User login
Weight loss, fatigue, and renal failure
A black 37-year-old man has gradually lost 100 lb (45 kg) over the past 2 years, and reports progressive fatigue and malaise as well. He has not noted swollen lymph nodes, fever, or night sweats. He denies dyspnea, cough, or chest pain. He has no skin rashes, and no dry or red eyes or visual changes. He reports no flank pain, dysuria, frank hematuria, foamy urine, decline in urine output, or difficulty voiding.
He has no history of significant medical conditions. He does not drink, smoke, or use recreational drugs. He is not taking any prescription medications and has not been using nonsteroidal anti-inflammatory drugs (NSAIDs) or combination analgesics. He does not have a family history of kidney disease.
Physical examination. He appears relaxed and comfortable. He does not have nasal polyps or signs of pharyngeal inflammation. He has no apparent lymphadenopathy. His breath sounds are normal without rales or wheezes. Cardiac examination reveals a regular rhythm, with no rub or murmurs. The abdomen is soft and nontender with no flank pain or groin tenderness. The skin is intact with no rash or nodules.
- Temperature 98.4ºF (36.9ºC)
- Blood pressure 125/70 mm Hg
- Heart rate 102 beats per minute
- Respiratory rate 19 per minute
- Oxygen saturation 99% while breathing room air
- Weight 194 lb (88 kg)
- Body mass index 28 kg/m2.
Laboratory testing (Table 1) reveals severe renal insufficiency with anemia:
- Serum creatinine 9 mg/dL (reference range 0.5–1.2)
- Estimated glomerular filtration rate (eGFR) 8 mL/min/1.73m2 (using the Modification of Diet in Renal Disease Study equation).
His serum calcium level is normal, but his serum phosphorus is 5.3 mg/dL (reference range 2.5–4.6), and his parathyroid hormone level is 317 pg/mL (12–88), consistent with hyperparathyroidism secondary to chronic kidney disease. His 25-hydroxyvitamin D level is less than 13 ng/mL (30–80), and angiotensin-converting enzyme (ACE) is 129 U/L (9–67 U/L). His urinary calcium level is less than 3.0 mg/dL.
Urinalysis:
- Urine protein 100 mg/dL (0–20)
- No urine crystals
- 3 to 5 coarse granular urine casts per high-power field
- No hematuria or pyuria.
Renal ultrasonography shows normal kidneys with no hydronephrosis.
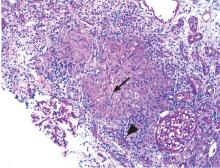
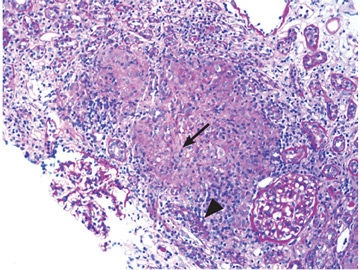
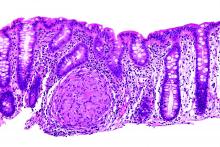
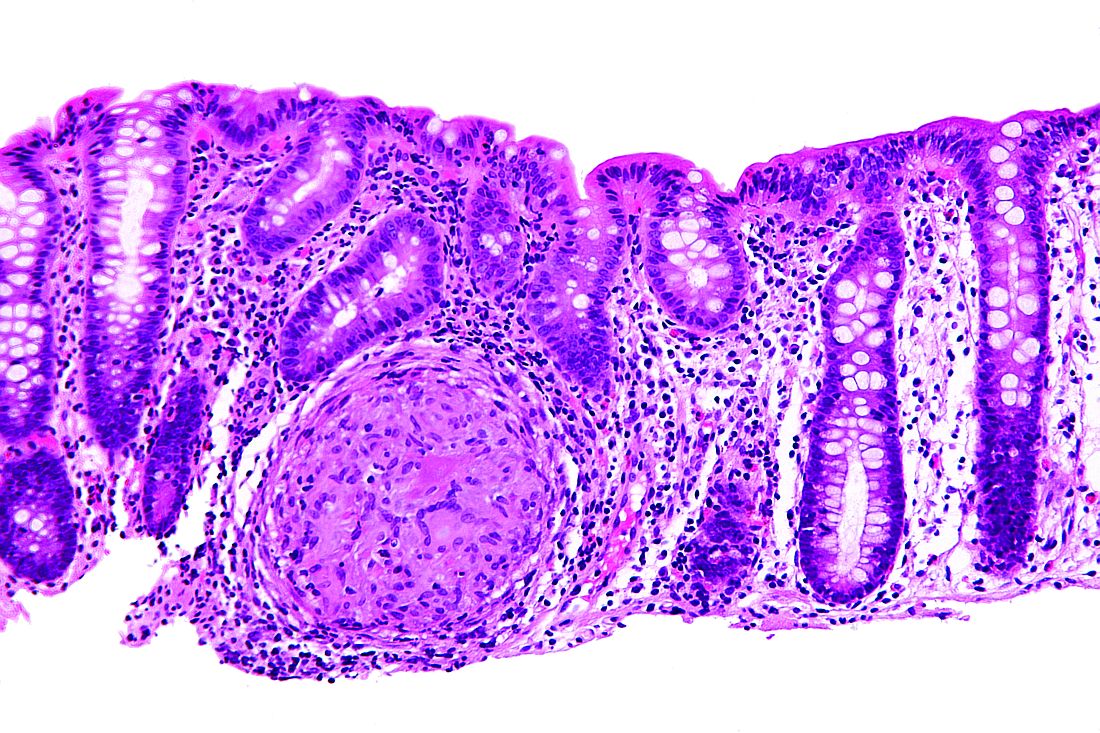
Renal biopsy study demonstrates noncaseating granulomatous interstitial nephritis (Figure 1).
GRANULOMATOUS INTERSTITIAL NEPHRITIS
1. Based on the information above, what is the most likely cause of this patient’s kidney disease?
- Medication
- Granulomatosis with polyangiitis
- Sarcoidosis
- Infection
Granulomatous interstitial nephritis is a histologic diagnosis that is present in up to 1% of renal biopsies. It has been associated with medications, infections, sarcoidosis, crystal deposits, paraproteinemia, and granulomatosis with polyangiitis and also is seen in an idiopathic form.
Medicines implicated include anticonvulsants, antibiotics, NSAIDs, allopurinol, and diuretics.
Mycobacteria and fungi are the main infective causes and seem to be the main causative factor in cases of renal transplant.1 Granulomas are usually not found on kidney biopsy in granulomatosis with polyangiitis, and that diagnosis is usually made by the presence of antiproteinase 3 antibodies.2
Sarcoidosis is the most likely diagnosis in this patient after excluding implicated medications, infection, and vasculitis and confirming the presence of granulomatous interstitial nephritis on renal biopsy.
SARCOIDOSIS: A MULTISYSTEM DISEASE
Sarcoidosis is a multisystem inflammatory disease of unknown cause, characterized by noncaseating epithelioid granulomas. It can involve any organ but most commonly the thoracic and peripheral lymph nodes.3,4 Involvement of the eyes and skin is also relatively common.
Extrapulmonary involvement occurs in more than 30% of cases of sarcoidosis, almost always with concomitant thoracic involvement.5,6 Isolated extrathoracic sarcoidosis is unusual, found in only 2% of patients in a sarcoidosis case-control study.5
Current theory suggests that sarcoidosis develops from a cell-mediated immune response triggered by one or more unidentified antigens in people with a genetic predisposition.7
Sarcoidosis affects men and women of all ages, most often adults ages 20 to 40; but more recently, it has increased in US adults over age 55.8 The condition is more prevalent in Northern Europe and Japan, and in blacks in the United States.7
HOW COMMON IS RENAL INVOLVEMENT IN SARCOIDOSIS?
2. What is the likelihood of finding clinically apparent renal involvement in a patient with sarcoidosis?
- Greater than 70%
- Greater than 50%
- Up to 50%
- Less than 10%
The prevalence of renal involvement in sarcoidosis is hard to determine due to differences in study design and patient populations included in the available reports, and because renal involvement may be silent for many years. Recent studies have reported impaired renal function in 0.7% to 9.7% of cases: eg, a case-control study of 736 patients reported clinically apparent renal involvement in 0.7% of patients,5 and in a series of 818 patients, the incidence was 1%.9 In earlier studies, depending on the diagnostic criteria, the incidence ranged from 1.1% to 9.7%.10
The prevalence of renal involvement may also be underestimated because it can be asymptomatic, and the number of granulomas may be so few that they are absent in a small biopsy specimen. A higher prevalence of renal involvement in sarcoidosis is reported from autopsy studies, although many cases remained clinically silent. These studies have reported renal noncaseating granulomas in 7% to 23% of sarcoidosis patients.11–13
PRESENTATION OF RENAL SARCOIDOSIS
3. What is the most common presentation in isolated renal sarcoidosis?
- Sterile pyuria
- Elevated urine eosinophils
- Renal insufficiency
- Painless hematuria
Renal manifestations of sarcoidosis include hypercalcemia, hypercalciuria, nephrocalcinosis, nephrolithiasis, and impaired renal function.14 Renal involvement can occur during the course of existing sarcoidosis, at the time of first presentation, or even as the sole presentation of the disease.1,11,15 In patients with isolated renal sarcoidosis, the most common presentation is renal insufficiency.15,16
Two main pathways for nephron insult that have been validated are granulomatous infiltration of the renal interstitium and disordered calcium homeostasis.11,17 Though extremely rare, various types of glomerular disease, renal tubular defects, and renal vascular involvement such as renal artery granulomatous angiitis have been documented.18
Hypercalcemia in sarcoidosis
Sarcoidosis is known to cause hypercalcemia by increasing calcium absorption secondary to 1,25-dihydroxyvitamin D production from granulomas. Our patient’s case is unusual, as renal failure was the sole extrapulmonary manifestation of sarcoidosis without hypercalcemia.
In sarcoidosis, extrarenal production of 1-alpha-hydroxylase by activated macrophages inappropriately increases levels of 1,25-dihydroxyvitamin D (calcitriol). Subsequently, serum calcium levels are increased. Unlike its renal equivalent, granulomatous 1-alpha-hydroxylase evades the normal negative feedback of hypercalcemia, so that increased calcitriol levels are sustained, leading to hypercalcemia, often accompanied by hypercalciuria.19
Disruption of calcium homeostasis affects renal function through several mechanisms. Hypercalcemia promotes vasoconstriction of the afferent arteriole, leading to a reduction in the GFR. Intracellular calcium overload can contribute to acute tubular necrosis and intratubular precipitation of calcium, leading to tubular obstruction. Hypercalciuria predisposes to nephrolithiasis and obstructive uropathy. Chronic hypercalcemia and hypercalciuria, if untreated, cause progressive interstitial inflammation and deposition of calcium in the kidney parenchyma and tubules, resulting in nephrocalcinosis. In some cases, nephrocalcinosis leads to chronic kidney injury and renal dysfunction.
HISTOLOGIC FEATURES
4. What is the characteristic histologic feature of renal sarcoidosis?
- Membranous glomerulonephritis
- Mesangioproliferative glomerulonephritis
- Minimal change disease
- Granulomatous interstitial nephritis
- Immunoglobulin (Ig) A nephropathy
Granulomatous interstitial nephritis is the most typical histologic feature of renal sarcoidosis.4,20–22 However, interstitial nephritis without granulomas is found in up to one-third of patients with sarcoid interstitial nephritis.15,23
Patients with sarcoid granulomatous interstitial nephritis usually present with elevated serum creatinine with or without mild proteinuria (< 1 g/24 hours).1,15,16 Advanced renal failure (stage 4 or 5 chronic kidney disease) is relatively common at the time of presentation.1,15,16 In the 2 largest case series of renal sarcoidosis to date, the mean presenting serum creatinine levels were 3.0 and 4.8 mg/dL.11,15 The most common clinical syndrome associated with sarcoidosis and granulomatous interstitial nephritis is chronic kidney disease with a decline in renal function, which if untreated can occur over weeks to months.21 Acute renal failure as an initial presentation is also well documented.15,24
Even though glomerular involvement in sarcoidosis is rare, different kinds of glomerulonephritis have been reported, including membranous glomerulonephritis, mesangioproliferative glomerulonephritis, IgA nephropathy, minimal change disease, focal segmental sclerosis, and crescentic glomerulonephritis.25
DIAGNOSIS OF RENAL SARCOIDOSIS
5. How is renal sarcoidosis diagnosed?
- By exclusion
- Complete urine analysis and renal function assessment
- Renal biopsy
- Computed tomography
- Renal ultrasonography
The diagnosis of renal sarcoidosis is one of exclusion. Sarcoidosis must be considered in the differential diagnosis of renal failure of unknown origin, especially if disordered calcium homeostasis is also present. If clinically suspected, diagnosis usually requires pathohistologic demonstration of typical granulomatous lesions in the kidneys or in one or more organ systems.26
In cases of sarcoidosis with granulomatous interstitial nephritis with isolated renal failure as a presenting feature, other causes of granulomatous interstitial nephritis must be ruled out. A number of drug reactions are associated with interstitial nephritis, most commonly with antibiotics, NSAIDs, and diuretics. Although granulomatous interstitial nephritis may develop as a reaction to some drugs, most cases of drug-induced interstitial nephritis do not involve granulomatous interstitial nephritis.
Other causes of granulomatous interstitial infiltrates include granulomatous infection by mycobacteria, fungi, or Brucella; foreign-body reaction such as cholesterol atheroemboli; heroin; lymphoma; or autoimmune disease such as tubulointerstitial nephritis with uveitis syndrome, granulomatosis with polyangiitis, or Crohn disease.27,28 The absence of characteristic kidney biopsy findings does not exclude the diagnosis because renal sarcoidosis can be focal and easily missed on biopsy.29
Urinary manifestations of renal sarcoidosis are usually not specific. In renal sarcoidosis with interstitial nephritis with or without granulomas, proteinuria is mild or absent, usually less than 1.0 g/day.11,15,16 Urine studies may show a “bland” sediment (ie, without red or white blood cells) or may show sterile pyuria or microscopic hematuria. In glomerular disease, more overt proteinuria or the presence of red blood cell casts is more typical.
Hypercalciuria, nephrocalcinosis, and nephrolithiasis are nonspecific abnormalities that may be present in patients with sarcoidosis. In this regard, an elevated urine calcium level may support the diagnosis of renal sarcoidosis.
Computed tomography and renal ultrasonography may aid in diagnosis by detecting nephrocalcinosis or nephrolithiasis.
The serum ACE level is elevated in 55% to 60% of patients with sarcoidosis, but it may also be elevated in other granulomatous diseases or in chronic kidney disease from various causes.5 Therefore, considering its nonspecificity, the serum ACE level has a limited role in the diagnosis of sarcoidosis.30 Using the ACE level as a marker for disease activity and response to treatment remains controversial because levels do not correlate with disease activity.5,11
TREATMENT OF RENAL SARCOIDOSIS
6. Which is a first-line therapy for renal sarcoidosis?
- Corticosteroids
- Azathioprine
- Mycophenolate mofetil
- Infliximab
- Adalimumab
Treatment of impaired calcium homeostasis in sarcoidosis includes hydration; reducing intake of calcium, vitamin D, and oxalate; and limiting sun exposure.11,31 For more significant hypercalcemia (eg, serum calcium levels > 11 mg/dL) or nephrolithiasis, corticosteroid therapy is the first choice and should be implemented at the first sign of renal involvement. Corticosteroids inhibit the activity of 1-alpha-hydroxylase in macrophages, thereby reducing the production of 1,25-dihydroxyvitamin D.
Chloroquine and hydroxychloroquine have been mentioned in the literature as alternatives to corticosteroids.32 But the effect of these agents is less predictable and is slower than treatment with corticosteroids. Ketoconazole has no effect on granuloma formation but corrects hypercalcemia by inhibiting calcitriol production, and can be used as an adjunct for treating hypercalcemia and hypercalciuria.
Corticosteroids are the mainstay of treatment for renal sarcoidosis, including granulomatous interstitial nephritis and interstitial nephritis without granulomas. Most patients experience significant improvement in renal function. However, full recovery is rare, likely as a result of long-standing disease with some degree of already established irreversible renal injury.16
Corticosteroid dosage
There is no standard dosing protocol, but patients with impaired renal function due to biopsy-proven renal sarcoidosis should receive prednisone 0.5 to 1 mg/kg/day, depending on the severity of the disease, in a single dose every morning.
The optimal dosing and duration of maintenance therapy are unknown. Based on studies to date, the initial dosing should be maintained for 4 weeks, after which it can be tapered by 5 mg each week down to a maintenance dosage of 5 to 10 mg/day.4
Patients with a poor response after 4 weeks tend to have a worse renal outcome and are more susceptible to relapse.15 Fortunately, relapse often responds to increased corticosteroid doses.11,15 In the case of relapse, the dose should be increased to the lowest effective dose and continued for 4 weeks, then tapered more gradually.
A total of 24 months of treatment seems necessary to be effective and to prevent relapse.15 Some authors have proposed a lifelong maintenance dose for patients with frequent relapses, and some propose it for all patients.4
Other agents
Tumor necrosis factor (TNF)-blocking agents. Considering the critical role TNF plays in granuloma formation, anti-TNF-alpha agents are useful in steroid-resistant sarcoidosis.33 A thorough workup is necessary before starting these agents because of the increased risk of serious infection, including reactivation of latent tuberculosis. Of the current TNF-blocking agents, infliximab is most often used in renal sarcoidosis.34 Experience with adalimumab is more limited, though promising results indicate it could be an alternative for patients who do not tolerate infliximab.35
Azathioprine, mycophenolate mofetil, or methotrexate may also be used as a second-line agent if treatment with corticosteroids is not tolerated or does not control the disease. The evidence in support of these agents is limited. In small series, they have allowed sustainable control of renal function while reducing the steroid dose. Currently, these agents are used for patients resistant to corticosteroid therapy, who would otherwise need prolonged high-dose corticosteroid treatment, or who have corticosteroid intolerance; they allow a more effective steroid taper and maintenance of stable renal function.15,36
The data supporting a standardized treatment of renal sarcoidosis are limited. For steroid intolerance or resistance, cytotoxic drugs and selected anti-TNF-alpha agents, as mentioned above, have shown promise in improving or stabilizing serum creatinine levels. Further exploration is required as to which agent or combination is better at limiting the disease process with fewer adverse effects.
Our patient was initially treated with corticosteroids and was ultimately weaned to a maintenance dose of 5 mg/day. He was followed as an outpatient and was started on mycophenolate mofetil in place of higher steroid doses. His renal function stabilized, but he was lost to follow-up after 2 years.
KEY POINTS
- Sarcoidosis is a multisystem granulomatous disease that most commonly involves the lungs, skin, and reticuloendothelial system.
- Renal involvement in sarcoidosis is likely underestimated due to its often clinically silent nature and the possibility of missing typical granulomatous lesions in a small or less-than-optimal biopsy sample.
- Manifestations of renal sarcoidosis include disrupted calcium homeostasis, nephrocalcinosis, nephrolithiasis, and renal failure.
- Because the clinical and histopathologic manifestations of renal sarcoidosis are nonspecific, the diagnosis is one of exclusion. In patients with renal failure or with hypercalcemia or hypercalciuria of unknown cause, renal sarcoidosis should be included in the differential diagnosis. Patients with chronic sarcoidosis should also be screened for renal impairment.
- Granulomatous interstitial nephritis is the classic histologic finding of renal sarcoidosis. Nonetheless, up to one-third of patients have interstitial nephritis without granulomas.
- Corticosteroids are the mainstay of treatment for renal sarcoidosis. An initial dose of oral prednisone 0.5 to 1 mg/kg/day should be maintained for 4 weeks and then gradually tapered to 5 to 10 mg/day for a total of 24 months. Some patients require lifelong therapy.
- Several immunosuppressive and cytotoxic agents may be used in cases of corticosteroid intolerance or to aid in effective taper of corticosteroids.
