User login
Endovascular device sustains blood pressure control after 3 years
WASHINGTON – As a result of remarkably sustained antihypertensive effect, interest is intensifying in the potential for a pivotal trial to associate a novel endovascular device with unprecedented blood pressure control in patients with treatment-resistant hypertension, according to an update presented at CRT 2019, sponsored by MedStar Heart & Vascular Institute.
With up to 3 years of follow-up, “systolic blood pressures have remained persistently reduced by as much as 24 mm Hg,” reported John P. Reilly, MD, an interventional cardiologist in Southampton, N.Y., who presented follow-up data for some of those enrolled in the first-in-human study of this device.
When the stent-like device is placed in the carotid artery, it alters its geometric shape, which increases pulsatile wall strain. The increase on wall strain alters an afferent signaling loop controlled by carotid baroreceptors that inhibits sympathetic outflow to lower blood pressure.
In the proof-of-principle, first-in-human CALM study, 47 patients were implanted with the device (MobiusHD, Vascular Dynamics). The initial study enrolled 30 subjects in Europe and 17 in the United States. Initial findings in the cohort of European patients, which included a mean 21–mm Hg reduction in systolic blood pressure and a 12–mm Hg reduction in diastolic blood pressure measured by ambulatory monitoring at 6 months, were published in the Lancet (2017 Dec 16;390[10113]:2655-661).
The patients enrolled in the proof-of-principle CALM trial were required to have highly-treatment-resistant hypertension, defined as a systolic blood pressure greater than or equal to 160 mm Hg despite at least three antihypertensive medications. The average number of medications was 4.4, according to Dr. Reilly. The mean blood pressure at entry was 165/98 mm Hg. Nearly 20% had previously undergone renal denervation.
The device was successfully deployed in all of the patients who participated in the open-label CALM study. Most of the 10 serious adverse events were related to hypotension, according to Dr. Reilly. Others included a wound infection and a case of intermittent claudication. Two instances of neurologic complaints, such as numbness and weakness, experienced within a day of device placement were considered potential transient ischemic attacks, but these resolved completely and no defects were observed on imaging.
In an update on CALM, Dr. Reilly reported that the large reductions in blood pressure previously reported at 6 months have been sustained. Follow-up is approximately 3 years in most patients, and the reductions previously reported have persisted in responders. When a clinically significant response is defined as a 10–mm Hg or more reduction in office blood pressure or 5–mm Hg or more reduction in ambulatory blood pressure, 75% of patients enrolled are still responding, but the more important point is that there has been no substantial reduction in blood pressure control over time in responders, according to Dr. Reilly.
When patients were stratified by a pulse pressure of greater or less than 70 mm Hg at study entry, response rates have been similar, he added.
The long-term responses are significant because there was concern about tachyphylaxis. In fact, coronary stents also produce a reduction in blood pressure immediately after placement that is likely caused by the same effect, but that effect “peters out in a day or 2,” noted Dr. Reilly. As opposed to the round shape of coronary stents, the rectangular shape of the novel device produces “an increase in the perceived strain on the carotid body” that does not appear to diminish over time.
CALM-2, which is designed to be a pivotal trial to support regulatory approval of the device, began enrolling in September 2018. An enrollment of 300 patients with treatment-resistant hypertension is planned. Participants will be randomized to receive the device or a sham procedure consisting of a carotid artery angiogram, according to Dr. Reilly. Although the initial CALM trial was small, open label, and conducted without a control, the persistent benefit over extended follow-up is driving excitement about the potential of this device.
“These are some of the greatest sustained reductions in ambulatory blood pressure we have ever seen,” according to Vasilios Papademetriou, MD, PhD, a professor of medicine at Georgetown University, Washington. Impressed by undiminished blood pressure control observed so far, he characterized the promise of this device as “very compelling.”
Dr. Reilly disclosed that he was a stockholder in Johnson & Johnson.
WASHINGTON – As a result of remarkably sustained antihypertensive effect, interest is intensifying in the potential for a pivotal trial to associate a novel endovascular device with unprecedented blood pressure control in patients with treatment-resistant hypertension, according to an update presented at CRT 2019, sponsored by MedStar Heart & Vascular Institute.
With up to 3 years of follow-up, “systolic blood pressures have remained persistently reduced by as much as 24 mm Hg,” reported John P. Reilly, MD, an interventional cardiologist in Southampton, N.Y., who presented follow-up data for some of those enrolled in the first-in-human study of this device.
When the stent-like device is placed in the carotid artery, it alters its geometric shape, which increases pulsatile wall strain. The increase on wall strain alters an afferent signaling loop controlled by carotid baroreceptors that inhibits sympathetic outflow to lower blood pressure.
In the proof-of-principle, first-in-human CALM study, 47 patients were implanted with the device (MobiusHD, Vascular Dynamics). The initial study enrolled 30 subjects in Europe and 17 in the United States. Initial findings in the cohort of European patients, which included a mean 21–mm Hg reduction in systolic blood pressure and a 12–mm Hg reduction in diastolic blood pressure measured by ambulatory monitoring at 6 months, were published in the Lancet (2017 Dec 16;390[10113]:2655-661).
The patients enrolled in the proof-of-principle CALM trial were required to have highly-treatment-resistant hypertension, defined as a systolic blood pressure greater than or equal to 160 mm Hg despite at least three antihypertensive medications. The average number of medications was 4.4, according to Dr. Reilly. The mean blood pressure at entry was 165/98 mm Hg. Nearly 20% had previously undergone renal denervation.
The device was successfully deployed in all of the patients who participated in the open-label CALM study. Most of the 10 serious adverse events were related to hypotension, according to Dr. Reilly. Others included a wound infection and a case of intermittent claudication. Two instances of neurologic complaints, such as numbness and weakness, experienced within a day of device placement were considered potential transient ischemic attacks, but these resolved completely and no defects were observed on imaging.
In an update on CALM, Dr. Reilly reported that the large reductions in blood pressure previously reported at 6 months have been sustained. Follow-up is approximately 3 years in most patients, and the reductions previously reported have persisted in responders. When a clinically significant response is defined as a 10–mm Hg or more reduction in office blood pressure or 5–mm Hg or more reduction in ambulatory blood pressure, 75% of patients enrolled are still responding, but the more important point is that there has been no substantial reduction in blood pressure control over time in responders, according to Dr. Reilly.
When patients were stratified by a pulse pressure of greater or less than 70 mm Hg at study entry, response rates have been similar, he added.
The long-term responses are significant because there was concern about tachyphylaxis. In fact, coronary stents also produce a reduction in blood pressure immediately after placement that is likely caused by the same effect, but that effect “peters out in a day or 2,” noted Dr. Reilly. As opposed to the round shape of coronary stents, the rectangular shape of the novel device produces “an increase in the perceived strain on the carotid body” that does not appear to diminish over time.
CALM-2, which is designed to be a pivotal trial to support regulatory approval of the device, began enrolling in September 2018. An enrollment of 300 patients with treatment-resistant hypertension is planned. Participants will be randomized to receive the device or a sham procedure consisting of a carotid artery angiogram, according to Dr. Reilly. Although the initial CALM trial was small, open label, and conducted without a control, the persistent benefit over extended follow-up is driving excitement about the potential of this device.
“These are some of the greatest sustained reductions in ambulatory blood pressure we have ever seen,” according to Vasilios Papademetriou, MD, PhD, a professor of medicine at Georgetown University, Washington. Impressed by undiminished blood pressure control observed so far, he characterized the promise of this device as “very compelling.”
Dr. Reilly disclosed that he was a stockholder in Johnson & Johnson.
WASHINGTON – As a result of remarkably sustained antihypertensive effect, interest is intensifying in the potential for a pivotal trial to associate a novel endovascular device with unprecedented blood pressure control in patients with treatment-resistant hypertension, according to an update presented at CRT 2019, sponsored by MedStar Heart & Vascular Institute.
With up to 3 years of follow-up, “systolic blood pressures have remained persistently reduced by as much as 24 mm Hg,” reported John P. Reilly, MD, an interventional cardiologist in Southampton, N.Y., who presented follow-up data for some of those enrolled in the first-in-human study of this device.
When the stent-like device is placed in the carotid artery, it alters its geometric shape, which increases pulsatile wall strain. The increase on wall strain alters an afferent signaling loop controlled by carotid baroreceptors that inhibits sympathetic outflow to lower blood pressure.
In the proof-of-principle, first-in-human CALM study, 47 patients were implanted with the device (MobiusHD, Vascular Dynamics). The initial study enrolled 30 subjects in Europe and 17 in the United States. Initial findings in the cohort of European patients, which included a mean 21–mm Hg reduction in systolic blood pressure and a 12–mm Hg reduction in diastolic blood pressure measured by ambulatory monitoring at 6 months, were published in the Lancet (2017 Dec 16;390[10113]:2655-661).
The patients enrolled in the proof-of-principle CALM trial were required to have highly-treatment-resistant hypertension, defined as a systolic blood pressure greater than or equal to 160 mm Hg despite at least three antihypertensive medications. The average number of medications was 4.4, according to Dr. Reilly. The mean blood pressure at entry was 165/98 mm Hg. Nearly 20% had previously undergone renal denervation.
The device was successfully deployed in all of the patients who participated in the open-label CALM study. Most of the 10 serious adverse events were related to hypotension, according to Dr. Reilly. Others included a wound infection and a case of intermittent claudication. Two instances of neurologic complaints, such as numbness and weakness, experienced within a day of device placement were considered potential transient ischemic attacks, but these resolved completely and no defects were observed on imaging.
In an update on CALM, Dr. Reilly reported that the large reductions in blood pressure previously reported at 6 months have been sustained. Follow-up is approximately 3 years in most patients, and the reductions previously reported have persisted in responders. When a clinically significant response is defined as a 10–mm Hg or more reduction in office blood pressure or 5–mm Hg or more reduction in ambulatory blood pressure, 75% of patients enrolled are still responding, but the more important point is that there has been no substantial reduction in blood pressure control over time in responders, according to Dr. Reilly.
When patients were stratified by a pulse pressure of greater or less than 70 mm Hg at study entry, response rates have been similar, he added.
The long-term responses are significant because there was concern about tachyphylaxis. In fact, coronary stents also produce a reduction in blood pressure immediately after placement that is likely caused by the same effect, but that effect “peters out in a day or 2,” noted Dr. Reilly. As opposed to the round shape of coronary stents, the rectangular shape of the novel device produces “an increase in the perceived strain on the carotid body” that does not appear to diminish over time.
CALM-2, which is designed to be a pivotal trial to support regulatory approval of the device, began enrolling in September 2018. An enrollment of 300 patients with treatment-resistant hypertension is planned. Participants will be randomized to receive the device or a sham procedure consisting of a carotid artery angiogram, according to Dr. Reilly. Although the initial CALM trial was small, open label, and conducted without a control, the persistent benefit over extended follow-up is driving excitement about the potential of this device.
“These are some of the greatest sustained reductions in ambulatory blood pressure we have ever seen,” according to Vasilios Papademetriou, MD, PhD, a professor of medicine at Georgetown University, Washington. Impressed by undiminished blood pressure control observed so far, he characterized the promise of this device as “very compelling.”
Dr. Reilly disclosed that he was a stockholder in Johnson & Johnson.
REPORTING FROM CRT 2019
FDA approves new valsartan generic
In response to a medication shortage, the Food and Drug Administration has approved a new generic of valsartan (Diovan), produced by Alkem Laboratories, for the treatment of high blood pressure and heart failure, the regulatory agency announced in a statement.
The FDA conducted an investigation into generic angiotensin II receptor blocker (ARB) products following reports of N-nitrosodimethylamine impurities being found in a separate valsartan product in the summer of 2018. Since that time, nitrosamine impurities have been detected in multiple ARBs, and as of March 1, 2019, hundreds of lots of ARBs produced by several companies have been recalled. The FDA has implemented new rules to prevent further contamination, but the ongoing recalls have caused a significant shortage.
“[To] address the public health consequences of these shortages, we’ve prioritized the review of generic applications for these valsartan products,” FDA commissioner Scott Gottlieb, MD, said in the statement.
For the new generic’s approval, the FDA assessed Alkem Laboratories’ manufacturing process and ensured that the company used proper testing methods to rule out the presence of nitrosamine impurities.
“We hope that today’s approval of this new generic will help reduce the valsartan shortage, and we remain committed to implementing measures to prevent the formation of these impurities during drug manufacturing processes for existing and future products,” Dr. Gottlieb said.
In response to a medication shortage, the Food and Drug Administration has approved a new generic of valsartan (Diovan), produced by Alkem Laboratories, for the treatment of high blood pressure and heart failure, the regulatory agency announced in a statement.
The FDA conducted an investigation into generic angiotensin II receptor blocker (ARB) products following reports of N-nitrosodimethylamine impurities being found in a separate valsartan product in the summer of 2018. Since that time, nitrosamine impurities have been detected in multiple ARBs, and as of March 1, 2019, hundreds of lots of ARBs produced by several companies have been recalled. The FDA has implemented new rules to prevent further contamination, but the ongoing recalls have caused a significant shortage.
“[To] address the public health consequences of these shortages, we’ve prioritized the review of generic applications for these valsartan products,” FDA commissioner Scott Gottlieb, MD, said in the statement.
