User login
Drug receives orphan designation for AML
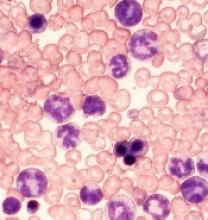
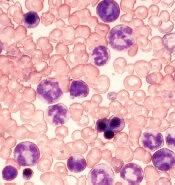
The US Food and Drug Administration (FDA) has granted annamycin orphan designation for the treatment of acute myeloid leukemia (AML).
Annamycin is a liposomal anthracycline under development by Moleculin Biotech, Inc.
The company said it is working with the FDA on an investigative new drug application for a phase 1/2 trial of annamycin in patients with relapsed or refractory AML.
Annamycin has already been tested in 6 clinical trials, 3 of which focused on leukemia.
Results from one of these trials, in adults with relapsed/refractory acute lymphoblastic leukemia, were published in Clinical Lymphoma, Myeloma & Leukemia in 2013.
Annamycin has been under development by several other pharmaceutical companies. Moleculin Biotech, Inc. acquired rights and development assets relating to the drug in 2015.
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent rare diseases/disorders affecting fewer than 200,000 people in the US.
Orphan designation provides companies with certain incentives to develop products for rare diseases. This includes a 50% tax break on research and development, a fee waiver, access to federal grants, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted annamycin orphan designation for the treatment of acute myeloid leukemia (AML).
Annamycin is a liposomal anthracycline under development by Moleculin Biotech, Inc.
The company said it is working with the FDA on an investigative new drug application for a phase 1/2 trial of annamycin in patients with relapsed or refractory AML.
Annamycin has already been tested in 6 clinical trials, 3 of which focused on leukemia.
Results from one of these trials, in adults with relapsed/refractory acute lymphoblastic leukemia, were published in Clinical Lymphoma, Myeloma & Leukemia in 2013.
Annamycin has been under development by several other pharmaceutical companies. Moleculin Biotech, Inc. acquired rights and development assets relating to the drug in 2015.
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent rare diseases/disorders affecting fewer than 200,000 people in the US.
Orphan designation provides companies with certain incentives to develop products for rare diseases. This includes a 50% tax break on research and development, a fee waiver, access to federal grants, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted annamycin orphan designation for the treatment of acute myeloid leukemia (AML).
Annamycin is a liposomal anthracycline under development by Moleculin Biotech, Inc.
The company said it is working with the FDA on an investigative new drug application for a phase 1/2 trial of annamycin in patients with relapsed or refractory AML.
Annamycin has already been tested in 6 clinical trials, 3 of which focused on leukemia.
Results from one of these trials, in adults with relapsed/refractory acute lymphoblastic leukemia, were published in Clinical Lymphoma, Myeloma & Leukemia in 2013.
Annamycin has been under development by several other pharmaceutical companies. Moleculin Biotech, Inc. acquired rights and development assets relating to the drug in 2015.
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent rare diseases/disorders affecting fewer than 200,000 people in the US.
Orphan designation provides companies with certain incentives to develop products for rare diseases. This includes a 50% tax break on research and development, a fee waiver, access to federal grants, and 7 years of market exclusivity if the product is approved. ![]()
Protein may prevent transformation from MDS to AML
The protein p300 may prevent the transformation from myelodysplastic syndromes (MDS) to acute myeloid leukemia (AML), according to research published in Leukemia.
Researchers found that loss of p300 “markedly” increased leukemogenesis in a mouse model of MDS.
“The loss of p300 allows these defective [MDS] cells to grow and become leukemic,” said study author Stephen Nimer, MD, of Sylvester Comprehensive Cancer Center in Miami, Florida.
“This work offers us a window into AML, which we are now going to try to exploit.”
Previous research suggested that p300 and CBP (both histone lysine acetyltransferases) may be tumor suppressors. The current study indicates that, in the context of MDS, that is only true for p300.
The researchers evaluated the effects of deleting both p300 and CBP in Nup98-HoxD13 (NHD13) transgenic mice, a model of human MDS.
The team found that p300 deletion, but not CBP deletion, accelerated leukemogenesis in the mice.
“When we eliminated p300, 100% of the mice developed leukemia,” Dr Nimer said. “It indicated that, under this specific circumstance, p300 is a tumor suppressor, offering great insight into how MDS converts to leukemia. It was quite surprising that CBP plays no role at all.”
The researchers also found that deleting p300 restored the ability of NHD13-expressing hematopoietic stem and progenitor cells (HSPCs) to self-renew, and p300 deletion decreased apoptosis.
“While investigating how p300 functions in MDS cells, we found that MDS cells do not grow well in the lab,” Dr Nimer said. “However, when you eliminate p300, suddenly, the cells continue to grow.”
On the other hand, deletion of p300 did not have a significant effect on wild-type hematopoiesis.
Finally, the researchers found that p300 deletion enhanced cytokine signaling in NHD13-expressing HSPCs. They observed enhanced activation of the MAPK and JAK/STAT pathways in HSPCs isolated from NHD13 transgenic mice.
The team said more research is needed to understand exactly how p300 controls MDS cells, but these findings could ultimately help MDS patients avoid AML.
“Other than chemotherapy, right now, there’s no way to prevent MDS from developing into myeloid leukemia,” Dr Nimer said. “However, drugs are being developed that can promote p300 function and possibly prevent MDS patients from developing leukemia.” ![]()
The protein p300 may prevent the transformation from myelodysplastic syndromes (MDS) to acute myeloid leukemia (AML), according to research published in Leukemia.
Researchers found that loss of p300 “markedly” increased leukemogenesis in a mouse model of MDS.
“The loss of p300 allows these defective [MDS] cells to grow and become leukemic,” said study author Stephen Nimer, MD, of Sylvester Comprehensive Cancer Center in Miami, Florida.
“This work offers us a window into AML, which we are now going to try to exploit.”
Previous research suggested that p300 and CBP (both histone lysine acetyltransferases) may be tumor suppressors. The current study indicates that, in the context of MDS, that is only true for p300.
The researchers evaluated the effects of deleting both p300 and CBP in Nup98-HoxD13 (NHD13) transgenic mice, a model of human MDS.
The team found that p300 deletion, but not CBP deletion, accelerated leukemogenesis in the mice.
“When we eliminated p300, 100% of the mice developed leukemia,” Dr Nimer said. “It indicated that, under this specific circumstance, p300 is a tumor suppressor, offering great insight into how MDS converts to leukemia. It was quite surprising that CBP plays no role at all.”
The researchers also found that deleting p300 restored the ability of NHD13-expressing hematopoietic stem and progenitor cells (HSPCs) to self-renew, and p300 deletion decreased apoptosis.
“While investigating how p300 functions in MDS cells, we found that MDS cells do not grow well in the lab,” Dr Nimer said. “However, when you eliminate p300, suddenly, the cells continue to grow.”
On the other hand, deletion of p300 did not have a significant effect on wild-type hematopoiesis.
Finally, the researchers found that p300 deletion enhanced cytokine signaling in NHD13-expressing HSPCs. They observed enhanced activation of the MAPK and JAK/STAT pathways in HSPCs isolated from NHD13 transgenic mice.
The team said more research is needed to understand exactly how p300 controls MDS cells, but these findings could ultimately help MDS patients avoid AML.
“Other than chemotherapy, right now, there’s no way to prevent MDS from developing into myeloid leukemia,” Dr Nimer said. “However, drugs are being developed that can promote p300 function and possibly prevent MDS patients from developing leukemia.” ![]()
The protein p300 may prevent the transformation from myelodysplastic syndromes (MDS) to acute myeloid leukemia (AML), according to research published in Leukemia.
Researchers found that loss of p300 “markedly” increased leukemogenesis in a mouse model of MDS.
“The loss of p300 allows these defective [MDS] cells to grow and become leukemic,” said study author Stephen Nimer, MD, of Sylvester Comprehensive Cancer Center in Miami, Florida.
“This work offers us a window into AML, which we are now going to try to exploit.”
Previous research suggested that p300 and CBP (both histone lysine acetyltransferases) may be tumor suppressors. The current study indicates that, in the context of MDS, that is only true for p300.
The researchers evaluated the effects of deleting both p300 and CBP in Nup98-HoxD13 (NHD13) transgenic mice, a model of human MDS.
The team found that p300 deletion, but not CBP deletion, accelerated leukemogenesis in the mice.
“When we eliminated p300, 100% of the mice developed leukemia,” Dr Nimer said. “It indicated that, under this specific circumstance, p300 is a tumor suppressor, offering great insight into how MDS converts to leukemia. It was quite surprising that CBP plays no role at all.”
The researchers also found that deleting p300 restored the ability of NHD13-expressing hematopoietic stem and progenitor cells (HSPCs) to self-renew, and p300 deletion decreased apoptosis.
“While investigating how p300 functions in MDS cells, we found that MDS cells do not grow well in the lab,” Dr Nimer said. “However, when you eliminate p300, suddenly, the cells continue to grow.”
On the other hand, deletion of p300 did not have a significant effect on wild-type hematopoiesis.
Finally, the researchers found that p300 deletion enhanced cytokine signaling in NHD13-expressing HSPCs. They observed enhanced activation of the MAPK and JAK/STAT pathways in HSPCs isolated from NHD13 transgenic mice.
The team said more research is needed to understand exactly how p300 controls MDS cells, but these findings could ultimately help MDS patients avoid AML.
“Other than chemotherapy, right now, there’s no way to prevent MDS from developing into myeloid leukemia,” Dr Nimer said. “However, drugs are being developed that can promote p300 function and possibly prevent MDS patients from developing leukemia.” ![]()
Advanced CLL treatment approach depends on comorbidity burden
ORLANDO – The choice of first-line therapy in symptomatic chronic lymphocytic leukemia patients depends largely on comorbidity burden, Andrew D. Zelenetz, MD, PhD, said at the annual conference of the National Comprehensive Cancer Network.
“This is a disease of elderly patients. Frequently they have comorbidities,” he said. Categorizing these patients as having a low or high comorbidity burden can be done with the Cumulative Index Rating Scale score, which involves scoring of all organ systems on a 0-5 scale representing “not affected” to “extremely disabled.”
“We use this to determine first-line therapy,” said Dr. Zelenetz of Memorial Sloan Kettering Cancer Center, New York. Dr. Zelenetz is chair of the NCCN Non-Hodgkin Lymphoma Guidelines panel.
