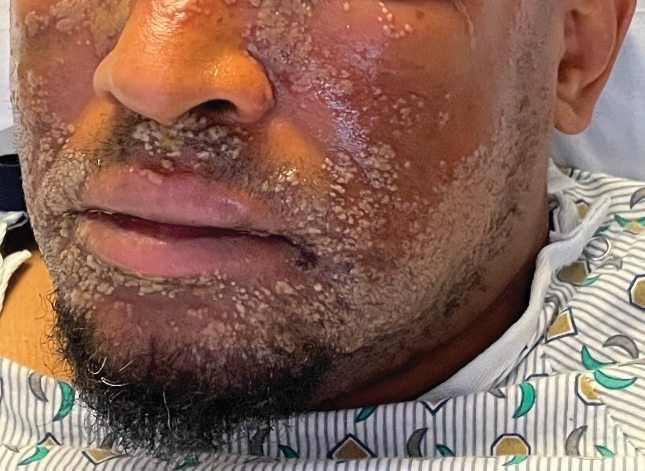
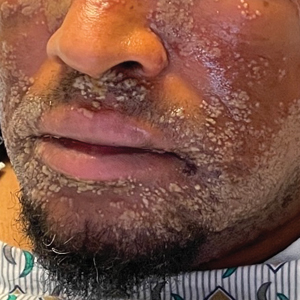
User login
Intense exercise may lead to colds. A new study tells us why
Can too much of a healthy habit become bad?
Lots of evidence shows that regular exercise wards off respiratory infections such as colds, flu, and COVID-19. However, according to a new study.
The findings come as we enter another possible tripledemic this winter, with an increase in COVID, flu, and respiratory syncytial virus (RSV). Public health officials are on alert for a potentially severe flu season, following high flu activity this year in Australia (which can help predict how bad the U.S. flu season will be).
Studies show that the risk for acute respiratory infections is lower in people who exercise regularly. Physically active people are also less likely to suffer severe outcomes from COVID.
But while inactivity has emerged as a potential risk factor for respiratory infections, scientists have long proposed that too much activity, particularly of a prolonged and highly intense nature, may also increase susceptibility.
“The theory suggests that a short-term suppression of the immune system following intense exercise leads to an increase in susceptibility to infection, especially upper respiratory illness,” said Choukri Ben Mamoun, PhD, professor of medicine (infectious diseases) and microbial pathogenesis at the Yale Institute for Global Health, New Haven, Conn. Researchers have documented a greater incidence of upper respiratory illness “among both highly trained and healthy untrained individuals following increased activity during competition or heaving training blocks.”
That’s important if you treat athletes or patients with physically demanding jobs that push them to their physical limits, such as firefighters, police officers, or military personnel.
The new study was small but sheds light on a possible mechanism. Researchers tested blood, saliva, and urine samples from 11 firefighters before and 10 minutes after intense exercise designed to mimic wildfire fighting. The firefighters hiked over hilly terrain for 45 minutes in humid weather wearing up to 44 pounds of wildland gear.
After the workout, subjects had fewer proinflammatory cytokines and ceramides, and more antimicrobial peptides, changes that indicate a greater susceptibility to infection, researchers said. A systematic review adds weight to their findings, revealing a handful of studies in marathon runners, firefighters, soldiers, and soccer players that found an increase in respiratory symptoms after strenuous workouts.
“The relationship between exercise and the immune system is complex and varies from person to person,” said Dr. Mamoun, who was not part of the study. “Physicians can use this study’s findings to provide individualized exercise recommendations.”
An adaptive mechanism gone awry
During intense exercise, the body may reduce airway inflammation to help you breathe, say the authors. The boost in antimicrobial peptides found in the saliva samples could be the body’s way of compensating for the diminished immune function.
Antimicrobial peptides are part of the immune response but they’re “usually not very effective for viral infections,” said lead author Ernesto Nakayasu, PhD, senior research scientist at the Pacific Northwest National Laboratory, a U.S. Department of Energy lab in Richland, Washington. “That’s why we think it may make you more exposed to respiratory infections.”
The drop in proinflammatory molecules had an inverse relationship with opiorphin, a peripheral tissue vasodilator thought to increase blood flow and improve oxygen delivery to the muscles during exercise. This may be an adaptive mechanism to improve gas exchange in response to greater oxygen demand.
But as with many adaptive mechanisms, this one may have an unintended consequence. Fewer proinflammatory molecules on patrol may leave you more vulnerable to infection. Plus, during intense exercise, people tend to breathe through their mouths, bypassing the nasal barriers and allowing more microbes – including viruses – to penetrate and deposit in the distal airways of the lungs.
Advice for patients
More research is needed to know exactly how long and how strenuously one needs to exercise to trigger these immune changes, Dr. Nakayasu said.
As shown by their lactate accumulation (an indicator of anaerobic metabolism), the firefighters in the study outpaced the average person’s aerobic respiratory capacity, meaning the average person doing moderate exercise likely wouldn’t trigger these changes.
“Regular moderate exercise is generally associated with better health outcomes [and] improved immune function,” said Dr. Mamoun. For those who exercise to the extreme, proper rest and recovery are “essential for maintaining a robust immune system,” Dr. Mamoun said.
And of course, you can encourage patients to get vaccinated. Young, healthy patients may assume they don’t need COVID-19 or flu shots, as indicated by a recent survey that found one-third of Americans feel they don’t need these vaccinations if they’re not high risk.
A version of this article first appeared on Medscape.com.
Can too much of a healthy habit become bad?
Lots of evidence shows that regular exercise wards off respiratory infections such as colds, flu, and COVID-19. However, according to a new study.
The findings come as we enter another possible tripledemic this winter, with an increase in COVID, flu, and respiratory syncytial virus (RSV). Public health officials are on alert for a potentially severe flu season, following high flu activity this year in Australia (which can help predict how bad the U.S. flu season will be).
Studies show that the risk for acute respiratory infections is lower in people who exercise regularly. Physically active people are also less likely to suffer severe outcomes from COVID.
But while inactivity has emerged as a potential risk factor for respiratory infections, scientists have long proposed that too much activity, particularly of a prolonged and highly intense nature, may also increase susceptibility.
“The theory suggests that a short-term suppression of the immune system following intense exercise leads to an increase in susceptibility to infection, especially upper respiratory illness,” said Choukri Ben Mamoun, PhD, professor of medicine (infectious diseases) and microbial pathogenesis at the Yale Institute for Global Health, New Haven, Conn. Researchers have documented a greater incidence of upper respiratory illness “among both highly trained and healthy untrained individuals following increased activity during competition or heaving training blocks.”
That’s important if you treat athletes or patients with physically demanding jobs that push them to their physical limits, such as firefighters, police officers, or military personnel.
The new study was small but sheds light on a possible mechanism. Researchers tested blood, saliva, and urine samples from 11 firefighters before and 10 minutes after intense exercise designed to mimic wildfire fighting. The firefighters hiked over hilly terrain for 45 minutes in humid weather wearing up to 44 pounds of wildland gear.
After the workout, subjects had fewer proinflammatory cytokines and ceramides, and more antimicrobial peptides, changes that indicate a greater susceptibility to infection, researchers said. A systematic review adds weight to their findings, revealing a handful of studies in marathon runners, firefighters, soldiers, and soccer players that found an increase in respiratory symptoms after strenuous workouts.
“The relationship between exercise and the immune system is complex and varies from person to person,” said Dr. Mamoun, who was not part of the study. “Physicians can use this study’s findings to provide individualized exercise recommendations.”
An adaptive mechanism gone awry
During intense exercise, the body may reduce airway inflammation to help you breathe, say the authors. The boost in antimicrobial peptides found in the saliva samples could be the body’s way of compensating for the diminished immune function.
Antimicrobial peptides are part of the immune response but they’re “usually not very effective for viral infections,” said lead author Ernesto Nakayasu, PhD, senior research scientist at the Pacific Northwest National Laboratory, a U.S. Department of Energy lab in Richland, Washington. “That’s why we think it may make you more exposed to respiratory infections.”
The drop in proinflammatory molecules had an inverse relationship with opiorphin, a peripheral tissue vasodilator thought to increase blood flow and improve oxygen delivery to the muscles during exercise. This may be an adaptive mechanism to improve gas exchange in response to greater oxygen demand.
But as with many adaptive mechanisms, this one may have an unintended consequence. Fewer proinflammatory molecules on patrol may leave you more vulnerable to infection. Plus, during intense exercise, people tend to breathe through their mouths, bypassing the nasal barriers and allowing more microbes – including viruses – to penetrate and deposit in the distal airways of the lungs.
Advice for patients
More research is needed to know exactly how long and how strenuously one needs to exercise to trigger these immune changes, Dr. Nakayasu said.
As shown by their lactate accumulation (an indicator of anaerobic metabolism), the firefighters in the study outpaced the average person’s aerobic respiratory capacity, meaning the average person doing moderate exercise likely wouldn’t trigger these changes.
“Regular moderate exercise is generally associated with better health outcomes [and] improved immune function,” said Dr. Mamoun. For those who exercise to the extreme, proper rest and recovery are “essential for maintaining a robust immune system,” Dr. Mamoun said.
And of course, you can encourage patients to get vaccinated. Young, healthy patients may assume they don’t need COVID-19 or flu shots, as indicated by a recent survey that found one-third of Americans feel they don’t need these vaccinations if they’re not high risk.
A version of this article first appeared on Medscape.com.
Can too much of a healthy habit become bad?
Lots of evidence shows that regular exercise wards off respiratory infections such as colds, flu, and COVID-19. However, according to a new study.
The findings come as we enter another possible tripledemic this winter, with an increase in COVID, flu, and respiratory syncytial virus (RSV). Public health officials are on alert for a potentially severe flu season, following high flu activity this year in Australia (which can help predict how bad the U.S. flu season will be).
Studies show that the risk for acute respiratory infections is lower in people who exercise regularly. Physically active people are also less likely to suffer severe outcomes from COVID.
But while inactivity has emerged as a potential risk factor for respiratory infections, scientists have long proposed that too much activity, particularly of a prolonged and highly intense nature, may also increase susceptibility.
“The theory suggests that a short-term suppression of the immune system following intense exercise leads to an increase in susceptibility to infection, especially upper respiratory illness,” said Choukri Ben Mamoun, PhD, professor of medicine (infectious diseases) and microbial pathogenesis at the Yale Institute for Global Health, New Haven, Conn. Researchers have documented a greater incidence of upper respiratory illness “among both highly trained and healthy untrained individuals following increased activity during competition or heaving training blocks.”
That’s important if you treat athletes or patients with physically demanding jobs that push them to their physical limits, such as firefighters, police officers, or military personnel.
The new study was small but sheds light on a possible mechanism. Researchers tested blood, saliva, and urine samples from 11 firefighters before and 10 minutes after intense exercise designed to mimic wildfire fighting. The firefighters hiked over hilly terrain for 45 minutes in humid weather wearing up to 44 pounds of wildland gear.
After the workout, subjects had fewer proinflammatory cytokines and ceramides, and more antimicrobial peptides, changes that indicate a greater susceptibility to infection, researchers said. A systematic review adds weight to their findings, revealing a handful of studies in marathon runners, firefighters, soldiers, and soccer players that found an increase in respiratory symptoms after strenuous workouts.
“The relationship between exercise and the immune system is complex and varies from person to person,” said Dr. Mamoun, who was not part of the study. “Physicians can use this study’s findings to provide individualized exercise recommendations.”
An adaptive mechanism gone awry
During intense exercise, the body may reduce airway inflammation to help you breathe, say the authors. The boost in antimicrobial peptides found in the saliva samples could be the body’s way of compensating for the diminished immune function.
Antimicrobial peptides are part of the immune response but they’re “usually not very effective for viral infections,” said lead author Ernesto Nakayasu, PhD, senior research scientist at the Pacific Northwest National Laboratory, a U.S. Department of Energy lab in Richland, Washington. “That’s why we think it may make you more exposed to respiratory infections.”
The drop in proinflammatory molecules had an inverse relationship with opiorphin, a peripheral tissue vasodilator thought to increase blood flow and improve oxygen delivery to the muscles during exercise. This may be an adaptive mechanism to improve gas exchange in response to greater oxygen demand.
But as with many adaptive mechanisms, this one may have an unintended consequence. Fewer proinflammatory molecules on patrol may leave you more vulnerable to infection. Plus, during intense exercise, people tend to breathe through their mouths, bypassing the nasal barriers and allowing more microbes – including viruses – to penetrate and deposit in the distal airways of the lungs.
Advice for patients
More research is needed to know exactly how long and how strenuously one needs to exercise to trigger these immune changes, Dr. Nakayasu said.
As shown by their lactate accumulation (an indicator of anaerobic metabolism), the firefighters in the study outpaced the average person’s aerobic respiratory capacity, meaning the average person doing moderate exercise likely wouldn’t trigger these changes.
“Regular moderate exercise is generally associated with better health outcomes [and] improved immune function,” said Dr. Mamoun. For those who exercise to the extreme, proper rest and recovery are “essential for maintaining a robust immune system,” Dr. Mamoun said.
And of course, you can encourage patients to get vaccinated. Young, healthy patients may assume they don’t need COVID-19 or flu shots, as indicated by a recent survey that found one-third of Americans feel they don’t need these vaccinations if they’re not high risk.
A version of this article first appeared on Medscape.com.
FROM MILITARY MEDICAL RESEARCH
Childhood immunization schedule includes new RSV, mpox, meningococcal, and pneumococcal vaccines
The immunization schedule for children and adolescents, summarized as an American Academy of Pediatrics policy statement in the journal Pediatrics, contains new entries for the monoclonal antibody immunization nirsevimab for respiratory syncytial virus in infants, the maternal RSV vaccine RSVpreF for pregnant people, the mpox vaccine for adolescents, the 2023-2024 COVID-19 vaccine, the 20-valent pneumococcal conjugate vaccine (PCV20), and the pentavalent meningococcal vaccine (MenACWY-TT/MenB-FHbp).
A number of immunizations have been deleted from the 2024 schedule, including the pentavalent meningococcal vaccine MenABCWY because of a discontinuation in its distribution in the United States, the bivalent mRNA COVID-19 vaccines, the diphtheria and tetanus toxoids adsorbed vaccine, the 13-valent pneumococcal conjugate vaccine (PCV13), and the pneumococcal polysaccharide vaccine (PPSV23).
The 2024 childhood and adolescent immunization schedule, also approved by the Centers for Disease Control and Prevention, American Academy of Family Physicians, American College of Obstetricians and Gynecologists, American College of Nurse-Midwives, American Academy of Physician Associates, and National Association of Pediatric Nurse Practitioners, is published each year based on current recommendations that have been approved for use by the Food and Drug Administration.
In a press release, the AAP said the CDC decided to publish the recommendations early to ensure health providers are able to administer immunizations and that they are covered by insurance. They also referenced CDC reports that found vaccination rates for kindergarteners have not bounced back since the beginning of the COVID-19 pandemic, and vaccine exemptions for the 2022-2023 school year were at an “all-time high.”
RSV
New to the schedule are the recently approved RSV monoclonal antibody nirsevimab for infants and the RSV vaccine RSVpreF for pregnant people. According to the CDC’s combined immunization schedule for 2024, the timing of the infant RSV immunization is heavily dependent upon when and whether a RSV vaccine was administered during pregnancy. The RSV vaccine should be routinely given between 32 weeks and 36 weeks of gestation between September and January in most of the United States with the caveat that either the maternal vaccine or the infant immunization is recommended.
Infants born between October and March in most of the United States are eligible for the RSV immunization within 14 days of birth if the pregnant parent did not receive an RSV vaccine during pregnancy, or if the parent received the vaccine in the 14 days prior to birth. For infants born between April and September RSV immunization is recommended prior to the start of RSV season.
The immunization is also recommended for infants who were hospitalized for conditions such as prematurity after birth between October and March, infants aged 8-19 months who are undergoing medical support related to prematurity, infants aged 8-19 months who are severely immunocompromised, and infants aged 9-19 months who are American Indian or Alaska Native, and infants undergoing cardiac surgery with cardiopulmonary bypass.
Mpox
Another new addition to the schedule is mpox, which is recommended for adolescents 18 years or older who are at risk for mpox infection, including gay, bisexual, nonbinary, transgender, or other individuals who have developed a sexually transmitted disease within the last 6 months, had more than one sexual partner, or engaged in sex in a commercial sex venue or public space with confirmed mpox transmission.
Currently, mpox vaccination during pregnancy is not recommended due to a lack of safety data on the vaccine during pregnancy; however, the CDC noted pregnant persons who have been exposed to any of the risk factors above may receive the vaccine.
COVID, influenza, pneumococcal vaccines
The COVID-19 vaccine recommendations were updated to reflect the 2023-2023 formulation of the vaccine. Unvaccinated children between 6 months and 4 years of age will now receive the 2023-2024 formula mRNA vaccines, which includes the two-dose Moderna vaccine and three-dose Pfizer vaccine for use in that age group. Children with a previous history of COVID-19 vaccination are eligible to receive an age-appropriate COVID-19 vaccine from the 2023-2024 formulation, and children between 5-11 years old and 12-18 years old can receive a single dose of an mRNA vaccine regardless of vaccine history; unvaccinated children 12-18 years old are also eligible to receive the two-dose Novavax vaccine.
For influenza, the schedule refers to the Advisory Committee on Immunization Practices recommendations released in August, with a note indicating that individuals with an egg allergy can receive another vaccine recommended for their age group without concerns for safety.
