User login
What is the future of para-aortic lymphadenectomy for endometrial cancer?
A landmark study of advanced endometrial cancer, GOG 258, was published in the New England Journal of Medicine this summer.1 This clinical trial compared the use of carboplatin and paclitaxel chemotherapy with a combination of chemotherapy with external beam radiation, exploring the notion of “more is better.” The results of the trial revealed that the “more” (chemotherapy with external beam radiation) was no better than chemotherapy alone with respect to overall survival. These results have challenged a creeping dogma in gynecologic oncology, which has seen many providers embrace combination therapy, particularly for patients with stage III (node-positive) endometrial cancer, a group of patients who made up approximately three-quarters of GOG 258’s study population. Many have been left searching for justification of their early adoption of combination therapy before the results of a trial such as this were available. For me it also raised a slightly different question: In the light of these results, what IS the role of para-aortic lymphadenectomy in the staging of endometrial cancers? If radiation to the nodal basins is no longer part of adjuvant therapy, then
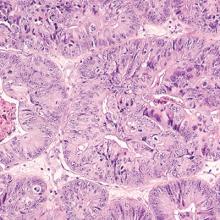
It was in the 1980s that the removal of clinically normal para-aortic lymph nodes (those residing between the renal and proximal common iliac vessels) became a part of surgical staging. This practice was endorsed by the International Federation of Gynecology and Obstetrics (FIGO) and the Gynecologic Oncology Group (GOG) surgical committee in response to findings that 11% of women with clinical stage I endometrial cancer had microscopic lymph node metastases which were discovered only with routine pathologic evaluation of these tissues. Among those with pelvic lymph node metastases (stage IIIC disease), approximately one-third also harbored disease in para-aortic nodal regions.2 Among all patients with endometrial cancer, including those with low-grade disease, only a small fraction (approximately 2%) have isolated para-aortic lymph nodes (positive para-aortic nodes, but negative pelvic nodes). However, among patients with deeply invasive higher-grade tumors, the likelihood of discovering isolated para-aortic metastases is higher at approximately 16%.3 Therefore, the dominant pattern of lymph node metastases and lymphatic dissemination of endometrial cancer appears to be via the parametrial channels laterally towards the pelvic basins, and then sequentially to the para-aortic regions. The direct lymphatic pathway to the para-aortic basins from the uterine fundus through the adnexal lymphatics misses the pelvic regions altogether and may seen logical, but actually is observed fairly infrequently.4
Over the subsequent decades, there have been debates and schools of thought regarding what is the optimal degree of lymphatic dissection for endometrial cancer staging. Some advocated for full pelvic and infrarenal para-aortic nodal dissections in all patients, including even those in the lowest risk for metastases. Others advocated for a more limited, inframesenteric para-aortic nodal dissection, although the origins of such a distinction appear to be largely arbitrary. The inferior mesenteric artery is not a physiologic landmark for lymphatic pathways, and approximately half of para-aortic metastases are located above the level of the inferior mesenteric artery. This limited sampling may have been preferred because of relative ease of dissection rather than diagnostic or therapeutic efficacy.
As the population became more obese, making para-aortic nodal dissections less feasible, and laparoscopic staging became accepted as the standard of care in endometrial cancer staging, there was a further push towards limiting the scope of lymphadenectomy. Selective algorithms, such as the so-called “Mayo clinic criteria,” were widely adopted. In this approach, gynecologic oncologists perform full pelvic and infrarenal para-aortic lymphadenectomies but only in the presence of a high-risk uterine feature such as tumor size greater than 2 cm, deep myometrial invasion, or grade 3 histology.3 While this reduced the number of para-aortic dissections being performed, it did not eliminate them, as approximately 40% of patients with endometrial cancer meet at least one of those criteria.
At this same time, we learned something else critical about the benefits, or lack thereof, of lymphadenectomy. Two landmark surgical-staging trials were published in 2009 which randomly assigned women to hysterectomy with lymphadenectomy or hysterectomy alone, and found no survival benefit for lymphadenectomy.5,6 While these trial results initially were met with noisy backlash, particularly from those who had legitimate concerns regarding study design and conclusions that reach beyond the scope of this column, ultimately their findings (that there is no therapeutic benefit to surgically removing clinically normal lymph nodes) has become largely accepted. The subsequent findings of the Laparoscopic Approach to Cancer of the Endometrium (LACE) trial further support this, as there was no difference in survival found between patients who were randomly assigned to laparoscopic versus open staging for endometrial cancer, even despite a significantly lower rate of lymphadenectomy among the laparoscopic arm.7
SLN biopsy, in which the specific nodes which drain the uterus are selectively removed, represents the most recent development in lymph node assessment for endometrial cancer. On average, only three lymph nodes are removed per patient, and para-aortic nodes infrequently are removed, because it is rare that lymphatic pathways drain directly into the aortic basins after cervical injection. Yet despite this more limited dissection of lymph nodes, especially para-aortic, with SLN biopsy, surgeons still observe similar rates of IIIC disease, compared with full lymphadenectomy, suggesting that the presence or absence of lymphatic metastases still is able to be adequately determined. SLN biopsy misses only 3% of lymphatic disease.8 What is of particular interest to practitioners of the SLN approach is that “atypical” pathways are discovered approximately 20% of the time, and nodes are harvested from locations such as the presacral space or medial to the internal iliac vessels. These nodes are in locations previously overlooked by even the most comprehensive pelvic and para-aortic lymphadenectomy. Therefore, while the para-aortic nodes may not be systematically removed with SLN biopsy, new and arguably more relevant regions are interrogated, which might explain its equivalent diagnostic virtue.
With this evolution in surgical-staging practice, what we have come to recognize is that the role of lymph node assessment is predominantly, if not exclusively, diagnostic. It can help us determine which patients are at risk for distant relapse and therefore candidates for systemic therapy (chemotherapy), versus those whose risk is predominantly of local relapse and can be adequately treated with local therapies alone, such as vaginal radiation. This brings us to the results of GOG 258. If defining the specific and complete extent of lymph node metastases (as if that was ever truly what surgeons were doing) is no longer necessary to guide the prescription and extent of external beam radiation, then lymph node dissection need only inform us of whether or not there are nodal metastases, not specifically the location of those nodal metastases. The prescription of chemotherapy is the same whether the disease is limited to the pelvic nodes or also includes the para-aortic nodes. While GOG 258 discovered more para-aortic failures among the chemotherapy-alone group, suggesting there may be some therapeutic role of radiation in preventing this, it should be noted that these para-aortic relapses did not negatively impact relapse-free survival, and these patients still can presumably be salvaged with external beam radiation to the site of para-aortic relapse.
It would seem logical that the results of GOG 258 further limit the potential role of para-aortic lymphadenectomy in women with clinical stage I disease. Perhaps para-aortic dissection can be limited to women who are at highest risk for isolated para-aortic disease, such as those with deeply invasive high-grade tumors not successfully mapped with the highly targeted sentinel node biopsy technique? Most clinicians look forward to an era in which we no longer rely on crude dissections of disease-free tissue just to prove they are disease free, but instead can utilize more sophisticated diagnostic methods to recognize disseminated disease.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Email her at [email protected].
References
1. N Engl J Med. 2019 Jun 13;380(24):2317-26.
2. Cancer. 1987 Oct 15;60(8 Suppl):2035-41.
3. Gynecol Oncol. 2008;109(1):11-8.
4. Int J Gynecol Cancer. 2019 Mar;29(3):613-21.
5. J Natl Cancer Inst. 2008 Dec 3;100(23):1707-16.
6. Lancet. 2009 Jan 10;373(9658):125-36.
7. JAMA. 2017 Mar 28;317(12):1224-33.
8. Lancet Oncol. 2017 Mar;18(3):384-92.
A landmark study of advanced endometrial cancer, GOG 258, was published in the New England Journal of Medicine this summer.1 This clinical trial compared the use of carboplatin and paclitaxel chemotherapy with a combination of chemotherapy with external beam radiation, exploring the notion of “more is better.” The results of the trial revealed that the “more” (chemotherapy with external beam radiation) was no better than chemotherapy alone with respect to overall survival. These results have challenged a creeping dogma in gynecologic oncology, which has seen many providers embrace combination therapy, particularly for patients with stage III (node-positive) endometrial cancer, a group of patients who made up approximately three-quarters of GOG 258’s study population. Many have been left searching for justification of their early adoption of combination therapy before the results of a trial such as this were available. For me it also raised a slightly different question: In the light of these results, what IS the role of para-aortic lymphadenectomy in the staging of endometrial cancers? If radiation to the nodal basins is no longer part of adjuvant therapy, then
It was in the 1980s that the removal of clinically normal para-aortic lymph nodes (those residing between the renal and proximal common iliac vessels) became a part of surgical staging. This practice was endorsed by the International Federation of Gynecology and Obstetrics (FIGO) and the Gynecologic Oncology Group (GOG) surgical committee in response to findings that 11% of women with clinical stage I endometrial cancer had microscopic lymph node metastases which were discovered only with routine pathologic evaluation of these tissues. Among those with pelvic lymph node metastases (stage IIIC disease), approximately one-third also harbored disease in para-aortic nodal regions.2 Among all patients with endometrial cancer, including those with low-grade disease, only a small fraction (approximately 2%) have isolated para-aortic lymph nodes (positive para-aortic nodes, but negative pelvic nodes). However, among patients with deeply invasive higher-grade tumors, the likelihood of discovering isolated para-aortic metastases is higher at approximately 16%.3 Therefore, the dominant pattern of lymph node metastases and lymphatic dissemination of endometrial cancer appears to be via the parametrial channels laterally towards the pelvic basins, and then sequentially to the para-aortic regions. The direct lymphatic pathway to the para-aortic basins from the uterine fundus through the adnexal lymphatics misses the pelvic regions altogether and may seen logical, but actually is observed fairly infrequently.4
Over the subsequent decades, there have been debates and schools of thought regarding what is the optimal degree of lymphatic dissection for endometrial cancer staging. Some advocated for full pelvic and infrarenal para-aortic nodal dissections in all patients, including even those in the lowest risk for metastases. Others advocated for a more limited, inframesenteric para-aortic nodal dissection, although the origins of such a distinction appear to be largely arbitrary. The inferior mesenteric artery is not a physiologic landmark for lymphatic pathways, and approximately half of para-aortic metastases are located above the level of the inferior mesenteric artery. This limited sampling may have been preferred because of relative ease of dissection rather than diagnostic or therapeutic efficacy.
As the population became more obese, making para-aortic nodal dissections less feasible, and laparoscopic staging became accepted as the standard of care in endometrial cancer staging, there was a further push towards limiting the scope of lymphadenectomy. Selective algorithms, such as the so-called “Mayo clinic criteria,” were widely adopted. In this approach, gynecologic oncologists perform full pelvic and infrarenal para-aortic lymphadenectomies but only in the presence of a high-risk uterine feature such as tumor size greater than 2 cm, deep myometrial invasion, or grade 3 histology.3 While this reduced the number of para-aortic dissections being performed, it did not eliminate them, as approximately 40% of patients with endometrial cancer meet at least one of those criteria.
At this same time, we learned something else critical about the benefits, or lack thereof, of lymphadenectomy. Two landmark surgical-staging trials were published in 2009 which randomly assigned women to hysterectomy with lymphadenectomy or hysterectomy alone, and found no survival benefit for lymphadenectomy.5,6 While these trial results initially were met with noisy backlash, particularly from those who had legitimate concerns regarding study design and conclusions that reach beyond the scope of this column, ultimately their findings (that there is no therapeutic benefit to surgically removing clinically normal lymph nodes) has become largely accepted. The subsequent findings of the Laparoscopic Approach to Cancer of the Endometrium (LACE) trial further support this, as there was no difference in survival found between patients who were randomly assigned to laparoscopic versus open staging for endometrial cancer, even despite a significantly lower rate of lymphadenectomy among the laparoscopic arm.7
SLN biopsy, in which the specific nodes which drain the uterus are selectively removed, represents the most recent development in lymph node assessment for endometrial cancer. On average, only three lymph nodes are removed per patient, and para-aortic nodes infrequently are removed, because it is rare that lymphatic pathways drain directly into the aortic basins after cervical injection. Yet despite this more limited dissection of lymph nodes, especially para-aortic, with SLN biopsy, surgeons still observe similar rates of IIIC disease, compared with full lymphadenectomy, suggesting that the presence or absence of lymphatic metastases still is able to be adequately determined. SLN biopsy misses only 3% of lymphatic disease.8 What is of particular interest to practitioners of the SLN approach is that “atypical” pathways are discovered approximately 20% of the time, and nodes are harvested from locations such as the presacral space or medial to the internal iliac vessels. These nodes are in locations previously overlooked by even the most comprehensive pelvic and para-aortic lymphadenectomy. Therefore, while the para-aortic nodes may not be systematically removed with SLN biopsy, new and arguably more relevant regions are interrogated, which might explain its equivalent diagnostic virtue.
With this evolution in surgical-staging practice, what we have come to recognize is that the role of lymph node assessment is predominantly, if not exclusively, diagnostic. It can help us determine which patients are at risk for distant relapse and therefore candidates for systemic therapy (chemotherapy), versus those whose risk is predominantly of local relapse and can be adequately treated with local therapies alone, such as vaginal radiation. This brings us to the results of GOG 258. If defining the specific and complete extent of lymph node metastases (as if that was ever truly what surgeons were doing) is no longer necessary to guide the prescription and extent of external beam radiation, then lymph node dissection need only inform us of whether or not there are nodal metastases, not specifically the location of those nodal metastases. The prescription of chemotherapy is the same whether the disease is limited to the pelvic nodes or also includes the para-aortic nodes. While GOG 258 discovered more para-aortic failures among the chemotherapy-alone group, suggesting there may be some therapeutic role of radiation in preventing this, it should be noted that these para-aortic relapses did not negatively impact relapse-free survival, and these patients still can presumably be salvaged with external beam radiation to the site of para-aortic relapse.
It would seem logical that the results of GOG 258 further limit the potential role of para-aortic lymphadenectomy in women with clinical stage I disease. Perhaps para-aortic dissection can be limited to women who are at highest risk for isolated para-aortic disease, such as those with deeply invasive high-grade tumors not successfully mapped with the highly targeted sentinel node biopsy technique? Most clinicians look forward to an era in which we no longer rely on crude dissections of disease-free tissue just to prove they are disease free, but instead can utilize more sophisticated diagnostic methods to recognize disseminated disease.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Email her at [email protected].
References
1. N Engl J Med. 2019 Jun 13;380(24):2317-26.
2. Cancer. 1987 Oct 15;60(8 Suppl):2035-41.
3. Gynecol Oncol. 2008;109(1):11-8.
4. Int J Gynecol Cancer. 2019 Mar;29(3):613-21.
5. J Natl Cancer Inst. 2008 Dec 3;100(23):1707-16.
6. Lancet. 2009 Jan 10;373(9658):125-36.
7. JAMA. 2017 Mar 28;317(12):1224-33.
8. Lancet Oncol. 2017 Mar;18(3):384-92.
A landmark study of advanced endometrial cancer, GOG 258, was published in the New England Journal of Medicine this summer.1 This clinical trial compared the use of carboplatin and paclitaxel chemotherapy with a combination of chemotherapy with external beam radiation, exploring the notion of “more is better.” The results of the trial revealed that the “more” (chemotherapy with external beam radiation) was no better than chemotherapy alone with respect to overall survival. These results have challenged a creeping dogma in gynecologic oncology, which has seen many providers embrace combination therapy, particularly for patients with stage III (node-positive) endometrial cancer, a group of patients who made up approximately three-quarters of GOG 258’s study population. Many have been left searching for justification of their early adoption of combination therapy before the results of a trial such as this were available. For me it also raised a slightly different question: In the light of these results, what IS the role of para-aortic lymphadenectomy in the staging of endometrial cancers? If radiation to the nodal basins is no longer part of adjuvant therapy, then
It was in the 1980s that the removal of clinically normal para-aortic lymph nodes (those residing between the renal and proximal common iliac vessels) became a part of surgical staging. This practice was endorsed by the International Federation of Gynecology and Obstetrics (FIGO) and the Gynecologic Oncology Group (GOG) surgical committee in response to findings that 11% of women with clinical stage I endometrial cancer had microscopic lymph node metastases which were discovered only with routine pathologic evaluation of these tissues. Among those with pelvic lymph node metastases (stage IIIC disease), approximately one-third also harbored disease in para-aortic nodal regions.2 Among all patients with endometrial cancer, including those with low-grade disease, only a small fraction (approximately 2%) have isolated para-aortic lymph nodes (positive para-aortic nodes, but negative pelvic nodes). However, among patients with deeply invasive higher-grade tumors, the likelihood of discovering isolated para-aortic metastases is higher at approximately 16%.3 Therefore, the dominant pattern of lymph node metastases and lymphatic dissemination of endometrial cancer appears to be via the parametrial channels laterally towards the pelvic basins, and then sequentially to the para-aortic regions. The direct lymphatic pathway to the para-aortic basins from the uterine fundus through the adnexal lymphatics misses the pelvic regions altogether and may seen logical, but actually is observed fairly infrequently.4
Over the subsequent decades, there have been debates and schools of thought regarding what is the optimal degree of lymphatic dissection for endometrial cancer staging. Some advocated for full pelvic and infrarenal para-aortic nodal dissections in all patients, including even those in the lowest risk for metastases. Others advocated for a more limited, inframesenteric para-aortic nodal dissection, although the origins of such a distinction appear to be largely arbitrary. The inferior mesenteric artery is not a physiologic landmark for lymphatic pathways, and approximately half of para-aortic metastases are located above the level of the inferior mesenteric artery. This limited sampling may have been preferred because of relative ease of dissection rather than diagnostic or therapeutic efficacy.