Patients with a score of greater than 12 on the 0- to 56-point scale, are “no-go” patients with respect to therapy, and are typically treated only with palliative approaches. Those with a score of 7-12 (“slow-go” patients) have a significant comorbidity burden, but can undergo treatment, thought typically to be at reduced intensity. Those with a score of 0-6 are “go-go” patients with respect to treatment, as they are physically fit, have excellent renal function, and have no significant comorbidities, he said.
Treatment options for ‘go-go’ CLL patients
Among the treatment options for the latter is FCR–the combination of fludarabine, cyclophosphamide, and rituximab, which was shown in the phase III CLL10 trial of patients with advanced CLL to be associated with improved complete response rates compared with the popular regimen of bendamustine and rituximab (BR), both overall and in patients under age 65. In older patients, the advantage disappeared, Dr. Zelenetz said.
FCR was also associated with improved outcomes vs. BR in patients with del(11q).
The primary endpoint of the study was progression-free survival, which favored FCR (median of 55.2 vs. 41.7 months; hazard ratio, 1.643), he said, noting that no difference was seen between the two regimens in terms of overall survival.
In a recent publication, MD Anderson Cancer Center reported its experience with its first 300 CLL patients treated with FCR. With long-term follow-up of at least 9-10 years (median of 12.8 years), patients in this trial have done extremely well.
“But interestingly, when you stratify these patients by whether they have IGHV [immunoglobulin heavy chain variable] mutated or unmutated [disease], the IGHV mutated patients have something that looks a whole lot like a survival plateau, and that survival plateau is not trivial – it’s about 60%,” he said. “So there is a group of patients with CLL who are, in fact, curable with conventional chemoimmunotherapy.
“This is an appropriate treatment for a young, fit, ‘go-go’ patient, and it has a big implication,” he said. That is, patients who are young and fit require IGHV mutation testing, as “you will absolutely choose FCR chemotherapy for the fit, young patients who has IGHV mutated disease.
“In that setting IGHV testing is now mandatory,” he stressed, noting that the benefits in this population extend to overall survival as well as progression-free survival.
Dr. Zelenetz also emphasized the need for increasing the single dose of rituximab from 375 mg/m2 during cycle 1 to 500 mg/m2 during cycles 2-6 in those receiving FCR, as this is often forgotten.
The data demonstrating the efficacy of FCR were based on this approach, he said.
Fludarabine is to be given at a dose of 25 mg/m2, and cyclophosphamide at a dose of 250 mg/m2 – both for 2-4 days during cycle 1 and for 1-3 days during cycles 2-6.
Treatment options for ‘slow-go’ CLL patients
In “slow-go” patients, an interesting approach is to use new anti-CD20 antibodies such as ofatumumab and obinutuzumab, which have features that are distinct from rituximab.
Both have been studied in CLL. The CLL11 trial compared chlorambucil, rituximab+chlorambucil, and obinutuzumab+chlorambucil, and the latest analysis showed substantial improvement in progression-free survival with obinutuzumab+chlorambucil vs. the other two regimens (26.7 months vs. 11.1 and 16.3 months, respectively), Dr. Zelenetz said, noting that rituximab+chlorambucil was also superior to chlorambucil alone, but that only the obinutuzumab regimen had an overall survival advantage vs. chlorambucil alone.
An updated analysis to be reported soon will show emerging evidence of a survival advantage of obinutuzumab+chlorambucil vs. rituximab+chlorambucil, he said.
“This suggests that obinutuzumab is a far better antibody,” he added, noting that the reasons for that are under debate, “but the way it’s given, it works better in CLL, and that, I think is unequivocal.”
A similar study looking at chlorambucil with and without ofatumumab in “slow-go” patients also demonstrated an improvement in PFS with ofatumumab, but showed “no difference whatsoever in overall survival.”
“This is actually very similar to the rituximab result, and I actually call this the ‘death of ofatumumab’ study, because clearly obinutuzumab in CLL is, I think, a superior anti-CD20 antibody,” Dr. Zelenetz said.
Studies in which obinutuzumab is substituted for rituximab in the FCR combination are currently underway as are a number of other studies of obinutuzumab, he noted.
Another treatment option in the up-front setting is ibrutinib, which was shown to be effective in the RESONATE 2 trial .
“But notice, a very, very small [complete response rate]. CRs are very difficult to achieve with ibrutinib alone, so this drug is given continuously, lifelong,” Dr. Zelenetz said, noting that it was, however, associated with an overall survival advantage vs. chlorambucil.
“Should this be the standard of care? I think it is in patients who have del(17p) or mutation of TP53. Outside of that setting, I’m still concerned about the cost of long-term tolerability of the agent,” he said.
Future of first-line CLL treatment
Avoidance of long-term therapy and conventional chemotherapy in patients with CLL is a goal, he added, noting that new understanding from studies in patients in the relapsed/refractory CLL setting – such as recent findings from a phase Ib study of venetoclax plus rituximab, which demonstrated potentially durable responses after treatment discontinuation in minimal residual disease (MRD)–negative patients – are providing insights into achieving MRD negativity that could be applied in the front line treatment setting.
“We’re still trying to figure out how to best use this. We want to try to use some of this knowledge about achievement of MRD negativity in the up-front setting so we don’t have to give patients long-term therapy, and we would like to avoid conventional chemotherapy,” he said. “So I’m hoping we’re going to be able to replace chronic long-term therapy of CLL with a defined course of treatment with high levels of MRD negativity.”
Dr. Zelenetz reported receiving consulting fees, honoraria, and/or grant/research support from Acerta Pharma, Amgen Inc., BeiGene, Bristol-Myers Squibb, Celgene Corporation, Genentech, Gilead Sciences, Janssen Pharmaceutica Products, MEI Pharma, NanoString Technologies, Pharmacyclics, Portola Pharmaceuticals, Roche Laboratories, and Takeda Pharmaceuticals North America.
ORLANDO – The choice of first-line therapy in symptomatic chronic lymphocytic leukemia patients depends largely on comorbidity burden, Andrew D. Zelenetz, MD, PhD, said at the annual conference of the National Comprehensive Cancer Network.
“This is a disease of elderly patients. Frequently they have comorbidities,” he said. Categorizing these patients as having a low or high comorbidity burden can be done with the Cumulative Index Rating Scale score, which involves scoring of all organ systems on a 0-5 scale representing “not affected” to “extremely disabled.”
“We use this to determine first-line therapy,” said Dr. Zelenetz of Memorial Sloan Kettering Cancer Center, New York. Dr. Zelenetz is chair of the NCCN Non-Hodgkin Lymphoma Guidelines panel.
Patients with a score of greater than 12 on the 0- to 56-point scale, are “no-go” patients with respect to therapy, and are typically treated only with palliative approaches. Those with a score of 7-12 (“slow-go” patients) have a significant comorbidity burden, but can undergo treatment, thought typically to be at reduced intensity. Those with a score of 0-6 are “go-go” patients with respect to treatment, as they are physically fit, have excellent renal function, and have no significant comorbidities, he said.
Treatment options for ‘go-go’ CLL patients
Among the treatment options for the latter is FCR–the combination of fludarabine, cyclophosphamide, and rituximab, which was shown in the phase III CLL10 trial of patients with advanced CLL to be associated with improved complete response rates compared with the popular regimen of bendamustine and rituximab (BR), both overall and in patients under age 65. In older patients, the advantage disappeared, Dr. Zelenetz said.
FCR was also associated with improved outcomes vs. BR in patients with del(11q).
The primary endpoint of the study was progression-free survival, which favored FCR (median of 55.2 vs. 41.7 months; hazard ratio, 1.643), he said, noting that no difference was seen between the two regimens in terms of overall survival.
In a recent publication, MD Anderson Cancer Center reported its experience with its first 300 CLL patients treated with FCR. With long-term follow-up of at least 9-10 years (median of 12.8 years), patients in this trial have done extremely well.
“But interestingly, when you stratify these patients by whether they have IGHV [immunoglobulin heavy chain variable] mutated or unmutated [disease], the IGHV mutated patients have something that looks a whole lot like a survival plateau, and that survival plateau is not trivial – it’s about 60%,” he said. “So there is a group of patients with CLL who are, in fact, curable with conventional chemoimmunotherapy.
“This is an appropriate treatment for a young, fit, ‘go-go’ patient, and it has a big implication,” he said. That is, patients who are young and fit require IGHV mutation testing, as “you will absolutely choose FCR chemotherapy for the fit, young patients who has IGHV mutated disease.
“In that setting IGHV testing is now mandatory,” he stressed, noting that the benefits in this population extend to overall survival as well as progression-free survival.
Dr. Zelenetz also emphasized the need for increasing the single dose of rituximab from 375 mg/m2 during cycle 1 to 500 mg/m2 during cycles 2-6 in those receiving FCR, as this is often forgotten.
The data demonstrating the efficacy of FCR were based on this approach, he said.
Fludarabine is to be given at a dose of 25 mg/m2, and cyclophosphamide at a dose of 250 mg/m2 – both for 2-4 days during cycle 1 and for 1-3 days during cycles 2-6.
Treatment options for ‘slow-go’ CLL patients
In “slow-go” patients, an interesting approach is to use new anti-CD20 antibodies such as ofatumumab and obinutuzumab, which have features that are distinct from rituximab.
Both have been studied in CLL. The CLL11 trial compared chlorambucil, rituximab+chlorambucil, and obinutuzumab+chlorambucil, and the latest analysis showed substantial improvement in progression-free survival with obinutuzumab+chlorambucil vs. the other two regimens (26.7 months vs. 11.1 and 16.3 months, respectively), Dr. Zelenetz said, noting that rituximab+chlorambucil was also superior to chlorambucil alone, but that only the obinutuzumab regimen had an overall survival advantage vs. chlorambucil alone.
An updated analysis to be reported soon will show emerging evidence of a survival advantage of obinutuzumab+chlorambucil vs. rituximab+chlorambucil, he said.
“This suggests that obinutuzumab is a far better antibody,” he added, noting that the reasons for that are under debate, “but the way it’s given, it works better in CLL, and that, I think is unequivocal.”
A similar study looking at chlorambucil with and without ofatumumab in “slow-go” patients also demonstrated an improvement in PFS with ofatumumab, but showed “no difference whatsoever in overall survival.”
