User login
Could an oral PCSK9 inhibitor be on the horizon?
The investigational PCSK9 inhibitor that Merck showcased recently would be more than a “me-too” drug if it ultimately wins approval, despite competition from several approved agents that slash elevated cholesterol levels by targeting the same protein.
In fact, it would be something of a breakthrough. The new agent under study – now called MK-0616 – comes in pill form, in contrast to the three currently available PCSK9-lowering drugs that must be given in injections separated by weeks to months.
The drug faces an uncertain road to regulatory review and any approval, but MK-0616 at least seems to be starting out in the right direction.
In two phase 1 studies with a total of 100 participants, plasma PCSK9 levels plunged more than 90% after a single dose of the drug; and low-density-lipoprotein cholesterol (LDL-C) levels dropped about 65% when MK-0616 was given daily for 2 weeks on a background of statin therapy.
Moreover, “MK-0616 was generally well tolerated at up to and including single doses of 300 milligrams,” the maximum tested in the studies, Douglas G. Johns, PhD, reported at the virtual American Heart Association scientific sessions.
The collective results from the oral agent’s earliest human experience are “definitely encouraging” and support MK-0616 as a potential LDL-lowering agent that would be more convenient and arguably more accessible to patients compared to current injectable PCSK9 inhibitors, proposed Dr. Johns, clinical director of translational medicine for Merck in Kenilworth, N.J.
Available PCSK9-targeting agents include alirocumab (Praluent, Sanofi/Regeneron), Food and Drug Administration–approved in July 2015, and evolocumab (Repatha, Amgen), approved by the agency the following month. Both are monoclonal antibodies with neutralizing specificity for the PCSK9 protein; whereas the third such agent, inclisiran (Leqvio, Novartis) is a small-molecule interfering-RNA that suppresses PCSK9 synthesis. Inclisiran is approved in the European Union but its case to the FDA was turned down in 2020.
Dr. Johns said MK-0616 is a cyclic peptide that is “about one-hundredth the size of a monoclonal antibody, but we’re able to achieve monoclonal antibody-like potency and selectivity with this much smaller footprint.”
Added to statin therapy, the current PCSK9-targeting agents reduce LDL-C by an additional one-half or more, and the two antibody-based agents “also decrease atherosclerotic cardiovascular events. They are, however, expensive and not always available, requiring insurance or other approval,” observed Anne C. Goldberg, MD, as invited discussant after Dr. Johns’ presentation.
“They require every 2- to 4-week injections. They’re generally reserved for secondary prevention, and sometimes primary prevention as in familial hypercholesterolemia,” said Dr. Goldberg, of Washington University, St. Louis. Inclisiran, she noted, requires injections every 6 months and has yet to show its mettle in cardiovascular outcomes trials.
“Certainly, an oral form would be easier to use,” she said. “This would be particularly helpful in patients averse to injections,” especially, perhaps, in children. “Children with familial hypercholesterolemia could benefit with greater cholesterol lowering and might be better off with a pill than an injection.” That would be good reason to emphasize the enrollment of children in the drug’s upcoming clinical trials, Dr. Goldberg said.
But cost could potentially become restrictive for MK-0616 as well, should it ever be approved. “If it’s priced too high, then are you really going to see the increased use?” she posed. “Certainly, there’s a high bar for therapies that are add-on to statins in terms of cost effectiveness.”
In the first of the two trials, 60 predominantly White male participants aged 50 or younger were randomly assigned to receive a single dose of MK-0616, at different levels ranging from 10 mg to 300 mg, or placebo. They subsequently crossed over to a different group for a second round of dosing. Both times, three participants took the drug for every one who received placebo.
Participants who took the active drug, regardless of dosage, showed greater than 90% reductions in circulating PCSK9 levels compared to baseline. Six participants discontinued the study before its completion.
In the second trial, 40 White adults aged 65 or younger (mean, 58), including 13 women, with LDL-C of 60 mg/dL to 160 mg/dL (mean, 87 mg/dL) on statin therapy for at least 3 months were randomly assigned 3-to-1 to add-on MK-0616, either 10 mg or 20 mg daily, or placebo for 14 days.
LDL-C levels fell an average of about 65% over the 2 weeks among those taking the active drug; they declined less than 5% for those who took placebo.
There were no deaths or serious adverse events in either trial, Dr. Johns reported. On the other hand, pharmacokinetics studies showed that exposure to the drug fell by “about 50%-60%” when dosing was preceded by food intake within the previous 30 minutes. “However, if a meal is consumed 30 minutes after the dose, this food effect is much, much less prominent, almost negligible.”
These preliminary results show the drug is “orally bioavailable and exerts a clinically meaningful effect,” Dr. Johns said. “However, there’s definitely more to be done. And we are planning the next phase of clinical development, a phase 2 trial, sometime next year.”
The research was funded by Merck. Dr. Johns disclosed employment with and equity ownership in Merck, as did all the study’s coauthors. Dr. Goldberg disclosed holding research contracts through her institution with Regeneron/Sanofi-Aventis, Amarin, Amgen, Pfizer, IONIS/Akcea, Regeneron, Novartis, Arrowroot Pharmaceuticals, and the FH Foundation; and consulting for Novartis, Akcea, Regeneron, and Esperion.
A version of this article first appeared on Medscape.com.
The investigational PCSK9 inhibitor that Merck showcased recently would be more than a “me-too” drug if it ultimately wins approval, despite competition from several approved agents that slash elevated cholesterol levels by targeting the same protein.
In fact, it would be something of a breakthrough. The new agent under study – now called MK-0616 – comes in pill form, in contrast to the three currently available PCSK9-lowering drugs that must be given in injections separated by weeks to months.
The drug faces an uncertain road to regulatory review and any approval, but MK-0616 at least seems to be starting out in the right direction.
In two phase 1 studies with a total of 100 participants, plasma PCSK9 levels plunged more than 90% after a single dose of the drug; and low-density-lipoprotein cholesterol (LDL-C) levels dropped about 65% when MK-0616 was given daily for 2 weeks on a background of statin therapy.
Moreover, “MK-0616 was generally well tolerated at up to and including single doses of 300 milligrams,” the maximum tested in the studies, Douglas G. Johns, PhD, reported at the virtual American Heart Association scientific sessions.
The collective results from the oral agent’s earliest human experience are “definitely encouraging” and support MK-0616 as a potential LDL-lowering agent that would be more convenient and arguably more accessible to patients compared to current injectable PCSK9 inhibitors, proposed Dr. Johns, clinical director of translational medicine for Merck in Kenilworth, N.J.
Available PCSK9-targeting agents include alirocumab (Praluent, Sanofi/Regeneron), Food and Drug Administration–approved in July 2015, and evolocumab (Repatha, Amgen), approved by the agency the following month. Both are monoclonal antibodies with neutralizing specificity for the PCSK9 protein; whereas the third such agent, inclisiran (Leqvio, Novartis) is a small-molecule interfering-RNA that suppresses PCSK9 synthesis. Inclisiran is approved in the European Union but its case to the FDA was turned down in 2020.
Dr. Johns said MK-0616 is a cyclic peptide that is “about one-hundredth the size of a monoclonal antibody, but we’re able to achieve monoclonal antibody-like potency and selectivity with this much smaller footprint.”
Added to statin therapy, the current PCSK9-targeting agents reduce LDL-C by an additional one-half or more, and the two antibody-based agents “also decrease atherosclerotic cardiovascular events. They are, however, expensive and not always available, requiring insurance or other approval,” observed Anne C. Goldberg, MD, as invited discussant after Dr. Johns’ presentation.
“They require every 2- to 4-week injections. They’re generally reserved for secondary prevention, and sometimes primary prevention as in familial hypercholesterolemia,” said Dr. Goldberg, of Washington University, St. Louis. Inclisiran, she noted, requires injections every 6 months and has yet to show its mettle in cardiovascular outcomes trials.
“Certainly, an oral form would be easier to use,” she said. “This would be particularly helpful in patients averse to injections,” especially, perhaps, in children. “Children with familial hypercholesterolemia could benefit with greater cholesterol lowering and might be better off with a pill than an injection.” That would be good reason to emphasize the enrollment of children in the drug’s upcoming clinical trials, Dr. Goldberg said.
But cost could potentially become restrictive for MK-0616 as well, should it ever be approved. “If it’s priced too high, then are you really going to see the increased use?” she posed. “Certainly, there’s a high bar for therapies that are add-on to statins in terms of cost effectiveness.”
In the first of the two trials, 60 predominantly White male participants aged 50 or younger were randomly assigned to receive a single dose of MK-0616, at different levels ranging from 10 mg to 300 mg, or placebo. They subsequently crossed over to a different group for a second round of dosing. Both times, three participants took the drug for every one who received placebo.
Participants who took the active drug, regardless of dosage, showed greater than 90% reductions in circulating PCSK9 levels compared to baseline. Six participants discontinued the study before its completion.
In the second trial, 40 White adults aged 65 or younger (mean, 58), including 13 women, with LDL-C of 60 mg/dL to 160 mg/dL (mean, 87 mg/dL) on statin therapy for at least 3 months were randomly assigned 3-to-1 to add-on MK-0616, either 10 mg or 20 mg daily, or placebo for 14 days.
LDL-C levels fell an average of about 65% over the 2 weeks among those taking the active drug; they declined less than 5% for those who took placebo.
There were no deaths or serious adverse events in either trial, Dr. Johns reported. On the other hand, pharmacokinetics studies showed that exposure to the drug fell by “about 50%-60%” when dosing was preceded by food intake within the previous 30 minutes. “However, if a meal is consumed 30 minutes after the dose, this food effect is much, much less prominent, almost negligible.”
These preliminary results show the drug is “orally bioavailable and exerts a clinically meaningful effect,” Dr. Johns said. “However, there’s definitely more to be done. And we are planning the next phase of clinical development, a phase 2 trial, sometime next year.”
The research was funded by Merck. Dr. Johns disclosed employment with and equity ownership in Merck, as did all the study’s coauthors. Dr. Goldberg disclosed holding research contracts through her institution with Regeneron/Sanofi-Aventis, Amarin, Amgen, Pfizer, IONIS/Akcea, Regeneron, Novartis, Arrowroot Pharmaceuticals, and the FH Foundation; and consulting for Novartis, Akcea, Regeneron, and Esperion.
A version of this article first appeared on Medscape.com.
The investigational PCSK9 inhibitor that Merck showcased recently would be more than a “me-too” drug if it ultimately wins approval, despite competition from several approved agents that slash elevated cholesterol levels by targeting the same protein.
In fact, it would be something of a breakthrough. The new agent under study – now called MK-0616 – comes in pill form, in contrast to the three currently available PCSK9-lowering drugs that must be given in injections separated by weeks to months.
The drug faces an uncertain road to regulatory review and any approval, but MK-0616 at least seems to be starting out in the right direction.
In two phase 1 studies with a total of 100 participants, plasma PCSK9 levels plunged more than 90% after a single dose of the drug; and low-density-lipoprotein cholesterol (LDL-C) levels dropped about 65% when MK-0616 was given daily for 2 weeks on a background of statin therapy.
Moreover, “MK-0616 was generally well tolerated at up to and including single doses of 300 milligrams,” the maximum tested in the studies, Douglas G. Johns, PhD, reported at the virtual American Heart Association scientific sessions.
The collective results from the oral agent’s earliest human experience are “definitely encouraging” and support MK-0616 as a potential LDL-lowering agent that would be more convenient and arguably more accessible to patients compared to current injectable PCSK9 inhibitors, proposed Dr. Johns, clinical director of translational medicine for Merck in Kenilworth, N.J.
Available PCSK9-targeting agents include alirocumab (Praluent, Sanofi/Regeneron), Food and Drug Administration–approved in July 2015, and evolocumab (Repatha, Amgen), approved by the agency the following month. Both are monoclonal antibodies with neutralizing specificity for the PCSK9 protein; whereas the third such agent, inclisiran (Leqvio, Novartis) is a small-molecule interfering-RNA that suppresses PCSK9 synthesis. Inclisiran is approved in the European Union but its case to the FDA was turned down in 2020.
Dr. Johns said MK-0616 is a cyclic peptide that is “about one-hundredth the size of a monoclonal antibody, but we’re able to achieve monoclonal antibody-like potency and selectivity with this much smaller footprint.”
Added to statin therapy, the current PCSK9-targeting agents reduce LDL-C by an additional one-half or more, and the two antibody-based agents “also decrease atherosclerotic cardiovascular events. They are, however, expensive and not always available, requiring insurance or other approval,” observed Anne C. Goldberg, MD, as invited discussant after Dr. Johns’ presentation.
“They require every 2- to 4-week injections. They’re generally reserved for secondary prevention, and sometimes primary prevention as in familial hypercholesterolemia,” said Dr. Goldberg, of Washington University, St. Louis. Inclisiran, she noted, requires injections every 6 months and has yet to show its mettle in cardiovascular outcomes trials.
“Certainly, an oral form would be easier to use,” she said. “This would be particularly helpful in patients averse to injections,” especially, perhaps, in children. “Children with familial hypercholesterolemia could benefit with greater cholesterol lowering and might be better off with a pill than an injection.” That would be good reason to emphasize the enrollment of children in the drug’s upcoming clinical trials, Dr. Goldberg said.
But cost could potentially become restrictive for MK-0616 as well, should it ever be approved. “If it’s priced too high, then are you really going to see the increased use?” she posed. “Certainly, there’s a high bar for therapies that are add-on to statins in terms of cost effectiveness.”
In the first of the two trials, 60 predominantly White male participants aged 50 or younger were randomly assigned to receive a single dose of MK-0616, at different levels ranging from 10 mg to 300 mg, or placebo. They subsequently crossed over to a different group for a second round of dosing. Both times, three participants took the drug for every one who received placebo.
Participants who took the active drug, regardless of dosage, showed greater than 90% reductions in circulating PCSK9 levels compared to baseline. Six participants discontinued the study before its completion.
In the second trial, 40 White adults aged 65 or younger (mean, 58), including 13 women, with LDL-C of 60 mg/dL to 160 mg/dL (mean, 87 mg/dL) on statin therapy for at least 3 months were randomly assigned 3-to-1 to add-on MK-0616, either 10 mg or 20 mg daily, or placebo for 14 days.
LDL-C levels fell an average of about 65% over the 2 weeks among those taking the active drug; they declined less than 5% for those who took placebo.
There were no deaths or serious adverse events in either trial, Dr. Johns reported. On the other hand, pharmacokinetics studies showed that exposure to the drug fell by “about 50%-60%” when dosing was preceded by food intake within the previous 30 minutes. “However, if a meal is consumed 30 minutes after the dose, this food effect is much, much less prominent, almost negligible.”
These preliminary results show the drug is “orally bioavailable and exerts a clinically meaningful effect,” Dr. Johns said. “However, there’s definitely more to be done. And we are planning the next phase of clinical development, a phase 2 trial, sometime next year.”
The research was funded by Merck. Dr. Johns disclosed employment with and equity ownership in Merck, as did all the study’s coauthors. Dr. Goldberg disclosed holding research contracts through her institution with Regeneron/Sanofi-Aventis, Amarin, Amgen, Pfizer, IONIS/Akcea, Regeneron, Novartis, Arrowroot Pharmaceuticals, and the FH Foundation; and consulting for Novartis, Akcea, Regeneron, and Esperion.
A version of this article first appeared on Medscape.com.
FROM AHA 2021
U.S. obesity rates soar in early adulthood
Obesity rates among “emerging adults” aged 18-25 have soared in the United States in recent decades with the mean body mass index (BMI) for these young adults now in the overweight category, according to research highlighting troubling trends in an often-overlooked age group.
While similar patterns have been observed in other age groups, including adolescents (ages 12-19) and young adults (ages 20-39) across recent decades, emerging adulthood tends to get less attention in the evaluation of obesity trends.
“Emerging adulthood may be a key period for preventing and treating obesity given that habits formed during this period often persist through the remainder of the life course,” write the authors of the study, which was published online Nov. 23 in JAMA.
“There is an urgent need for research on risk factors contributing to obesity during this developmental stage to inform the design of interventions as well as policies aimed at prevention,” they add.
They found that by 2018 a third of all young adults had obesity, compared with just 6% at the beginning of the study periods in 1976.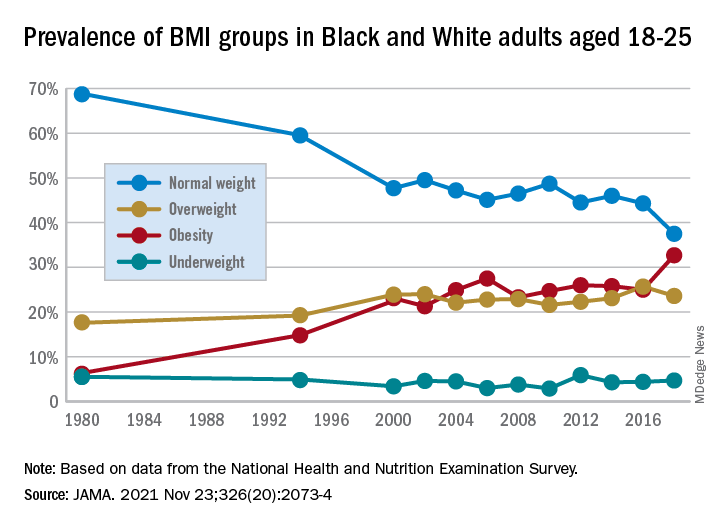
Studying the ages of transition
The findings are from an analysis of 8,015 emerging adults aged 18-25 in the cross-sectional National Health and Nutrition Examination Survey (NHANES), including NHANES II (1976-1980), NHANES III (1988-1994), and the continuous NHANES cycles from 1999 through 2018.
About half (3,965) of participants were female, 3,037 were non-Hispanic Black, and 2,386 met the criteria for household poverty.
The results showed substantial increases in mean BMI among emerging adults from a level in the normal range, at 23.1 kg/m2, in 1976-1980, increasing to 27.7 kg/m2 (overweight) in 2017-2018 (P = .006).
The prevalence of obesity (BMI 30.0 kg/m2 or higher) in the emerging adult age group soared from 6.2% between 1976-1980 to 32.7% in 2017-2018 (P = .007).
Meanwhile, the rate of those with normal/healthy weight (BMI 18.5-24.9 kg/m2) dropped from 68.7% to 37.5% (P = .005) over the same period.
Sensitivity analyses that were limited to continuous NHANES cycles showed similar results.
First author Alejandra Ellison-Barnes, MD, MPH, said the trends are consistent with rising obesity rates in the population as a whole – other studies have shown increases in obesity among children, adolescents, and adults over the same period – but are nevertheless striking, she stressed.
Young adults now fall into overweight category
“While we were not surprised by the general trend, given what is known about the increasing prevalence of obesity in both children and adults, we were surprised by the magnitude of the increase in prevalence and that the mean BMI in this age group now falls in the overweight range,” Dr. Ellison-Barnes, of the Division of General Internal Medicine, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
She said she is not aware of other studies that have looked at obesity trends specifically among emerging adults.
However, considering the substantial life changes and growing independence, the life stage is important to understand in terms of dietary/lifestyle patterns.
“We theorize that emerging adulthood is a critical period for obesity development given that it is a time when individuals are often undergoing major life transitions such as leaving home, attending higher education, entering the workforce, and developing new relationships,” she emphasized.
As far as causes are concerned, “societal and cultural trends in these areas over the past several decades may have played a role in the observed changes,” she speculated.
The study population was limited to non-Hispanic Black and non-Hispanic White individuals due to changes in how NHANES assessed race and ethnicity over time. Therefore, a study limitation is that the patterns observed may not be generalizable to other races and ethnicities, the authors note.
However, considering the influence lifestyle changes can have, early adulthood “may be an ideal time to intervene in the clinical setting to prevent, manage, or reverse obesity to prevent adverse health outcomes in the future,” Dr. Ellison-Barnes said.
Dr. Ellison-Barnes has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Obesity rates among “emerging adults” aged 18-25 have soared in the United States in recent decades with the mean body mass index (BMI) for these young adults now in the overweight category, according to research highlighting troubling trends in an often-overlooked age group.
While similar patterns have been observed in other age groups, including adolescents (ages 12-19) and young adults (ages 20-39) across recent decades, emerging adulthood tends to get less attention in the evaluation of obesity trends.
“Emerging adulthood may be a key period for preventing and treating obesity given that habits formed during this period often persist through the remainder of the life course,” write the authors of the study, which was published online Nov. 23 in JAMA.
“There is an urgent need for research on risk factors contributing to obesity during this developmental stage to inform the design of interventions as well as policies aimed at prevention,” they add.