As the population became more obese, making para-aortic nodal dissections less feasible, and laparoscopic staging became accepted as the standard of care in endometrial cancer staging, there was a further push towards limiting the scope of lymphadenectomy. Selective algorithms, such as the so-called “Mayo clinic criteria,” were widely adopted. In this approach, gynecologic oncologists perform full pelvic and infrarenal para-aortic lymphadenectomies but only in the presence of a high-risk uterine feature such as tumor size greater than 2 cm, deep myometrial invasion, or grade 3 histology.3 While this reduced the number of para-aortic dissections being performed, it did not eliminate them, as approximately 40% of patients with endometrial cancer meet at least one of those criteria.
At this same time, we learned something else critical about the benefits, or lack thereof, of lymphadenectomy. Two landmark surgical-staging trials were published in 2009 which randomly assigned women to hysterectomy with lymphadenectomy or hysterectomy alone, and found no survival benefit for lymphadenectomy.5,6 While these trial results initially were met with noisy backlash, particularly from those who had legitimate concerns regarding study design and conclusions that reach beyond the scope of this column, ultimately their findings (that there is no therapeutic benefit to surgically removing clinically normal lymph nodes) has become largely accepted. The subsequent findings of the Laparoscopic Approach to Cancer of the Endometrium (LACE) trial further support this, as there was no difference in survival found between patients who were randomly assigned to laparoscopic versus open staging for endometrial cancer, even despite a significantly lower rate of lymphadenectomy among the laparoscopic arm.7
SLN biopsy, in which the specific nodes which drain the uterus are selectively removed, represents the most recent development in lymph node assessment for endometrial cancer. On average, only three lymph nodes are removed per patient, and para-aortic nodes infrequently are removed, because it is rare that lymphatic pathways drain directly into the aortic basins after cervical injection. Yet despite this more limited dissection of lymph nodes, especially para-aortic, with SLN biopsy, surgeons still observe similar rates of IIIC disease, compared with full lymphadenectomy, suggesting that the presence or absence of lymphatic metastases still is able to be adequately determined. SLN biopsy misses only 3% of lymphatic disease.8 What is of particular interest to practitioners of the SLN approach is that “atypical” pathways are discovered approximately 20% of the time, and nodes are harvested from locations such as the presacral space or medial to the internal iliac vessels. These nodes are in locations previously overlooked by even the most comprehensive pelvic and para-aortic lymphadenectomy. Therefore, while the para-aortic nodes may not be systematically removed with SLN biopsy, new and arguably more relevant regions are interrogated, which might explain its equivalent diagnostic virtue.
With this evolution in surgical-staging practice, what we have come to recognize is that the role of lymph node assessment is predominantly, if not exclusively, diagnostic. It can help us determine which patients are at risk for distant relapse and therefore candidates for systemic therapy (chemotherapy), versus those whose risk is predominantly of local relapse and can be adequately treated with local therapies alone, such as vaginal radiation. This brings us to the results of GOG 258. If defining the specific and complete extent of lymph node metastases (as if that was ever truly what surgeons were doing) is no longer necessary to guide the prescription and extent of external beam radiation, then lymph node dissection need only inform us of whether or not there are nodal metastases, not specifically the location of those nodal metastases. The prescription of chemotherapy is the same whether the disease is limited to the pelvic nodes or also includes the para-aortic nodes. While GOG 258 discovered more para-aortic failures among the chemotherapy-alone group, suggesting there may be some therapeutic role of radiation in preventing this, it should be noted that these para-aortic relapses did not negatively impact relapse-free survival, and these patients still can presumably be salvaged with external beam radiation to the site of para-aortic relapse.
It would seem logical that the results of GOG 258 further limit the potential role of para-aortic lymphadenectomy in women with clinical stage I disease. Perhaps para-aortic dissection can be limited to women who are at highest risk for isolated para-aortic disease, such as those with deeply invasive high-grade tumors not successfully mapped with the highly targeted sentinel node biopsy technique? Most clinicians look forward to an era in which we no longer rely on crude dissections of disease-free tissue just to prove they are disease free, but instead can utilize more sophisticated diagnostic methods to recognize disseminated disease.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Email her at [email protected].
References
1. N Engl J Med. 2019 Jun 13;380(24):2317-26.
2. Cancer. 1987 Oct 15;60(8 Suppl):2035-41.
3. Gynecol Oncol. 2008;109(1):11-8.
4. Int J Gynecol Cancer. 2019 Mar;29(3):613-21.
5. J Natl Cancer Inst. 2008 Dec 3;100(23):1707-16.
6. Lancet. 2009 Jan 10;373(9658):125-36.
7. JAMA. 2017 Mar 28;317(12):1224-33.
8. Lancet Oncol. 2017 Mar;18(3):384-92.
Ovarian cancer diagnosed and treated earlier under ACA
CHICAGO – , according to a comparison of National Cancer Database surveys from 2006-2009 and 2011-2014.
During the post-Affordable Care Act (ACA) years of 2011-2014, compared with the pre-ACA years of 2004-2009, a treatment group of women aged 21-64 years with ovarian cancer was more likely to be diagnosed at an early stage, compared with a control group of women aged 65 years and older (difference-in-differences [DD]=1.7%), Anna Jo Smith, MD, reported at the annual meeting of the American Society of Clinical Oncology.
Also, the ACA was associated with more women aged 21-64 receiving treatment within 30 days of diagnosis (DD = 1.6%), said Dr. Smith, a 4th-year resident at Johns Hopkins University, Baltimore.
Among women with public insurance, the ACA was associated with even greater improvement in early-stage diagnosis and treatment within 30 days in the treatment group vs. the controls (DD = 2.5% for each), she said, noting that the improvements were seen across race, income, and education groups.
The findings are based on pre-ACA surveys from 35,842 women aged 21-64 years and 28,895 women aged 65 years, and from post-ACA surveys from 37,145 women and 30,604 women in those age groups, respectively, and were adjusted for patient race, living in a rural area, area-level household income and education level, Charlson comorbidity score, distance traveled for care, Census region, and care at an academic center.
The 2010 ACA expanded access to insurance and care for many Americans, and the objective of this study was to evaluate the impact of that access on women with ovarian cancer, Dr. Smith explained.
“Under the 2010 Affordable Care Act, women with ovarian cancer were more likely to be diagnosed at an early stage and receive treatment within 30 days of diagnosis,” she said. “As stage and treatment are the major determinants of survival advantage, these gains under the ACA may have long-term impacts on the survival, health, and well-being of women with ovarian cancer.”
Dr. Smith reported having no disclosures.
SOURCE: Smith A et al., ASCO 2019. Abstract LBA5563.
CHICAGO – , according to a comparison of National Cancer Database surveys from 2006-2009 and 2011-2014.
During the post-Affordable Care Act (ACA) years of 2011-2014, compared with the pre-ACA years of 2004-2009, a treatment group of women aged 21-64 years with ovarian cancer was more likely to be diagnosed at an early stage, compared with a control group of women aged 65 years and older (difference-in-differences [DD]=1.7%), Anna Jo Smith, MD, reported at the annual meeting of the American Society of Clinical Oncology.
Also, the ACA was associated with more women aged 21-64 receiving treatment within 30 days of diagnosis (DD = 1.6%), said Dr. Smith, a 4th-year resident at Johns Hopkins University, Baltimore.
Among women with public insurance, the ACA was associated with even greater improvement in early-stage diagnosis and treatment within 30 days in the treatment group vs. the controls (DD = 2.5% for each), she said, noting that the improvements were seen across race, income, and education groups.
The findings are based on pre-ACA surveys from 35,842 women aged 21-64 years and 28,895 women aged 65 years, and from post-ACA surveys from 37,145 women and 30,604 women in those age groups, respectively, and were adjusted for patient race, living in a rural area, area-level household income and education level, Charlson comorbidity score, distance traveled for care, Census region, and care at an academic center.
The 2010 ACA expanded access to insurance and care for many Americans, and the objective of this study was to evaluate the impact of that access on women with ovarian cancer, Dr. Smith explained.
“Under the 2010 Affordable Care Act, women with ovarian cancer were more likely to be diagnosed at an early stage and receive treatment within 30 days of diagnosis,” she said. “As stage and treatment are the major determinants of survival advantage, these gains under the ACA may have long-term impacts on the survival, health, and well-being of women with ovarian cancer.”
Dr. Smith reported having no disclosures.
SOURCE: Smith A et al., ASCO 2019. Abstract LBA5563.
CHICAGO – , according to a comparison of National Cancer Database surveys from 2006-2009 and 2011-2014.
During the post-Affordable Care Act (ACA) years of 2011-2014, compared with the pre-ACA years of 2004-2009, a treatment group of women aged 21-64 years with ovarian cancer was more likely to be diagnosed at an early stage, compared with a control group of women aged 65 years and older (difference-in-differences [DD]=1.7%), Anna Jo Smith, MD, reported at the annual meeting of the American Society of Clinical Oncology.
Also, the ACA was associated with more women aged 21-64 receiving treatment within 30 days of diagnosis (DD = 1.6%), said Dr. Smith, a 4th-year resident at Johns Hopkins University, Baltimore.
Among women with public insurance, the ACA was associated with even greater improvement in early-stage diagnosis and treatment within 30 days in the treatment group vs. the controls (DD = 2.5% for each), she said, noting that the improvements were seen across race, income, and education groups.
The findings are based on pre-ACA surveys from 35,842 women aged 21-64 years and 28,895 women aged 65 years, and from post-ACA surveys from 37,145 women and 30,604 women in those age groups, respectively, and were adjusted for patient race, living in a rural area, area-level household income and education level, Charlson comorbidity score, distance traveled for care, Census region, and care at an academic center.
The 2010 ACA expanded access to insurance and care for many Americans, and the objective of this study was to evaluate the impact of that access on women with ovarian cancer, Dr. Smith explained.
“Under the 2010 Affordable Care Act, women with ovarian cancer were more likely to be diagnosed at an early stage and receive treatment within 30 days of diagnosis,” she said. “As stage and treatment are the major determinants of survival advantage, these gains under the ACA may have long-term impacts on the survival, health, and well-being of women with ovarian cancer.”
Dr. Smith reported having no disclosures.
SOURCE: Smith A et al., ASCO 2019. Abstract LBA5563.
REPORTING FROM ASCO 2019
Vaginal microbiota composition linked to ovarian cancer risk
Women with epithelial ovarian cancer or BRCA1 mutational status were more likely to present with a community type O cervicovaginal microbiota relative to age-matched controls, according to a case-control analysis.
The results suggest that the composition of the cervicovaginal microbiome may have a key role in ovarian carcinogenesis. In addition, dysbiosis could be an important risk factor in women at high risk for the disease.
“Our aim was to establish whether women with, or at risk of developing, ovarian cancer have an imbalanced cervicovaginal microbiome,” wrote Nuno R Nené, PhD, of University College London, and colleagues. Their report is in The Lancet Oncology.
The researchers conducted a case-control study of adult females located in five European countries. Study participants were divided into two sets that consisted of 176 females with ovarian cancer and 109 females with a BRCA1 mutation, but without a diagnosis of ovarian cancer.
The two sets were matched with a combination of healthy controls and others with benign gynecologic disorders. Each cohort was split into females younger than 50 years and those over age 50.
In the analysis, females younger than 50 years with ovarian cancer were more likely to have a community type O microbiota relative to age-matched controls (adjusted odds ratio, 2.80; P = .020).
In the BRCA set, women with a BRCA1 mutation who were younger than 50 years were also more likely to present with a community type O microbiota than were wild type age-matched controls (adjusted odds ratio, 2.79; P = .012).
“In both sets, we noted that the younger the participants, the stronger the association between community type O microbiota and ovarian cancer or BRCA1 mutation status,” the researchers wrote.
They acknowledged that a key limitation of the study was the homogenous patient population. Since the vaginal microbiome can vary based on ethnicity, the generalizability of the results may be limited.
“Our findings warrant further detailed analyses of the vaginal microbiome, especially in high-risk women,” they concluded.
The study was funded by the EU’s Horizon 2020 Research and Innovation Programme, the EU’s Horizon 2020 European Research Council Programme, and The Eve Appeal. The authors reported financial affiliations with Eurofins, AstraZeneca, Biocad, Clovis, Pharmamar, Roche, Takeda, and Tesaro.
SOURCE: Nené NR et al. Lancet Oncol. 2019 Jul 9. doi: 10.1016/S1470-2045(19)30340-7.
One question that remains from the current study is whether the human microbiota is an important factor in the pathogenesis and development of ovarian cancer.
Currently, there has been no evidence directly linking the composition of the human microbiota to ovarian cancer. As a result, causation has yet to be established by means of randomized studies, since the majority of completed studies have been cross-sectional in nature. As a highly heterogeneous condition, several factors may be involved, including a variety of host reproductive, genetic, microbiota, and lifestyle considerations.
While various mechanisms have been proposed, other less familiar causes could also be implicated. For example, Dr. Nené and colleagues found an association between BRCA1 mutation carriers younger than 50 years of age and the presence of community type O microbiota, while only 10%-15% of females with incident ovarian cancers exhibit this mutation. These findings accentuate the complexities of ovarian cancer pathophysiology.
Despite the suggested benefits of probiotic therapy, there remains a call for systems biology strategies in ovarian cancer research. In a similar manner, the human microbiota needs to be considered in future research.
Hans Verstraelen, MD, MPH, PhD, is affiliated with Ghent University and the Ghent University Hospital in Belgium. Dr. Verstraelen reported no competing interests. These comments are adapted from his editorial (Lancet Oncol. 2019 Jul 9. doi: 10.1016/ S1470-2045[19]30340-7 ).
One question that remains from the current study is whether the human microbiota is an important factor in the pathogenesis and development of ovarian cancer.
Currently, there has been no evidence directly linking the composition of the human microbiota to ovarian cancer. As a result, causation has yet to be established by means of randomized studies, since the majority of completed studies have been cross-sectional in nature. As a highly heterogeneous condition, several factors may be involved, including a variety of host reproductive, genetic, microbiota, and lifestyle considerations.
While various mechanisms have been proposed, other less familiar causes could also be implicated. For example, Dr. Nené and colleagues found an association between BRCA1 mutation carriers younger than 50 years of age and the presence of community type O microbiota, while only 10%-15% of females with incident ovarian cancers exhibit this mutation. These findings accentuate the complexities of ovarian cancer pathophysiology.
Despite the suggested benefits of probiotic therapy, there remains a call for systems biology strategies in ovarian cancer research. In a similar manner, the human microbiota needs to be considered in future research.
Hans Verstraelen, MD, MPH, PhD, is affiliated with Ghent University and the Ghent University Hospital in Belgium. Dr. Verstraelen reported no competing interests. These comments are adapted from his editorial (Lancet Oncol. 2019 Jul 9. doi: 10.1016/ S1470-2045[19]30340-7 ).
One question that remains from the current study is whether the human microbiota is an important factor in the pathogenesis and development of ovarian cancer.
Currently, there has been no evidence directly linking the composition of the human microbiota to ovarian cancer. As a result, causation has yet to be established by means of randomized studies, since the majority of completed studies have been cross-sectional in nature. As a highly heterogeneous condition, several factors may be involved, including a variety of host reproductive, genetic, microbiota, and lifestyle considerations.
While various mechanisms have been proposed, other less familiar causes could also be implicated. For example, Dr. Nené and colleagues found an association between BRCA1 mutation carriers younger than 50 years of age and the presence of community type O microbiota, while only 10%-15% of females with incident ovarian cancers exhibit this mutation. These findings accentuate the complexities of ovarian cancer pathophysiology.
Despite the suggested benefits of probiotic therapy, there remains a call for systems biology strategies in ovarian cancer research. In a similar manner, the human microbiota needs to be considered in future research.
Hans Verstraelen, MD, MPH, PhD, is affiliated with Ghent University and the Ghent University Hospital in Belgium. Dr. Verstraelen reported no competing interests. These comments are adapted from his editorial (Lancet Oncol. 2019 Jul 9. doi: 10.1016/ S1470-2045[19]30340-7 ).
Women with epithelial ovarian cancer or BRCA1 mutational status were more likely to present with a community type O cervicovaginal microbiota relative to age-matched controls, according to a case-control analysis.
