User login
Most Important Elements of End-of-Life Care
An Australian team conducted a literature review of expected deaths in the hospital—where the majority of deaths in the developed world occur—and identified elements of end-of-life care that are important to patients and families.1 Published in the British journal Palliative Medicine, the review of nine electronic data bases and 1859 articles released between 1990 and 2014 identified eight quantitative studies that met inclusion criteria.
The authors, led by Claudia Virdun, RN, of the faculty of health at the University of Technology in Sydney, found four end-of-life domains that were most important to both patients and families:
- Effective communication and shared decision-making;
- Expert care;
- Respectful and compassionate care; and
- Trust and confidence in clinicians.
Not all patients dying in hospitals receive best evidence-based palliative care, the authors note, adding that the “challenge for healthcare services is to act on this evidence, reconfigure care systems accordingly and ensure universal access to optimal end-of-life care within hospitals.”
Reference
- Virdun C, Luckett T, Davidson PM, Phillips J. Dying in the hospital setting: A systematic review of quantitative studies identifying the elements of end-of-life care that patients and their families rank as being most important [published online ahead of print April 28, 2015]. Palliat Med.
An Australian team conducted a literature review of expected deaths in the hospital—where the majority of deaths in the developed world occur—and identified elements of end-of-life care that are important to patients and families.1 Published in the British journal Palliative Medicine, the review of nine electronic data bases and 1859 articles released between 1990 and 2014 identified eight quantitative studies that met inclusion criteria.
The authors, led by Claudia Virdun, RN, of the faculty of health at the University of Technology in Sydney, found four end-of-life domains that were most important to both patients and families:
- Effective communication and shared decision-making;
- Expert care;
- Respectful and compassionate care; and
- Trust and confidence in clinicians.
Not all patients dying in hospitals receive best evidence-based palliative care, the authors note, adding that the “challenge for healthcare services is to act on this evidence, reconfigure care systems accordingly and ensure universal access to optimal end-of-life care within hospitals.”
Reference
- Virdun C, Luckett T, Davidson PM, Phillips J. Dying in the hospital setting: A systematic review of quantitative studies identifying the elements of end-of-life care that patients and their families rank as being most important [published online ahead of print April 28, 2015]. Palliat Med.
An Australian team conducted a literature review of expected deaths in the hospital—where the majority of deaths in the developed world occur—and identified elements of end-of-life care that are important to patients and families.1 Published in the British journal Palliative Medicine, the review of nine electronic data bases and 1859 articles released between 1990 and 2014 identified eight quantitative studies that met inclusion criteria.
The authors, led by Claudia Virdun, RN, of the faculty of health at the University of Technology in Sydney, found four end-of-life domains that were most important to both patients and families:
- Effective communication and shared decision-making;
- Expert care;
- Respectful and compassionate care; and
- Trust and confidence in clinicians.
Not all patients dying in hospitals receive best evidence-based palliative care, the authors note, adding that the “challenge for healthcare services is to act on this evidence, reconfigure care systems accordingly and ensure universal access to optimal end-of-life care within hospitals.”
Reference
- Virdun C, Luckett T, Davidson PM, Phillips J. Dying in the hospital setting: A systematic review of quantitative studies identifying the elements of end-of-life care that patients and their families rank as being most important [published online ahead of print April 28, 2015]. Palliat Med.
Fellowship Training in Hospice and Palliative Care: New Pathways for Surgeons
Hospice and Palliative Medicine (HPM) has been a medical subspecialty recognized by the American Board of Medical Specialties since 2006. The American Board of Surgery (ABS) is one of 10 medical boards that offers board certification in HPM. Cosponsorship of HPM board certification by the ABS is significant because it signals an understanding and appreciation that patients in need of hospice and palliative care frequently require surgical services and also recognizes that many surgical patients benefit from palliative care.
Two specific domains of palliative care have been identified as core competencies for all surgeons: pain management and communication skills (Otolaryngology Clinics of North America 2009;42:1-13). Incorporation of these basic domains of care is considered primary palliative care for surgeons and inherent to good surgical care. For surgeons interested in obtaining subspecialty training in HPM, fellowship training is now required (the experiential pathway is no longer available).
Surgeons most likely to benefit from this additional training are those involved in the interdisciplinary care of chronically disease, critically ill, or terminally ill patients. Apparent in this description is the need for surgical palliative care providers across the full range of surgical subspecialties: pediatric to adult providers, trauma/critical care to oncology, cardiovascular surgery to transplant surgery. Currently, there are fewer than 100 surgeons with subspecialty board certification in HPM, constituting <1% of all physicians board certified in HPM.
The nascent field of HPM and needs surgeons as do the growing number of patients who require hospice and palliative medicine services. There currently exists a critical shortage of HPM physicians. A 2010 report by a task force appointed by the American Academy of Hospice and Palliative Medicine to study HPM physician workforce found that an estimated 6,000-18,000 additional physicians were needed to staff the then existing hospice and hospital-based palliative care programs (J. Pain Symptom Manage. 2010;40:899-91). The authors concluded that the capacity of fellowship programs at that time was insufficient to fill the shortage and changes in graduate medical education funding and structures were needed to increase the capacity to train sufficient numbers of HPM physicians. There are currently 108 Accreditation Council for Graduate Medical Education–accredited fellowships, up from 63 in 2009.
Surgeons interested in pursuing subspecialty training in HPM must complete a 1-year ACGME-accredited fellowship. Surgeons currently board-certified in surgery are eligible to apply. Many fellowship training programs have trained, or are willing to consider applications from, mid-career physicians, including surgeons (personal communication via HPM fellowship program directors listserv). Beginning July 1, 2015, an important change in eligibility for HPM fellowships goes into effect: Surgical residents with 3 years of training are now eligible to apply for ACGME-accredited HPM fellowships. This change in eligibility opens up an important pathway to HPM board certification, similar to that currently available for Surgical Critical Care. Trainees who complete HPM fellowship training through this pathway will not be eligible to obtain board certification in HPM until they have successfully achieved their primary board certification through the ABS.
Surgeons currently board certified in HPM incorporate their HPM training in a variety of ways: Some practice HPM full-time as members of a multidisciplinary in-patient palliative medicine consultation service while others integrate their training into their daily surgical practice, often serving as a resource on issues of surgical palliative care for their surgical colleagues. In my practice, I spend 1 day a week as a consultant on our in-patient palliative medicine consultation service in addition to providing faculty supervision to our palliative medicine fellows in our weekly outpatient clinic. For the remainder of the week, I am a practicing surgical oncologist and have found my training in palliative medicine invaluable in my daily care of patients with a variety of malignancies.
Like many of my surgical colleagues with HPM board certification, I am also actively engaged in teaching medical students, residents, and fellows about a variety of topics in palliative medicine, from evidence-based management of malignant bowel obstruction to communication skills for breaking bad news. Incorporating palliative medicine into my surgical practice has been incredibly rewarding, both personally and professionally.
In summary, HPM is in critical need of specialty trained physicians, including surgeons. Fellowship training is currently available to mid-career surgeons and, beginning July 1, 2015, surgical residents with 3 years of clinical training. Surgeons with subspecialty training in HPM are certain to find a wealth of clinical and academic opportunities as well as a path to a personally and professionally rewarding career.
For surgeons interested in obtaining more information about HPM fellowship training, go to the for a full list of programs and other training information.
Dr. Fahy is an associate professor of surgery at the University of New Mexico, Albuquerque. She is a surgical oncologist who is also board certified in hospice and palliative medicine. Dr. Fahy does not have any relevant conflicts of interest to disclose.
Hospice and Palliative Medicine (HPM) has been a medical subspecialty recognized by the American Board of Medical Specialties since 2006. The American Board of Surgery (ABS) is one of 10 medical boards that offers board certification in HPM. Cosponsorship of HPM board certification by the ABS is significant because it signals an understanding and appreciation that patients in need of hospice and palliative care frequently require surgical services and also recognizes that many surgical patients benefit from palliative care.
Two specific domains of palliative care have been identified as core competencies for all surgeons: pain management and communication skills (Otolaryngology Clinics of North America 2009;42:1-13). Incorporation of these basic domains of care is considered primary palliative care for surgeons and inherent to good surgical care. For surgeons interested in obtaining subspecialty training in HPM, fellowship training is now required (the experiential pathway is no longer available).
Surgeons most likely to benefit from this additional training are those involved in the interdisciplinary care of chronically disease, critically ill, or terminally ill patients. Apparent in this description is the need for surgical palliative care providers across the full range of surgical subspecialties: pediatric to adult providers, trauma/critical care to oncology, cardiovascular surgery to transplant surgery. Currently, there are fewer than 100 surgeons with subspecialty board certification in HPM, constituting <1% of all physicians board certified in HPM.
The nascent field of HPM and needs surgeons as do the growing number of patients who require hospice and palliative medicine services. There currently exists a critical shortage of HPM physicians. A 2010 report by a task force appointed by the American Academy of Hospice and Palliative Medicine to study HPM physician workforce found that an estimated 6,000-18,000 additional physicians were needed to staff the then existing hospice and hospital-based palliative care programs (J. Pain Symptom Manage. 2010;40:899-91). The authors concluded that the capacity of fellowship programs at that time was insufficient to fill the shortage and changes in graduate medical education funding and structures were needed to increase the capacity to train sufficient numbers of HPM physicians. There are currently 108 Accreditation Council for Graduate Medical Education–accredited fellowships, up from 63 in 2009.
Surgeons interested in pursuing subspecialty training in HPM must complete a 1-year ACGME-accredited fellowship. Surgeons currently board-certified in surgery are eligible to apply. Many fellowship training programs have trained, or are willing to consider applications from, mid-career physicians, including surgeons (personal communication via HPM fellowship program directors listserv). Beginning July 1, 2015, an important change in eligibility for HPM fellowships goes into effect: Surgical residents with 3 years of training are now eligible to apply for ACGME-accredited HPM fellowships. This change in eligibility opens up an important pathway to HPM board certification, similar to that currently available for Surgical Critical Care. Trainees who complete HPM fellowship training through this pathway will not be eligible to obtain board certification in HPM until they have successfully achieved their primary board certification through the ABS.
Surgeons currently board certified in HPM incorporate their HPM training in a variety of ways: Some practice HPM full-time as members of a multidisciplinary in-patient palliative medicine consultation service while others integrate their training into their daily surgical practice, often serving as a resource on issues of surgical palliative care for their surgical colleagues. In my practice, I spend 1 day a week as a consultant on our in-patient palliative medicine consultation service in addition to providing faculty supervision to our palliative medicine fellows in our weekly outpatient clinic. For the remainder of the week, I am a practicing surgical oncologist and have found my training in palliative medicine invaluable in my daily care of patients with a variety of malignancies.
Like many of my surgical colleagues with HPM board certification, I am also actively engaged in teaching medical students, residents, and fellows about a variety of topics in palliative medicine, from evidence-based management of malignant bowel obstruction to communication skills for breaking bad news. Incorporating palliative medicine into my surgical practice has been incredibly rewarding, both personally and professionally.
In summary, HPM is in critical need of specialty trained physicians, including surgeons. Fellowship training is currently available to mid-career surgeons and, beginning July 1, 2015, surgical residents with 3 years of clinical training. Surgeons with subspecialty training in HPM are certain to find a wealth of clinical and academic opportunities as well as a path to a personally and professionally rewarding career.
For surgeons interested in obtaining more information about HPM fellowship training, go to the for a full list of programs and other training information.
Dr. Fahy is an associate professor of surgery at the University of New Mexico, Albuquerque. She is a surgical oncologist who is also board certified in hospice and palliative medicine. Dr. Fahy does not have any relevant conflicts of interest to disclose.
Hospice and Palliative Medicine (HPM) has been a medical subspecialty recognized by the American Board of Medical Specialties since 2006. The American Board of Surgery (ABS) is one of 10 medical boards that offers board certification in HPM. Cosponsorship of HPM board certification by the ABS is significant because it signals an understanding and appreciation that patients in need of hospice and palliative care frequently require surgical services and also recognizes that many surgical patients benefit from palliative care.
Two specific domains of palliative care have been identified as core competencies for all surgeons: pain management and communication skills (Otolaryngology Clinics of North America 2009;42:1-13). Incorporation of these basic domains of care is considered primary palliative care for surgeons and inherent to good surgical care. For surgeons interested in obtaining subspecialty training in HPM, fellowship training is now required (the experiential pathway is no longer available).
Surgeons most likely to benefit from this additional training are those involved in the interdisciplinary care of chronically disease, critically ill, or terminally ill patients. Apparent in this description is the need for surgical palliative care providers across the full range of surgical subspecialties: pediatric to adult providers, trauma/critical care to oncology, cardiovascular surgery to transplant surgery. Currently, there are fewer than 100 surgeons with subspecialty board certification in HPM, constituting <1% of all physicians board certified in HPM.
The nascent field of HPM and needs surgeons as do the growing number of patients who require hospice and palliative medicine services. There currently exists a critical shortage of HPM physicians. A 2010 report by a task force appointed by the American Academy of Hospice and Palliative Medicine to study HPM physician workforce found that an estimated 6,000-18,000 additional physicians were needed to staff the then existing hospice and hospital-based palliative care programs (J. Pain Symptom Manage. 2010;40:899-91). The authors concluded that the capacity of fellowship programs at that time was insufficient to fill the shortage and changes in graduate medical education funding and structures were needed to increase the capacity to train sufficient numbers of HPM physicians. There are currently 108 Accreditation Council for Graduate Medical Education–accredited fellowships, up from 63 in 2009.
Surgeons interested in pursuing subspecialty training in HPM must complete a 1-year ACGME-accredited fellowship. Surgeons currently board-certified in surgery are eligible to apply. Many fellowship training programs have trained, or are willing to consider applications from, mid-career physicians, including surgeons (personal communication via HPM fellowship program directors listserv). Beginning July 1, 2015, an important change in eligibility for HPM fellowships goes into effect: Surgical residents with 3 years of training are now eligible to apply for ACGME-accredited HPM fellowships. This change in eligibility opens up an important pathway to HPM board certification, similar to that currently available for Surgical Critical Care. Trainees who complete HPM fellowship training through this pathway will not be eligible to obtain board certification in HPM until they have successfully achieved their primary board certification through the ABS.
Surgeons currently board certified in HPM incorporate their HPM training in a variety of ways: Some practice HPM full-time as members of a multidisciplinary in-patient palliative medicine consultation service while others integrate their training into their daily surgical practice, often serving as a resource on issues of surgical palliative care for their surgical colleagues. In my practice, I spend 1 day a week as a consultant on our in-patient palliative medicine consultation service in addition to providing faculty supervision to our palliative medicine fellows in our weekly outpatient clinic. For the remainder of the week, I am a practicing surgical oncologist and have found my training in palliative medicine invaluable in my daily care of patients with a variety of malignancies.
Like many of my surgical colleagues with HPM board certification, I am also actively engaged in teaching medical students, residents, and fellows about a variety of topics in palliative medicine, from evidence-based management of malignant bowel obstruction to communication skills for breaking bad news. Incorporating palliative medicine into my surgical practice has been incredibly rewarding, both personally and professionally.
In summary, HPM is in critical need of specialty trained physicians, including surgeons. Fellowship training is currently available to mid-career surgeons and, beginning July 1, 2015, surgical residents with 3 years of clinical training. Surgeons with subspecialty training in HPM are certain to find a wealth of clinical and academic opportunities as well as a path to a personally and professionally rewarding career.
For surgeons interested in obtaining more information about HPM fellowship training, go to the for a full list of programs and other training information.
Dr. Fahy is an associate professor of surgery at the University of New Mexico, Albuquerque. She is a surgical oncologist who is also board certified in hospice and palliative medicine. Dr. Fahy does not have any relevant conflicts of interest to disclose.
ICOO: Approach to opioids for cancer pain evolves
BOSTON – Opioid abuse might be as much of a problem in patients with cancer pain as in those who need analgesia for another reason, according to palliative care physicians at the Dana Farber Cancer Institute who outlined their safeguards at the International Conference of Opioids.
“Let’s not lose sight of the fact that the very access to these medications, which can do so much good, is in jeopardy,” said Dr. Douglas E. Brandoff, a palliative-care attending physician at the cancer institute. In the current era of “unprecedented regulation and scrutiny,” Dr. Brandoff said, pain practices in cancer care must evolve “to keep up with the times.”
Evidence that opioid abuse among cancer patients rivals that of other patients prescribed those agents is limited but reasonably consistent, according to Dr. Brandoff. He cited several published studies, including a survey of hospices in which substance abuse and diversion were considered a problem in 38% (J. Palliat. Med. 2013;16:237-42) of patients.
“It’s a little disconcerting. This is hospice, right? This doesn’t happen in hospice, but unfortunately, it does,” Dr. Brandoff reported.
At the cancer institute, a multidisciplinary task force convened in 2013 has now produced numerous specific policies designed to protect patients and institutions from abuse of controlled pain medications. Those steps are not much different from those being increasingly employed in clinics for nonmalignant chronic pain, but they are applied uniformly in essentially every patient – not just those singled out for high risk.
One required step is the implementation of a prescription-monitoring program for every patient started on a narcotic drug in controlled substances schedule II or III, a benzodiazepine, or a department of public health scheduled IV or V controlled substance. Another is the use of a medication management agreement designed to educate patients about the benefits and risks of controlled substances and outline expectations. All patients and their clinicians are required to sign the agreement.
“If we have someone who is imminently dying within hours or days, then, no, I would not impose a management agreement expectation on them or myself,” said Dr. Brandoff, but he said that there are essentially no other exceptions.
The agreement, crafted with nonjudgmental language aimed at clarifying the goals of chronic pain relief, is entered into the medical record. It generally has been well accepted, according to Dr. Lida Nabati, also a palliative care attending physician at the cancer institute, Dr. Nabati, who participated with Dr. Brandoff in presenting the cancer institute’s safeguards, noted that patient resistance to the agreement often is a red flag for potential problems with abuse.
