User login
Improving Access of Personalized Care: Piloting a Telegenetic Program
Purpose: The New Mexico Veterans Affairs Health Care System (NMVAHCS) is striving to improve personalized cancer care and prevention through early identification of hereditary cancer syndromes. Detection of genetic syndromes remains vital for the implementation of precise therapeutic options and prevention measures offering improved veteran-centered care.
Background: Numerous genomic discoveries have provided personalized therapeutic options for improving clinical management of hereditary disease. However, the NMVAHCS lacks professionally trained genetic counselors to appropriately assess and address genetic testing. Primary care physicians lack specialized knowledge regarding appropriate use, application of genetic architectures, and an understanding of result interpretation. This lack of knowledge leaves providers reluctant to apply genomics in clinical practice or utilize testing on inappropriate patients, which remains costly and increases risk for litigation.
Methods: Increasing NMVAHCS access to appropriate genetic counseling involved initiating telehealth consults through the Veteran Affairs Genomic Medicine Service (VAGMS) in Salt Lake, Utah. After seeking stakeholder input and addressing availability of telehealth equipment, VAGMS was contacted. Telehealth service agreements, memorandum of understanding, and information security was obtained to allow offsite access into veterans’ charts. Clinics and consults were built into the computerized patient record system (CPRS), and staff training occurred to learn the intricacies of coordinating virtual appointments and awareness of available service. Initially, consults were limited for breast cancer risk evaluation (BRCA1 and BRCA2 mutations), to establish process flow, before opening all genetic counseling consults.
Results: Five appropriately identified veterans received breast cancer risk consults within the first week with fee-based cost savings of over $25,000. Counseling empowered veterans and families with information providing personalized therapeutic options and improving satisfaction and overall outcomes. Although the cost of breast-conserving surgery vs mastectomy remains relatively equal, prophylactic therapies reduce overall associated costs and the psychosocial distress of treating breast cancer, which is estimated to be well over $100,000.
Implications: Increasing access to genetic counseling and testing through partnering with a proven VA genetic program provides veterans with personalized, proactive therapeutic options. The role of genetics will continue to evolve and require collaboration to insure optimal application of precision care for prevention and management of veterans and family members at risk for disease.
Purpose: The New Mexico Veterans Affairs Health Care System (NMVAHCS) is striving to improve personalized cancer care and prevention through early identification of hereditary cancer syndromes. Detection of genetic syndromes remains vital for the implementation of precise therapeutic options and prevention measures offering improved veteran-centered care.
Background: Numerous genomic discoveries have provided personalized therapeutic options for improving clinical management of hereditary disease. However, the NMVAHCS lacks professionally trained genetic counselors to appropriately assess and address genetic testing. Primary care physicians lack specialized knowledge regarding appropriate use, application of genetic architectures, and an understanding of result interpretation. This lack of knowledge leaves providers reluctant to apply genomics in clinical practice or utilize testing on inappropriate patients, which remains costly and increases risk for litigation.
Methods: Increasing NMVAHCS access to appropriate genetic counseling involved initiating telehealth consults through the Veteran Affairs Genomic Medicine Service (VAGMS) in Salt Lake, Utah. After seeking stakeholder input and addressing availability of telehealth equipment, VAGMS was contacted. Telehealth service agreements, memorandum of understanding, and information security was obtained to allow offsite access into veterans’ charts. Clinics and consults were built into the computerized patient record system (CPRS), and staff training occurred to learn the intricacies of coordinating virtual appointments and awareness of available service. Initially, consults were limited for breast cancer risk evaluation (BRCA1 and BRCA2 mutations), to establish process flow, before opening all genetic counseling consults.
Results: Five appropriately identified veterans received breast cancer risk consults within the first week with fee-based cost savings of over $25,000. Counseling empowered veterans and families with information providing personalized therapeutic options and improving satisfaction and overall outcomes. Although the cost of breast-conserving surgery vs mastectomy remains relatively equal, prophylactic therapies reduce overall associated costs and the psychosocial distress of treating breast cancer, which is estimated to be well over $100,000.
Implications: Increasing access to genetic counseling and testing through partnering with a proven VA genetic program provides veterans with personalized, proactive therapeutic options. The role of genetics will continue to evolve and require collaboration to insure optimal application of precision care for prevention and management of veterans and family members at risk for disease.
Purpose: The New Mexico Veterans Affairs Health Care System (NMVAHCS) is striving to improve personalized cancer care and prevention through early identification of hereditary cancer syndromes. Detection of genetic syndromes remains vital for the implementation of precise therapeutic options and prevention measures offering improved veteran-centered care.
Background: Numerous genomic discoveries have provided personalized therapeutic options for improving clinical management of hereditary disease. However, the NMVAHCS lacks professionally trained genetic counselors to appropriately assess and address genetic testing. Primary care physicians lack specialized knowledge regarding appropriate use, application of genetic architectures, and an understanding of result interpretation. This lack of knowledge leaves providers reluctant to apply genomics in clinical practice or utilize testing on inappropriate patients, which remains costly and increases risk for litigation.
Methods: Increasing NMVAHCS access to appropriate genetic counseling involved initiating telehealth consults through the Veteran Affairs Genomic Medicine Service (VAGMS) in Salt Lake, Utah. After seeking stakeholder input and addressing availability of telehealth equipment, VAGMS was contacted. Telehealth service agreements, memorandum of understanding, and information security was obtained to allow offsite access into veterans’ charts. Clinics and consults were built into the computerized patient record system (CPRS), and staff training occurred to learn the intricacies of coordinating virtual appointments and awareness of available service. Initially, consults were limited for breast cancer risk evaluation (BRCA1 and BRCA2 mutations), to establish process flow, before opening all genetic counseling consults.
Results: Five appropriately identified veterans received breast cancer risk consults within the first week with fee-based cost savings of over $25,000. Counseling empowered veterans and families with information providing personalized therapeutic options and improving satisfaction and overall outcomes. Although the cost of breast-conserving surgery vs mastectomy remains relatively equal, prophylactic therapies reduce overall associated costs and the psychosocial distress of treating breast cancer, which is estimated to be well over $100,000.
Implications: Increasing access to genetic counseling and testing through partnering with a proven VA genetic program provides veterans with personalized, proactive therapeutic options. The role of genetics will continue to evolve and require collaboration to insure optimal application of precision care for prevention and management of veterans and family members at risk for disease.
The Use of a Telehealth Clinic to Support Patients Receiving Radiation Therapy at a Site Distant From Their PCP
Purpose: To try to integrate primary care support from the “spoke” facility during the treatment of patients receiving radiation treatments at the “hub” facility.
Background: Twenty percent of the patients receiving radiation therapy at Richard L. Roudebush VA Medical Center must relocate for up to several months in order to receive their daily treatments due to their distance from the tertiary radiation oncology unit. This makes it impossible for the patients to easily access their primary care provider (PCP) while they are out of town. Patients run out of routine medications, lose weight, have changes in renal function, and require changes in medication during this time; they must then access care via the hub emergency department (ED) or admission. In addition, the provider at the “spoke” is not necessarily in the loop regarding these patients.
Methods: We performed an analysis of the satisfaction with the current process, ED visits, and admissions of radiation oncology caregivers and patients using the Veterans House.
Results: Of patients treated with radiotherapy from April 1,2013, to April 1, 2014, 106 veterans stayed in the Veterans House. Patients who received palliative care with local PCPs were currently being treated at the time of the analysis or declined radiotherapy prior to starting treatment were excluded, leaving 61 patients. Of the 61 patients, there were a total of 48 ED visits and 24 admissions accounting for 168 patient-days in the hospital. A root cause analysis was performed on these48 ED visits; 56% of those were felt to be preventable.
Discussion: After several PDSA (plan-do-study-act) cycles which did not work (involving hub PCPs, involving the ED), we were successful in setting up routine weekly telehealth visits between the patient in Indianapolis at the radiation oncology unit hub and the PCP in the distant facilities in Danville and Peoria, Illinois. This allowed the PCP to manage antihypertensives, diabetic medications, and so on, as the patient moved through the radiation process.
Implications: This pilot process should decrease ED visits and admissions during radiation therapy and also serve to tighten the relationship between the hub and spoke facilities during subspecialist treatment.
Purpose: To try to integrate primary care support from the “spoke” facility during the treatment of patients receiving radiation treatments at the “hub” facility.
Background: Twenty percent of the patients receiving radiation therapy at Richard L. Roudebush VA Medical Center must relocate for up to several months in order to receive their daily treatments due to their distance from the tertiary radiation oncology unit. This makes it impossible for the patients to easily access their primary care provider (PCP) while they are out of town. Patients run out of routine medications, lose weight, have changes in renal function, and require changes in medication during this time; they must then access care via the hub emergency department (ED) or admission. In addition, the provider at the “spoke” is not necessarily in the loop regarding these patients.
Methods: We performed an analysis of the satisfaction with the current process, ED visits, and admissions of radiation oncology caregivers and patients using the Veterans House.
Results: Of patients treated with radiotherapy from April 1,2013, to April 1, 2014, 106 veterans stayed in the Veterans House. Patients who received palliative care with local PCPs were currently being treated at the time of the analysis or declined radiotherapy prior to starting treatment were excluded, leaving 61 patients. Of the 61 patients, there were a total of 48 ED visits and 24 admissions accounting for 168 patient-days in the hospital. A root cause analysis was performed on these48 ED visits; 56% of those were felt to be preventable.
Discussion: After several PDSA (plan-do-study-act) cycles which did not work (involving hub PCPs, involving the ED), we were successful in setting up routine weekly telehealth visits between the patient in Indianapolis at the radiation oncology unit hub and the PCP in the distant facilities in Danville and Peoria, Illinois. This allowed the PCP to manage antihypertensives, diabetic medications, and so on, as the patient moved through the radiation process.
Implications: This pilot process should decrease ED visits and admissions during radiation therapy and also serve to tighten the relationship between the hub and spoke facilities during subspecialist treatment.
Purpose: To try to integrate primary care support from the “spoke” facility during the treatment of patients receiving radiation treatments at the “hub” facility.
Background: Twenty percent of the patients receiving radiation therapy at Richard L. Roudebush VA Medical Center must relocate for up to several months in order to receive their daily treatments due to their distance from the tertiary radiation oncology unit. This makes it impossible for the patients to easily access their primary care provider (PCP) while they are out of town. Patients run out of routine medications, lose weight, have changes in renal function, and require changes in medication during this time; they must then access care via the hub emergency department (ED) or admission. In addition, the provider at the “spoke” is not necessarily in the loop regarding these patients.
Methods: We performed an analysis of the satisfaction with the current process, ED visits, and admissions of radiation oncology caregivers and patients using the Veterans House.
Results: Of patients treated with radiotherapy from April 1,2013, to April 1, 2014, 106 veterans stayed in the Veterans House. Patients who received palliative care with local PCPs were currently being treated at the time of the analysis or declined radiotherapy prior to starting treatment were excluded, leaving 61 patients. Of the 61 patients, there were a total of 48 ED visits and 24 admissions accounting for 168 patient-days in the hospital. A root cause analysis was performed on these48 ED visits; 56% of those were felt to be preventable.
Discussion: After several PDSA (plan-do-study-act) cycles which did not work (involving hub PCPs, involving the ED), we were successful in setting up routine weekly telehealth visits between the patient in Indianapolis at the radiation oncology unit hub and the PCP in the distant facilities in Danville and Peoria, Illinois. This allowed the PCP to manage antihypertensives, diabetic medications, and so on, as the patient moved through the radiation process.
Implications: This pilot process should decrease ED visits and admissions during radiation therapy and also serve to tighten the relationship between the hub and spoke facilities during subspecialist treatment.
Split-Course Palliative Radiotherapy for Advanced Lung Cancer, Using Modern CT-Based Planning
Purpose: Palliative radiotherapy for metastatic and locally advanced lung cancer is commonly used among veterans. We report our institutional experience using a split-course radiotherapy schedule, utilizing modern planning techniques.
Methods: All patients diagnosed with carcinoma of the lung between January 1, 2006, and December 31, 2012, were identified in our database. Of these, 35 patients received palliative radiation for stage IV and advanced stage IIIB disease, using a split-course treatment delivery with 3D planning and repeat CT simulation prior to the second half of their treatment course. Radiation was commonly delivered in 25 to 30 Gy in 10 fractions, followed by a 2- to 3-week break with an additional 25 to 30 Gy delivered in10 fractions delivered after a repeat 3D CT simulation.
Results: There was at least a 50% reduction in tumor volume at the time of second simulation (initial tumor volume range: 47 cm3-301 cm3) in 15/35 patients. These were designated as responders and the rest as nonresponders. The median survival in the responder group was 332 days and 340 days in the nonrapid responder group (P = .94). The local failure on imaging was seen in 47% of the responder population and 45% of the nonresponders. The overall 1-year survival for both groups was 34%.
Conclusions: Split-course palliative radiation is a reasonable option for veterans with metastatic and locally advanced lung cancer. Our retrospective review suggests that tumor shrinkage between courses of palliative radiation in a split-course model does not predict survival or local control. A prospective, randomized study would be needed to confirm our findings and make definitive recommendations.
Purpose: Palliative radiotherapy for metastatic and locally advanced lung cancer is commonly used among veterans. We report our institutional experience using a split-course radiotherapy schedule, utilizing modern planning techniques.
Methods: All patients diagnosed with carcinoma of the lung between January 1, 2006, and December 31, 2012, were identified in our database. Of these, 35 patients received palliative radiation for stage IV and advanced stage IIIB disease, using a split-course treatment delivery with 3D planning and repeat CT simulation prior to the second half of their treatment course. Radiation was commonly delivered in 25 to 30 Gy in 10 fractions, followed by a 2- to 3-week break with an additional 25 to 30 Gy delivered in10 fractions delivered after a repeat 3D CT simulation.
Results: There was at least a 50% reduction in tumor volume at the time of second simulation (initial tumor volume range: 47 cm3-301 cm3) in 15/35 patients. These were designated as responders and the rest as nonresponders. The median survival in the responder group was 332 days and 340 days in the nonrapid responder group (P = .94). The local failure on imaging was seen in 47% of the responder population and 45% of the nonresponders. The overall 1-year survival for both groups was 34%.
Conclusions: Split-course palliative radiation is a reasonable option for veterans with metastatic and locally advanced lung cancer. Our retrospective review suggests that tumor shrinkage between courses of palliative radiation in a split-course model does not predict survival or local control. A prospective, randomized study would be needed to confirm our findings and make definitive recommendations.
Purpose: Palliative radiotherapy for metastatic and locally advanced lung cancer is commonly used among veterans. We report our institutional experience using a split-course radiotherapy schedule, utilizing modern planning techniques.
Methods: All patients diagnosed with carcinoma of the lung between January 1, 2006, and December 31, 2012, were identified in our database. Of these, 35 patients received palliative radiation for stage IV and advanced stage IIIB disease, using a split-course treatment delivery with 3D planning and repeat CT simulation prior to the second half of their treatment course. Radiation was commonly delivered in 25 to 30 Gy in 10 fractions, followed by a 2- to 3-week break with an additional 25 to 30 Gy delivered in10 fractions delivered after a repeat 3D CT simulation.
Results: There was at least a 50% reduction in tumor volume at the time of second simulation (initial tumor volume range: 47 cm3-301 cm3) in 15/35 patients. These were designated as responders and the rest as nonresponders. The median survival in the responder group was 332 days and 340 days in the nonrapid responder group (P = .94). The local failure on imaging was seen in 47% of the responder population and 45% of the nonresponders. The overall 1-year survival for both groups was 34%.
Conclusions: Split-course palliative radiation is a reasonable option for veterans with metastatic and locally advanced lung cancer. Our retrospective review suggests that tumor shrinkage between courses of palliative radiation in a split-course model does not predict survival or local control. A prospective, randomized study would be needed to confirm our findings and make definitive recommendations.
Palliative Care, Advance Care Planning Conversations Needed Between Patients, Hospitalists
Last week, the Centers for Medicare and Medicaid Services (CMS), the nation’s largest payer of healthcare services and the 800-pound gorilla in setting medical necessity and coverage policies, announced a proposal to begin paying for goals of care and advance care planning (ACP) discussions between medical providers and patients. Sound familiar? It should. This is the same, seemingly no-brainer proposal that in 2009 was stricken from the eventually approved Patient Protection and Affordable Care Act (PPACA, aka the ACA, aka “Obamacare”) in response to the intentional and patently false accusations of government-run “death panels,” in the hopes of salvaging some measure of bipartisan support. As we all know, the bill eventually passed the following year without a single Republican voting in favor in either the House or Senate, and without funding for ACP sessions!
The need for ACP and access to primary and specialty palliative care is so great and accepted in the healthcare community. In their Choosing Wisely recommendations, numerous medical specialty societies, including ACEP [American College of Emergency Physicians], AAHPM [American Academy of Hospice and Palliative Medicine], AGS [American Geriatrics Society], and AMDA [The Society for Post-Acute and Long-Term Care Medicine], have included early and reliable access to palliative care and avoidance of non-value added care, such as placement of feeding tubes in patients with advanced dementia, calling out the gap between quality, evidence-based, patient and family-centered care, and “usual care” (e.g. medical and disease-focused care) that patients receive too often near the end of life.
So where is the disconnect between what people want and what actually happens to them at end of life?
The answer is clear: We’re not having “The Conversation.”
And, though our primary care and even specialty care colleagues are involved regularly in the care of these patients, they may be inclined to postpone or avoid ACP with patients and families in the outpatient setting due to lack of comfort [or] skill or even recognizing that the person they’ve been trying valiantly to cure or at least prolong the inevitable [for] is on that downslope of life we all eventually experience—it’s called dying.
Our current reimbursement system throws another barrier in front of providers. Like many other “nonprocedural” activities, ACP is not only undervalued; there is currently a lack of value assigned to this important cognitive, empathic, and communication-based “procedure.” And I refer to it as a procedure because, like a surgical or invasive vascular procedure, when it goes badly, the consequences and sequelae can be just as damaging, and even irreparable.
Thus, intentionally or not, the can is kicked further down the proverbial road until the patient reaches the hospital in a state of crisis—sometimes in extremis—and the hospitalist is left to make sense of all the clinical, emotional, psychological, spiritual, and frequently familial history (baggage?) leading up to that hospital admission. We are expected to develop instant rapport and trust while simultaneously attempting to develop (in collaboration with our specialty care providers and, preferably, the patient’s primary care provider) a plan of care that takes into account the personal values and treatment preferences for that individual within the clinical realities of the patient’s illness and disease trajectory as they lie before us.
Sound familiar?
For the full blog post, including Dr. Epstein’s recommendations for what hospitalists can do to support the CMS proposal, visit Hospital Leader.
Last week, the Centers for Medicare and Medicaid Services (CMS), the nation’s largest payer of healthcare services and the 800-pound gorilla in setting medical necessity and coverage policies, announced a proposal to begin paying for goals of care and advance care planning (ACP) discussions between medical providers and patients. Sound familiar? It should. This is the same, seemingly no-brainer proposal that in 2009 was stricken from the eventually approved Patient Protection and Affordable Care Act (PPACA, aka the ACA, aka “Obamacare”) in response to the intentional and patently false accusations of government-run “death panels,” in the hopes of salvaging some measure of bipartisan support. As we all know, the bill eventually passed the following year without a single Republican voting in favor in either the House or Senate, and without funding for ACP sessions!
The need for ACP and access to primary and specialty palliative care is so great and accepted in the healthcare community. In their Choosing Wisely recommendations, numerous medical specialty societies, including ACEP [American College of Emergency Physicians], AAHPM [American Academy of Hospice and Palliative Medicine], AGS [American Geriatrics Society], and AMDA [The Society for Post-Acute and Long-Term Care Medicine], have included early and reliable access to palliative care and avoidance of non-value added care, such as placement of feeding tubes in patients with advanced dementia, calling out the gap between quality, evidence-based, patient and family-centered care, and “usual care” (e.g. medical and disease-focused care) that patients receive too often near the end of life.
So where is the disconnect between what people want and what actually happens to them at end of life?
The answer is clear: We’re not having “The Conversation.”
And, though our primary care and even specialty care colleagues are involved regularly in the care of these patients, they may be inclined to postpone or avoid ACP with patients and families in the outpatient setting due to lack of comfort [or] skill or even recognizing that the person they’ve been trying valiantly to cure or at least prolong the inevitable [for] is on that downslope of life we all eventually experience—it’s called dying.
Our current reimbursement system throws another barrier in front of providers. Like many other “nonprocedural” activities, ACP is not only undervalued; there is currently a lack of value assigned to this important cognitive, empathic, and communication-based “procedure.” And I refer to it as a procedure because, like a surgical or invasive vascular procedure, when it goes badly, the consequences and sequelae can be just as damaging, and even irreparable.
Thus, intentionally or not, the can is kicked further down the proverbial road until the patient reaches the hospital in a state of crisis—sometimes in extremis—and the hospitalist is left to make sense of all the clinical, emotional, psychological, spiritual, and frequently familial history (baggage?) leading up to that hospital admission. We are expected to develop instant rapport and trust while simultaneously attempting to develop (in collaboration with our specialty care providers and, preferably, the patient’s primary care provider) a plan of care that takes into account the personal values and treatment preferences for that individual within the clinical realities of the patient’s illness and disease trajectory as they lie before us.
Sound familiar?
For the full blog post, including Dr. Epstein’s recommendations for what hospitalists can do to support the CMS proposal, visit Hospital Leader.
Last week, the Centers for Medicare and Medicaid Services (CMS), the nation’s largest payer of healthcare services and the 800-pound gorilla in setting medical necessity and coverage policies, announced a proposal to begin paying for goals of care and advance care planning (ACP) discussions between medical providers and patients. Sound familiar? It should. This is the same, seemingly no-brainer proposal that in 2009 was stricken from the eventually approved Patient Protection and Affordable Care Act (PPACA, aka the ACA, aka “Obamacare”) in response to the intentional and patently false accusations of government-run “death panels,” in the hopes of salvaging some measure of bipartisan support. As we all know, the bill eventually passed the following year without a single Republican voting in favor in either the House or Senate, and without funding for ACP sessions!
The need for ACP and access to primary and specialty palliative care is so great and accepted in the healthcare community. In their Choosing Wisely recommendations, numerous medical specialty societies, including ACEP [American College of Emergency Physicians], AAHPM [American Academy of Hospice and Palliative Medicine], AGS [American Geriatrics Society], and AMDA [The Society for Post-Acute and Long-Term Care Medicine], have included early and reliable access to palliative care and avoidance of non-value added care, such as placement of feeding tubes in patients with advanced dementia, calling out the gap between quality, evidence-based, patient and family-centered care, and “usual care” (e.g. medical and disease-focused care) that patients receive too often near the end of life.
So where is the disconnect between what people want and what actually happens to them at end of life?
The answer is clear: We’re not having “The Conversation.”
And, though our primary care and even specialty care colleagues are involved regularly in the care of these patients, they may be inclined to postpone or avoid ACP with patients and families in the outpatient setting due to lack of comfort [or] skill or even recognizing that the person they’ve been trying valiantly to cure or at least prolong the inevitable [for] is on that downslope of life we all eventually experience—it’s called dying.
Our current reimbursement system throws another barrier in front of providers. Like many other “nonprocedural” activities, ACP is not only undervalued; there is currently a lack of value assigned to this important cognitive, empathic, and communication-based “procedure.” And I refer to it as a procedure because, like a surgical or invasive vascular procedure, when it goes badly, the consequences and sequelae can be just as damaging, and even irreparable.
Thus, intentionally or not, the can is kicked further down the proverbial road until the patient reaches the hospital in a state of crisis—sometimes in extremis—and the hospitalist is left to make sense of all the clinical, emotional, psychological, spiritual, and frequently familial history (baggage?) leading up to that hospital admission. We are expected to develop instant rapport and trust while simultaneously attempting to develop (in collaboration with our specialty care providers and, preferably, the patient’s primary care provider) a plan of care that takes into account the personal values and treatment preferences for that individual within the clinical realities of the patient’s illness and disease trajectory as they lie before us.
