User login
Vasculopathy Can Vary in Patients With Idiopathic Pulmonary Arterial Hypertension
Approximately half of adults with idiopathic pulmonary arterial hypertension (IPAH) had nonplexiform vasculopathy characterized in part by severe pulmonary microvascular remodeling, based on data from 50 individuals.
The clinical phenotype of IPAH was historically described as a rapidly progressive rare disease in young women and characterized by plexiform lesions, wrote Esther J. Nossent, MD, of Amsterdam University Medical Centers, Amsterdam, the Netherlands, and colleagues. However, the patient population with IPAH has become older and predominantly men, and the nature of vascular phenotypes and histologic patterns in patients with contemporary IPAH has not been well studied, the researchers said.
In a cross-sectional study published in CHEST, the researchers reviewed lung histology data from 50 adults with IPAH that had been assessed by two experienced pathologists. The mean age of the patients was 52 years and 58% were women. Based on a histopathologic evaluation, 24 patients had nonplexiform vasculopathy (48%) and 26 had plexiform vasculopathy (52%). Notably, microvascular remodeling involving arterioles and venules was substantial in patients with nonplexiform vasculopathy but mild or absent in those with plexiform vasculopathy, the researchers wrote.
The researchers also compared the clinical characteristics of patients with plexiform vs nonplexiform vasculopathy. Hemodynamic parameters were similar in both patient groups. However, those with nonplexiform vasculopathy were significantly older than those with plexiform vasculopathy (60 years vs 44 years), were more likely to be men (67% vs 20%), and had a lower diffusing capacity of the lungs for carbon monoxide (DLCO) at diagnosis (all P < .001). Patients with nonplexiform vasculopathy also were significantly more likely than those with plexiform vasculopathy to have a history of smoking (P = .03). Genetic testing revealed no mutations in established PAH genes in the nonplexiform group.
Low DLCO has been associated with worse outcomes regardless of hemodynamic response, the researchers noted. In the current study, “a DLCO of < 45% almost perfectly identified patients with nonplexiform vasculopathy with prominent pulmonary microvascular disease,” they said.
The findings were limited by several factors, including the small study population and the higher frequency of surgical lung biopsies in the nonplexiform group vs the plexiform group, which is not part of the general workup of patients with IPAH, the researchers noted.
, they said. However, the results suggest that differences between patients with IPAH with plexiform vasculopathy and those with nonplexiform vasculopathy could ultimately inform targeted treatment strategies.
“Recognizing these clinical phenotypes allows revisiting current datasets to understand better the potential future clinical consequences of the vascular phenotypes for treatment response and clinical outcome,” the researchers concluded.
Findings May Inform More Targeted Therapy
“Any investigation that adds substantive insight into a complex disease that can translate into a better understanding of clinical patient phenotypes and eventually into improved treatments and patient outcomes has relevance at any time,” Paul Forfia, MD, professor of medicine at the Lewis Katz School of Medicine at Temple University, Philadelphia, said in an interview.
“There is focus on the antiproliferative forms of pulmonary arterial hypertension–specific therapy, and the results of the current study may have implications to these therapies,” said Dr. Forfia, who was not involved in the current study.
“In the current study, the investigators show that 48% of patients that were traditionally categorized as IPAH had a vascular phenotype that is not considered ‘typical’ or classic for IPAH,” Dr. Forfia told this news organization. “These findings highlight a significant heterogeneity of the pulmonary vascular phenotype within IPAH, which raises the question of whether the nonplexiform patient would be less responsive to the novel, antiproliferative forms of therapy,” he said.
The new findings are quite interesting but not surprising, Dr. Forfia said. “The World Symposia diagnostic groupings for pulmonary hypertension are a very important and necessary form of categorization and differentiation amongst forms of PH [pulmonary hypertension], and these groupings make a best attempt based on available evidence to separate patients of varying PH pathophysiology, both in terms of diagnosis and in how PH patients are treated,” he explained.
“However, clinical experts in PH have known that subphenotypes of PH pathophysiology exist within group I PAH, as well as in PH related to left heart disease (group 2), chronic respiratory disease (group 3), and chronic thromboembolic disease (group 4),” he said.
Findings from the current study reinforce the importance of clinical and physiological phenotyping of each patient, which can help in terms of therapy selection and in managing expectations in response to therapy, Dr. Forfia added.
“Perhaps the most evident and important clinical implication from the current study is to remind clinicians treating patients with PH that heterogeneity exists within the vascular phenotype and clinical makeup of patients even within the same type of PAH,” Dr. Forfia said. “With this insight, clinicians are more informed and thus more apt to consider nuances in the diagnosis, treatment, and expectations for treatment response within PAH,” he said.
Dr. Forfia also highlighted the potential implications of the association between cigarette smoking and the nonplexiform vascular phenotype. “This association was present in the absence of radiographic evidence of emphysema and raises the provocative notion that cigarette smoking may lead to pulmonary vascular abnormalities, perhaps even PAH, in patients without a diagnosis of emphysema,” he said.
“An important limitation from the current study is that the vascular phenotypes observed within their cohort of IPAH patients were obtained from histopathology specimens at the time of autopsy, explant at the time of lung transplantation, and surgical lung biopsy spanning over a 22-year period,” Dr. Forfia noted. Additional research is needed to explore how vascular phenotypic differences can be appreciated in the absence of histopathology and how these differences could impact therapy selection and patient outcomes, he said.
The study received no outside funding. Dr. Nossent disclosed receiving speaker fees from Janssen, MSD, and United Therapeutics/Ferrer and consulting fees from Janssen and United Therapeutics/Ferrer. Dr. Forfia had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Approximately half of adults with idiopathic pulmonary arterial hypertension (IPAH) had nonplexiform vasculopathy characterized in part by severe pulmonary microvascular remodeling, based on data from 50 individuals.
The clinical phenotype of IPAH was historically described as a rapidly progressive rare disease in young women and characterized by plexiform lesions, wrote Esther J. Nossent, MD, of Amsterdam University Medical Centers, Amsterdam, the Netherlands, and colleagues. However, the patient population with IPAH has become older and predominantly men, and the nature of vascular phenotypes and histologic patterns in patients with contemporary IPAH has not been well studied, the researchers said.
In a cross-sectional study published in CHEST, the researchers reviewed lung histology data from 50 adults with IPAH that had been assessed by two experienced pathologists. The mean age of the patients was 52 years and 58% were women. Based on a histopathologic evaluation, 24 patients had nonplexiform vasculopathy (48%) and 26 had plexiform vasculopathy (52%). Notably, microvascular remodeling involving arterioles and venules was substantial in patients with nonplexiform vasculopathy but mild or absent in those with plexiform vasculopathy, the researchers wrote.
The researchers also compared the clinical characteristics of patients with plexiform vs nonplexiform vasculopathy. Hemodynamic parameters were similar in both patient groups. However, those with nonplexiform vasculopathy were significantly older than those with plexiform vasculopathy (60 years vs 44 years), were more likely to be men (67% vs 20%), and had a lower diffusing capacity of the lungs for carbon monoxide (DLCO) at diagnosis (all P < .001). Patients with nonplexiform vasculopathy also were significantly more likely than those with plexiform vasculopathy to have a history of smoking (P = .03). Genetic testing revealed no mutations in established PAH genes in the nonplexiform group.
Low DLCO has been associated with worse outcomes regardless of hemodynamic response, the researchers noted. In the current study, “a DLCO of < 45% almost perfectly identified patients with nonplexiform vasculopathy with prominent pulmonary microvascular disease,” they said.
The findings were limited by several factors, including the small study population and the higher frequency of surgical lung biopsies in the nonplexiform group vs the plexiform group, which is not part of the general workup of patients with IPAH, the researchers noted.
, they said. However, the results suggest that differences between patients with IPAH with plexiform vasculopathy and those with nonplexiform vasculopathy could ultimately inform targeted treatment strategies.
“Recognizing these clinical phenotypes allows revisiting current datasets to understand better the potential future clinical consequences of the vascular phenotypes for treatment response and clinical outcome,” the researchers concluded.
Findings May Inform More Targeted Therapy
“Any investigation that adds substantive insight into a complex disease that can translate into a better understanding of clinical patient phenotypes and eventually into improved treatments and patient outcomes has relevance at any time,” Paul Forfia, MD, professor of medicine at the Lewis Katz School of Medicine at Temple University, Philadelphia, said in an interview.
“There is focus on the antiproliferative forms of pulmonary arterial hypertension–specific therapy, and the results of the current study may have implications to these therapies,” said Dr. Forfia, who was not involved in the current study.
“In the current study, the investigators show that 48% of patients that were traditionally categorized as IPAH had a vascular phenotype that is not considered ‘typical’ or classic for IPAH,” Dr. Forfia told this news organization. “These findings highlight a significant heterogeneity of the pulmonary vascular phenotype within IPAH, which raises the question of whether the nonplexiform patient would be less responsive to the novel, antiproliferative forms of therapy,” he said.
The new findings are quite interesting but not surprising, Dr. Forfia said. “The World Symposia diagnostic groupings for pulmonary hypertension are a very important and necessary form of categorization and differentiation amongst forms of PH [pulmonary hypertension], and these groupings make a best attempt based on available evidence to separate patients of varying PH pathophysiology, both in terms of diagnosis and in how PH patients are treated,” he explained.
“However, clinical experts in PH have known that subphenotypes of PH pathophysiology exist within group I PAH, as well as in PH related to left heart disease (group 2), chronic respiratory disease (group 3), and chronic thromboembolic disease (group 4),” he said.
Findings from the current study reinforce the importance of clinical and physiological phenotyping of each patient, which can help in terms of therapy selection and in managing expectations in response to therapy, Dr. Forfia added.
“Perhaps the most evident and important clinical implication from the current study is to remind clinicians treating patients with PH that heterogeneity exists within the vascular phenotype and clinical makeup of patients even within the same type of PAH,” Dr. Forfia said. “With this insight, clinicians are more informed and thus more apt to consider nuances in the diagnosis, treatment, and expectations for treatment response within PAH,” he said.
Dr. Forfia also highlighted the potential implications of the association between cigarette smoking and the nonplexiform vascular phenotype. “This association was present in the absence of radiographic evidence of emphysema and raises the provocative notion that cigarette smoking may lead to pulmonary vascular abnormalities, perhaps even PAH, in patients without a diagnosis of emphysema,” he said.
“An important limitation from the current study is that the vascular phenotypes observed within their cohort of IPAH patients were obtained from histopathology specimens at the time of autopsy, explant at the time of lung transplantation, and surgical lung biopsy spanning over a 22-year period,” Dr. Forfia noted. Additional research is needed to explore how vascular phenotypic differences can be appreciated in the absence of histopathology and how these differences could impact therapy selection and patient outcomes, he said.
The study received no outside funding. Dr. Nossent disclosed receiving speaker fees from Janssen, MSD, and United Therapeutics/Ferrer and consulting fees from Janssen and United Therapeutics/Ferrer. Dr. Forfia had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Approximately half of adults with idiopathic pulmonary arterial hypertension (IPAH) had nonplexiform vasculopathy characterized in part by severe pulmonary microvascular remodeling, based on data from 50 individuals.
The clinical phenotype of IPAH was historically described as a rapidly progressive rare disease in young women and characterized by plexiform lesions, wrote Esther J. Nossent, MD, of Amsterdam University Medical Centers, Amsterdam, the Netherlands, and colleagues. However, the patient population with IPAH has become older and predominantly men, and the nature of vascular phenotypes and histologic patterns in patients with contemporary IPAH has not been well studied, the researchers said.
In a cross-sectional study published in CHEST, the researchers reviewed lung histology data from 50 adults with IPAH that had been assessed by two experienced pathologists. The mean age of the patients was 52 years and 58% were women. Based on a histopathologic evaluation, 24 patients had nonplexiform vasculopathy (48%) and 26 had plexiform vasculopathy (52%). Notably, microvascular remodeling involving arterioles and venules was substantial in patients with nonplexiform vasculopathy but mild or absent in those with plexiform vasculopathy, the researchers wrote.
The researchers also compared the clinical characteristics of patients with plexiform vs nonplexiform vasculopathy. Hemodynamic parameters were similar in both patient groups. However, those with nonplexiform vasculopathy were significantly older than those with plexiform vasculopathy (60 years vs 44 years), were more likely to be men (67% vs 20%), and had a lower diffusing capacity of the lungs for carbon monoxide (DLCO) at diagnosis (all P < .001). Patients with nonplexiform vasculopathy also were significantly more likely than those with plexiform vasculopathy to have a history of smoking (P = .03). Genetic testing revealed no mutations in established PAH genes in the nonplexiform group.
Low DLCO has been associated with worse outcomes regardless of hemodynamic response, the researchers noted. In the current study, “a DLCO of < 45% almost perfectly identified patients with nonplexiform vasculopathy with prominent pulmonary microvascular disease,” they said.
The findings were limited by several factors, including the small study population and the higher frequency of surgical lung biopsies in the nonplexiform group vs the plexiform group, which is not part of the general workup of patients with IPAH, the researchers noted.
, they said. However, the results suggest that differences between patients with IPAH with plexiform vasculopathy and those with nonplexiform vasculopathy could ultimately inform targeted treatment strategies.
“Recognizing these clinical phenotypes allows revisiting current datasets to understand better the potential future clinical consequences of the vascular phenotypes for treatment response and clinical outcome,” the researchers concluded.
Findings May Inform More Targeted Therapy
“Any investigation that adds substantive insight into a complex disease that can translate into a better understanding of clinical patient phenotypes and eventually into improved treatments and patient outcomes has relevance at any time,” Paul Forfia, MD, professor of medicine at the Lewis Katz School of Medicine at Temple University, Philadelphia, said in an interview.
“There is focus on the antiproliferative forms of pulmonary arterial hypertension–specific therapy, and the results of the current study may have implications to these therapies,” said Dr. Forfia, who was not involved in the current study.
“In the current study, the investigators show that 48% of patients that were traditionally categorized as IPAH had a vascular phenotype that is not considered ‘typical’ or classic for IPAH,” Dr. Forfia told this news organization. “These findings highlight a significant heterogeneity of the pulmonary vascular phenotype within IPAH, which raises the question of whether the nonplexiform patient would be less responsive to the novel, antiproliferative forms of therapy,” he said.
The new findings are quite interesting but not surprising, Dr. Forfia said. “The World Symposia diagnostic groupings for pulmonary hypertension are a very important and necessary form of categorization and differentiation amongst forms of PH [pulmonary hypertension], and these groupings make a best attempt based on available evidence to separate patients of varying PH pathophysiology, both in terms of diagnosis and in how PH patients are treated,” he explained.
“However, clinical experts in PH have known that subphenotypes of PH pathophysiology exist within group I PAH, as well as in PH related to left heart disease (group 2), chronic respiratory disease (group 3), and chronic thromboembolic disease (group 4),” he said.
Findings from the current study reinforce the importance of clinical and physiological phenotyping of each patient, which can help in terms of therapy selection and in managing expectations in response to therapy, Dr. Forfia added.
“Perhaps the most evident and important clinical implication from the current study is to remind clinicians treating patients with PH that heterogeneity exists within the vascular phenotype and clinical makeup of patients even within the same type of PAH,” Dr. Forfia said. “With this insight, clinicians are more informed and thus more apt to consider nuances in the diagnosis, treatment, and expectations for treatment response within PAH,” he said.
Dr. Forfia also highlighted the potential implications of the association between cigarette smoking and the nonplexiform vascular phenotype. “This association was present in the absence of radiographic evidence of emphysema and raises the provocative notion that cigarette smoking may lead to pulmonary vascular abnormalities, perhaps even PAH, in patients without a diagnosis of emphysema,” he said.
“An important limitation from the current study is that the vascular phenotypes observed within their cohort of IPAH patients were obtained from histopathology specimens at the time of autopsy, explant at the time of lung transplantation, and surgical lung biopsy spanning over a 22-year period,” Dr. Forfia noted. Additional research is needed to explore how vascular phenotypic differences can be appreciated in the absence of histopathology and how these differences could impact therapy selection and patient outcomes, he said.
The study received no outside funding. Dr. Nossent disclosed receiving speaker fees from Janssen, MSD, and United Therapeutics/Ferrer and consulting fees from Janssen and United Therapeutics/Ferrer. Dr. Forfia had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
New, Near-to-Market PCSK9s Could Help Patients Meet Cholesterol Targets
, experts said.
One new anti-PCSK9 agent — lerodalcibep from LIB Therapeutics — is a small protein molecule, which is expected to reach the market by early 2026. It is being positioned as a step forward from the two monoclonal antibody products already available — evolocumab (Repatha; Amgen) and alirocumab (Praluent; Sanofi/Regeneron).
The new option can be given less frequently than the antibodies with a once-a-month injection instead of every 2 weeks. It also does not need to be kept refrigerated like the antibodies, Evan Stein, MD, chief scientific and operating officer of LIB Therapeutics, said in an interview.
Two phase 3 trials have recently been reported, showing impressive reductions in LDL in patients already taking statins.
The LIBerate Trials
The LIBerate-HR trial, published in JAMA Cardiology, involved 922 patients who still had LDL above target despite taking maximally tolerated statin therapy plus other lipid-lowering agents in some cases.
The trial found a time-averaged mean reduction in LDL cholesterol of 62%.
“This large reduction resulted in more than 90% of patients reaching the new lower LDL targets set in recent guidelines of less than 55 mg/dL for patients with cardiovascular disease or at very high risk, and less than 70 mg/dL in patients at risk,” said Stein.
Another phase 3 trial, LIBerate-CVD, has also shown reductions in LDL cholesterol levels of more than 60% in patients at high risk for cardiovascular disease on maximally tolerated statins.
LIB Therapeutics plans to file approval applications for lerodalcibep in the United States and Europe later this year.
A Crowded Field
Dr. Stein said PCSK9 inhibitors have been underused so far, but this is starting to change.
“The monoclonal antibodies were way overpriced costing around $14,000 per year when they were first introduced, which resulted in huge pushback from insurance companies,” Dr. Stein said, which made the drugs difficult to prescribe. “Then a few years ago, the price dropped a bit, and now they’re probably running at about $4000 per year, which made them more accessible.”
He said the market is now rapidly taking off. Lerodalcibep will compete in the anti-PCSK9 market with not only the two monoclonal antibodies but also with the new short-interfering RNA agent, inclisiran (Leqvio; Novartis) , a novel injectable agent that is given just twice a year but has to be administered at a medical facility.
Despite the crowded field, there appears to be plenty of room in the market. “Last year, growth was just under 40%. The first quarter of this year, it has increased by 44%. While the introduction of inclisiran has added to this growth, it hasn’t dented the sales of the existing monoclonal antibodies,” said Dr. Stein.
He estimates that the anti-PCSK9 market will reach $3 billion globally this year, and by the time lerodalcibep is launched, it could be worth $5 billion.
As well as inclisiran and lerodalcibep, there are other innovations in the anti-PCSK9 field in development, with oral drugs now also in the pipeline. The first one of these, in development by Merck, is in early phase 3 trials, and AstraZeneca has an oral agent in earlier development.
Enthusiastic Response
Other experts in the lipid field are also enthusiastic about new developments in the PCSK9s.
Jorge Plutzky, MD, director of preventive cardiology at Brigham and Women’s Hospital in Boston, said he welcomes the prospect of new approaches to PCSK9 inhibition.
“The increase in the number of safe, effective tools for LDL lowering, whether through PCSK9 or other targets, is inevitably beneficial for patients and the field,” he said during an interview.
Dr. Plutzky pointed out that although the current agents are effective, cost and coverage remain issues despite some recent progress in these areas, and new agents will increase competition and should hopefully drive prices down. Having a variety of dosing methods and frequencies provides more options for patients to find the one that works best for them.
Lerodalcibep’s once-monthly dosing schedule and the lack of need for refrigeration may be appreciated by some patients, he said, particularly those who need to travel for long periods.
Connie Newman, MD, adjunct professor of medicine at NYU School of Medicine, New York City, said there is plenty of room in the market to accommodate patient’s needs and preferences.
“Despite the US FDA approval of three medications that target PCSK9, there is a need for more anti-PCSK9 agents that reduce LDL and cardiovascular events,” she said. “In the US alone, 25% of adults have LDL levels of 130 mg/dL and above. Of all the non-statin therapies, medications targeting PCSK9 produce the greatest reduction in LDL. However, some patients may not tolerate one or more of the medications available or may prefer a monthly injection of lower volume.”
