User login
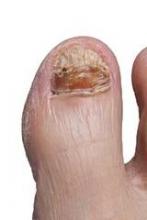
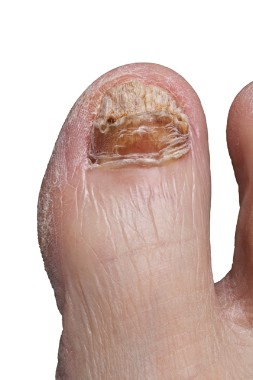
Nail complications of cancer therapies on the rise
NEW YORK – Nail complications are increasingly recognized as a problematic side effect of chemotherapies and biologic therapies for cancer patients.
In some cases these are cosmetic issues, but the side effects really do interfere with activities of daily living for many cancer patients, Dr. Patricia S. Myskowski, an attending dermatologist at Memorial Sloan Kettering Cancer Center, New York, reported at the American Academy of Dermatology summer meeting.
In a list of toxicities provided by the National Cancer Institute in 2006, only three categories of nail toxicities were listed, and these employed relatively vague descriptions, according to Dr. Myskowski. More recent summaries, including a literature review (J. Oncol. Pharm. Pract. 2009;15:143-55), have helped to classify and quantify nail complications as well as provide therapeutic guidance.
Taxanes, and specifically docetaxel, are "the worst offenders" of chemotherapies resulting in nails disorders, she said. More than 80% of patients treated with multiple cycles of docetaxel will develop some nail changes. Most are cosmetic reactions, such as depigmentation, but nearly one-third of patients have reactions that interfere with activities of daily living.
One of the most bothersome of these complications is subungual hematomas with hemopurulent discharge. In patients with nail infection, antibiotics may accelerate drainage and healing, but Dr. Myskowski suggested that this complication can be avoided by keeping the nails trimmed as short as possible.
Short nails are associated with a reduced risk of secondary infection, said Dr. Myskowski, who advised bacterial and fungal cultures when infection is suspected.
Biological therapies, particularly epidermal growth factor receptor (EGFR) inhibitors and tyrosine kinase inhibitors (TKIs), also are associated with a high rate of nail disorders, including paronychia, pyogenic granuloma, and infection. Although nail disorders are far less common than the characteristic rash associated with these agents, she cited one study suggesting a 12% incidence of symptomatic paronychia on EGFR inhibitors. Typically, nail complications emerge about 2 months after treatment is initiated.
To prevent paronychia associated with EGFR inhibitors, Dr. Myskowksi recommended following guidelines issued by the National Comprehensive Cancer Network (J. Natl. Compr. Canc. Netw. 2009;75:S5-S21).
Recommendations include avoiding frequent water immersion as well as contact with harsh chemicals. Applying petroleum jelly to the periungual soft tissue may be protective. In the event of nail infection, augmenting antibiotic therapy with topical therapies such as silver nitrate solution or white vinegar soaks may speed healing. In patients who have reinfections of toenails, disposing of shoes that may harbor bacteria sometimes resolves the problem.
Dr. Myskowski reported no financial disclosures relevant to her presentation.
NEW YORK – Nail complications are increasingly recognized as a problematic side effect of chemotherapies and biologic therapies for cancer patients.
In some cases these are cosmetic issues, but the side effects really do interfere with activities of daily living for many cancer patients, Dr. Patricia S. Myskowski, an attending dermatologist at Memorial Sloan Kettering Cancer Center, New York, reported at the American Academy of Dermatology summer meeting.
In a list of toxicities provided by the National Cancer Institute in 2006, only three categories of nail toxicities were listed, and these employed relatively vague descriptions, according to Dr. Myskowski. More recent summaries, including a literature review (J. Oncol. Pharm. Pract. 2009;15:143-55), have helped to classify and quantify nail complications as well as provide therapeutic guidance.
Taxanes, and specifically docetaxel, are "the worst offenders" of chemotherapies resulting in nails disorders, she said. More than 80% of patients treated with multiple cycles of docetaxel will develop some nail changes. Most are cosmetic reactions, such as depigmentation, but nearly one-third of patients have reactions that interfere with activities of daily living.
One of the most bothersome of these complications is subungual hematomas with hemopurulent discharge. In patients with nail infection, antibiotics may accelerate drainage and healing, but Dr. Myskowski suggested that this complication can be avoided by keeping the nails trimmed as short as possible.
Short nails are associated with a reduced risk of secondary infection, said Dr. Myskowski, who advised bacterial and fungal cultures when infection is suspected.
