment, 22 (33%) were identified by the direct sequencing panel as having Lynch syndrome mutations, and of those, 7 (10%) had mutations classified as high confidence inactivating mutations in either MLH1, MSH6, PMS2, or MSH2 genes, said Dr. Padron, a research scholar at Icahn School of Medicine at Mount Sinai, New York. The remaining 15 patients had rare mutations and met previously defined phenotypic criteria for Lynch syndrome pathogenicity, she reported.
The sequencing panel–based results were compared with commercially available gene tests, including immunohistochemistry (IHC) and microsatellite instability testing (MSI); 10 patients were identified by IHC to have loss of nuclear mismatch repair (MMR) protein expression, and 8 of those were Lynch syndrome mutation positive. In addition, two patients were MSI-high, and both of those were Lynch syndrome mutation positive.
Thus, two Lynch syndrome patients were missed by direct sequencing, noted Dr. Padron.
However, an additional 10 patients who were not identified as having Lynch syndrome by IHC and MSI testing were potentially identified as such using the sequencing panel, Dr. Padron said, noting that “the pathogenicity of these additional variants needs to be defined.”
Lynch syndrome is a hereditary cancer syndrome caused by germline mutations in DNA MMR genes; it is the third most common malignancy in women and it confers an increased risk of several types of cancer, including colorectal, ovarian, gastric, and endometrial cancer, among others.
“It is estimated that 3% to 5% of endometrial cancers will arise from Lynch syndrome,” Dr. Padron explained during the poster session.
Because the presence of Lynch syndrome directly affects immediate clinical management and future risk-reducing and surveillance strategies for patients and at-risk family members, screening is recommended in all women with endometrial cancer, she added, noting, however, that “the optimum screening method has yet to be established.”
The sequencing panel evaluated in this study – Swift’s Accel-Amplicon Plus Lynch Syndrome Panel – requires only low input amounts of DNA, and in an earlier test using 10 control samples, it exhibited greater than 90% on-target and coverage uniformity. The work flow allowed for data analysis within 2 days, Dr. Padron noted.
The panel then was tested in the current cohort of patients who were referred to a gynecology oncology clinic for either treatment of endometrial cancer or for evaluation of risk for endometrial cancer.
Germline/tumor DNA was isolated and 10 ng DNA was used for targeted exon-level hotspot coverage of MLH1, MSH2, MSH6, and PMS2.
The findings suggest that the prevalence of Lynch syndrome may be six to seven times greater than previously estimated, Dr. Padron said during the poster presentation.
“If confirmed, this would have huge implications for our patients and health care system,” she said, adding that the ability to perform and analyze the sequencing within 48 hours of sample collection using a very low DNA input also was of note.
Taken together, “the findings of this study support future larger studies that can be performed concurrently with current standard of care technologies,” she and her colleagues concluded, noting that such studies would better determine more robust estimates of the prevalence of Lynch syndrome in women with endometrial cancer, help define improved standard-of-care guidelines, and provide future guidance for possible universal/targeted screening programs – all with the goal of improving the clinical care of women.
Dr. Padron reported having no relevant financial disclosures.
NASHVILLE, TENN. – , according to findings in a prospective patient cohort.
The findings, which also suggest that the incidence of Lynch syndrome among endometrial cancer patients is higher than previously recognized, have “immediate and major implications for the individual patient with endometrial cancer ... and implications for related family members,” Maria Mercedes M. Padron, MD, reported during an e-poster session at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Of 71 patients included in the study, 67 were undergoing endometrial cancer treatment and 7 (3 among those undergoing endometrial cancer treatment and 4 who did not have endometrial cancer) were known to have Lynch syndrome.
Of the 67 undergoing treatment, 22 (33%) were identified by the direct sequencing panel as having Lynch syndrome mutations, and of those, 7 (10%) had mutations classified as high confidence inactivating mutations in either MLH1, MSH6, PMS2, or MSH2 genes, said Dr. Padron, a research scholar at Icahn School of Medicine at Mount Sinai, New York. The remaining 15 patients had rare mutations and met previously defined phenotypic criteria for Lynch syndrome pathogenicity, she reported.
The sequencing panel–based results were compared with commercially available gene tests, including immunohistochemistry (IHC) and microsatellite instability testing (MSI); 10 patients were identified by IHC to have loss of nuclear mismatch repair (MMR) protein expression, and 8 of those were Lynch syndrome mutation positive. In addition, two patients were MSI-high, and both of those were Lynch syndrome mutation positive.
Thus, two Lynch syndrome patients were missed by direct sequencing, noted Dr. Padron.
However, an additional 10 patients who were not identified as having Lynch syndrome by IHC and MSI testing were potentially identified as such using the sequencing panel, Dr. Padron said, noting that “the pathogenicity of these additional variants needs to be defined.”
Lynch syndrome is a hereditary cancer syndrome caused by germline mutations in DNA MMR genes; it is the third most common malignancy in women and it confers an increased risk of several types of cancer, including colorectal, ovarian, gastric, and endometrial cancer, among others.
“It is estimated that 3% to 5% of endometrial cancers will arise from Lynch syndrome,” Dr. Padron explained during the poster session.
Because the presence of Lynch syndrome directly affects immediate clinical management and future risk-reducing and surveillance strategies for patients and at-risk family members, screening is recommended in all women with endometrial cancer, she added, noting, however, that “the optimum screening method has yet to be established.”
The sequencing panel evaluated in this study – Swift’s Accel-Amplicon Plus Lynch Syndrome Panel – requires only low input amounts of DNA, and in an earlier test using 10 control samples, it exhibited greater than 90% on-target and coverage uniformity. The work flow allowed for data analysis within 2 days, Dr. Padron noted.
The panel then was tested in the current cohort of patients who were referred to a gynecology oncology clinic for either treatment of endometrial cancer or for evaluation of risk for endometrial cancer.
Germline/tumor DNA was isolated and 10 ng DNA was used for targeted exon-level hotspot coverage of MLH1, MSH2, MSH6, and PMS2.
The findings suggest that the prevalence of Lynch syndrome may be six to seven times greater than previously estimated, Dr. Padron said during the poster presentation.
“If confirmed, this would have huge implications for our patients and health care system,” she said, adding that the ability to perform and analyze the sequencing within 48 hours of sample collection using a very low DNA input also was of note.
Taken together, “the findings of this study support future larger studies that can be performed concurrently with current standard of care technologies,” she and her colleagues concluded, noting that such studies would better determine more robust estimates of the prevalence of Lynch syndrome in women with endometrial cancer, help define improved standard-of-care guidelines, and provide future guidance for possible universal/targeted screening programs – all with the goal of improving the clinical care of women.
Dr. Padron reported having no relevant financial disclosures.
NASHVILLE, TENN. – , according to findings in a prospective patient cohort.
The findings, which also suggest that the incidence of Lynch syndrome among endometrial cancer patients is higher than previously recognized, have “immediate and major implications for the individual patient with endometrial cancer ... and implications for related family members,” Maria Mercedes M. Padron, MD, reported during an e-poster session at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Of 71 patients included in the study, 67 were undergoing endometrial cancer treatment and 7 (3 among those undergoing endometrial cancer treatment and 4 who did not have endometrial cancer) were known to have Lynch syndrome.
Of the 67 undergoing treatment, 22 (33%) were identified by the direct sequencing panel as having Lynch syndrome mutations, and of those, 7 (10%) had mutations classified as high confidence inactivating mutations in either MLH1, MSH6, PMS2, or MSH2 genes, said Dr. Padron, a research scholar at Icahn School of Medicine at Mount Sinai, New York. The remaining 15 patients had rare mutations and met previously defined phenotypic criteria for Lynch syndrome pathogenicity, she reported.
The sequencing panel–based results were compared with commercially available gene tests, including immunohistochemistry (IHC) and microsatellite instability testing (MSI); 10 patients were identified by IHC to have loss of nuclear mismatch repair (MMR) protein expression, and 8 of those were Lynch syndrome mutation positive. In addition, two patients were MSI-high, and both of those were Lynch syndrome mutation positive.
Thus, two Lynch syndrome patients were missed by direct sequencing, noted Dr. Padron.
However, an additional 10 patients who were not identified as having Lynch syndrome by IHC and MSI testing were potentially identified as such using the sequencing panel, Dr. Padron said, noting that “the pathogenicity of these additional variants needs to be defined.”
Lynch syndrome is a hereditary cancer syndrome caused by germline mutations in DNA MMR genes; it is the third most common malignancy in women and it confers an increased risk of several types of cancer, including colorectal, ovarian, gastric, and endometrial cancer, among others.
“It is estimated that 3% to 5% of endometrial cancers will arise from Lynch syndrome,” Dr. Padron explained during the poster session.
Because the presence of Lynch syndrome directly affects immediate clinical management and future risk-reducing and surveillance strategies for patients and at-risk family members, screening is recommended in all women with endometrial cancer, she added, noting, however, that “the optimum screening method has yet to be established.”
The sequencing panel evaluated in this study – Swift’s Accel-Amplicon Plus Lynch Syndrome Panel – requires only low input amounts of DNA, and in an earlier test using 10 control samples, it exhibited greater than 90% on-target and coverage uniformity. The work flow allowed for data analysis within 2 days, Dr. Padron noted.
The panel then was tested in the current cohort of patients who were referred to a gynecology oncology clinic for either treatment of endometrial cancer or for evaluation of risk for endometrial cancer.
Germline/tumor DNA was isolated and 10 ng DNA was used for targeted exon-level hotspot coverage of MLH1, MSH2, MSH6, and PMS2.
The findings suggest that the prevalence of Lynch syndrome may be six to seven times greater than previously estimated, Dr. Padron said during the poster presentation.
“If confirmed, this would have huge implications for our patients and health care system,” she said, adding that the ability to perform and analyze the sequencing within 48 hours of sample collection using a very low DNA input also was of note.
Taken together, “the findings of this study support future larger studies that can be performed concurrently with current standard of care technologies,” she and her colleagues concluded, noting that such studies would better determine more robust estimates of the prevalence of Lynch syndrome in women with endometrial cancer, help define improved standard-of-care guidelines, and provide future guidance for possible universal/targeted screening programs – all with the goal of improving the clinical care of women.
Dr. Padron reported having no relevant financial disclosures.
REPORTING FROM ACOG 2019
Anomalous RT dose linked to lower survival in uterine cancer
As many as one in eight patients with uterine cancer who undergo adjuvant radiation therapy may have been treated with doses that are inconsistent with standard practice, a new study suggests.
Writing in JCO Clinical Cancer Informatics, Corbin D. Jacobs, MD, of Duke University, Durham, N.C., and coauthors analyzed National Cancer Database data from 14,298 women with stage IIIC1-C2 uterine cancer who underwent adjuvant radiation therapy after hysterectomy. The analysis included information on radiation therapy site, modality, dose, fractions, timing, duration, and stage, as well as details about the facilities at which the treatment was given.
Overall, 16% of the women had at least one ‘anomalous’ entry in their records of radiation therapy. The most common anomalies were that the combined total radiation therapy dose was insufficient, or there was an insufficient number of external beam radiation therapy fractions, both of which may have represented an incomplete course of radiation therapy.
Other anomalies were excessive brachytherapy fractions, inconsistency in the staging, and less than 100 days of radiation therapy.
The study showed that the 5-year overall survival rate in individuals who had at least one anomalous data entry was 51.3% compared with 58% among individuals without any anomalous entries (P less than .001). The difference in survival rates was entirely accounted for by insufficient, excessive, or unknown radiation therapy dose.
More than half of patients in the study had missing or unknown data for at least one entry, and this was associated with lower 5-year survival compared with patients with complete data entries.
The researchers also looked at facility-specific factors, such as the type of facility, its location, and its distance from the patient’s home, and how these impacted the frequency of anomalous data. They found that comprehensive community cancer programs had the lowest incidence of anomalous data (14.7%) compared with non–comprehensive community cancer programs, which had an incidence of 17.1%.
The incidence of anomalous data was highest in facilities in the south Atlantic, east south central and west south central regions of the United States. The further away from a patient’s home the reporting facility was, the higher the presence of anomalous data.
“Because an insufficient RT dose or fewer than 20 fractions accounted for such a large proportion of the anomalies, patients may potentially be more likely to have an incomplete RT course or complete RT in a hypofractionated manner when the facility is farther from their home,” the authors wrote.
One author was an employee of Bioventus, and three declared research funding from the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Jacobs C et al. JCO Clin Cancer Inform. 2019, May 3. doi: 10.1200/CCI.18.00118.
As many as one in eight patients with uterine cancer who undergo adjuvant radiation therapy may have been treated with doses that are inconsistent with standard practice, a new study suggests.
Writing in JCO Clinical Cancer Informatics, Corbin D. Jacobs, MD, of Duke University, Durham, N.C., and coauthors analyzed National Cancer Database data from 14,298 women with stage IIIC1-C2 uterine cancer who underwent adjuvant radiation therapy after hysterectomy. The analysis included information on radiation therapy site, modality, dose, fractions, timing, duration, and stage, as well as details about the facilities at which the treatment was given.
Overall, 16% of the women had at least one ‘anomalous’ entry in their records of radiation therapy. The most common anomalies were that the combined total radiation therapy dose was insufficient, or there was an insufficient number of external beam radiation therapy fractions, both of which may have represented an incomplete course of radiation therapy.
Other anomalies were excessive brachytherapy fractions, inconsistency in the staging, and less than 100 days of radiation therapy.
The study showed that the 5-year overall survival rate in individuals who had at least one anomalous data entry was 51.3% compared with 58% among individuals without any anomalous entries (P less than .001). The difference in survival rates was entirely accounted for by insufficient, excessive, or unknown radiation therapy dose.
More than half of patients in the study had missing or unknown data for at least one entry, and this was associated with lower 5-year survival compared with patients with complete data entries.
The researchers also looked at facility-specific factors, such as the type of facility, its location, and its distance from the patient’s home, and how these impacted the frequency of anomalous data. They found that comprehensive community cancer programs had the lowest incidence of anomalous data (14.7%) compared with non–comprehensive community cancer programs, which had an incidence of 17.1%.
The incidence of anomalous data was highest in facilities in the south Atlantic, east south central and west south central regions of the United States. The further away from a patient’s home the reporting facility was, the higher the presence of anomalous data.
“Because an insufficient RT dose or fewer than 20 fractions accounted for such a large proportion of the anomalies, patients may potentially be more likely to have an incomplete RT course or complete RT in a hypofractionated manner when the facility is farther from their home,” the authors wrote.
One author was an employee of Bioventus, and three declared research funding from the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Jacobs C et al. JCO Clin Cancer Inform. 2019, May 3. doi: 10.1200/CCI.18.00118.
As many as one in eight patients with uterine cancer who undergo adjuvant radiation therapy may have been treated with doses that are inconsistent with standard practice, a new study suggests.
Writing in JCO Clinical Cancer Informatics, Corbin D. Jacobs, MD, of Duke University, Durham, N.C., and coauthors analyzed National Cancer Database data from 14,298 women with stage IIIC1-C2 uterine cancer who underwent adjuvant radiation therapy after hysterectomy. The analysis included information on radiation therapy site, modality, dose, fractions, timing, duration, and stage, as well as details about the facilities at which the treatment was given.
