User login
Primary prevention statins cut mortality even in the very elderly: VHA study
Patients in the Veterans Health Administration (VHA) system 75 years or older, free of cardiovascular (CV) disease and prescribed statins for the first time, had a one-fourth lower risk for death and a 20% lower risk for CV death over an average 7 years than that of comparable patients not prescribed the drugs in an observational study.
Ariela R. Orkaby, MD, MPH, lead author on the study, published in the July 7 issue of JAMA, said in an interview.
The very elderly are frequently undertreated, particularly in primary prevention, as many physicians consider it unnecessary for them to initiate or continue preventive measures, said Dr. Orkaby, of VA Boston Healthcare System and Harvard Medical School, Boston.
“From available data, we don’t really expect statins to start providing benefit in primary prevention until they’ve been taken for about 2 to 5 years. So for people who have very limited life expectancy, it may not be a great idea to add to their pill burden or increase the possibility that they might decline functionally,” Dr. Orkaby said.
“But what we saw in this study is that there is benefit to prescribing statins even in elderly patients, even within 2 years” of follow-up.
Despite being among the most studied drugs in the world, statins are understudied in older people. Fewer than 2% of the 186,854 participants in 28 statin trials were aged 75 years or older, wrote Dr. Orkaby and associates.
Most of what is known about initiating statin therapy in the 75-and-older age group comes from underpowered subgroup analyses and a few observational studies, Steven J. Nicholls, MBBS, PhD, Monash University, Melbourne, and Adam J. Nelson, MBBS, PhD, Duke Clinical Research Institute, Durham, N.C., wrote in an accompanying editorial. As a result, the evidence is conflicting, with some reports suggesting marked benefit and others possible harm.
The current findings, they wrote, “provide additional support for treatment guidelines that have increasingly advocated for more widespread use of statin therapy for ASCVD prevention in older individuals.”
Of the 326,981 people in the analysis, 57,178 (17.5%) were new statin users or initiated a statin during the study period, usually simvastatin. Their mean age was about 81 years, and 97.3% of the patients were men, 90% were white, and 72% were former smokers.
Using propensity scoring, the authors compared statin users with the other remaining patients who had the same likelihood of being prescribed a statin based on clinical characteristics but did not receive a prescription for a statin.
Michael W. Rich, MD, Washington University, St. Louis, who was not involved in the study but has previously worked with Dr. Orkaby, praised the analysis.
“It’s one of the best studies I’ve seen addressing this particular issue. It’s a large sample size, the analysis was very well done, and I think that it comes to a pretty unequivocal conclusion that, at least in this population, those individuals who were started on statins for the first time, and having no known prior ASCVD, clearly had a lower all-cause mortality and cardiovascular mortality, as well as a lower risk of composite cardiovascular events,” he said in an interview.
But the data have limitations, he added. The findings are still observational and could be confounded by unknown variables, and the select population – mostly white, male veterans – is known to be at somewhat higher risk for events than the general population.
Perhaps even more impressive than the risk reductions seen at a mean 6.8 years of follow-up, Dr. Rich said, are the sensitivity analyses at 2, 4, and 6 years that showed the benefit manifesting early.
The researchers saw a 32% reduction in all-cause mortality risk (P < .05) at 2 years, 21% at 4 years, and 13% at 6 years (P < .05 for all). Risk reductions for CV death followed a similar pattern, they wrote.
Dr. Rich said that the trial, although not a “slam dunk,” has persuaded him to shift from being very conservative about prescribing statins to elderly patients to being much more willing to consider it.
“This doesn’t mean that I will be running to routinely prescribe my 90-plus patients a statin, nor should we should be starting statins in everyone over 75, not even in all male former smokers over 75 – the type of people in this study – but I do think that it provides a stronger basis for talking to these patients about the possibility of starting a statin.”
There are two ongoing trials that may provide greater clarity, the authors observed. The STAREE trial has enrolled adults 70 years and older in Australia and includes serial evaluation of cognitive scores. Also, PREVENTABLE will examine the role of statins for prevention of dementia and disability-free survival in adults 75 years and older.
However, neither trial may fully resolve the question of primary prevention statin use in the elderly, they wrote. “While these trials are necessary to broaden the evidence base for older adults, it is unlikely that any trial will enroll large numbers of individuals at very advanced ages, black individuals, and those with dementia, as were included in this study.”
Dr. Orkaby had no disclosures; potential conflicts for the other authors are in the report. Dr. Rich reported having no conflicts of interest. Dr. Nicholls disclosed receiving research support from AstraZeneca, Amgen, Anthera, Eli Lilly, Novartis, Cerenis, The Medicines Company, Resverlogix, InfraReDx, Roche, Sanofi-Regeneron, and LipoScience; and receiving consulting fees or honoraria from AstraZeneca, Eli Lilly, Anthera, Omthera, Merck, Takeda, Resverlogix, Sanofi-Regeneron, CSL Behring, Esperion, and Boehringer Ingelheim. Dr. Nelson had no disclosures.
A version of this article originally appeared on Medscape.com.
Patients in the Veterans Health Administration (VHA) system 75 years or older, free of cardiovascular (CV) disease and prescribed statins for the first time, had a one-fourth lower risk for death and a 20% lower risk for CV death over an average 7 years than that of comparable patients not prescribed the drugs in an observational study.
Ariela R. Orkaby, MD, MPH, lead author on the study, published in the July 7 issue of JAMA, said in an interview.
The very elderly are frequently undertreated, particularly in primary prevention, as many physicians consider it unnecessary for them to initiate or continue preventive measures, said Dr. Orkaby, of VA Boston Healthcare System and Harvard Medical School, Boston.
“From available data, we don’t really expect statins to start providing benefit in primary prevention until they’ve been taken for about 2 to 5 years. So for people who have very limited life expectancy, it may not be a great idea to add to their pill burden or increase the possibility that they might decline functionally,” Dr. Orkaby said.
“But what we saw in this study is that there is benefit to prescribing statins even in elderly patients, even within 2 years” of follow-up.
Despite being among the most studied drugs in the world, statins are understudied in older people. Fewer than 2% of the 186,854 participants in 28 statin trials were aged 75 years or older, wrote Dr. Orkaby and associates.
Most of what is known about initiating statin therapy in the 75-and-older age group comes from underpowered subgroup analyses and a few observational studies, Steven J. Nicholls, MBBS, PhD, Monash University, Melbourne, and Adam J. Nelson, MBBS, PhD, Duke Clinical Research Institute, Durham, N.C., wrote in an accompanying editorial. As a result, the evidence is conflicting, with some reports suggesting marked benefit and others possible harm.
The current findings, they wrote, “provide additional support for treatment guidelines that have increasingly advocated for more widespread use of statin therapy for ASCVD prevention in older individuals.”
Of the 326,981 people in the analysis, 57,178 (17.5%) were new statin users or initiated a statin during the study period, usually simvastatin. Their mean age was about 81 years, and 97.3% of the patients were men, 90% were white, and 72% were former smokers.
Using propensity scoring, the authors compared statin users with the other remaining patients who had the same likelihood of being prescribed a statin based on clinical characteristics but did not receive a prescription for a statin.
Michael W. Rich, MD, Washington University, St. Louis, who was not involved in the study but has previously worked with Dr. Orkaby, praised the analysis.
“It’s one of the best studies I’ve seen addressing this particular issue. It’s a large sample size, the analysis was very well done, and I think that it comes to a pretty unequivocal conclusion that, at least in this population, those individuals who were started on statins for the first time, and having no known prior ASCVD, clearly had a lower all-cause mortality and cardiovascular mortality, as well as a lower risk of composite cardiovascular events,” he said in an interview.
But the data have limitations, he added. The findings are still observational and could be confounded by unknown variables, and the select population – mostly white, male veterans – is known to be at somewhat higher risk for events than the general population.
Perhaps even more impressive than the risk reductions seen at a mean 6.8 years of follow-up, Dr. Rich said, are the sensitivity analyses at 2, 4, and 6 years that showed the benefit manifesting early.
The researchers saw a 32% reduction in all-cause mortality risk (P < .05) at 2 years, 21% at 4 years, and 13% at 6 years (P < .05 for all). Risk reductions for CV death followed a similar pattern, they wrote.
Dr. Rich said that the trial, although not a “slam dunk,” has persuaded him to shift from being very conservative about prescribing statins to elderly patients to being much more willing to consider it.
“This doesn’t mean that I will be running to routinely prescribe my 90-plus patients a statin, nor should we should be starting statins in everyone over 75, not even in all male former smokers over 75 – the type of people in this study – but I do think that it provides a stronger basis for talking to these patients about the possibility of starting a statin.”
There are two ongoing trials that may provide greater clarity, the authors observed. The STAREE trial has enrolled adults 70 years and older in Australia and includes serial evaluation of cognitive scores. Also, PREVENTABLE will examine the role of statins for prevention of dementia and disability-free survival in adults 75 years and older.
However, neither trial may fully resolve the question of primary prevention statin use in the elderly, they wrote. “While these trials are necessary to broaden the evidence base for older adults, it is unlikely that any trial will enroll large numbers of individuals at very advanced ages, black individuals, and those with dementia, as were included in this study.”
Dr. Orkaby had no disclosures; potential conflicts for the other authors are in the report. Dr. Rich reported having no conflicts of interest. Dr. Nicholls disclosed receiving research support from AstraZeneca, Amgen, Anthera, Eli Lilly, Novartis, Cerenis, The Medicines Company, Resverlogix, InfraReDx, Roche, Sanofi-Regeneron, and LipoScience; and receiving consulting fees or honoraria from AstraZeneca, Eli Lilly, Anthera, Omthera, Merck, Takeda, Resverlogix, Sanofi-Regeneron, CSL Behring, Esperion, and Boehringer Ingelheim. Dr. Nelson had no disclosures.
A version of this article originally appeared on Medscape.com.
Patients in the Veterans Health Administration (VHA) system 75 years or older, free of cardiovascular (CV) disease and prescribed statins for the first time, had a one-fourth lower risk for death and a 20% lower risk for CV death over an average 7 years than that of comparable patients not prescribed the drugs in an observational study.
Ariela R. Orkaby, MD, MPH, lead author on the study, published in the July 7 issue of JAMA, said in an interview.
The very elderly are frequently undertreated, particularly in primary prevention, as many physicians consider it unnecessary for them to initiate or continue preventive measures, said Dr. Orkaby, of VA Boston Healthcare System and Harvard Medical School, Boston.
“From available data, we don’t really expect statins to start providing benefit in primary prevention until they’ve been taken for about 2 to 5 years. So for people who have very limited life expectancy, it may not be a great idea to add to their pill burden or increase the possibility that they might decline functionally,” Dr. Orkaby said.
“But what we saw in this study is that there is benefit to prescribing statins even in elderly patients, even within 2 years” of follow-up.
Despite being among the most studied drugs in the world, statins are understudied in older people. Fewer than 2% of the 186,854 participants in 28 statin trials were aged 75 years or older, wrote Dr. Orkaby and associates.
Most of what is known about initiating statin therapy in the 75-and-older age group comes from underpowered subgroup analyses and a few observational studies, Steven J. Nicholls, MBBS, PhD, Monash University, Melbourne, and Adam J. Nelson, MBBS, PhD, Duke Clinical Research Institute, Durham, N.C., wrote in an accompanying editorial. As a result, the evidence is conflicting, with some reports suggesting marked benefit and others possible harm.
The current findings, they wrote, “provide additional support for treatment guidelines that have increasingly advocated for more widespread use of statin therapy for ASCVD prevention in older individuals.”
Of the 326,981 people in the analysis, 57,178 (17.5%) were new statin users or initiated a statin during the study period, usually simvastatin. Their mean age was about 81 years, and 97.3% of the patients were men, 90% were white, and 72% were former smokers.
Using propensity scoring, the authors compared statin users with the other remaining patients who had the same likelihood of being prescribed a statin based on clinical characteristics but did not receive a prescription for a statin.
Michael W. Rich, MD, Washington University, St. Louis, who was not involved in the study but has previously worked with Dr. Orkaby, praised the analysis.
“It’s one of the best studies I’ve seen addressing this particular issue. It’s a large sample size, the analysis was very well done, and I think that it comes to a pretty unequivocal conclusion that, at least in this population, those individuals who were started on statins for the first time, and having no known prior ASCVD, clearly had a lower all-cause mortality and cardiovascular mortality, as well as a lower risk of composite cardiovascular events,” he said in an interview.
But the data have limitations, he added. The findings are still observational and could be confounded by unknown variables, and the select population – mostly white, male veterans – is known to be at somewhat higher risk for events than the general population.
Perhaps even more impressive than the risk reductions seen at a mean 6.8 years of follow-up, Dr. Rich said, are the sensitivity analyses at 2, 4, and 6 years that showed the benefit manifesting early.
The researchers saw a 32% reduction in all-cause mortality risk (P < .05) at 2 years, 21% at 4 years, and 13% at 6 years (P < .05 for all). Risk reductions for CV death followed a similar pattern, they wrote.
Dr. Rich said that the trial, although not a “slam dunk,” has persuaded him to shift from being very conservative about prescribing statins to elderly patients to being much more willing to consider it.
“This doesn’t mean that I will be running to routinely prescribe my 90-plus patients a statin, nor should we should be starting statins in everyone over 75, not even in all male former smokers over 75 – the type of people in this study – but I do think that it provides a stronger basis for talking to these patients about the possibility of starting a statin.”
There are two ongoing trials that may provide greater clarity, the authors observed. The STAREE trial has enrolled adults 70 years and older in Australia and includes serial evaluation of cognitive scores. Also, PREVENTABLE will examine the role of statins for prevention of dementia and disability-free survival in adults 75 years and older.
However, neither trial may fully resolve the question of primary prevention statin use in the elderly, they wrote. “While these trials are necessary to broaden the evidence base for older adults, it is unlikely that any trial will enroll large numbers of individuals at very advanced ages, black individuals, and those with dementia, as were included in this study.”
Dr. Orkaby had no disclosures; potential conflicts for the other authors are in the report. Dr. Rich reported having no conflicts of interest. Dr. Nicholls disclosed receiving research support from AstraZeneca, Amgen, Anthera, Eli Lilly, Novartis, Cerenis, The Medicines Company, Resverlogix, InfraReDx, Roche, Sanofi-Regeneron, and LipoScience; and receiving consulting fees or honoraria from AstraZeneca, Eli Lilly, Anthera, Omthera, Merck, Takeda, Resverlogix, Sanofi-Regeneron, CSL Behring, Esperion, and Boehringer Ingelheim. Dr. Nelson had no disclosures.
A version of this article originally appeared on Medscape.com.
Bariatric embolotherapy helps shed pounds in obese patients
Transcatheter bariatric embolotherapy (TBE) provides sustained weight loss without serious adverse effects among obese patients, results of a pilot sham-controlled study suggest.
At 6-month follow-up, the patients receiving the intervention had lost 7.4 kg (16.3 lbs), compared with 3.0 kg (6.6 lbs) in those randomized to a sham procedure in an intention-to-treat analysis (P = .034).
Results were similar in a per-protocol analysis (9.4 kg/20.7 lbs vs. 1.9 kg/4.1 lbs; P = .0002).
Weight loss after embolotherapy was sustained over 12 months, falling 7.8 kg (17.1 lbs) from baseline in the intention-to-treat population (P = .0011) and 9.3 kg (20.5 lbs) in the per-protocol population (P = .0005).
Safety events after TBE were mild nausea or vomiting, reported Vivek Reddy, MD, Mount Sinai Hospital, New York City. Five participants had minor, asymptomatic ulcers that required no additional treatment.
“In this randomized pilot trial, we established the proof of principle that transcatheter bariatric embolotherapy of the left gastric artery is safe and it promotes clinically significant weight loss,” he concluded at PCR e-Course, the virtual meeting of the Congress of European Association of Percutaneous Cardiovascular Interventions 2020.
Although bariatric surgery is highly effective, he noted that the associated morbidity and mortality limit its use to the severely obese with a body mass index (BMI) typically over 40 kg/m2.
TBE is a minimally invasive approach that uses a custom occlusion balloon microcatheter and robotic manifold to inject 300- to 500-mcm beads to the left gastric artery. Preclinical and case studies suggest it promotes weight loss by reducing ghrelin, an appetite-stimulating hormone secreted from the gastric fundus, Dr. Reddy said.
The study enrolled 44 patients (aged 21-60 years) with a BMI of 35-55, excluding those with prior bariatric surgery and a history of ulcers, type 2 diabetes, chronic aspirin or nonsteroidal inflammatory use, and active Helicobacter pylori infection.
A total of 40 patients were randomly assigned to TBE or a sham procedure, in which lidocaine was applied to the femoral area and propofol infused for 1 hour. The two groups were well matched, with a mean age of 45 vs. 46 years, weight of 110 kg vs. 119 kg, and BMI of 39 vs. 40, Dr. Reddy noted.