“This is actually very similar to the rituximab result, and I actually call this the ‘death of ofatumumab’ study, because clearly obinutuzumab in CLL is, I think, a superior anti-CD20 antibody,” Dr. Zelenetz said.
Studies in which obinutuzumab is substituted for rituximab in the FCR combination are currently underway as are a number of other studies of obinutuzumab, he noted.
Another treatment option in the up-front setting is ibrutinib, which was shown to be effective in the RESONATE 2 trial .
“But notice, a very, very small [complete response rate]. CRs are very difficult to achieve with ibrutinib alone, so this drug is given continuously, lifelong,” Dr. Zelenetz said, noting that it was, however, associated with an overall survival advantage vs. chlorambucil.
“Should this be the standard of care? I think it is in patients who have del(17p) or mutation of TP53. Outside of that setting, I’m still concerned about the cost of long-term tolerability of the agent,” he said.
Future of first-line CLL treatment
Avoidance of long-term therapy and conventional chemotherapy in patients with CLL is a goal, he added, noting that new understanding from studies in patients in the relapsed/refractory CLL setting – such as recent findings from a phase Ib study of venetoclax plus rituximab, which demonstrated potentially durable responses after treatment discontinuation in minimal residual disease (MRD)–negative patients – are providing insights into achieving MRD negativity that could be applied in the front line treatment setting.
“We’re still trying to figure out how to best use this. We want to try to use some of this knowledge about achievement of MRD negativity in the up-front setting so we don’t have to give patients long-term therapy, and we would like to avoid conventional chemotherapy,” he said. “So I’m hoping we’re going to be able to replace chronic long-term therapy of CLL with a defined course of treatment with high levels of MRD negativity.”
Dr. Zelenetz reported receiving consulting fees, honoraria, and/or grant/research support from Acerta Pharma, Amgen Inc., BeiGene, Bristol-Myers Squibb, Celgene Corporation, Genentech, Gilead Sciences, Janssen Pharmaceutica Products, MEI Pharma, NanoString Technologies, Pharmacyclics, Portola Pharmaceuticals, Roche Laboratories, and Takeda Pharmaceuticals North America.
ORLANDO – The choice of first-line therapy in symptomatic chronic lymphocytic leukemia patients depends largely on comorbidity burden, Andrew D. Zelenetz, MD, PhD, said at the annual conference of the National Comprehensive Cancer Network.
“This is a disease of elderly patients. Frequently they have comorbidities,” he said. Categorizing these patients as having a low or high comorbidity burden can be done with the Cumulative Index Rating Scale score, which involves scoring of all organ systems on a 0-5 scale representing “not affected” to “extremely disabled.”
“We use this to determine first-line therapy,” said Dr. Zelenetz of Memorial Sloan Kettering Cancer Center, New York. Dr. Zelenetz is chair of the NCCN Non-Hodgkin Lymphoma Guidelines panel.
Patients with a score of greater than 12 on the 0- to 56-point scale, are “no-go” patients with respect to therapy, and are typically treated only with palliative approaches. Those with a score of 7-12 (“slow-go” patients) have a significant comorbidity burden, but can undergo treatment, thought typically to be at reduced intensity. Those with a score of 0-6 are “go-go” patients with respect to treatment, as they are physically fit, have excellent renal function, and have no significant comorbidities, he said.
Treatment options for ‘go-go’ CLL patients
Among the treatment options for the latter is FCR–the combination of fludarabine, cyclophosphamide, and rituximab, which was shown in the phase III CLL10 trial of patients with advanced CLL to be associated with improved complete response rates compared with the popular regimen of bendamustine and rituximab (BR), both overall and in patients under age 65. In older patients, the advantage disappeared, Dr. Zelenetz said.
FCR was also associated with improved outcomes vs. BR in patients with del(11q).
The primary endpoint of the study was progression-free survival, which favored FCR (median of 55.2 vs. 41.7 months; hazard ratio, 1.643), he said, noting that no difference was seen between the two regimens in terms of overall survival.
In a recent publication, MD Anderson Cancer Center reported its experience with its first 300 CLL patients treated with FCR. With long-term follow-up of at least 9-10 years (median of 12.8 years), patients in this trial have done extremely well.
“But interestingly, when you stratify these patients by whether they have IGHV [immunoglobulin heavy chain variable] mutated or unmutated [disease], the IGHV mutated patients have something that looks a whole lot like a survival plateau, and that survival plateau is not trivial – it’s about 60%,” he said. “So there is a group of patients with CLL who are, in fact, curable with conventional chemoimmunotherapy.
“This is an appropriate treatment for a young, fit, ‘go-go’ patient, and it has a big implication,” he said. That is, patients who are young and fit require IGHV mutation testing, as “you will absolutely choose FCR chemotherapy for the fit, young patients who has IGHV mutated disease.
“In that setting IGHV testing is now mandatory,” he stressed, noting that the benefits in this population extend to overall survival as well as progression-free survival.
Dr. Zelenetz also emphasized the need for increasing the single dose of rituximab from 375 mg/m2 during cycle 1 to 500 mg/m2 during cycles 2-6 in those receiving FCR, as this is often forgotten.
The data demonstrating the efficacy of FCR were based on this approach, he said.
Fludarabine is to be given at a dose of 25 mg/m2, and cyclophosphamide at a dose of 250 mg/m2 – both for 2-4 days during cycle 1 and for 1-3 days during cycles 2-6.
Treatment options for ‘slow-go’ CLL patients
In “slow-go” patients, an interesting approach is to use new anti-CD20 antibodies such as ofatumumab and obinutuzumab, which have features that are distinct from rituximab.
Both have been studied in CLL. The CLL11 trial compared chlorambucil, rituximab+chlorambucil, and obinutuzumab+chlorambucil, and the latest analysis showed substantial improvement in progression-free survival with obinutuzumab+chlorambucil vs. the other two regimens (26.7 months vs. 11.1 and 16.3 months, respectively), Dr. Zelenetz said, noting that rituximab+chlorambucil was also superior to chlorambucil alone, but that only the obinutuzumab regimen had an overall survival advantage vs. chlorambucil alone.
An updated analysis to be reported soon will show emerging evidence of a survival advantage of obinutuzumab+chlorambucil vs. rituximab+chlorambucil, he said.
“This suggests that obinutuzumab is a far better antibody,” he added, noting that the reasons for that are under debate, “but the way it’s given, it works better in CLL, and that, I think is unequivocal.”
A similar study looking at chlorambucil with and without ofatumumab in “slow-go” patients also demonstrated an improvement in PFS with ofatumumab, but showed “no difference whatsoever in overall survival.”
“This is actually very similar to the rituximab result, and I actually call this the ‘death of ofatumumab’ study, because clearly obinutuzumab in CLL is, I think, a superior anti-CD20 antibody,” Dr. Zelenetz said.
Studies in which obinutuzumab is substituted for rituximab in the FCR combination are currently underway as are a number of other studies of obinutuzumab, he noted.
Another treatment option in the up-front setting is ibrutinib, which was shown to be effective in the RESONATE 2 trial .
“But notice, a very, very small [complete response rate]. CRs are very difficult to achieve with ibrutinib alone, so this drug is given continuously, lifelong,” Dr. Zelenetz said, noting that it was, however, associated with an overall survival advantage vs. chlorambucil.
“Should this be the standard of care? I think it is in patients who have del(17p) or mutation of TP53. Outside of that setting, I’m still concerned about the cost of long-term tolerability of the agent,” he said.
Future of first-line CLL treatment
Avoidance of long-term therapy and conventional chemotherapy in patients with CLL is a goal, he added, noting that new understanding from studies in patients in the relapsed/refractory CLL setting – such as recent findings from a phase Ib study of venetoclax plus rituximab, which demonstrated potentially durable responses after treatment discontinuation in minimal residual disease (MRD)–negative patients – are providing insights into achieving MRD negativity that could be applied in the front line treatment setting.
“We’re still trying to figure out how to best use this. We want to try to use some of this knowledge about achievement of MRD negativity in the up-front setting so we don’t have to give patients long-term therapy, and we would like to avoid conventional chemotherapy,” he said. “So I’m hoping we’re going to be able to replace chronic long-term therapy of CLL with a defined course of treatment with high levels of MRD negativity.”
Dr. Zelenetz reported receiving consulting fees, honoraria, and/or grant/research support from Acerta Pharma, Amgen Inc., BeiGene, Bristol-Myers Squibb, Celgene Corporation, Genentech, Gilead Sciences, Janssen Pharmaceutica Products, MEI Pharma, NanoString Technologies, Pharmacyclics, Portola Pharmaceuticals, Roche Laboratories, and Takeda Pharmaceuticals North America.
EXPERT ANALYSIS FROM THE NCCN ANNUAL CONFERENCE
Most blood cancer mutations due to DNA replication errors
A new study supports the idea that most cancer-driving mutations are a result of DNA replication errors, not heredity or lifestyle/environmental factors.
For all 32 cancer types studied, researchers found that 66% of driver mutations resulted from DNA replication errors, 29% could be attributed to lifestyle or environmental factors, and the remaining 5% were inherited.
In hematologic malignancies, the percentage of mutations caused by DNA replication errors was even higher—70% in Hodgkin lymphoma, 85% in leukemias, 96% in non-Hodgkin lymphomas, and 99% in myeloma.
Cristian Tomasetti, PhD, of Johns Hopkins University School of Medicine in Baltimore, Maryland, and his colleagues reported these findings in Science.
“It is well-known that we must avoid environmental factors such as smoking to decrease our risk of getting cancer, but it is not as well-known that each time a normal cell divides and copies its DNA to produce 2 new cells, it makes multiple mistakes,” Dr Tomasetti said.
“These copying mistakes are a potent source of cancer mutations that, historically, have been scientifically undervalued, and this new work provides the first estimate of the fraction of mutations caused by these mistakes.”
In 2015, Dr Tomasetti and his colleagues reported that DNA replication errors could explain why certain cancers occur more often than others in the US.
The current study builds upon that research but includes additional cancers and encompasses an international population.
The researchers first studied the relationship between the number of normal stem cell divisions and the risk of 17 cancer types in 69 countries representing 4.8 billion people, or more than half of the world’s population.
The team said they observed a strong correlation between cancer incidence and normal stem cell divisions in all countries, regardless of their environment.