- Joss N, Morris S, Young B, Geddes C. Granulomatous interstitial nephritis. Clin J Am Soc Nephrol 2007; 2:222–230.
- Lutalo PM, D'Cruz DP. Diagnosis and classification of granulomatosis with polyangiitis (aka Wegener's granulomatosis). J Autoimmun 2014; 48–49:94–98.
- Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med 1997; 336:1224–1234.
- Rajakariar R, Sharples EJ, Raftery MJ, Sheaff M, Yaqoob MM. Sarcoid tubulo-interstitial nephritis: long-term outcome and response to corticosteroid therapy. Kidney Int 2006; 70:165–169.
- Baughman RP, Teirstein AS, Judson MA, et al; Case Control Etiologic Study of Sarcoidosis (ACCESS) research group. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med 2001; 164:1885–1889.
- Rizzato G, Palmieri G, Agrati AM, Zanussi C. The organ-specific extrapulmonary presentation of sarcoidosis: a frequent occurrence but a challenge to an early diagnosis. A 3-year-long prospective observational study. Sarcoidosis Vasc Diffuse Lung Dis 2004; 21:119–126.
- Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med 2007; 357:2153–2165.
- Baughman RP, Field S, Costabel U, et al. Sarcoidosis in America. Analysis based on health care use. Ann Am Thorac Soc 2016; 13:1244–1252.
- Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med 1983; 52:525–533.
- Mayock RL, Bertrand P, Morrison CE, Scott JH. Manifestations of sarcoidosis. Analysis of 145 patients, with a review of nine series selected from the literature. Am J Med 1963; 35:67–89.
- Berliner AR, Haas M, Choi MJ. Sarcoidosis: the nephrologist's perspective. Am J Kidney Dis 2006; 48:856–870.
- Longcope WT, Freiman DG. A study of sarcoidosis; based on a combined investigation of 160 cases including 30 autopsies from The Johns Hopkins Hospital and Massachusetts General Hospital. Medicine (Baltimore) 1952; 31:1–132.
- Branson JH, Park JH. Sarcoidosis hepatic involvement: presentation of a case with fatal liver involvement; including autopsy findings and review of the evidence for sarcoid involvement of the liver as found in the literature. Ann Intern Med 1954; 40:111–145.
- Muther RS, McCarron DA, Bennett WM. Renal manifestations of sarcoidosis. Arch Intern Med 1981; 141:643–645.
- Mahevas M, Lescure FX, Boffa JJ, et al. Renal sarcoidosis: clinical, laboratory, and histologic presentation and outcome in 47 patients. Medicine (Baltimore) 2009; 88:98–106.
- Robson MG, Banerjee D, Hopster D, Cairns HS. Seven cases of granulomatous interstitial nephritis in the absence of extrarenal sarcoid. Nephrol Dial Transplant 2003; 18:280–284.
- Casella FJ, Allon M. The kidney in sarcoidosis. J Am Soc Nephrol 1993; 3:1555–1562.
- Rafat C, Bobrie G, Chedid A, Nochy D, Hernigou A, Plouin PF. Sarcoidosis presenting as severe renin-dependent hypertension due to kidney vascular injury. Clin Kidney J 2014; 7:383–386.
- Reichel H, Koeffler HP, Barbers R, Norman AW. Regulation of 1,25-dihydroxyvitamin D3 production by cultured alveolar macrophages from normal human donors and from patients with pulmonary sarcoidosis. J Clin Endocrinol Metab 1987; 65:1201–1209.
- Brause M, Magnusson K, Degenhardt S, Helmchen U, Grabensee B. Renal involvement in sarcoidosis—a report of 6 cases. Clin Nephrol 2002; 57:142–148.
- Hannedouche T, Grateau G, Noel LH, et al. Renal granulomatous sarcoidosis: report of six cases. Nephrol Dial Transplant 1990; 5:18–24.
- Kettritz R, Goebel U, Fiebeler A, Schneider W, Luft F. The protean face of sarcoidosis revisited. Nephrol Dial Transplant 2006; 21:2690–2694.
- Bergner R, Hoffmann M, Waldherr R, Uppenkamp M. Frequency of kidney disease in chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2003; 20:126–132.
- O’Riordan E, Willert RP, Reeve R, et al. Isolated sarcoid granulomatous interstitial nephritis: review of five cases at one center. Clin Nephrol 2001; 55:297–302.
- Gobel U, Kettritz R, Schneider W, Luft F. The protean face of renal sarcoidosis. J Am Soc Nephrol 2001; 12:616–623.
- Statement on sarcoidosis. Joint statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999; 160:736–755.
- Bijol V, Mendez GP, Nose V, Rennke HG. Granulomatous interstitial nephritis: a clinicopathologic study of 46 cases from a single institution. Int J Surg Pathol 2006; 14:57–63.
- Mignon F, Mery JP, Mougenot B, Ronco P, Roland J, Morel-Maroger L. Granulomatous interstitial nephritis. Adv Nephrol Necker Hosp 1984; 13:219–245.
- Shah R, Shidham G, Agarwal A, Albawardi A, Nadasdy T. Diagnostic utility of kidney biopsy in patients with sarcoidosis and acute kidney injury. Int J Nephrol Renovasc Dis 2011; 4:131–136.
- Studdy PR, Bird R. Serum angiotensin converting enzyme in sarcoidosis—its value in present clinical practice. Ann Clin Biochem 1989; 26:13–18.
- Demetriou ET, Pietras SM, Holick MF. Hypercalcemia and soft tissue calcification owing to sarcoidosis: the sunlight-cola connection. J Bone Miner Res 2010; 25:1695–1699.
- Beegle SH, Barba K, Gobunsuy R, Judson MA. Current and emerging pharmacological treatments for sarcoidosis: a review. Drug Des Devel Ther 2013; 7:325–338.
- Roberts SD, Wilkes DS, Burgett RA, Knox KS. Refractory sarcoidosis responding to infliximab. Chest 2003; 124:2028–2031.
- Ahmed MM, Mubashir E, Dossabhoy NR. Isolated renal sarcoidosis: a rare presentation of a rare disease treated with infliximab. Clin Rheumatol 2007; 26:1346–1349.
- Gupta R, Beaudet L, Moore J, Mehta T. Treatment of sarcoid granulomatous interstitial nephritis with adalimumab. NDT Plus 2009; 2:139–142.
- Moudgil A, Przygodzki RM, Kher KK. Successful steroid-sparing treatment of renal limited sarcoidosis with mycophenolate mofetil. Pediatr Nephrol 2006; 21:281–285.
A black 37-year-old man has gradually lost 100 lb (45 kg) over the past 2 years, and reports progressive fatigue and malaise as well. He has not noted swollen lymph nodes, fever, or night sweats. He denies dyspnea, cough, or chest pain. He has no skin rashes, and no dry or red eyes or visual changes. He reports no flank pain, dysuria, frank hematuria, foamy urine, decline in urine output, or difficulty voiding.
He has no history of significant medical conditions. He does not drink, smoke, or use recreational drugs. He is not taking any prescription medications and has not been using nonsteroidal anti-inflammatory drugs (NSAIDs) or combination analgesics. He does not have a family history of kidney disease.
Physical examination. He appears relaxed and comfortable. He does not have nasal polyps or signs of pharyngeal inflammation. He has no apparent lymphadenopathy. His breath sounds are normal without rales or wheezes. Cardiac examination reveals a regular rhythm, with no rub or murmurs. The abdomen is soft and nontender with no flank pain or groin tenderness. The skin is intact with no rash or nodules.
- Temperature 98.4ºF (36.9ºC)
- Blood pressure 125/70 mm Hg
- Heart rate 102 beats per minute
- Respiratory rate 19 per minute
- Oxygen saturation 99% while breathing room air
- Weight 194 lb (88 kg)
- Body mass index 28 kg/m2.
Laboratory testing (Table 1) reveals severe renal insufficiency with anemia:
- Serum creatinine 9 mg/dL (reference range 0.5–1.2)
- Estimated glomerular filtration rate (eGFR) 8 mL/min/1.73m2 (using the Modification of Diet in Renal Disease Study equation).
His serum calcium level is normal, but his serum phosphorus is 5.3 mg/dL (reference range 2.5–4.6), and his parathyroid hormone level is 317 pg/mL (12–88), consistent with hyperparathyroidism secondary to chronic kidney disease. His 25-hydroxyvitamin D level is less than 13 ng/mL (30–80), and angiotensin-converting enzyme (ACE) is 129 U/L (9–67 U/L). His urinary calcium level is less than 3.0 mg/dL.
Urinalysis:
- Urine protein 100 mg/dL (0–20)
- No urine crystals
- 3 to 5 coarse granular urine casts per high-power field
- No hematuria or pyuria.
Renal ultrasonography shows normal kidneys with no hydronephrosis.
Renal biopsy study demonstrates noncaseating granulomatous interstitial nephritis (Figure 1).
GRANULOMATOUS INTERSTITIAL NEPHRITIS
1. Based on the information above, what is the most likely cause of this patient’s kidney disease?
- Medication
- Granulomatosis with polyangiitis
- Sarcoidosis
- Infection
Granulomatous interstitial nephritis is a histologic diagnosis that is present in up to 1% of renal biopsies. It has been associated with medications, infections, sarcoidosis, crystal deposits, paraproteinemia, and granulomatosis with polyangiitis and also is seen in an idiopathic form.
Medicines implicated include anticonvulsants, antibiotics, NSAIDs, allopurinol, and diuretics.
Mycobacteria and fungi are the main infective causes and seem to be the main causative factor in cases of renal transplant.1 Granulomas are usually not found on kidney biopsy in granulomatosis with polyangiitis, and that diagnosis is usually made by the presence of antiproteinase 3 antibodies.2
Sarcoidosis is the most likely diagnosis in this patient after excluding implicated medications, infection, and vasculitis and confirming the presence of granulomatous interstitial nephritis on renal biopsy.
SARCOIDOSIS: A MULTISYSTEM DISEASE
Sarcoidosis is a multisystem inflammatory disease of unknown cause, characterized by noncaseating epithelioid granulomas. It can involve any organ but most commonly the thoracic and peripheral lymph nodes.3,4 Involvement of the eyes and skin is also relatively common.
Extrapulmonary involvement occurs in more than 30% of cases of sarcoidosis, almost always with concomitant thoracic involvement.5,6 Isolated extrathoracic sarcoidosis is unusual, found in only 2% of patients in a sarcoidosis case-control study.5
Current theory suggests that sarcoidosis develops from a cell-mediated immune response triggered by one or more unidentified antigens in people with a genetic predisposition.7
Sarcoidosis affects men and women of all ages, most often adults ages 20 to 40; but more recently, it has increased in US adults over age 55.8 The condition is more prevalent in Northern Europe and Japan, and in blacks in the United States.7
HOW COMMON IS RENAL INVOLVEMENT IN SARCOIDOSIS?
2. What is the likelihood of finding clinically apparent renal involvement in a patient with sarcoidosis?
- Greater than 70%
- Greater than 50%
- Up to 50%
- Less than 10%
The prevalence of renal involvement in sarcoidosis is hard to determine due to differences in study design and patient populations included in the available reports, and because renal involvement may be silent for many years. Recent studies have reported impaired renal function in 0.7% to 9.7% of cases: eg, a case-control study of 736 patients reported clinically apparent renal involvement in 0.7% of patients,5 and in a series of 818 patients, the incidence was 1%.9 In earlier studies, depending on the diagnostic criteria, the incidence ranged from 1.1% to 9.7%.10
The prevalence of renal involvement may also be underestimated because it can be asymptomatic, and the number of granulomas may be so few that they are absent in a small biopsy specimen. A higher prevalence of renal involvement in sarcoidosis is reported from autopsy studies, although many cases remained clinically silent. These studies have reported renal noncaseating granulomas in 7% to 23% of sarcoidosis patients.11–13
PRESENTATION OF RENAL SARCOIDOSIS
3. What is the most common presentation in isolated renal sarcoidosis?
- Sterile pyuria
- Elevated urine eosinophils
- Renal insufficiency
- Painless hematuria
Renal manifestations of sarcoidosis include hypercalcemia, hypercalciuria, nephrocalcinosis, nephrolithiasis, and impaired renal function.14 Renal involvement can occur during the course of existing sarcoidosis, at the time of first presentation, or even as the sole presentation of the disease.1,11,15 In patients with isolated renal sarcoidosis, the most common presentation is renal insufficiency.15,16
Two main pathways for nephron insult that have been validated are granulomatous infiltration of the renal interstitium and disordered calcium homeostasis.11,17 Though extremely rare, various types of glomerular disease, renal tubular defects, and renal vascular involvement such as renal artery granulomatous angiitis have been documented.18
Hypercalcemia in sarcoidosis
Sarcoidosis is known to cause hypercalcemia by increasing calcium absorption secondary to 1,25-dihydroxyvitamin D production from granulomas. Our patient’s case is unusual, as renal failure was the sole extrapulmonary manifestation of sarcoidosis without hypercalcemia.
In sarcoidosis, extrarenal production of 1-alpha-hydroxylase by activated macrophages inappropriately increases levels of 1,25-dihydroxyvitamin D (calcitriol). Subsequently, serum calcium levels are increased. Unlike its renal equivalent, granulomatous 1-alpha-hydroxylase evades the normal negative feedback of hypercalcemia, so that increased calcitriol levels are sustained, leading to hypercalcemia, often accompanied by hypercalciuria.19
Disruption of calcium homeostasis affects renal function through several mechanisms. Hypercalcemia promotes vasoconstriction of the afferent arteriole, leading to a reduction in the GFR. Intracellular calcium overload can contribute to acute tubular necrosis and intratubular precipitation of calcium, leading to tubular obstruction. Hypercalciuria predisposes to nephrolithiasis and obstructive uropathy. Chronic hypercalcemia and hypercalciuria, if untreated, cause progressive interstitial inflammation and deposition of calcium in the kidney parenchyma and tubules, resulting in nephrocalcinosis. In some cases, nephrocalcinosis leads to chronic kidney injury and renal dysfunction.
HISTOLOGIC FEATURES
4. What is the characteristic histologic feature of renal sarcoidosis?
- Membranous glomerulonephritis
- Mesangioproliferative glomerulonephritis
- Minimal change disease
- Granulomatous interstitial nephritis
- Immunoglobulin (Ig) A nephropathy
Granulomatous interstitial nephritis is the most typical histologic feature of renal sarcoidosis.4,20–22 However, interstitial nephritis without granulomas is found in up to one-third of patients with sarcoid interstitial nephritis.15,23
Patients with sarcoid granulomatous interstitial nephritis usually present with elevated serum creatinine with or without mild proteinuria (< 1 g/24 hours).1,15,16 Advanced renal failure (stage 4 or 5 chronic kidney disease) is relatively common at the time of presentation.1,15,16 In the 2 largest case series of renal sarcoidosis to date, the mean presenting serum creatinine levels were 3.0 and 4.8 mg/dL.11,15 The most common clinical syndrome associated with sarcoidosis and granulomatous interstitial nephritis is chronic kidney disease with a decline in renal function, which if untreated can occur over weeks to months.21 Acute renal failure as an initial presentation is also well documented.15,24
Even though glomerular involvement in sarcoidosis is rare, different kinds of glomerulonephritis have been reported, including membranous glomerulonephritis, mesangioproliferative glomerulonephritis, IgA nephropathy, minimal change disease, focal segmental sclerosis, and crescentic glomerulonephritis.25
DIAGNOSIS OF RENAL SARCOIDOSIS
5. How is renal sarcoidosis diagnosed?
- By exclusion
- Complete urine analysis and renal function assessment
- Renal biopsy
- Computed tomography
- Renal ultrasonography
The diagnosis of renal sarcoidosis is one of exclusion. Sarcoidosis must be considered in the differential diagnosis of renal failure of unknown origin, especially if disordered calcium homeostasis is also present. If clinically suspected, diagnosis usually requires pathohistologic demonstration of typical granulomatous lesions in the kidneys or in one or more organ systems.26
In cases of sarcoidosis with granulomatous interstitial nephritis with isolated renal failure as a presenting feature, other causes of granulomatous interstitial nephritis must be ruled out. A number of drug reactions are associated with interstitial nephritis, most commonly with antibiotics, NSAIDs, and diuretics. Although granulomatous interstitial nephritis may develop as a reaction to some drugs, most cases of drug-induced interstitial nephritis do not involve granulomatous interstitial nephritis.
Other causes of granulomatous interstitial infiltrates include granulomatous infection by mycobacteria, fungi, or Brucella; foreign-body reaction such as cholesterol atheroemboli; heroin; lymphoma; or autoimmune disease such as tubulointerstitial nephritis with uveitis syndrome, granulomatosis with polyangiitis, or Crohn disease.27,28 The absence of characteristic kidney biopsy findings does not exclude the diagnosis because renal sarcoidosis can be focal and easily missed on biopsy.29
Urinary manifestations of renal sarcoidosis are usually not specific. In renal sarcoidosis with interstitial nephritis with or without granulomas, proteinuria is mild or absent, usually less than 1.0 g/day.11,15,16 Urine studies may show a “bland” sediment (ie, without red or white blood cells) or may show sterile pyuria or microscopic hematuria. In glomerular disease, more overt proteinuria or the presence of red blood cell casts is more typical.
Hypercalciuria, nephrocalcinosis, and nephrolithiasis are nonspecific abnormalities that may be present in patients with sarcoidosis. In this regard, an elevated urine calcium level may support the diagnosis of renal sarcoidosis.
Computed tomography and renal ultrasonography may aid in diagnosis by detecting nephrocalcinosis or nephrolithiasis.
The serum ACE level is elevated in 55% to 60% of patients with sarcoidosis, but it may also be elevated in other granulomatous diseases or in chronic kidney disease from various causes.5 Therefore, considering its nonspecificity, the serum ACE level has a limited role in the diagnosis of sarcoidosis.30 Using the ACE level as a marker for disease activity and response to treatment remains controversial because levels do not correlate with disease activity.5,11
TREATMENT OF RENAL SARCOIDOSIS
6. Which is a first-line therapy for renal sarcoidosis?
- Corticosteroids
- Azathioprine
- Mycophenolate mofetil
- Infliximab
- Adalimumab
Treatment of impaired calcium homeostasis in sarcoidosis includes hydration; reducing intake of calcium, vitamin D, and oxalate; and limiting sun exposure.11,31 For more significant hypercalcemia (eg, serum calcium levels > 11 mg/dL) or nephrolithiasis, corticosteroid therapy is the first choice and should be implemented at the first sign of renal involvement. Corticosteroids inhibit the activity of 1-alpha-hydroxylase in macrophages, thereby reducing the production of 1,25-dihydroxyvitamin D.
Chloroquine and hydroxychloroquine have been mentioned in the literature as alternatives to corticosteroids.32 But the effect of these agents is less predictable and is slower than treatment with corticosteroids. Ketoconazole has no effect on granuloma formation but corrects hypercalcemia by inhibiting calcitriol production, and can be used as an adjunct for treating hypercalcemia and hypercalciuria.
Corticosteroids are the mainstay of treatment for renal sarcoidosis, including granulomatous interstitial nephritis and interstitial nephritis without granulomas. Most patients experience significant improvement in renal function. However, full recovery is rare, likely as a result of long-standing disease with some degree of already established irreversible renal injury.16
Corticosteroid dosage
There is no standard dosing protocol, but patients with impaired renal function due to biopsy-proven renal sarcoidosis should receive prednisone 0.5 to 1 mg/kg/day, depending on the severity of the disease, in a single dose every morning.
The optimal dosing and duration of maintenance therapy are unknown. Based on studies to date, the initial dosing should be maintained for 4 weeks, after which it can be tapered by 5 mg each week down to a maintenance dosage of 5 to 10 mg/day.4
Patients with a poor response after 4 weeks tend to have a worse renal outcome and are more susceptible to relapse.15 Fortunately, relapse often responds to increased corticosteroid doses.11,15 In the case of relapse, the dose should be increased to the lowest effective dose and continued for 4 weeks, then tapered more gradually.
