User login
New mRNA Vaccines in Development for Cancer and Infections
Martina Prelog, MD, a pediatric and adolescent medicine specialist at the University Hospital of Würzburg in Germany, reported on the principles, research status, and perspectives for these vaccines at the 25th Travel and Health Forum of the Center for Travel Medicine in Berlin.
To understand the future, the immunologist first examined the past. “The induction of cellular and humoral immune responses by externally injected mRNA was discovered in the 1990s,” she said.
Instability Challenge
Significant hurdles in mRNA vaccinations included the instability of mRNA and the immune system’s ability to identify foreign mRNA as a threat and destroy mRNA fragments. “The breakthrough toward vaccination came through Dr. Katalin Karikó, who, along with Dr. Drew Weissman, both of the University of Pennsylvania School of Medicine, discovered in 2005 that modifications of mRNA (replacing the nucleoside uridine with pseudouridine) enable better stability of mRNA, reduced immunogenicity, and higher translational capacity at the ribosomes,” said Dr. Prelog.
With this discovery, the two researchers paved the way for the development of mRNA vaccines against COVID-19 and other diseases. They were awarded the Nobel Prize in medicine for their discovery last year.
Improved Scalability
“Since 2009, mRNA vaccines have been studied as a treatment option for cancer,” said Dr. Prelog. “Since 2012, they have been studied for the influenza virus and respiratory syncytial virus [RSV].” Consequently, several mRNA vaccines are currently in development or in approval studies. “The mRNA technology offers the advantage of quickly and flexibly responding to new variants of pathogens and the ability to scale up production when there is high demand for a particular vaccine.”
Different forms and designations of mRNA vaccines are used, depending on the application and desired effect, said Dr. Prelog.
In nucleoside-modified mRNA vaccines, modifications in the mRNA sequence enable the mRNA to remain in the body longer and to induce protein synthesis more effectively.
Lipid nanoparticle (LNP)–encapsulated mRNA vaccines protect the coding mRNA sequences against degradation by the body’s enzymes and facilitate the uptake of mRNA into cells, where it then triggers the production of the desired protein. In addition, LNPs are involved in cell stimulation and support the self-adjuvant effect of mRNA vaccines, thus eliminating the need for adjuvants.
Self-amplifying mRNA vaccines include a special mRNA that replicates itself in the cell and contains a sequence for RNA replicase, in addition to the coding sequence for the protein. This composition enables increased production of the target protein without the need for a high amount of external mRNA administration. Such vaccines could trigger a longer and stronger immune response because the immune system has more time to interact with the protein.
Cancer Immunotherapy
Dr. Prelog also discussed personalized vaccines for cancer immunotherapy. Personalized mRNA vaccines are tailored to the patient’s genetic characteristics and antigens. They could be used in cancer immunotherapy to activate the immune system selectively against tumor cells.
Multivalent mRNA vaccines contain mRNA that codes for multiple antigens rather than just one protein to generate an immune response. These vaccines could be particularly useful in fighting pathogens with variable or changing surface structures or in eliciting protection against multiple pathogens simultaneously.
The technology of mRNA-encoded antibodies involves introducing mRNA into the cell, which creates light and heavy chains of antibodies. This step leads to the formation of antibodies targeted against toxins (eg, diphtheria and tetanus), animal venoms, infectious agents, or tumor cells.
Genetic Engineering
Dr. Prelog also reviewed genetic engineering techniques. In regenerative therapy or protein replacement therapy, skin fibroblasts or other cells are transfected with mRNA to enable conversion into induced pluripotent stem cells. This approach avoids the risk for DNA integration into the genome and associated mutation risks.
Another approach is making post-transcriptional modifications through RNA interference. For example, RNA structures can be used to inhibit the translation of disease-causing proteins. This technique is currently being tested against HIV and tumors such as melanoma.
In addition, mRNA technologies can be combined with CRISPR/Cas9 technology (“gene scissors”) to influence the creation of gene products even more precisely. The advantage of this technique is that mRNA is only transiently expressed, thus preventing unwanted side effects. Furthermore, mRNA is translated directly in the cytoplasm, leading to a faster initiation of gene editing.
Of the numerous ongoing clinical mRNA vaccine studies, around 70% focus on infections, about 12% on cancer, and the rest on autoimmune diseases and neurodegenerative disorders, said Dr. Prelog.
Research in Infections
Research in the fields of infectious diseases and oncology is the most advanced: mRNA vaccines against influenza and RSV are already in advanced clinical trials, Dr. Prelog told this news organization.
“Conventional influenza vaccines contain immunogenic surface molecules against hemagglutinin and neuraminidase in various combinations of influenza strains A and B and are produced in egg or cell cultures,” she said. “This is a time-consuming manufacturing process that takes months and, particularly with the egg-based process, bears the risk of changing the vaccine strain.”
“Additionally, influenza viruses undergo antigenic shift and drift through recombination, thus requiring annual adjustments to the vaccines. Thus, these influenza vaccines often lose accuracy in targeting circulating seasonal influenza strains.”
Several mRNA vaccines being tested contain not only coding sequences against hemagglutinin and neuraminidase but also for structural proteins of influenza viruses. “These are more conserved and mutate less easily, meaning they could serve as the basis for universal pandemic influenza vaccines,” said Dr. Prelog.
An advantage of mRNA vaccines, she added, is the strong cellular immune response that they elicit. This response is intended to provide additional protection alongside specific antibodies. An mRNA vaccine with coding sequences for the pre-fusion protein of RSV is in phase 3 trials for approval for vaccination in patients aged 60 years and older. It shows high effectiveness even in older patients and those with comorbidities.
Elaborate Purification Process
Bacterial origin plasmid DNA is used to produce mRNA vaccines. The mRNA vaccines for COVID-19 raised concerns that production-related DNA residues could pose a safety risk and cause autoimmune diseases.
These vaccines “typically undergo a very elaborate purification process,” said Dr. Prelog. “This involves enzymatic digestion with DNase to fragment and deplete plasmid DNA, followed by purification using chromatography columns, so that no safety-relevant DNA fragments should remain afterward.”
Thus, the Paul-Ehrlich-Institut also pointed out the very small, fragmented plasmid DNA residues of bacterial origin in mRNA COVID-19 vaccines pose no risk, unlike residual DNA from animal cell culture might pose in other vaccines.
Prevention and Therapy
In addition to the numerous advantages of mRNA vaccines (such as rapid adaptability to new or mutated pathogens, scalability, rapid production capability, self-adjuvant effect, strong induction of cellular immune responses, and safety), there are also challenges in RNA technology as a preventive and therapeutic measure, according to Dr. Prelog.
“Stability and storability, as well as the costs of new vaccine developments, play a role, as do the long-term effects regarding the persistence of antibody and cellular responses,” she said. The COVID-19 mRNA vaccines, for example, showed a well-maintained cellular immune response despite a tendency toward a rapid decline in humoral immune response.
“The experience with COVID-19 mRNA vaccines and the new vaccine developments based on mRNA technology give hope for an efficient and safe preventive and therapeutic use, particularly in the fields of infectious diseases and oncology,” Dr. Prelog concluded.
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Martina Prelog, MD, a pediatric and adolescent medicine specialist at the University Hospital of Würzburg in Germany, reported on the principles, research status, and perspectives for these vaccines at the 25th Travel and Health Forum of the Center for Travel Medicine in Berlin.
To understand the future, the immunologist first examined the past. “The induction of cellular and humoral immune responses by externally injected mRNA was discovered in the 1990s,” she said.
Instability Challenge
Significant hurdles in mRNA vaccinations included the instability of mRNA and the immune system’s ability to identify foreign mRNA as a threat and destroy mRNA fragments. “The breakthrough toward vaccination came through Dr. Katalin Karikó, who, along with Dr. Drew Weissman, both of the University of Pennsylvania School of Medicine, discovered in 2005 that modifications of mRNA (replacing the nucleoside uridine with pseudouridine) enable better stability of mRNA, reduced immunogenicity, and higher translational capacity at the ribosomes,” said Dr. Prelog.
With this discovery, the two researchers paved the way for the development of mRNA vaccines against COVID-19 and other diseases. They were awarded the Nobel Prize in medicine for their discovery last year.
Improved Scalability
“Since 2009, mRNA vaccines have been studied as a treatment option for cancer,” said Dr. Prelog. “Since 2012, they have been studied for the influenza virus and respiratory syncytial virus [RSV].” Consequently, several mRNA vaccines are currently in development or in approval studies. “The mRNA technology offers the advantage of quickly and flexibly responding to new variants of pathogens and the ability to scale up production when there is high demand for a particular vaccine.”
Different forms and designations of mRNA vaccines are used, depending on the application and desired effect, said Dr. Prelog.
In nucleoside-modified mRNA vaccines, modifications in the mRNA sequence enable the mRNA to remain in the body longer and to induce protein synthesis more effectively.
Lipid nanoparticle (LNP)–encapsulated mRNA vaccines protect the coding mRNA sequences against degradation by the body’s enzymes and facilitate the uptake of mRNA into cells, where it then triggers the production of the desired protein. In addition, LNPs are involved in cell stimulation and support the self-adjuvant effect of mRNA vaccines, thus eliminating the need for adjuvants.
Self-amplifying mRNA vaccines include a special mRNA that replicates itself in the cell and contains a sequence for RNA replicase, in addition to the coding sequence for the protein. This composition enables increased production of the target protein without the need for a high amount of external mRNA administration. Such vaccines could trigger a longer and stronger immune response because the immune system has more time to interact with the protein.
Cancer Immunotherapy
Dr. Prelog also discussed personalized vaccines for cancer immunotherapy. Personalized mRNA vaccines are tailored to the patient’s genetic characteristics and antigens. They could be used in cancer immunotherapy to activate the immune system selectively against tumor cells.
Multivalent mRNA vaccines contain mRNA that codes for multiple antigens rather than just one protein to generate an immune response. These vaccines could be particularly useful in fighting pathogens with variable or changing surface structures or in eliciting protection against multiple pathogens simultaneously.
The technology of mRNA-encoded antibodies involves introducing mRNA into the cell, which creates light and heavy chains of antibodies. This step leads to the formation of antibodies targeted against toxins (eg, diphtheria and tetanus), animal venoms, infectious agents, or tumor cells.
Genetic Engineering
Dr. Prelog also reviewed genetic engineering techniques. In regenerative therapy or protein replacement therapy, skin fibroblasts or other cells are transfected with mRNA to enable conversion into induced pluripotent stem cells. This approach avoids the risk for DNA integration into the genome and associated mutation risks.
Another approach is making post-transcriptional modifications through RNA interference. For example, RNA structures can be used to inhibit the translation of disease-causing proteins. This technique is currently being tested against HIV and tumors such as melanoma.
In addition, mRNA technologies can be combined with CRISPR/Cas9 technology (“gene scissors”) to influence the creation of gene products even more precisely. The advantage of this technique is that mRNA is only transiently expressed, thus preventing unwanted side effects. Furthermore, mRNA is translated directly in the cytoplasm, leading to a faster initiation of gene editing.
Of the numerous ongoing clinical mRNA vaccine studies, around 70% focus on infections, about 12% on cancer, and the rest on autoimmune diseases and neurodegenerative disorders, said Dr. Prelog.
Research in Infections
Research in the fields of infectious diseases and oncology is the most advanced: mRNA vaccines against influenza and RSV are already in advanced clinical trials, Dr. Prelog told this news organization.
“Conventional influenza vaccines contain immunogenic surface molecules against hemagglutinin and neuraminidase in various combinations of influenza strains A and B and are produced in egg or cell cultures,” she said. “This is a time-consuming manufacturing process that takes months and, particularly with the egg-based process, bears the risk of changing the vaccine strain.”
“Additionally, influenza viruses undergo antigenic shift and drift through recombination, thus requiring annual adjustments to the vaccines. Thus, these influenza vaccines often lose accuracy in targeting circulating seasonal influenza strains.”
Several mRNA vaccines being tested contain not only coding sequences against hemagglutinin and neuraminidase but also for structural proteins of influenza viruses. “These are more conserved and mutate less easily, meaning they could serve as the basis for universal pandemic influenza vaccines,” said Dr. Prelog.
An advantage of mRNA vaccines, she added, is the strong cellular immune response that they elicit. This response is intended to provide additional protection alongside specific antibodies. An mRNA vaccine with coding sequences for the pre-fusion protein of RSV is in phase 3 trials for approval for vaccination in patients aged 60 years and older. It shows high effectiveness even in older patients and those with comorbidities.
Elaborate Purification Process
Bacterial origin plasmid DNA is used to produce mRNA vaccines. The mRNA vaccines for COVID-19 raised concerns that production-related DNA residues could pose a safety risk and cause autoimmune diseases.
These vaccines “typically undergo a very elaborate purification process,” said Dr. Prelog. “This involves enzymatic digestion with DNase to fragment and deplete plasmid DNA, followed by purification using chromatography columns, so that no safety-relevant DNA fragments should remain afterward.”
Thus, the Paul-Ehrlich-Institut also pointed out the very small, fragmented plasmid DNA residues of bacterial origin in mRNA COVID-19 vaccines pose no risk, unlike residual DNA from animal cell culture might pose in other vaccines.
Prevention and Therapy
In addition to the numerous advantages of mRNA vaccines (such as rapid adaptability to new or mutated pathogens, scalability, rapid production capability, self-adjuvant effect, strong induction of cellular immune responses, and safety), there are also challenges in RNA technology as a preventive and therapeutic measure, according to Dr. Prelog.
“Stability and storability, as well as the costs of new vaccine developments, play a role, as do the long-term effects regarding the persistence of antibody and cellular responses,” she said. The COVID-19 mRNA vaccines, for example, showed a well-maintained cellular immune response despite a tendency toward a rapid decline in humoral immune response.
“The experience with COVID-19 mRNA vaccines and the new vaccine developments based on mRNA technology give hope for an efficient and safe preventive and therapeutic use, particularly in the fields of infectious diseases and oncology,” Dr. Prelog concluded.
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Martina Prelog, MD, a pediatric and adolescent medicine specialist at the University Hospital of Würzburg in Germany, reported on the principles, research status, and perspectives for these vaccines at the 25th Travel and Health Forum of the Center for Travel Medicine in Berlin.
To understand the future, the immunologist first examined the past. “The induction of cellular and humoral immune responses by externally injected mRNA was discovered in the 1990s,” she said.
Instability Challenge
Significant hurdles in mRNA vaccinations included the instability of mRNA and the immune system’s ability to identify foreign mRNA as a threat and destroy mRNA fragments. “The breakthrough toward vaccination came through Dr. Katalin Karikó, who, along with Dr. Drew Weissman, both of the University of Pennsylvania School of Medicine, discovered in 2005 that modifications of mRNA (replacing the nucleoside uridine with pseudouridine) enable better stability of mRNA, reduced immunogenicity, and higher translational capacity at the ribosomes,” said Dr. Prelog.