Sound familiar?
For the full blog post, including Dr. Epstein’s recommendations for what hospitalists can do to support the CMS proposal, visit Hospital Leader.
VIDEO: Consistency is key to monitoring patients on opioids
ORLANDO – Take a consistent approach with all patients on opioid therapy, regardless of a patient’s perceived potential for abuse, Dr. Melissa B. Weimer advised.
“This is an area of medicine where I feel we need to apply universal precautions to all patients,” noted Dr. Weimer, assistant professor of medicine, Oregon Health and Science University, Portland. “We’re treating all patients as at some level of potential harm from this medication.”
In an interview at a meeting held by the American Pain Society and Global Academy for Medical Education, Dr. Weimer outlined a strategy that employs the same protocol to monitor therapy, even when the abuse potential is considered to be low.
Global Academy and this news organization are owned by the same company. Dr. Weimer reported no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Take a consistent approach with all patients on opioid therapy, regardless of a patient’s perceived potential for abuse, Dr. Melissa B. Weimer advised.
“This is an area of medicine where I feel we need to apply universal precautions to all patients,” noted Dr. Weimer, assistant professor of medicine, Oregon Health and Science University, Portland. “We’re treating all patients as at some level of potential harm from this medication.”
In an interview at a meeting held by the American Pain Society and Global Academy for Medical Education, Dr. Weimer outlined a strategy that employs the same protocol to monitor therapy, even when the abuse potential is considered to be low.
Global Academy and this news organization are owned by the same company. Dr. Weimer reported no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Take a consistent approach with all patients on opioid therapy, regardless of a patient’s perceived potential for abuse, Dr. Melissa B. Weimer advised.
“This is an area of medicine where I feel we need to apply universal precautions to all patients,” noted Dr. Weimer, assistant professor of medicine, Oregon Health and Science University, Portland. “We’re treating all patients as at some level of potential harm from this medication.”
In an interview at a meeting held by the American Pain Society and Global Academy for Medical Education, Dr. Weimer outlined a strategy that employs the same protocol to monitor therapy, even when the abuse potential is considered to be low.
Global Academy and this news organization are owned by the same company. Dr. Weimer reported no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM PAIN CARE FOR PRIMARY CARE
VIDEO: Use fiduciary duty to set pain medication boundaries
ORLANDO – Physicians should use the concept of fiduciary duty to set appropriate boundaries with patients taking pain medications, explained Dr. Louis Kuritzky.
Often, patients want treatments that are not in their best interests, noted Dr. Kuritzky of the department of community health and family medicine at the University of Florida, Gainesville.
In an interview at a meeting held by the American Pain Society and Global Academy for Medical Education, Dr. Kuritzky outlined how physicians can take a fiduciary duty approach to set boundaries with patients in a dispassionate manner.
Global Academy and this news organization are owned by the same company. Dr. Kuritzky reported a financial relationship with Lilly.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Physicians should use the concept of fiduciary duty to set appropriate boundaries with patients taking pain medications, explained Dr. Louis Kuritzky.
Often, patients want treatments that are not in their best interests, noted Dr. Kuritzky of the department of community health and family medicine at the University of Florida, Gainesville.
In an interview at a meeting held by the American Pain Society and Global Academy for Medical Education, Dr. Kuritzky outlined how physicians can take a fiduciary duty approach to set boundaries with patients in a dispassionate manner.
Global Academy and this news organization are owned by the same company. Dr. Kuritzky reported a financial relationship with Lilly.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Physicians should use the concept of fiduciary duty to set appropriate boundaries with patients taking pain medications, explained Dr. Louis Kuritzky.
Often, patients want treatments that are not in their best interests, noted Dr. Kuritzky of the department of community health and family medicine at the University of Florida, Gainesville.
In an interview at a meeting held by the American Pain Society and Global Academy for Medical Education, Dr. Kuritzky outlined how physicians can take a fiduciary duty approach to set boundaries with patients in a dispassionate manner.
Global Academy and this news organization are owned by the same company. Dr. Kuritzky reported a financial relationship with Lilly.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM PAIN CARE FOR PRIMARY CARE
Updates on Cancer Survivorship Care Planning
With advances in treatment, supportive care, and early diagnosis, the prevalence of cancer is increasing. An individual is considered a cancer survivor from the time of diagnosis to the end of his or her life.1 Although many patients with cancer are cured, they experience various short-term and long-term effects of cancer treatment, a high risk of recurrence and second cancer, anxiety, chronic pain, fatigue, depression, sexual dysfunction, and infertility.1
As of January 1, 2014, there were about 14.5 million cancer survivors in the U.S. The most common cancers in this population include prostate (43%), colon and rectal (9%), and melanoma (8%) in males; breast (41%), uterine corpus (8%), and colon and rectal (8%) in females.2 This estimate does not include noninvasive cancers, but does include bladder, basal cell, and squamous cell skin cancers. By January 1, 2024, the population of cancer survivors is predicted to increase to almost 19 million: 9.3 million males and 9.6 million females.1 Most of the cancer survivors (64%) were diagnosed 5 or more years ago, and 15% were diagnosed 20 or more years ago. Nearly half (46%) of cancer survivors are aged ≥ 70 years, and only 5% are aged < 40 years.2
Moye and colleagues reported that 524,052 (11%) of veterans treated in 2007 were cancer survivors.3 The most common types of cancers among these veterans were prostate, skin (nonmelanoma), and colorectal cancers. Compared with the general population of cancer survivors in the SEER database, veteran survivors were older.3 Because of the increasing prevalence of cancer survivors, greater attention is focused on long-term complications of cancer treatment. Recent studies have demonstrated that cancer survivors are less likely to receive general preventive care and care associated with noncancer-related medical conditions than are individuals without cancer.4
Survivorship Care Components
In 2005, the Institute of Medicine (IOM) published From Cancer Patient to Cancer Survivor: Lost to Transition.5 The report addresses 4 essential components of survivorship care: (1) prevention of recurrence, new cancers, and other late effects; (2) surveillance for cancer spread, recurrence, second cancers, and medical and psychosocial adverse events (AEs); (3) interventions for consequences of cancer and its treatment (medical problems, symptoms, psychological distress experienced by cancer survivors and their caregivers, and concerns related to employment, insurance, and disability); and (4) coordination between the specialist and primary care providers (PCPs) to ensure that all the survivors’ health needs are met.5
Cancer Treatment Summary
To ensure better transition, the IOM recommended that survivorship plans be made with a summary of treatment provided by the primary oncologist who treated the patient, to improve communication among all health care providers and between the providers and the patient.5 The summary should include the date of diagnosis; diagnostic tests; stage of diagnosis; a medical, surgical, and radiation treatment summary; and a detailed follow-up care plan. The IOM also recommends preventive practices to maintain health and well-being; information on legal protection regarding employment and health insurance; the availability of psychosocial services in the community; and screening for psychosocial distress in cancer survivors.3,6 However, studies have shown that gaps in adherence with IOM recommendations exist even in dedicated survivorship centers.7,8
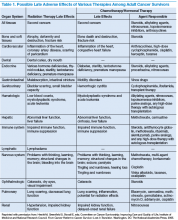
The American Society of Clinical Oncology (ASCO) and organizations such as Livestrong also have developed templates for survivorship care plans (SCPs). But there has been limited success in implementing SCPs.7,8 The American College of Surgeons Commission on Cancer (CoC) Standard 3.3 is scheduled to be implemented in 2015.9 The standard is expected to require that a cancer care committee develop and implement a process to disseminate a comprehensive care summary and follow-up plan for cancer survivors.9 To address this need, ASCO has formed a joint work group for improving cancer survivorship and a new version of an SCP template. This group recommended including contact information for the oncology providers who administered the treatment; basic diagnostic and staging information; and information on surgery, radiation therapy, systemic therapy (both chemotherapy and biologic therapy), and ongoing significant toxicities, including dates (Table 1).10
The ASCO also developed a follow-up care plan that includes a surveillance plan to detect recurrence and late AEs; interventions to manage ongoing problems resulting from cancer and its treatment; and age- and sex-appropriate health care, including cancer screening and general health promotion. It also recommended that the follow-up care plan should include a schedule of clinic visits in a table format, surveillance care testing to detect recurrence and second primary cancers, such as early breast cancer screening for women who underwent chest radiation for Hodgkin lymphoma. The person responsible for ordering these screening test should be included in the follow-up care plan.10 In addition, ASCO developed a cancer survivorship compendium, which included not only tools and resources, but also different models for cancer survivorship care.11 In addition, ASCO developed a Quality Oncology Practice Initiative (QOPI) to assess the quality of survivorship programs.9
The Central Arkansas Veterans Healthcare System (CAVHS) adopted National Comprehensive Cancer Network (NCCN), ASCO, and National Cancer Institute (NCI) guidelines and developed a template for a cancer treatment summary that includes the cancer type; grade; staging; and date of diagnosis and duration of treatment, including chemotherapy, surgery, and radiation with a disease-specific follow-up care plan for each type of cancer. The treatment summary is a useful tool to communicate a patient’s treatment and disease status to PCPs and patients.
Models of Care
Eight models for delivering survivorship care have been developed by ASCO11:
- Oncologist Specialist Care: Care occurs as a continuation in the oncology setting
- Multidisciplinary Survivor Clinic: Different specialists provide care
- General Survivorship Clinic: Care is provided by a physician or advanced care provider (APN) and implemented at a cancer center or private practice
- Consultative Survivorship Clinic: Initial follow-up is provided in an oncology setting with an eventual transition to a PCP; patients may be directed to the cancer center for needed services
- Integrated Survivorship Clinic: Care is provided by a physician or APN, and the care is coordinated with the PCP and other specialists as needed
- Community Generalist Model: The PCP, APN, or internist within the community provides care
- Shared-Care of Survivor: Care is coordinated and provided by any combination of specialists, PCPs, and nurses and is patient directed
Depending on the patients and the setting, practices can adopt various models to deliver survivorship care.
At CAVHS, cancer survivors are followed by an oncologist for their yearly examinations. This model is an illness model rather than a wellness model. A multidisciplinary clinic model can be initiated at CAVHS with the help of palliative care; complementary and alternative medicine for pain management; psychologists, chaplain services, and social workers for distress management; and coordination of survivorship care.
Implementation Barriers
There are many barriers to implementing SCPs, including time required by the providers to complete SCPs, inadequate reimbursement for the time and resources required to complete SCPs, challenges in coordinating care between survivors and providers, and lack of compatibility of the existing template with the electronic health record (EHR).7-10 A study regarding the barriers to implementation of SCPs, conducted at 14 NCI community cancer centers, demonstrated that the most common barrier is lack of personnel and time required to complete SCPs. The most widely used strategies was the use of a template with prespecified fields and delegating the completion of SCPs to one individual.12
Long-Term Complications for Survivors
Cancer survivors experience the physical and psychosocial effects of cancer treatment and have a very high risk of recurrence and second primary cancers. Common chronic AEs include fatigue, pain, neuropathy, infertility, sexual dysfunction, hypothyroidism, organ dysfunction, and urinary and bowel incontinence. In addition, patients also experience psychological AEs, including anxiety, depression, posttraumatic stress disorder, and sleep disturbances. Because of the AEs, cancer survivors have difficulty obtaining employment and insurance.1,5
Fatigue
Fatigue is the most common AE in cancer survivors.1 It may develop during treatment and persist for years. It is related to chemotherapy, radiation, surgical complications, depression, and insomnia.1 It is underrecognized and often untreated. It is important to assess and treat underlying comorbidities such as anemia, hypothyroidism, pain, depression and insomnia.13,14 Pharmacologic therapy with central nervous system stimulants and antidepressants have not shown any benefit.15 Studies on modafinil and armodafinil are ongoing.15 Exercise, treating underlying depression, sleep hygiene, behavioral and cognitive therapy, and yoga and mindfulness management of distress can help in treating fatigue.13 Meta-analyses showed that physical exercise helped reduce cancerrelated fatigue.15 A randomized controlled trial demonstrated that yoga led to significant improvement in fatigue in breast cancer survivors.16 Hence, it is important for providers to recognize and treat cancer-related fatigue and encourage patients to exercise.17
Psychological Adverse Effects
Cancer survivors also experience psychological AEs such as anxiety and depression because of the cancer diagnosis and the uncertainty of the outcome and the fear of relapse. Veterans may be at a higher risk of psychological AEs because of underlying mental illnesses. Counseling about the disease, psychotherapy interventions, and a mindfulness approach are recommended to treat anxiety and depression.14 The CAVHS cancer program has developed a mindfulness program as a multidisciplinary approach to manage psychological AEs.18
Sexual Dysfunction
Many patients may experience sexual dysfunction and infertility as a result of endocrine treatments, chemotherapy, radiation, and urologic and gynecologic surgeries.1 Over half of prostate cancer and breast cancer survivors report sexual dysfunction. Despite its high prevalence, sexual dysfunction often is not discussed with patients due to reluctance to discuss, lack of training, and lack of a standardized sexuality questionnaire.19 A brief sexual symptom checklist for women can be used as a primary screening tool. It is also recommended to screen for treatment-related infertility. Patients with sexual dysfunction should undergo screening for psychosocial problems such as anxiety, depression, and drug and alcohol use and treatment as these can contribute to sexual dysfunction.1
Vaginal lubricants are recommended to treat vaginal dryness. Vaginal estrogen creams are effective in patients with nonhormone-dependent gynecologic cancer without risk of breast cancer. The FDA approved the selective estrogen receptor modulator ospemifene for treating dyspareunia in postmenopausal women without risk of breast cancer.1 Oral phosphodiesterase-5 inhibitors, such as sildenafil, vacuum erection devices, penile prosthesis, and intracavernous injections, have shown to be effective in treating male erectile dysfunction (ED).19
Cardiovascular risk should be estimated in all patients with ED, because most of these patients will have common risk factors and need to be referred to a cardiologist before treating ED.1
Chronic Pain
Patients may also experience chronic pain as a late complication of chemotherapy, radiation, and surgery.1 More than one-third of cancer survivors experience pain, and it is often ineffectively managed due to lack of training, fear of AEs, and addiction.20 The goals of pain management are to increase comfort and improve quality of life. Short-acting and long-acting opioids remain an important treatment for cancer pain. Drug selection should be guided by previous exposure and comorbidities.15 Opioid therapy AEs, such as constipation, sleep apnea, and hypogonadism, should be recognized and treated. Antidepressants and anticonvulsants, such as gabapentin and pregabalin, may be used to manage neuropathic pain. A multidisciplinary approach using pharmacologic therapy, psychosocial therapy, behavioral interventions, exercise, and physical therapy is recommended.15 Patients with refractory pain are treated with interventional approaches, such as a neuronal blockade.
Survivorship Resources
Survivorship resources were developed by various cancer societies, such as NCCN, ASCO, Livestrong, and the Children’s Oncology Group. These resources help providers develop and deliver a quality survivorship care program (Table 2).5 The NCCN provides a template to help create cancer treatment summaries; guidelines for follow-up care for different cancers; and information for patients on legal and employment issues, smoking cessation, nutrition, and weight loss. In addition, the NCCN provides tools to assess anthracyclineinduced cardiac toxicity, anxiety, depression, cognitive dysfunction, pain, sexual dysfunction, and fatigue.
The CAVHS has several resources to develop a survivor ship progr am: It offers complementary and alternative therapy (a multidisciplinary approach) to help veterans with chronic pain and psychological distress. This resource incorporates acupuncture, yoga, hypnotherapy, biofeedback, mind-body approaches, stress management, nutritional counseling, physical therapy, and other mental and behavioral health support.
In addition, VA is implementing a patient-centered care and cultural transformation program to help veterans establish a relationship with their health care providers.21 The patient-centered approach along with the complementary and alternative therapy program, mindfulness program, and telehealth exercise motivation program can be incorporated to develop a multidisciplinary survivorship program.
Survivorship Research
Survivorship is an emerging field, and there is need for research to improve the implementation of quality survivorship care. Cancer survivorship research encompasses the physical, psychosocial, and economic sequelae of cancer diagnosis and its treatment among both pediatric and adult survivors of cancer. It also includes issues related to health care delivery, access, and follow-up care.
Mayer and colleagues reported that there were 42 published studies of SCPs in adult cancer survivors.22 Eleven studies reported that SCP use was limited and that < 25% of oncology providers never used an SCP. Research also showed that oncologists who have had training in long-term AEs of cancer and those who have used the EHRs were more likely to use SCPs.23 An integrated review of studies of SCPs recommended areas of research for survivorship. Thus the quality and quantity of SCP research are limited, and there is more need for quality research in survivorship to endorse the effective use of SCPs.
Conclusion
Cancer patients experience various AEs of cancer treatment, psychosocial distress, and can be lost to follow-up. It is important for health care providers to recognize and screen for the complications and provide SCPs, which includes a treatment summary and follow-up care to communicate and coordinate quality cancer survivorship care. Providers can use tools and resources developed by various organizations to develop and implement SCPs. The CoC surveys and accredits the cancer programs for quality measures, and has developed a new standard to provide SCPs for all patients effective 2015.
The CAVHS has already adopted this standard and has developed a template to complete a treatment summary and follow-up care planning in the EHR. The cancer treatment summary is given to the patient and is available to the PCP for review. There is also a need for more quality research in survivorship.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to continue reading.
1. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: survivorship. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf. Updated February 27, 2015. Accessed July 20, 2015.
2. American Cancer Society. Cancer treatment and survivorship facts and figures 2014-2015. American Cancer Society Website. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-042801.pdf. Accessed July 8, 2015.
3. Moye J, Schuster J, Latini D, Naik A. The future of cancer survivorship care for veterans. Fed Pract. 2010;27(3):36-43.
4. Snyder CF, Frick KD, Kantisiper ME, et al. Prevention, screening, and surveillance care for breast cancer survivors compared with controls: changes from 1998 to 2002. J Clin Oncol. 2009;27(7):1054-1061.
5. Hewitt M, Greenfield S, Stovall E, eds; Committee on Cancer Survivorship: Improving Care and Quality of Life, Institute of Medicine and National Research Council. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: The National Academies Press; 2005.
6. Adler NE, Page AEK, eds; Institute of Medicine (US) Committee on Psychosocial Services to Cancer Patients/Families in a Community Setting. Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. Washington, DC: National Academies Press; 2008. http://www.ncbi.nlm.nih.gov/books/NBK4015/. Accessed July 10, 2015.
7. Stricker CT, Jacobs LA, Risendal B, et al. Survivorship care planning after the institute of medicine recommendations: how are we faring? J Cancer Surviv. 2011;5(4):358-370.
8. Salz T, McCabe MS, Onstad EE, et al. Survivorship care plans: is there buy-in from community oncology providers? Cancer. 2014;120(5):722-730.
9. American College of Surgeons Commission on Cancer. Cancer Program Standards 2012: Ensuring Patient-Centered Care.V1.2.1. Chicago, IL: American College of Surgeons;2012. https://www.facs.org/~/media/files/quality%20programs/cancer
/coc/programstandards2012.ashx. Accessed July 8, 2015.
10. Mayer DK, Nekhlyudov L, Snyder CF, Merill JK, Wollins DS, Shulman LN. American Society of Clinical Oncology expert statement on cancer survivorship care planning. J Oncol Pract. 2014;10(6):345-351.
11. American Society of Clinical Oncology. ASCO cancer survivorship compendium. American Society of Clinical Oncology Website. http://www.asco.org/practice-research/asco-cancer-survivorship-compendium. Accessed July 10, 2015.
12. Forsythe LP, Alfano CM, Leach CR, Ganz PA, Stefanek ME, Rowland JH. Who provides psychosocial follow-up care for post-treatment cancer survivors? A survey of medical oncologists and primary care physicians. J Clin Oncol. 2012;30(23):2897-2905.
13. Mitchell SA, Hoffman AJ, Clark JC, et al. Putting evidence into practice: an update of evidence-based interventions for cancer-related fatigue during and following
treatment. Clin J Oncol Nurs. 2014;18(suppl):38-58.
14. Partridge AH, Jacobsen PB, Andersen BL. Challenges to standardizing the care for
adult cancer survivors: highlighing ASCO’s fatigue and anxiety and depression guidelines. Am Soc Clin Oncol Educ Book. 2015;35:188-194.
15. Pachman DR, Barton DL, Swetz KM, Loprinzi CL. Troublesome symptoms in cancer survivors: fatigue, insomnia, neuropathy, and pain. J Clin Oncol. 2012;30(30):3687-3696.
16. Cramp F, Daniel J. Exercise for the management of cancer–related fatigue in adults. Cochrane Database Syst Rev. 2008(2):CD006145.
17. Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors: A randomized controlled trial. Cancer. 2011;118(15):3766-3775.
18. Mesidor M, Kunthur A, Mehta P, et al. Mindfulness and cancer. Paper presented at: Annual meeting of Association of VA Hematologists/Oncologists; October 2015; Washington, DC.
19. Goncalves P, Groninger H. Sexual dysfunction in cancer patients and survivors #293. J Palliat Med. 2015 [Epub ahead of print].
20. Pargeon KL, Hailey BJ. Barriers to effective pain management: a review of the literature. J Pain Symptom Manage. 1999;18(5):358-368.
21. Heneghan C. The future of veteran care? Health Care Journal of Little Rock. May/June. http://www.healthcarejournallr.com/the-journal/contents-index/features/567-integrative-medicine.html. Accessed July 10, 2015.
22. Mayer DK, Birken SA, Check DK, Chen RC. Summing it up: an integrated
review of studies of cancer survivorship care plans (2006-2013). Cancer. 2015;121(7):978-996.
23. Blanch-Hartigan D, Forsythe LP, Alfano CM, et al. Provision and discussion of survivorship care plans among cancer survivors: results of a nationally representative survey of oncologists and primary care physicians. J Clin Oncol. 2014;32(15):1578-1585.