They found that by 2018 a third of all young adults had obesity, compared with just 6% at the beginning of the study periods in 1976.
Studying the ages of transition
The findings are from an analysis of 8,015 emerging adults aged 18-25 in the cross-sectional National Health and Nutrition Examination Survey (NHANES), including NHANES II (1976-1980), NHANES III (1988-1994), and the continuous NHANES cycles from 1999 through 2018.
About half (3,965) of participants were female, 3,037 were non-Hispanic Black, and 2,386 met the criteria for household poverty.
The results showed substantial increases in mean BMI among emerging adults from a level in the normal range, at 23.1 kg/m2, in 1976-1980, increasing to 27.7 kg/m2 (overweight) in 2017-2018 (P = .006).
The prevalence of obesity (BMI 30.0 kg/m2 or higher) in the emerging adult age group soared from 6.2% between 1976-1980 to 32.7% in 2017-2018 (P = .007).
Meanwhile, the rate of those with normal/healthy weight (BMI 18.5-24.9 kg/m2) dropped from 68.7% to 37.5% (P = .005) over the same period.
Sensitivity analyses that were limited to continuous NHANES cycles showed similar results.
First author Alejandra Ellison-Barnes, MD, MPH, said the trends are consistent with rising obesity rates in the population as a whole – other studies have shown increases in obesity among children, adolescents, and adults over the same period – but are nevertheless striking, she stressed.
Young adults now fall into overweight category
“While we were not surprised by the general trend, given what is known about the increasing prevalence of obesity in both children and adults, we were surprised by the magnitude of the increase in prevalence and that the mean BMI in this age group now falls in the overweight range,” Dr. Ellison-Barnes, of the Division of General Internal Medicine, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
She said she is not aware of other studies that have looked at obesity trends specifically among emerging adults.
However, considering the substantial life changes and growing independence, the life stage is important to understand in terms of dietary/lifestyle patterns.
“We theorize that emerging adulthood is a critical period for obesity development given that it is a time when individuals are often undergoing major life transitions such as leaving home, attending higher education, entering the workforce, and developing new relationships,” she emphasized.
As far as causes are concerned, “societal and cultural trends in these areas over the past several decades may have played a role in the observed changes,” she speculated.
The study population was limited to non-Hispanic Black and non-Hispanic White individuals due to changes in how NHANES assessed race and ethnicity over time. Therefore, a study limitation is that the patterns observed may not be generalizable to other races and ethnicities, the authors note.
However, considering the influence lifestyle changes can have, early adulthood “may be an ideal time to intervene in the clinical setting to prevent, manage, or reverse obesity to prevent adverse health outcomes in the future,” Dr. Ellison-Barnes said.
Dr. Ellison-Barnes has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Obesity rates among “emerging adults” aged 18-25 have soared in the United States in recent decades with the mean body mass index (BMI) for these young adults now in the overweight category, according to research highlighting troubling trends in an often-overlooked age group.
While similar patterns have been observed in other age groups, including adolescents (ages 12-19) and young adults (ages 20-39) across recent decades, emerging adulthood tends to get less attention in the evaluation of obesity trends.
“Emerging adulthood may be a key period for preventing and treating obesity given that habits formed during this period often persist through the remainder of the life course,” write the authors of the study, which was published online Nov. 23 in JAMA.
“There is an urgent need for research on risk factors contributing to obesity during this developmental stage to inform the design of interventions as well as policies aimed at prevention,” they add.
They found that by 2018 a third of all young adults had obesity, compared with just 6% at the beginning of the study periods in 1976.
Studying the ages of transition
The findings are from an analysis of 8,015 emerging adults aged 18-25 in the cross-sectional National Health and Nutrition Examination Survey (NHANES), including NHANES II (1976-1980), NHANES III (1988-1994), and the continuous NHANES cycles from 1999 through 2018.
About half (3,965) of participants were female, 3,037 were non-Hispanic Black, and 2,386 met the criteria for household poverty.
The results showed substantial increases in mean BMI among emerging adults from a level in the normal range, at 23.1 kg/m2, in 1976-1980, increasing to 27.7 kg/m2 (overweight) in 2017-2018 (P = .006).
The prevalence of obesity (BMI 30.0 kg/m2 or higher) in the emerging adult age group soared from 6.2% between 1976-1980 to 32.7% in 2017-2018 (P = .007).
Meanwhile, the rate of those with normal/healthy weight (BMI 18.5-24.9 kg/m2) dropped from 68.7% to 37.5% (P = .005) over the same period.
Sensitivity analyses that were limited to continuous NHANES cycles showed similar results.
First author Alejandra Ellison-Barnes, MD, MPH, said the trends are consistent with rising obesity rates in the population as a whole – other studies have shown increases in obesity among children, adolescents, and adults over the same period – but are nevertheless striking, she stressed.
Young adults now fall into overweight category
“While we were not surprised by the general trend, given what is known about the increasing prevalence of obesity in both children and adults, we were surprised by the magnitude of the increase in prevalence and that the mean BMI in this age group now falls in the overweight range,” Dr. Ellison-Barnes, of the Division of General Internal Medicine, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
She said she is not aware of other studies that have looked at obesity trends specifically among emerging adults.
However, considering the substantial life changes and growing independence, the life stage is important to understand in terms of dietary/lifestyle patterns.
“We theorize that emerging adulthood is a critical period for obesity development given that it is a time when individuals are often undergoing major life transitions such as leaving home, attending higher education, entering the workforce, and developing new relationships,” she emphasized.
As far as causes are concerned, “societal and cultural trends in these areas over the past several decades may have played a role in the observed changes,” she speculated.
The study population was limited to non-Hispanic Black and non-Hispanic White individuals due to changes in how NHANES assessed race and ethnicity over time. Therefore, a study limitation is that the patterns observed may not be generalizable to other races and ethnicities, the authors note.
However, considering the influence lifestyle changes can have, early adulthood “may be an ideal time to intervene in the clinical setting to prevent, manage, or reverse obesity to prevent adverse health outcomes in the future,” Dr. Ellison-Barnes said.
Dr. Ellison-Barnes has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In pill or food form, healthy fatty acids reduce liver fat
For patients with nonalcoholic fatty liver disease (NAFLD) who supplement their diets with polyunsaturated fatty acids (PUFA), liver and metabolic parameters improve, results of a systematic review and meta-analysis suggest.
Data from randomized clinical trials show that, for participants with NAFLD who used PUFA supplements with or without additional dietary interventions, hepatic steatosis and lobular inflammation decreased, and in one study, fibrosis decreased. There were also improvements in liver enzyme levels, said Saleh Alqahtani, MBChB, associate professor of medicine at Johns Hopkins University, Baltimore, during a presentation at the annual meeting of the American Association for the Study of Liver Diseases.
“Since there’s no effective medical therapy for NAFLD, weight loss through lifestyle modifications becomes the most important focused intervention for patients with NAFLD,” he said. “However, the majority of patients fail to achieve or to maintain weight loss for long-term therapy. Therefore, dietary intervention or supplementation might help reduce the prevalence of NAFLD and decrease the progression of nonalcoholic steatohepatitis [NASH] and liver cirrhosis.
“More clinical trials are warranted to determine the long-term efficacy of the Mediterranean diet and polyunsaturated fatty acid supplementation among adult patients with NAFLD,” he added.
RCTs and case-control studies
It’s well documented that consumption of PUFAs, found in fatty fish and in canola, grapeseed, corn, and soybean oils, as well as monounsaturated fatty acids, found in olive oil and peanut oil, can contribute to improvement of NALFD, Dr. Alqahtani said.
In contrast, foods high in saturated fatty acids, such as butter, as well as trans fats and cholesterol can contribute to NAFLD progression, he said.
In their studies of intrahepatic triglyceride content, Dr. Alqahtani and colleagues found that fatty acids in the liver come from three major sources: dietary fatty acids, which account for about 15% of liver fat, tissue lipolysis, and de novo hepatic lipogenesis.
Previous systematic reviews and meta-analyses of the relationship between diet and NAFLD have focused on marine-based (n-3) PUFAs, but “the data regarding the evidence of unsaturated fatty acids through supplements or monounsaturated fatty acids through dietary supplementation are lacking,” he said.
To summarize the effects of dietary or supplemental fatty acids on liver and metabolic parameters in adults with NAFLD, Dr. Alqahtani and colleagues conducted a systematic review and meta-analysis, concentrating on studies that included specifics about interventions and outcomes.
They identified a total of 18 randomized controlled trials and 4 case-control studies that met their criteria. The studies were published from 2008 to 2020.
Regarding the effects of interventions on the components of NASH, they found that, in 1 or more of 12 randomized trials of PUFA supplementation with or without dietary interventions, there were associations with decreased hepatic steatosis, lobular inflammation, and fibrosis and declines in ALT and AST levels.
In three trials of dietary-only interventions, there were decreases in hepatic steatosis and ALT and/or AST levels. In two studies of the effects of healthy cooking oils only, hepatic steatosis decreased, but there was no effect on ALT or AST levels.
All three interventions were associated with improvements in fasting glucose levels and insulin metabolism, as well as decreases in total cholesterol, triglycerides, and LDL cholesterol and increases in HDL cholesterol.
Better understanding of dietary composition
“We’ve known for a while that dietary composition may impact NAFLD and NASH,” said Manal F. Abdelmalek, MD, professor of medicine at Duke University, Durham, N.C., who commented on the study.
“What [Dr. Alqahtani and colleagues] have shown is that supplementation with healthy fatty acids improves fatty liver. This really does extend our knowledge of what we understand about dietary composition, particularly the recommendations that support higher fish consumption and a Mediterranean-style diet,” she said.
“It’s not just about the fat but the type of fat that’s consumed, and drilling down to the particulars of dietary composition beyond calories alone,” she added.
No source of funding for the study has been disclosed. Dr. Alqahtani and Dr. Abdelmalek have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For patients with nonalcoholic fatty liver disease (NAFLD) who supplement their diets with polyunsaturated fatty acids (PUFA), liver and metabolic parameters improve, results of a systematic review and meta-analysis suggest.
Data from randomized clinical trials show that, for participants with NAFLD who used PUFA supplements with or without additional dietary interventions, hepatic steatosis and lobular inflammation decreased, and in one study, fibrosis decreased. There were also improvements in liver enzyme levels, said Saleh Alqahtani, MBChB, associate professor of medicine at Johns Hopkins University, Baltimore, during a presentation at the annual meeting of the American Association for the Study of Liver Diseases.
“Since there’s no effective medical therapy for NAFLD, weight loss through lifestyle modifications becomes the most important focused intervention for patients with NAFLD,” he said. “However, the majority of patients fail to achieve or to maintain weight loss for long-term therapy. Therefore, dietary intervention or supplementation might help reduce the prevalence of NAFLD and decrease the progression of nonalcoholic steatohepatitis [NASH] and liver cirrhosis.
“More clinical trials are warranted to determine the long-term efficacy of the Mediterranean diet and polyunsaturated fatty acid supplementation among adult patients with NAFLD,” he added.
RCTs and case-control studies
It’s well documented that consumption of PUFAs, found in fatty fish and in canola, grapeseed, corn, and soybean oils, as well as monounsaturated fatty acids, found in olive oil and peanut oil, can contribute to improvement of NALFD, Dr. Alqahtani said.
In contrast, foods high in saturated fatty acids, such as butter, as well as trans fats and cholesterol can contribute to NAFLD progression, he said.
In their studies of intrahepatic triglyceride content, Dr. Alqahtani and colleagues found that fatty acids in the liver come from three major sources: dietary fatty acids, which account for about 15% of liver fat, tissue lipolysis, and de novo hepatic lipogenesis.
Previous systematic reviews and meta-analyses of the relationship between diet and NAFLD have focused on marine-based (n-3) PUFAs, but “the data regarding the evidence of unsaturated fatty acids through supplements or monounsaturated fatty acids through dietary supplementation are lacking,” he said.
To summarize the effects of dietary or supplemental fatty acids on liver and metabolic parameters in adults with NAFLD, Dr. Alqahtani and colleagues conducted a systematic review and meta-analysis, concentrating on studies that included specifics about interventions and outcomes.
They identified a total of 18 randomized controlled trials and 4 case-control studies that met their criteria. The studies were published from 2008 to 2020.
Regarding the effects of interventions on the components of NASH, they found that, in 1 or more of 12 randomized trials of PUFA supplementation with or without dietary interventions, there were associations with decreased hepatic steatosis, lobular inflammation, and fibrosis and declines in ALT and AST levels.
In three trials of dietary-only interventions, there were decreases in hepatic steatosis and ALT and/or AST levels. In two studies of the effects of healthy cooking oils only, hepatic steatosis decreased, but there was no effect on ALT or AST levels.
All three interventions were associated with improvements in fasting glucose levels and insulin metabolism, as well as decreases in total cholesterol, triglycerides, and LDL cholesterol and increases in HDL cholesterol.
Better understanding of dietary composition
“We’ve known for a while that dietary composition may impact NAFLD and NASH,” said Manal F. Abdelmalek, MD, professor of medicine at Duke University, Durham, N.C., who commented on the study.
“What [Dr. Alqahtani and colleagues] have shown is that supplementation with healthy fatty acids improves fatty liver. This really does extend our knowledge of what we understand about dietary composition, particularly the recommendations that support higher fish consumption and a Mediterranean-style diet,” she said.
“It’s not just about the fat but the type of fat that’s consumed, and drilling down to the particulars of dietary composition beyond calories alone,” she added.
No source of funding for the study has been disclosed. Dr. Alqahtani and Dr. Abdelmalek have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For patients with nonalcoholic fatty liver disease (NAFLD) who supplement their diets with polyunsaturated fatty acids (PUFA), liver and metabolic parameters improve, results of a systematic review and meta-analysis suggest.
Data from randomized clinical trials show that, for participants with NAFLD who used PUFA supplements with or without additional dietary interventions, hepatic steatosis and lobular inflammation decreased, and in one study, fibrosis decreased. There were also improvements in liver enzyme levels, said Saleh Alqahtani, MBChB, associate professor of medicine at Johns Hopkins University, Baltimore, during a presentation at the annual meeting of the American Association for the Study of Liver Diseases.
“Since there’s no effective medical therapy for NAFLD, weight loss through lifestyle modifications becomes the most important focused intervention for patients with NAFLD,” he said. “However, the majority of patients fail to achieve or to maintain weight loss for long-term therapy. Therefore, dietary intervention or supplementation might help reduce the prevalence of NAFLD and decrease the progression of nonalcoholic steatohepatitis [NASH] and liver cirrhosis.
“More clinical trials are warranted to determine the long-term efficacy of the Mediterranean diet and polyunsaturated fatty acid supplementation among adult patients with NAFLD,” he added.
RCTs and case-control studies
It’s well documented that consumption of PUFAs, found in fatty fish and in canola, grapeseed, corn, and soybean oils, as well as monounsaturated fatty acids, found in olive oil and peanut oil, can contribute to improvement of NALFD, Dr. Alqahtani said.
In contrast, foods high in saturated fatty acids, such as butter, as well as trans fats and cholesterol can contribute to NAFLD progression, he said.
In their studies of intrahepatic triglyceride content, Dr. Alqahtani and colleagues found that fatty acids in the liver come from three major sources: dietary fatty acids, which account for about 15% of liver fat, tissue lipolysis, and de novo hepatic lipogenesis.
Previous systematic reviews and meta-analyses of the relationship between diet and NAFLD have focused on marine-based (n-3) PUFAs, but “the data regarding the evidence of unsaturated fatty acids through supplements or monounsaturated fatty acids through dietary supplementation are lacking,” he said.
To summarize the effects of dietary or supplemental fatty acids on liver and metabolic parameters in adults with NAFLD, Dr. Alqahtani and colleagues conducted a systematic review and meta-analysis, concentrating on studies that included specifics about interventions and outcomes.
They identified a total of 18 randomized controlled trials and 4 case-control studies that met their criteria. The studies were published from 2008 to 2020.
Regarding the effects of interventions on the components of NASH, they found that, in 1 or more of 12 randomized trials of PUFA supplementation with or without dietary interventions, there were associations with decreased hepatic steatosis, lobular inflammation, and fibrosis and declines in ALT and AST levels.
In three trials of dietary-only interventions, there were decreases in hepatic steatosis and ALT and/or AST levels. In two studies of the effects of healthy cooking oils only, hepatic steatosis decreased, but there was no effect on ALT or AST levels.
All three interventions were associated with improvements in fasting glucose levels and insulin metabolism, as well as decreases in total cholesterol, triglycerides, and LDL cholesterol and increases in HDL cholesterol.
Better understanding of dietary composition
“We’ve known for a while that dietary composition may impact NAFLD and NASH,” said Manal F. Abdelmalek, MD, professor of medicine at Duke University, Durham, N.C., who commented on the study.
“What [Dr. Alqahtani and colleagues] have shown is that supplementation with healthy fatty acids improves fatty liver. This really does extend our knowledge of what we understand about dietary composition, particularly the recommendations that support higher fish consumption and a Mediterranean-style diet,” she said.
“It’s not just about the fat but the type of fat that’s consumed, and drilling down to the particulars of dietary composition beyond calories alone,” she added.
No source of funding for the study has been disclosed. Dr. Alqahtani and Dr. Abdelmalek have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LIVER MEETING 2021
High triglycerides in normal-weight men with obstructive sleep apnea
, according to results of a study published in Nature and Science of Sleep (2021:13 1771-82).
Layla B. Guscoth, MD, of the South Australian Health and Medical Research Institute and Faculty of Health and Medical Sciences, University of Adelaide, Australia, and colleagues assessed unselected male community-dwelling participants in the Men Androgen Inflammation Lifestyle Environment and Stress (MAILES) and the Florey Adelaide Male Aging Study (FAMAS) studies.
They examined the association of OSA and nocturnal hypoxemia with serum lipid profiles, and suggested that the cardiometabolic risk profiles of healthy weight individuals with OSA require clinical attention, according to the researchers.
The partial or complete obstruction of upper airways found in the OSA syndrome results in intermittent hypoxia, accompanied variably by sleep fragmentation and daytime sleepiness. While the prevalence of moderate to severe OSA was 49.7% in the Swiss HypnoLaus cohort, it was 74.7% in men aged 40 or older (or having OSA syndrome according to ICD-3 criteria). Dr. Guscoth and colleagues point out, however, that OSA is frequently underdiagnosed or unrecognized in clinical settings, and that OSA has been implicated in development of cardiovascular conditions. Furthermore, the nocturnal hypoxemia resulting from OSA during rapid eye movement (REM) sleep is longitudinally associated with cardiovascular disease and its risk factors (hypertension, insulin resistance, metabolic syndrome and carotid atherosclerosis).
Study details
Prior research suggests that intermittent hypoxemia activates the sympathetic nervous system, increases oxidative stress and systemic inflammation, and that when chronic, reduces clearance of triglyceride-rich lipoproteins and inhibits adipose tissue lipoprotein lipase activity. To clarify inconsistent results in studies investigating potential OSA-dyslipidemia associations, and to confirm research suggesting an independent association with severe OSA (apnea-hypopnea index [AHI] ≥ 30/h), the authors conducted analyses stratified by waist circumference to observe an obesity-independent association between OSA metrics and dyslipidemia.
The investigators assessed 753 MAILES participants (mean age 60.8 years) who underwent full in-home polysomnography (Embletta X100). They looked at triglycerides, high- (HDL) and low-density lipoprotein (LDL), total cholesterol, associations between lipids and continuous measures of nocturnal hypoxemia (oxygen desaturation index [ODI], AHI, and REM-AHI), and adjusted for chronic conditions, risk behavior, and sociodemographic factors.
Mean waist circumference was 99.3 cm and OSA (AHI ≥ 10) prevalence was 52.6%. No significant associations were found between OSA metrics and lipid measures in an overall analysis, nor in a sensitivity analysis excluding lipid-lowering therapies.
In a covariate adjusted analysis stratified according to waist circumference (< 95 cm, 95-100 cm, > 100 cm) to minimize the contribution of obesity to hypertriglyceridemia, triglyceride levels were positively associated with AHI, ODI and REM-AHI in the participants with a waist circumference < 95 cm (P < .05), but not in participants with waist circumferences of 95-100 cm or > 100 cm.
Worse during REM
The authors observed also that OSA during REM sleep is marked by longer obstructive events with greater oxygen desaturations. Obstructive events during REM sleep, research has shown, may be more harmful than obstructive events during non-REM sleep with respect to hypertension, cardiovascular disease, and glycemic control in type 2 diabetes.
Looking at clinical categories of OSA, Dr. Guscoth and colleagues found that severe OSA was significantly associated with higher likelihood of triglyceride levels ≥ 1.7 mmol/L (odds ratio, 4.1, 95% confidence interval, 1.1-15.5, P = .039). Analysis according to waist circumference confirmed the relationship only among men with waist circumference < 95 cm.