Next, the researchers set out to determine the percentage of driver mutations caused by DNA replication errors in 32 cancer types. The team developed a mathematical model using DNA sequencing data from The Cancer Genome Atlas and epidemiologic data from the Cancer Research UK database.
According to the researchers, it generally takes 2 or more critical mutations for cancer to occur. In an individual, these mutations can be due to random DNA replication errors, the environment, or inherited genes.
Knowing this, the researchers used their mathematical model to show, for example, that when critical mutations in leukemia are added together, 85.2% of them are due to random DNA replication errors, 14.3% to environmental factors, and 0.5% to heredity.
In Hodgkin lymphoma, 69.5% are due to DNA replication errors, 30% to environmental factors, and 0.5% to heredity. In non-Hodgkin lymphoma, 95.6% are due to random DNA replication errors, 3.9% to environmental factors, and 0.5% to heredity.
In myeloma, 99.3% are due to DNA replication errors, 0.2% to environmental factors, and 0.5% to heredity.
Dr Tomasetti said these random DNA replication errors will only get more important as aging populations continue to grow, prolonging the opportunity for cells to make more and more errors.
“We need to continue to encourage people to avoid environmental agents and lifestyles that increase their risk of developing cancer mutations,” said study author Bert Vogelstein, MD, of The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University.
“However, many people will still develop cancers due to these random DNA copying errors, and better methods to detect all cancers earlier, while they are still curable, are urgently needed.” ![]()
A new study supports the idea that most cancer-driving mutations are a result of DNA replication errors, not heredity or lifestyle/environmental factors.
For all 32 cancer types studied, researchers found that 66% of driver mutations resulted from DNA replication errors, 29% could be attributed to lifestyle or environmental factors, and the remaining 5% were inherited.
In hematologic malignancies, the percentage of mutations caused by DNA replication errors was even higher—70% in Hodgkin lymphoma, 85% in leukemias, 96% in non-Hodgkin lymphomas, and 99% in myeloma.
Cristian Tomasetti, PhD, of Johns Hopkins University School of Medicine in Baltimore, Maryland, and his colleagues reported these findings in Science.
“It is well-known that we must avoid environmental factors such as smoking to decrease our risk of getting cancer, but it is not as well-known that each time a normal cell divides and copies its DNA to produce 2 new cells, it makes multiple mistakes,” Dr Tomasetti said.
“These copying mistakes are a potent source of cancer mutations that, historically, have been scientifically undervalued, and this new work provides the first estimate of the fraction of mutations caused by these mistakes.”
In 2015, Dr Tomasetti and his colleagues reported that DNA replication errors could explain why certain cancers occur more often than others in the US.
The current study builds upon that research but includes additional cancers and encompasses an international population.
The researchers first studied the relationship between the number of normal stem cell divisions and the risk of 17 cancer types in 69 countries representing 4.8 billion people, or more than half of the world’s population.
The team said they observed a strong correlation between cancer incidence and normal stem cell divisions in all countries, regardless of their environment.
Next, the researchers set out to determine the percentage of driver mutations caused by DNA replication errors in 32 cancer types. The team developed a mathematical model using DNA sequencing data from The Cancer Genome Atlas and epidemiologic data from the Cancer Research UK database.
According to the researchers, it generally takes 2 or more critical mutations for cancer to occur. In an individual, these mutations can be due to random DNA replication errors, the environment, or inherited genes.
Knowing this, the researchers used their mathematical model to show, for example, that when critical mutations in leukemia are added together, 85.2% of them are due to random DNA replication errors, 14.3% to environmental factors, and 0.5% to heredity.
In Hodgkin lymphoma, 69.5% are due to DNA replication errors, 30% to environmental factors, and 0.5% to heredity. In non-Hodgkin lymphoma, 95.6% are due to random DNA replication errors, 3.9% to environmental factors, and 0.5% to heredity.
In myeloma, 99.3% are due to DNA replication errors, 0.2% to environmental factors, and 0.5% to heredity.
Dr Tomasetti said these random DNA replication errors will only get more important as aging populations continue to grow, prolonging the opportunity for cells to make more and more errors.
“We need to continue to encourage people to avoid environmental agents and lifestyles that increase their risk of developing cancer mutations,” said study author Bert Vogelstein, MD, of The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University.
“However, many people will still develop cancers due to these random DNA copying errors, and better methods to detect all cancers earlier, while they are still curable, are urgently needed.” ![]()
A new study supports the idea that most cancer-driving mutations are a result of DNA replication errors, not heredity or lifestyle/environmental factors.
For all 32 cancer types studied, researchers found that 66% of driver mutations resulted from DNA replication errors, 29% could be attributed to lifestyle or environmental factors, and the remaining 5% were inherited.
In hematologic malignancies, the percentage of mutations caused by DNA replication errors was even higher—70% in Hodgkin lymphoma, 85% in leukemias, 96% in non-Hodgkin lymphomas, and 99% in myeloma.
Cristian Tomasetti, PhD, of Johns Hopkins University School of Medicine in Baltimore, Maryland, and his colleagues reported these findings in Science.
“It is well-known that we must avoid environmental factors such as smoking to decrease our risk of getting cancer, but it is not as well-known that each time a normal cell divides and copies its DNA to produce 2 new cells, it makes multiple mistakes,” Dr Tomasetti said.
“These copying mistakes are a potent source of cancer mutations that, historically, have been scientifically undervalued, and this new work provides the first estimate of the fraction of mutations caused by these mistakes.”
In 2015, Dr Tomasetti and his colleagues reported that DNA replication errors could explain why certain cancers occur more often than others in the US.
The current study builds upon that research but includes additional cancers and encompasses an international population.
The researchers first studied the relationship between the number of normal stem cell divisions and the risk of 17 cancer types in 69 countries representing 4.8 billion people, or more than half of the world’s population.
The team said they observed a strong correlation between cancer incidence and normal stem cell divisions in all countries, regardless of their environment.
Next, the researchers set out to determine the percentage of driver mutations caused by DNA replication errors in 32 cancer types. The team developed a mathematical model using DNA sequencing data from The Cancer Genome Atlas and epidemiologic data from the Cancer Research UK database.
According to the researchers, it generally takes 2 or more critical mutations for cancer to occur. In an individual, these mutations can be due to random DNA replication errors, the environment, or inherited genes.
Knowing this, the researchers used their mathematical model to show, for example, that when critical mutations in leukemia are added together, 85.2% of them are due to random DNA replication errors, 14.3% to environmental factors, and 0.5% to heredity.
In Hodgkin lymphoma, 69.5% are due to DNA replication errors, 30% to environmental factors, and 0.5% to heredity. In non-Hodgkin lymphoma, 95.6% are due to random DNA replication errors, 3.9% to environmental factors, and 0.5% to heredity.
In myeloma, 99.3% are due to DNA replication errors, 0.2% to environmental factors, and 0.5% to heredity.
Dr Tomasetti said these random DNA replication errors will only get more important as aging populations continue to grow, prolonging the opportunity for cells to make more and more errors.
“We need to continue to encourage people to avoid environmental agents and lifestyles that increase their risk of developing cancer mutations,” said study author Bert Vogelstein, MD, of The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University.
“However, many people will still develop cancers due to these random DNA copying errors, and better methods to detect all cancers earlier, while they are still curable, are urgently needed.” ![]()
ASCO reports progress, challenges in cancer care
The US cancer care delivery system is undergoing changes to better meet the needs of cancer patients, but persistent hurdles threaten to slow progress, according to the American Society of Clinical Oncology (ASCO).
ASCO’s “The State of Cancer Care in America, 2017” report describes areas of progress, including new approaches for cancer diagnosis and treatment, improved data sharing to drive innovation, and an increased focus on value-based healthcare.
However, the report also suggests that access and affordability challenges, along with increased practice burdens, continue to pose barriers to high-value, high-quality cancer care.
The report was published in the Journal of Oncology Practice.
Challenges
The report notes that the US population is growing rapidly, changing demographically, and living longer. And all of these factors contribute to a record number of cancer cases/survivors.
It has been estimated that the number of cancer survivors in the US will grow from 15.5 million to 20.3 million by 2026.
Unfortunately, the report says, cancer care is unaffordable for many patients, even those with health insurance.
And significant health disparities persist that are independent of insurance status. Socioeconomic status, geography, and race/ethnicity all impact patient health outcomes.
The report also suggests that oncology practices are facing increased administrative burdens that divert time and resources from their patients.
Progress
Despite the aforementioned challenges, the report paints an optimistic vision about the future of cancer care and highlights activity in the past year aimed at improving care.
For instance, the Food and Drug Administration approved 5 new anticancer therapies, expanded the use of 13, and approved several diagnostic tests in 2016.
In addition, overall cancer incidence and mortality rates were lower in 2016 than in previous decades.
“Since 1991, we’ve been able to save 2.1 million lives because of significant advances in prevention, diagnosis, and treatment—something unimaginable even a decade ago,” said ASCO President Daniel F. Hayes, MD.
“But there’s still more work to be done to ensure that every patient with cancer, no matter who they are or where they live, has access to high-quality, high-value cancer care.”
The report includes a list of recommendations that, ASCO believes, could help bring the US closer to achieving that goal. ![]()
The US cancer care delivery system is undergoing changes to better meet the needs of cancer patients, but persistent hurdles threaten to slow progress, according to the American Society of Clinical Oncology (ASCO).
ASCO’s “The State of Cancer Care in America, 2017” report describes areas of progress, including new approaches for cancer diagnosis and treatment, improved data sharing to drive innovation, and an increased focus on value-based healthcare.
However, the report also suggests that access and affordability challenges, along with increased practice burdens, continue to pose barriers to high-value, high-quality cancer care.
The report was published in the Journal of Oncology Practice.
Challenges
The report notes that the US population is growing rapidly, changing demographically, and living longer. And all of these factors contribute to a record number of cancer cases/survivors.
It has been estimated that the number of cancer survivors in the US will grow from 15.5 million to 20.3 million by 2026.
Unfortunately, the report says, cancer care is unaffordable for many patients, even those with health insurance.
And significant health disparities persist that are independent of insurance status. Socioeconomic status, geography, and race/ethnicity all impact patient health outcomes.
The report also suggests that oncology practices are facing increased administrative burdens that divert time and resources from their patients.
