User login
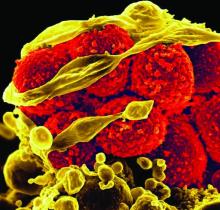
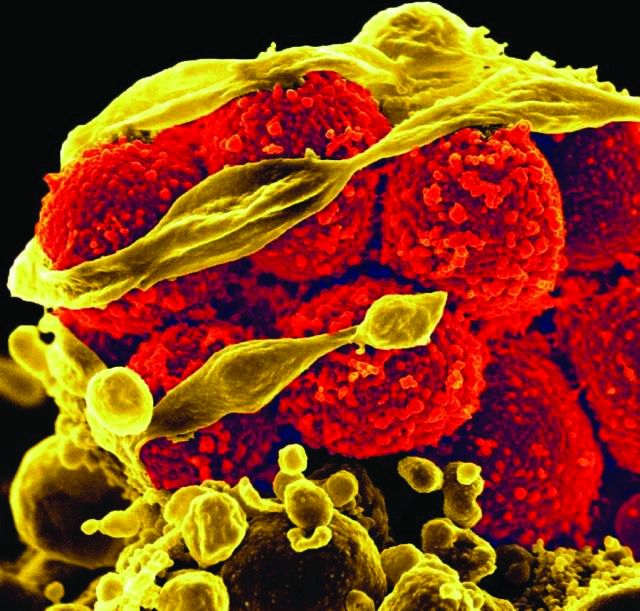
Fighting in a passive manner active against Clostridium difficile
Infections resulting from Clostridium difficile are a major clinical challenge. In hematology and oncology, the widespread use of broad-spectrum antibiotics is essential for patients with profound neutropenia and infectious complications, which are a high-risk factor for C. difficile enteritis.
C. difficile enteritis occurs in 5%-20% of cancer patients.1 With standard of care antibiotics, oral metronidazole or oral vancomycin, high C. difficile cure rates are possible, but up to 25% of these infections recur. Recently, oral fidaxomicin was approved for treatment of C. difficile enteritis and was associated with high cure rates and, more importantly, with significantly lower recurrence rates.2
Bezlotoxumab, a fully humanized monoclonal antibody against C. difficile toxin B, has been shown by x-ray crystallography to neutralize toxin B by blocking its ability to bind to host cells.7 Most recently, this new therapeutic approach was investigated in humans.8
Wilcox et al. used pooled data of 2655 adults treated in two double-blind, randomized, placebo-controlled phase III clinical trials (MODIFY I and MODIFY II) for primary or recurrent C. difficile enteritis. This industry-sponsored trial was conducted at 322 sites in 30 countries.
In one treatment group, patients received a single infusion of bezlotoxumab (781 patients) or placebo (773 patients) and one of the three oral standard-of-care C. difficile antibiotics. Importantly, the primary end point of this trial was recurrent infection within 12 weeks. About 28% of the patients in both the bezlotoxumab group and the placebo group previously had at least one episode of C. difficile enteritis. About 20% of the patients in both groups were immunocompromised.
Pooled data showed that recurrent infection was significantly lower (P less than 0.001) in the bezlotoxumab group (17%), compared with the placebo group (27%). The difference in recurrence rate (25% vs. 41%) was even more pronounced in patients with one or more episodes of recurrent C. difficile enteritis in the past 6 months. Furthermore, a benefit for bezlotoxumab was seen in immunocompromised patients, whose recurrence rates were 15% with bezlotoxumab, vs. 28% with placebo. After the 12 weeks of follow-up, the absolute difference in the Kaplan-Meier rates of recurrent infection was 13% (absolute rate, 21% in bezlotoxumab group vs. 34% in placebo group; P less than 0.001).
The results indicate that bezlotoxumab, which was approved in 2016 by the U.S. Food and Drug Administration, might improve the outcome of patients with C. difficile enteritis. However, bezlotoxumab is not a “magic bullet.” The number needed to treat to prevent one episode of C. difficile enteritis is 10.
It is conceivable that bezlotoxumab may find its role in high-risk patients – those older than 65 years or patients with recurrent C. difficile enteritis – since the number needed to treat is only 6 in these subgroups.8
This new agent could be an important treatment option for our high-risk patients in hematology. However, more studies concerning costs and real-life efficacy are needed.
The new approach of passive immunization for prevention of recurrent C. difficile enteritis shows the importance and the role of toxin B – not only the bacterium per se – in pathogenesis and virulence of C. difficile. This could mean that we have to renew our view on the role of antibiotics against C. difficile. However, in contrast, bezlotoxumab does not affect the efficacy of standard of care antibiotics since the initial cure rates were 80% for both the antibody and the placebo groups.8 Toxin B levels are not detectable in stool samples between days 4 and 10 of standard of care antibiotic treatment. Afterward, however, they increase again.9 Most of the patients had received bezlotoxumab 3 or more days after they began standard-of-care antibiotic treatment – in the time period when toxin B is undetectable in stool – which underlines the importance of toxin B in the pathogenesis of recurrent C. difficile enteritis.8
In summary, the introduction of bezlotoxumab in clinical care gives new and important insights and solutions not only for treatment options but also for our understanding of C. difficile pathogenesis.
Dr. Schalk is consultant of internal medicine at the department of hematology and oncology, Magdeburg University Hospital, Germany, with clinical and research focus on infectious diseases in hematology and oncology.
Dr. Fischer is professor of internal medicine, hematology and oncology, at the Otto-von-Guericke University Hospital Magdeburg, Germany. He is head of the department of hematology and oncology and a clinical/molecular researcher in myeloid neoplasms. He is a member of the editorial advisory board of Hematology News.
Contact Dr. Schalk at [email protected].
References
1. Vehreschild, MJ et al. Diagnosis and management of gastrointestinal complications in adult cancer patients: Wvidence-based guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Ann Oncol. 2013;24:1189-202
2. Cornely, OA. Current and emerging management options for Clostridium difficile infection: What is the role of fidaxomicin? Clin Microbiol Infect. 2012;18(Suppl 6):28-35.
3. Bartlett, JG et al. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978;298(10):531-4.
4. Lyras, D et al. Toxin B is essential for virulence of Clostridium difficile. Nature. 2009;458:1176-9.
5. Reineke, J et al. Autocatalytic cleavage of Clostridium difficile toxin B. Nature. 2007;446:415-9.
6. Leav, BA et al. Serum anti-toxin B antibody correlates with protection from recurrent Clostridium difficile infection (CDI). Vaccine. 2010;28:965-9.
7. Orth, P et al. Mechanism of action and epitopes of Clostridium difficile toxin B-neutralizing antibody bezlotoxumab revealed by X-ray crystallography. J Biol Chem. 2014;289:18008-21.
8. Wilcox, MH et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376:305-17.
9. Louie, TJ et al. Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin reexpression and recurrence of CDI. Clin Infect Dis. 2012;5(Suppl 2):S132-42.
Infections resulting from Clostridium difficile are a major clinical challenge. In hematology and oncology, the widespread use of broad-spectrum antibiotics is essential for patients with profound neutropenia and infectious complications, which are a high-risk factor for C. difficile enteritis.
C. difficile enteritis occurs in 5%-20% of cancer patients.1 With standard of care antibiotics, oral metronidazole or oral vancomycin, high C. difficile cure rates are possible, but up to 25% of these infections recur. Recently, oral fidaxomicin was approved for treatment of C. difficile enteritis and was associated with high cure rates and, more importantly, with significantly lower recurrence rates.2
Bezlotoxumab, a fully humanized monoclonal antibody against C. difficile toxin B, has been shown by x-ray crystallography to neutralize toxin B by blocking its ability to bind to host cells.7 Most recently, this new therapeutic approach was investigated in humans.8
Wilcox et al. used pooled data of 2655 adults treated in two double-blind, randomized, placebo-controlled phase III clinical trials (MODIFY I and MODIFY II) for primary or recurrent C. difficile enteritis. This industry-sponsored trial was conducted at 322 sites in 30 countries.
In one treatment group, patients received a single infusion of bezlotoxumab (781 patients) or placebo (773 patients) and one of the three oral standard-of-care C. difficile antibiotics. Importantly, the primary end point of this trial was recurrent infection within 12 weeks. About 28% of the patients in both the bezlotoxumab group and the placebo group previously had at least one episode of C. difficile enteritis. About 20% of the patients in both groups were immunocompromised.
Pooled data showed that recurrent infection was significantly lower (P less than 0.001) in the bezlotoxumab group (17%), compared with the placebo group (27%). The difference in recurrence rate (25% vs. 41%) was even more pronounced in patients with one or more episodes of recurrent C. difficile enteritis in the past 6 months. Furthermore, a benefit for bezlotoxumab was seen in immunocompromised patients, whose recurrence rates were 15% with bezlotoxumab, vs. 28% with placebo. After the 12 weeks of follow-up, the absolute difference in the Kaplan-Meier rates of recurrent infection was 13% (absolute rate, 21% in bezlotoxumab group vs. 34% in placebo group; P less than 0.001).
The results indicate that bezlotoxumab, which was approved in 2016 by the U.S. Food and Drug Administration, might improve the outcome of patients with C. difficile enteritis. However, bezlotoxumab is not a “magic bullet.” The number needed to treat to prevent one episode of C. difficile enteritis is 10.
It is conceivable that bezlotoxumab may find its role in high-risk patients – those older than 65 years or patients with recurrent C. difficile enteritis – since the number needed to treat is only 6 in these subgroups.8
This new agent could be an important treatment option for our high-risk patients in hematology. However, more studies concerning costs and real-life efficacy are needed.
The new approach of passive immunization for prevention of recurrent C. difficile enteritis shows the importance and the role of toxin B – not only the bacterium per se – in pathogenesis and virulence of C. difficile. This could mean that we have to renew our view on the role of antibiotics against C. difficile. However, in contrast, bezlotoxumab does not affect the efficacy of standard of care antibiotics since the initial cure rates were 80% for both the antibody and the placebo groups.8 Toxin B levels are not detectable in stool samples between days 4 and 10 of standard of care antibiotic treatment. Afterward, however, they increase again.9 Most of the patients had received bezlotoxumab 3 or more days after they began standard-of-care antibiotic treatment – in the time period when toxin B is undetectable in stool – which underlines the importance of toxin B in the pathogenesis of recurrent C. difficile enteritis.8
In summary, the introduction of bezlotoxumab in clinical care gives new and important insights and solutions not only for treatment options but also for our understanding of C. difficile pathogenesis.
Dr. Schalk is consultant of internal medicine at the department of hematology and oncology, Magdeburg University Hospital, Germany, with clinical and research focus on infectious diseases in hematology and oncology.
Dr. Fischer is professor of internal medicine, hematology and oncology, at the Otto-von-Guericke University Hospital Magdeburg, Germany. He is head of the department of hematology and oncology and a clinical/molecular researcher in myeloid neoplasms. He is a member of the editorial advisory board of Hematology News.
Contact Dr. Schalk at [email protected].
References
1. Vehreschild, MJ et al. Diagnosis and management of gastrointestinal complications in adult cancer patients: Wvidence-based guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Ann Oncol. 2013;24:1189-202
2. Cornely, OA. Current and emerging management options for Clostridium difficile infection: What is the role of fidaxomicin? Clin Microbiol Infect. 2012;18(Suppl 6):28-35.
3. Bartlett, JG et al. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978;298(10):531-4.
4. Lyras, D et al. Toxin B is essential for virulence of Clostridium difficile. Nature. 2009;458:1176-9.
5. Reineke, J et al. Autocatalytic cleavage of Clostridium difficile toxin B. Nature. 2007;446:415-9.
6. Leav, BA et al. Serum anti-toxin B antibody correlates with protection from recurrent Clostridium difficile infection (CDI). Vaccine. 2010;28:965-9.
7. Orth, P et al. Mechanism of action and epitopes of Clostridium difficile toxin B-neutralizing antibody bezlotoxumab revealed by X-ray crystallography. J Biol Chem. 2014;289:18008-21.
8. Wilcox, MH et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376:305-17.
9. Louie, TJ et al. Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin reexpression and recurrence of CDI. Clin Infect Dis. 2012;5(Suppl 2):S132-42.
Infections resulting from Clostridium difficile are a major clinical challenge. In hematology and oncology, the widespread use of broad-spectrum antibiotics is essential for patients with profound neutropenia and infectious complications, which are a high-risk factor for C. difficile enteritis.
C. difficile enteritis occurs in 5%-20% of cancer patients.1 With standard of care antibiotics, oral metronidazole or oral vancomycin, high C. difficile cure rates are possible, but up to 25% of these infections recur. Recently, oral fidaxomicin was approved for treatment of C. difficile enteritis and was associated with high cure rates and, more importantly, with significantly lower recurrence rates.2
Bezlotoxumab, a fully humanized monoclonal antibody against C. difficile toxin B, has been shown by x-ray crystallography to neutralize toxin B by blocking its ability to bind to host cells.7 Most recently, this new therapeutic approach was investigated in humans.8
Wilcox et al. used pooled data of 2655 adults treated in two double-blind, randomized, placebo-controlled phase III clinical trials (MODIFY I and MODIFY II) for primary or recurrent C. difficile enteritis. This industry-sponsored trial was conducted at 322 sites in 30 countries.
In one treatment group, patients received a single infusion of bezlotoxumab (781 patients) or placebo (773 patients) and one of the three oral standard-of-care C. difficile antibiotics. Importantly, the primary end point of this trial was recurrent infection within 12 weeks. About 28% of the patients in both the bezlotoxumab group and the placebo group previously had at least one episode of C. difficile enteritis. About 20% of the patients in both groups were immunocompromised.
Pooled data showed that recurrent infection was significantly lower (P less than 0.001) in the bezlotoxumab group (17%), compared with the placebo group (27%). The difference in recurrence rate (25% vs. 41%) was even more pronounced in patients with one or more episodes of recurrent C. difficile enteritis in the past 6 months. Furthermore, a benefit for bezlotoxumab was seen in immunocompromised patients, whose recurrence rates were 15% with bezlotoxumab, vs. 28% with placebo. After the 12 weeks of follow-up, the absolute difference in the Kaplan-Meier rates of recurrent infection was 13% (absolute rate, 21% in bezlotoxumab group vs. 34% in placebo group; P less than 0.001).
The results indicate that bezlotoxumab, which was approved in 2016 by the U.S. Food and Drug Administration, might improve the outcome of patients with C. difficile enteritis. However, bezlotoxumab is not a “magic bullet.” The number needed to treat to prevent one episode of C. difficile enteritis is 10.
It is conceivable that bezlotoxumab may find its role in high-risk patients – those older than 65 years or patients with recurrent C. difficile enteritis – since the number needed to treat is only 6 in these subgroups.8
This new agent could be an important treatment option for our high-risk patients in hematology. However, more studies concerning costs and real-life efficacy are needed.
The new approach of passive immunization for prevention of recurrent C. difficile enteritis shows the importance and the role of toxin B – not only the bacterium per se – in pathogenesis and virulence of C. difficile. This could mean that we have to renew our view on the role of antibiotics against C. difficile. However, in contrast, bezlotoxumab does not affect the efficacy of standard of care antibiotics since the initial cure rates were 80% for both the antibody and the placebo groups.8 Toxin B levels are not detectable in stool samples between days 4 and 10 of standard of care antibiotic treatment. Afterward, however, they increase again.9 Most of the patients had received bezlotoxumab 3 or more days after they began standard-of-care antibiotic treatment – in the time period when toxin B is undetectable in stool – which underlines the importance of toxin B in the pathogenesis of recurrent C. difficile enteritis.8
In summary, the introduction of bezlotoxumab in clinical care gives new and important insights and solutions not only for treatment options but also for our understanding of C. difficile pathogenesis.
Dr. Schalk is consultant of internal medicine at the department of hematology and oncology, Magdeburg University Hospital, Germany, with clinical and research focus on infectious diseases in hematology and oncology.