The pneumococcal vaccine recommendations have removed PCV13 completely, with updates on the PCV15, PCV20, and PPSV23 in sections on routine vaccination, catch-up vaccination, and special situations. The poliovirus section has also seen its catch-up section revised with a recommendation to complete a vaccination series in adolescents 18 years old known or suspected to have an incomplete series, and to count trivalent oral poliovirus vaccines and OPV administered before April 2016 toward U.S. vaccination requirements.
‘Timely and necessary’ changes
Michael Pichichero, MD, director of the Rochester (N.Y.) General Hospital Research Institute, said in an interview that the committee that developed the immunization schedule was thorough in its recommendations for children and adolescents.
“The additions are timely and necessary as the landscape of vaccines for children changes,” he said.
Bonnie M. Word, MD, director of the Houston Travel Medicine Clinic, said that the immunization schedule “sets the standard and provides clarification and uniformity for administration of all recommended vaccines for U.S. children.”
The U.S. immunization program “is one of the best success stories in medicine,” Dr. Wood said. She noted it is important for providers to become familiar with these vaccines and their indications “to provide advice and be able to respond to questions of parents and/or patients.
“Often patients spend more time with office staff than the physician. It is helpful to make sure everyone in the office understands the importance of and the rationale for immunizing, so families hear consistent messaging,” she said.
Dr. Pichichero and Dr. Word reported no relevant conflicts of interest.
The immunization schedule for children and adolescents, summarized as an American Academy of Pediatrics policy statement in the journal Pediatrics, contains new entries for the monoclonal antibody immunization nirsevimab for respiratory syncytial virus in infants, the maternal RSV vaccine RSVpreF for pregnant people, the mpox vaccine for adolescents, the 2023-2024 COVID-19 vaccine, the 20-valent pneumococcal conjugate vaccine (PCV20), and the pentavalent meningococcal vaccine (MenACWY-TT/MenB-FHbp).
A number of immunizations have been deleted from the 2024 schedule, including the pentavalent meningococcal vaccine MenABCWY because of a discontinuation in its distribution in the United States, the bivalent mRNA COVID-19 vaccines, the diphtheria and tetanus toxoids adsorbed vaccine, the 13-valent pneumococcal conjugate vaccine (PCV13), and the pneumococcal polysaccharide vaccine (PPSV23).
The 2024 childhood and adolescent immunization schedule, also approved by the Centers for Disease Control and Prevention, American Academy of Family Physicians, American College of Obstetricians and Gynecologists, American College of Nurse-Midwives, American Academy of Physician Associates, and National Association of Pediatric Nurse Practitioners, is published each year based on current recommendations that have been approved for use by the Food and Drug Administration.
In a press release, the AAP said the CDC decided to publish the recommendations early to ensure health providers are able to administer immunizations and that they are covered by insurance. They also referenced CDC reports that found vaccination rates for kindergarteners have not bounced back since the beginning of the COVID-19 pandemic, and vaccine exemptions for the 2022-2023 school year were at an “all-time high.”
RSV
New to the schedule are the recently approved RSV monoclonal antibody nirsevimab for infants and the RSV vaccine RSVpreF for pregnant people. According to the CDC’s combined immunization schedule for 2024, the timing of the infant RSV immunization is heavily dependent upon when and whether a RSV vaccine was administered during pregnancy. The RSV vaccine should be routinely given between 32 weeks and 36 weeks of gestation between September and January in most of the United States with the caveat that either the maternal vaccine or the infant immunization is recommended.
Infants born between October and March in most of the United States are eligible for the RSV immunization within 14 days of birth if the pregnant parent did not receive an RSV vaccine during pregnancy, or if the parent received the vaccine in the 14 days prior to birth. For infants born between April and September RSV immunization is recommended prior to the start of RSV season.
The immunization is also recommended for infants who were hospitalized for conditions such as prematurity after birth between October and March, infants aged 8-19 months who are undergoing medical support related to prematurity, infants aged 8-19 months who are severely immunocompromised, and infants aged 9-19 months who are American Indian or Alaska Native, and infants undergoing cardiac surgery with cardiopulmonary bypass.
Mpox
Another new addition to the schedule is mpox, which is recommended for adolescents 18 years or older who are at risk for mpox infection, including gay, bisexual, nonbinary, transgender, or other individuals who have developed a sexually transmitted disease within the last 6 months, had more than one sexual partner, or engaged in sex in a commercial sex venue or public space with confirmed mpox transmission.
Currently, mpox vaccination during pregnancy is not recommended due to a lack of safety data on the vaccine during pregnancy; however, the CDC noted pregnant persons who have been exposed to any of the risk factors above may receive the vaccine.
COVID, influenza, pneumococcal vaccines
The COVID-19 vaccine recommendations were updated to reflect the 2023-2023 formulation of the vaccine. Unvaccinated children between 6 months and 4 years of age will now receive the 2023-2024 formula mRNA vaccines, which includes the two-dose Moderna vaccine and three-dose Pfizer vaccine for use in that age group. Children with a previous history of COVID-19 vaccination are eligible to receive an age-appropriate COVID-19 vaccine from the 2023-2024 formulation, and children between 5-11 years old and 12-18 years old can receive a single dose of an mRNA vaccine regardless of vaccine history; unvaccinated children 12-18 years old are also eligible to receive the two-dose Novavax vaccine.
For influenza, the schedule refers to the Advisory Committee on Immunization Practices recommendations released in August, with a note indicating that individuals with an egg allergy can receive another vaccine recommended for their age group without concerns for safety.
The pneumococcal vaccine recommendations have removed PCV13 completely, with updates on the PCV15, PCV20, and PPSV23 in sections on routine vaccination, catch-up vaccination, and special situations. The poliovirus section has also seen its catch-up section revised with a recommendation to complete a vaccination series in adolescents 18 years old known or suspected to have an incomplete series, and to count trivalent oral poliovirus vaccines and OPV administered before April 2016 toward U.S. vaccination requirements.
‘Timely and necessary’ changes
Michael Pichichero, MD, director of the Rochester (N.Y.) General Hospital Research Institute, said in an interview that the committee that developed the immunization schedule was thorough in its recommendations for children and adolescents.
“The additions are timely and necessary as the landscape of vaccines for children changes,” he said.
Bonnie M. Word, MD, director of the Houston Travel Medicine Clinic, said that the immunization schedule “sets the standard and provides clarification and uniformity for administration of all recommended vaccines for U.S. children.”
The U.S. immunization program “is one of the best success stories in medicine,” Dr. Wood said. She noted it is important for providers to become familiar with these vaccines and their indications “to provide advice and be able to respond to questions of parents and/or patients.
“Often patients spend more time with office staff than the physician. It is helpful to make sure everyone in the office understands the importance of and the rationale for immunizing, so families hear consistent messaging,” she said.
Dr. Pichichero and Dr. Word reported no relevant conflicts of interest.
The immunization schedule for children and adolescents, summarized as an American Academy of Pediatrics policy statement in the journal Pediatrics, contains new entries for the monoclonal antibody immunization nirsevimab for respiratory syncytial virus in infants, the maternal RSV vaccine RSVpreF for pregnant people, the mpox vaccine for adolescents, the 2023-2024 COVID-19 vaccine, the 20-valent pneumococcal conjugate vaccine (PCV20), and the pentavalent meningococcal vaccine (MenACWY-TT/MenB-FHbp).
A number of immunizations have been deleted from the 2024 schedule, including the pentavalent meningococcal vaccine MenABCWY because of a discontinuation in its distribution in the United States, the bivalent mRNA COVID-19 vaccines, the diphtheria and tetanus toxoids adsorbed vaccine, the 13-valent pneumococcal conjugate vaccine (PCV13), and the pneumococcal polysaccharide vaccine (PPSV23).
The 2024 childhood and adolescent immunization schedule, also approved by the Centers for Disease Control and Prevention, American Academy of Family Physicians, American College of Obstetricians and Gynecologists, American College of Nurse-Midwives, American Academy of Physician Associates, and National Association of Pediatric Nurse Practitioners, is published each year based on current recommendations that have been approved for use by the Food and Drug Administration.
In a press release, the AAP said the CDC decided to publish the recommendations early to ensure health providers are able to administer immunizations and that they are covered by insurance. They also referenced CDC reports that found vaccination rates for kindergarteners have not bounced back since the beginning of the COVID-19 pandemic, and vaccine exemptions for the 2022-2023 school year were at an “all-time high.”
RSV
New to the schedule are the recently approved RSV monoclonal antibody nirsevimab for infants and the RSV vaccine RSVpreF for pregnant people. According to the CDC’s combined immunization schedule for 2024, the timing of the infant RSV immunization is heavily dependent upon when and whether a RSV vaccine was administered during pregnancy. The RSV vaccine should be routinely given between 32 weeks and 36 weeks of gestation between September and January in most of the United States with the caveat that either the maternal vaccine or the infant immunization is recommended.
Infants born between October and March in most of the United States are eligible for the RSV immunization within 14 days of birth if the pregnant parent did not receive an RSV vaccine during pregnancy, or if the parent received the vaccine in the 14 days prior to birth. For infants born between April and September RSV immunization is recommended prior to the start of RSV season.
The immunization is also recommended for infants who were hospitalized for conditions such as prematurity after birth between October and March, infants aged 8-19 months who are undergoing medical support related to prematurity, infants aged 8-19 months who are severely immunocompromised, and infants aged 9-19 months who are American Indian or Alaska Native, and infants undergoing cardiac surgery with cardiopulmonary bypass.
Mpox
Another new addition to the schedule is mpox, which is recommended for adolescents 18 years or older who are at risk for mpox infection, including gay, bisexual, nonbinary, transgender, or other individuals who have developed a sexually transmitted disease within the last 6 months, had more than one sexual partner, or engaged in sex in a commercial sex venue or public space with confirmed mpox transmission.
Currently, mpox vaccination during pregnancy is not recommended due to a lack of safety data on the vaccine during pregnancy; however, the CDC noted pregnant persons who have been exposed to any of the risk factors above may receive the vaccine.
COVID, influenza, pneumococcal vaccines
The COVID-19 vaccine recommendations were updated to reflect the 2023-2023 formulation of the vaccine. Unvaccinated children between 6 months and 4 years of age will now receive the 2023-2024 formula mRNA vaccines, which includes the two-dose Moderna vaccine and three-dose Pfizer vaccine for use in that age group. Children with a previous history of COVID-19 vaccination are eligible to receive an age-appropriate COVID-19 vaccine from the 2023-2024 formulation, and children between 5-11 years old and 12-18 years old can receive a single dose of an mRNA vaccine regardless of vaccine history; unvaccinated children 12-18 years old are also eligible to receive the two-dose Novavax vaccine.
For influenza, the schedule refers to the Advisory Committee on Immunization Practices recommendations released in August, with a note indicating that individuals with an egg allergy can receive another vaccine recommended for their age group without concerns for safety.
The pneumococcal vaccine recommendations have removed PCV13 completely, with updates on the PCV15, PCV20, and PPSV23 in sections on routine vaccination, catch-up vaccination, and special situations. The poliovirus section has also seen its catch-up section revised with a recommendation to complete a vaccination series in adolescents 18 years old known or suspected to have an incomplete series, and to count trivalent oral poliovirus vaccines and OPV administered before April 2016 toward U.S. vaccination requirements.
‘Timely and necessary’ changes
Michael Pichichero, MD, director of the Rochester (N.Y.) General Hospital Research Institute, said in an interview that the committee that developed the immunization schedule was thorough in its recommendations for children and adolescents.
“The additions are timely and necessary as the landscape of vaccines for children changes,” he said.
Bonnie M. Word, MD, director of the Houston Travel Medicine Clinic, said that the immunization schedule “sets the standard and provides clarification and uniformity for administration of all recommended vaccines for U.S. children.”
The U.S. immunization program “is one of the best success stories in medicine,” Dr. Wood said. She noted it is important for providers to become familiar with these vaccines and their indications “to provide advice and be able to respond to questions of parents and/or patients.
“Often patients spend more time with office staff than the physician. It is helpful to make sure everyone in the office understands the importance of and the rationale for immunizing, so families hear consistent messaging,” she said.
Dr. Pichichero and Dr. Word reported no relevant conflicts of interest.
FROM PEDIATRICS
Use the stool! Fecal microbiota transplants help kids with diarrheal infection
(AAP).
However, fecal microbiota transplants (FMTs) should not be used to treat other gastrointestinal ailments such as Crohn’s disease or ulcerative colitis, because scientific evidence falls short on effectiveness in treating these conditions, the group said.
C. difficile infections (CDIs) are major contributors to hospital-associated diarrhea and diarrhea caused by antibiotics. An FMT involves introducing the feces of a healthy person into the gastrointestinal tract, usually through a nasogastric tube but sometimes in capsules containing healthy stool. Serious adverse reactions associated with an FMT, such as hospitalization, are rare, occuring in roughly 2% of case, the AAP said.
An FMT “does have a place for treatment of recurrent CDIs in children,” said Maria Oliva-Hemker, MD, a pediatric gastroenterologist at Johns Hopkins University School of Medicine in Baltimore and the lead author of the report, which was online in Pediatrics.
The AAP strongly encourages people not to perform an FMT at home, although caregivers may be tempted due to a lack of medical facilities located nearby to deliver this care.
“People might see a video on YouTube and think they can do this themselves,” Dr. Oliva-Hemker said.
An FMT requires screening of donors for any infections, which involves administering questionnaires and analyzing donor blood and stool, which are tasks better suited for medical facilities than for a living room.
No controlled or prospective clinical trials on the efficacy of FMT for children exist, according to the AAP. But a retrospective study published in 2020 showed that one or two courses of FMT prevented CDI recurrence in children 87% of the time. Researchers defined the eradication of CDIs as no recurrence for at least 2 months after an FMT and noted the success rates in children were comaparable to those reported in adults.
Unlike pediatric data, adult data come from a randomized clinical trial.
“Sometimes, kids are the last people to be enrolled in these trials,” said Maribeth Nicholson, MD, MPH, a pediatric gastroenterologist at Vanderbilt University Medical Center in Nashville, Tenn., an author of the 2020 study.
Dr. Nicholson, who was not involved in the AAP report, said that the retrospective data are strong enough to justify using FMT to eradicate CDIs in children. But researchers are unclear about the biologic mechanisms that make FMTs work.
Dr. Nicholson said that many therapeutics meant to produce a healthier microbiome are being studied in clinical trials. Any clinical trials of such products should include children, Dr. Nicholson said. A child’s gastrointestinal microbiome is actively developing, Dr. Nicholson added, compared with the relatively stable microbiome of an adult.
“When we think about the microbiome it makes sense to target kids, because they’re more apt to respond to these therapies. I worry that somebody will say ‘this doesn’t work in adults,’ and it just stops there,” Dr. Nicholson said.
Though the AAP said that the benefits of FMT for treating CDIs are clear, the data available for treating other conditions such as ulcerative colitis or Crohn’s disease are less convincing. Any child receiving an FMT for these ailments should only do so as part of a clinical trial, the group said.
The AAP report endorses a joint position paper, published in 2019, about the benefits of FMTs for CDIs from North American and European pediatric gastroenterology societies. Dr. Nicholson was an author of this joint statement and hopes that the AAP report raises further awareness among pediatricians that FMTs are a safe and effective treatment for recurrent CDIs.
“This is something that maybe is not as discussed in pediatric circles. Kids need FMTs sometimes,” Dr. Nicholson said.
Dr. Oliva-Hemker and Dr. Nicholson report no relevant financial relationships.
A version of this article appeared on Medscape.com.
(AAP).
However, fecal microbiota transplants (FMTs) should not be used to treat other gastrointestinal ailments such as Crohn’s disease or ulcerative colitis, because scientific evidence falls short on effectiveness in treating these conditions, the group said.
C. difficile infections (CDIs) are major contributors to hospital-associated diarrhea and diarrhea caused by antibiotics. An FMT involves introducing the feces of a healthy person into the gastrointestinal tract, usually through a nasogastric tube but sometimes in capsules containing healthy stool. Serious adverse reactions associated with an FMT, such as hospitalization, are rare, occuring in roughly 2% of case, the AAP said.
An FMT “does have a place for treatment of recurrent CDIs in children,” said Maria Oliva-Hemker, MD, a pediatric gastroenterologist at Johns Hopkins University School of Medicine in Baltimore and the lead author of the report, which was online in Pediatrics.
The AAP strongly encourages people not to perform an FMT at home, although caregivers may be tempted due to a lack of medical facilities located nearby to deliver this care.
“People might see a video on YouTube and think they can do this themselves,” Dr. Oliva-Hemker said.
An FMT requires screening of donors for any infections, which involves administering questionnaires and analyzing donor blood and stool, which are tasks better suited for medical facilities than for a living room.
No controlled or prospective clinical trials on the efficacy of FMT for children exist, according to the AAP. But a retrospective study published in 2020 showed that one or two courses of FMT prevented CDI recurrence in children 87% of the time. Researchers defined the eradication of CDIs as no recurrence for at least 2 months after an FMT and noted the success rates in children were comaparable to those reported in adults.
Unlike pediatric data, adult data come from a randomized clinical trial.
“Sometimes, kids are the last people to be enrolled in these trials,” said Maribeth Nicholson, MD, MPH, a pediatric gastroenterologist at Vanderbilt University Medical Center in Nashville, Tenn., an author of the 2020 study.
Dr. Nicholson, who was not involved in the AAP report, said that the retrospective data are strong enough to justify using FMT to eradicate CDIs in children. But researchers are unclear about the biologic mechanisms that make FMTs work.