A total of 24 months of treatment seems necessary to be effective and to prevent relapse.15 Some authors have proposed a lifelong maintenance dose for patients with frequent relapses, and some propose it for all patients.4
Other agents
Tumor necrosis factor (TNF)-blocking agents. Considering the critical role TNF plays in granuloma formation, anti-TNF-alpha agents are useful in steroid-resistant sarcoidosis.33 A thorough workup is necessary before starting these agents because of the increased risk of serious infection, including reactivation of latent tuberculosis. Of the current TNF-blocking agents, infliximab is most often used in renal sarcoidosis.34 Experience with adalimumab is more limited, though promising results indicate it could be an alternative for patients who do not tolerate infliximab.35
Azathioprine, mycophenolate mofetil, or methotrexate may also be used as a second-line agent if treatment with corticosteroids is not tolerated or does not control the disease. The evidence in support of these agents is limited. In small series, they have allowed sustainable control of renal function while reducing the steroid dose. Currently, these agents are used for patients resistant to corticosteroid therapy, who would otherwise need prolonged high-dose corticosteroid treatment, or who have corticosteroid intolerance; they allow a more effective steroid taper and maintenance of stable renal function.15,36
The data supporting a standardized treatment of renal sarcoidosis are limited. For steroid intolerance or resistance, cytotoxic drugs and selected anti-TNF-alpha agents, as mentioned above, have shown promise in improving or stabilizing serum creatinine levels. Further exploration is required as to which agent or combination is better at limiting the disease process with fewer adverse effects.
Our patient was initially treated with corticosteroids and was ultimately weaned to a maintenance dose of 5 mg/day. He was followed as an outpatient and was started on mycophenolate mofetil in place of higher steroid doses. His renal function stabilized, but he was lost to follow-up after 2 years.
KEY POINTS
- Sarcoidosis is a multisystem granulomatous disease that most commonly involves the lungs, skin, and reticuloendothelial system.
- Renal involvement in sarcoidosis is likely underestimated due to its often clinically silent nature and the possibility of missing typical granulomatous lesions in a small or less-than-optimal biopsy sample.
- Manifestations of renal sarcoidosis include disrupted calcium homeostasis, nephrocalcinosis, nephrolithiasis, and renal failure.
- Because the clinical and histopathologic manifestations of renal sarcoidosis are nonspecific, the diagnosis is one of exclusion. In patients with renal failure or with hypercalcemia or hypercalciuria of unknown cause, renal sarcoidosis should be included in the differential diagnosis. Patients with chronic sarcoidosis should also be screened for renal impairment.
- Granulomatous interstitial nephritis is the classic histologic finding of renal sarcoidosis. Nonetheless, up to one-third of patients have interstitial nephritis without granulomas.
- Corticosteroids are the mainstay of treatment for renal sarcoidosis. An initial dose of oral prednisone 0.5 to 1 mg/kg/day should be maintained for 4 weeks and then gradually tapered to 5 to 10 mg/day for a total of 24 months. Some patients require lifelong therapy.
- Several immunosuppressive and cytotoxic agents may be used in cases of corticosteroid intolerance or to aid in effective taper of corticosteroids.
A black 37-year-old man has gradually lost 100 lb (45 kg) over the past 2 years, and reports progressive fatigue and malaise as well. He has not noted swollen lymph nodes, fever, or night sweats. He denies dyspnea, cough, or chest pain. He has no skin rashes, and no dry or red eyes or visual changes. He reports no flank pain, dysuria, frank hematuria, foamy urine, decline in urine output, or difficulty voiding.
He has no history of significant medical conditions. He does not drink, smoke, or use recreational drugs. He is not taking any prescription medications and has not been using nonsteroidal anti-inflammatory drugs (NSAIDs) or combination analgesics. He does not have a family history of kidney disease.
Physical examination. He appears relaxed and comfortable. He does not have nasal polyps or signs of pharyngeal inflammation. He has no apparent lymphadenopathy. His breath sounds are normal without rales or wheezes. Cardiac examination reveals a regular rhythm, with no rub or murmurs. The abdomen is soft and nontender with no flank pain or groin tenderness. The skin is intact with no rash or nodules.
- Temperature 98.4ºF (36.9ºC)
- Blood pressure 125/70 mm Hg
- Heart rate 102 beats per minute
- Respiratory rate 19 per minute
- Oxygen saturation 99% while breathing room air
- Weight 194 lb (88 kg)
- Body mass index 28 kg/m2.
Laboratory testing (Table 1) reveals severe renal insufficiency with anemia:
- Serum creatinine 9 mg/dL (reference range 0.5–1.2)
- Estimated glomerular filtration rate (eGFR) 8 mL/min/1.73m2 (using the Modification of Diet in Renal Disease Study equation).
His serum calcium level is normal, but his serum phosphorus is 5.3 mg/dL (reference range 2.5–4.6), and his parathyroid hormone level is 317 pg/mL (12–88), consistent with hyperparathyroidism secondary to chronic kidney disease. His 25-hydroxyvitamin D level is less than 13 ng/mL (30–80), and angiotensin-converting enzyme (ACE) is 129 U/L (9–67 U/L). His urinary calcium level is less than 3.0 mg/dL.
Urinalysis:
- Urine protein 100 mg/dL (0–20)
- No urine crystals
- 3 to 5 coarse granular urine casts per high-power field
- No hematuria or pyuria.
Renal ultrasonography shows normal kidneys with no hydronephrosis.
Renal biopsy study demonstrates noncaseating granulomatous interstitial nephritis (Figure 1).
GRANULOMATOUS INTERSTITIAL NEPHRITIS
1. Based on the information above, what is the most likely cause of this patient’s kidney disease?
- Medication
- Granulomatosis with polyangiitis
- Sarcoidosis
- Infection
Granulomatous interstitial nephritis is a histologic diagnosis that is present in up to 1% of renal biopsies. It has been associated with medications, infections, sarcoidosis, crystal deposits, paraproteinemia, and granulomatosis with polyangiitis and also is seen in an idiopathic form.
Medicines implicated include anticonvulsants, antibiotics, NSAIDs, allopurinol, and diuretics.
Mycobacteria and fungi are the main infective causes and seem to be the main causative factor in cases of renal transplant.1 Granulomas are usually not found on kidney biopsy in granulomatosis with polyangiitis, and that diagnosis is usually made by the presence of antiproteinase 3 antibodies.2
Sarcoidosis is the most likely diagnosis in this patient after excluding implicated medications, infection, and vasculitis and confirming the presence of granulomatous interstitial nephritis on renal biopsy.
SARCOIDOSIS: A MULTISYSTEM DISEASE
Sarcoidosis is a multisystem inflammatory disease of unknown cause, characterized by noncaseating epithelioid granulomas. It can involve any organ but most commonly the thoracic and peripheral lymph nodes.3,4 Involvement of the eyes and skin is also relatively common.
Extrapulmonary involvement occurs in more than 30% of cases of sarcoidosis, almost always with concomitant thoracic involvement.5,6 Isolated extrathoracic sarcoidosis is unusual, found in only 2% of patients in a sarcoidosis case-control study.5
Current theory suggests that sarcoidosis develops from a cell-mediated immune response triggered by one or more unidentified antigens in people with a genetic predisposition.7
Sarcoidosis affects men and women of all ages, most often adults ages 20 to 40; but more recently, it has increased in US adults over age 55.8 The condition is more prevalent in Northern Europe and Japan, and in blacks in the United States.7
HOW COMMON IS RENAL INVOLVEMENT IN SARCOIDOSIS?
2. What is the likelihood of finding clinically apparent renal involvement in a patient with sarcoidosis?
- Greater than 70%
- Greater than 50%
- Up to 50%
- Less than 10%
The prevalence of renal involvement in sarcoidosis is hard to determine due to differences in study design and patient populations included in the available reports, and because renal involvement may be silent for many years. Recent studies have reported impaired renal function in 0.7% to 9.7% of cases: eg, a case-control study of 736 patients reported clinically apparent renal involvement in 0.7% of patients,5 and in a series of 818 patients, the incidence was 1%.9 In earlier studies, depending on the diagnostic criteria, the incidence ranged from 1.1% to 9.7%.10
The prevalence of renal involvement may also be underestimated because it can be asymptomatic, and the number of granulomas may be so few that they are absent in a small biopsy specimen. A higher prevalence of renal involvement in sarcoidosis is reported from autopsy studies, although many cases remained clinically silent. These studies have reported renal noncaseating granulomas in 7% to 23% of sarcoidosis patients.11–13
PRESENTATION OF RENAL SARCOIDOSIS
3. What is the most common presentation in isolated renal sarcoidosis?
- Sterile pyuria
- Elevated urine eosinophils
- Renal insufficiency
- Painless hematuria
Renal manifestations of sarcoidosis include hypercalcemia, hypercalciuria, nephrocalcinosis, nephrolithiasis, and impaired renal function.14 Renal involvement can occur during the course of existing sarcoidosis, at the time of first presentation, or even as the sole presentation of the disease.1,11,15 In patients with isolated renal sarcoidosis, the most common presentation is renal insufficiency.15,16
Two main pathways for nephron insult that have been validated are granulomatous infiltration of the renal interstitium and disordered calcium homeostasis.11,17 Though extremely rare, various types of glomerular disease, renal tubular defects, and renal vascular involvement such as renal artery granulomatous angiitis have been documented.18
Hypercalcemia in sarcoidosis
Sarcoidosis is known to cause hypercalcemia by increasing calcium absorption secondary to 1,25-dihydroxyvitamin D production from granulomas. Our patient’s case is unusual, as renal failure was the sole extrapulmonary manifestation of sarcoidosis without hypercalcemia.
In sarcoidosis, extrarenal production of 1-alpha-hydroxylase by activated macrophages inappropriately increases levels of 1,25-dihydroxyvitamin D (calcitriol). Subsequently, serum calcium levels are increased. Unlike its renal equivalent, granulomatous 1-alpha-hydroxylase evades the normal negative feedback of hypercalcemia, so that increased calcitriol levels are sustained, leading to hypercalcemia, often accompanied by hypercalciuria.19
Disruption of calcium homeostasis affects renal function through several mechanisms. Hypercalcemia promotes vasoconstriction of the afferent arteriole, leading to a reduction in the GFR. Intracellular calcium overload can contribute to acute tubular necrosis and intratubular precipitation of calcium, leading to tubular obstruction. Hypercalciuria predisposes to nephrolithiasis and obstructive uropathy. Chronic hypercalcemia and hypercalciuria, if untreated, cause progressive interstitial inflammation and deposition of calcium in the kidney parenchyma and tubules, resulting in nephrocalcinosis. In some cases, nephrocalcinosis leads to chronic kidney injury and renal dysfunction.
HISTOLOGIC FEATURES
4. What is the characteristic histologic feature of renal sarcoidosis?
- Membranous glomerulonephritis
- Mesangioproliferative glomerulonephritis
- Minimal change disease
- Granulomatous interstitial nephritis
- Immunoglobulin (Ig) A nephropathy
Granulomatous interstitial nephritis is the most typical histologic feature of renal sarcoidosis.4,20–22 However, interstitial nephritis without granulomas is found in up to one-third of patients with sarcoid interstitial nephritis.15,23
Patients with sarcoid granulomatous interstitial nephritis usually present with elevated serum creatinine with or without mild proteinuria (< 1 g/24 hours).1,15,16 Advanced renal failure (stage 4 or 5 chronic kidney disease) is relatively common at the time of presentation.1,15,16 In the 2 largest case series of renal sarcoidosis to date, the mean presenting serum creatinine levels were 3.0 and 4.8 mg/dL.11,15 The most common clinical syndrome associated with sarcoidosis and granulomatous interstitial nephritis is chronic kidney disease with a decline in renal function, which if untreated can occur over weeks to months.21 Acute renal failure as an initial presentation is also well documented.15,24
Even though glomerular involvement in sarcoidosis is rare, different kinds of glomerulonephritis have been reported, including membranous glomerulonephritis, mesangioproliferative glomerulonephritis, IgA nephropathy, minimal change disease, focal segmental sclerosis, and crescentic glomerulonephritis.25
DIAGNOSIS OF RENAL SARCOIDOSIS
5. How is renal sarcoidosis diagnosed?
- By exclusion
- Complete urine analysis and renal function assessment
- Renal biopsy
- Computed tomography
- Renal ultrasonography
The diagnosis of renal sarcoidosis is one of exclusion. Sarcoidosis must be considered in the differential diagnosis of renal failure of unknown origin, especially if disordered calcium homeostasis is also present. If clinically suspected, diagnosis usually requires pathohistologic demonstration of typical granulomatous lesions in the kidneys or in one or more organ systems.26
In cases of sarcoidosis with granulomatous interstitial nephritis with isolated renal failure as a presenting feature, other causes of granulomatous interstitial nephritis must be ruled out. A number of drug reactions are associated with interstitial nephritis, most commonly with antibiotics, NSAIDs, and diuretics. Although granulomatous interstitial nephritis may develop as a reaction to some drugs, most cases of drug-induced interstitial nephritis do not involve granulomatous interstitial nephritis.
Other causes of granulomatous interstitial infiltrates include granulomatous infection by mycobacteria, fungi, or Brucella; foreign-body reaction such as cholesterol atheroemboli; heroin; lymphoma; or autoimmune disease such as tubulointerstitial nephritis with uveitis syndrome, granulomatosis with polyangiitis, or Crohn disease.27,28 The absence of characteristic kidney biopsy findings does not exclude the diagnosis because renal sarcoidosis can be focal and easily missed on biopsy.29
Urinary manifestations of renal sarcoidosis are usually not specific. In renal sarcoidosis with interstitial nephritis with or without granulomas, proteinuria is mild or absent, usually less than 1.0 g/day.11,15,16 Urine studies may show a “bland” sediment (ie, without red or white blood cells) or may show sterile pyuria or microscopic hematuria. In glomerular disease, more overt proteinuria or the presence of red blood cell casts is more typical.
Hypercalciuria, nephrocalcinosis, and nephrolithiasis are nonspecific abnormalities that may be present in patients with sarcoidosis. In this regard, an elevated urine calcium level may support the diagnosis of renal sarcoidosis.
Computed tomography and renal ultrasonography may aid in diagnosis by detecting nephrocalcinosis or nephrolithiasis.
The serum ACE level is elevated in 55% to 60% of patients with sarcoidosis, but it may also be elevated in other granulomatous diseases or in chronic kidney disease from various causes.5 Therefore, considering its nonspecificity, the serum ACE level has a limited role in the diagnosis of sarcoidosis.30 Using the ACE level as a marker for disease activity and response to treatment remains controversial because levels do not correlate with disease activity.5,11
TREATMENT OF RENAL SARCOIDOSIS
6. Which is a first-line therapy for renal sarcoidosis?
- Corticosteroids
- Azathioprine
- Mycophenolate mofetil
- Infliximab
- Adalimumab
Treatment of impaired calcium homeostasis in sarcoidosis includes hydration; reducing intake of calcium, vitamin D, and oxalate; and limiting sun exposure.11,31 For more significant hypercalcemia (eg, serum calcium levels > 11 mg/dL) or nephrolithiasis, corticosteroid therapy is the first choice and should be implemented at the first sign of renal involvement. Corticosteroids inhibit the activity of 1-alpha-hydroxylase in macrophages, thereby reducing the production of 1,25-dihydroxyvitamin D.
Chloroquine and hydroxychloroquine have been mentioned in the literature as alternatives to corticosteroids.32 But the effect of these agents is less predictable and is slower than treatment with corticosteroids. Ketoconazole has no effect on granuloma formation but corrects hypercalcemia by inhibiting calcitriol production, and can be used as an adjunct for treating hypercalcemia and hypercalciuria.
Corticosteroids are the mainstay of treatment for renal sarcoidosis, including granulomatous interstitial nephritis and interstitial nephritis without granulomas. Most patients experience significant improvement in renal function. However, full recovery is rare, likely as a result of long-standing disease with some degree of already established irreversible renal injury.16
Corticosteroid dosage
There is no standard dosing protocol, but patients with impaired renal function due to biopsy-proven renal sarcoidosis should receive prednisone 0.5 to 1 mg/kg/day, depending on the severity of the disease, in a single dose every morning.
The optimal dosing and duration of maintenance therapy are unknown. Based on studies to date, the initial dosing should be maintained for 4 weeks, after which it can be tapered by 5 mg each week down to a maintenance dosage of 5 to 10 mg/day.4
Patients with a poor response after 4 weeks tend to have a worse renal outcome and are more susceptible to relapse.15 Fortunately, relapse often responds to increased corticosteroid doses.11,15 In the case of relapse, the dose should be increased to the lowest effective dose and continued for 4 weeks, then tapered more gradually.
A total of 24 months of treatment seems necessary to be effective and to prevent relapse.15 Some authors have proposed a lifelong maintenance dose for patients with frequent relapses, and some propose it for all patients.4
Other agents
Tumor necrosis factor (TNF)-blocking agents. Considering the critical role TNF plays in granuloma formation, anti-TNF-alpha agents are useful in steroid-resistant sarcoidosis.33 A thorough workup is necessary before starting these agents because of the increased risk of serious infection, including reactivation of latent tuberculosis. Of the current TNF-blocking agents, infliximab is most often used in renal sarcoidosis.34 Experience with adalimumab is more limited, though promising results indicate it could be an alternative for patients who do not tolerate infliximab.35
Azathioprine, mycophenolate mofetil, or methotrexate may also be used as a second-line agent if treatment with corticosteroids is not tolerated or does not control the disease. The evidence in support of these agents is limited. In small series, they have allowed sustainable control of renal function while reducing the steroid dose. Currently, these agents are used for patients resistant to corticosteroid therapy, who would otherwise need prolonged high-dose corticosteroid treatment, or who have corticosteroid intolerance; they allow a more effective steroid taper and maintenance of stable renal function.15,36
The data supporting a standardized treatment of renal sarcoidosis are limited. For steroid intolerance or resistance, cytotoxic drugs and selected anti-TNF-alpha agents, as mentioned above, have shown promise in improving or stabilizing serum creatinine levels. Further exploration is required as to which agent or combination is better at limiting the disease process with fewer adverse effects.
Our patient was initially treated with corticosteroids and was ultimately weaned to a maintenance dose of 5 mg/day. He was followed as an outpatient and was started on mycophenolate mofetil in place of higher steroid doses. His renal function stabilized, but he was lost to follow-up after 2 years.
KEY POINTS
- Sarcoidosis is a multisystem granulomatous disease that most commonly involves the lungs, skin, and reticuloendothelial system.
- Renal involvement in sarcoidosis is likely underestimated due to its often clinically silent nature and the possibility of missing typical granulomatous lesions in a small or less-than-optimal biopsy sample.
- Manifestations of renal sarcoidosis include disrupted calcium homeostasis, nephrocalcinosis, nephrolithiasis, and renal failure.
- Because the clinical and histopathologic manifestations of renal sarcoidosis are nonspecific, the diagnosis is one of exclusion. In patients with renal failure or with hypercalcemia or hypercalciuria of unknown cause, renal sarcoidosis should be included in the differential diagnosis. Patients with chronic sarcoidosis should also be screened for renal impairment.
- Granulomatous interstitial nephritis is the classic histologic finding of renal sarcoidosis. Nonetheless, up to one-third of patients have interstitial nephritis without granulomas.
- Corticosteroids are the mainstay of treatment for renal sarcoidosis. An initial dose of oral prednisone 0.5 to 1 mg/kg/day should be maintained for 4 weeks and then gradually tapered to 5 to 10 mg/day for a total of 24 months. Some patients require lifelong therapy.
- Several immunosuppressive and cytotoxic agents may be used in cases of corticosteroid intolerance or to aid in effective taper of corticosteroids.