Dr. Fischer is professor of internal medicine, hematology and oncology, at the Otto-von-Guericke University Hospital Magdeburg, Germany. He is head of the department of hematology and oncology and a clinical/molecular researcher in myeloid neoplasms. He is a member of the editorial advisory board of Hematology News.
Contact Dr. Schalk at [email protected].
References
1. Vehreschild, MJ et al. Diagnosis and management of gastrointestinal complications in adult cancer patients: Wvidence-based guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Ann Oncol. 2013;24:1189-202
2. Cornely, OA. Current and emerging management options for Clostridium difficile infection: What is the role of fidaxomicin? Clin Microbiol Infect. 2012;18(Suppl 6):28-35.
3. Bartlett, JG et al. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978;298(10):531-4.
4. Lyras, D et al. Toxin B is essential for virulence of Clostridium difficile. Nature. 2009;458:1176-9.
5. Reineke, J et al. Autocatalytic cleavage of Clostridium difficile toxin B. Nature. 2007;446:415-9.
6. Leav, BA et al. Serum anti-toxin B antibody correlates with protection from recurrent Clostridium difficile infection (CDI). Vaccine. 2010;28:965-9.
7. Orth, P et al. Mechanism of action and epitopes of Clostridium difficile toxin B-neutralizing antibody bezlotoxumab revealed by X-ray crystallography. J Biol Chem. 2014;289:18008-21.
8. Wilcox, MH et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376:305-17.
9. Louie, TJ et al. Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin reexpression and recurrence of CDI. Clin Infect Dis. 2012;5(Suppl 2):S132-42.
For vertebral osteomyelitis, early switch to oral antibiotics is feasible
VIENNA – A 6-week course of antibiotics, with an early switch from intravenous to oral, appears to be a safe and appropriate option for some patients with pyogenic vertebral osteomyelitis.
A single-center retrospective study of 82 such patients found two treatment failures and two deaths over 1 year (4.8% failure rate). The patients who died were very elderly with serious comorbidities. The two treatment failures occurred in patients with methicillin-resistant coagulase-negative staphylococcal infections of a central catheter.
“Only two of the failures were due to inadequate antibiotic treatment,” Adrien Lemaignen, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress. “Both patients experienced a relapse of bacteremia with the same bacteria a few days after antibiotic cessation in a context of conservative treatment of a catheter-related infection.”
Guidelines recently adopted by the Infectious Diseases Society of America inspired the study, said Dr. Lemaignen of University Hospital of Tours, France. The 2015 document calls for 6-8 weeks of antibiotics, depending upon the infective organism and whether infective endocarditis complicates management. All suggested antibiotic regimens call for initial IV therapy followed by oral, but there are no cut-and-dried recommendations about when to switch. The guideline notes one study in which patients switched to oral after about 2.7 weeks, with a 97% success rate.
Dr. Lemaignen and his colleagues set out to determine cure rates of early oral relay in 82 patients with pyogenic vertebral osteomyelitis (PVO). All patients were treated at a single center from 2011 to 2016. The team defined treatment failure as death, or persistence or relapse of infection in the first year after treatment.
All patients had culture-proven PVO that also was visible on imaging. Patients were excluded if they had any brucellar, fungal, or mycobacterial coinfections, or if they had infected spinal implants.
The mean age of the patients in the cohort was 66 years; 39% had some neuropathology. The mean C-reactive protein level was 115 mg/L. More than half of the cases (56%) involved the lumbar-sacral spine; 30% were thoracic, and the remainder, cervical. About one-fifth had multiple level involvement. There was epidural inflammation in 68%, epidural abscess in 13%, and extradural abscess in 26%.
Staphylococcus aureus was the most common pathogen (34%); two infections were methicillin resistant. Other infective organisms were streptococci (27%), Gram-negative bacilli (15%), and coagulase-negative staph (12%). A few patients had enterococci (5%) or polymicrobial infections (7%).
Infective endocarditis was present in 16 patients; this was associated with enterococcal and streptococcal infections.
Treatment varied by pathogen. Patients with S. aureus received penicillin or cefazolin with an oral relay to fluoroquinolone/rifampicin or clindamycin. Those with streptococci received amoxicillin with or without an aminoglycoside, followed by oral amoxicillin or clindamycin. Those with coagulase-negative streptococci received a glycopeptide with or without blasticidin, followed by fluoroquinolone/rifampicin. Patients with enterococcal infections got a third generation cephalosporin followed by an oral third generation cephalosporin or a fluoroquinolone.
All but six patients received 6 weeks of treatment.
The mean oral relay occurred on day 12, but 30 patients (36%) were able to switch before 7 days elapsed. Thirteen patients had to stay on the IV route for their entire treatment; 25% of this group had infective endocarditis. Six patients, all of whom had motor symptoms, also needed surgery.
The median follow-up was 358 days. During this time, there were two deaths and two treatment failures.
One death was a 93-year-old who had a controlled sepsis, but died at day 79 of a massive hematemesis. The other was an 80-year-old with an amoxicillin-resistant staph infection and decompensated cirrhosis who died at day 49.
There were also two treatment failures. Both of these patients had methicillin-resistant coagulase-negative staph infections of indwelling central catheters. One had a relapse 70 days after the end of IV therapy; the other relapsed on day 26 of treatment, after a 2-week course of oral antibiotics.
Not all patients were able to succeed with 6 weeks of therapy. Three needed prolonged treatment: One of these had an infected vascular prosthesis and two were immunocompromised patients who had cervical osteomyelitis with multiple abscesses.
In light of these results, Dr. Lemaignen said, “We can say confirm the safety of short IV treatment with an early oral relay in pyogenic vertebral osteomyelitis under real-life conditions, with 95% success rate and good functional outcomes at 6 months.”
He had no relevant financial disclosures.
[email protected]
On Twitter @Alz_gal
VIENNA – A 6-week course of antibiotics, with an early switch from intravenous to oral, appears to be a safe and appropriate option for some patients with pyogenic vertebral osteomyelitis.
A single-center retrospective study of 82 such patients found two treatment failures and two deaths over 1 year (4.8% failure rate). The patients who died were very elderly with serious comorbidities. The two treatment failures occurred in patients with methicillin-resistant coagulase-negative staphylococcal infections of a central catheter.
“Only two of the failures were due to inadequate antibiotic treatment,” Adrien Lemaignen, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress. “Both patients experienced a relapse of bacteremia with the same bacteria a few days after antibiotic cessation in a context of conservative treatment of a catheter-related infection.”
Guidelines recently adopted by the Infectious Diseases Society of America inspired the study, said Dr. Lemaignen of University Hospital of Tours, France. The 2015 document calls for 6-8 weeks of antibiotics, depending upon the infective organism and whether infective endocarditis complicates management. All suggested antibiotic regimens call for initial IV therapy followed by oral, but there are no cut-and-dried recommendations about when to switch. The guideline notes one study in which patients switched to oral after about 2.7 weeks, with a 97% success rate.
Dr. Lemaignen and his colleagues set out to determine cure rates of early oral relay in 82 patients with pyogenic vertebral osteomyelitis (PVO). All patients were treated at a single center from 2011 to 2016. The team defined treatment failure as death, or persistence or relapse of infection in the first year after treatment.
All patients had culture-proven PVO that also was visible on imaging. Patients were excluded if they had any brucellar, fungal, or mycobacterial coinfections, or if they had infected spinal implants.
The mean age of the patients in the cohort was 66 years; 39% had some neuropathology. The mean C-reactive protein level was 115 mg/L. More than half of the cases (56%) involved the lumbar-sacral spine; 30% were thoracic, and the remainder, cervical. About one-fifth had multiple level involvement. There was epidural inflammation in 68%, epidural abscess in 13%, and extradural abscess in 26%.
Staphylococcus aureus was the most common pathogen (34%); two infections were methicillin resistant. Other infective organisms were streptococci (27%), Gram-negative bacilli (15%), and coagulase-negative staph (12%). A few patients had enterococci (5%) or polymicrobial infections (7%).
Infective endocarditis was present in 16 patients; this was associated with enterococcal and streptococcal infections.
Treatment varied by pathogen. Patients with S. aureus received penicillin or cefazolin with an oral relay to fluoroquinolone/rifampicin or clindamycin. Those with streptococci received amoxicillin with or without an aminoglycoside, followed by oral amoxicillin or clindamycin. Those with coagulase-negative streptococci received a glycopeptide with or without blasticidin, followed by fluoroquinolone/rifampicin. Patients with enterococcal infections got a third generation cephalosporin followed by an oral third generation cephalosporin or a fluoroquinolone.
All but six patients received 6 weeks of treatment.
The mean oral relay occurred on day 12, but 30 patients (36%) were able to switch before 7 days elapsed. Thirteen patients had to stay on the IV route for their entire treatment; 25% of this group had infective endocarditis. Six patients, all of whom had motor symptoms, also needed surgery.
The median follow-up was 358 days. During this time, there were two deaths and two treatment failures.
One death was a 93-year-old who had a controlled sepsis, but died at day 79 of a massive hematemesis. The other was an 80-year-old with an amoxicillin-resistant staph infection and decompensated cirrhosis who died at day 49.
There were also two treatment failures. Both of these patients had methicillin-resistant coagulase-negative staph infections of indwelling central catheters. One had a relapse 70 days after the end of IV therapy; the other relapsed on day 26 of treatment, after a 2-week course of oral antibiotics.
Not all patients were able to succeed with 6 weeks of therapy. Three needed prolonged treatment: One of these had an infected vascular prosthesis and two were immunocompromised patients who had cervical osteomyelitis with multiple abscesses.
In light of these results, Dr. Lemaignen said, “We can say confirm the safety of short IV treatment with an early oral relay in pyogenic vertebral osteomyelitis under real-life conditions, with 95% success rate and good functional outcomes at 6 months.”
He had no relevant financial disclosures.
[email protected]
On Twitter @Alz_gal
VIENNA – A 6-week course of antibiotics, with an early switch from intravenous to oral, appears to be a safe and appropriate option for some patients with pyogenic vertebral osteomyelitis.
A single-center retrospective study of 82 such patients found two treatment failures and two deaths over 1 year (4.8% failure rate). The patients who died were very elderly with serious comorbidities. The two treatment failures occurred in patients with methicillin-resistant coagulase-negative staphylococcal infections of a central catheter.
“Only two of the failures were due to inadequate antibiotic treatment,” Adrien Lemaignen, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress. “Both patients experienced a relapse of bacteremia with the same bacteria a few days after antibiotic cessation in a context of conservative treatment of a catheter-related infection.”
Guidelines recently adopted by the Infectious Diseases Society of America inspired the study, said Dr. Lemaignen of University Hospital of Tours, France. The 2015 document calls for 6-8 weeks of antibiotics, depending upon the infective organism and whether infective endocarditis complicates management. All suggested antibiotic regimens call for initial IV therapy followed by oral, but there are no cut-and-dried recommendations about when to switch. The guideline notes one study in which patients switched to oral after about 2.7 weeks, with a 97% success rate.
Dr. Lemaignen and his colleagues set out to determine cure rates of early oral relay in 82 patients with pyogenic vertebral osteomyelitis (PVO). All patients were treated at a single center from 2011 to 2016. The team defined treatment failure as death, or persistence or relapse of infection in the first year after treatment.
All patients had culture-proven PVO that also was visible on imaging. Patients were excluded if they had any brucellar, fungal, or mycobacterial coinfections, or if they had infected spinal implants.
The mean age of the patients in the cohort was 66 years; 39% had some neuropathology. The mean C-reactive protein level was 115 mg/L. More than half of the cases (56%) involved the lumbar-sacral spine; 30% were thoracic, and the remainder, cervical. About one-fifth had multiple level involvement. There was epidural inflammation in 68%, epidural abscess in 13%, and extradural abscess in 26%.
Staphylococcus aureus was the most common pathogen (34%); two infections were methicillin resistant. Other infective organisms were streptococci (27%), Gram-negative bacilli (15%), and coagulase-negative staph (12%). A few patients had enterococci (5%) or polymicrobial infections (7%).
Infective endocarditis was present in 16 patients; this was associated with enterococcal and streptococcal infections.
Treatment varied by pathogen. Patients with S. aureus received penicillin or cefazolin with an oral relay to fluoroquinolone/rifampicin or clindamycin. Those with streptococci received amoxicillin with or without an aminoglycoside, followed by oral amoxicillin or clindamycin. Those with coagulase-negative streptococci received a glycopeptide with or without blasticidin, followed by fluoroquinolone/rifampicin. Patients with enterococcal infections got a third generation cephalosporin followed by an oral third generation cephalosporin or a fluoroquinolone.
All but six patients received 6 weeks of treatment.
The mean oral relay occurred on day 12, but 30 patients (36%) were able to switch before 7 days elapsed. Thirteen patients had to stay on the IV route for their entire treatment; 25% of this group had infective endocarditis. Six patients, all of whom had motor symptoms, also needed surgery.
The median follow-up was 358 days. During this time, there were two deaths and two treatment failures.
One death was a 93-year-old who had a controlled sepsis, but died at day 79 of a massive hematemesis. The other was an 80-year-old with an amoxicillin-resistant staph infection and decompensated cirrhosis who died at day 49.
There were also two treatment failures. Both of these patients had methicillin-resistant coagulase-negative staph infections of indwelling central catheters. One had a relapse 70 days after the end of IV therapy; the other relapsed on day 26 of treatment, after a 2-week course of oral antibiotics.
Not all patients were able to succeed with 6 weeks of therapy. Three needed prolonged treatment: One of these had an infected vascular prosthesis and two were immunocompromised patients who had cervical osteomyelitis with multiple abscesses.
In light of these results, Dr. Lemaignen said, “We can say confirm the safety of short IV treatment with an early oral relay in pyogenic vertebral osteomyelitis under real-life conditions, with 95% success rate and good functional outcomes at 6 months.”
He had no relevant financial disclosures.
[email protected]
On Twitter @Alz_gal
AT ECCMID 2017
Key clinical point:
Major finding: There were two treatment failures attributable to the antibiotic regimen, and two deaths that were not, for a total treatment success rate of 95%.
Data source: A retrospective cohort comprising 82 patients.
Disclosures: Dr. Lemaignen had no financial disclosures.
Ribaxamase prevented C. difficile infections by protecting microbiome
VIENNA – An investigational beta-lactamase reduced Clostridium difficile infections by 71% in patients receiving extended antibiotic therapy for respiratory infections but not by killing the opportunistic bacteria.
Rather, ribaxamase prevented C. difficile infections (CDI) by breaking down excess therapeutic antibiotics in the gut before they could injure an otherwise healthy microbiome, John Kokai-Kun, PhD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
Ribaxamase is an oral enzyme that breaks the lactam ring in penicillins and cephalosporins. It’s formulated to release at a pH of 5.5 or higher, an environment that begins to develop in the upper small intestine near the bile duct – the same place that excess antibiotics are excreted.
“The drug is intended to be administered during, and for a short time after, intravenous administration of specific beta-lactam–containing antibiotics,” Dr. Kokai-Kun said. Ribaxamase doesn’t work on carbapenem-type antibiotics, he noted, and Synthetic Biologics is working on an effective enzyme for those as well.
In early human studies, ribaxamase was well tolerated and didn’t interfere with the pharmacokinetics of therapeutic antibiotics (Antimicrob Agents Chemother. 2017 Mar;61[3]:e02197-16). It’s also effective in patients who are taking a proton pump inhibitor, he said.
Dr. Kokai-Kun reported the results of a phase IIb study of 412 patients who received IV ceftriaxone for lower respiratory infections. They were assigned 1:1 to either 150 mg ribaxamase daily or placebo throughout the IV treatment and for 3 days after.
The primary endpoint was prevention of C. difficile infection. The secondary endpoint was prevention of non–C. difficile antibiotic-associated diarrhea. An exploratory endpoint examined the drug’s ability to protect the microbiome. Patients were monitored for 6 weeks after treatment stopped.