Dr. Nicholson said that many therapeutics meant to produce a healthier microbiome are being studied in clinical trials. Any clinical trials of such products should include children, Dr. Nicholson said. A child’s gastrointestinal microbiome is actively developing, Dr. Nicholson added, compared with the relatively stable microbiome of an adult.
“When we think about the microbiome it makes sense to target kids, because they’re more apt to respond to these therapies. I worry that somebody will say ‘this doesn’t work in adults,’ and it just stops there,” Dr. Nicholson said.
Though the AAP said that the benefits of FMT for treating CDIs are clear, the data available for treating other conditions such as ulcerative colitis or Crohn’s disease are less convincing. Any child receiving an FMT for these ailments should only do so as part of a clinical trial, the group said.
The AAP report endorses a joint position paper, published in 2019, about the benefits of FMTs for CDIs from North American and European pediatric gastroenterology societies. Dr. Nicholson was an author of this joint statement and hopes that the AAP report raises further awareness among pediatricians that FMTs are a safe and effective treatment for recurrent CDIs.
“This is something that maybe is not as discussed in pediatric circles. Kids need FMTs sometimes,” Dr. Nicholson said.
Dr. Oliva-Hemker and Dr. Nicholson report no relevant financial relationships.
A version of this article appeared on Medscape.com.
(AAP).
However, fecal microbiota transplants (FMTs) should not be used to treat other gastrointestinal ailments such as Crohn’s disease or ulcerative colitis, because scientific evidence falls short on effectiveness in treating these conditions, the group said.
C. difficile infections (CDIs) are major contributors to hospital-associated diarrhea and diarrhea caused by antibiotics. An FMT involves introducing the feces of a healthy person into the gastrointestinal tract, usually through a nasogastric tube but sometimes in capsules containing healthy stool. Serious adverse reactions associated with an FMT, such as hospitalization, are rare, occuring in roughly 2% of case, the AAP said.
An FMT “does have a place for treatment of recurrent CDIs in children,” said Maria Oliva-Hemker, MD, a pediatric gastroenterologist at Johns Hopkins University School of Medicine in Baltimore and the lead author of the report, which was online in Pediatrics.
The AAP strongly encourages people not to perform an FMT at home, although caregivers may be tempted due to a lack of medical facilities located nearby to deliver this care.
“People might see a video on YouTube and think they can do this themselves,” Dr. Oliva-Hemker said.
An FMT requires screening of donors for any infections, which involves administering questionnaires and analyzing donor blood and stool, which are tasks better suited for medical facilities than for a living room.
No controlled or prospective clinical trials on the efficacy of FMT for children exist, according to the AAP. But a retrospective study published in 2020 showed that one or two courses of FMT prevented CDI recurrence in children 87% of the time. Researchers defined the eradication of CDIs as no recurrence for at least 2 months after an FMT and noted the success rates in children were comaparable to those reported in adults.
Unlike pediatric data, adult data come from a randomized clinical trial.
“Sometimes, kids are the last people to be enrolled in these trials,” said Maribeth Nicholson, MD, MPH, a pediatric gastroenterologist at Vanderbilt University Medical Center in Nashville, Tenn., an author of the 2020 study.
Dr. Nicholson, who was not involved in the AAP report, said that the retrospective data are strong enough to justify using FMT to eradicate CDIs in children. But researchers are unclear about the biologic mechanisms that make FMTs work.
Dr. Nicholson said that many therapeutics meant to produce a healthier microbiome are being studied in clinical trials. Any clinical trials of such products should include children, Dr. Nicholson said. A child’s gastrointestinal microbiome is actively developing, Dr. Nicholson added, compared with the relatively stable microbiome of an adult.
“When we think about the microbiome it makes sense to target kids, because they’re more apt to respond to these therapies. I worry that somebody will say ‘this doesn’t work in adults,’ and it just stops there,” Dr. Nicholson said.
Though the AAP said that the benefits of FMT for treating CDIs are clear, the data available for treating other conditions such as ulcerative colitis or Crohn’s disease are less convincing. Any child receiving an FMT for these ailments should only do so as part of a clinical trial, the group said.
The AAP report endorses a joint position paper, published in 2019, about the benefits of FMTs for CDIs from North American and European pediatric gastroenterology societies. Dr. Nicholson was an author of this joint statement and hopes that the AAP report raises further awareness among pediatricians that FMTs are a safe and effective treatment for recurrent CDIs.
“This is something that maybe is not as discussed in pediatric circles. Kids need FMTs sometimes,” Dr. Nicholson said.
Dr. Oliva-Hemker and Dr. Nicholson report no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM PEDIATRICS
New at-home test approved for chlamydia and gonorrhea
Called Simple 2, it’s the first test approved by the Food and Drug Administration that uses a sample collected at home to test for an STD, other than tests for HIV. The test can be purchased over-the-counter in stores or ordered online and delivered in discreet packaging. A vaginal swab or urine sample is collected and then sent for laboratory testing using a prepaid shipping label.
The FDA issued the final needed approval on Nov. 15, and the product is already for sale on the website of the manufacturer, LetsGetChecked. The listed price is $99 with free shipping for a single test kit, and the site offers a discounted subscription to receive a kit every 3 months for $69.30 per kit.
Gonorrhea cases have surged 28% since 2017, reaching 700,000 cases during 2021, Centers for Disease Control and Prevention data show. Chlamydia has also been on the rise, up 4% from 2020 to 2021, with 1.6 million annual infections.
Previously, tests for the two STDs required that samples be taken at a health care location such as a doctor’s office. The Simple 2 test results can be retrieved online, and a health care provider will reach out to people whose tests are positive or invalid. Results are typically received in 2-5 days, according to a press release from LetsGetChecked, which also offers treatment services.
“This authorization marks an important public health milestone, giving patients more information about their health from the privacy of their own home,” said Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, in a statement. “We are eager to continue supporting greater consumer access to diagnostic tests, which helps further our goal of bringing more health care into the home.”
A version of this article first appeared on WebMD.com.
Called Simple 2, it’s the first test approved by the Food and Drug Administration that uses a sample collected at home to test for an STD, other than tests for HIV. The test can be purchased over-the-counter in stores or ordered online and delivered in discreet packaging. A vaginal swab or urine sample is collected and then sent for laboratory testing using a prepaid shipping label.
The FDA issued the final needed approval on Nov. 15, and the product is already for sale on the website of the manufacturer, LetsGetChecked. The listed price is $99 with free shipping for a single test kit, and the site offers a discounted subscription to receive a kit every 3 months for $69.30 per kit.
Gonorrhea cases have surged 28% since 2017, reaching 700,000 cases during 2021, Centers for Disease Control and Prevention data show. Chlamydia has also been on the rise, up 4% from 2020 to 2021, with 1.6 million annual infections.
Previously, tests for the two STDs required that samples be taken at a health care location such as a doctor’s office. The Simple 2 test results can be retrieved online, and a health care provider will reach out to people whose tests are positive or invalid. Results are typically received in 2-5 days, according to a press release from LetsGetChecked, which also offers treatment services.
“This authorization marks an important public health milestone, giving patients more information about their health from the privacy of their own home,” said Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, in a statement. “We are eager to continue supporting greater consumer access to diagnostic tests, which helps further our goal of bringing more health care into the home.”
A version of this article first appeared on WebMD.com.
Called Simple 2, it’s the first test approved by the Food and Drug Administration that uses a sample collected at home to test for an STD, other than tests for HIV. The test can be purchased over-the-counter in stores or ordered online and delivered in discreet packaging. A vaginal swab or urine sample is collected and then sent for laboratory testing using a prepaid shipping label.
The FDA issued the final needed approval on Nov. 15, and the product is already for sale on the website of the manufacturer, LetsGetChecked. The listed price is $99 with free shipping for a single test kit, and the site offers a discounted subscription to receive a kit every 3 months for $69.30 per kit.
Gonorrhea cases have surged 28% since 2017, reaching 700,000 cases during 2021, Centers for Disease Control and Prevention data show. Chlamydia has also been on the rise, up 4% from 2020 to 2021, with 1.6 million annual infections.
Previously, tests for the two STDs required that samples be taken at a health care location such as a doctor’s office. The Simple 2 test results can be retrieved online, and a health care provider will reach out to people whose tests are positive or invalid. Results are typically received in 2-5 days, according to a press release from LetsGetChecked, which also offers treatment services.
“This authorization marks an important public health milestone, giving patients more information about their health from the privacy of their own home,” said Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, in a statement. “We are eager to continue supporting greater consumer access to diagnostic tests, which helps further our goal of bringing more health care into the home.”
A version of this article first appeared on WebMD.com.
Breast implants used in double lung transplant post infection
An innovative surgical procedure combining breast implants and an artificial lung may help more patients with severe lung disease survive to receive transplants. The case was described in a press conference sponsored by Northwestern University, Evanston, Ill.
In May 2023, a surgical team at Northwestern removed both infected lungs from David “Davey” Bauer, aged 34 years, and temporarily used breast implants to hold his heart in place until new lungs were available.
In April 2023, Mr. Bauer, a longtime smoker and vaper, experienced shortness of breath. His girlfriend, Susan Gore, took him to an urgent care center, and he returned home, but “the next morning he couldn’t walk,” Ms. Gore said in the press conference. A trip to the ED yielded a diagnosis of influenza A, followed rapidly by a bacterial lung infection that proved resistant to antibiotics. Mr. Bauer had no prior medical history of serious illness, but he was soon in an intensive care unit. His condition continued to decline, and a double lung transplant was his only option.

The Northwestern Medicine Canning Thoracic Institute specializes in challenging cases, and Mr. Bauer was transferred there.
Back from the brink
Mr. Bauer made the transfer to Chicago despite being critically ill. He was in dire need of a lung transplant, and the only way to resolve his infection was to remove the lungs, said Ankit Bharat, MD, chief of thoracic surgery and director of Northwestern Medicine Canning Thoracic Institute, in the press conference.

“Something needed to be done right away,” Dr. Bharat said. Mr. Bauer’s lungs were removed and the chest cavity was extensively debrided to remove the infection.
Then it was time for outside-the-box thinking. “With the lungs taken out, we needed something to support the heart,” he said. Breast implants came to mind, and double Ds were the largest available.
In addition, the surgeons created an artificial lung system of conduits to keep Mr. Bauer’s blood pumping. “We wanted to maintain the natural blood flow in the body that would be present if the lungs were there,” Dr. Bharat explained.
Plastic surgeons at Northwestern gave Mr. Bauer’s surgical team “a crash course” in managing the breast implants, Dr. Bharat said. The team anticipated that their novel surgical solution would need to last for weeks, but Mr. Bauer’s condition improved immediately once the infected lungs were removed. He was placed on a double-lung transplant list, and the team received an offer of new lungs within 24 hours.
The breast implants were removed, the new lungs were implanted, and Bauer spent several months in the ICU before his discharge to rehabilitation therapy at the end of September, according to a Northwestern press release.
This type of procedure could help patients with infections who need transplants but are too sick to undergo them, Dr. Bharat said in the press conference. In Mr. Bauer’s case, “a lot of stars aligned,” including Bauer’s rapid improvement and the quick availability of a perfect lung match, Dr. Bharat said. Many patients don’t survive to the point of transplant.
“We were surprised how quickly he recovered once we removed the infected lungs,” Dr. Bharat noted. The quick recovery may be in part because of Bauer’s youth and relative good health, but “this was uncharted territory.”
Mr. Bauer’s case is the first use of this particular surgical technique, although the team drew on lessons learned in other surgical settings, such as removal of both lungs to prevent cross-contamination in patients with cancer, he added.
Causes and effects
As for the factors that contributed to Mr. Bauer’s initial infection, “there is a lot we don’t know, but we can try to put things together,” said Dr. Bharat. Just as many factors lined up to promote Mr. Bauer’s recovery, many factors lined up to cause the problem, including long-standing smoking and vaping. Although some still view vaping as a safer alternative to smoking, patient data and experiences do not support this claim. “We know for a fact that both of them cause harm,” he added.
Mr. Bauer started smoking cigarettes at age 21 and typically smoked a pack of cigarettes each day before switching to vaping in 2014. In addition, Mr. Bauer had not been vaccinated against the flu, and his flu infection was followed by a bacterial infection.
Bacterial infections followed by hospitalizations are not new as an effect of vaping; a series of articles described the ongoing epidemic of e-cigarette or vaping product use–associated lung injury (EVALI). Patients with EVALI often present at urgent care centers, as Bauer did, with symptoms of flu or pneumonia, and they are often given medication and sent home.
Looking ahead: “We expect that Davey will fully recover and live a normal life,” although he will remain in Chicago for another year for monitoring, said Rade Tomic, MD, pulmonologist and medical director of the Northwestern Medicine Canning Thoracic Institute lung transplant program, in the press conference.
Mr. Bauer expressed his thanks to the surgical team, who also presented him with another gift: a T-shirt with his newly chosen nickname, “DD Davey.” “I feel so blessed, I got a second chance at life,” Mr. Bauer said in the press conference. “You should not inhale anything into your lungs except oxygen.”
A version of this article first appeared on Medscape.com.
An innovative surgical procedure combining breast implants and an artificial lung may help more patients with severe lung disease survive to receive transplants. The case was described in a press conference sponsored by Northwestern University, Evanston, Ill.
In May 2023, a surgical team at Northwestern removed both infected lungs from David “Davey” Bauer, aged 34 years, and temporarily used breast implants to hold his heart in place until new lungs were available.
In April 2023, Mr. Bauer, a longtime smoker and vaper, experienced shortness of breath. His girlfriend, Susan Gore, took him to an urgent care center, and he returned home, but “the next morning he couldn’t walk,” Ms. Gore said in the press conference. A trip to the ED yielded a diagnosis of influenza A, followed rapidly by a bacterial lung infection that proved resistant to antibiotics. Mr. Bauer had no prior medical history of serious illness, but he was soon in an intensive care unit. His condition continued to decline, and a double lung transplant was his only option.

The Northwestern Medicine Canning Thoracic Institute specializes in challenging cases, and Mr. Bauer was transferred there.
Back from the brink
Mr. Bauer made the transfer to Chicago despite being critically ill. He was in dire need of a lung transplant, and the only way to resolve his infection was to remove the lungs, said Ankit Bharat, MD, chief of thoracic surgery and director of Northwestern Medicine Canning Thoracic Institute, in the press conference.

“Something needed to be done right away,” Dr. Bharat said. Mr. Bauer’s lungs were removed and the chest cavity was extensively debrided to remove the infection.
Then it was time for outside-the-box thinking. “With the lungs taken out, we needed something to support the heart,” he said. Breast implants came to mind, and double Ds were the largest available.
In addition, the surgeons created an artificial lung system of conduits to keep Mr. Bauer’s blood pumping. “We wanted to maintain the natural blood flow in the body that would be present if the lungs were there,” Dr. Bharat explained.
Plastic surgeons at Northwestern gave Mr. Bauer’s surgical team “a crash course” in managing the breast implants, Dr. Bharat said. The team anticipated that their novel surgical solution would need to last for weeks, but Mr. Bauer’s condition improved immediately once the infected lungs were removed. He was placed on a double-lung transplant list, and the team received an offer of new lungs within 24 hours.
The breast implants were removed, the new lungs were implanted, and Bauer spent several months in the ICU before his discharge to rehabilitation therapy at the end of September, according to a Northwestern press release.
This type of procedure could help patients with infections who need transplants but are too sick to undergo them, Dr. Bharat said in the press conference. In Mr. Bauer’s case, “a lot of stars aligned,” including Bauer’s rapid improvement and the quick availability of a perfect lung match, Dr. Bharat said. Many patients don’t survive to the point of transplant.
“We were surprised how quickly he recovered once we removed the infected lungs,” Dr. Bharat noted. The quick recovery may be in part because of Bauer’s youth and relative good health, but “this was uncharted territory.”
Mr. Bauer’s case is the first use of this particular surgical technique, although the team drew on lessons learned in other surgical settings, such as removal of both lungs to prevent cross-contamination in patients with cancer, he added.
Causes and effects
As for the factors that contributed to Mr. Bauer’s initial infection, “there is a lot we don’t know, but we can try to put things together,” said Dr. Bharat. Just as many factors lined up to promote Mr. Bauer’s recovery, many factors lined up to cause the problem, including long-standing smoking and vaping. Although some still view vaping as a safer alternative to smoking, patient data and experiences do not support this claim. “We know for a fact that both of them cause harm,” he added.
Mr. Bauer started smoking cigarettes at age 21 and typically smoked a pack of cigarettes each day before switching to vaping in 2014. In addition, Mr. Bauer had not been vaccinated against the flu, and his flu infection was followed by a bacterial infection.
Bacterial infections followed by hospitalizations are not new as an effect of vaping; a series of articles described the ongoing epidemic of e-cigarette or vaping product use–associated lung injury (EVALI). Patients with EVALI often present at urgent care centers, as Bauer did, with symptoms of flu or pneumonia, and they are often given medication and sent home.
Looking ahead: “We expect that Davey will fully recover and live a normal life,” although he will remain in Chicago for another year for monitoring, said Rade Tomic, MD, pulmonologist and medical director of the Northwestern Medicine Canning Thoracic Institute lung transplant program, in the press conference.
Mr. Bauer expressed his thanks to the surgical team, who also presented him with another gift: a T-shirt with his newly chosen nickname, “DD Davey.” “I feel so blessed, I got a second chance at life,” Mr. Bauer said in the press conference. “You should not inhale anything into your lungs except oxygen.”
