User login
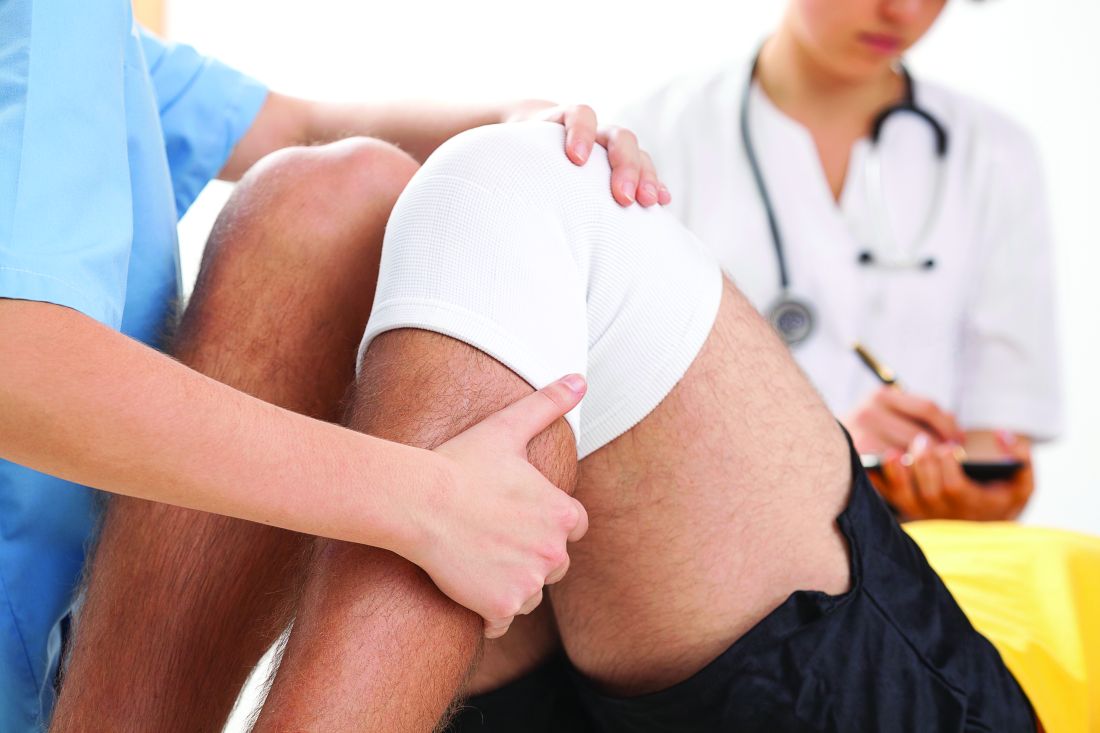
Preoperative tramadol fails to improve function after knee surgery
according to findings of a study based on pre- and postsurgery data.
Tramadol has become a popular choice for nonoperative knee pain relief because of its low potential for abuse and favorable safety profile, but its impact on postoperative outcomes when given before knee surgery has not been well studied, wrote Adam Driesman, MD, of the New York University Langone Orthopedic Hospital and colleagues.
In a study published in the Journal of Arthroplasty, the researchers compared patient-reported outcomes (PRO) after total knee arthroplasty among 136 patients who received no opiates, 21 who received tramadol, and 42 who received other opiates. All patients who did not have preoperative and postoperative PRO scores were excluded
All patients received the same multimodal perioperative pain protocol, and all were placed on oxycodone postoperatively for maintenance and breakthrough pain as needed, with discharge prescriptions for acetaminophen/oxycodone combination (Percocet) for breakthrough pain.
Patients preoperative assessment using the Knee Disability and Osteoarthritis Outcome Score Jr. (KOOS, JR.) were similar among the groups prior to surgery; baseline scores for the groups receiving either tramadol, no opiates, or other opiates were 49.95, 50.4, and 48.0, respectively. Demographics also were not significantly different among the groups.
At 3 months, the average KOOS, JR., score for the tramadol group (62.4) was significantly lower, compared with the other-opiate group (67.1) and treatment-naive group (70.1). In addition, patients in the tramadol group had the least change in scores on KOOS, JR., with an average of 12.5 points, compared with 19.1-point and 20.1-point improvements, respectively, in the alternate-opiate group and opiate-naive group.
The data expand on previous findings that patients given preoperative opioids had proportionally less postoperative pain relief than those not on opioids, the researchers said, but noted that they were surprised by the worse outcomes in the tramadol group given its demonstrated side-effect profile.
The study findings were limited by several factors including the retrospective design and relatively short follow-up period, as well as the inability to accurately determine outpatient medication use, not only of opioids, but of nonopioid postoperative pain medications that could have affected the results, the researchers said.
“However, given the conflicting evidence presented in this study and despite the 2013 American Academy of Orthopedic Surgeons Clinical Practice Guidelines, it is recommended providers remain very conservative in their administration of outpatient narcotics including tramadol prior to surgery,” they concluded.
SOURCE: Driesman A et al. J Arthroplasty. 2019;34(8):1662-66.
according to findings of a study based on pre- and postsurgery data.
Tramadol has become a popular choice for nonoperative knee pain relief because of its low potential for abuse and favorable safety profile, but its impact on postoperative outcomes when given before knee surgery has not been well studied, wrote Adam Driesman, MD, of the New York University Langone Orthopedic Hospital and colleagues.
In a study published in the Journal of Arthroplasty, the researchers compared patient-reported outcomes (PRO) after total knee arthroplasty among 136 patients who received no opiates, 21 who received tramadol, and 42 who received other opiates. All patients who did not have preoperative and postoperative PRO scores were excluded
All patients received the same multimodal perioperative pain protocol, and all were placed on oxycodone postoperatively for maintenance and breakthrough pain as needed, with discharge prescriptions for acetaminophen/oxycodone combination (Percocet) for breakthrough pain.
Patients preoperative assessment using the Knee Disability and Osteoarthritis Outcome Score Jr. (KOOS, JR.) were similar among the groups prior to surgery; baseline scores for the groups receiving either tramadol, no opiates, or other opiates were 49.95, 50.4, and 48.0, respectively. Demographics also were not significantly different among the groups.
At 3 months, the average KOOS, JR., score for the tramadol group (62.4) was significantly lower, compared with the other-opiate group (67.1) and treatment-naive group (70.1). In addition, patients in the tramadol group had the least change in scores on KOOS, JR., with an average of 12.5 points, compared with 19.1-point and 20.1-point improvements, respectively, in the alternate-opiate group and opiate-naive group.
The data expand on previous findings that patients given preoperative opioids had proportionally less postoperative pain relief than those not on opioids, the researchers said, but noted that they were surprised by the worse outcomes in the tramadol group given its demonstrated side-effect profile.
The study findings were limited by several factors including the retrospective design and relatively short follow-up period, as well as the inability to accurately determine outpatient medication use, not only of opioids, but of nonopioid postoperative pain medications that could have affected the results, the researchers said.
“However, given the conflicting evidence presented in this study and despite the 2013 American Academy of Orthopedic Surgeons Clinical Practice Guidelines, it is recommended providers remain very conservative in their administration of outpatient narcotics including tramadol prior to surgery,” they concluded.
SOURCE: Driesman A et al. J Arthroplasty. 2019;34(8):1662-66.
according to findings of a study based on pre- and postsurgery data.
Tramadol has become a popular choice for nonoperative knee pain relief because of its low potential for abuse and favorable safety profile, but its impact on postoperative outcomes when given before knee surgery has not been well studied, wrote Adam Driesman, MD, of the New York University Langone Orthopedic Hospital and colleagues.
In a study published in the Journal of Arthroplasty, the researchers compared patient-reported outcomes (PRO) after total knee arthroplasty among 136 patients who received no opiates, 21 who received tramadol, and 42 who received other opiates. All patients who did not have preoperative and postoperative PRO scores were excluded
All patients received the same multimodal perioperative pain protocol, and all were placed on oxycodone postoperatively for maintenance and breakthrough pain as needed, with discharge prescriptions for acetaminophen/oxycodone combination (Percocet) for breakthrough pain.
Patients preoperative assessment using the Knee Disability and Osteoarthritis Outcome Score Jr. (KOOS, JR.) were similar among the groups prior to surgery; baseline scores for the groups receiving either tramadol, no opiates, or other opiates were 49.95, 50.4, and 48.0, respectively. Demographics also were not significantly different among the groups.
At 3 months, the average KOOS, JR., score for the tramadol group (62.4) was significantly lower, compared with the other-opiate group (67.1) and treatment-naive group (70.1). In addition, patients in the tramadol group had the least change in scores on KOOS, JR., with an average of 12.5 points, compared with 19.1-point and 20.1-point improvements, respectively, in the alternate-opiate group and opiate-naive group.
The data expand on previous findings that patients given preoperative opioids had proportionally less postoperative pain relief than those not on opioids, the researchers said, but noted that they were surprised by the worse outcomes in the tramadol group given its demonstrated side-effect profile.
The study findings were limited by several factors including the retrospective design and relatively short follow-up period, as well as the inability to accurately determine outpatient medication use, not only of opioids, but of nonopioid postoperative pain medications that could have affected the results, the researchers said.
“However, given the conflicting evidence presented in this study and despite the 2013 American Academy of Orthopedic Surgeons Clinical Practice Guidelines, it is recommended providers remain very conservative in their administration of outpatient narcotics including tramadol prior to surgery,” they concluded.
SOURCE: Driesman A et al. J Arthroplasty. 2019;34(8):1662-66.
FROM THE JOURNAL OF ARTHROPLASTY
What’s hot in knee OA rehab research
TORONTO – Emerging evidence indicates that patients with knee osteoarthritis who engage in high-intensity interval training obtain significantly greater improvement in physical function than with conventionally prescribed moderate-intensity exercise, Monica R. Maly, PhD, said at the OARSI 2019 World Congress.
This was one of the key conclusions she and her coworkers drew from their analysis of the past year’s published research on diet and exercise interventions to improve outcomes in patients with OA, where obesity and physical inactivity figure prominently as modifiable lifestyle factors.
Another finding: Exercise interventions are where all the action is at present in the field of lifestyle-modification research aimed at achieving better health-related quality of life and other positive outcomes in OA. Dietary interventions are simply not a hot research topic. Indeed, her review of the past year’s literature included 38 randomized, controlled trials (RCTs) and 15 meta-analyses and systematic reviews – and all 38 RCTs addressed exercise interventions.
“It’s interesting to note that we found no new RCT data on diet to modify obesity in OA in the past year,” Dr. Maly said at the meeting sponsored by the Osteoarthritis Research Society International.
Additionally, 32 of the 38 RCTs devoted to exercise interventions for OA focused specifically on knee OA, noted Dr. Maly of the department of kinesiology at the University of Waterloo (Ont.).
Aerobic exercise dosage and intensity
Australian investigators conducted a pilot randomized trial of high-intensity interval training (HIIT) versus more conventional moderate-intensity exercise to improve health-related quality of life and physical function in 27 patients with knee OA. The exercise programs involved unsupervised home-based cycling, with participants requested to do four roughly 25-minute sessions per week for 8 weeks.
The two exercise intensity groups showed similar gains in health-related quality of life as assessed by the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). However, the HIIT group showed significantly greater improvement in physical function as measured on the Timed Up and Go test (PeerJ. 2018 May 9;6:e4738).
Dr. Maly noted that adherence to the home-based exercise programs was a challenge: Only 17 of the 27 patients completed the 8-week Australian study, for a 37% dropout rate. However, most study withdrawals were because of family-related issues, illness, or injuries unrelated to cycling, with no signal that HIIT placed knee OA patients at higher injury risk.
Other investigators performed a systematic review of 45 studies in an effort to generate evidence-based guidance about the optimal exercise dosing in order to improve outcomes in knee OA patients. They concluded that programs comprising 24 therapeutic exercise sessions over the course of 8-12 weeks resulted in the largest improvements in measures of pain and physical function. And, importantly, one exercise session per week conferred no benefits (J Orthop Sports Phys Ther. 2018 Mar;48[3]:146-61).
“Frequency probably matters,” Dr. Maly observed.