Clinical concern
“We therefore suggest that with our data unstratified by weight circumference, metabolic derangements associated with insulin resistance induced by intermittent hypoxia due to OSA cannot be separated from the predominant effect of visceral obesity. When stratified by weight circumference, our data show that these derangements in triglycerides are observed only in lean participants where obesity does not have a dominant effect,” the researchers concluded.
“These findings of high prevalence of metabolic risk in lean patients with OSA, I find very worrying,” coauthor Sarah Appleton, PhD, Flinders Medical Center, Adelaide, Australia, said in an interview. She cited a study showing a 61% risk of dyslipidemia in lean patients with OSA (AHI > 5/hr, body mass index < 25 kg/m2, and waist < 80 cm in women, < 90 cm in men), and two of three metabolic syndrome components in 64%. “Annual fasting blood tests would identify metabolic problems such as elevated fasting glucose and triglyceride levels,” she noted.
This work was supported by a National Health and Medical Research Council of Australia Project Grant (627227), the Hospital Research Foundation and ResMed Foundation. There were no relevant conflicts reported.
, according to results of a study published in Nature and Science of Sleep (2021:13 1771-82).
Layla B. Guscoth, MD, of the South Australian Health and Medical Research Institute and Faculty of Health and Medical Sciences, University of Adelaide, Australia, and colleagues assessed unselected male community-dwelling participants in the Men Androgen Inflammation Lifestyle Environment and Stress (MAILES) and the Florey Adelaide Male Aging Study (FAMAS) studies.
They examined the association of OSA and nocturnal hypoxemia with serum lipid profiles, and suggested that the cardiometabolic risk profiles of healthy weight individuals with OSA require clinical attention, according to the researchers.
The partial or complete obstruction of upper airways found in the OSA syndrome results in intermittent hypoxia, accompanied variably by sleep fragmentation and daytime sleepiness. While the prevalence of moderate to severe OSA was 49.7% in the Swiss HypnoLaus cohort, it was 74.7% in men aged 40 or older (or having OSA syndrome according to ICD-3 criteria). Dr. Guscoth and colleagues point out, however, that OSA is frequently underdiagnosed or unrecognized in clinical settings, and that OSA has been implicated in development of cardiovascular conditions. Furthermore, the nocturnal hypoxemia resulting from OSA during rapid eye movement (REM) sleep is longitudinally associated with cardiovascular disease and its risk factors (hypertension, insulin resistance, metabolic syndrome and carotid atherosclerosis).
Study details
Prior research suggests that intermittent hypoxemia activates the sympathetic nervous system, increases oxidative stress and systemic inflammation, and that when chronic, reduces clearance of triglyceride-rich lipoproteins and inhibits adipose tissue lipoprotein lipase activity. To clarify inconsistent results in studies investigating potential OSA-dyslipidemia associations, and to confirm research suggesting an independent association with severe OSA (apnea-hypopnea index [AHI] ≥ 30/h), the authors conducted analyses stratified by waist circumference to observe an obesity-independent association between OSA metrics and dyslipidemia.
The investigators assessed 753 MAILES participants (mean age 60.8 years) who underwent full in-home polysomnography (Embletta X100). They looked at triglycerides, high- (HDL) and low-density lipoprotein (LDL), total cholesterol, associations between lipids and continuous measures of nocturnal hypoxemia (oxygen desaturation index [ODI], AHI, and REM-AHI), and adjusted for chronic conditions, risk behavior, and sociodemographic factors.
Mean waist circumference was 99.3 cm and OSA (AHI ≥ 10) prevalence was 52.6%. No significant associations were found between OSA metrics and lipid measures in an overall analysis, nor in a sensitivity analysis excluding lipid-lowering therapies.
In a covariate adjusted analysis stratified according to waist circumference (< 95 cm, 95-100 cm, > 100 cm) to minimize the contribution of obesity to hypertriglyceridemia, triglyceride levels were positively associated with AHI, ODI and REM-AHI in the participants with a waist circumference < 95 cm (P < .05), but not in participants with waist circumferences of 95-100 cm or > 100 cm.
Worse during REM
The authors observed also that OSA during REM sleep is marked by longer obstructive events with greater oxygen desaturations. Obstructive events during REM sleep, research has shown, may be more harmful than obstructive events during non-REM sleep with respect to hypertension, cardiovascular disease, and glycemic control in type 2 diabetes.
Looking at clinical categories of OSA, Dr. Guscoth and colleagues found that severe OSA was significantly associated with higher likelihood of triglyceride levels ≥ 1.7 mmol/L (odds ratio, 4.1, 95% confidence interval, 1.1-15.5, P = .039). Analysis according to waist circumference confirmed the relationship only among men with waist circumference < 95 cm.
Clinical concern
“We therefore suggest that with our data unstratified by weight circumference, metabolic derangements associated with insulin resistance induced by intermittent hypoxia due to OSA cannot be separated from the predominant effect of visceral obesity. When stratified by weight circumference, our data show that these derangements in triglycerides are observed only in lean participants where obesity does not have a dominant effect,” the researchers concluded.
“These findings of high prevalence of metabolic risk in lean patients with OSA, I find very worrying,” coauthor Sarah Appleton, PhD, Flinders Medical Center, Adelaide, Australia, said in an interview. She cited a study showing a 61% risk of dyslipidemia in lean patients with OSA (AHI > 5/hr, body mass index < 25 kg/m2, and waist < 80 cm in women, < 90 cm in men), and two of three metabolic syndrome components in 64%. “Annual fasting blood tests would identify metabolic problems such as elevated fasting glucose and triglyceride levels,” she noted.
This work was supported by a National Health and Medical Research Council of Australia Project Grant (627227), the Hospital Research Foundation and ResMed Foundation. There were no relevant conflicts reported.
, according to results of a study published in Nature and Science of Sleep (2021:13 1771-82).
Layla B. Guscoth, MD, of the South Australian Health and Medical Research Institute and Faculty of Health and Medical Sciences, University of Adelaide, Australia, and colleagues assessed unselected male community-dwelling participants in the Men Androgen Inflammation Lifestyle Environment and Stress (MAILES) and the Florey Adelaide Male Aging Study (FAMAS) studies.
They examined the association of OSA and nocturnal hypoxemia with serum lipid profiles, and suggested that the cardiometabolic risk profiles of healthy weight individuals with OSA require clinical attention, according to the researchers.
The partial or complete obstruction of upper airways found in the OSA syndrome results in intermittent hypoxia, accompanied variably by sleep fragmentation and daytime sleepiness. While the prevalence of moderate to severe OSA was 49.7% in the Swiss HypnoLaus cohort, it was 74.7% in men aged 40 or older (or having OSA syndrome according to ICD-3 criteria). Dr. Guscoth and colleagues point out, however, that OSA is frequently underdiagnosed or unrecognized in clinical settings, and that OSA has been implicated in development of cardiovascular conditions. Furthermore, the nocturnal hypoxemia resulting from OSA during rapid eye movement (REM) sleep is longitudinally associated with cardiovascular disease and its risk factors (hypertension, insulin resistance, metabolic syndrome and carotid atherosclerosis).
Study details
Prior research suggests that intermittent hypoxemia activates the sympathetic nervous system, increases oxidative stress and systemic inflammation, and that when chronic, reduces clearance of triglyceride-rich lipoproteins and inhibits adipose tissue lipoprotein lipase activity. To clarify inconsistent results in studies investigating potential OSA-dyslipidemia associations, and to confirm research suggesting an independent association with severe OSA (apnea-hypopnea index [AHI] ≥ 30/h), the authors conducted analyses stratified by waist circumference to observe an obesity-independent association between OSA metrics and dyslipidemia.
The investigators assessed 753 MAILES participants (mean age 60.8 years) who underwent full in-home polysomnography (Embletta X100). They looked at triglycerides, high- (HDL) and low-density lipoprotein (LDL), total cholesterol, associations between lipids and continuous measures of nocturnal hypoxemia (oxygen desaturation index [ODI], AHI, and REM-AHI), and adjusted for chronic conditions, risk behavior, and sociodemographic factors.
Mean waist circumference was 99.3 cm and OSA (AHI ≥ 10) prevalence was 52.6%. No significant associations were found between OSA metrics and lipid measures in an overall analysis, nor in a sensitivity analysis excluding lipid-lowering therapies.
In a covariate adjusted analysis stratified according to waist circumference (< 95 cm, 95-100 cm, > 100 cm) to minimize the contribution of obesity to hypertriglyceridemia, triglyceride levels were positively associated with AHI, ODI and REM-AHI in the participants with a waist circumference < 95 cm (P < .05), but not in participants with waist circumferences of 95-100 cm or > 100 cm.
Worse during REM
The authors observed also that OSA during REM sleep is marked by longer obstructive events with greater oxygen desaturations. Obstructive events during REM sleep, research has shown, may be more harmful than obstructive events during non-REM sleep with respect to hypertension, cardiovascular disease, and glycemic control in type 2 diabetes.
Looking at clinical categories of OSA, Dr. Guscoth and colleagues found that severe OSA was significantly associated with higher likelihood of triglyceride levels ≥ 1.7 mmol/L (odds ratio, 4.1, 95% confidence interval, 1.1-15.5, P = .039). Analysis according to waist circumference confirmed the relationship only among men with waist circumference < 95 cm.
Clinical concern
“We therefore suggest that with our data unstratified by weight circumference, metabolic derangements associated with insulin resistance induced by intermittent hypoxia due to OSA cannot be separated from the predominant effect of visceral obesity. When stratified by weight circumference, our data show that these derangements in triglycerides are observed only in lean participants where obesity does not have a dominant effect,” the researchers concluded.
“These findings of high prevalence of metabolic risk in lean patients with OSA, I find very worrying,” coauthor Sarah Appleton, PhD, Flinders Medical Center, Adelaide, Australia, said in an interview. She cited a study showing a 61% risk of dyslipidemia in lean patients with OSA (AHI > 5/hr, body mass index < 25 kg/m2, and waist < 80 cm in women, < 90 cm in men), and two of three metabolic syndrome components in 64%. “Annual fasting blood tests would identify metabolic problems such as elevated fasting glucose and triglyceride levels,” she noted.
This work was supported by a National Health and Medical Research Council of Australia Project Grant (627227), the Hospital Research Foundation and ResMed Foundation. There were no relevant conflicts reported.
FROM NATURE AND SCIENCE OF SLEEP
High-dose fish oil: ‘Intriguing’ results in COVID-19
A high dose of the purified form of eicosapentaenoic acid, icosapent ethyl (Vascepa, Amarin), failed to significantly reduce hospitalizations or death in patients infected with COVID-19 in the PREPARE-IT 2 study.
The study did, however, show a favorable trend, with a 16% reduction in the primary endpoint of death or an indication for hospitalization. All secondary endpoints were also numerically reduced, but none reached statistical significance.
The product was also well tolerated over the 28 days of the study period, even though a new high-loading dose was used, with no increase in atrial fibrillation or bleeding or other adverse events versus placebo, although there was a slightly higher rate of discontinuation.
The trial was presented at the American Heart Association scientific sessions on Nov. 15 by Rafael Díaz, MD, director of Estudios Clínicos Latinoamérica in Rosario, Argentina.
“Larger, randomized trials powered for a relative risk reduction of around 15% with icosapent ethyl are needed to establish whether or not this product may have a role in the management of COVID-positive outpatients,” Dr. Diaz concluded.
‘Intriguing signals’
Commenting on the study, Manesh Patel, MD, chief of the division of cardiology and codirector of the Heart Center at Duke University, Durham, N.C., and chair of the Scientific Sessions scientific program, said that: “Certainly there are some intriguing signals.”
“I think the trend is valuable, but do we need a larger trial to confirm a benefit? I will leave that to the clinical community to decide,” Dr. Patel added. “But it is hard to power a trial to get that answer, and the world of COVID has changed since this trial started with vaccines now available and new therapeutics coming. So, there’s going to be a competing landscape.”
Discussing the trial at an AHA news briefing, Erin Michos, MD, associate professor of medicine within the division of cardiology at Johns Hopkins University, Baltimore, said: “Results showed that everything trended in the right direction, but did not reach statistical significance largely because there were fewer events than anticipated. COVID hospitalizations are going down because of the broad adoption of vaccines, which meant that this study didn’t quite meet its endpoint.”
But, she added: “Reassuringly, even with the higher loading dose, there was no increased risk of [atrial fibrillation] when used for just 28 days, and no increased risk in bleeding, so there was very good safety.”
“We need a larger trial to really definitely show whether icosapent ethyl can or cannot help COVID-positive outpatients, but I think a better prevention strategy would be the broad adoption of vaccinations globally,” Dr. Michos concluded.
‘A pretty big ask’
Donald Lloyd-Jones, MD, AHA president and designated discussant at the late-breaking science session, congratulated the investigators on conducting “a very nice pragmatic trial in the midst of the COVID pandemic.”
Dr. Lloyd-Jones concluded that the broad range of potentially beneficial actions of icosapent ethyl – including antitriglyceride, anti-inflammatory, antioxidant, and antithrombotic effects – leads to the possibility of it helping in COVID, but he added that “this is a pretty big ask for a fish oil supplement given short term.”
Presenting the study, Dr. Diaz noted that there are limited options for the outpatient treatment of patients with COVID-19 infection, and it is believed that inflammation plays a major role in worsening the severity of the infection.
He pointed out that previous data support a potential role of omega-3 fatty acids in reducing inflammation and infection, and that icosapent ethyl has shown a reduction in major cardiovascular events in the REDUCE-IT trial, with the mechanism thought to involve anti-inflammatory effects.
In the first trial to investigate the role of icosapent ethyl in COVID-19, PREPARE-IT, the product did not prevent uninfected individuals at risk from COVID from becoming infected with the virus, but there was no increase in side effects versus placebo with use over a 60-day period.
A small study last year in 100 COVID-positive patients showed icosapent ethyl reduced C-reactive protein, an inflammatory marker, and also improved symptoms.
PREPARE-IT 2, a pragmatic web-based trial, was conducted to investigate whether icosapent ethyl in nonhospitalized patients with a positive diagnosis of COVID-19 could reduce hospitalization rates and complications.
The trial enrolled 2,052 patients (mean age, 50 years), of whom 1,010 were allocated to the active group and 1,042 to the placebo group. Inclusion criteria included individuals aged 40 years or older with a confirmed COVID-19 diagnosis and no more than 7 days from the onset of symptoms and without a clear indication for hospitalization.
Patients who were allocated to the active arm received icosapent ethyl at a dose of 8 g (four capsules every 12 hours, morning and evening) for the first 3 days, followed by 4 g (two capsules every 12 hours) thereafter (days 4-28).
The primary outcome, COVID-19–related hospitalization (indication for hospitalization or hospitalization) or death at 28 days, occurred in 11.16% of the active group and 13.69% of the placebo group, giving a hazard ratio of 0.84 (95% confidence interval, 0.65-1.08; P = .166)
Secondary outcomes showed similar positive trends, but none were significant. These included: death or still hospitalized at 28 days (HR, 0.74), major events (MI, stroke, death; HR, 0.38), and total mortality (HR, 0.52).
In terms of safety, there was no significant difference in total adverse events between the two groups (16.5% in the active group vs. 14.8% in the placebo group). The most common adverse effects were constipation (2.7%), diarrhea (7.2%), and nausea (4%), but these were not significantly different from placebo. There were, however, more discontinuations in the active group (7% vs. 4%).
Dr. Diaz pointed out that the PREPARE-IT 2 trial was started in May 2020, when there wasn’t much known about the COVID-19 condition, and there were no vaccines or treatments, so hospitalization rates were high.
“We were hoping to see a 25%-30% reduction in hospitalizations with icosapent ethyl, and the trial was powered for that sort of reduction, but today we know we can expect a more modest reduction of about 15%,” Dr. Diaz concluded. “But to show that, we need a much larger trial with 8,000 or 9,000 patients, and that will be much more difficult to conduct.”
The PREPARE-IT 2 study was funded by Amarin. Dr. Diaz has received grants from Dalcor, Amarin, PHRI, and Lepetit.
A version of this article first appeared on Medscape.com.
A high dose of the purified form of eicosapentaenoic acid, icosapent ethyl (Vascepa, Amarin), failed to significantly reduce hospitalizations or death in patients infected with COVID-19 in the PREPARE-IT 2 study.
The study did, however, show a favorable trend, with a 16% reduction in the primary endpoint of death or an indication for hospitalization. All secondary endpoints were also numerically reduced, but none reached statistical significance.
The product was also well tolerated over the 28 days of the study period, even though a new high-loading dose was used, with no increase in atrial fibrillation or bleeding or other adverse events versus placebo, although there was a slightly higher rate of discontinuation.
The trial was presented at the American Heart Association scientific sessions on Nov. 15 by Rafael Díaz, MD, director of Estudios Clínicos Latinoamérica in Rosario, Argentina.
“Larger, randomized trials powered for a relative risk reduction of around 15% with icosapent ethyl are needed to establish whether or not this product may have a role in the management of COVID-positive outpatients,” Dr. Diaz concluded.
‘Intriguing signals’
Commenting on the study, Manesh Patel, MD, chief of the division of cardiology and codirector of the Heart Center at Duke University, Durham, N.C., and chair of the Scientific Sessions scientific program, said that: “Certainly there are some intriguing signals.”
“I think the trend is valuable, but do we need a larger trial to confirm a benefit? I will leave that to the clinical community to decide,” Dr. Patel added. “But it is hard to power a trial to get that answer, and the world of COVID has changed since this trial started with vaccines now available and new therapeutics coming. So, there’s going to be a competing landscape.”
Discussing the trial at an AHA news briefing, Erin Michos, MD, associate professor of medicine within the division of cardiology at Johns Hopkins University, Baltimore, said: “Results showed that everything trended in the right direction, but did not reach statistical significance largely because there were fewer events than anticipated. COVID hospitalizations are going down because of the broad adoption of vaccines, which meant that this study didn’t quite meet its endpoint.”
But, she added: “Reassuringly, even with the higher loading dose, there was no increased risk of [atrial fibrillation] when used for just 28 days, and no increased risk in bleeding, so there was very good safety.”
“We need a larger trial to really definitely show whether icosapent ethyl can or cannot help COVID-positive outpatients, but I think a better prevention strategy would be the broad adoption of vaccinations globally,” Dr. Michos concluded.
‘A pretty big ask’
Donald Lloyd-Jones, MD, AHA president and designated discussant at the late-breaking science session, congratulated the investigators on conducting “a very nice pragmatic trial in the midst of the COVID pandemic.”
Dr. Lloyd-Jones concluded that the broad range of potentially beneficial actions of icosapent ethyl – including antitriglyceride, anti-inflammatory, antioxidant, and antithrombotic effects – leads to the possibility of it helping in COVID, but he added that “this is a pretty big ask for a fish oil supplement given short term.”
Presenting the study, Dr. Diaz noted that there are limited options for the outpatient treatment of patients with COVID-19 infection, and it is believed that inflammation plays a major role in worsening the severity of the infection.
He pointed out that previous data support a potential role of omega-3 fatty acids in reducing inflammation and infection, and that icosapent ethyl has shown a reduction in major cardiovascular events in the REDUCE-IT trial, with the mechanism thought to involve anti-inflammatory effects.
In the first trial to investigate the role of icosapent ethyl in COVID-19, PREPARE-IT, the product did not prevent uninfected individuals at risk from COVID from becoming infected with the virus, but there was no increase in side effects versus placebo with use over a 60-day period.
A small study last year in 100 COVID-positive patients showed icosapent ethyl reduced C-reactive protein, an inflammatory marker, and also improved symptoms.
PREPARE-IT 2, a pragmatic web-based trial, was conducted to investigate whether icosapent ethyl in nonhospitalized patients with a positive diagnosis of COVID-19 could reduce hospitalization rates and complications.
The trial enrolled 2,052 patients (mean age, 50 years), of whom 1,010 were allocated to the active group and 1,042 to the placebo group. Inclusion criteria included individuals aged 40 years or older with a confirmed COVID-19 diagnosis and no more than 7 days from the onset of symptoms and without a clear indication for hospitalization.
Patients who were allocated to the active arm received icosapent ethyl at a dose of 8 g (four capsules every 12 hours, morning and evening) for the first 3 days, followed by 4 g (two capsules every 12 hours) thereafter (days 4-28).