With advances in treatment, supportive care, and early diagnosis, the prevalence of cancer is increasing. An individual is considered a cancer survivor from the time of diagnosis to the end of his or her life.1 Although many patients with cancer are cured, they experience various short-term and long-term effects of cancer treatment, a high risk of recurrence and second cancer, anxiety, chronic pain, fatigue, depression, sexual dysfunction, and infertility.1
As of January 1, 2014, there were about 14.5 million cancer survivors in the U.S. The most common cancers in this population include prostate (43%), colon and rectal (9%), and melanoma (8%) in males; breast (41%), uterine corpus (8%), and colon and rectal (8%) in females.2 This estimate does not include noninvasive cancers, but does include bladder, basal cell, and squamous cell skin cancers. By January 1, 2024, the population of cancer survivors is predicted to increase to almost 19 million: 9.3 million males and 9.6 million females.1 Most of the cancer survivors (64%) were diagnosed 5 or more years ago, and 15% were diagnosed 20 or more years ago. Nearly half (46%) of cancer survivors are aged ≥ 70 years, and only 5% are aged < 40 years.2
Moye and colleagues reported that 524,052 (11%) of veterans treated in 2007 were cancer survivors.3 The most common types of cancers among these veterans were prostate, skin (nonmelanoma), and colorectal cancers. Compared with the general population of cancer survivors in the SEER database, veteran survivors were older.3 Because of the increasing prevalence of cancer survivors, greater attention is focused on long-term complications of cancer treatment. Recent studies have demonstrated that cancer survivors are less likely to receive general preventive care and care associated with noncancer-related medical conditions than are individuals without cancer.4
Survivorship Care Components
In 2005, the Institute of Medicine (IOM) published From Cancer Patient to Cancer Survivor: Lost to Transition.5 The report addresses 4 essential components of survivorship care: (1) prevention of recurrence, new cancers, and other late effects; (2) surveillance for cancer spread, recurrence, second cancers, and medical and psychosocial adverse events (AEs); (3) interventions for consequences of cancer and its treatment (medical problems, symptoms, psychological distress experienced by cancer survivors and their caregivers, and concerns related to employment, insurance, and disability); and (4) coordination between the specialist and primary care providers (PCPs) to ensure that all the survivors’ health needs are met.5
Cancer Treatment Summary
To ensure better transition, the IOM recommended that survivorship plans be made with a summary of treatment provided by the primary oncologist who treated the patient, to improve communication among all health care providers and between the providers and the patient.5 The summary should include the date of diagnosis; diagnostic tests; stage of diagnosis; a medical, surgical, and radiation treatment summary; and a detailed follow-up care plan. The IOM also recommends preventive practices to maintain health and well-being; information on legal protection regarding employment and health insurance; the availability of psychosocial services in the community; and screening for psychosocial distress in cancer survivors.3,6 However, studies have shown that gaps in adherence with IOM recommendations exist even in dedicated survivorship centers.7,8
The American Society of Clinical Oncology (ASCO) and organizations such as Livestrong also have developed templates for survivorship care plans (SCPs). But there has been limited success in implementing SCPs.7,8 The American College of Surgeons Commission on Cancer (CoC) Standard 3.3 is scheduled to be implemented in 2015.9 The standard is expected to require that a cancer care committee develop and implement a process to disseminate a comprehensive care summary and follow-up plan for cancer survivors.9 To address this need, ASCO has formed a joint work group for improving cancer survivorship and a new version of an SCP template. This group recommended including contact information for the oncology providers who administered the treatment; basic diagnostic and staging information; and information on surgery, radiation therapy, systemic therapy (both chemotherapy and biologic therapy), and ongoing significant toxicities, including dates (Table 1).10
The ASCO also developed a follow-up care plan that includes a surveillance plan to detect recurrence and late AEs; interventions to manage ongoing problems resulting from cancer and its treatment; and age- and sex-appropriate health care, including cancer screening and general health promotion. It also recommended that the follow-up care plan should include a schedule of clinic visits in a table format, surveillance care testing to detect recurrence and second primary cancers, such as early breast cancer screening for women who underwent chest radiation for Hodgkin lymphoma. The person responsible for ordering these screening test should be included in the follow-up care plan.10 In addition, ASCO developed a cancer survivorship compendium, which included not only tools and resources, but also different models for cancer survivorship care.11 In addition, ASCO developed a Quality Oncology Practice Initiative (QOPI) to assess the quality of survivorship programs.9
The Central Arkansas Veterans Healthcare System (CAVHS) adopted National Comprehensive Cancer Network (NCCN), ASCO, and National Cancer Institute (NCI) guidelines and developed a template for a cancer treatment summary that includes the cancer type; grade; staging; and date of diagnosis and duration of treatment, including chemotherapy, surgery, and radiation with a disease-specific follow-up care plan for each type of cancer. The treatment summary is a useful tool to communicate a patient’s treatment and disease status to PCPs and patients.
Models of Care
Eight models for delivering survivorship care have been developed by ASCO11:
- Oncologist Specialist Care: Care occurs as a continuation in the oncology setting
- Multidisciplinary Survivor Clinic: Different specialists provide care
- General Survivorship Clinic: Care is provided by a physician or advanced care provider (APN) and implemented at a cancer center or private practice
- Consultative Survivorship Clinic: Initial follow-up is provided in an oncology setting with an eventual transition to a PCP; patients may be directed to the cancer center for needed services
- Integrated Survivorship Clinic: Care is provided by a physician or APN, and the care is coordinated with the PCP and other specialists as needed
- Community Generalist Model: The PCP, APN, or internist within the community provides care
- Shared-Care of Survivor: Care is coordinated and provided by any combination of specialists, PCPs, and nurses and is patient directed
Depending on the patients and the setting, practices can adopt various models to deliver survivorship care.
At CAVHS, cancer survivors are followed by an oncologist for their yearly examinations. This model is an illness model rather than a wellness model. A multidisciplinary clinic model can be initiated at CAVHS with the help of palliative care; complementary and alternative medicine for pain management; psychologists, chaplain services, and social workers for distress management; and coordination of survivorship care.
Implementation Barriers
There are many barriers to implementing SCPs, including time required by the providers to complete SCPs, inadequate reimbursement for the time and resources required to complete SCPs, challenges in coordinating care between survivors and providers, and lack of compatibility of the existing template with the electronic health record (EHR).7-10 A study regarding the barriers to implementation of SCPs, conducted at 14 NCI community cancer centers, demonstrated that the most common barrier is lack of personnel and time required to complete SCPs. The most widely used strategies was the use of a template with prespecified fields and delegating the completion of SCPs to one individual.12
Long-Term Complications for Survivors
Cancer survivors experience the physical and psychosocial effects of cancer treatment and have a very high risk of recurrence and second primary cancers. Common chronic AEs include fatigue, pain, neuropathy, infertility, sexual dysfunction, hypothyroidism, organ dysfunction, and urinary and bowel incontinence. In addition, patients also experience psychological AEs, including anxiety, depression, posttraumatic stress disorder, and sleep disturbances. Because of the AEs, cancer survivors have difficulty obtaining employment and insurance.1,5
Fatigue
Fatigue is the most common AE in cancer survivors.1 It may develop during treatment and persist for years. It is related to chemotherapy, radiation, surgical complications, depression, and insomnia.1 It is underrecognized and often untreated. It is important to assess and treat underlying comorbidities such as anemia, hypothyroidism, pain, depression and insomnia.13,14 Pharmacologic therapy with central nervous system stimulants and antidepressants have not shown any benefit.15 Studies on modafinil and armodafinil are ongoing.15 Exercise, treating underlying depression, sleep hygiene, behavioral and cognitive therapy, and yoga and mindfulness management of distress can help in treating fatigue.13 Meta-analyses showed that physical exercise helped reduce cancerrelated fatigue.15 A randomized controlled trial demonstrated that yoga led to significant improvement in fatigue in breast cancer survivors.16 Hence, it is important for providers to recognize and treat cancer-related fatigue and encourage patients to exercise.17
Psychological Adverse Effects
Cancer survivors also experience psychological AEs such as anxiety and depression because of the cancer diagnosis and the uncertainty of the outcome and the fear of relapse. Veterans may be at a higher risk of psychological AEs because of underlying mental illnesses. Counseling about the disease, psychotherapy interventions, and a mindfulness approach are recommended to treat anxiety and depression.14 The CAVHS cancer program has developed a mindfulness program as a multidisciplinary approach to manage psychological AEs.18
Sexual Dysfunction
Many patients may experience sexual dysfunction and infertility as a result of endocrine treatments, chemotherapy, radiation, and urologic and gynecologic surgeries.1 Over half of prostate cancer and breast cancer survivors report sexual dysfunction. Despite its high prevalence, sexual dysfunction often is not discussed with patients due to reluctance to discuss, lack of training, and lack of a standardized sexuality questionnaire.19 A brief sexual symptom checklist for women can be used as a primary screening tool. It is also recommended to screen for treatment-related infertility. Patients with sexual dysfunction should undergo screening for psychosocial problems such as anxiety, depression, and drug and alcohol use and treatment as these can contribute to sexual dysfunction.1
Vaginal lubricants are recommended to treat vaginal dryness. Vaginal estrogen creams are effective in patients with nonhormone-dependent gynecologic cancer without risk of breast cancer. The FDA approved the selective estrogen receptor modulator ospemifene for treating dyspareunia in postmenopausal women without risk of breast cancer.1 Oral phosphodiesterase-5 inhibitors, such as sildenafil, vacuum erection devices, penile prosthesis, and intracavernous injections, have shown to be effective in treating male erectile dysfunction (ED).19
Cardiovascular risk should be estimated in all patients with ED, because most of these patients will have common risk factors and need to be referred to a cardiologist before treating ED.1
Chronic Pain
Patients may also experience chronic pain as a late complication of chemotherapy, radiation, and surgery.1 More than one-third of cancer survivors experience pain, and it is often ineffectively managed due to lack of training, fear of AEs, and addiction.20 The goals of pain management are to increase comfort and improve quality of life. Short-acting and long-acting opioids remain an important treatment for cancer pain. Drug selection should be guided by previous exposure and comorbidities.15 Opioid therapy AEs, such as constipation, sleep apnea, and hypogonadism, should be recognized and treated. Antidepressants and anticonvulsants, such as gabapentin and pregabalin, may be used to manage neuropathic pain. A multidisciplinary approach using pharmacologic therapy, psychosocial therapy, behavioral interventions, exercise, and physical therapy is recommended.15 Patients with refractory pain are treated with interventional approaches, such as a neuronal blockade.
Survivorship Resources
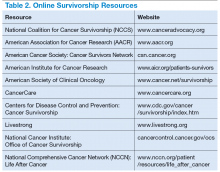
Survivorship resources were developed by various cancer societies, such as NCCN, ASCO, Livestrong, and the Children’s Oncology Group. These resources help providers develop and deliver a quality survivorship care program (Table 2).5 The NCCN provides a template to help create cancer treatment summaries; guidelines for follow-up care for different cancers; and information for patients on legal and employment issues, smoking cessation, nutrition, and weight loss. In addition, the NCCN provides tools to assess anthracyclineinduced cardiac toxicity, anxiety, depression, cognitive dysfunction, pain, sexual dysfunction, and fatigue.
The CAVHS has several resources to develop a survivor ship progr am: It offers complementary and alternative therapy (a multidisciplinary approach) to help veterans with chronic pain and psychological distress. This resource incorporates acupuncture, yoga, hypnotherapy, biofeedback, mind-body approaches, stress management, nutritional counseling, physical therapy, and other mental and behavioral health support.
In addition, VA is implementing a patient-centered care and cultural transformation program to help veterans establish a relationship with their health care providers.21 The patient-centered approach along with the complementary and alternative therapy program, mindfulness program, and telehealth exercise motivation program can be incorporated to develop a multidisciplinary survivorship program.
Survivorship Research
Survivorship is an emerging field, and there is need for research to improve the implementation of quality survivorship care. Cancer survivorship research encompasses the physical, psychosocial, and economic sequelae of cancer diagnosis and its treatment among both pediatric and adult survivors of cancer. It also includes issues related to health care delivery, access, and follow-up care.
Mayer and colleagues reported that there were 42 published studies of SCPs in adult cancer survivors.22 Eleven studies reported that SCP use was limited and that < 25% of oncology providers never used an SCP. Research also showed that oncologists who have had training in long-term AEs of cancer and those who have used the EHRs were more likely to use SCPs.23 An integrated review of studies of SCPs recommended areas of research for survivorship. Thus the quality and quantity of SCP research are limited, and there is more need for quality research in survivorship to endorse the effective use of SCPs.
Conclusion
Cancer patients experience various AEs of cancer treatment, psychosocial distress, and can be lost to follow-up. It is important for health care providers to recognize and screen for the complications and provide SCPs, which includes a treatment summary and follow-up care to communicate and coordinate quality cancer survivorship care. Providers can use tools and resources developed by various organizations to develop and implement SCPs. The CoC surveys and accredits the cancer programs for quality measures, and has developed a new standard to provide SCPs for all patients effective 2015.
The CAVHS has already adopted this standard and has developed a template to complete a treatment summary and follow-up care planning in the EHR. The cancer treatment summary is given to the patient and is available to the PCP for review. There is also a need for more quality research in survivorship.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to continue reading.
With advances in treatment, supportive care, and early diagnosis, the prevalence of cancer is increasing. An individual is considered a cancer survivor from the time of diagnosis to the end of his or her life.1 Although many patients with cancer are cured, they experience various short-term and long-term effects of cancer treatment, a high risk of recurrence and second cancer, anxiety, chronic pain, fatigue, depression, sexual dysfunction, and infertility.1
As of January 1, 2014, there were about 14.5 million cancer survivors in the U.S. The most common cancers in this population include prostate (43%), colon and rectal (9%), and melanoma (8%) in males; breast (41%), uterine corpus (8%), and colon and rectal (8%) in females.2 This estimate does not include noninvasive cancers, but does include bladder, basal cell, and squamous cell skin cancers. By January 1, 2024, the population of cancer survivors is predicted to increase to almost 19 million: 9.3 million males and 9.6 million females.1 Most of the cancer survivors (64%) were diagnosed 5 or more years ago, and 15% were diagnosed 20 or more years ago. Nearly half (46%) of cancer survivors are aged ≥ 70 years, and only 5% are aged < 40 years.2
Moye and colleagues reported that 524,052 (11%) of veterans treated in 2007 were cancer survivors.3 The most common types of cancers among these veterans were prostate, skin (nonmelanoma), and colorectal cancers. Compared with the general population of cancer survivors in the SEER database, veteran survivors were older.3 Because of the increasing prevalence of cancer survivors, greater attention is focused on long-term complications of cancer treatment. Recent studies have demonstrated that cancer survivors are less likely to receive general preventive care and care associated with noncancer-related medical conditions than are individuals without cancer.4
Survivorship Care Components
In 2005, the Institute of Medicine (IOM) published From Cancer Patient to Cancer Survivor: Lost to Transition.5 The report addresses 4 essential components of survivorship care: (1) prevention of recurrence, new cancers, and other late effects; (2) surveillance for cancer spread, recurrence, second cancers, and medical and psychosocial adverse events (AEs); (3) interventions for consequences of cancer and its treatment (medical problems, symptoms, psychological distress experienced by cancer survivors and their caregivers, and concerns related to employment, insurance, and disability); and (4) coordination between the specialist and primary care providers (PCPs) to ensure that all the survivors’ health needs are met.5
Cancer Treatment Summary
To ensure better transition, the IOM recommended that survivorship plans be made with a summary of treatment provided by the primary oncologist who treated the patient, to improve communication among all health care providers and between the providers and the patient.5 The summary should include the date of diagnosis; diagnostic tests; stage of diagnosis; a medical, surgical, and radiation treatment summary; and a detailed follow-up care plan. The IOM also recommends preventive practices to maintain health and well-being; information on legal protection regarding employment and health insurance; the availability of psychosocial services in the community; and screening for psychosocial distress in cancer survivors.3,6 However, studies have shown that gaps in adherence with IOM recommendations exist even in dedicated survivorship centers.7,8
The American Society of Clinical Oncology (ASCO) and organizations such as Livestrong also have developed templates for survivorship care plans (SCPs). But there has been limited success in implementing SCPs.7,8 The American College of Surgeons Commission on Cancer (CoC) Standard 3.3 is scheduled to be implemented in 2015.9 The standard is expected to require that a cancer care committee develop and implement a process to disseminate a comprehensive care summary and follow-up plan for cancer survivors.9 To address this need, ASCO has formed a joint work group for improving cancer survivorship and a new version of an SCP template. This group recommended including contact information for the oncology providers who administered the treatment; basic diagnostic and staging information; and information on surgery, radiation therapy, systemic therapy (both chemotherapy and biologic therapy), and ongoing significant toxicities, including dates (Table 1).10
The ASCO also developed a follow-up care plan that includes a surveillance plan to detect recurrence and late AEs; interventions to manage ongoing problems resulting from cancer and its treatment; and age- and sex-appropriate health care, including cancer screening and general health promotion. It also recommended that the follow-up care plan should include a schedule of clinic visits in a table format, surveillance care testing to detect recurrence and second primary cancers, such as early breast cancer screening for women who underwent chest radiation for Hodgkin lymphoma. The person responsible for ordering these screening test should be included in the follow-up care plan.10 In addition, ASCO developed a cancer survivorship compendium, which included not only tools and resources, but also different models for cancer survivorship care.11 In addition, ASCO developed a Quality Oncology Practice Initiative (QOPI) to assess the quality of survivorship programs.9
The Central Arkansas Veterans Healthcare System (CAVHS) adopted National Comprehensive Cancer Network (NCCN), ASCO, and National Cancer Institute (NCI) guidelines and developed a template for a cancer treatment summary that includes the cancer type; grade; staging; and date of diagnosis and duration of treatment, including chemotherapy, surgery, and radiation with a disease-specific follow-up care plan for each type of cancer. The treatment summary is a useful tool to communicate a patient’s treatment and disease status to PCPs and patients.
Models of Care
Eight models for delivering survivorship care have been developed by ASCO11:
- Oncologist Specialist Care: Care occurs as a continuation in the oncology setting
- Multidisciplinary Survivor Clinic: Different specialists provide care
- General Survivorship Clinic: Care is provided by a physician or advanced care provider (APN) and implemented at a cancer center or private practice
- Consultative Survivorship Clinic: Initial follow-up is provided in an oncology setting with an eventual transition to a PCP; patients may be directed to the cancer center for needed services
- Integrated Survivorship Clinic: Care is provided by a physician or APN, and the care is coordinated with the PCP and other specialists as needed
- Community Generalist Model: The PCP, APN, or internist within the community provides care
- Shared-Care of Survivor: Care is coordinated and provided by any combination of specialists, PCPs, and nurses and is patient directed
Depending on the patients and the setting, practices can adopt various models to deliver survivorship care.
At CAVHS, cancer survivors are followed by an oncologist for their yearly examinations. This model is an illness model rather than a wellness model. A multidisciplinary clinic model can be initiated at CAVHS with the help of palliative care; complementary and alternative medicine for pain management; psychologists, chaplain services, and social workers for distress management; and coordination of survivorship care.
Implementation Barriers
There are many barriers to implementing SCPs, including time required by the providers to complete SCPs, inadequate reimbursement for the time and resources required to complete SCPs, challenges in coordinating care between survivors and providers, and lack of compatibility of the existing template with the electronic health record (EHR).7-10 A study regarding the barriers to implementation of SCPs, conducted at 14 NCI community cancer centers, demonstrated that the most common barrier is lack of personnel and time required to complete SCPs. The most widely used strategies was the use of a template with prespecified fields and delegating the completion of SCPs to one individual.12
Long-Term Complications for Survivors
Cancer survivors experience the physical and psychosocial effects of cancer treatment and have a very high risk of recurrence and second primary cancers. Common chronic AEs include fatigue, pain, neuropathy, infertility, sexual dysfunction, hypothyroidism, organ dysfunction, and urinary and bowel incontinence. In addition, patients also experience psychological AEs, including anxiety, depression, posttraumatic stress disorder, and sleep disturbances. Because of the AEs, cancer survivors have difficulty obtaining employment and insurance.1,5
Fatigue
Fatigue is the most common AE in cancer survivors.1 It may develop during treatment and persist for years. It is related to chemotherapy, radiation, surgical complications, depression, and insomnia.1 It is underrecognized and often untreated. It is important to assess and treat underlying comorbidities such as anemia, hypothyroidism, pain, depression and insomnia.13,14 Pharmacologic therapy with central nervous system stimulants and antidepressants have not shown any benefit.15 Studies on modafinil and armodafinil are ongoing.15 Exercise, treating underlying depression, sleep hygiene, behavioral and cognitive therapy, and yoga and mindfulness management of distress can help in treating fatigue.13 Meta-analyses showed that physical exercise helped reduce cancerrelated fatigue.15 A randomized controlled trial demonstrated that yoga led to significant improvement in fatigue in breast cancer survivors.16 Hence, it is important for providers to recognize and treat cancer-related fatigue and encourage patients to exercise.17
Psychological Adverse Effects
Cancer survivors also experience psychological AEs such as anxiety and depression because of the cancer diagnosis and the uncertainty of the outcome and the fear of relapse. Veterans may be at a higher risk of psychological AEs because of underlying mental illnesses. Counseling about the disease, psychotherapy interventions, and a mindfulness approach are recommended to treat anxiety and depression.14 The CAVHS cancer program has developed a mindfulness program as a multidisciplinary approach to manage psychological AEs.18
Sexual Dysfunction
Many patients may experience sexual dysfunction and infertility as a result of endocrine treatments, chemotherapy, radiation, and urologic and gynecologic surgeries.1 Over half of prostate cancer and breast cancer survivors report sexual dysfunction. Despite its high prevalence, sexual dysfunction often is not discussed with patients due to reluctance to discuss, lack of training, and lack of a standardized sexuality questionnaire.19 A brief sexual symptom checklist for women can be used as a primary screening tool. It is also recommended to screen for treatment-related infertility. Patients with sexual dysfunction should undergo screening for psychosocial problems such as anxiety, depression, and drug and alcohol use and treatment as these can contribute to sexual dysfunction.1
Vaginal lubricants are recommended to treat vaginal dryness. Vaginal estrogen creams are effective in patients with nonhormone-dependent gynecologic cancer without risk of breast cancer. The FDA approved the selective estrogen receptor modulator ospemifene for treating dyspareunia in postmenopausal women without risk of breast cancer.1 Oral phosphodiesterase-5 inhibitors, such as sildenafil, vacuum erection devices, penile prosthesis, and intracavernous injections, have shown to be effective in treating male erectile dysfunction (ED).19
Cardiovascular risk should be estimated in all patients with ED, because most of these patients will have common risk factors and need to be referred to a cardiologist before treating ED.1
Chronic Pain
Patients may also experience chronic pain as a late complication of chemotherapy, radiation, and surgery.1 More than one-third of cancer survivors experience pain, and it is often ineffectively managed due to lack of training, fear of AEs, and addiction.20 The goals of pain management are to increase comfort and improve quality of life. Short-acting and long-acting opioids remain an important treatment for cancer pain. Drug selection should be guided by previous exposure and comorbidities.15 Opioid therapy AEs, such as constipation, sleep apnea, and hypogonadism, should be recognized and treated. Antidepressants and anticonvulsants, such as gabapentin and pregabalin, may be used to manage neuropathic pain. A multidisciplinary approach using pharmacologic therapy, psychosocial therapy, behavioral interventions, exercise, and physical therapy is recommended.15 Patients with refractory pain are treated with interventional approaches, such as a neuronal blockade.
Survivorship Resources
Survivorship resources were developed by various cancer societies, such as NCCN, ASCO, Livestrong, and the Children’s Oncology Group. These resources help providers develop and deliver a quality survivorship care program (Table 2).5 The NCCN provides a template to help create cancer treatment summaries; guidelines for follow-up care for different cancers; and information for patients on legal and employment issues, smoking cessation, nutrition, and weight loss. In addition, the NCCN provides tools to assess anthracyclineinduced cardiac toxicity, anxiety, depression, cognitive dysfunction, pain, sexual dysfunction, and fatigue.
The CAVHS has several resources to develop a survivor ship progr am: It offers complementary and alternative therapy (a multidisciplinary approach) to help veterans with chronic pain and psychological distress. This resource incorporates acupuncture, yoga, hypnotherapy, biofeedback, mind-body approaches, stress management, nutritional counseling, physical therapy, and other mental and behavioral health support.
In addition, VA is implementing a patient-centered care and cultural transformation program to help veterans establish a relationship with their health care providers.21 The patient-centered approach along with the complementary and alternative therapy program, mindfulness program, and telehealth exercise motivation program can be incorporated to develop a multidisciplinary survivorship program.
Survivorship Research
Survivorship is an emerging field, and there is need for research to improve the implementation of quality survivorship care. Cancer survivorship research encompasses the physical, psychosocial, and economic sequelae of cancer diagnosis and its treatment among both pediatric and adult survivors of cancer. It also includes issues related to health care delivery, access, and follow-up care.
Mayer and colleagues reported that there were 42 published studies of SCPs in adult cancer survivors.22 Eleven studies reported that SCP use was limited and that < 25% of oncology providers never used an SCP. Research also showed that oncologists who have had training in long-term AEs of cancer and those who have used the EHRs were more likely to use SCPs.23 An integrated review of studies of SCPs recommended areas of research for survivorship. Thus the quality and quantity of SCP research are limited, and there is more need for quality research in survivorship to endorse the effective use of SCPs.