The movement to control opioid abuse in cancer patients is relatively new. At the time that the task force began, Dr. Nabati noted that few other institutions had formal policies in place even though others also were beginning to review their approach. As recently as 2014, a directive from the Department of Veterans Affairs for opioid therapy in chronic pain patients specifically excluded those with cancer, Dr. Nabati reported.
Yet, cancer “does not afford some magical protective effect” from the very same risk factors associated with opioid use in noncancer patients, such as anxiety, depression, or history of substance use, according to Dr. Brandoff. Rather, he suggested that the added stress of a cancer diagnosis could exacerbate those factors.
The implementation of strategies aimed at reducing the risk of opioid abuse in patients with chronic cancer pain is needed and timely, according to Dr. Mellar P. Davis, the co-chair of the 2015 ICOO meeting and director of the palliative medicine fellowship program, Taussig Cancer Institute, Cleveland Clinic.
In an interview, Dr. Davis applauded the types of strategies implemented at the cancer institute, which he believes protect the patient, the physician, and the institution. He believes that the patients might be the greatest beneficiaries when appropriate opioid use permits a gain in quality of life through effective but nondebilitating pain control.
Dr. Brandoff and Dr. Nabati reported having no financial disclosures.
BOSTON – Opioid abuse might be as much of a problem in patients with cancer pain as in those who need analgesia for another reason, according to palliative care physicians at the Dana Farber Cancer Institute who outlined their safeguards at the International Conference of Opioids.
“Let’s not lose sight of the fact that the very access to these medications, which can do so much good, is in jeopardy,” said Dr. Douglas E. Brandoff, a palliative-care attending physician at the cancer institute. In the current era of “unprecedented regulation and scrutiny,” Dr. Brandoff said, pain practices in cancer care must evolve “to keep up with the times.”
Evidence that opioid abuse among cancer patients rivals that of other patients prescribed those agents is limited but reasonably consistent, according to Dr. Brandoff. He cited several published studies, including a survey of hospices in which substance abuse and diversion were considered a problem in 38% (J. Palliat. Med. 2013;16:237-42) of patients.
“It’s a little disconcerting. This is hospice, right? This doesn’t happen in hospice, but unfortunately, it does,” Dr. Brandoff reported.
At the cancer institute, a multidisciplinary task force convened in 2013 has now produced numerous specific policies designed to protect patients and institutions from abuse of controlled pain medications. Those steps are not much different from those being increasingly employed in clinics for nonmalignant chronic pain, but they are applied uniformly in essentially every patient – not just those singled out for high risk.
One required step is the implementation of a prescription-monitoring program for every patient started on a narcotic drug in controlled substances schedule II or III, a benzodiazepine, or a department of public health scheduled IV or V controlled substance. Another is the use of a medication management agreement designed to educate patients about the benefits and risks of controlled substances and outline expectations. All patients and their clinicians are required to sign the agreement.
“If we have someone who is imminently dying within hours or days, then, no, I would not impose a management agreement expectation on them or myself,” said Dr. Brandoff, but he said that there are essentially no other exceptions.
The agreement, crafted with nonjudgmental language aimed at clarifying the goals of chronic pain relief, is entered into the medical record. It generally has been well accepted, according to Dr. Lida Nabati, also a palliative care attending physician at the cancer institute, Dr. Nabati, who participated with Dr. Brandoff in presenting the cancer institute’s safeguards, noted that patient resistance to the agreement often is a red flag for potential problems with abuse.
The movement to control opioid abuse in cancer patients is relatively new. At the time that the task force began, Dr. Nabati noted that few other institutions had formal policies in place even though others also were beginning to review their approach. As recently as 2014, a directive from the Department of Veterans Affairs for opioid therapy in chronic pain patients specifically excluded those with cancer, Dr. Nabati reported.
Yet, cancer “does not afford some magical protective effect” from the very same risk factors associated with opioid use in noncancer patients, such as anxiety, depression, or history of substance use, according to Dr. Brandoff. Rather, he suggested that the added stress of a cancer diagnosis could exacerbate those factors.
The implementation of strategies aimed at reducing the risk of opioid abuse in patients with chronic cancer pain is needed and timely, according to Dr. Mellar P. Davis, the co-chair of the 2015 ICOO meeting and director of the palliative medicine fellowship program, Taussig Cancer Institute, Cleveland Clinic.
In an interview, Dr. Davis applauded the types of strategies implemented at the cancer institute, which he believes protect the patient, the physician, and the institution. He believes that the patients might be the greatest beneficiaries when appropriate opioid use permits a gain in quality of life through effective but nondebilitating pain control.
Dr. Brandoff and Dr. Nabati reported having no financial disclosures.
BOSTON – Opioid abuse might be as much of a problem in patients with cancer pain as in those who need analgesia for another reason, according to palliative care physicians at the Dana Farber Cancer Institute who outlined their safeguards at the International Conference of Opioids.
“Let’s not lose sight of the fact that the very access to these medications, which can do so much good, is in jeopardy,” said Dr. Douglas E. Brandoff, a palliative-care attending physician at the cancer institute. In the current era of “unprecedented regulation and scrutiny,” Dr. Brandoff said, pain practices in cancer care must evolve “to keep up with the times.”
Evidence that opioid abuse among cancer patients rivals that of other patients prescribed those agents is limited but reasonably consistent, according to Dr. Brandoff. He cited several published studies, including a survey of hospices in which substance abuse and diversion were considered a problem in 38% (J. Palliat. Med. 2013;16:237-42) of patients.
“It’s a little disconcerting. This is hospice, right? This doesn’t happen in hospice, but unfortunately, it does,” Dr. Brandoff reported.
At the cancer institute, a multidisciplinary task force convened in 2013 has now produced numerous specific policies designed to protect patients and institutions from abuse of controlled pain medications. Those steps are not much different from those being increasingly employed in clinics for nonmalignant chronic pain, but they are applied uniformly in essentially every patient – not just those singled out for high risk.
One required step is the implementation of a prescription-monitoring program for every patient started on a narcotic drug in controlled substances schedule II or III, a benzodiazepine, or a department of public health scheduled IV or V controlled substance. Another is the use of a medication management agreement designed to educate patients about the benefits and risks of controlled substances and outline expectations. All patients and their clinicians are required to sign the agreement.
“If we have someone who is imminently dying within hours or days, then, no, I would not impose a management agreement expectation on them or myself,” said Dr. Brandoff, but he said that there are essentially no other exceptions.
The agreement, crafted with nonjudgmental language aimed at clarifying the goals of chronic pain relief, is entered into the medical record. It generally has been well accepted, according to Dr. Lida Nabati, also a palliative care attending physician at the cancer institute, Dr. Nabati, who participated with Dr. Brandoff in presenting the cancer institute’s safeguards, noted that patient resistance to the agreement often is a red flag for potential problems with abuse.
The movement to control opioid abuse in cancer patients is relatively new. At the time that the task force began, Dr. Nabati noted that few other institutions had formal policies in place even though others also were beginning to review their approach. As recently as 2014, a directive from the Department of Veterans Affairs for opioid therapy in chronic pain patients specifically excluded those with cancer, Dr. Nabati reported.
Yet, cancer “does not afford some magical protective effect” from the very same risk factors associated with opioid use in noncancer patients, such as anxiety, depression, or history of substance use, according to Dr. Brandoff. Rather, he suggested that the added stress of a cancer diagnosis could exacerbate those factors.
The implementation of strategies aimed at reducing the risk of opioid abuse in patients with chronic cancer pain is needed and timely, according to Dr. Mellar P. Davis, the co-chair of the 2015 ICOO meeting and director of the palliative medicine fellowship program, Taussig Cancer Institute, Cleveland Clinic.
In an interview, Dr. Davis applauded the types of strategies implemented at the cancer institute, which he believes protect the patient, the physician, and the institution. He believes that the patients might be the greatest beneficiaries when appropriate opioid use permits a gain in quality of life through effective but nondebilitating pain control.
Dr. Brandoff and Dr. Nabati reported having no financial disclosures.
EXPERT ANALYSIS AT THE INTERNATIONAL CONFERENCE ON OPIOIDS
ICOO: Opioid self-dosing falls short of pain control
BOSTON – Many cancer patients do not pursue or at least do not achieve complete freedom from pain when permitted control over their opioid dose, according to a comprehensive analysis of published studies that evaluated patient-controlled analgesia.
“We do not know why. Patients were encouraged in these studies to titrate opioids until they were pain free or until they had side effects. Although this could be an issue of side effects, another interpretation is that complete pain control is not the goal for many individuals,” reported Dr. Brian H. Wetherington of the University of Kentucky, Lexington.
The data from this analysis were presented at the International Conference on Opioids from a comprehensive literature search that included 905 potentially relevant articles. Of these, 62 met inclusion criteria, particularly an assessment of patient-controlled opioids in patients with cancer pain. The studies also had to assess pain control with a visual analog scale (VAS) or the Neuropathy Pain Scale (NPS) using a 10-point system with 10 being the greatest level of pain imaginable.
“We were interested in evaluating whether patients, when given complete control over their opioids, would take sufficient doses to provide complete pain relief, which is often stated as the goal in pain management,” explained Dr. Wetherington, who was coauthor of a study led by his colleague at University of Kentucky, Dr. Michael Harned.
The answer was no. When the data from the 62 studies, which included 5,251 patients with cancer pain were collated, the average pain score at baseline was 5.4. At the time of assessment of pain control, the mean pain score was 2.7.
“The mean pain score for patients managing their own cancer pain on opioids was reduced from study entry but remained at the moderate to severe pain level or higher than what many health care providers would recommend,” Dr. Wetherington reported.
This review of published studies does not explain why lower pain scores are not reached, but the Dr. Wetherington and his coauthors hypothesized that patients are demonstrating their own benefit-to-risk ratio assessment.
This is thought to be the first systematic review to find that patients do not seek complete control of pain when given access to unrestricted analgesia, but several individual studies have made the same point. In one study cited by the authors, patients on a fentanyl patch only reduced their pain scores to 3.0 on average when given unlimited access to oral morphine for breakthroughs (J. Pain Symptom Manage. 1998;16:102-11).
“We think this deserves further study, because there may be lessons regarding how we think of optimal pain control. While the therapeutic target is often described as complete pain relief, these data suggest that this may not be the goal for patients when they are left to select their own level of pain control,” Dr. Wetherington explained.
The same observation regarding the failure of patients to eliminate all pain on patient-controlled analgesia has been made anecdotally by Dr. William G. Brose of Stanford (Calif.) University. However, he suggested in an interview that patients might be reluctant to rate themselves completely pain free on a subjective scale. He also believes that level of analgesia may not be the most relevant endpoint.
“We are increasingly evaluating change in patient function, which may be a more useful tool for evaluating the efficacy of pain control,” Dr. Brose said.
BOSTON – Many cancer patients do not pursue or at least do not achieve complete freedom from pain when permitted control over their opioid dose, according to a comprehensive analysis of published studies that evaluated patient-controlled analgesia.
“We do not know why. Patients were encouraged in these studies to titrate opioids until they were pain free or until they had side effects. Although this could be an issue of side effects, another interpretation is that complete pain control is not the goal for many individuals,” reported Dr. Brian H. Wetherington of the University of Kentucky, Lexington.
The data from this analysis were presented at the International Conference on Opioids from a comprehensive literature search that included 905 potentially relevant articles. Of these, 62 met inclusion criteria, particularly an assessment of patient-controlled opioids in patients with cancer pain. The studies also had to assess pain control with a visual analog scale (VAS) or the Neuropathy Pain Scale (NPS) using a 10-point system with 10 being the greatest level of pain imaginable.
“We were interested in evaluating whether patients, when given complete control over their opioids, would take sufficient doses to provide complete pain relief, which is often stated as the goal in pain management,” explained Dr. Wetherington, who was coauthor of a study led by his colleague at University of Kentucky, Dr. Michael Harned.
The answer was no. When the data from the 62 studies, which included 5,251 patients with cancer pain were collated, the average pain score at baseline was 5.4. At the time of assessment of pain control, the mean pain score was 2.7.
“The mean pain score for patients managing their own cancer pain on opioids was reduced from study entry but remained at the moderate to severe pain level or higher than what many health care providers would recommend,” Dr. Wetherington reported.
This review of published studies does not explain why lower pain scores are not reached, but the Dr. Wetherington and his coauthors hypothesized that patients are demonstrating their own benefit-to-risk ratio assessment.
This is thought to be the first systematic review to find that patients do not seek complete control of pain when given access to unrestricted analgesia, but several individual studies have made the same point. In one study cited by the authors, patients on a fentanyl patch only reduced their pain scores to 3.0 on average when given unlimited access to oral morphine for breakthroughs (J. Pain Symptom Manage. 1998;16:102-11).
“We think this deserves further study, because there may be lessons regarding how we think of optimal pain control. While the therapeutic target is often described as complete pain relief, these data suggest that this may not be the goal for patients when they are left to select their own level of pain control,” Dr. Wetherington explained.
The same observation regarding the failure of patients to eliminate all pain on patient-controlled analgesia has been made anecdotally by Dr. William G. Brose of Stanford (Calif.) University. However, he suggested in an interview that patients might be reluctant to rate themselves completely pain free on a subjective scale. He also believes that level of analgesia may not be the most relevant endpoint.
“We are increasingly evaluating change in patient function, which may be a more useful tool for evaluating the efficacy of pain control,” Dr. Brose said.
BOSTON – Many cancer patients do not pursue or at least do not achieve complete freedom from pain when permitted control over their opioid dose, according to a comprehensive analysis of published studies that evaluated patient-controlled analgesia.
“We do not know why. Patients were encouraged in these studies to titrate opioids until they were pain free or until they had side effects. Although this could be an issue of side effects, another interpretation is that complete pain control is not the goal for many individuals,” reported Dr. Brian H. Wetherington of the University of Kentucky, Lexington.
The data from this analysis were presented at the International Conference on Opioids from a comprehensive literature search that included 905 potentially relevant articles. Of these, 62 met inclusion criteria, particularly an assessment of patient-controlled opioids in patients with cancer pain. The studies also had to assess pain control with a visual analog scale (VAS) or the Neuropathy Pain Scale (NPS) using a 10-point system with 10 being the greatest level of pain imaginable.
“We were interested in evaluating whether patients, when given complete control over their opioids, would take sufficient doses to provide complete pain relief, which is often stated as the goal in pain management,” explained Dr. Wetherington, who was coauthor of a study led by his colleague at University of Kentucky, Dr. Michael Harned.
The answer was no. When the data from the 62 studies, which included 5,251 patients with cancer pain were collated, the average pain score at baseline was 5.4. At the time of assessment of pain control, the mean pain score was 2.7.
“The mean pain score for patients managing their own cancer pain on opioids was reduced from study entry but remained at the moderate to severe pain level or higher than what many health care providers would recommend,” Dr. Wetherington reported.
This review of published studies does not explain why lower pain scores are not reached, but the Dr. Wetherington and his coauthors hypothesized that patients are demonstrating their own benefit-to-risk ratio assessment.
This is thought to be the first systematic review to find that patients do not seek complete control of pain when given access to unrestricted analgesia, but several individual studies have made the same point. In one study cited by the authors, patients on a fentanyl patch only reduced their pain scores to 3.0 on average when given unlimited access to oral morphine for breakthroughs (J. Pain Symptom Manage. 1998;16:102-11).
“We think this deserves further study, because there may be lessons regarding how we think of optimal pain control. While the therapeutic target is often described as complete pain relief, these data suggest that this may not be the goal for patients when they are left to select their own level of pain control,” Dr. Wetherington explained.
The same observation regarding the failure of patients to eliminate all pain on patient-controlled analgesia has been made anecdotally by Dr. William G. Brose of Stanford (Calif.) University. However, he suggested in an interview that patients might be reluctant to rate themselves completely pain free on a subjective scale. He also believes that level of analgesia may not be the most relevant endpoint.
“We are increasingly evaluating change in patient function, which may be a more useful tool for evaluating the efficacy of pain control,” Dr. Brose said.
AT ICOO 2015
Key clinical point: Given the opportunity, patients do not titrate opioid therapy to a point of complete pain control, according to a comprehensive survey of published studies.
Major finding: In a survey of 62 published studies of cancer patients who were provided unlimited access to opioids for pain control, the average pain control was 2.7 on a scale of 10, indicating that most patients do not seek or are unable to achieve complete control with an acceptable benefit-to-risk ratio.
Data source: Retrospective data review.
Disclosures: The study was investigator initiated. Dr. Wetherington reported having no financial disclosures.
Palliative Radiotherapy for the Management of Metastatic Cancer
In recent years, there has been increasing interest in palliative care for patients with cancer at the end of life. Up to 23% of patients have metastatic disease at presentation, and symptoms from metastatic lesions can cause significant anxiety and impair patients’ quality of life (QOL).1
Palliative radiotherapy (RT) plays a valuable role in the management of metastatic disease to relieve tumor-related symptoms. Although palliative RT does not provide a chance for a cure, it improves QOL and may prolong survival time.2-4 An estimated 20% to 50% of radiation courses are prescribed with palliative intent, because RT is highly effective in providing symptom relief, and the toxicity associated with palliative doses is typically mild.5,6 Palliative RT can be used to manage bone and brain metastases, prevent or treat spinal cord compression, and manage numerous tumor-related symptoms, such as pain and bleeding in patients with terminal cancer.
Palliative RT for bone and brain metastases is supported by high-quality evidence and is considered one of the most effective and cost-effective options available.7,8 This article aims to review the role of RT in treating 3 conditions commonly encountered in patients with metastatic disease—bone metastases, spinal cord compression, and brain metastases—and to emphasize the importance of timely integration of RT for optimal results.