Dr. Newman said she believes there will still be a market for injectable formulations of PCSK9 inhibitors in the future, even if oral formulations are approved.
“Oral formulations usually require more frequent administration. Some people prefer longer-acting medications that can be taken less often. This might lead to better adherence,” she said.
Dr. Stein said he agrees there will always be room for different options. “And you only have to look at what is happening with the weight loss drugs to see that there is a market for injectables.” The ability to get patients to the new, more aggressive LDL goals is what is important, he added. “These drugs do that, and offering patients a choice of agents and delivery mechanisms is helpful.”
A version of this article appeared on Medscape.com.
, experts said.
One new anti-PCSK9 agent — lerodalcibep from LIB Therapeutics — is a small protein molecule, which is expected to reach the market by early 2026. It is being positioned as a step forward from the two monoclonal antibody products already available — evolocumab (Repatha; Amgen) and alirocumab (Praluent; Sanofi/Regeneron).
The new option can be given less frequently than the antibodies with a once-a-month injection instead of every 2 weeks. It also does not need to be kept refrigerated like the antibodies, Evan Stein, MD, chief scientific and operating officer of LIB Therapeutics, said in an interview.
Two phase 3 trials have recently been reported, showing impressive reductions in LDL in patients already taking statins.
The LIBerate Trials
The LIBerate-HR trial, published in JAMA Cardiology, involved 922 patients who still had LDL above target despite taking maximally tolerated statin therapy plus other lipid-lowering agents in some cases.
The trial found a time-averaged mean reduction in LDL cholesterol of 62%.
“This large reduction resulted in more than 90% of patients reaching the new lower LDL targets set in recent guidelines of less than 55 mg/dL for patients with cardiovascular disease or at very high risk, and less than 70 mg/dL in patients at risk,” said Stein.
Another phase 3 trial, LIBerate-CVD, has also shown reductions in LDL cholesterol levels of more than 60% in patients at high risk for cardiovascular disease on maximally tolerated statins.
LIB Therapeutics plans to file approval applications for lerodalcibep in the United States and Europe later this year.
A Crowded Field
Dr. Stein said PCSK9 inhibitors have been underused so far, but this is starting to change.
“The monoclonal antibodies were way overpriced costing around $14,000 per year when they were first introduced, which resulted in huge pushback from insurance companies,” Dr. Stein said, which made the drugs difficult to prescribe. “Then a few years ago, the price dropped a bit, and now they’re probably running at about $4000 per year, which made them more accessible.”
He said the market is now rapidly taking off. Lerodalcibep will compete in the anti-PCSK9 market with not only the two monoclonal antibodies but also with the new short-interfering RNA agent, inclisiran (Leqvio; Novartis) , a novel injectable agent that is given just twice a year but has to be administered at a medical facility.
Despite the crowded field, there appears to be plenty of room in the market. “Last year, growth was just under 40%. The first quarter of this year, it has increased by 44%. While the introduction of inclisiran has added to this growth, it hasn’t dented the sales of the existing monoclonal antibodies,” said Dr. Stein.
He estimates that the anti-PCSK9 market will reach $3 billion globally this year, and by the time lerodalcibep is launched, it could be worth $5 billion.
As well as inclisiran and lerodalcibep, there are other innovations in the anti-PCSK9 field in development, with oral drugs now also in the pipeline. The first one of these, in development by Merck, is in early phase 3 trials, and AstraZeneca has an oral agent in earlier development.
Enthusiastic Response
Other experts in the lipid field are also enthusiastic about new developments in the PCSK9s.
Jorge Plutzky, MD, director of preventive cardiology at Brigham and Women’s Hospital in Boston, said he welcomes the prospect of new approaches to PCSK9 inhibition.
“The increase in the number of safe, effective tools for LDL lowering, whether through PCSK9 or other targets, is inevitably beneficial for patients and the field,” he said during an interview.
Dr. Plutzky pointed out that although the current agents are effective, cost and coverage remain issues despite some recent progress in these areas, and new agents will increase competition and should hopefully drive prices down. Having a variety of dosing methods and frequencies provides more options for patients to find the one that works best for them.
Lerodalcibep’s once-monthly dosing schedule and the lack of need for refrigeration may be appreciated by some patients, he said, particularly those who need to travel for long periods.
Connie Newman, MD, adjunct professor of medicine at NYU School of Medicine, New York City, said there is plenty of room in the market to accommodate patient’s needs and preferences.
“Despite the US FDA approval of three medications that target PCSK9, there is a need for more anti-PCSK9 agents that reduce LDL and cardiovascular events,” she said. “In the US alone, 25% of adults have LDL levels of 130 mg/dL and above. Of all the non-statin therapies, medications targeting PCSK9 produce the greatest reduction in LDL. However, some patients may not tolerate one or more of the medications available or may prefer a monthly injection of lower volume.”
Dr. Newman said she believes there will still be a market for injectable formulations of PCSK9 inhibitors in the future, even if oral formulations are approved.
“Oral formulations usually require more frequent administration. Some people prefer longer-acting medications that can be taken less often. This might lead to better adherence,” she said.
Dr. Stein said he agrees there will always be room for different options. “And you only have to look at what is happening with the weight loss drugs to see that there is a market for injectables.” The ability to get patients to the new, more aggressive LDL goals is what is important, he added. “These drugs do that, and offering patients a choice of agents and delivery mechanisms is helpful.”
A version of this article appeared on Medscape.com.
, experts said.
One new anti-PCSK9 agent — lerodalcibep from LIB Therapeutics — is a small protein molecule, which is expected to reach the market by early 2026. It is being positioned as a step forward from the two monoclonal antibody products already available — evolocumab (Repatha; Amgen) and alirocumab (Praluent; Sanofi/Regeneron).
The new option can be given less frequently than the antibodies with a once-a-month injection instead of every 2 weeks. It also does not need to be kept refrigerated like the antibodies, Evan Stein, MD, chief scientific and operating officer of LIB Therapeutics, said in an interview.
Two phase 3 trials have recently been reported, showing impressive reductions in LDL in patients already taking statins.
The LIBerate Trials
The LIBerate-HR trial, published in JAMA Cardiology, involved 922 patients who still had LDL above target despite taking maximally tolerated statin therapy plus other lipid-lowering agents in some cases.
The trial found a time-averaged mean reduction in LDL cholesterol of 62%.
“This large reduction resulted in more than 90% of patients reaching the new lower LDL targets set in recent guidelines of less than 55 mg/dL for patients with cardiovascular disease or at very high risk, and less than 70 mg/dL in patients at risk,” said Stein.
Another phase 3 trial, LIBerate-CVD, has also shown reductions in LDL cholesterol levels of more than 60% in patients at high risk for cardiovascular disease on maximally tolerated statins.
LIB Therapeutics plans to file approval applications for lerodalcibep in the United States and Europe later this year.
A Crowded Field
Dr. Stein said PCSK9 inhibitors have been underused so far, but this is starting to change.
“The monoclonal antibodies were way overpriced costing around $14,000 per year when they were first introduced, which resulted in huge pushback from insurance companies,” Dr. Stein said, which made the drugs difficult to prescribe. “Then a few years ago, the price dropped a bit, and now they’re probably running at about $4000 per year, which made them more accessible.”
He said the market is now rapidly taking off. Lerodalcibep will compete in the anti-PCSK9 market with not only the two monoclonal antibodies but also with the new short-interfering RNA agent, inclisiran (Leqvio; Novartis) , a novel injectable agent that is given just twice a year but has to be administered at a medical facility.
Despite the crowded field, there appears to be plenty of room in the market. “Last year, growth was just under 40%. The first quarter of this year, it has increased by 44%. While the introduction of inclisiran has added to this growth, it hasn’t dented the sales of the existing monoclonal antibodies,” said Dr. Stein.
He estimates that the anti-PCSK9 market will reach $3 billion globally this year, and by the time lerodalcibep is launched, it could be worth $5 billion.
As well as inclisiran and lerodalcibep, there are other innovations in the anti-PCSK9 field in development, with oral drugs now also in the pipeline. The first one of these, in development by Merck, is in early phase 3 trials, and AstraZeneca has an oral agent in earlier development.
Enthusiastic Response
Other experts in the lipid field are also enthusiastic about new developments in the PCSK9s.
Jorge Plutzky, MD, director of preventive cardiology at Brigham and Women’s Hospital in Boston, said he welcomes the prospect of new approaches to PCSK9 inhibition.
“The increase in the number of safe, effective tools for LDL lowering, whether through PCSK9 or other targets, is inevitably beneficial for patients and the field,” he said during an interview.
Dr. Plutzky pointed out that although the current agents are effective, cost and coverage remain issues despite some recent progress in these areas, and new agents will increase competition and should hopefully drive prices down. Having a variety of dosing methods and frequencies provides more options for patients to find the one that works best for them.
Lerodalcibep’s once-monthly dosing schedule and the lack of need for refrigeration may be appreciated by some patients, he said, particularly those who need to travel for long periods.
Connie Newman, MD, adjunct professor of medicine at NYU School of Medicine, New York City, said there is plenty of room in the market to accommodate patient’s needs and preferences.
“Despite the US FDA approval of three medications that target PCSK9, there is a need for more anti-PCSK9 agents that reduce LDL and cardiovascular events,” she said. “In the US alone, 25% of adults have LDL levels of 130 mg/dL and above. Of all the non-statin therapies, medications targeting PCSK9 produce the greatest reduction in LDL. However, some patients may not tolerate one or more of the medications available or may prefer a monthly injection of lower volume.”
Dr. Newman said she believes there will still be a market for injectable formulations of PCSK9 inhibitors in the future, even if oral formulations are approved.
“Oral formulations usually require more frequent administration. Some people prefer longer-acting medications that can be taken less often. This might lead to better adherence,” she said.
Dr. Stein said he agrees there will always be room for different options. “And you only have to look at what is happening with the weight loss drugs to see that there is a market for injectables.” The ability to get patients to the new, more aggressive LDL goals is what is important, he added. “These drugs do that, and offering patients a choice of agents and delivery mechanisms is helpful.”
A version of this article appeared on Medscape.com.
Will Treating High Blood Pressure Curb Dementia Risk?
High blood pressure is an established risk factor for neurodegeneration and cognitive decline.
Valentin Fuster, MD, president of Mount Sinai Fuster Heart Hospital in New York City, told this news organization. “There is no question in the literature that untreated high blood pressure may lead to dementia,” he said. “The open question is whether treating blood pressure is sufficient to decrease or stop the progress of dementia.”
Studies are mixed, but recent research suggests that addressing hypertension does affect the risk for dementia. A secondary analysis of the China Rural Hypertension Control Project reported at the American Heart Association (AHA) Scientific Sessions in 2023 but not yet published showed that the 4-year blood pressure–lowering program in adults aged 40 or older significantly reduced the risk for all-cause dementia and cognitive impairment.
Similarly, a post hoc analysis of the SPRINT MIND trial found that participants aged 50 or older who underwent intensive (< 120 mm Hg) vs standard (< 140 mm Hg) blood pressure lowering had a lower rate of probable dementia or mild cognitive impairment.
Other studies pointing to a benefit included a pooled individual participant analysis of five randomized controlled trials, which found class I evidence to support antihypertensive treatment to reduce the risk for incident dementia, and an earlier systematic review and meta-analysis of the association of blood pressure lowering with newly diagnosed dementia or cognitive impairment.
How It Might Work
Some possible mechanisms underlying the connection have emerged.
“Vascular disease caused by hypertension is clearly implicated in one form of dementia, called vascular cognitive impairment and dementia,” Andrew Moran, MD, PhD, associate professor of medicine at Columbia University Vagelos College of Physicians and Surgeons in New York City, told this news organization. “This category includes dementia following a stroke caused by uncontrolled hypertension.”
“At the same time, we now know that hypertension and other vascular risk factors can also contribute, along with other factors, to developing Alzheimer dementia,” he said. “Even without causing clinically evident stroke, vascular disease from hypertension can lead to subtle damage to the brain via ischemia, microhemorrhage, and atrophy.”
“It is well known that hypertension affects the vasculature, and the vasculature of the brain is not spared,” agreed Eileen Handberg, PhD, ARNP, a member of the Hypertension Workgroup at the American College of Cardiology (ACC) and a professor of medicine and director of the Cardiovascular Clinical Trials Program in the University of Florida, Gainesville, Florida. “Combine this with other mechanisms like inflammation and endothelial dysfunction, and add amyloid accumulation, and there is a deterioration in vascular beds leading to decreased cerebral blood flow,” she said.
Treating hypertension likely helps lower dementia risk through “a combination of reduced risk of stroke and also benefits on blood flow, blood vessel health, and reduction in neurodegeneration,” suggested Mitchell S.V. Elkind, MD, chief clinical science officer and past president of the AHA and a professor of neurology and epidemiology at Columbia University Irving Medical Center in New York City. “Midlife blood pressure elevations are associated with deposition of amyloid in the brain, so controlling blood pressure may reduce amyloid deposits and neurodegeneration.”
Time in Range or Treat to Target?
With respect to dementia risk, does treating hypertension to a specific target make a difference, or is it the time spent in a healthy blood pressure range?
“Observational studies and a post hoc analysis of the SPRINT MIND trial suggest that more time spent in a healthy blood pressure range or more stable blood pressure are associated with lower dementia risk,” Dr. Moran said. Citing results of the CHRC program and SPRINT MIND trial, he suggested that while a dose-response effect (the lower the blood pressure, the lower the dementia risk) hasn’t been definitively demonstrated, it is likely the case.
In his practice, Dr. Moran follows ACC/AHA guidelines and prescribes antihypertensives to get blood pressure below 130/80 mm Hg in individuals with hypertension who have other high-risk factors (cardiovascular disease, diabetes, chronic kidney disease, or high risk for these conditions). “The treatment rule for people with hypertension without these other risk factors is less clear — lowering blood pressure below 140/90 mm Hg is a must; I will discuss with patients whether to go lower than that.”
“The relative contributions of time in range versus treating to a target for blood pressure require further study,” said Dr. Elkind. “It is likely that the cumulative effect of blood pressure over time has a big role to play — and it does seem clear that midlife blood pressure is even more important than blood pressure late in life.”
That said, he added, “In general and all things being equal, I would treat to a blood pressure of < 120/80 mmHg,” given the SPRINT trial findings of greater benefits when treating to this systolic blood pressure goal. “Of course, if patients have side effects such as lightheadedness or dizziness or other medical conditions that require a higher target, then one would need to adjust the treatment targets.”
According to Dr. Fuster, targets should not be the focus because they vary. For example, the ACC/AHA guidelines use < 130/80 mm Hg, whereas the European Society of Hypertension guidelines and those of the American Academy of Family Physicians specify < 140/90 mm Hg and include age-based criteria. Because there are no studies comparing the outcomes of one set of guidelines vs another, Dr. Fuster thinks the focus should be on starting treatment as early as possible to prevent hypertension leading to dementia.
He pointed to the ongoing PESA trial, which uses brain MRI and other tests to characterize longitudinal associations among cerebral glucose metabolism, subclinical atherosclerosis, and cardiovascular risk factors in asymptomatic individuals aged 40-54. Most did not have hypertension at baseline.
A recently published analysis of a subcohort of 370 PESA participants found that those with persistent high cardiovascular risk and subclinical carotid atherosclerosis already had signs of brain metabolic decline, “suggesting that maintenance of cardiovascular health during midlife could contribute to reductions in neurodegenerative disease burden later in life,” wrote the investigators.
Is It Ever Too Late?
If starting hypertension treatment in midlife can help reduce the risk for cognitive impairment later, can treating later in life also help? “It’s theoretically possible, but it has to be proven,” Dr. Fuster said. “There are no data on whether there’s less chance to prevent the development of dementia if you start treating hypertension at age 70, for example. And we have no idea whether hypertension treatment will prevent progression in those who already have dementia.”
“Treating high blood pressure in older adults could affect the course of further progressive cognitive decline by improving vascular health and preventing strokes, which likely exacerbate nonvascular dementia,” Dr. Elkind suggested. “Most people with dementia have a combination of vascular and nonvascular dementia, so treating reversible causes wherever possible makes a difference.”
Dr. Elkind treats older patients with this in mind, he said, “even though most of the evidence points to the fact that it is blood pressure in middle age, not older age, that seems to have the biggest impact on later-life cognitive decline and dementia.” Like Dr. Fuster, he said, “the best strategy is to identify and treat blood pressure in midlife, before damage to the brain has advanced.”
Dr. Moran noted, “The latest science on dementia causes suggests it is difficult to draw a border between vascular and nonvascular dementia. So, as a practical matter, healthcare providers should consider that hypertension treatment is one of the best ways to prevent any category of dementia. This dementia prevention is added to the well-known benefits of hypertension treatment to prevent heart attacks, strokes, and kidney disease: ‘Healthy heart, healthy brain.’ ”
“Our BP [blood pressure] control rates overall are still abysmal,” Dr. Handberg added. Currently around one in four US adults with hypertension have it under control. Studies have shown that blood pressure control rates of 70%-80% are achievable, she said. “We can’t let patient or provider inertia continue.”
Dr. Handberg, Dr. Elkind, Dr. Moran, and Dr. Fuster declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
High blood pressure is an established risk factor for neurodegeneration and cognitive decline.
Valentin Fuster, MD, president of Mount Sinai Fuster Heart Hospital in New York City, told this news organization. “There is no question in the literature that untreated high blood pressure may lead to dementia,” he said. “The open question is whether treating blood pressure is sufficient to decrease or stop the progress of dementia.”
Studies are mixed, but recent research suggests that addressing hypertension does affect the risk for dementia. A secondary analysis of the China Rural Hypertension Control Project reported at the American Heart Association (AHA) Scientific Sessions in 2023 but not yet published showed that the 4-year blood pressure–lowering program in adults aged 40 or older significantly reduced the risk for all-cause dementia and cognitive impairment.
Similarly, a post hoc analysis of the SPRINT MIND trial found that participants aged 50 or older who underwent intensive (< 120 mm Hg) vs standard (< 140 mm Hg) blood pressure lowering had a lower rate of probable dementia or mild cognitive impairment.
Other studies pointing to a benefit included a pooled individual participant analysis of five randomized controlled trials, which found class I evidence to support antihypertensive treatment to reduce the risk for incident dementia, and an earlier systematic review and meta-analysis of the association of blood pressure lowering with newly diagnosed dementia or cognitive impairment.
How It Might Work
Some possible mechanisms underlying the connection have emerged.
“Vascular disease caused by hypertension is clearly implicated in one form of dementia, called vascular cognitive impairment and dementia,” Andrew Moran, MD, PhD, associate professor of medicine at Columbia University Vagelos College of Physicians and Surgeons in New York City, told this news organization. “This category includes dementia following a stroke caused by uncontrolled hypertension.”
“At the same time, we now know that hypertension and other vascular risk factors can also contribute, along with other factors, to developing Alzheimer dementia,” he said. “Even without causing clinically evident stroke, vascular disease from hypertension can lead to subtle damage to the brain via ischemia, microhemorrhage, and atrophy.”
“It is well known that hypertension affects the vasculature, and the vasculature of the brain is not spared,” agreed Eileen Handberg, PhD, ARNP, a member of the Hypertension Workgroup at the American College of Cardiology (ACC) and a professor of medicine and director of the Cardiovascular Clinical Trials Program in the University of Florida, Gainesville, Florida. “Combine this with other mechanisms like inflammation and endothelial dysfunction, and add amyloid accumulation, and there is a deterioration in vascular beds leading to decreased cerebral blood flow,” she said.
Treating hypertension likely helps lower dementia risk through “a combination of reduced risk of stroke and also benefits on blood flow, blood vessel health, and reduction in neurodegeneration,” suggested Mitchell S.V. Elkind, MD, chief clinical science officer and past president of the AHA and a professor of neurology and epidemiology at Columbia University Irving Medical Center in New York City. “Midlife blood pressure elevations are associated with deposition of amyloid in the brain, so controlling blood pressure may reduce amyloid deposits and neurodegeneration.”
Time in Range or Treat to Target?
With respect to dementia risk, does treating hypertension to a specific target make a difference, or is it the time spent in a healthy blood pressure range?