Conclusion
Cancer patients experience various AEs of cancer treatment, psychosocial distress, and can be lost to follow-up. It is important for health care providers to recognize and screen for the complications and provide SCPs, which includes a treatment summary and follow-up care to communicate and coordinate quality cancer survivorship care. Providers can use tools and resources developed by various organizations to develop and implement SCPs. The CoC surveys and accredits the cancer programs for quality measures, and has developed a new standard to provide SCPs for all patients effective 2015.
The CAVHS has already adopted this standard and has developed a template to complete a treatment summary and follow-up care planning in the EHR. The cancer treatment summary is given to the patient and is available to the PCP for review. There is also a need for more quality research in survivorship.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to continue reading.
1. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: survivorship. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf. Updated February 27, 2015. Accessed July 20, 2015.
2. American Cancer Society. Cancer treatment and survivorship facts and figures 2014-2015. American Cancer Society Website. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-042801.pdf. Accessed July 8, 2015.
3. Moye J, Schuster J, Latini D, Naik A. The future of cancer survivorship care for veterans. Fed Pract. 2010;27(3):36-43.
4. Snyder CF, Frick KD, Kantisiper ME, et al. Prevention, screening, and surveillance care for breast cancer survivors compared with controls: changes from 1998 to 2002. J Clin Oncol. 2009;27(7):1054-1061.
5. Hewitt M, Greenfield S, Stovall E, eds; Committee on Cancer Survivorship: Improving Care and Quality of Life, Institute of Medicine and National Research Council. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: The National Academies Press; 2005.
6. Adler NE, Page AEK, eds; Institute of Medicine (US) Committee on Psychosocial Services to Cancer Patients/Families in a Community Setting. Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. Washington, DC: National Academies Press; 2008. http://www.ncbi.nlm.nih.gov/books/NBK4015/. Accessed July 10, 2015.
7. Stricker CT, Jacobs LA, Risendal B, et al. Survivorship care planning after the institute of medicine recommendations: how are we faring? J Cancer Surviv. 2011;5(4):358-370.
8. Salz T, McCabe MS, Onstad EE, et al. Survivorship care plans: is there buy-in from community oncology providers? Cancer. 2014;120(5):722-730.
9. American College of Surgeons Commission on Cancer. Cancer Program Standards 2012: Ensuring Patient-Centered Care.V1.2.1. Chicago, IL: American College of Surgeons;2012. https://www.facs.org/~/media/files/quality%20programs/cancer
/coc/programstandards2012.ashx. Accessed July 8, 2015.
10. Mayer DK, Nekhlyudov L, Snyder CF, Merill JK, Wollins DS, Shulman LN. American Society of Clinical Oncology expert statement on cancer survivorship care planning. J Oncol Pract. 2014;10(6):345-351.
11. American Society of Clinical Oncology. ASCO cancer survivorship compendium. American Society of Clinical Oncology Website. http://www.asco.org/practice-research/asco-cancer-survivorship-compendium. Accessed July 10, 2015.
12. Forsythe LP, Alfano CM, Leach CR, Ganz PA, Stefanek ME, Rowland JH. Who provides psychosocial follow-up care for post-treatment cancer survivors? A survey of medical oncologists and primary care physicians. J Clin Oncol. 2012;30(23):2897-2905.
13. Mitchell SA, Hoffman AJ, Clark JC, et al. Putting evidence into practice: an update of evidence-based interventions for cancer-related fatigue during and following
treatment. Clin J Oncol Nurs. 2014;18(suppl):38-58.
14. Partridge AH, Jacobsen PB, Andersen BL. Challenges to standardizing the care for
adult cancer survivors: highlighing ASCO’s fatigue and anxiety and depression guidelines. Am Soc Clin Oncol Educ Book. 2015;35:188-194.
15. Pachman DR, Barton DL, Swetz KM, Loprinzi CL. Troublesome symptoms in cancer survivors: fatigue, insomnia, neuropathy, and pain. J Clin Oncol. 2012;30(30):3687-3696.
16. Cramp F, Daniel J. Exercise for the management of cancer–related fatigue in adults. Cochrane Database Syst Rev. 2008(2):CD006145.
17. Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors: A randomized controlled trial. Cancer. 2011;118(15):3766-3775.
18. Mesidor M, Kunthur A, Mehta P, et al. Mindfulness and cancer. Paper presented at: Annual meeting of Association of VA Hematologists/Oncologists; October 2015; Washington, DC.
19. Goncalves P, Groninger H. Sexual dysfunction in cancer patients and survivors #293. J Palliat Med. 2015 [Epub ahead of print].
20. Pargeon KL, Hailey BJ. Barriers to effective pain management: a review of the literature. J Pain Symptom Manage. 1999;18(5):358-368.
21. Heneghan C. The future of veteran care? Health Care Journal of Little Rock. May/June. http://www.healthcarejournallr.com/the-journal/contents-index/features/567-integrative-medicine.html. Accessed July 10, 2015.
22. Mayer DK, Birken SA, Check DK, Chen RC. Summing it up: an integrated
review of studies of cancer survivorship care plans (2006-2013). Cancer. 2015;121(7):978-996.
23. Blanch-Hartigan D, Forsythe LP, Alfano CM, et al. Provision and discussion of survivorship care plans among cancer survivors: results of a nationally representative survey of oncologists and primary care physicians. J Clin Oncol. 2014;32(15):1578-1585.
1. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: survivorship. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf. Updated February 27, 2015. Accessed July 20, 2015.
2. American Cancer Society. Cancer treatment and survivorship facts and figures 2014-2015. American Cancer Society Website. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-042801.pdf. Accessed July 8, 2015.
3. Moye J, Schuster J, Latini D, Naik A. The future of cancer survivorship care for veterans. Fed Pract. 2010;27(3):36-43.
4. Snyder CF, Frick KD, Kantisiper ME, et al. Prevention, screening, and surveillance care for breast cancer survivors compared with controls: changes from 1998 to 2002. J Clin Oncol. 2009;27(7):1054-1061.
5. Hewitt M, Greenfield S, Stovall E, eds; Committee on Cancer Survivorship: Improving Care and Quality of Life, Institute of Medicine and National Research Council. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: The National Academies Press; 2005.
6. Adler NE, Page AEK, eds; Institute of Medicine (US) Committee on Psychosocial Services to Cancer Patients/Families in a Community Setting. Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. Washington, DC: National Academies Press; 2008. http://www.ncbi.nlm.nih.gov/books/NBK4015/. Accessed July 10, 2015.
7. Stricker CT, Jacobs LA, Risendal B, et al. Survivorship care planning after the institute of medicine recommendations: how are we faring? J Cancer Surviv. 2011;5(4):358-370.
8. Salz T, McCabe MS, Onstad EE, et al. Survivorship care plans: is there buy-in from community oncology providers? Cancer. 2014;120(5):722-730.
9. American College of Surgeons Commission on Cancer. Cancer Program Standards 2012: Ensuring Patient-Centered Care.V1.2.1. Chicago, IL: American College of Surgeons;2012. https://www.facs.org/~/media/files/quality%20programs/cancer
/coc/programstandards2012.ashx. Accessed July 8, 2015.
10. Mayer DK, Nekhlyudov L, Snyder CF, Merill JK, Wollins DS, Shulman LN. American Society of Clinical Oncology expert statement on cancer survivorship care planning. J Oncol Pract. 2014;10(6):345-351.
11. American Society of Clinical Oncology. ASCO cancer survivorship compendium. American Society of Clinical Oncology Website. http://www.asco.org/practice-research/asco-cancer-survivorship-compendium. Accessed July 10, 2015.
12. Forsythe LP, Alfano CM, Leach CR, Ganz PA, Stefanek ME, Rowland JH. Who provides psychosocial follow-up care for post-treatment cancer survivors? A survey of medical oncologists and primary care physicians. J Clin Oncol. 2012;30(23):2897-2905.
13. Mitchell SA, Hoffman AJ, Clark JC, et al. Putting evidence into practice: an update of evidence-based interventions for cancer-related fatigue during and following
treatment. Clin J Oncol Nurs. 2014;18(suppl):38-58.
14. Partridge AH, Jacobsen PB, Andersen BL. Challenges to standardizing the care for
adult cancer survivors: highlighing ASCO’s fatigue and anxiety and depression guidelines. Am Soc Clin Oncol Educ Book. 2015;35:188-194.
15. Pachman DR, Barton DL, Swetz KM, Loprinzi CL. Troublesome symptoms in cancer survivors: fatigue, insomnia, neuropathy, and pain. J Clin Oncol. 2012;30(30):3687-3696.
16. Cramp F, Daniel J. Exercise for the management of cancer–related fatigue in adults. Cochrane Database Syst Rev. 2008(2):CD006145.
17. Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors: A randomized controlled trial. Cancer. 2011;118(15):3766-3775.
18. Mesidor M, Kunthur A, Mehta P, et al. Mindfulness and cancer. Paper presented at: Annual meeting of Association of VA Hematologists/Oncologists; October 2015; Washington, DC.
19. Goncalves P, Groninger H. Sexual dysfunction in cancer patients and survivors #293. J Palliat Med. 2015 [Epub ahead of print].
20. Pargeon KL, Hailey BJ. Barriers to effective pain management: a review of the literature. J Pain Symptom Manage. 1999;18(5):358-368.
21. Heneghan C. The future of veteran care? Health Care Journal of Little Rock. May/June. http://www.healthcarejournallr.com/the-journal/contents-index/features/567-integrative-medicine.html. Accessed July 10, 2015.
22. Mayer DK, Birken SA, Check DK, Chen RC. Summing it up: an integrated
review of studies of cancer survivorship care plans (2006-2013). Cancer. 2015;121(7):978-996.
23. Blanch-Hartigan D, Forsythe LP, Alfano CM, et al. Provision and discussion of survivorship care plans among cancer survivors: results of a nationally representative survey of oncologists and primary care physicians. J Clin Oncol. 2014;32(15):1578-1585.
Establishing a Genetic Cancer Risk Assessment Clinic
Genetic cancers are relatively uncommon but not rare. Although there has not been a comprehensive study of the incidence of cancers that are caused by an identifiable single gene mutation, it is estimated that they account for approximately 5% to 10% of all cancers, or 50,000 to 100,000 patients annually in the U.S.1 The hallmarks of a genetic cancer syndrome are early onset, multiple family members in multiple generations with cancer, bilateral cancer, and multiple cancers in the same person.
Until recently, the VA has not had a significant interest in genetic cancer risk assessment (GCRA). This is changing, however, because veterans with identified genetic risks for cancer can benefit from targeted screening and intervention strategies to lower their risk of dying of cancer. The value of GCRA was also recognized in the 2015 standards for accreditation of the American College of Surgeons, which include a requirement for programs to include a provision for GCRA.2
The 2 most common familial cancer syndromes are hereditary breast and ovarian cancer (HBOC) syndrome, which occurs in about 5% of all patients with breast cancer, and Lynch syndrome (LS), or hereditary nonpolyposis colorectal cancer (CRC) syndrome, which occurs in about 3% of all patients with CRC.3,4 Other familial cancer syndromes are rare: For example, familial adenomatous polyposis (FAP) accounts for 0.2% to 0.5% of all CRC cases.5
The Raymond G. Murphy VAMC in Albuquerque is the sole VA hospital in New Mexico. Its catchment area extends into southern Colorado, eastern Arizona, and western Texas. About 40 CRCs and 8 breast cancers are diagnosed at this facility yearly. Given the incidence of these familial cancer syndromes, one might expect to see 1 LS case/year, 1 HBOC case every 2 years, and 1 FAP or attenuated FAP case every 5 to 10 years.
Note: Page numbers differ between the print issue and digital edition.
Genetic cancers are relatively uncommon but not rare. Although there has not been a comprehensive study of the incidence of cancers that are caused by an identifiable single gene mutation, it is estimated that they account for approximately 5% to 10% of all cancers, or 50,000 to 100,000 patients annually in the U.S.1 The hallmarks of a genetic cancer syndrome are early onset, multiple family members in multiple generations with cancer, bilateral cancer, and multiple cancers in the same person.
Until recently, the VA has not had a significant interest in genetic cancer risk assessment (GCRA). This is changing, however, because veterans with identified genetic risks for cancer can benefit from targeted screening and intervention strategies to lower their risk of dying of cancer. The value of GCRA was also recognized in the 2015 standards for accreditation of the American College of Surgeons, which include a requirement for programs to include a provision for GCRA.2
The 2 most common familial cancer syndromes are hereditary breast and ovarian cancer (HBOC) syndrome, which occurs in about 5% of all patients with breast cancer, and Lynch syndrome (LS), or hereditary nonpolyposis colorectal cancer (CRC) syndrome, which occurs in about 3% of all patients with CRC.3,4 Other familial cancer syndromes are rare: For example, familial adenomatous polyposis (FAP) accounts for 0.2% to 0.5% of all CRC cases.5
The Raymond G. Murphy VAMC in Albuquerque is the sole VA hospital in New Mexico. Its catchment area extends into southern Colorado, eastern Arizona, and western Texas. About 40 CRCs and 8 breast cancers are diagnosed at this facility yearly. Given the incidence of these familial cancer syndromes, one might expect to see 1 LS case/year, 1 HBOC case every 2 years, and 1 FAP or attenuated FAP case every 5 to 10 years.
Genetic cancers are relatively uncommon but not rare. Although there has not been a comprehensive study of the incidence of cancers that are caused by an identifiable single gene mutation, it is estimated that they account for approximately 5% to 10% of all cancers, or 50,000 to 100,000 patients annually in the U.S.1 The hallmarks of a genetic cancer syndrome are early onset, multiple family members in multiple generations with cancer, bilateral cancer, and multiple cancers in the same person.
Until recently, the VA has not had a significant interest in genetic cancer risk assessment (GCRA). This is changing, however, because veterans with identified genetic risks for cancer can benefit from targeted screening and intervention strategies to lower their risk of dying of cancer. The value of GCRA was also recognized in the 2015 standards for accreditation of the American College of Surgeons, which include a requirement for programs to include a provision for GCRA.2
The 2 most common familial cancer syndromes are hereditary breast and ovarian cancer (HBOC) syndrome, which occurs in about 5% of all patients with breast cancer, and Lynch syndrome (LS), or hereditary nonpolyposis colorectal cancer (CRC) syndrome, which occurs in about 3% of all patients with CRC.3,4 Other familial cancer syndromes are rare: For example, familial adenomatous polyposis (FAP) accounts for 0.2% to 0.5% of all CRC cases.5
The Raymond G. Murphy VAMC in Albuquerque is the sole VA hospital in New Mexico. Its catchment area extends into southern Colorado, eastern Arizona, and western Texas. About 40 CRCs and 8 breast cancers are diagnosed at this facility yearly. Given the incidence of these familial cancer syndromes, one might expect to see 1 LS case/year, 1 HBOC case every 2 years, and 1 FAP or attenuated FAP case every 5 to 10 years.
Note: Page numbers differ between the print issue and digital edition.
Note: Page numbers differ between the print issue and digital edition.
What is the difference between palliative care and hospice care?
Hospice care generally falls under the category of palliative care, despite being an older subspecialty. However, the two have different indications and goals and are often provided in different settings.
ORIGINS OF PALLIATIVE CARE
Prompted by what he perceived as neglect of dying patients in the acute care setting, Dr. Balfour Mount opened the first acute inpatient palliative care unit in Royal Victoria Hospital in Montréal, Québec, in 1976.1 His purpose was to provide a crisis-intervention service for patients who were actively dying, and this continues to be the main reason for consulting palliative care services in the hospital.
Palliative care has evolved since the 1970s and is now used in a variety of situations:
- A life-limiting illness in a patient who is not terminally ill
- A life-threatening illness in a patient who has symptoms but with the potential to recover
- A chronic illness such as heart failure or chronic obstructive pulmonary disease in a patient who is on disease-modifying therapy but has symptoms and will eventually succumb to the illness, but is expected to live longer than someone with advanced cancer.2
PALLIATIVE CARE IN CANCER PATIENTS
In patients with advanced cancer, palliative care is utilized earlier in the course of serious and life-limiting illness and is even involved in patient care when cure is the goal. Importantly, it now includes outpatient clinics to provide patients seamless care in conjunction with their oncologist’s care.3
Because palliative care focuses on the patient’s experience of the illness (sickness) rather than on disease itself (pathology), symptom management, psychosocial support, and assistance in decision-making are foremost. Initiating palliative care early in advanced cancer improves multiple outcomes and limits overly aggressive, ineffective therapies at the end of life (eg, late chemotherapy, late referral to hospice care, death in the intensive care unit), without hastening death. In fact, it may prolong life.3,4
Palliative care is indicated in a number of situations in oncology:
- Symptomatic presentations of cancer, even when curative treatments are available
- At the time of a sentinel event such as recurrence or unanticipated hospitalization
- When palliative radiation is needed
- When changes in chemotherapy are needed because of disease progression.
Also, cancer patients may develop symptoms that require a palliative procedure such as thoracentesis for pleural effusion, paracentesis for ascites, or surgery for a fracture or spinal cord compression. A palliative care consultation is also appropriate when patients change their goals of care (ie, palliation rather than cure), and when an oncologic crisis occurs and there is a need to offer support to the family and to clarify the goals of care.
PALLIATIVE CARE IN OTHER DISEASES
For patients with illnesses other than cancer, palliative care may be helpful when disease-modifying therapy becomes burdensome or ineffective, or when patients are symptomatic despite maximum therapy. Palliative care should also be considered when goals of care need to be explored, when a second opinion is needed on goals of care, or if the primary care provider and family are at odds.
WHEN A CONSULT IS INAPPROPRIATE
Palliative care consultation is inappropriate when used in lieu of an oncology consult in advanced cancer. Palliative care specialists are not experts in cancer care, whereas oncologists are familiar with rapid advancements in cancer care, including targeted agents that may offer benefit to patients with advanced cancer.
Palliative care consultation is also inappropriate if the patient does not want to see a palliative care specialist, or if the consult is used as a way to convince a patient to change advance directives or to choose not to be resuscitated. Also, cancer patients who are asymptomatic are unlikely to benefit from palliative care initially. The decision to consult palliative care should not depend on prognosis, and palliative care is more cost-effective when utilized early rather than as a crisis intervention near the end of life.3
THE PALLIATIVE CARE EVALUATION
The initial palliative care consultation usually involves an evaluation of the patient’s symptoms and concerns. Symptoms are targeted based on the patient’s priorities and on an assessment using validated questionnaires. A validated questionnaire is a better way to comprehensively gauge symptom burden than depending on patients to volunteer symptoms.5
As the relationship develops between patient, family, and palliative care specialist and as the disease takes its course, advance directives, prognosis, and end-of-life care goals can be addressed in follow-up consultations.3 Patients want to know about their prognosis, and they usually complete advance directives based on clinical circumstances rather than viewing them as an extension of patient autonomy, as originally intended.6
REIMBURSEMENT FOR PALLIATIVE CARE
Reimbursement for palliative care is similar to that for acute care and falls within the All Patient Refined Diagnosis-Related Group, or APR-DRG, system, and palliative care has its own V code for identification. Codes are used to designate disease, stage or location of metastases, disease complications, and symptoms, as well as for the discussion of goals of care.
WHAT PALLIATIVE CARE IS NOT
Palliative care has too often been tied to end-of-life care.7 The two often appear together in titles of reports in the literature. As a result, patients and physicians may be confused and, thus, reluctant to utilize palliative care services. To avoid the confusion, certain programs have included the term “supportive” oncology care in their title. This appears to facilitate palliative care referral, but may be misleading.8
WHAT IS HOSPICE CARE?
Hospice care is a service funded and capitated under Medicare part A and is largely provided as outpatient home care for those deemed terminally ill.9 An illness must be certified as terminal by two physicians. Medicare defines terminal illness as a life expectancy of 6 months or less if the illness runs its normal course.
The philosophy of hospice care is to provide comfort through intensive nurse management and home-based follow-up. In some cases, disease-modifying therapies are continued to control symptoms—eg, continuing angiotensin-converting enzyme inhibitors in heart failure patients. Hospice care is typically delivered at home, but it is also delivered in nursing homes, in hospital inpatient units, and at private or nonprofit hospice facilities.
Inpatient palliative care units are often mistaken for hospices. The purpose of hospice care is to provide quality of life and comfort and to avoid overly aggressive, expensive, and futile care at the end of life. The focus is on intensive, hands-on, personalized symptom care and family support at home. The goal is to provide a comfortable and dignified death among friends and family. The use of palliative radiation, transfusions, and antibiotics in hospice varies among hospice programs and is considered on a case-by-case basis.10
The Medicare per diem payment limits what hospices can afford, so they must be fiscally responsible. Hospice agencies are capitated and are responsible for providing medications and durable equipment necessary to treat symptoms related to the terminal illness. They also provide bereavement services for family members at no charge. Enrollment in hospice care can be revoked depending on circumstances and then reinstituted later as the goals of care change.
Care for nonterminal comorbid illnesses can be continued by a general practitioner or internist. This care is not covered under the Medicare hospice benefit, but it is covered under Medicare part B.
The patient and family can choose the hospice physician, who may be a family practitioner, internist, oncologist, or palliative care specialist, or may designate the hospice medical director as the hospice physician.
Criteria for hospice admission have been established for noncancer terminal illnesses and should be considered when practitioners decide to consult hospice.11–13
HOME-BASED PALLIATIVE CARE
Programs such as advanced illness management or home-based palliative care aim to improve the quality of care at home and prevent rehospitalization, particularly for patients with repeated hospitalizations.14 Home-based palliative care services are provided either by a clinician who makes home visits or by a certified home health care agency. Services are particularly useful for patients with serious illnesses who do not qualify for hospice services but are homebound. Consultations are obtained for ongoing supportive care at home, assessment for medication compliance, and disease monitoring at home. Consultations are scheduled at the time of hospital discharge.
Unlike hospice care, home-based palliative care does not include 24-hour on-call service. Comprehensive services (eg, home health aide, durable equipment, medications) are not provided as they are under hospice care: patients must qualify under Medicare stipulations for such services outside of hospice care. For example, home oxygen can only be supplied if the patient's oxygen saturation is less than 90%, while under the hospice benefit it is provided without regard to oxygen saturation and is based on symptom need. For home-based palliative care, patients must be largely homebound or unable to be seen regularly in the outpatient clinic. This type of care can be a bridge to hospice care for patients who feel they are not ready for hospice care at the time of discharge from acute care. Those who receive palliative care at home are less likely to be hospitalized at the end of life, are more likely to be transitioned to hospice at an appropriate time, and are more likely to have relief of symptoms.15
- Mount BM. The problem of caring for the dying in a general hospital; the palliative care unit as a possible solution. Can Med Assoc J 1976; 115:119–121.
- Higginson I. Palliative care: a review of past changes and future trends. J Public Health Med 1993; 15:3–8.
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010; 363:733–742.
- Zimmermann C, Riechelmann R, Krzyzanowska M, Rodin G, Tannock I. Effectiveness of specialized palliative care: a systematic review. JAMA 2008; 299:1698–1709.
- Homsi J, Walsh D, Rivera N, et al. Symptom evaluation in palliative medicine: patient report vs systematic assessment. Support Care Cancer 2006; 14:444–453.
- Tang ST, Liu TW, Lai MS, Liu LN, Chen CH, Koong SL. Congruence of knowledge, experiences, and p for disclosure of diagnosis and prognosis between terminally-ill cancer patients and their family caregivers in Taiwan. Cancer Invest 2006; 24:360–366.
- Bakitas M, Lyons KD, Hegel MT, Ahles T. Oncologists’ perspectives on concurrent palliative care in a National Cancer Institute-designated comprehensive cancer center. Palliat Support Care 2013; 11:415–423.
- Fadul N, Elsayem A, Palmer JL, et al. Supportive versus palliative care: what’s in a name: a survey of medical oncologists and midlevel providers at a comprehensive cancer center. Cancer 2009; 115:2013–2021.
- Rinaldo MJ. Medicare to cover hospice services. J Med Soc NJ 1982; 79:1015–1016.