With this discovery, the two researchers paved the way for the development of mRNA vaccines against COVID-19 and other diseases. They were awarded the Nobel Prize in medicine for their discovery last year.
Improved Scalability
“Since 2009, mRNA vaccines have been studied as a treatment option for cancer,” said Dr. Prelog. “Since 2012, they have been studied for the influenza virus and respiratory syncytial virus [RSV].” Consequently, several mRNA vaccines are currently in development or in approval studies. “The mRNA technology offers the advantage of quickly and flexibly responding to new variants of pathogens and the ability to scale up production when there is high demand for a particular vaccine.”
Different forms and designations of mRNA vaccines are used, depending on the application and desired effect, said Dr. Prelog.
In nucleoside-modified mRNA vaccines, modifications in the mRNA sequence enable the mRNA to remain in the body longer and to induce protein synthesis more effectively.
Lipid nanoparticle (LNP)–encapsulated mRNA vaccines protect the coding mRNA sequences against degradation by the body’s enzymes and facilitate the uptake of mRNA into cells, where it then triggers the production of the desired protein. In addition, LNPs are involved in cell stimulation and support the self-adjuvant effect of mRNA vaccines, thus eliminating the need for adjuvants.
Self-amplifying mRNA vaccines include a special mRNA that replicates itself in the cell and contains a sequence for RNA replicase, in addition to the coding sequence for the protein. This composition enables increased production of the target protein without the need for a high amount of external mRNA administration. Such vaccines could trigger a longer and stronger immune response because the immune system has more time to interact with the protein.
Cancer Immunotherapy
Dr. Prelog also discussed personalized vaccines for cancer immunotherapy. Personalized mRNA vaccines are tailored to the patient’s genetic characteristics and antigens. They could be used in cancer immunotherapy to activate the immune system selectively against tumor cells.
Multivalent mRNA vaccines contain mRNA that codes for multiple antigens rather than just one protein to generate an immune response. These vaccines could be particularly useful in fighting pathogens with variable or changing surface structures or in eliciting protection against multiple pathogens simultaneously.
The technology of mRNA-encoded antibodies involves introducing mRNA into the cell, which creates light and heavy chains of antibodies. This step leads to the formation of antibodies targeted against toxins (eg, diphtheria and tetanus), animal venoms, infectious agents, or tumor cells.
Genetic Engineering
Dr. Prelog also reviewed genetic engineering techniques. In regenerative therapy or protein replacement therapy, skin fibroblasts or other cells are transfected with mRNA to enable conversion into induced pluripotent stem cells. This approach avoids the risk for DNA integration into the genome and associated mutation risks.
Another approach is making post-transcriptional modifications through RNA interference. For example, RNA structures can be used to inhibit the translation of disease-causing proteins. This technique is currently being tested against HIV and tumors such as melanoma.
In addition, mRNA technologies can be combined with CRISPR/Cas9 technology (“gene scissors”) to influence the creation of gene products even more precisely. The advantage of this technique is that mRNA is only transiently expressed, thus preventing unwanted side effects. Furthermore, mRNA is translated directly in the cytoplasm, leading to a faster initiation of gene editing.
Of the numerous ongoing clinical mRNA vaccine studies, around 70% focus on infections, about 12% on cancer, and the rest on autoimmune diseases and neurodegenerative disorders, said Dr. Prelog.
Research in Infections
Research in the fields of infectious diseases and oncology is the most advanced: mRNA vaccines against influenza and RSV are already in advanced clinical trials, Dr. Prelog told this news organization.
“Conventional influenza vaccines contain immunogenic surface molecules against hemagglutinin and neuraminidase in various combinations of influenza strains A and B and are produced in egg or cell cultures,” she said. “This is a time-consuming manufacturing process that takes months and, particularly with the egg-based process, bears the risk of changing the vaccine strain.”
“Additionally, influenza viruses undergo antigenic shift and drift through recombination, thus requiring annual adjustments to the vaccines. Thus, these influenza vaccines often lose accuracy in targeting circulating seasonal influenza strains.”
Several mRNA vaccines being tested contain not only coding sequences against hemagglutinin and neuraminidase but also for structural proteins of influenza viruses. “These are more conserved and mutate less easily, meaning they could serve as the basis for universal pandemic influenza vaccines,” said Dr. Prelog.
An advantage of mRNA vaccines, she added, is the strong cellular immune response that they elicit. This response is intended to provide additional protection alongside specific antibodies. An mRNA vaccine with coding sequences for the pre-fusion protein of RSV is in phase 3 trials for approval for vaccination in patients aged 60 years and older. It shows high effectiveness even in older patients and those with comorbidities.
Elaborate Purification Process
Bacterial origin plasmid DNA is used to produce mRNA vaccines. The mRNA vaccines for COVID-19 raised concerns that production-related DNA residues could pose a safety risk and cause autoimmune diseases.
These vaccines “typically undergo a very elaborate purification process,” said Dr. Prelog. “This involves enzymatic digestion with DNase to fragment and deplete plasmid DNA, followed by purification using chromatography columns, so that no safety-relevant DNA fragments should remain afterward.”
Thus, the Paul-Ehrlich-Institut also pointed out the very small, fragmented plasmid DNA residues of bacterial origin in mRNA COVID-19 vaccines pose no risk, unlike residual DNA from animal cell culture might pose in other vaccines.
Prevention and Therapy
In addition to the numerous advantages of mRNA vaccines (such as rapid adaptability to new or mutated pathogens, scalability, rapid production capability, self-adjuvant effect, strong induction of cellular immune responses, and safety), there are also challenges in RNA technology as a preventive and therapeutic measure, according to Dr. Prelog.
“Stability and storability, as well as the costs of new vaccine developments, play a role, as do the long-term effects regarding the persistence of antibody and cellular responses,” she said. The COVID-19 mRNA vaccines, for example, showed a well-maintained cellular immune response despite a tendency toward a rapid decline in humoral immune response.
“The experience with COVID-19 mRNA vaccines and the new vaccine developments based on mRNA technology give hope for an efficient and safe preventive and therapeutic use, particularly in the fields of infectious diseases and oncology,” Dr. Prelog concluded.
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
COVID Vaccines and New-Onset Seizures: New Data
There is no association between the SARS-CoV-2 vaccine and the risk for new-onset seizure, data from a new meta-analysis of six randomized, placebo-controlled clinical trials (RCTs) showed.
Results of the pooled analysis that included 63,500 individuals vaccinated with SARS-CoV-2 and 55,000 who received a placebo vaccine showed there was no significant difference between the two groups with respect to new-onset seizures at 28- or 43-day follow-up.
Regarding new-onset seizures in the general population, there was no statistically significant difference in risk for seizure incidence among vaccinated individuals vs placebo recipients, according to our meta-analysis, wrote the investigators, led by Ali Rafati, MD, MPH, Iran University of Medical Sciences in Tehran.
The findings were published online in JAMA Neurology.
Mixed Results
Results from previous research have been mixed regarding the link between the SARS-CoV-2 vaccination and new-onset seizures, with some showing an association.
To learn more about the possible association between the vaccines and new-onset seizures, the researchers conducted a literature review and identified six RCTs that measured adverse events following SARS-CoV-2 vaccinations (including messenger RNA, viral vector, and inactivated virus) vs placebo or other vaccines.
While five of the studies defined new-onset seizures according to the Medical Dictionary for Regulatory Activities, trial investigators in the sixth RCT assessed and determined new-onset seizures in participants.
Participants received two vaccinations 28 days apart in five RCTs and only one vaccine in the sixth trial.
The research team searched the data for new-onset seizure in the 28 days following one or both COVID vaccinations.
No Link Found
After comparing the incidence of new-onset seizure between the 63,500 vaccine (nine new-onset seizures, 0.014%) and 55,000 placebo recipients (one new-onset seizure, 0.002%), investigators found no significant difference between the two groups (odds ratio [OR], 2.70; 95% CI, 0.76-9.57; P = .12)
Investigators also sliced the data several ways to see if it would yield different results. When they analyzed data by vaccine platform (viral vector) and age group (children), they didn’t observe significant differences in new-onset data.
The researchers also searched for data beyond the month following the injection to encompass the entire blinded phase, so they analyzed the results of three RCTs that reported adverse events up to 162 days after the vaccine.
After pooling the results from the three studies, investigators found no statistical difference between the vaccine and placebo groups in terms of the new-onset seizure (OR, 2.31; 95% CI, 0.86%-3.23; P > .99)
Study limitations included the missing information on vaccine doses or risk factors for the development of seizures. Also, the RCTs included in the meta-analysis were conducted at different times, so the SARS-CoV-2 vaccines may have differed in their composition and efficacy.
“The global vaccination drive against SARS-CoV-2 has been a monumental effort in combating the pandemic. SARS-CoV-2 vaccinations that are now available appear safe and appropriate,” the authors wrote.
There were no study funding sources or disclosures reported.
A version of this article appeared on Medscape.com.
There is no association between the SARS-CoV-2 vaccine and the risk for new-onset seizure, data from a new meta-analysis of six randomized, placebo-controlled clinical trials (RCTs) showed.
Results of the pooled analysis that included 63,500 individuals vaccinated with SARS-CoV-2 and 55,000 who received a placebo vaccine showed there was no significant difference between the two groups with respect to new-onset seizures at 28- or 43-day follow-up.
Regarding new-onset seizures in the general population, there was no statistically significant difference in risk for seizure incidence among vaccinated individuals vs placebo recipients, according to our meta-analysis, wrote the investigators, led by Ali Rafati, MD, MPH, Iran University of Medical Sciences in Tehran.
The findings were published online in JAMA Neurology.
Mixed Results
Results from previous research have been mixed regarding the link between the SARS-CoV-2 vaccination and new-onset seizures, with some showing an association.
To learn more about the possible association between the vaccines and new-onset seizures, the researchers conducted a literature review and identified six RCTs that measured adverse events following SARS-CoV-2 vaccinations (including messenger RNA, viral vector, and inactivated virus) vs placebo or other vaccines.
While five of the studies defined new-onset seizures according to the Medical Dictionary for Regulatory Activities, trial investigators in the sixth RCT assessed and determined new-onset seizures in participants.
Participants received two vaccinations 28 days apart in five RCTs and only one vaccine in the sixth trial.
The research team searched the data for new-onset seizure in the 28 days following one or both COVID vaccinations.
No Link Found
After comparing the incidence of new-onset seizure between the 63,500 vaccine (nine new-onset seizures, 0.014%) and 55,000 placebo recipients (one new-onset seizure, 0.002%), investigators found no significant difference between the two groups (odds ratio [OR], 2.70; 95% CI, 0.76-9.57; P = .12)
Investigators also sliced the data several ways to see if it would yield different results. When they analyzed data by vaccine platform (viral vector) and age group (children), they didn’t observe significant differences in new-onset data.
The researchers also searched for data beyond the month following the injection to encompass the entire blinded phase, so they analyzed the results of three RCTs that reported adverse events up to 162 days after the vaccine.
After pooling the results from the three studies, investigators found no statistical difference between the vaccine and placebo groups in terms of the new-onset seizure (OR, 2.31; 95% CI, 0.86%-3.23; P > .99)
Study limitations included the missing information on vaccine doses or risk factors for the development of seizures. Also, the RCTs included in the meta-analysis were conducted at different times, so the SARS-CoV-2 vaccines may have differed in their composition and efficacy.
“The global vaccination drive against SARS-CoV-2 has been a monumental effort in combating the pandemic. SARS-CoV-2 vaccinations that are now available appear safe and appropriate,” the authors wrote.
There were no study funding sources or disclosures reported.
A version of this article appeared on Medscape.com.
There is no association between the SARS-CoV-2 vaccine and the risk for new-onset seizure, data from a new meta-analysis of six randomized, placebo-controlled clinical trials (RCTs) showed.
Results of the pooled analysis that included 63,500 individuals vaccinated with SARS-CoV-2 and 55,000 who received a placebo vaccine showed there was no significant difference between the two groups with respect to new-onset seizures at 28- or 43-day follow-up.
Regarding new-onset seizures in the general population, there was no statistically significant difference in risk for seizure incidence among vaccinated individuals vs placebo recipients, according to our meta-analysis, wrote the investigators, led by Ali Rafati, MD, MPH, Iran University of Medical Sciences in Tehran.
The findings were published online in JAMA Neurology.
Mixed Results
Results from previous research have been mixed regarding the link between the SARS-CoV-2 vaccination and new-onset seizures, with some showing an association.
To learn more about the possible association between the vaccines and new-onset seizures, the researchers conducted a literature review and identified six RCTs that measured adverse events following SARS-CoV-2 vaccinations (including messenger RNA, viral vector, and inactivated virus) vs placebo or other vaccines.
While five of the studies defined new-onset seizures according to the Medical Dictionary for Regulatory Activities, trial investigators in the sixth RCT assessed and determined new-onset seizures in participants.
Participants received two vaccinations 28 days apart in five RCTs and only one vaccine in the sixth trial.
The research team searched the data for new-onset seizure in the 28 days following one or both COVID vaccinations.
No Link Found
After comparing the incidence of new-onset seizure between the 63,500 vaccine (nine new-onset seizures, 0.014%) and 55,000 placebo recipients (one new-onset seizure, 0.002%), investigators found no significant difference between the two groups (odds ratio [OR], 2.70; 95% CI, 0.76-9.57; P = .12)
Investigators also sliced the data several ways to see if it would yield different results. When they analyzed data by vaccine platform (viral vector) and age group (children), they didn’t observe significant differences in new-onset data.
The researchers also searched for data beyond the month following the injection to encompass the entire blinded phase, so they analyzed the results of three RCTs that reported adverse events up to 162 days after the vaccine.
After pooling the results from the three studies, investigators found no statistical difference between the vaccine and placebo groups in terms of the new-onset seizure (OR, 2.31; 95% CI, 0.86%-3.23; P > .99)
Study limitations included the missing information on vaccine doses or risk factors for the development of seizures. Also, the RCTs included in the meta-analysis were conducted at different times, so the SARS-CoV-2 vaccines may have differed in their composition and efficacy.
“The global vaccination drive against SARS-CoV-2 has been a monumental effort in combating the pandemic. SARS-CoV-2 vaccinations that are now available appear safe and appropriate,” the authors wrote.
There were no study funding sources or disclosures reported.
A version of this article appeared on Medscape.com.