“Observational studies and a post hoc analysis of the SPRINT MIND trial suggest that more time spent in a healthy blood pressure range or more stable blood pressure are associated with lower dementia risk,” Dr. Moran said. Citing results of the CHRC program and SPRINT MIND trial, he suggested that while a dose-response effect (the lower the blood pressure, the lower the dementia risk) hasn’t been definitively demonstrated, it is likely the case.
In his practice, Dr. Moran follows ACC/AHA guidelines and prescribes antihypertensives to get blood pressure below 130/80 mm Hg in individuals with hypertension who have other high-risk factors (cardiovascular disease, diabetes, chronic kidney disease, or high risk for these conditions). “The treatment rule for people with hypertension without these other risk factors is less clear — lowering blood pressure below 140/90 mm Hg is a must; I will discuss with patients whether to go lower than that.”
“The relative contributions of time in range versus treating to a target for blood pressure require further study,” said Dr. Elkind. “It is likely that the cumulative effect of blood pressure over time has a big role to play — and it does seem clear that midlife blood pressure is even more important than blood pressure late in life.”
That said, he added, “In general and all things being equal, I would treat to a blood pressure of < 120/80 mmHg,” given the SPRINT trial findings of greater benefits when treating to this systolic blood pressure goal. “Of course, if patients have side effects such as lightheadedness or dizziness or other medical conditions that require a higher target, then one would need to adjust the treatment targets.”
According to Dr. Fuster, targets should not be the focus because they vary. For example, the ACC/AHA guidelines use < 130/80 mm Hg, whereas the European Society of Hypertension guidelines and those of the American Academy of Family Physicians specify < 140/90 mm Hg and include age-based criteria. Because there are no studies comparing the outcomes of one set of guidelines vs another, Dr. Fuster thinks the focus should be on starting treatment as early as possible to prevent hypertension leading to dementia.
He pointed to the ongoing PESA trial, which uses brain MRI and other tests to characterize longitudinal associations among cerebral glucose metabolism, subclinical atherosclerosis, and cardiovascular risk factors in asymptomatic individuals aged 40-54. Most did not have hypertension at baseline.
A recently published analysis of a subcohort of 370 PESA participants found that those with persistent high cardiovascular risk and subclinical carotid atherosclerosis already had signs of brain metabolic decline, “suggesting that maintenance of cardiovascular health during midlife could contribute to reductions in neurodegenerative disease burden later in life,” wrote the investigators.
Is It Ever Too Late?
If starting hypertension treatment in midlife can help reduce the risk for cognitive impairment later, can treating later in life also help? “It’s theoretically possible, but it has to be proven,” Dr. Fuster said. “There are no data on whether there’s less chance to prevent the development of dementia if you start treating hypertension at age 70, for example. And we have no idea whether hypertension treatment will prevent progression in those who already have dementia.”
“Treating high blood pressure in older adults could affect the course of further progressive cognitive decline by improving vascular health and preventing strokes, which likely exacerbate nonvascular dementia,” Dr. Elkind suggested. “Most people with dementia have a combination of vascular and nonvascular dementia, so treating reversible causes wherever possible makes a difference.”
Dr. Elkind treats older patients with this in mind, he said, “even though most of the evidence points to the fact that it is blood pressure in middle age, not older age, that seems to have the biggest impact on later-life cognitive decline and dementia.” Like Dr. Fuster, he said, “the best strategy is to identify and treat blood pressure in midlife, before damage to the brain has advanced.”
Dr. Moran noted, “The latest science on dementia causes suggests it is difficult to draw a border between vascular and nonvascular dementia. So, as a practical matter, healthcare providers should consider that hypertension treatment is one of the best ways to prevent any category of dementia. This dementia prevention is added to the well-known benefits of hypertension treatment to prevent heart attacks, strokes, and kidney disease: ‘Healthy heart, healthy brain.’ ”
“Our BP [blood pressure] control rates overall are still abysmal,” Dr. Handberg added. Currently around one in four US adults with hypertension have it under control. Studies have shown that blood pressure control rates of 70%-80% are achievable, she said. “We can’t let patient or provider inertia continue.”
Dr. Handberg, Dr. Elkind, Dr. Moran, and Dr. Fuster declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
High blood pressure is an established risk factor for neurodegeneration and cognitive decline.
Valentin Fuster, MD, president of Mount Sinai Fuster Heart Hospital in New York City, told this news organization. “There is no question in the literature that untreated high blood pressure may lead to dementia,” he said. “The open question is whether treating blood pressure is sufficient to decrease or stop the progress of dementia.”
Studies are mixed, but recent research suggests that addressing hypertension does affect the risk for dementia. A secondary analysis of the China Rural Hypertension Control Project reported at the American Heart Association (AHA) Scientific Sessions in 2023 but not yet published showed that the 4-year blood pressure–lowering program in adults aged 40 or older significantly reduced the risk for all-cause dementia and cognitive impairment.
Similarly, a post hoc analysis of the SPRINT MIND trial found that participants aged 50 or older who underwent intensive (< 120 mm Hg) vs standard (< 140 mm Hg) blood pressure lowering had a lower rate of probable dementia or mild cognitive impairment.
Other studies pointing to a benefit included a pooled individual participant analysis of five randomized controlled trials, which found class I evidence to support antihypertensive treatment to reduce the risk for incident dementia, and an earlier systematic review and meta-analysis of the association of blood pressure lowering with newly diagnosed dementia or cognitive impairment.
How It Might Work
Some possible mechanisms underlying the connection have emerged.
“Vascular disease caused by hypertension is clearly implicated in one form of dementia, called vascular cognitive impairment and dementia,” Andrew Moran, MD, PhD, associate professor of medicine at Columbia University Vagelos College of Physicians and Surgeons in New York City, told this news organization. “This category includes dementia following a stroke caused by uncontrolled hypertension.”
“At the same time, we now know that hypertension and other vascular risk factors can also contribute, along with other factors, to developing Alzheimer dementia,” he said. “Even without causing clinically evident stroke, vascular disease from hypertension can lead to subtle damage to the brain via ischemia, microhemorrhage, and atrophy.”
“It is well known that hypertension affects the vasculature, and the vasculature of the brain is not spared,” agreed Eileen Handberg, PhD, ARNP, a member of the Hypertension Workgroup at the American College of Cardiology (ACC) and a professor of medicine and director of the Cardiovascular Clinical Trials Program in the University of Florida, Gainesville, Florida. “Combine this with other mechanisms like inflammation and endothelial dysfunction, and add amyloid accumulation, and there is a deterioration in vascular beds leading to decreased cerebral blood flow,” she said.
Treating hypertension likely helps lower dementia risk through “a combination of reduced risk of stroke and also benefits on blood flow, blood vessel health, and reduction in neurodegeneration,” suggested Mitchell S.V. Elkind, MD, chief clinical science officer and past president of the AHA and a professor of neurology and epidemiology at Columbia University Irving Medical Center in New York City. “Midlife blood pressure elevations are associated with deposition of amyloid in the brain, so controlling blood pressure may reduce amyloid deposits and neurodegeneration.”
Time in Range or Treat to Target?
With respect to dementia risk, does treating hypertension to a specific target make a difference, or is it the time spent in a healthy blood pressure range?
“Observational studies and a post hoc analysis of the SPRINT MIND trial suggest that more time spent in a healthy blood pressure range or more stable blood pressure are associated with lower dementia risk,” Dr. Moran said. Citing results of the CHRC program and SPRINT MIND trial, he suggested that while a dose-response effect (the lower the blood pressure, the lower the dementia risk) hasn’t been definitively demonstrated, it is likely the case.
In his practice, Dr. Moran follows ACC/AHA guidelines and prescribes antihypertensives to get blood pressure below 130/80 mm Hg in individuals with hypertension who have other high-risk factors (cardiovascular disease, diabetes, chronic kidney disease, or high risk for these conditions). “The treatment rule for people with hypertension without these other risk factors is less clear — lowering blood pressure below 140/90 mm Hg is a must; I will discuss with patients whether to go lower than that.”
“The relative contributions of time in range versus treating to a target for blood pressure require further study,” said Dr. Elkind. “It is likely that the cumulative effect of blood pressure over time has a big role to play — and it does seem clear that midlife blood pressure is even more important than blood pressure late in life.”
That said, he added, “In general and all things being equal, I would treat to a blood pressure of < 120/80 mmHg,” given the SPRINT trial findings of greater benefits when treating to this systolic blood pressure goal. “Of course, if patients have side effects such as lightheadedness or dizziness or other medical conditions that require a higher target, then one would need to adjust the treatment targets.”
According to Dr. Fuster, targets should not be the focus because they vary. For example, the ACC/AHA guidelines use < 130/80 mm Hg, whereas the European Society of Hypertension guidelines and those of the American Academy of Family Physicians specify < 140/90 mm Hg and include age-based criteria. Because there are no studies comparing the outcomes of one set of guidelines vs another, Dr. Fuster thinks the focus should be on starting treatment as early as possible to prevent hypertension leading to dementia.
He pointed to the ongoing PESA trial, which uses brain MRI and other tests to characterize longitudinal associations among cerebral glucose metabolism, subclinical atherosclerosis, and cardiovascular risk factors in asymptomatic individuals aged 40-54. Most did not have hypertension at baseline.
A recently published analysis of a subcohort of 370 PESA participants found that those with persistent high cardiovascular risk and subclinical carotid atherosclerosis already had signs of brain metabolic decline, “suggesting that maintenance of cardiovascular health during midlife could contribute to reductions in neurodegenerative disease burden later in life,” wrote the investigators.
Is It Ever Too Late?
If starting hypertension treatment in midlife can help reduce the risk for cognitive impairment later, can treating later in life also help? “It’s theoretically possible, but it has to be proven,” Dr. Fuster said. “There are no data on whether there’s less chance to prevent the development of dementia if you start treating hypertension at age 70, for example. And we have no idea whether hypertension treatment will prevent progression in those who already have dementia.”
“Treating high blood pressure in older adults could affect the course of further progressive cognitive decline by improving vascular health and preventing strokes, which likely exacerbate nonvascular dementia,” Dr. Elkind suggested. “Most people with dementia have a combination of vascular and nonvascular dementia, so treating reversible causes wherever possible makes a difference.”
Dr. Elkind treats older patients with this in mind, he said, “even though most of the evidence points to the fact that it is blood pressure in middle age, not older age, that seems to have the biggest impact on later-life cognitive decline and dementia.” Like Dr. Fuster, he said, “the best strategy is to identify and treat blood pressure in midlife, before damage to the brain has advanced.”
Dr. Moran noted, “The latest science on dementia causes suggests it is difficult to draw a border between vascular and nonvascular dementia. So, as a practical matter, healthcare providers should consider that hypertension treatment is one of the best ways to prevent any category of dementia. This dementia prevention is added to the well-known benefits of hypertension treatment to prevent heart attacks, strokes, and kidney disease: ‘Healthy heart, healthy brain.’ ”
“Our BP [blood pressure] control rates overall are still abysmal,” Dr. Handberg added. Currently around one in four US adults with hypertension have it under control. Studies have shown that blood pressure control rates of 70%-80% are achievable, she said. “We can’t let patient or provider inertia continue.”
Dr. Handberg, Dr. Elkind, Dr. Moran, and Dr. Fuster declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Are Beta-Blockers Safe for COPD?
Everyone takes a pharmacology class in medical school that includes a lecture on beta receptors. They’re in the heart (beta-1) and lungs (beta-2), and drug compounds agonize or antagonize one or both. The professor will caution against using antagonists (beta blockade) for patients with chronic obstructive pulmonary disease (COPD) lest they further impair the patient’s irreversibly narrowed airways. Obsequious students mature into obsequious doctors, intent on “doing no harm.” For better or worse, you withhold beta-blockers from your patient with COPD and comorbid cardiac disease.
Perhaps because the pulmonologist isn’t usually the one who decides whether a beta-blocker is prescribed, I’ve been napping on this topic since training. Early in fellowship, I read an ACP Journal Club article about a Cochrane systematic review (yes, I read a review of a review) that concluded that beta-blockers are fine in patients with COPD. The summary appealed to my bias towards evidence-based medicine (EBM) supplanting physiology, medical school, and everything else. I was more apt to believe my stodgy residency attendings than the stodgy pharmacology professor. Even though COPD and cardiovascular disease share multiple risk factors, I had never reinvestigated the relationship between beta-blockers and COPD.
Turns out that while I was sleeping, the debate continued. Go figure. Just last month a prospective, observational study published in JAMA Network Open found that beta-blockers did not increase the risk for cardiovascular or respiratory events among patients with COPD being discharged after hospitalization for acute myocardial infarction. Although this could be viewed as a triumph for EBM over physiology and a validation of my decade-plus of intellectual laziness, the results are actually pretty thin. These studies, in which patients with an indication for a therapy (a beta-blocker in this case) are analyzed by whether or not they received it, are problematic. The fanciest statistics — in this case, they used propensity scores — can’t control for residual confounding. What drove the physicians to prescribe in some cases but not others? We can only guess.
This might be okay if there hadn’t been a randomized controlled trial (RCT) published in 2019 in The New England Journal of Medicine that found that beta-blockers increase the risk for severe COPD exacerbations. In EBM, the RCT trumps all. Ironically, this trial was designed to test whether beta-blockers reduce severe COPD exacerbations. Yes, we’d come full circle. There was enough biologic plausibility to support a positive effect, or so thought the study authors and the Department of Defense (DOD) — for reasons I can’t possibly guess, the DOD funded this RCT. My pharmacology professor must be rolling over in his tenure.
The RCT did leave beta-blockers some wiggle room. The authors purposely excluded anyone with a cardiovascular indication for a beta-blocker. The intent was to ensure beneficial effects were isolated to respiratory and not cardiovascular outcomes. Of course, the reason I’m writing and you’re reading this is that COPD and cardiovascular disease co-occur at a high rate. The RCT notwithstanding, we prescribe beta-blockers to patients with COPD because they have a cardiac indication, not to reduce acute COPD exacerbations. So, it’s possible there’d be a net beta-blocker benefit in patients with COPD and comorbid heart disease.
That’s where the JAMA Network Open study comes in, but as discussed, methodologic weaknesses preclude its being the final word. That said, I think it’s unlikely we’ll see a COPD with comorbid cardiac disease RCT performed to assess whether beta-blockers provide a net benefit, unless maybe the DOD wants to fund another one of these. In the meantime, I’m calling clinical equipoise and punting. Fortunately for me, I don’t have to prescribe beta-blockers.
Dr. Holley is professor of medicine at Uniformed Services University in Bethesda, Maryland, and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center in Washington, DC. He reported conflicts of interest with Metapharm, CHEST College, and WebMD.
A version of this article first appeared on Medscape.com.
Everyone takes a pharmacology class in medical school that includes a lecture on beta receptors. They’re in the heart (beta-1) and lungs (beta-2), and drug compounds agonize or antagonize one or both. The professor will caution against using antagonists (beta blockade) for patients with chronic obstructive pulmonary disease (COPD) lest they further impair the patient’s irreversibly narrowed airways. Obsequious students mature into obsequious doctors, intent on “doing no harm.” For better or worse, you withhold beta-blockers from your patient with COPD and comorbid cardiac disease.
Perhaps because the pulmonologist isn’t usually the one who decides whether a beta-blocker is prescribed, I’ve been napping on this topic since training. Early in fellowship, I read an ACP Journal Club article about a Cochrane systematic review (yes, I read a review of a review) that concluded that beta-blockers are fine in patients with COPD. The summary appealed to my bias towards evidence-based medicine (EBM) supplanting physiology, medical school, and everything else. I was more apt to believe my stodgy residency attendings than the stodgy pharmacology professor. Even though COPD and cardiovascular disease share multiple risk factors, I had never reinvestigated the relationship between beta-blockers and COPD.
Turns out that while I was sleeping, the debate continued. Go figure. Just last month a prospective, observational study published in JAMA Network Open found that beta-blockers did not increase the risk for cardiovascular or respiratory events among patients with COPD being discharged after hospitalization for acute myocardial infarction. Although this could be viewed as a triumph for EBM over physiology and a validation of my decade-plus of intellectual laziness, the results are actually pretty thin. These studies, in which patients with an indication for a therapy (a beta-blocker in this case) are analyzed by whether or not they received it, are problematic. The fanciest statistics — in this case, they used propensity scores — can’t control for residual confounding. What drove the physicians to prescribe in some cases but not others? We can only guess.
This might be okay if there hadn’t been a randomized controlled trial (RCT) published in 2019 in The New England Journal of Medicine that found that beta-blockers increase the risk for severe COPD exacerbations. In EBM, the RCT trumps all. Ironically, this trial was designed to test whether beta-blockers reduce severe COPD exacerbations. Yes, we’d come full circle. There was enough biologic plausibility to support a positive effect, or so thought the study authors and the Department of Defense (DOD) — for reasons I can’t possibly guess, the DOD funded this RCT. My pharmacology professor must be rolling over in his tenure.
The RCT did leave beta-blockers some wiggle room. The authors purposely excluded anyone with a cardiovascular indication for a beta-blocker. The intent was to ensure beneficial effects were isolated to respiratory and not cardiovascular outcomes. Of course, the reason I’m writing and you’re reading this is that COPD and cardiovascular disease co-occur at a high rate. The RCT notwithstanding, we prescribe beta-blockers to patients with COPD because they have a cardiac indication, not to reduce acute COPD exacerbations. So, it’s possible there’d be a net beta-blocker benefit in patients with COPD and comorbid heart disease.
That’s where the JAMA Network Open study comes in, but as discussed, methodologic weaknesses preclude its being the final word. That said, I think it’s unlikely we’ll see a COPD with comorbid cardiac disease RCT performed to assess whether beta-blockers provide a net benefit, unless maybe the DOD wants to fund another one of these. In the meantime, I’m calling clinical equipoise and punting. Fortunately for me, I don’t have to prescribe beta-blockers.
Dr. Holley is professor of medicine at Uniformed Services University in Bethesda, Maryland, and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center in Washington, DC. He reported conflicts of interest with Metapharm, CHEST College, and WebMD.
A version of this article first appeared on Medscape.com.
Everyone takes a pharmacology class in medical school that includes a lecture on beta receptors. They’re in the heart (beta-1) and lungs (beta-2), and drug compounds agonize or antagonize one or both. The professor will caution against using antagonists (beta blockade) for patients with chronic obstructive pulmonary disease (COPD) lest they further impair the patient’s irreversibly narrowed airways. Obsequious students mature into obsequious doctors, intent on “doing no harm.” For better or worse, you withhold beta-blockers from your patient with COPD and comorbid cardiac disease.
Perhaps because the pulmonologist isn’t usually the one who decides whether a beta-blocker is prescribed, I’ve been napping on this topic since training. Early in fellowship, I read an ACP Journal Club article about a Cochrane systematic review (yes, I read a review of a review) that concluded that beta-blockers are fine in patients with COPD. The summary appealed to my bias towards evidence-based medicine (EBM) supplanting physiology, medical school, and everything else. I was more apt to believe my stodgy residency attendings than the stodgy pharmacology professor. Even though COPD and cardiovascular disease share multiple risk factors, I had never reinvestigated the relationship between beta-blockers and COPD.
Turns out that while I was sleeping, the debate continued. Go figure. Just last month a prospective, observational study published in JAMA Network Open found that beta-blockers did not increase the risk for cardiovascular or respiratory events among patients with COPD being discharged after hospitalization for acute myocardial infarction. Although this could be viewed as a triumph for EBM over physiology and a validation of my decade-plus of intellectual laziness, the results are actually pretty thin. These studies, in which patients with an indication for a therapy (a beta-blocker in this case) are analyzed by whether or not they received it, are problematic. The fanciest statistics — in this case, they used propensity scores — can’t control for residual confounding. What drove the physicians to prescribe in some cases but not others? We can only guess.
This might be okay if there hadn’t been a randomized controlled trial (RCT) published in 2019 in The New England Journal of Medicine that found that beta-blockers increase the risk for severe COPD exacerbations. In EBM, the RCT trumps all. Ironically, this trial was designed to test whether beta-blockers reduce severe COPD exacerbations. Yes, we’d come full circle. There was enough biologic plausibility to support a positive effect, or so thought the study authors and the Department of Defense (DOD) — for reasons I can’t possibly guess, the DOD funded this RCT. My pharmacology professor must be rolling over in his tenure.