The results suggest that the composition of the cervicovaginal microbiome may have a key role in ovarian carcinogenesis. In addition, dysbiosis could be an important risk factor in women at high risk for the disease.
“Our aim was to establish whether women with, or at risk of developing, ovarian cancer have an imbalanced cervicovaginal microbiome,” wrote Nuno R Nené, PhD, of University College London, and colleagues. Their report is in The Lancet Oncology.
The researchers conducted a case-control study of adult females located in five European countries. Study participants were divided into two sets that consisted of 176 females with ovarian cancer and 109 females with a BRCA1 mutation, but without a diagnosis of ovarian cancer.
The two sets were matched with a combination of healthy controls and others with benign gynecologic disorders. Each cohort was split into females younger than 50 years and those over age 50.
In the analysis, females younger than 50 years with ovarian cancer were more likely to have a community type O microbiota relative to age-matched controls (adjusted odds ratio, 2.80; P = .020).
In the BRCA set, women with a BRCA1 mutation who were younger than 50 years were also more likely to present with a community type O microbiota than were wild type age-matched controls (adjusted odds ratio, 2.79; P = .012).
“In both sets, we noted that the younger the participants, the stronger the association between community type O microbiota and ovarian cancer or BRCA1 mutation status,” the researchers wrote.
They acknowledged that a key limitation of the study was the homogenous patient population. Since the vaginal microbiome can vary based on ethnicity, the generalizability of the results may be limited.
“Our findings warrant further detailed analyses of the vaginal microbiome, especially in high-risk women,” they concluded.
The study was funded by the EU’s Horizon 2020 Research and Innovation Programme, the EU’s Horizon 2020 European Research Council Programme, and The Eve Appeal. The authors reported financial affiliations with Eurofins, AstraZeneca, Biocad, Clovis, Pharmamar, Roche, Takeda, and Tesaro.
SOURCE: Nené NR et al. Lancet Oncol. 2019 Jul 9. doi: 10.1016/S1470-2045(19)30340-7.
Women with epithelial ovarian cancer or BRCA1 mutational status were more likely to present with a community type O cervicovaginal microbiota relative to age-matched controls, according to a case-control analysis.
The results suggest that the composition of the cervicovaginal microbiome may have a key role in ovarian carcinogenesis. In addition, dysbiosis could be an important risk factor in women at high risk for the disease.
“Our aim was to establish whether women with, or at risk of developing, ovarian cancer have an imbalanced cervicovaginal microbiome,” wrote Nuno R Nené, PhD, of University College London, and colleagues. Their report is in The Lancet Oncology.
The researchers conducted a case-control study of adult females located in five European countries. Study participants were divided into two sets that consisted of 176 females with ovarian cancer and 109 females with a BRCA1 mutation, but without a diagnosis of ovarian cancer.
The two sets were matched with a combination of healthy controls and others with benign gynecologic disorders. Each cohort was split into females younger than 50 years and those over age 50.
In the analysis, females younger than 50 years with ovarian cancer were more likely to have a community type O microbiota relative to age-matched controls (adjusted odds ratio, 2.80; P = .020).
In the BRCA set, women with a BRCA1 mutation who were younger than 50 years were also more likely to present with a community type O microbiota than were wild type age-matched controls (adjusted odds ratio, 2.79; P = .012).
“In both sets, we noted that the younger the participants, the stronger the association between community type O microbiota and ovarian cancer or BRCA1 mutation status,” the researchers wrote.
They acknowledged that a key limitation of the study was the homogenous patient population. Since the vaginal microbiome can vary based on ethnicity, the generalizability of the results may be limited.
“Our findings warrant further detailed analyses of the vaginal microbiome, especially in high-risk women,” they concluded.
The study was funded by the EU’s Horizon 2020 Research and Innovation Programme, the EU’s Horizon 2020 European Research Council Programme, and The Eve Appeal. The authors reported financial affiliations with Eurofins, AstraZeneca, Biocad, Clovis, Pharmamar, Roche, Takeda, and Tesaro.
SOURCE: Nené NR et al. Lancet Oncol. 2019 Jul 9. doi: 10.1016/S1470-2045(19)30340-7.
Gaps in patient-provider survivorship communication persist
There has been little to no recent improvement in the large share of cancer patients who are not receiving detailed information about survivorship care, suggests a nationally representative cross-sectional survey.
In 2006, the Institute of Medicine issued a seminal report recommending survivorship care planning to address the special needs of this patient population, noted the investigators, led by Ashish Rai, PhD, American Cancer Society, Framingham, Mass. Other organizations have since issued guidelines and policies in this area.
For the study, Dr. Rai and colleagues analyzed data from 2,266 survivors who completed the 2011 or 2016 Medical Expenditure Panel Survey – Experiences with Cancer questionnaire. Survivors were asked whether any clinician had ever discussed various aspects of survivorship care; responses were dichotomized as having had detailed discussion versus not (brief or no discussion, or not remembering).
Between 2011 and 2016, there was minimal change in the percentage of survivors who reported not receiving detailed information on follow-up care (from 35.1% to 35.4%), late or long-term adverse effects (from 54.2% to 55.5%), lifestyle recommendations (from 58.9% to 57.8%), and emotional or social needs (from 69.2% to 68.2%), the investigators wrote. Their report is in Journal of Oncology Practice.
When analyses were restricted to only those survivors who had received cancer-directed treatment within 3 years of the survey, findings were essentially the same.
About one-quarter of survivors reported having detailed discussions about all four topics in both 2011 (24.4%) and 2016 (21.9%).
In 2016, nearly half of survivors, 47.6%, reported not having detailed discussions with their providers about a summary of their cancer treatments. (This question was not asked in 2011.)
“Despite national efforts and organizations promoting survivorship care planning and highlighting the need for improved quality of survivorship care delivery, clear gaps in quality of communication between survivors of cancer and providers persist,” Dr. Rai and colleagues said.
“Continued efforts are needed to promote communication about survivorship issues, including implementation and evaluation of targeted interventions in key survivorship care areas,” they recommended. “These interventions may consist of furnishing guidance on optimal ways to identify and address survivors’ communication needs, streamlining the flow of information across provider types, ensuring better integration of primary care providers with the survivorship care paradigm, and augmenting the use of health information technology for collection and dissemination of information across the cancer control continuum.”
Dr. Rai did not disclose any relevant conflicts of interest. The study did not receive specific funding.
SOURCE: Rai A et al. J Oncol Pract. 2019 July 2. doi: 10.1200/JOP.19.00157.
There has been little to no recent improvement in the large share of cancer patients who are not receiving detailed information about survivorship care, suggests a nationally representative cross-sectional survey.
In 2006, the Institute of Medicine issued a seminal report recommending survivorship care planning to address the special needs of this patient population, noted the investigators, led by Ashish Rai, PhD, American Cancer Society, Framingham, Mass. Other organizations have since issued guidelines and policies in this area.
For the study, Dr. Rai and colleagues analyzed data from 2,266 survivors who completed the 2011 or 2016 Medical Expenditure Panel Survey – Experiences with Cancer questionnaire. Survivors were asked whether any clinician had ever discussed various aspects of survivorship care; responses were dichotomized as having had detailed discussion versus not (brief or no discussion, or not remembering).
Between 2011 and 2016, there was minimal change in the percentage of survivors who reported not receiving detailed information on follow-up care (from 35.1% to 35.4%), late or long-term adverse effects (from 54.2% to 55.5%), lifestyle recommendations (from 58.9% to 57.8%), and emotional or social needs (from 69.2% to 68.2%), the investigators wrote. Their report is in Journal of Oncology Practice.
When analyses were restricted to only those survivors who had received cancer-directed treatment within 3 years of the survey, findings were essentially the same.
About one-quarter of survivors reported having detailed discussions about all four topics in both 2011 (24.4%) and 2016 (21.9%).
In 2016, nearly half of survivors, 47.6%, reported not having detailed discussions with their providers about a summary of their cancer treatments. (This question was not asked in 2011.)
“Despite national efforts and organizations promoting survivorship care planning and highlighting the need for improved quality of survivorship care delivery, clear gaps in quality of communication between survivors of cancer and providers persist,” Dr. Rai and colleagues said.
“Continued efforts are needed to promote communication about survivorship issues, including implementation and evaluation of targeted interventions in key survivorship care areas,” they recommended. “These interventions may consist of furnishing guidance on optimal ways to identify and address survivors’ communication needs, streamlining the flow of information across provider types, ensuring better integration of primary care providers with the survivorship care paradigm, and augmenting the use of health information technology for collection and dissemination of information across the cancer control continuum.”
Dr. Rai did not disclose any relevant conflicts of interest. The study did not receive specific funding.
SOURCE: Rai A et al. J Oncol Pract. 2019 July 2. doi: 10.1200/JOP.19.00157.
There has been little to no recent improvement in the large share of cancer patients who are not receiving detailed information about survivorship care, suggests a nationally representative cross-sectional survey.
In 2006, the Institute of Medicine issued a seminal report recommending survivorship care planning to address the special needs of this patient population, noted the investigators, led by Ashish Rai, PhD, American Cancer Society, Framingham, Mass. Other organizations have since issued guidelines and policies in this area.
For the study, Dr. Rai and colleagues analyzed data from 2,266 survivors who completed the 2011 or 2016 Medical Expenditure Panel Survey – Experiences with Cancer questionnaire. Survivors were asked whether any clinician had ever discussed various aspects of survivorship care; responses were dichotomized as having had detailed discussion versus not (brief or no discussion, or not remembering).
Between 2011 and 2016, there was minimal change in the percentage of survivors who reported not receiving detailed information on follow-up care (from 35.1% to 35.4%), late or long-term adverse effects (from 54.2% to 55.5%), lifestyle recommendations (from 58.9% to 57.8%), and emotional or social needs (from 69.2% to 68.2%), the investigators wrote. Their report is in Journal of Oncology Practice.
When analyses were restricted to only those survivors who had received cancer-directed treatment within 3 years of the survey, findings were essentially the same.
About one-quarter of survivors reported having detailed discussions about all four topics in both 2011 (24.4%) and 2016 (21.9%).
In 2016, nearly half of survivors, 47.6%, reported not having detailed discussions with their providers about a summary of their cancer treatments. (This question was not asked in 2011.)
“Despite national efforts and organizations promoting survivorship care planning and highlighting the need for improved quality of survivorship care delivery, clear gaps in quality of communication between survivors of cancer and providers persist,” Dr. Rai and colleagues said.
“Continued efforts are needed to promote communication about survivorship issues, including implementation and evaluation of targeted interventions in key survivorship care areas,” they recommended. “These interventions may consist of furnishing guidance on optimal ways to identify and address survivors’ communication needs, streamlining the flow of information across provider types, ensuring better integration of primary care providers with the survivorship care paradigm, and augmenting the use of health information technology for collection and dissemination of information across the cancer control continuum.”
Dr. Rai did not disclose any relevant conflicts of interest. The study did not receive specific funding.
SOURCE: Rai A et al. J Oncol Pract. 2019 July 2. doi: 10.1200/JOP.19.00157.
FROM THE JOURNAL OF ONCOLOGY PRACTICE
Abnormal uterine bleeding: When can you forgo biopsy?
NASHVILLE, TENN. – Ultrasound is a reasonable first step for the evaluation of postmenopausal women with abnormal uterine bleeding and endometrial thickness up to 5 mm, according to James Shwayder, MD, JD.
About a third of such patients presenting with abnormal uterine bleeding (AUB) will have an endometrial polyp or submucous myoma, Dr. Shwayder explained during a presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
However, recurrent bleeding warrants further work-up, he said.
The 5-mm value is based on a number of studies, which, in sum, show that the negative predictive value for cancer for endometrial thickness less than 4 mm is greater than 99%, said Dr. Shwayder, chief of the division of gynecology and director of the fellowship in advanced endoscopy obstetrics, gynecology, and women’s health at the University of Louisville (Ky.).
“In fact ... the risk of cancer is 1 in 917,” he added. “That’s pretty good ... that’s a great screening tool.”
The question is whether one can feel comfortable foregoing biopsy in such cases, he said, describing a 61-year-old patient with a 3.9-mm endometrium and AUB for 3 days, 3 weeks prior to her visit.
There are two possibilities: The patient will either have no further bleeding, or she will continue to bleed, he said, noting that a 2003 Swedish study provides some guidance in the case of continued bleeding (Am J Obstet Gynecol. 2003;188:401-8).
The investigators initially looked at histology associated with endometrial thickness in 394 postmenopausal women referred for AUB between 1987 and 1990. Both transvaginal ultrasound and dilatation and curettage were performed, and the findings were correlated.
“But they had the rare opportunity to take patients who had benign evaluations and bring them back 10 years later,” Dr. Shwayder said. “What they found was that, regardless of the endometrial thickness, if it was benign initially and they did not bleed over that 10-year period, no one had cancer.”
However, among the patients followed for 10 years who had recurrent bleeding, 10% had cancer and 12% had hyperplasia. Thus, deciding against a biopsy in this case is supported by good data; if the patient doesn’t bleed, she has an “incredibly low risk of cancer,” he said.
“If they bleed again, you’ve gotta work ‘em up,” he stressed. “Don’t continue to say, ‘Well, let me repeat the ultrasound and see if it’s thinner or thicker.’ No. They need to be evaluated.”
Keep in mind that if a biopsy is performed as the first step, the chances of the results coming back as tissue insufficient for diagnosis (TIFD) are increased, and a repeat biopsy will be necessary because of the inconclusive findings, Dr. Shwayder said. It helps to warn a patient in advance that their thin endometrium makes it highly likely that a repeat biopsy will be necessary, as 90% come back as TIFD or atrophy.
Importantly, though, ACOG says endometrial sampling should be performed first line in patients over age 45 years with AUB.
“I’ll be honest – I use ultrasound in these patients because of the fact that a third will have some sort of structural defect, and the focal abnormalities are things we’re not going to be able to pick up with a straight biopsy, but we have to be cognizant that the college recommends biopsy in this population,” Dr. Shwayder said.
Asymptomatic endometrial thickening
Postmenopausal women are increasingly being referred for asymptomatic endometrial thickening that is found incidentally during an unrelated evaluation, Dr. Shwayder said, noting that evidence to date on how to approach such cases is conflicting.
Although ACOG is working on a recommendation, none is currently available, he said.
However, a 2001 study comparing 123 asymptomatic patients with 90 symptomatic patients, all with an endometrial thickness of greater than 10 mm, found no prognostic advantage to screening versus waiting until bleeding occurred, he noted (Euro J Cancer. 2001;37:64-71).
Overall, 13% of the patients had cancer, 50% had polyps, and 17% had hyperplasia.
“But what they emphasized was ... the length [of time] the patients complained of abnormal uterine bleeding. If it was less than 8 weeks ... there was no statistical difference in outcome, but if it was over 8 weeks there was a statistically significant difference in the grade of disease – a prognostic advantage for those patients who were screened versus symptomatic.”
The overall 5-year disease-free survival was 86% for asymptomatic versus 77% for symptomatic patients; for those with bleeding for less than 8 weeks, it was 98% versus 83%, respectively. The differences were not statistically different. However, for those with bleeding for 8-16 weeks it was 90% versus 74%, and for those with bleeding for more than 16 weeks it was 69% versus 62%, respectively, and those differences were statistically significant.
The problem is that many patients put off coming in for a long time, which means they are in a category with a worse prognosis when they do come in, Dr. Shwayder said. That’s not to say everyone should be screened, but there is no prognostic advantage to screening asymptomatic patients versus symptomatic patients who had bleeding for less than 8 weeks.
“It’s a little clarification, but I think an important one,” he noted.
Another study of 1,607 patients with endometrial thickening, including 233 who were asymptomatic and 1,374 who were symptomatic, found a lower rate of deep invasion with stage 1 disease, but no difference in the rate of more advanced disease, and no association with a more favorable outcome between the groups. (Am J Obstet Gynecol. 2018;219[2]:183e1-6).
Additionally, a study of 42 asymptomatic patients, 95 symptomatic patients with bleeding for less than 3 months, and 83 symptomatic patients with bleeding for more than 3 months showed a nonsignificant trend toward poorer 5-year survival in patients with a longer history of bleeding prior to surgery (Arch Gynecol Obstet 2013;288:1361-4).
“So now the question becomes how thick is too thick [and whether there is] some threshold where we ought to be evaluating patients and some threshold where we’re not,” he said.
The risk of malignancy among symptomatic postmenopausal women with an endometrial thickness greater than 5 mm is 7.3%, and the risk is similar at 6.7% in asymptomatic patients with an endometrial thickness of 11 mm or greater, according to a 2004 study by Smith-Bindman et al. (Ultrasound Obstet Gynecol. 2004;24:558-65).
“So the thought process here is that if a patient is asymptomatic, but the endometrium is over 11 mm, maybe we ought to evaluate that patient, because her risk of cancer is equivalent to that of someone who presents with postmenopausal bleeding and has an endometrium greater than 5 mm,” he explained.