A version of this article first appeared on Medscape.com.
An innovative surgical procedure combining breast implants and an artificial lung may help more patients with severe lung disease survive to receive transplants. The case was described in a press conference sponsored by Northwestern University, Evanston, Ill.
In May 2023, a surgical team at Northwestern removed both infected lungs from David “Davey” Bauer, aged 34 years, and temporarily used breast implants to hold his heart in place until new lungs were available.
In April 2023, Mr. Bauer, a longtime smoker and vaper, experienced shortness of breath. His girlfriend, Susan Gore, took him to an urgent care center, and he returned home, but “the next morning he couldn’t walk,” Ms. Gore said in the press conference. A trip to the ED yielded a diagnosis of influenza A, followed rapidly by a bacterial lung infection that proved resistant to antibiotics. Mr. Bauer had no prior medical history of serious illness, but he was soon in an intensive care unit. His condition continued to decline, and a double lung transplant was his only option.

The Northwestern Medicine Canning Thoracic Institute specializes in challenging cases, and Mr. Bauer was transferred there.
Back from the brink
Mr. Bauer made the transfer to Chicago despite being critically ill. He was in dire need of a lung transplant, and the only way to resolve his infection was to remove the lungs, said Ankit Bharat, MD, chief of thoracic surgery and director of Northwestern Medicine Canning Thoracic Institute, in the press conference.

“Something needed to be done right away,” Dr. Bharat said. Mr. Bauer’s lungs were removed and the chest cavity was extensively debrided to remove the infection.
Then it was time for outside-the-box thinking. “With the lungs taken out, we needed something to support the heart,” he said. Breast implants came to mind, and double Ds were the largest available.
In addition, the surgeons created an artificial lung system of conduits to keep Mr. Bauer’s blood pumping. “We wanted to maintain the natural blood flow in the body that would be present if the lungs were there,” Dr. Bharat explained.
Plastic surgeons at Northwestern gave Mr. Bauer’s surgical team “a crash course” in managing the breast implants, Dr. Bharat said. The team anticipated that their novel surgical solution would need to last for weeks, but Mr. Bauer’s condition improved immediately once the infected lungs were removed. He was placed on a double-lung transplant list, and the team received an offer of new lungs within 24 hours.
The breast implants were removed, the new lungs were implanted, and Bauer spent several months in the ICU before his discharge to rehabilitation therapy at the end of September, according to a Northwestern press release.
This type of procedure could help patients with infections who need transplants but are too sick to undergo them, Dr. Bharat said in the press conference. In Mr. Bauer’s case, “a lot of stars aligned,” including Bauer’s rapid improvement and the quick availability of a perfect lung match, Dr. Bharat said. Many patients don’t survive to the point of transplant.
“We were surprised how quickly he recovered once we removed the infected lungs,” Dr. Bharat noted. The quick recovery may be in part because of Bauer’s youth and relative good health, but “this was uncharted territory.”
Mr. Bauer’s case is the first use of this particular surgical technique, although the team drew on lessons learned in other surgical settings, such as removal of both lungs to prevent cross-contamination in patients with cancer, he added.
Causes and effects
As for the factors that contributed to Mr. Bauer’s initial infection, “there is a lot we don’t know, but we can try to put things together,” said Dr. Bharat. Just as many factors lined up to promote Mr. Bauer’s recovery, many factors lined up to cause the problem, including long-standing smoking and vaping. Although some still view vaping as a safer alternative to smoking, patient data and experiences do not support this claim. “We know for a fact that both of them cause harm,” he added.
Mr. Bauer started smoking cigarettes at age 21 and typically smoked a pack of cigarettes each day before switching to vaping in 2014. In addition, Mr. Bauer had not been vaccinated against the flu, and his flu infection was followed by a bacterial infection.
Bacterial infections followed by hospitalizations are not new as an effect of vaping; a series of articles described the ongoing epidemic of e-cigarette or vaping product use–associated lung injury (EVALI). Patients with EVALI often present at urgent care centers, as Bauer did, with symptoms of flu or pneumonia, and they are often given medication and sent home.
Looking ahead: “We expect that Davey will fully recover and live a normal life,” although he will remain in Chicago for another year for monitoring, said Rade Tomic, MD, pulmonologist and medical director of the Northwestern Medicine Canning Thoracic Institute lung transplant program, in the press conference.
Mr. Bauer expressed his thanks to the surgical team, who also presented him with another gift: a T-shirt with his newly chosen nickname, “DD Davey.” “I feel so blessed, I got a second chance at life,” Mr. Bauer said in the press conference. “You should not inhale anything into your lungs except oxygen.”
A version of this article first appeared on Medscape.com.
Saltwater gargling may help avoid COVID hospitalization
ANAHEIM, CALIF. –
“The hypothesis was that interventions that target the upper respiratory tract may reduce the frequency and duration of upper respiratory symptoms associated with COVID-19,” said Sebastian Espinoza, first author of the study; he is with Trinity University, San Antonio.
Adults aged 18-65 years who tested positive for SARS-CoV-2 on polymerase chain reaction (PCR) testing between 2020 and 2022 were randomly selected to use low- or high-dose saltwater regimens for 14 days at the Harris Health System, Houston. For patients to be included in the study, 14 days had to have elapsed since the onset of any symptoms associated with COVID.
The low dose was 2.13 grams of salt dissolved in 8 ounces of warm water, and the high dose was 6 grams. Participants gargled the saltwater and used it as a nasal rinse for 5 minutes four times a day.
Primary outcomes included frequency and duration of symptoms associated with SARS-CoV-2 infection; secondary outcomes included admission to the hospital or the intensive care unit, mechanical ventilatory support, or death.
The findings were presented in a poster at the annual meeting of the American College of Allergy, Asthma, and Immunology.
Fifty-eight people were randomly assigned to either the low-saline (n = 27) or the high-saline (n = 28) group; three patients were lost to follow-up in both these groups. The reference control population consisted of 9,398 people with confirmed SARS-CoV-2 infection. Rates of vaccination were similar for all participants.
Hospitalization rates in the low- (18.5%) and high- (21.4%) saline groups were significantly lower than in the reference control population (58.8%; P < .001). No significant differences were noted in other outcomes among these groups.
The average age of patients in the control population (n = 9,398) was 45 years. The average age was similar in the low- and high-saline groups. In the low-saline group (n = 27), the average age was 39, and in the high-saline group, the average age was 41.
In all three groups, body mass index was between 29.6 and 31.7.
Exclusion criteria included chronic hypertension or participation in another interventional study.
‘Low risk, small potential benefit’
Allergist Zach Rubin, MD, a spokesperson for the ACAAI, said in an interview that the findings are in line with other small studies that previously reported some benefit in using nasal saline irrigation and gargling to treat a SARS-CoV-2 infection.
“This is a type of intervention that is low risk with some small potential benefit,” he said.
The researchers did not evaluate the potential reason for the saline regimen’s association with fewer hospitalizations, but Dr. Rubin said, “It may be possible that nasal saline irrigation and gargling help improve viral clearance and reduce the risk of microaspiration into the lungs, so it may be possible that this intervention could reduce the risk of pneumonia, which is a major cause of hospitalization.”
Dr. Rubin, who is an allergist at Oak Brook Allergists, Ill., said, “I generally recommend nasal saline irrigation to my patients for allergic rhinitis and viral upper respiratory infections already. It can help reduce symptoms such as nasal congestion, rhinorrhea, postnasal drip, and sinus pain and pressure.”
The intervention may be reasonable beyond an adult population, he said.
“This could be used for pediatric patients as well, if they are developmentally ready to try this intervention,” he said.
Mr. Espinoza said further study is warranted, but he said that if confirmed in later trials, the simple intervention may be particularly helpful in low-resource settings.
Mr. Espinoza and Dr. Rubin have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
ANAHEIM, CALIF. –
“The hypothesis was that interventions that target the upper respiratory tract may reduce the frequency and duration of upper respiratory symptoms associated with COVID-19,” said Sebastian Espinoza, first author of the study; he is with Trinity University, San Antonio.
Adults aged 18-65 years who tested positive for SARS-CoV-2 on polymerase chain reaction (PCR) testing between 2020 and 2022 were randomly selected to use low- or high-dose saltwater regimens for 14 days at the Harris Health System, Houston. For patients to be included in the study, 14 days had to have elapsed since the onset of any symptoms associated with COVID.
The low dose was 2.13 grams of salt dissolved in 8 ounces of warm water, and the high dose was 6 grams. Participants gargled the saltwater and used it as a nasal rinse for 5 minutes four times a day.
Primary outcomes included frequency and duration of symptoms associated with SARS-CoV-2 infection; secondary outcomes included admission to the hospital or the intensive care unit, mechanical ventilatory support, or death.
The findings were presented in a poster at the annual meeting of the American College of Allergy, Asthma, and Immunology.
Fifty-eight people were randomly assigned to either the low-saline (n = 27) or the high-saline (n = 28) group; three patients were lost to follow-up in both these groups. The reference control population consisted of 9,398 people with confirmed SARS-CoV-2 infection. Rates of vaccination were similar for all participants.
Hospitalization rates in the low- (18.5%) and high- (21.4%) saline groups were significantly lower than in the reference control population (58.8%; P < .001). No significant differences were noted in other outcomes among these groups.
The average age of patients in the control population (n = 9,398) was 45 years. The average age was similar in the low- and high-saline groups. In the low-saline group (n = 27), the average age was 39, and in the high-saline group, the average age was 41.
In all three groups, body mass index was between 29.6 and 31.7.
Exclusion criteria included chronic hypertension or participation in another interventional study.
‘Low risk, small potential benefit’
Allergist Zach Rubin, MD, a spokesperson for the ACAAI, said in an interview that the findings are in line with other small studies that previously reported some benefit in using nasal saline irrigation and gargling to treat a SARS-CoV-2 infection.
“This is a type of intervention that is low risk with some small potential benefit,” he said.
The researchers did not evaluate the potential reason for the saline regimen’s association with fewer hospitalizations, but Dr. Rubin said, “It may be possible that nasal saline irrigation and gargling help improve viral clearance and reduce the risk of microaspiration into the lungs, so it may be possible that this intervention could reduce the risk of pneumonia, which is a major cause of hospitalization.”
Dr. Rubin, who is an allergist at Oak Brook Allergists, Ill., said, “I generally recommend nasal saline irrigation to my patients for allergic rhinitis and viral upper respiratory infections already. It can help reduce symptoms such as nasal congestion, rhinorrhea, postnasal drip, and sinus pain and pressure.”
The intervention may be reasonable beyond an adult population, he said.
“This could be used for pediatric patients as well, if they are developmentally ready to try this intervention,” he said.
Mr. Espinoza said further study is warranted, but he said that if confirmed in later trials, the simple intervention may be particularly helpful in low-resource settings.
Mr. Espinoza and Dr. Rubin have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
ANAHEIM, CALIF. –
“The hypothesis was that interventions that target the upper respiratory tract may reduce the frequency and duration of upper respiratory symptoms associated with COVID-19,” said Sebastian Espinoza, first author of the study; he is with Trinity University, San Antonio.
Adults aged 18-65 years who tested positive for SARS-CoV-2 on polymerase chain reaction (PCR) testing between 2020 and 2022 were randomly selected to use low- or high-dose saltwater regimens for 14 days at the Harris Health System, Houston. For patients to be included in the study, 14 days had to have elapsed since the onset of any symptoms associated with COVID.
The low dose was 2.13 grams of salt dissolved in 8 ounces of warm water, and the high dose was 6 grams. Participants gargled the saltwater and used it as a nasal rinse for 5 minutes four times a day.
Primary outcomes included frequency and duration of symptoms associated with SARS-CoV-2 infection; secondary outcomes included admission to the hospital or the intensive care unit, mechanical ventilatory support, or death.
The findings were presented in a poster at the annual meeting of the American College of Allergy, Asthma, and Immunology.
Fifty-eight people were randomly assigned to either the low-saline (n = 27) or the high-saline (n = 28) group; three patients were lost to follow-up in both these groups. The reference control population consisted of 9,398 people with confirmed SARS-CoV-2 infection. Rates of vaccination were similar for all participants.
Hospitalization rates in the low- (18.5%) and high- (21.4%) saline groups were significantly lower than in the reference control population (58.8%; P < .001). No significant differences were noted in other outcomes among these groups.
The average age of patients in the control population (n = 9,398) was 45 years. The average age was similar in the low- and high-saline groups. In the low-saline group (n = 27), the average age was 39, and in the high-saline group, the average age was 41.
In all three groups, body mass index was between 29.6 and 31.7.
Exclusion criteria included chronic hypertension or participation in another interventional study.
‘Low risk, small potential benefit’
Allergist Zach Rubin, MD, a spokesperson for the ACAAI, said in an interview that the findings are in line with other small studies that previously reported some benefit in using nasal saline irrigation and gargling to treat a SARS-CoV-2 infection.
“This is a type of intervention that is low risk with some small potential benefit,” he said.
The researchers did not evaluate the potential reason for the saline regimen’s association with fewer hospitalizations, but Dr. Rubin said, “It may be possible that nasal saline irrigation and gargling help improve viral clearance and reduce the risk of microaspiration into the lungs, so it may be possible that this intervention could reduce the risk of pneumonia, which is a major cause of hospitalization.”
Dr. Rubin, who is an allergist at Oak Brook Allergists, Ill., said, “I generally recommend nasal saline irrigation to my patients for allergic rhinitis and viral upper respiratory infections already. It can help reduce symptoms such as nasal congestion, rhinorrhea, postnasal drip, and sinus pain and pressure.”
The intervention may be reasonable beyond an adult population, he said.
“This could be used for pediatric patients as well, if they are developmentally ready to try this intervention,” he said.
Mr. Espinoza said further study is warranted, but he said that if confirmed in later trials, the simple intervention may be particularly helpful in low-resource settings.
Mr. Espinoza and Dr. Rubin have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM ACAAI 2023
AI tool perfect in study of inflammatory diseases
Artificial intelligence can distinguish overlapping inflammatory conditions with total accuracy, according to a new study presented at the annual meeting of the American College of Rheumatology.
Texas pediatricians faced a conundrum during the pandemic. Endemic typhus, a flea-borne tropical infection common to the region, is nearly indistinguishable from multisystem inflammatory syndrome in children (MIS-C), a rare condition set in motion by SARS-CoV-2 infection. Children with either ailment had seemingly identical symptoms: fever, rash, gastrointestinal issues, and in need of swift treatment. A diagnosis of endemic typhus can take 4-6 days to confirm.
Tiphanie Vogel, MD, PhD, a pediatric rheumatologist at Texas Children’s Hospital, Houston, and colleagues sought to create a tool to hasten diagnosis and, ideally, treatment. To do so, they incorporated machine learning and clinical factors available within the first 6 hours of the onset of symptoms.
The team analyzed 49 demographic, clinical, and laboratory measures from the medical records of 133 children with MIS-C and 87 with endemic typhus. Using deep learning, they narrowed the model to 30 essential features that became the backbone of AI-MET, a two-phase clinical-decision support system.
Phase 1 uses 17 clinical factors and can be performed on paper. If a patient’s score in phase 1 is not determinative, clinicians proceed to phase 2, which uses an additional 13 weighted factors and machine learning.
In testing, the two-part tool classified each of the 220 test patients perfectly. And it diagnosed a second group of 111 patients with MIS-C with 99% (110/111) accuracy.
Of note, “that first step classifies [a patient] correctly half of the time,” Dr. Vogel said, so the second, AI phase of the tool was necessary for only half of cases. Dr. Vogel said that’s a good sign; it means that the tool is useful in settings where AI may not always be feasible, like in a busy ED.
Melissa Mizesko, MD, a pediatric rheumatologist at Driscoll Children’s Hospital in Corpus Christi, Tex., said that the new tool could help clinicians streamline care. When cases of MIS-C peaked in Texas, clinicians often would start sick children on doxycycline and treat for MIS-C at the same time, then wait to see whether the antibiotic brought the fever down.
“This [new tool] is helpful if you live in a part of the country that has typhus,” said Jane Burns, MD, director of the Kawasaki Disease Research Center at the University of California, San Diego, who helped develop a similar AI-based tool to distinguish MIS-C from Kawasaki disease. But she encouraged the researchers to expand their testing to include other conditions. Although the AI model Dr. Vogel’s group developed can pinpoint MIS-C or endemic typhus, what if a child has neither condition? “It’s not often you’re dealing with a diagnosis between just two specific diseases,” Dr. Burns said.
Dr. Vogel is also interested in making AI-MET more efficient. “This go-round we prioritized perfect accuracy,” she said. But 30 clinical factors, with 17 of them recorded and calculated by hand, is a lot. “Could we still get this to be very accurate, maybe not perfect, with less inputs?”
In addition to refining AI-MET, which Texas Children’s eventually hopes to make available to other institutions, Dr. Vogel and associates are also considering other use cases for AI. Lupus is one option. “Maybe with machine learning we could identify clues at diagnosis that would help recommend targeted treatment,” she said
Dr. Vogel disclosed potential conflicts of interest with Moderna, Novartis, Pfizer, and SOBI. Dr. Burns and Dr. Mizesko disclosed no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
Artificial intelligence can distinguish overlapping inflammatory conditions with total accuracy, according to a new study presented at the annual meeting of the American College of Rheumatology.