Biological therapies, particularly epidermal growth factor receptor (EGFR) inhibitors and tyrosine kinase inhibitors (TKIs), also are associated with a high rate of nail disorders, including paronychia, pyogenic granuloma, and infection. Although nail disorders are far less common than the characteristic rash associated with these agents, she cited one study suggesting a 12% incidence of symptomatic paronychia on EGFR inhibitors. Typically, nail complications emerge about 2 months after treatment is initiated.
To prevent paronychia associated with EGFR inhibitors, Dr. Myskowksi recommended following guidelines issued by the National Comprehensive Cancer Network (J. Natl. Compr. Canc. Netw. 2009;75:S5-S21).
Recommendations include avoiding frequent water immersion as well as contact with harsh chemicals. Applying petroleum jelly to the periungual soft tissue may be protective. In the event of nail infection, augmenting antibiotic therapy with topical therapies such as silver nitrate solution or white vinegar soaks may speed healing. In patients who have reinfections of toenails, disposing of shoes that may harbor bacteria sometimes resolves the problem.
Dr. Myskowski reported no financial disclosures relevant to her presentation.
NEW YORK – Nail complications are increasingly recognized as a problematic side effect of chemotherapies and biologic therapies for cancer patients.
In some cases these are cosmetic issues, but the side effects really do interfere with activities of daily living for many cancer patients, Dr. Patricia S. Myskowski, an attending dermatologist at Memorial Sloan Kettering Cancer Center, New York, reported at the American Academy of Dermatology summer meeting.
In a list of toxicities provided by the National Cancer Institute in 2006, only three categories of nail toxicities were listed, and these employed relatively vague descriptions, according to Dr. Myskowski. More recent summaries, including a literature review (J. Oncol. Pharm. Pract. 2009;15:143-55), have helped to classify and quantify nail complications as well as provide therapeutic guidance.
Taxanes, and specifically docetaxel, are "the worst offenders" of chemotherapies resulting in nails disorders, she said. More than 80% of patients treated with multiple cycles of docetaxel will develop some nail changes. Most are cosmetic reactions, such as depigmentation, but nearly one-third of patients have reactions that interfere with activities of daily living.
One of the most bothersome of these complications is subungual hematomas with hemopurulent discharge. In patients with nail infection, antibiotics may accelerate drainage and healing, but Dr. Myskowski suggested that this complication can be avoided by keeping the nails trimmed as short as possible.
Short nails are associated with a reduced risk of secondary infection, said Dr. Myskowski, who advised bacterial and fungal cultures when infection is suspected.
Biological therapies, particularly epidermal growth factor receptor (EGFR) inhibitors and tyrosine kinase inhibitors (TKIs), also are associated with a high rate of nail disorders, including paronychia, pyogenic granuloma, and infection. Although nail disorders are far less common than the characteristic rash associated with these agents, she cited one study suggesting a 12% incidence of symptomatic paronychia on EGFR inhibitors. Typically, nail complications emerge about 2 months after treatment is initiated.
To prevent paronychia associated with EGFR inhibitors, Dr. Myskowksi recommended following guidelines issued by the National Comprehensive Cancer Network (J. Natl. Compr. Canc. Netw. 2009;75:S5-S21).
Recommendations include avoiding frequent water immersion as well as contact with harsh chemicals. Applying petroleum jelly to the periungual soft tissue may be protective. In the event of nail infection, augmenting antibiotic therapy with topical therapies such as silver nitrate solution or white vinegar soaks may speed healing. In patients who have reinfections of toenails, disposing of shoes that may harbor bacteria sometimes resolves the problem.
Dr. Myskowski reported no financial disclosures relevant to her presentation.
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2013
PCR reveals multiple pathogens in onychomyosis
NEW YORK – Diagnosis of fungal nail infection with polymerase chain reaction is demonstrating that the rates of mixed infection, including coinfection with dermatophyte and nondermatophyte molds, are substantially higher than that produced by cultures, according to a series of studies with implications for treatment selection.
The data may explain why some infections persist despite therapy and could lead to more frequent use of PCR as a diagnostic tool, Dr. Aditya K. Gupta reported at the American Academy of Dermatology summer meeting.
PCR is far more sensitive than culture for identification of dermatophytes and nondermatophytes, and, unlike culture, PCR can detect the presence of multiple fungi in the same sample, according to Dr. Gupta, a dermatologist at Sunnybrook and Women’s College Health Sciences Center, Toronto.
Based on studies at his institution, he reported that two or more pathogens are more common than previously appreciated "and this is really important," because it has immediate implications for selecting broader spectrum agents to increase the likelihood of mycologic cure.
"A lot of treatment failures that we attribute to lack of efficacy of the agent may in fact be a reflection of the fact that there is a nondermatophyte mold that is present, evading therapy," Dr. Gupta said.
In a series of 155 patients, PCR was positive for dermatophytes in 44% of patients when culture was positive in 20%. Positive findings were 14% and 7% for PCR and culture, respectively, for nondermatophytes.