Bone Metastases
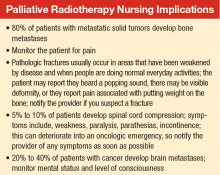
About 80% of patients with metastatic solid tumors develop bone metastases, and about 350,000 deaths are linked to bone metastases in the U.S. each year.9 Osseous
metastases can lead to pain, fracture, hypercalcemia, and spinal cord compression. The primary modality for treatment of pain and prevention of morbidity from bone metastases is external beam RT.10
The likelihood of bone pain relief with palliative RT is 60% to 80%, and 30% to 40% of patients achieving complete pain relief. Randomized studies have shown multiple-dose and fractionation regimens provided effective symptom relief for bone metastases. Most commonly used regimens include a single fraction of 8 gray (Gy) delivered in 1 treatment, 20 Gy in 5 fractions delivered daily over 1 week, and 30 Gy in 10 fractions delivered over 2 weeks. Treatment with a single fraction improves access to treatment and patient convenience, whereas more prolonged courses have been associated with lower rates of retreatment.11,12 Regarding the higher rate of retreatment with single-fraction RT, no clear evidence exists that this is due to a less durable pain response or lower level of pain relief.13
There has been recent interest in using predictive models to estimate life expectancy to avoid long courses of RT at the end of life.14,15 Shorter treatment courses of 8 Gyonce or 20 Gy in 5 fractions are particularly valuable for patients with a life expectancy < 3 months to avoid long courses of treatment, and thereby improve QOL as patients transition into hospice. A recent survey demonstrated that 93% of radiation oncologists within the VHA are willing to prescribe short courses of RT consisting of ≤ 6 fractions, and 76% have experience with single-fraction RT.16 These findings are in contradiction to the findings in the non-VA radiation oncology community, in which < 10% of patients with uncomplicated bone metastases are treated with a single fraction.17,18
In addition to providing pain relief, RT is used in the treatment of impending fractures either, adjuvant after surgical stabilization or alone for lower risk lesions.19 Factors that impact fracture risk include location of the metastasis (weight-bearing bones, such as femurs, which are at particularly high risk), length of bone involved, and extent of cortical involvement. Mirels’ scoring system was developed to predict fracture risk in patients with bone metastasis, based on 4 criteria: the
extent of cortical involvement, the location of the metastasis, the osteolytic vs osteoblastic appearance of the lesion, and the degree of pain.20 Surgical fixation can be considered, based on the total score and corresponding fracture risk. When appropriate, surgical stabilization should be considered by an orthopedic surgeon prior to initiating RT.
Postoperative RT after surgical stabilization has been associated with a reduced rate of secondary surgical procedures as well as with improved functional status.21 Radiotherapy promotes remineralization and bone healing and prevents the loss of surgical fixation by treating any residual tumor. A retrospective review of 60 patients with metastatic disease in weight-bearing bones with pathologic fracture or impending pathologic fracture demonstrated that surgery followed by RT was associated with improved functional status as well as with improved overall survival (OS).22,23 For patients in whom surgery is not indicated, the consulting radiation oncologist should consider factors such as the location of the metastasis in weight-bearing vs nonweight bearing bones, the size and extent of the metastasis, and associated symptoms when making a treatment recommendation. In patients at fracture risk from bone metastases, bisphosphonates should also be considered as part of the treatment regimen.24
Spinal Cord Compression
About 5% to 10% of patients diagnosed with cancer will develop spinal cord compression during the course of their disease.25 Spinal cord compression is considered a medical emergency that can result in significant pain and neurologic symptoms, including weakness, paralysis, parasthesias, and incontinence. Early treatment of spinal cord compression can prevent onset or progression of these symptoms; furthermore, early treatment prior to loss of ambulation is associated with improved long-term ambulatory function.26,27
Treatment decisions for spinal metastases with an associated concern for cord compression should be made after a consultation with both a neurosurgeon and a radiation oncologist. Early initiation of steroids is recommended to aid in tumor shrinkage for potential symptom relief.28 A standard way to administer dexamethasone is with a 10-mg loading dose followed by 16 mg per day, divided into 4 doses of 4 mg. Higher steroid doses showed no benefit in a prospective randomized trial comparing 96 mg with 16 mg of dexamethasone daily.29
Surgical decompression should be considered initial management of spinal cord compression. For patients treated surgically, local RT is indicated postoperatively as well. Randomized data show that surgery followed by RT provides better ambulatory function than does RT alone in patients with paralysis of < 2 days’ duration.30 Some patients with metastatic disease are not good candidates for surgery due to comorbidities, poor performance status, life expectancy < 3 months, or multilevel spinal involvement.
In patients who are not operative candidates, radiation alone is an appropriate alternative. However, several factors need consideration in deciding whether to manage cord compression with surgery followed by RT vs RT alone. These factors include life expectancy, tumor type (myeloma and lymphoma are more radiosensitive), interval since tumor diagnosis, and the presence of visceral metastases.31 Factors favoring surgical decompression plus postoperative RT over RT alone include spinal instability, KPS (Karnofsky Performance Status) > 70, radio-resistant tumor histology, minimal metastatic disease, and projected survival > 3 months.10
For patients managed with RT alone, early diagnosis and treatment is associated with improved outcomes. A prospective study of patients treated with RT without surgery for spinal cord compression demonstrated that 82% of patients experienced back pain relief, 76% achieved improvement in or preservation of ambulation, and 44% of patients with sphincter dysfunction experienced improvement with treatment.32 Patients with certain tumor histologies, such as myeloma, breast cancer, and prostate cancer, had better responses to RT.32
In the setting of spinal cord compression, longer courses of RT may provide better local control than do shorter courses.33 Therefore, longer courses of RT, such as 30 Gy in 10 fractions delivered over 2 weeks, are often preferred in cases of spinal cord compression treated with definitive RT as well as after surgical decompression. However, overall life expectancy is an important factor considered by the treating radiation oncologist when selecting a short course vs a longer course of RT.
In the instance of painful vertebral body metastases without spinal cord compression, a new subset analysis of the Radiation Therapy Oncology Group (RTOG) 9714 randomized trial indicated that single fraction RT (8 Gy) is just as effective as multiple fractions (30 Gy in 10 fractions), with this study demonstrating comparable rates of pain relief and narcotic use in both groups 3 months after RT.34 Advantages to the single-fraction plan compared with those of multiple fractions include mitigation of logistic concerns for patients and family at the end of life and less acute adverse effects.
Brain Metastases
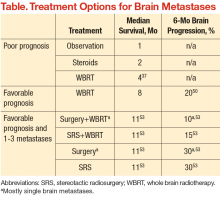
An estimated 20% to 40% of patients with cancer develop brain metastases.35 The incidence of brain metastases has been rising most likely due to improved detection rates with magnetic resonance imaging (MRI) and improved cancer survival, because treatment regimens have improved with targeted chemotherapy and radiation techniques. Currently, the annual incidence of brain metastases is 170,000 to 200,000 in the U.S.36 Prognosis for these patients is poor, with median survival of 1 month without treatment and about 4 months with whole brain RT (WBRT) (Table).25,37-39
The goal of management for patients with brain metastases is to prevent or treat neurologic symptoms and to prolong survival. Treatment options include corticosteroids, WBRT, surgery, and stereotactic radiosurgery (SRS). Recommendations for treatment should involve both a radiation oncologist and neurosurgeon to determine the best treatment for an individual based on patient age, performance status, extent of systemic disease, and number of brain metastases. These prognostic factors that may predict life expectancy and impact treatment recommendations.40
Factors that have been correlated with improved survival include younger age, better performance status, fewer brain metastases, and lower burden of systemic disease.41,42 Prognostic assessment tools such as the Graded Prognostic Assessment and RTOG-Recursive Partitioning Analysis can be used to predict life expectancy in patients with brain metastases.41,43 However, routine use of these tools is lagging, as evidenced by a recent survey of VHA radiation oncologists. Use of these tools in the clinic will enhance the quality of end of life care and decision making.
Corticosteroids have classically been used in the treatment of brain metastases either alone for supportive care or in combination with RT. Steroids are recommended to provide symptom relief in patients with symptoms related to cerebral edema or mass effect.44 Steroids have been shown to mitigate edema and improve neurologic deficits in about two-thirds of patients with brain metastases.36,45 The effect of corticosteroids is thought to be mediated through inhibition of prostaglandin synthesis, reduction in vascular permeability, and anti-inflammatory properties.46 A common corticosteroid regimen is a 10-mg loading dose of dexamethasone, followed by 16 mg daily in divided doses. For patients without neurologic deficits or cerebral edema, it is reasonable to defer corticosteroid use only when patients are symptomatic.
In general, WBRT is considered an appropriate treatment option for patients with multiple brain metastases based on data suggesting an improvement in OS compared with the use of corticosteroids alone.47 Whole brain radiation has been shown to result in the improvement of baseline neurologic deficits or the prevention of further symptom progression.48 The partial or complete metastasis response rates are on the order of 60%.38 Tumor regression after WBRT has been associated with preservation of neurocognitive function as well as prolonged survival.49
For good prognosis patients with a single brain metastasis and good performance status, the use of surgery or radiosurgery added to WBRT has been associated with improved OS (Table). The RTOG 9508 randomized trial of WBRT with or without SRS demonstrated a survival advantage with SRS, with median survival times of 6.5 months with WBRT + SRS vs 4.9 months with WBRT alone.50 Similarly, a randomized trial evaluating WBRT alone compared with surgery followed by WBRT in patients
with good prognosis demonstrated significantly improved OS in the surgery group (median 40 weeks vs 15 weeks).51 In general, WBRT or postoperative RT to the tumor bed is still indicated after surgical resection, based on randomized data showing a reduction in tumor bed recurrence with postoperative RT.52
For patients with only 1 to 3 brain metastases and a favorable prognosis, surgery and SRS can be considered treatment options, oftentimes with WBRT. The EORTC randomized trial of patients with 1 to 3 brain metastases was designed to determine the benefit of WBRT after treatment with surgery or SRS. In this study, 119 patients underwent SRS and 160 patients underwent surgical resection.53 Both groups of patients were randomized to observation vs adjuvant WBRT. This study demonstrated reduced rates of intracranial relapse with WBRT, however, without any change in OS. Although there is concern that WBRT may impair cognitive function with no clear survival benefit after surgery or SRS, WBRT does reduce recurrence rates in the brain and the need for further treatment.54 Therefore, decisions regarding WBRT in such a setting should be made only after a detailed discussion with a radiation oncologist regarding risks vs benefits of treatment as part of the informed decision-making process.
Conclusions
Palliative RT plays an important role in the management of metastatic cancer to provide symptom relief and is a cost-effective treatment option for bone and brain metastases. Life expectancy and tumor characteristics should be considered when making treatment recommendations to ensure selection of regimens that complement patients’ unique situations. Timely referrals for treatment are important to optimize treatment results.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Porter A and David M. Palliative care for bone, spinal cord, brain, and liver metastases. In: Gunderson LL, Tepper JE, eds. Clinical Radiation Oncology. 2nd ed. Philadelphia, PA: Elsevier Churchill Livingstone; 2007:437-451.
2. Yamaguchi S, Ohguri T, Matsuki Y, et al. Palliative radiotherapy in patients with a poor performance status: the palliative effect is correlated with prolongation of survival time. Radiat Oncol. 2013;8:166.
3. Mac Manus MP, Matthews JP, Wada M, Wirth A, Worotniuk V, Ball DL. Unexpected long-term survival after low-dose palliative radiotherapy for non-small cell lung cancer. Cancer. 2006;106(5):1110-1116.
4. Rastogi M, Revannasiddaiah S, Gupta MK, Seam RK, Thakur P, Gupta M. When palliative treatment achieves more than palliation: instances of long-term survival after palliative radiotherapy. Indian J Palliat Care. 2012;18(2):117-121.
5. Nieder C, Pawinski A, Haukland E, Dokmo R, Phillipi I, Dalhaug A. Estimating need for palliative external beam radiotherapy in adult cancer patients. Int J Radiat Oncol Biol Phys. 2010;76(1):207-211.
6. Hoegler D. Radiotherapy for palliation of symptoms in incurable cancer. Curr Probl Cancer. 1997;21(3):129-183.
7. Expósito J, Jaén J, Alonso E, Tovar I. Use of palliative radiotherapy in brain and bone metastases (VARA II study). Radiat Oncol. 2012;7:131.
8. Konski A. Radiotherapy is a cost-effective palliative treatment for patients with bone metastasis from prostate cancer. Int J Radiat Oncol Biol Phys. 2004;60(5):1373-1378.
9. Popovic M, den Hartogh M, Zhang L, et al. Review of international patterns of practice for the treatment of painful bone metastases with palliative radiotherapy from 1993 to 2013. Radiother Oncol. 2014;111(1):11-17.
10. Lutz S, Berk L, Chang E, et al; American Society for Radiation Oncology (ASTRO). Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;79(4):965-976.
11. Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systemic review. J Clin Oncol. 2007;25(11):1423-1436.
12. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy—a systemic review of the randomized trials. Cochrane Database Syst Rev. 2004;(2):CD004721.
13. Steenland E, Leer JW, van Houwelingen H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol. 1999;52(2):101-109.
14. Krishnan MS, Epstein-Peterson Z, Chen YH, et al. Predicting life expectance in patients with metastatic cancer receiving palliative radiotherapy: the TEACHH model. Cancer. 2014;120(1):134-141.
15. Guadagnolo BA, Liao KP, Elting L, Giordano S, Buccholz TA, Shih YC. Use of radiation therapy in the last 30 days of life among a large population-based cohort of elderly patients in the United States. J Clin Oncol. 2013;31(1):80-87.
16. Moghanaki D, Cheuk AV, Fosmire H, et al; U.S. Veterans Healthcare Administration National Palliative Radiotherapy Taskforce. Availability of single fraction palliative radiotherapy for cancer patients receiving end-of-life care within the Veterans Healthcare Administration. J Palliat Med. 2014;17(11):1221-1225.
17. Ellsworth SG, Alcorn SR, Hales RK, McNutt TR, DeWeese TL, Smith TJ. Patterns of care among patients receiving radiation therapy for bone metastases at a large academic institution. Int J Radiat Oncol Biol Phys. 2014;89(5):1100-1105.
18. Bradley NM, Husted J, Sey MS, et al. Review of patterns of practice and patients’ preferences in the treatment of bone metastases with palliative radiotherapy. Support Care Cancer. 2007;15(4):373-385.
19. Haidukewych GJ. Metastatic disease around the hip: maintaining quality of life. J Bone Joint Surg Br. 2012;94(11 suppl A):22-25.
20. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;(249):256-264.
21. Jacofsky DJ, Haidukewych GJ. Management of pathologic fractures of the proximal femur: state of the art. J Orthop Trauma. 2004;18(7):459-469.
22. Townsend PW, Rosenthal HG, Smalley SR, Cozad SC, Hassanein RE. Impact of postoperative radiation therapy and other perioperative factors on outcome after orthopedic stabilization of impending or pathologic fractures due to metastatic disease. J Clin Oncol. 1994;12(11):2345-2350.
23. Townsend PW, Smalley SR, Cozad SC, Rosenthal HG, Hassanein RE. Role of postoperative radiation therapy after stabilization of fractures caused by metastatic disease. Int J Radiat Oncol Biol Phys. 1995;31(1):43-49.
24. Farooki A. NCCN bone health task force: key recommendations. J Natl Compr Canc Netw. 2014;12(5 suppl):813-816.
25. Sejpal SV, Bhate A, Small W. Palliative radiation therapy in the management of brain metastases, spinal cord compression, and bone metastases. Semin Intervent Radiol. 2007;24(4):362-374.
26. Abrahm JL, Banffy MB, Harris MB. Spinal cord compression in patients with advanced metastatic cancer: “all I care about is walking and living my life.” JAMA. 2008;299(8):937-946.
27. Kim RY, Spencer SA, Meredith RF, et al. Extradural spinal cord compression: analysis of factors determining functional prognosis—prospective study. Radiology. 1990;176(1):279-282.
28. Kaloostian PE, Yurter A, Etame AB, Vrionis FD, Sciubba DM, Gokaslan ZL. Palliative strategies for the management of primary and metastatic spinal tumors. Cancer Control. 2014;21(2):140-143.
29. Graham PH, Capp A, Delaney G, et al. A pilot randomized comparison of dexamethasone 96 mg vs 16 mg per day for malignant spinal-cord compression treated by radiotherapy: TROG 01.05 Superdex study. Clin Oncol (R Coll Radiol). 2006;18(1):70-76.
30. Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643-648.
31. Rades D, Huttenlocher S, Bajrovic A, et al. Surgery followed by radiotherapy versus radiotherapy alone for metastatic spinal cord compression from unfavorable tumors. Int J Radiat Oncol Biol Phys. 2011;81(5):e861-e868.
32. Maranzano E, Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys. 1995;32(4):959-967.
33. Rades D, Fehlauer F, Schulte R, et al. Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. J Clin Oncol. 2006;24(21):3388-3393.
34. Howell DD, James JL, Hartsell WF, et al. Single-fraction radiotherapy versus multifraction radiotherapy for palliation of bone metastases-equivalent efficacy, less toxicity, more convenient: a subset analysis of Radiation Therapy Oncology Group trial 97-14. Cancer. 2013;119(4):888-896.
35. Wong J, Hird A, Kirou-Mauro, Napolskikh J, Chow E. Quality of life in brain metastases radiation trials: a literature review. Curr Oncol. 2008;15(5):25-45.
36. Nichols EM, Patchell RA, Regine WF, Kwok Y. Palliation of brain and spinal cord metastases. In: Halperin EC, Brady LW, Perez CA, Wazer DE, eds. Perez and Brady’s Principles and Practice of Radiation Oncology. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013:1974.
37. Zimm S, Wampler GL, Stablein D, Hazra T, Young HF. Intracerebral metastases in solid-tumor patients: natural history and results of treatment. Cancer. 1981;48(2):384-394.
38. Khuntia D, Brown P, Li J, Mehta MP. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24(8):1295-1304.
39. Sundström JT, Minn H, Lertola KK, Nordman E. Prognosis of patients treated for intracranial metastases with whole-brain irradiation. Ann Med. 1998;30(3):296-299.
40. Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210-225.
41. Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745-751.
42. Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG databases. Int J Radiat Oncol Biol Phys. 2008;70(2):510-514.
43. Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419-425.
44. Ryken TC, McDermott M, Robinson PD, et al. The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):103-114.
45. Ruderman NB, Hall TC. Use of glucocorticoids in the palliative treatment of metastatic brain tumors. Cancer. 1965;18:298-306.