The RCT did leave beta-blockers some wiggle room. The authors purposely excluded anyone with a cardiovascular indication for a beta-blocker. The intent was to ensure beneficial effects were isolated to respiratory and not cardiovascular outcomes. Of course, the reason I’m writing and you’re reading this is that COPD and cardiovascular disease co-occur at a high rate. The RCT notwithstanding, we prescribe beta-blockers to patients with COPD because they have a cardiac indication, not to reduce acute COPD exacerbations. So, it’s possible there’d be a net beta-blocker benefit in patients with COPD and comorbid heart disease.
That’s where the JAMA Network Open study comes in, but as discussed, methodologic weaknesses preclude its being the final word. That said, I think it’s unlikely we’ll see a COPD with comorbid cardiac disease RCT performed to assess whether beta-blockers provide a net benefit, unless maybe the DOD wants to fund another one of these. In the meantime, I’m calling clinical equipoise and punting. Fortunately for me, I don’t have to prescribe beta-blockers.
Dr. Holley is professor of medicine at Uniformed Services University in Bethesda, Maryland, and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center in Washington, DC. He reported conflicts of interest with Metapharm, CHEST College, and WebMD.
A version of this article first appeared on Medscape.com.
Vitamin B1 May Reduce Constipation in Adults
TOPLINE:
Increased dietary intake of vitamin B1 is associated with a lower prevalence of constipation, particularly among men and individuals without hypertension or diabetes.
METHODOLOGY:
- Researchers conducted a cross-sectional study using National Health and Nutrition Examination Survey data from 2005-2010 involving 10,371 adults aged ≥ 20 years.
- Participants provided information on fecal characteristics and bowel movement frequency, which was documented for 30 days prior to data collection.
- Constipation was established by either frequency of bowel movements (fewer than three per week) or stool consistency (Bristol Stool Scale type 1 or 2).
- Data on vitamin B1 intake were collected through 24-hour total nutritional intake recall interviews. Patients were divided into three groups based on their level of B1 intake: 0.064-1.21 mg, 1.21-1.76 mg, and 1.76-12.61 mg.
TAKEAWAY:
- Overall, 10.8% of participants were identified as having constipation.
- Greater dietary vitamin B1 intake was associated with a 23% reduction in constipation risk (P = .034).
- Additionally, a subgroup analysis found that higher B1 intake was associated with a reduction in constipation risk of 20% in men, 16% in people without hypertension, and 14% in those without diabetes.
IN PRACTICE:
“This association suggests that enhanced intake of vitamin B1 through diet may facilitate softer stools and heightened intestinal motility, thereby potentially alleviating constipation symptoms. Consequently, healthcare professionals are advised to prioritize the promotion of a well-balanced diet as an initial therapeutic approach, preceding medical interventions,” the authors wrote.
SOURCE:
The study, led by Wenyi Du, the Affiliated Stomatological Hospital of Soochow University, Suzhou Stomatological Hospital, Suzhou, China, and Wuxi People’s Hospital Affiliated to Nanjing Medical University, Wuxi Medical Center, Wuxi, China, was published online in BMC Gastroenterology.
LIMITATIONS:
A causal relationship could not be established between vitamin B1 intake and constipation owing to the cross-sectional nature of the study. The study relied on patient interviews and patient self-reported data. Additionally, 24-hour dietary recalls may not have accurately reflected the long-term eating habits of the participants.
DISCLOSURES:
The study had no specific funding source. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Increased dietary intake of vitamin B1 is associated with a lower prevalence of constipation, particularly among men and individuals without hypertension or diabetes.
METHODOLOGY:
- Researchers conducted a cross-sectional study using National Health and Nutrition Examination Survey data from 2005-2010 involving 10,371 adults aged ≥ 20 years.
- Participants provided information on fecal characteristics and bowel movement frequency, which was documented for 30 days prior to data collection.
- Constipation was established by either frequency of bowel movements (fewer than three per week) or stool consistency (Bristol Stool Scale type 1 or 2).
- Data on vitamin B1 intake were collected through 24-hour total nutritional intake recall interviews. Patients were divided into three groups based on their level of B1 intake: 0.064-1.21 mg, 1.21-1.76 mg, and 1.76-12.61 mg.
TAKEAWAY:
- Overall, 10.8% of participants were identified as having constipation.
- Greater dietary vitamin B1 intake was associated with a 23% reduction in constipation risk (P = .034).
- Additionally, a subgroup analysis found that higher B1 intake was associated with a reduction in constipation risk of 20% in men, 16% in people without hypertension, and 14% in those without diabetes.
IN PRACTICE:
“This association suggests that enhanced intake of vitamin B1 through diet may facilitate softer stools and heightened intestinal motility, thereby potentially alleviating constipation symptoms. Consequently, healthcare professionals are advised to prioritize the promotion of a well-balanced diet as an initial therapeutic approach, preceding medical interventions,” the authors wrote.
SOURCE:
The study, led by Wenyi Du, the Affiliated Stomatological Hospital of Soochow University, Suzhou Stomatological Hospital, Suzhou, China, and Wuxi People’s Hospital Affiliated to Nanjing Medical University, Wuxi Medical Center, Wuxi, China, was published online in BMC Gastroenterology.
LIMITATIONS:
A causal relationship could not be established between vitamin B1 intake and constipation owing to the cross-sectional nature of the study. The study relied on patient interviews and patient self-reported data. Additionally, 24-hour dietary recalls may not have accurately reflected the long-term eating habits of the participants.
DISCLOSURES:
The study had no specific funding source. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Increased dietary intake of vitamin B1 is associated with a lower prevalence of constipation, particularly among men and individuals without hypertension or diabetes.
METHODOLOGY:
- Researchers conducted a cross-sectional study using National Health and Nutrition Examination Survey data from 2005-2010 involving 10,371 adults aged ≥ 20 years.
- Participants provided information on fecal characteristics and bowel movement frequency, which was documented for 30 days prior to data collection.
- Constipation was established by either frequency of bowel movements (fewer than three per week) or stool consistency (Bristol Stool Scale type 1 or 2).
- Data on vitamin B1 intake were collected through 24-hour total nutritional intake recall interviews. Patients were divided into three groups based on their level of B1 intake: 0.064-1.21 mg, 1.21-1.76 mg, and 1.76-12.61 mg.
TAKEAWAY:
- Overall, 10.8% of participants were identified as having constipation.
- Greater dietary vitamin B1 intake was associated with a 23% reduction in constipation risk (P = .034).
- Additionally, a subgroup analysis found that higher B1 intake was associated with a reduction in constipation risk of 20% in men, 16% in people without hypertension, and 14% in those without diabetes.
IN PRACTICE:
“This association suggests that enhanced intake of vitamin B1 through diet may facilitate softer stools and heightened intestinal motility, thereby potentially alleviating constipation symptoms. Consequently, healthcare professionals are advised to prioritize the promotion of a well-balanced diet as an initial therapeutic approach, preceding medical interventions,” the authors wrote.
SOURCE:
The study, led by Wenyi Du, the Affiliated Stomatological Hospital of Soochow University, Suzhou Stomatological Hospital, Suzhou, China, and Wuxi People’s Hospital Affiliated to Nanjing Medical University, Wuxi Medical Center, Wuxi, China, was published online in BMC Gastroenterology.
LIMITATIONS:
A causal relationship could not be established between vitamin B1 intake and constipation owing to the cross-sectional nature of the study. The study relied on patient interviews and patient self-reported data. Additionally, 24-hour dietary recalls may not have accurately reflected the long-term eating habits of the participants.
DISCLOSURES:
The study had no specific funding source. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
For Richer, for Poorer: Low-Carb Diets Work for All Incomes
For 3 years, Ajala Efem’s type 2 diabetes was so poorly controlled that her blood sugar often soared northward of 500 mg/dL despite insulin shots three to five times a day. She would experience dizziness, vomiting, severe headaches, and the neuropathy in her feet made walking painful. She was also — literally — frothing at the mouth. The 47-year-old single mother of two adult children with mental disabilities feared that she would die.
Ms. Efem lives in the South Bronx, which is among the poorest areas of New York City, where the combined rate of prediabetes and diabetes is close to 30%, the highest rate of any borough in the city.
She had to wait 8 months for an appointment with an endocrinologist, but that visit proved to be life-changing. She lost 28 pounds and got off 15 medications in a single month. She did not join a gym or count calories; she simply changed the food she ate and adopted a low-carb diet.
“I went from being sick to feeling so great,” she told her endocrinologist recently: “My feet aren’t hurting; I’m not in pain; I’m eating as much as I want, and I really enjoy my food so much.”
Ms. Efem’s life-changing visit was with Mariela Glandt, MD, at the offices of Essen Health Care. One month earlier, Dr. Glandt’s company, OwnaHealth, was contracted by Essen to conduct a 100-person pilot program for endocrinology patients. Essen is the largest Medicaid provider in New York City, and “they were desperate for an endocrinologist,” said Dr. Glandt, who trained at Columbia University in New York. So she came — all the way from Madrid, Spain. She commutes monthly, staying for a week each visit.
Dr. Glandt keeps up this punishing schedule because, as she explains, “it’s such a high for me to see these incredible transformations.” Her mostly Black and Hispanic patients are poor and lack resources, yet they lose significant amounts of weight, and their health issues resolve.
“Food is medicine” is an idea very much in vogue. The concept was central to the landmark White House Conference on Hunger, Nutrition, and Health in 2022 and is now the focus of a number of a wide range of government programs. Recently, the Senate held a hearing aimed at further expanding food as medicine programs.
Still, only a single randomized controlled clinical trial has been conducted on this nutritional approach, with unexpectedly disappointing results. In the mid-Atlantic region, 456 food-insecure adults with type 2 diabetes were randomly assigned to usual care or the provision of weekly groceries for their entire families for about 1 year. Provisions for a Mediterranean-style diet included whole grains, fruits and vegetables, lean protein, low-fat dairy products, cereal, brown rice, and bread. In addition, participants received dietary consultations. Yet, those who got free food and coaching did not see improvements in their average blood sugar (the study’s primary outcome), and their low-density lipoprotein (LDL) cholesterol and high-density lipoprotein (HDL) cholesterol levels appeared to have worsened.
“To be honest, I was surprised,” the study’s lead author, Joseph Doyle, PhD, professor at the Sloan School of Management at MIT in Cambridge, Massachusetts, told me. “I was hoping we would show improved outcomes, but the way to make progress is to do well-randomized trials to find out what works.”
I was not surprised by these results because a recent rigorous systematic review and meta-analysis in The BMJ did not show a Mediterranean-style diet to be the most effective for glycemic control. And Ms. Efem was not in fact following a Mediterranean-style diet.
Ms. Efem’s low-carb success story is anecdotal, but Dr. Glandt has an established track record from her 9 years’ experience as the medical director of the eponymous diabetes center she founded in Tel Aviv. A recent audit of 344 patients from the center found that after 6 months of following a very low–carbohydrate diet, 96.3% of those with diabetes saw their A1c fall from a median 7.6% to 6.3%. Weight loss was significant, with a median drop of 6.5 kg (14 pounds) for patients with diabetes and 5.7 kg for those with prediabetes. The diet comprises 5%-10% of calories from carbs, but Dr. Glandt does not use numeric targets with her patients.
Blood pressure, triglycerides, and liver enzymes also improved. And though LDL cholesterol went up by 8%, this result may have been offset by an accompanying 13% rise in HDL cholesterol. Of the 78 patients initially on insulin, 62 were able to stop this medication entirely.
Although these results aren’t from a clinical trial, they’re still highly meaningful because the current dietary standard of care for type 2 diabetes can only slow the progression of the disease, not cause remission. Indeed, the idea that type 2 diabetes could be put into remission was not seriously considered by the American Diabetes Association (ADA) until 2009. By 2019, an ADA report concluded that “[r]educing overall carbohydrate intake for individuals with diabetes has demonstrated the most evidence for improving glycemia.” In other words, the best way to improve the key factor in diabetes is to reduce total carbohydrates. Yet, the ADA still advocates filling one quarter of one’s plate with carbohydrate-based foods, an amount that will prevent remission. Given that the ADA’s vision statement is “a life free of diabetes,” it seems negligent not to tell people with a deadly condition that they can reverse this diagnosis.
A 2023 meta-analysis of 42 controlled clinical trials on 4809 patients showed that a very low–carbohydrate ketogenic diet (keto) was “superior” to alternatives for glycemic control. A more recent review of 11 clinical trials found that this diet was equal but not superior to other nutritional approaches in terms of blood sugar control, but this review also concluded that keto led to greater increases in HDL cholesterol and lower triglycerides.
Dr. Glandt’s patients in the Bronx might not seem like obvious low-carb candidates. The diet is considered expensive and difficult to sustain. My interviews with a half dozen patients revealed some of these difficulties, but even for a woman living in a homeless shelter, the obstacles are not insurmountable.
Jerrilyn, who preferred that I use only her first name, lives in a shelter in Queens. While we strolled through a nearby park, she told me about her desire to lose weight and recover from polycystic ovary syndrome, which terrified her because it had caused dramatic hair loss. When she landed in Dr. Glandt’s office at age 28, she weighed 180 pounds.
Less than 5 months later, Jerrilyn had lost 25 pounds, and her period had returned with some regularity. She said she used “food stamps,” known as the Supplemental Nutrition Assistance Program (SNAP), to buy most of her food at local delis because the meals served at the shelter were too heavy in starches. She starts her day with eggs, turkey bacon, and avocado.
“It was hard to give up carbohydrates because in my culture [Latina], we have nothing but carbs: rice, potatoes, yuca,” Jerrilyn shared. She noticed that carbs make her hungrier, but after 3 days of going low-carb, her cravings diminished. “It was like getting over an addiction,” she said.
Jerrilyn told me she’d seen many doctors but none as involved as Dr. Glandt. “It feels awesome to know that I have a lot of really useful information coming from her all the time.” The OwnaHealth app tracks weight, blood pressure, blood sugar, ketones, meals, mood, and cravings. Patients wear continuous glucose monitors and enter other information manually. Ketone bodies are used to measure dietary adherence and are obtained through finger pricks and test strips provided by OwnaHealth. Dr. Glandt gives patients her own food plan, along with free visual guides to low-carbohydrate foods by dietdoctor.com.
Dr. Glandt also sends her patients for regular blood work. She says she does not frequently see a rise in LDL cholesterol, which can sometimes occur on a low-carbohydrate diet. This effect is most common among people who are lean and fit. She says she doesn’t discontinue statins unless cholesterol levels improve significantly.
Samuel Gonzalez, age 56, weighed 275 pounds when he walked into Dr. Glandt’s office this past November. His A1c was 9.2%, but none of his previous doctors had diagnosed him with diabetes. “I was like a walking bag of sugar!” he joked.
A low-carbohydrate diet seemed absurd to a Puerto Rican like himself: “Having coffee without sugar? That’s like sacrilegious in my culture!” exclaimed Mr. Gonzalez. Still, he managed, with SNAP, to cook eggs and bacon for breakfast and some kind of protein for dinner. He keeps lunch light, “like tuna fish,” and finds checking in with the OwnaHealth app to be very helpful. “Every day, I’m on it,” he said. In the past 7 months, he’s lost 50 pounds, normalized his cholesterol and blood pressure levels, and lowered his A1c to 5.5%.
Mr. Gonzalez gets disability payments due to a back injury, and Ms. Efem receives government payments because her husband died serving in the military. Ms. Efem says her new diet challenges her budget, but Mr. Gonzalez says he manages easily.
Mélissa Cruz, a 28-year-old studying to be a nail technician while also doing back office work at a physical therapy practice, says she’s stretched thin. “I end up sad because I can’t put energy into looking up recipes and cooking for me and my boyfriend,” she told me. She’ll often cook rice and plantains for him and meat for herself, but “it’s frustrating when I’m low on funds and can’t figure out what to eat.”
Low-carbohydrate diets have a reputation for being expensive because people often start eating pricier foods, like meat and cheese, to replace cheaper starchy foods such as pasta and rice.
A 2019 cost analysis published in Nutrition & Dietetics compared a low-carbohydrate dietary pattern with the New Zealand government’s recommended guidelines (which are almost identical to those in the United States) and found that it cost only an extra $1.27 in US dollars per person per day. One explanation is that protein and fat are more satiating than carbohydrates, so people who mostly consume these macronutrients often cut back on snacks like packaged chips, crackers, and even fruits. Also, those on a ketogenic diet usually cut down on medications, so the additional $1.27 daily is likely offset by reduced spending at the pharmacy.
It’s not just Bronx residents with low socioeconomic status (SES) who adapt well to low-carbohydrate diets. Among Alabama state employees with diabetes enrolled in a low-carbohydrate dietary program provided by a company called Virta, the low SES population had the best outcomes. Virta also published survey data in 2023 showing that participants in a program with the Veteran’s Administration did not find additional costs to be an obstacle to dietary adherence. In fact, some participants saw cost reductions due to decreased spending on processed snacks and fast foods.
Ms. Cruz told me she struggles financially, yet she’s still lost nearly 30 pounds in 5 months, and her A1c went from 7.1% down to 5.9%, putting her diabetes into remission. Equally motivating for her are the improvements she’s seen in other hormonal issues. Since childhood, she’s had acanthosis, a condition that causes the skin to darken in velvety patches, and more recently, she developed severe hirsutism to the point of growing sideburns. “I had tried going vegan and fasting, but these just weren’t sustainable for me, and I was so overwhelmed with counting calories all the time.” Now, on a low-carbohydrate diet, which doesn’t require calorie counting, she’s finally seeing both these conditions improve significantly.
When I last checked in with Ms. Cruz, she said she had “kind of ghosted” Dr. Glandt due to her work and school constraints, but she hadn’t abandoned the diet. She appreciated, too, that Dr. Glandt had not given up on her and kept calling and messaging. “She’s not at all like a typical doctor who would just tell me to lose weight and shake their head at me,” Ms. Cruz said.
Because Dr. Glandt’s approach is time-intensive and high-touch, it might seem impractical to scale up, but Dr. Glandt’s app uses artificial intelligence to help with communications thus allowing her, with help from part-time health coaches, to care for patients.
This early success in one of the United States’ poorest and sickest neighborhoods should give us hope that type 2 diabetes need not to be a progressive irreversible disease, even among the disadvantaged.
OwnaHealth’s track record, along with that of Virta and other similar low-carbohydrate medical practices also give hope to the food-is-medicine idea. Diabetes can go into remission, and people can be healed, provided that health practitioners prescribe the right foods. And in truth, it’s not a diet. It’s a way of eating that must be maintained. The sustainability of low-carbohydrate diets has been a point of contention, but the Virta trial, with 38% of patients sustaining remission at 2 years, showed that it’s possible. (OwnaHealth, for its part, offers long-term maintenance plans to help patients stay very low-carb permanently.)
Given the tremendous costs and health burden of diabetes, this approach should no doubt be the first line of treatment for doctors and the ADA. The past two decades of clinical trial research have demonstrated that remission of type 2 diabetes is possible through diet alone. It turns out that for metabolic diseases, only certain foods are truly medicine.
Tools and Tips for Clinicians:
- Free two-page keto starter’s guide by OwnaHealth; Dr. Glandt uses this guide with her patients.
- Illustrated low-carb guides by dietdoctor.com
- Free low-carbohydrate starter guide by the Michigan Collaborative for Type 2 Diabetes
- Low-Carb for Any Budget, a free digital booklet by Mark Cucuzzella, MD, and Kristie Sullivan, PhD
- Recipe and meal ideas from Ruled.me, Keto-Mojo.com, and
Dr. Teicholz is the founder of Nutrition Coalition, an independent nonprofit dedicated to ensuring that US dietary guidelines align with current science. She disclosed receiving book royalties from The Big Fat Surprise, and received honorarium not exceeding $2000 for speeches from various sources.
A version of this article appeared on Medscape.com.
For 3 years, Ajala Efem’s type 2 diabetes was so poorly controlled that her blood sugar often soared northward of 500 mg/dL despite insulin shots three to five times a day. She would experience dizziness, vomiting, severe headaches, and the neuropathy in her feet made walking painful. She was also — literally — frothing at the mouth. The 47-year-old single mother of two adult children with mental disabilities feared that she would die.