Texas pediatricians faced a conundrum during the pandemic. Endemic typhus, a flea-borne tropical infection common to the region, is nearly indistinguishable from multisystem inflammatory syndrome in children (MIS-C), a rare condition set in motion by SARS-CoV-2 infection. Children with either ailment had seemingly identical symptoms: fever, rash, gastrointestinal issues, and in need of swift treatment. A diagnosis of endemic typhus can take 4-6 days to confirm.
Tiphanie Vogel, MD, PhD, a pediatric rheumatologist at Texas Children’s Hospital, Houston, and colleagues sought to create a tool to hasten diagnosis and, ideally, treatment. To do so, they incorporated machine learning and clinical factors available within the first 6 hours of the onset of symptoms.
The team analyzed 49 demographic, clinical, and laboratory measures from the medical records of 133 children with MIS-C and 87 with endemic typhus. Using deep learning, they narrowed the model to 30 essential features that became the backbone of AI-MET, a two-phase clinical-decision support system.
Phase 1 uses 17 clinical factors and can be performed on paper. If a patient’s score in phase 1 is not determinative, clinicians proceed to phase 2, which uses an additional 13 weighted factors and machine learning.
In testing, the two-part tool classified each of the 220 test patients perfectly. And it diagnosed a second group of 111 patients with MIS-C with 99% (110/111) accuracy.
Of note, “that first step classifies [a patient] correctly half of the time,” Dr. Vogel said, so the second, AI phase of the tool was necessary for only half of cases. Dr. Vogel said that’s a good sign; it means that the tool is useful in settings where AI may not always be feasible, like in a busy ED.
Melissa Mizesko, MD, a pediatric rheumatologist at Driscoll Children’s Hospital in Corpus Christi, Tex., said that the new tool could help clinicians streamline care. When cases of MIS-C peaked in Texas, clinicians often would start sick children on doxycycline and treat for MIS-C at the same time, then wait to see whether the antibiotic brought the fever down.
“This [new tool] is helpful if you live in a part of the country that has typhus,” said Jane Burns, MD, director of the Kawasaki Disease Research Center at the University of California, San Diego, who helped develop a similar AI-based tool to distinguish MIS-C from Kawasaki disease. But she encouraged the researchers to expand their testing to include other conditions. Although the AI model Dr. Vogel’s group developed can pinpoint MIS-C or endemic typhus, what if a child has neither condition? “It’s not often you’re dealing with a diagnosis between just two specific diseases,” Dr. Burns said.
Dr. Vogel is also interested in making AI-MET more efficient. “This go-round we prioritized perfect accuracy,” she said. But 30 clinical factors, with 17 of them recorded and calculated by hand, is a lot. “Could we still get this to be very accurate, maybe not perfect, with less inputs?”
In addition to refining AI-MET, which Texas Children’s eventually hopes to make available to other institutions, Dr. Vogel and associates are also considering other use cases for AI. Lupus is one option. “Maybe with machine learning we could identify clues at diagnosis that would help recommend targeted treatment,” she said
Dr. Vogel disclosed potential conflicts of interest with Moderna, Novartis, Pfizer, and SOBI. Dr. Burns and Dr. Mizesko disclosed no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
Artificial intelligence can distinguish overlapping inflammatory conditions with total accuracy, according to a new study presented at the annual meeting of the American College of Rheumatology.
Texas pediatricians faced a conundrum during the pandemic. Endemic typhus, a flea-borne tropical infection common to the region, is nearly indistinguishable from multisystem inflammatory syndrome in children (MIS-C), a rare condition set in motion by SARS-CoV-2 infection. Children with either ailment had seemingly identical symptoms: fever, rash, gastrointestinal issues, and in need of swift treatment. A diagnosis of endemic typhus can take 4-6 days to confirm.
Tiphanie Vogel, MD, PhD, a pediatric rheumatologist at Texas Children’s Hospital, Houston, and colleagues sought to create a tool to hasten diagnosis and, ideally, treatment. To do so, they incorporated machine learning and clinical factors available within the first 6 hours of the onset of symptoms.
The team analyzed 49 demographic, clinical, and laboratory measures from the medical records of 133 children with MIS-C and 87 with endemic typhus. Using deep learning, they narrowed the model to 30 essential features that became the backbone of AI-MET, a two-phase clinical-decision support system.
Phase 1 uses 17 clinical factors and can be performed on paper. If a patient’s score in phase 1 is not determinative, clinicians proceed to phase 2, which uses an additional 13 weighted factors and machine learning.
In testing, the two-part tool classified each of the 220 test patients perfectly. And it diagnosed a second group of 111 patients with MIS-C with 99% (110/111) accuracy.
Of note, “that first step classifies [a patient] correctly half of the time,” Dr. Vogel said, so the second, AI phase of the tool was necessary for only half of cases. Dr. Vogel said that’s a good sign; it means that the tool is useful in settings where AI may not always be feasible, like in a busy ED.
Melissa Mizesko, MD, a pediatric rheumatologist at Driscoll Children’s Hospital in Corpus Christi, Tex., said that the new tool could help clinicians streamline care. When cases of MIS-C peaked in Texas, clinicians often would start sick children on doxycycline and treat for MIS-C at the same time, then wait to see whether the antibiotic brought the fever down.
“This [new tool] is helpful if you live in a part of the country that has typhus,” said Jane Burns, MD, director of the Kawasaki Disease Research Center at the University of California, San Diego, who helped develop a similar AI-based tool to distinguish MIS-C from Kawasaki disease. But she encouraged the researchers to expand their testing to include other conditions. Although the AI model Dr. Vogel’s group developed can pinpoint MIS-C or endemic typhus, what if a child has neither condition? “It’s not often you’re dealing with a diagnosis between just two specific diseases,” Dr. Burns said.
Dr. Vogel is also interested in making AI-MET more efficient. “This go-round we prioritized perfect accuracy,” she said. But 30 clinical factors, with 17 of them recorded and calculated by hand, is a lot. “Could we still get this to be very accurate, maybe not perfect, with less inputs?”
In addition to refining AI-MET, which Texas Children’s eventually hopes to make available to other institutions, Dr. Vogel and associates are also considering other use cases for AI. Lupus is one option. “Maybe with machine learning we could identify clues at diagnosis that would help recommend targeted treatment,” she said
Dr. Vogel disclosed potential conflicts of interest with Moderna, Novartis, Pfizer, and SOBI. Dr. Burns and Dr. Mizesko disclosed no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FROM ACR 2023
CDC says child vaccination exemptions hit all-time high
– the highest exemption rate ever reported in the United States.
Of the 3% of children who got exemptions, 0.2% were for medical reasons and 2.8% for nonmedical reasons, the CDC report said. The overall exemption rate was 2.6% for the previous school year.
Though more children received exemptions, the overall national vaccination rate remained steady at 93% for children entering kindergarten for the 2022-2023 school year. Before the COVID-19 pandemic, the overall rate was 95%, the CDC said.
“The bad news is that it’s gone down since the pandemic and still hasn’t rebounded,” Sean O’Leary, MD, a University of Colorado pediatric infectious diseases specialist, told The Associated Press. “The good news is that the vast majority of parents are still vaccinating their kids according to the recommended schedule.”
The CDC report did not offer a specific reason for higher vaccine exemptions. But it did note that the increase could be caused by the COVID-19 pandemic and COVID vaccine hesitancy.
“There is a rising distrust in the health care system,” Amna Husain, MD, a pediatrician in private practice in North Carolina and a spokesperson for the American Academy of Pediatrics, told NBC News. Vaccine exemptions “have unfortunately trended upward with it.”
Exemption rates varied across the nation. The CDC said 40 states reported a rise in exemptions and that the exemption rate went over 5% in 10 states: Alaska, Arizona, Hawaii, Idaho, Michigan, Nevada, North Dakota, Oregon, Utah, and Wisconsin. Idaho had the highest exemption rate in 2022 with 12%.
While requirements vary from state to state, most states require students entering kindergarten to receive four vaccines: MMR, DTaP, polio, and chickenpox.
A version of this article first appeared on WebMD.com.
– the highest exemption rate ever reported in the United States.
Of the 3% of children who got exemptions, 0.2% were for medical reasons and 2.8% for nonmedical reasons, the CDC report said. The overall exemption rate was 2.6% for the previous school year.
Though more children received exemptions, the overall national vaccination rate remained steady at 93% for children entering kindergarten for the 2022-2023 school year. Before the COVID-19 pandemic, the overall rate was 95%, the CDC said.
“The bad news is that it’s gone down since the pandemic and still hasn’t rebounded,” Sean O’Leary, MD, a University of Colorado pediatric infectious diseases specialist, told The Associated Press. “The good news is that the vast majority of parents are still vaccinating their kids according to the recommended schedule.”
The CDC report did not offer a specific reason for higher vaccine exemptions. But it did note that the increase could be caused by the COVID-19 pandemic and COVID vaccine hesitancy.
“There is a rising distrust in the health care system,” Amna Husain, MD, a pediatrician in private practice in North Carolina and a spokesperson for the American Academy of Pediatrics, told NBC News. Vaccine exemptions “have unfortunately trended upward with it.”
Exemption rates varied across the nation. The CDC said 40 states reported a rise in exemptions and that the exemption rate went over 5% in 10 states: Alaska, Arizona, Hawaii, Idaho, Michigan, Nevada, North Dakota, Oregon, Utah, and Wisconsin. Idaho had the highest exemption rate in 2022 with 12%.
While requirements vary from state to state, most states require students entering kindergarten to receive four vaccines: MMR, DTaP, polio, and chickenpox.
A version of this article first appeared on WebMD.com.
– the highest exemption rate ever reported in the United States.
Of the 3% of children who got exemptions, 0.2% were for medical reasons and 2.8% for nonmedical reasons, the CDC report said. The overall exemption rate was 2.6% for the previous school year.
Though more children received exemptions, the overall national vaccination rate remained steady at 93% for children entering kindergarten for the 2022-2023 school year. Before the COVID-19 pandemic, the overall rate was 95%, the CDC said.
“The bad news is that it’s gone down since the pandemic and still hasn’t rebounded,” Sean O’Leary, MD, a University of Colorado pediatric infectious diseases specialist, told The Associated Press. “The good news is that the vast majority of parents are still vaccinating their kids according to the recommended schedule.”
The CDC report did not offer a specific reason for higher vaccine exemptions. But it did note that the increase could be caused by the COVID-19 pandemic and COVID vaccine hesitancy.
“There is a rising distrust in the health care system,” Amna Husain, MD, a pediatrician in private practice in North Carolina and a spokesperson for the American Academy of Pediatrics, told NBC News. Vaccine exemptions “have unfortunately trended upward with it.”
Exemption rates varied across the nation. The CDC said 40 states reported a rise in exemptions and that the exemption rate went over 5% in 10 states: Alaska, Arizona, Hawaii, Idaho, Michigan, Nevada, North Dakota, Oregon, Utah, and Wisconsin. Idaho had the highest exemption rate in 2022 with 12%.
While requirements vary from state to state, most states require students entering kindergarten to receive four vaccines: MMR, DTaP, polio, and chickenpox.
A version of this article first appeared on WebMD.com.
The remaining frontiers in fighting hepatitis C
A, B, C, D, E: It’s a short, menacing alphabet representing the five types of virus causing viral hepatitis, a sickness afflicting some 400 million people around the world today.
Hepatitis viruses are a set of very different pathogens that kill 1.4 million people annually and infect more than HIV and the malaria pathogen do combined. Most of the deaths are from cirrhosis of the liver or hepatic cancer due to chronic infections with hepatitis viruses B or C, picked up through contact with contaminated blood.
Hepatitis B was the first of the five to be discovered, in the 1960s, by biochemist Baruch S. Blumberg, MD. Hepatitis A, which is most commonly spread through contaminated food and water, was next, discovered in 1973 by researchers Stephen Mark Feinstone, MD, Albert Kapikian, MD, and Robert Purcell, MD.
Screening tests for those two types of viruses paved the way to discovering a third. In the 1970s, hematologist Harvey J. Alter, MD, examined unexplained cases of hepatitis in patients after blood transfusions and found that only 25% of such cases were caused by the hepatitis B virus, and none were linked to the hepatitis A virus. The rest were caused by an unidentified transmissible agent that could persist in the body as a chronic infection and lead to liver cirrhosis and liver cancer.
The agent behind this disease, named non-A, non-B hepatitis, remained a mystery for a decade until Michael Houghton, PhD, a microbiologist working at the biotechnology company Chiron Corporation, and his team sequenced the agent’s genome in 1989 after years of intensive investigation. They identified it as a novel virus of the family to which yellow fever virus belongs: the flaviviruses, a group of RNA viruses often transmitted through the bite of infected arthropods.
But there was more to the story. Scientists needed to show that this new virus could, indeed, cause hepatitis C on its own – a feat achieved in 1997, when Charles M. Rice, PhD, then a virologist at Washington University in St. Louis, and others succeeded in creating a form of the virus in the lab that could replicate in the only animal model for hepatitis C, the chimpanzee. When they injected the virus into the liver of chimpanzees, it triggered clinical hepatitis, demonstrating the direct connection between hepatitis C and non-A, non-B hepatitis.
The findings led to lifesaving hepatitis C tests to avert infections through transfusions with contaminated blood, as well as for the development of effective antiviral medications to treat the disease. In 2020, in the thick of the SARS-CoV-2 pandemic, Dr. Alter, Dr. Houghton, and Dr. Rice received a Nobel Prize in Medicine for their work on identifying the virus.
This conversation has been edited for length and clarity.
What were the challenges at the time you began your research on hepatitis C?
The realization that an agent was behind non-A, non-B hepatitis had initiated a virus hunt to try to figure out what the causative agent was. Dr. Houghton and his group at Chiron won that race and reported the partial sequence of the virus in 1989 in Science.
It was an interesting kind of a dilemma for me as an early-stage assistant professor at Washington University in St. Louis, where I’d been working on yellow fever. All of a sudden, we had this new human virus that dropped into our laps and joined the flavivirus family; we had to decide if we were going to shift some of our attention to work on this virus. Initially, people in the viral hepatitis field invited us to meetings, but because we were doing work on the related virus, yellow fever, not because we were considered majors player in the field.
The main challenge was that we could not grow the virus in cell culture. And the only experimental model was the chimpanzee, so it was really difficult for laboratories to study this virus.
There were two major goals. One was to establish a cell culture system where you could replicate the virus and study it. And the other was try to create a system where we could do genetics on the virus. It was shown to be an RNA virus, and the collection of tools available for modifying RNA at that time, in the early 1990s, was not the same as it was for DNA. Now that’s changed to some extent, with modern editing technologies.
If there’s one lesson to be learned from this hepatitis C story, it’s that persistence pays off.
This journey started with an unknown virus and ended up with treatment in a relatively short period of time.
I don’t think it was a short period of time, between all of the failures to actually get a cell culture system and to show that we had a functional clone. From 1989, when the virus sequence was reported, to 2011, when the first antiviral compounds were produced, was 22 years.
And then, that initial generation of treatment compounds was not the greatest, and they were combined with the treatment that we were trying to get rid of – interferon – that made people quite ill and didn’t always cure them. They only had about a 50% cure rate.
It was 2014 when the interferon-free cocktails came about. And that was really amazing.
There were people who thought, “You are not going to be able to develop a drug cocktail that can eliminate this virus.” It was presumptuous to think that one could, but it was accomplished by biotech and the pharmaceutical industry. So it is really quite a success story, but I wish it could have been faster.
Over the last 50 years, researchers have identified five types of viruses that cause different forms of viral hepatitis. Each virus has its own mode of transmission and health impacts. Scientists around the world have worked to develop treatments and vaccines.
What are the current challenges in combating hepatitis C?
One thing that was a little sobering and disappointing for me was that when these medical advances are made and shown to be efficacious, it is not possible to get these drugs to everybody who needs them and successfully treat them. It’s a lot more complicated, in part because of the economics – how much the companies decide to charge for the drugs.
Also, it’s difficult to identify people who are infected with hepatitis C, because it’s often asymptomatic. Even when identified, getting people into treatment is challenging given differences in public health capabilities which vary at the local, national, and global levels. So we have wonderful drugs that can basically cure anybody, but I think we still could use a vaccine for hepatitis C.
During the first year of the COVID-19 pandemic, you won the Nobel Prize for the discovery of the hepatitis C virus. What was that experience like?
It was December 2020, and we were working on SARS-CoV-2 in the peak of the pandemic in New York City. My spouse and the dogs were off at our house in Connecticut, and I was living in the apartment in Manhattan. And I got this call at 4:30 in the morning. It was pretty shocking.
The pandemic made people more aware of what a highly infectious, disease-causing virus can do to our world. It encouraged the rapid dissemination of research results and more open publications. It also really made us appreciate how the same virus does different things depending upon who’s infected: In the case of COVID-19, it’s not good to be old, for example.
After many decades working with viruses, what would you say is the next frontier in virology?
There’s a lot that we don’t understand about these viruses. The more we study them, the more we understand about ourselves, our cells and our antiviral defense systems.
And there’s also great power in terms of being able to diagnose new viruses. The sequencing technology, the functional genomics technologies, all of those things, when applied to virology, give us a much richer picture of how these viruses interact with cells. I think it’s a golden age.
The five known types of viral hepatitis afflict hundreds of millions of people around the world, causing both acute and chronic liver diseases. Among them, types B and C are the most severe, and diagnosis often remains a challenge.
You have been working with flaviviruses (dengue, Zika, yellow fever, and hepatitis C) for many decades. Zika and dengue pose an ongoing threat worldwide and, in particular, Latin America. Based on the successful example of hepatitis C, what can scientific research do to mitigate the impact of these viruses?