- Joss N, Morris S, Young B, Geddes C. Granulomatous interstitial nephritis. Clin J Am Soc Nephrol 2007; 2:222–230.
- Lutalo PM, D'Cruz DP. Diagnosis and classification of granulomatosis with polyangiitis (aka Wegener's granulomatosis). J Autoimmun 2014; 48–49:94–98.
- Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med 1997; 336:1224–1234.
- Rajakariar R, Sharples EJ, Raftery MJ, Sheaff M, Yaqoob MM. Sarcoid tubulo-interstitial nephritis: long-term outcome and response to corticosteroid therapy. Kidney Int 2006; 70:165–169.
- Baughman RP, Teirstein AS, Judson MA, et al; Case Control Etiologic Study of Sarcoidosis (ACCESS) research group. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med 2001; 164:1885–1889.
- Rizzato G, Palmieri G, Agrati AM, Zanussi C. The organ-specific extrapulmonary presentation of sarcoidosis: a frequent occurrence but a challenge to an early diagnosis. A 3-year-long prospective observational study. Sarcoidosis Vasc Diffuse Lung Dis 2004; 21:119–126.
- Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med 2007; 357:2153–2165.
- Baughman RP, Field S, Costabel U, et al. Sarcoidosis in America. Analysis based on health care use. Ann Am Thorac Soc 2016; 13:1244–1252.
- Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med 1983; 52:525–533.
- Mayock RL, Bertrand P, Morrison CE, Scott JH. Manifestations of sarcoidosis. Analysis of 145 patients, with a review of nine series selected from the literature. Am J Med 1963; 35:67–89.
- Berliner AR, Haas M, Choi MJ. Sarcoidosis: the nephrologist's perspective. Am J Kidney Dis 2006; 48:856–870.
- Longcope WT, Freiman DG. A study of sarcoidosis; based on a combined investigation of 160 cases including 30 autopsies from The Johns Hopkins Hospital and Massachusetts General Hospital. Medicine (Baltimore) 1952; 31:1–132.
- Branson JH, Park JH. Sarcoidosis hepatic involvement: presentation of a case with fatal liver involvement; including autopsy findings and review of the evidence for sarcoid involvement of the liver as found in the literature. Ann Intern Med 1954; 40:111–145.
- Muther RS, McCarron DA, Bennett WM. Renal manifestations of sarcoidosis. Arch Intern Med 1981; 141:643–645.
- Mahevas M, Lescure FX, Boffa JJ, et al. Renal sarcoidosis: clinical, laboratory, and histologic presentation and outcome in 47 patients. Medicine (Baltimore) 2009; 88:98–106.
- Robson MG, Banerjee D, Hopster D, Cairns HS. Seven cases of granulomatous interstitial nephritis in the absence of extrarenal sarcoid. Nephrol Dial Transplant 2003; 18:280–284.
- Casella FJ, Allon M. The kidney in sarcoidosis. J Am Soc Nephrol 1993; 3:1555–1562.
- Rafat C, Bobrie G, Chedid A, Nochy D, Hernigou A, Plouin PF. Sarcoidosis presenting as severe renin-dependent hypertension due to kidney vascular injury. Clin Kidney J 2014; 7:383–386.
- Reichel H, Koeffler HP, Barbers R, Norman AW. Regulation of 1,25-dihydroxyvitamin D3 production by cultured alveolar macrophages from normal human donors and from patients with pulmonary sarcoidosis. J Clin Endocrinol Metab 1987; 65:1201–1209.
- Brause M, Magnusson K, Degenhardt S, Helmchen U, Grabensee B. Renal involvement in sarcoidosis—a report of 6 cases. Clin Nephrol 2002; 57:142–148.
- Hannedouche T, Grateau G, Noel LH, et al. Renal granulomatous sarcoidosis: report of six cases. Nephrol Dial Transplant 1990; 5:18–24.
- Kettritz R, Goebel U, Fiebeler A, Schneider W, Luft F. The protean face of sarcoidosis revisited. Nephrol Dial Transplant 2006; 21:2690–2694.
- Bergner R, Hoffmann M, Waldherr R, Uppenkamp M. Frequency of kidney disease in chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2003; 20:126–132.
- O’Riordan E, Willert RP, Reeve R, et al. Isolated sarcoid granulomatous interstitial nephritis: review of five cases at one center. Clin Nephrol 2001; 55:297–302.
- Gobel U, Kettritz R, Schneider W, Luft F. The protean face of renal sarcoidosis. J Am Soc Nephrol 2001; 12:616–623.
- Statement on sarcoidosis. Joint statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999; 160:736–755.
- Bijol V, Mendez GP, Nose V, Rennke HG. Granulomatous interstitial nephritis: a clinicopathologic study of 46 cases from a single institution. Int J Surg Pathol 2006; 14:57–63.
- Mignon F, Mery JP, Mougenot B, Ronco P, Roland J, Morel-Maroger L. Granulomatous interstitial nephritis. Adv Nephrol Necker Hosp 1984; 13:219–245.
- Shah R, Shidham G, Agarwal A, Albawardi A, Nadasdy T. Diagnostic utility of kidney biopsy in patients with sarcoidosis and acute kidney injury. Int J Nephrol Renovasc Dis 2011; 4:131–136.
- Studdy PR, Bird R. Serum angiotensin converting enzyme in sarcoidosis—its value in present clinical practice. Ann Clin Biochem 1989; 26:13–18.
- Demetriou ET, Pietras SM, Holick MF. Hypercalcemia and soft tissue calcification owing to sarcoidosis: the sunlight-cola connection. J Bone Miner Res 2010; 25:1695–1699.
- Beegle SH, Barba K, Gobunsuy R, Judson MA. Current and emerging pharmacological treatments for sarcoidosis: a review. Drug Des Devel Ther 2013; 7:325–338.
- Roberts SD, Wilkes DS, Burgett RA, Knox KS. Refractory sarcoidosis responding to infliximab. Chest 2003; 124:2028–2031.
- Ahmed MM, Mubashir E, Dossabhoy NR. Isolated renal sarcoidosis: a rare presentation of a rare disease treated with infliximab. Clin Rheumatol 2007; 26:1346–1349.
- Gupta R, Beaudet L, Moore J, Mehta T. Treatment of sarcoid granulomatous interstitial nephritis with adalimumab. NDT Plus 2009; 2:139–142.
- Moudgil A, Przygodzki RM, Kher KK. Successful steroid-sparing treatment of renal limited sarcoidosis with mycophenolate mofetil. Pediatr Nephrol 2006; 21:281–285.
- Joss N, Morris S, Young B, Geddes C. Granulomatous interstitial nephritis. Clin J Am Soc Nephrol 2007; 2:222–230.
- Lutalo PM, D'Cruz DP. Diagnosis and classification of granulomatosis with polyangiitis (aka Wegener's granulomatosis). J Autoimmun 2014; 48–49:94–98.
- Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med 1997; 336:1224–1234.
- Rajakariar R, Sharples EJ, Raftery MJ, Sheaff M, Yaqoob MM. Sarcoid tubulo-interstitial nephritis: long-term outcome and response to corticosteroid therapy. Kidney Int 2006; 70:165–169.
- Baughman RP, Teirstein AS, Judson MA, et al; Case Control Etiologic Study of Sarcoidosis (ACCESS) research group. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med 2001; 164:1885–1889.
- Rizzato G, Palmieri G, Agrati AM, Zanussi C. The organ-specific extrapulmonary presentation of sarcoidosis: a frequent occurrence but a challenge to an early diagnosis. A 3-year-long prospective observational study. Sarcoidosis Vasc Diffuse Lung Dis 2004; 21:119–126.
- Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med 2007; 357:2153–2165.
- Baughman RP, Field S, Costabel U, et al. Sarcoidosis in America. Analysis based on health care use. Ann Am Thorac Soc 2016; 13:1244–1252.
- Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med 1983; 52:525–533.
- Mayock RL, Bertrand P, Morrison CE, Scott JH. Manifestations of sarcoidosis. Analysis of 145 patients, with a review of nine series selected from the literature. Am J Med 1963; 35:67–89.
- Berliner AR, Haas M, Choi MJ. Sarcoidosis: the nephrologist's perspective. Am J Kidney Dis 2006; 48:856–870.
- Longcope WT, Freiman DG. A study of sarcoidosis; based on a combined investigation of 160 cases including 30 autopsies from The Johns Hopkins Hospital and Massachusetts General Hospital. Medicine (Baltimore) 1952; 31:1–132.
- Branson JH, Park JH. Sarcoidosis hepatic involvement: presentation of a case with fatal liver involvement; including autopsy findings and review of the evidence for sarcoid involvement of the liver as found in the literature. Ann Intern Med 1954; 40:111–145.
- Muther RS, McCarron DA, Bennett WM. Renal manifestations of sarcoidosis. Arch Intern Med 1981; 141:643–645.
- Mahevas M, Lescure FX, Boffa JJ, et al. Renal sarcoidosis: clinical, laboratory, and histologic presentation and outcome in 47 patients. Medicine (Baltimore) 2009; 88:98–106.
- Robson MG, Banerjee D, Hopster D, Cairns HS. Seven cases of granulomatous interstitial nephritis in the absence of extrarenal sarcoid. Nephrol Dial Transplant 2003; 18:280–284.
- Casella FJ, Allon M. The kidney in sarcoidosis. J Am Soc Nephrol 1993; 3:1555–1562.
- Rafat C, Bobrie G, Chedid A, Nochy D, Hernigou A, Plouin PF. Sarcoidosis presenting as severe renin-dependent hypertension due to kidney vascular injury. Clin Kidney J 2014; 7:383–386.
- Reichel H, Koeffler HP, Barbers R, Norman AW. Regulation of 1,25-dihydroxyvitamin D3 production by cultured alveolar macrophages from normal human donors and from patients with pulmonary sarcoidosis. J Clin Endocrinol Metab 1987; 65:1201–1209.
- Brause M, Magnusson K, Degenhardt S, Helmchen U, Grabensee B. Renal involvement in sarcoidosis—a report of 6 cases. Clin Nephrol 2002; 57:142–148.
- Hannedouche T, Grateau G, Noel LH, et al. Renal granulomatous sarcoidosis: report of six cases. Nephrol Dial Transplant 1990; 5:18–24.
- Kettritz R, Goebel U, Fiebeler A, Schneider W, Luft F. The protean face of sarcoidosis revisited. Nephrol Dial Transplant 2006; 21:2690–2694.
- Bergner R, Hoffmann M, Waldherr R, Uppenkamp M. Frequency of kidney disease in chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2003; 20:126–132.
- O’Riordan E, Willert RP, Reeve R, et al. Isolated sarcoid granulomatous interstitial nephritis: review of five cases at one center. Clin Nephrol 2001; 55:297–302.
- Gobel U, Kettritz R, Schneider W, Luft F. The protean face of renal sarcoidosis. J Am Soc Nephrol 2001; 12:616–623.
- Statement on sarcoidosis. Joint statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999; 160:736–755.
- Bijol V, Mendez GP, Nose V, Rennke HG. Granulomatous interstitial nephritis: a clinicopathologic study of 46 cases from a single institution. Int J Surg Pathol 2006; 14:57–63.
- Mignon F, Mery JP, Mougenot B, Ronco P, Roland J, Morel-Maroger L. Granulomatous interstitial nephritis. Adv Nephrol Necker Hosp 1984; 13:219–245.
- Shah R, Shidham G, Agarwal A, Albawardi A, Nadasdy T. Diagnostic utility of kidney biopsy in patients with sarcoidosis and acute kidney injury. Int J Nephrol Renovasc Dis 2011; 4:131–136.
- Studdy PR, Bird R. Serum angiotensin converting enzyme in sarcoidosis—its value in present clinical practice. Ann Clin Biochem 1989; 26:13–18.
- Demetriou ET, Pietras SM, Holick MF. Hypercalcemia and soft tissue calcification owing to sarcoidosis: the sunlight-cola connection. J Bone Miner Res 2010; 25:1695–1699.
- Beegle SH, Barba K, Gobunsuy R, Judson MA. Current and emerging pharmacological treatments for sarcoidosis: a review. Drug Des Devel Ther 2013; 7:325–338.
- Roberts SD, Wilkes DS, Burgett RA, Knox KS. Refractory sarcoidosis responding to infliximab. Chest 2003; 124:2028–2031.
- Ahmed MM, Mubashir E, Dossabhoy NR. Isolated renal sarcoidosis: a rare presentation of a rare disease treated with infliximab. Clin Rheumatol 2007; 26:1346–1349.
- Gupta R, Beaudet L, Moore J, Mehta T. Treatment of sarcoid granulomatous interstitial nephritis with adalimumab. NDT Plus 2009; 2:139–142.
- Moudgil A, Przygodzki RM, Kher KK. Successful steroid-sparing treatment of renal limited sarcoidosis with mycophenolate mofetil. Pediatr Nephrol 2006; 21:281–285.
Study indicates parent-reported penicillin allergy in question
, according to David Vyles, DO, and his associates at the Medical College of Wisconsin, Milwaukee.
Because there is no process to safely and rapidly diagnose true penicillin allergy in a critical care setting, pediatric providers are reluctant to prescribe penicillin to children with a reported allergy. In this study, a three-tier penicillin testing process was used to evaluate the accuracy of a parent-reported penicillin allergy questionnaire in identifying children likely to be at low risk for penicillin allergy in an ED setting.
“Our results in the current study highlight that the high percentage of patients reporting a penicillin allergy to medical providers are likely inconsistent with true allergy,” concluded Dr. Vyles and his associates.
Read more at Pediatrics (2017. doi: 10.1542/peds.2017-0471).
, according to David Vyles, DO, and his associates at the Medical College of Wisconsin, Milwaukee.
Because there is no process to safely and rapidly diagnose true penicillin allergy in a critical care setting, pediatric providers are reluctant to prescribe penicillin to children with a reported allergy. In this study, a three-tier penicillin testing process was used to evaluate the accuracy of a parent-reported penicillin allergy questionnaire in identifying children likely to be at low risk for penicillin allergy in an ED setting.
“Our results in the current study highlight that the high percentage of patients reporting a penicillin allergy to medical providers are likely inconsistent with true allergy,” concluded Dr. Vyles and his associates.
Read more at Pediatrics (2017. doi: 10.1542/peds.2017-0471).
, according to David Vyles, DO, and his associates at the Medical College of Wisconsin, Milwaukee.
Because there is no process to safely and rapidly diagnose true penicillin allergy in a critical care setting, pediatric providers are reluctant to prescribe penicillin to children with a reported allergy. In this study, a three-tier penicillin testing process was used to evaluate the accuracy of a parent-reported penicillin allergy questionnaire in identifying children likely to be at low risk for penicillin allergy in an ED setting.
“Our results in the current study highlight that the high percentage of patients reporting a penicillin allergy to medical providers are likely inconsistent with true allergy,” concluded Dr. Vyles and his associates.
Read more at Pediatrics (2017. doi: 10.1542/peds.2017-0471).
FROM PEDIATRICS
Studies provide insight into link between cancer immunotherapy and autoimmune disease
MADRID – Rheumatologists all over the world are beginning to find that the new class of anticancer immune checkpoint inhibitor therapies have the potential to elicit symptoms of rheumatoid arthritis (RA) and other rheumatic diseases in patients with no previous history of them, and two reports from the European Congress of Rheumatology provide typical examples.
These immune checkpoint inhibitor (ICI) agents, which include ipilimumab (Yervoy), nivolumab (Opdivo), and pembrolizumab (Keytruda), target regulatory pathways in T cells to boost antitumor immune responses, leading to improved survival for many cancer patients, but the induction of rheumatic disease can sometimes lead to the suspension of the agents, according to investigators.
“This phenomenon was unknown to me and my group before [February 2016], when we started noting referrals of patients from oncology,” Dr. Calabrese said. “We were seeing symptoms of everything from Sjögren’s syndrome to inflammatory arthritis and myositis in patients being treated with these drugs for their cancer.” The same year, Dr. Calabrese and her team began coordinating an ongoing study to assess these patients.
Dr. Calabrese said that the cohort has shown so far that patients who develop autoimmune disease after immune checkpoint inhibitors “require much higher doses – of steroids in particular – to treat their symptoms,” and this can all too often result in being taken out of a clinical trial or having to stop cancer treatment.
Most of the patients in the cohort were treated with steroids only, while three patients received biologic agents, and four received methotrexate or antimalarials.
Dr. Calabrese said that the serology results were available for all the patients in the cohort and “were largely unremarkable.”
She noted that the rheumatic symptoms did not always resolve after pausing or stopping the cancer treatment. “We have some patients that have been off their checkpoint inhibitors for over a year and still have symptoms, so it’s looking like it might be a more long-term effect,” she said.
“In my unit, we also manage patients with myeloma, and I developed a weekly consultation with a cancer center,” Dr. Belkhir said. In 2015, she saw her first patient with RA and no previous history who had been treated with checkpoint inhibitors. That patient’s symptoms resolved after treatment with nonsteroidal anti-inflammatory drugs alone.
Dr. Belkhir is sharing results from this and five other patients presenting with symptoms of RA after their cancer treatment with immune checkpoint inhibitors, taken from a larger cohort of patients (n = 13) with a spectrum of rheumatic disease–like adverse effects. None of the six patients in this study had a previous clinical history of RA. They manifested their RA symptoms after a median of 1 month on cancer immunotherapy.
Some were able to continue their checkpoint inhibitors and be treated simultaneously for RA with steroids, antimalarials, methotrexate, and NSAIDs, Dr. Belkhir said. None received biologic agents, and each medication strategy, she said, was arrived at in consultation with the treating oncologist.
Dr. Belkhir’s team also looked closely at serology and found all six patients to be at least weakly, and mostly strongly, seropositive for RA. Three patients underwent testing for anticyclic citrullinated protein antibodies prior to starting cancer immunotherapy and two of these three were anti-CCP positive. Now, she said, the oncologists she’s working with are testing for anticyclic citrullinated peptides and rheumatoid factor prior to initiating cancer immunotherapy, so that this relationship is better understood.
“It is possible that antibodies were already present and that the anti-PD1 immunotherapy,” one type of immune checkpoint inhibitor, “acted as a trigger for the disease.” Animal studies have suggested a role for PD1 in the development of autoimmune disease, “but it’s not well investigated,” Dr. Belkhir said.
Dr. Belkhir and Dr. Calabrese both acknowledged that the understanding of checkpoint inhibitor–induced autoimmune disease is in its infancy. Clinical trials largely missed the phenomenon, the researchers said, because the trials were not designed to capture musculoskeletal adverse effects with the same granularity as other serious adverse events.
“This will be a long discussion in the months and the years ahead with oncologists,” Dr. Belkhir said.
Neither Dr. Calabrese nor Dr. Belkhir reported having any relevant conflicts of interest.
MADRID – Rheumatologists all over the world are beginning to find that the new class of anticancer immune checkpoint inhibitor therapies have the potential to elicit symptoms of rheumatoid arthritis (RA) and other rheumatic diseases in patients with no previous history of them, and two reports from the European Congress of Rheumatology provide typical examples.
These immune checkpoint inhibitor (ICI) agents, which include ipilimumab (Yervoy), nivolumab (Opdivo), and pembrolizumab (Keytruda), target regulatory pathways in T cells to boost antitumor immune responses, leading to improved survival for many cancer patients, but the induction of rheumatic disease can sometimes lead to the suspension of the agents, according to investigators.
“This phenomenon was unknown to me and my group before [February 2016], when we started noting referrals of patients from oncology,” Dr. Calabrese said. “We were seeing symptoms of everything from Sjögren’s syndrome to inflammatory arthritis and myositis in patients being treated with these drugs for their cancer.” The same year, Dr. Calabrese and her team began coordinating an ongoing study to assess these patients.