Patients and their physicians often wonder if long-term, land-based exercise might have deleterious impacts on joint structure in patients with knee OA. Reassurance on this score was provided by a recent meta-analysis of RCTs that concluded, on the basis of moderate-strength evidence, that exercise therapy of longer than 6 months duration had no adverse effect on tibiofemoral radiographic disease severity, compared with no exercise. Nor was there evidence of a long-term-exercise–associated deterioration of tibiofemoral cartilage morphology or worsening of synovitis or effusion. Plus, the meta-analysis provided limited evidence to suggest long-term exercise had a protective effect on the composition of patellar cartilage (Semin Arthritis Rheum. 2019 Jun;48[6]:941-9).
“While there was a little bit of evidence suggesting that long-term exercise could worsen bone marrow lesions, really there was no other evidence that it could change the structure of a joint,” according to Dr. Maly.
Internet-based exercise training
Using the Internet to deliver an individually tailored exercise-training program for patients with symptomatic knee OA might sound like an efficient strategy, but in fact it proved fruitless in a large randomized trial. The 350 participants were assigned to an 8-visit, 4-month program of physical therapy, a wait-list control group, or an internet-based program that delivered tailored exercises and video demonstrations with no face-to-face contact. The bottom line is that improvement in WOMAC scores didn’t differ significantly between the three groups when evaluated at 4 and 12 months (Osteoarthritis Cartilage. 2018 Mar;26[3]:383-96).
“When we deliver exercise with the use of technology, it may require some support, including face to face,” Dr. Maly concluded from the study results.
Strength training
High-intensity resistance training such as weight lifting aimed at strengthening the quadriceps and other large muscles is often eschewed in patients with knee OA because of concern about possible damage to their already damaged joints. Intriguingly, Brazilian investigators may have found a workaround. They randomized 48 women with knee OA to 12 weeks of either supervised low-intensity resistance training performed with partial blood-flow restriction using an air cuff, to low-intensity resistance training alone, or to high-intensity resistance training. The low-intensity resistance workouts involved exercises such as leg presses and knee extensions performed at 30% of maximum effort.
The low-intensity resistance training performed with blood-flow restriction and the high-intensity strength training programs proved similarly effective in improving quadriceps muscle mass, muscle strength, and physical function to a significantly greater extent than with low-intensity resistance training alone. Moreover, low-intensity resistance training with blood-flow restriction also resulted in a significant improvement in pain scores. That finding, coupled with the fact that 4 of the 16 patients in the high-intensity resistance training group dropped out of the trial because of exercise-induced knee pain, suggests that low-intensity strength training carried out with partial blood-flow restriction may have a bright future (Med Sci Sports Exerc. 2018 May;50[5]:897-905).
Exercise plus diet-induced weight loss
How does the combination of dietary weight loss plus exercise stack up against diet alone in terms of benefits on pain and physical function in obese patients with knee OA? A systematic review and meta-analysis of nine RCTs aimed at answering that question concluded that diet-alone strategies are less effective. Both the diet-plus-exercise and diet-only interventions resulted in comparably moderate improvement in physical function. However, diet-only treatments didn’t reduce pain, whereas diet-plus-exercise interventions achieved moderate pain relief (Semin Arthritis Rheum. 2019 Apr;48[5]:765-77).
Dr. Maly reported having no financial conflicts of interest regarding her presentation.
TORONTO – Emerging evidence indicates that patients with knee osteoarthritis who engage in high-intensity interval training obtain significantly greater improvement in physical function than with conventionally prescribed moderate-intensity exercise, Monica R. Maly, PhD, said at the OARSI 2019 World Congress.
This was one of the key conclusions she and her coworkers drew from their analysis of the past year’s published research on diet and exercise interventions to improve outcomes in patients with OA, where obesity and physical inactivity figure prominently as modifiable lifestyle factors.
Another finding: Exercise interventions are where all the action is at present in the field of lifestyle-modification research aimed at achieving better health-related quality of life and other positive outcomes in OA. Dietary interventions are simply not a hot research topic. Indeed, her review of the past year’s literature included 38 randomized, controlled trials (RCTs) and 15 meta-analyses and systematic reviews – and all 38 RCTs addressed exercise interventions.
“It’s interesting to note that we found no new RCT data on diet to modify obesity in OA in the past year,” Dr. Maly said at the meeting sponsored by the Osteoarthritis Research Society International.
Additionally, 32 of the 38 RCTs devoted to exercise interventions for OA focused specifically on knee OA, noted Dr. Maly of the department of kinesiology at the University of Waterloo (Ont.).
Aerobic exercise dosage and intensity
Australian investigators conducted a pilot randomized trial of high-intensity interval training (HIIT) versus more conventional moderate-intensity exercise to improve health-related quality of life and physical function in 27 patients with knee OA. The exercise programs involved unsupervised home-based cycling, with participants requested to do four roughly 25-minute sessions per week for 8 weeks.
The two exercise intensity groups showed similar gains in health-related quality of life as assessed by the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). However, the HIIT group showed significantly greater improvement in physical function as measured on the Timed Up and Go test (PeerJ. 2018 May 9;6:e4738).
Dr. Maly noted that adherence to the home-based exercise programs was a challenge: Only 17 of the 27 patients completed the 8-week Australian study, for a 37% dropout rate. However, most study withdrawals were because of family-related issues, illness, or injuries unrelated to cycling, with no signal that HIIT placed knee OA patients at higher injury risk.
Other investigators performed a systematic review of 45 studies in an effort to generate evidence-based guidance about the optimal exercise dosing in order to improve outcomes in knee OA patients. They concluded that programs comprising 24 therapeutic exercise sessions over the course of 8-12 weeks resulted in the largest improvements in measures of pain and physical function. And, importantly, one exercise session per week conferred no benefits (J Orthop Sports Phys Ther. 2018 Mar;48[3]:146-61).
“Frequency probably matters,” Dr. Maly observed.
Patients and their physicians often wonder if long-term, land-based exercise might have deleterious impacts on joint structure in patients with knee OA. Reassurance on this score was provided by a recent meta-analysis of RCTs that concluded, on the basis of moderate-strength evidence, that exercise therapy of longer than 6 months duration had no adverse effect on tibiofemoral radiographic disease severity, compared with no exercise. Nor was there evidence of a long-term-exercise–associated deterioration of tibiofemoral cartilage morphology or worsening of synovitis or effusion. Plus, the meta-analysis provided limited evidence to suggest long-term exercise had a protective effect on the composition of patellar cartilage (Semin Arthritis Rheum. 2019 Jun;48[6]:941-9).
“While there was a little bit of evidence suggesting that long-term exercise could worsen bone marrow lesions, really there was no other evidence that it could change the structure of a joint,” according to Dr. Maly.
Internet-based exercise training
Using the Internet to deliver an individually tailored exercise-training program for patients with symptomatic knee OA might sound like an efficient strategy, but in fact it proved fruitless in a large randomized trial. The 350 participants were assigned to an 8-visit, 4-month program of physical therapy, a wait-list control group, or an internet-based program that delivered tailored exercises and video demonstrations with no face-to-face contact. The bottom line is that improvement in WOMAC scores didn’t differ significantly between the three groups when evaluated at 4 and 12 months (Osteoarthritis Cartilage. 2018 Mar;26[3]:383-96).
“When we deliver exercise with the use of technology, it may require some support, including face to face,” Dr. Maly concluded from the study results.
Strength training
High-intensity resistance training such as weight lifting aimed at strengthening the quadriceps and other large muscles is often eschewed in patients with knee OA because of concern about possible damage to their already damaged joints. Intriguingly, Brazilian investigators may have found a workaround. They randomized 48 women with knee OA to 12 weeks of either supervised low-intensity resistance training performed with partial blood-flow restriction using an air cuff, to low-intensity resistance training alone, or to high-intensity resistance training. The low-intensity resistance workouts involved exercises such as leg presses and knee extensions performed at 30% of maximum effort.
The low-intensity resistance training performed with blood-flow restriction and the high-intensity strength training programs proved similarly effective in improving quadriceps muscle mass, muscle strength, and physical function to a significantly greater extent than with low-intensity resistance training alone. Moreover, low-intensity resistance training with blood-flow restriction also resulted in a significant improvement in pain scores. That finding, coupled with the fact that 4 of the 16 patients in the high-intensity resistance training group dropped out of the trial because of exercise-induced knee pain, suggests that low-intensity strength training carried out with partial blood-flow restriction may have a bright future (Med Sci Sports Exerc. 2018 May;50[5]:897-905).
Exercise plus diet-induced weight loss
How does the combination of dietary weight loss plus exercise stack up against diet alone in terms of benefits on pain and physical function in obese patients with knee OA? A systematic review and meta-analysis of nine RCTs aimed at answering that question concluded that diet-alone strategies are less effective. Both the diet-plus-exercise and diet-only interventions resulted in comparably moderate improvement in physical function. However, diet-only treatments didn’t reduce pain, whereas diet-plus-exercise interventions achieved moderate pain relief (Semin Arthritis Rheum. 2019 Apr;48[5]:765-77).
Dr. Maly reported having no financial conflicts of interest regarding her presentation.
TORONTO – Emerging evidence indicates that patients with knee osteoarthritis who engage in high-intensity interval training obtain significantly greater improvement in physical function than with conventionally prescribed moderate-intensity exercise, Monica R. Maly, PhD, said at the OARSI 2019 World Congress.
This was one of the key conclusions she and her coworkers drew from their analysis of the past year’s published research on diet and exercise interventions to improve outcomes in patients with OA, where obesity and physical inactivity figure prominently as modifiable lifestyle factors.
Another finding: Exercise interventions are where all the action is at present in the field of lifestyle-modification research aimed at achieving better health-related quality of life and other positive outcomes in OA. Dietary interventions are simply not a hot research topic. Indeed, her review of the past year’s literature included 38 randomized, controlled trials (RCTs) and 15 meta-analyses and systematic reviews – and all 38 RCTs addressed exercise interventions.
“It’s interesting to note that we found no new RCT data on diet to modify obesity in OA in the past year,” Dr. Maly said at the meeting sponsored by the Osteoarthritis Research Society International.
Additionally, 32 of the 38 RCTs devoted to exercise interventions for OA focused specifically on knee OA, noted Dr. Maly of the department of kinesiology at the University of Waterloo (Ont.).
Aerobic exercise dosage and intensity
Australian investigators conducted a pilot randomized trial of high-intensity interval training (HIIT) versus more conventional moderate-intensity exercise to improve health-related quality of life and physical function in 27 patients with knee OA. The exercise programs involved unsupervised home-based cycling, with participants requested to do four roughly 25-minute sessions per week for 8 weeks.
The two exercise intensity groups showed similar gains in health-related quality of life as assessed by the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). However, the HIIT group showed significantly greater improvement in physical function as measured on the Timed Up and Go test (PeerJ. 2018 May 9;6:e4738).
Dr. Maly noted that adherence to the home-based exercise programs was a challenge: Only 17 of the 27 patients completed the 8-week Australian study, for a 37% dropout rate. However, most study withdrawals were because of family-related issues, illness, or injuries unrelated to cycling, with no signal that HIIT placed knee OA patients at higher injury risk.
Other investigators performed a systematic review of 45 studies in an effort to generate evidence-based guidance about the optimal exercise dosing in order to improve outcomes in knee OA patients. They concluded that programs comprising 24 therapeutic exercise sessions over the course of 8-12 weeks resulted in the largest improvements in measures of pain and physical function. And, importantly, one exercise session per week conferred no benefits (J Orthop Sports Phys Ther. 2018 Mar;48[3]:146-61).
“Frequency probably matters,” Dr. Maly observed.
Patients and their physicians often wonder if long-term, land-based exercise might have deleterious impacts on joint structure in patients with knee OA. Reassurance on this score was provided by a recent meta-analysis of RCTs that concluded, on the basis of moderate-strength evidence, that exercise therapy of longer than 6 months duration had no adverse effect on tibiofemoral radiographic disease severity, compared with no exercise. Nor was there evidence of a long-term-exercise–associated deterioration of tibiofemoral cartilage morphology or worsening of synovitis or effusion. Plus, the meta-analysis provided limited evidence to suggest long-term exercise had a protective effect on the composition of patellar cartilage (Semin Arthritis Rheum. 2019 Jun;48[6]:941-9).
“While there was a little bit of evidence suggesting that long-term exercise could worsen bone marrow lesions, really there was no other evidence that it could change the structure of a joint,” according to Dr. Maly.