The primary outcome, COVID-19–related hospitalization (indication for hospitalization or hospitalization) or death at 28 days, occurred in 11.16% of the active group and 13.69% of the placebo group, giving a hazard ratio of 0.84 (95% confidence interval, 0.65-1.08; P = .166)
Secondary outcomes showed similar positive trends, but none were significant. These included: death or still hospitalized at 28 days (HR, 0.74), major events (MI, stroke, death; HR, 0.38), and total mortality (HR, 0.52).
In terms of safety, there was no significant difference in total adverse events between the two groups (16.5% in the active group vs. 14.8% in the placebo group). The most common adverse effects were constipation (2.7%), diarrhea (7.2%), and nausea (4%), but these were not significantly different from placebo. There were, however, more discontinuations in the active group (7% vs. 4%).
Dr. Diaz pointed out that the PREPARE-IT 2 trial was started in May 2020, when there wasn’t much known about the COVID-19 condition, and there were no vaccines or treatments, so hospitalization rates were high.
“We were hoping to see a 25%-30% reduction in hospitalizations with icosapent ethyl, and the trial was powered for that sort of reduction, but today we know we can expect a more modest reduction of about 15%,” Dr. Diaz concluded. “But to show that, we need a much larger trial with 8,000 or 9,000 patients, and that will be much more difficult to conduct.”
The PREPARE-IT 2 study was funded by Amarin. Dr. Diaz has received grants from Dalcor, Amarin, PHRI, and Lepetit.
A version of this article first appeared on Medscape.com.
A high dose of the purified form of eicosapentaenoic acid, icosapent ethyl (Vascepa, Amarin), failed to significantly reduce hospitalizations or death in patients infected with COVID-19 in the PREPARE-IT 2 study.
The study did, however, show a favorable trend, with a 16% reduction in the primary endpoint of death or an indication for hospitalization. All secondary endpoints were also numerically reduced, but none reached statistical significance.
The product was also well tolerated over the 28 days of the study period, even though a new high-loading dose was used, with no increase in atrial fibrillation or bleeding or other adverse events versus placebo, although there was a slightly higher rate of discontinuation.
The trial was presented at the American Heart Association scientific sessions on Nov. 15 by Rafael Díaz, MD, director of Estudios Clínicos Latinoamérica in Rosario, Argentina.
“Larger, randomized trials powered for a relative risk reduction of around 15% with icosapent ethyl are needed to establish whether or not this product may have a role in the management of COVID-positive outpatients,” Dr. Diaz concluded.
‘Intriguing signals’
Commenting on the study, Manesh Patel, MD, chief of the division of cardiology and codirector of the Heart Center at Duke University, Durham, N.C., and chair of the Scientific Sessions scientific program, said that: “Certainly there are some intriguing signals.”
“I think the trend is valuable, but do we need a larger trial to confirm a benefit? I will leave that to the clinical community to decide,” Dr. Patel added. “But it is hard to power a trial to get that answer, and the world of COVID has changed since this trial started with vaccines now available and new therapeutics coming. So, there’s going to be a competing landscape.”
Discussing the trial at an AHA news briefing, Erin Michos, MD, associate professor of medicine within the division of cardiology at Johns Hopkins University, Baltimore, said: “Results showed that everything trended in the right direction, but did not reach statistical significance largely because there were fewer events than anticipated. COVID hospitalizations are going down because of the broad adoption of vaccines, which meant that this study didn’t quite meet its endpoint.”
But, she added: “Reassuringly, even with the higher loading dose, there was no increased risk of [atrial fibrillation] when used for just 28 days, and no increased risk in bleeding, so there was very good safety.”
“We need a larger trial to really definitely show whether icosapent ethyl can or cannot help COVID-positive outpatients, but I think a better prevention strategy would be the broad adoption of vaccinations globally,” Dr. Michos concluded.
‘A pretty big ask’
Donald Lloyd-Jones, MD, AHA president and designated discussant at the late-breaking science session, congratulated the investigators on conducting “a very nice pragmatic trial in the midst of the COVID pandemic.”
Dr. Lloyd-Jones concluded that the broad range of potentially beneficial actions of icosapent ethyl – including antitriglyceride, anti-inflammatory, antioxidant, and antithrombotic effects – leads to the possibility of it helping in COVID, but he added that “this is a pretty big ask for a fish oil supplement given short term.”
Presenting the study, Dr. Diaz noted that there are limited options for the outpatient treatment of patients with COVID-19 infection, and it is believed that inflammation plays a major role in worsening the severity of the infection.
He pointed out that previous data support a potential role of omega-3 fatty acids in reducing inflammation and infection, and that icosapent ethyl has shown a reduction in major cardiovascular events in the REDUCE-IT trial, with the mechanism thought to involve anti-inflammatory effects.
In the first trial to investigate the role of icosapent ethyl in COVID-19, PREPARE-IT, the product did not prevent uninfected individuals at risk from COVID from becoming infected with the virus, but there was no increase in side effects versus placebo with use over a 60-day period.
A small study last year in 100 COVID-positive patients showed icosapent ethyl reduced C-reactive protein, an inflammatory marker, and also improved symptoms.
PREPARE-IT 2, a pragmatic web-based trial, was conducted to investigate whether icosapent ethyl in nonhospitalized patients with a positive diagnosis of COVID-19 could reduce hospitalization rates and complications.
The trial enrolled 2,052 patients (mean age, 50 years), of whom 1,010 were allocated to the active group and 1,042 to the placebo group. Inclusion criteria included individuals aged 40 years or older with a confirmed COVID-19 diagnosis and no more than 7 days from the onset of symptoms and without a clear indication for hospitalization.
Patients who were allocated to the active arm received icosapent ethyl at a dose of 8 g (four capsules every 12 hours, morning and evening) for the first 3 days, followed by 4 g (two capsules every 12 hours) thereafter (days 4-28).
The primary outcome, COVID-19–related hospitalization (indication for hospitalization or hospitalization) or death at 28 days, occurred in 11.16% of the active group and 13.69% of the placebo group, giving a hazard ratio of 0.84 (95% confidence interval, 0.65-1.08; P = .166)
Secondary outcomes showed similar positive trends, but none were significant. These included: death or still hospitalized at 28 days (HR, 0.74), major events (MI, stroke, death; HR, 0.38), and total mortality (HR, 0.52).
In terms of safety, there was no significant difference in total adverse events between the two groups (16.5% in the active group vs. 14.8% in the placebo group). The most common adverse effects were constipation (2.7%), diarrhea (7.2%), and nausea (4%), but these were not significantly different from placebo. There were, however, more discontinuations in the active group (7% vs. 4%).
Dr. Diaz pointed out that the PREPARE-IT 2 trial was started in May 2020, when there wasn’t much known about the COVID-19 condition, and there were no vaccines or treatments, so hospitalization rates were high.
“We were hoping to see a 25%-30% reduction in hospitalizations with icosapent ethyl, and the trial was powered for that sort of reduction, but today we know we can expect a more modest reduction of about 15%,” Dr. Diaz concluded. “But to show that, we need a much larger trial with 8,000 or 9,000 patients, and that will be much more difficult to conduct.”
The PREPARE-IT 2 study was funded by Amarin. Dr. Diaz has received grants from Dalcor, Amarin, PHRI, and Lepetit.
A version of this article first appeared on Medscape.com.
FROM AHA 2021
Vegetable fats tied to lower stroke risk, animal fats to higher risk
Higher intake of vegetable fats from foods such as olive oil and nuts is associated with a lower risk for stroke, whereas people who eat more animal fats, especially processed red meats, may have a higher stroke risk, observational findings suggest.
In a study of more than 117,000 health professionals who were followed for 27 years, those whose diet was in the highest quintile for intake of vegetable fat had a 12% lower risk for stroke, compared with those who consumed the least amount of vegetable fats.
Conversely, having the highest intake of animal fat from nondairy sources was associated with a 16% increased risk of stroke.
Fenglei Wang, PhD, presented these results at the American Heart Association scientific sessions.
“Our findings support the Dietary Guidelines for Americans and dietary recommendations by AHA,” Dr. Wang, a postdoctoral fellow in the department of nutrition at Harvard University’s T.H. Chan School of Public Health in Boston, told this news organization.
“The main sources of vegetable fat have a large overlap with polyunsaturated fat, such as vegetable oils, nuts, walnuts, and peanut butter,” Dr. Wang noted, adding that fish, especially fatty fish, is a main source of polyunsaturated fat and is recommended for cardiovascular health.
“We would recommend that people reduce consumption of red and processed meat, minimize fatty parts of unprocessed meat if consumed, and replace lard or tallow (beef fat) with nontropical vegetable oils, such as olive oil, corn, or soybean oils in cooking, to lower their stroke risk,” she said.
Moreover, although the results from this study of dietary fat are informative, Dr. Wang continued, “there are other dietary factors (fruits, vegetables, salt, alcohol, et cetera), and lifestyle factors (physical activity, smoking, et cetera), that are associated with stroke risk and worthy of attention as well.”
“Many processed meats are high in salt and saturated fat, and low in vegetable fat,” Alice H. Lichtenstein, DSc, an AHA spokesperson who was not involved with this research, noted in a press release.
“Research shows that replacing processed meat with other protein sources, particularly plant sources, is associated with lower death rates,” added Dr. Lichtenstein, the Stanley N. Gershoff professor of nutrition science and policy at Tufts University in Boston, and lead author of the AHA’s 2021 scientific statement, Dietary Guidance to Improve Cardiovascular Health.
“Key features of a heart-healthy diet pattern,” she summarized, “are to balance calorie intake with calorie needs to achieve and maintain a healthy weight; choose whole grains, lean and plant-based protein, and a variety of fruits and vegetables; limit salt, sugar, animal fat, processed foods, and alcohol; and apply this guidance regardless of where the food is prepared or consumed.”
Replace processed meat with plant proteins
The focus on stroke in this study “is important” because, traditionally, studies of diet and cardiovascular health have focused on coronary heart disease, Andrew Mente, PhD, who also was not involved in this research, said in an email to this news organization.
“Overall, the take-home message from the study is that replacing processed meat with plant sources of protein in the diet is probably beneficial,” Dr. Mente, associate professor, health research methods, evidence, and impact, Faculty of Health Sciences, McMaster University, Hamilton, Ont., said.
The finding that people who ate the most vegetable fat had a modest 12% lower risk of stroke than those who ate the least vegetable fat “points to protective effects of foods like seeds, nuts, vegetables, and olive oil, which has been shown previously,” he continued.
The highest quintile of total red meat intake was associated with an 8% higher risk for stroke, but this was driven mainly by processed red meat (which was associated with a 12% higher risk for stroke). These findings are “generally consistent with cohort studies showing that processed meat, as with most highly processed foods for that matter, are associated with an increased risk of cardiovascular events,” Dr. Mente noted.
“Surprisingly, dairy products (such as cheese, butter, or milk) in the study were not connected with the risk of stroke,” he added. This finding differs from results of meta-analyses of multiple cohort studies of dairy intake and stroke and the recent large international PURE study, which showed that dairy intake was associated with a lower risk for stroke.
“What is needed to move the field forward,” according to Dr. Mente, “is to employ new methods that use cutting-edge technology to study nutritional biomarkers and health outcomes.”
“When dealing with modest associations as usually encountered in nutrition, it is a challenge to make causal connections based on dietary questionnaires, which are fraught with measurement error,” he added. “The use of novel methods is where the field is headed.”
Total dietary fat, different types, and different food sources
Dr. Wang and colleagues investigated how total dietary fat, different types of fat, and fats from different foods were associated with incident stroke in 73,867 women in the 1984-2016 Nurses’ Health Study and 43,269 men who participated in the 1986-2016 Health Professionals Follow-up Study.
The participants had an average age of 50 years, 63% were women, and 97% were White. They replied to food-frequency questionnaires every 4 years.
Total red meat included beef, pork, or lamb (as a main dish or in sandwiches or mixed dishes) as well as processed red meats (such as bacon, sausage, bologna, hot dogs, and salami).
Animal fat sources included meat, beef tallow, lard, and full-fat dairy products, such as full-fat milk and cheese.
The median percentage of total daily calories from different sources of fat ranged from 10% to 20% for vegetable fat, 3% to 10% for dairy fat, and 7% to 17% for nondairy animal fat (for lowest to highest quintiles).
The median percentage of total daily calories from different types of fat ranged from 5% to 8% for polyunsaturated fat, 4% to 7% for n-6 polyunsaturated fat, 9% to 15% for monounsaturated fat, 8% to 14% for saturated fat, and 1% to 2% for trans fat.
During follow-up, there were 6,189 incident strokes, including 2,967 ischemic strokes and 814 hemorrhagic strokes.
The researchers found that intake in the highest quintile of vegetable fat was associated with a lower risk for total stroke, compared with the lowest quintile (hazard ratio, 0.88; 95% confidence interval, 0.81-0.96; P for trend < .001).
Similarly, the highest intake of polyunsaturated fat was also associated with lower total stroke (HR, 0.88; 95% CI, 0.80-0.96; P for trend = .002).
Highest intake of nondairy animal fat, however, was associated with an increased risk for total stroke (HR, 1.16; 95% CI, 1.05-1.29; P for trend < .001). They observed “similar associations” for ischemic stroke, but the only positive association for nondairy animal fat was with hemorrhagic stroke, the abstract notes.
The risk for stroke was lower by 9% per serving per day for vegetable oil but increased by 8% and 12%, respectively, per serving of total red meat or processed red meat.
The association for vegetable oil was attenuated after adjustment for vegetable fat or polyunsaturated fat, whereas adjustment for nondairy animal fat rendered the association for total red meat and processed red meat nonsignificant.
The study was funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health. Dr. Wang has no relevant financial disclosures. Dr. Mente has received research funding from the Dairy Farmers of Canada and the National Dairy Council to analyze data on dairy consumption and health outcomes in the PURE study, which is funded by the Population Health Research Institute, Hamilton Health Sciences Research Institute, and more than 70 other sources (government and pharmaceutical).
A version of this article first appeared on Medscape.com.
Higher intake of vegetable fats from foods such as olive oil and nuts is associated with a lower risk for stroke, whereas people who eat more animal fats, especially processed red meats, may have a higher stroke risk, observational findings suggest.
In a study of more than 117,000 health professionals who were followed for 27 years, those whose diet was in the highest quintile for intake of vegetable fat had a 12% lower risk for stroke, compared with those who consumed the least amount of vegetable fats.
Conversely, having the highest intake of animal fat from nondairy sources was associated with a 16% increased risk of stroke.
Fenglei Wang, PhD, presented these results at the American Heart Association scientific sessions.
“Our findings support the Dietary Guidelines for Americans and dietary recommendations by AHA,” Dr. Wang, a postdoctoral fellow in the department of nutrition at Harvard University’s T.H. Chan School of Public Health in Boston, told this news organization.
“The main sources of vegetable fat have a large overlap with polyunsaturated fat, such as vegetable oils, nuts, walnuts, and peanut butter,” Dr. Wang noted, adding that fish, especially fatty fish, is a main source of polyunsaturated fat and is recommended for cardiovascular health.
“We would recommend that people reduce consumption of red and processed meat, minimize fatty parts of unprocessed meat if consumed, and replace lard or tallow (beef fat) with nontropical vegetable oils, such as olive oil, corn, or soybean oils in cooking, to lower their stroke risk,” she said.
Moreover, although the results from this study of dietary fat are informative, Dr. Wang continued, “there are other dietary factors (fruits, vegetables, salt, alcohol, et cetera), and lifestyle factors (physical activity, smoking, et cetera), that are associated with stroke risk and worthy of attention as well.”
“Many processed meats are high in salt and saturated fat, and low in vegetable fat,” Alice H. Lichtenstein, DSc, an AHA spokesperson who was not involved with this research, noted in a press release.
“Research shows that replacing processed meat with other protein sources, particularly plant sources, is associated with lower death rates,” added Dr. Lichtenstein, the Stanley N. Gershoff professor of nutrition science and policy at Tufts University in Boston, and lead author of the AHA’s 2021 scientific statement, Dietary Guidance to Improve Cardiovascular Health.
“Key features of a heart-healthy diet pattern,” she summarized, “are to balance calorie intake with calorie needs to achieve and maintain a healthy weight; choose whole grains, lean and plant-based protein, and a variety of fruits and vegetables; limit salt, sugar, animal fat, processed foods, and alcohol; and apply this guidance regardless of where the food is prepared or consumed.”
Replace processed meat with plant proteins
The focus on stroke in this study “is important” because, traditionally, studies of diet and cardiovascular health have focused on coronary heart disease, Andrew Mente, PhD, who also was not involved in this research, said in an email to this news organization.
“Overall, the take-home message from the study is that replacing processed meat with plant sources of protein in the diet is probably beneficial,” Dr. Mente, associate professor, health research methods, evidence, and impact, Faculty of Health Sciences, McMaster University, Hamilton, Ont., said.
The finding that people who ate the most vegetable fat had a modest 12% lower risk of stroke than those who ate the least vegetable fat “points to protective effects of foods like seeds, nuts, vegetables, and olive oil, which has been shown previously,” he continued.
The highest quintile of total red meat intake was associated with an 8% higher risk for stroke, but this was driven mainly by processed red meat (which was associated with a 12% higher risk for stroke). These findings are “generally consistent with cohort studies showing that processed meat, as with most highly processed foods for that matter, are associated with an increased risk of cardiovascular events,” Dr. Mente noted.
“Surprisingly, dairy products (such as cheese, butter, or milk) in the study were not connected with the risk of stroke,” he added. This finding differs from results of meta-analyses of multiple cohort studies of dairy intake and stroke and the recent large international PURE study, which showed that dairy intake was associated with a lower risk for stroke.
“What is needed to move the field forward,” according to Dr. Mente, “is to employ new methods that use cutting-edge technology to study nutritional biomarkers and health outcomes.”
“When dealing with modest associations as usually encountered in nutrition, it is a challenge to make causal connections based on dietary questionnaires, which are fraught with measurement error,” he added. “The use of novel methods is where the field is headed.”
Total dietary fat, different types, and different food sources
Dr. Wang and colleagues investigated how total dietary fat, different types of fat, and fats from different foods were associated with incident stroke in 73,867 women in the 1984-2016 Nurses’ Health Study and 43,269 men who participated in the 1986-2016 Health Professionals Follow-up Study.
The participants had an average age of 50 years, 63% were women, and 97% were White. They replied to food-frequency questionnaires every 4 years.
Total red meat included beef, pork, or lamb (as a main dish or in sandwiches or mixed dishes) as well as processed red meats (such as bacon, sausage, bologna, hot dogs, and salami).
Animal fat sources included meat, beef tallow, lard, and full-fat dairy products, such as full-fat milk and cheese.
The median percentage of total daily calories from different sources of fat ranged from 10% to 20% for vegetable fat, 3% to 10% for dairy fat, and 7% to 17% for nondairy animal fat (for lowest to highest quintiles).
The median percentage of total daily calories from different types of fat ranged from 5% to 8% for polyunsaturated fat, 4% to 7% for n-6 polyunsaturated fat, 9% to 15% for monounsaturated fat, 8% to 14% for saturated fat, and 1% to 2% for trans fat.
During follow-up, there were 6,189 incident strokes, including 2,967 ischemic strokes and 814 hemorrhagic strokes.
The researchers found that intake in the highest quintile of vegetable fat was associated with a lower risk for total stroke, compared with the lowest quintile (hazard ratio, 0.88; 95% confidence interval, 0.81-0.96; P for trend < .001).
Similarly, the highest intake of polyunsaturated fat was also associated with lower total stroke (HR, 0.88; 95% CI, 0.80-0.96; P for trend = .002).
Highest intake of nondairy animal fat, however, was associated with an increased risk for total stroke (HR, 1.16; 95% CI, 1.05-1.29; P for trend < .001). They observed “similar associations” for ischemic stroke, but the only positive association for nondairy animal fat was with hemorrhagic stroke, the abstract notes.
The risk for stroke was lower by 9% per serving per day for vegetable oil but increased by 8% and 12%, respectively, per serving of total red meat or processed red meat.
The association for vegetable oil was attenuated after adjustment for vegetable fat or polyunsaturated fat, whereas adjustment for nondairy animal fat rendered the association for total red meat and processed red meat nonsignificant.
The study was funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health. Dr. Wang has no relevant financial disclosures. Dr. Mente has received research funding from the Dairy Farmers of Canada and the National Dairy Council to analyze data on dairy consumption and health outcomes in the PURE study, which is funded by the Population Health Research Institute, Hamilton Health Sciences Research Institute, and more than 70 other sources (government and pharmaceutical).
A version of this article first appeared on Medscape.com.
Higher intake of vegetable fats from foods such as olive oil and nuts is associated with a lower risk for stroke, whereas people who eat more animal fats, especially processed red meats, may have a higher stroke risk, observational findings suggest.