Dr. Calabrese said that the cohort has shown so far that patients who develop autoimmune disease after immune checkpoint inhibitors “require much higher doses – of steroids in particular – to treat their symptoms,” and this can all too often result in being taken out of a clinical trial or having to stop cancer treatment.
Most of the patients in the cohort were treated with steroids only, while three patients received biologic agents, and four received methotrexate or antimalarials.
Dr. Calabrese said that the serology results were available for all the patients in the cohort and “were largely unremarkable.”
She noted that the rheumatic symptoms did not always resolve after pausing or stopping the cancer treatment. “We have some patients that have been off their checkpoint inhibitors for over a year and still have symptoms, so it’s looking like it might be a more long-term effect,” she said.
“In my unit, we also manage patients with myeloma, and I developed a weekly consultation with a cancer center,” Dr. Belkhir said. In 2015, she saw her first patient with RA and no previous history who had been treated with checkpoint inhibitors. That patient’s symptoms resolved after treatment with nonsteroidal anti-inflammatory drugs alone.
Dr. Belkhir is sharing results from this and five other patients presenting with symptoms of RA after their cancer treatment with immune checkpoint inhibitors, taken from a larger cohort of patients (n = 13) with a spectrum of rheumatic disease–like adverse effects. None of the six patients in this study had a previous clinical history of RA. They manifested their RA symptoms after a median of 1 month on cancer immunotherapy.
Some were able to continue their checkpoint inhibitors and be treated simultaneously for RA with steroids, antimalarials, methotrexate, and NSAIDs, Dr. Belkhir said. None received biologic agents, and each medication strategy, she said, was arrived at in consultation with the treating oncologist.
Dr. Belkhir’s team also looked closely at serology and found all six patients to be at least weakly, and mostly strongly, seropositive for RA. Three patients underwent testing for anticyclic citrullinated protein antibodies prior to starting cancer immunotherapy and two of these three were anti-CCP positive. Now, she said, the oncologists she’s working with are testing for anticyclic citrullinated peptides and rheumatoid factor prior to initiating cancer immunotherapy, so that this relationship is better understood.
“It is possible that antibodies were already present and that the anti-PD1 immunotherapy,” one type of immune checkpoint inhibitor, “acted as a trigger for the disease.” Animal studies have suggested a role for PD1 in the development of autoimmune disease, “but it’s not well investigated,” Dr. Belkhir said.
Dr. Belkhir and Dr. Calabrese both acknowledged that the understanding of checkpoint inhibitor–induced autoimmune disease is in its infancy. Clinical trials largely missed the phenomenon, the researchers said, because the trials were not designed to capture musculoskeletal adverse effects with the same granularity as other serious adverse events.
“This will be a long discussion in the months and the years ahead with oncologists,” Dr. Belkhir said.
Neither Dr. Calabrese nor Dr. Belkhir reported having any relevant conflicts of interest.
MADRID – Rheumatologists all over the world are beginning to find that the new class of anticancer immune checkpoint inhibitor therapies have the potential to elicit symptoms of rheumatoid arthritis (RA) and other rheumatic diseases in patients with no previous history of them, and two reports from the European Congress of Rheumatology provide typical examples.
These immune checkpoint inhibitor (ICI) agents, which include ipilimumab (Yervoy), nivolumab (Opdivo), and pembrolizumab (Keytruda), target regulatory pathways in T cells to boost antitumor immune responses, leading to improved survival for many cancer patients, but the induction of rheumatic disease can sometimes lead to the suspension of the agents, according to investigators.
“This phenomenon was unknown to me and my group before [February 2016], when we started noting referrals of patients from oncology,” Dr. Calabrese said. “We were seeing symptoms of everything from Sjögren’s syndrome to inflammatory arthritis and myositis in patients being treated with these drugs for their cancer.” The same year, Dr. Calabrese and her team began coordinating an ongoing study to assess these patients.
Dr. Calabrese said that the cohort has shown so far that patients who develop autoimmune disease after immune checkpoint inhibitors “require much higher doses – of steroids in particular – to treat their symptoms,” and this can all too often result in being taken out of a clinical trial or having to stop cancer treatment.
Most of the patients in the cohort were treated with steroids only, while three patients received biologic agents, and four received methotrexate or antimalarials.
Dr. Calabrese said that the serology results were available for all the patients in the cohort and “were largely unremarkable.”
She noted that the rheumatic symptoms did not always resolve after pausing or stopping the cancer treatment. “We have some patients that have been off their checkpoint inhibitors for over a year and still have symptoms, so it’s looking like it might be a more long-term effect,” she said.
“In my unit, we also manage patients with myeloma, and I developed a weekly consultation with a cancer center,” Dr. Belkhir said. In 2015, she saw her first patient with RA and no previous history who had been treated with checkpoint inhibitors. That patient’s symptoms resolved after treatment with nonsteroidal anti-inflammatory drugs alone.
Dr. Belkhir is sharing results from this and five other patients presenting with symptoms of RA after their cancer treatment with immune checkpoint inhibitors, taken from a larger cohort of patients (n = 13) with a spectrum of rheumatic disease–like adverse effects. None of the six patients in this study had a previous clinical history of RA. They manifested their RA symptoms after a median of 1 month on cancer immunotherapy.
Some were able to continue their checkpoint inhibitors and be treated simultaneously for RA with steroids, antimalarials, methotrexate, and NSAIDs, Dr. Belkhir said. None received biologic agents, and each medication strategy, she said, was arrived at in consultation with the treating oncologist.
Dr. Belkhir’s team also looked closely at serology and found all six patients to be at least weakly, and mostly strongly, seropositive for RA. Three patients underwent testing for anticyclic citrullinated protein antibodies prior to starting cancer immunotherapy and two of these three were anti-CCP positive. Now, she said, the oncologists she’s working with are testing for anticyclic citrullinated peptides and rheumatoid factor prior to initiating cancer immunotherapy, so that this relationship is better understood.
“It is possible that antibodies were already present and that the anti-PD1 immunotherapy,” one type of immune checkpoint inhibitor, “acted as a trigger for the disease.” Animal studies have suggested a role for PD1 in the development of autoimmune disease, “but it’s not well investigated,” Dr. Belkhir said.
Dr. Belkhir and Dr. Calabrese both acknowledged that the understanding of checkpoint inhibitor–induced autoimmune disease is in its infancy. Clinical trials largely missed the phenomenon, the researchers said, because the trials were not designed to capture musculoskeletal adverse effects with the same granularity as other serious adverse events.
“This will be a long discussion in the months and the years ahead with oncologists,” Dr. Belkhir said.
Neither Dr. Calabrese nor Dr. Belkhir reported having any relevant conflicts of interest.
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: Rheumatic symptoms did not always resolve after pausing or stopping the cancer treatment, and some were able to continue their checkpoint inhibitors and be treated simultaneously for RA.
Data source: Two retrospective cohort reviews of patients on immune checkpoint inhibitors.
Disclosures: Neither Dr. Calabrese nor Dr. Belkhir reported having any relevant conflicts of interest.
Common Variable Immunodeficiency: A Clinical Overview
IN THIS ARTICLE
- Diagnosis
- Treatment/management
- Physcial signs suggestive of CVID in patients with appropriate history
- Case outcome
A 60-year-old woman with a recent history of air and cruise ship travel presented with symptoms consistent with acute sinusitis. She had a 34–pack-year history of cigarette smoking but had quit at age 50. Her medical history was significant for hypothyroidism, hypertension, coronary artery disease, mild asthma, and COPD. Past surgical history included coronary artery bypass, abdominal hysterectomy, and cholecystectomy. Her medications included inhaled bronchodilators, thyroxin, hydrochlorothiazide, nitrates, ß-blockers, and calcium channel blockers.
Over the next five years, she presented with frequent episodes of respiratory illness for which she received multiple courses of antibiotics, inhaled bronchodilators, and oral as well as inhaled corticosteroids. She consequently became increasingly sensitized to multiple antibiotic classes and was frequently hospitalized for the treatment of her respiratory illnesses.
Common variable immunodeficiency disorders (collectively known as CVID) are the most common clinically significant immunodeficiency diseases among adults.1 Manifesting clinically as frequent, unusually severe or recalcitrant bacterial infections of the ear, sinus, respiratory tree, and/or gastrointestinal tract, CVID is genetically induced.2 Additionally, these disorders can predispose individuals to autoimmune conditions and to cancers involving B lymphocytes.3 Often thought to be a disease of younger people, CVID can occur across the age span.4
The immune dysfunction that characterizes CVID is believed to result from underlying genetic defects that affect the differentiation of B cells, leading to faulty immunoglobulin (Ig) synthesis. Recent advances that allow the detection of multiple novel susceptibility loci for CVID have dramatically increased our understanding of the pathophysiology and pathogenesis of this disorder.5 These advances are being used to refine the diagnostic parameters of CVID and in the future may help clinicians tailor treatment protocols to specific genetic defects.5,6
Although considered rare, CVID is often unrecognized; the incidence is likely much higher than the current estimates of 1:10,000 to 1:50,000.7 About 13% to 23% of individuals with chronic sinusitis are thought to be affected by CVID.8 While it is most commonly diagnosed during the second and third decades of life, it can be diagnosed at any time during the lifespan.4 A high burden of disease is associated with this disorder, as hospitalizations and costly, aggressive treatment regimens are needed to manage the resultant bacterial infections and sequelae.2
Increased awareness of CVID among primary care providers is needed to assure prompt diagnosis and to avoid unnecessary complications associated with delayed treatment. The diagnostic workup is complex, and referral to immunology for specific diagnosis and treatment is strongly advised. Recognition is the first step, and primary care providers must include primary immunodeficiency disorders, including CVID, in their differential to avert a missed diagnosis and to ensure optimal treatment.9
CLINICAL MANIFESTATIONS/PATIENT HISTORY
Frequent and severe infections are a hallmark of CVID. The most common types of infections seen in CVID are sinusitis, conjunctivitis, otitis media, bronchitis, pneumonia, and gastroenteritis.10 These primary bacterial infections can disseminate, causing septicemia and/or central nervous system infection.11 The usual infectious pathogens are encapsulated bacteria such as Streptococcus pneumoniae and Haemophilus influenzae, but atypical infections due to organisms such as Pneumocystis carinii and Mycoplasma pneumoniae also occur in some patients.12,13
Although the majority of CVID cases occur sporadically, family history is helpful in securing the correct diagnosis.15 Known immunodeficiency, unusual susceptibility to infections, autoimmune diseases, hematologic malignancy, or death caused by infection in other family members should increase the provider’s index of suspicion for CVID.16
Many genetic defects have been implicated in CVID, yet the wide phenotypic expression found even in persons with similar genetic profiles implies that CVID has a complex genetic transmission pattern.15 Known or suspected consanguinity in parents or grandparents increases the risk for CVID.6
Although these family history elements occur infrequently, they increase the likelihood of severe opportunistic infection, which can cause organ damage or even death.1,17 Being alert for these elements of family history can help to avoid delays in diagnosis and treatment and eventual organ damage.2

DIFFERENTIAL DIAGNOSIS
When considering the differential diagnosis for the primary features of CVID, other etiologies that should be considered include allergies, environmental exposures, uncontrolled gastroesophageal reflux disease, structural abnormalities of the upper respiratory tract, and celiac disease.5,10,18,19 Far less common but still worthy of consideration are other genetic conditions, such as primary ciliary dyskinesia, cystic fibrosis, thymic dysfunction or carcinoma, and protein-losing enteropathies.20,21
A number of conditions can cause immunosuppression. Transient reductions in serum Ig levels can occur in the presence of serious infections.22 Long-term, high-dose use of some medications, such as corticosteroids, or use of anticonvulsants may reduce antibody availability. Chronic illnesses, malignancy, and malnutrition can also play a role in immunosuppression.19 CVID shares features with a large number of primary immune diseases, and these as well as other causes of hypogammaglobulinemia must be excluded before the diagnosis of CVID can be made.1
DIAGNOSIS
While infectious disease is a common reason patients seek medical care, few patients presenting with one will have CVID. Nevertheless, immunologic evaluations should be performed and appropriate referral to an immunology specialist is strongly recommended when more than one severe infection arises in a year’s time; when a pattern of severe or unusual infections presents over a period of time; when bronchiectasis is present; or when infections do not resolve with conventional treatment.16 In addition, the physical findings noted in the Table, when combined with a history of recurrent infections, autoimmune disorders, or lymphocytic malignancy, should prompt evaluation for CVID.10,16,18,23
The diagnosis of CVID requires testing for low serum levels of total IgG, IgG subclasses, IgA, and IgM. In CVID, IgG and IgA levels will be reduced, and occasionally IgM levels will also be diminished.24 Unless an active infection is present, there will be no change in the patient’s routine blood tests, such as the complete blood count and total complement levels.
The diagnosis is also based on demonstration of a deficient antibody response to protein (tetanus) and polysaccharide (pneumonia) vaccine antigens.21 A minimal reaction to these vaccines should prompt referral to an immunology specialist for additional testing and a plan of care.25 However, whenever the index of suspicion for CVID is high, prompt referral to immunology should not be delayed to perform further testing.16
TREATMENT/MANAGEMENT
IgG replacement therapy, which treats the underlying pathophysiology of CVID by supplementing one of the deficient antibodies, is the standard treatment for CVID. IgG is considered a blood product since it is made from human plasma. Patients may experience untoward reactions to IgG replacement therapy, similar to transfusion reactions; such reactions commonly include back pain, low-grade fever, muscle and joint discomfort, and fatigue. These unpleasant effects can be minimized with the prophylactic use of antihistamines, antipyretics, or even glucocorticoids.26
Although IgG replacement therapy has high upfront costs, it increases patients’ well-being considerably by preventing multiple or recurrent infections and the resultant hospitalizations for antibiotic therapy.27 Home infusion of IgG can minimize costs as well as increase patient autonomy.28 With home infusions, IgG is administered via a multisite subcutaneous route using a slow-infusion mechanical pump. Subcutaneous infusions generally take four to six hours, depending on the number of sites used. Some patients can infuse while they sleep, which increases patient satisfaction with the treatment.27
Infections in persons with CVID can be severe and may lead to organ-system compromise, requiring aggressive therapy aimed at supporting the function of the affected organ systems. For example, patients with CVID can develop unrelenting vomiting and diarrhea, which may require inpatient admission for rehydration and stabilization until the infection can be treated adequately.32
Treatment options remain limited for the subset of CVID patients who develop severe complications, such as interstitial lung disease or neoplasms. These complications are associated with a significant increase in patient mortality, and allogeneic hematopoietic stem cell transplantation may be indicated for patients who develop them. This potentially curative treatment is being explored in ongoing research trials.33
PATIENT EDUCATION
Scrupulous hand hygiene, careful avoidance of infectious exposures, watchful food handling and preparation, and lifestyle choices that support good general health are key elements of self-care for patients who have CVID. Preventive measures serve this population well by helping to reduce some of the complications of this serious disease.
Patients with CVID should understand keys aspects regarding its diagnosis, treatment, and prognosis. Specifically, they should know that people who have CVID are born missing some of the body’s immune defenses, which increases their risk for infection, especially of the sinuses, lungs, and gut. Sometimes it takes years to make this diagnosis, because it is a rare cause of common symptoms.
The patient was referred to immunology, and a diagnosis of CVID was made. She was successfully treated with subcutaneous IgG replacement therapy. She died due to overwhelming sepsis after an episode of pneumonia at age 84.
CONCLUSION
The secret to prompt detection of CVID is adding it to the differential diagnosis of recurrent infections. Timely recognition and appropriate referral prevent serious complications, since successful treatment options are available.
Special thanks to Doug Bartelt, DNP, APNP, NP-C.
1. Bonilla FA, Barlan I, Chapel H, et al. International Consensus Document (ICON): Common Variable Immunodeficiency Disorders. J Allergy Clin Immunol Pract. 2016;4(1):38-59.
2. Jolles S. The variable in common variable immunodeficiency: a disease of complex phenotypes. J Allergy Clin Immunol Pract. 2013;1(6):545-556.
3. Barsotti NS, Almeida RR, Costa PR, et al. IL-10-Producing regulatory B cells are decreased in patients with common variable immunodeficiency. PLoS One. 2016;11(3): e0151761.
4. Rosenberg E, Dent PB, Denburg JA. Primary immune deficiencies in the adult: a previously underrecognized common condition. J Allergy Clin Immunol Pract. 2016;4(6):1101-1107.
5. Orange JS, Glessner JT, Resnick E,Genome-wide association identifies diverse causes of common variable immunodeficiency. J Allergy Clin Immunol. 2011;127(6):1360-1367.e6.
6. Stray-Pedersen A, Sorte HS, Samarakoon P, et al. Primary immunodeficiency diseases: genomic approaches delineate heterogeneous Mendelian disorders. J Allergy Clin Immunol. 2017;139(1):232-245.
7. Salzer U, Warnatz K, Peter HH. Common variable immunodeficiency—an update. Arthritis Res Ther. 2012;14(5):223.
8. Schwitzguébel AJ, Jandus P, Lacroix JS, et al. Immunoglobulin deficiency in patients with chronic rhinosinusitis: systematic review of the literature and meta-analysis. J Allergy Clin Immunol. 2015;136(6):1523-1531.
9. Chapel H. Common variable immunodeficiency disorders (CVID)—diagnoses of exclusion, especially combined immune defects. J Allergy Clin Immunol Pract. 2016;4(6):1158-1159.
10. Kakkas I. Clinical heterogeneity of common variable immunodeficiency. Hosp Chron. 2016;11(1):10-14.
11. Bonilla FA, Khan DA, Ballas ZK, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. 2015;136(5):1186-1205.
12. Schussler E, Beasley MB, Maglione PJ. Lung disease in primary antibody deficiencies. J Allergy Clin Immunol Pract. 2016;4(6):1039-1052.
13. Harville TO. Could better categorization of pulmonary disease in common variable immunodeficiency ultimately allow for better treatment outcomes? Ann Allergy Asthma Immunol. 2014;113(4):336-337.
14. Oliveira JB, Fleisher TA. Laboratory evaluation of primary immunodeficiencies. J Allergy Clin Immunol. 2010;125(2):S297-S305.
15. Bogaert DJ, Dullaers M, Lambrecht BN, et al. Genes associated with common variable immunodeficiency: one diagnosis to rule them all? J Med Genet. 2016;53(9):575-590.
16. De Vries E; European Society for Immunodeficiencies (ESID) members. Patient-centered screening for primary immunodeficiency, a multi-stage diagnostic protocol designed for non-immunologists: 2011 update. Clin Exp Immunol. 2012; 167(1):108-119.
17. Bertinchamp R, Gérard L, Boutboul D, et al. Exclusion of patients with a severe T-cell defect improves the definition of common variable immunodeficiency. J Allergy Clin Immunol Pract. 2016;4(6):1147-1157.
18. Fasano A, Catassi C. Celiac disease. N Engl J Med. 2012;367(25):2419-2426.
19. Park MA, Li JT, Hagan JB, et al. Common variable immunodeficiency: a new look at an old disease. Lancet. 2008;372(9637):489-502.
20. Cunningham-Rundles C, Maglione PJ. Common variable immunodeficiency. J Allergy Clin Immunol. 2012;129(5):1425-1426.
21. Bonilla FA, Barlan I, Chapel H, et al. International consensus document (ICON): Common variable immunodeficiency disorders. J Allergy Clin Immunol Pract. 2016;4(1):38-59.
22. Chinen J, Notarangelo LD, Shearer WT. Advances in basic and clinical immunology in 2014. J Allergy Clin Immunol Pract. 2015;135(5):1132-1141.
23. Verma N, Thaventhiran A, Gathmann B, et al. Therapeutic management of primary immunodeficiency in older patients. Drugs Aging. 2013;30(7):503-512.
24. Jolles S. The variable in common variable immunodeficiency: a disease of complex phenotypes. J Allergy Clin Immunol Pract. 2013;1(6):545-556.