Overall, 16% of the women had at least one ‘anomalous’ entry in their records of radiation therapy. The most common anomalies were that the combined total radiation therapy dose was insufficient, or there was an insufficient number of external beam radiation therapy fractions, both of which may have represented an incomplete course of radiation therapy.
Other anomalies were excessive brachytherapy fractions, inconsistency in the staging, and less than 100 days of radiation therapy.
The study showed that the 5-year overall survival rate in individuals who had at least one anomalous data entry was 51.3% compared with 58% among individuals without any anomalous entries (P less than .001). The difference in survival rates was entirely accounted for by insufficient, excessive, or unknown radiation therapy dose.
More than half of patients in the study had missing or unknown data for at least one entry, and this was associated with lower 5-year survival compared with patients with complete data entries.
The researchers also looked at facility-specific factors, such as the type of facility, its location, and its distance from the patient’s home, and how these impacted the frequency of anomalous data. They found that comprehensive community cancer programs had the lowest incidence of anomalous data (14.7%) compared with non–comprehensive community cancer programs, which had an incidence of 17.1%.
The incidence of anomalous data was highest in facilities in the south Atlantic, east south central and west south central regions of the United States. The further away from a patient’s home the reporting facility was, the higher the presence of anomalous data.
“Because an insufficient RT dose or fewer than 20 fractions accounted for such a large proportion of the anomalies, patients may potentially be more likely to have an incomplete RT course or complete RT in a hypofractionated manner when the facility is farther from their home,” the authors wrote.
One author was an employee of Bioventus, and three declared research funding from the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Jacobs C et al. JCO Clin Cancer Inform. 2019, May 3. doi: 10.1200/CCI.18.00118.
FROM JCO CLINICAL CANCER INFORMATICS
Thrice yearly cytologic testing may best annual cervical screenings
Less-frequent cytologic testing, followed by hrHPV tests
George F. Sawaya, MD, from the University of California, San Francisco, and colleagues enrolled 451 English-speaking or Spanish-speaking women aged 21-65 years from women’s health clinics between September 2014 and June 2016. The women were mean 38 years old, and 57% were nonwhite women. The researchers examined utilities for 23 different health states associated with cervical cancer, and created a Markov decision model of type-specific high-risk human papillomavirus (hrHPV)–induced cervical carcinogenesis.
The researchers evaluated 12 screening strategies, which included the following scenarios:
- For women aged 21-65 years, cytologic testing every 3 years; if atypical squamous cells of undetermined significance (ASCUS) are found, repeat cytologic testing in 1 year or switch to immediate hrHPV triage.
- For women 21-29 years, cytologic testing every 3 years, and then followed with cytologic testing plus hrHPV testing (cotesting) for women 30-65 years old; if a normal cytologic test result and positive hrHPV test results, move to cotesting in 1 year or immediate genotyping triage.
- For women 21-29 years, cytologic testing every 3 years, and then followed with hrHPV testing alone every 3-5 years for women 30-65 years; if there are positive hrHPV results, move to immediate cytologic testing triage or immediate genotyping triage. Women with positive hrHPV and negative genotyping results receive additional cytologic testing triage.
In the strategies that switched the women from cytologic testing to hrHPV tests, the study also tested doing the switch at age 25 years rather than 30 years, the investigators reported.
Overall, with regard to cost, screening resulted in more cost savings ($1,267-$2,577) than not screening ($2,891 per woman). Women received the most benefit as measured by lifetime quality-adjusted life-years (QALY) if they received cytologic test every 3 years and received repeat testing for ASCUS. The strategy with the lowest cost was cytologic testing every 3 years and hrHPV triage for ASCUS ($1,267), and the strategy of 3-year cytology testing with repeat testing for ASCUS had more QALY but at a higher cost ($2,166). Other higher-cost strategies relative to QALYs included cotesting and primary hrHPV and also annual cytologic testing ($2,577).
“Both the American College of Obstetricians and Gynecologists and the American Cancer Society consider cotesting the preferred cervical cancer screening strategy, and the U.S. Preventive Services Task Force considers it an alternative strategy,” Dr. Sawaya and colleagues noted. “Our findings challenge these endorsements.”
“Our analyses suggest that it is not cost effective to begin primary hrHPV testing prior to age 30 years, to perform hrHPV testing every 3 years, or to perform cytologic testing annually. Comparative modeling is needed to confirm these findings,” they concluded.
Dual stain vs. cytologic testing alone
In a second study, Nicolas Wentzensen, MD, PhD, from the National Cancer Institute and colleagues performed a prospective observational study of 3,225 women who tested positive for human papillomavirus (HPV) who underwent p16/Ki-67 dual stain (DS) and HPV16/18 genotyping.
p16/Ki-67 DS was more effective at risk stratification for cervical intraepithelial neoplasia grade 3 or more severe neoplasia (CIN3+) than cytologic testing alone, and women with positive DS results had a higher risk of developing CIN3+ (12%) than did women with cytologic testing alone (10%; P = .005). Women who were HPV16/18 negative were the most likely to not have CIN3+ if they had negative DS results, and DS strategies resulted in fewer overall colposcopies relative to CIN3+ detections, compared with cytologic testing alone.
“We found that, for primary HPV screening, DS has both higher sensitivity and specificity compared with cytologic testing for triage of HPV-positive women Because of the greater reassurance of negative DS results, screening intervals can be extended compared with the screening intervals after negative cytologic results. Dual stain reduces unnecessary colposcopy referral and unnecessary cervical biopsies, and may reduce unnecessary treatment compared with Papanicolaou cytologic testing,” Dr. Wentzensen and colleagues concluded. “Our estimates of sensitivity, absolute risk, and colposcopy referral for various triage strategies can guide implementation of primary HPV screening.”
Five authors of Sawaya et al. reported receiving grants from the National Cancer Institute, and Dr. Megan J. Huchko reported receiving a grant from the University of California, San Francisco, during that study. That study was funded by a grant from the NCI. Six authors from Wentzensen et al. reported receiving grants from the NCI or being employed by the NCI or NIH. Dr. Philip E. Castle reported receiving low-cost or free cervical screening tests from Roche, Becton Dickinson, Cepheid, and Arbor Vita Corp. The other authors from both studies reported no relevant conflicts of interest.
SOURCES: Sawaya GF et al. JAMA Intern Med. 2019. doi: 10.1001/jamainternmed.2019.0299; Wentzensen N et al. JAMA Intern Med. 2019. doi: 10.1001/jamainternmed.2019.0306.
Cervical cancer screening can be simplified and managed by reducing annual screening to every 3 years for women with normal cytological test results, Sarah Feldman, MD, MPH, wrote in a related editorial. There is evidence from large studies that this is possible for women with average risk of cervical cancer.
Primary human papillomavirus (HPV) screening also is an option for patients, and although there are no current guidelines, 2015 expert guidance states HPV16/18 genotyping and reflex cytologic testing should be used in cases of abnormal results. Transitioning from cytologic testing to primary HPV testing may require a period of using both tests in clinical practice, but this may raise issues with creating false positive results.
“The biggest challenge for cervical cancer screening, however, is likely not which test to use, but determining which women are at low enough risk of cervical cancer to undergo screening at less-frequent intervals,” wrote Dr. Feldman. In these cases, a better infrastructure where clinicians can access women’s prior screening results and make recommendations with decision support systems is needed.
But challenges remain. “These challenges include clinician and patient education and acceptance; access to primary HPV tests; the development of simple, easily implementable, and evidence-based management advice; and systems-based approaches to help clinicians implement optimal care.”
While women 30 years or older are likely to receive primary HPV testing as a standard of care, the risk of cervical cancer also should decrease as more children receive the HPV vaccine, concluded Dr. Feldman.
“Ultimately, once all children have received the HPV vaccination, the incidence of both cervical cancer and precancerous abnormalities should markedly diminish,” Dr. Feldman said. “Ultimately, we may hope to prevent all cervical cancer.”
Dr. Feldman is from the division of gynecologic oncology at Brigham and Women’s Hospital in Boston. Her editorial accompanied the reports by Sawaya et al. and Wentzensen et al. (JAMA Intern Med. 2019. doi: 10.1001/jamainternmed.2019.0298). She reported no relevant conflicts of interest.
Cervical cancer screening can be simplified and managed by reducing annual screening to every 3 years for women with normal cytological test results, Sarah Feldman, MD, MPH, wrote in a related editorial. There is evidence from large studies that this is possible for women with average risk of cervical cancer.
Primary human papillomavirus (HPV) screening also is an option for patients, and although there are no current guidelines, 2015 expert guidance states HPV16/18 genotyping and reflex cytologic testing should be used in cases of abnormal results. Transitioning from cytologic testing to primary HPV testing may require a period of using both tests in clinical practice, but this may raise issues with creating false positive results.
“The biggest challenge for cervical cancer screening, however, is likely not which test to use, but determining which women are at low enough risk of cervical cancer to undergo screening at less-frequent intervals,” wrote Dr. Feldman. In these cases, a better infrastructure where clinicians can access women’s prior screening results and make recommendations with decision support systems is needed.
But challenges remain. “These challenges include clinician and patient education and acceptance; access to primary HPV tests; the development of simple, easily implementable, and evidence-based management advice; and systems-based approaches to help clinicians implement optimal care.”
While women 30 years or older are likely to receive primary HPV testing as a standard of care, the risk of cervical cancer also should decrease as more children receive the HPV vaccine, concluded Dr. Feldman.
“Ultimately, once all children have received the HPV vaccination, the incidence of both cervical cancer and precancerous abnormalities should markedly diminish,” Dr. Feldman said. “Ultimately, we may hope to prevent all cervical cancer.”
Dr. Feldman is from the division of gynecologic oncology at Brigham and Women’s Hospital in Boston. Her editorial accompanied the reports by Sawaya et al. and Wentzensen et al. (JAMA Intern Med. 2019. doi: 10.1001/jamainternmed.2019.0298). She reported no relevant conflicts of interest.
Cervical cancer screening can be simplified and managed by reducing annual screening to every 3 years for women with normal cytological test results, Sarah Feldman, MD, MPH, wrote in a related editorial. There is evidence from large studies that this is possible for women with average risk of cervical cancer.
Primary human papillomavirus (HPV) screening also is an option for patients, and although there are no current guidelines, 2015 expert guidance states HPV16/18 genotyping and reflex cytologic testing should be used in cases of abnormal results. Transitioning from cytologic testing to primary HPV testing may require a period of using both tests in clinical practice, but this may raise issues with creating false positive results.
“The biggest challenge for cervical cancer screening, however, is likely not which test to use, but determining which women are at low enough risk of cervical cancer to undergo screening at less-frequent intervals,” wrote Dr. Feldman. In these cases, a better infrastructure where clinicians can access women’s prior screening results and make recommendations with decision support systems is needed.
But challenges remain. “These challenges include clinician and patient education and acceptance; access to primary HPV tests; the development of simple, easily implementable, and evidence-based management advice; and systems-based approaches to help clinicians implement optimal care.”
While women 30 years or older are likely to receive primary HPV testing as a standard of care, the risk of cervical cancer also should decrease as more children receive the HPV vaccine, concluded Dr. Feldman.
“Ultimately, once all children have received the HPV vaccination, the incidence of both cervical cancer and precancerous abnormalities should markedly diminish,” Dr. Feldman said. “Ultimately, we may hope to prevent all cervical cancer.”
Dr. Feldman is from the division of gynecologic oncology at Brigham and Women’s Hospital in Boston. Her editorial accompanied the reports by Sawaya et al. and Wentzensen et al. (JAMA Intern Med. 2019. doi: 10.1001/jamainternmed.2019.0298). She reported no relevant conflicts of interest.
Less-frequent cytologic testing, followed by hrHPV tests
George F. Sawaya, MD, from the University of California, San Francisco, and colleagues enrolled 451 English-speaking or Spanish-speaking women aged 21-65 years from women’s health clinics between September 2014 and June 2016. The women were mean 38 years old, and 57% were nonwhite women. The researchers examined utilities for 23 different health states associated with cervical cancer, and created a Markov decision model of type-specific high-risk human papillomavirus (hrHPV)–induced cervical carcinogenesis.
The researchers evaluated 12 screening strategies, which included the following scenarios:
- For women aged 21-65 years, cytologic testing every 3 years; if atypical squamous cells of undetermined significance (ASCUS) are found, repeat cytologic testing in 1 year or switch to immediate hrHPV triage.
- For women 21-29 years, cytologic testing every 3 years, and then followed with cytologic testing plus hrHPV testing (cotesting) for women 30-65 years old; if a normal cytologic test result and positive hrHPV test results, move to cotesting in 1 year or immediate genotyping triage.
- For women 21-29 years, cytologic testing every 3 years, and then followed with hrHPV testing alone every 3-5 years for women 30-65 years; if there are positive hrHPV results, move to immediate cytologic testing triage or immediate genotyping triage. Women with positive hrHPV and negative genotyping results receive additional cytologic testing triage.
In the strategies that switched the women from cytologic testing to hrHPV tests, the study also tested doing the switch at age 25 years rather than 30 years, the investigators reported.
Overall, with regard to cost, screening resulted in more cost savings ($1,267-$2,577) than not screening ($2,891 per woman). Women received the most benefit as measured by lifetime quality-adjusted life-years (QALY) if they received cytologic test every 3 years and received repeat testing for ASCUS. The strategy with the lowest cost was cytologic testing every 3 years and hrHPV triage for ASCUS ($1,267), and the strategy of 3-year cytology testing with repeat testing for ASCUS had more QALY but at a higher cost ($2,166). Other higher-cost strategies relative to QALYs included cotesting and primary hrHPV and also annual cytologic testing ($2,577).
“Both the American College of Obstetricians and Gynecologists and the American Cancer Society consider cotesting the preferred cervical cancer screening strategy, and the U.S. Preventive Services Task Force considers it an alternative strategy,” Dr. Sawaya and colleagues noted. “Our findings challenge these endorsements.”
“Our analyses suggest that it is not cost effective to begin primary hrHPV testing prior to age 30 years, to perform hrHPV testing every 3 years, or to perform cytologic testing annually. Comparative modeling is needed to confirm these findings,” they concluded.
Dual stain vs. cytologic testing alone
In a second study, Nicolas Wentzensen, MD, PhD, from the National Cancer Institute and colleagues performed a prospective observational study of 3,225 women who tested positive for human papillomavirus (HPV) who underwent p16/Ki-67 dual stain (DS) and HPV16/18 genotyping.
p16/Ki-67 DS was more effective at risk stratification for cervical intraepithelial neoplasia grade 3 or more severe neoplasia (CIN3+) than cytologic testing alone, and women with positive DS results had a higher risk of developing CIN3+ (12%) than did women with cytologic testing alone (10%; P = .005). Women who were HPV16/18 negative were the most likely to not have CIN3+ if they had negative DS results, and DS strategies resulted in fewer overall colposcopies relative to CIN3+ detections, compared with cytologic testing alone.
“We found that, for primary HPV screening, DS has both higher sensitivity and specificity compared with cytologic testing for triage of HPV-positive women Because of the greater reassurance of negative DS results, screening intervals can be extended compared with the screening intervals after negative cytologic results. Dual stain reduces unnecessary colposcopy referral and unnecessary cervical biopsies, and may reduce unnecessary treatment compared with Papanicolaou cytologic testing,” Dr. Wentzensen and colleagues concluded. “Our estimates of sensitivity, absolute risk, and colposcopy referral for various triage strategies can guide implementation of primary HPV screening.”