For the new generic’s approval, the FDA assessed Alkem Laboratories’ manufacturing process and ensured that the company used proper testing methods to rule out the presence of nitrosamine impurities.
“We hope that today’s approval of this new generic will help reduce the valsartan shortage, and we remain committed to implementing measures to prevent the formation of these impurities during drug manufacturing processes for existing and future products,” Dr. Gottlieb said.
In response to a medication shortage, the Food and Drug Administration has approved a new generic of valsartan (Diovan), produced by Alkem Laboratories, for the treatment of high blood pressure and heart failure, the regulatory agency announced in a statement.
The FDA conducted an investigation into generic angiotensin II receptor blocker (ARB) products following reports of N-nitrosodimethylamine impurities being found in a separate valsartan product in the summer of 2018. Since that time, nitrosamine impurities have been detected in multiple ARBs, and as of March 1, 2019, hundreds of lots of ARBs produced by several companies have been recalled. The FDA has implemented new rules to prevent further contamination, but the ongoing recalls have caused a significant shortage.
“[To] address the public health consequences of these shortages, we’ve prioritized the review of generic applications for these valsartan products,” FDA commissioner Scott Gottlieb, MD, said in the statement.
For the new generic’s approval, the FDA assessed Alkem Laboratories’ manufacturing process and ensured that the company used proper testing methods to rule out the presence of nitrosamine impurities.
“We hope that today’s approval of this new generic will help reduce the valsartan shortage, and we remain committed to implementing measures to prevent the formation of these impurities during drug manufacturing processes for existing and future products,” Dr. Gottlieb said.
Alcohol-mediated renal denervation appears safe for BP reduction
WASHINGTON – Injection of dehydrated alcohol through the wall of the renal artery can be added to a growing list of renal denervation strategies that have been associated with sustained blood pressure reductions, according to data presented as a latebreaker at 2019 CRT meeting.
For the primary efficacy endpoint of change in systolic blood pressure at six months, the mean reduction six months after denervation was 11 mmHg as measured with 24-hour ambulatory blood pressure monitoring (ABPM), according to Horst Sievert, MD, PhD, Director of the CardioVascular Center, Frankfurt, Germany.
“Alcohol denervation was associated with efficient and safe lowering of systolic blood pressure,” reported Dr. Sievert, who said these data have prompted a new set of studies, including a phase 2 controlled trial that will evaluate the effect of renal denervation for control of blood pressure off-medication.
After consent was withdrawn from one patient, study results were available from 44 patients with treatment resistant hypertension who were enrolled in this initial study. Entry requirements included a mean systolic blood pressure greater than 150 mmHg while taking at least three antihypertensive medications from different classes.
In this study, the alcohol was delivered with a proprietary device called the Peregrine System™ infusion catheter (Ablation Solutions). This catheter is equipped with microneedles that remain retracted until the catheter is navigated into position. Once in the renal artery, the microneedles are deployed to inject alcohol into the perivascular space, which produces a neurolytic effect.
The technical success for delivery of the alcohol was achieved in 100% of the study group. There were no serious adverse events associated with treatment. Minor adverse events included those involving the access site, such as pain, as well as two dissections that resolved without treatment. One patient complained of abdominal pain on the day of the procedure, but that also resolved, according to Dr. Sievert.
Over the course of followup, patients remained on the therapies they were taking prior to the intervention. There was no change in antihypertensive therapy during the first month of followup in 84% of treated patients. Of those who did have a change in medication, all but one involved a reduction in medication prompted by improved blood pressure control. At six months, there was no change in medication for 73% of those evaluated.
Following alcohol denervation, there was a mean 7 mmHg reduction in diastolic pressure as measured with 24-hour ABBM.
Based on these data, a trials program is being launched. In addition to the phase 2 multinational off-medication trial, which is enrolling 300 patients who are being randomized to the alcohol denervation therapy or a sham control, an open-label crossover trial will be conducted to confirm the safety and tolerability of this approach.
Delivery of alcohol through the catheter device used in this study requires a renal artery diameter of at least 4 mm. This is a potential limitation for smaller individuals, but several other devices used for denervation share this requirement, according to Dr. Sievert.
The potential advantage of this approach is that “you can stay proximal,” according to Dr. Sievert, contrasting this technique with renal denervation by radiofrequency ablation. He explained that radiofrequency renal denervation requires a relatively distal approach to achieve an appropriate energy penetration for target nerve ablation. Further study is needed to determine whether more proximal delivery has any clinical advantage.
SOURCE: 2019 Cardiovascular Research Technologies (CRT) Meeting.
WASHINGTON – Injection of dehydrated alcohol through the wall of the renal artery can be added to a growing list of renal denervation strategies that have been associated with sustained blood pressure reductions, according to data presented as a latebreaker at 2019 CRT meeting.
For the primary efficacy endpoint of change in systolic blood pressure at six months, the mean reduction six months after denervation was 11 mmHg as measured with 24-hour ambulatory blood pressure monitoring (ABPM), according to Horst Sievert, MD, PhD, Director of the CardioVascular Center, Frankfurt, Germany.
“Alcohol denervation was associated with efficient and safe lowering of systolic blood pressure,” reported Dr. Sievert, who said these data have prompted a new set of studies, including a phase 2 controlled trial that will evaluate the effect of renal denervation for control of blood pressure off-medication.
After consent was withdrawn from one patient, study results were available from 44 patients with treatment resistant hypertension who were enrolled in this initial study. Entry requirements included a mean systolic blood pressure greater than 150 mmHg while taking at least three antihypertensive medications from different classes.
In this study, the alcohol was delivered with a proprietary device called the Peregrine System™ infusion catheter (Ablation Solutions). This catheter is equipped with microneedles that remain retracted until the catheter is navigated into position. Once in the renal artery, the microneedles are deployed to inject alcohol into the perivascular space, which produces a neurolytic effect.
The technical success for delivery of the alcohol was achieved in 100% of the study group. There were no serious adverse events associated with treatment. Minor adverse events included those involving the access site, such as pain, as well as two dissections that resolved without treatment. One patient complained of abdominal pain on the day of the procedure, but that also resolved, according to Dr. Sievert.
Over the course of followup, patients remained on the therapies they were taking prior to the intervention. There was no change in antihypertensive therapy during the first month of followup in 84% of treated patients. Of those who did have a change in medication, all but one involved a reduction in medication prompted by improved blood pressure control. At six months, there was no change in medication for 73% of those evaluated.
Following alcohol denervation, there was a mean 7 mmHg reduction in diastolic pressure as measured with 24-hour ABBM.
Based on these data, a trials program is being launched. In addition to the phase 2 multinational off-medication trial, which is enrolling 300 patients who are being randomized to the alcohol denervation therapy or a sham control, an open-label crossover trial will be conducted to confirm the safety and tolerability of this approach.
Delivery of alcohol through the catheter device used in this study requires a renal artery diameter of at least 4 mm. This is a potential limitation for smaller individuals, but several other devices used for denervation share this requirement, according to Dr. Sievert.
The potential advantage of this approach is that “you can stay proximal,” according to Dr. Sievert, contrasting this technique with renal denervation by radiofrequency ablation. He explained that radiofrequency renal denervation requires a relatively distal approach to achieve an appropriate energy penetration for target nerve ablation. Further study is needed to determine whether more proximal delivery has any clinical advantage.
SOURCE: 2019 Cardiovascular Research Technologies (CRT) Meeting.
WASHINGTON – Injection of dehydrated alcohol through the wall of the renal artery can be added to a growing list of renal denervation strategies that have been associated with sustained blood pressure reductions, according to data presented as a latebreaker at 2019 CRT meeting.
For the primary efficacy endpoint of change in systolic blood pressure at six months, the mean reduction six months after denervation was 11 mmHg as measured with 24-hour ambulatory blood pressure monitoring (ABPM), according to Horst Sievert, MD, PhD, Director of the CardioVascular Center, Frankfurt, Germany.
“Alcohol denervation was associated with efficient and safe lowering of systolic blood pressure,” reported Dr. Sievert, who said these data have prompted a new set of studies, including a phase 2 controlled trial that will evaluate the effect of renal denervation for control of blood pressure off-medication.
After consent was withdrawn from one patient, study results were available from 44 patients with treatment resistant hypertension who were enrolled in this initial study. Entry requirements included a mean systolic blood pressure greater than 150 mmHg while taking at least three antihypertensive medications from different classes.
In this study, the alcohol was delivered with a proprietary device called the Peregrine System™ infusion catheter (Ablation Solutions). This catheter is equipped with microneedles that remain retracted until the catheter is navigated into position. Once in the renal artery, the microneedles are deployed to inject alcohol into the perivascular space, which produces a neurolytic effect.
The technical success for delivery of the alcohol was achieved in 100% of the study group. There were no serious adverse events associated with treatment. Minor adverse events included those involving the access site, such as pain, as well as two dissections that resolved without treatment. One patient complained of abdominal pain on the day of the procedure, but that also resolved, according to Dr. Sievert.
Over the course of followup, patients remained on the therapies they were taking prior to the intervention. There was no change in antihypertensive therapy during the first month of followup in 84% of treated patients. Of those who did have a change in medication, all but one involved a reduction in medication prompted by improved blood pressure control. At six months, there was no change in medication for 73% of those evaluated.
Following alcohol denervation, there was a mean 7 mmHg reduction in diastolic pressure as measured with 24-hour ABBM.
Based on these data, a trials program is being launched. In addition to the phase 2 multinational off-medication trial, which is enrolling 300 patients who are being randomized to the alcohol denervation therapy or a sham control, an open-label crossover trial will be conducted to confirm the safety and tolerability of this approach.
Delivery of alcohol through the catheter device used in this study requires a renal artery diameter of at least 4 mm. This is a potential limitation for smaller individuals, but several other devices used for denervation share this requirement, according to Dr. Sievert.
The potential advantage of this approach is that “you can stay proximal,” according to Dr. Sievert, contrasting this technique with renal denervation by radiofrequency ablation. He explained that radiofrequency renal denervation requires a relatively distal approach to achieve an appropriate energy penetration for target nerve ablation. Further study is needed to determine whether more proximal delivery has any clinical advantage.
SOURCE: 2019 Cardiovascular Research Technologies (CRT) Meeting.
REPORTING FROM CRT 2019
Individual pediatric hypertension trials support personalized care
The preferred medication in children with hypertension varied among three common medications in n-of-1 studies with repeated ambulatory blood pressure monitoring that allowed for individualized treatment plans.
“In usual care, the choice of specific antihypertensive regimen is based on physician preference with little or no systematic assessment of treatment benefits and hazards,” wrote Joyce P. Samuel, MD, of the University of Texas Health Science Center, Houston, and her colleagues.
In a study published in Pediatrics, the researchers assessed 32 hypertensive children who participated in n-of-1 studies at a single center. The children underwent repeated ambulatory blood pressure monitoring (APBM) to compare the effects of three medications: lisinopril, amlodipine, and hydrochlorothiazide. The children were at least 9 years old, and their primary referring physician had recommended antihypertensive treatment.
The preferred medication was defined as the one that yielded both a normal ambulatory blood pressure and the greatest reduction in average systolic blood pressure when awake for two treatment periods with no unacceptable side effects. If more than one medication met these criteria, the one with the least side effects was chosen.
Overall, the preferred medication was lisinopril for 16 patients (49%), amlodipine for 8 patients (24%), and hydrochlorothiazide for 4 patients (12%); 4 patients remained uncontrolled on monotherapy.
Each of the three medications was taken for 2 weeks, and patients were assessed with a 24-hour ABPM and a side-effect questionnaire during the final 24 hours of each treatment. A total of 27 patients reported at least one side effect during the study. Unacceptable side effects were most frequent on hydrochlorothiazide (25%), compared with 16% for lisinopril and 13% for amlodipine. None of the patients experienced hypertensive crisis, hypotension, hyperkalemia, or an increase in serum creatinine levels of more than 20% from baseline.
No single medication was preferred for at least 80% of the patients. “Within-patient variation in BP (blood pressure) response was considerable because the best-performing medication decreased the BP by about 12 mm Hg more than the worst-performing medication,” the researchers wrote.
The findings were limited by the possibility that a 2-week trial might be insufficient to evaluate medication effects, and more research is needed to refine the methods of the trials and the generalizability of the n-of-1 approach, the researchers noted. However, “this individualized approach to antihypertensive medication selection holds potential value by involving patients in their own care and facilitating informed treatment decisions,” they said.
“A randomized trial in which researchers compare usual care to routine use of n-of-1 trials with ABPM is needed to assess effects on treatment adherence, patient satisfaction, long-term BP control assessed with ABPM, and hypertensive target organ damage,” Dr. Samuel and her associates advised.
The study was supported in part by the National Center for Advancing Translational Sciences and the National Institutes of Health. The researchers had no financial conflicts to disclose.
SOURCE: Samuel JP et al. Pediatrics. 2019 Mar 6. doi: 10.1542/peds.2018-1818.
The preferred medication in children with hypertension varied among three common medications in n-of-1 studies with repeated ambulatory blood pressure monitoring that allowed for individualized treatment plans.
“In usual care, the choice of specific antihypertensive regimen is based on physician preference with little or no systematic assessment of treatment benefits and hazards,” wrote Joyce P. Samuel, MD, of the University of Texas Health Science Center, Houston, and her colleagues.