Embolotherapy was performed at a single center in Prague, and, on average, took 82.3 minutes and used 127 mL of contrast, 163 Gy/cm2 radiation, and 4.2 mL of microspheres. A single vessel was injected in 80% of cases.
The intention-to-treat population comprised 19 TBE and 18 control subjects, and the per-protocol population comprised 15 TBE and 16 control subjects, after the exclusion of patients in whom embolotherapy was unsuccessful or incomplete or who withdrew consent.
All patients received endoscopy at baseline and 1 week, as well as an intensive 19-session lifestyle and dietary education intervention out to 6 months.
Patients who underwent TBE had significant improvement in hunger scores at 6 and 12 months, compared with baseline. Similarly, quality of life improved across all six domains, including significant gains in physical function, self-esteem, and overall quality of life at both time points, Dr. Reddy reported.
Dr. Reddy disclosed receiving research support from Endobar Solutions.
This article first appeared on Medscape.com.
Transcatheter bariatric embolotherapy (TBE) provides sustained weight loss without serious adverse effects among obese patients, results of a pilot sham-controlled study suggest.
At 6-month follow-up, the patients receiving the intervention had lost 7.4 kg (16.3 lbs), compared with 3.0 kg (6.6 lbs) in those randomized to a sham procedure in an intention-to-treat analysis (P = .034).
Results were similar in a per-protocol analysis (9.4 kg/20.7 lbs vs. 1.9 kg/4.1 lbs; P = .0002).
Weight loss after embolotherapy was sustained over 12 months, falling 7.8 kg (17.1 lbs) from baseline in the intention-to-treat population (P = .0011) and 9.3 kg (20.5 lbs) in the per-protocol population (P = .0005).
Safety events after TBE were mild nausea or vomiting, reported Vivek Reddy, MD, Mount Sinai Hospital, New York City. Five participants had minor, asymptomatic ulcers that required no additional treatment.
“In this randomized pilot trial, we established the proof of principle that transcatheter bariatric embolotherapy of the left gastric artery is safe and it promotes clinically significant weight loss,” he concluded at PCR e-Course, the virtual meeting of the Congress of European Association of Percutaneous Cardiovascular Interventions 2020.
Although bariatric surgery is highly effective, he noted that the associated morbidity and mortality limit its use to the severely obese with a body mass index (BMI) typically over 40 kg/m2.
TBE is a minimally invasive approach that uses a custom occlusion balloon microcatheter and robotic manifold to inject 300- to 500-mcm beads to the left gastric artery. Preclinical and case studies suggest it promotes weight loss by reducing ghrelin, an appetite-stimulating hormone secreted from the gastric fundus, Dr. Reddy said.
The study enrolled 44 patients (aged 21-60 years) with a BMI of 35-55, excluding those with prior bariatric surgery and a history of ulcers, type 2 diabetes, chronic aspirin or nonsteroidal inflammatory use, and active Helicobacter pylori infection.
A total of 40 patients were randomly assigned to TBE or a sham procedure, in which lidocaine was applied to the femoral area and propofol infused for 1 hour. The two groups were well matched, with a mean age of 45 vs. 46 years, weight of 110 kg vs. 119 kg, and BMI of 39 vs. 40, Dr. Reddy noted.
Embolotherapy was performed at a single center in Prague, and, on average, took 82.3 minutes and used 127 mL of contrast, 163 Gy/cm2 radiation, and 4.2 mL of microspheres. A single vessel was injected in 80% of cases.
The intention-to-treat population comprised 19 TBE and 18 control subjects, and the per-protocol population comprised 15 TBE and 16 control subjects, after the exclusion of patients in whom embolotherapy was unsuccessful or incomplete or who withdrew consent.
All patients received endoscopy at baseline and 1 week, as well as an intensive 19-session lifestyle and dietary education intervention out to 6 months.
Patients who underwent TBE had significant improvement in hunger scores at 6 and 12 months, compared with baseline. Similarly, quality of life improved across all six domains, including significant gains in physical function, self-esteem, and overall quality of life at both time points, Dr. Reddy reported.
Dr. Reddy disclosed receiving research support from Endobar Solutions.
This article first appeared on Medscape.com.
Transcatheter bariatric embolotherapy (TBE) provides sustained weight loss without serious adverse effects among obese patients, results of a pilot sham-controlled study suggest.
At 6-month follow-up, the patients receiving the intervention had lost 7.4 kg (16.3 lbs), compared with 3.0 kg (6.6 lbs) in those randomized to a sham procedure in an intention-to-treat analysis (P = .034).
Results were similar in a per-protocol analysis (9.4 kg/20.7 lbs vs. 1.9 kg/4.1 lbs; P = .0002).
Weight loss after embolotherapy was sustained over 12 months, falling 7.8 kg (17.1 lbs) from baseline in the intention-to-treat population (P = .0011) and 9.3 kg (20.5 lbs) in the per-protocol population (P = .0005).
Safety events after TBE were mild nausea or vomiting, reported Vivek Reddy, MD, Mount Sinai Hospital, New York City. Five participants had minor, asymptomatic ulcers that required no additional treatment.
“In this randomized pilot trial, we established the proof of principle that transcatheter bariatric embolotherapy of the left gastric artery is safe and it promotes clinically significant weight loss,” he concluded at PCR e-Course, the virtual meeting of the Congress of European Association of Percutaneous Cardiovascular Interventions 2020.
Although bariatric surgery is highly effective, he noted that the associated morbidity and mortality limit its use to the severely obese with a body mass index (BMI) typically over 40 kg/m2.
TBE is a minimally invasive approach that uses a custom occlusion balloon microcatheter and robotic manifold to inject 300- to 500-mcm beads to the left gastric artery. Preclinical and case studies suggest it promotes weight loss by reducing ghrelin, an appetite-stimulating hormone secreted from the gastric fundus, Dr. Reddy said.
The study enrolled 44 patients (aged 21-60 years) with a BMI of 35-55, excluding those with prior bariatric surgery and a history of ulcers, type 2 diabetes, chronic aspirin or nonsteroidal inflammatory use, and active Helicobacter pylori infection.
A total of 40 patients were randomly assigned to TBE or a sham procedure, in which lidocaine was applied to the femoral area and propofol infused for 1 hour. The two groups were well matched, with a mean age of 45 vs. 46 years, weight of 110 kg vs. 119 kg, and BMI of 39 vs. 40, Dr. Reddy noted.
Embolotherapy was performed at a single center in Prague, and, on average, took 82.3 minutes and used 127 mL of contrast, 163 Gy/cm2 radiation, and 4.2 mL of microspheres. A single vessel was injected in 80% of cases.
The intention-to-treat population comprised 19 TBE and 18 control subjects, and the per-protocol population comprised 15 TBE and 16 control subjects, after the exclusion of patients in whom embolotherapy was unsuccessful or incomplete or who withdrew consent.
All patients received endoscopy at baseline and 1 week, as well as an intensive 19-session lifestyle and dietary education intervention out to 6 months.
Patients who underwent TBE had significant improvement in hunger scores at 6 and 12 months, compared with baseline. Similarly, quality of life improved across all six domains, including significant gains in physical function, self-esteem, and overall quality of life at both time points, Dr. Reddy reported.
Dr. Reddy disclosed receiving research support from Endobar Solutions.
This article first appeared on Medscape.com.
U.S. adults reach Healthy People 2020 cholesterol goal
Good news: High cholesterol is down in the United States. More good news: Low HDL cholesterol is down in the United States.
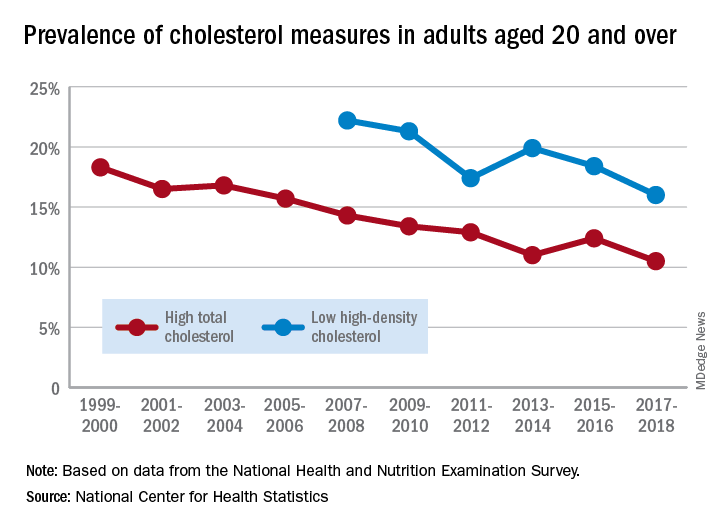
The prevalence of high total cholesterol in adults aged 20 years and older dropped from 18.3% in 1999-2000 to 10.5% in 2017-2018. And starting in 2007-2008, the prevalence of low HDL cholesterol declined from 22.2% to 16.0% in 2017-2018, the National Center for Health Statistics reported.
HDL cholesterol data before 2007 were not presented because of changes in laboratories and methods, but both trends are significant, and the decline in high total cholesterol means that the Healthy People 2020 goal of dropping prevalence to 13.5% has been met, said Margaret D. Carroll, MSPH, and Cheryl D. Fryar, MSPH, of the NCHS.
The demographic details, however, show some disparities hidden by the broader measures. The prevalence of low HDL cholesterol for women in 2015-2018 was 8.5%, but for men it was 26.6%, the NCHS investigators said.
And that Healthy People 2020 goal for total cholesterol? Age makes a difference: 7.5% of adults aged 20-39 years had high total cholesterol in 2015-2018, as did 11.4% of those aged 60 years and older, but those aged 40-59 years had a significantly higher prevalence of 15.7%, they reported.
Race/ethnicity was also a factor. Prevalence of low HDL was similar for white (16.6%) and Asian (15.8%) adults in 2015-2018, but black adults’ low HDL prevalence was significantly lower (11.9%) and Hispanics’ was significantly higher (21.9%), the researchers said.
The analysis was based on data from the National Health and Nutrition Examination Survey. The investigators defined high total cholesterol as a level of 240 mg/dL or more, and low HDL cholesterol as less than 40 mg/dL. LDL cholesterol was not included in the analysis.
Good news: High cholesterol is down in the United States. More good news: Low HDL cholesterol is down in the United States.

The prevalence of high total cholesterol in adults aged 20 years and older dropped from 18.3% in 1999-2000 to 10.5% in 2017-2018. And starting in 2007-2008, the prevalence of low HDL cholesterol declined from 22.2% to 16.0% in 2017-2018, the National Center for Health Statistics reported.
HDL cholesterol data before 2007 were not presented because of changes in laboratories and methods, but both trends are significant, and the decline in high total cholesterol means that the Healthy People 2020 goal of dropping prevalence to 13.5% has been met, said Margaret D. Carroll, MSPH, and Cheryl D. Fryar, MSPH, of the NCHS.
The demographic details, however, show some disparities hidden by the broader measures. The prevalence of low HDL cholesterol for women in 2015-2018 was 8.5%, but for men it was 26.6%, the NCHS investigators said.
And that Healthy People 2020 goal for total cholesterol? Age makes a difference: 7.5% of adults aged 20-39 years had high total cholesterol in 2015-2018, as did 11.4% of those aged 60 years and older, but those aged 40-59 years had a significantly higher prevalence of 15.7%, they reported.
Race/ethnicity was also a factor. Prevalence of low HDL was similar for white (16.6%) and Asian (15.8%) adults in 2015-2018, but black adults’ low HDL prevalence was significantly lower (11.9%) and Hispanics’ was significantly higher (21.9%), the researchers said.
The analysis was based on data from the National Health and Nutrition Examination Survey. The investigators defined high total cholesterol as a level of 240 mg/dL or more, and low HDL cholesterol as less than 40 mg/dL. LDL cholesterol was not included in the analysis.
Good news: High cholesterol is down in the United States. More good news: Low HDL cholesterol is down in the United States.

The prevalence of high total cholesterol in adults aged 20 years and older dropped from 18.3% in 1999-2000 to 10.5% in 2017-2018. And starting in 2007-2008, the prevalence of low HDL cholesterol declined from 22.2% to 16.0% in 2017-2018, the National Center for Health Statistics reported.
HDL cholesterol data before 2007 were not presented because of changes in laboratories and methods, but both trends are significant, and the decline in high total cholesterol means that the Healthy People 2020 goal of dropping prevalence to 13.5% has been met, said Margaret D. Carroll, MSPH, and Cheryl D. Fryar, MSPH, of the NCHS.
The demographic details, however, show some disparities hidden by the broader measures. The prevalence of low HDL cholesterol for women in 2015-2018 was 8.5%, but for men it was 26.6%, the NCHS investigators said.
And that Healthy People 2020 goal for total cholesterol? Age makes a difference: 7.5% of adults aged 20-39 years had high total cholesterol in 2015-2018, as did 11.4% of those aged 60 years and older, but those aged 40-59 years had a significantly higher prevalence of 15.7%, they reported.
Race/ethnicity was also a factor. Prevalence of low HDL was similar for white (16.6%) and Asian (15.8%) adults in 2015-2018, but black adults’ low HDL prevalence was significantly lower (11.9%) and Hispanics’ was significantly higher (21.9%), the researchers said.
The analysis was based on data from the National Health and Nutrition Examination Survey. The investigators defined high total cholesterol as a level of 240 mg/dL or more, and low HDL cholesterol as less than 40 mg/dL. LDL cholesterol was not included in the analysis.
Lipid-lowering bempedoic acid does not hasten or worsen diabetes
In an analysis of four phase 3 trials, the oral lipid-lowering drug bempedoic acid (Nexletol; Esperion) did not worsen glycemic control or increase the incidence of type 2 diabetes.
As previously reported, this first-in-class drug, which acts by inhibiting ATP-citrate lyase, was approved by the Food and Drug Administration in February 2020.
Lawrence A. Leiter MD, from the University of Toronto, delivered the findings of this latest analysis in an oral presentation at the virtual American Diabetes Association 80th Scientific Sessions.
“The current study is important as it shows overall consistent efficacy and safety regardless of glycemic status and no increase in new-onset diabetes,” Dr. Leiter said in an interview.
There is interest in how lipid-lowering drugs might affect glycemia because “meta-analyses have shown about a 10% increased risk of new-onset diabetes in statin users, although the absolute increased risk is 1 extra case per 255 treated patients [in whom one would expect 5.4 cardiovascular events to be prevented by the statin],” he noted.
In a comment, John R. Guyton, MD, from Duke University Medical Center, Durham, N.C., agreed that the new study demonstrates that “patients with diabetes and prediabetes respond to bempedoic acid with LDL cholesterol lowering that is similar to that in patients with normal glucose tolerance.”
Although “statins have a slight effect of worsening glucose tolerance and a modest effect of increasing cases of new-onset diabetes,” the current research shows that “bempedoic acid appears to be free of these effects,” said Dr. Guyton, who discussed this drug in another symposium at the meeting where he also discussed how the agent will “fit” into prescribing patterns.
How do patients with diabetes, prediabetes fare?
“Current guidelines support aggressive LDL cholesterol lowering in patients with diabetes, given the increased risk of cardiovascular morbidity and mortality,” said Dr. Leiter.
Bempedoic acid was approved as an adjunct to diet and maximally tolerated statin therapy to treat adults with atherosclerotic cardiovascular disease (ASCVD) and/or heterozygous familial hypercholesterolemia (HeFH) who require additional lowering of LDL cholesterol, although its effect on cardiovascular morbidity and mortality has not been determined, the prescribing information states.
However, it has been unknown how bempedoic acid affects LDL cholesterol or hemoglobin A1c levels in patients with diabetes, prediabetes, or normoglycemia.