Ms. Efem lives in the South Bronx, which is among the poorest areas of New York City, where the combined rate of prediabetes and diabetes is close to 30%, the highest rate of any borough in the city.
She had to wait 8 months for an appointment with an endocrinologist, but that visit proved to be life-changing. She lost 28 pounds and got off 15 medications in a single month. She did not join a gym or count calories; she simply changed the food she ate and adopted a low-carb diet.
“I went from being sick to feeling so great,” she told her endocrinologist recently: “My feet aren’t hurting; I’m not in pain; I’m eating as much as I want, and I really enjoy my food so much.”
Ms. Efem’s life-changing visit was with Mariela Glandt, MD, at the offices of Essen Health Care. One month earlier, Dr. Glandt’s company, OwnaHealth, was contracted by Essen to conduct a 100-person pilot program for endocrinology patients. Essen is the largest Medicaid provider in New York City, and “they were desperate for an endocrinologist,” said Dr. Glandt, who trained at Columbia University in New York. So she came — all the way from Madrid, Spain. She commutes monthly, staying for a week each visit.
Dr. Glandt keeps up this punishing schedule because, as she explains, “it’s such a high for me to see these incredible transformations.” Her mostly Black and Hispanic patients are poor and lack resources, yet they lose significant amounts of weight, and their health issues resolve.
“Food is medicine” is an idea very much in vogue. The concept was central to the landmark White House Conference on Hunger, Nutrition, and Health in 2022 and is now the focus of a number of a wide range of government programs. Recently, the Senate held a hearing aimed at further expanding food as medicine programs.
Still, only a single randomized controlled clinical trial has been conducted on this nutritional approach, with unexpectedly disappointing results. In the mid-Atlantic region, 456 food-insecure adults with type 2 diabetes were randomly assigned to usual care or the provision of weekly groceries for their entire families for about 1 year. Provisions for a Mediterranean-style diet included whole grains, fruits and vegetables, lean protein, low-fat dairy products, cereal, brown rice, and bread. In addition, participants received dietary consultations. Yet, those who got free food and coaching did not see improvements in their average blood sugar (the study’s primary outcome), and their low-density lipoprotein (LDL) cholesterol and high-density lipoprotein (HDL) cholesterol levels appeared to have worsened.
“To be honest, I was surprised,” the study’s lead author, Joseph Doyle, PhD, professor at the Sloan School of Management at MIT in Cambridge, Massachusetts, told me. “I was hoping we would show improved outcomes, but the way to make progress is to do well-randomized trials to find out what works.”
I was not surprised by these results because a recent rigorous systematic review and meta-analysis in The BMJ did not show a Mediterranean-style diet to be the most effective for glycemic control. And Ms. Efem was not in fact following a Mediterranean-style diet.
Ms. Efem’s low-carb success story is anecdotal, but Dr. Glandt has an established track record from her 9 years’ experience as the medical director of the eponymous diabetes center she founded in Tel Aviv. A recent audit of 344 patients from the center found that after 6 months of following a very low–carbohydrate diet, 96.3% of those with diabetes saw their A1c fall from a median 7.6% to 6.3%. Weight loss was significant, with a median drop of 6.5 kg (14 pounds) for patients with diabetes and 5.7 kg for those with prediabetes. The diet comprises 5%-10% of calories from carbs, but Dr. Glandt does not use numeric targets with her patients.
Blood pressure, triglycerides, and liver enzymes also improved. And though LDL cholesterol went up by 8%, this result may have been offset by an accompanying 13% rise in HDL cholesterol. Of the 78 patients initially on insulin, 62 were able to stop this medication entirely.
Although these results aren’t from a clinical trial, they’re still highly meaningful because the current dietary standard of care for type 2 diabetes can only slow the progression of the disease, not cause remission. Indeed, the idea that type 2 diabetes could be put into remission was not seriously considered by the American Diabetes Association (ADA) until 2009. By 2019, an ADA report concluded that “[r]educing overall carbohydrate intake for individuals with diabetes has demonstrated the most evidence for improving glycemia.” In other words, the best way to improve the key factor in diabetes is to reduce total carbohydrates. Yet, the ADA still advocates filling one quarter of one’s plate with carbohydrate-based foods, an amount that will prevent remission. Given that the ADA’s vision statement is “a life free of diabetes,” it seems negligent not to tell people with a deadly condition that they can reverse this diagnosis.
A 2023 meta-analysis of 42 controlled clinical trials on 4809 patients showed that a very low–carbohydrate ketogenic diet (keto) was “superior” to alternatives for glycemic control. A more recent review of 11 clinical trials found that this diet was equal but not superior to other nutritional approaches in terms of blood sugar control, but this review also concluded that keto led to greater increases in HDL cholesterol and lower triglycerides.
Dr. Glandt’s patients in the Bronx might not seem like obvious low-carb candidates. The diet is considered expensive and difficult to sustain. My interviews with a half dozen patients revealed some of these difficulties, but even for a woman living in a homeless shelter, the obstacles are not insurmountable.
Jerrilyn, who preferred that I use only her first name, lives in a shelter in Queens. While we strolled through a nearby park, she told me about her desire to lose weight and recover from polycystic ovary syndrome, which terrified her because it had caused dramatic hair loss. When she landed in Dr. Glandt’s office at age 28, she weighed 180 pounds.
Less than 5 months later, Jerrilyn had lost 25 pounds, and her period had returned with some regularity. She said she used “food stamps,” known as the Supplemental Nutrition Assistance Program (SNAP), to buy most of her food at local delis because the meals served at the shelter were too heavy in starches. She starts her day with eggs, turkey bacon, and avocado.
“It was hard to give up carbohydrates because in my culture [Latina], we have nothing but carbs: rice, potatoes, yuca,” Jerrilyn shared. She noticed that carbs make her hungrier, but after 3 days of going low-carb, her cravings diminished. “It was like getting over an addiction,” she said.
Jerrilyn told me she’d seen many doctors but none as involved as Dr. Glandt. “It feels awesome to know that I have a lot of really useful information coming from her all the time.” The OwnaHealth app tracks weight, blood pressure, blood sugar, ketones, meals, mood, and cravings. Patients wear continuous glucose monitors and enter other information manually. Ketone bodies are used to measure dietary adherence and are obtained through finger pricks and test strips provided by OwnaHealth. Dr. Glandt gives patients her own food plan, along with free visual guides to low-carbohydrate foods by dietdoctor.com.
Dr. Glandt also sends her patients for regular blood work. She says she does not frequently see a rise in LDL cholesterol, which can sometimes occur on a low-carbohydrate diet. This effect is most common among people who are lean and fit. She says she doesn’t discontinue statins unless cholesterol levels improve significantly.
Samuel Gonzalez, age 56, weighed 275 pounds when he walked into Dr. Glandt’s office this past November. His A1c was 9.2%, but none of his previous doctors had diagnosed him with diabetes. “I was like a walking bag of sugar!” he joked.
A low-carbohydrate diet seemed absurd to a Puerto Rican like himself: “Having coffee without sugar? That’s like sacrilegious in my culture!” exclaimed Mr. Gonzalez. Still, he managed, with SNAP, to cook eggs and bacon for breakfast and some kind of protein for dinner. He keeps lunch light, “like tuna fish,” and finds checking in with the OwnaHealth app to be very helpful. “Every day, I’m on it,” he said. In the past 7 months, he’s lost 50 pounds, normalized his cholesterol and blood pressure levels, and lowered his A1c to 5.5%.
Mr. Gonzalez gets disability payments due to a back injury, and Ms. Efem receives government payments because her husband died serving in the military. Ms. Efem says her new diet challenges her budget, but Mr. Gonzalez says he manages easily.
Mélissa Cruz, a 28-year-old studying to be a nail technician while also doing back office work at a physical therapy practice, says she’s stretched thin. “I end up sad because I can’t put energy into looking up recipes and cooking for me and my boyfriend,” she told me. She’ll often cook rice and plantains for him and meat for herself, but “it’s frustrating when I’m low on funds and can’t figure out what to eat.”
Low-carbohydrate diets have a reputation for being expensive because people often start eating pricier foods, like meat and cheese, to replace cheaper starchy foods such as pasta and rice.
A 2019 cost analysis published in Nutrition & Dietetics compared a low-carbohydrate dietary pattern with the New Zealand government’s recommended guidelines (which are almost identical to those in the United States) and found that it cost only an extra $1.27 in US dollars per person per day. One explanation is that protein and fat are more satiating than carbohydrates, so people who mostly consume these macronutrients often cut back on snacks like packaged chips, crackers, and even fruits. Also, those on a ketogenic diet usually cut down on medications, so the additional $1.27 daily is likely offset by reduced spending at the pharmacy.
It’s not just Bronx residents with low socioeconomic status (SES) who adapt well to low-carbohydrate diets. Among Alabama state employees with diabetes enrolled in a low-carbohydrate dietary program provided by a company called Virta, the low SES population had the best outcomes. Virta also published survey data in 2023 showing that participants in a program with the Veteran’s Administration did not find additional costs to be an obstacle to dietary adherence. In fact, some participants saw cost reductions due to decreased spending on processed snacks and fast foods.
Ms. Cruz told me she struggles financially, yet she’s still lost nearly 30 pounds in 5 months, and her A1c went from 7.1% down to 5.9%, putting her diabetes into remission. Equally motivating for her are the improvements she’s seen in other hormonal issues. Since childhood, she’s had acanthosis, a condition that causes the skin to darken in velvety patches, and more recently, she developed severe hirsutism to the point of growing sideburns. “I had tried going vegan and fasting, but these just weren’t sustainable for me, and I was so overwhelmed with counting calories all the time.” Now, on a low-carbohydrate diet, which doesn’t require calorie counting, she’s finally seeing both these conditions improve significantly.
When I last checked in with Ms. Cruz, she said she had “kind of ghosted” Dr. Glandt due to her work and school constraints, but she hadn’t abandoned the diet. She appreciated, too, that Dr. Glandt had not given up on her and kept calling and messaging. “She’s not at all like a typical doctor who would just tell me to lose weight and shake their head at me,” Ms. Cruz said.
Because Dr. Glandt’s approach is time-intensive and high-touch, it might seem impractical to scale up, but Dr. Glandt’s app uses artificial intelligence to help with communications thus allowing her, with help from part-time health coaches, to care for patients.
This early success in one of the United States’ poorest and sickest neighborhoods should give us hope that type 2 diabetes need not to be a progressive irreversible disease, even among the disadvantaged.
OwnaHealth’s track record, along with that of Virta and other similar low-carbohydrate medical practices also give hope to the food-is-medicine idea. Diabetes can go into remission, and people can be healed, provided that health practitioners prescribe the right foods. And in truth, it’s not a diet. It’s a way of eating that must be maintained. The sustainability of low-carbohydrate diets has been a point of contention, but the Virta trial, with 38% of patients sustaining remission at 2 years, showed that it’s possible. (OwnaHealth, for its part, offers long-term maintenance plans to help patients stay very low-carb permanently.)
Given the tremendous costs and health burden of diabetes, this approach should no doubt be the first line of treatment for doctors and the ADA. The past two decades of clinical trial research have demonstrated that remission of type 2 diabetes is possible through diet alone. It turns out that for metabolic diseases, only certain foods are truly medicine.
Tools and Tips for Clinicians:
- Free two-page keto starter’s guide by OwnaHealth; Dr. Glandt uses this guide with her patients.
- Illustrated low-carb guides by dietdoctor.com
- Free low-carbohydrate starter guide by the Michigan Collaborative for Type 2 Diabetes
- Low-Carb for Any Budget, a free digital booklet by Mark Cucuzzella, MD, and Kristie Sullivan, PhD
- Recipe and meal ideas from Ruled.me, Keto-Mojo.com, and
Dr. Teicholz is the founder of Nutrition Coalition, an independent nonprofit dedicated to ensuring that US dietary guidelines align with current science. She disclosed receiving book royalties from The Big Fat Surprise, and received honorarium not exceeding $2000 for speeches from various sources.
A version of this article appeared on Medscape.com.
For 3 years, Ajala Efem’s type 2 diabetes was so poorly controlled that her blood sugar often soared northward of 500 mg/dL despite insulin shots three to five times a day. She would experience dizziness, vomiting, severe headaches, and the neuropathy in her feet made walking painful. She was also — literally — frothing at the mouth. The 47-year-old single mother of two adult children with mental disabilities feared that she would die.
Ms. Efem lives in the South Bronx, which is among the poorest areas of New York City, where the combined rate of prediabetes and diabetes is close to 30%, the highest rate of any borough in the city.
She had to wait 8 months for an appointment with an endocrinologist, but that visit proved to be life-changing. She lost 28 pounds and got off 15 medications in a single month. She did not join a gym or count calories; she simply changed the food she ate and adopted a low-carb diet.
“I went from being sick to feeling so great,” she told her endocrinologist recently: “My feet aren’t hurting; I’m not in pain; I’m eating as much as I want, and I really enjoy my food so much.”
Ms. Efem’s life-changing visit was with Mariela Glandt, MD, at the offices of Essen Health Care. One month earlier, Dr. Glandt’s company, OwnaHealth, was contracted by Essen to conduct a 100-person pilot program for endocrinology patients. Essen is the largest Medicaid provider in New York City, and “they were desperate for an endocrinologist,” said Dr. Glandt, who trained at Columbia University in New York. So she came — all the way from Madrid, Spain. She commutes monthly, staying for a week each visit.
Dr. Glandt keeps up this punishing schedule because, as she explains, “it’s such a high for me to see these incredible transformations.” Her mostly Black and Hispanic patients are poor and lack resources, yet they lose significant amounts of weight, and their health issues resolve.
“Food is medicine” is an idea very much in vogue. The concept was central to the landmark White House Conference on Hunger, Nutrition, and Health in 2022 and is now the focus of a number of a wide range of government programs. Recently, the Senate held a hearing aimed at further expanding food as medicine programs.
Still, only a single randomized controlled clinical trial has been conducted on this nutritional approach, with unexpectedly disappointing results. In the mid-Atlantic region, 456 food-insecure adults with type 2 diabetes were randomly assigned to usual care or the provision of weekly groceries for their entire families for about 1 year. Provisions for a Mediterranean-style diet included whole grains, fruits and vegetables, lean protein, low-fat dairy products, cereal, brown rice, and bread. In addition, participants received dietary consultations. Yet, those who got free food and coaching did not see improvements in their average blood sugar (the study’s primary outcome), and their low-density lipoprotein (LDL) cholesterol and high-density lipoprotein (HDL) cholesterol levels appeared to have worsened.
“To be honest, I was surprised,” the study’s lead author, Joseph Doyle, PhD, professor at the Sloan School of Management at MIT in Cambridge, Massachusetts, told me. “I was hoping we would show improved outcomes, but the way to make progress is to do well-randomized trials to find out what works.”
I was not surprised by these results because a recent rigorous systematic review and meta-analysis in The BMJ did not show a Mediterranean-style diet to be the most effective for glycemic control. And Ms. Efem was not in fact following a Mediterranean-style diet.
Ms. Efem’s low-carb success story is anecdotal, but Dr. Glandt has an established track record from her 9 years’ experience as the medical director of the eponymous diabetes center she founded in Tel Aviv. A recent audit of 344 patients from the center found that after 6 months of following a very low–carbohydrate diet, 96.3% of those with diabetes saw their A1c fall from a median 7.6% to 6.3%. Weight loss was significant, with a median drop of 6.5 kg (14 pounds) for patients with diabetes and 5.7 kg for those with prediabetes. The diet comprises 5%-10% of calories from carbs, but Dr. Glandt does not use numeric targets with her patients.
Blood pressure, triglycerides, and liver enzymes also improved. And though LDL cholesterol went up by 8%, this result may have been offset by an accompanying 13% rise in HDL cholesterol. Of the 78 patients initially on insulin, 62 were able to stop this medication entirely.
Although these results aren’t from a clinical trial, they’re still highly meaningful because the current dietary standard of care for type 2 diabetes can only slow the progression of the disease, not cause remission. Indeed, the idea that type 2 diabetes could be put into remission was not seriously considered by the American Diabetes Association (ADA) until 2009. By 2019, an ADA report concluded that “[r]educing overall carbohydrate intake for individuals with diabetes has demonstrated the most evidence for improving glycemia.” In other words, the best way to improve the key factor in diabetes is to reduce total carbohydrates. Yet, the ADA still advocates filling one quarter of one’s plate with carbohydrate-based foods, an amount that will prevent remission. Given that the ADA’s vision statement is “a life free of diabetes,” it seems negligent not to tell people with a deadly condition that they can reverse this diagnosis.
A 2023 meta-analysis of 42 controlled clinical trials on 4809 patients showed that a very low–carbohydrate ketogenic diet (keto) was “superior” to alternatives for glycemic control. A more recent review of 11 clinical trials found that this diet was equal but not superior to other nutritional approaches in terms of blood sugar control, but this review also concluded that keto led to greater increases in HDL cholesterol and lower triglycerides.
Dr. Glandt’s patients in the Bronx might not seem like obvious low-carb candidates. The diet is considered expensive and difficult to sustain. My interviews with a half dozen patients revealed some of these difficulties, but even for a woman living in a homeless shelter, the obstacles are not insurmountable.
Jerrilyn, who preferred that I use only her first name, lives in a shelter in Queens. While we strolled through a nearby park, she told me about her desire to lose weight and recover from polycystic ovary syndrome, which terrified her because it had caused dramatic hair loss. When she landed in Dr. Glandt’s office at age 28, she weighed 180 pounds.
Less than 5 months later, Jerrilyn had lost 25 pounds, and her period had returned with some regularity. She said she used “food stamps,” known as the Supplemental Nutrition Assistance Program (SNAP), to buy most of her food at local delis because the meals served at the shelter were too heavy in starches. She starts her day with eggs, turkey bacon, and avocado.
“It was hard to give up carbohydrates because in my culture [Latina], we have nothing but carbs: rice, potatoes, yuca,” Jerrilyn shared. She noticed that carbs make her hungrier, but after 3 days of going low-carb, her cravings diminished. “It was like getting over an addiction,” she said.
Jerrilyn told me she’d seen many doctors but none as involved as Dr. Glandt. “It feels awesome to know that I have a lot of really useful information coming from her all the time.” The OwnaHealth app tracks weight, blood pressure, blood sugar, ketones, meals, mood, and cravings. Patients wear continuous glucose monitors and enter other information manually. Ketone bodies are used to measure dietary adherence and are obtained through finger pricks and test strips provided by OwnaHealth. Dr. Glandt gives patients her own food plan, along with free visual guides to low-carbohydrate foods by dietdoctor.com.
Dr. Glandt also sends her patients for regular blood work. She says she does not frequently see a rise in LDL cholesterol, which can sometimes occur on a low-carbohydrate diet. This effect is most common among people who are lean and fit. She says she doesn’t discontinue statins unless cholesterol levels improve significantly.
Samuel Gonzalez, age 56, weighed 275 pounds when he walked into Dr. Glandt’s office this past November. His A1c was 9.2%, but none of his previous doctors had diagnosed him with diabetes. “I was like a walking bag of sugar!” he joked.
A low-carbohydrate diet seemed absurd to a Puerto Rican like himself: “Having coffee without sugar? That’s like sacrilegious in my culture!” exclaimed Mr. Gonzalez. Still, he managed, with SNAP, to cook eggs and bacon for breakfast and some kind of protein for dinner. He keeps lunch light, “like tuna fish,” and finds checking in with the OwnaHealth app to be very helpful. “Every day, I’m on it,” he said. In the past 7 months, he’s lost 50 pounds, normalized his cholesterol and blood pressure levels, and lowered his A1c to 5.5%.
Mr. Gonzalez gets disability payments due to a back injury, and Ms. Efem receives government payments because her husband died serving in the military. Ms. Efem says her new diet challenges her budget, but Mr. Gonzalez says he manages easily.
Mélissa Cruz, a 28-year-old studying to be a nail technician while also doing back office work at a physical therapy practice, says she’s stretched thin. “I end up sad because I can’t put energy into looking up recipes and cooking for me and my boyfriend,” she told me. She’ll often cook rice and plantains for him and meat for herself, but “it’s frustrating when I’m low on funds and can’t figure out what to eat.”
Low-carbohydrate diets have a reputation for being expensive because people often start eating pricier foods, like meat and cheese, to replace cheaper starchy foods such as pasta and rice.