In fact, a practice guideline from the Society of Obstetricians and Gynecologists of Canada recommends that women with endometrial thickness over 11 mm and other risk factors for cancer – such as obesity, hypertension, or late menopause – should be referred to a gynecologist for investigation, Dr. Shwayder said, adding that he also considers increased vascularity, heterogeneity in the endometrium, and fluid seen on a scan as cause for further evaluation.
“But endometrial sampling without bleeding should not be routinely performed,” he said. “So don’t routinely [sample] but based on risk factors and ultrasound findings, you may want to consider evaluating these patients further.”
Dr. Shwayder is a consultant for GE Ultrasound.
NASHVILLE, TENN. – Ultrasound is a reasonable first step for the evaluation of postmenopausal women with abnormal uterine bleeding and endometrial thickness up to 5 mm, according to James Shwayder, MD, JD.
About a third of such patients presenting with abnormal uterine bleeding (AUB) will have an endometrial polyp or submucous myoma, Dr. Shwayder explained during a presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
However, recurrent bleeding warrants further work-up, he said.
The 5-mm value is based on a number of studies, which, in sum, show that the negative predictive value for cancer for endometrial thickness less than 4 mm is greater than 99%, said Dr. Shwayder, chief of the division of gynecology and director of the fellowship in advanced endoscopy obstetrics, gynecology, and women’s health at the University of Louisville (Ky.).
“In fact ... the risk of cancer is 1 in 917,” he added. “That’s pretty good ... that’s a great screening tool.”
The question is whether one can feel comfortable foregoing biopsy in such cases, he said, describing a 61-year-old patient with a 3.9-mm endometrium and AUB for 3 days, 3 weeks prior to her visit.
There are two possibilities: The patient will either have no further bleeding, or she will continue to bleed, he said, noting that a 2003 Swedish study provides some guidance in the case of continued bleeding (Am J Obstet Gynecol. 2003;188:401-8).
The investigators initially looked at histology associated with endometrial thickness in 394 postmenopausal women referred for AUB between 1987 and 1990. Both transvaginal ultrasound and dilatation and curettage were performed, and the findings were correlated.
“But they had the rare opportunity to take patients who had benign evaluations and bring them back 10 years later,” Dr. Shwayder said. “What they found was that, regardless of the endometrial thickness, if it was benign initially and they did not bleed over that 10-year period, no one had cancer.”
However, among the patients followed for 10 years who had recurrent bleeding, 10% had cancer and 12% had hyperplasia. Thus, deciding against a biopsy in this case is supported by good data; if the patient doesn’t bleed, she has an “incredibly low risk of cancer,” he said.
“If they bleed again, you’ve gotta work ‘em up,” he stressed. “Don’t continue to say, ‘Well, let me repeat the ultrasound and see if it’s thinner or thicker.’ No. They need to be evaluated.”
Keep in mind that if a biopsy is performed as the first step, the chances of the results coming back as tissue insufficient for diagnosis (TIFD) are increased, and a repeat biopsy will be necessary because of the inconclusive findings, Dr. Shwayder said. It helps to warn a patient in advance that their thin endometrium makes it highly likely that a repeat biopsy will be necessary, as 90% come back as TIFD or atrophy.
Importantly, though, ACOG says endometrial sampling should be performed first line in patients over age 45 years with AUB.
“I’ll be honest – I use ultrasound in these patients because of the fact that a third will have some sort of structural defect, and the focal abnormalities are things we’re not going to be able to pick up with a straight biopsy, but we have to be cognizant that the college recommends biopsy in this population,” Dr. Shwayder said.
Asymptomatic endometrial thickening
Postmenopausal women are increasingly being referred for asymptomatic endometrial thickening that is found incidentally during an unrelated evaluation, Dr. Shwayder said, noting that evidence to date on how to approach such cases is conflicting.
Although ACOG is working on a recommendation, none is currently available, he said.
However, a 2001 study comparing 123 asymptomatic patients with 90 symptomatic patients, all with an endometrial thickness of greater than 10 mm, found no prognostic advantage to screening versus waiting until bleeding occurred, he noted (Euro J Cancer. 2001;37:64-71).
Overall, 13% of the patients had cancer, 50% had polyps, and 17% had hyperplasia.
“But what they emphasized was ... the length [of time] the patients complained of abnormal uterine bleeding. If it was less than 8 weeks ... there was no statistical difference in outcome, but if it was over 8 weeks there was a statistically significant difference in the grade of disease – a prognostic advantage for those patients who were screened versus symptomatic.”
The overall 5-year disease-free survival was 86% for asymptomatic versus 77% for symptomatic patients; for those with bleeding for less than 8 weeks, it was 98% versus 83%, respectively. The differences were not statistically different. However, for those with bleeding for 8-16 weeks it was 90% versus 74%, and for those with bleeding for more than 16 weeks it was 69% versus 62%, respectively, and those differences were statistically significant.
The problem is that many patients put off coming in for a long time, which means they are in a category with a worse prognosis when they do come in, Dr. Shwayder said. That’s not to say everyone should be screened, but there is no prognostic advantage to screening asymptomatic patients versus symptomatic patients who had bleeding for less than 8 weeks.
“It’s a little clarification, but I think an important one,” he noted.
Another study of 1,607 patients with endometrial thickening, including 233 who were asymptomatic and 1,374 who were symptomatic, found a lower rate of deep invasion with stage 1 disease, but no difference in the rate of more advanced disease, and no association with a more favorable outcome between the groups. (Am J Obstet Gynecol. 2018;219[2]:183e1-6).
Additionally, a study of 42 asymptomatic patients, 95 symptomatic patients with bleeding for less than 3 months, and 83 symptomatic patients with bleeding for more than 3 months showed a nonsignificant trend toward poorer 5-year survival in patients with a longer history of bleeding prior to surgery (Arch Gynecol Obstet 2013;288:1361-4).
“So now the question becomes how thick is too thick [and whether there is] some threshold where we ought to be evaluating patients and some threshold where we’re not,” he said.
The risk of malignancy among symptomatic postmenopausal women with an endometrial thickness greater than 5 mm is 7.3%, and the risk is similar at 6.7% in asymptomatic patients with an endometrial thickness of 11 mm or greater, according to a 2004 study by Smith-Bindman et al. (Ultrasound Obstet Gynecol. 2004;24:558-65).
“So the thought process here is that if a patient is asymptomatic, but the endometrium is over 11 mm, maybe we ought to evaluate that patient, because her risk of cancer is equivalent to that of someone who presents with postmenopausal bleeding and has an endometrium greater than 5 mm,” he explained.
In fact, a practice guideline from the Society of Obstetricians and Gynecologists of Canada recommends that women with endometrial thickness over 11 mm and other risk factors for cancer – such as obesity, hypertension, or late menopause – should be referred to a gynecologist for investigation, Dr. Shwayder said, adding that he also considers increased vascularity, heterogeneity in the endometrium, and fluid seen on a scan as cause for further evaluation.
“But endometrial sampling without bleeding should not be routinely performed,” he said. “So don’t routinely [sample] but based on risk factors and ultrasound findings, you may want to consider evaluating these patients further.”
Dr. Shwayder is a consultant for GE Ultrasound.
NASHVILLE, TENN. – Ultrasound is a reasonable first step for the evaluation of postmenopausal women with abnormal uterine bleeding and endometrial thickness up to 5 mm, according to James Shwayder, MD, JD.
About a third of such patients presenting with abnormal uterine bleeding (AUB) will have an endometrial polyp or submucous myoma, Dr. Shwayder explained during a presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
However, recurrent bleeding warrants further work-up, he said.
The 5-mm value is based on a number of studies, which, in sum, show that the negative predictive value for cancer for endometrial thickness less than 4 mm is greater than 99%, said Dr. Shwayder, chief of the division of gynecology and director of the fellowship in advanced endoscopy obstetrics, gynecology, and women’s health at the University of Louisville (Ky.).
“In fact ... the risk of cancer is 1 in 917,” he added. “That’s pretty good ... that’s a great screening tool.”
The question is whether one can feel comfortable foregoing biopsy in such cases, he said, describing a 61-year-old patient with a 3.9-mm endometrium and AUB for 3 days, 3 weeks prior to her visit.
There are two possibilities: The patient will either have no further bleeding, or she will continue to bleed, he said, noting that a 2003 Swedish study provides some guidance in the case of continued bleeding (Am J Obstet Gynecol. 2003;188:401-8).
The investigators initially looked at histology associated with endometrial thickness in 394 postmenopausal women referred for AUB between 1987 and 1990. Both transvaginal ultrasound and dilatation and curettage were performed, and the findings were correlated.
“But they had the rare opportunity to take patients who had benign evaluations and bring them back 10 years later,” Dr. Shwayder said. “What they found was that, regardless of the endometrial thickness, if it was benign initially and they did not bleed over that 10-year period, no one had cancer.”
However, among the patients followed for 10 years who had recurrent bleeding, 10% had cancer and 12% had hyperplasia. Thus, deciding against a biopsy in this case is supported by good data; if the patient doesn’t bleed, she has an “incredibly low risk of cancer,” he said.
“If they bleed again, you’ve gotta work ‘em up,” he stressed. “Don’t continue to say, ‘Well, let me repeat the ultrasound and see if it’s thinner or thicker.’ No. They need to be evaluated.”
Keep in mind that if a biopsy is performed as the first step, the chances of the results coming back as tissue insufficient for diagnosis (TIFD) are increased, and a repeat biopsy will be necessary because of the inconclusive findings, Dr. Shwayder said. It helps to warn a patient in advance that their thin endometrium makes it highly likely that a repeat biopsy will be necessary, as 90% come back as TIFD or atrophy.
Importantly, though, ACOG says endometrial sampling should be performed first line in patients over age 45 years with AUB.
“I’ll be honest – I use ultrasound in these patients because of the fact that a third will have some sort of structural defect, and the focal abnormalities are things we’re not going to be able to pick up with a straight biopsy, but we have to be cognizant that the college recommends biopsy in this population,” Dr. Shwayder said.
Asymptomatic endometrial thickening
Postmenopausal women are increasingly being referred for asymptomatic endometrial thickening that is found incidentally during an unrelated evaluation, Dr. Shwayder said, noting that evidence to date on how to approach such cases is conflicting.
Although ACOG is working on a recommendation, none is currently available, he said.
However, a 2001 study comparing 123 asymptomatic patients with 90 symptomatic patients, all with an endometrial thickness of greater than 10 mm, found no prognostic advantage to screening versus waiting until bleeding occurred, he noted (Euro J Cancer. 2001;37:64-71).
Overall, 13% of the patients had cancer, 50% had polyps, and 17% had hyperplasia.
“But what they emphasized was ... the length [of time] the patients complained of abnormal uterine bleeding. If it was less than 8 weeks ... there was no statistical difference in outcome, but if it was over 8 weeks there was a statistically significant difference in the grade of disease – a prognostic advantage for those patients who were screened versus symptomatic.”
The overall 5-year disease-free survival was 86% for asymptomatic versus 77% for symptomatic patients; for those with bleeding for less than 8 weeks, it was 98% versus 83%, respectively. The differences were not statistically different. However, for those with bleeding for 8-16 weeks it was 90% versus 74%, and for those with bleeding for more than 16 weeks it was 69% versus 62%, respectively, and those differences were statistically significant.
The problem is that many patients put off coming in for a long time, which means they are in a category with a worse prognosis when they do come in, Dr. Shwayder said. That’s not to say everyone should be screened, but there is no prognostic advantage to screening asymptomatic patients versus symptomatic patients who had bleeding for less than 8 weeks.
“It’s a little clarification, but I think an important one,” he noted.
Another study of 1,607 patients with endometrial thickening, including 233 who were asymptomatic and 1,374 who were symptomatic, found a lower rate of deep invasion with stage 1 disease, but no difference in the rate of more advanced disease, and no association with a more favorable outcome between the groups. (Am J Obstet Gynecol. 2018;219[2]:183e1-6).
Additionally, a study of 42 asymptomatic patients, 95 symptomatic patients with bleeding for less than 3 months, and 83 symptomatic patients with bleeding for more than 3 months showed a nonsignificant trend toward poorer 5-year survival in patients with a longer history of bleeding prior to surgery (Arch Gynecol Obstet 2013;288:1361-4).
“So now the question becomes how thick is too thick [and whether there is] some threshold where we ought to be evaluating patients and some threshold where we’re not,” he said.
The risk of malignancy among symptomatic postmenopausal women with an endometrial thickness greater than 5 mm is 7.3%, and the risk is similar at 6.7% in asymptomatic patients with an endometrial thickness of 11 mm or greater, according to a 2004 study by Smith-Bindman et al. (Ultrasound Obstet Gynecol. 2004;24:558-65).
“So the thought process here is that if a patient is asymptomatic, but the endometrium is over 11 mm, maybe we ought to evaluate that patient, because her risk of cancer is equivalent to that of someone who presents with postmenopausal bleeding and has an endometrium greater than 5 mm,” he explained.
In fact, a practice guideline from the Society of Obstetricians and Gynecologists of Canada recommends that women with endometrial thickness over 11 mm and other risk factors for cancer – such as obesity, hypertension, or late menopause – should be referred to a gynecologist for investigation, Dr. Shwayder said, adding that he also considers increased vascularity, heterogeneity in the endometrium, and fluid seen on a scan as cause for further evaluation.
“But endometrial sampling without bleeding should not be routinely performed,” he said. “So don’t routinely [sample] but based on risk factors and ultrasound findings, you may want to consider evaluating these patients further.”
Dr. Shwayder is a consultant for GE Ultrasound.
EXPERT ANALYSIS FROM ACOG 2019
FDA approves bevacizumab-bvzr for several cancers
The Food and Drug Administration has approved bevacizumab-bvzr (Zirabev) – a biosimilar to bevacizumab (Avastin) – for the treatment of five cancers: metastatic colorectal cancer (mCRC); unresectable, locally advanced, recurrent or metastatic non-squamous non–small cell lung cancer (NSCLC); recurrent glioblastoma; metastatic renal cell carcinoma (RCC); and persistent, recurrent or metastatic cervical cancer.
Approval was based on “review of a comprehensive data package which demonstrated biosimilarity of [bevacizumab-bvzr] to the reference product,” Pfizer said in a statement announcing the approval.
Bevacizumab-bvzr is the second bevacizumab biosimilar to be approved, following approval of Amgen’s bevacizumab-awwb (Mvasi) in 2017.
Warnings and precautions with the biosimilars, as with bevacizumab, include serious and sometimes fatal gastrointestinal perforation, surgery and wound healing complications, and sometimes serious and fatal hemorrhage.
The most common adverse events observed in bevacizumab patients are epistaxis, headache, hypertension, rhinitis, proteinuria, taste alteration, dry skin, rectal hemorrhage, lacrimation disorder, back pain, and exfoliative dermatitis.
Specific indications for the biosimilar are as follows:
Metastatic colorectal cancer
Bevacizumab-bvzr, in combination with intravenous fluorouracil-based chemotherapy, is indicated for the first- or second-line treatment of patients with mCRC.
Bevacizumab-bvzr, in combination with fluoropyrimidine-irinotecan or fluoropyrimidine-oxaliplatin–based chemotherapy, is indicated for the second-line treatment of patients with mCRC who have progressed on a first-line bevacizumab product–containing regimen.
Bevacizumab-bvzr is not indicated for adjuvant treatment of colon cancer.
First-line nonsquamous non–small cell lung cancer
Bevacizumab-bvzr, in combination with carboplatin and paclitaxel, is indicated for the first-line treatment of patients with unresectable, locally advanced, recurrent or metastatic NSCLC.
Recurrent glioblastoma
Bevacizumab-bvzr is indicated for the treatment of recurrent glioblastoma in adults.
Metastatic renal cell carcinoma
Bevacizumab-bvzr, in combination with interferon alfa, is indicated for the treatment of metastatic RCC.
Persistent, recurrent, or metastatic cervical cancer
Bevacizumab-bvzr, in combination with paclitaxel and cisplatin or paclitaxel and topotecan, is indicated for the treatment of patients with persistent, recurrent, or metastatic cervical cancer.
Complete prescribing information can be found on the FDA website.
The Food and Drug Administration has approved bevacizumab-bvzr (Zirabev) – a biosimilar to bevacizumab (Avastin) – for the treatment of five cancers: metastatic colorectal cancer (mCRC); unresectable, locally advanced, recurrent or metastatic non-squamous non–small cell lung cancer (NSCLC); recurrent glioblastoma; metastatic renal cell carcinoma (RCC); and persistent, recurrent or metastatic cervical cancer.
Approval was based on “review of a comprehensive data package which demonstrated biosimilarity of [bevacizumab-bvzr] to the reference product,” Pfizer said in a statement announcing the approval.
Bevacizumab-bvzr is the second bevacizumab biosimilar to be approved, following approval of Amgen’s bevacizumab-awwb (Mvasi) in 2017.
Warnings and precautions with the biosimilars, as with bevacizumab, include serious and sometimes fatal gastrointestinal perforation, surgery and wound healing complications, and sometimes serious and fatal hemorrhage.