- Enck RE. Palliative radiation therapy in hospice care. Am J Hosp Palliat Care 2002; 19:151–152.
- Luchins DJ, Hanrahan P, Murphy K. Criteria for enrolling dementia patients in hospice. J Am Geriatr Soc 1997; 45:1054–1059.
- Fox E, Landrum-McNiff K, Zhong Z, Dawson NV, Wu AW, Lynn J. Evaluation of prognostic criteria for determining hospice eligibility in patients with advanced lung, heart, or liver disease. JAMA 1999; 282:1638–1645.
- Stuart B. The NHO medical guidelines for non-cancer disease and local medical review policy: hospice access for patients with diseases other than cancer. Hosp J 1999; 14:139–154.
- McKinney M. Beyond hospice. New models of care focus on advanced illnesses. Mod Healthc 2013; 43:14–15.
- Gomes B, Calanzani N, Curiale V, McCrone P, Higginson IJ. Effectiveness and cost-effectiveness of home palliative care services for adults with advanced illness and their caregivers. Cochrane Database Syst Rev 2013; 6:CD007760.
Hospice care generally falls under the category of palliative care, despite being an older subspecialty. However, the two have different indications and goals and are often provided in different settings.
ORIGINS OF PALLIATIVE CARE
Prompted by what he perceived as neglect of dying patients in the acute care setting, Dr. Balfour Mount opened the first acute inpatient palliative care unit in Royal Victoria Hospital in Montréal, Québec, in 1976.1 His purpose was to provide a crisis-intervention service for patients who were actively dying, and this continues to be the main reason for consulting palliative care services in the hospital.
Palliative care has evolved since the 1970s and is now used in a variety of situations:
- A life-limiting illness in a patient who is not terminally ill
- A life-threatening illness in a patient who has symptoms but with the potential to recover
- A chronic illness such as heart failure or chronic obstructive pulmonary disease in a patient who is on disease-modifying therapy but has symptoms and will eventually succumb to the illness, but is expected to live longer than someone with advanced cancer.2
PALLIATIVE CARE IN CANCER PATIENTS
In patients with advanced cancer, palliative care is utilized earlier in the course of serious and life-limiting illness and is even involved in patient care when cure is the goal. Importantly, it now includes outpatient clinics to provide patients seamless care in conjunction with their oncologist’s care.3
Because palliative care focuses on the patient’s experience of the illness (sickness) rather than on disease itself (pathology), symptom management, psychosocial support, and assistance in decision-making are foremost. Initiating palliative care early in advanced cancer improves multiple outcomes and limits overly aggressive, ineffective therapies at the end of life (eg, late chemotherapy, late referral to hospice care, death in the intensive care unit), without hastening death. In fact, it may prolong life.3,4
Palliative care is indicated in a number of situations in oncology:
- Symptomatic presentations of cancer, even when curative treatments are available
- At the time of a sentinel event such as recurrence or unanticipated hospitalization
- When palliative radiation is needed
- When changes in chemotherapy are needed because of disease progression.
Also, cancer patients may develop symptoms that require a palliative procedure such as thoracentesis for pleural effusion, paracentesis for ascites, or surgery for a fracture or spinal cord compression. A palliative care consultation is also appropriate when patients change their goals of care (ie, palliation rather than cure), and when an oncologic crisis occurs and there is a need to offer support to the family and to clarify the goals of care.
PALLIATIVE CARE IN OTHER DISEASES
For patients with illnesses other than cancer, palliative care may be helpful when disease-modifying therapy becomes burdensome or ineffective, or when patients are symptomatic despite maximum therapy. Palliative care should also be considered when goals of care need to be explored, when a second opinion is needed on goals of care, or if the primary care provider and family are at odds.
WHEN A CONSULT IS INAPPROPRIATE
Palliative care consultation is inappropriate when used in lieu of an oncology consult in advanced cancer. Palliative care specialists are not experts in cancer care, whereas oncologists are familiar with rapid advancements in cancer care, including targeted agents that may offer benefit to patients with advanced cancer.
Palliative care consultation is also inappropriate if the patient does not want to see a palliative care specialist, or if the consult is used as a way to convince a patient to change advance directives or to choose not to be resuscitated. Also, cancer patients who are asymptomatic are unlikely to benefit from palliative care initially. The decision to consult palliative care should not depend on prognosis, and palliative care is more cost-effective when utilized early rather than as a crisis intervention near the end of life.3
THE PALLIATIVE CARE EVALUATION
The initial palliative care consultation usually involves an evaluation of the patient’s symptoms and concerns. Symptoms are targeted based on the patient’s priorities and on an assessment using validated questionnaires. A validated questionnaire is a better way to comprehensively gauge symptom burden than depending on patients to volunteer symptoms.5
As the relationship develops between patient, family, and palliative care specialist and as the disease takes its course, advance directives, prognosis, and end-of-life care goals can be addressed in follow-up consultations.3 Patients want to know about their prognosis, and they usually complete advance directives based on clinical circumstances rather than viewing them as an extension of patient autonomy, as originally intended.6
REIMBURSEMENT FOR PALLIATIVE CARE
Reimbursement for palliative care is similar to that for acute care and falls within the All Patient Refined Diagnosis-Related Group, or APR-DRG, system, and palliative care has its own V code for identification. Codes are used to designate disease, stage or location of metastases, disease complications, and symptoms, as well as for the discussion of goals of care.
WHAT PALLIATIVE CARE IS NOT
Palliative care has too often been tied to end-of-life care.7 The two often appear together in titles of reports in the literature. As a result, patients and physicians may be confused and, thus, reluctant to utilize palliative care services. To avoid the confusion, certain programs have included the term “supportive” oncology care in their title. This appears to facilitate palliative care referral, but may be misleading.8
WHAT IS HOSPICE CARE?
Hospice care is a service funded and capitated under Medicare part A and is largely provided as outpatient home care for those deemed terminally ill.9 An illness must be certified as terminal by two physicians. Medicare defines terminal illness as a life expectancy of 6 months or less if the illness runs its normal course.
The philosophy of hospice care is to provide comfort through intensive nurse management and home-based follow-up. In some cases, disease-modifying therapies are continued to control symptoms—eg, continuing angiotensin-converting enzyme inhibitors in heart failure patients. Hospice care is typically delivered at home, but it is also delivered in nursing homes, in hospital inpatient units, and at private or nonprofit hospice facilities.
Inpatient palliative care units are often mistaken for hospices. The purpose of hospice care is to provide quality of life and comfort and to avoid overly aggressive, expensive, and futile care at the end of life. The focus is on intensive, hands-on, personalized symptom care and family support at home. The goal is to provide a comfortable and dignified death among friends and family. The use of palliative radiation, transfusions, and antibiotics in hospice varies among hospice programs and is considered on a case-by-case basis.10
The Medicare per diem payment limits what hospices can afford, so they must be fiscally responsible. Hospice agencies are capitated and are responsible for providing medications and durable equipment necessary to treat symptoms related to the terminal illness. They also provide bereavement services for family members at no charge. Enrollment in hospice care can be revoked depending on circumstances and then reinstituted later as the goals of care change.
Care for nonterminal comorbid illnesses can be continued by a general practitioner or internist. This care is not covered under the Medicare hospice benefit, but it is covered under Medicare part B.
The patient and family can choose the hospice physician, who may be a family practitioner, internist, oncologist, or palliative care specialist, or may designate the hospice medical director as the hospice physician.
Criteria for hospice admission have been established for noncancer terminal illnesses and should be considered when practitioners decide to consult hospice.11–13
HOME-BASED PALLIATIVE CARE
Programs such as advanced illness management or home-based palliative care aim to improve the quality of care at home and prevent rehospitalization, particularly for patients with repeated hospitalizations.14 Home-based palliative care services are provided either by a clinician who makes home visits or by a certified home health care agency. Services are particularly useful for patients with serious illnesses who do not qualify for hospice services but are homebound. Consultations are obtained for ongoing supportive care at home, assessment for medication compliance, and disease monitoring at home. Consultations are scheduled at the time of hospital discharge.
Unlike hospice care, home-based palliative care does not include 24-hour on-call service. Comprehensive services (eg, home health aide, durable equipment, medications) are not provided as they are under hospice care: patients must qualify under Medicare stipulations for such services outside of hospice care. For example, home oxygen can only be supplied if the patient's oxygen saturation is less than 90%, while under the hospice benefit it is provided without regard to oxygen saturation and is based on symptom need. For home-based palliative care, patients must be largely homebound or unable to be seen regularly in the outpatient clinic. This type of care can be a bridge to hospice care for patients who feel they are not ready for hospice care at the time of discharge from acute care. Those who receive palliative care at home are less likely to be hospitalized at the end of life, are more likely to be transitioned to hospice at an appropriate time, and are more likely to have relief of symptoms.15
Hospice care generally falls under the category of palliative care, despite being an older subspecialty. However, the two have different indications and goals and are often provided in different settings.
ORIGINS OF PALLIATIVE CARE
Prompted by what he perceived as neglect of dying patients in the acute care setting, Dr. Balfour Mount opened the first acute inpatient palliative care unit in Royal Victoria Hospital in Montréal, Québec, in 1976.1 His purpose was to provide a crisis-intervention service for patients who were actively dying, and this continues to be the main reason for consulting palliative care services in the hospital.
Palliative care has evolved since the 1970s and is now used in a variety of situations:
- A life-limiting illness in a patient who is not terminally ill
- A life-threatening illness in a patient who has symptoms but with the potential to recover
- A chronic illness such as heart failure or chronic obstructive pulmonary disease in a patient who is on disease-modifying therapy but has symptoms and will eventually succumb to the illness, but is expected to live longer than someone with advanced cancer.2
PALLIATIVE CARE IN CANCER PATIENTS
In patients with advanced cancer, palliative care is utilized earlier in the course of serious and life-limiting illness and is even involved in patient care when cure is the goal. Importantly, it now includes outpatient clinics to provide patients seamless care in conjunction with their oncologist’s care.3
Because palliative care focuses on the patient’s experience of the illness (sickness) rather than on disease itself (pathology), symptom management, psychosocial support, and assistance in decision-making are foremost. Initiating palliative care early in advanced cancer improves multiple outcomes and limits overly aggressive, ineffective therapies at the end of life (eg, late chemotherapy, late referral to hospice care, death in the intensive care unit), without hastening death. In fact, it may prolong life.3,4
Palliative care is indicated in a number of situations in oncology:
- Symptomatic presentations of cancer, even when curative treatments are available
- At the time of a sentinel event such as recurrence or unanticipated hospitalization
- When palliative radiation is needed
- When changes in chemotherapy are needed because of disease progression.
Also, cancer patients may develop symptoms that require a palliative procedure such as thoracentesis for pleural effusion, paracentesis for ascites, or surgery for a fracture or spinal cord compression. A palliative care consultation is also appropriate when patients change their goals of care (ie, palliation rather than cure), and when an oncologic crisis occurs and there is a need to offer support to the family and to clarify the goals of care.
PALLIATIVE CARE IN OTHER DISEASES
For patients with illnesses other than cancer, palliative care may be helpful when disease-modifying therapy becomes burdensome or ineffective, or when patients are symptomatic despite maximum therapy. Palliative care should also be considered when goals of care need to be explored, when a second opinion is needed on goals of care, or if the primary care provider and family are at odds.
WHEN A CONSULT IS INAPPROPRIATE
Palliative care consultation is inappropriate when used in lieu of an oncology consult in advanced cancer. Palliative care specialists are not experts in cancer care, whereas oncologists are familiar with rapid advancements in cancer care, including targeted agents that may offer benefit to patients with advanced cancer.
Palliative care consultation is also inappropriate if the patient does not want to see a palliative care specialist, or if the consult is used as a way to convince a patient to change advance directives or to choose not to be resuscitated. Also, cancer patients who are asymptomatic are unlikely to benefit from palliative care initially. The decision to consult palliative care should not depend on prognosis, and palliative care is more cost-effective when utilized early rather than as a crisis intervention near the end of life.3
THE PALLIATIVE CARE EVALUATION
The initial palliative care consultation usually involves an evaluation of the patient’s symptoms and concerns. Symptoms are targeted based on the patient’s priorities and on an assessment using validated questionnaires. A validated questionnaire is a better way to comprehensively gauge symptom burden than depending on patients to volunteer symptoms.5
As the relationship develops between patient, family, and palliative care specialist and as the disease takes its course, advance directives, prognosis, and end-of-life care goals can be addressed in follow-up consultations.3 Patients want to know about their prognosis, and they usually complete advance directives based on clinical circumstances rather than viewing them as an extension of patient autonomy, as originally intended.6
REIMBURSEMENT FOR PALLIATIVE CARE
Reimbursement for palliative care is similar to that for acute care and falls within the All Patient Refined Diagnosis-Related Group, or APR-DRG, system, and palliative care has its own V code for identification. Codes are used to designate disease, stage or location of metastases, disease complications, and symptoms, as well as for the discussion of goals of care.
WHAT PALLIATIVE CARE IS NOT
Palliative care has too often been tied to end-of-life care.7 The two often appear together in titles of reports in the literature. As a result, patients and physicians may be confused and, thus, reluctant to utilize palliative care services. To avoid the confusion, certain programs have included the term “supportive” oncology care in their title. This appears to facilitate palliative care referral, but may be misleading.8
WHAT IS HOSPICE CARE?
Hospice care is a service funded and capitated under Medicare part A and is largely provided as outpatient home care for those deemed terminally ill.9 An illness must be certified as terminal by two physicians. Medicare defines terminal illness as a life expectancy of 6 months or less if the illness runs its normal course.
The philosophy of hospice care is to provide comfort through intensive nurse management and home-based follow-up. In some cases, disease-modifying therapies are continued to control symptoms—eg, continuing angiotensin-converting enzyme inhibitors in heart failure patients. Hospice care is typically delivered at home, but it is also delivered in nursing homes, in hospital inpatient units, and at private or nonprofit hospice facilities.
Inpatient palliative care units are often mistaken for hospices. The purpose of hospice care is to provide quality of life and comfort and to avoid overly aggressive, expensive, and futile care at the end of life. The focus is on intensive, hands-on, personalized symptom care and family support at home. The goal is to provide a comfortable and dignified death among friends and family. The use of palliative radiation, transfusions, and antibiotics in hospice varies among hospice programs and is considered on a case-by-case basis.10
The Medicare per diem payment limits what hospices can afford, so they must be fiscally responsible. Hospice agencies are capitated and are responsible for providing medications and durable equipment necessary to treat symptoms related to the terminal illness. They also provide bereavement services for family members at no charge. Enrollment in hospice care can be revoked depending on circumstances and then reinstituted later as the goals of care change.
Care for nonterminal comorbid illnesses can be continued by a general practitioner or internist. This care is not covered under the Medicare hospice benefit, but it is covered under Medicare part B.
The patient and family can choose the hospice physician, who may be a family practitioner, internist, oncologist, or palliative care specialist, or may designate the hospice medical director as the hospice physician.
Criteria for hospice admission have been established for noncancer terminal illnesses and should be considered when practitioners decide to consult hospice.11–13
HOME-BASED PALLIATIVE CARE
Programs such as advanced illness management or home-based palliative care aim to improve the quality of care at home and prevent rehospitalization, particularly for patients with repeated hospitalizations.14 Home-based palliative care services are provided either by a clinician who makes home visits or by a certified home health care agency. Services are particularly useful for patients with serious illnesses who do not qualify for hospice services but are homebound. Consultations are obtained for ongoing supportive care at home, assessment for medication compliance, and disease monitoring at home. Consultations are scheduled at the time of hospital discharge.
Unlike hospice care, home-based palliative care does not include 24-hour on-call service. Comprehensive services (eg, home health aide, durable equipment, medications) are not provided as they are under hospice care: patients must qualify under Medicare stipulations for such services outside of hospice care. For example, home oxygen can only be supplied if the patient's oxygen saturation is less than 90%, while under the hospice benefit it is provided without regard to oxygen saturation and is based on symptom need. For home-based palliative care, patients must be largely homebound or unable to be seen regularly in the outpatient clinic. This type of care can be a bridge to hospice care for patients who feel they are not ready for hospice care at the time of discharge from acute care. Those who receive palliative care at home are less likely to be hospitalized at the end of life, are more likely to be transitioned to hospice at an appropriate time, and are more likely to have relief of symptoms.15
- Mount BM. The problem of caring for the dying in a general hospital; the palliative care unit as a possible solution. Can Med Assoc J 1976; 115:119–121.
- Higginson I. Palliative care: a review of past changes and future trends. J Public Health Med 1993; 15:3–8.
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010; 363:733–742.
- Zimmermann C, Riechelmann R, Krzyzanowska M, Rodin G, Tannock I. Effectiveness of specialized palliative care: a systematic review. JAMA 2008; 299:1698–1709.
- Homsi J, Walsh D, Rivera N, et al. Symptom evaluation in palliative medicine: patient report vs systematic assessment. Support Care Cancer 2006; 14:444–453.
- Tang ST, Liu TW, Lai MS, Liu LN, Chen CH, Koong SL. Congruence of knowledge, experiences, and p for disclosure of diagnosis and prognosis between terminally-ill cancer patients and their family caregivers in Taiwan. Cancer Invest 2006; 24:360–366.
- Bakitas M, Lyons KD, Hegel MT, Ahles T. Oncologists’ perspectives on concurrent palliative care in a National Cancer Institute-designated comprehensive cancer center. Palliat Support Care 2013; 11:415–423.
- Fadul N, Elsayem A, Palmer JL, et al. Supportive versus palliative care: what’s in a name: a survey of medical oncologists and midlevel providers at a comprehensive cancer center. Cancer 2009; 115:2013–2021.
- Rinaldo MJ. Medicare to cover hospice services. J Med Soc NJ 1982; 79:1015–1016.
- Enck RE. Palliative radiation therapy in hospice care. Am J Hosp Palliat Care 2002; 19:151–152.
- Luchins DJ, Hanrahan P, Murphy K. Criteria for enrolling dementia patients in hospice. J Am Geriatr Soc 1997; 45:1054–1059.
- Fox E, Landrum-McNiff K, Zhong Z, Dawson NV, Wu AW, Lynn J. Evaluation of prognostic criteria for determining hospice eligibility in patients with advanced lung, heart, or liver disease. JAMA 1999; 282:1638–1645.
- Stuart B. The NHO medical guidelines for non-cancer disease and local medical review policy: hospice access for patients with diseases other than cancer. Hosp J 1999; 14:139–154.
- McKinney M. Beyond hospice. New models of care focus on advanced illnesses. Mod Healthc 2013; 43:14–15.
- Gomes B, Calanzani N, Curiale V, McCrone P, Higginson IJ. Effectiveness and cost-effectiveness of home palliative care services for adults with advanced illness and their caregivers. Cochrane Database Syst Rev 2013; 6:CD007760.
- Mount BM. The problem of caring for the dying in a general hospital; the palliative care unit as a possible solution. Can Med Assoc J 1976; 115:119–121.
- Higginson I. Palliative care: a review of past changes and future trends. J Public Health Med 1993; 15:3–8.
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010; 363:733–742.
- Zimmermann C, Riechelmann R, Krzyzanowska M, Rodin G, Tannock I. Effectiveness of specialized palliative care: a systematic review. JAMA 2008; 299:1698–1709.
- Homsi J, Walsh D, Rivera N, et al. Symptom evaluation in palliative medicine: patient report vs systematic assessment. Support Care Cancer 2006; 14:444–453.
- Tang ST, Liu TW, Lai MS, Liu LN, Chen CH, Koong SL. Congruence of knowledge, experiences, and p for disclosure of diagnosis and prognosis between terminally-ill cancer patients and their family caregivers in Taiwan. Cancer Invest 2006; 24:360–366.
- Bakitas M, Lyons KD, Hegel MT, Ahles T. Oncologists’ perspectives on concurrent palliative care in a National Cancer Institute-designated comprehensive cancer center. Palliat Support Care 2013; 11:415–423.
- Fadul N, Elsayem A, Palmer JL, et al. Supportive versus palliative care: what’s in a name: a survey of medical oncologists and midlevel providers at a comprehensive cancer center. Cancer 2009; 115:2013–2021.
- Rinaldo MJ. Medicare to cover hospice services. J Med Soc NJ 1982; 79:1015–1016.
- Enck RE. Palliative radiation therapy in hospice care. Am J Hosp Palliat Care 2002; 19:151–152.
- Luchins DJ, Hanrahan P, Murphy K. Criteria for enrolling dementia patients in hospice. J Am Geriatr Soc 1997; 45:1054–1059.
- Fox E, Landrum-McNiff K, Zhong Z, Dawson NV, Wu AW, Lynn J. Evaluation of prognostic criteria for determining hospice eligibility in patients with advanced lung, heart, or liver disease. JAMA 1999; 282:1638–1645.
- Stuart B. The NHO medical guidelines for non-cancer disease and local medical review policy: hospice access for patients with diseases other than cancer. Hosp J 1999; 14:139–154.
- McKinney M. Beyond hospice. New models of care focus on advanced illnesses. Mod Healthc 2013; 43:14–15.
- Gomes B, Calanzani N, Curiale V, McCrone P, Higginson IJ. Effectiveness and cost-effectiveness of home palliative care services for adults with advanced illness and their caregivers. Cochrane Database Syst Rev 2013; 6:CD007760.
Comprehensive wound malodor management: Win the RACE
Wounds that fail to heal become more than mere skin lesions. Pain, malodor, and the accompanying psychological distress often complicate nonhealing wounds and impair quality of life.1 Management of malodor requires perseverance, sensitivity, and familiarity with tools and procedures that range from surgical debridement to medical-grade honey.
Chronic, nonhealing wounds are defined as persisting for more than 6 months.2 These lesions are incapable of undergoing anatomic and functional repair on their own. Commonly encountered nonhealing wounds include pressure ulcers, venous stasis ulcers, arterial insufficiency ulcers, and malignant cutaneous wounds.
Typically, the patient with a nonhealing wound is frail, debilitated, medically complex, and often faced with one or more life-limiting illnesses. Complete wound healing may therefore be unrealistic, and optimal wound management becomes the goal of care.3,4
Healthcare providers encounter nonhealing wounds in varied settings—acute inpatient, outpatient, long-term, and home care. For instance, in the home care setting, a study of 383 patients enrolled in hospice found that 35% had skin ulcers and wounds.3 Half of those affected had pressure ulcers, 20% had ischemic ulcers, and 30% had other skin disorders such as stasis ulcers, burns, skin tears, and tumors. A larger study, also in hospice patients, found that 26% had pressure ulcers and 10% more developed them within 6 months.5
While pressure ulcers are the most common nonhealing wounds, malignant or fungating wounds are found in 5% to 10% of patients with metastatic disease, usually with cancers of the breast, head, and neck.6
Maximizing wound care provides comfort, relieves suffering, and promotes quality of life.3,7 To achieve these goals, clinicians must be familiar with strategies to manage complications associated with nonhealing wounds such as pain, malodor, and psychosocial adverse effects. Of these complications, malodor has been pointed out by both patients and caregivers as the most distressing.8
This article focuses on wound malodor, discusses the processes that cause wounds to emit an offensive smell, and outlines a comprehensive management approach.
MRS. A., AGE 61, WITH STAGE IV BREAST CANCER
Mrs. A., 61 years old, had a fungating mass in her left breast, which began as a small nodule and progressively enlarged to deform her breast over several months. Her oncologist subsequently staged the extent of her cancer as stage IV after workup revealed lung metastasis. Mrs. A. and her family decided to forgo cancer treatment, including radiotherapy, and to transition to hospice care after discussions with the oncologist.
Mrs. A. lived at home with her husband. Her daughter and three grandchildren all lived nearby.
When her hospice physician arrived at her home to meet her, a strong, pungent, and nauseating smell greeted him as he entered her bedroom. The patient said that for the past few months she had been increasingly distressed by the revolting odor. She rarely left home and had been ashamed to have people visit her, including her family.
