User login
PET/CT Detects Early Recurrence of Head and Neck Cancer
PHOENIX – Routine use of positron emission tomography/computed tomography scans can detect locoregional recurrences of squamous cell carcinoma of the head and neck before they became clinically apparent, according to a retrospective chart review of 234 patients who had been treated with chemoradiation between 2006 and 2010.
The finding suggests that routine use of positron emission tomography/computed tomography (PET/CT) may improve the outcome of salvage therapy, said Dr. Yasir Rudha, who reported the study at the Multidisciplinary Head and Neck Symposium sponsored by the American Society for Radiation Oncology.
PET/CT was associated with a high false positive rate, which should be considered when ordering radiological exams and biopsies, but a negative posttherapy PET scan appears to be an excellent predictor of freedom from future locoregional recurrence, said Dr. Rudha of St. John Hospital/Van Elslander Cancer Center, Grosse Pointe Woods, Mich.
The technology is relatively new, and its use for routine follow-up in patients with head and neck cancer is still controversial, he acknowledged. "Only a few publications have reported the value of PET examination at a fixed time interval after the end of treatment," he said. "PET scan is often ordered in our hospital as a routine surveillance tool following successful completion of treatment."
The review of charts for all 234 patients identified 45 who had achieved clinical no-evidence-of-disease status at the time of posttreatment imaging. In this group, PET/CT scanning at 6-9 weeks identified 15 patients with abnormalities that required further evaluation. Of those, eight patients (53%) were proven to have malignancies based on biopsy findings: six showed occult persistent disease at the primary site, and two were diagnosed with regional lymph node recurrence and colon cancer, respectively.
In the remaining seven cases, imaging findings were shown to represent false positive results with unnecessary work-up and/or biopsy evaluation. All patients who had negative PET/CT scans remained free from locoregional relapse at the time of last follow-up.
Thus, Dr. Rudha said the true positive rate for routine PET/CT surveillance in head and neck cancer patients was estimated as 8/15, or 53%, and the false positive rate as 7/15, or 46%.
"With malignancies found in 53% of abnormal scans in this study, our research proves that PET/CT scans are valuable as routine follow-up and as a surveillance method for head and neck cancer patients ... However, since the rate of false positives was 46%, caution should be shown when ordering biopsies after abnormal scans to prevent excessive unnecessary biopsies," he said.
During a press briefing, Dr. Rudha said that the 46% false positive rate was lower than what he and his colleagues expected. "Actually we expected the false positive ratio to be about 90%," he said.
In an interview, he said that at his institution patients with positive PET/CT scans at 6-9 weeks postsurgery are followed in a variety of ways, depending primarily on the PET standard uptake volume (SUV). If low, the patient might undergo another PET scan at about 3 months. But if SUV is high, the patient would likely be referred for another test such as magnetic resonance imaging, ultrasound, or biopsy at the site of recurrence.
However, if the postsurgery PET/CT scan is negative, "according to this research, it gives a great indication that the patient is free from disease and the treatment is successful."
Dr. Rudha and his coauthors stated that they had no disclosures.
PET/CT is being evaluated in a variety of different situations. We’re still looking at programs that are combining PET/CT imaging with functional DCI/MRI imaging in part to help oncologists find the tumor volume and define how to plan our treatments in regard to what the target volumes are both on the ipsilateral and contralateral side of the neck.

|
|
In the posttreatment setting, it’s been absolutely critical for us in terms of determining who should go on to further evaluation or who could be watched carefully. For instance, a University of Maryland group has shown quite nicely that in patients who have a clinical complete response and a complete response by PET imaging, almost all those patients do not need a neck dissection (Head Neck 2010;32:46-52). ... That’s a significant savings in health care costs, whereas in the past, almost all of those patients 10-15 years ago would have gone on to a neck dissection.
The current study also offers important data. I believe 6 weeks is too early. We recommend a minimum of 8 weeks, preferably 12 weeks posttreatment, to allow inflammation to subside. That may help reduce the false positive rate. I think use of PET/CT continues to evolve, and hopefully additional functional imaging studies will help us nail down which patients need to go on to further treatment or biopsy.
Dr. David Raben is professor of radiation oncology at the University of Colorado, Denver. He had no disclosures
PET/CT is being evaluated in a variety of different situations. We’re still looking at programs that are combining PET/CT imaging with functional DCI/MRI imaging in part to help oncologists find the tumor volume and define how to plan our treatments in regard to what the target volumes are both on the ipsilateral and contralateral side of the neck.

|
|
In the posttreatment setting, it’s been absolutely critical for us in terms of determining who should go on to further evaluation or who could be watched carefully. For instance, a University of Maryland group has shown quite nicely that in patients who have a clinical complete response and a complete response by PET imaging, almost all those patients do not need a neck dissection (Head Neck 2010;32:46-52). ... That’s a significant savings in health care costs, whereas in the past, almost all of those patients 10-15 years ago would have gone on to a neck dissection.
The current study also offers important data. I believe 6 weeks is too early. We recommend a minimum of 8 weeks, preferably 12 weeks posttreatment, to allow inflammation to subside. That may help reduce the false positive rate. I think use of PET/CT continues to evolve, and hopefully additional functional imaging studies will help us nail down which patients need to go on to further treatment or biopsy.
Dr. David Raben is professor of radiation oncology at the University of Colorado, Denver. He had no disclosures
PET/CT is being evaluated in a variety of different situations. We’re still looking at programs that are combining PET/CT imaging with functional DCI/MRI imaging in part to help oncologists find the tumor volume and define how to plan our treatments in regard to what the target volumes are both on the ipsilateral and contralateral side of the neck.

|
|
In the posttreatment setting, it’s been absolutely critical for us in terms of determining who should go on to further evaluation or who could be watched carefully. For instance, a University of Maryland group has shown quite nicely that in patients who have a clinical complete response and a complete response by PET imaging, almost all those patients do not need a neck dissection (Head Neck 2010;32:46-52). ... That’s a significant savings in health care costs, whereas in the past, almost all of those patients 10-15 years ago would have gone on to a neck dissection.
The current study also offers important data. I believe 6 weeks is too early. We recommend a minimum of 8 weeks, preferably 12 weeks posttreatment, to allow inflammation to subside. That may help reduce the false positive rate. I think use of PET/CT continues to evolve, and hopefully additional functional imaging studies will help us nail down which patients need to go on to further treatment or biopsy.
Dr. David Raben is professor of radiation oncology at the University of Colorado, Denver. He had no disclosures
PHOENIX – Routine use of positron emission tomography/computed tomography scans can detect locoregional recurrences of squamous cell carcinoma of the head and neck before they became clinically apparent, according to a retrospective chart review of 234 patients who had been treated with chemoradiation between 2006 and 2010.
The finding suggests that routine use of positron emission tomography/computed tomography (PET/CT) may improve the outcome of salvage therapy, said Dr. Yasir Rudha, who reported the study at the Multidisciplinary Head and Neck Symposium sponsored by the American Society for Radiation Oncology.
PET/CT was associated with a high false positive rate, which should be considered when ordering radiological exams and biopsies, but a negative posttherapy PET scan appears to be an excellent predictor of freedom from future locoregional recurrence, said Dr. Rudha of St. John Hospital/Van Elslander Cancer Center, Grosse Pointe Woods, Mich.
The technology is relatively new, and its use for routine follow-up in patients with head and neck cancer is still controversial, he acknowledged. "Only a few publications have reported the value of PET examination at a fixed time interval after the end of treatment," he said. "PET scan is often ordered in our hospital as a routine surveillance tool following successful completion of treatment."
The review of charts for all 234 patients identified 45 who had achieved clinical no-evidence-of-disease status at the time of posttreatment imaging. In this group, PET/CT scanning at 6-9 weeks identified 15 patients with abnormalities that required further evaluation. Of those, eight patients (53%) were proven to have malignancies based on biopsy findings: six showed occult persistent disease at the primary site, and two were diagnosed with regional lymph node recurrence and colon cancer, respectively.
In the remaining seven cases, imaging findings were shown to represent false positive results with unnecessary work-up and/or biopsy evaluation. All patients who had negative PET/CT scans remained free from locoregional relapse at the time of last follow-up.
Thus, Dr. Rudha said the true positive rate for routine PET/CT surveillance in head and neck cancer patients was estimated as 8/15, or 53%, and the false positive rate as 7/15, or 46%.
"With malignancies found in 53% of abnormal scans in this study, our research proves that PET/CT scans are valuable as routine follow-up and as a surveillance method for head and neck cancer patients ... However, since the rate of false positives was 46%, caution should be shown when ordering biopsies after abnormal scans to prevent excessive unnecessary biopsies," he said.
During a press briefing, Dr. Rudha said that the 46% false positive rate was lower than what he and his colleagues expected. "Actually we expected the false positive ratio to be about 90%," he said.
In an interview, he said that at his institution patients with positive PET/CT scans at 6-9 weeks postsurgery are followed in a variety of ways, depending primarily on the PET standard uptake volume (SUV). If low, the patient might undergo another PET scan at about 3 months. But if SUV is high, the patient would likely be referred for another test such as magnetic resonance imaging, ultrasound, or biopsy at the site of recurrence.
However, if the postsurgery PET/CT scan is negative, "according to this research, it gives a great indication that the patient is free from disease and the treatment is successful."
Dr. Rudha and his coauthors stated that they had no disclosures.
PHOENIX – Routine use of positron emission tomography/computed tomography scans can detect locoregional recurrences of squamous cell carcinoma of the head and neck before they became clinically apparent, according to a retrospective chart review of 234 patients who had been treated with chemoradiation between 2006 and 2010.
The finding suggests that routine use of positron emission tomography/computed tomography (PET/CT) may improve the outcome of salvage therapy, said Dr. Yasir Rudha, who reported the study at the Multidisciplinary Head and Neck Symposium sponsored by the American Society for Radiation Oncology.
PET/CT was associated with a high false positive rate, which should be considered when ordering radiological exams and biopsies, but a negative posttherapy PET scan appears to be an excellent predictor of freedom from future locoregional recurrence, said Dr. Rudha of St. John Hospital/Van Elslander Cancer Center, Grosse Pointe Woods, Mich.
The technology is relatively new, and its use for routine follow-up in patients with head and neck cancer is still controversial, he acknowledged. "Only a few publications have reported the value of PET examination at a fixed time interval after the end of treatment," he said. "PET scan is often ordered in our hospital as a routine surveillance tool following successful completion of treatment."
The review of charts for all 234 patients identified 45 who had achieved clinical no-evidence-of-disease status at the time of posttreatment imaging. In this group, PET/CT scanning at 6-9 weeks identified 15 patients with abnormalities that required further evaluation. Of those, eight patients (53%) were proven to have malignancies based on biopsy findings: six showed occult persistent disease at the primary site, and two were diagnosed with regional lymph node recurrence and colon cancer, respectively.
In the remaining seven cases, imaging findings were shown to represent false positive results with unnecessary work-up and/or biopsy evaluation. All patients who had negative PET/CT scans remained free from locoregional relapse at the time of last follow-up.
Thus, Dr. Rudha said the true positive rate for routine PET/CT surveillance in head and neck cancer patients was estimated as 8/15, or 53%, and the false positive rate as 7/15, or 46%.
"With malignancies found in 53% of abnormal scans in this study, our research proves that PET/CT scans are valuable as routine follow-up and as a surveillance method for head and neck cancer patients ... However, since the rate of false positives was 46%, caution should be shown when ordering biopsies after abnormal scans to prevent excessive unnecessary biopsies," he said.
During a press briefing, Dr. Rudha said that the 46% false positive rate was lower than what he and his colleagues expected. "Actually we expected the false positive ratio to be about 90%," he said.
In an interview, he said that at his institution patients with positive PET/CT scans at 6-9 weeks postsurgery are followed in a variety of ways, depending primarily on the PET standard uptake volume (SUV). If low, the patient might undergo another PET scan at about 3 months. But if SUV is high, the patient would likely be referred for another test such as magnetic resonance imaging, ultrasound, or biopsy at the site of recurrence.
However, if the postsurgery PET/CT scan is negative, "according to this research, it gives a great indication that the patient is free from disease and the treatment is successful."
Dr. Rudha and his coauthors stated that they had no disclosures.
FROM A HEAD AND NECK CANCER SYMPOSIUM SPONSORED BY THE AMERICAN SOCIETY FOR RADIATION ONCOLOGY
Major Finding: The true positive rate for routine PET/CT surveillance in head and neck cancer patients was estimated as 8/15, or 53%, and the false positive rate as 7/15, or 46%.
Data Source: The data come from 45 patients with no evidence of disease after treatment with chemoradiation between 2006 and 2010.
Disclosures: Dr. Rudha and his coauthors reported having no disclosures.
Erlotinib Dose Doubled for Smokers With Head/Neck Cancer
PHOENIX – Giving smokers a higher, short-course dose of erlotinib before definitive surgery for squamous cell carcinoma of the head and neck resulted in favorable responses for the first patients evaluated in a small pilot study.