46. Kaloostian PE, Yurter A, Etame AB, Vrionis FD, Sciubba DM, Gokaslan ZL. Palliative strategies for the management of primary and metastatic spinal tumors. Cancer Control. 2014;21(2):140-143.
47. Horton J, Baxter DH, Olson KB. The management of metastases to the brain by irradiation and corticosteroids. Am J Roentgenol Radium Ther Nucl Med. 1971;111(2)334-336.
48. Wong J, Hird A, Zhang L, et al. Symptoms and quality of life in cancer patients with brain metastases following palliative radiotherapy. Int J Radiat Oncol Biol Phys. 2009;75(4):1125-1131.
49. Li J, Bentzen SM, Renschler M, Mehta MP. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol. 2007;25(10):1260-1266.
50. Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665-1672.
51. Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322(8):494-500.
52. Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485-1489.
53. Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134-141.
54. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037-1044.
In recent years, there has been increasing interest in palliative care for patients with cancer at the end of life. Up to 23% of patients have metastatic disease at presentation, and symptoms from metastatic lesions can cause significant anxiety and impair patients’ quality of life (QOL).1
Palliative radiotherapy (RT) plays a valuable role in the management of metastatic disease to relieve tumor-related symptoms. Although palliative RT does not provide a chance for a cure, it improves QOL and may prolong survival time.2-4 An estimated 20% to 50% of radiation courses are prescribed with palliative intent, because RT is highly effective in providing symptom relief, and the toxicity associated with palliative doses is typically mild.5,6 Palliative RT can be used to manage bone and brain metastases, prevent or treat spinal cord compression, and manage numerous tumor-related symptoms, such as pain and bleeding in patients with terminal cancer.
Palliative RT for bone and brain metastases is supported by high-quality evidence and is considered one of the most effective and cost-effective options available.7,8 This article aims to review the role of RT in treating 3 conditions commonly encountered in patients with metastatic disease—bone metastases, spinal cord compression, and brain metastases—and to emphasize the importance of timely integration of RT for optimal results.
Bone Metastases
About 80% of patients with metastatic solid tumors develop bone metastases, and about 350,000 deaths are linked to bone metastases in the U.S. each year.9 Osseous
metastases can lead to pain, fracture, hypercalcemia, and spinal cord compression. The primary modality for treatment of pain and prevention of morbidity from bone metastases is external beam RT.10
The likelihood of bone pain relief with palliative RT is 60% to 80%, and 30% to 40% of patients achieving complete pain relief. Randomized studies have shown multiple-dose and fractionation regimens provided effective symptom relief for bone metastases. Most commonly used regimens include a single fraction of 8 gray (Gy) delivered in 1 treatment, 20 Gy in 5 fractions delivered daily over 1 week, and 30 Gy in 10 fractions delivered over 2 weeks. Treatment with a single fraction improves access to treatment and patient convenience, whereas more prolonged courses have been associated with lower rates of retreatment.11,12 Regarding the higher rate of retreatment with single-fraction RT, no clear evidence exists that this is due to a less durable pain response or lower level of pain relief.13
There has been recent interest in using predictive models to estimate life expectancy to avoid long courses of RT at the end of life.14,15 Shorter treatment courses of 8 Gyonce or 20 Gy in 5 fractions are particularly valuable for patients with a life expectancy < 3 months to avoid long courses of treatment, and thereby improve QOL as patients transition into hospice. A recent survey demonstrated that 93% of radiation oncologists within the VHA are willing to prescribe short courses of RT consisting of ≤ 6 fractions, and 76% have experience with single-fraction RT.16 These findings are in contradiction to the findings in the non-VA radiation oncology community, in which < 10% of patients with uncomplicated bone metastases are treated with a single fraction.17,18
In addition to providing pain relief, RT is used in the treatment of impending fractures either, adjuvant after surgical stabilization or alone for lower risk lesions.19 Factors that impact fracture risk include location of the metastasis (weight-bearing bones, such as femurs, which are at particularly high risk), length of bone involved, and extent of cortical involvement. Mirels’ scoring system was developed to predict fracture risk in patients with bone metastasis, based on 4 criteria: the
extent of cortical involvement, the location of the metastasis, the osteolytic vs osteoblastic appearance of the lesion, and the degree of pain.20 Surgical fixation can be considered, based on the total score and corresponding fracture risk. When appropriate, surgical stabilization should be considered by an orthopedic surgeon prior to initiating RT.
Postoperative RT after surgical stabilization has been associated with a reduced rate of secondary surgical procedures as well as with improved functional status.21 Radiotherapy promotes remineralization and bone healing and prevents the loss of surgical fixation by treating any residual tumor. A retrospective review of 60 patients with metastatic disease in weight-bearing bones with pathologic fracture or impending pathologic fracture demonstrated that surgery followed by RT was associated with improved functional status as well as with improved overall survival (OS).22,23 For patients in whom surgery is not indicated, the consulting radiation oncologist should consider factors such as the location of the metastasis in weight-bearing vs nonweight bearing bones, the size and extent of the metastasis, and associated symptoms when making a treatment recommendation. In patients at fracture risk from bone metastases, bisphosphonates should also be considered as part of the treatment regimen.24
Spinal Cord Compression
About 5% to 10% of patients diagnosed with cancer will develop spinal cord compression during the course of their disease.25 Spinal cord compression is considered a medical emergency that can result in significant pain and neurologic symptoms, including weakness, paralysis, parasthesias, and incontinence. Early treatment of spinal cord compression can prevent onset or progression of these symptoms; furthermore, early treatment prior to loss of ambulation is associated with improved long-term ambulatory function.26,27
Treatment decisions for spinal metastases with an associated concern for cord compression should be made after a consultation with both a neurosurgeon and a radiation oncologist. Early initiation of steroids is recommended to aid in tumor shrinkage for potential symptom relief.28 A standard way to administer dexamethasone is with a 10-mg loading dose followed by 16 mg per day, divided into 4 doses of 4 mg. Higher steroid doses showed no benefit in a prospective randomized trial comparing 96 mg with 16 mg of dexamethasone daily.29
Surgical decompression should be considered initial management of spinal cord compression. For patients treated surgically, local RT is indicated postoperatively as well. Randomized data show that surgery followed by RT provides better ambulatory function than does RT alone in patients with paralysis of < 2 days’ duration.30 Some patients with metastatic disease are not good candidates for surgery due to comorbidities, poor performance status, life expectancy < 3 months, or multilevel spinal involvement.
In patients who are not operative candidates, radiation alone is an appropriate alternative. However, several factors need consideration in deciding whether to manage cord compression with surgery followed by RT vs RT alone. These factors include life expectancy, tumor type (myeloma and lymphoma are more radiosensitive), interval since tumor diagnosis, and the presence of visceral metastases.31 Factors favoring surgical decompression plus postoperative RT over RT alone include spinal instability, KPS (Karnofsky Performance Status) > 70, radio-resistant tumor histology, minimal metastatic disease, and projected survival > 3 months.10
For patients managed with RT alone, early diagnosis and treatment is associated with improved outcomes. A prospective study of patients treated with RT without surgery for spinal cord compression demonstrated that 82% of patients experienced back pain relief, 76% achieved improvement in or preservation of ambulation, and 44% of patients with sphincter dysfunction experienced improvement with treatment.32 Patients with certain tumor histologies, such as myeloma, breast cancer, and prostate cancer, had better responses to RT.32
In the setting of spinal cord compression, longer courses of RT may provide better local control than do shorter courses.33 Therefore, longer courses of RT, such as 30 Gy in 10 fractions delivered over 2 weeks, are often preferred in cases of spinal cord compression treated with definitive RT as well as after surgical decompression. However, overall life expectancy is an important factor considered by the treating radiation oncologist when selecting a short course vs a longer course of RT.
In the instance of painful vertebral body metastases without spinal cord compression, a new subset analysis of the Radiation Therapy Oncology Group (RTOG) 9714 randomized trial indicated that single fraction RT (8 Gy) is just as effective as multiple fractions (30 Gy in 10 fractions), with this study demonstrating comparable rates of pain relief and narcotic use in both groups 3 months after RT.34 Advantages to the single-fraction plan compared with those of multiple fractions include mitigation of logistic concerns for patients and family at the end of life and less acute adverse effects.
Brain Metastases
An estimated 20% to 40% of patients with cancer develop brain metastases.35 The incidence of brain metastases has been rising most likely due to improved detection rates with magnetic resonance imaging (MRI) and improved cancer survival, because treatment regimens have improved with targeted chemotherapy and radiation techniques. Currently, the annual incidence of brain metastases is 170,000 to 200,000 in the U.S.36 Prognosis for these patients is poor, with median survival of 1 month without treatment and about 4 months with whole brain RT (WBRT) (Table).25,37-39
The goal of management for patients with brain metastases is to prevent or treat neurologic symptoms and to prolong survival. Treatment options include corticosteroids, WBRT, surgery, and stereotactic radiosurgery (SRS). Recommendations for treatment should involve both a radiation oncologist and neurosurgeon to determine the best treatment for an individual based on patient age, performance status, extent of systemic disease, and number of brain metastases. These prognostic factors that may predict life expectancy and impact treatment recommendations.40
Factors that have been correlated with improved survival include younger age, better performance status, fewer brain metastases, and lower burden of systemic disease.41,42 Prognostic assessment tools such as the Graded Prognostic Assessment and RTOG-Recursive Partitioning Analysis can be used to predict life expectancy in patients with brain metastases.41,43 However, routine use of these tools is lagging, as evidenced by a recent survey of VHA radiation oncologists. Use of these tools in the clinic will enhance the quality of end of life care and decision making.
Corticosteroids have classically been used in the treatment of brain metastases either alone for supportive care or in combination with RT. Steroids are recommended to provide symptom relief in patients with symptoms related to cerebral edema or mass effect.44 Steroids have been shown to mitigate edema and improve neurologic deficits in about two-thirds of patients with brain metastases.36,45 The effect of corticosteroids is thought to be mediated through inhibition of prostaglandin synthesis, reduction in vascular permeability, and anti-inflammatory properties.46 A common corticosteroid regimen is a 10-mg loading dose of dexamethasone, followed by 16 mg daily in divided doses. For patients without neurologic deficits or cerebral edema, it is reasonable to defer corticosteroid use only when patients are symptomatic.
In general, WBRT is considered an appropriate treatment option for patients with multiple brain metastases based on data suggesting an improvement in OS compared with the use of corticosteroids alone.47 Whole brain radiation has been shown to result in the improvement of baseline neurologic deficits or the prevention of further symptom progression.48 The partial or complete metastasis response rates are on the order of 60%.38 Tumor regression after WBRT has been associated with preservation of neurocognitive function as well as prolonged survival.49
For good prognosis patients with a single brain metastasis and good performance status, the use of surgery or radiosurgery added to WBRT has been associated with improved OS (Table). The RTOG 9508 randomized trial of WBRT with or without SRS demonstrated a survival advantage with SRS, with median survival times of 6.5 months with WBRT + SRS vs 4.9 months with WBRT alone.50 Similarly, a randomized trial evaluating WBRT alone compared with surgery followed by WBRT in patients
with good prognosis demonstrated significantly improved OS in the surgery group (median 40 weeks vs 15 weeks).51 In general, WBRT or postoperative RT to the tumor bed is still indicated after surgical resection, based on randomized data showing a reduction in tumor bed recurrence with postoperative RT.52
For patients with only 1 to 3 brain metastases and a favorable prognosis, surgery and SRS can be considered treatment options, oftentimes with WBRT. The EORTC randomized trial of patients with 1 to 3 brain metastases was designed to determine the benefit of WBRT after treatment with surgery or SRS. In this study, 119 patients underwent SRS and 160 patients underwent surgical resection.53 Both groups of patients were randomized to observation vs adjuvant WBRT. This study demonstrated reduced rates of intracranial relapse with WBRT, however, without any change in OS. Although there is concern that WBRT may impair cognitive function with no clear survival benefit after surgery or SRS, WBRT does reduce recurrence rates in the brain and the need for further treatment.54 Therefore, decisions regarding WBRT in such a setting should be made only after a detailed discussion with a radiation oncologist regarding risks vs benefits of treatment as part of the informed decision-making process.
Conclusions
Palliative RT plays an important role in the management of metastatic cancer to provide symptom relief and is a cost-effective treatment option for bone and brain metastases. Life expectancy and tumor characteristics should be considered when making treatment recommendations to ensure selection of regimens that complement patients’ unique situations. Timely referrals for treatment are important to optimize treatment results.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
In recent years, there has been increasing interest in palliative care for patients with cancer at the end of life. Up to 23% of patients have metastatic disease at presentation, and symptoms from metastatic lesions can cause significant anxiety and impair patients’ quality of life (QOL).1
Palliative radiotherapy (RT) plays a valuable role in the management of metastatic disease to relieve tumor-related symptoms. Although palliative RT does not provide a chance for a cure, it improves QOL and may prolong survival time.2-4 An estimated 20% to 50% of radiation courses are prescribed with palliative intent, because RT is highly effective in providing symptom relief, and the toxicity associated with palliative doses is typically mild.5,6 Palliative RT can be used to manage bone and brain metastases, prevent or treat spinal cord compression, and manage numerous tumor-related symptoms, such as pain and bleeding in patients with terminal cancer.
Palliative RT for bone and brain metastases is supported by high-quality evidence and is considered one of the most effective and cost-effective options available.7,8 This article aims to review the role of RT in treating 3 conditions commonly encountered in patients with metastatic disease—bone metastases, spinal cord compression, and brain metastases—and to emphasize the importance of timely integration of RT for optimal results.
Bone Metastases
About 80% of patients with metastatic solid tumors develop bone metastases, and about 350,000 deaths are linked to bone metastases in the U.S. each year.9 Osseous
metastases can lead to pain, fracture, hypercalcemia, and spinal cord compression. The primary modality for treatment of pain and prevention of morbidity from bone metastases is external beam RT.10
The likelihood of bone pain relief with palliative RT is 60% to 80%, and 30% to 40% of patients achieving complete pain relief. Randomized studies have shown multiple-dose and fractionation regimens provided effective symptom relief for bone metastases. Most commonly used regimens include a single fraction of 8 gray (Gy) delivered in 1 treatment, 20 Gy in 5 fractions delivered daily over 1 week, and 30 Gy in 10 fractions delivered over 2 weeks. Treatment with a single fraction improves access to treatment and patient convenience, whereas more prolonged courses have been associated with lower rates of retreatment.11,12 Regarding the higher rate of retreatment with single-fraction RT, no clear evidence exists that this is due to a less durable pain response or lower level of pain relief.13
There has been recent interest in using predictive models to estimate life expectancy to avoid long courses of RT at the end of life.14,15 Shorter treatment courses of 8 Gyonce or 20 Gy in 5 fractions are particularly valuable for patients with a life expectancy < 3 months to avoid long courses of treatment, and thereby improve QOL as patients transition into hospice. A recent survey demonstrated that 93% of radiation oncologists within the VHA are willing to prescribe short courses of RT consisting of ≤ 6 fractions, and 76% have experience with single-fraction RT.16 These findings are in contradiction to the findings in the non-VA radiation oncology community, in which < 10% of patients with uncomplicated bone metastases are treated with a single fraction.17,18
In addition to providing pain relief, RT is used in the treatment of impending fractures either, adjuvant after surgical stabilization or alone for lower risk lesions.19 Factors that impact fracture risk include location of the metastasis (weight-bearing bones, such as femurs, which are at particularly high risk), length of bone involved, and extent of cortical involvement. Mirels’ scoring system was developed to predict fracture risk in patients with bone metastasis, based on 4 criteria: the
extent of cortical involvement, the location of the metastasis, the osteolytic vs osteoblastic appearance of the lesion, and the degree of pain.20 Surgical fixation can be considered, based on the total score and corresponding fracture risk. When appropriate, surgical stabilization should be considered by an orthopedic surgeon prior to initiating RT.
Postoperative RT after surgical stabilization has been associated with a reduced rate of secondary surgical procedures as well as with improved functional status.21 Radiotherapy promotes remineralization and bone healing and prevents the loss of surgical fixation by treating any residual tumor. A retrospective review of 60 patients with metastatic disease in weight-bearing bones with pathologic fracture or impending pathologic fracture demonstrated that surgery followed by RT was associated with improved functional status as well as with improved overall survival (OS).22,23 For patients in whom surgery is not indicated, the consulting radiation oncologist should consider factors such as the location of the metastasis in weight-bearing vs nonweight bearing bones, the size and extent of the metastasis, and associated symptoms when making a treatment recommendation. In patients at fracture risk from bone metastases, bisphosphonates should also be considered as part of the treatment regimen.24
Spinal Cord Compression
About 5% to 10% of patients diagnosed with cancer will develop spinal cord compression during the course of their disease.25 Spinal cord compression is considered a medical emergency that can result in significant pain and neurologic symptoms, including weakness, paralysis, parasthesias, and incontinence. Early treatment of spinal cord compression can prevent onset or progression of these symptoms; furthermore, early treatment prior to loss of ambulation is associated with improved long-term ambulatory function.26,27
Treatment decisions for spinal metastases with an associated concern for cord compression should be made after a consultation with both a neurosurgeon and a radiation oncologist. Early initiation of steroids is recommended to aid in tumor shrinkage for potential symptom relief.28 A standard way to administer dexamethasone is with a 10-mg loading dose followed by 16 mg per day, divided into 4 doses of 4 mg. Higher steroid doses showed no benefit in a prospective randomized trial comparing 96 mg with 16 mg of dexamethasone daily.29
Surgical decompression should be considered initial management of spinal cord compression. For patients treated surgically, local RT is indicated postoperatively as well. Randomized data show that surgery followed by RT provides better ambulatory function than does RT alone in patients with paralysis of < 2 days’ duration.30 Some patients with metastatic disease are not good candidates for surgery due to comorbidities, poor performance status, life expectancy < 3 months, or multilevel spinal involvement.