25. McCullagh BN, Comellas AP, Ballas ZK, et al. Antibody deficiency in patients with frequent exacerbations of chronic obstructive pulmonary disease (COPD). PLoS One. 2017;12(2):e0172437.
26. Wasserman RL. The nuts and bolts of immunoglobulin treatment for antibody deficiency. J Allergy Clin Immunol Pract. 2016;4(6):1076-1081.
27. Lingman-Framme J, Fasth A. Subcutaneous immunoglobulin for primary and secondary immunodeficiencies: an evidence-based review. Drugs. 2013;73(12):1307-1319.
28. Ducruet T, Levasseur M, Des Roches A, et al. Pharmacoeconomic advantages of subcutaneous versus intravenous immunoglobulin treatment in a Canadian pediatric center. J Allergy Clin Immunol Pract. 2013;131(2):585-587.
29. Driessen G, van der Burg M. Primary antibody deficiencies [educational paper]. Eur J Pediatr. 2011;170(6):693-702.
30. Kuruvilla M, de la Morena MT. Antibiotic prophylaxis in primary immune deficiency disorders. J Allergy Clin Immunol Pract. 2013;1(6):573-582.
31. Norlin AC, Hansen S, Wahren-Borgström E, et al. Vitamin D3 supplementation and antibiotic consumption—results from a prospective, observational study at an immune-deficiency unit in Sweden. PLoS One. 2016;11(9):e0163451.
32. Lougaris V, Ravelli A, Villanacci V, et al. Gastrointestinal pathologic abnormalities in pediatric- and adult-onset common variable immunodeficiency. Dig Dis Sci. 2015;60(8):2384-2389.
33. Wehr C, Gennery AR, Lindemans C, et al. Multicenter experience in hematopoietic stem cell transplantation for serious complications of common variable immunodeficiency. J Allergy Clin Immunol. 2015;135(4):988-997.
34. Shearer WT, Fleisher TA, Buckley RH, et al; Medical Advisory Committee of the Immune Deficiency Foundation. Recommendations for live viral and bacterial vaccines in immunodeficient patients and their close contacts. J Allergy Clin Immunol. 2014;133(4):961-966.
IN THIS ARTICLE
- Diagnosis
- Treatment/management
- Physcial signs suggestive of CVID in patients with appropriate history
- Case outcome
A 60-year-old woman with a recent history of air and cruise ship travel presented with symptoms consistent with acute sinusitis. She had a 34–pack-year history of cigarette smoking but had quit at age 50. Her medical history was significant for hypothyroidism, hypertension, coronary artery disease, mild asthma, and COPD. Past surgical history included coronary artery bypass, abdominal hysterectomy, and cholecystectomy. Her medications included inhaled bronchodilators, thyroxin, hydrochlorothiazide, nitrates, ß-blockers, and calcium channel blockers.
Over the next five years, she presented with frequent episodes of respiratory illness for which she received multiple courses of antibiotics, inhaled bronchodilators, and oral as well as inhaled corticosteroids. She consequently became increasingly sensitized to multiple antibiotic classes and was frequently hospitalized for the treatment of her respiratory illnesses.
Common variable immunodeficiency disorders (collectively known as CVID) are the most common clinically significant immunodeficiency diseases among adults.1 Manifesting clinically as frequent, unusually severe or recalcitrant bacterial infections of the ear, sinus, respiratory tree, and/or gastrointestinal tract, CVID is genetically induced.2 Additionally, these disorders can predispose individuals to autoimmune conditions and to cancers involving B lymphocytes.3 Often thought to be a disease of younger people, CVID can occur across the age span.4
The immune dysfunction that characterizes CVID is believed to result from underlying genetic defects that affect the differentiation of B cells, leading to faulty immunoglobulin (Ig) synthesis. Recent advances that allow the detection of multiple novel susceptibility loci for CVID have dramatically increased our understanding of the pathophysiology and pathogenesis of this disorder.5 These advances are being used to refine the diagnostic parameters of CVID and in the future may help clinicians tailor treatment protocols to specific genetic defects.5,6
Although considered rare, CVID is often unrecognized; the incidence is likely much higher than the current estimates of 1:10,000 to 1:50,000.7 About 13% to 23% of individuals with chronic sinusitis are thought to be affected by CVID.8 While it is most commonly diagnosed during the second and third decades of life, it can be diagnosed at any time during the lifespan.4 A high burden of disease is associated with this disorder, as hospitalizations and costly, aggressive treatment regimens are needed to manage the resultant bacterial infections and sequelae.2
Increased awareness of CVID among primary care providers is needed to assure prompt diagnosis and to avoid unnecessary complications associated with delayed treatment. The diagnostic workup is complex, and referral to immunology for specific diagnosis and treatment is strongly advised. Recognition is the first step, and primary care providers must include primary immunodeficiency disorders, including CVID, in their differential to avert a missed diagnosis and to ensure optimal treatment.9
CLINICAL MANIFESTATIONS/PATIENT HISTORY
Frequent and severe infections are a hallmark of CVID. The most common types of infections seen in CVID are sinusitis, conjunctivitis, otitis media, bronchitis, pneumonia, and gastroenteritis.10 These primary bacterial infections can disseminate, causing septicemia and/or central nervous system infection.11 The usual infectious pathogens are encapsulated bacteria such as Streptococcus pneumoniae and Haemophilus influenzae, but atypical infections due to organisms such as Pneumocystis carinii and Mycoplasma pneumoniae also occur in some patients.12,13
Although the majority of CVID cases occur sporadically, family history is helpful in securing the correct diagnosis.15 Known immunodeficiency, unusual susceptibility to infections, autoimmune diseases, hematologic malignancy, or death caused by infection in other family members should increase the provider’s index of suspicion for CVID.16
Many genetic defects have been implicated in CVID, yet the wide phenotypic expression found even in persons with similar genetic profiles implies that CVID has a complex genetic transmission pattern.15 Known or suspected consanguinity in parents or grandparents increases the risk for CVID.6
Although these family history elements occur infrequently, they increase the likelihood of severe opportunistic infection, which can cause organ damage or even death.1,17 Being alert for these elements of family history can help to avoid delays in diagnosis and treatment and eventual organ damage.2

DIFFERENTIAL DIAGNOSIS
When considering the differential diagnosis for the primary features of CVID, other etiologies that should be considered include allergies, environmental exposures, uncontrolled gastroesophageal reflux disease, structural abnormalities of the upper respiratory tract, and celiac disease.5,10,18,19 Far less common but still worthy of consideration are other genetic conditions, such as primary ciliary dyskinesia, cystic fibrosis, thymic dysfunction or carcinoma, and protein-losing enteropathies.20,21
A number of conditions can cause immunosuppression. Transient reductions in serum Ig levels can occur in the presence of serious infections.22 Long-term, high-dose use of some medications, such as corticosteroids, or use of anticonvulsants may reduce antibody availability. Chronic illnesses, malignancy, and malnutrition can also play a role in immunosuppression.19 CVID shares features with a large number of primary immune diseases, and these as well as other causes of hypogammaglobulinemia must be excluded before the diagnosis of CVID can be made.1
DIAGNOSIS
While infectious disease is a common reason patients seek medical care, few patients presenting with one will have CVID. Nevertheless, immunologic evaluations should be performed and appropriate referral to an immunology specialist is strongly recommended when more than one severe infection arises in a year’s time; when a pattern of severe or unusual infections presents over a period of time; when bronchiectasis is present; or when infections do not resolve with conventional treatment.16 In addition, the physical findings noted in the Table, when combined with a history of recurrent infections, autoimmune disorders, or lymphocytic malignancy, should prompt evaluation for CVID.10,16,18,23

The diagnosis of CVID requires testing for low serum levels of total IgG, IgG subclasses, IgA, and IgM. In CVID, IgG and IgA levels will be reduced, and occasionally IgM levels will also be diminished.24 Unless an active infection is present, there will be no change in the patient’s routine blood tests, such as the complete blood count and total complement levels.
The diagnosis is also based on demonstration of a deficient antibody response to protein (tetanus) and polysaccharide (pneumonia) vaccine antigens.21 A minimal reaction to these vaccines should prompt referral to an immunology specialist for additional testing and a plan of care.25 However, whenever the index of suspicion for CVID is high, prompt referral to immunology should not be delayed to perform further testing.16
TREATMENT/MANAGEMENT
IgG replacement therapy, which treats the underlying pathophysiology of CVID by supplementing one of the deficient antibodies, is the standard treatment for CVID. IgG is considered a blood product since it is made from human plasma. Patients may experience untoward reactions to IgG replacement therapy, similar to transfusion reactions; such reactions commonly include back pain, low-grade fever, muscle and joint discomfort, and fatigue. These unpleasant effects can be minimized with the prophylactic use of antihistamines, antipyretics, or even glucocorticoids.26
Although IgG replacement therapy has high upfront costs, it increases patients’ well-being considerably by preventing multiple or recurrent infections and the resultant hospitalizations for antibiotic therapy.27 Home infusion of IgG can minimize costs as well as increase patient autonomy.28 With home infusions, IgG is administered via a multisite subcutaneous route using a slow-infusion mechanical pump. Subcutaneous infusions generally take four to six hours, depending on the number of sites used. Some patients can infuse while they sleep, which increases patient satisfaction with the treatment.27
Infections in persons with CVID can be severe and may lead to organ-system compromise, requiring aggressive therapy aimed at supporting the function of the affected organ systems. For example, patients with CVID can develop unrelenting vomiting and diarrhea, which may require inpatient admission for rehydration and stabilization until the infection can be treated adequately.32
Treatment options remain limited for the subset of CVID patients who develop severe complications, such as interstitial lung disease or neoplasms. These complications are associated with a significant increase in patient mortality, and allogeneic hematopoietic stem cell transplantation may be indicated for patients who develop them. This potentially curative treatment is being explored in ongoing research trials.33
PATIENT EDUCATION
Scrupulous hand hygiene, careful avoidance of infectious exposures, watchful food handling and preparation, and lifestyle choices that support good general health are key elements of self-care for patients who have CVID. Preventive measures serve this population well by helping to reduce some of the complications of this serious disease.
Patients with CVID should understand keys aspects regarding its diagnosis, treatment, and prognosis. Specifically, they should know that people who have CVID are born missing some of the body’s immune defenses, which increases their risk for infection, especially of the sinuses, lungs, and gut. Sometimes it takes years to make this diagnosis, because it is a rare cause of common symptoms.
The patient was referred to immunology, and a diagnosis of CVID was made. She was successfully treated with subcutaneous IgG replacement therapy. She died due to overwhelming sepsis after an episode of pneumonia at age 84.
CONCLUSION
The secret to prompt detection of CVID is adding it to the differential diagnosis of recurrent infections. Timely recognition and appropriate referral prevent serious complications, since successful treatment options are available.
Special thanks to Doug Bartelt, DNP, APNP, NP-C.
IN THIS ARTICLE
- Diagnosis
- Treatment/management
- Physcial signs suggestive of CVID in patients with appropriate history
- Case outcome
A 60-year-old woman with a recent history of air and cruise ship travel presented with symptoms consistent with acute sinusitis. She had a 34–pack-year history of cigarette smoking but had quit at age 50. Her medical history was significant for hypothyroidism, hypertension, coronary artery disease, mild asthma, and COPD. Past surgical history included coronary artery bypass, abdominal hysterectomy, and cholecystectomy. Her medications included inhaled bronchodilators, thyroxin, hydrochlorothiazide, nitrates, ß-blockers, and calcium channel blockers.
Over the next five years, she presented with frequent episodes of respiratory illness for which she received multiple courses of antibiotics, inhaled bronchodilators, and oral as well as inhaled corticosteroids. She consequently became increasingly sensitized to multiple antibiotic classes and was frequently hospitalized for the treatment of her respiratory illnesses.
Common variable immunodeficiency disorders (collectively known as CVID) are the most common clinically significant immunodeficiency diseases among adults.1 Manifesting clinically as frequent, unusually severe or recalcitrant bacterial infections of the ear, sinus, respiratory tree, and/or gastrointestinal tract, CVID is genetically induced.2 Additionally, these disorders can predispose individuals to autoimmune conditions and to cancers involving B lymphocytes.3 Often thought to be a disease of younger people, CVID can occur across the age span.4
The immune dysfunction that characterizes CVID is believed to result from underlying genetic defects that affect the differentiation of B cells, leading to faulty immunoglobulin (Ig) synthesis. Recent advances that allow the detection of multiple novel susceptibility loci for CVID have dramatically increased our understanding of the pathophysiology and pathogenesis of this disorder.5 These advances are being used to refine the diagnostic parameters of CVID and in the future may help clinicians tailor treatment protocols to specific genetic defects.5,6
Although considered rare, CVID is often unrecognized; the incidence is likely much higher than the current estimates of 1:10,000 to 1:50,000.7 About 13% to 23% of individuals with chronic sinusitis are thought to be affected by CVID.8 While it is most commonly diagnosed during the second and third decades of life, it can be diagnosed at any time during the lifespan.4 A high burden of disease is associated with this disorder, as hospitalizations and costly, aggressive treatment regimens are needed to manage the resultant bacterial infections and sequelae.2
Increased awareness of CVID among primary care providers is needed to assure prompt diagnosis and to avoid unnecessary complications associated with delayed treatment. The diagnostic workup is complex, and referral to immunology for specific diagnosis and treatment is strongly advised. Recognition is the first step, and primary care providers must include primary immunodeficiency disorders, including CVID, in their differential to avert a missed diagnosis and to ensure optimal treatment.9
CLINICAL MANIFESTATIONS/PATIENT HISTORY
Frequent and severe infections are a hallmark of CVID. The most common types of infections seen in CVID are sinusitis, conjunctivitis, otitis media, bronchitis, pneumonia, and gastroenteritis.10 These primary bacterial infections can disseminate, causing septicemia and/or central nervous system infection.11 The usual infectious pathogens are encapsulated bacteria such as Streptococcus pneumoniae and Haemophilus influenzae, but atypical infections due to organisms such as Pneumocystis carinii and Mycoplasma pneumoniae also occur in some patients.12,13
Although the majority of CVID cases occur sporadically, family history is helpful in securing the correct diagnosis.15 Known immunodeficiency, unusual susceptibility to infections, autoimmune diseases, hematologic malignancy, or death caused by infection in other family members should increase the provider’s index of suspicion for CVID.16
Many genetic defects have been implicated in CVID, yet the wide phenotypic expression found even in persons with similar genetic profiles implies that CVID has a complex genetic transmission pattern.15 Known or suspected consanguinity in parents or grandparents increases the risk for CVID.6
Although these family history elements occur infrequently, they increase the likelihood of severe opportunistic infection, which can cause organ damage or even death.1,17 Being alert for these elements of family history can help to avoid delays in diagnosis and treatment and eventual organ damage.2

DIFFERENTIAL DIAGNOSIS
When considering the differential diagnosis for the primary features of CVID, other etiologies that should be considered include allergies, environmental exposures, uncontrolled gastroesophageal reflux disease, structural abnormalities of the upper respiratory tract, and celiac disease.5,10,18,19 Far less common but still worthy of consideration are other genetic conditions, such as primary ciliary dyskinesia, cystic fibrosis, thymic dysfunction or carcinoma, and protein-losing enteropathies.20,21
A number of conditions can cause immunosuppression. Transient reductions in serum Ig levels can occur in the presence of serious infections.22 Long-term, high-dose use of some medications, such as corticosteroids, or use of anticonvulsants may reduce antibody availability. Chronic illnesses, malignancy, and malnutrition can also play a role in immunosuppression.19 CVID shares features with a large number of primary immune diseases, and these as well as other causes of hypogammaglobulinemia must be excluded before the diagnosis of CVID can be made.1
DIAGNOSIS
While infectious disease is a common reason patients seek medical care, few patients presenting with one will have CVID. Nevertheless, immunologic evaluations should be performed and appropriate referral to an immunology specialist is strongly recommended when more than one severe infection arises in a year’s time; when a pattern of severe or unusual infections presents over a period of time; when bronchiectasis is present; or when infections do not resolve with conventional treatment.16 In addition, the physical findings noted in the Table, when combined with a history of recurrent infections, autoimmune disorders, or lymphocytic malignancy, should prompt evaluation for CVID.10,16,18,23

The diagnosis of CVID requires testing for low serum levels of total IgG, IgG subclasses, IgA, and IgM. In CVID, IgG and IgA levels will be reduced, and occasionally IgM levels will also be diminished.24 Unless an active infection is present, there will be no change in the patient’s routine blood tests, such as the complete blood count and total complement levels.
The diagnosis is also based on demonstration of a deficient antibody response to protein (tetanus) and polysaccharide (pneumonia) vaccine antigens.21 A minimal reaction to these vaccines should prompt referral to an immunology specialist for additional testing and a plan of care.25 However, whenever the index of suspicion for CVID is high, prompt referral to immunology should not be delayed to perform further testing.16
TREATMENT/MANAGEMENT
IgG replacement therapy, which treats the underlying pathophysiology of CVID by supplementing one of the deficient antibodies, is the standard treatment for CVID. IgG is considered a blood product since it is made from human plasma. Patients may experience untoward reactions to IgG replacement therapy, similar to transfusion reactions; such reactions commonly include back pain, low-grade fever, muscle and joint discomfort, and fatigue. These unpleasant effects can be minimized with the prophylactic use of antihistamines, antipyretics, or even glucocorticoids.26
Although IgG replacement therapy has high upfront costs, it increases patients’ well-being considerably by preventing multiple or recurrent infections and the resultant hospitalizations for antibiotic therapy.27 Home infusion of IgG can minimize costs as well as increase patient autonomy.28 With home infusions, IgG is administered via a multisite subcutaneous route using a slow-infusion mechanical pump. Subcutaneous infusions generally take four to six hours, depending on the number of sites used. Some patients can infuse while they sleep, which increases patient satisfaction with the treatment.27
Infections in persons with CVID can be severe and may lead to organ-system compromise, requiring aggressive therapy aimed at supporting the function of the affected organ systems. For example, patients with CVID can develop unrelenting vomiting and diarrhea, which may require inpatient admission for rehydration and stabilization until the infection can be treated adequately.32
Treatment options remain limited for the subset of CVID patients who develop severe complications, such as interstitial lung disease or neoplasms. These complications are associated with a significant increase in patient mortality, and allogeneic hematopoietic stem cell transplantation may be indicated for patients who develop them. This potentially curative treatment is being explored in ongoing research trials.33
PATIENT EDUCATION
Scrupulous hand hygiene, careful avoidance of infectious exposures, watchful food handling and preparation, and lifestyle choices that support good general health are key elements of self-care for patients who have CVID. Preventive measures serve this population well by helping to reduce some of the complications of this serious disease.
Patients with CVID should understand keys aspects regarding its diagnosis, treatment, and prognosis. Specifically, they should know that people who have CVID are born missing some of the body’s immune defenses, which increases their risk for infection, especially of the sinuses, lungs, and gut. Sometimes it takes years to make this diagnosis, because it is a rare cause of common symptoms.
The patient was referred to immunology, and a diagnosis of CVID was made. She was successfully treated with subcutaneous IgG replacement therapy. She died due to overwhelming sepsis after an episode of pneumonia at age 84.
CONCLUSION
The secret to prompt detection of CVID is adding it to the differential diagnosis of recurrent infections. Timely recognition and appropriate referral prevent serious complications, since successful treatment options are available.
Special thanks to Doug Bartelt, DNP, APNP, NP-C.
1. Bonilla FA, Barlan I, Chapel H, et al. International Consensus Document (ICON): Common Variable Immunodeficiency Disorders. J Allergy Clin Immunol Pract. 2016;4(1):38-59.
2. Jolles S. The variable in common variable immunodeficiency: a disease of complex phenotypes. J Allergy Clin Immunol Pract. 2013;1(6):545-556.
3. Barsotti NS, Almeida RR, Costa PR, et al. IL-10-Producing regulatory B cells are decreased in patients with common variable immunodeficiency. PLoS One. 2016;11(3): e0151761.