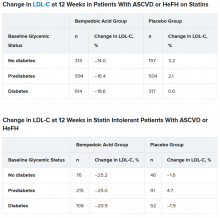
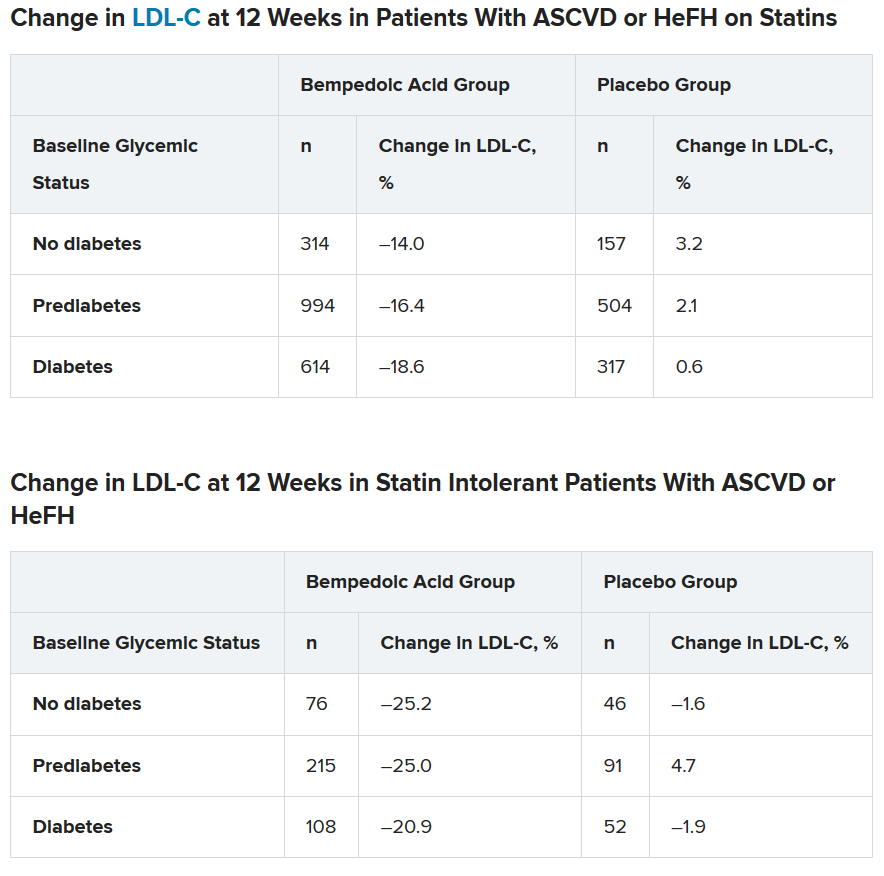
To examine this, the researchers pooled data from four phase 3 trials in 3623 patients with ASCVD or HeFH who had been randomized 2:1 to bempedoic acid 180 mg/day or placebo for 12 or 24 weeks (if they were statin intolerant) or 52 weeks (if they were also on statins).
In the pooled sample, about half the patients had prediabetes (52%), and the rest had diabetes (31%) or normoglycemia (17%). Overall, 75%-84% of patients had a history of ASCVD.
Mean LDL cholesterol levels were higher in patients with normoglycemia (119 mg/dL) or prediabetes (115 mg/dL) than in patients with diabetes (110 mg/dL).
The primary outcome was percent change in LDL cholesterol from baseline to week 12.
In the two types of patients (all with ASCVD or HeFH) – those on statins and those with statin intolerance – LDL cholesterol at 12 weeks was significantly lower in patients who received bempedoic acid, compared with placebo, regardless of whether they had no diabetes, prediabetes, or diabetes (all P < .001).
Similarly, patients who received bempedoic acid also had significant reductions in total cholesterol, non–HDL cholesterol, apolipoprotein B, and high-sensitivity C-reactive protein (hsCRP) at 12 weeks, compared with patients who received placebo (all P < .01).
The safety profile of bempedoic acid was similar to placebo and did not vary by glycemic status.
“Of course, with any lipid-lowering therapy, there’s lots of interest in changes in glycemic parameters,” said Dr. Leiter. “A1c did not increase. In fact, it was significantly lower in patients with prediabetes and diabetes on bempedoic acid versus placebo.”
In addition, “statin trials have shown small increases in body weight. We did not observe this,” he reported.
Where does bempedoic acid ‘fit?’
“Bempedoic acid will be a useful add-on to any patient who requires additional LDL cholesterol lowering,” according to Dr. Leiter. “It will typically be used as an add-on to statins, but will also be very useful in the statin-intolerant patient, especially when used in combination with ezetimib.”
The fixed-dose combination of bempedoic acid plus ezetimibe (Nexlizet; Esperion), was also approved in the United States in February, just days after bempedoic acid as a solo agent was cleared for marketing.
“Bempedoic acid would not be chosen in preference to a statin, ezetimibe, or PCSK9 inhibitor,” Dr. Guyton said. Rather, “its chief use will be in patients with statin intolerance and either FH or ASCVD when LDL-cholesterol is poorly controlled despite maximum tolerated lipid-lowering therapy.”
According to Dr. Guyton, “use of bempedoic acid should be undertaken only when provider-patient discussion acknowledges that it has not been shown to reduce cardiovascular events, although preliminary evidence from genetic analysis [Mendelian randomization study] suggests that it will,” as previously reported.
The CLEAR Outcomes cardiovascular outcomes trial of bempedoic acid completed enrollment in August 2019, involving 14,032 patients with hypercholesterolemia and high CVD risk according to a company statement.
The study was funded by Esperion. Dr. Leiter has reported being on advisory panels for Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, HLS Therapeutics, Janssen, Merck, Novo Nordisk, Sanofi, and Servier, receiving research support from Amgen, AstraZeneca, Kowa Pharmaceuticals, and the Medicines Company, and being on speakers bureaus for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, HLS Therapeutics, Janssen, Medscape, Merck, Novo Nordisk, Sanofi, and Servier. Disclosures for the other authors are listed with the abstract. Dr. Guyton has reported being a consultant for Amarin and receiving research support form Regeneron.
A version of this article originally appeared on Medscape.com.
In an analysis of four phase 3 trials, the oral lipid-lowering drug bempedoic acid (Nexletol; Esperion) did not worsen glycemic control or increase the incidence of type 2 diabetes.
As previously reported, this first-in-class drug, which acts by inhibiting ATP-citrate lyase, was approved by the Food and Drug Administration in February 2020.
Lawrence A. Leiter MD, from the University of Toronto, delivered the findings of this latest analysis in an oral presentation at the virtual American Diabetes Association 80th Scientific Sessions.
“The current study is important as it shows overall consistent efficacy and safety regardless of glycemic status and no increase in new-onset diabetes,” Dr. Leiter said in an interview.
There is interest in how lipid-lowering drugs might affect glycemia because “meta-analyses have shown about a 10% increased risk of new-onset diabetes in statin users, although the absolute increased risk is 1 extra case per 255 treated patients [in whom one would expect 5.4 cardiovascular events to be prevented by the statin],” he noted.
In a comment, John R. Guyton, MD, from Duke University Medical Center, Durham, N.C., agreed that the new study demonstrates that “patients with diabetes and prediabetes respond to bempedoic acid with LDL cholesterol lowering that is similar to that in patients with normal glucose tolerance.”
Although “statins have a slight effect of worsening glucose tolerance and a modest effect of increasing cases of new-onset diabetes,” the current research shows that “bempedoic acid appears to be free of these effects,” said Dr. Guyton, who discussed this drug in another symposium at the meeting where he also discussed how the agent will “fit” into prescribing patterns.
How do patients with diabetes, prediabetes fare?
“Current guidelines support aggressive LDL cholesterol lowering in patients with diabetes, given the increased risk of cardiovascular morbidity and mortality,” said Dr. Leiter.
Bempedoic acid was approved as an adjunct to diet and maximally tolerated statin therapy to treat adults with atherosclerotic cardiovascular disease (ASCVD) and/or heterozygous familial hypercholesterolemia (HeFH) who require additional lowering of LDL cholesterol, although its effect on cardiovascular morbidity and mortality has not been determined, the prescribing information states.
However, it has been unknown how bempedoic acid affects LDL cholesterol or hemoglobin A1c levels in patients with diabetes, prediabetes, or normoglycemia.
To examine this, the researchers pooled data from four phase 3 trials in 3623 patients with ASCVD or HeFH who had been randomized 2:1 to bempedoic acid 180 mg/day or placebo for 12 or 24 weeks (if they were statin intolerant) or 52 weeks (if they were also on statins).
In the pooled sample, about half the patients had prediabetes (52%), and the rest had diabetes (31%) or normoglycemia (17%). Overall, 75%-84% of patients had a history of ASCVD.
Mean LDL cholesterol levels were higher in patients with normoglycemia (119 mg/dL) or prediabetes (115 mg/dL) than in patients with diabetes (110 mg/dL).
The primary outcome was percent change in LDL cholesterol from baseline to week 12.
In the two types of patients (all with ASCVD or HeFH) – those on statins and those with statin intolerance – LDL cholesterol at 12 weeks was significantly lower in patients who received bempedoic acid, compared with placebo, regardless of whether they had no diabetes, prediabetes, or diabetes (all P < .001).
Similarly, patients who received bempedoic acid also had significant reductions in total cholesterol, non–HDL cholesterol, apolipoprotein B, and high-sensitivity C-reactive protein (hsCRP) at 12 weeks, compared with patients who received placebo (all P < .01).
The safety profile of bempedoic acid was similar to placebo and did not vary by glycemic status.
“Of course, with any lipid-lowering therapy, there’s lots of interest in changes in glycemic parameters,” said Dr. Leiter. “A1c did not increase. In fact, it was significantly lower in patients with prediabetes and diabetes on bempedoic acid versus placebo.”
In addition, “statin trials have shown small increases in body weight. We did not observe this,” he reported.
Where does bempedoic acid ‘fit?’
“Bempedoic acid will be a useful add-on to any patient who requires additional LDL cholesterol lowering,” according to Dr. Leiter. “It will typically be used as an add-on to statins, but will also be very useful in the statin-intolerant patient, especially when used in combination with ezetimib.”
The fixed-dose combination of bempedoic acid plus ezetimibe (Nexlizet; Esperion), was also approved in the United States in February, just days after bempedoic acid as a solo agent was cleared for marketing.
“Bempedoic acid would not be chosen in preference to a statin, ezetimibe, or PCSK9 inhibitor,” Dr. Guyton said. Rather, “its chief use will be in patients with statin intolerance and either FH or ASCVD when LDL-cholesterol is poorly controlled despite maximum tolerated lipid-lowering therapy.”
According to Dr. Guyton, “use of bempedoic acid should be undertaken only when provider-patient discussion acknowledges that it has not been shown to reduce cardiovascular events, although preliminary evidence from genetic analysis [Mendelian randomization study] suggests that it will,” as previously reported.
The CLEAR Outcomes cardiovascular outcomes trial of bempedoic acid completed enrollment in August 2019, involving 14,032 patients with hypercholesterolemia and high CVD risk according to a company statement.
The study was funded by Esperion. Dr. Leiter has reported being on advisory panels for Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, HLS Therapeutics, Janssen, Merck, Novo Nordisk, Sanofi, and Servier, receiving research support from Amgen, AstraZeneca, Kowa Pharmaceuticals, and the Medicines Company, and being on speakers bureaus for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, HLS Therapeutics, Janssen, Medscape, Merck, Novo Nordisk, Sanofi, and Servier. Disclosures for the other authors are listed with the abstract. Dr. Guyton has reported being a consultant for Amarin and receiving research support form Regeneron.
A version of this article originally appeared on Medscape.com.
In an analysis of four phase 3 trials, the oral lipid-lowering drug bempedoic acid (Nexletol; Esperion) did not worsen glycemic control or increase the incidence of type 2 diabetes.
As previously reported, this first-in-class drug, which acts by inhibiting ATP-citrate lyase, was approved by the Food and Drug Administration in February 2020.
Lawrence A. Leiter MD, from the University of Toronto, delivered the findings of this latest analysis in an oral presentation at the virtual American Diabetes Association 80th Scientific Sessions.
“The current study is important as it shows overall consistent efficacy and safety regardless of glycemic status and no increase in new-onset diabetes,” Dr. Leiter said in an interview.
There is interest in how lipid-lowering drugs might affect glycemia because “meta-analyses have shown about a 10% increased risk of new-onset diabetes in statin users, although the absolute increased risk is 1 extra case per 255 treated patients [in whom one would expect 5.4 cardiovascular events to be prevented by the statin],” he noted.
In a comment, John R. Guyton, MD, from Duke University Medical Center, Durham, N.C., agreed that the new study demonstrates that “patients with diabetes and prediabetes respond to bempedoic acid with LDL cholesterol lowering that is similar to that in patients with normal glucose tolerance.”
Although “statins have a slight effect of worsening glucose tolerance and a modest effect of increasing cases of new-onset diabetes,” the current research shows that “bempedoic acid appears to be free of these effects,” said Dr. Guyton, who discussed this drug in another symposium at the meeting where he also discussed how the agent will “fit” into prescribing patterns.
How do patients with diabetes, prediabetes fare?
“Current guidelines support aggressive LDL cholesterol lowering in patients with diabetes, given the increased risk of cardiovascular morbidity and mortality,” said Dr. Leiter.
Bempedoic acid was approved as an adjunct to diet and maximally tolerated statin therapy to treat adults with atherosclerotic cardiovascular disease (ASCVD) and/or heterozygous familial hypercholesterolemia (HeFH) who require additional lowering of LDL cholesterol, although its effect on cardiovascular morbidity and mortality has not been determined, the prescribing information states.
However, it has been unknown how bempedoic acid affects LDL cholesterol or hemoglobin A1c levels in patients with diabetes, prediabetes, or normoglycemia.
To examine this, the researchers pooled data from four phase 3 trials in 3623 patients with ASCVD or HeFH who had been randomized 2:1 to bempedoic acid 180 mg/day or placebo for 12 or 24 weeks (if they were statin intolerant) or 52 weeks (if they were also on statins).
In the pooled sample, about half the patients had prediabetes (52%), and the rest had diabetes (31%) or normoglycemia (17%). Overall, 75%-84% of patients had a history of ASCVD.
Mean LDL cholesterol levels were higher in patients with normoglycemia (119 mg/dL) or prediabetes (115 mg/dL) than in patients with diabetes (110 mg/dL).
The primary outcome was percent change in LDL cholesterol from baseline to week 12.
In the two types of patients (all with ASCVD or HeFH) – those on statins and those with statin intolerance – LDL cholesterol at 12 weeks was significantly lower in patients who received bempedoic acid, compared with placebo, regardless of whether they had no diabetes, prediabetes, or diabetes (all P < .001).
Similarly, patients who received bempedoic acid also had significant reductions in total cholesterol, non–HDL cholesterol, apolipoprotein B, and high-sensitivity C-reactive protein (hsCRP) at 12 weeks, compared with patients who received placebo (all P < .01).
The safety profile of bempedoic acid was similar to placebo and did not vary by glycemic status.
“Of course, with any lipid-lowering therapy, there’s lots of interest in changes in glycemic parameters,” said Dr. Leiter. “A1c did not increase. In fact, it was significantly lower in patients with prediabetes and diabetes on bempedoic acid versus placebo.”
In addition, “statin trials have shown small increases in body weight. We did not observe this,” he reported.
Where does bempedoic acid ‘fit?’
“Bempedoic acid will be a useful add-on to any patient who requires additional LDL cholesterol lowering,” according to Dr. Leiter. “It will typically be used as an add-on to statins, but will also be very useful in the statin-intolerant patient, especially when used in combination with ezetimib.”
The fixed-dose combination of bempedoic acid plus ezetimibe (Nexlizet; Esperion), was also approved in the United States in February, just days after bempedoic acid as a solo agent was cleared for marketing.
“Bempedoic acid would not be chosen in preference to a statin, ezetimibe, or PCSK9 inhibitor,” Dr. Guyton said. Rather, “its chief use will be in patients with statin intolerance and either FH or ASCVD when LDL-cholesterol is poorly controlled despite maximum tolerated lipid-lowering therapy.”
According to Dr. Guyton, “use of bempedoic acid should be undertaken only when provider-patient discussion acknowledges that it has not been shown to reduce cardiovascular events, although preliminary evidence from genetic analysis [Mendelian randomization study] suggests that it will,” as previously reported.
The CLEAR Outcomes cardiovascular outcomes trial of bempedoic acid completed enrollment in August 2019, involving 14,032 patients with hypercholesterolemia and high CVD risk according to a company statement.
The study was funded by Esperion. Dr. Leiter has reported being on advisory panels for Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, HLS Therapeutics, Janssen, Merck, Novo Nordisk, Sanofi, and Servier, receiving research support from Amgen, AstraZeneca, Kowa Pharmaceuticals, and the Medicines Company, and being on speakers bureaus for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, HLS Therapeutics, Janssen, Medscape, Merck, Novo Nordisk, Sanofi, and Servier. Disclosures for the other authors are listed with the abstract. Dr. Guyton has reported being a consultant for Amarin and receiving research support form Regeneron.
A version of this article originally appeared on Medscape.com.
Cognitive-behavioral therapy a standout for better immune function
Psychosocial interventions, particularly cognitive-behavioral therapy (CBT), are associated with enhanced immune system function, new research suggests.
Results of a systematic review and meta-analysis that included 56 randomized controlled trials and more than 4,000 participants showed that over time, psychosocial interventions appeared to augment beneficial immune system function while concurrently decreasing harmful immune system function in comparison with control conditions.
“These associations were most reliable for cognitive-behavioral therapy and multiple or combined interventions and for studies that assessed proinflammatory cytokines or markers, which are key indicators of inflammation in the body,” study investigator George M. Slavich, PhD, said in an interview.
“The analysis helps address the question of which types of psychosocial interventions are most consistently associated with changes in immune system function, under what conditions, and for whom. This knowledge could, in turn, be used to inform research efforts and public policy aimed at using psychosocial interventions to improve immune-related health outcomes,” added Dr. Slavich, director of the Laboratory for Stress Assessment and Research, University of California, Los Angeles.