Five authors of Sawaya et al. reported receiving grants from the National Cancer Institute, and Dr. Megan J. Huchko reported receiving a grant from the University of California, San Francisco, during that study. That study was funded by a grant from the NCI. Six authors from Wentzensen et al. reported receiving grants from the NCI or being employed by the NCI or NIH. Dr. Philip E. Castle reported receiving low-cost or free cervical screening tests from Roche, Becton Dickinson, Cepheid, and Arbor Vita Corp. The other authors from both studies reported no relevant conflicts of interest.
SOURCES: Sawaya GF et al. JAMA Intern Med. 2019. doi: 10.1001/jamainternmed.2019.0299; Wentzensen N et al. JAMA Intern Med. 2019. doi: 10.1001/jamainternmed.2019.0306.
Less-frequent cytologic testing, followed by hrHPV tests
George F. Sawaya, MD, from the University of California, San Francisco, and colleagues enrolled 451 English-speaking or Spanish-speaking women aged 21-65 years from women’s health clinics between September 2014 and June 2016. The women were mean 38 years old, and 57% were nonwhite women. The researchers examined utilities for 23 different health states associated with cervical cancer, and created a Markov decision model of type-specific high-risk human papillomavirus (hrHPV)–induced cervical carcinogenesis.
The researchers evaluated 12 screening strategies, which included the following scenarios:
- For women aged 21-65 years, cytologic testing every 3 years; if atypical squamous cells of undetermined significance (ASCUS) are found, repeat cytologic testing in 1 year or switch to immediate hrHPV triage.
- For women 21-29 years, cytologic testing every 3 years, and then followed with cytologic testing plus hrHPV testing (cotesting) for women 30-65 years old; if a normal cytologic test result and positive hrHPV test results, move to cotesting in 1 year or immediate genotyping triage.
- For women 21-29 years, cytologic testing every 3 years, and then followed with hrHPV testing alone every 3-5 years for women 30-65 years; if there are positive hrHPV results, move to immediate cytologic testing triage or immediate genotyping triage. Women with positive hrHPV and negative genotyping results receive additional cytologic testing triage.
In the strategies that switched the women from cytologic testing to hrHPV tests, the study also tested doing the switch at age 25 years rather than 30 years, the investigators reported.
Overall, with regard to cost, screening resulted in more cost savings ($1,267-$2,577) than not screening ($2,891 per woman). Women received the most benefit as measured by lifetime quality-adjusted life-years (QALY) if they received cytologic test every 3 years and received repeat testing for ASCUS. The strategy with the lowest cost was cytologic testing every 3 years and hrHPV triage for ASCUS ($1,267), and the strategy of 3-year cytology testing with repeat testing for ASCUS had more QALY but at a higher cost ($2,166). Other higher-cost strategies relative to QALYs included cotesting and primary hrHPV and also annual cytologic testing ($2,577).
“Both the American College of Obstetricians and Gynecologists and the American Cancer Society consider cotesting the preferred cervical cancer screening strategy, and the U.S. Preventive Services Task Force considers it an alternative strategy,” Dr. Sawaya and colleagues noted. “Our findings challenge these endorsements.”
“Our analyses suggest that it is not cost effective to begin primary hrHPV testing prior to age 30 years, to perform hrHPV testing every 3 years, or to perform cytologic testing annually. Comparative modeling is needed to confirm these findings,” they concluded.
Dual stain vs. cytologic testing alone
In a second study, Nicolas Wentzensen, MD, PhD, from the National Cancer Institute and colleagues performed a prospective observational study of 3,225 women who tested positive for human papillomavirus (HPV) who underwent p16/Ki-67 dual stain (DS) and HPV16/18 genotyping.
p16/Ki-67 DS was more effective at risk stratification for cervical intraepithelial neoplasia grade 3 or more severe neoplasia (CIN3+) than cytologic testing alone, and women with positive DS results had a higher risk of developing CIN3+ (12%) than did women with cytologic testing alone (10%; P = .005). Women who were HPV16/18 negative were the most likely to not have CIN3+ if they had negative DS results, and DS strategies resulted in fewer overall colposcopies relative to CIN3+ detections, compared with cytologic testing alone.
“We found that, for primary HPV screening, DS has both higher sensitivity and specificity compared with cytologic testing for triage of HPV-positive women Because of the greater reassurance of negative DS results, screening intervals can be extended compared with the screening intervals after negative cytologic results. Dual stain reduces unnecessary colposcopy referral and unnecessary cervical biopsies, and may reduce unnecessary treatment compared with Papanicolaou cytologic testing,” Dr. Wentzensen and colleagues concluded. “Our estimates of sensitivity, absolute risk, and colposcopy referral for various triage strategies can guide implementation of primary HPV screening.”
Five authors of Sawaya et al. reported receiving grants from the National Cancer Institute, and Dr. Megan J. Huchko reported receiving a grant from the University of California, San Francisco, during that study. That study was funded by a grant from the NCI. Six authors from Wentzensen et al. reported receiving grants from the NCI or being employed by the NCI or NIH. Dr. Philip E. Castle reported receiving low-cost or free cervical screening tests from Roche, Becton Dickinson, Cepheid, and Arbor Vita Corp. The other authors from both studies reported no relevant conflicts of interest.
SOURCES: Sawaya GF et al. JAMA Intern Med. 2019. doi: 10.1001/jamainternmed.2019.0299; Wentzensen N et al. JAMA Intern Med. 2019. doi: 10.1001/jamainternmed.2019.0306.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Current ways of testing women for cervical testing may be replaced in the near future.
Major finding: Two studies challenge existing recommendations on when women should be screened for cervical cancer and explore how to manage abnormal results.
Study details: It is not cost effective to begin primary hrHPV testing prior to age 30 years, to perform hrHPV testing every 3 years, or to perform cytologic testing annually. Dual stain reduces unnecessary colposcopy referral and unnecessary cervical biopsies, and may reduce unnecessary treatment, compared with Papanicolaou cytologic testing.
Disclosures: Five authors from Sawaya et al. reported receiving grants from the National Cancer Institute and Dr. Megan J. Huchko reported receiving a grant from the University of California, San Francisco, during that study. That study was funded by a grant from the NCI. Six authors from Wentzensen et al. reported receiving grants from the NCI or being employed by the NCI or National Institutes of Health. Dr. Philip E. Castle reported receiving low-cost or free cervical screening tests from Roche, Becton Dickinson, Cepheid, and Arbor Vita Corp. The other authors from both studies reported no relevant conflicts of interest.
Sources: Sawaya GF et al. JAMA Intern Med. 2019. doi: 10.1001/jamainternmed.2019.0299; Wentzensen N et al. JAMA Intern Med. 2019. doi: 10.1001/jamainternmed.2019.0306.
Ultrasound or biopsy for evaluation of endometrium? It depends
NASHVILLE, TENN. – Biopsy isn’t usually the first step in evaluating the endometrium of a reproductive-age woman who presents with abnormal uterine bleeding, but that’s not always the case, according to James M. Shwayder, MD.

“If we have young women come in, generally speaking, we don’t think much about doing biopsies, but there are those patients who really require a biopsy very early on: If they are obese and if they have long histories of oligomenorrhea ... they are at significantly greater risk for either endometrial hyperplasia or cancer, so in those patients I recommend biopsy very early on,” Dr. Shwayder said in this video interview about his presentation entitled “Modern Evaluation of the Endometrium: When to Use Ultrasound, When to Biopsy,” as presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Conversely, in some cases when biopsy is typically considered the first-line step in evaluation, ultrasound may actually be better, he argued.
“[ACOG] recommends that women over 45 ... should have a biopsy done as their first-line evaluation. I kind of take issue with that a little bit,” said Dr. Shwayder, a professor at the University of Mississippi Medical Center, Jackson, and president and chief executive officer of Shwayder Consulting in Venice, Fla.
Data suggest that a “blind biopsy” could miss up to 18% of cases involving either a submucous myoma or a polyp and that one-third to one-fourth of patients have a structural defect such as a polyp or fibroid that can’t be diagnosed with a biopsy, he explained, noting that sonohysterography is best for preoperative evaluation in such case.
Ultrasound also has utility for evaluating other abnormalities, and it can be a very simple way to evaluate the patient and decide whether they need further evaluation or further treatment, he said.
Dr. Shwayder also discussed evidence for making a choice between biopsy and ultrasound for initial evaluation in postmenopausal women and for assessing women with asymptomatic thickened endometrium.
Dr. Shwayder is a consultant for GE Ultrasound.
NASHVILLE, TENN. – Biopsy isn’t usually the first step in evaluating the endometrium of a reproductive-age woman who presents with abnormal uterine bleeding, but that’s not always the case, according to James M. Shwayder, MD.

“If we have young women come in, generally speaking, we don’t think much about doing biopsies, but there are those patients who really require a biopsy very early on: If they are obese and if they have long histories of oligomenorrhea ... they are at significantly greater risk for either endometrial hyperplasia or cancer, so in those patients I recommend biopsy very early on,” Dr. Shwayder said in this video interview about his presentation entitled “Modern Evaluation of the Endometrium: When to Use Ultrasound, When to Biopsy,” as presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Conversely, in some cases when biopsy is typically considered the first-line step in evaluation, ultrasound may actually be better, he argued.
“[ACOG] recommends that women over 45 ... should have a biopsy done as their first-line evaluation. I kind of take issue with that a little bit,” said Dr. Shwayder, a professor at the University of Mississippi Medical Center, Jackson, and president and chief executive officer of Shwayder Consulting in Venice, Fla.
Data suggest that a “blind biopsy” could miss up to 18% of cases involving either a submucous myoma or a polyp and that one-third to one-fourth of patients have a structural defect such as a polyp or fibroid that can’t be diagnosed with a biopsy, he explained, noting that sonohysterography is best for preoperative evaluation in such case.
Ultrasound also has utility for evaluating other abnormalities, and it can be a very simple way to evaluate the patient and decide whether they need further evaluation or further treatment, he said.
Dr. Shwayder also discussed evidence for making a choice between biopsy and ultrasound for initial evaluation in postmenopausal women and for assessing women with asymptomatic thickened endometrium.
Dr. Shwayder is a consultant for GE Ultrasound.
NASHVILLE, TENN. – Biopsy isn’t usually the first step in evaluating the endometrium of a reproductive-age woman who presents with abnormal uterine bleeding, but that’s not always the case, according to James M. Shwayder, MD.

“If we have young women come in, generally speaking, we don’t think much about doing biopsies, but there are those patients who really require a biopsy very early on: If they are obese and if they have long histories of oligomenorrhea ... they are at significantly greater risk for either endometrial hyperplasia or cancer, so in those patients I recommend biopsy very early on,” Dr. Shwayder said in this video interview about his presentation entitled “Modern Evaluation of the Endometrium: When to Use Ultrasound, When to Biopsy,” as presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Conversely, in some cases when biopsy is typically considered the first-line step in evaluation, ultrasound may actually be better, he argued.
“[ACOG] recommends that women over 45 ... should have a biopsy done as their first-line evaluation. I kind of take issue with that a little bit,” said Dr. Shwayder, a professor at the University of Mississippi Medical Center, Jackson, and president and chief executive officer of Shwayder Consulting in Venice, Fla.
Data suggest that a “blind biopsy” could miss up to 18% of cases involving either a submucous myoma or a polyp and that one-third to one-fourth of patients have a structural defect such as a polyp or fibroid that can’t be diagnosed with a biopsy, he explained, noting that sonohysterography is best for preoperative evaluation in such case.
Ultrasound also has utility for evaluating other abnormalities, and it can be a very simple way to evaluate the patient and decide whether they need further evaluation or further treatment, he said.
Dr. Shwayder also discussed evidence for making a choice between biopsy and ultrasound for initial evaluation in postmenopausal women and for assessing women with asymptomatic thickened endometrium.
Dr. Shwayder is a consultant for GE Ultrasound.
EXPERT ANALYSIS FROM ACOG 2019
2019 Update on cervical disease
Cervical cancer rates remain low in the United States, with the incidence having plateaued for decades. And yet, in 2019, more than 13,000 US women will be diagnosed with cervical cancer.1 Globally, in 2018 almost 600,000 women were diagnosed with cervical cancer2; it is the fourth most frequent cancer in women. This is despite the fact that we have adequate primary and secondary prevention tools available to minimize—and almost eliminate—cervical cancer. We must continue to raise the bar for preventing, screening for, and managing this disease.
Human papillomavirus (HPV) vaccines provide a highly effective primary prevention strategy, but we need to improve our ability to identify and diagnose dysplastic lesions prior to the development of cervical cancer. Highly sensitive HPV testing and cytology is a powerful secondary prevention approach that enables us to assess a woman’s risk of having precancerous cells both now and in the near future. These modalities have been very successful in decreasing the incidence of cervical cancer in the United States and other areas with organized screening programs. In low- and middle-income countries, however, access to, availability of, and performance with these modalities is not optimal. Innovative strategies and new technologies are being evaluated to overcome these limitations.
Advances in radiation and surgical technology have enabled us to vastly improve cervical cancer treatment. Women with early-stage cervical cancer are candidates for surgical management, which frequently includes a radical hysterectomy and lymph node dissection. While these surgeries traditionally have been performed via an exploratory laparotomy, minimally invasive techniques (laparoscopic and robot-assisted surgical techniques) have decreased the morbidity with these surgeries. Notable new studies have shed light on the comparative effectiveness of minimally invasive technologies and have shown us that new is not always better.
The US Preventive Services Task Force (USPSTF) recently released its updated cervical cancer screening guidelines. The suggested approach to screening differs from previous recommendations. HPV testing as a primary test (that is, HPV testing alone or followed by cytology) takes the spotlight now, according to the analysis by the Task Force.
In this Update, we highlight important studies published in the past year that address these issues.
Continue to: New tech's potential to identify high-grade...
New tech's potential to identify high-grade cervical dysplasia may be a boon to low-resource settings
Hu L, Bell D, Antani S, et al. An observational study of deep learning and automated evaluation of cervical images for cancer screening. J Natl Cancer Inst. 2019;doi:10.1093/jnci/djy225.
When cervical screening tests like cytology and HPV testing show abnormal results, colposcopy often is recommended. The goal of colposcopy is to identify the areas that might harbor a high-grade precancerous lesion or worse. The gold standard in this case, however, is histology, not colposcopic impression, as many studies have shown that colposcopy without biopsies is limited and that performance is improved with more biopsies.3,4
Visual inspection with acetic acid (VIA) is an approach used often in low-resource settings where visual impression is the gold standard. However, as with colposcopy, a visual evaluation without histology does not perform well, and often women are overtreated. Many attempts have been made with new technologies to overcome the limitations of time, cost, and workforce required for cytology and histology services. New disruptive technologies may be able to surmount human limitations and improve on not only VIA but also the need for histology.