A 2019 cost analysis published in Nutrition & Dietetics compared a low-carbohydrate dietary pattern with the New Zealand government’s recommended guidelines (which are almost identical to those in the United States) and found that it cost only an extra $1.27 in US dollars per person per day. One explanation is that protein and fat are more satiating than carbohydrates, so people who mostly consume these macronutrients often cut back on snacks like packaged chips, crackers, and even fruits. Also, those on a ketogenic diet usually cut down on medications, so the additional $1.27 daily is likely offset by reduced spending at the pharmacy.
It’s not just Bronx residents with low socioeconomic status (SES) who adapt well to low-carbohydrate diets. Among Alabama state employees with diabetes enrolled in a low-carbohydrate dietary program provided by a company called Virta, the low SES population had the best outcomes. Virta also published survey data in 2023 showing that participants in a program with the Veteran’s Administration did not find additional costs to be an obstacle to dietary adherence. In fact, some participants saw cost reductions due to decreased spending on processed snacks and fast foods.
Ms. Cruz told me she struggles financially, yet she’s still lost nearly 30 pounds in 5 months, and her A1c went from 7.1% down to 5.9%, putting her diabetes into remission. Equally motivating for her are the improvements she’s seen in other hormonal issues. Since childhood, she’s had acanthosis, a condition that causes the skin to darken in velvety patches, and more recently, she developed severe hirsutism to the point of growing sideburns. “I had tried going vegan and fasting, but these just weren’t sustainable for me, and I was so overwhelmed with counting calories all the time.” Now, on a low-carbohydrate diet, which doesn’t require calorie counting, she’s finally seeing both these conditions improve significantly.
When I last checked in with Ms. Cruz, she said she had “kind of ghosted” Dr. Glandt due to her work and school constraints, but she hadn’t abandoned the diet. She appreciated, too, that Dr. Glandt had not given up on her and kept calling and messaging. “She’s not at all like a typical doctor who would just tell me to lose weight and shake their head at me,” Ms. Cruz said.
Because Dr. Glandt’s approach is time-intensive and high-touch, it might seem impractical to scale up, but Dr. Glandt’s app uses artificial intelligence to help with communications thus allowing her, with help from part-time health coaches, to care for patients.
This early success in one of the United States’ poorest and sickest neighborhoods should give us hope that type 2 diabetes need not to be a progressive irreversible disease, even among the disadvantaged.
OwnaHealth’s track record, along with that of Virta and other similar low-carbohydrate medical practices also give hope to the food-is-medicine idea. Diabetes can go into remission, and people can be healed, provided that health practitioners prescribe the right foods. And in truth, it’s not a diet. It’s a way of eating that must be maintained. The sustainability of low-carbohydrate diets has been a point of contention, but the Virta trial, with 38% of patients sustaining remission at 2 years, showed that it’s possible. (OwnaHealth, for its part, offers long-term maintenance plans to help patients stay very low-carb permanently.)
Given the tremendous costs and health burden of diabetes, this approach should no doubt be the first line of treatment for doctors and the ADA. The past two decades of clinical trial research have demonstrated that remission of type 2 diabetes is possible through diet alone. It turns out that for metabolic diseases, only certain foods are truly medicine.
Tools and Tips for Clinicians:
- Free two-page keto starter’s guide by OwnaHealth; Dr. Glandt uses this guide with her patients.
- Illustrated low-carb guides by dietdoctor.com
- Free low-carbohydrate starter guide by the Michigan Collaborative for Type 2 Diabetes
- Low-Carb for Any Budget, a free digital booklet by Mark Cucuzzella, MD, and Kristie Sullivan, PhD
- Recipe and meal ideas from Ruled.me, Keto-Mojo.com, and
Dr. Teicholz is the founder of Nutrition Coalition, an independent nonprofit dedicated to ensuring that US dietary guidelines align with current science. She disclosed receiving book royalties from The Big Fat Surprise, and received honorarium not exceeding $2000 for speeches from various sources.
A version of this article appeared on Medscape.com.
Meat Alternatives May Benefit the Heart
Replacing meat with plant-based meat alternatives (PBMAs) can improve cardiovascular disease risk factors, including low-density lipoprotein cholesterol (LDL-C), a review of randomized controlled trials suggested.
Long-term randomized controlled trials and prospective cohort studies that evaluate cardiovascular disease events such as myocardial infarction and stroke are needed to draw definitive conclusions, according to the authors.
said senior author Ehud Ur, MB, professor of medicine at the University of British Columbia, Vancouver, in Canada, and an endocrinologist at St. Paul’s Hospital in Vancouver.
“However, we also found that there’s a lack of clinical outcome trials that would determine definitively whether plant-based meats are healthy. But certainly, everything points in the direction of cardiovascular benefit,” said Dr. Ur.
The review was published on June 25 in the Canadian Journal of Cardiology.
Ultraprocessed Foods
PBMAs are foods that mimic meats and contain ingredients such as protein derivatives from soy, pea, wheat, and fungi. A growing number of Canadians are limiting meat or excluding it from their diets. Some are opting to eat PBMAs instead.
But most PBMAs are classified as ultraprocessed foods. Such foods are produced primarily from substances extracted from whole food sources, such as sugar, salt, oil, and protein. Alternatively, they may be created in a laboratory using flavor enhancers and food coloring. This classification has caused the public and health professionals to question the potential health implications of PBMAs, said Dr. Ur.
“One of the concerns is that these products are highly processed, and things that are highly processed are considered bad. And so, are you swapping one set of risks for another?” he said.
To shed more light on this question, Dr. Ur’s team, which was led by Matthew Nagra, ND, of the Vancouver Naturopathic Clinic, assessed the literature on PBMAs and their impact on health.
“While the plant-based meat market has experienced significant growth in recent years and more and more Canadians are enjoying plant-based burgers, surprisingly little is known about how these meat alternatives may impact health and, in particular, cardiovascular disease risk,” Dr. Nagra said in a statement. “Thus, we sought to review the available literature on the topic to identify what is currently known and to provide direction for future research.”
Less Saturated Fat, Cholesterol
The researchers assessed the literature that was published from 1970 to 2023 on PBMAs, their contents, nutritional profiles, and impact on cardiovascular disease risk factors, such as cholesterol levels and blood pressure.
They found that, compared with meat, PBMAs had less saturated fat, less cholesterol, more fiber, more carbohydrates, fewer calories, less monounsaturated fat, more polyunsaturated fat, and more sodium.
In addition, several randomized controlled trials showed that PBMAs reduced total cholesterol and LDL-C, as well as apolipoprotein B-100, body weight, and waist circumference. PBMAs were not shown to raise blood pressure, despite some products’ high sodium content.
“No currently available evidence suggests that the concerning aspects of PBMAs (eg, food processing and high sodium content) negate the potential cardiovascular benefits,” wrote the researchers.
Unfortunately, no long-term research has evaluated how these alternatives may affect the risk of developing a myocardial infarction or stroke. Similarly, there is little research on the healthfulness of some common components of PBMAs, such as vital wheat gluten.
To shed light on these important issues would require large clinical trials, involving many patients, and great expense, said Dr. Ur. “Drug companies can afford to do large clinical trials, even if they are expensive to do, because they must do them to get approval for their drug. But these plant-based meats are produced by companies that most likely are not able to do clinical outcome trials. Such trials would have to be done by the National Institutes of Health in the United States, or in Canada, the National Research Council,” he said.
There are many reasons to avoid meat, Dr. Ur added. “There are ethical reasons against killing animals. Then there is the issue of global warming. Meat is a very expensive source of food energy. As an individual, the biggest impact you can make on global warming is to not eat meat. Then there is the argument about personal health, which is where our study comes in. For those people who like the taste of meat and who struggle with giving it up, the PBMAs allow them to have a reasonably diverse diet,” he said.
Are Eggs Healthy?
Meat substitutes are helpful for people who want to reduce their cardiovascular disease risk, J. David Spence, MD, professor emeritus of neurology and clinical pharmacology at the University of Western Ontario in London, Canada, wrote in an accompanying editorial.
“Eating too much meat and egg yolk increases cardiovascular risk, and it’s a challenge for patients to learn to eat less meat and cut out egg yolks. If we can find good substitutes that are tasty and enjoyable, that’s a good thing,” Dr. Spence told this news organization.
“Besides plant-based meat substitutes, there is great potential for reduction of cardiovascular risk with the use of egg substitutes,” he said.
Dr. Spence pointed out that two large egg yolks contain 474 mg of cholesterol, almost twice the amount contained in a Hardee’s Monster Thickburger (265 mg).
Cholesterol elevates plasma levels of toxic metabolites of the intestinal microbiome, such as trimethylamine N-oxide (TMAO). Plasma levels of TMAO increase in a linear fashion with egg consumption, and TMAO is bad for the arteries, said Dr. Spence.
“Eggs are terrible and should not be eaten by people at risk for cardiovascular disease. But people don’t understand that because the egg marketing propaganda has been so effective. The yolk is terrible. The egg marketing board is extremely effective in persuading people that eggs are healthy, and they’re not.”
Dr. Spence recommends using egg substitutes, such as Egg Beaters or Better’n Eggs, instead of whole eggs, and says it’s never too late to switch. “That’s the mistake people make, but the arteries can actually improve,” he said.
No funding source for the study was reported. Dr. Ur and Dr. Spence reported having no relevant financial relationships.
A version of this article appeared on Medscape.com.
Replacing meat with plant-based meat alternatives (PBMAs) can improve cardiovascular disease risk factors, including low-density lipoprotein cholesterol (LDL-C), a review of randomized controlled trials suggested.
Long-term randomized controlled trials and prospective cohort studies that evaluate cardiovascular disease events such as myocardial infarction and stroke are needed to draw definitive conclusions, according to the authors.
said senior author Ehud Ur, MB, professor of medicine at the University of British Columbia, Vancouver, in Canada, and an endocrinologist at St. Paul’s Hospital in Vancouver.
“However, we also found that there’s a lack of clinical outcome trials that would determine definitively whether plant-based meats are healthy. But certainly, everything points in the direction of cardiovascular benefit,” said Dr. Ur.
The review was published on June 25 in the Canadian Journal of Cardiology.
Ultraprocessed Foods
PBMAs are foods that mimic meats and contain ingredients such as protein derivatives from soy, pea, wheat, and fungi. A growing number of Canadians are limiting meat or excluding it from their diets. Some are opting to eat PBMAs instead.
But most PBMAs are classified as ultraprocessed foods. Such foods are produced primarily from substances extracted from whole food sources, such as sugar, salt, oil, and protein. Alternatively, they may be created in a laboratory using flavor enhancers and food coloring. This classification has caused the public and health professionals to question the potential health implications of PBMAs, said Dr. Ur.
“One of the concerns is that these products are highly processed, and things that are highly processed are considered bad. And so, are you swapping one set of risks for another?” he said.
To shed more light on this question, Dr. Ur’s team, which was led by Matthew Nagra, ND, of the Vancouver Naturopathic Clinic, assessed the literature on PBMAs and their impact on health.
“While the plant-based meat market has experienced significant growth in recent years and more and more Canadians are enjoying plant-based burgers, surprisingly little is known about how these meat alternatives may impact health and, in particular, cardiovascular disease risk,” Dr. Nagra said in a statement. “Thus, we sought to review the available literature on the topic to identify what is currently known and to provide direction for future research.”
Less Saturated Fat, Cholesterol
The researchers assessed the literature that was published from 1970 to 2023 on PBMAs, their contents, nutritional profiles, and impact on cardiovascular disease risk factors, such as cholesterol levels and blood pressure.
They found that, compared with meat, PBMAs had less saturated fat, less cholesterol, more fiber, more carbohydrates, fewer calories, less monounsaturated fat, more polyunsaturated fat, and more sodium.
In addition, several randomized controlled trials showed that PBMAs reduced total cholesterol and LDL-C, as well as apolipoprotein B-100, body weight, and waist circumference. PBMAs were not shown to raise blood pressure, despite some products’ high sodium content.
“No currently available evidence suggests that the concerning aspects of PBMAs (eg, food processing and high sodium content) negate the potential cardiovascular benefits,” wrote the researchers.
Unfortunately, no long-term research has evaluated how these alternatives may affect the risk of developing a myocardial infarction or stroke. Similarly, there is little research on the healthfulness of some common components of PBMAs, such as vital wheat gluten.
To shed light on these important issues would require large clinical trials, involving many patients, and great expense, said Dr. Ur. “Drug companies can afford to do large clinical trials, even if they are expensive to do, because they must do them to get approval for their drug. But these plant-based meats are produced by companies that most likely are not able to do clinical outcome trials. Such trials would have to be done by the National Institutes of Health in the United States, or in Canada, the National Research Council,” he said.
There are many reasons to avoid meat, Dr. Ur added. “There are ethical reasons against killing animals. Then there is the issue of global warming. Meat is a very expensive source of food energy. As an individual, the biggest impact you can make on global warming is to not eat meat. Then there is the argument about personal health, which is where our study comes in. For those people who like the taste of meat and who struggle with giving it up, the PBMAs allow them to have a reasonably diverse diet,” he said.
Are Eggs Healthy?
Meat substitutes are helpful for people who want to reduce their cardiovascular disease risk, J. David Spence, MD, professor emeritus of neurology and clinical pharmacology at the University of Western Ontario in London, Canada, wrote in an accompanying editorial.
“Eating too much meat and egg yolk increases cardiovascular risk, and it’s a challenge for patients to learn to eat less meat and cut out egg yolks. If we can find good substitutes that are tasty and enjoyable, that’s a good thing,” Dr. Spence told this news organization.
“Besides plant-based meat substitutes, there is great potential for reduction of cardiovascular risk with the use of egg substitutes,” he said.
Dr. Spence pointed out that two large egg yolks contain 474 mg of cholesterol, almost twice the amount contained in a Hardee’s Monster Thickburger (265 mg).
Cholesterol elevates plasma levels of toxic metabolites of the intestinal microbiome, such as trimethylamine N-oxide (TMAO). Plasma levels of TMAO increase in a linear fashion with egg consumption, and TMAO is bad for the arteries, said Dr. Spence.
“Eggs are terrible and should not be eaten by people at risk for cardiovascular disease. But people don’t understand that because the egg marketing propaganda has been so effective. The yolk is terrible. The egg marketing board is extremely effective in persuading people that eggs are healthy, and they’re not.”
Dr. Spence recommends using egg substitutes, such as Egg Beaters or Better’n Eggs, instead of whole eggs, and says it’s never too late to switch. “That’s the mistake people make, but the arteries can actually improve,” he said.
No funding source for the study was reported. Dr. Ur and Dr. Spence reported having no relevant financial relationships.
A version of this article appeared on Medscape.com.
Replacing meat with plant-based meat alternatives (PBMAs) can improve cardiovascular disease risk factors, including low-density lipoprotein cholesterol (LDL-C), a review of randomized controlled trials suggested.
Long-term randomized controlled trials and prospective cohort studies that evaluate cardiovascular disease events such as myocardial infarction and stroke are needed to draw definitive conclusions, according to the authors.
said senior author Ehud Ur, MB, professor of medicine at the University of British Columbia, Vancouver, in Canada, and an endocrinologist at St. Paul’s Hospital in Vancouver.
“However, we also found that there’s a lack of clinical outcome trials that would determine definitively whether plant-based meats are healthy. But certainly, everything points in the direction of cardiovascular benefit,” said Dr. Ur.
The review was published on June 25 in the Canadian Journal of Cardiology.
Ultraprocessed Foods
PBMAs are foods that mimic meats and contain ingredients such as protein derivatives from soy, pea, wheat, and fungi. A growing number of Canadians are limiting meat or excluding it from their diets. Some are opting to eat PBMAs instead.
But most PBMAs are classified as ultraprocessed foods. Such foods are produced primarily from substances extracted from whole food sources, such as sugar, salt, oil, and protein. Alternatively, they may be created in a laboratory using flavor enhancers and food coloring. This classification has caused the public and health professionals to question the potential health implications of PBMAs, said Dr. Ur.
“One of the concerns is that these products are highly processed, and things that are highly processed are considered bad. And so, are you swapping one set of risks for another?” he said.
To shed more light on this question, Dr. Ur’s team, which was led by Matthew Nagra, ND, of the Vancouver Naturopathic Clinic, assessed the literature on PBMAs and their impact on health.
“While the plant-based meat market has experienced significant growth in recent years and more and more Canadians are enjoying plant-based burgers, surprisingly little is known about how these meat alternatives may impact health and, in particular, cardiovascular disease risk,” Dr. Nagra said in a statement. “Thus, we sought to review the available literature on the topic to identify what is currently known and to provide direction for future research.”
Less Saturated Fat, Cholesterol
The researchers assessed the literature that was published from 1970 to 2023 on PBMAs, their contents, nutritional profiles, and impact on cardiovascular disease risk factors, such as cholesterol levels and blood pressure.
They found that, compared with meat, PBMAs had less saturated fat, less cholesterol, more fiber, more carbohydrates, fewer calories, less monounsaturated fat, more polyunsaturated fat, and more sodium.
In addition, several randomized controlled trials showed that PBMAs reduced total cholesterol and LDL-C, as well as apolipoprotein B-100, body weight, and waist circumference. PBMAs were not shown to raise blood pressure, despite some products’ high sodium content.
“No currently available evidence suggests that the concerning aspects of PBMAs (eg, food processing and high sodium content) negate the potential cardiovascular benefits,” wrote the researchers.
Unfortunately, no long-term research has evaluated how these alternatives may affect the risk of developing a myocardial infarction or stroke. Similarly, there is little research on the healthfulness of some common components of PBMAs, such as vital wheat gluten.
To shed light on these important issues would require large clinical trials, involving many patients, and great expense, said Dr. Ur. “Drug companies can afford to do large clinical trials, even if they are expensive to do, because they must do them to get approval for their drug. But these plant-based meats are produced by companies that most likely are not able to do clinical outcome trials. Such trials would have to be done by the National Institutes of Health in the United States, or in Canada, the National Research Council,” he said.
There are many reasons to avoid meat, Dr. Ur added. “There are ethical reasons against killing animals. Then there is the issue of global warming. Meat is a very expensive source of food energy. As an individual, the biggest impact you can make on global warming is to not eat meat. Then there is the argument about personal health, which is where our study comes in. For those people who like the taste of meat and who struggle with giving it up, the PBMAs allow them to have a reasonably diverse diet,” he said.
Are Eggs Healthy?
Meat substitutes are helpful for people who want to reduce their cardiovascular disease risk, J. David Spence, MD, professor emeritus of neurology and clinical pharmacology at the University of Western Ontario in London, Canada, wrote in an accompanying editorial.
“Eating too much meat and egg yolk increases cardiovascular risk, and it’s a challenge for patients to learn to eat less meat and cut out egg yolks. If we can find good substitutes that are tasty and enjoyable, that’s a good thing,” Dr. Spence told this news organization.
“Besides plant-based meat substitutes, there is great potential for reduction of cardiovascular risk with the use of egg substitutes,” he said.
Dr. Spence pointed out that two large egg yolks contain 474 mg of cholesterol, almost twice the amount contained in a Hardee’s Monster Thickburger (265 mg).
Cholesterol elevates plasma levels of toxic metabolites of the intestinal microbiome, such as trimethylamine N-oxide (TMAO). Plasma levels of TMAO increase in a linear fashion with egg consumption, and TMAO is bad for the arteries, said Dr. Spence.
“Eggs are terrible and should not be eaten by people at risk for cardiovascular disease. But people don’t understand that because the egg marketing propaganda has been so effective. The yolk is terrible. The egg marketing board is extremely effective in persuading people that eggs are healthy, and they’re not.”
Dr. Spence recommends using egg substitutes, such as Egg Beaters or Better’n Eggs, instead of whole eggs, and says it’s never too late to switch. “That’s the mistake people make, but the arteries can actually improve,” he said.
No funding source for the study was reported. Dr. Ur and Dr. Spence reported having no relevant financial relationships.
A version of this article appeared on Medscape.com.
Facial Temperature Can Reveal Age and Disease
This transcript has been edited for clarity.
My oldest daughter is at sleepaway camp for a couple of weeks, and the camp has a photographer who goes around all day taking pictures of the kids, which get uploaded to a private Facebook group. In the past, I would go online every day (or, okay, several times a day) and scroll through all those pictures looking for one that features my kid.