Internet-based exercise training
Using the Internet to deliver an individually tailored exercise-training program for patients with symptomatic knee OA might sound like an efficient strategy, but in fact it proved fruitless in a large randomized trial. The 350 participants were assigned to an 8-visit, 4-month program of physical therapy, a wait-list control group, or an internet-based program that delivered tailored exercises and video demonstrations with no face-to-face contact. The bottom line is that improvement in WOMAC scores didn’t differ significantly between the three groups when evaluated at 4 and 12 months (Osteoarthritis Cartilage. 2018 Mar;26[3]:383-96).
“When we deliver exercise with the use of technology, it may require some support, including face to face,” Dr. Maly concluded from the study results.
Strength training
High-intensity resistance training such as weight lifting aimed at strengthening the quadriceps and other large muscles is often eschewed in patients with knee OA because of concern about possible damage to their already damaged joints. Intriguingly, Brazilian investigators may have found a workaround. They randomized 48 women with knee OA to 12 weeks of either supervised low-intensity resistance training performed with partial blood-flow restriction using an air cuff, to low-intensity resistance training alone, or to high-intensity resistance training. The low-intensity resistance workouts involved exercises such as leg presses and knee extensions performed at 30% of maximum effort.
The low-intensity resistance training performed with blood-flow restriction and the high-intensity strength training programs proved similarly effective in improving quadriceps muscle mass, muscle strength, and physical function to a significantly greater extent than with low-intensity resistance training alone. Moreover, low-intensity resistance training with blood-flow restriction also resulted in a significant improvement in pain scores. That finding, coupled with the fact that 4 of the 16 patients in the high-intensity resistance training group dropped out of the trial because of exercise-induced knee pain, suggests that low-intensity strength training carried out with partial blood-flow restriction may have a bright future (Med Sci Sports Exerc. 2018 May;50[5]:897-905).
Exercise plus diet-induced weight loss
How does the combination of dietary weight loss plus exercise stack up against diet alone in terms of benefits on pain and physical function in obese patients with knee OA? A systematic review and meta-analysis of nine RCTs aimed at answering that question concluded that diet-alone strategies are less effective. Both the diet-plus-exercise and diet-only interventions resulted in comparably moderate improvement in physical function. However, diet-only treatments didn’t reduce pain, whereas diet-plus-exercise interventions achieved moderate pain relief (Semin Arthritis Rheum. 2019 Apr;48[5]:765-77).
Dr. Maly reported having no financial conflicts of interest regarding her presentation.
EXPERT ANALYSIS FROM OARSI 2019
Liposomal steroid brings durable pain relief in knee OA
TORONTO – A single intra-articular injection of a novel, sustained-release liposomal formulation of dexamethasone in patients with symptomatic knee osteoarthritis brought at least 6 months of pain control in a multicenter, phase 2a trial, David Hunter, MD, reported at the OARSI 2019 World Congress.
This is a product that could fill a significant unmet medical need. Current therapies for knee OA have modest efficacy, and the injectable ones provide only 2-4 weeks of benefit. The ability to obtain significant pain relief with just a couple of intra-articular injections per year would be an important therapeutic advance, observed Dr. Hunter, professor of medicine at the University of Sydney.
He presented a 24-week study of 75 patients with symptomatic knee OA randomized at 13 sites in Australia and Taiwan to a single intra-articular injection of either 12 or 18 mg of the liposomal dexamethasone or to normal saline. One knee per patient was treated.
The primary outcome was the change in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score from baseline to week 12. The 12-mg formulation of steroid significantly outperformed placebo at that time point as well as at all others. From a mean baseline WOMAC pain score of 1.49 on the 0-4 scale, patients in the 12-mg group averaged reductions of 0.83 points at 12 weeks, 0.85 at both weeks 16 and 20, and 0.87 at week 24. A statistically significant between-group difference was seen as early as day 3 after injection.
More than half (52%) of recipients of the 12-mg dose of liposomal dexamethasone, a product known for now simply as TLC599, maintained at least 30% pain relief at all visits through the study close at 24 weeks, as did 22% of controls, the rheumatologist reported at the meeting sponsored by the Osteoarthritis Research Society International.
The 12-mg injection also proved superior to placebo for the secondary endpoint of change in WOMAC function score. From a mean baseline score of 1.53, recipients of the 12-mg dose had improvements ranging from 0.82 points at week 12 to 0.85 points at week 24.
Of note, total acetaminophen intake over the course of the trial in the 12-mg steroid group was less than one-third of that in controls.
The 18-mg dose didn’t result in significantly greater reduction in pain scores than placebo. This is because dexamethasone release in the higher-dose formulation as presently constituted turned out to be less efficient, Dr. Hunter explained.
The safety profile was closely similar in all three study arms.
In phase 3 clinical trials, TLC599 will be compared with standard intra-articular triamcinolone, according to the rheumatologist.
He reported serving as a consultant to the Taiwan Liposome Company, which sponsored the phase 2a study, as well as to a handful of other pharmaceutical companies.
TORONTO – A single intra-articular injection of a novel, sustained-release liposomal formulation of dexamethasone in patients with symptomatic knee osteoarthritis brought at least 6 months of pain control in a multicenter, phase 2a trial, David Hunter, MD, reported at the OARSI 2019 World Congress.
This is a product that could fill a significant unmet medical need. Current therapies for knee OA have modest efficacy, and the injectable ones provide only 2-4 weeks of benefit. The ability to obtain significant pain relief with just a couple of intra-articular injections per year would be an important therapeutic advance, observed Dr. Hunter, professor of medicine at the University of Sydney.
He presented a 24-week study of 75 patients with symptomatic knee OA randomized at 13 sites in Australia and Taiwan to a single intra-articular injection of either 12 or 18 mg of the liposomal dexamethasone or to normal saline. One knee per patient was treated.
The primary outcome was the change in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score from baseline to week 12. The 12-mg formulation of steroid significantly outperformed placebo at that time point as well as at all others. From a mean baseline WOMAC pain score of 1.49 on the 0-4 scale, patients in the 12-mg group averaged reductions of 0.83 points at 12 weeks, 0.85 at both weeks 16 and 20, and 0.87 at week 24. A statistically significant between-group difference was seen as early as day 3 after injection.
More than half (52%) of recipients of the 12-mg dose of liposomal dexamethasone, a product known for now simply as TLC599, maintained at least 30% pain relief at all visits through the study close at 24 weeks, as did 22% of controls, the rheumatologist reported at the meeting sponsored by the Osteoarthritis Research Society International.
The 12-mg injection also proved superior to placebo for the secondary endpoint of change in WOMAC function score. From a mean baseline score of 1.53, recipients of the 12-mg dose had improvements ranging from 0.82 points at week 12 to 0.85 points at week 24.
Of note, total acetaminophen intake over the course of the trial in the 12-mg steroid group was less than one-third of that in controls.
The 18-mg dose didn’t result in significantly greater reduction in pain scores than placebo. This is because dexamethasone release in the higher-dose formulation as presently constituted turned out to be less efficient, Dr. Hunter explained.
The safety profile was closely similar in all three study arms.
In phase 3 clinical trials, TLC599 will be compared with standard intra-articular triamcinolone, according to the rheumatologist.
He reported serving as a consultant to the Taiwan Liposome Company, which sponsored the phase 2a study, as well as to a handful of other pharmaceutical companies.
TORONTO – A single intra-articular injection of a novel, sustained-release liposomal formulation of dexamethasone in patients with symptomatic knee osteoarthritis brought at least 6 months of pain control in a multicenter, phase 2a trial, David Hunter, MD, reported at the OARSI 2019 World Congress.
This is a product that could fill a significant unmet medical need. Current therapies for knee OA have modest efficacy, and the injectable ones provide only 2-4 weeks of benefit. The ability to obtain significant pain relief with just a couple of intra-articular injections per year would be an important therapeutic advance, observed Dr. Hunter, professor of medicine at the University of Sydney.
He presented a 24-week study of 75 patients with symptomatic knee OA randomized at 13 sites in Australia and Taiwan to a single intra-articular injection of either 12 or 18 mg of the liposomal dexamethasone or to normal saline. One knee per patient was treated.
The primary outcome was the change in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score from baseline to week 12. The 12-mg formulation of steroid significantly outperformed placebo at that time point as well as at all others. From a mean baseline WOMAC pain score of 1.49 on the 0-4 scale, patients in the 12-mg group averaged reductions of 0.83 points at 12 weeks, 0.85 at both weeks 16 and 20, and 0.87 at week 24. A statistically significant between-group difference was seen as early as day 3 after injection.
More than half (52%) of recipients of the 12-mg dose of liposomal dexamethasone, a product known for now simply as TLC599, maintained at least 30% pain relief at all visits through the study close at 24 weeks, as did 22% of controls, the rheumatologist reported at the meeting sponsored by the Osteoarthritis Research Society International.
The 12-mg injection also proved superior to placebo for the secondary endpoint of change in WOMAC function score. From a mean baseline score of 1.53, recipients of the 12-mg dose had improvements ranging from 0.82 points at week 12 to 0.85 points at week 24.
Of note, total acetaminophen intake over the course of the trial in the 12-mg steroid group was less than one-third of that in controls.
The 18-mg dose didn’t result in significantly greater reduction in pain scores than placebo. This is because dexamethasone release in the higher-dose formulation as presently constituted turned out to be less efficient, Dr. Hunter explained.
The safety profile was closely similar in all three study arms.
In phase 3 clinical trials, TLC599 will be compared with standard intra-articular triamcinolone, according to the rheumatologist.
He reported serving as a consultant to the Taiwan Liposome Company, which sponsored the phase 2a study, as well as to a handful of other pharmaceutical companies.
REPORTING FROM OARSI 2019
How common is accelerated knee OA?
TORONTO – Accelerated knee osteoarthritis – a particularly noxious form of the joint disease – occurred in more than one in seven women who developed knee osteoarthritis in the prospective, long-term Chingford Cohort Study, Jeffrey B. Driban, PhD, reported at the OARSI 2019 World Congress.
This finding from a unique prospective study of 1,003 middle-aged U.K. women who were followed for the development of knee osteoarthritis (OA) for 15 years is important because the participants represented a typical community-based population sample. And yet the Chingford results are consistent with and confirmatory of those found earlier in the Osteoarthritis Initiative, a U.S. cohort study of nearly 4,800 individuals, even though the Osteoarthritis Initiative featured a population enriched with established risk factors for knee OA, Dr. Driban explained at the meeting, sponsored by the Osteoarthritis Research Society International.
In Chingford, accelerated knee OA accounted for 15% of all incident cases of knee OA during follow-up, and for 17% of all newly affected knees, whereas 20% of incident knee OA in the Osteoarthritis Initiative was accelerated knee OA, noted Dr. Driban of Tufts University, Boston.
Accelerated knee OA is defined by rapidly progressive structural damage. Affected individuals streak from no radiographic evidence of knee OA to advanced-stage disease marked by a Kellgren-Lawrence score of 3 or more within 4 years, whereas the typical form of knee OA follows a more gradual course. Also, accelerated knee OA features greater pain and disability.
In the Chingford study, the cumulative incidence of accelerated knee OA was 3.9%, while typical knee OA occurred in 21.7% of women. During years 6-15 of follow-up, 21% of women with accelerated knee OA underwent total knee replacement, compared with 2% of those with typical knee OA and 0.9% of women without knee OA.
Dr. Driban reported having no financial conflicts regarding his analysis of the Chingford Cohort Study and the Osteoarthritis Initiative, supported by Arthritis Research UK and the National Institutes of Health, respectively.
SOURCE: Driban JB et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S250-S251, Abstract 352.
TORONTO – Accelerated knee osteoarthritis – a particularly noxious form of the joint disease – occurred in more than one in seven women who developed knee osteoarthritis in the prospective, long-term Chingford Cohort Study, Jeffrey B. Driban, PhD, reported at the OARSI 2019 World Congress.
This finding from a unique prospective study of 1,003 middle-aged U.K. women who were followed for the development of knee osteoarthritis (OA) for 15 years is important because the participants represented a typical community-based population sample. And yet the Chingford results are consistent with and confirmatory of those found earlier in the Osteoarthritis Initiative, a U.S. cohort study of nearly 4,800 individuals, even though the Osteoarthritis Initiative featured a population enriched with established risk factors for knee OA, Dr. Driban explained at the meeting, sponsored by the Osteoarthritis Research Society International.