Novel technology uses images to develop algorithm with predictive ability
In a recent observational study, Hu and colleagues used images that were collected during a large population study in Guanacaste, Costa Rica.5 More than 9,000 women were followed for up to 7 years, and cervical photographs (cervigrams) were obtained. Well-annotated histopathology results were obtained for women with abnormal screening, and 279 women had a high-grade dysplastic lesion or cancer.
Cervigrams from women with high-grade lesions and matched controls were collected, and a deep learning-based algorithm using artificial intelligence technology was developed using 70% of the images. The remaining 30% of images were used as a validation set to test the algorithm's ability to "predict" high-grade dysplasia without knowing the final result.
Findings. Termed automated visual evaluation (AVE), this new technology demonstrated a very accurate ability to identify high-grade dysplasia or worse, with an area under the curve (AUC) of 0.91 from merely a cervicogram (FIGURE). This outperformed conventional Pap smears (AUC, 0.71), liquid-based cytology (AUC, 0.79) and, surprisingly, highly sensitive HPV testing (AUC, 0.82) in women in the prime of their screening ages (>25 years of age).
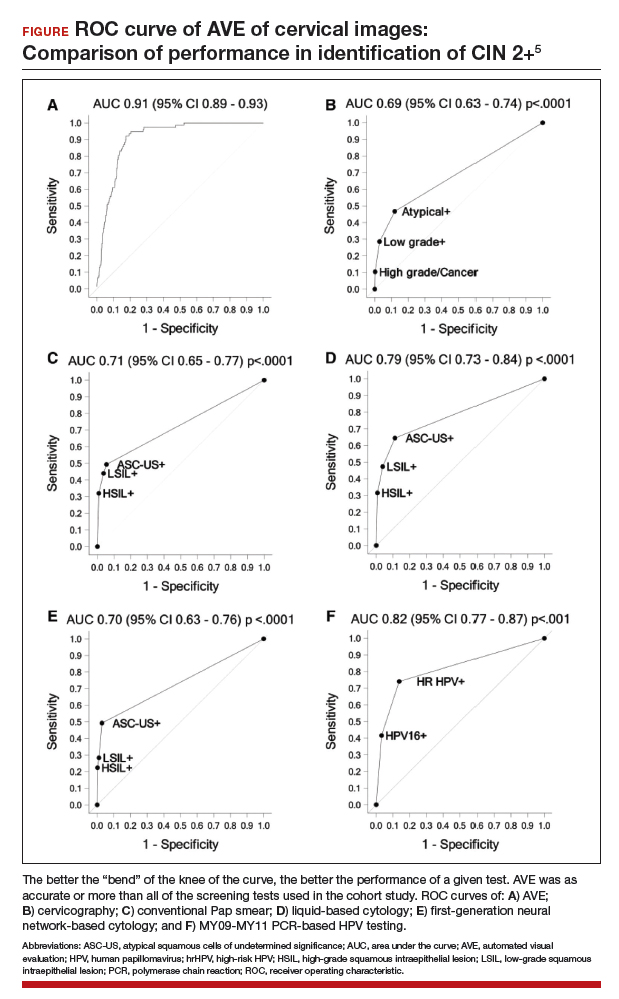
Colposcopy remains the gold standard for evaluating abnormal cervical cancer screening tests in the United States. But can we do better for our patients using new technologies like AVE? If validated in large-scale trials, AVE has the potential to revolutionize cervical cancer screening in low-resource settings where follow-up and adequate histology services are limited or nonexistent. Future large studies are necessary to evaluate the role of AVE alone versus in combination with other diagnostic testing (such as HPV testing) to detect cervical lesions globally.
Continue to: Data offer persuasive evidence...
Data offer persuasive evidence to abandon minimally invasive surgery in management of early-stage cervical cancer
Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
Over the past decade, gynecologic cancer surgery has shifted from what routinely were open procedures to the adoption of minimally invasive techniques. Recently, a large, well-designed prospective study and a large retrospective study both demonstrated worse outcomes with minimally invasive radical hysterectomy (MIRH) as compared with traditional open radical abdominal hysterectomy (RAH). These 2 landmark studies, initially presented at the Society of Gynecologic Oncology's 2018 annual meeting and later published in the New England Journal of Medicine, have really affected the gynecologic oncology community.
Shorter overall survival in women who had MIRH
Melamed and colleagues conducted a large, retrospective US-based study to evaluate all-cause mortality in women with cervical cancer who underwent MIRH compared with those who had RAH.6 The authors also sought to evaluate national trends in 4-year relative survival rates after minimally invasive surgery was adopted.
The study included 2,461 women who met the inclusion criteria; 49.8% (1,225) underwent MIRH procedures and, of those, 79.8% (978) had robot-assisted laparoscopy. Most women had stage IB1 tumors (88%), and most carcinomas were squamous cell (61%); 40.6% of tumors were less than 2 cm in size. There were no differences between the 2 groups with respect to rates of positive parametria, surgical margins, and lymph node involvement. Administration of adjuvant therapy, in those who qualified, was also similar between groups.
Results. At a median follow-up of 45 months, 94 deaths occurred in the minimally invasive group and 70 in the open surgery group. The risk of death at 4 years was 9.1% in the minimally invasive group versus 5.3% in the open surgery group, with a 65% higher risk of death from any cause, which was highly statistically significant.
Prospective trial showed MIRH was associated with lower survival rates
From 2008 to 2017, Ramirez and colleagues conducted a phase 3, multicenter, randomized controlled trial to prospectively establish the noninferiority of MIRH compared with RAH.7 The study included 631 women from 33 centers. The prespecified expected disease-free survival rate was 90% at 4.5 years.
To be included as a site, centers were required to submit details from 10 minimally invasive cases as well as 2 unedited videos for review by the trial management committee. In contrast to Melamed and colleagues' retrospective study, of the 319 procedures that were classified as minimally invasive, only 15.6% were robotically assisted. Similarly, most women had stage IB1 tumors (91.9%), and most were squamous cell carcinomas (67%). There were also no differences in the postoperative pathology findings or the need for adjuvant therapy administered between groups. The median follow-up was 2.5 years.
Results. At that time there were 27 recurrences in the MIRH group and 7 in the RAH group; there were also 19 deaths after MIRH and 3 after RAH. Disease-free survival at 4.5 years was 86% with MIRH versus 96.5% with RAH. Reported 3-year disease-free survival and overall survival were also significantily lower in the minimally invasive subgroup (91.2% vs 97.1%, 93.8% vs 99.0%, respectively).
Study limitations. Criticisms of this trial are that noninferiority could not be declared; in addition, the investigators were unable to complete enrollment secondary to early enrollment termination after the data and safety monitoring board raised survival concerns.
Many argue that subgroup analyses suggest a lower risk of poor outcomes in patients with smaller tumors (<2 cm); however, it is critical to note that this study was not powered to detect these differences.
The evidence is compelling and demonstrates potentially worse disease-related outcomes using MIRH when compared to traditional RAH with respect to cervical cancer recurrence, rates of death, and disease-free and overall survival. Several hypotheses have been proposed, and future research is needed to elucidate the differences in variables responsible for the outcomes demonstrated in these studies. Although there has been no ban on robot-assisted surgical devices or traditional minimally invasive techniques, the National Comprehensive Cancer Network has updated its recommendations to include careful counseling of patients who require a surgical approach for the management of early-stage cervical cancer.
Continue to: USPSTF updated guidance on cervical cancer screening...
USPSTF updated guidance on cervical cancer screening
Melnikow J, Henderson JT, Burda BU, et al. Screening for cervical cancer with high-risk human papillomavirus testing: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:687-705.
US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
Past guidelines for cervical cancer screening have included testing for high-risk HPV (hrHPV) as a cotest with cytology or for triage of atypical squamous cells of undetermined significance (ASCUS) in women aged 30 to 65 years.8 The American Society for Colposcopy and Cervical Pathology and the Society of Gynecologic Oncology, with other stakeholder organizations, issued interim guidance for primary HPV testing--that is, HPV test first and, in the case of non-16/18 hrHPV types, cytology as a triage. The most recent evidence report and systematic review by Melnikow and colleagues for the USPSTF offers an in-depth analysis of risks, benefits, harms, and value of cotesting and other management strategies.9
Focus on screening effectiveness
Large trials of cotesting were conducted in women aged 25 to 65.10-13 These studies all consistently showed that primary hrHPV screening led to a statistically significant increased detection of cervical intraepithelial neoplasia (CIN) 3+ in the initial round of screening, with a relative risk of detecting CIN 3+ ranging from 1.61 to 7.46 compared with cytology alone.
Four additional studies compared cotesting with conventional cytology for the detection of CIN 3+. None of these trials demonstrated a significantly higher detection rate of CIN 3+ with cotesting compared with conventional cytology testing alone. Notably, the studies reviewed were performed in European countries that had organized screening programs in place and a nationalized health care system. Thus, these data may not be as applicable to women in the United States, particularly to women who have limited health care access.
Risks of screening
In the same studies reviewed for screening effectiveness, the investigators found that overall, screening with hrHPV primary or cotesting was associated with more false-positive results and higher colposcopy rates. Women screened with hrHPV alone had a 7.9% referral rate to colposcopy, while those screened with cytology had a 2.8% referral rate to colposcopy. Similarly, the rate of biopsy was higher in the hrHPV-only group (3.2% vs 1.3%).
Overall, while cotesting might have some improvement in performance compared with hrHPV as a single modality, there might be risks of overreferral to colposcopy and overtreatment with additional cytology over hrHPV testing alone.
This evidence review also included an analysis of more potential harms. Very limited evidence suggests that positive hrHPV test results may be associated with greater psychological harm, including decreased sexual satisfaction, increased anxiety and distress, and worse feelings about sexual partners, than abnormal cytology results. These were assessed, however, 1 to 2 weeks after the test results were provided to the patients, and long-term assessment was not done.
New recommendations from the USPSTF
Based on these data, the USPSTF issued new recommendations regarding screening (TABLE).14 For women aged 21 to 29, cytology alone should be used for screening every 3 years. Women aged 30 to 65 can be screened with cytology alone every 3 years, with hrHPV testing alone every 5 years, or with cotesting every 5 years.
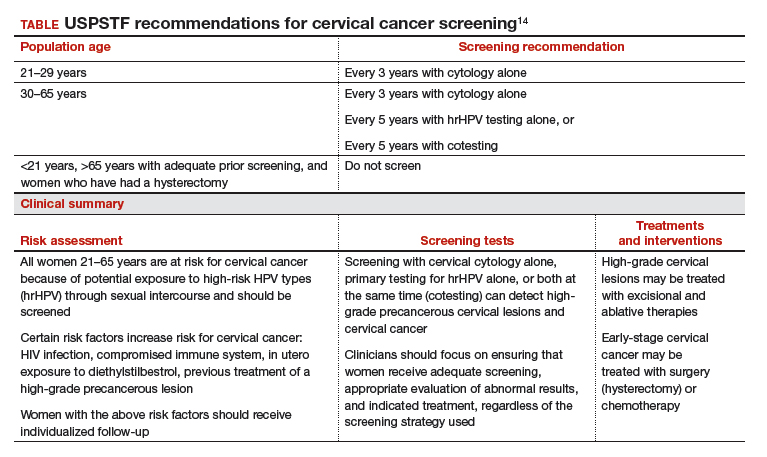
Primary screening with hrHPV is more effective in diagnosing a CIN 3+ than cytology alone. Cotesting with cytology and hrHPV testing appears to have limited performance improvement, with potential harm, compared with hrHPV testing alone in diagnosing CIN 3+. The Task Force recommendation is hrHPV testing alone or cotesting every 5 years.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
- World Health Organization website. Cervical cancer. https://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer/en/. Accessed April 17, 2019.
- Wentzensen N, Walker JL, Gold MA, et al. Multiple biopsies and detection of cervical cancer precursors at colposcopy. J Clin Oncol. 2015;33:83-89.
- Gage JC, Hanson VW, Abbey K, et al. Number of cervical biopsies and sensitivity of colposcopy. Obstet Gynecol. 2006;108:264-272.
- Hu L, Bell D, Antani S, et al. An observational study of deep learning and automated evaluation of cervical images for cancer screening. J Natl Cancer Inst. 2019;doi:10.1093/jnci/djy225.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147-172.
- Melnikow J, Henderson JT, Burda BU, et al. Screening for cervical cancer with high-risk human papillomavirus testing: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:687-705.
- Canfell K, Caruana M, Gebski V, et al. Cervical screening with primary HPV testing or cytology in a population of women in which those aged 33 years or younger had previously been offered HPV vaccination: results of the Compass pilot randomised trial. PLoS Med. 2017;14:e1002388. doi:10.1371/journal.pmed.1002388.
- Leinonen MK, Nieminen P, Lonnberg S, et al. Detection rates of precancerous and cancerous cervical lesions within one screening round of primary human papillomavirus DNA testing: prospective randomised trial in Finland. BMJ. 2012;345:e7789.
- Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52.
- Ronco G, Fioprgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257.
- US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
Cervical cancer rates remain low in the United States, with the incidence having plateaued for decades. And yet, in 2019, more than 13,000 US women will be diagnosed with cervical cancer.1 Globally, in 2018 almost 600,000 women were diagnosed with cervical cancer2; it is the fourth most frequent cancer in women. This is despite the fact that we have adequate primary and secondary prevention tools available to minimize—and almost eliminate—cervical cancer. We must continue to raise the bar for preventing, screening for, and managing this disease.
Human papillomavirus (HPV) vaccines provide a highly effective primary prevention strategy, but we need to improve our ability to identify and diagnose dysplastic lesions prior to the development of cervical cancer. Highly sensitive HPV testing and cytology is a powerful secondary prevention approach that enables us to assess a woman’s risk of having precancerous cells both now and in the near future. These modalities have been very successful in decreasing the incidence of cervical cancer in the United States and other areas with organized screening programs. In low- and middle-income countries, however, access to, availability of, and performance with these modalities is not optimal. Innovative strategies and new technologies are being evaluated to overcome these limitations.
Advances in radiation and surgical technology have enabled us to vastly improve cervical cancer treatment. Women with early-stage cervical cancer are candidates for surgical management, which frequently includes a radical hysterectomy and lymph node dissection. While these surgeries traditionally have been performed via an exploratory laparotomy, minimally invasive techniques (laparoscopic and robot-assisted surgical techniques) have decreased the morbidity with these surgeries. Notable new studies have shed light on the comparative effectiveness of minimally invasive technologies and have shown us that new is not always better.
The US Preventive Services Task Force (USPSTF) recently released its updated cervical cancer screening guidelines. The suggested approach to screening differs from previous recommendations. HPV testing as a primary test (that is, HPV testing alone or followed by cytology) takes the spotlight now, according to the analysis by the Task Force.
In this Update, we highlight important studies published in the past year that address these issues.
Continue to: New tech's potential to identify high-grade...
New tech's potential to identify high-grade cervical dysplasia may be a boon to low-resource settings
Hu L, Bell D, Antani S, et al. An observational study of deep learning and automated evaluation of cervical images for cancer screening. J Natl Cancer Inst. 2019;doi:10.1093/jnci/djy225.