The study was published online June 3 in JAMA Psychiatry.
Link to serious physical, mental illnesses
There is substantial evidence that the immune system plays a role in a variety of mental and physical health problems. Such problems include anxiety disorders, depression, suicide, schizophrenia, cardiovascular disease, autoimmune disorders, and neurodegenerative diseases. It has been recently suggested that more than half of all deaths worldwide are attributable to inflammation-related conditions.
Although pharmacologic interventions can play a role in addressing inflammation, they are not without drawbacks, most notably, cost and adverse side effects.
The World Health Organization, the National Academy of Medicine, the National Institutes of Health, and other groups have emphasized the importance of addressing global disease burden through psychosocial interventions when possible.
Such recommendations are supported by scientific evidence. Previous research has shown that immune system processes are influenced by a variety of social, neurocognitive, and behavioral factors.
Given such findings, researchers have examined the effects of interventions that reduce stress or bolster psychological resources on immune system function.
However, such research has yielded conflicting findings. Some studies show that psychosocial interventions clearly enhance immunity, whereas others do not.
In addition, questions remain regarding which types of interventions reliably improve immune system function, under what conditions, and for whom.
“Research has shown that psychological factors – such as life stress, negative emotions, and social support – are associated with changes in immune system function,” Dr. Slavich noted.
“In addition, there is growing appreciation that immune system processes involved in inflammation may contribute to peoples’ risk for several major mental and physical health problems, including anxiety disorders, depression, heart disease, and autoimmune and neurodegenerative disorders.”
First study of its kind
To shed light on these potential links, the researchers conducted what they believe is the first systematic review and meta-analysis of randomized clinical trials of the effects of psychosocial interventions on immune system outcomes.
As part of the review, Dr. Slavich and colleagues estimated the associations between eight psychosocial interventions and seven markers of immune system function.
The eight psychosocial interventions were behavior therapy, cognitive therapy, CBT, CBT plus additive treatment or mode of delivery, bereavement or supportive therapy, multiple or combined interventions, other psychotherapy, and psychoeducation.
The seven immune outcomes that might be influenced by these interventions are proinflammatory cytokines and markers, anti-inflammatory cytokines, antibodies, immune cell counts, natural killer cell activity, viral load, and other immune outcomes.
The researchers also examined nine potential factors that might moderate the associations between psychosocial interventions and immune system function.
They searched a variety of databases for all relevant randomized controlled trials published through Dec. 31, 2018. Studies were eligible for inclusion if they included a psychosocial intervention and immune outcome, as well as preintervention and postintervention immunologic assessments.
The researchers identified 4,621 studies. Of these studies, 62 were eligible for inclusion; 56, which included 4,060 patients, were included in the final meta-analysis.
Results showed that psychosocial interventions were associated with enhanced immune system function (P < .001). There was relatively low heterogeneity between studies in these effect sizes, which, the investigators said, indicates that the association was relatively consistent across studies and conditions.
The meta-analysis showed that individuals who were assigned to a psychosocial intervention condition demonstrated a 14.7% improvement (95% confidence interval [CI], 5.7%–23.8%) in beneficial immune system function compared with their counterparts who were assigned to a control condition.
Similarly, participants who received psychosocial interventions demonstrated an 18.0% decrease (95% CI, 7.2%–28.8%) in harmful immune system function over time.
A standout
Regarding the effect of the type of intervention on the association, only CBT (31 studies; P < .001) and multiple or combined interventions (seven studies; P = .01) were significantly associated with changes in immune system outcomes.
The analysis also found that interventions that included a group component were more consistently associated with enhanced immune function than were those that did not include a group component. Nevertheless, this difference did not reach statistical significance (P = .06).
Contrary to the researchers’ expectations, the analysis also revealed that intervention length did not moderate the association between psychosocial interventions and immune system function (P = .93).
With respect to the type of immune marker studied, the meta-analysis found that psychosocial interventions had significantly different associations with the various immune markers studied. Of the seven immune outcomes investigated, only proinflammatory cytokine or marker levels (33 studies; P < .001) and immune cell counts (27 studies; P < .001) were significantly associated with the psychosocial interventions examined.
and were robust across age, sex, and intervention duration.
These results suggest that psychosocial interventions – particularly CBT and multiple or combined psychotherapeutic modalities – may play an important role in improving immune-related health outcomes.
Such interventions may not only be effective, they may also prove to be affordable alternatives to current therapeutic options. The mean length of a CBT intervention in the meta-analysis was 10.4 weeks, which the investigators equated with a total cost of $1,560 per patient.
“By comparison, the cost of using infliximab to reduce inflammation in persons with an autoimmune disorder is approximately $25,000 per patient per year,” they wrote.
“The results suggest the possibility that psychotherapy may be helpful for reducing inflammation and improving immune-related health in certain circumstances,” Dr. Slavich concluded. “However, the studies that we examined differed in terms of their quality, and we did not examine health outcomes in the present investigation.
“Therefore, more research needs to be done to determine how the present findings might be translated into treatment options or public policy.”
A path to better health
In an accompanying editorial, Veronika Engert, PhD, Joshua A. Grant, PhD, and Bernhard Strauss, PhD, noted that although infectious disease was once the primary cause of death in society, it has been supplanted by other complex and chronic illnesses, which often do not follow simple cause-and-effect associations.
“Rather,” they wrote, “these illnesses develop from a complex milieu of biological, psychological, and social factors that may also influence the disease progress and its prognosis. Against this backdrop, the meta-analysis by Shields and colleagues is an important confirmation of the biopsychosocial model.”
The editorialists explained that recent psychophysiological, neurobiological, and epigenetic research offers a glimpse into the relationship between psychological and social factors in pathogenesis. Nevertheless, the authors noted that a comprehensive examination of the potential effects of psychosocial interventions on immune parameters in various physical health conditions has been lacking.
“The evidence provided by Shields et al. is exactly what is needed to more fully shift treatment from an illness-centered to a patient-centered approach,” they wrote. “To that end, this meta-analysis may serve as a guide for policy makers aiming to improve immune-associated health.”
The research was supported by a Society in Science–Branco Weiss Fellowship, Brain and Behavior Research, and the National Institutes of Health. Dr. Slavich, Dr. Engert, Dr. Grant, and Dr. Strauss have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Psychosocial interventions, particularly cognitive-behavioral therapy (CBT), are associated with enhanced immune system function, new research suggests.
Results of a systematic review and meta-analysis that included 56 randomized controlled trials and more than 4,000 participants showed that over time, psychosocial interventions appeared to augment beneficial immune system function while concurrently decreasing harmful immune system function in comparison with control conditions.
“These associations were most reliable for cognitive-behavioral therapy and multiple or combined interventions and for studies that assessed proinflammatory cytokines or markers, which are key indicators of inflammation in the body,” study investigator George M. Slavich, PhD, said in an interview.
“The analysis helps address the question of which types of psychosocial interventions are most consistently associated with changes in immune system function, under what conditions, and for whom. This knowledge could, in turn, be used to inform research efforts and public policy aimed at using psychosocial interventions to improve immune-related health outcomes,” added Dr. Slavich, director of the Laboratory for Stress Assessment and Research, University of California, Los Angeles.
The study was published online June 3 in JAMA Psychiatry.
Link to serious physical, mental illnesses
There is substantial evidence that the immune system plays a role in a variety of mental and physical health problems. Such problems include anxiety disorders, depression, suicide, schizophrenia, cardiovascular disease, autoimmune disorders, and neurodegenerative diseases. It has been recently suggested that more than half of all deaths worldwide are attributable to inflammation-related conditions.
Although pharmacologic interventions can play a role in addressing inflammation, they are not without drawbacks, most notably, cost and adverse side effects.
The World Health Organization, the National Academy of Medicine, the National Institutes of Health, and other groups have emphasized the importance of addressing global disease burden through psychosocial interventions when possible.
Such recommendations are supported by scientific evidence. Previous research has shown that immune system processes are influenced by a variety of social, neurocognitive, and behavioral factors.
Given such findings, researchers have examined the effects of interventions that reduce stress or bolster psychological resources on immune system function.
However, such research has yielded conflicting findings. Some studies show that psychosocial interventions clearly enhance immunity, whereas others do not.
In addition, questions remain regarding which types of interventions reliably improve immune system function, under what conditions, and for whom.
“Research has shown that psychological factors – such as life stress, negative emotions, and social support – are associated with changes in immune system function,” Dr. Slavich noted.
“In addition, there is growing appreciation that immune system processes involved in inflammation may contribute to peoples’ risk for several major mental and physical health problems, including anxiety disorders, depression, heart disease, and autoimmune and neurodegenerative disorders.”
First study of its kind
To shed light on these potential links, the researchers conducted what they believe is the first systematic review and meta-analysis of randomized clinical trials of the effects of psychosocial interventions on immune system outcomes.
As part of the review, Dr. Slavich and colleagues estimated the associations between eight psychosocial interventions and seven markers of immune system function.
The eight psychosocial interventions were behavior therapy, cognitive therapy, CBT, CBT plus additive treatment or mode of delivery, bereavement or supportive therapy, multiple or combined interventions, other psychotherapy, and psychoeducation.
The seven immune outcomes that might be influenced by these interventions are proinflammatory cytokines and markers, anti-inflammatory cytokines, antibodies, immune cell counts, natural killer cell activity, viral load, and other immune outcomes.
The researchers also examined nine potential factors that might moderate the associations between psychosocial interventions and immune system function.
They searched a variety of databases for all relevant randomized controlled trials published through Dec. 31, 2018. Studies were eligible for inclusion if they included a psychosocial intervention and immune outcome, as well as preintervention and postintervention immunologic assessments.
The researchers identified 4,621 studies. Of these studies, 62 were eligible for inclusion; 56, which included 4,060 patients, were included in the final meta-analysis.
Results showed that psychosocial interventions were associated with enhanced immune system function (P < .001). There was relatively low heterogeneity between studies in these effect sizes, which, the investigators said, indicates that the association was relatively consistent across studies and conditions.
The meta-analysis showed that individuals who were assigned to a psychosocial intervention condition demonstrated a 14.7% improvement (95% confidence interval [CI], 5.7%–23.8%) in beneficial immune system function compared with their counterparts who were assigned to a control condition.
Similarly, participants who received psychosocial interventions demonstrated an 18.0% decrease (95% CI, 7.2%–28.8%) in harmful immune system function over time.
A standout
Regarding the effect of the type of intervention on the association, only CBT (31 studies; P < .001) and multiple or combined interventions (seven studies; P = .01) were significantly associated with changes in immune system outcomes.
The analysis also found that interventions that included a group component were more consistently associated with enhanced immune function than were those that did not include a group component. Nevertheless, this difference did not reach statistical significance (P = .06).
Contrary to the researchers’ expectations, the analysis also revealed that intervention length did not moderate the association between psychosocial interventions and immune system function (P = .93).
With respect to the type of immune marker studied, the meta-analysis found that psychosocial interventions had significantly different associations with the various immune markers studied. Of the seven immune outcomes investigated, only proinflammatory cytokine or marker levels (33 studies; P < .001) and immune cell counts (27 studies; P < .001) were significantly associated with the psychosocial interventions examined.
and were robust across age, sex, and intervention duration.
These results suggest that psychosocial interventions – particularly CBT and multiple or combined psychotherapeutic modalities – may play an important role in improving immune-related health outcomes.
Such interventions may not only be effective, they may also prove to be affordable alternatives to current therapeutic options. The mean length of a CBT intervention in the meta-analysis was 10.4 weeks, which the investigators equated with a total cost of $1,560 per patient.
“By comparison, the cost of using infliximab to reduce inflammation in persons with an autoimmune disorder is approximately $25,000 per patient per year,” they wrote.
“The results suggest the possibility that psychotherapy may be helpful for reducing inflammation and improving immune-related health in certain circumstances,” Dr. Slavich concluded. “However, the studies that we examined differed in terms of their quality, and we did not examine health outcomes in the present investigation.
“Therefore, more research needs to be done to determine how the present findings might be translated into treatment options or public policy.”
A path to better health
In an accompanying editorial, Veronika Engert, PhD, Joshua A. Grant, PhD, and Bernhard Strauss, PhD, noted that although infectious disease was once the primary cause of death in society, it has been supplanted by other complex and chronic illnesses, which often do not follow simple cause-and-effect associations.
“Rather,” they wrote, “these illnesses develop from a complex milieu of biological, psychological, and social factors that may also influence the disease progress and its prognosis. Against this backdrop, the meta-analysis by Shields and colleagues is an important confirmation of the biopsychosocial model.”
The editorialists explained that recent psychophysiological, neurobiological, and epigenetic research offers a glimpse into the relationship between psychological and social factors in pathogenesis. Nevertheless, the authors noted that a comprehensive examination of the potential effects of psychosocial interventions on immune parameters in various physical health conditions has been lacking.
“The evidence provided by Shields et al. is exactly what is needed to more fully shift treatment from an illness-centered to a patient-centered approach,” they wrote. “To that end, this meta-analysis may serve as a guide for policy makers aiming to improve immune-associated health.”
The research was supported by a Society in Science–Branco Weiss Fellowship, Brain and Behavior Research, and the National Institutes of Health. Dr. Slavich, Dr. Engert, Dr. Grant, and Dr. Strauss have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Psychosocial interventions, particularly cognitive-behavioral therapy (CBT), are associated with enhanced immune system function, new research suggests.
Results of a systematic review and meta-analysis that included 56 randomized controlled trials and more than 4,000 participants showed that over time, psychosocial interventions appeared to augment beneficial immune system function while concurrently decreasing harmful immune system function in comparison with control conditions.
“These associations were most reliable for cognitive-behavioral therapy and multiple or combined interventions and for studies that assessed proinflammatory cytokines or markers, which are key indicators of inflammation in the body,” study investigator George M. Slavich, PhD, said in an interview.
“The analysis helps address the question of which types of psychosocial interventions are most consistently associated with changes in immune system function, under what conditions, and for whom. This knowledge could, in turn, be used to inform research efforts and public policy aimed at using psychosocial interventions to improve immune-related health outcomes,” added Dr. Slavich, director of the Laboratory for Stress Assessment and Research, University of California, Los Angeles.
The study was published online June 3 in JAMA Psychiatry.
Link to serious physical, mental illnesses
There is substantial evidence that the immune system plays a role in a variety of mental and physical health problems. Such problems include anxiety disorders, depression, suicide, schizophrenia, cardiovascular disease, autoimmune disorders, and neurodegenerative diseases. It has been recently suggested that more than half of all deaths worldwide are attributable to inflammation-related conditions.
Although pharmacologic interventions can play a role in addressing inflammation, they are not without drawbacks, most notably, cost and adverse side effects.
The World Health Organization, the National Academy of Medicine, the National Institutes of Health, and other groups have emphasized the importance of addressing global disease burden through psychosocial interventions when possible.
Such recommendations are supported by scientific evidence. Previous research has shown that immune system processes are influenced by a variety of social, neurocognitive, and behavioral factors.
Given such findings, researchers have examined the effects of interventions that reduce stress or bolster psychological resources on immune system function.
However, such research has yielded conflicting findings. Some studies show that psychosocial interventions clearly enhance immunity, whereas others do not.
In addition, questions remain regarding which types of interventions reliably improve immune system function, under what conditions, and for whom.
“Research has shown that psychological factors – such as life stress, negative emotions, and social support – are associated with changes in immune system function,” Dr. Slavich noted.
“In addition, there is growing appreciation that immune system processes involved in inflammation may contribute to peoples’ risk for several major mental and physical health problems, including anxiety disorders, depression, heart disease, and autoimmune and neurodegenerative disorders.”
First study of its kind
To shed light on these potential links, the researchers conducted what they believe is the first systematic review and meta-analysis of randomized clinical trials of the effects of psychosocial interventions on immune system outcomes.
As part of the review, Dr. Slavich and colleagues estimated the associations between eight psychosocial interventions and seven markers of immune system function.
The eight psychosocial interventions were behavior therapy, cognitive therapy, CBT, CBT plus additive treatment or mode of delivery, bereavement or supportive therapy, multiple or combined interventions, other psychotherapy, and psychoeducation.
The seven immune outcomes that might be influenced by these interventions are proinflammatory cytokines and markers, anti-inflammatory cytokines, antibodies, immune cell counts, natural killer cell activity, viral load, and other immune outcomes.
The researchers also examined nine potential factors that might moderate the associations between psychosocial interventions and immune system function.
They searched a variety of databases for all relevant randomized controlled trials published through Dec. 31, 2018. Studies were eligible for inclusion if they included a psychosocial intervention and immune outcome, as well as preintervention and postintervention immunologic assessments.
The researchers identified 4,621 studies. Of these studies, 62 were eligible for inclusion; 56, which included 4,060 patients, were included in the final meta-analysis.