PCR is now being used routinely at Dr. Gupta’s institution not only because of its ability to detect mixed infections but also because it is about twice as sensitive as culture for detecting nail fungi.
Dr. Gupta predicted wider use of PCR in onychomycosis because of its greater sensitivity, noting however, that cost may be an obstacle. The potential difficulty of obtaining third-party reimbursement for PCR, which is substantially more expensive than a culture is, was raised in the discussion period by several dermatologists who were impressed with these results. At Dr. Gupta’s center, cost has not been an issue because PCR is being performed as part of a research initiative, but the greater diagnostic accuracy may be relevant to a cost-benefit analysis that includes an opportunity to increase the proportion of patients initiated on an optimal therapy for the underlying pathogen, Dr. Gupta said.
Dr. Gupta reported that he has financial relationships with Valeant, NuvoLase, Bristol-Myers Squibb, Novartis, and Janssen. The study was not commercially sponsored.
NEW YORK – Diagnosis of fungal nail infection with polymerase chain reaction is demonstrating that the rates of mixed infection, including coinfection with dermatophyte and nondermatophyte molds, are substantially higher than that produced by cultures, according to a series of studies with implications for treatment selection.
The data may explain why some infections persist despite therapy and could lead to more frequent use of PCR as a diagnostic tool, Dr. Aditya K. Gupta reported at the American Academy of Dermatology summer meeting.
PCR is far more sensitive than culture for identification of dermatophytes and nondermatophytes, and, unlike culture, PCR can detect the presence of multiple fungi in the same sample, according to Dr. Gupta, a dermatologist at Sunnybrook and Women’s College Health Sciences Center, Toronto.
Based on studies at his institution, he reported that two or more pathogens are more common than previously appreciated "and this is really important," because it has immediate implications for selecting broader spectrum agents to increase the likelihood of mycologic cure.
"A lot of treatment failures that we attribute to lack of efficacy of the agent may in fact be a reflection of the fact that there is a nondermatophyte mold that is present, evading therapy," Dr. Gupta said.
In a series of 155 patients, PCR was positive for dermatophytes in 44% of patients when culture was positive in 20%. Positive findings were 14% and 7% for PCR and culture, respectively, for nondermatophytes.
PCR is now being used routinely at Dr. Gupta’s institution not only because of its ability to detect mixed infections but also because it is about twice as sensitive as culture for detecting nail fungi.
Dr. Gupta predicted wider use of PCR in onychomycosis because of its greater sensitivity, noting however, that cost may be an obstacle. The potential difficulty of obtaining third-party reimbursement for PCR, which is substantially more expensive than a culture is, was raised in the discussion period by several dermatologists who were impressed with these results. At Dr. Gupta’s center, cost has not been an issue because PCR is being performed as part of a research initiative, but the greater diagnostic accuracy may be relevant to a cost-benefit analysis that includes an opportunity to increase the proportion of patients initiated on an optimal therapy for the underlying pathogen, Dr. Gupta said.
Dr. Gupta reported that he has financial relationships with Valeant, NuvoLase, Bristol-Myers Squibb, Novartis, and Janssen. The study was not commercially sponsored.
NEW YORK – Diagnosis of fungal nail infection with polymerase chain reaction is demonstrating that the rates of mixed infection, including coinfection with dermatophyte and nondermatophyte molds, are substantially higher than that produced by cultures, according to a series of studies with implications for treatment selection.
The data may explain why some infections persist despite therapy and could lead to more frequent use of PCR as a diagnostic tool, Dr. Aditya K. Gupta reported at the American Academy of Dermatology summer meeting.
PCR is far more sensitive than culture for identification of dermatophytes and nondermatophytes, and, unlike culture, PCR can detect the presence of multiple fungi in the same sample, according to Dr. Gupta, a dermatologist at Sunnybrook and Women’s College Health Sciences Center, Toronto.
Based on studies at his institution, he reported that two or more pathogens are more common than previously appreciated "and this is really important," because it has immediate implications for selecting broader spectrum agents to increase the likelihood of mycologic cure.
"A lot of treatment failures that we attribute to lack of efficacy of the agent may in fact be a reflection of the fact that there is a nondermatophyte mold that is present, evading therapy," Dr. Gupta said.
In a series of 155 patients, PCR was positive for dermatophytes in 44% of patients when culture was positive in 20%. Positive findings were 14% and 7% for PCR and culture, respectively, for nondermatophytes.
PCR is now being used routinely at Dr. Gupta’s institution not only because of its ability to detect mixed infections but also because it is about twice as sensitive as culture for detecting nail fungi.