When cervical screening tests like cytology and HPV testing show abnormal results, colposcopy often is recommended. The goal of colposcopy is to identify the areas that might harbor a high-grade precancerous lesion or worse. The gold standard in this case, however, is histology, not colposcopic impression, as many studies have shown that colposcopy without biopsies is limited and that performance is improved with more biopsies.3,4
Visual inspection with acetic acid (VIA) is an approach used often in low-resource settings where visual impression is the gold standard. However, as with colposcopy, a visual evaluation without histology does not perform well, and often women are overtreated. Many attempts have been made with new technologies to overcome the limitations of time, cost, and workforce required for cytology and histology services. New disruptive technologies may be able to surmount human limitations and improve on not only VIA but also the need for histology.
Novel technology uses images to develop algorithm with predictive ability
In a recent observational study, Hu and colleagues used images that were collected during a large population study in Guanacaste, Costa Rica.5 More than 9,000 women were followed for up to 7 years, and cervical photographs (cervigrams) were obtained. Well-annotated histopathology results were obtained for women with abnormal screening, and 279 women had a high-grade dysplastic lesion or cancer.
Cervigrams from women with high-grade lesions and matched controls were collected, and a deep learning-based algorithm using artificial intelligence technology was developed using 70% of the images. The remaining 30% of images were used as a validation set to test the algorithm's ability to "predict" high-grade dysplasia without knowing the final result.
Findings. Termed automated visual evaluation (AVE), this new technology demonstrated a very accurate ability to identify high-grade dysplasia or worse, with an area under the curve (AUC) of 0.91 from merely a cervicogram (FIGURE). This outperformed conventional Pap smears (AUC, 0.71), liquid-based cytology (AUC, 0.79) and, surprisingly, highly sensitive HPV testing (AUC, 0.82) in women in the prime of their screening ages (>25 years of age).

Colposcopy remains the gold standard for evaluating abnormal cervical cancer screening tests in the United States. But can we do better for our patients using new technologies like AVE? If validated in large-scale trials, AVE has the potential to revolutionize cervical cancer screening in low-resource settings where follow-up and adequate histology services are limited or nonexistent. Future large studies are necessary to evaluate the role of AVE alone versus in combination with other diagnostic testing (such as HPV testing) to detect cervical lesions globally.
Continue to: Data offer persuasive evidence...
Data offer persuasive evidence to abandon minimally invasive surgery in management of early-stage cervical cancer
Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
Over the past decade, gynecologic cancer surgery has shifted from what routinely were open procedures to the adoption of minimally invasive techniques. Recently, a large, well-designed prospective study and a large retrospective study both demonstrated worse outcomes with minimally invasive radical hysterectomy (MIRH) as compared with traditional open radical abdominal hysterectomy (RAH). These 2 landmark studies, initially presented at the Society of Gynecologic Oncology's 2018 annual meeting and later published in the New England Journal of Medicine, have really affected the gynecologic oncology community.
Shorter overall survival in women who had MIRH
Melamed and colleagues conducted a large, retrospective US-based study to evaluate all-cause mortality in women with cervical cancer who underwent MIRH compared with those who had RAH.6 The authors also sought to evaluate national trends in 4-year relative survival rates after minimally invasive surgery was adopted.
The study included 2,461 women who met the inclusion criteria; 49.8% (1,225) underwent MIRH procedures and, of those, 79.8% (978) had robot-assisted laparoscopy. Most women had stage IB1 tumors (88%), and most carcinomas were squamous cell (61%); 40.6% of tumors were less than 2 cm in size. There were no differences between the 2 groups with respect to rates of positive parametria, surgical margins, and lymph node involvement. Administration of adjuvant therapy, in those who qualified, was also similar between groups.
Results. At a median follow-up of 45 months, 94 deaths occurred in the minimally invasive group and 70 in the open surgery group. The risk of death at 4 years was 9.1% in the minimally invasive group versus 5.3% in the open surgery group, with a 65% higher risk of death from any cause, which was highly statistically significant.
Prospective trial showed MIRH was associated with lower survival rates
From 2008 to 2017, Ramirez and colleagues conducted a phase 3, multicenter, randomized controlled trial to prospectively establish the noninferiority of MIRH compared with RAH.7 The study included 631 women from 33 centers. The prespecified expected disease-free survival rate was 90% at 4.5 years.
To be included as a site, centers were required to submit details from 10 minimally invasive cases as well as 2 unedited videos for review by the trial management committee. In contrast to Melamed and colleagues' retrospective study, of the 319 procedures that were classified as minimally invasive, only 15.6% were robotically assisted. Similarly, most women had stage IB1 tumors (91.9%), and most were squamous cell carcinomas (67%). There were also no differences in the postoperative pathology findings or the need for adjuvant therapy administered between groups. The median follow-up was 2.5 years.
Results. At that time there were 27 recurrences in the MIRH group and 7 in the RAH group; there were also 19 deaths after MIRH and 3 after RAH. Disease-free survival at 4.5 years was 86% with MIRH versus 96.5% with RAH. Reported 3-year disease-free survival and overall survival were also significantily lower in the minimally invasive subgroup (91.2% vs 97.1%, 93.8% vs 99.0%, respectively).
Study limitations. Criticisms of this trial are that noninferiority could not be declared; in addition, the investigators were unable to complete enrollment secondary to early enrollment termination after the data and safety monitoring board raised survival concerns.
Many argue that subgroup analyses suggest a lower risk of poor outcomes in patients with smaller tumors (<2 cm); however, it is critical to note that this study was not powered to detect these differences.
The evidence is compelling and demonstrates potentially worse disease-related outcomes using MIRH when compared to traditional RAH with respect to cervical cancer recurrence, rates of death, and disease-free and overall survival. Several hypotheses have been proposed, and future research is needed to elucidate the differences in variables responsible for the outcomes demonstrated in these studies. Although there has been no ban on robot-assisted surgical devices or traditional minimally invasive techniques, the National Comprehensive Cancer Network has updated its recommendations to include careful counseling of patients who require a surgical approach for the management of early-stage cervical cancer.
Continue to: USPSTF updated guidance on cervical cancer screening...
USPSTF updated guidance on cervical cancer screening
Melnikow J, Henderson JT, Burda BU, et al. Screening for cervical cancer with high-risk human papillomavirus testing: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:687-705.
US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
Past guidelines for cervical cancer screening have included testing for high-risk HPV (hrHPV) as a cotest with cytology or for triage of atypical squamous cells of undetermined significance (ASCUS) in women aged 30 to 65 years.8 The American Society for Colposcopy and Cervical Pathology and the Society of Gynecologic Oncology, with other stakeholder organizations, issued interim guidance for primary HPV testing--that is, HPV test first and, in the case of non-16/18 hrHPV types, cytology as a triage. The most recent evidence report and systematic review by Melnikow and colleagues for the USPSTF offers an in-depth analysis of risks, benefits, harms, and value of cotesting and other management strategies.9
Focus on screening effectiveness
Large trials of cotesting were conducted in women aged 25 to 65.10-13 These studies all consistently showed that primary hrHPV screening led to a statistically significant increased detection of cervical intraepithelial neoplasia (CIN) 3+ in the initial round of screening, with a relative risk of detecting CIN 3+ ranging from 1.61 to 7.46 compared with cytology alone.
Four additional studies compared cotesting with conventional cytology for the detection of CIN 3+. None of these trials demonstrated a significantly higher detection rate of CIN 3+ with cotesting compared with conventional cytology testing alone. Notably, the studies reviewed were performed in European countries that had organized screening programs in place and a nationalized health care system. Thus, these data may not be as applicable to women in the United States, particularly to women who have limited health care access.
Risks of screening
In the same studies reviewed for screening effectiveness, the investigators found that overall, screening with hrHPV primary or cotesting was associated with more false-positive results and higher colposcopy rates. Women screened with hrHPV alone had a 7.9% referral rate to colposcopy, while those screened with cytology had a 2.8% referral rate to colposcopy. Similarly, the rate of biopsy was higher in the hrHPV-only group (3.2% vs 1.3%).
Overall, while cotesting might have some improvement in performance compared with hrHPV as a single modality, there might be risks of overreferral to colposcopy and overtreatment with additional cytology over hrHPV testing alone.
This evidence review also included an analysis of more potential harms. Very limited evidence suggests that positive hrHPV test results may be associated with greater psychological harm, including decreased sexual satisfaction, increased anxiety and distress, and worse feelings about sexual partners, than abnormal cytology results. These were assessed, however, 1 to 2 weeks after the test results were provided to the patients, and long-term assessment was not done.
New recommendations from the USPSTF
Based on these data, the USPSTF issued new recommendations regarding screening (TABLE).14 For women aged 21 to 29, cytology alone should be used for screening every 3 years. Women aged 30 to 65 can be screened with cytology alone every 3 years, with hrHPV testing alone every 5 years, or with cotesting every 5 years.

Primary screening with hrHPV is more effective in diagnosing a CIN 3+ than cytology alone. Cotesting with cytology and hrHPV testing appears to have limited performance improvement, with potential harm, compared with hrHPV testing alone in diagnosing CIN 3+. The Task Force recommendation is hrHPV testing alone or cotesting every 5 years.
Cervical cancer rates remain low in the United States, with the incidence having plateaued for decades. And yet, in 2019, more than 13,000 US women will be diagnosed with cervical cancer.1 Globally, in 2018 almost 600,000 women were diagnosed with cervical cancer2; it is the fourth most frequent cancer in women. This is despite the fact that we have adequate primary and secondary prevention tools available to minimize—and almost eliminate—cervical cancer. We must continue to raise the bar for preventing, screening for, and managing this disease.
Human papillomavirus (HPV) vaccines provide a highly effective primary prevention strategy, but we need to improve our ability to identify and diagnose dysplastic lesions prior to the development of cervical cancer. Highly sensitive HPV testing and cytology is a powerful secondary prevention approach that enables us to assess a woman’s risk of having precancerous cells both now and in the near future. These modalities have been very successful in decreasing the incidence of cervical cancer in the United States and other areas with organized screening programs. In low- and middle-income countries, however, access to, availability of, and performance with these modalities is not optimal. Innovative strategies and new technologies are being evaluated to overcome these limitations.
Advances in radiation and surgical technology have enabled us to vastly improve cervical cancer treatment. Women with early-stage cervical cancer are candidates for surgical management, which frequently includes a radical hysterectomy and lymph node dissection. While these surgeries traditionally have been performed via an exploratory laparotomy, minimally invasive techniques (laparoscopic and robot-assisted surgical techniques) have decreased the morbidity with these surgeries. Notable new studies have shed light on the comparative effectiveness of minimally invasive technologies and have shown us that new is not always better.
The US Preventive Services Task Force (USPSTF) recently released its updated cervical cancer screening guidelines. The suggested approach to screening differs from previous recommendations. HPV testing as a primary test (that is, HPV testing alone or followed by cytology) takes the spotlight now, according to the analysis by the Task Force.
In this Update, we highlight important studies published in the past year that address these issues.
Continue to: New tech's potential to identify high-grade...
New tech's potential to identify high-grade cervical dysplasia may be a boon to low-resource settings
Hu L, Bell D, Antani S, et al. An observational study of deep learning and automated evaluation of cervical images for cancer screening. J Natl Cancer Inst. 2019;doi:10.1093/jnci/djy225.
When cervical screening tests like cytology and HPV testing show abnormal results, colposcopy often is recommended. The goal of colposcopy is to identify the areas that might harbor a high-grade precancerous lesion or worse. The gold standard in this case, however, is histology, not colposcopic impression, as many studies have shown that colposcopy without biopsies is limited and that performance is improved with more biopsies.3,4
Visual inspection with acetic acid (VIA) is an approach used often in low-resource settings where visual impression is the gold standard. However, as with colposcopy, a visual evaluation without histology does not perform well, and often women are overtreated. Many attempts have been made with new technologies to overcome the limitations of time, cost, and workforce required for cytology and histology services. New disruptive technologies may be able to surmount human limitations and improve on not only VIA but also the need for histology.
Novel technology uses images to develop algorithm with predictive ability
In a recent observational study, Hu and colleagues used images that were collected during a large population study in Guanacaste, Costa Rica.5 More than 9,000 women were followed for up to 7 years, and cervical photographs (cervigrams) were obtained. Well-annotated histopathology results were obtained for women with abnormal screening, and 279 women had a high-grade dysplastic lesion or cancer.
Cervigrams from women with high-grade lesions and matched controls were collected, and a deep learning-based algorithm using artificial intelligence technology was developed using 70% of the images. The remaining 30% of images were used as a validation set to test the algorithm's ability to "predict" high-grade dysplasia without knowing the final result.
Findings. Termed automated visual evaluation (AVE), this new technology demonstrated a very accurate ability to identify high-grade dysplasia or worse, with an area under the curve (AUC) of 0.91 from merely a cervicogram (FIGURE). This outperformed conventional Pap smears (AUC, 0.71), liquid-based cytology (AUC, 0.79) and, surprisingly, highly sensitive HPV testing (AUC, 0.82) in women in the prime of their screening ages (>25 years of age).

Colposcopy remains the gold standard for evaluating abnormal cervical cancer screening tests in the United States. But can we do better for our patients using new technologies like AVE? If validated in large-scale trials, AVE has the potential to revolutionize cervical cancer screening in low-resource settings where follow-up and adequate histology services are limited or nonexistent. Future large studies are necessary to evaluate the role of AVE alone versus in combination with other diagnostic testing (such as HPV testing) to detect cervical lesions globally.
Continue to: Data offer persuasive evidence...
Data offer persuasive evidence to abandon minimally invasive surgery in management of early-stage cervical cancer
Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
Over the past decade, gynecologic cancer surgery has shifted from what routinely were open procedures to the adoption of minimally invasive techniques. Recently, a large, well-designed prospective study and a large retrospective study both demonstrated worse outcomes with minimally invasive radical hysterectomy (MIRH) as compared with traditional open radical abdominal hysterectomy (RAH). These 2 landmark studies, initially presented at the Society of Gynecologic Oncology's 2018 annual meeting and later published in the New England Journal of Medicine, have really affected the gynecologic oncology community.
Shorter overall survival in women who had MIRH
Melamed and colleagues conducted a large, retrospective US-based study to evaluate all-cause mortality in women with cervical cancer who underwent MIRH compared with those who had RAH.6 The authors also sought to evaluate national trends in 4-year relative survival rates after minimally invasive surgery was adopted.
The study included 2,461 women who met the inclusion criteria; 49.8% (1,225) underwent MIRH procedures and, of those, 79.8% (978) had robot-assisted laparoscopy. Most women had stage IB1 tumors (88%), and most carcinomas were squamous cell (61%); 40.6% of tumors were less than 2 cm in size. There were no differences between the 2 groups with respect to rates of positive parametria, surgical margins, and lymph node involvement. Administration of adjuvant therapy, in those who qualified, was also similar between groups.
Results. At a median follow-up of 45 months, 94 deaths occurred in the minimally invasive group and 70 in the open surgery group. The risk of death at 4 years was 9.1% in the minimally invasive group versus 5.3% in the open surgery group, with a 65% higher risk of death from any cause, which was highly statistically significant.
Prospective trial showed MIRH was associated with lower survival rates
From 2008 to 2017, Ramirez and colleagues conducted a phase 3, multicenter, randomized controlled trial to prospectively establish the noninferiority of MIRH compared with RAH.7 The study included 631 women from 33 centers. The prespecified expected disease-free survival rate was 90% at 4.5 years.