In patients who are not operative candidates, radiation alone is an appropriate alternative. However, several factors need consideration in deciding whether to manage cord compression with surgery followed by RT vs RT alone. These factors include life expectancy, tumor type (myeloma and lymphoma are more radiosensitive), interval since tumor diagnosis, and the presence of visceral metastases.31 Factors favoring surgical decompression plus postoperative RT over RT alone include spinal instability, KPS (Karnofsky Performance Status) > 70, radio-resistant tumor histology, minimal metastatic disease, and projected survival > 3 months.10
For patients managed with RT alone, early diagnosis and treatment is associated with improved outcomes. A prospective study of patients treated with RT without surgery for spinal cord compression demonstrated that 82% of patients experienced back pain relief, 76% achieved improvement in or preservation of ambulation, and 44% of patients with sphincter dysfunction experienced improvement with treatment.32 Patients with certain tumor histologies, such as myeloma, breast cancer, and prostate cancer, had better responses to RT.32
In the setting of spinal cord compression, longer courses of RT may provide better local control than do shorter courses.33 Therefore, longer courses of RT, such as 30 Gy in 10 fractions delivered over 2 weeks, are often preferred in cases of spinal cord compression treated with definitive RT as well as after surgical decompression. However, overall life expectancy is an important factor considered by the treating radiation oncologist when selecting a short course vs a longer course of RT.
In the instance of painful vertebral body metastases without spinal cord compression, a new subset analysis of the Radiation Therapy Oncology Group (RTOG) 9714 randomized trial indicated that single fraction RT (8 Gy) is just as effective as multiple fractions (30 Gy in 10 fractions), with this study demonstrating comparable rates of pain relief and narcotic use in both groups 3 months after RT.34 Advantages to the single-fraction plan compared with those of multiple fractions include mitigation of logistic concerns for patients and family at the end of life and less acute adverse effects.
Brain Metastases
An estimated 20% to 40% of patients with cancer develop brain metastases.35 The incidence of brain metastases has been rising most likely due to improved detection rates with magnetic resonance imaging (MRI) and improved cancer survival, because treatment regimens have improved with targeted chemotherapy and radiation techniques. Currently, the annual incidence of brain metastases is 170,000 to 200,000 in the U.S.36 Prognosis for these patients is poor, with median survival of 1 month without treatment and about 4 months with whole brain RT (WBRT) (Table).25,37-39
The goal of management for patients with brain metastases is to prevent or treat neurologic symptoms and to prolong survival. Treatment options include corticosteroids, WBRT, surgery, and stereotactic radiosurgery (SRS). Recommendations for treatment should involve both a radiation oncologist and neurosurgeon to determine the best treatment for an individual based on patient age, performance status, extent of systemic disease, and number of brain metastases. These prognostic factors that may predict life expectancy and impact treatment recommendations.40
Factors that have been correlated with improved survival include younger age, better performance status, fewer brain metastases, and lower burden of systemic disease.41,42 Prognostic assessment tools such as the Graded Prognostic Assessment and RTOG-Recursive Partitioning Analysis can be used to predict life expectancy in patients with brain metastases.41,43 However, routine use of these tools is lagging, as evidenced by a recent survey of VHA radiation oncologists. Use of these tools in the clinic will enhance the quality of end of life care and decision making.
Corticosteroids have classically been used in the treatment of brain metastases either alone for supportive care or in combination with RT. Steroids are recommended to provide symptom relief in patients with symptoms related to cerebral edema or mass effect.44 Steroids have been shown to mitigate edema and improve neurologic deficits in about two-thirds of patients with brain metastases.36,45 The effect of corticosteroids is thought to be mediated through inhibition of prostaglandin synthesis, reduction in vascular permeability, and anti-inflammatory properties.46 A common corticosteroid regimen is a 10-mg loading dose of dexamethasone, followed by 16 mg daily in divided doses. For patients without neurologic deficits or cerebral edema, it is reasonable to defer corticosteroid use only when patients are symptomatic.
In general, WBRT is considered an appropriate treatment option for patients with multiple brain metastases based on data suggesting an improvement in OS compared with the use of corticosteroids alone.47 Whole brain radiation has been shown to result in the improvement of baseline neurologic deficits or the prevention of further symptom progression.48 The partial or complete metastasis response rates are on the order of 60%.38 Tumor regression after WBRT has been associated with preservation of neurocognitive function as well as prolonged survival.49
For good prognosis patients with a single brain metastasis and good performance status, the use of surgery or radiosurgery added to WBRT has been associated with improved OS (Table). The RTOG 9508 randomized trial of WBRT with or without SRS demonstrated a survival advantage with SRS, with median survival times of 6.5 months with WBRT + SRS vs 4.9 months with WBRT alone.50 Similarly, a randomized trial evaluating WBRT alone compared with surgery followed by WBRT in patients
with good prognosis demonstrated significantly improved OS in the surgery group (median 40 weeks vs 15 weeks).51 In general, WBRT or postoperative RT to the tumor bed is still indicated after surgical resection, based on randomized data showing a reduction in tumor bed recurrence with postoperative RT.52
For patients with only 1 to 3 brain metastases and a favorable prognosis, surgery and SRS can be considered treatment options, oftentimes with WBRT. The EORTC randomized trial of patients with 1 to 3 brain metastases was designed to determine the benefit of WBRT after treatment with surgery or SRS. In this study, 119 patients underwent SRS and 160 patients underwent surgical resection.53 Both groups of patients were randomized to observation vs adjuvant WBRT. This study demonstrated reduced rates of intracranial relapse with WBRT, however, without any change in OS. Although there is concern that WBRT may impair cognitive function with no clear survival benefit after surgery or SRS, WBRT does reduce recurrence rates in the brain and the need for further treatment.54 Therefore, decisions regarding WBRT in such a setting should be made only after a detailed discussion with a radiation oncologist regarding risks vs benefits of treatment as part of the informed decision-making process.
Conclusions
Palliative RT plays an important role in the management of metastatic cancer to provide symptom relief and is a cost-effective treatment option for bone and brain metastases. Life expectancy and tumor characteristics should be considered when making treatment recommendations to ensure selection of regimens that complement patients’ unique situations. Timely referrals for treatment are important to optimize treatment results.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Porter A and David M. Palliative care for bone, spinal cord, brain, and liver metastases. In: Gunderson LL, Tepper JE, eds. Clinical Radiation Oncology. 2nd ed. Philadelphia, PA: Elsevier Churchill Livingstone; 2007:437-451.
2. Yamaguchi S, Ohguri T, Matsuki Y, et al. Palliative radiotherapy in patients with a poor performance status: the palliative effect is correlated with prolongation of survival time. Radiat Oncol. 2013;8:166.
3. Mac Manus MP, Matthews JP, Wada M, Wirth A, Worotniuk V, Ball DL. Unexpected long-term survival after low-dose palliative radiotherapy for non-small cell lung cancer. Cancer. 2006;106(5):1110-1116.
4. Rastogi M, Revannasiddaiah S, Gupta MK, Seam RK, Thakur P, Gupta M. When palliative treatment achieves more than palliation: instances of long-term survival after palliative radiotherapy. Indian J Palliat Care. 2012;18(2):117-121.
5. Nieder C, Pawinski A, Haukland E, Dokmo R, Phillipi I, Dalhaug A. Estimating need for palliative external beam radiotherapy in adult cancer patients. Int J Radiat Oncol Biol Phys. 2010;76(1):207-211.
6. Hoegler D. Radiotherapy for palliation of symptoms in incurable cancer. Curr Probl Cancer. 1997;21(3):129-183.
7. Expósito J, Jaén J, Alonso E, Tovar I. Use of palliative radiotherapy in brain and bone metastases (VARA II study). Radiat Oncol. 2012;7:131.
8. Konski A. Radiotherapy is a cost-effective palliative treatment for patients with bone metastasis from prostate cancer. Int J Radiat Oncol Biol Phys. 2004;60(5):1373-1378.
9. Popovic M, den Hartogh M, Zhang L, et al. Review of international patterns of practice for the treatment of painful bone metastases with palliative radiotherapy from 1993 to 2013. Radiother Oncol. 2014;111(1):11-17.
10. Lutz S, Berk L, Chang E, et al; American Society for Radiation Oncology (ASTRO). Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;79(4):965-976.
11. Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systemic review. J Clin Oncol. 2007;25(11):1423-1436.
12. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy—a systemic review of the randomized trials. Cochrane Database Syst Rev. 2004;(2):CD004721.
13. Steenland E, Leer JW, van Houwelingen H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol. 1999;52(2):101-109.
14. Krishnan MS, Epstein-Peterson Z, Chen YH, et al. Predicting life expectance in patients with metastatic cancer receiving palliative radiotherapy: the TEACHH model. Cancer. 2014;120(1):134-141.
15. Guadagnolo BA, Liao KP, Elting L, Giordano S, Buccholz TA, Shih YC. Use of radiation therapy in the last 30 days of life among a large population-based cohort of elderly patients in the United States. J Clin Oncol. 2013;31(1):80-87.
16. Moghanaki D, Cheuk AV, Fosmire H, et al; U.S. Veterans Healthcare Administration National Palliative Radiotherapy Taskforce. Availability of single fraction palliative radiotherapy for cancer patients receiving end-of-life care within the Veterans Healthcare Administration. J Palliat Med. 2014;17(11):1221-1225.
17. Ellsworth SG, Alcorn SR, Hales RK, McNutt TR, DeWeese TL, Smith TJ. Patterns of care among patients receiving radiation therapy for bone metastases at a large academic institution. Int J Radiat Oncol Biol Phys. 2014;89(5):1100-1105.
18. Bradley NM, Husted J, Sey MS, et al. Review of patterns of practice and patients’ preferences in the treatment of bone metastases with palliative radiotherapy. Support Care Cancer. 2007;15(4):373-385.
19. Haidukewych GJ. Metastatic disease around the hip: maintaining quality of life. J Bone Joint Surg Br. 2012;94(11 suppl A):22-25.
20. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;(249):256-264.
21. Jacofsky DJ, Haidukewych GJ. Management of pathologic fractures of the proximal femur: state of the art. J Orthop Trauma. 2004;18(7):459-469.
22. Townsend PW, Rosenthal HG, Smalley SR, Cozad SC, Hassanein RE. Impact of postoperative radiation therapy and other perioperative factors on outcome after orthopedic stabilization of impending or pathologic fractures due to metastatic disease. J Clin Oncol. 1994;12(11):2345-2350.
23. Townsend PW, Smalley SR, Cozad SC, Rosenthal HG, Hassanein RE. Role of postoperative radiation therapy after stabilization of fractures caused by metastatic disease. Int J Radiat Oncol Biol Phys. 1995;31(1):43-49.
24. Farooki A. NCCN bone health task force: key recommendations. J Natl Compr Canc Netw. 2014;12(5 suppl):813-816.
25. Sejpal SV, Bhate A, Small W. Palliative radiation therapy in the management of brain metastases, spinal cord compression, and bone metastases. Semin Intervent Radiol. 2007;24(4):362-374.
26. Abrahm JL, Banffy MB, Harris MB. Spinal cord compression in patients with advanced metastatic cancer: “all I care about is walking and living my life.” JAMA. 2008;299(8):937-946.
27. Kim RY, Spencer SA, Meredith RF, et al. Extradural spinal cord compression: analysis of factors determining functional prognosis—prospective study. Radiology. 1990;176(1):279-282.
28. Kaloostian PE, Yurter A, Etame AB, Vrionis FD, Sciubba DM, Gokaslan ZL. Palliative strategies for the management of primary and metastatic spinal tumors. Cancer Control. 2014;21(2):140-143.
29. Graham PH, Capp A, Delaney G, et al. A pilot randomized comparison of dexamethasone 96 mg vs 16 mg per day for malignant spinal-cord compression treated by radiotherapy: TROG 01.05 Superdex study. Clin Oncol (R Coll Radiol). 2006;18(1):70-76.
30. Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643-648.
31. Rades D, Huttenlocher S, Bajrovic A, et al. Surgery followed by radiotherapy versus radiotherapy alone for metastatic spinal cord compression from unfavorable tumors. Int J Radiat Oncol Biol Phys. 2011;81(5):e861-e868.
32. Maranzano E, Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys. 1995;32(4):959-967.
33. Rades D, Fehlauer F, Schulte R, et al. Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. J Clin Oncol. 2006;24(21):3388-3393.
34. Howell DD, James JL, Hartsell WF, et al. Single-fraction radiotherapy versus multifraction radiotherapy for palliation of bone metastases-equivalent efficacy, less toxicity, more convenient: a subset analysis of Radiation Therapy Oncology Group trial 97-14. Cancer. 2013;119(4):888-896.
35. Wong J, Hird A, Kirou-Mauro, Napolskikh J, Chow E. Quality of life in brain metastases radiation trials: a literature review. Curr Oncol. 2008;15(5):25-45.
36. Nichols EM, Patchell RA, Regine WF, Kwok Y. Palliation of brain and spinal cord metastases. In: Halperin EC, Brady LW, Perez CA, Wazer DE, eds. Perez and Brady’s Principles and Practice of Radiation Oncology. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013:1974.
37. Zimm S, Wampler GL, Stablein D, Hazra T, Young HF. Intracerebral metastases in solid-tumor patients: natural history and results of treatment. Cancer. 1981;48(2):384-394.
38. Khuntia D, Brown P, Li J, Mehta MP. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24(8):1295-1304.
39. Sundström JT, Minn H, Lertola KK, Nordman E. Prognosis of patients treated for intracranial metastases with whole-brain irradiation. Ann Med. 1998;30(3):296-299.
40. Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210-225.
41. Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745-751.
42. Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG databases. Int J Radiat Oncol Biol Phys. 2008;70(2):510-514.
43. Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419-425.
44. Ryken TC, McDermott M, Robinson PD, et al. The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):103-114.
45. Ruderman NB, Hall TC. Use of glucocorticoids in the palliative treatment of metastatic brain tumors. Cancer. 1965;18:298-306.
46. Kaloostian PE, Yurter A, Etame AB, Vrionis FD, Sciubba DM, Gokaslan ZL. Palliative strategies for the management of primary and metastatic spinal tumors. Cancer Control. 2014;21(2):140-143.
47. Horton J, Baxter DH, Olson KB. The management of metastases to the brain by irradiation and corticosteroids. Am J Roentgenol Radium Ther Nucl Med. 1971;111(2)334-336.
48. Wong J, Hird A, Zhang L, et al. Symptoms and quality of life in cancer patients with brain metastases following palliative radiotherapy. Int J Radiat Oncol Biol Phys. 2009;75(4):1125-1131.
49. Li J, Bentzen SM, Renschler M, Mehta MP. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol. 2007;25(10):1260-1266.
50. Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665-1672.
51. Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322(8):494-500.
52. Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485-1489.
53. Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134-141.
54. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037-1044.
1. Porter A and David M. Palliative care for bone, spinal cord, brain, and liver metastases. In: Gunderson LL, Tepper JE, eds. Clinical Radiation Oncology. 2nd ed. Philadelphia, PA: Elsevier Churchill Livingstone; 2007:437-451.
2. Yamaguchi S, Ohguri T, Matsuki Y, et al. Palliative radiotherapy in patients with a poor performance status: the palliative effect is correlated with prolongation of survival time. Radiat Oncol. 2013;8:166.
3. Mac Manus MP, Matthews JP, Wada M, Wirth A, Worotniuk V, Ball DL. Unexpected long-term survival after low-dose palliative radiotherapy for non-small cell lung cancer. Cancer. 2006;106(5):1110-1116.
4. Rastogi M, Revannasiddaiah S, Gupta MK, Seam RK, Thakur P, Gupta M. When palliative treatment achieves more than palliation: instances of long-term survival after palliative radiotherapy. Indian J Palliat Care. 2012;18(2):117-121.
5. Nieder C, Pawinski A, Haukland E, Dokmo R, Phillipi I, Dalhaug A. Estimating need for palliative external beam radiotherapy in adult cancer patients. Int J Radiat Oncol Biol Phys. 2010;76(1):207-211.
6. Hoegler D. Radiotherapy for palliation of symptoms in incurable cancer. Curr Probl Cancer. 1997;21(3):129-183.
7. Expósito J, Jaén J, Alonso E, Tovar I. Use of palliative radiotherapy in brain and bone metastases (VARA II study). Radiat Oncol. 2012;7:131.
8. Konski A. Radiotherapy is a cost-effective palliative treatment for patients with bone metastasis from prostate cancer. Int J Radiat Oncol Biol Phys. 2004;60(5):1373-1378.
9. Popovic M, den Hartogh M, Zhang L, et al. Review of international patterns of practice for the treatment of painful bone metastases with palliative radiotherapy from 1993 to 2013. Radiother Oncol. 2014;111(1):11-17.
10. Lutz S, Berk L, Chang E, et al; American Society for Radiation Oncology (ASTRO). Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;79(4):965-976.
11. Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systemic review. J Clin Oncol. 2007;25(11):1423-1436.
12. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy—a systemic review of the randomized trials. Cochrane Database Syst Rev. 2004;(2):CD004721.
13. Steenland E, Leer JW, van Houwelingen H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol. 1999;52(2):101-109.
14. Krishnan MS, Epstein-Peterson Z, Chen YH, et al. Predicting life expectance in patients with metastatic cancer receiving palliative radiotherapy: the TEACHH model. Cancer. 2014;120(1):134-141.
15. Guadagnolo BA, Liao KP, Elting L, Giordano S, Buccholz TA, Shih YC. Use of radiation therapy in the last 30 days of life among a large population-based cohort of elderly patients in the United States. J Clin Oncol. 2013;31(1):80-87.
16. Moghanaki D, Cheuk AV, Fosmire H, et al; U.S. Veterans Healthcare Administration National Palliative Radiotherapy Taskforce. Availability of single fraction palliative radiotherapy for cancer patients receiving end-of-life care within the Veterans Healthcare Administration. J Palliat Med. 2014;17(11):1221-1225.
17. Ellsworth SG, Alcorn SR, Hales RK, McNutt TR, DeWeese TL, Smith TJ. Patterns of care among patients receiving radiation therapy for bone metastases at a large academic institution. Int J Radiat Oncol Biol Phys. 2014;89(5):1100-1105.