In Chingford, accelerated knee OA accounted for 15% of all incident cases of knee OA during follow-up, and for 17% of all newly affected knees, whereas 20% of incident knee OA in the Osteoarthritis Initiative was accelerated knee OA, noted Dr. Driban of Tufts University, Boston.
Accelerated knee OA is defined by rapidly progressive structural damage. Affected individuals streak from no radiographic evidence of knee OA to advanced-stage disease marked by a Kellgren-Lawrence score of 3 or more within 4 years, whereas the typical form of knee OA follows a more gradual course. Also, accelerated knee OA features greater pain and disability.
In the Chingford study, the cumulative incidence of accelerated knee OA was 3.9%, while typical knee OA occurred in 21.7% of women. During years 6-15 of follow-up, 21% of women with accelerated knee OA underwent total knee replacement, compared with 2% of those with typical knee OA and 0.9% of women without knee OA.
Dr. Driban reported having no financial conflicts regarding his analysis of the Chingford Cohort Study and the Osteoarthritis Initiative, supported by Arthritis Research UK and the National Institutes of Health, respectively.
SOURCE: Driban JB et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S250-S251, Abstract 352.
TORONTO – Accelerated knee osteoarthritis – a particularly noxious form of the joint disease – occurred in more than one in seven women who developed knee osteoarthritis in the prospective, long-term Chingford Cohort Study, Jeffrey B. Driban, PhD, reported at the OARSI 2019 World Congress.
This finding from a unique prospective study of 1,003 middle-aged U.K. women who were followed for the development of knee osteoarthritis (OA) for 15 years is important because the participants represented a typical community-based population sample. And yet the Chingford results are consistent with and confirmatory of those found earlier in the Osteoarthritis Initiative, a U.S. cohort study of nearly 4,800 individuals, even though the Osteoarthritis Initiative featured a population enriched with established risk factors for knee OA, Dr. Driban explained at the meeting, sponsored by the Osteoarthritis Research Society International.
In Chingford, accelerated knee OA accounted for 15% of all incident cases of knee OA during follow-up, and for 17% of all newly affected knees, whereas 20% of incident knee OA in the Osteoarthritis Initiative was accelerated knee OA, noted Dr. Driban of Tufts University, Boston.
Accelerated knee OA is defined by rapidly progressive structural damage. Affected individuals streak from no radiographic evidence of knee OA to advanced-stage disease marked by a Kellgren-Lawrence score of 3 or more within 4 years, whereas the typical form of knee OA follows a more gradual course. Also, accelerated knee OA features greater pain and disability.
In the Chingford study, the cumulative incidence of accelerated knee OA was 3.9%, while typical knee OA occurred in 21.7% of women. During years 6-15 of follow-up, 21% of women with accelerated knee OA underwent total knee replacement, compared with 2% of those with typical knee OA and 0.9% of women without knee OA.
Dr. Driban reported having no financial conflicts regarding his analysis of the Chingford Cohort Study and the Osteoarthritis Initiative, supported by Arthritis Research UK and the National Institutes of Health, respectively.
SOURCE: Driban JB et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S250-S251, Abstract 352.
REPORTING FROM OARSI 2019
Minor surgeries appear safe for hemophilia patients on emicizumab
MELBOURNE – A majority of minor surgeries can be performed in hemophilia A patients receiving emicizumab therapy without requiring prophylactic treatment with coagulation factors, according to data presented at the International Society on Thrombosis and Haemostasis congress.
Elena Santagostino, MD, PhD, from the Hemophilia and Thrombosis Center at Ospedale Maggiore Policlinico in Milan presented data from 399 patients involved in the four HAVEN trials of the humanized bispecific monoclonal antibody emicizumab (Hemlibra), which is Food and Drug Administration–approved for the prevention of bleeding episodes in individuals with hemophilia A, with or without inhibitors.
The analysis focused on the 126 patients (31.6%) who underwent at least one surgical procedure during the studies. Of the 233 surgeries, there were 215 minor procedures performed in 115 patients, and 18 major surgeries in 18 patients. All patients were receiving ongoing treatment with emicizumab, and there was no change to that treatment regimen during surgery.
“It is clear that surgery is a challenge for hemophilia,” Dr. Santagostino said. “It is a challenge for bleeding, it is a challenge for thrombosis, it is a challenge for any new drug, and this is why there is a lot of interest around this topic.”
Overall, 65.6% of minor surgeries were performed without any prophylactic coagulation factor treatment, and 90.8% of minor surgeries were conducted without postoperative bleeds requiring treatment. There were no cases of thrombosis reported.
The surgeries that did not require prophylactic coagulation factor included 42 dental procedures, 25 central venous access devices, 17 endoscopic procedures, and 12 joint procedures.
While the HAVEN studies did not allow for elective major surgery, there were still 18 unplanned major surgical situations that arose during the course of the studies. These included three hip, one knee, and one ankle arthroplasties; three synovectomies; and some dental, central venous line, and endoscopic biopsy procedures.
Of these, 15 involved prophylactic coagulant factor administration, but three procedures – including one synovectomy – were performed without prophylaxis and none resulted in a bleed.
There was one complicated bleed that occurred in a patient undergoing multiple procedures including a synovectomy, joint debridement and chondroplasty, who received prolonged treatment with recombinant Factor VIIa.
Dr. Santagostino said the findings showed surgery could be safely performed in patients who were being treated with emicizumab, both with and without inhibitors.
“A large number of minor procedures can be done without adding coagulation factors,” she said in an interview. “This is true for less invasive surgeries, such as catheter-related central venous line procedures. Even several endoscopic procedures, like a single biopsy, can be done reasonably safely.”
However she said there was still a lack of experience in dealing with hemophilia A patients who were undergoing cancer surgery, or who had significant comorbidities that might put them at higher risk of thrombosis.
“These are special patients populations that are still not investigated in the trial setting,” she said.
Commenting on the data, session cochair Liane Khoo, MD, from the Haemophilia Treatment Centre at Royal Prince Alfred Hospital in Sydney, said the results showed surgery could be performed in hemophilia A patients with and without inhibitors.
“The more we have the medication and the more experience we have, then we become more confident in using it,” she said.
The study was funded by F. Hoffman-La Roche and Chugai Pharmaceutical. Dr. Santagostino reported consultancies and speakers bureau engagements with the pharmaceutical sector.
SOURCE: Santagostino E et al. 2019 ISTH Congress, Abstract OC 60.1.
MELBOURNE – A majority of minor surgeries can be performed in hemophilia A patients receiving emicizumab therapy without requiring prophylactic treatment with coagulation factors, according to data presented at the International Society on Thrombosis and Haemostasis congress.
Elena Santagostino, MD, PhD, from the Hemophilia and Thrombosis Center at Ospedale Maggiore Policlinico in Milan presented data from 399 patients involved in the four HAVEN trials of the humanized bispecific monoclonal antibody emicizumab (Hemlibra), which is Food and Drug Administration–approved for the prevention of bleeding episodes in individuals with hemophilia A, with or without inhibitors.
The analysis focused on the 126 patients (31.6%) who underwent at least one surgical procedure during the studies. Of the 233 surgeries, there were 215 minor procedures performed in 115 patients, and 18 major surgeries in 18 patients. All patients were receiving ongoing treatment with emicizumab, and there was no change to that treatment regimen during surgery.
“It is clear that surgery is a challenge for hemophilia,” Dr. Santagostino said. “It is a challenge for bleeding, it is a challenge for thrombosis, it is a challenge for any new drug, and this is why there is a lot of interest around this topic.”
Overall, 65.6% of minor surgeries were performed without any prophylactic coagulation factor treatment, and 90.8% of minor surgeries were conducted without postoperative bleeds requiring treatment. There were no cases of thrombosis reported.
The surgeries that did not require prophylactic coagulation factor included 42 dental procedures, 25 central venous access devices, 17 endoscopic procedures, and 12 joint procedures.
While the HAVEN studies did not allow for elective major surgery, there were still 18 unplanned major surgical situations that arose during the course of the studies. These included three hip, one knee, and one ankle arthroplasties; three synovectomies; and some dental, central venous line, and endoscopic biopsy procedures.
Of these, 15 involved prophylactic coagulant factor administration, but three procedures – including one synovectomy – were performed without prophylaxis and none resulted in a bleed.
There was one complicated bleed that occurred in a patient undergoing multiple procedures including a synovectomy, joint debridement and chondroplasty, who received prolonged treatment with recombinant Factor VIIa.
Dr. Santagostino said the findings showed surgery could be safely performed in patients who were being treated with emicizumab, both with and without inhibitors.
“A large number of minor procedures can be done without adding coagulation factors,” she said in an interview. “This is true for less invasive surgeries, such as catheter-related central venous line procedures. Even several endoscopic procedures, like a single biopsy, can be done reasonably safely.”
However she said there was still a lack of experience in dealing with hemophilia A patients who were undergoing cancer surgery, or who had significant comorbidities that might put them at higher risk of thrombosis.
“These are special patients populations that are still not investigated in the trial setting,” she said.
Commenting on the data, session cochair Liane Khoo, MD, from the Haemophilia Treatment Centre at Royal Prince Alfred Hospital in Sydney, said the results showed surgery could be performed in hemophilia A patients with and without inhibitors.
“The more we have the medication and the more experience we have, then we become more confident in using it,” she said.
The study was funded by F. Hoffman-La Roche and Chugai Pharmaceutical. Dr. Santagostino reported consultancies and speakers bureau engagements with the pharmaceutical sector.
SOURCE: Santagostino E et al. 2019 ISTH Congress, Abstract OC 60.1.
MELBOURNE – A majority of minor surgeries can be performed in hemophilia A patients receiving emicizumab therapy without requiring prophylactic treatment with coagulation factors, according to data presented at the International Society on Thrombosis and Haemostasis congress.
Elena Santagostino, MD, PhD, from the Hemophilia and Thrombosis Center at Ospedale Maggiore Policlinico in Milan presented data from 399 patients involved in the four HAVEN trials of the humanized bispecific monoclonal antibody emicizumab (Hemlibra), which is Food and Drug Administration–approved for the prevention of bleeding episodes in individuals with hemophilia A, with or without inhibitors.
The analysis focused on the 126 patients (31.6%) who underwent at least one surgical procedure during the studies. Of the 233 surgeries, there were 215 minor procedures performed in 115 patients, and 18 major surgeries in 18 patients. All patients were receiving ongoing treatment with emicizumab, and there was no change to that treatment regimen during surgery.
“It is clear that surgery is a challenge for hemophilia,” Dr. Santagostino said. “It is a challenge for bleeding, it is a challenge for thrombosis, it is a challenge for any new drug, and this is why there is a lot of interest around this topic.”
Overall, 65.6% of minor surgeries were performed without any prophylactic coagulation factor treatment, and 90.8% of minor surgeries were conducted without postoperative bleeds requiring treatment. There were no cases of thrombosis reported.
The surgeries that did not require prophylactic coagulation factor included 42 dental procedures, 25 central venous access devices, 17 endoscopic procedures, and 12 joint procedures.
While the HAVEN studies did not allow for elective major surgery, there were still 18 unplanned major surgical situations that arose during the course of the studies. These included three hip, one knee, and one ankle arthroplasties; three synovectomies; and some dental, central venous line, and endoscopic biopsy procedures.
Of these, 15 involved prophylactic coagulant factor administration, but three procedures – including one synovectomy – were performed without prophylaxis and none resulted in a bleed.
There was one complicated bleed that occurred in a patient undergoing multiple procedures including a synovectomy, joint debridement and chondroplasty, who received prolonged treatment with recombinant Factor VIIa.
Dr. Santagostino said the findings showed surgery could be safely performed in patients who were being treated with emicizumab, both with and without inhibitors.
“A large number of minor procedures can be done without adding coagulation factors,” she said in an interview. “This is true for less invasive surgeries, such as catheter-related central venous line procedures. Even several endoscopic procedures, like a single biopsy, can be done reasonably safely.”
However she said there was still a lack of experience in dealing with hemophilia A patients who were undergoing cancer surgery, or who had significant comorbidities that might put them at higher risk of thrombosis.
“These are special patients populations that are still not investigated in the trial setting,” she said.