Progress
Despite the aforementioned challenges, the report paints an optimistic vision about the future of cancer care and highlights activity in the past year aimed at improving care.
For instance, the Food and Drug Administration approved 5 new anticancer therapies, expanded the use of 13, and approved several diagnostic tests in 2016.
In addition, overall cancer incidence and mortality rates were lower in 2016 than in previous decades.
“Since 1991, we’ve been able to save 2.1 million lives because of significant advances in prevention, diagnosis, and treatment—something unimaginable even a decade ago,” said ASCO President Daniel F. Hayes, MD.
“But there’s still more work to be done to ensure that every patient with cancer, no matter who they are or where they live, has access to high-quality, high-value cancer care.”
The report includes a list of recommendations that, ASCO believes, could help bring the US closer to achieving that goal. ![]()
The US cancer care delivery system is undergoing changes to better meet the needs of cancer patients, but persistent hurdles threaten to slow progress, according to the American Society of Clinical Oncology (ASCO).
ASCO’s “The State of Cancer Care in America, 2017” report describes areas of progress, including new approaches for cancer diagnosis and treatment, improved data sharing to drive innovation, and an increased focus on value-based healthcare.
However, the report also suggests that access and affordability challenges, along with increased practice burdens, continue to pose barriers to high-value, high-quality cancer care.
The report was published in the Journal of Oncology Practice.
Challenges
The report notes that the US population is growing rapidly, changing demographically, and living longer. And all of these factors contribute to a record number of cancer cases/survivors.
It has been estimated that the number of cancer survivors in the US will grow from 15.5 million to 20.3 million by 2026.
Unfortunately, the report says, cancer care is unaffordable for many patients, even those with health insurance.
And significant health disparities persist that are independent of insurance status. Socioeconomic status, geography, and race/ethnicity all impact patient health outcomes.
The report also suggests that oncology practices are facing increased administrative burdens that divert time and resources from their patients.
Progress
Despite the aforementioned challenges, the report paints an optimistic vision about the future of cancer care and highlights activity in the past year aimed at improving care.
For instance, the Food and Drug Administration approved 5 new anticancer therapies, expanded the use of 13, and approved several diagnostic tests in 2016.
In addition, overall cancer incidence and mortality rates were lower in 2016 than in previous decades.
“Since 1991, we’ve been able to save 2.1 million lives because of significant advances in prevention, diagnosis, and treatment—something unimaginable even a decade ago,” said ASCO President Daniel F. Hayes, MD.
“But there’s still more work to be done to ensure that every patient with cancer, no matter who they are or where they live, has access to high-quality, high-value cancer care.”
The report includes a list of recommendations that, ASCO believes, could help bring the US closer to achieving that goal. ![]()
New resource designed to help prevent CINV
The Hematology/Oncology Pharmacy Association (HOPA) has announced the release of a toolkit intended to help prevent chemotherapy-induced nausea and vomiting (CINV) in cancer patients.
The Time to Talk CINV™ toolkit was designed to facilitate dialogue between patients and their healthcare teams to ensure no patient is needlessly suffering from CINV.
The tools in the kit, which are targeted to both patients and healthcare providers, were created with guidance from patients, caregivers, pharmacists, and nurses.
The entire toolkit is available for free download at TimeToTalkCINV.com.
The toolkit is part of the Time to Talk CINV™ campaign, which is a collaboration between HOPA, Eisai Inc., and Helsinn Therapeutics (US), Inc. (funded by Eisai and Helsinn Therapeutics).
The campaign began in response to results from a survey of cancer patients.
“Our research revealed a vast majority of patients on chemotherapy who experience nausea and vomiting expect to have this side effect, and 95% of these patients said, at some point, chemo-induced nausea and vomiting had an impact on their daily lives,” said Sarah Peters, PharmD, president of HOPA.
“In response to these findings, as well as the finding that healthcare team members are looking for better communication tools, the Time to Talk CINV toolkit was created to facilitate efficient and effective conversations about nausea and vomiting from chemotherapy to ensure that each patient is receiving the right information and effective management approaches.”
The Time to Talk CINV toolkit includes the following resources:
- A list of myths and truths about CINV intended to eliminate common misperceptions
- A checklist of questions patients can ask healthcare providers to better understand CINV
- A chemotherapy side effect tracker, which enables patients to track their experience and report back to their healthcare team
- A communication checklist for the healthcare team outlining best practices for communicating with patients to prevent CINV.
Each tool can be printed or filled out digitally.
Additional information about CINV, including videos and infographics, can be found on the Time to Talk CINV website. ![]()
The Hematology/Oncology Pharmacy Association (HOPA) has announced the release of a toolkit intended to help prevent chemotherapy-induced nausea and vomiting (CINV) in cancer patients.
The Time to Talk CINV™ toolkit was designed to facilitate dialogue between patients and their healthcare teams to ensure no patient is needlessly suffering from CINV.
The tools in the kit, which are targeted to both patients and healthcare providers, were created with guidance from patients, caregivers, pharmacists, and nurses.
The entire toolkit is available for free download at TimeToTalkCINV.com.
The toolkit is part of the Time to Talk CINV™ campaign, which is a collaboration between HOPA, Eisai Inc., and Helsinn Therapeutics (US), Inc. (funded by Eisai and Helsinn Therapeutics).
The campaign began in response to results from a survey of cancer patients.
“Our research revealed a vast majority of patients on chemotherapy who experience nausea and vomiting expect to have this side effect, and 95% of these patients said, at some point, chemo-induced nausea and vomiting had an impact on their daily lives,” said Sarah Peters, PharmD, president of HOPA.
“In response to these findings, as well as the finding that healthcare team members are looking for better communication tools, the Time to Talk CINV toolkit was created to facilitate efficient and effective conversations about nausea and vomiting from chemotherapy to ensure that each patient is receiving the right information and effective management approaches.”
The Time to Talk CINV toolkit includes the following resources:
- A list of myths and truths about CINV intended to eliminate common misperceptions
- A checklist of questions patients can ask healthcare providers to better understand CINV
- A chemotherapy side effect tracker, which enables patients to track their experience and report back to their healthcare team
- A communication checklist for the healthcare team outlining best practices for communicating with patients to prevent CINV.
Each tool can be printed or filled out digitally.
Additional information about CINV, including videos and infographics, can be found on the Time to Talk CINV website. ![]()
The Hematology/Oncology Pharmacy Association (HOPA) has announced the release of a toolkit intended to help prevent chemotherapy-induced nausea and vomiting (CINV) in cancer patients.
The Time to Talk CINV™ toolkit was designed to facilitate dialogue between patients and their healthcare teams to ensure no patient is needlessly suffering from CINV.
The tools in the kit, which are targeted to both patients and healthcare providers, were created with guidance from patients, caregivers, pharmacists, and nurses.
The entire toolkit is available for free download at TimeToTalkCINV.com.
The toolkit is part of the Time to Talk CINV™ campaign, which is a collaboration between HOPA, Eisai Inc., and Helsinn Therapeutics (US), Inc. (funded by Eisai and Helsinn Therapeutics).
The campaign began in response to results from a survey of cancer patients.
“Our research revealed a vast majority of patients on chemotherapy who experience nausea and vomiting expect to have this side effect, and 95% of these patients said, at some point, chemo-induced nausea and vomiting had an impact on their daily lives,” said Sarah Peters, PharmD, president of HOPA.
“In response to these findings, as well as the finding that healthcare team members are looking for better communication tools, the Time to Talk CINV toolkit was created to facilitate efficient and effective conversations about nausea and vomiting from chemotherapy to ensure that each patient is receiving the right information and effective management approaches.”
The Time to Talk CINV toolkit includes the following resources:
- A list of myths and truths about CINV intended to eliminate common misperceptions
- A checklist of questions patients can ask healthcare providers to better understand CINV
- A chemotherapy side effect tracker, which enables patients to track their experience and report back to their healthcare team
- A communication checklist for the healthcare team outlining best practices for communicating with patients to prevent CINV.
Each tool can be printed or filled out digitally.
Additional information about CINV, including videos and infographics, can be found on the Time to Talk CINV website. ![]()
AYAs struggle socially in early years after cancer diagnosis
A new study indicates that adolescent and young adult (AYA) cancer survivors continue to face social difficulties for more than 2 years after their diagnosis.
The research, published in Cancer, suggests these patients may see some improvement in their social lives during the first year after diagnosis.
However, their social functioning tends to remain constant after that, leaving them socially impaired relative to their cancer-free peers.
Previous studies have shown that AYAs with cancer experience greater challenges in social functioning than their cancer-free peers or even compared to older cancer patients.
But few studies have examined this phenomenon by following the same patients over time.
Olga Husson, PhD, of the Radboud University Medical Center in The Netherlands, and her colleagues set out to examine changes in social functioning among AYAs in the early years after a cancer diagnosis.
The researchers asked AYA cancer patients at 5 US medical institutions to complete a survey about social functioning within 4 months of their diagnosis, 12 months later, and 24 months later.
There were 141 patients (ages 14 to 39 at diagnosis) who completed the surveys.
The researchers found that, when compared to population norms, the cancer patients had inferior social functioning at all the time points studied.
Among the cancer patients, the mean social functioning score from the Medical Outcomes Study Short Form 36 Health Survey (version 2) was 52.0 around the time of cancer diagnosis, 73.1 at the 12-month follow-up, and 69.2 at the 24-month follow-up. In comparison, the population norm (for people ages 18 to 44) is 85.1 (P<0.001 for all time points).
The researchers did note that cancer patients experienced significant improvements in social functioning from baseline to the 12-month follow-up, but there was no further improvement after that.
The researchers also examined the different trajectories of social functioning over time. They found that social functioning improved over time for 47% of the cancer patients but worsened for 13%. In addition, 32% of patients had consistently low social functioning, and 9% had consistently high social functioning.
The cancer patients with consistently low social functioning were more likely to be off treatment at the time of follow-up, report more physical symptoms and higher levels of psychological distress (at both baseline and follow-up), and perceive themselves to receive less social support.
“Reducing physical symptoms and psychological distress and enhancing social support by interventions in the period after treatment may potentially help these young survivors to better reintegrate into society,” Dr Husson said. ![]()
A new study indicates that adolescent and young adult (AYA) cancer survivors continue to face social difficulties for more than 2 years after their diagnosis.