Early Treatment of Lyme Disease Prompted by Histopathologic Analysis of the Abdomen of an Engorged Tick
To the Editor:
Lyme disease is caused by spirochetes of the Borrelia burgdorferi sensu lato species complex and transmitted to humans by the bite of the Ixodes scapularis tick. It was first classified as a nationally notifiable disease in 1991, and the incidence has risen remarkably since then.1 More than 63,000 cases are reported annually to the Centers for Disease Control and Prevention; however, this number reflects severe underreporting, as the true incidence of the disease is projected to be closer to 476,000 cases per year.2 Additionally, 95% of US cases occur in the Northeast and upper Midwest.3 Given the pervasiveness of Lyme disease, early and reliable diagnostic methodology is critical, especially in cases in which the timeline of inoculation is unclear. We present a case of Lyme disease that was discovered during a routine dermatologic visit.
A 77-year-old White man with no relevant medical history presented to a dermatology clinic in west-central Virginia for a routine skin check. Physical examination revealed a well-appearing patient without overt skin abnormalities. However, on closer evaluation, a 0.2×0.1-cm engorged black I scapularis tick was visualized on the left lateral upper back. There was a surrounding zone of erythema that measured less than the 5-cm-diameter criterion for erythema migrans.1
Upon questioning, the patient reported that he was unaware of the tick and could not provide a timeline for inoculation. To ensure proper treatment, the tick was removed in the office and a specimen was sent for histopathology. The arthropod was formalin fixed and paraffin embedded, and it was examined using hematoxylin and eosin and Warthin-Starry stains. Histopathology of the specimen revealed a blood-engorged arthropod. Warthin-Starry stain of the abdomen of the tick highlighted tiny strandlike spirochetes within the gut that were compatible with B burgdorferi (Figure). This finding prompted treatment with a 3-week course of doxycycline. Following treatment, erythema resolved. The patient experienced no sequelae.
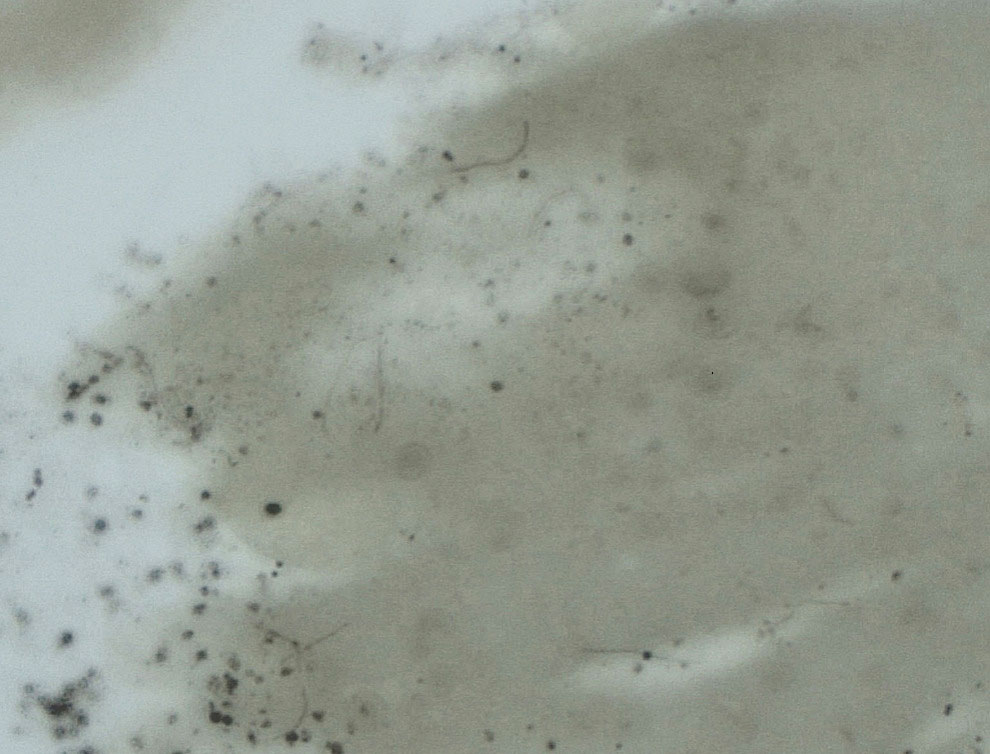
Lyme disease can cause a range of serious complications if left untreated, including arthritis, neurologic deficits, and heart block. During the early stages of disease, the sensitivity and specificity of diagnostic methods such as serologic testing are limited.4 The gold standard for the diagnosis of Lyme disease comprises culture and subsequent confirmation by polymerase chain reaction.1 However, cultivation of B burgdorferi is challenging.5 The Centers for Disease Control and Prevention recommends 2-tiered serologic antibody analysis, which has 27% sensitivity during the first week of cutaneous symptoms, and involves an enzyme-linked immunoassay followed by reflexive immunoblotting for positive or indeterminate cases.2,6 The precision of this method is limited by several variables; for example, seroconversion fails to occur in approximately 40% of cases, even after proven exposure to the spirochete.7 Furthermore, the sensitivity of the test is particularly low during the first 4 to 6 weeks of infection—before the body mounts a proper immune response; fewer than 50% of patients exhibit a positive response to the test at initial presentation.3
Clinical diagnosis of Lyme disease is possible, though the pathognomonic erythema migrans rash can be delayed for as long as 30 days and remains absent in 20% to 30% of patients.1 Prophylactic treatment can be offered to individuals who reside in a hyperendemic area and have a rash or have had an engorged Ixodes tick attached for longer than 36 hours.8
More definitive techniques for early diagnosis are needed to enable selective and accurate treatment. The standard of care for Lyme disease includes a 10-day course of doxycycline or a 14-day course of cefuroxime axetil or amoxicillin.9 Many patients tolerate treatment and achieve resolution of disease, but antibiotics are not benign, as some patients experience drug-related adverse effects such as photosensitivity, urticaria, diarrhea, nausea, vomiting, esophagitis, hepatotoxicity, and the Jarisch-Herxheimer reaction (fever, chills, rigors, nausea and vomiting, headache, tachycardia, hypotension, hyperventilation, flushing, myalgia, and exacerbation of lesions).10,11 In a group of 123 patients with Lyme disease, 30% treated with cefuroxime axetil and 32% treated with doxycycline had 1 or more drug-related adverse events.10 Additionally, avoidable antibiotic use is associated with increasing antibiotic resistance.12 Improved diagnostic accuracy would prevent unnecessary treatment. Galan and colleagues7 reported that Warthin-Starry staining of prepared sections of the abdomen of a tick allowed for detection of B burgdorferi with a sensitivity of 71% and specificity of 83%. This technique did not delay the final biopsy report and may be a promising adjunct to the diagnosis of early Lyme disease.7
Anecdotally, many patients who present with an attached and engorged tick are unaware of the timeline of their exposure. Histologic analysis of a removed tick could aid in early clinical decision-making—ie, when the diagnosis is unclear and treatment guidelines vary by region and circumstance. Improved sensitivity and specificity of diagnosis can prevent unnecessary antibiotic treatment, which is associated with adverse effects and escalation of antibiotic resistance.
- Borchers AT, Keen CL, Huntley AC, et al. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82-115. doi:10.1016/j.jaut.2014.09.004
- Centers for Disease Control and Prevention. Lyme disease: data and surveillance. February 14, 2024. Accessed March 5, 2024. https://www.cdc.gov/lyme/datasurveillance/index.html
- Marques AR. Laboratory diagnosis of Lyme disease. Infect Dis Clin North Am. 2015;29:295-307. doi:10.1016/j.idc.2015.02.005
- Bratton RL, Whiteside JW, Hovan MJ, et al. Diagnosis and treatment of Lyme disease. Mayo Clin Proc. 2008;83:566-571. doi:10.4065/83.5.566
- Berger B, Johnson R, Kodner C. Cultivation of Borrelia burgdorferi from human tick bite sites: a guide to the risk of infection. J Am Acad Dermatol. 1995;32(2 pt 1):184-187. doi:10.1016/0190-9622(95)90123-x
- Branda JA, Linskey K, Kim YA, et al. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis. 2011;53:541-547. doi:10.1093/cid/cir464
- Galan A, Kupernik P, Cowper SE. Detection of Borrelia in Ixodes scapularis ticks by silver stain, immunohistochemical and direct immunofluorescent methods. J Cutan Pathol. 2018;45:473-477. doi:10.1111/cup.13143
- Nadelman RB, Nowakowski J, Fish D, et al; Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N Engl J Med. 2001;345:79-84. doi:10.1056/NEJM200107123450201
- Lantos PM, Rumbaugh J, Bockenstedt LK, et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis, and treatment of Lyme disease. Arthritis Rheumatol. 2021;73:12-20. doi:10.1002/art.41562
- Nadelman RB, Luger SW, Frank E, et al. Comparison of cefuroxime axetil and doxycycline in the treatment of early Lyme disease. Ann Intern Med. 1992;117:273-280. doi:10.7326/0003-4819-117-4-273
- Gresser U. Amoxicillin–clavulanic acid therapy may be associated with severe side effects—review of the literature. Eur J Med Res. 2001;6:139-149.
- Nathan C, Cars O. Antibiotic resistance—problems, progress, and prospects. N Engl J Med. 2014;371:1761-1763. doi:10.1056/NEJMp1408040
To the Editor:
Lyme disease is caused by spirochetes of the Borrelia burgdorferi sensu lato species complex and transmitted to humans by the bite of the Ixodes scapularis tick. It was first classified as a nationally notifiable disease in 1991, and the incidence has risen remarkably since then.1 More than 63,000 cases are reported annually to the Centers for Disease Control and Prevention; however, this number reflects severe underreporting, as the true incidence of the disease is projected to be closer to 476,000 cases per year.2 Additionally, 95% of US cases occur in the Northeast and upper Midwest.3 Given the pervasiveness of Lyme disease, early and reliable diagnostic methodology is critical, especially in cases in which the timeline of inoculation is unclear. We present a case of Lyme disease that was discovered during a routine dermatologic visit.
A 77-year-old White man with no relevant medical history presented to a dermatology clinic in west-central Virginia for a routine skin check. Physical examination revealed a well-appearing patient without overt skin abnormalities. However, on closer evaluation, a 0.2×0.1-cm engorged black I scapularis tick was visualized on the left lateral upper back. There was a surrounding zone of erythema that measured less than the 5-cm-diameter criterion for erythema migrans.1
Upon questioning, the patient reported that he was unaware of the tick and could not provide a timeline for inoculation. To ensure proper treatment, the tick was removed in the office and a specimen was sent for histopathology. The arthropod was formalin fixed and paraffin embedded, and it was examined using hematoxylin and eosin and Warthin-Starry stains. Histopathology of the specimen revealed a blood-engorged arthropod. Warthin-Starry stain of the abdomen of the tick highlighted tiny strandlike spirochetes within the gut that were compatible with B burgdorferi (Figure). This finding prompted treatment with a 3-week course of doxycycline. Following treatment, erythema resolved. The patient experienced no sequelae.

Lyme disease can cause a range of serious complications if left untreated, including arthritis, neurologic deficits, and heart block. During the early stages of disease, the sensitivity and specificity of diagnostic methods such as serologic testing are limited.4 The gold standard for the diagnosis of Lyme disease comprises culture and subsequent confirmation by polymerase chain reaction.1 However, cultivation of B burgdorferi is challenging.5 The Centers for Disease Control and Prevention recommends 2-tiered serologic antibody analysis, which has 27% sensitivity during the first week of cutaneous symptoms, and involves an enzyme-linked immunoassay followed by reflexive immunoblotting for positive or indeterminate cases.2,6 The precision of this method is limited by several variables; for example, seroconversion fails to occur in approximately 40% of cases, even after proven exposure to the spirochete.7 Furthermore, the sensitivity of the test is particularly low during the first 4 to 6 weeks of infection—before the body mounts a proper immune response; fewer than 50% of patients exhibit a positive response to the test at initial presentation.3
Clinical diagnosis of Lyme disease is possible, though the pathognomonic erythema migrans rash can be delayed for as long as 30 days and remains absent in 20% to 30% of patients.1 Prophylactic treatment can be offered to individuals who reside in a hyperendemic area and have a rash or have had an engorged Ixodes tick attached for longer than 36 hours.8
More definitive techniques for early diagnosis are needed to enable selective and accurate treatment. The standard of care for Lyme disease includes a 10-day course of doxycycline or a 14-day course of cefuroxime axetil or amoxicillin.9 Many patients tolerate treatment and achieve resolution of disease, but antibiotics are not benign, as some patients experience drug-related adverse effects such as photosensitivity, urticaria, diarrhea, nausea, vomiting, esophagitis, hepatotoxicity, and the Jarisch-Herxheimer reaction (fever, chills, rigors, nausea and vomiting, headache, tachycardia, hypotension, hyperventilation, flushing, myalgia, and exacerbation of lesions).10,11 In a group of 123 patients with Lyme disease, 30% treated with cefuroxime axetil and 32% treated with doxycycline had 1 or more drug-related adverse events.10 Additionally, avoidable antibiotic use is associated with increasing antibiotic resistance.12 Improved diagnostic accuracy would prevent unnecessary treatment. Galan and colleagues7 reported that Warthin-Starry staining of prepared sections of the abdomen of a tick allowed for detection of B burgdorferi with a sensitivity of 71% and specificity of 83%. This technique did not delay the final biopsy report and may be a promising adjunct to the diagnosis of early Lyme disease.7
Anecdotally, many patients who present with an attached and engorged tick are unaware of the timeline of their exposure. Histologic analysis of a removed tick could aid in early clinical decision-making—ie, when the diagnosis is unclear and treatment guidelines vary by region and circumstance. Improved sensitivity and specificity of diagnosis can prevent unnecessary antibiotic treatment, which is associated with adverse effects and escalation of antibiotic resistance.
To the Editor:
Lyme disease is caused by spirochetes of the Borrelia burgdorferi sensu lato species complex and transmitted to humans by the bite of the Ixodes scapularis tick. It was first classified as a nationally notifiable disease in 1991, and the incidence has risen remarkably since then.1 More than 63,000 cases are reported annually to the Centers for Disease Control and Prevention; however, this number reflects severe underreporting, as the true incidence of the disease is projected to be closer to 476,000 cases per year.2 Additionally, 95% of US cases occur in the Northeast and upper Midwest.3 Given the pervasiveness of Lyme disease, early and reliable diagnostic methodology is critical, especially in cases in which the timeline of inoculation is unclear. We present a case of Lyme disease that was discovered during a routine dermatologic visit.