Investigators gave 300 mg of erlotinib (Tarceva) to smokers daily and 150 mg daily to nonsmokers who had a waiting period of more than 14 days before scheduled surgery for head and neck cancer. Seven of the 10 patients evaluated so far had partial responses and 3 had stable disease, according to a poster presented at a head and neck cancer symposium sponsored by the American Society for Radiation Oncology.
The study was based on recent data in non–small cell lung cancer (NSCLC) patients showing that smokers metabolize erlotinib, an epidermal growth factor receptor (EGFR) inhibitor, twice as quickly as do nonsmokers (J. Clin. Oncol. 2009;27:1220-6), said lead author Dr. Mercedes Porosnicu of Wake Forest Baptist Medical Center in Winston Salem, N.C. That study established the maximum tolerated dose of erlotinib at 300 mg daily in NSCLC patients who smoke.
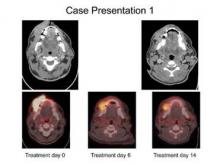
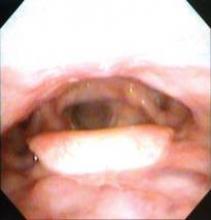
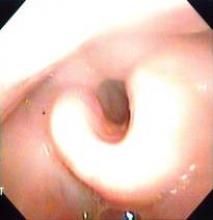
Dr. Poroniscu’s presentation included the case study of a smoker with a very large oral cavity tumor protruding through his lips. He was described as being in significant pain and unable to eat or chew. The first CT scan showed a tumor of at least 8 cm and there was "significant metabolic activity" on PET scan.
"At 6 days of erlotinib treatment, his tumor was obviously smaller and he could chew, eat, and talk. Metabolic activity on PET scan dropped to 44% compared to initial tumor metabolic activity," Dr. Porosnicu said. "At the end of 14 days’ treatment, his tumor was at least 20% smaller, and he had gained 5 pounds. His surgery wasn’t delayed, and the only treatment-related toxicity was a minimal skin rash."
A total of 12 patients have been treated to date, for an average of 18.2 days, she reported. Nine were smokers and three were nonsmokers. All patients, smokers and nonsmokers, tolerated the erlotinib dose well with no serious adverse events and no delays in the scheduled time of surgical intervention. There were no grade 3 or 4 toxicities.
Of 10 evaluable patients (including 8 smokers who received 300 mg), 7 (including 5 smokers) showed a partial response, as defined by at least a 20% reduction in maximum tumor diameter. The other three patients (all smokers) showed stable disease. Two of the 12 treated patients received shorter duration treatment but nonetheless displayed good responses.
Interestingly, all four treated female patients (including one smoker) had good responses, independent of the erlotinib dose received, Dr. Porosnicu said.
Early 18[F]-FDG PET scans taken 4-6 days after the start of neoadjuvant erlotinib showed a decrease in metabolic activity of 2% in maximum standardized uptake value (SUVmax) to 98.75% in patients with stable disease and a decrease to 48.06% in patients with partial response.
"Early changes in the PET scan uptake should be further investigated as a marker predictive of response to EGFR inhibition. This pilot trial will continue to enroll patients," Dr. Porosnicu said.
Erlotinib is approved for indications in non–small cell lung cancer and pancreatic cancer. Head and neck cancer would be an off-label use.
Dr. Porosnicu disclosed that she received financial support for this study from Astellas Pharma.
PHOENIX – Giving smokers a higher, short-course dose of erlotinib before definitive surgery for squamous cell carcinoma of the head and neck resulted in favorable responses for the first patients evaluated in a small pilot study.
Investigators gave 300 mg of erlotinib (Tarceva) to smokers daily and 150 mg daily to nonsmokers who had a waiting period of more than 14 days before scheduled surgery for head and neck cancer. Seven of the 10 patients evaluated so far had partial responses and 3 had stable disease, according to a poster presented at a head and neck cancer symposium sponsored by the American Society for Radiation Oncology.
The study was based on recent data in non–small cell lung cancer (NSCLC) patients showing that smokers metabolize erlotinib, an epidermal growth factor receptor (EGFR) inhibitor, twice as quickly as do nonsmokers (J. Clin. Oncol. 2009;27:1220-6), said lead author Dr. Mercedes Porosnicu of Wake Forest Baptist Medical Center in Winston Salem, N.C. That study established the maximum tolerated dose of erlotinib at 300 mg daily in NSCLC patients who smoke.
Dr. Poroniscu’s presentation included the case study of a smoker with a very large oral cavity tumor protruding through his lips. He was described as being in significant pain and unable to eat or chew. The first CT scan showed a tumor of at least 8 cm and there was "significant metabolic activity" on PET scan.
"At 6 days of erlotinib treatment, his tumor was obviously smaller and he could chew, eat, and talk. Metabolic activity on PET scan dropped to 44% compared to initial tumor metabolic activity," Dr. Porosnicu said. "At the end of 14 days’ treatment, his tumor was at least 20% smaller, and he had gained 5 pounds. His surgery wasn’t delayed, and the only treatment-related toxicity was a minimal skin rash."
A total of 12 patients have been treated to date, for an average of 18.2 days, she reported. Nine were smokers and three were nonsmokers. All patients, smokers and nonsmokers, tolerated the erlotinib dose well with no serious adverse events and no delays in the scheduled time of surgical intervention. There were no grade 3 or 4 toxicities.
Of 10 evaluable patients (including 8 smokers who received 300 mg), 7 (including 5 smokers) showed a partial response, as defined by at least a 20% reduction in maximum tumor diameter. The other three patients (all smokers) showed stable disease. Two of the 12 treated patients received shorter duration treatment but nonetheless displayed good responses.
Interestingly, all four treated female patients (including one smoker) had good responses, independent of the erlotinib dose received, Dr. Porosnicu said.
Early 18[F]-FDG PET scans taken 4-6 days after the start of neoadjuvant erlotinib showed a decrease in metabolic activity of 2% in maximum standardized uptake value (SUVmax) to 98.75% in patients with stable disease and a decrease to 48.06% in patients with partial response.
"Early changes in the PET scan uptake should be further investigated as a marker predictive of response to EGFR inhibition. This pilot trial will continue to enroll patients," Dr. Porosnicu said.
Erlotinib is approved for indications in non–small cell lung cancer and pancreatic cancer. Head and neck cancer would be an off-label use.
Dr. Porosnicu disclosed that she received financial support for this study from Astellas Pharma.
PHOENIX – Giving smokers a higher, short-course dose of erlotinib before definitive surgery for squamous cell carcinoma of the head and neck resulted in favorable responses for the first patients evaluated in a small pilot study.
Investigators gave 300 mg of erlotinib (Tarceva) to smokers daily and 150 mg daily to nonsmokers who had a waiting period of more than 14 days before scheduled surgery for head and neck cancer. Seven of the 10 patients evaluated so far had partial responses and 3 had stable disease, according to a poster presented at a head and neck cancer symposium sponsored by the American Society for Radiation Oncology.
The study was based on recent data in non–small cell lung cancer (NSCLC) patients showing that smokers metabolize erlotinib, an epidermal growth factor receptor (EGFR) inhibitor, twice as quickly as do nonsmokers (J. Clin. Oncol. 2009;27:1220-6), said lead author Dr. Mercedes Porosnicu of Wake Forest Baptist Medical Center in Winston Salem, N.C. That study established the maximum tolerated dose of erlotinib at 300 mg daily in NSCLC patients who smoke.
Dr. Poroniscu’s presentation included the case study of a smoker with a very large oral cavity tumor protruding through his lips. He was described as being in significant pain and unable to eat or chew. The first CT scan showed a tumor of at least 8 cm and there was "significant metabolic activity" on PET scan.
"At 6 days of erlotinib treatment, his tumor was obviously smaller and he could chew, eat, and talk. Metabolic activity on PET scan dropped to 44% compared to initial tumor metabolic activity," Dr. Porosnicu said. "At the end of 14 days’ treatment, his tumor was at least 20% smaller, and he had gained 5 pounds. His surgery wasn’t delayed, and the only treatment-related toxicity was a minimal skin rash."
A total of 12 patients have been treated to date, for an average of 18.2 days, she reported. Nine were smokers and three were nonsmokers. All patients, smokers and nonsmokers, tolerated the erlotinib dose well with no serious adverse events and no delays in the scheduled time of surgical intervention. There were no grade 3 or 4 toxicities.
Of 10 evaluable patients (including 8 smokers who received 300 mg), 7 (including 5 smokers) showed a partial response, as defined by at least a 20% reduction in maximum tumor diameter. The other three patients (all smokers) showed stable disease. Two of the 12 treated patients received shorter duration treatment but nonetheless displayed good responses.
Interestingly, all four treated female patients (including one smoker) had good responses, independent of the erlotinib dose received, Dr. Porosnicu said.
Early 18[F]-FDG PET scans taken 4-6 days after the start of neoadjuvant erlotinib showed a decrease in metabolic activity of 2% in maximum standardized uptake value (SUVmax) to 98.75% in patients with stable disease and a decrease to 48.06% in patients with partial response.
"Early changes in the PET scan uptake should be further investigated as a marker predictive of response to EGFR inhibition. This pilot trial will continue to enroll patients," Dr. Porosnicu said.
Erlotinib is approved for indications in non–small cell lung cancer and pancreatic cancer. Head and neck cancer would be an off-label use.
Dr. Porosnicu disclosed that she received financial support for this study from Astellas Pharma.
FROM A HEAD AND NECK CANCER SYMPOSIUM SPONSORED BY THE AMERICAN SOCIETY FOR RADIATION ONCOLOGY
Major Finding: Of 10 evaluable head and neck cancer patients, 7 (including 5 smokers) showed a partial response, as defined by at least a 20% reduction in maximum tumor diameter.
Data Source: In this small, ongoing pilot study smokers received 300 mg and nonsmokers 150 mg daily of neoadjuvant erlotinib.
Disclosures: Dr. Porosnicu disclosed that she received financial support for this study from Astellas Pharma.
Head/Neck Cancer Treatments Less Effective in HIV Patients
PHOENIX – Definitive radiation therapy with or without chemotherapy was less tolerated and less effective in HIV-positive patients with head and neck squamous cell carcinoma than in HIV-negative patients in a single-institution retrospective study of 71 HIV-positive patients.
"Head and neck squamous cell carcinoma with coexisting HIV remains a challenging clinical problem. ... Due to the advances in highly active antiretroviral therapy (HAART) – which prolongs HIV patients’ survival – the likelihood to develop HIV-related malignancy increases. It is of paramount importance to establish better-tolerated treatment strategies and regimens to improve tolerance, toxicity, and outcomes in this growing patient population," said Dr. Waleed Mourad, a radiation oncologist at Beth Israel Medical Center, New York.
The 71 HIV-positive patients with HNSCC were treated January 1997 through 2010. They had a median age of 34 years at the time of HIV diagnosis (range 25-50 years) with a median 11 years’ duration of seropositivity (6-20 years). Their median age at the time of radiation therapy was 51 years (32-72 years). All but one patient had squamous cell carcinoma, with the other having submandibular salivary duct carcinoma. That patient was treated with definitive surgery and received adjuvant radiation therapy without chemotherapy, Dr. Mourad noted.
Approximately one-third of the patients had cancer of the oropharynx (32%) and larynx (35%), and another 13% had cancer of the oral cavity. Other cancers among the patients included those located in the hypopharynx, nasopharynx, occult primary, and nasal cavity. American Joint Committee on Cancer 7th edition stages I-II, III and IVa/b were 22%, 27%, 51% respectively.
All patients were treated definitively with radiation therapy, with or without chemotherapy (cisplatin, carboplatin, or cetuximab). A total of 50 patients (70%) were on HAART during treatment, with a median CD4 count of 290 (range, 203-1,142). A median dose of 70 Gy (66-70) was delivered to the gross disease; high-risk neck 60-63 Gy; low-risk neck; and lateral retropharyngeal nodes 54 Gy. All fractions were given at the rate of 1.8-2 Gy/fraction. The median duration of treatment was 52 (49-64) days. A total of 12 patients (17%) underwent planned neck dissection for N3 disease.
In all, local control was achieved in 69% (49) and locoregional failure occurred in 31% (22). By site, local control rates were 70% (16) for the oropharynx, 76% (19) for the larynx, 78% (7) for the oral cavity, 0% (of 5) for hypopharynx, 67% (2) for the nasopharynx, 100% (3) for occult primary, 50% (1) for the nasal cavity, and 100% (1) for the submandibular duct.
Thus, locoregional failure rates ranged from 100% (all five patients with hypopharyngeal cancer) to 0 (for the three patients with occult primary cancer and the one with submandibular duct cancer). Seven patients developed second primary cancers, Dr. Mourad reported.
After a median follow up of 47 months (7-140), there were no fatalities related to radiation or chemotherapy. Treatment breaks in excess of 10, 7, and 5 days occurred in 6%, 13%, and 14% of patients, respectively. Acute dysphagia and odynophagia grades 1, 2, and 3 occurred in 31%, 52%, and 17%, respectively. All of the patients experienced dysgeusia, dysphagia, and xerostomia of grades 1-3. Acute desquamation of the skin of grades 1, 2, and 3 occurred in 66%, 20%, and 14%.