In a study of more than 117,000 health professionals who were followed for 27 years, those whose diet was in the highest quintile for intake of vegetable fat had a 12% lower risk for stroke, compared with those who consumed the least amount of vegetable fats.
Conversely, having the highest intake of animal fat from nondairy sources was associated with a 16% increased risk of stroke.
Fenglei Wang, PhD, presented these results at the American Heart Association scientific sessions.
“Our findings support the Dietary Guidelines for Americans and dietary recommendations by AHA,” Dr. Wang, a postdoctoral fellow in the department of nutrition at Harvard University’s T.H. Chan School of Public Health in Boston, told this news organization.
“The main sources of vegetable fat have a large overlap with polyunsaturated fat, such as vegetable oils, nuts, walnuts, and peanut butter,” Dr. Wang noted, adding that fish, especially fatty fish, is a main source of polyunsaturated fat and is recommended for cardiovascular health.
“We would recommend that people reduce consumption of red and processed meat, minimize fatty parts of unprocessed meat if consumed, and replace lard or tallow (beef fat) with nontropical vegetable oils, such as olive oil, corn, or soybean oils in cooking, to lower their stroke risk,” she said.
Moreover, although the results from this study of dietary fat are informative, Dr. Wang continued, “there are other dietary factors (fruits, vegetables, salt, alcohol, et cetera), and lifestyle factors (physical activity, smoking, et cetera), that are associated with stroke risk and worthy of attention as well.”
“Many processed meats are high in salt and saturated fat, and low in vegetable fat,” Alice H. Lichtenstein, DSc, an AHA spokesperson who was not involved with this research, noted in a press release.
“Research shows that replacing processed meat with other protein sources, particularly plant sources, is associated with lower death rates,” added Dr. Lichtenstein, the Stanley N. Gershoff professor of nutrition science and policy at Tufts University in Boston, and lead author of the AHA’s 2021 scientific statement, Dietary Guidance to Improve Cardiovascular Health.
“Key features of a heart-healthy diet pattern,” she summarized, “are to balance calorie intake with calorie needs to achieve and maintain a healthy weight; choose whole grains, lean and plant-based protein, and a variety of fruits and vegetables; limit salt, sugar, animal fat, processed foods, and alcohol; and apply this guidance regardless of where the food is prepared or consumed.”
Replace processed meat with plant proteins
The focus on stroke in this study “is important” because, traditionally, studies of diet and cardiovascular health have focused on coronary heart disease, Andrew Mente, PhD, who also was not involved in this research, said in an email to this news organization.
“Overall, the take-home message from the study is that replacing processed meat with plant sources of protein in the diet is probably beneficial,” Dr. Mente, associate professor, health research methods, evidence, and impact, Faculty of Health Sciences, McMaster University, Hamilton, Ont., said.
The finding that people who ate the most vegetable fat had a modest 12% lower risk of stroke than those who ate the least vegetable fat “points to protective effects of foods like seeds, nuts, vegetables, and olive oil, which has been shown previously,” he continued.
The highest quintile of total red meat intake was associated with an 8% higher risk for stroke, but this was driven mainly by processed red meat (which was associated with a 12% higher risk for stroke). These findings are “generally consistent with cohort studies showing that processed meat, as with most highly processed foods for that matter, are associated with an increased risk of cardiovascular events,” Dr. Mente noted.
“Surprisingly, dairy products (such as cheese, butter, or milk) in the study were not connected with the risk of stroke,” he added. This finding differs from results of meta-analyses of multiple cohort studies of dairy intake and stroke and the recent large international PURE study, which showed that dairy intake was associated with a lower risk for stroke.
“What is needed to move the field forward,” according to Dr. Mente, “is to employ new methods that use cutting-edge technology to study nutritional biomarkers and health outcomes.”
“When dealing with modest associations as usually encountered in nutrition, it is a challenge to make causal connections based on dietary questionnaires, which are fraught with measurement error,” he added. “The use of novel methods is where the field is headed.”
Total dietary fat, different types, and different food sources
Dr. Wang and colleagues investigated how total dietary fat, different types of fat, and fats from different foods were associated with incident stroke in 73,867 women in the 1984-2016 Nurses’ Health Study and 43,269 men who participated in the 1986-2016 Health Professionals Follow-up Study.
The participants had an average age of 50 years, 63% were women, and 97% were White. They replied to food-frequency questionnaires every 4 years.
Total red meat included beef, pork, or lamb (as a main dish or in sandwiches or mixed dishes) as well as processed red meats (such as bacon, sausage, bologna, hot dogs, and salami).
Animal fat sources included meat, beef tallow, lard, and full-fat dairy products, such as full-fat milk and cheese.
The median percentage of total daily calories from different sources of fat ranged from 10% to 20% for vegetable fat, 3% to 10% for dairy fat, and 7% to 17% for nondairy animal fat (for lowest to highest quintiles).
The median percentage of total daily calories from different types of fat ranged from 5% to 8% for polyunsaturated fat, 4% to 7% for n-6 polyunsaturated fat, 9% to 15% for monounsaturated fat, 8% to 14% for saturated fat, and 1% to 2% for trans fat.
During follow-up, there were 6,189 incident strokes, including 2,967 ischemic strokes and 814 hemorrhagic strokes.
The researchers found that intake in the highest quintile of vegetable fat was associated with a lower risk for total stroke, compared with the lowest quintile (hazard ratio, 0.88; 95% confidence interval, 0.81-0.96; P for trend < .001).
Similarly, the highest intake of polyunsaturated fat was also associated with lower total stroke (HR, 0.88; 95% CI, 0.80-0.96; P for trend = .002).
Highest intake of nondairy animal fat, however, was associated with an increased risk for total stroke (HR, 1.16; 95% CI, 1.05-1.29; P for trend < .001). They observed “similar associations” for ischemic stroke, but the only positive association for nondairy animal fat was with hemorrhagic stroke, the abstract notes.
The risk for stroke was lower by 9% per serving per day for vegetable oil but increased by 8% and 12%, respectively, per serving of total red meat or processed red meat.
The association for vegetable oil was attenuated after adjustment for vegetable fat or polyunsaturated fat, whereas adjustment for nondairy animal fat rendered the association for total red meat and processed red meat nonsignificant.
The study was funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health. Dr. Wang has no relevant financial disclosures. Dr. Mente has received research funding from the Dairy Farmers of Canada and the National Dairy Council to analyze data on dairy consumption and health outcomes in the PURE study, which is funded by the Population Health Research Institute, Hamilton Health Sciences Research Institute, and more than 70 other sources (government and pharmaceutical).
A version of this article first appeared on Medscape.com.
FROM AHA 2021
More STEP data: Semaglutide cuts weight, cravings, beats liraglutide
The STEP 5 clinical trial extends favorable weight loss from 1 year out to 2 years for the glucagon-like peptide-1 (GLP-1) agonist semaglutide (Wegovy, Novo Nordisk), given as a once-weekly 2.4-mg subcutaneous injection, and some food cravings were improved in a subgroup analysis.
In another study, STEP 8, weight loss was greater at 68 weeks with semaglutide subcutaneous injection than with a 3-mg daily subcutaneous injection of another GLP-1 agonist, liraglutide (Saxenda, Novo Nordisk), approved earlier for weight loss.
Researchers presented these promising outcomes, with no new safety signals, at ObesityWeek® 2021.
However, there is more to learn about the drug class, researchers agree. Follow-up is still relatively short for a chronic disease and many patients have gastrointestinal side effects with semaglutide, one expert cautions.
The key findings were:
In STEP 5, combined with lifestyle intervention (a reduced-calorie meal plan and advice about physical activity), weekly injection of 2.4 mg semaglutide led to:
- 15.2% weight loss, compared with 2.6% weight loss with placebo at 2 years (P < .0001);
- 77% of patients losing at least 5% of their weight, compared with 34% of patients in the placebo group at 2 years (P < .0001);
- Significantly greater improvement in overall control of cravings, and craving for savory foods, in a subset of patients, versus placebo, but questionnaire scores for positive mood and craving for sweet foods were similar in both groups.
- In STEP 8, mean body weight at 68 weeks was 15.8% lower with 2.4 mg/week subcutaneous semaglutide plus lifestyle changes versus 6.4% lower with 3.0 mg/day subcutaneous liraglutide plus lifestyle changes (P < .001).
Can treat to a target weight-loss range
The undiminished weight loss efficacy in the 2-year data for STEP 5 “portends well,” said W. Timothy Garvey, MD, following his presentation of the results.
“I think this is a new era in obesity care,” said Dr. Garvey, director of the diabetes research center at the University of Alabama at Birmingham. Semaglutide “essentially doubles weight loss efficacy” compared to the other approved pharmacotherapies for obesity.
With this degree of potential weight loss, clinicians “can use weight as a biomarker and treat to a target [weight-loss] range,” he said.
Expounding on this in an interview, Dr. Garvey noted that, as stated in the 2016 American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology (ACE) clinical practice guidelines for medical care of patients with obesity, of which he was lead author, “the objective of care in obesity is to increase health of patients and prevent or treat complications.”
Semaglutide “can treat to a range of weight loss of 10% to 20% in the majority of patients,” which is associated with improvements in cardiovascular and metabolic risk factors.
In STEP 5, of the 51% of patients in the semaglutide group who had prediabetes at enrollment, 80% had normal glycemia at 2 years; however, the trial was not powered nor designed to investigate this.
More data are needed to inform long-term care decisions. The ongoing SELECT cardiovascular outcomes trial of semaglutide, with expected primary study completion on Sept. 28, 2023, should provide more information.
Weight loss plus reduced cravings
In another presentation, Sean Wharton, MD, PharmD, said, “In adults with overweight or obesity, substantial weight loss with semaglutide 2.4 mg was accompanied by short- and long-term improvements in control of eating.”
“Most patients living with obesity who are attempting to decrease calories will have food cravings, based on the biological parameters of weight preservation,” Dr. Wharton, medical director at the Wharton Medical Clinic, in Hamilton, Ont., explained in an email.
The degree of craving varies from patient to patient, likely based on genetics, he added. Research in this field is still emerging.
“I believe that semaglutide 2.4 mg is a game-changer in the field of weight management, and it will change the dialogue for insurance plans and with policymakers regarding coverage for this medication,” said Dr. Wharton.
“The data from the STEP programs are very strong. I am certainly hoping for a change to bias against covering these medications that we have seen in the past,” he said.
Clinically meaningful weight loss
When presenting the STEP 8 findings, Domenica M. Rubino, MD, said: “Participants were significantly more likely to achieve clinically meaningful weight loss thresholds with semaglutide 2.4 mg versus liraglutide 3.0 mg, accompanied by greater improvements in cardiometabolic risk factors.”
For example, patients can have better mobility, which is important for quality of life, Dr. Rubino, director of the Washington Center for Weight Management and Research, Arlington, Virginia, noted.
A smaller percentage of patients respond to liraglutide, she added. Clinicians need to individualize treatment.
When asked, “How do you choose which medical therapy?” Dr. Rubino responded: “We sit and talk.” Finding the medical therapy that fits the patient depends on things such as the patient’s insurance coverage and ability to tolerate side effects such as dehydration, diarrhea, and nausea.
When asked, “How do you switch from liraglutide to semaglutide?” she noted that there are no current guidelines for this. “You have to be careful. Start on the lowest dose of Wegovy. Be cautious, conservative.”
Still early days, caveats remain
“The STEP trials as a group appear to be making the case that obesity may now be considered a medically manageable disease, based on the experience with semaglutide,” Julie R. Ingelfinger, MD, who was not involved with the research, commented in an email.
“STEP 5 and 8 may suggest that weight loss occurs and is sustainable in overweight persons without diabetes with one or more comorbidities or in obese persons without diabetes,” added Dr. Ingelfinger, professor of pediatrics, Harvard Medical School, consultant in pediatric nephrology, Massachusetts General Hospital, Boston, and deputy editor, The New England Journal of Medicine.
However, “even 2 years, in the case of STEP 5, and ~68 weeks in the case of STEP 8, may not be long enough to know whether semaglutide is as promising as these brief summaries (abstracts) suggest,” she cautioned.
“Obesity is a chronic condition, and very long-term therapy and management are required,” Dr. Ingelfinger continued.
“Further, it is hard to generalize when gastrointestinal adverse events are common in a study,” she said. For example, in STEP 8, they were just as common with semaglutide as with the comparator liraglutide, she noted.
“The racial and ethnic representativeness of these studies does not reflect population distributions in the U.S., limiting generalization,” she continued.
“So, there remain caveats in interpreting these data.”
STEP 5 weight loss efficacy and safety at 2 years
Garvey reported that STEP 5 was a phase 3b trial that randomized 304 adults in the United States, Canada, Hungary, Italy, and Spain, who were 18 years and older, with a body mass index (BMI) ≥27 kg/m2 with at least one weight-related comorbidity (hypertension, dyslipidemia, obstructive sleep apnea, or cardiovascular disease) or a BMI ≥30 kg/m2, without type 2 diabetes, to receive semaglutide or placebo plus lifestyle intervention.
Most participants were women (78%) and White (93%). On average, they were 47 years old, weighed 106 kg (223.7 pounds), had a BMI of 38.5 kg/m2, a waist circumference of 115.7 cm (45.6 inches), and an A1c of 5.7%.
A total of 87% of patients in the semaglutide group and 73% of patients in the placebo group completed the trial.
At 104 weeks, participants were more likely to lose ≥10%, ≥15%, and ≥20% of body weight with semaglutide versus placebo (61.8% vs. 13.3%, 52.1% vs. 7.0%, and 36.1% vs. 2.3%, respectively; P < .0001 for all).
Patients in the semaglutide group had greater health improvements in cardiovascular risk factors (waist circumference, systolic and diastolic blood pressure, and C-reactive protein) and metabolic risk factors (A1c, fasting plasma glucose, fasting serum insulin, and triglycerides) than those in the placebo group (P < .05 for all).
Safety and tolerability were consistent with adverse events seen with this drug class, with no new safety signals.
Control of eating questionnaire findings at 2 years in STEP 5
Dr. Wharton and colleagues assessed changes in responses to the Control of Eating questionnaire at baseline and at 20, 52, and 104 weeks in patients from the U.S. and Canada in the STEP 5 trial (88 patients in the semaglutide group and 86 patients in the placebo group).
The questionnaire consisted of 19 questions grouped into four categories: control of food cravings, craving for savory foods (salty and spicy, dairy, or starchy foods), craving for sweet foods (chocolate, sweet foods, or fruit/fruit juice), and positive mood.
At week 104, patients in the semaglutide group had significantly greater improvements in scores for craving for salty and spicy, dairy, and starchy foods, and resisting cravings.
Semaglutide versus liraglutide, 68-week efficacy and safety in STEP 8
STEP 8 randomized 338 U.S. adults without diabetes and a BMI of ≥27 kg/m2 plus one or more weight-related comorbidities or a BMI of ≥30 kg/m2 3:1 to semaglutide 2.4 mg once weekly (n = 126) or matching placebo, or 3:1 liraglutide 3.0 mg once daily (n = 127) or matching placebo, plus lifestyle intervention.
Most participants were women (78%) and were a mean age of 49, had a mean body weight of 104.5 kg, and had a mean BMI of 37.5 kg/m2.
In STEP 8, more participants achieved ≥10%, ≥15%, and ≥20% weight loss with semaglutide than with liraglutide (70.9% vs. 25.6%, 55.6% vs. 12.0%, and 38.5% vs. 6.0%, respectively; P < .001 for all odds ratios).
Semaglutide improved waist circumference, A1c, and C-reactive protein versus liraglutide (unadjusted P < .001 for all).
Gastrointestinal adverse events were reported by 84% and 83% of participants receiving semaglutide and liraglutide, respectively. Most events were mild/moderate and transient, with prevalence declining over time.
Fewer participants stopped treatment due to adverse events with semaglutide than liraglutide (3.2% vs. 12.6%).
Dr. Garvey has reported serving as a site principal investigator for multicentered clinical trials sponsored by his university and funded by Eli Lilly, Novo Nordisk, and Pfizer. Dr. Wharton has reported financial ties to Novo Nordisk, Bausch Health Canada, Eli Lily, and Boehringer Ingelheim Canada. Dr. Rubino has reported ties to Boehringer Ingelheim and AstraZeneca. Dr. Ingelfinger has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The STEP 5 clinical trial extends favorable weight loss from 1 year out to 2 years for the glucagon-like peptide-1 (GLP-1) agonist semaglutide (Wegovy, Novo Nordisk), given as a once-weekly 2.4-mg subcutaneous injection, and some food cravings were improved in a subgroup analysis.
In another study, STEP 8, weight loss was greater at 68 weeks with semaglutide subcutaneous injection than with a 3-mg daily subcutaneous injection of another GLP-1 agonist, liraglutide (Saxenda, Novo Nordisk), approved earlier for weight loss.
Researchers presented these promising outcomes, with no new safety signals, at ObesityWeek® 2021.
However, there is more to learn about the drug class, researchers agree. Follow-up is still relatively short for a chronic disease and many patients have gastrointestinal side effects with semaglutide, one expert cautions.
The key findings were:
In STEP 5, combined with lifestyle intervention (a reduced-calorie meal plan and advice about physical activity), weekly injection of 2.4 mg semaglutide led to:
- 15.2% weight loss, compared with 2.6% weight loss with placebo at 2 years (P < .0001);
- 77% of patients losing at least 5% of their weight, compared with 34% of patients in the placebo group at 2 years (P < .0001);
- Significantly greater improvement in overall control of cravings, and craving for savory foods, in a subset of patients, versus placebo, but questionnaire scores for positive mood and craving for sweet foods were similar in both groups.
- In STEP 8, mean body weight at 68 weeks was 15.8% lower with 2.4 mg/week subcutaneous semaglutide plus lifestyle changes versus 6.4% lower with 3.0 mg/day subcutaneous liraglutide plus lifestyle changes (P < .001).
Can treat to a target weight-loss range
The undiminished weight loss efficacy in the 2-year data for STEP 5 “portends well,” said W. Timothy Garvey, MD, following his presentation of the results.
“I think this is a new era in obesity care,” said Dr. Garvey, director of the diabetes research center at the University of Alabama at Birmingham. Semaglutide “essentially doubles weight loss efficacy” compared to the other approved pharmacotherapies for obesity.
With this degree of potential weight loss, clinicians “can use weight as a biomarker and treat to a target [weight-loss] range,” he said.
Expounding on this in an interview, Dr. Garvey noted that, as stated in the 2016 American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology (ACE) clinical practice guidelines for medical care of patients with obesity, of which he was lead author, “the objective of care in obesity is to increase health of patients and prevent or treat complications.”
Semaglutide “can treat to a range of weight loss of 10% to 20% in the majority of patients,” which is associated with improvements in cardiovascular and metabolic risk factors.
In STEP 5, of the 51% of patients in the semaglutide group who had prediabetes at enrollment, 80% had normal glycemia at 2 years; however, the trial was not powered nor designed to investigate this.
More data are needed to inform long-term care decisions. The ongoing SELECT cardiovascular outcomes trial of semaglutide, with expected primary study completion on Sept. 28, 2023, should provide more information.
Weight loss plus reduced cravings
In another presentation, Sean Wharton, MD, PharmD, said, “In adults with overweight or obesity, substantial weight loss with semaglutide 2.4 mg was accompanied by short- and long-term improvements in control of eating.”
“Most patients living with obesity who are attempting to decrease calories will have food cravings, based on the biological parameters of weight preservation,” Dr. Wharton, medical director at the Wharton Medical Clinic, in Hamilton, Ont., explained in an email.
The degree of craving varies from patient to patient, likely based on genetics, he added. Research in this field is still emerging.
“I believe that semaglutide 2.4 mg is a game-changer in the field of weight management, and it will change the dialogue for insurance plans and with policymakers regarding coverage for this medication,” said Dr. Wharton.
“The data from the STEP programs are very strong. I am certainly hoping for a change to bias against covering these medications that we have seen in the past,” he said.
Clinically meaningful weight loss
When presenting the STEP 8 findings, Domenica M. Rubino, MD, said: “Participants were significantly more likely to achieve clinically meaningful weight loss thresholds with semaglutide 2.4 mg versus liraglutide 3.0 mg, accompanied by greater improvements in cardiometabolic risk factors.”
For example, patients can have better mobility, which is important for quality of life, Dr. Rubino, director of the Washington Center for Weight Management and Research, Arlington, Virginia, noted.
A smaller percentage of patients respond to liraglutide, she added. Clinicians need to individualize treatment.
When asked, “How do you choose which medical therapy?” Dr. Rubino responded: “We sit and talk.” Finding the medical therapy that fits the patient depends on things such as the patient’s insurance coverage and ability to tolerate side effects such as dehydration, diarrhea, and nausea.