The cohort was a mean 70 years old. One-third of patients also received a macrolide during their hospitalization, and one-third were taking proton pump inhibitors. The respiratory infection cure rate was about 99% in both groups at both 72 hours and 4 weeks.
Eight patients in the placebo group (3.8%) and two in the active group (less than 1%) developed C. difficile infection. That translated to a statistically significant 71% risk reduction, with a P value of .027, Dr. Kokai-Kun said. Ribaxamase did not hit its secondary endpoint of preventing all-cause diarrhea or antibiotic-associated diarrhea that was not caused by C. difficile infection.
Although not a primary finding, ribaxamase also inhibited colonization by vancomycin-resistant enterococci, which occurred in about 70 (40%) patients in the placebo group and 40 (20%) in the ribaxamase group at both 72 hours and 4 weeks.
All patients contributed stool samples at baseline and after treatment for microbiome analysis. That portion of the study is still ongoing, Dr. Kokai-Kun said.
Synthetic Biologics sponsored the study and is developing ribaxamase. Dr. Kokai-Kun is the company’s vice president of nonclinical affairs.
[email protected]
On Twitter @alz_gal
VIENNA – An investigational beta-lactamase reduced Clostridium difficile infections by 71% in patients receiving extended antibiotic therapy for respiratory infections but not by killing the opportunistic bacteria.
Rather, ribaxamase prevented C. difficile infections (CDI) by breaking down excess therapeutic antibiotics in the gut before they could injure an otherwise healthy microbiome, John Kokai-Kun, PhD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
Ribaxamase is an oral enzyme that breaks the lactam ring in penicillins and cephalosporins. It’s formulated to release at a pH of 5.5 or higher, an environment that begins to develop in the upper small intestine near the bile duct – the same place that excess antibiotics are excreted.
“The drug is intended to be administered during, and for a short time after, intravenous administration of specific beta-lactam–containing antibiotics,” Dr. Kokai-Kun said. Ribaxamase doesn’t work on carbapenem-type antibiotics, he noted, and Synthetic Biologics is working on an effective enzyme for those as well.
In early human studies, ribaxamase was well tolerated and didn’t interfere with the pharmacokinetics of therapeutic antibiotics (Antimicrob Agents Chemother. 2017 Mar;61[3]:e02197-16). It’s also effective in patients who are taking a proton pump inhibitor, he said.
Dr. Kokai-Kun reported the results of a phase IIb study of 412 patients who received IV ceftriaxone for lower respiratory infections. They were assigned 1:1 to either 150 mg ribaxamase daily or placebo throughout the IV treatment and for 3 days after.
The primary endpoint was prevention of C. difficile infection. The secondary endpoint was prevention of non–C. difficile antibiotic-associated diarrhea. An exploratory endpoint examined the drug’s ability to protect the microbiome. Patients were monitored for 6 weeks after treatment stopped.
The cohort was a mean 70 years old. One-third of patients also received a macrolide during their hospitalization, and one-third were taking proton pump inhibitors. The respiratory infection cure rate was about 99% in both groups at both 72 hours and 4 weeks.
Eight patients in the placebo group (3.8%) and two in the active group (less than 1%) developed C. difficile infection. That translated to a statistically significant 71% risk reduction, with a P value of .027, Dr. Kokai-Kun said. Ribaxamase did not hit its secondary endpoint of preventing all-cause diarrhea or antibiotic-associated diarrhea that was not caused by C. difficile infection.
Although not a primary finding, ribaxamase also inhibited colonization by vancomycin-resistant enterococci, which occurred in about 70 (40%) patients in the placebo group and 40 (20%) in the ribaxamase group at both 72 hours and 4 weeks.
All patients contributed stool samples at baseline and after treatment for microbiome analysis. That portion of the study is still ongoing, Dr. Kokai-Kun said.
Synthetic Biologics sponsored the study and is developing ribaxamase. Dr. Kokai-Kun is the company’s vice president of nonclinical affairs.
[email protected]
On Twitter @alz_gal
VIENNA – An investigational beta-lactamase reduced Clostridium difficile infections by 71% in patients receiving extended antibiotic therapy for respiratory infections but not by killing the opportunistic bacteria.
Rather, ribaxamase prevented C. difficile infections (CDI) by breaking down excess therapeutic antibiotics in the gut before they could injure an otherwise healthy microbiome, John Kokai-Kun, PhD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
Ribaxamase is an oral enzyme that breaks the lactam ring in penicillins and cephalosporins. It’s formulated to release at a pH of 5.5 or higher, an environment that begins to develop in the upper small intestine near the bile duct – the same place that excess antibiotics are excreted.
“The drug is intended to be administered during, and for a short time after, intravenous administration of specific beta-lactam–containing antibiotics,” Dr. Kokai-Kun said. Ribaxamase doesn’t work on carbapenem-type antibiotics, he noted, and Synthetic Biologics is working on an effective enzyme for those as well.
In early human studies, ribaxamase was well tolerated and didn’t interfere with the pharmacokinetics of therapeutic antibiotics (Antimicrob Agents Chemother. 2017 Mar;61[3]:e02197-16). It’s also effective in patients who are taking a proton pump inhibitor, he said.
Dr. Kokai-Kun reported the results of a phase IIb study of 412 patients who received IV ceftriaxone for lower respiratory infections. They were assigned 1:1 to either 150 mg ribaxamase daily or placebo throughout the IV treatment and for 3 days after.
The primary endpoint was prevention of C. difficile infection. The secondary endpoint was prevention of non–C. difficile antibiotic-associated diarrhea. An exploratory endpoint examined the drug’s ability to protect the microbiome. Patients were monitored for 6 weeks after treatment stopped.
The cohort was a mean 70 years old. One-third of patients also received a macrolide during their hospitalization, and one-third were taking proton pump inhibitors. The respiratory infection cure rate was about 99% in both groups at both 72 hours and 4 weeks.
Eight patients in the placebo group (3.8%) and two in the active group (less than 1%) developed C. difficile infection. That translated to a statistically significant 71% risk reduction, with a P value of .027, Dr. Kokai-Kun said. Ribaxamase did not hit its secondary endpoint of preventing all-cause diarrhea or antibiotic-associated diarrhea that was not caused by C. difficile infection.
Although not a primary finding, ribaxamase also inhibited colonization by vancomycin-resistant enterococci, which occurred in about 70 (40%) patients in the placebo group and 40 (20%) in the ribaxamase group at both 72 hours and 4 weeks.
All patients contributed stool samples at baseline and after treatment for microbiome analysis. That portion of the study is still ongoing, Dr. Kokai-Kun said.
Synthetic Biologics sponsored the study and is developing ribaxamase. Dr. Kokai-Kun is the company’s vice president of nonclinical affairs.
[email protected]
On Twitter @alz_gal
AT ECCMID 2017
Key clinical point:
Major finding: Ribaxamase reduced C. difficile infections by 71%, relative to a placebo.
Data source: The study randomized 412 patients to either placebo or ribaxamase in addition to their therapeutic antibiotics.
Disclosures: Synthetic Biologics sponsored the study and is developing ribaxamase. Dr. Kokai-Kun is the company’s vice president of nonclinical affairs.
VIDEO: Registry study will follow 4,000 fecal transplant patients for 10 years
CHICAGO – A 10-year registry study aims to gather clinical and patient-reported outcomes on 4,000 adult and pediatric patients who undergo fecal microbiota transplant in the United States, officials of the American Gastroenterological Association announced during Digestive Disease Week®.
The AGA Fecal Microbiota Transplantation National Registry will be the first study to assess both short- and long-term patient outcomes associated with fecal microbiota transplant (FMT) in both adults and children, Colleen Kelly, MD, said in an video interview. Most subjects will have received FMT for recurrent or refractory Clostridium difficile infections – the only indication for which Food and Drug Administration currently allows independent clinician action. But the investigational uses of FMT are expanding rapidly, and patients who undergo the procedure during any registered study will be eligible for enrollment, said Dr. Kelly, co-chair of the study’s steering committee.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The study’s primary objectives are short- and long-term safety outcomes, said Dr. Kelly of Brown University, Providence, R.I. While generally considered quite safe, short-term adverse events have been reported with FMT, and some of them have been serious – including one death from aspiration pneumonia in a patient who received donor stool via nasogastric tube (Clin Infect Dis. 2015 Mar;61[1]:136-7). Other adverse events are usually self-limited but can include low-grade fever, abdominal pain, distention, bloating, and diarrhea.
Researchers seek to illuminate many of the unknowns associated with this relatively new procedure. Scientists are only now beginning to unravel the myriad ways the human microbiome promotes both health and disease. Specific alterations, for example, have been associated with obesity and other conditions; there is concern that transplanting a new microbial population could induce a disease phenotype in a recipient who might not have otherwise been at risk.
With the planned cohort size and follow-up period, the study should be able to detect any unanticipated adverse events that occur in more than 1% of the population, Dr. Kelly said. It will include a comparator group of patients with recurrent or refractory C. difficile infection from a large insurance claims database to allow comparison between patients treated with FMT and those treated with antibiotics only.
The registry study also aims to discover which method or methods of transplant material delivery are best, she said. Right now, there are a number of methods (colonoscopy/sigmoidoscopy, enema, upper gastrointestinal endoscopy, nasogastric or nasoduodenal tube, and capsules), and no consensus on which is the best. As indications for FMT expand, there may be no single best method. The approach will probably be matched to the disorder being treated, and the study may help illuminate this as well.
For the first 2 years after a transplant, clinicians will follow patients and enter data into the registry. After that, an electronic patient-reported outcomes system will automatically contact the patient annually for follow-up information by email or text message. When patients enter their data, they can access educational material that will help keep them up-to-date on potential adverse events.
The study will also include a biobank of stool samples obtained during the procedures, hosted by the American Gut Project and the Microbiome Initiative at the University of California, San Diego. This arm of the project will analyze the microbiome of 3,000 stool samples from recipients, both before and after their transplant, as well as the corresponding donors whose material was used in the fecal transplant.
The registry study, a project of the AGA Center for Gut Microbiome Research and Education, is funded by a $3.3 million grant from the National Institute of Allergy and Infectious Diseases. It will be conducted in partnership with the Crohn’s and Colitis Foundation, Infectious Diseases Society, and the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition.
The registry study currently is accepting applications. Physicians who perform FMT for C. difficile infections, and centers that conduct FMT research for other potential indications, can fill out a short survey to indicate their interest.
Digestive Disease Week® is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE) and the Society for Surgery of the Alimentary Tract (SSAT).
This article was updated June 8, 2017.
[email protected]
On Twitter @Alz_gal
CHICAGO – A 10-year registry study aims to gather clinical and patient-reported outcomes on 4,000 adult and pediatric patients who undergo fecal microbiota transplant in the United States, officials of the American Gastroenterological Association announced during Digestive Disease Week®.
The AGA Fecal Microbiota Transplantation National Registry will be the first study to assess both short- and long-term patient outcomes associated with fecal microbiota transplant (FMT) in both adults and children, Colleen Kelly, MD, said in an video interview. Most subjects will have received FMT for recurrent or refractory Clostridium difficile infections – the only indication for which Food and Drug Administration currently allows independent clinician action. But the investigational uses of FMT are expanding rapidly, and patients who undergo the procedure during any registered study will be eligible for enrollment, said Dr. Kelly, co-chair of the study’s steering committee.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The study’s primary objectives are short- and long-term safety outcomes, said Dr. Kelly of Brown University, Providence, R.I. While generally considered quite safe, short-term adverse events have been reported with FMT, and some of them have been serious – including one death from aspiration pneumonia in a patient who received donor stool via nasogastric tube (Clin Infect Dis. 2015 Mar;61[1]:136-7). Other adverse events are usually self-limited but can include low-grade fever, abdominal pain, distention, bloating, and diarrhea.
Researchers seek to illuminate many of the unknowns associated with this relatively new procedure. Scientists are only now beginning to unravel the myriad ways the human microbiome promotes both health and disease. Specific alterations, for example, have been associated with obesity and other conditions; there is concern that transplanting a new microbial population could induce a disease phenotype in a recipient who might not have otherwise been at risk.
With the planned cohort size and follow-up period, the study should be able to detect any unanticipated adverse events that occur in more than 1% of the population, Dr. Kelly said. It will include a comparator group of patients with recurrent or refractory C. difficile infection from a large insurance claims database to allow comparison between patients treated with FMT and those treated with antibiotics only.
The registry study also aims to discover which method or methods of transplant material delivery are best, she said. Right now, there are a number of methods (colonoscopy/sigmoidoscopy, enema, upper gastrointestinal endoscopy, nasogastric or nasoduodenal tube, and capsules), and no consensus on which is the best. As indications for FMT expand, there may be no single best method. The approach will probably be matched to the disorder being treated, and the study may help illuminate this as well.
For the first 2 years after a transplant, clinicians will follow patients and enter data into the registry. After that, an electronic patient-reported outcomes system will automatically contact the patient annually for follow-up information by email or text message. When patients enter their data, they can access educational material that will help keep them up-to-date on potential adverse events.
The study will also include a biobank of stool samples obtained during the procedures, hosted by the American Gut Project and the Microbiome Initiative at the University of California, San Diego. This arm of the project will analyze the microbiome of 3,000 stool samples from recipients, both before and after their transplant, as well as the corresponding donors whose material was used in the fecal transplant.
The registry study, a project of the AGA Center for Gut Microbiome Research and Education, is funded by a $3.3 million grant from the National Institute of Allergy and Infectious Diseases. It will be conducted in partnership with the Crohn’s and Colitis Foundation, Infectious Diseases Society, and the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition.
The registry study currently is accepting applications. Physicians who perform FMT for C. difficile infections, and centers that conduct FMT research for other potential indications, can fill out a short survey to indicate their interest.
Digestive Disease Week® is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE) and the Society for Surgery of the Alimentary Tract (SSAT).
This article was updated June 8, 2017.
[email protected]
On Twitter @Alz_gal
CHICAGO – A 10-year registry study aims to gather clinical and patient-reported outcomes on 4,000 adult and pediatric patients who undergo fecal microbiota transplant in the United States, officials of the American Gastroenterological Association announced during Digestive Disease Week®.
The AGA Fecal Microbiota Transplantation National Registry will be the first study to assess both short- and long-term patient outcomes associated with fecal microbiota transplant (FMT) in both adults and children, Colleen Kelly, MD, said in an video interview. Most subjects will have received FMT for recurrent or refractory Clostridium difficile infections – the only indication for which Food and Drug Administration currently allows independent clinician action. But the investigational uses of FMT are expanding rapidly, and patients who undergo the procedure during any registered study will be eligible for enrollment, said Dr. Kelly, co-chair of the study’s steering committee.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The study’s primary objectives are short- and long-term safety outcomes, said Dr. Kelly of Brown University, Providence, R.I. While generally considered quite safe, short-term adverse events have been reported with FMT, and some of them have been serious – including one death from aspiration pneumonia in a patient who received donor stool via nasogastric tube (Clin Infect Dis. 2015 Mar;61[1]:136-7). Other adverse events are usually self-limited but can include low-grade fever, abdominal pain, distention, bloating, and diarrhea.
Researchers seek to illuminate many of the unknowns associated with this relatively new procedure. Scientists are only now beginning to unravel the myriad ways the human microbiome promotes both health and disease. Specific alterations, for example, have been associated with obesity and other conditions; there is concern that transplanting a new microbial population could induce a disease phenotype in a recipient who might not have otherwise been at risk.
With the planned cohort size and follow-up period, the study should be able to detect any unanticipated adverse events that occur in more than 1% of the population, Dr. Kelly said. It will include a comparator group of patients with recurrent or refractory C. difficile infection from a large insurance claims database to allow comparison between patients treated with FMT and those treated with antibiotics only.
The registry study also aims to discover which method or methods of transplant material delivery are best, she said. Right now, there are a number of methods (colonoscopy/sigmoidoscopy, enema, upper gastrointestinal endoscopy, nasogastric or nasoduodenal tube, and capsules), and no consensus on which is the best. As indications for FMT expand, there may be no single best method. The approach will probably be matched to the disorder being treated, and the study may help illuminate this as well.