4. Rosenberg E, Dent PB, Denburg JA. Primary immune deficiencies in the adult: a previously underrecognized common condition. J Allergy Clin Immunol Pract. 2016;4(6):1101-1107.
5. Orange JS, Glessner JT, Resnick E,Genome-wide association identifies diverse causes of common variable immunodeficiency. J Allergy Clin Immunol. 2011;127(6):1360-1367.e6.
6. Stray-Pedersen A, Sorte HS, Samarakoon P, et al. Primary immunodeficiency diseases: genomic approaches delineate heterogeneous Mendelian disorders. J Allergy Clin Immunol. 2017;139(1):232-245.
7. Salzer U, Warnatz K, Peter HH. Common variable immunodeficiency—an update. Arthritis Res Ther. 2012;14(5):223.
8. Schwitzguébel AJ, Jandus P, Lacroix JS, et al. Immunoglobulin deficiency in patients with chronic rhinosinusitis: systematic review of the literature and meta-analysis. J Allergy Clin Immunol. 2015;136(6):1523-1531.
9. Chapel H. Common variable immunodeficiency disorders (CVID)—diagnoses of exclusion, especially combined immune defects. J Allergy Clin Immunol Pract. 2016;4(6):1158-1159.
10. Kakkas I. Clinical heterogeneity of common variable immunodeficiency. Hosp Chron. 2016;11(1):10-14.
11. Bonilla FA, Khan DA, Ballas ZK, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. 2015;136(5):1186-1205.
12. Schussler E, Beasley MB, Maglione PJ. Lung disease in primary antibody deficiencies. J Allergy Clin Immunol Pract. 2016;4(6):1039-1052.
13. Harville TO. Could better categorization of pulmonary disease in common variable immunodeficiency ultimately allow for better treatment outcomes? Ann Allergy Asthma Immunol. 2014;113(4):336-337.
14. Oliveira JB, Fleisher TA. Laboratory evaluation of primary immunodeficiencies. J Allergy Clin Immunol. 2010;125(2):S297-S305.
15. Bogaert DJ, Dullaers M, Lambrecht BN, et al. Genes associated with common variable immunodeficiency: one diagnosis to rule them all? J Med Genet. 2016;53(9):575-590.
16. De Vries E; European Society for Immunodeficiencies (ESID) members. Patient-centered screening for primary immunodeficiency, a multi-stage diagnostic protocol designed for non-immunologists: 2011 update. Clin Exp Immunol. 2012; 167(1):108-119.
17. Bertinchamp R, Gérard L, Boutboul D, et al. Exclusion of patients with a severe T-cell defect improves the definition of common variable immunodeficiency. J Allergy Clin Immunol Pract. 2016;4(6):1147-1157.
18. Fasano A, Catassi C. Celiac disease. N Engl J Med. 2012;367(25):2419-2426.
19. Park MA, Li JT, Hagan JB, et al. Common variable immunodeficiency: a new look at an old disease. Lancet. 2008;372(9637):489-502.
20. Cunningham-Rundles C, Maglione PJ. Common variable immunodeficiency. J Allergy Clin Immunol. 2012;129(5):1425-1426.
21. Bonilla FA, Barlan I, Chapel H, et al. International consensus document (ICON): Common variable immunodeficiency disorders. J Allergy Clin Immunol Pract. 2016;4(1):38-59.
22. Chinen J, Notarangelo LD, Shearer WT. Advances in basic and clinical immunology in 2014. J Allergy Clin Immunol Pract. 2015;135(5):1132-1141.
23. Verma N, Thaventhiran A, Gathmann B, et al. Therapeutic management of primary immunodeficiency in older patients. Drugs Aging. 2013;30(7):503-512.
24. Jolles S. The variable in common variable immunodeficiency: a disease of complex phenotypes. J Allergy Clin Immunol Pract. 2013;1(6):545-556.
25. McCullagh BN, Comellas AP, Ballas ZK, et al. Antibody deficiency in patients with frequent exacerbations of chronic obstructive pulmonary disease (COPD). PLoS One. 2017;12(2):e0172437.
26. Wasserman RL. The nuts and bolts of immunoglobulin treatment for antibody deficiency. J Allergy Clin Immunol Pract. 2016;4(6):1076-1081.
27. Lingman-Framme J, Fasth A. Subcutaneous immunoglobulin for primary and secondary immunodeficiencies: an evidence-based review. Drugs. 2013;73(12):1307-1319.
28. Ducruet T, Levasseur M, Des Roches A, et al. Pharmacoeconomic advantages of subcutaneous versus intravenous immunoglobulin treatment in a Canadian pediatric center. J Allergy Clin Immunol Pract. 2013;131(2):585-587.
29. Driessen G, van der Burg M. Primary antibody deficiencies [educational paper]. Eur J Pediatr. 2011;170(6):693-702.
30. Kuruvilla M, de la Morena MT. Antibiotic prophylaxis in primary immune deficiency disorders. J Allergy Clin Immunol Pract. 2013;1(6):573-582.
31. Norlin AC, Hansen S, Wahren-Borgström E, et al. Vitamin D3 supplementation and antibiotic consumption—results from a prospective, observational study at an immune-deficiency unit in Sweden. PLoS One. 2016;11(9):e0163451.
32. Lougaris V, Ravelli A, Villanacci V, et al. Gastrointestinal pathologic abnormalities in pediatric- and adult-onset common variable immunodeficiency. Dig Dis Sci. 2015;60(8):2384-2389.
33. Wehr C, Gennery AR, Lindemans C, et al. Multicenter experience in hematopoietic stem cell transplantation for serious complications of common variable immunodeficiency. J Allergy Clin Immunol. 2015;135(4):988-997.
34. Shearer WT, Fleisher TA, Buckley RH, et al; Medical Advisory Committee of the Immune Deficiency Foundation. Recommendations for live viral and bacterial vaccines in immunodeficient patients and their close contacts. J Allergy Clin Immunol. 2014;133(4):961-966.
1. Bonilla FA, Barlan I, Chapel H, et al. International Consensus Document (ICON): Common Variable Immunodeficiency Disorders. J Allergy Clin Immunol Pract. 2016;4(1):38-59.
2. Jolles S. The variable in common variable immunodeficiency: a disease of complex phenotypes. J Allergy Clin Immunol Pract. 2013;1(6):545-556.
3. Barsotti NS, Almeida RR, Costa PR, et al. IL-10-Producing regulatory B cells are decreased in patients with common variable immunodeficiency. PLoS One. 2016;11(3): e0151761.
4. Rosenberg E, Dent PB, Denburg JA. Primary immune deficiencies in the adult: a previously underrecognized common condition. J Allergy Clin Immunol Pract. 2016;4(6):1101-1107.
5. Orange JS, Glessner JT, Resnick E,Genome-wide association identifies diverse causes of common variable immunodeficiency. J Allergy Clin Immunol. 2011;127(6):1360-1367.e6.
6. Stray-Pedersen A, Sorte HS, Samarakoon P, et al. Primary immunodeficiency diseases: genomic approaches delineate heterogeneous Mendelian disorders. J Allergy Clin Immunol. 2017;139(1):232-245.
7. Salzer U, Warnatz K, Peter HH. Common variable immunodeficiency—an update. Arthritis Res Ther. 2012;14(5):223.
8. Schwitzguébel AJ, Jandus P, Lacroix JS, et al. Immunoglobulin deficiency in patients with chronic rhinosinusitis: systematic review of the literature and meta-analysis. J Allergy Clin Immunol. 2015;136(6):1523-1531.
9. Chapel H. Common variable immunodeficiency disorders (CVID)—diagnoses of exclusion, especially combined immune defects. J Allergy Clin Immunol Pract. 2016;4(6):1158-1159.
10. Kakkas I. Clinical heterogeneity of common variable immunodeficiency. Hosp Chron. 2016;11(1):10-14.
11. Bonilla FA, Khan DA, Ballas ZK, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. 2015;136(5):1186-1205.
12. Schussler E, Beasley MB, Maglione PJ. Lung disease in primary antibody deficiencies. J Allergy Clin Immunol Pract. 2016;4(6):1039-1052.
13. Harville TO. Could better categorization of pulmonary disease in common variable immunodeficiency ultimately allow for better treatment outcomes? Ann Allergy Asthma Immunol. 2014;113(4):336-337.
14. Oliveira JB, Fleisher TA. Laboratory evaluation of primary immunodeficiencies. J Allergy Clin Immunol. 2010;125(2):S297-S305.
15. Bogaert DJ, Dullaers M, Lambrecht BN, et al. Genes associated with common variable immunodeficiency: one diagnosis to rule them all? J Med Genet. 2016;53(9):575-590.
16. De Vries E; European Society for Immunodeficiencies (ESID) members. Patient-centered screening for primary immunodeficiency, a multi-stage diagnostic protocol designed for non-immunologists: 2011 update. Clin Exp Immunol. 2012; 167(1):108-119.
17. Bertinchamp R, Gérard L, Boutboul D, et al. Exclusion of patients with a severe T-cell defect improves the definition of common variable immunodeficiency. J Allergy Clin Immunol Pract. 2016;4(6):1147-1157.
18. Fasano A, Catassi C. Celiac disease. N Engl J Med. 2012;367(25):2419-2426.
19. Park MA, Li JT, Hagan JB, et al. Common variable immunodeficiency: a new look at an old disease. Lancet. 2008;372(9637):489-502.
20. Cunningham-Rundles C, Maglione PJ. Common variable immunodeficiency. J Allergy Clin Immunol. 2012;129(5):1425-1426.
21. Bonilla FA, Barlan I, Chapel H, et al. International consensus document (ICON): Common variable immunodeficiency disorders. J Allergy Clin Immunol Pract. 2016;4(1):38-59.
22. Chinen J, Notarangelo LD, Shearer WT. Advances in basic and clinical immunology in 2014. J Allergy Clin Immunol Pract. 2015;135(5):1132-1141.
23. Verma N, Thaventhiran A, Gathmann B, et al. Therapeutic management of primary immunodeficiency in older patients. Drugs Aging. 2013;30(7):503-512.
24. Jolles S. The variable in common variable immunodeficiency: a disease of complex phenotypes. J Allergy Clin Immunol Pract. 2013;1(6):545-556.
25. McCullagh BN, Comellas AP, Ballas ZK, et al. Antibody deficiency in patients with frequent exacerbations of chronic obstructive pulmonary disease (COPD). PLoS One. 2017;12(2):e0172437.
26. Wasserman RL. The nuts and bolts of immunoglobulin treatment for antibody deficiency. J Allergy Clin Immunol Pract. 2016;4(6):1076-1081.
27. Lingman-Framme J, Fasth A. Subcutaneous immunoglobulin for primary and secondary immunodeficiencies: an evidence-based review. Drugs. 2013;73(12):1307-1319.
28. Ducruet T, Levasseur M, Des Roches A, et al. Pharmacoeconomic advantages of subcutaneous versus intravenous immunoglobulin treatment in a Canadian pediatric center. J Allergy Clin Immunol Pract. 2013;131(2):585-587.
29. Driessen G, van der Burg M. Primary antibody deficiencies [educational paper]. Eur J Pediatr. 2011;170(6):693-702.
30. Kuruvilla M, de la Morena MT. Antibiotic prophylaxis in primary immune deficiency disorders. J Allergy Clin Immunol Pract. 2013;1(6):573-582.
31. Norlin AC, Hansen S, Wahren-Borgström E, et al. Vitamin D3 supplementation and antibiotic consumption—results from a prospective, observational study at an immune-deficiency unit in Sweden. PLoS One. 2016;11(9):e0163451.
32. Lougaris V, Ravelli A, Villanacci V, et al. Gastrointestinal pathologic abnormalities in pediatric- and adult-onset common variable immunodeficiency. Dig Dis Sci. 2015;60(8):2384-2389.
33. Wehr C, Gennery AR, Lindemans C, et al. Multicenter experience in hematopoietic stem cell transplantation for serious complications of common variable immunodeficiency. J Allergy Clin Immunol. 2015;135(4):988-997.
34. Shearer WT, Fleisher TA, Buckley RH, et al; Medical Advisory Committee of the Immune Deficiency Foundation. Recommendations for live viral and bacterial vaccines in immunodeficient patients and their close contacts. J Allergy Clin Immunol. 2014;133(4):961-966.
Meningococcal B, C vaccines together lead to adequate immune response
and it demonstrated adequate immune response to MenB, according to Marco Aurelio P. Safadi, MD, of Santa Casa de São Paulo (Brazil) School of Medical Sciences, and his associates.
Of 117 healthy infants who received 4CMenB concomitantly with MenC CRM and 111 who received MenC CRM alone, 99%-100% of infants had serum bactericidal antibody assay using human complement (hSBA) titres 1:8 or greater against MenC after the second vaccination of the primary series (3 months, 5 months, and 12 months of age), and 100% of infants reached these titres after the booster vaccination.
There were more local reactions, such as tenderness, with the combination MenB and MenC vaccinations than with the MenC vaccine alone, as well as systemic adverse reactions such as unusual crying and fever.
Read more in the journal Vaccine (2017 Apr 11;35[16]:2052-9).
and it demonstrated adequate immune response to MenB, according to Marco Aurelio P. Safadi, MD, of Santa Casa de São Paulo (Brazil) School of Medical Sciences, and his associates.
Of 117 healthy infants who received 4CMenB concomitantly with MenC CRM and 111 who received MenC CRM alone, 99%-100% of infants had serum bactericidal antibody assay using human complement (hSBA) titres 1:8 or greater against MenC after the second vaccination of the primary series (3 months, 5 months, and 12 months of age), and 100% of infants reached these titres after the booster vaccination.
There were more local reactions, such as tenderness, with the combination MenB and MenC vaccinations than with the MenC vaccine alone, as well as systemic adverse reactions such as unusual crying and fever.
Read more in the journal Vaccine (2017 Apr 11;35[16]:2052-9).
and it demonstrated adequate immune response to MenB, according to Marco Aurelio P. Safadi, MD, of Santa Casa de São Paulo (Brazil) School of Medical Sciences, and his associates.
Of 117 healthy infants who received 4CMenB concomitantly with MenC CRM and 111 who received MenC CRM alone, 99%-100% of infants had serum bactericidal antibody assay using human complement (hSBA) titres 1:8 or greater against MenC after the second vaccination of the primary series (3 months, 5 months, and 12 months of age), and 100% of infants reached these titres after the booster vaccination.
There were more local reactions, such as tenderness, with the combination MenB and MenC vaccinations than with the MenC vaccine alone, as well as systemic adverse reactions such as unusual crying and fever.
Read more in the journal Vaccine (2017 Apr 11;35[16]:2052-9).
FROM VACCINE
High allele level linked to lamotrigine-induced SCAR
, reported Byung-Keun Kim, MD, of Seoul National University and associates.
In a study of 18 Korean patients with lamotrigine-induced SCAR, a control group of Korean lamotrigine-tolerant patients, and a control group of the general Korean population, the frequency of the HLA-A*31:01 allele was significantly higher in the lamotrigine-induced SCAR patients than in the lamotrigine-tolerant patients (odds ratio, 11.43; P = .0037) or the other control group (OR, 7.27; P = .00034).
High levels of the HLA-A*31:01 allele also have been reported in Korean patients with carbamazepine-induced SCAR, suggesting an association with the HLA allele and drug-induced SCAR that is specific to ethnicity.
That idea is supported by reports that the HLA-B*15:02 allele is a well-known risk allele of carbamazepine-induced SCAR in Han Chinese and Southeast Asians and that other HLA alleles have been significantly associated with SCAR only with patients of European ancestry or only with patients of Mestizo Mexican ancestry, Dr. Kim and associates said.
The SCAR in this study were Stevens-Johnson syndrome, toxic epidermal necrolysis, and drug rash with eosinophilia and systemic syndrome, also known as DRESS.
Read more in the Annals of Allergy, Asthma & Immunology (2017 May;118[5]:629-30).
, reported Byung-Keun Kim, MD, of Seoul National University and associates.
In a study of 18 Korean patients with lamotrigine-induced SCAR, a control group of Korean lamotrigine-tolerant patients, and a control group of the general Korean population, the frequency of the HLA-A*31:01 allele was significantly higher in the lamotrigine-induced SCAR patients than in the lamotrigine-tolerant patients (odds ratio, 11.43; P = .0037) or the other control group (OR, 7.27; P = .00034).
High levels of the HLA-A*31:01 allele also have been reported in Korean patients with carbamazepine-induced SCAR, suggesting an association with the HLA allele and drug-induced SCAR that is specific to ethnicity.
That idea is supported by reports that the HLA-B*15:02 allele is a well-known risk allele of carbamazepine-induced SCAR in Han Chinese and Southeast Asians and that other HLA alleles have been significantly associated with SCAR only with patients of European ancestry or only with patients of Mestizo Mexican ancestry, Dr. Kim and associates said.
The SCAR in this study were Stevens-Johnson syndrome, toxic epidermal necrolysis, and drug rash with eosinophilia and systemic syndrome, also known as DRESS.
Read more in the Annals of Allergy, Asthma & Immunology (2017 May;118[5]:629-30).
, reported Byung-Keun Kim, MD, of Seoul National University and associates.
In a study of 18 Korean patients with lamotrigine-induced SCAR, a control group of Korean lamotrigine-tolerant patients, and a control group of the general Korean population, the frequency of the HLA-A*31:01 allele was significantly higher in the lamotrigine-induced SCAR patients than in the lamotrigine-tolerant patients (odds ratio, 11.43; P = .0037) or the other control group (OR, 7.27; P = .00034).
High levels of the HLA-A*31:01 allele also have been reported in Korean patients with carbamazepine-induced SCAR, suggesting an association with the HLA allele and drug-induced SCAR that is specific to ethnicity.
That idea is supported by reports that the HLA-B*15:02 allele is a well-known risk allele of carbamazepine-induced SCAR in Han Chinese and Southeast Asians and that other HLA alleles have been significantly associated with SCAR only with patients of European ancestry or only with patients of Mestizo Mexican ancestry, Dr. Kim and associates said.
The SCAR in this study were Stevens-Johnson syndrome, toxic epidermal necrolysis, and drug rash with eosinophilia and systemic syndrome, also known as DRESS.
Read more in the Annals of Allergy, Asthma & Immunology (2017 May;118[5]:629-30).
FROM THE ANNALS OF ALLERGY, ASTHMA & IMMUNOLOGY
Lower-dose aluminum hydroxide–adjuvanted polio vaccine noninferior to standard IPV
Aluminum hydroxide–adjuvanted vaccines with reduced doses of inactivated polio vaccine (IPV-Al) were noninferior to standard inactivated poliovirus vaccine (IPV) in a large trial in the Dominican Republic, said Luis Rivera, MD, of the Hospital Maternidad Nuestra Señora de la Altagracia, Santo Domingo, Dominican Republic, and his associates.
If these findings are replicated in phase III trials and regulatory approval follows, such vaccines could be low-cost replacements for standard IPV and oral poliovirus vaccines in low-resource countries, the study authors suggested.
In this phase II, blinded, randomized trial with three investigational IPV-Al groups and one IPV group, the vaccines were given at 6 weeks, 10 weeks, 14 weeks, and 18 weeks to 823 infants who had not previously received any polio vaccination. The three new IPV-Al vaccines all proved to be noninferior to IPV for poliovirus types 1, 2, and 3.
For 1/10 IPV-Al, the seroconversion rates for the different poliovirus types were 98.5% (type 1), 94.6% (type 2), and 99.5% (type 3), compared with 100% (type 1), 98.5% (type 2), and 100% (type 3) for IPV.
This and the results from other studies “have paved the way for further clinical investigations of IPV-Al in phase III trials,” Dr. Rivera and his associates wrote.
“The low frequency of adverse events in this phase II trial suggests that a safety evaluation is not necessarily justified,” they concluded.