In a study published in Pediatrics, the researchers assessed 32 hypertensive children who participated in n-of-1 studies at a single center. The children underwent repeated ambulatory blood pressure monitoring (APBM) to compare the effects of three medications: lisinopril, amlodipine, and hydrochlorothiazide. The children were at least 9 years old, and their primary referring physician had recommended antihypertensive treatment.
The preferred medication was defined as the one that yielded both a normal ambulatory blood pressure and the greatest reduction in average systolic blood pressure when awake for two treatment periods with no unacceptable side effects. If more than one medication met these criteria, the one with the least side effects was chosen.
Overall, the preferred medication was lisinopril for 16 patients (49%), amlodipine for 8 patients (24%), and hydrochlorothiazide for 4 patients (12%); 4 patients remained uncontrolled on monotherapy.
Each of the three medications was taken for 2 weeks, and patients were assessed with a 24-hour ABPM and a side-effect questionnaire during the final 24 hours of each treatment. A total of 27 patients reported at least one side effect during the study. Unacceptable side effects were most frequent on hydrochlorothiazide (25%), compared with 16% for lisinopril and 13% for amlodipine. None of the patients experienced hypertensive crisis, hypotension, hyperkalemia, or an increase in serum creatinine levels of more than 20% from baseline.
No single medication was preferred for at least 80% of the patients. “Within-patient variation in BP (blood pressure) response was considerable because the best-performing medication decreased the BP by about 12 mm Hg more than the worst-performing medication,” the researchers wrote.
The findings were limited by the possibility that a 2-week trial might be insufficient to evaluate medication effects, and more research is needed to refine the methods of the trials and the generalizability of the n-of-1 approach, the researchers noted. However, “this individualized approach to antihypertensive medication selection holds potential value by involving patients in their own care and facilitating informed treatment decisions,” they said.
“A randomized trial in which researchers compare usual care to routine use of n-of-1 trials with ABPM is needed to assess effects on treatment adherence, patient satisfaction, long-term BP control assessed with ABPM, and hypertensive target organ damage,” Dr. Samuel and her associates advised.
The study was supported in part by the National Center for Advancing Translational Sciences and the National Institutes of Health. The researchers had no financial conflicts to disclose.
SOURCE: Samuel JP et al. Pediatrics. 2019 Mar 6. doi: 10.1542/peds.2018-1818.
The preferred medication in children with hypertension varied among three common medications in n-of-1 studies with repeated ambulatory blood pressure monitoring that allowed for individualized treatment plans.
“In usual care, the choice of specific antihypertensive regimen is based on physician preference with little or no systematic assessment of treatment benefits and hazards,” wrote Joyce P. Samuel, MD, of the University of Texas Health Science Center, Houston, and her colleagues.
In a study published in Pediatrics, the researchers assessed 32 hypertensive children who participated in n-of-1 studies at a single center. The children underwent repeated ambulatory blood pressure monitoring (APBM) to compare the effects of three medications: lisinopril, amlodipine, and hydrochlorothiazide. The children were at least 9 years old, and their primary referring physician had recommended antihypertensive treatment.
The preferred medication was defined as the one that yielded both a normal ambulatory blood pressure and the greatest reduction in average systolic blood pressure when awake for two treatment periods with no unacceptable side effects. If more than one medication met these criteria, the one with the least side effects was chosen.
Overall, the preferred medication was lisinopril for 16 patients (49%), amlodipine for 8 patients (24%), and hydrochlorothiazide for 4 patients (12%); 4 patients remained uncontrolled on monotherapy.
Each of the three medications was taken for 2 weeks, and patients were assessed with a 24-hour ABPM and a side-effect questionnaire during the final 24 hours of each treatment. A total of 27 patients reported at least one side effect during the study. Unacceptable side effects were most frequent on hydrochlorothiazide (25%), compared with 16% for lisinopril and 13% for amlodipine. None of the patients experienced hypertensive crisis, hypotension, hyperkalemia, or an increase in serum creatinine levels of more than 20% from baseline.
No single medication was preferred for at least 80% of the patients. “Within-patient variation in BP (blood pressure) response was considerable because the best-performing medication decreased the BP by about 12 mm Hg more than the worst-performing medication,” the researchers wrote.
The findings were limited by the possibility that a 2-week trial might be insufficient to evaluate medication effects, and more research is needed to refine the methods of the trials and the generalizability of the n-of-1 approach, the researchers noted. However, “this individualized approach to antihypertensive medication selection holds potential value by involving patients in their own care and facilitating informed treatment decisions,” they said.
“A randomized trial in which researchers compare usual care to routine use of n-of-1 trials with ABPM is needed to assess effects on treatment adherence, patient satisfaction, long-term BP control assessed with ABPM, and hypertensive target organ damage,” Dr. Samuel and her associates advised.
The study was supported in part by the National Center for Advancing Translational Sciences and the National Institutes of Health. The researchers had no financial conflicts to disclose.
SOURCE: Samuel JP et al. Pediatrics. 2019 Mar 6. doi: 10.1542/peds.2018-1818.
FROM PEDIATRICS
Report: Cutting sodium consumption recommended
according to a report outlining the first-ever Dietary Reference Intakes (DRIs) recommendation intended to address chronic disease risk.
For individuals aged 14 years and older consuming sodium above that level, cutting back could reduce their risk for hypertension and other chronic diseases, according to the report from the National Academies of Sciences, Engineering, and Medicine.
That cutoff, referred to as a Chronic Disease Risk Reduction Intake (CDRR), represents an expansion of the DRIs model beyond the Recommended Dietary Allowance (RDA) and other measures of adequacy, and the Tolerable Upper Intake Level (UL), which is the maximum intake unlikely to result in adverse health effects, a report author said in a press conference.
“CDRR is a level now that will join all the rest of the alphabet soup and be a part of how we describe recommended dietary intakes,” said Virginia A. Stallings, MD, chair of the committee that reviewed the sodium and potassium DRIs. Most U.S. adults are already consuming sodium above the recommended CDRR, said Dr. Stallings, who is a professor of pediatrics at the University of Pennsylvania, Philadelphia, and the director of the Nutrition Center at Children’s Hospital of Philadelphia.
The recommendation to reduce sodium intakes to below 2,300 mg/day applies to both hypertensive and normotensive individuals, and it could be particularly beneficial in older adults, non-Hispanic blacks, and other groups at higher risk of cardiovascular disease, Dr. Stallings and her coauthors of the new report wrote. The DRIs established in 2005 for both sodium and potassium are also updated based on new methodology in this report
Lower sodium CDRRs were set for younger individuals. Children aged 1-3 years should cut back if sodium intake is above 1,200 mg/day, while the levels for children aged 4-8 years and 9-13 years were set at 1,500 and 1,800 mg/day, respectively, according to the report.
By contrast, authors of this report were unable to establish a CDRR level for potassium based on current evidence. However, that doesn’t rule out the possibility that changing potassium intake could be beneficial, they suggested, adding that the effects of potassium need to be further explored.
Sodium and potassium play important roles in maintaining physiologic homeostasis, and they have also been implicated in risk of cardiovascular disease, mainly through their effects on blood pressure, and other chronic diseases.
“The unique nature of potassium and sodium – that is, the coexistence of their essentiality with a relationship to adverse health effects, including chronic disease risk – necessitated a new approach to the review of intake recommendations for these nutrients within the [DRI] context,” the report reads. The report also affirms, and in some cases revises, levels of sodium and potassium assumed to be adequate in healthy individuals that had been established in 2005. The sodium Adequate Intake (AI) levels for individuals aged 14-50 years is unchanged at 1,500 mg daily, but that for individuals aged 51 years and older has been increased to the 1,500-mg level.
“As we examined the evidence and looked specifically at those age cuts, there was no evidence that older people needed less sodium,” Dr. Stallings said in the press conference.
Potassium AIs were decreased for most age groups. For adults, the new recommended potassium AIs are 3,400 mg/day in men and 2,600 mg/day in women, whereas the AI for potassium was 4,700 mg/day for all adults in the 2005 recommendations.
In 2005, much of the evidence used to establish the potassium AI values was based on research studies that included potassium supplementation, rather than simply potassium intake in the usual diet. “In our process, we did not use supplementation studies, but we used the intake of apparently healthy people,” Dr. Stallings said in the press conference.
Less than half of U.S. and Canadian adults have potassium intakes that meet or exceed the potassium AI, with intakes lowest among non-Hispanic blacks, Dr. Stallings said in the press conference.
Dietary potassium intake is related to fruit and vegetable consumption, which rarely meets the recommended servings per day, Dr. Stallings and her colleagues wrote in a preface to the National Academies report. Milk, white potatoes, and fruit are higher sources of dietary potassium, she said, while coffee is the leading source of potassium for Americans aged 51 years and older.
By contrast, most sodium in the modern diet comes from commercially prepared foods and beverages, instead of consumers adding salt at the time of cooking or eating, Dr. Stallings wrote.
“For the desired public health benefit of reduced sodium intake to be achieved, more attention must be paid by industry to reducing sodium in the food supply and by consumers who have the needed sodium content information and an understanding of how to make health-inspired food choices,” they wrote in the report.
The American Heart Association’s CEO, Nancy Brown, expressed her support for the new recommendation for sodium intake. The recommendation “aligns with what the American Heart Association and other prominent public health organizations have been saying for years: We must eat less salt,” she said.
“Our excessive sodium intake isn’t entirely driven by the salt shaker; it’s largely controlled by the food industry. More than 70 percent of sodium consumed is added to food before it reaches our plates. It is added in restaurants and during the manufacturing of processed and prepackaged foods,” she claimed. “We hope this report encourages the Food and Drug Administration to quickly release its voluntary sodium reduction targets for the food industry. School leaders should also take note and reject the recent U.S. Department of Agriculture decision to weaken sodium standards in school meals and continue their commitment to serve students healthier foods.”
The DRIs report on sodium and potassium was supported by contracts between the National Academy of Sciences and the Centers for Disease Control, Food and Drug Administration, Health Canada, National Institutes of Health, Public Health Agency of Canada, and the Department of Agriculture. Partial support also came from the National Academy of Sciences W. K. Kellogg Foundation Fund and the National Academy of Medicine.
SOURCE: National Academies of Sciences, Engineering, and Medicine. 2019. Dietary Reference Intakes for sodium and potassium. Washington: The National Academies Press. doi: 10.17226/25353.
according to a report outlining the first-ever Dietary Reference Intakes (DRIs) recommendation intended to address chronic disease risk.
For individuals aged 14 years and older consuming sodium above that level, cutting back could reduce their risk for hypertension and other chronic diseases, according to the report from the National Academies of Sciences, Engineering, and Medicine.
That cutoff, referred to as a Chronic Disease Risk Reduction Intake (CDRR), represents an expansion of the DRIs model beyond the Recommended Dietary Allowance (RDA) and other measures of adequacy, and the Tolerable Upper Intake Level (UL), which is the maximum intake unlikely to result in adverse health effects, a report author said in a press conference.
“CDRR is a level now that will join all the rest of the alphabet soup and be a part of how we describe recommended dietary intakes,” said Virginia A. Stallings, MD, chair of the committee that reviewed the sodium and potassium DRIs. Most U.S. adults are already consuming sodium above the recommended CDRR, said Dr. Stallings, who is a professor of pediatrics at the University of Pennsylvania, Philadelphia, and the director of the Nutrition Center at Children’s Hospital of Philadelphia.
The recommendation to reduce sodium intakes to below 2,300 mg/day applies to both hypertensive and normotensive individuals, and it could be particularly beneficial in older adults, non-Hispanic blacks, and other groups at higher risk of cardiovascular disease, Dr. Stallings and her coauthors of the new report wrote. The DRIs established in 2005 for both sodium and potassium are also updated based on new methodology in this report
Lower sodium CDRRs were set for younger individuals. Children aged 1-3 years should cut back if sodium intake is above 1,200 mg/day, while the levels for children aged 4-8 years and 9-13 years were set at 1,500 and 1,800 mg/day, respectively, according to the report.
By contrast, authors of this report were unable to establish a CDRR level for potassium based on current evidence. However, that doesn’t rule out the possibility that changing potassium intake could be beneficial, they suggested, adding that the effects of potassium need to be further explored.
Sodium and potassium play important roles in maintaining physiologic homeostasis, and they have also been implicated in risk of cardiovascular disease, mainly through their effects on blood pressure, and other chronic diseases.
“The unique nature of potassium and sodium – that is, the coexistence of their essentiality with a relationship to adverse health effects, including chronic disease risk – necessitated a new approach to the review of intake recommendations for these nutrients within the [DRI] context,” the report reads. The report also affirms, and in some cases revises, levels of sodium and potassium assumed to be adequate in healthy individuals that had been established in 2005. The sodium Adequate Intake (AI) levels for individuals aged 14-50 years is unchanged at 1,500 mg daily, but that for individuals aged 51 years and older has been increased to the 1,500-mg level.
“As we examined the evidence and looked specifically at those age cuts, there was no evidence that older people needed less sodium,” Dr. Stallings said in the press conference.
Potassium AIs were decreased for most age groups. For adults, the new recommended potassium AIs are 3,400 mg/day in men and 2,600 mg/day in women, whereas the AI for potassium was 4,700 mg/day for all adults in the 2005 recommendations.