One patient was hospitalized for grade 4 mucositis, dysphagia, and fever. Another developed osteoradionecrosis during concurrent chemoradiotherapy. Late dysphagia of grades 1-4 occurred in 46%, 28%, 15%, and 11%. Xerostomia of grades 1-3 occurred in 45%, 32%, and 23%, respectively, he said.
At 4 years, locoregional control was 69% and overall survival 55%. In an interview, Dr. Mourad noted that at his institution, the comparable rates for non-HIV patients were 85%-90% and 75%-85%, respectively.
The median CD4 count prior to chemotherapy was 370, and viral load was undetectable. The CD4 count dropped as chemoradiotherapy progressed to a nadir at 11 weeks, at which point it began to rise again and viral load decreased.
There was a statistically significant relationship between locoregional control and the duration of radiation therapy (P less than .001), and positive trends with weight loss of greater than 10% and absence of second malignancy. However, multivariate analysis did not show any statistically significant relationship because of the relatively small sample size with diverse subsites, Dr. Mourad noted.
"We believe that our data provide important information regarding treatment outcomes for newly diagnosed head and neck cancer with HIV. This is particularly important given the growing population of patients who live with HIV long term because of effective antiretroviral maintenance treatments," Dr. Mourad said in an interview.
Dr. Mourad and his nine coauthors declared that they had no financial disclosures.
PHOENIX – Definitive radiation therapy with or without chemotherapy was less tolerated and less effective in HIV-positive patients with head and neck squamous cell carcinoma than in HIV-negative patients in a single-institution retrospective study of 71 HIV-positive patients.
"Head and neck squamous cell carcinoma with coexisting HIV remains a challenging clinical problem. ... Due to the advances in highly active antiretroviral therapy (HAART) – which prolongs HIV patients’ survival – the likelihood to develop HIV-related malignancy increases. It is of paramount importance to establish better-tolerated treatment strategies and regimens to improve tolerance, toxicity, and outcomes in this growing patient population," said Dr. Waleed Mourad, a radiation oncologist at Beth Israel Medical Center, New York.
The 71 HIV-positive patients with HNSCC were treated January 1997 through 2010. They had a median age of 34 years at the time of HIV diagnosis (range 25-50 years) with a median 11 years’ duration of seropositivity (6-20 years). Their median age at the time of radiation therapy was 51 years (32-72 years). All but one patient had squamous cell carcinoma, with the other having submandibular salivary duct carcinoma. That patient was treated with definitive surgery and received adjuvant radiation therapy without chemotherapy, Dr. Mourad noted.
Approximately one-third of the patients had cancer of the oropharynx (32%) and larynx (35%), and another 13% had cancer of the oral cavity. Other cancers among the patients included those located in the hypopharynx, nasopharynx, occult primary, and nasal cavity. American Joint Committee on Cancer 7th edition stages I-II, III and IVa/b were 22%, 27%, 51% respectively.
All patients were treated definitively with radiation therapy, with or without chemotherapy (cisplatin, carboplatin, or cetuximab). A total of 50 patients (70%) were on HAART during treatment, with a median CD4 count of 290 (range, 203-1,142). A median dose of 70 Gy (66-70) was delivered to the gross disease; high-risk neck 60-63 Gy; low-risk neck; and lateral retropharyngeal nodes 54 Gy. All fractions were given at the rate of 1.8-2 Gy/fraction. The median duration of treatment was 52 (49-64) days. A total of 12 patients (17%) underwent planned neck dissection for N3 disease.
In all, local control was achieved in 69% (49) and locoregional failure occurred in 31% (22). By site, local control rates were 70% (16) for the oropharynx, 76% (19) for the larynx, 78% (7) for the oral cavity, 0% (of 5) for hypopharynx, 67% (2) for the nasopharynx, 100% (3) for occult primary, 50% (1) for the nasal cavity, and 100% (1) for the submandibular duct.
Thus, locoregional failure rates ranged from 100% (all five patients with hypopharyngeal cancer) to 0 (for the three patients with occult primary cancer and the one with submandibular duct cancer). Seven patients developed second primary cancers, Dr. Mourad reported.
After a median follow up of 47 months (7-140), there were no fatalities related to radiation or chemotherapy. Treatment breaks in excess of 10, 7, and 5 days occurred in 6%, 13%, and 14% of patients, respectively. Acute dysphagia and odynophagia grades 1, 2, and 3 occurred in 31%, 52%, and 17%, respectively. All of the patients experienced dysgeusia, dysphagia, and xerostomia of grades 1-3. Acute desquamation of the skin of grades 1, 2, and 3 occurred in 66%, 20%, and 14%.
One patient was hospitalized for grade 4 mucositis, dysphagia, and fever. Another developed osteoradionecrosis during concurrent chemoradiotherapy. Late dysphagia of grades 1-4 occurred in 46%, 28%, 15%, and 11%. Xerostomia of grades 1-3 occurred in 45%, 32%, and 23%, respectively, he said.
At 4 years, locoregional control was 69% and overall survival 55%. In an interview, Dr. Mourad noted that at his institution, the comparable rates for non-HIV patients were 85%-90% and 75%-85%, respectively.
The median CD4 count prior to chemotherapy was 370, and viral load was undetectable. The CD4 count dropped as chemoradiotherapy progressed to a nadir at 11 weeks, at which point it began to rise again and viral load decreased.
There was a statistically significant relationship between locoregional control and the duration of radiation therapy (P less than .001), and positive trends with weight loss of greater than 10% and absence of second malignancy. However, multivariate analysis did not show any statistically significant relationship because of the relatively small sample size with diverse subsites, Dr. Mourad noted.
"We believe that our data provide important information regarding treatment outcomes for newly diagnosed head and neck cancer with HIV. This is particularly important given the growing population of patients who live with HIV long term because of effective antiretroviral maintenance treatments," Dr. Mourad said in an interview.
Dr. Mourad and his nine coauthors declared that they had no financial disclosures.
PHOENIX – Definitive radiation therapy with or without chemotherapy was less tolerated and less effective in HIV-positive patients with head and neck squamous cell carcinoma than in HIV-negative patients in a single-institution retrospective study of 71 HIV-positive patients.
"Head and neck squamous cell carcinoma with coexisting HIV remains a challenging clinical problem. ... Due to the advances in highly active antiretroviral therapy (HAART) – which prolongs HIV patients’ survival – the likelihood to develop HIV-related malignancy increases. It is of paramount importance to establish better-tolerated treatment strategies and regimens to improve tolerance, toxicity, and outcomes in this growing patient population," said Dr. Waleed Mourad, a radiation oncologist at Beth Israel Medical Center, New York.
The 71 HIV-positive patients with HNSCC were treated January 1997 through 2010. They had a median age of 34 years at the time of HIV diagnosis (range 25-50 years) with a median 11 years’ duration of seropositivity (6-20 years). Their median age at the time of radiation therapy was 51 years (32-72 years). All but one patient had squamous cell carcinoma, with the other having submandibular salivary duct carcinoma. That patient was treated with definitive surgery and received adjuvant radiation therapy without chemotherapy, Dr. Mourad noted.
Approximately one-third of the patients had cancer of the oropharynx (32%) and larynx (35%), and another 13% had cancer of the oral cavity. Other cancers among the patients included those located in the hypopharynx, nasopharynx, occult primary, and nasal cavity. American Joint Committee on Cancer 7th edition stages I-II, III and IVa/b were 22%, 27%, 51% respectively.
All patients were treated definitively with radiation therapy, with or without chemotherapy (cisplatin, carboplatin, or cetuximab). A total of 50 patients (70%) were on HAART during treatment, with a median CD4 count of 290 (range, 203-1,142). A median dose of 70 Gy (66-70) was delivered to the gross disease; high-risk neck 60-63 Gy; low-risk neck; and lateral retropharyngeal nodes 54 Gy. All fractions were given at the rate of 1.8-2 Gy/fraction. The median duration of treatment was 52 (49-64) days. A total of 12 patients (17%) underwent planned neck dissection for N3 disease.
In all, local control was achieved in 69% (49) and locoregional failure occurred in 31% (22). By site, local control rates were 70% (16) for the oropharynx, 76% (19) for the larynx, 78% (7) for the oral cavity, 0% (of 5) for hypopharynx, 67% (2) for the nasopharynx, 100% (3) for occult primary, 50% (1) for the nasal cavity, and 100% (1) for the submandibular duct.
Thus, locoregional failure rates ranged from 100% (all five patients with hypopharyngeal cancer) to 0 (for the three patients with occult primary cancer and the one with submandibular duct cancer). Seven patients developed second primary cancers, Dr. Mourad reported.
After a median follow up of 47 months (7-140), there were no fatalities related to radiation or chemotherapy. Treatment breaks in excess of 10, 7, and 5 days occurred in 6%, 13%, and 14% of patients, respectively. Acute dysphagia and odynophagia grades 1, 2, and 3 occurred in 31%, 52%, and 17%, respectively. All of the patients experienced dysgeusia, dysphagia, and xerostomia of grades 1-3. Acute desquamation of the skin of grades 1, 2, and 3 occurred in 66%, 20%, and 14%.
One patient was hospitalized for grade 4 mucositis, dysphagia, and fever. Another developed osteoradionecrosis during concurrent chemoradiotherapy. Late dysphagia of grades 1-4 occurred in 46%, 28%, 15%, and 11%. Xerostomia of grades 1-3 occurred in 45%, 32%, and 23%, respectively, he said.
At 4 years, locoregional control was 69% and overall survival 55%. In an interview, Dr. Mourad noted that at his institution, the comparable rates for non-HIV patients were 85%-90% and 75%-85%, respectively.
The median CD4 count prior to chemotherapy was 370, and viral load was undetectable. The CD4 count dropped as chemoradiotherapy progressed to a nadir at 11 weeks, at which point it began to rise again and viral load decreased.
There was a statistically significant relationship between locoregional control and the duration of radiation therapy (P less than .001), and positive trends with weight loss of greater than 10% and absence of second malignancy. However, multivariate analysis did not show any statistically significant relationship because of the relatively small sample size with diverse subsites, Dr. Mourad noted.
"We believe that our data provide important information regarding treatment outcomes for newly diagnosed head and neck cancer with HIV. This is particularly important given the growing population of patients who live with HIV long term because of effective antiretroviral maintenance treatments," Dr. Mourad said in an interview.
Dr. Mourad and his nine coauthors declared that they had no financial disclosures.
FROM A HEAD AND NECK CANCER SYMPOSIUM BY THE AMERICAN SOCIETY FOR RADIATION ONCOLOGY
Major Finding: At 4 years, locoregional control was 69% and overall survival 55%.
Data Source: The findings come from a single-institution retrospective study of 71 HIV-positive patients with head and neck squamous cell carcinoma.
Disclosures: Dr. Mourad and his nine coauthors declared that they had no financial disclosures.
Oral HPV Infection More Prevalent in Men Than Women
The prevalence of oral human papillomavirus is nearly three times higher in men than in women, according to data from more than 5,000 individuals in the United States.
The findings were simultaneously published online in JAMA and presented at the Multidisciplinary Head and Neck Cancer Symposium in Phoenix on Jan. 26 (JAMA 2012;307: [doi: 10.1001/jama.2012/101]).
Oral HPV infection causes a subset of oropharyngeal squamous cell carcinoma (OSCC) that has increased in several countries, including the United States, over the past 3 decades, said Dr. Maura L. Gillison of the Ohio State University, Columbus, and her colleagues. "HPV has been directly implicated as the underlying cause," they noted. However, the epidemiology of OSCC has not been well studied.
The researchers conducted a cross-sectional study of HPV infection as part the National Health and Nutrition Examination Survey (NHANES) for 2009-2010. The study population included 5,579 men and women aged 14-69 years who were tested for HPV at mobile centers.
Overall, the prevalence of any HPV infection was 6.9%, and the prevalence of HPV type 16 (the type associated with OSCC) was 1%. The prevalence of any HPV infection was significantly higher in men than in women (10.1% vs. 3.6%, P less than .001). The peak prevalence of oral HPV occurred in people aged 30-34 years (7.3%) and 60-64 years (11.4%).
Infection in either gender was significantly more common in those with a history of any sexual contact (7.5%), compared with those who had no history of sexual contact (0.9%). The risk of infection also increased significantly as the number of sex partners for any type of sex increased. "One in five individuals with more than 20 lifetime sexual partners was infected," the researchers said.
Univariate associations between risk of oral HPV infection and alcohol and marijuana use did not remain significant in a multivariate analysis. However, cigarette smoking remained independently associated with an increased risk of oral HPV infection. The risk significantly increased with the number of cigarettes smoked daily for both men and women, but this trend was stronger in women.
In a multivariate analysis, age, gender, number of sexual partners, and number of cigarettes smoked daily remained independently associated with an increased risk of oral HPV infection.