A 77-year-old White man with no relevant medical history presented to a dermatology clinic in west-central Virginia for a routine skin check. Physical examination revealed a well-appearing patient without overt skin abnormalities. However, on closer evaluation, a 0.2×0.1-cm engorged black I scapularis tick was visualized on the left lateral upper back. There was a surrounding zone of erythema that measured less than the 5-cm-diameter criterion for erythema migrans.1
Upon questioning, the patient reported that he was unaware of the tick and could not provide a timeline for inoculation. To ensure proper treatment, the tick was removed in the office and a specimen was sent for histopathology. The arthropod was formalin fixed and paraffin embedded, and it was examined using hematoxylin and eosin and Warthin-Starry stains. Histopathology of the specimen revealed a blood-engorged arthropod. Warthin-Starry stain of the abdomen of the tick highlighted tiny strandlike spirochetes within the gut that were compatible with B burgdorferi (Figure). This finding prompted treatment with a 3-week course of doxycycline. Following treatment, erythema resolved. The patient experienced no sequelae.

Lyme disease can cause a range of serious complications if left untreated, including arthritis, neurologic deficits, and heart block. During the early stages of disease, the sensitivity and specificity of diagnostic methods such as serologic testing are limited.4 The gold standard for the diagnosis of Lyme disease comprises culture and subsequent confirmation by polymerase chain reaction.1 However, cultivation of B burgdorferi is challenging.5 The Centers for Disease Control and Prevention recommends 2-tiered serologic antibody analysis, which has 27% sensitivity during the first week of cutaneous symptoms, and involves an enzyme-linked immunoassay followed by reflexive immunoblotting for positive or indeterminate cases.2,6 The precision of this method is limited by several variables; for example, seroconversion fails to occur in approximately 40% of cases, even after proven exposure to the spirochete.7 Furthermore, the sensitivity of the test is particularly low during the first 4 to 6 weeks of infection—before the body mounts a proper immune response; fewer than 50% of patients exhibit a positive response to the test at initial presentation.3
Clinical diagnosis of Lyme disease is possible, though the pathognomonic erythema migrans rash can be delayed for as long as 30 days and remains absent in 20% to 30% of patients.1 Prophylactic treatment can be offered to individuals who reside in a hyperendemic area and have a rash or have had an engorged Ixodes tick attached for longer than 36 hours.8
More definitive techniques for early diagnosis are needed to enable selective and accurate treatment. The standard of care for Lyme disease includes a 10-day course of doxycycline or a 14-day course of cefuroxime axetil or amoxicillin.9 Many patients tolerate treatment and achieve resolution of disease, but antibiotics are not benign, as some patients experience drug-related adverse effects such as photosensitivity, urticaria, diarrhea, nausea, vomiting, esophagitis, hepatotoxicity, and the Jarisch-Herxheimer reaction (fever, chills, rigors, nausea and vomiting, headache, tachycardia, hypotension, hyperventilation, flushing, myalgia, and exacerbation of lesions).10,11 In a group of 123 patients with Lyme disease, 30% treated with cefuroxime axetil and 32% treated with doxycycline had 1 or more drug-related adverse events.10 Additionally, avoidable antibiotic use is associated with increasing antibiotic resistance.12 Improved diagnostic accuracy would prevent unnecessary treatment. Galan and colleagues7 reported that Warthin-Starry staining of prepared sections of the abdomen of a tick allowed for detection of B burgdorferi with a sensitivity of 71% and specificity of 83%. This technique did not delay the final biopsy report and may be a promising adjunct to the diagnosis of early Lyme disease.7
Anecdotally, many patients who present with an attached and engorged tick are unaware of the timeline of their exposure. Histologic analysis of a removed tick could aid in early clinical decision-making—ie, when the diagnosis is unclear and treatment guidelines vary by region and circumstance. Improved sensitivity and specificity of diagnosis can prevent unnecessary antibiotic treatment, which is associated with adverse effects and escalation of antibiotic resistance.
- Borchers AT, Keen CL, Huntley AC, et al. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82-115. doi:10.1016/j.jaut.2014.09.004
- Centers for Disease Control and Prevention. Lyme disease: data and surveillance. February 14, 2024. Accessed March 5, 2024. https://www.cdc.gov/lyme/datasurveillance/index.html
- Marques AR. Laboratory diagnosis of Lyme disease. Infect Dis Clin North Am. 2015;29:295-307. doi:10.1016/j.idc.2015.02.005
- Bratton RL, Whiteside JW, Hovan MJ, et al. Diagnosis and treatment of Lyme disease. Mayo Clin Proc. 2008;83:566-571. doi:10.4065/83.5.566
- Berger B, Johnson R, Kodner C. Cultivation of Borrelia burgdorferi from human tick bite sites: a guide to the risk of infection. J Am Acad Dermatol. 1995;32(2 pt 1):184-187. doi:10.1016/0190-9622(95)90123-x
- Branda JA, Linskey K, Kim YA, et al. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis. 2011;53:541-547. doi:10.1093/cid/cir464
- Galan A, Kupernik P, Cowper SE. Detection of Borrelia in Ixodes scapularis ticks by silver stain, immunohistochemical and direct immunofluorescent methods. J Cutan Pathol. 2018;45:473-477. doi:10.1111/cup.13143
- Nadelman RB, Nowakowski J, Fish D, et al; Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N Engl J Med. 2001;345:79-84. doi:10.1056/NEJM200107123450201
- Lantos PM, Rumbaugh J, Bockenstedt LK, et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis, and treatment of Lyme disease. Arthritis Rheumatol. 2021;73:12-20. doi:10.1002/art.41562
- Nadelman RB, Luger SW, Frank E, et al. Comparison of cefuroxime axetil and doxycycline in the treatment of early Lyme disease. Ann Intern Med. 1992;117:273-280. doi:10.7326/0003-4819-117-4-273
- Gresser U. Amoxicillin–clavulanic acid therapy may be associated with severe side effects—review of the literature. Eur J Med Res. 2001;6:139-149.
- Nathan C, Cars O. Antibiotic resistance—problems, progress, and prospects. N Engl J Med. 2014;371:1761-1763. doi:10.1056/NEJMp1408040
- Borchers AT, Keen CL, Huntley AC, et al. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82-115. doi:10.1016/j.jaut.2014.09.004
- Centers for Disease Control and Prevention. Lyme disease: data and surveillance. February 14, 2024. Accessed March 5, 2024. https://www.cdc.gov/lyme/datasurveillance/index.html
- Marques AR. Laboratory diagnosis of Lyme disease. Infect Dis Clin North Am. 2015;29:295-307. doi:10.1016/j.idc.2015.02.005
- Bratton RL, Whiteside JW, Hovan MJ, et al. Diagnosis and treatment of Lyme disease. Mayo Clin Proc. 2008;83:566-571. doi:10.4065/83.5.566
- Berger B, Johnson R, Kodner C. Cultivation of Borrelia burgdorferi from human tick bite sites: a guide to the risk of infection. J Am Acad Dermatol. 1995;32(2 pt 1):184-187. doi:10.1016/0190-9622(95)90123-x
- Branda JA, Linskey K, Kim YA, et al. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis. 2011;53:541-547. doi:10.1093/cid/cir464
- Galan A, Kupernik P, Cowper SE. Detection of Borrelia in Ixodes scapularis ticks by silver stain, immunohistochemical and direct immunofluorescent methods. J Cutan Pathol. 2018;45:473-477. doi:10.1111/cup.13143
- Nadelman RB, Nowakowski J, Fish D, et al; Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N Engl J Med. 2001;345:79-84. doi:10.1056/NEJM200107123450201
- Lantos PM, Rumbaugh J, Bockenstedt LK, et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis, and treatment of Lyme disease. Arthritis Rheumatol. 2021;73:12-20. doi:10.1002/art.41562
- Nadelman RB, Luger SW, Frank E, et al. Comparison of cefuroxime axetil and doxycycline in the treatment of early Lyme disease. Ann Intern Med. 1992;117:273-280. doi:10.7326/0003-4819-117-4-273
- Gresser U. Amoxicillin–clavulanic acid therapy may be associated with severe side effects—review of the literature. Eur J Med Res. 2001;6:139-149.
- Nathan C, Cars O. Antibiotic resistance—problems, progress, and prospects. N Engl J Med. 2014;371:1761-1763. doi:10.1056/NEJMp1408040
PRACTICE POINTS
- Lyme disease is increasingly common in the United States.
- Lyme disease can cause debilitating sequelae if left untreated, including arthritis, neurologic deficits, and heart block.
- Diagnostic methods for identifying early Lyme disease have limited sensitivity and specificity, necessitating alternative strategies for making an accurate diagnosis and initiating treatment.
Revamped Antibiotic May Treat Deadly Eye Infection
The relatively new antibiotic cefiderocol given in the form of eye drops may be a way to combat a type of ocular infection that broke out in the United States last year, according to research presented at the 2024 annual meeting of the Association for Research in Vision and Ophthalmology (ARVO).
The infections, linked to contaminated bottles of artificial tears, were detected in 81 patients in 18 states. The outbreak led to loss of vision in 14 patients, surgical removal of the eyeball in four patients, and four deaths, according to health officials.
An extensively drug-resistant strain of Pseudomonas aeruginosa that had not previously been reported in the country caused the infections. Scientists cautioned last year that the bacteria potentially could spread from person to person.
At ARVO on May 6, Eric G. Romanowski, MS, research director of the Charles T. Campbell Ophthalmic Microbiology Laboratory at the University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, described studies that his lab conducted evaluating topical cefiderocol as a potential treatment option for these infections (Abstract 2095).
Investigators had found that the bacterial strain was susceptible to this medication, which was approved by the US Food and Drug Administration in 2019 as a treatment for complicated urinary tract infections. But the antibiotic had not been tested as an eye drop.
“We showed that the ‘Trojan-horse’ antibiotic, cefiderocol … was non-toxic and effective against the highly resistant outbreak strain in an experimental model of infection,” Dr. Romanowski and co–lead investigator Robert M. Q. Shanks, PhD, said in a statement about their research. “These results demonstrate that topical cefiderocol could be a new weapon in the ophthalmologist’s arsenal for the treatment of corneal infections caused by highly antibiotic-resistant Pseudomonas aeruginosa.”
Experimental Models
Dr. Romanowski’s group, with colleagues at the Geisel School of Medicine at Dartmouth University, Hanover, New Hampshire, used minimum inhibitory concentration testing to evaluate the effectiveness of cefiderocol against 135 isolates from eye infections. They also tested ocular toxicity and antibiotic efficacy of cefiderocol eye drops in a rabbit model of keratitis caused by the bacterial strain.
Cefiderocol was “well tolerated on rabbit corneas,” they reported. It also was effective in vitro against the isolates and in vivo in the rabbit model of keratitis.
They first published their findings as a preprint in September 2023 and then in Ophthalmology Science in December.
A ‘Duty to the Profession’
Their paper noted that “there is no current consensus as to the most effective antimicrobial strategy to deal with” extensively drug-resistant keratitis.
During the outbreak, clinicians tried various treatment regimens, with mixed results. In one case, a combination of intravenous cefiderocol and other topical and oral medications appeared to be successful.
Dr. Romanowski’s team decided to test cefiderocol drops with their own resources “as a duty to the profession,” he said. “Not many labs do these types of studies.”
“We would like to see further development of this antibiotic for potential use,” Dr. Romanowski added. “It would be up to any individual clinician to determine whether to use this antibiotic in an emergency situation.”
A version of this article appeared on Medscape.com.
The relatively new antibiotic cefiderocol given in the form of eye drops may be a way to combat a type of ocular infection that broke out in the United States last year, according to research presented at the 2024 annual meeting of the Association for Research in Vision and Ophthalmology (ARVO).
The infections, linked to contaminated bottles of artificial tears, were detected in 81 patients in 18 states. The outbreak led to loss of vision in 14 patients, surgical removal of the eyeball in four patients, and four deaths, according to health officials.
An extensively drug-resistant strain of Pseudomonas aeruginosa that had not previously been reported in the country caused the infections. Scientists cautioned last year that the bacteria potentially could spread from person to person.
At ARVO on May 6, Eric G. Romanowski, MS, research director of the Charles T. Campbell Ophthalmic Microbiology Laboratory at the University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, described studies that his lab conducted evaluating topical cefiderocol as a potential treatment option for these infections (Abstract 2095).
Investigators had found that the bacterial strain was susceptible to this medication, which was approved by the US Food and Drug Administration in 2019 as a treatment for complicated urinary tract infections. But the antibiotic had not been tested as an eye drop.
“We showed that the ‘Trojan-horse’ antibiotic, cefiderocol … was non-toxic and effective against the highly resistant outbreak strain in an experimental model of infection,” Dr. Romanowski and co–lead investigator Robert M. Q. Shanks, PhD, said in a statement about their research. “These results demonstrate that topical cefiderocol could be a new weapon in the ophthalmologist’s arsenal for the treatment of corneal infections caused by highly antibiotic-resistant Pseudomonas aeruginosa.”
Experimental Models
Dr. Romanowski’s group, with colleagues at the Geisel School of Medicine at Dartmouth University, Hanover, New Hampshire, used minimum inhibitory concentration testing to evaluate the effectiveness of cefiderocol against 135 isolates from eye infections. They also tested ocular toxicity and antibiotic efficacy of cefiderocol eye drops in a rabbit model of keratitis caused by the bacterial strain.
Cefiderocol was “well tolerated on rabbit corneas,” they reported. It also was effective in vitro against the isolates and in vivo in the rabbit model of keratitis.
They first published their findings as a preprint in September 2023 and then in Ophthalmology Science in December.
A ‘Duty to the Profession’
Their paper noted that “there is no current consensus as to the most effective antimicrobial strategy to deal with” extensively drug-resistant keratitis.
During the outbreak, clinicians tried various treatment regimens, with mixed results. In one case, a combination of intravenous cefiderocol and other topical and oral medications appeared to be successful.
Dr. Romanowski’s team decided to test cefiderocol drops with their own resources “as a duty to the profession,” he said. “Not many labs do these types of studies.”
“We would like to see further development of this antibiotic for potential use,” Dr. Romanowski added. “It would be up to any individual clinician to determine whether to use this antibiotic in an emergency situation.”
A version of this article appeared on Medscape.com.
The relatively new antibiotic cefiderocol given in the form of eye drops may be a way to combat a type of ocular infection that broke out in the United States last year, according to research presented at the 2024 annual meeting of the Association for Research in Vision and Ophthalmology (ARVO).
The infections, linked to contaminated bottles of artificial tears, were detected in 81 patients in 18 states. The outbreak led to loss of vision in 14 patients, surgical removal of the eyeball in four patients, and four deaths, according to health officials.
An extensively drug-resistant strain of Pseudomonas aeruginosa that had not previously been reported in the country caused the infections. Scientists cautioned last year that the bacteria potentially could spread from person to person.