In 2005, much of the evidence used to establish the potassium AI values was based on research studies that included potassium supplementation, rather than simply potassium intake in the usual diet. “In our process, we did not use supplementation studies, but we used the intake of apparently healthy people,” Dr. Stallings said in the press conference.
Less than half of U.S. and Canadian adults have potassium intakes that meet or exceed the potassium AI, with intakes lowest among non-Hispanic blacks, Dr. Stallings said in the press conference.
Dietary potassium intake is related to fruit and vegetable consumption, which rarely meets the recommended servings per day, Dr. Stallings and her colleagues wrote in a preface to the National Academies report. Milk, white potatoes, and fruit are higher sources of dietary potassium, she said, while coffee is the leading source of potassium for Americans aged 51 years and older.
By contrast, most sodium in the modern diet comes from commercially prepared foods and beverages, instead of consumers adding salt at the time of cooking or eating, Dr. Stallings wrote.
“For the desired public health benefit of reduced sodium intake to be achieved, more attention must be paid by industry to reducing sodium in the food supply and by consumers who have the needed sodium content information and an understanding of how to make health-inspired food choices,” they wrote in the report.
The American Heart Association’s CEO, Nancy Brown, expressed her support for the new recommendation for sodium intake. The recommendation “aligns with what the American Heart Association and other prominent public health organizations have been saying for years: We must eat less salt,” she said.
“Our excessive sodium intake isn’t entirely driven by the salt shaker; it’s largely controlled by the food industry. More than 70 percent of sodium consumed is added to food before it reaches our plates. It is added in restaurants and during the manufacturing of processed and prepackaged foods,” she claimed. “We hope this report encourages the Food and Drug Administration to quickly release its voluntary sodium reduction targets for the food industry. School leaders should also take note and reject the recent U.S. Department of Agriculture decision to weaken sodium standards in school meals and continue their commitment to serve students healthier foods.”
The DRIs report on sodium and potassium was supported by contracts between the National Academy of Sciences and the Centers for Disease Control, Food and Drug Administration, Health Canada, National Institutes of Health, Public Health Agency of Canada, and the Department of Agriculture. Partial support also came from the National Academy of Sciences W. K. Kellogg Foundation Fund and the National Academy of Medicine.
SOURCE: National Academies of Sciences, Engineering, and Medicine. 2019. Dietary Reference Intakes for sodium and potassium. Washington: The National Academies Press. doi: 10.17226/25353.
according to a report outlining the first-ever Dietary Reference Intakes (DRIs) recommendation intended to address chronic disease risk.
For individuals aged 14 years and older consuming sodium above that level, cutting back could reduce their risk for hypertension and other chronic diseases, according to the report from the National Academies of Sciences, Engineering, and Medicine.
That cutoff, referred to as a Chronic Disease Risk Reduction Intake (CDRR), represents an expansion of the DRIs model beyond the Recommended Dietary Allowance (RDA) and other measures of adequacy, and the Tolerable Upper Intake Level (UL), which is the maximum intake unlikely to result in adverse health effects, a report author said in a press conference.
“CDRR is a level now that will join all the rest of the alphabet soup and be a part of how we describe recommended dietary intakes,” said Virginia A. Stallings, MD, chair of the committee that reviewed the sodium and potassium DRIs. Most U.S. adults are already consuming sodium above the recommended CDRR, said Dr. Stallings, who is a professor of pediatrics at the University of Pennsylvania, Philadelphia, and the director of the Nutrition Center at Children’s Hospital of Philadelphia.
The recommendation to reduce sodium intakes to below 2,300 mg/day applies to both hypertensive and normotensive individuals, and it could be particularly beneficial in older adults, non-Hispanic blacks, and other groups at higher risk of cardiovascular disease, Dr. Stallings and her coauthors of the new report wrote. The DRIs established in 2005 for both sodium and potassium are also updated based on new methodology in this report
Lower sodium CDRRs were set for younger individuals. Children aged 1-3 years should cut back if sodium intake is above 1,200 mg/day, while the levels for children aged 4-8 years and 9-13 years were set at 1,500 and 1,800 mg/day, respectively, according to the report.
By contrast, authors of this report were unable to establish a CDRR level for potassium based on current evidence. However, that doesn’t rule out the possibility that changing potassium intake could be beneficial, they suggested, adding that the effects of potassium need to be further explored.
Sodium and potassium play important roles in maintaining physiologic homeostasis, and they have also been implicated in risk of cardiovascular disease, mainly through their effects on blood pressure, and other chronic diseases.
“The unique nature of potassium and sodium – that is, the coexistence of their essentiality with a relationship to adverse health effects, including chronic disease risk – necessitated a new approach to the review of intake recommendations for these nutrients within the [DRI] context,” the report reads. The report also affirms, and in some cases revises, levels of sodium and potassium assumed to be adequate in healthy individuals that had been established in 2005. The sodium Adequate Intake (AI) levels for individuals aged 14-50 years is unchanged at 1,500 mg daily, but that for individuals aged 51 years and older has been increased to the 1,500-mg level.
“As we examined the evidence and looked specifically at those age cuts, there was no evidence that older people needed less sodium,” Dr. Stallings said in the press conference.
Potassium AIs were decreased for most age groups. For adults, the new recommended potassium AIs are 3,400 mg/day in men and 2,600 mg/day in women, whereas the AI for potassium was 4,700 mg/day for all adults in the 2005 recommendations.
In 2005, much of the evidence used to establish the potassium AI values was based on research studies that included potassium supplementation, rather than simply potassium intake in the usual diet. “In our process, we did not use supplementation studies, but we used the intake of apparently healthy people,” Dr. Stallings said in the press conference.
Less than half of U.S. and Canadian adults have potassium intakes that meet or exceed the potassium AI, with intakes lowest among non-Hispanic blacks, Dr. Stallings said in the press conference.
Dietary potassium intake is related to fruit and vegetable consumption, which rarely meets the recommended servings per day, Dr. Stallings and her colleagues wrote in a preface to the National Academies report. Milk, white potatoes, and fruit are higher sources of dietary potassium, she said, while coffee is the leading source of potassium for Americans aged 51 years and older.
By contrast, most sodium in the modern diet comes from commercially prepared foods and beverages, instead of consumers adding salt at the time of cooking or eating, Dr. Stallings wrote.
“For the desired public health benefit of reduced sodium intake to be achieved, more attention must be paid by industry to reducing sodium in the food supply and by consumers who have the needed sodium content information and an understanding of how to make health-inspired food choices,” they wrote in the report.
The American Heart Association’s CEO, Nancy Brown, expressed her support for the new recommendation for sodium intake. The recommendation “aligns with what the American Heart Association and other prominent public health organizations have been saying for years: We must eat less salt,” she said.
“Our excessive sodium intake isn’t entirely driven by the salt shaker; it’s largely controlled by the food industry. More than 70 percent of sodium consumed is added to food before it reaches our plates. It is added in restaurants and during the manufacturing of processed and prepackaged foods,” she claimed. “We hope this report encourages the Food and Drug Administration to quickly release its voluntary sodium reduction targets for the food industry. School leaders should also take note and reject the recent U.S. Department of Agriculture decision to weaken sodium standards in school meals and continue their commitment to serve students healthier foods.”
The DRIs report on sodium and potassium was supported by contracts between the National Academy of Sciences and the Centers for Disease Control, Food and Drug Administration, Health Canada, National Institutes of Health, Public Health Agency of Canada, and the Department of Agriculture. Partial support also came from the National Academy of Sciences W. K. Kellogg Foundation Fund and the National Academy of Medicine.
SOURCE: National Academies of Sciences, Engineering, and Medicine. 2019. Dietary Reference Intakes for sodium and potassium. Washington: The National Academies Press. doi: 10.17226/25353.
A couple of little known side effects of medications
A 46-year-old woman with diabetes and seizure disorder presents with nausea and fatigue. Her physical exam is unremarkable.
Meds: Glyburide 5 mg daily, metformin 850 mg b.i.d., phenytoin 300 mg daily, topiramate 400 mg daily, pantoprazole 40 mg daily.
Labs: Na 133, K 3.9, Cl 112, HCO3 13, Glu 158, Bun 18, Cr 1.0.
What is the most likely cause of this patient’s acidosis?
A. Phenytoin
B. Topiramate
C. Metformin
D. Pantoprazole
The correct answer to this question is topiramate.
Metformin has had warnings about risk of lactic acidosis occurring in patients with kidney disease, but there is no evidence that metformin is associated with lactic acidosis or raised serum lactate levels in patients with diabetes with normal renal function.1 and its use may decrease CV risk in patients with stage 3 CKD.2 This patient has a non–anion gap acidosis (anion gap is 8).
Topiramate acts as a carbonic anhydrase inhibitor, which causes impairment of both the normal reabsorption of filtered HCO3 by the proximal renal tubule and the excretion of hydrogen ion by the distal tubule.3 Acidosis occurs in most patients who are treated with topiramate. Dr. Ture and colleagues did a cross-sectional study to assess the frequency of metabolic acidosis in patients who were taking topiramate.4 Eighty patients who were on topiramate for seizure prevention prior to elective craniotomy were studied. Metabolic acidosis was present in 71% of the patients. Patients treated with topiramate also have a higher risk for kidney stones and uric acid elevation.
A 60-year-old patient presents with right great toe pain. On exam he has warmth and erythema of the 1st MTP joint. Aspiration of the joint shows uric acid crystals. He has had BP’s of 150-160 mm Hg systolic on his home BP monitoring over the past 6 months. In clinic today BP is 156/90 mm Hg. Labs: Bun 10, Cr 1.0, K 3.8, Uric acid 7.4.
Which blood pressure medication would you recommend?
A. Hydrochlorothiazide
B. Chlorthalidone
C. Lisinopril
D. Losartan
E. Irbesartan
In a patient with gout, diuretics should be avoided if possible, as they increase uric acid levels. Of the other three options, losartan offers the added benefit of lowering uric acid levels. Losartan has uricosuric effects and lowers uric acid levels, a property that is unique to losartan of the angiotensin receptor blockers (ARBs) that have been studied.5-6 The uric acid lowering appears to be a probenecid-like effect. Losartan has also been evaluated to see whether using it in combination with a thiazide diuretic can reduce the rise in uric acid that occurs with thiazides. Dr. Matsumura et al. looked at data from the COMFORT trial, focusing on the effect of combining losartan with hydrochlorothiazide on uric acid levels.7 They looked at a group of 118 patients on an ARB other than losartan plus a diuretic, who were then randomly assigned to losartan 50 mg/hydrochlorothiazide 12.5 mg or continuation of another ARB plus a diuretic. Blood pressure control was the same between groups, but the patients who received the losartan combination had lower uric acid levels (P = .01).
Pearls: Topiramate acts as a cerbonic anhydrase inhibitor and can cause a non–anion gap acidosis. Losartan has a modest uricosuric effect and can modestly lower uric acid levels. This is a unique property of losartan and is not shared by other ARBs.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Salpeter SR et al. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2010;4:CD002967.
2. Charytan DM et al. Metformin use and cardiovascular events in patients with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2019 Jan 22. doi: 10.1111/dom.13642.
3. Mirza N et al. Effect of topiramate on acid-base balance: extent, mechanism and effects. Br J Clin Pharmacol. 2009 Nov;68(5):655-61.
4. Ture H et al. The frequency and severity of metabolic acidosis related to topiramate. J Int Med Res. 2016;44(6):1376-80.
5. Würzner G et al. Comparative effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricaemia and gout. J Hypertens. 2001 Oct;19(10):1855-60.
6. Puig JG et al. Effect of eprosartan and losartan on uric acid metabolism in patients with essential hypertension. J Hypertens. 1999 Jul;17(7):1033-9.
7. Matsumura K et al. Effect of losartan on serum uric acid in hypertension treated with a diuretic: the COMFORT study. Clin Exp Hypertens. 2015;37(3):192-6.
A 46-year-old woman with diabetes and seizure disorder presents with nausea and fatigue. Her physical exam is unremarkable.
Meds: Glyburide 5 mg daily, metformin 850 mg b.i.d., phenytoin 300 mg daily, topiramate 400 mg daily, pantoprazole 40 mg daily.
Labs: Na 133, K 3.9, Cl 112, HCO3 13, Glu 158, Bun 18, Cr 1.0.
What is the most likely cause of this patient’s acidosis?
A. Phenytoin
B. Topiramate
C. Metformin
D. Pantoprazole
The correct answer to this question is topiramate.
Metformin has had warnings about risk of lactic acidosis occurring in patients with kidney disease, but there is no evidence that metformin is associated with lactic acidosis or raised serum lactate levels in patients with diabetes with normal renal function.1 and its use may decrease CV risk in patients with stage 3 CKD.2 This patient has a non–anion gap acidosis (anion gap is 8).
Topiramate acts as a carbonic anhydrase inhibitor, which causes impairment of both the normal reabsorption of filtered HCO3 by the proximal renal tubule and the excretion of hydrogen ion by the distal tubule.3 Acidosis occurs in most patients who are treated with topiramate. Dr. Ture and colleagues did a cross-sectional study to assess the frequency of metabolic acidosis in patients who were taking topiramate.4 Eighty patients who were on topiramate for seizure prevention prior to elective craniotomy were studied. Metabolic acidosis was present in 71% of the patients. Patients treated with topiramate also have a higher risk for kidney stones and uric acid elevation.