"Our data provide evidence that oral HPV infection is predominantly sexually transmitted," the researchers said. Although HPV-positive OSCC has been associated with oral sex in particular, this study could not associate infection with a particular sexual behavior, they added.
The findings were limited by the emphasis on Alpha-papillomaviruses only, which likely underestimates the prevalence of oral HPV infection, the researchers said. But the findings suggest that vaccine trials to test the efficacy of the current HPV vaccine against oral HPV may be warranted. "Such trials could inform ongoing discussions regarding the benefits of HPV vaccination for males, given the higher prevalence of oral HPV infection demonstrated here as well as higher incidence of HPV-positive OSCC among men," they said.
The Multidisciplinary Head and Neck Cancer Symposium is sponsored by the American Society for Radiation Oncology.
Study sponsors included the Ohio State University Comprehensive Cancer Center and the National Cancer Institute. Dr. Gillison has served as a consultant to study sponsor Merck and to GlaxoSmithKline.
The incidence of HPV-positive oropharyngeal cancers increased 225% – from 0.8/100,000 to 2.8/100,000 – between 1988 and 2004, according to a presentation by Dr. Gillison at the annual meeting of the American Society of Clinical Oncology in June 2011. Click here for a report on the earlier findings.
"Human papillomavirus–positive oropharyngeal tumors are increasing in incidence and exceed the number of tumors caused by the more traditional risk factors of tobacco and alcohol abuse," said Dr. Hans P. Schlecht in an editorial accompanying Dr. Gillison’s study (JAMA 2012 [doi: 10.1001/jama.2012.117]). The findings are noteworthy because they estimate oral HPV prevalence based on sexual experience, smoking history, and immune suppression, he said. In addition, the researchers found that HIV-negative individuals had lower rates of HPV infection of the mouth than at other sites, he said.
Additional research is needed to study how HPV-related oropharyngeal dysplastic lesions develop, said Dr. Schlecht. But "there is meaningful hope that prevention efforts will ameliorate the effects of HPV-related oropharyngeal cancer," he said.
Although HPV vaccination may eventually play a role in reducing cancers related to oral HPV infection, such an impact will take time, Dr. Schlecht noted. Meanwhile, "clinicians should encourage their patients who engage in oral sex to use barrier protection," he said. In addition, clinicians should be alert to signs of oropharyngeal cancer, including problems with speech or swallowing, ear pain, neck masses, and unexplained weight loss, he said.
Dr. Schlecht is affiliated with the division of infectious diseases and HIV medicine at Drexel University in Philadelphia. He reported no conflicts of interest.
"Human papillomavirus–positive oropharyngeal tumors are increasing in incidence and exceed the number of tumors caused by the more traditional risk factors of tobacco and alcohol abuse," said Dr. Hans P. Schlecht in an editorial accompanying Dr. Gillison’s study (JAMA 2012 [doi: 10.1001/jama.2012.117]). The findings are noteworthy because they estimate oral HPV prevalence based on sexual experience, smoking history, and immune suppression, he said. In addition, the researchers found that HIV-negative individuals had lower rates of HPV infection of the mouth than at other sites, he said.
Additional research is needed to study how HPV-related oropharyngeal dysplastic lesions develop, said Dr. Schlecht. But "there is meaningful hope that prevention efforts will ameliorate the effects of HPV-related oropharyngeal cancer," he said.
Although HPV vaccination may eventually play a role in reducing cancers related to oral HPV infection, such an impact will take time, Dr. Schlecht noted. Meanwhile, "clinicians should encourage their patients who engage in oral sex to use barrier protection," he said. In addition, clinicians should be alert to signs of oropharyngeal cancer, including problems with speech or swallowing, ear pain, neck masses, and unexplained weight loss, he said.
Dr. Schlecht is affiliated with the division of infectious diseases and HIV medicine at Drexel University in Philadelphia. He reported no conflicts of interest.
"Human papillomavirus–positive oropharyngeal tumors are increasing in incidence and exceed the number of tumors caused by the more traditional risk factors of tobacco and alcohol abuse," said Dr. Hans P. Schlecht in an editorial accompanying Dr. Gillison’s study (JAMA 2012 [doi: 10.1001/jama.2012.117]). The findings are noteworthy because they estimate oral HPV prevalence based on sexual experience, smoking history, and immune suppression, he said. In addition, the researchers found that HIV-negative individuals had lower rates of HPV infection of the mouth than at other sites, he said.
Additional research is needed to study how HPV-related oropharyngeal dysplastic lesions develop, said Dr. Schlecht. But "there is meaningful hope that prevention efforts will ameliorate the effects of HPV-related oropharyngeal cancer," he said.
Although HPV vaccination may eventually play a role in reducing cancers related to oral HPV infection, such an impact will take time, Dr. Schlecht noted. Meanwhile, "clinicians should encourage their patients who engage in oral sex to use barrier protection," he said. In addition, clinicians should be alert to signs of oropharyngeal cancer, including problems with speech or swallowing, ear pain, neck masses, and unexplained weight loss, he said.
Dr. Schlecht is affiliated with the division of infectious diseases and HIV medicine at Drexel University in Philadelphia. He reported no conflicts of interest.
The prevalence of oral human papillomavirus is nearly three times higher in men than in women, according to data from more than 5,000 individuals in the United States.
The findings were simultaneously published online in JAMA and presented at the Multidisciplinary Head and Neck Cancer Symposium in Phoenix on Jan. 26 (JAMA 2012;307: [doi: 10.1001/jama.2012/101]).
Oral HPV infection causes a subset of oropharyngeal squamous cell carcinoma (OSCC) that has increased in several countries, including the United States, over the past 3 decades, said Dr. Maura L. Gillison of the Ohio State University, Columbus, and her colleagues. "HPV has been directly implicated as the underlying cause," they noted. However, the epidemiology of OSCC has not been well studied.
The researchers conducted a cross-sectional study of HPV infection as part the National Health and Nutrition Examination Survey (NHANES) for 2009-2010. The study population included 5,579 men and women aged 14-69 years who were tested for HPV at mobile centers.
Overall, the prevalence of any HPV infection was 6.9%, and the prevalence of HPV type 16 (the type associated with OSCC) was 1%. The prevalence of any HPV infection was significantly higher in men than in women (10.1% vs. 3.6%, P less than .001). The peak prevalence of oral HPV occurred in people aged 30-34 years (7.3%) and 60-64 years (11.4%).
Infection in either gender was significantly more common in those with a history of any sexual contact (7.5%), compared with those who had no history of sexual contact (0.9%). The risk of infection also increased significantly as the number of sex partners for any type of sex increased. "One in five individuals with more than 20 lifetime sexual partners was infected," the researchers said.
Univariate associations between risk of oral HPV infection and alcohol and marijuana use did not remain significant in a multivariate analysis. However, cigarette smoking remained independently associated with an increased risk of oral HPV infection. The risk significantly increased with the number of cigarettes smoked daily for both men and women, but this trend was stronger in women.
In a multivariate analysis, age, gender, number of sexual partners, and number of cigarettes smoked daily remained independently associated with an increased risk of oral HPV infection.
"Our data provide evidence that oral HPV infection is predominantly sexually transmitted," the researchers said. Although HPV-positive OSCC has been associated with oral sex in particular, this study could not associate infection with a particular sexual behavior, they added.
The findings were limited by the emphasis on Alpha-papillomaviruses only, which likely underestimates the prevalence of oral HPV infection, the researchers said. But the findings suggest that vaccine trials to test the efficacy of the current HPV vaccine against oral HPV may be warranted. "Such trials could inform ongoing discussions regarding the benefits of HPV vaccination for males, given the higher prevalence of oral HPV infection demonstrated here as well as higher incidence of HPV-positive OSCC among men," they said.
The Multidisciplinary Head and Neck Cancer Symposium is sponsored by the American Society for Radiation Oncology.
Study sponsors included the Ohio State University Comprehensive Cancer Center and the National Cancer Institute. Dr. Gillison has served as a consultant to study sponsor Merck and to GlaxoSmithKline.
The incidence of HPV-positive oropharyngeal cancers increased 225% – from 0.8/100,000 to 2.8/100,000 – between 1988 and 2004, according to a presentation by Dr. Gillison at the annual meeting of the American Society of Clinical Oncology in June 2011. Click here for a report on the earlier findings.
The prevalence of oral human papillomavirus is nearly three times higher in men than in women, according to data from more than 5,000 individuals in the United States.
The findings were simultaneously published online in JAMA and presented at the Multidisciplinary Head and Neck Cancer Symposium in Phoenix on Jan. 26 (JAMA 2012;307: [doi: 10.1001/jama.2012/101]).
Oral HPV infection causes a subset of oropharyngeal squamous cell carcinoma (OSCC) that has increased in several countries, including the United States, over the past 3 decades, said Dr. Maura L. Gillison of the Ohio State University, Columbus, and her colleagues. "HPV has been directly implicated as the underlying cause," they noted. However, the epidemiology of OSCC has not been well studied.
The researchers conducted a cross-sectional study of HPV infection as part the National Health and Nutrition Examination Survey (NHANES) for 2009-2010. The study population included 5,579 men and women aged 14-69 years who were tested for HPV at mobile centers.
Overall, the prevalence of any HPV infection was 6.9%, and the prevalence of HPV type 16 (the type associated with OSCC) was 1%. The prevalence of any HPV infection was significantly higher in men than in women (10.1% vs. 3.6%, P less than .001). The peak prevalence of oral HPV occurred in people aged 30-34 years (7.3%) and 60-64 years (11.4%).
Infection in either gender was significantly more common in those with a history of any sexual contact (7.5%), compared with those who had no history of sexual contact (0.9%). The risk of infection also increased significantly as the number of sex partners for any type of sex increased. "One in five individuals with more than 20 lifetime sexual partners was infected," the researchers said.
Univariate associations between risk of oral HPV infection and alcohol and marijuana use did not remain significant in a multivariate analysis. However, cigarette smoking remained independently associated with an increased risk of oral HPV infection. The risk significantly increased with the number of cigarettes smoked daily for both men and women, but this trend was stronger in women.
In a multivariate analysis, age, gender, number of sexual partners, and number of cigarettes smoked daily remained independently associated with an increased risk of oral HPV infection.
"Our data provide evidence that oral HPV infection is predominantly sexually transmitted," the researchers said. Although HPV-positive OSCC has been associated with oral sex in particular, this study could not associate infection with a particular sexual behavior, they added.
The findings were limited by the emphasis on Alpha-papillomaviruses only, which likely underestimates the prevalence of oral HPV infection, the researchers said. But the findings suggest that vaccine trials to test the efficacy of the current HPV vaccine against oral HPV may be warranted. "Such trials could inform ongoing discussions regarding the benefits of HPV vaccination for males, given the higher prevalence of oral HPV infection demonstrated here as well as higher incidence of HPV-positive OSCC among men," they said.
The Multidisciplinary Head and Neck Cancer Symposium is sponsored by the American Society for Radiation Oncology.
Study sponsors included the Ohio State University Comprehensive Cancer Center and the National Cancer Institute. Dr. Gillison has served as a consultant to study sponsor Merck and to GlaxoSmithKline.
The incidence of HPV-positive oropharyngeal cancers increased 225% – from 0.8/100,000 to 2.8/100,000 – between 1988 and 2004, according to a presentation by Dr. Gillison at the annual meeting of the American Society of Clinical Oncology in June 2011. Click here for a report on the earlier findings.
FROM JAMA
Major Finding: The prevalence of HPV infection is nearly three times higher in men than in women (10.1% vs. 3.6%).
Data Source: The findings are based on data from 5,579 individuals aged 14-69 years who were part of the NHANES study cohort.
Disclosures: Study sponsors included the Ohio State University Comprehensive Cancer Center and the National Cancer Institute. Dr. Gillison has served as a consultant to study sponsor Merck and to GlaxoSmithKline.
Eruptive Collagenomas, Suprasellar Meningioma, and Elevated Prolactin in a Patient With a History of Papillary Thyroid Carcinoma
Lymphedema Common After Head & Neck Cancer
SAN FRANCISCO – Lymphedema is highly common and a source of considerable morbidity among patients who undergo treatment for head and neck cancer, finds a cross-sectional study among 103 survivors.
Fully three-fourths had developed some degree of lymphedema, according to results presented at the annual Oncology Congress presented by Reed Medical Education. The more severe it was, the more likely patients were to have symptoms, functional impairments, and poorer quality of life.
Disease and treatment-related factors such as high radiation dose and combined surgery and radiation therapy were risk factors for the development of lymphedema.
"This is the first study that we are aware of in the United States of this depth to systematically examine lymphedema" in this population, noted lead investigator Jie Deng, Ph.D., R.N., O.C.N., a postdoctoral fellow at the Vanderbilt University, Nashville, Tenn.
"Health care professionals should be aware that lymphedema is a frequent late effect in the head and neck cancer population," she advised. "We need to educate patients about the risk of lymphedema prior to treatment, during treatment, and posttreatment, and we need to conduct external and internal examinations to evaluate related signs and symptoms at each clinic visit."