For the first 2 years after a transplant, clinicians will follow patients and enter data into the registry. After that, an electronic patient-reported outcomes system will automatically contact the patient annually for follow-up information by email or text message. When patients enter their data, they can access educational material that will help keep them up-to-date on potential adverse events.
The study will also include a biobank of stool samples obtained during the procedures, hosted by the American Gut Project and the Microbiome Initiative at the University of California, San Diego. This arm of the project will analyze the microbiome of 3,000 stool samples from recipients, both before and after their transplant, as well as the corresponding donors whose material was used in the fecal transplant.
The registry study, a project of the AGA Center for Gut Microbiome Research and Education, is funded by a $3.3 million grant from the National Institute of Allergy and Infectious Diseases. It will be conducted in partnership with the Crohn’s and Colitis Foundation, Infectious Diseases Society, and the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition.
The registry study currently is accepting applications. Physicians who perform FMT for C. difficile infections, and centers that conduct FMT research for other potential indications, can fill out a short survey to indicate their interest.
Digestive Disease Week® is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE) and the Society for Surgery of the Alimentary Tract (SSAT).
This article was updated June 8, 2017.
[email protected]
On Twitter @Alz_gal
AT DDW
Each added day of pediatric MRSA bacteremia upped complication risk 50%
Every additional day of methicillin-resistant Staphylococcus aureus (MRSA) bacteremia in hospitalized children was associated with a 50% increased risk of developing a complication, reported Rana F. Hamdy, MD, of Children’s National Health System, Washington, and her associates.
That was one of the findings of a study performed to determine the epidemiology, clinical outcomes, and risk factors for treatment failure in pediatric MRSA bacteremia. It took place in three hospitals, one each in Philadelphia, Baltimore, and Salt Lake City.
“This finding is in contrast to the epidemiology of MRSA bacteremia in adults, in whom bacteremia is more frequently attributed to catheter-related infections (31%-36%), endovascular infections (13%-15%), or an unknown source (15%-20%), and the durations of MRSA bacteremia are typically more prolonged (median duration of bacteremia is 8-9 days),” Dr. Hamdy and her associates wrote.
“Differences in the epidemiology of MRSA bacteremia between children and adults emphasize the need for dedicated pediatric studies to better understand the clinical characteristics and outcomes specific to children,” the researchers noted.
Musculoskeletal infections and endovascular infections were linked with treatment failure, possibly reflecting “the relatively higher burden of bacteria and/or decreased drug penetration into bone and endovascular infection sites,” the investigators said. Catheter-related infections were tied to reduced odds of treatment failure, “these episodes being localized to the catheter and therefore potentially less-invasive S. aureus infections.”
Mortality among these children with MRSA bacteremia was low, at 2%, but “nearly one-quarter of all patients experienced complications,” the study authors said (Pediatrics. 2017 May 5. doi: 10.1542/peds.2017-0183).
There was progression of infection in 7% of cases, and hematogenous complications or sequelae occurred in 23%. Twenty percent of children developed septic emboli or another metastatic focus of infection.
“This association between the duration of bacteremia and the development of complications has been previously reported among adults with S. aureus bacteremia,” Dr. Hamdy noted, “and provides important epidemiologic data that could inform decisions relating to the timing of additional imaging, such as echocardiograms, to identify metastatic foci.”
The children were treated with vancomycin, and some received additional anti-MRSA antibiotics. “Vancomycin trough concentrations or [minimum inhibitory concentrations] were not associated with treatment failure,” the investigators said. “Future studies to determine the appropriate vancomycin dose, duration, and approach to therapeutic drug monitoring are warranted to optimize patient outcomes.”
The National Institutes of Health funded the study. Dr. Hamdy and her associates disclosed they have no relevant financial relationships.
Every additional day of methicillin-resistant Staphylococcus aureus (MRSA) bacteremia in hospitalized children was associated with a 50% increased risk of developing a complication, reported Rana F. Hamdy, MD, of Children’s National Health System, Washington, and her associates.
That was one of the findings of a study performed to determine the epidemiology, clinical outcomes, and risk factors for treatment failure in pediatric MRSA bacteremia. It took place in three hospitals, one each in Philadelphia, Baltimore, and Salt Lake City.
“This finding is in contrast to the epidemiology of MRSA bacteremia in adults, in whom bacteremia is more frequently attributed to catheter-related infections (31%-36%), endovascular infections (13%-15%), or an unknown source (15%-20%), and the durations of MRSA bacteremia are typically more prolonged (median duration of bacteremia is 8-9 days),” Dr. Hamdy and her associates wrote.
“Differences in the epidemiology of MRSA bacteremia between children and adults emphasize the need for dedicated pediatric studies to better understand the clinical characteristics and outcomes specific to children,” the researchers noted.
Musculoskeletal infections and endovascular infections were linked with treatment failure, possibly reflecting “the relatively higher burden of bacteria and/or decreased drug penetration into bone and endovascular infection sites,” the investigators said. Catheter-related infections were tied to reduced odds of treatment failure, “these episodes being localized to the catheter and therefore potentially less-invasive S. aureus infections.”
Mortality among these children with MRSA bacteremia was low, at 2%, but “nearly one-quarter of all patients experienced complications,” the study authors said (Pediatrics. 2017 May 5. doi: 10.1542/peds.2017-0183).
There was progression of infection in 7% of cases, and hematogenous complications or sequelae occurred in 23%. Twenty percent of children developed septic emboli or another metastatic focus of infection.
“This association between the duration of bacteremia and the development of complications has been previously reported among adults with S. aureus bacteremia,” Dr. Hamdy noted, “and provides important epidemiologic data that could inform decisions relating to the timing of additional imaging, such as echocardiograms, to identify metastatic foci.”
The children were treated with vancomycin, and some received additional anti-MRSA antibiotics. “Vancomycin trough concentrations or [minimum inhibitory concentrations] were not associated with treatment failure,” the investigators said. “Future studies to determine the appropriate vancomycin dose, duration, and approach to therapeutic drug monitoring are warranted to optimize patient outcomes.”
The National Institutes of Health funded the study. Dr. Hamdy and her associates disclosed they have no relevant financial relationships.
Every additional day of methicillin-resistant Staphylococcus aureus (MRSA) bacteremia in hospitalized children was associated with a 50% increased risk of developing a complication, reported Rana F. Hamdy, MD, of Children’s National Health System, Washington, and her associates.
That was one of the findings of a study performed to determine the epidemiology, clinical outcomes, and risk factors for treatment failure in pediatric MRSA bacteremia. It took place in three hospitals, one each in Philadelphia, Baltimore, and Salt Lake City.
“This finding is in contrast to the epidemiology of MRSA bacteremia in adults, in whom bacteremia is more frequently attributed to catheter-related infections (31%-36%), endovascular infections (13%-15%), or an unknown source (15%-20%), and the durations of MRSA bacteremia are typically more prolonged (median duration of bacteremia is 8-9 days),” Dr. Hamdy and her associates wrote.
“Differences in the epidemiology of MRSA bacteremia between children and adults emphasize the need for dedicated pediatric studies to better understand the clinical characteristics and outcomes specific to children,” the researchers noted.
Musculoskeletal infections and endovascular infections were linked with treatment failure, possibly reflecting “the relatively higher burden of bacteria and/or decreased drug penetration into bone and endovascular infection sites,” the investigators said. Catheter-related infections were tied to reduced odds of treatment failure, “these episodes being localized to the catheter and therefore potentially less-invasive S. aureus infections.”
Mortality among these children with MRSA bacteremia was low, at 2%, but “nearly one-quarter of all patients experienced complications,” the study authors said (Pediatrics. 2017 May 5. doi: 10.1542/peds.2017-0183).
There was progression of infection in 7% of cases, and hematogenous complications or sequelae occurred in 23%. Twenty percent of children developed septic emboli or another metastatic focus of infection.
“This association between the duration of bacteremia and the development of complications has been previously reported among adults with S. aureus bacteremia,” Dr. Hamdy noted, “and provides important epidemiologic data that could inform decisions relating to the timing of additional imaging, such as echocardiograms, to identify metastatic foci.”
The children were treated with vancomycin, and some received additional anti-MRSA antibiotics. “Vancomycin trough concentrations or [minimum inhibitory concentrations] were not associated with treatment failure,” the investigators said. “Future studies to determine the appropriate vancomycin dose, duration, and approach to therapeutic drug monitoring are warranted to optimize patient outcomes.”
The National Institutes of Health funded the study. Dr. Hamdy and her associates disclosed they have no relevant financial relationships.
FROM PEDIATRICS
Key clinical point:
Major finding: The primary sources of infection were osteomyelitis (31%), catheter-related bloodstream infections (22%), and skin and soft tissue infections (16%); endocarditis occurred in only 2% – a different epidemiology than in adults.
Data source: A study of 174 hospitalized children (younger than 19 years) with MRSA bacteremia at three hospitals in different states.
Disclosures: The National Institutes of Health funded the study. Dr. Hamdy and her associates disclosed they have no relevant financial relationships.
Hospital infections top WHO’s list of priority pathogens
The World Health Organization is urging governments to focus antibiotic research efforts on a list of urgent bacterial threats, topped by several increasingly powerful superbugs that cause hospital-based infections and other potentially deadly conditions.
The WHO listed the top 20 bacteria that it believes are most harmful to human health, other than mycobacteria such as Mycobacterium tuberculosis, which causes tuberculosis. The germ was not included in the list because it’s generally accepted to be the most urgent priority for new antibiotic research and development, Marie-Paule Kieny, PhD, a WHO assistant director, said at a press conference.
The priority list is needed because the antibiotic pipeline is “practically dry,” thanks to scientific research challenges and a lack of financial incentives, according to Dr. Kieny. “Antibiotics are generally used for the short term, unlike therapies for chronic diseases, which bring in much higher returns on investment,” she said. The list “is intended to signal to the scientific community and the pharmaceutical industry the areas they should focus on to address urgent public health threats.”
The WHO list begins with Priority 1/“Critical” pathogens that it believes most urgently need to be targeted through antibiotic research and development: Acinetobacter baumannii, carbapenem-resistant; Pseudomonas aeruginosa, carbapenem-resistant; and Enterobacteriaceae (including Klebsiella pneumonia, Escherichia coli, Enterobacter spp., Serratia spp., Proteus spp., Providencia spp., and Morganella spp.), carbapenem-resistant, extended-spectrum beta-lactamase–producing.
“These bacteria are responsible for severe infections and high mortality rates, mostly in hospitalized patients, transplant recipients, those receiving chemotherapy, or patients in intensive care units,” Dr. Kieny said. “While these bacteria are not widespread and do not generally affect healthy individuals, the burden for patients and society is now alarming – and new, effective therapies are imperative.”
Priority 2/”High” pathogens are Enterococcus faecium, vancomycin-resistant; Staphylococcus aureus, methicillin-resistant, vancomycin intermediate and resistant; Helicobacter pylori, clarithromycin-resistant; Campylobacter, fluoroquinolone-resistant; Salmonella spp., fluoroquinolone-resistant; Neisseria gonorrhoeae, third-generation cephalosporin-resistant and fluoroquinolone-resistant.
Pathogens in this category can infect healthy individuals, Dr. Kieny noted. “These infections, although not associated with significant mortality, have a dramatic health and economic impact on communities and, in particular, in low-income countries.”
Priority 3/”Medium” pathogens are Streptococcus pneumoniae, penicillin–non-susceptible; Haemophilus influenzae, ampicillin-resistant; and Shigella spp., fluoroquinolone-resistant.
These pathogens “represent a threat because of increasing resistance but still have some effective antibiotic options available,” Dr. Kieny said.
According to a statement provided by the WHO, the priority list doesn’t include streptococcus A and B or chlamydia, because resistance hasn’t reached the level of a public health threat.
One goal of the list is to focus attention on the development of small-market, gram-negative drugs that combat hospital-based infections, explained Nicola Magrini, MD, a WHO scientist who also spoke at the press conference.
Over the last decade, he said, the pipeline has instead focused more on gram-positive agents – mostly linked to beta-lactamase – that have wider market potential and generate less resistance.
“From a clinical point of view, these multidrug-resistant gram-negative clinical trials are very difficult and expensive to do, more than for gram-positive,” noted Evelina Tacconelli, MD, PhD, a contributor to the WHO report. “Because when we talk about gram-negative, we need to cover multiple pathogens and not just one or two, as in the case of gram-positive.”
Dr. Magrini said he couldn’t provide estimates about how many people worldwide are affected by the listed pathogens. However, he said a full report with numbers will be released by June.
It does appear that patients with severe infection from antibiotic-resistant germs face a mortality rate of up to 60%, while extended-spectrum beta-lactamase–positive E. coli accounts for up to 70% of urinary tract infections in many countries, explained Dr. Tacconelli, head of the division of infectious diseases at the University of Tübingen, Germany.
“Even if we don’t know exactly how many,” she said, “we are talking about millions of people affected.”
The World Health Organization is urging governments to focus antibiotic research efforts on a list of urgent bacterial threats, topped by several increasingly powerful superbugs that cause hospital-based infections and other potentially deadly conditions.
The WHO listed the top 20 bacteria that it believes are most harmful to human health, other than mycobacteria such as Mycobacterium tuberculosis, which causes tuberculosis. The germ was not included in the list because it’s generally accepted to be the most urgent priority for new antibiotic research and development, Marie-Paule Kieny, PhD, a WHO assistant director, said at a press conference.
The priority list is needed because the antibiotic pipeline is “practically dry,” thanks to scientific research challenges and a lack of financial incentives, according to Dr. Kieny. “Antibiotics are generally used for the short term, unlike therapies for chronic diseases, which bring in much higher returns on investment,” she said. The list “is intended to signal to the scientific community and the pharmaceutical industry the areas they should focus on to address urgent public health threats.”
The WHO list begins with Priority 1/“Critical” pathogens that it believes most urgently need to be targeted through antibiotic research and development: Acinetobacter baumannii, carbapenem-resistant; Pseudomonas aeruginosa, carbapenem-resistant; and Enterobacteriaceae (including Klebsiella pneumonia, Escherichia coli, Enterobacter spp., Serratia spp., Proteus spp., Providencia spp., and Morganella spp.), carbapenem-resistant, extended-spectrum beta-lactamase–producing.
“These bacteria are responsible for severe infections and high mortality rates, mostly in hospitalized patients, transplant recipients, those receiving chemotherapy, or patients in intensive care units,” Dr. Kieny said. “While these bacteria are not widespread and do not generally affect healthy individuals, the burden for patients and society is now alarming – and new, effective therapies are imperative.”
Priority 2/”High” pathogens are Enterococcus faecium, vancomycin-resistant; Staphylococcus aureus, methicillin-resistant, vancomycin intermediate and resistant; Helicobacter pylori, clarithromycin-resistant; Campylobacter, fluoroquinolone-resistant; Salmonella spp., fluoroquinolone-resistant; Neisseria gonorrhoeae, third-generation cephalosporin-resistant and fluoroquinolone-resistant.
Pathogens in this category can infect healthy individuals, Dr. Kieny noted. “These infections, although not associated with significant mortality, have a dramatic health and economic impact on communities and, in particular, in low-income countries.”
Priority 3/”Medium” pathogens are Streptococcus pneumoniae, penicillin–non-susceptible; Haemophilus influenzae, ampicillin-resistant; and Shigella spp., fluoroquinolone-resistant.
These pathogens “represent a threat because of increasing resistance but still have some effective antibiotic options available,” Dr. Kieny said.
According to a statement provided by the WHO, the priority list doesn’t include streptococcus A and B or chlamydia, because resistance hasn’t reached the level of a public health threat.
One goal of the list is to focus attention on the development of small-market, gram-negative drugs that combat hospital-based infections, explained Nicola Magrini, MD, a WHO scientist who also spoke at the press conference.
Over the last decade, he said, the pipeline has instead focused more on gram-positive agents – mostly linked to beta-lactamase – that have wider market potential and generate less resistance.