At ARVO on May 6, Eric G. Romanowski, MS, research director of the Charles T. Campbell Ophthalmic Microbiology Laboratory at the University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, described studies that his lab conducted evaluating topical cefiderocol as a potential treatment option for these infections (Abstract 2095).
Investigators had found that the bacterial strain was susceptible to this medication, which was approved by the US Food and Drug Administration in 2019 as a treatment for complicated urinary tract infections. But the antibiotic had not been tested as an eye drop.
“We showed that the ‘Trojan-horse’ antibiotic, cefiderocol … was non-toxic and effective against the highly resistant outbreak strain in an experimental model of infection,” Dr. Romanowski and co–lead investigator Robert M. Q. Shanks, PhD, said in a statement about their research. “These results demonstrate that topical cefiderocol could be a new weapon in the ophthalmologist’s arsenal for the treatment of corneal infections caused by highly antibiotic-resistant Pseudomonas aeruginosa.”
Experimental Models
Dr. Romanowski’s group, with colleagues at the Geisel School of Medicine at Dartmouth University, Hanover, New Hampshire, used minimum inhibitory concentration testing to evaluate the effectiveness of cefiderocol against 135 isolates from eye infections. They also tested ocular toxicity and antibiotic efficacy of cefiderocol eye drops in a rabbit model of keratitis caused by the bacterial strain.
Cefiderocol was “well tolerated on rabbit corneas,” they reported. It also was effective in vitro against the isolates and in vivo in the rabbit model of keratitis.
They first published their findings as a preprint in September 2023 and then in Ophthalmology Science in December.
A ‘Duty to the Profession’
Their paper noted that “there is no current consensus as to the most effective antimicrobial strategy to deal with” extensively drug-resistant keratitis.
During the outbreak, clinicians tried various treatment regimens, with mixed results. In one case, a combination of intravenous cefiderocol and other topical and oral medications appeared to be successful.
Dr. Romanowski’s team decided to test cefiderocol drops with their own resources “as a duty to the profession,” he said. “Not many labs do these types of studies.”
“We would like to see further development of this antibiotic for potential use,” Dr. Romanowski added. “It would be up to any individual clinician to determine whether to use this antibiotic in an emergency situation.”
A version of this article appeared on Medscape.com.
New HIV Infections After Vampire Facials at Unlicensed Spa
At least three clients of an unlicensed spa in New Mexico contracted HIV after receiving platelet-rich plasma (PRP) microneedling facials, according to an investigation by the US Centers for Disease Control and Prevention (CDC).
The investigation, spanning 5 years with parts of it still ongoing, has resulted in the closure of the spa and is raising questions about public safety in cosmetic clinics.
Though transmission of HIV by unsterile injection practices is a known risk, this is the first time it has been linked to cosmetic injection services, said Anna Stadelman-Behar, PhD, MPH, of the CDC’s Epidemic Intelligence Service.
Sometimes called a vampire facial, the PRP treatment involves taking a patient’s own blood and separating it in a centrifuge. The portion containing a high concentration of platelets is then reinjected with a syringe or microneedling device.
“The idea is that when you inject this concentrated amount of platelets, the growth factors that the platelets release help to stimulate the regenerative nature of that area,” said Anthony Rossi, MD, professor of dermatology at Weill Cornell Medical College in New York, and attending dermatologist at Memorial Sloan Kettering Cancer Center.
The infections under investigation first came to light when a woman was diagnosed with HIV with no known risk factors for the disease other than exposure to microneedling facials at a cosmetic spa.
The New Mexico Department of Health and the CDC launched an investigation of the spa and discovered a litany of “gross violations of infection control practices,” said Dr. Stadelman-Behar.
Infection-Control Violations
At the spa in New Mexico, investigators found:
- On a kitchen counter, a centrifuge, a heating dry bath, and a rack of unlabeled tubes containing blood
- In a refrigerator, unlabeled tubes of blood and medical injectables including botox and lidocaine stored along with food
- Unwrapped syringes in drawers, on counters, and discarded in regular trash cans
- No autoclave for steam sterilization on the premises
- Only surface cleaning for procedure equipment with ammonium chloride disinfecting spray and benzalkonium chloride disinfecting wipes after each client visit
- Disposable electric desiccator tips cleaned only by alcohol immersion to be reused
The spa’s owner operated without appropriate licenses at multiple locations and did not have an appointment scheduling system that stored client contact information.
Investigators contacted as many people as they could find and launched a large-scale community outreach effort to find more.
In total, four clients and one intimate partner of a client were diagnosed with HIV during the investigation, but one client and her partner were determined to likely have been infected before the spa visit.
It is not clear whether the infections were due to unlabeled contaminated blood products being given to the wrong client or contamination on shared needles. Investigators did not have the authority to collect specimens during their site visit that would have allowed them to study that.
“We can’t definitively say what the route of contamination was,” noted Dr. Stadelman-Behar.
Anne Chapas, MD, a board-certified dermatologist, and instructor at Mount Sinai Hospital in New York, added that just because a procedure is cosmetic, that doesn’t mean it is not medical. “Personally, I feel it should only be done by medical practitioners who understand the risks.”
A Medical Procedure
PRP microneedling has been used extensively in orthopedic surgery to promote joint regeneration. For the past 10 years, it has also been used in dermatology to treat hair loss from alopecia, to augment wound healing, and cosmetically to reduce facial wrinkles.
It is generally done in a doctor’s office or medical spa, and the procedure takes about half an hour.
Dr. Stadelman-Behar said that this ongoing investigation highlights the importance of front-line healthcare workers using their clinical expertise to help identify potential new routes of transmission for infections. “It was provider-led intuition that sparked this investigation, so it’s important to let the department of health know if there is something amiss with any of the exposures that the patient might have had,” she said.
A version of this article appeared on Medscape.com.
At least three clients of an unlicensed spa in New Mexico contracted HIV after receiving platelet-rich plasma (PRP) microneedling facials, according to an investigation by the US Centers for Disease Control and Prevention (CDC).
The investigation, spanning 5 years with parts of it still ongoing, has resulted in the closure of the spa and is raising questions about public safety in cosmetic clinics.
Though transmission of HIV by unsterile injection practices is a known risk, this is the first time it has been linked to cosmetic injection services, said Anna Stadelman-Behar, PhD, MPH, of the CDC’s Epidemic Intelligence Service.
Sometimes called a vampire facial, the PRP treatment involves taking a patient’s own blood and separating it in a centrifuge. The portion containing a high concentration of platelets is then reinjected with a syringe or microneedling device.
“The idea is that when you inject this concentrated amount of platelets, the growth factors that the platelets release help to stimulate the regenerative nature of that area,” said Anthony Rossi, MD, professor of dermatology at Weill Cornell Medical College in New York, and attending dermatologist at Memorial Sloan Kettering Cancer Center.
The infections under investigation first came to light when a woman was diagnosed with HIV with no known risk factors for the disease other than exposure to microneedling facials at a cosmetic spa.
The New Mexico Department of Health and the CDC launched an investigation of the spa and discovered a litany of “gross violations of infection control practices,” said Dr. Stadelman-Behar.
Infection-Control Violations
At the spa in New Mexico, investigators found:
- On a kitchen counter, a centrifuge, a heating dry bath, and a rack of unlabeled tubes containing blood
- In a refrigerator, unlabeled tubes of blood and medical injectables including botox and lidocaine stored along with food
- Unwrapped syringes in drawers, on counters, and discarded in regular trash cans
- No autoclave for steam sterilization on the premises
- Only surface cleaning for procedure equipment with ammonium chloride disinfecting spray and benzalkonium chloride disinfecting wipes after each client visit
- Disposable electric desiccator tips cleaned only by alcohol immersion to be reused
The spa’s owner operated without appropriate licenses at multiple locations and did not have an appointment scheduling system that stored client contact information.
Investigators contacted as many people as they could find and launched a large-scale community outreach effort to find more.
In total, four clients and one intimate partner of a client were diagnosed with HIV during the investigation, but one client and her partner were determined to likely have been infected before the spa visit.
It is not clear whether the infections were due to unlabeled contaminated blood products being given to the wrong client or contamination on shared needles. Investigators did not have the authority to collect specimens during their site visit that would have allowed them to study that.
“We can’t definitively say what the route of contamination was,” noted Dr. Stadelman-Behar.
Anne Chapas, MD, a board-certified dermatologist, and instructor at Mount Sinai Hospital in New York, added that just because a procedure is cosmetic, that doesn’t mean it is not medical. “Personally, I feel it should only be done by medical practitioners who understand the risks.”
A Medical Procedure
PRP microneedling has been used extensively in orthopedic surgery to promote joint regeneration. For the past 10 years, it has also been used in dermatology to treat hair loss from alopecia, to augment wound healing, and cosmetically to reduce facial wrinkles.
It is generally done in a doctor’s office or medical spa, and the procedure takes about half an hour.
Dr. Stadelman-Behar said that this ongoing investigation highlights the importance of front-line healthcare workers using their clinical expertise to help identify potential new routes of transmission for infections. “It was provider-led intuition that sparked this investigation, so it’s important to let the department of health know if there is something amiss with any of the exposures that the patient might have had,” she said.
A version of this article appeared on Medscape.com.
At least three clients of an unlicensed spa in New Mexico contracted HIV after receiving platelet-rich plasma (PRP) microneedling facials, according to an investigation by the US Centers for Disease Control and Prevention (CDC).
The investigation, spanning 5 years with parts of it still ongoing, has resulted in the closure of the spa and is raising questions about public safety in cosmetic clinics.
Though transmission of HIV by unsterile injection practices is a known risk, this is the first time it has been linked to cosmetic injection services, said Anna Stadelman-Behar, PhD, MPH, of the CDC’s Epidemic Intelligence Service.
Sometimes called a vampire facial, the PRP treatment involves taking a patient’s own blood and separating it in a centrifuge. The portion containing a high concentration of platelets is then reinjected with a syringe or microneedling device.
“The idea is that when you inject this concentrated amount of platelets, the growth factors that the platelets release help to stimulate the regenerative nature of that area,” said Anthony Rossi, MD, professor of dermatology at Weill Cornell Medical College in New York, and attending dermatologist at Memorial Sloan Kettering Cancer Center.
The infections under investigation first came to light when a woman was diagnosed with HIV with no known risk factors for the disease other than exposure to microneedling facials at a cosmetic spa.
The New Mexico Department of Health and the CDC launched an investigation of the spa and discovered a litany of “gross violations of infection control practices,” said Dr. Stadelman-Behar.
Infection-Control Violations
At the spa in New Mexico, investigators found:
- On a kitchen counter, a centrifuge, a heating dry bath, and a rack of unlabeled tubes containing blood
- In a refrigerator, unlabeled tubes of blood and medical injectables including botox and lidocaine stored along with food
- Unwrapped syringes in drawers, on counters, and discarded in regular trash cans
- No autoclave for steam sterilization on the premises
- Only surface cleaning for procedure equipment with ammonium chloride disinfecting spray and benzalkonium chloride disinfecting wipes after each client visit
- Disposable electric desiccator tips cleaned only by alcohol immersion to be reused
The spa’s owner operated without appropriate licenses at multiple locations and did not have an appointment scheduling system that stored client contact information.
Investigators contacted as many people as they could find and launched a large-scale community outreach effort to find more.
In total, four clients and one intimate partner of a client were diagnosed with HIV during the investigation, but one client and her partner were determined to likely have been infected before the spa visit.
It is not clear whether the infections were due to unlabeled contaminated blood products being given to the wrong client or contamination on shared needles. Investigators did not have the authority to collect specimens during their site visit that would have allowed them to study that.
“We can’t definitively say what the route of contamination was,” noted Dr. Stadelman-Behar.
Anne Chapas, MD, a board-certified dermatologist, and instructor at Mount Sinai Hospital in New York, added that just because a procedure is cosmetic, that doesn’t mean it is not medical. “Personally, I feel it should only be done by medical practitioners who understand the risks.”
A Medical Procedure
PRP microneedling has been used extensively in orthopedic surgery to promote joint regeneration. For the past 10 years, it has also been used in dermatology to treat hair loss from alopecia, to augment wound healing, and cosmetically to reduce facial wrinkles.
It is generally done in a doctor’s office or medical spa, and the procedure takes about half an hour.
Dr. Stadelman-Behar said that this ongoing investigation highlights the importance of front-line healthcare workers using their clinical expertise to help identify potential new routes of transmission for infections. “It was provider-led intuition that sparked this investigation, so it’s important to let the department of health know if there is something amiss with any of the exposures that the patient might have had,” she said.
A version of this article appeared on Medscape.com.
Mpox Presentation Compared in Different Racial, Ethnic Groups
TOPLINE:
.
METHODOLOGY:
- There is limited information on the populations disproportionately affected by the recent global mpox outbreak, particularly in individuals with HIV and racial and ethnic minorities.
- To investigate morphologic and clinical presentations of mpox in diverse populations, researchers conducted a review of the records of 54 individuals (mean age, 42.4 years) diagnosed with mpox at a San Francisco clinic for patients with HIV or at high risk for HIV, between June and October 2022.
- All patients were assigned male at birth, and three identified themselves as transgender women.
- Morphologic descriptions were documented through either photographic evidence or physical examination notes.
TAKEAWAY:
- Pustules or pseudopustules were the most common morphologic finding in 57.1% of the White non-Hispanic patients and 62.5% of the patients of color (P = .72).
- White non-Hispanic patients were more likely to have no prodromal symptoms (50.0% vs 17.5%; P = .02) and were more likely to have genital lesions (78.6% vs 40.0%; P = .01) than patients of color. These differences were significant or nearly significant when White non-Hispanic patients were compared with Hispanic patients but not in other ethnic or racial groups.
- There were no differences in HIV viral loads or CD4 counts between racial and ethnic groups, and no variations in clinical presentations were observed based on CD4 counts.
- Patients with higher HIV viral loads were more likely to have concurrent sexually transmitted infections (57.1% vs 25%; P = .03).
- Symptoms resolved in all patients, regardless of medical intervention, within weeks of initial presentation, and there were no hospitalizations or deaths.
IN PRACTICE:
Considering that HIV viral burden was not significantly different between White non-Hispanic patients and patients of color, the difference in presentation of the prodrome “may indicate disparities in vulnerable populations,” the authors wrote, noting that more research in large groups is needed to confirm their results.
SOURCE:
The study, led by Richard W. Kim, BS, from the University of California San Francisco, was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Inclusion of “other” racial category in the records highlighted potential inaccuracies in data representation.
DISCLOSURES:
The study received no external funding. The authors did not declare any competing interests.
A version of this article first appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- There is limited information on the populations disproportionately affected by the recent global mpox outbreak, particularly in individuals with HIV and racial and ethnic minorities.