Commenting on the data, session cochair Liane Khoo, MD, from the Haemophilia Treatment Centre at Royal Prince Alfred Hospital in Sydney, said the results showed surgery could be performed in hemophilia A patients with and without inhibitors.
“The more we have the medication and the more experience we have, then we become more confident in using it,” she said.
The study was funded by F. Hoffman-La Roche and Chugai Pharmaceutical. Dr. Santagostino reported consultancies and speakers bureau engagements with the pharmaceutical sector.
SOURCE: Santagostino E et al. 2019 ISTH Congress, Abstract OC 60.1.
REPORTING FROM 2019 ISTH CONGRESS
Tanezumab improves osteoarthritis pain, function in phase 3 trial
MADRID – Tanezumab, an investigational monoclonal antibody directed against nerve growth factor that is under development to treat osteoarthritis pain, met most of the coprimary efficacy endpoints set for the drug in a randomized, double-blind, parallel-group, placebo-controlled phase 3 study.
At the end of a 24-week, double-blind treatment period, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain and WOMAC physical function subscale scores were significantly improved, compared with placebo in the two tanezumab (2.5 mg and 5 mg) dose groups.
The least squares (ls) mean change from baseline in WOMAC pain scores were –2.24 for placebo, –2.70 for tanezumab 2.5 mg, and –2.85 for tanezumab 5 mg (P less than or equal to .01 and P less than or equal to .001 vs. placebo).
The ls mean change from baseline in WOMAC physical function scores were a respective –2.11, –2.70, and –2.82 (P less than or equal to .001 for both vs. placebo).
The coprimary endpoint of patients’ global assessment of OA (PGA-OA) was also significantly improved with tanezumab 5 mg (–0.90; P less than or equal to .05) but not 2.5 mg (–0.82) versus placebo (–0.72).
As the 2.5-mg dose of tanezumab didn’t meet one of the three coprimary endpoints, further hypothesis testing was not possible, but exploratory findings suggested that tanezumab at 2.5 mg or 5 mg yielded higher proportions of patients with reductions from baseline in WOMAC pain scores when compared against placebo. This was the case for reductions of at least 30% (65.6%, 68.7%, 56.6%, respectively), 50% (45.4%, 47.9%, 33.8%), or 70% (21.3%, 23.2%, 17.8%).
“I think that we have now a lot of studies with tanezumab showing a significant effect on hip and knee OA pain and function, so we have the studies in order to have the drug on the market,” study first author Francis Berenbaum, MD, PhD, of Saint-Antoine Hospital, Sorbonne Université in Paris, said in an interview at the European Congress of Rheumatology.
“Of course, because of the safety issue with rapid progressive osteoarthritis (RPOA), what we are discussing now is: ‘For which patients will there be an optimal benefit-to-risk?’ So, it’s now more a discussion around the population of patients who can benefit the most with the drug,” Dr. Berenbaum added.
A possible link between the use of tanezumab and a risk for developing RPOA was first suggested by preclinical and early clinical trial data, prompting the U.S. Food and Drug Administration to place partial holds on its clinical development in 2010, and again in 2012.
However, Dr. Berenbaum noted that a “mitigation plan” had been put in place for the phase 3 program to try to lower the likelihood of RPOA. This included: lowering the dose of the drug used and delivering it subcutaneously rather than intravenously; not prescribing it with NSAIDs and testing its possible effects and safety in a difficult-to-treat population of patients with no known risk factors for the potentially very serious adverse event.
“Based on this mitigation plan, the risk of rapid progressive osteoarthritis has considerably decreased,” Dr. Berenbaum observed. Indeed, in the phase 3 study he presented at the meeting, he said that around 2% of patients developed RPOA, which is “exactly in line with what has already been shown.” RPOA was reported in none of the placebo-treated patients, in 1.4% of those treated with tanezumab 2.5 mg, and in 2.8% in those treated with tanezumab 5 mg.
However, a “striking” finding of the current study was that despite the small increase in RPOA seen, there was no difference between the tanezumab and placebo groups in the number of patients needing total joint replacement (TJR). The percentages of patients undergoing at least one TJR was 6.7% in the placebo group, 7.8% in the tanezumab 2.5-mg group, and 7.0% in the tanezumab 5-mg group.
The joint safety events seen in the study, including TJRs, were adjudicated as being part of the normal progression of OA in the majority (73.4%) of cases. Other joint events of note were one case of subchondral insufficiency fracture occurring in a patient treated with tanezumab 2.5 mg and one case of primary osteonecrosis in a patient treated with tanezumab 5 mg.
During his presentation of the findings in a late-breaking oral abstract session, Dr. Berenbaum noted that this was a difficult-to-treat population of patients. All 849 patients who had been recruited had moderate to severe OA pain of the knee or hip and had a history of insufficient pain relief or intolerance to treatment with acetaminophen, oral NSAIDs, and tramadol and were also not responding to, or unwilling to take, opioid painkillers. Patients had to have no radiographic evidence of specified bone conditions, including RPOA.
Patients had been treated with subcutaneous tanezumab 2.5 mg (n = 283) or 5 mg (n = 284) or placebo (n = 282) at baseline, week 8, and week 16, with the three coprimary efficacy endpoints assessed at week 24.
Discussing the risk-to-benefit ratio of the drug after his presentation, Dr. Berenbaum said: “You have to keep in mind that, first, it was in very difficult-to-treat patients, compared to the other trials in the field of OA symptoms.”
He added: “Second, is that compared to the other trials, this one was able to include patients with Kellgren-Lawrence grade 4, meaning that this is a more serious population,” and third, “when you look at the responders – WOMAC 30%, 50%, 70% – there is a strong difference in terms of responders.”
Dr. Berenbaum and his coauthors noted on the poster that accompanied the late-breaking oral presentation that “an active-controlled study will provide data to further characterize the risk-benefit of tanezumab in patients with OA.”
The study was sponsored by Pfizer and Eli Lilly. Dr. Berenbaum disclosed receiving research funding through his institution from Pfizer and acting as a consultant to, and speaker for, the company as well as multiple other pharmaceutical companies. Coauthors of the study also disclosed research funding or consultancy agreements with Pfizer or Eli Lilly or were employees of the companies.
SOURCE: Berenbaum F et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):262-4. Abstract LB0007, doi: 10.1136/annrheumdis-2019-eular.8660
MADRID – Tanezumab, an investigational monoclonal antibody directed against nerve growth factor that is under development to treat osteoarthritis pain, met most of the coprimary efficacy endpoints set for the drug in a randomized, double-blind, parallel-group, placebo-controlled phase 3 study.
At the end of a 24-week, double-blind treatment period, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain and WOMAC physical function subscale scores were significantly improved, compared with placebo in the two tanezumab (2.5 mg and 5 mg) dose groups.
The least squares (ls) mean change from baseline in WOMAC pain scores were –2.24 for placebo, –2.70 for tanezumab 2.5 mg, and –2.85 for tanezumab 5 mg (P less than or equal to .01 and P less than or equal to .001 vs. placebo).
The ls mean change from baseline in WOMAC physical function scores were a respective –2.11, –2.70, and –2.82 (P less than or equal to .001 for both vs. placebo).
The coprimary endpoint of patients’ global assessment of OA (PGA-OA) was also significantly improved with tanezumab 5 mg (–0.90; P less than or equal to .05) but not 2.5 mg (–0.82) versus placebo (–0.72).
As the 2.5-mg dose of tanezumab didn’t meet one of the three coprimary endpoints, further hypothesis testing was not possible, but exploratory findings suggested that tanezumab at 2.5 mg or 5 mg yielded higher proportions of patients with reductions from baseline in WOMAC pain scores when compared against placebo. This was the case for reductions of at least 30% (65.6%, 68.7%, 56.6%, respectively), 50% (45.4%, 47.9%, 33.8%), or 70% (21.3%, 23.2%, 17.8%).
“I think that we have now a lot of studies with tanezumab showing a significant effect on hip and knee OA pain and function, so we have the studies in order to have the drug on the market,” study first author Francis Berenbaum, MD, PhD, of Saint-Antoine Hospital, Sorbonne Université in Paris, said in an interview at the European Congress of Rheumatology.
“Of course, because of the safety issue with rapid progressive osteoarthritis (RPOA), what we are discussing now is: ‘For which patients will there be an optimal benefit-to-risk?’ So, it’s now more a discussion around the population of patients who can benefit the most with the drug,” Dr. Berenbaum added.
A possible link between the use of tanezumab and a risk for developing RPOA was first suggested by preclinical and early clinical trial data, prompting the U.S. Food and Drug Administration to place partial holds on its clinical development in 2010, and again in 2012.
However, Dr. Berenbaum noted that a “mitigation plan” had been put in place for the phase 3 program to try to lower the likelihood of RPOA. This included: lowering the dose of the drug used and delivering it subcutaneously rather than intravenously; not prescribing it with NSAIDs and testing its possible effects and safety in a difficult-to-treat population of patients with no known risk factors for the potentially very serious adverse event.
“Based on this mitigation plan, the risk of rapid progressive osteoarthritis has considerably decreased,” Dr. Berenbaum observed. Indeed, in the phase 3 study he presented at the meeting, he said that around 2% of patients developed RPOA, which is “exactly in line with what has already been shown.” RPOA was reported in none of the placebo-treated patients, in 1.4% of those treated with tanezumab 2.5 mg, and in 2.8% in those treated with tanezumab 5 mg.
However, a “striking” finding of the current study was that despite the small increase in RPOA seen, there was no difference between the tanezumab and placebo groups in the number of patients needing total joint replacement (TJR). The percentages of patients undergoing at least one TJR was 6.7% in the placebo group, 7.8% in the tanezumab 2.5-mg group, and 7.0% in the tanezumab 5-mg group.
The joint safety events seen in the study, including TJRs, were adjudicated as being part of the normal progression of OA in the majority (73.4%) of cases. Other joint events of note were one case of subchondral insufficiency fracture occurring in a patient treated with tanezumab 2.5 mg and one case of primary osteonecrosis in a patient treated with tanezumab 5 mg.
During his presentation of the findings in a late-breaking oral abstract session, Dr. Berenbaum noted that this was a difficult-to-treat population of patients. All 849 patients who had been recruited had moderate to severe OA pain of the knee or hip and had a history of insufficient pain relief or intolerance to treatment with acetaminophen, oral NSAIDs, and tramadol and were also not responding to, or unwilling to take, opioid painkillers. Patients had to have no radiographic evidence of specified bone conditions, including RPOA.
Patients had been treated with subcutaneous tanezumab 2.5 mg (n = 283) or 5 mg (n = 284) or placebo (n = 282) at baseline, week 8, and week 16, with the three coprimary efficacy endpoints assessed at week 24.
Discussing the risk-to-benefit ratio of the drug after his presentation, Dr. Berenbaum said: “You have to keep in mind that, first, it was in very difficult-to-treat patients, compared to the other trials in the field of OA symptoms.”
He added: “Second, is that compared to the other trials, this one was able to include patients with Kellgren-Lawrence grade 4, meaning that this is a more serious population,” and third, “when you look at the responders – WOMAC 30%, 50%, 70% – there is a strong difference in terms of responders.”
Dr. Berenbaum and his coauthors noted on the poster that accompanied the late-breaking oral presentation that “an active-controlled study will provide data to further characterize the risk-benefit of tanezumab in patients with OA.”
The study was sponsored by Pfizer and Eli Lilly. Dr. Berenbaum disclosed receiving research funding through his institution from Pfizer and acting as a consultant to, and speaker for, the company as well as multiple other pharmaceutical companies. Coauthors of the study also disclosed research funding or consultancy agreements with Pfizer or Eli Lilly or were employees of the companies.
SOURCE: Berenbaum F et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):262-4. Abstract LB0007, doi: 10.1136/annrheumdis-2019-eular.8660
MADRID – Tanezumab, an investigational monoclonal antibody directed against nerve growth factor that is under development to treat osteoarthritis pain, met most of the coprimary efficacy endpoints set for the drug in a randomized, double-blind, parallel-group, placebo-controlled phase 3 study.
At the end of a 24-week, double-blind treatment period, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain and WOMAC physical function subscale scores were significantly improved, compared with placebo in the two tanezumab (2.5 mg and 5 mg) dose groups.