For viruses like Zika, developing a vaccine is probably going to be fairly straightforward – except that since Zika is so transient, it makes it hard to prove that your vaccine works. You would have to do a human challenge study, in which volunteers are deliberately exposed to an infection in a safe way with health-care support.
For dengue, it’s much more difficult, because there are four different serotypes – different versions of the same virus – and infection with one serotype can put you at increased risk of more severe disease if you get infected with a second serotype. Eliciting a balanced response that would protect you against all four dengue serotypes is the holy grail of trying to develop a dengue vaccine.
People are using various approaches to accomplish that. The classic one is to take live attenuated versions – weakened forms of viruses that have been modified so they can’t cause severe illness but can still stimulate the immune system – of each of the four serotypes and mix them together. Another is to make chimeric viruses: a combination of genetic material from different viruses, resulting in new viruses that have features of each of the four dengue serotypes, engineered into the backbone of the yellow fever vaccine. But this hasn’t worked as well as people have hoped. I think the cocktail of live, attenuated dengue variants is probably the most advanced approach. But I would guess that given the success of COVID-19 mRNA vaccines, the mRNA approach will also be tried out.
These diseases are not going to go away. You can’t eradicate every mosquito. And you can’t really immunize every susceptible vertebrate host. So occasionally there’s going to be spillover into the human population. We need to keep working on these because they are big problems.
You began your career at the California Institute of Technology studying RNA viruses, such as the mosquito-borne Sindbis virus, and then flaviviruses that cause encephalitis, polyarthritis, yellow fever, and dengue fever. Later on, you also studied hepatitis C virus. Is there any advantage for virologists in changing the viruses they study throughout their careers?
They’re all interesting, right? And they are all different in their own ways. I say that my career has been a downward spiral of tackling increasingly intricate viruses. Initially, the alphaviruses – a viral family that includes chikungunya virus, for example – were easy. The classical flaviviruses – like yellow fever, dengue fever, West Nile viruses, and Zika virus, among others – were a little more difficult, but the hepatitis C virus was impossible for 15 years, until we, and others, finally achieved a complete replication system in the laboratory.
We coexist daily with viruses, but the pandemic may have given people the idea that all these microorganisms are invariably life-threatening.
We have to treat them with respect. We’ve seen what can happen with the emergence of a novel coronavirus that can spread during an asymptomatic phase of infection. You can’t be prepared for everything, but in some respects our response was a lot slower and less effective than it could have been.
If there’s anything that we’ve learned over the last 10 years with the new nucleic acid sequencing technologies, it’s that our past view of the virosphere was very narrow. And if you really look at what’s out there, the estimated virus diversity is a staggering number, like 1,031 types. Although most of them are not pathogenic to humans, some are. We have to take this threat seriously.
Is science prepared?
I think so, but there has to be an investment, a societal investment. And that investment has to not only be an investment in infrastructure that can react quickly to something new, but also to establish a repository of protective antibodies and small molecules against viruses that we know could be future threats.
Often, these things go in cycles. There’s a disaster, like the COVID-19 pandemic, people are changed by the experience, but then they think “Oh, well, the virus has faded into the background, the threat is over.” And that’s just not the case. We need a more sustained plan rather than a reactive stance. And that’s hard to do when resources and money are limited.
What is the effect of science illiteracy, conspiracy theories, and lack of science information on the battle against viruses?
These are huge issues, and I don’t know the best way to combat them and educate people. Any combative, confrontational kind of response – it’s just not going to work. People will get more resolute in their entrenched beliefs and not hear or believe compelling evidence to the contrary.
It’s frustrating. I think that we have amazing tools and the power to make really significant advances to help people. It is more than a little discouraging for scientists when there’s a substantial fraction of people who don’t believe in things that are well-supported by facts.
It’s in large part an educational problem. I think we don’t put enough money into education, particularly early education. A lot of people don’t understand how much of what we take for granted today is underpinned by science. All this technology – good, bad or ugly – is all science.
This article originally appeared in Knowable Magazine on Oct. 30, 2023. Knowable Magazine is an independent journalistic endeavor from Annual Reviews, a nonprofit publisher dedicated to synthesizing and integrating knowledge for the progress of science and the benefit of society. Sign up for Knowable Magazine’s newsletter.
A, B, C, D, E: It’s a short, menacing alphabet representing the five types of virus causing viral hepatitis, a sickness afflicting some 400 million people around the world today.
Hepatitis viruses are a set of very different pathogens that kill 1.4 million people annually and infect more than HIV and the malaria pathogen do combined. Most of the deaths are from cirrhosis of the liver or hepatic cancer due to chronic infections with hepatitis viruses B or C, picked up through contact with contaminated blood.
Hepatitis B was the first of the five to be discovered, in the 1960s, by biochemist Baruch S. Blumberg, MD. Hepatitis A, which is most commonly spread through contaminated food and water, was next, discovered in 1973 by researchers Stephen Mark Feinstone, MD, Albert Kapikian, MD, and Robert Purcell, MD.
Screening tests for those two types of viruses paved the way to discovering a third. In the 1970s, hematologist Harvey J. Alter, MD, examined unexplained cases of hepatitis in patients after blood transfusions and found that only 25% of such cases were caused by the hepatitis B virus, and none were linked to the hepatitis A virus. The rest were caused by an unidentified transmissible agent that could persist in the body as a chronic infection and lead to liver cirrhosis and liver cancer.
The agent behind this disease, named non-A, non-B hepatitis, remained a mystery for a decade until Michael Houghton, PhD, a microbiologist working at the biotechnology company Chiron Corporation, and his team sequenced the agent’s genome in 1989 after years of intensive investigation. They identified it as a novel virus of the family to which yellow fever virus belongs: the flaviviruses, a group of RNA viruses often transmitted through the bite of infected arthropods.
But there was more to the story. Scientists needed to show that this new virus could, indeed, cause hepatitis C on its own – a feat achieved in 1997, when Charles M. Rice, PhD, then a virologist at Washington University in St. Louis, and others succeeded in creating a form of the virus in the lab that could replicate in the only animal model for hepatitis C, the chimpanzee. When they injected the virus into the liver of chimpanzees, it triggered clinical hepatitis, demonstrating the direct connection between hepatitis C and non-A, non-B hepatitis.
The findings led to lifesaving hepatitis C tests to avert infections through transfusions with contaminated blood, as well as for the development of effective antiviral medications to treat the disease. In 2020, in the thick of the SARS-CoV-2 pandemic, Dr. Alter, Dr. Houghton, and Dr. Rice received a Nobel Prize in Medicine for their work on identifying the virus.
This conversation has been edited for length and clarity.
What were the challenges at the time you began your research on hepatitis C?
The realization that an agent was behind non-A, non-B hepatitis had initiated a virus hunt to try to figure out what the causative agent was. Dr. Houghton and his group at Chiron won that race and reported the partial sequence of the virus in 1989 in Science.
It was an interesting kind of a dilemma for me as an early-stage assistant professor at Washington University in St. Louis, where I’d been working on yellow fever. All of a sudden, we had this new human virus that dropped into our laps and joined the flavivirus family; we had to decide if we were going to shift some of our attention to work on this virus. Initially, people in the viral hepatitis field invited us to meetings, but because we were doing work on the related virus, yellow fever, not because we were considered majors player in the field.
The main challenge was that we could not grow the virus in cell culture. And the only experimental model was the chimpanzee, so it was really difficult for laboratories to study this virus.
There were two major goals. One was to establish a cell culture system where you could replicate the virus and study it. And the other was try to create a system where we could do genetics on the virus. It was shown to be an RNA virus, and the collection of tools available for modifying RNA at that time, in the early 1990s, was not the same as it was for DNA. Now that’s changed to some extent, with modern editing technologies.
If there’s one lesson to be learned from this hepatitis C story, it’s that persistence pays off.
This journey started with an unknown virus and ended up with treatment in a relatively short period of time.
I don’t think it was a short period of time, between all of the failures to actually get a cell culture system and to show that we had a functional clone. From 1989, when the virus sequence was reported, to 2011, when the first antiviral compounds were produced, was 22 years.
And then, that initial generation of treatment compounds was not the greatest, and they were combined with the treatment that we were trying to get rid of – interferon – that made people quite ill and didn’t always cure them. They only had about a 50% cure rate.
It was 2014 when the interferon-free cocktails came about. And that was really amazing.
There were people who thought, “You are not going to be able to develop a drug cocktail that can eliminate this virus.” It was presumptuous to think that one could, but it was accomplished by biotech and the pharmaceutical industry. So it is really quite a success story, but I wish it could have been faster.
Over the last 50 years, researchers have identified five types of viruses that cause different forms of viral hepatitis. Each virus has its own mode of transmission and health impacts. Scientists around the world have worked to develop treatments and vaccines.
What are the current challenges in combating hepatitis C?
One thing that was a little sobering and disappointing for me was that when these medical advances are made and shown to be efficacious, it is not possible to get these drugs to everybody who needs them and successfully treat them. It’s a lot more complicated, in part because of the economics – how much the companies decide to charge for the drugs.
Also, it’s difficult to identify people who are infected with hepatitis C, because it’s often asymptomatic. Even when identified, getting people into treatment is challenging given differences in public health capabilities which vary at the local, national, and global levels. So we have wonderful drugs that can basically cure anybody, but I think we still could use a vaccine for hepatitis C.
During the first year of the COVID-19 pandemic, you won the Nobel Prize for the discovery of the hepatitis C virus. What was that experience like?
It was December 2020, and we were working on SARS-CoV-2 in the peak of the pandemic in New York City. My spouse and the dogs were off at our house in Connecticut, and I was living in the apartment in Manhattan. And I got this call at 4:30 in the morning. It was pretty shocking.
The pandemic made people more aware of what a highly infectious, disease-causing virus can do to our world. It encouraged the rapid dissemination of research results and more open publications. It also really made us appreciate how the same virus does different things depending upon who’s infected: In the case of COVID-19, it’s not good to be old, for example.
After many decades working with viruses, what would you say is the next frontier in virology?
There’s a lot that we don’t understand about these viruses. The more we study them, the more we understand about ourselves, our cells and our antiviral defense systems.
And there’s also great power in terms of being able to diagnose new viruses. The sequencing technology, the functional genomics technologies, all of those things, when applied to virology, give us a much richer picture of how these viruses interact with cells. I think it’s a golden age.
The five known types of viral hepatitis afflict hundreds of millions of people around the world, causing both acute and chronic liver diseases. Among them, types B and C are the most severe, and diagnosis often remains a challenge.
You have been working with flaviviruses (dengue, Zika, yellow fever, and hepatitis C) for many decades. Zika and dengue pose an ongoing threat worldwide and, in particular, Latin America. Based on the successful example of hepatitis C, what can scientific research do to mitigate the impact of these viruses?
For viruses like Zika, developing a vaccine is probably going to be fairly straightforward – except that since Zika is so transient, it makes it hard to prove that your vaccine works. You would have to do a human challenge study, in which volunteers are deliberately exposed to an infection in a safe way with health-care support.
For dengue, it’s much more difficult, because there are four different serotypes – different versions of the same virus – and infection with one serotype can put you at increased risk of more severe disease if you get infected with a second serotype. Eliciting a balanced response that would protect you against all four dengue serotypes is the holy grail of trying to develop a dengue vaccine.
People are using various approaches to accomplish that. The classic one is to take live attenuated versions – weakened forms of viruses that have been modified so they can’t cause severe illness but can still stimulate the immune system – of each of the four serotypes and mix them together. Another is to make chimeric viruses: a combination of genetic material from different viruses, resulting in new viruses that have features of each of the four dengue serotypes, engineered into the backbone of the yellow fever vaccine. But this hasn’t worked as well as people have hoped. I think the cocktail of live, attenuated dengue variants is probably the most advanced approach. But I would guess that given the success of COVID-19 mRNA vaccines, the mRNA approach will also be tried out.
These diseases are not going to go away. You can’t eradicate every mosquito. And you can’t really immunize every susceptible vertebrate host. So occasionally there’s going to be spillover into the human population. We need to keep working on these because they are big problems.
You began your career at the California Institute of Technology studying RNA viruses, such as the mosquito-borne Sindbis virus, and then flaviviruses that cause encephalitis, polyarthritis, yellow fever, and dengue fever. Later on, you also studied hepatitis C virus. Is there any advantage for virologists in changing the viruses they study throughout their careers?
They’re all interesting, right? And they are all different in their own ways. I say that my career has been a downward spiral of tackling increasingly intricate viruses. Initially, the alphaviruses – a viral family that includes chikungunya virus, for example – were easy. The classical flaviviruses – like yellow fever, dengue fever, West Nile viruses, and Zika virus, among others – were a little more difficult, but the hepatitis C virus was impossible for 15 years, until we, and others, finally achieved a complete replication system in the laboratory.
We coexist daily with viruses, but the pandemic may have given people the idea that all these microorganisms are invariably life-threatening.
We have to treat them with respect. We’ve seen what can happen with the emergence of a novel coronavirus that can spread during an asymptomatic phase of infection. You can’t be prepared for everything, but in some respects our response was a lot slower and less effective than it could have been.
If there’s anything that we’ve learned over the last 10 years with the new nucleic acid sequencing technologies, it’s that our past view of the virosphere was very narrow. And if you really look at what’s out there, the estimated virus diversity is a staggering number, like 1,031 types. Although most of them are not pathogenic to humans, some are. We have to take this threat seriously.
Is science prepared?
I think so, but there has to be an investment, a societal investment. And that investment has to not only be an investment in infrastructure that can react quickly to something new, but also to establish a repository of protective antibodies and small molecules against viruses that we know could be future threats.
Often, these things go in cycles. There’s a disaster, like the COVID-19 pandemic, people are changed by the experience, but then they think “Oh, well, the virus has faded into the background, the threat is over.” And that’s just not the case. We need a more sustained plan rather than a reactive stance. And that’s hard to do when resources and money are limited.
What is the effect of science illiteracy, conspiracy theories, and lack of science information on the battle against viruses?
These are huge issues, and I don’t know the best way to combat them and educate people. Any combative, confrontational kind of response – it’s just not going to work. People will get more resolute in their entrenched beliefs and not hear or believe compelling evidence to the contrary.
It’s frustrating. I think that we have amazing tools and the power to make really significant advances to help people. It is more than a little discouraging for scientists when there’s a substantial fraction of people who don’t believe in things that are well-supported by facts.
It’s in large part an educational problem. I think we don’t put enough money into education, particularly early education. A lot of people don’t understand how much of what we take for granted today is underpinned by science. All this technology – good, bad or ugly – is all science.
This article originally appeared in Knowable Magazine on Oct. 30, 2023. Knowable Magazine is an independent journalistic endeavor from Annual Reviews, a nonprofit publisher dedicated to synthesizing and integrating knowledge for the progress of science and the benefit of society. Sign up for Knowable Magazine’s newsletter.
A, B, C, D, E: It’s a short, menacing alphabet representing the five types of virus causing viral hepatitis, a sickness afflicting some 400 million people around the world today.
Hepatitis viruses are a set of very different pathogens that kill 1.4 million people annually and infect more than HIV and the malaria pathogen do combined. Most of the deaths are from cirrhosis of the liver or hepatic cancer due to chronic infections with hepatitis viruses B or C, picked up through contact with contaminated blood.
Hepatitis B was the first of the five to be discovered, in the 1960s, by biochemist Baruch S. Blumberg, MD. Hepatitis A, which is most commonly spread through contaminated food and water, was next, discovered in 1973 by researchers Stephen Mark Feinstone, MD, Albert Kapikian, MD, and Robert Purcell, MD.
Screening tests for those two types of viruses paved the way to discovering a third. In the 1970s, hematologist Harvey J. Alter, MD, examined unexplained cases of hepatitis in patients after blood transfusions and found that only 25% of such cases were caused by the hepatitis B virus, and none were linked to the hepatitis A virus. The rest were caused by an unidentified transmissible agent that could persist in the body as a chronic infection and lead to liver cirrhosis and liver cancer.
The agent behind this disease, named non-A, non-B hepatitis, remained a mystery for a decade until Michael Houghton, PhD, a microbiologist working at the biotechnology company Chiron Corporation, and his team sequenced the agent’s genome in 1989 after years of intensive investigation. They identified it as a novel virus of the family to which yellow fever virus belongs: the flaviviruses, a group of RNA viruses often transmitted through the bite of infected arthropods.
But there was more to the story. Scientists needed to show that this new virus could, indeed, cause hepatitis C on its own – a feat achieved in 1997, when Charles M. Rice, PhD, then a virologist at Washington University in St. Louis, and others succeeded in creating a form of the virus in the lab that could replicate in the only animal model for hepatitis C, the chimpanzee. When they injected the virus into the liver of chimpanzees, it triggered clinical hepatitis, demonstrating the direct connection between hepatitis C and non-A, non-B hepatitis.
The findings led to lifesaving hepatitis C tests to avert infections through transfusions with contaminated blood, as well as for the development of effective antiviral medications to treat the disease. In 2020, in the thick of the SARS-CoV-2 pandemic, Dr. Alter, Dr. Houghton, and Dr. Rice received a Nobel Prize in Medicine for their work on identifying the virus.
This conversation has been edited for length and clarity.
What were the challenges at the time you began your research on hepatitis C?
The realization that an agent was behind non-A, non-B hepatitis had initiated a virus hunt to try to figure out what the causative agent was. Dr. Houghton and his group at Chiron won that race and reported the partial sequence of the virus in 1989 in Science.