Dr. Gupta predicted wider use of PCR in onychomycosis because of its greater sensitivity, noting however, that cost may be an obstacle. The potential difficulty of obtaining third-party reimbursement for PCR, which is substantially more expensive than a culture is, was raised in the discussion period by several dermatologists who were impressed with these results. At Dr. Gupta’s center, cost has not been an issue because PCR is being performed as part of a research initiative, but the greater diagnostic accuracy may be relevant to a cost-benefit analysis that includes an opportunity to increase the proportion of patients initiated on an optimal therapy for the underlying pathogen, Dr. Gupta said.
Dr. Gupta reported that he has financial relationships with Valeant, NuvoLase, Bristol-Myers Squibb, Novartis, and Janssen. The study was not commercially sponsored.
AT THE AAD SUMMER ACADEMY 2013
Major finding: PCR is twice as effective as culture for the identification of pathogens causing onychomycosis.
Data source: Single-center study comparing diagnostic techniques in patient series
Disclosures: Dr. Gupta reported that he has financial relationships with Valeant, NuvoLase, Bristol-Myers Squibb, Novartis, and Janssen. The study was not commercially sponsored.
Fungal Diseases: How We Recognize and Treat Them
Watch Dr. Phoebe Rich present an insightful overview of several fungal diseases and available topical and oral medications. This webcast is sponsored by Merz Pharmaceuticals, LLC.
Watch Dr. Phoebe Rich present an insightful overview of several fungal diseases and available topical and oral medications. This webcast is sponsored by Merz Pharmaceuticals, LLC.
Watch Dr. Phoebe Rich present an insightful overview of several fungal diseases and available topical and oral medications. This webcast is sponsored by Merz Pharmaceuticals, LLC.
FDA issues strong warning about oral ketoconazole
Ketoconazole tablets should not be used as a first-line treatment for fungal infections because treatment has been associated with an increased risk of adrenal insufficiency, potentially fatal hepatotoxicity, and drug interactions, the Food and Drug Administration has announced.
Marketed as Nizoral, oral ketoconazole is no longer indicated for the treatment of Candida and dermatophyte infections and "should be used only for the treatment of certain life-threatening mycoses when the potential benefits outweigh the risks and alternative therapeutic options are not available or tolerated," according to the MedWatch safety alert issued on July 26.
In addition, oral ketoconazole should not be used to treat fungal infections of the skin and nails, and is only indicated for the treatment of blastomycosis, coccidioidomycosis, histoplasmosis, chromomycosis, and paracoccidioidomycosis "in patients in whom other treatments have failed or who are intolerant to other therapies," according to the label, which has been modified to reflect these risks and recommendations.
There is now a Medication Guide that will be provided to patients with each filled prescription of oral ketoconazole, explaining the risks.
Because oral ketoconazole has been associated with hepatoxicity that can result in liver transplantation or death, it is now contraindicated in patients with acute or chronic liver disease. The label also now recommends that patients be assessed and monitored for liver toxicity. Monitoring of adrenal function also is now recommended in patients who take the oral formulation of the drug and have adrenal problems or "are under prolonged periods of stress such as those who have had a recent major surgery or who are under intensive care in the hospital."
In addition, coadministration of ketoconazole – a potent inhibitor of the cytochrome P450 3A4 isoenzyme (CYP3A4) – with certain drugs is either restricted or contraindicated because of the increase in drug concentrations and increased risk of QT prolongation and other serious reactions. Contraindicated drugs include dofetilide, quinidine, pimozide, and cisapride.
The FDA changes are based on risk-benefit analyses of data that include reports made to the FDA’s Adverse Events Reporting System.
On July 26, the European Medicines Agency’s Committee on Medicinal Products for Human Use (CHMP) announced that it has concluded that the risk of hepatoxicity with oral ketoconazole products was greater than the benefits in treating fungal infections and recommended that these products no longer be marketed in the European Union.
Creams, shampoos, and other topical ketoconazole formulations have not been associated with these problems, according to the FDA.
The updated label is available here. Serious adverse events associated with ketoconazole should be reported to the FDA at 800-332-1088 or MedWatch.
Ketoconazole tablets should not be used as a first-line treatment for fungal infections because treatment has been associated with an increased risk of adrenal insufficiency, potentially fatal hepatotoxicity, and drug interactions, the Food and Drug Administration has announced.
Marketed as Nizoral, oral ketoconazole is no longer indicated for the treatment of Candida and dermatophyte infections and "should be used only for the treatment of certain life-threatening mycoses when the potential benefits outweigh the risks and alternative therapeutic options are not available or tolerated," according to the MedWatch safety alert issued on July 26.