“From a clinical point of view, these multidrug-resistant gram-negative clinical trials are very difficult and expensive to do, more than for gram-positive,” noted Evelina Tacconelli, MD, PhD, a contributor to the WHO report. “Because when we talk about gram-negative, we need to cover multiple pathogens and not just one or two, as in the case of gram-positive.”
Dr. Magrini said he couldn’t provide estimates about how many people worldwide are affected by the listed pathogens. However, he said a full report with numbers will be released by June.
It does appear that patients with severe infection from antibiotic-resistant germs face a mortality rate of up to 60%, while extended-spectrum beta-lactamase–positive E. coli accounts for up to 70% of urinary tract infections in many countries, explained Dr. Tacconelli, head of the division of infectious diseases at the University of Tübingen, Germany.
“Even if we don’t know exactly how many,” she said, “we are talking about millions of people affected.”
The World Health Organization is urging governments to focus antibiotic research efforts on a list of urgent bacterial threats, topped by several increasingly powerful superbugs that cause hospital-based infections and other potentially deadly conditions.
The WHO listed the top 20 bacteria that it believes are most harmful to human health, other than mycobacteria such as Mycobacterium tuberculosis, which causes tuberculosis. The germ was not included in the list because it’s generally accepted to be the most urgent priority for new antibiotic research and development, Marie-Paule Kieny, PhD, a WHO assistant director, said at a press conference.
The priority list is needed because the antibiotic pipeline is “practically dry,” thanks to scientific research challenges and a lack of financial incentives, according to Dr. Kieny. “Antibiotics are generally used for the short term, unlike therapies for chronic diseases, which bring in much higher returns on investment,” she said. The list “is intended to signal to the scientific community and the pharmaceutical industry the areas they should focus on to address urgent public health threats.”
The WHO list begins with Priority 1/“Critical” pathogens that it believes most urgently need to be targeted through antibiotic research and development: Acinetobacter baumannii, carbapenem-resistant; Pseudomonas aeruginosa, carbapenem-resistant; and Enterobacteriaceae (including Klebsiella pneumonia, Escherichia coli, Enterobacter spp., Serratia spp., Proteus spp., Providencia spp., and Morganella spp.), carbapenem-resistant, extended-spectrum beta-lactamase–producing.
“These bacteria are responsible for severe infections and high mortality rates, mostly in hospitalized patients, transplant recipients, those receiving chemotherapy, or patients in intensive care units,” Dr. Kieny said. “While these bacteria are not widespread and do not generally affect healthy individuals, the burden for patients and society is now alarming – and new, effective therapies are imperative.”
Priority 2/”High” pathogens are Enterococcus faecium, vancomycin-resistant; Staphylococcus aureus, methicillin-resistant, vancomycin intermediate and resistant; Helicobacter pylori, clarithromycin-resistant; Campylobacter, fluoroquinolone-resistant; Salmonella spp., fluoroquinolone-resistant; Neisseria gonorrhoeae, third-generation cephalosporin-resistant and fluoroquinolone-resistant.
Pathogens in this category can infect healthy individuals, Dr. Kieny noted. “These infections, although not associated with significant mortality, have a dramatic health and economic impact on communities and, in particular, in low-income countries.”
Priority 3/”Medium” pathogens are Streptococcus pneumoniae, penicillin–non-susceptible; Haemophilus influenzae, ampicillin-resistant; and Shigella spp., fluoroquinolone-resistant.
These pathogens “represent a threat because of increasing resistance but still have some effective antibiotic options available,” Dr. Kieny said.
According to a statement provided by the WHO, the priority list doesn’t include streptococcus A and B or chlamydia, because resistance hasn’t reached the level of a public health threat.
One goal of the list is to focus attention on the development of small-market, gram-negative drugs that combat hospital-based infections, explained Nicola Magrini, MD, a WHO scientist who also spoke at the press conference.
Over the last decade, he said, the pipeline has instead focused more on gram-positive agents – mostly linked to beta-lactamase – that have wider market potential and generate less resistance.
“From a clinical point of view, these multidrug-resistant gram-negative clinical trials are very difficult and expensive to do, more than for gram-positive,” noted Evelina Tacconelli, MD, PhD, a contributor to the WHO report. “Because when we talk about gram-negative, we need to cover multiple pathogens and not just one or two, as in the case of gram-positive.”
Dr. Magrini said he couldn’t provide estimates about how many people worldwide are affected by the listed pathogens. However, he said a full report with numbers will be released by June.
It does appear that patients with severe infection from antibiotic-resistant germs face a mortality rate of up to 60%, while extended-spectrum beta-lactamase–positive E. coli accounts for up to 70% of urinary tract infections in many countries, explained Dr. Tacconelli, head of the division of infectious diseases at the University of Tübingen, Germany.
“Even if we don’t know exactly how many,” she said, “we are talking about millions of people affected.”
Sepsis survivors may have high risk for seizures
BOSTON – Survivors of sepsis face a significantly increased risk of seizures following an index hospitalization, regardless of any previous history of seizures or seizures occurring during hospitalization, according to findings from a retrospective, population-based cohort study.
The risk for having subsequent seizures was highest for patients younger than 65 years but was still elevated above the general population for those aged 65 years or older, Michael Reznik, MD, reported at the annual meeting of the American Academy of Neurology.
Seizures are already a well-known complication of sepsis, and they also can occur alongside sepsis-associated encephalopathy, stroke, and neuromuscular disease. The frequency of sepsis-associated encephalopathy also has led to the recognition of postsepsis cognitive dysfunction, said Dr. Reznik, a neurocritical care fellow in the department of neurology at Weill Cornell Medicine and Columbia University Medical Center in New York.
It is unclear, however, how much of the risk for cognitive impairment after sepsis is due to pre-existing cognitive impairment, frailty, or lingering sedation effects, he said.
It’s possible, he noted, that “seizures may be more specific for structural brain injury, and I think our findings may support the hypothesis that sepsis could be associated with pathways leading to long-lasting brain injury that’s independent of other primary injuries that we have controlled for.”
Dr. Reznik and his coinvestigators used administrative claims data from all discharges from nonfederal emergency departments and acute care hospitals in California, New York, and Florida during 2005-2013 that had been collected as part of the federal Healthcare Cost and Utilization Project (HCUP). The HCUP assigns each patient a unique number that can be used to follow them anonymously through all subsequent hospitalizations. At each encounter, HCUP also tracks up to 25 discharge diagnoses that were present before hospital admission or developed during hospitalization, based on ICD-9-CM codes.
The investigators excluded patients with an ICD-9-CM diagnosis of seizures either before or during the index hospitalization for sepsis.
Overall, the 842,735 adult sepsis survivors in the study had a 6.67% cumulative rate of seizures over the 8-year period, compared with 1.27% in the general population. This translated to an incidence of about 1,288 per 100,000 patient-years in sepsis survivors, compared with 159 per 100,000 patient-years in the general population. The overall incidence rate ratio (IRR) for seizures among sepsis survivors was about 5, but was higher for those who also had neurologic dysfunction (such as encephalopathy, delirium, coma, or stupor) during their index hospitalization than in those without it (7.52 vs. 4.53). Sepsis survivors also had an elevated IRR of 5.42 for status epilepticus.
Sepsis survivors also had an elevated IRR of 4.35 for seizures when compared against control patients who were hospitalized for diagnoses other than sepsis and matched for age, sex, race, insurance, length of stay, discharge location, year of hospitalization, state, and the presence of codes for organ dysfunction.
The investigators confirmed the findings from the state-based HCUP analysis through inpatient and outpatient Medicare claims during 2008-1014 in a nationally representative sample of 5% of Medicare beneficiaries. These patients had an IRR for seizures of 2.72, and the IRR remained elevated (2.18) relative to patients who were hospitalized with diagnoses other than sepsis even when they excluded patients with ICD-9-CM codes for conditions that confer risk for seizures, including stroke, traumatic brain injury, CNS infection, or brain neoplasm. The seizure outcome in this analysis was defined as one or more inpatient claims for epilepsy or two or more outpatient claims within 3 months of each other.
Since the state-based HCUP data gave a much stronger association between sepsis and subsequent seizures than did the Medicare claims data, the investigators performed a post hoc stratified analysis according to age. Age proved to have a significant effect on the relationship between sepsis and subsequent seizures: Patients aged 65 years or older had an IRR of 2.83, compared with an IRR of 10.33 for those younger than 65.
In an interview, Dr. Reznik said that he sees the results as hypothesis generating and suggested they could serve as a “red flag” for neurologists that’s worth further investigation, given that studies suggest systemic infections and sepsis overall have long-term neurologic implications.
“I think there’s a possibility that, down the line, [sepsis] might be seen as a seizure risk factor, but unfortunately there are limitations from being based on an administrative data set,” he said.
The study was supported by a grant from the National Institute for Neurological Disorders and Stroke to one of the investigators and also by the Michael Goldberg Research Fund. Dr. Reznik had no disclosures to report.
BOSTON – Survivors of sepsis face a significantly increased risk of seizures following an index hospitalization, regardless of any previous history of seizures or seizures occurring during hospitalization, according to findings from a retrospective, population-based cohort study.
The risk for having subsequent seizures was highest for patients younger than 65 years but was still elevated above the general population for those aged 65 years or older, Michael Reznik, MD, reported at the annual meeting of the American Academy of Neurology.
Seizures are already a well-known complication of sepsis, and they also can occur alongside sepsis-associated encephalopathy, stroke, and neuromuscular disease. The frequency of sepsis-associated encephalopathy also has led to the recognition of postsepsis cognitive dysfunction, said Dr. Reznik, a neurocritical care fellow in the department of neurology at Weill Cornell Medicine and Columbia University Medical Center in New York.
It is unclear, however, how much of the risk for cognitive impairment after sepsis is due to pre-existing cognitive impairment, frailty, or lingering sedation effects, he said.
It’s possible, he noted, that “seizures may be more specific for structural brain injury, and I think our findings may support the hypothesis that sepsis could be associated with pathways leading to long-lasting brain injury that’s independent of other primary injuries that we have controlled for.”
Dr. Reznik and his coinvestigators used administrative claims data from all discharges from nonfederal emergency departments and acute care hospitals in California, New York, and Florida during 2005-2013 that had been collected as part of the federal Healthcare Cost and Utilization Project (HCUP). The HCUP assigns each patient a unique number that can be used to follow them anonymously through all subsequent hospitalizations. At each encounter, HCUP also tracks up to 25 discharge diagnoses that were present before hospital admission or developed during hospitalization, based on ICD-9-CM codes.
The investigators excluded patients with an ICD-9-CM diagnosis of seizures either before or during the index hospitalization for sepsis.
Overall, the 842,735 adult sepsis survivors in the study had a 6.67% cumulative rate of seizures over the 8-year period, compared with 1.27% in the general population. This translated to an incidence of about 1,288 per 100,000 patient-years in sepsis survivors, compared with 159 per 100,000 patient-years in the general population. The overall incidence rate ratio (IRR) for seizures among sepsis survivors was about 5, but was higher for those who also had neurologic dysfunction (such as encephalopathy, delirium, coma, or stupor) during their index hospitalization than in those without it (7.52 vs. 4.53). Sepsis survivors also had an elevated IRR of 5.42 for status epilepticus.
Sepsis survivors also had an elevated IRR of 4.35 for seizures when compared against control patients who were hospitalized for diagnoses other than sepsis and matched for age, sex, race, insurance, length of stay, discharge location, year of hospitalization, state, and the presence of codes for organ dysfunction.
The investigators confirmed the findings from the state-based HCUP analysis through inpatient and outpatient Medicare claims during 2008-1014 in a nationally representative sample of 5% of Medicare beneficiaries. These patients had an IRR for seizures of 2.72, and the IRR remained elevated (2.18) relative to patients who were hospitalized with diagnoses other than sepsis even when they excluded patients with ICD-9-CM codes for conditions that confer risk for seizures, including stroke, traumatic brain injury, CNS infection, or brain neoplasm. The seizure outcome in this analysis was defined as one or more inpatient claims for epilepsy or two or more outpatient claims within 3 months of each other.
Since the state-based HCUP data gave a much stronger association between sepsis and subsequent seizures than did the Medicare claims data, the investigators performed a post hoc stratified analysis according to age. Age proved to have a significant effect on the relationship between sepsis and subsequent seizures: Patients aged 65 years or older had an IRR of 2.83, compared with an IRR of 10.33 for those younger than 65.
In an interview, Dr. Reznik said that he sees the results as hypothesis generating and suggested they could serve as a “red flag” for neurologists that’s worth further investigation, given that studies suggest systemic infections and sepsis overall have long-term neurologic implications.
“I think there’s a possibility that, down the line, [sepsis] might be seen as a seizure risk factor, but unfortunately there are limitations from being based on an administrative data set,” he said.
The study was supported by a grant from the National Institute for Neurological Disorders and Stroke to one of the investigators and also by the Michael Goldberg Research Fund. Dr. Reznik had no disclosures to report.
BOSTON – Survivors of sepsis face a significantly increased risk of seizures following an index hospitalization, regardless of any previous history of seizures or seizures occurring during hospitalization, according to findings from a retrospective, population-based cohort study.
The risk for having subsequent seizures was highest for patients younger than 65 years but was still elevated above the general population for those aged 65 years or older, Michael Reznik, MD, reported at the annual meeting of the American Academy of Neurology.
Seizures are already a well-known complication of sepsis, and they also can occur alongside sepsis-associated encephalopathy, stroke, and neuromuscular disease. The frequency of sepsis-associated encephalopathy also has led to the recognition of postsepsis cognitive dysfunction, said Dr. Reznik, a neurocritical care fellow in the department of neurology at Weill Cornell Medicine and Columbia University Medical Center in New York.
It is unclear, however, how much of the risk for cognitive impairment after sepsis is due to pre-existing cognitive impairment, frailty, or lingering sedation effects, he said.
It’s possible, he noted, that “seizures may be more specific for structural brain injury, and I think our findings may support the hypothesis that sepsis could be associated with pathways leading to long-lasting brain injury that’s independent of other primary injuries that we have controlled for.”
Dr. Reznik and his coinvestigators used administrative claims data from all discharges from nonfederal emergency departments and acute care hospitals in California, New York, and Florida during 2005-2013 that had been collected as part of the federal Healthcare Cost and Utilization Project (HCUP). The HCUP assigns each patient a unique number that can be used to follow them anonymously through all subsequent hospitalizations. At each encounter, HCUP also tracks up to 25 discharge diagnoses that were present before hospital admission or developed during hospitalization, based on ICD-9-CM codes.
The investigators excluded patients with an ICD-9-CM diagnosis of seizures either before or during the index hospitalization for sepsis.
Overall, the 842,735 adult sepsis survivors in the study had a 6.67% cumulative rate of seizures over the 8-year period, compared with 1.27% in the general population. This translated to an incidence of about 1,288 per 100,000 patient-years in sepsis survivors, compared with 159 per 100,000 patient-years in the general population. The overall incidence rate ratio (IRR) for seizures among sepsis survivors was about 5, but was higher for those who also had neurologic dysfunction (such as encephalopathy, delirium, coma, or stupor) during their index hospitalization than in those without it (7.52 vs. 4.53). Sepsis survivors also had an elevated IRR of 5.42 for status epilepticus.
Sepsis survivors also had an elevated IRR of 4.35 for seizures when compared against control patients who were hospitalized for diagnoses other than sepsis and matched for age, sex, race, insurance, length of stay, discharge location, year of hospitalization, state, and the presence of codes for organ dysfunction.
The investigators confirmed the findings from the state-based HCUP analysis through inpatient and outpatient Medicare claims during 2008-1014 in a nationally representative sample of 5% of Medicare beneficiaries. These patients had an IRR for seizures of 2.72, and the IRR remained elevated (2.18) relative to patients who were hospitalized with diagnoses other than sepsis even when they excluded patients with ICD-9-CM codes for conditions that confer risk for seizures, including stroke, traumatic brain injury, CNS infection, or brain neoplasm. The seizure outcome in this analysis was defined as one or more inpatient claims for epilepsy or two or more outpatient claims within 3 months of each other.