The most common adverse events observed in bevacizumab patients are epistaxis, headache, hypertension, rhinitis, proteinuria, taste alteration, dry skin, rectal hemorrhage, lacrimation disorder, back pain, and exfoliative dermatitis.
Specific indications for the biosimilar are as follows:
Metastatic colorectal cancer
Bevacizumab-bvzr, in combination with intravenous fluorouracil-based chemotherapy, is indicated for the first- or second-line treatment of patients with mCRC.
Bevacizumab-bvzr, in combination with fluoropyrimidine-irinotecan or fluoropyrimidine-oxaliplatin–based chemotherapy, is indicated for the second-line treatment of patients with mCRC who have progressed on a first-line bevacizumab product–containing regimen.
Bevacizumab-bvzr is not indicated for adjuvant treatment of colon cancer.
First-line nonsquamous non–small cell lung cancer
Bevacizumab-bvzr, in combination with carboplatin and paclitaxel, is indicated for the first-line treatment of patients with unresectable, locally advanced, recurrent or metastatic NSCLC.
Recurrent glioblastoma
Bevacizumab-bvzr is indicated for the treatment of recurrent glioblastoma in adults.
Metastatic renal cell carcinoma
Bevacizumab-bvzr, in combination with interferon alfa, is indicated for the treatment of metastatic RCC.
Persistent, recurrent, or metastatic cervical cancer
Bevacizumab-bvzr, in combination with paclitaxel and cisplatin or paclitaxel and topotecan, is indicated for the treatment of patients with persistent, recurrent, or metastatic cervical cancer.
Complete prescribing information can be found on the FDA website.
The Food and Drug Administration has approved bevacizumab-bvzr (Zirabev) – a biosimilar to bevacizumab (Avastin) – for the treatment of five cancers: metastatic colorectal cancer (mCRC); unresectable, locally advanced, recurrent or metastatic non-squamous non–small cell lung cancer (NSCLC); recurrent glioblastoma; metastatic renal cell carcinoma (RCC); and persistent, recurrent or metastatic cervical cancer.
Approval was based on “review of a comprehensive data package which demonstrated biosimilarity of [bevacizumab-bvzr] to the reference product,” Pfizer said in a statement announcing the approval.
Bevacizumab-bvzr is the second bevacizumab biosimilar to be approved, following approval of Amgen’s bevacizumab-awwb (Mvasi) in 2017.
Warnings and precautions with the biosimilars, as with bevacizumab, include serious and sometimes fatal gastrointestinal perforation, surgery and wound healing complications, and sometimes serious and fatal hemorrhage.
The most common adverse events observed in bevacizumab patients are epistaxis, headache, hypertension, rhinitis, proteinuria, taste alteration, dry skin, rectal hemorrhage, lacrimation disorder, back pain, and exfoliative dermatitis.
Specific indications for the biosimilar are as follows:
Metastatic colorectal cancer
Bevacizumab-bvzr, in combination with intravenous fluorouracil-based chemotherapy, is indicated for the first- or second-line treatment of patients with mCRC.
Bevacizumab-bvzr, in combination with fluoropyrimidine-irinotecan or fluoropyrimidine-oxaliplatin–based chemotherapy, is indicated for the second-line treatment of patients with mCRC who have progressed on a first-line bevacizumab product–containing regimen.
Bevacizumab-bvzr is not indicated for adjuvant treatment of colon cancer.
First-line nonsquamous non–small cell lung cancer
Bevacizumab-bvzr, in combination with carboplatin and paclitaxel, is indicated for the first-line treatment of patients with unresectable, locally advanced, recurrent or metastatic NSCLC.
Recurrent glioblastoma
Bevacizumab-bvzr is indicated for the treatment of recurrent glioblastoma in adults.
Metastatic renal cell carcinoma
Bevacizumab-bvzr, in combination with interferon alfa, is indicated for the treatment of metastatic RCC.
Persistent, recurrent, or metastatic cervical cancer
Bevacizumab-bvzr, in combination with paclitaxel and cisplatin or paclitaxel and topotecan, is indicated for the treatment of patients with persistent, recurrent, or metastatic cervical cancer.
Complete prescribing information can be found on the FDA website.
Chemoradiotherapy no better than chemo alone in endometrial carcinoma
Chemotherapy plus radiation therapy (chemoradiotherapy) was not associated with improved relapse-free survival versus chemotherapy alone in patients with stage III or IVA endometrial cancer, according to results from a phase 3 trial.
“This combined approach has been studied, but its efficacy relative to that of chemotherapy alone is not known,” wrote Daniela Matei, MD, of Northwestern University, Chicago, and colleagues. The results were published in the New England Journal of Medicine.
The Gynecologic Oncology Group (GOG) 258 study included 736 patients with stage III or IVA endometrial carcinoma who were randomized in a 1:1 fashion to receive platinum-based chemotherapy plus volume–directed external-beam radiation therapy every 21 days for a total of four cycles or chemotherapy alone every 21 days for a total of six cycles.
The primary endpoint measured was relapse-free survival; secondary endpoints included safety, overall survival (OS), and quality of life.
At 60 months, the proportion of patients alive and relapse free was 59% (95% confidence interval, 53-65) and 58% (95% CI, 53-64) in the chemoradiotherapy and chemotherapy alone arms, respectively (hazard ratio, 0.90; 90% CI, 0.74-1.10).
“The data on overall survival are not sufficiently mature to allow comparison between the groups,” the researchers wrote.
With respect to safety, grade 3, 4, or 5 toxicities were reported in 58% and 63% of patients in the chemoradiotherapy and chemotherapy alone arms, respectively.
“Although acute toxic effects were more common in the chemoradiotherapy group than in the chemotherapy-only group in our trial, most were low-grade and reversible on treatment discontinuation,” Dr. Matei and colleagues explained.
A major strength of the study was the broad inclusion criteria, which included patients with nonperitoneal, lymph-node, pelvic, and adnexal metastasis, the researchers noted.
“Our data are compatible with the hypothesis from previous studies that completion of chemotherapy is important for the prevention of distant relapse,” they concluded.
The National Cancer Institute supported the study. The authors reported financial affiliations with AstraZeneca, Clovis, Genentech, the GOG Foundation, Tesaro, and several others.
SOURCE: Matei D et al. N Engl J Med. 2019 Jun 13. doi: 10.1056/NEJMoa1813181.
Chemotherapy plus radiation therapy (chemoradiotherapy) was not associated with improved relapse-free survival versus chemotherapy alone in patients with stage III or IVA endometrial cancer, according to results from a phase 3 trial.
“This combined approach has been studied, but its efficacy relative to that of chemotherapy alone is not known,” wrote Daniela Matei, MD, of Northwestern University, Chicago, and colleagues. The results were published in the New England Journal of Medicine.
The Gynecologic Oncology Group (GOG) 258 study included 736 patients with stage III or IVA endometrial carcinoma who were randomized in a 1:1 fashion to receive platinum-based chemotherapy plus volume–directed external-beam radiation therapy every 21 days for a total of four cycles or chemotherapy alone every 21 days for a total of six cycles.
The primary endpoint measured was relapse-free survival; secondary endpoints included safety, overall survival (OS), and quality of life.
At 60 months, the proportion of patients alive and relapse free was 59% (95% confidence interval, 53-65) and 58% (95% CI, 53-64) in the chemoradiotherapy and chemotherapy alone arms, respectively (hazard ratio, 0.90; 90% CI, 0.74-1.10).
“The data on overall survival are not sufficiently mature to allow comparison between the groups,” the researchers wrote.
With respect to safety, grade 3, 4, or 5 toxicities were reported in 58% and 63% of patients in the chemoradiotherapy and chemotherapy alone arms, respectively.
“Although acute toxic effects were more common in the chemoradiotherapy group than in the chemotherapy-only group in our trial, most were low-grade and reversible on treatment discontinuation,” Dr. Matei and colleagues explained.
A major strength of the study was the broad inclusion criteria, which included patients with nonperitoneal, lymph-node, pelvic, and adnexal metastasis, the researchers noted.
“Our data are compatible with the hypothesis from previous studies that completion of chemotherapy is important for the prevention of distant relapse,” they concluded.
The National Cancer Institute supported the study. The authors reported financial affiliations with AstraZeneca, Clovis, Genentech, the GOG Foundation, Tesaro, and several others.
SOURCE: Matei D et al. N Engl J Med. 2019 Jun 13. doi: 10.1056/NEJMoa1813181.
Chemotherapy plus radiation therapy (chemoradiotherapy) was not associated with improved relapse-free survival versus chemotherapy alone in patients with stage III or IVA endometrial cancer, according to results from a phase 3 trial.
“This combined approach has been studied, but its efficacy relative to that of chemotherapy alone is not known,” wrote Daniela Matei, MD, of Northwestern University, Chicago, and colleagues. The results were published in the New England Journal of Medicine.
The Gynecologic Oncology Group (GOG) 258 study included 736 patients with stage III or IVA endometrial carcinoma who were randomized in a 1:1 fashion to receive platinum-based chemotherapy plus volume–directed external-beam radiation therapy every 21 days for a total of four cycles or chemotherapy alone every 21 days for a total of six cycles.
The primary endpoint measured was relapse-free survival; secondary endpoints included safety, overall survival (OS), and quality of life.
At 60 months, the proportion of patients alive and relapse free was 59% (95% confidence interval, 53-65) and 58% (95% CI, 53-64) in the chemoradiotherapy and chemotherapy alone arms, respectively (hazard ratio, 0.90; 90% CI, 0.74-1.10).
“The data on overall survival are not sufficiently mature to allow comparison between the groups,” the researchers wrote.
With respect to safety, grade 3, 4, or 5 toxicities were reported in 58% and 63% of patients in the chemoradiotherapy and chemotherapy alone arms, respectively.
“Although acute toxic effects were more common in the chemoradiotherapy group than in the chemotherapy-only group in our trial, most were low-grade and reversible on treatment discontinuation,” Dr. Matei and colleagues explained.
A major strength of the study was the broad inclusion criteria, which included patients with nonperitoneal, lymph-node, pelvic, and adnexal metastasis, the researchers noted.
“Our data are compatible with the hypothesis from previous studies that completion of chemotherapy is important for the prevention of distant relapse,” they concluded.
The National Cancer Institute supported the study. The authors reported financial affiliations with AstraZeneca, Clovis, Genentech, the GOG Foundation, Tesaro, and several others.
SOURCE: Matei D et al. N Engl J Med. 2019 Jun 13. doi: 10.1056/NEJMoa1813181.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Niraparib-pembrolizumab combo finds niche in breast, ovarian cancers
The strategy of simultaneously exploiting deficient DNA damage repair and unleashing the immune response could expand treatment options for hard-to-treat breast and ovarian cancers, findings of the TOPACIO/KEYNOTE-162 trial suggest.
Triple-negative breast cancer (TNBC) and high-grade serous ovarian carcinoma share a number of genomic features, including a high frequency of BRCA1 and BRCA2 inactivation (Nature. 2012;490:61-70), as well as potential immunoreactivity (Lancet Oncol. 2018;19:40-50).
The open-label, single-arm phase 1/2 trial therefore tested the combination of niraparib (Zejula), an oral poly (ADP-ribose) polymerase (PARP) inhibitor, and pembrolizumab (Keytruda), an antibody to programmed death 1 (PD-1), among more than 100 patients with advanced or metastatic TNBC or recurrent platinum-resistant ovarian carcinoma. Patients were enrolled irrespective of BRCA mutation status or programmed death-ligand 1 (PD-L1) expression.
Main results, reported in JAMA Oncology, showed that the combination was safe, and about a fifth of patients with each type of cancer had an objective response. Median progression-free survival (PFS) was about 2 months in those with TNBC overall (although it exceeded 8 months in the subset with a tumor BRCA mutation) and about 3 months in those with ovarian cancer.
TNBC cohort
Investigators led by Shaveta Vinayak, MD, of the division of oncology at Fred Hutchinson Cancer Research Center, and University of Washington School of Medicine, Seattle Cancer Care Alliance, Seattle, studied 55 patients with TNBC treated with niraparib-pembrolizumab in the trial.
In the efficacy-evaluable population of 47 patients, the objective response rate (ORR) was 21%, and the disease control rate (DCR) was 49%. With a median duration of follow-up of 14.8 months, the median duration of response was not reached.
Activity of the combination varied by tumor BRCA mutation status. Compared with counterparts having BRCA wild-type tumors, patients having tumors with BRCA mutations had a numerically higher ORR (47% vs. 11%), DCR (80% vs. 33%), and PFS (8.3 vs. 2.1 months).
Some 18% of patients had treatment-related anemia, 15% thrombocytopenia, and 7% fatigue. In addition, 15% of patients had immune-related adverse events, with 4% having grade 3 immune-related adverse events.
“Combination niraparib plus pembrolizumab provides promising antitumor activity in patients with advanced or metastatic TNBC, with numerically higher response rates in those with tumor BRCA mutations,” Dr. Vinayak and colleagues conclude. “The combination therapy was safe with a tolerable safety profile, warranting further investigation.”
Ovarian cancer cohort
Investigators led by Panagiotis A. Konstantinopoulos, MD, PhD, of the division of gynecologic oncology, department of medical oncology at Dana-Farber Cancer Institute, Harvard Medical School, Boston, studied 62 patients with ovarian carcinoma treated with niraparib-pembrolizumab in the trial.
In the efficacy-evaluable population of 60 patients, the ORR was 18% and the DCR was 65%. The ORRs were similar regardless of patients’ platinum-based chemotherapy sensitivity, previous bevacizumab treatment, or tumor BRCA or homologous recombination deficiency (HRD) biomarker status.
With a median duration of follow-up of 12.4 months, the median duration of response was not reached, ranging from 4.2 to roughly 14.5 months. Median progression-free survival was 3.4 months.
The leading treatment-related adverse events of grade 3 or higher in this cohort were anemia (21%) and thrombocytopenia (9%). In addition, 19% of patients had immune-related adverse events, with 9% having grade 3 or higher immune-related adverse events.
“Niraparib in combination with pembrolizumab is tolerable, with promising antitumor activity for patients with ovarian carcinoma who have limited treatment options regardless of platinum status, biomarker status, or prior treatment with bevacizumab,” Dr. Konstantinopoulos and colleagues conclude. “Responses in patients without tumor BRCA mutations or non-HRD cancers were higher than expected with either agent as monotherapy.”
Dr. Vinayak disclosed receiving clinical trial funding from TESARO; serving on an advisory board for TESARO; and serving on an advisory board for OncoSec Medical (uncompensated). Dr. Konstantinopoulos disclosed serving on advisory boards for AstraZeneca, Pfizer, and Merck. The trial was supported by TESARO: a GSK company and Merck, and in part by Stand Up to Cancer (a program of the Entertainment Industry Foundation); the Ovarian Cancer Research Fund Alliance; and National Ovarian Cancer Coalition Dream Team Translational Research.
SOURCE: Vinayak A et al. JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1029. Konstantinopoulos PA et al. JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1048.
“Targeting DNA repair and immune checkpoint pathways has emerged as an important concept in cancer therapy, well supported by preclinical and clinical data in ovarian cancer and TNBC. However, there are some limitations to the two studies presented herein,” maintain editorialists Kunle Odunsi, MD, PhD, and Tanja Pejovic, MD, PhD.
Patients varied considerably with respect to number of prior chemotherapy regimens, they elaborate. Also, there may have been some misclassification of patients into DNA damage repair (DDR) groups, and small sample sizes precluded rigorous subgroup analyses.
“Because DDR and, by extension, tumor mutational burden and PD-L1 status do not fully explain the effects of the combination of PARP inhibitors and anti–PD-1 therapy, additional predictive biomarkers based on tumor intrinsic or adaptive mechanisms of resistance are needed for both cancer types,” the editorialists contend. In particular, knowledge of the tumor microenvironment could be used to tailor therapy for individual patients.
“The TOPACIO clinical studies are clearly steps in the right direction for patients with [platinum-resistant ovarian carcinoma] and TNBC,” they conclude. “However, larger confirmatory randomized clinical trials are needed that use panels of integrated biomarkers that would allow identification of patients most likely to respond.”
Dr. Odunsi is the deputy director and chair of the department of gynecologic oncology, executive director of the Center for Immunotherapy, and co-leader of the Tumor Immunology and Immunotherapy Research Program–Roswell Park Comprehensive Cancer Center, Buffalo, N.Y. Dr. Pejovic is associate professor, division of gynecologic oncology, department of obstetrics & gynecology, Knight Cancer Institute, Oregon Health & Science University, Portland, Ore. These remarks are adapted from a related editorial (JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1009 ).
“Targeting DNA repair and immune checkpoint pathways has emerged as an important concept in cancer therapy, well supported by preclinical and clinical data in ovarian cancer and TNBC. However, there are some limitations to the two studies presented herein,” maintain editorialists Kunle Odunsi, MD, PhD, and Tanja Pejovic, MD, PhD.
Patients varied considerably with respect to number of prior chemotherapy regimens, they elaborate. Also, there may have been some misclassification of patients into DNA damage repair (DDR) groups, and small sample sizes precluded rigorous subgroup analyses.