Patients found to have any signs or symptoms should be referred for lymphedema assessment. Furthermore, "it’s very important we have very detailed documentation so we can follow up on patients’ treatment effect and also identify potential issues in this population," Dr. Deng stressed. "An interdisciplinary approach is needed to best manage lymphedema."
She and her colleagues are now evaluating interventions to treat head and neck lymphedema. Manual lymphatic drainage is one possibility. Elevating the head of the bed at night is another, as anecdotal comments suggest that symptoms worsen in the recumbent position.
"Patients will mention in the morning they feel more tightness, more fluid accumulated in the submental area; around noontime or afternoon, they feel it has drained by itself," she explained.
There are more than half a million survivors of head and neck cancer in the United States, according to Dr. Deng. As a result of their disease and its treatment, these patients can develop both external lymphedema, causing symptoms such as facial puffiness, and internal lymphedema, causing issues such as epiglottal swelling.
The investigators studied adult patients treated for head and neck cancer at the Vanderbilt-Ingram Cancer Center who were at least 3 months out from the end of their treatment and had no evidence of cancer. External lymphedema was assessed with a clinical exam, using the Foldi scale. Internal lymphedema was assessed with an endoscopic exam, using the Patterson scale for edema of the larynx and pharynx.
The patients were 60 years old, on average. The majority were male (69%) and white (89%). In terms of health behaviors, 66% had a history of smoking and 38% had a history of alcohol use.
In all, 81% of the patients had had locally advanced cancer, and 90% had received at least two treatment modalities. The median time since end of treatment was 20 months.
Study results, reported at the meeting and also recently published, showed that 75% of the patients overall had lymphedema of the head and neck; of those with lymphedema (61 out of 81), 10% had only the external kind, 39% had only the internal kind, and 51% had both (J. Pain Symptom Manage. 2011 July 29 [doi:10.1016/j.jpainsymman.2011.03.019]).
The type and severity of lymphedema were associated with both physical and psychological symptoms, Dr. Deng reported.
As the severity of lymphedema increased, patients were significantly more likely to report difficulty swallowing, issues with mucus or dry mouth, nutritional problems, pain, and voice problems (P = .001 to P = .047, depending on the type of lymphedema and the specific symptom).
Additionally, increasing severity was associated with poorer body image (P = .028 to P = .049). "However, there was no statistically significant relationship between lymphedema and anxiety and depressive symptoms," she noted.
Lymphedema severity was also associated with hearing deficits, limitation of neck range of motion, and impaired quality of life (P = .004 to P = .045).
Analyses identified certain disease and treatment-related factors to be risk factors for the development of lymphedema, according to Dr. Deng.
Namely, patients were more likely to develop lymphedema if they had pharyngeal tumors; were a shorter time out from the end of treatment; received a high total dose of radiation, mirroring what has been seen in breast cancer; received a greater number of treatment modalities, a marker of treatment intensity; or had combined surgery and radiation therapy – specifically, either surgery plus postoperative radiation, or salvage surgery within the irradiated field – as compared with surgery alone.
The higher risk with shorter time since surgery hints that the lymphatics in the area may undergo regeneration over time, she speculated. "This was identified in the murine tail [model of lymphedema]; however, we didn’t know whether or not this phenomenon or similar exists in the head and neck cancer population."
None of the demographic factors or health behaviors assessed were found to be risk factors for the development of lymphedema. But the lack of association between smoking and alcohol consumption and lymphedema may have been related to the fact that patients were asked whether they smoked or drank but not the intensity, or to the cross-sectional nature of the study, Dr. Deng noted. "In the future, longitudinal study is needed to examine whether or not these are risk factors," she said.
Dr. Deng reported that she had no conflicts of interest related to the study. Reed Medical Education and this news organization are owned by Reed Elsevier.
SAN FRANCISCO – Lymphedema is highly common and a source of considerable morbidity among patients who undergo treatment for head and neck cancer, finds a cross-sectional study among 103 survivors.
Fully three-fourths had developed some degree of lymphedema, according to results presented at the annual Oncology Congress presented by Reed Medical Education. The more severe it was, the more likely patients were to have symptoms, functional impairments, and poorer quality of life.
Disease and treatment-related factors such as high radiation dose and combined surgery and radiation therapy were risk factors for the development of lymphedema.
"This is the first study that we are aware of in the United States of this depth to systematically examine lymphedema" in this population, noted lead investigator Jie Deng, Ph.D., R.N., O.C.N., a postdoctoral fellow at the Vanderbilt University, Nashville, Tenn.
"Health care professionals should be aware that lymphedema is a frequent late effect in the head and neck cancer population," she advised. "We need to educate patients about the risk of lymphedema prior to treatment, during treatment, and posttreatment, and we need to conduct external and internal examinations to evaluate related signs and symptoms at each clinic visit."
Patients found to have any signs or symptoms should be referred for lymphedema assessment. Furthermore, "it’s very important we have very detailed documentation so we can follow up on patients’ treatment effect and also identify potential issues in this population," Dr. Deng stressed. "An interdisciplinary approach is needed to best manage lymphedema."
She and her colleagues are now evaluating interventions to treat head and neck lymphedema. Manual lymphatic drainage is one possibility. Elevating the head of the bed at night is another, as anecdotal comments suggest that symptoms worsen in the recumbent position.
"Patients will mention in the morning they feel more tightness, more fluid accumulated in the submental area; around noontime or afternoon, they feel it has drained by itself," she explained.
There are more than half a million survivors of head and neck cancer in the United States, according to Dr. Deng. As a result of their disease and its treatment, these patients can develop both external lymphedema, causing symptoms such as facial puffiness, and internal lymphedema, causing issues such as epiglottal swelling.
The investigators studied adult patients treated for head and neck cancer at the Vanderbilt-Ingram Cancer Center who were at least 3 months out from the end of their treatment and had no evidence of cancer. External lymphedema was assessed with a clinical exam, using the Foldi scale. Internal lymphedema was assessed with an endoscopic exam, using the Patterson scale for edema of the larynx and pharynx.
The patients were 60 years old, on average. The majority were male (69%) and white (89%). In terms of health behaviors, 66% had a history of smoking and 38% had a history of alcohol use.
In all, 81% of the patients had had locally advanced cancer, and 90% had received at least two treatment modalities. The median time since end of treatment was 20 months.
Study results, reported at the meeting and also recently published, showed that 75% of the patients overall had lymphedema of the head and neck; of those with lymphedema (61 out of 81), 10% had only the external kind, 39% had only the internal kind, and 51% had both (J. Pain Symptom Manage. 2011 July 29 [doi:10.1016/j.jpainsymman.2011.03.019]).
The type and severity of lymphedema were associated with both physical and psychological symptoms, Dr. Deng reported.
As the severity of lymphedema increased, patients were significantly more likely to report difficulty swallowing, issues with mucus or dry mouth, nutritional problems, pain, and voice problems (P = .001 to P = .047, depending on the type of lymphedema and the specific symptom).
Additionally, increasing severity was associated with poorer body image (P = .028 to P = .049). "However, there was no statistically significant relationship between lymphedema and anxiety and depressive symptoms," she noted.
Lymphedema severity was also associated with hearing deficits, limitation of neck range of motion, and impaired quality of life (P = .004 to P = .045).
Analyses identified certain disease and treatment-related factors to be risk factors for the development of lymphedema, according to Dr. Deng.
Namely, patients were more likely to develop lymphedema if they had pharyngeal tumors; were a shorter time out from the end of treatment; received a high total dose of radiation, mirroring what has been seen in breast cancer; received a greater number of treatment modalities, a marker of treatment intensity; or had combined surgery and radiation therapy – specifically, either surgery plus postoperative radiation, or salvage surgery within the irradiated field – as compared with surgery alone.
The higher risk with shorter time since surgery hints that the lymphatics in the area may undergo regeneration over time, she speculated. "This was identified in the murine tail [model of lymphedema]; however, we didn’t know whether or not this phenomenon or similar exists in the head and neck cancer population."
None of the demographic factors or health behaviors assessed were found to be risk factors for the development of lymphedema. But the lack of association between smoking and alcohol consumption and lymphedema may have been related to the fact that patients were asked whether they smoked or drank but not the intensity, or to the cross-sectional nature of the study, Dr. Deng noted. "In the future, longitudinal study is needed to examine whether or not these are risk factors," she said.
Dr. Deng reported that she had no conflicts of interest related to the study. Reed Medical Education and this news organization are owned by Reed Elsevier.
SAN FRANCISCO – Lymphedema is highly common and a source of considerable morbidity among patients who undergo treatment for head and neck cancer, finds a cross-sectional study among 103 survivors.
Fully three-fourths had developed some degree of lymphedema, according to results presented at the annual Oncology Congress presented by Reed Medical Education. The more severe it was, the more likely patients were to have symptoms, functional impairments, and poorer quality of life.
Disease and treatment-related factors such as high radiation dose and combined surgery and radiation therapy were risk factors for the development of lymphedema.
"This is the first study that we are aware of in the United States of this depth to systematically examine lymphedema" in this population, noted lead investigator Jie Deng, Ph.D., R.N., O.C.N., a postdoctoral fellow at the Vanderbilt University, Nashville, Tenn.
"Health care professionals should be aware that lymphedema is a frequent late effect in the head and neck cancer population," she advised. "We need to educate patients about the risk of lymphedema prior to treatment, during treatment, and posttreatment, and we need to conduct external and internal examinations to evaluate related signs and symptoms at each clinic visit."
Patients found to have any signs or symptoms should be referred for lymphedema assessment. Furthermore, "it’s very important we have very detailed documentation so we can follow up on patients’ treatment effect and also identify potential issues in this population," Dr. Deng stressed. "An interdisciplinary approach is needed to best manage lymphedema."
She and her colleagues are now evaluating interventions to treat head and neck lymphedema. Manual lymphatic drainage is one possibility. Elevating the head of the bed at night is another, as anecdotal comments suggest that symptoms worsen in the recumbent position.
"Patients will mention in the morning they feel more tightness, more fluid accumulated in the submental area; around noontime or afternoon, they feel it has drained by itself," she explained.
There are more than half a million survivors of head and neck cancer in the United States, according to Dr. Deng. As a result of their disease and its treatment, these patients can develop both external lymphedema, causing symptoms such as facial puffiness, and internal lymphedema, causing issues such as epiglottal swelling.
The investigators studied adult patients treated for head and neck cancer at the Vanderbilt-Ingram Cancer Center who were at least 3 months out from the end of their treatment and had no evidence of cancer. External lymphedema was assessed with a clinical exam, using the Foldi scale. Internal lymphedema was assessed with an endoscopic exam, using the Patterson scale for edema of the larynx and pharynx.
The patients were 60 years old, on average. The majority were male (69%) and white (89%). In terms of health behaviors, 66% had a history of smoking and 38% had a history of alcohol use.
In all, 81% of the patients had had locally advanced cancer, and 90% had received at least two treatment modalities. The median time since end of treatment was 20 months.
Study results, reported at the meeting and also recently published, showed that 75% of the patients overall had lymphedema of the head and neck; of those with lymphedema (61 out of 81), 10% had only the external kind, 39% had only the internal kind, and 51% had both (J. Pain Symptom Manage. 2011 July 29 [doi:10.1016/j.jpainsymman.2011.03.019]).
The type and severity of lymphedema were associated with both physical and psychological symptoms, Dr. Deng reported.
As the severity of lymphedema increased, patients were significantly more likely to report difficulty swallowing, issues with mucus or dry mouth, nutritional problems, pain, and voice problems (P = .001 to P = .047, depending on the type of lymphedema and the specific symptom).
Additionally, increasing severity was associated with poorer body image (P = .028 to P = .049). "However, there was no statistically significant relationship between lymphedema and anxiety and depressive symptoms," she noted.
Lymphedema severity was also associated with hearing deficits, limitation of neck range of motion, and impaired quality of life (P = .004 to P = .045).
Analyses identified certain disease and treatment-related factors to be risk factors for the development of lymphedema, according to Dr. Deng.
Namely, patients were more likely to develop lymphedema if they had pharyngeal tumors; were a shorter time out from the end of treatment; received a high total dose of radiation, mirroring what has been seen in breast cancer; received a greater number of treatment modalities, a marker of treatment intensity; or had combined surgery and radiation therapy – specifically, either surgery plus postoperative radiation, or salvage surgery within the irradiated field – as compared with surgery alone.
The higher risk with shorter time since surgery hints that the lymphatics in the area may undergo regeneration over time, she speculated. "This was identified in the murine tail [model of lymphedema]; however, we didn’t know whether or not this phenomenon or similar exists in the head and neck cancer population."
None of the demographic factors or health behaviors assessed were found to be risk factors for the development of lymphedema. But the lack of association between smoking and alcohol consumption and lymphedema may have been related to the fact that patients were asked whether they smoked or drank but not the intensity, or to the cross-sectional nature of the study, Dr. Deng noted. "In the future, longitudinal study is needed to examine whether or not these are risk factors," she said.
Dr. Deng reported that she had no conflicts of interest related to the study. Reed Medical Education and this news organization are owned by Reed Elsevier.