It was an interesting kind of a dilemma for me as an early-stage assistant professor at Washington University in St. Louis, where I’d been working on yellow fever. All of a sudden, we had this new human virus that dropped into our laps and joined the flavivirus family; we had to decide if we were going to shift some of our attention to work on this virus. Initially, people in the viral hepatitis field invited us to meetings, but because we were doing work on the related virus, yellow fever, not because we were considered majors player in the field.
The main challenge was that we could not grow the virus in cell culture. And the only experimental model was the chimpanzee, so it was really difficult for laboratories to study this virus.
There were two major goals. One was to establish a cell culture system where you could replicate the virus and study it. And the other was try to create a system where we could do genetics on the virus. It was shown to be an RNA virus, and the collection of tools available for modifying RNA at that time, in the early 1990s, was not the same as it was for DNA. Now that’s changed to some extent, with modern editing technologies.
If there’s one lesson to be learned from this hepatitis C story, it’s that persistence pays off.
This journey started with an unknown virus and ended up with treatment in a relatively short period of time.
I don’t think it was a short period of time, between all of the failures to actually get a cell culture system and to show that we had a functional clone. From 1989, when the virus sequence was reported, to 2011, when the first antiviral compounds were produced, was 22 years.
And then, that initial generation of treatment compounds was not the greatest, and they were combined with the treatment that we were trying to get rid of – interferon – that made people quite ill and didn’t always cure them. They only had about a 50% cure rate.
It was 2014 when the interferon-free cocktails came about. And that was really amazing.
There were people who thought, “You are not going to be able to develop a drug cocktail that can eliminate this virus.” It was presumptuous to think that one could, but it was accomplished by biotech and the pharmaceutical industry. So it is really quite a success story, but I wish it could have been faster.
Over the last 50 years, researchers have identified five types of viruses that cause different forms of viral hepatitis. Each virus has its own mode of transmission and health impacts. Scientists around the world have worked to develop treatments and vaccines.
What are the current challenges in combating hepatitis C?
One thing that was a little sobering and disappointing for me was that when these medical advances are made and shown to be efficacious, it is not possible to get these drugs to everybody who needs them and successfully treat them. It’s a lot more complicated, in part because of the economics – how much the companies decide to charge for the drugs.
Also, it’s difficult to identify people who are infected with hepatitis C, because it’s often asymptomatic. Even when identified, getting people into treatment is challenging given differences in public health capabilities which vary at the local, national, and global levels. So we have wonderful drugs that can basically cure anybody, but I think we still could use a vaccine for hepatitis C.
During the first year of the COVID-19 pandemic, you won the Nobel Prize for the discovery of the hepatitis C virus. What was that experience like?
It was December 2020, and we were working on SARS-CoV-2 in the peak of the pandemic in New York City. My spouse and the dogs were off at our house in Connecticut, and I was living in the apartment in Manhattan. And I got this call at 4:30 in the morning. It was pretty shocking.
The pandemic made people more aware of what a highly infectious, disease-causing virus can do to our world. It encouraged the rapid dissemination of research results and more open publications. It also really made us appreciate how the same virus does different things depending upon who’s infected: In the case of COVID-19, it’s not good to be old, for example.
After many decades working with viruses, what would you say is the next frontier in virology?
There’s a lot that we don’t understand about these viruses. The more we study them, the more we understand about ourselves, our cells and our antiviral defense systems.
And there’s also great power in terms of being able to diagnose new viruses. The sequencing technology, the functional genomics technologies, all of those things, when applied to virology, give us a much richer picture of how these viruses interact with cells. I think it’s a golden age.
The five known types of viral hepatitis afflict hundreds of millions of people around the world, causing both acute and chronic liver diseases. Among them, types B and C are the most severe, and diagnosis often remains a challenge.
You have been working with flaviviruses (dengue, Zika, yellow fever, and hepatitis C) for many decades. Zika and dengue pose an ongoing threat worldwide and, in particular, Latin America. Based on the successful example of hepatitis C, what can scientific research do to mitigate the impact of these viruses?
For viruses like Zika, developing a vaccine is probably going to be fairly straightforward – except that since Zika is so transient, it makes it hard to prove that your vaccine works. You would have to do a human challenge study, in which volunteers are deliberately exposed to an infection in a safe way with health-care support.
For dengue, it’s much more difficult, because there are four different serotypes – different versions of the same virus – and infection with one serotype can put you at increased risk of more severe disease if you get infected with a second serotype. Eliciting a balanced response that would protect you against all four dengue serotypes is the holy grail of trying to develop a dengue vaccine.
People are using various approaches to accomplish that. The classic one is to take live attenuated versions – weakened forms of viruses that have been modified so they can’t cause severe illness but can still stimulate the immune system – of each of the four serotypes and mix them together. Another is to make chimeric viruses: a combination of genetic material from different viruses, resulting in new viruses that have features of each of the four dengue serotypes, engineered into the backbone of the yellow fever vaccine. But this hasn’t worked as well as people have hoped. I think the cocktail of live, attenuated dengue variants is probably the most advanced approach. But I would guess that given the success of COVID-19 mRNA vaccines, the mRNA approach will also be tried out.
These diseases are not going to go away. You can’t eradicate every mosquito. And you can’t really immunize every susceptible vertebrate host. So occasionally there’s going to be spillover into the human population. We need to keep working on these because they are big problems.
You began your career at the California Institute of Technology studying RNA viruses, such as the mosquito-borne Sindbis virus, and then flaviviruses that cause encephalitis, polyarthritis, yellow fever, and dengue fever. Later on, you also studied hepatitis C virus. Is there any advantage for virologists in changing the viruses they study throughout their careers?
They’re all interesting, right? And they are all different in their own ways. I say that my career has been a downward spiral of tackling increasingly intricate viruses. Initially, the alphaviruses – a viral family that includes chikungunya virus, for example – were easy. The classical flaviviruses – like yellow fever, dengue fever, West Nile viruses, and Zika virus, among others – were a little more difficult, but the hepatitis C virus was impossible for 15 years, until we, and others, finally achieved a complete replication system in the laboratory.
We coexist daily with viruses, but the pandemic may have given people the idea that all these microorganisms are invariably life-threatening.
We have to treat them with respect. We’ve seen what can happen with the emergence of a novel coronavirus that can spread during an asymptomatic phase of infection. You can’t be prepared for everything, but in some respects our response was a lot slower and less effective than it could have been.
If there’s anything that we’ve learned over the last 10 years with the new nucleic acid sequencing technologies, it’s that our past view of the virosphere was very narrow. And if you really look at what’s out there, the estimated virus diversity is a staggering number, like 1,031 types. Although most of them are not pathogenic to humans, some are. We have to take this threat seriously.
Is science prepared?
I think so, but there has to be an investment, a societal investment. And that investment has to not only be an investment in infrastructure that can react quickly to something new, but also to establish a repository of protective antibodies and small molecules against viruses that we know could be future threats.
Often, these things go in cycles. There’s a disaster, like the COVID-19 pandemic, people are changed by the experience, but then they think “Oh, well, the virus has faded into the background, the threat is over.” And that’s just not the case. We need a more sustained plan rather than a reactive stance. And that’s hard to do when resources and money are limited.
What is the effect of science illiteracy, conspiracy theories, and lack of science information on the battle against viruses?
These are huge issues, and I don’t know the best way to combat them and educate people. Any combative, confrontational kind of response – it’s just not going to work. People will get more resolute in their entrenched beliefs and not hear or believe compelling evidence to the contrary.
It’s frustrating. I think that we have amazing tools and the power to make really significant advances to help people. It is more than a little discouraging for scientists when there’s a substantial fraction of people who don’t believe in things that are well-supported by facts.
It’s in large part an educational problem. I think we don’t put enough money into education, particularly early education. A lot of people don’t understand how much of what we take for granted today is underpinned by science. All this technology – good, bad or ugly – is all science.
This article originally appeared in Knowable Magazine on Oct. 30, 2023. Knowable Magazine is an independent journalistic endeavor from Annual Reviews, a nonprofit publisher dedicated to synthesizing and integrating knowledge for the progress of science and the benefit of society. Sign up for Knowable Magazine’s newsletter.
Pustular Eruption on the Face
The Diagnosis: Eczema Herpeticum
The patient’s condition with worsening facial edema and notable pain prompted a bedside Tzanck smear using a sample from the base of a deroofed forehead vesicle. In addition, a swab of a deroofed lesion was sent for herpes simplex virus and varicella-zoster virus (VZV) polymerase chain reaction (PCR) testing. The Tzanck smear demonstrated ballooning multinucleated syncytial giant cells and eosinophilic inclusion bodies (Figure), which are characteristic of certain herpesviruses including herpes simplex virus and VZV. He was started on intravenous acyclovir while PCR results were pending; the PCR test later confirmed positivity for herpes simplex virus type 1. Treatment was transitioned to oral valacyclovir once the lesions started crusting over. Notable healing and epithelialization of the lesions occurred during his hospital stay, and he was discharged home 5 days after starting treatment. He was counseled on autoinoculation, advised that he was considered infectious until all lesions had crusted over, and encouraged to employ frequent handwashing. Complete resolution of eczema herpeticum (EH) was noted at 3-week follow-up.
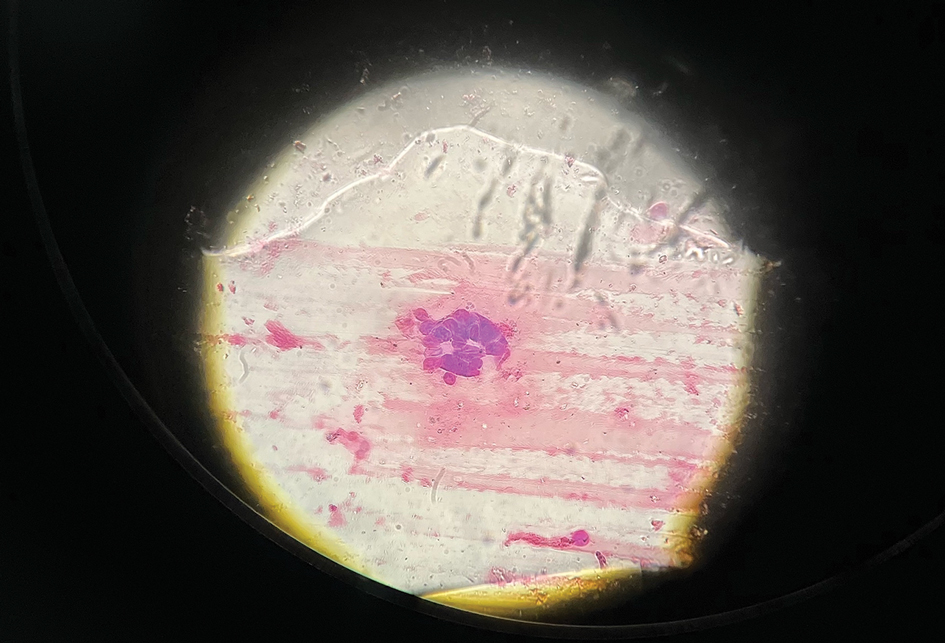
Eczema herpeticum (also known as Kaposi varicelliform eruption) is a potentially life-threatening disseminated cutaneous infection caused by herpes simplex virus types 1 and 2 in patients with pre-existing skin disease.1 It typically presents as a complication of atopic dermatitis (AD) but also has been identified as a rare complication in other conditions that disrupt the normal skin barrier, including mycosis fungoides, pemphigus foliaceus, pemphigus vulgaris, Darier disease, pityriasis rubra pilaris, contact dermatitis, and seborrheic dermatitis.1-4
The pathogenesis of EH is multifactorial. Disruption of the stratum corneum; impaired natural killer cell function; early-onset, untreated, or severe AD; disrupted skin microbiota with skewed colonization by Staphylococcus aureus; immunosuppressive AD therapies such as calcineurin inhibitors; eosinophilia; and helper T cell (TH2) cytokine predominance all have been suggested to play a role in the development of EH.5-8
As seen in our patient, EH presents with a sudden eruption of painful or pruritic, grouped, monomorphic, domeshaped vesicles with background swelling and erythema typically on the head, neck, and trunk. Vesicles then progress to punched-out erosions with overlying hemorrhagic crusting that can coalesce to form large denuded areas susceptible to superinfection with bacteria.9 Other accompanying symptoms include high fever, chills, malaise, and lymphadenopathy. Associated inflammation, classically described as erythema, may be difficult to discern in patients with darker skin and appears as hyperpigmentation; therefore, identification of clusters of monomorphic vesicles in areas of pre-existing dermatitis is particularly important for clinical diagnosis in people with darker skin types.
Various tests are available to confirm diagnosis in ambiguous cases. Bedside Tzanck smears can be performed rapidly and are considered positive if characteristic multinucleated giant cells are noted; however, they do not differentiate between the various herpesviruses. Direct fluorescent antibody testing of scraped lesions and viral cultures of swabbed vesicular fluid are equally effective in distinguishing between herpes simplex virus type 1, herpes simplex virus type 2, and VZV; PCR confirms the diagnosis with high specificity and sensitivity.10
In our patient, the initial differential diagnosis included EH, acute generalized exanthematous pustulosis, allergic contact dermatitis, and Orthopoxvirus infection. The positive Tzanck smear reduced the likelihood of a nonviral etiology. Additionally, worsening of the rash despite discontinuation of medications and utilization of topical steroids argued against acute generalized exanthematous pustulosis and allergic contact dermatitis. The laboratory findings reduced the likelihood of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, and PCR findings ultimately ruled out Orthopoxvirus infections. Additional differential diagnoses for EH include dermatitis herpetiformis; primary VZV infection; hand, foot, and mouth disease; disseminated zoster infection; disseminated molluscum contagiosum; and eczema coxsackium.
Complications of EH include scarring; herpetic keratitis due to corneal infection, which if left untreated can progress to blindness; and rarely death due to multiorgan failure or septicemia.11 The traditional smallpox vaccine (ACAM2000) is contraindicated in patients with AD and EH, even when AD is in remission. These patients should avoid contact with recently vaccinated individuals.12 An alternative vaccine—Jynneos (Bavarian Nordic)—is available for these patients and their family members.13 Clinicians should be aware of this guideline, especially given the recent mpox (monkeypox) outbreaks.
Mild cases of EH are more common, may sometimes go unnoticed, and self-resolve in healthy patients. Severe cases may require systemic antiviral therapy. Acyclovir and its prodrug valacyclovir are standard treatments for EH. Alternatively, foscarnet or cidofovir can be used in the treatment of acyclovir-resistant thymidine kinase– deficient herpes simplex virus and other acyclovirresistant cases.14 Any secondary bacterial superinfections, usually due to staphylococcal or streptococcal bacteria, should be treated with antibiotics. A thorough ophthalmologic evaluation should be performed for patients with periocular involvement of EH. Empiric treatment should be started immediately, given a relative low toxicity of systemic antiviral therapy and high morbidity and mortality associated with untreated widespread EH.
It is important to maintain a high index of clinical suspicion for EH, especially in patients with pre-existing conditions such as AD who present with systemic symptoms and facial vesicles, pustules, or erosions to ensure prompt diagnosis and appropriate treatment.
- Baaniya B, Agrawal S. Kaposi varicelliform eruption in a patient with pemphigus vulgaris: a case report and review of the literature. Case Rep Dermatol Med. 2020;2020:6695342. doi:10.1155/2020/6695342
- Tayabali K, Pothiwalla H, Lowitt M. Eczema herpeticum in Darier’s disease: a topical storm. J Community Hosp Intern Med Perspect. 2019;9:347. doi:10.1080/20009666.2019.1650590
- Cavalié M, Giacchero D, Cardot-Leccia N, et al. Kaposi’s varicelliform eruption in a patient with pityriasis rubra pilaris (pityriasis rubra pilaris herpeticum). J Eur Acad Dermatol Venereol. 2013;27:1585-1586. doi:10.1111/JDV.12120
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314. doi:10.5021/ad.2009.21.3.311
- Seegräber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum— a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients. J Eur Acad Dermatol Venereol. 2020;34:1074-1079. doi:10.1111/JDV.16090
- Kawakami Y, Ando T, Lee J-R, et al. Defective natural killer cell activity in a mouse model of eczema herpeticum. J Allergy Clin Immunol. 2017;139:997-1006.e10. doi:10.1016/j.jaci.2016.06.034
- Beck L, Latchney L, Zaccaro D, et al. Biomarkers of disease severity and Th2 polarity are predictors of risk for eczema herpeticum. J Allergy Clin Immunol. 2008;121:S37-S37. doi:10.1016/j.jaci.2007.12.152
- Kim M, Jung M, Hong SP, et al. Topical calcineurin inhibitors compromise stratum corneum integrity, epidermal permeability and antimicrobial barrier function. Exp Dermatol. 2010; 19:501-510. doi:10.1111/J.1600-0625.2009.00941.X
- Karray M, Kwan E, Souissi A. Kaposi varicelliform eruption. StatPearls [Internet]. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK482432/
- Dominguez SR, Pretty K, Hengartner R, et al. Comparison of herpes simplex virus PCR with culture for virus detection in multisource surface swab specimens from neonates [published online September 25, 2018]. J Clin Microbiol. doi:10.1128/JCM.00632-18
- Feye F, De Halleux C, Gillet JB, et al. Exacerbation of atopic dermatitis in the emergency department. Eur J Emerg Med. 2004;11:49-52. doi:10.1097/00063110-200412000-00014
- Casey C, Vellozzi C, Mootrey GT, et al; Vaccinia Case Definition Development Working Group; Advisory Committee on Immunization Practices-Armed Forces Epidemiological Board Smallpox Vaccine Safety Working Group. Surveillance guidelines for smallpox vaccine (vaccinia) adverse reactions. MMWR Recomm Rep. 2006;55:1-16.