Results showed that psychosocial interventions were associated with enhanced immune system function (P < .001). There was relatively low heterogeneity between studies in these effect sizes, which, the investigators said, indicates that the association was relatively consistent across studies and conditions.
The meta-analysis showed that individuals who were assigned to a psychosocial intervention condition demonstrated a 14.7% improvement (95% confidence interval [CI], 5.7%–23.8%) in beneficial immune system function compared with their counterparts who were assigned to a control condition.
Similarly, participants who received psychosocial interventions demonstrated an 18.0% decrease (95% CI, 7.2%–28.8%) in harmful immune system function over time.
A standout
Regarding the effect of the type of intervention on the association, only CBT (31 studies; P < .001) and multiple or combined interventions (seven studies; P = .01) were significantly associated with changes in immune system outcomes.
The analysis also found that interventions that included a group component were more consistently associated with enhanced immune function than were those that did not include a group component. Nevertheless, this difference did not reach statistical significance (P = .06).
Contrary to the researchers’ expectations, the analysis also revealed that intervention length did not moderate the association between psychosocial interventions and immune system function (P = .93).
With respect to the type of immune marker studied, the meta-analysis found that psychosocial interventions had significantly different associations with the various immune markers studied. Of the seven immune outcomes investigated, only proinflammatory cytokine or marker levels (33 studies; P < .001) and immune cell counts (27 studies; P < .001) were significantly associated with the psychosocial interventions examined.
and were robust across age, sex, and intervention duration.
These results suggest that psychosocial interventions – particularly CBT and multiple or combined psychotherapeutic modalities – may play an important role in improving immune-related health outcomes.
Such interventions may not only be effective, they may also prove to be affordable alternatives to current therapeutic options. The mean length of a CBT intervention in the meta-analysis was 10.4 weeks, which the investigators equated with a total cost of $1,560 per patient.
“By comparison, the cost of using infliximab to reduce inflammation in persons with an autoimmune disorder is approximately $25,000 per patient per year,” they wrote.
“The results suggest the possibility that psychotherapy may be helpful for reducing inflammation and improving immune-related health in certain circumstances,” Dr. Slavich concluded. “However, the studies that we examined differed in terms of their quality, and we did not examine health outcomes in the present investigation.
“Therefore, more research needs to be done to determine how the present findings might be translated into treatment options or public policy.”
A path to better health
In an accompanying editorial, Veronika Engert, PhD, Joshua A. Grant, PhD, and Bernhard Strauss, PhD, noted that although infectious disease was once the primary cause of death in society, it has been supplanted by other complex and chronic illnesses, which often do not follow simple cause-and-effect associations.
“Rather,” they wrote, “these illnesses develop from a complex milieu of biological, psychological, and social factors that may also influence the disease progress and its prognosis. Against this backdrop, the meta-analysis by Shields and colleagues is an important confirmation of the biopsychosocial model.”
The editorialists explained that recent psychophysiological, neurobiological, and epigenetic research offers a glimpse into the relationship between psychological and social factors in pathogenesis. Nevertheless, the authors noted that a comprehensive examination of the potential effects of psychosocial interventions on immune parameters in various physical health conditions has been lacking.
“The evidence provided by Shields et al. is exactly what is needed to more fully shift treatment from an illness-centered to a patient-centered approach,” they wrote. “To that end, this meta-analysis may serve as a guide for policy makers aiming to improve immune-associated health.”
The research was supported by a Society in Science–Branco Weiss Fellowship, Brain and Behavior Research, and the National Institutes of Health. Dr. Slavich, Dr. Engert, Dr. Grant, and Dr. Strauss have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Patients find CAC more persuasive than ASCVD risk score for statin decisions
Patients who received a protocol-driven recommendation to initiate statin therapy for primary prevention of cardiovascular disease based upon their CT angiography coronary artery calcium score were twice as likely to actually start on the drug than those whose recommendation was guided by the American College of Cardiology/American Heart Association Pooled Cohort Equations Risk Calculator, according to the results of the randomized CorCal Vanguard study.
These results suggest that patients – and their primary care physicians – find the conventional method of screening for cardiovascular risk using the Pooled Cohort Equations to estimate the 10-year risk of MI or stroke, as recommended in ACC/AHA guidelines, to be less persuasive than screening for the presence or absence of actual disease as captured by CT angiography images and the associated coronary artery calcium (CAC) score, Joseph B. Muhlestein, MD, said at the joint scientific sessions of the ACC and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
The CorCal Vanguard study included 601 patients with an average baseline LDL cholesterol of 120 mg/dL, an average age of 60 years, and no history of cardiovascular disease, diabetes, or prior statin therapy. They were randomized to decision-making regarding statin therapy based on either the ACC/AHA guideline–endorsed Pooled Cohort Equations, which use an estimated 10-year risk of 7.5% or more as the threshold for statin initiation, or their CAC score.
If a patient’s CAC score was 0, the recommendation was against starting a statin. Everyone with a CAC greater than 100 received a recommendation for high-intensity statin therapy. And for those with a CAC of 1-100, the decision defaulted to the results of the Pooled Cohort Equations. The screening results were provided to a patient’s primary physician so they could engage in joint decision-making regarding initiation of statin therapy. Adherence to a screening-based recommendation to start on a statin was assessed at 3 and 12 months of follow-up, explained Dr. Muhlestein, a cardiologist at the Intermountain Medical Center Heart Institute in Salt Lake City.
He noted that CorCal Vanguard was merely a feasibility study. Based on the study results he presented at ACC 2020, the full 9,000-patient CorCal primary prevention trial is now enrolling participants. CorCal is the first randomized trial to pit the Pooled Cohort Equations against the CAC score in a large study looking for differences in downstream clinical outcomes.
The rationale for this line of clinical research lies in the known limitations of the ACC/AHA risk calculator. “It may overestimate risk in some populations, patients aren’t always adherent to Pooled Cohort Equations Risk Calculator recommendations, and it doesn’t include novel risk markers such as C-reactive protein that some consider important for risk assessment. And the big question: Should we continue risk screening to determine potential benefit from drug therapy, or should we switch to disease screening?” the cardiologist commented.
The CorCal Vanguard results
A recommendation to start statin therapy was made in 48% of patients in the Pooled Cohort Equations group, versus 36% of the group randomized to CAC. However, only 17% of patients in the Pooled Cohort Equations group actually initiated a statin, a significantly lower rate than the 26% figure in the CAC arm. Fully 70% of patients who received a recommendation to start taking a statin on the basis of their CAC score actually did so, compared to just 36% of those whose recommendation was based upon their Pooled Cohort Equations Risk Calculator.
At 3 months of follow-up, 61% of patients who received an initial recommendation to start statin therapy based upon their CAC screening were actually taking a statin, compared with 41% of those whose recommendation was based upon the Pooled Cohort Equations. At 12 months, the figures were 64% and 49%.
In both groups, at 12 months of follow-up, the No. 1 reason patients weren’t taking a statin as recommended was that their personal physician had advised against it or never prescribed it. That accounted for roughly half of the nonadherence. Another quarter was because of a preference to try lifestyle change first. Fear of drug side effects was a less common reason.
Putting the CorCal Vanguard study results in perspective, Dr. Muhlestein observed that, prior to the screening study, none of the participants had ever been on a statin, yet 37% of them were found by one screening method or the other to be at high cardiovascular risk. Of those high-risk patients, 51% actually initiated statin therapy and the majority of them were still taking their medication 12 months later.
“That has to be a good thing. It emphasizes what can be done when proactive primary prevention is practiced,” the cardiologist said.
He reported having no financial conflicts regarding the CorCal study, which was funded by a grant from the Dell Loy Hansen Cardiovascular Research Fund.
SOURCE: Muhlestein JB et al. ACC 2020, Abstract 909-12.
Patients who received a protocol-driven recommendation to initiate statin therapy for primary prevention of cardiovascular disease based upon their CT angiography coronary artery calcium score were twice as likely to actually start on the drug than those whose recommendation was guided by the American College of Cardiology/American Heart Association Pooled Cohort Equations Risk Calculator, according to the results of the randomized CorCal Vanguard study.
These results suggest that patients – and their primary care physicians – find the conventional method of screening for cardiovascular risk using the Pooled Cohort Equations to estimate the 10-year risk of MI or stroke, as recommended in ACC/AHA guidelines, to be less persuasive than screening for the presence or absence of actual disease as captured by CT angiography images and the associated coronary artery calcium (CAC) score, Joseph B. Muhlestein, MD, said at the joint scientific sessions of the ACC and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
The CorCal Vanguard study included 601 patients with an average baseline LDL cholesterol of 120 mg/dL, an average age of 60 years, and no history of cardiovascular disease, diabetes, or prior statin therapy. They were randomized to decision-making regarding statin therapy based on either the ACC/AHA guideline–endorsed Pooled Cohort Equations, which use an estimated 10-year risk of 7.5% or more as the threshold for statin initiation, or their CAC score.
If a patient’s CAC score was 0, the recommendation was against starting a statin. Everyone with a CAC greater than 100 received a recommendation for high-intensity statin therapy. And for those with a CAC of 1-100, the decision defaulted to the results of the Pooled Cohort Equations. The screening results were provided to a patient’s primary physician so they could engage in joint decision-making regarding initiation of statin therapy. Adherence to a screening-based recommendation to start on a statin was assessed at 3 and 12 months of follow-up, explained Dr. Muhlestein, a cardiologist at the Intermountain Medical Center Heart Institute in Salt Lake City.
He noted that CorCal Vanguard was merely a feasibility study. Based on the study results he presented at ACC 2020, the full 9,000-patient CorCal primary prevention trial is now enrolling participants. CorCal is the first randomized trial to pit the Pooled Cohort Equations against the CAC score in a large study looking for differences in downstream clinical outcomes.
The rationale for this line of clinical research lies in the known limitations of the ACC/AHA risk calculator. “It may overestimate risk in some populations, patients aren’t always adherent to Pooled Cohort Equations Risk Calculator recommendations, and it doesn’t include novel risk markers such as C-reactive protein that some consider important for risk assessment. And the big question: Should we continue risk screening to determine potential benefit from drug therapy, or should we switch to disease screening?” the cardiologist commented.
The CorCal Vanguard results
A recommendation to start statin therapy was made in 48% of patients in the Pooled Cohort Equations group, versus 36% of the group randomized to CAC. However, only 17% of patients in the Pooled Cohort Equations group actually initiated a statin, a significantly lower rate than the 26% figure in the CAC arm. Fully 70% of patients who received a recommendation to start taking a statin on the basis of their CAC score actually did so, compared to just 36% of those whose recommendation was based upon their Pooled Cohort Equations Risk Calculator.
At 3 months of follow-up, 61% of patients who received an initial recommendation to start statin therapy based upon their CAC screening were actually taking a statin, compared with 41% of those whose recommendation was based upon the Pooled Cohort Equations. At 12 months, the figures were 64% and 49%.
In both groups, at 12 months of follow-up, the No. 1 reason patients weren’t taking a statin as recommended was that their personal physician had advised against it or never prescribed it. That accounted for roughly half of the nonadherence. Another quarter was because of a preference to try lifestyle change first. Fear of drug side effects was a less common reason.
Putting the CorCal Vanguard study results in perspective, Dr. Muhlestein observed that, prior to the screening study, none of the participants had ever been on a statin, yet 37% of them were found by one screening method or the other to be at high cardiovascular risk. Of those high-risk patients, 51% actually initiated statin therapy and the majority of them were still taking their medication 12 months later.
“That has to be a good thing. It emphasizes what can be done when proactive primary prevention is practiced,” the cardiologist said.
He reported having no financial conflicts regarding the CorCal study, which was funded by a grant from the Dell Loy Hansen Cardiovascular Research Fund.
SOURCE: Muhlestein JB et al. ACC 2020, Abstract 909-12.
Patients who received a protocol-driven recommendation to initiate statin therapy for primary prevention of cardiovascular disease based upon their CT angiography coronary artery calcium score were twice as likely to actually start on the drug than those whose recommendation was guided by the American College of Cardiology/American Heart Association Pooled Cohort Equations Risk Calculator, according to the results of the randomized CorCal Vanguard study.
These results suggest that patients – and their primary care physicians – find the conventional method of screening for cardiovascular risk using the Pooled Cohort Equations to estimate the 10-year risk of MI or stroke, as recommended in ACC/AHA guidelines, to be less persuasive than screening for the presence or absence of actual disease as captured by CT angiography images and the associated coronary artery calcium (CAC) score, Joseph B. Muhlestein, MD, said at the joint scientific sessions of the ACC and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
The CorCal Vanguard study included 601 patients with an average baseline LDL cholesterol of 120 mg/dL, an average age of 60 years, and no history of cardiovascular disease, diabetes, or prior statin therapy. They were randomized to decision-making regarding statin therapy based on either the ACC/AHA guideline–endorsed Pooled Cohort Equations, which use an estimated 10-year risk of 7.5% or more as the threshold for statin initiation, or their CAC score.
If a patient’s CAC score was 0, the recommendation was against starting a statin. Everyone with a CAC greater than 100 received a recommendation for high-intensity statin therapy. And for those with a CAC of 1-100, the decision defaulted to the results of the Pooled Cohort Equations. The screening results were provided to a patient’s primary physician so they could engage in joint decision-making regarding initiation of statin therapy. Adherence to a screening-based recommendation to start on a statin was assessed at 3 and 12 months of follow-up, explained Dr. Muhlestein, a cardiologist at the Intermountain Medical Center Heart Institute in Salt Lake City.
He noted that CorCal Vanguard was merely a feasibility study. Based on the study results he presented at ACC 2020, the full 9,000-patient CorCal primary prevention trial is now enrolling participants. CorCal is the first randomized trial to pit the Pooled Cohort Equations against the CAC score in a large study looking for differences in downstream clinical outcomes.
The rationale for this line of clinical research lies in the known limitations of the ACC/AHA risk calculator. “It may overestimate risk in some populations, patients aren’t always adherent to Pooled Cohort Equations Risk Calculator recommendations, and it doesn’t include novel risk markers such as C-reactive protein that some consider important for risk assessment. And the big question: Should we continue risk screening to determine potential benefit from drug therapy, or should we switch to disease screening?” the cardiologist commented.
The CorCal Vanguard results
A recommendation to start statin therapy was made in 48% of patients in the Pooled Cohort Equations group, versus 36% of the group randomized to CAC. However, only 17% of patients in the Pooled Cohort Equations group actually initiated a statin, a significantly lower rate than the 26% figure in the CAC arm. Fully 70% of patients who received a recommendation to start taking a statin on the basis of their CAC score actually did so, compared to just 36% of those whose recommendation was based upon their Pooled Cohort Equations Risk Calculator.
At 3 months of follow-up, 61% of patients who received an initial recommendation to start statin therapy based upon their CAC screening were actually taking a statin, compared with 41% of those whose recommendation was based upon the Pooled Cohort Equations. At 12 months, the figures were 64% and 49%.
In both groups, at 12 months of follow-up, the No. 1 reason patients weren’t taking a statin as recommended was that their personal physician had advised against it or never prescribed it. That accounted for roughly half of the nonadherence. Another quarter was because of a preference to try lifestyle change first. Fear of drug side effects was a less common reason.
Putting the CorCal Vanguard study results in perspective, Dr. Muhlestein observed that, prior to the screening study, none of the participants had ever been on a statin, yet 37% of them were found by one screening method or the other to be at high cardiovascular risk. Of those high-risk patients, 51% actually initiated statin therapy and the majority of them were still taking their medication 12 months later.
“That has to be a good thing. It emphasizes what can be done when proactive primary prevention is practiced,” the cardiologist said.
He reported having no financial conflicts regarding the CorCal study, which was funded by a grant from the Dell Loy Hansen Cardiovascular Research Fund.
SOURCE: Muhlestein JB et al. ACC 2020, Abstract 909-12.
FROM ACC 2020
More from REDUCE-IT: Icosapent ethyl cuts revascularization by a third
A new analysis from the REDUCE-IT trial has shown that the high-strength eicosapentaenoic acid product icosapent ethyl (Vascepa; Amarin) reduced the number of revascularization procedures by more than one-third in statin-treated patients whose triglyceride levels were elevated and who were at increased cardiovascular risk.
The new data were presented at the Society for Cardiovascular Angiography & Interventions virtual annual scientific sessions.
REDUCE-IT, a multicenter, double-blind, placebo-controlled trial, randomly assigned statin-treated patients whose triglyceride levels were elevated (135-499 mg/dL), whose LDL cholesterol levels were controlled (41-100 mg/dL), and who had established cardiovascular disease or diabetes plus risk factors to receive either icosapent ethyl 4 g daily or placebo.
The primary composite and other cardiovascular endpoints were substantially reduced. Prespecified analyses examined all coronary revascularizations, recurrent revascularizations, and revascularization subtypes.
“Compared with placebo, icosapent ethyl 4 g/day significantly reduced first and total revascularization events by 34% and 36%, respectively,” REDUCE-IT investigator Benjamin Peterson, MD, of Brigham and Women’s Hospital, Boston, concluded during his presentation.