“Because DDR and, by extension, tumor mutational burden and PD-L1 status do not fully explain the effects of the combination of PARP inhibitors and anti–PD-1 therapy, additional predictive biomarkers based on tumor intrinsic or adaptive mechanisms of resistance are needed for both cancer types,” the editorialists contend. In particular, knowledge of the tumor microenvironment could be used to tailor therapy for individual patients.
“The TOPACIO clinical studies are clearly steps in the right direction for patients with [platinum-resistant ovarian carcinoma] and TNBC,” they conclude. “However, larger confirmatory randomized clinical trials are needed that use panels of integrated biomarkers that would allow identification of patients most likely to respond.”
Dr. Odunsi is the deputy director and chair of the department of gynecologic oncology, executive director of the Center for Immunotherapy, and co-leader of the Tumor Immunology and Immunotherapy Research Program–Roswell Park Comprehensive Cancer Center, Buffalo, N.Y. Dr. Pejovic is associate professor, division of gynecologic oncology, department of obstetrics & gynecology, Knight Cancer Institute, Oregon Health & Science University, Portland, Ore. These remarks are adapted from a related editorial (JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1009 ).
“Targeting DNA repair and immune checkpoint pathways has emerged as an important concept in cancer therapy, well supported by preclinical and clinical data in ovarian cancer and TNBC. However, there are some limitations to the two studies presented herein,” maintain editorialists Kunle Odunsi, MD, PhD, and Tanja Pejovic, MD, PhD.
Patients varied considerably with respect to number of prior chemotherapy regimens, they elaborate. Also, there may have been some misclassification of patients into DNA damage repair (DDR) groups, and small sample sizes precluded rigorous subgroup analyses.
“Because DDR and, by extension, tumor mutational burden and PD-L1 status do not fully explain the effects of the combination of PARP inhibitors and anti–PD-1 therapy, additional predictive biomarkers based on tumor intrinsic or adaptive mechanisms of resistance are needed for both cancer types,” the editorialists contend. In particular, knowledge of the tumor microenvironment could be used to tailor therapy for individual patients.
“The TOPACIO clinical studies are clearly steps in the right direction for patients with [platinum-resistant ovarian carcinoma] and TNBC,” they conclude. “However, larger confirmatory randomized clinical trials are needed that use panels of integrated biomarkers that would allow identification of patients most likely to respond.”
Dr. Odunsi is the deputy director and chair of the department of gynecologic oncology, executive director of the Center for Immunotherapy, and co-leader of the Tumor Immunology and Immunotherapy Research Program–Roswell Park Comprehensive Cancer Center, Buffalo, N.Y. Dr. Pejovic is associate professor, division of gynecologic oncology, department of obstetrics & gynecology, Knight Cancer Institute, Oregon Health & Science University, Portland, Ore. These remarks are adapted from a related editorial (JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1009 ).
The strategy of simultaneously exploiting deficient DNA damage repair and unleashing the immune response could expand treatment options for hard-to-treat breast and ovarian cancers, findings of the TOPACIO/KEYNOTE-162 trial suggest.
Triple-negative breast cancer (TNBC) and high-grade serous ovarian carcinoma share a number of genomic features, including a high frequency of BRCA1 and BRCA2 inactivation (Nature. 2012;490:61-70), as well as potential immunoreactivity (Lancet Oncol. 2018;19:40-50).
The open-label, single-arm phase 1/2 trial therefore tested the combination of niraparib (Zejula), an oral poly (ADP-ribose) polymerase (PARP) inhibitor, and pembrolizumab (Keytruda), an antibody to programmed death 1 (PD-1), among more than 100 patients with advanced or metastatic TNBC or recurrent platinum-resistant ovarian carcinoma. Patients were enrolled irrespective of BRCA mutation status or programmed death-ligand 1 (PD-L1) expression.
Main results, reported in JAMA Oncology, showed that the combination was safe, and about a fifth of patients with each type of cancer had an objective response. Median progression-free survival (PFS) was about 2 months in those with TNBC overall (although it exceeded 8 months in the subset with a tumor BRCA mutation) and about 3 months in those with ovarian cancer.
TNBC cohort
Investigators led by Shaveta Vinayak, MD, of the division of oncology at Fred Hutchinson Cancer Research Center, and University of Washington School of Medicine, Seattle Cancer Care Alliance, Seattle, studied 55 patients with TNBC treated with niraparib-pembrolizumab in the trial.
In the efficacy-evaluable population of 47 patients, the objective response rate (ORR) was 21%, and the disease control rate (DCR) was 49%. With a median duration of follow-up of 14.8 months, the median duration of response was not reached.
Activity of the combination varied by tumor BRCA mutation status. Compared with counterparts having BRCA wild-type tumors, patients having tumors with BRCA mutations had a numerically higher ORR (47% vs. 11%), DCR (80% vs. 33%), and PFS (8.3 vs. 2.1 months).
Some 18% of patients had treatment-related anemia, 15% thrombocytopenia, and 7% fatigue. In addition, 15% of patients had immune-related adverse events, with 4% having grade 3 immune-related adverse events.
“Combination niraparib plus pembrolizumab provides promising antitumor activity in patients with advanced or metastatic TNBC, with numerically higher response rates in those with tumor BRCA mutations,” Dr. Vinayak and colleagues conclude. “The combination therapy was safe with a tolerable safety profile, warranting further investigation.”
Ovarian cancer cohort
Investigators led by Panagiotis A. Konstantinopoulos, MD, PhD, of the division of gynecologic oncology, department of medical oncology at Dana-Farber Cancer Institute, Harvard Medical School, Boston, studied 62 patients with ovarian carcinoma treated with niraparib-pembrolizumab in the trial.
In the efficacy-evaluable population of 60 patients, the ORR was 18% and the DCR was 65%. The ORRs were similar regardless of patients’ platinum-based chemotherapy sensitivity, previous bevacizumab treatment, or tumor BRCA or homologous recombination deficiency (HRD) biomarker status.
With a median duration of follow-up of 12.4 months, the median duration of response was not reached, ranging from 4.2 to roughly 14.5 months. Median progression-free survival was 3.4 months.
The leading treatment-related adverse events of grade 3 or higher in this cohort were anemia (21%) and thrombocytopenia (9%). In addition, 19% of patients had immune-related adverse events, with 9% having grade 3 or higher immune-related adverse events.
“Niraparib in combination with pembrolizumab is tolerable, with promising antitumor activity for patients with ovarian carcinoma who have limited treatment options regardless of platinum status, biomarker status, or prior treatment with bevacizumab,” Dr. Konstantinopoulos and colleagues conclude. “Responses in patients without tumor BRCA mutations or non-HRD cancers were higher than expected with either agent as monotherapy.”
Dr. Vinayak disclosed receiving clinical trial funding from TESARO; serving on an advisory board for TESARO; and serving on an advisory board for OncoSec Medical (uncompensated). Dr. Konstantinopoulos disclosed serving on advisory boards for AstraZeneca, Pfizer, and Merck. The trial was supported by TESARO: a GSK company and Merck, and in part by Stand Up to Cancer (a program of the Entertainment Industry Foundation); the Ovarian Cancer Research Fund Alliance; and National Ovarian Cancer Coalition Dream Team Translational Research.
SOURCE: Vinayak A et al. JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1029. Konstantinopoulos PA et al. JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1048.
The strategy of simultaneously exploiting deficient DNA damage repair and unleashing the immune response could expand treatment options for hard-to-treat breast and ovarian cancers, findings of the TOPACIO/KEYNOTE-162 trial suggest.
Triple-negative breast cancer (TNBC) and high-grade serous ovarian carcinoma share a number of genomic features, including a high frequency of BRCA1 and BRCA2 inactivation (Nature. 2012;490:61-70), as well as potential immunoreactivity (Lancet Oncol. 2018;19:40-50).
The open-label, single-arm phase 1/2 trial therefore tested the combination of niraparib (Zejula), an oral poly (ADP-ribose) polymerase (PARP) inhibitor, and pembrolizumab (Keytruda), an antibody to programmed death 1 (PD-1), among more than 100 patients with advanced or metastatic TNBC or recurrent platinum-resistant ovarian carcinoma. Patients were enrolled irrespective of BRCA mutation status or programmed death-ligand 1 (PD-L1) expression.
Main results, reported in JAMA Oncology, showed that the combination was safe, and about a fifth of patients with each type of cancer had an objective response. Median progression-free survival (PFS) was about 2 months in those with TNBC overall (although it exceeded 8 months in the subset with a tumor BRCA mutation) and about 3 months in those with ovarian cancer.
TNBC cohort
Investigators led by Shaveta Vinayak, MD, of the division of oncology at Fred Hutchinson Cancer Research Center, and University of Washington School of Medicine, Seattle Cancer Care Alliance, Seattle, studied 55 patients with TNBC treated with niraparib-pembrolizumab in the trial.
In the efficacy-evaluable population of 47 patients, the objective response rate (ORR) was 21%, and the disease control rate (DCR) was 49%. With a median duration of follow-up of 14.8 months, the median duration of response was not reached.
Activity of the combination varied by tumor BRCA mutation status. Compared with counterparts having BRCA wild-type tumors, patients having tumors with BRCA mutations had a numerically higher ORR (47% vs. 11%), DCR (80% vs. 33%), and PFS (8.3 vs. 2.1 months).
Some 18% of patients had treatment-related anemia, 15% thrombocytopenia, and 7% fatigue. In addition, 15% of patients had immune-related adverse events, with 4% having grade 3 immune-related adverse events.
“Combination niraparib plus pembrolizumab provides promising antitumor activity in patients with advanced or metastatic TNBC, with numerically higher response rates in those with tumor BRCA mutations,” Dr. Vinayak and colleagues conclude. “The combination therapy was safe with a tolerable safety profile, warranting further investigation.”
Ovarian cancer cohort
Investigators led by Panagiotis A. Konstantinopoulos, MD, PhD, of the division of gynecologic oncology, department of medical oncology at Dana-Farber Cancer Institute, Harvard Medical School, Boston, studied 62 patients with ovarian carcinoma treated with niraparib-pembrolizumab in the trial.
In the efficacy-evaluable population of 60 patients, the ORR was 18% and the DCR was 65%. The ORRs were similar regardless of patients’ platinum-based chemotherapy sensitivity, previous bevacizumab treatment, or tumor BRCA or homologous recombination deficiency (HRD) biomarker status.
With a median duration of follow-up of 12.4 months, the median duration of response was not reached, ranging from 4.2 to roughly 14.5 months. Median progression-free survival was 3.4 months.
The leading treatment-related adverse events of grade 3 or higher in this cohort were anemia (21%) and thrombocytopenia (9%). In addition, 19% of patients had immune-related adverse events, with 9% having grade 3 or higher immune-related adverse events.
“Niraparib in combination with pembrolizumab is tolerable, with promising antitumor activity for patients with ovarian carcinoma who have limited treatment options regardless of platinum status, biomarker status, or prior treatment with bevacizumab,” Dr. Konstantinopoulos and colleagues conclude. “Responses in patients without tumor BRCA mutations or non-HRD cancers were higher than expected with either agent as monotherapy.”
Dr. Vinayak disclosed receiving clinical trial funding from TESARO; serving on an advisory board for TESARO; and serving on an advisory board for OncoSec Medical (uncompensated). Dr. Konstantinopoulos disclosed serving on advisory boards for AstraZeneca, Pfizer, and Merck. The trial was supported by TESARO: a GSK company and Merck, and in part by Stand Up to Cancer (a program of the Entertainment Industry Foundation); the Ovarian Cancer Research Fund Alliance; and National Ovarian Cancer Coalition Dream Team Translational Research.
SOURCE: Vinayak A et al. JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1029. Konstantinopoulos PA et al. JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1048.
FROM JAMA ONCOLOGY
Nearly two-thirds of gynecologic oncology respondents experienced sexual harassment
CHICAGO – Nearly two-thirds of more than 400 U.S.-based physician members of the Society of Gynecologic Oncology who participated in a recent survey reported experiencing sexual harassment in training or practice.
Notably, of the 255 women and 147 men who responded, 71% and 51%, respectively, reported such sexual harassment – and only 15% overall reported it to officials, Marina Stasenko, MD, reported at the annual meeting of the American Society of Clinical Oncology.
The survey also addressed gender-based discrimination and disparities, including respondents’ views on pay disparities. In this video interview, Dr. Stasenko, a gynecologic oncology fellow at Memorial Sloan Kettering Cancer Center, New York, discusses the findings, their implications and context in this age of #MeToo and #TimesUp, and potential approaches to addressing the ongoing problem and the concerns victims have about reporting harassment.
“We need to start by setting an example, saying these things are not tolerated [and] put that into policy – really show folks how it can be reported and what is being done once that report is filed,” she said.
Dr. Stasenko reported having no disclosures.
SOURCE: Stasenko M et al. ASCO 2019, Abstract LBA10502.
CHICAGO – Nearly two-thirds of more than 400 U.S.-based physician members of the Society of Gynecologic Oncology who participated in a recent survey reported experiencing sexual harassment in training or practice.
Notably, of the 255 women and 147 men who responded, 71% and 51%, respectively, reported such sexual harassment – and only 15% overall reported it to officials, Marina Stasenko, MD, reported at the annual meeting of the American Society of Clinical Oncology.
The survey also addressed gender-based discrimination and disparities, including respondents’ views on pay disparities. In this video interview, Dr. Stasenko, a gynecologic oncology fellow at Memorial Sloan Kettering Cancer Center, New York, discusses the findings, their implications and context in this age of #MeToo and #TimesUp, and potential approaches to addressing the ongoing problem and the concerns victims have about reporting harassment.
“We need to start by setting an example, saying these things are not tolerated [and] put that into policy – really show folks how it can be reported and what is being done once that report is filed,” she said.
Dr. Stasenko reported having no disclosures.
SOURCE: Stasenko M et al. ASCO 2019, Abstract LBA10502.
CHICAGO – Nearly two-thirds of more than 400 U.S.-based physician members of the Society of Gynecologic Oncology who participated in a recent survey reported experiencing sexual harassment in training or practice.
Notably, of the 255 women and 147 men who responded, 71% and 51%, respectively, reported such sexual harassment – and only 15% overall reported it to officials, Marina Stasenko, MD, reported at the annual meeting of the American Society of Clinical Oncology.
The survey also addressed gender-based discrimination and disparities, including respondents’ views on pay disparities. In this video interview, Dr. Stasenko, a gynecologic oncology fellow at Memorial Sloan Kettering Cancer Center, New York, discusses the findings, their implications and context in this age of #MeToo and #TimesUp, and potential approaches to addressing the ongoing problem and the concerns victims have about reporting harassment.
“We need to start by setting an example, saying these things are not tolerated [and] put that into policy – really show folks how it can be reported and what is being done once that report is filed,” she said.
Dr. Stasenko reported having no disclosures.
SOURCE: Stasenko M et al. ASCO 2019, Abstract LBA10502.
REPORTING FROM ASCO 2019
2019 Update on menopause
Among peri- and postmenopausal women, abnormal bleeding, breast cancer, and mood disorders represent prevalent conditions. In this Update, we discuss data from a review that provides quantitative information on the likelihood of finding endometrial cancer among women with postmenopausal bleeding (PMB). We also summarize 2 recent consensus recommendations: One addresses the clinically important but controversial issue of the treatment of genitourinary syndrome of menopause (GSM) in breast cancer survivors, and the other provides guidance on the management of depression in perimenopausal women.
Endometrial cancer is associated with a high prevalence of PMB
Clarke MA, Long BJ, Del Mar Morillo A, et al. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:1210-1222.
Endometrial cancer is the most common gynecologic malignancy and the fourth most common cancer among US women. In recent years, the incidence of and mortality from endometrial cancer have increased.1 Despite the high prevalence of endometrial cancer, population-based screening currently is not recommended.
PMB affects up to 10% of women and can be caused by endometrial atrophy, endometrial polyps, uterine leiomyoma, and malignancy. While it is well known that PMB is a common presenting symptom of endometrial cancer, we do not have good data to guide counseling patients with PMB on the likelihood that endometrial cancer is present. Similarly, estimates are lacking regarding what proportion of women with endometrial cancer will present with PMB.
To address these 2 issues, Clarke and colleagues conducted a comprehensive systematic review and meta-analysis of the prevalence of PMB among women with endometrial cancer (sensitivity) and the risk of endometrial cancer among women with PMB (positive predictive value). The authors included 129 studies--with 34,432 women with PMB and 6,358 with endometrial cancer--in their report.
Cancer prevalence varied with HT use, geographic location
The study findings demonstrated that the prevalence of PMB in women with endometrial cancer was 90% (95% confidence interval [CI], 84%-94%), and there was no significant difference in the occurrence of PMB by cancer stage. The risk of endometrial cancer in women with PMB ranged from 0% to 48%, yielding an overall pooled estimate of 9% (95% CI, 8%-11%). As an editorialist pointed out, the risk of endometrial cancer in women with PMB is similar to that of colorectal cancer in individuals with rectal bleeding (8%) and breast cancer in women with a palpable mass (10%), supporting current guidance that recommends evaluation of women with PMB.2 Evaluating 100 women with PMB to diagnose 9 endometrial cancers does not seem excessive.