FROM THE ANNUAL ONCOLOGY CONGRESS
Major Finding: Fully 75% of patients had lymphedema. The severity of lymphedema was associated with symptoms, functional impairments, and poorer quality of life.
Data Source: A descriptive cross-sectional study among a convenience sample of 103 patients treated for head and neck cancer.
Disclosures: Dr. Deng reported that she had no conflicts of interest related to the study.
Mortality Greatest With Recurrence of Papillary Type Thyroid Cancer
SAN FRANCISCO – Thyroid cancer recurs in almost 40% of elderly patients, and while recurrence is accompanied by an increased mortality risk, this seems to be confined to the subset of patients with papillary thyroid cancer, according to researchers from Penn State Milton S. Hershey Medical Center in Hershey, Pa.
"Elderly patients with follicular disease and recurrence did not have a significantly different risk of death compared to patients without recurrences," said lead author Melissa M. Boltz, D.O., who presented the findings at the annual clinical congress of the American College of Surgeons.
About half of patients who develop recurrent disease will die from this, but little is known about the risk of recurrence. "We questioned whether the implications could be different for the elderly population," she said.
The researchers focused on recurrent well-differentiated thyroid cancer (WDTC) in patients aged 65 years or older and assessed its impact on 1-year and 5-year survival, controlling for patient-, disease-, and treatment-related variables.
From the SEER (Surveillance Epidemiology and End Results), Medicare-linked database, they identified 2,883 patients with primary WDTC treated between 1995 and 2007. They documented recurrence through billing codes, evidence of I-131 treatment, thyroid imaging, or the performance of additional thyroid procedures beyond 6 months of diagnosis.
Of these, 1,126 patients (39%) developed recurrent disease, and the recurrent group was not demographically different from the group of patients without recurrence. The majority recurred within the first 2 years of initial treatment, after which the probability of developing recurrence was never more than 45% over 10 years, Dr. Boltz said.
Risk factors associated with recurrence included older age, advanced stage, lack of surgical treatment, and regional disease, she reported.
Regional disease was present in 44% of the recurrent group, vs. only 24% of the nonrecurrent group, and thyroidectomy was performed on 33% vs. 60%, respectively.
At 10 years, of the total thyroid cancer population, 662 (23%) died of some form of cancer with thyroid cancer as the cause of death in 273 (41%).
"In the 1-year landmark analysis, patients with recurrence had a higher risk for cancer-specific mortality within 10 years, versus those without recurrence, and the trend was similar at the 5-year landmark," Dr. Boltz noted.
By histology, patients who recurred with papillary thyroid cancer were significantly more likely to die of thyroid cancer as compared to papillary thyroid cancer patients not experiencing recurrence. Papillary patients who were older, had regional or distant disease, and who did not undergo surgery were also at increased risk for cancer-specific death.
The hazard ratios for thyroid cancer death for papillary thyroid cancer patients were as follows:
• Recurrence: HR, 1.96 (P less than .001).
• Age, 5-year increases: HR, 1.46 (P less than .001).
• Regional disease: HR, 4.90 (P less than .001).
• Distant disease: HR, 16.97 (P less than .001).
• No surgery: HR, 7.98 (P less than .001).
• Treatment other than surgery: HR, 3.47 (P less than 0.001).
In contrast, patients with follicular thyroid cancer had an increase in cancer-specific mortality only in relation to the presence of distant disease (HR, 17.78; P less than 0.001). Older age was also associated with an increase in cancer-specific mortality (HR, 1.24; P = 0.04), but disease recurrence was not (HR 0.58; P = 0.16).
"Unlike papillary cancer, follicular cancer recurrence did not contribute to cancer-specific mortality. The only risks were related to older age and advanced stage," Dr. Boltz reported.
Dr. Boltz cautioned that this study pertained to elderly Medicare patients, and the results should not be generalized to a younger population, in which thyroid cancer is more prevalent.
Dr. Boltz reported no relevant conflicts of interest.
SAN FRANCISCO – Thyroid cancer recurs in almost 40% of elderly patients, and while recurrence is accompanied by an increased mortality risk, this seems to be confined to the subset of patients with papillary thyroid cancer, according to researchers from Penn State Milton S. Hershey Medical Center in Hershey, Pa.
"Elderly patients with follicular disease and recurrence did not have a significantly different risk of death compared to patients without recurrences," said lead author Melissa M. Boltz, D.O., who presented the findings at the annual clinical congress of the American College of Surgeons.
About half of patients who develop recurrent disease will die from this, but little is known about the risk of recurrence. "We questioned whether the implications could be different for the elderly population," she said.
The researchers focused on recurrent well-differentiated thyroid cancer (WDTC) in patients aged 65 years or older and assessed its impact on 1-year and 5-year survival, controlling for patient-, disease-, and treatment-related variables.
From the SEER (Surveillance Epidemiology and End Results), Medicare-linked database, they identified 2,883 patients with primary WDTC treated between 1995 and 2007. They documented recurrence through billing codes, evidence of I-131 treatment, thyroid imaging, or the performance of additional thyroid procedures beyond 6 months of diagnosis.
Of these, 1,126 patients (39%) developed recurrent disease, and the recurrent group was not demographically different from the group of patients without recurrence. The majority recurred within the first 2 years of initial treatment, after which the probability of developing recurrence was never more than 45% over 10 years, Dr. Boltz said.
Risk factors associated with recurrence included older age, advanced stage, lack of surgical treatment, and regional disease, she reported.
Regional disease was present in 44% of the recurrent group, vs. only 24% of the nonrecurrent group, and thyroidectomy was performed on 33% vs. 60%, respectively.
At 10 years, of the total thyroid cancer population, 662 (23%) died of some form of cancer with thyroid cancer as the cause of death in 273 (41%).
"In the 1-year landmark analysis, patients with recurrence had a higher risk for cancer-specific mortality within 10 years, versus those without recurrence, and the trend was similar at the 5-year landmark," Dr. Boltz noted.
By histology, patients who recurred with papillary thyroid cancer were significantly more likely to die of thyroid cancer as compared to papillary thyroid cancer patients not experiencing recurrence. Papillary patients who were older, had regional or distant disease, and who did not undergo surgery were also at increased risk for cancer-specific death.
The hazard ratios for thyroid cancer death for papillary thyroid cancer patients were as follows:
• Recurrence: HR, 1.96 (P less than .001).
• Age, 5-year increases: HR, 1.46 (P less than .001).
• Regional disease: HR, 4.90 (P less than .001).
• Distant disease: HR, 16.97 (P less than .001).
• No surgery: HR, 7.98 (P less than .001).
• Treatment other than surgery: HR, 3.47 (P less than 0.001).
In contrast, patients with follicular thyroid cancer had an increase in cancer-specific mortality only in relation to the presence of distant disease (HR, 17.78; P less than 0.001). Older age was also associated with an increase in cancer-specific mortality (HR, 1.24; P = 0.04), but disease recurrence was not (HR 0.58; P = 0.16).
"Unlike papillary cancer, follicular cancer recurrence did not contribute to cancer-specific mortality. The only risks were related to older age and advanced stage," Dr. Boltz reported.
Dr. Boltz cautioned that this study pertained to elderly Medicare patients, and the results should not be generalized to a younger population, in which thyroid cancer is more prevalent.
Dr. Boltz reported no relevant conflicts of interest.
SAN FRANCISCO – Thyroid cancer recurs in almost 40% of elderly patients, and while recurrence is accompanied by an increased mortality risk, this seems to be confined to the subset of patients with papillary thyroid cancer, according to researchers from Penn State Milton S. Hershey Medical Center in Hershey, Pa.
"Elderly patients with follicular disease and recurrence did not have a significantly different risk of death compared to patients without recurrences," said lead author Melissa M. Boltz, D.O., who presented the findings at the annual clinical congress of the American College of Surgeons.
About half of patients who develop recurrent disease will die from this, but little is known about the risk of recurrence. "We questioned whether the implications could be different for the elderly population," she said.
The researchers focused on recurrent well-differentiated thyroid cancer (WDTC) in patients aged 65 years or older and assessed its impact on 1-year and 5-year survival, controlling for patient-, disease-, and treatment-related variables.
From the SEER (Surveillance Epidemiology and End Results), Medicare-linked database, they identified 2,883 patients with primary WDTC treated between 1995 and 2007. They documented recurrence through billing codes, evidence of I-131 treatment, thyroid imaging, or the performance of additional thyroid procedures beyond 6 months of diagnosis.
Of these, 1,126 patients (39%) developed recurrent disease, and the recurrent group was not demographically different from the group of patients without recurrence. The majority recurred within the first 2 years of initial treatment, after which the probability of developing recurrence was never more than 45% over 10 years, Dr. Boltz said.
Risk factors associated with recurrence included older age, advanced stage, lack of surgical treatment, and regional disease, she reported.
Regional disease was present in 44% of the recurrent group, vs. only 24% of the nonrecurrent group, and thyroidectomy was performed on 33% vs. 60%, respectively.
At 10 years, of the total thyroid cancer population, 662 (23%) died of some form of cancer with thyroid cancer as the cause of death in 273 (41%).
"In the 1-year landmark analysis, patients with recurrence had a higher risk for cancer-specific mortality within 10 years, versus those without recurrence, and the trend was similar at the 5-year landmark," Dr. Boltz noted.
By histology, patients who recurred with papillary thyroid cancer were significantly more likely to die of thyroid cancer as compared to papillary thyroid cancer patients not experiencing recurrence. Papillary patients who were older, had regional or distant disease, and who did not undergo surgery were also at increased risk for cancer-specific death.
The hazard ratios for thyroid cancer death for papillary thyroid cancer patients were as follows:
• Recurrence: HR, 1.96 (P less than .001).
• Age, 5-year increases: HR, 1.46 (P less than .001).
• Regional disease: HR, 4.90 (P less than .001).
• Distant disease: HR, 16.97 (P less than .001).
• No surgery: HR, 7.98 (P less than .001).
• Treatment other than surgery: HR, 3.47 (P less than 0.001).
In contrast, patients with follicular thyroid cancer had an increase in cancer-specific mortality only in relation to the presence of distant disease (HR, 17.78; P less than 0.001). Older age was also associated with an increase in cancer-specific mortality (HR, 1.24; P = 0.04), but disease recurrence was not (HR 0.58; P = 0.16).
"Unlike papillary cancer, follicular cancer recurrence did not contribute to cancer-specific mortality. The only risks were related to older age and advanced stage," Dr. Boltz reported.
Dr. Boltz cautioned that this study pertained to elderly Medicare patients, and the results should not be generalized to a younger population, in which thyroid cancer is more prevalent.
Dr. Boltz reported no relevant conflicts of interest.
FROM THE ANNUAL CLINICAL CONGRESS OF THE AMERICAN COLLEGE OF SURGEONS
Major Finding: Patients who recurred with papillary thyroid cancer were significantly more likely to die of thyroid cancer as compared to papillary thyroid cancer patients not experiencing recurrence (HR, 1.96; P less than .001).
Data Source: An analysis of data from the SEER (Surveillance Epidemiology and End Results), Medicare-linked database, on 2,883 patients with primary well-differentiated thyroid cancer 5 years after initial treatment.
Disclosures: Dr. Boltz reported no relevant conflicts of interest.
FDA Approves Cetuximab for Late-Stage Head and Neck Cancer
The Food and Drug Administration on Nov. 7 approved cetuximab as an initial treatment of late-stage head and neck cancer in combination with chemotherapy.
Cetuximab, marketed as Erbitux by Bristol-Myers Squibb, is an epidermal growth factor receptor (EGFR) antagonist, administered as an intravenous infusion. Previously, it was approved in combination with radiation therapy for the initial treatment of locally or regionally advanced squamous cell carcinoma. It was also approved for use alone in patients with recurrent locoregional disease or metastatic disease whose disease has progressed following platinum-based chemotherapy.
The newly approved indication is for the treatment of these recurrent or metastatic patients as an initial therapy in combination with platinum-based therapy with 5-fluorouracil (5-FU), a BMS spokesperson said. (At press time, the company had not yet issued a statement on the approval.)
Erbitux was initially approved in 2004 to treat EGFR-positive late-stage colon cancer after patients stopped responding to chemotherapy and was approved in 2006 for the treatment of head and neck cancer. The newly approved indication is for "recurrent locoregional disease or metastatic squamous cell carcinoma of the head and neck in combination with platinum-based therapy with 5-FU," according to the revised label, posted on the FDA Web site.
The two previously approved indications for head and neck cancer were for "locally or regionally advanced squamous cell carcinoma of the head and neck in combination with radiation therapy," and for "recurrent or metastatic squamous cell carcinoma of the head and neck progressing after platinum-based therapy.
"Erbitux’s ability to extend the lives of patients with head and neck cancers is an important tool for oncologists who often rely on a multitreatment approach for patients," Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research said in the statement. "Given the aggressive nature of head and neck cancers that cannot be treated with surgery and radiation, it is important that patients have as many treatment options available as possible," he added.