When asked, “How do you switch from liraglutide to semaglutide?” she noted that there are no current guidelines for this. “You have to be careful. Start on the lowest dose of Wegovy. Be cautious, conservative.”
Still early days, caveats remain
“The STEP trials as a group appear to be making the case that obesity may now be considered a medically manageable disease, based on the experience with semaglutide,” Julie R. Ingelfinger, MD, who was not involved with the research, commented in an email.
“STEP 5 and 8 may suggest that weight loss occurs and is sustainable in overweight persons without diabetes with one or more comorbidities or in obese persons without diabetes,” added Dr. Ingelfinger, professor of pediatrics, Harvard Medical School, consultant in pediatric nephrology, Massachusetts General Hospital, Boston, and deputy editor, The New England Journal of Medicine.
However, “even 2 years, in the case of STEP 5, and ~68 weeks in the case of STEP 8, may not be long enough to know whether semaglutide is as promising as these brief summaries (abstracts) suggest,” she cautioned.
“Obesity is a chronic condition, and very long-term therapy and management are required,” Dr. Ingelfinger continued.
“Further, it is hard to generalize when gastrointestinal adverse events are common in a study,” she said. For example, in STEP 8, they were just as common with semaglutide as with the comparator liraglutide, she noted.
“The racial and ethnic representativeness of these studies does not reflect population distributions in the U.S., limiting generalization,” she continued.
“So, there remain caveats in interpreting these data.”
STEP 5 weight loss efficacy and safety at 2 years
Garvey reported that STEP 5 was a phase 3b trial that randomized 304 adults in the United States, Canada, Hungary, Italy, and Spain, who were 18 years and older, with a body mass index (BMI) ≥27 kg/m2 with at least one weight-related comorbidity (hypertension, dyslipidemia, obstructive sleep apnea, or cardiovascular disease) or a BMI ≥30 kg/m2, without type 2 diabetes, to receive semaglutide or placebo plus lifestyle intervention.
Most participants were women (78%) and White (93%). On average, they were 47 years old, weighed 106 kg (223.7 pounds), had a BMI of 38.5 kg/m2, a waist circumference of 115.7 cm (45.6 inches), and an A1c of 5.7%.
A total of 87% of patients in the semaglutide group and 73% of patients in the placebo group completed the trial.
At 104 weeks, participants were more likely to lose ≥10%, ≥15%, and ≥20% of body weight with semaglutide versus placebo (61.8% vs. 13.3%, 52.1% vs. 7.0%, and 36.1% vs. 2.3%, respectively; P < .0001 for all).
Patients in the semaglutide group had greater health improvements in cardiovascular risk factors (waist circumference, systolic and diastolic blood pressure, and C-reactive protein) and metabolic risk factors (A1c, fasting plasma glucose, fasting serum insulin, and triglycerides) than those in the placebo group (P < .05 for all).
Safety and tolerability were consistent with adverse events seen with this drug class, with no new safety signals.
Control of eating questionnaire findings at 2 years in STEP 5
Dr. Wharton and colleagues assessed changes in responses to the Control of Eating questionnaire at baseline and at 20, 52, and 104 weeks in patients from the U.S. and Canada in the STEP 5 trial (88 patients in the semaglutide group and 86 patients in the placebo group).
The questionnaire consisted of 19 questions grouped into four categories: control of food cravings, craving for savory foods (salty and spicy, dairy, or starchy foods), craving for sweet foods (chocolate, sweet foods, or fruit/fruit juice), and positive mood.
At week 104, patients in the semaglutide group had significantly greater improvements in scores for craving for salty and spicy, dairy, and starchy foods, and resisting cravings.
Semaglutide versus liraglutide, 68-week efficacy and safety in STEP 8
STEP 8 randomized 338 U.S. adults without diabetes and a BMI of ≥27 kg/m2 plus one or more weight-related comorbidities or a BMI of ≥30 kg/m2 3:1 to semaglutide 2.4 mg once weekly (n = 126) or matching placebo, or 3:1 liraglutide 3.0 mg once daily (n = 127) or matching placebo, plus lifestyle intervention.
Most participants were women (78%) and were a mean age of 49, had a mean body weight of 104.5 kg, and had a mean BMI of 37.5 kg/m2.
In STEP 8, more participants achieved ≥10%, ≥15%, and ≥20% weight loss with semaglutide than with liraglutide (70.9% vs. 25.6%, 55.6% vs. 12.0%, and 38.5% vs. 6.0%, respectively; P < .001 for all odds ratios).
Semaglutide improved waist circumference, A1c, and C-reactive protein versus liraglutide (unadjusted P < .001 for all).
Gastrointestinal adverse events were reported by 84% and 83% of participants receiving semaglutide and liraglutide, respectively. Most events were mild/moderate and transient, with prevalence declining over time.
Fewer participants stopped treatment due to adverse events with semaglutide than liraglutide (3.2% vs. 12.6%).
Dr. Garvey has reported serving as a site principal investigator for multicentered clinical trials sponsored by his university and funded by Eli Lilly, Novo Nordisk, and Pfizer. Dr. Wharton has reported financial ties to Novo Nordisk, Bausch Health Canada, Eli Lily, and Boehringer Ingelheim Canada. Dr. Rubino has reported ties to Boehringer Ingelheim and AstraZeneca. Dr. Ingelfinger has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The STEP 5 clinical trial extends favorable weight loss from 1 year out to 2 years for the glucagon-like peptide-1 (GLP-1) agonist semaglutide (Wegovy, Novo Nordisk), given as a once-weekly 2.4-mg subcutaneous injection, and some food cravings were improved in a subgroup analysis.
In another study, STEP 8, weight loss was greater at 68 weeks with semaglutide subcutaneous injection than with a 3-mg daily subcutaneous injection of another GLP-1 agonist, liraglutide (Saxenda, Novo Nordisk), approved earlier for weight loss.
Researchers presented these promising outcomes, with no new safety signals, at ObesityWeek® 2021.
However, there is more to learn about the drug class, researchers agree. Follow-up is still relatively short for a chronic disease and many patients have gastrointestinal side effects with semaglutide, one expert cautions.
The key findings were:
In STEP 5, combined with lifestyle intervention (a reduced-calorie meal plan and advice about physical activity), weekly injection of 2.4 mg semaglutide led to:
- 15.2% weight loss, compared with 2.6% weight loss with placebo at 2 years (P < .0001);
- 77% of patients losing at least 5% of their weight, compared with 34% of patients in the placebo group at 2 years (P < .0001);
- Significantly greater improvement in overall control of cravings, and craving for savory foods, in a subset of patients, versus placebo, but questionnaire scores for positive mood and craving for sweet foods were similar in both groups.
- In STEP 8, mean body weight at 68 weeks was 15.8% lower with 2.4 mg/week subcutaneous semaglutide plus lifestyle changes versus 6.4% lower with 3.0 mg/day subcutaneous liraglutide plus lifestyle changes (P < .001).
Can treat to a target weight-loss range
The undiminished weight loss efficacy in the 2-year data for STEP 5 “portends well,” said W. Timothy Garvey, MD, following his presentation of the results.
“I think this is a new era in obesity care,” said Dr. Garvey, director of the diabetes research center at the University of Alabama at Birmingham. Semaglutide “essentially doubles weight loss efficacy” compared to the other approved pharmacotherapies for obesity.
With this degree of potential weight loss, clinicians “can use weight as a biomarker and treat to a target [weight-loss] range,” he said.
Expounding on this in an interview, Dr. Garvey noted that, as stated in the 2016 American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology (ACE) clinical practice guidelines for medical care of patients with obesity, of which he was lead author, “the objective of care in obesity is to increase health of patients and prevent or treat complications.”
Semaglutide “can treat to a range of weight loss of 10% to 20% in the majority of patients,” which is associated with improvements in cardiovascular and metabolic risk factors.
In STEP 5, of the 51% of patients in the semaglutide group who had prediabetes at enrollment, 80% had normal glycemia at 2 years; however, the trial was not powered nor designed to investigate this.
More data are needed to inform long-term care decisions. The ongoing SELECT cardiovascular outcomes trial of semaglutide, with expected primary study completion on Sept. 28, 2023, should provide more information.
Weight loss plus reduced cravings
In another presentation, Sean Wharton, MD, PharmD, said, “In adults with overweight or obesity, substantial weight loss with semaglutide 2.4 mg was accompanied by short- and long-term improvements in control of eating.”
“Most patients living with obesity who are attempting to decrease calories will have food cravings, based on the biological parameters of weight preservation,” Dr. Wharton, medical director at the Wharton Medical Clinic, in Hamilton, Ont., explained in an email.
The degree of craving varies from patient to patient, likely based on genetics, he added. Research in this field is still emerging.
“I believe that semaglutide 2.4 mg is a game-changer in the field of weight management, and it will change the dialogue for insurance plans and with policymakers regarding coverage for this medication,” said Dr. Wharton.
“The data from the STEP programs are very strong. I am certainly hoping for a change to bias against covering these medications that we have seen in the past,” he said.
Clinically meaningful weight loss
When presenting the STEP 8 findings, Domenica M. Rubino, MD, said: “Participants were significantly more likely to achieve clinically meaningful weight loss thresholds with semaglutide 2.4 mg versus liraglutide 3.0 mg, accompanied by greater improvements in cardiometabolic risk factors.”
For example, patients can have better mobility, which is important for quality of life, Dr. Rubino, director of the Washington Center for Weight Management and Research, Arlington, Virginia, noted.
A smaller percentage of patients respond to liraglutide, she added. Clinicians need to individualize treatment.
When asked, “How do you choose which medical therapy?” Dr. Rubino responded: “We sit and talk.” Finding the medical therapy that fits the patient depends on things such as the patient’s insurance coverage and ability to tolerate side effects such as dehydration, diarrhea, and nausea.
When asked, “How do you switch from liraglutide to semaglutide?” she noted that there are no current guidelines for this. “You have to be careful. Start on the lowest dose of Wegovy. Be cautious, conservative.”
Still early days, caveats remain
“The STEP trials as a group appear to be making the case that obesity may now be considered a medically manageable disease, based on the experience with semaglutide,” Julie R. Ingelfinger, MD, who was not involved with the research, commented in an email.
“STEP 5 and 8 may suggest that weight loss occurs and is sustainable in overweight persons without diabetes with one or more comorbidities or in obese persons without diabetes,” added Dr. Ingelfinger, professor of pediatrics, Harvard Medical School, consultant in pediatric nephrology, Massachusetts General Hospital, Boston, and deputy editor, The New England Journal of Medicine.
However, “even 2 years, in the case of STEP 5, and ~68 weeks in the case of STEP 8, may not be long enough to know whether semaglutide is as promising as these brief summaries (abstracts) suggest,” she cautioned.
“Obesity is a chronic condition, and very long-term therapy and management are required,” Dr. Ingelfinger continued.
“Further, it is hard to generalize when gastrointestinal adverse events are common in a study,” she said. For example, in STEP 8, they were just as common with semaglutide as with the comparator liraglutide, she noted.
“The racial and ethnic representativeness of these studies does not reflect population distributions in the U.S., limiting generalization,” she continued.
“So, there remain caveats in interpreting these data.”
STEP 5 weight loss efficacy and safety at 2 years
Garvey reported that STEP 5 was a phase 3b trial that randomized 304 adults in the United States, Canada, Hungary, Italy, and Spain, who were 18 years and older, with a body mass index (BMI) ≥27 kg/m2 with at least one weight-related comorbidity (hypertension, dyslipidemia, obstructive sleep apnea, or cardiovascular disease) or a BMI ≥30 kg/m2, without type 2 diabetes, to receive semaglutide or placebo plus lifestyle intervention.
Most participants were women (78%) and White (93%). On average, they were 47 years old, weighed 106 kg (223.7 pounds), had a BMI of 38.5 kg/m2, a waist circumference of 115.7 cm (45.6 inches), and an A1c of 5.7%.
A total of 87% of patients in the semaglutide group and 73% of patients in the placebo group completed the trial.
At 104 weeks, participants were more likely to lose ≥10%, ≥15%, and ≥20% of body weight with semaglutide versus placebo (61.8% vs. 13.3%, 52.1% vs. 7.0%, and 36.1% vs. 2.3%, respectively; P < .0001 for all).
Patients in the semaglutide group had greater health improvements in cardiovascular risk factors (waist circumference, systolic and diastolic blood pressure, and C-reactive protein) and metabolic risk factors (A1c, fasting plasma glucose, fasting serum insulin, and triglycerides) than those in the placebo group (P < .05 for all).
Safety and tolerability were consistent with adverse events seen with this drug class, with no new safety signals.
Control of eating questionnaire findings at 2 years in STEP 5
Dr. Wharton and colleagues assessed changes in responses to the Control of Eating questionnaire at baseline and at 20, 52, and 104 weeks in patients from the U.S. and Canada in the STEP 5 trial (88 patients in the semaglutide group and 86 patients in the placebo group).
The questionnaire consisted of 19 questions grouped into four categories: control of food cravings, craving for savory foods (salty and spicy, dairy, or starchy foods), craving for sweet foods (chocolate, sweet foods, or fruit/fruit juice), and positive mood.
At week 104, patients in the semaglutide group had significantly greater improvements in scores for craving for salty and spicy, dairy, and starchy foods, and resisting cravings.
Semaglutide versus liraglutide, 68-week efficacy and safety in STEP 8
STEP 8 randomized 338 U.S. adults without diabetes and a BMI of ≥27 kg/m2 plus one or more weight-related comorbidities or a BMI of ≥30 kg/m2 3:1 to semaglutide 2.4 mg once weekly (n = 126) or matching placebo, or 3:1 liraglutide 3.0 mg once daily (n = 127) or matching placebo, plus lifestyle intervention.
Most participants were women (78%) and were a mean age of 49, had a mean body weight of 104.5 kg, and had a mean BMI of 37.5 kg/m2.
In STEP 8, more participants achieved ≥10%, ≥15%, and ≥20% weight loss with semaglutide than with liraglutide (70.9% vs. 25.6%, 55.6% vs. 12.0%, and 38.5% vs. 6.0%, respectively; P < .001 for all odds ratios).
Semaglutide improved waist circumference, A1c, and C-reactive protein versus liraglutide (unadjusted P < .001 for all).
Gastrointestinal adverse events were reported by 84% and 83% of participants receiving semaglutide and liraglutide, respectively. Most events were mild/moderate and transient, with prevalence declining over time.
Fewer participants stopped treatment due to adverse events with semaglutide than liraglutide (3.2% vs. 12.6%).
Dr. Garvey has reported serving as a site principal investigator for multicentered clinical trials sponsored by his university and funded by Eli Lilly, Novo Nordisk, and Pfizer. Dr. Wharton has reported financial ties to Novo Nordisk, Bausch Health Canada, Eli Lily, and Boehringer Ingelheim Canada. Dr. Rubino has reported ties to Boehringer Ingelheim and AstraZeneca. Dr. Ingelfinger has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treating young adults with high LDL may be cost-effective
Treating elevated low-density lipoprotein cholesterol (LDL-C) in adults younger than 40 with statins is highly cost-effective in men, and intermediately cost-effective in women, a new report suggests.
In a simulated model based on data from the U.S. National Health and Nutrition Examination Survey (NHANES), lipid lowering with statins or lifestyle interventions in this age group would prevent or reduce the risk of atherosclerotic cardiovascular disease (ASCVD) and improve quality of life in later years.
The findings were published online Nov. 8 in the Journal of the American College of Cardiology.
“My group does epidemiologic analyses with cohort studies as well as health economic analyses like this one, and if you have long-term longitudinal observation, you see that the early exposures are important for what happens later,” senior author Andrew E. Moran, MD, Columbia University Irving Medical Center, New York, told this news organization.
“But when it comes to treatment studies that a lot of the treatment guidelines are based on, those are usually short-term, and they usually enroll older people. We saw the gap in the evidence that this paper tries to fill,” Dr. Moran said.
His group used a computer simulation model to synthesize evidence from observational cohort studies and clinical trials of statin treatment, as well as health services data on the costs of medicines and treatments.
Combining information from these sources, the investigators made their best estimates of the potential health benefits and costs of treating high cholesterol earlier in life, compared with standard care, which was statin treatment at age 40, or if LDL-C was 190 mg/dL or greater.
Lipid lowering incremental to standard care with moderate-intensity statins or intensive lifestyle interventions was simulated starting when young adult LDL-C was either ≥160 mg/dL or ≥130 mg/dL.
They found that approximately 27% of young adults who are free of ASCVD have LDL-C ≥130 mg/dL, and 9% have LDL-C of ≥160 mg/dL.
Their model projected that treating adults younger than 40 with statins or lifestyle interventions would prevent lifetime ASCVD events and increase quality-adjusted life years (QALYs) compared with standard care, which would begin treatment at age 40.
Incremental cost-effectiveness ratios (ICERs) were $31,000/QALY for statin treatment in young adult men with LDL-C ≥130 mg/dL, and $106,000/QALY for statin treatment in young women with LDL-C ≥130 mg/dL.
Intensive lifestyle intervention was more costly and less effective than statin therapy.
“We are straining to find these young adults with very high cholesterol,” Dr. Moran noted. “A lot of young adults don’t even see a doctor. This is an argument for engaging them in their health care and getting them involved in some basic screening. Atherosclerosis is a long-term process that starts in childhood for a lot of people.”
More innovative approaches may be needed, because the traditional health care system is not doing a good job of reaching young adults, he added. “Many of them may not have adequate health insurance. They need health care in nontraditional ways; convenience is really important for them. Perhaps part of the solution here is to think about ways of reaching this particular group that is not engaged with health care generally.”
Time to relax the age 40 threshold
The U.S. Preventive Services Task Force and the American College of Cardiology/American Heart Association should emphasize lifetime risk of elevated cholesterol, Paul A. Heidenreich, MD, MS, Stanford University School of Medicine, California, and colleagues write in an accompanying editorial.
“In addition to calculating 10-year risk, we should calculate years of life lost (or QALYs lost) from unhealthy LDL-C levels, and both lifestyle and pharmacologic treatment should be considered to treat high LDL-C in adults regardless of age. We also need to communicate that the mantra ‘lower is better’ applies not only to a single measurement but to lifetime exposure to LDL-C,” the editorialists write.
“I think treatment should be earlier than age 40,” Dr. Heidenreich said in an interview.
“Part of the reason that 40 was chosen as a threshold was because everyone looked at 10-year, or even 20-year risk, and thought there was no reason to worry until you get older. It’s interesting that we never accepted that with high blood pressure. But more and more, we are learning that it is a lifelong process,” he said.
“Statins are getting less and less expensive, and their safety is more and more established with every decade that goes by. I definitely agree with this paper that it would actually make sense to be starting much earlier for those with elevated CVD risk from their high cholesterol.”
The study was supported by the U.S. National Heart, Lung, and Blood Institute (NHLBI), the Medical Research Council, Swindon, U.K. Dr. Moran and Dr. Heidenreich have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treating elevated low-density lipoprotein cholesterol (LDL-C) in adults younger than 40 with statins is highly cost-effective in men, and intermediately cost-effective in women, a new report suggests.
In a simulated model based on data from the U.S. National Health and Nutrition Examination Survey (NHANES), lipid lowering with statins or lifestyle interventions in this age group would prevent or reduce the risk of atherosclerotic cardiovascular disease (ASCVD) and improve quality of life in later years.
The findings were published online Nov. 8 in the Journal of the American College of Cardiology.
“My group does epidemiologic analyses with cohort studies as well as health economic analyses like this one, and if you have long-term longitudinal observation, you see that the early exposures are important for what happens later,” senior author Andrew E. Moran, MD, Columbia University Irving Medical Center, New York, told this news organization.
“But when it comes to treatment studies that a lot of the treatment guidelines are based on, those are usually short-term, and they usually enroll older people. We saw the gap in the evidence that this paper tries to fill,” Dr. Moran said.
His group used a computer simulation model to synthesize evidence from observational cohort studies and clinical trials of statin treatment, as well as health services data on the costs of medicines and treatments.
Combining information from these sources, the investigators made their best estimates of the potential health benefits and costs of treating high cholesterol earlier in life, compared with standard care, which was statin treatment at age 40, or if LDL-C was 190 mg/dL or greater.
Lipid lowering incremental to standard care with moderate-intensity statins or intensive lifestyle interventions was simulated starting when young adult LDL-C was either ≥160 mg/dL or ≥130 mg/dL.
They found that approximately 27% of young adults who are free of ASCVD have LDL-C ≥130 mg/dL, and 9% have LDL-C of ≥160 mg/dL.