The least squares (ls) mean change from baseline in WOMAC pain scores were –2.24 for placebo, –2.70 for tanezumab 2.5 mg, and –2.85 for tanezumab 5 mg (P less than or equal to .01 and P less than or equal to .001 vs. placebo).
The ls mean change from baseline in WOMAC physical function scores were a respective –2.11, –2.70, and –2.82 (P less than or equal to .001 for both vs. placebo).
The coprimary endpoint of patients’ global assessment of OA (PGA-OA) was also significantly improved with tanezumab 5 mg (–0.90; P less than or equal to .05) but not 2.5 mg (–0.82) versus placebo (–0.72).
As the 2.5-mg dose of tanezumab didn’t meet one of the three coprimary endpoints, further hypothesis testing was not possible, but exploratory findings suggested that tanezumab at 2.5 mg or 5 mg yielded higher proportions of patients with reductions from baseline in WOMAC pain scores when compared against placebo. This was the case for reductions of at least 30% (65.6%, 68.7%, 56.6%, respectively), 50% (45.4%, 47.9%, 33.8%), or 70% (21.3%, 23.2%, 17.8%).
“I think that we have now a lot of studies with tanezumab showing a significant effect on hip and knee OA pain and function, so we have the studies in order to have the drug on the market,” study first author Francis Berenbaum, MD, PhD, of Saint-Antoine Hospital, Sorbonne Université in Paris, said in an interview at the European Congress of Rheumatology.
“Of course, because of the safety issue with rapid progressive osteoarthritis (RPOA), what we are discussing now is: ‘For which patients will there be an optimal benefit-to-risk?’ So, it’s now more a discussion around the population of patients who can benefit the most with the drug,” Dr. Berenbaum added.
A possible link between the use of tanezumab and a risk for developing RPOA was first suggested by preclinical and early clinical trial data, prompting the U.S. Food and Drug Administration to place partial holds on its clinical development in 2010, and again in 2012.
However, Dr. Berenbaum noted that a “mitigation plan” had been put in place for the phase 3 program to try to lower the likelihood of RPOA. This included: lowering the dose of the drug used and delivering it subcutaneously rather than intravenously; not prescribing it with NSAIDs and testing its possible effects and safety in a difficult-to-treat population of patients with no known risk factors for the potentially very serious adverse event.
“Based on this mitigation plan, the risk of rapid progressive osteoarthritis has considerably decreased,” Dr. Berenbaum observed. Indeed, in the phase 3 study he presented at the meeting, he said that around 2% of patients developed RPOA, which is “exactly in line with what has already been shown.” RPOA was reported in none of the placebo-treated patients, in 1.4% of those treated with tanezumab 2.5 mg, and in 2.8% in those treated with tanezumab 5 mg.
However, a “striking” finding of the current study was that despite the small increase in RPOA seen, there was no difference between the tanezumab and placebo groups in the number of patients needing total joint replacement (TJR). The percentages of patients undergoing at least one TJR was 6.7% in the placebo group, 7.8% in the tanezumab 2.5-mg group, and 7.0% in the tanezumab 5-mg group.
The joint safety events seen in the study, including TJRs, were adjudicated as being part of the normal progression of OA in the majority (73.4%) of cases. Other joint events of note were one case of subchondral insufficiency fracture occurring in a patient treated with tanezumab 2.5 mg and one case of primary osteonecrosis in a patient treated with tanezumab 5 mg.
During his presentation of the findings in a late-breaking oral abstract session, Dr. Berenbaum noted that this was a difficult-to-treat population of patients. All 849 patients who had been recruited had moderate to severe OA pain of the knee or hip and had a history of insufficient pain relief or intolerance to treatment with acetaminophen, oral NSAIDs, and tramadol and were also not responding to, or unwilling to take, opioid painkillers. Patients had to have no radiographic evidence of specified bone conditions, including RPOA.
Patients had been treated with subcutaneous tanezumab 2.5 mg (n = 283) or 5 mg (n = 284) or placebo (n = 282) at baseline, week 8, and week 16, with the three coprimary efficacy endpoints assessed at week 24.
Discussing the risk-to-benefit ratio of the drug after his presentation, Dr. Berenbaum said: “You have to keep in mind that, first, it was in very difficult-to-treat patients, compared to the other trials in the field of OA symptoms.”
He added: “Second, is that compared to the other trials, this one was able to include patients with Kellgren-Lawrence grade 4, meaning that this is a more serious population,” and third, “when you look at the responders – WOMAC 30%, 50%, 70% – there is a strong difference in terms of responders.”
Dr. Berenbaum and his coauthors noted on the poster that accompanied the late-breaking oral presentation that “an active-controlled study will provide data to further characterize the risk-benefit of tanezumab in patients with OA.”
The study was sponsored by Pfizer and Eli Lilly. Dr. Berenbaum disclosed receiving research funding through his institution from Pfizer and acting as a consultant to, and speaker for, the company as well as multiple other pharmaceutical companies. Coauthors of the study also disclosed research funding or consultancy agreements with Pfizer or Eli Lilly or were employees of the companies.
SOURCE: Berenbaum F et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):262-4. Abstract LB0007, doi: 10.1136/annrheumdis-2019-eular.8660
REPORTING FROM EULAR 2019 CONGRESS
Opioid use curbed with patient education and lower prescription quantities
Patients given lower prescription quantities of opioid tablets with and without opioid education used significantly less of the medication compared with those given more tablets and no education, according to data from 264 adults and adolescents who underwent anterior cruciate ligament (ACL) surgery.
Although lower default prescription programs have been shown to reduce the number of tablets prescribed, “the effect of reduced prescription quantities on actual patient opioid consumption remains undetermined,” wrote Kevin X. Farley, BS, of Emory University, Atlanta, and colleagues.
In a study published in JAMA, the researchers examined whether patients took fewer tablets if given fewer, and whether patient education about opioids further reduced the number of tablets taken.
The study population included adults and adolescents who underwent ACL surgery at a single center. The patients were divided into three groups: 109 patients received 50 opioid tablets after surgery, 78 received 30 tablets plus education prior to surgery about appropriate opioid use and alternative pain management, and 77 received 30 tablets but no education on opioid use.
Patients given 50 tablets consumed an average of 25 tablets for an average of 5.8 days. By contrast, patients given 30 tablets but no opioid education consumed an average of 16 tablets for an average of 4.5 days, and those given 30 tablets and preoperative education consumed an average of 12 tablets for an average of 3.5 days.
In addition, patients given 30 tablets reported lower levels of constipation and fatigue compared with patients given 50 tablets. No differences were seen in medication refills among the groups.
The findings were limited by several factors including the use of data from a single center, the lack of randomization, and the potential for recall bias, the researchers noted. However, the results suggest that prescribing fewer tablets may further reduce use, as each group consumed approximately half of the tablets given, the researchers added.
“Further investigation should evaluate whether similar opioid stewardship and education protocols would be successful in other patient populations,” they said.
Corresponding author John Xerogeanes, MD, disclosed personal fees from Arthrex and stock options from Trice. The other researchers had no financial conflicts to disclose.
Patients given lower prescription quantities of opioid tablets with and without opioid education used significantly less of the medication compared with those given more tablets and no education, according to data from 264 adults and adolescents who underwent anterior cruciate ligament (ACL) surgery.
Although lower default prescription programs have been shown to reduce the number of tablets prescribed, “the effect of reduced prescription quantities on actual patient opioid consumption remains undetermined,” wrote Kevin X. Farley, BS, of Emory University, Atlanta, and colleagues.
In a study published in JAMA, the researchers examined whether patients took fewer tablets if given fewer, and whether patient education about opioids further reduced the number of tablets taken.
The study population included adults and adolescents who underwent ACL surgery at a single center. The patients were divided into three groups: 109 patients received 50 opioid tablets after surgery, 78 received 30 tablets plus education prior to surgery about appropriate opioid use and alternative pain management, and 77 received 30 tablets but no education on opioid use.
Patients given 50 tablets consumed an average of 25 tablets for an average of 5.8 days. By contrast, patients given 30 tablets but no opioid education consumed an average of 16 tablets for an average of 4.5 days, and those given 30 tablets and preoperative education consumed an average of 12 tablets for an average of 3.5 days.
In addition, patients given 30 tablets reported lower levels of constipation and fatigue compared with patients given 50 tablets. No differences were seen in medication refills among the groups.
The findings were limited by several factors including the use of data from a single center, the lack of randomization, and the potential for recall bias, the researchers noted. However, the results suggest that prescribing fewer tablets may further reduce use, as each group consumed approximately half of the tablets given, the researchers added.
“Further investigation should evaluate whether similar opioid stewardship and education protocols would be successful in other patient populations,” they said.
Corresponding author John Xerogeanes, MD, disclosed personal fees from Arthrex and stock options from Trice. The other researchers had no financial conflicts to disclose.
Patients given lower prescription quantities of opioid tablets with and without opioid education used significantly less of the medication compared with those given more tablets and no education, according to data from 264 adults and adolescents who underwent anterior cruciate ligament (ACL) surgery.
Although lower default prescription programs have been shown to reduce the number of tablets prescribed, “the effect of reduced prescription quantities on actual patient opioid consumption remains undetermined,” wrote Kevin X. Farley, BS, of Emory University, Atlanta, and colleagues.
In a study published in JAMA, the researchers examined whether patients took fewer tablets if given fewer, and whether patient education about opioids further reduced the number of tablets taken.
The study population included adults and adolescents who underwent ACL surgery at a single center. The patients were divided into three groups: 109 patients received 50 opioid tablets after surgery, 78 received 30 tablets plus education prior to surgery about appropriate opioid use and alternative pain management, and 77 received 30 tablets but no education on opioid use.
Patients given 50 tablets consumed an average of 25 tablets for an average of 5.8 days. By contrast, patients given 30 tablets but no opioid education consumed an average of 16 tablets for an average of 4.5 days, and those given 30 tablets and preoperative education consumed an average of 12 tablets for an average of 3.5 days.
In addition, patients given 30 tablets reported lower levels of constipation and fatigue compared with patients given 50 tablets. No differences were seen in medication refills among the groups.
The findings were limited by several factors including the use of data from a single center, the lack of randomization, and the potential for recall bias, the researchers noted. However, the results suggest that prescribing fewer tablets may further reduce use, as each group consumed approximately half of the tablets given, the researchers added.
“Further investigation should evaluate whether similar opioid stewardship and education protocols would be successful in other patient populations,” they said.
Corresponding author John Xerogeanes, MD, disclosed personal fees from Arthrex and stock options from Trice. The other researchers had no financial conflicts to disclose.
FROM JAMA
Key clinical point: Patient education and fewer tablets prescribed significantly reduced the amount of opioid tablets taken compared with no education and more tablets prescribed.
Major finding: Patients given 50 tablets and no patient education, 30 tablets and no patient education, and 30 tablets plus education consumed an average of 25, 16, and 12 tablets, respectively.
Study details: The data come from 264 adolescents and adults who underwent ACL surgery at a single center.
Disclosures: Corresponding author John Xerogeanes, MD, disclosed personal fees from Arthrex and stock options from Trice. The other researchers had no financial conflicts to disclose.
Source: Farley KX et al. JAMA. 2019 June 25.321(24):2465-7.
Weight loss in knee OA patients sustained with liraglutide over 1 year
MADRID – The glucagonlike peptide–1 receptor agonist liraglutide appears to be effective for keeping weight off following an intensive weight-loss program in patients with knee osteoarthritis, according to a randomized, double-blind, placebo-controlled trial presented at the European Congress of Rheumatology.
However, even though the 8-week intensive dietary program led to substantial weight loss and significant improvement in pain, additional weight loss of nearly 2.5 kg over 52 weeks of daily liraglutide treatment did not translate into more pain control.
According to study author Lars Erik Kristensen, MD, PhD, this is the first randomized trial to test the ability of liraglutide to provide a sustained weight loss in OA patients. The Food and Drug Administration indication for liraglutide is as an adjunct to diet and exercise for glycemic control in type 2 diabetes mellitus.
The study compared liraglutide against placebo in patients who had completed an intensive weight-control program in which the median loss was 12.46 kg. They were followed for 52 weeks.
At the end of follow-up, patients in the placebo group had gained a mean of 1.17 kg while those randomized to liraglutide lost an additional 2.76 kg. The between-group difference of 3.93 kg was statistically significant (P = .008).