The approval was based on a multicenter study of 442 patients who had metastatic or recurrent head and neck cancer, which was inoperable or widespread, and of those who had not been treated with chemotherapy. The study was conducted outside of the United States and used a version of cetuximab that is not approved in the United States, the statement said.
The median overall survival among patients who were treated with cetuximab and chemotherapy (cisplatin or carboplatin and 5-fluorouracil) combination was 10.1 months, compared with 7.4 months among those who received chemotherapy alone cisplatin or carboplatin and 5-fluorouracil, the FDA statement said.
The most common adverse events reported by patients in the cetuximab plus chemotherapy arm were rash; pruritus; nail changes; headache; diarrhea; and respiratory, skin, and mouth infections, according to the FDA.
Other adverse effects that have been associated with cetuximab include low serum levels of magnesium, potassium, and calcium; and potentially fatal infusion reactions and myocardial infarctions. Sun exposure should be limited during treatment.
Cetuximab available in the United States provides about 22% greater exposure than the European Union–approved cetuximab that was used in this study, but this pharmacokinetic data along with the results of this study "and other clinical trial data establish the efficacy of Erbitux at the recommended dose," according to the revised prescribing information posted on the FDA Web site.
The Food and Drug Administration on Nov. 7 approved cetuximab as an initial treatment of late-stage head and neck cancer in combination with chemotherapy.
Cetuximab, marketed as Erbitux by Bristol-Myers Squibb, is an epidermal growth factor receptor (EGFR) antagonist, administered as an intravenous infusion. Previously, it was approved in combination with radiation therapy for the initial treatment of locally or regionally advanced squamous cell carcinoma. It was also approved for use alone in patients with recurrent locoregional disease or metastatic disease whose disease has progressed following platinum-based chemotherapy.
The newly approved indication is for the treatment of these recurrent or metastatic patients as an initial therapy in combination with platinum-based therapy with 5-fluorouracil (5-FU), a BMS spokesperson said. (At press time, the company had not yet issued a statement on the approval.)
Erbitux was initially approved in 2004 to treat EGFR-positive late-stage colon cancer after patients stopped responding to chemotherapy and was approved in 2006 for the treatment of head and neck cancer. The newly approved indication is for "recurrent locoregional disease or metastatic squamous cell carcinoma of the head and neck in combination with platinum-based therapy with 5-FU," according to the revised label, posted on the FDA Web site.
The two previously approved indications for head and neck cancer were for "locally or regionally advanced squamous cell carcinoma of the head and neck in combination with radiation therapy," and for "recurrent or metastatic squamous cell carcinoma of the head and neck progressing after platinum-based therapy.
"Erbitux’s ability to extend the lives of patients with head and neck cancers is an important tool for oncologists who often rely on a multitreatment approach for patients," Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research said in the statement. "Given the aggressive nature of head and neck cancers that cannot be treated with surgery and radiation, it is important that patients have as many treatment options available as possible," he added.
The approval was based on a multicenter study of 442 patients who had metastatic or recurrent head and neck cancer, which was inoperable or widespread, and of those who had not been treated with chemotherapy. The study was conducted outside of the United States and used a version of cetuximab that is not approved in the United States, the statement said.
The median overall survival among patients who were treated with cetuximab and chemotherapy (cisplatin or carboplatin and 5-fluorouracil) combination was 10.1 months, compared with 7.4 months among those who received chemotherapy alone cisplatin or carboplatin and 5-fluorouracil, the FDA statement said.
The most common adverse events reported by patients in the cetuximab plus chemotherapy arm were rash; pruritus; nail changes; headache; diarrhea; and respiratory, skin, and mouth infections, according to the FDA.
Other adverse effects that have been associated with cetuximab include low serum levels of magnesium, potassium, and calcium; and potentially fatal infusion reactions and myocardial infarctions. Sun exposure should be limited during treatment.
Cetuximab available in the United States provides about 22% greater exposure than the European Union–approved cetuximab that was used in this study, but this pharmacokinetic data along with the results of this study "and other clinical trial data establish the efficacy of Erbitux at the recommended dose," according to the revised prescribing information posted on the FDA Web site.
The Food and Drug Administration on Nov. 7 approved cetuximab as an initial treatment of late-stage head and neck cancer in combination with chemotherapy.
Cetuximab, marketed as Erbitux by Bristol-Myers Squibb, is an epidermal growth factor receptor (EGFR) antagonist, administered as an intravenous infusion. Previously, it was approved in combination with radiation therapy for the initial treatment of locally or regionally advanced squamous cell carcinoma. It was also approved for use alone in patients with recurrent locoregional disease or metastatic disease whose disease has progressed following platinum-based chemotherapy.
The newly approved indication is for the treatment of these recurrent or metastatic patients as an initial therapy in combination with platinum-based therapy with 5-fluorouracil (5-FU), a BMS spokesperson said. (At press time, the company had not yet issued a statement on the approval.)
Erbitux was initially approved in 2004 to treat EGFR-positive late-stage colon cancer after patients stopped responding to chemotherapy and was approved in 2006 for the treatment of head and neck cancer. The newly approved indication is for "recurrent locoregional disease or metastatic squamous cell carcinoma of the head and neck in combination with platinum-based therapy with 5-FU," according to the revised label, posted on the FDA Web site.
The two previously approved indications for head and neck cancer were for "locally or regionally advanced squamous cell carcinoma of the head and neck in combination with radiation therapy," and for "recurrent or metastatic squamous cell carcinoma of the head and neck progressing after platinum-based therapy.
"Erbitux’s ability to extend the lives of patients with head and neck cancers is an important tool for oncologists who often rely on a multitreatment approach for patients," Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research said in the statement. "Given the aggressive nature of head and neck cancers that cannot be treated with surgery and radiation, it is important that patients have as many treatment options available as possible," he added.
The approval was based on a multicenter study of 442 patients who had metastatic or recurrent head and neck cancer, which was inoperable or widespread, and of those who had not been treated with chemotherapy. The study was conducted outside of the United States and used a version of cetuximab that is not approved in the United States, the statement said.
The median overall survival among patients who were treated with cetuximab and chemotherapy (cisplatin or carboplatin and 5-fluorouracil) combination was 10.1 months, compared with 7.4 months among those who received chemotherapy alone cisplatin or carboplatin and 5-fluorouracil, the FDA statement said.
The most common adverse events reported by patients in the cetuximab plus chemotherapy arm were rash; pruritus; nail changes; headache; diarrhea; and respiratory, skin, and mouth infections, according to the FDA.
Other adverse effects that have been associated with cetuximab include low serum levels of magnesium, potassium, and calcium; and potentially fatal infusion reactions and myocardial infarctions. Sun exposure should be limited during treatment.
Cetuximab available in the United States provides about 22% greater exposure than the European Union–approved cetuximab that was used in this study, but this pharmacokinetic data along with the results of this study "and other clinical trial data establish the efficacy of Erbitux at the recommended dose," according to the revised prescribing information posted on the FDA Web site.
FROM THE FOOD AND DRUG ADMINISTRATION
Early Trial Supports Bevacizumab in Head and Neck Cancer
MIAMI BEACH – Bevacizumab added to docetaxel and definitive radiotherapy was an effective and safe non–platinum-based regimen for locoregionally advanced squamous cell carcinoma of the head and neck in a small phase II trial that was presented at the annual meeting of the American Society for Radiation Oncology.
The disease-free survival rate at 2 years (the primary end point) was 75% among 28 patients who had completed the full course of chemoradiation in the phase II study, said Dr. Nicholas Galanopoulos of University Hospitals Seidman Cancer Center in Cleveland. In this as-treated population, 85% of patients were alive at 2 years, locoregional control was achieved in 85% of patients, and 81.5% were free of distant metastases at 2 years.
The numbers were slightly lower when two additional patients who dropped out of the study (one for unspecified reasons and one who developed aspiration pneumonia and was hospitalized) were included in an intention-to-treat analysis. Among all 30 patients, the disease-free survival rate at 2 years was 63% and overall survival was 72%, although locoregional control and distant-metastasis-free rates remained unchanged at 85% and 81.5%, respectively.
"Future trials of concurrent chemoradiotherapy with or without bevacizumab are justified for local-regionally advanced head and neck cancer," Dr. Galanopoulos said.
The rationale for trying bevacizumab (Avastin) in chemoradiation regimens for head and neck tumors is based on its demonstrated ability to inhibit endothelial cell proliferation and blood vessel formation, and to increase radiosensitivity of tumors when administered concurrently with radiation, he noted.
In all, 30 treatment-naive patients (26 men, 4 women) with locally advanced stage III (6 patients) or IV (24 patients) squamous cell carcinoma of the head and neck were enrolled. The patients had good performance status and a life expectancy greater than 12 weeks.
They received 70.2 Gy radiation in 1.8-Gy fractions, docetaxel (Taxotere) 20 mg/m2 per week, and bevacizumab 5 mg/kg every 2 weeks, with bevacizumab continued for up to 1 year following chemoradiation (median, 7 months).
"Future trials of concurrent chemo-radiotherapy with or without bevacizumab are justified for local-regionally advanced head and neck cancer."
Results were reported as of a median follow-up of 24.4 months.
Adjuvant bevacizumab was stopped 4 weeks before surgery in those patients who required neck dissection, and the drug was discontinued in three patients (one for radiation necrosis of the larynx requiring laryngectomy, one for severe pharyngoesophageal stenosis, and one for hemorrhagic cholecystits requiring cholecystectomy).
Major toxicities associated with the regimen included grade 4 bleeding in two patients (the episode of hemorrhagic cholecystitis already noted), and one oropharyngeal hemorrhage in field during chemoradiation.
Late grade 3 or 4 dysphagia occurred in 7 of 19 patients who had been treated with 3-D conformal radiation therapy, but in none of 11 patients who received intensity-modulated radiation (P less than .05). Patients with laryngeal primary tumors were more likely to develop serious late dysphagia, compared with patients with oropharyngeal primaries (58%% vs. 15%; P less than .05).
The study was funded in part by grants from the National Institutes of Health, Genentech, and Sanofi-Aventis. Dr. Galanopoulos reported having no relevant financial disclosures. Coauthor Dr. Panayiotis Savvides disclosed receiving research grants or support from Genentech and Sanofi-Aventis.
MIAMI BEACH – Bevacizumab added to docetaxel and definitive radiotherapy was an effective and safe non–platinum-based regimen for locoregionally advanced squamous cell carcinoma of the head and neck in a small phase II trial that was presented at the annual meeting of the American Society for Radiation Oncology.
The disease-free survival rate at 2 years (the primary end point) was 75% among 28 patients who had completed the full course of chemoradiation in the phase II study, said Dr. Nicholas Galanopoulos of University Hospitals Seidman Cancer Center in Cleveland. In this as-treated population, 85% of patients were alive at 2 years, locoregional control was achieved in 85% of patients, and 81.5% were free of distant metastases at 2 years.
The numbers were slightly lower when two additional patients who dropped out of the study (one for unspecified reasons and one who developed aspiration pneumonia and was hospitalized) were included in an intention-to-treat analysis. Among all 30 patients, the disease-free survival rate at 2 years was 63% and overall survival was 72%, although locoregional control and distant-metastasis-free rates remained unchanged at 85% and 81.5%, respectively.
"Future trials of concurrent chemoradiotherapy with or without bevacizumab are justified for local-regionally advanced head and neck cancer," Dr. Galanopoulos said.
The rationale for trying bevacizumab (Avastin) in chemoradiation regimens for head and neck tumors is based on its demonstrated ability to inhibit endothelial cell proliferation and blood vessel formation, and to increase radiosensitivity of tumors when administered concurrently with radiation, he noted.
In all, 30 treatment-naive patients (26 men, 4 women) with locally advanced stage III (6 patients) or IV (24 patients) squamous cell carcinoma of the head and neck were enrolled. The patients had good performance status and a life expectancy greater than 12 weeks.
They received 70.2 Gy radiation in 1.8-Gy fractions, docetaxel (Taxotere) 20 mg/m2 per week, and bevacizumab 5 mg/kg every 2 weeks, with bevacizumab continued for up to 1 year following chemoradiation (median, 7 months).
"Future trials of concurrent chemo-radiotherapy with or without bevacizumab are justified for local-regionally advanced head and neck cancer."
Results were reported as of a median follow-up of 24.4 months.
Adjuvant bevacizumab was stopped 4 weeks before surgery in those patients who required neck dissection, and the drug was discontinued in three patients (one for radiation necrosis of the larynx requiring laryngectomy, one for severe pharyngoesophageal stenosis, and one for hemorrhagic cholecystits requiring cholecystectomy).
Major toxicities associated with the regimen included grade 4 bleeding in two patients (the episode of hemorrhagic cholecystitis already noted), and one oropharyngeal hemorrhage in field during chemoradiation.
Late grade 3 or 4 dysphagia occurred in 7 of 19 patients who had been treated with 3-D conformal radiation therapy, but in none of 11 patients who received intensity-modulated radiation (P less than .05). Patients with laryngeal primary tumors were more likely to develop serious late dysphagia, compared with patients with oropharyngeal primaries (58%% vs. 15%; P less than .05).