Their model projected that treating adults younger than 40 with statins or lifestyle interventions would prevent lifetime ASCVD events and increase quality-adjusted life years (QALYs) compared with standard care, which would begin treatment at age 40.
Incremental cost-effectiveness ratios (ICERs) were $31,000/QALY for statin treatment in young adult men with LDL-C ≥130 mg/dL, and $106,000/QALY for statin treatment in young women with LDL-C ≥130 mg/dL.
Intensive lifestyle intervention was more costly and less effective than statin therapy.
“We are straining to find these young adults with very high cholesterol,” Dr. Moran noted. “A lot of young adults don’t even see a doctor. This is an argument for engaging them in their health care and getting them involved in some basic screening. Atherosclerosis is a long-term process that starts in childhood for a lot of people.”
More innovative approaches may be needed, because the traditional health care system is not doing a good job of reaching young adults, he added. “Many of them may not have adequate health insurance. They need health care in nontraditional ways; convenience is really important for them. Perhaps part of the solution here is to think about ways of reaching this particular group that is not engaged with health care generally.”
Time to relax the age 40 threshold
The U.S. Preventive Services Task Force and the American College of Cardiology/American Heart Association should emphasize lifetime risk of elevated cholesterol, Paul A. Heidenreich, MD, MS, Stanford University School of Medicine, California, and colleagues write in an accompanying editorial.
“In addition to calculating 10-year risk, we should calculate years of life lost (or QALYs lost) from unhealthy LDL-C levels, and both lifestyle and pharmacologic treatment should be considered to treat high LDL-C in adults regardless of age. We also need to communicate that the mantra ‘lower is better’ applies not only to a single measurement but to lifetime exposure to LDL-C,” the editorialists write.
“I think treatment should be earlier than age 40,” Dr. Heidenreich said in an interview.
“Part of the reason that 40 was chosen as a threshold was because everyone looked at 10-year, or even 20-year risk, and thought there was no reason to worry until you get older. It’s interesting that we never accepted that with high blood pressure. But more and more, we are learning that it is a lifelong process,” he said.
“Statins are getting less and less expensive, and their safety is more and more established with every decade that goes by. I definitely agree with this paper that it would actually make sense to be starting much earlier for those with elevated CVD risk from their high cholesterol.”
The study was supported by the U.S. National Heart, Lung, and Blood Institute (NHLBI), the Medical Research Council, Swindon, U.K. Dr. Moran and Dr. Heidenreich have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treating elevated low-density lipoprotein cholesterol (LDL-C) in adults younger than 40 with statins is highly cost-effective in men, and intermediately cost-effective in women, a new report suggests.
In a simulated model based on data from the U.S. National Health and Nutrition Examination Survey (NHANES), lipid lowering with statins or lifestyle interventions in this age group would prevent or reduce the risk of atherosclerotic cardiovascular disease (ASCVD) and improve quality of life in later years.
The findings were published online Nov. 8 in the Journal of the American College of Cardiology.
“My group does epidemiologic analyses with cohort studies as well as health economic analyses like this one, and if you have long-term longitudinal observation, you see that the early exposures are important for what happens later,” senior author Andrew E. Moran, MD, Columbia University Irving Medical Center, New York, told this news organization.
“But when it comes to treatment studies that a lot of the treatment guidelines are based on, those are usually short-term, and they usually enroll older people. We saw the gap in the evidence that this paper tries to fill,” Dr. Moran said.
His group used a computer simulation model to synthesize evidence from observational cohort studies and clinical trials of statin treatment, as well as health services data on the costs of medicines and treatments.
Combining information from these sources, the investigators made their best estimates of the potential health benefits and costs of treating high cholesterol earlier in life, compared with standard care, which was statin treatment at age 40, or if LDL-C was 190 mg/dL or greater.
Lipid lowering incremental to standard care with moderate-intensity statins or intensive lifestyle interventions was simulated starting when young adult LDL-C was either ≥160 mg/dL or ≥130 mg/dL.
They found that approximately 27% of young adults who are free of ASCVD have LDL-C ≥130 mg/dL, and 9% have LDL-C of ≥160 mg/dL.
Their model projected that treating adults younger than 40 with statins or lifestyle interventions would prevent lifetime ASCVD events and increase quality-adjusted life years (QALYs) compared with standard care, which would begin treatment at age 40.
Incremental cost-effectiveness ratios (ICERs) were $31,000/QALY for statin treatment in young adult men with LDL-C ≥130 mg/dL, and $106,000/QALY for statin treatment in young women with LDL-C ≥130 mg/dL.
Intensive lifestyle intervention was more costly and less effective than statin therapy.
“We are straining to find these young adults with very high cholesterol,” Dr. Moran noted. “A lot of young adults don’t even see a doctor. This is an argument for engaging them in their health care and getting them involved in some basic screening. Atherosclerosis is a long-term process that starts in childhood for a lot of people.”
More innovative approaches may be needed, because the traditional health care system is not doing a good job of reaching young adults, he added. “Many of them may not have adequate health insurance. They need health care in nontraditional ways; convenience is really important for them. Perhaps part of the solution here is to think about ways of reaching this particular group that is not engaged with health care generally.”
Time to relax the age 40 threshold
The U.S. Preventive Services Task Force and the American College of Cardiology/American Heart Association should emphasize lifetime risk of elevated cholesterol, Paul A. Heidenreich, MD, MS, Stanford University School of Medicine, California, and colleagues write in an accompanying editorial.
“In addition to calculating 10-year risk, we should calculate years of life lost (or QALYs lost) from unhealthy LDL-C levels, and both lifestyle and pharmacologic treatment should be considered to treat high LDL-C in adults regardless of age. We also need to communicate that the mantra ‘lower is better’ applies not only to a single measurement but to lifetime exposure to LDL-C,” the editorialists write.
“I think treatment should be earlier than age 40,” Dr. Heidenreich said in an interview.
“Part of the reason that 40 was chosen as a threshold was because everyone looked at 10-year, or even 20-year risk, and thought there was no reason to worry until you get older. It’s interesting that we never accepted that with high blood pressure. But more and more, we are learning that it is a lifelong process,” he said.
“Statins are getting less and less expensive, and their safety is more and more established with every decade that goes by. I definitely agree with this paper that it would actually make sense to be starting much earlier for those with elevated CVD risk from their high cholesterol.”
The study was supported by the U.S. National Heart, Lung, and Blood Institute (NHLBI), the Medical Research Council, Swindon, U.K. Dr. Moran and Dr. Heidenreich have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AHA 2021 puts scientific dialogue, health equity center stage
Virtual platforms democratized scientific meetings during the COVID-19 pandemic but, as any meeting-goer will tell you, it’s the questions from the floor and the back-and-forth of an expert panel that often reveal the importance of and/or problems with a presentation. It’s the scrutiny that makes the science resonate, especially in this postfactual era.
The all-virtual American Heart Association Scientific Sessions 2021 is looking to recreate the engagement of an in-person meeting by offering more live interactive events. They range from seven late-breaking science (LBS) sessions to Saturday’s fireside chat on the Pfizer and Moderna COVID-19 vaccines and Monday’s dive into the controversial new AHA/American College of Cardiology Chest Pain guidelines.
To help digest the latest science, attendees will be able to have their questions answered in real-time via Slido, meet with the trialists, and hear live commentary from key opinion leaders after the live events. A networking function will also allow attendees and exhibitors to chat or meet virtually.
“In this day and age, many people pretty quickly can get access to the science but it’s what I call the IC sort of phenomenon – the presentation of the information, the context of the information, putting it into how I’m going to use it in my practice, and then the critical appraisal – that’s what most people want at the Scientific Sessions,” program committee chair Manesh R. Patel, MD, of Duke University School of Medicine, said in an interview. “We’re all craving ways in which we can interact with one another to put things in context.”
Plans for a hybrid in-person meeting in Boston were scuttled in September because of the Delta variant surge, but the theme of the meeting remained: “One World. Together for Science.” Attendees will be able to access more than 500 live and on-demand sessions including 117 oral abstracts, 286 poster sessions, 59 moderated digital posters, and over a dozen sessions focused on strategies to promote health equity.
“Last year there was a Presidential Session and a statement on structural racism, so we wanted to take the next step and say, What are the ways in which people are starting to interact and do things to make a difference?” explained Dr. Patel. “So, this year, you’ll see different versions of that from the Main Event session, which has some case vignettes and a panel discussion, to other health equity sessions that describe not just COVID care, but blood pressure care, maternal-fetal medicine, and congenital kids. Wherever we can, we’ve tried to infuse it throughout the sessions and will continue to.”
Late-breaking science
The LBS sessions kick off at 9:30 a.m. ET Saturday with AVATAR, a randomized trial of aortic valve replacement vs. watchful waiting in severe aortic stenosis proved asymptomatic through exercise testing.
“The findings of that trial, depending on what they are, could certainly impact clinical practice because it’s a very common scenario in which we have elderly patients with aortic valve stenosis that might be severe but they may not be symptomatic,” he said.
It’s followed by a randomized trial from the Cardiothoracic Surgical Trials Network, examining whether tricuspid repair at the time of mitral valve surgery leads to beneficial outcomes. “I think it’s a pretty important study,” Dr. Patel said, “because it’ll again affect how we think about our clinical practice.”
Rounding out the LBS.01 session is RAPID CABG, comparing early vs. delayed coronary bypass graft surgery (CABG) in patients with acute coronary syndromes on ticagrelor, and the pivotal U.S. VEST trial of an external support device already approved in Europe for saphenous vein grafts during CABG.
Saturday’s LBS.02 at 3:00 p.m. ET is devoted to hypertension and looks at how the COVID-19 pandemic affected blood pressure control. There’s also a study of remotely delivered hypertension and lipid management in 10,000 patients across the Partners Healthcare System and a cluster randomized trial of a village doctor–led blood pressure intervention in rural China.
Sunday’s LBS.03 at 8:00 a.m. ET is focused on atrial arrhythmias, starting with the CRAVE trial examining the effect of caffeine consumption on cardiac ectopy burden in 108 patients using an N-of-1 design and 2-day blocks on and off caffeine. “There’s an ability to identify a dose response that you get arrhythmias when you increase the amount of coffee you drink vs. not in an individual, so I think that will be likely discussed a lot and worth paying attention to,” Dr. Patel said.
The session also includes GIRAF, a comparison of cognitive outcomes with dabigatran (Pradaxa) vs. warfarin (Coumadin) in nonvalvular atrial fibrillation (AF); PALACS, a randomized trial examining whether left-sided pericardiotomy prevents AF after cardiac surgery; and AMAZE, which study sponsor AtriCure revealed missed its primary efficacy endpoint of freedom from AF with the LARIAT suture delivery device for left atrial appendage closure plus pulmonary vein isolation.
LBS.04 at 3:30 p.m. ET Sunday takes on digital health, with results from the nonrandomized Fitbit Heart Study on AF notifications from 450,000 participants wearing a single-lead ECG patch. “A lot of technologies claim that they can detect things, and we should ask that people go through the rigorous evaluation to see if they in fact do. So, in that respect, I think it›s an important step,” observed Dr. Patel.
Also on tap is I-STOP-AFib, another N-of-1 study using mobile apps and the AliveCor device to identify individual AF triggers; and REVeAL-HF, a 4,000-patient study examining whether electronic alerts that provide clinicians with prognostic information on their heart failure (HF) patients will reduce mortality and 30-day HF hospitalizations.
LBS.05 at 5:00 p.m. ET provides new information from EMPEROR-Preserved in HF with preserved ejection fraction and main results from EMPULSE, also using the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) in 530 patients hospitalized for acute HF.
The session also features CHIEF-HF, a randomized trial leveraging mobile technologies to test whether 12 weeks of another SGLT2 inhibitor, canagliflozin (Invokana), is superior to placebo for improving HF symptoms; and DREAM-HF, a comparison of transendocardial delivery of allogeneic mesenchymal precursor cells vs. a sham comparator in chronic HF as a result of left ventricular systolic dysfunction.
Monday’s LBS.06 at 8:00 a.m. ET details the safety and cholesterol-lowering efficacy of MK-0616, an investigational oral PCSK9 inhibitor. “It’s just a phase 2 [trial], but there’s interest in an oral PCSK9 inhibitor, given that the current ones are subcutaneous,” Dr. Patel said.
Results will also be presented from PREPARE-IT 2, which tested icosapent ethyl vs. placebo in outpatients with COVID-19. In the recently reported PREPARE-IT 1, a loading dose of icosapent ethyl failed to reduce the risk of hospitalization with SARS-CoV-2 infection among at-risk individuals.
LBS.07 at 11:00 a.m. Monday completes the late-breakers with new results from ASCEND, this time examining the effect of aspirin on dementia and cognitive impairment in patients with diabetes.
Next up is a look at the effectiveness of P2Y12 inhibitors in hospitalized patients with COVID-19 in the adaptive ACTIV-4a trial, followed by results of the pivotal phase 3 REVERSE-IT trial of bentracimab, a recombinant human monoclonal antibody antigen fragment designed to reverse the antiplatelet activity of ticagrelor in the event of major bleeding or when urgent surgery is needed.
Closing out the session is AXIOMATIC-TKR, a double-blind comparison of the safety and efficacy of the investigational oral factor XI anticoagulant JNJ-70033093 vs. subcutaneous enoxaparin (Lovenox) in elective total knee replacement.
For those searching for more AHA-related science online, the Resuscitation Science Symposium (ReSS) will run from this Friday through Sunday and the Quality of Care and Outcomes Research (QCOR) Scientific Sessions will take the stage next Monday, Nov. 15.
A version of this article first appeared on Medscape.com.
Virtual platforms democratized scientific meetings during the COVID-19 pandemic but, as any meeting-goer will tell you, it’s the questions from the floor and the back-and-forth of an expert panel that often reveal the importance of and/or problems with a presentation. It’s the scrutiny that makes the science resonate, especially in this postfactual era.
The all-virtual American Heart Association Scientific Sessions 2021 is looking to recreate the engagement of an in-person meeting by offering more live interactive events. They range from seven late-breaking science (LBS) sessions to Saturday’s fireside chat on the Pfizer and Moderna COVID-19 vaccines and Monday’s dive into the controversial new AHA/American College of Cardiology Chest Pain guidelines.
To help digest the latest science, attendees will be able to have their questions answered in real-time via Slido, meet with the trialists, and hear live commentary from key opinion leaders after the live events. A networking function will also allow attendees and exhibitors to chat or meet virtually.
“In this day and age, many people pretty quickly can get access to the science but it’s what I call the IC sort of phenomenon – the presentation of the information, the context of the information, putting it into how I’m going to use it in my practice, and then the critical appraisal – that’s what most people want at the Scientific Sessions,” program committee chair Manesh R. Patel, MD, of Duke University School of Medicine, said in an interview. “We’re all craving ways in which we can interact with one another to put things in context.”
Plans for a hybrid in-person meeting in Boston were scuttled in September because of the Delta variant surge, but the theme of the meeting remained: “One World. Together for Science.” Attendees will be able to access more than 500 live and on-demand sessions including 117 oral abstracts, 286 poster sessions, 59 moderated digital posters, and over a dozen sessions focused on strategies to promote health equity.
“Last year there was a Presidential Session and a statement on structural racism, so we wanted to take the next step and say, What are the ways in which people are starting to interact and do things to make a difference?” explained Dr. Patel. “So, this year, you’ll see different versions of that from the Main Event session, which has some case vignettes and a panel discussion, to other health equity sessions that describe not just COVID care, but blood pressure care, maternal-fetal medicine, and congenital kids. Wherever we can, we’ve tried to infuse it throughout the sessions and will continue to.”
Late-breaking science
The LBS sessions kick off at 9:30 a.m. ET Saturday with AVATAR, a randomized trial of aortic valve replacement vs. watchful waiting in severe aortic stenosis proved asymptomatic through exercise testing.
“The findings of that trial, depending on what they are, could certainly impact clinical practice because it’s a very common scenario in which we have elderly patients with aortic valve stenosis that might be severe but they may not be symptomatic,” he said.
It’s followed by a randomized trial from the Cardiothoracic Surgical Trials Network, examining whether tricuspid repair at the time of mitral valve surgery leads to beneficial outcomes. “I think it’s a pretty important study,” Dr. Patel said, “because it’ll again affect how we think about our clinical practice.”
Rounding out the LBS.01 session is RAPID CABG, comparing early vs. delayed coronary bypass graft surgery (CABG) in patients with acute coronary syndromes on ticagrelor, and the pivotal U.S. VEST trial of an external support device already approved in Europe for saphenous vein grafts during CABG.
Saturday’s LBS.02 at 3:00 p.m. ET is devoted to hypertension and looks at how the COVID-19 pandemic affected blood pressure control. There’s also a study of remotely delivered hypertension and lipid management in 10,000 patients across the Partners Healthcare System and a cluster randomized trial of a village doctor–led blood pressure intervention in rural China.
Sunday’s LBS.03 at 8:00 a.m. ET is focused on atrial arrhythmias, starting with the CRAVE trial examining the effect of caffeine consumption on cardiac ectopy burden in 108 patients using an N-of-1 design and 2-day blocks on and off caffeine. “There’s an ability to identify a dose response that you get arrhythmias when you increase the amount of coffee you drink vs. not in an individual, so I think that will be likely discussed a lot and worth paying attention to,” Dr. Patel said.
The session also includes GIRAF, a comparison of cognitive outcomes with dabigatran (Pradaxa) vs. warfarin (Coumadin) in nonvalvular atrial fibrillation (AF); PALACS, a randomized trial examining whether left-sided pericardiotomy prevents AF after cardiac surgery; and AMAZE, which study sponsor AtriCure revealed missed its primary efficacy endpoint of freedom from AF with the LARIAT suture delivery device for left atrial appendage closure plus pulmonary vein isolation.
LBS.04 at 3:30 p.m. ET Sunday takes on digital health, with results from the nonrandomized Fitbit Heart Study on AF notifications from 450,000 participants wearing a single-lead ECG patch. “A lot of technologies claim that they can detect things, and we should ask that people go through the rigorous evaluation to see if they in fact do. So, in that respect, I think it›s an important step,” observed Dr. Patel.
Also on tap is I-STOP-AFib, another N-of-1 study using mobile apps and the AliveCor device to identify individual AF triggers; and REVeAL-HF, a 4,000-patient study examining whether electronic alerts that provide clinicians with prognostic information on their heart failure (HF) patients will reduce mortality and 30-day HF hospitalizations.
LBS.05 at 5:00 p.m. ET provides new information from EMPEROR-Preserved in HF with preserved ejection fraction and main results from EMPULSE, also using the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) in 530 patients hospitalized for acute HF.
The session also features CHIEF-HF, a randomized trial leveraging mobile technologies to test whether 12 weeks of another SGLT2 inhibitor, canagliflozin (Invokana), is superior to placebo for improving HF symptoms; and DREAM-HF, a comparison of transendocardial delivery of allogeneic mesenchymal precursor cells vs. a sham comparator in chronic HF as a result of left ventricular systolic dysfunction.
Monday’s LBS.06 at 8:00 a.m. ET details the safety and cholesterol-lowering efficacy of MK-0616, an investigational oral PCSK9 inhibitor. “It’s just a phase 2 [trial], but there’s interest in an oral PCSK9 inhibitor, given that the current ones are subcutaneous,” Dr. Patel said.
Results will also be presented from PREPARE-IT 2, which tested icosapent ethyl vs. placebo in outpatients with COVID-19. In the recently reported PREPARE-IT 1, a loading dose of icosapent ethyl failed to reduce the risk of hospitalization with SARS-CoV-2 infection among at-risk individuals.
LBS.07 at 11:00 a.m. Monday completes the late-breakers with new results from ASCEND, this time examining the effect of aspirin on dementia and cognitive impairment in patients with diabetes.
Next up is a look at the effectiveness of P2Y12 inhibitors in hospitalized patients with COVID-19 in the adaptive ACTIV-4a trial, followed by results of the pivotal phase 3 REVERSE-IT trial of bentracimab, a recombinant human monoclonal antibody antigen fragment designed to reverse the antiplatelet activity of ticagrelor in the event of major bleeding or when urgent surgery is needed.
Closing out the session is AXIOMATIC-TKR, a double-blind comparison of the safety and efficacy of the investigational oral factor XI anticoagulant JNJ-70033093 vs. subcutaneous enoxaparin (Lovenox) in elective total knee replacement.
For those searching for more AHA-related science online, the Resuscitation Science Symposium (ReSS) will run from this Friday through Sunday and the Quality of Care and Outcomes Research (QCOR) Scientific Sessions will take the stage next Monday, Nov. 15.
A version of this article first appeared on Medscape.com.