The research, published in Cancer, suggests these patients may see some improvement in their social lives during the first year after diagnosis.
However, their social functioning tends to remain constant after that, leaving them socially impaired relative to their cancer-free peers.
Previous studies have shown that AYAs with cancer experience greater challenges in social functioning than their cancer-free peers or even compared to older cancer patients.
But few studies have examined this phenomenon by following the same patients over time.
Olga Husson, PhD, of the Radboud University Medical Center in The Netherlands, and her colleagues set out to examine changes in social functioning among AYAs in the early years after a cancer diagnosis.
The researchers asked AYA cancer patients at 5 US medical institutions to complete a survey about social functioning within 4 months of their diagnosis, 12 months later, and 24 months later.
There were 141 patients (ages 14 to 39 at diagnosis) who completed the surveys.
The researchers found that, when compared to population norms, the cancer patients had inferior social functioning at all the time points studied.
Among the cancer patients, the mean social functioning score from the Medical Outcomes Study Short Form 36 Health Survey (version 2) was 52.0 around the time of cancer diagnosis, 73.1 at the 12-month follow-up, and 69.2 at the 24-month follow-up. In comparison, the population norm (for people ages 18 to 44) is 85.1 (P<0.001 for all time points).
The researchers did note that cancer patients experienced significant improvements in social functioning from baseline to the 12-month follow-up, but there was no further improvement after that.
The researchers also examined the different trajectories of social functioning over time. They found that social functioning improved over time for 47% of the cancer patients but worsened for 13%. In addition, 32% of patients had consistently low social functioning, and 9% had consistently high social functioning.
The cancer patients with consistently low social functioning were more likely to be off treatment at the time of follow-up, report more physical symptoms and higher levels of psychological distress (at both baseline and follow-up), and perceive themselves to receive less social support.
“Reducing physical symptoms and psychological distress and enhancing social support by interventions in the period after treatment may potentially help these young survivors to better reintegrate into society,” Dr Husson said. ![]()
A new study indicates that adolescent and young adult (AYA) cancer survivors continue to face social difficulties for more than 2 years after their diagnosis.
The research, published in Cancer, suggests these patients may see some improvement in their social lives during the first year after diagnosis.
However, their social functioning tends to remain constant after that, leaving them socially impaired relative to their cancer-free peers.
Previous studies have shown that AYAs with cancer experience greater challenges in social functioning than their cancer-free peers or even compared to older cancer patients.
But few studies have examined this phenomenon by following the same patients over time.
Olga Husson, PhD, of the Radboud University Medical Center in The Netherlands, and her colleagues set out to examine changes in social functioning among AYAs in the early years after a cancer diagnosis.
The researchers asked AYA cancer patients at 5 US medical institutions to complete a survey about social functioning within 4 months of their diagnosis, 12 months later, and 24 months later.
There were 141 patients (ages 14 to 39 at diagnosis) who completed the surveys.
The researchers found that, when compared to population norms, the cancer patients had inferior social functioning at all the time points studied.
Among the cancer patients, the mean social functioning score from the Medical Outcomes Study Short Form 36 Health Survey (version 2) was 52.0 around the time of cancer diagnosis, 73.1 at the 12-month follow-up, and 69.2 at the 24-month follow-up. In comparison, the population norm (for people ages 18 to 44) is 85.1 (P<0.001 for all time points).
The researchers did note that cancer patients experienced significant improvements in social functioning from baseline to the 12-month follow-up, but there was no further improvement after that.
The researchers also examined the different trajectories of social functioning over time. They found that social functioning improved over time for 47% of the cancer patients but worsened for 13%. In addition, 32% of patients had consistently low social functioning, and 9% had consistently high social functioning.
The cancer patients with consistently low social functioning were more likely to be off treatment at the time of follow-up, report more physical symptoms and higher levels of psychological distress (at both baseline and follow-up), and perceive themselves to receive less social support.
“Reducing physical symptoms and psychological distress and enhancing social support by interventions in the period after treatment may potentially help these young survivors to better reintegrate into society,” Dr Husson said. ![]()
Proteins may be therapeutic targets for TKI-resistant CML, ALL
Researchers say they have identified 2 signaling proteins that enable resistance to tyrosine kinase inhibitors (TKIs) and could be therapeutic targets for acute lymphoblastic leukemia (ALL) and chronic myeloid leukemia (CML).
The team found that by deleting these proteins—c-Fos and Dusp1—they could eradicate BCR-ABL-induced B-cell ALL in mice.
And treatment combining c-Fos and Dusp1 inhibitors with the TKI imatinib was able to cure mice with BCR-ABL-driven CML.
The researchers reported these findings in Nature Medicine.
“We think that, within the next 5 years, our data will change the way people think about cancer development and targeted therapy,” said study author Mohammad Azam, PhD, of Cincinnati Children’s Hospital Medical Center in Ohio.
“This study identifies a potential Achilles’ heel of kinase-driven cancers, and what we propose is intended to be curative, not just treatment.”
The potential Achilles’ heel is a common point of passage in cells—a signaling node—that appears to be required to generate cancer cells. The node is formed by the signaling proteins c-Fos and Dusp1, according to the researchers.
The team identified c-Fos and Dusp1 by conducting global gene-expression analysis of mouse leukemia cells and human CML cells. Analysis of the human cells revealed extremely high levels of c-FOS and DUSP1 in BCR-ABL-positive, TKI-resistant cells.
Dr Azam and his colleagues found that signaling from tyrosine kinase and growth factor proteins that support cell expansion (such as IL-3 and IL-6) converge to elevate c-Fos and Dusp1 levels in leukemia cells.
Working together, these molecules maintain the survival of leukemia stem cells (LSCs), which translates to minimal residual disease (MRD) after treatment.
Dr Azam said Dusp1 and c-Fos support the survival of LSCs by increasing the toxic threshold needed to kill them. This means imatinib and other TKIs cannot eliminate the residual LSCs.
After describing the roles of c-Fos and Dusp1, Dr Azam and his colleagues put their ideas to the test in mouse models of CML.
The team tested several treatments in these mice, including:
- monotherapy with imatinib
- inhibitors of c-Fos and Dusp1
- treatment with imatinib and inhibitors of c-Fos and Dusp1.
As suspected, treatment with imatinib alone initially stopped CML progression, but mice ultimately relapsed.
Treatment with c-Fos and Dusp1 inhibitors significantly slowed CML progression and prolonged survival in a majority of mice, but this treatment wasn’t curative.
However, a month of treatment with c-Fos and Dusp1 inhibitors as well as imatinib cured about 90% of mice with CML, and there were no signs of MRD.
The researchers also found that simply deleting c-Fos and Dusp1 was sufficient to block the development of B-cell ALL in mice.
The team said they are following up this study by testing c-Fos and Dusp1 as treatment targets for different kinase-fueled cancers. ![]()
Researchers say they have identified 2 signaling proteins that enable resistance to tyrosine kinase inhibitors (TKIs) and could be therapeutic targets for acute lymphoblastic leukemia (ALL) and chronic myeloid leukemia (CML).
The team found that by deleting these proteins—c-Fos and Dusp1—they could eradicate BCR-ABL-induced B-cell ALL in mice.
And treatment combining c-Fos and Dusp1 inhibitors with the TKI imatinib was able to cure mice with BCR-ABL-driven CML.
The researchers reported these findings in Nature Medicine.
“We think that, within the next 5 years, our data will change the way people think about cancer development and targeted therapy,” said study author Mohammad Azam, PhD, of Cincinnati Children’s Hospital Medical Center in Ohio.
“This study identifies a potential Achilles’ heel of kinase-driven cancers, and what we propose is intended to be curative, not just treatment.”
The potential Achilles’ heel is a common point of passage in cells—a signaling node—that appears to be required to generate cancer cells. The node is formed by the signaling proteins c-Fos and Dusp1, according to the researchers.
The team identified c-Fos and Dusp1 by conducting global gene-expression analysis of mouse leukemia cells and human CML cells. Analysis of the human cells revealed extremely high levels of c-FOS and DUSP1 in BCR-ABL-positive, TKI-resistant cells.
Dr Azam and his colleagues found that signaling from tyrosine kinase and growth factor proteins that support cell expansion (such as IL-3 and IL-6) converge to elevate c-Fos and Dusp1 levels in leukemia cells.
Working together, these molecules maintain the survival of leukemia stem cells (LSCs), which translates to minimal residual disease (MRD) after treatment.
Dr Azam said Dusp1 and c-Fos support the survival of LSCs by increasing the toxic threshold needed to kill them. This means imatinib and other TKIs cannot eliminate the residual LSCs.
After describing the roles of c-Fos and Dusp1, Dr Azam and his colleagues put their ideas to the test in mouse models of CML.
The team tested several treatments in these mice, including:
- monotherapy with imatinib
- inhibitors of c-Fos and Dusp1
- treatment with imatinib and inhibitors of c-Fos and Dusp1.
As suspected, treatment with imatinib alone initially stopped CML progression, but mice ultimately relapsed.
Treatment with c-Fos and Dusp1 inhibitors significantly slowed CML progression and prolonged survival in a majority of mice, but this treatment wasn’t curative.
However, a month of treatment with c-Fos and Dusp1 inhibitors as well as imatinib cured about 90% of mice with CML, and there were no signs of MRD.
The researchers also found that simply deleting c-Fos and Dusp1 was sufficient to block the development of B-cell ALL in mice.
The team said they are following up this study by testing c-Fos and Dusp1 as treatment targets for different kinase-fueled cancers. ![]()
Researchers say they have identified 2 signaling proteins that enable resistance to tyrosine kinase inhibitors (TKIs) and could be therapeutic targets for acute lymphoblastic leukemia (ALL) and chronic myeloid leukemia (CML).
The team found that by deleting these proteins—c-Fos and Dusp1—they could eradicate BCR-ABL-induced B-cell ALL in mice.
And treatment combining c-Fos and Dusp1 inhibitors with the TKI imatinib was able to cure mice with BCR-ABL-driven CML.
The researchers reported these findings in Nature Medicine.
“We think that, within the next 5 years, our data will change the way people think about cancer development and targeted therapy,” said study author Mohammad Azam, PhD, of Cincinnati Children’s Hospital Medical Center in Ohio.
“This study identifies a potential Achilles’ heel of kinase-driven cancers, and what we propose is intended to be curative, not just treatment.”