18. Bradley NM, Husted J, Sey MS, et al. Review of patterns of practice and patients’ preferences in the treatment of bone metastases with palliative radiotherapy. Support Care Cancer. 2007;15(4):373-385.
19. Haidukewych GJ. Metastatic disease around the hip: maintaining quality of life. J Bone Joint Surg Br. 2012;94(11 suppl A):22-25.
20. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;(249):256-264.
21. Jacofsky DJ, Haidukewych GJ. Management of pathologic fractures of the proximal femur: state of the art. J Orthop Trauma. 2004;18(7):459-469.
22. Townsend PW, Rosenthal HG, Smalley SR, Cozad SC, Hassanein RE. Impact of postoperative radiation therapy and other perioperative factors on outcome after orthopedic stabilization of impending or pathologic fractures due to metastatic disease. J Clin Oncol. 1994;12(11):2345-2350.
23. Townsend PW, Smalley SR, Cozad SC, Rosenthal HG, Hassanein RE. Role of postoperative radiation therapy after stabilization of fractures caused by metastatic disease. Int J Radiat Oncol Biol Phys. 1995;31(1):43-49.
24. Farooki A. NCCN bone health task force: key recommendations. J Natl Compr Canc Netw. 2014;12(5 suppl):813-816.
25. Sejpal SV, Bhate A, Small W. Palliative radiation therapy in the management of brain metastases, spinal cord compression, and bone metastases. Semin Intervent Radiol. 2007;24(4):362-374.
26. Abrahm JL, Banffy MB, Harris MB. Spinal cord compression in patients with advanced metastatic cancer: “all I care about is walking and living my life.” JAMA. 2008;299(8):937-946.
27. Kim RY, Spencer SA, Meredith RF, et al. Extradural spinal cord compression: analysis of factors determining functional prognosis—prospective study. Radiology. 1990;176(1):279-282.
28. Kaloostian PE, Yurter A, Etame AB, Vrionis FD, Sciubba DM, Gokaslan ZL. Palliative strategies for the management of primary and metastatic spinal tumors. Cancer Control. 2014;21(2):140-143.
29. Graham PH, Capp A, Delaney G, et al. A pilot randomized comparison of dexamethasone 96 mg vs 16 mg per day for malignant spinal-cord compression treated by radiotherapy: TROG 01.05 Superdex study. Clin Oncol (R Coll Radiol). 2006;18(1):70-76.
30. Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643-648.
31. Rades D, Huttenlocher S, Bajrovic A, et al. Surgery followed by radiotherapy versus radiotherapy alone for metastatic spinal cord compression from unfavorable tumors. Int J Radiat Oncol Biol Phys. 2011;81(5):e861-e868.
32. Maranzano E, Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys. 1995;32(4):959-967.
33. Rades D, Fehlauer F, Schulte R, et al. Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. J Clin Oncol. 2006;24(21):3388-3393.
34. Howell DD, James JL, Hartsell WF, et al. Single-fraction radiotherapy versus multifraction radiotherapy for palliation of bone metastases-equivalent efficacy, less toxicity, more convenient: a subset analysis of Radiation Therapy Oncology Group trial 97-14. Cancer. 2013;119(4):888-896.
35. Wong J, Hird A, Kirou-Mauro, Napolskikh J, Chow E. Quality of life in brain metastases radiation trials: a literature review. Curr Oncol. 2008;15(5):25-45.
36. Nichols EM, Patchell RA, Regine WF, Kwok Y. Palliation of brain and spinal cord metastases. In: Halperin EC, Brady LW, Perez CA, Wazer DE, eds. Perez and Brady’s Principles and Practice of Radiation Oncology. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013:1974.
37. Zimm S, Wampler GL, Stablein D, Hazra T, Young HF. Intracerebral metastases in solid-tumor patients: natural history and results of treatment. Cancer. 1981;48(2):384-394.
38. Khuntia D, Brown P, Li J, Mehta MP. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24(8):1295-1304.
39. Sundström JT, Minn H, Lertola KK, Nordman E. Prognosis of patients treated for intracranial metastases with whole-brain irradiation. Ann Med. 1998;30(3):296-299.
40. Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210-225.
41. Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745-751.
42. Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG databases. Int J Radiat Oncol Biol Phys. 2008;70(2):510-514.
43. Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419-425.
44. Ryken TC, McDermott M, Robinson PD, et al. The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):103-114.
45. Ruderman NB, Hall TC. Use of glucocorticoids in the palliative treatment of metastatic brain tumors. Cancer. 1965;18:298-306.
46. Kaloostian PE, Yurter A, Etame AB, Vrionis FD, Sciubba DM, Gokaslan ZL. Palliative strategies for the management of primary and metastatic spinal tumors. Cancer Control. 2014;21(2):140-143.
47. Horton J, Baxter DH, Olson KB. The management of metastases to the brain by irradiation and corticosteroids. Am J Roentgenol Radium Ther Nucl Med. 1971;111(2)334-336.
48. Wong J, Hird A, Zhang L, et al. Symptoms and quality of life in cancer patients with brain metastases following palliative radiotherapy. Int J Radiat Oncol Biol Phys. 2009;75(4):1125-1131.
49. Li J, Bentzen SM, Renschler M, Mehta MP. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol. 2007;25(10):1260-1266.
50. Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665-1672.
51. Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322(8):494-500.
52. Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485-1489.
53. Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134-141.
54. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037-1044.
Lessons from our dying patients
Dying patients teach us to think more carefully about whether or not our surgical interventions will be beneficial.
I work in palliative care, and my surgical colleagues, especially the residents, are often surprised when I call them and ask them to consult on my patients who are very ill and have a “Do Not Resuscitate” order in their charts. I’m also an anesthesiologist working in interventional pain management, and I regularly do procedures on patients who have prognoses that are extremely limited. For other patients, I recommend against any interventions at all.
How do we know when to intervene on patients who are dying? Perhaps more importantly, how do we know when NOT to intervene? Two recent cases of almost identical fractures illustrated for me the need to think beyond the anatomic problem when evaluating options for care.
Last year, I admitted a woman, “Donna,” with widely metastatic breast cancer to our inpatient palliative care service. She had fallen at home and hurt her arm about 2 months prior to admission. She had been confined to her bed for about 6 weeks. She was brought to the hospital because she was becoming delirious. She had many sources of pain that were relatively well controlled when she was lying down, but her worst pain was in her left arm. We found a fracture of her humerus. When her family learned that the fracture would not heal on its own because of the large metastasis there, they demanded surgery to fix it. Shortly thereafter, I re-admitted a patient, “Cindy,” with a very similar story. She also had widely metastatic breast cancer, and her pain had been very difficult to control. We had found a pain regimen that worked well for her on her previous admission, but she had fallen over her walker and broke her humerus after we had discharged her to a rehab facility. When I saw her back in the hospital, I told her that I thought she would need surgery to fix her arm. She was depressed by this setback, she was in pain again, and she told me that she would prefer not to have any intervention because she feared the additional pain that it would cause.
With Donna, we sat down with her and her family to hear what their hopes were for her care. They understood that she did not have further chemotherapy or radiation options for her cancer, but they thought if she got the surgery that at least she would be able to get out of bed and walk again. My colleague carefully explained that yes, he could fix the fracture and that this could mean that the pain in her left arm would improve. He went on to say, however, that he did not think that the surgery would allow her to walk again as she had not been able to walk for a few weeks after the injury. When the family heard that the surgery probably wouldn’t restore her mobility, they decided against the procedure. With Cindy, we had a very different conversation. She was not inclined to have the procedure, but I expressed my concern that she wouldn’t be able to walk again unless she had the procedure because she needed her arms to use her walker. Although she did not have any further chemotherapy or radiation options, her oncologist had told us that her prognosis could be several months. In this case, my surgical colleague explained that he could perform surgery for the fracture and that he thought that it would both help her pain and allow her to use her walker again. We recommended that she have the surgery given her hope to continue to live independently, as she had been, for as long as possible. She ultimately agreed to do so and was able to return home.
These two patients reminded me again of how important it is for us to understand what our patients’ hopes and expectations are for a procedure. It is very distressing for clinicians when desperate families want treatments that likely have little benefit. When patients have limited prognoses, aligning patient goals and procedure goals is especially important as the outcome of the procedure can define the patient’s remaining days.
Donna’s family demanded a surgery expecting a result that was very unlikely, and Cindy initially declined the same surgery that ultimately benefitted her greatly. Our job is to make and execute the medical recommendations that best fit with our patients’ goals and understanding. Sometimes this will mean performing procedures on patients who are extremely ill and have “Do Not Resuscitate” orders, and at other times, it will mean not doing procedures, even if a patient and family want them to be done.
Dr. Rickerson is an anesthesiologist at the Dana-Farber Cancer Institute and Brigham and Women’s Hospital, Boston.
Dying patients teach us to think more carefully about whether or not our surgical interventions will be beneficial.
I work in palliative care, and my surgical colleagues, especially the residents, are often surprised when I call them and ask them to consult on my patients who are very ill and have a “Do Not Resuscitate” order in their charts. I’m also an anesthesiologist working in interventional pain management, and I regularly do procedures on patients who have prognoses that are extremely limited. For other patients, I recommend against any interventions at all.
How do we know when to intervene on patients who are dying? Perhaps more importantly, how do we know when NOT to intervene? Two recent cases of almost identical fractures illustrated for me the need to think beyond the anatomic problem when evaluating options for care.
Last year, I admitted a woman, “Donna,” with widely metastatic breast cancer to our inpatient palliative care service. She had fallen at home and hurt her arm about 2 months prior to admission. She had been confined to her bed for about 6 weeks. She was brought to the hospital because she was becoming delirious. She had many sources of pain that were relatively well controlled when she was lying down, but her worst pain was in her left arm. We found a fracture of her humerus. When her family learned that the fracture would not heal on its own because of the large metastasis there, they demanded surgery to fix it. Shortly thereafter, I re-admitted a patient, “Cindy,” with a very similar story. She also had widely metastatic breast cancer, and her pain had been very difficult to control. We had found a pain regimen that worked well for her on her previous admission, but she had fallen over her walker and broke her humerus after we had discharged her to a rehab facility. When I saw her back in the hospital, I told her that I thought she would need surgery to fix her arm. She was depressed by this setback, she was in pain again, and she told me that she would prefer not to have any intervention because she feared the additional pain that it would cause.
With Donna, we sat down with her and her family to hear what their hopes were for her care. They understood that she did not have further chemotherapy or radiation options for her cancer, but they thought if she got the surgery that at least she would be able to get out of bed and walk again. My colleague carefully explained that yes, he could fix the fracture and that this could mean that the pain in her left arm would improve. He went on to say, however, that he did not think that the surgery would allow her to walk again as she had not been able to walk for a few weeks after the injury. When the family heard that the surgery probably wouldn’t restore her mobility, they decided against the procedure. With Cindy, we had a very different conversation. She was not inclined to have the procedure, but I expressed my concern that she wouldn’t be able to walk again unless she had the procedure because she needed her arms to use her walker. Although she did not have any further chemotherapy or radiation options, her oncologist had told us that her prognosis could be several months. In this case, my surgical colleague explained that he could perform surgery for the fracture and that he thought that it would both help her pain and allow her to use her walker again. We recommended that she have the surgery given her hope to continue to live independently, as she had been, for as long as possible. She ultimately agreed to do so and was able to return home.
These two patients reminded me again of how important it is for us to understand what our patients’ hopes and expectations are for a procedure. It is very distressing for clinicians when desperate families want treatments that likely have little benefit. When patients have limited prognoses, aligning patient goals and procedure goals is especially important as the outcome of the procedure can define the patient’s remaining days.
Donna’s family demanded a surgery expecting a result that was very unlikely, and Cindy initially declined the same surgery that ultimately benefitted her greatly. Our job is to make and execute the medical recommendations that best fit with our patients’ goals and understanding. Sometimes this will mean performing procedures on patients who are extremely ill and have “Do Not Resuscitate” orders, and at other times, it will mean not doing procedures, even if a patient and family want them to be done.
Dr. Rickerson is an anesthesiologist at the Dana-Farber Cancer Institute and Brigham and Women’s Hospital, Boston.
Dying patients teach us to think more carefully about whether or not our surgical interventions will be beneficial.
I work in palliative care, and my surgical colleagues, especially the residents, are often surprised when I call them and ask them to consult on my patients who are very ill and have a “Do Not Resuscitate” order in their charts. I’m also an anesthesiologist working in interventional pain management, and I regularly do procedures on patients who have prognoses that are extremely limited. For other patients, I recommend against any interventions at all.
How do we know when to intervene on patients who are dying? Perhaps more importantly, how do we know when NOT to intervene? Two recent cases of almost identical fractures illustrated for me the need to think beyond the anatomic problem when evaluating options for care.
Last year, I admitted a woman, “Donna,” with widely metastatic breast cancer to our inpatient palliative care service. She had fallen at home and hurt her arm about 2 months prior to admission. She had been confined to her bed for about 6 weeks. She was brought to the hospital because she was becoming delirious. She had many sources of pain that were relatively well controlled when she was lying down, but her worst pain was in her left arm. We found a fracture of her humerus. When her family learned that the fracture would not heal on its own because of the large metastasis there, they demanded surgery to fix it. Shortly thereafter, I re-admitted a patient, “Cindy,” with a very similar story. She also had widely metastatic breast cancer, and her pain had been very difficult to control. We had found a pain regimen that worked well for her on her previous admission, but she had fallen over her walker and broke her humerus after we had discharged her to a rehab facility. When I saw her back in the hospital, I told her that I thought she would need surgery to fix her arm. She was depressed by this setback, she was in pain again, and she told me that she would prefer not to have any intervention because she feared the additional pain that it would cause.
With Donna, we sat down with her and her family to hear what their hopes were for her care. They understood that she did not have further chemotherapy or radiation options for her cancer, but they thought if she got the surgery that at least she would be able to get out of bed and walk again. My colleague carefully explained that yes, he could fix the fracture and that this could mean that the pain in her left arm would improve. He went on to say, however, that he did not think that the surgery would allow her to walk again as she had not been able to walk for a few weeks after the injury. When the family heard that the surgery probably wouldn’t restore her mobility, they decided against the procedure. With Cindy, we had a very different conversation. She was not inclined to have the procedure, but I expressed my concern that she wouldn’t be able to walk again unless she had the procedure because she needed her arms to use her walker. Although she did not have any further chemotherapy or radiation options, her oncologist had told us that her prognosis could be several months. In this case, my surgical colleague explained that he could perform surgery for the fracture and that he thought that it would both help her pain and allow her to use her walker again. We recommended that she have the surgery given her hope to continue to live independently, as she had been, for as long as possible. She ultimately agreed to do so and was able to return home.
These two patients reminded me again of how important it is for us to understand what our patients’ hopes and expectations are for a procedure. It is very distressing for clinicians when desperate families want treatments that likely have little benefit. When patients have limited prognoses, aligning patient goals and procedure goals is especially important as the outcome of the procedure can define the patient’s remaining days.
Donna’s family demanded a surgery expecting a result that was very unlikely, and Cindy initially declined the same surgery that ultimately benefitted her greatly. Our job is to make and execute the medical recommendations that best fit with our patients’ goals and understanding. Sometimes this will mean performing procedures on patients who are extremely ill and have “Do Not Resuscitate” orders, and at other times, it will mean not doing procedures, even if a patient and family want them to be done.
Dr. Rickerson is an anesthesiologist at the Dana-Farber Cancer Institute and Brigham and Women’s Hospital, Boston.
Hospice, Palliative Care Groups Release Quality Care Measures
The American Academy of Hospice and Palliative Medicine (AAHPM) and the Hospice & Palliative Nurses Association (HPNA) recently published a list of performance measures to assess the quality of palliative and hospice patient care.
Refined over two years, the groups' Measuring What Matters recommendations [PDF] outline 10 clinically relevant measures to drive quality care. The list includes:
- Documenting patients’ preferences for life-sustaining treatments and their surrogate decision makers’ names;
- Screening patients for physical symptoms;
- Treating pain;
- Screening and managing dyspnea; and,
- Discussing patients' emotional and psychological needs.
"I'd say these things are relevant for hospitalists' patients, and for all seriously ill patients, whether or not a palliative care need has been identified," says Joe Rotella, MD, MBA, AAHPM's CMO and co-chair of the Measuring What Matters clinical user panel. The measures should make it possible to raise awareness about what constitutes quality of care for seriously ill patients and to compare quality between settings and between patients who receive palliative care and equally ill patients who do not, he notes.
The quality indicators, which have been reviewed by the National Quality Forum, focus on processes of providing palliative and hospice care and seek to achieve consistency in care quality among providers. For instance, do patients who screen positive for at least moderate pain receive treatments within 24 hours? Likewise, patients receiving hospice care should have a documented discussion of their spiritual concerns or of their preference not to have such a discussion, the recommendations state.
"It's worth looking at what really matters to these patients and maybe adapting a few measures for your hospital's quality improvement program," Dr. Rotella says.
Listen to our recent podcast on hospitalists and palliative care.
The American Academy of Hospice and Palliative Medicine (AAHPM) and the Hospice & Palliative Nurses Association (HPNA) recently published a list of performance measures to assess the quality of palliative and hospice patient care.
Refined over two years, the groups' Measuring What Matters recommendations [PDF] outline 10 clinically relevant measures to drive quality care. The list includes:
- Documenting patients’ preferences for life-sustaining treatments and their surrogate decision makers’ names;
- Screening patients for physical symptoms;
- Treating pain;
- Screening and managing dyspnea; and,
- Discussing patients' emotional and psychological needs.
"I'd say these things are relevant for hospitalists' patients, and for all seriously ill patients, whether or not a palliative care need has been identified," says Joe Rotella, MD, MBA, AAHPM's CMO and co-chair of the Measuring What Matters clinical user panel. The measures should make it possible to raise awareness about what constitutes quality of care for seriously ill patients and to compare quality between settings and between patients who receive palliative care and equally ill patients who do not, he notes.