- To investigate morphologic and clinical presentations of mpox in diverse populations, researchers conducted a review of the records of 54 individuals (mean age, 42.4 years) diagnosed with mpox at a San Francisco clinic for patients with HIV or at high risk for HIV, between June and October 2022.
- All patients were assigned male at birth, and three identified themselves as transgender women.
- Morphologic descriptions were documented through either photographic evidence or physical examination notes.
TAKEAWAY:
- Pustules or pseudopustules were the most common morphologic finding in 57.1% of the White non-Hispanic patients and 62.5% of the patients of color (P = .72).
- White non-Hispanic patients were more likely to have no prodromal symptoms (50.0% vs 17.5%; P = .02) and were more likely to have genital lesions (78.6% vs 40.0%; P = .01) than patients of color. These differences were significant or nearly significant when White non-Hispanic patients were compared with Hispanic patients but not in other ethnic or racial groups.
- There were no differences in HIV viral loads or CD4 counts between racial and ethnic groups, and no variations in clinical presentations were observed based on CD4 counts.
- Patients with higher HIV viral loads were more likely to have concurrent sexually transmitted infections (57.1% vs 25%; P = .03).
- Symptoms resolved in all patients, regardless of medical intervention, within weeks of initial presentation, and there were no hospitalizations or deaths.
IN PRACTICE:
Considering that HIV viral burden was not significantly different between White non-Hispanic patients and patients of color, the difference in presentation of the prodrome “may indicate disparities in vulnerable populations,” the authors wrote, noting that more research in large groups is needed to confirm their results.
SOURCE:
The study, led by Richard W. Kim, BS, from the University of California San Francisco, was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Inclusion of “other” racial category in the records highlighted potential inaccuracies in data representation.
DISCLOSURES:
The study received no external funding. The authors did not declare any competing interests.
A version of this article first appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- There is limited information on the populations disproportionately affected by the recent global mpox outbreak, particularly in individuals with HIV and racial and ethnic minorities.
- To investigate morphologic and clinical presentations of mpox in diverse populations, researchers conducted a review of the records of 54 individuals (mean age, 42.4 years) diagnosed with mpox at a San Francisco clinic for patients with HIV or at high risk for HIV, between June and October 2022.
- All patients were assigned male at birth, and three identified themselves as transgender women.
- Morphologic descriptions were documented through either photographic evidence or physical examination notes.
TAKEAWAY:
- Pustules or pseudopustules were the most common morphologic finding in 57.1% of the White non-Hispanic patients and 62.5% of the patients of color (P = .72).
- White non-Hispanic patients were more likely to have no prodromal symptoms (50.0% vs 17.5%; P = .02) and were more likely to have genital lesions (78.6% vs 40.0%; P = .01) than patients of color. These differences were significant or nearly significant when White non-Hispanic patients were compared with Hispanic patients but not in other ethnic or racial groups.
- There were no differences in HIV viral loads or CD4 counts between racial and ethnic groups, and no variations in clinical presentations were observed based on CD4 counts.
- Patients with higher HIV viral loads were more likely to have concurrent sexually transmitted infections (57.1% vs 25%; P = .03).
- Symptoms resolved in all patients, regardless of medical intervention, within weeks of initial presentation, and there were no hospitalizations or deaths.
IN PRACTICE:
Considering that HIV viral burden was not significantly different between White non-Hispanic patients and patients of color, the difference in presentation of the prodrome “may indicate disparities in vulnerable populations,” the authors wrote, noting that more research in large groups is needed to confirm their results.
SOURCE:
The study, led by Richard W. Kim, BS, from the University of California San Francisco, was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Inclusion of “other” racial category in the records highlighted potential inaccuracies in data representation.
DISCLOSURES:
The study received no external funding. The authors did not declare any competing interests.
A version of this article first appeared on Medscape.com.
In the Story of the Rubella Virus as a Source of Granulomas, the Plot Is Still Thickening
SAN DIEGO — Approximately 10 years ago in France, high throughput .
Based on accumulating evidence, the Centers for Disease Control and Prevention (CDC) through collaborations with others also recognized this in pediatric patients with inborn errors of immunity, and it is now appropriate for clinicians to consider this etiology when no other infectious agents can be identified, according to Karolyn A. Wanat, MD, professor of dermatology, Medical College of Wisconsin, Milwaukee, who spoke about rubella as a trigger in granulomatous disease at the meeting. “This is a huge evolving area of interest,” said Dr. Wanat, who has been the first author or coauthor on several published papers, including a review article published earlier this year.
In the earliest cases, including those reported in 2014, the cutaneous granulomas presumed to be causally related to vaccine-derived rubella virus were found only in those with a primary immunodeficiency. This is no longer the case. In a collaboration among US clinics, granulomas that had persisted for years in immunocompetent adults were identified, according to Dr. Wanat, the first author of a report on these findings in four adults in 2022. In addition, it now appears that wild-type rubella virus, like vaccine-derived rubella virus, can be the source of the antigenic response that underlies the development of rubella-associated cutaneous granulomas.
The phenotype of these granulomas is comparable to granulomas associated with other infectious agents. On dermatopathology, these commonly feature a robust granulomatous inflammation with multinucleated giant cells and lymphocytic infiltrate. Necrosis and fibrosis are also common.
“These are the types of granulomas that we would be thinking infection. If tissue cultures are negative, we would probably repeat them,” she said, suggesting that suspicion of an infectious etiology would probably remain high even after multiple negative tests.
Of the cases accruing in the United States and elsewhere, most but not all have been linked to inborn errors of immunity. In a 2020 CDC review, the risk of granulomas caused by compromised immunity, such as defects in T cell function, was estimated to be in the range of 0.6% to 2.5%, Dr. Wanat said.
It is now known that primary immunodeficiency is not a prerequisite, but this should not change the perception that the rubella vaccine, which was introduced in 1979, is effective and safe, according to Dr. Wanat. The vaccine is associated with few serious adverse events and is so effective that rubella was eliminated from the United States in 2004 and from the Americas in 2015.
This makes cases of granuloma associated with wild-type rubella virus surprising, but they appear to be exceedingly rare. Whether caused by vaccine exposure or another source, the mechanism of latent development of cutaneous granulomas is consistent with other infectious sources, and is not well understood.
“Rubella is a sneaky virus that can persist in some immunoprivileged sites indefinitely,” Dr. Wanat said. These sites include the eyes, joints, and placenta.
Many initial cases of rubella-associated granulomas occurred on the arms, presumably where the vaccine was administered, despite long intervals between exposure and lesion growth. This interval is often measured in years.
With more cases, it is now understood that involvement of other organs does occur even if the skin is the most common site of antigenic response in patients with immunodeficiency. The liver and lymph nodes represent other tissues that have been affected. Even lesions in the brain have been seen on autopsy.
Based on the benefit-to-risk ratio of a highly effective and successful vaccine, however, the association with a risk of granulomas “should not raise questions” about the value of the vaccine itself, Dr. Wanat noted.
“The proportion of patients who develop these granulomas is very, very low. Yet, the vaccine provides life-long immunity,” she said.
The discovery of granulomas associated with wild type rubella infection was “shocking” based on the supposition that the rubella virus had been eliminated, but this is just one of the unexpected discoveries as the still-evolving science has traced the story of rubella-associated granulomas over the past 10 years.
Cases now include children and adults through advanced ages.
Shedding of the virus and risk of infection to others has been studied but so far, the risk — if it exists — is very low. The evidence includes the many patients who have lived with the granulomas for years, even decades, without any known spread to others.
As for ongoing work in this area, Dr. Wanat said that a histopathological case definition for rubella-associated granulomas is being developed, and she and other investigators are actively seeking new cases to better characterize the disease.
So far, optimal treatment is not well defined. A number of strategies have had limited success or are considered impractical for routine use. One example is a stem cell transplant. In a case Dr. Wanat cited, complete resolution of the skin lesions was achieved with a transplant.
“I am not suggesting that those with localized disease in the skin should undergo a transplant, but it does support the role of the immune system and the potential for a reboot to clear the skin,” she said.
Other therapies associated with benefit in at least some patients include tumor necrosis factor (TNF) inhibitors with dapsone and ribavirin. The risk of adverse events for the latter might again limit its use, Dr. Wanat said.
With awareness, the number of granulomas found to be associated with rubella virus is expected to grow. Dr. Wanat speculated that those areas of the country that not yet have documented a case will do so over time. For idiopathic cases of cutaneous granulomas, rubella should be kept in mind, she said.
Characterizing rubella-associated cutaneous granulomas as “a public health concern,” Dr. Wanat urged clinicians to consider this etiology in lesions that match the phenotype, particularly when other more common infectious agents cannot be identified.
Asked for his perspective, Jeffrey P. North, MD, managing director of the UCSF Dermatopathology, and professor of dermatology and pathology at the University of California, San Francisco, agreed that rubella should be considered as a source of granulomas with a suspected infectious etiology when a pathogen cannot be found.
“It is likely much more common than we know as it has only been recently described and testing for it is limited. I suspected there are a lot of undiagnosed patients suffering from this disease,” Dr. North said in an interview.
“One of the important points for clinicians to consider is that while this has been reported mostly in patients with some form of immunodeficiency, there have also been patients reported to have this condition with no immunodeficiency,” he added. Even though the association between rubella and granulomas was made 10 years ago, awareness is only now spreading, which means the frequency with which rubella leads to granulomas remains uncertain.
“I think we will start to get a better idea of how common this is as more people learn about and testing for it expands,” Dr. North said.
Dr. Wanat reports no potential conflicts of interest. Dr. North reports financial relationships with AdviNow and Kiniksa Pharmaceuticals.
SAN DIEGO — Approximately 10 years ago in France, high throughput .
Based on accumulating evidence, the Centers for Disease Control and Prevention (CDC) through collaborations with others also recognized this in pediatric patients with inborn errors of immunity, and it is now appropriate for clinicians to consider this etiology when no other infectious agents can be identified, according to Karolyn A. Wanat, MD, professor of dermatology, Medical College of Wisconsin, Milwaukee, who spoke about rubella as a trigger in granulomatous disease at the meeting. “This is a huge evolving area of interest,” said Dr. Wanat, who has been the first author or coauthor on several published papers, including a review article published earlier this year.
In the earliest cases, including those reported in 2014, the cutaneous granulomas presumed to be causally related to vaccine-derived rubella virus were found only in those with a primary immunodeficiency. This is no longer the case. In a collaboration among US clinics, granulomas that had persisted for years in immunocompetent adults were identified, according to Dr. Wanat, the first author of a report on these findings in four adults in 2022. In addition, it now appears that wild-type rubella virus, like vaccine-derived rubella virus, can be the source of the antigenic response that underlies the development of rubella-associated cutaneous granulomas.
The phenotype of these granulomas is comparable to granulomas associated with other infectious agents. On dermatopathology, these commonly feature a robust granulomatous inflammation with multinucleated giant cells and lymphocytic infiltrate. Necrosis and fibrosis are also common.
“These are the types of granulomas that we would be thinking infection. If tissue cultures are negative, we would probably repeat them,” she said, suggesting that suspicion of an infectious etiology would probably remain high even after multiple negative tests.
Of the cases accruing in the United States and elsewhere, most but not all have been linked to inborn errors of immunity. In a 2020 CDC review, the risk of granulomas caused by compromised immunity, such as defects in T cell function, was estimated to be in the range of 0.6% to 2.5%, Dr. Wanat said.
It is now known that primary immunodeficiency is not a prerequisite, but this should not change the perception that the rubella vaccine, which was introduced in 1979, is effective and safe, according to Dr. Wanat. The vaccine is associated with few serious adverse events and is so effective that rubella was eliminated from the United States in 2004 and from the Americas in 2015.
This makes cases of granuloma associated with wild-type rubella virus surprising, but they appear to be exceedingly rare. Whether caused by vaccine exposure or another source, the mechanism of latent development of cutaneous granulomas is consistent with other infectious sources, and is not well understood.
“Rubella is a sneaky virus that can persist in some immunoprivileged sites indefinitely,” Dr. Wanat said. These sites include the eyes, joints, and placenta.
Many initial cases of rubella-associated granulomas occurred on the arms, presumably where the vaccine was administered, despite long intervals between exposure and lesion growth. This interval is often measured in years.
With more cases, it is now understood that involvement of other organs does occur even if the skin is the most common site of antigenic response in patients with immunodeficiency. The liver and lymph nodes represent other tissues that have been affected. Even lesions in the brain have been seen on autopsy.
Based on the benefit-to-risk ratio of a highly effective and successful vaccine, however, the association with a risk of granulomas “should not raise questions” about the value of the vaccine itself, Dr. Wanat noted.
“The proportion of patients who develop these granulomas is very, very low. Yet, the vaccine provides life-long immunity,” she said.
The discovery of granulomas associated with wild type rubella infection was “shocking” based on the supposition that the rubella virus had been eliminated, but this is just one of the unexpected discoveries as the still-evolving science has traced the story of rubella-associated granulomas over the past 10 years.
Cases now include children and adults through advanced ages.
Shedding of the virus and risk of infection to others has been studied but so far, the risk — if it exists — is very low. The evidence includes the many patients who have lived with the granulomas for years, even decades, without any known spread to others.
As for ongoing work in this area, Dr. Wanat said that a histopathological case definition for rubella-associated granulomas is being developed, and she and other investigators are actively seeking new cases to better characterize the disease.
So far, optimal treatment is not well defined. A number of strategies have had limited success or are considered impractical for routine use. One example is a stem cell transplant. In a case Dr. Wanat cited, complete resolution of the skin lesions was achieved with a transplant.
“I am not suggesting that those with localized disease in the skin should undergo a transplant, but it does support the role of the immune system and the potential for a reboot to clear the skin,” she said.
Other therapies associated with benefit in at least some patients include tumor necrosis factor (TNF) inhibitors with dapsone and ribavirin. The risk of adverse events for the latter might again limit its use, Dr. Wanat said.
With awareness, the number of granulomas found to be associated with rubella virus is expected to grow. Dr. Wanat speculated that those areas of the country that not yet have documented a case will do so over time. For idiopathic cases of cutaneous granulomas, rubella should be kept in mind, she said.
Characterizing rubella-associated cutaneous granulomas as “a public health concern,” Dr. Wanat urged clinicians to consider this etiology in lesions that match the phenotype, particularly when other more common infectious agents cannot be identified.
Asked for his perspective, Jeffrey P. North, MD, managing director of the UCSF Dermatopathology, and professor of dermatology and pathology at the University of California, San Francisco, agreed that rubella should be considered as a source of granulomas with a suspected infectious etiology when a pathogen cannot be found.
“It is likely much more common than we know as it has only been recently described and testing for it is limited. I suspected there are a lot of undiagnosed patients suffering from this disease,” Dr. North said in an interview.