Since the state-based HCUP data gave a much stronger association between sepsis and subsequent seizures than did the Medicare claims data, the investigators performed a post hoc stratified analysis according to age. Age proved to have a significant effect on the relationship between sepsis and subsequent seizures: Patients aged 65 years or older had an IRR of 2.83, compared with an IRR of 10.33 for those younger than 65.
In an interview, Dr. Reznik said that he sees the results as hypothesis generating and suggested they could serve as a “red flag” for neurologists that’s worth further investigation, given that studies suggest systemic infections and sepsis overall have long-term neurologic implications.
“I think there’s a possibility that, down the line, [sepsis] might be seen as a seizure risk factor, but unfortunately there are limitations from being based on an administrative data set,” he said.
The study was supported by a grant from the National Institute for Neurological Disorders and Stroke to one of the investigators and also by the Michael Goldberg Research Fund. Dr. Reznik had no disclosures to report.
Key clinical point:
Major finding: The overall incidence rate ratio for seizures among sepsis survivors was about 5, compared with the general population.
Data source: A retrospective, population-based cohort study of 842,735 sepsis survivors from three states during 2005-2013.
Disclosures: The study was supported by a grant from the National Institute for Neurological Disorders and Stroke to one of the investigators and also the Michael Goldberg Research Fund. Dr. Reznik had no disclosures to report.
Plazomicin beats gold standard antibiotics in complex, gram-negative bacterial infections
VIENNA – An investigational antibiotic effective against several types of gram-negative antibiotic-resistant bacteria has proved its mettle against serious infections of the urinary tract, respiratory tract, and bloodstream.
Plazomicin (Achaogen, San Francisco) posted good results in two phase III studies, handily besting comparator drugs considered gold standard for treating complicated urinary tract infections and pyelonephritis, as well as bloodstream infections and hospital- and ventilator-associated bacterial pneumonia.
Both the EPIC (Evaluating Plazomicin In cUTI) and CARE (Combating Antibiotic Resistant Enterobacteriaceae) trials have provided enough positive data for the company to move forward with a new drug application later this year. The company also plans to seek European Medicines Agency approval in 2018.
Plazomicin is an aminoglycoside that has been modified with several side chains that block aminoglycoside-modifying enzymes, Daniel Cloutier, PhD, principal clinical scientist of Achaogen said at the European Society of Clinical Microbiology and Infectious Disease annual congress.
“Aminoglycoside enzymes tend to travel along with beta-lactamases and carbapenemases as well, so this drug retains potent bactericidal activity against extended spectrum beta-lactamase producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and aminoglycoside-resistant Enterobacteriaceae,” said Dr. Cloutier, who presented the results of the EPIC trial. The drug is given once-daily as a 30-minute intravenous infusion.
EPIC enrolled 609 adult patients with complicated urinary tract infections or acute pyelonephritis; Dr. Cloutier presented a modified intent-to-treat analysis comprising 388 of these. They were randomized to plazomicin 15 mg/kg every 25 hours or to IV meropenem 1 gram every 24 hours. Treatment proceeded for 4-7 days, after which patients could step down to oral therapy (levofloxacin 500 m/day), for a total of 7-10 days of treatment. About 80% of patients had both IV and oral therapy. At 15-19 days from the first dose, patients had a test of cure; at 24-32 days from first dose, they returned for a safety follow-up.
Patients were a mean of 60 years old. About 60% had a complicated UTI; the rest had acute pyelonephritis. About 35% had a mean creatinine clearance of more than 30-60 mL/min; the rest had a mean clearance of more than 60-90 mL/min.
The primary efficacy endpoint was microbial eradication. Plazomicin performed significantly better than meropenem in this measure (87% vs.72%), a mean difference of about 15%. Patients with pyelonephritis responded marginally better than those with complicated UTI, both groups favoring plazomicin (mean treatment differences of 17.5% and 13.7%, respectively).
The results were similar when the investigators examined groups by whether they needed oral step-down treatment. In the IV-only groups, plazomicin bested meropenem in microbiological eradication by almost 19% (84% vs. 65%). In the IV plus oral therapy group, the mean difference was 14%, also in favor of plazomicin (88% vs. 74%).
Plazomicin was significantly more effective then meropenem in all of the resistant Enterobacteriaceae groups (ESBL-positive, and levofloxacin- and aminoglycoside-resistant). It was also significantly more effective against E. coli (17% treatment difference), Klebsiella pneumonia (9%), and Proteus mirabilis (25%). However, it was 20% less effective than meropenem against E. cloacae.
At late follow-up, 2% of the plazomicin group and 8% of the meropenem group had relapsed – a significant difference.
Plazomicin and meropenem had similar safety profiles. Diarrhea and hypertension were the most common adverse events (about 2% in each group). Headache occurred in 3% of the meropenem patients and 1% of the plazomicin patients. Nausea, vomiting, and anemia occurred in about 1% of each group.
More patients taking plazomicin experienced a serum creatinine clearance increase of at least 0.5 mg/dL during treatment (7% vs. 4%). All but two patients taking plazomicin experienced a full recovery by the last follow-up visit.
The CARE trial was much smaller, comprised of 39 patients who had either bloodstream infections, or hospital-acquired or ventilator-associated bacterial pneumonia caused by carbapenem-resistant Enterobacteriaceae. Lynn Connolly, MD, senior medical director and head of late development at Achaogen, presented the data. Recruitment for such a narrow diagnosis was difficult, and hampered patient accrual, she noted.
CARE’s primary endpoints were a combination of all-cause mortality and significant disease-related complications, and all-cause mortality only, both at 28 days.
Patients were randomized 1:1 to either plazomicin 15 mg/kg every 24 hours, or colistin in a 300-mg loading dose, followed by daily infusions of 5 mg/kg. Everyone, regardless of treatment group, could also receive meropenem or tigecycline if deemed necessary. Treatment lasted 7-14 days. There was a test of cure at 7 days from the last IV dose, a safety assessment at 28 days, and a long-term follow-up at 60 days.
The patients’ mean age was about 65 years. Most (about 80%) had a bloodstream infection; bacterial pneumonias were present in the remainder. Most infections (85%) were monomicrobial, with polymicrobial infections making up the balance. Tigecycline was the favored adjunctive therapy (60%), followed by meropenem.
At day 28, plazomicin was associated with significantly better overall outcomes than colistin. It reduced the combination mortality/significant disease complications endpoint by 26% (23% vs. 50% meropenem). This translated to a 53% relative reduction in the risk of death.
Plazomicin also reduced all-cause mortality only by 28% (12% vs. 40% meropenem). This translated to a relative risk reduction of 70.5%.
The study drug performed well in the subgroup of patients with bloodstream infections, reducing the occurrence of the composite endpoint by 39% (14% vs. 53%), and of the mortality-only endpoint by 33% (7% vs. 40%). This translated to a 63% increased chance of 60-day survival in the plazomicin group.
Almost all patients in each group experienced at least one adverse event; 28% were deemed related to plazomicin and 43% to colistin. Many of these events were related to renal function (33% plazomicin, 52% colistin). Serum creatinine increases of at least 0.5 mg/dL during IV treatment occurred in fewer taking plazomicin (1 vs. 6 taking colistin). Full renal recovery occurred in the patient taking plazomicin, but only in three taking colistin.
“These data suggest that plazomicin could offer an important new treatment option for patients with serious infections due to carbapenem-resistant Enterobacteriaceae,” Dr. Connolly said.
[email protected]
On Twitter @Alz_gal
VIENNA – An investigational antibiotic effective against several types of gram-negative antibiotic-resistant bacteria has proved its mettle against serious infections of the urinary tract, respiratory tract, and bloodstream.
Plazomicin (Achaogen, San Francisco) posted good results in two phase III studies, handily besting comparator drugs considered gold standard for treating complicated urinary tract infections and pyelonephritis, as well as bloodstream infections and hospital- and ventilator-associated bacterial pneumonia.
Both the EPIC (Evaluating Plazomicin In cUTI) and CARE (Combating Antibiotic Resistant Enterobacteriaceae) trials have provided enough positive data for the company to move forward with a new drug application later this year. The company also plans to seek European Medicines Agency approval in 2018.
Plazomicin is an aminoglycoside that has been modified with several side chains that block aminoglycoside-modifying enzymes, Daniel Cloutier, PhD, principal clinical scientist of Achaogen said at the European Society of Clinical Microbiology and Infectious Disease annual congress.
“Aminoglycoside enzymes tend to travel along with beta-lactamases and carbapenemases as well, so this drug retains potent bactericidal activity against extended spectrum beta-lactamase producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and aminoglycoside-resistant Enterobacteriaceae,” said Dr. Cloutier, who presented the results of the EPIC trial. The drug is given once-daily as a 30-minute intravenous infusion.
EPIC enrolled 609 adult patients with complicated urinary tract infections or acute pyelonephritis; Dr. Cloutier presented a modified intent-to-treat analysis comprising 388 of these. They were randomized to plazomicin 15 mg/kg every 25 hours or to IV meropenem 1 gram every 24 hours. Treatment proceeded for 4-7 days, after which patients could step down to oral therapy (levofloxacin 500 m/day), for a total of 7-10 days of treatment. About 80% of patients had both IV and oral therapy. At 15-19 days from the first dose, patients had a test of cure; at 24-32 days from first dose, they returned for a safety follow-up.
Patients were a mean of 60 years old. About 60% had a complicated UTI; the rest had acute pyelonephritis. About 35% had a mean creatinine clearance of more than 30-60 mL/min; the rest had a mean clearance of more than 60-90 mL/min.
The primary efficacy endpoint was microbial eradication. Plazomicin performed significantly better than meropenem in this measure (87% vs.72%), a mean difference of about 15%. Patients with pyelonephritis responded marginally better than those with complicated UTI, both groups favoring plazomicin (mean treatment differences of 17.5% and 13.7%, respectively).
The results were similar when the investigators examined groups by whether they needed oral step-down treatment. In the IV-only groups, plazomicin bested meropenem in microbiological eradication by almost 19% (84% vs. 65%). In the IV plus oral therapy group, the mean difference was 14%, also in favor of plazomicin (88% vs. 74%).
Plazomicin was significantly more effective then meropenem in all of the resistant Enterobacteriaceae groups (ESBL-positive, and levofloxacin- and aminoglycoside-resistant). It was also significantly more effective against E. coli (17% treatment difference), Klebsiella pneumonia (9%), and Proteus mirabilis (25%). However, it was 20% less effective than meropenem against E. cloacae.
At late follow-up, 2% of the plazomicin group and 8% of the meropenem group had relapsed – a significant difference.
Plazomicin and meropenem had similar safety profiles. Diarrhea and hypertension were the most common adverse events (about 2% in each group). Headache occurred in 3% of the meropenem patients and 1% of the plazomicin patients. Nausea, vomiting, and anemia occurred in about 1% of each group.
More patients taking plazomicin experienced a serum creatinine clearance increase of at least 0.5 mg/dL during treatment (7% vs. 4%). All but two patients taking plazomicin experienced a full recovery by the last follow-up visit.
The CARE trial was much smaller, comprised of 39 patients who had either bloodstream infections, or hospital-acquired or ventilator-associated bacterial pneumonia caused by carbapenem-resistant Enterobacteriaceae. Lynn Connolly, MD, senior medical director and head of late development at Achaogen, presented the data. Recruitment for such a narrow diagnosis was difficult, and hampered patient accrual, she noted.
CARE’s primary endpoints were a combination of all-cause mortality and significant disease-related complications, and all-cause mortality only, both at 28 days.
Patients were randomized 1:1 to either plazomicin 15 mg/kg every 24 hours, or colistin in a 300-mg loading dose, followed by daily infusions of 5 mg/kg. Everyone, regardless of treatment group, could also receive meropenem or tigecycline if deemed necessary. Treatment lasted 7-14 days. There was a test of cure at 7 days from the last IV dose, a safety assessment at 28 days, and a long-term follow-up at 60 days.
The patients’ mean age was about 65 years. Most (about 80%) had a bloodstream infection; bacterial pneumonias were present in the remainder. Most infections (85%) were monomicrobial, with polymicrobial infections making up the balance. Tigecycline was the favored adjunctive therapy (60%), followed by meropenem.
At day 28, plazomicin was associated with significantly better overall outcomes than colistin. It reduced the combination mortality/significant disease complications endpoint by 26% (23% vs. 50% meropenem). This translated to a 53% relative reduction in the risk of death.
Plazomicin also reduced all-cause mortality only by 28% (12% vs. 40% meropenem). This translated to a relative risk reduction of 70.5%.
The study drug performed well in the subgroup of patients with bloodstream infections, reducing the occurrence of the composite endpoint by 39% (14% vs. 53%), and of the mortality-only endpoint by 33% (7% vs. 40%). This translated to a 63% increased chance of 60-day survival in the plazomicin group.
Almost all patients in each group experienced at least one adverse event; 28% were deemed related to plazomicin and 43% to colistin. Many of these events were related to renal function (33% plazomicin, 52% colistin). Serum creatinine increases of at least 0.5 mg/dL during IV treatment occurred in fewer taking plazomicin (1 vs. 6 taking colistin). Full renal recovery occurred in the patient taking plazomicin, but only in three taking colistin.
“These data suggest that plazomicin could offer an important new treatment option for patients with serious infections due to carbapenem-resistant Enterobacteriaceae,” Dr. Connolly said.
[email protected]
On Twitter @Alz_gal
VIENNA – An investigational antibiotic effective against several types of gram-negative antibiotic-resistant bacteria has proved its mettle against serious infections of the urinary tract, respiratory tract, and bloodstream.
Plazomicin (Achaogen, San Francisco) posted good results in two phase III studies, handily besting comparator drugs considered gold standard for treating complicated urinary tract infections and pyelonephritis, as well as bloodstream infections and hospital- and ventilator-associated bacterial pneumonia.
Both the EPIC (Evaluating Plazomicin In cUTI) and CARE (Combating Antibiotic Resistant Enterobacteriaceae) trials have provided enough positive data for the company to move forward with a new drug application later this year. The company also plans to seek European Medicines Agency approval in 2018.
Plazomicin is an aminoglycoside that has been modified with several side chains that block aminoglycoside-modifying enzymes, Daniel Cloutier, PhD, principal clinical scientist of Achaogen said at the European Society of Clinical Microbiology and Infectious Disease annual congress.
“Aminoglycoside enzymes tend to travel along with beta-lactamases and carbapenemases as well, so this drug retains potent bactericidal activity against extended spectrum beta-lactamase producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and aminoglycoside-resistant Enterobacteriaceae,” said Dr. Cloutier, who presented the results of the EPIC trial. The drug is given once-daily as a 30-minute intravenous infusion.
EPIC enrolled 609 adult patients with complicated urinary tract infections or acute pyelonephritis; Dr. Cloutier presented a modified intent-to-treat analysis comprising 388 of these. They were randomized to plazomicin 15 mg/kg every 25 hours or to IV meropenem 1 gram every 24 hours. Treatment proceeded for 4-7 days, after which patients could step down to oral therapy (levofloxacin 500 m/day), for a total of 7-10 days of treatment. About 80% of patients had both IV and oral therapy. At 15-19 days from the first dose, patients had a test of cure; at 24-32 days from first dose, they returned for a safety follow-up.
Patients were a mean of 60 years old. About 60% had a complicated UTI; the rest had acute pyelonephritis. About 35% had a mean creatinine clearance of more than 30-60 mL/min; the rest had a mean clearance of more than 60-90 mL/min.
The primary efficacy endpoint was microbial eradication. Plazomicin performed significantly better than meropenem in this measure (87% vs.72%), a mean difference of about 15%. Patients with pyelonephritis responded marginally better than those with complicated UTI, both groups favoring plazomicin (mean treatment differences of 17.5% and 13.7%, respectively).