A 60-year-old patient presents with right great toe pain. On exam he has warmth and erythema of the 1st MTP joint. Aspiration of the joint shows uric acid crystals. He has had BP’s of 150-160 mm Hg systolic on his home BP monitoring over the past 6 months. In clinic today BP is 156/90 mm Hg. Labs: Bun 10, Cr 1.0, K 3.8, Uric acid 7.4.
Which blood pressure medication would you recommend?
A. Hydrochlorothiazide
B. Chlorthalidone
C. Lisinopril
D. Losartan
E. Irbesartan
In a patient with gout, diuretics should be avoided if possible, as they increase uric acid levels. Of the other three options, losartan offers the added benefit of lowering uric acid levels. Losartan has uricosuric effects and lowers uric acid levels, a property that is unique to losartan of the angiotensin receptor blockers (ARBs) that have been studied.5-6 The uric acid lowering appears to be a probenecid-like effect. Losartan has also been evaluated to see whether using it in combination with a thiazide diuretic can reduce the rise in uric acid that occurs with thiazides. Dr. Matsumura et al. looked at data from the COMFORT trial, focusing on the effect of combining losartan with hydrochlorothiazide on uric acid levels.7 They looked at a group of 118 patients on an ARB other than losartan plus a diuretic, who were then randomly assigned to losartan 50 mg/hydrochlorothiazide 12.5 mg or continuation of another ARB plus a diuretic. Blood pressure control was the same between groups, but the patients who received the losartan combination had lower uric acid levels (P = .01).
Pearls: Topiramate acts as a cerbonic anhydrase inhibitor and can cause a non–anion gap acidosis. Losartan has a modest uricosuric effect and can modestly lower uric acid levels. This is a unique property of losartan and is not shared by other ARBs.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Salpeter SR et al. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2010;4:CD002967.
2. Charytan DM et al. Metformin use and cardiovascular events in patients with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2019 Jan 22. doi: 10.1111/dom.13642.
3. Mirza N et al. Effect of topiramate on acid-base balance: extent, mechanism and effects. Br J Clin Pharmacol. 2009 Nov;68(5):655-61.
4. Ture H et al. The frequency and severity of metabolic acidosis related to topiramate. J Int Med Res. 2016;44(6):1376-80.
5. Würzner G et al. Comparative effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricaemia and gout. J Hypertens. 2001 Oct;19(10):1855-60.
6. Puig JG et al. Effect of eprosartan and losartan on uric acid metabolism in patients with essential hypertension. J Hypertens. 1999 Jul;17(7):1033-9.
7. Matsumura K et al. Effect of losartan on serum uric acid in hypertension treated with a diuretic: the COMFORT study. Clin Exp Hypertens. 2015;37(3):192-6.
A 46-year-old woman with diabetes and seizure disorder presents with nausea and fatigue. Her physical exam is unremarkable.
Meds: Glyburide 5 mg daily, metformin 850 mg b.i.d., phenytoin 300 mg daily, topiramate 400 mg daily, pantoprazole 40 mg daily.
Labs: Na 133, K 3.9, Cl 112, HCO3 13, Glu 158, Bun 18, Cr 1.0.
What is the most likely cause of this patient’s acidosis?
A. Phenytoin
B. Topiramate
C. Metformin
D. Pantoprazole
The correct answer to this question is topiramate.
Metformin has had warnings about risk of lactic acidosis occurring in patients with kidney disease, but there is no evidence that metformin is associated with lactic acidosis or raised serum lactate levels in patients with diabetes with normal renal function.1 and its use may decrease CV risk in patients with stage 3 CKD.2 This patient has a non–anion gap acidosis (anion gap is 8).
Topiramate acts as a carbonic anhydrase inhibitor, which causes impairment of both the normal reabsorption of filtered HCO3 by the proximal renal tubule and the excretion of hydrogen ion by the distal tubule.3 Acidosis occurs in most patients who are treated with topiramate. Dr. Ture and colleagues did a cross-sectional study to assess the frequency of metabolic acidosis in patients who were taking topiramate.4 Eighty patients who were on topiramate for seizure prevention prior to elective craniotomy were studied. Metabolic acidosis was present in 71% of the patients. Patients treated with topiramate also have a higher risk for kidney stones and uric acid elevation.
A 60-year-old patient presents with right great toe pain. On exam he has warmth and erythema of the 1st MTP joint. Aspiration of the joint shows uric acid crystals. He has had BP’s of 150-160 mm Hg systolic on his home BP monitoring over the past 6 months. In clinic today BP is 156/90 mm Hg. Labs: Bun 10, Cr 1.0, K 3.8, Uric acid 7.4.
Which blood pressure medication would you recommend?
A. Hydrochlorothiazide
B. Chlorthalidone
C. Lisinopril
D. Losartan
E. Irbesartan
In a patient with gout, diuretics should be avoided if possible, as they increase uric acid levels. Of the other three options, losartan offers the added benefit of lowering uric acid levels. Losartan has uricosuric effects and lowers uric acid levels, a property that is unique to losartan of the angiotensin receptor blockers (ARBs) that have been studied.5-6 The uric acid lowering appears to be a probenecid-like effect. Losartan has also been evaluated to see whether using it in combination with a thiazide diuretic can reduce the rise in uric acid that occurs with thiazides. Dr. Matsumura et al. looked at data from the COMFORT trial, focusing on the effect of combining losartan with hydrochlorothiazide on uric acid levels.7 They looked at a group of 118 patients on an ARB other than losartan plus a diuretic, who were then randomly assigned to losartan 50 mg/hydrochlorothiazide 12.5 mg or continuation of another ARB plus a diuretic. Blood pressure control was the same between groups, but the patients who received the losartan combination had lower uric acid levels (P = .01).
Pearls: Topiramate acts as a cerbonic anhydrase inhibitor and can cause a non–anion gap acidosis. Losartan has a modest uricosuric effect and can modestly lower uric acid levels. This is a unique property of losartan and is not shared by other ARBs.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Salpeter SR et al. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2010;4:CD002967.
2. Charytan DM et al. Metformin use and cardiovascular events in patients with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2019 Jan 22. doi: 10.1111/dom.13642.
3. Mirza N et al. Effect of topiramate on acid-base balance: extent, mechanism and effects. Br J Clin Pharmacol. 2009 Nov;68(5):655-61.
4. Ture H et al. The frequency and severity of metabolic acidosis related to topiramate. J Int Med Res. 2016;44(6):1376-80.
5. Würzner G et al. Comparative effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricaemia and gout. J Hypertens. 2001 Oct;19(10):1855-60.
6. Puig JG et al. Effect of eprosartan and losartan on uric acid metabolism in patients with essential hypertension. J Hypertens. 1999 Jul;17(7):1033-9.
7. Matsumura K et al. Effect of losartan on serum uric acid in hypertension treated with a diuretic: the COMFORT study. Clin Exp Hypertens. 2015;37(3):192-6.
Automated office BP readings best routine measures
Automated office blood pressure readings appear to be more accurate than routine office readings and BP readings in research settings, according to a recent systematic review and meta-analysis.
Based on the evidence, automated office BP (AOBP) readings should now be the preferred method of reading a patient’s BP in clinical practice despite initial reluctance to incorporate this technique over other methods, the researchers wrote in JAMA Internal Medicine.
“The existing evidence supports the use of AOBP to screen patients for possible hypertension in clinical practice, especially if one takes into account the white coat effect associated with current manual or oscillometric techniques for office BP measurement,” wrote Michael Roerecke, PhD, of the University of Toronto, and his colleagues.
Dr. Roerecke and his colleagues identified 31 articles with 9,279 participants (4,736 men, 4,543 women) where AOBP was compared with another method of BP reading, such as awake ambulatory, routine office, and research BP readings. The AOBP reading was performed with a fully automated oscillometric sphygmomanometer with the patient resting in a quiet area.
The researchers found systolic AOBP of 130 mm Hg was associated with significantly higher readings from routine office (mean difference, 14.5 mm Hg) or research BP readings (7.0 mm Hg), while participants had similar AOBP and awake ambulatory BP readings (0.3 mm Hg). All differences were statistically significant (P less than .001).
“If AOBP is to be used in clinical practice, readings must closely adhere to the procedures used in the AOBP studies in this meta-analysis, including multiple BP readings recorded with a fully automated oscillometric sphygmomanometer while the patient rests alone in a quiet place,” the researchers wrote.
Potential limitations of the study were the large statistical heterogeneity of the sample, though the researchers noted little clinical heterogeneity, and that most studies measured AOBP and awake ambulatory BP on the same day to limit differences in timing.
The authors reported no relevant conflicts of interest.
SOURCE: Roerecke M et al. JAMA Intern Med. 2019 Feb 4. doi: 10.1001/jamainternmed.2018.6551.
Automated office blood pressure readings appear to be more accurate than routine office readings and BP readings in research settings, according to a recent systematic review and meta-analysis.
Based on the evidence, automated office BP (AOBP) readings should now be the preferred method of reading a patient’s BP in clinical practice despite initial reluctance to incorporate this technique over other methods, the researchers wrote in JAMA Internal Medicine.
“The existing evidence supports the use of AOBP to screen patients for possible hypertension in clinical practice, especially if one takes into account the white coat effect associated with current manual or oscillometric techniques for office BP measurement,” wrote Michael Roerecke, PhD, of the University of Toronto, and his colleagues.
Dr. Roerecke and his colleagues identified 31 articles with 9,279 participants (4,736 men, 4,543 women) where AOBP was compared with another method of BP reading, such as awake ambulatory, routine office, and research BP readings. The AOBP reading was performed with a fully automated oscillometric sphygmomanometer with the patient resting in a quiet area.
The researchers found systolic AOBP of 130 mm Hg was associated with significantly higher readings from routine office (mean difference, 14.5 mm Hg) or research BP readings (7.0 mm Hg), while participants had similar AOBP and awake ambulatory BP readings (0.3 mm Hg). All differences were statistically significant (P less than .001).
“If AOBP is to be used in clinical practice, readings must closely adhere to the procedures used in the AOBP studies in this meta-analysis, including multiple BP readings recorded with a fully automated oscillometric sphygmomanometer while the patient rests alone in a quiet place,” the researchers wrote.
Potential limitations of the study were the large statistical heterogeneity of the sample, though the researchers noted little clinical heterogeneity, and that most studies measured AOBP and awake ambulatory BP on the same day to limit differences in timing.
The authors reported no relevant conflicts of interest.
SOURCE: Roerecke M et al. JAMA Intern Med. 2019 Feb 4. doi: 10.1001/jamainternmed.2018.6551.
Automated office blood pressure readings appear to be more accurate than routine office readings and BP readings in research settings, according to a recent systematic review and meta-analysis.
Based on the evidence, automated office BP (AOBP) readings should now be the preferred method of reading a patient’s BP in clinical practice despite initial reluctance to incorporate this technique over other methods, the researchers wrote in JAMA Internal Medicine.
“The existing evidence supports the use of AOBP to screen patients for possible hypertension in clinical practice, especially if one takes into account the white coat effect associated with current manual or oscillometric techniques for office BP measurement,” wrote Michael Roerecke, PhD, of the University of Toronto, and his colleagues.
Dr. Roerecke and his colleagues identified 31 articles with 9,279 participants (4,736 men, 4,543 women) where AOBP was compared with another method of BP reading, such as awake ambulatory, routine office, and research BP readings. The AOBP reading was performed with a fully automated oscillometric sphygmomanometer with the patient resting in a quiet area.
The researchers found systolic AOBP of 130 mm Hg was associated with significantly higher readings from routine office (mean difference, 14.5 mm Hg) or research BP readings (7.0 mm Hg), while participants had similar AOBP and awake ambulatory BP readings (0.3 mm Hg). All differences were statistically significant (P less than .001).
“If AOBP is to be used in clinical practice, readings must closely adhere to the procedures used in the AOBP studies in this meta-analysis, including multiple BP readings recorded with a fully automated oscillometric sphygmomanometer while the patient rests alone in a quiet place,” the researchers wrote.
Potential limitations of the study were the large statistical heterogeneity of the sample, though the researchers noted little clinical heterogeneity, and that most studies measured AOBP and awake ambulatory BP on the same day to limit differences in timing.
The authors reported no relevant conflicts of interest.
SOURCE: Roerecke M et al. JAMA Intern Med. 2019 Feb 4. doi: 10.1001/jamainternmed.2018.6551.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Automated office BP readings are lower than those taken in routine office or research settings and are similar to awake ambulatory BP readings.
Major finding: The mean difference between automated office BP readings was 14.5 mm Hg, compared with routine office systolic BP, and 7.0 mm Hg, compared with research systolic BP readings.
Study details: A systematic review and meta-analysis of 31 articles with 9,279 patients comparing automated office BP readings with awake ambulatory, routine office, and research BP readings.
Disclosures: The authors reported no relevant conflicts of interest.
Source: Roerecke M et al. JAMA Intern Med. 2019 Feb 4. doi: 10.1001/jamainternmed.2018.6551.