“We believe that liraglutide is a promising agent for sustained weight loss in OA patients,” concluded Dr. Kristensen, a clinical researcher in rheumatology in the Parker Institute at Bispebjerg-Frederiksberg Hospital in Copenhagen.
In the single-center study, 156 patients were enrolled and randomized. In an initial 8-week diet intervention undertaken by both groups, an intensive program for weight loss included average daily calorie intakes of less than 800 kcal along with dietetic counseling. Patients were monitored for daily activities.
The majority of patients achieved a 10% or greater loss of total body weight during the intensive program before initiating 3 mg of once-daily liraglutide or a placebo.
Over the course of 52 weeks, the attrition from the study was relatively low. Among the 80 patients randomized to liraglutide, only 2 were lost because of noncompliance. Another 12 participants left the study before completion, 10 of whom did so for treatment-associated adverse effects. In the placebo arm, four patients were noncompliant, four left for treatment-associated adverse effects, and five left for other reasons.
Following the 8-week intensive dietary program, there was 11.86-point improvement in the pain subscale of the Knee and Osteoarthritis Outcome Score, confirming a substantial symptomatic benefit from this degree of weight loss. While this improvement in pain score was sustained at 52 weeks in both groups, the additional weight loss in the liraglutide arm did not lead to additional pain control.
The lack of additional pain control in the liraglutide group was disappointing, and the reason is unclear, but Dr. Kristensen emphasized that the persistent improvement in pain control was a positive result. In patients who are overweight or obese, regardless of whether they have concomitant OA, weight loss is not only difficult to achieve but difficult to sustain even after a successful intervention.
Dr. Kristensen reported financial relationships with multiple pharmaceutical companies. The trial received funding from Novo Nordisk.
SOURCE: Kristensen LE et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):71-2. Abstract OP0011. doi: 10.1136/annrheumdis-2019-eular.1375.
MADRID – The glucagonlike peptide–1 receptor agonist liraglutide appears to be effective for keeping weight off following an intensive weight-loss program in patients with knee osteoarthritis, according to a randomized, double-blind, placebo-controlled trial presented at the European Congress of Rheumatology.
However, even though the 8-week intensive dietary program led to substantial weight loss and significant improvement in pain, additional weight loss of nearly 2.5 kg over 52 weeks of daily liraglutide treatment did not translate into more pain control.
According to study author Lars Erik Kristensen, MD, PhD, this is the first randomized trial to test the ability of liraglutide to provide a sustained weight loss in OA patients. The Food and Drug Administration indication for liraglutide is as an adjunct to diet and exercise for glycemic control in type 2 diabetes mellitus.
The study compared liraglutide against placebo in patients who had completed an intensive weight-control program in which the median loss was 12.46 kg. They were followed for 52 weeks.
At the end of follow-up, patients in the placebo group had gained a mean of 1.17 kg while those randomized to liraglutide lost an additional 2.76 kg. The between-group difference of 3.93 kg was statistically significant (P = .008).
“We believe that liraglutide is a promising agent for sustained weight loss in OA patients,” concluded Dr. Kristensen, a clinical researcher in rheumatology in the Parker Institute at Bispebjerg-Frederiksberg Hospital in Copenhagen.
In the single-center study, 156 patients were enrolled and randomized. In an initial 8-week diet intervention undertaken by both groups, an intensive program for weight loss included average daily calorie intakes of less than 800 kcal along with dietetic counseling. Patients were monitored for daily activities.
The majority of patients achieved a 10% or greater loss of total body weight during the intensive program before initiating 3 mg of once-daily liraglutide or a placebo.
Over the course of 52 weeks, the attrition from the study was relatively low. Among the 80 patients randomized to liraglutide, only 2 were lost because of noncompliance. Another 12 participants left the study before completion, 10 of whom did so for treatment-associated adverse effects. In the placebo arm, four patients were noncompliant, four left for treatment-associated adverse effects, and five left for other reasons.
Following the 8-week intensive dietary program, there was 11.86-point improvement in the pain subscale of the Knee and Osteoarthritis Outcome Score, confirming a substantial symptomatic benefit from this degree of weight loss. While this improvement in pain score was sustained at 52 weeks in both groups, the additional weight loss in the liraglutide arm did not lead to additional pain control.
The lack of additional pain control in the liraglutide group was disappointing, and the reason is unclear, but Dr. Kristensen emphasized that the persistent improvement in pain control was a positive result. In patients who are overweight or obese, regardless of whether they have concomitant OA, weight loss is not only difficult to achieve but difficult to sustain even after a successful intervention.
Dr. Kristensen reported financial relationships with multiple pharmaceutical companies. The trial received funding from Novo Nordisk.
SOURCE: Kristensen LE et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):71-2. Abstract OP0011. doi: 10.1136/annrheumdis-2019-eular.1375.
MADRID – The glucagonlike peptide–1 receptor agonist liraglutide appears to be effective for keeping weight off following an intensive weight-loss program in patients with knee osteoarthritis, according to a randomized, double-blind, placebo-controlled trial presented at the European Congress of Rheumatology.
However, even though the 8-week intensive dietary program led to substantial weight loss and significant improvement in pain, additional weight loss of nearly 2.5 kg over 52 weeks of daily liraglutide treatment did not translate into more pain control.
According to study author Lars Erik Kristensen, MD, PhD, this is the first randomized trial to test the ability of liraglutide to provide a sustained weight loss in OA patients. The Food and Drug Administration indication for liraglutide is as an adjunct to diet and exercise for glycemic control in type 2 diabetes mellitus.
The study compared liraglutide against placebo in patients who had completed an intensive weight-control program in which the median loss was 12.46 kg. They were followed for 52 weeks.
At the end of follow-up, patients in the placebo group had gained a mean of 1.17 kg while those randomized to liraglutide lost an additional 2.76 kg. The between-group difference of 3.93 kg was statistically significant (P = .008).
“We believe that liraglutide is a promising agent for sustained weight loss in OA patients,” concluded Dr. Kristensen, a clinical researcher in rheumatology in the Parker Institute at Bispebjerg-Frederiksberg Hospital in Copenhagen.
In the single-center study, 156 patients were enrolled and randomized. In an initial 8-week diet intervention undertaken by both groups, an intensive program for weight loss included average daily calorie intakes of less than 800 kcal along with dietetic counseling. Patients were monitored for daily activities.
The majority of patients achieved a 10% or greater loss of total body weight during the intensive program before initiating 3 mg of once-daily liraglutide or a placebo.
Over the course of 52 weeks, the attrition from the study was relatively low. Among the 80 patients randomized to liraglutide, only 2 were lost because of noncompliance. Another 12 participants left the study before completion, 10 of whom did so for treatment-associated adverse effects. In the placebo arm, four patients were noncompliant, four left for treatment-associated adverse effects, and five left for other reasons.
Following the 8-week intensive dietary program, there was 11.86-point improvement in the pain subscale of the Knee and Osteoarthritis Outcome Score, confirming a substantial symptomatic benefit from this degree of weight loss. While this improvement in pain score was sustained at 52 weeks in both groups, the additional weight loss in the liraglutide arm did not lead to additional pain control.
The lack of additional pain control in the liraglutide group was disappointing, and the reason is unclear, but Dr. Kristensen emphasized that the persistent improvement in pain control was a positive result. In patients who are overweight or obese, regardless of whether they have concomitant OA, weight loss is not only difficult to achieve but difficult to sustain even after a successful intervention.
Dr. Kristensen reported financial relationships with multiple pharmaceutical companies. The trial received funding from Novo Nordisk.
SOURCE: Kristensen LE et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):71-2. Abstract OP0011. doi: 10.1136/annrheumdis-2019-eular.1375.
REPORTING FROM EULAR 2019 CONGRESS
Pain coping skills training doesn’t improve knee arthroplasty outcomes
TORONTO – A high level of pain catastrophizing prior to scheduled knee arthroplasty is not, as previously thought, a harbinger of poor outcomes, and affected patients don’t benefit from cognitive-behavioral therapy–based training in pain coping skills, Daniel L. Riddle, PhD, reported at the OARSI 2019 World Congress.
“The take-home message for us is knee arthroplasty is incredibly effective and there really is no reason to do pain coping skills training in these high–pain catastrophizing patients because the great majority of them have such good outcomes,” said Dr. Riddle, professor of physical therapy at Virginia Commonwealth University, Richmond.
“The other clear message from our trial is that, when you have pain-catastrophizing patients and you lower their pain, their catastrophizing is also lowered. So pain catastrophizing is clearly a response to pain and not a personality trait per se,” he said at the meeting sponsored by the Osteoarthritis Research Society International.
He presented the results of a 402-patient, randomized, three-arm, single-blind trial conducted at five U.S. medical centers. All participants were scheduled for knee arthroplasty for osteoarthritis, and all had moderate- to high-level pain catastrophizing as reflected in the group’s average Pain Catastrophizing Score of 30. They were assigned to an arthritis education active control group, usual care, or an intervention developed specifically for this study: a cognitive-behavioral therapy–based training program for pain coping skills. Similar pain coping skills training interventions have been shown to be beneficial in patients with medically treated knee OA but hadn’t previously been evaluated in surgically treated patients. The primary study endpoint was change in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Pain Scale at 2, 6, and 12 months after surgery.
The improvement in WOMAC pain in the three study arms was virtually superimposable, going from an average pain score of about 12 preoperatively to 2 postoperatively.
“This was a clear no-effect trial,” Dr. Riddle observed. “These are patients we thought to be at increased risk for poor outcome, but indeed they’re not.”
Pain Catastrophizing Scores improved from 30 preoperatively to roughly 7 at 1 year. “We’ve never seen pain catastrophizing improvements of this magnitude,” the researcher commented.
The study participants typically had a large number of chronically painful areas, but only minimal change in pain scores occurred except in the surgically treated knee.
Of note, even with the impressively large improvements in knee pain, function, and other secondary endpoints in the study group as a whole, roughly 20% of study participants experienced essentially no improvement in their function-limiting knee pain during the first year after arthroplasty. These nonresponders were spread equally across all three study arms. Further research will be needed to develop interventions to help this challenging patient subgroup.
The pain coping skills training consisted of 8 weekly sessions, each an hour long, which began prior to surgery and continued afterward. The intervention was delivered by physical therapists who had been trained by pain psychologists with expertise in cognitive-behavioral therapy. The intervention was delivered by telephone and in face-to-face sessions. The trainers were tracked over the course of the study to make sure that the structured intervention was delivered as planned.
Dr. Riddle reported having no financial conflicts regarding the National Institutes of Health-funded study, the full details of which have been published (J Bone Joint Surg Am. 2019 Feb 6;101[3]:218-227).
TORONTO – A high level of pain catastrophizing prior to scheduled knee arthroplasty is not, as previously thought, a harbinger of poor outcomes, and affected patients don’t benefit from cognitive-behavioral therapy–based training in pain coping skills, Daniel L. Riddle, PhD, reported at the OARSI 2019 World Congress.
“The take-home message for us is knee arthroplasty is incredibly effective and there really is no reason to do pain coping skills training in these high–pain catastrophizing patients because the great majority of them have such good outcomes,” said Dr. Riddle, professor of physical therapy at Virginia Commonwealth University, Richmond.
“The other clear message from our trial is that, when you have pain-catastrophizing patients and you lower their pain, their catastrophizing is also lowered. So pain catastrophizing is clearly a response to pain and not a personality trait per se,” he said at the meeting sponsored by the Osteoarthritis Research Society International.
He presented the results of a 402-patient, randomized, three-arm, single-blind trial conducted at five U.S. medical centers. All participants were scheduled for knee arthroplasty for osteoarthritis, and all had moderate- to high-level pain catastrophizing as reflected in the group’s average Pain Catastrophizing Score of 30. They were assigned to an arthritis education active control group, usual care, or an intervention developed specifically for this study: a cognitive-behavioral therapy–based training program for pain coping skills. Similar pain coping skills training interventions have been shown to be beneficial in patients with medically treated knee OA but hadn’t previously been evaluated in surgically treated patients. The primary study endpoint was change in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Pain Scale at 2, 6, and 12 months after surgery.
The improvement in WOMAC pain in the three study arms was virtually superimposable, going from an average pain score of about 12 preoperatively to 2 postoperatively.