Interestingly, among women with PMB, the prevalence of endometrial cancer was significantly higher among women not using hormone therapy (HT) than among users of HT (12% and 7%, respectively). In 7 studies restricted to women with PMB and polyps (n = 2,801), the pooled risk of endometrial cancer was 3% (95% CI, 3%-4%). In an analysis stratified by geographic region, a striking difference was noted in the risk of endometrial cancer among women with PMB in North America (5%), Northern Europe (7%), and in Western Europe (13%). This finding may be explained by regional differences in the approach to evaluating PMB, cultural perceptions of PMB that can affect thresholds to present for care, and differences in risk factors between these populations.
The study had several limitations, including an inability to evaluate the number of years since menopause and the effects of body mass index. Additionally, the study did not address endometrial hyperplasia or endometrial intraepithelial neoplasia.
PMB accounts for two-thirds of all gynecologic visits among perimenopausal and postmenopausal women.3 This study revealed a 9% risk of endometrial cancer in patients experiencing PMB, which supports current practice guidelines to further evaluate and rule out endometrial cancer among all women presenting with PMB4; it also provides reassurance that targeting this high-risk group of women for early detection and prevention strategies will capture most cases of endometrial cancers. However, the relatively low positive predictive value of PMB emphasizes the need for additional triage tests with high specificity to improve management of PMB and minimize unnecessary biopsies in low-risk women.
Treating GSM in breast cancer survivors: New guidance targets QoL and sexuality
Faubion SS, Larkin LC, Stuenkel CA, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendations from The North American Menopause Society and The International Society for the Study of Women's Sexual Health. Menopause. 2018;25:596-608.
Given that there is little evidence addressing the safety of vaginal estrogen, other hormonal therapies, and nonprescription treatments for GSM in breast cancer survivors, many survivors with bothersome GSM symptoms are not appropriately treated.
Continue to: Expert panel creates evidence-based guidance...
Expert panel creates evidence-based guidance
Against this backdrop, The North American Menopause Society and the International Society for the Study of Women's Sexual Health convened a group comprised of menopause specialists (ObGyns, internists, and nurse practitioners), specialists in sexuality, medical oncologists specializing in breast cancer, and a psychologist to create evidence-based interdisciplinary consensus guidelines for enhancing quality of life and sexuality for breast cancer survivors with GSM.
Measures to help enhance quality of life and sexuality
The group's key recommendations for clinicians include:
- Sexual function and quality of life (QoL) should be assessed in all women with or at high risk for breast cancer.
- Management of GSM should be individualized based on shared decision-making involving the patient and her oncologist.
- Initial treatment options include:
—over-the-counter vaginal moisturizers used several times weekly on a regular basis
—lubricants used with intercourse
—vaginal dilator therapy
—pelvic floor physical therapy.
- Low-dose vaginal estrogen therapy may be appropriate for select women who have been treated for breast cancer:
—With use of vaginal estrogen, serum estradiol levels remain in the postmenopausal range.
—Based on limited data, use of vaginal estrogen is associated with a minimal risk for recurrence of breast cancer.
—Because their use is associated with the lowest serum estradiol levels, vaginal tablets, rings, or inserts may be preferable to creams.
—Decisions regarding use of vaginal estrogen in breast cancer survivors should involve the woman's oncologist. Appropriate candidates for off-label use of vaginal estrogen may be survivors:
–who are at relatively low risk for recurrence
–with hormone receptor-negative disease
–using tamoxifen rather than an AI
–who are particularly concerned about quality of life.
—Given that AIs prevent recurrence by lowering estrogen levels, oncologists may be reluctant to consider use of vaginal estrogen in survivors using adjuvant agents.
—With respect to use of vaginal estrogen, oncologists may be more comfortable with use in patients taking tamoxifen.
- Neither intravaginal dehydroepiandrosterone (DHEA; prasterone) nor the oral selective estrogen receptor modulator ospemifene has been studied in breast cancer survivors.
In women with metastatic disease, QoL, comfort, and sexual intimacy are key considerations when weighing potential therapies; optimal choices will vary with probability of long-term survival.
Although more data addressing the safety of vaginal estrogen as well as prasterone and ospemifene in breast cancer survivors clearly are needed, these guidelines should help clinicians who care for breast cancer survivors with GSM.
Framework provided for managing depressive disorders in perimenopausal women
Maki PM, Kornstein SG, Joffe H, et al; Board of Trustees for The North American Menopause Society (NAMS) and the Women and Mood Disorders Task Force of the National Network of Depression Centers. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. Menopause. 2018;25:1069-1085.
Although perimenopausal women are more susceptible to the development of depressive symptoms and major depressive episodes (MDE), there is a lack of consensus regarding how to evaluate and treat depression in women during the menopausal transition and postmenopausal period.
Recently, an expert panel comprised of representatives from The North American Menopause Society and the National Network of Depression Centers Women and Mood Disorders Task Group developed clinical guidelines addressing epidemiology, clinical presentation, therapeutic effects of antidepressants, effects of HT, and efficacy of other therapies. Here we provide a summary of the expert panel's findings and guidelines.
Continue to: Certain factors are associated with higher risk for depression...
Certain factors are associated with higher risk for depression
The perimenopause represents a time of increased risk for depressive symptoms and major depressive disorder (MDD), even in women with no prior history of depression. Several characteristics and health factors are associated with the increased risk during the menopause transition. These include a prior history of MDD, current antidepressant use, anxiety, premenstrual depressive symptoms, African American race, high body mass index, younger age, social isolation, upsetting life events, and menopausal sleep disturbances.
Although data are inconclusive on whether surgical menopause increases or decreases the risk for developing depression compared with women who transition through menopause naturally, recent studies show an elevated risk of depression in women following hysterectomy with and without oophorectomy.6,7
Menopausal and depressive symptoms can overlap
Midlife depression presents with classic depressive symptoms that commonly occur in combination with menopausal symptoms, including vasomotor symptoms, sleep and sexual disturbances, and weight and energy changes. These menopausal symptoms can complicate, co-occur, and overlap with the clinical presentation of depression.
Conversely, depression may affect an individual's judgment of the degree of bother from menopausal somatic symptoms, thereby further magnifying the effect of symptoms on quality of life. The interrelationship between depressive symptoms and menopausal symptoms may pose a challenge when attempting to parse out contributing etiologies, relative contributions of each etiology, and the potential additive effects.
Diagnosis and treatment options
Diagnosis involves identifying the menopausal stage, assessing for co-existing psychiatric and menopause symptoms, appreciating the psychosocial factors common in midlife, and considering the differential diagnosis. Validated screening instruments can be helpful. Although a menopause-specific mood disorder scale does not yet exist, several general validated screening measures, such as the Patient Health Questionnaire-9, or PHQ-9, can be used for categorical determination of mood disorder diagnoses during the menopause transition.
Antidepressants, cognitive-behavioral therapy, and other psychotherapies are considered first-line treatments for perimenopausal major depressive episodes. Only desvenlafaxine has been studied in large randomized placebo-controlled trials and has proven efficacious for the treatment of MDD in perimenopausal and postmenopausal women.
A number of small open-label studies of other selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), and mirtazapine to treat MDD in perimenopausal and postmenopausal women have demonstrated a positive effect on mood, and several SSRIs and SNRIs also have the added benefit of improving menopause-related symptoms.
In women with a history of MDD, a prior adequate response to a particular antidepressant should guide treatment selection when MDD recurs during the midlife years.
Although estrogen is not approved by the US Food and Drug Administration specifically for the treatment of mood disturbances, some evidence suggests that unopposed estrogen therapy has efficacy similar to that of antidepressant medications in treating depressive disorders in perimenopausal women,8-11 but it is ineffective in treating depressive disorders in postmenopausal women. Estrogen therapy also may augment the clinical response to antidepressants in midlife and older women.12,13 The data on combined HT (estrogen plus progestogen) or for different progestogens in treating depressive disorders in perimenopausal women are lacking and inconclusive.
The findings from this expert review panel demonstrate that women in the perimenopausal transition are at increased risk for depressive symptoms, major depressive episodes, and major depressive disorder. The interrelationship between symptoms of depression and menopause can complicate, co-occur, overlap, and magnify one another. Clinicians treating perimenopausal women with depression that is unresponsive to conventional antidepressant therapy should consider concurrent use of estrogen-based hormone therapy or referring the patient to a clinician comfortable doing so.

- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Matteson KA, Robison K, Jacoby VL. Opportunities for early detection of endometrial cancer in women with postmenopausal bleeding. JAMA Intern Med. 2018;178:1222-1223.
- van Hanegem N, Breijer MC, Khan KS, et al. Diagnostic evaluation of the endometrium in postmenopausal bleeding: an evidence-based approach. Maturitas. 2011;68:155-164.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 734 summary. The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018; 131:945-946.
- Baumgart J, Nilsson K, Evers AS, et al. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013;20:162-168.
- Chou PH, Lin CH, Cheng C, et al. Risk of depressive disorders in women undergoing hysterectomy: a population-based follow-up study. J Psychiatr Res. 2015;68:186-191.
- Wilson L, Pandeya N, Byles J, et al. Hysterectomy and incidence of depressive symptoms in midlife women: the Australian Longitudinal Study on Women's Health. Epidemiol Psychiatr Sci. 2018;27:381-392.
- Schmidt PJ, Nieman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183:414-420.
- Rasgon NL, Altshuler LL, Fairbanks L. Estrogen-replacement therapy for depression. Am J Psychiatry. 2001;158:1738.
- Soares CN, Almeida OP, Joffe H, et al. Efficacy of estradiol for the treatment of major depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529-534.
- Cohen LS, Soares CN, Poitras JR, et al. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: a preliminary report. Am J Psychiatry. 2003;160:1519-1522.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Schneider LS, Small GW, Clary CM. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. Am J Geriatr Psychiatry. 2001;9:393-399.
Among peri- and postmenopausal women, abnormal bleeding, breast cancer, and mood disorders represent prevalent conditions. In this Update, we discuss data from a review that provides quantitative information on the likelihood of finding endometrial cancer among women with postmenopausal bleeding (PMB). We also summarize 2 recent consensus recommendations: One addresses the clinically important but controversial issue of the treatment of genitourinary syndrome of menopause (GSM) in breast cancer survivors, and the other provides guidance on the management of depression in perimenopausal women.
Endometrial cancer is associated with a high prevalence of PMB
Clarke MA, Long BJ, Del Mar Morillo A, et al. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:1210-1222.
Endometrial cancer is the most common gynecologic malignancy and the fourth most common cancer among US women. In recent years, the incidence of and mortality from endometrial cancer have increased.1 Despite the high prevalence of endometrial cancer, population-based screening currently is not recommended.
PMB affects up to 10% of women and can be caused by endometrial atrophy, endometrial polyps, uterine leiomyoma, and malignancy. While it is well known that PMB is a common presenting symptom of endometrial cancer, we do not have good data to guide counseling patients with PMB on the likelihood that endometrial cancer is present. Similarly, estimates are lacking regarding what proportion of women with endometrial cancer will present with PMB.
To address these 2 issues, Clarke and colleagues conducted a comprehensive systematic review and meta-analysis of the prevalence of PMB among women with endometrial cancer (sensitivity) and the risk of endometrial cancer among women with PMB (positive predictive value). The authors included 129 studies--with 34,432 women with PMB and 6,358 with endometrial cancer--in their report.
Cancer prevalence varied with HT use, geographic location
The study findings demonstrated that the prevalence of PMB in women with endometrial cancer was 90% (95% confidence interval [CI], 84%-94%), and there was no significant difference in the occurrence of PMB by cancer stage. The risk of endometrial cancer in women with PMB ranged from 0% to 48%, yielding an overall pooled estimate of 9% (95% CI, 8%-11%). As an editorialist pointed out, the risk of endometrial cancer in women with PMB is similar to that of colorectal cancer in individuals with rectal bleeding (8%) and breast cancer in women with a palpable mass (10%), supporting current guidance that recommends evaluation of women with PMB.2 Evaluating 100 women with PMB to diagnose 9 endometrial cancers does not seem excessive.
Interestingly, among women with PMB, the prevalence of endometrial cancer was significantly higher among women not using hormone therapy (HT) than among users of HT (12% and 7%, respectively). In 7 studies restricted to women with PMB and polyps (n = 2,801), the pooled risk of endometrial cancer was 3% (95% CI, 3%-4%). In an analysis stratified by geographic region, a striking difference was noted in the risk of endometrial cancer among women with PMB in North America (5%), Northern Europe (7%), and in Western Europe (13%). This finding may be explained by regional differences in the approach to evaluating PMB, cultural perceptions of PMB that can affect thresholds to present for care, and differences in risk factors between these populations.
The study had several limitations, including an inability to evaluate the number of years since menopause and the effects of body mass index. Additionally, the study did not address endometrial hyperplasia or endometrial intraepithelial neoplasia.
PMB accounts for two-thirds of all gynecologic visits among perimenopausal and postmenopausal women.3 This study revealed a 9% risk of endometrial cancer in patients experiencing PMB, which supports current practice guidelines to further evaluate and rule out endometrial cancer among all women presenting with PMB4; it also provides reassurance that targeting this high-risk group of women for early detection and prevention strategies will capture most cases of endometrial cancers. However, the relatively low positive predictive value of PMB emphasizes the need for additional triage tests with high specificity to improve management of PMB and minimize unnecessary biopsies in low-risk women.
Treating GSM in breast cancer survivors: New guidance targets QoL and sexuality
Faubion SS, Larkin LC, Stuenkel CA, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendations from The North American Menopause Society and The International Society for the Study of Women's Sexual Health. Menopause. 2018;25:596-608.
Given that there is little evidence addressing the safety of vaginal estrogen, other hormonal therapies, and nonprescription treatments for GSM in breast cancer survivors, many survivors with bothersome GSM symptoms are not appropriately treated.
Continue to: Expert panel creates evidence-based guidance...
Expert panel creates evidence-based guidance
Against this backdrop, The North American Menopause Society and the International Society for the Study of Women's Sexual Health convened a group comprised of menopause specialists (ObGyns, internists, and nurse practitioners), specialists in sexuality, medical oncologists specializing in breast cancer, and a psychologist to create evidence-based interdisciplinary consensus guidelines for enhancing quality of life and sexuality for breast cancer survivors with GSM.
Measures to help enhance quality of life and sexuality
The group's key recommendations for clinicians include:
- Sexual function and quality of life (QoL) should be assessed in all women with or at high risk for breast cancer.
- Management of GSM should be individualized based on shared decision-making involving the patient and her oncologist.
- Initial treatment options include:
—over-the-counter vaginal moisturizers used several times weekly on a regular basis
—lubricants used with intercourse
—vaginal dilator therapy
—pelvic floor physical therapy.
- Low-dose vaginal estrogen therapy may be appropriate for select women who have been treated for breast cancer:
—With use of vaginal estrogen, serum estradiol levels remain in the postmenopausal range.
—Based on limited data, use of vaginal estrogen is associated with a minimal risk for recurrence of breast cancer.
—Because their use is associated with the lowest serum estradiol levels, vaginal tablets, rings, or inserts may be preferable to creams.
—Decisions regarding use of vaginal estrogen in breast cancer survivors should involve the woman's oncologist. Appropriate candidates for off-label use of vaginal estrogen may be survivors:
–who are at relatively low risk for recurrence
–with hormone receptor-negative disease
–using tamoxifen rather than an AI
–who are particularly concerned about quality of life.
—Given that AIs prevent recurrence by lowering estrogen levels, oncologists may be reluctant to consider use of vaginal estrogen in survivors using adjuvant agents.
—With respect to use of vaginal estrogen, oncologists may be more comfortable with use in patients taking tamoxifen.
- Neither intravaginal dehydroepiandrosterone (DHEA; prasterone) nor the oral selective estrogen receptor modulator ospemifene has been studied in breast cancer survivors.
In women with metastatic disease, QoL, comfort, and sexual intimacy are key considerations when weighing potential therapies; optimal choices will vary with probability of long-term survival.
Although more data addressing the safety of vaginal estrogen as well as prasterone and ospemifene in breast cancer survivors clearly are needed, these guidelines should help clinicians who care for breast cancer survivors with GSM.
Framework provided for managing depressive disorders in perimenopausal women
Maki PM, Kornstein SG, Joffe H, et al; Board of Trustees for The North American Menopause Society (NAMS) and the Women and Mood Disorders Task Force of the National Network of Depression Centers. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. Menopause. 2018;25:1069-1085.
Although perimenopausal women are more susceptible to the development of depressive symptoms and major depressive episodes (MDE), there is a lack of consensus regarding how to evaluate and treat depression in women during the menopausal transition and postmenopausal period.