Virtual platforms democratized scientific meetings during the COVID-19 pandemic but, as any meeting-goer will tell you, it’s the questions from the floor and the back-and-forth of an expert panel that often reveal the importance of and/or problems with a presentation. It’s the scrutiny that makes the science resonate, especially in this postfactual era.
The all-virtual American Heart Association Scientific Sessions 2021 is looking to recreate the engagement of an in-person meeting by offering more live interactive events. They range from seven late-breaking science (LBS) sessions to Saturday’s fireside chat on the Pfizer and Moderna COVID-19 vaccines and Monday’s dive into the controversial new AHA/American College of Cardiology Chest Pain guidelines.
To help digest the latest science, attendees will be able to have their questions answered in real-time via Slido, meet with the trialists, and hear live commentary from key opinion leaders after the live events. A networking function will also allow attendees and exhibitors to chat or meet virtually.
“In this day and age, many people pretty quickly can get access to the science but it’s what I call the IC sort of phenomenon – the presentation of the information, the context of the information, putting it into how I’m going to use it in my practice, and then the critical appraisal – that’s what most people want at the Scientific Sessions,” program committee chair Manesh R. Patel, MD, of Duke University School of Medicine, said in an interview. “We’re all craving ways in which we can interact with one another to put things in context.”
Plans for a hybrid in-person meeting in Boston were scuttled in September because of the Delta variant surge, but the theme of the meeting remained: “One World. Together for Science.” Attendees will be able to access more than 500 live and on-demand sessions including 117 oral abstracts, 286 poster sessions, 59 moderated digital posters, and over a dozen sessions focused on strategies to promote health equity.
“Last year there was a Presidential Session and a statement on structural racism, so we wanted to take the next step and say, What are the ways in which people are starting to interact and do things to make a difference?” explained Dr. Patel. “So, this year, you’ll see different versions of that from the Main Event session, which has some case vignettes and a panel discussion, to other health equity sessions that describe not just COVID care, but blood pressure care, maternal-fetal medicine, and congenital kids. Wherever we can, we’ve tried to infuse it throughout the sessions and will continue to.”
Late-breaking science
The LBS sessions kick off at 9:30 a.m. ET Saturday with AVATAR, a randomized trial of aortic valve replacement vs. watchful waiting in severe aortic stenosis proved asymptomatic through exercise testing.
“The findings of that trial, depending on what they are, could certainly impact clinical practice because it’s a very common scenario in which we have elderly patients with aortic valve stenosis that might be severe but they may not be symptomatic,” he said.
It’s followed by a randomized trial from the Cardiothoracic Surgical Trials Network, examining whether tricuspid repair at the time of mitral valve surgery leads to beneficial outcomes. “I think it’s a pretty important study,” Dr. Patel said, “because it’ll again affect how we think about our clinical practice.”
Rounding out the LBS.01 session is RAPID CABG, comparing early vs. delayed coronary bypass graft surgery (CABG) in patients with acute coronary syndromes on ticagrelor, and the pivotal U.S. VEST trial of an external support device already approved in Europe for saphenous vein grafts during CABG.
Saturday’s LBS.02 at 3:00 p.m. ET is devoted to hypertension and looks at how the COVID-19 pandemic affected blood pressure control. There’s also a study of remotely delivered hypertension and lipid management in 10,000 patients across the Partners Healthcare System and a cluster randomized trial of a village doctor–led blood pressure intervention in rural China.
Sunday’s LBS.03 at 8:00 a.m. ET is focused on atrial arrhythmias, starting with the CRAVE trial examining the effect of caffeine consumption on cardiac ectopy burden in 108 patients using an N-of-1 design and 2-day blocks on and off caffeine. “There’s an ability to identify a dose response that you get arrhythmias when you increase the amount of coffee you drink vs. not in an individual, so I think that will be likely discussed a lot and worth paying attention to,” Dr. Patel said.
The session also includes GIRAF, a comparison of cognitive outcomes with dabigatran (Pradaxa) vs. warfarin (Coumadin) in nonvalvular atrial fibrillation (AF); PALACS, a randomized trial examining whether left-sided pericardiotomy prevents AF after cardiac surgery; and AMAZE, which study sponsor AtriCure revealed missed its primary efficacy endpoint of freedom from AF with the LARIAT suture delivery device for left atrial appendage closure plus pulmonary vein isolation.
LBS.04 at 3:30 p.m. ET Sunday takes on digital health, with results from the nonrandomized Fitbit Heart Study on AF notifications from 450,000 participants wearing a single-lead ECG patch. “A lot of technologies claim that they can detect things, and we should ask that people go through the rigorous evaluation to see if they in fact do. So, in that respect, I think it›s an important step,” observed Dr. Patel.
Also on tap is I-STOP-AFib, another N-of-1 study using mobile apps and the AliveCor device to identify individual AF triggers; and REVeAL-HF, a 4,000-patient study examining whether electronic alerts that provide clinicians with prognostic information on their heart failure (HF) patients will reduce mortality and 30-day HF hospitalizations.
LBS.05 at 5:00 p.m. ET provides new information from EMPEROR-Preserved in HF with preserved ejection fraction and main results from EMPULSE, also using the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) in 530 patients hospitalized for acute HF.
The session also features CHIEF-HF, a randomized trial leveraging mobile technologies to test whether 12 weeks of another SGLT2 inhibitor, canagliflozin (Invokana), is superior to placebo for improving HF symptoms; and DREAM-HF, a comparison of transendocardial delivery of allogeneic mesenchymal precursor cells vs. a sham comparator in chronic HF as a result of left ventricular systolic dysfunction.
Monday’s LBS.06 at 8:00 a.m. ET details the safety and cholesterol-lowering efficacy of MK-0616, an investigational oral PCSK9 inhibitor. “It’s just a phase 2 [trial], but there’s interest in an oral PCSK9 inhibitor, given that the current ones are subcutaneous,” Dr. Patel said.
Results will also be presented from PREPARE-IT 2, which tested icosapent ethyl vs. placebo in outpatients with COVID-19. In the recently reported PREPARE-IT 1, a loading dose of icosapent ethyl failed to reduce the risk of hospitalization with SARS-CoV-2 infection among at-risk individuals.
LBS.07 at 11:00 a.m. Monday completes the late-breakers with new results from ASCEND, this time examining the effect of aspirin on dementia and cognitive impairment in patients with diabetes.
Next up is a look at the effectiveness of P2Y12 inhibitors in hospitalized patients with COVID-19 in the adaptive ACTIV-4a trial, followed by results of the pivotal phase 3 REVERSE-IT trial of bentracimab, a recombinant human monoclonal antibody antigen fragment designed to reverse the antiplatelet activity of ticagrelor in the event of major bleeding or when urgent surgery is needed.
Closing out the session is AXIOMATIC-TKR, a double-blind comparison of the safety and efficacy of the investigational oral factor XI anticoagulant JNJ-70033093 vs. subcutaneous enoxaparin (Lovenox) in elective total knee replacement.
For those searching for more AHA-related science online, the Resuscitation Science Symposium (ReSS) will run from this Friday through Sunday and the Quality of Care and Outcomes Research (QCOR) Scientific Sessions will take the stage next Monday, Nov. 15.
A version of this article first appeared on Medscape.com.
FROM AHA 2021
Liraglutide effective against weight regain after gastric bypass
The glucagon-like peptide-1 (GLP-1) receptor agonist liraglutide (Saxenda, Novo Nordisk) was safe and effective for treating weight regain after Roux-en-Y gastric bypass (RYGB), in a randomized controlled trial.
That is, 132 patients who had lost at least 25% of their initial weight after RYGB and then gained at least 10% back were randomized 2:1 to receive liraglutide plus frequent lifestyle advice from a registered dietitian or lifestyle advice alone.
After a year, 69%, 48%, and 24% of patients who had received liraglutide lost at least 5%, 10%, and 15% of their study entry weight, respectively. In contrast, only 5% of patients in the control group lost at least 5% of their weight and none lost at least 10% of their weight.
“Liraglutide 3.0 mg/day, with lifestyle modification, was significantly more effective than placebo in treating weight regain after RYGB without increased risk of serious adverse events,” Holly F. Lofton, MD, summarized this week in an oral session at ObesityWeek®, the annual meeting of The Obesity Society.
Dr. Lofton, a clinical associate professor of surgery and medicine, and director, weight management program, NYU, Langone Health, explained to this news organization that she initiated the study after attending a “packed” session about post bariatric surgery weight regain at a prior American Society of Metabolic and Bariatric Surgery conference.
“The lecturers recommended conservative measures (such as reiterating the diet recommendations, exercise, [and] counseling), and revisional surgeries,” she said in an email, but at the time “there was no literature that provided direction on which pharmacotherapies are best for this population.”
It was known that decreases in endogenous GLP-1 levels coincide with weight regain, and liraglutide (Saxenda) was the only GLP-1 agonist approved for chronic weight management at the time, so she devised the current study protocol.
The findings are especially helpful for patients who are not candidates for bariatric surgery revisions, she noted. Further research is needed to investigate the effect of newer GLP-1 agonists, such as semaglutide (Wegovy), on weight regain following different types of bariatric surgery.
Asked to comment, Wendy C. King, PhD, who was not involved with this research, said that more than two-thirds of patients treated with 3 mg/day subcutaneous liraglutide injections in the current study lost at least 5% of their initial weight a year later, and 20% of them attained a weight as low as, or lower than, their lowest weight after bariatric surgery (nadir weight).
“The fact that both groups received lifestyle counseling from registered dietitians for just over a year, but only patients in the liraglutide group lost weight, on average, speaks to the difficulty of losing weight following weight regain post–bariatric surgery,” added Dr. King, an associate professor of epidemiology at the University of Pittsburgh, Pennsylvania.
This study “provides data that may help clinicians and patients understand the potential effect of adding liraglutide 3.0 mg/day to their weight loss efforts,” she told this news organization in an email.
However, “given that 42% of those on liraglutide reported gastrointestinal-related side effects, patients should also be counseled on this potential outcome and given suggestions for how to minimize such side effects,” Dr. King suggested.
Weight regain common, repeat surgery entails risk
Weight regain is common even years after bariatric surgery. Repeat surgery entails some risk, and lifestyle approaches alone are rarely successful in reversing weight regain, Dr. Lofton told the audience.
The researchers enrolled 132 adults who had a mean weight of 134 kg (295 pounds) when they underwent RYGB, and who lost at least 25% of their initial weight (mean weight loss of 38%) after the surgery, but who also regained at least 10% of their initial weight.
At enrollment of the current study (baseline), the patients had had RYGB 18 months to 10 years earlier (mean 5.7 years earlier) and now had a mean weight of 99 kg (218 pounds) and a mean BMI of 35.6 kg/m2. None of the patients had diabetes.
The patients were randomized to receive liraglutide (n = 89, 84% women) or placebo (n = 43, 88% women) for 56 weeks.
They were a mean age of 48 years, and about 59% were White and 25% were Black.
All patients had clinic visits every 3 months where they received lifestyle counseling from a registered dietitian.
At 12 months, patients in the liraglutide group had lost a mean of 8.8% of their baseline weight, whereas those in the placebo group had gained a mean of 1.48% of their baseline weight.
There were no significant between-group differences in cardiometabolic variables.
None of the patients in the control group attained a weight that was as low as their nadir weight after RYGB.
The rates of nausea (25%), constipation (16%), and abdominal pain (10%) in the liraglutide group were higher than in the placebo group (7%, 14%, and 5%, respectively) but similar to rates of gastrointestinal side effects in other trials of this agent.
Dr. Lofton has disclosed receiving consulting fees and being on a speaker bureau for Novo Nordisk and receiving research funds from Boehringer Ingelheim, Eli Lilly, and Novo Nordisk. Dr. King has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The glucagon-like peptide-1 (GLP-1) receptor agonist liraglutide (Saxenda, Novo Nordisk) was safe and effective for treating weight regain after Roux-en-Y gastric bypass (RYGB), in a randomized controlled trial.
That is, 132 patients who had lost at least 25% of their initial weight after RYGB and then gained at least 10% back were randomized 2:1 to receive liraglutide plus frequent lifestyle advice from a registered dietitian or lifestyle advice alone.
After a year, 69%, 48%, and 24% of patients who had received liraglutide lost at least 5%, 10%, and 15% of their study entry weight, respectively. In contrast, only 5% of patients in the control group lost at least 5% of their weight and none lost at least 10% of their weight.
“Liraglutide 3.0 mg/day, with lifestyle modification, was significantly more effective than placebo in treating weight regain after RYGB without increased risk of serious adverse events,” Holly F. Lofton, MD, summarized this week in an oral session at ObesityWeek®, the annual meeting of The Obesity Society.
Dr. Lofton, a clinical associate professor of surgery and medicine, and director, weight management program, NYU, Langone Health, explained to this news organization that she initiated the study after attending a “packed” session about post bariatric surgery weight regain at a prior American Society of Metabolic and Bariatric Surgery conference.
“The lecturers recommended conservative measures (such as reiterating the diet recommendations, exercise, [and] counseling), and revisional surgeries,” she said in an email, but at the time “there was no literature that provided direction on which pharmacotherapies are best for this population.”
It was known that decreases in endogenous GLP-1 levels coincide with weight regain, and liraglutide (Saxenda) was the only GLP-1 agonist approved for chronic weight management at the time, so she devised the current study protocol.
The findings are especially helpful for patients who are not candidates for bariatric surgery revisions, she noted. Further research is needed to investigate the effect of newer GLP-1 agonists, such as semaglutide (Wegovy), on weight regain following different types of bariatric surgery.
Asked to comment, Wendy C. King, PhD, who was not involved with this research, said that more than two-thirds of patients treated with 3 mg/day subcutaneous liraglutide injections in the current study lost at least 5% of their initial weight a year later, and 20% of them attained a weight as low as, or lower than, their lowest weight after bariatric surgery (nadir weight).
“The fact that both groups received lifestyle counseling from registered dietitians for just over a year, but only patients in the liraglutide group lost weight, on average, speaks to the difficulty of losing weight following weight regain post–bariatric surgery,” added Dr. King, an associate professor of epidemiology at the University of Pittsburgh, Pennsylvania.
This study “provides data that may help clinicians and patients understand the potential effect of adding liraglutide 3.0 mg/day to their weight loss efforts,” she told this news organization in an email.
However, “given that 42% of those on liraglutide reported gastrointestinal-related side effects, patients should also be counseled on this potential outcome and given suggestions for how to minimize such side effects,” Dr. King suggested.
Weight regain common, repeat surgery entails risk
Weight regain is common even years after bariatric surgery. Repeat surgery entails some risk, and lifestyle approaches alone are rarely successful in reversing weight regain, Dr. Lofton told the audience.
The researchers enrolled 132 adults who had a mean weight of 134 kg (295 pounds) when they underwent RYGB, and who lost at least 25% of their initial weight (mean weight loss of 38%) after the surgery, but who also regained at least 10% of their initial weight.
At enrollment of the current study (baseline), the patients had had RYGB 18 months to 10 years earlier (mean 5.7 years earlier) and now had a mean weight of 99 kg (218 pounds) and a mean BMI of 35.6 kg/m2. None of the patients had diabetes.
The patients were randomized to receive liraglutide (n = 89, 84% women) or placebo (n = 43, 88% women) for 56 weeks.
They were a mean age of 48 years, and about 59% were White and 25% were Black.
All patients had clinic visits every 3 months where they received lifestyle counseling from a registered dietitian.
At 12 months, patients in the liraglutide group had lost a mean of 8.8% of their baseline weight, whereas those in the placebo group had gained a mean of 1.48% of their baseline weight.
There were no significant between-group differences in cardiometabolic variables.
None of the patients in the control group attained a weight that was as low as their nadir weight after RYGB.
The rates of nausea (25%), constipation (16%), and abdominal pain (10%) in the liraglutide group were higher than in the placebo group (7%, 14%, and 5%, respectively) but similar to rates of gastrointestinal side effects in other trials of this agent.
Dr. Lofton has disclosed receiving consulting fees and being on a speaker bureau for Novo Nordisk and receiving research funds from Boehringer Ingelheim, Eli Lilly, and Novo Nordisk. Dr. King has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The glucagon-like peptide-1 (GLP-1) receptor agonist liraglutide (Saxenda, Novo Nordisk) was safe and effective for treating weight regain after Roux-en-Y gastric bypass (RYGB), in a randomized controlled trial.
That is, 132 patients who had lost at least 25% of their initial weight after RYGB and then gained at least 10% back were randomized 2:1 to receive liraglutide plus frequent lifestyle advice from a registered dietitian or lifestyle advice alone.
After a year, 69%, 48%, and 24% of patients who had received liraglutide lost at least 5%, 10%, and 15% of their study entry weight, respectively. In contrast, only 5% of patients in the control group lost at least 5% of their weight and none lost at least 10% of their weight.
“Liraglutide 3.0 mg/day, with lifestyle modification, was significantly more effective than placebo in treating weight regain after RYGB without increased risk of serious adverse events,” Holly F. Lofton, MD, summarized this week in an oral session at ObesityWeek®, the annual meeting of The Obesity Society.
Dr. Lofton, a clinical associate professor of surgery and medicine, and director, weight management program, NYU, Langone Health, explained to this news organization that she initiated the study after attending a “packed” session about post bariatric surgery weight regain at a prior American Society of Metabolic and Bariatric Surgery conference.
“The lecturers recommended conservative measures (such as reiterating the diet recommendations, exercise, [and] counseling), and revisional surgeries,” she said in an email, but at the time “there was no literature that provided direction on which pharmacotherapies are best for this population.”
It was known that decreases in endogenous GLP-1 levels coincide with weight regain, and liraglutide (Saxenda) was the only GLP-1 agonist approved for chronic weight management at the time, so she devised the current study protocol.
The findings are especially helpful for patients who are not candidates for bariatric surgery revisions, she noted. Further research is needed to investigate the effect of newer GLP-1 agonists, such as semaglutide (Wegovy), on weight regain following different types of bariatric surgery.
Asked to comment, Wendy C. King, PhD, who was not involved with this research, said that more than two-thirds of patients treated with 3 mg/day subcutaneous liraglutide injections in the current study lost at least 5% of their initial weight a year later, and 20% of them attained a weight as low as, or lower than, their lowest weight after bariatric surgery (nadir weight).
“The fact that both groups received lifestyle counseling from registered dietitians for just over a year, but only patients in the liraglutide group lost weight, on average, speaks to the difficulty of losing weight following weight regain post–bariatric surgery,” added Dr. King, an associate professor of epidemiology at the University of Pittsburgh, Pennsylvania.
This study “provides data that may help clinicians and patients understand the potential effect of adding liraglutide 3.0 mg/day to their weight loss efforts,” she told this news organization in an email.
However, “given that 42% of those on liraglutide reported gastrointestinal-related side effects, patients should also be counseled on this potential outcome and given suggestions for how to minimize such side effects,” Dr. King suggested.
Weight regain common, repeat surgery entails risk
Weight regain is common even years after bariatric surgery. Repeat surgery entails some risk, and lifestyle approaches alone are rarely successful in reversing weight regain, Dr. Lofton told the audience.
The researchers enrolled 132 adults who had a mean weight of 134 kg (295 pounds) when they underwent RYGB, and who lost at least 25% of their initial weight (mean weight loss of 38%) after the surgery, but who also regained at least 10% of their initial weight.
At enrollment of the current study (baseline), the patients had had RYGB 18 months to 10 years earlier (mean 5.7 years earlier) and now had a mean weight of 99 kg (218 pounds) and a mean BMI of 35.6 kg/m2. None of the patients had diabetes.
The patients were randomized to receive liraglutide (n = 89, 84% women) or placebo (n = 43, 88% women) for 56 weeks.
They were a mean age of 48 years, and about 59% were White and 25% were Black.
All patients had clinic visits every 3 months where they received lifestyle counseling from a registered dietitian.
At 12 months, patients in the liraglutide group had lost a mean of 8.8% of their baseline weight, whereas those in the placebo group had gained a mean of 1.48% of their baseline weight.
There were no significant between-group differences in cardiometabolic variables.
None of the patients in the control group attained a weight that was as low as their nadir weight after RYGB.
The rates of nausea (25%), constipation (16%), and abdominal pain (10%) in the liraglutide group were higher than in the placebo group (7%, 14%, and 5%, respectively) but similar to rates of gastrointestinal side effects in other trials of this agent.
Dr. Lofton has disclosed receiving consulting fees and being on a speaker bureau for Novo Nordisk and receiving research funds from Boehringer Ingelheim, Eli Lilly, and Novo Nordisk. Dr. King has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM OBESITY WEEK 2021