On examination, the physician noticed a large fungating mass with yellowish discharge and necrotic tissue in her left breast. In addition to mild pain, she was immensely bothered by the strong odor coming from her breast.
THE IMPACT OF MALODOR
As seen in the case of Mrs. A., malodor has grave effects, both physical and psychological. Patients experience impaired or socially unacceptable body image, social rejection, personal shame, and embarrassment.9,10 Feelings of fear, anxiety, and depression are common. If left uncontrolled, malodor results in social isolation, reluctance to engage in social activities, diminished appetite, and nausea. In addition, malodor is a constant reminder of patients’ pain and cancer, and it results in further suffering.11
Reactions of family members and caregivers can worsen the situation.9,12 Expressions of revulsion limit contact and inhibit intimacy, especially near the end of life. Caregivers are often frustrated and distressed over their inability to control the malodor. The environment becomes uninhabitable, and the malodor can permeate clothing, furniture, and living quarters.
Managing malodor can be emotionally draining, physically daunting, and frustrating for healthcare professionals, as several methods are usually employed, often in a trial-and-error approach, to achieve an acceptable degree of odor control. In addition, clinicians must face the challenge of treating malodorous wounds at very close distance without reacting in a way that offends or alarms patients and family members.13
MALODOR PRODUCTION: WHERE IS THAT SMELL COMING FROM?
All wounds can produce an odor.14 Wounds that are expected to heal typically emit a faint but not unpleasant odor, akin to fresh blood. Wounds colonized by Pseudomonas aeruginosa produce a fruity or grapelike odor that is tolerable. Malodor occurs with wounds infected by other gram-negative organisms or anaerobic bacteria.15 Similarly, wounds covered by necrotic tissue smell like decaying flesh.
Three major causes
The three major causes of wound malodor are slough, infection, and exudate (Figure 1).
Slough is dead or necrotic tissue, usually resulting from vascular compromise. Arterial ulcers, pressure ulcers, and malignant wounds all form slough from capillary occlusion, subsequent ischemia, and tissue necrosis.
Infection. Devitalized tissue, an ideal medium in which bacteria thrive, becomes the source of infection. Anaerobic bacteria are usually implicated in malodor. These include Bacteroides fragilis, Bacteroides prevotella, Clostridium perfringens, and Fusobacterium nucleatum.16,17 Anaerobic organisms produce putrescine and cadaverine, which are largely responsible for the offensive odor.16,18 Volatile fatty acids such as propionic, butyric, isovaleric, and valeric acid are formed from lipid catabolism by anaerobes and add to malodor.17 Aerobic bacteria such as Proteus, Klebsiella, and Pseudomonas species supercolonize necrotic tissue as well and contribute to malodor.17,18
Exudate. Since nonhealing wounds undergo repeated cycles of inflammation, infection, and necrosis, accumulation of exudate becomes inevitable. Exudate typically is a pus-like fluid containing serum, fibrin, and white blood cells, which leak from blood vessels. In addition, bacteria that colonize chronic wounds filled with necrotic tissue activate proteases that degrade and liquefy dead tissue, thereby forming extensive amounts of exudate.19
Apart from slough, infection, and exudate, poor general hygiene and dressings left on for too long may contribute to malodor.16 Moisture-retentive dressings such as hydrocolloids leave an odor after removal. Dressings that liquefy upon contact with the wound surface leave a pus-like, potentially malodorous material.
MALODOR ASSESSMENT: DO YOU SMELL SOMETHING?
Various ways to document wound malodor can prove useful in guiding assessment and treatment. Descriptions such as “foul,” “putrid,” “fishy,” or “filled the room” vividly portray the initial presentation. A 10-point numerical scale similar to a numerical pain scale or a visual analogue scale can be used as a subjective measure.
Other grading methods, which to the authors’ knowledge are not validated, may be helpful. In a study that focused on patients suffering from malodorous gynecologic malignancies, von Gruenigen et al20 used a 0-to-3 scale:
- 0 Absent
- 1 Not offensive
- 2 Offensive but tolerable
- 3 Offensive and intolerable.
A scale often adapted by other authors was devised by Baker and Haig,21 which clearly defines four classes:
- 1 Strong—odor is evident upon entering the room (6 to 10 feet from the patient) with the dressing intact
- 2 Moderate—odor is evident upon entering the room with dressing removed
- 3 Slight—odor is evident at close proximity to the patient when the dressing is removed
- 4 No odor—no odor is evident, even at the patient’s bedside with the dressing removed.
COMPREHENSIVE MANAGEMENT: HOW DO WE WIN THE ‘RACE’?
The acronym RACE outlines an approach to dealing with malodor. It stands for removal of necrotic tissue; antibacterials; odor concealers; and education and support (Table 1).
Remove necrotic tissue
An important step in eliminating malodor is to remove necrotic tissue. This starts with debridement, which decreases the incidence of infection and hastens wound closure.22,23 Table 2 compares the different types of debridement.
Sharp or surgical debridement involves the use of a scalpel or scissors. This type of debridement may increase the risk of bleeding, pain, and malignant cell seeding in fungating wounds.4,24
Enzymatic debridement employs chemicals with proteolytic action (eg, collagenase) to digest extracellular proteins in wounds.18,25
Mechanical debridement involves aggressive therapies such as forceful irrigation and hydrotherapy, which may fail to discriminate between necrotic and viable tissues.18,26
Biological debridement using maggots, which ingest bacteria and devitalized tissue, may cause increased wound bleeding and may be unacceptable for patients and families.24,27
Autolytic debridement is often recommended, particularly if complete healing is not the primary goal.17,24,28,29 Autolysis uses proteolytic enzymes and phagocytic cells present in the wound bed and wound fluid to clear devitalized tissue. It is easy, inexpensive, noninvasive, and painless,4 and it requires less frequent dressing changes relative to standard dressing or wet-to-dry dressing.
Autolytic debridement is commonly accomplished using hydrocolloid and hydrogel dressings.15,29 Hydrocolloids are adhesive, occlusive, and conformable dressings that are suitable for wounds with low to moderate amounts of exudate. Upon contact with the wound surface, the dressing absorbs the exudate, forms a gel layer, and maintains a moist environment. Hydrocolloids are not recommended for infected wounds or for those with copious exudate as they may lead to maceration around the wound. A disadvantage of hydrocolloid dressings is their tendency to generate brown, often malodorous exudate when removed.
On the other hand, hydrogels in amorphous gel, dressing, sheet, or impregnated gauze form are water-based products that create a moist environment similar to hydrocolloids. Aside from causing minimal trauma to the wound bed when removed, the dressing’s cooling effect may bring some pain relief. Hydrogels are appropriate for dry wounds and for those with minimal exudate.
After debridement, the wound is cleansed and irrigated. A number of cleansers and solutions are available, but normal saline is a cheap alternative. To irrigate, experts recommend an 18- or 20-gauge intravenous catheter attached to a 30- or 60-mL syringe.15 This technique provides 8 to 15 psi of pressure, enough to cleanse the wound without causing tissue trauma.
Antibacterials and absorption
Antibacterials. Topical antibiotics have several advantages over systemic antibiotics in treating chronic wounds.30,31 These include a high and sustained concentration of the antimicrobial at the site of infection, limited potential for systemic absorption and toxicity, reduced potential for antibiotic resistance, and drawing of the patient’s and caregiver’s attention to the wound.
Metronidazole is the most widely used topical antibacterial for malodor management. Its efficacy is likely due to the predominant involvement of anaerobic bacteria in foul-smelling wounds. Topical metronidazole is available as a gel and as a cream. A systematic review showed that on average, topical metronidazole was used once daily for 14 consecutive days.19 The layer of topical metronidazole is typically covered with a nonadherent primary dressing followed by an absorbent secondary dressing.
The best clinical evidence for topical metronidazole consists of case reports and series.32–35 The largest of these studies was done by Finlay et al, who treated 47 patients with malodorous benign and malignant cutaneous wounds with 0.75% metronidazole gel daily.32 Forty-five (96%) of the patients reported significantly decreased odor by 14 days, as well as decreased pain, discharge, and surrounding cellulitis.
A randomized, placebo-controlled trial conducted by Bale et al had equivocal findings.9 All 41 patients who received metronidazole gel reported a decrease in malodor within 3 days of starting it. However, 76% of patients who received placebo also reported malodor control; in the final analysis, no significant difference was noted in the success rate between the two groups.
Metronidazole tablets can be crushed and sprinkled over the wound. As with metronidazole gel or cream, the crushed tablets are applied daily and covered by a primary nonadherent dressing and an absorbent secondary dressing. This off-label use of metronidazole serves as a cheaper alternative to commercially available topical preparations. To our knowledge, there has been no head-to-head trial comparing the two topical strategies.
Systemic metronidazole, often given orally, has been recommended if evidence of deep tissue or systemic infection is noted15 and in cases of fungating wounds with fistulas invading either the gastrointestinal or genitourinary tracts.18 Side effects such as nausea, neuropathy, and alcohol intolerance (ie, disulfiram reaction) may occur, which are not seen with topical metronidazole.
Both topical and systemic metronidazole can be used together on a time-limited basis for extensive malodorous wounds, such as fungating malignant wounds or stage IV sacral pressure ulcers.
Other antimicrobial agents used to treat malodor include silver-containing products, iodine-containing topical agents, mupirocin, bacitracin, neomycin, and polymyxin B.
Honey was used for wound care by the ancient Egyptians, and it is still used.36 Its beneficial effects include antimicrobial, debriding, deodorizing, anti-inflammatory, and granulation tissue-stimulating. Honey has even been shown to significantly decrease skin colonization with various kinds of bacteria, including methicillin-resistant Staphylococcus aureus.37 Medical-grade honey is preferred over table honey, as the latter is nonsterile and can contain Clostridium spores, which contaminate the wound.38
Yogurt and buttermilk lower the pH of the wound and control bacterial proliferation to control malodor.39,40 Either is applied for 10 to 15 minutes after the wound is cleansed and is then washed off thoroughly.
Absorbent dressings are used either over a layer of topical metronidazole and a nonadherent primary dressing or as a primary dressing itself. An absorbent dressing containing activated charcoal is used for rapid improvement, although cost may be prohibitive, especially in developing countries.13,19 Another type of absorbent dressing, composed of polyester impregnated with sodium chloride, has been found to be useful in malodor control.41 An important pointer is to maintain a tight seal around the absorbent dressing to prevent leakage of exudate.
Concealers
Aromatics used to conceal malodor include scented candles, incense, fragrant flowers and plants, and air-freshener sprays. When circumstances allow, candles are good options since they conceal malodor by emitting fragrance, and the flame burns off foul-smelling chemicals. Aromatics such as coffee beans, vanilla beans, and cider vinegar can be placed in a pan and left under the patient’s bed or close to it. Drops of peppermint oil or oil of wintergreen can be placed on wound dressings.
Other odor concealers are adsorbent materials that attract and cause ions and molecules to adhere to their surface. Examples are charcoal, baking soda, and cat litter. As with other aromatics, these materials are placed in pans and left under the bed or near the patient.
Aromatics can have disadvantages, as certain scents, especially strong ones, can be nauseating for patients. Some fragrances trigger asthma or skin irritation. Patients and caregivers can be left with an unpleasant association of certain fragrances with malodor by conditioning.15,17,18
Education and support
Concerns of the patient and family members need to be heard, addressed promptly, and reassessed with each visit, since uncontrolled malodor can be a chief source of caregiver fatigue.
Foremost in formulating a patient- and family-centered malodor management strategy is to commit to controlling malodor as much as possible. Regular follow-up appointments should be made, whether in the office or at home, to check on the patient’s progress and address new and ongoing concerns. Symptoms accompanying malodor, such as pain, bleeding, and sleep disturbance, need to be addressed, as they all affect quality of life.1 Audience-appropriate educational materials should be made available.26 Online resources that patients and families can explore include the websites of the Wound Ostomy and Continence Nurses Society (www.wocn.org) and the Association for the Advancement of Wound Care (aawconline.org).
Healthcare professionals need to be prepared to deal with problems and complications involving patients and family members that may arise in the course of treatment.12 Problems include the cost and local unavailability of dressing supplies, insurance coverage for dressings and topical agents, lack of assistance at home, and fear of changing dressings. A cardinal rule for healthcare providers is to avoid expressing distress at odors in front of or within hearing of patients and families.
OTHER STRATEGIES: WHAT ELSE CAN WE DO?
Curcumin, the main biologically active compound in the herb turmeric, applied directly to wounds three times daily as an ointment, has been shown to have odor-controlling properties.42
Sugar paste has been reported to control malodor by drawing out exudative and tissue fluid osmotically, and inhibiting bacterial growth.16,17 Water is mixed with sugar (ie, granulated, caster, or powdered) to form a paste, with additives like glycerin and polyethylene glycol used to alter the consistency. Thick clay-like paste is good for wounds with large cavities, while thin paste is useful for wounds with small or superficial openings. The paste is applied twice daily and is covered by an absorbent dressing.
Pressure relief is vital in managing pressure ulcers.18,43 Repositioning every 2 hours and using special devices, such as mattress overlays, alternating pressure mattresses, and low air loss mattresses, are frequently employed techniques.
If circumstances permit and when congruent with the patient’s goals of care, intra-arterial chemotherapy and radiotherapy can be contemplated for malignant fungating wounds.44,45
Other strategies include opening the windows during dressing changes, increasing the frequency of dressing changes, promptly removing used dressings from the house, and ensuring good general hygiene.
CASE RESOLUTION
After telling her that he was committed to control the malodor or, if possible, eliminate it, Mrs. A.’s doctor prepared two lists of materials—one for himself and one for Mrs. A.’s husband. He returned the next day, brought out his supplies, asked Mrs. A. to lie in bed, and invited her husband to assist him.
He cleansed and irrigated the breast lesion with normal saline, making sure to remove as much dead tissue as he could. He applied a layer of metronidazole cream to the wound cavity, then covered it with a nonadherent dressing. He then covered the wound with gauze, sealed the edges with medical adhesive tape, and applied a few drops of oil of wintergreen to the surface. A pan of charcoal briquettes was put under the bed, and a candle with Mrs. A.’s favorite scent was lit by the bedside. The physician then instructed Mrs. A.’s husband to repeat the procedure once daily for 1 week.
After 2 weeks, Mrs. A. and her husband said the foul odor had greatly decreased. She appeared more cheerful and energetic, especially after her grandchildren visited a few days earlier. The physician then instructed the husband to stop using metronidazole cream and to apply a hydrocolloid dressing every 3 days instead. He advised them to continue the rest of the process of applying a few drops of oil of wintergreen on the dressing surface, placing a pan of charcoal briquettes under the bed, and lighting a scented candle by the bedside.
FINISH THE RACE!
Complex nonhealing wounds are encountered across various healthcare settings. Wound malodor is an important component of nonhealing wounds, which adversely affects patients, families, and healthcare providers. Infection, slough, and exudate are the major causes of wound malodor. The essential steps to reduce malodor are to remove necrotic tissue, use antibacterial and odor-absorbing agents, apply appropriate odor “concealers,” educate families, and formulate a patient- and family-centered strategy (Table 1).
Acknowledgment: The authors would like to thank Sue Reif, CNP, for her assistance in completing the manuscript.
- Lo SF, Hayter M, Hu WY, Tai CY, Hsu MY, Li YF. Symptom burden and quality of life in patients with malignant fungating wounds. J Adv Nurs 2012; 68:1312–1321.
- Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol 1994; 130:489–493.
- Tippett AW. Wounds at the end of life. Wounds 2005; 17:91–98.
- Burt T. Palliative care of pressure ulcers in long-term care. Ann Long-Term Care 2013; 21:20–28.
- Reifsnyder J, Magee HS. Development of pressure ulcers in patients receiving home hospice care. Wounds 2005; 17:74–79.
- Haisfield-Wolfe ME, Rund C. Malignant cutaneous wounds: a management protocol. Ostomy Wound Manage 1997; 43:56–66.
- O’Brien C. Malignant wounds: managing odour. Can Fam Physician 2012; 58:272–274.
- Gethin G, Grocott P, Probst S, Clarke E. Current practice in the management of wound odour: an international survey. Int J Nurs Stud 2014; 51:865–874.
- Bale S, Tebble N, Price P. A topical metronidazole gel used to treat malodorous wounds. Br J Nurs 2004; 13:S4–S11.
- Hack A. Malodorous wounds—taking the patient’s perspective into account. J Wound Care 2003; 12:319–321.
- Price E. Wound care. The stigma of smell. Nurs Times 1996; 92:71–72.
- Paul JC, Pieper BA. Topical metronidazole for the treatment of wound odor: a review of the literature. Ostomy Wound Manage 2008; 54:18–27.
- Lee G, Anand SC, Rajendran S, Walker I. Overview of current practice and future trends in the evaluation of dressings for malodorous wounds. J Wound Care 2006; 15:344–346.
- Cutting K, Harding K. Criteria for identifying wound infection. J Wound Care 1994; 3:198–201.
- McDonald A, Lesage P. Palliative management of pressure ulcers and malignant wounds in patients with advanced illness. J Palliat Med 2006; 9:285–295.
- Holloway S. Recognising and treating the causes of chronic malodorous wounds. Prof Nurse 2004; 19:380–384.
- Haughton W, Young T. Common problems in wound care: malodorous wounds. Br J Nurs 1995; 4:959–963.
- Alvarez OM, Kalinski C, Nusbaum J, et al. Incorporating wound healing strategies to improve palliation (symptom management) in patients with chronic wounds. J Palliat Med 2007; 10:1161–1189.
- da Costa Santos CM, de Mattos Pimenta CA, Nobre MR. A systematic review of topical treatments to control the odor of malignant fungating wounds. J Pain Symptom Manage 2010; 39:1065–1076.
- Von Gruenigen VE, Coleman RL, et al. Bacteriology and treatment of malodorous lower reproductive tract in gynecologic cancer patients. Obstet Gynecol 2000; 96:23–27.
- Baker PG, Haig G. Metronidazole in the treatment of chronic pressure sores and ulcers: a comparison with standard treatment in general practice. Practitioner 1981; 225:569–573.
- Whitney J, Phillips L, Aslam R, et al. Guidelines for the treatment of pressure ulcers. Wound Repair Regen 2006; 14:663–679.
- Williams D, Enoch S, Miller D, Harris K, Price P, Harding KG. Effect of sharp debridement using curette on recalcitrant nonhealing venous ulcers: a concurrently controlled, prospective cohort study. Wound Repair Regen 2005; 13:131–137.
- Bergstrom KJ. Assessment and management of fungating wounds. J Wound Ostomy Continence Nurs 2011: 38:31–37.
- Sinclair RD, Ryan TJ. Proteolytic enzymes in wound healing: the role of enzymatic debridement. Australas J Dermatol 1994; 35:35–41.
- Enoch S, Harding KG. Wound bed preparation: the science behind the removal of barriers to healing. Wounds 2003;15:213–229.
- Mumcuoglu KY. Clinical applications for maggots in wound care. Am J Clin Dermatol 2001; 2:219–227.
- Langemo DK, Black J; National Pressure Ulcer Advisory Panel. Pressure ulcers in individuals receiving palliative care: a National Pressure Ulcer Advisory Panel white paper. Adv Skin Wound Care 2010; 23:59–72.
- Fonder MA, Lazarus GS, Cowan DA, Aronson-Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol 2008; 58:185–206.
- Lio PA, Kaye ET. Topical antibacterial agents. Infect Dis Clin North Am 2004; 18:717–733.
- Gelmetti C. Local antibiotics in dermatology. Dermatol Ther 2008; 21:187–195.
- Finlay IG, Bowszyc J, Ramlau C, Gwiezdzinski Z. The effect of topical 0.75% metronidazole gel on malodorous cutaneous ulcers. J Pain Symptom Manage 1996; 11:158–162.
- Bower M, Stein R, Evans TR, Hedley A, Pert P, Coombes RC. A double-blind study of the efficacy of metronidazole gel in the treatment of malodorous fungating tumours. Eur J Cancer 1992; 28A:888–889.
- Kalinski C, Schnepf M, Laboy D, et al. Effectiveness of a topical formulation containing metronidazole for wound odor and exudate control. Wounds 2005; 17:84–90.
- Kuge S, Tokuda Y, Ohta M, et al. Use of metronidazole gel to control malodor in advanced and recurrent breast cancer. Jpn J Clin Oncol 1996; 26:207–210.
- Belcher J. A review of medical-grade honey in wound care. Br J Nurs 2012: 21:S4–S9.
- Kwakman PH, Van den Akker JP, Güçlü A, et al. Medical-grade honey kills antibiotic-resistant bacteria in vitro and eradicates skin colonization. Clin Infect Dis 2008; 46:1677–1682.
- Cooper RA, Jenkins L. A comparison between medical grade honey and table honeys in relation to antimicrobial efficacy. Wounds 2009; 21:29–36.
- Patel B, Cox-Hayley D. Managing wound odor #218. J Palliat Med 2010; 13:1286–1287.
- Schulte MJ. Yogurt helps to control wound odor. Oncol Nurs Forum 1993; 20:1262.
- Upright CA, Salton C, Roberts F, Murphy J. Evaluation of Mesalt dressings and continuous wet saline dressings in ulcerating metastatic skin lesions. Cancer Nurs 1994; 17:149–155.
- Kuttan R, Sudheeran PC, Josph CD. Turmeric and curcumin as topical agents in cancer therapy. Tumori 1987; 73:29–31.
- Bass MJ, Phillips LG. Pressure sores. Curr Probl Surg 2007; 44:101–143.
- Bufill JA, Grace WR, Neff R. Intra-arterial chemotherapy for palliation of fungating breast cancer: a case report and review of the literature. Am J Clin Oncol 1994; 17:118–124.
- Murakami M, Kuroda Y, Sano A, et al. Validity of local treatment including intraarterial infusion chemotherapy and radiotherapy for fungating adenocarcinoma of the breast: case report of more than 8-year survival. Am J Clin Oncol 2001; 24:388–391.
Wounds that fail to heal become more than mere skin lesions. Pain, malodor, and the accompanying psychological distress often complicate nonhealing wounds and impair quality of life.1 Management of malodor requires perseverance, sensitivity, and familiarity with tools and procedures that range from surgical debridement to medical-grade honey.
Chronic, nonhealing wounds are defined as persisting for more than 6 months.2 These lesions are incapable of undergoing anatomic and functional repair on their own. Commonly encountered nonhealing wounds include pressure ulcers, venous stasis ulcers, arterial insufficiency ulcers, and malignant cutaneous wounds.
Typically, the patient with a nonhealing wound is frail, debilitated, medically complex, and often faced with one or more life-limiting illnesses. Complete wound healing may therefore be unrealistic, and optimal wound management becomes the goal of care.3,4
Healthcare providers encounter nonhealing wounds in varied settings—acute inpatient, outpatient, long-term, and home care. For instance, in the home care setting, a study of 383 patients enrolled in hospice found that 35% had skin ulcers and wounds.3 Half of those affected had pressure ulcers, 20% had ischemic ulcers, and 30% had other skin disorders such as stasis ulcers, burns, skin tears, and tumors. A larger study, also in hospice patients, found that 26% had pressure ulcers and 10% more developed them within 6 months.5
While pressure ulcers are the most common nonhealing wounds, malignant or fungating wounds are found in 5% to 10% of patients with metastatic disease, usually with cancers of the breast, head, and neck.6
Maximizing wound care provides comfort, relieves suffering, and promotes quality of life.3,7 To achieve these goals, clinicians must be familiar with strategies to manage complications associated with nonhealing wounds such as pain, malodor, and psychosocial adverse effects. Of these complications, malodor has been pointed out by both patients and caregivers as the most distressing.8
This article focuses on wound malodor, discusses the processes that cause wounds to emit an offensive smell, and outlines a comprehensive management approach.