AHA report highlights CVD burden, declines in smoking, sleep importance
Almost half of U.S. adults now have some form of cardiovascular disease, according to the latest annual statistical update from the American Heart Association.
The prevalence is driven in part by the recently changed definition of hypertension, from 140/90 to 130/80 mm Hg, said authors of the American Heart Association Heart Disease and Stroke Statistics–2019 Update.
Cardiovascular disease (CVD) deaths are up, though smoking rates continue to decline, and adults are getting more exercise (Circulation. 2019;139. doi: 10.1161/CIR.0000000000000659).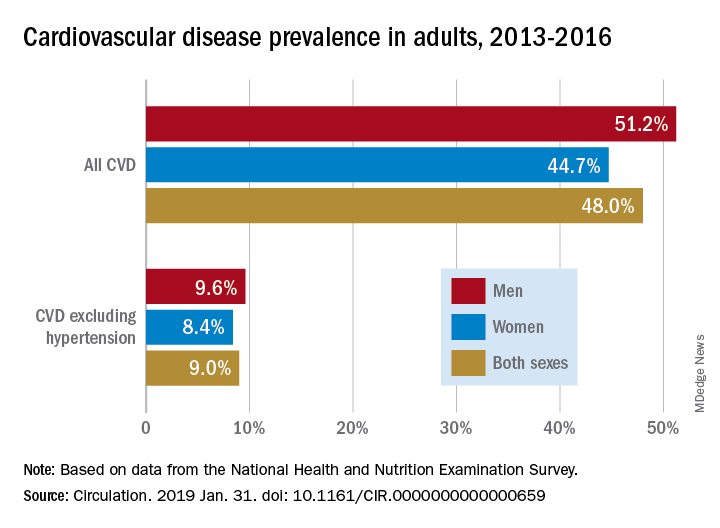
The update includes a new section on sleep and cardiovascular health, an enhanced focus on social determinants of health, and further evidence-based approaches to behavior change, according to the update’s authors, led by chair Emelia J. Benjamin, MD, professor of medicine and epidemiology at Boston University, and vice chair Paul Muntner, PhD, professor of epidemiology at the University of Alabama, Birmingham.
High blood pressure is an “overwhelming presence” that drives heart disease and stroke and can’t be dismissed in the fight against cardiovascular disease, AHA President Ivor J. Benjamin, MD, said in a statement. “Eliminating high blood pressure could have a larger impact on CVD deaths than the elimination of all other risk factors among women, and all except smoking among men.”
Using data from 2013 to 2016, 46% of adults in the United States had hypertension, and in 2016 there were 82,735 deaths attributable primarily to high blood pressure, according to the update.
Total direct costs of hypertension could approach $221 billion by 2035, according to projections in the report.
After decades of decline, U.S. cardiovascular disease deaths increased to 840,678 in 2016, up from 836,546 in 2015, the report says.
Smoking rate declines represent some of the most significant improvements outlined in the report, according to an AHA news release.
Ninety-four percent of adolescents were nonsmokers in the 2015-2016 period, which is up from 76% in 1999-2000, according to the report. The proportion of adult nonsmokers increased to 79% in 2015-2016, up from 73% in 1999-2000.
The new chapter on the importance of sleep cites data from the Centers for Disease Control and Prevention that only 65.2% of Americans have a healthy sleep duration (at least 7 hours), with even lower rates among non-Hispanic blacks, native Hawaiians and Pacific Islanders, and multiracial non-Hispanic individuals.
Short sleep duration is associated with a higher risk of all-cause mortality, total CVD, and coronary heart disease, according to a meta-analysis cited in the report. Long sleep duration, defined as greater than 8 hours, also was associated with higher risk of all-cause mortality, total CVD, coronary heart disease, and stroke.
Members of the statistical update writing group reported disclosures related to the American Heart Association, National Institutes of Health, Amgen, Sanofi, Roche, Abbott, Biogen, Medtronic, and others.
SOURCE: Benjamin EJ et al. Circulation. 2019 Jan 31.
The latest statistics on heart disease and stroke include some metrics that indicate progress, and others that suggest opportunities for improvement.
Tobacco use continues to decline; however, among high school students, e-cigarette use is up to 11.3%, which is concerning.
One bright spot is that the proportion of inactive adults has dropped to 30% in 2016, down from 40% in 2007. Despite that improvement, however, the prevalence of obesity increased significantly over the decade, to the point where nearly 40% of adults are obese and 7.7% are severely obese.
Although 48% of U.S. adults now have cardiovascular disease, according to this latest update, the number drops to just 9% when hypertension is excluded. Even so, 9% represents more than 24.3 million Americans who have coronary artery disease, stroke, or heart failure.
The cost of cardiovascular disease is astronomical, exceeding $351 billion in 2014-1205, with costs projected to increase sharply for older adults over the next few decades.
Starting in 2020, the AHA will begin charting progress in CVD using a metric called health-adjusted life expectancy (HALE), which relies on morbidity and mortality patterns to reflect the number of years a person can expect to live. Patients and the general public may find this metric more understandable than statistics about death rates and cardiovascular risk factors.
Mariell Jessup, MD, is chief science and medical officer for the American Heart Association. Her view on the latest statistical update was derived from a commentary that accompanied the update.
The latest statistics on heart disease and stroke include some metrics that indicate progress, and others that suggest opportunities for improvement.
Tobacco use continues to decline; however, among high school students, e-cigarette use is up to 11.3%, which is concerning.
One bright spot is that the proportion of inactive adults has dropped to 30% in 2016, down from 40% in 2007. Despite that improvement, however, the prevalence of obesity increased significantly over the decade, to the point where nearly 40% of adults are obese and 7.7% are severely obese.
Although 48% of U.S. adults now have cardiovascular disease, according to this latest update, the number drops to just 9% when hypertension is excluded. Even so, 9% represents more than 24.3 million Americans who have coronary artery disease, stroke, or heart failure.
The cost of cardiovascular disease is astronomical, exceeding $351 billion in 2014-1205, with costs projected to increase sharply for older adults over the next few decades.
Starting in 2020, the AHA will begin charting progress in CVD using a metric called health-adjusted life expectancy (HALE), which relies on morbidity and mortality patterns to reflect the number of years a person can expect to live. Patients and the general public may find this metric more understandable than statistics about death rates and cardiovascular risk factors.
Mariell Jessup, MD, is chief science and medical officer for the American Heart Association. Her view on the latest statistical update was derived from a commentary that accompanied the update.
The latest statistics on heart disease and stroke include some metrics that indicate progress, and others that suggest opportunities for improvement.
Tobacco use continues to decline; however, among high school students, e-cigarette use is up to 11.3%, which is concerning.
One bright spot is that the proportion of inactive adults has dropped to 30% in 2016, down from 40% in 2007. Despite that improvement, however, the prevalence of obesity increased significantly over the decade, to the point where nearly 40% of adults are obese and 7.7% are severely obese.
Although 48% of U.S. adults now have cardiovascular disease, according to this latest update, the number drops to just 9% when hypertension is excluded. Even so, 9% represents more than 24.3 million Americans who have coronary artery disease, stroke, or heart failure.
The cost of cardiovascular disease is astronomical, exceeding $351 billion in 2014-1205, with costs projected to increase sharply for older adults over the next few decades.
Starting in 2020, the AHA will begin charting progress in CVD using a metric called health-adjusted life expectancy (HALE), which relies on morbidity and mortality patterns to reflect the number of years a person can expect to live. Patients and the general public may find this metric more understandable than statistics about death rates and cardiovascular risk factors.
Mariell Jessup, MD, is chief science and medical officer for the American Heart Association. Her view on the latest statistical update was derived from a commentary that accompanied the update.
Almost half of U.S. adults now have some form of cardiovascular disease, according to the latest annual statistical update from the American Heart Association.
The prevalence is driven in part by the recently changed definition of hypertension, from 140/90 to 130/80 mm Hg, said authors of the American Heart Association Heart Disease and Stroke Statistics–2019 Update.
Cardiovascular disease (CVD) deaths are up, though smoking rates continue to decline, and adults are getting more exercise (Circulation. 2019;139. doi: 10.1161/CIR.0000000000000659).
The update includes a new section on sleep and cardiovascular health, an enhanced focus on social determinants of health, and further evidence-based approaches to behavior change, according to the update’s authors, led by chair Emelia J. Benjamin, MD, professor of medicine and epidemiology at Boston University, and vice chair Paul Muntner, PhD, professor of epidemiology at the University of Alabama, Birmingham.
High blood pressure is an “overwhelming presence” that drives heart disease and stroke and can’t be dismissed in the fight against cardiovascular disease, AHA President Ivor J. Benjamin, MD, said in a statement. “Eliminating high blood pressure could have a larger impact on CVD deaths than the elimination of all other risk factors among women, and all except smoking among men.”
Using data from 2013 to 2016, 46% of adults in the United States had hypertension, and in 2016 there were 82,735 deaths attributable primarily to high blood pressure, according to the update.
Total direct costs of hypertension could approach $221 billion by 2035, according to projections in the report.
After decades of decline, U.S. cardiovascular disease deaths increased to 840,678 in 2016, up from 836,546 in 2015, the report says.
Smoking rate declines represent some of the most significant improvements outlined in the report, according to an AHA news release.
Ninety-four percent of adolescents were nonsmokers in the 2015-2016 period, which is up from 76% in 1999-2000, according to the report. The proportion of adult nonsmokers increased to 79% in 2015-2016, up from 73% in 1999-2000.
The new chapter on the importance of sleep cites data from the Centers for Disease Control and Prevention that only 65.2% of Americans have a healthy sleep duration (at least 7 hours), with even lower rates among non-Hispanic blacks, native Hawaiians and Pacific Islanders, and multiracial non-Hispanic individuals.
Short sleep duration is associated with a higher risk of all-cause mortality, total CVD, and coronary heart disease, according to a meta-analysis cited in the report. Long sleep duration, defined as greater than 8 hours, also was associated with higher risk of all-cause mortality, total CVD, coronary heart disease, and stroke.
Members of the statistical update writing group reported disclosures related to the American Heart Association, National Institutes of Health, Amgen, Sanofi, Roche, Abbott, Biogen, Medtronic, and others.
SOURCE: Benjamin EJ et al. Circulation. 2019 Jan 31.
Almost half of U.S. adults now have some form of cardiovascular disease, according to the latest annual statistical update from the American Heart Association.
The prevalence is driven in part by the recently changed definition of hypertension, from 140/90 to 130/80 mm Hg, said authors of the American Heart Association Heart Disease and Stroke Statistics–2019 Update.
Cardiovascular disease (CVD) deaths are up, though smoking rates continue to decline, and adults are getting more exercise (Circulation. 2019;139. doi: 10.1161/CIR.0000000000000659).
The update includes a new section on sleep and cardiovascular health, an enhanced focus on social determinants of health, and further evidence-based approaches to behavior change, according to the update’s authors, led by chair Emelia J. Benjamin, MD, professor of medicine and epidemiology at Boston University, and vice chair Paul Muntner, PhD, professor of epidemiology at the University of Alabama, Birmingham.
High blood pressure is an “overwhelming presence” that drives heart disease and stroke and can’t be dismissed in the fight against cardiovascular disease, AHA President Ivor J. Benjamin, MD, said in a statement. “Eliminating high blood pressure could have a larger impact on CVD deaths than the elimination of all other risk factors among women, and all except smoking among men.”
Using data from 2013 to 2016, 46% of adults in the United States had hypertension, and in 2016 there were 82,735 deaths attributable primarily to high blood pressure, according to the update.
Total direct costs of hypertension could approach $221 billion by 2035, according to projections in the report.
After decades of decline, U.S. cardiovascular disease deaths increased to 840,678 in 2016, up from 836,546 in 2015, the report says.
Smoking rate declines represent some of the most significant improvements outlined in the report, according to an AHA news release.
Ninety-four percent of adolescents were nonsmokers in the 2015-2016 period, which is up from 76% in 1999-2000, according to the report. The proportion of adult nonsmokers increased to 79% in 2015-2016, up from 73% in 1999-2000.
The new chapter on the importance of sleep cites data from the Centers for Disease Control and Prevention that only 65.2% of Americans have a healthy sleep duration (at least 7 hours), with even lower rates among non-Hispanic blacks, native Hawaiians and Pacific Islanders, and multiracial non-Hispanic individuals.
Short sleep duration is associated with a higher risk of all-cause mortality, total CVD, and coronary heart disease, according to a meta-analysis cited in the report. Long sleep duration, defined as greater than 8 hours, also was associated with higher risk of all-cause mortality, total CVD, coronary heart disease, and stroke.
Members of the statistical update writing group reported disclosures related to the American Heart Association, National Institutes of Health, Amgen, Sanofi, Roche, Abbott, Biogen, Medtronic, and others.
SOURCE: Benjamin EJ et al. Circulation. 2019 Jan 31.
FROM CIRCULATION
SPRINT MIND published: Extension trial to add 2 years’ follow-up
A new iteration of the SPRINT MIND hypertension trial will seek to prove conclusively the original study’s tantalizing suggestion: that intensive blood pressure control decreases the risk of developing mild cognitive impairment (MCI) and, eventually, dementia.