The quality indicators, which have been reviewed by the National Quality Forum, focus on processes of providing palliative and hospice care and seek to achieve consistency in care quality among providers. For instance, do patients who screen positive for at least moderate pain receive treatments within 24 hours? Likewise, patients receiving hospice care should have a documented discussion of their spiritual concerns or of their preference not to have such a discussion, the recommendations state.
"It's worth looking at what really matters to these patients and maybe adapting a few measures for your hospital's quality improvement program," Dr. Rotella says.
Listen to our recent podcast on hospitalists and palliative care.
The American Academy of Hospice and Palliative Medicine (AAHPM) and the Hospice & Palliative Nurses Association (HPNA) recently published a list of performance measures to assess the quality of palliative and hospice patient care.
Refined over two years, the groups' Measuring What Matters recommendations [PDF] outline 10 clinically relevant measures to drive quality care. The list includes:
- Documenting patients’ preferences for life-sustaining treatments and their surrogate decision makers’ names;
- Screening patients for physical symptoms;
- Treating pain;
- Screening and managing dyspnea; and,
- Discussing patients' emotional and psychological needs.
"I'd say these things are relevant for hospitalists' patients, and for all seriously ill patients, whether or not a palliative care need has been identified," says Joe Rotella, MD, MBA, AAHPM's CMO and co-chair of the Measuring What Matters clinical user panel. The measures should make it possible to raise awareness about what constitutes quality of care for seriously ill patients and to compare quality between settings and between patients who receive palliative care and equally ill patients who do not, he notes.
The quality indicators, which have been reviewed by the National Quality Forum, focus on processes of providing palliative and hospice care and seek to achieve consistency in care quality among providers. For instance, do patients who screen positive for at least moderate pain receive treatments within 24 hours? Likewise, patients receiving hospice care should have a documented discussion of their spiritual concerns or of their preference not to have such a discussion, the recommendations state.
"It's worth looking at what really matters to these patients and maybe adapting a few measures for your hospital's quality improvement program," Dr. Rotella says.
Listen to our recent podcast on hospitalists and palliative care.
Using Genetics to Fight Disease
In January, President Obama launched the Precision Medicine Initiative, a far-reaching national program aimed at incorporating individual variability in genes, environment, and lifestyle into the treatment of diseases. The initiative incorporates genomic medicine, a growing medical field that applies individual genetic information and variation to better tailor clinical care for each patient. The goal: to make a difference for the millions of Americans facing illness, improve treatment options, and revolutionize everyday clinical practice.
Federal Practitioner talked with Eric Green, MD, PhD, the director of NIH’s National Human Genome Research Institute about how the NIH is using genomics to meet the goals of the Precision Medicine Initiative and its impact on the future of medicine.
Click below to hear the full discussion.
Federal Practitioner: What is precision and personalized medicine?
Dr. Eric Green: Genomic medicine is something that reflects the use of genomic information about an individual patient to tailor their clinical care. That is very much focused on genomic information, but of course, there are other components of disease beyond genomics and genetics. It is not all in our DNA. There are also aspects of everyday life that influence our health and our well-being and our risk for diseases such as our diet, our lifestyle, what things we are exposed to in the environment, and so forth.
A larger framing of the notion of individualizing medical care around each individual’s makeup in terms of their blueprint and what they are exposed to brings us to phrases like personalized medicine or individualized medicine. More recently, the phrase that has been used is precision medicine. Precision medicine, essentially, takes a broader view of what are the things that contribute to health and disease. It is genomics, it is lifestyle, it is diet, it is environmental exposures, and then thinking about how to tailor medical treatment based on taking those individual aspects of a person into account.
FP: How can genomics be applied to hematology and oncology?
EG: Some of the earliest applications of using genomic information to tailor medical care come in the area of cancer; in particular, some of the leukemias that are studied and treated every day in hospitals around the world where we have learned by taking samples from individuals who have certain types of leukemia and opening up their genomes. In those tumor samples, in those leukemia samples, there turns out to be some very characteristic genomic changes that have taken place that are basically driving those cancers and making them behave the way they do.
It is now standard practice for some hematologic disorders, in particular types of leukemia, to consider getting genomic information on that specific patient—a blood sample—in order to help guide the best way to treat the patient and to get information about prognosis. It is only going to get more exciting and more advanced over the next 5 and 10 years.
FP: Why do you think this topic warrants discussion?
EG: I think this warrants discussing in almost any clinical group, because I completely believe that over the next 5 to 10 years, many aspects of medicine are going to be changed because of genomics. It is not going to just be in certain areas of medicine; I think it is going to be in almost all areas of medicine. This makes it very relevant to talk to clinical groups about this fast-moving area and how it will find its way into clinical practice in the next few years.
FP: You will be presenting the keynote presentation at the 2015 AVAHO Meeting in Washington, DC, in October. Are you excited to bring the discussion of genomics to AVAHO?
EG: I talk to clinical groups all the time, because I firmly believe this is going to be really important for them to know. I also fully appreciate that the field has moved so quickly that a lot of people who are out there in practice—whether they are physicians, pharmacists, nurses, physician assistants, or genetic counselors—when they were trained even 5, or 10, or 20 years ago, the things that we can do know using genomic information simply weren’t known about then. It is very important for us to use professional meetings as venues for furthering the education of professionals, especially in fast-moving fields where this is a great opportunity for them to catch up on what the latest is.
FP: Is there anything else you would like to say?
EG: The Precision Medicine Initiative aims to accelerate progress in precision medicine so that people in the United States can be the beneficiaries of these exciting developments. I think it is going to be an important partnership with scientists and practicing physicians to make this a reality, so it is really important for us to be talking about these things.
Don't have time to listen to the entire discussion? We understand. Use this guide to skip ahead to the topic that most interests you.
1:04 What is genomic medicine?
2:36 What is precision medicine?
3:48 How can genomics be used in hematology and oncology?
6:25 How is genomics changing the future of medicine?
7:22 Why should clinical groups like AVAHO learn about genomics?
8:20 How is the Precision Medicine Initiative using genomics?
In January, President Obama launched the Precision Medicine Initiative, a far-reaching national program aimed at incorporating individual variability in genes, environment, and lifestyle into the treatment of diseases. The initiative incorporates genomic medicine, a growing medical field that applies individual genetic information and variation to better tailor clinical care for each patient. The goal: to make a difference for the millions of Americans facing illness, improve treatment options, and revolutionize everyday clinical practice.
Federal Practitioner talked with Eric Green, MD, PhD, the director of NIH’s National Human Genome Research Institute about how the NIH is using genomics to meet the goals of the Precision Medicine Initiative and its impact on the future of medicine.
Click below to hear the full discussion.
Federal Practitioner: What is precision and personalized medicine?
Dr. Eric Green: Genomic medicine is something that reflects the use of genomic information about an individual patient to tailor their clinical care. That is very much focused on genomic information, but of course, there are other components of disease beyond genomics and genetics. It is not all in our DNA. There are also aspects of everyday life that influence our health and our well-being and our risk for diseases such as our diet, our lifestyle, what things we are exposed to in the environment, and so forth.
A larger framing of the notion of individualizing medical care around each individual’s makeup in terms of their blueprint and what they are exposed to brings us to phrases like personalized medicine or individualized medicine. More recently, the phrase that has been used is precision medicine. Precision medicine, essentially, takes a broader view of what are the things that contribute to health and disease. It is genomics, it is lifestyle, it is diet, it is environmental exposures, and then thinking about how to tailor medical treatment based on taking those individual aspects of a person into account.
FP: How can genomics be applied to hematology and oncology?
EG: Some of the earliest applications of using genomic information to tailor medical care come in the area of cancer; in particular, some of the leukemias that are studied and treated every day in hospitals around the world where we have learned by taking samples from individuals who have certain types of leukemia and opening up their genomes. In those tumor samples, in those leukemia samples, there turns out to be some very characteristic genomic changes that have taken place that are basically driving those cancers and making them behave the way they do.
It is now standard practice for some hematologic disorders, in particular types of leukemia, to consider getting genomic information on that specific patient—a blood sample—in order to help guide the best way to treat the patient and to get information about prognosis. It is only going to get more exciting and more advanced over the next 5 and 10 years.
FP: Why do you think this topic warrants discussion?
EG: I think this warrants discussing in almost any clinical group, because I completely believe that over the next 5 to 10 years, many aspects of medicine are going to be changed because of genomics. It is not going to just be in certain areas of medicine; I think it is going to be in almost all areas of medicine. This makes it very relevant to talk to clinical groups about this fast-moving area and how it will find its way into clinical practice in the next few years.
FP: You will be presenting the keynote presentation at the 2015 AVAHO Meeting in Washington, DC, in October. Are you excited to bring the discussion of genomics to AVAHO?
EG: I talk to clinical groups all the time, because I firmly believe this is going to be really important for them to know. I also fully appreciate that the field has moved so quickly that a lot of people who are out there in practice—whether they are physicians, pharmacists, nurses, physician assistants, or genetic counselors—when they were trained even 5, or 10, or 20 years ago, the things that we can do know using genomic information simply weren’t known about then. It is very important for us to use professional meetings as venues for furthering the education of professionals, especially in fast-moving fields where this is a great opportunity for them to catch up on what the latest is.
FP: Is there anything else you would like to say?
EG: The Precision Medicine Initiative aims to accelerate progress in precision medicine so that people in the United States can be the beneficiaries of these exciting developments. I think it is going to be an important partnership with scientists and practicing physicians to make this a reality, so it is really important for us to be talking about these things.
Don't have time to listen to the entire discussion? We understand. Use this guide to skip ahead to the topic that most interests you.
1:04 What is genomic medicine?
2:36 What is precision medicine?
3:48 How can genomics be used in hematology and oncology?
6:25 How is genomics changing the future of medicine?
7:22 Why should clinical groups like AVAHO learn about genomics?
8:20 How is the Precision Medicine Initiative using genomics?
In January, President Obama launched the Precision Medicine Initiative, a far-reaching national program aimed at incorporating individual variability in genes, environment, and lifestyle into the treatment of diseases. The initiative incorporates genomic medicine, a growing medical field that applies individual genetic information and variation to better tailor clinical care for each patient. The goal: to make a difference for the millions of Americans facing illness, improve treatment options, and revolutionize everyday clinical practice.
Federal Practitioner talked with Eric Green, MD, PhD, the director of NIH’s National Human Genome Research Institute about how the NIH is using genomics to meet the goals of the Precision Medicine Initiative and its impact on the future of medicine.
Click below to hear the full discussion.
Federal Practitioner: What is precision and personalized medicine?
Dr. Eric Green: Genomic medicine is something that reflects the use of genomic information about an individual patient to tailor their clinical care. That is very much focused on genomic information, but of course, there are other components of disease beyond genomics and genetics. It is not all in our DNA. There are also aspects of everyday life that influence our health and our well-being and our risk for diseases such as our diet, our lifestyle, what things we are exposed to in the environment, and so forth.
A larger framing of the notion of individualizing medical care around each individual’s makeup in terms of their blueprint and what they are exposed to brings us to phrases like personalized medicine or individualized medicine. More recently, the phrase that has been used is precision medicine. Precision medicine, essentially, takes a broader view of what are the things that contribute to health and disease. It is genomics, it is lifestyle, it is diet, it is environmental exposures, and then thinking about how to tailor medical treatment based on taking those individual aspects of a person into account.
FP: How can genomics be applied to hematology and oncology?
EG: Some of the earliest applications of using genomic information to tailor medical care come in the area of cancer; in particular, some of the leukemias that are studied and treated every day in hospitals around the world where we have learned by taking samples from individuals who have certain types of leukemia and opening up their genomes. In those tumor samples, in those leukemia samples, there turns out to be some very characteristic genomic changes that have taken place that are basically driving those cancers and making them behave the way they do.
It is now standard practice for some hematologic disorders, in particular types of leukemia, to consider getting genomic information on that specific patient—a blood sample—in order to help guide the best way to treat the patient and to get information about prognosis. It is only going to get more exciting and more advanced over the next 5 and 10 years.
FP: Why do you think this topic warrants discussion?
EG: I think this warrants discussing in almost any clinical group, because I completely believe that over the next 5 to 10 years, many aspects of medicine are going to be changed because of genomics. It is not going to just be in certain areas of medicine; I think it is going to be in almost all areas of medicine. This makes it very relevant to talk to clinical groups about this fast-moving area and how it will find its way into clinical practice in the next few years.
FP: You will be presenting the keynote presentation at the 2015 AVAHO Meeting in Washington, DC, in October. Are you excited to bring the discussion of genomics to AVAHO?
EG: I talk to clinical groups all the time, because I firmly believe this is going to be really important for them to know. I also fully appreciate that the field has moved so quickly that a lot of people who are out there in practice—whether they are physicians, pharmacists, nurses, physician assistants, or genetic counselors—when they were trained even 5, or 10, or 20 years ago, the things that we can do know using genomic information simply weren’t known about then. It is very important for us to use professional meetings as venues for furthering the education of professionals, especially in fast-moving fields where this is a great opportunity for them to catch up on what the latest is.
FP: Is there anything else you would like to say?
EG: The Precision Medicine Initiative aims to accelerate progress in precision medicine so that people in the United States can be the beneficiaries of these exciting developments. I think it is going to be an important partnership with scientists and practicing physicians to make this a reality, so it is really important for us to be talking about these things.
Don't have time to listen to the entire discussion? We understand. Use this guide to skip ahead to the topic that most interests you.
1:04 What is genomic medicine?
2:36 What is precision medicine?
3:48 How can genomics be used in hematology and oncology?
6:25 How is genomics changing the future of medicine?
7:22 Why should clinical groups like AVAHO learn about genomics?
8:20 How is the Precision Medicine Initiative using genomics?
Palliative Care and Last-Minute Heroics
4/8/15
HM15 Presenter: Tammie Quest, MD
Summation: Heroics- a set of medical actions that attempt to prolong life with a low likelihood of success.
Palliative care- an approach of care provided to patients and families suffering from serious and/or life limiting illness; focus on physical, spiritual, psychological and social aspects of distress.
Hospice care- intense palliative care provided when the patient has terminal illness with a prognosis of 6 months or less if the disease runs its usual course.
We underutilize Palliative and Hospice care in the US. Here in the US fewer than 50% of all persons receive hospice care at EOL, of those who receive hospice care more than half receive care for less than 20 days, and 1 in 5 patients die in an ICU. Palliative Care can/should co-exist with life prolonging care following the diagnosis of serious illness.
Common therapies/interventions to be contemplated and discussed with patient at end of life: cpr, mechanical ventilation, central venous/arterial access, renal replacement therapy, surgical procedures, valve therapies, ventricular assist devices, continuous infusions, IV fluids, supplemental oxygen, artificial nutrition, antimicrobials, blood products, cancer directed therapy, antithrombotics, anticoagulation.
Practical Elements of Palliative Care: pain and symptom management, advance care planning, communication/goals of care, truth-telling, social support, spiritual support, psychological support, risk/burden assessment of treatments.
Key Points/HM Takeaways:
1-Palliative Care Bedside Talking Points-
- Cardiac arrest is the moment of death, very few people survive an attempt at reversing death
- If you are one of the few who survive to discharge, you may do well but few will survive to discharge
- Antibiotics DO improve survival, antibiotics DO NOT improve comfort
- No evidence to show that dying from pneumonia, or other infection, is painful
- Allowing natural death includes permitting the body to shut itself down through natural mechanisms, including infection
- Dialysis may extend life, but there will be progressive functional decline
2-Goals of Care define what therapies are indicated. Balance prolongation of life with illness experience.
Julianna Lindsey is a hospitalist and physician leader based in the Dallas-Fort Worth Metroplex. Her focus is patient safety/quality and physician leadership. She is a member of TeamHospitalist.
4/8/15
HM15 Presenter: Tammie Quest, MD
Summation: Heroics- a set of medical actions that attempt to prolong life with a low likelihood of success.
Palliative care- an approach of care provided to patients and families suffering from serious and/or life limiting illness; focus on physical, spiritual, psychological and social aspects of distress.
Hospice care- intense palliative care provided when the patient has terminal illness with a prognosis of 6 months or less if the disease runs its usual course.
We underutilize Palliative and Hospice care in the US. Here in the US fewer than 50% of all persons receive hospice care at EOL, of those who receive hospice care more than half receive care for less than 20 days, and 1 in 5 patients die in an ICU. Palliative Care can/should co-exist with life prolonging care following the diagnosis of serious illness.
Common therapies/interventions to be contemplated and discussed with patient at end of life: cpr, mechanical ventilation, central venous/arterial access, renal replacement therapy, surgical procedures, valve therapies, ventricular assist devices, continuous infusions, IV fluids, supplemental oxygen, artificial nutrition, antimicrobials, blood products, cancer directed therapy, antithrombotics, anticoagulation.
Practical Elements of Palliative Care: pain and symptom management, advance care planning, communication/goals of care, truth-telling, social support, spiritual support, psychological support, risk/burden assessment of treatments.
Key Points/HM Takeaways:
1-Palliative Care Bedside Talking Points-
- Cardiac arrest is the moment of death, very few people survive an attempt at reversing death
- If you are one of the few who survive to discharge, you may do well but few will survive to discharge
- Antibiotics DO improve survival, antibiotics DO NOT improve comfort
- No evidence to show that dying from pneumonia, or other infection, is painful
- Allowing natural death includes permitting the body to shut itself down through natural mechanisms, including infection
- Dialysis may extend life, but there will be progressive functional decline
2-Goals of Care define what therapies are indicated. Balance prolongation of life with illness experience.
Julianna Lindsey is a hospitalist and physician leader based in the Dallas-Fort Worth Metroplex. Her focus is patient safety/quality and physician leadership. She is a member of TeamHospitalist.
4/8/15
HM15 Presenter: Tammie Quest, MD
Summation: Heroics- a set of medical actions that attempt to prolong life with a low likelihood of success.
Palliative care- an approach of care provided to patients and families suffering from serious and/or life limiting illness; focus on physical, spiritual, psychological and social aspects of distress.
Hospice care- intense palliative care provided when the patient has terminal illness with a prognosis of 6 months or less if the disease runs its usual course.