“One of the important points for clinicians to consider is that while this has been reported mostly in patients with some form of immunodeficiency, there have also been patients reported to have this condition with no immunodeficiency,” he added. Even though the association between rubella and granulomas was made 10 years ago, awareness is only now spreading, which means the frequency with which rubella leads to granulomas remains uncertain.
“I think we will start to get a better idea of how common this is as more people learn about and testing for it expands,” Dr. North said.
Dr. Wanat reports no potential conflicts of interest. Dr. North reports financial relationships with AdviNow and Kiniksa Pharmaceuticals.
SAN DIEGO — Approximately 10 years ago in France, high throughput .
Based on accumulating evidence, the Centers for Disease Control and Prevention (CDC) through collaborations with others also recognized this in pediatric patients with inborn errors of immunity, and it is now appropriate for clinicians to consider this etiology when no other infectious agents can be identified, according to Karolyn A. Wanat, MD, professor of dermatology, Medical College of Wisconsin, Milwaukee, who spoke about rubella as a trigger in granulomatous disease at the meeting. “This is a huge evolving area of interest,” said Dr. Wanat, who has been the first author or coauthor on several published papers, including a review article published earlier this year.
In the earliest cases, including those reported in 2014, the cutaneous granulomas presumed to be causally related to vaccine-derived rubella virus were found only in those with a primary immunodeficiency. This is no longer the case. In a collaboration among US clinics, granulomas that had persisted for years in immunocompetent adults were identified, according to Dr. Wanat, the first author of a report on these findings in four adults in 2022. In addition, it now appears that wild-type rubella virus, like vaccine-derived rubella virus, can be the source of the antigenic response that underlies the development of rubella-associated cutaneous granulomas.
The phenotype of these granulomas is comparable to granulomas associated with other infectious agents. On dermatopathology, these commonly feature a robust granulomatous inflammation with multinucleated giant cells and lymphocytic infiltrate. Necrosis and fibrosis are also common.
“These are the types of granulomas that we would be thinking infection. If tissue cultures are negative, we would probably repeat them,” she said, suggesting that suspicion of an infectious etiology would probably remain high even after multiple negative tests.
Of the cases accruing in the United States and elsewhere, most but not all have been linked to inborn errors of immunity. In a 2020 CDC review, the risk of granulomas caused by compromised immunity, such as defects in T cell function, was estimated to be in the range of 0.6% to 2.5%, Dr. Wanat said.
It is now known that primary immunodeficiency is not a prerequisite, but this should not change the perception that the rubella vaccine, which was introduced in 1979, is effective and safe, according to Dr. Wanat. The vaccine is associated with few serious adverse events and is so effective that rubella was eliminated from the United States in 2004 and from the Americas in 2015.
This makes cases of granuloma associated with wild-type rubella virus surprising, but they appear to be exceedingly rare. Whether caused by vaccine exposure or another source, the mechanism of latent development of cutaneous granulomas is consistent with other infectious sources, and is not well understood.
“Rubella is a sneaky virus that can persist in some immunoprivileged sites indefinitely,” Dr. Wanat said. These sites include the eyes, joints, and placenta.
Many initial cases of rubella-associated granulomas occurred on the arms, presumably where the vaccine was administered, despite long intervals between exposure and lesion growth. This interval is often measured in years.
With more cases, it is now understood that involvement of other organs does occur even if the skin is the most common site of antigenic response in patients with immunodeficiency. The liver and lymph nodes represent other tissues that have been affected. Even lesions in the brain have been seen on autopsy.
Based on the benefit-to-risk ratio of a highly effective and successful vaccine, however, the association with a risk of granulomas “should not raise questions” about the value of the vaccine itself, Dr. Wanat noted.
“The proportion of patients who develop these granulomas is very, very low. Yet, the vaccine provides life-long immunity,” she said.
The discovery of granulomas associated with wild type rubella infection was “shocking” based on the supposition that the rubella virus had been eliminated, but this is just one of the unexpected discoveries as the still-evolving science has traced the story of rubella-associated granulomas over the past 10 years.
Cases now include children and adults through advanced ages.
Shedding of the virus and risk of infection to others has been studied but so far, the risk — if it exists — is very low. The evidence includes the many patients who have lived with the granulomas for years, even decades, without any known spread to others.
As for ongoing work in this area, Dr. Wanat said that a histopathological case definition for rubella-associated granulomas is being developed, and she and other investigators are actively seeking new cases to better characterize the disease.
So far, optimal treatment is not well defined. A number of strategies have had limited success or are considered impractical for routine use. One example is a stem cell transplant. In a case Dr. Wanat cited, complete resolution of the skin lesions was achieved with a transplant.
“I am not suggesting that those with localized disease in the skin should undergo a transplant, but it does support the role of the immune system and the potential for a reboot to clear the skin,” she said.
Other therapies associated with benefit in at least some patients include tumor necrosis factor (TNF) inhibitors with dapsone and ribavirin. The risk of adverse events for the latter might again limit its use, Dr. Wanat said.
With awareness, the number of granulomas found to be associated with rubella virus is expected to grow. Dr. Wanat speculated that those areas of the country that not yet have documented a case will do so over time. For idiopathic cases of cutaneous granulomas, rubella should be kept in mind, she said.
Characterizing rubella-associated cutaneous granulomas as “a public health concern,” Dr. Wanat urged clinicians to consider this etiology in lesions that match the phenotype, particularly when other more common infectious agents cannot be identified.
Asked for his perspective, Jeffrey P. North, MD, managing director of the UCSF Dermatopathology, and professor of dermatology and pathology at the University of California, San Francisco, agreed that rubella should be considered as a source of granulomas with a suspected infectious etiology when a pathogen cannot be found.
“It is likely much more common than we know as it has only been recently described and testing for it is limited. I suspected there are a lot of undiagnosed patients suffering from this disease,” Dr. North said in an interview.
“One of the important points for clinicians to consider is that while this has been reported mostly in patients with some form of immunodeficiency, there have also been patients reported to have this condition with no immunodeficiency,” he added. Even though the association between rubella and granulomas was made 10 years ago, awareness is only now spreading, which means the frequency with which rubella leads to granulomas remains uncertain.
“I think we will start to get a better idea of how common this is as more people learn about and testing for it expands,” Dr. North said.
Dr. Wanat reports no potential conflicts of interest. Dr. North reports financial relationships with AdviNow and Kiniksa Pharmaceuticals.
FROM AAD 2024
New Contraindications to Coadministration of Atazanavir
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) this week recommended new contraindications on the coadministration of the protease inhibitor atazanavir (Reyataz, Bristol-Myers Squibb) with antineoplastic agents encorafenib and ivosidenib (atazanavir may significantly increase blood levels and thus side effects), and with the anticonvulsants carbamazepine, phenobarbital, and phenytoin (which may decrease serum levels of atazanavir).
The new rules alter sections 4.3 and 4.5 of the summary of product characteristics (SmPC) to reclassify drug–drug interactions with the new contraindications.
Atazanavir is an orally administered drug, used in combination with low-dose ritonavir (Norvir) to boost its pharmacokinetics. It is indicated for the treatment of HIV-1 infected adults and pediatric patients 3 months of age and older in combination with other antiretroviral medicinal products. A combination preparation boosted with cobicistat (Evotaz) is also available.
The drug is an azapeptide HIV-1 protease inhibitor (PI) that selectively inhibits the virus-specific processing of viral Gag-Pol proteins in HIV-1 infected cells, thus preventing formation of mature virions and infection of other cells. This prevents the virus from multiplying and slows the spread of infection. Based on available virological and clinical data from adult patients, no benefit is expected in patients with HIV strains resistant to multiple protease inhibitors (four or more PI mutations).
Therapy with atazanavir is intended to be initiated by a physician experienced in the management of HIV infection, with the choice of atazanavir in treatment-experienced adult and pediatric patients based on individual viral resistance testing and the patient’s treatment history. The standard dose is 300 mg atazanavir taken with 100 mg ritonavir once daily with food.
Atazanavir is already contraindicated in combination or coadministration with a wide variety of other agents:
- Coadministration with simvastatin or lovastatin [statins – risk of increased blood levels with atazanavir].
- Combination with the anti-TB antibiotic rifampicin.
- Combination with the PDE5 inhibitor sildenafil when used for the treatment of pulmonary arterial hypertension only.
- Coadministration with substrates of the CYP3A4 isoform of cytochrome P450 that have narrow therapeutic windows (eg, quetiapine, lurasidone, alfuzosin, astemizole, terfenadine, cisapride, pimozide, quinidine, bepridil, triazolam, oral midazolam, lomitapide, and ergot alkaloids).
- Coadministration with grazoprevir-containing products, including elbasvir/grazoprevir fixed dose combination (hepatitis C drug combination; atazanavir increases its blood levels).
- Coadministration with glecaprevir/pibrentasvir fixed dose combination (hepatitis C drug combination; increased hepatotoxicity due to increased bilirubin concentration).
- Coadministration with products containing St. John’s wort (Hypericum perforatum).
The EMA said detailed recommendations for the use of atazanavir will be described in the updated SmPC, which will be published in the revised European public assessment report after a decision on this change to the marketing authorization has been granted by the European Commission.
A version of this article appeared on Medscape.com.
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) this week recommended new contraindications on the coadministration of the protease inhibitor atazanavir (Reyataz, Bristol-Myers Squibb) with antineoplastic agents encorafenib and ivosidenib (atazanavir may significantly increase blood levels and thus side effects), and with the anticonvulsants carbamazepine, phenobarbital, and phenytoin (which may decrease serum levels of atazanavir).
The new rules alter sections 4.3 and 4.5 of the summary of product characteristics (SmPC) to reclassify drug–drug interactions with the new contraindications.
Atazanavir is an orally administered drug, used in combination with low-dose ritonavir (Norvir) to boost its pharmacokinetics. It is indicated for the treatment of HIV-1 infected adults and pediatric patients 3 months of age and older in combination with other antiretroviral medicinal products. A combination preparation boosted with cobicistat (Evotaz) is also available.
The drug is an azapeptide HIV-1 protease inhibitor (PI) that selectively inhibits the virus-specific processing of viral Gag-Pol proteins in HIV-1 infected cells, thus preventing formation of mature virions and infection of other cells. This prevents the virus from multiplying and slows the spread of infection. Based on available virological and clinical data from adult patients, no benefit is expected in patients with HIV strains resistant to multiple protease inhibitors (four or more PI mutations).
Therapy with atazanavir is intended to be initiated by a physician experienced in the management of HIV infection, with the choice of atazanavir in treatment-experienced adult and pediatric patients based on individual viral resistance testing and the patient’s treatment history. The standard dose is 300 mg atazanavir taken with 100 mg ritonavir once daily with food.
Atazanavir is already contraindicated in combination or coadministration with a wide variety of other agents:
- Coadministration with simvastatin or lovastatin [statins – risk of increased blood levels with atazanavir].
- Combination with the anti-TB antibiotic rifampicin.
- Combination with the PDE5 inhibitor sildenafil when used for the treatment of pulmonary arterial hypertension only.
- Coadministration with substrates of the CYP3A4 isoform of cytochrome P450 that have narrow therapeutic windows (eg, quetiapine, lurasidone, alfuzosin, astemizole, terfenadine, cisapride, pimozide, quinidine, bepridil, triazolam, oral midazolam, lomitapide, and ergot alkaloids).
- Coadministration with grazoprevir-containing products, including elbasvir/grazoprevir fixed dose combination (hepatitis C drug combination; atazanavir increases its blood levels).
- Coadministration with glecaprevir/pibrentasvir fixed dose combination (hepatitis C drug combination; increased hepatotoxicity due to increased bilirubin concentration).
- Coadministration with products containing St. John’s wort (Hypericum perforatum).
The EMA said detailed recommendations for the use of atazanavir will be described in the updated SmPC, which will be published in the revised European public assessment report after a decision on this change to the marketing authorization has been granted by the European Commission.
A version of this article appeared on Medscape.com.
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) this week recommended new contraindications on the coadministration of the protease inhibitor atazanavir (Reyataz, Bristol-Myers Squibb) with antineoplastic agents encorafenib and ivosidenib (atazanavir may significantly increase blood levels and thus side effects), and with the anticonvulsants carbamazepine, phenobarbital, and phenytoin (which may decrease serum levels of atazanavir).
The new rules alter sections 4.3 and 4.5 of the summary of product characteristics (SmPC) to reclassify drug–drug interactions with the new contraindications.
Atazanavir is an orally administered drug, used in combination with low-dose ritonavir (Norvir) to boost its pharmacokinetics. It is indicated for the treatment of HIV-1 infected adults and pediatric patients 3 months of age and older in combination with other antiretroviral medicinal products. A combination preparation boosted with cobicistat (Evotaz) is also available.
The drug is an azapeptide HIV-1 protease inhibitor (PI) that selectively inhibits the virus-specific processing of viral Gag-Pol proteins in HIV-1 infected cells, thus preventing formation of mature virions and infection of other cells. This prevents the virus from multiplying and slows the spread of infection. Based on available virological and clinical data from adult patients, no benefit is expected in patients with HIV strains resistant to multiple protease inhibitors (four or more PI mutations).
Therapy with atazanavir is intended to be initiated by a physician experienced in the management of HIV infection, with the choice of atazanavir in treatment-experienced adult and pediatric patients based on individual viral resistance testing and the patient’s treatment history. The standard dose is 300 mg atazanavir taken with 100 mg ritonavir once daily with food.
Atazanavir is already contraindicated in combination or coadministration with a wide variety of other agents:
- Coadministration with simvastatin or lovastatin [statins – risk of increased blood levels with atazanavir].
- Combination with the anti-TB antibiotic rifampicin.
- Combination with the PDE5 inhibitor sildenafil when used for the treatment of pulmonary arterial hypertension only.
- Coadministration with substrates of the CYP3A4 isoform of cytochrome P450 that have narrow therapeutic windows (eg, quetiapine, lurasidone, alfuzosin, astemizole, terfenadine, cisapride, pimozide, quinidine, bepridil, triazolam, oral midazolam, lomitapide, and ergot alkaloids).
- Coadministration with grazoprevir-containing products, including elbasvir/grazoprevir fixed dose combination (hepatitis C drug combination; atazanavir increases its blood levels).
- Coadministration with glecaprevir/pibrentasvir fixed dose combination (hepatitis C drug combination; increased hepatotoxicity due to increased bilirubin concentration).
- Coadministration with products containing St. John’s wort (Hypericum perforatum).
The EMA said detailed recommendations for the use of atazanavir will be described in the updated SmPC, which will be published in the revised European public assessment report after a decision on this change to the marketing authorization has been granted by the European Commission.
A version of this article appeared on Medscape.com.