SPRINT MIND 2.0 will re-recruit SPRINT MIND subjects and enable another follow-up cognitive assessment and other clinical tests as they remain on their standard of care blood pressure regimen. It is largely funded by an $800,000 grant from the Alzheimer’s Association.
Initially released last July at the Alzheimer’s Association International Conference, the results of the SPRINT MIND have now appeared online in JAMA. Although it failed to meet its primary endpoint of reducing dementia incidence, the study did score on two secondary endpoints. Patients who reduced their systolic blood pressure to less than 120 mm Hg were 19% less likely to develop MCI and 17% less likely to be diagnosed with all-cause dementia than were those who achieved a hypertension target of less than 140 mm Hg.
The secondary results, and positive movement in the primary results, sparked excitement in the dementia research community last summer. They have suggested that the median 5-year follow-up just wasn’t long enough to show any significant effects on dementia, which can take years to fully manifest. Adding 2 more years with SPRINT MIND 2.0 should be long enough to discern those benefits, if indeed they exist.
“SPRINT MIND 2.0 and the work leading up to it offers genuine, concrete hope,” Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association, said in a press statement. “MCI is a known risk factor for dementia, and everyone who experiences dementia passes through MCI. When you prevent new cases of MCI, you are preventing new cases of dementia. The Alzheimer’s Association finds these data to be compelling and is committed to getting clarity and certainty on the dementia outcome by following participants for a longer period of time.”
The study strengthens the new and energetic push to find ways to prevent dementia, which has proven itself intractable in every drug study to date.
“This study is in line with where the field of dementia research is going: preventing memory loss earlier,” said Laurie Ryan, PhD, chief of the dementias of aging branch in the National Institute on Aging. “Much like we have research-based interventions for heart health and cancer prevention, we hope to have guidance based on this and subsequent studies that will more definitively show how to slow or even stop dementia well before symptoms appear.”
NIA director Richard J. Hodes, MD, agreed.
“Dementia continues to be a large public health challenge, and based on the primary results of this study, we still have yet to find an intervention strategy proven to reduce the risk of dementia,” he said in a press statement. “Nevertheless, the secondary results showing that intensive lowering of blood pressure may reduce risk for MCI, a known risk factor for dementia, gives us additional avenues to explore on the path to prevention.”
SPRINT MIND was a substudy of the Systolic Blood Pressure Intervention Trial (SPRINT). It compared two strategies for managing hypertension in older adults. The intensive strategy had a target of less than 120 mm Hg, while standard care had a target of less than 140 mm Hg. SPRINT showed that more intensive blood pressure control produced a 25% reduction in the composite primary composite endpoint of cardiovascular events, stroke, and cardiovascular death. The intensive arm was so successful that SPRINT helped inform the 2017 high blood pressure clinical guidelines from the American Heart Association and American College of Cardiology.
The SPRINT MIND substudy, headed by Jeff D. Williamson, MD, of Wake Forest University, Winston-Salem, NC, asked whether intensive management had any effect on probable all-cause dementia or MCI, as well as imaging evidence of changes in white matter lesions and brain volume. It followed patients for up to 7 years and comprised 9,361 SPRINT subjects at least 50 years old (mean, 68 years) with at least one cardiovascular risk factor. Nearly a third (30%) were black, and 10% Hispanic. The primary outcome was incident probable dementia. Secondary outcomes were MCI and a composite of MCI and/or probable dementia. About a third had a SBP of 132 mm Hg or less, another third had a systolic pressure of 132-145 mm Hg, and the remainder had a systolic pressure greater than 145 mm Hg.
Physicians could use their choice of antihypertensive treatments. The study protocol encouraged, but did not mandate, thiazide-type diuretics as a first-line agent, followed by loop diuretics and beta-adrenergic blockers. Chlorthalidone was encouraged as the primary thiazide-type diuretic, and amlodipine as the preferred calcium-channel blocker.
The interventions did successfully control blood pressure, with a significant difference between the treatment groups. The mean SBP was 121.6 mm Hg in the intensive therapy group and 134.8 mm Hg in the standard group – a statistically significant difference of 13.3 mm Hg.
Dementia developed in 149 in the aggressive control group and 176 in the standard group – a nonsignificant difference of 17% (hazard ratio, 0.83). MCI developed in 287 in the intensive group and 353 in the standard treatment group. This amounted to a statistically significant 19% reduction. There was also a significant 15% reduction in the composite outcome of MCI or probable dementia in favor of intensive treatment.
As evidenced by the Alzheimer’s Association grant, dementia researchers chose to focus on SPRINT MIND’s positive secondary endpoints. At the AAIC meeting, Dr. Williamson even suggested that antihypertensive medications could be seen as disease-modifying agents for cognitive decline. Data support his claim: No dementia intervention yet tested has approached this level of success.
“I think we can say this is the first disease-modifying strategy to reduce the risk of MCI,” Dr. Williamson said during a press briefing. And although the primary endpoint – the 17% relative risk reduction for probable all-cause dementia – didn’t meet statistical significance, “It’s comforting to see that the benefit went in the same direction and was of the same magnitude..”
SOURCE: Williamson JD et al. JAMA 2019 Jan 28. doi:10.1001/jama.2018.21442.
SPRINT MIND offers hope that a very achievable blood pressure goal can dramatically alter the trajectory from mild cognitive impairment to dementia, Kristine Yaffe, MD, wrote in an accompanying editorial. But at this point, it’s impossible to make specific clinical recommendations.
Additionally it is not possible, right now, to know which hypertension treatment regimens were most effective in improved cognitive outcomes.
“Information necessary to compare the effects of classes of antihypertensive agents on cognitive outcomes is also not provided. SPRINT used a quasi-pragmatic approach with suggestions for treatment choice, but practitioners approached SBP control individually, and most participants were taking multiple drugs.”
Nevertheless, the positive secondary findings and the encouraging trajectory on dementia risk should fix blood pressure management squarely into a cornerstone of dementia prevention algorithms.
“The SPRINT MIND study may not be the final approach for prevention of AD or other cognitive impairment, but it represents a major leap forward in what has emerged as a marathon journey.”
Dr. Kristine Yaffe is professor of psychiatry, neurology and epidemiology and the Roy and Marie Scola Endowed Chair at the University of California, San Francisco.
SPRINT MIND offers hope that a very achievable blood pressure goal can dramatically alter the trajectory from mild cognitive impairment to dementia, Kristine Yaffe, MD, wrote in an accompanying editorial. But at this point, it’s impossible to make specific clinical recommendations.
Additionally it is not possible, right now, to know which hypertension treatment regimens were most effective in improved cognitive outcomes.
“Information necessary to compare the effects of classes of antihypertensive agents on cognitive outcomes is also not provided. SPRINT used a quasi-pragmatic approach with suggestions for treatment choice, but practitioners approached SBP control individually, and most participants were taking multiple drugs.”
Nevertheless, the positive secondary findings and the encouraging trajectory on dementia risk should fix blood pressure management squarely into a cornerstone of dementia prevention algorithms.
“The SPRINT MIND study may not be the final approach for prevention of AD or other cognitive impairment, but it represents a major leap forward in what has emerged as a marathon journey.”
Dr. Kristine Yaffe is professor of psychiatry, neurology and epidemiology and the Roy and Marie Scola Endowed Chair at the University of California, San Francisco.
SPRINT MIND offers hope that a very achievable blood pressure goal can dramatically alter the trajectory from mild cognitive impairment to dementia, Kristine Yaffe, MD, wrote in an accompanying editorial. But at this point, it’s impossible to make specific clinical recommendations.
Additionally it is not possible, right now, to know which hypertension treatment regimens were most effective in improved cognitive outcomes.
“Information necessary to compare the effects of classes of antihypertensive agents on cognitive outcomes is also not provided. SPRINT used a quasi-pragmatic approach with suggestions for treatment choice, but practitioners approached SBP control individually, and most participants were taking multiple drugs.”
Nevertheless, the positive secondary findings and the encouraging trajectory on dementia risk should fix blood pressure management squarely into a cornerstone of dementia prevention algorithms.
“The SPRINT MIND study may not be the final approach for prevention of AD or other cognitive impairment, but it represents a major leap forward in what has emerged as a marathon journey.”
Dr. Kristine Yaffe is professor of psychiatry, neurology and epidemiology and the Roy and Marie Scola Endowed Chair at the University of California, San Francisco.
A new iteration of the SPRINT MIND hypertension trial will seek to prove conclusively the original study’s tantalizing suggestion: that intensive blood pressure control decreases the risk of developing mild cognitive impairment (MCI) and, eventually, dementia.
SPRINT MIND 2.0 will re-recruit SPRINT MIND subjects and enable another follow-up cognitive assessment and other clinical tests as they remain on their standard of care blood pressure regimen. It is largely funded by an $800,000 grant from the Alzheimer’s Association.
Initially released last July at the Alzheimer’s Association International Conference, the results of the SPRINT MIND have now appeared online in JAMA. Although it failed to meet its primary endpoint of reducing dementia incidence, the study did score on two secondary endpoints. Patients who reduced their systolic blood pressure to less than 120 mm Hg were 19% less likely to develop MCI and 17% less likely to be diagnosed with all-cause dementia than were those who achieved a hypertension target of less than 140 mm Hg.
The secondary results, and positive movement in the primary results, sparked excitement in the dementia research community last summer. They have suggested that the median 5-year follow-up just wasn’t long enough to show any significant effects on dementia, which can take years to fully manifest. Adding 2 more years with SPRINT MIND 2.0 should be long enough to discern those benefits, if indeed they exist.
“SPRINT MIND 2.0 and the work leading up to it offers genuine, concrete hope,” Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association, said in a press statement. “MCI is a known risk factor for dementia, and everyone who experiences dementia passes through MCI. When you prevent new cases of MCI, you are preventing new cases of dementia. The Alzheimer’s Association finds these data to be compelling and is committed to getting clarity and certainty on the dementia outcome by following participants for a longer period of time.”
The study strengthens the new and energetic push to find ways to prevent dementia, which has proven itself intractable in every drug study to date.
“This study is in line with where the field of dementia research is going: preventing memory loss earlier,” said Laurie Ryan, PhD, chief of the dementias of aging branch in the National Institute on Aging. “Much like we have research-based interventions for heart health and cancer prevention, we hope to have guidance based on this and subsequent studies that will more definitively show how to slow or even stop dementia well before symptoms appear.”
NIA director Richard J. Hodes, MD, agreed.
“Dementia continues to be a large public health challenge, and based on the primary results of this study, we still have yet to find an intervention strategy proven to reduce the risk of dementia,” he said in a press statement. “Nevertheless, the secondary results showing that intensive lowering of blood pressure may reduce risk for MCI, a known risk factor for dementia, gives us additional avenues to explore on the path to prevention.”
SPRINT MIND was a substudy of the Systolic Blood Pressure Intervention Trial (SPRINT). It compared two strategies for managing hypertension in older adults. The intensive strategy had a target of less than 120 mm Hg, while standard care had a target of less than 140 mm Hg. SPRINT showed that more intensive blood pressure control produced a 25% reduction in the composite primary composite endpoint of cardiovascular events, stroke, and cardiovascular death. The intensive arm was so successful that SPRINT helped inform the 2017 high blood pressure clinical guidelines from the American Heart Association and American College of Cardiology.
The SPRINT MIND substudy, headed by Jeff D. Williamson, MD, of Wake Forest University, Winston-Salem, NC, asked whether intensive management had any effect on probable all-cause dementia or MCI, as well as imaging evidence of changes in white matter lesions and brain volume. It followed patients for up to 7 years and comprised 9,361 SPRINT subjects at least 50 years old (mean, 68 years) with at least one cardiovascular risk factor. Nearly a third (30%) were black, and 10% Hispanic. The primary outcome was incident probable dementia. Secondary outcomes were MCI and a composite of MCI and/or probable dementia. About a third had a SBP of 132 mm Hg or less, another third had a systolic pressure of 132-145 mm Hg, and the remainder had a systolic pressure greater than 145 mm Hg.
Physicians could use their choice of antihypertensive treatments. The study protocol encouraged, but did not mandate, thiazide-type diuretics as a first-line agent, followed by loop diuretics and beta-adrenergic blockers. Chlorthalidone was encouraged as the primary thiazide-type diuretic, and amlodipine as the preferred calcium-channel blocker.
The interventions did successfully control blood pressure, with a significant difference between the treatment groups. The mean SBP was 121.6 mm Hg in the intensive therapy group and 134.8 mm Hg in the standard group – a statistically significant difference of 13.3 mm Hg.
Dementia developed in 149 in the aggressive control group and 176 in the standard group – a nonsignificant difference of 17% (hazard ratio, 0.83). MCI developed in 287 in the intensive group and 353 in the standard treatment group. This amounted to a statistically significant 19% reduction. There was also a significant 15% reduction in the composite outcome of MCI or probable dementia in favor of intensive treatment.
As evidenced by the Alzheimer’s Association grant, dementia researchers chose to focus on SPRINT MIND’s positive secondary endpoints. At the AAIC meeting, Dr. Williamson even suggested that antihypertensive medications could be seen as disease-modifying agents for cognitive decline. Data support his claim: No dementia intervention yet tested has approached this level of success.
“I think we can say this is the first disease-modifying strategy to reduce the risk of MCI,” Dr. Williamson said during a press briefing. And although the primary endpoint – the 17% relative risk reduction for probable all-cause dementia – didn’t meet statistical significance, “It’s comforting to see that the benefit went in the same direction and was of the same magnitude..”