To be included as a site, centers were required to submit details from 10 minimally invasive cases as well as 2 unedited videos for review by the trial management committee. In contrast to Melamed and colleagues' retrospective study, of the 319 procedures that were classified as minimally invasive, only 15.6% were robotically assisted. Similarly, most women had stage IB1 tumors (91.9%), and most were squamous cell carcinomas (67%). There were also no differences in the postoperative pathology findings or the need for adjuvant therapy administered between groups. The median follow-up was 2.5 years.
Results. At that time there were 27 recurrences in the MIRH group and 7 in the RAH group; there were also 19 deaths after MIRH and 3 after RAH. Disease-free survival at 4.5 years was 86% with MIRH versus 96.5% with RAH. Reported 3-year disease-free survival and overall survival were also significantily lower in the minimally invasive subgroup (91.2% vs 97.1%, 93.8% vs 99.0%, respectively).
Study limitations. Criticisms of this trial are that noninferiority could not be declared; in addition, the investigators were unable to complete enrollment secondary to early enrollment termination after the data and safety monitoring board raised survival concerns.
Many argue that subgroup analyses suggest a lower risk of poor outcomes in patients with smaller tumors (<2 cm); however, it is critical to note that this study was not powered to detect these differences.
The evidence is compelling and demonstrates potentially worse disease-related outcomes using MIRH when compared to traditional RAH with respect to cervical cancer recurrence, rates of death, and disease-free and overall survival. Several hypotheses have been proposed, and future research is needed to elucidate the differences in variables responsible for the outcomes demonstrated in these studies. Although there has been no ban on robot-assisted surgical devices or traditional minimally invasive techniques, the National Comprehensive Cancer Network has updated its recommendations to include careful counseling of patients who require a surgical approach for the management of early-stage cervical cancer.
Continue to: USPSTF updated guidance on cervical cancer screening...
USPSTF updated guidance on cervical cancer screening
Melnikow J, Henderson JT, Burda BU, et al. Screening for cervical cancer with high-risk human papillomavirus testing: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:687-705.
US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
Past guidelines for cervical cancer screening have included testing for high-risk HPV (hrHPV) as a cotest with cytology or for triage of atypical squamous cells of undetermined significance (ASCUS) in women aged 30 to 65 years.8 The American Society for Colposcopy and Cervical Pathology and the Society of Gynecologic Oncology, with other stakeholder organizations, issued interim guidance for primary HPV testing--that is, HPV test first and, in the case of non-16/18 hrHPV types, cytology as a triage. The most recent evidence report and systematic review by Melnikow and colleagues for the USPSTF offers an in-depth analysis of risks, benefits, harms, and value of cotesting and other management strategies.9
Focus on screening effectiveness
Large trials of cotesting were conducted in women aged 25 to 65.10-13 These studies all consistently showed that primary hrHPV screening led to a statistically significant increased detection of cervical intraepithelial neoplasia (CIN) 3+ in the initial round of screening, with a relative risk of detecting CIN 3+ ranging from 1.61 to 7.46 compared with cytology alone.
Four additional studies compared cotesting with conventional cytology for the detection of CIN 3+. None of these trials demonstrated a significantly higher detection rate of CIN 3+ with cotesting compared with conventional cytology testing alone. Notably, the studies reviewed were performed in European countries that had organized screening programs in place and a nationalized health care system. Thus, these data may not be as applicable to women in the United States, particularly to women who have limited health care access.
Risks of screening
In the same studies reviewed for screening effectiveness, the investigators found that overall, screening with hrHPV primary or cotesting was associated with more false-positive results and higher colposcopy rates. Women screened with hrHPV alone had a 7.9% referral rate to colposcopy, while those screened with cytology had a 2.8% referral rate to colposcopy. Similarly, the rate of biopsy was higher in the hrHPV-only group (3.2% vs 1.3%).
Overall, while cotesting might have some improvement in performance compared with hrHPV as a single modality, there might be risks of overreferral to colposcopy and overtreatment with additional cytology over hrHPV testing alone.
This evidence review also included an analysis of more potential harms. Very limited evidence suggests that positive hrHPV test results may be associated with greater psychological harm, including decreased sexual satisfaction, increased anxiety and distress, and worse feelings about sexual partners, than abnormal cytology results. These were assessed, however, 1 to 2 weeks after the test results were provided to the patients, and long-term assessment was not done.
New recommendations from the USPSTF
Based on these data, the USPSTF issued new recommendations regarding screening (TABLE).14 For women aged 21 to 29, cytology alone should be used for screening every 3 years. Women aged 30 to 65 can be screened with cytology alone every 3 years, with hrHPV testing alone every 5 years, or with cotesting every 5 years.

Primary screening with hrHPV is more effective in diagnosing a CIN 3+ than cytology alone. Cotesting with cytology and hrHPV testing appears to have limited performance improvement, with potential harm, compared with hrHPV testing alone in diagnosing CIN 3+. The Task Force recommendation is hrHPV testing alone or cotesting every 5 years.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
- World Health Organization website. Cervical cancer. https://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer/en/. Accessed April 17, 2019.
- Wentzensen N, Walker JL, Gold MA, et al. Multiple biopsies and detection of cervical cancer precursors at colposcopy. J Clin Oncol. 2015;33:83-89.
- Gage JC, Hanson VW, Abbey K, et al. Number of cervical biopsies and sensitivity of colposcopy. Obstet Gynecol. 2006;108:264-272.
- Hu L, Bell D, Antani S, et al. An observational study of deep learning and automated evaluation of cervical images for cancer screening. J Natl Cancer Inst. 2019;doi:10.1093/jnci/djy225.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147-172.
- Melnikow J, Henderson JT, Burda BU, et al. Screening for cervical cancer with high-risk human papillomavirus testing: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:687-705.
- Canfell K, Caruana M, Gebski V, et al. Cervical screening with primary HPV testing or cytology in a population of women in which those aged 33 years or younger had previously been offered HPV vaccination: results of the Compass pilot randomised trial. PLoS Med. 2017;14:e1002388. doi:10.1371/journal.pmed.1002388.
- Leinonen MK, Nieminen P, Lonnberg S, et al. Detection rates of precancerous and cancerous cervical lesions within one screening round of primary human papillomavirus DNA testing: prospective randomised trial in Finland. BMJ. 2012;345:e7789.
- Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52.
- Ronco G, Fioprgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257.
- US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
- World Health Organization website. Cervical cancer. https://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer/en/. Accessed April 17, 2019.
- Wentzensen N, Walker JL, Gold MA, et al. Multiple biopsies and detection of cervical cancer precursors at colposcopy. J Clin Oncol. 2015;33:83-89.
- Gage JC, Hanson VW, Abbey K, et al. Number of cervical biopsies and sensitivity of colposcopy. Obstet Gynecol. 2006;108:264-272.
- Hu L, Bell D, Antani S, et al. An observational study of deep learning and automated evaluation of cervical images for cancer screening. J Natl Cancer Inst. 2019;doi:10.1093/jnci/djy225.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147-172.
- Melnikow J, Henderson JT, Burda BU, et al. Screening for cervical cancer with high-risk human papillomavirus testing: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:687-705.
- Canfell K, Caruana M, Gebski V, et al. Cervical screening with primary HPV testing or cytology in a population of women in which those aged 33 years or younger had previously been offered HPV vaccination: results of the Compass pilot randomised trial. PLoS Med. 2017;14:e1002388. doi:10.1371/journal.pmed.1002388.
- Leinonen MK, Nieminen P, Lonnberg S, et al. Detection rates of precancerous and cancerous cervical lesions within one screening round of primary human papillomavirus DNA testing: prospective randomised trial in Finland. BMJ. 2012;345:e7789.
- Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52.
- Ronco G, Fioprgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257.
- US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
Vaginal brachytherapy more toxic than pelvic RT in endometrial carcinoma
, according to results from a phase 3 trial.
The GOG-0249 study comprised 601 patients with high-intermediate– and high-risk early-stage endometrial carcinoma who were randomized in a 1:1 fashion to receive vaginal cuff brachytherapy plus paclitaxel and carboplatin or pelvic RT every 21 days for a total of three cycles.
Those in the brachytherapy group received treatment at both high and low-dosing rates. Paclitaxel was given at a dose of 175 mg/m2 infused over 3 hours, succeeded by carboplatin (area under the curve, 6) infused over 45 minutes. Pelvic RT was provided at a dose of 45 to 50.4 Gy over a period of 5-6 weeks.
“The primary objective was to determine if vaginal cuff brachytherapy and chemotherapy increases recurrence-free survival (RFS) compared with pelvic RT,” wrote Marcus E. Randall, MD, of the University of Kentucky, Lexington, and colleagues. The report is in the Journal of Clinical Oncology.
Additional outcomes measured were overall survival and acute toxicity.
After analysis, the researchers found that vaginal brachytherapy plus chemotherapy did not show superiority over pelvic RT in terms of 60-month RFS (hazard ratio, 0.92; 90% confidence limit, 0.69-1.23). Also, there was no significant difference for overall survival (HR, 1.04; 90% confidence limit, 0.71-1.52).
With respect to safety, acute adverse events were more common and severe in the vaginal brachytherapy plus chemotherapy group. However, “differences in late toxicity were minimal,” Dr. Randall and colleagues reported.
“Pelvic RT remains an appropriate treatment for high-risk early-stage endometrial carcinoma,” they wrote. “Novel combinations, dose intensification, and additional translational research represent potential paths forward.”
The study was supported by grant funding from the National Cancer Institute and the Memorial Sloan Kettering Cancer Center. The authors reported financial affiliations with AstraZeneca, Genentech, Genmab, Janssen, Johnson & Johnson, Tesaro, and several others.
SOURCE: Randall ME et al. J Clin Oncol. 2019 Apr 17. doi: 10.1200/JCO.18.01575.
, according to results from a phase 3 trial.
The GOG-0249 study comprised 601 patients with high-intermediate– and high-risk early-stage endometrial carcinoma who were randomized in a 1:1 fashion to receive vaginal cuff brachytherapy plus paclitaxel and carboplatin or pelvic RT every 21 days for a total of three cycles.
Those in the brachytherapy group received treatment at both high and low-dosing rates. Paclitaxel was given at a dose of 175 mg/m2 infused over 3 hours, succeeded by carboplatin (area under the curve, 6) infused over 45 minutes. Pelvic RT was provided at a dose of 45 to 50.4 Gy over a period of 5-6 weeks.
“The primary objective was to determine if vaginal cuff brachytherapy and chemotherapy increases recurrence-free survival (RFS) compared with pelvic RT,” wrote Marcus E. Randall, MD, of the University of Kentucky, Lexington, and colleagues. The report is in the Journal of Clinical Oncology.
Additional outcomes measured were overall survival and acute toxicity.
After analysis, the researchers found that vaginal brachytherapy plus chemotherapy did not show superiority over pelvic RT in terms of 60-month RFS (hazard ratio, 0.92; 90% confidence limit, 0.69-1.23). Also, there was no significant difference for overall survival (HR, 1.04; 90% confidence limit, 0.71-1.52).
With respect to safety, acute adverse events were more common and severe in the vaginal brachytherapy plus chemotherapy group. However, “differences in late toxicity were minimal,” Dr. Randall and colleagues reported.
“Pelvic RT remains an appropriate treatment for high-risk early-stage endometrial carcinoma,” they wrote. “Novel combinations, dose intensification, and additional translational research represent potential paths forward.”
The study was supported by grant funding from the National Cancer Institute and the Memorial Sloan Kettering Cancer Center. The authors reported financial affiliations with AstraZeneca, Genentech, Genmab, Janssen, Johnson & Johnson, Tesaro, and several others.
SOURCE: Randall ME et al. J Clin Oncol. 2019 Apr 17. doi: 10.1200/JCO.18.01575.
, according to results from a phase 3 trial.
The GOG-0249 study comprised 601 patients with high-intermediate– and high-risk early-stage endometrial carcinoma who were randomized in a 1:1 fashion to receive vaginal cuff brachytherapy plus paclitaxel and carboplatin or pelvic RT every 21 days for a total of three cycles.
Those in the brachytherapy group received treatment at both high and low-dosing rates. Paclitaxel was given at a dose of 175 mg/m2 infused over 3 hours, succeeded by carboplatin (area under the curve, 6) infused over 45 minutes. Pelvic RT was provided at a dose of 45 to 50.4 Gy over a period of 5-6 weeks.
“The primary objective was to determine if vaginal cuff brachytherapy and chemotherapy increases recurrence-free survival (RFS) compared with pelvic RT,” wrote Marcus E. Randall, MD, of the University of Kentucky, Lexington, and colleagues. The report is in the Journal of Clinical Oncology.
Additional outcomes measured were overall survival and acute toxicity.
After analysis, the researchers found that vaginal brachytherapy plus chemotherapy did not show superiority over pelvic RT in terms of 60-month RFS (hazard ratio, 0.92; 90% confidence limit, 0.69-1.23). Also, there was no significant difference for overall survival (HR, 1.04; 90% confidence limit, 0.71-1.52).
With respect to safety, acute adverse events were more common and severe in the vaginal brachytherapy plus chemotherapy group. However, “differences in late toxicity were minimal,” Dr. Randall and colleagues reported.
“Pelvic RT remains an appropriate treatment for high-risk early-stage endometrial carcinoma,” they wrote. “Novel combinations, dose intensification, and additional translational research represent potential paths forward.”
The study was supported by grant funding from the National Cancer Institute and the Memorial Sloan Kettering Cancer Center. The authors reported financial affiliations with AstraZeneca, Genentech, Genmab, Janssen, Johnson & Johnson, Tesaro, and several others.
SOURCE: Randall ME et al. J Clin Oncol. 2019 Apr 17. doi: 10.1200/JCO.18.01575.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Discuss compounded bioidentical hormones and cancer risk
The clinical scenario is as follows: A 62-year-old woman comes to see me for a new diagnosis of grade 1 endometrial cancer. She has a normal body mass index of 24 kg/m2, a history of four prior full-term pregnancies, no family history of malignancy, and no medical comorbidities. She is otherwise a specimen of good health, and has no clear identifiable risk factors for this malignancy. She then reports that she transitioned through menopause at age 52 years and developed severe hot flashes with sleep and mood disturbance. She did not wish to take conventional hormone replacement therapy (HT) because she had heard it causes cancer. She subsequently researched the Internet and found a provider who has been prescribing compounded bioidentical hormone therapy (CBHT) for her for the past 10 years. She submits saliva for testing of her estrogen levels, and the provider uses this data to compound the appropriate doses of “natural” estrogens and testosterone for her which she applies via vaginal or transdermal creams. She has been prescribed a progesterone suppository, but she doesn’t always take that because she doesn’t notice that it has any effect on how she feels.
My answer is, of course, I don’t know. Cancer is a complex disease with a complex array of causative and promoting factors. However, we do know that taking estrogen unopposed with adequate progesterone can cause the development of uterine cancer and its precursor state.1 If those bioidentical estrogens were effective at controlling her menopausal symptoms, they likely were effective at stimulating her endometrium at the same time.
What are compounded bioidentical hormones?