MRS. A., AGE 61, WITH STAGE IV BREAST CANCER
Mrs. A., 61 years old, had a fungating mass in her left breast, which began as a small nodule and progressively enlarged to deform her breast over several months. Her oncologist subsequently staged the extent of her cancer as stage IV after workup revealed lung metastasis. Mrs. A. and her family decided to forgo cancer treatment, including radiotherapy, and to transition to hospice care after discussions with the oncologist.
Mrs. A. lived at home with her husband. Her daughter and three grandchildren all lived nearby.
When her hospice physician arrived at her home to meet her, a strong, pungent, and nauseating smell greeted him as he entered her bedroom. The patient said that for the past few months she had been increasingly distressed by the revolting odor. She rarely left home and had been ashamed to have people visit her, including her family.
On examination, the physician noticed a large fungating mass with yellowish discharge and necrotic tissue in her left breast. In addition to mild pain, she was immensely bothered by the strong odor coming from her breast.
THE IMPACT OF MALODOR
As seen in the case of Mrs. A., malodor has grave effects, both physical and psychological. Patients experience impaired or socially unacceptable body image, social rejection, personal shame, and embarrassment.9,10 Feelings of fear, anxiety, and depression are common. If left uncontrolled, malodor results in social isolation, reluctance to engage in social activities, diminished appetite, and nausea. In addition, malodor is a constant reminder of patients’ pain and cancer, and it results in further suffering.11
Reactions of family members and caregivers can worsen the situation.9,12 Expressions of revulsion limit contact and inhibit intimacy, especially near the end of life. Caregivers are often frustrated and distressed over their inability to control the malodor. The environment becomes uninhabitable, and the malodor can permeate clothing, furniture, and living quarters.
Managing malodor can be emotionally draining, physically daunting, and frustrating for healthcare professionals, as several methods are usually employed, often in a trial-and-error approach, to achieve an acceptable degree of odor control. In addition, clinicians must face the challenge of treating malodorous wounds at very close distance without reacting in a way that offends or alarms patients and family members.13
MALODOR PRODUCTION: WHERE IS THAT SMELL COMING FROM?
All wounds can produce an odor.14 Wounds that are expected to heal typically emit a faint but not unpleasant odor, akin to fresh blood. Wounds colonized by Pseudomonas aeruginosa produce a fruity or grapelike odor that is tolerable. Malodor occurs with wounds infected by other gram-negative organisms or anaerobic bacteria.15 Similarly, wounds covered by necrotic tissue smell like decaying flesh.
Three major causes
The three major causes of wound malodor are slough, infection, and exudate (Figure 1).
Slough is dead or necrotic tissue, usually resulting from vascular compromise. Arterial ulcers, pressure ulcers, and malignant wounds all form slough from capillary occlusion, subsequent ischemia, and tissue necrosis.
Infection. Devitalized tissue, an ideal medium in which bacteria thrive, becomes the source of infection. Anaerobic bacteria are usually implicated in malodor. These include Bacteroides fragilis, Bacteroides prevotella, Clostridium perfringens, and Fusobacterium nucleatum.16,17 Anaerobic organisms produce putrescine and cadaverine, which are largely responsible for the offensive odor.16,18 Volatile fatty acids such as propionic, butyric, isovaleric, and valeric acid are formed from lipid catabolism by anaerobes and add to malodor.17 Aerobic bacteria such as Proteus, Klebsiella, and Pseudomonas species supercolonize necrotic tissue as well and contribute to malodor.17,18
Exudate. Since nonhealing wounds undergo repeated cycles of inflammation, infection, and necrosis, accumulation of exudate becomes inevitable. Exudate typically is a pus-like fluid containing serum, fibrin, and white blood cells, which leak from blood vessels. In addition, bacteria that colonize chronic wounds filled with necrotic tissue activate proteases that degrade and liquefy dead tissue, thereby forming extensive amounts of exudate.19
Apart from slough, infection, and exudate, poor general hygiene and dressings left on for too long may contribute to malodor.16 Moisture-retentive dressings such as hydrocolloids leave an odor after removal. Dressings that liquefy upon contact with the wound surface leave a pus-like, potentially malodorous material.
MALODOR ASSESSMENT: DO YOU SMELL SOMETHING?
Various ways to document wound malodor can prove useful in guiding assessment and treatment. Descriptions such as “foul,” “putrid,” “fishy,” or “filled the room” vividly portray the initial presentation. A 10-point numerical scale similar to a numerical pain scale or a visual analogue scale can be used as a subjective measure.
Other grading methods, which to the authors’ knowledge are not validated, may be helpful. In a study that focused on patients suffering from malodorous gynecologic malignancies, von Gruenigen et al20 used a 0-to-3 scale:
- 0 Absent
- 1 Not offensive
- 2 Offensive but tolerable
- 3 Offensive and intolerable.
A scale often adapted by other authors was devised by Baker and Haig,21 which clearly defines four classes:
- 1 Strong—odor is evident upon entering the room (6 to 10 feet from the patient) with the dressing intact
- 2 Moderate—odor is evident upon entering the room with dressing removed
- 3 Slight—odor is evident at close proximity to the patient when the dressing is removed
- 4 No odor—no odor is evident, even at the patient’s bedside with the dressing removed.
COMPREHENSIVE MANAGEMENT: HOW DO WE WIN THE ‘RACE’?
The acronym RACE outlines an approach to dealing with malodor. It stands for removal of necrotic tissue; antibacterials; odor concealers; and education and support (Table 1).
Remove necrotic tissue
An important step in eliminating malodor is to remove necrotic tissue. This starts with debridement, which decreases the incidence of infection and hastens wound closure.22,23 Table 2 compares the different types of debridement.
Sharp or surgical debridement involves the use of a scalpel or scissors. This type of debridement may increase the risk of bleeding, pain, and malignant cell seeding in fungating wounds.4,24
Enzymatic debridement employs chemicals with proteolytic action (eg, collagenase) to digest extracellular proteins in wounds.18,25
Mechanical debridement involves aggressive therapies such as forceful irrigation and hydrotherapy, which may fail to discriminate between necrotic and viable tissues.18,26
Biological debridement using maggots, which ingest bacteria and devitalized tissue, may cause increased wound bleeding and may be unacceptable for patients and families.24,27
Autolytic debridement is often recommended, particularly if complete healing is not the primary goal.17,24,28,29 Autolysis uses proteolytic enzymes and phagocytic cells present in the wound bed and wound fluid to clear devitalized tissue. It is easy, inexpensive, noninvasive, and painless,4 and it requires less frequent dressing changes relative to standard dressing or wet-to-dry dressing.
Autolytic debridement is commonly accomplished using hydrocolloid and hydrogel dressings.15,29 Hydrocolloids are adhesive, occlusive, and conformable dressings that are suitable for wounds with low to moderate amounts of exudate. Upon contact with the wound surface, the dressing absorbs the exudate, forms a gel layer, and maintains a moist environment. Hydrocolloids are not recommended for infected wounds or for those with copious exudate as they may lead to maceration around the wound. A disadvantage of hydrocolloid dressings is their tendency to generate brown, often malodorous exudate when removed.
On the other hand, hydrogels in amorphous gel, dressing, sheet, or impregnated gauze form are water-based products that create a moist environment similar to hydrocolloids. Aside from causing minimal trauma to the wound bed when removed, the dressing’s cooling effect may bring some pain relief. Hydrogels are appropriate for dry wounds and for those with minimal exudate.
After debridement, the wound is cleansed and irrigated. A number of cleansers and solutions are available, but normal saline is a cheap alternative. To irrigate, experts recommend an 18- or 20-gauge intravenous catheter attached to a 30- or 60-mL syringe.15 This technique provides 8 to 15 psi of pressure, enough to cleanse the wound without causing tissue trauma.
Antibacterials and absorption
Antibacterials. Topical antibiotics have several advantages over systemic antibiotics in treating chronic wounds.30,31 These include a high and sustained concentration of the antimicrobial at the site of infection, limited potential for systemic absorption and toxicity, reduced potential for antibiotic resistance, and drawing of the patient’s and caregiver’s attention to the wound.
Metronidazole is the most widely used topical antibacterial for malodor management. Its efficacy is likely due to the predominant involvement of anaerobic bacteria in foul-smelling wounds. Topical metronidazole is available as a gel and as a cream. A systematic review showed that on average, topical metronidazole was used once daily for 14 consecutive days.19 The layer of topical metronidazole is typically covered with a nonadherent primary dressing followed by an absorbent secondary dressing.
The best clinical evidence for topical metronidazole consists of case reports and series.32–35 The largest of these studies was done by Finlay et al, who treated 47 patients with malodorous benign and malignant cutaneous wounds with 0.75% metronidazole gel daily.32 Forty-five (96%) of the patients reported significantly decreased odor by 14 days, as well as decreased pain, discharge, and surrounding cellulitis.
A randomized, placebo-controlled trial conducted by Bale et al had equivocal findings.9 All 41 patients who received metronidazole gel reported a decrease in malodor within 3 days of starting it. However, 76% of patients who received placebo also reported malodor control; in the final analysis, no significant difference was noted in the success rate between the two groups.
Metronidazole tablets can be crushed and sprinkled over the wound. As with metronidazole gel or cream, the crushed tablets are applied daily and covered by a primary nonadherent dressing and an absorbent secondary dressing. This off-label use of metronidazole serves as a cheaper alternative to commercially available topical preparations. To our knowledge, there has been no head-to-head trial comparing the two topical strategies.
Systemic metronidazole, often given orally, has been recommended if evidence of deep tissue or systemic infection is noted15 and in cases of fungating wounds with fistulas invading either the gastrointestinal or genitourinary tracts.18 Side effects such as nausea, neuropathy, and alcohol intolerance (ie, disulfiram reaction) may occur, which are not seen with topical metronidazole.
Both topical and systemic metronidazole can be used together on a time-limited basis for extensive malodorous wounds, such as fungating malignant wounds or stage IV sacral pressure ulcers.
Other antimicrobial agents used to treat malodor include silver-containing products, iodine-containing topical agents, mupirocin, bacitracin, neomycin, and polymyxin B.
Honey was used for wound care by the ancient Egyptians, and it is still used.36 Its beneficial effects include antimicrobial, debriding, deodorizing, anti-inflammatory, and granulation tissue-stimulating. Honey has even been shown to significantly decrease skin colonization with various kinds of bacteria, including methicillin-resistant Staphylococcus aureus.37 Medical-grade honey is preferred over table honey, as the latter is nonsterile and can contain Clostridium spores, which contaminate the wound.38
Yogurt and buttermilk lower the pH of the wound and control bacterial proliferation to control malodor.39,40 Either is applied for 10 to 15 minutes after the wound is cleansed and is then washed off thoroughly.
Absorbent dressings are used either over a layer of topical metronidazole and a nonadherent primary dressing or as a primary dressing itself. An absorbent dressing containing activated charcoal is used for rapid improvement, although cost may be prohibitive, especially in developing countries.13,19 Another type of absorbent dressing, composed of polyester impregnated with sodium chloride, has been found to be useful in malodor control.41 An important pointer is to maintain a tight seal around the absorbent dressing to prevent leakage of exudate.
Concealers
Aromatics used to conceal malodor include scented candles, incense, fragrant flowers and plants, and air-freshener sprays. When circumstances allow, candles are good options since they conceal malodor by emitting fragrance, and the flame burns off foul-smelling chemicals. Aromatics such as coffee beans, vanilla beans, and cider vinegar can be placed in a pan and left under the patient’s bed or close to it. Drops of peppermint oil or oil of wintergreen can be placed on wound dressings.
Other odor concealers are adsorbent materials that attract and cause ions and molecules to adhere to their surface. Examples are charcoal, baking soda, and cat litter. As with other aromatics, these materials are placed in pans and left under the bed or near the patient.
Aromatics can have disadvantages, as certain scents, especially strong ones, can be nauseating for patients. Some fragrances trigger asthma or skin irritation. Patients and caregivers can be left with an unpleasant association of certain fragrances with malodor by conditioning.15,17,18
Education and support
Concerns of the patient and family members need to be heard, addressed promptly, and reassessed with each visit, since uncontrolled malodor can be a chief source of caregiver fatigue.
Foremost in formulating a patient- and family-centered malodor management strategy is to commit to controlling malodor as much as possible. Regular follow-up appointments should be made, whether in the office or at home, to check on the patient’s progress and address new and ongoing concerns. Symptoms accompanying malodor, such as pain, bleeding, and sleep disturbance, need to be addressed, as they all affect quality of life.1 Audience-appropriate educational materials should be made available.26 Online resources that patients and families can explore include the websites of the Wound Ostomy and Continence Nurses Society (www.wocn.org) and the Association for the Advancement of Wound Care (aawconline.org).
Healthcare professionals need to be prepared to deal with problems and complications involving patients and family members that may arise in the course of treatment.12 Problems include the cost and local unavailability of dressing supplies, insurance coverage for dressings and topical agents, lack of assistance at home, and fear of changing dressings. A cardinal rule for healthcare providers is to avoid expressing distress at odors in front of or within hearing of patients and families.
OTHER STRATEGIES: WHAT ELSE CAN WE DO?
Curcumin, the main biologically active compound in the herb turmeric, applied directly to wounds three times daily as an ointment, has been shown to have odor-controlling properties.42
Sugar paste has been reported to control malodor by drawing out exudative and tissue fluid osmotically, and inhibiting bacterial growth.16,17 Water is mixed with sugar (ie, granulated, caster, or powdered) to form a paste, with additives like glycerin and polyethylene glycol used to alter the consistency. Thick clay-like paste is good for wounds with large cavities, while thin paste is useful for wounds with small or superficial openings. The paste is applied twice daily and is covered by an absorbent dressing.
Pressure relief is vital in managing pressure ulcers.18,43 Repositioning every 2 hours and using special devices, such as mattress overlays, alternating pressure mattresses, and low air loss mattresses, are frequently employed techniques.
If circumstances permit and when congruent with the patient’s goals of care, intra-arterial chemotherapy and radiotherapy can be contemplated for malignant fungating wounds.44,45
Other strategies include opening the windows during dressing changes, increasing the frequency of dressing changes, promptly removing used dressings from the house, and ensuring good general hygiene.
CASE RESOLUTION
After telling her that he was committed to control the malodor or, if possible, eliminate it, Mrs. A.’s doctor prepared two lists of materials—one for himself and one for Mrs. A.’s husband. He returned the next day, brought out his supplies, asked Mrs. A. to lie in bed, and invited her husband to assist him.
He cleansed and irrigated the breast lesion with normal saline, making sure to remove as much dead tissue as he could. He applied a layer of metronidazole cream to the wound cavity, then covered it with a nonadherent dressing. He then covered the wound with gauze, sealed the edges with medical adhesive tape, and applied a few drops of oil of wintergreen to the surface. A pan of charcoal briquettes was put under the bed, and a candle with Mrs. A.’s favorite scent was lit by the bedside. The physician then instructed Mrs. A.’s husband to repeat the procedure once daily for 1 week.
After 2 weeks, Mrs. A. and her husband said the foul odor had greatly decreased. She appeared more cheerful and energetic, especially after her grandchildren visited a few days earlier. The physician then instructed the husband to stop using metronidazole cream and to apply a hydrocolloid dressing every 3 days instead. He advised them to continue the rest of the process of applying a few drops of oil of wintergreen on the dressing surface, placing a pan of charcoal briquettes under the bed, and lighting a scented candle by the bedside.
FINISH THE RACE!
Complex nonhealing wounds are encountered across various healthcare settings. Wound malodor is an important component of nonhealing wounds, which adversely affects patients, families, and healthcare providers. Infection, slough, and exudate are the major causes of wound malodor. The essential steps to reduce malodor are to remove necrotic tissue, use antibacterial and odor-absorbing agents, apply appropriate odor “concealers,” educate families, and formulate a patient- and family-centered strategy (Table 1).
Acknowledgment: The authors would like to thank Sue Reif, CNP, for her assistance in completing the manuscript.
Wounds that fail to heal become more than mere skin lesions. Pain, malodor, and the accompanying psychological distress often complicate nonhealing wounds and impair quality of life.1 Management of malodor requires perseverance, sensitivity, and familiarity with tools and procedures that range from surgical debridement to medical-grade honey.
Chronic, nonhealing wounds are defined as persisting for more than 6 months.2 These lesions are incapable of undergoing anatomic and functional repair on their own. Commonly encountered nonhealing wounds include pressure ulcers, venous stasis ulcers, arterial insufficiency ulcers, and malignant cutaneous wounds.
Typically, the patient with a nonhealing wound is frail, debilitated, medically complex, and often faced with one or more life-limiting illnesses. Complete wound healing may therefore be unrealistic, and optimal wound management becomes the goal of care.3,4
Healthcare providers encounter nonhealing wounds in varied settings—acute inpatient, outpatient, long-term, and home care. For instance, in the home care setting, a study of 383 patients enrolled in hospice found that 35% had skin ulcers and wounds.3 Half of those affected had pressure ulcers, 20% had ischemic ulcers, and 30% had other skin disorders such as stasis ulcers, burns, skin tears, and tumors. A larger study, also in hospice patients, found that 26% had pressure ulcers and 10% more developed them within 6 months.5
While pressure ulcers are the most common nonhealing wounds, malignant or fungating wounds are found in 5% to 10% of patients with metastatic disease, usually with cancers of the breast, head, and neck.6
Maximizing wound care provides comfort, relieves suffering, and promotes quality of life.3,7 To achieve these goals, clinicians must be familiar with strategies to manage complications associated with nonhealing wounds such as pain, malodor, and psychosocial adverse effects. Of these complications, malodor has been pointed out by both patients and caregivers as the most distressing.8
This article focuses on wound malodor, discusses the processes that cause wounds to emit an offensive smell, and outlines a comprehensive management approach.
MRS. A., AGE 61, WITH STAGE IV BREAST CANCER
Mrs. A., 61 years old, had a fungating mass in her left breast, which began as a small nodule and progressively enlarged to deform her breast over several months. Her oncologist subsequently staged the extent of her cancer as stage IV after workup revealed lung metastasis. Mrs. A. and her family decided to forgo cancer treatment, including radiotherapy, and to transition to hospice care after discussions with the oncologist.
Mrs. A. lived at home with her husband. Her daughter and three grandchildren all lived nearby.
When her hospice physician arrived at her home to meet her, a strong, pungent, and nauseating smell greeted him as he entered her bedroom. The patient said that for the past few months she had been increasingly distressed by the revolting odor. She rarely left home and had been ashamed to have people visit her, including her family.
On examination, the physician noticed a large fungating mass with yellowish discharge and necrotic tissue in her left breast. In addition to mild pain, she was immensely bothered by the strong odor coming from her breast.
THE IMPACT OF MALODOR
As seen in the case of Mrs. A., malodor has grave effects, both physical and psychological. Patients experience impaired or socially unacceptable body image, social rejection, personal shame, and embarrassment.9,10 Feelings of fear, anxiety, and depression are common. If left uncontrolled, malodor results in social isolation, reluctance to engage in social activities, diminished appetite, and nausea. In addition, malodor is a constant reminder of patients’ pain and cancer, and it results in further suffering.11
Reactions of family members and caregivers can worsen the situation.9,12 Expressions of revulsion limit contact and inhibit intimacy, especially near the end of life. Caregivers are often frustrated and distressed over their inability to control the malodor. The environment becomes uninhabitable, and the malodor can permeate clothing, furniture, and living quarters.
Managing malodor can be emotionally draining, physically daunting, and frustrating for healthcare professionals, as several methods are usually employed, often in a trial-and-error approach, to achieve an acceptable degree of odor control. In addition, clinicians must face the challenge of treating malodorous wounds at very close distance without reacting in a way that offends or alarms patients and family members.13
MALODOR PRODUCTION: WHERE IS THAT SMELL COMING FROM?
All wounds can produce an odor.14 Wounds that are expected to heal typically emit a faint but not unpleasant odor, akin to fresh blood. Wounds colonized by Pseudomonas aeruginosa produce a fruity or grapelike odor that is tolerable. Malodor occurs with wounds infected by other gram-negative organisms or anaerobic bacteria.15 Similarly, wounds covered by necrotic tissue smell like decaying flesh.
Three major causes
The three major causes of wound malodor are slough, infection, and exudate (Figure 1).
Slough is dead or necrotic tissue, usually resulting from vascular compromise. Arterial ulcers, pressure ulcers, and malignant wounds all form slough from capillary occlusion, subsequent ischemia, and tissue necrosis.
Infection. Devitalized tissue, an ideal medium in which bacteria thrive, becomes the source of infection. Anaerobic bacteria are usually implicated in malodor. These include Bacteroides fragilis, Bacteroides prevotella, Clostridium perfringens, and Fusobacterium nucleatum.16,17 Anaerobic organisms produce putrescine and cadaverine, which are largely responsible for the offensive odor.16,18 Volatile fatty acids such as propionic, butyric, isovaleric, and valeric acid are formed from lipid catabolism by anaerobes and add to malodor.17 Aerobic bacteria such as Proteus, Klebsiella, and Pseudomonas species supercolonize necrotic tissue as well and contribute to malodor.17,18
Exudate. Since nonhealing wounds undergo repeated cycles of inflammation, infection, and necrosis, accumulation of exudate becomes inevitable. Exudate typically is a pus-like fluid containing serum, fibrin, and white blood cells, which leak from blood vessels. In addition, bacteria that colonize chronic wounds filled with necrotic tissue activate proteases that degrade and liquefy dead tissue, thereby forming extensive amounts of exudate.19
Apart from slough, infection, and exudate, poor general hygiene and dressings left on for too long may contribute to malodor.16 Moisture-retentive dressings such as hydrocolloids leave an odor after removal. Dressings that liquefy upon contact with the wound surface leave a pus-like, potentially malodorous material.
MALODOR ASSESSMENT: DO YOU SMELL SOMETHING?
Various ways to document wound malodor can prove useful in guiding assessment and treatment. Descriptions such as “foul,” “putrid,” “fishy,” or “filled the room” vividly portray the initial presentation. A 10-point numerical scale similar to a numerical pain scale or a visual analogue scale can be used as a subjective measure.
Other grading methods, which to the authors’ knowledge are not validated, may be helpful. In a study that focused on patients suffering from malodorous gynecologic malignancies, von Gruenigen et al20 used a 0-to-3 scale:
- 0 Absent
- 1 Not offensive
- 2 Offensive but tolerable
- 3 Offensive and intolerable.
A scale often adapted by other authors was devised by Baker and Haig,21 which clearly defines four classes:
- 1 Strong—odor is evident upon entering the room (6 to 10 feet from the patient) with the dressing intact
- 2 Moderate—odor is evident upon entering the room with dressing removed
- 3 Slight—odor is evident at close proximity to the patient when the dressing is removed
- 4 No odor—no odor is evident, even at the patient’s bedside with the dressing removed.
COMPREHENSIVE MANAGEMENT: HOW DO WE WIN THE ‘RACE’?
The acronym RACE outlines an approach to dealing with malodor. It stands for removal of necrotic tissue; antibacterials; odor concealers; and education and support (Table 1).
Remove necrotic tissue
An important step in eliminating malodor is to remove necrotic tissue. This starts with debridement, which decreases the incidence of infection and hastens wound closure.22,23 Table 2 compares the different types of debridement.
Sharp or surgical debridement involves the use of a scalpel or scissors. This type of debridement may increase the risk of bleeding, pain, and malignant cell seeding in fungating wounds.4,24
Enzymatic debridement employs chemicals with proteolytic action (eg, collagenase) to digest extracellular proteins in wounds.18,25
Mechanical debridement involves aggressive therapies such as forceful irrigation and hydrotherapy, which may fail to discriminate between necrotic and viable tissues.18,26
Biological debridement using maggots, which ingest bacteria and devitalized tissue, may cause increased wound bleeding and may be unacceptable for patients and families.24,27
Autolytic debridement is often recommended, particularly if complete healing is not the primary goal.17,24,28,29 Autolysis uses proteolytic enzymes and phagocytic cells present in the wound bed and wound fluid to clear devitalized tissue. It is easy, inexpensive, noninvasive, and painless,4 and it requires less frequent dressing changes relative to standard dressing or wet-to-dry dressing.