In addition, oral ketoconazole should not be used to treat fungal infections of the skin and nails, and is only indicated for the treatment of blastomycosis, coccidioidomycosis, histoplasmosis, chromomycosis, and paracoccidioidomycosis "in patients in whom other treatments have failed or who are intolerant to other therapies," according to the label, which has been modified to reflect these risks and recommendations.
There is now a Medication Guide that will be provided to patients with each filled prescription of oral ketoconazole, explaining the risks.
Because oral ketoconazole has been associated with hepatoxicity that can result in liver transplantation or death, it is now contraindicated in patients with acute or chronic liver disease. The label also now recommends that patients be assessed and monitored for liver toxicity. Monitoring of adrenal function also is now recommended in patients who take the oral formulation of the drug and have adrenal problems or "are under prolonged periods of stress such as those who have had a recent major surgery or who are under intensive care in the hospital."
In addition, coadministration of ketoconazole – a potent inhibitor of the cytochrome P450 3A4 isoenzyme (CYP3A4) – with certain drugs is either restricted or contraindicated because of the increase in drug concentrations and increased risk of QT prolongation and other serious reactions. Contraindicated drugs include dofetilide, quinidine, pimozide, and cisapride.
The FDA changes are based on risk-benefit analyses of data that include reports made to the FDA’s Adverse Events Reporting System.
On July 26, the European Medicines Agency’s Committee on Medicinal Products for Human Use (CHMP) announced that it has concluded that the risk of hepatoxicity with oral ketoconazole products was greater than the benefits in treating fungal infections and recommended that these products no longer be marketed in the European Union.
Creams, shampoos, and other topical ketoconazole formulations have not been associated with these problems, according to the FDA.
The updated label is available here. Serious adverse events associated with ketoconazole should be reported to the FDA at 800-332-1088 or MedWatch.
Ketoconazole tablets should not be used as a first-line treatment for fungal infections because treatment has been associated with an increased risk of adrenal insufficiency, potentially fatal hepatotoxicity, and drug interactions, the Food and Drug Administration has announced.
Marketed as Nizoral, oral ketoconazole is no longer indicated for the treatment of Candida and dermatophyte infections and "should be used only for the treatment of certain life-threatening mycoses when the potential benefits outweigh the risks and alternative therapeutic options are not available or tolerated," according to the MedWatch safety alert issued on July 26.
In addition, oral ketoconazole should not be used to treat fungal infections of the skin and nails, and is only indicated for the treatment of blastomycosis, coccidioidomycosis, histoplasmosis, chromomycosis, and paracoccidioidomycosis "in patients in whom other treatments have failed or who are intolerant to other therapies," according to the label, which has been modified to reflect these risks and recommendations.
There is now a Medication Guide that will be provided to patients with each filled prescription of oral ketoconazole, explaining the risks.
Because oral ketoconazole has been associated with hepatoxicity that can result in liver transplantation or death, it is now contraindicated in patients with acute or chronic liver disease. The label also now recommends that patients be assessed and monitored for liver toxicity. Monitoring of adrenal function also is now recommended in patients who take the oral formulation of the drug and have adrenal problems or "are under prolonged periods of stress such as those who have had a recent major surgery or who are under intensive care in the hospital."
In addition, coadministration of ketoconazole – a potent inhibitor of the cytochrome P450 3A4 isoenzyme (CYP3A4) – with certain drugs is either restricted or contraindicated because of the increase in drug concentrations and increased risk of QT prolongation and other serious reactions. Contraindicated drugs include dofetilide, quinidine, pimozide, and cisapride.
The FDA changes are based on risk-benefit analyses of data that include reports made to the FDA’s Adverse Events Reporting System.
On July 26, the European Medicines Agency’s Committee on Medicinal Products for Human Use (CHMP) announced that it has concluded that the risk of hepatoxicity with oral ketoconazole products was greater than the benefits in treating fungal infections and recommended that these products no longer be marketed in the European Union.
Creams, shampoos, and other topical ketoconazole formulations have not been associated with these problems, according to the FDA.
The updated label is available here. Serious adverse events associated with ketoconazole should be reported to the FDA at 800-332-1088 or MedWatch.
Make your scalp surgery seamless
WASHINGTON – To make scalp surgery seamless, remember what makes the scalp unique: hair and tension, Dr. Mark Welch said.
The scalp is "a bloodless plain," said Dr. Welch of the Skin Cancer Surgery Center in Bethesda, Md.
Also, the scalp is painless, so it’s possible to go beyond the field of anesthesia, he noted at the Atlantic Dermatological Conference.
To keep hair out of the surgical field, Dr. Welch uses a razor to shave the immediate area, and then tapes down the surrounding hair. "The surrounding hair will find its way into your surgery site and wound," he said. Alternatively, moistening the hair with saline or water can keep it away from the surgical field. Tubular bandaging also can be used to hold the hair away from the surgery site.