I don’t have to do that anymore. This year, I simply uploaded a picture of my daughter to an app and artificial intelligence (AI) takes care of the rest, recognizing her face amidst the sea of smiling children, and flagging just those photos for me to peruse. It’s amazing, really. And a bit scary.
The fact that facial recognition has penetrated the summer camp market should tell you that the tech is truly ubiquitous. But today we’re going to think a bit more about what AI can do with a picture of your face, because the power of facial recognition is not just skin deep.
What’s got me hot and bothered about facial images is this paper, appearing in Cell Metabolism, which adds a new layer to the standard facial-analysis playbook: facial temperature.
To understand this paper, you need to understand a whole field of research that is developing various different “clocks” for age.
It turns out that age really is just a number. Our cells, our proteins, our biochemistry can be analyzed to give different numbers. These “clocks,” as distinct from the calendar we usually use to measure our age, might have more predictive power than the number itself.
There are numerous molecular clocks, such as telomere length, that not only correlate with calendar age but are superior to calendar age in predicting age-related complications. Testing telomere length typically requires a blood sample — and remains costly. But we can use other sources to estimate age; how about a photo?
I mean, we do this all the time when we meet someone new or, as a physician, when we meet a new patient. I have often written that a patient “appears younger than their stated age,” and we’ve all had the experience of hearing how old someone is and being shocked. I mean, have you seen Sharon Stone recently? She’s 66 years old. Okay — to be fair, there might be some outside help there. But you get the point.
Back to the Cell Metabolism paper. Researchers report on multiple algorithms to obtain an “age” from a picture of an individual’s face.
The first algorithm is pretty straightforward. Researchers collected 2811 images, all of Han Chinese individuals ranging in age from 20 to 90 years, and reconstructed a 3D facial map from those. 
They then trained a convolutional neural network to predict the individuals’ ages from the pictures. It was quite accurate, as you can see here.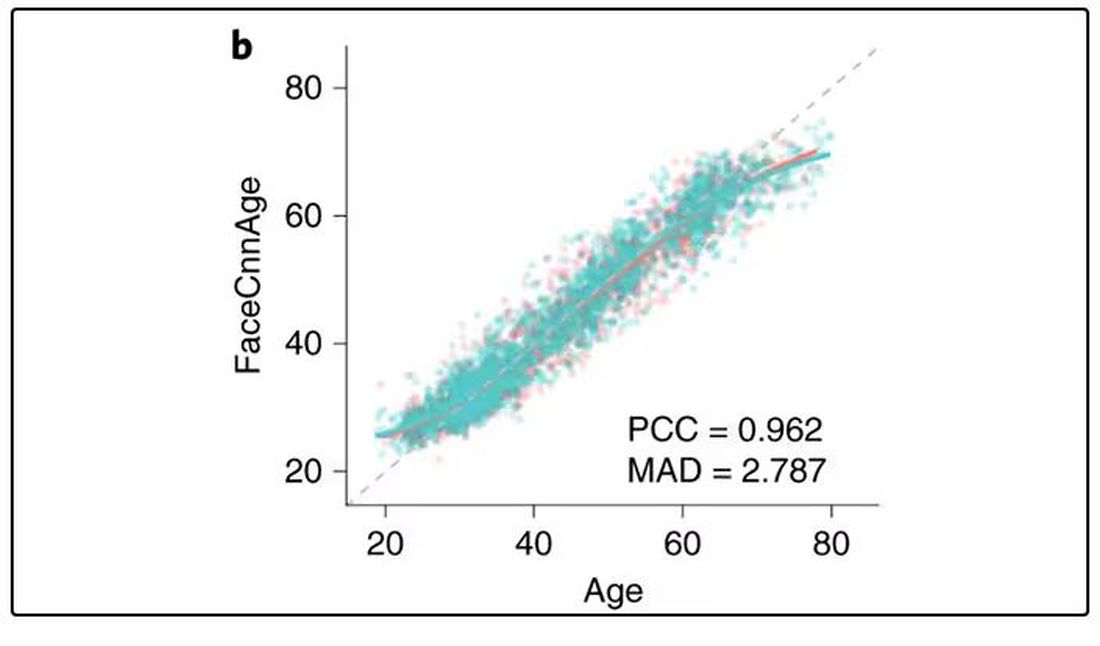
In the AI age, this may not seem that impressive. A brief search online turned up dozens of apps that promised to guess my age from a photo.
I sent this rather unflattering picture of myself to ChatGPT which, after initially demurring and saying it was not designed to guess ages, pegged me at somewhere between 35 and 45, which I am taking as a major victory.
But the Cell Metabolism paper goes deeper. Literally. 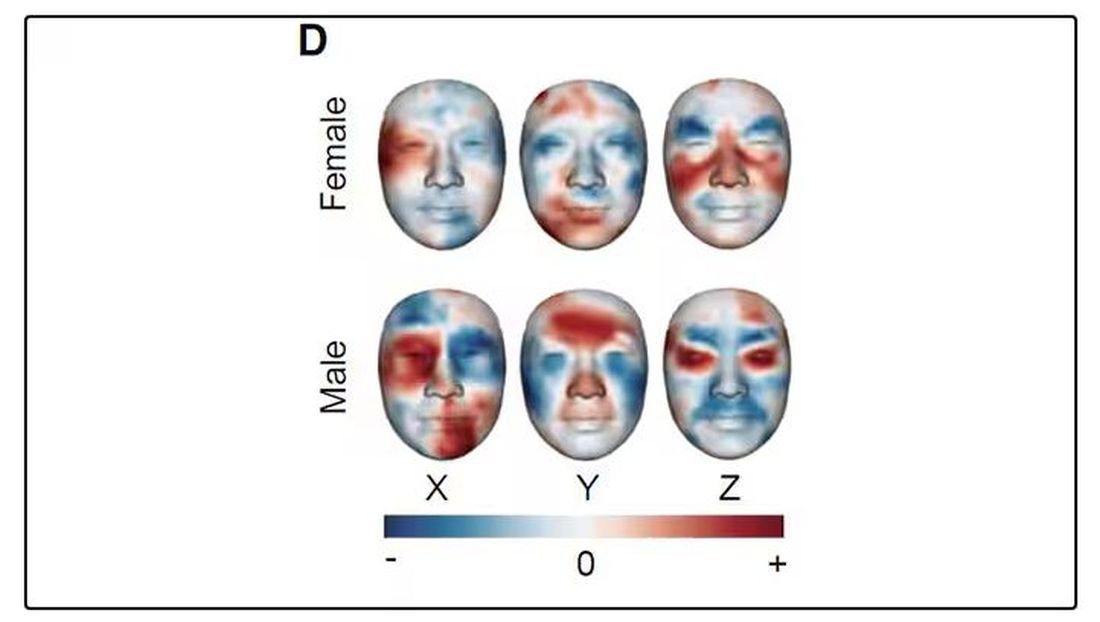
And this is where things start to get interesting. Because sure, the visible part of your face can change depending on makeup, expression, plastic surgery, and the like. But the temperature? That’s harder to fake.
It turns out that the temperature distribution in your face changes as you get older. There is a cooling of the nose and the cheeks, for example.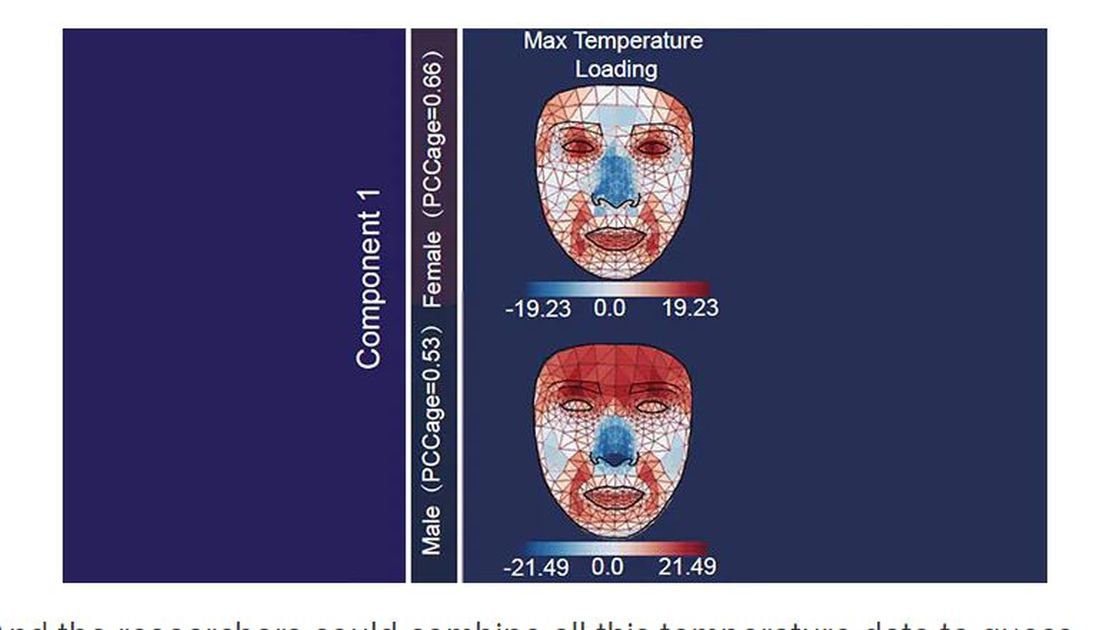
And the researchers could combine all this temperature data to guess someone’s calendar age fairly accurately, though notably not as accurately as the model that just looks at the pictures.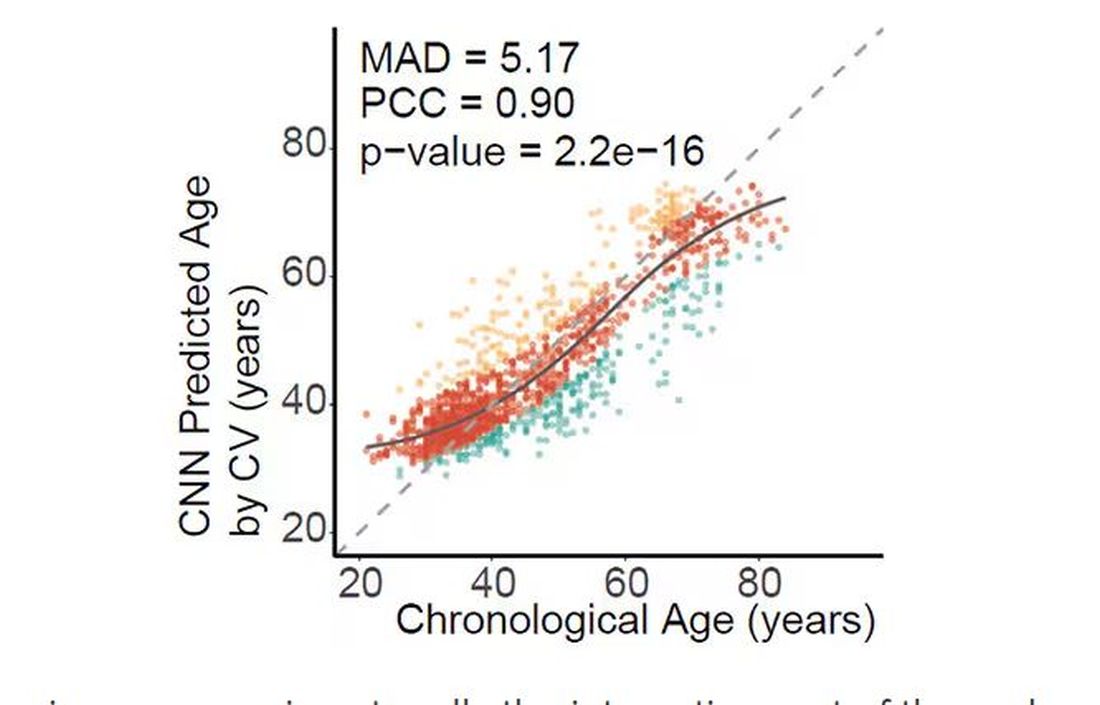
But guessing your age is not really the interesting part of thermal imaging of the face. It’s guessing — or, rather, predicting — the state of your metabolism. All these study participants had extensive metabolic testing performed, as well as detailed analysis of their lifestyle behaviors. And facial images could be used to predict those factors.
For example, the 3D reconstruction of the faces could predict who ate seafood (they tend to look younger than their actual age) compared with who ate poultry and meat (they tend to look older). The thermal imaging could predict who got more sleep (they look younger from a temperature perspective) and who ate more yogurt (also younger-appearing, temperature-wise). Facial temperature patterns could identify those with higher BMI, higher blood pressure, higher fasting glucose.
The researchers used the difference between actual and predicted age as a metric to measure illness as well. You can see here how, on average, individuals with hypertension, diabetes, and even liver cysts are “older,” at least by face temperature.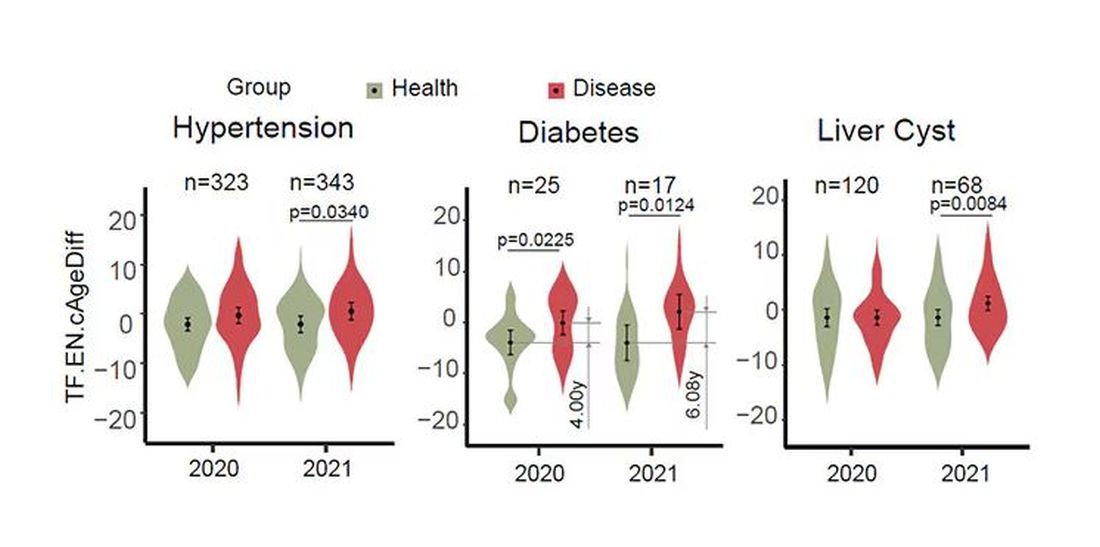
It may even be possible to use facial temperature as biofeedback. In a small study, the researchers measured the difference between facial temperature age and real age before and after 2 weeks of jump-roping. It turns out that 2 weeks of jump-roping can make you look about 5 years younger, at least as judged by a thermal camera. Or like the Predator.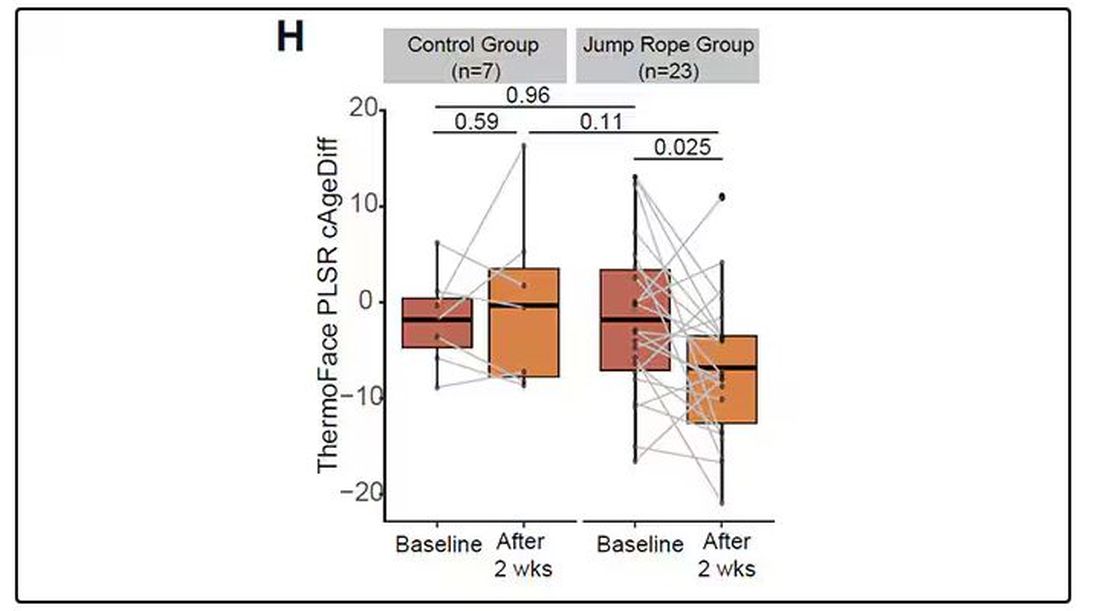
Okay, this is all very cool, but I’m not saying we’ll all be doing facial temperature tests in the near future. No; what this study highlights for me is how much information about ourselves is available to those who know how to decode it. Maybe those data come from the wrinkles in our faces, or the angles of our smiles, or the speed with which we type, or the temperature of our elbows. The data have always been there, actually, but we’ve never had the tools powerful enough to analyze them until now.
When I was a kid, I was obsessed with Star Trek — I know, you’re shocked — and, of course, the famous tricorder, a scanner that could tell everything about someone’s state of health in 5 seconds from 3 feet away. That’s how I thought medicine really would be in the future. Once I got to medical school, I was disabused of that notion. But the age of data, the age of AI, may mean the tricorder age is not actually that far away.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
My oldest daughter is at sleepaway camp for a couple of weeks, and the camp has a photographer who goes around all day taking pictures of the kids, which get uploaded to a private Facebook group. In the past, I would go online every day (or, okay, several times a day) and scroll through all those pictures looking for one that features my kid.
I don’t have to do that anymore. This year, I simply uploaded a picture of my daughter to an app and artificial intelligence (AI) takes care of the rest, recognizing her face amidst the sea of smiling children, and flagging just those photos for me to peruse. It’s amazing, really. And a bit scary.
The fact that facial recognition has penetrated the summer camp market should tell you that the tech is truly ubiquitous. But today we’re going to think a bit more about what AI can do with a picture of your face, because the power of facial recognition is not just skin deep.
What’s got me hot and bothered about facial images is this paper, appearing in Cell Metabolism, which adds a new layer to the standard facial-analysis playbook: facial temperature.
To understand this paper, you need to understand a whole field of research that is developing various different “clocks” for age.
It turns out that age really is just a number. Our cells, our proteins, our biochemistry can be analyzed to give different numbers. These “clocks,” as distinct from the calendar we usually use to measure our age, might have more predictive power than the number itself.
There are numerous molecular clocks, such as telomere length, that not only correlate with calendar age but are superior to calendar age in predicting age-related complications. Testing telomere length typically requires a blood sample — and remains costly. But we can use other sources to estimate age; how about a photo?
I mean, we do this all the time when we meet someone new or, as a physician, when we meet a new patient. I have often written that a patient “appears younger than their stated age,” and we’ve all had the experience of hearing how old someone is and being shocked. I mean, have you seen Sharon Stone recently? She’s 66 years old. Okay — to be fair, there might be some outside help there. But you get the point.
Back to the Cell Metabolism paper. Researchers report on multiple algorithms to obtain an “age” from a picture of an individual’s face.
The first algorithm is pretty straightforward. Researchers collected 2811 images, all of Han Chinese individuals ranging in age from 20 to 90 years, and reconstructed a 3D facial map from those. 
They then trained a convolutional neural network to predict the individuals’ ages from the pictures. It was quite accurate, as you can see here.
In the AI age, this may not seem that impressive. A brief search online turned up dozens of apps that promised to guess my age from a photo.
I sent this rather unflattering picture of myself to ChatGPT which, after initially demurring and saying it was not designed to guess ages, pegged me at somewhere between 35 and 45, which I am taking as a major victory.