The potential Achilles’ heel is a common point of passage in cells—a signaling node—that appears to be required to generate cancer cells. The node is formed by the signaling proteins c-Fos and Dusp1, according to the researchers.
The team identified c-Fos and Dusp1 by conducting global gene-expression analysis of mouse leukemia cells and human CML cells. Analysis of the human cells revealed extremely high levels of c-FOS and DUSP1 in BCR-ABL-positive, TKI-resistant cells.
Dr Azam and his colleagues found that signaling from tyrosine kinase and growth factor proteins that support cell expansion (such as IL-3 and IL-6) converge to elevate c-Fos and Dusp1 levels in leukemia cells.
Working together, these molecules maintain the survival of leukemia stem cells (LSCs), which translates to minimal residual disease (MRD) after treatment.
Dr Azam said Dusp1 and c-Fos support the survival of LSCs by increasing the toxic threshold needed to kill them. This means imatinib and other TKIs cannot eliminate the residual LSCs.
After describing the roles of c-Fos and Dusp1, Dr Azam and his colleagues put their ideas to the test in mouse models of CML.
The team tested several treatments in these mice, including:
- monotherapy with imatinib
- inhibitors of c-Fos and Dusp1
- treatment with imatinib and inhibitors of c-Fos and Dusp1.
As suspected, treatment with imatinib alone initially stopped CML progression, but mice ultimately relapsed.
Treatment with c-Fos and Dusp1 inhibitors significantly slowed CML progression and prolonged survival in a majority of mice, but this treatment wasn’t curative.
However, a month of treatment with c-Fos and Dusp1 inhibitors as well as imatinib cured about 90% of mice with CML, and there were no signs of MRD.
The researchers also found that simply deleting c-Fos and Dusp1 was sufficient to block the development of B-cell ALL in mice.
The team said they are following up this study by testing c-Fos and Dusp1 as treatment targets for different kinase-fueled cancers.
New insight into high-hyperdiploid ALL
New research appears to explain how 10q21.2 influences the risk of high-hyperdiploid acute lymphoblastic leukemia (HD-ALL).
Previous research indicated that variation in the gene ARID5B at 10q21.2 is associated with HD-ALL.
Now, researchers have reported that the 10q21.2 risk locus for HD-ALL is mediated through the single nucleotide polymorphism (SNP) rs7090445, which disrupts RUNX3 transcription factor binding.
Specifically, the rs7090445-C allele confers an increased risk of HD-ALL through reduced RUNX3-mediated expression of ARID5B.
The researchers described these findings in Nature Communications.
“This study expands our understanding of how genetic risk factors can influence the development of acute lymphoblastic leukemia . . .,” said study author Richard Houlston, MD, PhD, of The Institute of Cancer Research in London, UK.
Dr Houlston and his colleagues focused this research on 10q21.2 because it had previously been implicated in HD-ALL, but it wasn’t clear how the region affects the risk of HD-ALL.
The team said they found that a SNP in the region, rs7090445, is “highly associated” with HD-ALL.
Further investigation revealed that variation at rs7090445 disrupts RUNX3 binding and reduces the expression of ARID5B, as RUNX3 regulates ARID5B expression.
The researchers also discovered that the rs7090445-C risk allele, which is associated with reduced ARID5B expression, is amplified in HD-ALL. The risk allele is “preferentially retained” on additional copies of chromosome 10 in HD-ALL blasts.
“We implicate reduced expression of a gene called ARID5B in the production and release of the immature ‘blast’ cells that characterize [HD-ALL],” Dr Houlston said. “Our study gives a new insight into the causes of the disease and may open up new strategies for prevention.”
New research appears to explain how 10q21.2 influences the risk of high-hyperdiploid acute lymphoblastic leukemia (HD-ALL).
Previous research indicated that variation in the gene ARID5B at 10q21.2 is associated with HD-ALL.
Now, researchers have reported that the 10q21.2 risk locus for HD-ALL is mediated through the single nucleotide polymorphism (SNP) rs7090445, which disrupts RUNX3 transcription factor binding.
Specifically, the rs7090445-C allele confers an increased risk of HD-ALL through reduced RUNX3-mediated expression of ARID5B.
The researchers described these findings in Nature Communications.
“This study expands our understanding of how genetic risk factors can influence the development of acute lymphoblastic leukemia . . .,” said study author Richard Houlston, MD, PhD, of The Institute of Cancer Research in London, UK.
Dr Houlston and his colleagues focused this research on 10q21.2 because it had previously been implicated in HD-ALL, but it wasn’t clear how the region affects the risk of HD-ALL.
The team said they found that a SNP in the region, rs7090445, is “highly associated” with HD-ALL.
Further investigation revealed that variation at rs7090445 disrupts RUNX3 binding and reduces the expression of ARID5B, as RUNX3 regulates ARID5B expression.
The researchers also discovered that the rs7090445-C risk allele, which is associated with reduced ARID5B expression, is amplified in HD-ALL. The risk allele is “preferentially retained” on additional copies of chromosome 10 in HD-ALL blasts.
“We implicate reduced expression of a gene called ARID5B in the production and release of the immature ‘blast’ cells that characterize [HD-ALL],” Dr Houlston said. “Our study gives a new insight into the causes of the disease and may open up new strategies for prevention.”
New research appears to explain how 10q21.2 influences the risk of high-hyperdiploid acute lymphoblastic leukemia (HD-ALL).
Previous research indicated that variation in the gene ARID5B at 10q21.2 is associated with HD-ALL.
Now, researchers have reported that the 10q21.2 risk locus for HD-ALL is mediated through the single nucleotide polymorphism (SNP) rs7090445, which disrupts RUNX3 transcription factor binding.
Specifically, the rs7090445-C allele confers an increased risk of HD-ALL through reduced RUNX3-mediated expression of ARID5B.
The researchers described these findings in Nature Communications.
“This study expands our understanding of how genetic risk factors can influence the development of acute lymphoblastic leukemia . . .,” said study author Richard Houlston, MD, PhD, of The Institute of Cancer Research in London, UK.
Dr Houlston and his colleagues focused this research on 10q21.2 because it had previously been implicated in HD-ALL, but it wasn’t clear how the region affects the risk of HD-ALL.
The team said they found that a SNP in the region, rs7090445, is “highly associated” with HD-ALL.
Further investigation revealed that variation at rs7090445 disrupts RUNX3 binding and reduces the expression of ARID5B, as RUNX3 regulates ARID5B expression.
The researchers also discovered that the rs7090445-C risk allele, which is associated with reduced ARID5B expression, is amplified in HD-ALL. The risk allele is “preferentially retained” on additional copies of chromosome 10 in HD-ALL blasts.
“We implicate reduced expression of a gene called ARID5B in the production and release of the immature ‘blast’ cells that characterize [HD-ALL],” Dr Houlston said. “Our study gives a new insight into the causes of the disease and may open up new strategies for prevention.”
Auto-HCT patients run high risks for myeloid neoplasms
ORLANDO – For post–autologous hematopoietic cell transplant (auto-HCT) patients, the 10-year risk of developing a myeloid neoplasm was as high as 6%, based on a recent review of two large cancer databases.
Older age at transplant, receiving total body irradiation, and receiving multiple lines of chemotherapy before transplant all upped the risk of later cancers, according to a study presented by Shahrukh Hashmi, MD, and his collaborators at the combined annual meetings of the Center for International Blood & Marrow Transplant Research (CIBMTR) and the American Society for Blood and Marrow Transplantation.
“The guidelines for autologous stem cell transplantation for surveillance for AML [acute myeloid leukemia] and MDS [myelodysplastic syndrome] need to be clearly formulated. We are doing 30,000 autologous transplants a year globally and these patients are at risk for the most feared cancer, which is leukemia and MDS, for which outcomes are very poor,” said Dr. Hashmi of the Mayo Clinic in Rochester, Minn.
The researchers examined data from auto-HCT patients with diagnoses of non-Hodgkin lymphoma (NHL), Hodgkin lymphoma, and multiple myeloma to determine the relative risks of developing AML and MDS. The study also explored which patient characteristics and aspects of the conditioning regimen might affect risk for later myeloid neoplasms.
In the dataset of 9,108 patients that Dr. Hashmi and his colleagues obtained from CIBMTR, 3,540 patients had NHL.
“As age progresses, the risk of acquiring myeloid neoplasms increases significantly,” he said, noting that the relative risk (RR) rose to 4.52 for patients aged 55 years and older at the time of transplant (95% confidence interval [CI], 2.63-7.77; P less than .0001).
Patients with NHL who received more than two lines of chemotherapy had approximately double the rate of myeloid cancers (RR, 1.93; 95% CI, 1.34-2.78; P = .0004).
The type of conditioning regimen made a difference for NHL patients as well. With total-body irradiation set as the reference at RR = 1, carmustine-etoposide-cytarabine-melphalan (BEAM) or similar therapies were relatively protective, with an RR of 0.59 (95% CI, 0.40-0.87; P = .0083). Also protective were cyclophosphamide-carmustine-etoposide (CBV) and similar therapies (RR, 0.57; 95% CI, 0.33-0.99; P = .0463).
Age at transplant was a factor among the 4,653 patients with multiple myeloma, with an RR of 2.47 for those transplanted at age 55 years or older (95% CI, 1.55-3.93; P = .0001). Multiple lines of chemotherapy also increased risk, with patients who received more than two lines having an RR of 1.77 for neoplasm (95% CI, 0.04-2.06; P = .0302). Women had less than half the risk of recurrence as men (RR, 0.44; 95% CI, 0.28-0.69; P = .0003).
Among the 915 study patients with Hodgkin lymphoma, patients aged 45 years and older at the time of transplant carried an RR of 5.59 for new myeloid neoplasms (95% CI, 2.98-11.70; P less than .0001).
Total-body irradiation was received by 14% of patients with non-Hodgkin lymphoma and by 5% of patients with multiple myeloma and Hodgkin lymphoma. Total-body irradiation was associated with a fourfold increase in neoplasm risk (RR, 4.02; 95% CI, 1.40-11.55; P = .0096).