“This was a clear no-effect trial,” Dr. Riddle observed. “These are patients we thought to be at increased risk for poor outcome, but indeed they’re not.”
Pain Catastrophizing Scores improved from 30 preoperatively to roughly 7 at 1 year. “We’ve never seen pain catastrophizing improvements of this magnitude,” the researcher commented.
The study participants typically had a large number of chronically painful areas, but only minimal change in pain scores occurred except in the surgically treated knee.
Of note, even with the impressively large improvements in knee pain, function, and other secondary endpoints in the study group as a whole, roughly 20% of study participants experienced essentially no improvement in their function-limiting knee pain during the first year after arthroplasty. These nonresponders were spread equally across all three study arms. Further research will be needed to develop interventions to help this challenging patient subgroup.
The pain coping skills training consisted of 8 weekly sessions, each an hour long, which began prior to surgery and continued afterward. The intervention was delivered by physical therapists who had been trained by pain psychologists with expertise in cognitive-behavioral therapy. The intervention was delivered by telephone and in face-to-face sessions. The trainers were tracked over the course of the study to make sure that the structured intervention was delivered as planned.
Dr. Riddle reported having no financial conflicts regarding the National Institutes of Health-funded study, the full details of which have been published (J Bone Joint Surg Am. 2019 Feb 6;101[3]:218-227).
TORONTO – A high level of pain catastrophizing prior to scheduled knee arthroplasty is not, as previously thought, a harbinger of poor outcomes, and affected patients don’t benefit from cognitive-behavioral therapy–based training in pain coping skills, Daniel L. Riddle, PhD, reported at the OARSI 2019 World Congress.
“The take-home message for us is knee arthroplasty is incredibly effective and there really is no reason to do pain coping skills training in these high–pain catastrophizing patients because the great majority of them have such good outcomes,” said Dr. Riddle, professor of physical therapy at Virginia Commonwealth University, Richmond.
“The other clear message from our trial is that, when you have pain-catastrophizing patients and you lower their pain, their catastrophizing is also lowered. So pain catastrophizing is clearly a response to pain and not a personality trait per se,” he said at the meeting sponsored by the Osteoarthritis Research Society International.
He presented the results of a 402-patient, randomized, three-arm, single-blind trial conducted at five U.S. medical centers. All participants were scheduled for knee arthroplasty for osteoarthritis, and all had moderate- to high-level pain catastrophizing as reflected in the group’s average Pain Catastrophizing Score of 30. They were assigned to an arthritis education active control group, usual care, or an intervention developed specifically for this study: a cognitive-behavioral therapy–based training program for pain coping skills. Similar pain coping skills training interventions have been shown to be beneficial in patients with medically treated knee OA but hadn’t previously been evaluated in surgically treated patients. The primary study endpoint was change in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Pain Scale at 2, 6, and 12 months after surgery.
The improvement in WOMAC pain in the three study arms was virtually superimposable, going from an average pain score of about 12 preoperatively to 2 postoperatively.
“This was a clear no-effect trial,” Dr. Riddle observed. “These are patients we thought to be at increased risk for poor outcome, but indeed they’re not.”
Pain Catastrophizing Scores improved from 30 preoperatively to roughly 7 at 1 year. “We’ve never seen pain catastrophizing improvements of this magnitude,” the researcher commented.
The study participants typically had a large number of chronically painful areas, but only minimal change in pain scores occurred except in the surgically treated knee.
Of note, even with the impressively large improvements in knee pain, function, and other secondary endpoints in the study group as a whole, roughly 20% of study participants experienced essentially no improvement in their function-limiting knee pain during the first year after arthroplasty. These nonresponders were spread equally across all three study arms. Further research will be needed to develop interventions to help this challenging patient subgroup.
The pain coping skills training consisted of 8 weekly sessions, each an hour long, which began prior to surgery and continued afterward. The intervention was delivered by physical therapists who had been trained by pain psychologists with expertise in cognitive-behavioral therapy. The intervention was delivered by telephone and in face-to-face sessions. The trainers were tracked over the course of the study to make sure that the structured intervention was delivered as planned.
Dr. Riddle reported having no financial conflicts regarding the National Institutes of Health-funded study, the full details of which have been published (J Bone Joint Surg Am. 2019 Feb 6;101[3]:218-227).
REPORTING FROM OARSI 2019
Scandinavian studies shed light on OA inheritance
TORONTO – Patients with osteoarthritis often want to know if their debilitating disease is likely to be passed on to their children. Karin Magnusson, PhD, believes she can answer that question based upon an analysis of two large Nordic studies.
“OA in the mother, but not in the father, increases the risk of surgical and clinically defined hip, knee, and hand OA in the offspring, and particularly in daughters,” she reported at the OARSI 2019 World Congress.
Dr. Magnusson, an epidemiologist at Lund (Sweden) University, and her coinvestigators, turned to the Musculoskeletal Pain in Ullensaker Study (MUST) of 630 individuals aged 40-79 with rheumatologist-diagnosed hand, hip, or knee OA by American College of Rheumatology clinical criteria and their offspring, as well as the Nor-Twin OA Study of 7,184 twins, aged 30-75, and their children. Linkage with a national registry that records virtually all joint arthroplasties performed in Norway enabled the investigators to identify which subjects in the two studies had joint surgery for OA, she explained at the meeting, sponsored by the Osteoarthritis Research Society International.
The main outcome in this analysis was the relative risk of hip, knee, or hand OA in the sons and daughters of families in which a parent had OA at those sites, compared with the rate when neither parent had OA. The key finding: If the mother had OA, her daughters had a 13% increased risk of OA in MUST and a 44% increased risk in the Nor-Twin OA Study when compared with daughters of women without OA. In contrast, the sons of a mother with OA had no significant increase in risk of OA. And when OA was present in the father, there was no increased risk of OA at any site in his daughters or sons.
“The implication is the heredity of OA is linked to maternal genes and/or maternal-specific factors, such as the fetal environment,” according to Dr. Magnusson.
And for clinical practice, the implication is that it’s important to ask about family history of OA, and in which parent, to better predict future risk of disease transmission to the children, she added.
These Norwegian study results open the door to exploration of the possible role of mitochondrial DNA in familial clustering of OA, since mitochondrial DNA is inherited only from the mother, Dr. Magnusson noted.
David T. Felson, MD, rose from the audience to say, “I’m a little bit worried” about the fact that when he and other Framingham Heart Study investigators looked specifically for possible mother/daughter, mother/son, father/daughter, and father/son associations for knee and hip OA, “we really didn’t find any.
“You can go through all of the explanations that you want about maternal inheritance, but I’m not sure that’s the best explanation. It might just be that what’s going on here is you’re seeing guys who are relatively young and who got their OA through injury or sports, which is fairly common in young men, and not through inheritance,” said Dr. Felson, professor of medicine and epidemiology at Boston University.
So a third observational study in an independent cohort might be needed as a tie breaker regarding the issue of OA inheritance.
Dr. Magnusson reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Magnusson K et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S47, Abstract 33
TORONTO – Patients with osteoarthritis often want to know if their debilitating disease is likely to be passed on to their children. Karin Magnusson, PhD, believes she can answer that question based upon an analysis of two large Nordic studies.
“OA in the mother, but not in the father, increases the risk of surgical and clinically defined hip, knee, and hand OA in the offspring, and particularly in daughters,” she reported at the OARSI 2019 World Congress.
Dr. Magnusson, an epidemiologist at Lund (Sweden) University, and her coinvestigators, turned to the Musculoskeletal Pain in Ullensaker Study (MUST) of 630 individuals aged 40-79 with rheumatologist-diagnosed hand, hip, or knee OA by American College of Rheumatology clinical criteria and their offspring, as well as the Nor-Twin OA Study of 7,184 twins, aged 30-75, and their children. Linkage with a national registry that records virtually all joint arthroplasties performed in Norway enabled the investigators to identify which subjects in the two studies had joint surgery for OA, she explained at the meeting, sponsored by the Osteoarthritis Research Society International.
The main outcome in this analysis was the relative risk of hip, knee, or hand OA in the sons and daughters of families in which a parent had OA at those sites, compared with the rate when neither parent had OA. The key finding: If the mother had OA, her daughters had a 13% increased risk of OA in MUST and a 44% increased risk in the Nor-Twin OA Study when compared with daughters of women without OA. In contrast, the sons of a mother with OA had no significant increase in risk of OA. And when OA was present in the father, there was no increased risk of OA at any site in his daughters or sons.
“The implication is the heredity of OA is linked to maternal genes and/or maternal-specific factors, such as the fetal environment,” according to Dr. Magnusson.
And for clinical practice, the implication is that it’s important to ask about family history of OA, and in which parent, to better predict future risk of disease transmission to the children, she added.
These Norwegian study results open the door to exploration of the possible role of mitochondrial DNA in familial clustering of OA, since mitochondrial DNA is inherited only from the mother, Dr. Magnusson noted.
David T. Felson, MD, rose from the audience to say, “I’m a little bit worried” about the fact that when he and other Framingham Heart Study investigators looked specifically for possible mother/daughter, mother/son, father/daughter, and father/son associations for knee and hip OA, “we really didn’t find any.
“You can go through all of the explanations that you want about maternal inheritance, but I’m not sure that’s the best explanation. It might just be that what’s going on here is you’re seeing guys who are relatively young and who got their OA through injury or sports, which is fairly common in young men, and not through inheritance,” said Dr. Felson, professor of medicine and epidemiology at Boston University.
So a third observational study in an independent cohort might be needed as a tie breaker regarding the issue of OA inheritance.
Dr. Magnusson reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Magnusson K et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S47, Abstract 33
TORONTO – Patients with osteoarthritis often want to know if their debilitating disease is likely to be passed on to their children. Karin Magnusson, PhD, believes she can answer that question based upon an analysis of two large Nordic studies.
“OA in the mother, but not in the father, increases the risk of surgical and clinically defined hip, knee, and hand OA in the offspring, and particularly in daughters,” she reported at the OARSI 2019 World Congress.
Dr. Magnusson, an epidemiologist at Lund (Sweden) University, and her coinvestigators, turned to the Musculoskeletal Pain in Ullensaker Study (MUST) of 630 individuals aged 40-79 with rheumatologist-diagnosed hand, hip, or knee OA by American College of Rheumatology clinical criteria and their offspring, as well as the Nor-Twin OA Study of 7,184 twins, aged 30-75, and their children. Linkage with a national registry that records virtually all joint arthroplasties performed in Norway enabled the investigators to identify which subjects in the two studies had joint surgery for OA, she explained at the meeting, sponsored by the Osteoarthritis Research Society International.
The main outcome in this analysis was the relative risk of hip, knee, or hand OA in the sons and daughters of families in which a parent had OA at those sites, compared with the rate when neither parent had OA. The key finding: If the mother had OA, her daughters had a 13% increased risk of OA in MUST and a 44% increased risk in the Nor-Twin OA Study when compared with daughters of women without OA. In contrast, the sons of a mother with OA had no significant increase in risk of OA. And when OA was present in the father, there was no increased risk of OA at any site in his daughters or sons.
“The implication is the heredity of OA is linked to maternal genes and/or maternal-specific factors, such as the fetal environment,” according to Dr. Magnusson.
And for clinical practice, the implication is that it’s important to ask about family history of OA, and in which parent, to better predict future risk of disease transmission to the children, she added.
These Norwegian study results open the door to exploration of the possible role of mitochondrial DNA in familial clustering of OA, since mitochondrial DNA is inherited only from the mother, Dr. Magnusson noted.
David T. Felson, MD, rose from the audience to say, “I’m a little bit worried” about the fact that when he and other Framingham Heart Study investigators looked specifically for possible mother/daughter, mother/son, father/daughter, and father/son associations for knee and hip OA, “we really didn’t find any.
“You can go through all of the explanations that you want about maternal inheritance, but I’m not sure that’s the best explanation. It might just be that what’s going on here is you’re seeing guys who are relatively young and who got their OA through injury or sports, which is fairly common in young men, and not through inheritance,” said Dr. Felson, professor of medicine and epidemiology at Boston University.
So a third observational study in an independent cohort might be needed as a tie breaker regarding the issue of OA inheritance.
Dr. Magnusson reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Magnusson K et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S47, Abstract 33
REPORTING FROM OARSI 2019