Recently, an expert panel comprised of representatives from The North American Menopause Society and the National Network of Depression Centers Women and Mood Disorders Task Group developed clinical guidelines addressing epidemiology, clinical presentation, therapeutic effects of antidepressants, effects of HT, and efficacy of other therapies. Here we provide a summary of the expert panel's findings and guidelines.
Continue to: Certain factors are associated with higher risk for depression...
Certain factors are associated with higher risk for depression
The perimenopause represents a time of increased risk for depressive symptoms and major depressive disorder (MDD), even in women with no prior history of depression. Several characteristics and health factors are associated with the increased risk during the menopause transition. These include a prior history of MDD, current antidepressant use, anxiety, premenstrual depressive symptoms, African American race, high body mass index, younger age, social isolation, upsetting life events, and menopausal sleep disturbances.
Although data are inconclusive on whether surgical menopause increases or decreases the risk for developing depression compared with women who transition through menopause naturally, recent studies show an elevated risk of depression in women following hysterectomy with and without oophorectomy.6,7
Menopausal and depressive symptoms can overlap
Midlife depression presents with classic depressive symptoms that commonly occur in combination with menopausal symptoms, including vasomotor symptoms, sleep and sexual disturbances, and weight and energy changes. These menopausal symptoms can complicate, co-occur, and overlap with the clinical presentation of depression.
Conversely, depression may affect an individual's judgment of the degree of bother from menopausal somatic symptoms, thereby further magnifying the effect of symptoms on quality of life. The interrelationship between depressive symptoms and menopausal symptoms may pose a challenge when attempting to parse out contributing etiologies, relative contributions of each etiology, and the potential additive effects.
Diagnosis and treatment options
Diagnosis involves identifying the menopausal stage, assessing for co-existing psychiatric and menopause symptoms, appreciating the psychosocial factors common in midlife, and considering the differential diagnosis. Validated screening instruments can be helpful. Although a menopause-specific mood disorder scale does not yet exist, several general validated screening measures, such as the Patient Health Questionnaire-9, or PHQ-9, can be used for categorical determination of mood disorder diagnoses during the menopause transition.
Antidepressants, cognitive-behavioral therapy, and other psychotherapies are considered first-line treatments for perimenopausal major depressive episodes. Only desvenlafaxine has been studied in large randomized placebo-controlled trials and has proven efficacious for the treatment of MDD in perimenopausal and postmenopausal women.
A number of small open-label studies of other selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), and mirtazapine to treat MDD in perimenopausal and postmenopausal women have demonstrated a positive effect on mood, and several SSRIs and SNRIs also have the added benefit of improving menopause-related symptoms.
In women with a history of MDD, a prior adequate response to a particular antidepressant should guide treatment selection when MDD recurs during the midlife years.
Although estrogen is not approved by the US Food and Drug Administration specifically for the treatment of mood disturbances, some evidence suggests that unopposed estrogen therapy has efficacy similar to that of antidepressant medications in treating depressive disorders in perimenopausal women,8-11 but it is ineffective in treating depressive disorders in postmenopausal women. Estrogen therapy also may augment the clinical response to antidepressants in midlife and older women.12,13 The data on combined HT (estrogen plus progestogen) or for different progestogens in treating depressive disorders in perimenopausal women are lacking and inconclusive.
The findings from this expert review panel demonstrate that women in the perimenopausal transition are at increased risk for depressive symptoms, major depressive episodes, and major depressive disorder. The interrelationship between symptoms of depression and menopause can complicate, co-occur, overlap, and magnify one another. Clinicians treating perimenopausal women with depression that is unresponsive to conventional antidepressant therapy should consider concurrent use of estrogen-based hormone therapy or referring the patient to a clinician comfortable doing so.

Among peri- and postmenopausal women, abnormal bleeding, breast cancer, and mood disorders represent prevalent conditions. In this Update, we discuss data from a review that provides quantitative information on the likelihood of finding endometrial cancer among women with postmenopausal bleeding (PMB). We also summarize 2 recent consensus recommendations: One addresses the clinically important but controversial issue of the treatment of genitourinary syndrome of menopause (GSM) in breast cancer survivors, and the other provides guidance on the management of depression in perimenopausal women.
Endometrial cancer is associated with a high prevalence of PMB
Clarke MA, Long BJ, Del Mar Morillo A, et al. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:1210-1222.
Endometrial cancer is the most common gynecologic malignancy and the fourth most common cancer among US women. In recent years, the incidence of and mortality from endometrial cancer have increased.1 Despite the high prevalence of endometrial cancer, population-based screening currently is not recommended.
PMB affects up to 10% of women and can be caused by endometrial atrophy, endometrial polyps, uterine leiomyoma, and malignancy. While it is well known that PMB is a common presenting symptom of endometrial cancer, we do not have good data to guide counseling patients with PMB on the likelihood that endometrial cancer is present. Similarly, estimates are lacking regarding what proportion of women with endometrial cancer will present with PMB.
To address these 2 issues, Clarke and colleagues conducted a comprehensive systematic review and meta-analysis of the prevalence of PMB among women with endometrial cancer (sensitivity) and the risk of endometrial cancer among women with PMB (positive predictive value). The authors included 129 studies--with 34,432 women with PMB and 6,358 with endometrial cancer--in their report.
Cancer prevalence varied with HT use, geographic location
The study findings demonstrated that the prevalence of PMB in women with endometrial cancer was 90% (95% confidence interval [CI], 84%-94%), and there was no significant difference in the occurrence of PMB by cancer stage. The risk of endometrial cancer in women with PMB ranged from 0% to 48%, yielding an overall pooled estimate of 9% (95% CI, 8%-11%). As an editorialist pointed out, the risk of endometrial cancer in women with PMB is similar to that of colorectal cancer in individuals with rectal bleeding (8%) and breast cancer in women with a palpable mass (10%), supporting current guidance that recommends evaluation of women with PMB.2 Evaluating 100 women with PMB to diagnose 9 endometrial cancers does not seem excessive.
Interestingly, among women with PMB, the prevalence of endometrial cancer was significantly higher among women not using hormone therapy (HT) than among users of HT (12% and 7%, respectively). In 7 studies restricted to women with PMB and polyps (n = 2,801), the pooled risk of endometrial cancer was 3% (95% CI, 3%-4%). In an analysis stratified by geographic region, a striking difference was noted in the risk of endometrial cancer among women with PMB in North America (5%), Northern Europe (7%), and in Western Europe (13%). This finding may be explained by regional differences in the approach to evaluating PMB, cultural perceptions of PMB that can affect thresholds to present for care, and differences in risk factors between these populations.
The study had several limitations, including an inability to evaluate the number of years since menopause and the effects of body mass index. Additionally, the study did not address endometrial hyperplasia or endometrial intraepithelial neoplasia.
PMB accounts for two-thirds of all gynecologic visits among perimenopausal and postmenopausal women.3 This study revealed a 9% risk of endometrial cancer in patients experiencing PMB, which supports current practice guidelines to further evaluate and rule out endometrial cancer among all women presenting with PMB4; it also provides reassurance that targeting this high-risk group of women for early detection and prevention strategies will capture most cases of endometrial cancers. However, the relatively low positive predictive value of PMB emphasizes the need for additional triage tests with high specificity to improve management of PMB and minimize unnecessary biopsies in low-risk women.
Treating GSM in breast cancer survivors: New guidance targets QoL and sexuality
Faubion SS, Larkin LC, Stuenkel CA, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendations from The North American Menopause Society and The International Society for the Study of Women's Sexual Health. Menopause. 2018;25:596-608.
Given that there is little evidence addressing the safety of vaginal estrogen, other hormonal therapies, and nonprescription treatments for GSM in breast cancer survivors, many survivors with bothersome GSM symptoms are not appropriately treated.
Continue to: Expert panel creates evidence-based guidance...
Expert panel creates evidence-based guidance
Against this backdrop, The North American Menopause Society and the International Society for the Study of Women's Sexual Health convened a group comprised of menopause specialists (ObGyns, internists, and nurse practitioners), specialists in sexuality, medical oncologists specializing in breast cancer, and a psychologist to create evidence-based interdisciplinary consensus guidelines for enhancing quality of life and sexuality for breast cancer survivors with GSM.
Measures to help enhance quality of life and sexuality
The group's key recommendations for clinicians include:
- Sexual function and quality of life (QoL) should be assessed in all women with or at high risk for breast cancer.
- Management of GSM should be individualized based on shared decision-making involving the patient and her oncologist.
- Initial treatment options include:
—over-the-counter vaginal moisturizers used several times weekly on a regular basis
—lubricants used with intercourse
—vaginal dilator therapy
—pelvic floor physical therapy.
- Low-dose vaginal estrogen therapy may be appropriate for select women who have been treated for breast cancer:
—With use of vaginal estrogen, serum estradiol levels remain in the postmenopausal range.
—Based on limited data, use of vaginal estrogen is associated with a minimal risk for recurrence of breast cancer.
—Because their use is associated with the lowest serum estradiol levels, vaginal tablets, rings, or inserts may be preferable to creams.
—Decisions regarding use of vaginal estrogen in breast cancer survivors should involve the woman's oncologist. Appropriate candidates for off-label use of vaginal estrogen may be survivors:
–who are at relatively low risk for recurrence
–with hormone receptor-negative disease
–using tamoxifen rather than an AI
–who are particularly concerned about quality of life.
—Given that AIs prevent recurrence by lowering estrogen levels, oncologists may be reluctant to consider use of vaginal estrogen in survivors using adjuvant agents.
—With respect to use of vaginal estrogen, oncologists may be more comfortable with use in patients taking tamoxifen.
- Neither intravaginal dehydroepiandrosterone (DHEA; prasterone) nor the oral selective estrogen receptor modulator ospemifene has been studied in breast cancer survivors.
In women with metastatic disease, QoL, comfort, and sexual intimacy are key considerations when weighing potential therapies; optimal choices will vary with probability of long-term survival.
Although more data addressing the safety of vaginal estrogen as well as prasterone and ospemifene in breast cancer survivors clearly are needed, these guidelines should help clinicians who care for breast cancer survivors with GSM.
Framework provided for managing depressive disorders in perimenopausal women
Maki PM, Kornstein SG, Joffe H, et al; Board of Trustees for The North American Menopause Society (NAMS) and the Women and Mood Disorders Task Force of the National Network of Depression Centers. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. Menopause. 2018;25:1069-1085.
Although perimenopausal women are more susceptible to the development of depressive symptoms and major depressive episodes (MDE), there is a lack of consensus regarding how to evaluate and treat depression in women during the menopausal transition and postmenopausal period.
Recently, an expert panel comprised of representatives from The North American Menopause Society and the National Network of Depression Centers Women and Mood Disorders Task Group developed clinical guidelines addressing epidemiology, clinical presentation, therapeutic effects of antidepressants, effects of HT, and efficacy of other therapies. Here we provide a summary of the expert panel's findings and guidelines.
Continue to: Certain factors are associated with higher risk for depression...
Certain factors are associated with higher risk for depression
The perimenopause represents a time of increased risk for depressive symptoms and major depressive disorder (MDD), even in women with no prior history of depression. Several characteristics and health factors are associated with the increased risk during the menopause transition. These include a prior history of MDD, current antidepressant use, anxiety, premenstrual depressive symptoms, African American race, high body mass index, younger age, social isolation, upsetting life events, and menopausal sleep disturbances.
Although data are inconclusive on whether surgical menopause increases or decreases the risk for developing depression compared with women who transition through menopause naturally, recent studies show an elevated risk of depression in women following hysterectomy with and without oophorectomy.6,7
Menopausal and depressive symptoms can overlap
Midlife depression presents with classic depressive symptoms that commonly occur in combination with menopausal symptoms, including vasomotor symptoms, sleep and sexual disturbances, and weight and energy changes. These menopausal symptoms can complicate, co-occur, and overlap with the clinical presentation of depression.
Conversely, depression may affect an individual's judgment of the degree of bother from menopausal somatic symptoms, thereby further magnifying the effect of symptoms on quality of life. The interrelationship between depressive symptoms and menopausal symptoms may pose a challenge when attempting to parse out contributing etiologies, relative contributions of each etiology, and the potential additive effects.
Diagnosis and treatment options
Diagnosis involves identifying the menopausal stage, assessing for co-existing psychiatric and menopause symptoms, appreciating the psychosocial factors common in midlife, and considering the differential diagnosis. Validated screening instruments can be helpful. Although a menopause-specific mood disorder scale does not yet exist, several general validated screening measures, such as the Patient Health Questionnaire-9, or PHQ-9, can be used for categorical determination of mood disorder diagnoses during the menopause transition.
Antidepressants, cognitive-behavioral therapy, and other psychotherapies are considered first-line treatments for perimenopausal major depressive episodes. Only desvenlafaxine has been studied in large randomized placebo-controlled trials and has proven efficacious for the treatment of MDD in perimenopausal and postmenopausal women.
A number of small open-label studies of other selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), and mirtazapine to treat MDD in perimenopausal and postmenopausal women have demonstrated a positive effect on mood, and several SSRIs and SNRIs also have the added benefit of improving menopause-related symptoms.
In women with a history of MDD, a prior adequate response to a particular antidepressant should guide treatment selection when MDD recurs during the midlife years.
Although estrogen is not approved by the US Food and Drug Administration specifically for the treatment of mood disturbances, some evidence suggests that unopposed estrogen therapy has efficacy similar to that of antidepressant medications in treating depressive disorders in perimenopausal women,8-11 but it is ineffective in treating depressive disorders in postmenopausal women. Estrogen therapy also may augment the clinical response to antidepressants in midlife and older women.12,13 The data on combined HT (estrogen plus progestogen) or for different progestogens in treating depressive disorders in perimenopausal women are lacking and inconclusive.
The findings from this expert review panel demonstrate that women in the perimenopausal transition are at increased risk for depressive symptoms, major depressive episodes, and major depressive disorder. The interrelationship between symptoms of depression and menopause can complicate, co-occur, overlap, and magnify one another. Clinicians treating perimenopausal women with depression that is unresponsive to conventional antidepressant therapy should consider concurrent use of estrogen-based hormone therapy or referring the patient to a clinician comfortable doing so.

- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Matteson KA, Robison K, Jacoby VL. Opportunities for early detection of endometrial cancer in women with postmenopausal bleeding. JAMA Intern Med. 2018;178:1222-1223.
- van Hanegem N, Breijer MC, Khan KS, et al. Diagnostic evaluation of the endometrium in postmenopausal bleeding: an evidence-based approach. Maturitas. 2011;68:155-164.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 734 summary. The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018; 131:945-946.
- Baumgart J, Nilsson K, Evers AS, et al. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013;20:162-168.
- Chou PH, Lin CH, Cheng C, et al. Risk of depressive disorders in women undergoing hysterectomy: a population-based follow-up study. J Psychiatr Res. 2015;68:186-191.
- Wilson L, Pandeya N, Byles J, et al. Hysterectomy and incidence of depressive symptoms in midlife women: the Australian Longitudinal Study on Women's Health. Epidemiol Psychiatr Sci. 2018;27:381-392.
- Schmidt PJ, Nieman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183:414-420.
- Rasgon NL, Altshuler LL, Fairbanks L. Estrogen-replacement therapy for depression. Am J Psychiatry. 2001;158:1738.
- Soares CN, Almeida OP, Joffe H, et al. Efficacy of estradiol for the treatment of major depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529-534.
- Cohen LS, Soares CN, Poitras JR, et al. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: a preliminary report. Am J Psychiatry. 2003;160:1519-1522.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Schneider LS, Small GW, Clary CM. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. Am J Geriatr Psychiatry. 2001;9:393-399.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Matteson KA, Robison K, Jacoby VL. Opportunities for early detection of endometrial cancer in women with postmenopausal bleeding. JAMA Intern Med. 2018;178:1222-1223.
- van Hanegem N, Breijer MC, Khan KS, et al. Diagnostic evaluation of the endometrium in postmenopausal bleeding: an evidence-based approach. Maturitas. 2011;68:155-164.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 734 summary. The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018; 131:945-946.
- Baumgart J, Nilsson K, Evers AS, et al. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013;20:162-168.
- Chou PH, Lin CH, Cheng C, et al. Risk of depressive disorders in women undergoing hysterectomy: a population-based follow-up study. J Psychiatr Res. 2015;68:186-191.
- Wilson L, Pandeya N, Byles J, et al. Hysterectomy and incidence of depressive symptoms in midlife women: the Australian Longitudinal Study on Women's Health. Epidemiol Psychiatr Sci. 2018;27:381-392.
- Schmidt PJ, Nieman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183:414-420.
- Rasgon NL, Altshuler LL, Fairbanks L. Estrogen-replacement therapy for depression. Am J Psychiatry. 2001;158:1738.
- Soares CN, Almeida OP, Joffe H, et al. Efficacy of estradiol for the treatment of major depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529-534.
- Cohen LS, Soares CN, Poitras JR, et al. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: a preliminary report. Am J Psychiatry. 2003;160:1519-1522.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Schneider LS, Small GW, Clary CM. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. Am J Geriatr Psychiatry. 2001;9:393-399.