The results were similar when the investigators examined groups by whether they needed oral step-down treatment. In the IV-only groups, plazomicin bested meropenem in microbiological eradication by almost 19% (84% vs. 65%). In the IV plus oral therapy group, the mean difference was 14%, also in favor of plazomicin (88% vs. 74%).
Plazomicin was significantly more effective then meropenem in all of the resistant Enterobacteriaceae groups (ESBL-positive, and levofloxacin- and aminoglycoside-resistant). It was also significantly more effective against E. coli (17% treatment difference), Klebsiella pneumonia (9%), and Proteus mirabilis (25%). However, it was 20% less effective than meropenem against E. cloacae.
At late follow-up, 2% of the plazomicin group and 8% of the meropenem group had relapsed – a significant difference.
Plazomicin and meropenem had similar safety profiles. Diarrhea and hypertension were the most common adverse events (about 2% in each group). Headache occurred in 3% of the meropenem patients and 1% of the plazomicin patients. Nausea, vomiting, and anemia occurred in about 1% of each group.
More patients taking plazomicin experienced a serum creatinine clearance increase of at least 0.5 mg/dL during treatment (7% vs. 4%). All but two patients taking plazomicin experienced a full recovery by the last follow-up visit.
The CARE trial was much smaller, comprised of 39 patients who had either bloodstream infections, or hospital-acquired or ventilator-associated bacterial pneumonia caused by carbapenem-resistant Enterobacteriaceae. Lynn Connolly, MD, senior medical director and head of late development at Achaogen, presented the data. Recruitment for such a narrow diagnosis was difficult, and hampered patient accrual, she noted.
CARE’s primary endpoints were a combination of all-cause mortality and significant disease-related complications, and all-cause mortality only, both at 28 days.
Patients were randomized 1:1 to either plazomicin 15 mg/kg every 24 hours, or colistin in a 300-mg loading dose, followed by daily infusions of 5 mg/kg. Everyone, regardless of treatment group, could also receive meropenem or tigecycline if deemed necessary. Treatment lasted 7-14 days. There was a test of cure at 7 days from the last IV dose, a safety assessment at 28 days, and a long-term follow-up at 60 days.
The patients’ mean age was about 65 years. Most (about 80%) had a bloodstream infection; bacterial pneumonias were present in the remainder. Most infections (85%) were monomicrobial, with polymicrobial infections making up the balance. Tigecycline was the favored adjunctive therapy (60%), followed by meropenem.
At day 28, plazomicin was associated with significantly better overall outcomes than colistin. It reduced the combination mortality/significant disease complications endpoint by 26% (23% vs. 50% meropenem). This translated to a 53% relative reduction in the risk of death.
Plazomicin also reduced all-cause mortality only by 28% (12% vs. 40% meropenem). This translated to a relative risk reduction of 70.5%.
The study drug performed well in the subgroup of patients with bloodstream infections, reducing the occurrence of the composite endpoint by 39% (14% vs. 53%), and of the mortality-only endpoint by 33% (7% vs. 40%). This translated to a 63% increased chance of 60-day survival in the plazomicin group.
Almost all patients in each group experienced at least one adverse event; 28% were deemed related to plazomicin and 43% to colistin. Many of these events were related to renal function (33% plazomicin, 52% colistin). Serum creatinine increases of at least 0.5 mg/dL during IV treatment occurred in fewer taking plazomicin (1 vs. 6 taking colistin). Full renal recovery occurred in the patient taking plazomicin, but only in three taking colistin.
“These data suggest that plazomicin could offer an important new treatment option for patients with serious infections due to carbapenem-resistant Enterobacteriaceae,” Dr. Connolly said.
[email protected]
On Twitter @Alz_gal
AATS publishes guidelines for infective endocarditis
Infective endocarditis (IE) is a devastating complication of heart valve disease that, left untreated, can be fatal. Management requires a multidisciplinary approach, and many of the respective medical societies that represent the participating specialties have developed guidelines. Now, the American Association for Thoracic Surgery has published “Consensus Guidelines for the Surgical Treatment of Infective Endocarditis” to guide thoracic and cardiovascular surgeons in making decisions of when to operate in cases of IE (J Thorac Cardiovasc Surg. 2017 Jan 24. doi: 10.1016/j.jtcvs.2016.09.093).
The rationale for developing the guidelines is a growing prevalence of IE, including in patients with normal valves and no previous diagnosis of heart disease. “These new AATS consensus guidelines primarily address questions related to active and suspected active IE affective valves and intracardiac structures,” Dr. Pettersson and his coauthors said. The AATS guidelines for infective endocarditis address complications including risk of embolism and the timing of surgery in patients with neurological complications, while acknowledging the the need for additional research into these topics.* “It is understood that surgery is beneficial only if the patient’s complications and other comorbidities do not preclude survival and meaningful recovery,” the guideline authors said.
The guidelines confirm the team approach for managing patients with IE. The team should include cardiology, infectious disease, cardiac surgery, and other specialties needed to handle IE-related complications (class of recommendation [COR] I, level of evidence [LOE] B). Before surgery, the surgeon should know the patient is on effective antimicrobial therapy (COR I, LOE B). Transesophageal echocardiography (TEE) is indicated to yield the clearest understanding of the pathology (COR I, LOE B).
Dr. Pettersson and the guideline writing team also clarified indications for surgery in patients with IE. They include when valve dysfunction causes heart failure (COR I, LOE B); when, after a full course of antibiotics, the patient has signs of heart block, annular or aortic abscess or destructive penetrating lesions (COR I, LOE B); and in the setting of recurrent emboli and persistent vegetations despite appropriate antibiotic therapy (COR IIA, LOE B).
The guideline writers acknowledged potential disagreement between the AATS guidelines and those of the American College of Cardiology/American Heart Association with regard to early surgery in IE. Debate surrounds whether to operate early or wait for symptoms of heart failure to manifest in patients with native valve endocarditis (NVE). The AATS guideline authors cite work by Duk-Hyun Kang, MD, PhD, and coauthors in South Korea (N Engl J Med. 2012;366;2466-73) and others advocating for early surgery. “For this reason, once a surgical indication is evident, surgery should not be delayed,” Dr. Pettersson and his coauthors said.
Several conditions can influence the timing of surgery. Patients with cerebral mycotic aneurysm should be managed closely with neurology or neurosurgery (COR I, LOE C). Patients with a recent intracranial hemorrhage should wait at least 3 weeks for surgery (COR IIA, LOE B), but those with nonhemorrhagic strokes could go in for urgent surgery (COR IIA, LOE B). Brain imaging is indicated for IE patients with neurological symptoms (COR I, LOE B), but anticoagulation management requires a nuanced approach that takes all risks and benefits into consideration (COR I, LOE C).
Key steps during surgery involve mandatory intraoperative TEE (COR I, LOE B), median sternotomy with few exceptions (COR I, LOE B), and radical debridement and removal of all infected and necrotic tissue (COR I, LOE B). The writers also provided four guidelines for reconstruction and valve replacement:
- Repair when possible for patients with NVE (COR I, LOE B).
- When replacement is indicated, the surgeon should base valve choice on normal criteria – age, life expectancy, comorbidities, and expected compliance with anticoagulation (COR I, LOE B).
- Avoid use of mechanical valves in patients with intracranial bleeding or who have had a major stroke (COR IIA, LOE C).
- In patients with invasive disease and deconstruction, reconstruction should depend on the involved valve, severity of destruction, and available options for cardiac reconstruction (COR I, LOE B).
The AATS guidelines also challenge conventional thinking on the practice of soaking a gel-impregnated graft with antimicrobials targeting a specific organism. “We found no evidence to support this practice,” Dr. Pettersson and his coauthors said (COR IIB, LOE B). They came to the same conclusion with regard to the use of local antimicrobials or antiseptics during irrigation after debridement and local injection of antimicrobials around the infected area (COR I, LOE C).
The guidelines provide direction on a host of other surgical issues in IE: use of aortic valve grafts; when to remove or replace noninfected grafts; when to remove pacemakers; the role of drainage; postoperative management; follow-up; and additional screening. They also shed insight into what the guideline authors call “residual controversies,” including surgery for injection drug users (use “all available resources and options for drug rehabilitation”) and dialysis patients (“it is reasonable to offer surgery when the additional burden of comorbidities is not overwhelming”). They also acknowledge seven different scenarios that lack clear evidence for intervention but require the surgeon to determine the need for surgery, ranging from timing of surgery for IE in patients with neurologic complications to how to treat patients with functional valve issues after being cured of IE.
The guideline writers acknowledged that institutional funds supported the work. Dr. Pettersson had no financial relationships to disclose.
*Correction 5/172017: It was incorrectly stated that these complications were not addressed in the guidelines due to lack of evidence
Whether they’re intended to or not, guidelines like the AATS Consensus Guidelines for the Surgical Treatment of Infected Endocarditis “can evolve into hard and fast principles, sometimes leading to incorrect decision making and even creating medicolegal problems for treating physicians,” Gus J. Vlahakes, MD, of Harvard Medical School and Massachusetts General Hospital, Boston, said in his invited commentary (J Thorac Cardiovasc Surg. 2016 Nov 3. doi: 10.1016/j.jtcvs.2016.10.041).
Guidelines cannot “integrate all the necessary considerations,” Dr. Vlahakes said, so surgeons should view them as “a set of general principles to guide decision making.” In IE, that means having an experienced cardiac surgeon who can apply the guidelines on a case-by-case basis and a multidisciplinary team that includes an infectious disease specialist, he said. The surgeon must participate in preoperative management.
Dr. Vlahakes had no relevant financial disclosures.
Whether they’re intended to or not, guidelines like the AATS Consensus Guidelines for the Surgical Treatment of Infected Endocarditis “can evolve into hard and fast principles, sometimes leading to incorrect decision making and even creating medicolegal problems for treating physicians,” Gus J. Vlahakes, MD, of Harvard Medical School and Massachusetts General Hospital, Boston, said in his invited commentary (J Thorac Cardiovasc Surg. 2016 Nov 3. doi: 10.1016/j.jtcvs.2016.10.041).
Guidelines cannot “integrate all the necessary considerations,” Dr. Vlahakes said, so surgeons should view them as “a set of general principles to guide decision making.” In IE, that means having an experienced cardiac surgeon who can apply the guidelines on a case-by-case basis and a multidisciplinary team that includes an infectious disease specialist, he said. The surgeon must participate in preoperative management.
Dr. Vlahakes had no relevant financial disclosures.
Whether they’re intended to or not, guidelines like the AATS Consensus Guidelines for the Surgical Treatment of Infected Endocarditis “can evolve into hard and fast principles, sometimes leading to incorrect decision making and even creating medicolegal problems for treating physicians,” Gus J. Vlahakes, MD, of Harvard Medical School and Massachusetts General Hospital, Boston, said in his invited commentary (J Thorac Cardiovasc Surg. 2016 Nov 3. doi: 10.1016/j.jtcvs.2016.10.041).
Guidelines cannot “integrate all the necessary considerations,” Dr. Vlahakes said, so surgeons should view them as “a set of general principles to guide decision making.” In IE, that means having an experienced cardiac surgeon who can apply the guidelines on a case-by-case basis and a multidisciplinary team that includes an infectious disease specialist, he said. The surgeon must participate in preoperative management.
Dr. Vlahakes had no relevant financial disclosures.
Infective endocarditis (IE) is a devastating complication of heart valve disease that, left untreated, can be fatal. Management requires a multidisciplinary approach, and many of the respective medical societies that represent the participating specialties have developed guidelines. Now, the American Association for Thoracic Surgery has published “Consensus Guidelines for the Surgical Treatment of Infective Endocarditis” to guide thoracic and cardiovascular surgeons in making decisions of when to operate in cases of IE (J Thorac Cardiovasc Surg. 2017 Jan 24. doi: 10.1016/j.jtcvs.2016.09.093).
The rationale for developing the guidelines is a growing prevalence of IE, including in patients with normal valves and no previous diagnosis of heart disease. “These new AATS consensus guidelines primarily address questions related to active and suspected active IE affective valves and intracardiac structures,” Dr. Pettersson and his coauthors said. The AATS guidelines for infective endocarditis address complications including risk of embolism and the timing of surgery in patients with neurological complications, while acknowledging the the need for additional research into these topics.* “It is understood that surgery is beneficial only if the patient’s complications and other comorbidities do not preclude survival and meaningful recovery,” the guideline authors said.
The guidelines confirm the team approach for managing patients with IE. The team should include cardiology, infectious disease, cardiac surgery, and other specialties needed to handle IE-related complications (class of recommendation [COR] I, level of evidence [LOE] B). Before surgery, the surgeon should know the patient is on effective antimicrobial therapy (COR I, LOE B). Transesophageal echocardiography (TEE) is indicated to yield the clearest understanding of the pathology (COR I, LOE B).
Dr. Pettersson and the guideline writing team also clarified indications for surgery in patients with IE. They include when valve dysfunction causes heart failure (COR I, LOE B); when, after a full course of antibiotics, the patient has signs of heart block, annular or aortic abscess or destructive penetrating lesions (COR I, LOE B); and in the setting of recurrent emboli and persistent vegetations despite appropriate antibiotic therapy (COR IIA, LOE B).
The guideline writers acknowledged potential disagreement between the AATS guidelines and those of the American College of Cardiology/American Heart Association with regard to early surgery in IE. Debate surrounds whether to operate early or wait for symptoms of heart failure to manifest in patients with native valve endocarditis (NVE). The AATS guideline authors cite work by Duk-Hyun Kang, MD, PhD, and coauthors in South Korea (N Engl J Med. 2012;366;2466-73) and others advocating for early surgery. “For this reason, once a surgical indication is evident, surgery should not be delayed,” Dr. Pettersson and his coauthors said.
Several conditions can influence the timing of surgery. Patients with cerebral mycotic aneurysm should be managed closely with neurology or neurosurgery (COR I, LOE C). Patients with a recent intracranial hemorrhage should wait at least 3 weeks for surgery (COR IIA, LOE B), but those with nonhemorrhagic strokes could go in for urgent surgery (COR IIA, LOE B). Brain imaging is indicated for IE patients with neurological symptoms (COR I, LOE B), but anticoagulation management requires a nuanced approach that takes all risks and benefits into consideration (COR I, LOE C).
Key steps during surgery involve mandatory intraoperative TEE (COR I, LOE B), median sternotomy with few exceptions (COR I, LOE B), and radical debridement and removal of all infected and necrotic tissue (COR I, LOE B). The writers also provided four guidelines for reconstruction and valve replacement:
- Repair when possible for patients with NVE (COR I, LOE B).
- When replacement is indicated, the surgeon should base valve choice on normal criteria – age, life expectancy, comorbidities, and expected compliance with anticoagulation (COR I, LOE B).
- Avoid use of mechanical valves in patients with intracranial bleeding or who have had a major stroke (COR IIA, LOE C).
- In patients with invasive disease and deconstruction, reconstruction should depend on the involved valve, severity of destruction, and available options for cardiac reconstruction (COR I, LOE B).
The AATS guidelines also challenge conventional thinking on the practice of soaking a gel-impregnated graft with antimicrobials targeting a specific organism. “We found no evidence to support this practice,” Dr. Pettersson and his coauthors said (COR IIB, LOE B). They came to the same conclusion with regard to the use of local antimicrobials or antiseptics during irrigation after debridement and local injection of antimicrobials around the infected area (COR I, LOE C).
The guidelines provide direction on a host of other surgical issues in IE: use of aortic valve grafts; when to remove or replace noninfected grafts; when to remove pacemakers; the role of drainage; postoperative management; follow-up; and additional screening. They also shed insight into what the guideline authors call “residual controversies,” including surgery for injection drug users (use “all available resources and options for drug rehabilitation”) and dialysis patients (“it is reasonable to offer surgery when the additional burden of comorbidities is not overwhelming”). They also acknowledge seven different scenarios that lack clear evidence for intervention but require the surgeon to determine the need for surgery, ranging from timing of surgery for IE in patients with neurologic complications to how to treat patients with functional valve issues after being cured of IE.