Dr. Hashmi and his colleagues then examined the incidence rates for myelodysplastic syndrome and acute myelogenous leukemia in the Surveillance, Epidemiology, and End Results (SEER) database , finding that, even at baseline, the rates of myeloid neoplasms were higher for patients with NHL, Hodgkin lymphoma, or MM patients than for the general population of cancer survivors. “Post NHL, Hodgkin lymphoma, and myeloma, the risks are significantly higher to begin with. … We saw a high risk of AML and MDS compared to the SEER controls – risks as high as 100 times greater for auto-transplant patients,” said Dr. Hashmi. “A risk of one hundred times more for MDS was astounding, surprising, unexpected,” he said. The risk of AML, he said, was elevated about 10-50 times in the CIBMTR data.
The cumulative incidence of MDS or AML for NHL was 6% at 10 years post transplant, 4% for Hodgkin lymphoma, and 3% for multiple myeloma.
A limitation of the study, said Dr. Hashmi, was that the investigators did not assess for post-transplant maintenance chemotherapy.
“We have to prospectively assess our transplant patients in a fashion to detect changes early. Or maybe they were present at the time of transplant and we never did sophisticated methods [like] next-generation sequencing” to detect them, he said.
Dr. Hashmi reported no conflicts of interest.
[email protected]
On Twitter @karioakes
ORLANDO – For post–autologous hematopoietic cell transplant (auto-HCT) patients, the 10-year risk of developing a myeloid neoplasm was as high as 6%, based on a recent review of two large cancer databases.
Older age at transplant, receiving total body irradiation, and receiving multiple lines of chemotherapy before transplant all upped the risk of later cancers, according to a study presented by Shahrukh Hashmi, MD, and his collaborators at the combined annual meetings of the Center for International Blood & Marrow Transplant Research (CIBMTR) and the American Society for Blood and Marrow Transplantation.
“The guidelines for autologous stem cell transplantation for surveillance for AML [acute myeloid leukemia] and MDS [myelodysplastic syndrome] need to be clearly formulated. We are doing 30,000 autologous transplants a year globally and these patients are at risk for the most feared cancer, which is leukemia and MDS, for which outcomes are very poor,” said Dr. Hashmi of the Mayo Clinic in Rochester, Minn.
The researchers examined data from auto-HCT patients with diagnoses of non-Hodgkin lymphoma (NHL), Hodgkin lymphoma, and multiple myeloma to determine the relative risks of developing AML and MDS. The study also explored which patient characteristics and aspects of the conditioning regimen might affect risk for later myeloid neoplasms.
In the dataset of 9,108 patients that Dr. Hashmi and his colleagues obtained from CIBMTR, 3,540 patients had NHL.
“As age progresses, the risk of acquiring myeloid neoplasms increases significantly,” he said, noting that the relative risk (RR) rose to 4.52 for patients aged 55 years and older at the time of transplant (95% confidence interval [CI], 2.63-7.77; P less than .0001).
Patients with NHL who received more than two lines of chemotherapy had approximately double the rate of myeloid cancers (RR, 1.93; 95% CI, 1.34-2.78; P = .0004).
The type of conditioning regimen made a difference for NHL patients as well. With total-body irradiation set as the reference at RR = 1, carmustine-etoposide-cytarabine-melphalan (BEAM) or similar therapies were relatively protective, with an RR of 0.59 (95% CI, 0.40-0.87; P = .0083). Also protective were cyclophosphamide-carmustine-etoposide (CBV) and similar therapies (RR, 0.57; 95% CI, 0.33-0.99; P = .0463).
Age at transplant was a factor among the 4,653 patients with multiple myeloma, with an RR of 2.47 for those transplanted at age 55 years or older (95% CI, 1.55-3.93; P = .0001). Multiple lines of chemotherapy also increased risk, with patients who received more than two lines having an RR of 1.77 for neoplasm (95% CI, 0.04-2.06; P = .0302). Women had less than half the risk of recurrence as men (RR, 0.44; 95% CI, 0.28-0.69; P = .0003).
Among the 915 study patients with Hodgkin lymphoma, patients aged 45 years and older at the time of transplant carried an RR of 5.59 for new myeloid neoplasms (95% CI, 2.98-11.70; P less than .0001).
Total-body irradiation was received by 14% of patients with non-Hodgkin lymphoma and by 5% of patients with multiple myeloma and Hodgkin lymphoma. Total-body irradiation was associated with a fourfold increase in neoplasm risk (RR, 4.02; 95% CI, 1.40-11.55; P = .0096).
Dr. Hashmi and his colleagues then examined the incidence rates for myelodysplastic syndrome and acute myelogenous leukemia in the Surveillance, Epidemiology, and End Results (SEER) database , finding that, even at baseline, the rates of myeloid neoplasms were higher for patients with NHL, Hodgkin lymphoma, or MM patients than for the general population of cancer survivors. “Post NHL, Hodgkin lymphoma, and myeloma, the risks are significantly higher to begin with. … We saw a high risk of AML and MDS compared to the SEER controls – risks as high as 100 times greater for auto-transplant patients,” said Dr. Hashmi. “A risk of one hundred times more for MDS was astounding, surprising, unexpected,” he said. The risk of AML, he said, was elevated about 10-50 times in the CIBMTR data.
The cumulative incidence of MDS or AML for NHL was 6% at 10 years post transplant, 4% for Hodgkin lymphoma, and 3% for multiple myeloma.
A limitation of the study, said Dr. Hashmi, was that the investigators did not assess for post-transplant maintenance chemotherapy.
“We have to prospectively assess our transplant patients in a fashion to detect changes early. Or maybe they were present at the time of transplant and we never did sophisticated methods [like] next-generation sequencing” to detect them, he said.
Dr. Hashmi reported no conflicts of interest.
[email protected]
On Twitter @karioakes
ORLANDO – For post–autologous hematopoietic cell transplant (auto-HCT) patients, the 10-year risk of developing a myeloid neoplasm was as high as 6%, based on a recent review of two large cancer databases.
Older age at transplant, receiving total body irradiation, and receiving multiple lines of chemotherapy before transplant all upped the risk of later cancers, according to a study presented by Shahrukh Hashmi, MD, and his collaborators at the combined annual meetings of the Center for International Blood & Marrow Transplant Research (CIBMTR) and the American Society for Blood and Marrow Transplantation.
“The guidelines for autologous stem cell transplantation for surveillance for AML [acute myeloid leukemia] and MDS [myelodysplastic syndrome] need to be clearly formulated. We are doing 30,000 autologous transplants a year globally and these patients are at risk for the most feared cancer, which is leukemia and MDS, for which outcomes are very poor,” said Dr. Hashmi of the Mayo Clinic in Rochester, Minn.
The researchers examined data from auto-HCT patients with diagnoses of non-Hodgkin lymphoma (NHL), Hodgkin lymphoma, and multiple myeloma to determine the relative risks of developing AML and MDS. The study also explored which patient characteristics and aspects of the conditioning regimen might affect risk for later myeloid neoplasms.
In the dataset of 9,108 patients that Dr. Hashmi and his colleagues obtained from CIBMTR, 3,540 patients had NHL.
“As age progresses, the risk of acquiring myeloid neoplasms increases significantly,” he said, noting that the relative risk (RR) rose to 4.52 for patients aged 55 years and older at the time of transplant (95% confidence interval [CI], 2.63-7.77; P less than .0001).
Patients with NHL who received more than two lines of chemotherapy had approximately double the rate of myeloid cancers (RR, 1.93; 95% CI, 1.34-2.78; P = .0004).
The type of conditioning regimen made a difference for NHL patients as well. With total-body irradiation set as the reference at RR = 1, carmustine-etoposide-cytarabine-melphalan (BEAM) or similar therapies were relatively protective, with an RR of 0.59 (95% CI, 0.40-0.87; P = .0083). Also protective were cyclophosphamide-carmustine-etoposide (CBV) and similar therapies (RR, 0.57; 95% CI, 0.33-0.99; P = .0463).
Age at transplant was a factor among the 4,653 patients with multiple myeloma, with an RR of 2.47 for those transplanted at age 55 years or older (95% CI, 1.55-3.93; P = .0001). Multiple lines of chemotherapy also increased risk, with patients who received more than two lines having an RR of 1.77 for neoplasm (95% CI, 0.04-2.06; P = .0302). Women had less than half the risk of recurrence as men (RR, 0.44; 95% CI, 0.28-0.69; P = .0003).
Among the 915 study patients with Hodgkin lymphoma, patients aged 45 years and older at the time of transplant carried an RR of 5.59 for new myeloid neoplasms (95% CI, 2.98-11.70; P less than .0001).
Total-body irradiation was received by 14% of patients with non-Hodgkin lymphoma and by 5% of patients with multiple myeloma and Hodgkin lymphoma. Total-body irradiation was associated with a fourfold increase in neoplasm risk (RR, 4.02; 95% CI, 1.40-11.55; P = .0096).
Dr. Hashmi and his colleagues then examined the incidence rates for myelodysplastic syndrome and acute myelogenous leukemia in the Surveillance, Epidemiology, and End Results (SEER) database , finding that, even at baseline, the rates of myeloid neoplasms were higher for patients with NHL, Hodgkin lymphoma, or MM patients than for the general population of cancer survivors. “Post NHL, Hodgkin lymphoma, and myeloma, the risks are significantly higher to begin with. … We saw a high risk of AML and MDS compared to the SEER controls – risks as high as 100 times greater for auto-transplant patients,” said Dr. Hashmi. “A risk of one hundred times more for MDS was astounding, surprising, unexpected,” he said. The risk of AML, he said, was elevated about 10-50 times in the CIBMTR data.
The cumulative incidence of MDS or AML for NHL was 6% at 10 years post transplant, 4% for Hodgkin lymphoma, and 3% for multiple myeloma.
A limitation of the study, said Dr. Hashmi, was that the investigators did not assess for post-transplant maintenance chemotherapy.
“We have to prospectively assess our transplant patients in a fashion to detect changes early. Or maybe they were present at the time of transplant and we never did sophisticated methods [like] next-generation sequencing” to detect them, he said.
Dr. Hashmi reported no conflicts of interest.
[email protected]
On Twitter @karioakes
AT THE BMT TANDEM MEETINGS
Key clinical point:
Major finding: The 10-year cumulative risk for auto-HCT patients with Hodgkin or non-Hodgkin lymphoma or multiple myeloma was as high at 6%.
Data source: Review of 9,108 patients from an international transplant database.
Disclosures: Dr. Hashmi reported no conflicts of interest.