The Bill & Melinda Gates Foundation funded the study. The authors had no disclosures.
Read more in the Lancet Infectious Diseases (2017 Apr 25. doi: 10.1016/S1473-3099(17)30177-9).
Aluminum hydroxide–adjuvanted vaccines with reduced doses of inactivated polio vaccine (IPV-Al) were noninferior to standard inactivated poliovirus vaccine (IPV) in a large trial in the Dominican Republic, said Luis Rivera, MD, of the Hospital Maternidad Nuestra Señora de la Altagracia, Santo Domingo, Dominican Republic, and his associates.
If these findings are replicated in phase III trials and regulatory approval follows, such vaccines could be low-cost replacements for standard IPV and oral poliovirus vaccines in low-resource countries, the study authors suggested.
In this phase II, blinded, randomized trial with three investigational IPV-Al groups and one IPV group, the vaccines were given at 6 weeks, 10 weeks, 14 weeks, and 18 weeks to 823 infants who had not previously received any polio vaccination. The three new IPV-Al vaccines all proved to be noninferior to IPV for poliovirus types 1, 2, and 3.
For 1/10 IPV-Al, the seroconversion rates for the different poliovirus types were 98.5% (type 1), 94.6% (type 2), and 99.5% (type 3), compared with 100% (type 1), 98.5% (type 2), and 100% (type 3) for IPV.
This and the results from other studies “have paved the way for further clinical investigations of IPV-Al in phase III trials,” Dr. Rivera and his associates wrote.
“The low frequency of adverse events in this phase II trial suggests that a safety evaluation is not necessarily justified,” they concluded.
The Bill & Melinda Gates Foundation funded the study. The authors had no disclosures.
Read more in the Lancet Infectious Diseases (2017 Apr 25. doi: 10.1016/S1473-3099(17)30177-9).
Aluminum hydroxide–adjuvanted vaccines with reduced doses of inactivated polio vaccine (IPV-Al) were noninferior to standard inactivated poliovirus vaccine (IPV) in a large trial in the Dominican Republic, said Luis Rivera, MD, of the Hospital Maternidad Nuestra Señora de la Altagracia, Santo Domingo, Dominican Republic, and his associates.
If these findings are replicated in phase III trials and regulatory approval follows, such vaccines could be low-cost replacements for standard IPV and oral poliovirus vaccines in low-resource countries, the study authors suggested.
In this phase II, blinded, randomized trial with three investigational IPV-Al groups and one IPV group, the vaccines were given at 6 weeks, 10 weeks, 14 weeks, and 18 weeks to 823 infants who had not previously received any polio vaccination. The three new IPV-Al vaccines all proved to be noninferior to IPV for poliovirus types 1, 2, and 3.
For 1/10 IPV-Al, the seroconversion rates for the different poliovirus types were 98.5% (type 1), 94.6% (type 2), and 99.5% (type 3), compared with 100% (type 1), 98.5% (type 2), and 100% (type 3) for IPV.
This and the results from other studies “have paved the way for further clinical investigations of IPV-Al in phase III trials,” Dr. Rivera and his associates wrote.
“The low frequency of adverse events in this phase II trial suggests that a safety evaluation is not necessarily justified,” they concluded.
The Bill & Melinda Gates Foundation funded the study. The authors had no disclosures.
Read more in the Lancet Infectious Diseases (2017 Apr 25. doi: 10.1016/S1473-3099(17)30177-9).
FROM THE LANCET INFECTIOUS DISEASES
Adalimumab is good first-line anti-TNF therapy for pediatric Crohn’s disease
Adalimumab (ADA) as a first-line anti–tumor necrosis factor therapy induced and maintained clinical remission in children with Crohn’s disease, said Víctor Manuel Navas-López, MD, PhD, of the Hospital Materno Infantil, Málaga, Spain, and his associates.
Infliximab is the usual first-line anti–tumor necrosis factor treatment given to children with Crohn’s disease, with ADA used in patients who don’t respond or who develop tolerance to infliximab.
Dose escalation was necessary for 26% of the 62 patients. Thirty-nine percent of patients had growth retardation.
“ADA treatment significantly improved z-score growth rate in children with Crohn’s disease, especially in those with severe growth failure at baseline,” the researchers said. Only 13% of patients reported adverse events, none of them severe.
Read more in the Anales de Pediatría (2017 Apr 14. doi: 10.1016/j.anpedi.2017.01.013).
Adalimumab (ADA) as a first-line anti–tumor necrosis factor therapy induced and maintained clinical remission in children with Crohn’s disease, said Víctor Manuel Navas-López, MD, PhD, of the Hospital Materno Infantil, Málaga, Spain, and his associates.
Infliximab is the usual first-line anti–tumor necrosis factor treatment given to children with Crohn’s disease, with ADA used in patients who don’t respond or who develop tolerance to infliximab.
Dose escalation was necessary for 26% of the 62 patients. Thirty-nine percent of patients had growth retardation.
“ADA treatment significantly improved z-score growth rate in children with Crohn’s disease, especially in those with severe growth failure at baseline,” the researchers said. Only 13% of patients reported adverse events, none of them severe.
Read more in the Anales de Pediatría (2017 Apr 14. doi: 10.1016/j.anpedi.2017.01.013).
Adalimumab (ADA) as a first-line anti–tumor necrosis factor therapy induced and maintained clinical remission in children with Crohn’s disease, said Víctor Manuel Navas-López, MD, PhD, of the Hospital Materno Infantil, Málaga, Spain, and his associates.
Infliximab is the usual first-line anti–tumor necrosis factor treatment given to children with Crohn’s disease, with ADA used in patients who don’t respond or who develop tolerance to infliximab.
Dose escalation was necessary for 26% of the 62 patients. Thirty-nine percent of patients had growth retardation.
“ADA treatment significantly improved z-score growth rate in children with Crohn’s disease, especially in those with severe growth failure at baseline,” the researchers said. Only 13% of patients reported adverse events, none of them severe.
Read more in the Anales de Pediatría (2017 Apr 14. doi: 10.1016/j.anpedi.2017.01.013).
FROM ANALES DE PEDIATRIA
HMGB1 might be new biomarker of celiac disease in children
, said Sara Manti, MD, of the unit of pediatric genetics and immunology at the University of Messina, Italy, and her associates.
Serum HMGB1 levels were significantly higher in 49 children with celiac disease, compared with 44 healthy children in the control group (16.4 ng/mL vs. 6.23 ng/mL; P less than .001). Children with typical form celiac disease had significantly higher serum HMGB1 levels (22.03 ng/mL) than both children with atypical form (14.83 ng/mL) and silent form celiac disease (12.3 ng/mL). There was no statistically significant difference in serum HMGB1 levels between children with atypical form and silent form celiac disease.
Higher serum HMGB1 levels were correlated with severity of Marsh-Oberhüber classification.
These data, which need to be confirmed in further studies, suggest that HMGB1 is upregulated and linked to the severity of histologic damage in celiac disease. If other studies confirm these findings, it could be hypothesized that “asymptomatic children only with positive familial history and abnormal serum anti–tTG-IgA levels as well as normal serum HMGB1 levels need not be subjected to endoscopy to rule out the CD diagnosis,” the investigators said.
Perhaps, “neutralizing HMGB1 activity might be identified as a potential therapeutic target,” Dr. Manti and her associates noted.
Read more in the journal Nutrition (2017 May;37:18-21).
, said Sara Manti, MD, of the unit of pediatric genetics and immunology at the University of Messina, Italy, and her associates.
Serum HMGB1 levels were significantly higher in 49 children with celiac disease, compared with 44 healthy children in the control group (16.4 ng/mL vs. 6.23 ng/mL; P less than .001). Children with typical form celiac disease had significantly higher serum HMGB1 levels (22.03 ng/mL) than both children with atypical form (14.83 ng/mL) and silent form celiac disease (12.3 ng/mL). There was no statistically significant difference in serum HMGB1 levels between children with atypical form and silent form celiac disease.
Higher serum HMGB1 levels were correlated with severity of Marsh-Oberhüber classification.
These data, which need to be confirmed in further studies, suggest that HMGB1 is upregulated and linked to the severity of histologic damage in celiac disease. If other studies confirm these findings, it could be hypothesized that “asymptomatic children only with positive familial history and abnormal serum anti–tTG-IgA levels as well as normal serum HMGB1 levels need not be subjected to endoscopy to rule out the CD diagnosis,” the investigators said.
Perhaps, “neutralizing HMGB1 activity might be identified as a potential therapeutic target,” Dr. Manti and her associates noted.
Read more in the journal Nutrition (2017 May;37:18-21).
, said Sara Manti, MD, of the unit of pediatric genetics and immunology at the University of Messina, Italy, and her associates.
Serum HMGB1 levels were significantly higher in 49 children with celiac disease, compared with 44 healthy children in the control group (16.4 ng/mL vs. 6.23 ng/mL; P less than .001). Children with typical form celiac disease had significantly higher serum HMGB1 levels (22.03 ng/mL) than both children with atypical form (14.83 ng/mL) and silent form celiac disease (12.3 ng/mL). There was no statistically significant difference in serum HMGB1 levels between children with atypical form and silent form celiac disease.
Higher serum HMGB1 levels were correlated with severity of Marsh-Oberhüber classification.
These data, which need to be confirmed in further studies, suggest that HMGB1 is upregulated and linked to the severity of histologic damage in celiac disease. If other studies confirm these findings, it could be hypothesized that “asymptomatic children only with positive familial history and abnormal serum anti–tTG-IgA levels as well as normal serum HMGB1 levels need not be subjected to endoscopy to rule out the CD diagnosis,” the investigators said.
Perhaps, “neutralizing HMGB1 activity might be identified as a potential therapeutic target,” Dr. Manti and her associates noted.
Read more in the journal Nutrition (2017 May;37:18-21).
FROM NUTRITION
Flu shots may spark immune adverse events in PD-1 blockade for NSCLC
GENEVA – The influenza vaccine may interact with immune checkpoint inhibitors in patients with lung cancer, results of a small study suggest.
Among 23 patients with non–small cell lung cancer (NSCLC) treated with a drug targeted against programmed death-1 (PD-1), the seasonal flu vaccine appeared to produce good serologic protection against infection, but at the possible cost of an increase in the rate of immune-related adverse events (IrAE), reported Sacha Rothschild, MD, PhD, of University Hospital Basel (Switzerland) at the European Lung Cancer Conference.
Among 23 patients with lung cancer treated with a PD-1 inhibitor, 12 (52.2%) had one or more IrAEs. In contrast, the most frequent IrAE in a key registration trial for nivolumab (Opdivo) was skin rash, which occurred in 9% of patients (N Engl J Med. 2015 Jul 9;373:1627-39).
“It’s a very small study, but it raises some concern that there might be an interaction between the vaccine and PD-1 blockade,” Dr. Rothschild said.
To see whether blocking the PD-1/PD–ligand-1 (PD-L1) axis might induce an overactive immune response, the investigators prospectively studied 23 patients with NSCLC who were undergoing treatment with a PD-1 inhibitor – 22 with nivolumab and 1 with pembrolizumab (Keytruda) – who were also vaccinated with a trivalent influenza vaccine in October or November 2015. They used the partners of the patients, also vaccinated, for an age-matched cohort of healthy controls.
The investigators looked at antibody titers against flu strains covered by the vaccine, measured inflammatory chemokines and assessed the vaccine’s safety and the frequency of IrAEs.
None of the patients came down with the flu during the 2015-2016 season. There were no major differences over time in the generation of antibodies against all three viral strains tested.
However, at both 30 and 60 days after vaccination, a hemagglutination inhibition assay showed slightly elevated antibody titers among patients, compared with controls. Antibody titers against H1N1 virus also appeared to increase somewhat more rapidly among patients than among controls, the authors found.
The patients appeared to tolerate the vaccine well, and no serious adverse events were reported within 30 days of vaccination.
When they looked at the incidence of IrAEs, however, the investigators found that six patients had grade 1 or 2 IrAEs, and six had grade 3 or 4 events.
The events included skin rash and arthritis in three patients each, colitis and encephalitis in two patients each, and hypothyroidism, pneumonitis, and neuropathy in one patient each.
“We looked into inflammatory chemokines to understand if there was a high rate of systemic inflammation, and we didn’t find any differences in this regard. So far, we have no clue about why the immune-related adverse event rate in this group is higher,” Dr. Rothschild said.
Although the sample size was small, the IrAE effect they saw was large enough to warrant concern, and it should be studied in a larger population sample, he said.
Egbert Smit, MD, PhD, of the Netherlands Cancer Institute in Amsterdam, who was not involved in the study, commented that “it shows how much we still have to learn about the optimal use of checkpoint inhibitors in lung cancer patients. The study is important as it is the first to investigate the impact of influenza vaccination in such patients, and there is a hint that we actually put them at increased risk for serious toxicities, including encephalitis. However, until we have data on a larger cohort, preferably in a controlled, prospective study, in my institution, we advocate influenza vaccination irrespective of concurrent treatment with immune-checkpoint inhibitors.”
The study was supported by institutional funding. The investigators and Dr. Smit reported no relevant conflicts of interest.
GENEVA – The influenza vaccine may interact with immune checkpoint inhibitors in patients with lung cancer, results of a small study suggest.
Among 23 patients with non–small cell lung cancer (NSCLC) treated with a drug targeted against programmed death-1 (PD-1), the seasonal flu vaccine appeared to produce good serologic protection against infection, but at the possible cost of an increase in the rate of immune-related adverse events (IrAE), reported Sacha Rothschild, MD, PhD, of University Hospital Basel (Switzerland) at the European Lung Cancer Conference.
Among 23 patients with lung cancer treated with a PD-1 inhibitor, 12 (52.2%) had one or more IrAEs. In contrast, the most frequent IrAE in a key registration trial for nivolumab (Opdivo) was skin rash, which occurred in 9% of patients (N Engl J Med. 2015 Jul 9;373:1627-39).
“It’s a very small study, but it raises some concern that there might be an interaction between the vaccine and PD-1 blockade,” Dr. Rothschild said.
To see whether blocking the PD-1/PD–ligand-1 (PD-L1) axis might induce an overactive immune response, the investigators prospectively studied 23 patients with NSCLC who were undergoing treatment with a PD-1 inhibitor – 22 with nivolumab and 1 with pembrolizumab (Keytruda) – who were also vaccinated with a trivalent influenza vaccine in October or November 2015. They used the partners of the patients, also vaccinated, for an age-matched cohort of healthy controls.
The investigators looked at antibody titers against flu strains covered by the vaccine, measured inflammatory chemokines and assessed the vaccine’s safety and the frequency of IrAEs.
None of the patients came down with the flu during the 2015-2016 season. There were no major differences over time in the generation of antibodies against all three viral strains tested.
However, at both 30 and 60 days after vaccination, a hemagglutination inhibition assay showed slightly elevated antibody titers among patients, compared with controls. Antibody titers against H1N1 virus also appeared to increase somewhat more rapidly among patients than among controls, the authors found.
The patients appeared to tolerate the vaccine well, and no serious adverse events were reported within 30 days of vaccination.
When they looked at the incidence of IrAEs, however, the investigators found that six patients had grade 1 or 2 IrAEs, and six had grade 3 or 4 events.
The events included skin rash and arthritis in three patients each, colitis and encephalitis in two patients each, and hypothyroidism, pneumonitis, and neuropathy in one patient each.
“We looked into inflammatory chemokines to understand if there was a high rate of systemic inflammation, and we didn’t find any differences in this regard. So far, we have no clue about why the immune-related adverse event rate in this group is higher,” Dr. Rothschild said.
Although the sample size was small, the IrAE effect they saw was large enough to warrant concern, and it should be studied in a larger population sample, he said.
Egbert Smit, MD, PhD, of the Netherlands Cancer Institute in Amsterdam, who was not involved in the study, commented that “it shows how much we still have to learn about the optimal use of checkpoint inhibitors in lung cancer patients. The study is important as it is the first to investigate the impact of influenza vaccination in such patients, and there is a hint that we actually put them at increased risk for serious toxicities, including encephalitis. However, until we have data on a larger cohort, preferably in a controlled, prospective study, in my institution, we advocate influenza vaccination irrespective of concurrent treatment with immune-checkpoint inhibitors.”
The study was supported by institutional funding. The investigators and Dr. Smit reported no relevant conflicts of interest.
GENEVA – The influenza vaccine may interact with immune checkpoint inhibitors in patients with lung cancer, results of a small study suggest.
Among 23 patients with non–small cell lung cancer (NSCLC) treated with a drug targeted against programmed death-1 (PD-1), the seasonal flu vaccine appeared to produce good serologic protection against infection, but at the possible cost of an increase in the rate of immune-related adverse events (IrAE), reported Sacha Rothschild, MD, PhD, of University Hospital Basel (Switzerland) at the European Lung Cancer Conference.
Among 23 patients with lung cancer treated with a PD-1 inhibitor, 12 (52.2%) had one or more IrAEs. In contrast, the most frequent IrAE in a key registration trial for nivolumab (Opdivo) was skin rash, which occurred in 9% of patients (N Engl J Med. 2015 Jul 9;373:1627-39).
“It’s a very small study, but it raises some concern that there might be an interaction between the vaccine and PD-1 blockade,” Dr. Rothschild said.
To see whether blocking the PD-1/PD–ligand-1 (PD-L1) axis might induce an overactive immune response, the investigators prospectively studied 23 patients with NSCLC who were undergoing treatment with a PD-1 inhibitor – 22 with nivolumab and 1 with pembrolizumab (Keytruda) – who were also vaccinated with a trivalent influenza vaccine in October or November 2015. They used the partners of the patients, also vaccinated, for an age-matched cohort of healthy controls.
The investigators looked at antibody titers against flu strains covered by the vaccine, measured inflammatory chemokines and assessed the vaccine’s safety and the frequency of IrAEs.
None of the patients came down with the flu during the 2015-2016 season. There were no major differences over time in the generation of antibodies against all three viral strains tested.
However, at both 30 and 60 days after vaccination, a hemagglutination inhibition assay showed slightly elevated antibody titers among patients, compared with controls. Antibody titers against H1N1 virus also appeared to increase somewhat more rapidly among patients than among controls, the authors found.
The patients appeared to tolerate the vaccine well, and no serious adverse events were reported within 30 days of vaccination.
When they looked at the incidence of IrAEs, however, the investigators found that six patients had grade 1 or 2 IrAEs, and six had grade 3 or 4 events.
The events included skin rash and arthritis in three patients each, colitis and encephalitis in two patients each, and hypothyroidism, pneumonitis, and neuropathy in one patient each.
“We looked into inflammatory chemokines to understand if there was a high rate of systemic inflammation, and we didn’t find any differences in this regard. So far, we have no clue about why the immune-related adverse event rate in this group is higher,” Dr. Rothschild said.
Although the sample size was small, the IrAE effect they saw was large enough to warrant concern, and it should be studied in a larger population sample, he said.
Egbert Smit, MD, PhD, of the Netherlands Cancer Institute in Amsterdam, who was not involved in the study, commented that “it shows how much we still have to learn about the optimal use of checkpoint inhibitors in lung cancer patients. The study is important as it is the first to investigate the impact of influenza vaccination in such patients, and there is a hint that we actually put them at increased risk for serious toxicities, including encephalitis. However, until we have data on a larger cohort, preferably in a controlled, prospective study, in my institution, we advocate influenza vaccination irrespective of concurrent treatment with immune-checkpoint inhibitors.”
The study was supported by institutional funding. The investigators and Dr. Smit reported no relevant conflicts of interest.
AT ELCC
Key clinical point:
Major finding: Of 23 patients who were vaccinated, 12 developed IrAEs, including 6 grade 1/2 and 6 grade 3/4 events.
Data source: Prospective study of 23 patients with NSCLC and 23 healthy controls.
Disclosures: The study was supported by institutional funding. The investigators reported no relevant conflicts of interest.