The guideline writers acknowledged that institutional funds supported the work. Dr. Pettersson had no financial relationships to disclose.
*Correction 5/172017: It was incorrectly stated that these complications were not addressed in the guidelines due to lack of evidence
Infective endocarditis (IE) is a devastating complication of heart valve disease that, left untreated, can be fatal. Management requires a multidisciplinary approach, and many of the respective medical societies that represent the participating specialties have developed guidelines. Now, the American Association for Thoracic Surgery has published “Consensus Guidelines for the Surgical Treatment of Infective Endocarditis” to guide thoracic and cardiovascular surgeons in making decisions of when to operate in cases of IE (J Thorac Cardiovasc Surg. 2017 Jan 24. doi: 10.1016/j.jtcvs.2016.09.093).
The rationale for developing the guidelines is a growing prevalence of IE, including in patients with normal valves and no previous diagnosis of heart disease. “These new AATS consensus guidelines primarily address questions related to active and suspected active IE affective valves and intracardiac structures,” Dr. Pettersson and his coauthors said. The AATS guidelines for infective endocarditis address complications including risk of embolism and the timing of surgery in patients with neurological complications, while acknowledging the the need for additional research into these topics.* “It is understood that surgery is beneficial only if the patient’s complications and other comorbidities do not preclude survival and meaningful recovery,” the guideline authors said.
The guidelines confirm the team approach for managing patients with IE. The team should include cardiology, infectious disease, cardiac surgery, and other specialties needed to handle IE-related complications (class of recommendation [COR] I, level of evidence [LOE] B). Before surgery, the surgeon should know the patient is on effective antimicrobial therapy (COR I, LOE B). Transesophageal echocardiography (TEE) is indicated to yield the clearest understanding of the pathology (COR I, LOE B).
Dr. Pettersson and the guideline writing team also clarified indications for surgery in patients with IE. They include when valve dysfunction causes heart failure (COR I, LOE B); when, after a full course of antibiotics, the patient has signs of heart block, annular or aortic abscess or destructive penetrating lesions (COR I, LOE B); and in the setting of recurrent emboli and persistent vegetations despite appropriate antibiotic therapy (COR IIA, LOE B).
The guideline writers acknowledged potential disagreement between the AATS guidelines and those of the American College of Cardiology/American Heart Association with regard to early surgery in IE. Debate surrounds whether to operate early or wait for symptoms of heart failure to manifest in patients with native valve endocarditis (NVE). The AATS guideline authors cite work by Duk-Hyun Kang, MD, PhD, and coauthors in South Korea (N Engl J Med. 2012;366;2466-73) and others advocating for early surgery. “For this reason, once a surgical indication is evident, surgery should not be delayed,” Dr. Pettersson and his coauthors said.
Several conditions can influence the timing of surgery. Patients with cerebral mycotic aneurysm should be managed closely with neurology or neurosurgery (COR I, LOE C). Patients with a recent intracranial hemorrhage should wait at least 3 weeks for surgery (COR IIA, LOE B), but those with nonhemorrhagic strokes could go in for urgent surgery (COR IIA, LOE B). Brain imaging is indicated for IE patients with neurological symptoms (COR I, LOE B), but anticoagulation management requires a nuanced approach that takes all risks and benefits into consideration (COR I, LOE C).
Key steps during surgery involve mandatory intraoperative TEE (COR I, LOE B), median sternotomy with few exceptions (COR I, LOE B), and radical debridement and removal of all infected and necrotic tissue (COR I, LOE B). The writers also provided four guidelines for reconstruction and valve replacement:
- Repair when possible for patients with NVE (COR I, LOE B).
- When replacement is indicated, the surgeon should base valve choice on normal criteria – age, life expectancy, comorbidities, and expected compliance with anticoagulation (COR I, LOE B).
- Avoid use of mechanical valves in patients with intracranial bleeding or who have had a major stroke (COR IIA, LOE C).
- In patients with invasive disease and deconstruction, reconstruction should depend on the involved valve, severity of destruction, and available options for cardiac reconstruction (COR I, LOE B).
The AATS guidelines also challenge conventional thinking on the practice of soaking a gel-impregnated graft with antimicrobials targeting a specific organism. “We found no evidence to support this practice,” Dr. Pettersson and his coauthors said (COR IIB, LOE B). They came to the same conclusion with regard to the use of local antimicrobials or antiseptics during irrigation after debridement and local injection of antimicrobials around the infected area (COR I, LOE C).
The guidelines provide direction on a host of other surgical issues in IE: use of aortic valve grafts; when to remove or replace noninfected grafts; when to remove pacemakers; the role of drainage; postoperative management; follow-up; and additional screening. They also shed insight into what the guideline authors call “residual controversies,” including surgery for injection drug users (use “all available resources and options for drug rehabilitation”) and dialysis patients (“it is reasonable to offer surgery when the additional burden of comorbidities is not overwhelming”). They also acknowledge seven different scenarios that lack clear evidence for intervention but require the surgeon to determine the need for surgery, ranging from timing of surgery for IE in patients with neurologic complications to how to treat patients with functional valve issues after being cured of IE.
The guideline writers acknowledged that institutional funds supported the work. Dr. Pettersson had no financial relationships to disclose.
*Correction 5/172017: It was incorrectly stated that these complications were not addressed in the guidelines due to lack of evidence
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: The American Association for Thoracic Surgery charged a committee of eight members to author “Consensus Guidelines: Surgical Treatment of Infective Endocarditis.”
Major finding: Patients with infective endocarditis need early input from the responsible cardiac surgeon, who must also lead the care team in evaluation, decision- making, and ultimately carrying out surgery as needed.
Data source: The writing committee followed Institute of Medicine standards for clinical practice guidelines, invited comment from a group of 12 multidisciplinary specialists and reviewed 288 articles in drafting the guidelines.
Disclosures: Institutional funds supported the work. Dr. Pettersson and his coauthors had no financial relationships to disclose.
Hospital floors are an overlooked reservoir for pathogens
Floors in hospital patients’ rooms are frequently contaminated with pathogens such as Clostridium difficile, methicillin-resistant Staphylococcus aureus, and vancomycin-resistant Enterococci, which are easily transmitted to the hands of patients, care providers, and visitors, according to a report published in the American Journal of Infection Control.
Disinfection usually focuses on surfaces that are frequently touched by patients’ or health care workers’ hands, such as bed rails and call buttons. Floor disinfection has received limited attention.
However, floors are frequently touched by objects that are then handled, such as shoes and socks, said Abhishek Deshpande, MD, PhD, of the Cleveland Clinic, and his associates.
To examine the extent of floor contamination and the potential for transfer of pathogens to hands, the investigators surveyed five Cleveland-area hospitals.
They collected samples from 1-square-foot areas of floors adjacent to beds and in bathrooms in C. difficile isolation rooms, and in 2-3 randomly selected nonisolation rooms on the same wards. At least 30 rooms at each hospital were cultured for C. difficile, MRSA, and VRE, either during a patient stay or after the rooms had been cleaned at patient discharge.
In addition, the researchers performed a point-prevalence survey of the number and type of high-touch objects contacting floors in 10-25 randomly selected occupied patient rooms at each hospital. After they handled these objects, their hands also were cultured.
Floor contamination was common with all of the pathogens, particularly with C. difficile. The frequency of contamination was similar across the five hospitals, in both bedroom and bathroom sites, and even in the 50 rooms that had been cleaned at the last patient discharge.
C. difficile spores were recovered from the floors of 47%-55% of rooms, MRSA was recovered from the floors of 8%-32% of rooms, and VRE were recovered from the floors of 13%-30% of rooms.
In addition, 41 of 100 occupied rooms had 1-4 “high-touch” objects in direct contact with the floors, including personal items such as clothing, canes, or cellphone chargers; medical supplies or devices such as pulse oximeters, call buttons, heating pads, urinals, blood pressure cuffs, and wash basins; and linens such as bed sheets, pillows, and towels.
Of the 31 cultures taken from both bare and gloved hands that handled these items, MRSA was recovered from 18%, VRE were recovered from 6%, and C. difficile was recovered from 3%.
“These results suggest that floors in hospital rooms could be an underappreciated source for dissemination of pathogens,” Dr. Deshpande and his associates noted (Am J Infect Control. 2017 Mar 1;45[3]:336-8).
“It would be reasonable to educate health care personnel and patients that they should avoid placing high-touch objects on the floor when possible,” they added.
Moreover, the efficacy of current floor-cleaning and disinfection techniques should be reexamined, particularly with regard to eliminating C. difficile spores.
Other modes of transmission from floors also should be assessed, such as contamination of wheelchairs and wheeled equipment. And transmission of other pathogens, such as gram-negative organisms and viruses, should be examined, the investigators said.
The Agency for Healthcare Research and Quality and the U.S. Department of Veterans Affairs funded the study. Dr. Deshpande reported receiving research grants from 3M, Clorox, and Steris, and one of his associates reported receiving research grants from Clorox, Ecolab, GOJO, Merck, Pfizer, and Steris.
Floors in hospital patients’ rooms are frequently contaminated with pathogens such as Clostridium difficile, methicillin-resistant Staphylococcus aureus, and vancomycin-resistant Enterococci, which are easily transmitted to the hands of patients, care providers, and visitors, according to a report published in the American Journal of Infection Control.
Disinfection usually focuses on surfaces that are frequently touched by patients’ or health care workers’ hands, such as bed rails and call buttons. Floor disinfection has received limited attention.
However, floors are frequently touched by objects that are then handled, such as shoes and socks, said Abhishek Deshpande, MD, PhD, of the Cleveland Clinic, and his associates.
To examine the extent of floor contamination and the potential for transfer of pathogens to hands, the investigators surveyed five Cleveland-area hospitals.
They collected samples from 1-square-foot areas of floors adjacent to beds and in bathrooms in C. difficile isolation rooms, and in 2-3 randomly selected nonisolation rooms on the same wards. At least 30 rooms at each hospital were cultured for C. difficile, MRSA, and VRE, either during a patient stay or after the rooms had been cleaned at patient discharge.
In addition, the researchers performed a point-prevalence survey of the number and type of high-touch objects contacting floors in 10-25 randomly selected occupied patient rooms at each hospital. After they handled these objects, their hands also were cultured.
Floor contamination was common with all of the pathogens, particularly with C. difficile. The frequency of contamination was similar across the five hospitals, in both bedroom and bathroom sites, and even in the 50 rooms that had been cleaned at the last patient discharge.
C. difficile spores were recovered from the floors of 47%-55% of rooms, MRSA was recovered from the floors of 8%-32% of rooms, and VRE were recovered from the floors of 13%-30% of rooms.
In addition, 41 of 100 occupied rooms had 1-4 “high-touch” objects in direct contact with the floors, including personal items such as clothing, canes, or cellphone chargers; medical supplies or devices such as pulse oximeters, call buttons, heating pads, urinals, blood pressure cuffs, and wash basins; and linens such as bed sheets, pillows, and towels.
Of the 31 cultures taken from both bare and gloved hands that handled these items, MRSA was recovered from 18%, VRE were recovered from 6%, and C. difficile was recovered from 3%.
“These results suggest that floors in hospital rooms could be an underappreciated source for dissemination of pathogens,” Dr. Deshpande and his associates noted (Am J Infect Control. 2017 Mar 1;45[3]:336-8).
“It would be reasonable to educate health care personnel and patients that they should avoid placing high-touch objects on the floor when possible,” they added.
Moreover, the efficacy of current floor-cleaning and disinfection techniques should be reexamined, particularly with regard to eliminating C. difficile spores.
Other modes of transmission from floors also should be assessed, such as contamination of wheelchairs and wheeled equipment. And transmission of other pathogens, such as gram-negative organisms and viruses, should be examined, the investigators said.
The Agency for Healthcare Research and Quality and the U.S. Department of Veterans Affairs funded the study. Dr. Deshpande reported receiving research grants from 3M, Clorox, and Steris, and one of his associates reported receiving research grants from Clorox, Ecolab, GOJO, Merck, Pfizer, and Steris.
Floors in hospital patients’ rooms are frequently contaminated with pathogens such as Clostridium difficile, methicillin-resistant Staphylococcus aureus, and vancomycin-resistant Enterococci, which are easily transmitted to the hands of patients, care providers, and visitors, according to a report published in the American Journal of Infection Control.
Disinfection usually focuses on surfaces that are frequently touched by patients’ or health care workers’ hands, such as bed rails and call buttons. Floor disinfection has received limited attention.
However, floors are frequently touched by objects that are then handled, such as shoes and socks, said Abhishek Deshpande, MD, PhD, of the Cleveland Clinic, and his associates.
To examine the extent of floor contamination and the potential for transfer of pathogens to hands, the investigators surveyed five Cleveland-area hospitals.
They collected samples from 1-square-foot areas of floors adjacent to beds and in bathrooms in C. difficile isolation rooms, and in 2-3 randomly selected nonisolation rooms on the same wards. At least 30 rooms at each hospital were cultured for C. difficile, MRSA, and VRE, either during a patient stay or after the rooms had been cleaned at patient discharge.
In addition, the researchers performed a point-prevalence survey of the number and type of high-touch objects contacting floors in 10-25 randomly selected occupied patient rooms at each hospital. After they handled these objects, their hands also were cultured.
Floor contamination was common with all of the pathogens, particularly with C. difficile. The frequency of contamination was similar across the five hospitals, in both bedroom and bathroom sites, and even in the 50 rooms that had been cleaned at the last patient discharge.
C. difficile spores were recovered from the floors of 47%-55% of rooms, MRSA was recovered from the floors of 8%-32% of rooms, and VRE were recovered from the floors of 13%-30% of rooms.
In addition, 41 of 100 occupied rooms had 1-4 “high-touch” objects in direct contact with the floors, including personal items such as clothing, canes, or cellphone chargers; medical supplies or devices such as pulse oximeters, call buttons, heating pads, urinals, blood pressure cuffs, and wash basins; and linens such as bed sheets, pillows, and towels.
Of the 31 cultures taken from both bare and gloved hands that handled these items, MRSA was recovered from 18%, VRE were recovered from 6%, and C. difficile was recovered from 3%.
“These results suggest that floors in hospital rooms could be an underappreciated source for dissemination of pathogens,” Dr. Deshpande and his associates noted (Am J Infect Control. 2017 Mar 1;45[3]:336-8).
“It would be reasonable to educate health care personnel and patients that they should avoid placing high-touch objects on the floor when possible,” they added.
Moreover, the efficacy of current floor-cleaning and disinfection techniques should be reexamined, particularly with regard to eliminating C. difficile spores.
Other modes of transmission from floors also should be assessed, such as contamination of wheelchairs and wheeled equipment. And transmission of other pathogens, such as gram-negative organisms and viruses, should be examined, the investigators said.
The Agency for Healthcare Research and Quality and the U.S. Department of Veterans Affairs funded the study. Dr. Deshpande reported receiving research grants from 3M, Clorox, and Steris, and one of his associates reported receiving research grants from Clorox, Ecolab, GOJO, Merck, Pfizer, and Steris.
FROM THE AMERICAN JOURNAL OF INFECTION CONTROL
Key clinical point: Floors in hospital patients’ rooms are frequently contaminated with pathogens that are easily transmitted to the hands of patients, care providers, and visitors.
Major finding: C. difficile spores were recovered from the floors of 47%-55% of rooms, MRSA was recovered from the floors of 8%-32% of rooms, and VRE were recovered from the floors of 13%-30% of rooms.
Data source: A survey of five Cleveland-area hospitals in which 318 samples were collected from floors in patient rooms and bathrooms.
Disclosures: The Agency for Healthcare Research and Quality and the U.S. Department of Veterans Affairs funded the study. Dr. Deshpande reported receiving research grants from 3M, Clorox, and Steris, and one of his associates reported receiving research grants from Clorox, Ecolab, Gojo, Merck, Pfizer, and Steris.