This reduction was consistent with respect to urgent, emergent, and elective revascularization procedures overall, as well as percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) individually, he reported.
“Prior therapies aimed at patients with elevated triglycerides have not demonstrated a consistent benefit in reducing coronary revascularization, and to the best of our knowledge, this is the first non–LDL cholesterol intervention in a major randomized trial in which statin-treated patients underwent fewer CABG surgeries,” Dr. Peterson stated.
“These data highlight the substantial impact of icosapent ethyl on the underlying atherothrombotic burden in the at-risk REDUCE-IT population,” he added.
Detailed results showed that the percentage of patients who underwent first revascularizations was 9.2% with icosapent ethyl versus 13.3% with placebo (hazard ratio, 0.66; P < .0001; number needed to treat, 25).
Similar reductions were observed in total (first and subsequent) revascularizations (risk ratio, 0.64; P < .0001) and across urgent, emergent, and elective revascularizations. Icosapent ethyl significantly reduced the need for PCI (HR, 0.68; P < .0001) and CABG (HR, 0.61; P = .0005).
The moderator of a SCAI press conference, Kirk Garratt, MD, of the Center for Heart and Vascular Health at Christiana Care Health System in Wilmington, Del., said that “this is an impressive impact. I couldn’t count the number of zeros in the P value.”
Timothy Henry, MD, of Christ Hospital in Cincinnati, said that REDUCE-IT was an important trial. “It showed a very impressive effect on revascularizations along with all the other benefits.” But he suggested that the uptake in usage of icosapent ethyl in the United States has been slow, and he asked what could be done to enhance this.
REDUCE-IT senior investigator Deepak Bhatt, MD, replied that the product was only approved for the REDUCE-IT indication in December 2019, and he suggested that initial uptake may have been affected by the current COVID-19 pandemic.
“This new REDUCE-IT indication ― patients with established cardiovascular disease or diabetes plus risk factors who have moderately elevated triglycerides and controlled LDL ― includes a lot of patients, between 15% and 50% of all cardiovascular patients,” he said.
“We wanted to present this revascularization data at the SCAI meeting, as it is superimportant that interventional cardiologists know about this. Interventionalists have now taken ownership of LDL and make sure patients are on statins, and we hope they will now do the same thing for triglycerides,” Dr. Bhatt commented.
Dr. Henry agreed. “It should be a simple thing to take all secondary prevention patients with eligible triglycerides and be aggressive with this new therapy.”
Dr. Bhatt noted that a cost-effectiveness analysis of the REDUCE-IT trial that was presented at last year’s American Heart Association meeting “has shown the drug to be highly cost effective and actually cost saving at the current list price.”
Asked what the mechanism of benefit is, Dr. Bhatt said that “there has been good basic science showing that EPA [eicosapentaenoic acid] stabilizes cell membranes and reduces plaque vulnerability and progression.”
REDUCE-IT was sponsored by Amarin. Brigham and Women’s Hospital receives research funding from Amarin for Dr. Bhatt’s role as chair of the trial.
A version of this article originally appeared on Medscape.com.
A new analysis from the REDUCE-IT trial has shown that the high-strength eicosapentaenoic acid product icosapent ethyl (Vascepa; Amarin) reduced the number of revascularization procedures by more than one-third in statin-treated patients whose triglyceride levels were elevated and who were at increased cardiovascular risk.
The new data were presented at the Society for Cardiovascular Angiography & Interventions virtual annual scientific sessions.
REDUCE-IT, a multicenter, double-blind, placebo-controlled trial, randomly assigned statin-treated patients whose triglyceride levels were elevated (135-499 mg/dL), whose LDL cholesterol levels were controlled (41-100 mg/dL), and who had established cardiovascular disease or diabetes plus risk factors to receive either icosapent ethyl 4 g daily or placebo.
The primary composite and other cardiovascular endpoints were substantially reduced. Prespecified analyses examined all coronary revascularizations, recurrent revascularizations, and revascularization subtypes.
“Compared with placebo, icosapent ethyl 4 g/day significantly reduced first and total revascularization events by 34% and 36%, respectively,” REDUCE-IT investigator Benjamin Peterson, MD, of Brigham and Women’s Hospital, Boston, concluded during his presentation.
This reduction was consistent with respect to urgent, emergent, and elective revascularization procedures overall, as well as percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) individually, he reported.
“Prior therapies aimed at patients with elevated triglycerides have not demonstrated a consistent benefit in reducing coronary revascularization, and to the best of our knowledge, this is the first non–LDL cholesterol intervention in a major randomized trial in which statin-treated patients underwent fewer CABG surgeries,” Dr. Peterson stated.
“These data highlight the substantial impact of icosapent ethyl on the underlying atherothrombotic burden in the at-risk REDUCE-IT population,” he added.
Detailed results showed that the percentage of patients who underwent first revascularizations was 9.2% with icosapent ethyl versus 13.3% with placebo (hazard ratio, 0.66; P < .0001; number needed to treat, 25).
Similar reductions were observed in total (first and subsequent) revascularizations (risk ratio, 0.64; P < .0001) and across urgent, emergent, and elective revascularizations. Icosapent ethyl significantly reduced the need for PCI (HR, 0.68; P < .0001) and CABG (HR, 0.61; P = .0005).
The moderator of a SCAI press conference, Kirk Garratt, MD, of the Center for Heart and Vascular Health at Christiana Care Health System in Wilmington, Del., said that “this is an impressive impact. I couldn’t count the number of zeros in the P value.”
Timothy Henry, MD, of Christ Hospital in Cincinnati, said that REDUCE-IT was an important trial. “It showed a very impressive effect on revascularizations along with all the other benefits.” But he suggested that the uptake in usage of icosapent ethyl in the United States has been slow, and he asked what could be done to enhance this.
REDUCE-IT senior investigator Deepak Bhatt, MD, replied that the product was only approved for the REDUCE-IT indication in December 2019, and he suggested that initial uptake may have been affected by the current COVID-19 pandemic.
“This new REDUCE-IT indication ― patients with established cardiovascular disease or diabetes plus risk factors who have moderately elevated triglycerides and controlled LDL ― includes a lot of patients, between 15% and 50% of all cardiovascular patients,” he said.
“We wanted to present this revascularization data at the SCAI meeting, as it is superimportant that interventional cardiologists know about this. Interventionalists have now taken ownership of LDL and make sure patients are on statins, and we hope they will now do the same thing for triglycerides,” Dr. Bhatt commented.
Dr. Henry agreed. “It should be a simple thing to take all secondary prevention patients with eligible triglycerides and be aggressive with this new therapy.”
Dr. Bhatt noted that a cost-effectiveness analysis of the REDUCE-IT trial that was presented at last year’s American Heart Association meeting “has shown the drug to be highly cost effective and actually cost saving at the current list price.”
Asked what the mechanism of benefit is, Dr. Bhatt said that “there has been good basic science showing that EPA [eicosapentaenoic acid] stabilizes cell membranes and reduces plaque vulnerability and progression.”
REDUCE-IT was sponsored by Amarin. Brigham and Women’s Hospital receives research funding from Amarin for Dr. Bhatt’s role as chair of the trial.
A version of this article originally appeared on Medscape.com.
A new analysis from the REDUCE-IT trial has shown that the high-strength eicosapentaenoic acid product icosapent ethyl (Vascepa; Amarin) reduced the number of revascularization procedures by more than one-third in statin-treated patients whose triglyceride levels were elevated and who were at increased cardiovascular risk.
The new data were presented at the Society for Cardiovascular Angiography & Interventions virtual annual scientific sessions.
REDUCE-IT, a multicenter, double-blind, placebo-controlled trial, randomly assigned statin-treated patients whose triglyceride levels were elevated (135-499 mg/dL), whose LDL cholesterol levels were controlled (41-100 mg/dL), and who had established cardiovascular disease or diabetes plus risk factors to receive either icosapent ethyl 4 g daily or placebo.
The primary composite and other cardiovascular endpoints were substantially reduced. Prespecified analyses examined all coronary revascularizations, recurrent revascularizations, and revascularization subtypes.
“Compared with placebo, icosapent ethyl 4 g/day significantly reduced first and total revascularization events by 34% and 36%, respectively,” REDUCE-IT investigator Benjamin Peterson, MD, of Brigham and Women’s Hospital, Boston, concluded during his presentation.
This reduction was consistent with respect to urgent, emergent, and elective revascularization procedures overall, as well as percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) individually, he reported.
“Prior therapies aimed at patients with elevated triglycerides have not demonstrated a consistent benefit in reducing coronary revascularization, and to the best of our knowledge, this is the first non–LDL cholesterol intervention in a major randomized trial in which statin-treated patients underwent fewer CABG surgeries,” Dr. Peterson stated.
“These data highlight the substantial impact of icosapent ethyl on the underlying atherothrombotic burden in the at-risk REDUCE-IT population,” he added.
Detailed results showed that the percentage of patients who underwent first revascularizations was 9.2% with icosapent ethyl versus 13.3% with placebo (hazard ratio, 0.66; P < .0001; number needed to treat, 25).
Similar reductions were observed in total (first and subsequent) revascularizations (risk ratio, 0.64; P < .0001) and across urgent, emergent, and elective revascularizations. Icosapent ethyl significantly reduced the need for PCI (HR, 0.68; P < .0001) and CABG (HR, 0.61; P = .0005).
The moderator of a SCAI press conference, Kirk Garratt, MD, of the Center for Heart and Vascular Health at Christiana Care Health System in Wilmington, Del., said that “this is an impressive impact. I couldn’t count the number of zeros in the P value.”
Timothy Henry, MD, of Christ Hospital in Cincinnati, said that REDUCE-IT was an important trial. “It showed a very impressive effect on revascularizations along with all the other benefits.” But he suggested that the uptake in usage of icosapent ethyl in the United States has been slow, and he asked what could be done to enhance this.
REDUCE-IT senior investigator Deepak Bhatt, MD, replied that the product was only approved for the REDUCE-IT indication in December 2019, and he suggested that initial uptake may have been affected by the current COVID-19 pandemic.
“This new REDUCE-IT indication ― patients with established cardiovascular disease or diabetes plus risk factors who have moderately elevated triglycerides and controlled LDL ― includes a lot of patients, between 15% and 50% of all cardiovascular patients,” he said.
“We wanted to present this revascularization data at the SCAI meeting, as it is superimportant that interventional cardiologists know about this. Interventionalists have now taken ownership of LDL and make sure patients are on statins, and we hope they will now do the same thing for triglycerides,” Dr. Bhatt commented.
Dr. Henry agreed. “It should be a simple thing to take all secondary prevention patients with eligible triglycerides and be aggressive with this new therapy.”
Dr. Bhatt noted that a cost-effectiveness analysis of the REDUCE-IT trial that was presented at last year’s American Heart Association meeting “has shown the drug to be highly cost effective and actually cost saving at the current list price.”
Asked what the mechanism of benefit is, Dr. Bhatt said that “there has been good basic science showing that EPA [eicosapentaenoic acid] stabilizes cell membranes and reduces plaque vulnerability and progression.”
REDUCE-IT was sponsored by Amarin. Brigham and Women’s Hospital receives research funding from Amarin for Dr. Bhatt’s role as chair of the trial.
A version of this article originally appeared on Medscape.com.
Health care costs nearly doubled for patients with NAFLD
The health care costs of patients with nonalcoholic fatty liver disease (NAFLD) were nearly twice that of matched population controls, according to the results of a longitudinal cohort study.
Patients with biopsy-confirmed nonalcoholic steatohepatitis (NASH) were hospitalized an average of 0.27 times per year versus 0.16 times for controls (P < .001), for an annual incremental cost of $635, reported Hannes Hagström, MD, PhD, of Karolinska University Hospital in Stockholm. Patients with NAFLD also made significantly more outpatient care visits than controls (P < .001), he said. “Patients with advanced fibrosis [had] the highest costs, suggesting that reducing fibrosis progression is important to reduce future health care costs” among patients with NASH, Dr. Hagström and his associates wrote in Clinical Gastroenterology and Hepatology.
The retrospective longitudinal cohort study included all 646 patients diagnosed with biopsy-confirmed NAFLD at two hospitals in Sweden between 1971 and 2019. Patients with other liver diseases were excluded, as were heavy drinkers: men who drank more than 30 g of alcohol (just under four units) daily and women who drank more than 20 g daily. Each patient with NAFLD was matched with 10 population controls matched by age, sex, and county of residence.
Over a mean of 19.9 years of follow-up (range, 0-40 years), patients with NASH were hospitalized a total of 3,478 times, an average of 5.4 hospitalizations per patient. Controls were hospitalized an average of 3.2 times during the same time period (P < .001 vs. NASH patients). “This corresponded to a higher incremental cost in NAFLD patients of $635 per year (95% confidence interval, $407-$864; P < .001),” the researchers reported.
Between 2001 and 2009, patients with NAFLD averaged 5.4 more outpatient visits than controls (P < .001), with annual averages of 1.46 versus 0.86 visits (P < .001). Consequently, patient with NASH incurred $255 more per year in annual outpatient care costs. Liver disease accounted for 6% of outpatient care costs among NASH patients versus 0.2% of costs among controls.
“Cumulative costs in the [fibrosis stage 3 and 4] subgroup were relatively matched with the control population until around year 4 after biopsy, when costs diverged,” the researchers said. “This could possibly be an effect of the larger F3 population developing cirrhosis and increasing costs due to decompensation events.”
They noted that the rising prevalence of NAFLD will further burden health care budgets. “Costs [among patients with NASH] were higher in conjunction with liver biopsy, which is why using noninvasive diagnostic methods (e.g., transient elastography) is likely to reduce total costs,” they added. Of note, although patients with NAFLD also incurred somewhat more per year in prescription costs, the difference was not statistically significant.
The study was supported by Stockholm City Council, the Bengt Ihre Foundation, the County Council of Östergötland, and Gilead. The researchers reported having no conflicts of interest.
SOURCE: Kim H et al. Clin Gastroenterol Hepatol. 2019 Sep 12. doi: 10.1016/j.cgh.2019.10.023.
The possibility of FDA approval of NASH-modifying drugs later this year brings the hope of improving outcomes for patients with NAFLD. Inevitably, the cost effectiveness of those drugs also will be scrutinized as we evaluate their impact in the coming years. To that end, Hagstrom et al. provide useful insight regarding the real-world costs of medical care among patients with histologically staged NAFLD in Sweden.
Their main finding is that medical costs for a patient with NAFLD over 20 years is double that for a random control patient from the general population.
It is worth taking a deeper dive into the factors that drove the cost differential. First, higher inpatient and outpatient specialty care costs accounted for the incremental cost of NAFLD care; drug costs were materially similar in the two groups, albeit examined over a very short time period in the study due to limited national registry data. Second, the cost differential was largest in the first year of diagnosis and attributed to the cost of liver biopsy and related expenses. Last, as one would expect, the cost differential was largest between patients who had stage 3-4 fibrosis, possibly explained by the costs of NASH-related complications.
While we hope that NASH-modifying drugs will reduce the risk of liver-specific complications, the cumulative financial impact of such therapies remains to be seen. On the one hand, short-term costs may increase because of the direct expense of the NASH-modifying drugs plus additional expenses related to management of side effects. In addition, it is likely patients treated with NASH-modifying drugs will need more frequent assessments of liver disease severity to evaluate whether the medication is working, which even if done noninvasively, is likely the add to medical costs. In the long term however, NASH-modifying treatments may reduce the risk of NAFLD complications over time, mitigating the cumulative cost of NAFLD care. The true net effect remains to be seen. In the meantime, we need further studies that quantify costs of NAFLD care - ideally by disease severity and that provide greater insight into the cost of caring for the complications of NASH progression, including liver disease clinical decompensations and transplant.
Maya Balakrishnan, MD, MPH, is an assistant professor, department of medicine, section of gastroenterology & hepatology, Baylor College of Medicine, Houston, and director of hepatology at Ben Taub General Hospital, Houston. She has no conflicts of interest.
The possibility of FDA approval of NASH-modifying drugs later this year brings the hope of improving outcomes for patients with NAFLD. Inevitably, the cost effectiveness of those drugs also will be scrutinized as we evaluate their impact in the coming years. To that end, Hagstrom et al. provide useful insight regarding the real-world costs of medical care among patients with histologically staged NAFLD in Sweden.
Their main finding is that medical costs for a patient with NAFLD over 20 years is double that for a random control patient from the general population.
It is worth taking a deeper dive into the factors that drove the cost differential. First, higher inpatient and outpatient specialty care costs accounted for the incremental cost of NAFLD care; drug costs were materially similar in the two groups, albeit examined over a very short time period in the study due to limited national registry data. Second, the cost differential was largest in the first year of diagnosis and attributed to the cost of liver biopsy and related expenses. Last, as one would expect, the cost differential was largest between patients who had stage 3-4 fibrosis, possibly explained by the costs of NASH-related complications.