The tension on the scalp presents a unique surgical challenge, said Dr. Welch. "The scalp skin is holding the weight of the body; there’s lots of tension up there."
Dr. Welch said he starts with a temporary pulley stitch to decrease the distance across the wound, which allows easier placement of subcutaneous stitches. "Then the pulley stitch can come out," he said.
"One technique I use a lot is preplaced subcutaneous sutures," Dr. Welch said. "You leave yourself a tail long enough to tie, then go posterior to the first subcutaneous stitch, and then go back and tie the first stitch, then the second, then go to the external stitches."
Dr. Welch said he uses a running stitch for external stitches. "On the last external running stitch, go out and come back in on the same side, and angle it back slightly." This technique allows for a more perpendicular closing to the wound edge, he explained.
Dr. Welch cited one case of a large defect in a patient with skin cancer on the scalp. He opted for a pulley stitch with gel foam in the center, and some silver nitrate. The wound was essentially healed in 8 weeks, even without the defect being completely closed.
For scalp dressings, Dr. Welch said he often prefers a Xeroform gauze bolster, which he sews in place, "so we don’t have to use any tape." When the stitches come out after a week, flexible collodion can be used. "It hardens, and over the next 3 or 4 weeks of shampooing, it flakes off."
When using wraps, Dr. Welch recommends combining vertical and horizontal wraps to create tension and promote healing.
He said he had no financial conflicts to disclose.
WASHINGTON – To make scalp surgery seamless, remember what makes the scalp unique: hair and tension, Dr. Mark Welch said.
The scalp is "a bloodless plain," said Dr. Welch of the Skin Cancer Surgery Center in Bethesda, Md.
Also, the scalp is painless, so it’s possible to go beyond the field of anesthesia, he noted at the Atlantic Dermatological Conference.
To keep hair out of the surgical field, Dr. Welch uses a razor to shave the immediate area, and then tapes down the surrounding hair. "The surrounding hair will find its way into your surgery site and wound," he said. Alternatively, moistening the hair with saline or water can keep it away from the surgical field. Tubular bandaging also can be used to hold the hair away from the surgery site.
The tension on the scalp presents a unique surgical challenge, said Dr. Welch. "The scalp skin is holding the weight of the body; there’s lots of tension up there."
Dr. Welch said he starts with a temporary pulley stitch to decrease the distance across the wound, which allows easier placement of subcutaneous stitches. "Then the pulley stitch can come out," he said.
"One technique I use a lot is preplaced subcutaneous sutures," Dr. Welch said. "You leave yourself a tail long enough to tie, then go posterior to the first subcutaneous stitch, and then go back and tie the first stitch, then the second, then go to the external stitches."
Dr. Welch said he uses a running stitch for external stitches. "On the last external running stitch, go out and come back in on the same side, and angle it back slightly." This technique allows for a more perpendicular closing to the wound edge, he explained.
Dr. Welch cited one case of a large defect in a patient with skin cancer on the scalp. He opted for a pulley stitch with gel foam in the center, and some silver nitrate. The wound was essentially healed in 8 weeks, even without the defect being completely closed.
For scalp dressings, Dr. Welch said he often prefers a Xeroform gauze bolster, which he sews in place, "so we don’t have to use any tape." When the stitches come out after a week, flexible collodion can be used. "It hardens, and over the next 3 or 4 weeks of shampooing, it flakes off."
When using wraps, Dr. Welch recommends combining vertical and horizontal wraps to create tension and promote healing.
He said he had no financial conflicts to disclose.
WASHINGTON – To make scalp surgery seamless, remember what makes the scalp unique: hair and tension, Dr. Mark Welch said.
The scalp is "a bloodless plain," said Dr. Welch of the Skin Cancer Surgery Center in Bethesda, Md.
Also, the scalp is painless, so it’s possible to go beyond the field of anesthesia, he noted at the Atlantic Dermatological Conference.
To keep hair out of the surgical field, Dr. Welch uses a razor to shave the immediate area, and then tapes down the surrounding hair. "The surrounding hair will find its way into your surgery site and wound," he said. Alternatively, moistening the hair with saline or water can keep it away from the surgical field. Tubular bandaging also can be used to hold the hair away from the surgery site.
The tension on the scalp presents a unique surgical challenge, said Dr. Welch. "The scalp skin is holding the weight of the body; there’s lots of tension up there."
Dr. Welch said he starts with a temporary pulley stitch to decrease the distance across the wound, which allows easier placement of subcutaneous stitches. "Then the pulley stitch can come out," he said.
"One technique I use a lot is preplaced subcutaneous sutures," Dr. Welch said. "You leave yourself a tail long enough to tie, then go posterior to the first subcutaneous stitch, and then go back and tie the first stitch, then the second, then go to the external stitches."