The study was funded in part by grants from the National Institutes of Health, Genentech, and Sanofi-Aventis. Dr. Galanopoulos reported having no relevant financial disclosures. Coauthor Dr. Panayiotis Savvides disclosed receiving research grants or support from Genentech and Sanofi-Aventis.
MIAMI BEACH – Bevacizumab added to docetaxel and definitive radiotherapy was an effective and safe non–platinum-based regimen for locoregionally advanced squamous cell carcinoma of the head and neck in a small phase II trial that was presented at the annual meeting of the American Society for Radiation Oncology.
The disease-free survival rate at 2 years (the primary end point) was 75% among 28 patients who had completed the full course of chemoradiation in the phase II study, said Dr. Nicholas Galanopoulos of University Hospitals Seidman Cancer Center in Cleveland. In this as-treated population, 85% of patients were alive at 2 years, locoregional control was achieved in 85% of patients, and 81.5% were free of distant metastases at 2 years.
The numbers were slightly lower when two additional patients who dropped out of the study (one for unspecified reasons and one who developed aspiration pneumonia and was hospitalized) were included in an intention-to-treat analysis. Among all 30 patients, the disease-free survival rate at 2 years was 63% and overall survival was 72%, although locoregional control and distant-metastasis-free rates remained unchanged at 85% and 81.5%, respectively.
"Future trials of concurrent chemoradiotherapy with or without bevacizumab are justified for local-regionally advanced head and neck cancer," Dr. Galanopoulos said.
The rationale for trying bevacizumab (Avastin) in chemoradiation regimens for head and neck tumors is based on its demonstrated ability to inhibit endothelial cell proliferation and blood vessel formation, and to increase radiosensitivity of tumors when administered concurrently with radiation, he noted.
In all, 30 treatment-naive patients (26 men, 4 women) with locally advanced stage III (6 patients) or IV (24 patients) squamous cell carcinoma of the head and neck were enrolled. The patients had good performance status and a life expectancy greater than 12 weeks.
They received 70.2 Gy radiation in 1.8-Gy fractions, docetaxel (Taxotere) 20 mg/m2 per week, and bevacizumab 5 mg/kg every 2 weeks, with bevacizumab continued for up to 1 year following chemoradiation (median, 7 months).
"Future trials of concurrent chemo-radiotherapy with or without bevacizumab are justified for local-regionally advanced head and neck cancer."
Results were reported as of a median follow-up of 24.4 months.
Adjuvant bevacizumab was stopped 4 weeks before surgery in those patients who required neck dissection, and the drug was discontinued in three patients (one for radiation necrosis of the larynx requiring laryngectomy, one for severe pharyngoesophageal stenosis, and one for hemorrhagic cholecystits requiring cholecystectomy).
Major toxicities associated with the regimen included grade 4 bleeding in two patients (the episode of hemorrhagic cholecystitis already noted), and one oropharyngeal hemorrhage in field during chemoradiation.
Late grade 3 or 4 dysphagia occurred in 7 of 19 patients who had been treated with 3-D conformal radiation therapy, but in none of 11 patients who received intensity-modulated radiation (P less than .05). Patients with laryngeal primary tumors were more likely to develop serious late dysphagia, compared with patients with oropharyngeal primaries (58%% vs. 15%; P less than .05).
The study was funded in part by grants from the National Institutes of Health, Genentech, and Sanofi-Aventis. Dr. Galanopoulos reported having no relevant financial disclosures. Coauthor Dr. Panayiotis Savvides disclosed receiving research grants or support from Genentech and Sanofi-Aventis.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR RADIATION ONCOLOGY
Major Finding: The 2-year disease-free survival rate was 75% in an as-treated population with locoregionally advanced squamous cell carcinoma of the head and neck, and 63% in an intention-to-treat analysis.
Data Source: A phase II clinical trial evaluating the addition of bevacizumab to chemoradiation with docetaxel.
Disclosures: The study was funded in part by grants from the National Institutes of Health, Genentech, and Sanofi-Aventis. Dr. Galanopoulos reported having no relevant financial disclosures. Coauthor Dr. Panayiotis Savvides disclosed receiving research grants or support from Genentech and Sanofi-Aventis.
Screen Carotids After Head and Neck Radiation
MIAMI BEACH – Head and neck cancer patients treated with radiation should be screened routinely for carotid artery stenosis, investigators recommended at the annual meeting of the American Society for Radiation Oncology.
Among 225 patients who had received radiation and were screened, an estimated 18% had significant asymptomatic stenosis (50% or greater narrowing) of one or both carotid arteries 3 years after treatment said Dr. Jennifer Dorth, a resident in radiation oncology at Duke University Medical Center in Durham, N.C.
"We recommend screening for head and neck cancer patients given that there are high rates of stenosis as well as high rates of progression of stenosis," she said.
Factors significantly associated with risk for stenosis included Framingham risk factors (smoking history, hypertension, hyperlipidemia, diabetes mellitus, cardiovascular/peripheral vascular disease, and atrial fibrillation) and radiation dose.
The investigators retrospectively reviewed outcomes of asymptomatic, disease-free head and neck cancer patients who had received radiation with curative intent to the neck. The patients were screened with carotid Doppler ultrasound at or after the 1-year follow-up visit, and this was repeated every 2-3 years. Patients with ultrasound evidence of 50% or greater stenosis were referred to vascular surgery.
The study identified 225 patients, 139 of whom had received intensity-modulated radiation therapy (IMRT), with the dose calculated separately for each side of the neck. Because of the separate treatment planning, the investigators analyzed the data by creating two separate models: one looking at all patients, and the other looking at 278 treatments in the 139 patients with IMRT.
In each model, about 85% of patients had stage III or IV disease and about 58% had cancer in the oropharynx, followed in order of frequency by the larynx, oral cavity, nasopharynx, or other sites.
A total of 33 patients had stenosis in 51 arteries. The median time between completion of radiation therapy and the last follow-up screening was 2 years. The median time to stenosis was 3 years.
Actuarial estimates of carotid artery stenosis were 2% at 1 year, 6% at 2 years, and 18% at 3 years.
In univariate analysis, factors associated with stenosis included male gender (P = .02), hypertension (P = .003), vascular disease (P less than .001), and Framingham score (P less than .001).
In the multivariate model looking at all patients, each Framingham risk factor was associated with a near doubling of stenosis risk (hazard ratio 1.8, P = .0003). In the model focusing on the IMRT population (adjusted for Framingham score), only radiation dose was significantly associated with stenosis (HR 1.07/Gy, P = .02).
Of the 33 patients with stenosis, 8 had no further follow-up imaging, 8 had stable stenosis, and 17 had progressive stenosis, 2 of whom had a cerebrovascular event. Eight patients with progressive stenosis received medical management only, and nine went on to surgery (three endarterectomies and six stent placements).
"Of the nine patients who underwent surgical management, there was a high rate of restenosis in 30% of patients at a year median follow-up, and this is consistent, unfortunately, with other series looking at rates of restenosis," Dr. Dorth said.
The study was internally funded. Dr. Dorth reported having no relevant financial disclosures.
MIAMI BEACH – Head and neck cancer patients treated with radiation should be screened routinely for carotid artery stenosis, investigators recommended at the annual meeting of the American Society for Radiation Oncology.
Among 225 patients who had received radiation and were screened, an estimated 18% had significant asymptomatic stenosis (50% or greater narrowing) of one or both carotid arteries 3 years after treatment said Dr. Jennifer Dorth, a resident in radiation oncology at Duke University Medical Center in Durham, N.C.
"We recommend screening for head and neck cancer patients given that there are high rates of stenosis as well as high rates of progression of stenosis," she said.
Factors significantly associated with risk for stenosis included Framingham risk factors (smoking history, hypertension, hyperlipidemia, diabetes mellitus, cardiovascular/peripheral vascular disease, and atrial fibrillation) and radiation dose.
The investigators retrospectively reviewed outcomes of asymptomatic, disease-free head and neck cancer patients who had received radiation with curative intent to the neck. The patients were screened with carotid Doppler ultrasound at or after the 1-year follow-up visit, and this was repeated every 2-3 years. Patients with ultrasound evidence of 50% or greater stenosis were referred to vascular surgery.
The study identified 225 patients, 139 of whom had received intensity-modulated radiation therapy (IMRT), with the dose calculated separately for each side of the neck. Because of the separate treatment planning, the investigators analyzed the data by creating two separate models: one looking at all patients, and the other looking at 278 treatments in the 139 patients with IMRT.
In each model, about 85% of patients had stage III or IV disease and about 58% had cancer in the oropharynx, followed in order of frequency by the larynx, oral cavity, nasopharynx, or other sites.
A total of 33 patients had stenosis in 51 arteries. The median time between completion of radiation therapy and the last follow-up screening was 2 years. The median time to stenosis was 3 years.
Actuarial estimates of carotid artery stenosis were 2% at 1 year, 6% at 2 years, and 18% at 3 years.
In univariate analysis, factors associated with stenosis included male gender (P = .02), hypertension (P = .003), vascular disease (P less than .001), and Framingham score (P less than .001).
In the multivariate model looking at all patients, each Framingham risk factor was associated with a near doubling of stenosis risk (hazard ratio 1.8, P = .0003). In the model focusing on the IMRT population (adjusted for Framingham score), only radiation dose was significantly associated with stenosis (HR 1.07/Gy, P = .02).
Of the 33 patients with stenosis, 8 had no further follow-up imaging, 8 had stable stenosis, and 17 had progressive stenosis, 2 of whom had a cerebrovascular event. Eight patients with progressive stenosis received medical management only, and nine went on to surgery (three endarterectomies and six stent placements).
"Of the nine patients who underwent surgical management, there was a high rate of restenosis in 30% of patients at a year median follow-up, and this is consistent, unfortunately, with other series looking at rates of restenosis," Dr. Dorth said.
The study was internally funded. Dr. Dorth reported having no relevant financial disclosures.
MIAMI BEACH – Head and neck cancer patients treated with radiation should be screened routinely for carotid artery stenosis, investigators recommended at the annual meeting of the American Society for Radiation Oncology.
Among 225 patients who had received radiation and were screened, an estimated 18% had significant asymptomatic stenosis (50% or greater narrowing) of one or both carotid arteries 3 years after treatment said Dr. Jennifer Dorth, a resident in radiation oncology at Duke University Medical Center in Durham, N.C.
"We recommend screening for head and neck cancer patients given that there are high rates of stenosis as well as high rates of progression of stenosis," she said.
Factors significantly associated with risk for stenosis included Framingham risk factors (smoking history, hypertension, hyperlipidemia, diabetes mellitus, cardiovascular/peripheral vascular disease, and atrial fibrillation) and radiation dose.
The investigators retrospectively reviewed outcomes of asymptomatic, disease-free head and neck cancer patients who had received radiation with curative intent to the neck. The patients were screened with carotid Doppler ultrasound at or after the 1-year follow-up visit, and this was repeated every 2-3 years. Patients with ultrasound evidence of 50% or greater stenosis were referred to vascular surgery.
The study identified 225 patients, 139 of whom had received intensity-modulated radiation therapy (IMRT), with the dose calculated separately for each side of the neck. Because of the separate treatment planning, the investigators analyzed the data by creating two separate models: one looking at all patients, and the other looking at 278 treatments in the 139 patients with IMRT.
In each model, about 85% of patients had stage III or IV disease and about 58% had cancer in the oropharynx, followed in order of frequency by the larynx, oral cavity, nasopharynx, or other sites.
A total of 33 patients had stenosis in 51 arteries. The median time between completion of radiation therapy and the last follow-up screening was 2 years. The median time to stenosis was 3 years.
Actuarial estimates of carotid artery stenosis were 2% at 1 year, 6% at 2 years, and 18% at 3 years.
In univariate analysis, factors associated with stenosis included male gender (P = .02), hypertension (P = .003), vascular disease (P less than .001), and Framingham score (P less than .001).
In the multivariate model looking at all patients, each Framingham risk factor was associated with a near doubling of stenosis risk (hazard ratio 1.8, P = .0003). In the model focusing on the IMRT population (adjusted for Framingham score), only radiation dose was significantly associated with stenosis (HR 1.07/Gy, P = .02).
Of the 33 patients with stenosis, 8 had no further follow-up imaging, 8 had stable stenosis, and 17 had progressive stenosis, 2 of whom had a cerebrovascular event. Eight patients with progressive stenosis received medical management only, and nine went on to surgery (three endarterectomies and six stent placements).
"Of the nine patients who underwent surgical management, there was a high rate of restenosis in 30% of patients at a year median follow-up, and this is consistent, unfortunately, with other series looking at rates of restenosis," Dr. Dorth said.
The study was internally funded. Dr. Dorth reported having no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR RADIATION ONCOLOGY
Major Finding: The estimated risk for stenosis of the carotid arteries 3 years after irradiation for head and neck cancer was 18%.
Data Source: Retrospective review of data on 225 patients who received irradiation for head and neck cancer.
Disclosures: The study was internally funded. Dr. Dorth reported having no relevant financial disclosures.