We underutilize Palliative and Hospice care in the US. Here in the US fewer than 50% of all persons receive hospice care at EOL, of those who receive hospice care more than half receive care for less than 20 days, and 1 in 5 patients die in an ICU. Palliative Care can/should co-exist with life prolonging care following the diagnosis of serious illness.
Common therapies/interventions to be contemplated and discussed with patient at end of life: cpr, mechanical ventilation, central venous/arterial access, renal replacement therapy, surgical procedures, valve therapies, ventricular assist devices, continuous infusions, IV fluids, supplemental oxygen, artificial nutrition, antimicrobials, blood products, cancer directed therapy, antithrombotics, anticoagulation.
Practical Elements of Palliative Care: pain and symptom management, advance care planning, communication/goals of care, truth-telling, social support, spiritual support, psychological support, risk/burden assessment of treatments.
Key Points/HM Takeaways:
1-Palliative Care Bedside Talking Points-
- Cardiac arrest is the moment of death, very few people survive an attempt at reversing death
- If you are one of the few who survive to discharge, you may do well but few will survive to discharge
- Antibiotics DO improve survival, antibiotics DO NOT improve comfort
- No evidence to show that dying from pneumonia, or other infection, is painful
- Allowing natural death includes permitting the body to shut itself down through natural mechanisms, including infection
- Dialysis may extend life, but there will be progressive functional decline
2-Goals of Care define what therapies are indicated. Balance prolongation of life with illness experience.
Julianna Lindsey is a hospitalist and physician leader based in the Dallas-Fort Worth Metroplex. Her focus is patient safety/quality and physician leadership. She is a member of TeamHospitalist.
Smaller tubes take bite out of blood draws in critically ill
CHICAGO – Switching from conventional to small-volume phlebotomy tubes is an easy step toward reducing iatrogenic blood loss in critically ill adults, a new study suggests.
“We were looking at the amount of blood we were drawing off these patients and when we asked the nurses, the numbers were crazy. It could be as high as 20 mL per time that they drew off the patient and we felt we had to do better. The common sense dictum is the more blood you draw off, the more harm you are causing the patient,” principal investigator Dr. Heather Dolman from Detroit Receiving Hospital, Wayne State University, said in an interview.
For patients staying only a day or 2 at the hospital, the type of blood tube used may not make a difference. But for the critically ill, who studies suggest can have an average of 5 to more than 24 samples drawn a day, the cumulative blood loss over an extended stay can be sizable.
Clinicians are also inclined to order more diagnostic tests as the severity of illness increases, thus putting their sickest patients at the greatest risk of iatrogenic anemia and transfusion. Anemia secondary to phlebotomy accounts for up to 40% of packed red blood cells transfused, Dr. Dolman noted at the annual meeting of the Central Surgical Association.
The process of blood sampling itself also involves a fair amount of waste. Conventional arterial line systems require that an initial blood sample be removed to “clear the line.” This typically results in 2-10 mL of blood being discarded before a second sample of undiluted blood can be obtained.
Some hospitals have turned to closed blood sampling devices that avoid the need for a second sample. The impact of blood-conserving devices on transfusion rates has been underwhelming, with only one study showing a positive impact leading to reduced blood product use.
As part of their blood-conserving strategy, Dr. Dolman and her colleagues asked the hospital to invest in small-volume phlebotomy tubes (SVTs), which are sized somewhere between a conventional-volume tube (CVT) and a pediatric blood tube.
SVTs reduce the amount of blood needed from 8.5 mL with a conventional tube to 5.0 mL for a basic metabolic panel, from 6.0 mL to 2.0 mL for a complete blood count (CBC) or cross-matching, and from 2.7 mL to 1.8 mL for a prothrombin time /internationalized ratio/partial thromboplastin time, Dr. Dolman said. The cost of an SVT is the same as a CVT, as is the cost of the machinery needed to analyze the samples.
“Everyone is worried about missing out on data, but if you look at the research on the amount of blood the machine really needs, it is only 0.1 mL, that’s less than a cc,” she said. “The technology has been there for a while, I just think the common sense aspect of all this, no one has ever thought of.”
The investigators then retrospectively compared 248 critically ill patients in the ICU, of whom 116 had blood drawn with an SVT and 132 with a CVT. The two groups were well matched with respect to age (55 years vs. 57 years), admission to the emergency surgery/trauma service (63% vs. 64%), and mean APACHE II scores (14.1 vs. 12.7).
Transfusion was at the discretion of the primary team using a restrictive hemoglobin threshold of < 7.0 gm/dL, unless hemodynamic instability or active bleeding were present.
Utilizing an SVT significantly reduced daily blood loss from phlebotomy from 31.7 mL with a CVT to 22.5 mL (P < .0001) and overall phlebotomy blood loss from 299 mL to 174 mL (P < .001), Dr. Dolman reported.
This translated into a nonsignificant trend for fewer units of packed red blood cells transfused in the SVT group (mean 4.4 vs. 6.0; P = .16).
The same pattern was observed in the 158 patients admitted to the emergency surgery/trauma service, with SVT also leading to significantly fewer episodes of severe anemia (6 vs. 20; P = .01) and a trend toward shorter ICU stays (9.2 days vs. 10.6 days; P = .46), she said.
Patients with an APACHE score of at least 20, a group one would anticipate to derive greater advantage from a blood-conserving strategy, did not benefit from use of an SVT vs. a CVT, but the number of patients was very low at just 27 and 19, respectively, Dr. Dolman noted.
Anemia, however, had a profound impact on the critically ill cohort. Patients with severe anemia were significantly more likely than those with a hemoglobin level of at least 7 gm/dL to have longer ICU stays (16 days vs. 7.7 days; P < .001), longer hospital stays (23.3 days vs. 13.6 days; P < .001), and to die in the hospital (29% vs. 13%; P = .01).
Using a small-volume tube cut the number of patients with more than one episode of severe anemia from 22 to 11 (P = .01) and those with more than two episodes from 6 to 4 (P = .53).
“Anemia in the critically ill is a significant problem,” Dr. Dolman said. “Phlebotomy waste contributes to anemia and should be recorded to decrease this hidden loss.”
The impact of transfusion vs. no transfusion was less pronounced with respect to ICU stay (12 days vs. 6 days; P < .001), hospital stay (19 days vs. 11 days; P = .44), and in-hospital mortality (17% vs. 15%; P = .60), but can lead to other negative sequelae such as increased risk of infection, circulatory overload transfusion reactions, and immune modulation, she added.
Detroit Receiving Hospital continues to use conventional tubes in its ICU and other units, although a switch to small-volume tubes is expected to be considered following peer review of the full results, Dr. Dolman said.
Dr. Dolman reported having no financial disclosures.
On Twitter @pwendl
“This is something that should be replicated at institutions across the country,” discussant William C. Cirocco said in an interview. “Why not? It may not have clinical implications for the patient who is only in the hospital for 2 or 3 days, but for the ICU patient, it will have big impact. It’s a no-brainer.”
Dr. William C. Cirocco is a professor of surgery at Ohio State University in Columbus. He reported no relevant conflicts of interest.
“This is something that should be replicated at institutions across the country,” discussant William C. Cirocco said in an interview. “Why not? It may not have clinical implications for the patient who is only in the hospital for 2 or 3 days, but for the ICU patient, it will have big impact. It’s a no-brainer.”
Dr. William C. Cirocco is a professor of surgery at Ohio State University in Columbus. He reported no relevant conflicts of interest.
“This is something that should be replicated at institutions across the country,” discussant William C. Cirocco said in an interview. “Why not? It may not have clinical implications for the patient who is only in the hospital for 2 or 3 days, but for the ICU patient, it will have big impact. It’s a no-brainer.”
Dr. William C. Cirocco is a professor of surgery at Ohio State University in Columbus. He reported no relevant conflicts of interest.
CHICAGO – Switching from conventional to small-volume phlebotomy tubes is an easy step toward reducing iatrogenic blood loss in critically ill adults, a new study suggests.
“We were looking at the amount of blood we were drawing off these patients and when we asked the nurses, the numbers were crazy. It could be as high as 20 mL per time that they drew off the patient and we felt we had to do better. The common sense dictum is the more blood you draw off, the more harm you are causing the patient,” principal investigator Dr. Heather Dolman from Detroit Receiving Hospital, Wayne State University, said in an interview.
For patients staying only a day or 2 at the hospital, the type of blood tube used may not make a difference. But for the critically ill, who studies suggest can have an average of 5 to more than 24 samples drawn a day, the cumulative blood loss over an extended stay can be sizable.
Clinicians are also inclined to order more diagnostic tests as the severity of illness increases, thus putting their sickest patients at the greatest risk of iatrogenic anemia and transfusion. Anemia secondary to phlebotomy accounts for up to 40% of packed red blood cells transfused, Dr. Dolman noted at the annual meeting of the Central Surgical Association.
The process of blood sampling itself also involves a fair amount of waste. Conventional arterial line systems require that an initial blood sample be removed to “clear the line.” This typically results in 2-10 mL of blood being discarded before a second sample of undiluted blood can be obtained.
Some hospitals have turned to closed blood sampling devices that avoid the need for a second sample. The impact of blood-conserving devices on transfusion rates has been underwhelming, with only one study showing a positive impact leading to reduced blood product use.
As part of their blood-conserving strategy, Dr. Dolman and her colleagues asked the hospital to invest in small-volume phlebotomy tubes (SVTs), which are sized somewhere between a conventional-volume tube (CVT) and a pediatric blood tube.
SVTs reduce the amount of blood needed from 8.5 mL with a conventional tube to 5.0 mL for a basic metabolic panel, from 6.0 mL to 2.0 mL for a complete blood count (CBC) or cross-matching, and from 2.7 mL to 1.8 mL for a prothrombin time /internationalized ratio/partial thromboplastin time, Dr. Dolman said. The cost of an SVT is the same as a CVT, as is the cost of the machinery needed to analyze the samples.
“Everyone is worried about missing out on data, but if you look at the research on the amount of blood the machine really needs, it is only 0.1 mL, that’s less than a cc,” she said. “The technology has been there for a while, I just think the common sense aspect of all this, no one has ever thought of.”
The investigators then retrospectively compared 248 critically ill patients in the ICU, of whom 116 had blood drawn with an SVT and 132 with a CVT. The two groups were well matched with respect to age (55 years vs. 57 years), admission to the emergency surgery/trauma service (63% vs. 64%), and mean APACHE II scores (14.1 vs. 12.7).
Transfusion was at the discretion of the primary team using a restrictive hemoglobin threshold of < 7.0 gm/dL, unless hemodynamic instability or active bleeding were present.
Utilizing an SVT significantly reduced daily blood loss from phlebotomy from 31.7 mL with a CVT to 22.5 mL (P < .0001) and overall phlebotomy blood loss from 299 mL to 174 mL (P < .001), Dr. Dolman reported.
This translated into a nonsignificant trend for fewer units of packed red blood cells transfused in the SVT group (mean 4.4 vs. 6.0; P = .16).
The same pattern was observed in the 158 patients admitted to the emergency surgery/trauma service, with SVT also leading to significantly fewer episodes of severe anemia (6 vs. 20; P = .01) and a trend toward shorter ICU stays (9.2 days vs. 10.6 days; P = .46), she said.
Patients with an APACHE score of at least 20, a group one would anticipate to derive greater advantage from a blood-conserving strategy, did not benefit from use of an SVT vs. a CVT, but the number of patients was very low at just 27 and 19, respectively, Dr. Dolman noted.
Anemia, however, had a profound impact on the critically ill cohort. Patients with severe anemia were significantly more likely than those with a hemoglobin level of at least 7 gm/dL to have longer ICU stays (16 days vs. 7.7 days; P < .001), longer hospital stays (23.3 days vs. 13.6 days; P < .001), and to die in the hospital (29% vs. 13%; P = .01).
Using a small-volume tube cut the number of patients with more than one episode of severe anemia from 22 to 11 (P = .01) and those with more than two episodes from 6 to 4 (P = .53).
“Anemia in the critically ill is a significant problem,” Dr. Dolman said. “Phlebotomy waste contributes to anemia and should be recorded to decrease this hidden loss.”
The impact of transfusion vs. no transfusion was less pronounced with respect to ICU stay (12 days vs. 6 days; P < .001), hospital stay (19 days vs. 11 days; P = .44), and in-hospital mortality (17% vs. 15%; P = .60), but can lead to other negative sequelae such as increased risk of infection, circulatory overload transfusion reactions, and immune modulation, she added.
Detroit Receiving Hospital continues to use conventional tubes in its ICU and other units, although a switch to small-volume tubes is expected to be considered following peer review of the full results, Dr. Dolman said.
Dr. Dolman reported having no financial disclosures.
On Twitter @pwendl
CHICAGO – Switching from conventional to small-volume phlebotomy tubes is an easy step toward reducing iatrogenic blood loss in critically ill adults, a new study suggests.
“We were looking at the amount of blood we were drawing off these patients and when we asked the nurses, the numbers were crazy. It could be as high as 20 mL per time that they drew off the patient and we felt we had to do better. The common sense dictum is the more blood you draw off, the more harm you are causing the patient,” principal investigator Dr. Heather Dolman from Detroit Receiving Hospital, Wayne State University, said in an interview.
For patients staying only a day or 2 at the hospital, the type of blood tube used may not make a difference. But for the critically ill, who studies suggest can have an average of 5 to more than 24 samples drawn a day, the cumulative blood loss over an extended stay can be sizable.
Clinicians are also inclined to order more diagnostic tests as the severity of illness increases, thus putting their sickest patients at the greatest risk of iatrogenic anemia and transfusion. Anemia secondary to phlebotomy accounts for up to 40% of packed red blood cells transfused, Dr. Dolman noted at the annual meeting of the Central Surgical Association.
The process of blood sampling itself also involves a fair amount of waste. Conventional arterial line systems require that an initial blood sample be removed to “clear the line.” This typically results in 2-10 mL of blood being discarded before a second sample of undiluted blood can be obtained.
Some hospitals have turned to closed blood sampling devices that avoid the need for a second sample. The impact of blood-conserving devices on transfusion rates has been underwhelming, with only one study showing a positive impact leading to reduced blood product use.
As part of their blood-conserving strategy, Dr. Dolman and her colleagues asked the hospital to invest in small-volume phlebotomy tubes (SVTs), which are sized somewhere between a conventional-volume tube (CVT) and a pediatric blood tube.
SVTs reduce the amount of blood needed from 8.5 mL with a conventional tube to 5.0 mL for a basic metabolic panel, from 6.0 mL to 2.0 mL for a complete blood count (CBC) or cross-matching, and from 2.7 mL to 1.8 mL for a prothrombin time /internationalized ratio/partial thromboplastin time, Dr. Dolman said. The cost of an SVT is the same as a CVT, as is the cost of the machinery needed to analyze the samples.
“Everyone is worried about missing out on data, but if you look at the research on the amount of blood the machine really needs, it is only 0.1 mL, that’s less than a cc,” she said. “The technology has been there for a while, I just think the common sense aspect of all this, no one has ever thought of.”
The investigators then retrospectively compared 248 critically ill patients in the ICU, of whom 116 had blood drawn with an SVT and 132 with a CVT. The two groups were well matched with respect to age (55 years vs. 57 years), admission to the emergency surgery/trauma service (63% vs. 64%), and mean APACHE II scores (14.1 vs. 12.7).
Transfusion was at the discretion of the primary team using a restrictive hemoglobin threshold of < 7.0 gm/dL, unless hemodynamic instability or active bleeding were present.
Utilizing an SVT significantly reduced daily blood loss from phlebotomy from 31.7 mL with a CVT to 22.5 mL (P < .0001) and overall phlebotomy blood loss from 299 mL to 174 mL (P < .001), Dr. Dolman reported.
This translated into a nonsignificant trend for fewer units of packed red blood cells transfused in the SVT group (mean 4.4 vs. 6.0; P = .16).
The same pattern was observed in the 158 patients admitted to the emergency surgery/trauma service, with SVT also leading to significantly fewer episodes of severe anemia (6 vs. 20; P = .01) and a trend toward shorter ICU stays (9.2 days vs. 10.6 days; P = .46), she said.
Patients with an APACHE score of at least 20, a group one would anticipate to derive greater advantage from a blood-conserving strategy, did not benefit from use of an SVT vs. a CVT, but the number of patients was very low at just 27 and 19, respectively, Dr. Dolman noted.
Anemia, however, had a profound impact on the critically ill cohort. Patients with severe anemia were significantly more likely than those with a hemoglobin level of at least 7 gm/dL to have longer ICU stays (16 days vs. 7.7 days; P < .001), longer hospital stays (23.3 days vs. 13.6 days; P < .001), and to die in the hospital (29% vs. 13%; P = .01).
Using a small-volume tube cut the number of patients with more than one episode of severe anemia from 22 to 11 (P = .01) and those with more than two episodes from 6 to 4 (P = .53).
“Anemia in the critically ill is a significant problem,” Dr. Dolman said. “Phlebotomy waste contributes to anemia and should be recorded to decrease this hidden loss.”
The impact of transfusion vs. no transfusion was less pronounced with respect to ICU stay (12 days vs. 6 days; P < .001), hospital stay (19 days vs. 11 days; P = .44), and in-hospital mortality (17% vs. 15%; P = .60), but can lead to other negative sequelae such as increased risk of infection, circulatory overload transfusion reactions, and immune modulation, she added.
Detroit Receiving Hospital continues to use conventional tubes in its ICU and other units, although a switch to small-volume tubes is expected to be considered following peer review of the full results, Dr. Dolman said.
Dr. Dolman reported having no financial disclosures.
On Twitter @pwendl
AT THE ANNUAL MEETING OF THE CENTRAL SURGICAL ASSOCIATION
Key clinical point: Utilizing small-volume phlebotomy tubes minimizes diagnostic blood loss in the critically ill.
Major finding: Small tubes vs. conventional tubes reduced overall phlebotomy blood loss (174 mL vs. 299 mL; P < .001) and transfused packed RBCs (mean 4.4 units vs. 6.0 units; P = .16).
Data source: Retrospective case cohort in 248 critically ill patients.
Disclosures: Dr. Dolman reported having no financial disclosures.