Dr. Welch said he uses a running stitch for external stitches. "On the last external running stitch, go out and come back in on the same side, and angle it back slightly." This technique allows for a more perpendicular closing to the wound edge, he explained.
Dr. Welch cited one case of a large defect in a patient with skin cancer on the scalp. He opted for a pulley stitch with gel foam in the center, and some silver nitrate. The wound was essentially healed in 8 weeks, even without the defect being completely closed.
For scalp dressings, Dr. Welch said he often prefers a Xeroform gauze bolster, which he sews in place, "so we don’t have to use any tape." When the stitches come out after a week, flexible collodion can be used. "It hardens, and over the next 3 or 4 weeks of shampooing, it flakes off."
When using wraps, Dr. Welch recommends combining vertical and horizontal wraps to create tension and promote healing.
He said he had no financial conflicts to disclose.
EXPERT ANALYSIS FROM THE ATLANTIC DERMATOLOGICAL CONFERENCE
Nail surgery made simple
WASHINGTON – There’s a lot of anxiety about nail surgery, particularly nail biopsies, for both physicians and patients, according to Dr. Maral K. Skelsey.
The goals of successful nail surgery are threefold: avoid complications, reduce patient pain and anxiety, and optimize pathologic diagnosis, said Dr. Skelsey of Georgetown University Medical Center in Washington.
Because nail surgery is often performed to obtain a clinical diagnosis, a good specimen is needed to allow the dermatopathologist to make a diagnosis, she noted at the Atlantic Dermatological Conference.
Approach preoperative assessment for nail surgery as any other surgery, said Dr. Skelsey. Take a full history, including information about vascular impairment, arterial disease, latex allergies, and a history of anticoagulant use. "We don’t stop anticoagulants, usually," Dr. Skelsey noted, but she does assess the prothrombin time (PT/INR) within 1 week.
Also, don’t underestimate the value of an x-ray. "One thing I have found physicians don’t do often" is to x-ray to check for bony changes and any anatomic abnormalities, she said.
To help optimize nail surgery outcomes, Dr. Skelsey recommended the following preoperative instructions for patients: Remove nail polish, scrub the area with povidone-iodine twice daily for 3 days prior to surgery, bring open-toed shoes (for toenail surgeries), arrange for a ride home, and plan to elevate the hand or foot as much as possible for the first 48 hours following the procedure.
Also, it "will help reduce morbidity if you tell your patients ahead of time to reduce their exercise and activity" immediately after the procedure, she said.
The right tools "will make your nail surgery much more successful," Dr. Skelsey said. Her essential tools: a nail splitter, nail nipper, and nail elevator.
Allow the patient to recline with goggles and ear phones to reduce anxiety during the procedure, she said.
For anesthesia, "I always use a 30-gauge needle, injecting very slowly," she said. She prefers a wing block, injecting slowly at a 45-degree angle towards the bone. This injection also acts as a volumetric tourniquet.
When obtaining the specimen during nail surgery, "visualize the location of the pathology by reflecting the proximal nail fold with a suture of skin hook and full or partial nail avulsion," said Dr. Skelsey.
"You can use a punch biopsy for longitudinal melanonychia less than 3 mm," she noted, but for anything more than 3 mm, a transverse excision or shave biopsy with a tangential excision is needed.
After the biopsy, Dr. Skelsey said that she applies an absorbable gelatin sponge saturated in aluminum chloride.
"What’s very important is giving your dermatopathologist a good specimen," she said. Don’t forget to ink the margins and orient the specimen. "You don’t want to go through all this trouble and have someone tell you there is nothing there," she added. She also recommended using separate, labelled formalin jars for the nail plate, bed, and matrix.
Dr. Skelsey said that she had no financial conflicts to disclose.
WASHINGTON – There’s a lot of anxiety about nail surgery, particularly nail biopsies, for both physicians and patients, according to Dr. Maral K. Skelsey.
The goals of successful nail surgery are threefold: avoid complications, reduce patient pain and anxiety, and optimize pathologic diagnosis, said Dr. Skelsey of Georgetown University Medical Center in Washington.
Because nail surgery is often performed to obtain a clinical diagnosis, a good specimen is needed to allow the dermatopathologist to make a diagnosis, she noted at the Atlantic Dermatological Conference.
Approach preoperative assessment for nail surgery as any other surgery, said Dr. Skelsey. Take a full history, including information about vascular impairment, arterial disease, latex allergies, and a history of anticoagulant use. "We don’t stop anticoagulants, usually," Dr. Skelsey noted, but she does assess the prothrombin time (PT/INR) within 1 week.