While we hope that NASH-modifying drugs will reduce the risk of liver-specific complications, the cumulative financial impact of such therapies remains to be seen. On the one hand, short-term costs may increase because of the direct expense of the NASH-modifying drugs plus additional expenses related to management of side effects. In addition, it is likely patients treated with NASH-modifying drugs will need more frequent assessments of liver disease severity to evaluate whether the medication is working, which even if done noninvasively, is likely the add to medical costs. In the long term however, NASH-modifying treatments may reduce the risk of NAFLD complications over time, mitigating the cumulative cost of NAFLD care. The true net effect remains to be seen. In the meantime, we need further studies that quantify costs of NAFLD care - ideally by disease severity and that provide greater insight into the cost of caring for the complications of NASH progression, including liver disease clinical decompensations and transplant.
Maya Balakrishnan, MD, MPH, is an assistant professor, department of medicine, section of gastroenterology & hepatology, Baylor College of Medicine, Houston, and director of hepatology at Ben Taub General Hospital, Houston. She has no conflicts of interest.
The possibility of FDA approval of NASH-modifying drugs later this year brings the hope of improving outcomes for patients with NAFLD. Inevitably, the cost effectiveness of those drugs also will be scrutinized as we evaluate their impact in the coming years. To that end, Hagstrom et al. provide useful insight regarding the real-world costs of medical care among patients with histologically staged NAFLD in Sweden.
Their main finding is that medical costs for a patient with NAFLD over 20 years is double that for a random control patient from the general population.
It is worth taking a deeper dive into the factors that drove the cost differential. First, higher inpatient and outpatient specialty care costs accounted for the incremental cost of NAFLD care; drug costs were materially similar in the two groups, albeit examined over a very short time period in the study due to limited national registry data. Second, the cost differential was largest in the first year of diagnosis and attributed to the cost of liver biopsy and related expenses. Last, as one would expect, the cost differential was largest between patients who had stage 3-4 fibrosis, possibly explained by the costs of NASH-related complications.
While we hope that NASH-modifying drugs will reduce the risk of liver-specific complications, the cumulative financial impact of such therapies remains to be seen. On the one hand, short-term costs may increase because of the direct expense of the NASH-modifying drugs plus additional expenses related to management of side effects. In addition, it is likely patients treated with NASH-modifying drugs will need more frequent assessments of liver disease severity to evaluate whether the medication is working, which even if done noninvasively, is likely the add to medical costs. In the long term however, NASH-modifying treatments may reduce the risk of NAFLD complications over time, mitigating the cumulative cost of NAFLD care. The true net effect remains to be seen. In the meantime, we need further studies that quantify costs of NAFLD care - ideally by disease severity and that provide greater insight into the cost of caring for the complications of NASH progression, including liver disease clinical decompensations and transplant.
Maya Balakrishnan, MD, MPH, is an assistant professor, department of medicine, section of gastroenterology & hepatology, Baylor College of Medicine, Houston, and director of hepatology at Ben Taub General Hospital, Houston. She has no conflicts of interest.
The health care costs of patients with nonalcoholic fatty liver disease (NAFLD) were nearly twice that of matched population controls, according to the results of a longitudinal cohort study.
Patients with biopsy-confirmed nonalcoholic steatohepatitis (NASH) were hospitalized an average of 0.27 times per year versus 0.16 times for controls (P < .001), for an annual incremental cost of $635, reported Hannes Hagström, MD, PhD, of Karolinska University Hospital in Stockholm. Patients with NAFLD also made significantly more outpatient care visits than controls (P < .001), he said. “Patients with advanced fibrosis [had] the highest costs, suggesting that reducing fibrosis progression is important to reduce future health care costs” among patients with NASH, Dr. Hagström and his associates wrote in Clinical Gastroenterology and Hepatology.
The retrospective longitudinal cohort study included all 646 patients diagnosed with biopsy-confirmed NAFLD at two hospitals in Sweden between 1971 and 2019. Patients with other liver diseases were excluded, as were heavy drinkers: men who drank more than 30 g of alcohol (just under four units) daily and women who drank more than 20 g daily. Each patient with NAFLD was matched with 10 population controls matched by age, sex, and county of residence.
Over a mean of 19.9 years of follow-up (range, 0-40 years), patients with NASH were hospitalized a total of 3,478 times, an average of 5.4 hospitalizations per patient. Controls were hospitalized an average of 3.2 times during the same time period (P < .001 vs. NASH patients). “This corresponded to a higher incremental cost in NAFLD patients of $635 per year (95% confidence interval, $407-$864; P < .001),” the researchers reported.
Between 2001 and 2009, patients with NAFLD averaged 5.4 more outpatient visits than controls (P < .001), with annual averages of 1.46 versus 0.86 visits (P < .001). Consequently, patient with NASH incurred $255 more per year in annual outpatient care costs. Liver disease accounted for 6% of outpatient care costs among NASH patients versus 0.2% of costs among controls.
“Cumulative costs in the [fibrosis stage 3 and 4] subgroup were relatively matched with the control population until around year 4 after biopsy, when costs diverged,” the researchers said. “This could possibly be an effect of the larger F3 population developing cirrhosis and increasing costs due to decompensation events.”
They noted that the rising prevalence of NAFLD will further burden health care budgets. “Costs [among patients with NASH] were higher in conjunction with liver biopsy, which is why using noninvasive diagnostic methods (e.g., transient elastography) is likely to reduce total costs,” they added. Of note, although patients with NAFLD also incurred somewhat more per year in prescription costs, the difference was not statistically significant.
The study was supported by Stockholm City Council, the Bengt Ihre Foundation, the County Council of Östergötland, and Gilead. The researchers reported having no conflicts of interest.
SOURCE: Kim H et al. Clin Gastroenterol Hepatol. 2019 Sep 12. doi: 10.1016/j.cgh.2019.10.023.
The health care costs of patients with nonalcoholic fatty liver disease (NAFLD) were nearly twice that of matched population controls, according to the results of a longitudinal cohort study.
Patients with biopsy-confirmed nonalcoholic steatohepatitis (NASH) were hospitalized an average of 0.27 times per year versus 0.16 times for controls (P < .001), for an annual incremental cost of $635, reported Hannes Hagström, MD, PhD, of Karolinska University Hospital in Stockholm. Patients with NAFLD also made significantly more outpatient care visits than controls (P < .001), he said. “Patients with advanced fibrosis [had] the highest costs, suggesting that reducing fibrosis progression is important to reduce future health care costs” among patients with NASH, Dr. Hagström and his associates wrote in Clinical Gastroenterology and Hepatology.
The retrospective longitudinal cohort study included all 646 patients diagnosed with biopsy-confirmed NAFLD at two hospitals in Sweden between 1971 and 2019. Patients with other liver diseases were excluded, as were heavy drinkers: men who drank more than 30 g of alcohol (just under four units) daily and women who drank more than 20 g daily. Each patient with NAFLD was matched with 10 population controls matched by age, sex, and county of residence.
Over a mean of 19.9 years of follow-up (range, 0-40 years), patients with NASH were hospitalized a total of 3,478 times, an average of 5.4 hospitalizations per patient. Controls were hospitalized an average of 3.2 times during the same time period (P < .001 vs. NASH patients). “This corresponded to a higher incremental cost in NAFLD patients of $635 per year (95% confidence interval, $407-$864; P < .001),” the researchers reported.
Between 2001 and 2009, patients with NAFLD averaged 5.4 more outpatient visits than controls (P < .001), with annual averages of 1.46 versus 0.86 visits (P < .001). Consequently, patient with NASH incurred $255 more per year in annual outpatient care costs. Liver disease accounted for 6% of outpatient care costs among NASH patients versus 0.2% of costs among controls.
“Cumulative costs in the [fibrosis stage 3 and 4] subgroup were relatively matched with the control population until around year 4 after biopsy, when costs diverged,” the researchers said. “This could possibly be an effect of the larger F3 population developing cirrhosis and increasing costs due to decompensation events.”
They noted that the rising prevalence of NAFLD will further burden health care budgets. “Costs [among patients with NASH] were higher in conjunction with liver biopsy, which is why using noninvasive diagnostic methods (e.g., transient elastography) is likely to reduce total costs,” they added. Of note, although patients with NAFLD also incurred somewhat more per year in prescription costs, the difference was not statistically significant.
The study was supported by Stockholm City Council, the Bengt Ihre Foundation, the County Council of Östergötland, and Gilead. The researchers reported having no conflicts of interest.
SOURCE: Kim H et al. Clin Gastroenterol Hepatol. 2019 Sep 12. doi: 10.1016/j.cgh.2019.10.023.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Obesity can shift severe COVID-19 to younger age groups
published in The Lancet.
“By itself, obesity seems to be a sufficient risk factor to start seeing younger people landing in the ICU,” said the study’s lead author, David Kass, MD, a professor of cardiology and medicine at Johns Hopkins University School of Medicine in Baltimore, Maryland.
“In that sense, there’s a simple message: If you’re very, very overweight, don’t think that if you’re 35 you’re that much safer [from severe COVID-19] than your mother or grandparents or others in their 60s or 70s,” Kass told Medscape Medical News.
The findings, which Kass describes as a “2-week snapshot” of 265 patients (58% male) in late March and early April at a handful of university hospitals in the United States reinforces other recent research indicating that obesity is one of the biggest risk factors for severe COVID-19 disease, particularly among younger patients. In addition, a large British study showed that, after adjusting for comorbidities, obesity was a significant factor associated with in-hospital death in COVID-19.
But this new analysis stands out as the only dataset to date that specifically “asks the question relative to age” of whether severe COVID-19 disease correlates to ICU treatment, he said.
The mean age of his study population of ICU patients was 55, Kass said, “and that was young, not what we were expecting.”
“Even with the first 20 patients, we were already seeing younger people and they definitely were heavier, with plenty of patients with a BMI over 35 kg/m2,” he added. “The relationship was pretty tight, pretty quick.”
“Just don’t make the assumption that any of us are too young to be vulnerable if, in fact, this is an aspect of our bodies,” he said.
Steven Heymsfield, MD, past president and a spokesperson for the Obesity Society, agrees with Kass’ conclusions.
“One thing we’ve had on our minds is that the prototype of a person with this disease is older...but now if we get [a patient] who’s symptomatic and 40 and obese, we shouldn’t assume they have some other disease,” Heymsfield told Medscape Medical News.
“We should think of them as a susceptible population.”
Kass and colleagues agree. “Public messaging to younger adults, reducing the threshold for virus testing in obese individuals, and maintaining greater vigilance for this at-risk population should reduce the prevalence of severe COVID-19 disease [among those with obesity],” they state.
“I think it’s a mental adjustment from a health care standpoint, which might hopefully help target the folks who are at higher risk before they get into trouble,” Kass told Medscape Medical News.
Trio of mechanisms explain obesity’s extra COVID-19 risks
Kass and coauthors write that, in analyzing their data, they anticipated similar results to the largest study of 1591 ICU patients from Italy in which only 203 were younger than 51 years. Common comorbidities among those patients included hypertension, cardiovascular disease, and type 2 diabetes, with similar data reported from China.
When the COVID-19 epidemic accelerated in the United States, older age was also identified as a risk factor. Obesity had not yet been added to this list, Kass noted. But following informal discussions with colleagues in other ICUs around the country, he decided to investigate further as to whether it was an underappreciated risk factor.
Kass and colleagues did a quick evaluation of the link between BMI and age of patients with COVID-19 admitted to ICUs at Johns Hopkins, University of Cincinnati, New York University, University of Washington, Florida Health, and University of Pennsylvania.
The “significant inverse correlation between age and BMI” showed younger ICU patients were more likely to be obese, with no difference by gender.
Median BMI among study participants was 29.3 kg/m2, with only a quarter having a BMI lower than 26 kg/m2 and another 25% having a BMI higher than 34.7 kg/m2.
Kass acknowledged that it wasn’t possible with this simple dataset to account for any other potential confounders, but he told Medscape Medical News that, “while diabetes, cardiovascular disease, and hypertension, for example, can occur with obesity, this is generally less so in younger populations as it takes time for the other comorbidities to develop.”
He said several mechanisms could explain why obesity predisposes patients with COVID-19 to severe disease.
For one, obesity places extra pressure on the diaphragm while lying on the back, restricting breathing.
“Morbid obesity itself is sort of proinflammatory,” he continued.
“Here we’ve got a viral infection where the early reports suggest that cytokine storms and immune mishandling of the virus are why it’s so much more severe than other forms of coronavirus we’ve seen before. So if you have someone with an already underlying proinflammatory state, this could be a reason there’s higher risk.”
Additionally, the angiotensin-converting enzyme-2 (ACE-2) receptor to which the SARS-CoV-2 virus that causes COVID-19 attaches is expressed in higher amounts in adipose tissue than the lungs, Kass noted.
“This could turn into kind of a viral replication depot,” he explained. “You may well be brewing more virus as a component of obesity.”
Sensitivity needed in public messaging about risks, but test sooner
With an obesity rate of about 40% in the United States, the results are particularly relevant for Americans, Kass and Heymsfield say, noting that the country’s “obesity belt” runs through the South.
Heymsfield, who wasn’t part of the new analysis, notes that public messaging around severe COVID-19 risks to younger adults with obesity is “tricky,” especially because the virus is “still pretty common in nonobese people.”
Kass agrees, noting, “it’s difficult to turn to 40% of the population and say: ‘You guys have to watch it.’ ”
But the mounting research findings necessitate linking obesity with severe COVID-19 disease and perhaps testing patients in this category for the virus sooner before symptoms become severe.
And of note, since shortness of breath is common among people with obesity regardless of illness, similar COVID-19 symptoms might catch these individuals unaware, pointed out Heymsfield, who is also a professor in the Metabolism and Body Composition Lab at Pennington Biomedical Research Center at Louisiana State University, Baton Rouge.
“They may find themselves literally unable to breathe, and the concern would be that they wait much too long to come in” for treatment, he said. Typically, people can deteriorate between day 7 and 10 of the COVID-19 infection.
Individuals with obesity “need to be educated to recognize the serious complications of COVID-19 often appear suddenly, although the virus has sometimes been working its way through the body for a long time,” he concluded.
Kass and Heymsfield have declared no relevant financial relationships.
This article first appeared on Medscape.com.
published in The Lancet.
“By itself, obesity seems to be a sufficient risk factor to start seeing younger people landing in the ICU,” said the study’s lead author, David Kass, MD, a professor of cardiology and medicine at Johns Hopkins University School of Medicine in Baltimore, Maryland.
“In that sense, there’s a simple message: If you’re very, very overweight, don’t think that if you’re 35 you’re that much safer [from severe COVID-19] than your mother or grandparents or others in their 60s or 70s,” Kass told Medscape Medical News.
The findings, which Kass describes as a “2-week snapshot” of 265 patients (58% male) in late March and early April at a handful of university hospitals in the United States reinforces other recent research indicating that obesity is one of the biggest risk factors for severe COVID-19 disease, particularly among younger patients. In addition, a large British study showed that, after adjusting for comorbidities, obesity was a significant factor associated with in-hospital death in COVID-19.
But this new analysis stands out as the only dataset to date that specifically “asks the question relative to age” of whether severe COVID-19 disease correlates to ICU treatment, he said.
The mean age of his study population of ICU patients was 55, Kass said, “and that was young, not what we were expecting.”
“Even with the first 20 patients, we were already seeing younger people and they definitely were heavier, with plenty of patients with a BMI over 35 kg/m2,” he added. “The relationship was pretty tight, pretty quick.”
“Just don’t make the assumption that any of us are too young to be vulnerable if, in fact, this is an aspect of our bodies,” he said.
Steven Heymsfield, MD, past president and a spokesperson for the Obesity Society, agrees with Kass’ conclusions.
“One thing we’ve had on our minds is that the prototype of a person with this disease is older...but now if we get [a patient] who’s symptomatic and 40 and obese, we shouldn’t assume they have some other disease,” Heymsfield told Medscape Medical News.
“We should think of them as a susceptible population.”
Kass and colleagues agree. “Public messaging to younger adults, reducing the threshold for virus testing in obese individuals, and maintaining greater vigilance for this at-risk population should reduce the prevalence of severe COVID-19 disease [among those with obesity],” they state.
“I think it’s a mental adjustment from a health care standpoint, which might hopefully help target the folks who are at higher risk before they get into trouble,” Kass told Medscape Medical News.
Trio of mechanisms explain obesity’s extra COVID-19 risks
Kass and coauthors write that, in analyzing their data, they anticipated similar results to the largest study of 1591 ICU patients from Italy in which only 203 were younger than 51 years. Common comorbidities among those patients included hypertension, cardiovascular disease, and type 2 diabetes, with similar data reported from China.