But the Cell Metabolism paper goes deeper. Literally. 
And this is where things start to get interesting. Because sure, the visible part of your face can change depending on makeup, expression, plastic surgery, and the like. But the temperature? That’s harder to fake.
It turns out that the temperature distribution in your face changes as you get older. There is a cooling of the nose and the cheeks, for example.
And the researchers could combine all this temperature data to guess someone’s calendar age fairly accurately, though notably not as accurately as the model that just looks at the pictures.
But guessing your age is not really the interesting part of thermal imaging of the face. It’s guessing — or, rather, predicting — the state of your metabolism. All these study participants had extensive metabolic testing performed, as well as detailed analysis of their lifestyle behaviors. And facial images could be used to predict those factors.
For example, the 3D reconstruction of the faces could predict who ate seafood (they tend to look younger than their actual age) compared with who ate poultry and meat (they tend to look older). The thermal imaging could predict who got more sleep (they look younger from a temperature perspective) and who ate more yogurt (also younger-appearing, temperature-wise). Facial temperature patterns could identify those with higher BMI, higher blood pressure, higher fasting glucose.
The researchers used the difference between actual and predicted age as a metric to measure illness as well. You can see here how, on average, individuals with hypertension, diabetes, and even liver cysts are “older,” at least by face temperature.
It may even be possible to use facial temperature as biofeedback. In a small study, the researchers measured the difference between facial temperature age and real age before and after 2 weeks of jump-roping. It turns out that 2 weeks of jump-roping can make you look about 5 years younger, at least as judged by a thermal camera. Or like the Predator.
Okay, this is all very cool, but I’m not saying we’ll all be doing facial temperature tests in the near future. No; what this study highlights for me is how much information about ourselves is available to those who know how to decode it. Maybe those data come from the wrinkles in our faces, or the angles of our smiles, or the speed with which we type, or the temperature of our elbows. The data have always been there, actually, but we’ve never had the tools powerful enough to analyze them until now.
When I was a kid, I was obsessed with Star Trek — I know, you’re shocked — and, of course, the famous tricorder, a scanner that could tell everything about someone’s state of health in 5 seconds from 3 feet away. That’s how I thought medicine really would be in the future. Once I got to medical school, I was disabused of that notion. But the age of data, the age of AI, may mean the tricorder age is not actually that far away.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
My oldest daughter is at sleepaway camp for a couple of weeks, and the camp has a photographer who goes around all day taking pictures of the kids, which get uploaded to a private Facebook group. In the past, I would go online every day (or, okay, several times a day) and scroll through all those pictures looking for one that features my kid.
I don’t have to do that anymore. This year, I simply uploaded a picture of my daughter to an app and artificial intelligence (AI) takes care of the rest, recognizing her face amidst the sea of smiling children, and flagging just those photos for me to peruse. It’s amazing, really. And a bit scary.
The fact that facial recognition has penetrated the summer camp market should tell you that the tech is truly ubiquitous. But today we’re going to think a bit more about what AI can do with a picture of your face, because the power of facial recognition is not just skin deep.
What’s got me hot and bothered about facial images is this paper, appearing in Cell Metabolism, which adds a new layer to the standard facial-analysis playbook: facial temperature.
To understand this paper, you need to understand a whole field of research that is developing various different “clocks” for age.
It turns out that age really is just a number. Our cells, our proteins, our biochemistry can be analyzed to give different numbers. These “clocks,” as distinct from the calendar we usually use to measure our age, might have more predictive power than the number itself.
There are numerous molecular clocks, such as telomere length, that not only correlate with calendar age but are superior to calendar age in predicting age-related complications. Testing telomere length typically requires a blood sample — and remains costly. But we can use other sources to estimate age; how about a photo?
I mean, we do this all the time when we meet someone new or, as a physician, when we meet a new patient. I have often written that a patient “appears younger than their stated age,” and we’ve all had the experience of hearing how old someone is and being shocked. I mean, have you seen Sharon Stone recently? She’s 66 years old. Okay — to be fair, there might be some outside help there. But you get the point.
Back to the Cell Metabolism paper. Researchers report on multiple algorithms to obtain an “age” from a picture of an individual’s face.
The first algorithm is pretty straightforward. Researchers collected 2811 images, all of Han Chinese individuals ranging in age from 20 to 90 years, and reconstructed a 3D facial map from those. 
They then trained a convolutional neural network to predict the individuals’ ages from the pictures. It was quite accurate, as you can see here.
In the AI age, this may not seem that impressive. A brief search online turned up dozens of apps that promised to guess my age from a photo.
I sent this rather unflattering picture of myself to ChatGPT which, after initially demurring and saying it was not designed to guess ages, pegged me at somewhere between 35 and 45, which I am taking as a major victory.
But the Cell Metabolism paper goes deeper. Literally. 
And this is where things start to get interesting. Because sure, the visible part of your face can change depending on makeup, expression, plastic surgery, and the like. But the temperature? That’s harder to fake.
It turns out that the temperature distribution in your face changes as you get older. There is a cooling of the nose and the cheeks, for example.
And the researchers could combine all this temperature data to guess someone’s calendar age fairly accurately, though notably not as accurately as the model that just looks at the pictures.
But guessing your age is not really the interesting part of thermal imaging of the face. It’s guessing — or, rather, predicting — the state of your metabolism. All these study participants had extensive metabolic testing performed, as well as detailed analysis of their lifestyle behaviors. And facial images could be used to predict those factors.
For example, the 3D reconstruction of the faces could predict who ate seafood (they tend to look younger than their actual age) compared with who ate poultry and meat (they tend to look older). The thermal imaging could predict who got more sleep (they look younger from a temperature perspective) and who ate more yogurt (also younger-appearing, temperature-wise). Facial temperature patterns could identify those with higher BMI, higher blood pressure, higher fasting glucose.
The researchers used the difference between actual and predicted age as a metric to measure illness as well. You can see here how, on average, individuals with hypertension, diabetes, and even liver cysts are “older,” at least by face temperature.
It may even be possible to use facial temperature as biofeedback. In a small study, the researchers measured the difference between facial temperature age and real age before and after 2 weeks of jump-roping. It turns out that 2 weeks of jump-roping can make you look about 5 years younger, at least as judged by a thermal camera. Or like the Predator.
Okay, this is all very cool, but I’m not saying we’ll all be doing facial temperature tests in the near future. No; what this study highlights for me is how much information about ourselves is available to those who know how to decode it. Maybe those data come from the wrinkles in our faces, or the angles of our smiles, or the speed with which we type, or the temperature of our elbows. The data have always been there, actually, but we’ve never had the tools powerful enough to analyze them until now.
When I was a kid, I was obsessed with Star Trek — I know, you’re shocked — and, of course, the famous tricorder, a scanner that could tell everything about someone’s state of health in 5 seconds from 3 feet away. That’s how I thought medicine really would be in the future. Once I got to medical school, I was disabused of that notion. But the age of data, the age of AI, may mean the tricorder age is not actually that far away.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiovascular Health Becoming a Major Risk Factor for Dementia
That’s according to researchers from University College London (UCL) in the United Kingdom who analyzed 27 papers about dementia that had data collected over more than 70 years. They calculated what share of dementia cases were due to different risk factors. Their findings were recently published in the Lancet Public Health.
Top risk factors for dementia over the years have been hypertension, obesity, diabetes, education, and smoking, according to a news release on the findings. But the prevalence of risk factors has changed over the decades.
Researchers said smoking and education have become less important risk factors because of “population-level interventions,” such as stop-smoking campaigns and compulsory public education. On the other hand, obesity and diabetes rates have increased and become bigger risk factors.
Hypertension remains the greatest risk factor, even though doctors and public health groups are putting more emphasis on managing the condition, the study said.
“Cardiovascular risk factors may have contributed more to dementia risk over time, so these deserve more targeted action for future dementia prevention efforts,” said Naaheed Mukadam, PhD, an associate professor at UCL and the lead author of the study.
Eliminating modifiable risk factors could theoretically prevent 40% of dementia cases, the release said.
The CDC says that an estimated 5.8 million people in the United States have Alzheimer’s disease and related dementias, including 5.6 million people ages 65 and older and about 200,000 under age 65. The UCL release said an estimated 944,000 in the U.K. have dementia.
A version of this article first appeared on WebMD.com.
That’s according to researchers from University College London (UCL) in the United Kingdom who analyzed 27 papers about dementia that had data collected over more than 70 years. They calculated what share of dementia cases were due to different risk factors. Their findings were recently published in the Lancet Public Health.
Top risk factors for dementia over the years have been hypertension, obesity, diabetes, education, and smoking, according to a news release on the findings. But the prevalence of risk factors has changed over the decades.
Researchers said smoking and education have become less important risk factors because of “population-level interventions,” such as stop-smoking campaigns and compulsory public education. On the other hand, obesity and diabetes rates have increased and become bigger risk factors.
Hypertension remains the greatest risk factor, even though doctors and public health groups are putting more emphasis on managing the condition, the study said.
“Cardiovascular risk factors may have contributed more to dementia risk over time, so these deserve more targeted action for future dementia prevention efforts,” said Naaheed Mukadam, PhD, an associate professor at UCL and the lead author of the study.
Eliminating modifiable risk factors could theoretically prevent 40% of dementia cases, the release said.
The CDC says that an estimated 5.8 million people in the United States have Alzheimer’s disease and related dementias, including 5.6 million people ages 65 and older and about 200,000 under age 65. The UCL release said an estimated 944,000 in the U.K. have dementia.
A version of this article first appeared on WebMD.com.
That’s according to researchers from University College London (UCL) in the United Kingdom who analyzed 27 papers about dementia that had data collected over more than 70 years. They calculated what share of dementia cases were due to different risk factors. Their findings were recently published in the Lancet Public Health.
Top risk factors for dementia over the years have been hypertension, obesity, diabetes, education, and smoking, according to a news release on the findings. But the prevalence of risk factors has changed over the decades.
Researchers said smoking and education have become less important risk factors because of “population-level interventions,” such as stop-smoking campaigns and compulsory public education. On the other hand, obesity and diabetes rates have increased and become bigger risk factors.
Hypertension remains the greatest risk factor, even though doctors and public health groups are putting more emphasis on managing the condition, the study said.
“Cardiovascular risk factors may have contributed more to dementia risk over time, so these deserve more targeted action for future dementia prevention efforts,” said Naaheed Mukadam, PhD, an associate professor at UCL and the lead author of the study.
Eliminating modifiable risk factors could theoretically prevent 40% of dementia cases, the release said.
The CDC says that an estimated 5.8 million people in the United States have Alzheimer’s disease and related dementias, including 5.6 million people ages 65 and older and about 200,000 under age 65. The UCL release said an estimated 944,000 in the U.K. have dementia.
A version of this article first appeared on WebMD.com.
FROM THE LANCET PUBLIC HEALTH
Similar Outcomes With Labetalol, Nifedipine for Chronic Hypertension in Pregnancy
Treatment for chronic hypertension in pregnancy with labetalol showed no significant differences in maternal or neonatal outcomes, compared with treatment with nifedipine, new research indicates.
The open-label, multicenter, randomized CHAP (Chronic Hypertension in Pregnancy) trial showed that treating mild chronic hypertension was better than delaying treatment until severe hypertension developed, but still unclear was whether, or to what extent, the choice of first-line treatment affected outcomes.
Researchers, led by Ayodeji A. Sanusi, MD, MPH, with the Division of Maternal and Fetal Medicine at the University of Alabama at Birmingham, conducted a secondary analysis of CHAP to compare the primary treatments. Mild chronic hypertension in the study was defined as blood pressure of 140-159/90-104 mmHg before 20 weeks of gestation.
Three Comparisons
Three comparisons were performed in 2292 participants based on medications prescribed at enrollment: 720 (31.4%) received labetalol; 417 (18.2%) initially received nifedipine; and 1155 (50.4%) had standard care. Labetalol was compared with standard care; nifedipine was compared with standard care; and labetalol was compared with nifedipine.
The primary outcome was occurrence of superimposed preeclampsia with severe features; preterm birth before 35 weeks of gestation; placental abruption; or fetal or neonatal death. The key secondary outcome was a small-for-gestational age neonate. Researchers also compared adverse effects between groups.
Among the results were the following:
- The primary outcome occurred in 30.1% in the labetalol group; 31.2% in the nifedipine group; and 37% in the standard care group.
- Risk of the primary outcome was lower among those receiving treatment. For labetalol vs standard care, the adjusted relative risk (RR) was 0.82; 95% confidence interval (CI), 0.72-0.94. For nifedipine vs standard care, the adjusted RR was 0.84; 95% CI, 0.71-0.99. There was no significant difference in risk when labetalol was compared with nifedipine (adjusted RR, 0.98; 95% CI, 0.82-1.18).
- There were no significant differences in numbers of small-for-gestational age neonates or serious adverse events between those who received labetalol and those using nifedipine.
Any adverse events were significantly more common with nifedipine, compared with labetalol (35.7% vs 28.3%, P = .009), and with nifedipine, compared with standard care (35.7% vs 26.3%, P = .0003). Adverse event rates were not significantly higher with labetalol when compared with standard care (28.3% vs 26.3%, P = .34). The most frequently reported adverse events were headache, medication intolerance, dizziness, nausea, dyspepsia, neonatal jaundice, and vomiting.
“Thus, labetalol compared with nifedipine appeared to have fewer adverse events and to be better tolerated,” the authors write. They note that labetalol, a third-generation mixed alpha- and beta-adrenergic antagonist, is contraindicated for those who have obstructive pulmonary disease and nifedipine, a dihydropyridine calcium channel blocker, is contraindicated in people with tachycardia.
The authors write that their results align with other studies that have not found differences between labetalol and nifedipine. “[O]ur findings support the use of either labetalol or nifedipine as initial first-line agents for the management of mild chronic hypertension in pregnancy to reduce the risk of adverse maternal and other perinatal outcomes with no increased risk of fetal harm,” the authors write.
Dr. Sanusi reports no relevant financial relationships. Full coauthor disclosures are available with the full text of the paper.
Treatment for chronic hypertension in pregnancy with labetalol showed no significant differences in maternal or neonatal outcomes, compared with treatment with nifedipine, new research indicates.
The open-label, multicenter, randomized CHAP (Chronic Hypertension in Pregnancy) trial showed that treating mild chronic hypertension was better than delaying treatment until severe hypertension developed, but still unclear was whether, or to what extent, the choice of first-line treatment affected outcomes.
Researchers, led by Ayodeji A. Sanusi, MD, MPH, with the Division of Maternal and Fetal Medicine at the University of Alabama at Birmingham, conducted a secondary analysis of CHAP to compare the primary treatments. Mild chronic hypertension in the study was defined as blood pressure of 140-159/90-104 mmHg before 20 weeks of gestation.
Three Comparisons
Three comparisons were performed in 2292 participants based on medications prescribed at enrollment: 720 (31.4%) received labetalol; 417 (18.2%) initially received nifedipine; and 1155 (50.4%) had standard care. Labetalol was compared with standard care; nifedipine was compared with standard care; and labetalol was compared with nifedipine.
The primary outcome was occurrence of superimposed preeclampsia with severe features; preterm birth before 35 weeks of gestation; placental abruption; or fetal or neonatal death. The key secondary outcome was a small-for-gestational age neonate. Researchers also compared adverse effects between groups.
Among the results were the following:
- The primary outcome occurred in 30.1% in the labetalol group; 31.2% in the nifedipine group; and 37% in the standard care group.
- Risk of the primary outcome was lower among those receiving treatment. For labetalol vs standard care, the adjusted relative risk (RR) was 0.82; 95% confidence interval (CI), 0.72-0.94. For nifedipine vs standard care, the adjusted RR was 0.84; 95% CI, 0.71-0.99. There was no significant difference in risk when labetalol was compared with nifedipine (adjusted RR, 0.98; 95% CI, 0.82-1.18).
- There were no significant differences in numbers of small-for-gestational age neonates or serious adverse events between those who received labetalol and those using nifedipine.
Any adverse events were significantly more common with nifedipine, compared with labetalol (35.7% vs 28.3%, P = .009), and with nifedipine, compared with standard care (35.7% vs 26.3%, P = .0003). Adverse event rates were not significantly higher with labetalol when compared with standard care (28.3% vs 26.3%, P = .34). The most frequently reported adverse events were headache, medication intolerance, dizziness, nausea, dyspepsia, neonatal jaundice, and vomiting.
“Thus, labetalol compared with nifedipine appeared to have fewer adverse events and to be better tolerated,” the authors write. They note that labetalol, a third-generation mixed alpha- and beta-adrenergic antagonist, is contraindicated for those who have obstructive pulmonary disease and nifedipine, a dihydropyridine calcium channel blocker, is contraindicated in people with tachycardia.
The authors write that their results align with other studies that have not found differences between labetalol and nifedipine. “[O]ur findings support the use of either labetalol or nifedipine as initial first-line agents for the management of mild chronic hypertension in pregnancy to reduce the risk of adverse maternal and other perinatal outcomes with no increased risk of fetal harm,” the authors write.
Dr. Sanusi reports no relevant financial relationships. Full coauthor disclosures are available with the full text of the paper.
Treatment for chronic hypertension in pregnancy with labetalol showed no significant differences in maternal or neonatal outcomes, compared with treatment with nifedipine, new research indicates.
The open-label, multicenter, randomized CHAP (Chronic Hypertension in Pregnancy) trial showed that treating mild chronic hypertension was better than delaying treatment until severe hypertension developed, but still unclear was whether, or to what extent, the choice of first-line treatment affected outcomes.
Researchers, led by Ayodeji A. Sanusi, MD, MPH, with the Division of Maternal and Fetal Medicine at the University of Alabama at Birmingham, conducted a secondary analysis of CHAP to compare the primary treatments. Mild chronic hypertension in the study was defined as blood pressure of 140-159/90-104 mmHg before 20 weeks of gestation.
Three Comparisons
Three comparisons were performed in 2292 participants based on medications prescribed at enrollment: 720 (31.4%) received labetalol; 417 (18.2%) initially received nifedipine; and 1155 (50.4%) had standard care. Labetalol was compared with standard care; nifedipine was compared with standard care; and labetalol was compared with nifedipine.
The primary outcome was occurrence of superimposed preeclampsia with severe features; preterm birth before 35 weeks of gestation; placental abruption; or fetal or neonatal death. The key secondary outcome was a small-for-gestational age neonate. Researchers also compared adverse effects between groups.
Among the results were the following:
- The primary outcome occurred in 30.1% in the labetalol group; 31.2% in the nifedipine group; and 37% in the standard care group.
- Risk of the primary outcome was lower among those receiving treatment. For labetalol vs standard care, the adjusted relative risk (RR) was 0.82; 95% confidence interval (CI), 0.72-0.94. For nifedipine vs standard care, the adjusted RR was 0.84; 95% CI, 0.71-0.99. There was no significant difference in risk when labetalol was compared with nifedipine (adjusted RR, 0.98; 95% CI, 0.82-1.18).
- There were no significant differences in numbers of small-for-gestational age neonates or serious adverse events between those who received labetalol and those using nifedipine.
Any adverse events were significantly more common with nifedipine, compared with labetalol (35.7% vs 28.3%, P = .009), and with nifedipine, compared with standard care (35.7% vs 26.3%, P = .0003). Adverse event rates were not significantly higher with labetalol when compared with standard care (28.3% vs 26.3%, P = .34). The most frequently reported adverse events were headache, medication intolerance, dizziness, nausea, dyspepsia, neonatal jaundice, and vomiting.
“Thus, labetalol compared with nifedipine appeared to have fewer adverse events and to be better tolerated,” the authors write. They note that labetalol, a third-generation mixed alpha- and beta-adrenergic antagonist, is contraindicated for those who have obstructive pulmonary disease and nifedipine, a dihydropyridine calcium channel blocker, is contraindicated in people with tachycardia.
The authors write that their results align with other studies that have not found differences between labetalol and nifedipine. “[O]ur findings support the use of either labetalol or nifedipine as initial first-line agents for the management of mild chronic hypertension in pregnancy to reduce the risk of adverse maternal and other perinatal outcomes with no increased risk of fetal harm,” the authors write.
Dr. Sanusi reports no relevant financial relationships. Full coauthor disclosures are available with the full text of the paper.
FROM OBSTETRICS & GYNECOLOGY