SOURCE: Williamson JD et al. JAMA 2019 Jan 28. doi:10.1001/jama.2018.21442.
A new iteration of the SPRINT MIND hypertension trial will seek to prove conclusively the original study’s tantalizing suggestion: that intensive blood pressure control decreases the risk of developing mild cognitive impairment (MCI) and, eventually, dementia.
SPRINT MIND 2.0 will re-recruit SPRINT MIND subjects and enable another follow-up cognitive assessment and other clinical tests as they remain on their standard of care blood pressure regimen. It is largely funded by an $800,000 grant from the Alzheimer’s Association.
Initially released last July at the Alzheimer’s Association International Conference, the results of the SPRINT MIND have now appeared online in JAMA. Although it failed to meet its primary endpoint of reducing dementia incidence, the study did score on two secondary endpoints. Patients who reduced their systolic blood pressure to less than 120 mm Hg were 19% less likely to develop MCI and 17% less likely to be diagnosed with all-cause dementia than were those who achieved a hypertension target of less than 140 mm Hg.
The secondary results, and positive movement in the primary results, sparked excitement in the dementia research community last summer. They have suggested that the median 5-year follow-up just wasn’t long enough to show any significant effects on dementia, which can take years to fully manifest. Adding 2 more years with SPRINT MIND 2.0 should be long enough to discern those benefits, if indeed they exist.
“SPRINT MIND 2.0 and the work leading up to it offers genuine, concrete hope,” Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association, said in a press statement. “MCI is a known risk factor for dementia, and everyone who experiences dementia passes through MCI. When you prevent new cases of MCI, you are preventing new cases of dementia. The Alzheimer’s Association finds these data to be compelling and is committed to getting clarity and certainty on the dementia outcome by following participants for a longer period of time.”
The study strengthens the new and energetic push to find ways to prevent dementia, which has proven itself intractable in every drug study to date.
“This study is in line with where the field of dementia research is going: preventing memory loss earlier,” said Laurie Ryan, PhD, chief of the dementias of aging branch in the National Institute on Aging. “Much like we have research-based interventions for heart health and cancer prevention, we hope to have guidance based on this and subsequent studies that will more definitively show how to slow or even stop dementia well before symptoms appear.”
NIA director Richard J. Hodes, MD, agreed.
“Dementia continues to be a large public health challenge, and based on the primary results of this study, we still have yet to find an intervention strategy proven to reduce the risk of dementia,” he said in a press statement. “Nevertheless, the secondary results showing that intensive lowering of blood pressure may reduce risk for MCI, a known risk factor for dementia, gives us additional avenues to explore on the path to prevention.”
SPRINT MIND was a substudy of the Systolic Blood Pressure Intervention Trial (SPRINT). It compared two strategies for managing hypertension in older adults. The intensive strategy had a target of less than 120 mm Hg, while standard care had a target of less than 140 mm Hg. SPRINT showed that more intensive blood pressure control produced a 25% reduction in the composite primary composite endpoint of cardiovascular events, stroke, and cardiovascular death. The intensive arm was so successful that SPRINT helped inform the 2017 high blood pressure clinical guidelines from the American Heart Association and American College of Cardiology.
The SPRINT MIND substudy, headed by Jeff D. Williamson, MD, of Wake Forest University, Winston-Salem, NC, asked whether intensive management had any effect on probable all-cause dementia or MCI, as well as imaging evidence of changes in white matter lesions and brain volume. It followed patients for up to 7 years and comprised 9,361 SPRINT subjects at least 50 years old (mean, 68 years) with at least one cardiovascular risk factor. Nearly a third (30%) were black, and 10% Hispanic. The primary outcome was incident probable dementia. Secondary outcomes were MCI and a composite of MCI and/or probable dementia. About a third had a SBP of 132 mm Hg or less, another third had a systolic pressure of 132-145 mm Hg, and the remainder had a systolic pressure greater than 145 mm Hg.
Physicians could use their choice of antihypertensive treatments. The study protocol encouraged, but did not mandate, thiazide-type diuretics as a first-line agent, followed by loop diuretics and beta-adrenergic blockers. Chlorthalidone was encouraged as the primary thiazide-type diuretic, and amlodipine as the preferred calcium-channel blocker.
The interventions did successfully control blood pressure, with a significant difference between the treatment groups. The mean SBP was 121.6 mm Hg in the intensive therapy group and 134.8 mm Hg in the standard group – a statistically significant difference of 13.3 mm Hg.
Dementia developed in 149 in the aggressive control group and 176 in the standard group – a nonsignificant difference of 17% (hazard ratio, 0.83). MCI developed in 287 in the intensive group and 353 in the standard treatment group. This amounted to a statistically significant 19% reduction. There was also a significant 15% reduction in the composite outcome of MCI or probable dementia in favor of intensive treatment.
As evidenced by the Alzheimer’s Association grant, dementia researchers chose to focus on SPRINT MIND’s positive secondary endpoints. At the AAIC meeting, Dr. Williamson even suggested that antihypertensive medications could be seen as disease-modifying agents for cognitive decline. Data support his claim: No dementia intervention yet tested has approached this level of success.
“I think we can say this is the first disease-modifying strategy to reduce the risk of MCI,” Dr. Williamson said during a press briefing. And although the primary endpoint – the 17% relative risk reduction for probable all-cause dementia – didn’t meet statistical significance, “It’s comforting to see that the benefit went in the same direction and was of the same magnitude..”
SOURCE: Williamson JD et al. JAMA 2019 Jan 28. doi:10.1001/jama.2018.21442.
FROM JAMA
Key clinical point: Keeping systolic blood pressure lower than 120 mm Hg did not significantly reduce the risk of all-cause dementia in patients with hypertension, but it did lower the risk of mild cognitive impairment and probable dementia.
Major finding: The intensively treated group had a nonsignificant 17% lower risk of dementia, and significant reductions in the risk of MCI (19%) and probable dementia (15%).
Study details: SPRINT MIND was a substudy of the SPRINT antihypertension trial.
Source: Williamson JD et al. JAMA 2019 Jan 28. doi:10.1001/jama.2018.21442.
FDA: Nitrosamine-contaminated ARBs marketed for 4 years
Although first detected in summer 2018, angiotensin II receptor blockers (ARBs) contaminated with nitrosamines have been on the market in the United States for 4 years, according to the Food and Drug Administration.
“FDA scientists estimate that, if 8,000 people took the highest daily valsartan dose (320 mg) that contained NDMA [N-Nitrosodimethylamine] for 4 years (the time we think the affected products had been on the U.S. market), there may be one additional case of cancer beyond the average cancer rate among those 8,000 Americans,” the agency said in a Jan. 25 update from Commissioner Scott Gottlieb, MD, and Janet Woodcock, MD, director of the Center for Drug Evaluation and Research.
“The vast majority of patients exposed to NDMA through ARBs received much smaller amounts of the impurity than this worst-case scenario. Since not all ARBs are affected, it’s very likely that a patient taking an ARB for 4 years would not have always received one of the affected products. We’re still seeking to similarly quantify the risk from NDEA [N-Nitrosodiethylamine] and plan to communicate our findings as soon as possible,” they said.
“While the total exposure to these impurities for most patients was small, we are deeply concerned that patients were exposed to this impurity in the first place and that the presence of nitrosamines went undetected for a period of time,” Dr. Gottlieb and Dr. Woodcock said in the statement. Through ongoing and “exhaustive” efforts, they pledged the agency will resolve the problem and ensure it never happens again.
Meanwhile, the ongoing recalls have led to a shortage of valsartan, and other ARBs may soon follow suit. The agency hoped the one case of cancer per 8,000 patients analysis would help providers “balance the risk of patients ingesting low levels of the impurities ... for a short period of time” during shortages until a suitable replacement or alternative is found.
The problem surfaced last year when FDA was alerted to nitrosamine contamination in valsartan manufactured in China and marketed in the U.S. by generic pharmaceutical companies. Contamination has since been detected in generic irbesartan and losartan.
The impurities are generated “when specific chemicals and reaction conditions are present in the manufacturing process ... and may also result from the reuse of materials, such as solvents,” something “neither regulators nor industry fully understood” before. The agency has developed and shared new tests to detect nitrosamines. “Manufacturers using processes at risk for these impurities are expected to test for them to ensure that active ingredients and finished products are free of detectable levels,” it said.
Meanwhile, the recalls keep coming, the latest for losartan and hydrochlorothiazide combination tablets from Torrent Pharmaceuticals. The recalls are listed on FDA’s website.
Although first detected in summer 2018, angiotensin II receptor blockers (ARBs) contaminated with nitrosamines have been on the market in the United States for 4 years, according to the Food and Drug Administration.
“FDA scientists estimate that, if 8,000 people took the highest daily valsartan dose (320 mg) that contained NDMA [N-Nitrosodimethylamine] for 4 years (the time we think the affected products had been on the U.S. market), there may be one additional case of cancer beyond the average cancer rate among those 8,000 Americans,” the agency said in a Jan. 25 update from Commissioner Scott Gottlieb, MD, and Janet Woodcock, MD, director of the Center for Drug Evaluation and Research.
“The vast majority of patients exposed to NDMA through ARBs received much smaller amounts of the impurity than this worst-case scenario. Since not all ARBs are affected, it’s very likely that a patient taking an ARB for 4 years would not have always received one of the affected products. We’re still seeking to similarly quantify the risk from NDEA [N-Nitrosodiethylamine] and plan to communicate our findings as soon as possible,” they said.
“While the total exposure to these impurities for most patients was small, we are deeply concerned that patients were exposed to this impurity in the first place and that the presence of nitrosamines went undetected for a period of time,” Dr. Gottlieb and Dr. Woodcock said in the statement. Through ongoing and “exhaustive” efforts, they pledged the agency will resolve the problem and ensure it never happens again.
Meanwhile, the ongoing recalls have led to a shortage of valsartan, and other ARBs may soon follow suit. The agency hoped the one case of cancer per 8,000 patients analysis would help providers “balance the risk of patients ingesting low levels of the impurities ... for a short period of time” during shortages until a suitable replacement or alternative is found.
The problem surfaced last year when FDA was alerted to nitrosamine contamination in valsartan manufactured in China and marketed in the U.S. by generic pharmaceutical companies. Contamination has since been detected in generic irbesartan and losartan.
The impurities are generated “when specific chemicals and reaction conditions are present in the manufacturing process ... and may also result from the reuse of materials, such as solvents,” something “neither regulators nor industry fully understood” before. The agency has developed and shared new tests to detect nitrosamines. “Manufacturers using processes at risk for these impurities are expected to test for them to ensure that active ingredients and finished products are free of detectable levels,” it said.
Meanwhile, the recalls keep coming, the latest for losartan and hydrochlorothiazide combination tablets from Torrent Pharmaceuticals. The recalls are listed on FDA’s website.
Although first detected in summer 2018, angiotensin II receptor blockers (ARBs) contaminated with nitrosamines have been on the market in the United States for 4 years, according to the Food and Drug Administration.
“FDA scientists estimate that, if 8,000 people took the highest daily valsartan dose (320 mg) that contained NDMA [N-Nitrosodimethylamine] for 4 years (the time we think the affected products had been on the U.S. market), there may be one additional case of cancer beyond the average cancer rate among those 8,000 Americans,” the agency said in a Jan. 25 update from Commissioner Scott Gottlieb, MD, and Janet Woodcock, MD, director of the Center for Drug Evaluation and Research.
“The vast majority of patients exposed to NDMA through ARBs received much smaller amounts of the impurity than this worst-case scenario. Since not all ARBs are affected, it’s very likely that a patient taking an ARB for 4 years would not have always received one of the affected products. We’re still seeking to similarly quantify the risk from NDEA [N-Nitrosodiethylamine] and plan to communicate our findings as soon as possible,” they said.
“While the total exposure to these impurities for most patients was small, we are deeply concerned that patients were exposed to this impurity in the first place and that the presence of nitrosamines went undetected for a period of time,” Dr. Gottlieb and Dr. Woodcock said in the statement. Through ongoing and “exhaustive” efforts, they pledged the agency will resolve the problem and ensure it never happens again.
Meanwhile, the ongoing recalls have led to a shortage of valsartan, and other ARBs may soon follow suit. The agency hoped the one case of cancer per 8,000 patients analysis would help providers “balance the risk of patients ingesting low levels of the impurities ... for a short period of time” during shortages until a suitable replacement or alternative is found.
The problem surfaced last year when FDA was alerted to nitrosamine contamination in valsartan manufactured in China and marketed in the U.S. by generic pharmaceutical companies. Contamination has since been detected in generic irbesartan and losartan.
The impurities are generated “when specific chemicals and reaction conditions are present in the manufacturing process ... and may also result from the reuse of materials, such as solvents,” something “neither regulators nor industry fully understood” before. The agency has developed and shared new tests to detect nitrosamines. “Manufacturers using processes at risk for these impurities are expected to test for them to ensure that active ingredients and finished products are free of detectable levels,” it said.
Meanwhile, the recalls keep coming, the latest for losartan and hydrochlorothiazide combination tablets from Torrent Pharmaceuticals. The recalls are listed on FDA’s website.