Autolytic debridement is commonly accomplished using hydrocolloid and hydrogel dressings.15,29 Hydrocolloids are adhesive, occlusive, and conformable dressings that are suitable for wounds with low to moderate amounts of exudate. Upon contact with the wound surface, the dressing absorbs the exudate, forms a gel layer, and maintains a moist environment. Hydrocolloids are not recommended for infected wounds or for those with copious exudate as they may lead to maceration around the wound. A disadvantage of hydrocolloid dressings is their tendency to generate brown, often malodorous exudate when removed.
On the other hand, hydrogels in amorphous gel, dressing, sheet, or impregnated gauze form are water-based products that create a moist environment similar to hydrocolloids. Aside from causing minimal trauma to the wound bed when removed, the dressing’s cooling effect may bring some pain relief. Hydrogels are appropriate for dry wounds and for those with minimal exudate.
After debridement, the wound is cleansed and irrigated. A number of cleansers and solutions are available, but normal saline is a cheap alternative. To irrigate, experts recommend an 18- or 20-gauge intravenous catheter attached to a 30- or 60-mL syringe.15 This technique provides 8 to 15 psi of pressure, enough to cleanse the wound without causing tissue trauma.
Antibacterials and absorption
Antibacterials. Topical antibiotics have several advantages over systemic antibiotics in treating chronic wounds.30,31 These include a high and sustained concentration of the antimicrobial at the site of infection, limited potential for systemic absorption and toxicity, reduced potential for antibiotic resistance, and drawing of the patient’s and caregiver’s attention to the wound.
Metronidazole is the most widely used topical antibacterial for malodor management. Its efficacy is likely due to the predominant involvement of anaerobic bacteria in foul-smelling wounds. Topical metronidazole is available as a gel and as a cream. A systematic review showed that on average, topical metronidazole was used once daily for 14 consecutive days.19 The layer of topical metronidazole is typically covered with a nonadherent primary dressing followed by an absorbent secondary dressing.
The best clinical evidence for topical metronidazole consists of case reports and series.32–35 The largest of these studies was done by Finlay et al, who treated 47 patients with malodorous benign and malignant cutaneous wounds with 0.75% metronidazole gel daily.32 Forty-five (96%) of the patients reported significantly decreased odor by 14 days, as well as decreased pain, discharge, and surrounding cellulitis.
A randomized, placebo-controlled trial conducted by Bale et al had equivocal findings.9 All 41 patients who received metronidazole gel reported a decrease in malodor within 3 days of starting it. However, 76% of patients who received placebo also reported malodor control; in the final analysis, no significant difference was noted in the success rate between the two groups.
Metronidazole tablets can be crushed and sprinkled over the wound. As with metronidazole gel or cream, the crushed tablets are applied daily and covered by a primary nonadherent dressing and an absorbent secondary dressing. This off-label use of metronidazole serves as a cheaper alternative to commercially available topical preparations. To our knowledge, there has been no head-to-head trial comparing the two topical strategies.
Systemic metronidazole, often given orally, has been recommended if evidence of deep tissue or systemic infection is noted15 and in cases of fungating wounds with fistulas invading either the gastrointestinal or genitourinary tracts.18 Side effects such as nausea, neuropathy, and alcohol intolerance (ie, disulfiram reaction) may occur, which are not seen with topical metronidazole.
Both topical and systemic metronidazole can be used together on a time-limited basis for extensive malodorous wounds, such as fungating malignant wounds or stage IV sacral pressure ulcers.
Other antimicrobial agents used to treat malodor include silver-containing products, iodine-containing topical agents, mupirocin, bacitracin, neomycin, and polymyxin B.
Honey was used for wound care by the ancient Egyptians, and it is still used.36 Its beneficial effects include antimicrobial, debriding, deodorizing, anti-inflammatory, and granulation tissue-stimulating. Honey has even been shown to significantly decrease skin colonization with various kinds of bacteria, including methicillin-resistant Staphylococcus aureus.37 Medical-grade honey is preferred over table honey, as the latter is nonsterile and can contain Clostridium spores, which contaminate the wound.38
Yogurt and buttermilk lower the pH of the wound and control bacterial proliferation to control malodor.39,40 Either is applied for 10 to 15 minutes after the wound is cleansed and is then washed off thoroughly.
Absorbent dressings are used either over a layer of topical metronidazole and a nonadherent primary dressing or as a primary dressing itself. An absorbent dressing containing activated charcoal is used for rapid improvement, although cost may be prohibitive, especially in developing countries.13,19 Another type of absorbent dressing, composed of polyester impregnated with sodium chloride, has been found to be useful in malodor control.41 An important pointer is to maintain a tight seal around the absorbent dressing to prevent leakage of exudate.
Concealers
Aromatics used to conceal malodor include scented candles, incense, fragrant flowers and plants, and air-freshener sprays. When circumstances allow, candles are good options since they conceal malodor by emitting fragrance, and the flame burns off foul-smelling chemicals. Aromatics such as coffee beans, vanilla beans, and cider vinegar can be placed in a pan and left under the patient’s bed or close to it. Drops of peppermint oil or oil of wintergreen can be placed on wound dressings.
Other odor concealers are adsorbent materials that attract and cause ions and molecules to adhere to their surface. Examples are charcoal, baking soda, and cat litter. As with other aromatics, these materials are placed in pans and left under the bed or near the patient.
Aromatics can have disadvantages, as certain scents, especially strong ones, can be nauseating for patients. Some fragrances trigger asthma or skin irritation. Patients and caregivers can be left with an unpleasant association of certain fragrances with malodor by conditioning.15,17,18
Education and support
Concerns of the patient and family members need to be heard, addressed promptly, and reassessed with each visit, since uncontrolled malodor can be a chief source of caregiver fatigue.
Foremost in formulating a patient- and family-centered malodor management strategy is to commit to controlling malodor as much as possible. Regular follow-up appointments should be made, whether in the office or at home, to check on the patient’s progress and address new and ongoing concerns. Symptoms accompanying malodor, such as pain, bleeding, and sleep disturbance, need to be addressed, as they all affect quality of life.1 Audience-appropriate educational materials should be made available.26 Online resources that patients and families can explore include the websites of the Wound Ostomy and Continence Nurses Society (www.wocn.org) and the Association for the Advancement of Wound Care (aawconline.org).
Healthcare professionals need to be prepared to deal with problems and complications involving patients and family members that may arise in the course of treatment.12 Problems include the cost and local unavailability of dressing supplies, insurance coverage for dressings and topical agents, lack of assistance at home, and fear of changing dressings. A cardinal rule for healthcare providers is to avoid expressing distress at odors in front of or within hearing of patients and families.
OTHER STRATEGIES: WHAT ELSE CAN WE DO?
Curcumin, the main biologically active compound in the herb turmeric, applied directly to wounds three times daily as an ointment, has been shown to have odor-controlling properties.42
Sugar paste has been reported to control malodor by drawing out exudative and tissue fluid osmotically, and inhibiting bacterial growth.16,17 Water is mixed with sugar (ie, granulated, caster, or powdered) to form a paste, with additives like glycerin and polyethylene glycol used to alter the consistency. Thick clay-like paste is good for wounds with large cavities, while thin paste is useful for wounds with small or superficial openings. The paste is applied twice daily and is covered by an absorbent dressing.
Pressure relief is vital in managing pressure ulcers.18,43 Repositioning every 2 hours and using special devices, such as mattress overlays, alternating pressure mattresses, and low air loss mattresses, are frequently employed techniques.
If circumstances permit and when congruent with the patient’s goals of care, intra-arterial chemotherapy and radiotherapy can be contemplated for malignant fungating wounds.44,45
Other strategies include opening the windows during dressing changes, increasing the frequency of dressing changes, promptly removing used dressings from the house, and ensuring good general hygiene.
CASE RESOLUTION
After telling her that he was committed to control the malodor or, if possible, eliminate it, Mrs. A.’s doctor prepared two lists of materials—one for himself and one for Mrs. A.’s husband. He returned the next day, brought out his supplies, asked Mrs. A. to lie in bed, and invited her husband to assist him.
He cleansed and irrigated the breast lesion with normal saline, making sure to remove as much dead tissue as he could. He applied a layer of metronidazole cream to the wound cavity, then covered it with a nonadherent dressing. He then covered the wound with gauze, sealed the edges with medical adhesive tape, and applied a few drops of oil of wintergreen to the surface. A pan of charcoal briquettes was put under the bed, and a candle with Mrs. A.’s favorite scent was lit by the bedside. The physician then instructed Mrs. A.’s husband to repeat the procedure once daily for 1 week.
After 2 weeks, Mrs. A. and her husband said the foul odor had greatly decreased. She appeared more cheerful and energetic, especially after her grandchildren visited a few days earlier. The physician then instructed the husband to stop using metronidazole cream and to apply a hydrocolloid dressing every 3 days instead. He advised them to continue the rest of the process of applying a few drops of oil of wintergreen on the dressing surface, placing a pan of charcoal briquettes under the bed, and lighting a scented candle by the bedside.
FINISH THE RACE!
Complex nonhealing wounds are encountered across various healthcare settings. Wound malodor is an important component of nonhealing wounds, which adversely affects patients, families, and healthcare providers. Infection, slough, and exudate are the major causes of wound malodor. The essential steps to reduce malodor are to remove necrotic tissue, use antibacterial and odor-absorbing agents, apply appropriate odor “concealers,” educate families, and formulate a patient- and family-centered strategy (Table 1).
Acknowledgment: The authors would like to thank Sue Reif, CNP, for her assistance in completing the manuscript.
- Lo SF, Hayter M, Hu WY, Tai CY, Hsu MY, Li YF. Symptom burden and quality of life in patients with malignant fungating wounds. J Adv Nurs 2012; 68:1312–1321.
- Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol 1994; 130:489–493.
- Tippett AW. Wounds at the end of life. Wounds 2005; 17:91–98.
- Burt T. Palliative care of pressure ulcers in long-term care. Ann Long-Term Care 2013; 21:20–28.
- Reifsnyder J, Magee HS. Development of pressure ulcers in patients receiving home hospice care. Wounds 2005; 17:74–79.
- Haisfield-Wolfe ME, Rund C. Malignant cutaneous wounds: a management protocol. Ostomy Wound Manage 1997; 43:56–66.
- O’Brien C. Malignant wounds: managing odour. Can Fam Physician 2012; 58:272–274.
- Gethin G, Grocott P, Probst S, Clarke E. Current practice in the management of wound odour: an international survey. Int J Nurs Stud 2014; 51:865–874.
- Bale S, Tebble N, Price P. A topical metronidazole gel used to treat malodorous wounds. Br J Nurs 2004; 13:S4–S11.
- Hack A. Malodorous wounds—taking the patient’s perspective into account. J Wound Care 2003; 12:319–321.
- Price E. Wound care. The stigma of smell. Nurs Times 1996; 92:71–72.
- Paul JC, Pieper BA. Topical metronidazole for the treatment of wound odor: a review of the literature. Ostomy Wound Manage 2008; 54:18–27.
- Lee G, Anand SC, Rajendran S, Walker I. Overview of current practice and future trends in the evaluation of dressings for malodorous wounds. J Wound Care 2006; 15:344–346.
- Cutting K, Harding K. Criteria for identifying wound infection. J Wound Care 1994; 3:198–201.
- McDonald A, Lesage P. Palliative management of pressure ulcers and malignant wounds in patients with advanced illness. J Palliat Med 2006; 9:285–295.
- Holloway S. Recognising and treating the causes of chronic malodorous wounds. Prof Nurse 2004; 19:380–384.
- Haughton W, Young T. Common problems in wound care: malodorous wounds. Br J Nurs 1995; 4:959–963.
- Alvarez OM, Kalinski C, Nusbaum J, et al. Incorporating wound healing strategies to improve palliation (symptom management) in patients with chronic wounds. J Palliat Med 2007; 10:1161–1189.
- da Costa Santos CM, de Mattos Pimenta CA, Nobre MR. A systematic review of topical treatments to control the odor of malignant fungating wounds. J Pain Symptom Manage 2010; 39:1065–1076.
- Von Gruenigen VE, Coleman RL, et al. Bacteriology and treatment of malodorous lower reproductive tract in gynecologic cancer patients. Obstet Gynecol 2000; 96:23–27.
- Baker PG, Haig G. Metronidazole in the treatment of chronic pressure sores and ulcers: a comparison with standard treatment in general practice. Practitioner 1981; 225:569–573.
- Whitney J, Phillips L, Aslam R, et al. Guidelines for the treatment of pressure ulcers. Wound Repair Regen 2006; 14:663–679.
- Williams D, Enoch S, Miller D, Harris K, Price P, Harding KG. Effect of sharp debridement using curette on recalcitrant nonhealing venous ulcers: a concurrently controlled, prospective cohort study. Wound Repair Regen 2005; 13:131–137.
- Bergstrom KJ. Assessment and management of fungating wounds. J Wound Ostomy Continence Nurs 2011: 38:31–37.
- Sinclair RD, Ryan TJ. Proteolytic enzymes in wound healing: the role of enzymatic debridement. Australas J Dermatol 1994; 35:35–41.
- Enoch S, Harding KG. Wound bed preparation: the science behind the removal of barriers to healing. Wounds 2003;15:213–229.
- Mumcuoglu KY. Clinical applications for maggots in wound care. Am J Clin Dermatol 2001; 2:219–227.
- Langemo DK, Black J; National Pressure Ulcer Advisory Panel. Pressure ulcers in individuals receiving palliative care: a National Pressure Ulcer Advisory Panel white paper. Adv Skin Wound Care 2010; 23:59–72.
- Fonder MA, Lazarus GS, Cowan DA, Aronson-Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol 2008; 58:185–206.
- Lio PA, Kaye ET. Topical antibacterial agents. Infect Dis Clin North Am 2004; 18:717–733.
- Gelmetti C. Local antibiotics in dermatology. Dermatol Ther 2008; 21:187–195.
- Finlay IG, Bowszyc J, Ramlau C, Gwiezdzinski Z. The effect of topical 0.75% metronidazole gel on malodorous cutaneous ulcers. J Pain Symptom Manage 1996; 11:158–162.
- Bower M, Stein R, Evans TR, Hedley A, Pert P, Coombes RC. A double-blind study of the efficacy of metronidazole gel in the treatment of malodorous fungating tumours. Eur J Cancer 1992; 28A:888–889.
- Kalinski C, Schnepf M, Laboy D, et al. Effectiveness of a topical formulation containing metronidazole for wound odor and exudate control. Wounds 2005; 17:84–90.
- Kuge S, Tokuda Y, Ohta M, et al. Use of metronidazole gel to control malodor in advanced and recurrent breast cancer. Jpn J Clin Oncol 1996; 26:207–210.
- Belcher J. A review of medical-grade honey in wound care. Br J Nurs 2012: 21:S4–S9.
- Kwakman PH, Van den Akker JP, Güçlü A, et al. Medical-grade honey kills antibiotic-resistant bacteria in vitro and eradicates skin colonization. Clin Infect Dis 2008; 46:1677–1682.
- Cooper RA, Jenkins L. A comparison between medical grade honey and table honeys in relation to antimicrobial efficacy. Wounds 2009; 21:29–36.
- Patel B, Cox-Hayley D. Managing wound odor #218. J Palliat Med 2010; 13:1286–1287.
- Schulte MJ. Yogurt helps to control wound odor. Oncol Nurs Forum 1993; 20:1262.
- Upright CA, Salton C, Roberts F, Murphy J. Evaluation of Mesalt dressings and continuous wet saline dressings in ulcerating metastatic skin lesions. Cancer Nurs 1994; 17:149–155.
- Kuttan R, Sudheeran PC, Josph CD. Turmeric and curcumin as topical agents in cancer therapy. Tumori 1987; 73:29–31.
- Bass MJ, Phillips LG. Pressure sores. Curr Probl Surg 2007; 44:101–143.
- Bufill JA, Grace WR, Neff R. Intra-arterial chemotherapy for palliation of fungating breast cancer: a case report and review of the literature. Am J Clin Oncol 1994; 17:118–124.
- Murakami M, Kuroda Y, Sano A, et al. Validity of local treatment including intraarterial infusion chemotherapy and radiotherapy for fungating adenocarcinoma of the breast: case report of more than 8-year survival. Am J Clin Oncol 2001; 24:388–391.
- Lo SF, Hayter M, Hu WY, Tai CY, Hsu MY, Li YF. Symptom burden and quality of life in patients with malignant fungating wounds. J Adv Nurs 2012; 68:1312–1321.
- Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol 1994; 130:489–493.
- Tippett AW. Wounds at the end of life. Wounds 2005; 17:91–98.
- Burt T. Palliative care of pressure ulcers in long-term care. Ann Long-Term Care 2013; 21:20–28.
- Reifsnyder J, Magee HS. Development of pressure ulcers in patients receiving home hospice care. Wounds 2005; 17:74–79.
- Haisfield-Wolfe ME, Rund C. Malignant cutaneous wounds: a management protocol. Ostomy Wound Manage 1997; 43:56–66.
- O’Brien C. Malignant wounds: managing odour. Can Fam Physician 2012; 58:272–274.
- Gethin G, Grocott P, Probst S, Clarke E. Current practice in the management of wound odour: an international survey. Int J Nurs Stud 2014; 51:865–874.
- Bale S, Tebble N, Price P. A topical metronidazole gel used to treat malodorous wounds. Br J Nurs 2004; 13:S4–S11.
- Hack A. Malodorous wounds—taking the patient’s perspective into account. J Wound Care 2003; 12:319–321.
- Price E. Wound care. The stigma of smell. Nurs Times 1996; 92:71–72.
- Paul JC, Pieper BA. Topical metronidazole for the treatment of wound odor: a review of the literature. Ostomy Wound Manage 2008; 54:18–27.
- Lee G, Anand SC, Rajendran S, Walker I. Overview of current practice and future trends in the evaluation of dressings for malodorous wounds. J Wound Care 2006; 15:344–346.
- Cutting K, Harding K. Criteria for identifying wound infection. J Wound Care 1994; 3:198–201.
- McDonald A, Lesage P. Palliative management of pressure ulcers and malignant wounds in patients with advanced illness. J Palliat Med 2006; 9:285–295.
- Holloway S. Recognising and treating the causes of chronic malodorous wounds. Prof Nurse 2004; 19:380–384.
- Haughton W, Young T. Common problems in wound care: malodorous wounds. Br J Nurs 1995; 4:959–963.
- Alvarez OM, Kalinski C, Nusbaum J, et al. Incorporating wound healing strategies to improve palliation (symptom management) in patients with chronic wounds. J Palliat Med 2007; 10:1161–1189.
- da Costa Santos CM, de Mattos Pimenta CA, Nobre MR. A systematic review of topical treatments to control the odor of malignant fungating wounds. J Pain Symptom Manage 2010; 39:1065–1076.
- Von Gruenigen VE, Coleman RL, et al. Bacteriology and treatment of malodorous lower reproductive tract in gynecologic cancer patients. Obstet Gynecol 2000; 96:23–27.
- Baker PG, Haig G. Metronidazole in the treatment of chronic pressure sores and ulcers: a comparison with standard treatment in general practice. Practitioner 1981; 225:569–573.
- Whitney J, Phillips L, Aslam R, et al. Guidelines for the treatment of pressure ulcers. Wound Repair Regen 2006; 14:663–679.
- Williams D, Enoch S, Miller D, Harris K, Price P, Harding KG. Effect of sharp debridement using curette on recalcitrant nonhealing venous ulcers: a concurrently controlled, prospective cohort study. Wound Repair Regen 2005; 13:131–137.
- Bergstrom KJ. Assessment and management of fungating wounds. J Wound Ostomy Continence Nurs 2011: 38:31–37.
- Sinclair RD, Ryan TJ. Proteolytic enzymes in wound healing: the role of enzymatic debridement. Australas J Dermatol 1994; 35:35–41.
- Enoch S, Harding KG. Wound bed preparation: the science behind the removal of barriers to healing. Wounds 2003;15:213–229.
- Mumcuoglu KY. Clinical applications for maggots in wound care. Am J Clin Dermatol 2001; 2:219–227.
- Langemo DK, Black J; National Pressure Ulcer Advisory Panel. Pressure ulcers in individuals receiving palliative care: a National Pressure Ulcer Advisory Panel white paper. Adv Skin Wound Care 2010; 23:59–72.
- Fonder MA, Lazarus GS, Cowan DA, Aronson-Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol 2008; 58:185–206.
- Lio PA, Kaye ET. Topical antibacterial agents. Infect Dis Clin North Am 2004; 18:717–733.
- Gelmetti C. Local antibiotics in dermatology. Dermatol Ther 2008; 21:187–195.
- Finlay IG, Bowszyc J, Ramlau C, Gwiezdzinski Z. The effect of topical 0.75% metronidazole gel on malodorous cutaneous ulcers. J Pain Symptom Manage 1996; 11:158–162.
- Bower M, Stein R, Evans TR, Hedley A, Pert P, Coombes RC. A double-blind study of the efficacy of metronidazole gel in the treatment of malodorous fungating tumours. Eur J Cancer 1992; 28A:888–889.
- Kalinski C, Schnepf M, Laboy D, et al. Effectiveness of a topical formulation containing metronidazole for wound odor and exudate control. Wounds 2005; 17:84–90.
- Kuge S, Tokuda Y, Ohta M, et al. Use of metronidazole gel to control malodor in advanced and recurrent breast cancer. Jpn J Clin Oncol 1996; 26:207–210.
- Belcher J. A review of medical-grade honey in wound care. Br J Nurs 2012: 21:S4–S9.
- Kwakman PH, Van den Akker JP, Güçlü A, et al. Medical-grade honey kills antibiotic-resistant bacteria in vitro and eradicates skin colonization. Clin Infect Dis 2008; 46:1677–1682.
- Cooper RA, Jenkins L. A comparison between medical grade honey and table honeys in relation to antimicrobial efficacy. Wounds 2009; 21:29–36.
- Patel B, Cox-Hayley D. Managing wound odor #218. J Palliat Med 2010; 13:1286–1287.
- Schulte MJ. Yogurt helps to control wound odor. Oncol Nurs Forum 1993; 20:1262.
- Upright CA, Salton C, Roberts F, Murphy J. Evaluation of Mesalt dressings and continuous wet saline dressings in ulcerating metastatic skin lesions. Cancer Nurs 1994; 17:149–155.
- Kuttan R, Sudheeran PC, Josph CD. Turmeric and curcumin as topical agents in cancer therapy. Tumori 1987; 73:29–31.
- Bass MJ, Phillips LG. Pressure sores. Curr Probl Surg 2007; 44:101–143.
- Bufill JA, Grace WR, Neff R. Intra-arterial chemotherapy for palliation of fungating breast cancer: a case report and review of the literature. Am J Clin Oncol 1994; 17:118–124.
- Murakami M, Kuroda Y, Sano A, et al. Validity of local treatment including intraarterial infusion chemotherapy and radiotherapy for fungating adenocarcinoma of the breast: case report of more than 8-year survival. Am J Clin Oncol 2001; 24:388–391.
KEY POINTS
- Necrotic tissue is a substrate for bacterial growth and should be debrided. A variety of methods can be used.
- Malodor is most often from infection with anaerobic organisms, which topical metronidazole and other agents can help control.
- An absorbent dressing should be used either as a primary dressing, or over a layer of topical metronidazole and a nonadherent primary dressing.
- Foremost in formulating a patient- and family-centered malodor management strategy is to commit to controlling it as much as possible.