The term “bioidentical” refers to having the same molecular structure as that which is found in the human body. Examples of bioidentical estrogens include 17-beta-estradiol, estrone, and estriol – which are produced from yams and soy. Micronized progesterone is an example of a bioidentical progesterone. Many of these drugs are approved by the Food and Drug Administration, and prescribed and dispensed by conventional pharmacies.
An alternative, and increasingly popular, version of bioidentical hormones are CBHs. It should be recognized that this is a marketing, and not a scientific, term. These products utilize hormones, in some cases FDA-approved bioidentical hormones, that are broken down and blended by specialized pharmacies and reconstituted (compounded) into different, and sometimes “customized,” dosing and delivery methods (such as capsules, patches, gels, creams, lozenges, suppositories). Frequently used compounded products utilize multiple formulations of estrogens in doublets and triplets as well as progesterone, testosterone, and dehydroepiandrosterone.
How do they differ from synthetic hormones?
Distributors of CBHs state that they differ from conventional HT (synthetic and bioidentical) because of the customization process from which they promise greater efficacy and a sense of personalized medicine. The distributors frequently utilize assays from saliva, blood, vaginal secretions, and urine to measure a woman’s hormone levels, and titrate her compounded formulation based on those results. It should be noted that there is no data to support that titration of hormones to blood, salivary, or urine levels is efficacious or ensures greater safety than titration based on symptom management.
Critics of CBHT, which includes the North American Menopause Society2 and the American College of Obstetricians and Gynecologists,3 highlight that the main difference between CBHT and HT is lack of FDA regulation over the CBHT industry. Many of these agents are delivered transdermally and therefore are classified as “dietary supplements.” As such, they do not require FDA regulation or proof of safety or efficacy.
Lack of FDA approval allows CBHs to be distributed without package inserts and boxed warnings (such as the standard warnings about MI, venous thromboembolic events, and breast cancer). The absence of FDA approval also allows them to avoid FDA-regulated guarantees about purity, potency, and efficacy. Audits of CBHs have shown high rates of discrepancy between stated and measured potency, including observations of both much lower and much higher than stated strength.4
Why would dosing accuracy be important in hormone therapy prescription? If a woman taking estrogen therapy is not receiving adequate cotreatment with progesterone because of either omission or a subtherapeutic product, she increases her risk for endometrial cancer.
What drives patients’ decision to use compounded bioidentical hormones?
After the Womens’ Health Initiative study was published in 20025, all FDA-regulated estrogen preparations were required to carry specific warnings, particularly in relation to the increased risk for MI, venous thromboembolic events, and breast cancer. There was a clear uptake in use of CBHT after this study was reported. By avoiding FDA regulations, distributors of CBHTs may have avoided providing Womens’ Health Initiative information to patients. The absence of an insert with a written warning, in and of itself, makes these preparations seem safer to the patient.
But is it entirely a lack of information that drives demand for CBHTs? Surveys of current or former users suggest the motivations are more complex than that. A survey of 21 past or present CBHT users inquired about reasons for use of CBHT over conventional HT.6 Their responses were categorized as either push motivations away from conventional therapy versus pull motivations toward CBHT. About 95% of current and former users cited distrust of the biomedicine and pharmaceutical industry as reasons for use of CBHT. Fear about the safety of conventional HT, particularly with respect to cancer risk, also was strongly cited at 81%. Motivations pulling toward CBHT included its efficacy (76%) and perception that CBHT is “safer” than conventional HT (76%).
Women in this study also appreciated the tailored, individualized approach that often is associated with CBHT, in which providers spend long consultations discussing individual patient needs and concerns. They enjoy the idea of a customized blend that is created, as opposed to a standard dosing regimen, and intuitively trust the reliability of blood and saliva testing as a prescriptive tool.
Are bioidentical hormones safe with respect to cancer risk?
Hormones themselves are not inert substances, including those derived in vivo and those from plants. They have powerful effects in the human body and can promote malignant transformation or proliferation, alter metabolic pathways, stimulate vascular tone, influence coagulation pathways, along with many other effects. A hormone’s potential for deleterious effect can be present regardless of how that hormone is synthesized, procured, or prepared. While there are no data to suggest that CBHT is associated with increased cancer risk, compared with conventional HT, there are by no means any data to suggest it is safer. Unopposed compounded estrogens place women at increased risk for endometrial cancers, and the prolonged use of hormonal therapy, compounded or otherwise, after menopause increases the risk for breast cancer.
How should we counsel patients?
Patients who desire compounded bioidentical hormone preparations should be counseled that little is known about the safety of these preparations, compared with conventional hormone preparations. The fact that the components are often plant based rather than synthetic does not inherently alter their potential negative impact on biologic pathways. Patients should be educated regarding the difference between FDA-regulated products and nonregulated products so that they can understand that lack of a boxed warning on a non-FDA regulated product does not mean an absence of risk. Women should be informed of the potential inaccuracies in dosing and strength of the CBH preparations they receive.
We should recognize that our patients strongly desire a relationship with their provider in which they are listened to, understood, and treated as individuals. If conversations regarding hormone use are approached with these principles, we will optimize the likelihood our patients are receptive to the highest quality information and not pulled in the direction of unregulated products.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported that she had no conflicts of interest. Email Dr. Rossi at [email protected].
References:
1. Maturitas. 2014 Jan;77(1):4-6.
2. Menopause. 2014 Dec;21(12):1298-300.
3. Fertil Steril. 2012 Aug;98(2):308-12.
4. Report: Limited FDA survey of compounded drug products (Silver Spring, Md.: U.S. Food and Drug Administration, 2009).
5. JAMA. 2002;288(3):321-33.
6. BMC Womens Health. 2017 Oct 2;17(1):97.
The clinical scenario is as follows: A 62-year-old woman comes to see me for a new diagnosis of grade 1 endometrial cancer. She has a normal body mass index of 24 kg/m2, a history of four prior full-term pregnancies, no family history of malignancy, and no medical comorbidities. She is otherwise a specimen of good health, and has no clear identifiable risk factors for this malignancy. She then reports that she transitioned through menopause at age 52 years and developed severe hot flashes with sleep and mood disturbance. She did not wish to take conventional hormone replacement therapy (HT) because she had heard it causes cancer. She subsequently researched the Internet and found a provider who has been prescribing compounded bioidentical hormone therapy (CBHT) for her for the past 10 years. She submits saliva for testing of her estrogen levels, and the provider uses this data to compound the appropriate doses of “natural” estrogens and testosterone for her which she applies via vaginal or transdermal creams. She has been prescribed a progesterone suppository, but she doesn’t always take that because she doesn’t notice that it has any effect on how she feels.
My answer is, of course, I don’t know. Cancer is a complex disease with a complex array of causative and promoting factors. However, we do know that taking estrogen unopposed with adequate progesterone can cause the development of uterine cancer and its precursor state.1 If those bioidentical estrogens were effective at controlling her menopausal symptoms, they likely were effective at stimulating her endometrium at the same time.
What are compounded bioidentical hormones?
The term “bioidentical” refers to having the same molecular structure as that which is found in the human body. Examples of bioidentical estrogens include 17-beta-estradiol, estrone, and estriol – which are produced from yams and soy. Micronized progesterone is an example of a bioidentical progesterone. Many of these drugs are approved by the Food and Drug Administration, and prescribed and dispensed by conventional pharmacies.
An alternative, and increasingly popular, version of bioidentical hormones are CBHs. It should be recognized that this is a marketing, and not a scientific, term. These products utilize hormones, in some cases FDA-approved bioidentical hormones, that are broken down and blended by specialized pharmacies and reconstituted (compounded) into different, and sometimes “customized,” dosing and delivery methods (such as capsules, patches, gels, creams, lozenges, suppositories). Frequently used compounded products utilize multiple formulations of estrogens in doublets and triplets as well as progesterone, testosterone, and dehydroepiandrosterone.
How do they differ from synthetic hormones?
Distributors of CBHs state that they differ from conventional HT (synthetic and bioidentical) because of the customization process from which they promise greater efficacy and a sense of personalized medicine. The distributors frequently utilize assays from saliva, blood, vaginal secretions, and urine to measure a woman’s hormone levels, and titrate her compounded formulation based on those results. It should be noted that there is no data to support that titration of hormones to blood, salivary, or urine levels is efficacious or ensures greater safety than titration based on symptom management.
Critics of CBHT, which includes the North American Menopause Society2 and the American College of Obstetricians and Gynecologists,3 highlight that the main difference between CBHT and HT is lack of FDA regulation over the CBHT industry. Many of these agents are delivered transdermally and therefore are classified as “dietary supplements.” As such, they do not require FDA regulation or proof of safety or efficacy.
Lack of FDA approval allows CBHs to be distributed without package inserts and boxed warnings (such as the standard warnings about MI, venous thromboembolic events, and breast cancer). The absence of FDA approval also allows them to avoid FDA-regulated guarantees about purity, potency, and efficacy. Audits of CBHs have shown high rates of discrepancy between stated and measured potency, including observations of both much lower and much higher than stated strength.4
Why would dosing accuracy be important in hormone therapy prescription? If a woman taking estrogen therapy is not receiving adequate cotreatment with progesterone because of either omission or a subtherapeutic product, she increases her risk for endometrial cancer.
What drives patients’ decision to use compounded bioidentical hormones?
After the Womens’ Health Initiative study was published in 20025, all FDA-regulated estrogen preparations were required to carry specific warnings, particularly in relation to the increased risk for MI, venous thromboembolic events, and breast cancer. There was a clear uptake in use of CBHT after this study was reported. By avoiding FDA regulations, distributors of CBHTs may have avoided providing Womens’ Health Initiative information to patients. The absence of an insert with a written warning, in and of itself, makes these preparations seem safer to the patient.
But is it entirely a lack of information that drives demand for CBHTs? Surveys of current or former users suggest the motivations are more complex than that. A survey of 21 past or present CBHT users inquired about reasons for use of CBHT over conventional HT.6 Their responses were categorized as either push motivations away from conventional therapy versus pull motivations toward CBHT. About 95% of current and former users cited distrust of the biomedicine and pharmaceutical industry as reasons for use of CBHT. Fear about the safety of conventional HT, particularly with respect to cancer risk, also was strongly cited at 81%. Motivations pulling toward CBHT included its efficacy (76%) and perception that CBHT is “safer” than conventional HT (76%).
Women in this study also appreciated the tailored, individualized approach that often is associated with CBHT, in which providers spend long consultations discussing individual patient needs and concerns. They enjoy the idea of a customized blend that is created, as opposed to a standard dosing regimen, and intuitively trust the reliability of blood and saliva testing as a prescriptive tool.
Are bioidentical hormones safe with respect to cancer risk?
Hormones themselves are not inert substances, including those derived in vivo and those from plants. They have powerful effects in the human body and can promote malignant transformation or proliferation, alter metabolic pathways, stimulate vascular tone, influence coagulation pathways, along with many other effects. A hormone’s potential for deleterious effect can be present regardless of how that hormone is synthesized, procured, or prepared. While there are no data to suggest that CBHT is associated with increased cancer risk, compared with conventional HT, there are by no means any data to suggest it is safer. Unopposed compounded estrogens place women at increased risk for endometrial cancers, and the prolonged use of hormonal therapy, compounded or otherwise, after menopause increases the risk for breast cancer.
How should we counsel patients?
Patients who desire compounded bioidentical hormone preparations should be counseled that little is known about the safety of these preparations, compared with conventional hormone preparations. The fact that the components are often plant based rather than synthetic does not inherently alter their potential negative impact on biologic pathways. Patients should be educated regarding the difference between FDA-regulated products and nonregulated products so that they can understand that lack of a boxed warning on a non-FDA regulated product does not mean an absence of risk. Women should be informed of the potential inaccuracies in dosing and strength of the CBH preparations they receive.
We should recognize that our patients strongly desire a relationship with their provider in which they are listened to, understood, and treated as individuals. If conversations regarding hormone use are approached with these principles, we will optimize the likelihood our patients are receptive to the highest quality information and not pulled in the direction of unregulated products.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported that she had no conflicts of interest. Email Dr. Rossi at [email protected].
References:
1. Maturitas. 2014 Jan;77(1):4-6.
2. Menopause. 2014 Dec;21(12):1298-300.
3. Fertil Steril. 2012 Aug;98(2):308-12.
4. Report: Limited FDA survey of compounded drug products (Silver Spring, Md.: U.S. Food and Drug Administration, 2009).
5. JAMA. 2002;288(3):321-33.
6. BMC Womens Health. 2017 Oct 2;17(1):97.
The clinical scenario is as follows: A 62-year-old woman comes to see me for a new diagnosis of grade 1 endometrial cancer. She has a normal body mass index of 24 kg/m2, a history of four prior full-term pregnancies, no family history of malignancy, and no medical comorbidities. She is otherwise a specimen of good health, and has no clear identifiable risk factors for this malignancy. She then reports that she transitioned through menopause at age 52 years and developed severe hot flashes with sleep and mood disturbance. She did not wish to take conventional hormone replacement therapy (HT) because she had heard it causes cancer. She subsequently researched the Internet and found a provider who has been prescribing compounded bioidentical hormone therapy (CBHT) for her for the past 10 years. She submits saliva for testing of her estrogen levels, and the provider uses this data to compound the appropriate doses of “natural” estrogens and testosterone for her which she applies via vaginal or transdermal creams. She has been prescribed a progesterone suppository, but she doesn’t always take that because she doesn’t notice that it has any effect on how she feels.
My answer is, of course, I don’t know. Cancer is a complex disease with a complex array of causative and promoting factors. However, we do know that taking estrogen unopposed with adequate progesterone can cause the development of uterine cancer and its precursor state.1 If those bioidentical estrogens were effective at controlling her menopausal symptoms, they likely were effective at stimulating her endometrium at the same time.
What are compounded bioidentical hormones?
The term “bioidentical” refers to having the same molecular structure as that which is found in the human body. Examples of bioidentical estrogens include 17-beta-estradiol, estrone, and estriol – which are produced from yams and soy. Micronized progesterone is an example of a bioidentical progesterone. Many of these drugs are approved by the Food and Drug Administration, and prescribed and dispensed by conventional pharmacies.
An alternative, and increasingly popular, version of bioidentical hormones are CBHs. It should be recognized that this is a marketing, and not a scientific, term. These products utilize hormones, in some cases FDA-approved bioidentical hormones, that are broken down and blended by specialized pharmacies and reconstituted (compounded) into different, and sometimes “customized,” dosing and delivery methods (such as capsules, patches, gels, creams, lozenges, suppositories). Frequently used compounded products utilize multiple formulations of estrogens in doublets and triplets as well as progesterone, testosterone, and dehydroepiandrosterone.
How do they differ from synthetic hormones?
Distributors of CBHs state that they differ from conventional HT (synthetic and bioidentical) because of the customization process from which they promise greater efficacy and a sense of personalized medicine. The distributors frequently utilize assays from saliva, blood, vaginal secretions, and urine to measure a woman’s hormone levels, and titrate her compounded formulation based on those results. It should be noted that there is no data to support that titration of hormones to blood, salivary, or urine levels is efficacious or ensures greater safety than titration based on symptom management.
Critics of CBHT, which includes the North American Menopause Society2 and the American College of Obstetricians and Gynecologists,3 highlight that the main difference between CBHT and HT is lack of FDA regulation over the CBHT industry. Many of these agents are delivered transdermally and therefore are classified as “dietary supplements.” As such, they do not require FDA regulation or proof of safety or efficacy.