When the COVID-19 epidemic accelerated in the United States, older age was also identified as a risk factor. Obesity had not yet been added to this list, Kass noted. But following informal discussions with colleagues in other ICUs around the country, he decided to investigate further as to whether it was an underappreciated risk factor.
Kass and colleagues did a quick evaluation of the link between BMI and age of patients with COVID-19 admitted to ICUs at Johns Hopkins, University of Cincinnati, New York University, University of Washington, Florida Health, and University of Pennsylvania.
The “significant inverse correlation between age and BMI” showed younger ICU patients were more likely to be obese, with no difference by gender.
Median BMI among study participants was 29.3 kg/m2, with only a quarter having a BMI lower than 26 kg/m2 and another 25% having a BMI higher than 34.7 kg/m2.
Kass acknowledged that it wasn’t possible with this simple dataset to account for any other potential confounders, but he told Medscape Medical News that, “while diabetes, cardiovascular disease, and hypertension, for example, can occur with obesity, this is generally less so in younger populations as it takes time for the other comorbidities to develop.”
He said several mechanisms could explain why obesity predisposes patients with COVID-19 to severe disease.
For one, obesity places extra pressure on the diaphragm while lying on the back, restricting breathing.
“Morbid obesity itself is sort of proinflammatory,” he continued.
“Here we’ve got a viral infection where the early reports suggest that cytokine storms and immune mishandling of the virus are why it’s so much more severe than other forms of coronavirus we’ve seen before. So if you have someone with an already underlying proinflammatory state, this could be a reason there’s higher risk.”
Additionally, the angiotensin-converting enzyme-2 (ACE-2) receptor to which the SARS-CoV-2 virus that causes COVID-19 attaches is expressed in higher amounts in adipose tissue than the lungs, Kass noted.
“This could turn into kind of a viral replication depot,” he explained. “You may well be brewing more virus as a component of obesity.”
Sensitivity needed in public messaging about risks, but test sooner
With an obesity rate of about 40% in the United States, the results are particularly relevant for Americans, Kass and Heymsfield say, noting that the country’s “obesity belt” runs through the South.
Heymsfield, who wasn’t part of the new analysis, notes that public messaging around severe COVID-19 risks to younger adults with obesity is “tricky,” especially because the virus is “still pretty common in nonobese people.”
Kass agrees, noting, “it’s difficult to turn to 40% of the population and say: ‘You guys have to watch it.’ ”
But the mounting research findings necessitate linking obesity with severe COVID-19 disease and perhaps testing patients in this category for the virus sooner before symptoms become severe.
And of note, since shortness of breath is common among people with obesity regardless of illness, similar COVID-19 symptoms might catch these individuals unaware, pointed out Heymsfield, who is also a professor in the Metabolism and Body Composition Lab at Pennington Biomedical Research Center at Louisiana State University, Baton Rouge.
“They may find themselves literally unable to breathe, and the concern would be that they wait much too long to come in” for treatment, he said. Typically, people can deteriorate between day 7 and 10 of the COVID-19 infection.
Individuals with obesity “need to be educated to recognize the serious complications of COVID-19 often appear suddenly, although the virus has sometimes been working its way through the body for a long time,” he concluded.
Kass and Heymsfield have declared no relevant financial relationships.
This article first appeared on Medscape.com.
published in The Lancet.
“By itself, obesity seems to be a sufficient risk factor to start seeing younger people landing in the ICU,” said the study’s lead author, David Kass, MD, a professor of cardiology and medicine at Johns Hopkins University School of Medicine in Baltimore, Maryland.
“In that sense, there’s a simple message: If you’re very, very overweight, don’t think that if you’re 35 you’re that much safer [from severe COVID-19] than your mother or grandparents or others in their 60s or 70s,” Kass told Medscape Medical News.
The findings, which Kass describes as a “2-week snapshot” of 265 patients (58% male) in late March and early April at a handful of university hospitals in the United States reinforces other recent research indicating that obesity is one of the biggest risk factors for severe COVID-19 disease, particularly among younger patients. In addition, a large British study showed that, after adjusting for comorbidities, obesity was a significant factor associated with in-hospital death in COVID-19.
But this new analysis stands out as the only dataset to date that specifically “asks the question relative to age” of whether severe COVID-19 disease correlates to ICU treatment, he said.
The mean age of his study population of ICU patients was 55, Kass said, “and that was young, not what we were expecting.”
“Even with the first 20 patients, we were already seeing younger people and they definitely were heavier, with plenty of patients with a BMI over 35 kg/m2,” he added. “The relationship was pretty tight, pretty quick.”
“Just don’t make the assumption that any of us are too young to be vulnerable if, in fact, this is an aspect of our bodies,” he said.
Steven Heymsfield, MD, past president and a spokesperson for the Obesity Society, agrees with Kass’ conclusions.
“One thing we’ve had on our minds is that the prototype of a person with this disease is older...but now if we get [a patient] who’s symptomatic and 40 and obese, we shouldn’t assume they have some other disease,” Heymsfield told Medscape Medical News.
“We should think of them as a susceptible population.”
Kass and colleagues agree. “Public messaging to younger adults, reducing the threshold for virus testing in obese individuals, and maintaining greater vigilance for this at-risk population should reduce the prevalence of severe COVID-19 disease [among those with obesity],” they state.
“I think it’s a mental adjustment from a health care standpoint, which might hopefully help target the folks who are at higher risk before they get into trouble,” Kass told Medscape Medical News.
Trio of mechanisms explain obesity’s extra COVID-19 risks
Kass and coauthors write that, in analyzing their data, they anticipated similar results to the largest study of 1591 ICU patients from Italy in which only 203 were younger than 51 years. Common comorbidities among those patients included hypertension, cardiovascular disease, and type 2 diabetes, with similar data reported from China.
When the COVID-19 epidemic accelerated in the United States, older age was also identified as a risk factor. Obesity had not yet been added to this list, Kass noted. But following informal discussions with colleagues in other ICUs around the country, he decided to investigate further as to whether it was an underappreciated risk factor.
Kass and colleagues did a quick evaluation of the link between BMI and age of patients with COVID-19 admitted to ICUs at Johns Hopkins, University of Cincinnati, New York University, University of Washington, Florida Health, and University of Pennsylvania.
The “significant inverse correlation between age and BMI” showed younger ICU patients were more likely to be obese, with no difference by gender.
Median BMI among study participants was 29.3 kg/m2, with only a quarter having a BMI lower than 26 kg/m2 and another 25% having a BMI higher than 34.7 kg/m2.
Kass acknowledged that it wasn’t possible with this simple dataset to account for any other potential confounders, but he told Medscape Medical News that, “while diabetes, cardiovascular disease, and hypertension, for example, can occur with obesity, this is generally less so in younger populations as it takes time for the other comorbidities to develop.”
He said several mechanisms could explain why obesity predisposes patients with COVID-19 to severe disease.
For one, obesity places extra pressure on the diaphragm while lying on the back, restricting breathing.
“Morbid obesity itself is sort of proinflammatory,” he continued.
“Here we’ve got a viral infection where the early reports suggest that cytokine storms and immune mishandling of the virus are why it’s so much more severe than other forms of coronavirus we’ve seen before. So if you have someone with an already underlying proinflammatory state, this could be a reason there’s higher risk.”
Additionally, the angiotensin-converting enzyme-2 (ACE-2) receptor to which the SARS-CoV-2 virus that causes COVID-19 attaches is expressed in higher amounts in adipose tissue than the lungs, Kass noted.
“This could turn into kind of a viral replication depot,” he explained. “You may well be brewing more virus as a component of obesity.”
Sensitivity needed in public messaging about risks, but test sooner
With an obesity rate of about 40% in the United States, the results are particularly relevant for Americans, Kass and Heymsfield say, noting that the country’s “obesity belt” runs through the South.
Heymsfield, who wasn’t part of the new analysis, notes that public messaging around severe COVID-19 risks to younger adults with obesity is “tricky,” especially because the virus is “still pretty common in nonobese people.”
Kass agrees, noting, “it’s difficult to turn to 40% of the population and say: ‘You guys have to watch it.’ ”
But the mounting research findings necessitate linking obesity with severe COVID-19 disease and perhaps testing patients in this category for the virus sooner before symptoms become severe.
And of note, since shortness of breath is common among people with obesity regardless of illness, similar COVID-19 symptoms might catch these individuals unaware, pointed out Heymsfield, who is also a professor in the Metabolism and Body Composition Lab at Pennington Biomedical Research Center at Louisiana State University, Baton Rouge.
“They may find themselves literally unable to breathe, and the concern would be that they wait much too long to come in” for treatment, he said. Typically, people can deteriorate between day 7 and 10 of the COVID-19 infection.
Individuals with obesity “need to be educated to recognize the serious complications of COVID-19 often appear suddenly, although the virus has sometimes been working its way through the body for a long time,” he concluded.
Kass and Heymsfield have declared no relevant financial relationships.
This article first appeared on Medscape.com.
Evolocumab safe, well-tolerated in HIV+ patients
Evolocumab proved effective, well tolerated, and safe for the treatment of refractory dyslipidemia in persons living with HIV in the phase 3, randomized, double-blind BEIJERINCK study.
At 24 weeks, nearly three-quarters of patients randomized to evolocumab (Repatha) achieved at least a 50% reduction in LDL cholesterol while on maximally tolerated background lipid lowering with a statin and/or other drugs. This was accompanied by significant reductions in other atherogenic lipids, Franck Boccara, MD, PhD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
Evolocumab thus shows the potential to help fill a major unmet need for more effective treatment of dyslipidemia in HIV-positive patients, who number an estimated 38 million worldwide, including 1.1 million in the United States. Access to highly active antiretroviral therapies has transformed HIV infection into a chronic manageable disease, but this major advance has been accompanied by a rate of premature atherosclerotic cardiovascular disease that’s nearly twice that of the general population, observed Dr. Boccara, a cardiologist at Sorbonne University, Paris.
The BEIJERINCK study included 464 HIV-infected patients in the United States and 14 other countries on five continents. Participants had a mean baseline LDL cholesterol of 133 mg/dL and triglycerides of about 190 mg/dL while on maximally tolerated lipid-lowering therapy. They had been diagnosed with HIV an average of 18 years earlier. One-third of them had known atherosclerotic cardiovascular disease. More than one-quarter of participants were cigarette smokers. Patients were randomized 2:1 to 24 weeks of double-blind subcutaneous evolocumab at 420 mg once monthly or placebo, then an additional 24 weeks of open-label evolocumab for all.
The primary endpoint was change in LDL from baseline to week 24: a 56.2% reduction in the evolocumab group and a 0.7% increase with placebo. About 73% of patients on evolocumab achieved at least a 50% reduction in LDL cholesterol, as did less than 1% of controls. Likewise, 73% of the evolocumab group got their LDL cholesterol below 70 mg/dL, compared with 7.9% with placebo.
The evolocumab group also experienced favorable placebo-subtracted differences from baseline of 23% in triglycerides, 27% in lipoprotein(a), and 22% in very-low-density lipoprotein cholesterol.
As was the case in the earlier, much larger landmark clinical trials, evolocumab was well tolerated in BEIJERINCK, with a side effect profile similar to placebo. Notably, there was no increase in liver abnormalities in evolocumab-treated patients on highly active antiretroviral therapy, and no one developed evolocumab neutralizing antibodies.
Dr. Boccara reported receiving a research grant from Amgen, the study sponsor, as well as lecture fees from several other pharmaceutical companies.
Simultaneous with the presentation at ACC 2020, the primary results of the BEIJERINCK study were published online (J Am Coll Cardiol. 2020 Mar 19. doi: 10.1016/j.jacc.2020.03.025).
Evolocumab proved effective, well tolerated, and safe for the treatment of refractory dyslipidemia in persons living with HIV in the phase 3, randomized, double-blind BEIJERINCK study.
At 24 weeks, nearly three-quarters of patients randomized to evolocumab (Repatha) achieved at least a 50% reduction in LDL cholesterol while on maximally tolerated background lipid lowering with a statin and/or other drugs. This was accompanied by significant reductions in other atherogenic lipids, Franck Boccara, MD, PhD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
Evolocumab thus shows the potential to help fill a major unmet need for more effective treatment of dyslipidemia in HIV-positive patients, who number an estimated 38 million worldwide, including 1.1 million in the United States. Access to highly active antiretroviral therapies has transformed HIV infection into a chronic manageable disease, but this major advance has been accompanied by a rate of premature atherosclerotic cardiovascular disease that’s nearly twice that of the general population, observed Dr. Boccara, a cardiologist at Sorbonne University, Paris.
The BEIJERINCK study included 464 HIV-infected patients in the United States and 14 other countries on five continents. Participants had a mean baseline LDL cholesterol of 133 mg/dL and triglycerides of about 190 mg/dL while on maximally tolerated lipid-lowering therapy. They had been diagnosed with HIV an average of 18 years earlier. One-third of them had known atherosclerotic cardiovascular disease. More than one-quarter of participants were cigarette smokers. Patients were randomized 2:1 to 24 weeks of double-blind subcutaneous evolocumab at 420 mg once monthly or placebo, then an additional 24 weeks of open-label evolocumab for all.
The primary endpoint was change in LDL from baseline to week 24: a 56.2% reduction in the evolocumab group and a 0.7% increase with placebo. About 73% of patients on evolocumab achieved at least a 50% reduction in LDL cholesterol, as did less than 1% of controls. Likewise, 73% of the evolocumab group got their LDL cholesterol below 70 mg/dL, compared with 7.9% with placebo.
The evolocumab group also experienced favorable placebo-subtracted differences from baseline of 23% in triglycerides, 27% in lipoprotein(a), and 22% in very-low-density lipoprotein cholesterol.
As was the case in the earlier, much larger landmark clinical trials, evolocumab was well tolerated in BEIJERINCK, with a side effect profile similar to placebo. Notably, there was no increase in liver abnormalities in evolocumab-treated patients on highly active antiretroviral therapy, and no one developed evolocumab neutralizing antibodies.
Dr. Boccara reported receiving a research grant from Amgen, the study sponsor, as well as lecture fees from several other pharmaceutical companies.
Simultaneous with the presentation at ACC 2020, the primary results of the BEIJERINCK study were published online (J Am Coll Cardiol. 2020 Mar 19. doi: 10.1016/j.jacc.2020.03.025).
Evolocumab proved effective, well tolerated, and safe for the treatment of refractory dyslipidemia in persons living with HIV in the phase 3, randomized, double-blind BEIJERINCK study.
At 24 weeks, nearly three-quarters of patients randomized to evolocumab (Repatha) achieved at least a 50% reduction in LDL cholesterol while on maximally tolerated background lipid lowering with a statin and/or other drugs. This was accompanied by significant reductions in other atherogenic lipids, Franck Boccara, MD, PhD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
Evolocumab thus shows the potential to help fill a major unmet need for more effective treatment of dyslipidemia in HIV-positive patients, who number an estimated 38 million worldwide, including 1.1 million in the United States. Access to highly active antiretroviral therapies has transformed HIV infection into a chronic manageable disease, but this major advance has been accompanied by a rate of premature atherosclerotic cardiovascular disease that’s nearly twice that of the general population, observed Dr. Boccara, a cardiologist at Sorbonne University, Paris.
The BEIJERINCK study included 464 HIV-infected patients in the United States and 14 other countries on five continents. Participants had a mean baseline LDL cholesterol of 133 mg/dL and triglycerides of about 190 mg/dL while on maximally tolerated lipid-lowering therapy. They had been diagnosed with HIV an average of 18 years earlier. One-third of them had known atherosclerotic cardiovascular disease. More than one-quarter of participants were cigarette smokers. Patients were randomized 2:1 to 24 weeks of double-blind subcutaneous evolocumab at 420 mg once monthly or placebo, then an additional 24 weeks of open-label evolocumab for all.
The primary endpoint was change in LDL from baseline to week 24: a 56.2% reduction in the evolocumab group and a 0.7% increase with placebo. About 73% of patients on evolocumab achieved at least a 50% reduction in LDL cholesterol, as did less than 1% of controls. Likewise, 73% of the evolocumab group got their LDL cholesterol below 70 mg/dL, compared with 7.9% with placebo.
The evolocumab group also experienced favorable placebo-subtracted differences from baseline of 23% in triglycerides, 27% in lipoprotein(a), and 22% in very-low-density lipoprotein cholesterol.
As was the case in the earlier, much larger landmark clinical trials, evolocumab was well tolerated in BEIJERINCK, with a side effect profile similar to placebo. Notably, there was no increase in liver abnormalities in evolocumab-treated patients on highly active antiretroviral therapy, and no one developed evolocumab neutralizing antibodies.
Dr. Boccara reported receiving a research grant from Amgen, the study sponsor, as well as lecture fees from several other pharmaceutical companies.
Simultaneous with the presentation at ACC 2020, the primary results of the BEIJERINCK study were published online (J Am Coll Cardiol. 2020 Mar 19. doi: 10.1016/j.jacc.2020.03.025).
FROM ACC 2020