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585 /MMWR.MM7122E1
- Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011;55:459. doi:10.1128/AAC.00615-10
The Diagnosis: Eczema Herpeticum
The patient’s condition with worsening facial edema and notable pain prompted a bedside Tzanck smear using a sample from the base of a deroofed forehead vesicle. In addition, a swab of a deroofed lesion was sent for herpes simplex virus and varicella-zoster virus (VZV) polymerase chain reaction (PCR) testing. The Tzanck smear demonstrated ballooning multinucleated syncytial giant cells and eosinophilic inclusion bodies (Figure), which are characteristic of certain herpesviruses including herpes simplex virus and VZV. He was started on intravenous acyclovir while PCR results were pending; the PCR test later confirmed positivity for herpes simplex virus type 1. Treatment was transitioned to oral valacyclovir once the lesions started crusting over. Notable healing and epithelialization of the lesions occurred during his hospital stay, and he was discharged home 5 days after starting treatment. He was counseled on autoinoculation, advised that he was considered infectious until all lesions had crusted over, and encouraged to employ frequent handwashing. Complete resolution of eczema herpeticum (EH) was noted at 3-week follow-up.

Eczema herpeticum (also known as Kaposi varicelliform eruption) is a potentially life-threatening disseminated cutaneous infection caused by herpes simplex virus types 1 and 2 in patients with pre-existing skin disease.1 It typically presents as a complication of atopic dermatitis (AD) but also has been identified as a rare complication in other conditions that disrupt the normal skin barrier, including mycosis fungoides, pemphigus foliaceus, pemphigus vulgaris, Darier disease, pityriasis rubra pilaris, contact dermatitis, and seborrheic dermatitis.1-4
The pathogenesis of EH is multifactorial. Disruption of the stratum corneum; impaired natural killer cell function; early-onset, untreated, or severe AD; disrupted skin microbiota with skewed colonization by Staphylococcus aureus; immunosuppressive AD therapies such as calcineurin inhibitors; eosinophilia; and helper T cell (TH2) cytokine predominance all have been suggested to play a role in the development of EH.5-8
As seen in our patient, EH presents with a sudden eruption of painful or pruritic, grouped, monomorphic, domeshaped vesicles with background swelling and erythema typically on the head, neck, and trunk. Vesicles then progress to punched-out erosions with overlying hemorrhagic crusting that can coalesce to form large denuded areas susceptible to superinfection with bacteria.9 Other accompanying symptoms include high fever, chills, malaise, and lymphadenopathy. Associated inflammation, classically described as erythema, may be difficult to discern in patients with darker skin and appears as hyperpigmentation; therefore, identification of clusters of monomorphic vesicles in areas of pre-existing dermatitis is particularly important for clinical diagnosis in people with darker skin types.
Various tests are available to confirm diagnosis in ambiguous cases. Bedside Tzanck smears can be performed rapidly and are considered positive if characteristic multinucleated giant cells are noted; however, they do not differentiate between the various herpesviruses. Direct fluorescent antibody testing of scraped lesions and viral cultures of swabbed vesicular fluid are equally effective in distinguishing between herpes simplex virus type 1, herpes simplex virus type 2, and VZV; PCR confirms the diagnosis with high specificity and sensitivity.10
In our patient, the initial differential diagnosis included EH, acute generalized exanthematous pustulosis, allergic contact dermatitis, and Orthopoxvirus infection. The positive Tzanck smear reduced the likelihood of a nonviral etiology. Additionally, worsening of the rash despite discontinuation of medications and utilization of topical steroids argued against acute generalized exanthematous pustulosis and allergic contact dermatitis. The laboratory findings reduced the likelihood of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, and PCR findings ultimately ruled out Orthopoxvirus infections. Additional differential diagnoses for EH include dermatitis herpetiformis; primary VZV infection; hand, foot, and mouth disease; disseminated zoster infection; disseminated molluscum contagiosum; and eczema coxsackium.
Complications of EH include scarring; herpetic keratitis due to corneal infection, which if left untreated can progress to blindness; and rarely death due to multiorgan failure or septicemia.11 The traditional smallpox vaccine (ACAM2000) is contraindicated in patients with AD and EH, even when AD is in remission. These patients should avoid contact with recently vaccinated individuals.12 An alternative vaccine—Jynneos (Bavarian Nordic)—is available for these patients and their family members.13 Clinicians should be aware of this guideline, especially given the recent mpox (monkeypox) outbreaks.
Mild cases of EH are more common, may sometimes go unnoticed, and self-resolve in healthy patients. Severe cases may require systemic antiviral therapy. Acyclovir and its prodrug valacyclovir are standard treatments for EH. Alternatively, foscarnet or cidofovir can be used in the treatment of acyclovir-resistant thymidine kinase– deficient herpes simplex virus and other acyclovirresistant cases.14 Any secondary bacterial superinfections, usually due to staphylococcal or streptococcal bacteria, should be treated with antibiotics. A thorough ophthalmologic evaluation should be performed for patients with periocular involvement of EH. Empiric treatment should be started immediately, given a relative low toxicity of systemic antiviral therapy and high morbidity and mortality associated with untreated widespread EH.
It is important to maintain a high index of clinical suspicion for EH, especially in patients with pre-existing conditions such as AD who present with systemic symptoms and facial vesicles, pustules, or erosions to ensure prompt diagnosis and appropriate treatment.
The Diagnosis: Eczema Herpeticum
The patient’s condition with worsening facial edema and notable pain prompted a bedside Tzanck smear using a sample from the base of a deroofed forehead vesicle. In addition, a swab of a deroofed lesion was sent for herpes simplex virus and varicella-zoster virus (VZV) polymerase chain reaction (PCR) testing. The Tzanck smear demonstrated ballooning multinucleated syncytial giant cells and eosinophilic inclusion bodies (Figure), which are characteristic of certain herpesviruses including herpes simplex virus and VZV. He was started on intravenous acyclovir while PCR results were pending; the PCR test later confirmed positivity for herpes simplex virus type 1. Treatment was transitioned to oral valacyclovir once the lesions started crusting over. Notable healing and epithelialization of the lesions occurred during his hospital stay, and he was discharged home 5 days after starting treatment. He was counseled on autoinoculation, advised that he was considered infectious until all lesions had crusted over, and encouraged to employ frequent handwashing. Complete resolution of eczema herpeticum (EH) was noted at 3-week follow-up.

Eczema herpeticum (also known as Kaposi varicelliform eruption) is a potentially life-threatening disseminated cutaneous infection caused by herpes simplex virus types 1 and 2 in patients with pre-existing skin disease.1 It typically presents as a complication of atopic dermatitis (AD) but also has been identified as a rare complication in other conditions that disrupt the normal skin barrier, including mycosis fungoides, pemphigus foliaceus, pemphigus vulgaris, Darier disease, pityriasis rubra pilaris, contact dermatitis, and seborrheic dermatitis.1-4
The pathogenesis of EH is multifactorial. Disruption of the stratum corneum; impaired natural killer cell function; early-onset, untreated, or severe AD; disrupted skin microbiota with skewed colonization by Staphylococcus aureus; immunosuppressive AD therapies such as calcineurin inhibitors; eosinophilia; and helper T cell (TH2) cytokine predominance all have been suggested to play a role in the development of EH.5-8
As seen in our patient, EH presents with a sudden eruption of painful or pruritic, grouped, monomorphic, domeshaped vesicles with background swelling and erythema typically on the head, neck, and trunk. Vesicles then progress to punched-out erosions with overlying hemorrhagic crusting that can coalesce to form large denuded areas susceptible to superinfection with bacteria.9 Other accompanying symptoms include high fever, chills, malaise, and lymphadenopathy. Associated inflammation, classically described as erythema, may be difficult to discern in patients with darker skin and appears as hyperpigmentation; therefore, identification of clusters of monomorphic vesicles in areas of pre-existing dermatitis is particularly important for clinical diagnosis in people with darker skin types.
Various tests are available to confirm diagnosis in ambiguous cases. Bedside Tzanck smears can be performed rapidly and are considered positive if characteristic multinucleated giant cells are noted; however, they do not differentiate between the various herpesviruses. Direct fluorescent antibody testing of scraped lesions and viral cultures of swabbed vesicular fluid are equally effective in distinguishing between herpes simplex virus type 1, herpes simplex virus type 2, and VZV; PCR confirms the diagnosis with high specificity and sensitivity.10
In our patient, the initial differential diagnosis included EH, acute generalized exanthematous pustulosis, allergic contact dermatitis, and Orthopoxvirus infection. The positive Tzanck smear reduced the likelihood of a nonviral etiology. Additionally, worsening of the rash despite discontinuation of medications and utilization of topical steroids argued against acute generalized exanthematous pustulosis and allergic contact dermatitis. The laboratory findings reduced the likelihood of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, and PCR findings ultimately ruled out Orthopoxvirus infections. Additional differential diagnoses for EH include dermatitis herpetiformis; primary VZV infection; hand, foot, and mouth disease; disseminated zoster infection; disseminated molluscum contagiosum; and eczema coxsackium.
Complications of EH include scarring; herpetic keratitis due to corneal infection, which if left untreated can progress to blindness; and rarely death due to multiorgan failure or septicemia.11 The traditional smallpox vaccine (ACAM2000) is contraindicated in patients with AD and EH, even when AD is in remission. These patients should avoid contact with recently vaccinated individuals.12 An alternative vaccine—Jynneos (Bavarian Nordic)—is available for these patients and their family members.13 Clinicians should be aware of this guideline, especially given the recent mpox (monkeypox) outbreaks.
Mild cases of EH are more common, may sometimes go unnoticed, and self-resolve in healthy patients. Severe cases may require systemic antiviral therapy. Acyclovir and its prodrug valacyclovir are standard treatments for EH. Alternatively, foscarnet or cidofovir can be used in the treatment of acyclovir-resistant thymidine kinase– deficient herpes simplex virus and other acyclovirresistant cases.14 Any secondary bacterial superinfections, usually due to staphylococcal or streptococcal bacteria, should be treated with antibiotics. A thorough ophthalmologic evaluation should be performed for patients with periocular involvement of EH. Empiric treatment should be started immediately, given a relative low toxicity of systemic antiviral therapy and high morbidity and mortality associated with untreated widespread EH.
It is important to maintain a high index of clinical suspicion for EH, especially in patients with pre-existing conditions such as AD who present with systemic symptoms and facial vesicles, pustules, or erosions to ensure prompt diagnosis and appropriate treatment.
- Baaniya B, Agrawal S. Kaposi varicelliform eruption in a patient with pemphigus vulgaris: a case report and review of the literature. Case Rep Dermatol Med. 2020;2020:6695342. doi:10.1155/2020/6695342
- Tayabali K, Pothiwalla H, Lowitt M. Eczema herpeticum in Darier’s disease: a topical storm. J Community Hosp Intern Med Perspect. 2019;9:347. doi:10.1080/20009666.2019.1650590
- Cavalié M, Giacchero D, Cardot-Leccia N, et al. Kaposi’s varicelliform eruption in a patient with pityriasis rubra pilaris (pityriasis rubra pilaris herpeticum). J Eur Acad Dermatol Venereol. 2013;27:1585-1586. doi:10.1111/JDV.12120
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314. doi:10.5021/ad.2009.21.3.311
- Seegräber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum— a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients. J Eur Acad Dermatol Venereol. 2020;34:1074-1079. doi:10.1111/JDV.16090
- Kawakami Y, Ando T, Lee J-R, et al. Defective natural killer cell activity in a mouse model of eczema herpeticum. J Allergy Clin Immunol. 2017;139:997-1006.e10. doi:10.1016/j.jaci.2016.06.034
- Beck L, Latchney L, Zaccaro D, et al. Biomarkers of disease severity and Th2 polarity are predictors of risk for eczema herpeticum. J Allergy Clin Immunol. 2008;121:S37-S37. doi:10.1016/j.jaci.2007.12.152
- Kim M, Jung M, Hong SP, et al. Topical calcineurin inhibitors compromise stratum corneum integrity, epidermal permeability and antimicrobial barrier function. Exp Dermatol. 2010; 19:501-510. doi:10.1111/J.1600-0625.2009.00941.X
- Karray M, Kwan E, Souissi A. Kaposi varicelliform eruption. StatPearls [Internet]. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK482432/
- Dominguez SR, Pretty K, Hengartner R, et al. Comparison of herpes simplex virus PCR with culture for virus detection in multisource surface swab specimens from neonates [published online September 25, 2018]. J Clin Microbiol. doi:10.1128/JCM.00632-18
- Feye F, De Halleux C, Gillet JB, et al. Exacerbation of atopic dermatitis in the emergency department. Eur J Emerg Med. 2004;11:49-52. doi:10.1097/00063110-200412000-00014
- Casey C, Vellozzi C, Mootrey GT, et al; Vaccinia Case Definition Development Working Group; Advisory Committee on Immunization Practices-Armed Forces Epidemiological Board Smallpox Vaccine Safety Working Group. Surveillance guidelines for smallpox vaccine (vaccinia) adverse reactions. MMWR Recomm Rep. 2006;55:1-16.
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585 /MMWR.MM7122E1
- Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011;55:459. doi:10.1128/AAC.00615-10
- Baaniya B, Agrawal S. Kaposi varicelliform eruption in a patient with pemphigus vulgaris: a case report and review of the literature. Case Rep Dermatol Med. 2020;2020:6695342. doi:10.1155/2020/6695342
- Tayabali K, Pothiwalla H, Lowitt M. Eczema herpeticum in Darier’s disease: a topical storm. J Community Hosp Intern Med Perspect. 2019;9:347. doi:10.1080/20009666.2019.1650590
- Cavalié M, Giacchero D, Cardot-Leccia N, et al. Kaposi’s varicelliform eruption in a patient with pityriasis rubra pilaris (pityriasis rubra pilaris herpeticum). J Eur Acad Dermatol Venereol. 2013;27:1585-1586. doi:10.1111/JDV.12120
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314. doi:10.5021/ad.2009.21.3.311
- Seegräber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum— a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients. J Eur Acad Dermatol Venereol. 2020;34:1074-1079. doi:10.1111/JDV.16090
- Kawakami Y, Ando T, Lee J-R, et al. Defective natural killer cell activity in a mouse model of eczema herpeticum. J Allergy Clin Immunol. 2017;139:997-1006.e10. doi:10.1016/j.jaci.2016.06.034
- Beck L, Latchney L, Zaccaro D, et al. Biomarkers of disease severity and Th2 polarity are predictors of risk for eczema herpeticum. J Allergy Clin Immunol. 2008;121:S37-S37. doi:10.1016/j.jaci.2007.12.152
- Kim M, Jung M, Hong SP, et al. Topical calcineurin inhibitors compromise stratum corneum integrity, epidermal permeability and antimicrobial barrier function. Exp Dermatol. 2010; 19:501-510. doi:10.1111/J.1600-0625.2009.00941.X
- Karray M, Kwan E, Souissi A. Kaposi varicelliform eruption. StatPearls [Internet]. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK482432/
- Dominguez SR, Pretty K, Hengartner R, et al. Comparison of herpes simplex virus PCR with culture for virus detection in multisource surface swab specimens from neonates [published online September 25, 2018]. J Clin Microbiol. doi:10.1128/JCM.00632-18
- Feye F, De Halleux C, Gillet JB, et al. Exacerbation of atopic dermatitis in the emergency department. Eur J Emerg Med. 2004;11:49-52. doi:10.1097/00063110-200412000-00014
- Casey C, Vellozzi C, Mootrey GT, et al; Vaccinia Case Definition Development Working Group; Advisory Committee on Immunization Practices-Armed Forces Epidemiological Board Smallpox Vaccine Safety Working Group. Surveillance guidelines for smallpox vaccine (vaccinia) adverse reactions. MMWR Recomm Rep. 2006;55:1-16.
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585 /MMWR.MM7122E1
- Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011;55:459. doi:10.1128/AAC.00615-10
A 52-year-old man developed a sudden eruption of small pustules on background erythema and edema covering the forehead, nasal bridge, periorbital region, cheeks, and perioral region on day 3 of hospitalization in the intensive care unit for management of septic shock secondary to a complicated urinary tract infection. He had a medical history of benign prostatic hyperplasia, sarcoidosis, and atopic dermatitis. He initially presented to the emergency department with fever, chills, and dysuria of 2 days’ duration. Because he received ceftriaxone, vancomycin, ciprofloxacin, and tamsulosin while hospitalized for the infection, the primary medical team suspected a drug reaction and empirically started applying hydrocortisone cream 2.5%. The rash continued to spread over the ensuing day, prompting a dermatology consultation to rule out a drug eruption and to help guide further management. The patient was in substantial distress and pain. Physical examination revealed numerous discrete and confluent monomorphic pustules on background erythema with faint collarettes of scale covering most of the face. Substantial periorbital and facial edema forced the eyes closed. There was no mucous membrane involvement. A review of systems was negative for dyspnea and dysphagia, and the rash was not present elsewhere on the body. Ophthalmologic evaluation revealed no ocular involvement or vision changes. Laboratory studies demonstrated neutrophilia (17.27×109 cells/L [reference range, 2.0–6.9×109 cells/L]). The eosinophil count, blood urea nitrogen/creatinine, and liver function tests were within reference range.