Lack of FDA approval allows CBHs to be distributed without package inserts and boxed warnings (such as the standard warnings about MI, venous thromboembolic events, and breast cancer). The absence of FDA approval also allows them to avoid FDA-regulated guarantees about purity, potency, and efficacy. Audits of CBHs have shown high rates of discrepancy between stated and measured potency, including observations of both much lower and much higher than stated strength.4
Why would dosing accuracy be important in hormone therapy prescription? If a woman taking estrogen therapy is not receiving adequate cotreatment with progesterone because of either omission or a subtherapeutic product, she increases her risk for endometrial cancer.
What drives patients’ decision to use compounded bioidentical hormones?
After the Womens’ Health Initiative study was published in 20025, all FDA-regulated estrogen preparations were required to carry specific warnings, particularly in relation to the increased risk for MI, venous thromboembolic events, and breast cancer. There was a clear uptake in use of CBHT after this study was reported. By avoiding FDA regulations, distributors of CBHTs may have avoided providing Womens’ Health Initiative information to patients. The absence of an insert with a written warning, in and of itself, makes these preparations seem safer to the patient.
But is it entirely a lack of information that drives demand for CBHTs? Surveys of current or former users suggest the motivations are more complex than that. A survey of 21 past or present CBHT users inquired about reasons for use of CBHT over conventional HT.6 Their responses were categorized as either push motivations away from conventional therapy versus pull motivations toward CBHT. About 95% of current and former users cited distrust of the biomedicine and pharmaceutical industry as reasons for use of CBHT. Fear about the safety of conventional HT, particularly with respect to cancer risk, also was strongly cited at 81%. Motivations pulling toward CBHT included its efficacy (76%) and perception that CBHT is “safer” than conventional HT (76%).
Women in this study also appreciated the tailored, individualized approach that often is associated with CBHT, in which providers spend long consultations discussing individual patient needs and concerns. They enjoy the idea of a customized blend that is created, as opposed to a standard dosing regimen, and intuitively trust the reliability of blood and saliva testing as a prescriptive tool.
Are bioidentical hormones safe with respect to cancer risk?
Hormones themselves are not inert substances, including those derived in vivo and those from plants. They have powerful effects in the human body and can promote malignant transformation or proliferation, alter metabolic pathways, stimulate vascular tone, influence coagulation pathways, along with many other effects. A hormone’s potential for deleterious effect can be present regardless of how that hormone is synthesized, procured, or prepared. While there are no data to suggest that CBHT is associated with increased cancer risk, compared with conventional HT, there are by no means any data to suggest it is safer. Unopposed compounded estrogens place women at increased risk for endometrial cancers, and the prolonged use of hormonal therapy, compounded or otherwise, after menopause increases the risk for breast cancer.
How should we counsel patients?
Patients who desire compounded bioidentical hormone preparations should be counseled that little is known about the safety of these preparations, compared with conventional hormone preparations. The fact that the components are often plant based rather than synthetic does not inherently alter their potential negative impact on biologic pathways. Patients should be educated regarding the difference between FDA-regulated products and nonregulated products so that they can understand that lack of a boxed warning on a non-FDA regulated product does not mean an absence of risk. Women should be informed of the potential inaccuracies in dosing and strength of the CBH preparations they receive.
We should recognize that our patients strongly desire a relationship with their provider in which they are listened to, understood, and treated as individuals. If conversations regarding hormone use are approached with these principles, we will optimize the likelihood our patients are receptive to the highest quality information and not pulled in the direction of unregulated products.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported that she had no conflicts of interest. Email Dr. Rossi at [email protected].
References:
1. Maturitas. 2014 Jan;77(1):4-6.
2. Menopause. 2014 Dec;21(12):1298-300.
3. Fertil Steril. 2012 Aug;98(2):308-12.
4. Report: Limited FDA survey of compounded drug products (Silver Spring, Md.: U.S. Food and Drug Administration, 2009).
5. JAMA. 2002;288(3):321-33.
6. BMC Womens Health. 2017 Oct 2;17(1):97.
Lessons from KEYNOTE-158 and the role of R-CHOP
In this edition of “How I will treat my next patient,” I take a look at two recent trials – one offers potential in previously-treated cervical cancer patients with poor prognosis and the other confirms the role of R-CHOP as the standard of care in diffuse large B-cell lymphoma.
Pembrolizumab in KEYNOTE-158
In an international phase 2 “basket trial,” Hyun Cheol Chung, MD, PhD, and colleagues used pembrolizumab 200 mg every 3 weeks in 98 previously treated patients with advanced cervical cancer. Almost 84% of o the patients had PD-L1 positive tumors (greater than 1%). The authors said that viral induction of malignancy leads to antigen production and upregulation of PD-1. Therefore, advanced cervical cancer patients would likely express PD-L1 on tumor cells and respond to immune checkpoint inhibitor therapy.
In this interim report, there were 12 responses (all in PD-L1 positive patients), with three complete responses. Median response duration had not been reached at median follow-up of 10.2 months. Seven of 12 responses were ongoing at 12 months. There were grade 3-4 adverse events in 12.2% of patients and no treatment-related deaths.
The study – “Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study” – was published in the Journal of Clinical Oncology (2019 April 3. doi: 10.1200/JCO.18.01265).
The encouraging results of pembrolizumab in this generally chemotherapy-refractory patient population were consistent with other small, early-phase studies investigating immune checkpoint inhibitors that led to the accelerated approval of pembrolizumab in previously treated PD-L1 advanced cervical cancer patients with progressive disease after chemotherapy.
What this means in practice
Although excitement should be tempered about an interim report of an organ-specific subset of a phase 2 international basket trial that was heavily populated by young PS 0-1 patients and generated an overall response rate of less than 15%, no conventional chemotherapy or biologic agent offers the potential of complete or prolonged response, and disease control rates of 30%.
Clinical trials should always be the first choice, but immune checkpoint inhibitors offer an attractive off-study option.
Among many single agents in National Comprehensive Cancer Network guidelines for recurrent advanced cervical cancer after first-line cisplatin-based chemotherapy, there is a reason why pembrolizumab is listed first. For patients with PD-L1 expressing tumors or MSI-H/dMMR tumors, I would use it.
Frontline therapy in DLBCL
In a large, randomized phase 3 trial, close to 500 stage III-IV patients with diffuse large B-cell lymphoma (DLBCL), including primary mediastinal B-cell lymphoma and intravascular large B-cell lymphoma, were assigned to receive either conventional R-CHOP chemotherapy or the more complex, more toxic DA-EPOCH-R regimen that appeared superior in single-institution studies and was feasible in multi-institutional phase 2 trials.
The study – “Dose-Adjusted EPOCH-R Compared With R-CHOP as Frontline Therapy for Diffuse Large B-Cell Lymphoma: Clinical Outcomes of the Phase III Intergroup Trial Alliance/CALGB 50303” – was published in the Journal of Clinical Oncology (2019 Apr 2. doi: 10.1200/JCO.18.01994).
In the study, progression-free survival and overall survival were no different for R-CHOP and DA-EPOCH-R, but – predictably – DA-EPOCH-R was more toxic and had more treatment discontinuations.
R-CHOP had better outcomes than expected. This suggests that patient-selection bias (more favorable histology, fewer high-risk subsets who required urgent therapy) may have been at work.
Further study of DA-EPOCH-R in higher IPI patients or in patients selected because of more adverse molecular features (DE phenotype, MYC+, double hit) is warranted given the poor outcomes with R-CHOP in high-risk patients, intriguing results in single institution trials of DA-EPOCH-R, and the underrepresentation of high-risk patients in the current study.
What this means in practice
Whether by virtue of the types of patients enrolled or because it is the best regimen in all DLBCL patients, R-CHOP remains the standard of care outside of a clinical trial.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers, and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I will treat my next patient,” I take a look at two recent trials – one offers potential in previously-treated cervical cancer patients with poor prognosis and the other confirms the role of R-CHOP as the standard of care in diffuse large B-cell lymphoma.
Pembrolizumab in KEYNOTE-158
In an international phase 2 “basket trial,” Hyun Cheol Chung, MD, PhD, and colleagues used pembrolizumab 200 mg every 3 weeks in 98 previously treated patients with advanced cervical cancer. Almost 84% of o the patients had PD-L1 positive tumors (greater than 1%). The authors said that viral induction of malignancy leads to antigen production and upregulation of PD-1. Therefore, advanced cervical cancer patients would likely express PD-L1 on tumor cells and respond to immune checkpoint inhibitor therapy.
In this interim report, there were 12 responses (all in PD-L1 positive patients), with three complete responses. Median response duration had not been reached at median follow-up of 10.2 months. Seven of 12 responses were ongoing at 12 months. There were grade 3-4 adverse events in 12.2% of patients and no treatment-related deaths.
The study – “Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study” – was published in the Journal of Clinical Oncology (2019 April 3. doi: 10.1200/JCO.18.01265).
The encouraging results of pembrolizumab in this generally chemotherapy-refractory patient population were consistent with other small, early-phase studies investigating immune checkpoint inhibitors that led to the accelerated approval of pembrolizumab in previously treated PD-L1 advanced cervical cancer patients with progressive disease after chemotherapy.
What this means in practice
Although excitement should be tempered about an interim report of an organ-specific subset of a phase 2 international basket trial that was heavily populated by young PS 0-1 patients and generated an overall response rate of less than 15%, no conventional chemotherapy or biologic agent offers the potential of complete or prolonged response, and disease control rates of 30%.
Clinical trials should always be the first choice, but immune checkpoint inhibitors offer an attractive off-study option.
Among many single agents in National Comprehensive Cancer Network guidelines for recurrent advanced cervical cancer after first-line cisplatin-based chemotherapy, there is a reason why pembrolizumab is listed first. For patients with PD-L1 expressing tumors or MSI-H/dMMR tumors, I would use it.
Frontline therapy in DLBCL
In a large, randomized phase 3 trial, close to 500 stage III-IV patients with diffuse large B-cell lymphoma (DLBCL), including primary mediastinal B-cell lymphoma and intravascular large B-cell lymphoma, were assigned to receive either conventional R-CHOP chemotherapy or the more complex, more toxic DA-EPOCH-R regimen that appeared superior in single-institution studies and was feasible in multi-institutional phase 2 trials.
The study – “Dose-Adjusted EPOCH-R Compared With R-CHOP as Frontline Therapy for Diffuse Large B-Cell Lymphoma: Clinical Outcomes of the Phase III Intergroup Trial Alliance/CALGB 50303” – was published in the Journal of Clinical Oncology (2019 Apr 2. doi: 10.1200/JCO.18.01994).
In the study, progression-free survival and overall survival were no different for R-CHOP and DA-EPOCH-R, but – predictably – DA-EPOCH-R was more toxic and had more treatment discontinuations.
R-CHOP had better outcomes than expected. This suggests that patient-selection bias (more favorable histology, fewer high-risk subsets who required urgent therapy) may have been at work.
Further study of DA-EPOCH-R in higher IPI patients or in patients selected because of more adverse molecular features (DE phenotype, MYC+, double hit) is warranted given the poor outcomes with R-CHOP in high-risk patients, intriguing results in single institution trials of DA-EPOCH-R, and the underrepresentation of high-risk patients in the current study.
What this means in practice
Whether by virtue of the types of patients enrolled or because it is the best regimen in all DLBCL patients, R-CHOP remains the standard of care outside of a clinical trial.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers, and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I will treat my next patient,” I take a look at two recent trials – one offers potential in previously-treated cervical cancer patients with poor prognosis and the other confirms the role of R-CHOP as the standard of care in diffuse large B-cell lymphoma.
Pembrolizumab in KEYNOTE-158
In an international phase 2 “basket trial,” Hyun Cheol Chung, MD, PhD, and colleagues used pembrolizumab 200 mg every 3 weeks in 98 previously treated patients with advanced cervical cancer. Almost 84% of o the patients had PD-L1 positive tumors (greater than 1%). The authors said that viral induction of malignancy leads to antigen production and upregulation of PD-1. Therefore, advanced cervical cancer patients would likely express PD-L1 on tumor cells and respond to immune checkpoint inhibitor therapy.
In this interim report, there were 12 responses (all in PD-L1 positive patients), with three complete responses. Median response duration had not been reached at median follow-up of 10.2 months. Seven of 12 responses were ongoing at 12 months. There were grade 3-4 adverse events in 12.2% of patients and no treatment-related deaths.
The study – “Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study” – was published in the Journal of Clinical Oncology (2019 April 3. doi: 10.1200/JCO.18.01265).
The encouraging results of pembrolizumab in this generally chemotherapy-refractory patient population were consistent with other small, early-phase studies investigating immune checkpoint inhibitors that led to the accelerated approval of pembrolizumab in previously treated PD-L1 advanced cervical cancer patients with progressive disease after chemotherapy.
What this means in practice
Although excitement should be tempered about an interim report of an organ-specific subset of a phase 2 international basket trial that was heavily populated by young PS 0-1 patients and generated an overall response rate of less than 15%, no conventional chemotherapy or biologic agent offers the potential of complete or prolonged response, and disease control rates of 30%.
Clinical trials should always be the first choice, but immune checkpoint inhibitors offer an attractive off-study option.
Among many single agents in National Comprehensive Cancer Network guidelines for recurrent advanced cervical cancer after first-line cisplatin-based chemotherapy, there is a reason why pembrolizumab is listed first. For patients with PD-L1 expressing tumors or MSI-H/dMMR tumors, I would use it.
Frontline therapy in DLBCL
In a large, randomized phase 3 trial, close to 500 stage III-IV patients with diffuse large B-cell lymphoma (DLBCL), including primary mediastinal B-cell lymphoma and intravascular large B-cell lymphoma, were assigned to receive either conventional R-CHOP chemotherapy or the more complex, more toxic DA-EPOCH-R regimen that appeared superior in single-institution studies and was feasible in multi-institutional phase 2 trials.
The study – “Dose-Adjusted EPOCH-R Compared With R-CHOP as Frontline Therapy for Diffuse Large B-Cell Lymphoma: Clinical Outcomes of the Phase III Intergroup Trial Alliance/CALGB 50303” – was published in the Journal of Clinical Oncology (2019 Apr 2. doi: 10.1200/JCO.18.01994).
In the study, progression-free survival and overall survival were no different for R-CHOP and DA-EPOCH-R, but – predictably – DA-EPOCH-R was more toxic and had more treatment discontinuations.
R-CHOP had better outcomes than expected. This suggests that patient-selection bias (more favorable histology, fewer high-risk subsets who required urgent therapy) may have been at work.
Further study of DA-EPOCH-R in higher IPI patients or in patients selected because of more adverse molecular features (DE phenotype, MYC+, double hit) is warranted given the poor outcomes with R-CHOP in high-risk patients, intriguing results in single institution trials of DA-EPOCH-R, and the underrepresentation of high-risk patients in the current study.
What this means in practice
Whether by virtue of the types of patients enrolled or because it is the best regimen in all DLBCL patients, R-CHOP remains the standard of care outside of a clinical trial.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers, and in expanding access to clinical trials to medically underserved populations.