Also, don’t underestimate the value of an x-ray. "One thing I have found physicians don’t do often" is to x-ray to check for bony changes and any anatomic abnormalities, she said.
To help optimize nail surgery outcomes, Dr. Skelsey recommended the following preoperative instructions for patients: Remove nail polish, scrub the area with povidone-iodine twice daily for 3 days prior to surgery, bring open-toed shoes (for toenail surgeries), arrange for a ride home, and plan to elevate the hand or foot as much as possible for the first 48 hours following the procedure.
Also, it "will help reduce morbidity if you tell your patients ahead of time to reduce their exercise and activity" immediately after the procedure, she said.
The right tools "will make your nail surgery much more successful," Dr. Skelsey said. Her essential tools: a nail splitter, nail nipper, and nail elevator.
Allow the patient to recline with goggles and ear phones to reduce anxiety during the procedure, she said.
For anesthesia, "I always use a 30-gauge needle, injecting very slowly," she said. She prefers a wing block, injecting slowly at a 45-degree angle towards the bone. This injection also acts as a volumetric tourniquet.
When obtaining the specimen during nail surgery, "visualize the location of the pathology by reflecting the proximal nail fold with a suture of skin hook and full or partial nail avulsion," said Dr. Skelsey.
"You can use a punch biopsy for longitudinal melanonychia less than 3 mm," she noted, but for anything more than 3 mm, a transverse excision or shave biopsy with a tangential excision is needed.
After the biopsy, Dr. Skelsey said that she applies an absorbable gelatin sponge saturated in aluminum chloride.
"What’s very important is giving your dermatopathologist a good specimen," she said. Don’t forget to ink the margins and orient the specimen. "You don’t want to go through all this trouble and have someone tell you there is nothing there," she added. She also recommended using separate, labelled formalin jars for the nail plate, bed, and matrix.
Dr. Skelsey said that she had no financial conflicts to disclose.
WASHINGTON – There’s a lot of anxiety about nail surgery, particularly nail biopsies, for both physicians and patients, according to Dr. Maral K. Skelsey.
The goals of successful nail surgery are threefold: avoid complications, reduce patient pain and anxiety, and optimize pathologic diagnosis, said Dr. Skelsey of Georgetown University Medical Center in Washington.
Because nail surgery is often performed to obtain a clinical diagnosis, a good specimen is needed to allow the dermatopathologist to make a diagnosis, she noted at the Atlantic Dermatological Conference.
Approach preoperative assessment for nail surgery as any other surgery, said Dr. Skelsey. Take a full history, including information about vascular impairment, arterial disease, latex allergies, and a history of anticoagulant use. "We don’t stop anticoagulants, usually," Dr. Skelsey noted, but she does assess the prothrombin time (PT/INR) within 1 week.
Also, don’t underestimate the value of an x-ray. "One thing I have found physicians don’t do often" is to x-ray to check for bony changes and any anatomic abnormalities, she said.
To help optimize nail surgery outcomes, Dr. Skelsey recommended the following preoperative instructions for patients: Remove nail polish, scrub the area with povidone-iodine twice daily for 3 days prior to surgery, bring open-toed shoes (for toenail surgeries), arrange for a ride home, and plan to elevate the hand or foot as much as possible for the first 48 hours following the procedure.
Also, it "will help reduce morbidity if you tell your patients ahead of time to reduce their exercise and activity" immediately after the procedure, she said.
The right tools "will make your nail surgery much more successful," Dr. Skelsey said. Her essential tools: a nail splitter, nail nipper, and nail elevator.
Allow the patient to recline with goggles and ear phones to reduce anxiety during the procedure, she said.
For anesthesia, "I always use a 30-gauge needle, injecting very slowly," she said. She prefers a wing block, injecting slowly at a 45-degree angle towards the bone. This injection also acts as a volumetric tourniquet.
When obtaining the specimen during nail surgery, "visualize the location of the pathology by reflecting the proximal nail fold with a suture of skin hook and full or partial nail avulsion," said Dr. Skelsey.
"You can use a punch biopsy for longitudinal melanonychia less than 3 mm," she noted, but for anything more than 3 mm, a transverse excision or shave biopsy with a tangential excision is needed.
After the biopsy, Dr. Skelsey said that she applies an absorbable gelatin sponge saturated in aluminum chloride.
"What’s very important is giving your dermatopathologist a good specimen," she said. Don’t forget to ink the margins and orient the specimen. "You don’t want to go through all this trouble and have someone tell you there is nothing there," she added. She also recommended using separate, labelled formalin jars for the nail plate, bed, and matrix.
Dr. Skelsey said that she had no financial conflicts to disclose.
EXPERT ANALYSIS FROM THE ATLANTIC DERMATOLOGICAL CONFERENCE