Vaccine Against Urinary Tract Infections in Development
Urinary tract infections are among the most common bacterial infections. They can be painful, require antibiotic treatments, and recur in 20%-30% of cases. With the risk for the emergence or increase of resistance to antibiotics, it is important to search for potential therapeutic alternatives to treat or prevent urinary tract infections.
The MV140 Vaccine
The MV140 vaccine is produced by the Spanish pharmaceutical company Immunotek. MV140, known as Uromune, consists of a suspension of whole heat-inactivated bacteria in glycerol, sodium chloride, an artificial pineapple flavor, and water. It includes equal percentages of strains from four bacterial species (V121 Escherichia coli, V113 Klebsiella pneumoniae, V125 Enterococcus faecalis, and V127 Proteus vulgaris). MV140 is administered sublingually by spraying two 100-µL doses daily for 3 months.
The vaccine is in phase 2-3 of development. It is available under special access programs outside of marketing authorization in 26 countries, including Spain, Portugal, the United Kingdom, Lithuania, the Netherlands, Sweden, Norway, Australia, New Zealand, and Chile. Recently, MV140 was approved in Mexico and the Dominican Republic and submitted to Health Canada for registration.
A randomized study published in 2022 showed the vaccine›s efficacy in preventing urinary tract infections over 9 months. In total, 240 women with a urinary tract infection received MV140 for either 3 or 6 months or a placebo for 6 months. The primary outcome was the number of urinary tract infection episodes during the 9-month study period after vaccination.
In this pivotal study, MV140 administration for 3 and 6 months was associated with a significant reduction in the median number of urinary tract infection episodes, from 3.0 to 0.0 compared with the placebo during the 9-month efficacy period. The median time to the first urinary tract infection after 3 months of treatment was 275.0 days in the MV140 groups compared with 48.0 days in the placebo group.
Nine-Year Follow-Up
On April 6 at the 2024 congress of The European Association of Urology, urologists from the Royal Berkshire NHS Foundation Trust presented the results of a study evaluating the MV140 vaccine spray for long-term prevention of bacterial urinary tract infections.
This was a prospective cohort study involving 89 participants (72 women and 17 men) older than 18 years with recurrent urinary tract infections who received a course of MV140 for 3 months. Participants had no urinary tract infection when offered the vaccine and had no other urinary abnormalities (such as tumors, stones, or kidney infections).
Postvaccination follow-up was conducted over a 9-year period, during which researchers analyzed the data from the electronic health records of their initial cohort. They queried participants about the occurrence of urinary tract infections since receiving the vaccine and about potential related side effects. Thus, the results were self-reported.
Long-Term Efficacy
In this cohort, 48 participants (59%) reported having no infections during the 9-year follow-up. In the cohort of 89 participants, the average period without infection was 54.7 months (4.5 years; 56.7 months for women and 44.3 months for men). No vaccine-related side effects were observed.
The study’s limitations included the small number of participants and the collection of self-reported data. Furthermore, all cases were simple urinary tract infections without complications.
The authors concluded that “9 years after first receiving the sublingual spray MV140 vaccine, 54% of participants remained free from urinary tract infection.” For them, “this vaccine is safe in the long-term, and our participants reported fewer urinary tract infections and, if any, they were less severe.”
Vaccination could thus be an alternative to antibiotic treatments and could help combat the emergence of antibiotic resistance. The full study results should be published by the end of 2024.
Other studies are planned to evaluate the efficacy and safety of the MV140 vaccine in older patients residing in long-term care homes, in children suffering from acute urinary tract infections, and in adults suffering from complicated acute urinary tract infections (for example, patients with a catheter or with a neurogenic bladder).
This story was translated from JIM, which is part of the Medscape Professional Network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Urinary tract infections are among the most common bacterial infections. They can be painful, require antibiotic treatments, and recur in 20%-30% of cases. With the risk for the emergence or increase of resistance to antibiotics, it is important to search for potential therapeutic alternatives to treat or prevent urinary tract infections.
The MV140 Vaccine
The MV140 vaccine is produced by the Spanish pharmaceutical company Immunotek. MV140, known as Uromune, consists of a suspension of whole heat-inactivated bacteria in glycerol, sodium chloride, an artificial pineapple flavor, and water. It includes equal percentages of strains from four bacterial species (V121 Escherichia coli, V113 Klebsiella pneumoniae, V125 Enterococcus faecalis, and V127 Proteus vulgaris). MV140 is administered sublingually by spraying two 100-µL doses daily for 3 months.
The vaccine is in phase 2-3 of development. It is available under special access programs outside of marketing authorization in 26 countries, including Spain, Portugal, the United Kingdom, Lithuania, the Netherlands, Sweden, Norway, Australia, New Zealand, and Chile. Recently, MV140 was approved in Mexico and the Dominican Republic and submitted to Health Canada for registration.
A randomized study published in 2022 showed the vaccine›s efficacy in preventing urinary tract infections over 9 months. In total, 240 women with a urinary tract infection received MV140 for either 3 or 6 months or a placebo for 6 months. The primary outcome was the number of urinary tract infection episodes during the 9-month study period after vaccination.
In this pivotal study, MV140 administration for 3 and 6 months was associated with a significant reduction in the median number of urinary tract infection episodes, from 3.0 to 0.0 compared with the placebo during the 9-month efficacy period. The median time to the first urinary tract infection after 3 months of treatment was 275.0 days in the MV140 groups compared with 48.0 days in the placebo group.
Nine-Year Follow-Up
On April 6 at the 2024 congress of The European Association of Urology, urologists from the Royal Berkshire NHS Foundation Trust presented the results of a study evaluating the MV140 vaccine spray for long-term prevention of bacterial urinary tract infections.
This was a prospective cohort study involving 89 participants (72 women and 17 men) older than 18 years with recurrent urinary tract infections who received a course of MV140 for 3 months. Participants had no urinary tract infection when offered the vaccine and had no other urinary abnormalities (such as tumors, stones, or kidney infections).
Postvaccination follow-up was conducted over a 9-year period, during which researchers analyzed the data from the electronic health records of their initial cohort. They queried participants about the occurrence of urinary tract infections since receiving the vaccine and about potential related side effects. Thus, the results were self-reported.
Long-Term Efficacy
In this cohort, 48 participants (59%) reported having no infections during the 9-year follow-up. In the cohort of 89 participants, the average period without infection was 54.7 months (4.5 years; 56.7 months for women and 44.3 months for men). No vaccine-related side effects were observed.
The study’s limitations included the small number of participants and the collection of self-reported data. Furthermore, all cases were simple urinary tract infections without complications.
The authors concluded that “9 years after first receiving the sublingual spray MV140 vaccine, 54% of participants remained free from urinary tract infection.” For them, “this vaccine is safe in the long-term, and our participants reported fewer urinary tract infections and, if any, they were less severe.”
Vaccination could thus be an alternative to antibiotic treatments and could help combat the emergence of antibiotic resistance. The full study results should be published by the end of 2024.
Other studies are planned to evaluate the efficacy and safety of the MV140 vaccine in older patients residing in long-term care homes, in children suffering from acute urinary tract infections, and in adults suffering from complicated acute urinary tract infections (for example, patients with a catheter or with a neurogenic bladder).
This story was translated from JIM, which is part of the Medscape Professional Network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Urinary tract infections are among the most common bacterial infections. They can be painful, require antibiotic treatments, and recur in 20%-30% of cases. With the risk for the emergence or increase of resistance to antibiotics, it is important to search for potential therapeutic alternatives to treat or prevent urinary tract infections.
The MV140 Vaccine
The MV140 vaccine is produced by the Spanish pharmaceutical company Immunotek. MV140, known as Uromune, consists of a suspension of whole heat-inactivated bacteria in glycerol, sodium chloride, an artificial pineapple flavor, and water. It includes equal percentages of strains from four bacterial species (V121 Escherichia coli, V113 Klebsiella pneumoniae, V125 Enterococcus faecalis, and V127 Proteus vulgaris). MV140 is administered sublingually by spraying two 100-µL doses daily for 3 months.
The vaccine is in phase 2-3 of development. It is available under special access programs outside of marketing authorization in 26 countries, including Spain, Portugal, the United Kingdom, Lithuania, the Netherlands, Sweden, Norway, Australia, New Zealand, and Chile. Recently, MV140 was approved in Mexico and the Dominican Republic and submitted to Health Canada for registration.
A randomized study published in 2022 showed the vaccine›s efficacy in preventing urinary tract infections over 9 months. In total, 240 women with a urinary tract infection received MV140 for either 3 or 6 months or a placebo for 6 months. The primary outcome was the number of urinary tract infection episodes during the 9-month study period after vaccination.
In this pivotal study, MV140 administration for 3 and 6 months was associated with a significant reduction in the median number of urinary tract infection episodes, from 3.0 to 0.0 compared with the placebo during the 9-month efficacy period. The median time to the first urinary tract infection after 3 months of treatment was 275.0 days in the MV140 groups compared with 48.0 days in the placebo group.
Nine-Year Follow-Up
On April 6 at the 2024 congress of The European Association of Urology, urologists from the Royal Berkshire NHS Foundation Trust presented the results of a study evaluating the MV140 vaccine spray for long-term prevention of bacterial urinary tract infections.
This was a prospective cohort study involving 89 participants (72 women and 17 men) older than 18 years with recurrent urinary tract infections who received a course of MV140 for 3 months. Participants had no urinary tract infection when offered the vaccine and had no other urinary abnormalities (such as tumors, stones, or kidney infections).
Postvaccination follow-up was conducted over a 9-year period, during which researchers analyzed the data from the electronic health records of their initial cohort. They queried participants about the occurrence of urinary tract infections since receiving the vaccine and about potential related side effects. Thus, the results were self-reported.
Long-Term Efficacy
In this cohort, 48 participants (59%) reported having no infections during the 9-year follow-up. In the cohort of 89 participants, the average period without infection was 54.7 months (4.5 years; 56.7 months for women and 44.3 months for men). No vaccine-related side effects were observed.
The study’s limitations included the small number of participants and the collection of self-reported data. Furthermore, all cases were simple urinary tract infections without complications.
The authors concluded that “9 years after first receiving the sublingual spray MV140 vaccine, 54% of participants remained free from urinary tract infection.” For them, “this vaccine is safe in the long-term, and our participants reported fewer urinary tract infections and, if any, they were less severe.”
Vaccination could thus be an alternative to antibiotic treatments and could help combat the emergence of antibiotic resistance. The full study results should be published by the end of 2024.
Other studies are planned to evaluate the efficacy and safety of the MV140 vaccine in older patients residing in long-term care homes, in children suffering from acute urinary tract infections, and in adults suffering from complicated acute urinary tract infections (for example, patients with a catheter or with a neurogenic bladder).
This story was translated from JIM, which is part of the Medscape Professional Network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
FDA Approves New Antibiotic for Uncomplicated UTIs
The US Food and Drug Administration (FDA) has approved a new treatment for uncomplicated urinary tract infections (UTIs).
The agency on April 24 approved pivmecillinam tablets to treat women aged 18 years or older with UTIs caused by bacteria susceptible to the drug.
The beta-lactam antibiotic already is approved in Europe and has been used for more than 40 years outside of the United States to treat infections, according to the drug’s manufacturer, Utility Therapeutics.
The drug is an aminopenicillin that rapidly converts to mecillinam, according to the company, which is marketing the medication as Pivya.
Pivmecillinam is intended to treat UTIs caused by susceptible isolates of Escherichia coli, Proteus mirabilis, and Staphylococcus saprophyticus.
Researchers studied the treatment in three clinical trials. One study found women who received the new antibiotic were more likely to have resolution of symptoms and a reduction in bacteria in urine compared with placebo (62% vs 10%). Similar results were seen in a trial that used ibuprofen as the comparator (66% vs 22%).
In a third study that assessed two oral antibacterial drugs, 72% of women who received pivmecillinam and 76% who received the other drug achieved resolution of symptoms and a reduction in bacteria, according to the FDA.
The most common side effects of pivmecillinam include nausea and diarrhea.
About half of all women will experience at least one UTI in their lifetime, and the infections are one the top reasons for antibiotic prescriptions, the FDA noted.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved a new treatment for uncomplicated urinary tract infections (UTIs).
The agency on April 24 approved pivmecillinam tablets to treat women aged 18 years or older with UTIs caused by bacteria susceptible to the drug.
The beta-lactam antibiotic already is approved in Europe and has been used for more than 40 years outside of the United States to treat infections, according to the drug’s manufacturer, Utility Therapeutics.
The drug is an aminopenicillin that rapidly converts to mecillinam, according to the company, which is marketing the medication as Pivya.
Pivmecillinam is intended to treat UTIs caused by susceptible isolates of Escherichia coli, Proteus mirabilis, and Staphylococcus saprophyticus.
Researchers studied the treatment in three clinical trials. One study found women who received the new antibiotic were more likely to have resolution of symptoms and a reduction in bacteria in urine compared with placebo (62% vs 10%). Similar results were seen in a trial that used ibuprofen as the comparator (66% vs 22%).
In a third study that assessed two oral antibacterial drugs, 72% of women who received pivmecillinam and 76% who received the other drug achieved resolution of symptoms and a reduction in bacteria, according to the FDA.
The most common side effects of pivmecillinam include nausea and diarrhea.
About half of all women will experience at least one UTI in their lifetime, and the infections are one the top reasons for antibiotic prescriptions, the FDA noted.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved a new treatment for uncomplicated urinary tract infections (UTIs).
The agency on April 24 approved pivmecillinam tablets to treat women aged 18 years or older with UTIs caused by bacteria susceptible to the drug.
The beta-lactam antibiotic already is approved in Europe and has been used for more than 40 years outside of the United States to treat infections, according to the drug’s manufacturer, Utility Therapeutics.
The drug is an aminopenicillin that rapidly converts to mecillinam, according to the company, which is marketing the medication as Pivya.
Pivmecillinam is intended to treat UTIs caused by susceptible isolates of Escherichia coli, Proteus mirabilis, and Staphylococcus saprophyticus.
Researchers studied the treatment in three clinical trials. One study found women who received the new antibiotic were more likely to have resolution of symptoms and a reduction in bacteria in urine compared with placebo (62% vs 10%). Similar results were seen in a trial that used ibuprofen as the comparator (66% vs 22%).
In a third study that assessed two oral antibacterial drugs, 72% of women who received pivmecillinam and 76% who received the other drug achieved resolution of symptoms and a reduction in bacteria, according to the FDA.
The most common side effects of pivmecillinam include nausea and diarrhea.
About half of all women will experience at least one UTI in their lifetime, and the infections are one the top reasons for antibiotic prescriptions, the FDA noted.
A version of this article appeared on Medscape.com.
